User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Cost of Diagnosing Psoriasis and Rosacea for Dermatologists Versus Primary Care Physicians
Growing incentives to control health care costs may cause accountable care organizations (ACOs) to reconsider how diseases are best managed. Few studies have examined the cost difference between primary care providers (PCPs) and specialists in managing the same disease. Limited data have suggested that management of some diseases by a PCP may be less costly compared to a specialist1,2; however, it is not clear if this finding extends to skin disease. This study sought to assess the cost of seeing a dermatologist versus a PCP for diagnosis of the common skin diseases psoriasis and rosacea.
Methods
Patient data were obtained from the Humana database, a large commercial data set for claims and reimbursed costs encompassing 18,162,539 patients covered between January 2007 and December 2014. Our study population consisted of 3,944,465 patients with claims that included International Classification of Diseases, Ninth Revision (ICD-9), codes for dermatological diagnoses (680.0–709.9). We searched by ICD-9 code for US patients with primary diagnoses of psoriasis (696.1) and rosacea (695.3). We narrowed the search to include patients aged 30 to 64 years, as the diagnoses for these diseases are most common in patients older than 30 years. Patients who were older than 64 years were not included in the study, as most are covered by Medicare and therefore costs covered by Humana in this age group would not be as representative as in younger age groups. Total and average diagnosis-related costs per patient were compared between dermatologists and PCPs. Diagnosis-related costs encompassed physician reimbursement; laboratory and imaging costs, including skin biopsies; inpatient hospitalization cost; and any other charge that could be coded or billed by providers and reimbursed by the insurance company. To be eligible for reimbursement from Humana, dermatologists and PCPs must be registered with the insurer according to specialty board certification and practice credentialing, and they are reimbursed differently based on specialty. Drug costs, which would possibly skew the data toward providers using more expensive systemic medications (ie, dermatologists), were not included in this study, as the discussion is better reserved for long-term management of disease rather than diagnosis-related costs. All diagnoses of psoriasis were included in the study, which likely includes all severities of psoriasis, though we did not have the ability to further break down these diagnoses by severity.
Results
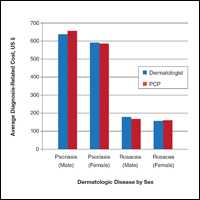
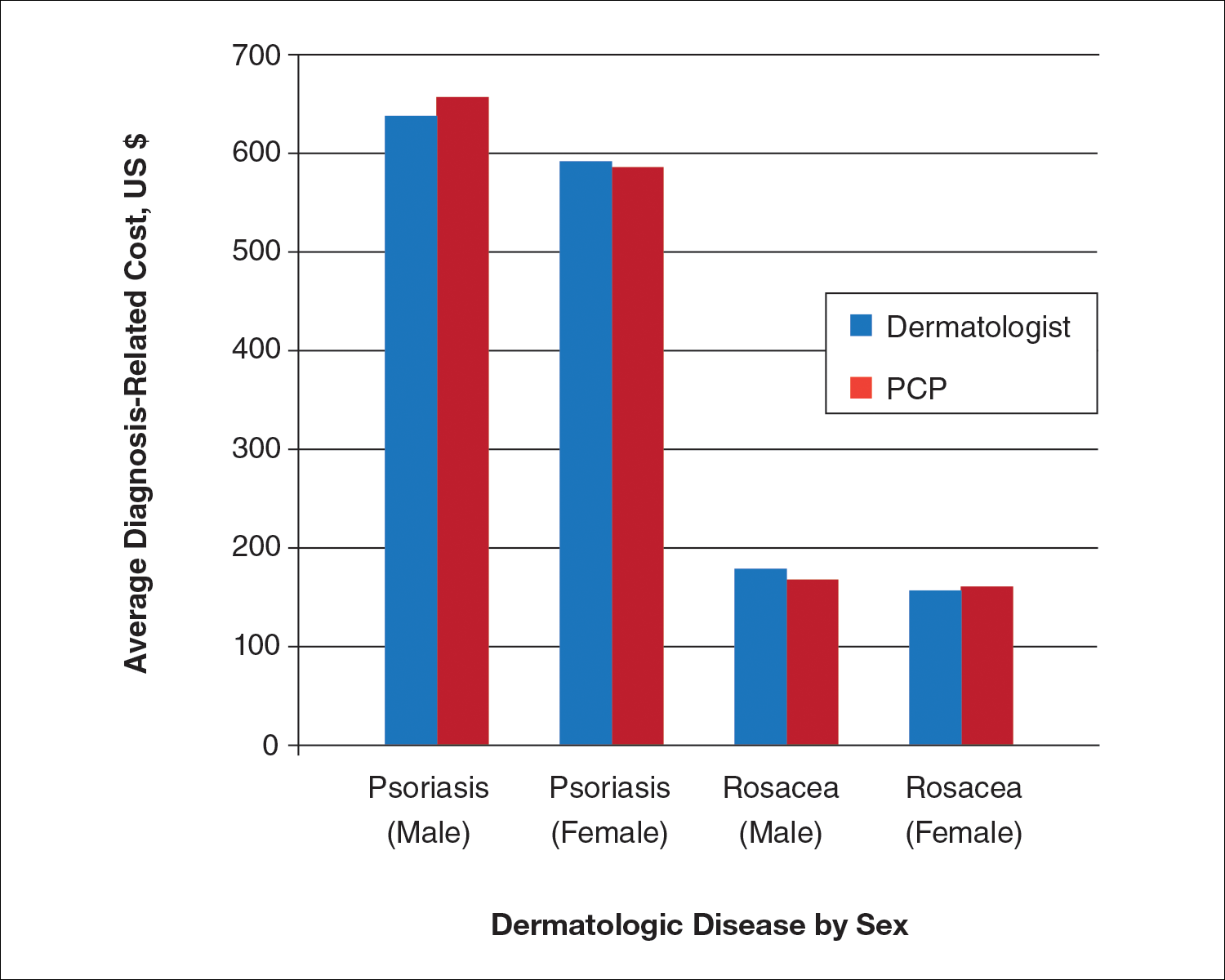
We identified 30,217 psoriasis patients and 37,561 rosacea patients. Of those patients with a primary diagnosis of psoriasis, 26,112 (86%) were seen by a dermatologist and 4105 (14%) were seen by a PCP (Table). Of those patients with a primary diagnosis of rosacea, 34,694 (92%) were seen by a dermatologist and 2867 (8%) were seen by a PCP (Table). There was little difference in the average diagnosis-related cost per patient for psoriasis in males (dermatologists, $638; PCPs, $657) versus females (dermatologists, $592; PCPs, $586) or between specialties (Figure). Findings were similar for rosacea in males (dermatologists, $179; PCPs, $168) versus females (dermatologists, $157; PCPs, $161). For these skin diseases, i

Comment
For the management of common skin disorders such as psoriasis and rosacea, there is little cost difference in seeing a dermatologist versus a PCP. Through extensive training and repeated exposure to many skin diseases, dermatologists are expected to be more comfortable in diagnosing and managing psoriasis and rosacea. Compared to PCPs, dermatologists have demonstrated increased diagnostic accuracy and efficiency when examining pigmented lesions and other dermatologic diseases in several studies.3-6 Although the current study shows that diagnosis-related costs for psoriasis and rosacea are essentially equal between dermatologists and PCPs, it actually may be less expensive for patients to see a dermatologist, as unnecessary tests, biopsies, or medications are more likely to be ordered/prescribed when there is less clinical diagnostic certainty.7,8 Additionally, seeing a PCP for diagnosis of a skin disease may be inefficient if subsequent referral to a dermatologist is needed, a common scenario that occurs when patients see a PCP for skin conditions.9
Our study had limitations, which is typical of a study using a claims database. We used ICD-9 codes recorded in patients’ medical claims to determine diagnosis of psoriasis and rosacea; therefore, our study and data are subject to coding errors. We could not assess the severity of disease, only the presence of disease. Further confirmation of diagnosis could have been made through searching for a second ICD-9 code in the patient’s history. Our data also are from a limited time period and may not represent costs from other time periods.
Conclusion
Given the lack of cost difference between both specialties, we conclude that ACOs should consider encouraging patients to seek care for dermatologic diseases by dermatologists who generally are more accurate and efficient skin diagnosticians, particularly if there is a shortage of PCPs within the ACO network.
- Wimo A, Religa D, Spångberg K, et al. Costs of diagnosing dementia: results from SveDem, the Swedish Dementia Registry. Int J Geriatr Psychiatry. 2013;28:1039-1044.
- Grunfeld E, Fitzpatrick R, Mant D, et al. Comparison of breast cancer patient satisfaction with follow-up in primary care versus specialist care: results from a randomized controlled trial. Br J Gen Pract. 1999;49:705-710.
- Chen SC, Pennie ML, Kolm P, et al. Diagnosing and managing cutaneous pigmented lesions: primary care physicians versus dermatologists. J Gen Intern Med. 2006;21:678-682.
- Federman D, Hogan D, Taylor JR, et al. A comparison of diagnosis, evaluation, and treatment of patients with dermatologic disorders. J Am Acad Dermatol. 1995;32:726-729.
- Feldman SR, Fleischer AB, Young AC, et al. Time-efficiency of nondermatologists compared with dermatologists in the care of skin disease. J Am Acad Dermatol. 1999;40:194-199.
- Feldman SR, Peterson SR, Fleischer AB Jr. Dermatologists meet the primary care standard for first contact management of skin disease. J Am Acad Dermatol. 1998;39(2, pt 1):182-186.
- Smith ES, Fleischer AB, Feldman SR. Nondermatologists are more likely than dermatologists to prescribe antifungal/corticosteroid products: an analysis of office visits for cutaneous fungal infections, 1990-1994. J Am Acad Dermatol. 1998;39:43-47.
- Shaffer MP, Feldman SR, Fleischer AB. Use of clotrimazole/betamethasone diproprionate by family physicians. Fam Med. 2000;32:561-565.
- Feldman SR, Fleischer AB, Chen JG. The gatekeeper model is inefficient for the delivery of dermatologic services. J Am Acad Dermatol. 1999;40:426-432.
Growing incentives to control health care costs may cause accountable care organizations (ACOs) to reconsider how diseases are best managed. Few studies have examined the cost difference between primary care providers (PCPs) and specialists in managing the same disease. Limited data have suggested that management of some diseases by a PCP may be less costly compared to a specialist1,2; however, it is not clear if this finding extends to skin disease. This study sought to assess the cost of seeing a dermatologist versus a PCP for diagnosis of the common skin diseases psoriasis and rosacea.
Methods
Patient data were obtained from the Humana database, a large commercial data set for claims and reimbursed costs encompassing 18,162,539 patients covered between January 2007 and December 2014. Our study population consisted of 3,944,465 patients with claims that included International Classification of Diseases, Ninth Revision (ICD-9), codes for dermatological diagnoses (680.0–709.9). We searched by ICD-9 code for US patients with primary diagnoses of psoriasis (696.1) and rosacea (695.3). We narrowed the search to include patients aged 30 to 64 years, as the diagnoses for these diseases are most common in patients older than 30 years. Patients who were older than 64 years were not included in the study, as most are covered by Medicare and therefore costs covered by Humana in this age group would not be as representative as in younger age groups. Total and average diagnosis-related costs per patient were compared between dermatologists and PCPs. Diagnosis-related costs encompassed physician reimbursement; laboratory and imaging costs, including skin biopsies; inpatient hospitalization cost; and any other charge that could be coded or billed by providers and reimbursed by the insurance company. To be eligible for reimbursement from Humana, dermatologists and PCPs must be registered with the insurer according to specialty board certification and practice credentialing, and they are reimbursed differently based on specialty. Drug costs, which would possibly skew the data toward providers using more expensive systemic medications (ie, dermatologists), were not included in this study, as the discussion is better reserved for long-term management of disease rather than diagnosis-related costs. All diagnoses of psoriasis were included in the study, which likely includes all severities of psoriasis, though we did not have the ability to further break down these diagnoses by severity.
Results
We identified 30,217 psoriasis patients and 37,561 rosacea patients. Of those patients with a primary diagnosis of psoriasis, 26,112 (86%) were seen by a dermatologist and 4105 (14%) were seen by a PCP (Table). Of those patients with a primary diagnosis of rosacea, 34,694 (92%) were seen by a dermatologist and 2867 (8%) were seen by a PCP (Table). There was little difference in the average diagnosis-related cost per patient for psoriasis in males (dermatologists, $638; PCPs, $657) versus females (dermatologists, $592; PCPs, $586) or between specialties (Figure). Findings were similar for rosacea in males (dermatologists, $179; PCPs, $168) versus females (dermatologists, $157; PCPs, $161). For these skin diseases, i


Comment
For the management of common skin disorders such as psoriasis and rosacea, there is little cost difference in seeing a dermatologist versus a PCP. Through extensive training and repeated exposure to many skin diseases, dermatologists are expected to be more comfortable in diagnosing and managing psoriasis and rosacea. Compared to PCPs, dermatologists have demonstrated increased diagnostic accuracy and efficiency when examining pigmented lesions and other dermatologic diseases in several studies.3-6 Although the current study shows that diagnosis-related costs for psoriasis and rosacea are essentially equal between dermatologists and PCPs, it actually may be less expensive for patients to see a dermatologist, as unnecessary tests, biopsies, or medications are more likely to be ordered/prescribed when there is less clinical diagnostic certainty.7,8 Additionally, seeing a PCP for diagnosis of a skin disease may be inefficient if subsequent referral to a dermatologist is needed, a common scenario that occurs when patients see a PCP for skin conditions.9
Our study had limitations, which is typical of a study using a claims database. We used ICD-9 codes recorded in patients’ medical claims to determine diagnosis of psoriasis and rosacea; therefore, our study and data are subject to coding errors. We could not assess the severity of disease, only the presence of disease. Further confirmation of diagnosis could have been made through searching for a second ICD-9 code in the patient’s history. Our data also are from a limited time period and may not represent costs from other time periods.
Conclusion
Given the lack of cost difference between both specialties, we conclude that ACOs should consider encouraging patients to seek care for dermatologic diseases by dermatologists who generally are more accurate and efficient skin diagnosticians, particularly if there is a shortage of PCPs within the ACO network.
Growing incentives to control health care costs may cause accountable care organizations (ACOs) to reconsider how diseases are best managed. Few studies have examined the cost difference between primary care providers (PCPs) and specialists in managing the same disease. Limited data have suggested that management of some diseases by a PCP may be less costly compared to a specialist1,2; however, it is not clear if this finding extends to skin disease. This study sought to assess the cost of seeing a dermatologist versus a PCP for diagnosis of the common skin diseases psoriasis and rosacea.
Methods
Patient data were obtained from the Humana database, a large commercial data set for claims and reimbursed costs encompassing 18,162,539 patients covered between January 2007 and December 2014. Our study population consisted of 3,944,465 patients with claims that included International Classification of Diseases, Ninth Revision (ICD-9), codes for dermatological diagnoses (680.0–709.9). We searched by ICD-9 code for US patients with primary diagnoses of psoriasis (696.1) and rosacea (695.3). We narrowed the search to include patients aged 30 to 64 years, as the diagnoses for these diseases are most common in patients older than 30 years. Patients who were older than 64 years were not included in the study, as most are covered by Medicare and therefore costs covered by Humana in this age group would not be as representative as in younger age groups. Total and average diagnosis-related costs per patient were compared between dermatologists and PCPs. Diagnosis-related costs encompassed physician reimbursement; laboratory and imaging costs, including skin biopsies; inpatient hospitalization cost; and any other charge that could be coded or billed by providers and reimbursed by the insurance company. To be eligible for reimbursement from Humana, dermatologists and PCPs must be registered with the insurer according to specialty board certification and practice credentialing, and they are reimbursed differently based on specialty. Drug costs, which would possibly skew the data toward providers using more expensive systemic medications (ie, dermatologists), were not included in this study, as the discussion is better reserved for long-term management of disease rather than diagnosis-related costs. All diagnoses of psoriasis were included in the study, which likely includes all severities of psoriasis, though we did not have the ability to further break down these diagnoses by severity.
Results
We identified 30,217 psoriasis patients and 37,561 rosacea patients. Of those patients with a primary diagnosis of psoriasis, 26,112 (86%) were seen by a dermatologist and 4105 (14%) were seen by a PCP (Table). Of those patients with a primary diagnosis of rosacea, 34,694 (92%) were seen by a dermatologist and 2867 (8%) were seen by a PCP (Table). There was little difference in the average diagnosis-related cost per patient for psoriasis in males (dermatologists, $638; PCPs, $657) versus females (dermatologists, $592; PCPs, $586) or between specialties (Figure). Findings were similar for rosacea in males (dermatologists, $179; PCPs, $168) versus females (dermatologists, $157; PCPs, $161). For these skin diseases, i


Comment
For the management of common skin disorders such as psoriasis and rosacea, there is little cost difference in seeing a dermatologist versus a PCP. Through extensive training and repeated exposure to many skin diseases, dermatologists are expected to be more comfortable in diagnosing and managing psoriasis and rosacea. Compared to PCPs, dermatologists have demonstrated increased diagnostic accuracy and efficiency when examining pigmented lesions and other dermatologic diseases in several studies.3-6 Although the current study shows that diagnosis-related costs for psoriasis and rosacea are essentially equal between dermatologists and PCPs, it actually may be less expensive for patients to see a dermatologist, as unnecessary tests, biopsies, or medications are more likely to be ordered/prescribed when there is less clinical diagnostic certainty.7,8 Additionally, seeing a PCP for diagnosis of a skin disease may be inefficient if subsequent referral to a dermatologist is needed, a common scenario that occurs when patients see a PCP for skin conditions.9
Our study had limitations, which is typical of a study using a claims database. We used ICD-9 codes recorded in patients’ medical claims to determine diagnosis of psoriasis and rosacea; therefore, our study and data are subject to coding errors. We could not assess the severity of disease, only the presence of disease. Further confirmation of diagnosis could have been made through searching for a second ICD-9 code in the patient’s history. Our data also are from a limited time period and may not represent costs from other time periods.
Conclusion
Given the lack of cost difference between both specialties, we conclude that ACOs should consider encouraging patients to seek care for dermatologic diseases by dermatologists who generally are more accurate and efficient skin diagnosticians, particularly if there is a shortage of PCPs within the ACO network.
- Wimo A, Religa D, Spångberg K, et al. Costs of diagnosing dementia: results from SveDem, the Swedish Dementia Registry. Int J Geriatr Psychiatry. 2013;28:1039-1044.
- Grunfeld E, Fitzpatrick R, Mant D, et al. Comparison of breast cancer patient satisfaction with follow-up in primary care versus specialist care: results from a randomized controlled trial. Br J Gen Pract. 1999;49:705-710.
- Chen SC, Pennie ML, Kolm P, et al. Diagnosing and managing cutaneous pigmented lesions: primary care physicians versus dermatologists. J Gen Intern Med. 2006;21:678-682.
- Federman D, Hogan D, Taylor JR, et al. A comparison of diagnosis, evaluation, and treatment of patients with dermatologic disorders. J Am Acad Dermatol. 1995;32:726-729.
- Feldman SR, Fleischer AB, Young AC, et al. Time-efficiency of nondermatologists compared with dermatologists in the care of skin disease. J Am Acad Dermatol. 1999;40:194-199.
- Feldman SR, Peterson SR, Fleischer AB Jr. Dermatologists meet the primary care standard for first contact management of skin disease. J Am Acad Dermatol. 1998;39(2, pt 1):182-186.
- Smith ES, Fleischer AB, Feldman SR. Nondermatologists are more likely than dermatologists to prescribe antifungal/corticosteroid products: an analysis of office visits for cutaneous fungal infections, 1990-1994. J Am Acad Dermatol. 1998;39:43-47.
- Shaffer MP, Feldman SR, Fleischer AB. Use of clotrimazole/betamethasone diproprionate by family physicians. Fam Med. 2000;32:561-565.
- Feldman SR, Fleischer AB, Chen JG. The gatekeeper model is inefficient for the delivery of dermatologic services. J Am Acad Dermatol. 1999;40:426-432.
- Wimo A, Religa D, Spångberg K, et al. Costs of diagnosing dementia: results from SveDem, the Swedish Dementia Registry. Int J Geriatr Psychiatry. 2013;28:1039-1044.
- Grunfeld E, Fitzpatrick R, Mant D, et al. Comparison of breast cancer patient satisfaction with follow-up in primary care versus specialist care: results from a randomized controlled trial. Br J Gen Pract. 1999;49:705-710.
- Chen SC, Pennie ML, Kolm P, et al. Diagnosing and managing cutaneous pigmented lesions: primary care physicians versus dermatologists. J Gen Intern Med. 2006;21:678-682.
- Federman D, Hogan D, Taylor JR, et al. A comparison of diagnosis, evaluation, and treatment of patients with dermatologic disorders. J Am Acad Dermatol. 1995;32:726-729.
- Feldman SR, Fleischer AB, Young AC, et al. Time-efficiency of nondermatologists compared with dermatologists in the care of skin disease. J Am Acad Dermatol. 1999;40:194-199.
- Feldman SR, Peterson SR, Fleischer AB Jr. Dermatologists meet the primary care standard for first contact management of skin disease. J Am Acad Dermatol. 1998;39(2, pt 1):182-186.
- Smith ES, Fleischer AB, Feldman SR. Nondermatologists are more likely than dermatologists to prescribe antifungal/corticosteroid products: an analysis of office visits for cutaneous fungal infections, 1990-1994. J Am Acad Dermatol. 1998;39:43-47.
- Shaffer MP, Feldman SR, Fleischer AB. Use of clotrimazole/betamethasone diproprionate by family physicians. Fam Med. 2000;32:561-565.
- Feldman SR, Fleischer AB, Chen JG. The gatekeeper model is inefficient for the delivery of dermatologic services. J Am Acad Dermatol. 1999;40:426-432.
Practice Points
- Growing health care costs are causing accountable care organizations (ACOs) to reconsider how to best manage skin disease.
- There is little difference in average diagnosis-related cost between primary care physicians and dermatologists in diagnosing psoriasis or rosacea.
- With diagnosis costs essentially equal and increased dermatologist diagnostic accuracy, ACOs may encourage skin disease to be managed by dermatologists.
New Biologics in Psoriasis: An Update on IL-23 and IL-17 Inhibitors
The role of current biologic therapies in psoriasis predicates on the pathogenic role of upregulated, immune-related mechanisms that result in the activation of myeloid dendritic cells, which release IL-17, IL-23, and other cytokines to activate T cells, including helper T cell TH17. Along with other immune cells, TH17 produces IL-17. This proinflammatory cascade results in keratinocyte proliferation, angiogenesis, and migration of immune cells toward psoriatic lesions.1 Thus, the newest classes of biologics target IL-12, IL-23, and IL-17 to disrupt this inflammatory cascade.
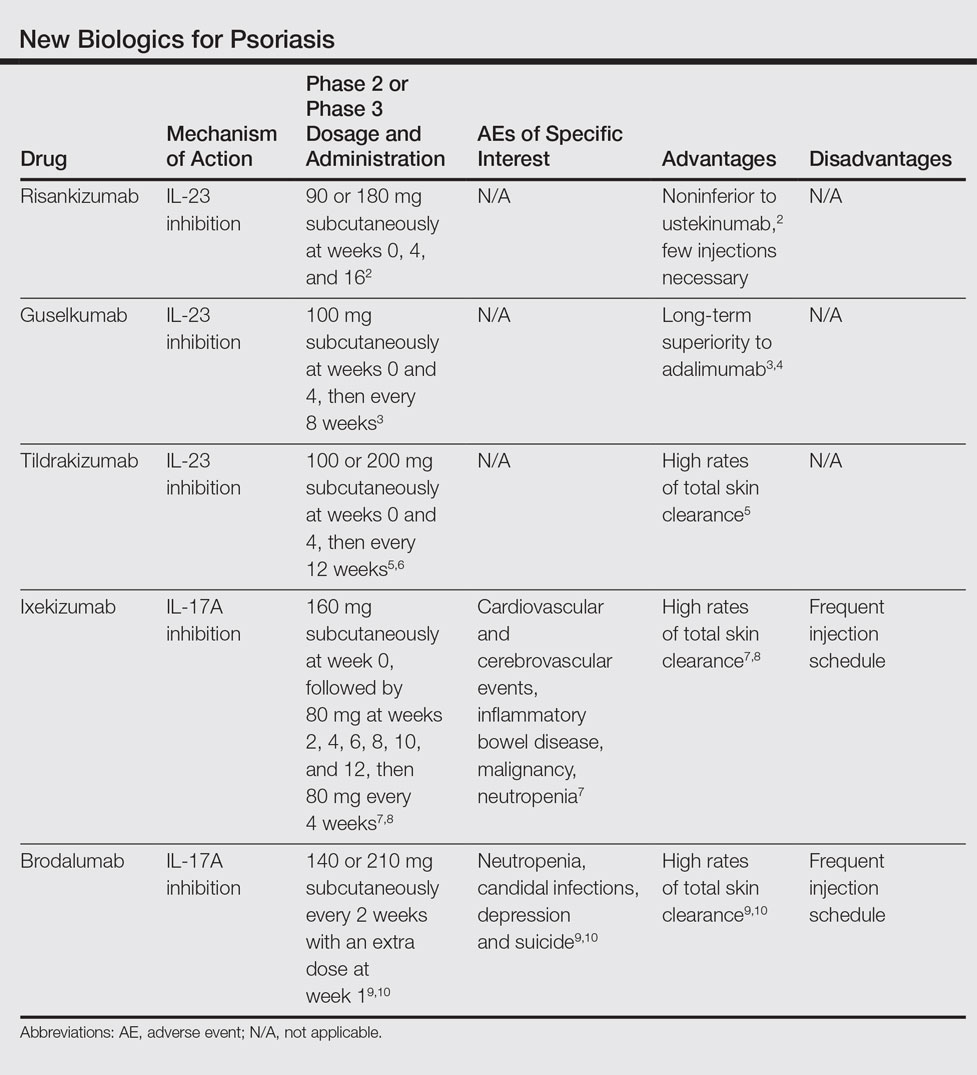
We provide an updated review of the most recent clinical efficacy and safety data on the newest IL-23 and IL-17 inhibitors in the pipeline or approved for psoriasis, including risankizumab, guselkumab, tildrakizumab, ixekizumab, and brodalumab (Table). Ustekinumab and adalimumab, which have been previously approved by the US Food and Drug Administration (FDA), will be discussed here only as comparators.
IL-23 Inhibitors
Risankizumab
Risankizumab (formerly known as BI 655066)(Boehringer Ingelheim) is a selective human monoclonal antibody targeting the p19 subunit of IL-23 and currently is undergoing phase 3 trials for psoriasis. A proof-of-concept phase 1 study of 39 participants demonstrated efficacy after 12 weeks of treatment at varying subcutaneous and intravenous doses with placebo control.11 At week 12, 87% (27/31)(P<.001) of all risankizumab-treated participants achieved 75% reduction in psoriasis area and severity index (PASI) score compared to 0% of 8 placebo-treated participants. Common adverse effects (AEs) occurred in 65% (20/31) of risankizumab-treated participants, including non–dose-dependent upper respiratory tract infections, nasopharyngitis, and headache. Serious adverse events (SAEs) that occurred were considered unrelated to the study medication.11
A phase 2 trial of 166 participants compared 3 dosing regimens of subcutaneous risankizumab (single 18-mg dose at week 0; single 90-mg dose at weeks 0, 4, and 16; or single 180-mg dose at weeks 0, 4, and 16) and ustekinumab (weight-based single 45- or 90-mg dose at weeks 0, 4, and 16), demonstrating noninferiority at higher doses of risankizumab.2 Preliminary primary end point results at week 12 showed PASI 90 in 32.6% (P=.4667), 73.2% (P=.0013), 81.0% (P<.0001), and 40.0% of the treatment groups, respectively. Participants in the 180-mg risankizumab group achieved PASI 90 eight weeks faster than those on ustekinumab, lasting more than 2 months longer. Adverse effects were similar across all treatment groups and SAEs were unrelated to the study medications.2
Guselkumab
Guselkumab (Janssen Biotech, Inc) is a selective human monoclonal antibody against the p19 subunit of IL-23. The 52-week phase 2 X-PLORE trial compared dose-ranging subcutaneous guselkumab (5 mg at weeks 0 and 4, then every 12 weeks; 15 mg every 8 weeks; 50 mg at weeks 0 and 4, then every 12 weeks; 100 mg every 8 weeks; or 200 mg at weeks 0 and 4, then every 12 weeks), adalimumab (80-mg loading dose, followed by 40 mg at week 1, then every other week), and placebo in 293 randomized participants.4 At week 16, 34% (P=.002) of participants in the 5-mg guselkumab group, 61% (P<.001) in the 15-mg group, 79% (P<.001) in the 50-mg group, 86% (P<.001) in the 100-mg group, 83% (P<.001) in the 200-mg group, and 58% (P<.001) in the adalimumab group achieved physician global assessment (PGA) scores of 0 (clear) or 1 (minimal psoriasis) compared to 7% of the placebo group. Achievement of PASI 75 similarly favored the guselkumab (44% [P<.001]; 76% [no P value given]; 81% [P<.001]; 79% [P<.001]; and 81% [P<.001], respectively) and adalimumab treatment arms (70% [P<.001]) compared to 5% in the placebo group. In longer-term comparisons to week 40, participants in the 50-, 100-, and 200-mg guselkumab groups showed significantly greater remission of psoriatic lesions, measured by a PGA score of 0 or 1, than participants in the adalimumab group (71% [P=.05]; 77% [P=.005]; 81% [P=.01]; and 49%, respectively).4
Preliminary results from VOYAGE 1 (N=837), the first of several phase 3 trials, further demonstrate the superiority of guselkumab 100 mg at weeks 0 and 4 and then every 8 weeks over adalimumab (standard dosing) and placebo; at week 16, 73.3% (P<.001 for both comparisons) versus 49.7% and 2.9% of participants, respectively, achieved PASI 90, with sustained superiority of skin clearance in guselkumab-treated participants compared to adalimumab and placebo through week 48.3
Long-term safety data showed no dose dependence or trend from 0 to 16 weeks and 16 to 52 weeks of treatment regarding rates of AEs, SAEs, or serious infections.4 Between weeks 16 and 52, 48.9% of all guselkumab-treated participants exhibited AEs compared to 60.5% of adalimumab-treated participants and 51.3% of placebo participants. Overall infection rates also were lowest in the guselkumab group at 29.8% compared to 36.8% and 35.9%, respectively. Three participants treated with guselkumab had major cardiovascular events, including a fatal myocardial infarction. No cases of tuberculosis or serious opportunistic infections were reported.4
Tildrakizumab
Tildrakizumab (formerly known as MK-3222)(Sun Pharmaceutical Industries Ltd) is a human monoclonal antibody also targeting the p19 subunit of IL-23. In a phase 2 study of 355 participants with chronic plaque psoriasis, participants received 5-, 25-, 100-, or 200-mg subcutaneous tildrakizumab or placebo at weeks 0 and 4 and then every 12 weeks for a total of 52 weeks.6 At week 16, PASI 75 results were 33.3%, 64.4%, 66.3%, 74.4%, and 4.4%, respectively (P<.001 for each comparison). Improvement began within the first month of treatment, with median times to PASI 75 of 57 days at 200-mg dosing and 84 days at 100-mg dosing. Of those participants achieving PASI 75 by drug discontinuation at week 52, 96% of the 100-mg group and 93% of the 200-mg group maintained PASI 75 through week 72, suggesting low relapse rates after treatment cessation.6
In October 2016, the efficacy results of 2 pivotal phase 3 trials (reSURFACE 1 and reSURFACE 2) involving more than 1800 participants combined revealed PASI 90 achievement in an average of 54% of participants on tildrakizumab 100 mg and 59% of participants on tildrakizumab 200 mg at week 28.5 Achievement of PASI 100 occurred in 24% and 30% of participants at week 28, respectively. The second of these trials included an etanercept comparison group and demonstrated head-to-head superiority of 100 and 200 mg subcutaneous tildrakizumab at week 12 by end point measures.5
Treatment-related AEs occurred at rates of 25% in tildrakizumab-treated participants and 22% in placebo-treated participants, most frequently nasopharyngitis and headache.6 At least 1 AE occurred in 64% of tildrakizumab-treated participants without dose dependence compared to 69% of placebo-treated participants. Severe AEs thought to be drug treatment related were bacterial arthritis, lymphedema, melanoma, stroke, and epiglottitis.6
IL-17 Inhibitors
Ixekizumab
Ixekizumab (Eli Lilly and Company), a monoclonal inhibitor of IL-17A, is the most recently approved psoriasis biologic on the market and has been cleared for use in adults with moderate to severe plaque psoriasis. Recommended dosing is 160 mg (given in two 80-mg subcutaneous injections via an autoinjector or prefilled syringe) at week 0, followed by an 80-mg injection at weeks 2, 4, 6, 8, 10, and 12, and then 80 mg every 4 weeks thereafter. The FDA approved ixekizumab in March 2016 following favorable results of several phase 3 trials: UNCOVER-1, UNCOVER-2, and UNCOVER-3.7,8
In UNCOVER-1, 1296 participants were randomized to 1 of 2 ixekizumab treatment arms—160 mg starting dose at week 0, 80 mg every 2 or 4 weeks thereafter—or placebo.7 At week 12, 89.1%, 82.6%, and 3.9% achieved PASI 75, respectively (P<.001 for both). Importantly, high numbers of participants also achieved PASI 90 (70.9% in the 2-week group and 64.6% in the 4-week group vs 0.5% in the placebo group [P<.001]) and PASI 100 (35.3% and 33.6% vs 0%, respectively [P<.001]), suggesting high rates of disease clearance.7
UNCOVER-2 (N=1224) and UNCOVER-3 (N=1346) investigated the same 2 dosing regimens of ixekizumab compared to etanercept 50 mg biweekly and placebo.8 At week 12, the percentage of participants achieving PASI 90 in UNCOVER-2 was 70.7%, 59.7%, 18.7%, and 0.6%, respectively, and 68.1%, 65.3%, 25.7%, and 3.1%, respectively, in UNCOVER-3 (P<.0001 for all comparisons to placebo and etanercept). At week 12, PASI 100 results also showed striking superiority, with 40.5%, 30.8%, 5.3%, and 0.6% of participants, respectively, in UNCOVER-2, and 37.7%, 35%, 7.3%, and 0%, respectively, in UNCOVER-3, achieving complete clearance of disease (P<.0001 for all comparisons to placebo and etanercept). Responses to ixekizumab were observed as early as weeks 1 and 2, while no participants in the etanercept and placebo treatment groups achieved comparative efficiency.8
In an extension of UNCOVER-3, efficacy increased from week 12 to week 60 according to PASI 90 (68%–73% in the 2-week group; 65%–72% in the 4-week group) and PASI 100 measures (38%–55% in the 2-week group; 35%–52% in the 4-week group).7
The most common AEs associated with ixekizumab treatment from weeks 0 to 12 occurred at higher rates in the 2-week and 4-week ixekizumab groups compared to placebo, including nasopharyngitis (9.5% and 9% vs 8.7%, respectively), upper respiratory tract infection (4.4% and 3.9% vs 3.5%, respectively), injection-site reaction (10% and 7.7% vs 1%, respectively), arthralgia (4.4% and 4.3% vs 2.9%, respectively), and headache (2.5% and 1.9% vs 2.1%, respectively). Infections, including candidal, oral, vulvovaginal, and cutaneous, occurred in 27% of the 2-week dosing group and 27.4% of the 4-week dosing group compared to 22.9% of the placebo group during weeks 0 to 12, with candidal infections in particular occurring more frequently in the active treatment groups and exhibiting dose dependence. Other AEs of special interest that occurred among all ixekizumab-treated participants (n=3736) from weeks 0 to 60 were cardiovascular and cerebrovascular events (22 [0.6%]), inflammatory bowel disease (11 [0.3%]), non–skin cancer malignancy (14 [0.4%]), and nonmelanoma skin cancer (20 [0.5%]). Neutropenia occurred at higher rates in ixekizumab-treated participants (9.3% in the 2-week group and 8.6% in the 4-week group) compared to placebo (3.3%) and occurred in 11.5% of all ixekizumab participants over 60 weeks.7
Brodalumab
Brodalumab (Valeant Pharmaceuticals International, Inc) is a human monoclonal antibody targeting the IL-17A receptor currently under review for FDA approval after undergoing phase 3 trials. The first of these trials, AMAGINE-1, showed efficacy of subcutaneous brodalumab (140 or 210 mg administered every 2 weeks with an extra dose at week 1) compared to placebo in 661 participants.9 At week 12, 60%, 83%, and 3%, respectively, achieved PASI 75; 43%, 70%, and 1%, respectively, achieved PASI 90; and 23%, 42%, and 1%, respectively, achieved PASI 100 (P<.001 for all respective comparisons to placebo). These effects were retained through 52 weeks of treatment. The median time to complete disease clearance in participants reaching PASI 100 was 12 weeks. Conversely, participants who were re-randomized to placebo after week 12 of brodalumab treatment relapsed within weeks to months.9
AMAGINE-2 and AMAGINE-3 further demonstrated the efficacy of brodalumab (140 or 210 mg every 2 weeks with extra dose at week 1) compared to ustekinumab (45 or 90 mg weight-based standard dosing) and placebo in 1831 participants, respectively.10 In AMAGINE-2, 49% of participants in the 140-mg group (P<.001 vs placebo), 70% in the 210-mg group (P<.001 vs placebo), 47% in the ustekinumab group, and 3% in the placebo group achieved PASI 90 at week 12. Similarly, in AMAGINE-3, 52% of participants in the 140-mg group (P<.001), 69% in the 210-mg group (P<.001), 48% in the ustekinumab group, and 2% in the placebo group achieved PASI 90. Impressively, complete clearance (PASI 100) at week 12 occurred in 26% of the 140-mg group (P<.001 vs placebo), 44% of the 210-mg group (P<.001 vs placebo), and 22% of the ustekinumab group compared to 2% of the placebo group in AMAGINE-2, with similar rates in AMAGINE-3. Brodalumab was significantly superior to ustekinumab at the 210-mg dose by PASI 90 measures (P<.001) in both studies and at the 140-mg dose by PASI 100 measures (P=.007) in AMAGINE-3 only.10
Common AEs were nasopharyngitis, upper respiratory tract infection, headache, and arthralgia, all occurring at grossly similar rates (49%–60%) across all experimental groups in AMAGINE-1, AMAGINE-2, and AMAGINE-3 during the first 12-week treatment period.9,10 Brodalumab treatment groups had high rates of specific interest AEs compared to ustekinumab and placebo groups, including neutropenia (0.8%, 1.1%, 0.3%, and 0%, respectively) and candidal infections (0.8%, 1.3%, 0.3%, and 0.3%, respectively). Induction phase (weeks 0–12) depression rates were concerning, with 6 cases each in AMAGINE-2 (4 [0.7%] in the 140-mg group, 2 [0.3%] in the 210-mg group) and AMAGINE-3 (4 [0.6%] in the 140-mg group, 2 [0.3%] in the 210-mg group). Cases of neutropenia were mild, were not associated with major infection, and were transient or reversible. Depression rates after 52 weeks of treatment were 1.7% (23/1567) of brodalumab participants in AMAGINE-2 and 1.8% (21/1613) in AMAGINE-3. Three participants, all on constant 210-mg dosing through week 52, attempted suicide with 1 completion10; however, because no other IL-17 inhibitors were associated with depression or suicide in other trials, it has been suggested that these cases were incidental and not treatment related.12 An FDA advisory panel recommended approval of brodalumab in July 2016 despite ongoing concerns of depression and suicide.13
Conclusion
The robust investigation into IL-23 and IL-17 inhibitors to treat plaque psoriasis has yielded promising results, including the unprecedented rates of PASI 100 achievement with these new biologics. Risankizumab, ixekizumab, and brodalumab have demonstrated superior efficacy in trials compared to ustekinumab. Tildrakizumab has shown low disease relapse after drug cessation. Ixekizumab and brodalumab have shown high rates of total disease clearance. Thus far, safety findings for these pipeline biologics have been consistent with those of ustekinumab. With ixekizumab approved in 2016 and brodalumab under review, new options in biologic therapy will offer patients and clinicians greater choices in treating severe and recalcitrant psoriasis.
- Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361:496-509.
- Papp K, Menter A, Sofen H, et al. Efficacy and safety of different dose regimens of a selective IL-23p19 inhibitor (BI 655066) compared with ustekinumab in patients with moderate-to-severe plaque psoriasis with and without psoriatic arthritis. Paper presented at: 2015 American College of Rheumatology/Association of Rheumatology Health Professionals Annual Meeting; November 6-11, 2015; San Francisco, CA.
- New phase 3 data show significant efficacy versus placebo and superiority of guselkumab versus Humira in treatment of moderate to severe plaque psoriasis [press release]. Vienna, Austria; Janssen Research & Development, LLC: October 1, 2016.
- Gordon KB, Duffin KC, Bissonnette R, et al. A phase 2 trial of guselkumab versus adalimumab for plaque psoriasis. N Engl J Med. 2015;373:136-144.
- Sun Pharma to announce late-breaking results for investigational IL-23p19 inhibitor, Tildrakizumab, achieves primary end point in both phase-3 studies in patients with moderate-to-severe plaque psoriasis [press release]. Mumbai, India; Sun Pharmaceutical Industries Ltd: October 1, 2016.
- Papp K, Thaci D, Reich K, et al. Tildrakizumab (MK-3222), an anti-interleukin-23p19 monoclonal antibody, improves psoriasis in a phase IIb randomized placebo-controlled trial. Br J Dermatol. 2015;173:930-939.
- Gordon KB, Blauvelt A, Papp KA, et al; UNCOVER-1 Study Group, UNCOVER-2 Study Group, UNCOVER-3 Study Group. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375:345-356.
- Griffiths CE, Reich K, Lebwohl M, et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet. 2015;386:541-551.
- Papp KA, Reich K, Paul C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis [published online June 23, 2016]. Br J Dermatol. 2016;175:273-286.
- Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373:1318-1328.
- Krueger JG, Ferris LK, Menter A, et al. Anti-IL-23A mAb BI 655066 for treatment of moderate-to-severe psoriasis: safety, efficacy, pharmacokinetics, and biomarker results of a single-rising-dose, randomized, double-blind, placebo-controlled trial [published online March 1, 2015]. J Allergy Clin Immunol. 2015;136:116-124.e7.
- Chiricozzi A, Romanelli M, Saraceno R, et al. No meaningful association between suicidal behavior and the use of IL-17A-neutralizing or IL-17RA-blocking agents [published online August 31, 2016]. Expert Opin Drug Saf. 2016;15:1653-1659.
- FDA advisory committee recommends approval of brodalumab for treatment of moderate-to-severe plaque psoriasis [news release]. Laval, Quebec: Valeant Pharmaceuticals International, Inc; July 19, 2016.
The role of current biologic therapies in psoriasis predicates on the pathogenic role of upregulated, immune-related mechanisms that result in the activation of myeloid dendritic cells, which release IL-17, IL-23, and other cytokines to activate T cells, including helper T cell TH17. Along with other immune cells, TH17 produces IL-17. This proinflammatory cascade results in keratinocyte proliferation, angiogenesis, and migration of immune cells toward psoriatic lesions.1 Thus, the newest classes of biologics target IL-12, IL-23, and IL-17 to disrupt this inflammatory cascade.
We provide an updated review of the most recent clinical efficacy and safety data on the newest IL-23 and IL-17 inhibitors in the pipeline or approved for psoriasis, including risankizumab, guselkumab, tildrakizumab, ixekizumab, and brodalumab (Table). Ustekinumab and adalimumab, which have been previously approved by the US Food and Drug Administration (FDA), will be discussed here only as comparators.

