User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
The Effects of Sunscreen on Marine Environments
Coastal travel accounts for 80% of all tourism worldwide, a number that continues to grow. The number of travelers to the Mediterranean Sea alone is expected to rise to 350 million individuals per year within the next 20 years.1 As the number of tourists visiting the world’s oceans increases, the rate of sunscreen unintentionally washed into these marine environments also rises. One study estimated that approximately one-quarter of the sunscreen applied to the skin is washed off over a 20-minute period spent in the water.2 Four of the most common sunscreen agents—benzophenone-3 (BP-3),
Benzophenone-3
4-Methylbenzylidene Camphor
Environmental concerns have also been raised about another common chemical UV filter: 4-MBC, or enzacamene. In laboratory studies, 4-MBC has been shown to cause oxidative stress to Tetrahymena thermophila, an aquatic protozoan, which results in inhibited growth. At higher concentrations, damage to the cellular membrane was seen as soon as 4 hours after exposure.6 In embryonic zebrafish, elevated 4-MBC levels were correlated to improper nerve and muscular development, resulting in developmental defects.7 Another study demonstrated that 4-MBC was toxic to Mytilus galloprovincialis, known as the Mediterranean mussel, and Paracentrotus lividus, a species of sea urchin.8 Although these studies utilized highly controlled laboratory settings, further studies are needed to examine the effects of 4-MBC on these species at environmentally relevant concentrations.
Physical Sunscreens
Physical sunscreens, as compared to the chemical filters referenced above, use either zinc or titanium to protect the skin from the sun’s rays. Nanoparticles, in particular, are preferred because they do not leave a white film on the skin.9 Both titanium dioxide and zinc oxide nanoparticles have been found to inhibit the growth and photosynthesis of marine phytoplankton, the most abundant primary producers on Earth.10,11 These metal contaminants can be transferred to organisms of higher trophic levels, including zooplankton,12 and filter-feeding organisms, including marine abalone13 and the Mediterranean mussel.14 These nanoparticles have been shown to cause oxidative stress to these organisms, making them less fit to withstand environmental stressors. It is difficult to show their true impact, however, as it is challenging to accurately detect and quantify nanoparticle concentrations in vivo.15
Final Thoughts
- Marine problems: tourism & coastal development. World Wide Fund for Nature website. http://wwf.panda.org/about_our_earth/blue_planet/problems/tourism/. Published 2017. Accessed November 14, 2017.
- Danovaro R, Bongiorni L, Corinaldesi C, et al. Sunscreens cause coral bleaching by promoting viral infections. Environ Health Perspect. 2008;116:441-447.
- Downs C, Kramarsky-Winter E, Segal R, et al. Toxicopathological effects of the sunscreen UV filter, oxybenzone (benzophenone-3), on coral planulae and cultured primary cells and its environmental contamination in Hawaii and the US Virgin Islands. Arch Environ Contam Toxicol. 2016;70:265-288.
- Sánchez Rodríguez A, Rodrigo Sanz M, Betancort Rodríguez JR. Occurrence of eight UV filters in beaches of Gran Canaria (Canary Islands)[published online March 17, 2015]. Chemosphere. 2015;131:85-90.
- Bratkovics S, Sapozhnikova Y. Determination of seven commonly used organic UV filters in fresh and saline waters by liquid chromatography-tandem mass spectrometry. Analytical Methods. 2011;3:2943-2950.
- Gao L, Yuan T, Zhou C, et al. Effects of four commonly used UV filters on the growth, cell viability and oxidative stress responses of the Tetrahymena thermophila. Chemosphere. 2013;93:2507-2513.
- Li VW, Tsui MP, Chen X, et al. Effects of 4-methylbenzylidene camphor (4-MBC) on neuronal and muscular development in zebrafish (Danio rerio) embryos [published online February 18, 2016]. Environ Sci Pollut Res Int. 2016;23:8275-8285.
- Paredes E, Perez S, Rodil R, et al. Ecotoxicological evaluation of four UV filters using marine organisms from different trophic levels Isochrysis galbana, Mytilus galloprovincialis, Paracentrotus lividus, and Siriella armata. Chemosphere. 2014;104:44-50.
- Osterwalder U, Sohn M, Herzog B. Global state of sunscreens. Photodermatol Photoimmunol Photomed. 2014;30:62-80.
- Miller RJ, Bennett S, Keller AA, et al. TiO2 nanoparticles are phototoxic to marine phytoplankton. PloS One. 2012;7:E30321.
- Spisni E. Toxicity Assessment of Industrial- and Sunscreen-derived ZnO Nanoparticles [master’s thesis]. Coral Gables, FL: University of Miami Libraries Scholarly Repository; 2016. http://scholarlyrepository.miami.edu/cgi/viewcontent.cgi?article=1625&context=oa_theses. Accessed November 10, 2017.
- Jarvis TA, Miller RJ, Lenihan HS, et al. Toxicity of ZnO nanoparticles to the copepod Acartia tonsa, exposed through a phytoplankton diet [published online April 15, 2013]. Environ Toxicol Chem. 2013;32:1264-1269.
- Zhu X, Zhou J, Cai Z. The toxicity and oxidative stress of TiO2 nanoparticles in marine abalone (Haliotis diversicolor supertexta). Mar Pollut Bull. 2011;63:334-338.
- Barmo C, Ciacci C, Canonico B, et al. In vivo effects of n-TiO2 on digestive gland and immune function of the marine bivalve Mytilus galloprovincialis. Aquatic Toxicol. 2013;132:9-18.
- Sánchez-Quiles D, Tovar-Sánchez A. Are sunscreens a new environmental risk associated with coastal tourism? Environ Int. 2015;83:158-170.
- Xu S, Kwa M, Agarwal A, et al. Sunscreen product performance and other determinants of consumer preferences. JAMA Dermatol. 2016;152:920-927.
- Vesper I. Hawaii seeks to ban ‘reef-unfriendly’ sunscreen. Nature. February 3, 2017. https://www.nature.com/news/hawaii-seeks-to-ban-reef-unfriendly-sunscreen-1.21332. Accessed November 16, 2017.
Coastal travel accounts for 80% of all tourism worldwide, a number that continues to grow. The number of travelers to the Mediterranean Sea alone is expected to rise to 350 million individuals per year within the next 20 years.1 As the number of tourists visiting the world’s oceans increases, the rate of sunscreen unintentionally washed into these marine environments also rises. One study estimated that approximately one-quarter of the sunscreen applied to the skin is washed off over a 20-minute period spent in the water.2 Four of the most common sunscreen agents—benzophenone-3 (BP-3),
Benzophenone-3
4-Methylbenzylidene Camphor
Environmental concerns have also been raised about another common chemical UV filter: 4-MBC, or enzacamene. In laboratory studies, 4-MBC has been shown to cause oxidative stress to Tetrahymena thermophila, an aquatic protozoan, which results in inhibited growth. At higher concentrations, damage to the cellular membrane was seen as soon as 4 hours after exposure.6 In embryonic zebrafish, elevated 4-MBC levels were correlated to improper nerve and muscular development, resulting in developmental defects.7 Another study demonstrated that 4-MBC was toxic to Mytilus galloprovincialis, known as the Mediterranean mussel, and Paracentrotus lividus, a species of sea urchin.8 Although these studies utilized highly controlled laboratory settings, further studies are needed to examine the effects of 4-MBC on these species at environmentally relevant concentrations.
Physical Sunscreens
Physical sunscreens, as compared to the chemical filters referenced above, use either zinc or titanium to protect the skin from the sun’s rays. Nanoparticles, in particular, are preferred because they do not leave a white film on the skin.9 Both titanium dioxide and zinc oxide nanoparticles have been found to inhibit the growth and photosynthesis of marine phytoplankton, the most abundant primary producers on Earth.10,11 These metal contaminants can be transferred to organisms of higher trophic levels, including zooplankton,12 and filter-feeding organisms, including marine abalone13 and the Mediterranean mussel.14 These nanoparticles have been shown to cause oxidative stress to these organisms, making them less fit to withstand environmental stressors. It is difficult to show their true impact, however, as it is challenging to accurately detect and quantify nanoparticle concentrations in vivo.15
Final Thoughts
Coastal travel accounts for 80% of all tourism worldwide, a number that continues to grow. The number of travelers to the Mediterranean Sea alone is expected to rise to 350 million individuals per year within the next 20 years.1 As the number of tourists visiting the world’s oceans increases, the rate of sunscreen unintentionally washed into these marine environments also rises. One study estimated that approximately one-quarter of the sunscreen applied to the skin is washed off over a 20-minute period spent in the water.2 Four of the most common sunscreen agents—benzophenone-3 (BP-3),
Benzophenone-3
4-Methylbenzylidene Camphor
Environmental concerns have also been raised about another common chemical UV filter: 4-MBC, or enzacamene. In laboratory studies, 4-MBC has been shown to cause oxidative stress to Tetrahymena thermophila, an aquatic protozoan, which results in inhibited growth. At higher concentrations, damage to the cellular membrane was seen as soon as 4 hours after exposure.6 In embryonic zebrafish, elevated 4-MBC levels were correlated to improper nerve and muscular development, resulting in developmental defects.7 Another study demonstrated that 4-MBC was toxic to Mytilus galloprovincialis, known as the Mediterranean mussel, and Paracentrotus lividus, a species of sea urchin.8 Although these studies utilized highly controlled laboratory settings, further studies are needed to examine the effects of 4-MBC on these species at environmentally relevant concentrations.
Physical Sunscreens
Physical sunscreens, as compared to the chemical filters referenced above, use either zinc or titanium to protect the skin from the sun’s rays. Nanoparticles, in particular, are preferred because they do not leave a white film on the skin.9 Both titanium dioxide and zinc oxide nanoparticles have been found to inhibit the growth and photosynthesis of marine phytoplankton, the most abundant primary producers on Earth.10,11 These metal contaminants can be transferred to organisms of higher trophic levels, including zooplankton,12 and filter-feeding organisms, including marine abalone13 and the Mediterranean mussel.14 These nanoparticles have been shown to cause oxidative stress to these organisms, making them less fit to withstand environmental stressors. It is difficult to show their true impact, however, as it is challenging to accurately detect and quantify nanoparticle concentrations in vivo.15
Final Thoughts
- Marine problems: tourism & coastal development. World Wide Fund for Nature website. http://wwf.panda.org/about_our_earth/blue_planet/problems/tourism/. Published 2017. Accessed November 14, 2017.
- Danovaro R, Bongiorni L, Corinaldesi C, et al. Sunscreens cause coral bleaching by promoting viral infections. Environ Health Perspect. 2008;116:441-447.
- Downs C, Kramarsky-Winter E, Segal R, et al. Toxicopathological effects of the sunscreen UV filter, oxybenzone (benzophenone-3), on coral planulae and cultured primary cells and its environmental contamination in Hawaii and the US Virgin Islands. Arch Environ Contam Toxicol. 2016;70:265-288.
- Sánchez Rodríguez A, Rodrigo Sanz M, Betancort Rodríguez JR. Occurrence of eight UV filters in beaches of Gran Canaria (Canary Islands)[published online March 17, 2015]. Chemosphere. 2015;131:85-90.
- Bratkovics S, Sapozhnikova Y. Determination of seven commonly used organic UV filters in fresh and saline waters by liquid chromatography-tandem mass spectrometry. Analytical Methods. 2011;3:2943-2950.
- Gao L, Yuan T, Zhou C, et al. Effects of four commonly used UV filters on the growth, cell viability and oxidative stress responses of the Tetrahymena thermophila. Chemosphere. 2013;93:2507-2513.
- Li VW, Tsui MP, Chen X, et al. Effects of 4-methylbenzylidene camphor (4-MBC) on neuronal and muscular development in zebrafish (Danio rerio) embryos [published online February 18, 2016]. Environ Sci Pollut Res Int. 2016;23:8275-8285.
- Paredes E, Perez S, Rodil R, et al. Ecotoxicological evaluation of four UV filters using marine organisms from different trophic levels Isochrysis galbana, Mytilus galloprovincialis, Paracentrotus lividus, and Siriella armata. Chemosphere. 2014;104:44-50.
- Osterwalder U, Sohn M, Herzog B. Global state of sunscreens. Photodermatol Photoimmunol Photomed. 2014;30:62-80.
- Miller RJ, Bennett S, Keller AA, et al. TiO2 nanoparticles are phototoxic to marine phytoplankton. PloS One. 2012;7:E30321.
- Spisni E. Toxicity Assessment of Industrial- and Sunscreen-derived ZnO Nanoparticles [master’s thesis]. Coral Gables, FL: University of Miami Libraries Scholarly Repository; 2016. http://scholarlyrepository.miami.edu/cgi/viewcontent.cgi?article=1625&context=oa_theses. Accessed November 10, 2017.
- Jarvis TA, Miller RJ, Lenihan HS, et al. Toxicity of ZnO nanoparticles to the copepod Acartia tonsa, exposed through a phytoplankton diet [published online April 15, 2013]. Environ Toxicol Chem. 2013;32:1264-1269.
- Zhu X, Zhou J, Cai Z. The toxicity and oxidative stress of TiO2 nanoparticles in marine abalone (Haliotis diversicolor supertexta). Mar Pollut Bull. 2011;63:334-338.
- Barmo C, Ciacci C, Canonico B, et al. In vivo effects of n-TiO2 on digestive gland and immune function of the marine bivalve Mytilus galloprovincialis. Aquatic Toxicol. 2013;132:9-18.
- Sánchez-Quiles D, Tovar-Sánchez A. Are sunscreens a new environmental risk associated with coastal tourism? Environ Int. 2015;83:158-170.
- Xu S, Kwa M, Agarwal A, et al. Sunscreen product performance and other determinants of consumer preferences. JAMA Dermatol. 2016;152:920-927.
- Vesper I. Hawaii seeks to ban ‘reef-unfriendly’ sunscreen. Nature. February 3, 2017. https://www.nature.com/news/hawaii-seeks-to-ban-reef-unfriendly-sunscreen-1.21332. Accessed November 16, 2017.
- Marine problems: tourism & coastal development. World Wide Fund for Nature website. http://wwf.panda.org/about_our_earth/blue_planet/problems/tourism/. Published 2017. Accessed November 14, 2017.
- Danovaro R, Bongiorni L, Corinaldesi C, et al. Sunscreens cause coral bleaching by promoting viral infections. Environ Health Perspect. 2008;116:441-447.
- Downs C, Kramarsky-Winter E, Segal R, et al. Toxicopathological effects of the sunscreen UV filter, oxybenzone (benzophenone-3), on coral planulae and cultured primary cells and its environmental contamination in Hawaii and the US Virgin Islands. Arch Environ Contam Toxicol. 2016;70:265-288.
- Sánchez Rodríguez A, Rodrigo Sanz M, Betancort Rodríguez JR. Occurrence of eight UV filters in beaches of Gran Canaria (Canary Islands)[published online March 17, 2015]. Chemosphere. 2015;131:85-90.
- Bratkovics S, Sapozhnikova Y. Determination of seven commonly used organic UV filters in fresh and saline waters by liquid chromatography-tandem mass spectrometry. Analytical Methods. 2011;3:2943-2950.
- Gao L, Yuan T, Zhou C, et al. Effects of four commonly used UV filters on the growth, cell viability and oxidative stress responses of the Tetrahymena thermophila. Chemosphere. 2013;93:2507-2513.
- Li VW, Tsui MP, Chen X, et al. Effects of 4-methylbenzylidene camphor (4-MBC) on neuronal and muscular development in zebrafish (Danio rerio) embryos [published online February 18, 2016]. Environ Sci Pollut Res Int. 2016;23:8275-8285.
