User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Idiopathic Livedo Racemosa Presenting With Splenomegaly and Diffuse Lymphadenopathy
Sneddon syndrome (SS) was first described in 1965 in patients with persistent livedo racemosa and neurological events.1 Because the other manifestations of SS are nonspecific (eg, hypertension, cardiac valvulopathy, arterial and venous occlusion), the diagnosis often is delayed. Many patients who experience prodromal neurologic symptoms such as headaches, depression, anxiety, dizziness, and neuropathy often present to a physician prior to developing ischemic brain manifestations2 but seldom receive the correct diagnosis. Onset of cerebral occlusive events typically occurs in patients younger than 45 years and may present as a transient ischemic attack, stroke, or intracranial hemorrhage.3 The disease is more prevalent in females than males (2:1 ratio). The exact pathogenesis of SS is still unknown, and although it has been thought of as a separate entity from systemic lupus erythematosus and other antiphospholipid disorders, it has been postulated that an immunological dysfunction damages vessel walls leading to thrombosis.
Cutaneous findings associated with SS involve small- to medium-sized dermal-subdermal arteries. Histopathology in some patients demonstrates proliferation of the endothelium and fibrin deposits with subsequent obliteration of involved arteries.4 In many patients including our patient, histopathologic examination of involved skin fails to show specific abnormalities.1 Zelger et al5 reported the sequence of histopathologic skin events in a series of antiphospholipid-negative SS patients. The authors reported that only small arteries at the dermis-subcutis junction were involved and a progression of endothelial dysfunction was observed. The authors believed there were several nonspecific stages prior to fibrin occlusion of involved arteries.5 Stage I involved loosening of endothelial cells with nonspecific perivascular lymphocytic infiltration with perivascular inflammation and lymphocytic infiltration representing the prime mover of the disease.5,6 This stage is thought to be short lived, thus the reason why it has gone undetected for many years in SS patients. Stages II to IV progress through fibrin deposition and occlusion.5 Histological features of stages I to II have not been reported because of late diagnosis of SS. Stage I patients typically present with an average duration of symptoms of 6 months with few neurologic symptoms, the most common being paresthesia of the legs.5
Case Report
A 37-year-old woman with epigastric tenderness on the left side and splenomegaly seen on computed tomography was referred by a hematologist for evaluation of a reticular rash on the left side of the flank of 9 months’ duration with a presumed diagnosis of focal melanoderma. Her medical history was remarkable for a congenital ventricular septal defect and coarctation of the aorta, as well as endometriosis, myalgia, and joint stiffness that had all developed over the last year. Her medical history also was remarkable for nephrolithiasis, irritable bowel syndrome, and chronic sinusitis, as well as psychiatric depression and anxiety disorders. She recently had been diagnosed with moderate hypertension and had experienced difficulty getting pregnant for the last several years with 3 consecutive miscarriages in the first trimester. Neurologic symptoms included neuropathy involving the feet, intermittent paresthesia of the legs, and a history of chronic migraine headaches for several months.
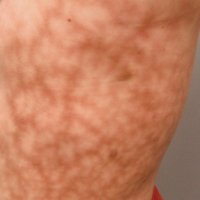
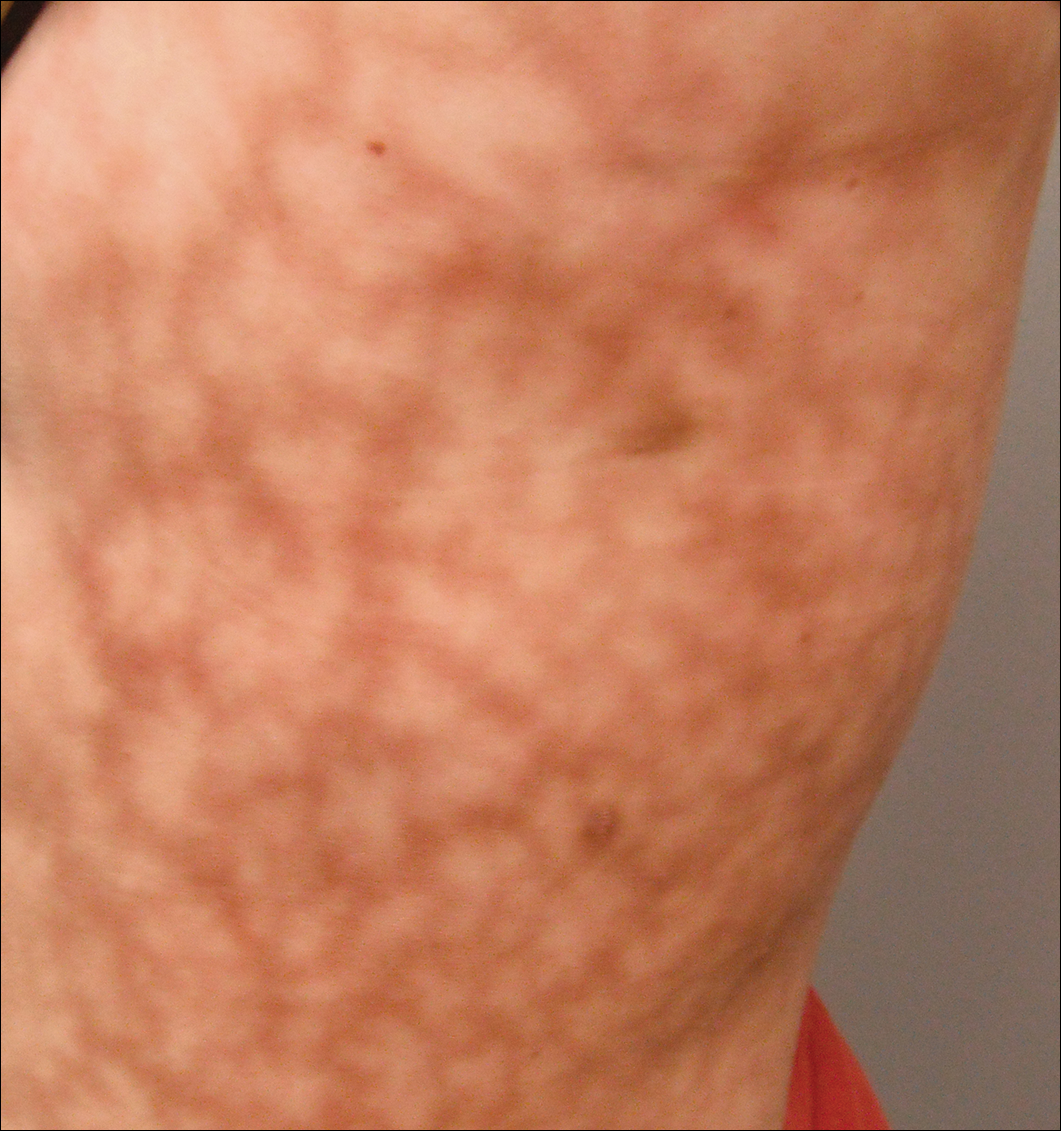
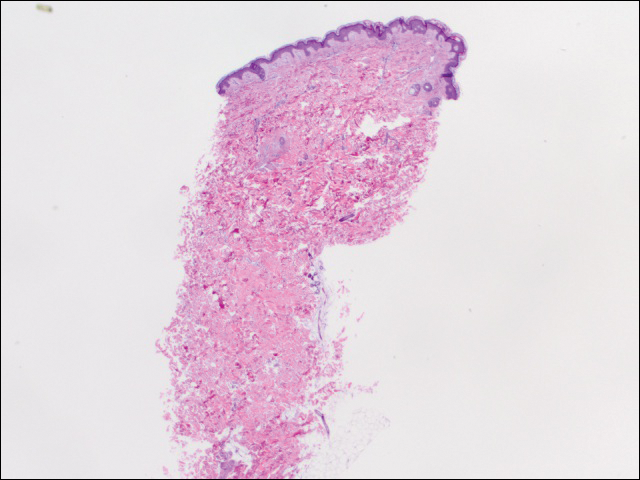
Dermatologic examination revealed a slightly overweight woman with a 25×30-cm dusky, erythematous, irregular, netlike pattern on the left side of the upper and lower trunk (Figure 1). Extensive livedo racemosa was not altered by changes in temperature and had been unchanged for more than 9 months. There were no signs of pruritus or ulcerations, and areas of livedo racemosa were slightly tender to palpation.
We performed 2 sets of three 4-mm biopsies. The first set targeted areas within the violaceous pattern, while the second set targeted areas of normal tissue between the mottled areas. All 6 specimens demonstrated superficial perivascular lymphocytic infiltrate with no evidence of vasculitis or connective tissue disease. The vessels showed no microthrombi or surrounding fibrosis. No eosinophils were identified within the epidermis. There was no evidence of increased dermal mucin. Both the superficial and deep vascular plexuses were unremarkable and showed no evidence of damage to the walls (Figure 2).
To rule out other possible causes of livedo racemosa, complete blood cell count, comprehensive metabolic panel, coagulation profile, lipase test, urinalysis, serologic testing, and immunologic workup were performed. Lipase was within reference range. The complete blood cell count revealed mild anemia, while the rest of the values were within reference range. An immunologic workup included Sjögren syndrome antigen A, Sjögren syndrome antigen B, anticardiolipin antibodies, and antinuclear antibody, which were all negative. Family history was remarkable for first-degree relatives with systemic lupus erythematosus and Crohn disease.
Computed tomography revealed enlargement of the spleen, as well as periaortic, portacaval, and porta hepatis lymphadenopathy. Based on the laboratory findings and clinical presentation as well as the patient’s medical history, the diagnosis of exclusion was idiopathic livedo racemosa with unknown progression to full-blown SS. The patient did not meet the current diagnostic criteria for SS, and her immunologic studies failed to confirm any present antibodies, but involvement of the reticuloendothelial system pointed to production of antibodies that were not yet detectable on laboratory testing.
Comment
More than 50 years after the first case of SS was diagnosed, better laboratory workup is available and more information is known about the pathophysiology. Sneddon syndrome is a rare disorder, affecting only approximately 4 patients per million each year worldwide. Seronegative antiphospholipid antibody syndrome (SNAPS) describes patients with clinical presentations of antiphospholipid syndrome (APS) without detectable serological markers.7 Antiphospholipid-negative SS, which was seen in our patient, would be categorized under SNAPS. A PubMed search of articles indexed for MEDLINE using the terms livedo racemosa, Sneddon syndrome, and SNAPS and splenomegaly revealed there currently are no known cases of SNAPS that have been reported with splenomegaly and lymphadenopathy. Our patient presented with the following clinical features of SS: livedo racemosa, history of miscarriage, psychiatric disturbances, and hypertension. Surprisingly, biopsies from affected skin did not show any fibrin deposition or microthrombi but did reveal perivascular lymphocytic infiltrations. Magnetic resonance imaging did not show any pathological lesions or vascular changes.
Sneddon syndrome and APS share a common pathway to occlusive arteriolopathy for which 4 stages have been described by Zelger et al.5 Stage I involves a nonspecific Langerhans cell infiltrate with polymorphonuclear leukocytes. The tunica media and elastic lamina usually are unaltered at this early stage, while the surrounding connective tissue may appear edematous.5 This early stage of histopathology has not been evaluated in SS patients, primarily because of delay of diagnosis. Late stages III and IV will show fibrin deposition and shrinkage of affected vessels.7
A PubMed search using the terms Sneddon syndrome, lymphadenopathy and livedo racemosa, and Sneddon syndrome and lymphadenopathy revealed that splenomegaly and lymphadenopathy have not been reported in patients with SS. In patients with antiphospholipid-negative SS, one can assume that antibodies to other phospholipids not tested must exist because of striking similarities between APS and antiphospholipid-negative SS.8 Although our patient did not test positive for any of these antibodies, she did present with lymphadenopathy and splenic enlargement, leading us to believe that involvement of the reticuloendothelial system may be a feature of SS that has not been previously reported. Further studies are required to name specific antigens responsible for clinical manifestations in SS.
Currently, no single diagnostic test for SS exists, thus delaying both diagnosis and initiation of treatment. Histopathologic examination may be helpful, but in many cases it is nonspecific, as are serologic markers. Neuroradiological confirmation of involvement usually is the confirmatory feature in many patients with late-stage diagnosis.2 A diagnostic schematic for SS, which was first described by Daoud et al,2 illustrates classification of symptoms and aids in diagnosis. A working diagnosis of idiopathic livedo racemosa is made after ruling out other causes of SS in a patient with nonspecific biopsy findings and negative magnetic resonance imaging results with prodromal symptoms. The prognosis for such patients progressing to full SS is unknown with or without management using anticoagulant therapy.
Conclusion
Early diagnosis of livedo racemosa and SS is essential, as prevention of cerebrovascular accidents, myocardial infarction, and other thromboembolic diseases can be minimized by attacking risk factors such as smoking, taking oral contraceptive pills, becoming pregnant,9 and by initiating either antiplatelet or anticoagulation treatments. These treatments have been shown to delay the development of neurovascular damage and early-onset dementia. We present this case to demonstrate the variability of early-presenting symptoms in idiopathic livedo racemosa. Recognizing some of the early manifestations can lead to early diagnosis and initiation of treatment.
- Sneddon IB. Cerebro-vascular lesions and livedo reticularis. Br J Dermatol. 1965;77:180-185.
- Daoud MS, Wilmoth GJ, Su WP, et al. Sneddon syndrome. Semin Dermatol. 1995;14:166-172.
- Besnier R, Francès C, Ankri A, et al. Factor V Leiden mutation in Sneddon syndrome. Lupus. 2003;12:406-408.
- K aragülle AT, Karadağ D, Erden A, et al. Sneddon’s syndrome: MR imaging findings. Eur Radiol. 2002;12:144-146.
- Zelg er B, Sepp N, Schmid KW, et al. Life-history of cutaneous vascular-lesions in Sneddon’s syndrome. Hum Pathol. 1992;23:668-675.
- Ayoub N, Esposito G, Barete S, et al. Protein Z deficiency in antiphospholipid-negative Sneddon’s syndrome. Stroke. 2004;35:1329-1332.
- Duva l A, Darnige L, Glowacki F, et al. Livedo, dementia, thrombocytopenia, and endotheliitis without antiphospholipid antibodies: seronegative antiphospholipid-like syndrome. J Am Acad Dermatol. 2009;61:1076-1078.
- Kala shnikova LA, Nasonov EL, Kushekbaeva AE, et al. Anticardiolipin antibodies in Sneddon’s syndrome. Neurology. 1990;40:464-467.
- Wohl rab J, Fischer M, Wolter M, et al. Diagnostic impact and sensitivity of skin biopsies in Sneddon’s syndrome. a report of 15 cases. Br J Dermatol. 2001;145:285-288.
Sneddon syndrome (SS) was first described in 1965 in patients with persistent livedo racemosa and neurological events.1 Because the other manifestations of SS are nonspecific (eg, hypertension, cardiac valvulopathy, arterial and venous occlusion), the diagnosis often is delayed. Many patients who experience prodromal neurologic symptoms such as headaches, depression, anxiety, dizziness, and neuropathy often present to a physician prior to developing ischemic brain manifestations2 but seldom receive the correct diagnosis. Onset of cerebral occlusive events typically occurs in patients younger than 45 years and may present as a transient ischemic attack, stroke, or intracranial hemorrhage.3 The disease is more prevalent in females than males (2:1 ratio). The exact pathogenesis of SS is still unknown, and although it has been thought of as a separate entity from systemic lupus erythematosus and other antiphospholipid disorders, it has been postulated that an immunological dysfunction damages vessel walls leading to thrombosis.
Cutaneous findings associated with SS involve small- to medium-sized dermal-subdermal arteries. Histopathology in some patients demonstrates proliferation of the endothelium and fibrin deposits with subsequent obliteration of involved arteries.4 In many patients including our patient, histopathologic examination of involved skin fails to show specific abnormalities.1 Zelger et al5 reported the sequence of histopathologic skin events in a series of antiphospholipid-negative SS patients. The authors reported that only small arteries at the dermis-subcutis junction were involved and a progression of endothelial dysfunction was observed. The authors believed there were several nonspecific stages prior to fibrin occlusion of involved arteries.5 Stage I involved loosening of endothelial cells with nonspecific perivascular lymphocytic infiltration with perivascular inflammation and lymphocytic infiltration representing the prime mover of the disease.5,6 This stage is thought to be short lived, thus the reason why it has gone undetected for many years in SS patients. Stages II to IV progress through fibrin deposition and occlusion.5 Histological features of stages I to II have not been reported because of late diagnosis of SS. Stage I patients typically present with an average duration of symptoms of 6 months with few neurologic symptoms, the most common being paresthesia of the legs.5
Case Report
A 37-year-old woman with epigastric tenderness on the left side and splenomegaly seen on computed tomography was referred by a hematologist for evaluation of a reticular rash on the left side of the flank of 9 months’ duration with a presumed diagnosis of focal melanoderma. Her medical history was remarkable for a congenital ventricular septal defect and coarctation of the aorta, as well as endometriosis, myalgia, and joint stiffness that had all developed over the last year. Her medical history also was remarkable for nephrolithiasis, irritable bowel syndrome, and chronic sinusitis, as well as psychiatric depression and anxiety disorders. She recently had been diagnosed with moderate hypertension and had experienced difficulty getting pregnant for the last several years with 3 consecutive miscarriages in the first trimester. Neurologic symptoms included neuropathy involving the feet, intermittent paresthesia of the legs, and a history of chronic migraine headaches for several months.
Dermatologic examination revealed a slightly overweight woman with a 25×30-cm dusky, erythematous, irregular, netlike pattern on the left side of the upper and lower trunk (Figure 1). Extensive livedo racemosa was not altered by changes in temperature and had been unchanged for more than 9 months. There were no signs of pruritus or ulcerations, and areas of livedo racemosa were slightly tender to palpation.

We performed 2 sets of three 4-mm biopsies. The first set targeted areas within the violaceous pattern, while the second set targeted areas of normal tissue between the mottled areas. All 6 specimens demonstrated superficial perivascular lymphocytic infiltrate with no evidence of vasculitis or connective tissue disease. The vessels showed no microthrombi or surrounding fibrosis. No eosinophils were identified within the epidermis. There was no evidence of increased dermal mucin. Both the superficial and deep vascular plexuses were unremarkable and showed no evidence of damage to the walls (Figure 2).

To rule out other possible causes of livedo racemosa, complete blood cell count, comprehensive metabolic panel, coagulation profile, lipase test, urinalysis, serologic testing, and immunologic workup were performed. Lipase was within reference range. The complete blood cell count revealed mild anemia, while the rest of the values were within reference range. An immunologic workup included Sjögren syndrome antigen A, Sjögren syndrome antigen B, anticardiolipin antibodies, and antinuclear antibody, which were all negative. Family history was remarkable for first-degree relatives with systemic lupus erythematosus and Crohn disease.
Computed tomography revealed enlargement of the spleen, as well as periaortic, portacaval, and porta hepatis lymphadenopathy. Based on the laboratory findings and clinical presentation as well as the patient’s medical history, the diagnosis of exclusion was idiopathic livedo racemosa with unknown progression to full-blown SS. The patient did not meet the current diagnostic criteria for SS, and her immunologic studies failed to confirm any present antibodies, but involvement of the reticuloendothelial system pointed to production of antibodies that were not yet detectable on laboratory testing.
Comment
More than 50 years after the first case of SS was diagnosed, better laboratory workup is available and more information is known about the pathophysiology. Sneddon syndrome is a rare disorder, affecting only approximately 4 patients per million each year worldwide. Seronegative antiphospholipid antibody syndrome (SNAPS) describes patients with clinical presentations of antiphospholipid syndrome (APS) without detectable serological markers.7 Antiphospholipid-negative SS, which was seen in our patient, would be categorized under SNAPS. A PubMed search of articles indexed for MEDLINE using the terms livedo racemosa, Sneddon syndrome, and SNAPS and splenomegaly revealed there currently are no known cases of SNAPS that have been reported with splenomegaly and lymphadenopathy. Our patient presented with the following clinical features of SS: livedo racemosa, history of miscarriage, psychiatric disturbances, and hypertension. Surprisingly, biopsies from affected skin did not show any fibrin deposition or microthrombi but did reveal perivascular lymphocytic infiltrations. Magnetic resonance imaging did not show any pathological lesions or vascular changes.
Sneddon syndrome and APS share a common pathway to occlusive arteriolopathy for which 4 stages have been described by Zelger et al.5 Stage I involves a nonspecific Langerhans cell infiltrate with polymorphonuclear leukocytes. The tunica media and elastic lamina usually are unaltered at this early stage, while the surrounding connective tissue may appear edematous.5 This early stage of histopathology has not been evaluated in SS patients, primarily because of delay of diagnosis. Late stages III and IV will show fibrin deposition and shrinkage of affected vessels.7
A PubMed search using the terms Sneddon syndrome, lymphadenopathy and livedo racemosa, and Sneddon syndrome and lymphadenopathy revealed that splenomegaly and lymphadenopathy have not been reported in patients with SS. In patients with antiphospholipid-negative SS, one can assume that antibodies to other phospholipids not tested must exist because of striking similarities between APS and antiphospholipid-negative SS.8 Although our patient did not test positive for any of these antibodies, she did present with lymphadenopathy and splenic enlargement, leading us to believe that involvement of the reticuloendothelial system may be a feature of SS that has not been previously reported. Further studies are required to name specific antigens responsible for clinical manifestations in SS.
Currently, no single diagnostic test for SS exists, thus delaying both diagnosis and initiation of treatment. Histopathologic examination may be helpful, but in many cases it is nonspecific, as are serologic markers. Neuroradiological confirmation of involvement usually is the confirmatory feature in many patients with late-stage diagnosis.2 A diagnostic schematic for SS, which was first described by Daoud et al,2 illustrates classification of symptoms and aids in diagnosis. A working diagnosis of idiopathic livedo racemosa is made after ruling out other causes of SS in a patient with nonspecific biopsy findings and negative magnetic resonance imaging results with prodromal symptoms. The prognosis for such patients progressing to full SS is unknown with or without management using anticoagulant therapy.
Conclusion
Early diagnosis of livedo racemosa and SS is essential, as prevention of cerebrovascular accidents, myocardial infarction, and other thromboembolic diseases can be minimized by attacking risk factors such as smoking, taking oral contraceptive pills, becoming pregnant,9 and by initiating either antiplatelet or anticoagulation treatments. These treatments have been shown to delay the development of neurovascular damage and early-onset dementia. We present this case to demonstrate the variability of early-presenting symptoms in idiopathic livedo racemosa. Recognizing some of the early manifestations can lead to early diagnosis and initiation of treatment.
Sneddon syndrome (SS) was first described in 1965 in patients with persistent livedo racemosa and neurological events.1 Because the other manifestations of SS are nonspecific (eg, hypertension, cardiac valvulopathy, arterial and venous occlusion), the diagnosis often is delayed. Many patients who experience prodromal neurologic symptoms such as headaches, depression, anxiety, dizziness, and neuropathy often present to a physician prior to developing ischemic brain manifestations2 but seldom receive the correct diagnosis. Onset of cerebral occlusive events typically occurs in patients younger than 45 years and may present as a transient ischemic attack, stroke, or intracranial hemorrhage.3 The disease is more prevalent in females than males (2:1 ratio). The exact pathogenesis of SS is still unknown, and although it has been thought of as a separate entity from systemic lupus erythematosus and other antiphospholipid disorders, it has been postulated that an immunological dysfunction damages vessel walls leading to thrombosis.
Cutaneous findings associated with SS involve small- to medium-sized dermal-subdermal arteries. Histopathology in some patients demonstrates proliferation of the endothelium and fibrin deposits with subsequent obliteration of involved arteries.4 In many patients including our patient, histopathologic examination of involved skin fails to show specific abnormalities.1 Zelger et al5 reported the sequence of histopathologic skin events in a series of antiphospholipid-negative SS patients. The authors reported that only small arteries at the dermis-subcutis junction were involved and a progression of endothelial dysfunction was observed. The authors believed there were several nonspecific stages prior to fibrin occlusion of involved arteries.5 Stage I involved loosening of endothelial cells with nonspecific perivascular lymphocytic infiltration with perivascular inflammation and lymphocytic infiltration representing the prime mover of the disease.5,6 This stage is thought to be short lived, thus the reason why it has gone undetected for many years in SS patients. Stages II to IV progress through fibrin deposition and occlusion.5 Histological features of stages I to II have not been reported because of late diagnosis of SS. Stage I patients typically present with an average duration of symptoms of 6 months with few neurologic symptoms, the most common being paresthesia of the legs.5
Case Report
A 37-year-old woman with epigastric tenderness on the left side and splenomegaly seen on computed tomography was referred by a hematologist for evaluation of a reticular rash on the left side of the flank of 9 months’ duration with a presumed diagnosis of focal melanoderma. Her medical history was remarkable for a congenital ventricular septal defect and coarctation of the aorta, as well as endometriosis, myalgia, and joint stiffness that had all developed over the last year. Her medical history also was remarkable for nephrolithiasis, irritable bowel syndrome, and chronic sinusitis, as well as psychiatric depression and anxiety disorders. She recently had been diagnosed with moderate hypertension and had experienced difficulty getting pregnant for the last several years with 3 consecutive miscarriages in the first trimester. Neurologic symptoms included neuropathy involving the feet, intermittent paresthesia of the legs, and a history of chronic migraine headaches for several months.
Dermatologic examination revealed a slightly overweight woman with a 25×30-cm dusky, erythematous, irregular, netlike pattern on the left side of the upper and lower trunk (Figure 1). Extensive livedo racemosa was not altered by changes in temperature and had been unchanged for more than 9 months. There were no signs of pruritus or ulcerations, and areas of livedo racemosa were slightly tender to palpation.

We performed 2 sets of three 4-mm biopsies. The first set targeted areas within the violaceous pattern, while the second set targeted areas of normal tissue between the mottled areas. All 6 specimens demonstrated superficial perivascular lymphocytic infiltrate with no evidence of vasculitis or connective tissue disease. The vessels showed no microthrombi or surrounding fibrosis. No eosinophils were identified within the epidermis. There was no evidence of increased dermal mucin. Both the superficial and deep vascular plexuses were unremarkable and showed no evidence of damage to the walls (Figure 2).

