User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Knowledge of the Platysma Muscle Anatomy in the Face Can Improve Cosmetic Outcomes
The platysma muscle has been widely studied in its course through the cervical region in an attempt to develop more effective surgical approaches for rejuvenation. As a person ages, the platysma muscle becomes thinner, less defined, and ptotic, which results in the clinical appearance of a sagging neck that prompts patients to undergo neck-lift procedures. However, little is known about the course of the platysma in the mid and lower face and the implications on facial aging.
Bae et al (Plast Reconstr Surg. 2016;138:365-371) performed a cadaveric dissection of the facial portion of the platysma muscle to delineate the morphology and extension of the muscle in the mid and lower face. Thirty-four adult hemifaces were dissected. The demographics were broken down by age (mean, 71.4 years; range, 41–93 years), gender (29 men; 5 women), and ethnicity (14 Korean; 20 Thai). The extension of the platysma was documented according to a grid the authors created. The grid was divided along 3 horizontal lines (H1–H3) and 3 vertical lines (V1–V3). The intersection of these lines created a grid that was comprised of 3×3 squares and was labeled superior (S1–S3), middle (M1–M3), and inferior (I1–I3). H1 was a line drawn horizontally from the tragus to the lateral orbit, H2 was a line from the earlobe to the nasal ala, and H3 was a line from the angle of the mandible to the corner of the mouth.
The extension pattern of the facial portion of the platysma muscle was classified into patterns: A (n=3; S1–S2, M1–M3, and I1-I3 were covered by the muscle), B-1 (n=20; M1–M3, I1–I3), B-2 (n=9; M1–M2, I1–I3), and C (n=2; I1-I3).
The platysma muscle is divided into anterior and posterior portions. The anterior platysma ascends superiorly and medially in the face to interdigitate with the depressor anguli oris and the depressor labii inferioris muscles. The morphology pattern of the posterior platysma varied and was classified into 1 of 3 patterns: straight (n=13), straight-curved (n=18), and curved (n=3). The straight pattern showed platysma fibers that travel parallel to the mandibular malar line and head toward the zygomaticus major or the orbicularis oculi muscles. The straight-curved type has fibers that travel straight from the neck to the face but then curve, heading toward the risorius, zygomaticus major, or orbicularis oculi muscle depending on the extent of the platysma muscle in the area. The curved type has fibers that run parallel to the zygomaticus arch and interdigitate with the orbicularis oculi muscle and either the risorius or zygomaticus major muscle.
What’s the issue?
For many years, the cervical platysma muscle was studied and surgical approaches were modified to enhance results. The authors of this study have taken the study of the platysma muscle further to delineate its course through the face. Knowledge of the various pathways of the muscle can help us determine the most natural direction to resuspend ptotic facial tissue during surgery and minimally invasive procedures such as thread-lifting. The most salient points include: (1) the lower one-third of the masseter muscle is covered by the platysma, and (2) straight-curved was the most common morphology type, suggesting that the vector for repositioning the platysma muscle is vertical.
Although Bae et al do not discuss the use of botulinum toxin (BTX) in the treatment of the platysma, the results of this study further support the importance of BTX to the lower face. We can look back at more than 14 years of cosmetic use of BTX for the glabella and see the reduced number of brow-lift surgeries nationwide. Although the preventative effects of BTX on brow depression still need to be scientifically proven, it may behoove us to think preventatively on the use of BTX in the platysma to minimize ptosis of the lower face and neck. The results of this anatomic study support the importance of tailoring the approach to the dynamic lines caused by the platysma muscle in each patient to address jowls and neck bands. Have you been offering lower face BTX treatments to patients in an effort to reduce lower face aging?
The platysma muscle has been widely studied in its course through the cervical region in an attempt to develop more effective surgical approaches for rejuvenation. As a person ages, the platysma muscle becomes thinner, less defined, and ptotic, which results in the clinical appearance of a sagging neck that prompts patients to undergo neck-lift procedures. However, little is known about the course of the platysma in the mid and lower face and the implications on facial aging.
Bae et al (Plast Reconstr Surg. 2016;138:365-371) performed a cadaveric dissection of the facial portion of the platysma muscle to delineate the morphology and extension of the muscle in the mid and lower face. Thirty-four adult hemifaces were dissected. The demographics were broken down by age (mean, 71.4 years; range, 41–93 years), gender (29 men; 5 women), and ethnicity (14 Korean; 20 Thai). The extension of the platysma was documented according to a grid the authors created. The grid was divided along 3 horizontal lines (H1–H3) and 3 vertical lines (V1–V3). The intersection of these lines created a grid that was comprised of 3×3 squares and was labeled superior (S1–S3), middle (M1–M3), and inferior (I1–I3). H1 was a line drawn horizontally from the tragus to the lateral orbit, H2 was a line from the earlobe to the nasal ala, and H3 was a line from the angle of the mandible to the corner of the mouth.
The extension pattern of the facial portion of the platysma muscle was classified into patterns: A (n=3; S1–S2, M1–M3, and I1-I3 were covered by the muscle), B-1 (n=20; M1–M3, I1–I3), B-2 (n=9; M1–M2, I1–I3), and C (n=2; I1-I3).
The platysma muscle is divided into anterior and posterior portions. The anterior platysma ascends superiorly and medially in the face to interdigitate with the depressor anguli oris and the depressor labii inferioris muscles. The morphology pattern of the posterior platysma varied and was classified into 1 of 3 patterns: straight (n=13), straight-curved (n=18), and curved (n=3). The straight pattern showed platysma fibers that travel parallel to the mandibular malar line and head toward the zygomaticus major or the orbicularis oculi muscles. The straight-curved type has fibers that travel straight from the neck to the face but then curve, heading toward the risorius, zygomaticus major, or orbicularis oculi muscle depending on the extent of the platysma muscle in the area. The curved type has fibers that run parallel to the zygomaticus arch and interdigitate with the orbicularis oculi muscle and either the risorius or zygomaticus major muscle.
What’s the issue?
For many years, the cervical platysma muscle was studied and surgical approaches were modified to enhance results. The authors of this study have taken the study of the platysma muscle further to delineate its course through the face. Knowledge of the various pathways of the muscle can help us determine the most natural direction to resuspend ptotic facial tissue during surgery and minimally invasive procedures such as thread-lifting. The most salient points include: (1) the lower one-third of the masseter muscle is covered by the platysma, and (2) straight-curved was the most common morphology type, suggesting that the vector for repositioning the platysma muscle is vertical.
Although Bae et al do not discuss the use of botulinum toxin (BTX) in the treatment of the platysma, the results of this study further support the importance of BTX to the lower face. We can look back at more than 14 years of cosmetic use of BTX for the glabella and see the reduced number of brow-lift surgeries nationwide. Although the preventative effects of BTX on brow depression still need to be scientifically proven, it may behoove us to think preventatively on the use of BTX in the platysma to minimize ptosis of the lower face and neck. The results of this anatomic study support the importance of tailoring the approach to the dynamic lines caused by the platysma muscle in each patient to address jowls and neck bands. Have you been offering lower face BTX treatments to patients in an effort to reduce lower face aging?
The platysma muscle has been widely studied in its course through the cervical region in an attempt to develop more effective surgical approaches for rejuvenation. As a person ages, the platysma muscle becomes thinner, less defined, and ptotic, which results in the clinical appearance of a sagging neck that prompts patients to undergo neck-lift procedures. However, little is known about the course of the platysma in the mid and lower face and the implications on facial aging.
Bae et al (Plast Reconstr Surg. 2016;138:365-371) performed a cadaveric dissection of the facial portion of the platysma muscle to delineate the morphology and extension of the muscle in the mid and lower face. Thirty-four adult hemifaces were dissected. The demographics were broken down by age (mean, 71.4 years; range, 41–93 years), gender (29 men; 5 women), and ethnicity (14 Korean; 20 Thai). The extension of the platysma was documented according to a grid the authors created. The grid was divided along 3 horizontal lines (H1–H3) and 3 vertical lines (V1–V3). The intersection of these lines created a grid that was comprised of 3×3 squares and was labeled superior (S1–S3), middle (M1–M3), and inferior (I1–I3). H1 was a line drawn horizontally from the tragus to the lateral orbit, H2 was a line from the earlobe to the nasal ala, and H3 was a line from the angle of the mandible to the corner of the mouth.
The extension pattern of the facial portion of the platysma muscle was classified into patterns: A (n=3; S1–S2, M1–M3, and I1-I3 were covered by the muscle), B-1 (n=20; M1–M3, I1–I3), B-2 (n=9; M1–M2, I1–I3), and C (n=2; I1-I3).
The platysma muscle is divided into anterior and posterior portions. The anterior platysma ascends superiorly and medially in the face to interdigitate with the depressor anguli oris and the depressor labii inferioris muscles. The morphology pattern of the posterior platysma varied and was classified into 1 of 3 patterns: straight (n=13), straight-curved (n=18), and curved (n=3). The straight pattern showed platysma fibers that travel parallel to the mandibular malar line and head toward the zygomaticus major or the orbicularis oculi muscles. The straight-curved type has fibers that travel straight from the neck to the face but then curve, heading toward the risorius, zygomaticus major, or orbicularis oculi muscle depending on the extent of the platysma muscle in the area. The curved type has fibers that run parallel to the zygomaticus arch and interdigitate with the orbicularis oculi muscle and either the risorius or zygomaticus major muscle.
What’s the issue?
For many years, the cervical platysma muscle was studied and surgical approaches were modified to enhance results. The authors of this study have taken the study of the platysma muscle further to delineate its course through the face. Knowledge of the various pathways of the muscle can help us determine the most natural direction to resuspend ptotic facial tissue during surgery and minimally invasive procedures such as thread-lifting. The most salient points include: (1) the lower one-third of the masseter muscle is covered by the platysma, and (2) straight-curved was the most common morphology type, suggesting that the vector for repositioning the platysma muscle is vertical.
Although Bae et al do not discuss the use of botulinum toxin (BTX) in the treatment of the platysma, the results of this study further support the importance of BTX to the lower face. We can look back at more than 14 years of cosmetic use of BTX for the glabella and see the reduced number of brow-lift surgeries nationwide. Although the preventative effects of BTX on brow depression still need to be scientifically proven, it may behoove us to think preventatively on the use of BTX in the platysma to minimize ptosis of the lower face and neck. The results of this anatomic study support the importance of tailoring the approach to the dynamic lines caused by the platysma muscle in each patient to address jowls and neck bands. Have you been offering lower face BTX treatments to patients in an effort to reduce lower face aging?
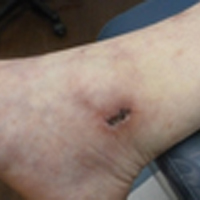
Painful Ulcerations Above the Malleoli
The Diagnosis: Livedoid Vasculopathy
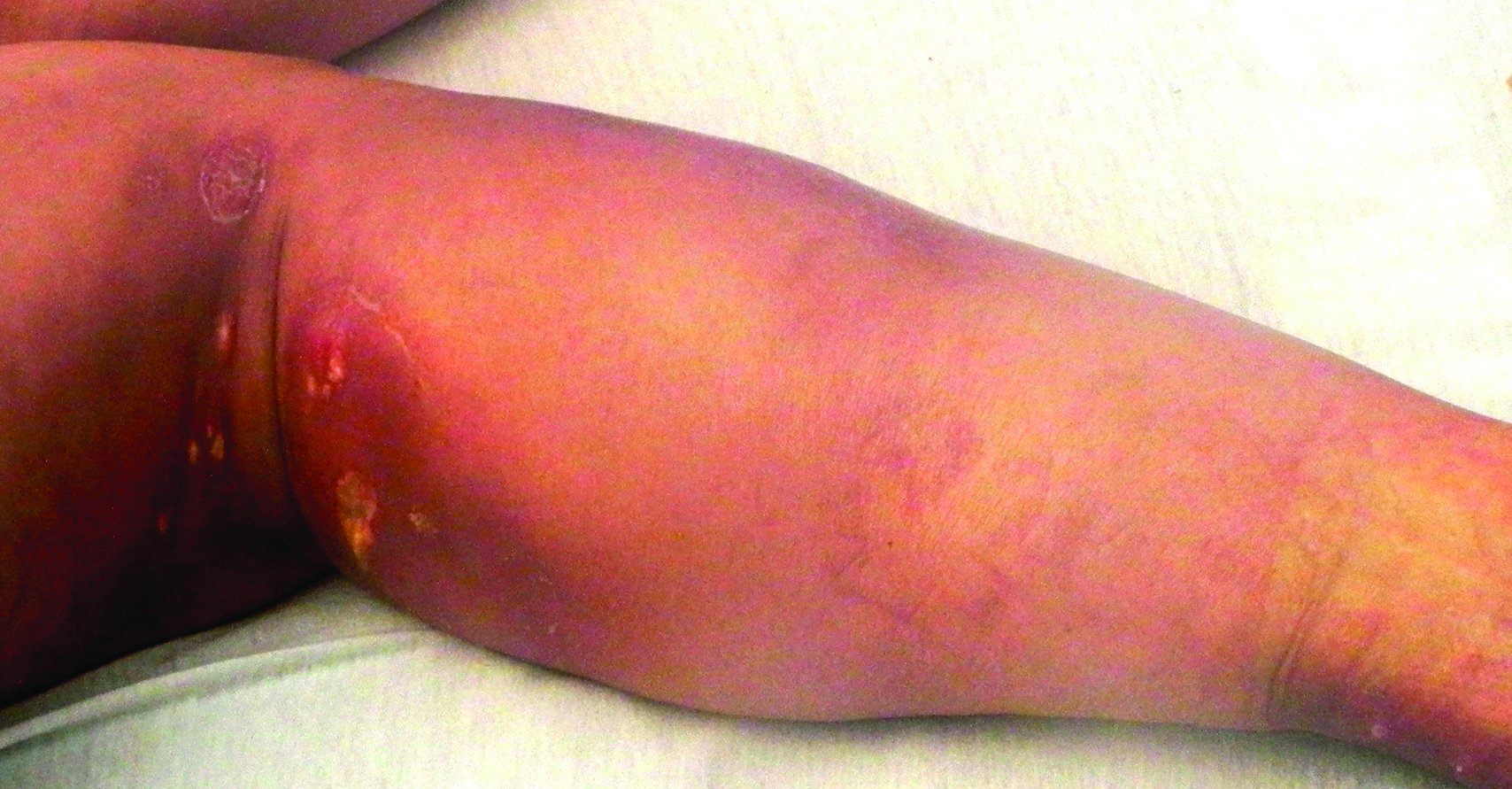
Livedoid vasculopathy (LV) is a rare cutaneous disorder that most commonly affects the lower legs. It has an estimated incidence of 1 case per 100,000 per year and predominantly affects women.1 The disease pathogenesis is not fully understood but is thought to involve thrombosis and occlusion of dermal vessels resulting in tissue hypoxia.2 Both inherited and acquired thrombophilic conditions frequently are seen in patients with LV.3,4 Livedoid vasculopathy also has been described as idiopathic5 and is associated with immune complex deposition.6 However, the number of cases of idiopathic LV may be overestimated; as technological advancements to detect coagulation abnormalities improve, it is hypothesized that this entity will be identified less often.2,4
Livedoid vasculopathy has been described in the literature using the term PPURPLE (painful purpuric ulcers with reticular pattern of lower extremities).7 The triad of livedo racemosa, recurrent painful ulcerations, and residual healing with atrophie blanche characterizes the clinical manifestations of LV; however, all 3 characteristics do not need to appear simultaneously for a diagnosis to be made. The condition has a chronic course with spontaneous remissions and exacerbations. Episodic ulcerations occur, especially in the summertime, and heal slowly, leaving behind atrophic, porcelain white, stellate-shaped scars called atrophie blanche. Livedo racemosa also may be seen in Sneddon syndrome; however, these patients experience neurologic symptoms secondary to cerebrovascular occlusion. In contrast to livedo racemosa, acquired livedo reticularis represents a physiologic hypoperfusion pattern that occurs in response to cold exposure.8 A localized sharp pain, known as angina cutis, typically precedes the clinical symptom of painful ulcerations.9 Atrophie blanche once was thought to be specific to LV but has been seen in other diseases such as systemic lupus erythematosus and chronic venous insufficiency.2
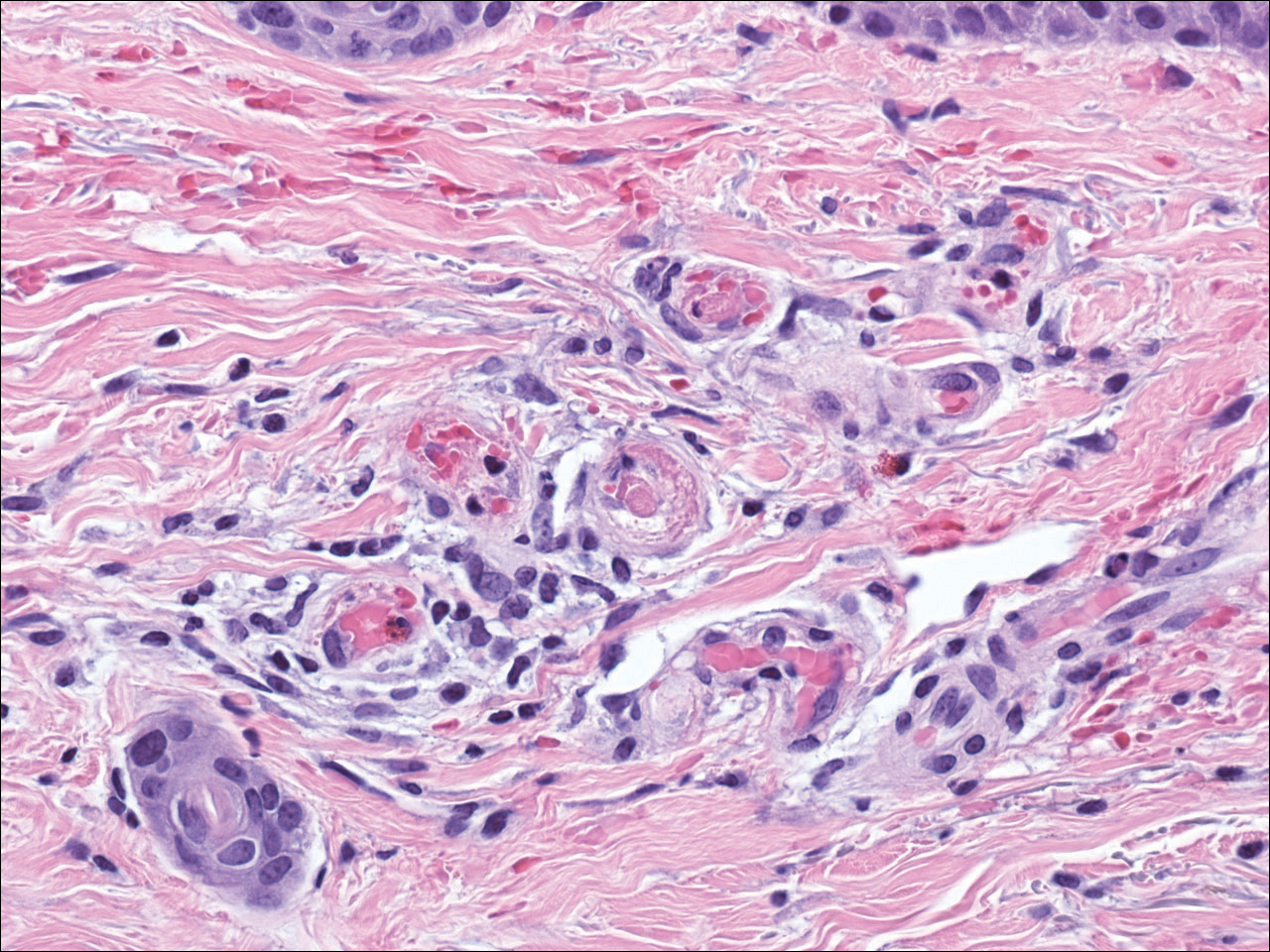
The diagnosis of LV is based on identification of characteristic clinical features and skin biopsy. In almost all biopsy specimens, histopathology reveals fibrinoid occlusion of vessels in the superficial and mid dermis.4 Other findings may include epidermal necrosis and vessel wall hyalinization and infarction2 (Figure). Because LV is commonly misdiagnosed as vasculitis, the absence of hallmark features of vasculitis such as neutrophilic infiltrate of blood vessel walls and fibrinoid necrosis suggest the diagnosis. Extensive laboratory evaluation for inherited and acquired coagulation abnormalities should be performed.
Treatment of LV is difficult, as there is currently no consensus on optimal therapy. The mainstay of therapy is to reduce pain, prevent infection, and reduce ulceration and development of atrophie blanche. Underlying causes should be identified and appropriately treated. Because the primary pathogenesis of LV is considered to be a hypercoagulable state, first-line treatment often includes therapies to enhance blood flow and prevent thrombosis such as smoking cessation, antiplatelet therapy, and pentoxifylline. Vasodilating agents, anti-inflammatory agents, anticoagulation, and fibrinolytic therapy also have been used with varying degrees of success.7
- Fritsch P, Zelger B. Livedo vasculitis [in German]. Hautarzt. 1995;46:215-224; quiz 222-223.
- Kerk N, Goerge T. Livedoid vasculopathy—a thrombotic disease. Vasa. 2013;42:317-322.
- Stevanovic DV. Atrophie blanche. a sign of dermal blood occlusion. Arch Dermatol. 1974;109:858-862.
- Hairston BR, Davis MD, Pittelkow MR, et al. Livedoid vasculopathy: further evidence for procoagulant pathogenesis. Arch Dermatol. 2006;142:1413-1418.
- Shornick JK, Nicholes BK, Bergstresser PR, et al. Idiopathic atrophie blanche. J Am Acad Dermatol. 1983;8:792-798.
- Feldaker M, Hines EA Jr, Kierland RR. Livedo reticularis with ulcerations. Circulation. 1956;13:196-216.
- Callen JP. Livedoid vasculopathy: what it is and how the patient should be evaluated and treated. Arch Dermatol. 2006;142:1481-1482.
- Copeman PW. Livedo reticularis. signs in the skin of disturbance of blood viscosity and of blood flow. Br J Dermatol. 1975;93:519-529.
- Goerge T. Livedoid vasculopathy. pathogenesis, diagnosis and treatment of cutaneous infarction [in German]. Hautarzt. 2011;62:627-634; quiz 635.
The Diagnosis: Livedoid Vasculopathy
Livedoid vasculopathy (LV) is a rare cutaneous disorder that most commonly affects the lower legs. It has an estimated incidence of 1 case per 100,000 per year and predominantly affects women.1 The disease pathogenesis is not fully understood but is thought to involve thrombosis and occlusion of dermal vessels resulting in tissue hypoxia.2 Both inherited and acquired thrombophilic conditions frequently are seen in patients with LV.3,4 Livedoid vasculopathy also has been described as idiopathic5 and is associated with immune complex deposition.6 However, the number of cases of idiopathic LV may be overestimated; as technological advancements to detect coagulation abnormalities improve, it is hypothesized that this entity will be identified less often.2,4
Livedoid vasculopathy has been described in the literature using the term PPURPLE (painful purpuric ulcers with reticular pattern of lower extremities).7 The triad of livedo racemosa, recurrent painful ulcerations, and residual healing with atrophie blanche characterizes the clinical manifestations of LV; however, all 3 characteristics do not need to appear simultaneously for a diagnosis to be made. The condition has a chronic course with spontaneous remissions and exacerbations. Episodic ulcerations occur, especially in the summertime, and heal slowly, leaving behind atrophic, porcelain white, stellate-shaped scars called atrophie blanche. Livedo racemosa also may be seen in Sneddon syndrome; however, these patients experience neurologic symptoms secondary to cerebrovascular occlusion. In contrast to livedo racemosa, acquired livedo reticularis represents a physiologic hypoperfusion pattern that occurs in response to cold exposure.8 A localized sharp pain, known as angina cutis, typically precedes the clinical symptom of painful ulcerations.9 Atrophie blanche once was thought to be specific to LV but has been seen in other diseases such as systemic lupus erythematosus and chronic venous insufficiency.2
The diagnosis of LV is based on identification of characteristic clinical features and skin biopsy. In almost all biopsy specimens, histopathology reveals fibrinoid occlusion of vessels in the superficial and mid dermis.4 Other findings may include epidermal necrosis and vessel wall hyalinization and infarction2 (Figure). Because LV is commonly misdiagnosed as vasculitis, the absence of hallmark features of vasculitis such as neutrophilic infiltrate of blood vessel walls and fibrinoid necrosis suggest the diagnosis. Extensive laboratory evaluation for inherited and acquired coagulation abnormalities should be performed.

Treatment of LV is difficult, as there is currently no consensus on optimal therapy. The mainstay of therapy is to reduce pain, prevent infection, and reduce ulceration and development of atrophie blanche. Underlying causes should be identified and appropriately treated. Because the primary pathogenesis of LV is considered to be a hypercoagulable state, first-line treatment often includes therapies to enhance blood flow and prevent thrombosis such as smoking cessation, antiplatelet therapy, and pentoxifylline. Vasodilating agents, anti-inflammatory agents, anticoagulation, and fibrinolytic therapy also have been used with varying degrees of success.7
The Diagnosis: Livedoid Vasculopathy
Livedoid vasculopathy (LV) is a rare cutaneous disorder that most commonly affects the lower legs. It has an estimated incidence of 1 case per 100,000 per year and predominantly affects women.1 The disease pathogenesis is not fully understood but is thought to involve thrombosis and occlusion of dermal vessels resulting in tissue hypoxia.2 Both inherited and acquired thrombophilic conditions frequently are seen in patients with LV.3,4 Livedoid vasculopathy also has been described as idiopathic5 and is associated with immune complex deposition.6 However, the number of cases of idiopathic LV may be overestimated; as technological advancements to detect coagulation abnormalities improve, it is hypothesized that this entity will be identified less often.2,4
Livedoid vasculopathy has been described in the literature using the term PPURPLE (painful purpuric ulcers with reticular pattern of lower extremities).7 The triad of livedo racemosa, recurrent painful ulcerations, and residual healing with atrophie blanche characterizes the clinical manifestations of LV; however, all 3 characteristics do not need to appear simultaneously for a diagnosis to be made. The condition has a chronic course with spontaneous remissions and exacerbations. Episodic ulcerations occur, especially in the summertime, and heal slowly, leaving behind atrophic, porcelain white, stellate-shaped scars called atrophie blanche. Livedo racemosa also may be seen in Sneddon syndrome; however, these patients experience neurologic symptoms secondary to cerebrovascular occlusion. In contrast to livedo racemosa, acquired livedo reticularis represents a physiologic hypoperfusion pattern that occurs in response to cold exposure.8 A localized sharp pain, known as angina cutis, typically precedes the clinical symptom of painful ulcerations.9 Atrophie blanche once was thought to be specific to LV but has been seen in other diseases such as systemic lupus erythematosus and chronic venous insufficiency.2
The diagnosis of LV is based on identification of characteristic clinical features and skin biopsy. In almost all biopsy specimens, histopathology reveals fibrinoid occlusion of vessels in the superficial and mid dermis.4 Other findings may include epidermal necrosis and vessel wall hyalinization and infarction2 (Figure). Because LV is commonly misdiagnosed as vasculitis, the absence of hallmark features of vasculitis such as neutrophilic infiltrate of blood vessel walls and fibrinoid necrosis suggest the diagnosis. Extensive laboratory evaluation for inherited and acquired coagulation abnormalities should be performed.

