User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Erratum
Due to a submission error, the article “A Boxed Warning for Inadequate Psoriasis Treatment” (Cutis. 2016;98:206-207) did not contain the complete author disclosure information. The corrected disclosure statement appears below:
Ms. Kagha and Ms. Anderson report no conflict of interest. Dr. Blauvelt has served as a clinical study investigator and scientific adviser for AbbVie Inc; Amgen, Inc; Boehringer Ingelheim; Celgene Corporation; Dermira Inc; Eli Lilly and Company; Genentech, Inc; GlaxoSmithKline; Janssen Biotech, Inc; Merck & Co; Novartis; Pfizer Inc; Regeneron Pharmaceuticals, Inc; Sandoz, a Novartis Division; Sanofi; Sun Pharmaceutical Industries, Ltd; UCB; and Valeant Pharmaceuticals International, Inc, as well as a paid speaker for Eli Lilly and Company. Dr. Leonardi has served as an advisory board member and consultant for AbbVie Inc; Amgen, Inc; Boehringer Ingelheim; Dermira Inc; Eli Lilly and Company; Janssen Biotech, Inc; LEO Pharma; Pfizer Inc; Sandoz, a Novartis Division; UCB; and Vitae Pharmaceuticals. He also has been an investigator for AbbVie Inc; Actavis Pharma, Inc; Amgen, Inc; Boehringer Ingelheim; Celgene Corporation; Coherus BioSciences; Corrona, LLC; Dermira Inc; Eli Lilly and Company; Galderma Laboratories, LP; Glenmark Pharmaceuticals Inc; Janssen Biotech, Inc; LEO Pharma; Merck & Co; Novartis; Pfizer Inc; Sandoz, a Novartis Division; Stiefel, a GSK company; and Wyeth Pharmaceuticals, Inc. Dr. Leonardi also has been on the speaker’s bureau for AbbVie Inc; Celgene Corporation; Eli Lilly and Company; and Novartis. Dr. Feldman is a consultant, researcher, and/or speaker for AbbVie Inc; Amgen, Inc; Baxter; Boehringer Ingelheim; Celgene Corporation; Janssen Biotech, Inc; Merck & Co; Mylan; Novartis; Pfizer Inc; and Valeant Pharmaceuticals International, Inc.
The staff of Cutis® makes every possible effort to ensure accuracy in its articles and apologizes for the mistake.
Due to a submission error, the article “A Boxed Warning for Inadequate Psoriasis Treatment” (Cutis. 2016;98:206-207) did not contain the complete author disclosure information. The corrected disclosure statement appears below:
Ms. Kagha and Ms. Anderson report no conflict of interest. Dr. Blauvelt has served as a clinical study investigator and scientific adviser for AbbVie Inc; Amgen, Inc; Boehringer Ingelheim; Celgene Corporation; Dermira Inc; Eli Lilly and Company; Genentech, Inc; GlaxoSmithKline; Janssen Biotech, Inc; Merck & Co; Novartis; Pfizer Inc; Regeneron Pharmaceuticals, Inc; Sandoz, a Novartis Division; Sanofi; Sun Pharmaceutical Industries, Ltd; UCB; and Valeant Pharmaceuticals International, Inc, as well as a paid speaker for Eli Lilly and Company. Dr. Leonardi has served as an advisory board member and consultant for AbbVie Inc; Amgen, Inc; Boehringer Ingelheim; Dermira Inc; Eli Lilly and Company; Janssen Biotech, Inc; LEO Pharma; Pfizer Inc; Sandoz, a Novartis Division; UCB; and Vitae Pharmaceuticals. He also has been an investigator for AbbVie Inc; Actavis Pharma, Inc; Amgen, Inc; Boehringer Ingelheim; Celgene Corporation; Coherus BioSciences; Corrona, LLC; Dermira Inc; Eli Lilly and Company; Galderma Laboratories, LP; Glenmark Pharmaceuticals Inc; Janssen Biotech, Inc; LEO Pharma; Merck & Co; Novartis; Pfizer Inc; Sandoz, a Novartis Division; Stiefel, a GSK company; and Wyeth Pharmaceuticals, Inc. Dr. Leonardi also has been on the speaker’s bureau for AbbVie Inc; Celgene Corporation; Eli Lilly and Company; and Novartis. Dr. Feldman is a consultant, researcher, and/or speaker for AbbVie Inc; Amgen, Inc; Baxter; Boehringer Ingelheim; Celgene Corporation; Janssen Biotech, Inc; Merck & Co; Mylan; Novartis; Pfizer Inc; and Valeant Pharmaceuticals International, Inc.
The staff of Cutis® makes every possible effort to ensure accuracy in its articles and apologizes for the mistake.
Due to a submission error, the article “A Boxed Warning for Inadequate Psoriasis Treatment” (Cutis. 2016;98:206-207) did not contain the complete author disclosure information. The corrected disclosure statement appears below:
Ms. Kagha and Ms. Anderson report no conflict of interest. Dr. Blauvelt has served as a clinical study investigator and scientific adviser for AbbVie Inc; Amgen, Inc; Boehringer Ingelheim; Celgene Corporation; Dermira Inc; Eli Lilly and Company; Genentech, Inc; GlaxoSmithKline; Janssen Biotech, Inc; Merck & Co; Novartis; Pfizer Inc; Regeneron Pharmaceuticals, Inc; Sandoz, a Novartis Division; Sanofi; Sun Pharmaceutical Industries, Ltd; UCB; and Valeant Pharmaceuticals International, Inc, as well as a paid speaker for Eli Lilly and Company. Dr. Leonardi has served as an advisory board member and consultant for AbbVie Inc; Amgen, Inc; Boehringer Ingelheim; Dermira Inc; Eli Lilly and Company; Janssen Biotech, Inc; LEO Pharma; Pfizer Inc; Sandoz, a Novartis Division; UCB; and Vitae Pharmaceuticals. He also has been an investigator for AbbVie Inc; Actavis Pharma, Inc; Amgen, Inc; Boehringer Ingelheim; Celgene Corporation; Coherus BioSciences; Corrona, LLC; Dermira Inc; Eli Lilly and Company; Galderma Laboratories, LP; Glenmark Pharmaceuticals Inc; Janssen Biotech, Inc; LEO Pharma; Merck & Co; Novartis; Pfizer Inc; Sandoz, a Novartis Division; Stiefel, a GSK company; and Wyeth Pharmaceuticals, Inc. Dr. Leonardi also has been on the speaker’s bureau for AbbVie Inc; Celgene Corporation; Eli Lilly and Company; and Novartis. Dr. Feldman is a consultant, researcher, and/or speaker for AbbVie Inc; Amgen, Inc; Baxter; Boehringer Ingelheim; Celgene Corporation; Janssen Biotech, Inc; Merck & Co; Mylan; Novartis; Pfizer Inc; and Valeant Pharmaceuticals International, Inc.
The staff of Cutis® makes every possible effort to ensure accuracy in its articles and apologizes for the mistake.
Renewal in Cosmetic Dermatology
It is an exciting time for dermatologists. In the 16 years that I have been in practice our knowledge of disease pathogenesis has increased and has shaped treatments that can now offer life-changing improvement for patients with extensive dermatologic disease. The everyday practice of cosmetic dermatology also has advanced. Sixteen years ago the only cosmetic options we had for our patients were bovine or human collagen injections, traditional CO2 laser resurfacing, and the older generations of pulsed dye lasers. Neuromodulators were just being introduced. We quickly learned the limitations and complications associated with these modalities. Collagen injections were directed at fine perioral lines and lasted 3 to 4 months. The worst complication would be a bruise or an allergic reaction to the bovine collagen. If we really needed to restore volume beyond the perioral regions, our only option was autologous fat transfer. CO2 laser resurfacing was used to firm skin, erase deep wrinkles, and improve sun damage, but its use was limited to older, fair-skinned individuals due to the inherent risk for hypopigmentation and depigmentation. Pulsed dye lasers similarly had no means of cooling the skin, thus they were limited to lighter-skinned individuals and had notable risk for blisters, burns, and hypopigmentation. Since then, our understanding of skin healing, laser-tissue interaction, and facial aging has driven the field to new heights of technological advances and safety. Just as I tell my patients and residents, there is no better time to be in this field than at this moment. Dermatologists have driven the advances behind many of the technologies that are now in widespread use among physicians in a variety of specialties.
Our understanding of facial aging has evolved to include the complex interplay of skeletal change, fat atrophy, and skin aging, which must all be considered when improving a patient’s appearance. Fillers have evolved in physical characteristics to give us the ability to choose between lift, spread, neocollagenesis, or water absorption. Thus, we can select the proper filler for the specific anatomic area we are rejuvenating.
Our understanding of photoaging and laser-tissue physics has allowed for the development of a newer generation of lasers ranging from fractionated lasers to noninvasive modalities that can safely be used in a variety of ethnic skin types to address acne scars, wrinkles, and inflammatory processes such as acne and rosacea. Similarly, the desire to tighten redundant skin has continued to drive ultrasound and radiofrequency technology and sparked growth in a newer field of cryolipolysis and chemical lipolysis agents.
Our dialogue with patients also has evolved to include the 4 R’s of antiaging: resurfacing the skin (eg, lasers, peels), refilling the lost volume (eg, fillers, fat), redraping the excess skin (eg, radiofrequency, ultrasound, laser, surgery), and relaxing dynamic lines (eg, neuromodulators). I propose an additional R: renewal! We must focus on the need for constant renewal so that patients maintain the results we have achieved. I apply the analogy of exercising to get into shape. One must go to the gym regularly to get to the desired level of fitness, but you do not stop exercising, otherwise you will quickly relapse to your former lack of fitness. Similarly, patients should receive the appropriate treatments to bring them to the desired level of rejuvenation, but then some form of constant renewal process is needed to maintain them at that level. Without the stimulation of the skin, the aging process continues and the cycle begins all over again.
In my practice, renewal is achieved by driving the skin to maintain the glow and smoothness that enhances the results of the fillers, neuromodulators, lasers, and peels that we have used. The skin is the first thing people notice. Without the glow, the patient will look good but not great. I tell patients that this part of the process is their responsibility. They must adhere to the skin care regimen specifically designed to address their needs. By incorporating the patient in the rejuvenation process, he/she is empowered to take control over the aging process and has grown more confident in you as a physician.
These are exciting times and there is still so much in the pipeline. By continually learning, reading, and attending workshops and meetings, you can make sure our specialty continue to be the leader in the antiaging field.
It is an exciting time for dermatologists. In the 16 years that I have been in practice our knowledge of disease pathogenesis has increased and has shaped treatments that can now offer life-changing improvement for patients with extensive dermatologic disease. The everyday practice of cosmetic dermatology also has advanced. Sixteen years ago the only cosmetic options we had for our patients were bovine or human collagen injections, traditional CO2 laser resurfacing, and the older generations of pulsed dye lasers. Neuromodulators were just being introduced. We quickly learned the limitations and complications associated with these modalities. Collagen injections were directed at fine perioral lines and lasted 3 to 4 months. The worst complication would be a bruise or an allergic reaction to the bovine collagen. If we really needed to restore volume beyond the perioral regions, our only option was autologous fat transfer. CO2 laser resurfacing was used to firm skin, erase deep wrinkles, and improve sun damage, but its use was limited to older, fair-skinned individuals due to the inherent risk for hypopigmentation and depigmentation. Pulsed dye lasers similarly had no means of cooling the skin, thus they were limited to lighter-skinned individuals and had notable risk for blisters, burns, and hypopigmentation. Since then, our understanding of skin healing, laser-tissue interaction, and facial aging has driven the field to new heights of technological advances and safety. Just as I tell my patients and residents, there is no better time to be in this field than at this moment. Dermatologists have driven the advances behind many of the technologies that are now in widespread use among physicians in a variety of specialties.
Our understanding of facial aging has evolved to include the complex interplay of skeletal change, fat atrophy, and skin aging, which must all be considered when improving a patient’s appearance. Fillers have evolved in physical characteristics to give us the ability to choose between lift, spread, neocollagenesis, or water absorption. Thus, we can select the proper filler for the specific anatomic area we are rejuvenating.
Our understanding of photoaging and laser-tissue physics has allowed for the development of a newer generation of lasers ranging from fractionated lasers to noninvasive modalities that can safely be used in a variety of ethnic skin types to address acne scars, wrinkles, and inflammatory processes such as acne and rosacea. Similarly, the desire to tighten redundant skin has continued to drive ultrasound and radiofrequency technology and sparked growth in a newer field of cryolipolysis and chemical lipolysis agents.
Our dialogue with patients also has evolved to include the 4 R’s of antiaging: resurfacing the skin (eg, lasers, peels), refilling the lost volume (eg, fillers, fat), redraping the excess skin (eg, radiofrequency, ultrasound, laser, surgery), and relaxing dynamic lines (eg, neuromodulators). I propose an additional R: renewal! We must focus on the need for constant renewal so that patients maintain the results we have achieved. I apply the analogy of exercising to get into shape. One must go to the gym regularly to get to the desired level of fitness, but you do not stop exercising, otherwise you will quickly relapse to your former lack of fitness. Similarly, patients should receive the appropriate treatments to bring them to the desired level of rejuvenation, but then some form of constant renewal process is needed to maintain them at that level. Without the stimulation of the skin, the aging process continues and the cycle begins all over again.
In my practice, renewal is achieved by driving the skin to maintain the glow and smoothness that enhances the results of the fillers, neuromodulators, lasers, and peels that we have used. The skin is the first thing people notice. Without the glow, the patient will look good but not great. I tell patients that this part of the process is their responsibility. They must adhere to the skin care regimen specifically designed to address their needs. By incorporating the patient in the rejuvenation process, he/she is empowered to take control over the aging process and has grown more confident in you as a physician.
These are exciting times and there is still so much in the pipeline. By continually learning, reading, and attending workshops and meetings, you can make sure our specialty continue to be the leader in the antiaging field.
It is an exciting time for dermatologists. In the 16 years that I have been in practice our knowledge of disease pathogenesis has increased and has shaped treatments that can now offer life-changing improvement for patients with extensive dermatologic disease. The everyday practice of cosmetic dermatology also has advanced. Sixteen years ago the only cosmetic options we had for our patients were bovine or human collagen injections, traditional CO2 laser resurfacing, and the older generations of pulsed dye lasers. Neuromodulators were just being introduced. We quickly learned the limitations and complications associated with these modalities. Collagen injections were directed at fine perioral lines and lasted 3 to 4 months. The worst complication would be a bruise or an allergic reaction to the bovine collagen. If we really needed to restore volume beyond the perioral regions, our only option was autologous fat transfer. CO2 laser resurfacing was used to firm skin, erase deep wrinkles, and improve sun damage, but its use was limited to older, fair-skinned individuals due to the inherent risk for hypopigmentation and depigmentation. Pulsed dye lasers similarly had no means of cooling the skin, thus they were limited to lighter-skinned individuals and had notable risk for blisters, burns, and hypopigmentation. Since then, our understanding of skin healing, laser-tissue interaction, and facial aging has driven the field to new heights of technological advances and safety. Just as I tell my patients and residents, there is no better time to be in this field than at this moment. Dermatologists have driven the advances behind many of the technologies that are now in widespread use among physicians in a variety of specialties.