To rule out other possible causes of livedo racemosa, complete blood cell count, comprehensive metabolic panel, coagulation profile, lipase test, urinalysis, serologic testing, and immunologic workup were performed. Lipase was within reference range. The complete blood cell count revealed mild anemia, while the rest of the values were within reference range. An immunologic workup included Sjögren syndrome antigen A, Sjögren syndrome antigen B, anticardiolipin antibodies, and antinuclear antibody, which were all negative. Family history was remarkable for first-degree relatives with systemic lupus erythematosus and Crohn disease.
Computed tomography revealed enlargement of the spleen, as well as periaortic, portacaval, and porta hepatis lymphadenopathy. Based on the laboratory findings and clinical presentation as well as the patient’s medical history, the diagnosis of exclusion was idiopathic livedo racemosa with unknown progression to full-blown SS. The patient did not meet the current diagnostic criteria for SS, and her immunologic studies failed to confirm any present antibodies, but involvement of the reticuloendothelial system pointed to production of antibodies that were not yet detectable on laboratory testing.
Comment
More than 50 years after the first case of SS was diagnosed, better laboratory workup is available and more information is known about the pathophysiology. Sneddon syndrome is a rare disorder, affecting only approximately 4 patients per million each year worldwide. Seronegative antiphospholipid antibody syndrome (SNAPS) describes patients with clinical presentations of antiphospholipid syndrome (APS) without detectable serological markers.7 Antiphospholipid-negative SS, which was seen in our patient, would be categorized under SNAPS. A PubMed search of articles indexed for MEDLINE using the terms livedo racemosa, Sneddon syndrome, and SNAPS and splenomegaly revealed there currently are no known cases of SNAPS that have been reported with splenomegaly and lymphadenopathy. Our patient presented with the following clinical features of SS: livedo racemosa, history of miscarriage, psychiatric disturbances, and hypertension. Surprisingly, biopsies from affected skin did not show any fibrin deposition or microthrombi but did reveal perivascular lymphocytic infiltrations. Magnetic resonance imaging did not show any pathological lesions or vascular changes.
Sneddon syndrome and APS share a common pathway to occlusive arteriolopathy for which 4 stages have been described by Zelger et al.5 Stage I involves a nonspecific Langerhans cell infiltrate with polymorphonuclear leukocytes. The tunica media and elastic lamina usually are unaltered at this early stage, while the surrounding connective tissue may appear edematous.5 This early stage of histopathology has not been evaluated in SS patients, primarily because of delay of diagnosis. Late stages III and IV will show fibrin deposition and shrinkage of affected vessels.7
A PubMed search using the terms Sneddon syndrome, lymphadenopathy and livedo racemosa, and Sneddon syndrome and lymphadenopathy revealed that splenomegaly and lymphadenopathy have not been reported in patients with SS. In patients with antiphospholipid-negative SS, one can assume that antibodies to other phospholipids not tested must exist because of striking similarities between APS and antiphospholipid-negative SS.8 Although our patient did not test positive for any of these antibodies, she did present with lymphadenopathy and splenic enlargement, leading us to believe that involvement of the reticuloendothelial system may be a feature of SS that has not been previously reported. Further studies are required to name specific antigens responsible for clinical manifestations in SS.
Currently, no single diagnostic test for SS exists, thus delaying both diagnosis and initiation of treatment. Histopathologic examination may be helpful, but in many cases it is nonspecific, as are serologic markers. Neuroradiological confirmation of involvement usually is the confirmatory feature in many patients with late-stage diagnosis.2 A diagnostic schematic for SS, which was first described by Daoud et al,2 illustrates classification of symptoms and aids in diagnosis. A working diagnosis of idiopathic livedo racemosa is made after ruling out other causes of SS in a patient with nonspecific biopsy findings and negative magnetic resonance imaging results with prodromal symptoms. The prognosis for such patients progressing to full SS is unknown with or without management using anticoagulant therapy.
Conclusion
Early diagnosis of livedo racemosa and SS is essential, as prevention of cerebrovascular accidents, myocardial infarction, and other thromboembolic diseases can be minimized by attacking risk factors such as smoking, taking oral contraceptive pills, becoming pregnant,9 and by initiating either antiplatelet or anticoagulation treatments. These treatments have been shown to delay the development of neurovascular damage and early-onset dementia. We present this case to demonstrate the variability of early-presenting symptoms in idiopathic livedo racemosa. Recognizing some of the early manifestations can lead to early diagnosis and initiation of treatment.
- Sneddon IB. Cerebro-vascular lesions and livedo reticularis. Br J Dermatol. 1965;77:180-185.
- Daoud MS, Wilmoth GJ, Su WP, et al. Sneddon syndrome. Semin Dermatol. 1995;14:166-172.
- Besnier R, Francès C, Ankri A, et al. Factor V Leiden mutation in Sneddon syndrome. Lupus. 2003;12:406-408.
- K aragülle AT, Karadağ D, Erden A, et al. Sneddon’s syndrome: MR imaging findings. Eur Radiol. 2002;12:144-146.
- Zelg er B, Sepp N, Schmid KW, et al. Life-history of cutaneous vascular-lesions in Sneddon’s syndrome. Hum Pathol. 1992;23:668-675.
- Ayoub N, Esposito G, Barete S, et al. Protein Z deficiency in antiphospholipid-negative Sneddon’s syndrome. Stroke. 2004;35:1329-1332.
- Duva l A, Darnige L, Glowacki F, et al. Livedo, dementia, thrombocytopenia, and endotheliitis without antiphospholipid antibodies: seronegative antiphospholipid-like syndrome. J Am Acad Dermatol. 2009;61:1076-1078.
- Kala shnikova LA, Nasonov EL, Kushekbaeva AE, et al. Anticardiolipin antibodies in Sneddon’s syndrome. Neurology. 1990;40:464-467.
- Wohl rab J, Fischer M, Wolter M, et al. Diagnostic impact and sensitivity of skin biopsies in Sneddon’s syndrome. a report of 15 cases. Br J Dermatol. 2001;145:285-288.
- Sneddon IB. Cerebro-vascular lesions and livedo reticularis. Br J Dermatol. 1965;77:180-185.
- Daoud MS, Wilmoth GJ, Su WP, et al. Sneddon syndrome. Semin Dermatol. 1995;14:166-172.
- Besnier R, Francès C, Ankri A, et al. Factor V Leiden mutation in Sneddon syndrome. Lupus. 2003;12:406-408.
- K aragülle AT, Karadağ D, Erden A, et al. Sneddon’s syndrome: MR imaging findings. Eur Radiol. 2002;12:144-146.
- Zelg er B, Sepp N, Schmid KW, et al. Life-history of cutaneous vascular-lesions in Sneddon’s syndrome. Hum Pathol. 1992;23:668-675.
- Ayoub N, Esposito G, Barete S, et al. Protein Z deficiency in antiphospholipid-negative Sneddon’s syndrome. Stroke. 2004;35:1329-1332.
- Duva l A, Darnige L, Glowacki F, et al. Livedo, dementia, thrombocytopenia, and endotheliitis without antiphospholipid antibodies: seronegative antiphospholipid-like syndrome. J Am Acad Dermatol. 2009;61:1076-1078.
- Kala shnikova LA, Nasonov EL, Kushekbaeva AE, et al. Anticardiolipin antibodies in Sneddon’s syndrome. Neurology. 1990;40:464-467.
- Wohl rab J, Fischer M, Wolter M, et al. Diagnostic impact and sensitivity of skin biopsies in Sneddon’s syndrome. a report of 15 cases. Br J Dermatol. 2001;145:285-288.
Practice Points
- The classic physical diagnostic finding of Sneddon syndrome (SS) is livedo racemosa.
- Early identification and treatment of SS can prevent serious morbidity due to stroke, myocardial infarction, and other thrombotic events.
- Preventive care in SS should include antiplatelet therapy or anticoagulants and smoking cessation along with avoidance of birth control pills.
Nominate a Colleague for a NORD 2017 Rare Impact Award
Continuing a 31-year tradition, the National Organization for Rare Disorders (NORD) will honor medical researchers, clinicians, patients, caregivers, and others who have had a positive impact on the rare disease community at its annual Rare Impact Awards celebration in May 2017. Nominations are now open and may be submitted online. Friday, Jan. 13, is the deadline for submitting nominations.
Nominees can include individuals or organizations. Past honorees have been recognized for their work in advocacy, research, patient care, awareness, ethics, and public policy. The 2017 honorees will be determined by a nominations committee comprised of NORD’s Scientific and Medical Advisory Committee, Board of Directors, and advocates.
The awards ceremony will take place on May 18 in the amphitheater of the Ronald Reagan Building and International Trade Center in Washington, DC. The 2017 Rare Impact Awards will continue NORD’s longstanding tradition of bringing together medical professionals, patients, caregivers, federal officials, and others to celebrate those who have had a positive impact on the community.
Continuing a 31-year tradition, the National Organization for Rare Disorders (NORD) will honor medical researchers, clinicians, patients, caregivers, and others who have had a positive impact on the rare disease community at its annual Rare Impact Awards celebration in May 2017. Nominations are now open and may be submitted online. Friday, Jan. 13, is the deadline for submitting nominations.
Nominees can include individuals or organizations. Past honorees have been recognized for their work in advocacy, research, patient care, awareness, ethics, and public policy. The 2017 honorees will be determined by a nominations committee comprised of NORD’s Scientific and Medical Advisory Committee, Board of Directors, and advocates.
The awards ceremony will take place on May 18 in the amphitheater of the Ronald Reagan Building and International Trade Center in Washington, DC. The 2017 Rare Impact Awards will continue NORD’s longstanding tradition of bringing together medical professionals, patients, caregivers, federal officials, and others to celebrate those who have had a positive impact on the community.
Continuing a 31-year tradition, the National Organization for Rare Disorders (NORD) will honor medical researchers, clinicians, patients, caregivers, and others who have had a positive impact on the rare disease community at its annual Rare Impact Awards celebration in May 2017. Nominations are now open and may be submitted online. Friday, Jan. 13, is the deadline for submitting nominations.
Nominees can include individuals or organizations. Past honorees have been recognized for their work in advocacy, research, patient care, awareness, ethics, and public policy. The 2017 honorees will be determined by a nominations committee comprised of NORD’s Scientific and Medical Advisory Committee, Board of Directors, and advocates.
The awards ceremony will take place on May 18 in the amphitheater of the Ronald Reagan Building and International Trade Center in Washington, DC. The 2017 Rare Impact Awards will continue NORD’s longstanding tradition of bringing together medical professionals, patients, caregivers, federal officials, and others to celebrate those who have had a positive impact on the community.
Verrucous Plaque on the Leg
Blastomycosis
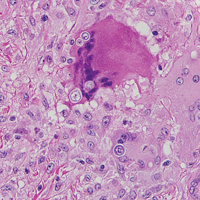
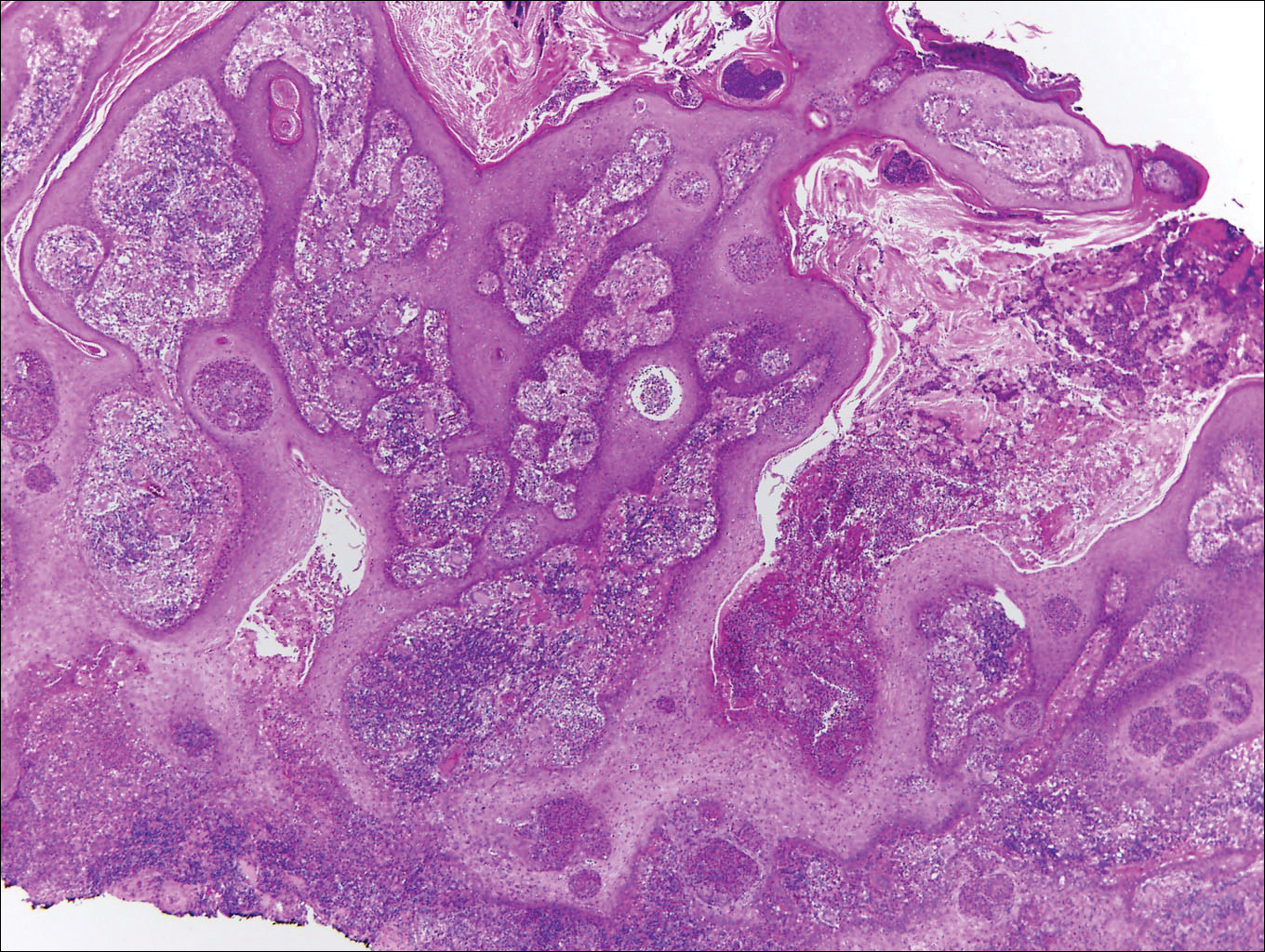
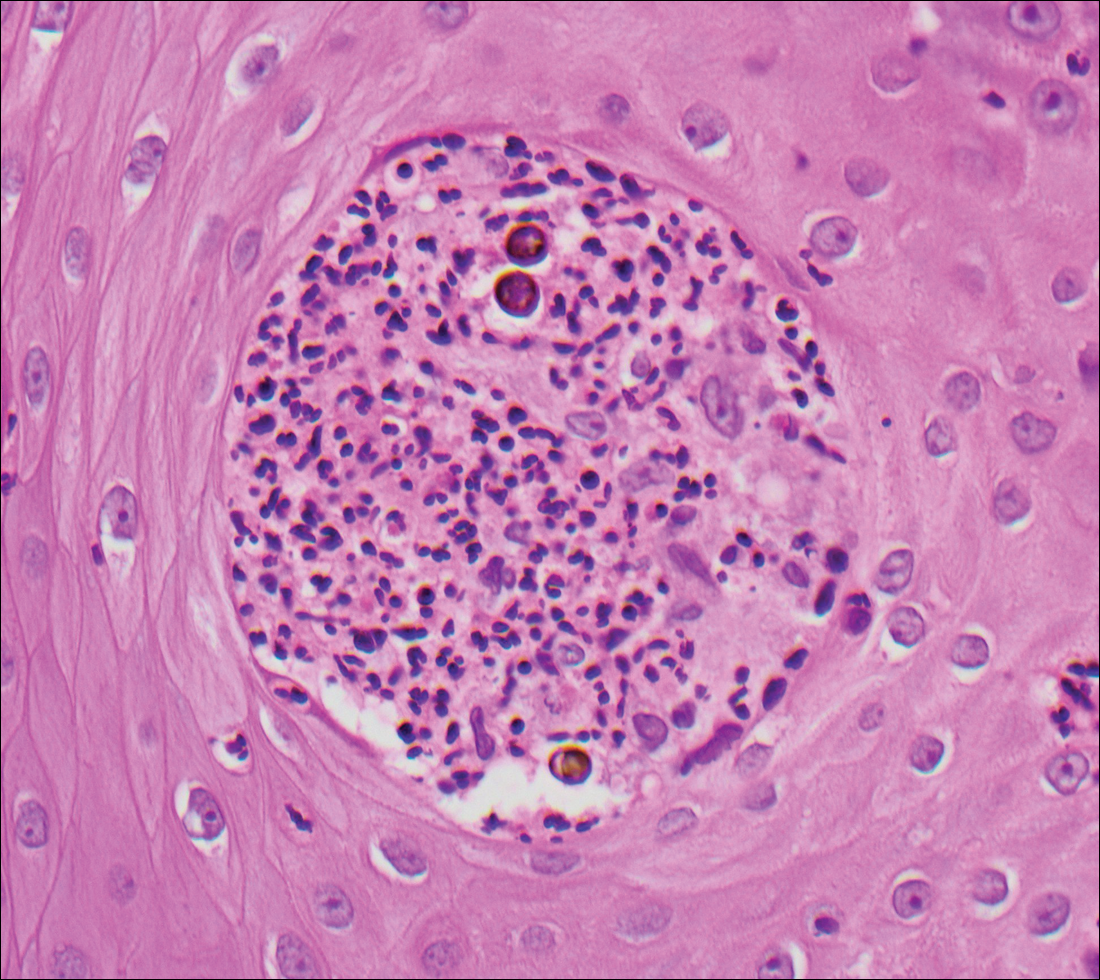
Blastomycosis is caused by Blastomyces dermatitidis, which is endemic in the Midwestern and southeastern United States where it occurs environmentally in wood and soil. Unlike many fungal infections, blastomycosis most often develops in immunocompetent hosts. Infection is usually acquired via inhalation,1 and cutaneous disease typically is secondary to pulmonary infection. Although not common, traumatic inoculation also can cause cutaneous blastomycosis. Skin lesions include crusted verrucous nodules and plaques with elevated borders.1,2 Histologic features include pseudoepitheliomatous hyperplasia with intraepidermal neutrophilic microabscesses (Figure 1), and a neutrophilic and granulomatous dermal infiltrate. Organisms often are found within histiocytes (quiz image) or small abscesses. The yeasts usually are 8 to 15 µm in diameter with a thick cell wall and occasionally display broad-based budding.
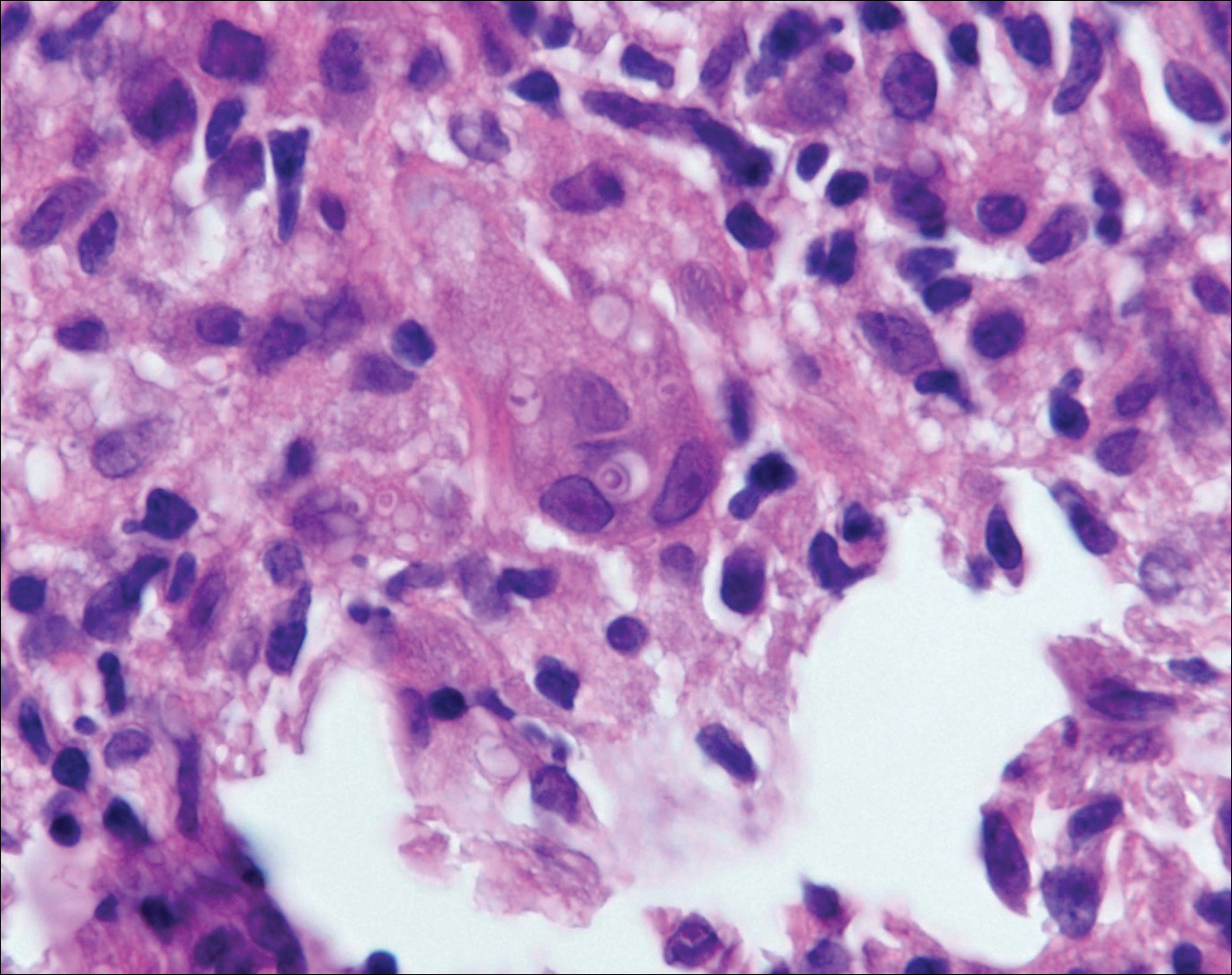
Chromoblastomycosis is caused by dematiaceous (pigmented) fungi, including Fonsecaea, Phialophora, Cladophialophora, and Rhinocladiella species,3 which are present in soil and vegetable debris in tropical and subtropical regions. Infection typically occurs in the foot or lower leg from traumatic inoculation, such as a thorn or splinter injury.2 Histologically, chromoblastomycosis is characterized by pseudoepitheliomatous hyperplasia; suppurative and granulomatous dermatitis; and sclerotic (Medlar) bodies, which are 5 to 12 µm in diameter, round, brown, sometimes septate cells resembling copper pennies (Figure 2).2
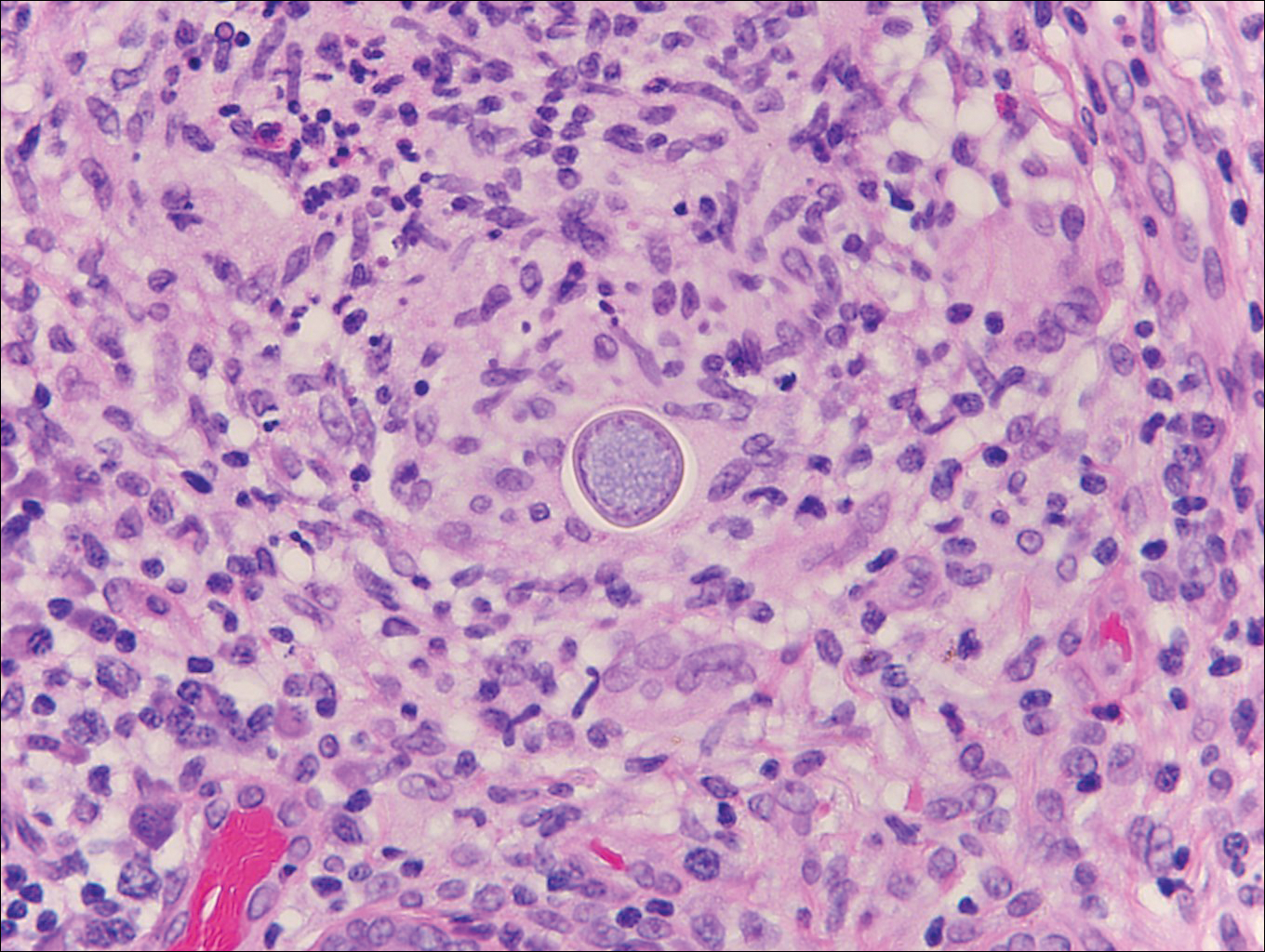
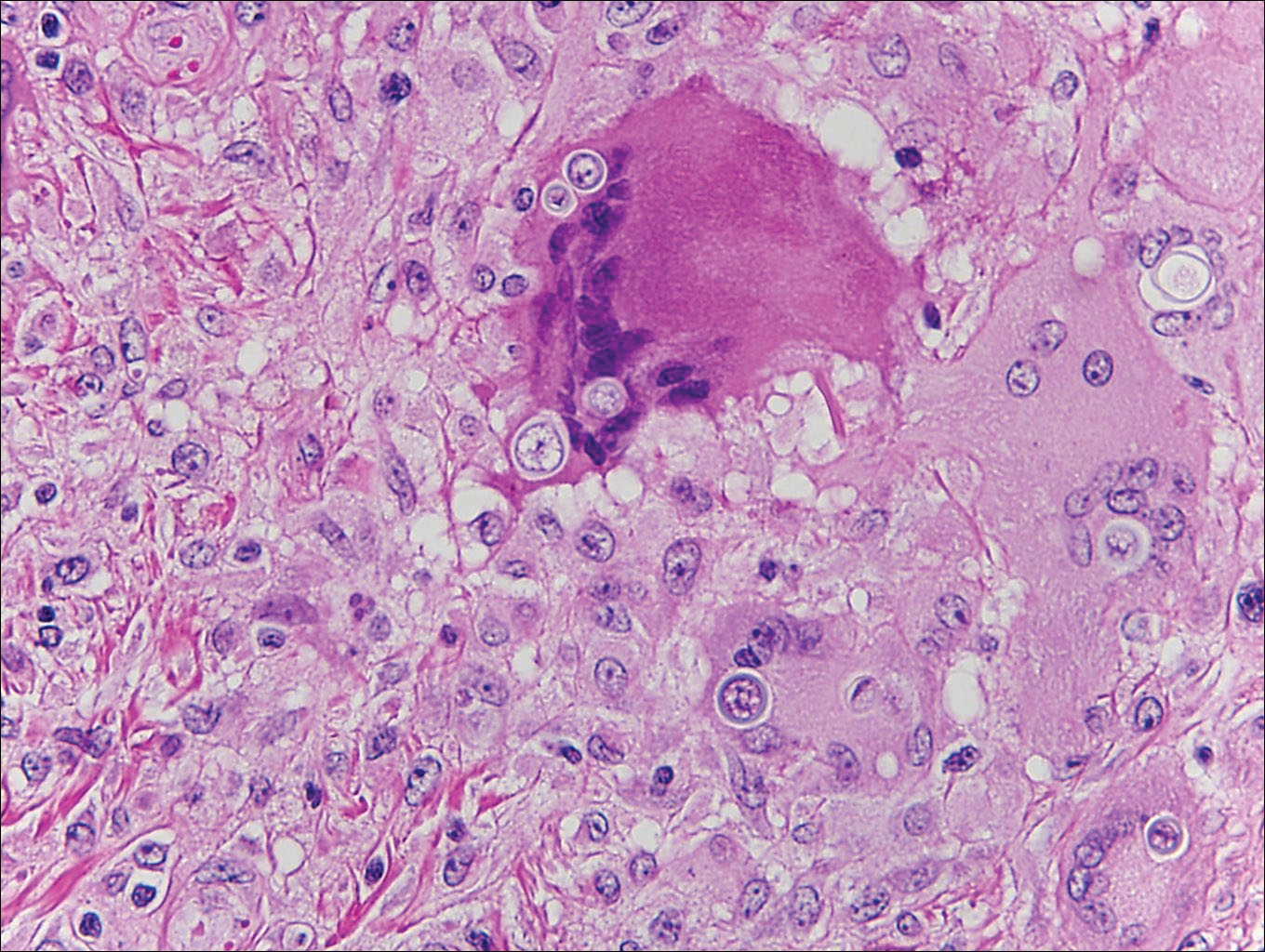
Coccidioidomycosis is caused by Coccidioides immitis, which is found in soil in the southwestern United States. Infection most often occurs via inhalation of airborne arthrospores.2 Cutaneous lesions occasionally are observed following dissemination or rarely following primary inoculation injury. They may present as papules, nodules, pustules, plaques, and ulcers, with the face being the most commonly affected site.1 Histologically, coccidioidomycosis is characterized by pseudoepitheliomatous hyperplasia, suppurative and granulomatous dermatitis, and large spherules (up to 100 µm in diameter) containing numerous small endospores (Figure 3).
Cryptococcosis is caused by Cryptococcus neoformans, a fungus found in soil, fruit, and pigeon droppings throughout the world.2,3 The most common route of infection is via the respiratory tract. Systemic spread and central nervous system involvement may occur in immunocompromised hosts.2 Skin involvement is uncommon and may present on the head and neck with umbilicated papules, pustules, nodules, plaques, or ulcers. Histologically, Cryptococcus is a spherical yeast, often 4 to 20 µm in diameter. Replication is by narrow-based budding. A characteristic feature is a mucoid capsule, which retracts during processing, leaving a clear space around the yeast (Figure 4). When present, the mucoid capsule can be highlighted on mucicarmine or Alcian blue staining. Histologic variants of cryptococcosis include granulomatous (high host immune response), gelatinous (low host immune response), and suppurative types.3
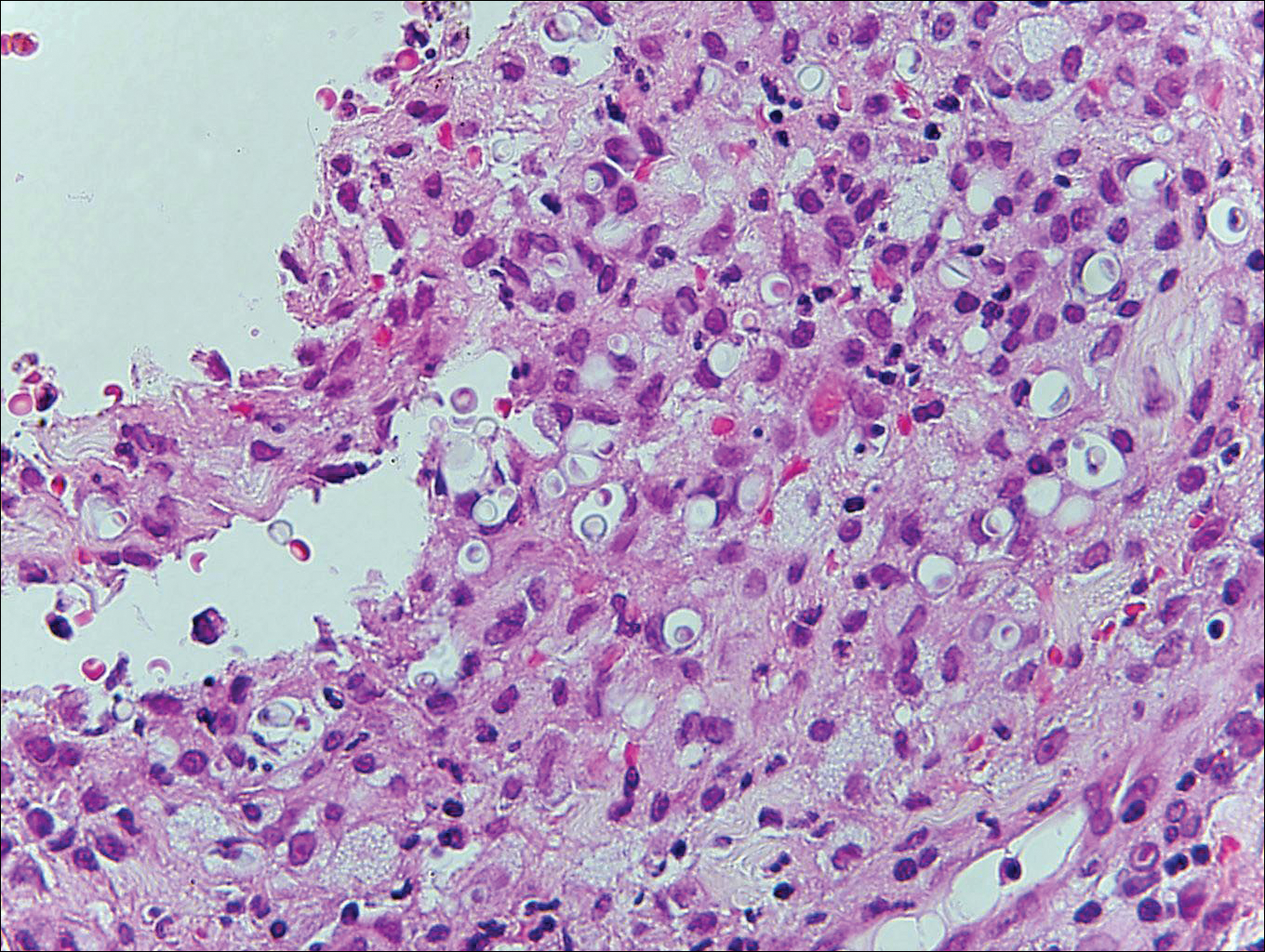
Histoplasmosis is caused by Histoplasma capsulatum, which occurs in soil and bird and bat droppings, with exposure primarily via inhalation. Cutaneous histoplasmosis is almost always a feature of disseminated disease, which occurs most commonly in immunosuppressed individuals.1 Skin lesions may present as macules, papules, indurated plaques, ulcers, purpura, panniculitis, and subcutaneous nodules.2 Histologically, there is a granulomatous and neutrophilic infiltrate within the dermis and subcutis. Yeasts are small (2-4 µm in diameter) and are observed within the cytoplasm of macrophages (Figure 5) where they appear as basophilic dots, sometimes surrounded by an artifactual clear space (pseudocapsule).2
- Bolognia JL, Jorizzo JL, Shaffer JV. Dermatology. 3rd ed. Vol 2. Philadelphia, PA: Elsevier/Saunders; 2012.
- Calonje JE, Brenn T, Lazar AJ, et al. McKee's Pathology of the Skin. 4th ed. St. Louis, MO: Elsevier/Saunders; 2012.
- Schwarzenberger K, Werchniak A, Ko C. Requisites in Dermatology: General Dermatology. Philadelphia, PA: Elsevier/Saunders; 2009.
Blastomycosis
Blastomycosis is caused by Blastomyces dermatitidis, which is endemic in the Midwestern and southeastern United States where it occurs environmentally in wood and soil. Unlike many fungal infections, blastomycosis most often develops in immunocompetent hosts. Infection is usually acquired via inhalation,1 and cutaneous disease typically is secondary to pulmonary infection. Although not common, traumatic inoculation also can cause cutaneous blastomycosis. Skin lesions include crusted verrucous nodules and plaques with elevated borders.1,2 Histologic features include pseudoepitheliomatous hyperplasia with intraepidermal neutrophilic microabscesses (Figure 1), and a neutrophilic and granulomatous dermal infiltrate. Organisms often are found within histiocytes (quiz image) or small abscesses. The yeasts usually are 8 to 15 µm in diameter with a thick cell wall and occasionally display broad-based budding.

Chromoblastomycosis is caused by dematiaceous (pigmented) fungi, including Fonsecaea, Phialophora, Cladophialophora, and Rhinocladiella species,3 which are present in soil and vegetable debris in tropical and subtropical regions. Infection typically occurs in the foot or lower leg from traumatic inoculation, such as a thorn or splinter injury.2 Histologically, chromoblastomycosis is characterized by pseudoepitheliomatous hyperplasia; suppurative and granulomatous dermatitis; and sclerotic (Medlar) bodies, which are 5 to 12 µm in diameter, round, brown, sometimes septate cells resembling copper pennies (Figure 2).2

Coccidioidomycosis is caused by Coccidioides immitis, which is found in soil in the southwestern United States. Infection most often occurs via inhalation of airborne arthrospores.2 Cutaneous lesions occasionally are observed following dissemination or rarely following primary inoculation injury. They may present as papules, nodules, pustules, plaques, and ulcers, with the face being the most commonly affected site.1 Histologically, coccidioidomycosis is characterized by pseudoepitheliomatous hyperplasia, suppurative and granulomatous dermatitis, and large spherules (up to 100 µm in diameter) containing numerous small endospores (Figure 3).

Cryptococcosis is caused by Cryptococcus neoformans, a fungus found in soil, fruit, and pigeon droppings throughout the world.2,3 The most common route of infection is via the respiratory tract. Systemic spread and central nervous system involvement may occur in immunocompromised hosts.2 Skin involvement is uncommon and may present on the head and neck with umbilicated papules, pustules, nodules, plaques, or ulcers. Histologically, Cryptococcus is a spherical yeast, often 4 to 20 µm in diameter. Replication is by narrow-based budding. A characteristic feature is a mucoid capsule, which retracts during processing, leaving a clear space around the yeast (Figure 4). When present, the mucoid capsule can be highlighted on mucicarmine or Alcian blue staining. Histologic variants of cryptococcosis include granulomatous (high host immune response), gelatinous (low host immune response), and suppurative types.3

Histoplasmosis is caused by Histoplasma capsulatum, which occurs in soil and bird and bat droppings, with exposure primarily via inhalation. Cutaneous histoplasmosis is almost always a feature of disseminated disease, which occurs most commonly in immunosuppressed individuals.1 Skin lesions may present as macules, papules, indurated plaques, ulcers, purpura, panniculitis, and subcutaneous nodules.2 Histologically, there is a granulomatous and neutrophilic infiltrate within the dermis and subcutis. Yeasts are small (2-4 µm in diameter) and are observed within the cytoplasm of macrophages (Figure 5) where they appear as basophilic dots, sometimes surrounded by an artifactual clear space (pseudocapsule).2

Blastomycosis
Blastomycosis is caused by Blastomyces dermatitidis, which is endemic in the Midwestern and southeastern United States where it occurs environmentally in wood and soil. Unlike many fungal infections, blastomycosis most often develops in immunocompetent hosts. Infection is usually acquired via inhalation,1 and cutaneous disease typically is secondary to pulmonary infection. Although not common, traumatic inoculation also can cause cutaneous blastomycosis. Skin lesions include crusted verrucous nodules and plaques with elevated borders.1,2 Histologic features include pseudoepitheliomatous hyperplasia with intraepidermal neutrophilic microabscesses (Figure 1), and a neutrophilic and granulomatous dermal infiltrate. Organisms often are found within histiocytes (quiz image) or small abscesses. The yeasts usually are 8 to 15 µm in diameter with a thick cell wall and occasionally display broad-based budding.

Chromoblastomycosis is caused by dematiaceous (pigmented) fungi, including Fonsecaea, Phialophora, Cladophialophora, and Rhinocladiella species,3 which are present in soil and vegetable debris in tropical and subtropical regions. Infection typically occurs in the foot or lower leg from traumatic inoculation, such as a thorn or splinter injury.2 Histologically, chromoblastomycosis is characterized by pseudoepitheliomatous hyperplasia; suppurative and granulomatous dermatitis; and sclerotic (Medlar) bodies, which are 5 to 12 µm in diameter, round, brown, sometimes septate cells resembling copper pennies (Figure 2).2

Coccidioidomycosis is caused by Coccidioides immitis, which is found in soil in the southwestern United States. Infection most often occurs via inhalation of airborne arthrospores.2 Cutaneous lesions occasionally are observed following dissemination or rarely following primary inoculation injury. They may present as papules, nodules, pustules, plaques, and ulcers, with the face being the most commonly affected site.1 Histologically, coccidioidomycosis is characterized by pseudoepitheliomatous hyperplasia, suppurative and granulomatous dermatitis, and large spherules (up to 100 µm in diameter) containing numerous small endospores (Figure 3).

Cryptococcosis is caused by Cryptococcus neoformans, a fungus found in soil, fruit, and pigeon droppings throughout the world.2,3 The most common route of infection is via the respiratory tract. Systemic spread and central nervous system involvement may occur in immunocompromised hosts.2 Skin involvement is uncommon and may present on the head and neck with umbilicated papules, pustules, nodules, plaques, or ulcers. Histologically, Cryptococcus is a spherical yeast, often 4 to 20 µm in diameter. Replication is by narrow-based budding. A characteristic feature is a mucoid capsule, which retracts during processing, leaving a clear space around the yeast (Figure 4). When present, the mucoid capsule can be highlighted on mucicarmine or Alcian blue staining. Histologic variants of cryptococcosis include granulomatous (high host immune response), gelatinous (low host immune response), and suppurative types.3

Histoplasmosis is caused by Histoplasma capsulatum, which occurs in soil and bird and bat droppings, with exposure primarily via inhalation. Cutaneous histoplasmosis is almost always a feature of disseminated disease, which occurs most commonly in immunosuppressed individuals.1 Skin lesions may present as macules, papules, indurated plaques, ulcers, purpura, panniculitis, and subcutaneous nodules.2 Histologically, there is a granulomatous and neutrophilic infiltrate within the dermis and subcutis. Yeasts are small (2-4 µm in diameter) and are observed within the cytoplasm of macrophages (Figure 5) where they appear as basophilic dots, sometimes surrounded by an artifactual clear space (pseudocapsule).2