Treatment of LV is difficult, as there is currently no consensus on optimal therapy. The mainstay of therapy is to reduce pain, prevent infection, and reduce ulceration and development of atrophie blanche. Underlying causes should be identified and appropriately treated. Because the primary pathogenesis of LV is considered to be a hypercoagulable state, first-line treatment often includes therapies to enhance blood flow and prevent thrombosis such as smoking cessation, antiplatelet therapy, and pentoxifylline. Vasodilating agents, anti-inflammatory agents, anticoagulation, and fibrinolytic therapy also have been used with varying degrees of success.7
- Fritsch P, Zelger B. Livedo vasculitis [in German]. Hautarzt. 1995;46:215-224; quiz 222-223.
- Kerk N, Goerge T. Livedoid vasculopathy—a thrombotic disease. Vasa. 2013;42:317-322.
- Stevanovic DV. Atrophie blanche. a sign of dermal blood occlusion. Arch Dermatol. 1974;109:858-862.
- Hairston BR, Davis MD, Pittelkow MR, et al. Livedoid vasculopathy: further evidence for procoagulant pathogenesis. Arch Dermatol. 2006;142:1413-1418.
- Shornick JK, Nicholes BK, Bergstresser PR, et al. Idiopathic atrophie blanche. J Am Acad Dermatol. 1983;8:792-798.
- Feldaker M, Hines EA Jr, Kierland RR. Livedo reticularis with ulcerations. Circulation. 1956;13:196-216.
- Callen JP. Livedoid vasculopathy: what it is and how the patient should be evaluated and treated. Arch Dermatol. 2006;142:1481-1482.
- Copeman PW. Livedo reticularis. signs in the skin of disturbance of blood viscosity and of blood flow. Br J Dermatol. 1975;93:519-529.
- Goerge T. Livedoid vasculopathy. pathogenesis, diagnosis and treatment of cutaneous infarction [in German]. Hautarzt. 2011;62:627-634; quiz 635.
- Fritsch P, Zelger B. Livedo vasculitis [in German]. Hautarzt. 1995;46:215-224; quiz 222-223.
- Kerk N, Goerge T. Livedoid vasculopathy—a thrombotic disease. Vasa. 2013;42:317-322.
- Stevanovic DV. Atrophie blanche. a sign of dermal blood occlusion. Arch Dermatol. 1974;109:858-862.
- Hairston BR, Davis MD, Pittelkow MR, et al. Livedoid vasculopathy: further evidence for procoagulant pathogenesis. Arch Dermatol. 2006;142:1413-1418.
- Shornick JK, Nicholes BK, Bergstresser PR, et al. Idiopathic atrophie blanche. J Am Acad Dermatol. 1983;8:792-798.
- Feldaker M, Hines EA Jr, Kierland RR. Livedo reticularis with ulcerations. Circulation. 1956;13:196-216.
- Callen JP. Livedoid vasculopathy: what it is and how the patient should be evaluated and treated. Arch Dermatol. 2006;142:1481-1482.
- Copeman PW. Livedo reticularis. signs in the skin of disturbance of blood viscosity and of blood flow. Br J Dermatol. 1975;93:519-529.
- Goerge T. Livedoid vasculopathy. pathogenesis, diagnosis and treatment of cutaneous infarction [in German]. Hautarzt. 2011;62:627-634; quiz 635.
A 58-year-old woman presented in the summertime with skin discoloration of the bilateral lower legs and painful ulcerations above the medial and lateral malleoli of 15 years’ duration. She denied any recent trauma to the area or change in skin lesion appearance with cold exposure. Extensive laboratory evaluation for inherited and acquired coagulation abnormalities was negative. A punch biopsy specimen obtained from the left anterior lower leg revealed vascular thrombi with extravasated erythrocytes and a sparse perivascular inflammatory cell infiltrate.
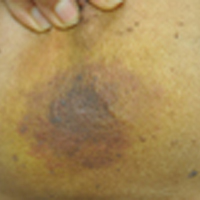
Enlarging Breast Lesion
The Diagnosis: Radiation-Associated Angiosarcoma
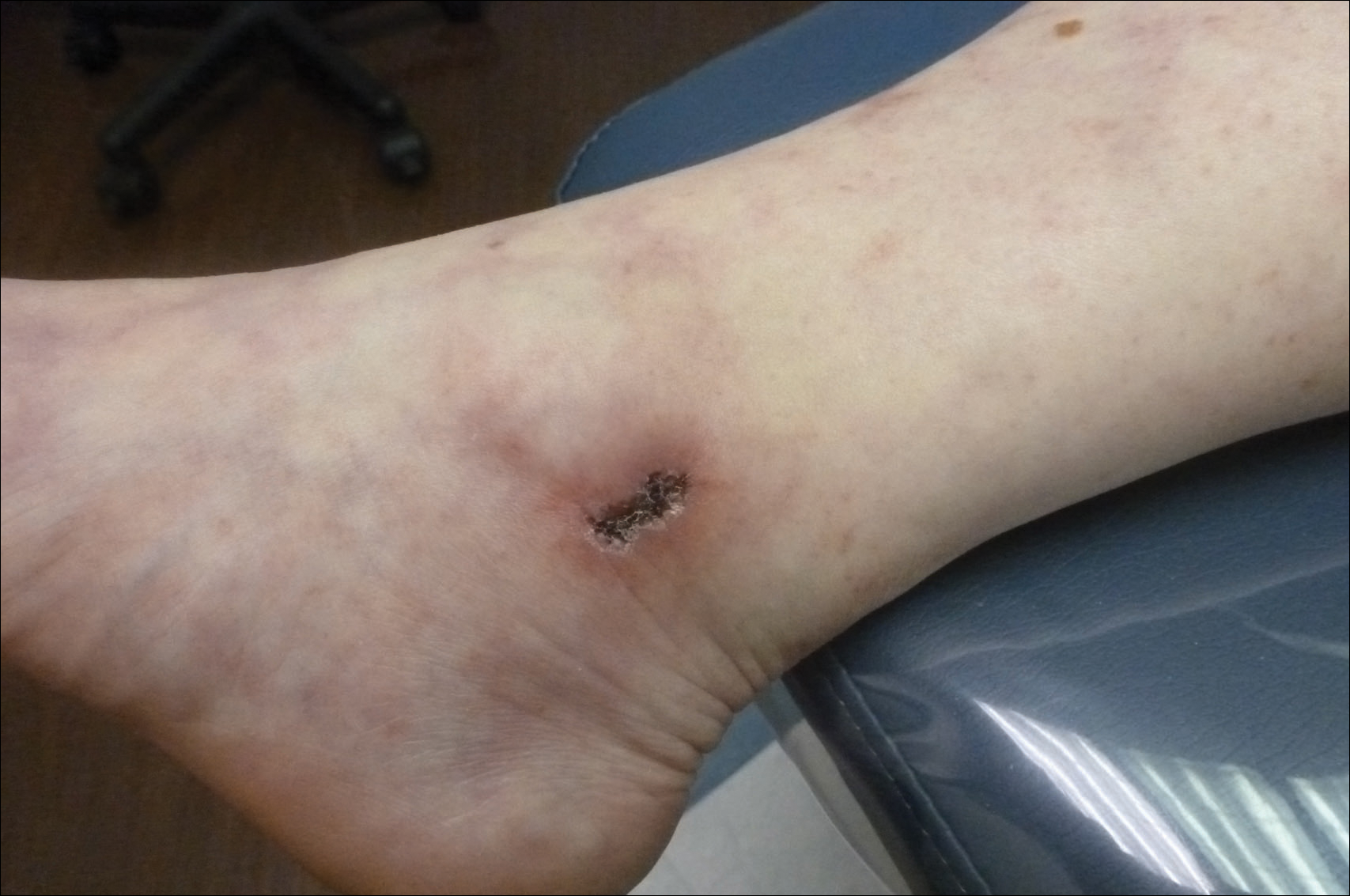
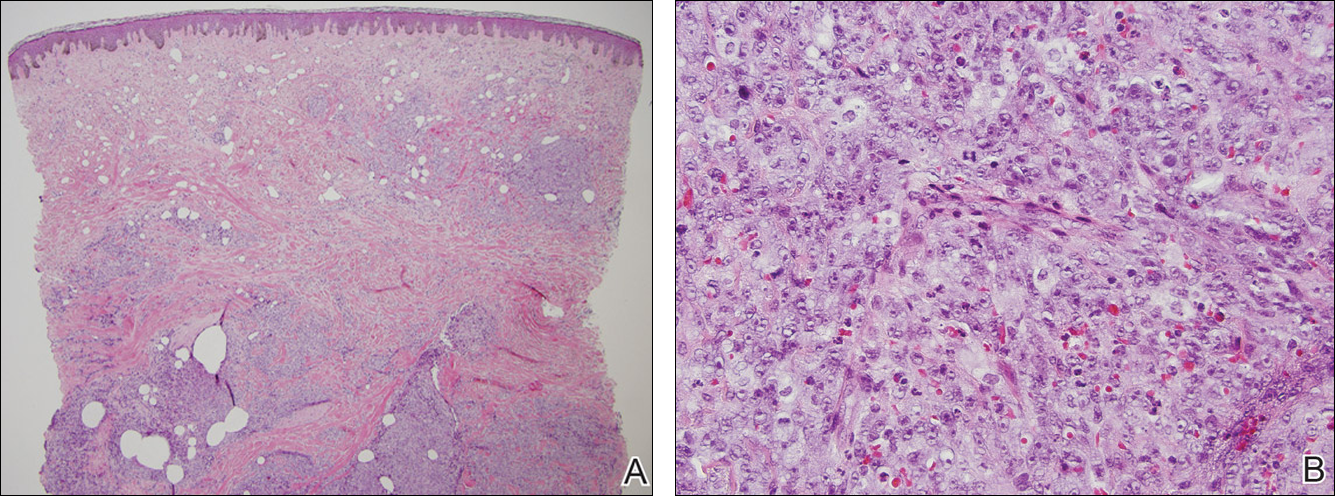
At the time of presentation, a 4-mm lesional punch biopsy was obtained (Figure), which revealed an epithelioid neoplasm within the dermis expressing CD31 and CD34, and staining negatively for S-100, CD45, and estrogen and progesterone receptors. The histologic and immunophenotypic findings were compatible with the diagnosis of angiosarcoma. Given the patient’s history of radiation for breast carcinoma several years ago, this tumor was consistent with radiation-associated angiosarcoma (RAAS).
Development of secondary angiosarcoma has been linked to both prior radiation (RAAS) and chronic lymphedema (Stewart-Treves syndrome).1 Radiation-associated angiosarcoma is defined as a “pathologically confirmed breast or chest wall angiosarcoma arising within a previously irradiated field.”2 The incidence of RAAS is estimated to be 0.9 per 1000 individuals following radiation treatment of breast cancer over the subsequent 15 years and a mean time from radiation to development of 7 years.1 Incidence is expected to increase in the future due to improved likelihood of surviving early-stage breast carcinoma and the increased use of external beam radiation therapy for management of breast cancer.
Differentiating between primary and secondary angiosarcoma of the breast is important. Although primary breast angiosarcoma usually arises in women aged 30 to 40 years, RAAS tends to arise in older women (mean age, 68 years) and is seen only in those women with prior radiation.2 Additionally, high-level amplification of MYC, a known photo-oncogene, on chromosome 8 is a key genetic alteration of RAAS that helps to distinguish it from primary angiosarcoma, though this variance may be present in only half of RAAS cases.3 Immunohistochemical analysis of tumor cells for MYC expression correlates well with this amplification and also is helpful in distinguishing atypical vascular lesions from RAAS.4 Atypical vascular lesions, similar to RAAS, occur years after radiation exposure and may have a similar clinical presentation. Atypical vascular lesions do not progress to angiosarcoma in reported cases, but clinical and histologic overlap with RAAS make the diagnosis difficult.5 In these cases, analysis with fluorescence in situ hybridization or immunohistochemistry for the MYC amplification is important to differentiate these tumors.6
At the time of presentation, the majority of patients with RAAS of the breast have localized disease, often with a variable presentation. In all known cases, there have been skin changes present, emphasizing the importance of both patient and clinician vigilance on a regular basis in at-risk individuals. In one study, the most common presentation was breast ecchymosis, which was observed in 55% of patients.7 These lesions involve the dermis and are commonly mistaken for benign conditions such as infection or hemorrhage.2 In 2 other studies, RAAS most often manifested as a skin nodule or apparent tumor, closely followed by either a rash or bruiselike presentation.1,2
The overall recommendation for management of patients with ecchymotic skin lesions in previously irradiated regions is to obtain a biopsy specimen for tissue diagnosis. Although there is no standard of care for the management of RAAS, a multidisciplinary approach involving specialists from oncology, surgical oncology, and radiation oncology is recommended. Most often, radical surgery encompassing both the breast parenchyma and the at-risk radiated skin is performed. Extensive surgery has demonstrated the best survival benefits compared to mastectomy alone.7 Chemotherapeutics also may be used as adjuncts to surgery, which have been determined to decrease local recurrence rates but have no proven survival benefits.2 Adverse prognostic factors for survival are tumor size greater than 10 cm and development of local and/or distant metastases.2 Following the diagnosis of RAAS, our patient underwent radical mastectomy with adjuvant chemotherapy and remained disease free 6 months after surgery.
In summary, RAAS is a well-known, albeit relatively uncommon, consequence of radiation therapy. Dermatologists, oncologists, and primary care providers play an important role in recognizing this entity when evaluating patients with ecchymotic lesions as well as nodules or tumors within an irradiated field. Biopsy should be obtained promptly to prevent delay in diagnosis and to expedite referral to appropriate specialists for further evaluation and treatment.
- Seinen JM, Emelie S, Verstappen V, et al. Radiation-associated angiosarcoma after breast cancer: high recurrence rate and poor survival despite surgical treatment with R0 resection. Ann Surg Oncol. 2012;19:2700-2706.
- Torres KE, Ravi V, Kin K, et al. Long-term outcomes in patients with radiation-associated angiosarcomas of the breast following surgery and radiotherapy for breast cancer. Ann Surg Oncol. 2013;20:1267-1274.
- Manner J, Radlwimmer B, Hohenberger P, et al. MYC high level gene amplification is a distinctive feature of angiosarcomas after irradiation or chronic lymphedema. Am J Pathol. 2010;176:34-39.
- Ginter PS, Mosquera JM, MacDonald TY, et al. Diagnostic utility of MYC amplification and anti-MYC immunohistochemistry in atypical vascular lesions, primary or radiation-induced mammary angiosarcomas, and primary angiosarcomas of other sites. Hum Pathol. 2014;45:709-716.
- Mentzel T, Schildhaus HU, Palmedo G, et al. Postradiation cutaneous angiosarcoma after treatment of breast carcinoma is characterized by MYC amplification in contrast to atypical vascular lesions after radiotherapy and control cases: clinicopathological immunohistochemical and molecular analysis of 66 cases. Mod Pathol. 2012;25:75-85.
- Fernandez AP, Sun Y, Tubbs RR, et al. FISH for MYC amplification and anti-MYC immunohistochemistry: useful diagnostic tools in the assessment of secondary angiosarcoma and atypical vascular proliferations. J Cutan Pathol. 2012;39:234-242.
- Morgan EA, Kozono DE, Wang Q, et al. Cutaneous radiation-associated angiosarcoma of the breast: poor prognosis in a rare secondary malignancy. Ann Surg Oncol. 2012;19:3801-3808.
The Diagnosis: Radiation-Associated Angiosarcoma
At the time of presentation, a 4-mm lesional punch biopsy was obtained (Figure), which revealed an epithelioid neoplasm within the dermis expressing CD31 and CD34, and staining negatively for S-100, CD45, and estrogen and progesterone receptors. The histologic and immunophenotypic findings were compatible with the diagnosis of angiosarcoma. Given the patient’s history of radiation for breast carcinoma several years ago, this tumor was consistent with radiation-associated angiosarcoma (RAAS).

Development of secondary angiosarcoma has been linked to both prior radiation (RAAS) and chronic lymphedema (Stewart-Treves syndrome).1 Radiation-associated angiosarcoma is defined as a “pathologically confirmed breast or chest wall angiosarcoma arising within a previously irradiated field.”2 The incidence of RAAS is estimated to be 0.9 per 1000 individuals following radiation treatment of breast cancer over the subsequent 15 years and a mean time from radiation to development of 7 years.1 Incidence is expected to increase in the future due to improved likelihood of surviving early-stage breast carcinoma and the increased use of external beam radiation therapy for management of breast cancer.
Differentiating between primary and secondary angiosarcoma of the breast is important. Although primary breast angiosarcoma usually arises in women aged 30 to 40 years, RAAS tends to arise in older women (mean age, 68 years) and is seen only in those women with prior radiation.2 Additionally, high-level amplification of MYC, a known photo-oncogene, on chromosome 8 is a key genetic alteration of RAAS that helps to distinguish it from primary angiosarcoma, though this variance may be present in only half of RAAS cases.3 Immunohistochemical analysis of tumor cells for MYC expression correlates well with this amplification and also is helpful in distinguishing atypical vascular lesions from RAAS.4 Atypical vascular lesions, similar to RAAS, occur years after radiation exposure and may have a similar clinical presentation. Atypical vascular lesions do not progress to angiosarcoma in reported cases, but clinical and histologic overlap with RAAS make the diagnosis difficult.5 In these cases, analysis with fluorescence in situ hybridization or immunohistochemistry for the MYC amplification is important to differentiate these tumors.6
At the time of presentation, the majority of patients with RAAS of the breast have localized disease, often with a variable presentation. In all known cases, there have been skin changes present, emphasizing the importance of both patient and clinician vigilance on a regular basis in at-risk individuals. In one study, the most common presentation was breast ecchymosis, which was observed in 55% of patients.7 These lesions involve the dermis and are commonly mistaken for benign conditions such as infection or hemorrhage.2 In 2 other studies, RAAS most often manifested as a skin nodule or apparent tumor, closely followed by either a rash or bruiselike presentation.1,2
The overall recommendation for management of patients with ecchymotic skin lesions in previously irradiated regions is to obtain a biopsy specimen for tissue diagnosis. Although there is no standard of care for the management of RAAS, a multidisciplinary approach involving specialists from oncology, surgical oncology, and radiation oncology is recommended. Most often, radical surgery encompassing both the breast parenchyma and the at-risk radiated skin is performed. Extensive surgery has demonstrated the best survival benefits compared to mastectomy alone.7 Chemotherapeutics also may be used as adjuncts to surgery, which have been determined to decrease local recurrence rates but have no proven survival benefits.2 Adverse prognostic factors for survival are tumor size greater than 10 cm and development of local and/or distant metastases.2 Following the diagnosis of RAAS, our patient underwent radical mastectomy with adjuvant chemotherapy and remained disease free 6 months after surgery.
In summary, RAAS is a well-known, albeit relatively uncommon, consequence of radiation therapy. Dermatologists, oncologists, and primary care providers play an important role in recognizing this entity when evaluating patients with ecchymotic lesions as well as nodules or tumors within an irradiated field. Biopsy should be obtained promptly to prevent delay in diagnosis and to expedite referral to appropriate specialists for further evaluation and treatment.
The Diagnosis: Radiation-Associated Angiosarcoma
At the time of presentation, a 4-mm lesional punch biopsy was obtained (Figure), which revealed an epithelioid neoplasm within the dermis expressing CD31 and CD34, and staining negatively for S-100, CD45, and estrogen and progesterone receptors. The histologic and immunophenotypic findings were compatible with the diagnosis of angiosarcoma. Given the patient’s history of radiation for breast carcinoma several years ago, this tumor was consistent with radiation-associated angiosarcoma (RAAS).

Development of secondary angiosarcoma has been linked to both prior radiation (RAAS) and chronic lymphedema (Stewart-Treves syndrome).1 Radiation-associated angiosarcoma is defined as a “pathologically confirmed breast or chest wall angiosarcoma arising within a previously irradiated field.”2 The incidence of RAAS is estimated to be 0.9 per 1000 individuals following radiation treatment of breast cancer over the subsequent 15 years and a mean time from radiation to development of 7 years.1 Incidence is expected to increase in the future due to improved likelihood of surviving early-stage breast carcinoma and the increased use of external beam radiation therapy for management of breast cancer.
Differentiating between primary and secondary angiosarcoma of the breast is important. Although primary breast angiosarcoma usually arises in women aged 30 to 40 years, RAAS tends to arise in older women (mean age, 68 years) and is seen only in those women with prior radiation.2 Additionally, high-level amplification of MYC, a known photo-oncogene, on chromosome 8 is a key genetic alteration of RAAS that helps to distinguish it from primary angiosarcoma, though this variance may be present in only half of RAAS cases.3 Immunohistochemical analysis of tumor cells for MYC expression correlates well with this amplification and also is helpful in distinguishing atypical vascular lesions from RAAS.4 Atypical vascular lesions, similar to RAAS, occur years after radiation exposure and may have a similar clinical presentation. Atypical vascular lesions do not progress to angiosarcoma in reported cases, but clinical and histologic overlap with RAAS make the diagnosis difficult.5 In these cases, analysis with fluorescence in situ hybridization or immunohistochemistry for the MYC amplification is important to differentiate these tumors.6
At the time of presentation, the majority of patients with RAAS of the breast have localized disease, often with a variable presentation. In all known cases, there have been skin changes present, emphasizing the importance of both patient and clinician vigilance on a regular basis in at-risk individuals. In one study, the most common presentation was breast ecchymosis, which was observed in 55% of patients.7 These lesions involve the dermis and are commonly mistaken for benign conditions such as infection or hemorrhage.2 In 2 other studies, RAAS most often manifested as a skin nodule or apparent tumor, closely followed by either a rash or bruiselike presentation.1,2
The overall recommendation for management of patients with ecchymotic skin lesions in previously irradiated regions is to obtain a biopsy specimen for tissue diagnosis. Although there is no standard of care for the management of RAAS, a multidisciplinary approach involving specialists from oncology, surgical oncology, and radiation oncology is recommended. Most often, radical surgery encompassing both the breast parenchyma and the at-risk radiated skin is performed. Extensive surgery has demonstrated the best survival benefits compared to mastectomy alone.7 Chemotherapeutics also may be used as adjuncts to surgery, which have been determined to decrease local recurrence rates but have no proven survival benefits.2 Adverse prognostic factors for survival are tumor size greater than 10 cm and development of local and/or distant metastases.2 Following the diagnosis of RAAS, our patient underwent radical mastectomy with adjuvant chemotherapy and remained disease free 6 months after surgery.
In summary, RAAS is a well-known, albeit relatively uncommon, consequence of radiation therapy. Dermatologists, oncologists, and primary care providers play an important role in recognizing this entity when evaluating patients with ecchymotic lesions as well as nodules or tumors within an irradiated field. Biopsy should be obtained promptly to prevent delay in diagnosis and to expedite referral to appropriate specialists for further evaluation and treatment.
- Seinen JM, Emelie S, Verstappen V, et al. Radiation-associated angiosarcoma after breast cancer: high recurrence rate and poor survival despite surgical treatment with R0 resection. Ann Surg Oncol. 2012;19:2700-2706.
- Torres KE, Ravi V, Kin K, et al. Long-term outcomes in patients with radiation-associated angiosarcomas of the breast following surgery and radiotherapy for breast cancer. Ann Surg Oncol. 2013;20:1267-1274.
- Manner J, Radlwimmer B, Hohenberger P, et al. MYC high level gene amplification is a distinctive feature of angiosarcomas after irradiation or chronic lymphedema. Am J Pathol. 2010;176:34-39.
- Ginter PS, Mosquera JM, MacDonald TY, et al. Diagnostic utility of MYC amplification and anti-MYC immunohistochemistry in atypical vascular lesions, primary or radiation-induced mammary angiosarcomas, and primary angiosarcomas of other sites. Hum Pathol. 2014;45:709-716.
- Mentzel T, Schildhaus HU, Palmedo G, et al. Postradiation cutaneous angiosarcoma after treatment of breast carcinoma is characterized by MYC amplification in contrast to atypical vascular lesions after radiotherapy and control cases: clinicopathological immunohistochemical and molecular analysis of 66 cases. Mod Pathol. 2012;25:75-85.
- Fernandez AP, Sun Y, Tubbs RR, et al. FISH for MYC amplification and anti-MYC immunohistochemistry: useful diagnostic tools in the assessment of secondary angiosarcoma and atypical vascular proliferations. J Cutan Pathol. 2012;39:234-242.
- Morgan EA, Kozono DE, Wang Q, et al. Cutaneous radiation-associated angiosarcoma of the breast: poor prognosis in a rare secondary malignancy. Ann Surg Oncol. 2012;19:3801-3808.
- Seinen JM, Emelie S, Verstappen V, et al. Radiation-associated angiosarcoma after breast cancer: high recurrence rate and poor survival despite surgical treatment with R0 resection. Ann Surg Oncol. 2012;19:2700-2706.
- Torres KE, Ravi V, Kin K, et al. Long-term outcomes in patients with radiation-associated angiosarcomas of the breast following surgery and radiotherapy for breast cancer. Ann Surg Oncol. 2013;20:1267-1274.
- Manner J, Radlwimmer B, Hohenberger P, et al. MYC high level gene amplification is a distinctive feature of angiosarcomas after irradiation or chronic lymphedema. Am J Pathol. 2010;176:34-39.
- Ginter PS, Mosquera JM, MacDonald TY, et al. Diagnostic utility of MYC amplification and anti-MYC immunohistochemistry in atypical vascular lesions, primary or radiation-induced mammary angiosarcomas, and primary angiosarcomas of other sites. Hum Pathol. 2014;45:709-716.
- Mentzel T, Schildhaus HU, Palmedo G, et al. Postradiation cutaneous angiosarcoma after treatment of breast carcinoma is characterized by MYC amplification in contrast to atypical vascular lesions after radiotherapy and control cases: clinicopathological immunohistochemical and molecular analysis of 66 cases. Mod Pathol. 2012;25:75-85.
- Fernandez AP, Sun Y, Tubbs RR, et al. FISH for MYC amplification and anti-MYC immunohistochemistry: useful diagnostic tools in the assessment of secondary angiosarcoma and atypical vascular proliferations. J Cutan Pathol. 2012;39:234-242.
- Morgan EA, Kozono DE, Wang Q, et al. Cutaneous radiation-associated angiosarcoma of the breast: poor prognosis in a rare secondary malignancy. Ann Surg Oncol. 2012;19:3801-3808.

A 75-year-old woman with a history of stage II invasive ductal carcinoma of the right breast presented to the dermatology clinic with an enlarging, indurated, ecchymotic plaque on the inferior aspect of the right breast of 2 months’ duration. The patient underwent a lumpectomy, radiation, and adjuvant chemotherapy 13 years prior to presentation. Review of systems was otherwise noncontributory.
Anastrozole-Induced Subacute Cutaneous Lupus Erythematosus
Drug-induced subacute cutaneous lupus erythematosus (DI-SCLE) was first described in 1985 in 5 patients who had been taking hydrochlorothiazide.1 The skin lesions in these patients were identical to those seen in idiopathic subacute cutaneous lupus erythematosus (SCLE) and were accompanied by the same autoantibodies (anti-Ro/Sjögren syndrome antigen A [SS-A] and anti-La/Sjögren syndrome antigen B [SS-B]) and HLA type (HLA-DR2/DR3) that are known to be associated with idiopathic SCLE. The skin lesions of SCLE in these 5 patients resolved spontaneously after discontinuing hydrochlorothiazide; however, anti-Ro/SS-A antibodies persisted in all except 1 patient.1 Over the last decade, an increasing number of drugs from different classes have been implicated to be associated with DI-SCLE. Since the concept of DI-SCLE was introduced, it has been reported to look identical to idiopathic SCLE, both clinically and histopathologically; however, one report suggested that the 2 entities can be distinguished based on clinical variations.2 In general, patients with DI-SCLE develop the same anti-Ro antibodies as seen in idiopathic SCLE. In addition, although the rash in DI-SCLE typically resolves with withdrawal of the offending drug, the antibodies tend to persist. Herein, we report a case of a patient being treated with an aromatase inhibitor who presented with clinical, serologic, and histopathologic evidence of DI-SCLE.
Case Report
A 69-year-old woman diagnosed with breast cancer 4 years prior to her presentation to dermatology initially underwent a lumpectomy and radiation treatment. She was subsequently started on anastrozole 2 years later. After 16 months of treatment with anastrozole, she developed an erythematous scaly rash on sun-exposed areas of the skin. The patient was seen by an outside dermatologist who treated her for a patient-perceived drug rash based on biopsy results that simply demonstrated interface dermatitis. She was treated with both topical and oral steroids with little improvement and therefore presented to our office approximately 6 months after starting treatment seeking a second opinion.
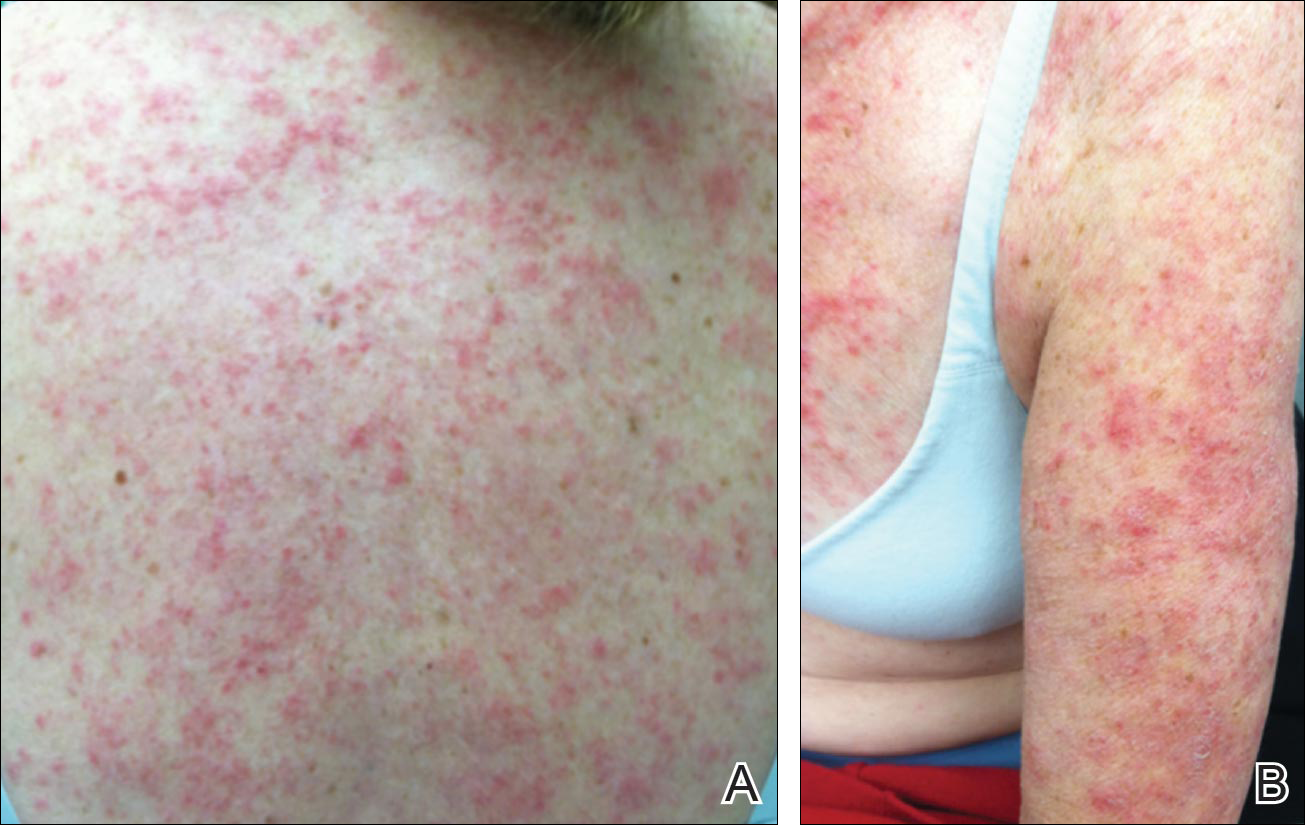
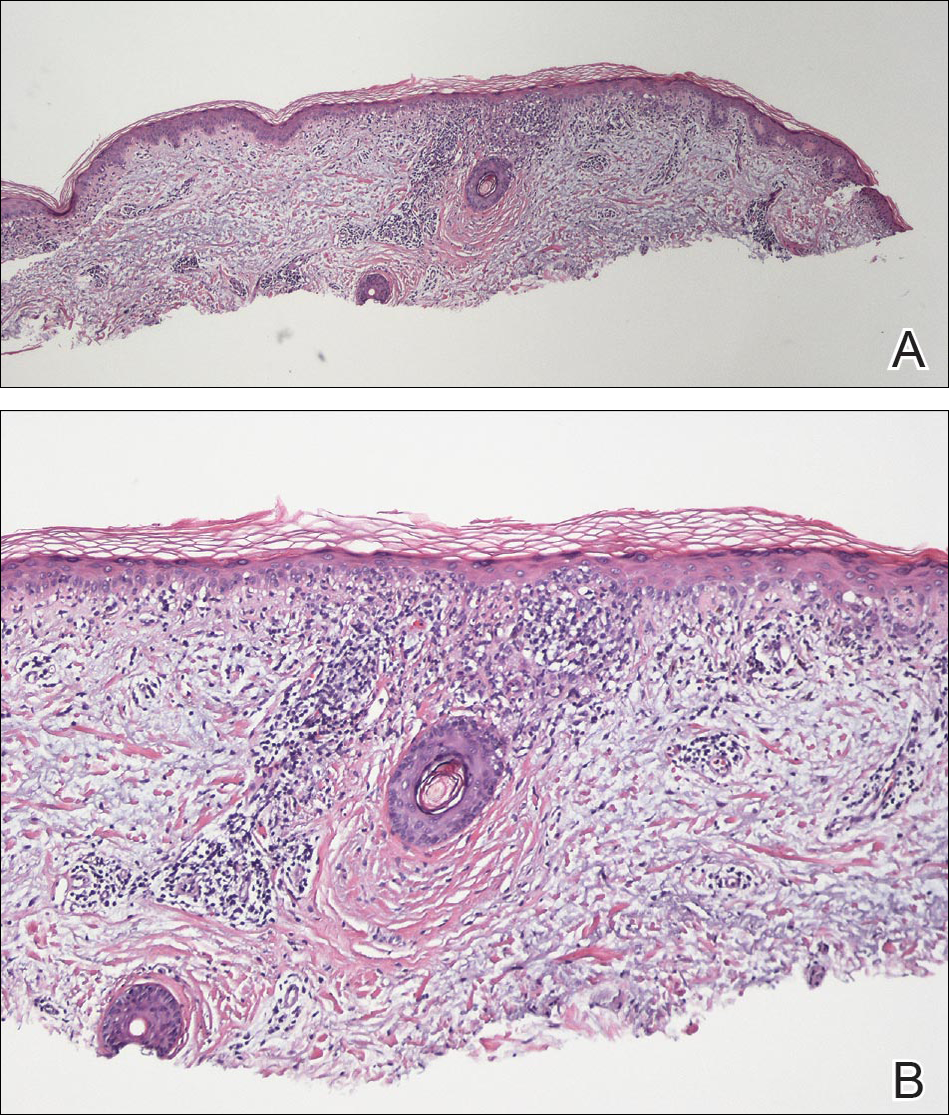
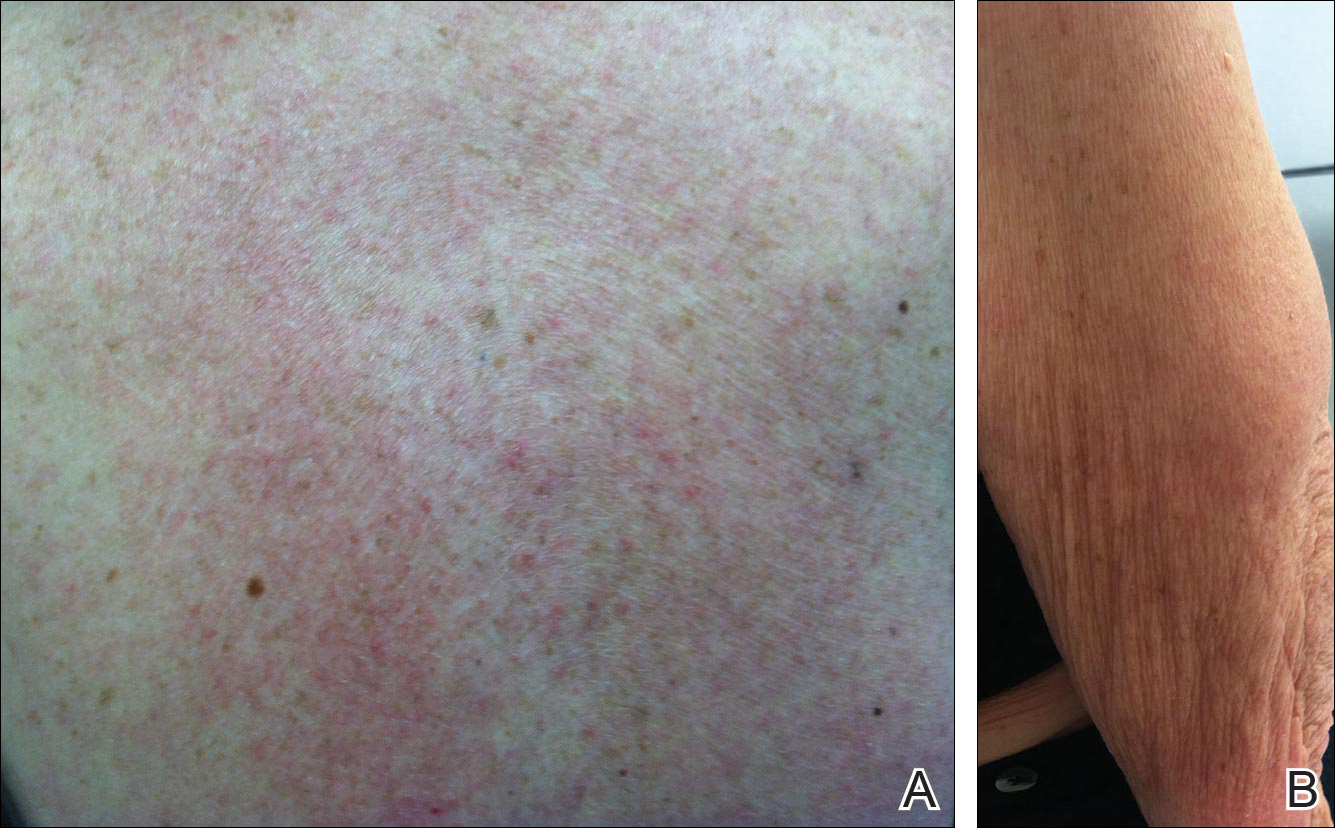
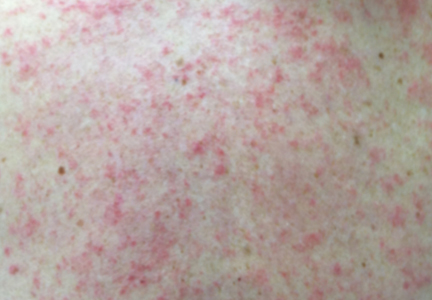
Physical examination revealed numerous erythematous scaly papules and plaques in a photodistributed pattern on the chest, back, legs, and arms (Figure 1). On further questioning, the patient noted that the rash became worse when she was at the beach or playing tennis outside as well as under indoor lights. A repeat biopsy was performed, revealing interface and perivascular dermatitis with an infiltrate composed of lymphocytes, histiocytes, and scattered pigment-laden macrophages (Figure 2). Given the appearance and distribution of the rash as well as the clinical scenario, drug-induced lupus was suspected. Anastrozole was the only medication being taken. Laboratory evaluation was performed and was negative for antinuclear antibodies, antihistone antibodies, and anti-La/SS-B antibodies but was positive for anti-Ro/SS-A antibodies (>8.0 U [reference range, <1.0 U]). Based on these findings, anastrozole-induced SCLE was the most likely explanation for this presentation. The patient was started on a sun-protective regimen (ie, wide-brimmed hat, daily sunscreen) and anastrozole was discontinued by her oncologist; the combination led to moderate improvement in symptoms. One week later, oral hydroxychloroquine 200 mg twice daily was started, which led to notable improvement (Figure 3). The patient was seen for 2 additional follow-up visits, each time with sustained resolution of the rash. The hydroxychloroquine was then stopped at her last visit 3 months after diagnosis. The patient was subsequently lost to follow-up.
Comment
Presentation of SCLE
Subacute cutaneous lupus erythematosus is a form of lupus erythematosus characterized by nonscarring, annular, scaly, erythematous plaques that occur on sun-exposed skin. The lesions are classically distributed on the upper back, chest, dorsal arms, and lateral neck but also can be found in other locations.3,4 Subacute cutaneous lupus erythematosus may be idiopathic; may occur in patients with systemic lupus erythematosus, Sjögren syndrome, or deficiency of the second component of complement (C2d); or may be drug induced.5 On histology SCLE presents as a lichenoid tissue reaction with focal vacuolization of the epidermal basal layer and perivascular lymphocytic infiltrate. On direct immunofluorescence, both idiopathic and drug-induced SCLE present with granular deposition of IgM, IgG, and C3 in a bandlike array at the dermoepidermal junction and circulating anti-Ro/SS-A antibodies. Therefore, histopathologically and immunologically, DI-SCLE is indistinguishable from idiopathic cases.6
Differential Diagnosis
It was previously thought that the clinical presentation of DI-SCLE and idiopathic SCLE were indistinguishable; however, Marzano et al2 described remarkable differences in the cutaneous manifestations of the 2 diseases. Drug-induced SCLE lesions are more widespread, occur more frequently on the legs, and may be bullous or erythema multiforme–like versus the idiopathic lesions, which tend to be more concentrated on the upper body and classically present as scaly erythematous plaques. Additionally, malar rash and vasculitic lesions, such as purpura and necrotic-ulcerative lesions, are seen more often in DI-SCLE.
Drug-induced systemic lupus erythematosus (DI-SLE) is a lupuslike syndrome that can be differentiated from DI-SCLE by virtue of its clinical and serological presentation. It differs from DI-SCLE in that DI-SLE typically does not present with skin symptoms; rather, systemic symptoms such as fever, weight loss, arthralgia, polyarthritis, pericarditis, and pleuritis are more commonly seen. Additionally, it has been associated with antihistone antibodies.4 More than 80 drugs have been reported to cause DI-SLE, including procainamide, hydralazine, and quinidine.7
To be classified as either DI-SCLE or DI-SLE, symptoms need to present after administration of the triggering drug and must resolve after the drug is discontinued.7 The drugs most commonly associated with DI-SCLE are thiazides, calcium channel blockers, tumor necrosis factor α inhibitors, angiotensin-converting enzyme inhibitors, and terbinafine, with few cases citing anastrozole as the inciting agent.4,6,8,9 The incubation period for DI-SCLE varies substantially. Thiazide diuretics and calcium channel blockers typically have the longest incubation period, ranging from 6 months to 5 years for thiazides,1,6,10,11 while calcium channel blockers have an average incubation period of 3 years.12 Drug-induced SCLE associated with antifungals, however, usually is much more rapid in onset; the incubation period on average is 5 weeks for terbinafine and 2 weeks for griseofulvin.13-15
Antiestrogen Drugs and SCLE
Anastrozole, the inciting agent in our case, is a third-generation, selective, nonsteroidal, aromatase inhibitor with no progestogenic, androgenic, or estrogenic activity. Anastrozole, when taken at its recommended dosage of 1 mg daily, will suppress estradiol. It is used as an adjuvant treatment of estrogen-sensitive breast cancer in postmenopausal women. In contrast to a prior case of DI-SCLE secondary to anastrozole in which the incubation period was approximately 1 month,8 our patient had an incubation period of approximately 16 months. Tamoxifen, another antiestrogen drug, also has been associated with DI-SCLE.9 In cases of tamoxifen-induced SCLE, the incubation period was several years, which is more similar to our patient.Although these drugs do not have the same mechanism of action, they both have antiestrogen properties.9 A systemic review of DI-SCLE reported that incubation periods between drug exposure and appearance of DI-SCLE varied greatly and were drug class dependent. It is possible that reactions associated with antiestrogen medications have a delayed presentation; however, given there are limited cases of anastrozole-induced DI-SCLE, we cannot make a clear statement on incubation periods.6
Reports of DI-SCLE caused by antiestrogen drugs are particularly interesting because sex hormones in relation to lupus disease activity have been the subject of debate for decades. Women are considerably more likely to develop autoimmune diseases than men, suggesting that steroid hormones, especially estrogen and progesterone, influence the immune system.16 Estrogen actions are proinflammatory, while the actions of progesterone, androgens, and glucocorticoids are anti-inflammatory.17 Studies in women with lupus revealed an increased rate of mild- to moderate-intensity disease flares associated with estrogen-containing hormone replace-ment therapy.18-20
Over the years, several antiestrogen therapies have been used in murine models, which showed remarkable clinical improvement in the course of SLE. The precise mechanisms involved in disease immunomodulation by these therapies have not been elucidated.21-23 It is thought that estrogen plays a role in the synthesis and expression of Ro antigens on the surface of keratinocytes, increasing the fixation of anti-Ro antibodies in keratinocytes and provoking the appearance of a cutaneous eruption in patients with a susceptible HLA profile.6
Conclusion
We report a rare case of SCLE induced by anastrozole use. Cases such as ours and others that implicate antiestrogen drugs in association with DI-SCLE are particularly noteworthy, considering many studies are looking at the potential usefulness of antiestrogen therapy in the treatment of SLE. Further research on this relationship is warranted.
- Reed B, Huff J, Jones S, et al. Subacute cutaneous lupus erythematosus associated with hydrochlorothiazide therapy. Ann Intern Med. 1985;103:49-51.
- Marzano A, Lazzari R, Polloni I, et al. Drug-induced subacute cutaneous lupus erythematosus: evidence for differences from its idiopathic counterpart. Br J Dermatol. 2011;165:335-341.
- Bonsmann G, Schiller M, Luger T, et al. Terbinafine-induced subacute cutaneous lupus erythematosus. J Am Acad Dermatol. 2001;44:925-931.
- Callen J. Review: drug induced subacute cutaneous lupus erythematosus. Lupus. 2010;19:1107-1111.
- Lin J, Callen JP. Subacute cutaneous lupus erythematosus (SCLE). Medscape website. http://emedicine.medscape.com/article/1065657-overview. Updated March 7, 2016. Accessed April 29, 2016.
- Lowe GC, Henderson CL, Grau RH, et al. A systematic review of drug-induced subacute cutaneous lupus erythematosus. Br J Dermatol. 2011;164:465-472.
- Vedove C, Giglio M, Schena D, et al. Drug-induced lupus erythematosus. Arch Dermatol Res. 2009;301:99-105.
- Trancart M, Cavailhes A, Balme B, et al. Anastrozole-induced subacute cutaneous lupus erythematosus [published online December 6, 2007]. Br J Dermatol. 2008;158:628-629.
- Fumal I, Danchin A, Cosserat F, et al. Subacute cutaneous lupus erythematosus associated with tamoxifen therapy: two cases. Dermatology. 2005;210:251-252.
- Brown C, Deng J. Thiazide diuretics induce cutaneous lupus-like adverse reaction. J Toxicol Clin Toxicol. 1995;33:729-733.
- Sontheimer R. Subacute cutaneous lupus erythematosus: 25-year evolution of a prototypic subset (subphenotype) of lupus erythematosus defined by characteristic cutaneous, pathological, immunological, and genetic findings. Autoimmun Rev. 2005;4:253-263.
- Crowson A, Magro C. Subacute cutaneous lupus erythematosus arising in the setting of calcium channel blocker therapy. Hum Pathol. 1997;28:67-73.
- Lorentz K, Booken N, Goerdt S, et al. Subacute cutaneous lupus erythematosus induced by terbinafine: case report and review of literature. J Dtsch Dermatol Ges. 2008;6:823-837.
- Kasperkiewicz M, Anemüller W, Angelova-Fischer I, et al. Subacute cutaneous lupus erythematosus associated with terbinafine. Clin Exp Dermatol. 2009;34:403-404.
- Miyagawa S, Okuchi T, Shiomi Y, et al. Subacute cutaneous lupus erythematosus lesions precipitated by griseofulvin. J Am Acad Dermatol. 1989;21:343-346.
- Inman RD. Immunologic sex differences and the female predominance in systemic lupus erythematosus. Arthritis Rheum. 1978;21:849-854.
- Cutolo M, Wilder RL. Different roles of androgens and estrogens in the susceptibility to autoimmune rheumatic diseases. Rheum Dis Clin North Am. 2000;26:825-839.
- Petri M. Sex hormones and systemic lupus erythematosus. Lupus. 2008;17:412-415.
- Lateef A, Petri M. Hormone replacement and contraceptive therapy in autoimmune diseases [published online January 18, 2012]. J Autoimmun. 2012;38:J170-J176.
- Buyon JP, Petri M, Kim MY, et al. The effect of combined estrogen and progesterone hormone replacement therapy on disease activity in systemic lupus erythematosus: a randomized trial. Ann Intern Med. 2005;142:954-962.
- Wu W, Suen J, Lin B, et al. Tamoxifen alleviates disease severity and decreases double negative T cells in autoimmune MRL-lpr/lpr mice. Immunology. 2000;100:110-118.
- Dayan M, Zinger H, Kalush F, et al. The beneficial effects of treatment with tamoxifen and anti-oestradiol antibody on experimental systemic lupus erythematosus are associated with cytokine modulations. Immunology. 1997;90:101-108.
- Sthoeger Z, Zinger H, Mozes E. Beneficial effects of the anti-oestrogen tamoxifen on systemic lupus erythematosus of (NZBxNZW)F1 female mice are associated with specific reduction of IgG3 autoantibodies. Ann Rheum Dis. 2003;62:341-346.
Drug-induced subacute cutaneous lupus erythematosus (DI-SCLE) was first described in 1985 in 5 patients who had been taking hydrochlorothiazide.1 The skin lesions in these patients were identical to those seen in idiopathic subacute cutaneous lupus erythematosus (SCLE) and were accompanied by the same autoantibodies (anti-Ro/Sjögren syndrome antigen A [SS-A] and anti-La/Sjögren syndrome antigen B [SS-B]) and HLA type (HLA-DR2/DR3) that are known to be associated with idiopathic SCLE. The skin lesions of SCLE in these 5 patients resolved spontaneously after discontinuing hydrochlorothiazide; however, anti-Ro/SS-A antibodies persisted in all except 1 patient.1 Over the last decade, an increasing number of drugs from different classes have been implicated to be associated with DI-SCLE. Since the concept of DI-SCLE was introduced, it has been reported to look identical to idiopathic SCLE, both clinically and histopathologically; however, one report suggested that the 2 entities can be distinguished based on clinical variations.2 In general, patients with DI-SCLE develop the same anti-Ro antibodies as seen in idiopathic SCLE. In addition, although the rash in DI-SCLE typically resolves with withdrawal of the offending drug, the antibodies tend to persist. Herein, we report a case of a patient being treated with an aromatase inhibitor who presented with clinical, serologic, and histopathologic evidence of DI-SCLE.
Case Report
A 69-year-old woman diagnosed with breast cancer 4 years prior to her presentation to dermatology initially underwent a lumpectomy and radiation treatment. She was subsequently started on anastrozole 2 years later. After 16 months of treatment with anastrozole, she developed an erythematous scaly rash on sun-exposed areas of the skin. The patient was seen by an outside dermatologist who treated her for a patient-perceived drug rash based on biopsy results that simply demonstrated interface dermatitis. She was treated with both topical and oral steroids with little improvement and therefore presented to our office approximately 6 months after starting treatment seeking a second opinion.