Our understanding of facial aging has evolved to include the complex interplay of skeletal change, fat atrophy, and skin aging, which must all be considered when improving a patient’s appearance. Fillers have evolved in physical characteristics to give us the ability to choose between lift, spread, neocollagenesis, or water absorption. Thus, we can select the proper filler for the specific anatomic area we are rejuvenating.
Our understanding of photoaging and laser-tissue physics has allowed for the development of a newer generation of lasers ranging from fractionated lasers to noninvasive modalities that can safely be used in a variety of ethnic skin types to address acne scars, wrinkles, and inflammatory processes such as acne and rosacea. Similarly, the desire to tighten redundant skin has continued to drive ultrasound and radiofrequency technology and sparked growth in a newer field of cryolipolysis and chemical lipolysis agents.
Our dialogue with patients also has evolved to include the 4 R’s of antiaging: resurfacing the skin (eg, lasers, peels), refilling the lost volume (eg, fillers, fat), redraping the excess skin (eg, radiofrequency, ultrasound, laser, surgery), and relaxing dynamic lines (eg, neuromodulators). I propose an additional R: renewal! We must focus on the need for constant renewal so that patients maintain the results we have achieved. I apply the analogy of exercising to get into shape. One must go to the gym regularly to get to the desired level of fitness, but you do not stop exercising, otherwise you will quickly relapse to your former lack of fitness. Similarly, patients should receive the appropriate treatments to bring them to the desired level of rejuvenation, but then some form of constant renewal process is needed to maintain them at that level. Without the stimulation of the skin, the aging process continues and the cycle begins all over again.
In my practice, renewal is achieved by driving the skin to maintain the glow and smoothness that enhances the results of the fillers, neuromodulators, lasers, and peels that we have used. The skin is the first thing people notice. Without the glow, the patient will look good but not great. I tell patients that this part of the process is their responsibility. They must adhere to the skin care regimen specifically designed to address their needs. By incorporating the patient in the rejuvenation process, he/she is empowered to take control over the aging process and has grown more confident in you as a physician.
These are exciting times and there is still so much in the pipeline. By continually learning, reading, and attending workshops and meetings, you can make sure our specialty continue to be the leader in the antiaging field.
How to Increase Patient Adherence to Therapy
How do we increase patient adherence to therapy? This question fascinates me. As dermatologists, we will see thousands of patients over the course of our careers, most with treatable conditions that will improve with therapy and others with chronic or genetic conditions that will at least be made more tolerable with therapy. Only 50% of patients with a chronic condition are adherent to therapy.1 Why some patients adhere to treatment and others do not can be difficult to understand. The emotional makeup, culture, family background, socioeconomic status, and motivation of each person is unique, which leads to complexity. This column is not meant to answer a question that is both complex and broad; rather, it is meant to survey and summarize the literature on this topic.
Education
Health literacy is defined as cognitive and social skills that determine the motivation and ability of individuals to gain access to, understand, and use information in ways that promote and maintain good health.2 Greater health literacy leads to improved compliance and health outcomes.3,4 When we take the time to educate patients about their condition, it improves health literacy, treatment compliance, and patient safety and satisfaction, factors that ultimately are linked to better health outcomes.3-8
There are many practical ways of educating patients. Interestingly, one meta-analysis found that no single strategy is more effective than another.6 This analysis found that "[c]omprehensive interventions combining cognitive, behavioral, and affective components were more effective than single-focus interventions."6 The Centers for Disease Control and Prevention (CDC) website is an excellent source of information on how to educate patients and increase patient treatment compliance.2 The CDC website offers a free tool kit on how to design educational information to your target audience, resources for children, a database of health-related educational images, an electronic textbook on teaching patients with low literacy skills, a summary of evidence-based ideas on how to improve patient adherence to medications used long-term, and more.2
Facilitating Adherence
The World Health Organization (WHO) emphasizes 5 dimensions of patient adherence: health system, socioeconomic, condition-related, therapy-related, and patient-related factors.9 Becker and Maiman5 summarized it eloquently when they wrote that we must take "clinically appropriate steps to reduce the cost, complexity, duration, and amount of behavioral change required by the regimen and increasing the regimen's convenience through 'tailoring' and other approaches." It is a broad ultimatum that will require creativity and persistence on the part of the dermatology community.
Some common patient-related factors associated with nonadherence to treatment are lack of information and skills as they pertain to self-management, difficulty with motivation and self-efficacy, and lack of support for behavioral changes.9 It is interesting that low socioeconomic status has not been consistently shown to portend low treatment adherence. It has been shown that children, especially adolescents, and elderly patients tend to be the least adherent.9-11
Dermatologists Take Action
As dermatologists, the WHO encourages us (physicians) to promote optimism, provide enthusiasm, and encourage maintenance of healthy behaviors.9 Comprehensive interventions that have had a positive impact on patient adherence to therapy for diseases such as diabetes mellitus, asthma, and hypertension may serve as motivating examples.9 Some specific dermatologic conditions that will benefit from increased patient adherence include acne, vesiculobullous disease, psoriasis, and atopic dermatitis. We can lend support to efforts to reduce the cost of dermatologic medications and be aware of the populations most at risk for low adherence to treatment.9-12
Final Thoughts
As we work to increase patient adherence to therapy in dermatology, we will help improve health literacy, patient safety, and patient satisfaction. These factors are ultimately linked to better health outcomes. The CDC and WHO websites are excellent sources of information on practical methods for doing so.2,9
- Haynes RB, McDonald H, Garg AX, et al. Interventions for helping patients to follow prescriptions for medications. Cochrane Database Syst Rev. 2002:CD000011.
- Centers for Disease Control and Prevention. Health literacy. http://www.cdc.gov/healthliteracy/index.html. Updated January 13, 2016. Accessed September 23, 2016.
- Berkman ND, Sheridan SL, Donahue KE, et al. Low health literacy and health outcomes: an updated systematic review. Ann Intern Med. 2011;155:97-107.
- Pignone MP, DeWalt DA. Literacy and health outcomes: is adherence the missing link? J Gen Intern Med. 2006;21:896-897.
- Becker MH, Maiman LA. Strategies for enhancing patient compliance. J Community Health. 1980;6:113-135.
- Roter DL, Hall JA, Merisca R, et al. Effectiveness of interventions to improve patient compliance: a meta-analysis. Med Care. 1998;36:1138-1161.
- Renzi C, Abeni D, Picardi A, et al. Factors associated with patient satisfaction with care among dermatological outpatients. Br J Dermatol. 2001;145:617-623.
- Stewart MA. Effective physician-patient communication and health outcomes: a review. CMAJ. 1995;152:1423-1433.
- World Health Organization. Adherence to long-term therapies: evidence for action. http://www.who.int/chp/knowledge/publications/adherence_full_report.pdf. Posted 2003. Accessed September 23, 2016.
- Lee IA, Maibach HI. Pharmionics in dermatology: a review of topical medication adherence. Am J Clin Dermatol. 2006;7:231-236.
- Burkhart P, Dunbar-Jacob J. Adherence research in the pediatric and adolescent populations: a decade in review. In: Hayman L, Mahon M, Turner R, eds. Chronic Illness in Children: An Evidence-Based Approach. New York, NY: Springer Publishing Company; 2002:199-229.
- Rosenberg ME, Rosenberg SP. Changes in retail prices of prescription dermatologic drugs from 2009 to 2015. JAMA Dermatol. 2016;152:158-163.
How do we increase patient adherence to therapy? This question fascinates me. As dermatologists, we will see thousands of patients over the course of our careers, most with treatable conditions that will improve with therapy and others with chronic or genetic conditions that will at least be made more tolerable with therapy. Only 50% of patients with a chronic condition are adherent to therapy.1 Why some patients adhere to treatment and others do not can be difficult to understand. The emotional makeup, culture, family background, socioeconomic status, and motivation of each person is unique, which leads to complexity. This column is not meant to answer a question that is both complex and broad; rather, it is meant to survey and summarize the literature on this topic.
Education
Health literacy is defined as cognitive and social skills that determine the motivation and ability of individuals to gain access to, understand, and use information in ways that promote and maintain good health.2 Greater health literacy leads to improved compliance and health outcomes.3,4 When we take the time to educate patients about their condition, it improves health literacy, treatment compliance, and patient safety and satisfaction, factors that ultimately are linked to better health outcomes.3-8
There are many practical ways of educating patients. Interestingly, one meta-analysis found that no single strategy is more effective than another.6 This analysis found that "[c]omprehensive interventions combining cognitive, behavioral, and affective components were more effective than single-focus interventions."6 The Centers for Disease Control and Prevention (CDC) website is an excellent source of information on how to educate patients and increase patient treatment compliance.2 The CDC website offers a free tool kit on how to design educational information to your target audience, resources for children, a database of health-related educational images, an electronic textbook on teaching patients with low literacy skills, a summary of evidence-based ideas on how to improve patient adherence to medications used long-term, and more.2
Facilitating Adherence
The World Health Organization (WHO) emphasizes 5 dimensions of patient adherence: health system, socioeconomic, condition-related, therapy-related, and patient-related factors.9 Becker and Maiman5 summarized it eloquently when they wrote that we must take "clinically appropriate steps to reduce the cost, complexity, duration, and amount of behavioral change required by the regimen and increasing the regimen's convenience through 'tailoring' and other approaches." It is a broad ultimatum that will require creativity and persistence on the part of the dermatology community.
Some common patient-related factors associated with nonadherence to treatment are lack of information and skills as they pertain to self-management, difficulty with motivation and self-efficacy, and lack of support for behavioral changes.9 It is interesting that low socioeconomic status has not been consistently shown to portend low treatment adherence. It has been shown that children, especially adolescents, and elderly patients tend to be the least adherent.9-11
Dermatologists Take Action
As dermatologists, the WHO encourages us (physicians) to promote optimism, provide enthusiasm, and encourage maintenance of healthy behaviors.9 Comprehensive interventions that have had a positive impact on patient adherence to therapy for diseases such as diabetes mellitus, asthma, and hypertension may serve as motivating examples.9 Some specific dermatologic conditions that will benefit from increased patient adherence include acne, vesiculobullous disease, psoriasis, and atopic dermatitis. We can lend support to efforts to reduce the cost of dermatologic medications and be aware of the populations most at risk for low adherence to treatment.9-12
Final Thoughts
As we work to increase patient adherence to therapy in dermatology, we will help improve health literacy, patient safety, and patient satisfaction. These factors are ultimately linked to better health outcomes. The CDC and WHO websites are excellent sources of information on practical methods for doing so.2,9
How do we increase patient adherence to therapy? This question fascinates me. As dermatologists, we will see thousands of patients over the course of our careers, most with treatable conditions that will improve with therapy and others with chronic or genetic conditions that will at least be made more tolerable with therapy. Only 50% of patients with a chronic condition are adherent to therapy.1 Why some patients adhere to treatment and others do not can be difficult to understand. The emotional makeup, culture, family background, socioeconomic status, and motivation of each person is unique, which leads to complexity. This column is not meant to answer a question that is both complex and broad; rather, it is meant to survey and summarize the literature on this topic.
Education
Health literacy is defined as cognitive and social skills that determine the motivation and ability of individuals to gain access to, understand, and use information in ways that promote and maintain good health.2 Greater health literacy leads to improved compliance and health outcomes.3,4 When we take the time to educate patients about their condition, it improves health literacy, treatment compliance, and patient safety and satisfaction, factors that ultimately are linked to better health outcomes.3-8
There are many practical ways of educating patients. Interestingly, one meta-analysis found that no single strategy is more effective than another.6 This analysis found that "[c]omprehensive interventions combining cognitive, behavioral, and affective components were more effective than single-focus interventions."6 The Centers for Disease Control and Prevention (CDC) website is an excellent source of information on how to educate patients and increase patient treatment compliance.2 The CDC website offers a free tool kit on how to design educational information to your target audience, resources for children, a database of health-related educational images, an electronic textbook on teaching patients with low literacy skills, a summary of evidence-based ideas on how to improve patient adherence to medications used long-term, and more.2
Facilitating Adherence
The World Health Organization (WHO) emphasizes 5 dimensions of patient adherence: health system, socioeconomic, condition-related, therapy-related, and patient-related factors.9 Becker and Maiman5 summarized it eloquently when they wrote that we must take "clinically appropriate steps to reduce the cost, complexity, duration, and amount of behavioral change required by the regimen and increasing the regimen's convenience through 'tailoring' and other approaches." It is a broad ultimatum that will require creativity and persistence on the part of the dermatology community.
Some common patient-related factors associated with nonadherence to treatment are lack of information and skills as they pertain to self-management, difficulty with motivation and self-efficacy, and lack of support for behavioral changes.9 It is interesting that low socioeconomic status has not been consistently shown to portend low treatment adherence. It has been shown that children, especially adolescents, and elderly patients tend to be the least adherent.9-11
Dermatologists Take Action
As dermatologists, the WHO encourages us (physicians) to promote optimism, provide enthusiasm, and encourage maintenance of healthy behaviors.9 Comprehensive interventions that have had a positive impact on patient adherence to therapy for diseases such as diabetes mellitus, asthma, and hypertension may serve as motivating examples.9 Some specific dermatologic conditions that will benefit from increased patient adherence include acne, vesiculobullous disease, psoriasis, and atopic dermatitis. We can lend support to efforts to reduce the cost of dermatologic medications and be aware of the populations most at risk for low adherence to treatment.9-12
Final Thoughts
As we work to increase patient adherence to therapy in dermatology, we will help improve health literacy, patient safety, and patient satisfaction. These factors are ultimately linked to better health outcomes. The CDC and WHO websites are excellent sources of information on practical methods for doing so.2,9
- Haynes RB, McDonald H, Garg AX, et al. Interventions for helping patients to follow prescriptions for medications. Cochrane Database Syst Rev. 2002:CD000011.
- Centers for Disease Control and Prevention. Health literacy. http://www.cdc.gov/healthliteracy/index.html. Updated January 13, 2016. Accessed September 23, 2016.
- Berkman ND, Sheridan SL, Donahue KE, et al. Low health literacy and health outcomes: an updated systematic review. Ann Intern Med. 2011;155:97-107.
- Pignone MP, DeWalt DA. Literacy and health outcomes: is adherence the missing link? J Gen Intern Med. 2006;21:896-897.
- Becker MH, Maiman LA. Strategies for enhancing patient compliance. J Community Health. 1980;6:113-135.
- Roter DL, Hall JA, Merisca R, et al. Effectiveness of interventions to improve patient compliance: a meta-analysis. Med Care. 1998;36:1138-1161.
- Renzi C, Abeni D, Picardi A, et al. Factors associated with patient satisfaction with care among dermatological outpatients. Br J Dermatol. 2001;145:617-623.
- Stewart MA. Effective physician-patient communication and health outcomes: a review. CMAJ. 1995;152:1423-1433.
- World Health Organization. Adherence to long-term therapies: evidence for action. http://www.who.int/chp/knowledge/publications/adherence_full_report.pdf. Posted 2003. Accessed September 23, 2016.
- Lee IA, Maibach HI. Pharmionics in dermatology: a review of topical medication adherence. Am J Clin Dermatol. 2006;7:231-236.
