User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Psoriatic Arthritis Treatment: The Dermatologist’s Role
Resolution of Psoriatic Lesions on the Gingiva and Hard Palate Following Administration of Adalimumab for Cutaneous Psoriasis
Psoriasis is a chronic, relapsing, inflammatory systemic disorder of the skin with an incidence of 2% to 3% and is estimated to affect 125 million individuals worldwide.1 Environmental triggers of disease modulation may include cutaneous microbiota, smoking, alcohol use, drugs (ie, beta-blockers, lithium, antimalarials), stress, and trauma.2 Comorbidities associated with cutaneous lesions include psoriatic arthritis, Crohn disease, type 2 diabetes mellitus, metabolic syndrome, stroke, and cardiovascular disease.3 In some studies, patients with psoriasis also had a 24% to 27% increased propensity for periodontal bone loss versus 10% of controls.4,5
Oral psoriasis is rare and case reports have been preferentially published in dental journals, usually with regard to glossal lesions, leaving gingival and palatal psoriatic involvement infrequently reported in the dermatologic literature.6,7 In fact, oral assessments involving 535 psoriatic patients from a dermatology center only yielded cases of geographic and fissured tongue.8 Another study at a psoriasis clinic found 3.8% (21/547) of patients with geographic tongue, 3.1% (17/547) with buccal mucosal plaques, and only 0.4% (2/547) with palatal lesions.9 To extend the knowledge of oral psoriasis, we provide the clinical and histopathologic findings of a patient with synchronous oral and cutaneous psoriatic lesions that responded well to the administration of adalimumab for management of recurrent cutaneous disease.
Case Report
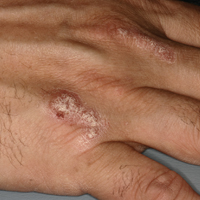
A 51-year-old man presented to the attending periodontist for comprehensive treatment of multiple quadrants of gingival recession. His medical history was remarkable for psoriasis; Prinzmetal angina, which led to myocardial infarction; and diverticulitis. The cutaneous psoriasis began approximately 18 years prior to the current presentation and was initially managed with various topical therapeutics. At an 11-year follow-up, the patient was experiencing poor lesional control as well as severe pruritus and was prescribed etanercept by a dermatologist. His inconsistent compliance with frequency and dosing failed to achieve satisfactory disease suppression and etanercept was discontinued after approximately 2.5 years. Two years later the patient was switched to adalimumab by a dermatologist, and around this time he had developed psoriatic arthritis of the hands and knees and pitting of the nail plates. The patient elected to discontinue adalimumab usage after 3 years due to successful management of the skin lesions, cost considerations, and his perception that the psoriasis could “remain in remission.” After a 6-month lapse, the patient resumed adalimumab due to cutaneous lesional recurrence (Figure 1A).
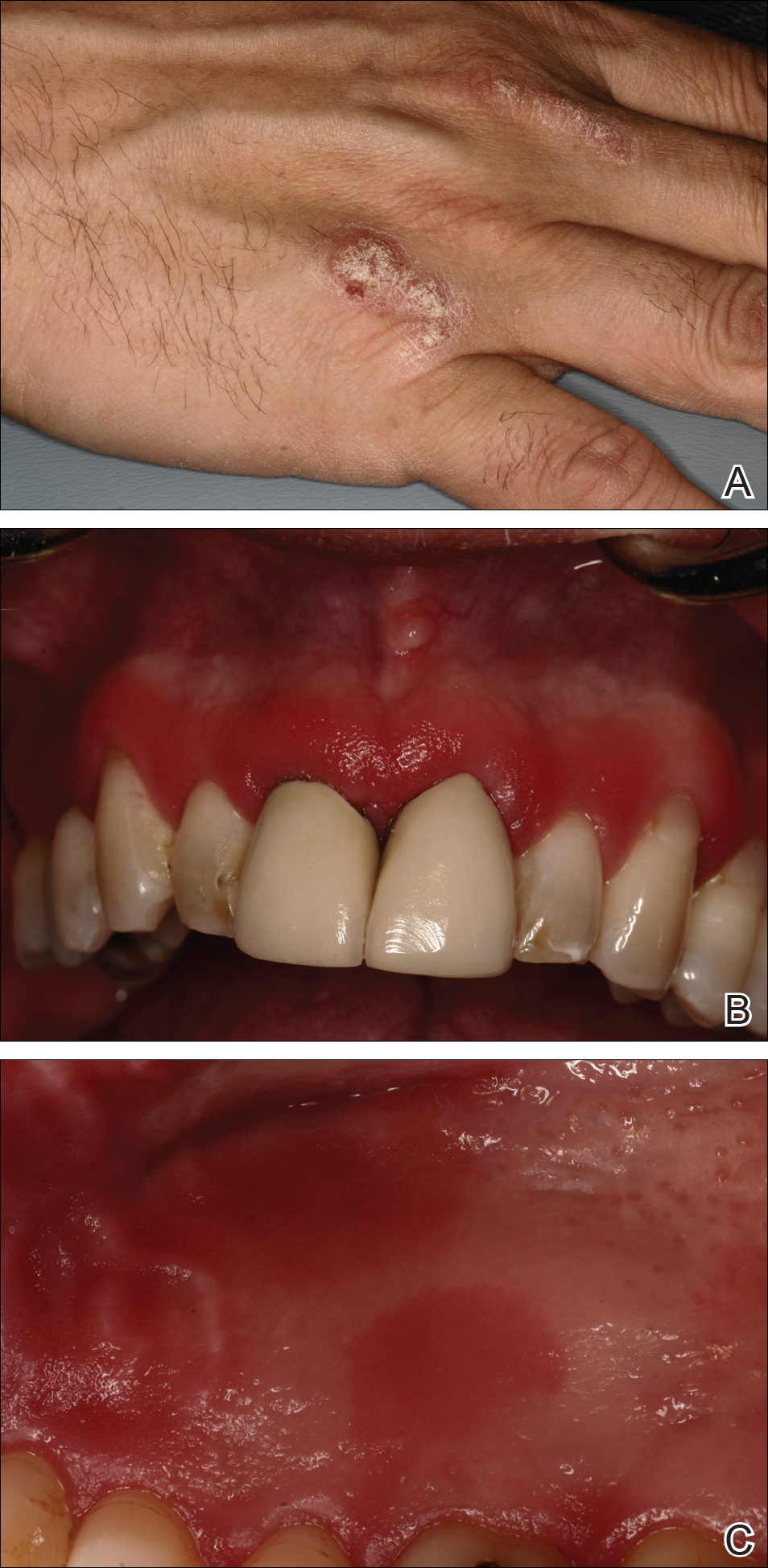
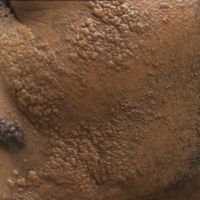
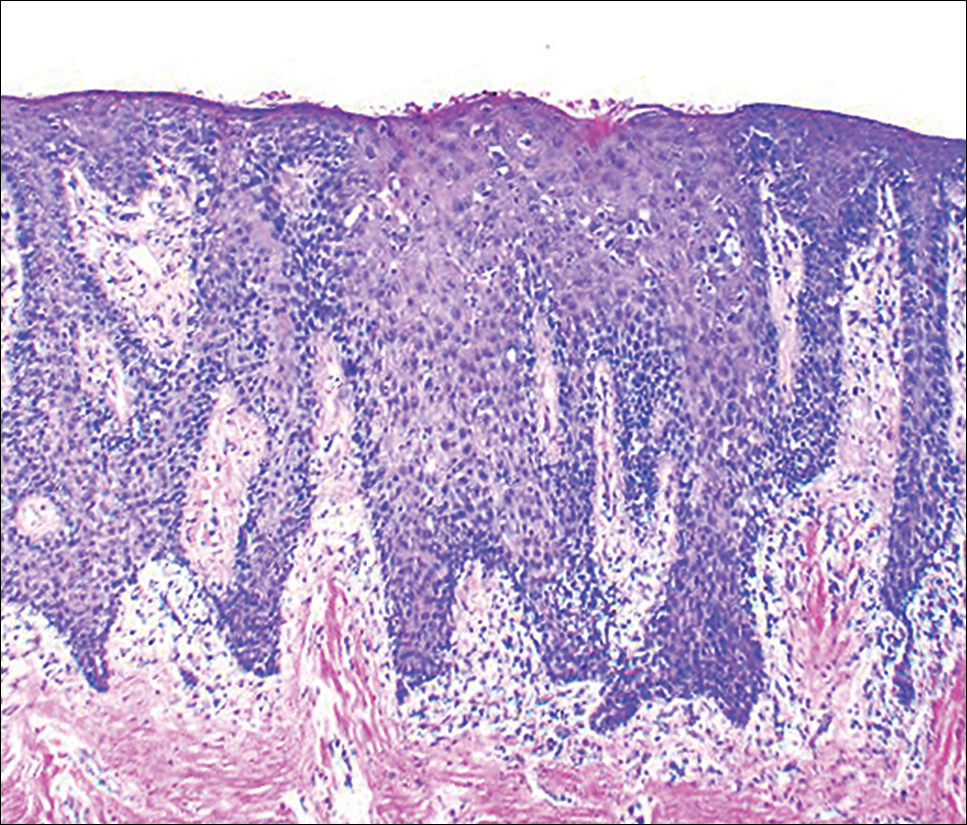
At the current presentation, an oral examination performed 2 days after the reinstitution of adalim-umab revealed generalized severe gingivitis with an atypical inflammatory response that extended from just beyond the mucogingival junction to the marginal gingiva. The gingiva also appeared edematous with a conspicuously granular surface (Figure 1B). The hard palate displayed multiple red macules of varying sizes (Figure 1C). A maxillary gingival biopsy demonstrated hyperkeratosis, parakeratosis, spongiosis, acanthosis, elongation of the rete ridges, numerous collections of neutrophils (Munro microabscesses), and abundant lymphocytes in the subjacent connective tissue (Figure 2). Periodic acid–Schiff staining was negative for fungal hyphae. These features were consistent with oral mucosal psoriasis.
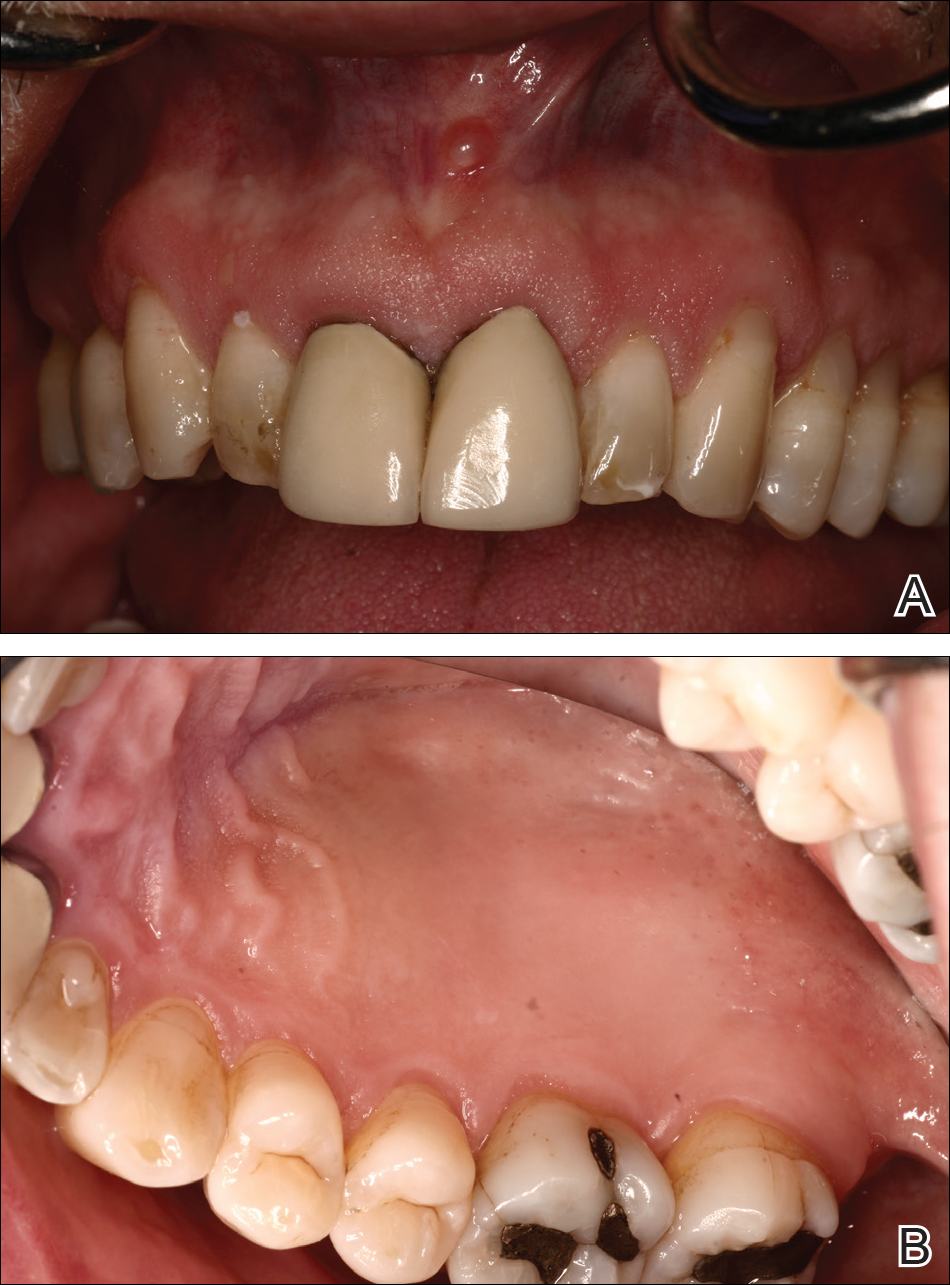
At a 2-month follow-up, the biopsy site had healed without incident and without loss of the gingival architecture. There was an almost-complete resolution of the gingival erythema (Figure 3A) and the patient has since noticed a lack of bleeding using floss. Additionally, the red macules on the palate were no longer present (Figure 3B). The cutaneous plaques were greatly reduced in size and the patient experienced a proportionate decline in pruritus. Based on the uneventful surgical biopsy procedure, the patient was advised to undergo gingival grafting and has not returned for periodontal care.
Comment
Psoriasis of the oral cavity is rare and typically occurs on the tongue and less frequently on the hard palate, lip, buccal mucosa, and gingiva.2,7 The lesions are almost always concordant with cutaneous psoriasis, and only sporadic examples exclusive to the oral mucosa have been recognized.7,10 Gingival psoriasis usually is described as intensely erythematous and occasionally laced with white scaly streaks involving the marginal gingiva that extend toward the mucogingival junction. In general, the erythematous presentation of gingival psoriasis may not be commensurate with the degree of inflammation induced by dental plaque-based periodontal disease. Doben11 documented gingival psoriasis as appearing “deeply stippled and grainy” and commented that the tissue was “friable” and incapable of maintaining a “clean incision line” during periodontal surgery. In our patient, the gingiva also had exhibited a granular surface. Patients with oral psoriasis often report soreness or a burning sensation of the gingiva, which may easily bleed on manipulation or brushing the teeth, whereas other patients are asymptomatic,12 as in our case. Psoriasis of the hard palate usually presents as multiple painless red macules. Unlike cutaneous psoriasis, oral lesions rarely evoke pruritus.10 Histopathologically, oral psoriasis bears a striking resemblance to its cutaneous counterpart. The epithelium has a pronounced parakeratinized surface with elongated rete ridges and aggregations of Munro microabscesses. The connective tissue often is composed of dilated capillaries that closely approximate the epithelium as well as infiltrations of lymphocytes. Specimens suspected for oral psoriasis should routinely be stained with periodic acid–Schiff to rule out candidiasis coinfection. The microscopic findings of our patient were congruent with prior reports of oral psoriasis.7,10-12 Some clinicians have questioned if psoriasis can actually occur in the oral cavity, but most authorities in the field have recognized its true existence, as evidenced by various shared HLA antigens, specifically HLA-Cw.13
Another group of oral lesions collectively referred to as psoriasiform mucositis, notably geographic tongue (benign migratory glossitis, erythema migrans) and its extraglossal variant geographic stomatitis,14,15 have histopathologic features and HLAs similar to those seen in cutaneous psoriasis.13 Interestingly, geographic tongue has been found in 3.8% to 9.1% of cohorts with cutaneous psoriasis,8,9 but in the extant population, the vast majority of patients with oral psoriasiform mucositis do not have cutaneous psoriasis. Other differential diagnoses for gingival psoriasis are lichen planus, human immunodeficiency virus–associated periodontitis, desquamative gingivitis, plasma cell gingivitis, erythematous candidiasis, mucous membrane pemphigoid, pemphigus vulgaris, leukemia, systemic lupus erythematosus, granulomatosis with polyangiitis, orofacial granulomatosis, localized juvenile spongiotic gingivitis hyperplasia, and primary gingivostomatitis.
Management of gingival psoriasis focuses on strategies to reduce inflammation and discomfort and measures to achieve meticulous oral plaque control. Judicious efforts should be exercised to avoid oral soft-tissue injury when performing periodontal scaling, although it has not been established whether gingival psoriasis is associated with the Köbner phenomenon, as seen with cutaneous lesions. Adjunctive measures employed for symptomatic patients have involved the use of corticosteroids (eg, lesional injection, oral rinse, systemic) and oral rinses with retinoic acid, chlorhexidine gluconate, and warm saline.7,10,16 Prolonged utilization of corticosteroids, however, may necessitate supplemental administration of antifungal agents.
This case report represents a rare documentation of a successful outcome of gingival and palatal psoriasis subsequent to the reinstitution of adalimumab solely for treatment of recurrent cutaneous disease. There likely is a pharmacologic basis for the amelioration of oral psoriasis in our patient. Adalimumab is a bivalent IgG monoclonal antibody that binds to activated dermal dendritic cell receptors of tumor necrosis factor α, thereby attenuating a cytokine-derived inflammatory response and apoptosis.17 In fact, patients with rheumatoid arthritis showed notable reductions in both gingival inflammation and bleeding following a 3-month regimen of adalimumab.18
Conclusion
Practitioners should be aware of the phenotypic overlap of cutaneous and oral psoriasis, particularly involving the gingiva and palate. It is recommended that psoriasis patients routinely receive a dental prophylaxis and engage in oral hygiene efforts to reduce the presence of oral microbiota. Furthermore, it is emphasized that psoriatic patients who maintain an atypical erythematous presentation on the oral mucosa undergo a biopsy for identification of the lesions and correlation with disease dissemination. Prospective studies are needed to characterize the clinical courses of oral psoriasis, ascertain their correlative behavior with cutaneous flares, and determine if lesional improvement can be achieved with the use of biologic agents or other therapeutic modalities.
- Gupta R, Debbaneh MG, Liao W. Genetic epidemiology of psoriasis. Curr Dermatol Rep. 2014;3:61-78.
- Younai FS, Phelan JA. Oral mucositis with features of psoriasis: report of a case and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;84:61-67.
- Xu T, Zhang YH. Association of psoriasis with stroke and myocardial infarction: meta-analysis of cohort studies. Br J Dermatol. 2012;167:1345-1350.
- Lazaridou E, Tsikrikoni A, Fotiadou C, et al. Association of chronic plaque psoriasis and severe periodontitis: a hospital based case-control study. J Eur Acad Dermatol Venereol. 2013;27:967-972.
- Skudutyte-Rysstad R, Slevolden EM, Hansen BF, et al. Association between moderate to severe psoriasis and periodontitis in a Scandinavian population. BMC Oral Health. 2014;14:139.
- Zunt SL, Tomich CE. Erythema migrans—a psoriasiform lesion of the oral mucosa. J Dermatol Surg Oncol. 1989;15:1067-1070.
- Reis V, Artico G, Seo J, et al. Psoriasiform mucositis on the gingival and palatal mucosae treated with retinoic-acid mouthwash. Int J Dermatol. 2013;52:113-115.
- Germi L, De Giorgi V, Bergamo F, et al. Psoriasis and oral lesions: multicentric study of oral mucosa diseases Italian group (GIPMO). Dermatol Online J. 2012;18:11.
- Kaur I, Handa S, Kumar B. Oral lesions in psoriasis. Int J Dermatol. 1997;36:78-79.
- Brayshaw HA, Orban B. Psoriasis gingivae. J Periodontol. 1953;24:156-160.
- Doben DI. Psoriasis of the attached gingiva. J Periodontol. 1976;47:38-40.
- Mattsson U, Warfvinge G, Jontell M. Oral psoriasis—a diagnostic dilemma: a report of two cases and a review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;120:e183-e189.
- Dermatologic diseases. In: Neville BW, Damm DD, Allen CM, et al, eds. Oral and Maxillofacial Pathology. 3rd ed. St. Louis, MO: Saunders/Elsevier; 2009:792-794.
- Brooks JK, Balciunas BA. Geographic stomatitis: review of the literature and report of five cases. J Am Dent Assoc. 1987;115:421-424.
- Brooks JK, Nikitakis NG. Multiple mucosal lesions. erythema migrans. Gen Dent. 2007;55:160, 163.
- Ulmansky M, Michelle R, Azaz B. Oral psoriasis: report of six new cases. J Oral Pathol Med. 1995;24:42-45.
- Lis K, Kuzawinska O, Bałkowiec-Iskra E. Tumor necrosis factor inhibitors—state of knowledge. Arch Med Sci. 2014;10:1175-1185.
- Kobayashi T, Yokoyama T, Ito S, et al. Periodontal and serum protein profiles in patients with rheumatoid arthritis treated with tumor necrosis factor inhibitor adalimumab. J Periodontol. 2014;85:1480-1488.
Psoriasis is a chronic, relapsing, inflammatory systemic disorder of the skin with an incidence of 2% to 3% and is estimated to affect 125 million individuals worldwide.1 Environmental triggers of disease modulation may include cutaneous microbiota, smoking, alcohol use, drugs (ie, beta-blockers, lithium, antimalarials), stress, and trauma.2 Comorbidities associated with cutaneous lesions include psoriatic arthritis, Crohn disease, type 2 diabetes mellitus, metabolic syndrome, stroke, and cardiovascular disease.3 In some studies, patients with psoriasis also had a 24% to 27% increased propensity for periodontal bone loss versus 10% of controls.4,5
Oral psoriasis is rare and case reports have been preferentially published in dental journals, usually with regard to glossal lesions, leaving gingival and palatal psoriatic involvement infrequently reported in the dermatologic literature.6,7 In fact, oral assessments involving 535 psoriatic patients from a dermatology center only yielded cases of geographic and fissured tongue.8 Another study at a psoriasis clinic found 3.8% (21/547) of patients with geographic tongue, 3.1% (17/547) with buccal mucosal plaques, and only 0.4% (2/547) with palatal lesions.9 To extend the knowledge of oral psoriasis, we provide the clinical and histopathologic findings of a patient with synchronous oral and cutaneous psoriatic lesions that responded well to the administration of adalimumab for management of recurrent cutaneous disease.
Case Report
A 51-year-old man presented to the attending periodontist for comprehensive treatment of multiple quadrants of gingival recession. His medical history was remarkable for psoriasis; Prinzmetal angina, which led to myocardial infarction; and diverticulitis. The cutaneous psoriasis began approximately 18 years prior to the current presentation and was initially managed with various topical therapeutics. At an 11-year follow-up, the patient was experiencing poor lesional control as well as severe pruritus and was prescribed etanercept by a dermatologist. His inconsistent compliance with frequency and dosing failed to achieve satisfactory disease suppression and etanercept was discontinued after approximately 2.5 years. Two years later the patient was switched to adalimumab by a dermatologist, and around this time he had developed psoriatic arthritis of the hands and knees and pitting of the nail plates. The patient elected to discontinue adalimumab usage after 3 years due to successful management of the skin lesions, cost considerations, and his perception that the psoriasis could “remain in remission.” After a 6-month lapse, the patient resumed adalimumab due to cutaneous lesional recurrence (Figure 1A).

At the current presentation, an oral examination performed 2 days after the reinstitution of adalim-umab revealed generalized severe gingivitis with an atypical inflammatory response that extended from just beyond the mucogingival junction to the marginal gingiva. The gingiva also appeared edematous with a conspicuously granular surface (Figure 1B). The hard palate displayed multiple red macules of varying sizes (Figure 1C). A maxillary gingival biopsy demonstrated hyperkeratosis, parakeratosis, spongiosis, acanthosis, elongation of the rete ridges, numerous collections of neutrophils (Munro microabscesses), and abundant lymphocytes in the subjacent connective tissue (Figure 2). Periodic acid–Schiff staining was negative for fungal hyphae. These features were consistent with oral mucosal psoriasis.

At a 2-month follow-up, the biopsy site had healed without incident and without loss of the gingival architecture. There was an almost-complete resolution of the gingival erythema (Figure 3A) and the patient has since noticed a lack of bleeding using floss. Additionally, the red macules on the palate were no longer present (Figure 3B). The cutaneous plaques were greatly reduced in size and the patient experienced a proportionate decline in pruritus. Based on the uneventful surgical biopsy procedure, the patient was advised to undergo gingival grafting and has not returned for periodontal care.