Physical examination revealed numerous erythematous scaly papules and plaques in a photodistributed pattern on the chest, back, legs, and arms (Figure 1). On further questioning, the patient noted that the rash became worse when she was at the beach or playing tennis outside as well as under indoor lights. A repeat biopsy was performed, revealing interface and perivascular dermatitis with an infiltrate composed of lymphocytes, histiocytes, and scattered pigment-laden macrophages (Figure 2). Given the appearance and distribution of the rash as well as the clinical scenario, drug-induced lupus was suspected. Anastrozole was the only medication being taken. Laboratory evaluation was performed and was negative for antinuclear antibodies, antihistone antibodies, and anti-La/SS-B antibodies but was positive for anti-Ro/SS-A antibodies (>8.0 U [reference range, <1.0 U]). Based on these findings, anastrozole-induced SCLE was the most likely explanation for this presentation. The patient was started on a sun-protective regimen (ie, wide-brimmed hat, daily sunscreen) and anastrozole was discontinued by her oncologist; the combination led to moderate improvement in symptoms. One week later, oral hydroxychloroquine 200 mg twice daily was started, which led to notable improvement (Figure 3). The patient was seen for 2 additional follow-up visits, each time with sustained resolution of the rash. The hydroxychloroquine was then stopped at her last visit 3 months after diagnosis. The patient was subsequently lost to follow-up.
Comment
Presentation of SCLE
Subacute cutaneous lupus erythematosus is a form of lupus erythematosus characterized by nonscarring, annular, scaly, erythematous plaques that occur on sun-exposed skin. The lesions are classically distributed on the upper back, chest, dorsal arms, and lateral neck but also can be found in other locations.3,4 Subacute cutaneous lupus erythematosus may be idiopathic; may occur in patients with systemic lupus erythematosus, Sjögren syndrome, or deficiency of the second component of complement (C2d); or may be drug induced.5 On histology SCLE presents as a lichenoid tissue reaction with focal vacuolization of the epidermal basal layer and perivascular lymphocytic infiltrate. On direct immunofluorescence, both idiopathic and drug-induced SCLE present with granular deposition of IgM, IgG, and C3 in a bandlike array at the dermoepidermal junction and circulating anti-Ro/SS-A antibodies. Therefore, histopathologically and immunologically, DI-SCLE is indistinguishable from idiopathic cases.6
Differential Diagnosis
It was previously thought that the clinical presentation of DI-SCLE and idiopathic SCLE were indistinguishable; however, Marzano et al2 described remarkable differences in the cutaneous manifestations of the 2 diseases. Drug-induced SCLE lesions are more widespread, occur more frequently on the legs, and may be bullous or erythema multiforme–like versus the idiopathic lesions, which tend to be more concentrated on the upper body and classically present as scaly erythematous plaques. Additionally, malar rash and vasculitic lesions, such as purpura and necrotic-ulcerative lesions, are seen more often in DI-SCLE.
Drug-induced systemic lupus erythematosus (DI-SLE) is a lupuslike syndrome that can be differentiated from DI-SCLE by virtue of its clinical and serological presentation. It differs from DI-SCLE in that DI-SLE typically does not present with skin symptoms; rather, systemic symptoms such as fever, weight loss, arthralgia, polyarthritis, pericarditis, and pleuritis are more commonly seen. Additionally, it has been associated with antihistone antibodies.4 More than 80 drugs have been reported to cause DI-SLE, including procainamide, hydralazine, and quinidine.7
To be classified as either DI-SCLE or DI-SLE, symptoms need to present after administration of the triggering drug and must resolve after the drug is discontinued.7 The drugs most commonly associated with DI-SCLE are thiazides, calcium channel blockers, tumor necrosis factor α inhibitors, angiotensin-converting enzyme inhibitors, and terbinafine, with few cases citing anastrozole as the inciting agent.4,6,8,9 The incubation period for DI-SCLE varies substantially. Thiazide diuretics and calcium channel blockers typically have the longest incubation period, ranging from 6 months to 5 years for thiazides,1,6,10,11 while calcium channel blockers have an average incubation period of 3 years.12 Drug-induced SCLE associated with antifungals, however, usually is much more rapid in onset; the incubation period on average is 5 weeks for terbinafine and 2 weeks for griseofulvin.13-15
Antiestrogen Drugs and SCLE
Anastrozole, the inciting agent in our case, is a third-generation, selective, nonsteroidal, aromatase inhibitor with no progestogenic, androgenic, or estrogenic activity. Anastrozole, when taken at its recommended dosage of 1 mg daily, will suppress estradiol. It is used as an adjuvant treatment of estrogen-sensitive breast cancer in postmenopausal women. In contrast to a prior case of DI-SCLE secondary to anastrozole in which the incubation period was approximately 1 month,8 our patient had an incubation period of approximately 16 months. Tamoxifen, another antiestrogen drug, also has been associated with DI-SCLE.9 In cases of tamoxifen-induced SCLE, the incubation period was several years, which is more similar to our patient.Although these drugs do not have the same mechanism of action, they both have antiestrogen properties.9 A systemic review of DI-SCLE reported that incubation periods between drug exposure and appearance of DI-SCLE varied greatly and were drug class dependent. It is possible that reactions associated with antiestrogen medications have a delayed presentation; however, given there are limited cases of anastrozole-induced DI-SCLE, we cannot make a clear statement on incubation periods.6
Reports of DI-SCLE caused by antiestrogen drugs are particularly interesting because sex hormones in relation to lupus disease activity have been the subject of debate for decades. Women are considerably more likely to develop autoimmune diseases than men, suggesting that steroid hormones, especially estrogen and progesterone, influence the immune system.16 Estrogen actions are proinflammatory, while the actions of progesterone, androgens, and glucocorticoids are anti-inflammatory.17 Studies in women with lupus revealed an increased rate of mild- to moderate-intensity disease flares associated with estrogen-containing hormone replace-ment therapy.18-20
Over the years, several antiestrogen therapies have been used in murine models, which showed remarkable clinical improvement in the course of SLE. The precise mechanisms involved in disease immunomodulation by these therapies have not been elucidated.21-23 It is thought that estrogen plays a role in the synthesis and expression of Ro antigens on the surface of keratinocytes, increasing the fixation of anti-Ro antibodies in keratinocytes and provoking the appearance of a cutaneous eruption in patients with a susceptible HLA profile.6
Conclusion
We report a rare case of SCLE induced by anastrozole use. Cases such as ours and others that implicate antiestrogen drugs in association with DI-SCLE are particularly noteworthy, considering many studies are looking at the potential usefulness of antiestrogen therapy in the treatment of SLE. Further research on this relationship is warranted.
Drug-induced subacute cutaneous lupus erythematosus (DI-SCLE) was first described in 1985 in 5 patients who had been taking hydrochlorothiazide.1 The skin lesions in these patients were identical to those seen in idiopathic subacute cutaneous lupus erythematosus (SCLE) and were accompanied by the same autoantibodies (anti-Ro/Sjögren syndrome antigen A [SS-A] and anti-La/Sjögren syndrome antigen B [SS-B]) and HLA type (HLA-DR2/DR3) that are known to be associated with idiopathic SCLE. The skin lesions of SCLE in these 5 patients resolved spontaneously after discontinuing hydrochlorothiazide; however, anti-Ro/SS-A antibodies persisted in all except 1 patient.1 Over the last decade, an increasing number of drugs from different classes have been implicated to be associated with DI-SCLE. Since the concept of DI-SCLE was introduced, it has been reported to look identical to idiopathic SCLE, both clinically and histopathologically; however, one report suggested that the 2 entities can be distinguished based on clinical variations.2 In general, patients with DI-SCLE develop the same anti-Ro antibodies as seen in idiopathic SCLE. In addition, although the rash in DI-SCLE typically resolves with withdrawal of the offending drug, the antibodies tend to persist. Herein, we report a case of a patient being treated with an aromatase inhibitor who presented with clinical, serologic, and histopathologic evidence of DI-SCLE.
Case Report
A 69-year-old woman diagnosed with breast cancer 4 years prior to her presentation to dermatology initially underwent a lumpectomy and radiation treatment. She was subsequently started on anastrozole 2 years later. After 16 months of treatment with anastrozole, she developed an erythematous scaly rash on sun-exposed areas of the skin. The patient was seen by an outside dermatologist who treated her for a patient-perceived drug rash based on biopsy results that simply demonstrated interface dermatitis. She was treated with both topical and oral steroids with little improvement and therefore presented to our office approximately 6 months after starting treatment seeking a second opinion.