- Burkhart P, Dunbar-Jacob J. Adherence research in the pediatric and adolescent populations: a decade in review. In: Hayman L, Mahon M, Turner R, eds. Chronic Illness in Children: An Evidence-Based Approach. New York, NY: Springer Publishing Company; 2002:199-229.
- Rosenberg ME, Rosenberg SP. Changes in retail prices of prescription dermatologic drugs from 2009 to 2015. JAMA Dermatol. 2016;152:158-163.
- Haynes RB, McDonald H, Garg AX, et al. Interventions for helping patients to follow prescriptions for medications. Cochrane Database Syst Rev. 2002:CD000011.
- Centers for Disease Control and Prevention. Health literacy. http://www.cdc.gov/healthliteracy/index.html. Updated January 13, 2016. Accessed September 23, 2016.
- Berkman ND, Sheridan SL, Donahue KE, et al. Low health literacy and health outcomes: an updated systematic review. Ann Intern Med. 2011;155:97-107.
- Pignone MP, DeWalt DA. Literacy and health outcomes: is adherence the missing link? J Gen Intern Med. 2006;21:896-897.
- Becker MH, Maiman LA. Strategies for enhancing patient compliance. J Community Health. 1980;6:113-135.
- Roter DL, Hall JA, Merisca R, et al. Effectiveness of interventions to improve patient compliance: a meta-analysis. Med Care. 1998;36:1138-1161.
- Renzi C, Abeni D, Picardi A, et al. Factors associated with patient satisfaction with care among dermatological outpatients. Br J Dermatol. 2001;145:617-623.
- Stewart MA. Effective physician-patient communication and health outcomes: a review. CMAJ. 1995;152:1423-1433.
- World Health Organization. Adherence to long-term therapies: evidence for action. http://www.who.int/chp/knowledge/publications/adherence_full_report.pdf. Posted 2003. Accessed September 23, 2016.
- Lee IA, Maibach HI. Pharmionics in dermatology: a review of topical medication adherence. Am J Clin Dermatol. 2006;7:231-236.
- Burkhart P, Dunbar-Jacob J. Adherence research in the pediatric and adolescent populations: a decade in review. In: Hayman L, Mahon M, Turner R, eds. Chronic Illness in Children: An Evidence-Based Approach. New York, NY: Springer Publishing Company; 2002:199-229.
- Rosenberg ME, Rosenberg SP. Changes in retail prices of prescription dermatologic drugs from 2009 to 2015. JAMA Dermatol. 2016;152:158-163.
Chemical Peels
Review the PDF of the fact sheet on Chemical Peels with board-relevant, easy-to-review material. This fact sheet will review the use of chemical peels for dermatologic indications.
Practice Questions
1. Which peel requires neutralization?
a. Baker-Gordon
b. glycolic acid
c. Jessner
d. salicylic acid
e. trichloroacetic acid
2. Which peel contains resorcinol?
a. Baker-Gordon
b. glycolic acid
c. Jessner
d. salicylic acid
e. trichloroacetic acid
3. Which peel would be the best treatment of severe actinic photodamage?
a. Baker-Gordon
b. glycolic acid
c. Jessner
d. salicylic acid
e. trichloroacetic acid
4. Which peel would not be indicated for treatment of melasma in a patient with Fitzpatrick skin type IV?
a. Baker-Gordon
b. glycolic acid
c. Jessner
d. salicylic acid
e. trichloroacetic acid
5. Which peel is a β-hydroxy acid?
a. Baker-Gordon
b. glycolic acid
c. Jessner
d. salicylic acid
e. trichloroacetic acid
Answers to practice questions provided on next page
Practice Question Answers
1. Which peel requires neutralization?
a. Baker-Gordon
b. glycolic acid
c. Jessner
d. salicylic acid
e. trichloroacetic acid
2. Which peel contains resorcinol?
a. Baker-Gordon
b. glycolic acid
c. Jessner
d. salicylic acid
e. trichloroacetic acid
3. Which peel would be the best treatment of severe actinic photodamage?
a. Baker-Gordon
b. glycolic acid
c. Jessner
d. salicylic acid
e. trichloroacetic acid
4. Which peel would not be indicated for treatment of melasma in a patient with Fitzpatrick skin type IV?
a. Baker-Gordon
b. glycolic acid
c. Jessner
d. salicylic acid
e. trichloroacetic acid
5. Which peel is a β-hydroxy acid?
a. Baker-Gordon
b. glycolic acid
c. Jessner
d. salicylic acid
e. trichloroacetic acid
Review the PDF of the fact sheet on Chemical Peels with board-relevant, easy-to-review material. This fact sheet will review the use of chemical peels for dermatologic indications.
Practice Questions
1. Which peel requires neutralization?
a. Baker-Gordon
b. glycolic acid
c. Jessner
d. salicylic acid
e. trichloroacetic acid
2. Which peel contains resorcinol?
a. Baker-Gordon
b. glycolic acid
c. Jessner
d. salicylic acid
e. trichloroacetic acid
3. Which peel would be the best treatment of severe actinic photodamage?
a. Baker-Gordon
b. glycolic acid
c. Jessner
d. salicylic acid
e. trichloroacetic acid
4. Which peel would not be indicated for treatment of melasma in a patient with Fitzpatrick skin type IV?
a. Baker-Gordon
b. glycolic acid
c. Jessner
d. salicylic acid
e. trichloroacetic acid
5. Which peel is a β-hydroxy acid?
a. Baker-Gordon
b. glycolic acid
c. Jessner
d. salicylic acid
e. trichloroacetic acid
Answers to practice questions provided on next page
Practice Question Answers
1. Which peel requires neutralization?
a. Baker-Gordon
b. glycolic acid
c. Jessner
d. salicylic acid
e. trichloroacetic acid
2. Which peel contains resorcinol?
a. Baker-Gordon
b. glycolic acid
c. Jessner
d. salicylic acid
e. trichloroacetic acid
3. Which peel would be the best treatment of severe actinic photodamage?
a. Baker-Gordon
b. glycolic acid
c. Jessner
d. salicylic acid
e. trichloroacetic acid
4. Which peel would not be indicated for treatment of melasma in a patient with Fitzpatrick skin type IV?
a. Baker-Gordon
b. glycolic acid
c. Jessner
d. salicylic acid
e. trichloroacetic acid
5. Which peel is a β-hydroxy acid?
a. Baker-Gordon
b. glycolic acid
c. Jessner
d. salicylic acid
e. trichloroacetic acid
Review the PDF of the fact sheet on Chemical Peels with board-relevant, easy-to-review material. This fact sheet will review the use of chemical peels for dermatologic indications.
Practice Questions
1. Which peel requires neutralization?
a. Baker-Gordon
b. glycolic acid
c. Jessner
d. salicylic acid
e. trichloroacetic acid
2. Which peel contains resorcinol?
a. Baker-Gordon
b. glycolic acid
c. Jessner
d. salicylic acid
e. trichloroacetic acid
3. Which peel would be the best treatment of severe actinic photodamage?
a. Baker-Gordon
b. glycolic acid
c. Jessner
d. salicylic acid
e. trichloroacetic acid
4. Which peel would not be indicated for treatment of melasma in a patient with Fitzpatrick skin type IV?
a. Baker-Gordon
b. glycolic acid
c. Jessner
d. salicylic acid
e. trichloroacetic acid
5. Which peel is a β-hydroxy acid?
a. Baker-Gordon
b. glycolic acid
c. Jessner
d. salicylic acid
e. trichloroacetic acid
Answers to practice questions provided on next page
Practice Question Answers
1. Which peel requires neutralization?
a. Baker-Gordon
b. glycolic acid
c. Jessner
d. salicylic acid
e. trichloroacetic acid
2. Which peel contains resorcinol?
a. Baker-Gordon
b. glycolic acid
c. Jessner
d. salicylic acid
e. trichloroacetic acid
3. Which peel would be the best treatment of severe actinic photodamage?
a. Baker-Gordon
b. glycolic acid
c. Jessner
d. salicylic acid
e. trichloroacetic acid
4. Which peel would not be indicated for treatment of melasma in a patient with Fitzpatrick skin type IV?
a. Baker-Gordon
b. glycolic acid
c. Jessner
d. salicylic acid
e. trichloroacetic acid
5. Which peel is a β-hydroxy acid?
a. Baker-Gordon
b. glycolic acid
c. Jessner
d. salicylic acid
e. trichloroacetic acid
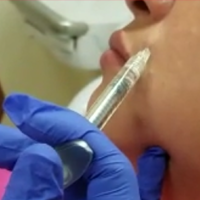
Lip Augmentation With Juvéderm Ultra XC



Evaluating the Malignant Potential of Nevus Spilus
Abnormal Wound Healing Related to High-Dose Systemic Corticosteroid Therapy in a Patient With Ehlers-Danlos Syndrome Benign Hypermobility Type
The process of wound healing has been well characterized. Immediately following injury, neutrophils arrive at the site in response to chemotactic factors produced by the coagulation cascade. Monocytes follow 24 to 36 hours later; transform into macrophages; and begin to phagocytose tissue debris, organisms, and any remaining neutrophils. In turn, macrophages release chemotactic factors such as basic fibroblast growth factor to attract fibroblasts to the wound, which then begin the process of synthesizing collagen and ground substance. Fibroblasts then take over as the dominant cell type, with collagen synthesis continuing for approximately 6 weeks. Keratinocytes and endothelial cells also proliferate during this time. After approximately 6 weeks, collagen remodeling begins. Tensile strength of the wound may continue to increase up to one year after the injury.1,2
Corticosteroids inhibit wound healing in several ways. Notably, they decrease the number of circulating monocytes, leading to fewer macrophages in the tissue at the site of injury, which then leads to impaired phagocytosis and reduced release of chemotactic factors that attract fibroblasts. Additionally, corticosteroids can inhibit collagen synthesis and remodeling, leading to delayed wound healing and decreased tensile strength of the wound as well as impacting capillary proliferation.3
The subtypes of EDS were reclassified in 1998 by Beighton et al,4 and the benign hypermobility type (EDS-BHT)(formerly type III) is considered the least severe. There is some controversy as to whether this subtype constitutes a separate diagnosis from the benign familial joint hypermobility syndrome. It is characterized by hypermobility of the joints (objectively measured with the Beighton scale) and mild hyperextensibility of the skin, and patients often have a history of joint subluxations and dislocations with resultant degenerative joint disease and chronic pain. Manifestations of fragile skin and soft tissue (eg, abnormal wound healing or scarring; spontaneous tearing of the skin, ligaments, tendons, or organs) are notably absent from the findings in this syndrome.5 The genetic basis for EDS is unknown in the majority of patients, although a deficiency in tenascin X (secondary to defects in the tenascin XB gene [TNXB]) has been identified in a small subset (<5%) of patients, leading to elastic fiber abnormalities, reduced collagen deposition, and impaired cross-linking of collagen.6,7 Inheritance usually is autosomal dominant but also can be autosomal recessive. In contrast, the classic type of EDS (formerly types I and II) is associated with atrophic scarring and tissue fragility, in addition to joint hypermobility and skin hyperextensibility. Type V collagen mutations are found in more than half of patients with this disorder.8
We present the case of a patient with EDS-BHT who developed large nonhealing cutaneous ulcerations with initiation of high-dose systemic corticosteroids for treatment of dermatomyositis. This case provides a dramatic illustration of the effects of the use of chronic systemic corticosteroids on skin fragility and wound healing in patients with an underlying inherited defect in collagen or connective tissue.
Case Report
A 23-year-old man with an unremarkable medical history was admitted to our inpatient cardiology service with palpitations attributable to new-onset atrial fibrillation. Dermatology was consulted to evaluate a rash of approximately 4 months’ duration that started on the dorsal aspect of the hands, then progressed to involve the extensor elbows and knees. The rash also was associated with fatigue, arthralgia, and proximal muscle weakness. A taper of prednisone that was prescribed approximately 2 months prior to admission by a rheumatologist for presumed dermatomyositis improved his symptoms, but they recurred with discontinuation of the medication.
Physical examination revealed reddish, violaceous and hyperpigmented patches on the dorsal aspect of the hands and digits and the extensor aspect of the knees and elbows. A skin biopsy from the right elbow showed a mild interface reaction and nonspecific direct immunofluorescence, consistent with a diagnosis of dermatomyositis. Autoimmune serologies were negative, including antinuclear, anti–Jo-1, anti–Mi-2, anti–Sjögren syndrome antigen A, anti–Sjögren syndrome antigen B, anti-Smith, and antiribonucleoprotein antibodies. Creatine kinase and rheumatoid factor levels were within reference range. Electromyogram was supportive of the diagnosis of dermatomyositis, showing an irritable myopathy. Cardiac magnetic resonance imaging showed an acute inflammatory process of the myocardium, and a transthoracic echocardiogram revealed a depressed left ventricular ejection fraction of 35% to 40% (reference range, 55%–70%). His cardiac disease also was attributed to dermatomyositis, and he was managed by cardiology with anangiotensin-converting enzyme inhibitor and antiarrhythmic therapy. Rheumatology was consulted and prednisone 60 mg once daily was started, with the patient reporting improvement in his muscle weakness and the rash.
Interestingly, the patient also noted a history of joint hypermobility, and a genetics consultation was obtained during the current hospitalization. He denied a history of abnormal scarring or skin problems, but he did note dislocation of the patella on 2 occasions and an umbilical hernia repair at 3 years of age. A paternal uncle had a history of similar joint hypermobility. His Beighton score was noted to be 8/8 (bending at the waist was unable to be tested due to recent lumbar puncture obtained during this hospitalization). The patient was diagnosed with EDS-BHT, and no further workup was recommended.