Comment
Psoriasis of the oral cavity is rare and typically occurs on the tongue and less frequently on the hard palate, lip, buccal mucosa, and gingiva.2,7 The lesions are almost always concordant with cutaneous psoriasis, and only sporadic examples exclusive to the oral mucosa have been recognized.7,10 Gingival psoriasis usually is described as intensely erythematous and occasionally laced with white scaly streaks involving the marginal gingiva that extend toward the mucogingival junction. In general, the erythematous presentation of gingival psoriasis may not be commensurate with the degree of inflammation induced by dental plaque-based periodontal disease. Doben11 documented gingival psoriasis as appearing “deeply stippled and grainy” and commented that the tissue was “friable” and incapable of maintaining a “clean incision line” during periodontal surgery. In our patient, the gingiva also had exhibited a granular surface. Patients with oral psoriasis often report soreness or a burning sensation of the gingiva, which may easily bleed on manipulation or brushing the teeth, whereas other patients are asymptomatic,12 as in our case. Psoriasis of the hard palate usually presents as multiple painless red macules. Unlike cutaneous psoriasis, oral lesions rarely evoke pruritus.10 Histopathologically, oral psoriasis bears a striking resemblance to its cutaneous counterpart. The epithelium has a pronounced parakeratinized surface with elongated rete ridges and aggregations of Munro microabscesses. The connective tissue often is composed of dilated capillaries that closely approximate the epithelium as well as infiltrations of lymphocytes. Specimens suspected for oral psoriasis should routinely be stained with periodic acid–Schiff to rule out candidiasis coinfection. The microscopic findings of our patient were congruent with prior reports of oral psoriasis.7,10-12 Some clinicians have questioned if psoriasis can actually occur in the oral cavity, but most authorities in the field have recognized its true existence, as evidenced by various shared HLA antigens, specifically HLA-Cw.13
Another group of oral lesions collectively referred to as psoriasiform mucositis, notably geographic tongue (benign migratory glossitis, erythema migrans) and its extraglossal variant geographic stomatitis,14,15 have histopathologic features and HLAs similar to those seen in cutaneous psoriasis.13 Interestingly, geographic tongue has been found in 3.8% to 9.1% of cohorts with cutaneous psoriasis,8,9 but in the extant population, the vast majority of patients with oral psoriasiform mucositis do not have cutaneous psoriasis. Other differential diagnoses for gingival psoriasis are lichen planus, human immunodeficiency virus–associated periodontitis, desquamative gingivitis, plasma cell gingivitis, erythematous candidiasis, mucous membrane pemphigoid, pemphigus vulgaris, leukemia, systemic lupus erythematosus, granulomatosis with polyangiitis, orofacial granulomatosis, localized juvenile spongiotic gingivitis hyperplasia, and primary gingivostomatitis.
Management of gingival psoriasis focuses on strategies to reduce inflammation and discomfort and measures to achieve meticulous oral plaque control. Judicious efforts should be exercised to avoid oral soft-tissue injury when performing periodontal scaling, although it has not been established whether gingival psoriasis is associated with the Köbner phenomenon, as seen with cutaneous lesions. Adjunctive measures employed for symptomatic patients have involved the use of corticosteroids (eg, lesional injection, oral rinse, systemic) and oral rinses with retinoic acid, chlorhexidine gluconate, and warm saline.7,10,16 Prolonged utilization of corticosteroids, however, may necessitate supplemental administration of antifungal agents.
This case report represents a rare documentation of a successful outcome of gingival and palatal psoriasis subsequent to the reinstitution of adalimumab solely for treatment of recurrent cutaneous disease. There likely is a pharmacologic basis for the amelioration of oral psoriasis in our patient. Adalimumab is a bivalent IgG monoclonal antibody that binds to activated dermal dendritic cell receptors of tumor necrosis factor α, thereby attenuating a cytokine-derived inflammatory response and apoptosis.17 In fact, patients with rheumatoid arthritis showed notable reductions in both gingival inflammation and bleeding following a 3-month regimen of adalimumab.18
Conclusion
Practitioners should be aware of the phenotypic overlap of cutaneous and oral psoriasis, particularly involving the gingiva and palate. It is recommended that psoriasis patients routinely receive a dental prophylaxis and engage in oral hygiene efforts to reduce the presence of oral microbiota. Furthermore, it is emphasized that psoriatic patients who maintain an atypical erythematous presentation on the oral mucosa undergo a biopsy for identification of the lesions and correlation with disease dissemination. Prospective studies are needed to characterize the clinical courses of oral psoriasis, ascertain their correlative behavior with cutaneous flares, and determine if lesional improvement can be achieved with the use of biologic agents or other therapeutic modalities.
Psoriasis is a chronic, relapsing, inflammatory systemic disorder of the skin with an incidence of 2% to 3% and is estimated to affect 125 million individuals worldwide.1 Environmental triggers of disease modulation may include cutaneous microbiota, smoking, alcohol use, drugs (ie, beta-blockers, lithium, antimalarials), stress, and trauma.2 Comorbidities associated with cutaneous lesions include psoriatic arthritis, Crohn disease, type 2 diabetes mellitus, metabolic syndrome, stroke, and cardiovascular disease.3 In some studies, patients with psoriasis also had a 24% to 27% increased propensity for periodontal bone loss versus 10% of controls.4,5
Oral psoriasis is rare and case reports have been preferentially published in dental journals, usually with regard to glossal lesions, leaving gingival and palatal psoriatic involvement infrequently reported in the dermatologic literature.6,7 In fact, oral assessments involving 535 psoriatic patients from a dermatology center only yielded cases of geographic and fissured tongue.8 Another study at a psoriasis clinic found 3.8% (21/547) of patients with geographic tongue, 3.1% (17/547) with buccal mucosal plaques, and only 0.4% (2/547) with palatal lesions.9 To extend the knowledge of oral psoriasis, we provide the clinical and histopathologic findings of a patient with synchronous oral and cutaneous psoriatic lesions that responded well to the administration of adalimumab for management of recurrent cutaneous disease.
Case Report
A 51-year-old man presented to the attending periodontist for comprehensive treatment of multiple quadrants of gingival recession. His medical history was remarkable for psoriasis; Prinzmetal angina, which led to myocardial infarction; and diverticulitis. The cutaneous psoriasis began approximately 18 years prior to the current presentation and was initially managed with various topical therapeutics. At an 11-year follow-up, the patient was experiencing poor lesional control as well as severe pruritus and was prescribed etanercept by a dermatologist. His inconsistent compliance with frequency and dosing failed to achieve satisfactory disease suppression and etanercept was discontinued after approximately 2.5 years. Two years later the patient was switched to adalimumab by a dermatologist, and around this time he had developed psoriatic arthritis of the hands and knees and pitting of the nail plates. The patient elected to discontinue adalimumab usage after 3 years due to successful management of the skin lesions, cost considerations, and his perception that the psoriasis could “remain in remission.” After a 6-month lapse, the patient resumed adalimumab due to cutaneous lesional recurrence (Figure 1A).

At the current presentation, an oral examination performed 2 days after the reinstitution of adalim-umab revealed generalized severe gingivitis with an atypical inflammatory response that extended from just beyond the mucogingival junction to the marginal gingiva. The gingiva also appeared edematous with a conspicuously granular surface (Figure 1B). The hard palate displayed multiple red macules of varying sizes (Figure 1C). A maxillary gingival biopsy demonstrated hyperkeratosis, parakeratosis, spongiosis, acanthosis, elongation of the rete ridges, numerous collections of neutrophils (Munro microabscesses), and abundant lymphocytes in the subjacent connective tissue (Figure 2). Periodic acid–Schiff staining was negative for fungal hyphae. These features were consistent with oral mucosal psoriasis.

At a 2-month follow-up, the biopsy site had healed without incident and without loss of the gingival architecture. There was an almost-complete resolution of the gingival erythema (Figure 3A) and the patient has since noticed a lack of bleeding using floss. Additionally, the red macules on the palate were no longer present (Figure 3B). The cutaneous plaques were greatly reduced in size and the patient experienced a proportionate decline in pruritus. Based on the uneventful surgical biopsy procedure, the patient was advised to undergo gingival grafting and has not returned for periodontal care.

Comment
Psoriasis of the oral cavity is rare and typically occurs on the tongue and less frequently on the hard palate, lip, buccal mucosa, and gingiva.2,7 The lesions are almost always concordant with cutaneous psoriasis, and only sporadic examples exclusive to the oral mucosa have been recognized.7,10 Gingival psoriasis usually is described as intensely erythematous and occasionally laced with white scaly streaks involving the marginal gingiva that extend toward the mucogingival junction. In general, the erythematous presentation of gingival psoriasis may not be commensurate with the degree of inflammation induced by dental plaque-based periodontal disease. Doben11 documented gingival psoriasis as appearing “deeply stippled and grainy” and commented that the tissue was “friable” and incapable of maintaining a “clean incision line” during periodontal surgery. In our patient, the gingiva also had exhibited a granular surface. Patients with oral psoriasis often report soreness or a burning sensation of the gingiva, which may easily bleed on manipulation or brushing the teeth, whereas other patients are asymptomatic,12 as in our case. Psoriasis of the hard palate usually presents as multiple painless red macules. Unlike cutaneous psoriasis, oral lesions rarely evoke pruritus.10 Histopathologically, oral psoriasis bears a striking resemblance to its cutaneous counterpart. The epithelium has a pronounced parakeratinized surface with elongated rete ridges and aggregations of Munro microabscesses. The connective tissue often is composed of dilated capillaries that closely approximate the epithelium as well as infiltrations of lymphocytes. Specimens suspected for oral psoriasis should routinely be stained with periodic acid–Schiff to rule out candidiasis coinfection. The microscopic findings of our patient were congruent with prior reports of oral psoriasis.7,10-12 Some clinicians have questioned if psoriasis can actually occur in the oral cavity, but most authorities in the field have recognized its true existence, as evidenced by various shared HLA antigens, specifically HLA-Cw.13
Another group of oral lesions collectively referred to as psoriasiform mucositis, notably geographic tongue (benign migratory glossitis, erythema migrans) and its extraglossal variant geographic stomatitis,14,15 have histopathologic features and HLAs similar to those seen in cutaneous psoriasis.13 Interestingly, geographic tongue has been found in 3.8% to 9.1% of cohorts with cutaneous psoriasis,8,9 but in the extant population, the vast majority of patients with oral psoriasiform mucositis do not have cutaneous psoriasis. Other differential diagnoses for gingival psoriasis are lichen planus, human immunodeficiency virus–associated periodontitis, desquamative gingivitis, plasma cell gingivitis, erythematous candidiasis, mucous membrane pemphigoid, pemphigus vulgaris, leukemia, systemic lupus erythematosus, granulomatosis with polyangiitis, orofacial granulomatosis, localized juvenile spongiotic gingivitis hyperplasia, and primary gingivostomatitis.
Management of gingival psoriasis focuses on strategies to reduce inflammation and discomfort and measures to achieve meticulous oral plaque control. Judicious efforts should be exercised to avoid oral soft-tissue injury when performing periodontal scaling, although it has not been established whether gingival psoriasis is associated with the Köbner phenomenon, as seen with cutaneous lesions. Adjunctive measures employed for symptomatic patients have involved the use of corticosteroids (eg, lesional injection, oral rinse, systemic) and oral rinses with retinoic acid, chlorhexidine gluconate, and warm saline.7,10,16 Prolonged utilization of corticosteroids, however, may necessitate supplemental administration of antifungal agents.
This case report represents a rare documentation of a successful outcome of gingival and palatal psoriasis subsequent to the reinstitution of adalimumab solely for treatment of recurrent cutaneous disease. There likely is a pharmacologic basis for the amelioration of oral psoriasis in our patient. Adalimumab is a bivalent IgG monoclonal antibody that binds to activated dermal dendritic cell receptors of tumor necrosis factor α, thereby attenuating a cytokine-derived inflammatory response and apoptosis.17 In fact, patients with rheumatoid arthritis showed notable reductions in both gingival inflammation and bleeding following a 3-month regimen of adalimumab.18
Conclusion
Practitioners should be aware of the phenotypic overlap of cutaneous and oral psoriasis, particularly involving the gingiva and palate. It is recommended that psoriasis patients routinely receive a dental prophylaxis and engage in oral hygiene efforts to reduce the presence of oral microbiota. Furthermore, it is emphasized that psoriatic patients who maintain an atypical erythematous presentation on the oral mucosa undergo a biopsy for identification of the lesions and correlation with disease dissemination. Prospective studies are needed to characterize the clinical courses of oral psoriasis, ascertain their correlative behavior with cutaneous flares, and determine if lesional improvement can be achieved with the use of biologic agents or other therapeutic modalities.
- Gupta R, Debbaneh MG, Liao W. Genetic epidemiology of psoriasis. Curr Dermatol Rep. 2014;3:61-78.
- Younai FS, Phelan JA. Oral mucositis with features of psoriasis: report of a case and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;84:61-67.
- Xu T, Zhang YH. Association of psoriasis with stroke and myocardial infarction: meta-analysis of cohort studies. Br J Dermatol. 2012;167:1345-1350.
- Lazaridou E, Tsikrikoni A, Fotiadou C, et al. Association of chronic plaque psoriasis and severe periodontitis: a hospital based case-control study. J Eur Acad Dermatol Venereol. 2013;27:967-972.
- Skudutyte-Rysstad R, Slevolden EM, Hansen BF, et al. Association between moderate to severe psoriasis and periodontitis in a Scandinavian population. BMC Oral Health. 2014;14:139.
- Zunt SL, Tomich CE. Erythema migrans—a psoriasiform lesion of the oral mucosa. J Dermatol Surg Oncol. 1989;15:1067-1070.
- Reis V, Artico G, Seo J, et al. Psoriasiform mucositis on the gingival and palatal mucosae treated with retinoic-acid mouthwash. Int J Dermatol. 2013;52:113-115.
- Germi L, De Giorgi V, Bergamo F, et al. Psoriasis and oral lesions: multicentric study of oral mucosa diseases Italian group (GIPMO). Dermatol Online J. 2012;18:11.
- Kaur I, Handa S, Kumar B. Oral lesions in psoriasis. Int J Dermatol. 1997;36:78-79.
- Brayshaw HA, Orban B. Psoriasis gingivae. J Periodontol. 1953;24:156-160.
- Doben DI. Psoriasis of the attached gingiva. J Periodontol. 1976;47:38-40.
- Mattsson U, Warfvinge G, Jontell M. Oral psoriasis—a diagnostic dilemma: a report of two cases and a review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;120:e183-e189.
- Dermatologic diseases. In: Neville BW, Damm DD, Allen CM, et al, eds. Oral and Maxillofacial Pathology. 3rd ed. St. Louis, MO: Saunders/Elsevier; 2009:792-794.
- Brooks JK, Balciunas BA. Geographic stomatitis: review of the literature and report of five cases. J Am Dent Assoc. 1987;115:421-424.
- Brooks JK, Nikitakis NG. Multiple mucosal lesions. erythema migrans. Gen Dent. 2007;55:160, 163.
- Ulmansky M, Michelle R, Azaz B. Oral psoriasis: report of six new cases. J Oral Pathol Med. 1995;24:42-45.
- Lis K, Kuzawinska O, Bałkowiec-Iskra E. Tumor necrosis factor inhibitors—state of knowledge. Arch Med Sci. 2014;10:1175-1185.
- Kobayashi T, Yokoyama T, Ito S, et al. Periodontal and serum protein profiles in patients with rheumatoid arthritis treated with tumor necrosis factor inhibitor adalimumab. J Periodontol. 2014;85:1480-1488.
- Gupta R, Debbaneh MG, Liao W. Genetic epidemiology of psoriasis. Curr Dermatol Rep. 2014;3:61-78.
- Younai FS, Phelan JA. Oral mucositis with features of psoriasis: report of a case and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;84:61-67.
- Xu T, Zhang YH. Association of psoriasis with stroke and myocardial infarction: meta-analysis of cohort studies. Br J Dermatol. 2012;167:1345-1350.
- Lazaridou E, Tsikrikoni A, Fotiadou C, et al. Association of chronic plaque psoriasis and severe periodontitis: a hospital based case-control study. J Eur Acad Dermatol Venereol. 2013;27:967-972.
- Skudutyte-Rysstad R, Slevolden EM, Hansen BF, et al. Association between moderate to severe psoriasis and periodontitis in a Scandinavian population. BMC Oral Health. 2014;14:139.
- Zunt SL, Tomich CE. Erythema migrans—a psoriasiform lesion of the oral mucosa. J Dermatol Surg Oncol. 1989;15:1067-1070.
- Reis V, Artico G, Seo J, et al. Psoriasiform mucositis on the gingival and palatal mucosae treated with retinoic-acid mouthwash. Int J Dermatol. 2013;52:113-115.
- Germi L, De Giorgi V, Bergamo F, et al. Psoriasis and oral lesions: multicentric study of oral mucosa diseases Italian group (GIPMO). Dermatol Online J. 2012;18:11.
- Kaur I, Handa S, Kumar B. Oral lesions in psoriasis. Int J Dermatol. 1997;36:78-79.
- Brayshaw HA, Orban B. Psoriasis gingivae. J Periodontol. 1953;24:156-160.
- Doben DI. Psoriasis of the attached gingiva. J Periodontol. 1976;47:38-40.
- Mattsson U, Warfvinge G, Jontell M. Oral psoriasis—a diagnostic dilemma: a report of two cases and a review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;120:e183-e189.
- Dermatologic diseases. In: Neville BW, Damm DD, Allen CM, et al, eds. Oral and Maxillofacial Pathology. 3rd ed. St. Louis, MO: Saunders/Elsevier; 2009:792-794.
- Brooks JK, Balciunas BA. Geographic stomatitis: review of the literature and report of five cases. J Am Dent Assoc. 1987;115:421-424.
- Brooks JK, Nikitakis NG. Multiple mucosal lesions. erythema migrans. Gen Dent. 2007;55:160, 163.
- Ulmansky M, Michelle R, Azaz B. Oral psoriasis: report of six new cases. J Oral Pathol Med. 1995;24:42-45.
- Lis K, Kuzawinska O, Bałkowiec-Iskra E. Tumor necrosis factor inhibitors—state of knowledge. Arch Med Sci. 2014;10:1175-1185.
- Kobayashi T, Yokoyama T, Ito S, et al. Periodontal and serum protein profiles in patients with rheumatoid arthritis treated with tumor necrosis factor inhibitor adalimumab. J Periodontol. 2014;85:1480-1488.
Practice Points
- A subset of patients with cutaneous psoriasis may be associated with oral psoriatic outbreaks.
- Oral psoriasis presents as an atypical inflammatory response, and histopathologic assessment is recommended for lesional identity.
- Use of adalimumab for management of cutaneous psoriasis may demonstrate efficacy for oral psoriasis.
What’s Eating You? Lone Star Tick (Amblyomma americanum)
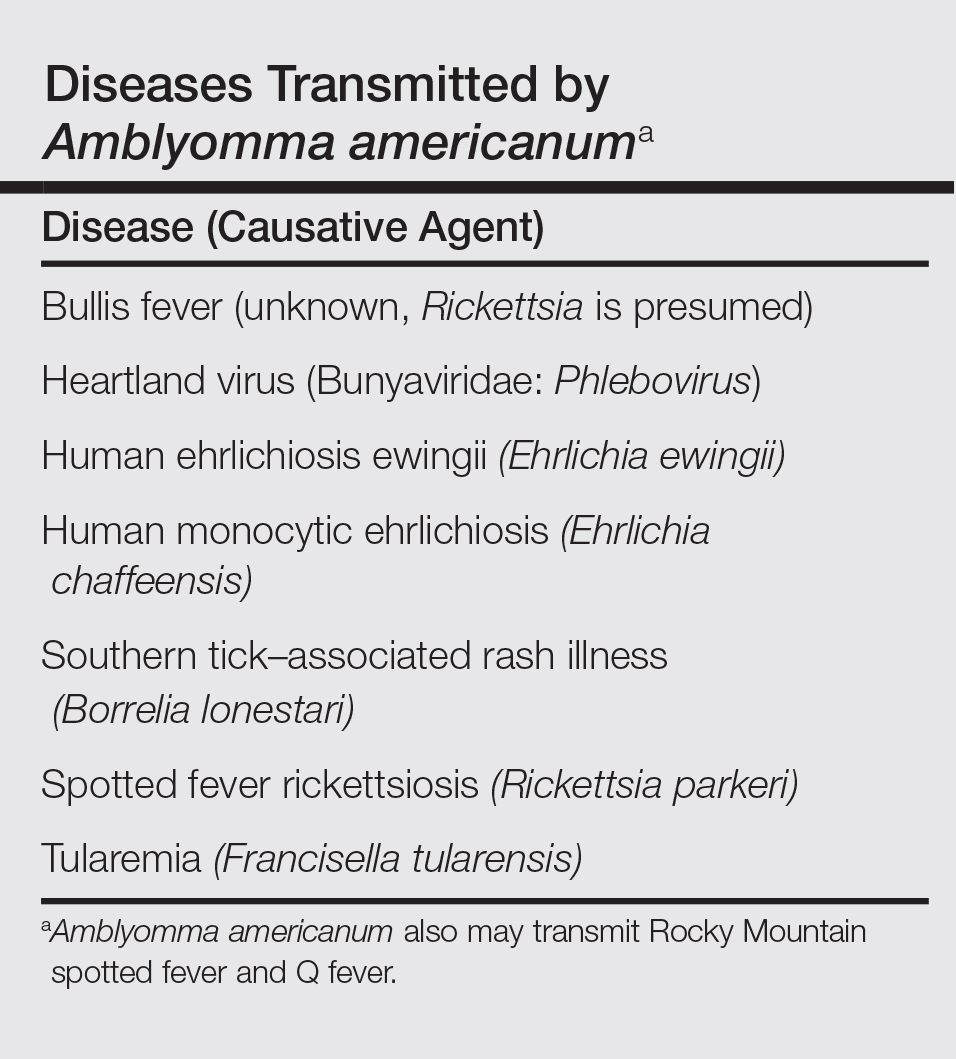
The lone star tick (Amblyomma americanum) is distributed throughout much of the eastern United States. It serves as a vector for species of Rickettsia, Ehrlichia, and Borrelia that are an important cause of tick-borne illness (Table). In addition, the bite of the lone star tick can cause impressive local and systemic reactions. Delayed anaphylaxis to ingestion of red meat has been attributed to the bite of A americanum.1 Herein, we discuss human disease associated with the lone star tick as well as potential tick-control measures.
Tick Characteristics
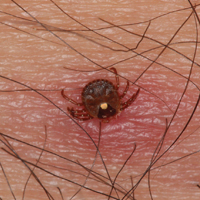
Lone star ticks are characterized by long anterior mouthparts and an ornate scutum (hard dorsal plate). Widely spaced eyes and posterior festoons also are present. In contrast to some other ticks, adanal plates are absent on the ventral surface in male lone star ticks. Amblyomma americanum demonstrates a single white spot on the female’s scutum (Figure 1). The male has inverted horseshoe markings on the posterior scutum. The female’s scutum often covers only a portion of the body to allow room for engorgement.
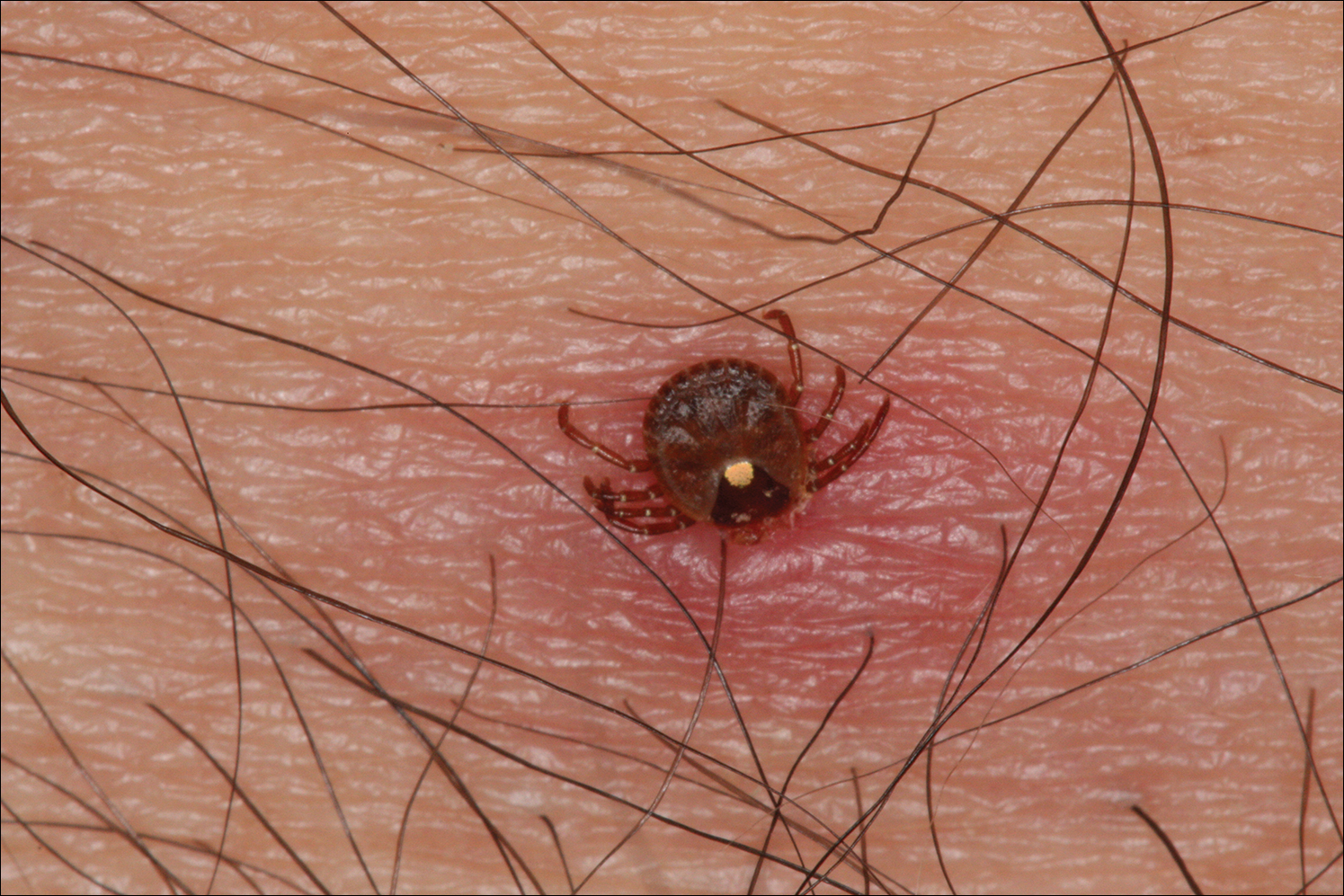
Patients usually become aware of tick bites while the tick is still attached to the skin, which provides the physician with an opportunity to identify the tick and discuss tick-control measures as well as symptoms of tick-borne disease. Once the tick has been removed, delayed-type hypersensitivity to the tick antigens continues at the attachment site. Erythema and pruritus can be dramatic. Nodules with a pseudolymphomatous histology can occur. Milder reactions respond to application of topical corticosteroids. More intense reactions may require intralesional corticosteroid injection or even surgical excision.
Most hard ticks have a 3-host life cycle, meaning they attach for one long blood meal during each phase of the life cycle. Because they search for a new host for each blood meal, they are efficient disease vectors. The larval ticks, so-called seed ticks, have 6 legs and feed on small animals. Nymphs and adults feed on larger animals. Nymphs resemble small adult ticks with 8 legs but are sexually immature.
Distribution
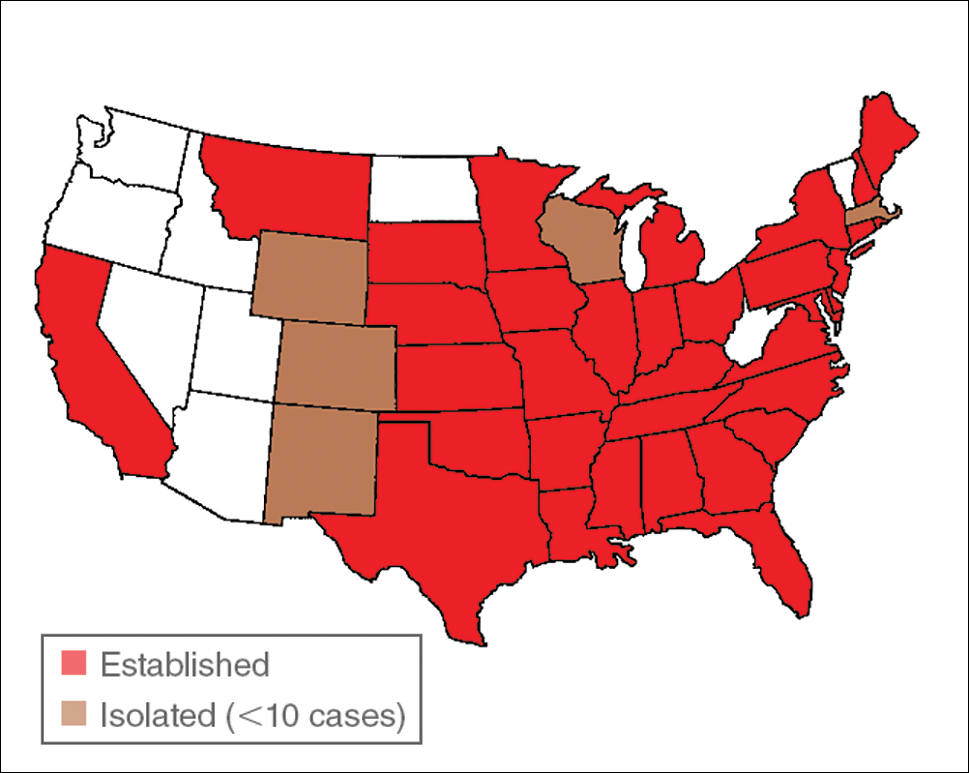
Amblyomma americanum has a wide distribution in the United States from Texas to Iowa and as far north as Maine (Figure 2).2 Tick attachments often are seen in individuals who work outdoors, especially in areas where new commercial or residential development disrupts the environment and the tick’s usual hosts move out of the area. Hungry ticks are left behind in search of a host.
Disease Transmission
Lone star ticks have been implicated as vectors of Ehrlichia chaffeensis, the agent of human monocytic ehrlichiosis (HME),3 which has been documented from the mid-Atlantic to south-central United States. It may present as a somewhat milder Rocky Mountain spotted fever–like illness with fever and headache or as a life-threatening systemic illness with organ failure. Prompt diagnosis and treatment with a tetracycline has been correlated with a better prognosis.4 Immunofluorescent antibody testing and polymerase chain reaction can be used to establish the diagnosis.5 Two tick species—A americanum and Dermacentor variabilis—have been implicated as vectors, but A americanum appears to be the major vector.6,7
The lone star tick also is a vector for Erlichia ewingii, the cause of human ehrlichiosis ewingii. Human ehrlichiosis ewingii is a rare disease that presents similar to HME, with most reported cases occurring in immunocompromised hosts.8
A novel member of the Phlebovirus genus, the Heartland virus, was first described in 2 Missouri farmers who presented with symptoms similar to HME but did not respond to doxycycline treatment.9 The virus has since been isolated from A americanum adult ticks, implicating them as the major vectors of the disease.10
Rickettsia parkeri, a cause of spotted fever rickettsiosis, is responsible for an eschar-associated illness in affected individuals.11 The organism has been detected in A americanum ticks collected from the wild. Experiments show the tick is capable of transmitting R parkeri to animals in the laboratory. It is unclear, however, what role A americanum plays in the natural transmission of the disease.12
In Missouri, strains of Borrelia have been isolated from A americanum ticks that feed on cottontail rabbits, but it seems unlikely that the tick plays any role in transmission of true Lyme disease13,14; Borrelia has been shown to have poor survival in the saliva of A americanum beyond 24 hours.15 Southern tick–associated rash illness is a Lyme disease–like illness with several reported cases due to A americanum.16 Patients generally present with an erythema migrans–like rash and may have headache, fever, arthralgia, or myalgia.16 The causative organism remains unclear, though Borrelia lonestari has been implicated.17 Lone star ticks also transmit tularemia and may transmit Rocky Mountain spotted fever and Q fever.13
Bullis fever (first reported at Camp Bullis near San Antonio, Texas) affected huge numbers of military personnel from 1942 to 1943.18 The causative organism appears to be rickettsial. During one outbreak of Bullis fever, it was noted that A americanum was so numerous that more than 4000 adult ticks were collected under a single juniper tree and more than 1000 ticks were removed from a single soldier who sat in a thicket for 2 hours.12 No cases of Bullis fever have been reported in recent years,12 which probably relates to the introduction of fire ants.
Disease Hosts
At Little Rock Air Force Base in Arkansas, A americanum has been a source of Ehrlichia infection. During one outbreak, deer in the area were found to have as many as 2550 ticks per ear,19 which demonstrates the magnitude of tick infestation in some areas of the United States. Tick infestation is not merely of concern to the US military. Ticks are ubiquitous and can be found on neatly trimmed suburban lawns as well as in rough thickets.
More recently, bites from A americanum have been found to induce allergies to red meat in some patients.1 IgE antibodies directed against galactose-alpha-1,3-galactose (alpha gal) have been implicated as the cause of this reaction. These antibodies cause delayed-onset anaphylaxis occurring 3 to 6 hours after ingestion of red meat. Tick bites appear to be the most important and perhaps the only cause of IgE antibodies to alpha gal in the United States.1
Wild white-tailed deer serve as reservoir hosts for several diseases transmitted by A americanum, including HME, human ehrlichiosis ewingii, and Southern tick–associated rash illness.12,20 Communities located close to wildlife reserves may have higher rates of infection.21 Application of acaricides to corn contained in deer feeders has been shown to be an effective method of decreasing local tick populations, which is a potential method for disease control in at-risk areas, though it is costly and time consuming.22
Tick-Control Measures
Hard ticks produce little urine. Instead, excess water is eliminated via salivation back into the host. Loss of water also occurs through spiracles. Absorption of water from the atmosphere is important for the tick to maintain hydration. The tick produces intensely hygroscopic saliva that absorbs water from surrounding moist air. The humidified saliva is then reingested by the tick. In hot climates, ticks are prone to dehydration unless they can find a source of moist air, usually within a layer of leaf debris.23 When the leaf debris is stirred by a human walking through the area, the tick can make contact with the human. Therefore, removal of leaf debris is a critical part of tick-control efforts, as it reduces tick numbers by means of dehydration. Tick eggs also require sufficient humidity to hatch. Leaf removal increases the effectiveness of insecticide applications, which would otherwise do little harm to the ticks below if sprayed on top of leaf debris.
Some lone star ticks attach to birds and disseminate widely. Attachments to animal hosts with long-range migration patterns complicate tick-control efforts.24 Animal migration may contribute to the spread of disease from one geographic region to another.
Imported fire ants are voracious eaters that gather and consume ticks eggs. Fire ants provide an excellent natural means of tick control. Tick numbers in places such as Camp Bullis have declined dramatically since the introduction of imported fire ants.25
- Commins SP, Platts-Mills TA. Tick bites and red meat allergy. Curr Opin Allergy Clin Immunol. 2013;13:354-359.
- Springer YP, Eisen L, Beati L, et al. Spatial distribution of counties in the continental United States with records of occurrence of Amblyomma americanum (Ixodida: Ixodidae). J Med Entomol. 2014;51:342-351.
- Yu X, Piesman JF, Olson JG, et al. Geographic distribution of different genetic types of Ehrlichia chaffeensis. Am J Trop Med Hyg. 1997;56:679-680.
- Dumler JS, Bakken JS. Human ehrlichiosis: newly recognized infections transmitted by ticks. An Rev Med. 1998;49:201-213.
- Dumler JS, Madigan JE, Pusterla N, et al. Ehrlichioses in humans: epidemiology, clinical presentation, diagnosis, and treatment. Clin Infect Dis. 2007;45(suppl 1):S45-S51.
- Lockhart JM, Davidson WR, Stallknecht DE, et al. Natural history of Ehrlichia chaffeensis (Ricketsiales: Ehrlichiea) in the piedmont physiographic province of Georgia. J Parasitol. 1997;83:887-894.
- Centers for Disease Control and Prevention (CDC). Human ehrlichiosis—Maryland, 1994. MMWR Morb Mortal Wkly Rep. 1996;45:798-802.
- Ismail N, Bloch KC, McBride JW. Human ehrlichiosis and anaplasmosis. Clin Lab Med. 2010;30:261-292.
- McMullan LK, Folk SM, Kelly AJ, et al. A new phlebovirus associated with severe febrile illness in Missouri. N Engl J Med. 2012;367:834-841.
- Savage HM, Godsey MS Jr, Panella NA, et al. Surveillance for heartland virus (Bunyaviridae: Phlebovirus) in Missouri during 2013: first detection of virus in adults of Amblyomma americanum (Acari: Ixodidae) [published online March 30, 2016]. J Med Entomol. pii:tjw028.
- Cragun WC, Bartlett BL, Ellis MW, et al. The expanding spectrum of eschar-associated rickettsioses in the United States. Arch Dermatol. 2010;146:641-648.
- Paddock CD, Sumner JW, Comer JA, et al. Rickettsia parkeri: a newly recognized cause of spotted fever rickettsiosis in the United States. Clin Infect Dis. 2004;38:805-811.
- Goddard J, Varela-Stokes AS. Role of the lone star tick, Amblyomma americanum (L.) in human and animal diseases. Vet Parasitol. 2009;160:1-12.
- Oliver JH, Kollars TM, Chandler FW, et al. First isolation and cultivation of Borrelia burgdorferi sensu lato from Missouri. J Clin Microbiol. 1998;36:1-5.
- Ledin KE, Zeidner NS, Ribeiro JM, et al. Borreliacidal activity of saliva of the tick Amblyomma americanum. Med Vet Entomol. 2005;19:90-95.
- Feder HM Jr, Hoss DM, Zemel L, et al. Southern tick-associated rash illness (STARI) in the North: STARI following a tick bite in Long Island, New York. Clin Infect Dis. 2011;53:e142-e146.
- Varela AS, Luttrell MP, Howerth EW, et al. First culture isolation of Borrelia lonestari, putative agent of southern tick-associated rash illness. J Clin Microbiol. 2004;42:1163-1169.
- Livesay HR, Pollard M. Laboratory report on a clinical syndrome referred to as “Bullis Fever.” Am J Trop Med. 1943;23:475-479.
- Goddard J. Ticks and tickborne diseases affecting military personnel. US Air Force School of Aerospace Medicine USAFSAM-SR-89-2. http://www.dtic.mil/dtic/tr/fulltext/u2/a221956.pdf. Published September 1989. Accessed January 19, 2017.
- Lockhart JM, Davidson WR, Stallkneeckt DE, et al. Isolation of Ehrlichia chaffeensis from wild white tailed deer (Odocoileus virginianus) confirms their role as natural reservoir hosts. J Clin Microbiol. 1997;35:1681-1686.
- Standaert SM, Dawson JE, Schaffner W, et al. Ehrlichiosis in a golf-oriented retirement community. N Engl J Med. 1995;333:420-425.
- Schulze TL, Jordan RA, Hung RW, et al. Effectiveness of the 4-Poster passive topical treatment device in the control of Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae) in New Jersey. Vector Borne Zoonotic Dis. 2009;9:389-400.
- Strey OF, Teel PD, Longnecker MT, et al. Survival and water-balance characteristics of unfed Amblyomma cajennense (Acari: Ixodidae). J Med Entomol. 1996;33:63-73.
- Popham TW, Garris GI, Barre N. Development of a computer model of the population dynamics of Amblyomma variegatum and simulations of eradication strategies for use in the Caribbean. Ann New York Acad Sci. 1996;791:452-465.
- Burns EC, Melancon DG. Effect of important fire ant (Hymenoptera: Formicidae) invasion on lone star tick (Acarina: Ixodidae) populations. J Med Entomol. 1977;14:247-249.
The lone star tick (Amblyomma americanum) is distributed throughout much of the eastern United States. It serves as a vector for species of Rickettsia, Ehrlichia, and Borrelia that are an important cause of tick-borne illness (Table). In addition, the bite of the lone star tick can cause impressive local and systemic reactions. Delayed anaphylaxis to ingestion of red meat has been attributed to the bite of A americanum.1 Herein, we discuss human disease associated with the lone star tick as well as potential tick-control measures.