Physical examination revealed numerous erythematous scaly papules and plaques in a photodistributed pattern on the chest, back, legs, and arms (Figure 1). On further questioning, the patient noted that the rash became worse when she was at the beach or playing tennis outside as well as under indoor lights. A repeat biopsy was performed, revealing interface and perivascular dermatitis with an infiltrate composed of lymphocytes, histiocytes, and scattered pigment-laden macrophages (Figure 2). Given the appearance and distribution of the rash as well as the clinical scenario, drug-induced lupus was suspected. Anastrozole was the only medication being taken. Laboratory evaluation was performed and was negative for antinuclear antibodies, antihistone antibodies, and anti-La/SS-B antibodies but was positive for anti-Ro/SS-A antibodies (>8.0 U [reference range, <1.0 U]). Based on these findings, anastrozole-induced SCLE was the most likely explanation for this presentation. The patient was started on a sun-protective regimen (ie, wide-brimmed hat, daily sunscreen) and anastrozole was discontinued by her oncologist; the combination led to moderate improvement in symptoms. One week later, oral hydroxychloroquine 200 mg twice daily was started, which led to notable improvement (Figure 3). The patient was seen for 2 additional follow-up visits, each time with sustained resolution of the rash. The hydroxychloroquine was then stopped at her last visit 3 months after diagnosis. The patient was subsequently lost to follow-up.
Comment
Presentation of SCLE
Subacute cutaneous lupus erythematosus is a form of lupus erythematosus characterized by nonscarring, annular, scaly, erythematous plaques that occur on sun-exposed skin. The lesions are classically distributed on the upper back, chest, dorsal arms, and lateral neck but also can be found in other locations.3,4 Subacute cutaneous lupus erythematosus may be idiopathic; may occur in patients with systemic lupus erythematosus, Sjögren syndrome, or deficiency of the second component of complement (C2d); or may be drug induced.5 On histology SCLE presents as a lichenoid tissue reaction with focal vacuolization of the epidermal basal layer and perivascular lymphocytic infiltrate. On direct immunofluorescence, both idiopathic and drug-induced SCLE present with granular deposition of IgM, IgG, and C3 in a bandlike array at the dermoepidermal junction and circulating anti-Ro/SS-A antibodies. Therefore, histopathologically and immunologically, DI-SCLE is indistinguishable from idiopathic cases.6
Differential Diagnosis
It was previously thought that the clinical presentation of DI-SCLE and idiopathic SCLE were indistinguishable; however, Marzano et al2 described remarkable differences in the cutaneous manifestations of the 2 diseases. Drug-induced SCLE lesions are more widespread, occur more frequently on the legs, and may be bullous or erythema multiforme–like versus the idiopathic lesions, which tend to be more concentrated on the upper body and classically present as scaly erythematous plaques. Additionally, malar rash and vasculitic lesions, such as purpura and necrotic-ulcerative lesions, are seen more often in DI-SCLE.
Drug-induced systemic lupus erythematosus (DI-SLE) is a lupuslike syndrome that can be differentiated from DI-SCLE by virtue of its clinical and serological presentation. It differs from DI-SCLE in that DI-SLE typically does not present with skin symptoms; rather, systemic symptoms such as fever, weight loss, arthralgia, polyarthritis, pericarditis, and pleuritis are more commonly seen. Additionally, it has been associated with antihistone antibodies.4 More than 80 drugs have been reported to cause DI-SLE, including procainamide, hydralazine, and quinidine.7
To be classified as either DI-SCLE or DI-SLE, symptoms need to present after administration of the triggering drug and must resolve after the drug is discontinued.7 The drugs most commonly associated with DI-SCLE are thiazides, calcium channel blockers, tumor necrosis factor α inhibitors, angiotensin-converting enzyme inhibitors, and terbinafine, with few cases citing anastrozole as the inciting agent.4,6,8,9 The incubation period for DI-SCLE varies substantially. Thiazide diuretics and calcium channel blockers typically have the longest incubation period, ranging from 6 months to 5 years for thiazides,1,6,10,11 while calcium channel blockers have an average incubation period of 3 years.12 Drug-induced SCLE associated with antifungals, however, usually is much more rapid in onset; the incubation period on average is 5 weeks for terbinafine and 2 weeks for griseofulvin.13-15
Antiestrogen Drugs and SCLE
Anastrozole, the inciting agent in our case, is a third-generation, selective, nonsteroidal, aromatase inhibitor with no progestogenic, androgenic, or estrogenic activity. Anastrozole, when taken at its recommended dosage of 1 mg daily, will suppress estradiol. It is used as an adjuvant treatment of estrogen-sensitive breast cancer in postmenopausal women. In contrast to a prior case of DI-SCLE secondary to anastrozole in which the incubation period was approximately 1 month,8 our patient had an incubation period of approximately 16 months. Tamoxifen, another antiestrogen drug, also has been associated with DI-SCLE.9 In cases of tamoxifen-induced SCLE, the incubation period was several years, which is more similar to our patient.Although these drugs do not have the same mechanism of action, they both have antiestrogen properties.9 A systemic review of DI-SCLE reported that incubation periods between drug exposure and appearance of DI-SCLE varied greatly and were drug class dependent. It is possible that reactions associated with antiestrogen medications have a delayed presentation; however, given there are limited cases of anastrozole-induced DI-SCLE, we cannot make a clear statement on incubation periods.6
Reports of DI-SCLE caused by antiestrogen drugs are particularly interesting because sex hormones in relation to lupus disease activity have been the subject of debate for decades. Women are considerably more likely to develop autoimmune diseases than men, suggesting that steroid hormones, especially estrogen and progesterone, influence the immune system.16 Estrogen actions are proinflammatory, while the actions of progesterone, androgens, and glucocorticoids are anti-inflammatory.17 Studies in women with lupus revealed an increased rate of mild- to moderate-intensity disease flares associated with estrogen-containing hormone replace-ment therapy.18-20
Over the years, several antiestrogen therapies have been used in murine models, which showed remarkable clinical improvement in the course of SLE. The precise mechanisms involved in disease immunomodulation by these therapies have not been elucidated.21-23 It is thought that estrogen plays a role in the synthesis and expression of Ro antigens on the surface of keratinocytes, increasing the fixation of anti-Ro antibodies in keratinocytes and provoking the appearance of a cutaneous eruption in patients with a susceptible HLA profile.6
Conclusion
We report a rare case of SCLE induced by anastrozole use. Cases such as ours and others that implicate antiestrogen drugs in association with DI-SCLE are particularly noteworthy, considering many studies are looking at the potential usefulness of antiestrogen therapy in the treatment of SLE. Further research on this relationship is warranted.
- Reed B, Huff J, Jones S, et al. Subacute cutaneous lupus erythematosus associated with hydrochlorothiazide therapy. Ann Intern Med. 1985;103:49-51.
- Marzano A, Lazzari R, Polloni I, et al. Drug-induced subacute cutaneous lupus erythematosus: evidence for differences from its idiopathic counterpart. Br J Dermatol. 2011;165:335-341.
- Bonsmann G, Schiller M, Luger T, et al. Terbinafine-induced subacute cutaneous lupus erythematosus. J Am Acad Dermatol. 2001;44:925-931.
- Callen J. Review: drug induced subacute cutaneous lupus erythematosus. Lupus. 2010;19:1107-1111.
- Lin J, Callen JP. Subacute cutaneous lupus erythematosus (SCLE). Medscape website. http://emedicine.medscape.com/article/1065657-overview. Updated March 7, 2016. Accessed April 29, 2016.
- Lowe GC, Henderson CL, Grau RH, et al. A systematic review of drug-induced subacute cutaneous lupus erythematosus. Br J Dermatol. 2011;164:465-472.
- Vedove C, Giglio M, Schena D, et al. Drug-induced lupus erythematosus. Arch Dermatol Res. 2009;301:99-105.
- Trancart M, Cavailhes A, Balme B, et al. Anastrozole-induced subacute cutaneous lupus erythematosus [published online December 6, 2007]. Br J Dermatol. 2008;158:628-629.
- Fumal I, Danchin A, Cosserat F, et al. Subacute cutaneous lupus erythematosus associated with tamoxifen therapy: two cases. Dermatology. 2005;210:251-252.
- Brown C, Deng J. Thiazide diuretics induce cutaneous lupus-like adverse reaction. J Toxicol Clin Toxicol. 1995;33:729-733.
- Sontheimer R. Subacute cutaneous lupus erythematosus: 25-year evolution of a prototypic subset (subphenotype) of lupus erythematosus defined by characteristic cutaneous, pathological, immunological, and genetic findings. Autoimmun Rev. 2005;4:253-263.
- Crowson A, Magro C. Subacute cutaneous lupus erythematosus arising in the setting of calcium channel blocker therapy. Hum Pathol. 1997;28:67-73.
- Lorentz K, Booken N, Goerdt S, et al. Subacute cutaneous lupus erythematosus induced by terbinafine: case report and review of literature. J Dtsch Dermatol Ges. 2008;6:823-837.
- Kasperkiewicz M, Anemüller W, Angelova-Fischer I, et al. Subacute cutaneous lupus erythematosus associated with terbinafine. Clin Exp Dermatol. 2009;34:403-404.
- Miyagawa S, Okuchi T, Shiomi Y, et al. Subacute cutaneous lupus erythematosus lesions precipitated by griseofulvin. J Am Acad Dermatol. 1989;21:343-346.
- Inman RD. Immunologic sex differences and the female predominance in systemic lupus erythematosus. Arthritis Rheum. 1978;21:849-854.
- Cutolo M, Wilder RL. Different roles of androgens and estrogens in the susceptibility to autoimmune rheumatic diseases. Rheum Dis Clin North Am. 2000;26:825-839.
- Petri M. Sex hormones and systemic lupus erythematosus. Lupus. 2008;17:412-415.
- Lateef A, Petri M. Hormone replacement and contraceptive therapy in autoimmune diseases [published online January 18, 2012]. J Autoimmun. 2012;38:J170-J176.
- Buyon JP, Petri M, Kim MY, et al. The effect of combined estrogen and progesterone hormone replacement therapy on disease activity in systemic lupus erythematosus: a randomized trial. Ann Intern Med. 2005;142:954-962.
- Wu W, Suen J, Lin B, et al. Tamoxifen alleviates disease severity and decreases double negative T cells in autoimmune MRL-lpr/lpr mice. Immunology. 2000;100:110-118.
- Dayan M, Zinger H, Kalush F, et al. The beneficial effects of treatment with tamoxifen and anti-oestradiol antibody on experimental systemic lupus erythematosus are associated with cytokine modulations. Immunology. 1997;90:101-108.
- Sthoeger Z, Zinger H, Mozes E. Beneficial effects of the anti-oestrogen tamoxifen on systemic lupus erythematosus of (NZBxNZW)F1 female mice are associated with specific reduction of IgG3 autoantibodies. Ann Rheum Dis. 2003;62:341-346.
- Reed B, Huff J, Jones S, et al. Subacute cutaneous lupus erythematosus associated with hydrochlorothiazide therapy. Ann Intern Med. 1985;103:49-51.
- Marzano A, Lazzari R, Polloni I, et al. Drug-induced subacute cutaneous lupus erythematosus: evidence for differences from its idiopathic counterpart. Br J Dermatol. 2011;165:335-341.
- Bonsmann G, Schiller M, Luger T, et al. Terbinafine-induced subacute cutaneous lupus erythematosus. J Am Acad Dermatol. 2001;44:925-931.
- Callen J. Review: drug induced subacute cutaneous lupus erythematosus. Lupus. 2010;19:1107-1111.
- Lin J, Callen JP. Subacute cutaneous lupus erythematosus (SCLE). Medscape website. http://emedicine.medscape.com/article/1065657-overview. Updated March 7, 2016. Accessed April 29, 2016.
- Lowe GC, Henderson CL, Grau RH, et al. A systematic review of drug-induced subacute cutaneous lupus erythematosus. Br J Dermatol. 2011;164:465-472.
- Vedove C, Giglio M, Schena D, et al. Drug-induced lupus erythematosus. Arch Dermatol Res. 2009;301:99-105.
- Trancart M, Cavailhes A, Balme B, et al. Anastrozole-induced subacute cutaneous lupus erythematosus [published online December 6, 2007]. Br J Dermatol. 2008;158:628-629.
- Fumal I, Danchin A, Cosserat F, et al. Subacute cutaneous lupus erythematosus associated with tamoxifen therapy: two cases. Dermatology. 2005;210:251-252.
- Brown C, Deng J. Thiazide diuretics induce cutaneous lupus-like adverse reaction. J Toxicol Clin Toxicol. 1995;33:729-733.
- Sontheimer R. Subacute cutaneous lupus erythematosus: 25-year evolution of a prototypic subset (subphenotype) of lupus erythematosus defined by characteristic cutaneous, pathological, immunological, and genetic findings. Autoimmun Rev. 2005;4:253-263.
- Crowson A, Magro C. Subacute cutaneous lupus erythematosus arising in the setting of calcium channel blocker therapy. Hum Pathol. 1997;28:67-73.
- Lorentz K, Booken N, Goerdt S, et al. Subacute cutaneous lupus erythematosus induced by terbinafine: case report and review of literature. J Dtsch Dermatol Ges. 2008;6:823-837.
- Kasperkiewicz M, Anemüller W, Angelova-Fischer I, et al. Subacute cutaneous lupus erythematosus associated with terbinafine. Clin Exp Dermatol. 2009;34:403-404.
- Miyagawa S, Okuchi T, Shiomi Y, et al. Subacute cutaneous lupus erythematosus lesions precipitated by griseofulvin. J Am Acad Dermatol. 1989;21:343-346.
- Inman RD. Immunologic sex differences and the female predominance in systemic lupus erythematosus. Arthritis Rheum. 1978;21:849-854.
- Cutolo M, Wilder RL. Different roles of androgens and estrogens in the susceptibility to autoimmune rheumatic diseases. Rheum Dis Clin North Am. 2000;26:825-839.
- Petri M. Sex hormones and systemic lupus erythematosus. Lupus. 2008;17:412-415.
- Lateef A, Petri M. Hormone replacement and contraceptive therapy in autoimmune diseases [published online January 18, 2012]. J Autoimmun. 2012;38:J170-J176.
- Buyon JP, Petri M, Kim MY, et al. The effect of combined estrogen and progesterone hormone replacement therapy on disease activity in systemic lupus erythematosus: a randomized trial. Ann Intern Med. 2005;142:954-962.
- Wu W, Suen J, Lin B, et al. Tamoxifen alleviates disease severity and decreases double negative T cells in autoimmune MRL-lpr/lpr mice. Immunology. 2000;100:110-118.
- Dayan M, Zinger H, Kalush F, et al. The beneficial effects of treatment with tamoxifen and anti-oestradiol antibody on experimental systemic lupus erythematosus are associated with cytokine modulations. Immunology. 1997;90:101-108.
- Sthoeger Z, Zinger H, Mozes E. Beneficial effects of the anti-oestrogen tamoxifen on systemic lupus erythematosus of (NZBxNZW)F1 female mice are associated with specific reduction of IgG3 autoantibodies. Ann Rheum Dis. 2003;62:341-346.
Practice Points
- There are numerous cases of drug-induced subacute cutaneous lupus erythematosus (DI-SCLE) published in the literature; however, there are limited reports with anastrozole implicated as the causative agent.
- Cases of DI-SCLE are clinically and histologically indistinguishable from idiopathic cases. It is important to recognize and withdraw the offending agent.
Prednisone and Vardenafil Hydrochloride for Refractory Levamisole-Induced Vasculitis
Levamisole is an immunomodulatory drug that had been used to treat various medical conditions, including parasitic infections, nephrotic syndrome, and colorectal cancer,1 before being withdrawn from the US market in 2000.The most common reasons for levamisole discontinuation were leukopenia and rashes (1%–2%),1 many of which included leg ulcers and necrotizing purpura of the ears.1,2 The drug is currently available only as a deworming agent in veterinary medicine.
Since 2007, increasing amounts of levamisole have been used as an adulterant in cocaine. In 2007, less than 10% of cocaine was contaminated with levamisole, with an increase to 77% by 2010.3 In addition, 78% of 249 urine toxicology screens that were positive for cocaine in an inner city hospital also tested positive for levamisole.4 Levamisole-cut cocaine has become a concern because it is associated with a life-threatening syndrome involving a necrotizing purpuric rash, autoantibody production, and leukopenia.5
Levamisole-induced vasculitis is an independent entity from cocaine-induced vasculitis, which is associated with skin findings ranging from palpable purpura and chronic ulcers to digital infarction secondary to its vasospastic activity.6-8 Cocaine-induced vasculopathy has been related to cytoplasmic antineutrophil cytoplasmic antibody positivity and often resembles Wegener granulomatosis.6 Although both cocaine and levamisole have reportedly caused acrally distributed purpura and vasculopathy, levamisole is specifically associated with retiform purpura, ear involvement, and leukopenia.6,9 In addition, levamisole-induced skin reactions have been linked to specific antibodies, including antinuclear, antiphospholipid, and perinuclear antineutrophil cytoplasmic antibody (p-ANCA).2,5-7,9-14
We present a case of refractory levamisole-induced vasculitis and review its clinical presentation, diagnostic approach, laboratory findings, histology, and management. Furthermore, we discuss the possibility of a new treatment option for levamisole-induced vasculitis for patients with refractory disease or for patients who continue to use levamisole.
Case Report
A 49-year-old man with a history of polysubstance abuse presented with intermittent fevers and painful swollen ears as well as joint pain of 3 weeks’ duration. One week after the lesions developed on the ears, similar lesions were seen on the legs, arms, and trunk. He admitted to cocaine use 3 weeks prior to presentation when the symptoms began.
On physical examination, violaceous patches with necrotic bleeding edges and overlying black eschars were noted on the helices, antihelices, and ear lobules bilaterally (Figure 1). Retiform, purpuric to dark brown patches, some with signs of epidermal necrosis, were scattered on the arms, legs, and chest (Figure 2).
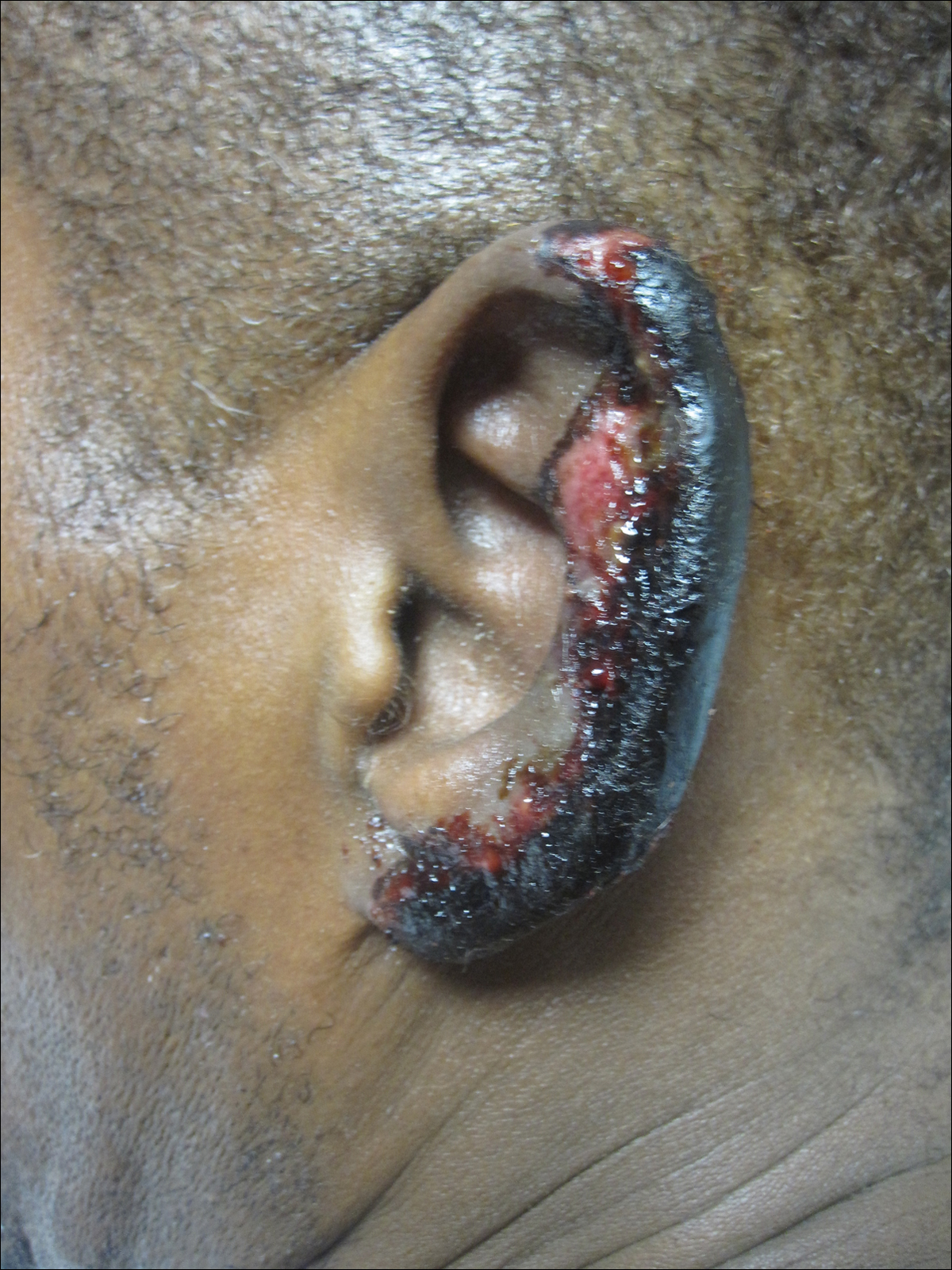
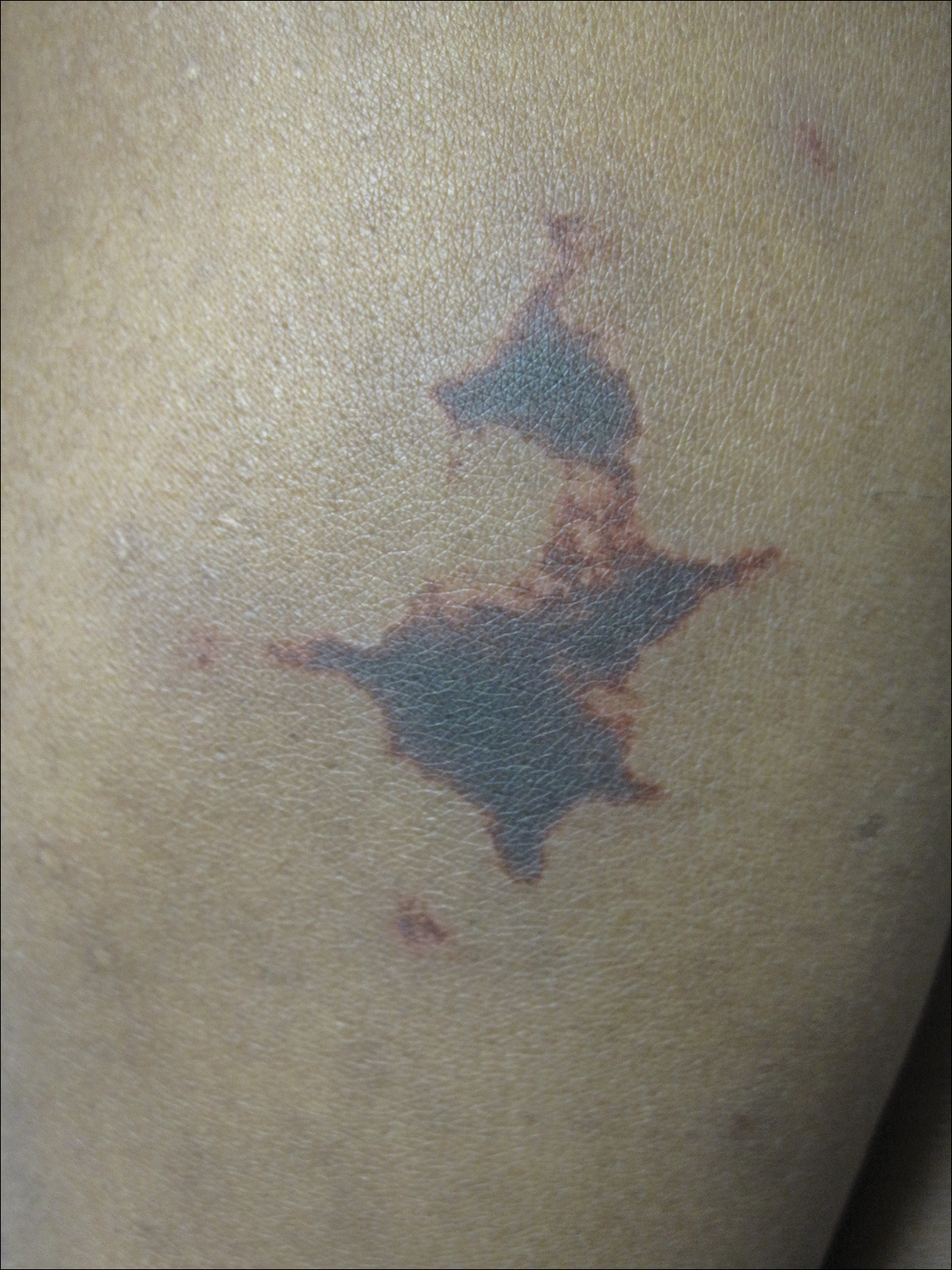
Laboratory examination revealed renal failure, anemia of chronic disease, and thrombocytosis (Table). The patient also screened positive for lupus anticoagulant and antinuclear antibodies and had elevated p-ANCA and anti–double-stranded DNA (Table). He also had an elevated sedimentation rate (109 mm/h [reference range, 0–20 mm/h]) and C-reactive protein level (11.3 mg/dL [reference range, 0–1.0 mg/dL])(Table). Urine toxicology was positive for cocaine.
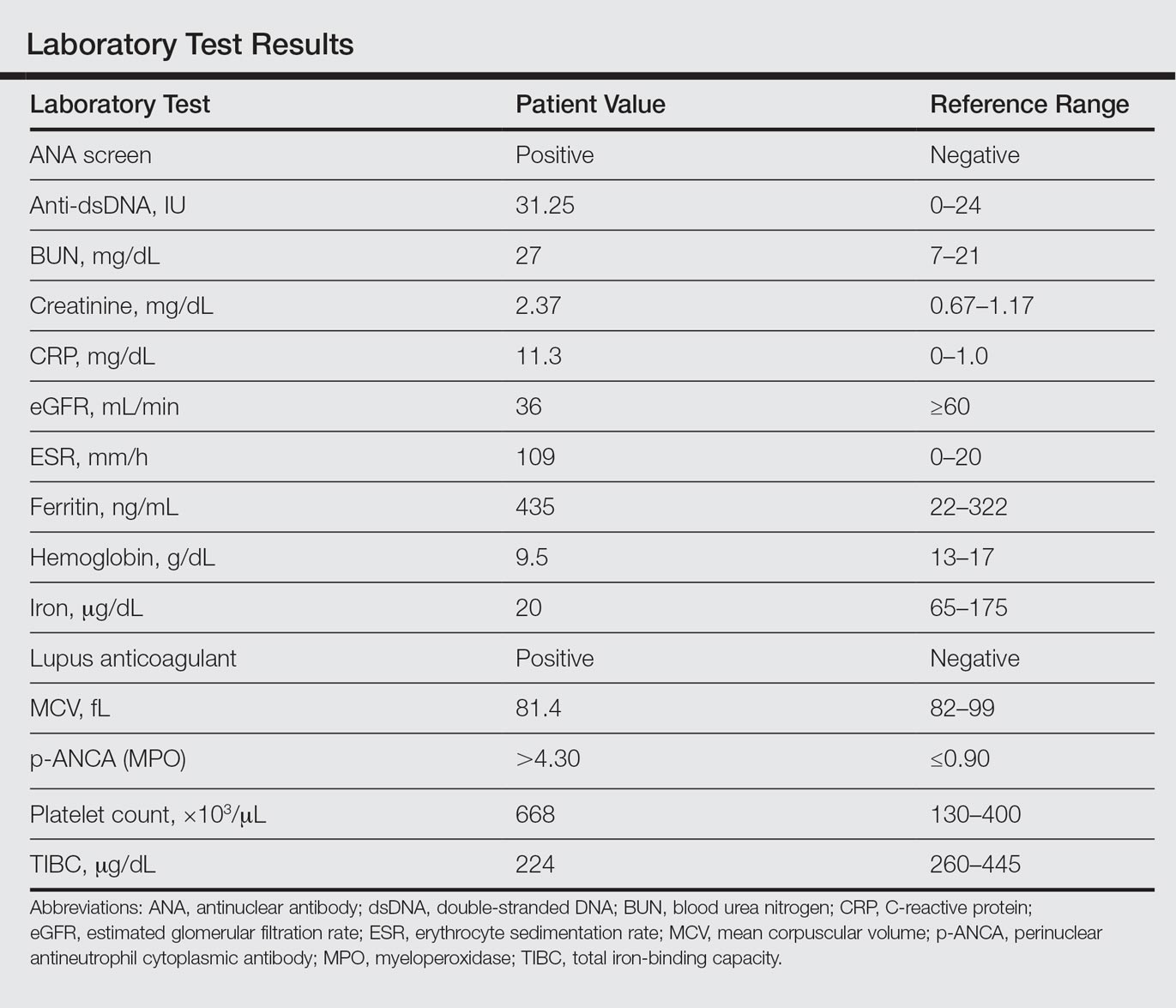
A punch biopsy of the left thigh was performed on the edge of a retiform purpuric patch. Histopathologic examination revealed epidermal necrosis with subjacent intraluminal vascular thrombi along with extravasated red blood cells and neutrophilic debris (leukocytoclasis) and fibrin in and around vessel walls, consistent with vasculitis (Figure 3).
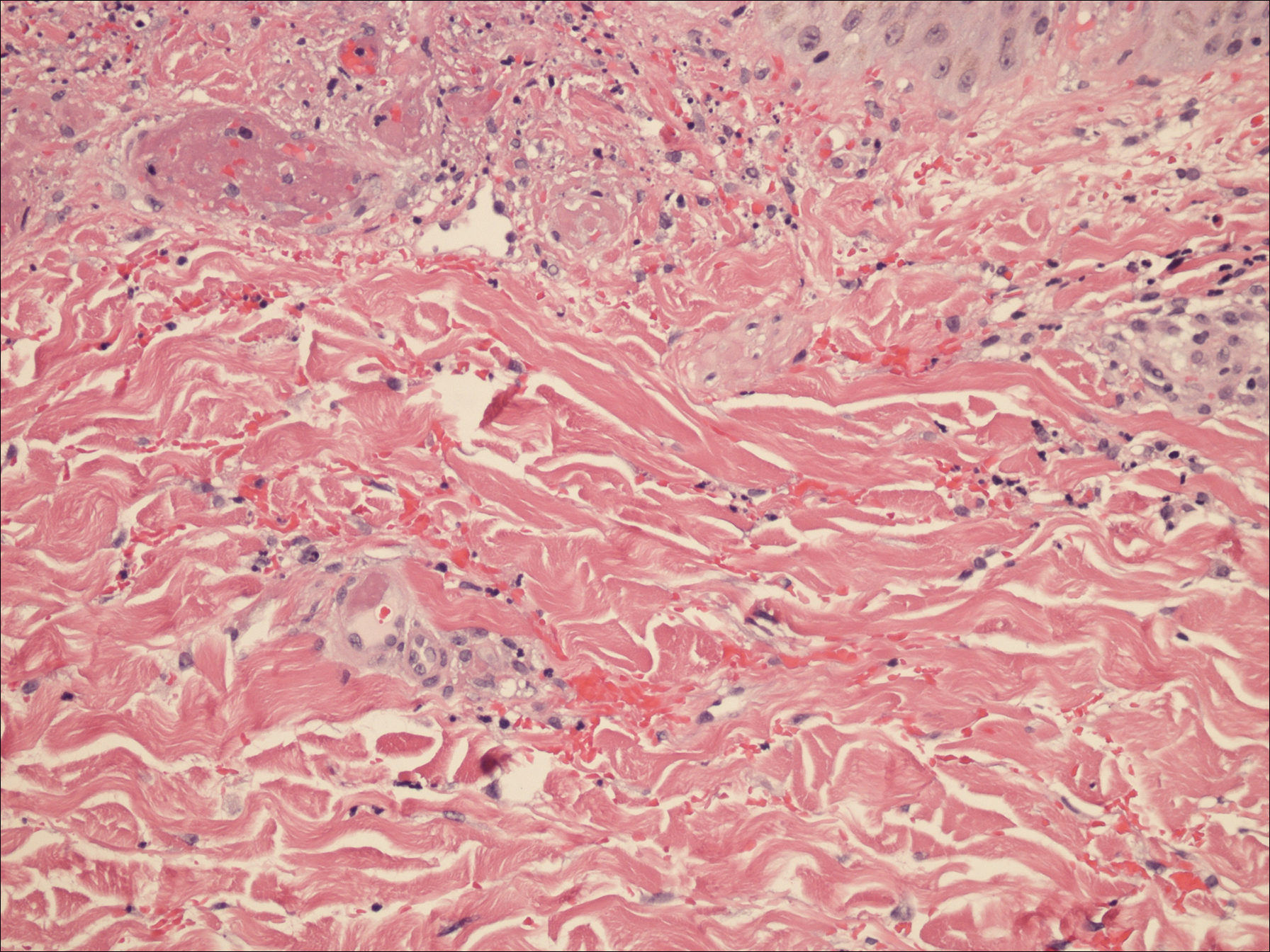
The patient was admitted to the hospital for pain management and wound care. Despite cocaine cessation and oral prednisone taper, the lesions on the legs worsened over the next several weeks. His condition was further complicated by wound infections, nonhealing ulcers, and subjective fevers and chills requiring frequent hospitalization. The patient was managed by the dermatology department as an outpatient and in clinic between hospital visits. He was treated with antibiotics, ulcer debridement, compression wraps, and aspirin (81 mg once daily) with moderate improvement.
Ten weeks after the first visit, the patient returned with worsening and recurrent leg and ear lesions. He denied any cocaine use since the initial hospital admission; however, a toxicology screen was never obtained. It was decided that the patient would need additional treatment along with traditional trigger (cocaine) avoidance and wound care. Combined treatment with aspirin (81 mg once daily), oral prednisone (40 mg once daily), and vardenafil hydrochloride (20 mg twice weekly) was initiated. At the end of week 1, the patient began to exhibit signs of improvement, which continued over the next 4 weeks. He was then lost to follow-up.
Comment
Our patient presented with severe necrotizing cutaneous vasculitis, likely secondary to levamisole exposure. Some of our patient’s cutaneous findings may be explained exclusively on the basis of cocaine exposure, but the characteristic lesion distribution and histopathologic findings along with the evidence of autoantibody positivity and concurrent arthralgias make the combination of levamisole and cocaine a more likely cause. Similar skin lesions were first described in children treated with levamisole for nephrotic syndrome.2 The most common site of clinical involvement in these children was the ears, as seen in our patient. Our patient tested positive for p-ANCA, which is the most commonly reported autoantibody associated with this patient population. Sixty-one percent (20/33) of patients with levamisole-induced vasculitis from 2 separate reviews showed p-ANCA positivity.7,10
On histopathology, our patient’s skin biopsy findings were consistent with those of prior reports of levamisole-induced vasculitis, which describe patterns of thrombotic vasculitis, leukocytoclasis, and fibrin deposition or occlusive disease.2,6,7,9-14 Mixed histologic findings of vasculitis and thrombosis, usually with varying ages of thrombi, are characteristic of levamisole-induced purpura. In addition, the disease can present nonspecifically with pure microvascular thrombosis without vasculitis, especially later in the course.9
The recommended management of levamisole-induced vasculitis currently involves the withdrawal of the culprit adulterated cocaine along with supportive treatment. Spontaneous and complete clinical resolution of lesions has been reported within 2 to 3 weeks and serology normalization within 2 to 14 months of levamisole cessation.2,6 A 2011 review of patients with levamisole-induced vasculitis reported 66% (19/29) of cases with either full cutaneous resolution after levamisole withdrawal or recurrence with resumed use, supporting a causal relationship.7 Walsh et al9 described 2 patients with recurrent and exacerbated retiform purpura following cocaine binges. Both of these patients had urine samples that tested positive for levamisole.9 In more severe cases, medications shown to be effective include colchicine, polidocanol, antibiotics, methotrexate, anticoagulants, and most commonly systemic corticosteroids.7,10,11,15 Nonsteroidal anti-inflammatory drugs were successful in treating lesions in 2 patients with concurrent arthralgia.7 Rarely, patients have required surgical debridement or skin grafting due to advanced disease at initial presentation.9,12-14 One of the most severe cases of levamisole-induced vasculitis reported in the literature involved 52% of the patient’s total body surface area with skin, soft tissue, and bony necrosis requiring nasal amputation, upper lip excision, skin grafting, and extremity amputation.14 Another severe case with widespread skin involvement was recently reported.16
For unclear reasons, our patient continued to develop cutaneous lesions despite self-reported cocaine cessation. Complete resolution required the combination of vardenafil, prednisone, and aspirin, along with debridement and wound care. Vardenafil, a selective phosphodiesterase 5 inhibitor, enhances the effect of nitrous oxide by increasing levels of cyclic guanosine monophosphate,17 which results in smooth muscle relaxation and vasodilatation. The primary indication for vardenafil is the treatment of erectile dysfunction, but it often is used off label in diseases that may benefit from vasodilatation. Because of its mechanism of action, it is understandable that a vasodilator such as vardenafil could be therapeutic in a condition associated with thrombosis. Moreover, the autoinflammatory nature of levamisole-induced vasculitis makes corticosteroid treatment effective. Given the 10-week delay in improvement, we suspect that it was the combination of treatment or an individual agent that led to our patient’s eventual recovery.
There are few reports in the literature focusing on optimal treatment of levamisole-induced vasculitis and none that mention alternative management for patients who continue to develop new lesions despite cocaine avoidance. Although the discontinuation of levamisole seems to be imperative for resolution of cutaneous lesions, it may not always be enough. It is possible that there is a subpopulation of patients that may not respond to the simple withdrawal of cocaine. It also should be mentioned that there was no urine toxicology screen obtained to support our patient’s reported cocaine cessation. Therefore, it is possible that his worsening condition was secondary to continued cocaine use. However, the patient successfully responded to the combination of vardenafil and prednisone, regardless of whether his condition persisted due to continued use of cocaine or not. This case suggests the possibility of a new treatment option for levamisole-induced vasculitis for patients who continue to use levamisole despite instruction for cessation or for patients with refractory disease.
Conclusion
A trial of prednisone and vardenafil should be considered for patients with refractory levamisole-induced vasculitis. Further studies and discussions of disease course are needed to identify the best treatment of this skin condition, especially for patients with refractory lesions.
- Scheinfeld N, Rosenberg JD, Weinberg JM. Levamisole in dermatology: a review. Am J Clin Dermatol. 2004;5:97-104.
- Rongioletti F, Ghio L, Ginevri F, et al. Purpura of the ears: a distinctive vasculopathy with circulating autoantibodies complicating long-term treatment with levamisole in children. Br J Dermatol. 1999;140:948-951.
- National Drug Threat Assessment 2011. US Department of Justice National Drug Intelligence Center website. https://www.justice.gov/archive/ndic/pubs44/44849/44849p.pdf. Published August 2011. Accessed August 7, 2016.
- Buchanan JA, Heard K, Burbach C, et al. Prevalence of levamisole in urine toxicology screens positive for cocaine in an inner-city hospital. JAMA. 2011;305:1657-1658.
- Gross RL, Brucker J, Bahce-Altuntas A, et al. A novel cutaneous vasculitis syndrome induced by levamisole-contaminated cocaine. Clin Rheumatol. 2011;30:1385-1392.
- Waller JM, Feramisco JD, Alberta-Wszolek L, et al. Cocaine-associated retiform purpura and neutropenia: is levamisole the culprit? J Am Acad Dermatol. 2010;63:530-535.
- Poon SH, Baliog CR, Sams RN, et al. Syndrome of cocaine-levamisole-induced cutaneous vasculitis and immune-mediated leukopenia. Semin Arthritis Rheum. 2011;41:434-444.
- Brewer JD, Meves A, Bostwick JM, et al. Cocaine abuse: dermatologic manifestations and therapeutic approaches. J Am Acad Dermatol. 2008;59:483-487.
- Walsh NMG, Green PJ, Burlingame RW, et al. Cocaine-related retiform purpura: evidence to incriminate the adulterant, levamisole. J Cutan Pathol. 2010;37:1212-1219.
- Chung C, Tumeh PC, Birnbaum R, et al. Characteristic purpura of the ears, vasculitis, and neutropenia—a potential public health epidemic associated with levamisole adultered cocaine. J Am Acad Dermatol. 2011;65:722-725.
- Kahn TA, Cuchacovich R, Espinoza LR, et al. Vasculopathy, hematological, and immune abnormalities associated with levamisole-contaminated cocaine use. Semin Arthritis Rheum. 2011;41:445-454.
- Graf J, Lynch K, Yeh CL, et al. Purpura, cutaneous necrosis, and antineutrophil cytoplasmic antibodies associated with levamisole-adulterated cocaine. Arthritis Rheum. 2011;63:3998-4001.
- Farmer RW, Malhotra PS, Mays MP, et al. Necrotizing peripheral vasculitis/vasculopathy following the use of cocaine laced with levamisole. J Burn Care Res. 2012;33:e6-e11.
- Ching JA, Smith DJ Jr. Levamisole-induced skin necrosis of skin, soft tissue, and bone: case report and review of literature. J Burn Care Res. 2012;33:e1-e5.
- Buchanan JA, Vogel JA, Eberhardt AM. Levamisole-induced occlusive necrotizing vasculitis of the ears after use of cocaine contaminated with levamisole. J Med Toxicol. 2011;7:83-84.
- Graff N, Whitworth K, Trigger C. Purpuric skin eruption in an illicit drug user: levamisole-induced vasculitis. Am J Emer Med. 2016;34:1321.
- Schwartz BG, Kloner RA. Drug interactions with phosphodiesterase-5 inhibitors used for the treatment of erectile dysfunction or pulmonary hypertension. Circulation. 2010;122:88-95.
Levamisole is an immunomodulatory drug that had been used to treat various medical conditions, including parasitic infections, nephrotic syndrome, and colorectal cancer,1 before being withdrawn from the US market in 2000.The most common reasons for levamisole discontinuation were leukopenia and rashes (1%–2%),1 many of which included leg ulcers and necrotizing purpura of the ears.1,2 The drug is currently available only as a deworming agent in veterinary medicine.
Since 2007, increasing amounts of levamisole have been used as an adulterant in cocaine. In 2007, less than 10% of cocaine was contaminated with levamisole, with an increase to 77% by 2010.3 In addition, 78% of 249 urine toxicology screens that were positive for cocaine in an inner city hospital also tested positive for levamisole.4 Levamisole-cut cocaine has become a concern because it is associated with a life-threatening syndrome involving a necrotizing purpuric rash, autoantibody production, and leukopenia.5
Levamisole-induced vasculitis is an independent entity from cocaine-induced vasculitis, which is associated with skin findings ranging from palpable purpura and chronic ulcers to digital infarction secondary to its vasospastic activity.6-8 Cocaine-induced vasculopathy has been related to cytoplasmic antineutrophil cytoplasmic antibody positivity and often resembles Wegener granulomatosis.6 Although both cocaine and levamisole have reportedly caused acrally distributed purpura and vasculopathy, levamisole is specifically associated with retiform purpura, ear involvement, and leukopenia.6,9 In addition, levamisole-induced skin reactions have been linked to specific antibodies, including antinuclear, antiphospholipid, and perinuclear antineutrophil cytoplasmic antibody (p-ANCA).2,5-7,9-14
We present a case of refractory levamisole-induced vasculitis and review its clinical presentation, diagnostic approach, laboratory findings, histology, and management. Furthermore, we discuss the possibility of a new treatment option for levamisole-induced vasculitis for patients with refractory disease or for patients who continue to use levamisole.
Case Report
A 49-year-old man with a history of polysubstance abuse presented with intermittent fevers and painful swollen ears as well as joint pain of 3 weeks’ duration. One week after the lesions developed on the ears, similar lesions were seen on the legs, arms, and trunk. He admitted to cocaine use 3 weeks prior to presentation when the symptoms began.
On physical examination, violaceous patches with necrotic bleeding edges and overlying black eschars were noted on the helices, antihelices, and ear lobules bilaterally (Figure 1). Retiform, purpuric to dark brown patches, some with signs of epidermal necrosis, were scattered on the arms, legs, and chest (Figure 2).


Laboratory examination revealed renal failure, anemia of chronic disease, and thrombocytosis (Table). The patient also screened positive for lupus anticoagulant and antinuclear antibodies and had elevated p-ANCA and anti–double-stranded DNA (Table). He also had an elevated sedimentation rate (109 mm/h [reference range, 0–20 mm/h]) and C-reactive protein level (11.3 mg/dL [reference range, 0–1.0 mg/dL])(Table). Urine toxicology was positive for cocaine.

A punch biopsy of the left thigh was performed on the edge of a retiform purpuric patch. Histopathologic examination revealed epidermal necrosis with subjacent intraluminal vascular thrombi along with extravasated red blood cells and neutrophilic debris (leukocytoclasis) and fibrin in and around vessel walls, consistent with vasculitis (Figure 3).

The patient was admitted to the hospital for pain management and wound care. Despite cocaine cessation and oral prednisone taper, the lesions on the legs worsened over the next several weeks. His condition was further complicated by wound infections, nonhealing ulcers, and subjective fevers and chills requiring frequent hospitalization. The patient was managed by the dermatology department as an outpatient and in clinic between hospital visits. He was treated with antibiotics, ulcer debridement, compression wraps, and aspirin (81 mg once daily) with moderate improvement.
Ten weeks after the first visit, the patient returned with worsening and recurrent leg and ear lesions. He denied any cocaine use since the initial hospital admission; however, a toxicology screen was never obtained. It was decided that the patient would need additional treatment along with traditional trigger (cocaine) avoidance and wound care. Combined treatment with aspirin (81 mg once daily), oral prednisone (40 mg once daily), and vardenafil hydrochloride (20 mg twice weekly) was initiated. At the end of week 1, the patient began to exhibit signs of improvement, which continued over the next 4 weeks. He was then lost to follow-up.
Comment
Our patient presented with severe necrotizing cutaneous vasculitis, likely secondary to levamisole exposure. Some of our patient’s cutaneous findings may be explained exclusively on the basis of cocaine exposure, but the characteristic lesion distribution and histopathologic findings along with the evidence of autoantibody positivity and concurrent arthralgias make the combination of levamisole and cocaine a more likely cause. Similar skin lesions were first described in children treated with levamisole for nephrotic syndrome.2 The most common site of clinical involvement in these children was the ears, as seen in our patient. Our patient tested positive for p-ANCA, which is the most commonly reported autoantibody associated with this patient population. Sixty-one percent (20/33) of patients with levamisole-induced vasculitis from 2 separate reviews showed p-ANCA positivity.7,10
On histopathology, our patient’s skin biopsy findings were consistent with those of prior reports of levamisole-induced vasculitis, which describe patterns of thrombotic vasculitis, leukocytoclasis, and fibrin deposition or occlusive disease.2,6,7,9-14 Mixed histologic findings of vasculitis and thrombosis, usually with varying ages of thrombi, are characteristic of levamisole-induced purpura. In addition, the disease can present nonspecifically with pure microvascular thrombosis without vasculitis, especially later in the course.9
The recommended management of levamisole-induced vasculitis currently involves the withdrawal of the culprit adulterated cocaine along with supportive treatment. Spontaneous and complete clinical resolution of lesions has been reported within 2 to 3 weeks and serology normalization within 2 to 14 months of levamisole cessation.2,6 A 2011 review of patients with levamisole-induced vasculitis reported 66% (19/29) of cases with either full cutaneous resolution after levamisole withdrawal or recurrence with resumed use, supporting a causal relationship.7 Walsh et al9 described 2 patients with recurrent and exacerbated retiform purpura following cocaine binges. Both of these patients had urine samples that tested positive for levamisole.9 In more severe cases, medications shown to be effective include colchicine, polidocanol, antibiotics, methotrexate, anticoagulants, and most commonly systemic corticosteroids.7,10,11,15 Nonsteroidal anti-inflammatory drugs were successful in treating lesions in 2 patients with concurrent arthralgia.7 Rarely, patients have required surgical debridement or skin grafting due to advanced disease at initial presentation.9,12-14 One of the most severe cases of levamisole-induced vasculitis reported in the literature involved 52% of the patient’s total body surface area with skin, soft tissue, and bony necrosis requiring nasal amputation, upper lip excision, skin grafting, and extremity amputation.14 Another severe case with widespread skin involvement was recently reported.16
For unclear reasons, our patient continued to develop cutaneous lesions despite self-reported cocaine cessation. Complete resolution required the combination of vardenafil, prednisone, and aspirin, along with debridement and wound care. Vardenafil, a selective phosphodiesterase 5 inhibitor, enhances the effect of nitrous oxide by increasing levels of cyclic guanosine monophosphate,17 which results in smooth muscle relaxation and vasodilatation. The primary indication for vardenafil is the treatment of erectile dysfunction, but it often is used off label in diseases that may benefit from vasodilatation. Because of its mechanism of action, it is understandable that a vasodilator such as vardenafil could be therapeutic in a condition associated with thrombosis. Moreover, the autoinflammatory nature of levamisole-induced vasculitis makes corticosteroid treatment effective. Given the 10-week delay in improvement, we suspect that it was the combination of treatment or an individual agent that led to our patient’s eventual recovery.
There are few reports in the literature focusing on optimal treatment of levamisole-induced vasculitis and none that mention alternative management for patients who continue to develop new lesions despite cocaine avoidance. Although the discontinuation of levamisole seems to be imperative for resolution of cutaneous lesions, it may not always be enough. It is possible that there is a subpopulation of patients that may not respond to the simple withdrawal of cocaine. It also should be mentioned that there was no urine toxicology screen obtained to support our patient’s reported cocaine cessation. Therefore, it is possible that his worsening condition was secondary to continued cocaine use. However, the patient successfully responded to the combination of vardenafil and prednisone, regardless of whether his condition persisted due to continued use of cocaine or not. This case suggests the possibility of a new treatment option for levamisole-induced vasculitis for patients who continue to use levamisole despite instruction for cessation or for patients with refractory disease.
Conclusion
A trial of prednisone and vardenafil should be considered for patients with refractory levamisole-induced vasculitis. Further studies and discussions of disease course are needed to identify the best treatment of this skin condition, especially for patients with refractory lesions.
Levamisole is an immunomodulatory drug that had been used to treat various medical conditions, including parasitic infections, nephrotic syndrome, and colorectal cancer,1 before being withdrawn from the US market in 2000.The most common reasons for levamisole discontinuation were leukopenia and rashes (1%–2%),1 many of which included leg ulcers and necrotizing purpura of the ears.1,2 The drug is currently available only as a deworming agent in veterinary medicine.
Since 2007, increasing amounts of levamisole have been used as an adulterant in cocaine. In 2007, less than 10% of cocaine was contaminated with levamisole, with an increase to 77% by 2010.3 In addition, 78% of 249 urine toxicology screens that were positive for cocaine in an inner city hospital also tested positive for levamisole.4 Levamisole-cut cocaine has become a concern because it is associated with a life-threatening syndrome involving a necrotizing purpuric rash, autoantibody production, and leukopenia.5
Levamisole-induced vasculitis is an independent entity from cocaine-induced vasculitis, which is associated with skin findings ranging from palpable purpura and chronic ulcers to digital infarction secondary to its vasospastic activity.6-8 Cocaine-induced vasculopathy has been related to cytoplasmic antineutrophil cytoplasmic antibody positivity and often resembles Wegener granulomatosis.6 Although both cocaine and levamisole have reportedly caused acrally distributed purpura and vasculopathy, levamisole is specifically associated with retiform purpura, ear involvement, and leukopenia.6,9 In addition, levamisole-induced skin reactions have been linked to specific antibodies, including antinuclear, antiphospholipid, and perinuclear antineutrophil cytoplasmic antibody (p-ANCA).2,5-7,9-14
We present a case of refractory levamisole-induced vasculitis and review its clinical presentation, diagnostic approach, laboratory findings, histology, and management. Furthermore, we discuss the possibility of a new treatment option for levamisole-induced vasculitis for patients with refractory disease or for patients who continue to use levamisole.
Case Report
A 49-year-old man with a history of polysubstance abuse presented with intermittent fevers and painful swollen ears as well as joint pain of 3 weeks’ duration. One week after the lesions developed on the ears, similar lesions were seen on the legs, arms, and trunk. He admitted to cocaine use 3 weeks prior to presentation when the symptoms began.
On physical examination, violaceous patches with necrotic bleeding edges and overlying black eschars were noted on the helices, antihelices, and ear lobules bilaterally (Figure 1). Retiform, purpuric to dark brown patches, some with signs of epidermal necrosis, were scattered on the arms, legs, and chest (Figure 2).