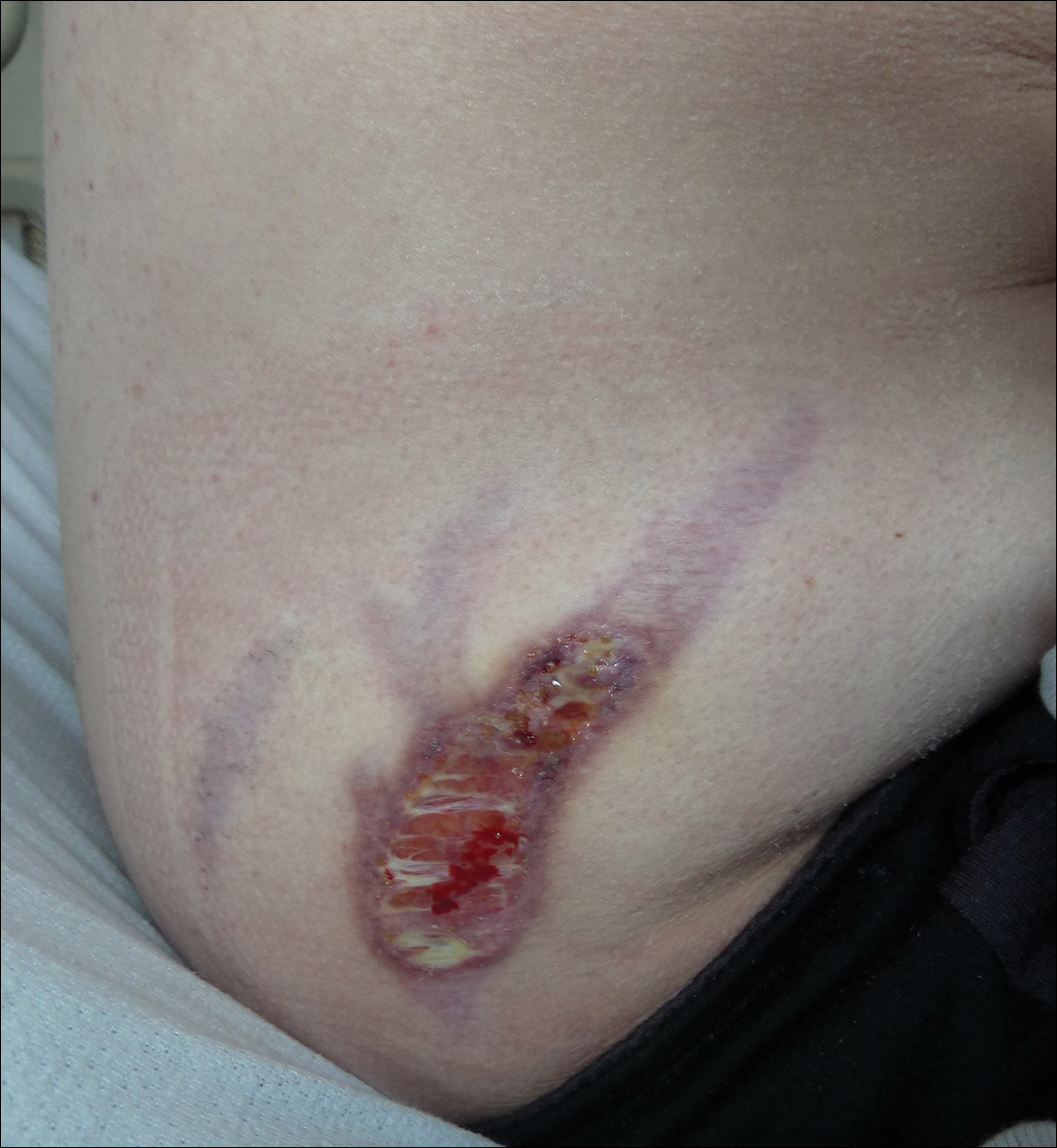
Subsequent to his hospitalization for several days, the patient’s prednisone was slowly tapered down from 60 mg once daily to 12.5 mg once daily, and azathioprine was started and titrated up to 150 mg once daily. Approximately 6 months after his initial hospitalization, he was readmitted due to increased pain of the right knee with concern for osteomyelitis. Dermatology was again consulted, and at this time, the patient reported a 4-month history of nonhealing ulcers to the knees and elbows (Figure 1). He stated that the ulcers were initially about the size of a pencil eraser and had started approximately 2 months after the prednisone was started, with subsequent slow enlargement. He noted a stinging sensation with enlargement of the ulcers, but otherwise they were not painful. He denied major trauma to the areas. He noted that his prior rash from the dermatomyositis seemed to have resolved, along with his muscle weakness, and he reported weight gain and improvement in his energy levels. Physical examination at this time revealed several stigmata of chronic systemic corticosteroids, including fatty deposits in the face (moon facies) and between the shoulders (buffalo hump), facial acne, and numerous erythematous striae on the trunk and proximal extremities (Figure 2). Multiple noninflammatory ulcers with punched-out borders ranging in size from 0.5 to 6 cm were seen at sites overlying bony prominences, including the bilateral extensor elbows and knees and the right plantar foot. Similar ulcers were noted on the trunk within the striae. Some of the ulcers were covered with a thick hyperkeratotic crust. A biopsy from the edge of an ulcer on the right side of the flank showed only dermal fibrosis. Workup by orthopedic surgery was felt to be inconsistent with osteomyelitis, and plastic surgery was consulted to consider surgical options for repair. Consequently, the patient was taken to the operating room for primary closure of the ulcers to the bilateral knees and right elbow. He has been followed closely by plastic surgery, with the use of joint immobilization to promote wound healing.
Comment
This case represents a dramatic illustration of the effects of chronic systemic corticosteroids on skin fragility and wound healing in a patient with an underlying genetic defect in the connective tissue. The ulcers were all located within striae or overlying bony prominences where the skin was subjected to increased tension; however, the patient reported no problems with wound healing or scarring at these sites prior to the initiation of corticosteroids, suggesting that the addition of this medication was disruptive to the cutaneous wound healing mechanisms. This case is unique because abnormal wound healing in an EDS patient was so clearly linked to the initiation of systemic steroids.
The exact pathogenesis of the patient’s ulcers is unclear. The diagnosis of EDS was primarily clinical, and without genetic testing, we cannot state with certainty the underlying molecular problem in this patient. Although tenascin X deficiency has been found in a few patients, a genetic defect remains uncharacterized in most patients with EDS-BHT, and in most situations, EDS-BHT remains a clinical diagnosis. In 2001, Schalkwijk et al9 first described the association of tenascin X deficiency and EDS in 5 patients, and they noted delayed wound healing in 1 patient who had received systemic corticosteroids for congenital adrenal hyperplasia. The authors remarked that it was not clear whether the abnormality was linked to the patient’s EDS or to his treatment with systemic corticosteroids.9 Furthermore, it is possible that our patient in fact has a milder variant of classic type EDS and that the manifestations of tissue fragility remained subclinical until the addition of systemic corticosteroids. It also is interesting to note that muscle weakness can be a symptom of EDS, both classic and BHT of EDS, but our patient’s muscle weakness improved with immunosuppression, supporting an underlying autoimmune disease as the cause for it.10 Skin ulcerations have been reported as a rare manifestation of dermatomyositis, but it is remarkable that his ulcers progressed as his other dermatomyositis symptoms improved with therapy, suggesting that his autoimmune disease was not the underlying cause for the ulcers.11-13 This case points to the need to thoughtfully consider the adverse effects of corticosteroids on wound healing in patients with an inherited disorder of collagen or connective tissue such as EDS.
- Bolognia JL, Jorizzo JL, Rapini RP, et al. Dermatology. 2nd ed. Philadelphia, PA: Mosby Elsevier; 2008.
- Gurtner GC, Werner S, Barrandon Y, et al. Wound repair and regeneration. Nature. 2008;453:314-321.
- Poetker DM, Reh DD. A comprehensive review of the adverse effects of systemic corticosteroids. Otolaryng Clin N Am. 2010;43:753-768.
- Beighton P, De Paepe A, Steinmann B, et al. Ehlers-Danlos syndromes: revised nosology, Villefranche, 1997. Ehlers-Danlos National Foundation (USA) and Ehlers-Danlos Support Group (UK). Am J Med Genet. 1998;77:31-37.
- Levy HP. Ehlers-Danlos syndrome, hypermobility type. In: Pagon RA, Bird TD, Dolan CR, et al, es. GeneReviews. Seattle, WA: University of Washington, Seattle; 1993-2015. http://www.ncbi.nlm.nih.gov/books/NBK1279/. Accessed August 5, 2015.
- Zweers MC, Bristow J, Steijlen PM, et al. Haploinsufficiency of TNXB is associated with hypermobility type of Ehlers-Danlos syndrome. Am J Hum Genet. 2003;73:214-217.
- Brellier F, Tucker RP, Chiquet-Ehrismann R. Tenascins and their implications in diseases and tissue mechanics. Scand J Med Sci Spor. 2009;19:511-519.
- Malfait F, Wenstrup R, De Paepe A. Ehlers-Danlos syndrome, classic type. In: Pagon RA, Bird TD, Dolan CR, et al, eds. GeneReviews. Seattle,WA: University of Washington, Seattle; 1993-2015. http://www.ncbi.nlm.nih.gov/books/NBK1244/. Accessed August 5, 2015.
- Schalkwijk J, Zweers MC, Steijlen PM, et al. A recessive form of the Ehlers-Danlos syndrome caused by tenascin X deficiency. N Engl J Med. 2001;345:1167-1175.
- Voermans NC, Alfen NV, Pillen S, et al. Neuromuscular involvement in various types of Ehlers-Danlos syndrome. Ann Neurol. 2009;65:687-697.
- Scheinfeld NS. Ulcerative paraneoplastic dermatomyositis secondary to metastatic breast cancer. Skinmed. 2006;5:94-96.
- Tomb R, Stephan F. Perforating skin ulcers occurring in an adult with dermatomyositis [in French]. Ann Dermatol Venerol. 2002;129:1383-1385.
- Yosipovitch G, Feinmesser M, David M. Adult dermatomyositis with livedo reticularis and multiple skin ulcers. J Eur Acad Dermatol. 1998;11:48-50.
The process of wound healing has been well characterized. Immediately following injury, neutrophils arrive at the site in response to chemotactic factors produced by the coagulation cascade. Monocytes follow 24 to 36 hours later; transform into macrophages; and begin to phagocytose tissue debris, organisms, and any remaining neutrophils. In turn, macrophages release chemotactic factors such as basic fibroblast growth factor to attract fibroblasts to the wound, which then begin the process of synthesizing collagen and ground substance. Fibroblasts then take over as the dominant cell type, with collagen synthesis continuing for approximately 6 weeks. Keratinocytes and endothelial cells also proliferate during this time. After approximately 6 weeks, collagen remodeling begins. Tensile strength of the wound may continue to increase up to one year after the injury.1,2
Corticosteroids inhibit wound healing in several ways. Notably, they decrease the number of circulating monocytes, leading to fewer macrophages in the tissue at the site of injury, which then leads to impaired phagocytosis and reduced release of chemotactic factors that attract fibroblasts. Additionally, corticosteroids can inhibit collagen synthesis and remodeling, leading to delayed wound healing and decreased tensile strength of the wound as well as impacting capillary proliferation.3
The subtypes of EDS were reclassified in 1998 by Beighton et al,4 and the benign hypermobility type (EDS-BHT)(formerly type III) is considered the least severe. There is some controversy as to whether this subtype constitutes a separate diagnosis from the benign familial joint hypermobility syndrome. It is characterized by hypermobility of the joints (objectively measured with the Beighton scale) and mild hyperextensibility of the skin, and patients often have a history of joint subluxations and dislocations with resultant degenerative joint disease and chronic pain. Manifestations of fragile skin and soft tissue (eg, abnormal wound healing or scarring; spontaneous tearing of the skin, ligaments, tendons, or organs) are notably absent from the findings in this syndrome.5 The genetic basis for EDS is unknown in the majority of patients, although a deficiency in tenascin X (secondary to defects in the tenascin XB gene [TNXB]) has been identified in a small subset (<5%) of patients, leading to elastic fiber abnormalities, reduced collagen deposition, and impaired cross-linking of collagen.6,7 Inheritance usually is autosomal dominant but also can be autosomal recessive. In contrast, the classic type of EDS (formerly types I and II) is associated with atrophic scarring and tissue fragility, in addition to joint hypermobility and skin hyperextensibility. Type V collagen mutations are found in more than half of patients with this disorder.8
We present the case of a patient with EDS-BHT who developed large nonhealing cutaneous ulcerations with initiation of high-dose systemic corticosteroids for treatment of dermatomyositis. This case provides a dramatic illustration of the effects of the use of chronic systemic corticosteroids on skin fragility and wound healing in patients with an underlying inherited defect in collagen or connective tissue.
Case Report
A 23-year-old man with an unremarkable medical history was admitted to our inpatient cardiology service with palpitations attributable to new-onset atrial fibrillation. Dermatology was consulted to evaluate a rash of approximately 4 months’ duration that started on the dorsal aspect of the hands, then progressed to involve the extensor elbows and knees. The rash also was associated with fatigue, arthralgia, and proximal muscle weakness. A taper of prednisone that was prescribed approximately 2 months prior to admission by a rheumatologist for presumed dermatomyositis improved his symptoms, but they recurred with discontinuation of the medication.
Physical examination revealed reddish, violaceous and hyperpigmented patches on the dorsal aspect of the hands and digits and the extensor aspect of the knees and elbows. A skin biopsy from the right elbow showed a mild interface reaction and nonspecific direct immunofluorescence, consistent with a diagnosis of dermatomyositis. Autoimmune serologies were negative, including antinuclear, anti–Jo-1, anti–Mi-2, anti–Sjögren syndrome antigen A, anti–Sjögren syndrome antigen B, anti-Smith, and antiribonucleoprotein antibodies. Creatine kinase and rheumatoid factor levels were within reference range. Electromyogram was supportive of the diagnosis of dermatomyositis, showing an irritable myopathy. Cardiac magnetic resonance imaging showed an acute inflammatory process of the myocardium, and a transthoracic echocardiogram revealed a depressed left ventricular ejection fraction of 35% to 40% (reference range, 55%–70%). His cardiac disease also was attributed to dermatomyositis, and he was managed by cardiology with anangiotensin-converting enzyme inhibitor and antiarrhythmic therapy. Rheumatology was consulted and prednisone 60 mg once daily was started, with the patient reporting improvement in his muscle weakness and the rash.
Interestingly, the patient also noted a history of joint hypermobility, and a genetics consultation was obtained during the current hospitalization. He denied a history of abnormal scarring or skin problems, but he did note dislocation of the patella on 2 occasions and an umbilical hernia repair at 3 years of age. A paternal uncle had a history of similar joint hypermobility. His Beighton score was noted to be 8/8 (bending at the waist was unable to be tested due to recent lumbar puncture obtained during this hospitalization). The patient was diagnosed with EDS-BHT, and no further workup was recommended.