- Paredes E, Perez S, Rodil R, et al. Ecotoxicological evaluation of four UV filters using marine organisms from different trophic levels Isochrysis galbana, Mytilus galloprovincialis, Paracentrotus lividus, and Siriella armata. Chemosphere. 2014;104:44-50.
- Osterwalder U, Sohn M, Herzog B. Global state of sunscreens. Photodermatol Photoimmunol Photomed. 2014;30:62-80.
- Miller RJ, Bennett S, Keller AA, et al. TiO2 nanoparticles are phototoxic to marine phytoplankton. PloS One. 2012;7:E30321.
- Spisni E. Toxicity Assessment of Industrial- and Sunscreen-derived ZnO Nanoparticles [master’s thesis]. Coral Gables, FL: University of Miami Libraries Scholarly Repository; 2016. http://scholarlyrepository.miami.edu/cgi/viewcontent.cgi?article=1625&context=oa_theses. Accessed November 10, 2017.
- Jarvis TA, Miller RJ, Lenihan HS, et al. Toxicity of ZnO nanoparticles to the copepod Acartia tonsa, exposed through a phytoplankton diet [published online April 15, 2013]. Environ Toxicol Chem. 2013;32:1264-1269.
- Zhu X, Zhou J, Cai Z. The toxicity and oxidative stress of TiO2 nanoparticles in marine abalone (Haliotis diversicolor supertexta). Mar Pollut Bull. 2011;63:334-338.
- Barmo C, Ciacci C, Canonico B, et al. In vivo effects of n-TiO2 on digestive gland and immune function of the marine bivalve Mytilus galloprovincialis. Aquatic Toxicol. 2013;132:9-18.
- Sánchez-Quiles D, Tovar-Sánchez A. Are sunscreens a new environmental risk associated with coastal tourism? Environ Int. 2015;83:158-170.
- Xu S, Kwa M, Agarwal A, et al. Sunscreen product performance and other determinants of consumer preferences. JAMA Dermatol. 2016;152:920-927.
- Vesper I. Hawaii seeks to ban ‘reef-unfriendly’ sunscreen. Nature. February 3, 2017. https://www.nature.com/news/hawaii-seeks-to-ban-reef-unfriendly-sunscreen-1.21332. Accessed November 16, 2017.
Cordlike Dermal Plaques and Nodules on the Neck and Hands
The Diagnosis: Fibroblastic Rheumatism
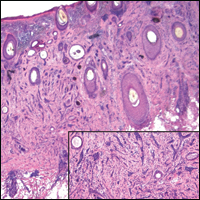
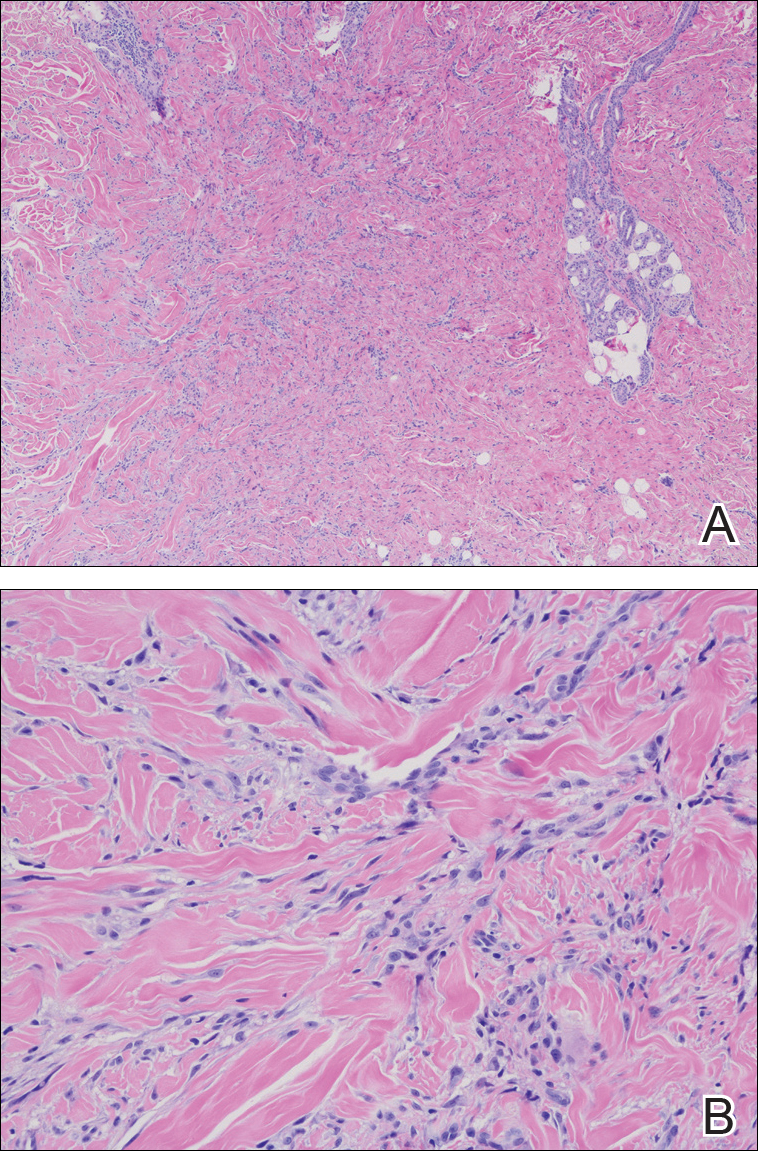
Routine histologic sections stained with hematoxylin and eosin demonstrated a noncircumscribed dermal proliferation of fibroblasts and myofibroblasts with thickened collagen bundles (Figure, A and B). Focally fragmented elastin fibers were noted with Verhoeff elastic tissue stain. Alcian blue stain did not show increased dermal mucin. With the clinical presentation and histologic findings described, we diagnosed the patient with fibroblastic rheumatism (FR). To date, the patient's condition has stabilized overall with skin lesions fading and minimal to no joint pain. Current therapies include adalimumab, mycophenolate mofetil 500 mg 3 times daily, and low-dose prednisone.
Fibroblastic rheumatism is a rare arthropathy with cutaneous findings initially described by Chaouat et al1 in 1980. Age of onset varies, and the condition also has been observed in pediatric patients.2 Fibroblastic rheumatism is characterized by sudden onset of firm, flesh-colored, subcutaneous nodules on periungual and periarticular surfaces.2 Neck lesions rarely are described,2-4 and cordlike plaques previously have not been reported in FR. Typically, patients develop diffusely swollen fingers, palmar thickening, sclerodactyly, and contractures. The eruption may be accompanied by Raynaud phenomenon as well as a progressive symmetric erosive arthropathy.2,5
The clinical course in FR is variable. The cutaneous findings spontaneously may regress in months to years.3,4 However, polyarthropathy often is destructive and progresses to disability.3 Response to therapy has been unpredictable, and the following treatments have been tried, generally with poor efficacy: aspirin, nonsteroidal anti-inflammatory drugs, hydroxychloroquine, colchicine, methotrexate, prednisone, infliximab, D-penicillamine, interferon alfa, and intensive physical therapy.2-4,6 Histologic characteristics may include thickened collagen bundles along with a fibroblastic and myofibroblastic proliferation. Elastic fibers may be decreased or absent.2,3,5
Clinical and histologic features in FR may mimic other entities; thus, clinical pathological correlation is essential in determining the correct diagnosis. Considerations in the differential diagnoses include multicentric reticulohistiocytosis (MRH), palisaded neutrophilic and granulomatous dermatitis, and scleroderma.
In MRH, a symmetric erosive arthritis of mainly distal interphalangeal joints typically precedes the cutaneous disease. Occurrence of arthritis mutilans is reported in approximately half of patients.4 Cutaneous manifestations typically include the presence of coral bead-like papules and nodules over the dorsal aspect of the hands, face, and neck. Unlike FR, MRH has a concomitant autoimmune disease in up to 20% of cases and an associated malignancy in up to 31% of cases, with breast and ovarian carcinomas most common. On histology, MRH is characterized by a nodular infiltrate of histiocytes and multinucleated giant cells with eosinophilic ground-glass cytoplasm.4 No notable collagen changes or fibroblastic proliferations typically are present.
Palisaded neutrophilic and granulomatous dermatitis, usually associated with rheumatoid arthritis or connective tissue disease, classically presents as annular plaques and indurated linear bands over the trunk and extremities. However, its clinical presentation is quite variable and may include pink to violaceous urticarialike; livedoid-appearing; or nonspecific papules, plaques, or nodules. Histology in palisaded neutrophilic and granulomatous dermatitis shows a dense dermal neutrophilic infiltrate associated with interstitial histiocytes having a palisading arrangement around degenerated collagen.7 No fibroblastic proliferation typically is present.
Scleroderma can be distinguished based on additional clinical and laboratory findings as well as histology showing thickened collagen bundles without fibroblastic proliferation.2 The histologic findings also may suggest inclusion of dermatofibroma or a scar in the differential diagnosis, though the clinical presentation of these entities would not support these diagnoses.
Acknowledgments
We thank the patient for granting permission to share this information. We also thank Sheng Chen, MD, PhD (Lake Success, New York), for his dermatopathological contributions to the case.
- Chaouat Y, Aron-Brunetiere R, Faures B, et al. Une nouvelle entité: le rhumatisme fibroblastique. a propos d'une observation [in French]. Rev Rhum Mal Osteoartic. 1980;47:34-35.
- Jurado SA, Alvin GG, Selim MA, et al. Fibroblastic rheumatism: a report of 4 cases with potential therapeutic implications. J Am Acad Dermatol. 2012;66:959-965.
- Colonna L, Barbieri C, Di Lella G, et al. Fibroblastic rheumatism: a case without rheumatological symptoms. Acta Derm Venereol. 2002;82:200-203.
- Trotta F, Colina M. Multicentric reticulohistiocytosis and fibroblastic rheumatism. Best Pract Res Clin Rheumatol. 2012;26:543-557.
- Lee JM, Sundel RP, Liang MG. Fibroblastic rheumatism: case report and review of the literature. Pediatr Dermatol. 2002;19:532-535.
- Kluger N, Dumas-Tesici A, Hamel D, et al. Fibroblastic rheumatism: fibromatosis rather than non-Langerhans cell histiocytosis. J Cutan Pathol. 2010;37:587-592.
- Stephenson SR, Campbell M, Dre GS, et al. Palisaded neutrophilic and granulomatous dermatitis presenting in a patient with rheumatoid arthritis on adalimumab. J Cutan Pathol. 2011;38:644-648.
The Diagnosis: Fibroblastic Rheumatism
Routine histologic sections stained with hematoxylin and eosin demonstrated a noncircumscribed dermal proliferation of fibroblasts and myofibroblasts with thickened collagen bundles (Figure, A and B). Focally fragmented elastin fibers were noted with Verhoeff elastic tissue stain. Alcian blue stain did not show increased dermal mucin. With the clinical presentation and histologic findings described, we diagnosed the patient with fibroblastic rheumatism (FR). To date, the patient's condition has stabilized overall with skin lesions fading and minimal to no joint pain. Current therapies include adalimumab, mycophenolate mofetil 500 mg 3 times daily, and low-dose prednisone.

Fibroblastic rheumatism is a rare arthropathy with cutaneous findings initially described by Chaouat et al1 in 1980. Age of onset varies, and the condition also has been observed in pediatric patients.2 Fibroblastic rheumatism is characterized by sudden onset of firm, flesh-colored, subcutaneous nodules on periungual and periarticular surfaces.2 Neck lesions rarely are described,2-4 and cordlike plaques previously have not been reported in FR. Typically, patients develop diffusely swollen fingers, palmar thickening, sclerodactyly, and contractures. The eruption may be accompanied by Raynaud phenomenon as well as a progressive symmetric erosive arthropathy.2,5
The clinical course in FR is variable. The cutaneous findings spontaneously may regress in months to years.3,4 However, polyarthropathy often is destructive and progresses to disability.3 Response to therapy has been unpredictable, and the following treatments have been tried, generally with poor efficacy: aspirin, nonsteroidal anti-inflammatory drugs, hydroxychloroquine, colchicine, methotrexate, prednisone, infliximab, D-penicillamine, interferon alfa, and intensive physical therapy.2-4,6 Histologic characteristics may include thickened collagen bundles along with a fibroblastic and myofibroblastic proliferation. Elastic fibers may be decreased or absent.2,3,5
Clinical and histologic features in FR may mimic other entities; thus, clinical pathological correlation is essential in determining the correct diagnosis. Considerations in the differential diagnoses include multicentric reticulohistiocytosis (MRH), palisaded neutrophilic and granulomatous dermatitis, and scleroderma.
In MRH, a symmetric erosive arthritis of mainly distal interphalangeal joints typically precedes the cutaneous disease. Occurrence of arthritis mutilans is reported in approximately half of patients.4 Cutaneous manifestations typically include the presence of coral bead-like papules and nodules over the dorsal aspect of the hands, face, and neck. Unlike FR, MRH has a concomitant autoimmune disease in up to 20% of cases and an associated malignancy in up to 31% of cases, with breast and ovarian carcinomas most common. On histology, MRH is characterized by a nodular infiltrate of histiocytes and multinucleated giant cells with eosinophilic ground-glass cytoplasm.4 No notable collagen changes or fibroblastic proliferations typically are present.
Palisaded neutrophilic and granulomatous dermatitis, usually associated with rheumatoid arthritis or connective tissue disease, classically presents as annular plaques and indurated linear bands over the trunk and extremities. However, its clinical presentation is quite variable and may include pink to violaceous urticarialike; livedoid-appearing; or nonspecific papules, plaques, or nodules. Histology in palisaded neutrophilic and granulomatous dermatitis shows a dense dermal neutrophilic infiltrate associated with interstitial histiocytes having a palisading arrangement around degenerated collagen.7 No fibroblastic proliferation typically is present.
Scleroderma can be distinguished based on additional clinical and laboratory findings as well as histology showing thickened collagen bundles without fibroblastic proliferation.2 The histologic findings also may suggest inclusion of dermatofibroma or a scar in the differential diagnosis, though the clinical presentation of these entities would not support these diagnoses.
Acknowledgments
We thank the patient for granting permission to share this information. We also thank Sheng Chen, MD, PhD (Lake Success, New York), for his dermatopathological contributions to the case.
The Diagnosis: Fibroblastic Rheumatism
Routine histologic sections stained with hematoxylin and eosin demonstrated a noncircumscribed dermal proliferation of fibroblasts and myofibroblasts with thickened collagen bundles (Figure, A and B). Focally fragmented elastin fibers were noted with Verhoeff elastic tissue stain. Alcian blue stain did not show increased dermal mucin. With the clinical presentation and histologic findings described, we diagnosed the patient with fibroblastic rheumatism (FR). To date, the patient's condition has stabilized overall with skin lesions fading and minimal to no joint pain. Current therapies include adalimumab, mycophenolate mofetil 500 mg 3 times daily, and low-dose prednisone.