Laboratory examination revealed renal failure, anemia of chronic disease, and thrombocytosis (Table). The patient also screened positive for lupus anticoagulant and antinuclear antibodies and had elevated p-ANCA and anti–double-stranded DNA (Table). He also had an elevated sedimentation rate (109 mm/h [reference range, 0–20 mm/h]) and C-reactive protein level (11.3 mg/dL [reference range, 0–1.0 mg/dL])(Table). Urine toxicology was positive for cocaine.

A punch biopsy of the left thigh was performed on the edge of a retiform purpuric patch. Histopathologic examination revealed epidermal necrosis with subjacent intraluminal vascular thrombi along with extravasated red blood cells and neutrophilic debris (leukocytoclasis) and fibrin in and around vessel walls, consistent with vasculitis (Figure 3).

The patient was admitted to the hospital for pain management and wound care. Despite cocaine cessation and oral prednisone taper, the lesions on the legs worsened over the next several weeks. His condition was further complicated by wound infections, nonhealing ulcers, and subjective fevers and chills requiring frequent hospitalization. The patient was managed by the dermatology department as an outpatient and in clinic between hospital visits. He was treated with antibiotics, ulcer debridement, compression wraps, and aspirin (81 mg once daily) with moderate improvement.
Ten weeks after the first visit, the patient returned with worsening and recurrent leg and ear lesions. He denied any cocaine use since the initial hospital admission; however, a toxicology screen was never obtained. It was decided that the patient would need additional treatment along with traditional trigger (cocaine) avoidance and wound care. Combined treatment with aspirin (81 mg once daily), oral prednisone (40 mg once daily), and vardenafil hydrochloride (20 mg twice weekly) was initiated. At the end of week 1, the patient began to exhibit signs of improvement, which continued over the next 4 weeks. He was then lost to follow-up.
Comment
Our patient presented with severe necrotizing cutaneous vasculitis, likely secondary to levamisole exposure. Some of our patient’s cutaneous findings may be explained exclusively on the basis of cocaine exposure, but the characteristic lesion distribution and histopathologic findings along with the evidence of autoantibody positivity and concurrent arthralgias make the combination of levamisole and cocaine a more likely cause. Similar skin lesions were first described in children treated with levamisole for nephrotic syndrome.2 The most common site of clinical involvement in these children was the ears, as seen in our patient. Our patient tested positive for p-ANCA, which is the most commonly reported autoantibody associated with this patient population. Sixty-one percent (20/33) of patients with levamisole-induced vasculitis from 2 separate reviews showed p-ANCA positivity.7,10
On histopathology, our patient’s skin biopsy findings were consistent with those of prior reports of levamisole-induced vasculitis, which describe patterns of thrombotic vasculitis, leukocytoclasis, and fibrin deposition or occlusive disease.2,6,7,9-14 Mixed histologic findings of vasculitis and thrombosis, usually with varying ages of thrombi, are characteristic of levamisole-induced purpura. In addition, the disease can present nonspecifically with pure microvascular thrombosis without vasculitis, especially later in the course.9
The recommended management of levamisole-induced vasculitis currently involves the withdrawal of the culprit adulterated cocaine along with supportive treatment. Spontaneous and complete clinical resolution of lesions has been reported within 2 to 3 weeks and serology normalization within 2 to 14 months of levamisole cessation.2,6 A 2011 review of patients with levamisole-induced vasculitis reported 66% (19/29) of cases with either full cutaneous resolution after levamisole withdrawal or recurrence with resumed use, supporting a causal relationship.7 Walsh et al9 described 2 patients with recurrent and exacerbated retiform purpura following cocaine binges. Both of these patients had urine samples that tested positive for levamisole.9 In more severe cases, medications shown to be effective include colchicine, polidocanol, antibiotics, methotrexate, anticoagulants, and most commonly systemic corticosteroids.7,10,11,15 Nonsteroidal anti-inflammatory drugs were successful in treating lesions in 2 patients with concurrent arthralgia.7 Rarely, patients have required surgical debridement or skin grafting due to advanced disease at initial presentation.9,12-14 One of the most severe cases of levamisole-induced vasculitis reported in the literature involved 52% of the patient’s total body surface area with skin, soft tissue, and bony necrosis requiring nasal amputation, upper lip excision, skin grafting, and extremity amputation.14 Another severe case with widespread skin involvement was recently reported.16
For unclear reasons, our patient continued to develop cutaneous lesions despite self-reported cocaine cessation. Complete resolution required the combination of vardenafil, prednisone, and aspirin, along with debridement and wound care. Vardenafil, a selective phosphodiesterase 5 inhibitor, enhances the effect of nitrous oxide by increasing levels of cyclic guanosine monophosphate,17 which results in smooth muscle relaxation and vasodilatation. The primary indication for vardenafil is the treatment of erectile dysfunction, but it often is used off label in diseases that may benefit from vasodilatation. Because of its mechanism of action, it is understandable that a vasodilator such as vardenafil could be therapeutic in a condition associated with thrombosis. Moreover, the autoinflammatory nature of levamisole-induced vasculitis makes corticosteroid treatment effective. Given the 10-week delay in improvement, we suspect that it was the combination of treatment or an individual agent that led to our patient’s eventual recovery.
There are few reports in the literature focusing on optimal treatment of levamisole-induced vasculitis and none that mention alternative management for patients who continue to develop new lesions despite cocaine avoidance. Although the discontinuation of levamisole seems to be imperative for resolution of cutaneous lesions, it may not always be enough. It is possible that there is a subpopulation of patients that may not respond to the simple withdrawal of cocaine. It also should be mentioned that there was no urine toxicology screen obtained to support our patient’s reported cocaine cessation. Therefore, it is possible that his worsening condition was secondary to continued cocaine use. However, the patient successfully responded to the combination of vardenafil and prednisone, regardless of whether his condition persisted due to continued use of cocaine or not. This case suggests the possibility of a new treatment option for levamisole-induced vasculitis for patients who continue to use levamisole despite instruction for cessation or for patients with refractory disease.
Conclusion
A trial of prednisone and vardenafil should be considered for patients with refractory levamisole-induced vasculitis. Further studies and discussions of disease course are needed to identify the best treatment of this skin condition, especially for patients with refractory lesions.
- Scheinfeld N, Rosenberg JD, Weinberg JM. Levamisole in dermatology: a review. Am J Clin Dermatol. 2004;5:97-104.
- Rongioletti F, Ghio L, Ginevri F, et al. Purpura of the ears: a distinctive vasculopathy with circulating autoantibodies complicating long-term treatment with levamisole in children. Br J Dermatol. 1999;140:948-951.
- National Drug Threat Assessment 2011. US Department of Justice National Drug Intelligence Center website. https://www.justice.gov/archive/ndic/pubs44/44849/44849p.pdf. Published August 2011. Accessed August 7, 2016.
- Buchanan JA, Heard K, Burbach C, et al. Prevalence of levamisole in urine toxicology screens positive for cocaine in an inner-city hospital. JAMA. 2011;305:1657-1658.
- Gross RL, Brucker J, Bahce-Altuntas A, et al. A novel cutaneous vasculitis syndrome induced by levamisole-contaminated cocaine. Clin Rheumatol. 2011;30:1385-1392.
- Waller JM, Feramisco JD, Alberta-Wszolek L, et al. Cocaine-associated retiform purpura and neutropenia: is levamisole the culprit? J Am Acad Dermatol. 2010;63:530-535.
- Poon SH, Baliog CR, Sams RN, et al. Syndrome of cocaine-levamisole-induced cutaneous vasculitis and immune-mediated leukopenia. Semin Arthritis Rheum. 2011;41:434-444.
- Brewer JD, Meves A, Bostwick JM, et al. Cocaine abuse: dermatologic manifestations and therapeutic approaches. J Am Acad Dermatol. 2008;59:483-487.
- Walsh NMG, Green PJ, Burlingame RW, et al. Cocaine-related retiform purpura: evidence to incriminate the adulterant, levamisole. J Cutan Pathol. 2010;37:1212-1219.
- Chung C, Tumeh PC, Birnbaum R, et al. Characteristic purpura of the ears, vasculitis, and neutropenia—a potential public health epidemic associated with levamisole adultered cocaine. J Am Acad Dermatol. 2011;65:722-725.
- Kahn TA, Cuchacovich R, Espinoza LR, et al. Vasculopathy, hematological, and immune abnormalities associated with levamisole-contaminated cocaine use. Semin Arthritis Rheum. 2011;41:445-454.
- Graf J, Lynch K, Yeh CL, et al. Purpura, cutaneous necrosis, and antineutrophil cytoplasmic antibodies associated with levamisole-adulterated cocaine. Arthritis Rheum. 2011;63:3998-4001.
- Farmer RW, Malhotra PS, Mays MP, et al. Necrotizing peripheral vasculitis/vasculopathy following the use of cocaine laced with levamisole. J Burn Care Res. 2012;33:e6-e11.
- Ching JA, Smith DJ Jr. Levamisole-induced skin necrosis of skin, soft tissue, and bone: case report and review of literature. J Burn Care Res. 2012;33:e1-e5.
- Buchanan JA, Vogel JA, Eberhardt AM. Levamisole-induced occlusive necrotizing vasculitis of the ears after use of cocaine contaminated with levamisole. J Med Toxicol. 2011;7:83-84.
- Graff N, Whitworth K, Trigger C. Purpuric skin eruption in an illicit drug user: levamisole-induced vasculitis. Am J Emer Med. 2016;34:1321.
- Schwartz BG, Kloner RA. Drug interactions with phosphodiesterase-5 inhibitors used for the treatment of erectile dysfunction or pulmonary hypertension. Circulation. 2010;122:88-95.
- Scheinfeld N, Rosenberg JD, Weinberg JM. Levamisole in dermatology: a review. Am J Clin Dermatol. 2004;5:97-104.
- Rongioletti F, Ghio L, Ginevri F, et al. Purpura of the ears: a distinctive vasculopathy with circulating autoantibodies complicating long-term treatment with levamisole in children. Br J Dermatol. 1999;140:948-951.
- National Drug Threat Assessment 2011. US Department of Justice National Drug Intelligence Center website. https://www.justice.gov/archive/ndic/pubs44/44849/44849p.pdf. Published August 2011. Accessed August 7, 2016.
- Buchanan JA, Heard K, Burbach C, et al. Prevalence of levamisole in urine toxicology screens positive for cocaine in an inner-city hospital. JAMA. 2011;305:1657-1658.
- Gross RL, Brucker J, Bahce-Altuntas A, et al. A novel cutaneous vasculitis syndrome induced by levamisole-contaminated cocaine. Clin Rheumatol. 2011;30:1385-1392.
- Waller JM, Feramisco JD, Alberta-Wszolek L, et al. Cocaine-associated retiform purpura and neutropenia: is levamisole the culprit? J Am Acad Dermatol. 2010;63:530-535.
- Poon SH, Baliog CR, Sams RN, et al. Syndrome of cocaine-levamisole-induced cutaneous vasculitis and immune-mediated leukopenia. Semin Arthritis Rheum. 2011;41:434-444.
- Brewer JD, Meves A, Bostwick JM, et al. Cocaine abuse: dermatologic manifestations and therapeutic approaches. J Am Acad Dermatol. 2008;59:483-487.
- Walsh NMG, Green PJ, Burlingame RW, et al. Cocaine-related retiform purpura: evidence to incriminate the adulterant, levamisole. J Cutan Pathol. 2010;37:1212-1219.
- Chung C, Tumeh PC, Birnbaum R, et al. Characteristic purpura of the ears, vasculitis, and neutropenia—a potential public health epidemic associated with levamisole adultered cocaine. J Am Acad Dermatol. 2011;65:722-725.
- Kahn TA, Cuchacovich R, Espinoza LR, et al. Vasculopathy, hematological, and immune abnormalities associated with levamisole-contaminated cocaine use. Semin Arthritis Rheum. 2011;41:445-454.
- Graf J, Lynch K, Yeh CL, et al. Purpura, cutaneous necrosis, and antineutrophil cytoplasmic antibodies associated with levamisole-adulterated cocaine. Arthritis Rheum. 2011;63:3998-4001.
- Farmer RW, Malhotra PS, Mays MP, et al. Necrotizing peripheral vasculitis/vasculopathy following the use of cocaine laced with levamisole. J Burn Care Res. 2012;33:e6-e11.
- Ching JA, Smith DJ Jr. Levamisole-induced skin necrosis of skin, soft tissue, and bone: case report and review of literature. J Burn Care Res. 2012;33:e1-e5.
- Buchanan JA, Vogel JA, Eberhardt AM. Levamisole-induced occlusive necrotizing vasculitis of the ears after use of cocaine contaminated with levamisole. J Med Toxicol. 2011;7:83-84.
- Graff N, Whitworth K, Trigger C. Purpuric skin eruption in an illicit drug user: levamisole-induced vasculitis. Am J Emer Med. 2016;34:1321.
- Schwartz BG, Kloner RA. Drug interactions with phosphodiesterase-5 inhibitors used for the treatment of erectile dysfunction or pulmonary hypertension. Circulation. 2010;122:88-95.
Practice Points
- Levamisole is an immunomodulatory drug that, before being withdrawn from the US market in 2000, was previously used to treat various medical conditions.
- A majority of the cocaine in the United States is contaminated with levamisole, which is added as an adulterant or bulking agent.
- Levamisole-cut cocaine is a concern because it is associated with a life-threatening syndrome involving a necrotizing purpuric rash, autoantibody production, and leukopenia.
- Although treatment of levamisole toxicity is primarily supportive and includes cessation of levamisole-cut cocaine, a trial of prednisone and vardenafil hydrochloride can be considered for refractory levamisole-induced vasculopathy or for patients who continue to use the drug.
Multiple Eruptive Dermatofibromas in a Patient With Sarcoidosis
To the Editor:
Dermatofibromas, the most common fibrohistiocytic tumors of the skin, are typically solitary lesions. Clustering of and multiple dermatofibromas (multiple eruptive dermatofibromas [MEDFs]) are relatively less common. The association between MEDF and systemic immunoaltered disease states such as systemic lupus erythematosus (SLE) or human immunodeficiency virus infection has been described and led to speculation that MEDF might be a result of an abnormal immune response. We report a patient with sarcoidosis who developed multiple large dermatofibromas, some clustered, on the neck, left shoulder, and back.
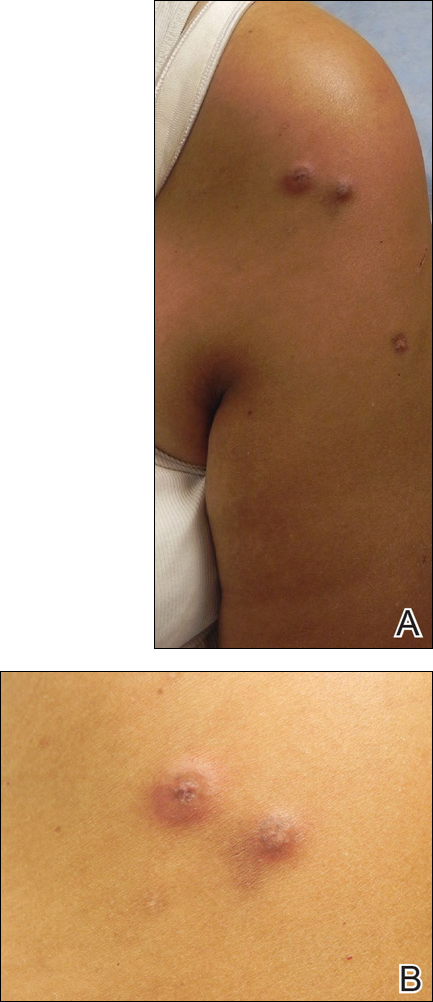
A 61-year-old woman with a history of mild pulmonary sarcoidosis confirmed by transbronchial biopsies presented to our clinic with a 2-year history of hyperpigmented papules on the trunk and extremities with subjective enlargement and increased erythema of a papule on the left shoulder over the last 6 months. She had associated pain and pruritus in the area. She was not on any systemic medications for sarcoidosis at the time. Physical examination revealed 2 large, firm, hyperpigmented nodules on the left shoulder, one with overlying erythema and mild scale (Figure 1). There also were multiple scattered hyperpigmented papules on the back, chest, and right arm that dimpled when compressed. A biopsy was obtained because of clinical concern for cutaneous sarcoidosis. Histopathologic evaluation of the largest nodule demonstrated epidermal hyperplasia with effacement of the rete ridges and a proliferation of spindle cells that wrapped around collagen fibers in the dermis, consistent with a dermatofibroma (Figure 2).
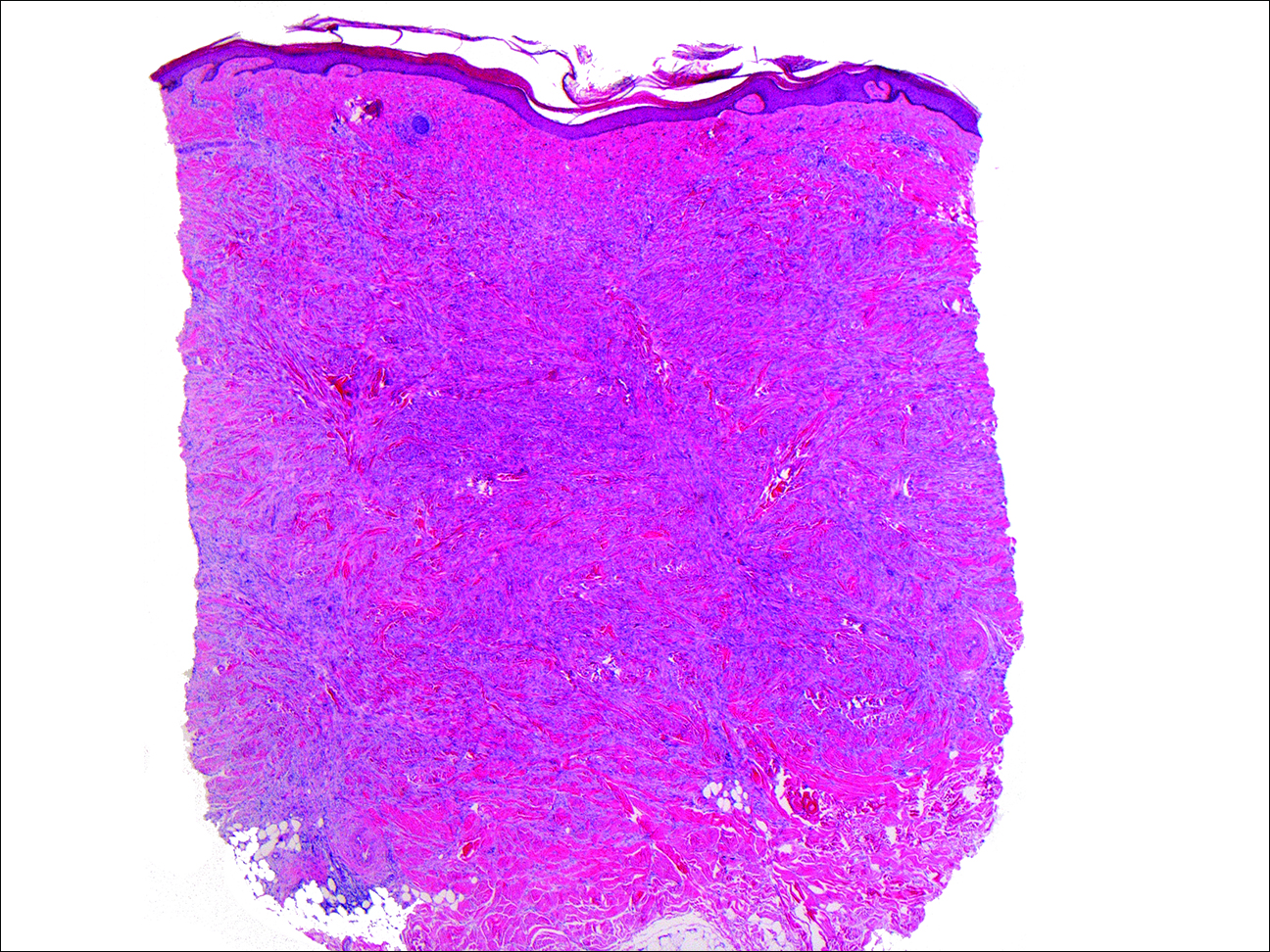
Dermatofibromas are common fibrohistiocytic neoplasms in the skin that typically present as a solitary lesion. A clustering of dermatofibromas, MEDFs, is relatively less common, representing only 0.3% of all dermatofibromas.1,2 Histopathologically, similar to solitary dermatofibromas,3 MEDF has classically been defined as more than 15 lesions, though another definition includes the appearance of several dermatofibromas over a relatively short period of time.2
Multiple eruptive dermatofibromas have been described in association with several underlying diseases. A strong association between MEDFs and immune dysregulation appears to exist, with 69% of reported cases of MEDF associated with an underlying disease, 83% of which were related to dysregulated immunity. Systemic lupus erythematosus was the most common underlying disease associated with MEDF, representing 25% of published cases.3 Multiple eruptive dermatofibromas also have been linked to other autoimmune disorders such as myasthenia gravis,4,5 Hashimoto thyroiditis,4 diabetes mellitus,6 Sjögren syndrome,7,8 dermatomyositis,9 and Graves-Basedow disease.10
Multiple eruptive dermatofibromas also have been linked to immunosuppression, including human immunodeficiency virus11; malignancy12,13; and immunosuppressive or immunomodulatory drugs such as corticosteroids,14 cyclophosphamide,5 methotrexate,9 efalizumab,15 and interferon alfa.12 The degree of immunosuppression, however, does not seem to correlate to the number of MEDFs.3 In addition, MEDFs have been reported in pregnancy and a variety of other systemic disorders including atopic dermatitis,1,16,17 hypertriglyceridemia,8 and pulmonary hypertension.18 We report a case of MEDF in a patient with sarcoidosis who was not treated with immunosuppressive medication. A report of sarcoidosis and MEDF was previously published, but the patient had been treated for many years with prednisone.19 Most reports of SLE-associated MEDF occurred in the setting of steroid use.
Although the etiology of dermatofibromas is unclear, the link between MEDFs and altered immunity has led to speculation that dermatofibromas could be a manifestation of defective autoimmune inflammatory regulation. This hypothesis has been supported by the observation that the lesions are often associated with cells that express class II MHC molecules and also bear morphologic similarity to dermal antigen-presenting cells.20 Reports of familial cases of MEDF suggest that there could be a genetic predisposition.1
The association of MEDFs and underlying immune disorders is important for clinicians to know for appropriate evaluation of potential systemic associations, including sarcoidosis. In addition, biopsy should be considered to confirm the diagnosis with large or atypical lesions to exclude other potential diagnoses. Given the protean nature of sarcoidosis, skin biopsy often is indicated to identify whether cutaneous findings are granulomatous sarcoid-related manifestations. The association of MEDFs with sarcoidosis requires further evaluation but might provide keys to understanding the pathophysiology of these lesions.
- Yazici AC, Baz K, Ikizoglu G, et al. Familial eruptive dermatofibromas in atopic dermatitis. J Eur Acad Dermatol Venereol. 2006;20:90-92.
- Niiyama S, Katsuoka K, Happle R, et al. Multiple eruptive dermatofibromas: a review of the literature. Acta Derm Venereol. 2002;82:241-244.
- Zaccaria E, Rebora A, Rongioletti F. Multiple eruptive dermatofibromas and immunosuppression: report of two cases and review of the literature. Int J Dermatol. 2008;47:723-727.
- Kimura Y, Kaneko T, Akasaka E, et al. Multiple eruptive dermatofibromas associated with Hashimoto’s thyroiditis and myasthenia gravis. Eur J Dermatol. 2010;20:538-539.
- Bargman HB, Fefferman I. Multiple eruptive dermatofibromas in a patient with myasthenia gravis treated with prednisone and cyclophosphamide. J Am Acad Dermatol. 1986;14:351-352.
- Gelfarb M, Hyman AB. Multiple noduli cutanei. an unusual case of multiple noduli cutanei in a patient with hydronephrosis. Arch Dermatol. 1962;85:89-94.
- Yamamoto T, Katayama I, Nishioka K. Mast cell numbers in multiple dermatofibromas. Dermatology. 1995;190:9-13.
- Tsunemi Y, Tada Y, Saeki H, et al. Multiple dermatofibromas in a patient with systemic lupus erythematosus and Sjögren’s syndrome. Clin Exp Dermatol. 2004;29:483-485.
- Huang PY, Chu CY, Hsiao CH. Multiple eruptive dermatofibromas in a patient with dermatomyositis taking prednisolone and methotrexate. J Am Acad Dermatol. 2007;57(suppl 5):S81-S84.
- Lopez N, Fernandez A, Bosch RJ, et al. Multiple eruptive dermatofibromas in a patient with Graves-Basedow disease. J Eur Acad Dermatol Venereol. 2008;22:402-403.
- Gualandri L, Betti R, Cerri A, et al. Eruptive dermatofibromas and immunosuppression. Eur J Dermatol. 1999;9:45-47.
- Alexandrescu DT, Wiernik PH. Multiple eruptive dermatofibromas occurring in a patient with chronic myelogenous leukemia. Arch Dermatol. 2005;141:397-398.
- Chang SE, Choi JH, Sung KJ, et al. Multiple eruptive dermatofibromas occurring in a patient with acute myeloid leukaemia. Br J Dermatol. 2000;142:1062-1063.
- Cohen PR. Multiple dermatofibromas in patients with autoimmune disorders receiving immunosuppressive therapy. Int J Dermatol. 1991;30:266-270.
- Santos-Juanes J, Coto-Segura P, Mallo S, et al. Multiple eruptive dermatofibromas in a patient receiving efalizumab. Dermatology. 2008;216:363.
- Stainforth J, Goodfield MJ. Multiple dermatofibromata developing during pregnancy. Clin Exp Dermatol. 1994;19:59-60.
- Ashworth J, Archard L, Woodrow D, et al. Multiple eruptive histiocytoma cutis in an atopic. Clin Exp Dermatol. 1990;15:454-456.
- Lee HW, Lee DK, Oh SH, et al. Multiple eruptive dermatofibromas in a patient with primary pulmonary hypertension. Br J Dermatol. 2005;153:845-847.
- Veraldi S, Drudi E, Gianotti R. Multiple, eruptive dermatofibromas. Eur J Dermatol. 1996;6:523-524.
- Nestle FO, Nickeloff BJ, Burg G. Dermatofibroma: an abortive immunoreactive process mediated by dermal dendritic cells? Dermatology. 1995;190:265-268.
To the Editor:
Dermatofibromas, the most common fibrohistiocytic tumors of the skin, are typically solitary lesions. Clustering of and multiple dermatofibromas (multiple eruptive dermatofibromas [MEDFs]) are relatively less common. The association between MEDF and systemic immunoaltered disease states such as systemic lupus erythematosus (SLE) or human immunodeficiency virus infection has been described and led to speculation that MEDF might be a result of an abnormal immune response. We report a patient with sarcoidosis who developed multiple large dermatofibromas, some clustered, on the neck, left shoulder, and back.

A 61-year-old woman with a history of mild pulmonary sarcoidosis confirmed by transbronchial biopsies presented to our clinic with a 2-year history of hyperpigmented papules on the trunk and extremities with subjective enlargement and increased erythema of a papule on the left shoulder over the last 6 months. She had associated pain and pruritus in the area. She was not on any systemic medications for sarcoidosis at the time. Physical examination revealed 2 large, firm, hyperpigmented nodules on the left shoulder, one with overlying erythema and mild scale (Figure 1). There also were multiple scattered hyperpigmented papules on the back, chest, and right arm that dimpled when compressed. A biopsy was obtained because of clinical concern for cutaneous sarcoidosis. Histopathologic evaluation of the largest nodule demonstrated epidermal hyperplasia with effacement of the rete ridges and a proliferation of spindle cells that wrapped around collagen fibers in the dermis, consistent with a dermatofibroma (Figure 2).

Dermatofibromas are common fibrohistiocytic neoplasms in the skin that typically present as a solitary lesion. A clustering of dermatofibromas, MEDFs, is relatively less common, representing only 0.3% of all dermatofibromas.1,2 Histopathologically, similar to solitary dermatofibromas,3 MEDF has classically been defined as more than 15 lesions, though another definition includes the appearance of several dermatofibromas over a relatively short period of time.2
Multiple eruptive dermatofibromas have been described in association with several underlying diseases. A strong association between MEDFs and immune dysregulation appears to exist, with 69% of reported cases of MEDF associated with an underlying disease, 83% of which were related to dysregulated immunity. Systemic lupus erythematosus was the most common underlying disease associated with MEDF, representing 25% of published cases.3 Multiple eruptive dermatofibromas also have been linked to other autoimmune disorders such as myasthenia gravis,4,5 Hashimoto thyroiditis,4 diabetes mellitus,6 Sjögren syndrome,7,8 dermatomyositis,9 and Graves-Basedow disease.10
Multiple eruptive dermatofibromas also have been linked to immunosuppression, including human immunodeficiency virus11; malignancy12,13; and immunosuppressive or immunomodulatory drugs such as corticosteroids,14 cyclophosphamide,5 methotrexate,9 efalizumab,15 and interferon alfa.12 The degree of immunosuppression, however, does not seem to correlate to the number of MEDFs.3 In addition, MEDFs have been reported in pregnancy and a variety of other systemic disorders including atopic dermatitis,1,16,17 hypertriglyceridemia,8 and pulmonary hypertension.18 We report a case of MEDF in a patient with sarcoidosis who was not treated with immunosuppressive medication. A report of sarcoidosis and MEDF was previously published, but the patient had been treated for many years with prednisone.19 Most reports of SLE-associated MEDF occurred in the setting of steroid use.
Although the etiology of dermatofibromas is unclear, the link between MEDFs and altered immunity has led to speculation that dermatofibromas could be a manifestation of defective autoimmune inflammatory regulation. This hypothesis has been supported by the observation that the lesions are often associated with cells that express class II MHC molecules and also bear morphologic similarity to dermal antigen-presenting cells.20 Reports of familial cases of MEDF suggest that there could be a genetic predisposition.1
The association of MEDFs and underlying immune disorders is important for clinicians to know for appropriate evaluation of potential systemic associations, including sarcoidosis. In addition, biopsy should be considered to confirm the diagnosis with large or atypical lesions to exclude other potential diagnoses. Given the protean nature of sarcoidosis, skin biopsy often is indicated to identify whether cutaneous findings are granulomatous sarcoid-related manifestations. The association of MEDFs with sarcoidosis requires further evaluation but might provide keys to understanding the pathophysiology of these lesions.
To the Editor:
Dermatofibromas, the most common fibrohistiocytic tumors of the skin, are typically solitary lesions. Clustering of and multiple dermatofibromas (multiple eruptive dermatofibromas [MEDFs]) are relatively less common. The association between MEDF and systemic immunoaltered disease states such as systemic lupus erythematosus (SLE) or human immunodeficiency virus infection has been described and led to speculation that MEDF might be a result of an abnormal immune response. We report a patient with sarcoidosis who developed multiple large dermatofibromas, some clustered, on the neck, left shoulder, and back.