- Bolognia JL, Jorizzo JL, Shaffer JV. Dermatology. 3rd ed. Vol 2. Philadelphia, PA: Elsevier/Saunders; 2012.
- Calonje JE, Brenn T, Lazar AJ, et al. McKee's Pathology of the Skin. 4th ed. St. Louis, MO: Elsevier/Saunders; 2012.
- Schwarzenberger K, Werchniak A, Ko C. Requisites in Dermatology: General Dermatology. Philadelphia, PA: Elsevier/Saunders; 2009.
- Bolognia JL, Jorizzo JL, Shaffer JV. Dermatology. 3rd ed. Vol 2. Philadelphia, PA: Elsevier/Saunders; 2012.
- Calonje JE, Brenn T, Lazar AJ, et al. McKee's Pathology of the Skin. 4th ed. St. Louis, MO: Elsevier/Saunders; 2012.
- Schwarzenberger K, Werchniak A, Ko C. Requisites in Dermatology: General Dermatology. Philadelphia, PA: Elsevier/Saunders; 2009.
Aquatic Antagonists: Cutaneous Sea Urchin Spine Injury
Sea urchin injuries are commonly seen in coastal regions near both warm and cold salt water with frequent recreational water activities or fishing. Sea urchins belong to the class Echinoidea with approximately 600 species, of which roughly 80 are poisonous to humans.1,2 When a human comes in contact with a sea urchin, the spines of the sea urchin (made of calcium carbonate) can penetrate the skin and break off from the sea urchin, becoming embedded in the skin. Injuries from sea urchin spines are most commonly seen on the hands and feet, as the likelihood of contact with a sea urchin is greater on these sites. The severity of sea urchin spine injuries can vary widely, from minimal local trauma and pain to arthritis, synovitis, and occasionally systemic illness.1,3 It is important to recognize the wide variety of responses to sea urchin spine injuries and the impact of prompt treatment. Many published reports on injuries from sea urchin spines describe arthritis and synovitis from spines in the joints.1,2,4-6 Fewer reports discuss nonjoint injuries and the dermatologic aspects of sea urchin spine injuries.3,7,8 We pre-sent a case of a patient with a puncture injury from sea urchin spines that resulted in painful granulomas.
Case Report
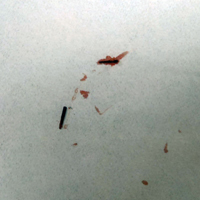
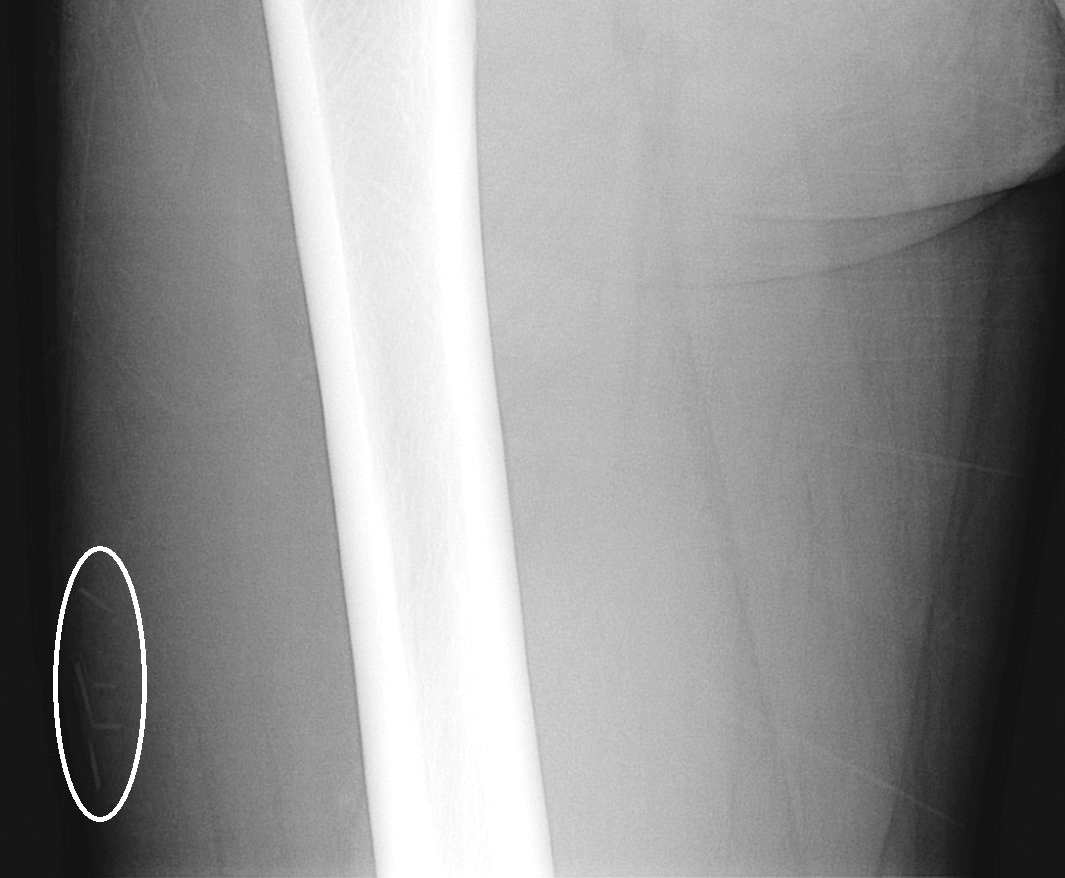
A 29-year-old otherwise healthy man was referred to our dermatology clinic by the university student health center due to continued pain in the right thigh. Five weeks prior to presentation to the student health center, the patient had fallen on a sea urchin while snorkeling in Hawaii. Sea urchin spines became lodged in the right thigh, some of which were removed in a local medical clinic in Hawaii. He was given oral antibiotics prior to his return home. A plain film radiograph of the affected area ordered by the student health center showed several punctate and linear densities in the lateral aspect of the right mid thigh (Figure 1). These findings were consistent with sea urchin spines within the superficial soft tissues of the lateral thigh.
At the time of presentation to our dermatology clinic, the patient reported sharp intermittent pain localized to the right thigh. The patient denied any fever, chills, or pain in the joints. On physical examination, there were several firm nodules on the right thigh, ranging from 4 to 20 mm in diameter (Figure 2). The nodules were tender to palpation with some surrounding edema. Drainage was not noted. Several scars were visible at sites of the original puncture injuries and removal of the spines.
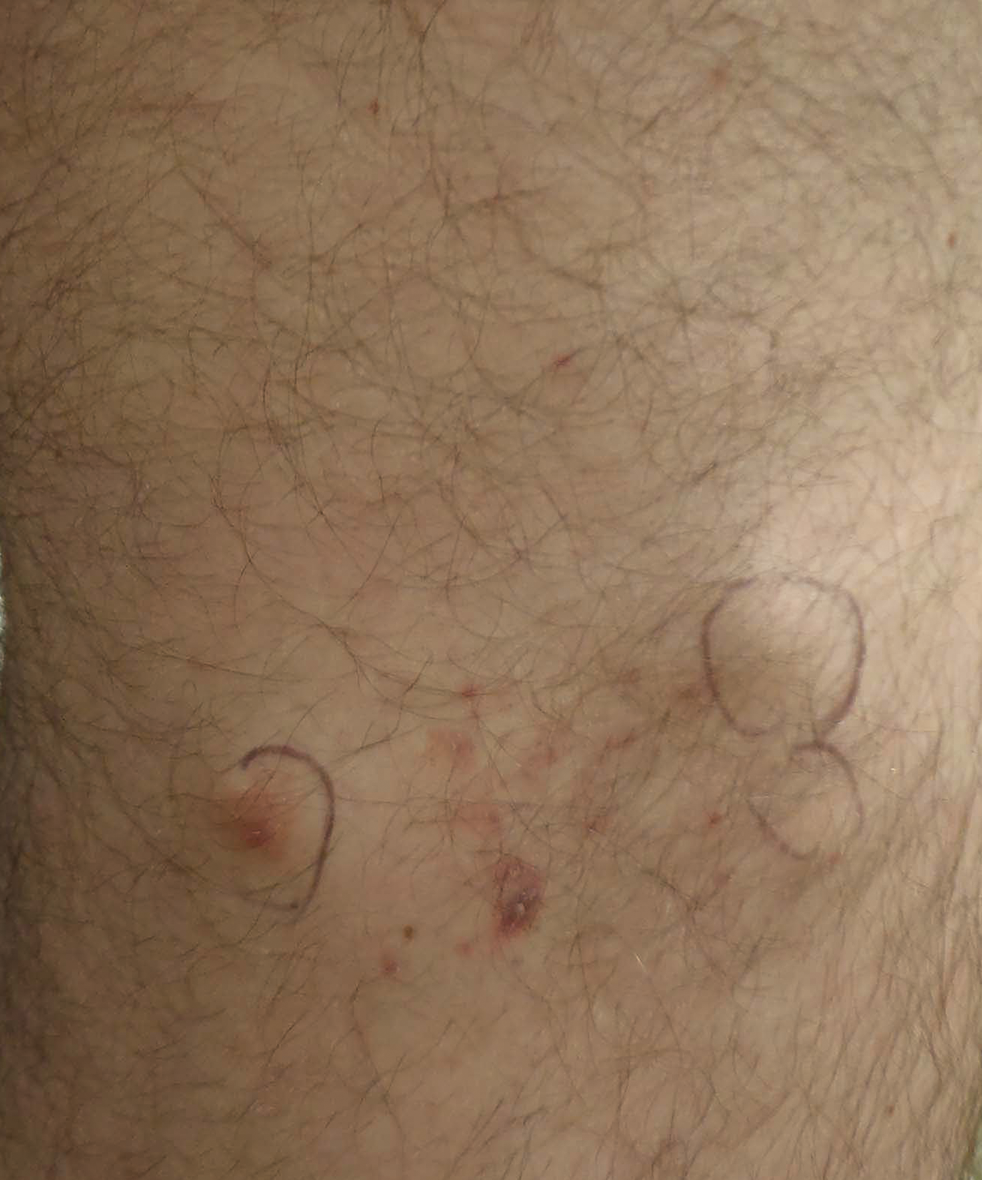
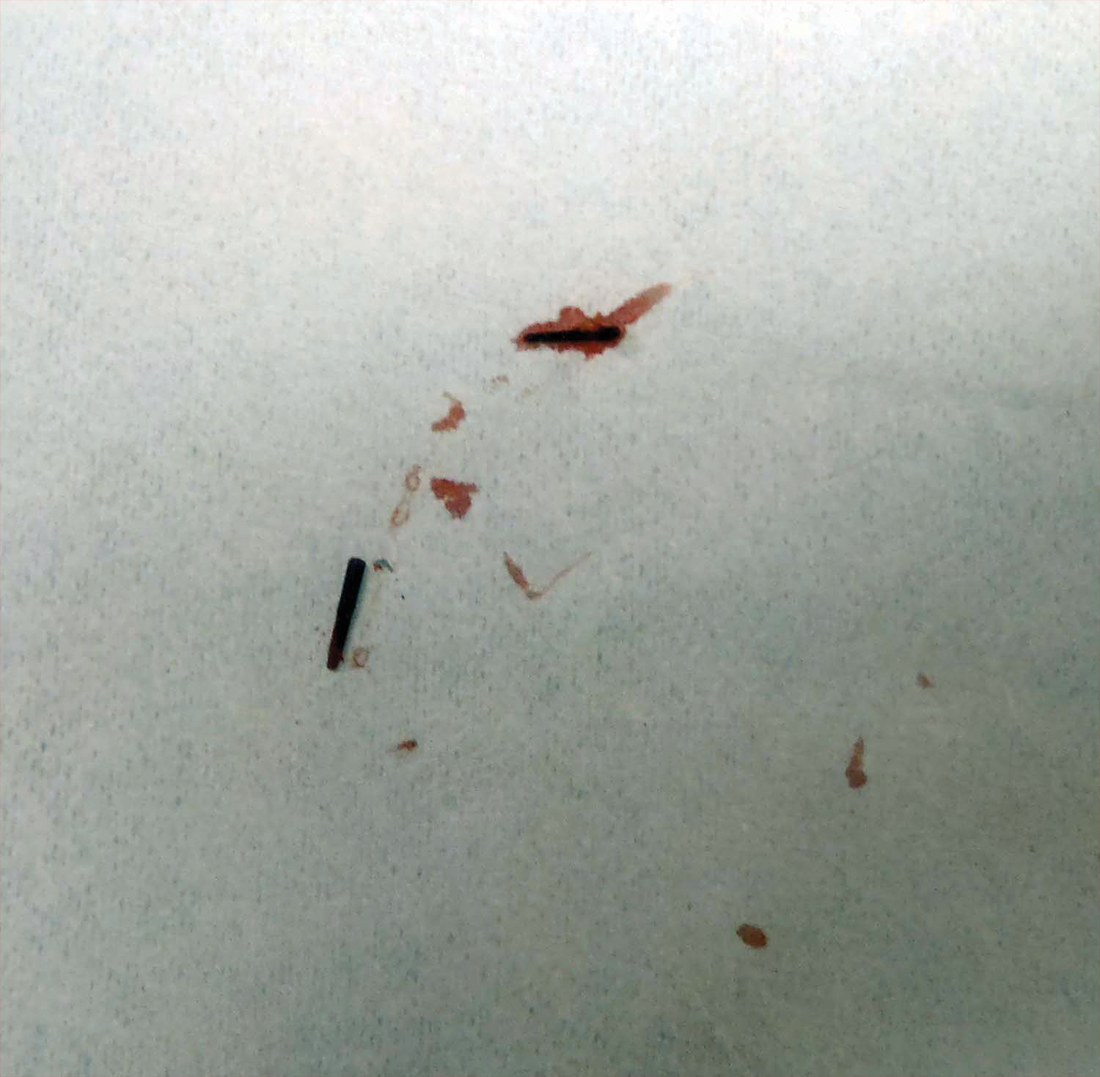
Two 6-mm punch biopsies were performed on representative nodules on the right thigh for histopathologic examination. Along with the biopsy tissue, firm, brown-black, linear foreign bodies consistent with sea urchin spines were extracted with forceps (Figure 3). Histopathologic examination revealed a dense, diffuse, mixed inflammatory cell infiltrate in the dermis predominantly composed of lymphocytes, histiocytes, and numerous eosinophils. Proliferation of small vessels was noted. In one of the biopsies, small fragments of necrotic tissue were present. These findings were consistent with granulomatous inflammation and granulation tissue due to a foreign body.
At the time of suture removal 2 weeks later, the biopsied areas were well healed with minimal erythema. The patient reported decreased pain in the involved areas. He was not seen in clinic again due to resolution of the nodules and associated pain.
Comment
Sea urchin spine injuries are commonly seen in coastal regions with frequent participation in recreational and occupational water activities. A wide variety of responses can be seen in sea urchin spine injuries. There generally are 2 types of cutaneous reaction patterns to sea urchin spines: a primary initial reaction and a secondary delayed/granulomatous reaction. When the spines initially penetrate the skin, the primary initial reaction consists of sharp localized pain that worsens with applied pressure. In addition to pain, bleeding, erythema, edema, and myalgia can occur.3 These symptoms typically subside a few hours after complete removal of the spines from the skin.6 If some spines remain in the skin, a secondary delayed/granulomatous reaction can occur, which can lead to the formation of granulomas that can manifest as nodules or papules and can be diffuse.
Many patients may think their painful encounter with a sea urchin was just an unfortunate event, but depending on the location of the injury, more serious extracutaneous reactions and chronic symptoms may occur. Some cases have described the development of arthritis and synovitis from the implantation of spines into joints.1,2,4-6 Other extracutaneous complications include neuropathy and paresthesia, local bone destruction, radiating pain, muscular weakness, and hypotension.3
The severity of the injury also can depend on the sea urchin species and the number of spines implanted. There are approximately 80 poisonous sea urchin species possessing toxins in venomous spines, resulting in edema and change in the leukocyte-endothelial interaction.9 Substances identified in the spines include proteins, steroids, serotonin, histamine, and glycosides.3,9 The number of spines implanted, particularly the number of venomous spines, can lead to more severe complications. Penetration of 15 or more venomous spines can commonly lead to extracutaneous symptoms.3 Another concern, irrespective of species type, is the potential for secondary infection associated with the spine penetration or implantation into the skin. Mycobacterium marinum infections have been reported in some sea urchin granulomas,10 as well as fungal infection, bacterial infection, and tetanus.3
The diagnosis of sea urchin spine injuries starts with a thorough history and physical examination. A positive history of sea urchin contact suggests the diagnosis, and radiographs can be useful to find the location of the spine(s), especially if there are no visible nodules on the skin. However, small fragments of spine may not be completely observed on plain radiographs. Any signs or symptoms of infection should prompt a culture for confirmation and guidance for management. Cutaneous biopsies can be helpful for both diagnosis confirmation and symptomatic relief. Reported cases have described granulomatous reactions in the vast majority of the histologic specimens, with necrosis an additional common finding.7,8 Sea urchin granulomas can be of varying types, the majority being foreign-body and sarcoid types.3,6,7
Treatment of sea urchin spine injuries primarily involves removal of the spines by a physician. Patients may soak the affected areas in warm water prior to the removal of the spines to aid in pain relief. Surgical removal with local anesthesia and cutaneous extraction is a common treatment method, and more extensive surgical removal of the spines is another option, especially in areas around the joints.2 The use of liquid nitrogen or skin punch biopsy also have been described as possible methods to remove the spines.11,12
Conclusion
Sea urchin spine injuries can result in a wide range of cutaneous and systemic complications. Prompt diagnosis and treatment to remove the sea urchin spines can lessen the associated pain and is important in the prevention of more serious complications.
- Liram N, Gomori M, Perouansky M. Sea urchin puncture resulting in PIP joint synovial arthritis: case report and MRI study. J Travel Med. 2000;7:43-45.
- Dahl WJ, Jebson P, Louis DS. Sea urchin injuries to the hand: a case report and review of the literature. Iowa Orthop J. 2010;30:153-156.
- Rossetto AL, de Macedo Mora J, Haddad Junior V. Sea urchin granuloma. Rev Inst Med Trop Sao Paulo. 2006;48:303-306.
- Ahmad R, McCann PA, Barakat M, et al. Sea urchin spine injuries of the hand. J Hand Surg Eur Vol. 2008;33:670-671.
- Schefflein J, Umans H, Ellenbogen D, et al. Sea urchin spine arthritis in the foot. Skeletal Radiol. 2012;41:1327-1331.
- Wada T, Soma T, Gaman K, et al. Sea urchin spine arthritis of the hand. J Hand Surg. 2008;33:398-401.
- Suárez-Peñaranda JM, Vieites B, Del Río E, et al. Histopathologic and immunohistochemical features of sea urchin granulomas. J Cutan Pathol. 2013;40:550-556.
- De La Torre C, Toribio J. Sea-urchin granuloma: histologic profile. a pathologic study of 50 biopsies. J Cutan Pathol. 2001;28:223-228.
- Sciani JM, Zychar BC, Gonçalves LR, et al. Pro-inflammatory effects of the aqueous extract of Echinometra lucunter sea urchin spines. Exp Biol Med (Maywood). 2011;236:277-280.
- De la Torre C, Vega A, Carracedo A, et al. Identification of Mycobacterium marinum in sea-urchin granulomas. Br J Dermatol. 2001;145:114-116.
- Gargus MD, Morohashi DK. A sea-urchin spine chilling remedy. N Engl J Med. 2012;367:1867-1868.
- Sjøberg T, de Weerd L. The usefulness of a skin biopsy punch to remove sea urchin spines. ANZ J Surg. 2010;80:383.
Sea urchin injuries are commonly seen in coastal regions near both warm and cold salt water with frequent recreational water activities or fishing. Sea urchins belong to the class Echinoidea with approximately 600 species, of which roughly 80 are poisonous to humans.1,2 When a human comes in contact with a sea urchin, the spines of the sea urchin (made of calcium carbonate) can penetrate the skin and break off from the sea urchin, becoming embedded in the skin. Injuries from sea urchin spines are most commonly seen on the hands and feet, as the likelihood of contact with a sea urchin is greater on these sites. The severity of sea urchin spine injuries can vary widely, from minimal local trauma and pain to arthritis, synovitis, and occasionally systemic illness.1,3 It is important to recognize the wide variety of responses to sea urchin spine injuries and the impact of prompt treatment. Many published reports on injuries from sea urchin spines describe arthritis and synovitis from spines in the joints.1,2,4-6 Fewer reports discuss nonjoint injuries and the dermatologic aspects of sea urchin spine injuries.3,7,8 We pre-sent a case of a patient with a puncture injury from sea urchin spines that resulted in painful granulomas.
Case Report
A 29-year-old otherwise healthy man was referred to our dermatology clinic by the university student health center due to continued pain in the right thigh. Five weeks prior to presentation to the student health center, the patient had fallen on a sea urchin while snorkeling in Hawaii. Sea urchin spines became lodged in the right thigh, some of which were removed in a local medical clinic in Hawaii. He was given oral antibiotics prior to his return home. A plain film radiograph of the affected area ordered by the student health center showed several punctate and linear densities in the lateral aspect of the right mid thigh (Figure 1). These findings were consistent with sea urchin spines within the superficial soft tissues of the lateral thigh.

At the time of presentation to our dermatology clinic, the patient reported sharp intermittent pain localized to the right thigh. The patient denied any fever, chills, or pain in the joints. On physical examination, there were several firm nodules on the right thigh, ranging from 4 to 20 mm in diameter (Figure 2). The nodules were tender to palpation with some surrounding edema. Drainage was not noted. Several scars were visible at sites of the original puncture injuries and removal of the spines.

Two 6-mm punch biopsies were performed on representative nodules on the right thigh for histopathologic examination. Along with the biopsy tissue, firm, brown-black, linear foreign bodies consistent with sea urchin spines were extracted with forceps (Figure 3). Histopathologic examination revealed a dense, diffuse, mixed inflammatory cell infiltrate in the dermis predominantly composed of lymphocytes, histiocytes, and numerous eosinophils. Proliferation of small vessels was noted. In one of the biopsies, small fragments of necrotic tissue were present. These findings were consistent with granulomatous inflammation and granulation tissue due to a foreign body.

At the time of suture removal 2 weeks later, the biopsied areas were well healed with minimal erythema. The patient reported decreased pain in the involved areas. He was not seen in clinic again due to resolution of the nodules and associated pain.
Comment
Sea urchin spine injuries are commonly seen in coastal regions with frequent participation in recreational and occupational water activities. A wide variety of responses can be seen in sea urchin spine injuries. There generally are 2 types of cutaneous reaction patterns to sea urchin spines: a primary initial reaction and a secondary delayed/granulomatous reaction. When the spines initially penetrate the skin, the primary initial reaction consists of sharp localized pain that worsens with applied pressure. In addition to pain, bleeding, erythema, edema, and myalgia can occur.3 These symptoms typically subside a few hours after complete removal of the spines from the skin.6 If some spines remain in the skin, a secondary delayed/granulomatous reaction can occur, which can lead to the formation of granulomas that can manifest as nodules or papules and can be diffuse.
Many patients may think their painful encounter with a sea urchin was just an unfortunate event, but depending on the location of the injury, more serious extracutaneous reactions and chronic symptoms may occur. Some cases have described the development of arthritis and synovitis from the implantation of spines into joints.1,2,4-6 Other extracutaneous complications include neuropathy and paresthesia, local bone destruction, radiating pain, muscular weakness, and hypotension.3
The severity of the injury also can depend on the sea urchin species and the number of spines implanted. There are approximately 80 poisonous sea urchin species possessing toxins in venomous spines, resulting in edema and change in the leukocyte-endothelial interaction.9 Substances identified in the spines include proteins, steroids, serotonin, histamine, and glycosides.3,9 The number of spines implanted, particularly the number of venomous spines, can lead to more severe complications. Penetration of 15 or more venomous spines can commonly lead to extracutaneous symptoms.3 Another concern, irrespective of species type, is the potential for secondary infection associated with the spine penetration or implantation into the skin. Mycobacterium marinum infections have been reported in some sea urchin granulomas,10 as well as fungal infection, bacterial infection, and tetanus.3
The diagnosis of sea urchin spine injuries starts with a thorough history and physical examination. A positive history of sea urchin contact suggests the diagnosis, and radiographs can be useful to find the location of the spine(s), especially if there are no visible nodules on the skin. However, small fragments of spine may not be completely observed on plain radiographs. Any signs or symptoms of infection should prompt a culture for confirmation and guidance for management. Cutaneous biopsies can be helpful for both diagnosis confirmation and symptomatic relief. Reported cases have described granulomatous reactions in the vast majority of the histologic specimens, with necrosis an additional common finding.7,8 Sea urchin granulomas can be of varying types, the majority being foreign-body and sarcoid types.3,6,7
Treatment of sea urchin spine injuries primarily involves removal of the spines by a physician. Patients may soak the affected areas in warm water prior to the removal of the spines to aid in pain relief. Surgical removal with local anesthesia and cutaneous extraction is a common treatment method, and more extensive surgical removal of the spines is another option, especially in areas around the joints.2 The use of liquid nitrogen or skin punch biopsy also have been described as possible methods to remove the spines.11,12
Conclusion
Sea urchin spine injuries can result in a wide range of cutaneous and systemic complications. Prompt diagnosis and treatment to remove the sea urchin spines can lessen the associated pain and is important in the prevention of more serious complications.
Sea urchin injuries are commonly seen in coastal regions near both warm and cold salt water with frequent recreational water activities or fishing. Sea urchins belong to the class Echinoidea with approximately 600 species, of which roughly 80 are poisonous to humans.1,2 When a human comes in contact with a sea urchin, the spines of the sea urchin (made of calcium carbonate) can penetrate the skin and break off from the sea urchin, becoming embedded in the skin. Injuries from sea urchin spines are most commonly seen on the hands and feet, as the likelihood of contact with a sea urchin is greater on these sites. The severity of sea urchin spine injuries can vary widely, from minimal local trauma and pain to arthritis, synovitis, and occasionally systemic illness.1,3 It is important to recognize the wide variety of responses to sea urchin spine injuries and the impact of prompt treatment. Many published reports on injuries from sea urchin spines describe arthritis and synovitis from spines in the joints.1,2,4-6 Fewer reports discuss nonjoint injuries and the dermatologic aspects of sea urchin spine injuries.3,7,8 We pre-sent a case of a patient with a puncture injury from sea urchin spines that resulted in painful granulomas.
Case Report
A 29-year-old otherwise healthy man was referred to our dermatology clinic by the university student health center due to continued pain in the right thigh. Five weeks prior to presentation to the student health center, the patient had fallen on a sea urchin while snorkeling in Hawaii. Sea urchin spines became lodged in the right thigh, some of which were removed in a local medical clinic in Hawaii. He was given oral antibiotics prior to his return home. A plain film radiograph of the affected area ordered by the student health center showed several punctate and linear densities in the lateral aspect of the right mid thigh (Figure 1). These findings were consistent with sea urchin spines within the superficial soft tissues of the lateral thigh.

At the time of presentation to our dermatology clinic, the patient reported sharp intermittent pain localized to the right thigh. The patient denied any fever, chills, or pain in the joints. On physical examination, there were several firm nodules on the right thigh, ranging from 4 to 20 mm in diameter (Figure 2). The nodules were tender to palpation with some surrounding edema. Drainage was not noted. Several scars were visible at sites of the original puncture injuries and removal of the spines.

Two 6-mm punch biopsies were performed on representative nodules on the right thigh for histopathologic examination. Along with the biopsy tissue, firm, brown-black, linear foreign bodies consistent with sea urchin spines were extracted with forceps (Figure 3). Histopathologic examination revealed a dense, diffuse, mixed inflammatory cell infiltrate in the dermis predominantly composed of lymphocytes, histiocytes, and numerous eosinophils. Proliferation of small vessels was noted. In one of the biopsies, small fragments of necrotic tissue were present. These findings were consistent with granulomatous inflammation and granulation tissue due to a foreign body.

At the time of suture removal 2 weeks later, the biopsied areas were well healed with minimal erythema. The patient reported decreased pain in the involved areas. He was not seen in clinic again due to resolution of the nodules and associated pain.
Comment
Sea urchin spine injuries are commonly seen in coastal regions with frequent participation in recreational and occupational water activities. A wide variety of responses can be seen in sea urchin spine injuries. There generally are 2 types of cutaneous reaction patterns to sea urchin spines: a primary initial reaction and a secondary delayed/granulomatous reaction. When the spines initially penetrate the skin, the primary initial reaction consists of sharp localized pain that worsens with applied pressure. In addition to pain, bleeding, erythema, edema, and myalgia can occur.3 These symptoms typically subside a few hours after complete removal of the spines from the skin.6 If some spines remain in the skin, a secondary delayed/granulomatous reaction can occur, which can lead to the formation of granulomas that can manifest as nodules or papules and can be diffuse.
Many patients may think their painful encounter with a sea urchin was just an unfortunate event, but depending on the location of the injury, more serious extracutaneous reactions and chronic symptoms may occur. Some cases have described the development of arthritis and synovitis from the implantation of spines into joints.1,2,4-6 Other extracutaneous complications include neuropathy and paresthesia, local bone destruction, radiating pain, muscular weakness, and hypotension.3
The severity of the injury also can depend on the sea urchin species and the number of spines implanted. There are approximately 80 poisonous sea urchin species possessing toxins in venomous spines, resulting in edema and change in the leukocyte-endothelial interaction.9 Substances identified in the spines include proteins, steroids, serotonin, histamine, and glycosides.3,9 The number of spines implanted, particularly the number of venomous spines, can lead to more severe complications. Penetration of 15 or more venomous spines can commonly lead to extracutaneous symptoms.3 Another concern, irrespective of species type, is the potential for secondary infection associated with the spine penetration or implantation into the skin. Mycobacterium marinum infections have been reported in some sea urchin granulomas,10 as well as fungal infection, bacterial infection, and tetanus.3
The diagnosis of sea urchin spine injuries starts with a thorough history and physical examination. A positive history of sea urchin contact suggests the diagnosis, and radiographs can be useful to find the location of the spine(s), especially if there are no visible nodules on the skin. However, small fragments of spine may not be completely observed on plain radiographs. Any signs or symptoms of infection should prompt a culture for confirmation and guidance for management. Cutaneous biopsies can be helpful for both diagnosis confirmation and symptomatic relief. Reported cases have described granulomatous reactions in the vast majority of the histologic specimens, with necrosis an additional common finding.7,8 Sea urchin granulomas can be of varying types, the majority being foreign-body and sarcoid types.3,6,7
Treatment of sea urchin spine injuries primarily involves removal of the spines by a physician. Patients may soak the affected areas in warm water prior to the removal of the spines to aid in pain relief. Surgical removal with local anesthesia and cutaneous extraction is a common treatment method, and more extensive surgical removal of the spines is another option, especially in areas around the joints.2 The use of liquid nitrogen or skin punch biopsy also have been described as possible methods to remove the spines.11,12
Conclusion
Sea urchin spine injuries can result in a wide range of cutaneous and systemic complications. Prompt diagnosis and treatment to remove the sea urchin spines can lessen the associated pain and is important in the prevention of more serious complications.
- Liram N, Gomori M, Perouansky M. Sea urchin puncture resulting in PIP joint synovial arthritis: case report and MRI study. J Travel Med. 2000;7:43-45.
- Dahl WJ, Jebson P, Louis DS. Sea urchin injuries to the hand: a case report and review of the literature. Iowa Orthop J. 2010;30:153-156.
- Rossetto AL, de Macedo Mora J, Haddad Junior V. Sea urchin granuloma. Rev Inst Med Trop Sao Paulo. 2006;48:303-306.
- Ahmad R, McCann PA, Barakat M, et al. Sea urchin spine injuries of the hand. J Hand Surg Eur Vol. 2008;33:670-671.
- Schefflein J, Umans H, Ellenbogen D, et al. Sea urchin spine arthritis in the foot. Skeletal Radiol. 2012;41:1327-1331.
- Wada T, Soma T, Gaman K, et al. Sea urchin spine arthritis of the hand. J Hand Surg. 2008;33:398-401.
- Suárez-Peñaranda JM, Vieites B, Del Río E, et al. Histopathologic and immunohistochemical features of sea urchin granulomas. J Cutan Pathol. 2013;40:550-556.
- De La Torre C, Toribio J. Sea-urchin granuloma: histologic profile. a pathologic study of 50 biopsies. J Cutan Pathol. 2001;28:223-228.
- Sciani JM, Zychar BC, Gonçalves LR, et al. Pro-inflammatory effects of the aqueous extract of Echinometra lucunter sea urchin spines. Exp Biol Med (Maywood). 2011;236:277-280.
- De la Torre C, Vega A, Carracedo A, et al. Identification of Mycobacterium marinum in sea-urchin granulomas. Br J Dermatol. 2001;145:114-116.
- Gargus MD, Morohashi DK. A sea-urchin spine chilling remedy. N Engl J Med. 2012;367:1867-1868.
- Sjøberg T, de Weerd L. The usefulness of a skin biopsy punch to remove sea urchin spines. ANZ J Surg. 2010;80:383.
- Liram N, Gomori M, Perouansky M. Sea urchin puncture resulting in PIP joint synovial arthritis: case report and MRI study. J Travel Med. 2000;7:43-45.
- Dahl WJ, Jebson P, Louis DS. Sea urchin injuries to the hand: a case report and review of the literature. Iowa Orthop J. 2010;30:153-156.
- Rossetto AL, de Macedo Mora J, Haddad Junior V. Sea urchin granuloma. Rev Inst Med Trop Sao Paulo. 2006;48:303-306.
- Ahmad R, McCann PA, Barakat M, et al. Sea urchin spine injuries of the hand. J Hand Surg Eur Vol. 2008;33:670-671.
- Schefflein J, Umans H, Ellenbogen D, et al. Sea urchin spine arthritis in the foot. Skeletal Radiol. 2012;41:1327-1331.
- Wada T, Soma T, Gaman K, et al. Sea urchin spine arthritis of the hand. J Hand Surg. 2008;33:398-401.
- Suárez-Peñaranda JM, Vieites B, Del Río E, et al. Histopathologic and immunohistochemical features of sea urchin granulomas. J Cutan Pathol. 2013;40:550-556.
- De La Torre C, Toribio J. Sea-urchin granuloma: histologic profile. a pathologic study of 50 biopsies. J Cutan Pathol. 2001;28:223-228.
- Sciani JM, Zychar BC, Gonçalves LR, et al. Pro-inflammatory effects of the aqueous extract of Echinometra lucunter sea urchin spines. Exp Biol Med (Maywood). 2011;236:277-280.
- De la Torre C, Vega A, Carracedo A, et al. Identification of Mycobacterium marinum in sea-urchin granulomas. Br J Dermatol. 2001;145:114-116.
- Gargus MD, Morohashi DK. A sea-urchin spine chilling remedy. N Engl J Med. 2012;367:1867-1868.
- Sjøberg T, de Weerd L. The usefulness of a skin biopsy punch to remove sea urchin spines. ANZ J Surg. 2010;80:383.
Practice Points
- Radiographic imaging may aid in the identification of sea urchin spines, especially if there are no visible or palpable skin nodules.
- Treatment of sea urchin spine injuries typically involves surgical removal of the spines with local anesthesia and cutaneous extraction.
- Prompt extraction of sea urchin spines can improve pain symptoms and decrease the likelihood of granuloma formation, infection, and extracutaneous complications.
Efficacy and Safety of New Dermal Fillers
Facial aging is the result of the interplay between loss of skin elasticity, changes in subcutaneous fat and other soft-tissue layers, and skeletal remodeling with chronological age.1 Dermal fillers are effective for the treatment of rhytides, facial scars, and lipoatrophy, as well as facial contouring and augmentation. Given that multiple filler options exist, updated reviews are necessary to inform clinicians of the choices that are available. We provide a detailed review of the clinical efficacy and safety of the dermal fillers with the most recent approvals by the US Food and Drug Administration (FDA).
Polymethylmethacrylate
Polymethylmethacrylate (PMMA) microspheres suspended in bovine collagen and lidocaine 0.3% were approved in 2006 for use in nasolabial folds (NLFs) and in 2014 for acne scars. Now branded as Bellafill (Suneva Medical, Inc), it is the only permanent injectable filler currently available. Once injected, the particles are not reabsorbed and can only be removed by procedural extraction (eg, liposuction of the surrounding fat); however, the permanence of PMMA does not extend to facial rejuvenation, which can last up to 5 years. Prior to use, skin testing for bovine collagen reaction is necessary. In a clinical trial of 147 patients with moderate to severe acne scarring, patients were randomized to receive PMMA in collagen (n=97) or saline (n=50).2 Injections were administered using a linear threading or serial puncture technique, and patients were reevaluated after 4 weeks for touch-up injections. After 6 months, 64% of patients treated with PMMA in collagen achieved improvement in acne scars by 2 points or more on the acne scar rating scale versus 33% of the control group (P=.0005).2
Treatment-related adverse events (AEs) include injection-site pain, bruising, swelling, erythema, and more rarely pruritus and lumps/granulomas.3 A 5-year longitudinal safety investigation of 871 patients initially treated with PMMA in collagen for NLF correction revealed that 17 patients (2.0%) had biopsy-confirmed granulomas with half of these retained at study end.4 Fifteen of these patients were treated with intralesional corticosteroids alone or in combination with intralesional 5-fluorouracil, oral antibiotics, or topical calcineurin inhibitors; 1 patient was untreated and another used topical corticosteroids. The authors noted no correlation between treatment method and granuloma response.4 Polymethylmethacrylate in collagen is contraindicated in patients with lidocaine or bovine collagen sensitivity and is not indicated for use in lip augmentation due to high rates of nodule formation.3
Hyaluronic Acid
Hyaluronic acid (HA) is a naturally occurring glycosaminoglycan polymer found in the extracellular matrix of the dermis. Hyaluronic acid fillers are bacteria derived and come in gel form. A useful advantage of HA fillers compared to other dermal fillers is the commercial availability of hyaluronidase to correct injections. Preinjection skin testing is not necessary.5
This category of nonpermanent dermal fillers has the most robust market choices. Older HA dermal fillers with reliable and proven efficacy are Restylane (Galderma Laboratories, LP)(facial rhytides, lip augmentation), Juvéderm (Ultra/Ultra XC/Ultra Plus/Ultra Plus XC [Allergan, Inc])(facial rhytides, lip augmentation), Hydrelle (Anika Therapeutics, Inc)(facial rhytides), and Prevelle Silk (Mentor Corporation)(facial rhytides); they will not be reviewed here. Newer agents include Belotero Balance (Merz Aesthetics), Juvéderm Voluma XC (Allergan, Inc), Restylane Silk (Galderma Laboratories, LP), and Restylane Lyft (Galderma Laboratories, LP).
Belotero Balance
Belotero Balance is used to treat fine lines and wrinkles, especially NLFs.6 The initial pivotal studies that led to FDA approval in 2011 demonstrated noninferiority and superiority to bovine collagen for use in the treatment of NLFs.7,8 One hundred eighteen patients with bilateral NLFs that were rated as 2 (moderate) or 3 (severe) on the wrinkle severity rating scale (WSRS) were randomized to split-face injection of Belotero Balance in one NLF and bovine collagen in the contralateral NLF.7 An additional injection at week 2 was allowed for optimal correction. Belotero Balance was noninferior to bovine collagen at week 2, with mean improvement in WSRS of 1.52 versus 1.57 (P=.50). Belotero Balance was superior to bovine collagen in mean WSRS improvement at weeks 12 (1.25 vs 0.26; P<.001), 16 (1.09 vs 0.66; P<.001), and 24 (1.08 vs 0.50; P<.001).7 In a subsequent open-label extension study, which included 95 of 118 patients who received Belotero Balance injections in both NLFs at week 24, 80.2% of patients showed sustained improvement in WSRS from baseline for 48 weeks without further injection.8
The first comparative study of Belotero Balance with other established HA fillers at the time—Restylane and Juvéderm Ultra 3/Ultra Plus XC—to treat NLFs demonstrated noninferiority.9 Forty patients with bilateral, moderate to severe NLFs (rated 3 or 4 on the Merz severity scale) were randomized to split-face groups of Belotero Balance versus Restylane or Belotero Balance versus Juvéderm. At 12 months, NLF severity improved from 2.3 to 1.5 in the Restylane group and from 2.3 to 1.6 in the Juvéderm group.9
Belotero Balance has been compared to Juvéderm Ultra XC for use in perioral lines.10 The study included 136 patients with moderate to severe perioral lines, according to the perioral lines severity scale, who were randomized (1:1 ratio) to receive injections of Belotero Balance or Juvéderm Ultra XC to correct upper and lower perioral lines, with assessment at week 2 for optimization. After 6 months, 87% of Juvéderm-treated patients compared to 72% of Belotero Balance–treated patients had 1-point improvement in perioral lines (P<.04). Juvéderm-treated patients also reported significantly less pain than Belotero Balance–treated patients (P<.001).10
Treatment-related AEs are described in the Table, with the majority occurring at lower rates compared to a collagen control group and self-resolving within 2 weeks.7
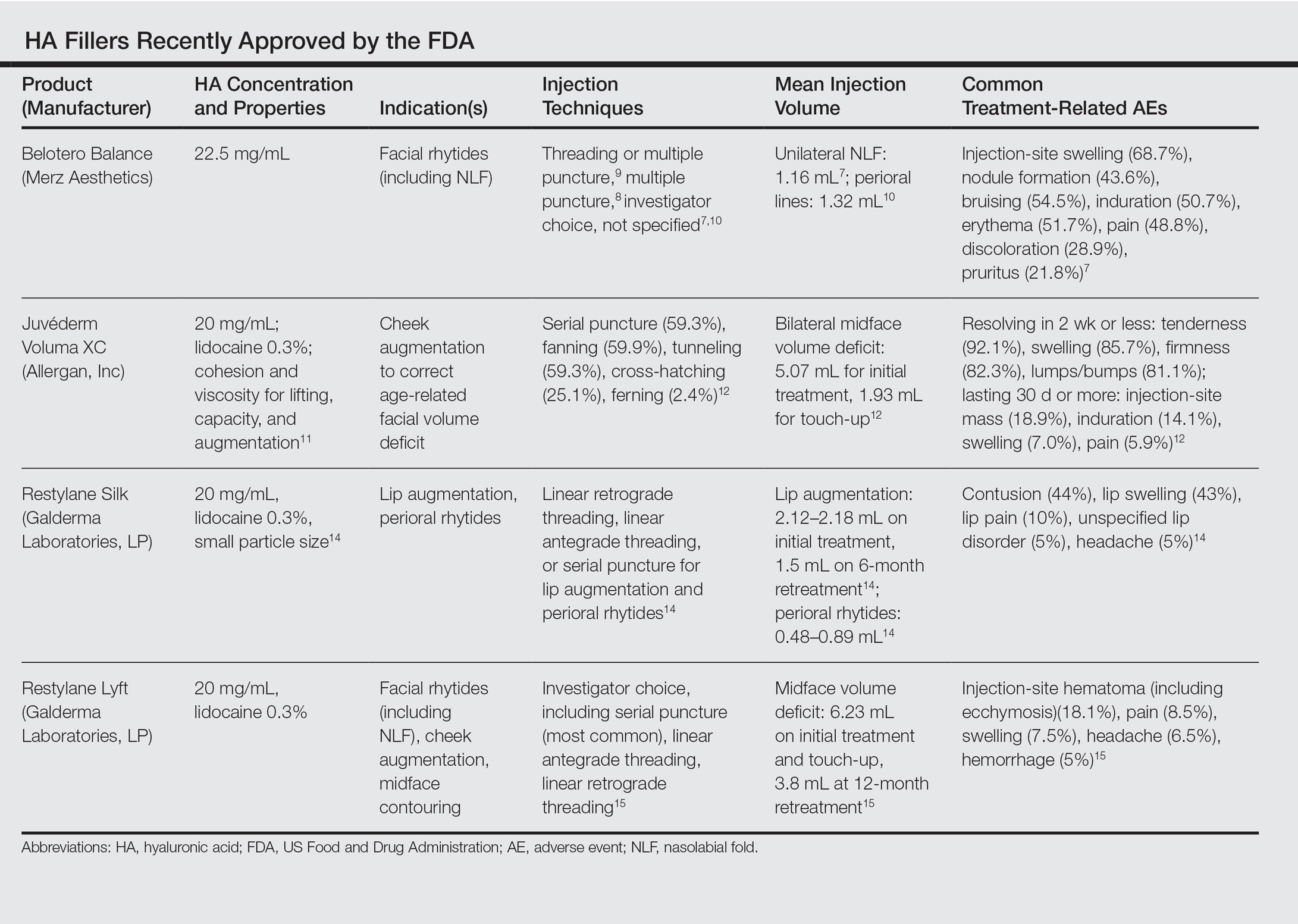
Juvéderm Voluma XC
Juvéderm Voluma XC was FDA approved in 2013 for cheek augmentation to correct age-related volume deficit restoration by subcutaneous or subperiosteal injections. In its landmark multicenter investigation, 282 patients with moderate to severe midface (eg, zygomaticomalar, anteromedial cheek, submalar regions) volume deficit measured on a validated midface volume deficit scale (MFVDS) were treated with Juvéderm Voluma XC (n=235) or control (n=47).11 Patients were reevaluated at 30 days and 81.9% received touch-up injections. At a 6-month primary evaluation, 86% of the Juvéderm-treated patients versus 39% of the control patients showed 1-point improvement on the MFVDS (P<.001). At 24-months’ follow-up, 44.6% of patients sustained efficacy.11 Of these aforementioned patients, 167 received repeat treatment due to lost correction or patient request and 91.1% improved by 1 point or more on the MFVDS on evaluation 12 months after repeat treatment.12 For this same population of patients, a 2-year extended follow-up of patient-reported outcomes revealed that 49% of patients felt fulfilled in their treatment goals 2 years after treatment and 79% of patients rated improvement from baseline based on the global aesthetic improvement scale.13 Efficacy studies involving Juvéderm Voluma XC are currently ongoing for facial temporal aging (registered at www.clinicaltrials.gov with the identifier NCT02437903) and recruiting for mandibular hypoplasia (NCT02330016).
Common treatment-related AEs are detailed in the Table. Two patients required treatment with hyaluronidase for chronic lumpiness and nodularity following non–treatment-related cellulitis.11 The product is contraindicated in patients with allergy to lidocaine.
Restylane Silk
Restylane Silk was approved in 2014 for lip augmentation and perioral rhytides. Efficacy and safety was demonstrated in a large multicenter randomized investigation in which 221 patients seeking lip augmentation received either Restylane Silk (n=177) injected submucosally for treatment of the upper and lower lips and/or intradermally for perioral rhytides or no treatment (n=44).14 Restylane treatment group patients optionally received touch-up at 2 weeks for optimization. All patients, including the control group, received injections at 6 months. At the 2-month primary end point, 80.2% of the treatment group exhibited at least 1-point improvement in upper lip fullness on the Medicis lip fullness scale compared to 11.9% (P<.001) of the control group; response rates for the lower lips were 84.2% versus 18.4% (P<.001). Patients in the treatment group receiving injections for perioral rhytides showed significant improvement in perioral rhytides through week 24 compared to patients treated for lip augmentation only (P<.001).14 Restylane Silk currently is undergoing investigation for cheek rejuvenation (NCT02636894, NCT02679924) and treatment of hand photoaging (NCT02780258).
The most common AEs are listed in the Table. No lip disorders were considered clinically concerning on evaluation. Concomitant lip augmentation and treatment of perioral rhytides yielded similar rates of AEs.14 Restylane Silk is not to be used in patients with known lidocaine allergy.
Restylane Lyft
Restylane Lyft (formerly known as Perlane-L) was approved in 2010 for use in facial rhytides, including NLFs, and gained approval in 2015 for use in cheek augmentation and midface contouring. Only its efficacy and safety for the more recent indication will be reviewed here.
In an evaluator-blinded investigation of 200 patients with mild to substantial bilateral midface deficiency based on the Medicis midface volume scale (MMVS), patients were randomized to receive supraperiosteal and subcutaneous treatment with Restylane Lyft (n=150) or no treatment (n=50).15 Touch-up injections at week 2 or month 12 were available to treatment group patients and all patients were given either an initial treatment or retreatment at 12 months. Primary end point evaluation at week 8 showed that 89% of treatment group patients had at least 1 grade MMVS improvement compared to 16% of the control group (P<.001). Although the percentage of these MMVS responders in the treatment group decreased with each follow-up period to 54.3% at month 12, retreatment was effective in reproducing a similar MMVS response rate as with initial treatment.15 Restylane Lyft is under ongoing investigation for dorsal hand rejuvenation (NCT02650921).
In addition to the common treatment-related AEs listed in the Table, 2 patients reported serious AEs, including bilateral implant-site inflammation and unilateral implant-site hematoma and infection (organism not described), all of which resolved with unspecified treatment.15 Lidocaine allergies are contraindications for use.
Conclusion
Several new options in dermal fillers have been approved in recent years and have demonstrated efficacy and acceptable safety in various cosmetic rejuvenation applications. Restylane Silk and Restylane Lyft are undergoing further studies to evaluate use in hand rejuvenation, an area that currently has few cosmetic filler treatment options. As technology continues to progress and new formulations of dermal fillers with varied properties and benefits are available, clinicians should expect multiple options for use in rhytides, volume deficits, and contouring.
ADDENDUM
After the manuscript was accepted for publication, Juvéderm Volbella XC (Allergan, Inc) was approved by the FDA for use in lip augmentation and thus is not included in this review.
- Fitzgerald R, Graivier MH, Kane M, et al. Update on facial aging. Aesthet Surg J. 2010;30(suppl):S11-S24.
- Karnik J, Baumann L, Bruce S, et al. A double-blind, randomized, multicenter, controlled trial of suspended polymethylmethacrylate microspheres for the correction of atrophic facial acne scars. J Am Acad Dermatol. 2014;71:77-83.
- Bellafill [package insert]. San Diego, CA: Suneva Medical, Inc; 2015.
- Cohen S, Dover J, Monheit G, et al. Five-year safety and satisfaction study of PMMA-collagen in the correction of nasolabial folds. Dermatol Surg. 2015;41(suppl 1):S302-S313.
- Greene JJ, Sidle DM. The hyaluronic acid fillers: current understanding of the tissue device interface. Facial Plast Surg Clin North Am. 2015;23:423-432.
- Lorenc ZP, Fagien S, Flynn TC, et al. Review of key Belotero Balance safety and efficacy trials. Plast Reconstr Surg. 2013;132(4, suppl 2):33S-40S.
- Narins RS, Coleman W, Donofrio L, et al. Nonanimal sourced hyaluronic acid–based dermal filler using a cohesive polydensified matrix technology is superior to bovine collagen in the correction of moderate to severe nasolabial folds: results from a 6-month, randomized, blinded, controlled, multicenter study. Dermatol Surg. 2010;36(suppl 1):730-740.
- Narins RS, Coleman WP 3rd, Donofrio LM, et al. Improvement in nasolabial folds with a hyaluronic acid filler using a cohesive polydensified matrix technology: results from an 18-month open-label extension trial. Dermatol Surg. 2010;36(suppl 3):1800-1808.
- Prager W, Wissmueller E, Havermann I, et al. A prospective, split-face, randomized, comparative study of safety and 12-month longevity of three formulations of hyaluronic acid dermal filler for treatment of nasolabial folds. Dermatol Surg. 2012;38(7, pt 2):1143-1150.
- Butterwick K, Marmur E, Narurkar V, et al. HYC-24L demonstrates greater effectiveness with less pain than CPM-22.5 for treatment of perioral lines in a randomized controlled trial. Dermatol Surg. 2015;41:1351-1360.
- Jones D, Murphy DK. Volumizing hyaluronic acid filler for midface volume deficit: 2-year results from a pivotal single-blind randomized controlled study. Dermatol Surg. 2013;39:1602-1612.
- Baumann L, Narins RS, Beer K, et al. Volumizing hyaluronic acid filler for midface volume deficit: results after repeat treatment. Dermatol Surg. 2015;41(suppl 1):S284-S292.
- Few J, Cox SE, Paradkar-Mitragotri D, et al. A multicenter, single-blind randomized, controlled study of a volumizing hyaluronic acid filler for midface volume deficit: patient-reported outcomes at 2 years. Aesthet Surg J. 2015;35:589-599.
- Beer K, Glogau RG, Dover JS, et al. A randomized, evaluator-blinded, controlled study of effectiveness and safety of small particle hyaluronic acid plus lidocaine for lip augmentation and perioral rhytides. Dermatol Surg. 2015;41(suppl 1):S127-S136.
- Weiss RA, Moradi A, Bank D, et al. Effectiveness and safety of large gel particle hyaluronic acid with lidocaine for correction of midface volume deficit or contour deficiency. Dermatol Surg. 2016;42:699-709.
Facial aging is the result of the interplay between loss of skin elasticity, changes in subcutaneous fat and other soft-tissue layers, and skeletal remodeling with chronological age.1 Dermal fillers are effective for the treatment of rhytides, facial scars, and lipoatrophy, as well as facial contouring and augmentation. Given that multiple filler options exist, updated reviews are necessary to inform clinicians of the choices that are available. We provide a detailed review of the clinical efficacy and safety of the dermal fillers with the most recent approvals by the US Food and Drug Administration (FDA).
Polymethylmethacrylate
Polymethylmethacrylate (PMMA) microspheres suspended in bovine collagen and lidocaine 0.3% were approved in 2006 for use in nasolabial folds (NLFs) and in 2014 for acne scars. Now branded as Bellafill (Suneva Medical, Inc), it is the only permanent injectable filler currently available. Once injected, the particles are not reabsorbed and can only be removed by procedural extraction (eg, liposuction of the surrounding fat); however, the permanence of PMMA does not extend to facial rejuvenation, which can last up to 5 years. Prior to use, skin testing for bovine collagen reaction is necessary. In a clinical trial of 147 patients with moderate to severe acne scarring, patients were randomized to receive PMMA in collagen (n=97) or saline (n=50).2 Injections were administered using a linear threading or serial puncture technique, and patients were reevaluated after 4 weeks for touch-up injections. After 6 months, 64% of patients treated with PMMA in collagen achieved improvement in acne scars by 2 points or more on the acne scar rating scale versus 33% of the control group (P=.0005).2
Treatment-related adverse events (AEs) include injection-site pain, bruising, swelling, erythema, and more rarely pruritus and lumps/granulomas.3 A 5-year longitudinal safety investigation of 871 patients initially treated with PMMA in collagen for NLF correction revealed that 17 patients (2.0%) had biopsy-confirmed granulomas with half of these retained at study end.4 Fifteen of these patients were treated with intralesional corticosteroids alone or in combination with intralesional 5-fluorouracil, oral antibiotics, or topical calcineurin inhibitors; 1 patient was untreated and another used topical corticosteroids. The authors noted no correlation between treatment method and granuloma response.4 Polymethylmethacrylate in collagen is contraindicated in patients with lidocaine or bovine collagen sensitivity and is not indicated for use in lip augmentation due to high rates of nodule formation.3
Hyaluronic Acid
Hyaluronic acid (HA) is a naturally occurring glycosaminoglycan polymer found in the extracellular matrix of the dermis. Hyaluronic acid fillers are bacteria derived and come in gel form. A useful advantage of HA fillers compared to other dermal fillers is the commercial availability of hyaluronidase to correct injections. Preinjection skin testing is not necessary.5
This category of nonpermanent dermal fillers has the most robust market choices. Older HA dermal fillers with reliable and proven efficacy are Restylane (Galderma Laboratories, LP)(facial rhytides, lip augmentation), Juvéderm (Ultra/Ultra XC/Ultra Plus/Ultra Plus XC [Allergan, Inc])(facial rhytides, lip augmentation), Hydrelle (Anika Therapeutics, Inc)(facial rhytides), and Prevelle Silk (Mentor Corporation)(facial rhytides); they will not be reviewed here. Newer agents include Belotero Balance (Merz Aesthetics), Juvéderm Voluma XC (Allergan, Inc), Restylane Silk (Galderma Laboratories, LP), and Restylane Lyft (Galderma Laboratories, LP).
Belotero Balance
Belotero Balance is used to treat fine lines and wrinkles, especially NLFs.6 The initial pivotal studies that led to FDA approval in 2011 demonstrated noninferiority and superiority to bovine collagen for use in the treatment of NLFs.7,8 One hundred eighteen patients with bilateral NLFs that were rated as 2 (moderate) or 3 (severe) on the wrinkle severity rating scale (WSRS) were randomized to split-face injection of Belotero Balance in one NLF and bovine collagen in the contralateral NLF.7 An additional injection at week 2 was allowed for optimal correction. Belotero Balance was noninferior to bovine collagen at week 2, with mean improvement in WSRS of 1.52 versus 1.57 (P=.50). Belotero Balance was superior to bovine collagen in mean WSRS improvement at weeks 12 (1.25 vs 0.26; P<.001), 16 (1.09 vs 0.66; P<.001), and 24 (1.08 vs 0.50; P<.001).7 In a subsequent open-label extension study, which included 95 of 118 patients who received Belotero Balance injections in both NLFs at week 24, 80.2% of patients showed sustained improvement in WSRS from baseline for 48 weeks without further injection.8
The first comparative study of Belotero Balance with other established HA fillers at the time—Restylane and Juvéderm Ultra 3/Ultra Plus XC—to treat NLFs demonstrated noninferiority.9 Forty patients with bilateral, moderate to severe NLFs (rated 3 or 4 on the Merz severity scale) were randomized to split-face groups of Belotero Balance versus Restylane or Belotero Balance versus Juvéderm. At 12 months, NLF severity improved from 2.3 to 1.5 in the Restylane group and from 2.3 to 1.6 in the Juvéderm group.9
Belotero Balance has been compared to Juvéderm Ultra XC for use in perioral lines.10 The study included 136 patients with moderate to severe perioral lines, according to the perioral lines severity scale, who were randomized (1:1 ratio) to receive injections of Belotero Balance or Juvéderm Ultra XC to correct upper and lower perioral lines, with assessment at week 2 for optimization. After 6 months, 87% of Juvéderm-treated patients compared to 72% of Belotero Balance–treated patients had 1-point improvement in perioral lines (P<.04). Juvéderm-treated patients also reported significantly less pain than Belotero Balance–treated patients (P<.001).10
Treatment-related AEs are described in the Table, with the majority occurring at lower rates compared to a collagen control group and self-resolving within 2 weeks.7