Fibroblastic rheumatism is a rare arthropathy with cutaneous findings initially described by Chaouat et al1 in 1980. Age of onset varies, and the condition also has been observed in pediatric patients.2 Fibroblastic rheumatism is characterized by sudden onset of firm, flesh-colored, subcutaneous nodules on periungual and periarticular surfaces.2 Neck lesions rarely are described,2-4 and cordlike plaques previously have not been reported in FR. Typically, patients develop diffusely swollen fingers, palmar thickening, sclerodactyly, and contractures. The eruption may be accompanied by Raynaud phenomenon as well as a progressive symmetric erosive arthropathy.2,5
The clinical course in FR is variable. The cutaneous findings spontaneously may regress in months to years.3,4 However, polyarthropathy often is destructive and progresses to disability.3 Response to therapy has been unpredictable, and the following treatments have been tried, generally with poor efficacy: aspirin, nonsteroidal anti-inflammatory drugs, hydroxychloroquine, colchicine, methotrexate, prednisone, infliximab, D-penicillamine, interferon alfa, and intensive physical therapy.2-4,6 Histologic characteristics may include thickened collagen bundles along with a fibroblastic and myofibroblastic proliferation. Elastic fibers may be decreased or absent.2,3,5
Clinical and histologic features in FR may mimic other entities; thus, clinical pathological correlation is essential in determining the correct diagnosis. Considerations in the differential diagnoses include multicentric reticulohistiocytosis (MRH), palisaded neutrophilic and granulomatous dermatitis, and scleroderma.
In MRH, a symmetric erosive arthritis of mainly distal interphalangeal joints typically precedes the cutaneous disease. Occurrence of arthritis mutilans is reported in approximately half of patients.4 Cutaneous manifestations typically include the presence of coral bead-like papules and nodules over the dorsal aspect of the hands, face, and neck. Unlike FR, MRH has a concomitant autoimmune disease in up to 20% of cases and an associated malignancy in up to 31% of cases, with breast and ovarian carcinomas most common. On histology, MRH is characterized by a nodular infiltrate of histiocytes and multinucleated giant cells with eosinophilic ground-glass cytoplasm.4 No notable collagen changes or fibroblastic proliferations typically are present.
Palisaded neutrophilic and granulomatous dermatitis, usually associated with rheumatoid arthritis or connective tissue disease, classically presents as annular plaques and indurated linear bands over the trunk and extremities. However, its clinical presentation is quite variable and may include pink to violaceous urticarialike; livedoid-appearing; or nonspecific papules, plaques, or nodules. Histology in palisaded neutrophilic and granulomatous dermatitis shows a dense dermal neutrophilic infiltrate associated with interstitial histiocytes having a palisading arrangement around degenerated collagen.7 No fibroblastic proliferation typically is present.
Scleroderma can be distinguished based on additional clinical and laboratory findings as well as histology showing thickened collagen bundles without fibroblastic proliferation.2 The histologic findings also may suggest inclusion of dermatofibroma or a scar in the differential diagnosis, though the clinical presentation of these entities would not support these diagnoses.
Acknowledgments
We thank the patient for granting permission to share this information. We also thank Sheng Chen, MD, PhD (Lake Success, New York), for his dermatopathological contributions to the case.
- Chaouat Y, Aron-Brunetiere R, Faures B, et al. Une nouvelle entité: le rhumatisme fibroblastique. a propos d'une observation [in French]. Rev Rhum Mal Osteoartic. 1980;47:34-35.
- Jurado SA, Alvin GG, Selim MA, et al. Fibroblastic rheumatism: a report of 4 cases with potential therapeutic implications. J Am Acad Dermatol. 2012;66:959-965.
- Colonna L, Barbieri C, Di Lella G, et al. Fibroblastic rheumatism: a case without rheumatological symptoms. Acta Derm Venereol. 2002;82:200-203.
- Trotta F, Colina M. Multicentric reticulohistiocytosis and fibroblastic rheumatism. Best Pract Res Clin Rheumatol. 2012;26:543-557.
- Lee JM, Sundel RP, Liang MG. Fibroblastic rheumatism: case report and review of the literature. Pediatr Dermatol. 2002;19:532-535.
- Kluger N, Dumas-Tesici A, Hamel D, et al. Fibroblastic rheumatism: fibromatosis rather than non-Langerhans cell histiocytosis. J Cutan Pathol. 2010;37:587-592.
- Stephenson SR, Campbell M, Dre GS, et al. Palisaded neutrophilic and granulomatous dermatitis presenting in a patient with rheumatoid arthritis on adalimumab. J Cutan Pathol. 2011;38:644-648.
- Chaouat Y, Aron-Brunetiere R, Faures B, et al. Une nouvelle entité: le rhumatisme fibroblastique. a propos d'une observation [in French]. Rev Rhum Mal Osteoartic. 1980;47:34-35.
- Jurado SA, Alvin GG, Selim MA, et al. Fibroblastic rheumatism: a report of 4 cases with potential therapeutic implications. J Am Acad Dermatol. 2012;66:959-965.
- Colonna L, Barbieri C, Di Lella G, et al. Fibroblastic rheumatism: a case without rheumatological symptoms. Acta Derm Venereol. 2002;82:200-203.
- Trotta F, Colina M. Multicentric reticulohistiocytosis and fibroblastic rheumatism. Best Pract Res Clin Rheumatol. 2012;26:543-557.
- Lee JM, Sundel RP, Liang MG. Fibroblastic rheumatism: case report and review of the literature. Pediatr Dermatol. 2002;19:532-535.
- Kluger N, Dumas-Tesici A, Hamel D, et al. Fibroblastic rheumatism: fibromatosis rather than non-Langerhans cell histiocytosis. J Cutan Pathol. 2010;37:587-592.
- Stephenson SR, Campbell M, Dre GS, et al. Palisaded neutrophilic and granulomatous dermatitis presenting in a patient with rheumatoid arthritis on adalimumab. J Cutan Pathol. 2011;38:644-648.
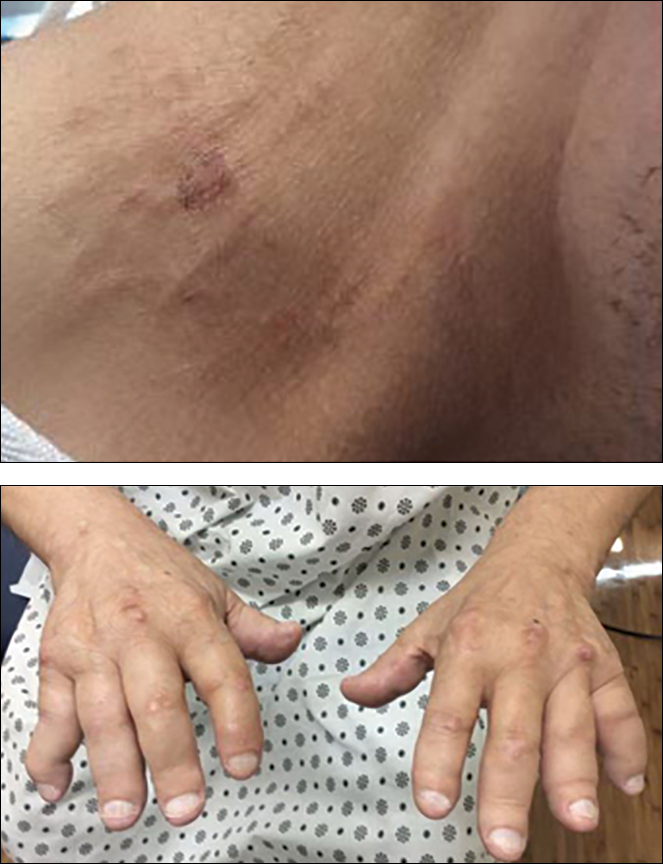
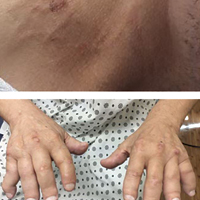
A 67-year-old man presented with asymptomatic plaques on the neck of 4 months' duration and nodules scattered over the hands, elbows, ears, and forehead of 3 years' duration. The eruption was associated with progressive thickening and contractures of the fingers, hand morning stiffness lasting less than 45 minutes, and Raynaud phenomenon. Physical examination revealed flesh-colored, firm, cordlike plaques on the neck bilaterally (top), with firm subcutaneous nodules on the helix and antihelix of the ears, forehead, elbows, and on the dorsal and ventral aspects of the hands (bottom). The largest nodules were approximately 5 cm. All fingers and first toes were thickened and firm with few contractile bands on the fingers. The patient had a persistently elevated erythrocyte sedimentation rate (80 mm/h)(reference range, 0-20 mm/h) and C-reactive protein level (3.27 mg/dL)(reference range, 0.00-0.40 mg/dL). Serologic workup was remarkable only for an antinuclear antibody titer of 1:80 (speckled). Plain radiographs confirmed an erosive arthropathy of the hands and feet. Erosions on the hands predominantly involved distal interphalangeal articulations, as well as, to a lesser extent, the proximal interphalangeal articulations, carpus, and the left distal radius. Erosive changes on the feet involved metatarsophalangeal, proximal interphalangeal, and distal interphalangeal articulations. Biopsies from the neck were performed for histopathologic correlation.
Indurated Plaque on the Eyebrow
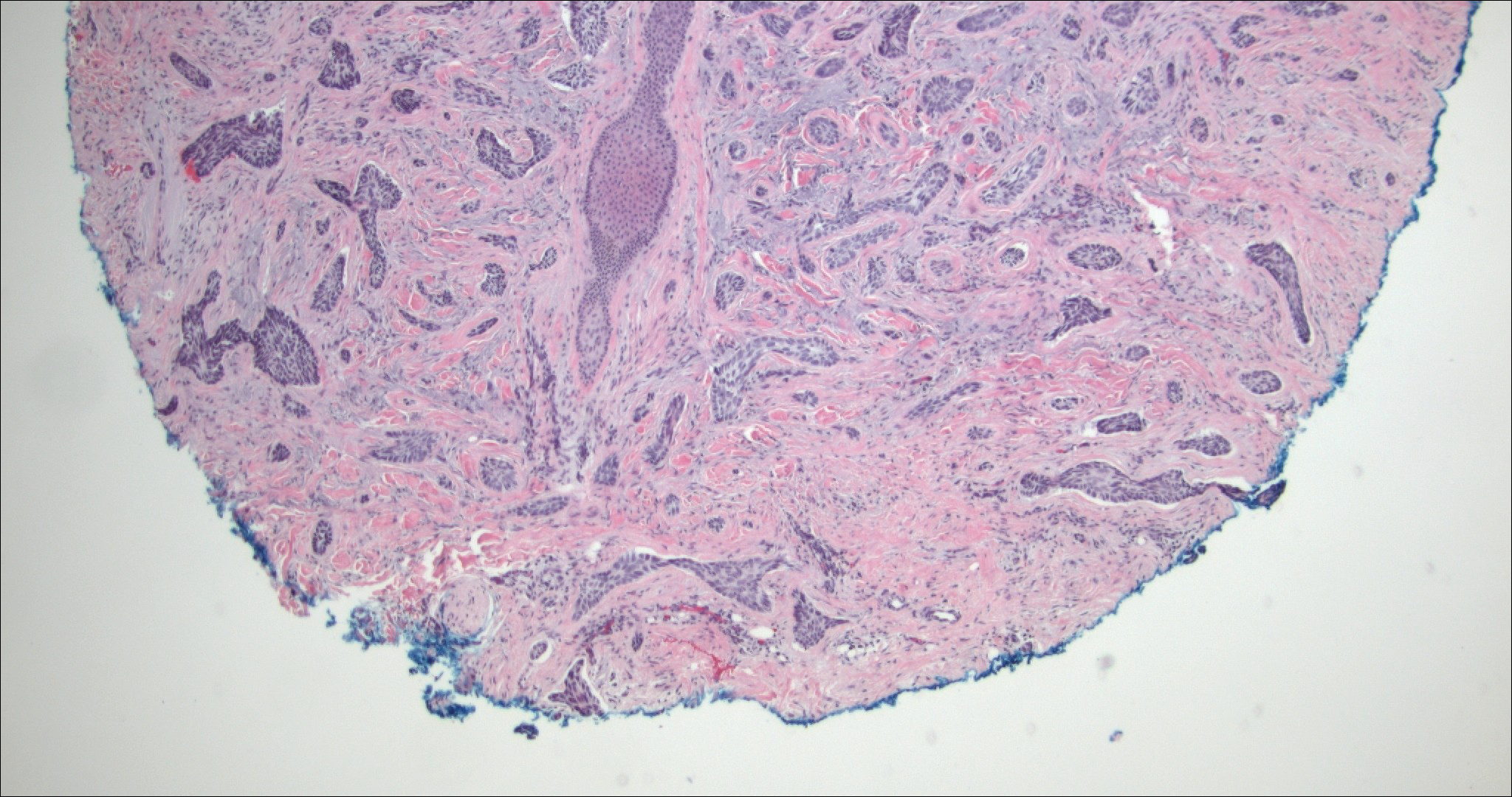
The Diagnosis: Microcystic Adnexal Carcinoma
Microcystic adnexal carcinoma (MAC) is a rare, low-grade adnexal carcinoma consisting of both ductal and pilar differentiation.1 It typically presents in young to middle-aged adults as a flesh-colored or yellow indurated plaque on the upper lip, medial cheek, or chin. Histologically, MACs exhibit a biphasic pattern consisting of epithelial islands of cords and lumina creating tadpolelike ducts intermixed with basaloid nests (quiz image). Keratin horn cysts are common superficially. A dense red sclerotic stroma is seen interspersed between the ducts and epithelial islands creating a "paisley tie" appearance. The lesion displays an infiltrative pattern and can be deeply invasive, extending down to the fat and muscle (quiz image, inset). Perineural invasion is common. Atypia, when present, is minimal or mild and mitoses are rare. Although this tumor's histologic pattern appears aggressive in nature, it lacks immunohistochemical staining such as p53, Ki-67, bcl-2, and c-erbB-2 that correlate with malignant behavior.2 A common diagnostic pitfall is examination of a superficial biopsy in which an MAC may be mistakenly identified as another entity.