IL-23 Inhibitors
Risankizumab
Risankizumab (formerly known as BI 655066)(Boehringer Ingelheim) is a selective human monoclonal antibody targeting the p19 subunit of IL-23 and currently is undergoing phase 3 trials for psoriasis. A proof-of-concept phase 1 study of 39 participants demonstrated efficacy after 12 weeks of treatment at varying subcutaneous and intravenous doses with placebo control.11 At week 12, 87% (27/31)(P<.001) of all risankizumab-treated participants achieved 75% reduction in psoriasis area and severity index (PASI) score compared to 0% of 8 placebo-treated participants. Common adverse effects (AEs) occurred in 65% (20/31) of risankizumab-treated participants, including non–dose-dependent upper respiratory tract infections, nasopharyngitis, and headache. Serious adverse events (SAEs) that occurred were considered unrelated to the study medication.11
A phase 2 trial of 166 participants compared 3 dosing regimens of subcutaneous risankizumab (single 18-mg dose at week 0; single 90-mg dose at weeks 0, 4, and 16; or single 180-mg dose at weeks 0, 4, and 16) and ustekinumab (weight-based single 45- or 90-mg dose at weeks 0, 4, and 16), demonstrating noninferiority at higher doses of risankizumab.2 Preliminary primary end point results at week 12 showed PASI 90 in 32.6% (P=.4667), 73.2% (P=.0013), 81.0% (P<.0001), and 40.0% of the treatment groups, respectively. Participants in the 180-mg risankizumab group achieved PASI 90 eight weeks faster than those on ustekinumab, lasting more than 2 months longer. Adverse effects were similar across all treatment groups and SAEs were unrelated to the study medications.2
Guselkumab
Guselkumab (Janssen Biotech, Inc) is a selective human monoclonal antibody against the p19 subunit of IL-23. The 52-week phase 2 X-PLORE trial compared dose-ranging subcutaneous guselkumab (5 mg at weeks 0 and 4, then every 12 weeks; 15 mg every 8 weeks; 50 mg at weeks 0 and 4, then every 12 weeks; 100 mg every 8 weeks; or 200 mg at weeks 0 and 4, then every 12 weeks), adalimumab (80-mg loading dose, followed by 40 mg at week 1, then every other week), and placebo in 293 randomized participants.4 At week 16, 34% (P=.002) of participants in the 5-mg guselkumab group, 61% (P<.001) in the 15-mg group, 79% (P<.001) in the 50-mg group, 86% (P<.001) in the 100-mg group, 83% (P<.001) in the 200-mg group, and 58% (P<.001) in the adalimumab group achieved physician global assessment (PGA) scores of 0 (clear) or 1 (minimal psoriasis) compared to 7% of the placebo group. Achievement of PASI 75 similarly favored the guselkumab (44% [P<.001]; 76% [no P value given]; 81% [P<.001]; 79% [P<.001]; and 81% [P<.001], respectively) and adalimumab treatment arms (70% [P<.001]) compared to 5% in the placebo group. In longer-term comparisons to week 40, participants in the 50-, 100-, and 200-mg guselkumab groups showed significantly greater remission of psoriatic lesions, measured by a PGA score of 0 or 1, than participants in the adalimumab group (71% [P=.05]; 77% [P=.005]; 81% [P=.01]; and 49%, respectively).4
Preliminary results from VOYAGE 1 (N=837), the first of several phase 3 trials, further demonstrate the superiority of guselkumab 100 mg at weeks 0 and 4 and then every 8 weeks over adalimumab (standard dosing) and placebo; at week 16, 73.3% (P<.001 for both comparisons) versus 49.7% and 2.9% of participants, respectively, achieved PASI 90, with sustained superiority of skin clearance in guselkumab-treated participants compared to adalimumab and placebo through week 48.3
Long-term safety data showed no dose dependence or trend from 0 to 16 weeks and 16 to 52 weeks of treatment regarding rates of AEs, SAEs, or serious infections.4 Between weeks 16 and 52, 48.9% of all guselkumab-treated participants exhibited AEs compared to 60.5% of adalimumab-treated participants and 51.3% of placebo participants. Overall infection rates also were lowest in the guselkumab group at 29.8% compared to 36.8% and 35.9%, respectively. Three participants treated with guselkumab had major cardiovascular events, including a fatal myocardial infarction. No cases of tuberculosis or serious opportunistic infections were reported.4
Tildrakizumab
Tildrakizumab (formerly known as MK-3222)(Sun Pharmaceutical Industries Ltd) is a human monoclonal antibody also targeting the p19 subunit of IL-23. In a phase 2 study of 355 participants with chronic plaque psoriasis, participants received 5-, 25-, 100-, or 200-mg subcutaneous tildrakizumab or placebo at weeks 0 and 4 and then every 12 weeks for a total of 52 weeks.6 At week 16, PASI 75 results were 33.3%, 64.4%, 66.3%, 74.4%, and 4.4%, respectively (P<.001 for each comparison). Improvement began within the first month of treatment, with median times to PASI 75 of 57 days at 200-mg dosing and 84 days at 100-mg dosing. Of those participants achieving PASI 75 by drug discontinuation at week 52, 96% of the 100-mg group and 93% of the 200-mg group maintained PASI 75 through week 72, suggesting low relapse rates after treatment cessation.6
In October 2016, the efficacy results of 2 pivotal phase 3 trials (reSURFACE 1 and reSURFACE 2) involving more than 1800 participants combined revealed PASI 90 achievement in an average of 54% of participants on tildrakizumab 100 mg and 59% of participants on tildrakizumab 200 mg at week 28.5 Achievement of PASI 100 occurred in 24% and 30% of participants at week 28, respectively. The second of these trials included an etanercept comparison group and demonstrated head-to-head superiority of 100 and 200 mg subcutaneous tildrakizumab at week 12 by end point measures.5
Treatment-related AEs occurred at rates of 25% in tildrakizumab-treated participants and 22% in placebo-treated participants, most frequently nasopharyngitis and headache.6 At least 1 AE occurred in 64% of tildrakizumab-treated participants without dose dependence compared to 69% of placebo-treated participants. Severe AEs thought to be drug treatment related were bacterial arthritis, lymphedema, melanoma, stroke, and epiglottitis.6
IL-17 Inhibitors
Ixekizumab
Ixekizumab (Eli Lilly and Company), a monoclonal inhibitor of IL-17A, is the most recently approved psoriasis biologic on the market and has been cleared for use in adults with moderate to severe plaque psoriasis. Recommended dosing is 160 mg (given in two 80-mg subcutaneous injections via an autoinjector or prefilled syringe) at week 0, followed by an 80-mg injection at weeks 2, 4, 6, 8, 10, and 12, and then 80 mg every 4 weeks thereafter. The FDA approved ixekizumab in March 2016 following favorable results of several phase 3 trials: UNCOVER-1, UNCOVER-2, and UNCOVER-3.7,8
In UNCOVER-1, 1296 participants were randomized to 1 of 2 ixekizumab treatment arms—160 mg starting dose at week 0, 80 mg every 2 or 4 weeks thereafter—or placebo.7 At week 12, 89.1%, 82.6%, and 3.9% achieved PASI 75, respectively (P<.001 for both). Importantly, high numbers of participants also achieved PASI 90 (70.9% in the 2-week group and 64.6% in the 4-week group vs 0.5% in the placebo group [P<.001]) and PASI 100 (35.3% and 33.6% vs 0%, respectively [P<.001]), suggesting high rates of disease clearance.7
UNCOVER-2 (N=1224) and UNCOVER-3 (N=1346) investigated the same 2 dosing regimens of ixekizumab compared to etanercept 50 mg biweekly and placebo.8 At week 12, the percentage of participants achieving PASI 90 in UNCOVER-2 was 70.7%, 59.7%, 18.7%, and 0.6%, respectively, and 68.1%, 65.3%, 25.7%, and 3.1%, respectively, in UNCOVER-3 (P<.0001 for all comparisons to placebo and etanercept). At week 12, PASI 100 results also showed striking superiority, with 40.5%, 30.8%, 5.3%, and 0.6% of participants, respectively, in UNCOVER-2, and 37.7%, 35%, 7.3%, and 0%, respectively, in UNCOVER-3, achieving complete clearance of disease (P<.0001 for all comparisons to placebo and etanercept). Responses to ixekizumab were observed as early as weeks 1 and 2, while no participants in the etanercept and placebo treatment groups achieved comparative efficiency.8
In an extension of UNCOVER-3, efficacy increased from week 12 to week 60 according to PASI 90 (68%–73% in the 2-week group; 65%–72% in the 4-week group) and PASI 100 measures (38%–55% in the 2-week group; 35%–52% in the 4-week group).7
The most common AEs associated with ixekizumab treatment from weeks 0 to 12 occurred at higher rates in the 2-week and 4-week ixekizumab groups compared to placebo, including nasopharyngitis (9.5% and 9% vs 8.7%, respectively), upper respiratory tract infection (4.4% and 3.9% vs 3.5%, respectively), injection-site reaction (10% and 7.7% vs 1%, respectively), arthralgia (4.4% and 4.3% vs 2.9%, respectively), and headache (2.5% and 1.9% vs 2.1%, respectively). Infections, including candidal, oral, vulvovaginal, and cutaneous, occurred in 27% of the 2-week dosing group and 27.4% of the 4-week dosing group compared to 22.9% of the placebo group during weeks 0 to 12, with candidal infections in particular occurring more frequently in the active treatment groups and exhibiting dose dependence. Other AEs of special interest that occurred among all ixekizumab-treated participants (n=3736) from weeks 0 to 60 were cardiovascular and cerebrovascular events (22 [0.6%]), inflammatory bowel disease (11 [0.3%]), non–skin cancer malignancy (14 [0.4%]), and nonmelanoma skin cancer (20 [0.5%]). Neutropenia occurred at higher rates in ixekizumab-treated participants (9.3% in the 2-week group and 8.6% in the 4-week group) compared to placebo (3.3%) and occurred in 11.5% of all ixekizumab participants over 60 weeks.7
Brodalumab
Brodalumab (Valeant Pharmaceuticals International, Inc) is a human monoclonal antibody targeting the IL-17A receptor currently under review for FDA approval after undergoing phase 3 trials. The first of these trials, AMAGINE-1, showed efficacy of subcutaneous brodalumab (140 or 210 mg administered every 2 weeks with an extra dose at week 1) compared to placebo in 661 participants.9 At week 12, 60%, 83%, and 3%, respectively, achieved PASI 75; 43%, 70%, and 1%, respectively, achieved PASI 90; and 23%, 42%, and 1%, respectively, achieved PASI 100 (P<.001 for all respective comparisons to placebo). These effects were retained through 52 weeks of treatment. The median time to complete disease clearance in participants reaching PASI 100 was 12 weeks. Conversely, participants who were re-randomized to placebo after week 12 of brodalumab treatment relapsed within weeks to months.9
AMAGINE-2 and AMAGINE-3 further demonstrated the efficacy of brodalumab (140 or 210 mg every 2 weeks with extra dose at week 1) compared to ustekinumab (45 or 90 mg weight-based standard dosing) and placebo in 1831 participants, respectively.10 In AMAGINE-2, 49% of participants in the 140-mg group (P<.001 vs placebo), 70% in the 210-mg group (P<.001 vs placebo), 47% in the ustekinumab group, and 3% in the placebo group achieved PASI 90 at week 12. Similarly, in AMAGINE-3, 52% of participants in the 140-mg group (P<.001), 69% in the 210-mg group (P<.001), 48% in the ustekinumab group, and 2% in the placebo group achieved PASI 90. Impressively, complete clearance (PASI 100) at week 12 occurred in 26% of the 140-mg group (P<.001 vs placebo), 44% of the 210-mg group (P<.001 vs placebo), and 22% of the ustekinumab group compared to 2% of the placebo group in AMAGINE-2, with similar rates in AMAGINE-3. Brodalumab was significantly superior to ustekinumab at the 210-mg dose by PASI 90 measures (P<.001) in both studies and at the 140-mg dose by PASI 100 measures (P=.007) in AMAGINE-3 only.10
Common AEs were nasopharyngitis, upper respiratory tract infection, headache, and arthralgia, all occurring at grossly similar rates (49%–60%) across all experimental groups in AMAGINE-1, AMAGINE-2, and AMAGINE-3 during the first 12-week treatment period.9,10 Brodalumab treatment groups had high rates of specific interest AEs compared to ustekinumab and placebo groups, including neutropenia (0.8%, 1.1%, 0.3%, and 0%, respectively) and candidal infections (0.8%, 1.3%, 0.3%, and 0.3%, respectively). Induction phase (weeks 0–12) depression rates were concerning, with 6 cases each in AMAGINE-2 (4 [0.7%] in the 140-mg group, 2 [0.3%] in the 210-mg group) and AMAGINE-3 (4 [0.6%] in the 140-mg group, 2 [0.3%] in the 210-mg group). Cases of neutropenia were mild, were not associated with major infection, and were transient or reversible. Depression rates after 52 weeks of treatment were 1.7% (23/1567) of brodalumab participants in AMAGINE-2 and 1.8% (21/1613) in AMAGINE-3. Three participants, all on constant 210-mg dosing through week 52, attempted suicide with 1 completion10; however, because no other IL-17 inhibitors were associated with depression or suicide in other trials, it has been suggested that these cases were incidental and not treatment related.12 An FDA advisory panel recommended approval of brodalumab in July 2016 despite ongoing concerns of depression and suicide.13
Conclusion
The robust investigation into IL-23 and IL-17 inhibitors to treat plaque psoriasis has yielded promising results, including the unprecedented rates of PASI 100 achievement with these new biologics. Risankizumab, ixekizumab, and brodalumab have demonstrated superior efficacy in trials compared to ustekinumab. Tildrakizumab has shown low disease relapse after drug cessation. Ixekizumab and brodalumab have shown high rates of total disease clearance. Thus far, safety findings for these pipeline biologics have been consistent with those of ustekinumab. With ixekizumab approved in 2016 and brodalumab under review, new options in biologic therapy will offer patients and clinicians greater choices in treating severe and recalcitrant psoriasis.
The role of current biologic therapies in psoriasis predicates on the pathogenic role of upregulated, immune-related mechanisms that result in the activation of myeloid dendritic cells, which release IL-17, IL-23, and other cytokines to activate T cells, including helper T cell TH17. Along with other immune cells, TH17 produces IL-17. This proinflammatory cascade results in keratinocyte proliferation, angiogenesis, and migration of immune cells toward psoriatic lesions.1 Thus, the newest classes of biologics target IL-12, IL-23, and IL-17 to disrupt this inflammatory cascade.
We provide an updated review of the most recent clinical efficacy and safety data on the newest IL-23 and IL-17 inhibitors in the pipeline or approved for psoriasis, including risankizumab, guselkumab, tildrakizumab, ixekizumab, and brodalumab (Table). Ustekinumab and adalimumab, which have been previously approved by the US Food and Drug Administration (FDA), will be discussed here only as comparators.