Tick Characteristics
Lone star ticks are characterized by long anterior mouthparts and an ornate scutum (hard dorsal plate). Widely spaced eyes and posterior festoons also are present. In contrast to some other ticks, adanal plates are absent on the ventral surface in male lone star ticks. Amblyomma americanum demonstrates a single white spot on the female’s scutum (Figure 1). The male has inverted horseshoe markings on the posterior scutum. The female’s scutum often covers only a portion of the body to allow room for engorgement.

Patients usually become aware of tick bites while the tick is still attached to the skin, which provides the physician with an opportunity to identify the tick and discuss tick-control measures as well as symptoms of tick-borne disease. Once the tick has been removed, delayed-type hypersensitivity to the tick antigens continues at the attachment site. Erythema and pruritus can be dramatic. Nodules with a pseudolymphomatous histology can occur. Milder reactions respond to application of topical corticosteroids. More intense reactions may require intralesional corticosteroid injection or even surgical excision.
Most hard ticks have a 3-host life cycle, meaning they attach for one long blood meal during each phase of the life cycle. Because they search for a new host for each blood meal, they are efficient disease vectors. The larval ticks, so-called seed ticks, have 6 legs and feed on small animals. Nymphs and adults feed on larger animals. Nymphs resemble small adult ticks with 8 legs but are sexually immature.
Distribution
Amblyomma americanum has a wide distribution in the United States from Texas to Iowa and as far north as Maine (Figure 2).2 Tick attachments often are seen in individuals who work outdoors, especially in areas where new commercial or residential development disrupts the environment and the tick’s usual hosts move out of the area. Hungry ticks are left behind in search of a host.

Disease Transmission
Lone star ticks have been implicated as vectors of Ehrlichia chaffeensis, the agent of human monocytic ehrlichiosis (HME),3 which has been documented from the mid-Atlantic to south-central United States. It may present as a somewhat milder Rocky Mountain spotted fever–like illness with fever and headache or as a life-threatening systemic illness with organ failure. Prompt diagnosis and treatment with a tetracycline has been correlated with a better prognosis.4 Immunofluorescent antibody testing and polymerase chain reaction can be used to establish the diagnosis.5 Two tick species—A americanum and Dermacentor variabilis—have been implicated as vectors, but A americanum appears to be the major vector.6,7
The lone star tick also is a vector for Erlichia ewingii, the cause of human ehrlichiosis ewingii. Human ehrlichiosis ewingii is a rare disease that presents similar to HME, with most reported cases occurring in immunocompromised hosts.8
A novel member of the Phlebovirus genus, the Heartland virus, was first described in 2 Missouri farmers who presented with symptoms similar to HME but did not respond to doxycycline treatment.9 The virus has since been isolated from A americanum adult ticks, implicating them as the major vectors of the disease.10
Rickettsia parkeri, a cause of spotted fever rickettsiosis, is responsible for an eschar-associated illness in affected individuals.11 The organism has been detected in A americanum ticks collected from the wild. Experiments show the tick is capable of transmitting R parkeri to animals in the laboratory. It is unclear, however, what role A americanum plays in the natural transmission of the disease.12
In Missouri, strains of Borrelia have been isolated from A americanum ticks that feed on cottontail rabbits, but it seems unlikely that the tick plays any role in transmission of true Lyme disease13,14; Borrelia has been shown to have poor survival in the saliva of A americanum beyond 24 hours.15 Southern tick–associated rash illness is a Lyme disease–like illness with several reported cases due to A americanum.16 Patients generally present with an erythema migrans–like rash and may have headache, fever, arthralgia, or myalgia.16 The causative organism remains unclear, though Borrelia lonestari has been implicated.17 Lone star ticks also transmit tularemia and may transmit Rocky Mountain spotted fever and Q fever.13
Bullis fever (first reported at Camp Bullis near San Antonio, Texas) affected huge numbers of military personnel from 1942 to 1943.18 The causative organism appears to be rickettsial. During one outbreak of Bullis fever, it was noted that A americanum was so numerous that more than 4000 adult ticks were collected under a single juniper tree and more than 1000 ticks were removed from a single soldier who sat in a thicket for 2 hours.12 No cases of Bullis fever have been reported in recent years,12 which probably relates to the introduction of fire ants.
Disease Hosts
At Little Rock Air Force Base in Arkansas, A americanum has been a source of Ehrlichia infection. During one outbreak, deer in the area were found to have as many as 2550 ticks per ear,19 which demonstrates the magnitude of tick infestation in some areas of the United States. Tick infestation is not merely of concern to the US military. Ticks are ubiquitous and can be found on neatly trimmed suburban lawns as well as in rough thickets.
More recently, bites from A americanum have been found to induce allergies to red meat in some patients.1 IgE antibodies directed against galactose-alpha-1,3-galactose (alpha gal) have been implicated as the cause of this reaction. These antibodies cause delayed-onset anaphylaxis occurring 3 to 6 hours after ingestion of red meat. Tick bites appear to be the most important and perhaps the only cause of IgE antibodies to alpha gal in the United States.1
Wild white-tailed deer serve as reservoir hosts for several diseases transmitted by A americanum, including HME, human ehrlichiosis ewingii, and Southern tick–associated rash illness.12,20 Communities located close to wildlife reserves may have higher rates of infection.21 Application of acaricides to corn contained in deer feeders has been shown to be an effective method of decreasing local tick populations, which is a potential method for disease control in at-risk areas, though it is costly and time consuming.22
Tick-Control Measures
Hard ticks produce little urine. Instead, excess water is eliminated via salivation back into the host. Loss of water also occurs through spiracles. Absorption of water from the atmosphere is important for the tick to maintain hydration. The tick produces intensely hygroscopic saliva that absorbs water from surrounding moist air. The humidified saliva is then reingested by the tick. In hot climates, ticks are prone to dehydration unless they can find a source of moist air, usually within a layer of leaf debris.23 When the leaf debris is stirred by a human walking through the area, the tick can make contact with the human. Therefore, removal of leaf debris is a critical part of tick-control efforts, as it reduces tick numbers by means of dehydration. Tick eggs also require sufficient humidity to hatch. Leaf removal increases the effectiveness of insecticide applications, which would otherwise do little harm to the ticks below if sprayed on top of leaf debris.
Some lone star ticks attach to birds and disseminate widely. Attachments to animal hosts with long-range migration patterns complicate tick-control efforts.24 Animal migration may contribute to the spread of disease from one geographic region to another.
Imported fire ants are voracious eaters that gather and consume ticks eggs. Fire ants provide an excellent natural means of tick control. Tick numbers in places such as Camp Bullis have declined dramatically since the introduction of imported fire ants.25
The lone star tick (Amblyomma americanum) is distributed throughout much of the eastern United States. It serves as a vector for species of Rickettsia, Ehrlichia, and Borrelia that are an important cause of tick-borne illness (Table). In addition, the bite of the lone star tick can cause impressive local and systemic reactions. Delayed anaphylaxis to ingestion of red meat has been attributed to the bite of A americanum.1 Herein, we discuss human disease associated with the lone star tick as well as potential tick-control measures.

Tick Characteristics
Lone star ticks are characterized by long anterior mouthparts and an ornate scutum (hard dorsal plate). Widely spaced eyes and posterior festoons also are present. In contrast to some other ticks, adanal plates are absent on the ventral surface in male lone star ticks. Amblyomma americanum demonstrates a single white spot on the female’s scutum (Figure 1). The male has inverted horseshoe markings on the posterior scutum. The female’s scutum often covers only a portion of the body to allow room for engorgement.

Patients usually become aware of tick bites while the tick is still attached to the skin, which provides the physician with an opportunity to identify the tick and discuss tick-control measures as well as symptoms of tick-borne disease. Once the tick has been removed, delayed-type hypersensitivity to the tick antigens continues at the attachment site. Erythema and pruritus can be dramatic. Nodules with a pseudolymphomatous histology can occur. Milder reactions respond to application of topical corticosteroids. More intense reactions may require intralesional corticosteroid injection or even surgical excision.
Most hard ticks have a 3-host life cycle, meaning they attach for one long blood meal during each phase of the life cycle. Because they search for a new host for each blood meal, they are efficient disease vectors. The larval ticks, so-called seed ticks, have 6 legs and feed on small animals. Nymphs and adults feed on larger animals. Nymphs resemble small adult ticks with 8 legs but are sexually immature.
Distribution
Amblyomma americanum has a wide distribution in the United States from Texas to Iowa and as far north as Maine (Figure 2).2 Tick attachments often are seen in individuals who work outdoors, especially in areas where new commercial or residential development disrupts the environment and the tick’s usual hosts move out of the area. Hungry ticks are left behind in search of a host.