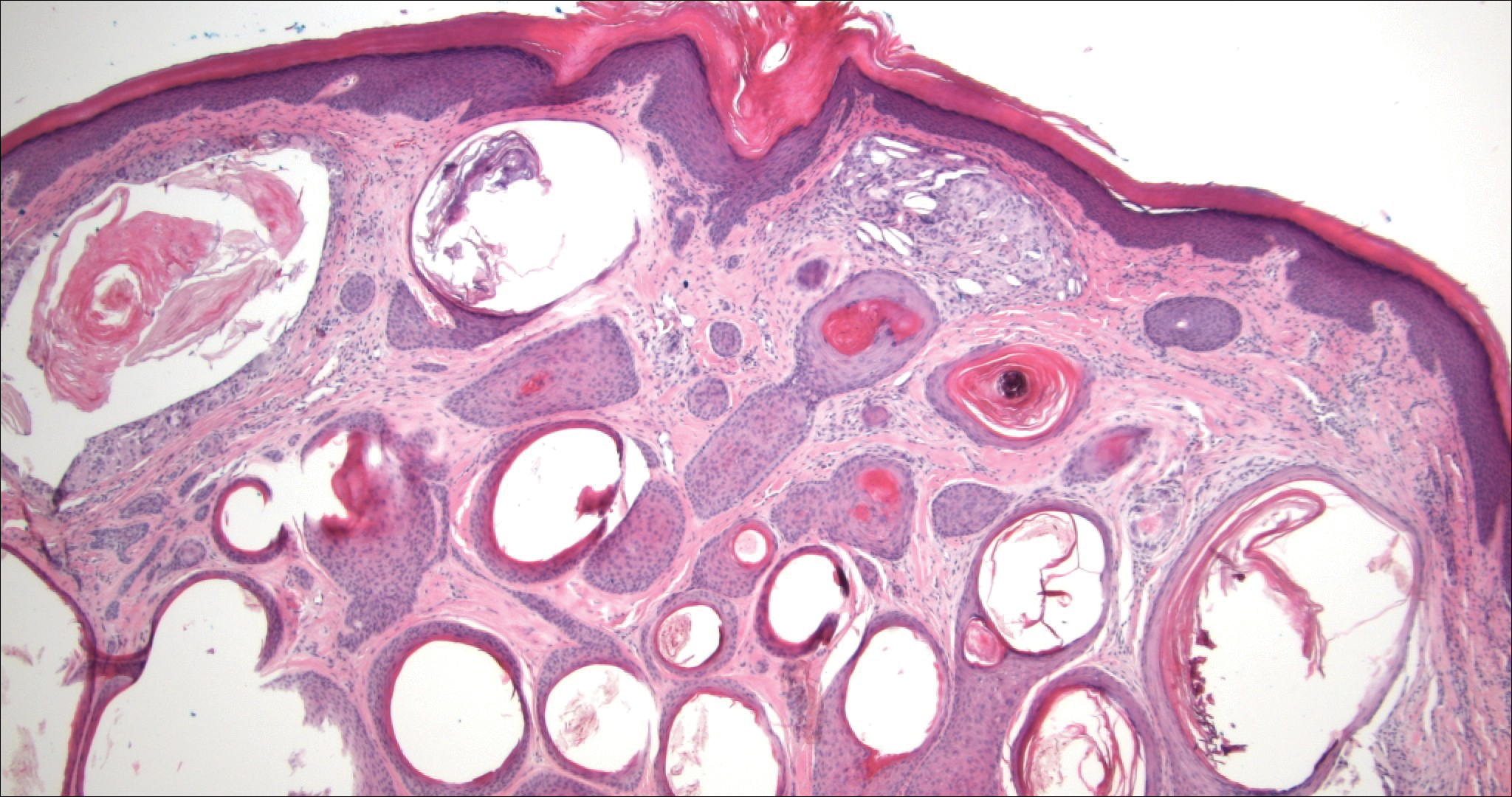
Syringomas are benign adnexal neoplasms with ductal differentiation.3 They are more common in women, especially those of Asian descent, and in patients with Down syndrome. They typically present as multiple small, firm, flesh-colored papules in the periorbital area or upper trunk. Histologically, syringomas also display comma-shaped tubules and ducts with a tadpolelike appearance and a dense red stroma creating a paisley tie-like pattern. Ductal cells have an abundant pink cytoplasm. Syringomas are well-circumscribed and more superficial than MACs without an infiltrative pattern. They lack mitotic activity or perineural invasion (Figure 1).

Desmoplastic trichoepithelioma (DTE) is a benign follicular neoplasm.4 It presents in adulthood with a female predominance. Clinically, it appears as a solitary flesh-colored to yellow annular plaque with raised borders and a depressed central area, often on the medial cheek. Histologically, DTEs are well-circumscribed with narrow branching cords lined with polygonal cells. A dense red stroma in combination with the epithelioid aggregates also creates the paisley tie-like pattern in this lesion. Retraction between collagen bundles within the stroma can be seen, helping distinguish this lesion from a morpheaform basal cell carcinoma (BCC), which has retraction between the epithelium and stroma. Immunohistochemistry also can be a useful tool to help differentiate DTEs from morpheaform BCCs in that sparse cytokeratin 20-positive Merkel cells can be seen within the basaloid islands of DTE but not BCC.5 Also seen with DTEs are numerous keratin horn cysts that commonly are filled with dystrophic calcifications. Cellular atypia and mitoses are not seen (Figure 2). Compared to MACs, DTEs lack abundant ductal structures and also contain papillary mesenchymal bodies and a more fibroblast-rich stroma.
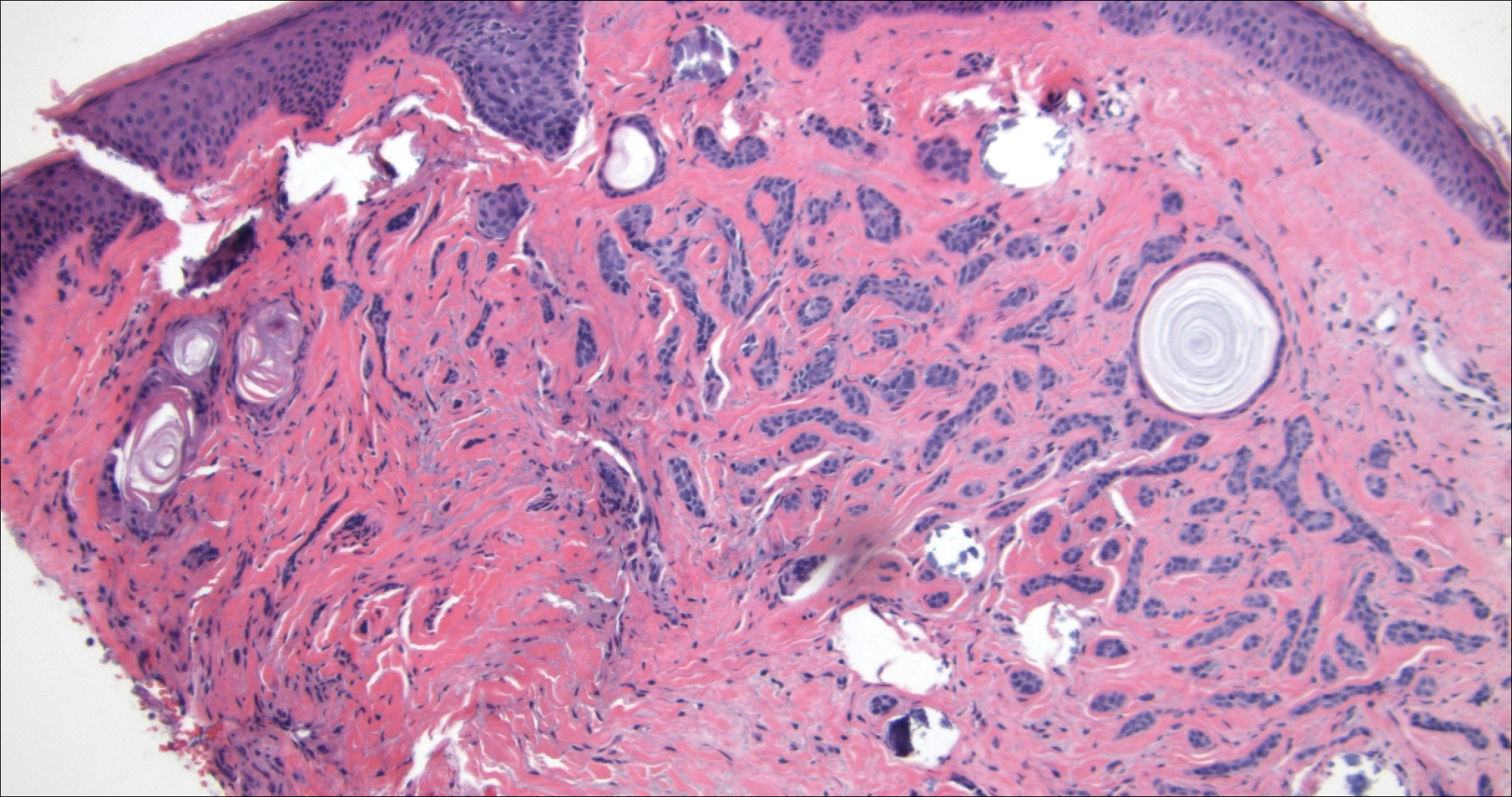
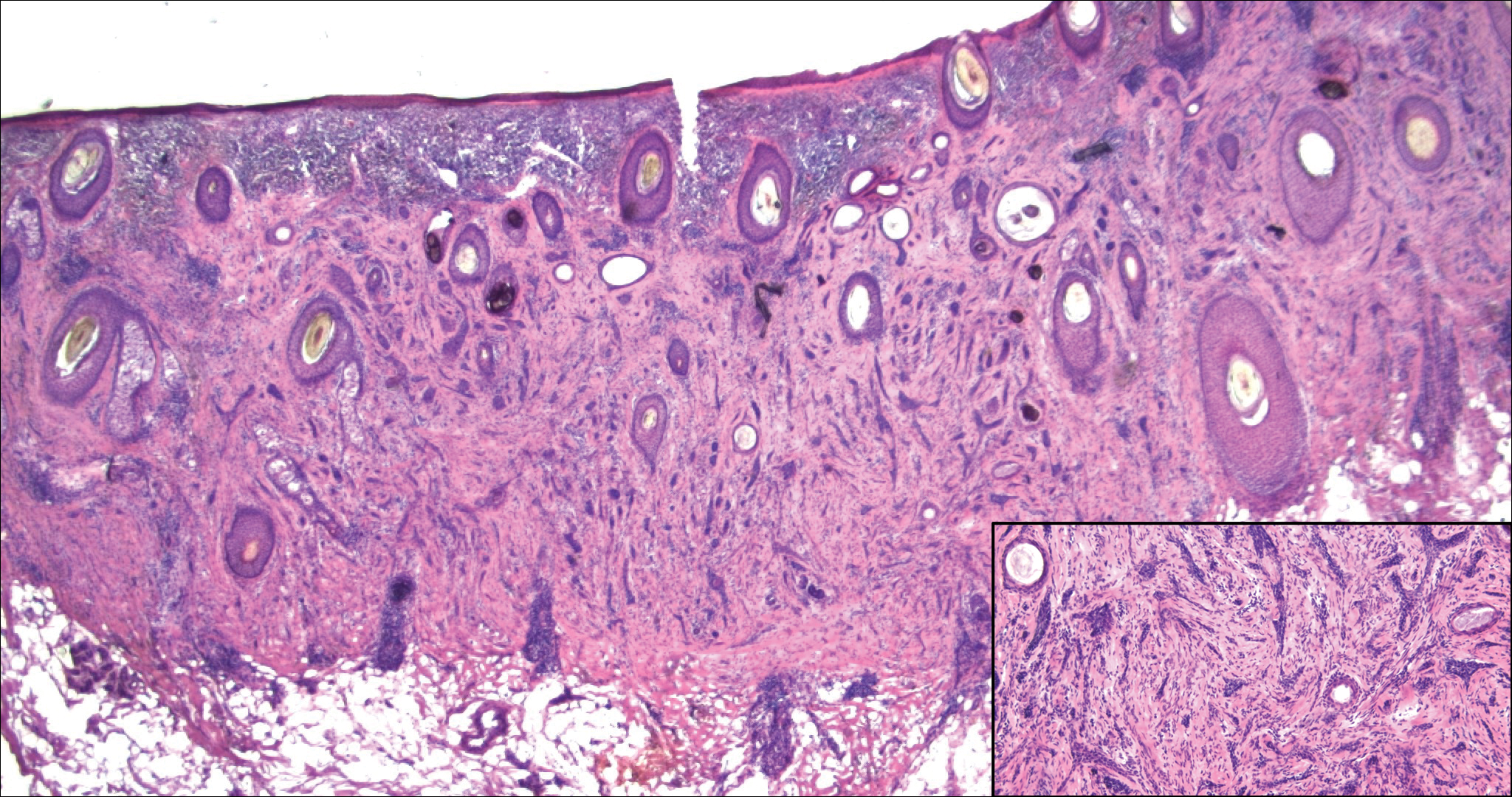
Morpheaform BCC is an aggressive subtype of BCC. It presents as a scarlike plaque that gradually expands. Thin infiltrating strands of basaloid cells are seen haphazardly throughout a pink sclerotic stroma. Tadpolelike basaloid islands and rarely horn cysts can be seen scattered superficially, creating the paisley tie-like pattern. This lesion is more infiltrating than a syringoma or a DTE, and perineural invasion is common. Retraction is uncommon, but when present, it is seen between the epithelial cords and adjacent stroma (Figure 3).
Trichoadenoma is another benign neoplasm of follicular differentiation.6 It typically presents as a dome-shaped papule or plaque on the head or neck. Histologically it displays numerous dilated cystic spaces that reflect its origin from isthmic and infundibular differentiation. There is no attachment to the overlying epidermis. It can be distinguished from MAC, DTE, and syringoma due to a lack of basaloid aggregates and only a small number of non-cyst-forming epithelial cells (Figure 4).
- Nickoloff BJ, Fleischmann HE, Carmel J. Microcystic adnexal carcinoma: immunohistologic observations suggesting dual (pilar and eccrine) differentiation. Arch Dermatol. 1986;122:290-294.
- Smith KJ, Williams J, Corbett D, et al. Microcystic adnexal carcinoma: an immunohistochemical study including markers of proliferation and apoptosis. Am J Surg Pathol. 2001;25:464-471.
- Hashimoto K, Lever WF. Histogenesis of skin appendage tumors. Arch Dermatol. 1969;100:356-369.
- Brownstein MH, Shapiro L. Desmoplastic trichoepithelioma. Cancer. 1977;40:2979-2986.
- Hartschuh W, Schulz T. Merkel cells are integral constituents of desmoplastic trichoepithelioma: an immunohistochemical and electron microscopy study. J Cutan Pathol. 1995;22:413-421.
- Rahbari H, Mehregan A, Pinkus A. Trichoadenoma of Nikolowski. J Cutan Pathol. 1977;4:90-98.
The Diagnosis: Microcystic Adnexal Carcinoma
Microcystic adnexal carcinoma (MAC) is a rare, low-grade adnexal carcinoma consisting of both ductal and pilar differentiation.1 It typically presents in young to middle-aged adults as a flesh-colored or yellow indurated plaque on the upper lip, medial cheek, or chin. Histologically, MACs exhibit a biphasic pattern consisting of epithelial islands of cords and lumina creating tadpolelike ducts intermixed with basaloid nests (quiz image). Keratin horn cysts are common superficially. A dense red sclerotic stroma is seen interspersed between the ducts and epithelial islands creating a "paisley tie" appearance. The lesion displays an infiltrative pattern and can be deeply invasive, extending down to the fat and muscle (quiz image, inset). Perineural invasion is common. Atypia, when present, is minimal or mild and mitoses are rare. Although this tumor's histologic pattern appears aggressive in nature, it lacks immunohistochemical staining such as p53, Ki-67, bcl-2, and c-erbB-2 that correlate with malignant behavior.2 A common diagnostic pitfall is examination of a superficial biopsy in which an MAC may be mistakenly identified as another entity.
Syringomas are benign adnexal neoplasms with ductal differentiation.3 They are more common in women, especially those of Asian descent, and in patients with Down syndrome. They typically present as multiple small, firm, flesh-colored papules in the periorbital area or upper trunk. Histologically, syringomas also display comma-shaped tubules and ducts with a tadpolelike appearance and a dense red stroma creating a paisley tie-like pattern. Ductal cells have an abundant pink cytoplasm. Syringomas are well-circumscribed and more superficial than MACs without an infiltrative pattern. They lack mitotic activity or perineural invasion (Figure 1).

Desmoplastic trichoepithelioma (DTE) is a benign follicular neoplasm.4 It presents in adulthood with a female predominance. Clinically, it appears as a solitary flesh-colored to yellow annular plaque with raised borders and a depressed central area, often on the medial cheek. Histologically, DTEs are well-circumscribed with narrow branching cords lined with polygonal cells. A dense red stroma in combination with the epithelioid aggregates also creates the paisley tie-like pattern in this lesion. Retraction between collagen bundles within the stroma can be seen, helping distinguish this lesion from a morpheaform basal cell carcinoma (BCC), which has retraction between the epithelium and stroma. Immunohistochemistry also can be a useful tool to help differentiate DTEs from morpheaform BCCs in that sparse cytokeratin 20-positive Merkel cells can be seen within the basaloid islands of DTE but not BCC.5 Also seen with DTEs are numerous keratin horn cysts that commonly are filled with dystrophic calcifications. Cellular atypia and mitoses are not seen (Figure 2). Compared to MACs, DTEs lack abundant ductal structures and also contain papillary mesenchymal bodies and a more fibroblast-rich stroma.