IL-23 Inhibitors
Risankizumab
Risankizumab (formerly known as BI 655066)(Boehringer Ingelheim) is a selective human monoclonal antibody targeting the p19 subunit of IL-23 and currently is undergoing phase 3 trials for psoriasis. A proof-of-concept phase 1 study of 39 participants demonstrated efficacy after 12 weeks of treatment at varying subcutaneous and intravenous doses with placebo control.11 At week 12, 87% (27/31)(P<.001) of all risankizumab-treated participants achieved 75% reduction in psoriasis area and severity index (PASI) score compared to 0% of 8 placebo-treated participants. Common adverse effects (AEs) occurred in 65% (20/31) of risankizumab-treated participants, including non–dose-dependent upper respiratory tract infections, nasopharyngitis, and headache. Serious adverse events (SAEs) that occurred were considered unrelated to the study medication.11
A phase 2 trial of 166 participants compared 3 dosing regimens of subcutaneous risankizumab (single 18-mg dose at week 0; single 90-mg dose at weeks 0, 4, and 16; or single 180-mg dose at weeks 0, 4, and 16) and ustekinumab (weight-based single 45- or 90-mg dose at weeks 0, 4, and 16), demonstrating noninferiority at higher doses of risankizumab.2 Preliminary primary end point results at week 12 showed PASI 90 in 32.6% (P=.4667), 73.2% (P=.0013), 81.0% (P<.0001), and 40.0% of the treatment groups, respectively. Participants in the 180-mg risankizumab group achieved PASI 90 eight weeks faster than those on ustekinumab, lasting more than 2 months longer. Adverse effects were similar across all treatment groups and SAEs were unrelated to the study medications.2
Guselkumab
Guselkumab (Janssen Biotech, Inc) is a selective human monoclonal antibody against the p19 subunit of IL-23. The 52-week phase 2 X-PLORE trial compared dose-ranging subcutaneous guselkumab (5 mg at weeks 0 and 4, then every 12 weeks; 15 mg every 8 weeks; 50 mg at weeks 0 and 4, then every 12 weeks; 100 mg every 8 weeks; or 200 mg at weeks 0 and 4, then every 12 weeks), adalimumab (80-mg loading dose, followed by 40 mg at week 1, then every other week), and placebo in 293 randomized participants.4 At week 16, 34% (P=.002) of participants in the 5-mg guselkumab group, 61% (P<.001) in the 15-mg group, 79% (P<.001) in the 50-mg group, 86% (P<.001) in the 100-mg group, 83% (P<.001) in the 200-mg group, and 58% (P<.001) in the adalimumab group achieved physician global assessment (PGA) scores of 0 (clear) or 1 (minimal psoriasis) compared to 7% of the placebo group. Achievement of PASI 75 similarly favored the guselkumab (44% [P<.001]; 76% [no P value given]; 81% [P<.001]; 79% [P<.001]; and 81% [P<.001], respectively) and adalimumab treatment arms (70% [P<.001]) compared to 5% in the placebo group. In longer-term comparisons to week 40, participants in the 50-, 100-, and 200-mg guselkumab groups showed significantly greater remission of psoriatic lesions, measured by a PGA score of 0 or 1, than participants in the adalimumab group (71% [P=.05]; 77% [P=.005]; 81% [P=.01]; and 49%, respectively).4
Preliminary results from VOYAGE 1 (N=837), the first of several phase 3 trials, further demonstrate the superiority of guselkumab 100 mg at weeks 0 and 4 and then every 8 weeks over adalimumab (standard dosing) and placebo; at week 16, 73.3% (P<.001 for both comparisons) versus 49.7% and 2.9% of participants, respectively, achieved PASI 90, with sustained superiority of skin clearance in guselkumab-treated participants compared to adalimumab and placebo through week 48.3
Long-term safety data showed no dose dependence or trend from 0 to 16 weeks and 16 to 52 weeks of treatment regarding rates of AEs, SAEs, or serious infections.4 Between weeks 16 and 52, 48.9% of all guselkumab-treated participants exhibited AEs compared to 60.5% of adalimumab-treated participants and 51.3% of placebo participants. Overall infection rates also were lowest in the guselkumab group at 29.8% compared to 36.8% and 35.9%, respectively. Three participants treated with guselkumab had major cardiovascular events, including a fatal myocardial infarction. No cases of tuberculosis or serious opportunistic infections were reported.4
Tildrakizumab
Tildrakizumab (formerly known as MK-3222)(Sun Pharmaceutical Industries Ltd) is a human monoclonal antibody also targeting the p19 subunit of IL-23. In a phase 2 study of 355 participants with chronic plaque psoriasis, participants received 5-, 25-, 100-, or 200-mg subcutaneous tildrakizumab or placebo at weeks 0 and 4 and then every 12 weeks for a total of 52 weeks.6 At week 16, PASI 75 results were 33.3%, 64.4%, 66.3%, 74.4%, and 4.4%, respectively (P<.001 for each comparison). Improvement began within the first month of treatment, with median times to PASI 75 of 57 days at 200-mg dosing and 84 days at 100-mg dosing. Of those participants achieving PASI 75 by drug discontinuation at week 52, 96% of the 100-mg group and 93% of the 200-mg group maintained PASI 75 through week 72, suggesting low relapse rates after treatment cessation.6
In October 2016, the efficacy results of 2 pivotal phase 3 trials (reSURFACE 1 and reSURFACE 2) involving more than 1800 participants combined revealed PASI 90 achievement in an average of 54% of participants on tildrakizumab 100 mg and 59% of participants on tildrakizumab 200 mg at week 28.5 Achievement of PASI 100 occurred in 24% and 30% of participants at week 28, respectively. The second of these trials included an etanercept comparison group and demonstrated head-to-head superiority of 100 and 200 mg subcutaneous tildrakizumab at week 12 by end point measures.5
Treatment-related AEs occurred at rates of 25% in tildrakizumab-treated participants and 22% in placebo-treated participants, most frequently nasopharyngitis and headache.6 At least 1 AE occurred in 64% of tildrakizumab-treated participants without dose dependence compared to 69% of placebo-treated participants. Severe AEs thought to be drug treatment related were bacterial arthritis, lymphedema, melanoma, stroke, and epiglottitis.6
IL-17 Inhibitors
Ixekizumab
Ixekizumab (Eli Lilly and Company), a monoclonal inhibitor of IL-17A, is the most recently approved psoriasis biologic on the market and has been cleared for use in adults with moderate to severe plaque psoriasis. Recommended dosing is 160 mg (given in two 80-mg subcutaneous injections via an autoinjector or prefilled syringe) at week 0, followed by an 80-mg injection at weeks 2, 4, 6, 8, 10, and 12, and then 80 mg every 4 weeks thereafter. The FDA approved ixekizumab in March 2016 following favorable results of several phase 3 trials: UNCOVER-1, UNCOVER-2, and UNCOVER-3.7,8
In UNCOVER-1, 1296 participants were randomized to 1 of 2 ixekizumab treatment arms—160 mg starting dose at week 0, 80 mg every 2 or 4 weeks thereafter—or placebo.7 At week 12, 89.1%, 82.6%, and 3.9% achieved PASI 75, respectively (P<.001 for both). Importantly, high numbers of participants also achieved PASI 90 (70.9% in the 2-week group and 64.6% in the 4-week group vs 0.5% in the placebo group [P<.001]) and PASI 100 (35.3% and 33.6% vs 0%, respectively [P<.001]), suggesting high rates of disease clearance.7
UNCOVER-2 (N=1224) and UNCOVER-3 (N=1346) investigated the same 2 dosing regimens of ixekizumab compared to etanercept 50 mg biweekly and placebo.8 At week 12, the percentage of participants achieving PASI 90 in UNCOVER-2 was 70.7%, 59.7%, 18.7%, and 0.6%, respectively, and 68.1%, 65.3%, 25.7%, and 3.1%, respectively, in UNCOVER-3 (P<.0001 for all comparisons to placebo and etanercept). At week 12, PASI 100 results also showed striking superiority, with 40.5%, 30.8%, 5.3%, and 0.6% of participants, respectively, in UNCOVER-2, and 37.7%, 35%, 7.3%, and 0%, respectively, in UNCOVER-3, achieving complete clearance of disease (P<.0001 for all comparisons to placebo and etanercept). Responses to ixekizumab were observed as early as weeks 1 and 2, while no participants in the etanercept and placebo treatment groups achieved comparative efficiency.8
In an extension of UNCOVER-3, efficacy increased from week 12 to week 60 according to PASI 90 (68%–73% in the 2-week group; 65%–72% in the 4-week group) and PASI 100 measures (38%–55% in the 2-week group; 35%–52% in the 4-week group).7
The most common AEs associated with ixekizumab treatment from weeks 0 to 12 occurred at higher rates in the 2-week and 4-week ixekizumab groups compared to placebo, including nasopharyngitis (9.5% and 9% vs 8.7%, respectively), upper respiratory tract infection (4.4% and 3.9% vs 3.5%, respectively), injection-site reaction (10% and 7.7% vs 1%, respectively), arthralgia (4.4% and 4.3% vs 2.9%, respectively), and headache (2.5% and 1.9% vs 2.1%, respectively). Infections, including candidal, oral, vulvovaginal, and cutaneous, occurred in 27% of the 2-week dosing group and 27.4% of the 4-week dosing group compared to 22.9% of the placebo group during weeks 0 to 12, with candidal infections in particular occurring more frequently in the active treatment groups and exhibiting dose dependence. Other AEs of special interest that occurred among all ixekizumab-treated participants (n=3736) from weeks 0 to 60 were cardiovascular and cerebrovascular events (22 [0.6%]), inflammatory bowel disease (11 [0.3%]), non–skin cancer malignancy (14 [0.4%]), and nonmelanoma skin cancer (20 [0.5%]). Neutropenia occurred at higher rates in ixekizumab-treated participants (9.3% in the 2-week group and 8.6% in the 4-week group) compared to placebo (3.3%) and occurred in 11.5% of all ixekizumab participants over 60 weeks.7
Brodalumab
Brodalumab (Valeant Pharmaceuticals International, Inc) is a human monoclonal antibody targeting the IL-17A receptor currently under review for FDA approval after undergoing phase 3 trials. The first of these trials, AMAGINE-1, showed efficacy of subcutaneous brodalumab (140 or 210 mg administered every 2 weeks with an extra dose at week 1) compared to placebo in 661 participants.9 At week 12, 60%, 83%, and 3%, respectively, achieved PASI 75; 43%, 70%, and 1%, respectively, achieved PASI 90; and 23%, 42%, and 1%, respectively, achieved PASI 100 (P<.001 for all respective comparisons to placebo). These effects were retained through 52 weeks of treatment. The median time to complete disease clearance in participants reaching PASI 100 was 12 weeks. Conversely, participants who were re-randomized to placebo after week 12 of brodalumab treatment relapsed within weeks to months.9
AMAGINE-2 and AMAGINE-3 further demonstrated the efficacy of brodalumab (140 or 210 mg every 2 weeks with extra dose at week 1) compared to ustekinumab (45 or 90 mg weight-based standard dosing) and placebo in 1831 participants, respectively.10 In AMAGINE-2, 49% of participants in the 140-mg group (P<.001 vs placebo), 70% in the 210-mg group (P<.001 vs placebo), 47% in the ustekinumab group, and 3% in the placebo group achieved PASI 90 at week 12. Similarly, in AMAGINE-3, 52% of participants in the 140-mg group (P<.001), 69% in the 210-mg group (P<.001), 48% in the ustekinumab group, and 2% in the placebo group achieved PASI 90. Impressively, complete clearance (PASI 100) at week 12 occurred in 26% of the 140-mg group (P<.001 vs placebo), 44% of the 210-mg group (P<.001 vs placebo), and 22% of the ustekinumab group compared to 2% of the placebo group in AMAGINE-2, with similar rates in AMAGINE-3. Brodalumab was significantly superior to ustekinumab at the 210-mg dose by PASI 90 measures (P<.001) in both studies and at the 140-mg dose by PASI 100 measures (P=.007) in AMAGINE-3 only.10
Common AEs were nasopharyngitis, upper respiratory tract infection, headache, and arthralgia, all occurring at grossly similar rates (49%–60%) across all experimental groups in AMAGINE-1, AMAGINE-2, and AMAGINE-3 during the first 12-week treatment period.9,10 Brodalumab treatment groups had high rates of specific interest AEs compared to ustekinumab and placebo groups, including neutropenia (0.8%, 1.1%, 0.3%, and 0%, respectively) and candidal infections (0.8%, 1.3%, 0.3%, and 0.3%, respectively). Induction phase (weeks 0–12) depression rates were concerning, with 6 cases each in AMAGINE-2 (4 [0.7%] in the 140-mg group, 2 [0.3%] in the 210-mg group) and AMAGINE-3 (4 [0.6%] in the 140-mg group, 2 [0.3%] in the 210-mg group). Cases of neutropenia were mild, were not associated with major infection, and were transient or reversible. Depression rates after 52 weeks of treatment were 1.7% (23/1567) of brodalumab participants in AMAGINE-2 and 1.8% (21/1613) in AMAGINE-3. Three participants, all on constant 210-mg dosing through week 52, attempted suicide with 1 completion10; however, because no other IL-17 inhibitors were associated with depression or suicide in other trials, it has been suggested that these cases were incidental and not treatment related.12 An FDA advisory panel recommended approval of brodalumab in July 2016 despite ongoing concerns of depression and suicide.13
Conclusion
The robust investigation into IL-23 and IL-17 inhibitors to treat plaque psoriasis has yielded promising results, including the unprecedented rates of PASI 100 achievement with these new biologics. Risankizumab, ixekizumab, and brodalumab have demonstrated superior efficacy in trials compared to ustekinumab. Tildrakizumab has shown low disease relapse after drug cessation. Ixekizumab and brodalumab have shown high rates of total disease clearance. Thus far, safety findings for these pipeline biologics have been consistent with those of ustekinumab. With ixekizumab approved in 2016 and brodalumab under review, new options in biologic therapy will offer patients and clinicians greater choices in treating severe and recalcitrant psoriasis.
- Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361:496-509.
- Papp K, Menter A, Sofen H, et al. Efficacy and safety of different dose regimens of a selective IL-23p19 inhibitor (BI 655066) compared with ustekinumab in patients with moderate-to-severe plaque psoriasis with and without psoriatic arthritis. Paper presented at: 2015 American College of Rheumatology/Association of Rheumatology Health Professionals Annual Meeting; November 6-11, 2015; San Francisco, CA.
- New phase 3 data show significant efficacy versus placebo and superiority of guselkumab versus Humira in treatment of moderate to severe plaque psoriasis [press release]. Vienna, Austria; Janssen Research & Development, LLC: October 1, 2016.
- Gordon KB, Duffin KC, Bissonnette R, et al. A phase 2 trial of guselkumab versus adalimumab for plaque psoriasis. N Engl J Med. 2015;373:136-144.
- Sun Pharma to announce late-breaking results for investigational IL-23p19 inhibitor, Tildrakizumab, achieves primary end point in both phase-3 studies in patients with moderate-to-severe plaque psoriasis [press release]. Mumbai, India; Sun Pharmaceutical Industries Ltd: October 1, 2016.
- Papp K, Thaci D, Reich K, et al. Tildrakizumab (MK-3222), an anti-interleukin-23p19 monoclonal antibody, improves psoriasis in a phase IIb randomized placebo-controlled trial. Br J Dermatol. 2015;173:930-939.
- Gordon KB, Blauvelt A, Papp KA, et al; UNCOVER-1 Study Group, UNCOVER-2 Study Group, UNCOVER-3 Study Group. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375:345-356.
- Griffiths CE, Reich K, Lebwohl M, et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet. 2015;386:541-551.
- Papp KA, Reich K, Paul C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis [published online June 23, 2016]. Br J Dermatol. 2016;175:273-286.
- Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373:1318-1328.
- Krueger JG, Ferris LK, Menter A, et al. Anti-IL-23A mAb BI 655066 for treatment of moderate-to-severe psoriasis: safety, efficacy, pharmacokinetics, and biomarker results of a single-rising-dose, randomized, double-blind, placebo-controlled trial [published online March 1, 2015]. J Allergy Clin Immunol. 2015;136:116-124.e7.
- Chiricozzi A, Romanelli M, Saraceno R, et al. No meaningful association between suicidal behavior and the use of IL-17A-neutralizing or IL-17RA-blocking agents [published online August 31, 2016]. Expert Opin Drug Saf. 2016;15:1653-1659.
- FDA advisory committee recommends approval of brodalumab for treatment of moderate-to-severe plaque psoriasis [news release]. Laval, Quebec: Valeant Pharmaceuticals International, Inc; July 19, 2016.
- Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361:496-509.
- Papp K, Menter A, Sofen H, et al. Efficacy and safety of different dose regimens of a selective IL-23p19 inhibitor (BI 655066) compared with ustekinumab in patients with moderate-to-severe plaque psoriasis with and without psoriatic arthritis. Paper presented at: 2015 American College of Rheumatology/Association of Rheumatology Health Professionals Annual Meeting; November 6-11, 2015; San Francisco, CA.
- New phase 3 data show significant efficacy versus placebo and superiority of guselkumab versus Humira in treatment of moderate to severe plaque psoriasis [press release]. Vienna, Austria; Janssen Research & Development, LLC: October 1, 2016.
- Gordon KB, Duffin KC, Bissonnette R, et al. A phase 2 trial of guselkumab versus adalimumab for plaque psoriasis. N Engl J Med. 2015;373:136-144.
- Sun Pharma to announce late-breaking results for investigational IL-23p19 inhibitor, Tildrakizumab, achieves primary end point in both phase-3 studies in patients with moderate-to-severe plaque psoriasis [press release]. Mumbai, India; Sun Pharmaceutical Industries Ltd: October 1, 2016.
- Papp K, Thaci D, Reich K, et al. Tildrakizumab (MK-3222), an anti-interleukin-23p19 monoclonal antibody, improves psoriasis in a phase IIb randomized placebo-controlled trial. Br J Dermatol. 2015;173:930-939.
- Gordon KB, Blauvelt A, Papp KA, et al; UNCOVER-1 Study Group, UNCOVER-2 Study Group, UNCOVER-3 Study Group. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375:345-356.
- Griffiths CE, Reich K, Lebwohl M, et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet. 2015;386:541-551.
- Papp KA, Reich K, Paul C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis [published online June 23, 2016]. Br J Dermatol. 2016;175:273-286.
- Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373:1318-1328.
- Krueger JG, Ferris LK, Menter A, et al. Anti-IL-23A mAb BI 655066 for treatment of moderate-to-severe psoriasis: safety, efficacy, pharmacokinetics, and biomarker results of a single-rising-dose, randomized, double-blind, placebo-controlled trial [published online March 1, 2015]. J Allergy Clin Immunol. 2015;136:116-124.e7.
- Chiricozzi A, Romanelli M, Saraceno R, et al. No meaningful association between suicidal behavior and the use of IL-17A-neutralizing or IL-17RA-blocking agents [published online August 31, 2016]. Expert Opin Drug Saf. 2016;15:1653-1659.
- FDA advisory committee recommends approval of brodalumab for treatment of moderate-to-severe plaque psoriasis [news release]. Laval, Quebec: Valeant Pharmaceuticals International, Inc; July 19, 2016.
Practice Points
- The newest biologics for treatment of moderate to severe plaque psoriasis are IL-23 and IL-17 inhibitors with unprecedented efficacy of complete skin clearance compared to older biologics.
- Risankizumab, guselkumab, and tildrakizumab are new IL-23 inhibitors currently in phase 3 trials with promising early efficacy and safety results.
- Ixekizumab, which recently was approved, and brodalumab, which is pending US Food and Drug Administration review, are new IL-17 inhibitors that achieved total skin clearance in more than one-quarter of phase 3 participants after 12 weeks of treatment.
Coding Changes for 2017
All physicians will see changes in reimbursement in 2017. A new president with a new agenda makes for an interesting time ahead for health care in the United States. However, in this time of flux, there is one constant: the Final Rule, an informal term for the annual update on how the Medicare system will function and how much you will get paid for what you do.1 The document is 393 pages and outlines what is new in the Medicare system, with lots of supplements giving granular details about physician work, overhead, and supply and labor costs. In this column, I have taken the liberty of dissecting the Final Rule for you and to bring attention to its high and low points for dermatologists.
Changes in Relative Value Units
The conversion factor has gone up, meaning you will be paid a bit more this year for what you do; it is not enough to account for inflation or the increasing cost of unfunded mandates, but it is better than nothing. Although the conversion factor was $35.8043 in 2016, it increased by more than 0.2% on January 1, 2017, to $35.8887.1 How is this conversion factor calculated? We go up 0.5% due to MACRA (Medicare Access and CHIP Reauthorization Act), down 0.013% due to budget neutrality, down 0.07% due to multiple procedure payment reduction changes, and down another 0.18% due to the misvalued code target.1 The misvalued code target is related to targets established by statute for 2016 to 2018 and payment rates are reduced across the board if they are not met.
If payments suffer from reductions in work value, they may not happen all at once. If the Centers for Medicare & Medicaid Services (CMS) reduce total relative value units (RVUs) by more than 20%, reductions will take place over at least 2 years with a single year drop maximum of 19%.1 Unfortunately, such limits do not apply to revised codes, which can take as big a hit as the CMS cares to make.
Changes to Global Periods
In 2015, we learned that 10- and 90-day global periods would be eliminated in 2017 and 2018, respectively, with great concern on the part of the government about the number and level of evaluation and management services embedded in these codes. The implementation of global policy elimination was prohibited by MACRA and the CMS was required to develop and implement a process to gather data on services furnished in the global period from a representative sample of physicians, which they will use to value surgical services beginningin 2019.1 The CMS decided to capture this data with a new set of time-based G codes (which would be onerous for all practicing physicians), not just the unlucky folks who were to be the sample mandated under MACRA.2 During the comment period, it became obvious to the CMS that this concept was flawed for many reasons and it decided to hold a town hall meeting at the CMS headquarters on August 25, 2016, on data collection on resources used in furnishing global services in which 90 minutes of live testimony in the morning was followed by another 90 minutes by telephone in the afternoon.3 This meeting, which I attended, resulted in the CMS changing the all-practitioner reporting program to a specified sample with others allowed to opt in. Practitioners in groups of less than 10 are exempt, and only physicians in Florida, Kentucky, Louisiana, Nevada, New Jersey, North Dakota, Ohio, Oregon, and Rhode Island must capture data beginning in July 2017.1 These data only have to be captured on codes that are used by more than 100 practitioners and are furnished at least 10,000 times or have allowed charges of greater than $10,000,000 annually. If you are lucky enough to live in one of the testing states, you must start on July 1 but can start before July 1 if you wish. Practitioners in smaller practices or in other geographic areas are encouraged to report data if feasible but are not required to do so. Current Procedural Terminology (CPT) code 99024 will be used for reporting postoperative services rather than the proposed onerous set of G codes, and reporting will not be required for preoperative visits included in the global package or for services not related to the patient’s visit.
Changes to Chronic Care Management
There are new and modified chronic care management codes that are not of use to you unless you are the primary provider for the patient and you and the patient meet multiple stringent requirements.4 The patient must have multiple illnesses, use multiple medications, be unable to perform activities of daily living, require a caregiver, and/or have repeat admissions or emergency department visits. Typical adult patients who receive complex chronic care management services are treated with 3 or more prescription medications and may be receiving other types of therapeutic interventions (eg, physical therapy, occupational therapy). Typical pediatric patients receive 3 or more therapeutic interventions (eg, medications, nutritional support, respiratory therapy). All patients have 2 or more chronic continuous or episodic health conditions that are expected to last at least 12 months or until the death of the patient and place the patient at serious risk for death, acute exacerbation/decompensation, or functional decline.4
Changes to Moderate Sedation Codes
The economic value of providing moderate sedation (eg, drug-induced depression of consciousness during which patients respond purposefully to verbal commands, either alone or accompanied by light tactile stimulation) used to be embedded in a variety of CPT codes, which is no longer the case in 2017. Diazepam or similar drugs swallowed or dissolved under the tongue are not included. The new CPT codes 99151, 99152, 99153, 99155, 99156, and 99157 are not to be used to report administration of medications for pain control or minimal sedation (anxiolysis). An independent trained observer, an individual who is qualified to monitor the patient during the procedure and who has no other duties (eg, assisting at surgery) during the procedure, must be present. If you are thinking of using these codes, read the entire section in the CPT manual,4 check your state laws, and consult your malpractice carrier and perhaps even your health care attorney.
Changes to Nail Procedure Codes
Current Procedural Terminology code 11752 (excision of nail and nail matrix, partial or complete [eg, ingrown or deformed nail], for permanent removal; with amputation of tuft of distal phalanx) is now gone, while base code 11750 remains. If you are doing nail surgery and removing underlying bone, instead use code 26236 (partial excision [craterization, saucerization, or diaphysectomy] bone [eg, osteomyelitis]; distal phalanx of finger), 28124 (partial excision [craterization, saucerization, sequestrectomy, or diaphysectomy] bone [eg, osteomyelitis or bossing]; phalanx of toe), or other codes in the same section of the CPT manual if they more precisely describe the procedure performed.
Changes to Slide Consultation Codes
The slide consultation codes 88321 (consultation and report on referred slides prepared elsewhere), 88323 (consultation and report on referred material requiring preparation of slides), and 88325 (consultation, comprehensive, with review of records and specimens, with report on referred material) were revalued this year, with the first 2 showing no change but the latter showing an increase in value from 2.50 to 2.85 RVUs.1 None are meant to be routine. If you have every slide looked at by someone else for “quality assurance reasons,” the consultation is not reportable. If you use these consultation codes too often, the CMS might have concerns about fraud and abuse. Visit http://data.cms.gov to see how you compare to your peers.
Changes to Reflectance Confocal Microscopy Codes
Reflectance confocal microscopy had new codes for 2016, which were carrier priced, and in 2017 they have real RVUs per the CMS. The payments for these codes have a national average reimbursement of $161.85 for 96931 (reflectance confocal microscopy for cellular and subcellular imaging of skin; image acquisition and interpretation and report, first lesion), $104.80 for 96932 (image acquisition only, first lesion), and $45.94 for 96933 (interpretation and report only, first lesion).5 The respective add-on codes have values of $83.26 for 96934 (image acquisition and interpretation and report, each additional lesion [list separately in addition to code for primary procedure]), $35.17 for 96935 (image acquisition only, each additional lesion [list separately in addition to code for primary procedure]), and $43.78 for 96936 (interpretation and report only, each additional lesion [list separately in addition to code for primary procedure]).
Other Coding Changes
There are a whole bunch of new codes in the “Genomic Sequencing Procedures and Other Molecular Multianalyte Assays” (MMAAs) section of CPT. The important thing for you to remember is these codes are for the laboratory performing the assay to report, not the physician ordering it. There is a new Appendix O for proprietary laboratory analysis MMAAs, including those that do not have a Category I code. These MMAAs are identified in Appendix O by a 4-digit number followed by the letter M.4
There are some revisions to psychotherapy codes 90832 to 90847. These codes are outside our scope of practice and should only be used by psychiatrists, social workers, psychologists, or other appropriate mental health workers.
Final Thoughts
It has not been a breakout year for telehealth and we still do not have payment for store-and-forward teledermatology, except in a few designated rural areas. With the advent of the rhetoric we have heard after the presidential election, any speculation on what will happen to the brave new world of the merit-based incentive payment system, alternative payment models, and other regulations are anyone’s guess.
- Medicare Program; Revisions to Payment Policies Under the Physician Fee Schedule and Other Revisions to Part B for CY 2017; Medicare Advantage Bid Pricing Data Release; Medicare Advantage and Part D Medical Loss Ratio Data Release; Medicare Advantage Provider Network Requirements; Expansion of Medicare Diabetes Prevention Program Model; Medicare Shared Savings Program Requirements. Fed Regist. 2016;81(220):80170-80562. To be codified at 42 CFR § 405, 410, 411, 414, 417, 422, 423, 424, 425, and 460.
- Siegel DM. The Proposed Rule and payments for 2017: the good, the bad, and the ugly. Cutis. 2016;98:245-248.
- Data collection on resources used in furnishing global services town hall CY 2017 Medicare physician fee schedule Proposed Rule. Centers for Medicare & Medicaid Services website. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/Downloads/CY2017-PFS-FR-Townhall.pdf. Published August 25, 2016. Accessed January 4, 2017.
- Current Procedural Terminology 2017, Professional Edition. Chicago, IL: American Medical Association; 2016.
- Addendum B—relative value units and related information used in CY 2017 final rule. Centers for Medicare & Medicaid Services website. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/Downloads/CY2017-PFS-FR-Addenda.zip. Accessed January 23, 2017.
All physicians will see changes in reimbursement in 2017. A new president with a new agenda makes for an interesting time ahead for health care in the United States. However, in this time of flux, there is one constant: the Final Rule, an informal term for the annual update on how the Medicare system will function and how much you will get paid for what you do.1 The document is 393 pages and outlines what is new in the Medicare system, with lots of supplements giving granular details about physician work, overhead, and supply and labor costs. In this column, I have taken the liberty of dissecting the Final Rule for you and to bring attention to its high and low points for dermatologists.
Changes in Relative Value Units
The conversion factor has gone up, meaning you will be paid a bit more this year for what you do; it is not enough to account for inflation or the increasing cost of unfunded mandates, but it is better than nothing. Although the conversion factor was $35.8043 in 2016, it increased by more than 0.2% on January 1, 2017, to $35.8887.1 How is this conversion factor calculated? We go up 0.5% due to MACRA (Medicare Access and CHIP Reauthorization Act), down 0.013% due to budget neutrality, down 0.07% due to multiple procedure payment reduction changes, and down another 0.18% due to the misvalued code target.1 The misvalued code target is related to targets established by statute for 2016 to 2018 and payment rates are reduced across the board if they are not met.
If payments suffer from reductions in work value, they may not happen all at once. If the Centers for Medicare & Medicaid Services (CMS) reduce total relative value units (RVUs) by more than 20%, reductions will take place over at least 2 years with a single year drop maximum of 19%.1 Unfortunately, such limits do not apply to revised codes, which can take as big a hit as the CMS cares to make.
Changes to Global Periods
In 2015, we learned that 10- and 90-day global periods would be eliminated in 2017 and 2018, respectively, with great concern on the part of the government about the number and level of evaluation and management services embedded in these codes. The implementation of global policy elimination was prohibited by MACRA and the CMS was required to develop and implement a process to gather data on services furnished in the global period from a representative sample of physicians, which they will use to value surgical services beginningin 2019.1 The CMS decided to capture this data with a new set of time-based G codes (which would be onerous for all practicing physicians), not just the unlucky folks who were to be the sample mandated under MACRA.2 During the comment period, it became obvious to the CMS that this concept was flawed for many reasons and it decided to hold a town hall meeting at the CMS headquarters on August 25, 2016, on data collection on resources used in furnishing global services in which 90 minutes of live testimony in the morning was followed by another 90 minutes by telephone in the afternoon.3 This meeting, which I attended, resulted in the CMS changing the all-practitioner reporting program to a specified sample with others allowed to opt in. Practitioners in groups of less than 10 are exempt, and only physicians in Florida, Kentucky, Louisiana, Nevada, New Jersey, North Dakota, Ohio, Oregon, and Rhode Island must capture data beginning in July 2017.1 These data only have to be captured on codes that are used by more than 100 practitioners and are furnished at least 10,000 times or have allowed charges of greater than $10,000,000 annually. If you are lucky enough to live in one of the testing states, you must start on July 1 but can start before July 1 if you wish. Practitioners in smaller practices or in other geographic areas are encouraged to report data if feasible but are not required to do so. Current Procedural Terminology (CPT) code 99024 will be used for reporting postoperative services rather than the proposed onerous set of G codes, and reporting will not be required for preoperative visits included in the global package or for services not related to the patient’s visit.
Changes to Chronic Care Management
There are new and modified chronic care management codes that are not of use to you unless you are the primary provider for the patient and you and the patient meet multiple stringent requirements.4 The patient must have multiple illnesses, use multiple medications, be unable to perform activities of daily living, require a caregiver, and/or have repeat admissions or emergency department visits. Typical adult patients who receive complex chronic care management services are treated with 3 or more prescription medications and may be receiving other types of therapeutic interventions (eg, physical therapy, occupational therapy). Typical pediatric patients receive 3 or more therapeutic interventions (eg, medications, nutritional support, respiratory therapy). All patients have 2 or more chronic continuous or episodic health conditions that are expected to last at least 12 months or until the death of the patient and place the patient at serious risk for death, acute exacerbation/decompensation, or functional decline.4
Changes to Moderate Sedation Codes
The economic value of providing moderate sedation (eg, drug-induced depression of consciousness during which patients respond purposefully to verbal commands, either alone or accompanied by light tactile stimulation) used to be embedded in a variety of CPT codes, which is no longer the case in 2017. Diazepam or similar drugs swallowed or dissolved under the tongue are not included. The new CPT codes 99151, 99152, 99153, 99155, 99156, and 99157 are not to be used to report administration of medications for pain control or minimal sedation (anxiolysis). An independent trained observer, an individual who is qualified to monitor the patient during the procedure and who has no other duties (eg, assisting at surgery) during the procedure, must be present. If you are thinking of using these codes, read the entire section in the CPT manual,4 check your state laws, and consult your malpractice carrier and perhaps even your health care attorney.
Changes to Nail Procedure Codes
Current Procedural Terminology code 11752 (excision of nail and nail matrix, partial or complete [eg, ingrown or deformed nail], for permanent removal; with amputation of tuft of distal phalanx) is now gone, while base code 11750 remains. If you are doing nail surgery and removing underlying bone, instead use code 26236 (partial excision [craterization, saucerization, or diaphysectomy] bone [eg, osteomyelitis]; distal phalanx of finger), 28124 (partial excision [craterization, saucerization, sequestrectomy, or diaphysectomy] bone [eg, osteomyelitis or bossing]; phalanx of toe), or other codes in the same section of the CPT manual if they more precisely describe the procedure performed.
Changes to Slide Consultation Codes
The slide consultation codes 88321 (consultation and report on referred slides prepared elsewhere), 88323 (consultation and report on referred material requiring preparation of slides), and 88325 (consultation, comprehensive, with review of records and specimens, with report on referred material) were revalued this year, with the first 2 showing no change but the latter showing an increase in value from 2.50 to 2.85 RVUs.1 None are meant to be routine. If you have every slide looked at by someone else for “quality assurance reasons,” the consultation is not reportable. If you use these consultation codes too often, the CMS might have concerns about fraud and abuse. Visit http://data.cms.gov to see how you compare to your peers.
Changes to Reflectance Confocal Microscopy Codes
Reflectance confocal microscopy had new codes for 2016, which were carrier priced, and in 2017 they have real RVUs per the CMS. The payments for these codes have a national average reimbursement of $161.85 for 96931 (reflectance confocal microscopy for cellular and subcellular imaging of skin; image acquisition and interpretation and report, first lesion), $104.80 for 96932 (image acquisition only, first lesion), and $45.94 for 96933 (interpretation and report only, first lesion).5 The respective add-on codes have values of $83.26 for 96934 (image acquisition and interpretation and report, each additional lesion [list separately in addition to code for primary procedure]), $35.17 for 96935 (image acquisition only, each additional lesion [list separately in addition to code for primary procedure]), and $43.78 for 96936 (interpretation and report only, each additional lesion [list separately in addition to code for primary procedure]).
Other Coding Changes
There are a whole bunch of new codes in the “Genomic Sequencing Procedures and Other Molecular Multianalyte Assays” (MMAAs) section of CPT. The important thing for you to remember is these codes are for the laboratory performing the assay to report, not the physician ordering it. There is a new Appendix O for proprietary laboratory analysis MMAAs, including those that do not have a Category I code. These MMAAs are identified in Appendix O by a 4-digit number followed by the letter M.4
There are some revisions to psychotherapy codes 90832 to 90847. These codes are outside our scope of practice and should only be used by psychiatrists, social workers, psychologists, or other appropriate mental health workers.
Final Thoughts
It has not been a breakout year for telehealth and we still do not have payment for store-and-forward teledermatology, except in a few designated rural areas. With the advent of the rhetoric we have heard after the presidential election, any speculation on what will happen to the brave new world of the merit-based incentive payment system, alternative payment models, and other regulations are anyone’s guess.
All physicians will see changes in reimbursement in 2017. A new president with a new agenda makes for an interesting time ahead for health care in the United States. However, in this time of flux, there is one constant: the Final Rule, an informal term for the annual update on how the Medicare system will function and how much you will get paid for what you do.1 The document is 393 pages and outlines what is new in the Medicare system, with lots of supplements giving granular details about physician work, overhead, and supply and labor costs. In this column, I have taken the liberty of dissecting the Final Rule for you and to bring attention to its high and low points for dermatologists.
Changes in Relative Value Units
The conversion factor has gone up, meaning you will be paid a bit more this year for what you do; it is not enough to account for inflation or the increasing cost of unfunded mandates, but it is better than nothing. Although the conversion factor was $35.8043 in 2016, it increased by more than 0.2% on January 1, 2017, to $35.8887.1 How is this conversion factor calculated? We go up 0.5% due to MACRA (Medicare Access and CHIP Reauthorization Act), down 0.013% due to budget neutrality, down 0.07% due to multiple procedure payment reduction changes, and down another 0.18% due to the misvalued code target.1 The misvalued code target is related to targets established by statute for 2016 to 2018 and payment rates are reduced across the board if they are not met.
If payments suffer from reductions in work value, they may not happen all at once. If the Centers for Medicare & Medicaid Services (CMS) reduce total relative value units (RVUs) by more than 20%, reductions will take place over at least 2 years with a single year drop maximum of 19%.1 Unfortunately, such limits do not apply to revised codes, which can take as big a hit as the CMS cares to make.
Changes to Global Periods
In 2015, we learned that 10- and 90-day global periods would be eliminated in 2017 and 2018, respectively, with great concern on the part of the government about the number and level of evaluation and management services embedded in these codes. The implementation of global policy elimination was prohibited by MACRA and the CMS was required to develop and implement a process to gather data on services furnished in the global period from a representative sample of physicians, which they will use to value surgical services beginningin 2019.1 The CMS decided to capture this data with a new set of time-based G codes (which would be onerous for all practicing physicians), not just the unlucky folks who were to be the sample mandated under MACRA.2 During the comment period, it became obvious to the CMS that this concept was flawed for many reasons and it decided to hold a town hall meeting at the CMS headquarters on August 25, 2016, on data collection on resources used in furnishing global services in which 90 minutes of live testimony in the morning was followed by another 90 minutes by telephone in the afternoon.3 This meeting, which I attended, resulted in the CMS changing the all-practitioner reporting program to a specified sample with others allowed to opt in. Practitioners in groups of less than 10 are exempt, and only physicians in Florida, Kentucky, Louisiana, Nevada, New Jersey, North Dakota, Ohio, Oregon, and Rhode Island must capture data beginning in July 2017.1 These data only have to be captured on codes that are used by more than 100 practitioners and are furnished at least 10,000 times or have allowed charges of greater than $10,000,000 annually. If you are lucky enough to live in one of the testing states, you must start on July 1 but can start before July 1 if you wish. Practitioners in smaller practices or in other geographic areas are encouraged to report data if feasible but are not required to do so. Current Procedural Terminology (CPT) code 99024 will be used for reporting postoperative services rather than the proposed onerous set of G codes, and reporting will not be required for preoperative visits included in the global package or for services not related to the patient’s visit.
Changes to Chronic Care Management
There are new and modified chronic care management codes that are not of use to you unless you are the primary provider for the patient and you and the patient meet multiple stringent requirements.4 The patient must have multiple illnesses, use multiple medications, be unable to perform activities of daily living, require a caregiver, and/or have repeat admissions or emergency department visits. Typical adult patients who receive complex chronic care management services are treated with 3 or more prescription medications and may be receiving other types of therapeutic interventions (eg, physical therapy, occupational therapy). Typical pediatric patients receive 3 or more therapeutic interventions (eg, medications, nutritional support, respiratory therapy). All patients have 2 or more chronic continuous or episodic health conditions that are expected to last at least 12 months or until the death of the patient and place the patient at serious risk for death, acute exacerbation/decompensation, or functional decline.4
Changes to Moderate Sedation Codes
The economic value of providing moderate sedation (eg, drug-induced depression of consciousness during which patients respond purposefully to verbal commands, either alone or accompanied by light tactile stimulation) used to be embedded in a variety of CPT codes, which is no longer the case in 2017. Diazepam or similar drugs swallowed or dissolved under the tongue are not included. The new CPT codes 99151, 99152, 99153, 99155, 99156, and 99157 are not to be used to report administration of medications for pain control or minimal sedation (anxiolysis). An independent trained observer, an individual who is qualified to monitor the patient during the procedure and who has no other duties (eg, assisting at surgery) during the procedure, must be present. If you are thinking of using these codes, read the entire section in the CPT manual,4 check your state laws, and consult your malpractice carrier and perhaps even your health care attorney.
Changes to Nail Procedure Codes
Current Procedural Terminology code 11752 (excision of nail and nail matrix, partial or complete [eg, ingrown or deformed nail], for permanent removal; with amputation of tuft of distal phalanx) is now gone, while base code 11750 remains. If you are doing nail surgery and removing underlying bone, instead use code 26236 (partial excision [craterization, saucerization, or diaphysectomy] bone [eg, osteomyelitis]; distal phalanx of finger), 28124 (partial excision [craterization, saucerization, sequestrectomy, or diaphysectomy] bone [eg, osteomyelitis or bossing]; phalanx of toe), or other codes in the same section of the CPT manual if they more precisely describe the procedure performed.
Changes to Slide Consultation Codes
The slide consultation codes 88321 (consultation and report on referred slides prepared elsewhere), 88323 (consultation and report on referred material requiring preparation of slides), and 88325 (consultation, comprehensive, with review of records and specimens, with report on referred material) were revalued this year, with the first 2 showing no change but the latter showing an increase in value from 2.50 to 2.85 RVUs.1 None are meant to be routine. If you have every slide looked at by someone else for “quality assurance reasons,” the consultation is not reportable. If you use these consultation codes too often, the CMS might have concerns about fraud and abuse. Visit http://data.cms.gov to see how you compare to your peers.
Changes to Reflectance Confocal Microscopy Codes
Reflectance confocal microscopy had new codes for 2016, which were carrier priced, and in 2017 they have real RVUs per the CMS. The payments for these codes have a national average reimbursement of $161.85 for 96931 (reflectance confocal microscopy for cellular and subcellular imaging of skin; image acquisition and interpretation and report, first lesion), $104.80 for 96932 (image acquisition only, first lesion), and $45.94 for 96933 (interpretation and report only, first lesion).5 The respective add-on codes have values of $83.26 for 96934 (image acquisition and interpretation and report, each additional lesion [list separately in addition to code for primary procedure]), $35.17 for 96935 (image acquisition only, each additional lesion [list separately in addition to code for primary procedure]), and $43.78 for 96936 (interpretation and report only, each additional lesion [list separately in addition to code for primary procedure]).
Other Coding Changes
There are a whole bunch of new codes in the “Genomic Sequencing Procedures and Other Molecular Multianalyte Assays” (MMAAs) section of CPT. The important thing for you to remember is these codes are for the laboratory performing the assay to report, not the physician ordering it. There is a new Appendix O for proprietary laboratory analysis MMAAs, including those that do not have a Category I code. These MMAAs are identified in Appendix O by a 4-digit number followed by the letter M.4
There are some revisions to psychotherapy codes 90832 to 90847. These codes are outside our scope of practice and should only be used by psychiatrists, social workers, psychologists, or other appropriate mental health workers.
Final Thoughts
It has not been a breakout year for telehealth and we still do not have payment for store-and-forward teledermatology, except in a few designated rural areas. With the advent of the rhetoric we have heard after the presidential election, any speculation on what will happen to the brave new world of the merit-based incentive payment system, alternative payment models, and other regulations are anyone’s guess.
- Medicare Program; Revisions to Payment Policies Under the Physician Fee Schedule and Other Revisions to Part B for CY 2017; Medicare Advantage Bid Pricing Data Release; Medicare Advantage and Part D Medical Loss Ratio Data Release; Medicare Advantage Provider Network Requirements; Expansion of Medicare Diabetes Prevention Program Model; Medicare Shared Savings Program Requirements. Fed Regist. 2016;81(220):80170-80562. To be codified at 42 CFR § 405, 410, 411, 414, 417, 422, 423, 424, 425, and 460.
- Siegel DM. The Proposed Rule and payments for 2017: the good, the bad, and the ugly. Cutis. 2016;98:245-248.
- Data collection on resources used in furnishing global services town hall CY 2017 Medicare physician fee schedule Proposed Rule. Centers for Medicare & Medicaid Services website. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/Downloads/CY2017-PFS-FR-Townhall.pdf. Published August 25, 2016. Accessed January 4, 2017.
- Current Procedural Terminology 2017, Professional Edition. Chicago, IL: American Medical Association; 2016.
- Addendum B—relative value units and related information used in CY 2017 final rule. Centers for Medicare & Medicaid Services website. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/Downloads/CY2017-PFS-FR-Addenda.zip. Accessed January 23, 2017.
- Medicare Program; Revisions to Payment Policies Under the Physician Fee Schedule and Other Revisions to Part B for CY 2017; Medicare Advantage Bid Pricing Data Release; Medicare Advantage and Part D Medical Loss Ratio Data Release; Medicare Advantage Provider Network Requirements; Expansion of Medicare Diabetes Prevention Program Model; Medicare Shared Savings Program Requirements. Fed Regist. 2016;81(220):80170-80562. To be codified at 42 CFR § 405, 410, 411, 414, 417, 422, 423, 424, 425, and 460.
- Siegel DM. The Proposed Rule and payments for 2017: the good, the bad, and the ugly. Cutis. 2016;98:245-248.
- Data collection on resources used in furnishing global services town hall CY 2017 Medicare physician fee schedule Proposed Rule. Centers for Medicare & Medicaid Services website. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/Downloads/CY2017-PFS-FR-Townhall.pdf. Published August 25, 2016. Accessed January 4, 2017.
- Current Procedural Terminology 2017, Professional Edition. Chicago, IL: American Medical Association; 2016.
- Addendum B—relative value units and related information used in CY 2017 final rule. Centers for Medicare & Medicaid Services website. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/Downloads/CY2017-PFS-FR-Addenda.zip. Accessed January 23, 2017.
Practice Points
- The conversion factor has increased more than 0.2%, which means you will be paid a bit more this year.
- Review Current Procedural Terminology codes carefully for pain control or moderate sedation as well as nail surgery and slide consultation.
- Reflectance confocal microscopy now has relative value units assigned by the Centers for Medicare & Medicaid Services.
Photoprotection Prevents Skin Cancer: Let’s Make It Fashionable to Wear Sun-Protective Clothing
Photoprotection is the foundation of all skin cancer prevention, as UV radiation (UVR) exposure is the only known modifiable risk factor for skin cancer. With the majority of UVR exposure–induced skin cancers found on the scalp, ears, face, and neck, public health initiatives call for wise choices in personal fashion that emphasize the importance of covering these areas.1-3 From a science of fashion perspective, research has shown that wide-brimmed hats provide better means of ensuring the largest area of coverage compared to standard baseball-style hats.4 Thus, for maximum protection, wide-brimmed hats should be favored. However, in academic and military settings, individual style is not optional and is instead influenced or directed by policy, which may not be aligned with the goal of providing photoprotection and raises additional concern for individuals working in environments with longer periods of peak daylight UVR exposure.
In all military branches, service members don uniforms that include head coverage when operating outdoors; however, the choice of headgear is not always aimed at reducing UVR exposure. Similarly, in our counterpart civilian populations, wearing hats that provide the best photoprotection may be influenced by school policies, which frequently mandate clothing choices for children, or by the press or fashion industry in the general public, which might portray sun-protective garments as unfashionable or in some cases threatening if perceived as demonstrating gang affiliation.5 This article serves to encourage health care providers to not only discuss the use of sunscreen when educating patients on sun protection but also to emphasize the benefits of wearing photoprotective garments, particularly wide-brimmed hats given their simplicity, reusability, and affordability. Hat use is particularly important for men with comorbid androgenetic alopecia.6
Skin Cancer Risk
Unfortunately, the incidence of most common types of skin cancer, specifically nonmelanoma skin cancers such basal cell carcinomas and squamous cell carcinomas (ie, keratinocyte carcinomas [KCs]), is difficult to estimate properly, as these cases are not required to be reported to worldwide cancer registries. However, more than 5.4 million cases of skin cancers were diagnosed among 3.3 million Americans in 2016, with an estimated 13,650 deaths associated with skin cancers (not including KCs).3 Tracking and data analyses of cases diagnosed in the active and reserve component populations of the US Armed Forces reflect parallel findings.7 Keratinocyte carcinomas could be considered largely preventable, as most are the result of UVR exposure.1 Additionally, it has been suggested that the vast majority of mutations in melanoma skin cancers (up to 86%) are caused by UVR exposure.8
Prevention
United States–based national public health services such as the American Cancer Society, the Centers for Disease Control and Prevention, and the American Academy of Dermatology embrace photoprotection as the central practice in reducing risk factors for skin cancers. Guidelines put forth by these and other national preventive medical institutions specifically recommend the use of wide-brimmed hats as the best option for protection of the face, head, ears, and neck, in addition to more common recommendations such as seeking shade, avoiding sunlight during peak hours of the day, and using sunscreen.1-3 At state and local levels, policies should be adapted from these recommendations to support protective practices and skin cancer education that begins early for school-aged children. Unfortunately, in some school districts, wearing hats of any kind may be perceived as disruptive or in some cases baseball hats may be a sign of gang affiliation and are therefore banned in the schoolyard.5 The opposite is true in certain parts of the world where sun protection is embraced by the population as a whole, such as Australia where the widely accepted “slip, slop and slap, seek and slide” campaign has extended to some school policymakers who have considered adopting a “no hat, no play” policy.9,10
Sunscreen use as a primary component of photoprotection has its disadvantages in comparison to wearing protective clothing, as sunscreen cannot be reused and proper usage requires reapplication after swimming, when sweating, and following 2 hours of application.1-3 The need for reapplication of sunscreen can lead to considerable expense as well as time spent in application and reapplication. Additionally, for individuals who are physically active (eg, operationally engaged service members, outdoor athletes), sunscreen applied to the face may become a hindrance to function, as it may drip or enter the eyes with excessive sweating, possibly impairing vision. Some individuals may be averse to applying lotions or creams to the skin in general, as they do not prefer the textural changes or appearance of the skin after application. The application of sunscreen also could impair use of lifesaving military gear (eg, gas masks, helmets) from fitting or securing appropriately.
Patient Education
From a military perspective, a review of a recent targeted pilot study in which skin cancer patients at a US Veterans Administration hospital were surveyed on personal knowledge of UVR protection showed that respondents who had a history of skin cancer diagnosis did not feel that they had ever been at an increased risk for skin cancers and did not receive skin cancer prevention education during their tours of service. The overwhelming majority of all participants in this study agreed that the military should issue sun-protective clothing and sunscreen to active-duty personnel.11 Another 2015 survey of 356 current US Air Force flight line personnel noted that active-duty service members tend not to use sunscreen when at work or while at home, and 43% of participants reported using no sun-protective methods while working outdoors.12 Although these studies focused on military personal, the data mirror findings within the general public, as it was shown in a survey by the Centers for Disease Control and Prevention that Americans do not fully take advantage of the benefits of UVR protection, specifically with regard to sunscreen use. Little to no usage was correlated with low socioeconomic status, suggesting that a reusable form of protection could be preferred.13
Public health initiatives typically promote education on the use of sunscreen in populations that spend a considerable amount of time working outdoors (eg, construction workers, farmers, military personnel); however, we feel emphasis should be placed on the benefits of wearing hats, as the UVR exposure protection they provide does not wear off, is cost effective, does not require reapplication, and has the advantage of being a recyclable and affordable form of photoprotection.
History of the Military-Grade Wide-Brimmed Hat
One military-specific example of a sun-protective hat is the boonie hat, known at the time of its inception as the tropical or hot-weather hat, which first became popular during the Vietnam War. This hat option was initially proposed on April 7, 1966, when it was realized that a full-brimmed field hat was needed to protect soldiers’ faces and necks from rain and sun in harsh tropical climates.14 Unfortunately, despite the protective advantages of this style of head covering and favorable support from service members themselves, the boonie hat was not widely accepted, as commanders disliked its “unmilitary appearance.” Fervent protests by units throughout Vietnam eventually led to a compromise in policy that allowed unit-level commanders to authorize the use of boonie hats for units in combat or combat support field operations.14 Today, the boonie hat continues to garnish mixed emotions from unit commanders, as wearing this garment often is interpreted as not being in line with an appropriate military appearance, which is similar to the public fashion zeitgeist that also does not openly endorse the use of sun-protective garments. A change in fashion culture and policy (both military and civilian) that promotes sun-protective measures is needed.
Wide-Brimmed Hats Are Superior to Baseball Hats
The distribution of skin cancers across anatomic sites is consistent and proportional with the level and frequency of chronic UVR exposure, with the occurrence of most skin cancers being greatest on the nose, forehead/temples, cheeks/perioral areas, and ears.15 Additionally, higher incidences of skin cancers have been noted in chronically sun-exposed areas of the head and neck in men versus women. It is thought that hair distribution in these areas may be the causal factor.6
Baseball-style hats are worn by all branches of the US military as part of standard training and work duty uniform requirements, primarily for the sake of tradition by maintaining a standard appearance and uniform dress code but also to provide photoprotection to these vulnerable areas of the body. Standard, nonmilitary, baseball-style hats have been shown to provide UV protection factor (UPF) equivalents ranging from 2 to 10 on sites known for the highest levels of exposure.16 Military “patrol caps,” fashioned similar to the baseball-style hat but constructed from military-grade textiles, provide greater levels of photoprotection with UPF ratings from 35 to 50 and higher depending on the fabric color.17 Although patrol caps have a favorable UPF rating and are advantageous compared to former military headgear styles (eg, berets), wide-brimmed hats would provide greater overall coverage.4,6 Studies in school environments also revealed that wide-brimmed hats come out ahead in side-by-side testing against baseball hats and are shown to provide greater photoprotection for the cheeks, chin, ears, and neck.16
Final Thoughts
The battle to educate the public about adequate photoprotection to prevent skin cancers caused by UVR exposure applies to all providers, both military and civilian. Our ongoing initiatives should not only sustain current practices but should further stress the importance of wearing wide-brimmed hats as a vital part of coverage of the skin and protection from UVR. We must combat the public perception that wearing wide-brimmed hats is a detractor of personal fashion and that instead it is desirable to reduce the risk for skin cancer. The wide-brimmed hat is a simple, reusable, and easily executed recommendation that should be made to all patients, both military and civilian, young and old. In conclusion, by improving patients’ perceptions and acknowledgment of the importance of photoprotection as well as making a concerted effort to integrate our knowledge in the fashion industry, in policies at schools, in the military, and in popular culture, we will undoubtedly come to agree that it is not unfashionable to wear a wide-brimmed hat, but it is unfashionable to risk developing skin cancer.
- Prevent skin cancer. American Academy of Dermatology website. https://www.aad.org/public/spot-skin-cancer/learn-about-skin-cancer/prevent. Accessed January 4, 2017.
- What can I do to reduce my risk of skin cancer? Centers for Disease Control and Prevention website. http://www.cdc.gov/cancer/skin/basic_info/prevention.htm. Accessed January 4, 2017.
- Cancer facts & figures 2016. American Cancer Society website. http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-047079.pdf. Accessed January 4, 2017.
- Diffey BL, Cheeseman J. Sun protection with hats. Br J Dermatol. 1992;127:10-12.
- Bray FN. Florida school boards restrict access to outdoor sun protection: an observational study. J Am Acad Dermatol. 2016;75:642-644.
- Yeung H, Luk KM, Chen SC. Focal photodamage on the occipital scalp. JAMA Dermatol. 2016;152:1060-1062.
- Lee T, Williams VF, Clark LL. Incident diagnoses of cancers in the active component and cancer-related deaths in the active and reserve components, U.S. Armed Forces, 2005-2014. MSMR. 2016;23:23-31.
- Parkin DM, Mesher D, Sasieni P. Cancers attributable to solar (ultraviolet) radiation exposure in the UK in 2010. Br J Cancer. 2011;105(suppl 2):S66-S69.
- Casper K. Elementary schools consider “no hat no play policy.” Coolibar website. http://blog.coolibar.com/elementary-schools-consider-no-hat-no-play-policy/. Published March 27, 2012. Accessed January 4, 2017.
- Slip, slop, slap, seek & slide: Sid Seagull. SunSmart Victoria website. http://www.sunsmart.com.au/tools/videos/current-tv-campaigns/slip-slop-slap-seek-slide-sid-seagull.html. Accessed January 4, 2017.
- McGrath JM, Fisher V, Krejci-Manwaring J. Skin cancer warnings and the need for new preventive campaigns - a pilot study. Am J Prev Med. 2016;50:E62-E63.
- Parker G, Williams B, Driggers P. Sun exposure knowledge and practices survey of maintenance squadrons at Travis AFB. Mil Med. 2015;180:26-31.
- Holman DM, Berkowitz Z, Guy GP Jr, et al. Patterns of sunscreen use on the face and other exposed skin among US adults [published online May 19, 2015]. J Am Acad Dermatol. 2015;73:83-92.e1.
- Stanton SL. Headgear. In: Stanton SL. U.S. Army Uniforms of the Vietnam War. Harrisburg, PA: Stackpole Books; 1992:26-61.
- Richmond-Sinclair NM, Pandeya N, Ware RS, et al. Incidence of basal cell carcinoma multiplicity and detailed anatomic distribution: longitudinal study of an Australian population [published online July 31, 2008]. J Invest Dermatol. 2009;129:323-328.
- Gies P, Javorniczky J, Roy C, et al. Measurements of the UVR protection provided by hats used at school. Photochem Photobiol. 2006;82:750-754.
- Winterhalter C, DiLuna K, Bide M. Characterization of the Ultraviolet Protection of Combat Uniform Fabrics. Natick, MA: US Army Solider and Biological Chemical Command; 2002. Technical report 02/006.
Photoprotection is the foundation of all skin cancer prevention, as UV radiation (UVR) exposure is the only known modifiable risk factor for skin cancer. With the majority of UVR exposure–induced skin cancers found on the scalp, ears, face, and neck, public health initiatives call for wise choices in personal fashion that emphasize the importance of covering these areas.1-3 From a science of fashion perspective, research has shown that wide-brimmed hats provide better means of ensuring the largest area of coverage compared to standard baseball-style hats.4 Thus, for maximum protection, wide-brimmed hats should be favored. However, in academic and military settings, individual style is not optional and is instead influenced or directed by policy, which may not be aligned with the goal of providing photoprotection and raises additional concern for individuals working in environments with longer periods of peak daylight UVR exposure.
In all military branches, service members don uniforms that include head coverage when operating outdoors; however, the choice of headgear is not always aimed at reducing UVR exposure. Similarly, in our counterpart civilian populations, wearing hats that provide the best photoprotection may be influenced by school policies, which frequently mandate clothing choices for children, or by the press or fashion industry in the general public, which might portray sun-protective garments as unfashionable or in some cases threatening if perceived as demonstrating gang affiliation.5 This article serves to encourage health care providers to not only discuss the use of sunscreen when educating patients on sun protection but also to emphasize the benefits of wearing photoprotective garments, particularly wide-brimmed hats given their simplicity, reusability, and affordability. Hat use is particularly important for men with comorbid androgenetic alopecia.6
Skin Cancer Risk
Unfortunately, the incidence of most common types of skin cancer, specifically nonmelanoma skin cancers such basal cell carcinomas and squamous cell carcinomas (ie, keratinocyte carcinomas [KCs]), is difficult to estimate properly, as these cases are not required to be reported to worldwide cancer registries. However, more than 5.4 million cases of skin cancers were diagnosed among 3.3 million Americans in 2016, with an estimated 13,650 deaths associated with skin cancers (not including KCs).3 Tracking and data analyses of cases diagnosed in the active and reserve component populations of the US Armed Forces reflect parallel findings.7 Keratinocyte carcinomas could be considered largely preventable, as most are the result of UVR exposure.1 Additionally, it has been suggested that the vast majority of mutations in melanoma skin cancers (up to 86%) are caused by UVR exposure.8
Prevention
United States–based national public health services such as the American Cancer Society, the Centers for Disease Control and Prevention, and the American Academy of Dermatology embrace photoprotection as the central practice in reducing risk factors for skin cancers. Guidelines put forth by these and other national preventive medical institutions specifically recommend the use of wide-brimmed hats as the best option for protection of the face, head, ears, and neck, in addition to more common recommendations such as seeking shade, avoiding sunlight during peak hours of the day, and using sunscreen.1-3 At state and local levels, policies should be adapted from these recommendations to support protective practices and skin cancer education that begins early for school-aged children. Unfortunately, in some school districts, wearing hats of any kind may be perceived as disruptive or in some cases baseball hats may be a sign of gang affiliation and are therefore banned in the schoolyard.5 The opposite is true in certain parts of the world where sun protection is embraced by the population as a whole, such as Australia where the widely accepted “slip, slop and slap, seek and slide” campaign has extended to some school policymakers who have considered adopting a “no hat, no play” policy.9,10
Sunscreen use as a primary component of photoprotection has its disadvantages in comparison to wearing protective clothing, as sunscreen cannot be reused and proper usage requires reapplication after swimming, when sweating, and following 2 hours of application.1-3 The need for reapplication of sunscreen can lead to considerable expense as well as time spent in application and reapplication. Additionally, for individuals who are physically active (eg, operationally engaged service members, outdoor athletes), sunscreen applied to the face may become a hindrance to function, as it may drip or enter the eyes with excessive sweating, possibly impairing vision. Some individuals may be averse to applying lotions or creams to the skin in general, as they do not prefer the textural changes or appearance of the skin after application. The application of sunscreen also could impair use of lifesaving military gear (eg, gas masks, helmets) from fitting or securing appropriately.
Patient Education
From a military perspective, a review of a recent targeted pilot study in which skin cancer patients at a US Veterans Administration hospital were surveyed on personal knowledge of UVR protection showed that respondents who had a history of skin cancer diagnosis did not feel that they had ever been at an increased risk for skin cancers and did not receive skin cancer prevention education during their tours of service. The overwhelming majority of all participants in this study agreed that the military should issue sun-protective clothing and sunscreen to active-duty personnel.11 Another 2015 survey of 356 current US Air Force flight line personnel noted that active-duty service members tend not to use sunscreen when at work or while at home, and 43% of participants reported using no sun-protective methods while working outdoors.12 Although these studies focused on military personal, the data mirror findings within the general public, as it was shown in a survey by the Centers for Disease Control and Prevention that Americans do not fully take advantage of the benefits of UVR protection, specifically with regard to sunscreen use. Little to no usage was correlated with low socioeconomic status, suggesting that a reusable form of protection could be preferred.13
Public health initiatives typically promote education on the use of sunscreen in populations that spend a considerable amount of time working outdoors (eg, construction workers, farmers, military personnel); however, we feel emphasis should be placed on the benefits of wearing hats, as the UVR exposure protection they provide does not wear off, is cost effective, does not require reapplication, and has the advantage of being a recyclable and affordable form of photoprotection.
History of the Military-Grade Wide-Brimmed Hat
One military-specific example of a sun-protective hat is the boonie hat, known at the time of its inception as the tropical or hot-weather hat, which first became popular during the Vietnam War. This hat option was initially proposed on April 7, 1966, when it was realized that a full-brimmed field hat was needed to protect soldiers’ faces and necks from rain and sun in harsh tropical climates.14 Unfortunately, despite the protective advantages of this style of head covering and favorable support from service members themselves, the boonie hat was not widely accepted, as commanders disliked its “unmilitary appearance.” Fervent protests by units throughout Vietnam eventually led to a compromise in policy that allowed unit-level commanders to authorize the use of boonie hats for units in combat or combat support field operations.14 Today, the boonie hat continues to garnish mixed emotions from unit commanders, as wearing this garment often is interpreted as not being in line with an appropriate military appearance, which is similar to the public fashion zeitgeist that also does not openly endorse the use of sun-protective garments. A change in fashion culture and policy (both military and civilian) that promotes sun-protective measures is needed.
Wide-Brimmed Hats Are Superior to Baseball Hats
The distribution of skin cancers across anatomic sites is consistent and proportional with the level and frequency of chronic UVR exposure, with the occurrence of most skin cancers being greatest on the nose, forehead/temples, cheeks/perioral areas, and ears.15 Additionally, higher incidences of skin cancers have been noted in chronically sun-exposed areas of the head and neck in men versus women. It is thought that hair distribution in these areas may be the causal factor.6
Baseball-style hats are worn by all branches of the US military as part of standard training and work duty uniform requirements, primarily for the sake of tradition by maintaining a standard appearance and uniform dress code but also to provide photoprotection to these vulnerable areas of the body. Standard, nonmilitary, baseball-style hats have been shown to provide UV protection factor (UPF) equivalents ranging from 2 to 10 on sites known for the highest levels of exposure.16 Military “patrol caps,” fashioned similar to the baseball-style hat but constructed from military-grade textiles, provide greater levels of photoprotection with UPF ratings from 35 to 50 and higher depending on the fabric color.17 Although patrol caps have a favorable UPF rating and are advantageous compared to former military headgear styles (eg, berets), wide-brimmed hats would provide greater overall coverage.4,6 Studies in school environments also revealed that wide-brimmed hats come out ahead in side-by-side testing against baseball hats and are shown to provide greater photoprotection for the cheeks, chin, ears, and neck.16
Final Thoughts
The battle to educate the public about adequate photoprotection to prevent skin cancers caused by UVR exposure applies to all providers, both military and civilian. Our ongoing initiatives should not only sustain current practices but should further stress the importance of wearing wide-brimmed hats as a vital part of coverage of the skin and protection from UVR. We must combat the public perception that wearing wide-brimmed hats is a detractor of personal fashion and that instead it is desirable to reduce the risk for skin cancer. The wide-brimmed hat is a simple, reusable, and easily executed recommendation that should be made to all patients, both military and civilian, young and old. In conclusion, by improving patients’ perceptions and acknowledgment of the importance of photoprotection as well as making a concerted effort to integrate our knowledge in the fashion industry, in policies at schools, in the military, and in popular culture, we will undoubtedly come to agree that it is not unfashionable to wear a wide-brimmed hat, but it is unfashionable to risk developing skin cancer.
Photoprotection is the foundation of all skin cancer prevention, as UV radiation (UVR) exposure is the only known modifiable risk factor for skin cancer. With the majority of UVR exposure–induced skin cancers found on the scalp, ears, face, and neck, public health initiatives call for wise choices in personal fashion that emphasize the importance of covering these areas.1-3 From a science of fashion perspective, research has shown that wide-brimmed hats provide better means of ensuring the largest area of coverage compared to standard baseball-style hats.4 Thus, for maximum protection, wide-brimmed hats should be favored. However, in academic and military settings, individual style is not optional and is instead influenced or directed by policy, which may not be aligned with the goal of providing photoprotection and raises additional concern for individuals working in environments with longer periods of peak daylight UVR exposure.
In all military branches, service members don uniforms that include head coverage when operating outdoors; however, the choice of headgear is not always aimed at reducing UVR exposure. Similarly, in our counterpart civilian populations, wearing hats that provide the best photoprotection may be influenced by school policies, which frequently mandate clothing choices for children, or by the press or fashion industry in the general public, which might portray sun-protective garments as unfashionable or in some cases threatening if perceived as demonstrating gang affiliation.5 This article serves to encourage health care providers to not only discuss the use of sunscreen when educating patients on sun protection but also to emphasize the benefits of wearing photoprotective garments, particularly wide-brimmed hats given their simplicity, reusability, and affordability. Hat use is particularly important for men with comorbid androgenetic alopecia.6
Skin Cancer Risk
Unfortunately, the incidence of most common types of skin cancer, specifically nonmelanoma skin cancers such basal cell carcinomas and squamous cell carcinomas (ie, keratinocyte carcinomas [KCs]), is difficult to estimate properly, as these cases are not required to be reported to worldwide cancer registries. However, more than 5.4 million cases of skin cancers were diagnosed among 3.3 million Americans in 2016, with an estimated 13,650 deaths associated with skin cancers (not including KCs).3 Tracking and data analyses of cases diagnosed in the active and reserve component populations of the US Armed Forces reflect parallel findings.7 Keratinocyte carcinomas could be considered largely preventable, as most are the result of UVR exposure.1 Additionally, it has been suggested that the vast majority of mutations in melanoma skin cancers (up to 86%) are caused by UVR exposure.8
Prevention
United States–based national public health services such as the American Cancer Society, the Centers for Disease Control and Prevention, and the American Academy of Dermatology embrace photoprotection as the central practice in reducing risk factors for skin cancers. Guidelines put forth by these and other national preventive medical institutions specifically recommend the use of wide-brimmed hats as the best option for protection of the face, head, ears, and neck, in addition to more common recommendations such as seeking shade, avoiding sunlight during peak hours of the day, and using sunscreen.1-3 At state and local levels, policies should be adapted from these recommendations to support protective practices and skin cancer education that begins early for school-aged children. Unfortunately, in some school districts, wearing hats of any kind may be perceived as disruptive or in some cases baseball hats may be a sign of gang affiliation and are therefore banned in the schoolyard.5 The opposite is true in certain parts of the world where sun protection is embraced by the population as a whole, such as Australia where the widely accepted “slip, slop and slap, seek and slide” campaign has extended to some school policymakers who have considered adopting a “no hat, no play” policy.9,10
Sunscreen use as a primary component of photoprotection has its disadvantages in comparison to wearing protective clothing, as sunscreen cannot be reused and proper usage requires reapplication after swimming, when sweating, and following 2 hours of application.1-3 The need for reapplication of sunscreen can lead to considerable expense as well as time spent in application and reapplication. Additionally, for individuals who are physically active (eg, operationally engaged service members, outdoor athletes), sunscreen applied to the face may become a hindrance to function, as it may drip or enter the eyes with excessive sweating, possibly impairing vision. Some individuals may be averse to applying lotions or creams to the skin in general, as they do not prefer the textural changes or appearance of the skin after application. The application of sunscreen also could impair use of lifesaving military gear (eg, gas masks, helmets) from fitting or securing appropriately.
Patient Education
From a military perspective, a review of a recent targeted pilot study in which skin cancer patients at a US Veterans Administration hospital were surveyed on personal knowledge of UVR protection showed that respondents who had a history of skin cancer diagnosis did not feel that they had ever been at an increased risk for skin cancers and did not receive skin cancer prevention education during their tours of service. The overwhelming majority of all participants in this study agreed that the military should issue sun-protective clothing and sunscreen to active-duty personnel.11 Another 2015 survey of 356 current US Air Force flight line personnel noted that active-duty service members tend not to use sunscreen when at work or while at home, and 43% of participants reported using no sun-protective methods while working outdoors.12 Although these studies focused on military personal, the data mirror findings within the general public, as it was shown in a survey by the Centers for Disease Control and Prevention that Americans do not fully take advantage of the benefits of UVR protection, specifically with regard to sunscreen use. Little to no usage was correlated with low socioeconomic status, suggesting that a reusable form of protection could be preferred.13
Public health initiatives typically promote education on the use of sunscreen in populations that spend a considerable amount of time working outdoors (eg, construction workers, farmers, military personnel); however, we feel emphasis should be placed on the benefits of wearing hats, as the UVR exposure protection they provide does not wear off, is cost effective, does not require reapplication, and has the advantage of being a recyclable and affordable form of photoprotection.
History of the Military-Grade Wide-Brimmed Hat
One military-specific example of a sun-protective hat is the boonie hat, known at the time of its inception as the tropical or hot-weather hat, which first became popular during the Vietnam War. This hat option was initially proposed on April 7, 1966, when it was realized that a full-brimmed field hat was needed to protect soldiers’ faces and necks from rain and sun in harsh tropical climates.14 Unfortunately, despite the protective advantages of this style of head covering and favorable support from service members themselves, the boonie hat was not widely accepted, as commanders disliked its “unmilitary appearance.” Fervent protests by units throughout Vietnam eventually led to a compromise in policy that allowed unit-level commanders to authorize the use of boonie hats for units in combat or combat support field operations.14 Today, the boonie hat continues to garnish mixed emotions from unit commanders, as wearing this garment often is interpreted as not being in line with an appropriate military appearance, which is similar to the public fashion zeitgeist that also does not openly endorse the use of sun-protective garments. A change in fashion culture and policy (both military and civilian) that promotes sun-protective measures is needed.
Wide-Brimmed Hats Are Superior to Baseball Hats
The distribution of skin cancers across anatomic sites is consistent and proportional with the level and frequency of chronic UVR exposure, with the occurrence of most skin cancers being greatest on the nose, forehead/temples, cheeks/perioral areas, and ears.15 Additionally, higher incidences of skin cancers have been noted in chronically sun-exposed areas of the head and neck in men versus women. It is thought that hair distribution in these areas may be the causal factor.6
Baseball-style hats are worn by all branches of the US military as part of standard training and work duty uniform requirements, primarily for the sake of tradition by maintaining a standard appearance and uniform dress code but also to provide photoprotection to these vulnerable areas of the body. Standard, nonmilitary, baseball-style hats have been shown to provide UV protection factor (UPF) equivalents ranging from 2 to 10 on sites known for the highest levels of exposure.16 Military “patrol caps,” fashioned similar to the baseball-style hat but constructed from military-grade textiles, provide greater levels of photoprotection with UPF ratings from 35 to 50 and higher depending on the fabric color.17 Although patrol caps have a favorable UPF rating and are advantageous compared to former military headgear styles (eg, berets), wide-brimmed hats would provide greater overall coverage.4,6 Studies in school environments also revealed that wide-brimmed hats come out ahead in side-by-side testing against baseball hats and are shown to provide greater photoprotection for the cheeks, chin, ears, and neck.16
Final Thoughts
The battle to educate the public about adequate photoprotection to prevent skin cancers caused by UVR exposure applies to all providers, both military and civilian. Our ongoing initiatives should not only sustain current practices but should further stress the importance of wearing wide-brimmed hats as a vital part of coverage of the skin and protection from UVR. We must combat the public perception that wearing wide-brimmed hats is a detractor of personal fashion and that instead it is desirable to reduce the risk for skin cancer. The wide-brimmed hat is a simple, reusable, and easily executed recommendation that should be made to all patients, both military and civilian, young and old. In conclusion, by improving patients’ perceptions and acknowledgment of the importance of photoprotection as well as making a concerted effort to integrate our knowledge in the fashion industry, in policies at schools, in the military, and in popular culture, we will undoubtedly come to agree that it is not unfashionable to wear a wide-brimmed hat, but it is unfashionable to risk developing skin cancer.
- Prevent skin cancer. American Academy of Dermatology website. https://www.aad.org/public/spot-skin-cancer/learn-about-skin-cancer/prevent. Accessed January 4, 2017.
- What can I do to reduce my risk of skin cancer? Centers for Disease Control and Prevention website. http://www.cdc.gov/cancer/skin/basic_info/prevention.htm. Accessed January 4, 2017.
- Cancer facts & figures 2016. American Cancer Society website. http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-047079.pdf. Accessed January 4, 2017.
- Diffey BL, Cheeseman J. Sun protection with hats. Br J Dermatol. 1992;127:10-12.
- Bray FN. Florida school boards restrict access to outdoor sun protection: an observational study. J Am Acad Dermatol. 2016;75:642-644.
- Yeung H, Luk KM, Chen SC. Focal photodamage on the occipital scalp. JAMA Dermatol. 2016;152:1060-1062.
- Lee T, Williams VF, Clark LL. Incident diagnoses of cancers in the active component and cancer-related deaths in the active and reserve components, U.S. Armed Forces, 2005-2014. MSMR. 2016;23:23-31.
- Parkin DM, Mesher D, Sasieni P. Cancers attributable to solar (ultraviolet) radiation exposure in the UK in 2010. Br J Cancer. 2011;105(suppl 2):S66-S69.
- Casper K. Elementary schools consider “no hat no play policy.” Coolibar website. http://blog.coolibar.com/elementary-schools-consider-no-hat-no-play-policy/. Published March 27, 2012. Accessed January 4, 2017.
- Slip, slop, slap, seek & slide: Sid Seagull. SunSmart Victoria website. http://www.sunsmart.com.au/tools/videos/current-tv-campaigns/slip-slop-slap-seek-slide-sid-seagull.html. Accessed January 4, 2017.
- McGrath JM, Fisher V, Krejci-Manwaring J. Skin cancer warnings and the need for new preventive campaigns - a pilot study. Am J Prev Med. 2016;50:E62-E63.
- Parker G, Williams B, Driggers P. Sun exposure knowledge and practices survey of maintenance squadrons at Travis AFB. Mil Med. 2015;180:26-31.
- Holman DM, Berkowitz Z, Guy GP Jr, et al. Patterns of sunscreen use on the face and other exposed skin among US adults [published online May 19, 2015]. J Am Acad Dermatol. 2015;73:83-92.e1.
- Stanton SL. Headgear. In: Stanton SL. U.S. Army Uniforms of the Vietnam War. Harrisburg, PA: Stackpole Books; 1992:26-61.
- Richmond-Sinclair NM, Pandeya N, Ware RS, et al. Incidence of basal cell carcinoma multiplicity and detailed anatomic distribution: longitudinal study of an Australian population [published online July 31, 2008]. J Invest Dermatol. 2009;129:323-328.
- Gies P, Javorniczky J, Roy C, et al. Measurements of the UVR protection provided by hats used at school. Photochem Photobiol. 2006;82:750-754.
- Winterhalter C, DiLuna K, Bide M. Characterization of the Ultraviolet Protection of Combat Uniform Fabrics. Natick, MA: US Army Solider and Biological Chemical Command; 2002. Technical report 02/006.
- Prevent skin cancer. American Academy of Dermatology website. https://www.aad.org/public/spot-skin-cancer/learn-about-skin-cancer/prevent. Accessed January 4, 2017.
- What can I do to reduce my risk of skin cancer? Centers for Disease Control and Prevention website. http://www.cdc.gov/cancer/skin/basic_info/prevention.htm. Accessed January 4, 2017.
- Cancer facts & figures 2016. American Cancer Society website. http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-047079.pdf. Accessed January 4, 2017.
- Diffey BL, Cheeseman J. Sun protection with hats. Br J Dermatol. 1992;127:10-12.
- Bray FN. Florida school boards restrict access to outdoor sun protection: an observational study. J Am Acad Dermatol. 2016;75:642-644.
- Yeung H, Luk KM, Chen SC. Focal photodamage on the occipital scalp. JAMA Dermatol. 2016;152:1060-1062.
- Lee T, Williams VF, Clark LL. Incident diagnoses of cancers in the active component and cancer-related deaths in the active and reserve components, U.S. Armed Forces, 2005-2014. MSMR. 2016;23:23-31.
- Parkin DM, Mesher D, Sasieni P. Cancers attributable to solar (ultraviolet) radiation exposure in the UK in 2010. Br J Cancer. 2011;105(suppl 2):S66-S69.
- Casper K. Elementary schools consider “no hat no play policy.” Coolibar website. http://blog.coolibar.com/elementary-schools-consider-no-hat-no-play-policy/. Published March 27, 2012. Accessed January 4, 2017.
- Slip, slop, slap, seek & slide: Sid Seagull. SunSmart Victoria website. http://www.sunsmart.com.au/tools/videos/current-tv-campaigns/slip-slop-slap-seek-slide-sid-seagull.html. Accessed January 4, 2017.
- McGrath JM, Fisher V, Krejci-Manwaring J. Skin cancer warnings and the need for new preventive campaigns - a pilot study. Am J Prev Med. 2016;50:E62-E63.
- Parker G, Williams B, Driggers P. Sun exposure knowledge and practices survey of maintenance squadrons at Travis AFB. Mil Med. 2015;180:26-31.
- Holman DM, Berkowitz Z, Guy GP Jr, et al. Patterns of sunscreen use on the face and other exposed skin among US adults [published online May 19, 2015]. J Am Acad Dermatol. 2015;73:83-92.e1.
- Stanton SL. Headgear. In: Stanton SL. U.S. Army Uniforms of the Vietnam War. Harrisburg, PA: Stackpole Books; 1992:26-61.
- Richmond-Sinclair NM, Pandeya N, Ware RS, et al. Incidence of basal cell carcinoma multiplicity and detailed anatomic distribution: longitudinal study of an Australian population [published online July 31, 2008]. J Invest Dermatol. 2009;129:323-328.
- Gies P, Javorniczky J, Roy C, et al. Measurements of the UVR protection provided by hats used at school. Photochem Photobiol. 2006;82:750-754.
- Winterhalter C, DiLuna K, Bide M. Characterization of the Ultraviolet Protection of Combat Uniform Fabrics. Natick, MA: US Army Solider and Biological Chemical Command; 2002. Technical report 02/006.
Practice Points
- Routine wear of wide-brimmed hats is the simplest, most inexpensive, and only reusable form of photoprotection for the head and neck and should be an everyday practice for reducing the risk for preventable skin cancers.
- The regular wear of clothing and head cover with adequate UV protection factor is equally as important to utilize in the prevention of UV-induced skin cancers as the application of topical sunscreens and sunblocks.
- The medical community should make a concerted effort to dispel any public policy or fashion trend that does not promote personal protection from sun-induced skin cancers. Policies that restrict wearing photoprotective garments, such as in schools and in the military, need to be changed.
The Role of Biologic Therapy for Psoriasis in Cardiovascular Risk Reduction
The cardiovascular comorbidities associated with psoriasis have been well documented; however, the mechanism by which psoriasis increases the risk for cardiovascular disease (CVD) remains unclear. Elevated systemic inflammatory cytokines and mediators may play a key role in their association, which prompts the questions: Do systemic medications have a protective effect? Do patients on systemic antipsoriatic treatment have a decreased risk for major adverse cardiovascular events (MACEs) compared with untreated patients?
We believe the shared inflammatory processes involved in psoriasis and atherosclerosis formation are potential targets for therapy in reducing the incidence of CVD and its associated complications. A growing amount of evidence suggests cardioprotective effects associated with antipsoriatic treatments such as tumor necrosis factor (TNF) inhibitors and methotrexate. Gkalpakiotis et al1 demonstrated a reduction in serum E-selectin (mean [standard deviation], 53.04 [23.54] ng/mL vs 35.32 [8.70] ng/mL; P<.001) and IL-22 (25.11 [19.9] pg/mL vs 12.83 [8.42] pg/mL; P<.001) after 3 months of adalimumab administration in patients with moderate to severe psoriasis. Both E-selectin and IL-22 are associated with the development of atherosclerosis, endothelial dysfunction, and an increased incidence of CVD. Similarly, Wu et al2 demonstrated a statistically significant reduction (–5.04 mg/dL [95% confidence interval [CI], –8.24 to –2.12; P<.01) in C-reactive protein in patients with psoriasis, psoriatic arthritis, and rheumatoid arthritis after concurrent use of methotrexate and TNF inhibitors.
Solomon et al3 compared the rate of newly diagnosed diabetes mellitus among psoriasis and rheumatoid arthritis patients treated with TNF inhibitors, methotrexate, hydroxychloroquine, and other nonbiologic disease-modifying antirheumatic drugs. The authors’ findings suggest that those who take a TNF inhibitor (hazard ratio [HR], 0.62; 95% CI, 0.42-0.91) and hydroxychloroquine (HR, 0.54; 95% CI, 0.36-0.80) are at lower risk for diabetes mellitus compared to those treated with nonbiologic disease-modifying antirheumatic drugs. Conversely, the methotrexate (HR, 0.77; 95% CI, 0.53-1.13) cohort did not show a statistically significant reduction in diabetes risk.3
Pina et al4 revealed improvement in endothelial function after 6 months of adalimumab use in patients with moderate to severe psoriasis. To evaluate the presence of subclinical endothelial dysfunction, the authors assessed brachial artery reactivity by measuring flow-mediated dilation and carotid artery stiffness by pulse wave velocity. Patients showed an increase in flow-mediated dilation (mean [SD], 6.19% [2.44%] vs 7.46% [2.43%]; P=.008) and reduction in pulse wave velocity (6.28 [1.04] m/s vs 5.69 [1.31] m/s; P=.03) compared to baseline measurements, indicating an improvement of endothelial function.4
Ahlehoff et al5 observed for improvements in subclinical left ventricular dysfunction in psoriasis patients after treatment with biologics. Using echocardiography, they assessed for changes in diastolic function and left ventricular systolic deformation (defined by global longitudinal strain). Of patients who received 3 months of biologic therapy (TNF inhibitor orIL-12/23 inhibitor) and maintained at minimum a psoriasis area and severity index 50 response, all demonstrated an improvement in diastolic function (mean [SD], 8.1 [2.1] vs 6.7 [1.9]; P<.001) and global longitudinal strain (mean [SD], –16.8% [2.1%] vs –18.3% [2.3%]; P<.001). Of note, patients who did not achieve a psoriasis area and severity index 50 response at follow-up did not exhibit an improvement in subclinical myocardial function.5
Moreover, a Danish nationwide study with up to 5-year follow-up evaluated the risk for MACE (ie, cardiovascular death, myocardial infarction, stroke) in patients with severe psoriasis receiving systemic anti-inflammatory medications and nonsystemic therapies including topical treatments, phototherapy, and climate therapy.6 Compared to nonsystemic therapies, methotrexate use (HR, 0.53; 95% CI, 0.34-0.83) was associated with a decreased risk for cardiovascular events. However, a protective decreased risk was not found among patients who used systemic cyclosporine (HR, 1.06; 95% CI, 0.26-4.27) or retinoids (HR, 1.80; 95% CI, 1.03-2.96). Any biological drug use had a comparable but nonsignificant reduction of cardiovascular events (HR, 0.58; 95% CI, 0.30-1.10). After multivariable adjustment, TNF inhibitors were associated with a statistically significant decreased risk for cardiovascular events (HR, 0.46; 95% CI, 0.22-0.98; P=.04) compared to nonsystemic therapies. The IL-12/23 inhibitor did not demonstrate this relationship (HR, 1.52; 95% CI, 0.47-4.94).6
Lastly, Wu et al7 compared the risk for MACE (ie, myocardial infarction, stroke, unstable angina, transient ischemic attack) between patients with psoriasis who received TNF inhibitors or methotrexate. The TNF inhibitor and methotrexate cohorts were observed for a median of 12 months and 9 months, respectively. After adjusting for potential confounding factors, they found a 45% reduction (HR, 0.55; 95% CI, 0.45-0.67) in cardiovascular event risk in the TNF inhibitor cohort compared with the methotrexate cohort. Notably, analyses also showed comparatively fewer cardiovascular events in the TNF inhibitor cohort throughout all time points—6, 12, 18, 24, 60 months—in the observation period. Regression analysis revealed an 11% reduction in cardiovascular events (HR, 0.89; 95% CI, 0.80-0.98) with each additional 6 months of cumulative TNF inhibitor exposure.
The current sum of evidence suggests cardioprotective effects of TNF inhibitor and methotrexate use. However, given the cumulative systemic toxicity and inferior cutaneous efficacy of methotrexate, TNF inhibitors will likely play a more significant role going forward. The role of methotrexate may be for its simultaneous use with biologic therapies to limit immunogenicity. Newer biologic agents such as IL-12/23 and IL-17 inhibitors have not yet been as extensively studied for their effects on cardiovascular risk as their TNF inhibitor counterparts. However, because of their shared ability to target specific immunological pathways, it is plausible that IL-12/23 and IL-17 agents may exhibit cardioprotective effects.8
Patients with psoriasis should be counseled and educated about the increased risk for CVD and its associated morbidity and mortality risk. Screening for modifiable risk factors and recommending therapeutic lifestyle changes also is appropriate. Future studies should help define the role of specific systemic drugs in reducing the risk for CVD in patients with psoriasis.
- Gkalpakiotis S, Arenbergerova M, Gkalpakioti P, et al. Impact of adalimumab treatment on cardiovascular risk biomarkers in psoriasis: results of a pilot study [published online October 24, 2016]. J Dermatol. doi:10.1111/1346-8138.13661.
- Wu JJ, Rowan CG, Bebchuk JD, et al. Association between tumor necrosis factor inhibitor (TNFi) therapy and changes in C-reactive protein (CRP), blood pressure, and alanine aminotransferase (ALT) among patients with psoriasis, psoriatic arthritis, or rheumatoid arthritis [published online March 5, 2015]. J Am Acad Dermatol. 2015;72:917-919.
- Solomon DH, Massarotti E, Garg R, et al. Association between disease-modifying antirheumatic drugs and diabetes risk in patients with rheumatoid arthritis and psoriasis. JAMA. 2011;305:2525-2531.
- Pina T, Corrales A, Lopez-Mejias R, et al. Anti-tumor necrosis factor-alpha therapy improves endothelial function and arterial stiffness in patients with moderate to severe psoriasis: a 6-month prospective study. J Dermatol. 2016;43:1267-1272.
- Ahlehoff O, Hansen PR, Gislason GH, et al. Myocardial function and effects of biologic therapy in patients with severe psoriasis: a prospective echocardiographic study [published online April 6, 2015]. J Eur Acad Dermatol Venereol. 2016;30:819-823.
- Ahlehoff O, Skov L, Gislason G, et al. Cardiovascular outcomes and systemic anti-inflammatory drugs in patients with severe psoriasis: 5-year follow-up of a Danish nationwide cohort [published online October 10, 2014]. J Eur Acad Dermatol Venereol. 2015;29:1128-1134.
- Wu JJ, Guérin A, Sundaram M, et al. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-α inhibitors versus methotrexate [published online October 26, 2016]. J Am Acad Dermatol. 2017;76:81-90.
- Egeberg A, Skov L. Management of cardiovascular disease in patients with psoriasis. Expert Opin Pharmacother. 2016;17:1509-1516.
The cardiovascular comorbidities associated with psoriasis have been well documented; however, the mechanism by which psoriasis increases the risk for cardiovascular disease (CVD) remains unclear. Elevated systemic inflammatory cytokines and mediators may play a key role in their association, which prompts the questions: Do systemic medications have a protective effect? Do patients on systemic antipsoriatic treatment have a decreased risk for major adverse cardiovascular events (MACEs) compared with untreated patients?
We believe the shared inflammatory processes involved in psoriasis and atherosclerosis formation are potential targets for therapy in reducing the incidence of CVD and its associated complications. A growing amount of evidence suggests cardioprotective effects associated with antipsoriatic treatments such as tumor necrosis factor (TNF) inhibitors and methotrexate. Gkalpakiotis et al1 demonstrated a reduction in serum E-selectin (mean [standard deviation], 53.04 [23.54] ng/mL vs 35.32 [8.70] ng/mL; P<.001) and IL-22 (25.11 [19.9] pg/mL vs 12.83 [8.42] pg/mL; P<.001) after 3 months of adalimumab administration in patients with moderate to severe psoriasis. Both E-selectin and IL-22 are associated with the development of atherosclerosis, endothelial dysfunction, and an increased incidence of CVD. Similarly, Wu et al2 demonstrated a statistically significant reduction (–5.04 mg/dL [95% confidence interval [CI], –8.24 to –2.12; P<.01) in C-reactive protein in patients with psoriasis, psoriatic arthritis, and rheumatoid arthritis after concurrent use of methotrexate and TNF inhibitors.
Solomon et al3 compared the rate of newly diagnosed diabetes mellitus among psoriasis and rheumatoid arthritis patients treated with TNF inhibitors, methotrexate, hydroxychloroquine, and other nonbiologic disease-modifying antirheumatic drugs. The authors’ findings suggest that those who take a TNF inhibitor (hazard ratio [HR], 0.62; 95% CI, 0.42-0.91) and hydroxychloroquine (HR, 0.54; 95% CI, 0.36-0.80) are at lower risk for diabetes mellitus compared to those treated with nonbiologic disease-modifying antirheumatic drugs. Conversely, the methotrexate (HR, 0.77; 95% CI, 0.53-1.13) cohort did not show a statistically significant reduction in diabetes risk.3
Pina et al4 revealed improvement in endothelial function after 6 months of adalimumab use in patients with moderate to severe psoriasis. To evaluate the presence of subclinical endothelial dysfunction, the authors assessed brachial artery reactivity by measuring flow-mediated dilation and carotid artery stiffness by pulse wave velocity. Patients showed an increase in flow-mediated dilation (mean [SD], 6.19% [2.44%] vs 7.46% [2.43%]; P=.008) and reduction in pulse wave velocity (6.28 [1.04] m/s vs 5.69 [1.31] m/s; P=.03) compared to baseline measurements, indicating an improvement of endothelial function.4
Ahlehoff et al5 observed for improvements in subclinical left ventricular dysfunction in psoriasis patients after treatment with biologics. Using echocardiography, they assessed for changes in diastolic function and left ventricular systolic deformation (defined by global longitudinal strain). Of patients who received 3 months of biologic therapy (TNF inhibitor orIL-12/23 inhibitor) and maintained at minimum a psoriasis area and severity index 50 response, all demonstrated an improvement in diastolic function (mean [SD], 8.1 [2.1] vs 6.7 [1.9]; P<.001) and global longitudinal strain (mean [SD], –16.8% [2.1%] vs –18.3% [2.3%]; P<.001). Of note, patients who did not achieve a psoriasis area and severity index 50 response at follow-up did not exhibit an improvement in subclinical myocardial function.5
Moreover, a Danish nationwide study with up to 5-year follow-up evaluated the risk for MACE (ie, cardiovascular death, myocardial infarction, stroke) in patients with severe psoriasis receiving systemic anti-inflammatory medications and nonsystemic therapies including topical treatments, phototherapy, and climate therapy.6 Compared to nonsystemic therapies, methotrexate use (HR, 0.53; 95% CI, 0.34-0.83) was associated with a decreased risk for cardiovascular events. However, a protective decreased risk was not found among patients who used systemic cyclosporine (HR, 1.06; 95% CI, 0.26-4.27) or retinoids (HR, 1.80; 95% CI, 1.03-2.96). Any biological drug use had a comparable but nonsignificant reduction of cardiovascular events (HR, 0.58; 95% CI, 0.30-1.10). After multivariable adjustment, TNF inhibitors were associated with a statistically significant decreased risk for cardiovascular events (HR, 0.46; 95% CI, 0.22-0.98; P=.04) compared to nonsystemic therapies. The IL-12/23 inhibitor did not demonstrate this relationship (HR, 1.52; 95% CI, 0.47-4.94).6
Lastly, Wu et al7 compared the risk for MACE (ie, myocardial infarction, stroke, unstable angina, transient ischemic attack) between patients with psoriasis who received TNF inhibitors or methotrexate. The TNF inhibitor and methotrexate cohorts were observed for a median of 12 months and 9 months, respectively. After adjusting for potential confounding factors, they found a 45% reduction (HR, 0.55; 95% CI, 0.45-0.67) in cardiovascular event risk in the TNF inhibitor cohort compared with the methotrexate cohort. Notably, analyses also showed comparatively fewer cardiovascular events in the TNF inhibitor cohort throughout all time points—6, 12, 18, 24, 60 months—in the observation period. Regression analysis revealed an 11% reduction in cardiovascular events (HR, 0.89; 95% CI, 0.80-0.98) with each additional 6 months of cumulative TNF inhibitor exposure.
The current sum of evidence suggests cardioprotective effects of TNF inhibitor and methotrexate use. However, given the cumulative systemic toxicity and inferior cutaneous efficacy of methotrexate, TNF inhibitors will likely play a more significant role going forward. The role of methotrexate may be for its simultaneous use with biologic therapies to limit immunogenicity. Newer biologic agents such as IL-12/23 and IL-17 inhibitors have not yet been as extensively studied for their effects on cardiovascular risk as their TNF inhibitor counterparts. However, because of their shared ability to target specific immunological pathways, it is plausible that IL-12/23 and IL-17 agents may exhibit cardioprotective effects.8
Patients with psoriasis should be counseled and educated about the increased risk for CVD and its associated morbidity and mortality risk. Screening for modifiable risk factors and recommending therapeutic lifestyle changes also is appropriate. Future studies should help define the role of specific systemic drugs in reducing the risk for CVD in patients with psoriasis.
The cardiovascular comorbidities associated with psoriasis have been well documented; however, the mechanism by which psoriasis increases the risk for cardiovascular disease (CVD) remains unclear. Elevated systemic inflammatory cytokines and mediators may play a key role in their association, which prompts the questions: Do systemic medications have a protective effect? Do patients on systemic antipsoriatic treatment have a decreased risk for major adverse cardiovascular events (MACEs) compared with untreated patients?
We believe the shared inflammatory processes involved in psoriasis and atherosclerosis formation are potential targets for therapy in reducing the incidence of CVD and its associated complications. A growing amount of evidence suggests cardioprotective effects associated with antipsoriatic treatments such as tumor necrosis factor (TNF) inhibitors and methotrexate. Gkalpakiotis et al1 demonstrated a reduction in serum E-selectin (mean [standard deviation], 53.04 [23.54] ng/mL vs 35.32 [8.70] ng/mL; P<.001) and IL-22 (25.11 [19.9] pg/mL vs 12.83 [8.42] pg/mL; P<.001) after 3 months of adalimumab administration in patients with moderate to severe psoriasis. Both E-selectin and IL-22 are associated with the development of atherosclerosis, endothelial dysfunction, and an increased incidence of CVD. Similarly, Wu et al2 demonstrated a statistically significant reduction (–5.04 mg/dL [95% confidence interval [CI], –8.24 to –2.12; P<.01) in C-reactive protein in patients with psoriasis, psoriatic arthritis, and rheumatoid arthritis after concurrent use of methotrexate and TNF inhibitors.
Solomon et al3 compared the rate of newly diagnosed diabetes mellitus among psoriasis and rheumatoid arthritis patients treated with TNF inhibitors, methotrexate, hydroxychloroquine, and other nonbiologic disease-modifying antirheumatic drugs. The authors’ findings suggest that those who take a TNF inhibitor (hazard ratio [HR], 0.62; 95% CI, 0.42-0.91) and hydroxychloroquine (HR, 0.54; 95% CI, 0.36-0.80) are at lower risk for diabetes mellitus compared to those treated with nonbiologic disease-modifying antirheumatic drugs. Conversely, the methotrexate (HR, 0.77; 95% CI, 0.53-1.13) cohort did not show a statistically significant reduction in diabetes risk.3
Pina et al4 revealed improvement in endothelial function after 6 months of adalimumab use in patients with moderate to severe psoriasis. To evaluate the presence of subclinical endothelial dysfunction, the authors assessed brachial artery reactivity by measuring flow-mediated dilation and carotid artery stiffness by pulse wave velocity. Patients showed an increase in flow-mediated dilation (mean [SD], 6.19% [2.44%] vs 7.46% [2.43%]; P=.008) and reduction in pulse wave velocity (6.28 [1.04] m/s vs 5.69 [1.31] m/s; P=.03) compared to baseline measurements, indicating an improvement of endothelial function.4
Ahlehoff et al5 observed for improvements in subclinical left ventricular dysfunction in psoriasis patients after treatment with biologics. Using echocardiography, they assessed for changes in diastolic function and left ventricular systolic deformation (defined by global longitudinal strain). Of patients who received 3 months of biologic therapy (TNF inhibitor orIL-12/23 inhibitor) and maintained at minimum a psoriasis area and severity index 50 response, all demonstrated an improvement in diastolic function (mean [SD], 8.1 [2.1] vs 6.7 [1.9]; P<.001) and global longitudinal strain (mean [SD], –16.8% [2.1%] vs –18.3% [2.3%]; P<.001). Of note, patients who did not achieve a psoriasis area and severity index 50 response at follow-up did not exhibit an improvement in subclinical myocardial function.5
Moreover, a Danish nationwide study with up to 5-year follow-up evaluated the risk for MACE (ie, cardiovascular death, myocardial infarction, stroke) in patients with severe psoriasis receiving systemic anti-inflammatory medications and nonsystemic therapies including topical treatments, phototherapy, and climate therapy.6 Compared to nonsystemic therapies, methotrexate use (HR, 0.53; 95% CI, 0.34-0.83) was associated with a decreased risk for cardiovascular events. However, a protective decreased risk was not found among patients who used systemic cyclosporine (HR, 1.06; 95% CI, 0.26-4.27) or retinoids (HR, 1.80; 95% CI, 1.03-2.96). Any biological drug use had a comparable but nonsignificant reduction of cardiovascular events (HR, 0.58; 95% CI, 0.30-1.10). After multivariable adjustment, TNF inhibitors were associated with a statistically significant decreased risk for cardiovascular events (HR, 0.46; 95% CI, 0.22-0.98; P=.04) compared to nonsystemic therapies. The IL-12/23 inhibitor did not demonstrate this relationship (HR, 1.52; 95% CI, 0.47-4.94).6
Lastly, Wu et al7 compared the risk for MACE (ie, myocardial infarction, stroke, unstable angina, transient ischemic attack) between patients with psoriasis who received TNF inhibitors or methotrexate. The TNF inhibitor and methotrexate cohorts were observed for a median of 12 months and 9 months, respectively. After adjusting for potential confounding factors, they found a 45% reduction (HR, 0.55; 95% CI, 0.45-0.67) in cardiovascular event risk in the TNF inhibitor cohort compared with the methotrexate cohort. Notably, analyses also showed comparatively fewer cardiovascular events in the TNF inhibitor cohort throughout all time points—6, 12, 18, 24, 60 months—in the observation period. Regression analysis revealed an 11% reduction in cardiovascular events (HR, 0.89; 95% CI, 0.80-0.98) with each additional 6 months of cumulative TNF inhibitor exposure.
The current sum of evidence suggests cardioprotective effects of TNF inhibitor and methotrexate use. However, given the cumulative systemic toxicity and inferior cutaneous efficacy of methotrexate, TNF inhibitors will likely play a more significant role going forward. The role of methotrexate may be for its simultaneous use with biologic therapies to limit immunogenicity. Newer biologic agents such as IL-12/23 and IL-17 inhibitors have not yet been as extensively studied for their effects on cardiovascular risk as their TNF inhibitor counterparts. However, because of their shared ability to target specific immunological pathways, it is plausible that IL-12/23 and IL-17 agents may exhibit cardioprotective effects.8
Patients with psoriasis should be counseled and educated about the increased risk for CVD and its associated morbidity and mortality risk. Screening for modifiable risk factors and recommending therapeutic lifestyle changes also is appropriate. Future studies should help define the role of specific systemic drugs in reducing the risk for CVD in patients with psoriasis.
- Gkalpakiotis S, Arenbergerova M, Gkalpakioti P, et al. Impact of adalimumab treatment on cardiovascular risk biomarkers in psoriasis: results of a pilot study [published online October 24, 2016]. J Dermatol. doi:10.1111/1346-8138.13661.
- Wu JJ, Rowan CG, Bebchuk JD, et al. Association between tumor necrosis factor inhibitor (TNFi) therapy and changes in C-reactive protein (CRP), blood pressure, and alanine aminotransferase (ALT) among patients with psoriasis, psoriatic arthritis, or rheumatoid arthritis [published online March 5, 2015]. J Am Acad Dermatol. 2015;72:917-919.
- Solomon DH, Massarotti E, Garg R, et al. Association between disease-modifying antirheumatic drugs and diabetes risk in patients with rheumatoid arthritis and psoriasis. JAMA. 2011;305:2525-2531.
- Pina T, Corrales A, Lopez-Mejias R, et al. Anti-tumor necrosis factor-alpha therapy improves endothelial function and arterial stiffness in patients with moderate to severe psoriasis: a 6-month prospective study. J Dermatol. 2016;43:1267-1272.
- Ahlehoff O, Hansen PR, Gislason GH, et al. Myocardial function and effects of biologic therapy in patients with severe psoriasis: a prospective echocardiographic study [published online April 6, 2015]. J Eur Acad Dermatol Venereol. 2016;30:819-823.
- Ahlehoff O, Skov L, Gislason G, et al. Cardiovascular outcomes and systemic anti-inflammatory drugs in patients with severe psoriasis: 5-year follow-up of a Danish nationwide cohort [published online October 10, 2014]. J Eur Acad Dermatol Venereol. 2015;29:1128-1134.
- Wu JJ, Guérin A, Sundaram M, et al. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-α inhibitors versus methotrexate [published online October 26, 2016]. J Am Acad Dermatol. 2017;76:81-90.
- Egeberg A, Skov L. Management of cardiovascular disease in patients with psoriasis. Expert Opin Pharmacother. 2016;17:1509-1516.
- Gkalpakiotis S, Arenbergerova M, Gkalpakioti P, et al. Impact of adalimumab treatment on cardiovascular risk biomarkers in psoriasis: results of a pilot study [published online October 24, 2016]. J Dermatol. doi:10.1111/1346-8138.13661.
- Wu JJ, Rowan CG, Bebchuk JD, et al. Association between tumor necrosis factor inhibitor (TNFi) therapy and changes in C-reactive protein (CRP), blood pressure, and alanine aminotransferase (ALT) among patients with psoriasis, psoriatic arthritis, or rheumatoid arthritis [published online March 5, 2015]. J Am Acad Dermatol. 2015;72:917-919.
- Solomon DH, Massarotti E, Garg R, et al. Association between disease-modifying antirheumatic drugs and diabetes risk in patients with rheumatoid arthritis and psoriasis. JAMA. 2011;305:2525-2531.
- Pina T, Corrales A, Lopez-Mejias R, et al. Anti-tumor necrosis factor-alpha therapy improves endothelial function and arterial stiffness in patients with moderate to severe psoriasis: a 6-month prospective study. J Dermatol. 2016;43:1267-1272.
- Ahlehoff O, Hansen PR, Gislason GH, et al. Myocardial function and effects of biologic therapy in patients with severe psoriasis: a prospective echocardiographic study [published online April 6, 2015]. J Eur Acad Dermatol Venereol. 2016;30:819-823.
- Ahlehoff O, Skov L, Gislason G, et al. Cardiovascular outcomes and systemic anti-inflammatory drugs in patients with severe psoriasis: 5-year follow-up of a Danish nationwide cohort [published online October 10, 2014]. J Eur Acad Dermatol Venereol. 2015;29:1128-1134.
- Wu JJ, Guérin A, Sundaram M, et al. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-α inhibitors versus methotrexate [published online October 26, 2016]. J Am Acad Dermatol. 2017;76:81-90.
- Egeberg A, Skov L. Management of cardiovascular disease in patients with psoriasis. Expert Opin Pharmacother. 2016;17:1509-1516.
Congenital Hemangioma
Hemangiomas are the most common benign tumors of childhood. In recent years, subsets of hemangiomas that are fully formed at birth have been recognized as clinically and biologically distinct from the classic infantile hemangioma (IH). Congenital hemangiomas (CHs) are classified based on clinical course as rapidly involuting CHs (RICHs) or noninvoluting CHs (NICHs). The aim of this retrospective study was to describe the epidemiology, clinical aspects, and clinical outcome of CH over a 5-year period.
Methods
Using electronic medical records from the department of dermatology (Hedi Chaker Hospital, Sfax, Tunisia) for a 5-year period (2008-2012), we searched for hemangioma. After collecting those records, we identified patients with CHs. We studied the epidemiologic (eg, sex, age), clinical course (eg, location, size, number, color, surrounding skin), and evolutionary aspects in these patients.
Results
Twenty IHs were identified, 6 (30%) of which were considered CHs. The clinical characteristics of the 6 patients are summarized in the Table. We identified 2 females and 4 males aged 2 to 60 days (mean age, 16 days). Four patients had CHs involving the limbs (knee [n=2]; ankle [n=1]; elbow [n=1]) and 2 patients had CHs involving the trunk. Congenital hemangiomas were singular, oval shaped, and surrounded by a clear halo in all 6 patients. They presented as exophytic masses (n=3) or bossed plaques (n=3). A blue hue was noted in 5 patients and a purple hue in 1 patient. In some cases, telangiectasia (n=2) or small areas of necrosis (n=1) were noted at the center of the CHs. The CHs ranged in size from 3 to 6 cm (mean, 4 cm). Doppler ultrasonography was performed in 2 patients and showed fast blood flow. It is well known that manipulating a CH when it is ulcerative may cause a fatal hemorrhage. Thus, parents/guardians should be cautious when cleaning and dressing the lesions. Regular follow-up was recommended to all patients as noted in the medical records. The lesion involuted in4 patients after a mean period of 6 months, which allowed us to classify the lesions as RICHs (Figure, A). Two CHs were persistent after 2-year (Figure, B) and 4-year (Figure, C) follow-up, which was consistent with NICH classification.
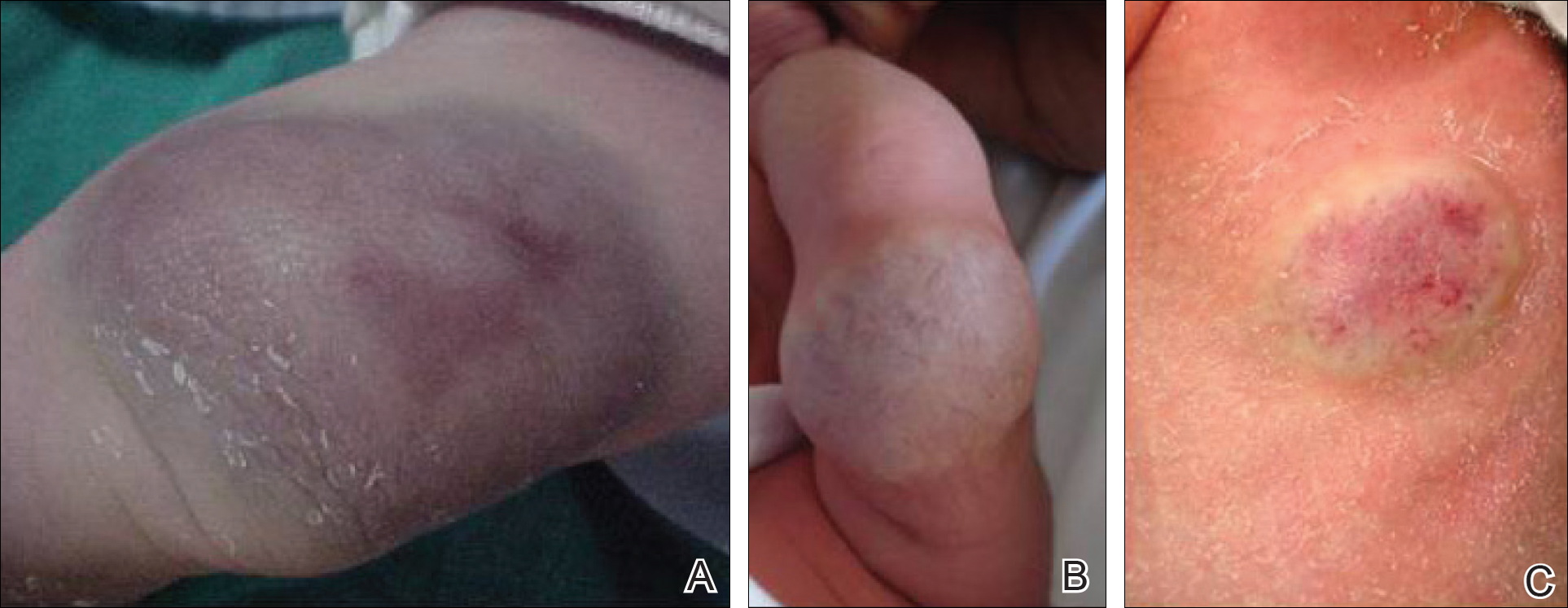
Comment
Since 1996, vascular anomalies have been classified either as tumors or malformations.1 Infantile hemangioma is the most common vascular tumor and presents as an endothelial cellular proliferation that develops within days after birth. Congenital hemangiomas are fully developed at birth2,3 and are classified as RICHs and NICHs according to their clinical outcome.
As expected, our analysis revealed that CH usually is solitary and may present as a small lesion (eg, a few millimeters) but also may be large in size.4 Congenital hemangioma has an equal sex distribution and a predilection for the head and limbs near a joint. In contrast, IH exhibits female predilection and can occur anywhere on the body.4-6 In our study, CHs were more common in males and had a predilection for the limbs. Three patients presented with exophytic masses with a clear halo and overlying telangiectasia, which are commonly described features in CH.4,6
In the classification of vascular anomalies, RICHs and NICHs are fast-flow lesions that are indistinguishable at birth.7,8 Untreated, RICHs usually resolve in the first 14 months of life, often resulting in an area of atrophic or excess skin.8,9 Noninvoluting CHs persist and grow in proportion with the patient.10-12
When Doppler ultrasonography findings are inconsistent with a CH, an early biopsy from the periphery of the lesion may be performed to exclude an uncommon soft-tissue tumor such as infantile myofibromatosis or sarcoma.8,9,12 Because of the presence of a clear halo in all cases and mainly rapid involution of CHs, these differential diagnoses were dismissed. The histologic appearance of RICH differed from NICH and common IH, but some overlap was noted among the 3 lesions. Rapidly involuting CH was composed of small to large lobules of capillaries with moderately plump endothelial cells and pericytes; the lobules were surrounded by abundant fibrous tissue.9
Despite the notable differences in natural history between RICHs and NICHs, as RICHs regress within months while NICHs do not, both classes of CH share an important immunohistochemical phenotype; they do not express glucose transporter 1, the marker of IH.13 Tests for this marker were not performed in our study. The prognosis of CH generally is good, and special management is not required.
Conclusion
Rapidly involuting CHs and NICHs have many similarities, such as appearance, location, and sex distribution. The obvious differences in behavior serve to differentiate RICHs, NICHs, and common IHs. Infantile hemangiomas are not fully developed at birth and need many years to regress.
- Boon LM, Enjolras O, Mulliken JB. Congenital hemangioma: evidence of accelerated involution. J Pediatr. 1996;128:329-335.
- Neri I, Balestri R, Patrizi A. Hemangiomas: new insight and medical treatment. Dermatol Ther. 2012;25:322-334.
- Enjolras O, Mulliken JB. Vascular tumors and vascular malformations (new issues). Adv Dermatol. 1997;13:375-423.
- Mulliken JB, Enjolras O. Congenital hemangiomas and infantile hemangioma: missing links. J Am Acad Dermatol. 2004:50:875-882.
- Frieden IJ, Haggstrom AN, Drolet BA, et al. Infantile hemangiomas: current knowledge, future directions. proceedings of a research workshop on infantile hemangiomas, April 7-9, 2005, Bethesda, Maryland, USA. Pediatr Dermatol. 2005;22:383-406.
- Enjolras O, Picard A, Soupre V. Congenital haemangiomas and other rare infantile vascular tumours [in French]. Ann Chir Plast Esthet. 2006;51:339-346.
- Gorincour G, Kokta V, Rypens F, et al. Imaging characteristics of two subtypes of congenital hemangiomas: rapidly involuting congenital hemangiomas and non-involuting congenital hemangiomas. Pediatr Radiol. 2005;35:1178-1185.
- Rogers M, Lam A, Fischer G. Sonographic findings in a series of rapidly involuting congenital hemangiomas (RICH). Pediatr Dermatol. 2002;19:5-11.
- Berenguer B, Mulliken JB, Enjolras O, et al. Rapidly involuting congenital hemangioma: clinical and histopathologic features. Pediatr Dev Pathol. 2003;6:495-510.
- North PE, Waner M, James CA, et al. Congenital nonprogressive hemangioma: a distinct clinicopathologic entity unlike infantile hemangioma. Arch Dermatol. 2001;137:1607-1620.
- Chiavérini C, Kurzenne JY, Rogopoulos A, et al. Noninvoluting congenital hemangioma: 2 cases [in French]. Ann Dermatol Venerol. 2002;129:735-737.
- Enjolras O, Mulliken JB, Boon LM, et al. Noninvoluting congenital hemangioma: a rare cutaneous vascular anomaly. Plast Reconstr Surg. 2001;107:1647-1654.
- North PE, Waner M, Mizeracki A, et al. GLUT1: a newly discovered immunohistochemical marker for juvenile hemangiomas. Hum Pathol. 2000;31:11-22.
Hemangiomas are the most common benign tumors of childhood. In recent years, subsets of hemangiomas that are fully formed at birth have been recognized as clinically and biologically distinct from the classic infantile hemangioma (IH). Congenital hemangiomas (CHs) are classified based on clinical course as rapidly involuting CHs (RICHs) or noninvoluting CHs (NICHs). The aim of this retrospective study was to describe the epidemiology, clinical aspects, and clinical outcome of CH over a 5-year period.
Methods
Using electronic medical records from the department of dermatology (Hedi Chaker Hospital, Sfax, Tunisia) for a 5-year period (2008-2012), we searched for hemangioma. After collecting those records, we identified patients with CHs. We studied the epidemiologic (eg, sex, age), clinical course (eg, location, size, number, color, surrounding skin), and evolutionary aspects in these patients.
Results
Twenty IHs were identified, 6 (30%) of which were considered CHs. The clinical characteristics of the 6 patients are summarized in the Table. We identified 2 females and 4 males aged 2 to 60 days (mean age, 16 days). Four patients had CHs involving the limbs (knee [n=2]; ankle [n=1]; elbow [n=1]) and 2 patients had CHs involving the trunk. Congenital hemangiomas were singular, oval shaped, and surrounded by a clear halo in all 6 patients. They presented as exophytic masses (n=3) or bossed plaques (n=3). A blue hue was noted in 5 patients and a purple hue in 1 patient. In some cases, telangiectasia (n=2) or small areas of necrosis (n=1) were noted at the center of the CHs. The CHs ranged in size from 3 to 6 cm (mean, 4 cm). Doppler ultrasonography was performed in 2 patients and showed fast blood flow. It is well known that manipulating a CH when it is ulcerative may cause a fatal hemorrhage. Thus, parents/guardians should be cautious when cleaning and dressing the lesions. Regular follow-up was recommended to all patients as noted in the medical records. The lesion involuted in4 patients after a mean period of 6 months, which allowed us to classify the lesions as RICHs (Figure, A). Two CHs were persistent after 2-year (Figure, B) and 4-year (Figure, C) follow-up, which was consistent with NICH classification.