A 61-year-old woman with a history of mild pulmonary sarcoidosis confirmed by transbronchial biopsies presented to our clinic with a 2-year history of hyperpigmented papules on the trunk and extremities with subjective enlargement and increased erythema of a papule on the left shoulder over the last 6 months. She had associated pain and pruritus in the area. She was not on any systemic medications for sarcoidosis at the time. Physical examination revealed 2 large, firm, hyperpigmented nodules on the left shoulder, one with overlying erythema and mild scale (Figure 1). There also were multiple scattered hyperpigmented papules on the back, chest, and right arm that dimpled when compressed. A biopsy was obtained because of clinical concern for cutaneous sarcoidosis. Histopathologic evaluation of the largest nodule demonstrated epidermal hyperplasia with effacement of the rete ridges and a proliferation of spindle cells that wrapped around collagen fibers in the dermis, consistent with a dermatofibroma (Figure 2).

Dermatofibromas are common fibrohistiocytic neoplasms in the skin that typically present as a solitary lesion. A clustering of dermatofibromas, MEDFs, is relatively less common, representing only 0.3% of all dermatofibromas.1,2 Histopathologically, similar to solitary dermatofibromas,3 MEDF has classically been defined as more than 15 lesions, though another definition includes the appearance of several dermatofibromas over a relatively short period of time.2
Multiple eruptive dermatofibromas have been described in association with several underlying diseases. A strong association between MEDFs and immune dysregulation appears to exist, with 69% of reported cases of MEDF associated with an underlying disease, 83% of which were related to dysregulated immunity. Systemic lupus erythematosus was the most common underlying disease associated with MEDF, representing 25% of published cases.3 Multiple eruptive dermatofibromas also have been linked to other autoimmune disorders such as myasthenia gravis,4,5 Hashimoto thyroiditis,4 diabetes mellitus,6 Sjögren syndrome,7,8 dermatomyositis,9 and Graves-Basedow disease.10
Multiple eruptive dermatofibromas also have been linked to immunosuppression, including human immunodeficiency virus11; malignancy12,13; and immunosuppressive or immunomodulatory drugs such as corticosteroids,14 cyclophosphamide,5 methotrexate,9 efalizumab,15 and interferon alfa.12 The degree of immunosuppression, however, does not seem to correlate to the number of MEDFs.3 In addition, MEDFs have been reported in pregnancy and a variety of other systemic disorders including atopic dermatitis,1,16,17 hypertriglyceridemia,8 and pulmonary hypertension.18 We report a case of MEDF in a patient with sarcoidosis who was not treated with immunosuppressive medication. A report of sarcoidosis and MEDF was previously published, but the patient had been treated for many years with prednisone.19 Most reports of SLE-associated MEDF occurred in the setting of steroid use.
Although the etiology of dermatofibromas is unclear, the link between MEDFs and altered immunity has led to speculation that dermatofibromas could be a manifestation of defective autoimmune inflammatory regulation. This hypothesis has been supported by the observation that the lesions are often associated with cells that express class II MHC molecules and also bear morphologic similarity to dermal antigen-presenting cells.20 Reports of familial cases of MEDF suggest that there could be a genetic predisposition.1
The association of MEDFs and underlying immune disorders is important for clinicians to know for appropriate evaluation of potential systemic associations, including sarcoidosis. In addition, biopsy should be considered to confirm the diagnosis with large or atypical lesions to exclude other potential diagnoses. Given the protean nature of sarcoidosis, skin biopsy often is indicated to identify whether cutaneous findings are granulomatous sarcoid-related manifestations. The association of MEDFs with sarcoidosis requires further evaluation but might provide keys to understanding the pathophysiology of these lesions.
- Yazici AC, Baz K, Ikizoglu G, et al. Familial eruptive dermatofibromas in atopic dermatitis. J Eur Acad Dermatol Venereol. 2006;20:90-92.
- Niiyama S, Katsuoka K, Happle R, et al. Multiple eruptive dermatofibromas: a review of the literature. Acta Derm Venereol. 2002;82:241-244.
- Zaccaria E, Rebora A, Rongioletti F. Multiple eruptive dermatofibromas and immunosuppression: report of two cases and review of the literature. Int J Dermatol. 2008;47:723-727.
- Kimura Y, Kaneko T, Akasaka E, et al. Multiple eruptive dermatofibromas associated with Hashimoto’s thyroiditis and myasthenia gravis. Eur J Dermatol. 2010;20:538-539.
- Bargman HB, Fefferman I. Multiple eruptive dermatofibromas in a patient with myasthenia gravis treated with prednisone and cyclophosphamide. J Am Acad Dermatol. 1986;14:351-352.
- Gelfarb M, Hyman AB. Multiple noduli cutanei. an unusual case of multiple noduli cutanei in a patient with hydronephrosis. Arch Dermatol. 1962;85:89-94.
- Yamamoto T, Katayama I, Nishioka K. Mast cell numbers in multiple dermatofibromas. Dermatology. 1995;190:9-13.
- Tsunemi Y, Tada Y, Saeki H, et al. Multiple dermatofibromas in a patient with systemic lupus erythematosus and Sjögren’s syndrome. Clin Exp Dermatol. 2004;29:483-485.
- Huang PY, Chu CY, Hsiao CH. Multiple eruptive dermatofibromas in a patient with dermatomyositis taking prednisolone and methotrexate. J Am Acad Dermatol. 2007;57(suppl 5):S81-S84.
- Lopez N, Fernandez A, Bosch RJ, et al. Multiple eruptive dermatofibromas in a patient with Graves-Basedow disease. J Eur Acad Dermatol Venereol. 2008;22:402-403.
- Gualandri L, Betti R, Cerri A, et al. Eruptive dermatofibromas and immunosuppression. Eur J Dermatol. 1999;9:45-47.
- Alexandrescu DT, Wiernik PH. Multiple eruptive dermatofibromas occurring in a patient with chronic myelogenous leukemia. Arch Dermatol. 2005;141:397-398.
- Chang SE, Choi JH, Sung KJ, et al. Multiple eruptive dermatofibromas occurring in a patient with acute myeloid leukaemia. Br J Dermatol. 2000;142:1062-1063.
- Cohen PR. Multiple dermatofibromas in patients with autoimmune disorders receiving immunosuppressive therapy. Int J Dermatol. 1991;30:266-270.
- Santos-Juanes J, Coto-Segura P, Mallo S, et al. Multiple eruptive dermatofibromas in a patient receiving efalizumab. Dermatology. 2008;216:363.
- Stainforth J, Goodfield MJ. Multiple dermatofibromata developing during pregnancy. Clin Exp Dermatol. 1994;19:59-60.
- Ashworth J, Archard L, Woodrow D, et al. Multiple eruptive histiocytoma cutis in an atopic. Clin Exp Dermatol. 1990;15:454-456.
- Lee HW, Lee DK, Oh SH, et al. Multiple eruptive dermatofibromas in a patient with primary pulmonary hypertension. Br J Dermatol. 2005;153:845-847.
- Veraldi S, Drudi E, Gianotti R. Multiple, eruptive dermatofibromas. Eur J Dermatol. 1996;6:523-524.
- Nestle FO, Nickeloff BJ, Burg G. Dermatofibroma: an abortive immunoreactive process mediated by dermal dendritic cells? Dermatology. 1995;190:265-268.
- Yazici AC, Baz K, Ikizoglu G, et al. Familial eruptive dermatofibromas in atopic dermatitis. J Eur Acad Dermatol Venereol. 2006;20:90-92.
- Niiyama S, Katsuoka K, Happle R, et al. Multiple eruptive dermatofibromas: a review of the literature. Acta Derm Venereol. 2002;82:241-244.
- Zaccaria E, Rebora A, Rongioletti F. Multiple eruptive dermatofibromas and immunosuppression: report of two cases and review of the literature. Int J Dermatol. 2008;47:723-727.
- Kimura Y, Kaneko T, Akasaka E, et al. Multiple eruptive dermatofibromas associated with Hashimoto’s thyroiditis and myasthenia gravis. Eur J Dermatol. 2010;20:538-539.
- Bargman HB, Fefferman I. Multiple eruptive dermatofibromas in a patient with myasthenia gravis treated with prednisone and cyclophosphamide. J Am Acad Dermatol. 1986;14:351-352.
- Gelfarb M, Hyman AB. Multiple noduli cutanei. an unusual case of multiple noduli cutanei in a patient with hydronephrosis. Arch Dermatol. 1962;85:89-94.
- Yamamoto T, Katayama I, Nishioka K. Mast cell numbers in multiple dermatofibromas. Dermatology. 1995;190:9-13.
- Tsunemi Y, Tada Y, Saeki H, et al. Multiple dermatofibromas in a patient with systemic lupus erythematosus and Sjögren’s syndrome. Clin Exp Dermatol. 2004;29:483-485.
- Huang PY, Chu CY, Hsiao CH. Multiple eruptive dermatofibromas in a patient with dermatomyositis taking prednisolone and methotrexate. J Am Acad Dermatol. 2007;57(suppl 5):S81-S84.
- Lopez N, Fernandez A, Bosch RJ, et al. Multiple eruptive dermatofibromas in a patient with Graves-Basedow disease. J Eur Acad Dermatol Venereol. 2008;22:402-403.
- Gualandri L, Betti R, Cerri A, et al. Eruptive dermatofibromas and immunosuppression. Eur J Dermatol. 1999;9:45-47.
- Alexandrescu DT, Wiernik PH. Multiple eruptive dermatofibromas occurring in a patient with chronic myelogenous leukemia. Arch Dermatol. 2005;141:397-398.
- Chang SE, Choi JH, Sung KJ, et al. Multiple eruptive dermatofibromas occurring in a patient with acute myeloid leukaemia. Br J Dermatol. 2000;142:1062-1063.
- Cohen PR. Multiple dermatofibromas in patients with autoimmune disorders receiving immunosuppressive therapy. Int J Dermatol. 1991;30:266-270.
- Santos-Juanes J, Coto-Segura P, Mallo S, et al. Multiple eruptive dermatofibromas in a patient receiving efalizumab. Dermatology. 2008;216:363.
- Stainforth J, Goodfield MJ. Multiple dermatofibromata developing during pregnancy. Clin Exp Dermatol. 1994;19:59-60.
- Ashworth J, Archard L, Woodrow D, et al. Multiple eruptive histiocytoma cutis in an atopic. Clin Exp Dermatol. 1990;15:454-456.
- Lee HW, Lee DK, Oh SH, et al. Multiple eruptive dermatofibromas in a patient with primary pulmonary hypertension. Br J Dermatol. 2005;153:845-847.
- Veraldi S, Drudi E, Gianotti R. Multiple, eruptive dermatofibromas. Eur J Dermatol. 1996;6:523-524.
- Nestle FO, Nickeloff BJ, Burg G. Dermatofibroma: an abortive immunoreactive process mediated by dermal dendritic cells? Dermatology. 1995;190:265-268.
Practice Points
- Sarcoidosis can present with multiple cutaneous morphologies and dermatologists should have a low threshold to perform skin biopsy to confirm sarcoidal granulomatous inflammation.
- Dermatofibromas can occur in greater numbers in patients with immune dysregulation such as human immunodeficiency virus and systemic lupus erythematosus.
Herpes Zoster in Children
Herpes zoster (HZ) is commonly seen in immunocompromised patients but is quite uncommon in immunocompetent children. Pediatric cases have been attributed to 1 of 3 primary exposures: intrauterine exposure to the varicella-zoster virus (VZV), postuterine exposure to wild-type VZV, or exposure due to vaccination with the live-attenuated strain of the virus.1
We report a case of HZ in an immunocompetent pediatric patient soon after routine VZV vaccination. We also review the literature on the incidence, clinical characteristics, and diagnostic aids for pediatric cases of HZ.
Case Report
A 15-month-old boy who was previously healthy presented with a red vesicular rash on the right upper cheek of 3 days’ duration. The patient was otherwise asymptomatic and had no constitutional symptoms. The patient’s mother reported an uncomplicated pregnancy and delivery with no history of maternal VZV infection. There was no known exposure to other individuals with VZV or a history of a similar rash. The patient was up-to-date on his immunizations, which included the VZV vaccine at 12 months of age.
Physical examination revealed vesicles and pustules with an erythematous base on the right zygoma extending to the right lateral canthus and upper eyelid in a dermatomal distribution (Figure). No lesions were present on any other area of the body. One group of vesicles was ruptured with a polyester-tipped applicator and submitted for polymerase chain reaction (PCR) analysis for suspected VZV infection. An ophthalmology evaluation revealed no ocular involvement.
Although no complications were noted on the ophthalmologist’s initial examination or at the follow-up visit, 1 month later the patient’s father noted a “cloudy change” to the right eye. The patient had several subsequent evaluations with ophthalmologists and was treated for HZ ophthalmicus with acyclovir over the following 10 months. The patient’s mother reported eventual clearance of the eye findings without permanent visual sequelae. She stated that the PCR results documenting VZV positivity were extremely helpful for the ophthalmologist in establishing a diagnosis and treatment plan.
Comment
Varicella-zoster virus is 1 of 8 viruses in the Herpesviridae family known to infect humans. It is known to cause 2 distinct disease states: varicella (chickenpox) due to a primary infection from the virus, and HZ (shingles) caused by a reactivation of the latent virus in the dorsal root ganglion, which then travels the neural pathway and manifests cutaneously along 1 to 2 dermatomes.1 This recurrence is possible in infants, children, and adults via 1 of 3 routes of exposure.
The overall incidence of HZ is lower in children compared to adults, and the risk dramatically increases in individuals older than 50 years. Evidence also shows that exposure to VZV before 1 year of age increases the lifetime risk for HZ.2,3 Children aged 1 to 18 years who were evaluated for HZ demonstrated a decreased incidence among those who were vaccinated versus those who were not.4,5 Interestingly, there was an increased incidence of HZ among children aged 1 to 2 years who had been vaccinated. Based on PCR analysis, it was noted that HZ was attributed to the vaccine-related strain of VZV in 92% of 1- to 2-year-old patients.4
There is some concern that the varicella vaccination program implemented in 1995 has led to increased rates of HZ. The literature presents mixed reports. Some studies showed an overall increased incidence of HZ,6,7 and 2 other studies showed no increase in the incidence of HZ.4,8 More recent studies have demonstrated that vaccination may have a protective effect against HZ.4-6,9 In a 2013 study in which HZ samples were tested by PCR analysis to determine the strains of VZV that were responsible for an HZ outbreak in children aged 1 to 18 years, the HZ incidence was 48 per 100,000 person-years in patients who were vaccinated versus 230 per 100,000 person-years in patients who were not vaccinated.Among the subset of patients who were vaccinated (n=118), 52% of the HZ lesions were from the wild-type strain.4
Clinical Characteristics
The typical presentation of HZ includes grouped vesicles or small bullae on an erythematous base that occur unilaterally within the distribution of a cranial or spinal sensory nerve, occasionally with overflow into the dermatomes above and below, typically without crossing the midline.8 The most frequent distributions in descending order are thoracic, cranial (mostly trigeminal), lumbar, and sacral. Pain in the dermatome may never occur, may precede, may occur during, or may even occur after the eruption. The initial presentation involves papules and plaques that develop blisters within hours of their development. Lesions continue to appear for several days and may coalesce. The lesions may become hemorrhagic, necrotic, or bullous, with or without adenopathy. Rarely, there can be pain without the associated skin eruption (zoster sine herpete). Lesions tend to crust by days 7 to 10.8
Herpes zoster typically affects children to a lesser extent than adults. The disease state often is milder in children with a decreased likelihood of postherpetic neuralgia.10 However, there are documented cases of severe sequelae secondary to zoster infection in pediatric patients, including but not limited to disseminated HZ,8 HZ ophthalmicus,11,12 Ramsay Hunt syndrome,8 and chronic encephalitis.8 In the adult population, ocular involvement will present in 33% to 50% of cases that involve the ophthalmic branch of the trigeminal nerve without clinical involvement of the nasociliary branch of the ophthalmic nerve. Involvement of the nasociliary branch will lead to ocular pathology in an estimated 76% to 100% of adult cases.13,14 It is unknown if this rate is the same in the pediatric population, but it highlights the importance of educating patients and/or guardians about possible complications. It also demonstrates the importance of including HZ in the differential diagnosis for pediatric patients presenting with papular or vesicular skin eruptions, particularly in the area of the ophthalmic branch of the trigeminal nerve.
Diagnosis
Herpes zoster usually is diagnosed based on its clinical presentation. Human herpesvirus 1 or 2 also may present with similar lesions and should be included in the differential diagnosis. To confirm a clinical diagnosis, additional testing may be done. A Tzanck smear historically has been the least expensive and most rapid test. Scrapings can be taken from the base of a vesicle, stained, and examined for multinucleated giant cells; however, a Tzanck smear cannot help in distinguishing herpes simplex virus from VZV. Direct fluorescent antibody testing and viral culture are less rapid but are standard tests that may help with the diagnosis. Direct fluorescent antibody testing can have a high false-negative rate, and viral cultures typically take 2 weeks for completion. These tests have largely been replaced by PCR analysis. Polymerase chain reaction has been the most sensitive test developed yet. With recent advances, real-time PCR, which can be performed within 1 hour in small hospital laboratories,15 has become more readily available and much more rapid than standard PCR. Further PCR testing can differentiate between the 2 possible infective strains (wild-type vs vaccine related).16 Real-time PCR is now commonly used as the first-line ancillary diagnostic test after physical examination.17
Conclusion
Although uncommon, HZ does occur in immunocompetent children and should be included in the differential diagnosis in children with vesicular lesions. Herpes zoster is a reactivation of VZV and initial exposure may be from the wild-type or vaccine-related strains. Clinicians must be vigilant in their evaluation of vesicular lesions in children even without known varicella exposure. Polymerase chain reaction testing can be helpful to distinguish between herpes simplex lesions and VZV. Polymerase chain reaction testing also may be of benefit to determine the strain of VZV infection.
- Myers MG, Seward JF, LaRussa PS. Varicella-zoster virus. In: Kliegman RM, Behrman RE, Jenson HB, et al, eds. Nelson Textbook of Pediatrics. 19th ed. Philadelphia, PA: Saunders; 2011:1104-1105.
- Terada K, Kawano S, Yoshihiro K, et al. Varicella-zoster virus (VZV) reactivation is related to the low response of VZV-specific immunity after chickenpox in infancy. J Infect Dis. 1994;169:650-652.
- Takayama N, Yamada H, Kaku H, et al. Herpes zoster in immunocompetent and immunocompromised Japanese children. Pediatr Int. 2000;42:275-279.
- Weinmann S, Chun C, Schmid DS, et al. Incidence and clinical characteristics of herpes zoster among children in the varicella vaccine era, 2005-2009. J Infect Dis. 2013;208:1859-1868.
- Civen R, Lopez AS, Zhang J, et al. Varicella outbreak epidemiology in an active surveillance site, 1995–2005. J Infect Dis. 2008;197(suppl 2):S114-S119.
- Russell ML, Dover DC, Simmonds KA, et al. Shingles in Alberta: before and after publicly funded varicella vaccination. Vaccine. 2014;32:6319-6324.
- Goldman GS, King PG. Review of the United States universal varicella vaccination program: herpes zoster incidence rates, cost-effectiveness, and vaccine efficacy based primarily on the Antelope Valley Varicella Active Surveillance Project data. Vaccine. 2013;31:1680-1694.
- Arikawa J, Asahi T, Au WY, et al. Zoster (shingles, herpes zoster). In: James WD, Berger TG, Elston DM, eds. Andrews’ Diseases of the Skin. 11th ed. Philadelphia, PA: Saunders/Elsevier; 2011:372-376.
- Guris D, Jumaan AO, Mascola L, et al. Changing varicella epidemiology in active surveillance sites–United States, 1995-2005. J Infect Dis. 2008;197(suppl 2):S71-S75.
- Petursson G, Helgason S, Gudmundsson S, et al. Herpes zoster in children and adolescents. Pediatr Infect Dis J. 1998;17:905-908.
- Oladokun RE, Olomukoro CN, Owa AB. Disseminated herpes zoster ophthalmicus in an immunocompetent 8-year-old boy. Clin Pract. 2013;3:e16.
- Lewkonia IK, Jackson AA. Infantile herpes zoster after intrauterine exposure to varicella. Br Med J. 1973;3:149.
- Zaal MJ, Völker-Dieben HJ, D’Amaro J. Prognostic value of Hutchinson’s sign in acute herpes zoster ophthalmicus. Graefes Arch Clin Exp Ophthalmol. 2003;241:187-191.
- Harding SP, Lipton JR, Wells JC. Natural history of herpes zoster ophthalmicus: predictors of postherpetic neuralgia and ocular involvement. Br J Ophthalmol. 1987;71:353-358.
- Higashimoto Y, Ihira M, Ohta A, et al. Discriminating between varicella-zoster virus vaccine and wild-type strains by loop-mediated isothermal amplification. J Clin Microbiol. 2008;46:2665-2670.
- Harbecke R, Oxman MN, Arnold, et al. A real-time PCR assay to identify and discriminate among wild-type and vaccine strains of varicella-zoster virus and herpes simplex virus in clinical specimens, and comparison with the clinical diagnoses. J Med Virol. 2009;81:1310-1322.
- Albrecht MA. Diagnosis of varicella-zoster infection. UpToDate website. http://www.uptodate.com/contents/diagnosis-of-varicella-zoster-virus-infection. Updated July 6, 2015. Accessed July 19, 2016.
Herpes zoster (HZ) is commonly seen in immunocompromised patients but is quite uncommon in immunocompetent children. Pediatric cases have been attributed to 1 of 3 primary exposures: intrauterine exposure to the varicella-zoster virus (VZV), postuterine exposure to wild-type VZV, or exposure due to vaccination with the live-attenuated strain of the virus.1
We report a case of HZ in an immunocompetent pediatric patient soon after routine VZV vaccination. We also review the literature on the incidence, clinical characteristics, and diagnostic aids for pediatric cases of HZ.
Case Report
A 15-month-old boy who was previously healthy presented with a red vesicular rash on the right upper cheek of 3 days’ duration. The patient was otherwise asymptomatic and had no constitutional symptoms. The patient’s mother reported an uncomplicated pregnancy and delivery with no history of maternal VZV infection. There was no known exposure to other individuals with VZV or a history of a similar rash. The patient was up-to-date on his immunizations, which included the VZV vaccine at 12 months of age.
Physical examination revealed vesicles and pustules with an erythematous base on the right zygoma extending to the right lateral canthus and upper eyelid in a dermatomal distribution (Figure). No lesions were present on any other area of the body. One group of vesicles was ruptured with a polyester-tipped applicator and submitted for polymerase chain reaction (PCR) analysis for suspected VZV infection. An ophthalmology evaluation revealed no ocular involvement.
Although no complications were noted on the ophthalmologist’s initial examination or at the follow-up visit, 1 month later the patient’s father noted a “cloudy change” to the right eye. The patient had several subsequent evaluations with ophthalmologists and was treated for HZ ophthalmicus with acyclovir over the following 10 months. The patient’s mother reported eventual clearance of the eye findings without permanent visual sequelae. She stated that the PCR results documenting VZV positivity were extremely helpful for the ophthalmologist in establishing a diagnosis and treatment plan.
Comment
Varicella-zoster virus is 1 of 8 viruses in the Herpesviridae family known to infect humans. It is known to cause 2 distinct disease states: varicella (chickenpox) due to a primary infection from the virus, and HZ (shingles) caused by a reactivation of the latent virus in the dorsal root ganglion, which then travels the neural pathway and manifests cutaneously along 1 to 2 dermatomes.1 This recurrence is possible in infants, children, and adults via 1 of 3 routes of exposure.
The overall incidence of HZ is lower in children compared to adults, and the risk dramatically increases in individuals older than 50 years. Evidence also shows that exposure to VZV before 1 year of age increases the lifetime risk for HZ.2,3 Children aged 1 to 18 years who were evaluated for HZ demonstrated a decreased incidence among those who were vaccinated versus those who were not.4,5 Interestingly, there was an increased incidence of HZ among children aged 1 to 2 years who had been vaccinated. Based on PCR analysis, it was noted that HZ was attributed to the vaccine-related strain of VZV in 92% of 1- to 2-year-old patients.4
There is some concern that the varicella vaccination program implemented in 1995 has led to increased rates of HZ. The literature presents mixed reports. Some studies showed an overall increased incidence of HZ,6,7 and 2 other studies showed no increase in the incidence of HZ.4,8 More recent studies have demonstrated that vaccination may have a protective effect against HZ.4-6,9 In a 2013 study in which HZ samples were tested by PCR analysis to determine the strains of VZV that were responsible for an HZ outbreak in children aged 1 to 18 years, the HZ incidence was 48 per 100,000 person-years in patients who were vaccinated versus 230 per 100,000 person-years in patients who were not vaccinated.Among the subset of patients who were vaccinated (n=118), 52% of the HZ lesions were from the wild-type strain.4
Clinical Characteristics
The typical presentation of HZ includes grouped vesicles or small bullae on an erythematous base that occur unilaterally within the distribution of a cranial or spinal sensory nerve, occasionally with overflow into the dermatomes above and below, typically without crossing the midline.8 The most frequent distributions in descending order are thoracic, cranial (mostly trigeminal), lumbar, and sacral. Pain in the dermatome may never occur, may precede, may occur during, or may even occur after the eruption. The initial presentation involves papules and plaques that develop blisters within hours of their development. Lesions continue to appear for several days and may coalesce. The lesions may become hemorrhagic, necrotic, or bullous, with or without adenopathy. Rarely, there can be pain without the associated skin eruption (zoster sine herpete). Lesions tend to crust by days 7 to 10.8
Herpes zoster typically affects children to a lesser extent than adults. The disease state often is milder in children with a decreased likelihood of postherpetic neuralgia.10 However, there are documented cases of severe sequelae secondary to zoster infection in pediatric patients, including but not limited to disseminated HZ,8 HZ ophthalmicus,11,12 Ramsay Hunt syndrome,8 and chronic encephalitis.8 In the adult population, ocular involvement will present in 33% to 50% of cases that involve the ophthalmic branch of the trigeminal nerve without clinical involvement of the nasociliary branch of the ophthalmic nerve. Involvement of the nasociliary branch will lead to ocular pathology in an estimated 76% to 100% of adult cases.13,14 It is unknown if this rate is the same in the pediatric population, but it highlights the importance of educating patients and/or guardians about possible complications. It also demonstrates the importance of including HZ in the differential diagnosis for pediatric patients presenting with papular or vesicular skin eruptions, particularly in the area of the ophthalmic branch of the trigeminal nerve.
Diagnosis
Herpes zoster usually is diagnosed based on its clinical presentation. Human herpesvirus 1 or 2 also may present with similar lesions and should be included in the differential diagnosis. To confirm a clinical diagnosis, additional testing may be done. A Tzanck smear historically has been the least expensive and most rapid test. Scrapings can be taken from the base of a vesicle, stained, and examined for multinucleated giant cells; however, a Tzanck smear cannot help in distinguishing herpes simplex virus from VZV. Direct fluorescent antibody testing and viral culture are less rapid but are standard tests that may help with the diagnosis. Direct fluorescent antibody testing can have a high false-negative rate, and viral cultures typically take 2 weeks for completion. These tests have largely been replaced by PCR analysis. Polymerase chain reaction has been the most sensitive test developed yet. With recent advances, real-time PCR, which can be performed within 1 hour in small hospital laboratories,15 has become more readily available and much more rapid than standard PCR. Further PCR testing can differentiate between the 2 possible infective strains (wild-type vs vaccine related).16 Real-time PCR is now commonly used as the first-line ancillary diagnostic test after physical examination.17
Conclusion
Although uncommon, HZ does occur in immunocompetent children and should be included in the differential diagnosis in children with vesicular lesions. Herpes zoster is a reactivation of VZV and initial exposure may be from the wild-type or vaccine-related strains. Clinicians must be vigilant in their evaluation of vesicular lesions in children even without known varicella exposure. Polymerase chain reaction testing can be helpful to distinguish between herpes simplex lesions and VZV. Polymerase chain reaction testing also may be of benefit to determine the strain of VZV infection.
Herpes zoster (HZ) is commonly seen in immunocompromised patients but is quite uncommon in immunocompetent children. Pediatric cases have been attributed to 1 of 3 primary exposures: intrauterine exposure to the varicella-zoster virus (VZV), postuterine exposure to wild-type VZV, or exposure due to vaccination with the live-attenuated strain of the virus.1
We report a case of HZ in an immunocompetent pediatric patient soon after routine VZV vaccination. We also review the literature on the incidence, clinical characteristics, and diagnostic aids for pediatric cases of HZ.
Case Report
A 15-month-old boy who was previously healthy presented with a red vesicular rash on the right upper cheek of 3 days’ duration. The patient was otherwise asymptomatic and had no constitutional symptoms. The patient’s mother reported an uncomplicated pregnancy and delivery with no history of maternal VZV infection. There was no known exposure to other individuals with VZV or a history of a similar rash. The patient was up-to-date on his immunizations, which included the VZV vaccine at 12 months of age.
Physical examination revealed vesicles and pustules with an erythematous base on the right zygoma extending to the right lateral canthus and upper eyelid in a dermatomal distribution (Figure). No lesions were present on any other area of the body. One group of vesicles was ruptured with a polyester-tipped applicator and submitted for polymerase chain reaction (PCR) analysis for suspected VZV infection. An ophthalmology evaluation revealed no ocular involvement.
Although no complications were noted on the ophthalmologist’s initial examination or at the follow-up visit, 1 month later the patient’s father noted a “cloudy change” to the right eye. The patient had several subsequent evaluations with ophthalmologists and was treated for HZ ophthalmicus with acyclovir over the following 10 months. The patient’s mother reported eventual clearance of the eye findings without permanent visual sequelae. She stated that the PCR results documenting VZV positivity were extremely helpful for the ophthalmologist in establishing a diagnosis and treatment plan.
Comment
Varicella-zoster virus is 1 of 8 viruses in the Herpesviridae family known to infect humans. It is known to cause 2 distinct disease states: varicella (chickenpox) due to a primary infection from the virus, and HZ (shingles) caused by a reactivation of the latent virus in the dorsal root ganglion, which then travels the neural pathway and manifests cutaneously along 1 to 2 dermatomes.1 This recurrence is possible in infants, children, and adults via 1 of 3 routes of exposure.
The overall incidence of HZ is lower in children compared to adults, and the risk dramatically increases in individuals older than 50 years. Evidence also shows that exposure to VZV before 1 year of age increases the lifetime risk for HZ.2,3 Children aged 1 to 18 years who were evaluated for HZ demonstrated a decreased incidence among those who were vaccinated versus those who were not.4,5 Interestingly, there was an increased incidence of HZ among children aged 1 to 2 years who had been vaccinated. Based on PCR analysis, it was noted that HZ was attributed to the vaccine-related strain of VZV in 92% of 1- to 2-year-old patients.4
There is some concern that the varicella vaccination program implemented in 1995 has led to increased rates of HZ. The literature presents mixed reports. Some studies showed an overall increased incidence of HZ,6,7 and 2 other studies showed no increase in the incidence of HZ.4,8 More recent studies have demonstrated that vaccination may have a protective effect against HZ.4-6,9 In a 2013 study in which HZ samples were tested by PCR analysis to determine the strains of VZV that were responsible for an HZ outbreak in children aged 1 to 18 years, the HZ incidence was 48 per 100,000 person-years in patients who were vaccinated versus 230 per 100,000 person-years in patients who were not vaccinated.Among the subset of patients who were vaccinated (n=118), 52% of the HZ lesions were from the wild-type strain.4
Clinical Characteristics
The typical presentation of HZ includes grouped vesicles or small bullae on an erythematous base that occur unilaterally within the distribution of a cranial or spinal sensory nerve, occasionally with overflow into the dermatomes above and below, typically without crossing the midline.8 The most frequent distributions in descending order are thoracic, cranial (mostly trigeminal), lumbar, and sacral. Pain in the dermatome may never occur, may precede, may occur during, or may even occur after the eruption. The initial presentation involves papules and plaques that develop blisters within hours of their development. Lesions continue to appear for several days and may coalesce. The lesions may become hemorrhagic, necrotic, or bullous, with or without adenopathy. Rarely, there can be pain without the associated skin eruption (zoster sine herpete). Lesions tend to crust by days 7 to 10.8
Herpes zoster typically affects children to a lesser extent than adults. The disease state often is milder in children with a decreased likelihood of postherpetic neuralgia.10 However, there are documented cases of severe sequelae secondary to zoster infection in pediatric patients, including but not limited to disseminated HZ,8 HZ ophthalmicus,11,12 Ramsay Hunt syndrome,8 and chronic encephalitis.8 In the adult population, ocular involvement will present in 33% to 50% of cases that involve the ophthalmic branch of the trigeminal nerve without clinical involvement of the nasociliary branch of the ophthalmic nerve. Involvement of the nasociliary branch will lead to ocular pathology in an estimated 76% to 100% of adult cases.13,14 It is unknown if this rate is the same in the pediatric population, but it highlights the importance of educating patients and/or guardians about possible complications. It also demonstrates the importance of including HZ in the differential diagnosis for pediatric patients presenting with papular or vesicular skin eruptions, particularly in the area of the ophthalmic branch of the trigeminal nerve.
Diagnosis
Herpes zoster usually is diagnosed based on its clinical presentation. Human herpesvirus 1 or 2 also may present with similar lesions and should be included in the differential diagnosis. To confirm a clinical diagnosis, additional testing may be done. A Tzanck smear historically has been the least expensive and most rapid test. Scrapings can be taken from the base of a vesicle, stained, and examined for multinucleated giant cells; however, a Tzanck smear cannot help in distinguishing herpes simplex virus from VZV. Direct fluorescent antibody testing and viral culture are less rapid but are standard tests that may help with the diagnosis. Direct fluorescent antibody testing can have a high false-negative rate, and viral cultures typically take 2 weeks for completion. These tests have largely been replaced by PCR analysis. Polymerase chain reaction has been the most sensitive test developed yet. With recent advances, real-time PCR, which can be performed within 1 hour in small hospital laboratories,15 has become more readily available and much more rapid than standard PCR. Further PCR testing can differentiate between the 2 possible infective strains (wild-type vs vaccine related).16 Real-time PCR is now commonly used as the first-line ancillary diagnostic test after physical examination.17
Conclusion
Although uncommon, HZ does occur in immunocompetent children and should be included in the differential diagnosis in children with vesicular lesions. Herpes zoster is a reactivation of VZV and initial exposure may be from the wild-type or vaccine-related strains. Clinicians must be vigilant in their evaluation of vesicular lesions in children even without known varicella exposure. Polymerase chain reaction testing can be helpful to distinguish between herpes simplex lesions and VZV. Polymerase chain reaction testing also may be of benefit to determine the strain of VZV infection.
- Myers MG, Seward JF, LaRussa PS. Varicella-zoster virus. In: Kliegman RM, Behrman RE, Jenson HB, et al, eds. Nelson Textbook of Pediatrics. 19th ed. Philadelphia, PA: Saunders; 2011:1104-1105.
- Terada K, Kawano S, Yoshihiro K, et al. Varicella-zoster virus (VZV) reactivation is related to the low response of VZV-specific immunity after chickenpox in infancy. J Infect Dis. 1994;169:650-652.
- Takayama N, Yamada H, Kaku H, et al. Herpes zoster in immunocompetent and immunocompromised Japanese children. Pediatr Int. 2000;42:275-279.
- Weinmann S, Chun C, Schmid DS, et al. Incidence and clinical characteristics of herpes zoster among children in the varicella vaccine era, 2005-2009. J Infect Dis. 2013;208:1859-1868.
- Civen R, Lopez AS, Zhang J, et al. Varicella outbreak epidemiology in an active surveillance site, 1995–2005. J Infect Dis. 2008;197(suppl 2):S114-S119.
- Russell ML, Dover DC, Simmonds KA, et al. Shingles in Alberta: before and after publicly funded varicella vaccination. Vaccine. 2014;32:6319-6324.
- Goldman GS, King PG. Review of the United States universal varicella vaccination program: herpes zoster incidence rates, cost-effectiveness, and vaccine efficacy based primarily on the Antelope Valley Varicella Active Surveillance Project data. Vaccine. 2013;31:1680-1694.
- Arikawa J, Asahi T, Au WY, et al. Zoster (shingles, herpes zoster). In: James WD, Berger TG, Elston DM, eds. Andrews’ Diseases of the Skin. 11th ed. Philadelphia, PA: Saunders/Elsevier; 2011:372-376.
- Guris D, Jumaan AO, Mascola L, et al. Changing varicella epidemiology in active surveillance sites–United States, 1995-2005. J Infect Dis. 2008;197(suppl 2):S71-S75.
- Petursson G, Helgason S, Gudmundsson S, et al. Herpes zoster in children and adolescents. Pediatr Infect Dis J. 1998;17:905-908.
- Oladokun RE, Olomukoro CN, Owa AB. Disseminated herpes zoster ophthalmicus in an immunocompetent 8-year-old boy. Clin Pract. 2013;3:e16.
- Lewkonia IK, Jackson AA. Infantile herpes zoster after intrauterine exposure to varicella. Br Med J. 1973;3:149.
- Zaal MJ, Völker-Dieben HJ, D’Amaro J. Prognostic value of Hutchinson’s sign in acute herpes zoster ophthalmicus. Graefes Arch Clin Exp Ophthalmol. 2003;241:187-191.
- Harding SP, Lipton JR, Wells JC. Natural history of herpes zoster ophthalmicus: predictors of postherpetic neuralgia and ocular involvement. Br J Ophthalmol. 1987;71:353-358.
- Higashimoto Y, Ihira M, Ohta A, et al. Discriminating between varicella-zoster virus vaccine and wild-type strains by loop-mediated isothermal amplification. J Clin Microbiol. 2008;46:2665-2670.
- Harbecke R, Oxman MN, Arnold, et al. A real-time PCR assay to identify and discriminate among wild-type and vaccine strains of varicella-zoster virus and herpes simplex virus in clinical specimens, and comparison with the clinical diagnoses. J Med Virol. 2009;81:1310-1322.
- Albrecht MA. Diagnosis of varicella-zoster infection. UpToDate website. http://www.uptodate.com/contents/diagnosis-of-varicella-zoster-virus-infection. Updated July 6, 2015. Accessed July 19, 2016.
- Myers MG, Seward JF, LaRussa PS. Varicella-zoster virus. In: Kliegman RM, Behrman RE, Jenson HB, et al, eds. Nelson Textbook of Pediatrics. 19th ed. Philadelphia, PA: Saunders; 2011:1104-1105.
- Terada K, Kawano S, Yoshihiro K, et al. Varicella-zoster virus (VZV) reactivation is related to the low response of VZV-specific immunity after chickenpox in infancy. J Infect Dis. 1994;169:650-652.
- Takayama N, Yamada H, Kaku H, et al. Herpes zoster in immunocompetent and immunocompromised Japanese children. Pediatr Int. 2000;42:275-279.
- Weinmann S, Chun C, Schmid DS, et al. Incidence and clinical characteristics of herpes zoster among children in the varicella vaccine era, 2005-2009. J Infect Dis. 2013;208:1859-1868.
- Civen R, Lopez AS, Zhang J, et al. Varicella outbreak epidemiology in an active surveillance site, 1995–2005. J Infect Dis. 2008;197(suppl 2):S114-S119.
- Russell ML, Dover DC, Simmonds KA, et al. Shingles in Alberta: before and after publicly funded varicella vaccination. Vaccine. 2014;32:6319-6324.
- Goldman GS, King PG. Review of the United States universal varicella vaccination program: herpes zoster incidence rates, cost-effectiveness, and vaccine efficacy based primarily on the Antelope Valley Varicella Active Surveillance Project data. Vaccine. 2013;31:1680-1694.
- Arikawa J, Asahi T, Au WY, et al. Zoster (shingles, herpes zoster). In: James WD, Berger TG, Elston DM, eds. Andrews’ Diseases of the Skin. 11th ed. Philadelphia, PA: Saunders/Elsevier; 2011:372-376.
- Guris D, Jumaan AO, Mascola L, et al. Changing varicella epidemiology in active surveillance sites–United States, 1995-2005. J Infect Dis. 2008;197(suppl 2):S71-S75.
- Petursson G, Helgason S, Gudmundsson S, et al. Herpes zoster in children and adolescents. Pediatr Infect Dis J. 1998;17:905-908.
- Oladokun RE, Olomukoro CN, Owa AB. Disseminated herpes zoster ophthalmicus in an immunocompetent 8-year-old boy. Clin Pract. 2013;3:e16.
- Lewkonia IK, Jackson AA. Infantile herpes zoster after intrauterine exposure to varicella. Br Med J. 1973;3:149.
- Zaal MJ, Völker-Dieben HJ, D’Amaro J. Prognostic value of Hutchinson’s sign in acute herpes zoster ophthalmicus. Graefes Arch Clin Exp Ophthalmol. 2003;241:187-191.
- Harding SP, Lipton JR, Wells JC. Natural history of herpes zoster ophthalmicus: predictors of postherpetic neuralgia and ocular involvement. Br J Ophthalmol. 1987;71:353-358.
- Higashimoto Y, Ihira M, Ohta A, et al. Discriminating between varicella-zoster virus vaccine and wild-type strains by loop-mediated isothermal amplification. J Clin Microbiol. 2008;46:2665-2670.
- Harbecke R, Oxman MN, Arnold, et al. A real-time PCR assay to identify and discriminate among wild-type and vaccine strains of varicella-zoster virus and herpes simplex virus in clinical specimens, and comparison with the clinical diagnoses. J Med Virol. 2009;81:1310-1322.
- Albrecht MA. Diagnosis of varicella-zoster infection. UpToDate website. http://www.uptodate.com/contents/diagnosis-of-varicella-zoster-virus-infection. Updated July 6, 2015. Accessed July 19, 2016.
Practice Points
- Herpes zoster (HZ) should be included in the differential diagnosis for children presenting with vesicular lesions in a dermatomal distribution and a history of varicella exposure.
- Clinical diagnosis of HZ and herpes simplex virus can be aided by the use of viral polymerase chain reaction testing.
- Children with HZ should be monitored for the same possible complications as adults.
Sporotrichoid Fluctuant Nodules
The Diagnosis: Atypical Mycobacterial Infection
Punch biopsy specimens demonstrated necrotizing granulomatous inflammation in the dermis and subcutis (Figure). Special staining for microorganisms was negative. Tissue culture grew Mycobacterium avium-intracellulare (MAI). The patient began treatment with azithromycin, ethambutol, and rifabutin. Tissue susceptibilities later showed resistance to rifabutin and sensitivity to clarithromycin, moxifloxacin, and clofazimine. She subsequently was switched to azithromycin, clofazimine, and moxifloxacin with good response.