Morpheaform BCC is an aggressive subtype of BCC. It presents as a scarlike plaque that gradually expands. Thin infiltrating strands of basaloid cells are seen haphazardly throughout a pink sclerotic stroma. Tadpolelike basaloid islands and rarely horn cysts can be seen scattered superficially, creating the paisley tie-like pattern. This lesion is more infiltrating than a syringoma or a DTE, and perineural invasion is common. Retraction is uncommon, but when present, it is seen between the epithelial cords and adjacent stroma (Figure 3).

Trichoadenoma is another benign neoplasm of follicular differentiation.6 It typically presents as a dome-shaped papule or plaque on the head or neck. Histologically it displays numerous dilated cystic spaces that reflect its origin from isthmic and infundibular differentiation. There is no attachment to the overlying epidermis. It can be distinguished from MAC, DTE, and syringoma due to a lack of basaloid aggregates and only a small number of non-cyst-forming epithelial cells (Figure 4).

The Diagnosis: Microcystic Adnexal Carcinoma
Microcystic adnexal carcinoma (MAC) is a rare, low-grade adnexal carcinoma consisting of both ductal and pilar differentiation.1 It typically presents in young to middle-aged adults as a flesh-colored or yellow indurated plaque on the upper lip, medial cheek, or chin. Histologically, MACs exhibit a biphasic pattern consisting of epithelial islands of cords and lumina creating tadpolelike ducts intermixed with basaloid nests (quiz image). Keratin horn cysts are common superficially. A dense red sclerotic stroma is seen interspersed between the ducts and epithelial islands creating a "paisley tie" appearance. The lesion displays an infiltrative pattern and can be deeply invasive, extending down to the fat and muscle (quiz image, inset). Perineural invasion is common. Atypia, when present, is minimal or mild and mitoses are rare. Although this tumor's histologic pattern appears aggressive in nature, it lacks immunohistochemical staining such as p53, Ki-67, bcl-2, and c-erbB-2 that correlate with malignant behavior.2 A common diagnostic pitfall is examination of a superficial biopsy in which an MAC may be mistakenly identified as another entity.
Syringomas are benign adnexal neoplasms with ductal differentiation.3 They are more common in women, especially those of Asian descent, and in patients with Down syndrome. They typically present as multiple small, firm, flesh-colored papules in the periorbital area or upper trunk. Histologically, syringomas also display comma-shaped tubules and ducts with a tadpolelike appearance and a dense red stroma creating a paisley tie-like pattern. Ductal cells have an abundant pink cytoplasm. Syringomas are well-circumscribed and more superficial than MACs without an infiltrative pattern. They lack mitotic activity or perineural invasion (Figure 1).

Desmoplastic trichoepithelioma (DTE) is a benign follicular neoplasm.4 It presents in adulthood with a female predominance. Clinically, it appears as a solitary flesh-colored to yellow annular plaque with raised borders and a depressed central area, often on the medial cheek. Histologically, DTEs are well-circumscribed with narrow branching cords lined with polygonal cells. A dense red stroma in combination with the epithelioid aggregates also creates the paisley tie-like pattern in this lesion. Retraction between collagen bundles within the stroma can be seen, helping distinguish this lesion from a morpheaform basal cell carcinoma (BCC), which has retraction between the epithelium and stroma. Immunohistochemistry also can be a useful tool to help differentiate DTEs from morpheaform BCCs in that sparse cytokeratin 20-positive Merkel cells can be seen within the basaloid islands of DTE but not BCC.5 Also seen with DTEs are numerous keratin horn cysts that commonly are filled with dystrophic calcifications. Cellular atypia and mitoses are not seen (Figure 2). Compared to MACs, DTEs lack abundant ductal structures and also contain papillary mesenchymal bodies and a more fibroblast-rich stroma.

Morpheaform BCC is an aggressive subtype of BCC. It presents as a scarlike plaque that gradually expands. Thin infiltrating strands of basaloid cells are seen haphazardly throughout a pink sclerotic stroma. Tadpolelike basaloid islands and rarely horn cysts can be seen scattered superficially, creating the paisley tie-like pattern. This lesion is more infiltrating than a syringoma or a DTE, and perineural invasion is common. Retraction is uncommon, but when present, it is seen between the epithelial cords and adjacent stroma (Figure 3).

Trichoadenoma is another benign neoplasm of follicular differentiation.6 It typically presents as a dome-shaped papule or plaque on the head or neck. Histologically it displays numerous dilated cystic spaces that reflect its origin from isthmic and infundibular differentiation. There is no attachment to the overlying epidermis. It can be distinguished from MAC, DTE, and syringoma due to a lack of basaloid aggregates and only a small number of non-cyst-forming epithelial cells (Figure 4).

- Nickoloff BJ, Fleischmann HE, Carmel J. Microcystic adnexal carcinoma: immunohistologic observations suggesting dual (pilar and eccrine) differentiation. Arch Dermatol. 1986;122:290-294.
- Smith KJ, Williams J, Corbett D, et al. Microcystic adnexal carcinoma: an immunohistochemical study including markers of proliferation and apoptosis. Am J Surg Pathol. 2001;25:464-471.
- Hashimoto K, Lever WF. Histogenesis of skin appendage tumors. Arch Dermatol. 1969;100:356-369.
- Brownstein MH, Shapiro L. Desmoplastic trichoepithelioma. Cancer. 1977;40:2979-2986.
- Hartschuh W, Schulz T. Merkel cells are integral constituents of desmoplastic trichoepithelioma: an immunohistochemical and electron microscopy study. J Cutan Pathol. 1995;22:413-421.
- Rahbari H, Mehregan A, Pinkus A. Trichoadenoma of Nikolowski. J Cutan Pathol. 1977;4:90-98.
- Nickoloff BJ, Fleischmann HE, Carmel J. Microcystic adnexal carcinoma: immunohistologic observations suggesting dual (pilar and eccrine) differentiation. Arch Dermatol. 1986;122:290-294.
- Smith KJ, Williams J, Corbett D, et al. Microcystic adnexal carcinoma: an immunohistochemical study including markers of proliferation and apoptosis. Am J Surg Pathol. 2001;25:464-471.
- Hashimoto K, Lever WF. Histogenesis of skin appendage tumors. Arch Dermatol. 1969;100:356-369.
- Brownstein MH, Shapiro L. Desmoplastic trichoepithelioma. Cancer. 1977;40:2979-2986.
- Hartschuh W, Schulz T. Merkel cells are integral constituents of desmoplastic trichoepithelioma: an immunohistochemical and electron microscopy study. J Cutan Pathol. 1995;22:413-421.
- Rahbari H, Mehregan A, Pinkus A. Trichoadenoma of Nikolowski. J Cutan Pathol. 1977;4:90-98.
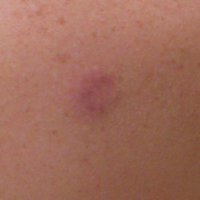
A 52-year-old woman presented with an indurated plaque on the right lateral eyebrow that had been slowly enlarging over the last 4 months.
Over-the-counter Topical Musculoskeletal Pain Relievers Used With a Heat Source: A Dangerous Combination
To the Editor:
The combination of menthol and methyl salicylate found in a variety of over-the-counter (OTC) creams in conjunction with a heat source such as a heating pad used for musculoskeletal symptoms can be a dire combination due to increased systemic absorption with associated toxicity and localized effects ranging from contact dermatitis or irritation to burn or necrosis.1-6 We present a case of localized burn due a combination of topical methyl salicylate and heating pad use. We also discuss 2 commonly encountered side effects in the literature—localized burns and systemic toxicity associated with percutaneous absorption—and provide specific considerations related to the geriatric and pediatric populations.
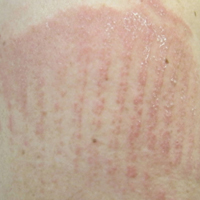
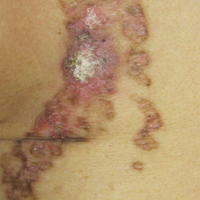
A 62-year-old woman with a history of eczematous dermatitis and osteoarthritis with pain of the left shoulder presented to the dermatology clinic with painful skin-related changes on the left arm of 1 week’s duration. She was prescribed acetaminophen and ibuprofen. However, she self-medicated the left shoulder pain with 2 OTC products containing topical menthol and/or methyl salicylate in combination with a heating pad and likely fell asleep with this combination therapy applied. She noticed the burn the next morning. On examination, the left arm exhibited a geometric, irregularly shaped, erythematous, scaly plaque with a sharp transverse linear demarcation proximally and numerous erythematous linear scaly plaques oriented in an axial orientation with less-defined borders distally (Figure). The patient was diagnosed with burn secondary to combination of topical methyl salicylate and heating pad use. The patient was advised to discontinue the topical medication and to use caution with the heating pad in the future. She was prescribed pramoxine-hydrocortisone lotion to be applied to the affected area twice daily up to 5 days weekly until resolution. Subsequent evaluations revealed progressive improvement with only mild postinflammatory hyperpigmentation noted at 6 months after the burn.
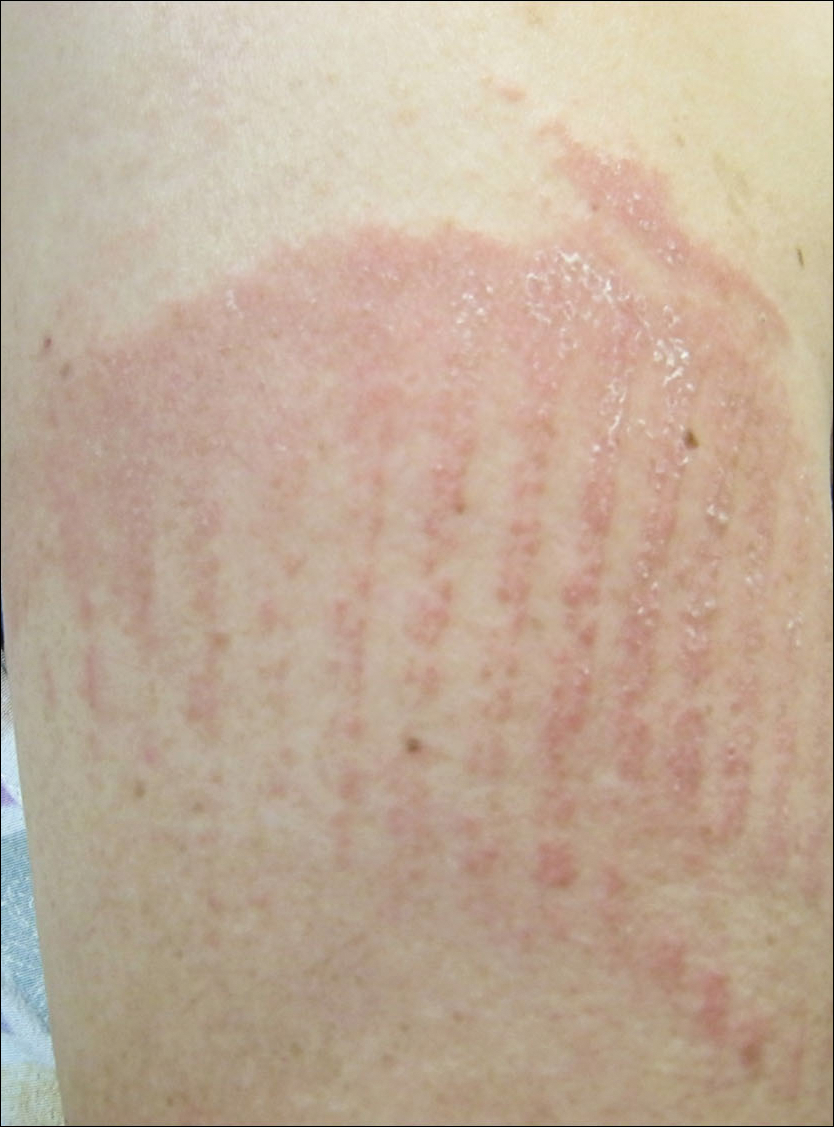
The US Food and Drug Administration (FDA) released statements in 2012 regarding concern for burns related to use of OTC musculoskeletal pain relievers, with 43 cases of burns reported due to methyl salicylate and menthol from 2004 to 2010. Most of the second- and third-degree burns occurred following topical applications of products containing either menthol monotherapy or a combination of methyl salicylate and menthol.1,2 In 2006, the FDA had already ordered 5 firms to stop compounding topical pain relief formulations containing these ingredients, with concerns that it puts patients at increased risk because the compounded formulations had not received FDA approval.3 Despite package warnings, patients may not be aware of the concerning side effects and risks associated with use of OTC creams, especially in combination with occlusion or heating pad use. Our case highlights the importance of ongoing patient education and physician counseling when encountering patients with arthritis or musculoskeletal pain who may often try various OTC self-treatments for pain relief.7
In 2012, the FDA reports stated that the cases of mild to serious burns were associated with methyl salicylate and menthol usage, in some cases 24 hours after first usage. Typically, these effects occur when concentrations are more than either 3% menthol alone or a combination of more than 3% menthol and more than 10% methyl salicylate.1,2 In our case, the patient had been using 2 different OTC products that may have contained as much as 11% menthol and/or 30% methyl salicylate. Electronic resources are available that disclose safety instructions including not to occlude the site, not to use on wounds, and not to be used in conjunction with a heating pad.8,9 Skin breakdown and vasodilation are more likely to occur in a setting of heat and occlusion, which allows for more absorption and localized side effects.4,10 Localized reactions may range from contact dermatitis4 to muscle necrosis.5
The most noteworthy case of localized destruction described a 62-year-old man who had applied topical methyl salicylate and menthol to the forearms, calves, and thighs, then intermittently used a heating pad for 15 to 20 minutes (total duration).5 He subsequently developed erythema and numerous 7.62- to 10.16-cm bullae, which was thought to be consistent with contact dermatitis. Three days later, he was found to have full-thickness cutaneous, fascial, and muscle necrosis in a linear pattern. He was hospitalized for approximately 1 year and treated with extensive debridement and a skin graft. His serum creatinine level increased from 0.7 mg per 100 mL to 2.7 mg per 100 mL (reference range, 0.6–1.2 mg/dL) with evidence of toxic nephrosis and persistent interstitial nephritis, demonstrating the severity of localized destruction that may result when combining these products with direct heat and potential subsequent systemic consequences of this combination.5
The systemic absorption of OTC formulations also has been studied. Morra et al10 studied 12 volunteers (6 women, 6 men) who applied either 5 g of methyl salicylate ointment 12.5% twice daily for 4 days to an area on the thigh (approximately equal to 567 mg salicylate) or trolamine cream 10% twice for 1 day. The participants underwent a break for 7 days and then switched to the alternate treatment. They found that 0.31 to 0.91 mg/L methyl salicylate was detected in the serum 1 hour after applying the ointment consisting of methyl salicylate, and 2 to 6 mg/L methyl salicylate was detected on day 4. Therapeutic serum salicylate levels are 150 to 300 mg/L. They found that approximately 22% of the methyl salicylate also was found in urine samples on day 4. Although these figures may appear small, this study was prompted when a 62-year-old man presented to the emergency department with symptoms of salicylate toxicity and a serum concentration of 518 mg/L from twice-daily use of an OTC formulation containing methyl salicylate over the course of multiple weeks.10 Additionally, those who have aspirin hypersensitivity should be cautious when using such products due to the risk for reported angioedema.4
Providers must exercise extreme caution while caring for geriatric patients, especially if patients are taking warfarin. The combined effects of warfarin and methyl salicylate have previously caused cutaneous purpura, gastrointestinal bleeding, and elevated international normalized ratio values.4,10 Older individuals also have increased skin fragility, allowing microtraumatic insult to easily develop. This fragility, along with an overall decreased intactness of the skin barrier, may lead to increased skin absorption. Furthermore, the addition of applying any heat source places the geriatric patient at greater risk for adverse events.10
In considering the limits of age, the pediatric population also has been studied regarding salicylate toxicity. Most commonly, oral ingestion has caused fatalities, as oil of wintergreen has been cited as extremely dangerous for children if swallowed; doses as small as a teaspoon (5 mL: 7000 mg salicylate) have resulted in fatalities.4,6 Although the consumption of a large amount of a cream- or ointment-based product is unlikely due to the consistency of the medication,6 the thought does merit consideration in the inquisitive toddler age group. For a 15-kg toddler, 150 mg/kg of aspirin or 2250 mg of aspirin, is considered the toxic level, which upon conversion to methyl salicylate levels using a 1.4 factor equates to 1607 mg of methyl salicylate to reach toxicity.6 If using a product with methyl salicylate 30% composition, 1 g of the product contains 300 mg of methyl salicylate; therefore if the toddler consumed approximately 5.3 g of the product (1607 mg methyl salicylate [toxic level] divided by 300 mg methyl salicylate per 1 g of product), he/she would reach toxic levels.6,11 To put this into perspective, a 2-oz tube contains 57 g (approximately 10 times the toxic dose) of the product.8 Thus, although there is less concern overall for consumption of cream- or ointment-based methyl salicylate, there still is potential for harm if a small child were to ingest such a product containing higher percentages of methyl salicylate.6
There also have been reports of pediatric toxicity related to percutaneous absorption, even leading to pediatric fatality.4,6 In particular, there was a case of a young boy hospitalized with ichthyosis who received escalating doses of percutaneous salicylate, which resulted in toxicity; when therapy was discontinued, he experienced full recovery.12 In 2007, a 17-year-old adolescent girl died from methyl salicylate toxicity after numerous applications of salicylate-containing products in conjunction with medicated pads.7
Although the FDA has drawn attention and encouraged caution with use of OTC topical musculoskeletal pain relievers, the importance of ensuring patients are fully aware of potential burns, permanent skin or muscle damage, and even death if used inappropriately cannot be overstated. The FDA consumer health information website has 2 patient-directed handouts2,3 that may be useful to post in patient waiting areas to increase overall understanding of the risks associated with OTC products containing methyl salicylate and menthol ingredients. Fortunately, our patient suffered only mild postinflammatory hyperpigmentation without substantial sustained consequences.