Disease Transmission
Lone star ticks have been implicated as vectors of Ehrlichia chaffeensis, the agent of human monocytic ehrlichiosis (HME),3 which has been documented from the mid-Atlantic to south-central United States. It may present as a somewhat milder Rocky Mountain spotted fever–like illness with fever and headache or as a life-threatening systemic illness with organ failure. Prompt diagnosis and treatment with a tetracycline has been correlated with a better prognosis.4 Immunofluorescent antibody testing and polymerase chain reaction can be used to establish the diagnosis.5 Two tick species—A americanum and Dermacentor variabilis—have been implicated as vectors, but A americanum appears to be the major vector.6,7
The lone star tick also is a vector for Erlichia ewingii, the cause of human ehrlichiosis ewingii. Human ehrlichiosis ewingii is a rare disease that presents similar to HME, with most reported cases occurring in immunocompromised hosts.8
A novel member of the Phlebovirus genus, the Heartland virus, was first described in 2 Missouri farmers who presented with symptoms similar to HME but did not respond to doxycycline treatment.9 The virus has since been isolated from A americanum adult ticks, implicating them as the major vectors of the disease.10
Rickettsia parkeri, a cause of spotted fever rickettsiosis, is responsible for an eschar-associated illness in affected individuals.11 The organism has been detected in A americanum ticks collected from the wild. Experiments show the tick is capable of transmitting R parkeri to animals in the laboratory. It is unclear, however, what role A americanum plays in the natural transmission of the disease.12
In Missouri, strains of Borrelia have been isolated from A americanum ticks that feed on cottontail rabbits, but it seems unlikely that the tick plays any role in transmission of true Lyme disease13,14; Borrelia has been shown to have poor survival in the saliva of A americanum beyond 24 hours.15 Southern tick–associated rash illness is a Lyme disease–like illness with several reported cases due to A americanum.16 Patients generally present with an erythema migrans–like rash and may have headache, fever, arthralgia, or myalgia.16 The causative organism remains unclear, though Borrelia lonestari has been implicated.17 Lone star ticks also transmit tularemia and may transmit Rocky Mountain spotted fever and Q fever.13
Bullis fever (first reported at Camp Bullis near San Antonio, Texas) affected huge numbers of military personnel from 1942 to 1943.18 The causative organism appears to be rickettsial. During one outbreak of Bullis fever, it was noted that A americanum was so numerous that more than 4000 adult ticks were collected under a single juniper tree and more than 1000 ticks were removed from a single soldier who sat in a thicket for 2 hours.12 No cases of Bullis fever have been reported in recent years,12 which probably relates to the introduction of fire ants.
Disease Hosts
At Little Rock Air Force Base in Arkansas, A americanum has been a source of Ehrlichia infection. During one outbreak, deer in the area were found to have as many as 2550 ticks per ear,19 which demonstrates the magnitude of tick infestation in some areas of the United States. Tick infestation is not merely of concern to the US military. Ticks are ubiquitous and can be found on neatly trimmed suburban lawns as well as in rough thickets.
More recently, bites from A americanum have been found to induce allergies to red meat in some patients.1 IgE antibodies directed against galactose-alpha-1,3-galactose (alpha gal) have been implicated as the cause of this reaction. These antibodies cause delayed-onset anaphylaxis occurring 3 to 6 hours after ingestion of red meat. Tick bites appear to be the most important and perhaps the only cause of IgE antibodies to alpha gal in the United States.1
Wild white-tailed deer serve as reservoir hosts for several diseases transmitted by A americanum, including HME, human ehrlichiosis ewingii, and Southern tick–associated rash illness.12,20 Communities located close to wildlife reserves may have higher rates of infection.21 Application of acaricides to corn contained in deer feeders has been shown to be an effective method of decreasing local tick populations, which is a potential method for disease control in at-risk areas, though it is costly and time consuming.22
Tick-Control Measures
Hard ticks produce little urine. Instead, excess water is eliminated via salivation back into the host. Loss of water also occurs through spiracles. Absorption of water from the atmosphere is important for the tick to maintain hydration. The tick produces intensely hygroscopic saliva that absorbs water from surrounding moist air. The humidified saliva is then reingested by the tick. In hot climates, ticks are prone to dehydration unless they can find a source of moist air, usually within a layer of leaf debris.23 When the leaf debris is stirred by a human walking through the area, the tick can make contact with the human. Therefore, removal of leaf debris is a critical part of tick-control efforts, as it reduces tick numbers by means of dehydration. Tick eggs also require sufficient humidity to hatch. Leaf removal increases the effectiveness of insecticide applications, which would otherwise do little harm to the ticks below if sprayed on top of leaf debris.
Some lone star ticks attach to birds and disseminate widely. Attachments to animal hosts with long-range migration patterns complicate tick-control efforts.24 Animal migration may contribute to the spread of disease from one geographic region to another.
Imported fire ants are voracious eaters that gather and consume ticks eggs. Fire ants provide an excellent natural means of tick control. Tick numbers in places such as Camp Bullis have declined dramatically since the introduction of imported fire ants.25
- Commins SP, Platts-Mills TA. Tick bites and red meat allergy. Curr Opin Allergy Clin Immunol. 2013;13:354-359.
- Springer YP, Eisen L, Beati L, et al. Spatial distribution of counties in the continental United States with records of occurrence of Amblyomma americanum (Ixodida: Ixodidae). J Med Entomol. 2014;51:342-351.
- Yu X, Piesman JF, Olson JG, et al. Geographic distribution of different genetic types of Ehrlichia chaffeensis. Am J Trop Med Hyg. 1997;56:679-680.
- Dumler JS, Bakken JS. Human ehrlichiosis: newly recognized infections transmitted by ticks. An Rev Med. 1998;49:201-213.
- Dumler JS, Madigan JE, Pusterla N, et al. Ehrlichioses in humans: epidemiology, clinical presentation, diagnosis, and treatment. Clin Infect Dis. 2007;45(suppl 1):S45-S51.
- Lockhart JM, Davidson WR, Stallknecht DE, et al. Natural history of Ehrlichia chaffeensis (Ricketsiales: Ehrlichiea) in the piedmont physiographic province of Georgia. J Parasitol. 1997;83:887-894.
- Centers for Disease Control and Prevention (CDC). Human ehrlichiosis—Maryland, 1994. MMWR Morb Mortal Wkly Rep. 1996;45:798-802.
- Ismail N, Bloch KC, McBride JW. Human ehrlichiosis and anaplasmosis. Clin Lab Med. 2010;30:261-292.
- McMullan LK, Folk SM, Kelly AJ, et al. A new phlebovirus associated with severe febrile illness in Missouri. N Engl J Med. 2012;367:834-841.
- Savage HM, Godsey MS Jr, Panella NA, et al. Surveillance for heartland virus (Bunyaviridae: Phlebovirus) in Missouri during 2013: first detection of virus in adults of Amblyomma americanum (Acari: Ixodidae) [published online March 30, 2016]. J Med Entomol. pii:tjw028.
- Cragun WC, Bartlett BL, Ellis MW, et al. The expanding spectrum of eschar-associated rickettsioses in the United States. Arch Dermatol. 2010;146:641-648.
- Paddock CD, Sumner JW, Comer JA, et al. Rickettsia parkeri: a newly recognized cause of spotted fever rickettsiosis in the United States. Clin Infect Dis. 2004;38:805-811.
- Goddard J, Varela-Stokes AS. Role of the lone star tick, Amblyomma americanum (L.) in human and animal diseases. Vet Parasitol. 2009;160:1-12.
- Oliver JH, Kollars TM, Chandler FW, et al. First isolation and cultivation of Borrelia burgdorferi sensu lato from Missouri. J Clin Microbiol. 1998;36:1-5.
- Ledin KE, Zeidner NS, Ribeiro JM, et al. Borreliacidal activity of saliva of the tick Amblyomma americanum. Med Vet Entomol. 2005;19:90-95.
- Feder HM Jr, Hoss DM, Zemel L, et al. Southern tick-associated rash illness (STARI) in the North: STARI following a tick bite in Long Island, New York. Clin Infect Dis. 2011;53:e142-e146.
- Varela AS, Luttrell MP, Howerth EW, et al. First culture isolation of Borrelia lonestari, putative agent of southern tick-associated rash illness. J Clin Microbiol. 2004;42:1163-1169.
- Livesay HR, Pollard M. Laboratory report on a clinical syndrome referred to as “Bullis Fever.” Am J Trop Med. 1943;23:475-479.
- Goddard J. Ticks and tickborne diseases affecting military personnel. US Air Force School of Aerospace Medicine USAFSAM-SR-89-2. http://www.dtic.mil/dtic/tr/fulltext/u2/a221956.pdf. Published September 1989. Accessed January 19, 2017.
- Lockhart JM, Davidson WR, Stallkneeckt DE, et al. Isolation of Ehrlichia chaffeensis from wild white tailed deer (Odocoileus virginianus) confirms their role as natural reservoir hosts. J Clin Microbiol. 1997;35:1681-1686.
- Standaert SM, Dawson JE, Schaffner W, et al. Ehrlichiosis in a golf-oriented retirement community. N Engl J Med. 1995;333:420-425.
- Schulze TL, Jordan RA, Hung RW, et al. Effectiveness of the 4-Poster passive topical treatment device in the control of Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae) in New Jersey. Vector Borne Zoonotic Dis. 2009;9:389-400.
- Strey OF, Teel PD, Longnecker MT, et al. Survival and water-balance characteristics of unfed Amblyomma cajennense (Acari: Ixodidae). J Med Entomol. 1996;33:63-73.
- Popham TW, Garris GI, Barre N. Development of a computer model of the population dynamics of Amblyomma variegatum and simulations of eradication strategies for use in the Caribbean. Ann New York Acad Sci. 1996;791:452-465.
- Burns EC, Melancon DG. Effect of important fire ant (Hymenoptera: Formicidae) invasion on lone star tick (Acarina: Ixodidae) populations. J Med Entomol. 1977;14:247-249.
- Commins SP, Platts-Mills TA. Tick bites and red meat allergy. Curr Opin Allergy Clin Immunol. 2013;13:354-359.
- Springer YP, Eisen L, Beati L, et al. Spatial distribution of counties in the continental United States with records of occurrence of Amblyomma americanum (Ixodida: Ixodidae). J Med Entomol. 2014;51:342-351.
- Yu X, Piesman JF, Olson JG, et al. Geographic distribution of different genetic types of Ehrlichia chaffeensis. Am J Trop Med Hyg. 1997;56:679-680.
- Dumler JS, Bakken JS. Human ehrlichiosis: newly recognized infections transmitted by ticks. An Rev Med. 1998;49:201-213.
- Dumler JS, Madigan JE, Pusterla N, et al. Ehrlichioses in humans: epidemiology, clinical presentation, diagnosis, and treatment. Clin Infect Dis. 2007;45(suppl 1):S45-S51.
- Lockhart JM, Davidson WR, Stallknecht DE, et al. Natural history of Ehrlichia chaffeensis (Ricketsiales: Ehrlichiea) in the piedmont physiographic province of Georgia. J Parasitol. 1997;83:887-894.
- Centers for Disease Control and Prevention (CDC). Human ehrlichiosis—Maryland, 1994. MMWR Morb Mortal Wkly Rep. 1996;45:798-802.
- Ismail N, Bloch KC, McBride JW. Human ehrlichiosis and anaplasmosis. Clin Lab Med. 2010;30:261-292.
- McMullan LK, Folk SM, Kelly AJ, et al. A new phlebovirus associated with severe febrile illness in Missouri. N Engl J Med. 2012;367:834-841.
- Savage HM, Godsey MS Jr, Panella NA, et al. Surveillance for heartland virus (Bunyaviridae: Phlebovirus) in Missouri during 2013: first detection of virus in adults of Amblyomma americanum (Acari: Ixodidae) [published online March 30, 2016]. J Med Entomol. pii:tjw028.
- Cragun WC, Bartlett BL, Ellis MW, et al. The expanding spectrum of eschar-associated rickettsioses in the United States. Arch Dermatol. 2010;146:641-648.
- Paddock CD, Sumner JW, Comer JA, et al. Rickettsia parkeri: a newly recognized cause of spotted fever rickettsiosis in the United States. Clin Infect Dis. 2004;38:805-811.
- Goddard J, Varela-Stokes AS. Role of the lone star tick, Amblyomma americanum (L.) in human and animal diseases. Vet Parasitol. 2009;160:1-12.
- Oliver JH, Kollars TM, Chandler FW, et al. First isolation and cultivation of Borrelia burgdorferi sensu lato from Missouri. J Clin Microbiol. 1998;36:1-5.
- Ledin KE, Zeidner NS, Ribeiro JM, et al. Borreliacidal activity of saliva of the tick Amblyomma americanum. Med Vet Entomol. 2005;19:90-95.
- Feder HM Jr, Hoss DM, Zemel L, et al. Southern tick-associated rash illness (STARI) in the North: STARI following a tick bite in Long Island, New York. Clin Infect Dis. 2011;53:e142-e146.
- Varela AS, Luttrell MP, Howerth EW, et al. First culture isolation of Borrelia lonestari, putative agent of southern tick-associated rash illness. J Clin Microbiol. 2004;42:1163-1169.
- Livesay HR, Pollard M. Laboratory report on a clinical syndrome referred to as “Bullis Fever.” Am J Trop Med. 1943;23:475-479.
- Goddard J. Ticks and tickborne diseases affecting military personnel. US Air Force School of Aerospace Medicine USAFSAM-SR-89-2. http://www.dtic.mil/dtic/tr/fulltext/u2/a221956.pdf. Published September 1989. Accessed January 19, 2017.
- Lockhart JM, Davidson WR, Stallkneeckt DE, et al. Isolation of Ehrlichia chaffeensis from wild white tailed deer (Odocoileus virginianus) confirms their role as natural reservoir hosts. J Clin Microbiol. 1997;35:1681-1686.
- Standaert SM, Dawson JE, Schaffner W, et al. Ehrlichiosis in a golf-oriented retirement community. N Engl J Med. 1995;333:420-425.
- Schulze TL, Jordan RA, Hung RW, et al. Effectiveness of the 4-Poster passive topical treatment device in the control of Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae) in New Jersey. Vector Borne Zoonotic Dis. 2009;9:389-400.
- Strey OF, Teel PD, Longnecker MT, et al. Survival and water-balance characteristics of unfed Amblyomma cajennense (Acari: Ixodidae). J Med Entomol. 1996;33:63-73.
- Popham TW, Garris GI, Barre N. Development of a computer model of the population dynamics of Amblyomma variegatum and simulations of eradication strategies for use in the Caribbean. Ann New York Acad Sci. 1996;791:452-465.
- Burns EC, Melancon DG. Effect of important fire ant (Hymenoptera: Formicidae) invasion on lone star tick (Acarina: Ixodidae) populations. J Med Entomol. 1977;14:247-249.
Practice Points
- Amblyomma americanum (lone star tick) is widely distributed throughout the United States and is an important cause of several tick-borne illnesses.
- Prompt diagnosis and treatment of tick-borne disease improves patient outcomes.
- In some cases, tick bites may cause the human host to develop certain IgE antibodies that result in a delayed-onset anaphylaxis after ingestion of red meat.
Biosimilars in Psoriasis: The Future or Not?
According to the US Food and Drug Administration (FDA), a biosimilar is “highly similar to an FDA-approved biological product, . . . and has no clinically meaningful differences in terms of safety and effectiveness.”1 The Biologics Price Competition and Innovation (BPCI) Act of 2009 created an expedited pathway for the approval of products shown to be biosimilar to FDA-licensed reference products.2 In 2013, the European Medicines Agency approved the first biosimilar modeled on infliximab (Remsima [formerly known as CT-P13], Celltrion Healthcare Co, Ltd) for the same indications as its reference product.3 In 2016, the FDA approved Inflectra (Hospira, a Pfizer Company), an infliximab biosimilar; Erelzi (Sandoz, a Novartis Division), an etanercept biosimilar; and Amjevita (Amgen Inc), an adalimumab biosimilar, all for numerous clinical indications including plaque psoriasis and psoriatic arthritis.4-6
There has been a substantial amount of distrust surrounding the biosimilars; however, as the patents for the biologic agents expire, new biosimilars will undoubtedly flood the market. In this article, we provide information that will help dermatologists understand the need for and use of these agents.
Biosimilars Versus Generic Drugs
Small-molecule generics can be made in a process that is relatively inexpensive, reproducible, and able to yield identical products with each lot.7 In contrast, biosimilars are large complex proteins made in living cells. They differ from their reference product because of changes that occur during manufacturing (eg, purification system, posttranslational modifications).7-9 Glycosylation is particularly sensitive to manufacturing and can affect the immunogenicity of the product.9 The impact of manufacturing can be substantial; for example, during phase 3 trials for efalizumab, a change in the manufacturing facility affected pharmacokinetic properties to such a degree that the FDA required a repeat of the trials.10
FDA Guidelines on Biosimilarity
The FDA outlines the following approach to demonstrate biosimilarity.2 The first step is structural characterization to evaluate the primary, secondary, tertiary, and quaternary structures and posttranslational modifications. The next step utilizes in vivo and/or in vitro functional assays to compare the biosimilar and reference product. The third step is a focus on toxicity and immunogenicity. The fourth step involves clinical studies to study pharmacokinetic and pharmacodynamic data, immunogenicity, safety, and efficacy. After the biosimilar has been approved, there must be a system in place to monitor postmarketing safety. If a biosimilar is tested in one patient population (eg, patients with plaque psoriasis), a request can be made to approve the drug for all the conditions that the reference product was approved for, such as plaque psoriasis, rheumatoid arthritis, and inflammatory bowel disease, even though clinical trials were not performed in all of these patient populations.2 The BPCI Act leaves it up to the FDA to determine how much and what type of data (eg, in vitro, in vivo, clinical) are required.11
Extrapolation and Interchangeability
Once a biosimilar has been approved, 2 questions must be answered: First, can its use be extrapolated to all indications for the reference product? The infliximab biosimilar approved by the European Medicines Agency and the FDA had only been studied in patients with ankylosing spondylitis12 and rheumatoid arthritis,13 yet it was granted all the indications for infliximab, including severe plaque psoriasis.14 As of now, the various regulatory agencies differ on their policies regarding extrapolation. Extrapolation is not automatically bestowed on a biosimilar in the United States but can be requested by the manufacturer.2
Second, can the biosimilar be seamlessly switched with its reference product at the pharmacy level? The BPCI Act allows for the substitution of biosimilars that are deemed interchangeable without notifying the provider, yet individual states ultimately can pass laws regarding this issue.15,16 An interchangeable agent would “produce the same clinical result as the reference product,” and “the risk in terms of safety or diminished efficacy of alternating or switching between use of the biological product and the reference product is not greater than the risk of using the reference product.”15 Generic drugs are allowed to be substituted without notifying the patient or prescriber16; however, biosimilars that are not deemed interchangeable would require permission from the prescriber before substitution.11
Biosimilars for Psoriasis
In April 2016, an infliximab biosimilar (Inflectra) became the second biosimilar approved by the FDA.4 Inflectra was studied in clinical trials for patients with ankylosing spondylitis17 and rheumatoid arthritis,18 and in both trials the biosimilar was found to have similar efficacy and safety profiles to that of the reference product. In August 2016, an etanercept biosimilar (Erelzi) was approved,5 and in September 2016, an adalimumab biosimilar (Amjevita) was approved.6
The Table summarizes clinical trials (both completed and ongoing) evaluating biosimilars in adults with plaque psoriasis; thus far, there are 2464 participants enrolled across 5 different studies of adalimumab biosimilars (registered at www.clinicaltrials.gov with the identifiers NCT01970488, NCT02016105, NCT02489227, NCT02714322, NCT02581345) and 531 participants in an etanercept biosimilar study (NCT01891864).
A phase 3 double-blind study compared adalimumab to an adalimumab biosimilar (ABP 501) in 350 adults with plaque psoriasis (NCT01970488). Participants received an initial loading dose of adalimumab (n=175) or ABP 501 (n=175) 80 mg subcutaneously on week 1/day 1, followed by 40 mg at week 2 every 2 weeks thereafter. At week 16, participants with psoriasis area and severity index (PASI) 50 or greater remained in the study for up to 52 weeks; those who were receiving adalimumab were re-randomized to receive either ABP 501 or adalimumab. Participants receiving ABP 501 continued to receive the biosimilar. The mean PASI improvement at weeks 16, 32, and 50 was 86.6, 87.6, and 87.2, respectively, in the ABP 501/ABP 501 group (A/A) compared to 88.0, 88.2, and 88.1, respectively, in the adalimumab/adalimumab group (B/B).19 Autoantibodies developed in 68.4% of participants in the A/A group compared to 74.7% in the B/B group. The incidence of treatment-emergent adverse events (TEAEs) was 86.2% in the A/A group and 78.5% in the B/B group. The most common TEAEs were nasopharyngitis, headache, and upper respiratory tract infection. The incidence of serious TEAEs was 4.6% in the A/A group compared to 5.1% in the B/B group. Overall, the efficacy, safety, and immunogenicity of the adalimumab biosimilar was comparable to the reference product.19
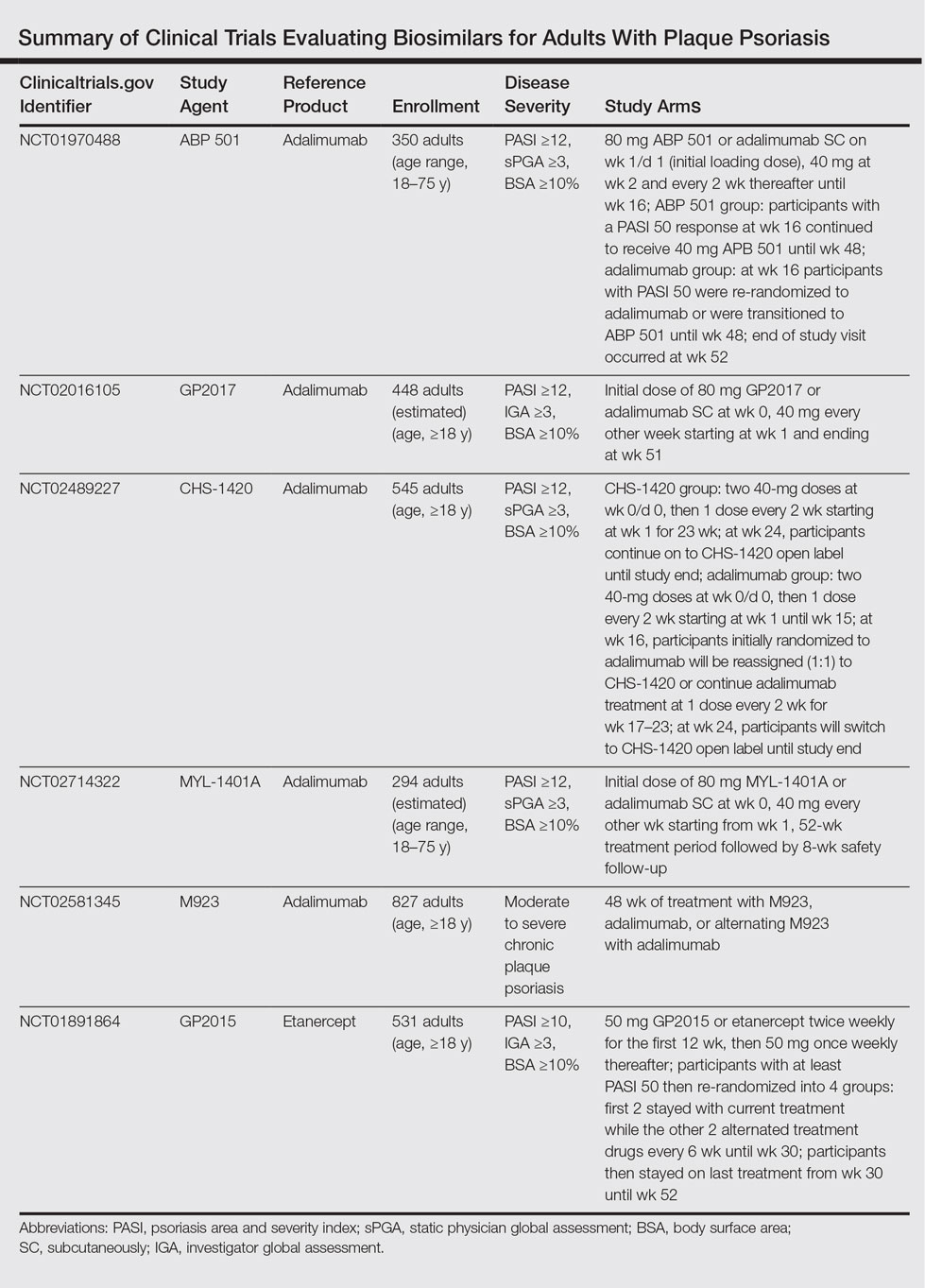
A second phase 3 trial (ADACCESS) evaluated the adalimumab biosimilar GP2017 (NCT02016105). Participants received an initial dose of 80 mg subcutaneously of either GP2017 or adalimumab at week 0, followed by 40 mg every other week starting at week 1 and ending at week 51. The study has been completed but results are not yet available.
The third trial is evaluating the adalimumab biosimilar CHS-1420 (NCT02489227). Participants in the experimental arm receive two 40-mg doses of CHS-1420 at week 0/day 0, and then 1 dose every 2 weeks from week 1 for 23 weeks. At week 24, participants continue with an open-label study. Participants in the adalimumab group receive two 40-mg doses at week 0/day 0, and then 1 dose every 2 weeks from week 1 to week 15. At week 16, participants will be re-randomized (1:1) to continue adalimumab or start CHS-1420 at one 40-mg dose every 2 weeks during weeks 17 to 23. At week 24, participants will switch to CHS-1420 open label until the end of the study. Study results are not yet available; the study is ongoing but not recruiting.
The fourth ongoing trial is evaluating the adalimumab biosimilar MYL-1401A (NCT02714322). Participants receive an initial dose of 80 mg subcutaneously of either MYL-1401A or adalimumab (2:1), followed by 40 mg every other week starting 1 week after the initial dose. After the 52-week treatment period, there is an 8-week safety follow-up period. Study results are not yet available; the study is ongoing but not recruiting.
A fifth adalimumab biosimilar, M923, also is currently being tested in clinical trials (NCT02581345). Participants receive either M923, adalimumab, or alternate between the 2 agents. Although the study is still ongoing, data released from the manufacturer state that the proportion of participants who achieved PASI 75 after 16 weeks of treatment was equivalent in the 2 treatment groups. The proportion of participants who achieved PASI 90, as well as the type, frequency, and severity of adverse events, also were comparable.20
The EGALITY trial, completed in March 2015, compared the etanercept biosimilar GP2015 to etanercept over a 52-week period (NCT01891864). Participants received either GP2015 or etanercept 50 mg twice weekly for the first 12 weeks. Participants with at least PASI 50 were then re-randomized into 4 groups: the first 2 groups stayed with their current treatments while the other 2 groups alternated treatments every 6 weeks until week 30. Participants then stayed on their last treatment from week 30 to week 52. The adjusted PASI 75 response rate at week 12 was 73.4% in the group receiving GP2015 and 75.7% in the group receiving etanercept.21 The percentage change in PASI score at all time points was found to be comparable from baseline until week 52. Importantly, the incidence of TEAEs up to week 52 was comparable and no new safety issues were reported. Additionally, switching participants from etanercept to the biosimilar during the subsequent treatment periods did not cause an increase in formation of antidrug antibodies.21
There are 2 upcoming studies involving biosimilars that are not yet recruiting patients. The first (NCT02925338) will analyze the characteristics of patients treated with Inflectra as well as their response to treatment. The second (NCT02762955) will be comparing the efficacy and safety of an adalimumab biosimilar (BCD-057, BIOCAD) to adalimumab.
Economic Advantages of Biosimilars
The annual economic burden of psoriasis in the United States is substantial, with estimates between $35.2 billion22 and $112 billion.23 Biosimilars can be 25% to 30% cheaper than their reference products9,11,24 and have the potential to save the US health care system billions of dollars.25 Furthermore, the developers of biosimilars could offer patient assistance programs.11 That being said, drug developers can extend patents for their branded drugs; for instance, 2 patents for Enbrel (Amgen Inc) could protect the drug until 2029.26,27
Although cost is an important factor in deciding which medications to prescribe for patients, it should never take precedence over safety and efficacy. Manufacturers can develop new drugs with greater efficacy, fewer side effects, or more convenient dosing schedules,26,27 or they could offer co-payment assistance programs.26,28 Physicians also must consider how the biosimilars will be integrated into drug formularies. Would patients be required to use a biosimilar before a branded drug?11,29 Will patients already taking a branded drug be grandfathered in?11 Would they have to pay a premium to continue taking their drug? And finally, could changes in formularies and employer-payer relationships destabilize patient regimens?30
Conclusion
Preliminary results suggest that biosimilars can have similar safety, efficacy, and immunogenicity data compared to their reference products.19,21 Biosimilars have the potential to greatly reduce the cost burden associated with psoriasis. However, how similar is “highly similar”? Although cost is an important consideration in selecting drug therapies, the reason for using a biosimilar should never be based on cost alone.
- Information on biosimilars. US Food and Drug Administration website. http://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/TherapeuticBiologicApplications/Biosimilars/. Updated May 10, 2016. Accessed July 5, 2016.
- US Department of Health and Human Services. Scientific Considerations in Demonstrating Biosimilarity to a Reference Product: Guidance for Industry. Silver Spring, MD: US Food and Drug Administration; 2015.
- McKeage K. A review of CT-P13: an infliximab biosimilar. BioDrugs. 2014;28:313-321.
- FDA approves Inflectra, a biosimilar to Remicade [news release]. Silver Spring, MD: US Food and Drug Administration; April 5, 2016. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm494227.htm. Updated April 20, 2016. Accessed January 23, 2017.
- FDA approves Erelzi, a biosimilar to Enbrel [news release]. Silver Spring, MD: US Food and Drug Administration; August 30, 2016. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm518639.htm. Accessed January 23, 2017.
- FDA approves Amjevita, a biosimilar to Humira [news release]. Silver Spring, MD: US Food and Drug Administration; September 23, 2016. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm522243.htm. Accessed January 23, 2017.
- Scott BJ, Klein AV, Wang J. Biosimilar monoclonal antibodies: a Canadian regulatory perspective on the assessment of clinically relevant differences and indication extrapolation [published online June 26, 2014]. J Clin Pharmacol. 2015;55(suppl 3):S123-S132.
- Mellstedt H, Niederwieser D, Ludwig H. The challenge of biosimilars [published online September 14, 2007]. Ann Oncol. 2008;19:411-419.
- Puig L. Biosimilars and reference biologics: decisions on biosimilar interchangeability require the involvement of dermatologists [published online October 2, 2013]. Actas Dermosifiliogr. 2014;105:435-437.
- Strober BE, Armour K, Romiti R, et al. Biopharmaceuticals and biosimilars in psoriasis: what the dermatologist needs to know. J Am Acad Dermatol. 2012;66:317-322.
- Falit BP, Singh SC, Brennan TA. Biosimilar competition in the United States: statutory incentives, payers, and pharmacy benefit managers. Health Aff (Millwood). 2015;34:294-301.
- Park W, Hrycaj P, Jeka S, et al. A randomised, double-blind, multicentre, parallel-group, prospective study comparing the pharmacokinetics, safety, and efficacy of CT-P13 and innovator infliximab in patients with ankylosing spondylitis: the PLANETAS study. Ann Rheum Dis. 2013;72:1605-1612.
- Yoo DH, Hrycaj P, Miranda P, et al. A randomised, double-blind, parallel-group study to demonstrate equivalence in efficacy and safety of CT-P13 compared with innovator infliximab when coadministered with methotrexate in patients with active rheumatoid arthritis: the PLANETRA study. Ann Rheum Dis. 2013;72:1613-1620.
- Carretero Hernandez G, Puig L. The use of biosimilar drugs in psoriasis: a position paper. Actas Dermosifiliogr. 2015;106:249-251.
- Regulation of Biological Products, 42 USC §262 (2013).
- Ventola CL. Evaluation of biosimilars for formulary inclusion: factors for consideration by P&T committees. P T. 2015;40:680-689.
- Park W, Yoo DH, Jaworski J, et al. Comparable long-term efficacy, as assessed by patient-reported outcomes, safety and pharmacokinetics, of CT-P13 and reference infliximab in patients with ankylosing spondylitis: 54-week results from the randomized, parallel-group PLANETAS study. Arthritis Res Ther. 2016;18:25.
- Yoo DH, Racewicz A, Brzezicki J, et al. A phase III randomized study to evaluate the efficacy and safety of CT-P13 compared with reference infliximab in patients with active rheumatoid arthritis: 54-week results from the PLANETRA study. Arthritis Res Ther. 2015;18:82.
- Strober B, Foley P, Philipp S, et al. Evaluation of efficacy and safety of ABP 501 in a phase 3 study in subjects with moderate to severe plaque psoriasis: 52-week results. J Am Acad Dermatol. 2016;74(5, suppl 1):AB249.
- Momenta Pharmaceuticals announces positive top-line phase 3 results for M923, a proposed Humira (adalimumab) biosimilar [news release]. Cambridge, MA: Momenta Pharmaceuticals, Inc; November 29, 2016. http://ir.momentapharma.com/releasedetail.cfm?ReleaseID=1001255. Accessed January 25, 2017.
- Griffiths CE, Thaci D, Gerdes S, et al. The EGALITY study: a confirmatory, randomised, double-blind study comparing the efficacy, safety and immunogenicity of GP2015, a proposed etanercept biosimilar, versus the originator product in patients with moderate to severe chronic plaque-type psoriasis [published online October 27, 2016]. Br J Dermatol. doi:10.1111/bjd.15152.
- Vanderpuye-Orgle J, Zhao Y, Lu J, et al. Evaluating the economic burden of psoriasis in the United States [published online April 14, 2015]. J Am Acad Dermatol. 2015;72:961-967.
- Brezinski EA, Dhillon JS, Armstrong AW. Economic burden of psoriasis in the United States: a systematic review. JAMA Dermatol. 2015;151:651-658.
- Menter MA, Griffiths CE. Psoriasis: the future. Dermatol Clin. 2015;33:161-166.
- Hackbarth GM, Crosson FJ, Miller ME. Report to the Congress: improving incentives in the Medicare program. Medicare Payment Advisory Commission, Washington, DC; 2009.
- Lovenworth SJ. The new biosimilar era: the basics, the landscape, and the future. Bloomberg website. http://about.bloomberglaw.com/practitioner-contributions/the-new-biosimilar-era-the-basics-the-landscape-and-the-future. Published September 21, 2012. Accessed July 6, 2016.
- Blackstone EA, Joseph PF. The economics of biosimilars. Am Health Drug Benefits. 2013;6:469-478.
- Calvo B, Zuniga L. The US approach to biosimilars: the long-awaited FDA approval pathway. BioDrugs. 2012;26:357-361.
- Lucio SD, Stevenson JG, Hoffman JM. Biosimilars: implications for health-system pharmacists. Am J Health Syst Pharm. 2013;70:2004-2017.
- Barriers to access attributed to formulary changes. Manag Care. 2012;21:41.
According to the US Food and Drug Administration (FDA), a biosimilar is “highly similar to an FDA-approved biological product, . . . and has no clinically meaningful differences in terms of safety and effectiveness.”1 The Biologics Price Competition and Innovation (BPCI) Act of 2009 created an expedited pathway for the approval of products shown to be biosimilar to FDA-licensed reference products.2 In 2013, the European Medicines Agency approved the first biosimilar modeled on infliximab (Remsima [formerly known as CT-P13], Celltrion Healthcare Co, Ltd) for the same indications as its reference product.3 In 2016, the FDA approved Inflectra (Hospira, a Pfizer Company), an infliximab biosimilar; Erelzi (Sandoz, a Novartis Division), an etanercept biosimilar; and Amjevita (Amgen Inc), an adalimumab biosimilar, all for numerous clinical indications including plaque psoriasis and psoriatic arthritis.4-6
There has been a substantial amount of distrust surrounding the biosimilars; however, as the patents for the biologic agents expire, new biosimilars will undoubtedly flood the market. In this article, we provide information that will help dermatologists understand the need for and use of these agents.
Biosimilars Versus Generic Drugs
Small-molecule generics can be made in a process that is relatively inexpensive, reproducible, and able to yield identical products with each lot.7 In contrast, biosimilars are large complex proteins made in living cells. They differ from their reference product because of changes that occur during manufacturing (eg, purification system, posttranslational modifications).7-9 Glycosylation is particularly sensitive to manufacturing and can affect the immunogenicity of the product.9 The impact of manufacturing can be substantial; for example, during phase 3 trials for efalizumab, a change in the manufacturing facility affected pharmacokinetic properties to such a degree that the FDA required a repeat of the trials.10
FDA Guidelines on Biosimilarity
The FDA outlines the following approach to demonstrate biosimilarity.2 The first step is structural characterization to evaluate the primary, secondary, tertiary, and quaternary structures and posttranslational modifications. The next step utilizes in vivo and/or in vitro functional assays to compare the biosimilar and reference product. The third step is a focus on toxicity and immunogenicity. The fourth step involves clinical studies to study pharmacokinetic and pharmacodynamic data, immunogenicity, safety, and efficacy. After the biosimilar has been approved, there must be a system in place to monitor postmarketing safety. If a biosimilar is tested in one patient population (eg, patients with plaque psoriasis), a request can be made to approve the drug for all the conditions that the reference product was approved for, such as plaque psoriasis, rheumatoid arthritis, and inflammatory bowel disease, even though clinical trials were not performed in all of these patient populations.2 The BPCI Act leaves it up to the FDA to determine how much and what type of data (eg, in vitro, in vivo, clinical) are required.11
Extrapolation and Interchangeability
Once a biosimilar has been approved, 2 questions must be answered: First, can its use be extrapolated to all indications for the reference product? The infliximab biosimilar approved by the European Medicines Agency and the FDA had only been studied in patients with ankylosing spondylitis12 and rheumatoid arthritis,13 yet it was granted all the indications for infliximab, including severe plaque psoriasis.14 As of now, the various regulatory agencies differ on their policies regarding extrapolation. Extrapolation is not automatically bestowed on a biosimilar in the United States but can be requested by the manufacturer.2
Second, can the biosimilar be seamlessly switched with its reference product at the pharmacy level? The BPCI Act allows for the substitution of biosimilars that are deemed interchangeable without notifying the provider, yet individual states ultimately can pass laws regarding this issue.15,16 An interchangeable agent would “produce the same clinical result as the reference product,” and “the risk in terms of safety or diminished efficacy of alternating or switching between use of the biological product and the reference product is not greater than the risk of using the reference product.”15 Generic drugs are allowed to be substituted without notifying the patient or prescriber16; however, biosimilars that are not deemed interchangeable would require permission from the prescriber before substitution.11
Biosimilars for Psoriasis
In April 2016, an infliximab biosimilar (Inflectra) became the second biosimilar approved by the FDA.4 Inflectra was studied in clinical trials for patients with ankylosing spondylitis17 and rheumatoid arthritis,18 and in both trials the biosimilar was found to have similar efficacy and safety profiles to that of the reference product. In August 2016, an etanercept biosimilar (Erelzi) was approved,5 and in September 2016, an adalimumab biosimilar (Amjevita) was approved.6
The Table summarizes clinical trials (both completed and ongoing) evaluating biosimilars in adults with plaque psoriasis; thus far, there are 2464 participants enrolled across 5 different studies of adalimumab biosimilars (registered at www.clinicaltrials.gov with the identifiers NCT01970488, NCT02016105, NCT02489227, NCT02714322, NCT02581345) and 531 participants in an etanercept biosimilar study (NCT01891864).
A phase 3 double-blind study compared adalimumab to an adalimumab biosimilar (ABP 501) in 350 adults with plaque psoriasis (NCT01970488). Participants received an initial loading dose of adalimumab (n=175) or ABP 501 (n=175) 80 mg subcutaneously on week 1/day 1, followed by 40 mg at week 2 every 2 weeks thereafter. At week 16, participants with psoriasis area and severity index (PASI) 50 or greater remained in the study for up to 52 weeks; those who were receiving adalimumab were re-randomized to receive either ABP 501 or adalimumab. Participants receiving ABP 501 continued to receive the biosimilar. The mean PASI improvement at weeks 16, 32, and 50 was 86.6, 87.6, and 87.2, respectively, in the ABP 501/ABP 501 group (A/A) compared to 88.0, 88.2, and 88.1, respectively, in the adalimumab/adalimumab group (B/B).19 Autoantibodies developed in 68.4% of participants in the A/A group compared to 74.7% in the B/B group. The incidence of treatment-emergent adverse events (TEAEs) was 86.2% in the A/A group and 78.5% in the B/B group. The most common TEAEs were nasopharyngitis, headache, and upper respiratory tract infection. The incidence of serious TEAEs was 4.6% in the A/A group compared to 5.1% in the B/B group. Overall, the efficacy, safety, and immunogenicity of the adalimumab biosimilar was comparable to the reference product.19