Comment
Since 1996, vascular anomalies have been classified either as tumors or malformations.1 Infantile hemangioma is the most common vascular tumor and presents as an endothelial cellular proliferation that develops within days after birth. Congenital hemangiomas are fully developed at birth2,3 and are classified as RICHs and NICHs according to their clinical outcome.
As expected, our analysis revealed that CH usually is solitary and may present as a small lesion (eg, a few millimeters) but also may be large in size.4 Congenital hemangioma has an equal sex distribution and a predilection for the head and limbs near a joint. In contrast, IH exhibits female predilection and can occur anywhere on the body.4-6 In our study, CHs were more common in males and had a predilection for the limbs. Three patients presented with exophytic masses with a clear halo and overlying telangiectasia, which are commonly described features in CH.4,6
In the classification of vascular anomalies, RICHs and NICHs are fast-flow lesions that are indistinguishable at birth.7,8 Untreated, RICHs usually resolve in the first 14 months of life, often resulting in an area of atrophic or excess skin.8,9 Noninvoluting CHs persist and grow in proportion with the patient.10-12
When Doppler ultrasonography findings are inconsistent with a CH, an early biopsy from the periphery of the lesion may be performed to exclude an uncommon soft-tissue tumor such as infantile myofibromatosis or sarcoma.8,9,12 Because of the presence of a clear halo in all cases and mainly rapid involution of CHs, these differential diagnoses were dismissed. The histologic appearance of RICH differed from NICH and common IH, but some overlap was noted among the 3 lesions. Rapidly involuting CH was composed of small to large lobules of capillaries with moderately plump endothelial cells and pericytes; the lobules were surrounded by abundant fibrous tissue.9
Despite the notable differences in natural history between RICHs and NICHs, as RICHs regress within months while NICHs do not, both classes of CH share an important immunohistochemical phenotype; they do not express glucose transporter 1, the marker of IH.13 Tests for this marker were not performed in our study. The prognosis of CH generally is good, and special management is not required.
Conclusion
Rapidly involuting CHs and NICHs have many similarities, such as appearance, location, and sex distribution. The obvious differences in behavior serve to differentiate RICHs, NICHs, and common IHs. Infantile hemangiomas are not fully developed at birth and need many years to regress.
Hemangiomas are the most common benign tumors of childhood. In recent years, subsets of hemangiomas that are fully formed at birth have been recognized as clinically and biologically distinct from the classic infantile hemangioma (IH). Congenital hemangiomas (CHs) are classified based on clinical course as rapidly involuting CHs (RICHs) or noninvoluting CHs (NICHs). The aim of this retrospective study was to describe the epidemiology, clinical aspects, and clinical outcome of CH over a 5-year period.
Methods
Using electronic medical records from the department of dermatology (Hedi Chaker Hospital, Sfax, Tunisia) for a 5-year period (2008-2012), we searched for hemangioma. After collecting those records, we identified patients with CHs. We studied the epidemiologic (eg, sex, age), clinical course (eg, location, size, number, color, surrounding skin), and evolutionary aspects in these patients.
Results
Twenty IHs were identified, 6 (30%) of which were considered CHs. The clinical characteristics of the 6 patients are summarized in the Table. We identified 2 females and 4 males aged 2 to 60 days (mean age, 16 days). Four patients had CHs involving the limbs (knee [n=2]; ankle [n=1]; elbow [n=1]) and 2 patients had CHs involving the trunk. Congenital hemangiomas were singular, oval shaped, and surrounded by a clear halo in all 6 patients. They presented as exophytic masses (n=3) or bossed plaques (n=3). A blue hue was noted in 5 patients and a purple hue in 1 patient. In some cases, telangiectasia (n=2) or small areas of necrosis (n=1) were noted at the center of the CHs. The CHs ranged in size from 3 to 6 cm (mean, 4 cm). Doppler ultrasonography was performed in 2 patients and showed fast blood flow. It is well known that manipulating a CH when it is ulcerative may cause a fatal hemorrhage. Thus, parents/guardians should be cautious when cleaning and dressing the lesions. Regular follow-up was recommended to all patients as noted in the medical records. The lesion involuted in4 patients after a mean period of 6 months, which allowed us to classify the lesions as RICHs (Figure, A). Two CHs were persistent after 2-year (Figure, B) and 4-year (Figure, C) follow-up, which was consistent with NICH classification.