- US Food and Drug Administration. FDA Drug Safety Communication: rare cases of serious burns with the use of over-the-counter topical muscle and joint pain relievers. http://www.fda.gov/Drugs/DrugSafety/ucm318858.htm. Published September 13, 2012. Updated February 11, 2016. Accessed October 31, 2017.
- US Food and Drug Administration. Topical pain relievers may cause burns. http://www.fda.gov/ForConsumers/ConsumerUpdates/ucm318674.htm. Published September 13, 2012. Updated November 5, 2015. Accessed October 31, 2017.
- US Food and Drug Administration. Use caution with over-the-counter creams, ointments. http://www.fda.gov/forconsumers/consumerupdates/ucm049367.htm. Updated October 17, 2017. Accessed October 31, 2017.
- Chan TY. Potential dangers from topical preparations containing methyl salicylate. Hum Exp Toxicol. 1996;15:747-750.
- Heng MC. Local necrosis and interstitial nephritis due to topical methyl salicylate and menthol. Cutis. 1987;39:442-444.
- Davis JE. Are one or two dangerous? methyl salicylate exposure in toddlers. J Emerg Med. 2007;32:63-69.
- Associated Press. Sports cream warnings urged after teen’s death: track star’s overdose points to risks of popular muscle salve. NBC News. http://www.nbcnews.com/id/19208195. Updated June 13, 2007. Accessed October 31, 2017.
- Ultra Strength Bengay Cream. Bengay website. http://www.bengay.com/bengay-ultra-strength-cream. Accessed November 1, 2017.
- Tiger Balm Arthritis Rub. Tiger Balm website. http://www.tigerbalm.com/us/pages/tb_product?product_id=6. Accessed November 1, 2017.
- Morra P, Bartle WR, Walker SE, et al. Serum concentrations of salicylic acid following topically applied salicylate derivatives. Ann Pharmacother. 1996;9:935-940.
- US National Library of Medicine. Bengay Ultra Strength non greasy pain relieving- camphor (synthetic), menthol, and methyl salicylate cream. Daily Med website. http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=5aa265f8-ab45-47b2-b5ab-d4df54daed01. Updated November 3, 2016. Accessed November 1, 2017.
- Aspinall JB, Goel KM. Salicylate poisoning in dermatological therapy. Br Med J. 1978;2:1373.
To the Editor:
The combination of menthol and methyl salicylate found in a variety of over-the-counter (OTC) creams in conjunction with a heat source such as a heating pad used for musculoskeletal symptoms can be a dire combination due to increased systemic absorption with associated toxicity and localized effects ranging from contact dermatitis or irritation to burn or necrosis.1-6 We present a case of localized burn due a combination of topical methyl salicylate and heating pad use. We also discuss 2 commonly encountered side effects in the literature—localized burns and systemic toxicity associated with percutaneous absorption—and provide specific considerations related to the geriatric and pediatric populations.
A 62-year-old woman with a history of eczematous dermatitis and osteoarthritis with pain of the left shoulder presented to the dermatology clinic with painful skin-related changes on the left arm of 1 week’s duration. She was prescribed acetaminophen and ibuprofen. However, she self-medicated the left shoulder pain with 2 OTC products containing topical menthol and/or methyl salicylate in combination with a heating pad and likely fell asleep with this combination therapy applied. She noticed the burn the next morning. On examination, the left arm exhibited a geometric, irregularly shaped, erythematous, scaly plaque with a sharp transverse linear demarcation proximally and numerous erythematous linear scaly plaques oriented in an axial orientation with less-defined borders distally (Figure). The patient was diagnosed with burn secondary to combination of topical methyl salicylate and heating pad use. The patient was advised to discontinue the topical medication and to use caution with the heating pad in the future. She was prescribed pramoxine-hydrocortisone lotion to be applied to the affected area twice daily up to 5 days weekly until resolution. Subsequent evaluations revealed progressive improvement with only mild postinflammatory hyperpigmentation noted at 6 months after the burn.

The US Food and Drug Administration (FDA) released statements in 2012 regarding concern for burns related to use of OTC musculoskeletal pain relievers, with 43 cases of burns reported due to methyl salicylate and menthol from 2004 to 2010. Most of the second- and third-degree burns occurred following topical applications of products containing either menthol monotherapy or a combination of methyl salicylate and menthol.1,2 In 2006, the FDA had already ordered 5 firms to stop compounding topical pain relief formulations containing these ingredients, with concerns that it puts patients at increased risk because the compounded formulations had not received FDA approval.3 Despite package warnings, patients may not be aware of the concerning side effects and risks associated with use of OTC creams, especially in combination with occlusion or heating pad use. Our case highlights the importance of ongoing patient education and physician counseling when encountering patients with arthritis or musculoskeletal pain who may often try various OTC self-treatments for pain relief.7
In 2012, the FDA reports stated that the cases of mild to serious burns were associated with methyl salicylate and menthol usage, in some cases 24 hours after first usage. Typically, these effects occur when concentrations are more than either 3% menthol alone or a combination of more than 3% menthol and more than 10% methyl salicylate.1,2 In our case, the patient had been using 2 different OTC products that may have contained as much as 11% menthol and/or 30% methyl salicylate. Electronic resources are available that disclose safety instructions including not to occlude the site, not to use on wounds, and not to be used in conjunction with a heating pad.8,9 Skin breakdown and vasodilation are more likely to occur in a setting of heat and occlusion, which allows for more absorption and localized side effects.4,10 Localized reactions may range from contact dermatitis4 to muscle necrosis.5
The most noteworthy case of localized destruction described a 62-year-old man who had applied topical methyl salicylate and menthol to the forearms, calves, and thighs, then intermittently used a heating pad for 15 to 20 minutes (total duration).5 He subsequently developed erythema and numerous 7.62- to 10.16-cm bullae, which was thought to be consistent with contact dermatitis. Three days later, he was found to have full-thickness cutaneous, fascial, and muscle necrosis in a linear pattern. He was hospitalized for approximately 1 year and treated with extensive debridement and a skin graft. His serum creatinine level increased from 0.7 mg per 100 mL to 2.7 mg per 100 mL (reference range, 0.6–1.2 mg/dL) with evidence of toxic nephrosis and persistent interstitial nephritis, demonstrating the severity of localized destruction that may result when combining these products with direct heat and potential subsequent systemic consequences of this combination.5
The systemic absorption of OTC formulations also has been studied. Morra et al10 studied 12 volunteers (6 women, 6 men) who applied either 5 g of methyl salicylate ointment 12.5% twice daily for 4 days to an area on the thigh (approximately equal to 567 mg salicylate) or trolamine cream 10% twice for 1 day. The participants underwent a break for 7 days and then switched to the alternate treatment. They found that 0.31 to 0.91 mg/L methyl salicylate was detected in the serum 1 hour after applying the ointment consisting of methyl salicylate, and 2 to 6 mg/L methyl salicylate was detected on day 4. Therapeutic serum salicylate levels are 150 to 300 mg/L. They found that approximately 22% of the methyl salicylate also was found in urine samples on day 4. Although these figures may appear small, this study was prompted when a 62-year-old man presented to the emergency department with symptoms of salicylate toxicity and a serum concentration of 518 mg/L from twice-daily use of an OTC formulation containing methyl salicylate over the course of multiple weeks.10 Additionally, those who have aspirin hypersensitivity should be cautious when using such products due to the risk for reported angioedema.4
Providers must exercise extreme caution while caring for geriatric patients, especially if patients are taking warfarin. The combined effects of warfarin and methyl salicylate have previously caused cutaneous purpura, gastrointestinal bleeding, and elevated international normalized ratio values.4,10 Older individuals also have increased skin fragility, allowing microtraumatic insult to easily develop. This fragility, along with an overall decreased intactness of the skin barrier, may lead to increased skin absorption. Furthermore, the addition of applying any heat source places the geriatric patient at greater risk for adverse events.10
In considering the limits of age, the pediatric population also has been studied regarding salicylate toxicity. Most commonly, oral ingestion has caused fatalities, as oil of wintergreen has been cited as extremely dangerous for children if swallowed; doses as small as a teaspoon (5 mL: 7000 mg salicylate) have resulted in fatalities.4,6 Although the consumption of a large amount of a cream- or ointment-based product is unlikely due to the consistency of the medication,6 the thought does merit consideration in the inquisitive toddler age group. For a 15-kg toddler, 150 mg/kg of aspirin or 2250 mg of aspirin, is considered the toxic level, which upon conversion to methyl salicylate levels using a 1.4 factor equates to 1607 mg of methyl salicylate to reach toxicity.6 If using a product with methyl salicylate 30% composition, 1 g of the product contains 300 mg of methyl salicylate; therefore if the toddler consumed approximately 5.3 g of the product (1607 mg methyl salicylate [toxic level] divided by 300 mg methyl salicylate per 1 g of product), he/she would reach toxic levels.6,11 To put this into perspective, a 2-oz tube contains 57 g (approximately 10 times the toxic dose) of the product.8 Thus, although there is less concern overall for consumption of cream- or ointment-based methyl salicylate, there still is potential for harm if a small child were to ingest such a product containing higher percentages of methyl salicylate.6
There also have been reports of pediatric toxicity related to percutaneous absorption, even leading to pediatric fatality.4,6 In particular, there was a case of a young boy hospitalized with ichthyosis who received escalating doses of percutaneous salicylate, which resulted in toxicity; when therapy was discontinued, he experienced full recovery.12 In 2007, a 17-year-old adolescent girl died from methyl salicylate toxicity after numerous applications of salicylate-containing products in conjunction with medicated pads.7
Although the FDA has drawn attention and encouraged caution with use of OTC topical musculoskeletal pain relievers, the importance of ensuring patients are fully aware of potential burns, permanent skin or muscle damage, and even death if used inappropriately cannot be overstated. The FDA consumer health information website has 2 patient-directed handouts2,3 that may be useful to post in patient waiting areas to increase overall understanding of the risks associated with OTC products containing methyl salicylate and menthol ingredients. Fortunately, our patient suffered only mild postinflammatory hyperpigmentation without substantial sustained consequences.
To the Editor:
The combination of menthol and methyl salicylate found in a variety of over-the-counter (OTC) creams in conjunction with a heat source such as a heating pad used for musculoskeletal symptoms can be a dire combination due to increased systemic absorption with associated toxicity and localized effects ranging from contact dermatitis or irritation to burn or necrosis.1-6 We present a case of localized burn due a combination of topical methyl salicylate and heating pad use. We also discuss 2 commonly encountered side effects in the literature—localized burns and systemic toxicity associated with percutaneous absorption—and provide specific considerations related to the geriatric and pediatric populations.
A 62-year-old woman with a history of eczematous dermatitis and osteoarthritis with pain of the left shoulder presented to the dermatology clinic with painful skin-related changes on the left arm of 1 week’s duration. She was prescribed acetaminophen and ibuprofen. However, she self-medicated the left shoulder pain with 2 OTC products containing topical menthol and/or methyl salicylate in combination with a heating pad and likely fell asleep with this combination therapy applied. She noticed the burn the next morning. On examination, the left arm exhibited a geometric, irregularly shaped, erythematous, scaly plaque with a sharp transverse linear demarcation proximally and numerous erythematous linear scaly plaques oriented in an axial orientation with less-defined borders distally (Figure). The patient was diagnosed with burn secondary to combination of topical methyl salicylate and heating pad use. The patient was advised to discontinue the topical medication and to use caution with the heating pad in the future. She was prescribed pramoxine-hydrocortisone lotion to be applied to the affected area twice daily up to 5 days weekly until resolution. Subsequent evaluations revealed progressive improvement with only mild postinflammatory hyperpigmentation noted at 6 months after the burn.