A second phase 3 trial (ADACCESS) evaluated the adalimumab biosimilar GP2017 (NCT02016105). Participants received an initial dose of 80 mg subcutaneously of either GP2017 or adalimumab at week 0, followed by 40 mg every other week starting at week 1 and ending at week 51. The study has been completed but results are not yet available.
The third trial is evaluating the adalimumab biosimilar CHS-1420 (NCT02489227). Participants in the experimental arm receive two 40-mg doses of CHS-1420 at week 0/day 0, and then 1 dose every 2 weeks from week 1 for 23 weeks. At week 24, participants continue with an open-label study. Participants in the adalimumab group receive two 40-mg doses at week 0/day 0, and then 1 dose every 2 weeks from week 1 to week 15. At week 16, participants will be re-randomized (1:1) to continue adalimumab or start CHS-1420 at one 40-mg dose every 2 weeks during weeks 17 to 23. At week 24, participants will switch to CHS-1420 open label until the end of the study. Study results are not yet available; the study is ongoing but not recruiting.
The fourth ongoing trial is evaluating the adalimumab biosimilar MYL-1401A (NCT02714322). Participants receive an initial dose of 80 mg subcutaneously of either MYL-1401A or adalimumab (2:1), followed by 40 mg every other week starting 1 week after the initial dose. After the 52-week treatment period, there is an 8-week safety follow-up period. Study results are not yet available; the study is ongoing but not recruiting.
A fifth adalimumab biosimilar, M923, also is currently being tested in clinical trials (NCT02581345). Participants receive either M923, adalimumab, or alternate between the 2 agents. Although the study is still ongoing, data released from the manufacturer state that the proportion of participants who achieved PASI 75 after 16 weeks of treatment was equivalent in the 2 treatment groups. The proportion of participants who achieved PASI 90, as well as the type, frequency, and severity of adverse events, also were comparable.20
The EGALITY trial, completed in March 2015, compared the etanercept biosimilar GP2015 to etanercept over a 52-week period (NCT01891864). Participants received either GP2015 or etanercept 50 mg twice weekly for the first 12 weeks. Participants with at least PASI 50 were then re-randomized into 4 groups: the first 2 groups stayed with their current treatments while the other 2 groups alternated treatments every 6 weeks until week 30. Participants then stayed on their last treatment from week 30 to week 52. The adjusted PASI 75 response rate at week 12 was 73.4% in the group receiving GP2015 and 75.7% in the group receiving etanercept.21 The percentage change in PASI score at all time points was found to be comparable from baseline until week 52. Importantly, the incidence of TEAEs up to week 52 was comparable and no new safety issues were reported. Additionally, switching participants from etanercept to the biosimilar during the subsequent treatment periods did not cause an increase in formation of antidrug antibodies.21
There are 2 upcoming studies involving biosimilars that are not yet recruiting patients. The first (NCT02925338) will analyze the characteristics of patients treated with Inflectra as well as their response to treatment. The second (NCT02762955) will be comparing the efficacy and safety of an adalimumab biosimilar (BCD-057, BIOCAD) to adalimumab.
Economic Advantages of Biosimilars
The annual economic burden of psoriasis in the United States is substantial, with estimates between $35.2 billion22 and $112 billion.23 Biosimilars can be 25% to 30% cheaper than their reference products9,11,24 and have the potential to save the US health care system billions of dollars.25 Furthermore, the developers of biosimilars could offer patient assistance programs.11 That being said, drug developers can extend patents for their branded drugs; for instance, 2 patents for Enbrel (Amgen Inc) could protect the drug until 2029.26,27
Although cost is an important factor in deciding which medications to prescribe for patients, it should never take precedence over safety and efficacy. Manufacturers can develop new drugs with greater efficacy, fewer side effects, or more convenient dosing schedules,26,27 or they could offer co-payment assistance programs.26,28 Physicians also must consider how the biosimilars will be integrated into drug formularies. Would patients be required to use a biosimilar before a branded drug?11,29 Will patients already taking a branded drug be grandfathered in?11 Would they have to pay a premium to continue taking their drug? And finally, could changes in formularies and employer-payer relationships destabilize patient regimens?30
Conclusion
Preliminary results suggest that biosimilars can have similar safety, efficacy, and immunogenicity data compared to their reference products.19,21 Biosimilars have the potential to greatly reduce the cost burden associated with psoriasis. However, how similar is “highly similar”? Although cost is an important consideration in selecting drug therapies, the reason for using a biosimilar should never be based on cost alone.
According to the US Food and Drug Administration (FDA), a biosimilar is “highly similar to an FDA-approved biological product, . . . and has no clinically meaningful differences in terms of safety and effectiveness.”1 The Biologics Price Competition and Innovation (BPCI) Act of 2009 created an expedited pathway for the approval of products shown to be biosimilar to FDA-licensed reference products.2 In 2013, the European Medicines Agency approved the first biosimilar modeled on infliximab (Remsima [formerly known as CT-P13], Celltrion Healthcare Co, Ltd) for the same indications as its reference product.3 In 2016, the FDA approved Inflectra (Hospira, a Pfizer Company), an infliximab biosimilar; Erelzi (Sandoz, a Novartis Division), an etanercept biosimilar; and Amjevita (Amgen Inc), an adalimumab biosimilar, all for numerous clinical indications including plaque psoriasis and psoriatic arthritis.4-6
There has been a substantial amount of distrust surrounding the biosimilars; however, as the patents for the biologic agents expire, new biosimilars will undoubtedly flood the market. In this article, we provide information that will help dermatologists understand the need for and use of these agents.
Biosimilars Versus Generic Drugs
Small-molecule generics can be made in a process that is relatively inexpensive, reproducible, and able to yield identical products with each lot.7 In contrast, biosimilars are large complex proteins made in living cells. They differ from their reference product because of changes that occur during manufacturing (eg, purification system, posttranslational modifications).7-9 Glycosylation is particularly sensitive to manufacturing and can affect the immunogenicity of the product.9 The impact of manufacturing can be substantial; for example, during phase 3 trials for efalizumab, a change in the manufacturing facility affected pharmacokinetic properties to such a degree that the FDA required a repeat of the trials.10
FDA Guidelines on Biosimilarity
The FDA outlines the following approach to demonstrate biosimilarity.2 The first step is structural characterization to evaluate the primary, secondary, tertiary, and quaternary structures and posttranslational modifications. The next step utilizes in vivo and/or in vitro functional assays to compare the biosimilar and reference product. The third step is a focus on toxicity and immunogenicity. The fourth step involves clinical studies to study pharmacokinetic and pharmacodynamic data, immunogenicity, safety, and efficacy. After the biosimilar has been approved, there must be a system in place to monitor postmarketing safety. If a biosimilar is tested in one patient population (eg, patients with plaque psoriasis), a request can be made to approve the drug for all the conditions that the reference product was approved for, such as plaque psoriasis, rheumatoid arthritis, and inflammatory bowel disease, even though clinical trials were not performed in all of these patient populations.2 The BPCI Act leaves it up to the FDA to determine how much and what type of data (eg, in vitro, in vivo, clinical) are required.11
Extrapolation and Interchangeability
Once a biosimilar has been approved, 2 questions must be answered: First, can its use be extrapolated to all indications for the reference product? The infliximab biosimilar approved by the European Medicines Agency and the FDA had only been studied in patients with ankylosing spondylitis12 and rheumatoid arthritis,13 yet it was granted all the indications for infliximab, including severe plaque psoriasis.14 As of now, the various regulatory agencies differ on their policies regarding extrapolation. Extrapolation is not automatically bestowed on a biosimilar in the United States but can be requested by the manufacturer.2
Second, can the biosimilar be seamlessly switched with its reference product at the pharmacy level? The BPCI Act allows for the substitution of biosimilars that are deemed interchangeable without notifying the provider, yet individual states ultimately can pass laws regarding this issue.15,16 An interchangeable agent would “produce the same clinical result as the reference product,” and “the risk in terms of safety or diminished efficacy of alternating or switching between use of the biological product and the reference product is not greater than the risk of using the reference product.”15 Generic drugs are allowed to be substituted without notifying the patient or prescriber16; however, biosimilars that are not deemed interchangeable would require permission from the prescriber before substitution.11
Biosimilars for Psoriasis
In April 2016, an infliximab biosimilar (Inflectra) became the second biosimilar approved by the FDA.4 Inflectra was studied in clinical trials for patients with ankylosing spondylitis17 and rheumatoid arthritis,18 and in both trials the biosimilar was found to have similar efficacy and safety profiles to that of the reference product. In August 2016, an etanercept biosimilar (Erelzi) was approved,5 and in September 2016, an adalimumab biosimilar (Amjevita) was approved.6
The Table summarizes clinical trials (both completed and ongoing) evaluating biosimilars in adults with plaque psoriasis; thus far, there are 2464 participants enrolled across 5 different studies of adalimumab biosimilars (registered at www.clinicaltrials.gov with the identifiers NCT01970488, NCT02016105, NCT02489227, NCT02714322, NCT02581345) and 531 participants in an etanercept biosimilar study (NCT01891864).
A phase 3 double-blind study compared adalimumab to an adalimumab biosimilar (ABP 501) in 350 adults with plaque psoriasis (NCT01970488). Participants received an initial loading dose of adalimumab (n=175) or ABP 501 (n=175) 80 mg subcutaneously on week 1/day 1, followed by 40 mg at week 2 every 2 weeks thereafter. At week 16, participants with psoriasis area and severity index (PASI) 50 or greater remained in the study for up to 52 weeks; those who were receiving adalimumab were re-randomized to receive either ABP 501 or adalimumab. Participants receiving ABP 501 continued to receive the biosimilar. The mean PASI improvement at weeks 16, 32, and 50 was 86.6, 87.6, and 87.2, respectively, in the ABP 501/ABP 501 group (A/A) compared to 88.0, 88.2, and 88.1, respectively, in the adalimumab/adalimumab group (B/B).19 Autoantibodies developed in 68.4% of participants in the A/A group compared to 74.7% in the B/B group. The incidence of treatment-emergent adverse events (TEAEs) was 86.2% in the A/A group and 78.5% in the B/B group. The most common TEAEs were nasopharyngitis, headache, and upper respiratory tract infection. The incidence of serious TEAEs was 4.6% in the A/A group compared to 5.1% in the B/B group. Overall, the efficacy, safety, and immunogenicity of the adalimumab biosimilar was comparable to the reference product.19