Juvéderm Voluma XC
Juvéderm Voluma XC was FDA approved in 2013 for cheek augmentation to correct age-related volume deficit restoration by subcutaneous or subperiosteal injections. In its landmark multicenter investigation, 282 patients with moderate to severe midface (eg, zygomaticomalar, anteromedial cheek, submalar regions) volume deficit measured on a validated midface volume deficit scale (MFVDS) were treated with Juvéderm Voluma XC (n=235) or control (n=47).11 Patients were reevaluated at 30 days and 81.9% received touch-up injections. At a 6-month primary evaluation, 86% of the Juvéderm-treated patients versus 39% of the control patients showed 1-point improvement on the MFVDS (P<.001). At 24-months’ follow-up, 44.6% of patients sustained efficacy.11 Of these aforementioned patients, 167 received repeat treatment due to lost correction or patient request and 91.1% improved by 1 point or more on the MFVDS on evaluation 12 months after repeat treatment.12 For this same population of patients, a 2-year extended follow-up of patient-reported outcomes revealed that 49% of patients felt fulfilled in their treatment goals 2 years after treatment and 79% of patients rated improvement from baseline based on the global aesthetic improvement scale.13 Efficacy studies involving Juvéderm Voluma XC are currently ongoing for facial temporal aging (registered at www.clinicaltrials.gov with the identifier NCT02437903) and recruiting for mandibular hypoplasia (NCT02330016).
Common treatment-related AEs are detailed in the Table. Two patients required treatment with hyaluronidase for chronic lumpiness and nodularity following non–treatment-related cellulitis.11 The product is contraindicated in patients with allergy to lidocaine.
Restylane Silk
Restylane Silk was approved in 2014 for lip augmentation and perioral rhytides. Efficacy and safety was demonstrated in a large multicenter randomized investigation in which 221 patients seeking lip augmentation received either Restylane Silk (n=177) injected submucosally for treatment of the upper and lower lips and/or intradermally for perioral rhytides or no treatment (n=44).14 Restylane treatment group patients optionally received touch-up at 2 weeks for optimization. All patients, including the control group, received injections at 6 months. At the 2-month primary end point, 80.2% of the treatment group exhibited at least 1-point improvement in upper lip fullness on the Medicis lip fullness scale compared to 11.9% (P<.001) of the control group; response rates for the lower lips were 84.2% versus 18.4% (P<.001). Patients in the treatment group receiving injections for perioral rhytides showed significant improvement in perioral rhytides through week 24 compared to patients treated for lip augmentation only (P<.001).14 Restylane Silk currently is undergoing investigation for cheek rejuvenation (NCT02636894, NCT02679924) and treatment of hand photoaging (NCT02780258).
The most common AEs are listed in the Table. No lip disorders were considered clinically concerning on evaluation. Concomitant lip augmentation and treatment of perioral rhytides yielded similar rates of AEs.14 Restylane Silk is not to be used in patients with known lidocaine allergy.
Restylane Lyft
Restylane Lyft (formerly known as Perlane-L) was approved in 2010 for use in facial rhytides, including NLFs, and gained approval in 2015 for use in cheek augmentation and midface contouring. Only its efficacy and safety for the more recent indication will be reviewed here.
In an evaluator-blinded investigation of 200 patients with mild to substantial bilateral midface deficiency based on the Medicis midface volume scale (MMVS), patients were randomized to receive supraperiosteal and subcutaneous treatment with Restylane Lyft (n=150) or no treatment (n=50).15 Touch-up injections at week 2 or month 12 were available to treatment group patients and all patients were given either an initial treatment or retreatment at 12 months. Primary end point evaluation at week 8 showed that 89% of treatment group patients had at least 1 grade MMVS improvement compared to 16% of the control group (P<.001). Although the percentage of these MMVS responders in the treatment group decreased with each follow-up period to 54.3% at month 12, retreatment was effective in reproducing a similar MMVS response rate as with initial treatment.15 Restylane Lyft is under ongoing investigation for dorsal hand rejuvenation (NCT02650921).
In addition to the common treatment-related AEs listed in the Table, 2 patients reported serious AEs, including bilateral implant-site inflammation and unilateral implant-site hematoma and infection (organism not described), all of which resolved with unspecified treatment.15 Lidocaine allergies are contraindications for use.
Conclusion
Several new options in dermal fillers have been approved in recent years and have demonstrated efficacy and acceptable safety in various cosmetic rejuvenation applications. Restylane Silk and Restylane Lyft are undergoing further studies to evaluate use in hand rejuvenation, an area that currently has few cosmetic filler treatment options. As technology continues to progress and new formulations of dermal fillers with varied properties and benefits are available, clinicians should expect multiple options for use in rhytides, volume deficits, and contouring.
ADDENDUM
After the manuscript was accepted for publication, Juvéderm Volbella XC (Allergan, Inc) was approved by the FDA for use in lip augmentation and thus is not included in this review.
Facial aging is the result of the interplay between loss of skin elasticity, changes in subcutaneous fat and other soft-tissue layers, and skeletal remodeling with chronological age.1 Dermal fillers are effective for the treatment of rhytides, facial scars, and lipoatrophy, as well as facial contouring and augmentation. Given that multiple filler options exist, updated reviews are necessary to inform clinicians of the choices that are available. We provide a detailed review of the clinical efficacy and safety of the dermal fillers with the most recent approvals by the US Food and Drug Administration (FDA).
Polymethylmethacrylate
Polymethylmethacrylate (PMMA) microspheres suspended in bovine collagen and lidocaine 0.3% were approved in 2006 for use in nasolabial folds (NLFs) and in 2014 for acne scars. Now branded as Bellafill (Suneva Medical, Inc), it is the only permanent injectable filler currently available. Once injected, the particles are not reabsorbed and can only be removed by procedural extraction (eg, liposuction of the surrounding fat); however, the permanence of PMMA does not extend to facial rejuvenation, which can last up to 5 years. Prior to use, skin testing for bovine collagen reaction is necessary. In a clinical trial of 147 patients with moderate to severe acne scarring, patients were randomized to receive PMMA in collagen (n=97) or saline (n=50).2 Injections were administered using a linear threading or serial puncture technique, and patients were reevaluated after 4 weeks for touch-up injections. After 6 months, 64% of patients treated with PMMA in collagen achieved improvement in acne scars by 2 points or more on the acne scar rating scale versus 33% of the control group (P=.0005).2
Treatment-related adverse events (AEs) include injection-site pain, bruising, swelling, erythema, and more rarely pruritus and lumps/granulomas.3 A 5-year longitudinal safety investigation of 871 patients initially treated with PMMA in collagen for NLF correction revealed that 17 patients (2.0%) had biopsy-confirmed granulomas with half of these retained at study end.4 Fifteen of these patients were treated with intralesional corticosteroids alone or in combination with intralesional 5-fluorouracil, oral antibiotics, or topical calcineurin inhibitors; 1 patient was untreated and another used topical corticosteroids. The authors noted no correlation between treatment method and granuloma response.4 Polymethylmethacrylate in collagen is contraindicated in patients with lidocaine or bovine collagen sensitivity and is not indicated for use in lip augmentation due to high rates of nodule formation.3
Hyaluronic Acid
Hyaluronic acid (HA) is a naturally occurring glycosaminoglycan polymer found in the extracellular matrix of the dermis. Hyaluronic acid fillers are bacteria derived and come in gel form. A useful advantage of HA fillers compared to other dermal fillers is the commercial availability of hyaluronidase to correct injections. Preinjection skin testing is not necessary.5
This category of nonpermanent dermal fillers has the most robust market choices. Older HA dermal fillers with reliable and proven efficacy are Restylane (Galderma Laboratories, LP)(facial rhytides, lip augmentation), Juvéderm (Ultra/Ultra XC/Ultra Plus/Ultra Plus XC [Allergan, Inc])(facial rhytides, lip augmentation), Hydrelle (Anika Therapeutics, Inc)(facial rhytides), and Prevelle Silk (Mentor Corporation)(facial rhytides); they will not be reviewed here. Newer agents include Belotero Balance (Merz Aesthetics), Juvéderm Voluma XC (Allergan, Inc), Restylane Silk (Galderma Laboratories, LP), and Restylane Lyft (Galderma Laboratories, LP).
Belotero Balance
Belotero Balance is used to treat fine lines and wrinkles, especially NLFs.6 The initial pivotal studies that led to FDA approval in 2011 demonstrated noninferiority and superiority to bovine collagen for use in the treatment of NLFs.7,8 One hundred eighteen patients with bilateral NLFs that were rated as 2 (moderate) or 3 (severe) on the wrinkle severity rating scale (WSRS) were randomized to split-face injection of Belotero Balance in one NLF and bovine collagen in the contralateral NLF.7 An additional injection at week 2 was allowed for optimal correction. Belotero Balance was noninferior to bovine collagen at week 2, with mean improvement in WSRS of 1.52 versus 1.57 (P=.50). Belotero Balance was superior to bovine collagen in mean WSRS improvement at weeks 12 (1.25 vs 0.26; P<.001), 16 (1.09 vs 0.66; P<.001), and 24 (1.08 vs 0.50; P<.001).7 In a subsequent open-label extension study, which included 95 of 118 patients who received Belotero Balance injections in both NLFs at week 24, 80.2% of patients showed sustained improvement in WSRS from baseline for 48 weeks without further injection.8
The first comparative study of Belotero Balance with other established HA fillers at the time—Restylane and Juvéderm Ultra 3/Ultra Plus XC—to treat NLFs demonstrated noninferiority.9 Forty patients with bilateral, moderate to severe NLFs (rated 3 or 4 on the Merz severity scale) were randomized to split-face groups of Belotero Balance versus Restylane or Belotero Balance versus Juvéderm. At 12 months, NLF severity improved from 2.3 to 1.5 in the Restylane group and from 2.3 to 1.6 in the Juvéderm group.9
Belotero Balance has been compared to Juvéderm Ultra XC for use in perioral lines.10 The study included 136 patients with moderate to severe perioral lines, according to the perioral lines severity scale, who were randomized (1:1 ratio) to receive injections of Belotero Balance or Juvéderm Ultra XC to correct upper and lower perioral lines, with assessment at week 2 for optimization. After 6 months, 87% of Juvéderm-treated patients compared to 72% of Belotero Balance–treated patients had 1-point improvement in perioral lines (P<.04). Juvéderm-treated patients also reported significantly less pain than Belotero Balance–treated patients (P<.001).10
Treatment-related AEs are described in the Table, with the majority occurring at lower rates compared to a collagen control group and self-resolving within 2 weeks.7

Juvéderm Voluma XC
Juvéderm Voluma XC was FDA approved in 2013 for cheek augmentation to correct age-related volume deficit restoration by subcutaneous or subperiosteal injections. In its landmark multicenter investigation, 282 patients with moderate to severe midface (eg, zygomaticomalar, anteromedial cheek, submalar regions) volume deficit measured on a validated midface volume deficit scale (MFVDS) were treated with Juvéderm Voluma XC (n=235) or control (n=47).11 Patients were reevaluated at 30 days and 81.9% received touch-up injections. At a 6-month primary evaluation, 86% of the Juvéderm-treated patients versus 39% of the control patients showed 1-point improvement on the MFVDS (P<.001). At 24-months’ follow-up, 44.6% of patients sustained efficacy.11 Of these aforementioned patients, 167 received repeat treatment due to lost correction or patient request and 91.1% improved by 1 point or more on the MFVDS on evaluation 12 months after repeat treatment.12 For this same population of patients, a 2-year extended follow-up of patient-reported outcomes revealed that 49% of patients felt fulfilled in their treatment goals 2 years after treatment and 79% of patients rated improvement from baseline based on the global aesthetic improvement scale.13 Efficacy studies involving Juvéderm Voluma XC are currently ongoing for facial temporal aging (registered at www.clinicaltrials.gov with the identifier NCT02437903) and recruiting for mandibular hypoplasia (NCT02330016).
Common treatment-related AEs are detailed in the Table. Two patients required treatment with hyaluronidase for chronic lumpiness and nodularity following non–treatment-related cellulitis.11 The product is contraindicated in patients with allergy to lidocaine.
Restylane Silk
Restylane Silk was approved in 2014 for lip augmentation and perioral rhytides. Efficacy and safety was demonstrated in a large multicenter randomized investigation in which 221 patients seeking lip augmentation received either Restylane Silk (n=177) injected submucosally for treatment of the upper and lower lips and/or intradermally for perioral rhytides or no treatment (n=44).14 Restylane treatment group patients optionally received touch-up at 2 weeks for optimization. All patients, including the control group, received injections at 6 months. At the 2-month primary end point, 80.2% of the treatment group exhibited at least 1-point improvement in upper lip fullness on the Medicis lip fullness scale compared to 11.9% (P<.001) of the control group; response rates for the lower lips were 84.2% versus 18.4% (P<.001). Patients in the treatment group receiving injections for perioral rhytides showed significant improvement in perioral rhytides through week 24 compared to patients treated for lip augmentation only (P<.001).14 Restylane Silk currently is undergoing investigation for cheek rejuvenation (NCT02636894, NCT02679924) and treatment of hand photoaging (NCT02780258).
The most common AEs are listed in the Table. No lip disorders were considered clinically concerning on evaluation. Concomitant lip augmentation and treatment of perioral rhytides yielded similar rates of AEs.14 Restylane Silk is not to be used in patients with known lidocaine allergy.
Restylane Lyft
Restylane Lyft (formerly known as Perlane-L) was approved in 2010 for use in facial rhytides, including NLFs, and gained approval in 2015 for use in cheek augmentation and midface contouring. Only its efficacy and safety for the more recent indication will be reviewed here.
In an evaluator-blinded investigation of 200 patients with mild to substantial bilateral midface deficiency based on the Medicis midface volume scale (MMVS), patients were randomized to receive supraperiosteal and subcutaneous treatment with Restylane Lyft (n=150) or no treatment (n=50).15 Touch-up injections at week 2 or month 12 were available to treatment group patients and all patients were given either an initial treatment or retreatment at 12 months. Primary end point evaluation at week 8 showed that 89% of treatment group patients had at least 1 grade MMVS improvement compared to 16% of the control group (P<.001). Although the percentage of these MMVS responders in the treatment group decreased with each follow-up period to 54.3% at month 12, retreatment was effective in reproducing a similar MMVS response rate as with initial treatment.15 Restylane Lyft is under ongoing investigation for dorsal hand rejuvenation (NCT02650921).
In addition to the common treatment-related AEs listed in the Table, 2 patients reported serious AEs, including bilateral implant-site inflammation and unilateral implant-site hematoma and infection (organism not described), all of which resolved with unspecified treatment.15 Lidocaine allergies are contraindications for use.
Conclusion
Several new options in dermal fillers have been approved in recent years and have demonstrated efficacy and acceptable safety in various cosmetic rejuvenation applications. Restylane Silk and Restylane Lyft are undergoing further studies to evaluate use in hand rejuvenation, an area that currently has few cosmetic filler treatment options. As technology continues to progress and new formulations of dermal fillers with varied properties and benefits are available, clinicians should expect multiple options for use in rhytides, volume deficits, and contouring.
ADDENDUM
After the manuscript was accepted for publication, Juvéderm Volbella XC (Allergan, Inc) was approved by the FDA for use in lip augmentation and thus is not included in this review.
- Fitzgerald R, Graivier MH, Kane M, et al. Update on facial aging. Aesthet Surg J. 2010;30(suppl):S11-S24.
- Karnik J, Baumann L, Bruce S, et al. A double-blind, randomized, multicenter, controlled trial of suspended polymethylmethacrylate microspheres for the correction of atrophic facial acne scars. J Am Acad Dermatol. 2014;71:77-83.
- Bellafill [package insert]. San Diego, CA: Suneva Medical, Inc; 2015.
- Cohen S, Dover J, Monheit G, et al. Five-year safety and satisfaction study of PMMA-collagen in the correction of nasolabial folds. Dermatol Surg. 2015;41(suppl 1):S302-S313.
- Greene JJ, Sidle DM. The hyaluronic acid fillers: current understanding of the tissue device interface. Facial Plast Surg Clin North Am. 2015;23:423-432.
- Lorenc ZP, Fagien S, Flynn TC, et al. Review of key Belotero Balance safety and efficacy trials. Plast Reconstr Surg. 2013;132(4, suppl 2):33S-40S.
- Narins RS, Coleman W, Donofrio L, et al. Nonanimal sourced hyaluronic acid–based dermal filler using a cohesive polydensified matrix technology is superior to bovine collagen in the correction of moderate to severe nasolabial folds: results from a 6-month, randomized, blinded, controlled, multicenter study. Dermatol Surg. 2010;36(suppl 1):730-740.
- Narins RS, Coleman WP 3rd, Donofrio LM, et al. Improvement in nasolabial folds with a hyaluronic acid filler using a cohesive polydensified matrix technology: results from an 18-month open-label extension trial. Dermatol Surg. 2010;36(suppl 3):1800-1808.
- Prager W, Wissmueller E, Havermann I, et al. A prospective, split-face, randomized, comparative study of safety and 12-month longevity of three formulations of hyaluronic acid dermal filler for treatment of nasolabial folds. Dermatol Surg. 2012;38(7, pt 2):1143-1150.
- Butterwick K, Marmur E, Narurkar V, et al. HYC-24L demonstrates greater effectiveness with less pain than CPM-22.5 for treatment of perioral lines in a randomized controlled trial. Dermatol Surg. 2015;41:1351-1360.
- Jones D, Murphy DK. Volumizing hyaluronic acid filler for midface volume deficit: 2-year results from a pivotal single-blind randomized controlled study. Dermatol Surg. 2013;39:1602-1612.
- Baumann L, Narins RS, Beer K, et al. Volumizing hyaluronic acid filler for midface volume deficit: results after repeat treatment. Dermatol Surg. 2015;41(suppl 1):S284-S292.
- Few J, Cox SE, Paradkar-Mitragotri D, et al. A multicenter, single-blind randomized, controlled study of a volumizing hyaluronic acid filler for midface volume deficit: patient-reported outcomes at 2 years. Aesthet Surg J. 2015;35:589-599.
- Beer K, Glogau RG, Dover JS, et al. A randomized, evaluator-blinded, controlled study of effectiveness and safety of small particle hyaluronic acid plus lidocaine for lip augmentation and perioral rhytides. Dermatol Surg. 2015;41(suppl 1):S127-S136.
- Weiss RA, Moradi A, Bank D, et al. Effectiveness and safety of large gel particle hyaluronic acid with lidocaine for correction of midface volume deficit or contour deficiency. Dermatol Surg. 2016;42:699-709.
- Fitzgerald R, Graivier MH, Kane M, et al. Update on facial aging. Aesthet Surg J. 2010;30(suppl):S11-S24.
- Karnik J, Baumann L, Bruce S, et al. A double-blind, randomized, multicenter, controlled trial of suspended polymethylmethacrylate microspheres for the correction of atrophic facial acne scars. J Am Acad Dermatol. 2014;71:77-83.
- Bellafill [package insert]. San Diego, CA: Suneva Medical, Inc; 2015.
- Cohen S, Dover J, Monheit G, et al. Five-year safety and satisfaction study of PMMA-collagen in the correction of nasolabial folds. Dermatol Surg. 2015;41(suppl 1):S302-S313.
- Greene JJ, Sidle DM. The hyaluronic acid fillers: current understanding of the tissue device interface. Facial Plast Surg Clin North Am. 2015;23:423-432.
- Lorenc ZP, Fagien S, Flynn TC, et al. Review of key Belotero Balance safety and efficacy trials. Plast Reconstr Surg. 2013;132(4, suppl 2):33S-40S.
- Narins RS, Coleman W, Donofrio L, et al. Nonanimal sourced hyaluronic acid–based dermal filler using a cohesive polydensified matrix technology is superior to bovine collagen in the correction of moderate to severe nasolabial folds: results from a 6-month, randomized, blinded, controlled, multicenter study. Dermatol Surg. 2010;36(suppl 1):730-740.
- Narins RS, Coleman WP 3rd, Donofrio LM, et al. Improvement in nasolabial folds with a hyaluronic acid filler using a cohesive polydensified matrix technology: results from an 18-month open-label extension trial. Dermatol Surg. 2010;36(suppl 3):1800-1808.
- Prager W, Wissmueller E, Havermann I, et al. A prospective, split-face, randomized, comparative study of safety and 12-month longevity of three formulations of hyaluronic acid dermal filler for treatment of nasolabial folds. Dermatol Surg. 2012;38(7, pt 2):1143-1150.
- Butterwick K, Marmur E, Narurkar V, et al. HYC-24L demonstrates greater effectiveness with less pain than CPM-22.5 for treatment of perioral lines in a randomized controlled trial. Dermatol Surg. 2015;41:1351-1360.
- Jones D, Murphy DK. Volumizing hyaluronic acid filler for midface volume deficit: 2-year results from a pivotal single-blind randomized controlled study. Dermatol Surg. 2013;39:1602-1612.
- Baumann L, Narins RS, Beer K, et al. Volumizing hyaluronic acid filler for midface volume deficit: results after repeat treatment. Dermatol Surg. 2015;41(suppl 1):S284-S292.
- Few J, Cox SE, Paradkar-Mitragotri D, et al. A multicenter, single-blind randomized, controlled study of a volumizing hyaluronic acid filler for midface volume deficit: patient-reported outcomes at 2 years. Aesthet Surg J. 2015;35:589-599.
- Beer K, Glogau RG, Dover JS, et al. A randomized, evaluator-blinded, controlled study of effectiveness and safety of small particle hyaluronic acid plus lidocaine for lip augmentation and perioral rhytides. Dermatol Surg. 2015;41(suppl 1):S127-S136.
- Weiss RA, Moradi A, Bank D, et al. Effectiveness and safety of large gel particle hyaluronic acid with lidocaine for correction of midface volume deficit or contour deficiency. Dermatol Surg. 2016;42:699-709.
Practice Points
- The merits of new dermal fillers approved by the US Food and Drug Administration should be weighed with an understanding of aesthetic indications of use, duration of efficacy, and common adverse effects, in line with patient preference.
- The most common adverse effects are injection-site contusion, swelling, and pain, usually self-resolving within days to 2 weeks. Patient quality of care can be improved with forewarning and emphasis on alleviating symptoms.
Comment on “Merkel Cell Carcinoma in a Vein Graft Donor Site”
To the Editor:
A recent Cutis article, “Merkel Cell Carcinoma in a Vein Graft Donor Site” (Cutis. 2016;97:364-367), highlighted the localization of a Merkel cell carcinoma (MCC) within a well-healed scar resulting from a vein harvesting procedure performed 18 years prior to presentation. Their discussion focused on factors that may have contributed to the development of the MCC at that specific location. As noted by the authors, this case does not classically fit under the umbrella of a Marjolin ulcer given the stable, well-healed clinical appearance of the scar. We agree and believe it is not secondary to chance either but consistent with Wolf isotopic response.
This concept was originally described by Wyburn-Mason in 19551 and later revived by Wolf et al.2 Wolf isotopic response describes the development of dermatologic disorders that localize to a site of another distinct and clinically healed skin disorder. Originally, it was reserved for infections, malignancies, and immune conditions restricted to a site of a prior herpetic infection but recently has been expanded to encompass other primary nonherpesvirus-related skin disorders. The pathophysiology behind this phenomenon is unknown but thought to be the interplay of several key elements including immune dysregulation, neural, vascular, and locus minoris resistentiae (ie, a site of lessened resistance).3 Immunosuppression is a known risk factor in the development of MCCs,4 thus the proposed local immune dysregulation within a scar may alter the virus-host balance and foster the oncogenic nature of the MCC polyomavirus. A recent article describes another case of an MCC arising within a sternotomy scar,5 lending further credibility to a skin vulnerability philosophy. These cases provide further insight into the pathomechanisms involved in the development of this rare and aggressive neoplasm and sheds light on an intriguing dermatologic phenomenon.
- Wyburn-Mason R. Malignant change arising in tissues affected by herpes. Br Med J. 1955;2:1106-1109.
- Wolf R, Brenner S, Ruocco V, et al. Isotopic response. Int J Dermatol. 1995;34:341-348.
- Liu CI, Hsu CH. Leukaemia cutis at the site of striae distensae: an isotopic response? Acta Derm Venereol. 2010;90:422-423.
- Heath M, Jaimes N, Lemos B, et al. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the AEIOU features. J Am Acad Dermatol. 2008;58:375-381.
- Grippaudo FR, Costantino B, Santanelli F. Merkel cell carcinoma on a sternotomy scar: atypical clinical presentation. J Clin Oncol. 2015;33:e22-e24.
To the Editor:
A recent Cutis article, “Merkel Cell Carcinoma in a Vein Graft Donor Site” (Cutis. 2016;97:364-367), highlighted the localization of a Merkel cell carcinoma (MCC) within a well-healed scar resulting from a vein harvesting procedure performed 18 years prior to presentation. Their discussion focused on factors that may have contributed to the development of the MCC at that specific location. As noted by the authors, this case does not classically fit under the umbrella of a Marjolin ulcer given the stable, well-healed clinical appearance of the scar. We agree and believe it is not secondary to chance either but consistent with Wolf isotopic response.
This concept was originally described by Wyburn-Mason in 19551 and later revived by Wolf et al.2 Wolf isotopic response describes the development of dermatologic disorders that localize to a site of another distinct and clinically healed skin disorder. Originally, it was reserved for infections, malignancies, and immune conditions restricted to a site of a prior herpetic infection but recently has been expanded to encompass other primary nonherpesvirus-related skin disorders. The pathophysiology behind this phenomenon is unknown but thought to be the interplay of several key elements including immune dysregulation, neural, vascular, and locus minoris resistentiae (ie, a site of lessened resistance).3 Immunosuppression is a known risk factor in the development of MCCs,4 thus the proposed local immune dysregulation within a scar may alter the virus-host balance and foster the oncogenic nature of the MCC polyomavirus. A recent article describes another case of an MCC arising within a sternotomy scar,5 lending further credibility to a skin vulnerability philosophy. These cases provide further insight into the pathomechanisms involved in the development of this rare and aggressive neoplasm and sheds light on an intriguing dermatologic phenomenon.
To the Editor:
A recent Cutis article, “Merkel Cell Carcinoma in a Vein Graft Donor Site” (Cutis. 2016;97:364-367), highlighted the localization of a Merkel cell carcinoma (MCC) within a well-healed scar resulting from a vein harvesting procedure performed 18 years prior to presentation. Their discussion focused on factors that may have contributed to the development of the MCC at that specific location. As noted by the authors, this case does not classically fit under the umbrella of a Marjolin ulcer given the stable, well-healed clinical appearance of the scar. We agree and believe it is not secondary to chance either but consistent with Wolf isotopic response.
This concept was originally described by Wyburn-Mason in 19551 and later revived by Wolf et al.2 Wolf isotopic response describes the development of dermatologic disorders that localize to a site of another distinct and clinically healed skin disorder. Originally, it was reserved for infections, malignancies, and immune conditions restricted to a site of a prior herpetic infection but recently has been expanded to encompass other primary nonherpesvirus-related skin disorders. The pathophysiology behind this phenomenon is unknown but thought to be the interplay of several key elements including immune dysregulation, neural, vascular, and locus minoris resistentiae (ie, a site of lessened resistance).3 Immunosuppression is a known risk factor in the development of MCCs,4 thus the proposed local immune dysregulation within a scar may alter the virus-host balance and foster the oncogenic nature of the MCC polyomavirus. A recent article describes another case of an MCC arising within a sternotomy scar,5 lending further credibility to a skin vulnerability philosophy. These cases provide further insight into the pathomechanisms involved in the development of this rare and aggressive neoplasm and sheds light on an intriguing dermatologic phenomenon.
- Wyburn-Mason R. Malignant change arising in tissues affected by herpes. Br Med J. 1955;2:1106-1109.
- Wolf R, Brenner S, Ruocco V, et al. Isotopic response. Int J Dermatol. 1995;34:341-348.
- Liu CI, Hsu CH. Leukaemia cutis at the site of striae distensae: an isotopic response? Acta Derm Venereol. 2010;90:422-423.
- Heath M, Jaimes N, Lemos B, et al. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the AEIOU features. J Am Acad Dermatol. 2008;58:375-381.
- Grippaudo FR, Costantino B, Santanelli F. Merkel cell carcinoma on a sternotomy scar: atypical clinical presentation. J Clin Oncol. 2015;33:e22-e24.
- Wyburn-Mason R. Malignant change arising in tissues affected by herpes. Br Med J. 1955;2:1106-1109.
- Wolf R, Brenner S, Ruocco V, et al. Isotopic response. Int J Dermatol. 1995;34:341-348.
- Liu CI, Hsu CH. Leukaemia cutis at the site of striae distensae: an isotopic response? Acta Derm Venereol. 2010;90:422-423.
- Heath M, Jaimes N, Lemos B, et al. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the AEIOU features. J Am Acad Dermatol. 2008;58:375-381.
- Grippaudo FR, Costantino B, Santanelli F. Merkel cell carcinoma on a sternotomy scar: atypical clinical presentation. J Clin Oncol. 2015;33:e22-e24.
Necrotic Lesion of the Ear
The Diagnosis: Chondrodermatitis Nodularis Chronica Helicis
Histopathologic examination revealed focal epidermal erosion and ulceration directly overlying the hyaline cartilage with degenerative changes (Figure). The dermis was relatively noninflamed with fibroplasia of the vasculature. The blood vessels indirectly beneath the ulceration were found to be unremarkable with no indications of fibrinoid necrosis, vasculitis, or the presence of thrombi. The patient was informed of the diagnosis, at which point she reported that she slept on the right side. The excisional biopsy site healed well without recurrence of chondrodermatitis nodularis chronica helicis (CNH).
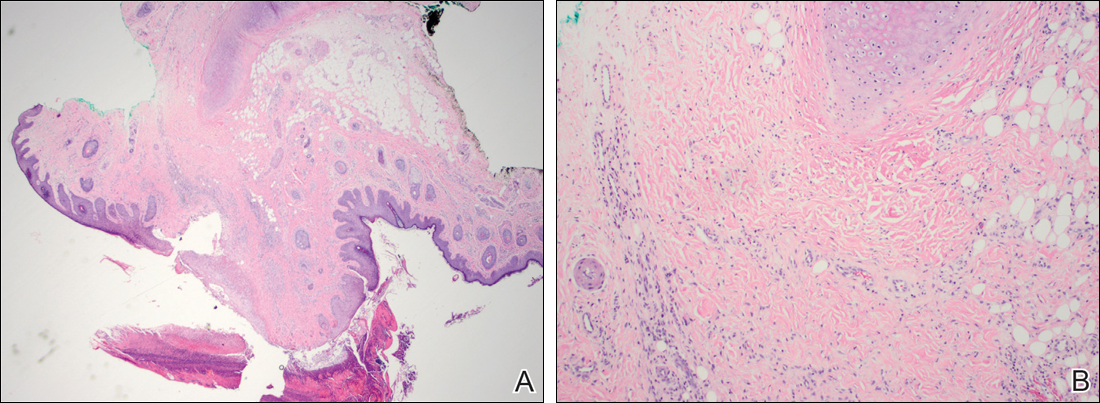
Chondrodermatitis nodularis chronica helicis, also known as clavus helicis, is a benign, usually solitary, painful lesion. Historically, it was first described in 1915 by Winkler1 and in the 1960s the most common documented cases were attributed to the headpieces of telephone operators and nuns.2 In the early 2000s, cell phones were determined to be a growing cause.3 Chondrodermatitis nodularis chronica helicis is most commonly found on the helix with the antihelix being affected less often.4 The condition is more common in men, with a male to female ratio being reported as high as 10:1. Possible causes of this disorder stem from damage to cartilage associated with pressure, sun exposure, cold temperatures, and microvascular disease. Additionally, some researchers have hypothesized that the cartilaginous damage resulting from solar elastosis and minor trauma leaves a susceptibility to CNH. This disorder usually presents as a small, exquisitely tender nodule that may ulcerate and crust.4 Chondrodermatitis nodularis chronica helicis may be mistaken for basal cell carcinoma, squamous cell carcinoma, actinic keratosis, and weathering nodules, though CNH tends to be more painful.
The diagnosis of CNH often is clinical but may require a skin biopsy. Histopathology of CNH shows a benign inflammatory lesion with an acanthotic hyperkeratotic epidermis that may be ulcerated. A primarily lymphocytic infiltrate usually is observed with variable presence of histiocytes and neutrophils. Cartilaginous changes range from simple perichondral thickening to notable areas of degeneration with calcification and ossification.4
Although the diagnosis of CNH often is straightforward, the remarkable necrosis present in our case made for an interesting differential diagnosis. Pernio, cryoglobulinemia, and levamisole-induced vasculopathy were all considered. Pernio, caused by cold-induced vasoconstriction and hypoxemia, classically presents as erythematous lesions with a symmetrical distribution on acral sites.5 Cryoglobulinemia involves proteins that precipitate at cold temperatures causing damage via an occlusive vasculopathy or an immune complex-mediated vasculitis. The presence of cryoglobulinemia is strongly associated with concomitant hepatitis C virus infection.6 Ulcerated and purpuric lesions of cryoglobulinemia may become necrotic. Levamisole is a veterinary antihelminthic drug and common cocaine contaminant, often added to cocaine as a cutting agent. Levamisole-induced vasculopathy favors acral sites and often is noted on the ears as purpuric patches, sometimes with necrosis.7
Several therapies for CNH have been reported with variable effectiveness.8 First-line treatments are the use of pressure-relieving devices including a doughnut-shaped pillow during sleep and intralesional corticosteroids.9 Surgical treatments including cryotherapy, simple excision, electrodesiccation and curettage, wedge resection with helical rim advancement flap, punch and graft technique, and CO2 laser have been tried.8 Photodynamic therapy and topical nitroglycerine also have shown to be of benefit.8,9
Our case of CNH is unique because of the remarkable degree of necrosis present on clinical examination. Chondrodermatitis nodularis chronica helicis with such an impressive necrotic presentation is rare. We speculate that the patient's underlying hypercoagulable state may have contributed to the dramatic presentation. It is important to keep CNH in mind when evaluating any necrotic lesion on the ear.
- Winkler M. Knötcehnformige Erkrankung am helix. chondrodermatitis nodularis chronic helicis. Arch für Dermatologie und Syphilis. 1915;121:278-285.
- Barker L, Young AW, Sachs W. Chondrodermatitis of the ears: a differential study of nodules of the helix and antihelix. Arch Dermatol. 1960;81:15-25.
- Elgart M. Cell phone chondrodermatitis. Arch Dermatol. 2000;136:1568.
- Cribier B, Scrivener Y, Peltre B. Neural hyperplasia in chondrodermatitis nodularis chronica helicis. J Am Acad Dermatol. 2006;55:844-848.
- King JM, Plotner AN, Adams BB. Perniosis induced by a cold-therapy system. Arch Dermatol. 2012;148:1101-1102.
- Berk DR, Mallory SB, Keeffe EB, et al. Dermatologic disorders associated with chronic hepatitis C: effect of interferon therapy. Clin Gastroenterol Hepatol. 2007;5:142-151.
- Hennings C, Miller J. Illicit drugs: what dermatologists need to know. J Am Acad Dermatol. 2013;69:135-142.
- Flynn V, Chisholm C, Grimwood R. Topical nitroglycerin: a promising treatment option for chondrodermatitis nodularis helicis. J Am Acad Dermatol. 2011;64:531-536.
- Gilaberte Y, Frias M, Pérez-Lorenz J. Chondrodermatitis nodularis helicis successfully treated with photodynamic therapy. Arch Dermatol. 2010;146:1080-1082.
The Diagnosis: Chondrodermatitis Nodularis Chronica Helicis
Histopathologic examination revealed focal epidermal erosion and ulceration directly overlying the hyaline cartilage with degenerative changes (Figure). The dermis was relatively noninflamed with fibroplasia of the vasculature. The blood vessels indirectly beneath the ulceration were found to be unremarkable with no indications of fibrinoid necrosis, vasculitis, or the presence of thrombi. The patient was informed of the diagnosis, at which point she reported that she slept on the right side. The excisional biopsy site healed well without recurrence of chondrodermatitis nodularis chronica helicis (CNH).