Comment
Since 1996, vascular anomalies have been classified either as tumors or malformations.1 Infantile hemangioma is the most common vascular tumor and presents as an endothelial cellular proliferation that develops within days after birth. Congenital hemangiomas are fully developed at birth2,3 and are classified as RICHs and NICHs according to their clinical outcome.
As expected, our analysis revealed that CH usually is solitary and may present as a small lesion (eg, a few millimeters) but also may be large in size.4 Congenital hemangioma has an equal sex distribution and a predilection for the head and limbs near a joint. In contrast, IH exhibits female predilection and can occur anywhere on the body.4-6 In our study, CHs were more common in males and had a predilection for the limbs. Three patients presented with exophytic masses with a clear halo and overlying telangiectasia, which are commonly described features in CH.4,6
In the classification of vascular anomalies, RICHs and NICHs are fast-flow lesions that are indistinguishable at birth.7,8 Untreated, RICHs usually resolve in the first 14 months of life, often resulting in an area of atrophic or excess skin.8,9 Noninvoluting CHs persist and grow in proportion with the patient.10-12
When Doppler ultrasonography findings are inconsistent with a CH, an early biopsy from the periphery of the lesion may be performed to exclude an uncommon soft-tissue tumor such as infantile myofibromatosis or sarcoma.8,9,12 Because of the presence of a clear halo in all cases and mainly rapid involution of CHs, these differential diagnoses were dismissed. The histologic appearance of RICH differed from NICH and common IH, but some overlap was noted among the 3 lesions. Rapidly involuting CH was composed of small to large lobules of capillaries with moderately plump endothelial cells and pericytes; the lobules were surrounded by abundant fibrous tissue.9
Despite the notable differences in natural history between RICHs and NICHs, as RICHs regress within months while NICHs do not, both classes of CH share an important immunohistochemical phenotype; they do not express glucose transporter 1, the marker of IH.13 Tests for this marker were not performed in our study. The prognosis of CH generally is good, and special management is not required.
Conclusion
Rapidly involuting CHs and NICHs have many similarities, such as appearance, location, and sex distribution. The obvious differences in behavior serve to differentiate RICHs, NICHs, and common IHs. Infantile hemangiomas are not fully developed at birth and need many years to regress.
- Boon LM, Enjolras O, Mulliken JB. Congenital hemangioma: evidence of accelerated involution. J Pediatr. 1996;128:329-335.
- Neri I, Balestri R, Patrizi A. Hemangiomas: new insight and medical treatment. Dermatol Ther. 2012;25:322-334.
- Enjolras O, Mulliken JB. Vascular tumors and vascular malformations (new issues). Adv Dermatol. 1997;13:375-423.
- Mulliken JB, Enjolras O. Congenital hemangiomas and infantile hemangioma: missing links. J Am Acad Dermatol. 2004:50:875-882.
- Frieden IJ, Haggstrom AN, Drolet BA, et al. Infantile hemangiomas: current knowledge, future directions. proceedings of a research workshop on infantile hemangiomas, April 7-9, 2005, Bethesda, Maryland, USA. Pediatr Dermatol. 2005;22:383-406.
- Enjolras O, Picard A, Soupre V. Congenital haemangiomas and other rare infantile vascular tumours [in French]. Ann Chir Plast Esthet. 2006;51:339-346.
- Gorincour G, Kokta V, Rypens F, et al. Imaging characteristics of two subtypes of congenital hemangiomas: rapidly involuting congenital hemangiomas and non-involuting congenital hemangiomas. Pediatr Radiol. 2005;35:1178-1185.
- Rogers M, Lam A, Fischer G. Sonographic findings in a series of rapidly involuting congenital hemangiomas (RICH). Pediatr Dermatol. 2002;19:5-11.
- Berenguer B, Mulliken JB, Enjolras O, et al. Rapidly involuting congenital hemangioma: clinical and histopathologic features. Pediatr Dev Pathol. 2003;6:495-510.
- North PE, Waner M, James CA, et al. Congenital nonprogressive hemangioma: a distinct clinicopathologic entity unlike infantile hemangioma. Arch Dermatol. 2001;137:1607-1620.
- Chiavérini C, Kurzenne JY, Rogopoulos A, et al. Noninvoluting congenital hemangioma: 2 cases [in French]. Ann Dermatol Venerol. 2002;129:735-737.
- Enjolras O, Mulliken JB, Boon LM, et al. Noninvoluting congenital hemangioma: a rare cutaneous vascular anomaly. Plast Reconstr Surg. 2001;107:1647-1654.
- North PE, Waner M, Mizeracki A, et al. GLUT1: a newly discovered immunohistochemical marker for juvenile hemangiomas. Hum Pathol. 2000;31:11-22.
- Boon LM, Enjolras O, Mulliken JB. Congenital hemangioma: evidence of accelerated involution. J Pediatr. 1996;128:329-335.
- Neri I, Balestri R, Patrizi A. Hemangiomas: new insight and medical treatment. Dermatol Ther. 2012;25:322-334.
- Enjolras O, Mulliken JB. Vascular tumors and vascular malformations (new issues). Adv Dermatol. 1997;13:375-423.
- Mulliken JB, Enjolras O. Congenital hemangiomas and infantile hemangioma: missing links. J Am Acad Dermatol. 2004:50:875-882.
- Frieden IJ, Haggstrom AN, Drolet BA, et al. Infantile hemangiomas: current knowledge, future directions. proceedings of a research workshop on infantile hemangiomas, April 7-9, 2005, Bethesda, Maryland, USA. Pediatr Dermatol. 2005;22:383-406.
- Enjolras O, Picard A, Soupre V. Congenital haemangiomas and other rare infantile vascular tumours [in French]. Ann Chir Plast Esthet. 2006;51:339-346.
- Gorincour G, Kokta V, Rypens F, et al. Imaging characteristics of two subtypes of congenital hemangiomas: rapidly involuting congenital hemangiomas and non-involuting congenital hemangiomas. Pediatr Radiol. 2005;35:1178-1185.
- Rogers M, Lam A, Fischer G. Sonographic findings in a series of rapidly involuting congenital hemangiomas (RICH). Pediatr Dermatol. 2002;19:5-11.
- Berenguer B, Mulliken JB, Enjolras O, et al. Rapidly involuting congenital hemangioma: clinical and histopathologic features. Pediatr Dev Pathol. 2003;6:495-510.
- North PE, Waner M, James CA, et al. Congenital nonprogressive hemangioma: a distinct clinicopathologic entity unlike infantile hemangioma. Arch Dermatol. 2001;137:1607-1620.
- Chiavérini C, Kurzenne JY, Rogopoulos A, et al. Noninvoluting congenital hemangioma: 2 cases [in French]. Ann Dermatol Venerol. 2002;129:735-737.
- Enjolras O, Mulliken JB, Boon LM, et al. Noninvoluting congenital hemangioma: a rare cutaneous vascular anomaly. Plast Reconstr Surg. 2001;107:1647-1654.
- North PE, Waner M, Mizeracki A, et al. GLUT1: a newly discovered immunohistochemical marker for juvenile hemangiomas. Hum Pathol. 2000;31:11-22.
Practice Points
- Congenital hemangiomas (CHs) are fully developed hemangiomas that are present at birth.
- In our study, CHs were more common in males, with a predilection for the limbs.
- Infantile hemangiomas are not fully developed at birth and need many years to regress.
Localized Pemphigus Foliaceus
To the Editor:
Pemphigus foliaceus is a rare autoimmune blistering disorder that typically presents with crusted scaly erosions in a seborrheic distribution. We describe a case of pemphigus foliaceus localized to the right cheek of 10 years’ duration that spread to other areas. With a PubMed search of articles indexed for MEDLINE yielding only 14 cases of localized pemphigus foliaceus (Table), it represents an extremely rare entity that often is a diagnostic challenge and may be a harbinger for disseminated disease months to years after the inciting lesion appears.
A 51-year-old woman presented with an asymptomatic cutaneous eruption that had remained localized to the right cheek for 10 years before it increased in size and new lesions developed on the left cheek, chest, and upper back. No inciting factors, such as contactants, insect bites, infections, medications, or recent travel were identified. On physical examination a well-demarcated, hypertrophic, verrucouslike plaque with central pink atrophy and exfoliative scale involved the right malar and submalar regions but spared the mucocutaneous junctions of the face (Figure 1). Subtle dark brown papules, some with overlying scale, speckled the left cheek, right jawline, chest, and upper back. The oral cavity was clear.
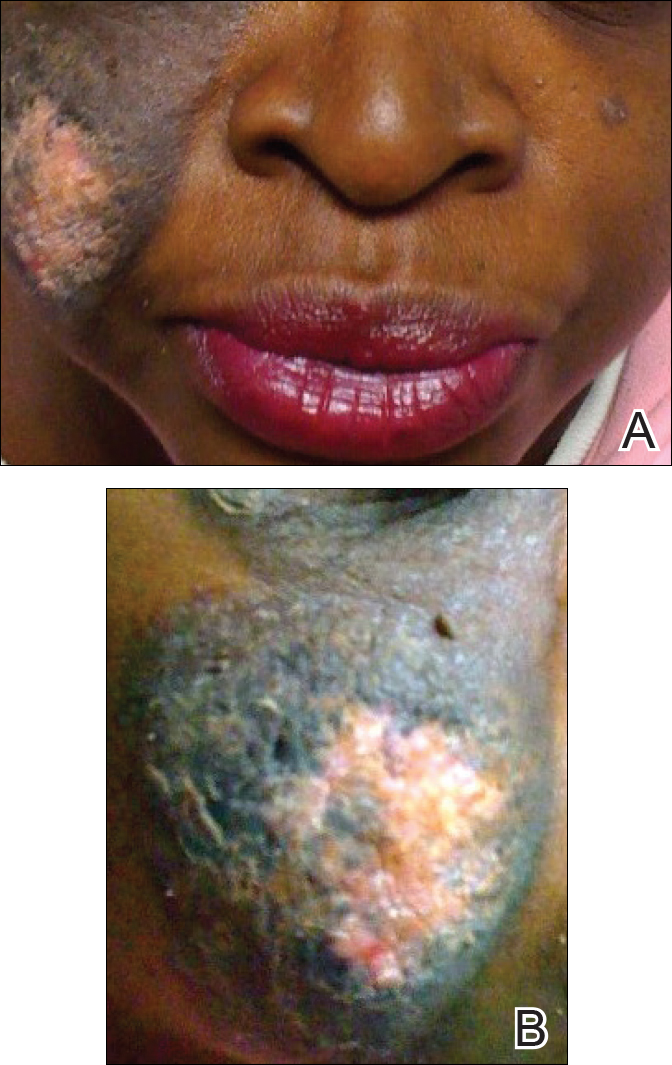
Leading differentials included hypertrophic discoid lupus erythematosus and pemphigus vegetans. Other considerations included sarcoidosis, granuloma faciale, lupus vulgaris, disseminated coccidioidomycosis or blastomycosis, and squamous cell carcinoma.
An initial biopsy revealed a lymphocytic lichenoid dermatitis with epidermal hyperplasia and scattered eosinophils for which the following differentials were provided: insect bite, hypertrophic lichen planus, prurigo nodularis superimposed on rosacea, and allergic contact dermatitis. Under these histologic diagnoses, tacrolimus ointment 0.03%, topical mid-potency corticosteroid, and a combination of oral doxycycline and metronidazole gel 1% were prescribed but failed to ameliorate her condition.
Because the clinical differentials were vast and noncorrelative with the original pathology, additional biopsies were performed: one from the edge of the large malar plaque, which was transected for hematoxylin and eosin (H&E) and tissue cultures; one perilesional to the large malar plaque for direct immunofluorescence (DIF); and one from the papule on the right jawline for H&E. Tissue cultures were negative for fungal and mycobacterial organisms. Both specimens submitted for H&E showed the prominent epidermal hyperplasia and lymphocytic dermal infiltrate noted on the original H&E but also demonstrated intragranular acantholysis (Figure 2). The DIF revealed intercellular IgG and C3 deposition throughout the epidermis (Figure 3). Indirect immunofluorescence was negative, but enzyme-linked immunosorbent assay detected circulating antidesmoglein-1 but not antidesmoglein-3 autoantibodies. Other serologies including antinuclear antibody, anti–double-stranded DNA, antihistone, anti–Sjögren syndrome A, and anti–Sjögren syndrome B antibodies were negative.
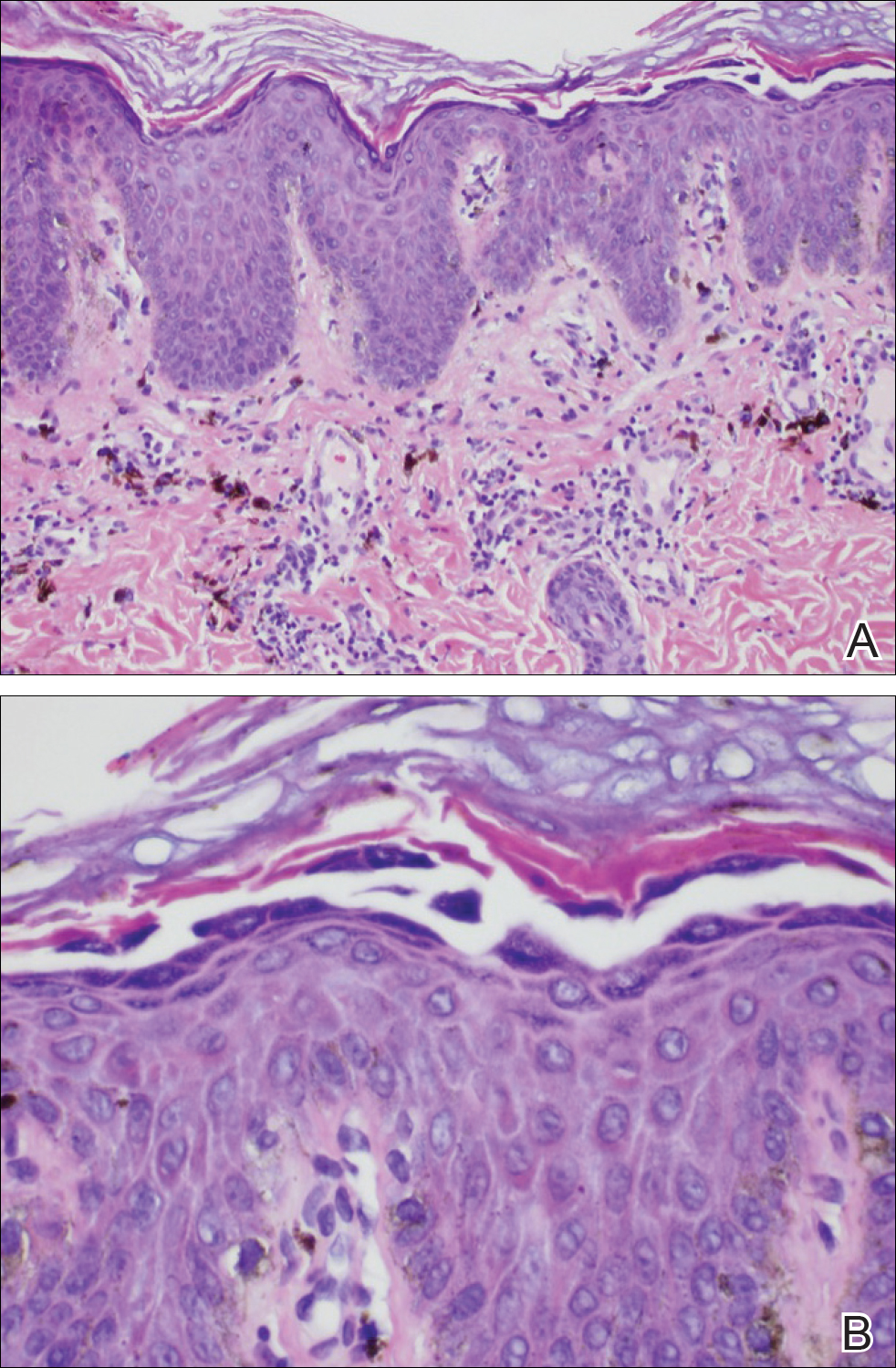