A second phase 3 trial (ADACCESS) evaluated the adalimumab biosimilar GP2017 (NCT02016105). Participants received an initial dose of 80 mg subcutaneously of either GP2017 or adalimumab at week 0, followed by 40 mg every other week starting at week 1 and ending at week 51. The study has been completed but results are not yet available.
The third trial is evaluating the adalimumab biosimilar CHS-1420 (NCT02489227). Participants in the experimental arm receive two 40-mg doses of CHS-1420 at week 0/day 0, and then 1 dose every 2 weeks from week 1 for 23 weeks. At week 24, participants continue with an open-label study. Participants in the adalimumab group receive two 40-mg doses at week 0/day 0, and then 1 dose every 2 weeks from week 1 to week 15. At week 16, participants will be re-randomized (1:1) to continue adalimumab or start CHS-1420 at one 40-mg dose every 2 weeks during weeks 17 to 23. At week 24, participants will switch to CHS-1420 open label until the end of the study. Study results are not yet available; the study is ongoing but not recruiting.
The fourth ongoing trial is evaluating the adalimumab biosimilar MYL-1401A (NCT02714322). Participants receive an initial dose of 80 mg subcutaneously of either MYL-1401A or adalimumab (2:1), followed by 40 mg every other week starting 1 week after the initial dose. After the 52-week treatment period, there is an 8-week safety follow-up period. Study results are not yet available; the study is ongoing but not recruiting.
A fifth adalimumab biosimilar, M923, also is currently being tested in clinical trials (NCT02581345). Participants receive either M923, adalimumab, or alternate between the 2 agents. Although the study is still ongoing, data released from the manufacturer state that the proportion of participants who achieved PASI 75 after 16 weeks of treatment was equivalent in the 2 treatment groups. The proportion of participants who achieved PASI 90, as well as the type, frequency, and severity of adverse events, also were comparable.20
The EGALITY trial, completed in March 2015, compared the etanercept biosimilar GP2015 to etanercept over a 52-week period (NCT01891864). Participants received either GP2015 or etanercept 50 mg twice weekly for the first 12 weeks. Participants with at least PASI 50 were then re-randomized into 4 groups: the first 2 groups stayed with their current treatments while the other 2 groups alternated treatments every 6 weeks until week 30. Participants then stayed on their last treatment from week 30 to week 52. The adjusted PASI 75 response rate at week 12 was 73.4% in the group receiving GP2015 and 75.7% in the group receiving etanercept.21 The percentage change in PASI score at all time points was found to be comparable from baseline until week 52. Importantly, the incidence of TEAEs up to week 52 was comparable and no new safety issues were reported. Additionally, switching participants from etanercept to the biosimilar during the subsequent treatment periods did not cause an increase in formation of antidrug antibodies.21
There are 2 upcoming studies involving biosimilars that are not yet recruiting patients. The first (NCT02925338) will analyze the characteristics of patients treated with Inflectra as well as their response to treatment. The second (NCT02762955) will be comparing the efficacy and safety of an adalimumab biosimilar (BCD-057, BIOCAD) to adalimumab.
Economic Advantages of Biosimilars
The annual economic burden of psoriasis in the United States is substantial, with estimates between $35.2 billion22 and $112 billion.23 Biosimilars can be 25% to 30% cheaper than their reference products9,11,24 and have the potential to save the US health care system billions of dollars.25 Furthermore, the developers of biosimilars could offer patient assistance programs.11 That being said, drug developers can extend patents for their branded drugs; for instance, 2 patents for Enbrel (Amgen Inc) could protect the drug until 2029.26,27
Although cost is an important factor in deciding which medications to prescribe for patients, it should never take precedence over safety and efficacy. Manufacturers can develop new drugs with greater efficacy, fewer side effects, or more convenient dosing schedules,26,27 or they could offer co-payment assistance programs.26,28 Physicians also must consider how the biosimilars will be integrated into drug formularies. Would patients be required to use a biosimilar before a branded drug?11,29 Will patients already taking a branded drug be grandfathered in?11 Would they have to pay a premium to continue taking their drug? And finally, could changes in formularies and employer-payer relationships destabilize patient regimens?30
Conclusion
Preliminary results suggest that biosimilars can have similar safety, efficacy, and immunogenicity data compared to their reference products.19,21 Biosimilars have the potential to greatly reduce the cost burden associated with psoriasis. However, how similar is “highly similar”? Although cost is an important consideration in selecting drug therapies, the reason for using a biosimilar should never be based on cost alone.
- Information on biosimilars. US Food and Drug Administration website. http://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/TherapeuticBiologicApplications/Biosimilars/. Updated May 10, 2016. Accessed July 5, 2016.
- US Department of Health and Human Services. Scientific Considerations in Demonstrating Biosimilarity to a Reference Product: Guidance for Industry. Silver Spring, MD: US Food and Drug Administration; 2015.
- McKeage K. A review of CT-P13: an infliximab biosimilar. BioDrugs. 2014;28:313-321.
- FDA approves Inflectra, a biosimilar to Remicade [news release]. Silver Spring, MD: US Food and Drug Administration; April 5, 2016. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm494227.htm. Updated April 20, 2016. Accessed January 23, 2017.
- FDA approves Erelzi, a biosimilar to Enbrel [news release]. Silver Spring, MD: US Food and Drug Administration; August 30, 2016. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm518639.htm. Accessed January 23, 2017.
- FDA approves Amjevita, a biosimilar to Humira [news release]. Silver Spring, MD: US Food and Drug Administration; September 23, 2016. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm522243.htm. Accessed January 23, 2017.
- Scott BJ, Klein AV, Wang J. Biosimilar monoclonal antibodies: a Canadian regulatory perspective on the assessment of clinically relevant differences and indication extrapolation [published online June 26, 2014]. J Clin Pharmacol. 2015;55(suppl 3):S123-S132.
- Mellstedt H, Niederwieser D, Ludwig H. The challenge of biosimilars [published online September 14, 2007]. Ann Oncol. 2008;19:411-419.
- Puig L. Biosimilars and reference biologics: decisions on biosimilar interchangeability require the involvement of dermatologists [published online October 2, 2013]. Actas Dermosifiliogr. 2014;105:435-437.
- Strober BE, Armour K, Romiti R, et al. Biopharmaceuticals and biosimilars in psoriasis: what the dermatologist needs to know. J Am Acad Dermatol. 2012;66:317-322.
- Falit BP, Singh SC, Brennan TA. Biosimilar competition in the United States: statutory incentives, payers, and pharmacy benefit managers. Health Aff (Millwood). 2015;34:294-301.
- Park W, Hrycaj P, Jeka S, et al. A randomised, double-blind, multicentre, parallel-group, prospective study comparing the pharmacokinetics, safety, and efficacy of CT-P13 and innovator infliximab in patients with ankylosing spondylitis: the PLANETAS study. Ann Rheum Dis. 2013;72:1605-1612.
- Yoo DH, Hrycaj P, Miranda P, et al. A randomised, double-blind, parallel-group study to demonstrate equivalence in efficacy and safety of CT-P13 compared with innovator infliximab when coadministered with methotrexate in patients with active rheumatoid arthritis: the PLANETRA study. Ann Rheum Dis. 2013;72:1613-1620.
- Carretero Hernandez G, Puig L. The use of biosimilar drugs in psoriasis: a position paper. Actas Dermosifiliogr. 2015;106:249-251.
- Regulation of Biological Products, 42 USC §262 (2013).
- Ventola CL. Evaluation of biosimilars for formulary inclusion: factors for consideration by P&T committees. P T. 2015;40:680-689.
- Park W, Yoo DH, Jaworski J, et al. Comparable long-term efficacy, as assessed by patient-reported outcomes, safety and pharmacokinetics, of CT-P13 and reference infliximab in patients with ankylosing spondylitis: 54-week results from the randomized, parallel-group PLANETAS study. Arthritis Res Ther. 2016;18:25.
- Yoo DH, Racewicz A, Brzezicki J, et al. A phase III randomized study to evaluate the efficacy and safety of CT-P13 compared with reference infliximab in patients with active rheumatoid arthritis: 54-week results from the PLANETRA study. Arthritis Res Ther. 2015;18:82.
- Strober B, Foley P, Philipp S, et al. Evaluation of efficacy and safety of ABP 501 in a phase 3 study in subjects with moderate to severe plaque psoriasis: 52-week results. J Am Acad Dermatol. 2016;74(5, suppl 1):AB249.
- Momenta Pharmaceuticals announces positive top-line phase 3 results for M923, a proposed Humira (adalimumab) biosimilar [news release]. Cambridge, MA: Momenta Pharmaceuticals, Inc; November 29, 2016. http://ir.momentapharma.com/releasedetail.cfm?ReleaseID=1001255. Accessed January 25, 2017.
- Griffiths CE, Thaci D, Gerdes S, et al. The EGALITY study: a confirmatory, randomised, double-blind study comparing the efficacy, safety and immunogenicity of GP2015, a proposed etanercept biosimilar, versus the originator product in patients with moderate to severe chronic plaque-type psoriasis [published online October 27, 2016]. Br J Dermatol. doi:10.1111/bjd.15152.
- Vanderpuye-Orgle J, Zhao Y, Lu J, et al. Evaluating the economic burden of psoriasis in the United States [published online April 14, 2015]. J Am Acad Dermatol. 2015;72:961-967.
- Brezinski EA, Dhillon JS, Armstrong AW. Economic burden of psoriasis in the United States: a systematic review. JAMA Dermatol. 2015;151:651-658.
- Menter MA, Griffiths CE. Psoriasis: the future. Dermatol Clin. 2015;33:161-166.
- Hackbarth GM, Crosson FJ, Miller ME. Report to the Congress: improving incentives in the Medicare program. Medicare Payment Advisory Commission, Washington, DC; 2009.
- Lovenworth SJ. The new biosimilar era: the basics, the landscape, and the future. Bloomberg website. http://about.bloomberglaw.com/practitioner-contributions/the-new-biosimilar-era-the-basics-the-landscape-and-the-future. Published September 21, 2012. Accessed July 6, 2016.
- Blackstone EA, Joseph PF. The economics of biosimilars. Am Health Drug Benefits. 2013;6:469-478.
- Calvo B, Zuniga L. The US approach to biosimilars: the long-awaited FDA approval pathway. BioDrugs. 2012;26:357-361.
- Lucio SD, Stevenson JG, Hoffman JM. Biosimilars: implications for health-system pharmacists. Am J Health Syst Pharm. 2013;70:2004-2017.
- Barriers to access attributed to formulary changes. Manag Care. 2012;21:41.
- Information on biosimilars. US Food and Drug Administration website. http://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/TherapeuticBiologicApplications/Biosimilars/. Updated May 10, 2016. Accessed July 5, 2016.
- US Department of Health and Human Services. Scientific Considerations in Demonstrating Biosimilarity to a Reference Product: Guidance for Industry. Silver Spring, MD: US Food and Drug Administration; 2015.
- McKeage K. A review of CT-P13: an infliximab biosimilar. BioDrugs. 2014;28:313-321.
- FDA approves Inflectra, a biosimilar to Remicade [news release]. Silver Spring, MD: US Food and Drug Administration; April 5, 2016. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm494227.htm. Updated April 20, 2016. Accessed January 23, 2017.
- FDA approves Erelzi, a biosimilar to Enbrel [news release]. Silver Spring, MD: US Food and Drug Administration; August 30, 2016. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm518639.htm. Accessed January 23, 2017.
- FDA approves Amjevita, a biosimilar to Humira [news release]. Silver Spring, MD: US Food and Drug Administration; September 23, 2016. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm522243.htm. Accessed January 23, 2017.
- Scott BJ, Klein AV, Wang J. Biosimilar monoclonal antibodies: a Canadian regulatory perspective on the assessment of clinically relevant differences and indication extrapolation [published online June 26, 2014]. J Clin Pharmacol. 2015;55(suppl 3):S123-S132.
- Mellstedt H, Niederwieser D, Ludwig H. The challenge of biosimilars [published online September 14, 2007]. Ann Oncol. 2008;19:411-419.
- Puig L. Biosimilars and reference biologics: decisions on biosimilar interchangeability require the involvement of dermatologists [published online October 2, 2013]. Actas Dermosifiliogr. 2014;105:435-437.
- Strober BE, Armour K, Romiti R, et al. Biopharmaceuticals and biosimilars in psoriasis: what the dermatologist needs to know. J Am Acad Dermatol. 2012;66:317-322.
- Falit BP, Singh SC, Brennan TA. Biosimilar competition in the United States: statutory incentives, payers, and pharmacy benefit managers. Health Aff (Millwood). 2015;34:294-301.
- Park W, Hrycaj P, Jeka S, et al. A randomised, double-blind, multicentre, parallel-group, prospective study comparing the pharmacokinetics, safety, and efficacy of CT-P13 and innovator infliximab in patients with ankylosing spondylitis: the PLANETAS study. Ann Rheum Dis. 2013;72:1605-1612.
- Yoo DH, Hrycaj P, Miranda P, et al. A randomised, double-blind, parallel-group study to demonstrate equivalence in efficacy and safety of CT-P13 compared with innovator infliximab when coadministered with methotrexate in patients with active rheumatoid arthritis: the PLANETRA study. Ann Rheum Dis. 2013;72:1613-1620.
- Carretero Hernandez G, Puig L. The use of biosimilar drugs in psoriasis: a position paper. Actas Dermosifiliogr. 2015;106:249-251.
- Regulation of Biological Products, 42 USC §262 (2013).
- Ventola CL. Evaluation of biosimilars for formulary inclusion: factors for consideration by P&T committees. P T. 2015;40:680-689.
- Park W, Yoo DH, Jaworski J, et al. Comparable long-term efficacy, as assessed by patient-reported outcomes, safety and pharmacokinetics, of CT-P13 and reference infliximab in patients with ankylosing spondylitis: 54-week results from the randomized, parallel-group PLANETAS study. Arthritis Res Ther. 2016;18:25.
- Yoo DH, Racewicz A, Brzezicki J, et al. A phase III randomized study to evaluate the efficacy and safety of CT-P13 compared with reference infliximab in patients with active rheumatoid arthritis: 54-week results from the PLANETRA study. Arthritis Res Ther. 2015;18:82.
- Strober B, Foley P, Philipp S, et al. Evaluation of efficacy and safety of ABP 501 in a phase 3 study in subjects with moderate to severe plaque psoriasis: 52-week results. J Am Acad Dermatol. 2016;74(5, suppl 1):AB249.
- Momenta Pharmaceuticals announces positive top-line phase 3 results for M923, a proposed Humira (adalimumab) biosimilar [news release]. Cambridge, MA: Momenta Pharmaceuticals, Inc; November 29, 2016. http://ir.momentapharma.com/releasedetail.cfm?ReleaseID=1001255. Accessed January 25, 2017.
- Griffiths CE, Thaci D, Gerdes S, et al. The EGALITY study: a confirmatory, randomised, double-blind study comparing the efficacy, safety and immunogenicity of GP2015, a proposed etanercept biosimilar, versus the originator product in patients with moderate to severe chronic plaque-type psoriasis [published online October 27, 2016]. Br J Dermatol. doi:10.1111/bjd.15152.
- Vanderpuye-Orgle J, Zhao Y, Lu J, et al. Evaluating the economic burden of psoriasis in the United States [published online April 14, 2015]. J Am Acad Dermatol. 2015;72:961-967.
- Brezinski EA, Dhillon JS, Armstrong AW. Economic burden of psoriasis in the United States: a systematic review. JAMA Dermatol. 2015;151:651-658.
- Menter MA, Griffiths CE. Psoriasis: the future. Dermatol Clin. 2015;33:161-166.
- Hackbarth GM, Crosson FJ, Miller ME. Report to the Congress: improving incentives in the Medicare program. Medicare Payment Advisory Commission, Washington, DC; 2009.
- Lovenworth SJ. The new biosimilar era: the basics, the landscape, and the future. Bloomberg website. http://about.bloomberglaw.com/practitioner-contributions/the-new-biosimilar-era-the-basics-the-landscape-and-the-future. Published September 21, 2012. Accessed July 6, 2016.
- Blackstone EA, Joseph PF. The economics of biosimilars. Am Health Drug Benefits. 2013;6:469-478.
- Calvo B, Zuniga L. The US approach to biosimilars: the long-awaited FDA approval pathway. BioDrugs. 2012;26:357-361.
- Lucio SD, Stevenson JG, Hoffman JM. Biosimilars: implications for health-system pharmacists. Am J Health Syst Pharm. 2013;70:2004-2017.
- Barriers to access attributed to formulary changes. Manag Care. 2012;21:41.
Practice Points
- Three biosimilars have been approved by the US Food and Drug Administration to treat adult patients with plaque psoriasis and psoriatic arthritis.
- By virtue of their production, biosimilars are not identical to their reference products, and we must ensure that their safety is comparable.
Microneedling With Platelet-Rich Plasma



Progressive Papular Eruption on the Face and Groin
The Diagnosis: Xanthoma Disseminatum
Genital examination revealed approximately 1.5×3-cm soft, yellow-pink plaques extending from the bilateral inguinal folds to the proximal medial thighs (Figure 1). There was no mucosal, axillary, extensor extremity, or palmoplantar involvement. Histopathologic examination of a biopsy from a plaque on the left side of the lower abdomen revealed sheets of foamy histiocytes distributed throughout a fibrotic dermis. Both mononucleated and multinucleated histiocytes were present, including many Touton giant cells (Figure 2). A patchy infiltrate of lymphocytes and rare eosinophils also was noted. The histiocytes labeled with factor XIIIa but not with S-100. Laboratory tests were performed with the following pertinent findings: low-density lipoprotein, 150 mg/dL (reference range, <130 mg/dL); high-density lipoprotein, 30 mg/dL (reference range, >40 mg/dL). Total cholesterol and triglyceride levels were within reference range, and complete blood cell count and basic metabolic panel were normal.
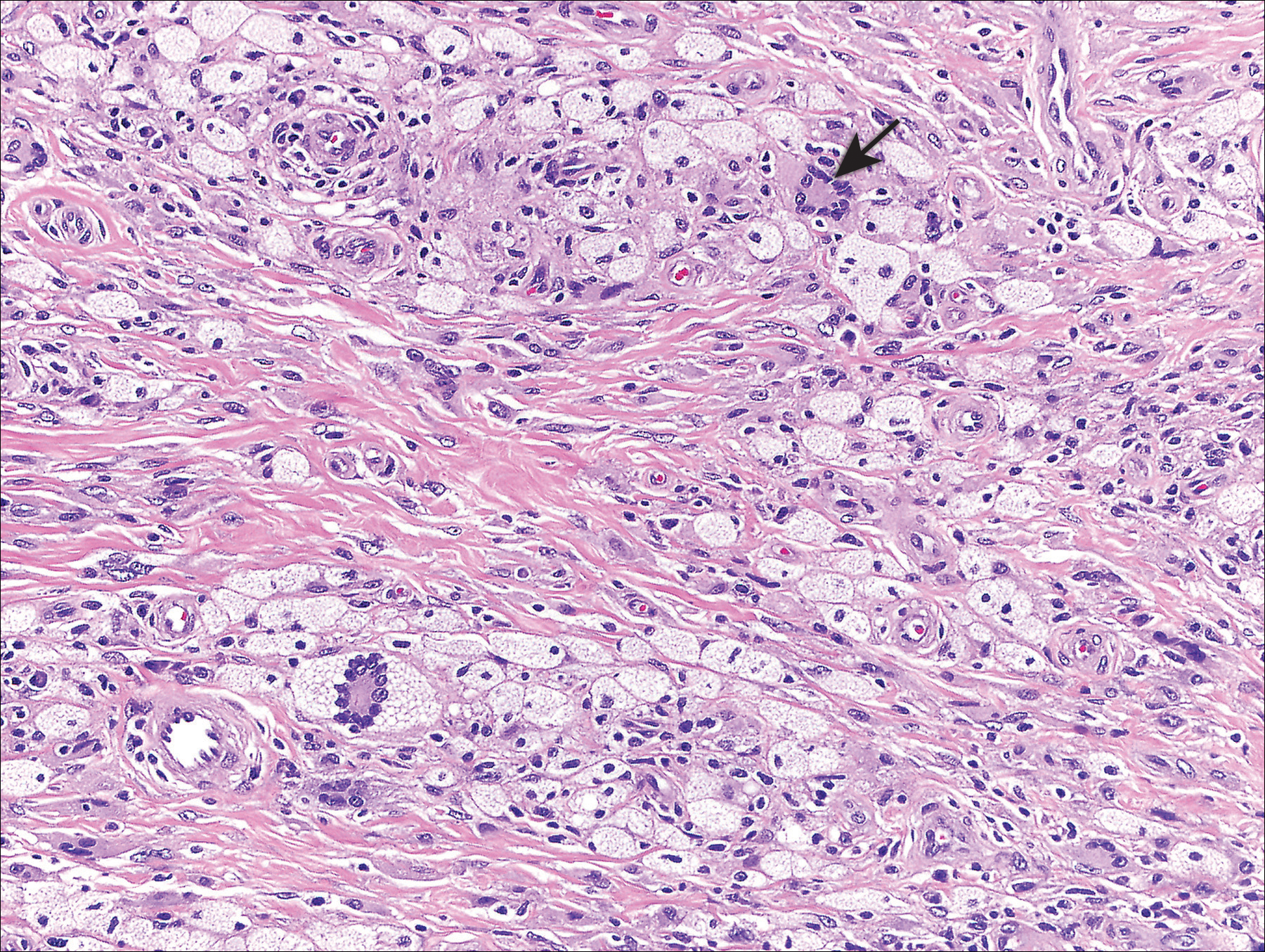
Xanthoma disseminatum (XD)(also known as Montgomery syndrome) is a rare, nonfamilial, normolipemic non-Langerhans cell histiocytosis characterized by extensive lipid deposition in the skin, mucous membranes, and internal organs. The pathogenesis of XD is poorly understood, but it may represent a macrophage-mediated reactive process triggered by superantigens.1
Xanthoma disseminatum most commonly affects males aged 5 to 25 years.2 Clinically, it is characterized by red-brown to yellowish papules and plaques symmetrically distributed over the eyelids, trunk, face, and proximal extremities. There is a predilection for involvement of flexural and intertriginous surfaces and tendency for extension along Langer lines. Extracutaneous involvement can be a notable cause of morbidity and mortality, underscoring the importance of distinguishing XD from other clinically similar xanthomatoses. Mucous membrane involvement occurs in 40% to 60% of patients.3 The oropharynx, larynx, and corneal and conjunctival membranes are most commonly affected, resulting in dysphagia, dysphonia or dyspnea, and visual impairment, respectively. Symptoms of internal organ involvement can be manifold, including pain or limited mobility secondary to osteolytic bone lesions or muscle or synovial membrane involvement, as well as seizures, strabismus, and cerebellar ataxia due to central nervous system lesions.2-4 Approximately 40% of patients develop diabetes insipidus secondary to involvement of the pituitary meninges.3
The differential diagnosis of XD includes juvenile xanthogranuloma, papular xanthomas, eruptive xanthomas, generalized eruptive histiocytosis, progressive nodular histiocytosis, multicentric reticulohistiocytosis, eruptive syringomas, sarcoidosis, and Langerhans cell histiocytosis; the latter should be considered, especially when there is concomitant diabetes insipidus.5 Laboratory studies typically are unremarkable. Although the majority of patients are normolipemic, rates of hyperlipemia within this group are comparable to the general population, occasionally rendering it difficult for the clinician to distinguish XD from hyperlipemic xanthomatoses. As such, diagnosis and differentiation from other xanthomatous processes rests on clinicopathological correlation. Histopathology reveals dermal collections of histiocytes, some with foamy cytoplasm, that range in appearance from spindled to scalloped to Touton-like. Early histopathology demonstrates scalloped macrophages with few foamy cells; a mixture of foamy cells, scalloped cells, inflammatory cells, and Touton and foreign body giant cells is characteristic of late lesions. Immunohistochemistry stains positive for non-Langerhans cell surface markers CD68 and factor XIIIa. Electron microscopy demonstrates dense and myeloid bodies, cholesterol crystals, and lipid vacuoles.5
Three subtypes of XD have been described based on the distinct clinical courses that have been observed in patients: a common, persistent, cutaneous form; a self-limited form with spontaneous resolution; and a progressive subtype with internal organ involvement. No consistently efficacious therapies have been identified, but isolated case reports attest to the efficacy of various agents, including azathioprine, clofibrate, cyclophosphamide, glucocorticoids, chlorambucil, and combination or monotherapy with lipid-lowering agents.3,5,6 Surgical resection, cryotherapy, radiotherapy, and CO2 laser therapy may offer some temporary benefit but do not alter the typically relapsing course of the disease.7,8 Remission and long-term control of lesions was reported with use of 2-chlorodeoxyadenosine, a purine nucleoside analogue, for 5 of 8 patients in a case series.3
- Zelger B, Cerio R, Orchard G, et al. Histologic and immunohistochemical study comparing xanthoma disseminatum and histiocytosis X. Arch Dermatol. 1992;128:1207-1212.
- Mahajan V, Sharma A, Chauhan P, et al. Xanthoma disseminatum: a red herring xanthomatosis. Indian J Dermatol Venereol Leprol. 2013;79:253-254.
- Khezri F, Gibson LE, Tefferi A. Xanthoma disseminatum: effective therapy with 2-chlorodeoxyadenosine in a case series. Arch Dermatol. 2011;147:459-464.
- Weiss N, Keller C. Xanthoma disseminatum: a rare normolipemic xanthomatosis. Clin Investig. 1993;71:233-238.
- Park HY, Cho DH, Joe DH, et al. A case of xanthoma disseminatum with spontaneous resolution over 10 years: review of the literature on long-term follow-up [published online May 26, 2011]. Dermatology. 2011;222:236-243.
- Kim SM, Waters P, Vincent A, et al. Sjogren's syndrome myelopathy: spinal cord involvement in Sjogren's syndrome might be a manifestation of neuromyelitis optica. Mult Scler. 2009;15:1062-1068.
- Eisendle K, Linder D, Ratzinger G, et al. Inflammation and lipid accumulation in xanthoma disseminatum: therapeutic considerations. J Am Acad Dermatol. 2008;58(2 suppl):S47-S49.
- Kim JY, Jung HD, Choe YS, et al. A case of xanthoma disseminatum accentuating over the eyelids. Ann Dermatol. 2010;22:353-357.
The Diagnosis: Xanthoma Disseminatum
Genital examination revealed approximately 1.5×3-cm soft, yellow-pink plaques extending from the bilateral inguinal folds to the proximal medial thighs (Figure 1). There was no mucosal, axillary, extensor extremity, or palmoplantar involvement. Histopathologic examination of a biopsy from a plaque on the left side of the lower abdomen revealed sheets of foamy histiocytes distributed throughout a fibrotic dermis. Both mononucleated and multinucleated histiocytes were present, including many Touton giant cells (Figure 2). A patchy infiltrate of lymphocytes and rare eosinophils also was noted. The histiocytes labeled with factor XIIIa but not with S-100. Laboratory tests were performed with the following pertinent findings: low-density lipoprotein, 150 mg/dL (reference range, <130 mg/dL); high-density lipoprotein, 30 mg/dL (reference range, >40 mg/dL). Total cholesterol and triglyceride levels were within reference range, and complete blood cell count and basic metabolic panel were normal.


Xanthoma disseminatum (XD)(also known as Montgomery syndrome) is a rare, nonfamilial, normolipemic non-Langerhans cell histiocytosis characterized by extensive lipid deposition in the skin, mucous membranes, and internal organs. The pathogenesis of XD is poorly understood, but it may represent a macrophage-mediated reactive process triggered by superantigens.1
Xanthoma disseminatum most commonly affects males aged 5 to 25 years.2 Clinically, it is characterized by red-brown to yellowish papules and plaques symmetrically distributed over the eyelids, trunk, face, and proximal extremities. There is a predilection for involvement of flexural and intertriginous surfaces and tendency for extension along Langer lines. Extracutaneous involvement can be a notable cause of morbidity and mortality, underscoring the importance of distinguishing XD from other clinically similar xanthomatoses. Mucous membrane involvement occurs in 40% to 60% of patients.3 The oropharynx, larynx, and corneal and conjunctival membranes are most commonly affected, resulting in dysphagia, dysphonia or dyspnea, and visual impairment, respectively. Symptoms of internal organ involvement can be manifold, including pain or limited mobility secondary to osteolytic bone lesions or muscle or synovial membrane involvement, as well as seizures, strabismus, and cerebellar ataxia due to central nervous system lesions.2-4 Approximately 40% of patients develop diabetes insipidus secondary to involvement of the pituitary meninges.3
The differential diagnosis of XD includes juvenile xanthogranuloma, papular xanthomas, eruptive xanthomas, generalized eruptive histiocytosis, progressive nodular histiocytosis, multicentric reticulohistiocytosis, eruptive syringomas, sarcoidosis, and Langerhans cell histiocytosis; the latter should be considered, especially when there is concomitant diabetes insipidus.5 Laboratory studies typically are unremarkable. Although the majority of patients are normolipemic, rates of hyperlipemia within this group are comparable to the general population, occasionally rendering it difficult for the clinician to distinguish XD from hyperlipemic xanthomatoses. As such, diagnosis and differentiation from other xanthomatous processes rests on clinicopathological correlation. Histopathology reveals dermal collections of histiocytes, some with foamy cytoplasm, that range in appearance from spindled to scalloped to Touton-like. Early histopathology demonstrates scalloped macrophages with few foamy cells; a mixture of foamy cells, scalloped cells, inflammatory cells, and Touton and foreign body giant cells is characteristic of late lesions. Immunohistochemistry stains positive for non-Langerhans cell surface markers CD68 and factor XIIIa. Electron microscopy demonstrates dense and myeloid bodies, cholesterol crystals, and lipid vacuoles.5
Three subtypes of XD have been described based on the distinct clinical courses that have been observed in patients: a common, persistent, cutaneous form; a self-limited form with spontaneous resolution; and a progressive subtype with internal organ involvement. No consistently efficacious therapies have been identified, but isolated case reports attest to the efficacy of various agents, including azathioprine, clofibrate, cyclophosphamide, glucocorticoids, chlorambucil, and combination or monotherapy with lipid-lowering agents.3,5,6 Surgical resection, cryotherapy, radiotherapy, and CO2 laser therapy may offer some temporary benefit but do not alter the typically relapsing course of the disease.7,8 Remission and long-term control of lesions was reported with use of 2-chlorodeoxyadenosine, a purine nucleoside analogue, for 5 of 8 patients in a case series.3
The Diagnosis: Xanthoma Disseminatum
Genital examination revealed approximately 1.5×3-cm soft, yellow-pink plaques extending from the bilateral inguinal folds to the proximal medial thighs (Figure 1). There was no mucosal, axillary, extensor extremity, or palmoplantar involvement. Histopathologic examination of a biopsy from a plaque on the left side of the lower abdomen revealed sheets of foamy histiocytes distributed throughout a fibrotic dermis. Both mononucleated and multinucleated histiocytes were present, including many Touton giant cells (Figure 2). A patchy infiltrate of lymphocytes and rare eosinophils also was noted. The histiocytes labeled with factor XIIIa but not with S-100. Laboratory tests were performed with the following pertinent findings: low-density lipoprotein, 150 mg/dL (reference range, <130 mg/dL); high-density lipoprotein, 30 mg/dL (reference range, >40 mg/dL). Total cholesterol and triglyceride levels were within reference range, and complete blood cell count and basic metabolic panel were normal.