Chondrodermatitis nodularis chronica helicis, also known as clavus helicis, is a benign, usually solitary, painful lesion. Historically, it was first described in 1915 by Winkler1 and in the 1960s the most common documented cases were attributed to the headpieces of telephone operators and nuns.2 In the early 2000s, cell phones were determined to be a growing cause.3 Chondrodermatitis nodularis chronica helicis is most commonly found on the helix with the antihelix being affected less often.4 The condition is more common in men, with a male to female ratio being reported as high as 10:1. Possible causes of this disorder stem from damage to cartilage associated with pressure, sun exposure, cold temperatures, and microvascular disease. Additionally, some researchers have hypothesized that the cartilaginous damage resulting from solar elastosis and minor trauma leaves a susceptibility to CNH. This disorder usually presents as a small, exquisitely tender nodule that may ulcerate and crust.4 Chondrodermatitis nodularis chronica helicis may be mistaken for basal cell carcinoma, squamous cell carcinoma, actinic keratosis, and weathering nodules, though CNH tends to be more painful.
The diagnosis of CNH often is clinical but may require a skin biopsy. Histopathology of CNH shows a benign inflammatory lesion with an acanthotic hyperkeratotic epidermis that may be ulcerated. A primarily lymphocytic infiltrate usually is observed with variable presence of histiocytes and neutrophils. Cartilaginous changes range from simple perichondral thickening to notable areas of degeneration with calcification and ossification.4
Although the diagnosis of CNH often is straightforward, the remarkable necrosis present in our case made for an interesting differential diagnosis. Pernio, cryoglobulinemia, and levamisole-induced vasculopathy were all considered. Pernio, caused by cold-induced vasoconstriction and hypoxemia, classically presents as erythematous lesions with a symmetrical distribution on acral sites.5 Cryoglobulinemia involves proteins that precipitate at cold temperatures causing damage via an occlusive vasculopathy or an immune complex-mediated vasculitis. The presence of cryoglobulinemia is strongly associated with concomitant hepatitis C virus infection.6 Ulcerated and purpuric lesions of cryoglobulinemia may become necrotic. Levamisole is a veterinary antihelminthic drug and common cocaine contaminant, often added to cocaine as a cutting agent. Levamisole-induced vasculopathy favors acral sites and often is noted on the ears as purpuric patches, sometimes with necrosis.7
Several therapies for CNH have been reported with variable effectiveness.8 First-line treatments are the use of pressure-relieving devices including a doughnut-shaped pillow during sleep and intralesional corticosteroids.9 Surgical treatments including cryotherapy, simple excision, electrodesiccation and curettage, wedge resection with helical rim advancement flap, punch and graft technique, and CO2 laser have been tried.8 Photodynamic therapy and topical nitroglycerine also have shown to be of benefit.8,9
Our case of CNH is unique because of the remarkable degree of necrosis present on clinical examination. Chondrodermatitis nodularis chronica helicis with such an impressive necrotic presentation is rare. We speculate that the patient's underlying hypercoagulable state may have contributed to the dramatic presentation. It is important to keep CNH in mind when evaluating any necrotic lesion on the ear.
The Diagnosis: Chondrodermatitis Nodularis Chronica Helicis
Histopathologic examination revealed focal epidermal erosion and ulceration directly overlying the hyaline cartilage with degenerative changes (Figure). The dermis was relatively noninflamed with fibroplasia of the vasculature. The blood vessels indirectly beneath the ulceration were found to be unremarkable with no indications of fibrinoid necrosis, vasculitis, or the presence of thrombi. The patient was informed of the diagnosis, at which point she reported that she slept on the right side. The excisional biopsy site healed well without recurrence of chondrodermatitis nodularis chronica helicis (CNH).

Chondrodermatitis nodularis chronica helicis, also known as clavus helicis, is a benign, usually solitary, painful lesion. Historically, it was first described in 1915 by Winkler1 and in the 1960s the most common documented cases were attributed to the headpieces of telephone operators and nuns.2 In the early 2000s, cell phones were determined to be a growing cause.3 Chondrodermatitis nodularis chronica helicis is most commonly found on the helix with the antihelix being affected less often.4 The condition is more common in men, with a male to female ratio being reported as high as 10:1. Possible causes of this disorder stem from damage to cartilage associated with pressure, sun exposure, cold temperatures, and microvascular disease. Additionally, some researchers have hypothesized that the cartilaginous damage resulting from solar elastosis and minor trauma leaves a susceptibility to CNH. This disorder usually presents as a small, exquisitely tender nodule that may ulcerate and crust.4 Chondrodermatitis nodularis chronica helicis may be mistaken for basal cell carcinoma, squamous cell carcinoma, actinic keratosis, and weathering nodules, though CNH tends to be more painful.
The diagnosis of CNH often is clinical but may require a skin biopsy. Histopathology of CNH shows a benign inflammatory lesion with an acanthotic hyperkeratotic epidermis that may be ulcerated. A primarily lymphocytic infiltrate usually is observed with variable presence of histiocytes and neutrophils. Cartilaginous changes range from simple perichondral thickening to notable areas of degeneration with calcification and ossification.4
Although the diagnosis of CNH often is straightforward, the remarkable necrosis present in our case made for an interesting differential diagnosis. Pernio, cryoglobulinemia, and levamisole-induced vasculopathy were all considered. Pernio, caused by cold-induced vasoconstriction and hypoxemia, classically presents as erythematous lesions with a symmetrical distribution on acral sites.5 Cryoglobulinemia involves proteins that precipitate at cold temperatures causing damage via an occlusive vasculopathy or an immune complex-mediated vasculitis. The presence of cryoglobulinemia is strongly associated with concomitant hepatitis C virus infection.6 Ulcerated and purpuric lesions of cryoglobulinemia may become necrotic. Levamisole is a veterinary antihelminthic drug and common cocaine contaminant, often added to cocaine as a cutting agent. Levamisole-induced vasculopathy favors acral sites and often is noted on the ears as purpuric patches, sometimes with necrosis.7
Several therapies for CNH have been reported with variable effectiveness.8 First-line treatments are the use of pressure-relieving devices including a doughnut-shaped pillow during sleep and intralesional corticosteroids.9 Surgical treatments including cryotherapy, simple excision, electrodesiccation and curettage, wedge resection with helical rim advancement flap, punch and graft technique, and CO2 laser have been tried.8 Photodynamic therapy and topical nitroglycerine also have shown to be of benefit.8,9
Our case of CNH is unique because of the remarkable degree of necrosis present on clinical examination. Chondrodermatitis nodularis chronica helicis with such an impressive necrotic presentation is rare. We speculate that the patient's underlying hypercoagulable state may have contributed to the dramatic presentation. It is important to keep CNH in mind when evaluating any necrotic lesion on the ear.
- Winkler M. Knötcehnformige Erkrankung am helix. chondrodermatitis nodularis chronic helicis. Arch für Dermatologie und Syphilis. 1915;121:278-285.
- Barker L, Young AW, Sachs W. Chondrodermatitis of the ears: a differential study of nodules of the helix and antihelix. Arch Dermatol. 1960;81:15-25.
- Elgart M. Cell phone chondrodermatitis. Arch Dermatol. 2000;136:1568.
- Cribier B, Scrivener Y, Peltre B. Neural hyperplasia in chondrodermatitis nodularis chronica helicis. J Am Acad Dermatol. 2006;55:844-848.
- King JM, Plotner AN, Adams BB. Perniosis induced by a cold-therapy system. Arch Dermatol. 2012;148:1101-1102.
- Berk DR, Mallory SB, Keeffe EB, et al. Dermatologic disorders associated with chronic hepatitis C: effect of interferon therapy. Clin Gastroenterol Hepatol. 2007;5:142-151.
- Hennings C, Miller J. Illicit drugs: what dermatologists need to know. J Am Acad Dermatol. 2013;69:135-142.
- Flynn V, Chisholm C, Grimwood R. Topical nitroglycerin: a promising treatment option for chondrodermatitis nodularis helicis. J Am Acad Dermatol. 2011;64:531-536.
- Gilaberte Y, Frias M, Pérez-Lorenz J. Chondrodermatitis nodularis helicis successfully treated with photodynamic therapy. Arch Dermatol. 2010;146:1080-1082.
- Winkler M. Knötcehnformige Erkrankung am helix. chondrodermatitis nodularis chronic helicis. Arch für Dermatologie und Syphilis. 1915;121:278-285.
- Barker L, Young AW, Sachs W. Chondrodermatitis of the ears: a differential study of nodules of the helix and antihelix. Arch Dermatol. 1960;81:15-25.
- Elgart M. Cell phone chondrodermatitis. Arch Dermatol. 2000;136:1568.
- Cribier B, Scrivener Y, Peltre B. Neural hyperplasia in chondrodermatitis nodularis chronica helicis. J Am Acad Dermatol. 2006;55:844-848.
- King JM, Plotner AN, Adams BB. Perniosis induced by a cold-therapy system. Arch Dermatol. 2012;148:1101-1102.
- Berk DR, Mallory SB, Keeffe EB, et al. Dermatologic disorders associated with chronic hepatitis C: effect of interferon therapy. Clin Gastroenterol Hepatol. 2007;5:142-151.
- Hennings C, Miller J. Illicit drugs: what dermatologists need to know. J Am Acad Dermatol. 2013;69:135-142.
- Flynn V, Chisholm C, Grimwood R. Topical nitroglycerin: a promising treatment option for chondrodermatitis nodularis helicis. J Am Acad Dermatol. 2011;64:531-536.
- Gilaberte Y, Frias M, Pérez-Lorenz J. Chondrodermatitis nodularis helicis successfully treated with photodynamic therapy. Arch Dermatol. 2010;146:1080-1082.
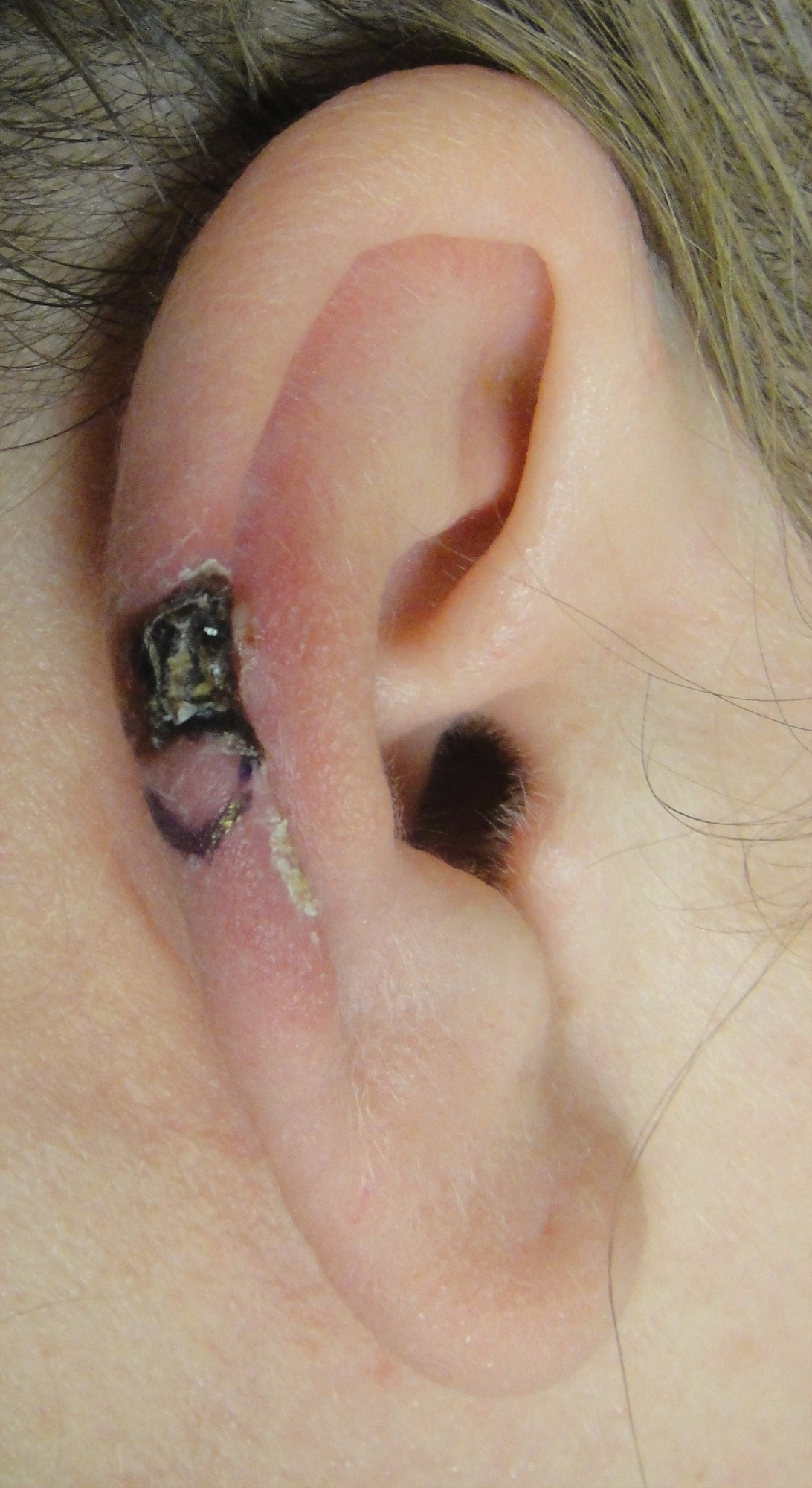
A 43-year-old woman presented with a painful necrotic lesion on the right ear of 1 month's duration. She denied trauma to the ear and had no other skin lesions elsewhere on the body. A course of doxycycline prior to presentation did not result in improvement. Her medical history was remarkable for diabetes mellitus, deep vein thrombosis, depression, and gastroesophageal reflux disease. She had been taking warfarin regularly for years. She denied using recreational drugs. On physical examination, the right ear demonstrated a 6-mm necrotic area with surrounding tender erythema. Examinations of the left ear, face, and legs were normal. An excisional biopsy was performed.
Periocular Fillers and Related Anatomy
Rejuvenation of the periocular area is in high demand among patients who want to look and feel their best. Physicians should understand the complicated anatomy surrounding the eyes before attempting to inject this area with facial fillers, both to understand the aging process and to minimize treatment complications.
Basic Oculoplastic and Orbital Anatomy
The injector should understand the anatomy of the periocular muscles, the orbital osteology, and the secretory and lacrimal system, in addition to the fat, ligaments, and vascular anatomy in this area.1
The eyes are surrounded by fat compartments that provide glide planes for the motion of the eyelids and globe. There are 2 upper eyelid fat-pads—nasal and central [preaponeurotic])—in the upper lid, leaving room for the lacrimal gland laterally. There are 3 fat compartments—nasal, central, and lateral—in the lower eyelid. The nasal and central compartments are separated by the inferior oblique muscle, which elevates and extorts the eye. The orbital septum holds the fat-pads in place in the orbit. The brow fat-pad is the retro-orbicularis oculi fat-pad (ROOF). There are fat compartments that lie in the subcutaneous space along the entire forehead and in the temple. The suborbicularis oculi fat-pad (SOOF) lies over the malar eminence. Superficial and deep submuscular fat compartments of the face have been described.2 Deep fat compartments also have been examined on computed tomography.3
Orbital circulation comes from the internal carotid artery and anastomoses with the supply from the external carotid artery to supply the orbit. The first branch off of the carotid artery is the ophthalmic artery, and the first branch off of the ophthalmic artery is the central retinal artery that enters the optic nerve sheath 1 cm behind the globe to supply the retina. The supraorbital and supratrochlear arteries branch off of the ophthalmic artery and supply the forehead. The supraorbital artery runs through the supraorbital notch (foramen in 8%)1 and can usually be palpated with one’s finger. There are 15 to 20 short posterior ciliary arteries leading to the choroid, 2 long posterior ciliary arteries to the iris circle, and 7 anterior ciliary arteries to the extraocular muscles. The superior and inferior venous systems drain into the cavernous sinus.4
The ligaments are important to signs of facial aging because tissue atrophy occurs along them. The main orbital ligaments are the lateral orbital thickening (known as the LOT) that adheres the eyelids to the lateral orbital rim and the orbitomalar ligament (orbicularis retaining ligament), which is a condensation fibrous tissue that attaches the skin to the inferior orbital rim and orbital septum along the arcus marginalis and defines the superior edge of the SOOF.5 The zygomatic ligament not only suspends the zygomaticus major and zygomaticus minor muscles to the malar eminence but there are osseocutaneous attachments that connect the skin over the zygoma’s malar eminence and demarcate the inferior edge of the SOOF.6
Periocular Aging
The skin, fat, muscles, and bones change and rotate with aging, and not all orbits age in the same manner. Older patients with dermatochalasis (excess skin fat and muscle) often undergo rejuvenation with blepharoplasty, a brow-lift, and a midface-lift, but many atrophic changes can be improved with facial fillers.7,8
As adults age, the soft tissue along the ligaments begins to show atrophy, prime signs of aging that are often improved with fillers. Atrophy along the orbitomalar ligament along the infraorbital rim creates a depressed tear trough, which is an early sign of aging. A 3-point grading system reported by Hirmand8 describes the severity of progressive hallowing. There also is atrophy along the zygomatic cutaneous ligament that creates the malar hollow. The SOOF appears to be more prominent when these areas above and below show atrophy, which creates the look of an unwanted bag known as a festoon. Additionally, there is atrophy along the superior orbital notch where the ophthalmic branch of the trigeminal nerve (V1) and the supraorbital artery traverse. Soft-tissue atrophy along the supraorbital notch resembles the peak at the top of the letter A, giving the slang term A-frame deformity.
Periocular fat can atrophy, hypertrophy, herniate forward as the septum weakens, or become ptotic. Some patients develop hypertrophy and herniation of the superior and inferior orbital fat-pads, while others develop unwanted atrophy leaving a hollow superior orbit and loss of support to the levator muscle that contributes to eyelid ptosis. The frontalis fat deflates, leaving veins, arteries, and the hypertrophied corrugators unwantedly visible. Loss of subcutaneous fat in the glabella contributes to the formation of frown lines between the brows (also called number 11’s). The ROOF deflates in some patients adding to brow ptosis. Loss of the facial frame occurs when temple fat atrophies.
Skeletal rotation also occurs. Throughout a patient’s life, the skeleton remodels itself via activity of osteoclasts and osteoblasts. Pessa et al9,10 has described the expansion of the anterior orbital aperture superomedially and inferolaterally as well as maxillary retrusion that results in angular changes of the midface in relation to the orbital rim. Lambros’ algorithm describes the rotational changes of the cranium where the superior orbit protrudes as the maxilla retreats posteriorly.9-11 The equator of the globe does not change its distance from the ROOF of the orbit, presumably because of its suspension in the orbit by the optic nerve after it passes through the optic canal and trochlea via the superior oblique muscle, but the distance of the inferior equator of the globe to the floor of the orbit increases as the floor of the orbit descends.12
Dermal Fillers for Periocular Rejuvenation
Hyaluronic acid (HA) was first pioneered for use in humans in the late 1970s by ophthalmologists for anterior segment surgery.13-15 Biocompatibility for orthopedic and dermal applications was explored in the early 1990s.16
At this time, no dermal filler is approved by the US Food and Drug Administration for use in the periorbital area. Some fillers are approved for subdermal areas extending to the preperiosteal plane and can be used in the midface such as HA fillers (eg, Restylane Lyft [Galderma Laboratories, LP]), Juvéderm Voluma XC [Allergan, Inc]), poly-L-lactic acid (PLLA), and calcium hydroxylapatite (CaHA). No dermal fillers are approved for use in the forehead, glabella, or temples. Their use is becoming increasingly popular but is considered off label. In addition, cannulas are not approved for use in these areas. Cannulas may be beneficial in that they are thought to create less bruising and have less chance of entering a vessel than needles, but some injectors prefer needles because they are stiffer and therefore more precise.
The ideal filler for the tear trough, superior sulcus, ROOF, over the orbitomalar ligament, forehead, and glabella is one that is somewhat moldable but does not migrate, is not hydrophilic, is smooth to inject, and is reversible should there be any complications. No single filler fits this ideal description, but HAs typically are the first choice.
In vitro studies to determine the stiffness (G') and the ability to flow (viscosity) have been performed.17,18 Calcium hydroxylapatite has the most stiffness, while Belotero Balance (Merz Aesthetics) and Juvéderm Ultra XC (Allergan, Inc) are more soft17 (Table). These guidelines are important but may not correlate directly with how the fillers behave in vivo as demonstrated in animal models.18
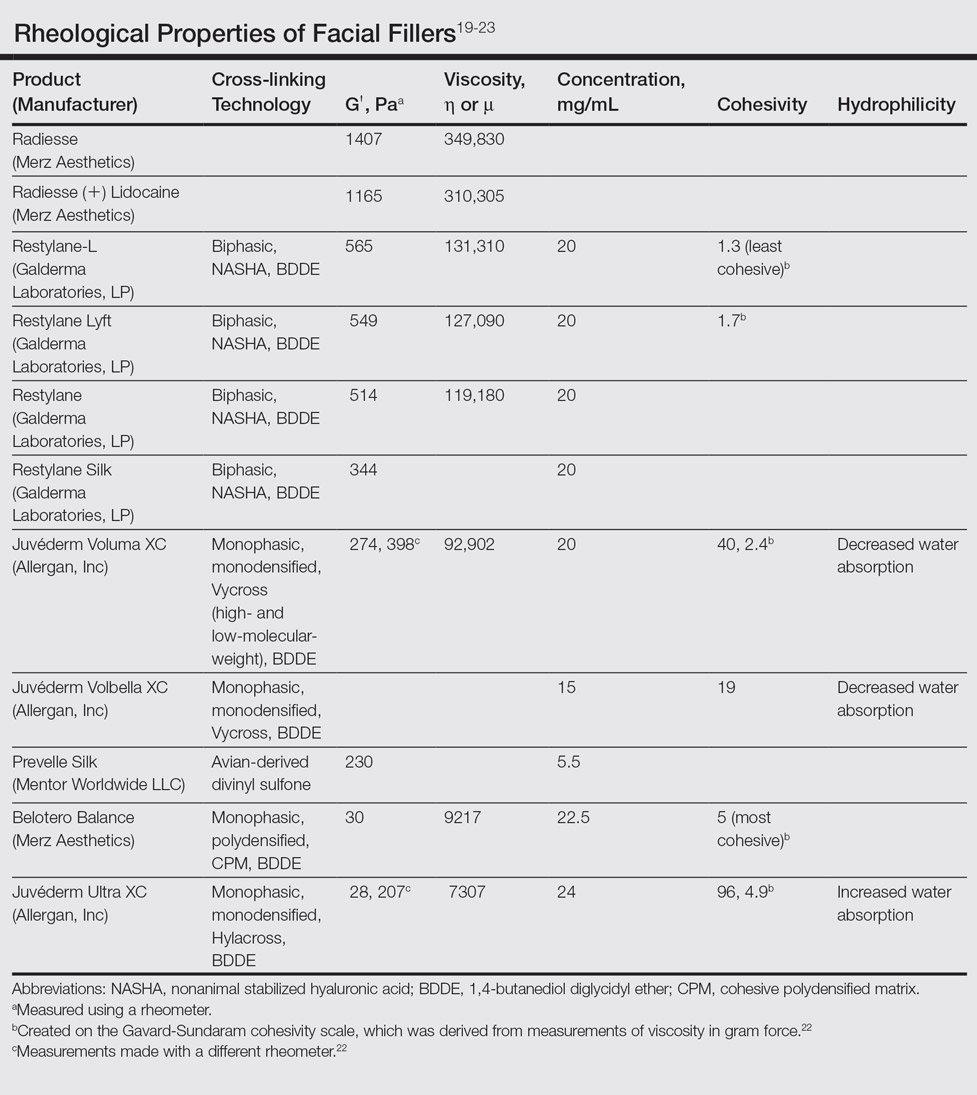
Hyaluronic acid fillers are produced by different technologies to create their cross-link patterns with 1,4-butanediol diglycidyl ether, which determines, to some degree, their behavior in human tissue. Fillers are either monophasic; monodensified; formed by Hylacross (Juvéderm), Vycross (Juvéderm Voluma XC, Juvéderm Volbella XC), or cohesive polydensified matrix technology (Belotero Balance), or biphasic, formed by nonanimal stabilized HA sieving technology (Restylane family). Biopsy has demonstrated that monophasic fillers tend to percolate through and integrate into the tissue, while biphasic fillers dissect tissue to the sides to create a potential space for the filler to reside (Table).24
Periocular Injection Considerations
An experienced injector is one who has developed not only an artistic eye for the face and excellent sense of anatomy but also has a sensitive ability to predict the filler-tissue interaction based on tactile feedback dependent on 3 main qualities: (1) stiffness and viscosity of the filler, (2) gauge of the needle or cannula, and (3) depth of the needle in the tissue. Periocular injections of dermal fillers can be delivered with needles or cannulas, diluted or undiluted. Smaller-gauge needles require more force than larger-gauge needles and cannulas that flow more freely. A needle in the dense dermis will require more force than one placed in the loose subcutaneous space.
The tear trough is generally preferable to fill with a mid-level G' HA filler that is less apt to migrate. A neutral gaze during the injection is preferred because closing or moving the eyes can distort the position of the inferior orbital fat-pads (Figure 1). A needle or cannula can be used, diluted or undiluted. The tear trough can be filled with the injection directed horizontally or vertically via a fanning technique. If needles are used, the skin should be stretched to view the 3 to 5 vertical veins and then the needle should be advanced beneath them to avoid bruising. Avoidance of hydrophilic fillers in the tear trough is important to avoid edema. The superior sulcus can be filled both anteriorly and posteriorly to the septum, which is a highly advanced injection for experienced injectors because of the proximity to the supratrochlear and supraorbital arteries as well as the superior ophthalmic vein (Figure 2). Sharp creases such as deep lateral periocular rhytides known as crow’s-feet are nicely filled with intradermal HAs with a low G'.
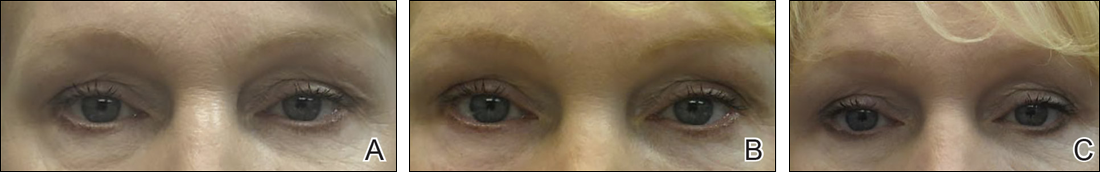
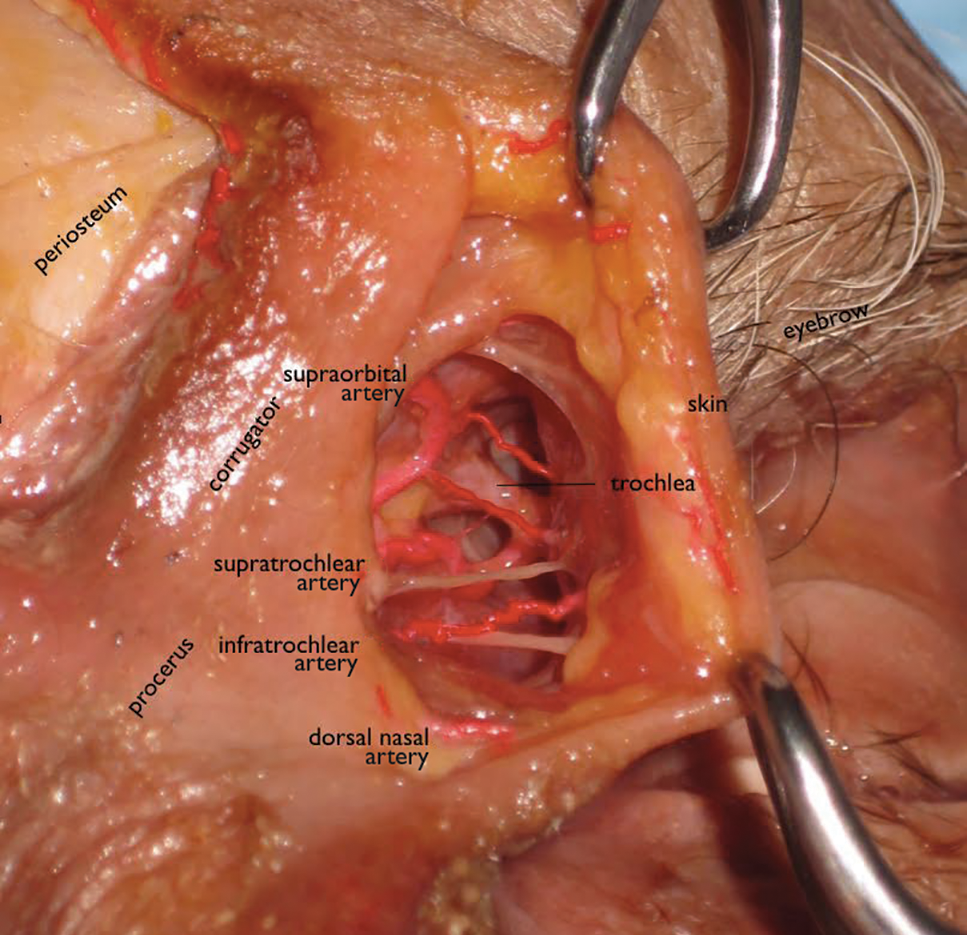
Adding volume to the midface is important because it is the continuum of the lower eyelid. Fillers can be injected into multiple levels in this area: deep (to act as pillars to lift the malar eminence and replace bone that has rotated and soft tissue that has become atrophic or descended) and subcutaneous (to efface soft tissue along the zygomatic cutaneous ligament). Higher G' HA fillers and CaHA often are used in the midface along with PLLA. Facial framing of the temples, lateral cheeks, and preauricular area is often accomplished with PLLA but also can be done with mid to high G' HA fillers or CaHA. A cannula may be used to undermine and break apart the zygomatic cutaneous ligament’s cutaneous attachments prior to delivery of the filler in the subcutaneous plane.26 If not done, filler may track away from the hollow area where the ligament is attached and instead move to adjacent areas that will accentuate the hollow and make it look worse.
The temples and lateral face often are filled with PLLA for framing. Mid or high G' HA fillers and CaHA also are used in the temples both beneath the temporalis muscle and also above the deep temporalis fascia or sometimes in the subcutaneous plane.27
Prevention and Management of Periocular Complications
Blindness is the most devastating periocular complication of facial fillers, which is caused by retrograde arterial embolization followed by anterograde flow into the ophthalmic then central retinal arteries. Injectables that have caused blindness include (in descending order of frequency) fat, HA, collagen, paraffin, polymethyl methacrylate, silicone, PLLA, CaHA, polyacrylamide hydrogel, and micronized acellular dermal matrix. Of the 98 cases of blindness from periocular complications from dermal fillers reported in the world literature, the order of affected sites include the glabella (38 cases), nose (25), nasolabial folds (13), superior forehead (12), infraorbital rim (6), temples (1), malar area (1), lip (1), and chin (1). Prevention includes avoidance of danger zone arteries including the supratrochlear, supraorbital, dorsal nasal, angular, infraorbital, zygomaticofacial and zygomaticotemporal arteries.28
Avoiding the average critical volume of 0.84 in any single aliquot dispensed is key to avoid filling of these periocular arteries to the critical bifurcation point that can result in anterograde flow into the eye (Freudenthal Nicolau syndrome). The smallest supratrochlear artery’s volume in this study was 0.04 cc, so aliquots that do not exceed 0.03 cc are ideal.29,30
The injector should always be thinking about the anatomy of the danger zones (eg, infratrochlear and supratrochlear arteries, supraorbital artery, frontal branch of the superficial temporal artery, lacrimal artery, dorsal nasal artery, infraorbital artery, angular artery, zygomaticofacial artery, zygomaticotemporal artery)(Figure 3).
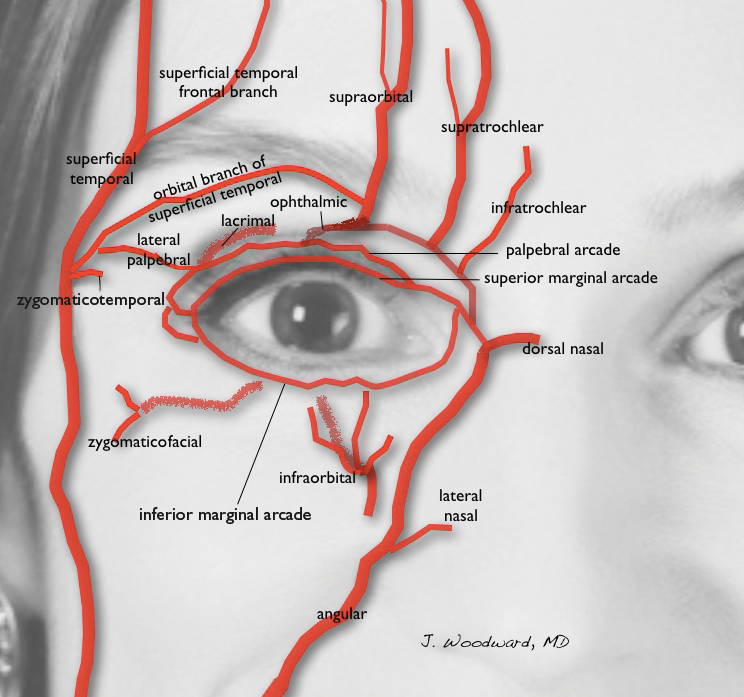
Hyaluronidase can be used off label to hydrolyze unwanted HA. It was first used to aid transcutaneous hydration and was used by ophthalmologists in the 1960s and 1970s to promote the spread of anesthetics by retrobulbar injection.31,32 It can penetrate through soft tissues and blood vessels.33 It is therefore hypothesized that a retrobulbar injection of hyaluronidase could aid in a case of impending blindness34 but has not been successfully accomplished to date. If vision is confirmed to be poor or there is no light perception, a retrobulbar injection of 300 U of hyaluronidase should be given immediately and then repeated in approximately 30 to 45 minutes. The retina begins to show permanent loss of function after being deprived of blood flow for just 97 minutes,35 so there may not be time for an immediate ophthalmology consultation, though such a consultation would be ideal.
Aside from common complications such as bruising and swelling, granulomas and biofilms are well documented in the literature. There are a variety of algorithms to treat such complications, which can happen many weeks after the injection of a dermal filler or years after the injection of a semipermanent filler.36 Postinjection periocular edema can occur years after the initial injection.37,38 Other periocular complications of dermal fillers include nonischemic (eg, bluish hue, filler migration, infection, inflammation, lumps) and ischemic (eg, blindness, necrosis, ophthalmoplegia, ptosis) disturbances.
Conclusion
In summary, periocular injections of facial fillers are useful tools for rejuvenation of the upper face when used with great caution and respect for anatomy.
- Foster J, ed. Orbit, Eyelids, and Lacrimal System. San Francisco, CA: American Academy of Ophthalmology; 2016. 2016-2017 Basic and Clinical Science Course; section 7.
- Rohrich RJ, Pessa JE. The fat compartments of the face: anatomy and clinical implications for cosmetic surgery. Plast Reconstr Surg. 2007;119:2219-2227; discussion 2228-2231.
- Gierloff M, Stöhring C, Buder T, et al. Aging changes of the midfacial fat compartments: a computed tomographic study. Plast Reconst Surg. 2012;129:263-273.
- Zide BM, Jelks GW. Surgical Anatomy of the Orbit: The System of Zones. Philadelphia, PA: Lippincott Williams & Wilkins; 2006.
- Kikkawa DO, Lemke BN, Dortzbach RK. Relations of the superficial musculoaponeurotic system to the orbit and characterization of the oribitomalar ligament. Ophthal Plast Reconstr Surg. 1996;12:77-88.
- Furnas DW. The retaining ligaments of the cheek. Plast Reconstr Surg. 1989;83:11-16.
- Morley AM, Taban M, Malhotra R, et al. Use of hyaluronic acid gel for upper eyelid filling and contouring. Ophthal Plast Reconstr Surg. 2009;25:440-444.
- Hirmand H. Anatomy and nonsurgical correction of the tear trough deformity. Plast Reconstr Surg. 2010;125:699-708.
- Pessa JE, Zadoo VP, Mutimer KL, et al. Relative maxillary retrusion as a natural consequence of aging. Plast Reconstr Surg. 1998;102:205-212.
- Pessa JE, Desvigne LD, Lambros VS, et al. Changes in ocular globe-to-orbital rim position with age: implications for aesthetic blepharoplasty of the lower eyelids. Aesthet Plast Surg. 1999;23:337-345.
- Goldberg RA, Relan A, Hoenig J. Relationship of the eye to the bony orbit, with clinical correlations. Aust N Z J Ophthalmol. 1999;27:398-403.
- Richard MJ, Morris C, Deen BF, et al. Analysis of the anatomic changes of the aging facial skeleton using computer-assisted tomography. Ophthal Plast Reconstr Surg. 2009;25:382-386.
- Miller D, O’Connor P, Williams J. Use of Na-hyaluronate during intraocular lens implantation in rabbits. Ophthalmic Surg. 1977;8:58-61.
- Miller D, Stegmann R. Use of Na-hyaluronate in anterior segment eye surgery. J Am Intraocul Implant Soc. 1980;6:13-15.
- Pape LG, Balazs EA. The use of sodium hyaluronate (Healon) in human anterior segment surgery. Ophthalmology. 1980;87:699-705.
- Larsen NE, Pollak CT, Reiner K, et al. Hylan gel biomaterial: dermal and immunologic compatibility. J Biomed Mater Res. 1993;27:1129-1134.
- Sundaram H, Cassuto D. Biophysical characteristics of hyaluronic acid soft-tissue fillers and their relevance to aesthetic applications. Plast Reconstr Surg. 2013;132(4, suppl 2):5S-21S.
- Hee CK, Shumate GT, Narurkar V, et al. Rheological properties and in vivo performance characteristics of soft tissue fillers. Dermatol Surg. 2015;41(suppl 1):S373-S381.
- Sundaram H, Voigts B, Beer K, et al. Comparison of the rheological properties of viscosity and elasticity in two categories of soft tissue fillers: calcium hydroxylapatite and hyaluronic acid. Dermatol Surg. 2010;36(suppl 3):1859-1865.
- Sundaram H. The new face of fillers: a multi-specialty CME initiative: supplement part II of II. J Drugs Dermatol. 2012;11(suppl 8):S8.
- Stocks D, Sundaram H, Michaels J, et al. Rheological evaluation of the physical properties of hyaluronic acid dermal fillers. J Drugs Dermatol. 2011;10:974-980.
- Goodman GJ, Swift A, Remington BK. Current concepts in the use of Voluma, Volift, and Volbella. Plast Reconstr Surg. 2015;136(suppl 5):139S-148S.
- Sundaram H, Rohrich RJ, Liew S, et al. Cohesivity of hyaluronic acid fillers: development and clinical implications of a novel assay, pilot validation with a five-point grading scale and evaluation of six U.S. Food and Drug Administration–approved fillers. Plast Reconstr Surg. 2015;136:678-686.
- Flynn TC, Sarazin D, Bezzola A, et al. Comparative histology of intradermal implantation of mono and biphasic hyaluronic acid fillers. Dermatol Surg. 2011;37:637-643.
- Woodward JA, Langelier N. Filler enhancement of the superior periocular area [published online Jun 23, 2016]. JAMA Facial Plast Surg. doi:10.1001/jamafacial.2016.0636.
- Cotofana S, Schenck TL, Trevidic P, et al. Midface: clinical anatomy and regional approaches with injectable fillers. Plast Reconstr Surg. 2015;136(suppl 5):219S-234S.
- Buckingham ED, Glasgold R, Kontis T, et al. Volume rejuvenation of the facial upper third. Facial Plast Surg. 2015;31:43-54.
- Beleznay K, Carruthers JD, Humphrey S, et al. Avoiding and treating blindness from fillers: a review of the world literature. Dermatol Surg. 2015;41:1097-1117.
- Coleman SR. Avoidance of arterial occlusion from injection of soft tissue fillers. Aesthet Surg J. 2002;22:555-557.
- Khan T, Colon-Acevedo B, Mettu P, et al. An anatomical analysis of the supratrochlear artery: considerations in facial filler injections and preventing vision loss [published online August 16, 2016]. Aesthet Surg J. pii: sjw132.
- Iserle J, Kumstat Z. Retrobulbar injections of hyaluronidase as a method of increasing safety in cataract surgery [in Czech]. Cesk Oftalmol. 1960;15:126-130.
- Wojtowicz S. Effect of retrobulbar injections of novocaine and lignocaine with adrenalin and hyaluronidase for the immobilization of the eye in electromyography [in Polish]. Klin Oczna. 1964;34:285-296.
- Delorenzi C. Transarterial degradation of hyaluronic acid filler by hyaluronidase. Dermatol Surg. 2014;40:832-841.
- Carruthers J, Fagien S, Dolman P. Retro or peribulbar injections techniques to reverse visual loss after filler injections. 2015;41(suppl 1):S354-S357.
- Hayreh SS, Zimmerman MB, Kimura A, et al. Central retinal artery occlusion. retinal survival time. Exp Eye Res. 2004;78:723-736.
- Woodward J, Khan T, Martin J. Facial filler complications. Facial Plast Surg Clin North Am. 2015;23:447-458.
- Khan TT, Woodward JA. Retained dermal filler in the upper eyelid masquerading as periorbital edema. Dermatol Surg. 2015;41:1182-1184.
- Chang JR, Baharestani S, Salek SS, et al. Delayed superficial migration of retained hyaluronic acid years following periocular injection [published online April 20, 2015]. Ophthal Plast Reconstr Surg. doi:10.1097/IOP.0000000000000434.
Rejuvenation of the periocular area is in high demand among patients who want to look and feel their best. Physicians should understand the complicated anatomy surrounding the eyes before attempting to inject this area with facial fillers, both to understand the aging process and to minimize treatment complications.
Basic Oculoplastic and Orbital Anatomy
The injector should understand the anatomy of the periocular muscles, the orbital osteology, and the secretory and lacrimal system, in addition to the fat, ligaments, and vascular anatomy in this area.1
The eyes are surrounded by fat compartments that provide glide planes for the motion of the eyelids and globe. There are 2 upper eyelid fat-pads—nasal and central [preaponeurotic])—in the upper lid, leaving room for the lacrimal gland laterally. There are 3 fat compartments—nasal, central, and lateral—in the lower eyelid. The nasal and central compartments are separated by the inferior oblique muscle, which elevates and extorts the eye. The orbital septum holds the fat-pads in place in the orbit. The brow fat-pad is the retro-orbicularis oculi fat-pad (ROOF). There are fat compartments that lie in the subcutaneous space along the entire forehead and in the temple. The suborbicularis oculi fat-pad (SOOF) lies over the malar eminence. Superficial and deep submuscular fat compartments of the face have been described.2 Deep fat compartments also have been examined on computed tomography.3
Orbital circulation comes from the internal carotid artery and anastomoses with the supply from the external carotid artery to supply the orbit. The first branch off of the carotid artery is the ophthalmic artery, and the first branch off of the ophthalmic artery is the central retinal artery that enters the optic nerve sheath 1 cm behind the globe to supply the retina. The supraorbital and supratrochlear arteries branch off of the ophthalmic artery and supply the forehead. The supraorbital artery runs through the supraorbital notch (foramen in 8%)1 and can usually be palpated with one’s finger. There are 15 to 20 short posterior ciliary arteries leading to the choroid, 2 long posterior ciliary arteries to the iris circle, and 7 anterior ciliary arteries to the extraocular muscles. The superior and inferior venous systems drain into the cavernous sinus.4
The ligaments are important to signs of facial aging because tissue atrophy occurs along them. The main orbital ligaments are the lateral orbital thickening (known as the LOT) that adheres the eyelids to the lateral orbital rim and the orbitomalar ligament (orbicularis retaining ligament), which is a condensation fibrous tissue that attaches the skin to the inferior orbital rim and orbital septum along the arcus marginalis and defines the superior edge of the SOOF.5 The zygomatic ligament not only suspends the zygomaticus major and zygomaticus minor muscles to the malar eminence but there are osseocutaneous attachments that connect the skin over the zygoma’s malar eminence and demarcate the inferior edge of the SOOF.6
Periocular Aging
The skin, fat, muscles, and bones change and rotate with aging, and not all orbits age in the same manner. Older patients with dermatochalasis (excess skin fat and muscle) often undergo rejuvenation with blepharoplasty, a brow-lift, and a midface-lift, but many atrophic changes can be improved with facial fillers.7,8
As adults age, the soft tissue along the ligaments begins to show atrophy, prime signs of aging that are often improved with fillers. Atrophy along the orbitomalar ligament along the infraorbital rim creates a depressed tear trough, which is an early sign of aging. A 3-point grading system reported by Hirmand8 describes the severity of progressive hallowing. There also is atrophy along the zygomatic cutaneous ligament that creates the malar hollow. The SOOF appears to be more prominent when these areas above and below show atrophy, which creates the look of an unwanted bag known as a festoon. Additionally, there is atrophy along the superior orbital notch where the ophthalmic branch of the trigeminal nerve (V1) and the supraorbital artery traverse. Soft-tissue atrophy along the supraorbital notch resembles the peak at the top of the letter A, giving the slang term A-frame deformity.
Periocular fat can atrophy, hypertrophy, herniate forward as the septum weakens, or become ptotic. Some patients develop hypertrophy and herniation of the superior and inferior orbital fat-pads, while others develop unwanted atrophy leaving a hollow superior orbit and loss of support to the levator muscle that contributes to eyelid ptosis. The frontalis fat deflates, leaving veins, arteries, and the hypertrophied corrugators unwantedly visible. Loss of subcutaneous fat in the glabella contributes to the formation of frown lines between the brows (also called number 11’s). The ROOF deflates in some patients adding to brow ptosis. Loss of the facial frame occurs when temple fat atrophies.
Skeletal rotation also occurs. Throughout a patient’s life, the skeleton remodels itself via activity of osteoclasts and osteoblasts. Pessa et al9,10 has described the expansion of the anterior orbital aperture superomedially and inferolaterally as well as maxillary retrusion that results in angular changes of the midface in relation to the orbital rim. Lambros’ algorithm describes the rotational changes of the cranium where the superior orbit protrudes as the maxilla retreats posteriorly.9-11 The equator of the globe does not change its distance from the ROOF of the orbit, presumably because of its suspension in the orbit by the optic nerve after it passes through the optic canal and trochlea via the superior oblique muscle, but the distance of the inferior equator of the globe to the floor of the orbit increases as the floor of the orbit descends.12
Dermal Fillers for Periocular Rejuvenation
Hyaluronic acid (HA) was first pioneered for use in humans in the late 1970s by ophthalmologists for anterior segment surgery.13-15 Biocompatibility for orthopedic and dermal applications was explored in the early 1990s.16
At this time, no dermal filler is approved by the US Food and Drug Administration for use in the periorbital area. Some fillers are approved for subdermal areas extending to the preperiosteal plane and can be used in the midface such as HA fillers (eg, Restylane Lyft [Galderma Laboratories, LP]), Juvéderm Voluma XC [Allergan, Inc]), poly-L-lactic acid (PLLA), and calcium hydroxylapatite (CaHA). No dermal fillers are approved for use in the forehead, glabella, or temples. Their use is becoming increasingly popular but is considered off label. In addition, cannulas are not approved for use in these areas. Cannulas may be beneficial in that they are thought to create less bruising and have less chance of entering a vessel than needles, but some injectors prefer needles because they are stiffer and therefore more precise.
The ideal filler for the tear trough, superior sulcus, ROOF, over the orbitomalar ligament, forehead, and glabella is one that is somewhat moldable but does not migrate, is not hydrophilic, is smooth to inject, and is reversible should there be any complications. No single filler fits this ideal description, but HAs typically are the first choice.
In vitro studies to determine the stiffness (G') and the ability to flow (viscosity) have been performed.17,18 Calcium hydroxylapatite has the most stiffness, while Belotero Balance (Merz Aesthetics) and Juvéderm Ultra XC (Allergan, Inc) are more soft17 (Table). These guidelines are important but may not correlate directly with how the fillers behave in vivo as demonstrated in animal models.18