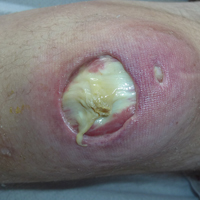
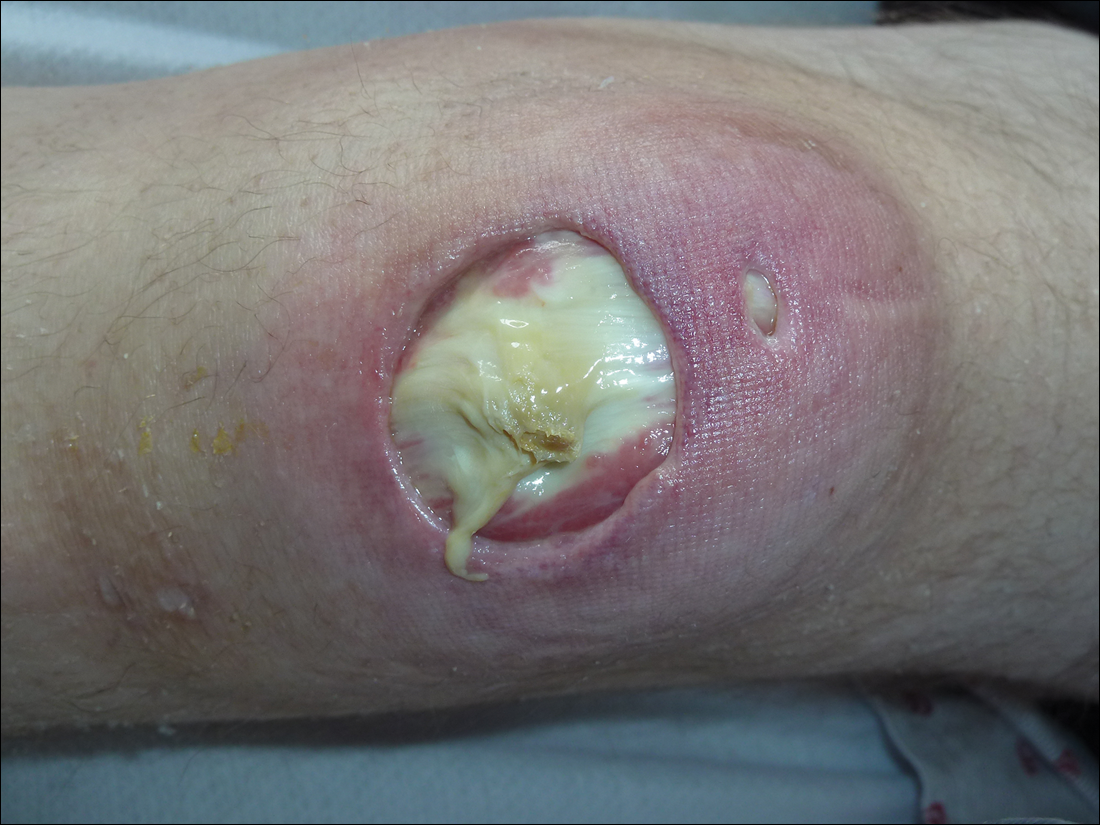
Subsequent to his hospitalization for several days, the patient’s prednisone was slowly tapered down from 60 mg once daily to 12.5 mg once daily, and azathioprine was started and titrated up to 150 mg once daily. Approximately 6 months after his initial hospitalization, he was readmitted due to increased pain of the right knee with concern for osteomyelitis. Dermatology was again consulted, and at this time, the patient reported a 4-month history of nonhealing ulcers to the knees and elbows (Figure 1). He stated that the ulcers were initially about the size of a pencil eraser and had started approximately 2 months after the prednisone was started, with subsequent slow enlargement. He noted a stinging sensation with enlargement of the ulcers, but otherwise they were not painful. He denied major trauma to the areas. He noted that his prior rash from the dermatomyositis seemed to have resolved, along with his muscle weakness, and he reported weight gain and improvement in his energy levels. Physical examination at this time revealed several stigmata of chronic systemic corticosteroids, including fatty deposits in the face (moon facies) and between the shoulders (buffalo hump), facial acne, and numerous erythematous striae on the trunk and proximal extremities (Figure 2). Multiple noninflammatory ulcers with punched-out borders ranging in size from 0.5 to 6 cm were seen at sites overlying bony prominences, including the bilateral extensor elbows and knees and the right plantar foot. Similar ulcers were noted on the trunk within the striae. Some of the ulcers were covered with a thick hyperkeratotic crust. A biopsy from the edge of an ulcer on the right side of the flank showed only dermal fibrosis. Workup by orthopedic surgery was felt to be inconsistent with osteomyelitis, and plastic surgery was consulted to consider surgical options for repair. Consequently, the patient was taken to the operating room for primary closure of the ulcers to the bilateral knees and right elbow. He has been followed closely by plastic surgery, with the use of joint immobilization to promote wound healing.


Comment
This case represents a dramatic illustration of the effects of chronic systemic corticosteroids on skin fragility and wound healing in a patient with an underlying genetic defect in the connective tissue. The ulcers were all located within striae or overlying bony prominences where the skin was subjected to increased tension; however, the patient reported no problems with wound healing or scarring at these sites prior to the initiation of corticosteroids, suggesting that the addition of this medication was disruptive to the cutaneous wound healing mechanisms. This case is unique because abnormal wound healing in an EDS patient was so clearly linked to the initiation of systemic steroids.
The exact pathogenesis of the patient’s ulcers is unclear. The diagnosis of EDS was primarily clinical, and without genetic testing, we cannot state with certainty the underlying molecular problem in this patient. Although tenascin X deficiency has been found in a few patients, a genetic defect remains uncharacterized in most patients with EDS-BHT, and in most situations, EDS-BHT remains a clinical diagnosis. In 2001, Schalkwijk et al9 first described the association of tenascin X deficiency and EDS in 5 patients, and they noted delayed wound healing in 1 patient who had received systemic corticosteroids for congenital adrenal hyperplasia. The authors remarked that it was not clear whether the abnormality was linked to the patient’s EDS or to his treatment with systemic corticosteroids.9 Furthermore, it is possible that our patient in fact has a milder variant of classic type EDS and that the manifestations of tissue fragility remained subclinical until the addition of systemic corticosteroids. It also is interesting to note that muscle weakness can be a symptom of EDS, both classic and BHT of EDS, but our patient’s muscle weakness improved with immunosuppression, supporting an underlying autoimmune disease as the cause for it.10 Skin ulcerations have been reported as a rare manifestation of dermatomyositis, but it is remarkable that his ulcers progressed as his other dermatomyositis symptoms improved with therapy, suggesting that his autoimmune disease was not the underlying cause for the ulcers.11-13 This case points to the need to thoughtfully consider the adverse effects of corticosteroids on wound healing in patients with an inherited disorder of collagen or connective tissue such as EDS.
The process of wound healing has been well characterized. Immediately following injury, neutrophils arrive at the site in response to chemotactic factors produced by the coagulation cascade. Monocytes follow 24 to 36 hours later; transform into macrophages; and begin to phagocytose tissue debris, organisms, and any remaining neutrophils. In turn, macrophages release chemotactic factors such as basic fibroblast growth factor to attract fibroblasts to the wound, which then begin the process of synthesizing collagen and ground substance. Fibroblasts then take over as the dominant cell type, with collagen synthesis continuing for approximately 6 weeks. Keratinocytes and endothelial cells also proliferate during this time. After approximately 6 weeks, collagen remodeling begins. Tensile strength of the wound may continue to increase up to one year after the injury.1,2
Corticosteroids inhibit wound healing in several ways. Notably, they decrease the number of circulating monocytes, leading to fewer macrophages in the tissue at the site of injury, which then leads to impaired phagocytosis and reduced release of chemotactic factors that attract fibroblasts. Additionally, corticosteroids can inhibit collagen synthesis and remodeling, leading to delayed wound healing and decreased tensile strength of the wound as well as impacting capillary proliferation.3
The subtypes of EDS were reclassified in 1998 by Beighton et al,4 and the benign hypermobility type (EDS-BHT)(formerly type III) is considered the least severe. There is some controversy as to whether this subtype constitutes a separate diagnosis from the benign familial joint hypermobility syndrome. It is characterized by hypermobility of the joints (objectively measured with the Beighton scale) and mild hyperextensibility of the skin, and patients often have a history of joint subluxations and dislocations with resultant degenerative joint disease and chronic pain. Manifestations of fragile skin and soft tissue (eg, abnormal wound healing or scarring; spontaneous tearing of the skin, ligaments, tendons, or organs) are notably absent from the findings in this syndrome.5 The genetic basis for EDS is unknown in the majority of patients, although a deficiency in tenascin X (secondary to defects in the tenascin XB gene [TNXB]) has been identified in a small subset (<5%) of patients, leading to elastic fiber abnormalities, reduced collagen deposition, and impaired cross-linking of collagen.6,7 Inheritance usually is autosomal dominant but also can be autosomal recessive. In contrast, the classic type of EDS (formerly types I and II) is associated with atrophic scarring and tissue fragility, in addition to joint hypermobility and skin hyperextensibility. Type V collagen mutations are found in more than half of patients with this disorder.8
We present the case of a patient with EDS-BHT who developed large nonhealing cutaneous ulcerations with initiation of high-dose systemic corticosteroids for treatment of dermatomyositis. This case provides a dramatic illustration of the effects of the use of chronic systemic corticosteroids on skin fragility and wound healing in patients with an underlying inherited defect in collagen or connective tissue.
Case Report
A 23-year-old man with an unremarkable medical history was admitted to our inpatient cardiology service with palpitations attributable to new-onset atrial fibrillation. Dermatology was consulted to evaluate a rash of approximately 4 months’ duration that started on the dorsal aspect of the hands, then progressed to involve the extensor elbows and knees. The rash also was associated with fatigue, arthralgia, and proximal muscle weakness. A taper of prednisone that was prescribed approximately 2 months prior to admission by a rheumatologist for presumed dermatomyositis improved his symptoms, but they recurred with discontinuation of the medication.
Physical examination revealed reddish, violaceous and hyperpigmented patches on the dorsal aspect of the hands and digits and the extensor aspect of the knees and elbows. A skin biopsy from the right elbow showed a mild interface reaction and nonspecific direct immunofluorescence, consistent with a diagnosis of dermatomyositis. Autoimmune serologies were negative, including antinuclear, anti–Jo-1, anti–Mi-2, anti–Sjögren syndrome antigen A, anti–Sjögren syndrome antigen B, anti-Smith, and antiribonucleoprotein antibodies. Creatine kinase and rheumatoid factor levels were within reference range. Electromyogram was supportive of the diagnosis of dermatomyositis, showing an irritable myopathy. Cardiac magnetic resonance imaging showed an acute inflammatory process of the myocardium, and a transthoracic echocardiogram revealed a depressed left ventricular ejection fraction of 35% to 40% (reference range, 55%–70%). His cardiac disease also was attributed to dermatomyositis, and he was managed by cardiology with anangiotensin-converting enzyme inhibitor and antiarrhythmic therapy. Rheumatology was consulted and prednisone 60 mg once daily was started, with the patient reporting improvement in his muscle weakness and the rash.
Interestingly, the patient also noted a history of joint hypermobility, and a genetics consultation was obtained during the current hospitalization. He denied a history of abnormal scarring or skin problems, but he did note dislocation of the patella on 2 occasions and an umbilical hernia repair at 3 years of age. A paternal uncle had a history of similar joint hypermobility. His Beighton score was noted to be 8/8 (bending at the waist was unable to be tested due to recent lumbar puncture obtained during this hospitalization). The patient was diagnosed with EDS-BHT, and no further workup was recommended.
Subsequent to his hospitalization for several days, the patient’s prednisone was slowly tapered down from 60 mg once daily to 12.5 mg once daily, and azathioprine was started and titrated up to 150 mg once daily. Approximately 6 months after his initial hospitalization, he was readmitted due to increased pain of the right knee with concern for osteomyelitis. Dermatology was again consulted, and at this time, the patient reported a 4-month history of nonhealing ulcers to the knees and elbows (Figure 1). He stated that the ulcers were initially about the size of a pencil eraser and had started approximately 2 months after the prednisone was started, with subsequent slow enlargement. He noted a stinging sensation with enlargement of the ulcers, but otherwise they were not painful. He denied major trauma to the areas. He noted that his prior rash from the dermatomyositis seemed to have resolved, along with his muscle weakness, and he reported weight gain and improvement in his energy levels. Physical examination at this time revealed several stigmata of chronic systemic corticosteroids, including fatty deposits in the face (moon facies) and between the shoulders (buffalo hump), facial acne, and numerous erythematous striae on the trunk and proximal extremities (Figure 2). Multiple noninflammatory ulcers with punched-out borders ranging in size from 0.5 to 6 cm were seen at sites overlying bony prominences, including the bilateral extensor elbows and knees and the right plantar foot. Similar ulcers were noted on the trunk within the striae. Some of the ulcers were covered with a thick hyperkeratotic crust. A biopsy from the edge of an ulcer on the right side of the flank showed only dermal fibrosis. Workup by orthopedic surgery was felt to be inconsistent with osteomyelitis, and plastic surgery was consulted to consider surgical options for repair. Consequently, the patient was taken to the operating room for primary closure of the ulcers to the bilateral knees and right elbow. He has been followed closely by plastic surgery, with the use of joint immobilization to promote wound healing.


Comment
This case represents a dramatic illustration of the effects of chronic systemic corticosteroids on skin fragility and wound healing in a patient with an underlying genetic defect in the connective tissue. The ulcers were all located within striae or overlying bony prominences where the skin was subjected to increased tension; however, the patient reported no problems with wound healing or scarring at these sites prior to the initiation of corticosteroids, suggesting that the addition of this medication was disruptive to the cutaneous wound healing mechanisms. This case is unique because abnormal wound healing in an EDS patient was so clearly linked to the initiation of systemic steroids.
The exact pathogenesis of the patient’s ulcers is unclear. The diagnosis of EDS was primarily clinical, and without genetic testing, we cannot state with certainty the underlying molecular problem in this patient. Although tenascin X deficiency has been found in a few patients, a genetic defect remains uncharacterized in most patients with EDS-BHT, and in most situations, EDS-BHT remains a clinical diagnosis. In 2001, Schalkwijk et al9 first described the association of tenascin X deficiency and EDS in 5 patients, and they noted delayed wound healing in 1 patient who had received systemic corticosteroids for congenital adrenal hyperplasia. The authors remarked that it was not clear whether the abnormality was linked to the patient’s EDS or to his treatment with systemic corticosteroids.9 Furthermore, it is possible that our patient in fact has a milder variant of classic type EDS and that the manifestations of tissue fragility remained subclinical until the addition of systemic corticosteroids. It also is interesting to note that muscle weakness can be a symptom of EDS, both classic and BHT of EDS, but our patient’s muscle weakness improved with immunosuppression, supporting an underlying autoimmune disease as the cause for it.10 Skin ulcerations have been reported as a rare manifestation of dermatomyositis, but it is remarkable that his ulcers progressed as his other dermatomyositis symptoms improved with therapy, suggesting that his autoimmune disease was not the underlying cause for the ulcers.11-13 This case points to the need to thoughtfully consider the adverse effects of corticosteroids on wound healing in patients with an inherited disorder of collagen or connective tissue such as EDS.
- Bolognia JL, Jorizzo JL, Rapini RP, et al. Dermatology. 2nd ed. Philadelphia, PA: Mosby Elsevier; 2008.
- Gurtner GC, Werner S, Barrandon Y, et al. Wound repair and regeneration. Nature. 2008;453:314-321.
- Poetker DM, Reh DD. A comprehensive review of the adverse effects of systemic corticosteroids. Otolaryng Clin N Am. 2010;43:753-768.
- Beighton P, De Paepe A, Steinmann B, et al. Ehlers-Danlos syndromes: revised nosology, Villefranche, 1997. Ehlers-Danlos National Foundation (USA) and Ehlers-Danlos Support Group (UK). Am J Med Genet. 1998;77:31-37.
- Levy HP. Ehlers-Danlos syndrome, hypermobility type. In: Pagon RA, Bird TD, Dolan CR, et al, es. GeneReviews. Seattle, WA: University of Washington, Seattle; 1993-2015. http://www.ncbi.nlm.nih.gov/books/NBK1279/. Accessed August 5, 2015.
- Zweers MC, Bristow J, Steijlen PM, et al. Haploinsufficiency of TNXB is associated with hypermobility type of Ehlers-Danlos syndrome. Am J Hum Genet. 2003;73:214-217.
- Brellier F, Tucker RP, Chiquet-Ehrismann R. Tenascins and their implications in diseases and tissue mechanics. Scand J Med Sci Spor. 2009;19:511-519.