Xanthoma disseminatum (XD)(also known as Montgomery syndrome) is a rare, nonfamilial, normolipemic non-Langerhans cell histiocytosis characterized by extensive lipid deposition in the skin, mucous membranes, and internal organs. The pathogenesis of XD is poorly understood, but it may represent a macrophage-mediated reactive process triggered by superantigens.1
Xanthoma disseminatum most commonly affects males aged 5 to 25 years.2 Clinically, it is characterized by red-brown to yellowish papules and plaques symmetrically distributed over the eyelids, trunk, face, and proximal extremities. There is a predilection for involvement of flexural and intertriginous surfaces and tendency for extension along Langer lines. Extracutaneous involvement can be a notable cause of morbidity and mortality, underscoring the importance of distinguishing XD from other clinically similar xanthomatoses. Mucous membrane involvement occurs in 40% to 60% of patients.3 The oropharynx, larynx, and corneal and conjunctival membranes are most commonly affected, resulting in dysphagia, dysphonia or dyspnea, and visual impairment, respectively. Symptoms of internal organ involvement can be manifold, including pain or limited mobility secondary to osteolytic bone lesions or muscle or synovial membrane involvement, as well as seizures, strabismus, and cerebellar ataxia due to central nervous system lesions.2-4 Approximately 40% of patients develop diabetes insipidus secondary to involvement of the pituitary meninges.3
The differential diagnosis of XD includes juvenile xanthogranuloma, papular xanthomas, eruptive xanthomas, generalized eruptive histiocytosis, progressive nodular histiocytosis, multicentric reticulohistiocytosis, eruptive syringomas, sarcoidosis, and Langerhans cell histiocytosis; the latter should be considered, especially when there is concomitant diabetes insipidus.5 Laboratory studies typically are unremarkable. Although the majority of patients are normolipemic, rates of hyperlipemia within this group are comparable to the general population, occasionally rendering it difficult for the clinician to distinguish XD from hyperlipemic xanthomatoses. As such, diagnosis and differentiation from other xanthomatous processes rests on clinicopathological correlation. Histopathology reveals dermal collections of histiocytes, some with foamy cytoplasm, that range in appearance from spindled to scalloped to Touton-like. Early histopathology demonstrates scalloped macrophages with few foamy cells; a mixture of foamy cells, scalloped cells, inflammatory cells, and Touton and foreign body giant cells is characteristic of late lesions. Immunohistochemistry stains positive for non-Langerhans cell surface markers CD68 and factor XIIIa. Electron microscopy demonstrates dense and myeloid bodies, cholesterol crystals, and lipid vacuoles.5
Three subtypes of XD have been described based on the distinct clinical courses that have been observed in patients: a common, persistent, cutaneous form; a self-limited form with spontaneous resolution; and a progressive subtype with internal organ involvement. No consistently efficacious therapies have been identified, but isolated case reports attest to the efficacy of various agents, including azathioprine, clofibrate, cyclophosphamide, glucocorticoids, chlorambucil, and combination or monotherapy with lipid-lowering agents.3,5,6 Surgical resection, cryotherapy, radiotherapy, and CO2 laser therapy may offer some temporary benefit but do not alter the typically relapsing course of the disease.7,8 Remission and long-term control of lesions was reported with use of 2-chlorodeoxyadenosine, a purine nucleoside analogue, for 5 of 8 patients in a case series.3
- Zelger B, Cerio R, Orchard G, et al. Histologic and immunohistochemical study comparing xanthoma disseminatum and histiocytosis X. Arch Dermatol. 1992;128:1207-1212.
- Mahajan V, Sharma A, Chauhan P, et al. Xanthoma disseminatum: a red herring xanthomatosis. Indian J Dermatol Venereol Leprol. 2013;79:253-254.
- Khezri F, Gibson LE, Tefferi A. Xanthoma disseminatum: effective therapy with 2-chlorodeoxyadenosine in a case series. Arch Dermatol. 2011;147:459-464.
- Weiss N, Keller C. Xanthoma disseminatum: a rare normolipemic xanthomatosis. Clin Investig. 1993;71:233-238.
- Park HY, Cho DH, Joe DH, et al. A case of xanthoma disseminatum with spontaneous resolution over 10 years: review of the literature on long-term follow-up [published online May 26, 2011]. Dermatology. 2011;222:236-243.
- Kim SM, Waters P, Vincent A, et al. Sjogren's syndrome myelopathy: spinal cord involvement in Sjogren's syndrome might be a manifestation of neuromyelitis optica. Mult Scler. 2009;15:1062-1068.
- Eisendle K, Linder D, Ratzinger G, et al. Inflammation and lipid accumulation in xanthoma disseminatum: therapeutic considerations. J Am Acad Dermatol. 2008;58(2 suppl):S47-S49.
- Kim JY, Jung HD, Choe YS, et al. A case of xanthoma disseminatum accentuating over the eyelids. Ann Dermatol. 2010;22:353-357.
- Zelger B, Cerio R, Orchard G, et al. Histologic and immunohistochemical study comparing xanthoma disseminatum and histiocytosis X. Arch Dermatol. 1992;128:1207-1212.
- Mahajan V, Sharma A, Chauhan P, et al. Xanthoma disseminatum: a red herring xanthomatosis. Indian J Dermatol Venereol Leprol. 2013;79:253-254.
- Khezri F, Gibson LE, Tefferi A. Xanthoma disseminatum: effective therapy with 2-chlorodeoxyadenosine in a case series. Arch Dermatol. 2011;147:459-464.
- Weiss N, Keller C. Xanthoma disseminatum: a rare normolipemic xanthomatosis. Clin Investig. 1993;71:233-238.
- Park HY, Cho DH, Joe DH, et al. A case of xanthoma disseminatum with spontaneous resolution over 10 years: review of the literature on long-term follow-up [published online May 26, 2011]. Dermatology. 2011;222:236-243.
- Kim SM, Waters P, Vincent A, et al. Sjogren's syndrome myelopathy: spinal cord involvement in Sjogren's syndrome might be a manifestation of neuromyelitis optica. Mult Scler. 2009;15:1062-1068.
- Eisendle K, Linder D, Ratzinger G, et al. Inflammation and lipid accumulation in xanthoma disseminatum: therapeutic considerations. J Am Acad Dermatol. 2008;58(2 suppl):S47-S49.
- Kim JY, Jung HD, Choe YS, et al. A case of xanthoma disseminatum accentuating over the eyelids. Ann Dermatol. 2010;22:353-357.

A 28-year-old man presented for evaluation of numerous papules on the face and groin that first appeared in adolescence and had been increasing in size and number over the last several years. The lesions occasionally were pruritic. Review of systems was noncontributory. His medical history was notable for asthma, and there were no affected family members. Physical examination revealed numerous symmetrically distributed, soft, yellow-pink, 1- to 5-mm papules coalescing into plaques on the bilateral malar cheeks extending to the medial canthi and the maxillary, mandibular, zygomatic, and submental regions, as well as the bilateral external auditory meatus.
Hyperpigmented Papules and Plaques
The Diagnosis: Persistent Still Disease
At the time of presentation, the patient had not taken systemic medications for a year. Laboratory studies revealed leukocytosis with neutrophilia and a serum ferritin level of 5493 ng/mL (reference range, 15-200 ng/mL). Rheumatoid factor and antinuclear antibody serologies were within reference range. Microbiologic workup was negative. Lymph node and bone marrow biopsies were negative for a lymphoproliferative disorder. Skin biopsies were performed on the back and forearm. Histologic evaluation revealed orthokeratosis, slight acanthosis, and dyskeratosis confined to the upper layers of the epidermis without evidence of interface dermatitis. There was a mixed perivascular infiltrate composed of lymphocytes and neutrophils with no attendant vasculitic change (Figure).
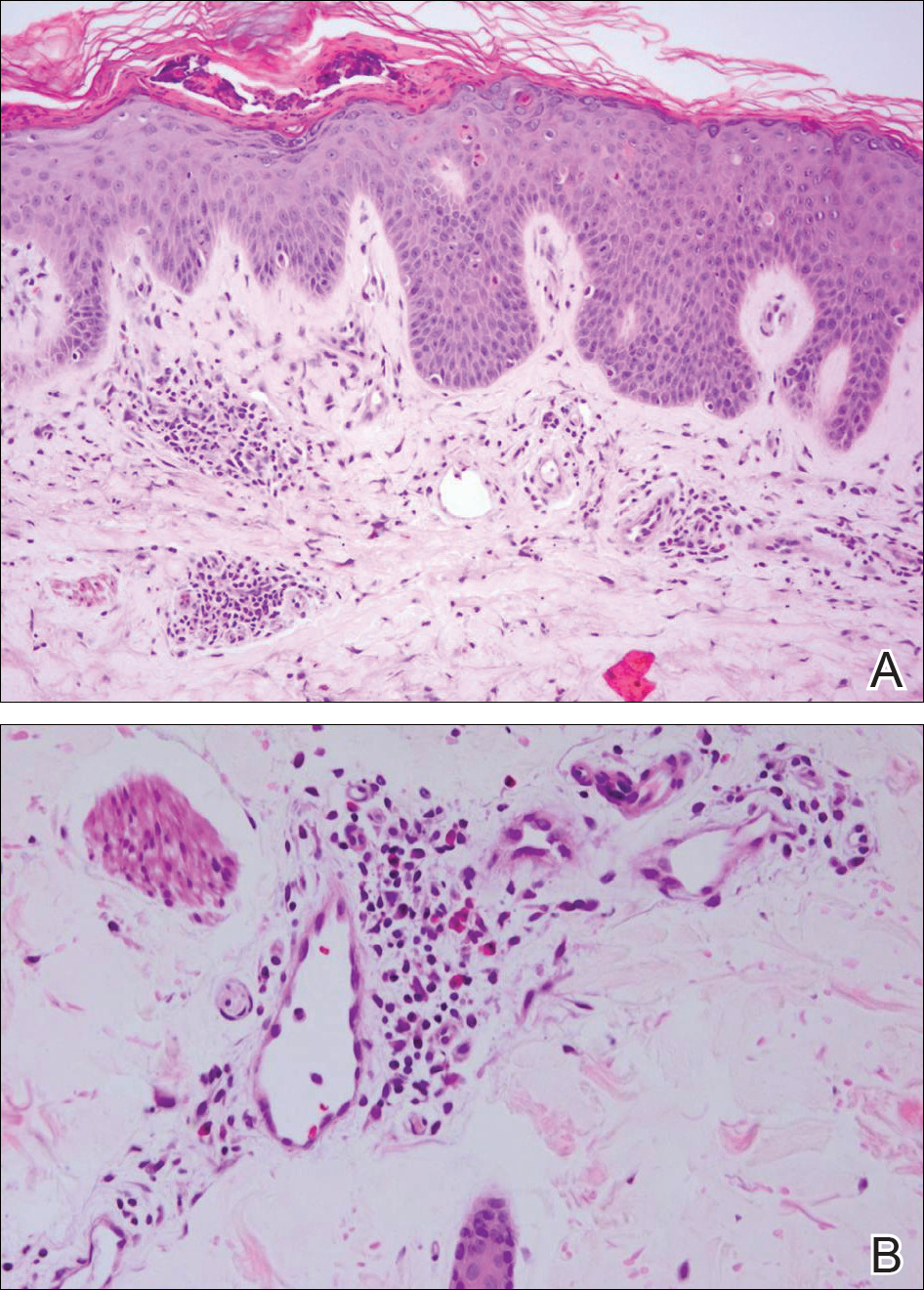
The patient was discharged on prednisone and seen for outpatient follow-up weeks later. Six weeks later, the cutaneous eruption remained unchanged. The patient was unable to start other systemic medications due to lack of insurance and ineligibility for the local patient-assistance program; he was subsequently lost to follow-up.
Adult-onset Still disease is a rare, systemic, inflammatory condition with a broad spectrum of clinical presentations.1-3 Still disease affects all age groups, and children with Still disease (<16 years) usually have a concurrent diagnosis of juvenile idiopathic arthritis (formerly known as juvenile rheumatoid arthritis).1,2,4 Still disease preferentially affects adolescents and adults aged 16 to 35 years, with more than 75% of new cases occurring in this age range.1 Worldwide, the incidence and prevalence of Still disease is disputed with no conclusive rates established.1,3
Still disease is characterized by 4 cardinal signs: high spiking fevers (temperature, ≥39°C); leukocytosis with a predominance of neutrophils (≥10,000 cells/mm3 with ≥80% neutrophils); arthralgia or arthritis; and an evanescent, nonpruritic, salmon-colored morbilliform eruption of the skin, typically on the trunk or extremities.2 Histologic evaluation of the classic Still disease eruption displays perivascular inflammation of the superficial dermis with infiltration by lymphocytes and histiocytes.3
In 1992, major and minor diagnostic criteria were established for adult-onset Still disease. For diagnosis, patients must meet 5 criteria, including 2 major criteria.5 Major criteria include arthralgia or arthritis present for more than 2 weeks, fever (temperature, >39°C) for at least 1 week, the classic Still disease morbilliform eruption (ie, salmon colored, evanescent, morbilliform), and leukocytosis with more than 80% neutrophils. Minor criteria include sore throat, lymphadenopathy and/or splenomegaly, negative rheumatoid factor and antinuclear antibody serologies, and abnormal liver function (defined as elevated transaminases).5 Although not included in the diagnostic criteria, there have been reports of elevated serum ferritin levels in patients with Still disease, a finding that potentially is useful in distinguishing between active and inactive rheumatic conditions.6,7
Several case reports have described persistent Still disease, a subtype of Still disease in which patients present with brown-red, persistent, pruritic macules, papules, and plaques that are widespread and oddly shaped.8,9 Histologically, this subtype is characterized by necrotic keratinocytes in the epidermis and dermal perivascular inflammation composed of neutrophils and lymphocytes.10 This histology differs from classic Still disease in that the latter typically does not have superficial epidermal dyskeratosis. Our case is consistent with reports of persistent Still disease.
Although the etiology of Still disease remains to be elucidated, HLA-B17, -B18, -B35, and -DR2 have been associated with the disease.3 Furthermore, helper T cell TH1, IL-2, IFN-γ, and tumor necrosis factor α have been implicated in disease pathology, enabling the use of newer targeted pharmacologic therapies. Canakinumab, an IL-1β inhibitor, has been found to improve arthritis, fever, and rash in patients with Still disease.11 These findings are particularly encouraging for patients who have not experienced improvement with traditional antirheumatic drugs, such as our patient who was not steroid responsive.3
Although a salmon-colored, evanescent, morbilliform eruption in the context of other systemic signs and symptoms readily evokes consideration of Still disease, the less common fixed cutaneous eruption seen in our case may evade accurate diagnosis. Our case aims to increase awareness of this unusual and rare subtype of the cutaneous eruption of Still disease, as a timely diagnosis may prevent potentially life-threatening sequelae including cardiopulmonary disease and respiratory failure.3,5,9
- Efthimiou P, Paik PK, Bielory L. Diagnosis and management of adult onset Still's disease [published online October 11, 2005]. Ann Rheum Dis. 2006;65:564-572.
- Fautrel B. Adult-onset Still disease. Best Pract Res Clin Rheumatol. 2008;22:773-792.
- Bagnari V, Colina M, Ciancio G, et al. Adult-onset Still's disease. Rheumatol Int. 2010;30:855-862.
- Ravelli A, Martini A. Juvenile idiopathic arthritis. Lancet. 2007;369:767-778.
- Yamaguchi M, Ohta A, Tsunematsu, T, et al. Preliminary criteria for classification of adult Still's disease. J Rheumatol. 1992;19:424-430.
- Van Reeth C, Le Moel G, Lasne Y, et al. Serum ferritin and isoferritins are tools for diagnosis of active adult Still's disease. J Rheumatol. 1994;21:890-895.
- Novak S, Anic F, Luke-Vrbanic TS. Extremely high serum ferritin levels as a main diagnostic tool of adult-onset Still's disease. Rheumatol Int. 2012;32:1091-1094.
- Fortna RR, Gudjonsson JE, Seidel G, et al. Persistent pruritic papules and plaques: a characteristic histopathologic presentation seen in a subset of patients with adult-onset and juvenile Still's disease. J Cutan Pathol. 2010;37:932-937.
- Yang CC, Lee JY, Liu MF, et al. Adult-onset Still's disease with persistent skin eruption and fatal respiratory failure in a Taiwanese woman. Eur J Dermatol. 2006;16:593-594.
- Lee JY, Yang CC, Hsu MM. Histopathology of persistent papules and plaques in adult-onset Still's disease. J Am Acad Dermatol. 2005;52:1003-1008.
- Kontzias A, Efthimiou P. The use of canakinumab, a novel IL-1β long-acting inhibitor in refractory adult-onset Still's disease. Sem Arthritis Rheum. 2012;42:201-205.
The Diagnosis: Persistent Still Disease
At the time of presentation, the patient had not taken systemic medications for a year. Laboratory studies revealed leukocytosis with neutrophilia and a serum ferritin level of 5493 ng/mL (reference range, 15-200 ng/mL). Rheumatoid factor and antinuclear antibody serologies were within reference range. Microbiologic workup was negative. Lymph node and bone marrow biopsies were negative for a lymphoproliferative disorder. Skin biopsies were performed on the back and forearm. Histologic evaluation revealed orthokeratosis, slight acanthosis, and dyskeratosis confined to the upper layers of the epidermis without evidence of interface dermatitis. There was a mixed perivascular infiltrate composed of lymphocytes and neutrophils with no attendant vasculitic change (Figure).

The patient was discharged on prednisone and seen for outpatient follow-up weeks later. Six weeks later, the cutaneous eruption remained unchanged. The patient was unable to start other systemic medications due to lack of insurance and ineligibility for the local patient-assistance program; he was subsequently lost to follow-up.
Adult-onset Still disease is a rare, systemic, inflammatory condition with a broad spectrum of clinical presentations.1-3 Still disease affects all age groups, and children with Still disease (<16 years) usually have a concurrent diagnosis of juvenile idiopathic arthritis (formerly known as juvenile rheumatoid arthritis).1,2,4 Still disease preferentially affects adolescents and adults aged 16 to 35 years, with more than 75% of new cases occurring in this age range.1 Worldwide, the incidence and prevalence of Still disease is disputed with no conclusive rates established.1,3
Still disease is characterized by 4 cardinal signs: high spiking fevers (temperature, ≥39°C); leukocytosis with a predominance of neutrophils (≥10,000 cells/mm3 with ≥80% neutrophils); arthralgia or arthritis; and an evanescent, nonpruritic, salmon-colored morbilliform eruption of the skin, typically on the trunk or extremities.2 Histologic evaluation of the classic Still disease eruption displays perivascular inflammation of the superficial dermis with infiltration by lymphocytes and histiocytes.3
In 1992, major and minor diagnostic criteria were established for adult-onset Still disease. For diagnosis, patients must meet 5 criteria, including 2 major criteria.5 Major criteria include arthralgia or arthritis present for more than 2 weeks, fever (temperature, >39°C) for at least 1 week, the classic Still disease morbilliform eruption (ie, salmon colored, evanescent, morbilliform), and leukocytosis with more than 80% neutrophils. Minor criteria include sore throat, lymphadenopathy and/or splenomegaly, negative rheumatoid factor and antinuclear antibody serologies, and abnormal liver function (defined as elevated transaminases).5 Although not included in the diagnostic criteria, there have been reports of elevated serum ferritin levels in patients with Still disease, a finding that potentially is useful in distinguishing between active and inactive rheumatic conditions.6,7
Several case reports have described persistent Still disease, a subtype of Still disease in which patients present with brown-red, persistent, pruritic macules, papules, and plaques that are widespread and oddly shaped.8,9 Histologically, this subtype is characterized by necrotic keratinocytes in the epidermis and dermal perivascular inflammation composed of neutrophils and lymphocytes.10 This histology differs from classic Still disease in that the latter typically does not have superficial epidermal dyskeratosis. Our case is consistent with reports of persistent Still disease.
Although the etiology of Still disease remains to be elucidated, HLA-B17, -B18, -B35, and -DR2 have been associated with the disease.3 Furthermore, helper T cell TH1, IL-2, IFN-γ, and tumor necrosis factor α have been implicated in disease pathology, enabling the use of newer targeted pharmacologic therapies. Canakinumab, an IL-1β inhibitor, has been found to improve arthritis, fever, and rash in patients with Still disease.11 These findings are particularly encouraging for patients who have not experienced improvement with traditional antirheumatic drugs, such as our patient who was not steroid responsive.3
Although a salmon-colored, evanescent, morbilliform eruption in the context of other systemic signs and symptoms readily evokes consideration of Still disease, the less common fixed cutaneous eruption seen in our case may evade accurate diagnosis. Our case aims to increase awareness of this unusual and rare subtype of the cutaneous eruption of Still disease, as a timely diagnosis may prevent potentially life-threatening sequelae including cardiopulmonary disease and respiratory failure.3,5,9
The Diagnosis: Persistent Still Disease
At the time of presentation, the patient had not taken systemic medications for a year. Laboratory studies revealed leukocytosis with neutrophilia and a serum ferritin level of 5493 ng/mL (reference range, 15-200 ng/mL). Rheumatoid factor and antinuclear antibody serologies were within reference range. Microbiologic workup was negative. Lymph node and bone marrow biopsies were negative for a lymphoproliferative disorder. Skin biopsies were performed on the back and forearm. Histologic evaluation revealed orthokeratosis, slight acanthosis, and dyskeratosis confined to the upper layers of the epidermis without evidence of interface dermatitis. There was a mixed perivascular infiltrate composed of lymphocytes and neutrophils with no attendant vasculitic change (Figure).