Hyaluronic acid fillers are produced by different technologies to create their cross-link patterns with 1,4-butanediol diglycidyl ether, which determines, to some degree, their behavior in human tissue. Fillers are either monophasic; monodensified; formed by Hylacross (Juvéderm), Vycross (Juvéderm Voluma XC, Juvéderm Volbella XC), or cohesive polydensified matrix technology (Belotero Balance), or biphasic, formed by nonanimal stabilized HA sieving technology (Restylane family). Biopsy has demonstrated that monophasic fillers tend to percolate through and integrate into the tissue, while biphasic fillers dissect tissue to the sides to create a potential space for the filler to reside (Table).24
Periocular Injection Considerations
An experienced injector is one who has developed not only an artistic eye for the face and excellent sense of anatomy but also has a sensitive ability to predict the filler-tissue interaction based on tactile feedback dependent on 3 main qualities: (1) stiffness and viscosity of the filler, (2) gauge of the needle or cannula, and (3) depth of the needle in the tissue. Periocular injections of dermal fillers can be delivered with needles or cannulas, diluted or undiluted. Smaller-gauge needles require more force than larger-gauge needles and cannulas that flow more freely. A needle in the dense dermis will require more force than one placed in the loose subcutaneous space.
The tear trough is generally preferable to fill with a mid-level G' HA filler that is less apt to migrate. A neutral gaze during the injection is preferred because closing or moving the eyes can distort the position of the inferior orbital fat-pads (Figure 1). A needle or cannula can be used, diluted or undiluted. The tear trough can be filled with the injection directed horizontally or vertically via a fanning technique. If needles are used, the skin should be stretched to view the 3 to 5 vertical veins and then the needle should be advanced beneath them to avoid bruising. Avoidance of hydrophilic fillers in the tear trough is important to avoid edema. The superior sulcus can be filled both anteriorly and posteriorly to the septum, which is a highly advanced injection for experienced injectors because of the proximity to the supratrochlear and supraorbital arteries as well as the superior ophthalmic vein (Figure 2). Sharp creases such as deep lateral periocular rhytides known as crow’s-feet are nicely filled with intradermal HAs with a low G'.


Adding volume to the midface is important because it is the continuum of the lower eyelid. Fillers can be injected into multiple levels in this area: deep (to act as pillars to lift the malar eminence and replace bone that has rotated and soft tissue that has become atrophic or descended) and subcutaneous (to efface soft tissue along the zygomatic cutaneous ligament). Higher G' HA fillers and CaHA often are used in the midface along with PLLA. Facial framing of the temples, lateral cheeks, and preauricular area is often accomplished with PLLA but also can be done with mid to high G' HA fillers or CaHA. A cannula may be used to undermine and break apart the zygomatic cutaneous ligament’s cutaneous attachments prior to delivery of the filler in the subcutaneous plane.26 If not done, filler may track away from the hollow area where the ligament is attached and instead move to adjacent areas that will accentuate the hollow and make it look worse.
The temples and lateral face often are filled with PLLA for framing. Mid or high G' HA fillers and CaHA also are used in the temples both beneath the temporalis muscle and also above the deep temporalis fascia or sometimes in the subcutaneous plane.27
Prevention and Management of Periocular Complications
Blindness is the most devastating periocular complication of facial fillers, which is caused by retrograde arterial embolization followed by anterograde flow into the ophthalmic then central retinal arteries. Injectables that have caused blindness include (in descending order of frequency) fat, HA, collagen, paraffin, polymethyl methacrylate, silicone, PLLA, CaHA, polyacrylamide hydrogel, and micronized acellular dermal matrix. Of the 98 cases of blindness from periocular complications from dermal fillers reported in the world literature, the order of affected sites include the glabella (38 cases), nose (25), nasolabial folds (13), superior forehead (12), infraorbital rim (6), temples (1), malar area (1), lip (1), and chin (1). Prevention includes avoidance of danger zone arteries including the supratrochlear, supraorbital, dorsal nasal, angular, infraorbital, zygomaticofacial and zygomaticotemporal arteries.28
Avoiding the average critical volume of 0.84 in any single aliquot dispensed is key to avoid filling of these periocular arteries to the critical bifurcation point that can result in anterograde flow into the eye (Freudenthal Nicolau syndrome). The smallest supratrochlear artery’s volume in this study was 0.04 cc, so aliquots that do not exceed 0.03 cc are ideal.29,30
The injector should always be thinking about the anatomy of the danger zones (eg, infratrochlear and supratrochlear arteries, supraorbital artery, frontal branch of the superficial temporal artery, lacrimal artery, dorsal nasal artery, infraorbital artery, angular artery, zygomaticofacial artery, zygomaticotemporal artery)(Figure 3).

Hyaluronidase can be used off label to hydrolyze unwanted HA. It was first used to aid transcutaneous hydration and was used by ophthalmologists in the 1960s and 1970s to promote the spread of anesthetics by retrobulbar injection.31,32 It can penetrate through soft tissues and blood vessels.33 It is therefore hypothesized that a retrobulbar injection of hyaluronidase could aid in a case of impending blindness34 but has not been successfully accomplished to date. If vision is confirmed to be poor or there is no light perception, a retrobulbar injection of 300 U of hyaluronidase should be given immediately and then repeated in approximately 30 to 45 minutes. The retina begins to show permanent loss of function after being deprived of blood flow for just 97 minutes,35 so there may not be time for an immediate ophthalmology consultation, though such a consultation would be ideal.
Aside from common complications such as bruising and swelling, granulomas and biofilms are well documented in the literature. There are a variety of algorithms to treat such complications, which can happen many weeks after the injection of a dermal filler or years after the injection of a semipermanent filler.36 Postinjection periocular edema can occur years after the initial injection.37,38 Other periocular complications of dermal fillers include nonischemic (eg, bluish hue, filler migration, infection, inflammation, lumps) and ischemic (eg, blindness, necrosis, ophthalmoplegia, ptosis) disturbances.
Conclusion
In summary, periocular injections of facial fillers are useful tools for rejuvenation of the upper face when used with great caution and respect for anatomy.
Rejuvenation of the periocular area is in high demand among patients who want to look and feel their best. Physicians should understand the complicated anatomy surrounding the eyes before attempting to inject this area with facial fillers, both to understand the aging process and to minimize treatment complications.
Basic Oculoplastic and Orbital Anatomy
The injector should understand the anatomy of the periocular muscles, the orbital osteology, and the secretory and lacrimal system, in addition to the fat, ligaments, and vascular anatomy in this area.1
The eyes are surrounded by fat compartments that provide glide planes for the motion of the eyelids and globe. There are 2 upper eyelid fat-pads—nasal and central [preaponeurotic])—in the upper lid, leaving room for the lacrimal gland laterally. There are 3 fat compartments—nasal, central, and lateral—in the lower eyelid. The nasal and central compartments are separated by the inferior oblique muscle, which elevates and extorts the eye. The orbital septum holds the fat-pads in place in the orbit. The brow fat-pad is the retro-orbicularis oculi fat-pad (ROOF). There are fat compartments that lie in the subcutaneous space along the entire forehead and in the temple. The suborbicularis oculi fat-pad (SOOF) lies over the malar eminence. Superficial and deep submuscular fat compartments of the face have been described.2 Deep fat compartments also have been examined on computed tomography.3
Orbital circulation comes from the internal carotid artery and anastomoses with the supply from the external carotid artery to supply the orbit. The first branch off of the carotid artery is the ophthalmic artery, and the first branch off of the ophthalmic artery is the central retinal artery that enters the optic nerve sheath 1 cm behind the globe to supply the retina. The supraorbital and supratrochlear arteries branch off of the ophthalmic artery and supply the forehead. The supraorbital artery runs through the supraorbital notch (foramen in 8%)1 and can usually be palpated with one’s finger. There are 15 to 20 short posterior ciliary arteries leading to the choroid, 2 long posterior ciliary arteries to the iris circle, and 7 anterior ciliary arteries to the extraocular muscles. The superior and inferior venous systems drain into the cavernous sinus.4
The ligaments are important to signs of facial aging because tissue atrophy occurs along them. The main orbital ligaments are the lateral orbital thickening (known as the LOT) that adheres the eyelids to the lateral orbital rim and the orbitomalar ligament (orbicularis retaining ligament), which is a condensation fibrous tissue that attaches the skin to the inferior orbital rim and orbital septum along the arcus marginalis and defines the superior edge of the SOOF.5 The zygomatic ligament not only suspends the zygomaticus major and zygomaticus minor muscles to the malar eminence but there are osseocutaneous attachments that connect the skin over the zygoma’s malar eminence and demarcate the inferior edge of the SOOF.6
Periocular Aging
The skin, fat, muscles, and bones change and rotate with aging, and not all orbits age in the same manner. Older patients with dermatochalasis (excess skin fat and muscle) often undergo rejuvenation with blepharoplasty, a brow-lift, and a midface-lift, but many atrophic changes can be improved with facial fillers.7,8
As adults age, the soft tissue along the ligaments begins to show atrophy, prime signs of aging that are often improved with fillers. Atrophy along the orbitomalar ligament along the infraorbital rim creates a depressed tear trough, which is an early sign of aging. A 3-point grading system reported by Hirmand8 describes the severity of progressive hallowing. There also is atrophy along the zygomatic cutaneous ligament that creates the malar hollow. The SOOF appears to be more prominent when these areas above and below show atrophy, which creates the look of an unwanted bag known as a festoon. Additionally, there is atrophy along the superior orbital notch where the ophthalmic branch of the trigeminal nerve (V1) and the supraorbital artery traverse. Soft-tissue atrophy along the supraorbital notch resembles the peak at the top of the letter A, giving the slang term A-frame deformity.
Periocular fat can atrophy, hypertrophy, herniate forward as the septum weakens, or become ptotic. Some patients develop hypertrophy and herniation of the superior and inferior orbital fat-pads, while others develop unwanted atrophy leaving a hollow superior orbit and loss of support to the levator muscle that contributes to eyelid ptosis. The frontalis fat deflates, leaving veins, arteries, and the hypertrophied corrugators unwantedly visible. Loss of subcutaneous fat in the glabella contributes to the formation of frown lines between the brows (also called number 11’s). The ROOF deflates in some patients adding to brow ptosis. Loss of the facial frame occurs when temple fat atrophies.
Skeletal rotation also occurs. Throughout a patient’s life, the skeleton remodels itself via activity of osteoclasts and osteoblasts. Pessa et al9,10 has described the expansion of the anterior orbital aperture superomedially and inferolaterally as well as maxillary retrusion that results in angular changes of the midface in relation to the orbital rim. Lambros’ algorithm describes the rotational changes of the cranium where the superior orbit protrudes as the maxilla retreats posteriorly.9-11 The equator of the globe does not change its distance from the ROOF of the orbit, presumably because of its suspension in the orbit by the optic nerve after it passes through the optic canal and trochlea via the superior oblique muscle, but the distance of the inferior equator of the globe to the floor of the orbit increases as the floor of the orbit descends.12
Dermal Fillers for Periocular Rejuvenation
Hyaluronic acid (HA) was first pioneered for use in humans in the late 1970s by ophthalmologists for anterior segment surgery.13-15 Biocompatibility for orthopedic and dermal applications was explored in the early 1990s.16
At this time, no dermal filler is approved by the US Food and Drug Administration for use in the periorbital area. Some fillers are approved for subdermal areas extending to the preperiosteal plane and can be used in the midface such as HA fillers (eg, Restylane Lyft [Galderma Laboratories, LP]), Juvéderm Voluma XC [Allergan, Inc]), poly-L-lactic acid (PLLA), and calcium hydroxylapatite (CaHA). No dermal fillers are approved for use in the forehead, glabella, or temples. Their use is becoming increasingly popular but is considered off label. In addition, cannulas are not approved for use in these areas. Cannulas may be beneficial in that they are thought to create less bruising and have less chance of entering a vessel than needles, but some injectors prefer needles because they are stiffer and therefore more precise.
The ideal filler for the tear trough, superior sulcus, ROOF, over the orbitomalar ligament, forehead, and glabella is one that is somewhat moldable but does not migrate, is not hydrophilic, is smooth to inject, and is reversible should there be any complications. No single filler fits this ideal description, but HAs typically are the first choice.
In vitro studies to determine the stiffness (G') and the ability to flow (viscosity) have been performed.17,18 Calcium hydroxylapatite has the most stiffness, while Belotero Balance (Merz Aesthetics) and Juvéderm Ultra XC (Allergan, Inc) are more soft17 (Table). These guidelines are important but may not correlate directly with how the fillers behave in vivo as demonstrated in animal models.18

Hyaluronic acid fillers are produced by different technologies to create their cross-link patterns with 1,4-butanediol diglycidyl ether, which determines, to some degree, their behavior in human tissue. Fillers are either monophasic; monodensified; formed by Hylacross (Juvéderm), Vycross (Juvéderm Voluma XC, Juvéderm Volbella XC), or cohesive polydensified matrix technology (Belotero Balance), or biphasic, formed by nonanimal stabilized HA sieving technology (Restylane family). Biopsy has demonstrated that monophasic fillers tend to percolate through and integrate into the tissue, while biphasic fillers dissect tissue to the sides to create a potential space for the filler to reside (Table).24
Periocular Injection Considerations
An experienced injector is one who has developed not only an artistic eye for the face and excellent sense of anatomy but also has a sensitive ability to predict the filler-tissue interaction based on tactile feedback dependent on 3 main qualities: (1) stiffness and viscosity of the filler, (2) gauge of the needle or cannula, and (3) depth of the needle in the tissue. Periocular injections of dermal fillers can be delivered with needles or cannulas, diluted or undiluted. Smaller-gauge needles require more force than larger-gauge needles and cannulas that flow more freely. A needle in the dense dermis will require more force than one placed in the loose subcutaneous space.
The tear trough is generally preferable to fill with a mid-level G' HA filler that is less apt to migrate. A neutral gaze during the injection is preferred because closing or moving the eyes can distort the position of the inferior orbital fat-pads (Figure 1). A needle or cannula can be used, diluted or undiluted. The tear trough can be filled with the injection directed horizontally or vertically via a fanning technique. If needles are used, the skin should be stretched to view the 3 to 5 vertical veins and then the needle should be advanced beneath them to avoid bruising. Avoidance of hydrophilic fillers in the tear trough is important to avoid edema. The superior sulcus can be filled both anteriorly and posteriorly to the septum, which is a highly advanced injection for experienced injectors because of the proximity to the supratrochlear and supraorbital arteries as well as the superior ophthalmic vein (Figure 2). Sharp creases such as deep lateral periocular rhytides known as crow’s-feet are nicely filled with intradermal HAs with a low G'.


Adding volume to the midface is important because it is the continuum of the lower eyelid. Fillers can be injected into multiple levels in this area: deep (to act as pillars to lift the malar eminence and replace bone that has rotated and soft tissue that has become atrophic or descended) and subcutaneous (to efface soft tissue along the zygomatic cutaneous ligament). Higher G' HA fillers and CaHA often are used in the midface along with PLLA. Facial framing of the temples, lateral cheeks, and preauricular area is often accomplished with PLLA but also can be done with mid to high G' HA fillers or CaHA. A cannula may be used to undermine and break apart the zygomatic cutaneous ligament’s cutaneous attachments prior to delivery of the filler in the subcutaneous plane.26 If not done, filler may track away from the hollow area where the ligament is attached and instead move to adjacent areas that will accentuate the hollow and make it look worse.
The temples and lateral face often are filled with PLLA for framing. Mid or high G' HA fillers and CaHA also are used in the temples both beneath the temporalis muscle and also above the deep temporalis fascia or sometimes in the subcutaneous plane.27
Prevention and Management of Periocular Complications
Blindness is the most devastating periocular complication of facial fillers, which is caused by retrograde arterial embolization followed by anterograde flow into the ophthalmic then central retinal arteries. Injectables that have caused blindness include (in descending order of frequency) fat, HA, collagen, paraffin, polymethyl methacrylate, silicone, PLLA, CaHA, polyacrylamide hydrogel, and micronized acellular dermal matrix. Of the 98 cases of blindness from periocular complications from dermal fillers reported in the world literature, the order of affected sites include the glabella (38 cases), nose (25), nasolabial folds (13), superior forehead (12), infraorbital rim (6), temples (1), malar area (1), lip (1), and chin (1). Prevention includes avoidance of danger zone arteries including the supratrochlear, supraorbital, dorsal nasal, angular, infraorbital, zygomaticofacial and zygomaticotemporal arteries.28
Avoiding the average critical volume of 0.84 in any single aliquot dispensed is key to avoid filling of these periocular arteries to the critical bifurcation point that can result in anterograde flow into the eye (Freudenthal Nicolau syndrome). The smallest supratrochlear artery’s volume in this study was 0.04 cc, so aliquots that do not exceed 0.03 cc are ideal.29,30
The injector should always be thinking about the anatomy of the danger zones (eg, infratrochlear and supratrochlear arteries, supraorbital artery, frontal branch of the superficial temporal artery, lacrimal artery, dorsal nasal artery, infraorbital artery, angular artery, zygomaticofacial artery, zygomaticotemporal artery)(Figure 3).