The US Food and Drug Administration (FDA) released statements in 2012 regarding concern for burns related to use of OTC musculoskeletal pain relievers, with 43 cases of burns reported due to methyl salicylate and menthol from 2004 to 2010. Most of the second- and third-degree burns occurred following topical applications of products containing either menthol monotherapy or a combination of methyl salicylate and menthol.1,2 In 2006, the FDA had already ordered 5 firms to stop compounding topical pain relief formulations containing these ingredients, with concerns that it puts patients at increased risk because the compounded formulations had not received FDA approval.3 Despite package warnings, patients may not be aware of the concerning side effects and risks associated with use of OTC creams, especially in combination with occlusion or heating pad use. Our case highlights the importance of ongoing patient education and physician counseling when encountering patients with arthritis or musculoskeletal pain who may often try various OTC self-treatments for pain relief.7
In 2012, the FDA reports stated that the cases of mild to serious burns were associated with methyl salicylate and menthol usage, in some cases 24 hours after first usage. Typically, these effects occur when concentrations are more than either 3% menthol alone or a combination of more than 3% menthol and more than 10% methyl salicylate.1,2 In our case, the patient had been using 2 different OTC products that may have contained as much as 11% menthol and/or 30% methyl salicylate. Electronic resources are available that disclose safety instructions including not to occlude the site, not to use on wounds, and not to be used in conjunction with a heating pad.8,9 Skin breakdown and vasodilation are more likely to occur in a setting of heat and occlusion, which allows for more absorption and localized side effects.4,10 Localized reactions may range from contact dermatitis4 to muscle necrosis.5
The most noteworthy case of localized destruction described a 62-year-old man who had applied topical methyl salicylate and menthol to the forearms, calves, and thighs, then intermittently used a heating pad for 15 to 20 minutes (total duration).5 He subsequently developed erythema and numerous 7.62- to 10.16-cm bullae, which was thought to be consistent with contact dermatitis. Three days later, he was found to have full-thickness cutaneous, fascial, and muscle necrosis in a linear pattern. He was hospitalized for approximately 1 year and treated with extensive debridement and a skin graft. His serum creatinine level increased from 0.7 mg per 100 mL to 2.7 mg per 100 mL (reference range, 0.6–1.2 mg/dL) with evidence of toxic nephrosis and persistent interstitial nephritis, demonstrating the severity of localized destruction that may result when combining these products with direct heat and potential subsequent systemic consequences of this combination.5
The systemic absorption of OTC formulations also has been studied. Morra et al10 studied 12 volunteers (6 women, 6 men) who applied either 5 g of methyl salicylate ointment 12.5% twice daily for 4 days to an area on the thigh (approximately equal to 567 mg salicylate) or trolamine cream 10% twice for 1 day. The participants underwent a break for 7 days and then switched to the alternate treatment. They found that 0.31 to 0.91 mg/L methyl salicylate was detected in the serum 1 hour after applying the ointment consisting of methyl salicylate, and 2 to 6 mg/L methyl salicylate was detected on day 4. Therapeutic serum salicylate levels are 150 to 300 mg/L. They found that approximately 22% of the methyl salicylate also was found in urine samples on day 4. Although these figures may appear small, this study was prompted when a 62-year-old man presented to the emergency department with symptoms of salicylate toxicity and a serum concentration of 518 mg/L from twice-daily use of an OTC formulation containing methyl salicylate over the course of multiple weeks.10 Additionally, those who have aspirin hypersensitivity should be cautious when using such products due to the risk for reported angioedema.4
Providers must exercise extreme caution while caring for geriatric patients, especially if patients are taking warfarin. The combined effects of warfarin and methyl salicylate have previously caused cutaneous purpura, gastrointestinal bleeding, and elevated international normalized ratio values.4,10 Older individuals also have increased skin fragility, allowing microtraumatic insult to easily develop. This fragility, along with an overall decreased intactness of the skin barrier, may lead to increased skin absorption. Furthermore, the addition of applying any heat source places the geriatric patient at greater risk for adverse events.10
In considering the limits of age, the pediatric population also has been studied regarding salicylate toxicity. Most commonly, oral ingestion has caused fatalities, as oil of wintergreen has been cited as extremely dangerous for children if swallowed; doses as small as a teaspoon (5 mL: 7000 mg salicylate) have resulted in fatalities.4,6 Although the consumption of a large amount of a cream- or ointment-based product is unlikely due to the consistency of the medication,6 the thought does merit consideration in the inquisitive toddler age group. For a 15-kg toddler, 150 mg/kg of aspirin or 2250 mg of aspirin, is considered the toxic level, which upon conversion to methyl salicylate levels using a 1.4 factor equates to 1607 mg of methyl salicylate to reach toxicity.6 If using a product with methyl salicylate 30% composition, 1 g of the product contains 300 mg of methyl salicylate; therefore if the toddler consumed approximately 5.3 g of the product (1607 mg methyl salicylate [toxic level] divided by 300 mg methyl salicylate per 1 g of product), he/she would reach toxic levels.6,11 To put this into perspective, a 2-oz tube contains 57 g (approximately 10 times the toxic dose) of the product.8 Thus, although there is less concern overall for consumption of cream- or ointment-based methyl salicylate, there still is potential for harm if a small child were to ingest such a product containing higher percentages of methyl salicylate.6
There also have been reports of pediatric toxicity related to percutaneous absorption, even leading to pediatric fatality.4,6 In particular, there was a case of a young boy hospitalized with ichthyosis who received escalating doses of percutaneous salicylate, which resulted in toxicity; when therapy was discontinued, he experienced full recovery.12 In 2007, a 17-year-old adolescent girl died from methyl salicylate toxicity after numerous applications of salicylate-containing products in conjunction with medicated pads.7
Although the FDA has drawn attention and encouraged caution with use of OTC topical musculoskeletal pain relievers, the importance of ensuring patients are fully aware of potential burns, permanent skin or muscle damage, and even death if used inappropriately cannot be overstated. The FDA consumer health information website has 2 patient-directed handouts2,3 that may be useful to post in patient waiting areas to increase overall understanding of the risks associated with OTC products containing methyl salicylate and menthol ingredients. Fortunately, our patient suffered only mild postinflammatory hyperpigmentation without substantial sustained consequences.
- US Food and Drug Administration. FDA Drug Safety Communication: rare cases of serious burns with the use of over-the-counter topical muscle and joint pain relievers. http://www.fda.gov/Drugs/DrugSafety/ucm318858.htm. Published September 13, 2012. Updated February 11, 2016. Accessed October 31, 2017.
- US Food and Drug Administration. Topical pain relievers may cause burns. http://www.fda.gov/ForConsumers/ConsumerUpdates/ucm318674.htm. Published September 13, 2012. Updated November 5, 2015. Accessed October 31, 2017.
- US Food and Drug Administration. Use caution with over-the-counter creams, ointments. http://www.fda.gov/forconsumers/consumerupdates/ucm049367.htm. Updated October 17, 2017. Accessed October 31, 2017.
- Chan TY. Potential dangers from topical preparations containing methyl salicylate. Hum Exp Toxicol. 1996;15:747-750.
- Heng MC. Local necrosis and interstitial nephritis due to topical methyl salicylate and menthol. Cutis. 1987;39:442-444.
- Davis JE. Are one or two dangerous? methyl salicylate exposure in toddlers. J Emerg Med. 2007;32:63-69.
- Associated Press. Sports cream warnings urged after teen’s death: track star’s overdose points to risks of popular muscle salve. NBC News. http://www.nbcnews.com/id/19208195. Updated June 13, 2007. Accessed October 31, 2017.
- Ultra Strength Bengay Cream. Bengay website. http://www.bengay.com/bengay-ultra-strength-cream. Accessed November 1, 2017.
- Tiger Balm Arthritis Rub. Tiger Balm website. http://www.tigerbalm.com/us/pages/tb_product?product_id=6. Accessed November 1, 2017.
- Morra P, Bartle WR, Walker SE, et al. Serum concentrations of salicylic acid following topically applied salicylate derivatives. Ann Pharmacother. 1996;9:935-940.
- US National Library of Medicine. Bengay Ultra Strength non greasy pain relieving- camphor (synthetic), menthol, and methyl salicylate cream. Daily Med website. http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=5aa265f8-ab45-47b2-b5ab-d4df54daed01. Updated November 3, 2016. Accessed November 1, 2017.
- Aspinall JB, Goel KM. Salicylate poisoning in dermatological therapy. Br Med J. 1978;2:1373.
- US Food and Drug Administration. FDA Drug Safety Communication: rare cases of serious burns with the use of over-the-counter topical muscle and joint pain relievers. http://www.fda.gov/Drugs/DrugSafety/ucm318858.htm. Published September 13, 2012. Updated February 11, 2016. Accessed October 31, 2017.
- US Food and Drug Administration. Topical pain relievers may cause burns. http://www.fda.gov/ForConsumers/ConsumerUpdates/ucm318674.htm. Published September 13, 2012. Updated November 5, 2015. Accessed October 31, 2017.
- US Food and Drug Administration. Use caution with over-the-counter creams, ointments. http://www.fda.gov/forconsumers/consumerupdates/ucm049367.htm. Updated October 17, 2017. Accessed October 31, 2017.
- Chan TY. Potential dangers from topical preparations containing methyl salicylate. Hum Exp Toxicol. 1996;15:747-750.
- Heng MC. Local necrosis and interstitial nephritis due to topical methyl salicylate and menthol. Cutis. 1987;39:442-444.
- Davis JE. Are one or two dangerous? methyl salicylate exposure in toddlers. J Emerg Med. 2007;32:63-69.
- Associated Press. Sports cream warnings urged after teen’s death: track star’s overdose points to risks of popular muscle salve. NBC News. http://www.nbcnews.com/id/19208195. Updated June 13, 2007. Accessed October 31, 2017.
- Ultra Strength Bengay Cream. Bengay website. http://www.bengay.com/bengay-ultra-strength-cream. Accessed November 1, 2017.
- Tiger Balm Arthritis Rub. Tiger Balm website. http://www.tigerbalm.com/us/pages/tb_product?product_id=6. Accessed November 1, 2017.
- Morra P, Bartle WR, Walker SE, et al. Serum concentrations of salicylic acid following topically applied salicylate derivatives. Ann Pharmacother. 1996;9:935-940.
- US National Library of Medicine. Bengay Ultra Strength non greasy pain relieving- camphor (synthetic), menthol, and methyl salicylate cream. Daily Med website. http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=5aa265f8-ab45-47b2-b5ab-d4df54daed01. Updated November 3, 2016. Accessed November 1, 2017.
- Aspinall JB, Goel KM. Salicylate poisoning in dermatological therapy. Br Med J. 1978;2:1373.
Practice Points
- Recognize the potential complication of burn from use of over-the-counter (OTC) musculoskeletal relievers in combination with a heat source.
- Screen for OTC product use as well as device application when evaluating an atypically patterned cutaneous eruption.
- Recognize potential toxicity associated with both topical application and accidental ingestion in the pediatric population.
- Physicians should become familiar with resources available, including patient handouts that describe risks associated with use of OTC musculoskeletal relievers containing methyl salicylate and menthol ingredients.
Cellular Versus Acellular Grafts for Diabetic Foot Ulcers: Altering the Protocol to Improve Recruitment to a Comparative Efficacy Trial
Chronic diabetic foot ulcers (DFUs) remain a serious therapeutic challenge worldwide.1-2 Patients with DFUs are at higher risk for infections, which may lead to limb loss.1-5 In fact, 1 in 6 patients with DFUs will undergo an amputation.6 The long-term consequences of DFUs are numerous and can severely affect patients’ quality of life, including loss of productivity.7 The current standard of care for DFUs consists of debridement of the necrotic tissue, application of a moist dressing, and use of an off-loading device that protects the wound from pressure or trauma related to ambulation and other acts of daily living.4-6,8 Unfortunately, studies have shown that the best standard of care (SOC) only heals 30% of DFUs after 20 weeks of therapy.9 With the estimated cost per episode of care approaching $40,000, DFUs remain a costly and important problem.10
The altered extracellular matrix (ECM) in DFUs has been a target for the development of new therapeutic devices that provide a new matrix that is either devoid of cells or can be enriched with fibroblasts.8,11 These bioengineered skin substitutes stimulate the growth of new vessels and generate cytokines essential for tissue repair. In 2013, Lev-Tov et al12 published this study protocol (Dermagraft Oasis Longitudinal Comparative Efficacy [DOLCE] trial) to compare the effectiveness of 2 advanced wound care devices, specifically to evaluate the clinical efficacy of a cellular matrix versus an acellular matrix, which we have amended. The cellular matrix used in the study is a dermal substitute composed of viable newborn foreskin fibroblasts seeded onto a bioabsorbable polyglactin mesh on which fibroblasts generate an ECM.13,14 It is supplied frozen and requires specific thawing steps prior to application. The recommended regimen for treatment of DFUs for this cellular matrix is 8 weekly applications.13,14 In 2016, the cost of the product was reported as $1411 per 5.0×7.5-cm sheet.15 The acellular matrix product used in the study is a bioabsorbable ECM that is derived from porcine small intestinal submucosa.16,17 It is stored at room temperature and has a long shelf life, with a current price of $112.6 for a 3.0×3.5-cm single-layer fenestrated sheet ($1126.60 per box of 10 sheets). The industry-supported randomized controlled trials for each of these devices have reported a 20% added benefit in the rate of wound closure at week 12 compared to SOC.14,17
This article provides the interim report of the trial (registered at www.clinicaltrials.gov with the identifier NCT01450943) described in the published protocol and initiated in 2011,12 focusing on elements that required modification during the trial’s duration.
Methods
Study Protocol
The clinical trial was approved by the Veterans’ Affairs Institutional Research and Development Committee and their institutional review board. This study was funded by the Veteran’s Administration Merit Award (#10554640), which was awarded to 2 of the investigators (S.E.D. and R.R.I.). Eligible veterans were recruited from all 7 sites of the VA Northern California Healthcare System. This trial is a randomized, single-blinded, 3-armed, controlled clinical equivalence trial comparing the effectiveness of an SOC treatment, cellular ECM, and acellular ECM.
Study Products
The SOC dressing applied in the clinical trial included a sterile antimicrobial gel, a nonadherent dressing, and gauze.12 The SOC dressing also was used as a secondary dressing for the active treatment arms. Bacitracin antibiotic ointment was used as an alternative for patients with allergy to iodine.12
Randomization
The inclusion and exclusion criteria were previously outlined.12 After a 2-week screening phase to exclude rapid healers, patients were randomized into a treatment arm and entered the active phase for 12 weeks.
Primary and Secondary Outcomes
The primary outcome was complete wound closure by week 12.12 Complete healing was defined as full reepithelialization with no drainage or dressing requirement. The secondary outcomes included healing at 28 weeks, rate of healing, ulcer recurrence at week 20, association of wound healing with ulcer characteristics or patients’ characteristic, incidence of adverse events, and cost-effectiveness of each treatment compared to the SOC arm.12
Statistical Analysis
To detect a 25% difference in the incidence of ulcer closure between the 2 study groups and the SOC group, the estimation of the sample size was based on 80% power with a significance level of 0.05. Specifically, it was expected that 50% of the cellular and acellular matrix groups and 25% of the SOC group would reach complete wound closure. The protocol indicated that 57 participants would be enrolled in each arm (total of 171 participants). Lev-Tov et al12 discussed the statistical analysis in more detail.
Results
Study Protocol Amendments
Given the number of diabetic patients in the US veteran population, we anticipated that there would be enough participants meeting the inclusion and exclusion criteria; however, because of the difficulty with recruitment, the initial study criteria were modified. The study was initially designed to incorporate DFUs with a minimum size of 1.0 cm2.12
Another limiting criterion was the percentage of total hemoglobin level for hemoglobin A1C (HbA1C). The study was originally established to include participants with an HbA1C level of 10% of total hemoglobin or below.12 Unfortunately, the majority of the potential participants had values substantially higher, and thus could not be enrolled in the trial, requiring another amendment to the study protocol in 2014, which was approved to include patients with an HbA1C level less than 12% of total hemoglobin. This change contributed considerably to the noted increase in enrollment rates in 2015, which almost doubled relative to enrollment under the original exclusion criteria (Figure).