The diagnosis of localized pemphigus foliaceus was made and management with oral prednisone and mycophenolate mofetil resulted in improvement within weeks.
Localized pemphigus foliaceus is extremely rare with only 14 cases reported in the literature (Table).1-10 Its diagnosis is challenging, as the clinical presentation simulates various entities and the histological features and serological markers are difficult to capture.
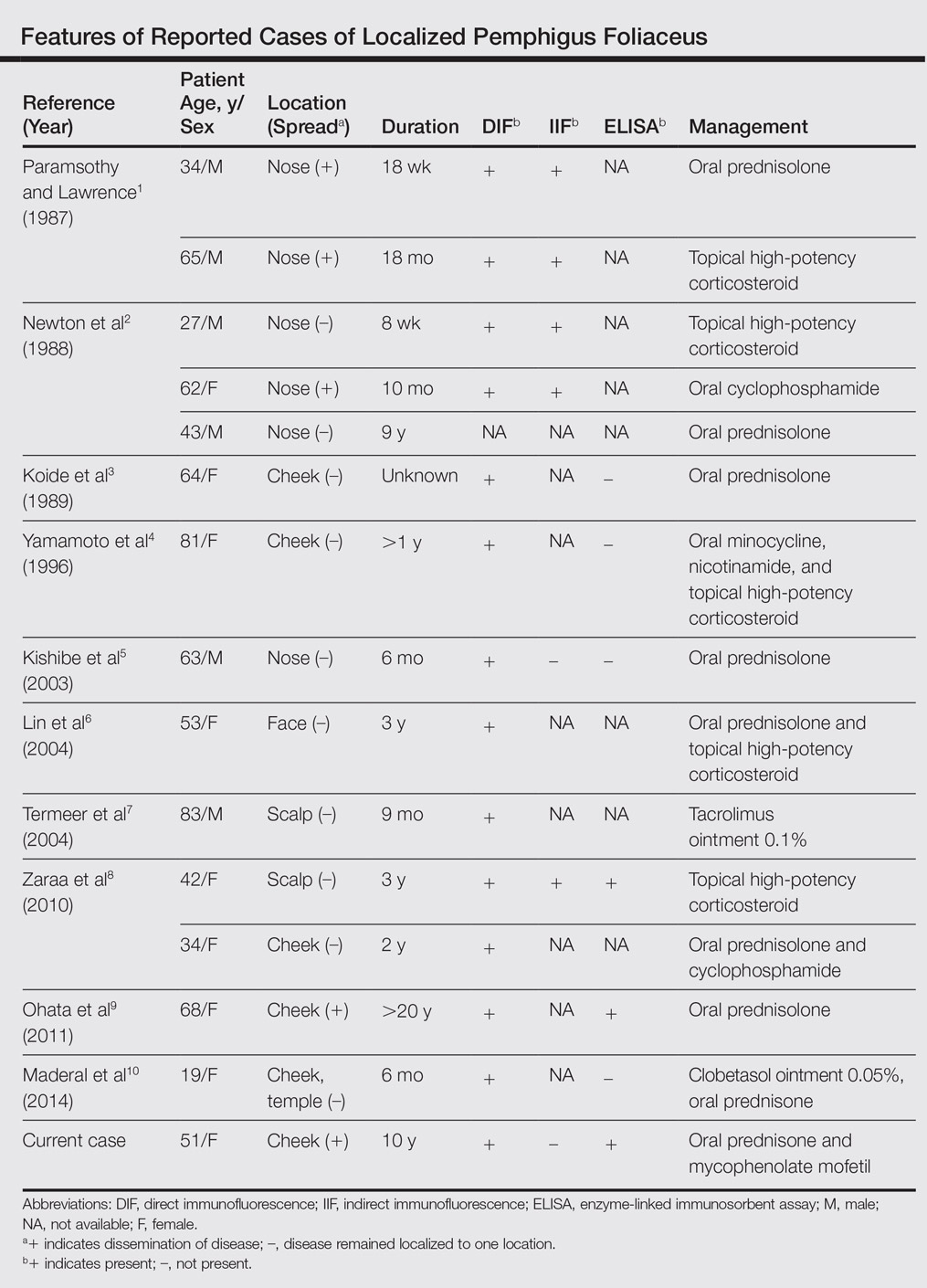
Localized pemphigus foliaceus typically presents as an isolated, erythematous, scaly, crusted plaque involving the nose, cheek, or scalp and may mimic several conditions including contact dermatitis, seborrheic dermatitis, rosacea, cutaneous sarcoidosis, discoid lupus erythematosus, lupus vulgaris, impetigo contagiosa, solar keratosis, and nonmelanoma skin cancer.1-10
The predilection for sun-exposed areas suggests UV radiation may induce binding of antidesmoglein-1 autoantibodies with subsequent cytokine-mediated inflammation and acantholysis at these sites.11-13 Similarly, the immunomodulatory agent imiquimod has been reported to induce pemphigus foliaceus at its application sites.6
When pemphigus foliaceus is clinically discernible, the histology and DIF are in accordance with the clinical diagnosis 53.8% of the time.13 In cases of localized pemphigus foliaceus in which the diagnosis is more elusive, many biopsies often are needed to capture the characteristic intragranular acantholysis; this feature often is so subtle that unless the diagnosis is suspected, it is underappreciated or undetectable. In chronic lesions, it may be masked by secondary changes such as acanthosis, hyperkeratosis, and parakeratosis.14
In pemphigus foliaceus, detection of circulating antidesmoglein-1 autoantibodies by enzyme-linked immunosorbent assay is slightly more sensitive and specific compared to indirect immunofluorescence, but both correlate with disease activity.15,16 The low or absent autoantibody titers in localized pemphigus foliaceus may reflect its limited involvement, but dissemination of the disease with subsequent elevation of autoantibody titers may occur months to years after initial presentation,1,2,9 as was the case with our patient.
The majority of localized pemphigus foliaceus cases require systemic prednisone, sometimes in conjunction with nonsteroidal immunosuppressants or topical high-potency corticosteroids.1-3,5,6,8-10 One case was efficaciously managed with tacrolimus ointment 0.1%.7
Localized pemphigus foliaceus is a rare and challenging entity that must be a diagnostic consideration for any chronic focal plaque on the face or scalp, as it may herald disseminated disease.
- Paramsothy Y, Lawrence CM. “Tin-tack” sign in localized pemphigus foliaceus. Br J Dermatol. 1987;116:127-129.
- Newton JA, McGibbon DH, Monk B, et al. Pemphigus foliaceus localized to the nose. Br J Dermatol. 1988;118:303-312.
- Koide M, Kokura N, Takano N. Pemphigus foliaceus localized on the face [in Japanese]. Jpn J Dermatol. 1989;97:1262.
- Yamamoto S, Kanekura T, Gushi A, et al. A case of localized pemphigus foliaceus. J Dermatol. 1996;23:893-895.
- Kishibe M, Kinouchi M, Ishida-Yamamoto A, et al. Pemphigus foliaceus localized to the nose. Clin Exp Dermatol. 2003;28:560-562.
- Lin R, Ladd DJ, Powell DJ, et al. Localized pemphigus foliaceus induced by topical imiquimod treatment. Arch Dermatol. 2004;140:889-890.
- Termeer CC, Technau K, Augustin M, et al. Topical tacrolimus (Protopic) for the treatment of a localized pemphigus foliaceus. J Eur Acad Dermatol Venereol. 2004;18:636-637.
- Zaraa I, El Euch D, Kort R, et al. Localized pemphigus: a report of three cases. Int J Dermatol 2010;49:715-716.
- Ohata C, Akamatsu K, Imai N, et al. Localized pemphigus foliaceus exclusively involving the follicular infundibulum: a novel peau d’orange appearance. Eur J Dermatol. 2011;21:392-395.
- Maderal AD, Miner A, Nousari C, et al. Localized pemphigus foliaceus with unilateral facial involvement. Actas Dermosifiliogr. 2014;105:413-417.
- Cram DL, Winkelmann RK. Ultraviolet-induced acantholysis in pemphigus. Arch Dermatol. 1965;92:7-13.
- Kano Y, Shimosegawa M, Mizukawa Y, et al. Pemphigus foliaceus induced by exposure to sunlight. Dermatology. 2000;201:132-138.
- Lebe B, Gül Nıflıoğlu G, Seyrek S, et al. Evaluation of clinical and histopathologic/direct immunofluorescence diagnosis in autoimmune vesiculobullous dermatitis: utility of direct immunofluorescence. Turk Patoloji Derg. 2012;28:11-16.
- Joly P, Litrowski N. Pemphigus group (vulgaris, vegetans, foliaceus, herpetiformis, brasiliensis). Clin Dermatol. 2011;29:432-436.
- Ishii K, Amagai M, Hall RP, et al. Characterization of autoantibodies in pemphigus using antigen specific enzyme-linked immunosorbent assays with baculovirus-expressed recombinant desmogleins. J Immunol. 1997;159:2010-2017.
- Ng PP, Thng ST, Mohamed K, et al. Comparison of desmoglein ELISA and indirect immunofluorescence using two substrates (monkey esophagus and normal human skin) in the diagnosis of pemphigus. Australas J Dermatol. 2005;46:239-241.
To the Editor:
Pemphigus foliaceus is a rare autoimmune blistering disorder that typically presents with crusted scaly erosions in a seborrheic distribution. We describe a case of pemphigus foliaceus localized to the right cheek of 10 years’ duration that spread to other areas. With a PubMed search of articles indexed for MEDLINE yielding only 14 cases of localized pemphigus foliaceus (Table), it represents an extremely rare entity that often is a diagnostic challenge and may be a harbinger for disseminated disease months to years after the inciting lesion appears.
A 51-year-old woman presented with an asymptomatic cutaneous eruption that had remained localized to the right cheek for 10 years before it increased in size and new lesions developed on the left cheek, chest, and upper back. No inciting factors, such as contactants, insect bites, infections, medications, or recent travel were identified. On physical examination a well-demarcated, hypertrophic, verrucouslike plaque with central pink atrophy and exfoliative scale involved the right malar and submalar regions but spared the mucocutaneous junctions of the face (Figure 1). Subtle dark brown papules, some with overlying scale, speckled the left cheek, right jawline, chest, and upper back. The oral cavity was clear.

Leading differentials included hypertrophic discoid lupus erythematosus and pemphigus vegetans. Other considerations included sarcoidosis, granuloma faciale, lupus vulgaris, disseminated coccidioidomycosis or blastomycosis, and squamous cell carcinoma.
An initial biopsy revealed a lymphocytic lichenoid dermatitis with epidermal hyperplasia and scattered eosinophils for which the following differentials were provided: insect bite, hypertrophic lichen planus, prurigo nodularis superimposed on rosacea, and allergic contact dermatitis. Under these histologic diagnoses, tacrolimus ointment 0.03%, topical mid-potency corticosteroid, and a combination of oral doxycycline and metronidazole gel 1% were prescribed but failed to ameliorate her condition.
Because the clinical differentials were vast and noncorrelative with the original pathology, additional biopsies were performed: one from the edge of the large malar plaque, which was transected for hematoxylin and eosin (H&E) and tissue cultures; one perilesional to the large malar plaque for direct immunofluorescence (DIF); and one from the papule on the right jawline for H&E. Tissue cultures were negative for fungal and mycobacterial organisms. Both specimens submitted for H&E showed the prominent epidermal hyperplasia and lymphocytic dermal infiltrate noted on the original H&E but also demonstrated intragranular acantholysis (Figure 2). The DIF revealed intercellular IgG and C3 deposition throughout the epidermis (Figure 3). Indirect immunofluorescence was negative, but enzyme-linked immunosorbent assay detected circulating antidesmoglein-1 but not antidesmoglein-3 autoantibodies. Other serologies including antinuclear antibody, anti–double-stranded DNA, antihistone, anti–Sjögren syndrome A, and anti–Sjögren syndrome B antibodies were negative.


The diagnosis of localized pemphigus foliaceus was made and management with oral prednisone and mycophenolate mofetil resulted in improvement within weeks.
Localized pemphigus foliaceus is extremely rare with only 14 cases reported in the literature (Table).1-10 Its diagnosis is challenging, as the clinical presentation simulates various entities and the histological features and serological markers are difficult to capture.

Localized pemphigus foliaceus typically presents as an isolated, erythematous, scaly, crusted plaque involving the nose, cheek, or scalp and may mimic several conditions including contact dermatitis, seborrheic dermatitis, rosacea, cutaneous sarcoidosis, discoid lupus erythematosus, lupus vulgaris, impetigo contagiosa, solar keratosis, and nonmelanoma skin cancer.1-10
The predilection for sun-exposed areas suggests UV radiation may induce binding of antidesmoglein-1 autoantibodies with subsequent cytokine-mediated inflammation and acantholysis at these sites.11-13 Similarly, the immunomodulatory agent imiquimod has been reported to induce pemphigus foliaceus at its application sites.6
When pemphigus foliaceus is clinically discernible, the histology and DIF are in accordance with the clinical diagnosis 53.8% of the time.13 In cases of localized pemphigus foliaceus in which the diagnosis is more elusive, many biopsies often are needed to capture the characteristic intragranular acantholysis; this feature often is so subtle that unless the diagnosis is suspected, it is underappreciated or undetectable. In chronic lesions, it may be masked by secondary changes such as acanthosis, hyperkeratosis, and parakeratosis.14
In pemphigus foliaceus, detection of circulating antidesmoglein-1 autoantibodies by enzyme-linked immunosorbent assay is slightly more sensitive and specific compared to indirect immunofluorescence, but both correlate with disease activity.15,16 The low or absent autoantibody titers in localized pemphigus foliaceus may reflect its limited involvement, but dissemination of the disease with subsequent elevation of autoantibody titers may occur months to years after initial presentation,1,2,9 as was the case with our patient.
The majority of localized pemphigus foliaceus cases require systemic prednisone, sometimes in conjunction with nonsteroidal immunosuppressants or topical high-potency corticosteroids.1-3,5,6,8-10 One case was efficaciously managed with tacrolimus ointment 0.1%.7
Localized pemphigus foliaceus is a rare and challenging entity that must be a diagnostic consideration for any chronic focal plaque on the face or scalp, as it may herald disseminated disease.
To the Editor:
Pemphigus foliaceus is a rare autoimmune blistering disorder that typically presents with crusted scaly erosions in a seborrheic distribution. We describe a case of pemphigus foliaceus localized to the right cheek of 10 years’ duration that spread to other areas. With a PubMed search of articles indexed for MEDLINE yielding only 14 cases of localized pemphigus foliaceus (Table), it represents an extremely rare entity that often is a diagnostic challenge and may be a harbinger for disseminated disease months to years after the inciting lesion appears.
A 51-year-old woman presented with an asymptomatic cutaneous eruption that had remained localized to the right cheek for 10 years before it increased in size and new lesions developed on the left cheek, chest, and upper back. No inciting factors, such as contactants, insect bites, infections, medications, or recent travel were identified. On physical examination a well-demarcated, hypertrophic, verrucouslike plaque with central pink atrophy and exfoliative scale involved the right malar and submalar regions but spared the mucocutaneous junctions of the face (Figure 1). Subtle dark brown papules, some with overlying scale, speckled the left cheek, right jawline, chest, and upper back. The oral cavity was clear.

Leading differentials included hypertrophic discoid lupus erythematosus and pemphigus vegetans. Other considerations included sarcoidosis, granuloma faciale, lupus vulgaris, disseminated coccidioidomycosis or blastomycosis, and squamous cell carcinoma.
An initial biopsy revealed a lymphocytic lichenoid dermatitis with epidermal hyperplasia and scattered eosinophils for which the following differentials were provided: insect bite, hypertrophic lichen planus, prurigo nodularis superimposed on rosacea, and allergic contact dermatitis. Under these histologic diagnoses, tacrolimus ointment 0.03%, topical mid-potency corticosteroid, and a combination of oral doxycycline and metronidazole gel 1% were prescribed but failed to ameliorate her condition.
Because the clinical differentials were vast and noncorrelative with the original pathology, additional biopsies were performed: one from the edge of the large malar plaque, which was transected for hematoxylin and eosin (H&E) and tissue cultures; one perilesional to the large malar plaque for direct immunofluorescence (DIF); and one from the papule on the right jawline for H&E. Tissue cultures were negative for fungal and mycobacterial organisms. Both specimens submitted for H&E showed the prominent epidermal hyperplasia and lymphocytic dermal infiltrate noted on the original H&E but also demonstrated intragranular acantholysis (Figure 2). The DIF revealed intercellular IgG and C3 deposition throughout the epidermis (Figure 3). Indirect immunofluorescence was negative, but enzyme-linked immunosorbent assay detected circulating antidesmoglein-1 but not antidesmoglein-3 autoantibodies. Other serologies including antinuclear antibody, anti–double-stranded DNA, antihistone, anti–Sjögren syndrome A, and anti–Sjögren syndrome B antibodies were negative.


The diagnosis of localized pemphigus foliaceus was made and management with oral prednisone and mycophenolate mofetil resulted in improvement within weeks.
Localized pemphigus foliaceus is extremely rare with only 14 cases reported in the literature (Table).1-10 Its diagnosis is challenging, as the clinical presentation simulates various entities and the histological features and serological markers are difficult to capture.