Hyaluronidase can be used off label to hydrolyze unwanted HA. It was first used to aid transcutaneous hydration and was used by ophthalmologists in the 1960s and 1970s to promote the spread of anesthetics by retrobulbar injection.31,32 It can penetrate through soft tissues and blood vessels.33 It is therefore hypothesized that a retrobulbar injection of hyaluronidase could aid in a case of impending blindness34 but has not been successfully accomplished to date. If vision is confirmed to be poor or there is no light perception, a retrobulbar injection of 300 U of hyaluronidase should be given immediately and then repeated in approximately 30 to 45 minutes. The retina begins to show permanent loss of function after being deprived of blood flow for just 97 minutes,35 so there may not be time for an immediate ophthalmology consultation, though such a consultation would be ideal.
Aside from common complications such as bruising and swelling, granulomas and biofilms are well documented in the literature. There are a variety of algorithms to treat such complications, which can happen many weeks after the injection of a dermal filler or years after the injection of a semipermanent filler.36 Postinjection periocular edema can occur years after the initial injection.37,38 Other periocular complications of dermal fillers include nonischemic (eg, bluish hue, filler migration, infection, inflammation, lumps) and ischemic (eg, blindness, necrosis, ophthalmoplegia, ptosis) disturbances.
Conclusion
In summary, periocular injections of facial fillers are useful tools for rejuvenation of the upper face when used with great caution and respect for anatomy.
- Foster J, ed. Orbit, Eyelids, and Lacrimal System. San Francisco, CA: American Academy of Ophthalmology; 2016. 2016-2017 Basic and Clinical Science Course; section 7.
- Rohrich RJ, Pessa JE. The fat compartments of the face: anatomy and clinical implications for cosmetic surgery. Plast Reconstr Surg. 2007;119:2219-2227; discussion 2228-2231.
- Gierloff M, Stöhring C, Buder T, et al. Aging changes of the midfacial fat compartments: a computed tomographic study. Plast Reconst Surg. 2012;129:263-273.
- Zide BM, Jelks GW. Surgical Anatomy of the Orbit: The System of Zones. Philadelphia, PA: Lippincott Williams & Wilkins; 2006.
- Kikkawa DO, Lemke BN, Dortzbach RK. Relations of the superficial musculoaponeurotic system to the orbit and characterization of the oribitomalar ligament. Ophthal Plast Reconstr Surg. 1996;12:77-88.
- Furnas DW. The retaining ligaments of the cheek. Plast Reconstr Surg. 1989;83:11-16.
- Morley AM, Taban M, Malhotra R, et al. Use of hyaluronic acid gel for upper eyelid filling and contouring. Ophthal Plast Reconstr Surg. 2009;25:440-444.
- Hirmand H. Anatomy and nonsurgical correction of the tear trough deformity. Plast Reconstr Surg. 2010;125:699-708.
- Pessa JE, Zadoo VP, Mutimer KL, et al. Relative maxillary retrusion as a natural consequence of aging. Plast Reconstr Surg. 1998;102:205-212.
- Pessa JE, Desvigne LD, Lambros VS, et al. Changes in ocular globe-to-orbital rim position with age: implications for aesthetic blepharoplasty of the lower eyelids. Aesthet Plast Surg. 1999;23:337-345.
- Goldberg RA, Relan A, Hoenig J. Relationship of the eye to the bony orbit, with clinical correlations. Aust N Z J Ophthalmol. 1999;27:398-403.
- Richard MJ, Morris C, Deen BF, et al. Analysis of the anatomic changes of the aging facial skeleton using computer-assisted tomography. Ophthal Plast Reconstr Surg. 2009;25:382-386.
- Miller D, O’Connor P, Williams J. Use of Na-hyaluronate during intraocular lens implantation in rabbits. Ophthalmic Surg. 1977;8:58-61.
- Miller D, Stegmann R. Use of Na-hyaluronate in anterior segment eye surgery. J Am Intraocul Implant Soc. 1980;6:13-15.
- Pape LG, Balazs EA. The use of sodium hyaluronate (Healon) in human anterior segment surgery. Ophthalmology. 1980;87:699-705.
- Larsen NE, Pollak CT, Reiner K, et al. Hylan gel biomaterial: dermal and immunologic compatibility. J Biomed Mater Res. 1993;27:1129-1134.
- Sundaram H, Cassuto D. Biophysical characteristics of hyaluronic acid soft-tissue fillers and their relevance to aesthetic applications. Plast Reconstr Surg. 2013;132(4, suppl 2):5S-21S.
- Hee CK, Shumate GT, Narurkar V, et al. Rheological properties and in vivo performance characteristics of soft tissue fillers. Dermatol Surg. 2015;41(suppl 1):S373-S381.
- Sundaram H, Voigts B, Beer K, et al. Comparison of the rheological properties of viscosity and elasticity in two categories of soft tissue fillers: calcium hydroxylapatite and hyaluronic acid. Dermatol Surg. 2010;36(suppl 3):1859-1865.
- Sundaram H. The new face of fillers: a multi-specialty CME initiative: supplement part II of II. J Drugs Dermatol. 2012;11(suppl 8):S8.
- Stocks D, Sundaram H, Michaels J, et al. Rheological evaluation of the physical properties of hyaluronic acid dermal fillers. J Drugs Dermatol. 2011;10:974-980.
- Goodman GJ, Swift A, Remington BK. Current concepts in the use of Voluma, Volift, and Volbella. Plast Reconstr Surg. 2015;136(suppl 5):139S-148S.
- Sundaram H, Rohrich RJ, Liew S, et al. Cohesivity of hyaluronic acid fillers: development and clinical implications of a novel assay, pilot validation with a five-point grading scale and evaluation of six U.S. Food and Drug Administration–approved fillers. Plast Reconstr Surg. 2015;136:678-686.
- Flynn TC, Sarazin D, Bezzola A, et al. Comparative histology of intradermal implantation of mono and biphasic hyaluronic acid fillers. Dermatol Surg. 2011;37:637-643.
- Woodward JA, Langelier N. Filler enhancement of the superior periocular area [published online Jun 23, 2016]. JAMA Facial Plast Surg. doi:10.1001/jamafacial.2016.0636.
- Cotofana S, Schenck TL, Trevidic P, et al. Midface: clinical anatomy and regional approaches with injectable fillers. Plast Reconstr Surg. 2015;136(suppl 5):219S-234S.
- Buckingham ED, Glasgold R, Kontis T, et al. Volume rejuvenation of the facial upper third. Facial Plast Surg. 2015;31:43-54.
- Beleznay K, Carruthers JD, Humphrey S, et al. Avoiding and treating blindness from fillers: a review of the world literature. Dermatol Surg. 2015;41:1097-1117.
- Coleman SR. Avoidance of arterial occlusion from injection of soft tissue fillers. Aesthet Surg J. 2002;22:555-557.
- Khan T, Colon-Acevedo B, Mettu P, et al. An anatomical analysis of the supratrochlear artery: considerations in facial filler injections and preventing vision loss [published online August 16, 2016]. Aesthet Surg J. pii: sjw132.
- Iserle J, Kumstat Z. Retrobulbar injections of hyaluronidase as a method of increasing safety in cataract surgery [in Czech]. Cesk Oftalmol. 1960;15:126-130.
- Wojtowicz S. Effect of retrobulbar injections of novocaine and lignocaine with adrenalin and hyaluronidase for the immobilization of the eye in electromyography [in Polish]. Klin Oczna. 1964;34:285-296.
- Delorenzi C. Transarterial degradation of hyaluronic acid filler by hyaluronidase. Dermatol Surg. 2014;40:832-841.
- Carruthers J, Fagien S, Dolman P. Retro or peribulbar injections techniques to reverse visual loss after filler injections. 2015;41(suppl 1):S354-S357.
- Hayreh SS, Zimmerman MB, Kimura A, et al. Central retinal artery occlusion. retinal survival time. Exp Eye Res. 2004;78:723-736.
- Woodward J, Khan T, Martin J. Facial filler complications. Facial Plast Surg Clin North Am. 2015;23:447-458.
- Khan TT, Woodward JA. Retained dermal filler in the upper eyelid masquerading as periorbital edema. Dermatol Surg. 2015;41:1182-1184.
- Chang JR, Baharestani S, Salek SS, et al. Delayed superficial migration of retained hyaluronic acid years following periocular injection [published online April 20, 2015]. Ophthal Plast Reconstr Surg. doi:10.1097/IOP.0000000000000434.
- Foster J, ed. Orbit, Eyelids, and Lacrimal System. San Francisco, CA: American Academy of Ophthalmology; 2016. 2016-2017 Basic and Clinical Science Course; section 7.
- Rohrich RJ, Pessa JE. The fat compartments of the face: anatomy and clinical implications for cosmetic surgery. Plast Reconstr Surg. 2007;119:2219-2227; discussion 2228-2231.
- Gierloff M, Stöhring C, Buder T, et al. Aging changes of the midfacial fat compartments: a computed tomographic study. Plast Reconst Surg. 2012;129:263-273.
- Zide BM, Jelks GW. Surgical Anatomy of the Orbit: The System of Zones. Philadelphia, PA: Lippincott Williams & Wilkins; 2006.
- Kikkawa DO, Lemke BN, Dortzbach RK. Relations of the superficial musculoaponeurotic system to the orbit and characterization of the oribitomalar ligament. Ophthal Plast Reconstr Surg. 1996;12:77-88.
- Furnas DW. The retaining ligaments of the cheek. Plast Reconstr Surg. 1989;83:11-16.
- Morley AM, Taban M, Malhotra R, et al. Use of hyaluronic acid gel for upper eyelid filling and contouring. Ophthal Plast Reconstr Surg. 2009;25:440-444.
- Hirmand H. Anatomy and nonsurgical correction of the tear trough deformity. Plast Reconstr Surg. 2010;125:699-708.
- Pessa JE, Zadoo VP, Mutimer KL, et al. Relative maxillary retrusion as a natural consequence of aging. Plast Reconstr Surg. 1998;102:205-212.
- Pessa JE, Desvigne LD, Lambros VS, et al. Changes in ocular globe-to-orbital rim position with age: implications for aesthetic blepharoplasty of the lower eyelids. Aesthet Plast Surg. 1999;23:337-345.
- Goldberg RA, Relan A, Hoenig J. Relationship of the eye to the bony orbit, with clinical correlations. Aust N Z J Ophthalmol. 1999;27:398-403.
- Richard MJ, Morris C, Deen BF, et al. Analysis of the anatomic changes of the aging facial skeleton using computer-assisted tomography. Ophthal Plast Reconstr Surg. 2009;25:382-386.
- Miller D, O’Connor P, Williams J. Use of Na-hyaluronate during intraocular lens implantation in rabbits. Ophthalmic Surg. 1977;8:58-61.
- Miller D, Stegmann R. Use of Na-hyaluronate in anterior segment eye surgery. J Am Intraocul Implant Soc. 1980;6:13-15.
- Pape LG, Balazs EA. The use of sodium hyaluronate (Healon) in human anterior segment surgery. Ophthalmology. 1980;87:699-705.
- Larsen NE, Pollak CT, Reiner K, et al. Hylan gel biomaterial: dermal and immunologic compatibility. J Biomed Mater Res. 1993;27:1129-1134.
- Sundaram H, Cassuto D. Biophysical characteristics of hyaluronic acid soft-tissue fillers and their relevance to aesthetic applications. Plast Reconstr Surg. 2013;132(4, suppl 2):5S-21S.
- Hee CK, Shumate GT, Narurkar V, et al. Rheological properties and in vivo performance characteristics of soft tissue fillers. Dermatol Surg. 2015;41(suppl 1):S373-S381.
- Sundaram H, Voigts B, Beer K, et al. Comparison of the rheological properties of viscosity and elasticity in two categories of soft tissue fillers: calcium hydroxylapatite and hyaluronic acid. Dermatol Surg. 2010;36(suppl 3):1859-1865.
- Sundaram H. The new face of fillers: a multi-specialty CME initiative: supplement part II of II. J Drugs Dermatol. 2012;11(suppl 8):S8.
- Stocks D, Sundaram H, Michaels J, et al. Rheological evaluation of the physical properties of hyaluronic acid dermal fillers. J Drugs Dermatol. 2011;10:974-980.
- Goodman GJ, Swift A, Remington BK. Current concepts in the use of Voluma, Volift, and Volbella. Plast Reconstr Surg. 2015;136(suppl 5):139S-148S.
- Sundaram H, Rohrich RJ, Liew S, et al. Cohesivity of hyaluronic acid fillers: development and clinical implications of a novel assay, pilot validation with a five-point grading scale and evaluation of six U.S. Food and Drug Administration–approved fillers. Plast Reconstr Surg. 2015;136:678-686.
- Flynn TC, Sarazin D, Bezzola A, et al. Comparative histology of intradermal implantation of mono and biphasic hyaluronic acid fillers. Dermatol Surg. 2011;37:637-643.
- Woodward JA, Langelier N. Filler enhancement of the superior periocular area [published online Jun 23, 2016]. JAMA Facial Plast Surg. doi:10.1001/jamafacial.2016.0636.
- Cotofana S, Schenck TL, Trevidic P, et al. Midface: clinical anatomy and regional approaches with injectable fillers. Plast Reconstr Surg. 2015;136(suppl 5):219S-234S.
- Buckingham ED, Glasgold R, Kontis T, et al. Volume rejuvenation of the facial upper third. Facial Plast Surg. 2015;31:43-54.
- Beleznay K, Carruthers JD, Humphrey S, et al. Avoiding and treating blindness from fillers: a review of the world literature. Dermatol Surg. 2015;41:1097-1117.
- Coleman SR. Avoidance of arterial occlusion from injection of soft tissue fillers. Aesthet Surg J. 2002;22:555-557.
- Khan T, Colon-Acevedo B, Mettu P, et al. An anatomical analysis of the supratrochlear artery: considerations in facial filler injections and preventing vision loss [published online August 16, 2016]. Aesthet Surg J. pii: sjw132.
- Iserle J, Kumstat Z. Retrobulbar injections of hyaluronidase as a method of increasing safety in cataract surgery [in Czech]. Cesk Oftalmol. 1960;15:126-130.
- Wojtowicz S. Effect of retrobulbar injections of novocaine and lignocaine with adrenalin and hyaluronidase for the immobilization of the eye in electromyography [in Polish]. Klin Oczna. 1964;34:285-296.
- Delorenzi C. Transarterial degradation of hyaluronic acid filler by hyaluronidase. Dermatol Surg. 2014;40:832-841.
- Carruthers J, Fagien S, Dolman P. Retro or peribulbar injections techniques to reverse visual loss after filler injections. 2015;41(suppl 1):S354-S357.
- Hayreh SS, Zimmerman MB, Kimura A, et al. Central retinal artery occlusion. retinal survival time. Exp Eye Res. 2004;78:723-736.
- Woodward J, Khan T, Martin J. Facial filler complications. Facial Plast Surg Clin North Am. 2015;23:447-458.
- Khan TT, Woodward JA. Retained dermal filler in the upper eyelid masquerading as periorbital edema. Dermatol Surg. 2015;41:1182-1184.
- Chang JR, Baharestani S, Salek SS, et al. Delayed superficial migration of retained hyaluronic acid years following periocular injection [published online April 20, 2015]. Ophthal Plast Reconstr Surg. doi:10.1097/IOP.0000000000000434.
Practice Points
- When performing periocular dermal injections, physicians should understand the complicated anatomy surrounding the eyes and related changes with upper face aging.
- The different rheological properties of facial fillers impact product selection for various areas of the upper face.
- Physicians should be aware of the anatomical danger zones to avoid intravascular embolization.
Current Concepts in Lip Augmentation
Historically, a variety of tools have been used to alter one’s appearance for cultural or religious purposes or to conform to standards of beauty. As a defining feature of the face, the lips provide a unique opportunity for facial aesthetic enhancement. There has been a paradigm shift in medicine favoring preventative health and a desire to slow and even reverse the aging process.1 Acknowledging that product technology, skill sets, and cultural ideals continually evolve, this article highlights perioral anatomy, explains aging of the lower face, and reviews techniques to achieve perioral rejuvenation through volume restoration and muscle control.
Perioral Anatomy
The layers of the lips include the epidermis, subcutaneous tissue, orbicularis oris muscle fibers, and mucosa. The upper lip extends from the base of the nose to the mucosa inferiorly and to the nasolabial folds laterally. The curvilinear lower lip extends from the mucosa to the mandible inferiorly and to the oral commissures laterally.2 Circumferential at the vermilion-cutaneous junction, a raised area of pale skin known as the white roll accentuates the vermilion border and provides an important landmark during lip augmentation.3 At the upper lip, this elevation of the vermilion joins at a V-shaped depression centrally to form the Cupid’s bow. The cutaneous upper lip has 2 raised vertical pillars known as the philtral columns, which are formed from decussating fibers of the orbicularis oris muscle.2 The resultant midline depression is the philtrum. These defining features of the upper lip are to be preserved during augmentation procedures (Figure 1).4
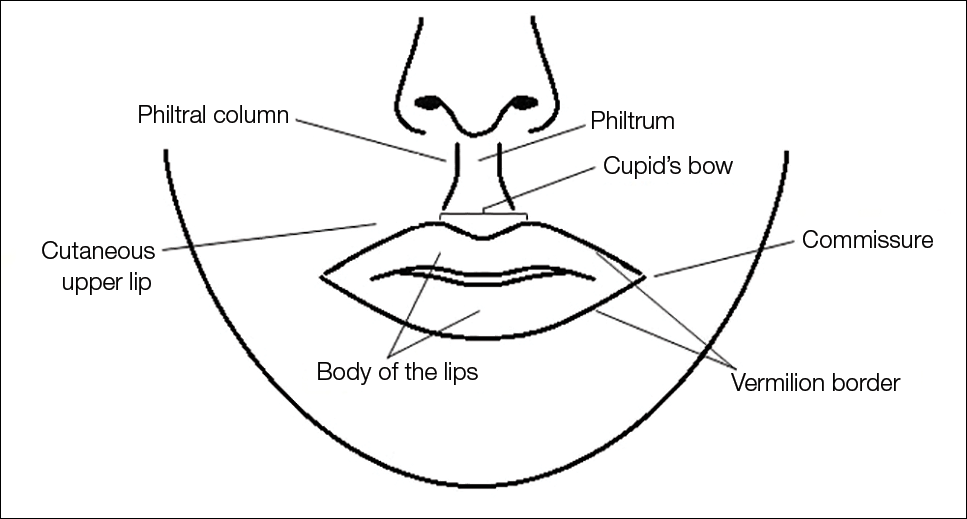
The superior and inferior labial arteries, both branches of the facial artery, supply the upper and lower lip, respectively. The anastomotic arch of the superior labial artery is susceptible to injury from deep injection of the upper lip between the muscle layer and mucosa; therefore, caution must be exercised in this area.5 Injections into the vermilion and lower lip can be safely performed with less concern for vascular compromise. The vermilion derives its red color from the translucency of capillaries in the superficial papillae.2 The capillary plexus at the papillae and rich sensory nerve network render the lip a highly vascular and sensitive structure.
Aging of the Lower Face
Subcutaneous fat atrophy, loss of elasticity, gravitational forces, and remodeling of the skeletal foundation all contribute to aging of the lower face. Starting as early as the third decade of life, intrinsic factors including hormonal changes and genetically determined processes produce alterations in skin quality and structure. Similarly, extrinsic aging through environmental influences, namely exposure to UV radiation and smoking, accelerate the loss of skin integrity.6
The decreased laxity of the skin in combination with repeated contraction of the orbicularis oris muscle results in perioral rhytides.7 For women in particular, vertically oriented perioral rhytides develop above the vermilion; terminal hair follicles, thicker skin, and a greater density of subcutaneous fat are presumptive protective factors for males.8 With time, the cutaneous portion of the upper lip lengthens and there is redistribution of volume with effacement of the upper lip vermilion.9 Additionally, the demarcation of the vermilion becomes blurred secondary to pallor, flattening of the philtral columns, and loss of projection of the Cupid’s bow.10
Downturning of the oral commissures is observed secondary to a combination of gravity, bone resorption, and soft tissue volume loss. Hyperactivity of the depressor anguli oris muscle exacerbates the mesolabial folds, producing marionette lines and a saddened expression.7 With ongoing volume loss and ligament laxity, tissue redistributes near the jaws and chin, giving rise to jowls. Similarly, perioral volume loss and descent of the malar fat-pad deepen the nasolabial folds in the aging midface.6
The main objective of perioral rejuvenation is to reinstate a harmonious refreshed look to the lower face; however, aesthetic analysis should occur within the context of the face as a whole, as the lips should complement the surrounding perioral cosmetic unit and overall skeletal foundation of the face. To accomplish this goal, the dermatologist’s armamentarium contains a broad variety of approaches including restriction of muscle movement, volume restoration, and surface contouring.
Volume Restoration
Treatment Options
In 2015, hyaluronic acid (HA) fillers constituted 80% of all injectable soft-tissue fillers, an 8% increase from 2014.11 Hyaluronic acid has achieved immense popularity as a temporary dermal filler given its biocompatibility, longevity, and reversibility via hyaluronidase.12
Hyaluronic acid is a naturally occurring glycosaminoglycan that comprises the connective tissue matrix. The molecular composition affords HA its hydrophilic property, which augments dermal volume.7 Endogenous HA has a short half-life, and chemical modification by a cross-linking process extends longevity by 6 to 12 months. The various HA fillers are distinguished by method of purification, size of molecules, concentration and degree of cross-linking, and viscosity.7,13,14 These differences dictate overall clinical performance such as flow properties, longevity, and stability. As a general rule, a high-viscosity product is more appropriate for deeper augmentation; fillers with low viscosity are more appropriate for correction of shallow defects.1 Table 1 lists the HA fillers that are currently approved by the US Food and Drug Administration for lip augmentation and/or perioral rhytides in adults 21 years and older.15-17
Randomized controlled trials comparing the efficacy, longevity, and tolerability of different HA products are lacking in the literature and, where present, have strong industry influence.18,19 The advent of assessment scales has provided an objective evaluation of perioral and lip augmentation, facilitating comparisons between products in both clinical research and practice.20
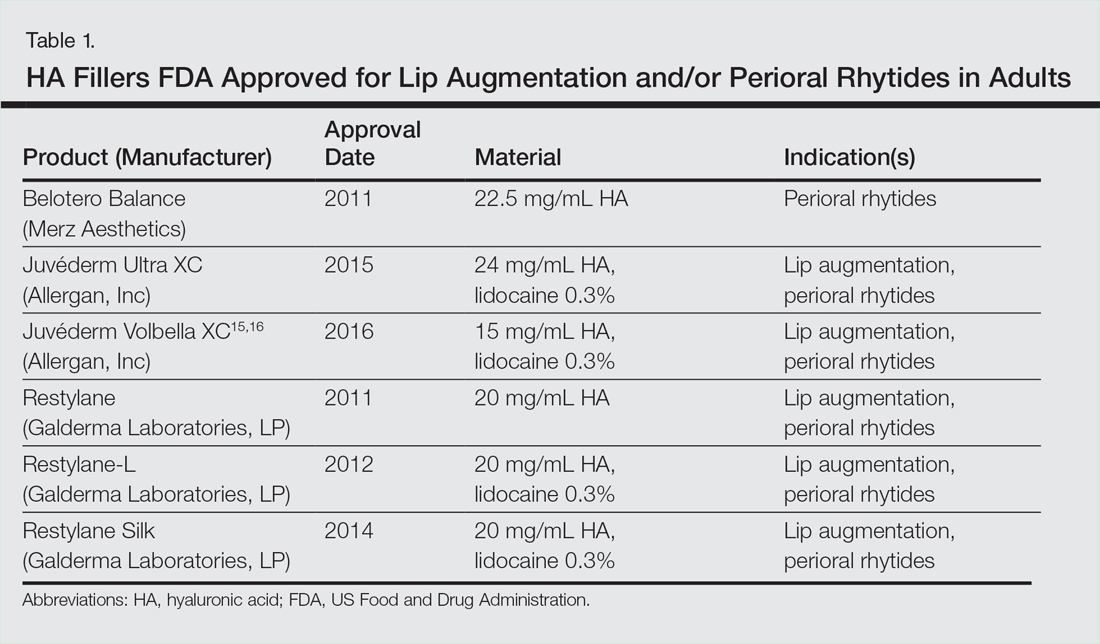
Semipermanent biostimulatory dermal fillers such as calcium hydroxylapatite and poly-L-lactic acid are not recommended for lip augmentation due to an increased incidence of submucosal nodule formation.6,14,21 Likewise, permanent fillers are not recommended given their irreversibility and risk of nodule formation around the lips.14,22 Nonetheless, liquid silicone (purified polydimethylsiloxane) administered via a microdroplet technique (0.01 mL of silicone at a time, no more than 1 cc per lip per session) has been used off label as a permanent filling agent for lip augmentation with limited complications.23 Regardless, trepidations about its use with respect to reported risks continue to limit its application.22
Similarly, surgical lip implants such as expanded polytetrafluoroethylene is an option for a subset of patients desiring permanent enhancement but are less commonly utilized given the side-effect profile, irreversibility, and relatively invasive nature of the procedure.22 Lastly, autologous fat transfer has been used in correction of the nasolabial and mesolabial folds as well as in lip augmentation; however, irregular surface contours and unpredictable longevity secondary to postinjection resorption (20%–90%) has limited its popularity.3,14,21
HA Injection Technique
With respect to HA fillers in the perioral area, numerous approaches have been described.10,22 The techniques in Table 2 provide a foundation for lip rejuvenation.

Several injection techniques exist, including serial puncture, linear threading, cross-hatching, and fanning in a retrograde or anterograde manner.24 A blunt microcannula (27 gauge, 38 mm) may be used in place of sharp needles and offers the benefit of increased patient comfort, reduced edema and ecchymosis, and shortened recovery period.25,26 Gentle massage of the product after injection can assist with an even contour. Lastly, a key determinant of successful outcomes is using an adequate volume of HA filler (1–2 mL for shaping the vermilion border and volumizing the lips).27 Figure 2 highlights a clinical example of HA filler for lip augmentation.
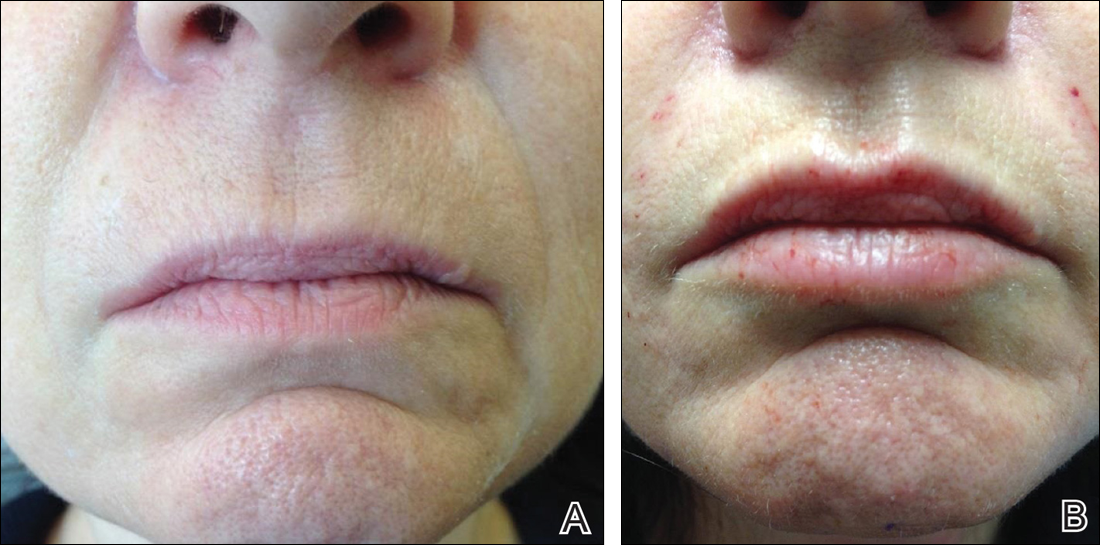
Fortunately, most complications encountered with HA lip augmentation are mild and transient. The most commonly observed side effects include injection-site reactions such as pain, erythema, and edema. Similarly, most adverse effects are related to injection technique. All HA fillers are prone to the Tyndall effect, a consequence of too superficial an injection plane. Patients with history of recurrent herpes simplex virus infections should receive prophylactic antiviral therapy.12
Muscle Control
An emerging concept in rejuvenation of the lower face recognizes not only restoration of volume but also control of muscle movement. Local injection of botulinum toxin type A induces relaxation of hyperfunctional facial muscles through temporary inhibition of neurotransmitter release.6 The potential for paralysis of the oral cavity may limit the application of botulinum toxin type A in that region.7 Nonetheless, the off-label potential of botulinum toxin type A has expanded to include several targets in the lower face. The orbicularis oris muscle is targeted to soften perioral rhytides. Conservative dosing (1–2 U per lip quadrant or approximately 5 U total) and superficial injection is emphasized in this area.27 Similarly, the depressor anguli oris muscle is targeted by injection of 4 U bilaterally to soften the marionette lines. In the chin area, the mentalis muscle can be targeted by injection of 2 U deep into each belly of the muscle to reduce the mental crease and dimpling.28 Combination treatment with dermal filler and neurotoxin demonstrates effects that last longer than either modality alone without additional adverse events.29 With combination therapy, guidelines suggest treating with filler first.27
Conclusion
A greater understanding of the extrinsic and intrinsic factors that contribute to the structural and surface changes of the aging face coupled with a preference for minimally invasive procedures has revolutionized the dermatologist’s approach to perioral rejuvenation. Serving as a focal point of the face, the lips and perioral skin are well poised to benefit from this paradigm shift. A multifaceted approach utilizing dermal fillers and neurotoxins may be most appropriate and has demonstrated optimal outcomes in facial aesthetics.
- Buck DW, Alam M, Kim JYS. Injectable fillers for facial rejuvenation: a review. J Plast Reconstr Aesthet Surg. 2009;62:11-18.
- Guareschi M, Stella E. Lips. In: Goisis M, ed. Injections in Aesthetic Medicine. Milan, Italy: Springer; 2014:125-136.
- Byrne PJ, Hilger PA. Lip augmentation. Facial Plast Surg. 2004;20:31-38.
- Niamtu J. Rejuvenation of the lip and perioral areas. In: Bell WH, Guerroro CA, eds. Distraction Osteogenesis of the Facial Skeleton. Ontario, Canada: BC Decker Inc; 2007:38-48.
- Tansatit T, Apinuntrum P, Phetudom T. A typical pattern of the labial arteries with implication for lip augmentation with injectable fillers. Aesthet Plast Surg. 2014;38:1083-1089.
- Sadick NS, Karcher C, Palmisano L. Cosmetic dermatology of the aging face. Clin Dermatol. 2009;27(suppl):S3-S12.
- Ali MJ, Ende K, Mass CS. Perioral rejuvenation and lip augmentation. Facial Plast Surg Clin N Am. 2007;15:491-500.
- Chien AL, Qi J, Cheng N, et al. Perioral wrinkles are associated with female gender, aging, and smoking: development of a gender-specific photonumeric scale. J Am Acad Dermatol. 2016;74:924-930.
- Iblher N, Stark GB, Penna V. The aging perioral region—do we really know what is happening? J Nutr Health Aging. 2012;16:581-585.
- Sarnoff DS, Gotkin RH. Six steps to the “perfect” lip. J Drugs Dermatol. 2012;11:1081-1088.
- American Society of Plastic Surgeons. 2015 Cosmetic plastic surgery statistics. https://d2wirczt3b6wjm.cloudfront.net/News/Statistics/2015/cosmetic-procedure-trends-2015.pdf. Published February 26, 2015. Accessed October 5, 2016.
- Abduljabbar MH, Basendwh MA. Complications of hyaluronic acid fillers and their managements. J Dermatol Surg. 2016;20:1-7.
- Luebberding S, Alexiades-Armenakas M. Facial volume augmentation in 2014: overview of different filler options. J Drugs Dermatol. 2013;12:1339-1344.
- Huang Attenello N, Mass CS. Injectable fillers: review of material and properties. Facial Plast Surg. 2015;31:29-34.
- Eccleston D, Murphy DK. Juvéderm Volbella in the perioral area: a 12-month perspective, multicenter, open-label study. Clin Cosmet Investig Dermatol. 2012;5:167-172.
- Raspaldo H, Chantrey J, Belhaouari L, et al. Lip and perioral enhancement: a 12-month prospective, randomized, controlled study. J Drugs Dermatol. 2015;14:1444-1452.
- Soft tissue fillers approved by the center for devices and radiological health. US Food and Drug Administration website. http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/CosmeticDevices/WrinkleFillers/ucm227749.htm. Updated July 27, 2015. Accessed October 5, 2016.
- Butterwick K, Marmur E, Narurkar V, et al. HYC-24L demonstrates greater effectiveness with less pain than CPM-22.5 for treatment of perioral lines in a randomized controlled trial. Dermatol Surg. 2015;41:1351-1360.
- San Miguel Moragas J, Reddy RR, Hernández Alfaro F, et al. Systematic review of “filling” procedures for lip augmentation regarding types of material, outcomes and complications. J Craniomaxillofac Surg. 2015;43:883-906.
- Cohen JL, Thomas J, Paradkar D, et al. An interrater and intrarater reliability study of 3 photographic scales for the classification of perioral aesthetic features. Dermatol Surg. 2014;40:663-670.
- Broder KW, Cohen SR. An overview of permanent and semipermanent fillers. Plast Reconstr Surg. 2006;118(3 suppl):7S-14S.
- Sarnoff DS, Saini R, Gotkin RH. Comparison of filling agents for lip augmentation. Aesthet Surg J. 2008;28:556-563.
- Moscona RA, Fodor L. A retrospective study on liquid injectable silicone for lip augmentation: long-term results and patient satisfaction. J Plast Reconstr Aesthet Surg. 2010;63:1694-1698.
- Bertucci V, Lynde CB. Current concepts in the use of small-particle hyaluronic acid. Plast Reconstr Surg. 2015;136(5 suppl):132S-138S.
- Wilson AJ, Taglienti AJ, Chang CS, et al. Current applications of facial volumization with fillers. Plast Reconstr Surg. 2016;137:E872-E889.
- Dewandre L, Caperton C, Fulton J. Filler injections with the blunt-tip microcannula compared to the sharp hypodermic needle. J Drugs Dermatol. 2012;11:1098-1103.
- Carruthers JD, Glogau RG, Blitzer A; Facial Aesthetics Consensus Group Faculty. Advances in facial rejuvenation: botulinum toxin type A, hyaluronic acid dermal fillers, and combination therapies-consensus recommendations. Plast Reconstr Surg. 2008;121(5 suppl):5S-30S.
- Wu DC, Fabi SG, Goldman MP. Neurotoxins: current concepts in cosmetic use on the face and neck-lower face. Plast Reconstr Surg. 2015;136(5 suppl):76S-79S.
- Carruthers A, Carruthers J, Monheit GD, et al. Multicenter, randomized, parallel-group study of the safety and effectiveness of onabotulinumtoxin A and hyaluronic acid dermal fillers (24-mg/mL smooth, cohesive gel) alone and in combination for lower facial rejuvenation. Dermatol Surg. 2010;36:2121-2134.
Historically, a variety of tools have been used to alter one’s appearance for cultural or religious purposes or to conform to standards of beauty. As a defining feature of the face, the lips provide a unique opportunity for facial aesthetic enhancement. There has been a paradigm shift in medicine favoring preventative health and a desire to slow and even reverse the aging process.1 Acknowledging that product technology, skill sets, and cultural ideals continually evolve, this article highlights perioral anatomy, explains aging of the lower face, and reviews techniques to achieve perioral rejuvenation through volume restoration and muscle control.
Perioral Anatomy
The layers of the lips include the epidermis, subcutaneous tissue, orbicularis oris muscle fibers, and mucosa. The upper lip extends from the base of the nose to the mucosa inferiorly and to the nasolabial folds laterally. The curvilinear lower lip extends from the mucosa to the mandible inferiorly and to the oral commissures laterally.2 Circumferential at the vermilion-cutaneous junction, a raised area of pale skin known as the white roll accentuates the vermilion border and provides an important landmark during lip augmentation.3 At the upper lip, this elevation of the vermilion joins at a V-shaped depression centrally to form the Cupid’s bow. The cutaneous upper lip has 2 raised vertical pillars known as the philtral columns, which are formed from decussating fibers of the orbicularis oris muscle.2 The resultant midline depression is the philtrum. These defining features of the upper lip are to be preserved during augmentation procedures (Figure 1).4

The superior and inferior labial arteries, both branches of the facial artery, supply the upper and lower lip, respectively. The anastomotic arch of the superior labial artery is susceptible to injury from deep injection of the upper lip between the muscle layer and mucosa; therefore, caution must be exercised in this area.5 Injections into the vermilion and lower lip can be safely performed with less concern for vascular compromise. The vermilion derives its red color from the translucency of capillaries in the superficial papillae.2 The capillary plexus at the papillae and rich sensory nerve network render the lip a highly vascular and sensitive structure.
Aging of the Lower Face
Subcutaneous fat atrophy, loss of elasticity, gravitational forces, and remodeling of the skeletal foundation all contribute to aging of the lower face. Starting as early as the third decade of life, intrinsic factors including hormonal changes and genetically determined processes produce alterations in skin quality and structure. Similarly, extrinsic aging through environmental influences, namely exposure to UV radiation and smoking, accelerate the loss of skin integrity.6
The decreased laxity of the skin in combination with repeated contraction of the orbicularis oris muscle results in perioral rhytides.7 For women in particular, vertically oriented perioral rhytides develop above the vermilion; terminal hair follicles, thicker skin, and a greater density of subcutaneous fat are presumptive protective factors for males.8 With time, the cutaneous portion of the upper lip lengthens and there is redistribution of volume with effacement of the upper lip vermilion.9 Additionally, the demarcation of the vermilion becomes blurred secondary to pallor, flattening of the philtral columns, and loss of projection of the Cupid’s bow.10
Downturning of the oral commissures is observed secondary to a combination of gravity, bone resorption, and soft tissue volume loss. Hyperactivity of the depressor anguli oris muscle exacerbates the mesolabial folds, producing marionette lines and a saddened expression.7 With ongoing volume loss and ligament laxity, tissue redistributes near the jaws and chin, giving rise to jowls. Similarly, perioral volume loss and descent of the malar fat-pad deepen the nasolabial folds in the aging midface.6
The main objective of perioral rejuvenation is to reinstate a harmonious refreshed look to the lower face; however, aesthetic analysis should occur within the context of the face as a whole, as the lips should complement the surrounding perioral cosmetic unit and overall skeletal foundation of the face. To accomplish this goal, the dermatologist’s armamentarium contains a broad variety of approaches including restriction of muscle movement, volume restoration, and surface contouring.
Volume Restoration
Treatment Options
In 2015, hyaluronic acid (HA) fillers constituted 80% of all injectable soft-tissue fillers, an 8% increase from 2014.11 Hyaluronic acid has achieved immense popularity as a temporary dermal filler given its biocompatibility, longevity, and reversibility via hyaluronidase.12
Hyaluronic acid is a naturally occurring glycosaminoglycan that comprises the connective tissue matrix. The molecular composition affords HA its hydrophilic property, which augments dermal volume.7 Endogenous HA has a short half-life, and chemical modification by a cross-linking process extends longevity by 6 to 12 months. The various HA fillers are distinguished by method of purification, size of molecules, concentration and degree of cross-linking, and viscosity.7,13,14 These differences dictate overall clinical performance such as flow properties, longevity, and stability. As a general rule, a high-viscosity product is more appropriate for deeper augmentation; fillers with low viscosity are more appropriate for correction of shallow defects.1 Table 1 lists the HA fillers that are currently approved by the US Food and Drug Administration for lip augmentation and/or perioral rhytides in adults 21 years and older.15-17
Randomized controlled trials comparing the efficacy, longevity, and tolerability of different HA products are lacking in the literature and, where present, have strong industry influence.18,19 The advent of assessment scales has provided an objective evaluation of perioral and lip augmentation, facilitating comparisons between products in both clinical research and practice.20

Semipermanent biostimulatory dermal fillers such as calcium hydroxylapatite and poly-L-lactic acid are not recommended for lip augmentation due to an increased incidence of submucosal nodule formation.6,14,21 Likewise, permanent fillers are not recommended given their irreversibility and risk of nodule formation around the lips.14,22 Nonetheless, liquid silicone (purified polydimethylsiloxane) administered via a microdroplet technique (0.01 mL of silicone at a time, no more than 1 cc per lip per session) has been used off label as a permanent filling agent for lip augmentation with limited complications.23 Regardless, trepidations about its use with respect to reported risks continue to limit its application.22
Similarly, surgical lip implants such as expanded polytetrafluoroethylene is an option for a subset of patients desiring permanent enhancement but are less commonly utilized given the side-effect profile, irreversibility, and relatively invasive nature of the procedure.22 Lastly, autologous fat transfer has been used in correction of the nasolabial and mesolabial folds as well as in lip augmentation; however, irregular surface contours and unpredictable longevity secondary to postinjection resorption (20%–90%) has limited its popularity.3,14,21
HA Injection Technique
With respect to HA fillers in the perioral area, numerous approaches have been described.10,22 The techniques in Table 2 provide a foundation for lip rejuvenation.