Mycobacterium avium-intracellulare is a slow-growing, nonchromogenic, atypical mycobacteria. Although ubiquitous, it tends to only cause serious infection in the setting of immunosuppression. Transmission usually is through the respiratory or gastrointestinal tract.1 Skin infections with MAI are uncommon and usually are secondary to seeding from disseminated infection or from direct inoculation.2
The clinical presentations of primary cutaneous MAI are myriad, including an isolated red nodule, multiple ulcers, abscesses, draining sinuses, facial nodules, granulomatous plaques, and panniculitis.2,3 Of 3 reported cases of primary cutaneous MAI in the form of sporotrichoid lesions, 2 involved patients with AIDS2 and 1 involved a cardiac transplant recipient.4
Cutaneous MAI is typically diagnosed with skin biopsy and tissue culture. Tissue culture is critical for determining the specific mycobacterial species and antibiotic susceptibilities. Polymerase chain reaction has been utilized to rapidly diagnose cutaneous MAI infection from an acid-fast bacilli–positive tissue sample in which the tissue culture was negative.5
Recommended treatment protocols for MAI involve multidrug regimens because of the intrinsic resistance of MAI and the concern for development of resistance with monotherapy.2 No definitive guidelines exist for treatment of primary cutaneous MAI infections. However, regimens for the treatment of pulmonary infection that also have been successfully utilized for cutaneous infection include a macrolide, ethambutol, and a rifamycin.6 Clinicians should be aware of MAI as a cause of primary cutaneous infections presenting as lymphocutaneous suppurative nodules and ulcerations.
- Hautmann G, Lotti T. Atypical mycobacterial infections of the skin. Dermatol Clin. 1994;12:657-668.
- Kayal JD, McCall CO. Sporotrichoid cutaneous Mycobacterium avium complex infection. J Am Acad Dermatol. 2002;47(5 suppl):S249-S250.
- Kullavanijaya P, Sirimachan S, Surarak S. Primary cutaneous infection with Mycobacterium avium-intracellulare complex resembling lupus vulgaris. Br J Dermatol. 1997;136:264-266.
- Wood C, Nickoloff BJ, Todes-Taylor NR. Pseudotumor resulting from atypical mycobacterial infection: a “histoid” variety of Mycobacterium avium-intracellulare complex infection. Am J Clin Pathol. 1985;83:524-527.
- Carlos CA, Tang YW, Adler DJ, et al. Mycobacterial infection identified with broad-range PCR amplification and suspension array identification. J Cutan Pathol. 2012;39:795-797.
- Griffith DE, Aksamit T, Brown-Elliot BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416.
The Diagnosis: Atypical Mycobacterial Infection
Punch biopsy specimens demonstrated necrotizing granulomatous inflammation in the dermis and subcutis (Figure). Special staining for microorganisms was negative. Tissue culture grew Mycobacterium avium-intracellulare (MAI). The patient began treatment with azithromycin, ethambutol, and rifabutin. Tissue susceptibilities later showed resistance to rifabutin and sensitivity to clarithromycin, moxifloxacin, and clofazimine. She subsequently was switched to azithromycin, clofazimine, and moxifloxacin with good response.

Mycobacterium avium-intracellulare is a slow-growing, nonchromogenic, atypical mycobacteria. Although ubiquitous, it tends to only cause serious infection in the setting of immunosuppression. Transmission usually is through the respiratory or gastrointestinal tract.1 Skin infections with MAI are uncommon and usually are secondary to seeding from disseminated infection or from direct inoculation.2
The clinical presentations of primary cutaneous MAI are myriad, including an isolated red nodule, multiple ulcers, abscesses, draining sinuses, facial nodules, granulomatous plaques, and panniculitis.2,3 Of 3 reported cases of primary cutaneous MAI in the form of sporotrichoid lesions, 2 involved patients with AIDS2 and 1 involved a cardiac transplant recipient.4
Cutaneous MAI is typically diagnosed with skin biopsy and tissue culture. Tissue culture is critical for determining the specific mycobacterial species and antibiotic susceptibilities. Polymerase chain reaction has been utilized to rapidly diagnose cutaneous MAI infection from an acid-fast bacilli–positive tissue sample in which the tissue culture was negative.5
Recommended treatment protocols for MAI involve multidrug regimens because of the intrinsic resistance of MAI and the concern for development of resistance with monotherapy.2 No definitive guidelines exist for treatment of primary cutaneous MAI infections. However, regimens for the treatment of pulmonary infection that also have been successfully utilized for cutaneous infection include a macrolide, ethambutol, and a rifamycin.6 Clinicians should be aware of MAI as a cause of primary cutaneous infections presenting as lymphocutaneous suppurative nodules and ulcerations.
The Diagnosis: Atypical Mycobacterial Infection
Punch biopsy specimens demonstrated necrotizing granulomatous inflammation in the dermis and subcutis (Figure). Special staining for microorganisms was negative. Tissue culture grew Mycobacterium avium-intracellulare (MAI). The patient began treatment with azithromycin, ethambutol, and rifabutin. Tissue susceptibilities later showed resistance to rifabutin and sensitivity to clarithromycin, moxifloxacin, and clofazimine. She subsequently was switched to azithromycin, clofazimine, and moxifloxacin with good response.

Mycobacterium avium-intracellulare is a slow-growing, nonchromogenic, atypical mycobacteria. Although ubiquitous, it tends to only cause serious infection in the setting of immunosuppression. Transmission usually is through the respiratory or gastrointestinal tract.1 Skin infections with MAI are uncommon and usually are secondary to seeding from disseminated infection or from direct inoculation.2
The clinical presentations of primary cutaneous MAI are myriad, including an isolated red nodule, multiple ulcers, abscesses, draining sinuses, facial nodules, granulomatous plaques, and panniculitis.2,3 Of 3 reported cases of primary cutaneous MAI in the form of sporotrichoid lesions, 2 involved patients with AIDS2 and 1 involved a cardiac transplant recipient.4
Cutaneous MAI is typically diagnosed with skin biopsy and tissue culture. Tissue culture is critical for determining the specific mycobacterial species and antibiotic susceptibilities. Polymerase chain reaction has been utilized to rapidly diagnose cutaneous MAI infection from an acid-fast bacilli–positive tissue sample in which the tissue culture was negative.5
Recommended treatment protocols for MAI involve multidrug regimens because of the intrinsic resistance of MAI and the concern for development of resistance with monotherapy.2 No definitive guidelines exist for treatment of primary cutaneous MAI infections. However, regimens for the treatment of pulmonary infection that also have been successfully utilized for cutaneous infection include a macrolide, ethambutol, and a rifamycin.6 Clinicians should be aware of MAI as a cause of primary cutaneous infections presenting as lymphocutaneous suppurative nodules and ulcerations.
- Hautmann G, Lotti T. Atypical mycobacterial infections of the skin. Dermatol Clin. 1994;12:657-668.
- Kayal JD, McCall CO. Sporotrichoid cutaneous Mycobacterium avium complex infection. J Am Acad Dermatol. 2002;47(5 suppl):S249-S250.
- Kullavanijaya P, Sirimachan S, Surarak S. Primary cutaneous infection with Mycobacterium avium-intracellulare complex resembling lupus vulgaris. Br J Dermatol. 1997;136:264-266.
- Wood C, Nickoloff BJ, Todes-Taylor NR. Pseudotumor resulting from atypical mycobacterial infection: a “histoid” variety of Mycobacterium avium-intracellulare complex infection. Am J Clin Pathol. 1985;83:524-527.
- Carlos CA, Tang YW, Adler DJ, et al. Mycobacterial infection identified with broad-range PCR amplification and suspension array identification. J Cutan Pathol. 2012;39:795-797.
- Griffith DE, Aksamit T, Brown-Elliot BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416.
- Hautmann G, Lotti T. Atypical mycobacterial infections of the skin. Dermatol Clin. 1994;12:657-668.
- Kayal JD, McCall CO. Sporotrichoid cutaneous Mycobacterium avium complex infection. J Am Acad Dermatol. 2002;47(5 suppl):S249-S250.
- Kullavanijaya P, Sirimachan S, Surarak S. Primary cutaneous infection with Mycobacterium avium-intracellulare complex resembling lupus vulgaris. Br J Dermatol. 1997;136:264-266.
- Wood C, Nickoloff BJ, Todes-Taylor NR. Pseudotumor resulting from atypical mycobacterial infection: a “histoid” variety of Mycobacterium avium-intracellulare complex infection. Am J Clin Pathol. 1985;83:524-527.
- Carlos CA, Tang YW, Adler DJ, et al. Mycobacterial infection identified with broad-range PCR amplification and suspension array identification. J Cutan Pathol. 2012;39:795-797.
- Griffith DE, Aksamit T, Brown-Elliot BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416.
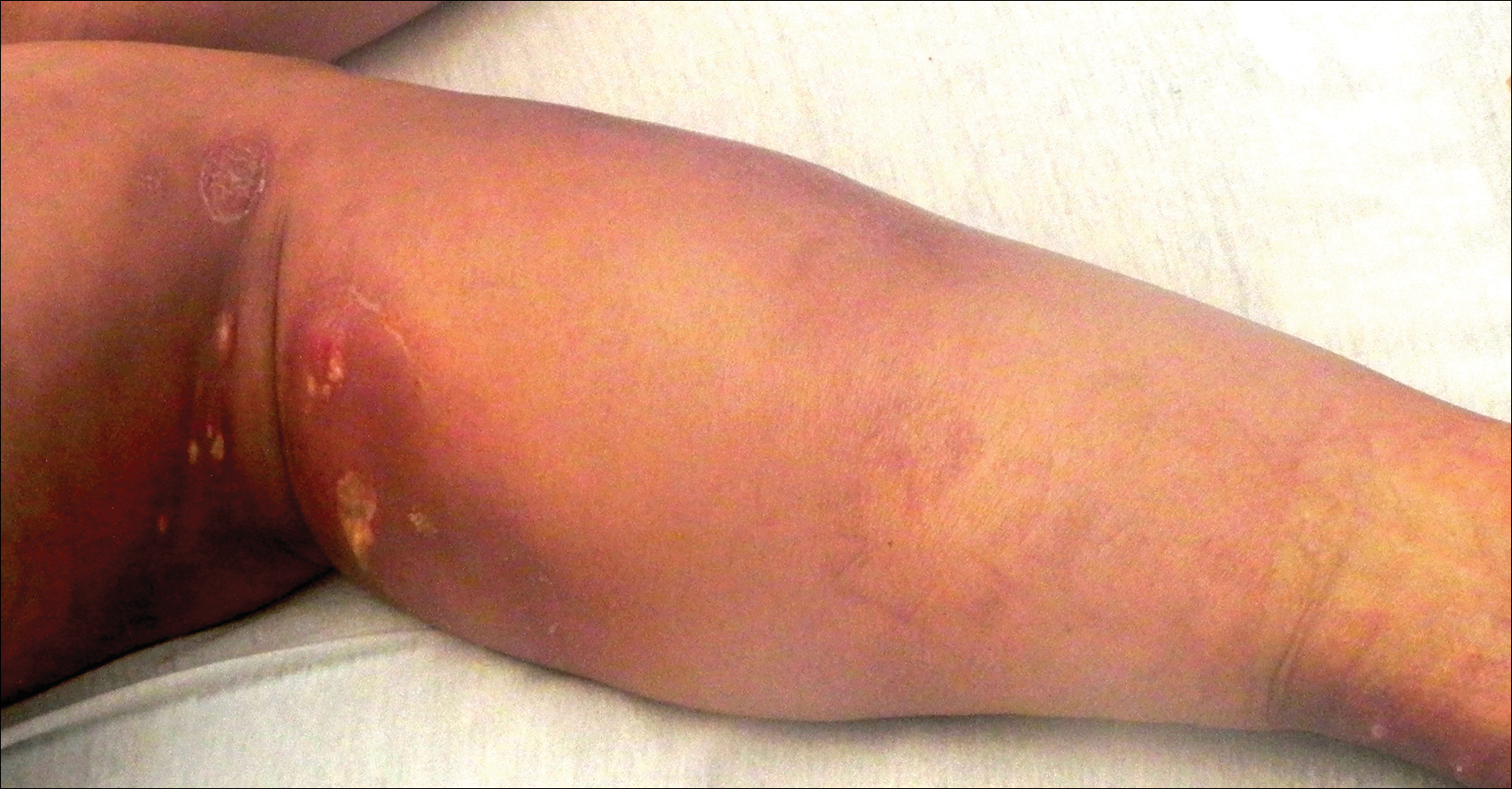
A woman in her 50s presented with low-grade subjective intermittent fevers and painful draining ulcerations on the legs of 7 months’ duration. Her medical history was remarkable for polymyositis and interstitial lung disease managed with prednisone and mycophenolate mofetil. While living in Taiwan, she developed lower extremity abscesses and persistent fevers. The patient denied any skin injuries or exposure to animals or brackish water. Mycophenolate mofetil was discontinued, and she was treated with multiple antibiotics alone and in combination without improvement, including amoxicillin–clavulanic acid, levofloxacin, azithromycin, moxifloxacin, rifampin, rifabutin, and ethambutol. She returned to the United States for evaluation. Physical examination revealed ulcerations with purulent drainage and interconnected sinus tracts with rare fluctuant nodules along the right leg. A single similar lesion was present on the right chest wall. There was no clinical evidence of disseminated disease.
Product News: 08 2016
CeraVeValeant Pharmaceuticals North America LLC receives The Skin Cancer Foundation (SCF) Seal of Recommendation for 2 products in the CeraVe skin care line. The CeraVe AM Facial Moisturizing Lotion With SPF 30 has earned the daily use SCF seal and the CeraVe SPF 50 Sunscreen Face Lotion has earned the active SCF seal. Products earning the SCF seal have fulfilled strict criteria from photobiologists, and consumers can purchase these products with the added reassurance that they perform as claimed. “We believe that more and more people are becoming aware of the dangers posed by unprotected sun exposure and for these individuals, particularly, the Seal of Recommendation is very meaningful,” said Joe Gordon, general manager of consumer health care at Valeant Pharmaceuticals. Both products are available over-the-counter at major retailers. For more information, visit www.cerave.com.
Differin Gel Galderma Laboratories, LP, announces approval from the US Food and Drug Administration for
over-the-counter use of Differin Gel (adapalene gel 0.1%) for the treatment of acne. “We’re excited to deliver this new level of efficacy without a prescription to consumers, and to bring the first new active ingredient to the category in over 30 years,” said Miles Harrison, president and general manager of Galderma Laboratories. With Differin Gel now available over-the-counter versus by prescription, acne patients will have easier access to this prescription-strength product containing the retinoid adapalene. For more information, visit www.differin.com.
UltraShape Power Syneron Medical Ltd announces US Food and Drug Administration clearance of UltraShape Power, a noninvasive fat destruction device for reduction of abdominal circumference. UltraShape Power uses pulsed mechanical ultrasound energy to target and destroy fat in both large and small areas and the advanced transducer delivers 20% more energy than its predecessor. UltraShape Power offers patients a 32% reduction in subcutaneous fat thickness. Patients have reported favorable results with little pain. A typical regimen is 3 treatments spaced 2 weeks apart. For more information, visit www.syneron-candela.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at [email protected].
CeraVeValeant Pharmaceuticals North America LLC receives The Skin Cancer Foundation (SCF) Seal of Recommendation for 2 products in the CeraVe skin care line. The CeraVe AM Facial Moisturizing Lotion With SPF 30 has earned the daily use SCF seal and the CeraVe SPF 50 Sunscreen Face Lotion has earned the active SCF seal. Products earning the SCF seal have fulfilled strict criteria from photobiologists, and consumers can purchase these products with the added reassurance that they perform as claimed. “We believe that more and more people are becoming aware of the dangers posed by unprotected sun exposure and for these individuals, particularly, the Seal of Recommendation is very meaningful,” said Joe Gordon, general manager of consumer health care at Valeant Pharmaceuticals. Both products are available over-the-counter at major retailers. For more information, visit www.cerave.com.
Differin Gel Galderma Laboratories, LP, announces approval from the US Food and Drug Administration for
over-the-counter use of Differin Gel (adapalene gel 0.1%) for the treatment of acne. “We’re excited to deliver this new level of efficacy without a prescription to consumers, and to bring the first new active ingredient to the category in over 30 years,” said Miles Harrison, president and general manager of Galderma Laboratories. With Differin Gel now available over-the-counter versus by prescription, acne patients will have easier access to this prescription-strength product containing the retinoid adapalene. For more information, visit www.differin.com.
UltraShape Power Syneron Medical Ltd announces US Food and Drug Administration clearance of UltraShape Power, a noninvasive fat destruction device for reduction of abdominal circumference. UltraShape Power uses pulsed mechanical ultrasound energy to target and destroy fat in both large and small areas and the advanced transducer delivers 20% more energy than its predecessor. UltraShape Power offers patients a 32% reduction in subcutaneous fat thickness. Patients have reported favorable results with little pain. A typical regimen is 3 treatments spaced 2 weeks apart. For more information, visit www.syneron-candela.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at [email protected].
CeraVeValeant Pharmaceuticals North America LLC receives The Skin Cancer Foundation (SCF) Seal of Recommendation for 2 products in the CeraVe skin care line. The CeraVe AM Facial Moisturizing Lotion With SPF 30 has earned the daily use SCF seal and the CeraVe SPF 50 Sunscreen Face Lotion has earned the active SCF seal. Products earning the SCF seal have fulfilled strict criteria from photobiologists, and consumers can purchase these products with the added reassurance that they perform as claimed. “We believe that more and more people are becoming aware of the dangers posed by unprotected sun exposure and for these individuals, particularly, the Seal of Recommendation is very meaningful,” said Joe Gordon, general manager of consumer health care at Valeant Pharmaceuticals. Both products are available over-the-counter at major retailers. For more information, visit www.cerave.com.
Differin Gel Galderma Laboratories, LP, announces approval from the US Food and Drug Administration for
over-the-counter use of Differin Gel (adapalene gel 0.1%) for the treatment of acne. “We’re excited to deliver this new level of efficacy without a prescription to consumers, and to bring the first new active ingredient to the category in over 30 years,” said Miles Harrison, president and general manager of Galderma Laboratories. With Differin Gel now available over-the-counter versus by prescription, acne patients will have easier access to this prescription-strength product containing the retinoid adapalene. For more information, visit www.differin.com.
UltraShape Power Syneron Medical Ltd announces US Food and Drug Administration clearance of UltraShape Power, a noninvasive fat destruction device for reduction of abdominal circumference. UltraShape Power uses pulsed mechanical ultrasound energy to target and destroy fat in both large and small areas and the advanced transducer delivers 20% more energy than its predecessor. UltraShape Power offers patients a 32% reduction in subcutaneous fat thickness. Patients have reported favorable results with little pain. A typical regimen is 3 treatments spaced 2 weeks apart. For more information, visit www.syneron-candela.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at [email protected].
Impact of Acne Vulgaris on Quality of Life and Self-esteem
Acne vulgaris predominantly occurs during puberty and can persist beyond 25 years of age, most commonly in women.1,2 Although acne does not cause physical impairment, it can be associated with a considerable psychosocial burden including increased levels of anxiety, anger, depression, and frustration, which in turn can affect vocational and academic performance, quality of life (QOL), and self-esteem.3
Quality of life measures provide valuable insight into the debilitating effects of acne.1 It has been suggested that acne patients may experience poor body image and low self-esteem as well as social isolation and constriction of activities.4 Self-esteem is a favorable and unfavorable attitude toward oneself.5 A marked emphasis has been placed on body image in society, fueled by external cues such as the media.3,6 This study was carried out to assess QOL and self-esteem in acne patients.
Methods
This prospective, hospital-based, cross-sectional, case-control study was conducted at The Oxford Medical College, Hospital & Research Center (Bangalore, India), over a period of 3 months. One hundred consecutive acne cases (age range, 12–45 years) and 100 age- and gender-matched controls who did not have any skin disease provided consent and were included in the analysis. Guardians gave consent for individuals who were younger than 18 years. Exclusion criteria for cases included a medical disorder (eg, epilepsy, diabetes mellitus, hypertension) or medications that would likely interfere with acne assessment.
The cases and controls were administered a semistructured questionnaire to collect sociodemographic details. Acne was graded for the predominant lesions, QOL was assessed using the Cardiff Acne Disability Index (CADI) and World Health Organization Quality of Life–BREF (WHOQOL-BREF) scale, and self-esteem was measured using the Rosenberg self-esteem scale (RSES). The study was approved by the institutional review board.
Acne Grading
Acne was graded according to the predominant lesions using the following criteria: grade 1=comedones and occasional papules; grade 2=papules, comedones, and few pustules; grade 3=predominant pustules, nodules, and abscesses; and grade 4=mainly cysts, abscesses, and widespread scarring.1
Quality of Life Assessment
The CADI questionnaire was used to assess the level of disability caused by acne.6 It is a 5-item questionnaire with scores ranging from 0 to 3 for a total maximum score of 15 and minimum score of 0. Total scores were classified as low (0–4), medium (5–9), and high (10–15).7
The WHOQOL-BREF is a self-reported questionnaire containing 26 items that make up the 4 domains of physical health (7 items), psychological health (6 items), social relationships (3 items), and environment (8 items); there also are 2 single questions regarding the overall perception of QOL and health. Questions were scored on aseries of 5-point scales with higher scores denoting better QOL.8
Self-esteem Assessment
The RSES uses a 5-point Likert scale from strongly agree to strongly disagree to rate a series of 10 statements. The total score ranges from 0 to 30. Scores less than 15 suggest low self-esteem, while scores of 15 and greater indicate high self-esteem.5
Statistical Analysis
Results were analyzed using descriptive and inferential statistical methods. A χ2 test was used for categorical data, and a Student t test and an analysis of variance were used for continuous data.
Results
The study consisted of 100 cases and 100 controls. The mean age was 21 years. The majority of cases reported an age of onset of acne of 11 to 20 years (66%), were predominantly female (58%) from rural backgrounds, and had a family history of acne (68%). The majority of lesions ceased within 24 months (60%). The face was the most commonly involved area (80%) and papules were the most prevalent lesion type (62%).
Cases predominantly had grade 2 acne (46%), and there was medium to high impairment in QOL according to CADI scores.
The scores for all the domains of the WHOQOL-BREF as well as the total score were lower in cases compared to controls (Table). There was a statistically significant difference between the 2 groups in the psychological (P=.0402) and environment (P=.006) domains.
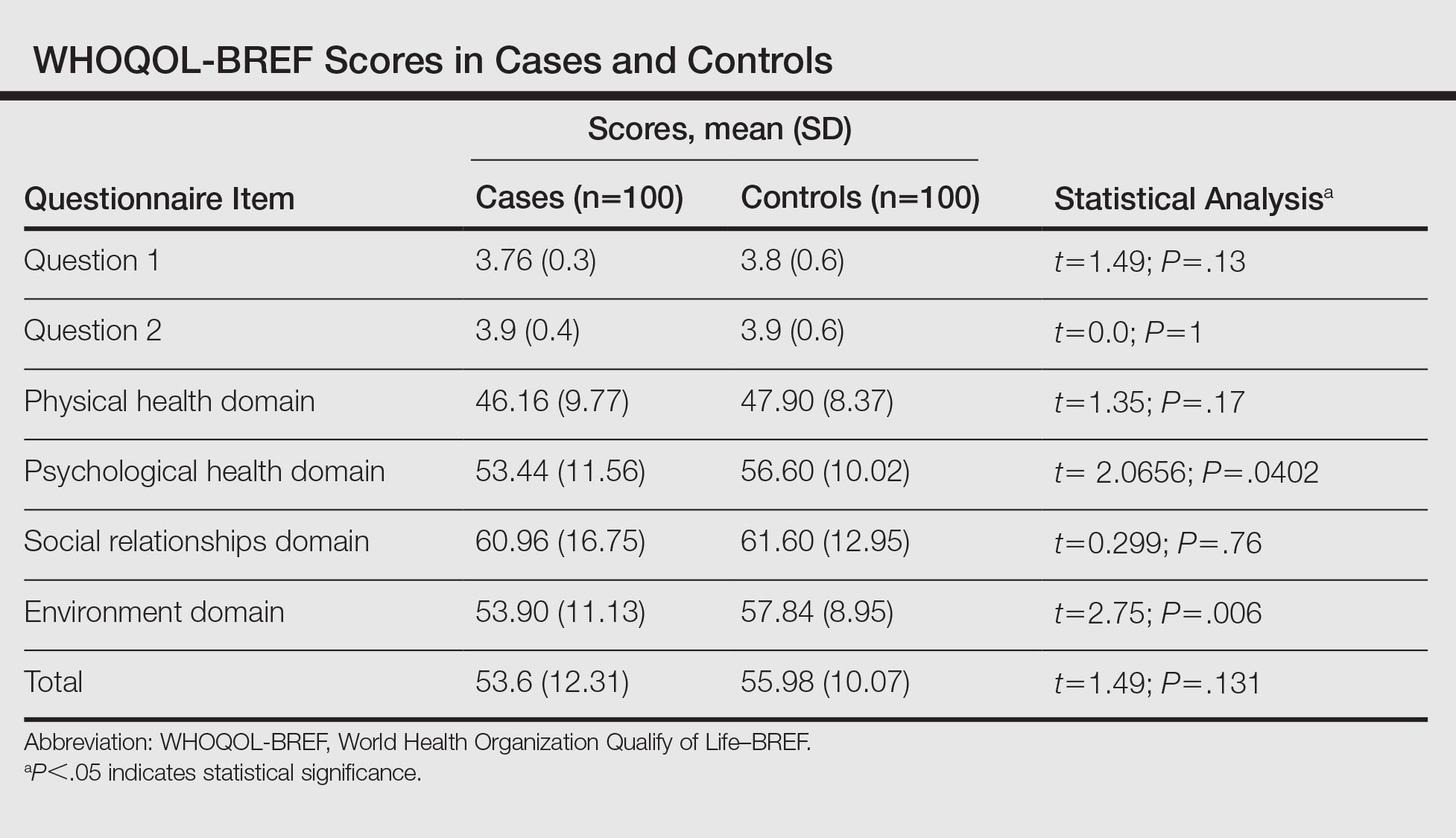
The RSES mean (SD) score was higher in controls (19.74 [4.23]) than in cases (15.72 [5.06]) and was statistically significant (P<.0001). Low self-esteem was noted in 38% of cases and 16% of controls, and high self-esteem was noted in 62% and 84%, respectively.
In reviewing the correlation between acne severity, CADI, WHOQOL-BREF, and RSES scores, we found a positive correlation between acne severity and CADI scores (R=0.51), which implies that as the severity of acne worsens, the QOL impairment increases. There was a negative correlation between acne severity, WHOQOL-BREF score (R=–0.13), and RSES score (R=–0.18), which showed that as the severity of acne increases, QOL and self-esteem decrease. We observed that as the grade of acne increases, there is a statistically significant impairment in the QOL according to CADI (P<.001), while there is a reduction in QOL and self-esteem according to WHOQOL-BREF and RSES, respectively (P>.05).
Comment
Patients are more likely to develop acne than any other skin disease in their lifetime. Only in recent years has the psychodermatologic literature begun to address the possibility of acne having a psychological and emotional impact.4 Although the cause-and-effect relationship between acne and psychological trauma has been debated for decades, only recently has the measurement focus shifted from psychological correlates (eg, personality) and emotional triggers (eg, stress) to the effect of acne on patients’ QOL and self-esteem. This shift occurred as validated instruments for measuring disability, QOL, and self-esteem, specifically in patients with skin diseases, became available.9
In our study, the age of onset of acne was 11 to 20 years and it affected predominantly females (58%), which is in concordance with other studies, as acne develops in adolescence and subsides in adulthood.1,10 Acne is more common in females due to hormonal factors and use of cosmetics. We observed that the face (80%) was most frequently affected, followed by the back (14%) and chest (6%), which is similar to prior studies.1,10 Because the face plays an important role in body image, the presence of facial lesions may be unacceptable for patients and therefore they may present more frequently to dermatologists.
In our study, 68% of cases and 22% of controls had a family history of acne. A similar correlation also was noted in other studies, which suggests acne has an inherited predisposition due to involvement of the cytochrome P450-1A1 gene, CYP1A1, and steroid 21-hydroxylase, P-450-c21.1,11 We found 46% of cases had grade 2 acne and 36% had grade 1 acne, which was congruent with prior studies.12,13 Patients with severe acne are more likely to seek medical intervention in hospitals.
In our study, 58% of the cases had medium to high impairment in QOL according to CADI scores. We noticed as the severity of acne increased there was severe impairment in QOL. Similar findings have been found in studies that used other scales to assess QOL.1,6,9
In our study, 38% of cases and 16% of controls had low self-esteem, which was statistically significant (P<.0001). There was a negative correlation between the severity of acne and self-esteem. In a prior study of 240 professional college students, 53% had feelings of low self-esteem and 40% revealed they avoided social gatherings and interactions with the opposite sex because of their acne.14 In a questionnaire-based survey of 3775 students, it was observed that the presence of acne correlated with poor self-attitude in boys and poor self-worth in girls.3 We found patients with grade 1 acne had higher self-esteem as compared to other grades of acne. Similarly, a cross-sectional study by Uslu et al15 found a direct correlation between acne severity and lower self-esteem using the RSES questionnaire. Although acne may be viewed as a minor cosmetic issue, it can have a negative impact on self-esteem and interpersonal relationships. Many of the studies had not used a validated structured questionnaire to assess self-esteem and there is a paucity of literature in relation to acne and self-esteem.3,16,17
According to the WHOQOL-BREF, the psychological domain was affected more in cases than in controls, which was a statistically significant difference. One study observed that patients experience immediate psychological consequences of acne such as reduced self-esteem, poor self-image, self-consciousness, and embarrassment.3 These effects are exacerbated by taunting, stigmatization, and perceptions of scrutiny and being judged, causing patients to avoid interaction and social situations. Similarly, Pruthi and Babu18 observed that acne had an impact on the psychosocial aspects of adult females using the Dermatology Life Quality Index and CADI.
Financial resources, health and social care accessibility, and opportunities for acquiring new information and skills were the factors that were considered in the environment domain of the WHOQOL-BREF.8 We noted that the environment domain scores were significantly lower in cases than in controls. The cases could have had a detrimental effect on the latest opportunities in occupational functioning due to acne, and as most of the population was from a rural area, they were having less favorable circumstances in acquiring new information about the management of acne.
There was no statistically significant difference between cases and controls in the social and physical domains of the WHOQOL-BREF, which suggests that these fields do not influence QOL. Similarly, patients in Sarawak, Malaysia, were least affected in the domain of social functioning, which was likely attributed to the upbringing of this population encouraging stoicism.19
In the current study, QOL impairment showed a positive correlation with acne severity according to CADI scores; however, there was no significant difference between WHOQOL-BREF score and acne grading, which suggests that QOL impairment does not depend on severity of acne alone. Physical, psychological, social, and environment domains play an important role in impaired QOL. Hence, by using the WHOQOL-BREF we can evaluate the actual domain that is adversely affected by acne and can be treated with a holistic approach. This point must be stressed in the training of medical faculty, as the treatment of acne should not be based on acne severity alone but also on the degree of QOL impairment.19
These results indicate that more data are required and there is a need to consider other variables that could play a role. This study was a hospital-based, cross-sectional study with a small sample group that cannot be generalized, which are limitations. Longitudinal follow-up of the cases before and after treatment was not done. The questionnaires helped us to detect psychosocial aspects but were insufficient to diagnose psychiatric comorbidity.
The strengths of this study include the use of a specific scale for the assessment of self-esteem. The usage of comprehensive (WHOQOL-BREF) and specific (CADI) scales to evaluate QOL has mutual advantage.
Conclusion
Acne vulgaris is a disease that can adversely affect an individual’s QOL and self-esteem. This study suggested the importance of screening for psychosocial problems in those who present for management of acne. It is important for dermatologists to be cautious about psychological problems in acne patients and be aware of the importance of basic psychosomatic treatment in conjunction with medical treatment in the management of acne.
- Durai PC, Nair DG. Acne vulgaris and quality of life among young adults in South India. Indian J Dermatol. 2015;60:33-40.
- Karciauskiene J, Valiukeviciene S, Gollnick H, et al. The prevalence and risk factors of adolescent acne among schoolchildren in Lithuania: a cross-sectional study. J Eur Acad Dermatol Venereol. 2014;28:733-740.
- Dunn LK, O’Neill JL, Feldman SR. Acne in adolescents: quality of life, self-esteem, mood, and psychological disorders. Dermatol Online J. 2011;17:1.
- Do JE, Cho SM, In SI, et al. Psychosocial aspects of acne vulgaris: a community-based study with Korean adolescents. Ann Dermatol. 2009;21:125-129.
- Rosenberg M. Society and the Adolescent Self-Image. Princeton, NJ: Princeton University Press; 1965.
- Ogedegbe EE, Henshaw EB. Severity and impact of acne vulgaris on the quality of life of adolescents in Nigeria. Clin Cosmet Investig Dermatol. 2014;7:329-334.
- Cardiff Acne Disability Index (CADI). Cardiff University website. sites.cardiff.ac.uk/dermatology/…of…/Cardiff-acne-disability-index-cadi/. Accessed July 21, 2016.
- WHO QOL-BREF: Introduction, administration, scoring and generic version of the assessment. World Health Organization website. http://www.who.int/mental_health/media/en/76.pdf. Published December 1996. Accessed June 6, 2016.
- Lasek RJ, Chren MM. Acne vulgaris and the quality of life of adult dermatology patients. Arch Dermatol. 1998;134:454-458.
- Adityan B, Thappa DM. Profile of acne vulgaris—a hospital-based study from South India. Indian J Dermatol Venereol Leprol. 2009;75:272-278.
- Tasoula E, Gregoriou S, Chalikias J, et al. The impact of acne vulgaris on quality of life and psychic health in young adolescents in Greece. results of a population survey. An Bras Dermatol. 2012;87:862-869.
- Agheai S, Mazaharinia N, Jafari P, et al. The Persian version of the Cardiff Acne Disability Index. reliability and validity study. Saudi Med J. 2006;27:80-82.
- Mallon E, Newton JN, Klassen A, et al. The quality of life in acne: a comparison with general medical conditions using generic questionnaires. Br J Dermatol. 1999;140:672-676.
- Goel S, Goel S. Clinico-psychological profile of acne vulgaris among professional students. Indian J Public Health Res Dev. 2012;3:175-178.
- Uslu G, Sendur N, Uslu M, et al. Acne: prevalence, perceptions and effects on psychological health among adolescents in Aydin, Turkey. J Eur Acad Dermatol Venereol. 2008;22:462-469.
- Ayer J, Burrows N. Acne: more than skin deep. Postgrad Med J. 2006;82:500-506.
- Fried RG, Gupta MA, Gupta AK. Depression and skin disease. Dermatol Clin. 2005;23:657-664.
- Pruthi GK, Babu N. Physical and psychosocial impact of acne in adult females. Indian J Dermatol. 2012;57:26-29.
- Yap FB. Cardiff Acne Disability Index in Sarawak, Malaysia. Ann Dermatol. 2012;24:158-161.
Acne vulgaris predominantly occurs during puberty and can persist beyond 25 years of age, most commonly in women.1,2 Although acne does not cause physical impairment, it can be associated with a considerable psychosocial burden including increased levels of anxiety, anger, depression, and frustration, which in turn can affect vocational and academic performance, quality of life (QOL), and self-esteem.3
Quality of life measures provide valuable insight into the debilitating effects of acne.1 It has been suggested that acne patients may experience poor body image and low self-esteem as well as social isolation and constriction of activities.4 Self-esteem is a favorable and unfavorable attitude toward oneself.5 A marked emphasis has been placed on body image in society, fueled by external cues such as the media.3,6 This study was carried out to assess QOL and self-esteem in acne patients.
Methods
This prospective, hospital-based, cross-sectional, case-control study was conducted at The Oxford Medical College, Hospital & Research Center (Bangalore, India), over a period of 3 months. One hundred consecutive acne cases (age range, 12–45 years) and 100 age- and gender-matched controls who did not have any skin disease provided consent and were included in the analysis. Guardians gave consent for individuals who were younger than 18 years. Exclusion criteria for cases included a medical disorder (eg, epilepsy, diabetes mellitus, hypertension) or medications that would likely interfere with acne assessment.
The cases and controls were administered a semistructured questionnaire to collect sociodemographic details. Acne was graded for the predominant lesions, QOL was assessed using the Cardiff Acne Disability Index (CADI) and World Health Organization Quality of Life–BREF (WHOQOL-BREF) scale, and self-esteem was measured using the Rosenberg self-esteem scale (RSES). The study was approved by the institutional review board.
Acne Grading
Acne was graded according to the predominant lesions using the following criteria: grade 1=comedones and occasional papules; grade 2=papules, comedones, and few pustules; grade 3=predominant pustules, nodules, and abscesses; and grade 4=mainly cysts, abscesses, and widespread scarring.1
Quality of Life Assessment
The CADI questionnaire was used to assess the level of disability caused by acne.6 It is a 5-item questionnaire with scores ranging from 0 to 3 for a total maximum score of 15 and minimum score of 0. Total scores were classified as low (0–4), medium (5–9), and high (10–15).7
The WHOQOL-BREF is a self-reported questionnaire containing 26 items that make up the 4 domains of physical health (7 items), psychological health (6 items), social relationships (3 items), and environment (8 items); there also are 2 single questions regarding the overall perception of QOL and health. Questions were scored on aseries of 5-point scales with higher scores denoting better QOL.8
Self-esteem Assessment
The RSES uses a 5-point Likert scale from strongly agree to strongly disagree to rate a series of 10 statements. The total score ranges from 0 to 30. Scores less than 15 suggest low self-esteem, while scores of 15 and greater indicate high self-esteem.5
Statistical Analysis
Results were analyzed using descriptive and inferential statistical methods. A χ2 test was used for categorical data, and a Student t test and an analysis of variance were used for continuous data.
Results
The study consisted of 100 cases and 100 controls. The mean age was 21 years. The majority of cases reported an age of onset of acne of 11 to 20 years (66%), were predominantly female (58%) from rural backgrounds, and had a family history of acne (68%). The majority of lesions ceased within 24 months (60%). The face was the most commonly involved area (80%) and papules were the most prevalent lesion type (62%).
Cases predominantly had grade 2 acne (46%), and there was medium to high impairment in QOL according to CADI scores.
The scores for all the domains of the WHOQOL-BREF as well as the total score were lower in cases compared to controls (Table). There was a statistically significant difference between the 2 groups in the psychological (P=.0402) and environment (P=.006) domains.