Localized pemphigus foliaceus typically presents as an isolated, erythematous, scaly, crusted plaque involving the nose, cheek, or scalp and may mimic several conditions including contact dermatitis, seborrheic dermatitis, rosacea, cutaneous sarcoidosis, discoid lupus erythematosus, lupus vulgaris, impetigo contagiosa, solar keratosis, and nonmelanoma skin cancer.1-10
The predilection for sun-exposed areas suggests UV radiation may induce binding of antidesmoglein-1 autoantibodies with subsequent cytokine-mediated inflammation and acantholysis at these sites.11-13 Similarly, the immunomodulatory agent imiquimod has been reported to induce pemphigus foliaceus at its application sites.6
When pemphigus foliaceus is clinically discernible, the histology and DIF are in accordance with the clinical diagnosis 53.8% of the time.13 In cases of localized pemphigus foliaceus in which the diagnosis is more elusive, many biopsies often are needed to capture the characteristic intragranular acantholysis; this feature often is so subtle that unless the diagnosis is suspected, it is underappreciated or undetectable. In chronic lesions, it may be masked by secondary changes such as acanthosis, hyperkeratosis, and parakeratosis.14
In pemphigus foliaceus, detection of circulating antidesmoglein-1 autoantibodies by enzyme-linked immunosorbent assay is slightly more sensitive and specific compared to indirect immunofluorescence, but both correlate with disease activity.15,16 The low or absent autoantibody titers in localized pemphigus foliaceus may reflect its limited involvement, but dissemination of the disease with subsequent elevation of autoantibody titers may occur months to years after initial presentation,1,2,9 as was the case with our patient.
The majority of localized pemphigus foliaceus cases require systemic prednisone, sometimes in conjunction with nonsteroidal immunosuppressants or topical high-potency corticosteroids.1-3,5,6,8-10 One case was efficaciously managed with tacrolimus ointment 0.1%.7
Localized pemphigus foliaceus is a rare and challenging entity that must be a diagnostic consideration for any chronic focal plaque on the face or scalp, as it may herald disseminated disease.
- Paramsothy Y, Lawrence CM. “Tin-tack” sign in localized pemphigus foliaceus. Br J Dermatol. 1987;116:127-129.
- Newton JA, McGibbon DH, Monk B, et al. Pemphigus foliaceus localized to the nose. Br J Dermatol. 1988;118:303-312.
- Koide M, Kokura N, Takano N. Pemphigus foliaceus localized on the face [in Japanese]. Jpn J Dermatol. 1989;97:1262.
- Yamamoto S, Kanekura T, Gushi A, et al. A case of localized pemphigus foliaceus. J Dermatol. 1996;23:893-895.
- Kishibe M, Kinouchi M, Ishida-Yamamoto A, et al. Pemphigus foliaceus localized to the nose. Clin Exp Dermatol. 2003;28:560-562.
- Lin R, Ladd DJ, Powell DJ, et al. Localized pemphigus foliaceus induced by topical imiquimod treatment. Arch Dermatol. 2004;140:889-890.
- Termeer CC, Technau K, Augustin M, et al. Topical tacrolimus (Protopic) for the treatment of a localized pemphigus foliaceus. J Eur Acad Dermatol Venereol. 2004;18:636-637.
- Zaraa I, El Euch D, Kort R, et al. Localized pemphigus: a report of three cases. Int J Dermatol 2010;49:715-716.
- Ohata C, Akamatsu K, Imai N, et al. Localized pemphigus foliaceus exclusively involving the follicular infundibulum: a novel peau d’orange appearance. Eur J Dermatol. 2011;21:392-395.
- Maderal AD, Miner A, Nousari C, et al. Localized pemphigus foliaceus with unilateral facial involvement. Actas Dermosifiliogr. 2014;105:413-417.
- Cram DL, Winkelmann RK. Ultraviolet-induced acantholysis in pemphigus. Arch Dermatol. 1965;92:7-13.
- Kano Y, Shimosegawa M, Mizukawa Y, et al. Pemphigus foliaceus induced by exposure to sunlight. Dermatology. 2000;201:132-138.
- Lebe B, Gül Nıflıoğlu G, Seyrek S, et al. Evaluation of clinical and histopathologic/direct immunofluorescence diagnosis in autoimmune vesiculobullous dermatitis: utility of direct immunofluorescence. Turk Patoloji Derg. 2012;28:11-16.
- Joly P, Litrowski N. Pemphigus group (vulgaris, vegetans, foliaceus, herpetiformis, brasiliensis). Clin Dermatol. 2011;29:432-436.
- Ishii K, Amagai M, Hall RP, et al. Characterization of autoantibodies in pemphigus using antigen specific enzyme-linked immunosorbent assays with baculovirus-expressed recombinant desmogleins. J Immunol. 1997;159:2010-2017.
- Ng PP, Thng ST, Mohamed K, et al. Comparison of desmoglein ELISA and indirect immunofluorescence using two substrates (monkey esophagus and normal human skin) in the diagnosis of pemphigus. Australas J Dermatol. 2005;46:239-241.
- Paramsothy Y, Lawrence CM. “Tin-tack” sign in localized pemphigus foliaceus. Br J Dermatol. 1987;116:127-129.
- Newton JA, McGibbon DH, Monk B, et al. Pemphigus foliaceus localized to the nose. Br J Dermatol. 1988;118:303-312.
- Koide M, Kokura N, Takano N. Pemphigus foliaceus localized on the face [in Japanese]. Jpn J Dermatol. 1989;97:1262.
- Yamamoto S, Kanekura T, Gushi A, et al. A case of localized pemphigus foliaceus. J Dermatol. 1996;23:893-895.
- Kishibe M, Kinouchi M, Ishida-Yamamoto A, et al. Pemphigus foliaceus localized to the nose. Clin Exp Dermatol. 2003;28:560-562.
- Lin R, Ladd DJ, Powell DJ, et al. Localized pemphigus foliaceus induced by topical imiquimod treatment. Arch Dermatol. 2004;140:889-890.
- Termeer CC, Technau K, Augustin M, et al. Topical tacrolimus (Protopic) for the treatment of a localized pemphigus foliaceus. J Eur Acad Dermatol Venereol. 2004;18:636-637.
- Zaraa I, El Euch D, Kort R, et al. Localized pemphigus: a report of three cases. Int J Dermatol 2010;49:715-716.
- Ohata C, Akamatsu K, Imai N, et al. Localized pemphigus foliaceus exclusively involving the follicular infundibulum: a novel peau d’orange appearance. Eur J Dermatol. 2011;21:392-395.
- Maderal AD, Miner A, Nousari C, et al. Localized pemphigus foliaceus with unilateral facial involvement. Actas Dermosifiliogr. 2014;105:413-417.
- Cram DL, Winkelmann RK. Ultraviolet-induced acantholysis in pemphigus. Arch Dermatol. 1965;92:7-13.
- Kano Y, Shimosegawa M, Mizukawa Y, et al. Pemphigus foliaceus induced by exposure to sunlight. Dermatology. 2000;201:132-138.
- Lebe B, Gül Nıflıoğlu G, Seyrek S, et al. Evaluation of clinical and histopathologic/direct immunofluorescence diagnosis in autoimmune vesiculobullous dermatitis: utility of direct immunofluorescence. Turk Patoloji Derg. 2012;28:11-16.
- Joly P, Litrowski N. Pemphigus group (vulgaris, vegetans, foliaceus, herpetiformis, brasiliensis). Clin Dermatol. 2011;29:432-436.
- Ishii K, Amagai M, Hall RP, et al. Characterization of autoantibodies in pemphigus using antigen specific enzyme-linked immunosorbent assays with baculovirus-expressed recombinant desmogleins. J Immunol. 1997;159:2010-2017.
- Ng PP, Thng ST, Mohamed K, et al. Comparison of desmoglein ELISA and indirect immunofluorescence using two substrates (monkey esophagus and normal human skin) in the diagnosis of pemphigus. Australas J Dermatol. 2005;46:239-241.
Practice Points
- The diagnosis of pemphigus foliceus is challenging, as the clinical presentation simulates various entities.
- Clinicopathological correlation is important. If pathology and other diagnostics do not support clinical findings, trust your clinical assessment and consider repeating or adjusting the workup.
Severe Henoch-Schönlein Purpura Complicating Infliximab Therapy for Ulcerative Colitis
To the Editor:
Anti–tumor necrosis factor (TNF) α treatments have radically improved the management of chronic inflammatory conditions, including rheumatoid arthritis, ankylosing spondylitis, psoriasis and psoriatic arthritis, and bowel diseases (eg, Crohn disease, ulcerative colitis [UC]). Because the number of patients treated with these agents has increased, uncommon adverse reactions have increasingly occurred. Cutaneous adverse reactions that have been reported with anti-TNF agents include immediate injection-site reaction, systemic infusion reactions, and delayed reactions.1 Among the delayed adverse reactions, psoriatic and eczematous eruptions as well as cutaneous infections are the most common, while cutaneous adverse effects related to an immune imbalance syndrome including vasculitis; lupuslike, lichenlike, and granulomatous eruptions; and skin cancer rarely are observed.1 Although most of the cutaneous adverse effects do not require anti-TNF treatment discontinuation and are resolved with symptomatic treatment, anti-TNF therapy must be stopped in more severe cases. We report the case of severe Henoch-Schönlein purpura (HSP) following treatment with infliximab.
A 46-year-old man who was a nonsmoker with quiescent UC on infliximab for 30 months presented with palpable necrotic purpura on both legs (Figure) and arms as well as the abdomen of 10 days’ duration, along with diffuse joint pain and swelling. He had no history of infectious or gastrointestinal symptoms. The last infliximab infusion was performed 6 weeks prior to developing the purpura. His UC was diagnosed 10 years prior to the current presentation and was not associated with any extragastrointestinal manifestations. Since diagnosis, UC had failed to respond to therapies such as azathioprine, cyclosporine, and purinethol. The complete blood cell count was normal. The C-reactive protein level was 18.7 mg/L (reference range, <5 mg/L) and the erythrocyte sedimentation rate was 30 mm/h (reference range, 0–20 mm/h). Electrolytes, urea, creatinine clearance, and liver function were normal, and a chest radiograph and radiographs of the swollen joints were unremarkable. The total IgA level was elevated at 4 g/L (reference range, 0.7–4 g/L), with IgG and IgM levels within reference range. There was no hematuria or proteinuria on urinalysis. Tests for antinuclear antibodies, rheumatoid factor, circulating immune complexes, and antineutrophil cytoplasmic antibody were negative. Total complement, C3, and C4 levels also were normal. A skin biopsy confirmed a leukocytoclastic vasculitis of small vessels with C3 deposition. Serologic tests for hepatitis B virus, hepatitis C virus, and human immunodeficiency virus were negative. Based on these findings, the diagnosis of HSP was made. Systemic corticosteroids—120 mg daily of intravenous methylprednisolone for 3 days, followed by 1 mg/kg daily of oral prednisone for 2 weeks—were then introduced with rapid clinical improvement. Henoch-Schönlein purpura and joint symptoms completely resolved, but UC relapsed with bloody diarrhea and severe abdominal pain. Oral prednisone was maintained (1 mg/kg daily). Because of the severity of cutaneous vasculitis (HSP), a multidisciplinary decision was taken to definitively stop the anti-TNF agents and to first add azathioprine (2 mg/kg daily for 2 months), then subcutaneous methotrexate (25 mg weekly). Colonoscopy did not show any dysplasia or adenocarcinoma and confirmed the diagnosis of UC. After 6 months of combined therapy, UC was still active and we decided to perform a total colectomy with ileostomy formation. Complete remission of UC was obtained and maintained after 28 months of follow-up.
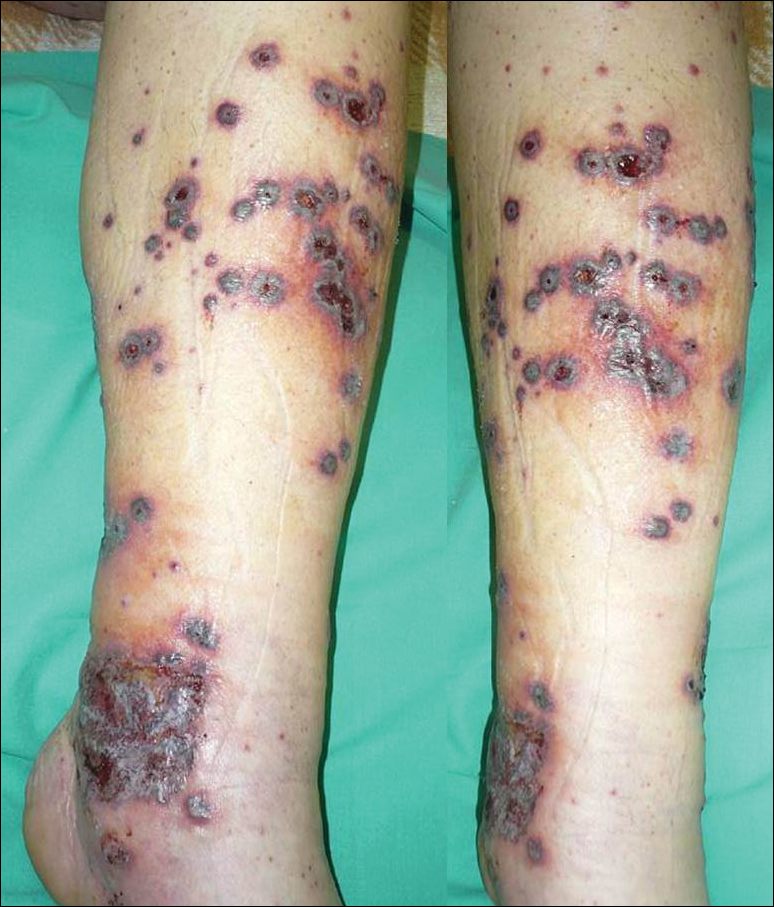
Henoch-Schönlein purpura is a multisystem small vessel leukocytoclastic vasculitis with the deposition of immune complexes containing IgA. Clinical manifestations may include palpable purpura, arthritis, enteritis, and nephritis. Henoch-Schönlein purpura usually affects children. Adult onset is rare but associated with more severe symptoms and a poor prognosis.2 The criteria for HSP, as defined by the American College of Rheumatology,3 include palpable purpura, 20 years or younger at disease onset, bowel angina, and presence of vascular wall granulocytes on biopsy. At least 2 of these criteria are required for HSP diagnosis. Various viral or bacterial infections and drugs can trigger HSP, which also can be associated with autoinflammatory or autoimmune diseases. The association of HSP and UC is a rare event, as demonstrated by de Oliveira et al.4 Although only 2 cases of cutaneous vasculitis mimicking HSP have been described in UC,4 we cannot exclude a possible association between HSP and UC. However, our patient had UC for 10 years and never had clinical manifestations of vasculitis.
There are 5 reports of HSP following etanercept5,6 or adalimumab7-9 therapy and 1 following infliximab therapy.10 In all cases, HSP occurred after several months of anti-TNF therapy. However, there also are reports of cutaneous vasculitis associated with arthralgia and glomerulonephritis that resolved after withdrawal of anti-TNF agents.11,12 It is possible that some of these reactions may have been manifestations of undiagnosed HSP. In a series of 113 patients who developed cutaneous vasculitis after anti-TNF agents, visceral vasculitis was observed in 24% of patients. Treatment of vasculitis involved withdrawal of the anti-TNF therapy in 101 cases (89%).13 In these UC patients with few therapeutic alternatives, the continuation of anti-TNF agents should be discussed. In the previous series,13 of 16 patients who were rechallenged with the same or a different TNF antagonist, 12 (75%) experienced vasculitis relapse, suggesting a class effect of TNF inhibition. Because of the severity of cutaneous vasculitis and as previously suggested in a recent analytical and comprehensive overview on paradoxical reactions under TNF blockers,1 we decided not to re-expose our patient to infliximab or to other anti-TNF agents.
In conclusion, HSP may occur during anti-TNF therapy and physicians need to be aware of this potentially serious complication.
- Toussirot É, Aubin F. Paradoxical reactions under TNF-α blocking agents and other biological agents given for chronic immune-mediated diseases: an analytical and comprehensive overview. RMD Open. 2016;2:e000239.
- Saulsbury FT. Henoch-Schönlein purpura. Curr Opin Rheumatol. 2001;13:35-40.
- Ortiz-Sanjuán F, Blanco R, Hernández JL, et al. Applicability of the 2006 European League Against Rheumatism (EULAR) criteria for the classification ofHenoch-Schönlein purpura. an analysis based on 766 patients with cutaneous vasculitis. Clin Exp Rheumatol. 2015;33(2, suppl 89):S44-S47.
- de Oliveira GT, Martins SS, Deboni M, et al. Cutaneous vasculitis in ulcerative colitis mimicking Henoch-Schönlein purpura [published online May 22, 2012]. J Crohns Colitis. 2013;7:e69-e73.
- Marques I, Lagos A, Reis J, et al. Reversible Henoch-Schönlein purpura complicating adalimumab therapy. J Crohns Colitis. 2012;6:796-799.
- Rahman FZ, Takhar GK, Roy O, et al. Henoch-Schönlein purpura complicating adalimumab therapy for Crohn’s disease. World J Gastrointest Pharmacol Ther. 2010;1:119-122.
- Lee A, Kasama R, Evangelisto A, et al. Henoch-Schönlein purpura after etanercept therapy for psoriasis. J Clin Rheumatol. 2006;12:249-251.
- Duffy TN, Genta M, Moll S, et al. Henoch Schönlein purpura following etanercept treatment of rheumatoid arthritis. Clin Exp Rheumatol. 2006;24(2, suppl 41):S106.
- LaConti JJ, Donet JA, Cho-Vega JH, et al. Henoch-Schönlein purpura with adalimumab therapy for ulcerative colitis: a case report and review of the literature. Case Rep Rheumatol. 2016:2812980.
- Nobile S, Catassi C, Felici L. Herpes zoster infection followed by Henoch-Schönlein purpura in a girl receiving infliximab for ulcerative colitis. J Clin Rheumatol. 2009;15:101.
- Mohan N, Edwards ET, Cupps TR, et al. Leukocytoclastic vasculitis associated with tumor necrosis factor-alpha blocking agents. J Rheumatol. 2004;31:1955-1958.
- Simms R, Kipgen D, Dahill S, et al. ANCA-associated renal vasculitis following anti-tumor necrosis factor alpha therapy. Am J Kidney Dis. 2008;51:e11-e14.
- Ramos-Casals M, Brito-Zerón P, Muñoz S, et al. Autoimmune diseases induced by TNF-targeted therapies: analysis of 233 cases. Medicine (Baltimore). 2007;86:242-251.
To the Editor:
Anti–tumor necrosis factor (TNF) α treatments have radically improved the management of chronic inflammatory conditions, including rheumatoid arthritis, ankylosing spondylitis, psoriasis and psoriatic arthritis, and bowel diseases (eg, Crohn disease, ulcerative colitis [UC]). Because the number of patients treated with these agents has increased, uncommon adverse reactions have increasingly occurred. Cutaneous adverse reactions that have been reported with anti-TNF agents include immediate injection-site reaction, systemic infusion reactions, and delayed reactions.1 Among the delayed adverse reactions, psoriatic and eczematous eruptions as well as cutaneous infections are the most common, while cutaneous adverse effects related to an immune imbalance syndrome including vasculitis; lupuslike, lichenlike, and granulomatous eruptions; and skin cancer rarely are observed.1 Although most of the cutaneous adverse effects do not require anti-TNF treatment discontinuation and are resolved with symptomatic treatment, anti-TNF therapy must be stopped in more severe cases. We report the case of severe Henoch-Schönlein purpura (HSP) following treatment with infliximab.
A 46-year-old man who was a nonsmoker with quiescent UC on infliximab for 30 months presented with palpable necrotic purpura on both legs (Figure) and arms as well as the abdomen of 10 days’ duration, along with diffuse joint pain and swelling. He had no history of infectious or gastrointestinal symptoms. The last infliximab infusion was performed 6 weeks prior to developing the purpura. His UC was diagnosed 10 years prior to the current presentation and was not associated with any extragastrointestinal manifestations. Since diagnosis, UC had failed to respond to therapies such as azathioprine, cyclosporine, and purinethol. The complete blood cell count was normal. The C-reactive protein level was 18.7 mg/L (reference range, <5 mg/L) and the erythrocyte sedimentation rate was 30 mm/h (reference range, 0–20 mm/h). Electrolytes, urea, creatinine clearance, and liver function were normal, and a chest radiograph and radiographs of the swollen joints were unremarkable. The total IgA level was elevated at 4 g/L (reference range, 0.7–4 g/L), with IgG and IgM levels within reference range. There was no hematuria or proteinuria on urinalysis. Tests for antinuclear antibodies, rheumatoid factor, circulating immune complexes, and antineutrophil cytoplasmic antibody were negative. Total complement, C3, and C4 levels also were normal. A skin biopsy confirmed a leukocytoclastic vasculitis of small vessels with C3 deposition. Serologic tests for hepatitis B virus, hepatitis C virus, and human immunodeficiency virus were negative. Based on these findings, the diagnosis of HSP was made. Systemic corticosteroids—120 mg daily of intravenous methylprednisolone for 3 days, followed by 1 mg/kg daily of oral prednisone for 2 weeks—were then introduced with rapid clinical improvement. Henoch-Schönlein purpura and joint symptoms completely resolved, but UC relapsed with bloody diarrhea and severe abdominal pain. Oral prednisone was maintained (1 mg/kg daily). Because of the severity of cutaneous vasculitis (HSP), a multidisciplinary decision was taken to definitively stop the anti-TNF agents and to first add azathioprine (2 mg/kg daily for 2 months), then subcutaneous methotrexate (25 mg weekly). Colonoscopy did not show any dysplasia or adenocarcinoma and confirmed the diagnosis of UC. After 6 months of combined therapy, UC was still active and we decided to perform a total colectomy with ileostomy formation. Complete remission of UC was obtained and maintained after 28 months of follow-up.

Henoch-Schönlein purpura is a multisystem small vessel leukocytoclastic vasculitis with the deposition of immune complexes containing IgA. Clinical manifestations may include palpable purpura, arthritis, enteritis, and nephritis. Henoch-Schönlein purpura usually affects children. Adult onset is rare but associated with more severe symptoms and a poor prognosis.2 The criteria for HSP, as defined by the American College of Rheumatology,3 include palpable purpura, 20 years or younger at disease onset, bowel angina, and presence of vascular wall granulocytes on biopsy. At least 2 of these criteria are required for HSP diagnosis. Various viral or bacterial infections and drugs can trigger HSP, which also can be associated with autoinflammatory or autoimmune diseases. The association of HSP and UC is a rare event, as demonstrated by de Oliveira et al.4 Although only 2 cases of cutaneous vasculitis mimicking HSP have been described in UC,4 we cannot exclude a possible association between HSP and UC. However, our patient had UC for 10 years and never had clinical manifestations of vasculitis.
There are 5 reports of HSP following etanercept5,6 or adalimumab7-9 therapy and 1 following infliximab therapy.10 In all cases, HSP occurred after several months of anti-TNF therapy. However, there also are reports of cutaneous vasculitis associated with arthralgia and glomerulonephritis that resolved after withdrawal of anti-TNF agents.11,12 It is possible that some of these reactions may have been manifestations of undiagnosed HSP. In a series of 113 patients who developed cutaneous vasculitis after anti-TNF agents, visceral vasculitis was observed in 24% of patients. Treatment of vasculitis involved withdrawal of the anti-TNF therapy in 101 cases (89%).13 In these UC patients with few therapeutic alternatives, the continuation of anti-TNF agents should be discussed. In the previous series,13 of 16 patients who were rechallenged with the same or a different TNF antagonist, 12 (75%) experienced vasculitis relapse, suggesting a class effect of TNF inhibition. Because of the severity of cutaneous vasculitis and as previously suggested in a recent analytical and comprehensive overview on paradoxical reactions under TNF blockers,1 we decided not to re-expose our patient to infliximab or to other anti-TNF agents.
In conclusion, HSP may occur during anti-TNF therapy and physicians need to be aware of this potentially serious complication.
To the Editor:
Anti–tumor necrosis factor (TNF) α treatments have radically improved the management of chronic inflammatory conditions, including rheumatoid arthritis, ankylosing spondylitis, psoriasis and psoriatic arthritis, and bowel diseases (eg, Crohn disease, ulcerative colitis [UC]). Because the number of patients treated with these agents has increased, uncommon adverse reactions have increasingly occurred. Cutaneous adverse reactions that have been reported with anti-TNF agents include immediate injection-site reaction, systemic infusion reactions, and delayed reactions.1 Among the delayed adverse reactions, psoriatic and eczematous eruptions as well as cutaneous infections are the most common, while cutaneous adverse effects related to an immune imbalance syndrome including vasculitis; lupuslike, lichenlike, and granulomatous eruptions; and skin cancer rarely are observed.1 Although most of the cutaneous adverse effects do not require anti-TNF treatment discontinuation and are resolved with symptomatic treatment, anti-TNF therapy must be stopped in more severe cases. We report the case of severe Henoch-Schönlein purpura (HSP) following treatment with infliximab.
A 46-year-old man who was a nonsmoker with quiescent UC on infliximab for 30 months presented with palpable necrotic purpura on both legs (Figure) and arms as well as the abdomen of 10 days’ duration, along with diffuse joint pain and swelling. He had no history of infectious or gastrointestinal symptoms. The last infliximab infusion was performed 6 weeks prior to developing the purpura. His UC was diagnosed 10 years prior to the current presentation and was not associated with any extragastrointestinal manifestations. Since diagnosis, UC had failed to respond to therapies such as azathioprine, cyclosporine, and purinethol. The complete blood cell count was normal. The C-reactive protein level was 18.7 mg/L (reference range, <5 mg/L) and the erythrocyte sedimentation rate was 30 mm/h (reference range, 0–20 mm/h). Electrolytes, urea, creatinine clearance, and liver function were normal, and a chest radiograph and radiographs of the swollen joints were unremarkable. The total IgA level was elevated at 4 g/L (reference range, 0.7–4 g/L), with IgG and IgM levels within reference range. There was no hematuria or proteinuria on urinalysis. Tests for antinuclear antibodies, rheumatoid factor, circulating immune complexes, and antineutrophil cytoplasmic antibody were negative. Total complement, C3, and C4 levels also were normal. A skin biopsy confirmed a leukocytoclastic vasculitis of small vessels with C3 deposition. Serologic tests for hepatitis B virus, hepatitis C virus, and human immunodeficiency virus were negative. Based on these findings, the diagnosis of HSP was made. Systemic corticosteroids—120 mg daily of intravenous methylprednisolone for 3 days, followed by 1 mg/kg daily of oral prednisone for 2 weeks—were then introduced with rapid clinical improvement. Henoch-Schönlein purpura and joint symptoms completely resolved, but UC relapsed with bloody diarrhea and severe abdominal pain. Oral prednisone was maintained (1 mg/kg daily). Because of the severity of cutaneous vasculitis (HSP), a multidisciplinary decision was taken to definitively stop the anti-TNF agents and to first add azathioprine (2 mg/kg daily for 2 months), then subcutaneous methotrexate (25 mg weekly). Colonoscopy did not show any dysplasia or adenocarcinoma and confirmed the diagnosis of UC. After 6 months of combined therapy, UC was still active and we decided to perform a total colectomy with ileostomy formation. Complete remission of UC was obtained and maintained after 28 months of follow-up.

Henoch-Schönlein purpura is a multisystem small vessel leukocytoclastic vasculitis with the deposition of immune complexes containing IgA. Clinical manifestations may include palpable purpura, arthritis, enteritis, and nephritis. Henoch-Schönlein purpura usually affects children. Adult onset is rare but associated with more severe symptoms and a poor prognosis.2 The criteria for HSP, as defined by the American College of Rheumatology,3 include palpable purpura, 20 years or younger at disease onset, bowel angina, and presence of vascular wall granulocytes on biopsy. At least 2 of these criteria are required for HSP diagnosis. Various viral or bacterial infections and drugs can trigger HSP, which also can be associated with autoinflammatory or autoimmune diseases. The association of HSP and UC is a rare event, as demonstrated by de Oliveira et al.4 Although only 2 cases of cutaneous vasculitis mimicking HSP have been described in UC,4 we cannot exclude a possible association between HSP and UC. However, our patient had UC for 10 years and never had clinical manifestations of vasculitis.
There are 5 reports of HSP following etanercept5,6 or adalimumab7-9 therapy and 1 following infliximab therapy.10 In all cases, HSP occurred after several months of anti-TNF therapy. However, there also are reports of cutaneous vasculitis associated with arthralgia and glomerulonephritis that resolved after withdrawal of anti-TNF agents.11,12 It is possible that some of these reactions may have been manifestations of undiagnosed HSP. In a series of 113 patients who developed cutaneous vasculitis after anti-TNF agents, visceral vasculitis was observed in 24% of patients. Treatment of vasculitis involved withdrawal of the anti-TNF therapy in 101 cases (89%).13 In these UC patients with few therapeutic alternatives, the continuation of anti-TNF agents should be discussed. In the previous series,13 of 16 patients who were rechallenged with the same or a different TNF antagonist, 12 (75%) experienced vasculitis relapse, suggesting a class effect of TNF inhibition. Because of the severity of cutaneous vasculitis and as previously suggested in a recent analytical and comprehensive overview on paradoxical reactions under TNF blockers,1 we decided not to re-expose our patient to infliximab or to other anti-TNF agents.
In conclusion, HSP may occur during anti-TNF therapy and physicians need to be aware of this potentially serious complication.
- Toussirot É, Aubin F. Paradoxical reactions under TNF-α blocking agents and other biological agents given for chronic immune-mediated diseases: an analytical and comprehensive overview. RMD Open. 2016;2:e000239.
- Saulsbury FT. Henoch-Schönlein purpura. Curr Opin Rheumatol. 2001;13:35-40.
- Ortiz-Sanjuán F, Blanco R, Hernández JL, et al. Applicability of the 2006 European League Against Rheumatism (EULAR) criteria for the classification ofHenoch-Schönlein purpura. an analysis based on 766 patients with cutaneous vasculitis. Clin Exp Rheumatol. 2015;33(2, suppl 89):S44-S47.
- de Oliveira GT, Martins SS, Deboni M, et al. Cutaneous vasculitis in ulcerative colitis mimicking Henoch-Schönlein purpura [published online May 22, 2012]. J Crohns Colitis. 2013;7:e69-e73.
- Marques I, Lagos A, Reis J, et al. Reversible Henoch-Schönlein purpura complicating adalimumab therapy. J Crohns Colitis. 2012;6:796-799.
- Rahman FZ, Takhar GK, Roy O, et al. Henoch-Schönlein purpura complicating adalimumab therapy for Crohn’s disease. World J Gastrointest Pharmacol Ther. 2010;1:119-122.
- Lee A, Kasama R, Evangelisto A, et al. Henoch-Schönlein purpura after etanercept therapy for psoriasis. J Clin Rheumatol. 2006;12:249-251.
- Duffy TN, Genta M, Moll S, et al. Henoch Schönlein purpura following etanercept treatment of rheumatoid arthritis. Clin Exp Rheumatol. 2006;24(2, suppl 41):S106.
- LaConti JJ, Donet JA, Cho-Vega JH, et al. Henoch-Schönlein purpura with adalimumab therapy for ulcerative colitis: a case report and review of the literature. Case Rep Rheumatol. 2016:2812980.
- Nobile S, Catassi C, Felici L. Herpes zoster infection followed by Henoch-Schönlein purpura in a girl receiving infliximab for ulcerative colitis. J Clin Rheumatol. 2009;15:101.
- Mohan N, Edwards ET, Cupps TR, et al. Leukocytoclastic vasculitis associated with tumor necrosis factor-alpha blocking agents. J Rheumatol. 2004;31:1955-1958.
- Simms R, Kipgen D, Dahill S, et al. ANCA-associated renal vasculitis following anti-tumor necrosis factor alpha therapy. Am J Kidney Dis. 2008;51:e11-e14.
- Ramos-Casals M, Brito-Zerón P, Muñoz S, et al. Autoimmune diseases induced by TNF-targeted therapies: analysis of 233 cases. Medicine (Baltimore). 2007;86:242-251.
- Toussirot É, Aubin F. Paradoxical reactions under TNF-α blocking agents and other biological agents given for chronic immune-mediated diseases: an analytical and comprehensive overview. RMD Open. 2016;2:e000239.
- Saulsbury FT. Henoch-Schönlein purpura. Curr Opin Rheumatol. 2001;13:35-40.
- Ortiz-Sanjuán F, Blanco R, Hernández JL, et al. Applicability of the 2006 European League Against Rheumatism (EULAR) criteria for the classification ofHenoch-Schönlein purpura. an analysis based on 766 patients with cutaneous vasculitis. Clin Exp Rheumatol. 2015;33(2, suppl 89):S44-S47.
- de Oliveira GT, Martins SS, Deboni M, et al. Cutaneous vasculitis in ulcerative colitis mimicking Henoch-Schönlein purpura [published online May 22, 2012]. J Crohns Colitis. 2013;7:e69-e73.
- Marques I, Lagos A, Reis J, et al. Reversible Henoch-Schönlein purpura complicating adalimumab therapy. J Crohns Colitis. 2012;6:796-799.
- Rahman FZ, Takhar GK, Roy O, et al. Henoch-Schönlein purpura complicating adalimumab therapy for Crohn’s disease. World J Gastrointest Pharmacol Ther. 2010;1:119-122.
- Lee A, Kasama R, Evangelisto A, et al. Henoch-Schönlein purpura after etanercept therapy for psoriasis. J Clin Rheumatol. 2006;12:249-251.
- Duffy TN, Genta M, Moll S, et al. Henoch Schönlein purpura following etanercept treatment of rheumatoid arthritis. Clin Exp Rheumatol. 2006;24(2, suppl 41):S106.
- LaConti JJ, Donet JA, Cho-Vega JH, et al. Henoch-Schönlein purpura with adalimumab therapy for ulcerative colitis: a case report and review of the literature. Case Rep Rheumatol. 2016:2812980.
- Nobile S, Catassi C, Felici L. Herpes zoster infection followed by Henoch-Schönlein purpura in a girl receiving infliximab for ulcerative colitis. J Clin Rheumatol. 2009;15:101.
- Mohan N, Edwards ET, Cupps TR, et al. Leukocytoclastic vasculitis associated with tumor necrosis factor-alpha blocking agents. J Rheumatol. 2004;31:1955-1958.
- Simms R, Kipgen D, Dahill S, et al. ANCA-associated renal vasculitis following anti-tumor necrosis factor alpha therapy. Am J Kidney Dis. 2008;51:e11-e14.
- Ramos-Casals M, Brito-Zerón P, Muñoz S, et al. Autoimmune diseases induced by TNF-targeted therapies: analysis of 233 cases. Medicine (Baltimore). 2007;86:242-251.
Practice Points
- Cutaneous adverse effects may occur in approximately 20% of patients treated with anti–tumor necrosis factor (TNF) drugs.
- Henoch-Schönlein purpura (HSP), a small-vessel vasculitis, is an extremely rare complication of anti-TNF treatment.
- Although most cutaneous adverse effects do not require anti-TNF treatment discontinuation and are resolved with symptomatic treatment, anti-TNF therapy must be stopped in more severe cases.
Questioning the Specificity and Sensitivity of ELISA for Bullous Pemphigoid Diagnosis
Bullous pemphigoid (BP) is the most common autoimmune blistering disease. The classic presentation of BP is a generalized, pruritic, bullous eruption in elderly patients, which is occasionally preceded by an urticarial prodrome. Immunopathologically, BP is characterized by IgG and sometimes IgE autoantibodies that target basement membrane zone proteins BP180 and BP230 of the epidermis.1
The diagnosis of BP should be suspected when an elderly patient presents with tense blisters and can be confirmed via diagnostic testing, including tissue histology and direct immunofluorescence (DIF) as the gold standard, as well as indirect immunofluorescence (IIF), enzyme-linked immunosorbent assay (ELISA), and most recently biochip technology as supportive tests.2 Since its advent, ELISA has gained popularity as a trustworthy diagnostic test for BP. The specificity of ELISA for BP diagnosis is reported to be 98% to 100%, which leads clinicians to believe that a positive ELISA equals certain diagnosis of BP; however, misdiagnosis of BP based on a positive ELISA result can occur.3-13 The treatment of BP often involves lifelong immunosuppressive therapy. Complications of immunosuppressive therapy contribute to morbidity and mortality in these patients, thus an accurate diagnosis is paramount before introducing therapy.14
We present the case of a 74-year-old man with a history of a pruritic nonbullous eruption who was diagnosed with BP and treated for 3 years based on positive ELISA results in the absence of confirmatory histology or DIF.
Case Report
A 74-year-old man with diabetes mellitus, hypertension, hyperlipidemia, benign prostatic hypertrophy, and obstructive sleep apnea presented for further evaluation and confirmation of a prior diagnosis of BP by an outside dermatologist. He reported a pruritic rash on the trunk, back, and extremities of 3 years’ duration. He denied occurrence of blisters at any time.
On presentation to an outside dermatologist 3 years ago, a biopsy was performed along with serologic studies due to the patient’s age and the possibility of an urticarial prodrome in BP. The biopsy revealed epidermal acanthosis, subepidermal separation, and a perivascular and interstitial infiltrate of lymphocytes and eosinophils in the papillary dermis. Direct immunofluorescence was nondiagnostic with a weak discontinuous pattern of IgG and IgA linearly along the basement membrane zone as well as few scattered and clumped cytoid bodies of IgM and IgA. Indirect immunofluoresence revealed a positive IgG titer of 1:40 on monkey esophagus substrate and a positive epidermal pattern on human split-skin substrate with a titer of 1:80. An ELISA for IgG autoantibodies against BP180 and BP230 yielded 15 U and 6 U, respectively (cut off value, 9 U). Based on the positive ELISA for IgG against BP180, a diagnosis of BP was made.
Over the following 3 years, the treatment included prednisone, tetracycline, nicotinamide, doxycycline, and dapsone. Therapy was suboptimal due to the patient’s comorbidities and socioeconomic status. Poorly controlled diabetes mellitus precluded consistent use of prednisone as recommended for BP. Tetracycline and nicotinamide were transiently effective in controlling the patient’s symptoms but were discontinued due to changes in his health insurance. Doxycycline and dapsone were ineffective. Throughout this 3-year period, the patient remained blister free, but the pruritic eruption was persistent.
The patient presented to our clinic due to his frustration with the lack of improvement and doubts about the BP diagnosis given the persistent absence of bullous lesions. Physical examination revealed numerous eroded, scaly, crusted papules on erythematous edematous plaques on all extremities, trunk, and back (Figure 1). The head, neck, face, and oral mucosa were spared. His history and clinical findings were atypical for BP and skin biopsies were performed. Histology revealed epidermal erosion with parakeratosis, spongiosis, and superficial perivascular lymphocytic inflammation with rare eosinophils without subepidermal split (Figure 2). Direct immunofluorescence was negative for IgG, IgA, IgM, C3, and C1q. Additionally, further review of the initial histology by another dermatopathologist revealed that the subepidermal separation reported was more likely artifactual clefts. These findings were not consistent with BP.
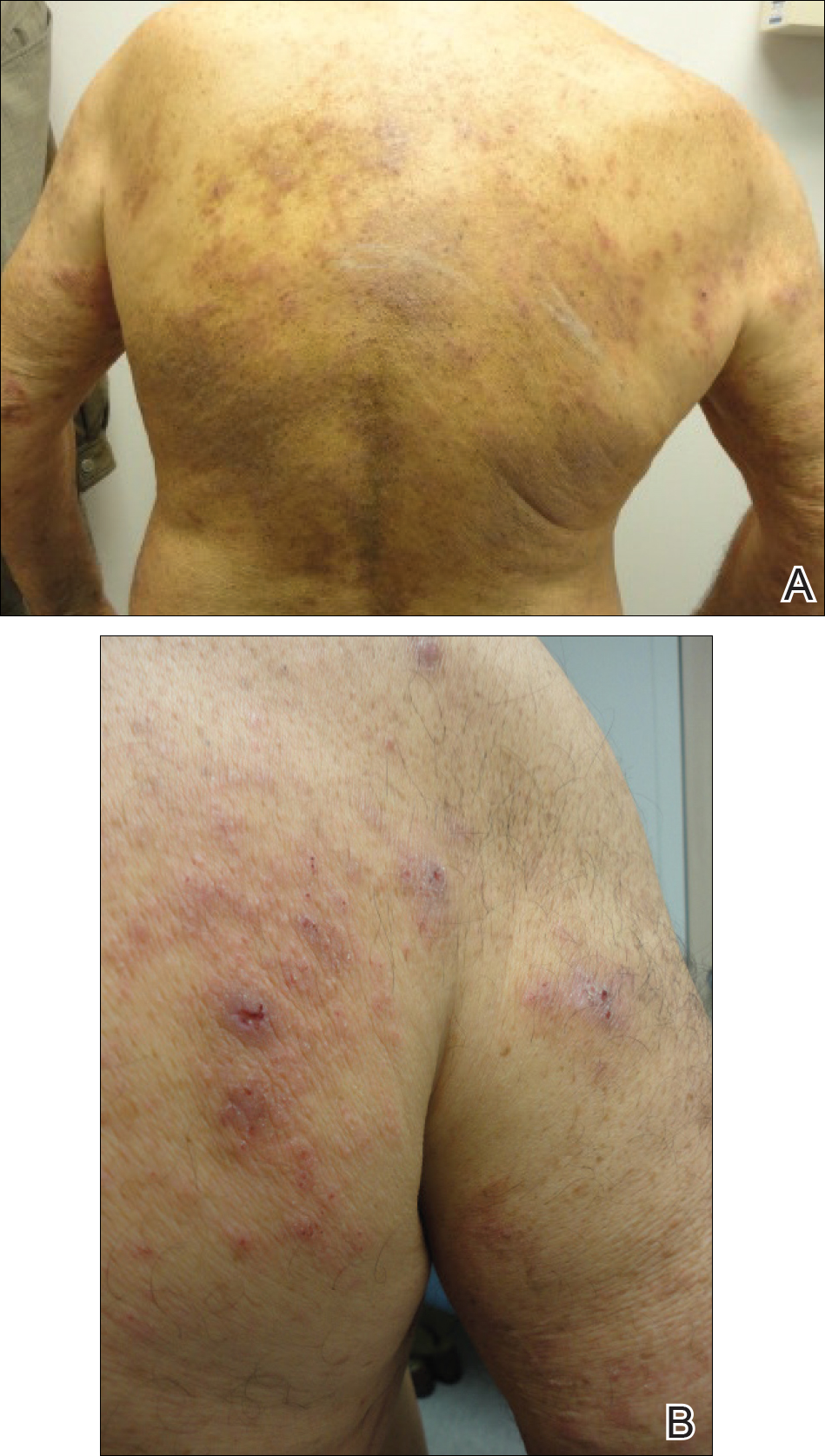