Several injection techniques exist, including serial puncture, linear threading, cross-hatching, and fanning in a retrograde or anterograde manner.24 A blunt microcannula (27 gauge, 38 mm) may be used in place of sharp needles and offers the benefit of increased patient comfort, reduced edema and ecchymosis, and shortened recovery period.25,26 Gentle massage of the product after injection can assist with an even contour. Lastly, a key determinant of successful outcomes is using an adequate volume of HA filler (1–2 mL for shaping the vermilion border and volumizing the lips).27 Figure 2 highlights a clinical example of HA filler for lip augmentation.

Fortunately, most complications encountered with HA lip augmentation are mild and transient. The most commonly observed side effects include injection-site reactions such as pain, erythema, and edema. Similarly, most adverse effects are related to injection technique. All HA fillers are prone to the Tyndall effect, a consequence of too superficial an injection plane. Patients with history of recurrent herpes simplex virus infections should receive prophylactic antiviral therapy.12
Muscle Control
An emerging concept in rejuvenation of the lower face recognizes not only restoration of volume but also control of muscle movement. Local injection of botulinum toxin type A induces relaxation of hyperfunctional facial muscles through temporary inhibition of neurotransmitter release.6 The potential for paralysis of the oral cavity may limit the application of botulinum toxin type A in that region.7 Nonetheless, the off-label potential of botulinum toxin type A has expanded to include several targets in the lower face. The orbicularis oris muscle is targeted to soften perioral rhytides. Conservative dosing (1–2 U per lip quadrant or approximately 5 U total) and superficial injection is emphasized in this area.27 Similarly, the depressor anguli oris muscle is targeted by injection of 4 U bilaterally to soften the marionette lines. In the chin area, the mentalis muscle can be targeted by injection of 2 U deep into each belly of the muscle to reduce the mental crease and dimpling.28 Combination treatment with dermal filler and neurotoxin demonstrates effects that last longer than either modality alone without additional adverse events.29 With combination therapy, guidelines suggest treating with filler first.27
Conclusion
A greater understanding of the extrinsic and intrinsic factors that contribute to the structural and surface changes of the aging face coupled with a preference for minimally invasive procedures has revolutionized the dermatologist’s approach to perioral rejuvenation. Serving as a focal point of the face, the lips and perioral skin are well poised to benefit from this paradigm shift. A multifaceted approach utilizing dermal fillers and neurotoxins may be most appropriate and has demonstrated optimal outcomes in facial aesthetics.
Historically, a variety of tools have been used to alter one’s appearance for cultural or religious purposes or to conform to standards of beauty. As a defining feature of the face, the lips provide a unique opportunity for facial aesthetic enhancement. There has been a paradigm shift in medicine favoring preventative health and a desire to slow and even reverse the aging process.1 Acknowledging that product technology, skill sets, and cultural ideals continually evolve, this article highlights perioral anatomy, explains aging of the lower face, and reviews techniques to achieve perioral rejuvenation through volume restoration and muscle control.
Perioral Anatomy
The layers of the lips include the epidermis, subcutaneous tissue, orbicularis oris muscle fibers, and mucosa. The upper lip extends from the base of the nose to the mucosa inferiorly and to the nasolabial folds laterally. The curvilinear lower lip extends from the mucosa to the mandible inferiorly and to the oral commissures laterally.2 Circumferential at the vermilion-cutaneous junction, a raised area of pale skin known as the white roll accentuates the vermilion border and provides an important landmark during lip augmentation.3 At the upper lip, this elevation of the vermilion joins at a V-shaped depression centrally to form the Cupid’s bow. The cutaneous upper lip has 2 raised vertical pillars known as the philtral columns, which are formed from decussating fibers of the orbicularis oris muscle.2 The resultant midline depression is the philtrum. These defining features of the upper lip are to be preserved during augmentation procedures (Figure 1).4

The superior and inferior labial arteries, both branches of the facial artery, supply the upper and lower lip, respectively. The anastomotic arch of the superior labial artery is susceptible to injury from deep injection of the upper lip between the muscle layer and mucosa; therefore, caution must be exercised in this area.5 Injections into the vermilion and lower lip can be safely performed with less concern for vascular compromise. The vermilion derives its red color from the translucency of capillaries in the superficial papillae.2 The capillary plexus at the papillae and rich sensory nerve network render the lip a highly vascular and sensitive structure.
Aging of the Lower Face
Subcutaneous fat atrophy, loss of elasticity, gravitational forces, and remodeling of the skeletal foundation all contribute to aging of the lower face. Starting as early as the third decade of life, intrinsic factors including hormonal changes and genetically determined processes produce alterations in skin quality and structure. Similarly, extrinsic aging through environmental influences, namely exposure to UV radiation and smoking, accelerate the loss of skin integrity.6
The decreased laxity of the skin in combination with repeated contraction of the orbicularis oris muscle results in perioral rhytides.7 For women in particular, vertically oriented perioral rhytides develop above the vermilion; terminal hair follicles, thicker skin, and a greater density of subcutaneous fat are presumptive protective factors for males.8 With time, the cutaneous portion of the upper lip lengthens and there is redistribution of volume with effacement of the upper lip vermilion.9 Additionally, the demarcation of the vermilion becomes blurred secondary to pallor, flattening of the philtral columns, and loss of projection of the Cupid’s bow.10
Downturning of the oral commissures is observed secondary to a combination of gravity, bone resorption, and soft tissue volume loss. Hyperactivity of the depressor anguli oris muscle exacerbates the mesolabial folds, producing marionette lines and a saddened expression.7 With ongoing volume loss and ligament laxity, tissue redistributes near the jaws and chin, giving rise to jowls. Similarly, perioral volume loss and descent of the malar fat-pad deepen the nasolabial folds in the aging midface.6
The main objective of perioral rejuvenation is to reinstate a harmonious refreshed look to the lower face; however, aesthetic analysis should occur within the context of the face as a whole, as the lips should complement the surrounding perioral cosmetic unit and overall skeletal foundation of the face. To accomplish this goal, the dermatologist’s armamentarium contains a broad variety of approaches including restriction of muscle movement, volume restoration, and surface contouring.
Volume Restoration
Treatment Options
In 2015, hyaluronic acid (HA) fillers constituted 80% of all injectable soft-tissue fillers, an 8% increase from 2014.11 Hyaluronic acid has achieved immense popularity as a temporary dermal filler given its biocompatibility, longevity, and reversibility via hyaluronidase.12
Hyaluronic acid is a naturally occurring glycosaminoglycan that comprises the connective tissue matrix. The molecular composition affords HA its hydrophilic property, which augments dermal volume.7 Endogenous HA has a short half-life, and chemical modification by a cross-linking process extends longevity by 6 to 12 months. The various HA fillers are distinguished by method of purification, size of molecules, concentration and degree of cross-linking, and viscosity.7,13,14 These differences dictate overall clinical performance such as flow properties, longevity, and stability. As a general rule, a high-viscosity product is more appropriate for deeper augmentation; fillers with low viscosity are more appropriate for correction of shallow defects.1 Table 1 lists the HA fillers that are currently approved by the US Food and Drug Administration for lip augmentation and/or perioral rhytides in adults 21 years and older.15-17
Randomized controlled trials comparing the efficacy, longevity, and tolerability of different HA products are lacking in the literature and, where present, have strong industry influence.18,19 The advent of assessment scales has provided an objective evaluation of perioral and lip augmentation, facilitating comparisons between products in both clinical research and practice.20

Semipermanent biostimulatory dermal fillers such as calcium hydroxylapatite and poly-L-lactic acid are not recommended for lip augmentation due to an increased incidence of submucosal nodule formation.6,14,21 Likewise, permanent fillers are not recommended given their irreversibility and risk of nodule formation around the lips.14,22 Nonetheless, liquid silicone (purified polydimethylsiloxane) administered via a microdroplet technique (0.01 mL of silicone at a time, no more than 1 cc per lip per session) has been used off label as a permanent filling agent for lip augmentation with limited complications.23 Regardless, trepidations about its use with respect to reported risks continue to limit its application.22
Similarly, surgical lip implants such as expanded polytetrafluoroethylene is an option for a subset of patients desiring permanent enhancement but are less commonly utilized given the side-effect profile, irreversibility, and relatively invasive nature of the procedure.22 Lastly, autologous fat transfer has been used in correction of the nasolabial and mesolabial folds as well as in lip augmentation; however, irregular surface contours and unpredictable longevity secondary to postinjection resorption (20%–90%) has limited its popularity.3,14,21
HA Injection Technique
With respect to HA fillers in the perioral area, numerous approaches have been described.10,22 The techniques in Table 2 provide a foundation for lip rejuvenation.

Several injection techniques exist, including serial puncture, linear threading, cross-hatching, and fanning in a retrograde or anterograde manner.24 A blunt microcannula (27 gauge, 38 mm) may be used in place of sharp needles and offers the benefit of increased patient comfort, reduced edema and ecchymosis, and shortened recovery period.25,26 Gentle massage of the product after injection can assist with an even contour. Lastly, a key determinant of successful outcomes is using an adequate volume of HA filler (1–2 mL for shaping the vermilion border and volumizing the lips).27 Figure 2 highlights a clinical example of HA filler for lip augmentation.

Fortunately, most complications encountered with HA lip augmentation are mild and transient. The most commonly observed side effects include injection-site reactions such as pain, erythema, and edema. Similarly, most adverse effects are related to injection technique. All HA fillers are prone to the Tyndall effect, a consequence of too superficial an injection plane. Patients with history of recurrent herpes simplex virus infections should receive prophylactic antiviral therapy.12
Muscle Control
An emerging concept in rejuvenation of the lower face recognizes not only restoration of volume but also control of muscle movement. Local injection of botulinum toxin type A induces relaxation of hyperfunctional facial muscles through temporary inhibition of neurotransmitter release.6 The potential for paralysis of the oral cavity may limit the application of botulinum toxin type A in that region.7 Nonetheless, the off-label potential of botulinum toxin type A has expanded to include several targets in the lower face. The orbicularis oris muscle is targeted to soften perioral rhytides. Conservative dosing (1–2 U per lip quadrant or approximately 5 U total) and superficial injection is emphasized in this area.27 Similarly, the depressor anguli oris muscle is targeted by injection of 4 U bilaterally to soften the marionette lines. In the chin area, the mentalis muscle can be targeted by injection of 2 U deep into each belly of the muscle to reduce the mental crease and dimpling.28 Combination treatment with dermal filler and neurotoxin demonstrates effects that last longer than either modality alone without additional adverse events.29 With combination therapy, guidelines suggest treating with filler first.27
Conclusion
A greater understanding of the extrinsic and intrinsic factors that contribute to the structural and surface changes of the aging face coupled with a preference for minimally invasive procedures has revolutionized the dermatologist’s approach to perioral rejuvenation. Serving as a focal point of the face, the lips and perioral skin are well poised to benefit from this paradigm shift. A multifaceted approach utilizing dermal fillers and neurotoxins may be most appropriate and has demonstrated optimal outcomes in facial aesthetics.
- Buck DW, Alam M, Kim JYS. Injectable fillers for facial rejuvenation: a review. J Plast Reconstr Aesthet Surg. 2009;62:11-18.
- Guareschi M, Stella E. Lips. In: Goisis M, ed. Injections in Aesthetic Medicine. Milan, Italy: Springer; 2014:125-136.
- Byrne PJ, Hilger PA. Lip augmentation. Facial Plast Surg. 2004;20:31-38.
- Niamtu J. Rejuvenation of the lip and perioral areas. In: Bell WH, Guerroro CA, eds. Distraction Osteogenesis of the Facial Skeleton. Ontario, Canada: BC Decker Inc; 2007:38-48.
- Tansatit T, Apinuntrum P, Phetudom T. A typical pattern of the labial arteries with implication for lip augmentation with injectable fillers. Aesthet Plast Surg. 2014;38:1083-1089.
- Sadick NS, Karcher C, Palmisano L. Cosmetic dermatology of the aging face. Clin Dermatol. 2009;27(suppl):S3-S12.
- Ali MJ, Ende K, Mass CS. Perioral rejuvenation and lip augmentation. Facial Plast Surg Clin N Am. 2007;15:491-500.
- Chien AL, Qi J, Cheng N, et al. Perioral wrinkles are associated with female gender, aging, and smoking: development of a gender-specific photonumeric scale. J Am Acad Dermatol. 2016;74:924-930.
- Iblher N, Stark GB, Penna V. The aging perioral region—do we really know what is happening? J Nutr Health Aging. 2012;16:581-585.
- Sarnoff DS, Gotkin RH. Six steps to the “perfect” lip. J Drugs Dermatol. 2012;11:1081-1088.
- American Society of Plastic Surgeons. 2015 Cosmetic plastic surgery statistics. https://d2wirczt3b6wjm.cloudfront.net/News/Statistics/2015/cosmetic-procedure-trends-2015.pdf. Published February 26, 2015. Accessed October 5, 2016.
- Abduljabbar MH, Basendwh MA. Complications of hyaluronic acid fillers and their managements. J Dermatol Surg. 2016;20:1-7.
- Luebberding S, Alexiades-Armenakas M. Facial volume augmentation in 2014: overview of different filler options. J Drugs Dermatol. 2013;12:1339-1344.
- Huang Attenello N, Mass CS. Injectable fillers: review of material and properties. Facial Plast Surg. 2015;31:29-34.
- Eccleston D, Murphy DK. Juvéderm Volbella in the perioral area: a 12-month perspective, multicenter, open-label study. Clin Cosmet Investig Dermatol. 2012;5:167-172.
- Raspaldo H, Chantrey J, Belhaouari L, et al. Lip and perioral enhancement: a 12-month prospective, randomized, controlled study. J Drugs Dermatol. 2015;14:1444-1452.
- Soft tissue fillers approved by the center for devices and radiological health. US Food and Drug Administration website. http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/CosmeticDevices/WrinkleFillers/ucm227749.htm. Updated July 27, 2015. Accessed October 5, 2016.
- Butterwick K, Marmur E, Narurkar V, et al. HYC-24L demonstrates greater effectiveness with less pain than CPM-22.5 for treatment of perioral lines in a randomized controlled trial. Dermatol Surg. 2015;41:1351-1360.
- San Miguel Moragas J, Reddy RR, Hernández Alfaro F, et al. Systematic review of “filling” procedures for lip augmentation regarding types of material, outcomes and complications. J Craniomaxillofac Surg. 2015;43:883-906.
- Cohen JL, Thomas J, Paradkar D, et al. An interrater and intrarater reliability study of 3 photographic scales for the classification of perioral aesthetic features. Dermatol Surg. 2014;40:663-670.
- Broder KW, Cohen SR. An overview of permanent and semipermanent fillers. Plast Reconstr Surg. 2006;118(3 suppl):7S-14S.
- Sarnoff DS, Saini R, Gotkin RH. Comparison of filling agents for lip augmentation. Aesthet Surg J. 2008;28:556-563.
- Moscona RA, Fodor L. A retrospective study on liquid injectable silicone for lip augmentation: long-term results and patient satisfaction. J Plast Reconstr Aesthet Surg. 2010;63:1694-1698.
- Bertucci V, Lynde CB. Current concepts in the use of small-particle hyaluronic acid. Plast Reconstr Surg. 2015;136(5 suppl):132S-138S.
- Wilson AJ, Taglienti AJ, Chang CS, et al. Current applications of facial volumization with fillers. Plast Reconstr Surg. 2016;137:E872-E889.
- Dewandre L, Caperton C, Fulton J. Filler injections with the blunt-tip microcannula compared to the sharp hypodermic needle. J Drugs Dermatol. 2012;11:1098-1103.
- Carruthers JD, Glogau RG, Blitzer A; Facial Aesthetics Consensus Group Faculty. Advances in facial rejuvenation: botulinum toxin type A, hyaluronic acid dermal fillers, and combination therapies-consensus recommendations. Plast Reconstr Surg. 2008;121(5 suppl):5S-30S.
- Wu DC, Fabi SG, Goldman MP. Neurotoxins: current concepts in cosmetic use on the face and neck-lower face. Plast Reconstr Surg. 2015;136(5 suppl):76S-79S.
- Carruthers A, Carruthers J, Monheit GD, et al. Multicenter, randomized, parallel-group study of the safety and effectiveness of onabotulinumtoxin A and hyaluronic acid dermal fillers (24-mg/mL smooth, cohesive gel) alone and in combination for lower facial rejuvenation. Dermatol Surg. 2010;36:2121-2134.
- Buck DW, Alam M, Kim JYS. Injectable fillers for facial rejuvenation: a review. J Plast Reconstr Aesthet Surg. 2009;62:11-18.
- Guareschi M, Stella E. Lips. In: Goisis M, ed. Injections in Aesthetic Medicine. Milan, Italy: Springer; 2014:125-136.
- Byrne PJ, Hilger PA. Lip augmentation. Facial Plast Surg. 2004;20:31-38.
- Niamtu J. Rejuvenation of the lip and perioral areas. In: Bell WH, Guerroro CA, eds. Distraction Osteogenesis of the Facial Skeleton. Ontario, Canada: BC Decker Inc; 2007:38-48.
- Tansatit T, Apinuntrum P, Phetudom T. A typical pattern of the labial arteries with implication for lip augmentation with injectable fillers. Aesthet Plast Surg. 2014;38:1083-1089.
- Sadick NS, Karcher C, Palmisano L. Cosmetic dermatology of the aging face. Clin Dermatol. 2009;27(suppl):S3-S12.
- Ali MJ, Ende K, Mass CS. Perioral rejuvenation and lip augmentation. Facial Plast Surg Clin N Am. 2007;15:491-500.
- Chien AL, Qi J, Cheng N, et al. Perioral wrinkles are associated with female gender, aging, and smoking: development of a gender-specific photonumeric scale. J Am Acad Dermatol. 2016;74:924-930.
- Iblher N, Stark GB, Penna V. The aging perioral region—do we really know what is happening? J Nutr Health Aging. 2012;16:581-585.
- Sarnoff DS, Gotkin RH. Six steps to the “perfect” lip. J Drugs Dermatol. 2012;11:1081-1088.
- American Society of Plastic Surgeons. 2015 Cosmetic plastic surgery statistics. https://d2wirczt3b6wjm.cloudfront.net/News/Statistics/2015/cosmetic-procedure-trends-2015.pdf. Published February 26, 2015. Accessed October 5, 2016.
- Abduljabbar MH, Basendwh MA. Complications of hyaluronic acid fillers and their managements. J Dermatol Surg. 2016;20:1-7.
- Luebberding S, Alexiades-Armenakas M. Facial volume augmentation in 2014: overview of different filler options. J Drugs Dermatol. 2013;12:1339-1344.
- Huang Attenello N, Mass CS. Injectable fillers: review of material and properties. Facial Plast Surg. 2015;31:29-34.
- Eccleston D, Murphy DK. Juvéderm Volbella in the perioral area: a 12-month perspective, multicenter, open-label study. Clin Cosmet Investig Dermatol. 2012;5:167-172.
- Raspaldo H, Chantrey J, Belhaouari L, et al. Lip and perioral enhancement: a 12-month prospective, randomized, controlled study. J Drugs Dermatol. 2015;14:1444-1452.
- Soft tissue fillers approved by the center for devices and radiological health. US Food and Drug Administration website. http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/CosmeticDevices/WrinkleFillers/ucm227749.htm. Updated July 27, 2015. Accessed October 5, 2016.
- Butterwick K, Marmur E, Narurkar V, et al. HYC-24L demonstrates greater effectiveness with less pain than CPM-22.5 for treatment of perioral lines in a randomized controlled trial. Dermatol Surg. 2015;41:1351-1360.
- San Miguel Moragas J, Reddy RR, Hernández Alfaro F, et al. Systematic review of “filling” procedures for lip augmentation regarding types of material, outcomes and complications. J Craniomaxillofac Surg. 2015;43:883-906.
- Cohen JL, Thomas J, Paradkar D, et al. An interrater and intrarater reliability study of 3 photographic scales for the classification of perioral aesthetic features. Dermatol Surg. 2014;40:663-670.
- Broder KW, Cohen SR. An overview of permanent and semipermanent fillers. Plast Reconstr Surg. 2006;118(3 suppl):7S-14S.
- Sarnoff DS, Saini R, Gotkin RH. Comparison of filling agents for lip augmentation. Aesthet Surg J. 2008;28:556-563.
- Moscona RA, Fodor L. A retrospective study on liquid injectable silicone for lip augmentation: long-term results and patient satisfaction. J Plast Reconstr Aesthet Surg. 2010;63:1694-1698.
- Bertucci V, Lynde CB. Current concepts in the use of small-particle hyaluronic acid. Plast Reconstr Surg. 2015;136(5 suppl):132S-138S.
- Wilson AJ, Taglienti AJ, Chang CS, et al. Current applications of facial volumization with fillers. Plast Reconstr Surg. 2016;137:E872-E889.
- Dewandre L, Caperton C, Fulton J. Filler injections with the blunt-tip microcannula compared to the sharp hypodermic needle. J Drugs Dermatol. 2012;11:1098-1103.
- Carruthers JD, Glogau RG, Blitzer A; Facial Aesthetics Consensus Group Faculty. Advances in facial rejuvenation: botulinum toxin type A, hyaluronic acid dermal fillers, and combination therapies-consensus recommendations. Plast Reconstr Surg. 2008;121(5 suppl):5S-30S.
- Wu DC, Fabi SG, Goldman MP. Neurotoxins: current concepts in cosmetic use on the face and neck-lower face. Plast Reconstr Surg. 2015;136(5 suppl):76S-79S.
- Carruthers A, Carruthers J, Monheit GD, et al. Multicenter, randomized, parallel-group study of the safety and effectiveness of onabotulinumtoxin A and hyaluronic acid dermal fillers (24-mg/mL smooth, cohesive gel) alone and in combination for lower facial rejuvenation. Dermatol Surg. 2010;36:2121-2134.
Practice Points
- Hyaluronic acid (HA) fillers are approved by the US Food and Drug Administration for lip augmentation and/or treatment of perioral rhytides in adults 21 years and older.
- Most complications encountered with HA lip augmentation are mild and transient and can include injection-site reactions such as pain, erythema, and edema.
- Combination treatment with dermal fillers and neurotoxins (off label) may demonstrate effects that last longer than either modality alone without additional adverse events.
Reasons Behind the Ink
Tattoos have been viewed as one of the most exotic forms of art for thousands of years. In ancient times, tattoos were used mainly for therapeutic and status purposes. According to British archeologist Joann Fletcher, the oldest evidence of tattoo use was found on the famous “Iceman,” a 5200-year-old frozen mummy that was discovered more than 20 years ago.1 Tattoos were thought to be a form of therapy used to decrease joint pain. On the other hand, the ancient Egyptians used tattoos as symbols of wealth and high status; surprisingly, only women were tattooed. Fletcher also reported that tattoos were used as a form of therapy during pregnancy in upper-class women.1
Tattoos have served different purposes in the last few centuries, making their way to the United States at the start of the 20th century.2 New York City became the tattoo capital of the country. During this early period, male artists often would tattoo their wives so that they could advertise their work. After the Prohibition era, tattoos became widely used within the US Military, becoming a way to show pride and patriotism.2
Due to the permanent nature of tattoos, we sought to understand the reasons for obtaining this particular genre of body art. The purpose of this study was to provide a greater understanding of the current demographics of individuals who get tattoos, looking at specific trends in age and level of education of those who get tattoos as well as the motivation for tattoo placement. As dermatologists, it is essential to understand this patient population to be able to provide services (ie, tattoo removal) in the safe setting of a physician’s office.
Methods
The study was conducted at a private dermatology clinic in the Chicago (Illinois) metropolitan area with no institutional review board approval. Between January 2011 and December 2012, local patients with at least 1 tattoo were asked, with assumed consent, to fill out an investigator-developed survey containing 18 multiple-choice questions regarding age, educational and family background, and other factors. The race and gender of the respondents as well as the number of patients who declined to complete the survey were not recorded.
Results
A total of 363 patients completed the in-person survey. Responses were tabulated and converted into percentages for comparison (N=363). Data analysis was divided into 3 parameters: education level, health concerns, and motivation for getting a tattoo. Figure 1 shows that 70% of respondents had obtained a college degree or higher.
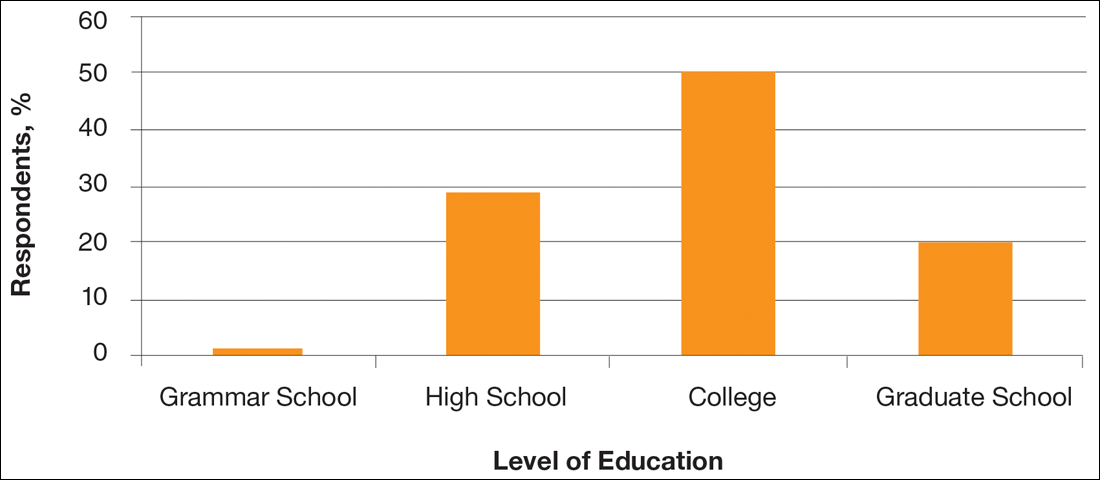
With regard to health concerns associated with tattoos, the majority of respondents (71%) claimed they were not concerned with the health risks (eg, infection with human immunodeficiency virus or hepatitis C virus) associated with getting a tattoo. Also, only 6% of respondents admitted to being under the influence of drugs or alcohol at the time of getting a tattoo. Of 21 respondents who claimed drugs and/or alcohol were part of their tattoo experience, the highest level of education was high school in 7 respondents and 2 got their first tattoo when they were younger than 14 years.
Survey results revealed that the majority of respondents got a tattoo as an act of rememberance (Figure 2). For example, one respondent reported getting a tattoo for religious purposes, while another got a tattoo to celebrate and mark each level of completed education (ie, high school, college, graduate school). However, a high percentage of respondents (26%) got a tattoo for fun.
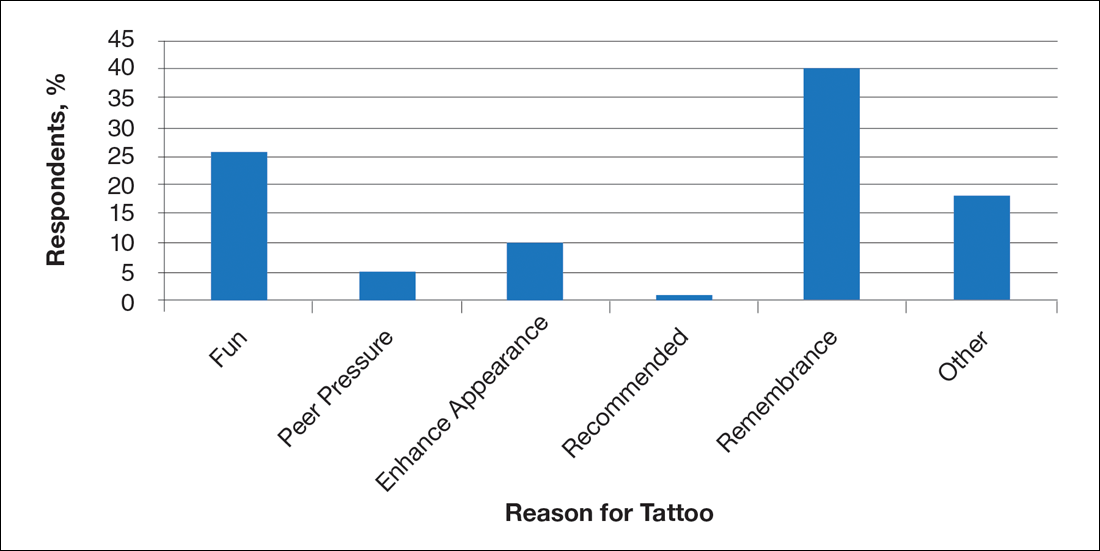
Comment
Although ancient tattoos were used for therapeutic purposes, this study revealed that tattoos are now obtained by individuals with higher levels of education to remember a loved one or purely for enjoyment. The potential health risks associated with getting a tattoo did not deter the respondents in this study. Converse to the popular belief that individuals are under the influence of drugs and/or alcohol when getting a tattoo, our study found that only 6% of respondents were under the influence. A comparable trend was found among US military service members in a similar study.3 The majority of respondents did not regret their tattoos and did not report taking a mind-altering substance. Tattoos serve as a symbol of one’s proud individualism.3 However, a 2001 study found a correlation between greater use of alcohol and marijuana among college students with tattoos and piecings.4 These circumstances may lead patients to seek consultation from a dermatologist for tattoo removal. Therefore, it is important to have a better understanding of this particular patient population to facilitate care in an efficient manner.
Evaluation of the gender and race of survey respondents would be useful in the future. Financial status of respondents also may be explored, as wealth and status were used by the ancient Egyptians to determine who could get a tattoo. A follow-up analysis on removal of tattoos also will be explored in the future.
- Lineberry C. Tattoos: the ancient and mysterious history. Smithsonian. http://www.smithsonianmag.com/history-archaeology/tattoo.html. Published January 1, 2007. Accessed April 29, 2016.
- Bickerstaff L. Tattoos: fad, fashion, or folly? Odyssey. 2005;14:34-36.
- Lande RG, Bahroo BA, Soumoff A. United States military service members and their tattoos: a descriptive study. Mil Med. 2013;178:921-925.
- Forbes GB. College students with tattoos and piercings: motives, family experiences, personality factors, and perception by others. Psychol Rep. 2001;89:774-786.
Tattoos have been viewed as one of the most exotic forms of art for thousands of years. In ancient times, tattoos were used mainly for therapeutic and status purposes. According to British archeologist Joann Fletcher, the oldest evidence of tattoo use was found on the famous “Iceman,” a 5200-year-old frozen mummy that was discovered more than 20 years ago.1 Tattoos were thought to be a form of therapy used to decrease joint pain. On the other hand, the ancient Egyptians used tattoos as symbols of wealth and high status; surprisingly, only women were tattooed. Fletcher also reported that tattoos were used as a form of therapy during pregnancy in upper-class women.1
Tattoos have served different purposes in the last few centuries, making their way to the United States at the start of the 20th century.2 New York City became the tattoo capital of the country. During this early period, male artists often would tattoo their wives so that they could advertise their work. After the Prohibition era, tattoos became widely used within the US Military, becoming a way to show pride and patriotism.2
Due to the permanent nature of tattoos, we sought to understand the reasons for obtaining this particular genre of body art. The purpose of this study was to provide a greater understanding of the current demographics of individuals who get tattoos, looking at specific trends in age and level of education of those who get tattoos as well as the motivation for tattoo placement. As dermatologists, it is essential to understand this patient population to be able to provide services (ie, tattoo removal) in the safe setting of a physician’s office.
Methods
The study was conducted at a private dermatology clinic in the Chicago (Illinois) metropolitan area with no institutional review board approval. Between January 2011 and December 2012, local patients with at least 1 tattoo were asked, with assumed consent, to fill out an investigator-developed survey containing 18 multiple-choice questions regarding age, educational and family background, and other factors. The race and gender of the respondents as well as the number of patients who declined to complete the survey were not recorded.
Results
A total of 363 patients completed the in-person survey. Responses were tabulated and converted into percentages for comparison (N=363). Data analysis was divided into 3 parameters: education level, health concerns, and motivation for getting a tattoo. Figure 1 shows that 70% of respondents had obtained a college degree or higher.

With regard to health concerns associated with tattoos, the majority of respondents (71%) claimed they were not concerned with the health risks (eg, infection with human immunodeficiency virus or hepatitis C virus) associated with getting a tattoo. Also, only 6% of respondents admitted to being under the influence of drugs or alcohol at the time of getting a tattoo. Of 21 respondents who claimed drugs and/or alcohol were part of their tattoo experience, the highest level of education was high school in 7 respondents and 2 got their first tattoo when they were younger than 14 years.
Survey results revealed that the majority of respondents got a tattoo as an act of rememberance (Figure 2). For example, one respondent reported getting a tattoo for religious purposes, while another got a tattoo to celebrate and mark each level of completed education (ie, high school, college, graduate school). However, a high percentage of respondents (26%) got a tattoo for fun.

Comment
Although ancient tattoos were used for therapeutic purposes, this study revealed that tattoos are now obtained by individuals with higher levels of education to remember a loved one or purely for enjoyment. The potential health risks associated with getting a tattoo did not deter the respondents in this study. Converse to the popular belief that individuals are under the influence of drugs and/or alcohol when getting a tattoo, our study found that only 6% of respondents were under the influence. A comparable trend was found among US military service members in a similar study.3 The majority of respondents did not regret their tattoos and did not report taking a mind-altering substance. Tattoos serve as a symbol of one’s proud individualism.3 However, a 2001 study found a correlation between greater use of alcohol and marijuana among college students with tattoos and piecings.4 These circumstances may lead patients to seek consultation from a dermatologist for tattoo removal. Therefore, it is important to have a better understanding of this particular patient population to facilitate care in an efficient manner.
Evaluation of the gender and race of survey respondents would be useful in the future. Financial status of respondents also may be explored, as wealth and status were used by the ancient Egyptians to determine who could get a tattoo. A follow-up analysis on removal of tattoos also will be explored in the future.
Tattoos have been viewed as one of the most exotic forms of art for thousands of years. In ancient times, tattoos were used mainly for therapeutic and status purposes. According to British archeologist Joann Fletcher, the oldest evidence of tattoo use was found on the famous “Iceman,” a 5200-year-old frozen mummy that was discovered more than 20 years ago.1 Tattoos were thought to be a form of therapy used to decrease joint pain. On the other hand, the ancient Egyptians used tattoos as symbols of wealth and high status; surprisingly, only women were tattooed. Fletcher also reported that tattoos were used as a form of therapy during pregnancy in upper-class women.1
Tattoos have served different purposes in the last few centuries, making their way to the United States at the start of the 20th century.2 New York City became the tattoo capital of the country. During this early period, male artists often would tattoo their wives so that they could advertise their work. After the Prohibition era, tattoos became widely used within the US Military, becoming a way to show pride and patriotism.2
Due to the permanent nature of tattoos, we sought to understand the reasons for obtaining this particular genre of body art. The purpose of this study was to provide a greater understanding of the current demographics of individuals who get tattoos, looking at specific trends in age and level of education of those who get tattoos as well as the motivation for tattoo placement. As dermatologists, it is essential to understand this patient population to be able to provide services (ie, tattoo removal) in the safe setting of a physician’s office.
Methods
The study was conducted at a private dermatology clinic in the Chicago (Illinois) metropolitan area with no institutional review board approval. Between January 2011 and December 2012, local patients with at least 1 tattoo were asked, with assumed consent, to fill out an investigator-developed survey containing 18 multiple-choice questions regarding age, educational and family background, and other factors. The race and gender of the respondents as well as the number of patients who declined to complete the survey were not recorded.
Results
A total of 363 patients completed the in-person survey. Responses were tabulated and converted into percentages for comparison (N=363). Data analysis was divided into 3 parameters: education level, health concerns, and motivation for getting a tattoo. Figure 1 shows that 70% of respondents had obtained a college degree or higher.

With regard to health concerns associated with tattoos, the majority of respondents (71%) claimed they were not concerned with the health risks (eg, infection with human immunodeficiency virus or hepatitis C virus) associated with getting a tattoo. Also, only 6% of respondents admitted to being under the influence of drugs or alcohol at the time of getting a tattoo. Of 21 respondents who claimed drugs and/or alcohol were part of their tattoo experience, the highest level of education was high school in 7 respondents and 2 got their first tattoo when they were younger than 14 years.
Survey results revealed that the majority of respondents got a tattoo as an act of rememberance (Figure 2). For example, one respondent reported getting a tattoo for religious purposes, while another got a tattoo to celebrate and mark each level of completed education (ie, high school, college, graduate school). However, a high percentage of respondents (26%) got a tattoo for fun.

Comment
Although ancient tattoos were used for therapeutic purposes, this study revealed that tattoos are now obtained by individuals with higher levels of education to remember a loved one or purely for enjoyment. The potential health risks associated with getting a tattoo did not deter the respondents in this study. Converse to the popular belief that individuals are under the influence of drugs and/or alcohol when getting a tattoo, our study found that only 6% of respondents were under the influence. A comparable trend was found among US military service members in a similar study.3 The majority of respondents did not regret their tattoos and did not report taking a mind-altering substance. Tattoos serve as a symbol of one’s proud individualism.3 However, a 2001 study found a correlation between greater use of alcohol and marijuana among college students with tattoos and piecings.4 These circumstances may lead patients to seek consultation from a dermatologist for tattoo removal. Therefore, it is important to have a better understanding of this particular patient population to facilitate care in an efficient manner.
Evaluation of the gender and race of survey respondents would be useful in the future. Financial status of respondents also may be explored, as wealth and status were used by the ancient Egyptians to determine who could get a tattoo. A follow-up analysis on removal of tattoos also will be explored in the future.
- Lineberry C. Tattoos: the ancient and mysterious history. Smithsonian. http://www.smithsonianmag.com/history-archaeology/tattoo.html. Published January 1, 2007. Accessed April 29, 2016.
- Bickerstaff L. Tattoos: fad, fashion, or folly? Odyssey. 2005;14:34-36.
- Lande RG, Bahroo BA, Soumoff A. United States military service members and their tattoos: a descriptive study. Mil Med. 2013;178:921-925.
- Forbes GB. College students with tattoos and piercings: motives, family experiences, personality factors, and perception by others. Psychol Rep. 2001;89:774-786.
- Lineberry C. Tattoos: the ancient and mysterious history. Smithsonian. http://www.smithsonianmag.com/history-archaeology/tattoo.html. Published January 1, 2007. Accessed April 29, 2016.
- Bickerstaff L. Tattoos: fad, fashion, or folly? Odyssey. 2005;14:34-36.
- Lande RG, Bahroo BA, Soumoff A. United States military service members and their tattoos: a descriptive study. Mil Med. 2013;178:921-925.
- Forbes GB. College students with tattoos and piercings: motives, family experiences, personality factors, and perception by others. Psychol Rep. 2001;89:774-786.
Practice Points
- Individuals who get tattoos often are more educated and well informed than previously thought, more likely leading them to seek removal if desired.
- Our results indicate that tattoos are not regretted as often as previously speculated.