The patient was discharged on prednisone and seen for outpatient follow-up weeks later. Six weeks later, the cutaneous eruption remained unchanged. The patient was unable to start other systemic medications due to lack of insurance and ineligibility for the local patient-assistance program; he was subsequently lost to follow-up.
Adult-onset Still disease is a rare, systemic, inflammatory condition with a broad spectrum of clinical presentations.1-3 Still disease affects all age groups, and children with Still disease (<16 years) usually have a concurrent diagnosis of juvenile idiopathic arthritis (formerly known as juvenile rheumatoid arthritis).1,2,4 Still disease preferentially affects adolescents and adults aged 16 to 35 years, with more than 75% of new cases occurring in this age range.1 Worldwide, the incidence and prevalence of Still disease is disputed with no conclusive rates established.1,3
Still disease is characterized by 4 cardinal signs: high spiking fevers (temperature, ≥39°C); leukocytosis with a predominance of neutrophils (≥10,000 cells/mm3 with ≥80% neutrophils); arthralgia or arthritis; and an evanescent, nonpruritic, salmon-colored morbilliform eruption of the skin, typically on the trunk or extremities.2 Histologic evaluation of the classic Still disease eruption displays perivascular inflammation of the superficial dermis with infiltration by lymphocytes and histiocytes.3
In 1992, major and minor diagnostic criteria were established for adult-onset Still disease. For diagnosis, patients must meet 5 criteria, including 2 major criteria.5 Major criteria include arthralgia or arthritis present for more than 2 weeks, fever (temperature, >39°C) for at least 1 week, the classic Still disease morbilliform eruption (ie, salmon colored, evanescent, morbilliform), and leukocytosis with more than 80% neutrophils. Minor criteria include sore throat, lymphadenopathy and/or splenomegaly, negative rheumatoid factor and antinuclear antibody serologies, and abnormal liver function (defined as elevated transaminases).5 Although not included in the diagnostic criteria, there have been reports of elevated serum ferritin levels in patients with Still disease, a finding that potentially is useful in distinguishing between active and inactive rheumatic conditions.6,7
Several case reports have described persistent Still disease, a subtype of Still disease in which patients present with brown-red, persistent, pruritic macules, papules, and plaques that are widespread and oddly shaped.8,9 Histologically, this subtype is characterized by necrotic keratinocytes in the epidermis and dermal perivascular inflammation composed of neutrophils and lymphocytes.10 This histology differs from classic Still disease in that the latter typically does not have superficial epidermal dyskeratosis. Our case is consistent with reports of persistent Still disease.
Although the etiology of Still disease remains to be elucidated, HLA-B17, -B18, -B35, and -DR2 have been associated with the disease.3 Furthermore, helper T cell TH1, IL-2, IFN-γ, and tumor necrosis factor α have been implicated in disease pathology, enabling the use of newer targeted pharmacologic therapies. Canakinumab, an IL-1β inhibitor, has been found to improve arthritis, fever, and rash in patients with Still disease.11 These findings are particularly encouraging for patients who have not experienced improvement with traditional antirheumatic drugs, such as our patient who was not steroid responsive.3
Although a salmon-colored, evanescent, morbilliform eruption in the context of other systemic signs and symptoms readily evokes consideration of Still disease, the less common fixed cutaneous eruption seen in our case may evade accurate diagnosis. Our case aims to increase awareness of this unusual and rare subtype of the cutaneous eruption of Still disease, as a timely diagnosis may prevent potentially life-threatening sequelae including cardiopulmonary disease and respiratory failure.3,5,9
- Efthimiou P, Paik PK, Bielory L. Diagnosis and management of adult onset Still's disease [published online October 11, 2005]. Ann Rheum Dis. 2006;65:564-572.
- Fautrel B. Adult-onset Still disease. Best Pract Res Clin Rheumatol. 2008;22:773-792.
- Bagnari V, Colina M, Ciancio G, et al. Adult-onset Still's disease. Rheumatol Int. 2010;30:855-862.
- Ravelli A, Martini A. Juvenile idiopathic arthritis. Lancet. 2007;369:767-778.
- Yamaguchi M, Ohta A, Tsunematsu, T, et al. Preliminary criteria for classification of adult Still's disease. J Rheumatol. 1992;19:424-430.
- Van Reeth C, Le Moel G, Lasne Y, et al. Serum ferritin and isoferritins are tools for diagnosis of active adult Still's disease. J Rheumatol. 1994;21:890-895.
- Novak S, Anic F, Luke-Vrbanic TS. Extremely high serum ferritin levels as a main diagnostic tool of adult-onset Still's disease. Rheumatol Int. 2012;32:1091-1094.
- Fortna RR, Gudjonsson JE, Seidel G, et al. Persistent pruritic papules and plaques: a characteristic histopathologic presentation seen in a subset of patients with adult-onset and juvenile Still's disease. J Cutan Pathol. 2010;37:932-937.
- Yang CC, Lee JY, Liu MF, et al. Adult-onset Still's disease with persistent skin eruption and fatal respiratory failure in a Taiwanese woman. Eur J Dermatol. 2006;16:593-594.
- Lee JY, Yang CC, Hsu MM. Histopathology of persistent papules and plaques in adult-onset Still's disease. J Am Acad Dermatol. 2005;52:1003-1008.
- Kontzias A, Efthimiou P. The use of canakinumab, a novel IL-1β long-acting inhibitor in refractory adult-onset Still's disease. Sem Arthritis Rheum. 2012;42:201-205.
- Efthimiou P, Paik PK, Bielory L. Diagnosis and management of adult onset Still's disease [published online October 11, 2005]. Ann Rheum Dis. 2006;65:564-572.
- Fautrel B. Adult-onset Still disease. Best Pract Res Clin Rheumatol. 2008;22:773-792.
- Bagnari V, Colina M, Ciancio G, et al. Adult-onset Still's disease. Rheumatol Int. 2010;30:855-862.
- Ravelli A, Martini A. Juvenile idiopathic arthritis. Lancet. 2007;369:767-778.
- Yamaguchi M, Ohta A, Tsunematsu, T, et al. Preliminary criteria for classification of adult Still's disease. J Rheumatol. 1992;19:424-430.
- Van Reeth C, Le Moel G, Lasne Y, et al. Serum ferritin and isoferritins are tools for diagnosis of active adult Still's disease. J Rheumatol. 1994;21:890-895.
- Novak S, Anic F, Luke-Vrbanic TS. Extremely high serum ferritin levels as a main diagnostic tool of adult-onset Still's disease. Rheumatol Int. 2012;32:1091-1094.
- Fortna RR, Gudjonsson JE, Seidel G, et al. Persistent pruritic papules and plaques: a characteristic histopathologic presentation seen in a subset of patients with adult-onset and juvenile Still's disease. J Cutan Pathol. 2010;37:932-937.
- Yang CC, Lee JY, Liu MF, et al. Adult-onset Still's disease with persistent skin eruption and fatal respiratory failure in a Taiwanese woman. Eur J Dermatol. 2006;16:593-594.
- Lee JY, Yang CC, Hsu MM. Histopathology of persistent papules and plaques in adult-onset Still's disease. J Am Acad Dermatol. 2005;52:1003-1008.
- Kontzias A, Efthimiou P. The use of canakinumab, a novel IL-1β long-acting inhibitor in refractory adult-onset Still's disease. Sem Arthritis Rheum. 2012;42:201-205.
A 25-year-old Hispanic man with a history of juvenile idiopathic arthritis was admitted with a high-grade fever (temperature, >38.9°C) and diffuse nonlocalized abdominal pain of 2 days' duration. Physical examination revealed tachycardia, axillary lymphadenopathy, and hepatosplenomegaly. Cutaneous findings consisted of striking hyperpigmented patches on the chest and back, and hyperpigmented scaly lichenoid papules and plaques on the upper and lower extremities. The plaques on the lower extremities exhibited koebnerization. The patient reported that the eruption initially presented at 16 years of age as pruritic papules on the legs, which gradually spread to involve the arms, chest, and back. Prior treatments of juvenile idiopathic arthritis included prednisone, methotrexate, infliximab, and etanercept, though they were intermittent and temporary. Over time, the cutaneous eruption evolved into its current morphology and distribution, with periods of clearance observed while receiving systemic medications.
Hyperkeratotic Papules on the Medial Aspects of the Feet
To the Editor:
A 43-year-old woman with recently diagnosed diabetes mellitus and a history of thrombotic thrombocytopenic purpura on chronic oral steroids presented with a several-year history of small bumps and bilateral hyperpigmentation on the feet. On physical examination 2- to 3-mm dark brown, hyperkeratotic, firm papules were present on the medial aspects of the feet as well as the dorsal and medial aspects of the thumbs (Figure 1). There also were brown thickened firm plaques on the heels and soles of the feet.
A punch biopsy of the medial aspect of the right foot was performed (Figure 2). Microscopic examination revealed acral skin with hyperkeratosis, parakeratosis, mild hypergranulosis, mild basilar pigmentation, and mild dermal fibrosis (Figure 2A). A periodic acid–Schiff stain for fungus was negative. An elastic van Gieson stain showed fragmentation of the dermal elastic fibers (Figure 2B). The patient was diagnosed with acrokeratoelastoidosis (AKE).
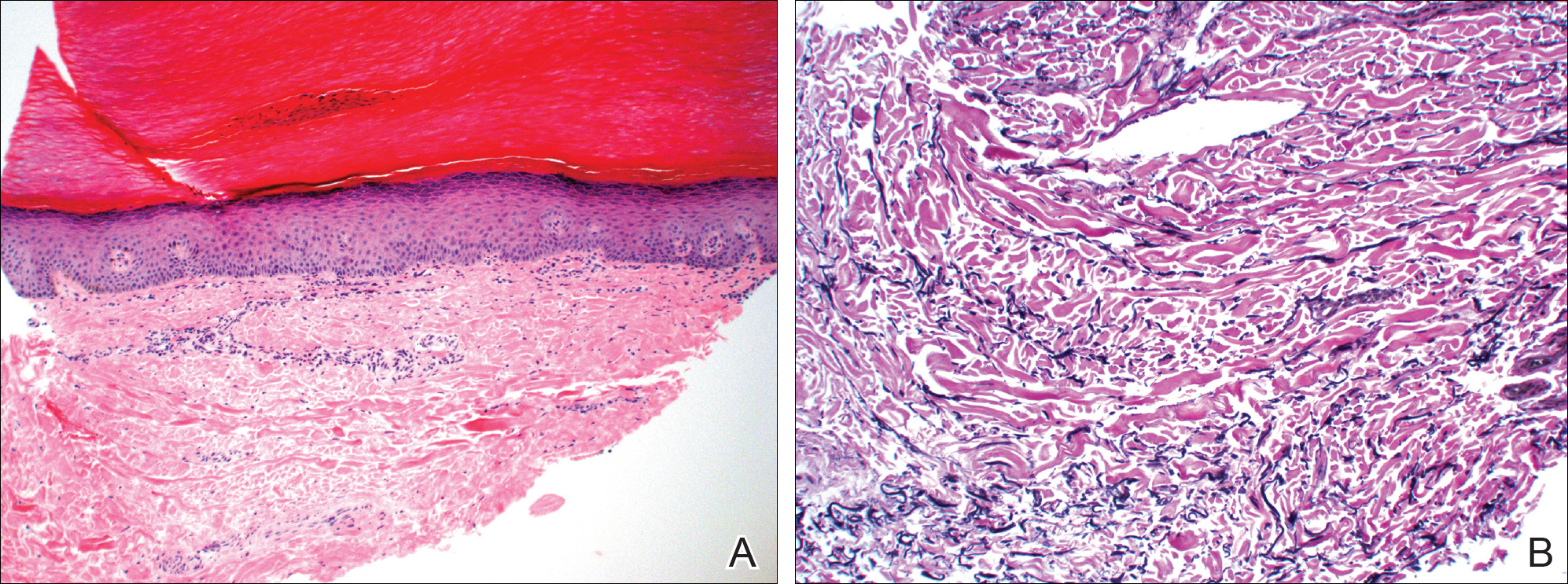
Acrokeratoelastoidosis is a rare autosomal-dominant genodermatosis characterized by firm yellow papules and plaques that appear along the margins of the hands and feet and increase in number over time.1 Histopathologically, hyperkeratosis with hypergranulosis and acanthosis can be seen. Elastorrhexis, resulting in fragmentation of elastic fibers within the dermis, typically is present, a feature that distinguishes AKE from focal acral hyperkeratosis.2 Also, the dermis may be normal with hematoxylin and eosin stain or slightly thickened with mild depression and thin elastic fibers. There is no reported racial or sex predilection, but rapid progression of the disease during pregnancy has been observed.3
The pathogenesis of AKE is not completely understood. However, it has been implicated that abnormalities in the secretion of elastic fibers from fibroblasts may be involved in disease pathogenesis.4,5 Electron microscopy has demonstrated fibroblasts with dense granules at the periphery of their cytoplasm and an absence of surrounding elastic fibers. Genetic studies have linked underlying mutations in chromosome 2 to the disease.6 Defects in keratinization and overproduction of filaggrin also may be involved in the disease process.7
Most therapies generally are ineffective but have included urea, salicylic acid, prednisone, and tretinoin.8 Six-month treatment with etretinate 25 to 50 mg has shown promising results, though recurrences occurred with dosage reduction or discontinuation.9 Our patient demonstrated mild improvement with urea cream 30%.
- Meziane M, Senouci K, Ouidane Y, et al. Acrokeratoelastoidosis. Dermatol Online J. 2008;14:11.
- Lewis KG, Bercovitch L, Dill SW, et al. Acquired disorders of elastic tissue: part II. decreased elastic tissue. J Am Acad Dermatol. 2004;51:165-185; quiz 186-188.
- Tsai S, Kageyama N, Warthan M, et al. Acrokeratoelastoidosis. Int J Dermatol. 2005;44:406-407.
- Johansson EA, Kariniemi AL, Niemi KM. Palmoplantar keratoderma of punctate type: acrokeratoelastoidosis Costa. Acta Derm Venereol. 1980;60:149-153.
- Fiallo P, Pesce C, Brusasco A, et al. Acrokeratoelastoidosis of Costa: a primary disease of the elastic tissue? J Cutan Pathol. 1998;25:580-582.
- Shbaklo Z, Jamaleddine NF, Kibbi AG, et al. Acrokeratoelastoidosis. Int J Dermatol. 1990;29:333-336.
- Abulafia J, Vignale RA. Degenerative collagenous plaques of the hands and acrokeratoelastoidosis: pathogenesis and relationship with knuckle pads. Int J Dermatol. 2000;39:424-432.
- Hu W, Cook TF, Vicki GJ, et al. Acrokeratoelastoidosis. Pediatr Dermatol. 2002;19:320-322.
- Handfield-Jones S, Kennedy CT. Acrokeratoelastoidosis treated with etretinate. J Am Acad Dermatol. 1987;17(5, pt 2):881-882.
To the Editor:
A 43-year-old woman with recently diagnosed diabetes mellitus and a history of thrombotic thrombocytopenic purpura on chronic oral steroids presented with a several-year history of small bumps and bilateral hyperpigmentation on the feet. On physical examination 2- to 3-mm dark brown, hyperkeratotic, firm papules were present on the medial aspects of the feet as well as the dorsal and medial aspects of the thumbs (Figure 1). There also were brown thickened firm plaques on the heels and soles of the feet.
A punch biopsy of the medial aspect of the right foot was performed (Figure 2). Microscopic examination revealed acral skin with hyperkeratosis, parakeratosis, mild hypergranulosis, mild basilar pigmentation, and mild dermal fibrosis (Figure 2A). A periodic acid–Schiff stain for fungus was negative. An elastic van Gieson stain showed fragmentation of the dermal elastic fibers (Figure 2B). The patient was diagnosed with acrokeratoelastoidosis (AKE).


Acrokeratoelastoidosis is a rare autosomal-dominant genodermatosis characterized by firm yellow papules and plaques that appear along the margins of the hands and feet and increase in number over time.1 Histopathologically, hyperkeratosis with hypergranulosis and acanthosis can be seen. Elastorrhexis, resulting in fragmentation of elastic fibers within the dermis, typically is present, a feature that distinguishes AKE from focal acral hyperkeratosis.2 Also, the dermis may be normal with hematoxylin and eosin stain or slightly thickened with mild depression and thin elastic fibers. There is no reported racial or sex predilection, but rapid progression of the disease during pregnancy has been observed.3
The pathogenesis of AKE is not completely understood. However, it has been implicated that abnormalities in the secretion of elastic fibers from fibroblasts may be involved in disease pathogenesis.4,5 Electron microscopy has demonstrated fibroblasts with dense granules at the periphery of their cytoplasm and an absence of surrounding elastic fibers. Genetic studies have linked underlying mutations in chromosome 2 to the disease.6 Defects in keratinization and overproduction of filaggrin also may be involved in the disease process.7
Most therapies generally are ineffective but have included urea, salicylic acid, prednisone, and tretinoin.8 Six-month treatment with etretinate 25 to 50 mg has shown promising results, though recurrences occurred with dosage reduction or discontinuation.9 Our patient demonstrated mild improvement with urea cream 30%.
To the Editor:
A 43-year-old woman with recently diagnosed diabetes mellitus and a history of thrombotic thrombocytopenic purpura on chronic oral steroids presented with a several-year history of small bumps and bilateral hyperpigmentation on the feet. On physical examination 2- to 3-mm dark brown, hyperkeratotic, firm papules were present on the medial aspects of the feet as well as the dorsal and medial aspects of the thumbs (Figure 1). There also were brown thickened firm plaques on the heels and soles of the feet.
A punch biopsy of the medial aspect of the right foot was performed (Figure 2). Microscopic examination revealed acral skin with hyperkeratosis, parakeratosis, mild hypergranulosis, mild basilar pigmentation, and mild dermal fibrosis (Figure 2A). A periodic acid–Schiff stain for fungus was negative. An elastic van Gieson stain showed fragmentation of the dermal elastic fibers (Figure 2B). The patient was diagnosed with acrokeratoelastoidosis (AKE).


Acrokeratoelastoidosis is a rare autosomal-dominant genodermatosis characterized by firm yellow papules and plaques that appear along the margins of the hands and feet and increase in number over time.1 Histopathologically, hyperkeratosis with hypergranulosis and acanthosis can be seen. Elastorrhexis, resulting in fragmentation of elastic fibers within the dermis, typically is present, a feature that distinguishes AKE from focal acral hyperkeratosis.2 Also, the dermis may be normal with hematoxylin and eosin stain or slightly thickened with mild depression and thin elastic fibers. There is no reported racial or sex predilection, but rapid progression of the disease during pregnancy has been observed.3
The pathogenesis of AKE is not completely understood. However, it has been implicated that abnormalities in the secretion of elastic fibers from fibroblasts may be involved in disease pathogenesis.4,5 Electron microscopy has demonstrated fibroblasts with dense granules at the periphery of their cytoplasm and an absence of surrounding elastic fibers. Genetic studies have linked underlying mutations in chromosome 2 to the disease.6 Defects in keratinization and overproduction of filaggrin also may be involved in the disease process.7
Most therapies generally are ineffective but have included urea, salicylic acid, prednisone, and tretinoin.8 Six-month treatment with etretinate 25 to 50 mg has shown promising results, though recurrences occurred with dosage reduction or discontinuation.9 Our patient demonstrated mild improvement with urea cream 30%.
- Meziane M, Senouci K, Ouidane Y, et al. Acrokeratoelastoidosis. Dermatol Online J. 2008;14:11.
- Lewis KG, Bercovitch L, Dill SW, et al. Acquired disorders of elastic tissue: part II. decreased elastic tissue. J Am Acad Dermatol. 2004;51:165-185; quiz 186-188.
- Tsai S, Kageyama N, Warthan M, et al. Acrokeratoelastoidosis. Int J Dermatol. 2005;44:406-407.
- Johansson EA, Kariniemi AL, Niemi KM. Palmoplantar keratoderma of punctate type: acrokeratoelastoidosis Costa. Acta Derm Venereol. 1980;60:149-153.
- Fiallo P, Pesce C, Brusasco A, et al. Acrokeratoelastoidosis of Costa: a primary disease of the elastic tissue? J Cutan Pathol. 1998;25:580-582.
- Shbaklo Z, Jamaleddine NF, Kibbi AG, et al. Acrokeratoelastoidosis. Int J Dermatol. 1990;29:333-336.
- Abulafia J, Vignale RA. Degenerative collagenous plaques of the hands and acrokeratoelastoidosis: pathogenesis and relationship with knuckle pads. Int J Dermatol. 2000;39:424-432.
- Hu W, Cook TF, Vicki GJ, et al. Acrokeratoelastoidosis. Pediatr Dermatol. 2002;19:320-322.
- Handfield-Jones S, Kennedy CT. Acrokeratoelastoidosis treated with etretinate. J Am Acad Dermatol. 1987;17(5, pt 2):881-882.
- Meziane M, Senouci K, Ouidane Y, et al. Acrokeratoelastoidosis. Dermatol Online J. 2008;14:11.
- Lewis KG, Bercovitch L, Dill SW, et al. Acquired disorders of elastic tissue: part II. decreased elastic tissue. J Am Acad Dermatol. 2004;51:165-185; quiz 186-188.
- Tsai S, Kageyama N, Warthan M, et al. Acrokeratoelastoidosis. Int J Dermatol. 2005;44:406-407.
- Johansson EA, Kariniemi AL, Niemi KM. Palmoplantar keratoderma of punctate type: acrokeratoelastoidosis Costa. Acta Derm Venereol. 1980;60:149-153.
- Fiallo P, Pesce C, Brusasco A, et al. Acrokeratoelastoidosis of Costa: a primary disease of the elastic tissue? J Cutan Pathol. 1998;25:580-582.
- Shbaklo Z, Jamaleddine NF, Kibbi AG, et al. Acrokeratoelastoidosis. Int J Dermatol. 1990;29:333-336.
- Abulafia J, Vignale RA. Degenerative collagenous plaques of the hands and acrokeratoelastoidosis: pathogenesis and relationship with knuckle pads. Int J Dermatol. 2000;39:424-432.
- Hu W, Cook TF, Vicki GJ, et al. Acrokeratoelastoidosis. Pediatr Dermatol. 2002;19:320-322.
- Handfield-Jones S, Kennedy CT. Acrokeratoelastoidosis treated with etretinate. J Am Acad Dermatol. 1987;17(5, pt 2):881-882.
Practice Points
- Acrokeratoelastoidosis is a rare autosomal-dominant genodermatosis characterized by firm yellow papules along the margins of the hands and feet.
- Most therapies generally are ineffective but have included urea, salicylic acid, and tretinoin.
Clinical Pearl: Early Diagnosis of Nail Psoriasis and Psoriatic Arthritis
Practice Gap
Early diagnosis of nail psoriasis is challenging because nail changes, including pitting, subungual hyperkeratosis, crumbling, oil spots, salmon patches, onycholysis, and splinter hemorrhages, may be subtle and nonspecific. Furthermore, 5% to 10% of psoriasis patients do not have skin findings, making the diagnosis of nail psoriasis even more difficult. Psoriatic arthritis (PsA) is more common in patients with nail psoriasis than in those with cutaneous psoriasis, and early joint damage may be asymptomatic.1 Both nail psoriasis and PsA may progress rapidly, leading to functional impairment with poor quality of life.2
Diagnostic Tool
A 36-year-old man presented with a 4-year history of abnormal fingernails. He denied nail pain but stated that the nails felt sensitive at times and it was difficult to pick up small objects. His medical history was notable for type 2 diabetes mellitus, hypertension, and attention deficit disorder. He denied joint pain or skin rash.
Physical examination revealed pitting and onycholysis of the fingernails (Figure, A) without involvement of the toenails. A nail clipping was negative for fungus but revealed an incompletely keratinized nail plate with subungual parakeratotic scale, consistent with nail psoriasis. A radiograph showed erosive changes of the third finger of the right hand that were compatible with PsA (Figure, B).
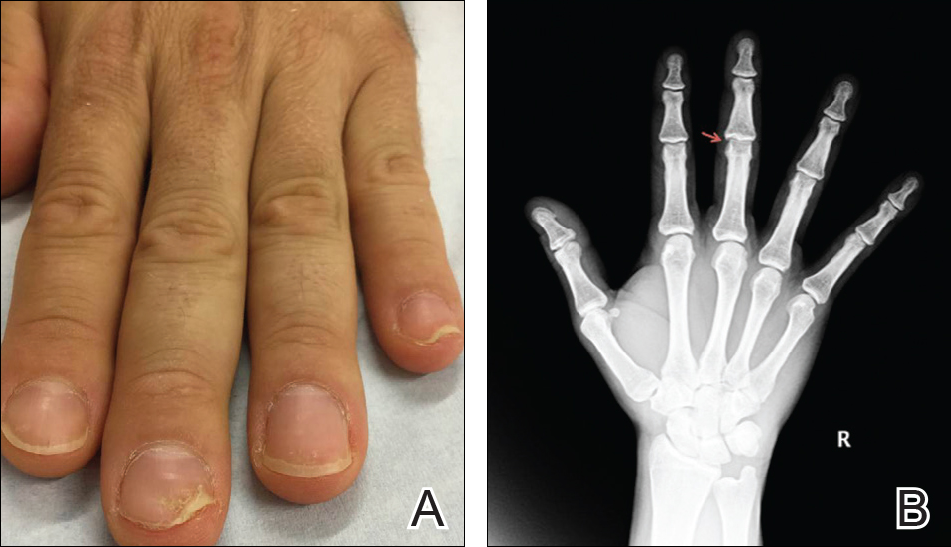
Practice Implications
A nail clipping may be performed to diagnose nail psoriasis. Imaging and/or referral to a rheumatologist should be performed in all patients with isolated nail psoriasis to evaluate for early arthritic changes. If present, appropriate therapy is initiated to prevent further joint damage. In patients with nail psoriasis with or without associated joint pain, dermatologists should consider using radiograph imaging to screen patients for PsA.
- 1. Balestri R, Rech G, Rossi E, et al. Natural history of isolated nail psoriasis and its role as a risk factor for the development of psoriatic arthritis: a single center cross sectional study [published online September 2, 2016]. Br J Dermatol. doi:10.1111/bjd.15026.
- Klaassen KM, van de Kerkhof PC, Pasch MC. Nail psoriasis, the unknown burden of disease [published online January 15, 2014]. J Eur Acad Dermatol Venereol. 2014;28:1690-1695.
Practice Gap
Early diagnosis of nail psoriasis is challenging because nail changes, including pitting, subungual hyperkeratosis, crumbling, oil spots, salmon patches, onycholysis, and splinter hemorrhages, may be subtle and nonspecific. Furthermore, 5% to 10% of psoriasis patients do not have skin findings, making the diagnosis of nail psoriasis even more difficult. Psoriatic arthritis (PsA) is more common in patients with nail psoriasis than in those with cutaneous psoriasis, and early joint damage may be asymptomatic.1 Both nail psoriasis and PsA may progress rapidly, leading to functional impairment with poor quality of life.2
Diagnostic Tool
A 36-year-old man presented with a 4-year history of abnormal fingernails. He denied nail pain but stated that the nails felt sensitive at times and it was difficult to pick up small objects. His medical history was notable for type 2 diabetes mellitus, hypertension, and attention deficit disorder. He denied joint pain or skin rash.
Physical examination revealed pitting and onycholysis of the fingernails (Figure, A) without involvement of the toenails. A nail clipping was negative for fungus but revealed an incompletely keratinized nail plate with subungual parakeratotic scale, consistent with nail psoriasis. A radiograph showed erosive changes of the third finger of the right hand that were compatible with PsA (Figure, B).

Practice Implications
A nail clipping may be performed to diagnose nail psoriasis. Imaging and/or referral to a rheumatologist should be performed in all patients with isolated nail psoriasis to evaluate for early arthritic changes. If present, appropriate therapy is initiated to prevent further joint damage. In patients with nail psoriasis with or without associated joint pain, dermatologists should consider using radiograph imaging to screen patients for PsA.
Practice Gap
Early diagnosis of nail psoriasis is challenging because nail changes, including pitting, subungual hyperkeratosis, crumbling, oil spots, salmon patches, onycholysis, and splinter hemorrhages, may be subtle and nonspecific. Furthermore, 5% to 10% of psoriasis patients do not have skin findings, making the diagnosis of nail psoriasis even more difficult. Psoriatic arthritis (PsA) is more common in patients with nail psoriasis than in those with cutaneous psoriasis, and early joint damage may be asymptomatic.1 Both nail psoriasis and PsA may progress rapidly, leading to functional impairment with poor quality of life.2
Diagnostic Tool
A 36-year-old man presented with a 4-year history of abnormal fingernails. He denied nail pain but stated that the nails felt sensitive at times and it was difficult to pick up small objects. His medical history was notable for type 2 diabetes mellitus, hypertension, and attention deficit disorder. He denied joint pain or skin rash.
Physical examination revealed pitting and onycholysis of the fingernails (Figure, A) without involvement of the toenails. A nail clipping was negative for fungus but revealed an incompletely keratinized nail plate with subungual parakeratotic scale, consistent with nail psoriasis. A radiograph showed erosive changes of the third finger of the right hand that were compatible with PsA (Figure, B).

Practice Implications
A nail clipping may be performed to diagnose nail psoriasis. Imaging and/or referral to a rheumatologist should be performed in all patients with isolated nail psoriasis to evaluate for early arthritic changes. If present, appropriate therapy is initiated to prevent further joint damage. In patients with nail psoriasis with or without associated joint pain, dermatologists should consider using radiograph imaging to screen patients for PsA.
- 1. Balestri R, Rech G, Rossi E, et al. Natural history of isolated nail psoriasis and its role as a risk factor for the development of psoriatic arthritis: a single center cross sectional study [published online September 2, 2016]. Br J Dermatol. doi:10.1111/bjd.15026.
- Klaassen KM, van de Kerkhof PC, Pasch MC. Nail psoriasis, the unknown burden of disease [published online January 15, 2014]. J Eur Acad Dermatol Venereol. 2014;28:1690-1695.
- 1. Balestri R, Rech G, Rossi E, et al. Natural history of isolated nail psoriasis and its role as a risk factor for the development of psoriatic arthritis: a single center cross sectional study [published online September 2, 2016]. Br J Dermatol. doi:10.1111/bjd.15026.
- Klaassen KM, van de Kerkhof PC, Pasch MC. Nail psoriasis, the unknown burden of disease [published online January 15, 2014]. J Eur Acad Dermatol Venereol. 2014;28:1690-1695.