- Malfait F, Wenstrup R, De Paepe A. Ehlers-Danlos syndrome, classic type. In: Pagon RA, Bird TD, Dolan CR, et al, eds. GeneReviews. Seattle,WA: University of Washington, Seattle; 1993-2015. http://www.ncbi.nlm.nih.gov/books/NBK1244/. Accessed August 5, 2015.
- Schalkwijk J, Zweers MC, Steijlen PM, et al. A recessive form of the Ehlers-Danlos syndrome caused by tenascin X deficiency. N Engl J Med. 2001;345:1167-1175.
- Voermans NC, Alfen NV, Pillen S, et al. Neuromuscular involvement in various types of Ehlers-Danlos syndrome. Ann Neurol. 2009;65:687-697.
- Scheinfeld NS. Ulcerative paraneoplastic dermatomyositis secondary to metastatic breast cancer. Skinmed. 2006;5:94-96.
- Tomb R, Stephan F. Perforating skin ulcers occurring in an adult with dermatomyositis [in French]. Ann Dermatol Venerol. 2002;129:1383-1385.
- Yosipovitch G, Feinmesser M, David M. Adult dermatomyositis with livedo reticularis and multiple skin ulcers. J Eur Acad Dermatol. 1998;11:48-50.
- Bolognia JL, Jorizzo JL, Rapini RP, et al. Dermatology. 2nd ed. Philadelphia, PA: Mosby Elsevier; 2008.
- Gurtner GC, Werner S, Barrandon Y, et al. Wound repair and regeneration. Nature. 2008;453:314-321.
- Poetker DM, Reh DD. A comprehensive review of the adverse effects of systemic corticosteroids. Otolaryng Clin N Am. 2010;43:753-768.
- Beighton P, De Paepe A, Steinmann B, et al. Ehlers-Danlos syndromes: revised nosology, Villefranche, 1997. Ehlers-Danlos National Foundation (USA) and Ehlers-Danlos Support Group (UK). Am J Med Genet. 1998;77:31-37.
- Levy HP. Ehlers-Danlos syndrome, hypermobility type. In: Pagon RA, Bird TD, Dolan CR, et al, es. GeneReviews. Seattle, WA: University of Washington, Seattle; 1993-2015. http://www.ncbi.nlm.nih.gov/books/NBK1279/. Accessed August 5, 2015.
- Zweers MC, Bristow J, Steijlen PM, et al. Haploinsufficiency of TNXB is associated with hypermobility type of Ehlers-Danlos syndrome. Am J Hum Genet. 2003;73:214-217.
- Brellier F, Tucker RP, Chiquet-Ehrismann R. Tenascins and their implications in diseases and tissue mechanics. Scand J Med Sci Spor. 2009;19:511-519.
- Malfait F, Wenstrup R, De Paepe A. Ehlers-Danlos syndrome, classic type. In: Pagon RA, Bird TD, Dolan CR, et al, eds. GeneReviews. Seattle,WA: University of Washington, Seattle; 1993-2015. http://www.ncbi.nlm.nih.gov/books/NBK1244/. Accessed August 5, 2015.
- Schalkwijk J, Zweers MC, Steijlen PM, et al. A recessive form of the Ehlers-Danlos syndrome caused by tenascin X deficiency. N Engl J Med. 2001;345:1167-1175.
- Voermans NC, Alfen NV, Pillen S, et al. Neuromuscular involvement in various types of Ehlers-Danlos syndrome. Ann Neurol. 2009;65:687-697.
- Scheinfeld NS. Ulcerative paraneoplastic dermatomyositis secondary to metastatic breast cancer. Skinmed. 2006;5:94-96.
- Tomb R, Stephan F. Perforating skin ulcers occurring in an adult with dermatomyositis [in French]. Ann Dermatol Venerol. 2002;129:1383-1385.
- Yosipovitch G, Feinmesser M, David M. Adult dermatomyositis with livedo reticularis and multiple skin ulcers. J Eur Acad Dermatol. 1998;11:48-50.
Practice Points
- Chronic corticosteroids have profound effects on the wound-healing process, and their detrimental effects may be amplified in patients with underlying connective tissue defects.
- Although genetic testing is available, the diagnosis of Ehlers-Danlos syndrome benign hypermobility type usually is made clinically.
Recurrent Cerebriform Connective Tissue Nevus on the Foot of a Patient With Proteus Syndrome
To the Editor:
A 12-year-old girl presented with discomfort and walking limitation caused by cutaneous masses on the plantar aspects of the feet with associated bone abnormalities that had started as several flesh-colored papules on the plantar surface of both feet at the age of 1 year. Over time the lesions gradually enlarged and formed irregular masses, more prominently on the right foot. At the age of 6 years, surgical correction was performed due to increased walking impairment and a skin examination that suggested connective tissue nevus. The results were good. However, the local tissue overgrowth recurred after 1 year. Slowly growing lesions were found at the surgical site, which necessitated hospitalization. Her medical history was negative for other disease. There was no family history of similar skin conditions and her parents were nonconsanguineous.
Physical examination revealed malnutrition and poor development in height as well as difficulty walking. She also had moderate scoliosis with a curve to the left. Dermatological examination showed multiple reddish cerebriform hyperplasia in both plantar areas; the right side was more severely involved (Figure 1A). There was macrodactyly of 2 toes on the right foot (Figure 1B). All results of routine blood examinations were within reference range. There were no abnormalities noted in the abdominal ultrasound and cardiac examinations. Plain radiographs of the spine and feet demonstrated scoliosis and exostosis on the calcaneus and bottom of the scaphoid. Histopathologic examination of tissue from the plantar cerebriform hyperplasia revealed hyperkeratosis, slight acanthosis and papillomatosis in the epidermis, and dense collagen bands and sparse elastic fibers in the dermis (Figure 2).

Given the clinical and radiologic manifestation, the diagnosis of Proteus syndrome (PS) was established. After taking into account the severe discomfort and the success of the first surgery, we performed a resection and full-thickness skin graft surgery once again. The feet recovered without any discomfort in daily life. The appearance of the skin graft area was normal 1 year following surgery (Figure 3). She was treated with spinal plate fixation at another institution, progressed well for 2 years, and was subsequently lost to follow-up.
Proteus syndrome is a multisystem disorder with a difficult diagnosis due to the variability of its manifestations. The worldwide incidence of this rare disorder is less than 1 per 1 million individuals, and it is thought to be caused by a somatic genetic alteration.1 Clinical characteristics include bone abnormalities, vascular malformations, dysregulation of fatty tissue, linear verrucous epidermal nevus, and cerebriform connective tissue nevus (CCTN). Although CCTN is not a common finding in patients with PS, it is considered a fairly specific sign with the greatest impact for the diagnosis of PS.2
The general feature of PS--asymmetric disproportionate overgrowth of tissues--appears at 6 to 18 months of age, which makes it challenging to diagnose disease earlier. The CCTN in our patient was present since 1 year of age.
To make a diagnosis of PS, one must have all the general criteria and various specific criteria. The revised diagnostic criteria for PS are given in the Table.3 According to the diagnostic criteria, our patient fulfilled the mandatory general criteria and had plantar CCTN, epidermal nevus, and dysregulated adipose tissue. The CCTN has notable diagnostic value in mildly affected patients, as it is absent in diseases included in the differential diagnosis such as neurofibromatosis, Klippel-Trenaunay-Weber syndrome, Maffucci syndrome, and Bannayan-Riley-Ruvalcaba syndrome. Hemihyperplasia-multiple lipomatosis syndrome and CLOVES (congenital, lipomatous overgrowth, vascular malformations, epidermal nevi, and scoliosis/spinal/skeletal anomalies) syndrome also can present on the plantar surfaces, and lesions may be overgrown at birth but are softer and compressible, have wrinkles instead of deep folds, and tend to grow with the child rather than disproportionately as in PS.4
The epidermal nevi and vascular malformations generally do not spread or increase in number. In contrast, CCTN in PS grows throughout childhood but tends to remain stable in adulthood.4 Postponing surgical treatment until skin lesions stabilize appears to be the best option. However, for practical purposes, surgical intervention may be required at an earlier phase to address the severe functional and cosmetic consequences. Some patients require multiple orthopedic procedures over the ensuing years or decades to control the hyperplasia.3 New CCTN that developed from the prior surgical incision, macrodactyly of the fourth and fifth right toes, and scoliosis appeared when the patient came to our clinic for retreatment 1 year after the initial presentation. The asymmetrical and disproportionate overgrowth of tissues had moderately accelerated in that period. Considering the increasingly impaired walking, we performed a second surgery. On follow-up visits, the patient expressed improvement in daily life.

Studies had been performed to clarify the genetic bases of PS, and the somatic activating mutation in AKT1 (AKT serine/threonine kinase 1) was reported to be the cause of the disease.5,6 Germline PTEN (phosphatase and tensin homolog) mutations have been identified in some patients with overgrowth abnormalities of PS. However, given the misdiagnosis of PS with PTEN mutations and the notion that a gene alone cannot result in PS, the loss-of-function mutations of LEMD3 that have been reported in familial cutaneous collagenomas also may be related to the abnormal growth of connective and bone tissues that are typical of PS.7,8 Lindhurst et al5 concluded that PS is caused by a somatic activating mutation in AKT1, which proved the hypothesis of somatic mosaicism and implicated activation of the PI3K-AKT pathway in the characteristic clinical findings of overgrowth and tumor susceptibility in this disorder. AKT1 is activated by loss-of-function mutations in PTEN, which explains why patients with these mutations (eg, those with the segmental overgrowth, lipomatosis, arteriovenous malformation, epidermal nevus, SOLAMEN [segmental overgrowth, lipomatosis, arteriovenous malformation, and epidermal nevus] syndrome) and patients with activating mutations in AKT1 (eg, those with PS) have overlapping but distinct clinical manifestations. Molecular genetic testing may be useful to confirm the diagnosis in individuals who meet clinical criteria and to establish the diagnosis in individuals with clinical findings that are ambiguous or mild. Further studies are necessary to progress the understanding and management of PS, which will require cooperation of geneticists, surgeons, and other specialists.
- Popescu MD, Burnei G, Draghici L, et al. Proteus syndrome: a difficult diagnosis and management plan. J Med Life. 2014;7:563-566.
- Schepis C, Greco D, Siragusa M, et al. Cerebriform plantar hyperplasia: the major cutaneous feature of Proteus syndrome. Int J Dermatol. 2008;47:374-376.
- Biesecker L. The challenges of Proteus syndrome: diagnosis and management. Eur J Hum Genet. 2006;14:1151-1157.
- Beachkofsky TM, Sapp JC, Biesecker LG, et al. Progressive overgrowth of the cerebriform connective tissue nevus in patients with Proteus syndrome. J Am Acad Dermatol. 2010;63:799-804.
- Lindhurst MJ, Sapp JC, Teer JK, et al. A mosaic activating mutation in AKT1 associated with the Proteus syndrome. N Engl J Med. 2011;365:611-619.
- Wieland I, Tinschert S, Zenker M. High-level somatic mosaicism of AKT1 c.49G>A mutation in skin scrapings from epidermal nevi enables non-invasive molecular diagnosis in patients with Proteus syndrome. Am J Med Genet A. 2013;161A:889-891.
- Cohen MJ, Turner JT, Biesecker LG. Proteus syndrome: misdiagnosis with PTEN mutations. Am J Med Genet A. 2003;122A:323-324.
- Di Stefani A, Gabellini M, Ferlosio A, et al. Cerebriform plantar hyperplasia: the clinico-pathological hallmark of Proteus syndrome. Acta Derm Venereol. 2011;91:580-581.
To the Editor:
A 12-year-old girl presented with discomfort and walking limitation caused by cutaneous masses on the plantar aspects of the feet with associated bone abnormalities that had started as several flesh-colored papules on the plantar surface of both feet at the age of 1 year. Over time the lesions gradually enlarged and formed irregular masses, more prominently on the right foot. At the age of 6 years, surgical correction was performed due to increased walking impairment and a skin examination that suggested connective tissue nevus. The results were good. However, the local tissue overgrowth recurred after 1 year. Slowly growing lesions were found at the surgical site, which necessitated hospitalization. Her medical history was negative for other disease. There was no family history of similar skin conditions and her parents were nonconsanguineous.
Physical examination revealed malnutrition and poor development in height as well as difficulty walking. She also had moderate scoliosis with a curve to the left. Dermatological examination showed multiple reddish cerebriform hyperplasia in both plantar areas; the right side was more severely involved (Figure 1A). There was macrodactyly of 2 toes on the right foot (Figure 1B). All results of routine blood examinations were within reference range. There were no abnormalities noted in the abdominal ultrasound and cardiac examinations. Plain radiographs of the spine and feet demonstrated scoliosis and exostosis on the calcaneus and bottom of the scaphoid. Histopathologic examination of tissue from the plantar cerebriform hyperplasia revealed hyperkeratosis, slight acanthosis and papillomatosis in the epidermis, and dense collagen bands and sparse elastic fibers in the dermis (Figure 2).


Given the clinical and radiologic manifestation, the diagnosis of Proteus syndrome (PS) was established. After taking into account the severe discomfort and the success of the first surgery, we performed a resection and full-thickness skin graft surgery once again. The feet recovered without any discomfort in daily life. The appearance of the skin graft area was normal 1 year following surgery (Figure 3). She was treated with spinal plate fixation at another institution, progressed well for 2 years, and was subsequently lost to follow-up.

Proteus syndrome is a multisystem disorder with a difficult diagnosis due to the variability of its manifestations. The worldwide incidence of this rare disorder is less than 1 per 1 million individuals, and it is thought to be caused by a somatic genetic alteration.1 Clinical characteristics include bone abnormalities, vascular malformations, dysregulation of fatty tissue, linear verrucous epidermal nevus, and cerebriform connective tissue nevus (CCTN). Although CCTN is not a common finding in patients with PS, it is considered a fairly specific sign with the greatest impact for the diagnosis of PS.2
The general feature of PS--asymmetric disproportionate overgrowth of tissues--appears at 6 to 18 months of age, which makes it challenging to diagnose disease earlier. The CCTN in our patient was present since 1 year of age.
To make a diagnosis of PS, one must have all the general criteria and various specific criteria. The revised diagnostic criteria for PS are given in the Table.3 According to the diagnostic criteria, our patient fulfilled the mandatory general criteria and had plantar CCTN, epidermal nevus, and dysregulated adipose tissue. The CCTN has notable diagnostic value in mildly affected patients, as it is absent in diseases included in the differential diagnosis such as neurofibromatosis, Klippel-Trenaunay-Weber syndrome, Maffucci syndrome, and Bannayan-Riley-Ruvalcaba syndrome. Hemihyperplasia-multiple lipomatosis syndrome and CLOVES (congenital, lipomatous overgrowth, vascular malformations, epidermal nevi, and scoliosis/spinal/skeletal anomalies) syndrome also can present on the plantar surfaces, and lesions may be overgrown at birth but are softer and compressible, have wrinkles instead of deep folds, and tend to grow with the child rather than disproportionately as in PS.4
The epidermal nevi and vascular malformations generally do not spread or increase in number. In contrast, CCTN in PS grows throughout childhood but tends to remain stable in adulthood.4 Postponing surgical treatment until skin lesions stabilize appears to be the best option. However, for practical purposes, surgical intervention may be required at an earlier phase to address the severe functional and cosmetic consequences. Some patients require multiple orthopedic procedures over the ensuing years or decades to control the hyperplasia.3 New CCTN that developed from the prior surgical incision, macrodactyly of the fourth and fifth right toes, and scoliosis appeared when the patient came to our clinic for retreatment 1 year after the initial presentation. The asymmetrical and disproportionate overgrowth of tissues had moderately accelerated in that period. Considering the increasingly impaired walking, we performed a second surgery. On follow-up visits, the patient expressed improvement in daily life.