Given the patient’s clinical history, lack of bullae, and twice-negative DIF, the diagnosis was determined to be more consistent with eczematous spongiotic dermatitis. He refused a referral for phototherapy due to scheduling inconvenience. The patient was started on cyclosporine 0.5 mg/kg twice daily. After 10 days of treatment, he returned for follow-up and reported notable improvement in the pruritus. On physical examination, his dermatitis was improved with decreased erythema and inflammation.
The patient is being continued on extensive dry skin care with thick moisturizers and additional topical corticosteroid application on an as-needed basis.
Comment
Chronic immunosuppression contributes to morbidity and mortality in patients with BP; therefore, accurate diagnosis of BP is of utmost importance.14 A meta-analysis described ELISA as a test with high sensitivity and specificity (87% and 98%–100%, respectively) for diagnosis of BP.3 Nevertheless, there are opportunities for misdiagnosis using ELISA, as demonstrated in our case. To determine if the reported sensitivity and specificity of ELISA is accurate and reliable for clinical use, individual studies from the meta-analysis were reviewed.4,5,7-10,13,15 Issues identified in our review included dissimilar diagnostic procedures and patient populations among individual studies, several reports of positive ELISA in patients without BP, and a lack of explanation for these false-positive results.
There are notable differences in diagnostic procedures and patient populations among reports that establish the sensitivity and specificity of ELISA for BP diagnosis.3-13 Studies have detected IgG that targets the NC16A domain of the BP180 kD antigen, the C-terminal of the BP180 kD antigen, or the entire ectodomain of the BP180 kD antigen. Study patient populations varied in disease activity, stage, and treatment. Control patients included healthy patients as well as those with many dermatoses, including pemphigus vulgaris, systemic scleroderma, systemic lupus erythematosus, rheumatoid arthritis, lichen planus, and discoid lupus erythematosus.3-13 Due to these differences between individual studies, we believe the results that determine the overall sensitivity and specificity of ELISA for BP diagnosis must be interpreted with caution. For ELISA statistics to be clinically applicable to a specific patient, he/she should be similar to the patients studied. Therefore, we believe each study must be evaluated individually for applicability, given the differences that exist between them.
Furthermore, there have been several reports of false-positive ELISA results in patients with other dermatologic disorders, specifically in elderly patients with pruritus who do not fulfill clinical criteria for diagnosis with BP.16-18 In a population of elderly patients with pruritus for which no specific dermatological or systemic cause was identified, Hofmann et al18 found that 12% (3/25) of patients showed IgG reactivity to BP180 despite having negative DIF results. In another study of elderly patients with pruritic dermatoses, Feliciani et al17 found that 33% (5/15) of patients had IgG reactivity against BP230 or BP180, though they did not fulfill BP criteria based on clinical presentation and showed negative DIF and IIF results. These findings suggest that IgG reactivity against BP autoantibodies as determined by ELISA is not uncommon in pruritic diseases of the elderly.
Explanations for false-positive ELISA results were rare. A few authors suggested that false-positives could be attributed to an excessively low cutoff value,7-9 which was consistent with reports that the titer of autoantibodies to BP180 correlates with disease severity, suggesting that the higher titer of antibodies correlates with more severe disease and likely more accurate diagnosis.10,19,20 It is important to consider that patients who have low titers of BP180 autoantibodies with inconsistent clinical characteristics and DIF results may not truly have BP. Furthermore, to determine the clinical value of ELISA in identifying patients in the initial phase of BP, sera of BP patients should be compared with sera of elderly patients with pruritic skin disorders because they comprise the patient population that often requires diagnosis.18
Given the issues identified in our review of the literature, the published sensitivity and specificity of ELISA for BP diagnosis are likely overstated. In conclusion, ELISA should not be relied on as a single criterion adequate for diagnosis of BP.12,21 Rather, the diagnosis of BP can be obtained with a positive predictive value of 95% when a patient meets 3 of 4 clinical criteria (ie, absence of atrophic scars, absence of head and neck involvement, absence of mucosal involvement, and older than 70 years) and demonstrates linear deposits of predominantly IgG and/or C3 along the basement membrane zone of a perilesional biopsy on DIF.15 The gold standard for diagnosis of BP remains clinical presentation along with DIF, which can be supported by histology, IIF, and ELISA.22
- Delaporte E, Dubost-Brama A, Ghohestani R, et al. IgE autoantibodies directed against the major bullous pemphigoid antigen in patients with a severe form of pemphigoid. J Immunol. 1996;157:3642-3647.
- Schmidt E, Zillikens D. Diagnosis and clinical severity markers of bullous pemphigoid. F1000 Med Rep. 2009;1:15.
- Tampoia M, Giavarina D, Di Giorgio C, et al. Diagnostic accuracy of enzyme-linked immunosorbent assays (ELISA) to detect anti-skin autoantibodies in autoimmune blistering diseases: a systematic review and meta-analysis. Autoimmun Rev. 2012;12:121-126.
- Zillikens D, Mascaro JM, Rose PA, et al. A highly sensitive enzyme-linked immunosorbent assay for the detection of circulating anti-BP180 autoantibodies in patients with bullous pemphigoid. J Invest Dermatol. 1997;109:679-683.
- Sitaru C, Dahnrich C, Probst C, et al. Enzyme-linked immunosorbent assay using multimers of the 16th non-collagenous domain of the BP180 antigen for sensitive and specific detection of pemphigoid autoantibodies. Exp Dermatol. 2007;16:770-777.
- Yang B, Wang C, Chen S, et al. Evaluation of the combination of BP180-NC16a enzyme-linked immunosorbent assay and BP230 enzyme-linked immunosorbent assay in the diagnosis of bullous pemphigoid. Indian J Dermatol Venereol Leprol. 2012;78:722-727.
- Sakuma-Oyama Y, Powell AM, Oyama N, et al. Evaluation of a BP180-NC16a enzyme-linked immunosorbent assay in the initial diagnosis of bullous pemphigoid. Br J Dermatol. 2004;151:126-131.
- Tampoia M, Lattanzi V, Zucano A, et al. Evaluation of a new ELISA assay for detection of BP230 autoantibodies in bullous pemphigoid. Ann N Y Acad Sci. 2009;1173:15-20.
- Feng S, Lin L, Jin P, et al. Role of BP180NC16a-enzyme-linked immunosorbent assay (ELISA) in the diagnosis of bullous pemphigoid in China. Int J Dermatol. 2008;47:24-28.
- Kobayashi M, Amagai M, Kuroda-Kinoshita K, et al. BP180 ELISA using bacterial recombinant NC16a protein as a diagnostic and monitoring tool for bullous pemphigoid. J Dermatol Sci. 2002;30:224-232.
- Roussel A, Benichou J, Arivelo Randriamanantany Z, et al. Enzyme-linked immunosorbent assay for the combination of bullous pemphigoid antigens 1 and 2 in the diagnosis of bullous pemphigoid. Arch Dermatol. 2011;147:293-298.
- Chan, Lawrence S. ELISA instead of indirect IF in patients with BP. Arch Dermatol. 2011;147:291-292.
- Barnadas MA, Rubiales V, González J, et al. Enzyme-linked immunosorbent assay (ELISA) and indirect immunofluorescence testing in a bullous pemphigoid and pemphigoid gestationis. Int J Dermatol. 2008;47:1245-1249.
- Borradori L, Bernard P. Pemphigoid group. In: Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. New York, NY: Mosby; 2003:469.
- Vaillant L, Bernard P, Joly P, et al. Evaluation of clinical criteria for diagnosis of bullous pemphigoid. Arch Dermatol. 1998;134:1075-1080.
- Fania L, Caldarola G, Muller R, et al. IgE recognition of bullous pemphigoid (BP)180 and BP230 in BP patients and elderly individuals with pruritic dermatoses. Clin Immunol. 2012;143:236-245.
- Feliciani C, Caldarola G, Kneisel A, et al. IgG autoantibody reactivity against bullous pemphigoid (BP) 180 and BP230 in elderly patients with pruritic dermatoses. Br J Dermatol. 2009;61:306-312.
- Hofmann SC, Tamm K, Hertl M, et al. Diagnostic value of an enzyme-linked immunosorbent assay using BP180 recombinant proteins in elderly patients with pruritic skin disorders. Br J Dermatol. 2003;149:910-911.
- Schmidt E, Obe K, Brocker EB, et al. Serum levels of autoantibodies to BP180 correlate with disease activity in patients with bullous pemphigoid. Arch Dermatol. 2000;136:174-178.
- Feng S, Wu Q, Jin P, et al. Serum levels of autoantibodies to BP180 correlate with disease activity in patients with bullous pemphigoid. Int J Dermatol. 2008;47:225-228.
- Di Zenzo G, Joly P, Zambruno G, et al. Sensitivity of immunofluorescence studies vs enzyme-linked immunosorbent assay for diagnosis of bullous pemphigoid. Arch Dermatol. 2011;147:1454-1456.
- Schmidt E, Zillikens D. Modern diagnosis of autoimmune blistering skin diseases. Autoimmun Rev. 2010;10:84-89.
Bullous pemphigoid (BP) is the most common autoimmune blistering disease. The classic presentation of BP is a generalized, pruritic, bullous eruption in elderly patients, which is occasionally preceded by an urticarial prodrome. Immunopathologically, BP is characterized by IgG and sometimes IgE autoantibodies that target basement membrane zone proteins BP180 and BP230 of the epidermis.1
The diagnosis of BP should be suspected when an elderly patient presents with tense blisters and can be confirmed via diagnostic testing, including tissue histology and direct immunofluorescence (DIF) as the gold standard, as well as indirect immunofluorescence (IIF), enzyme-linked immunosorbent assay (ELISA), and most recently biochip technology as supportive tests.2 Since its advent, ELISA has gained popularity as a trustworthy diagnostic test for BP. The specificity of ELISA for BP diagnosis is reported to be 98% to 100%, which leads clinicians to believe that a positive ELISA equals certain diagnosis of BP; however, misdiagnosis of BP based on a positive ELISA result can occur.3-13 The treatment of BP often involves lifelong immunosuppressive therapy. Complications of immunosuppressive therapy contribute to morbidity and mortality in these patients, thus an accurate diagnosis is paramount before introducing therapy.14
We present the case of a 74-year-old man with a history of a pruritic nonbullous eruption who was diagnosed with BP and treated for 3 years based on positive ELISA results in the absence of confirmatory histology or DIF.
Case Report
A 74-year-old man with diabetes mellitus, hypertension, hyperlipidemia, benign prostatic hypertrophy, and obstructive sleep apnea presented for further evaluation and confirmation of a prior diagnosis of BP by an outside dermatologist. He reported a pruritic rash on the trunk, back, and extremities of 3 years’ duration. He denied occurrence of blisters at any time.
On presentation to an outside dermatologist 3 years ago, a biopsy was performed along with serologic studies due to the patient’s age and the possibility of an urticarial prodrome in BP. The biopsy revealed epidermal acanthosis, subepidermal separation, and a perivascular and interstitial infiltrate of lymphocytes and eosinophils in the papillary dermis. Direct immunofluorescence was nondiagnostic with a weak discontinuous pattern of IgG and IgA linearly along the basement membrane zone as well as few scattered and clumped cytoid bodies of IgM and IgA. Indirect immunofluoresence revealed a positive IgG titer of 1:40 on monkey esophagus substrate and a positive epidermal pattern on human split-skin substrate with a titer of 1:80. An ELISA for IgG autoantibodies against BP180 and BP230 yielded 15 U and 6 U, respectively (cut off value, 9 U). Based on the positive ELISA for IgG against BP180, a diagnosis of BP was made.
Over the following 3 years, the treatment included prednisone, tetracycline, nicotinamide, doxycycline, and dapsone. Therapy was suboptimal due to the patient’s comorbidities and socioeconomic status. Poorly controlled diabetes mellitus precluded consistent use of prednisone as recommended for BP. Tetracycline and nicotinamide were transiently effective in controlling the patient’s symptoms but were discontinued due to changes in his health insurance. Doxycycline and dapsone were ineffective. Throughout this 3-year period, the patient remained blister free, but the pruritic eruption was persistent.
The patient presented to our clinic due to his frustration with the lack of improvement and doubts about the BP diagnosis given the persistent absence of bullous lesions. Physical examination revealed numerous eroded, scaly, crusted papules on erythematous edematous plaques on all extremities, trunk, and back (Figure 1). The head, neck, face, and oral mucosa were spared. His history and clinical findings were atypical for BP and skin biopsies were performed. Histology revealed epidermal erosion with parakeratosis, spongiosis, and superficial perivascular lymphocytic inflammation with rare eosinophils without subepidermal split (Figure 2). Direct immunofluorescence was negative for IgG, IgA, IgM, C3, and C1q. Additionally, further review of the initial histology by another dermatopathologist revealed that the subepidermal separation reported was more likely artifactual clefts. These findings were not consistent with BP.


Given the patient’s clinical history, lack of bullae, and twice-negative DIF, the diagnosis was determined to be more consistent with eczematous spongiotic dermatitis. He refused a referral for phototherapy due to scheduling inconvenience. The patient was started on cyclosporine 0.5 mg/kg twice daily. After 10 days of treatment, he returned for follow-up and reported notable improvement in the pruritus. On physical examination, his dermatitis was improved with decreased erythema and inflammation.
The patient is being continued on extensive dry skin care with thick moisturizers and additional topical corticosteroid application on an as-needed basis.
Comment
Chronic immunosuppression contributes to morbidity and mortality in patients with BP; therefore, accurate diagnosis of BP is of utmost importance.14 A meta-analysis described ELISA as a test with high sensitivity and specificity (87% and 98%–100%, respectively) for diagnosis of BP.3 Nevertheless, there are opportunities for misdiagnosis using ELISA, as demonstrated in our case. To determine if the reported sensitivity and specificity of ELISA is accurate and reliable for clinical use, individual studies from the meta-analysis were reviewed.4,5,7-10,13,15 Issues identified in our review included dissimilar diagnostic procedures and patient populations among individual studies, several reports of positive ELISA in patients without BP, and a lack of explanation for these false-positive results.
There are notable differences in diagnostic procedures and patient populations among reports that establish the sensitivity and specificity of ELISA for BP diagnosis.3-13 Studies have detected IgG that targets the NC16A domain of the BP180 kD antigen, the C-terminal of the BP180 kD antigen, or the entire ectodomain of the BP180 kD antigen. Study patient populations varied in disease activity, stage, and treatment. Control patients included healthy patients as well as those with many dermatoses, including pemphigus vulgaris, systemic scleroderma, systemic lupus erythematosus, rheumatoid arthritis, lichen planus, and discoid lupus erythematosus.3-13 Due to these differences between individual studies, we believe the results that determine the overall sensitivity and specificity of ELISA for BP diagnosis must be interpreted with caution. For ELISA statistics to be clinically applicable to a specific patient, he/she should be similar to the patients studied. Therefore, we believe each study must be evaluated individually for applicability, given the differences that exist between them.
Furthermore, there have been several reports of false-positive ELISA results in patients with other dermatologic disorders, specifically in elderly patients with pruritus who do not fulfill clinical criteria for diagnosis with BP.16-18 In a population of elderly patients with pruritus for which no specific dermatological or systemic cause was identified, Hofmann et al18 found that 12% (3/25) of patients showed IgG reactivity to BP180 despite having negative DIF results. In another study of elderly patients with pruritic dermatoses, Feliciani et al17 found that 33% (5/15) of patients had IgG reactivity against BP230 or BP180, though they did not fulfill BP criteria based on clinical presentation and showed negative DIF and IIF results. These findings suggest that IgG reactivity against BP autoantibodies as determined by ELISA is not uncommon in pruritic diseases of the elderly.
Explanations for false-positive ELISA results were rare. A few authors suggested that false-positives could be attributed to an excessively low cutoff value,7-9 which was consistent with reports that the titer of autoantibodies to BP180 correlates with disease severity, suggesting that the higher titer of antibodies correlates with more severe disease and likely more accurate diagnosis.10,19,20 It is important to consider that patients who have low titers of BP180 autoantibodies with inconsistent clinical characteristics and DIF results may not truly have BP. Furthermore, to determine the clinical value of ELISA in identifying patients in the initial phase of BP, sera of BP patients should be compared with sera of elderly patients with pruritic skin disorders because they comprise the patient population that often requires diagnosis.18
Given the issues identified in our review of the literature, the published sensitivity and specificity of ELISA for BP diagnosis are likely overstated. In conclusion, ELISA should not be relied on as a single criterion adequate for diagnosis of BP.12,21 Rather, the diagnosis of BP can be obtained with a positive predictive value of 95% when a patient meets 3 of 4 clinical criteria (ie, absence of atrophic scars, absence of head and neck involvement, absence of mucosal involvement, and older than 70 years) and demonstrates linear deposits of predominantly IgG and/or C3 along the basement membrane zone of a perilesional biopsy on DIF.15 The gold standard for diagnosis of BP remains clinical presentation along with DIF, which can be supported by histology, IIF, and ELISA.22
Bullous pemphigoid (BP) is the most common autoimmune blistering disease. The classic presentation of BP is a generalized, pruritic, bullous eruption in elderly patients, which is occasionally preceded by an urticarial prodrome. Immunopathologically, BP is characterized by IgG and sometimes IgE autoantibodies that target basement membrane zone proteins BP180 and BP230 of the epidermis.1
The diagnosis of BP should be suspected when an elderly patient presents with tense blisters and can be confirmed via diagnostic testing, including tissue histology and direct immunofluorescence (DIF) as the gold standard, as well as indirect immunofluorescence (IIF), enzyme-linked immunosorbent assay (ELISA), and most recently biochip technology as supportive tests.2 Since its advent, ELISA has gained popularity as a trustworthy diagnostic test for BP. The specificity of ELISA for BP diagnosis is reported to be 98% to 100%, which leads clinicians to believe that a positive ELISA equals certain diagnosis of BP; however, misdiagnosis of BP based on a positive ELISA result can occur.3-13 The treatment of BP often involves lifelong immunosuppressive therapy. Complications of immunosuppressive therapy contribute to morbidity and mortality in these patients, thus an accurate diagnosis is paramount before introducing therapy.14
We present the case of a 74-year-old man with a history of a pruritic nonbullous eruption who was diagnosed with BP and treated for 3 years based on positive ELISA results in the absence of confirmatory histology or DIF.
Case Report
A 74-year-old man with diabetes mellitus, hypertension, hyperlipidemia, benign prostatic hypertrophy, and obstructive sleep apnea presented for further evaluation and confirmation of a prior diagnosis of BP by an outside dermatologist. He reported a pruritic rash on the trunk, back, and extremities of 3 years’ duration. He denied occurrence of blisters at any time.
On presentation to an outside dermatologist 3 years ago, a biopsy was performed along with serologic studies due to the patient’s age and the possibility of an urticarial prodrome in BP. The biopsy revealed epidermal acanthosis, subepidermal separation, and a perivascular and interstitial infiltrate of lymphocytes and eosinophils in the papillary dermis. Direct immunofluorescence was nondiagnostic with a weak discontinuous pattern of IgG and IgA linearly along the basement membrane zone as well as few scattered and clumped cytoid bodies of IgM and IgA. Indirect immunofluoresence revealed a positive IgG titer of 1:40 on monkey esophagus substrate and a positive epidermal pattern on human split-skin substrate with a titer of 1:80. An ELISA for IgG autoantibodies against BP180 and BP230 yielded 15 U and 6 U, respectively (cut off value, 9 U). Based on the positive ELISA for IgG against BP180, a diagnosis of BP was made.
Over the following 3 years, the treatment included prednisone, tetracycline, nicotinamide, doxycycline, and dapsone. Therapy was suboptimal due to the patient’s comorbidities and socioeconomic status. Poorly controlled diabetes mellitus precluded consistent use of prednisone as recommended for BP. Tetracycline and nicotinamide were transiently effective in controlling the patient’s symptoms but were discontinued due to changes in his health insurance. Doxycycline and dapsone were ineffective. Throughout this 3-year period, the patient remained blister free, but the pruritic eruption was persistent.
The patient presented to our clinic due to his frustration with the lack of improvement and doubts about the BP diagnosis given the persistent absence of bullous lesions. Physical examination revealed numerous eroded, scaly, crusted papules on erythematous edematous plaques on all extremities, trunk, and back (Figure 1). The head, neck, face, and oral mucosa were spared. His history and clinical findings were atypical for BP and skin biopsies were performed. Histology revealed epidermal erosion with parakeratosis, spongiosis, and superficial perivascular lymphocytic inflammation with rare eosinophils without subepidermal split (Figure 2). Direct immunofluorescence was negative for IgG, IgA, IgM, C3, and C1q. Additionally, further review of the initial histology by another dermatopathologist revealed that the subepidermal separation reported was more likely artifactual clefts. These findings were not consistent with BP.


Given the patient’s clinical history, lack of bullae, and twice-negative DIF, the diagnosis was determined to be more consistent with eczematous spongiotic dermatitis. He refused a referral for phototherapy due to scheduling inconvenience. The patient was started on cyclosporine 0.5 mg/kg twice daily. After 10 days of treatment, he returned for follow-up and reported notable improvement in the pruritus. On physical examination, his dermatitis was improved with decreased erythema and inflammation.
The patient is being continued on extensive dry skin care with thick moisturizers and additional topical corticosteroid application on an as-needed basis.
Comment
Chronic immunosuppression contributes to morbidity and mortality in patients with BP; therefore, accurate diagnosis of BP is of utmost importance.14 A meta-analysis described ELISA as a test with high sensitivity and specificity (87% and 98%–100%, respectively) for diagnosis of BP.3 Nevertheless, there are opportunities for misdiagnosis using ELISA, as demonstrated in our case. To determine if the reported sensitivity and specificity of ELISA is accurate and reliable for clinical use, individual studies from the meta-analysis were reviewed.4,5,7-10,13,15 Issues identified in our review included dissimilar diagnostic procedures and patient populations among individual studies, several reports of positive ELISA in patients without BP, and a lack of explanation for these false-positive results.
There are notable differences in diagnostic procedures and patient populations among reports that establish the sensitivity and specificity of ELISA for BP diagnosis.3-13 Studies have detected IgG that targets the NC16A domain of the BP180 kD antigen, the C-terminal of the BP180 kD antigen, or the entire ectodomain of the BP180 kD antigen. Study patient populations varied in disease activity, stage, and treatment. Control patients included healthy patients as well as those with many dermatoses, including pemphigus vulgaris, systemic scleroderma, systemic lupus erythematosus, rheumatoid arthritis, lichen planus, and discoid lupus erythematosus.3-13 Due to these differences between individual studies, we believe the results that determine the overall sensitivity and specificity of ELISA for BP diagnosis must be interpreted with caution. For ELISA statistics to be clinically applicable to a specific patient, he/she should be similar to the patients studied. Therefore, we believe each study must be evaluated individually for applicability, given the differences that exist between them.
Furthermore, there have been several reports of false-positive ELISA results in patients with other dermatologic disorders, specifically in elderly patients with pruritus who do not fulfill clinical criteria for diagnosis with BP.16-18 In a population of elderly patients with pruritus for which no specific dermatological or systemic cause was identified, Hofmann et al18 found that 12% (3/25) of patients showed IgG reactivity to BP180 despite having negative DIF results. In another study of elderly patients with pruritic dermatoses, Feliciani et al17 found that 33% (5/15) of patients had IgG reactivity against BP230 or BP180, though they did not fulfill BP criteria based on clinical presentation and showed negative DIF and IIF results. These findings suggest that IgG reactivity against BP autoantibodies as determined by ELISA is not uncommon in pruritic diseases of the elderly.
Explanations for false-positive ELISA results were rare. A few authors suggested that false-positives could be attributed to an excessively low cutoff value,7-9 which was consistent with reports that the titer of autoantibodies to BP180 correlates with disease severity, suggesting that the higher titer of antibodies correlates with more severe disease and likely more accurate diagnosis.10,19,20 It is important to consider that patients who have low titers of BP180 autoantibodies with inconsistent clinical characteristics and DIF results may not truly have BP. Furthermore, to determine the clinical value of ELISA in identifying patients in the initial phase of BP, sera of BP patients should be compared with sera of elderly patients with pruritic skin disorders because they comprise the patient population that often requires diagnosis.18
Given the issues identified in our review of the literature, the published sensitivity and specificity of ELISA for BP diagnosis are likely overstated. In conclusion, ELISA should not be relied on as a single criterion adequate for diagnosis of BP.12,21 Rather, the diagnosis of BP can be obtained with a positive predictive value of 95% when a patient meets 3 of 4 clinical criteria (ie, absence of atrophic scars, absence of head and neck involvement, absence of mucosal involvement, and older than 70 years) and demonstrates linear deposits of predominantly IgG and/or C3 along the basement membrane zone of a perilesional biopsy on DIF.15 The gold standard for diagnosis of BP remains clinical presentation along with DIF, which can be supported by histology, IIF, and ELISA.22
- Delaporte E, Dubost-Brama A, Ghohestani R, et al. IgE autoantibodies directed against the major bullous pemphigoid antigen in patients with a severe form of pemphigoid. J Immunol. 1996;157:3642-3647.
- Schmidt E, Zillikens D. Diagnosis and clinical severity markers of bullous pemphigoid. F1000 Med Rep. 2009;1:15.
- Tampoia M, Giavarina D, Di Giorgio C, et al. Diagnostic accuracy of enzyme-linked immunosorbent assays (ELISA) to detect anti-skin autoantibodies in autoimmune blistering diseases: a systematic review and meta-analysis. Autoimmun Rev. 2012;12:121-126.
- Zillikens D, Mascaro JM, Rose PA, et al. A highly sensitive enzyme-linked immunosorbent assay for the detection of circulating anti-BP180 autoantibodies in patients with bullous pemphigoid. J Invest Dermatol. 1997;109:679-683.
- Sitaru C, Dahnrich C, Probst C, et al. Enzyme-linked immunosorbent assay using multimers of the 16th non-collagenous domain of the BP180 antigen for sensitive and specific detection of pemphigoid autoantibodies. Exp Dermatol. 2007;16:770-777.
- Yang B, Wang C, Chen S, et al. Evaluation of the combination of BP180-NC16a enzyme-linked immunosorbent assay and BP230 enzyme-linked immunosorbent assay in the diagnosis of bullous pemphigoid. Indian J Dermatol Venereol Leprol. 2012;78:722-727.
- Sakuma-Oyama Y, Powell AM, Oyama N, et al. Evaluation of a BP180-NC16a enzyme-linked immunosorbent assay in the initial diagnosis of bullous pemphigoid. Br J Dermatol. 2004;151:126-131.
- Tampoia M, Lattanzi V, Zucano A, et al. Evaluation of a new ELISA assay for detection of BP230 autoantibodies in bullous pemphigoid. Ann N Y Acad Sci. 2009;1173:15-20.
- Feng S, Lin L, Jin P, et al. Role of BP180NC16a-enzyme-linked immunosorbent assay (ELISA) in the diagnosis of bullous pemphigoid in China. Int J Dermatol. 2008;47:24-28.
- Kobayashi M, Amagai M, Kuroda-Kinoshita K, et al. BP180 ELISA using bacterial recombinant NC16a protein as a diagnostic and monitoring tool for bullous pemphigoid. J Dermatol Sci. 2002;30:224-232.
- Roussel A, Benichou J, Arivelo Randriamanantany Z, et al. Enzyme-linked immunosorbent assay for the combination of bullous pemphigoid antigens 1 and 2 in the diagnosis of bullous pemphigoid. Arch Dermatol. 2011;147:293-298.
- Chan, Lawrence S. ELISA instead of indirect IF in patients with BP. Arch Dermatol. 2011;147:291-292.
- Barnadas MA, Rubiales V, González J, et al. Enzyme-linked immunosorbent assay (ELISA) and indirect immunofluorescence testing in a bullous pemphigoid and pemphigoid gestationis. Int J Dermatol. 2008;47:1245-1249.
- Borradori L, Bernard P. Pemphigoid group. In: Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. New York, NY: Mosby; 2003:469.
- Vaillant L, Bernard P, Joly P, et al. Evaluation of clinical criteria for diagnosis of bullous pemphigoid. Arch Dermatol. 1998;134:1075-1080.
- Fania L, Caldarola G, Muller R, et al. IgE recognition of bullous pemphigoid (BP)180 and BP230 in BP patients and elderly individuals with pruritic dermatoses. Clin Immunol. 2012;143:236-245.
- Feliciani C, Caldarola G, Kneisel A, et al. IgG autoantibody reactivity against bullous pemphigoid (BP) 180 and BP230 in elderly patients with pruritic dermatoses. Br J Dermatol. 2009;61:306-312.
- Hofmann SC, Tamm K, Hertl M, et al. Diagnostic value of an enzyme-linked immunosorbent assay using BP180 recombinant proteins in elderly patients with pruritic skin disorders. Br J Dermatol. 2003;149:910-911.
- Schmidt E, Obe K, Brocker EB, et al. Serum levels of autoantibodies to BP180 correlate with disease activity in patients with bullous pemphigoid. Arch Dermatol. 2000;136:174-178.
- Feng S, Wu Q, Jin P, et al. Serum levels of autoantibodies to BP180 correlate with disease activity in patients with bullous pemphigoid. Int J Dermatol. 2008;47:225-228.
- Di Zenzo G, Joly P, Zambruno G, et al. Sensitivity of immunofluorescence studies vs enzyme-linked immunosorbent assay for diagnosis of bullous pemphigoid. Arch Dermatol. 2011;147:1454-1456.
- Schmidt E, Zillikens D. Modern diagnosis of autoimmune blistering skin diseases. Autoimmun Rev. 2010;10:84-89.
- Delaporte E, Dubost-Brama A, Ghohestani R, et al. IgE autoantibodies directed against the major bullous pemphigoid antigen in patients with a severe form of pemphigoid. J Immunol. 1996;157:3642-3647.
- Schmidt E, Zillikens D. Diagnosis and clinical severity markers of bullous pemphigoid. F1000 Med Rep. 2009;1:15.
- Tampoia M, Giavarina D, Di Giorgio C, et al. Diagnostic accuracy of enzyme-linked immunosorbent assays (ELISA) to detect anti-skin autoantibodies in autoimmune blistering diseases: a systematic review and meta-analysis. Autoimmun Rev. 2012;12:121-126.
- Zillikens D, Mascaro JM, Rose PA, et al. A highly sensitive enzyme-linked immunosorbent assay for the detection of circulating anti-BP180 autoantibodies in patients with bullous pemphigoid. J Invest Dermatol. 1997;109:679-683.
- Sitaru C, Dahnrich C, Probst C, et al. Enzyme-linked immunosorbent assay using multimers of the 16th non-collagenous domain of the BP180 antigen for sensitive and specific detection of pemphigoid autoantibodies. Exp Dermatol. 2007;16:770-777.
- Yang B, Wang C, Chen S, et al. Evaluation of the combination of BP180-NC16a enzyme-linked immunosorbent assay and BP230 enzyme-linked immunosorbent assay in the diagnosis of bullous pemphigoid. Indian J Dermatol Venereol Leprol. 2012;78:722-727.
- Sakuma-Oyama Y, Powell AM, Oyama N, et al. Evaluation of a BP180-NC16a enzyme-linked immunosorbent assay in the initial diagnosis of bullous pemphigoid. Br J Dermatol. 2004;151:126-131.
- Tampoia M, Lattanzi V, Zucano A, et al. Evaluation of a new ELISA assay for detection of BP230 autoantibodies in bullous pemphigoid. Ann N Y Acad Sci. 2009;1173:15-20.
- Feng S, Lin L, Jin P, et al. Role of BP180NC16a-enzyme-linked immunosorbent assay (ELISA) in the diagnosis of bullous pemphigoid in China. Int J Dermatol. 2008;47:24-28.
- Kobayashi M, Amagai M, Kuroda-Kinoshita K, et al. BP180 ELISA using bacterial recombinant NC16a protein as a diagnostic and monitoring tool for bullous pemphigoid. J Dermatol Sci. 2002;30:224-232.
- Roussel A, Benichou J, Arivelo Randriamanantany Z, et al. Enzyme-linked immunosorbent assay for the combination of bullous pemphigoid antigens 1 and 2 in the diagnosis of bullous pemphigoid. Arch Dermatol. 2011;147:293-298.
- Chan, Lawrence S. ELISA instead of indirect IF in patients with BP. Arch Dermatol. 2011;147:291-292.
- Barnadas MA, Rubiales V, González J, et al. Enzyme-linked immunosorbent assay (ELISA) and indirect immunofluorescence testing in a bullous pemphigoid and pemphigoid gestationis. Int J Dermatol. 2008;47:1245-1249.
- Borradori L, Bernard P. Pemphigoid group. In: Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. New York, NY: Mosby; 2003:469.
- Vaillant L, Bernard P, Joly P, et al. Evaluation of clinical criteria for diagnosis of bullous pemphigoid. Arch Dermatol. 1998;134:1075-1080.
- Fania L, Caldarola G, Muller R, et al. IgE recognition of bullous pemphigoid (BP)180 and BP230 in BP patients and elderly individuals with pruritic dermatoses. Clin Immunol. 2012;143:236-245.
- Feliciani C, Caldarola G, Kneisel A, et al. IgG autoantibody reactivity against bullous pemphigoid (BP) 180 and BP230 in elderly patients with pruritic dermatoses. Br J Dermatol. 2009;61:306-312.
- Hofmann SC, Tamm K, Hertl M, et al. Diagnostic value of an enzyme-linked immunosorbent assay using BP180 recombinant proteins in elderly patients with pruritic skin disorders. Br J Dermatol. 2003;149:910-911.
- Schmidt E, Obe K, Brocker EB, et al. Serum levels of autoantibodies to BP180 correlate with disease activity in patients with bullous pemphigoid. Arch Dermatol. 2000;136:174-178.
- Feng S, Wu Q, Jin P, et al. Serum levels of autoantibodies to BP180 correlate with disease activity in patients with bullous pemphigoid. Int J Dermatol. 2008;47:225-228.
- Di Zenzo G, Joly P, Zambruno G, et al. Sensitivity of immunofluorescence studies vs enzyme-linked immunosorbent assay for diagnosis of bullous pemphigoid. Arch Dermatol. 2011;147:1454-1456.
- Schmidt E, Zillikens D. Modern diagnosis of autoimmune blistering skin diseases. Autoimmun Rev. 2010;10:84-89.
Practice Points
- A low serum level of autoantibodies to BP180 should be interpreted with caution because it is more likely to represent a false-positive than a high serum level.
- Rely on the gold standard for diagnosis of bullous pemphigoid: clinical presentation along with direct immunofluorescence, which can be supported by histology, indirect immunofluorescence, and enzyme-linked immunosorbent assay (ELISA) rather than ELISA alone.