The RSES mean (SD) score was higher in controls (19.74 [4.23]) than in cases (15.72 [5.06]) and was statistically significant (P<.0001). Low self-esteem was noted in 38% of cases and 16% of controls, and high self-esteem was noted in 62% and 84%, respectively.
In reviewing the correlation between acne severity, CADI, WHOQOL-BREF, and RSES scores, we found a positive correlation between acne severity and CADI scores (R=0.51), which implies that as the severity of acne worsens, the QOL impairment increases. There was a negative correlation between acne severity, WHOQOL-BREF score (R=–0.13), and RSES score (R=–0.18), which showed that as the severity of acne increases, QOL and self-esteem decrease. We observed that as the grade of acne increases, there is a statistically significant impairment in the QOL according to CADI (P<.001), while there is a reduction in QOL and self-esteem according to WHOQOL-BREF and RSES, respectively (P>.05).
Comment
Patients are more likely to develop acne than any other skin disease in their lifetime. Only in recent years has the psychodermatologic literature begun to address the possibility of acne having a psychological and emotional impact.4 Although the cause-and-effect relationship between acne and psychological trauma has been debated for decades, only recently has the measurement focus shifted from psychological correlates (eg, personality) and emotional triggers (eg, stress) to the effect of acne on patients’ QOL and self-esteem. This shift occurred as validated instruments for measuring disability, QOL, and self-esteem, specifically in patients with skin diseases, became available.9
In our study, the age of onset of acne was 11 to 20 years and it affected predominantly females (58%), which is in concordance with other studies, as acne develops in adolescence and subsides in adulthood.1,10 Acne is more common in females due to hormonal factors and use of cosmetics. We observed that the face (80%) was most frequently affected, followed by the back (14%) and chest (6%), which is similar to prior studies.1,10 Because the face plays an important role in body image, the presence of facial lesions may be unacceptable for patients and therefore they may present more frequently to dermatologists.
In our study, 68% of cases and 22% of controls had a family history of acne. A similar correlation also was noted in other studies, which suggests acne has an inherited predisposition due to involvement of the cytochrome P450-1A1 gene, CYP1A1, and steroid 21-hydroxylase, P-450-c21.1,11 We found 46% of cases had grade 2 acne and 36% had grade 1 acne, which was congruent with prior studies.12,13 Patients with severe acne are more likely to seek medical intervention in hospitals.
In our study, 58% of the cases had medium to high impairment in QOL according to CADI scores. We noticed as the severity of acne increased there was severe impairment in QOL. Similar findings have been found in studies that used other scales to assess QOL.1,6,9
In our study, 38% of cases and 16% of controls had low self-esteem, which was statistically significant (P<.0001). There was a negative correlation between the severity of acne and self-esteem. In a prior study of 240 professional college students, 53% had feelings of low self-esteem and 40% revealed they avoided social gatherings and interactions with the opposite sex because of their acne.14 In a questionnaire-based survey of 3775 students, it was observed that the presence of acne correlated with poor self-attitude in boys and poor self-worth in girls.3 We found patients with grade 1 acne had higher self-esteem as compared to other grades of acne. Similarly, a cross-sectional study by Uslu et al15 found a direct correlation between acne severity and lower self-esteem using the RSES questionnaire. Although acne may be viewed as a minor cosmetic issue, it can have a negative impact on self-esteem and interpersonal relationships. Many of the studies had not used a validated structured questionnaire to assess self-esteem and there is a paucity of literature in relation to acne and self-esteem.3,16,17
According to the WHOQOL-BREF, the psychological domain was affected more in cases than in controls, which was a statistically significant difference. One study observed that patients experience immediate psychological consequences of acne such as reduced self-esteem, poor self-image, self-consciousness, and embarrassment.3 These effects are exacerbated by taunting, stigmatization, and perceptions of scrutiny and being judged, causing patients to avoid interaction and social situations. Similarly, Pruthi and Babu18 observed that acne had an impact on the psychosocial aspects of adult females using the Dermatology Life Quality Index and CADI.
Financial resources, health and social care accessibility, and opportunities for acquiring new information and skills were the factors that were considered in the environment domain of the WHOQOL-BREF.8 We noted that the environment domain scores were significantly lower in cases than in controls. The cases could have had a detrimental effect on the latest opportunities in occupational functioning due to acne, and as most of the population was from a rural area, they were having less favorable circumstances in acquiring new information about the management of acne.
There was no statistically significant difference between cases and controls in the social and physical domains of the WHOQOL-BREF, which suggests that these fields do not influence QOL. Similarly, patients in Sarawak, Malaysia, were least affected in the domain of social functioning, which was likely attributed to the upbringing of this population encouraging stoicism.19
In the current study, QOL impairment showed a positive correlation with acne severity according to CADI scores; however, there was no significant difference between WHOQOL-BREF score and acne grading, which suggests that QOL impairment does not depend on severity of acne alone. Physical, psychological, social, and environment domains play an important role in impaired QOL. Hence, by using the WHOQOL-BREF we can evaluate the actual domain that is adversely affected by acne and can be treated with a holistic approach. This point must be stressed in the training of medical faculty, as the treatment of acne should not be based on acne severity alone but also on the degree of QOL impairment.19
These results indicate that more data are required and there is a need to consider other variables that could play a role. This study was a hospital-based, cross-sectional study with a small sample group that cannot be generalized, which are limitations. Longitudinal follow-up of the cases before and after treatment was not done. The questionnaires helped us to detect psychosocial aspects but were insufficient to diagnose psychiatric comorbidity.
The strengths of this study include the use of a specific scale for the assessment of self-esteem. The usage of comprehensive (WHOQOL-BREF) and specific (CADI) scales to evaluate QOL has mutual advantage.
Conclusion
Acne vulgaris is a disease that can adversely affect an individual’s QOL and self-esteem. This study suggested the importance of screening for psychosocial problems in those who present for management of acne. It is important for dermatologists to be cautious about psychological problems in acne patients and be aware of the importance of basic psychosomatic treatment in conjunction with medical treatment in the management of acne.
Acne vulgaris predominantly occurs during puberty and can persist beyond 25 years of age, most commonly in women.1,2 Although acne does not cause physical impairment, it can be associated with a considerable psychosocial burden including increased levels of anxiety, anger, depression, and frustration, which in turn can affect vocational and academic performance, quality of life (QOL), and self-esteem.3
Quality of life measures provide valuable insight into the debilitating effects of acne.1 It has been suggested that acne patients may experience poor body image and low self-esteem as well as social isolation and constriction of activities.4 Self-esteem is a favorable and unfavorable attitude toward oneself.5 A marked emphasis has been placed on body image in society, fueled by external cues such as the media.3,6 This study was carried out to assess QOL and self-esteem in acne patients.
Methods
This prospective, hospital-based, cross-sectional, case-control study was conducted at The Oxford Medical College, Hospital & Research Center (Bangalore, India), over a period of 3 months. One hundred consecutive acne cases (age range, 12–45 years) and 100 age- and gender-matched controls who did not have any skin disease provided consent and were included in the analysis. Guardians gave consent for individuals who were younger than 18 years. Exclusion criteria for cases included a medical disorder (eg, epilepsy, diabetes mellitus, hypertension) or medications that would likely interfere with acne assessment.
The cases and controls were administered a semistructured questionnaire to collect sociodemographic details. Acne was graded for the predominant lesions, QOL was assessed using the Cardiff Acne Disability Index (CADI) and World Health Organization Quality of Life–BREF (WHOQOL-BREF) scale, and self-esteem was measured using the Rosenberg self-esteem scale (RSES). The study was approved by the institutional review board.
Acne Grading
Acne was graded according to the predominant lesions using the following criteria: grade 1=comedones and occasional papules; grade 2=papules, comedones, and few pustules; grade 3=predominant pustules, nodules, and abscesses; and grade 4=mainly cysts, abscesses, and widespread scarring.1
Quality of Life Assessment
The CADI questionnaire was used to assess the level of disability caused by acne.6 It is a 5-item questionnaire with scores ranging from 0 to 3 for a total maximum score of 15 and minimum score of 0. Total scores were classified as low (0–4), medium (5–9), and high (10–15).7
The WHOQOL-BREF is a self-reported questionnaire containing 26 items that make up the 4 domains of physical health (7 items), psychological health (6 items), social relationships (3 items), and environment (8 items); there also are 2 single questions regarding the overall perception of QOL and health. Questions were scored on aseries of 5-point scales with higher scores denoting better QOL.8
Self-esteem Assessment
The RSES uses a 5-point Likert scale from strongly agree to strongly disagree to rate a series of 10 statements. The total score ranges from 0 to 30. Scores less than 15 suggest low self-esteem, while scores of 15 and greater indicate high self-esteem.5
Statistical Analysis
Results were analyzed using descriptive and inferential statistical methods. A χ2 test was used for categorical data, and a Student t test and an analysis of variance were used for continuous data.
Results
The study consisted of 100 cases and 100 controls. The mean age was 21 years. The majority of cases reported an age of onset of acne of 11 to 20 years (66%), were predominantly female (58%) from rural backgrounds, and had a family history of acne (68%). The majority of lesions ceased within 24 months (60%). The face was the most commonly involved area (80%) and papules were the most prevalent lesion type (62%).
Cases predominantly had grade 2 acne (46%), and there was medium to high impairment in QOL according to CADI scores.
The scores for all the domains of the WHOQOL-BREF as well as the total score were lower in cases compared to controls (Table). There was a statistically significant difference between the 2 groups in the psychological (P=.0402) and environment (P=.006) domains.

The RSES mean (SD) score was higher in controls (19.74 [4.23]) than in cases (15.72 [5.06]) and was statistically significant (P<.0001). Low self-esteem was noted in 38% of cases and 16% of controls, and high self-esteem was noted in 62% and 84%, respectively.
In reviewing the correlation between acne severity, CADI, WHOQOL-BREF, and RSES scores, we found a positive correlation between acne severity and CADI scores (R=0.51), which implies that as the severity of acne worsens, the QOL impairment increases. There was a negative correlation between acne severity, WHOQOL-BREF score (R=–0.13), and RSES score (R=–0.18), which showed that as the severity of acne increases, QOL and self-esteem decrease. We observed that as the grade of acne increases, there is a statistically significant impairment in the QOL according to CADI (P<.001), while there is a reduction in QOL and self-esteem according to WHOQOL-BREF and RSES, respectively (P>.05).
Comment
Patients are more likely to develop acne than any other skin disease in their lifetime. Only in recent years has the psychodermatologic literature begun to address the possibility of acne having a psychological and emotional impact.4 Although the cause-and-effect relationship between acne and psychological trauma has been debated for decades, only recently has the measurement focus shifted from psychological correlates (eg, personality) and emotional triggers (eg, stress) to the effect of acne on patients’ QOL and self-esteem. This shift occurred as validated instruments for measuring disability, QOL, and self-esteem, specifically in patients with skin diseases, became available.9
In our study, the age of onset of acne was 11 to 20 years and it affected predominantly females (58%), which is in concordance with other studies, as acne develops in adolescence and subsides in adulthood.1,10 Acne is more common in females due to hormonal factors and use of cosmetics. We observed that the face (80%) was most frequently affected, followed by the back (14%) and chest (6%), which is similar to prior studies.1,10 Because the face plays an important role in body image, the presence of facial lesions may be unacceptable for patients and therefore they may present more frequently to dermatologists.
In our study, 68% of cases and 22% of controls had a family history of acne. A similar correlation also was noted in other studies, which suggests acne has an inherited predisposition due to involvement of the cytochrome P450-1A1 gene, CYP1A1, and steroid 21-hydroxylase, P-450-c21.1,11 We found 46% of cases had grade 2 acne and 36% had grade 1 acne, which was congruent with prior studies.12,13 Patients with severe acne are more likely to seek medical intervention in hospitals.
In our study, 58% of the cases had medium to high impairment in QOL according to CADI scores. We noticed as the severity of acne increased there was severe impairment in QOL. Similar findings have been found in studies that used other scales to assess QOL.1,6,9
In our study, 38% of cases and 16% of controls had low self-esteem, which was statistically significant (P<.0001). There was a negative correlation between the severity of acne and self-esteem. In a prior study of 240 professional college students, 53% had feelings of low self-esteem and 40% revealed they avoided social gatherings and interactions with the opposite sex because of their acne.14 In a questionnaire-based survey of 3775 students, it was observed that the presence of acne correlated with poor self-attitude in boys and poor self-worth in girls.3 We found patients with grade 1 acne had higher self-esteem as compared to other grades of acne. Similarly, a cross-sectional study by Uslu et al15 found a direct correlation between acne severity and lower self-esteem using the RSES questionnaire. Although acne may be viewed as a minor cosmetic issue, it can have a negative impact on self-esteem and interpersonal relationships. Many of the studies had not used a validated structured questionnaire to assess self-esteem and there is a paucity of literature in relation to acne and self-esteem.3,16,17
According to the WHOQOL-BREF, the psychological domain was affected more in cases than in controls, which was a statistically significant difference. One study observed that patients experience immediate psychological consequences of acne such as reduced self-esteem, poor self-image, self-consciousness, and embarrassment.3 These effects are exacerbated by taunting, stigmatization, and perceptions of scrutiny and being judged, causing patients to avoid interaction and social situations. Similarly, Pruthi and Babu18 observed that acne had an impact on the psychosocial aspects of adult females using the Dermatology Life Quality Index and CADI.
Financial resources, health and social care accessibility, and opportunities for acquiring new information and skills were the factors that were considered in the environment domain of the WHOQOL-BREF.8 We noted that the environment domain scores were significantly lower in cases than in controls. The cases could have had a detrimental effect on the latest opportunities in occupational functioning due to acne, and as most of the population was from a rural area, they were having less favorable circumstances in acquiring new information about the management of acne.
There was no statistically significant difference between cases and controls in the social and physical domains of the WHOQOL-BREF, which suggests that these fields do not influence QOL. Similarly, patients in Sarawak, Malaysia, were least affected in the domain of social functioning, which was likely attributed to the upbringing of this population encouraging stoicism.19
In the current study, QOL impairment showed a positive correlation with acne severity according to CADI scores; however, there was no significant difference between WHOQOL-BREF score and acne grading, which suggests that QOL impairment does not depend on severity of acne alone. Physical, psychological, social, and environment domains play an important role in impaired QOL. Hence, by using the WHOQOL-BREF we can evaluate the actual domain that is adversely affected by acne and can be treated with a holistic approach. This point must be stressed in the training of medical faculty, as the treatment of acne should not be based on acne severity alone but also on the degree of QOL impairment.19
These results indicate that more data are required and there is a need to consider other variables that could play a role. This study was a hospital-based, cross-sectional study with a small sample group that cannot be generalized, which are limitations. Longitudinal follow-up of the cases before and after treatment was not done. The questionnaires helped us to detect psychosocial aspects but were insufficient to diagnose psychiatric comorbidity.
The strengths of this study include the use of a specific scale for the assessment of self-esteem. The usage of comprehensive (WHOQOL-BREF) and specific (CADI) scales to evaluate QOL has mutual advantage.
Conclusion
Acne vulgaris is a disease that can adversely affect an individual’s QOL and self-esteem. This study suggested the importance of screening for psychosocial problems in those who present for management of acne. It is important for dermatologists to be cautious about psychological problems in acne patients and be aware of the importance of basic psychosomatic treatment in conjunction with medical treatment in the management of acne.
- Durai PC, Nair DG. Acne vulgaris and quality of life among young adults in South India. Indian J Dermatol. 2015;60:33-40.
- Karciauskiene J, Valiukeviciene S, Gollnick H, et al. The prevalence and risk factors of adolescent acne among schoolchildren in Lithuania: a cross-sectional study. J Eur Acad Dermatol Venereol. 2014;28:733-740.
- Dunn LK, O’Neill JL, Feldman SR. Acne in adolescents: quality of life, self-esteem, mood, and psychological disorders. Dermatol Online J. 2011;17:1.
- Do JE, Cho SM, In SI, et al. Psychosocial aspects of acne vulgaris: a community-based study with Korean adolescents. Ann Dermatol. 2009;21:125-129.
- Rosenberg M. Society and the Adolescent Self-Image. Princeton, NJ: Princeton University Press; 1965.
- Ogedegbe EE, Henshaw EB. Severity and impact of acne vulgaris on the quality of life of adolescents in Nigeria. Clin Cosmet Investig Dermatol. 2014;7:329-334.
- Cardiff Acne Disability Index (CADI). Cardiff University website. sites.cardiff.ac.uk/dermatology/…of…/Cardiff-acne-disability-index-cadi/. Accessed July 21, 2016.
- WHO QOL-BREF: Introduction, administration, scoring and generic version of the assessment. World Health Organization website. http://www.who.int/mental_health/media/en/76.pdf. Published December 1996. Accessed June 6, 2016.
- Lasek RJ, Chren MM. Acne vulgaris and the quality of life of adult dermatology patients. Arch Dermatol. 1998;134:454-458.
- Adityan B, Thappa DM. Profile of acne vulgaris—a hospital-based study from South India. Indian J Dermatol Venereol Leprol. 2009;75:272-278.
- Tasoula E, Gregoriou S, Chalikias J, et al. The impact of acne vulgaris on quality of life and psychic health in young adolescents in Greece. results of a population survey. An Bras Dermatol. 2012;87:862-869.
- Agheai S, Mazaharinia N, Jafari P, et al. The Persian version of the Cardiff Acne Disability Index. reliability and validity study. Saudi Med J. 2006;27:80-82.
- Mallon E, Newton JN, Klassen A, et al. The quality of life in acne: a comparison with general medical conditions using generic questionnaires. Br J Dermatol. 1999;140:672-676.
- Goel S, Goel S. Clinico-psychological profile of acne vulgaris among professional students. Indian J Public Health Res Dev. 2012;3:175-178.
- Uslu G, Sendur N, Uslu M, et al. Acne: prevalence, perceptions and effects on psychological health among adolescents in Aydin, Turkey. J Eur Acad Dermatol Venereol. 2008;22:462-469.
- Ayer J, Burrows N. Acne: more than skin deep. Postgrad Med J. 2006;82:500-506.
- Fried RG, Gupta MA, Gupta AK. Depression and skin disease. Dermatol Clin. 2005;23:657-664.
- Pruthi GK, Babu N. Physical and psychosocial impact of acne in adult females. Indian J Dermatol. 2012;57:26-29.
- Yap FB. Cardiff Acne Disability Index in Sarawak, Malaysia. Ann Dermatol. 2012;24:158-161.
- Durai PC, Nair DG. Acne vulgaris and quality of life among young adults in South India. Indian J Dermatol. 2015;60:33-40.
- Karciauskiene J, Valiukeviciene S, Gollnick H, et al. The prevalence and risk factors of adolescent acne among schoolchildren in Lithuania: a cross-sectional study. J Eur Acad Dermatol Venereol. 2014;28:733-740.
- Dunn LK, O’Neill JL, Feldman SR. Acne in adolescents: quality of life, self-esteem, mood, and psychological disorders. Dermatol Online J. 2011;17:1.
- Do JE, Cho SM, In SI, et al. Psychosocial aspects of acne vulgaris: a community-based study with Korean adolescents. Ann Dermatol. 2009;21:125-129.
- Rosenberg M. Society and the Adolescent Self-Image. Princeton, NJ: Princeton University Press; 1965.
- Ogedegbe EE, Henshaw EB. Severity and impact of acne vulgaris on the quality of life of adolescents in Nigeria. Clin Cosmet Investig Dermatol. 2014;7:329-334.
- Cardiff Acne Disability Index (CADI). Cardiff University website. sites.cardiff.ac.uk/dermatology/…of…/Cardiff-acne-disability-index-cadi/. Accessed July 21, 2016.
- WHO QOL-BREF: Introduction, administration, scoring and generic version of the assessment. World Health Organization website. http://www.who.int/mental_health/media/en/76.pdf. Published December 1996. Accessed June 6, 2016.
- Lasek RJ, Chren MM. Acne vulgaris and the quality of life of adult dermatology patients. Arch Dermatol. 1998;134:454-458.
- Adityan B, Thappa DM. Profile of acne vulgaris—a hospital-based study from South India. Indian J Dermatol Venereol Leprol. 2009;75:272-278.
- Tasoula E, Gregoriou S, Chalikias J, et al. The impact of acne vulgaris on quality of life and psychic health in young adolescents in Greece. results of a population survey. An Bras Dermatol. 2012;87:862-869.
- Agheai S, Mazaharinia N, Jafari P, et al. The Persian version of the Cardiff Acne Disability Index. reliability and validity study. Saudi Med J. 2006;27:80-82.
- Mallon E, Newton JN, Klassen A, et al. The quality of life in acne: a comparison with general medical conditions using generic questionnaires. Br J Dermatol. 1999;140:672-676.
- Goel S, Goel S. Clinico-psychological profile of acne vulgaris among professional students. Indian J Public Health Res Dev. 2012;3:175-178.
- Uslu G, Sendur N, Uslu M, et al. Acne: prevalence, perceptions and effects on psychological health among adolescents in Aydin, Turkey. J Eur Acad Dermatol Venereol. 2008;22:462-469.
- Ayer J, Burrows N. Acne: more than skin deep. Postgrad Med J. 2006;82:500-506.
- Fried RG, Gupta MA, Gupta AK. Depression and skin disease. Dermatol Clin. 2005;23:657-664.
- Pruthi GK, Babu N. Physical and psychosocial impact of acne in adult females. Indian J Dermatol. 2012;57:26-29.
- Yap FB. Cardiff Acne Disability Index in Sarawak, Malaysia. Ann Dermatol. 2012;24:158-161.
Practice Points
- Grading of acne will help with appropriate treatment, thus reducing the adverse psychological effects of the condition.
- Acne severity has a negative impact on quality of life and self-esteem.
- A sympathetic approach and basic psychosomatic treatment are necessary in the management of acne.