Studies had been performed to clarify the genetic bases of PS, and the somatic activating mutation in AKT1 (AKT serine/threonine kinase 1) was reported to be the cause of the disease.5,6 Germline PTEN (phosphatase and tensin homolog) mutations have been identified in some patients with overgrowth abnormalities of PS. However, given the misdiagnosis of PS with PTEN mutations and the notion that a gene alone cannot result in PS, the loss-of-function mutations of LEMD3 that have been reported in familial cutaneous collagenomas also may be related to the abnormal growth of connective and bone tissues that are typical of PS.7,8 Lindhurst et al5 concluded that PS is caused by a somatic activating mutation in AKT1, which proved the hypothesis of somatic mosaicism and implicated activation of the PI3K-AKT pathway in the characteristic clinical findings of overgrowth and tumor susceptibility in this disorder. AKT1 is activated by loss-of-function mutations in PTEN, which explains why patients with these mutations (eg, those with the segmental overgrowth, lipomatosis, arteriovenous malformation, epidermal nevus, SOLAMEN [segmental overgrowth, lipomatosis, arteriovenous malformation, and epidermal nevus] syndrome) and patients with activating mutations in AKT1 (eg, those with PS) have overlapping but distinct clinical manifestations. Molecular genetic testing may be useful to confirm the diagnosis in individuals who meet clinical criteria and to establish the diagnosis in individuals with clinical findings that are ambiguous or mild. Further studies are necessary to progress the understanding and management of PS, which will require cooperation of geneticists, surgeons, and other specialists.
To the Editor:
A 12-year-old girl presented with discomfort and walking limitation caused by cutaneous masses on the plantar aspects of the feet with associated bone abnormalities that had started as several flesh-colored papules on the plantar surface of both feet at the age of 1 year. Over time the lesions gradually enlarged and formed irregular masses, more prominently on the right foot. At the age of 6 years, surgical correction was performed due to increased walking impairment and a skin examination that suggested connective tissue nevus. The results were good. However, the local tissue overgrowth recurred after 1 year. Slowly growing lesions were found at the surgical site, which necessitated hospitalization. Her medical history was negative for other disease. There was no family history of similar skin conditions and her parents were nonconsanguineous.
Physical examination revealed malnutrition and poor development in height as well as difficulty walking. She also had moderate scoliosis with a curve to the left. Dermatological examination showed multiple reddish cerebriform hyperplasia in both plantar areas; the right side was more severely involved (Figure 1A). There was macrodactyly of 2 toes on the right foot (Figure 1B). All results of routine blood examinations were within reference range. There were no abnormalities noted in the abdominal ultrasound and cardiac examinations. Plain radiographs of the spine and feet demonstrated scoliosis and exostosis on the calcaneus and bottom of the scaphoid. Histopathologic examination of tissue from the plantar cerebriform hyperplasia revealed hyperkeratosis, slight acanthosis and papillomatosis in the epidermis, and dense collagen bands and sparse elastic fibers in the dermis (Figure 2).


Given the clinical and radiologic manifestation, the diagnosis of Proteus syndrome (PS) was established. After taking into account the severe discomfort and the success of the first surgery, we performed a resection and full-thickness skin graft surgery once again. The feet recovered without any discomfort in daily life. The appearance of the skin graft area was normal 1 year following surgery (Figure 3). She was treated with spinal plate fixation at another institution, progressed well for 2 years, and was subsequently lost to follow-up.

Proteus syndrome is a multisystem disorder with a difficult diagnosis due to the variability of its manifestations. The worldwide incidence of this rare disorder is less than 1 per 1 million individuals, and it is thought to be caused by a somatic genetic alteration.1 Clinical characteristics include bone abnormalities, vascular malformations, dysregulation of fatty tissue, linear verrucous epidermal nevus, and cerebriform connective tissue nevus (CCTN). Although CCTN is not a common finding in patients with PS, it is considered a fairly specific sign with the greatest impact for the diagnosis of PS.2
The general feature of PS--asymmetric disproportionate overgrowth of tissues--appears at 6 to 18 months of age, which makes it challenging to diagnose disease earlier. The CCTN in our patient was present since 1 year of age.
To make a diagnosis of PS, one must have all the general criteria and various specific criteria. The revised diagnostic criteria for PS are given in the Table.3 According to the diagnostic criteria, our patient fulfilled the mandatory general criteria and had plantar CCTN, epidermal nevus, and dysregulated adipose tissue. The CCTN has notable diagnostic value in mildly affected patients, as it is absent in diseases included in the differential diagnosis such as neurofibromatosis, Klippel-Trenaunay-Weber syndrome, Maffucci syndrome, and Bannayan-Riley-Ruvalcaba syndrome. Hemihyperplasia-multiple lipomatosis syndrome and CLOVES (congenital, lipomatous overgrowth, vascular malformations, epidermal nevi, and scoliosis/spinal/skeletal anomalies) syndrome also can present on the plantar surfaces, and lesions may be overgrown at birth but are softer and compressible, have wrinkles instead of deep folds, and tend to grow with the child rather than disproportionately as in PS.4
The epidermal nevi and vascular malformations generally do not spread or increase in number. In contrast, CCTN in PS grows throughout childhood but tends to remain stable in adulthood.4 Postponing surgical treatment until skin lesions stabilize appears to be the best option. However, for practical purposes, surgical intervention may be required at an earlier phase to address the severe functional and cosmetic consequences. Some patients require multiple orthopedic procedures over the ensuing years or decades to control the hyperplasia.3 New CCTN that developed from the prior surgical incision, macrodactyly of the fourth and fifth right toes, and scoliosis appeared when the patient came to our clinic for retreatment 1 year after the initial presentation. The asymmetrical and disproportionate overgrowth of tissues had moderately accelerated in that period. Considering the increasingly impaired walking, we performed a second surgery. On follow-up visits, the patient expressed improvement in daily life.

Studies had been performed to clarify the genetic bases of PS, and the somatic activating mutation in AKT1 (AKT serine/threonine kinase 1) was reported to be the cause of the disease.5,6 Germline PTEN (phosphatase and tensin homolog) mutations have been identified in some patients with overgrowth abnormalities of PS. However, given the misdiagnosis of PS with PTEN mutations and the notion that a gene alone cannot result in PS, the loss-of-function mutations of LEMD3 that have been reported in familial cutaneous collagenomas also may be related to the abnormal growth of connective and bone tissues that are typical of PS.7,8 Lindhurst et al5 concluded that PS is caused by a somatic activating mutation in AKT1, which proved the hypothesis of somatic mosaicism and implicated activation of the PI3K-AKT pathway in the characteristic clinical findings of overgrowth and tumor susceptibility in this disorder. AKT1 is activated by loss-of-function mutations in PTEN, which explains why patients with these mutations (eg, those with the segmental overgrowth, lipomatosis, arteriovenous malformation, epidermal nevus, SOLAMEN [segmental overgrowth, lipomatosis, arteriovenous malformation, and epidermal nevus] syndrome) and patients with activating mutations in AKT1 (eg, those with PS) have overlapping but distinct clinical manifestations. Molecular genetic testing may be useful to confirm the diagnosis in individuals who meet clinical criteria and to establish the diagnosis in individuals with clinical findings that are ambiguous or mild. Further studies are necessary to progress the understanding and management of PS, which will require cooperation of geneticists, surgeons, and other specialists.
- Popescu MD, Burnei G, Draghici L, et al. Proteus syndrome: a difficult diagnosis and management plan. J Med Life. 2014;7:563-566.
- Schepis C, Greco D, Siragusa M, et al. Cerebriform plantar hyperplasia: the major cutaneous feature of Proteus syndrome. Int J Dermatol. 2008;47:374-376.
- Biesecker L. The challenges of Proteus syndrome: diagnosis and management. Eur J Hum Genet. 2006;14:1151-1157.
- Beachkofsky TM, Sapp JC, Biesecker LG, et al. Progressive overgrowth of the cerebriform connective tissue nevus in patients with Proteus syndrome. J Am Acad Dermatol. 2010;63:799-804.
- Lindhurst MJ, Sapp JC, Teer JK, et al. A mosaic activating mutation in AKT1 associated with the Proteus syndrome. N Engl J Med. 2011;365:611-619.
- Wieland I, Tinschert S, Zenker M. High-level somatic mosaicism of AKT1 c.49G>A mutation in skin scrapings from epidermal nevi enables non-invasive molecular diagnosis in patients with Proteus syndrome. Am J Med Genet A. 2013;161A:889-891.
- Cohen MJ, Turner JT, Biesecker LG. Proteus syndrome: misdiagnosis with PTEN mutations. Am J Med Genet A. 2003;122A:323-324.
- Di Stefani A, Gabellini M, Ferlosio A, et al. Cerebriform plantar hyperplasia: the clinico-pathological hallmark of Proteus syndrome. Acta Derm Venereol. 2011;91:580-581.
- Popescu MD, Burnei G, Draghici L, et al. Proteus syndrome: a difficult diagnosis and management plan. J Med Life. 2014;7:563-566.
- Schepis C, Greco D, Siragusa M, et al. Cerebriform plantar hyperplasia: the major cutaneous feature of Proteus syndrome. Int J Dermatol. 2008;47:374-376.
- Biesecker L. The challenges of Proteus syndrome: diagnosis and management. Eur J Hum Genet. 2006;14:1151-1157.
- Beachkofsky TM, Sapp JC, Biesecker LG, et al. Progressive overgrowth of the cerebriform connective tissue nevus in patients with Proteus syndrome. J Am Acad Dermatol. 2010;63:799-804.
- Lindhurst MJ, Sapp JC, Teer JK, et al. A mosaic activating mutation in AKT1 associated with the Proteus syndrome. N Engl J Med. 2011;365:611-619.
- Wieland I, Tinschert S, Zenker M. High-level somatic mosaicism of AKT1 c.49G>A mutation in skin scrapings from epidermal nevi enables non-invasive molecular diagnosis in patients with Proteus syndrome. Am J Med Genet A. 2013;161A:889-891.
- Cohen MJ, Turner JT, Biesecker LG. Proteus syndrome: misdiagnosis with PTEN mutations. Am J Med Genet A. 2003;122A:323-324.
- Di Stefani A, Gabellini M, Ferlosio A, et al. Cerebriform plantar hyperplasia: the clinico-pathological hallmark of Proteus syndrome. Acta Derm Venereol. 2011;91:580-581.
Practice Points
- Proteus syndrome (PS) is a rare mosaic condition characterized by progressive overgrowth of skin, connective tissue, brain tissue, and other tissues.
- A somatic activating mutation of the AKT1 gene has been identified as a cause for developing PS.
- Distinct cutaneous features, including cerebriform connective tissue nevi (CCTN), epidermal nevi, vascular malformations, and adipose abnormalities, can alert the dermatologist to the underlying condition before the onset of asymmetric skeletal overgrowth.
- The CCTN in PS grows throughout childhood but tends to remain stable in adulthood. Postponing surgical treatment until skin lesions stabilize appears to be the best option. However, for practical purposes, surgical intervention may be required at an earlier phase to address the severe functional and cosmetic consequences.
Crusted Plaque in the Umbilicus
The Diagnosis: Sister Mary Joseph Nodule
The umbilical skin biopsy revealed a moderately differentiated adenocarcinoma (Figure) that was positive for cytokeratin 20 and CDX2 and negative for cytokeratin 7 and transcription termination factor 1. The patient subsequently underwent computed tomography of the abdomen and pelvis, which showed multiple soft-tissue nodules on the greater omentum, a soft-tissue density at the umbilicus, and thickening of the gastric mucosa. An upper endoscopy was then performed, which revealed a large fungating ulcerated mass in the stomach. Biopsy of this mass showed an invasive moderately differentiated adenocarcinoma, which was ERBB2 (formerly HER2) negative. Histopathologically, these pleomorphic glands looked similar to the glands seen in the original skin biopsy. With this diagnosis of metastatic gastric adenocarcinoma, our patient chose palliative chemotherapy but declined precipitously and died 2 months after the initial skin biopsy of the umbilical lesion.
When encountering a patient with an umbilical lesion, it is important to consider benign and malignant lesions in the differential diagnosis. A benign lesion may include scar, cyst, pyogenic granuloma, hemangioma, umbilical hernia, endometriosis, polyp, abscess, or the presence of an omphalith.1 Inflammatory dermatoses such as psoriasis or eczema also should be considered. Malignant lesions could be either primary or secondary, with metastatic disease being the most common.2 Sister Mary Joseph nodule (SMJN) is the eponymgiven to an umbilical lesion representing metastatic disease. Sister Mary Joseph was a nurse and surgical assistant to Dr. William Mayo in Rochester, Minnesota, in what is now known as the Mayo Clinic. She is credited to be the first to observe and note the association between an umbilical nodule and intra-abdominal malignancy. Metastasis to the umbilicus is thought to occur by way of contiguous, hematogenous, lymphatic, or direct spread through embryologic remnants from primary cancers of nearby gastrointestinal or pelvic viscera. It is a rare cutaneous sign of internal malignancy, with an estimated prevalence of 1% to 3%.3 The most common primary cancer is gastric adenocarcinoma, though cases of metastasis from pancreatic, endometrial, and less commonly hematopoietic or supradiaphragmatic cancers have been reported.4 It is more common in women, likely due to the addition of gynecologic malignancies.1
The use of dermoscopy has been advocated as an adjuvant tool in delineating benign and malignant umbilical lesions when an atypical polymorphous vascular pattern indicating neovascularization has been observed with neoplastic growth.5 Once a suspicious umbilical lesion is identified, the first step should be to obtain a skin biopsy or to use fine needle aspiration for cytology.6 Biopsy is especially relevant in the background of cancer history because SMJN may present with cancer recurrence.3 Once one of these is obtained, histological and immunohistochemical analysis will guide further workup and diagnosis of the umbilical lesion.
The importance of reviewing such cases lies in the variable presentation of cutaneous metastases such as SMJN and the grim prognosis that accompanies this finding. It presents as a firm indurated plaque or nodule that may present with systemic symptoms suggestive of malignancy, though in 30% of cases it is the sole initial sign.7 The nodule may be painful if ulcerated or fissured. Bloody, serous, or purulent discharge may be present. After diagnosis of an SMJN, most patients succumb to the disease within 12 months. Thus, it is vital for dermatologists to investigate umbilical lesions with great caution and a high index of suspicion.
- Chalya PL, Mabula JB, Rambau PF, et al. Sister Mary Joseph's nodule at a University teaching hospital in northwestern Tanzania: a retrospective review of 34 cases. World J Surg Oncol. 2013;11:151.
- Papalas JA, Selim MA. Metastatic vs primary malignant neoplasms affecting the umbilicus: clinicopathologic features of 77 tumors. Ann Diagn Pathol. 2011;15:237-242.
- Palaniappan M, Jose WM, Mehta A, et al. Umbilical metastasis: a case series of four Sister Joseph nodules from four different visceral malignancies. Curr Oncol. 2010;17:78-81.
- Zhang YL, Selvaggi SM. Metastatic islet cell carcinoma to the umbilicus: diagnosis by fine-needle aspiration. Diagn Cytopathol. 2003;29:91-94.
- Mun JH, Kim JM, Ko HC, et al. Dermoscopy of a Sister Mary Joseph nodule. J Am Acad Dermatol. 2013;68:e190-e192.
- Handa U, Garg S, Mohan H. Fine-needle aspiration of Sister Mary Joseph's (paraumbilical) nodules. Diagn Cytopathol. 2008;36:348-350.
- Abu-Hilal M, Newman JS. Sister Mary Joseph and her nodule: historical and clinical perspective. Am J Med Sci. 2009;337:271-273.
The Diagnosis: Sister Mary Joseph Nodule
The umbilical skin biopsy revealed a moderately differentiated adenocarcinoma (Figure) that was positive for cytokeratin 20 and CDX2 and negative for cytokeratin 7 and transcription termination factor 1. The patient subsequently underwent computed tomography of the abdomen and pelvis, which showed multiple soft-tissue nodules on the greater omentum, a soft-tissue density at the umbilicus, and thickening of the gastric mucosa. An upper endoscopy was then performed, which revealed a large fungating ulcerated mass in the stomach. Biopsy of this mass showed an invasive moderately differentiated adenocarcinoma, which was ERBB2 (formerly HER2) negative. Histopathologically, these pleomorphic glands looked similar to the glands seen in the original skin biopsy. With this diagnosis of metastatic gastric adenocarcinoma, our patient chose palliative chemotherapy but declined precipitously and died 2 months after the initial skin biopsy of the umbilical lesion.

When encountering a patient with an umbilical lesion, it is important to consider benign and malignant lesions in the differential diagnosis. A benign lesion may include scar, cyst, pyogenic granuloma, hemangioma, umbilical hernia, endometriosis, polyp, abscess, or the presence of an omphalith.1 Inflammatory dermatoses such as psoriasis or eczema also should be considered. Malignant lesions could be either primary or secondary, with metastatic disease being the most common.2 Sister Mary Joseph nodule (SMJN) is the eponymgiven to an umbilical lesion representing metastatic disease. Sister Mary Joseph was a nurse and surgical assistant to Dr. William Mayo in Rochester, Minnesota, in what is now known as the Mayo Clinic. She is credited to be the first to observe and note the association between an umbilical nodule and intra-abdominal malignancy. Metastasis to the umbilicus is thought to occur by way of contiguous, hematogenous, lymphatic, or direct spread through embryologic remnants from primary cancers of nearby gastrointestinal or pelvic viscera. It is a rare cutaneous sign of internal malignancy, with an estimated prevalence of 1% to 3%.3 The most common primary cancer is gastric adenocarcinoma, though cases of metastasis from pancreatic, endometrial, and less commonly hematopoietic or supradiaphragmatic cancers have been reported.4 It is more common in women, likely due to the addition of gynecologic malignancies.1
The use of dermoscopy has been advocated as an adjuvant tool in delineating benign and malignant umbilical lesions when an atypical polymorphous vascular pattern indicating neovascularization has been observed with neoplastic growth.5 Once a suspicious umbilical lesion is identified, the first step should be to obtain a skin biopsy or to use fine needle aspiration for cytology.6 Biopsy is especially relevant in the background of cancer history because SMJN may present with cancer recurrence.3 Once one of these is obtained, histological and immunohistochemical analysis will guide further workup and diagnosis of the umbilical lesion.
The importance of reviewing such cases lies in the variable presentation of cutaneous metastases such as SMJN and the grim prognosis that accompanies this finding. It presents as a firm indurated plaque or nodule that may present with systemic symptoms suggestive of malignancy, though in 30% of cases it is the sole initial sign.7 The nodule may be painful if ulcerated or fissured. Bloody, serous, or purulent discharge may be present. After diagnosis of an SMJN, most patients succumb to the disease within 12 months. Thus, it is vital for dermatologists to investigate umbilical lesions with great caution and a high index of suspicion.
The Diagnosis: Sister Mary Joseph Nodule
The umbilical skin biopsy revealed a moderately differentiated adenocarcinoma (Figure) that was positive for cytokeratin 20 and CDX2 and negative for cytokeratin 7 and transcription termination factor 1. The patient subsequently underwent computed tomography of the abdomen and pelvis, which showed multiple soft-tissue nodules on the greater omentum, a soft-tissue density at the umbilicus, and thickening of the gastric mucosa. An upper endoscopy was then performed, which revealed a large fungating ulcerated mass in the stomach. Biopsy of this mass showed an invasive moderately differentiated adenocarcinoma, which was ERBB2 (formerly HER2) negative. Histopathologically, these pleomorphic glands looked similar to the glands seen in the original skin biopsy. With this diagnosis of metastatic gastric adenocarcinoma, our patient chose palliative chemotherapy but declined precipitously and died 2 months after the initial skin biopsy of the umbilical lesion.

When encountering a patient with an umbilical lesion, it is important to consider benign and malignant lesions in the differential diagnosis. A benign lesion may include scar, cyst, pyogenic granuloma, hemangioma, umbilical hernia, endometriosis, polyp, abscess, or the presence of an omphalith.1 Inflammatory dermatoses such as psoriasis or eczema also should be considered. Malignant lesions could be either primary or secondary, with metastatic disease being the most common.2 Sister Mary Joseph nodule (SMJN) is the eponymgiven to an umbilical lesion representing metastatic disease. Sister Mary Joseph was a nurse and surgical assistant to Dr. William Mayo in Rochester, Minnesota, in what is now known as the Mayo Clinic. She is credited to be the first to observe and note the association between an umbilical nodule and intra-abdominal malignancy. Metastasis to the umbilicus is thought to occur by way of contiguous, hematogenous, lymphatic, or direct spread through embryologic remnants from primary cancers of nearby gastrointestinal or pelvic viscera. It is a rare cutaneous sign of internal malignancy, with an estimated prevalence of 1% to 3%.3 The most common primary cancer is gastric adenocarcinoma, though cases of metastasis from pancreatic, endometrial, and less commonly hematopoietic or supradiaphragmatic cancers have been reported.4 It is more common in women, likely due to the addition of gynecologic malignancies.1
The use of dermoscopy has been advocated as an adjuvant tool in delineating benign and malignant umbilical lesions when an atypical polymorphous vascular pattern indicating neovascularization has been observed with neoplastic growth.5 Once a suspicious umbilical lesion is identified, the first step should be to obtain a skin biopsy or to use fine needle aspiration for cytology.6 Biopsy is especially relevant in the background of cancer history because SMJN may present with cancer recurrence.3 Once one of these is obtained, histological and immunohistochemical analysis will guide further workup and diagnosis of the umbilical lesion.
The importance of reviewing such cases lies in the variable presentation of cutaneous metastases such as SMJN and the grim prognosis that accompanies this finding. It presents as a firm indurated plaque or nodule that may present with systemic symptoms suggestive of malignancy, though in 30% of cases it is the sole initial sign.7 The nodule may be painful if ulcerated or fissured. Bloody, serous, or purulent discharge may be present. After diagnosis of an SMJN, most patients succumb to the disease within 12 months. Thus, it is vital for dermatologists to investigate umbilical lesions with great caution and a high index of suspicion.
- Chalya PL, Mabula JB, Rambau PF, et al. Sister Mary Joseph's nodule at a University teaching hospital in northwestern Tanzania: a retrospective review of 34 cases. World J Surg Oncol. 2013;11:151.
- Papalas JA, Selim MA. Metastatic vs primary malignant neoplasms affecting the umbilicus: clinicopathologic features of 77 tumors. Ann Diagn Pathol. 2011;15:237-242.
- Palaniappan M, Jose WM, Mehta A, et al. Umbilical metastasis: a case series of four Sister Joseph nodules from four different visceral malignancies. Curr Oncol. 2010;17:78-81.
- Zhang YL, Selvaggi SM. Metastatic islet cell carcinoma to the umbilicus: diagnosis by fine-needle aspiration. Diagn Cytopathol. 2003;29:91-94.
- Mun JH, Kim JM, Ko HC, et al. Dermoscopy of a Sister Mary Joseph nodule. J Am Acad Dermatol. 2013;68:e190-e192.
- Handa U, Garg S, Mohan H. Fine-needle aspiration of Sister Mary Joseph's (paraumbilical) nodules. Diagn Cytopathol. 2008;36:348-350.
- Abu-Hilal M, Newman JS. Sister Mary Joseph and her nodule: historical and clinical perspective. Am J Med Sci. 2009;337:271-273.
- Chalya PL, Mabula JB, Rambau PF, et al. Sister Mary Joseph's nodule at a University teaching hospital in northwestern Tanzania: a retrospective review of 34 cases. World J Surg Oncol. 2013;11:151.
- Papalas JA, Selim MA. Metastatic vs primary malignant neoplasms affecting the umbilicus: clinicopathologic features of 77 tumors. Ann Diagn Pathol. 2011;15:237-242.
- Palaniappan M, Jose WM, Mehta A, et al. Umbilical metastasis: a case series of four Sister Joseph nodules from four different visceral malignancies. Curr Oncol. 2010;17:78-81.
- Zhang YL, Selvaggi SM. Metastatic islet cell carcinoma to the umbilicus: diagnosis by fine-needle aspiration. Diagn Cytopathol. 2003;29:91-94.
- Mun JH, Kim JM, Ko HC, et al. Dermoscopy of a Sister Mary Joseph nodule. J Am Acad Dermatol. 2013;68:e190-e192.
- Handa U, Garg S, Mohan H. Fine-needle aspiration of Sister Mary Joseph's (paraumbilical) nodules. Diagn Cytopathol. 2008;36:348-350.
- Abu-Hilal M, Newman JS. Sister Mary Joseph and her nodule: historical and clinical perspective. Am J Med Sci. 2009;337:271-273.
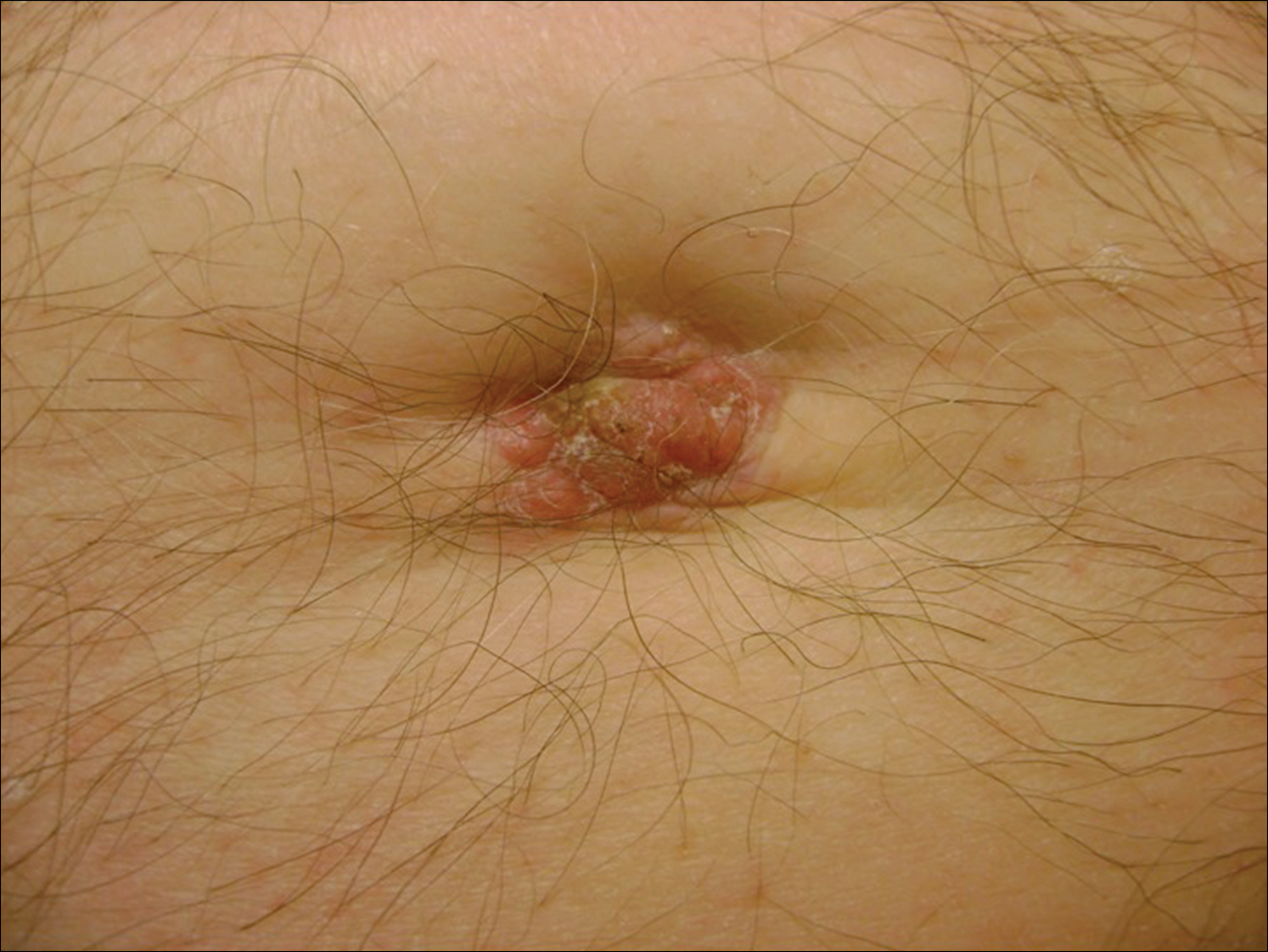
A 74-year-old man presented to our outpatient dermatology clinic with an asymptomatic umbilical lesion of unknown duration. The patient believed the lesion was a scar resulting from a prior laparoscopic repair of an umbilical hernia. However, the patient reported epigastric abdominal pain and diarrhea of 1 month's duration that he believed was due to the stomach flu. The patient denied fever, chills, loss of appetite, or weight loss. History was remarkable for hypertension, hyperlipidemia, coronary artery disease, chronic kidney disease, and emphysema. The patient had a surgical history of percutaneous transluminal coronary angioplasty in addition to the laparoscopic umbilical hernia repair. The patient's medications included pantoprazole, ondansetron, diphenoxylate-atropine as needed, amlodipine, lisinopril-hydrochlorothiazide, simvastatin, and aspirin. Physical examination revealed a 1×2-cm pink, nodular, firm plaque with crust at the umbilicus that was tender on palpation. A shave biopsy of the umbilicus was performed and sent for both pathological and immunohistochemical analysis.