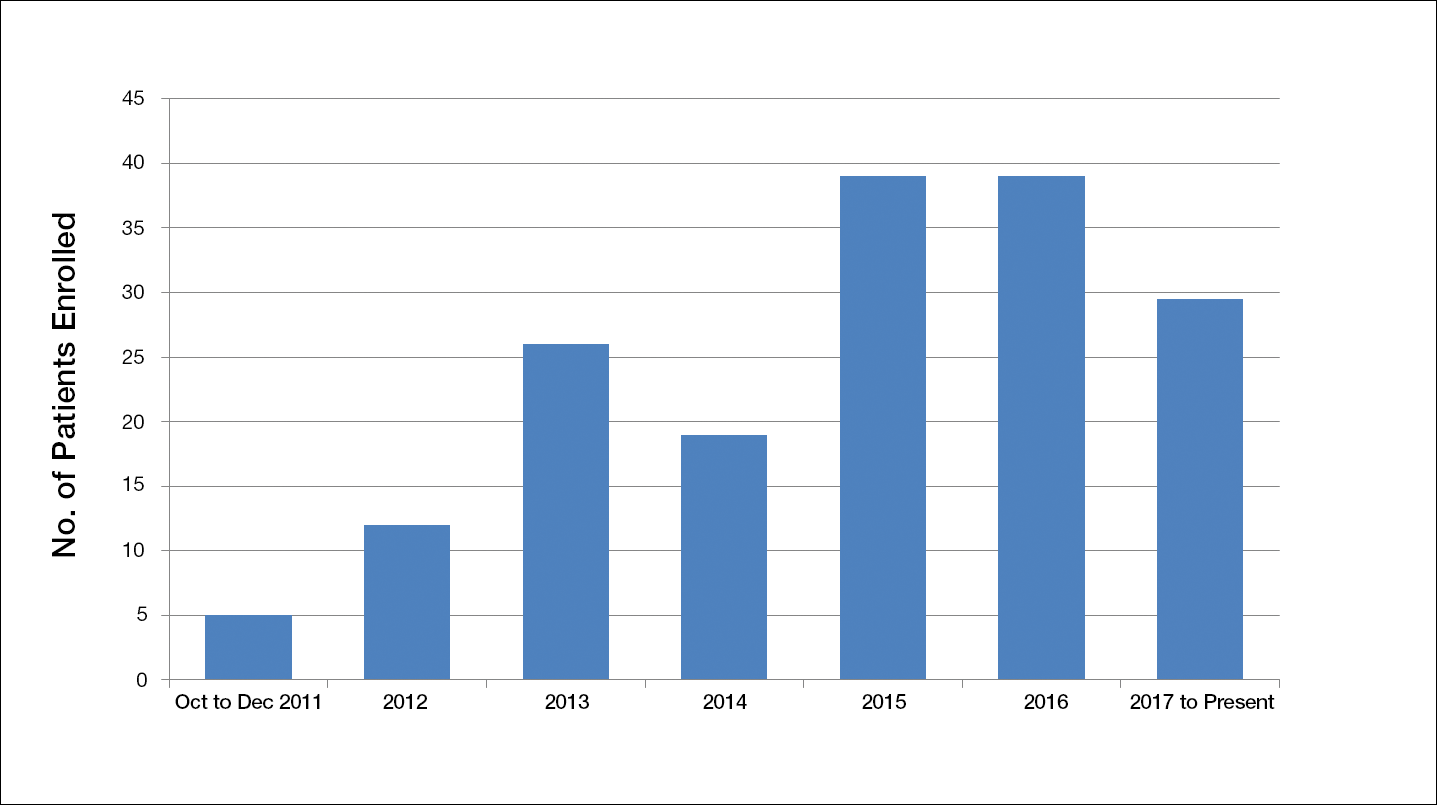
The study has screened more than 600 patients. Among them, 137 were assessed for eligibility; 71 were excluded for various reasons, including screen failure (eg, decrease in wound size by >40% during the 2-week screening phase), loss to follow-up, and adverse events. Sixty-six participants reached the primary outcome at week 12, while 55 participants completed the study (19 in the SOC group; 18 in the cellular matrix group; 18 in the acellular matrix group).
We have stopped enrolling patients from all sites and the community, as we have reached our target enrollment.
Comment
One of the challenges of clinical trials is the recruitment of an adequate number of participants within an appropriate time frame, which is explained by Lasagna’s Law,18 a well-described phenomenon whereby the investigator overestimates the number of potential participants available to meet the inclusion criteria. This so-called funnel-effect was partly encountered in our selection process. A review of the veteran population with DFUs seemed to be more than adequate to fulfill the sample size; however, some important participant-related factors also played a substantial role.
In addition, the Veterans’ Affairs network centralizes health information, making it readily available to all providers participating in their care. As a result, patients with diabetes mellitus typically are seen by a primary care physician along with an endocrinologist, a diabetic nurse, and/or a dietician. Despite the collaboration with an interdisciplinary team, the glycemic control of the participants remains an issue along with other psychosocial factors that are deterrents in patient compliance. As a result, patients with poorly controlled diabetes and an HbA1C level above 10% (and less than 12%) of total hemoglobin who were initially excluded from the study were reincluded after modifying the inclusion criteria. Some patients were interested in joining the study, but physical limitations (eg, impaired mobility) prompted their decision not to join the trial, even though they met all the inclusion criteria.
As far as research-related factors that could affect participation, it is notable that most of the patients were retired; thus, the interventions did not cause additional burden of taking time off from work or loss of productivity. Although randomization could be a deterrent in many clinical trials, the majority of patients were willing to participate without demanding to be assigned to a particular treatment group.
There are many factors that are intertwined and can lead to enrollment and/or attrition rates. It was critical for our team to make some adjustment without compromising the controlled nature of a randomized trial.
Acknowledgment
The authors wish to acknowledge Huong Le, DPM, MPH, who was the coauthor of the study protocol.
- Sen CK, Gordillo GM, Roy S, et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen. 2009;17:763-771.
- Gurtner GC, Werner S, Barrandon Y, et al. Wound repair and regeneration. Nature. 2008;453:314-321.
- Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366:1736-1743.
- Boulton AJ. The diabetic foot: grand overview, epidemiology and pathogenesis. Diabetes Metab Res Rev. 2008;24(suppl 1):S3-S6.
- Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA. 2005;293:217-228.
- Vuorisalo S, Venermo M, Lepäntalo M. Treatment of diabetic foot ulcers. J Cardiovasc Surg (Torino). 2009;50:275-291.
- Meijer JW, Trip J, Jaegers SM, et al. Quality of life in patients with diabetic foot ulcers. Disabil Rehabil. 2001;23:336-340.
- Santema TB, Poyck PP, Ubbink DT. Skin grafting and tissue replacement for treating foot ulcers in people with diabetes. Cochrane Database Syst Rev. 2016;2:CD011255.
- Margolis DJ, Kantor J, Berlin JA. Healing of diabetic neuropathic foot ulcers receiving standard treatment. a meta-analysis. Diabetes Care. 1999;22:692-695.
- Cavanagh P, Attinger C, Abbas Z, et al. Cost of treating diabetic foot ulcers in five different countries. Diabetes Metab Res Rev. 2012;2(suppl 1):107-111.
- Panuncialman J, Falanga V. The science of wound bed preparation. Surg Clin North Am. 2009;89:611-626.
- Lev-Tov H, Li CS, Dahle S, et al. Cellular versus acellular matrix devices in treatment of diabetic foot ulcers: study protocol for a comparative efficacy randomized controlled trial. Trials. 2013;14:8.
- Gentzkow GD, Iwasaki SD, Hershon KS, et al. Use of dermagraft, a cultured human dermis, to treat diabetic foot ulcers. Diabetes Care. 1996;19:350-354.
- Marston WA, Hanft J, Norwood P, et al; Dermagraft Diabetic Foot Ulcer Study Group. The efficacy and safety of Dermagraft in improving the healing of chronic diabetic foot ulcers: results of a prospective randomized trial. Diabetes Care. 2003;26:1701-1705.
- 2016 Dermagraft® Medicare Product and Related Procedure Payment. http://www.dermagraft.com/wp-content/uploads/sites/1/Dermagraft_Hotsheet%202016%20Q1%20HOSPITAL_FINAL.pdf. Accessed November 23, 2017.
- Oasis® Wound Matrix. http://www.oasiswoundmatrix.com/aboutowm. Accessed November 23, 2017.
- Niezgoda JA, Van Gils CC, Frykberg RG, et al. Randomized clinical trial comparing OASIS Wound Matrix to Regranex Gel for diabetic ulcers. Adv Skin Wound Care. 2005;18(5, pt 1):258-266.
- Torgerson JS, Arlinger K, Käppi M, et al. Principles for enhanced recruitment of subjects in a large clinical trial. the XENDOS (XENical in the prevention of Diabetes in Obese Subjects) study experience. Controlled Clin Trials. 2001;22:515-525.
Chronic diabetic foot ulcers (DFUs) remain a serious therapeutic challenge worldwide.1-2 Patients with DFUs are at higher risk for infections, which may lead to limb loss.1-5 In fact, 1 in 6 patients with DFUs will undergo an amputation.6 The long-term consequences of DFUs are numerous and can severely affect patients’ quality of life, including loss of productivity.7 The current standard of care for DFUs consists of debridement of the necrotic tissue, application of a moist dressing, and use of an off-loading device that protects the wound from pressure or trauma related to ambulation and other acts of daily living.4-6,8 Unfortunately, studies have shown that the best standard of care (SOC) only heals 30% of DFUs after 20 weeks of therapy.9 With the estimated cost per episode of care approaching $40,000, DFUs remain a costly and important problem.10
The altered extracellular matrix (ECM) in DFUs has been a target for the development of new therapeutic devices that provide a new matrix that is either devoid of cells or can be enriched with fibroblasts.8,11 These bioengineered skin substitutes stimulate the growth of new vessels and generate cytokines essential for tissue repair. In 2013, Lev-Tov et al12 published this study protocol (Dermagraft Oasis Longitudinal Comparative Efficacy [DOLCE] trial) to compare the effectiveness of 2 advanced wound care devices, specifically to evaluate the clinical efficacy of a cellular matrix versus an acellular matrix, which we have amended. The cellular matrix used in the study is a dermal substitute composed of viable newborn foreskin fibroblasts seeded onto a bioabsorbable polyglactin mesh on which fibroblasts generate an ECM.13,14 It is supplied frozen and requires specific thawing steps prior to application. The recommended regimen for treatment of DFUs for this cellular matrix is 8 weekly applications.13,14 In 2016, the cost of the product was reported as $1411 per 5.0×7.5-cm sheet.15 The acellular matrix product used in the study is a bioabsorbable ECM that is derived from porcine small intestinal submucosa.16,17 It is stored at room temperature and has a long shelf life, with a current price of $112.6 for a 3.0×3.5-cm single-layer fenestrated sheet ($1126.60 per box of 10 sheets). The industry-supported randomized controlled trials for each of these devices have reported a 20% added benefit in the rate of wound closure at week 12 compared to SOC.14,17
This article provides the interim report of the trial (registered at www.clinicaltrials.gov with the identifier NCT01450943) described in the published protocol and initiated in 2011,12 focusing on elements that required modification during the trial’s duration.
Methods
Study Protocol
The clinical trial was approved by the Veterans’ Affairs Institutional Research and Development Committee and their institutional review board. This study was funded by the Veteran’s Administration Merit Award (#10554640), which was awarded to 2 of the investigators (S.E.D. and R.R.I.). Eligible veterans were recruited from all 7 sites of the VA Northern California Healthcare System. This trial is a randomized, single-blinded, 3-armed, controlled clinical equivalence trial comparing the effectiveness of an SOC treatment, cellular ECM, and acellular ECM.
Study Products
The SOC dressing applied in the clinical trial included a sterile antimicrobial gel, a nonadherent dressing, and gauze.12 The SOC dressing also was used as a secondary dressing for the active treatment arms. Bacitracin antibiotic ointment was used as an alternative for patients with allergy to iodine.12
Randomization
The inclusion and exclusion criteria were previously outlined.12 After a 2-week screening phase to exclude rapid healers, patients were randomized into a treatment arm and entered the active phase for 12 weeks.
Primary and Secondary Outcomes
The primary outcome was complete wound closure by week 12.12 Complete healing was defined as full reepithelialization with no drainage or dressing requirement. The secondary outcomes included healing at 28 weeks, rate of healing, ulcer recurrence at week 20, association of wound healing with ulcer characteristics or patients’ characteristic, incidence of adverse events, and cost-effectiveness of each treatment compared to the SOC arm.12
Statistical Analysis
To detect a 25% difference in the incidence of ulcer closure between the 2 study groups and the SOC group, the estimation of the sample size was based on 80% power with a significance level of 0.05. Specifically, it was expected that 50% of the cellular and acellular matrix groups and 25% of the SOC group would reach complete wound closure. The protocol indicated that 57 participants would be enrolled in each arm (total of 171 participants). Lev-Tov et al12 discussed the statistical analysis in more detail.
Results
Study Protocol Amendments
Given the number of diabetic patients in the US veteran population, we anticipated that there would be enough participants meeting the inclusion and exclusion criteria; however, because of the difficulty with recruitment, the initial study criteria were modified. The study was initially designed to incorporate DFUs with a minimum size of 1.0 cm2.12
Another limiting criterion was the percentage of total hemoglobin level for hemoglobin A1C (HbA1C). The study was originally established to include participants with an HbA1C level of 10% of total hemoglobin or below.12 Unfortunately, the majority of the potential participants had values substantially higher, and thus could not be enrolled in the trial, requiring another amendment to the study protocol in 2014, which was approved to include patients with an HbA1C level less than 12% of total hemoglobin. This change contributed considerably to the noted increase in enrollment rates in 2015, which almost doubled relative to enrollment under the original exclusion criteria (Figure).

The study has screened more than 600 patients. Among them, 137 were assessed for eligibility; 71 were excluded for various reasons, including screen failure (eg, decrease in wound size by >40% during the 2-week screening phase), loss to follow-up, and adverse events. Sixty-six participants reached the primary outcome at week 12, while 55 participants completed the study (19 in the SOC group; 18 in the cellular matrix group; 18 in the acellular matrix group).
We have stopped enrolling patients from all sites and the community, as we have reached our target enrollment.
Comment
One of the challenges of clinical trials is the recruitment of an adequate number of participants within an appropriate time frame, which is explained by Lasagna’s Law,18 a well-described phenomenon whereby the investigator overestimates the number of potential participants available to meet the inclusion criteria. This so-called funnel-effect was partly encountered in our selection process. A review of the veteran population with DFUs seemed to be more than adequate to fulfill the sample size; however, some important participant-related factors also played a substantial role.
In addition, the Veterans’ Affairs network centralizes health information, making it readily available to all providers participating in their care. As a result, patients with diabetes mellitus typically are seen by a primary care physician along with an endocrinologist, a diabetic nurse, and/or a dietician. Despite the collaboration with an interdisciplinary team, the glycemic control of the participants remains an issue along with other psychosocial factors that are deterrents in patient compliance. As a result, patients with poorly controlled diabetes and an HbA1C level above 10% (and less than 12%) of total hemoglobin who were initially excluded from the study were reincluded after modifying the inclusion criteria. Some patients were interested in joining the study, but physical limitations (eg, impaired mobility) prompted their decision not to join the trial, even though they met all the inclusion criteria.
As far as research-related factors that could affect participation, it is notable that most of the patients were retired; thus, the interventions did not cause additional burden of taking time off from work or loss of productivity. Although randomization could be a deterrent in many clinical trials, the majority of patients were willing to participate without demanding to be assigned to a particular treatment group.
There are many factors that are intertwined and can lead to enrollment and/or attrition rates. It was critical for our team to make some adjustment without compromising the controlled nature of a randomized trial.
Acknowledgment
The authors wish to acknowledge Huong Le, DPM, MPH, who was the coauthor of the study protocol.
Chronic diabetic foot ulcers (DFUs) remain a serious therapeutic challenge worldwide.1-2 Patients with DFUs are at higher risk for infections, which may lead to limb loss.1-5 In fact, 1 in 6 patients with DFUs will undergo an amputation.6 The long-term consequences of DFUs are numerous and can severely affect patients’ quality of life, including loss of productivity.7 The current standard of care for DFUs consists of debridement of the necrotic tissue, application of a moist dressing, and use of an off-loading device that protects the wound from pressure or trauma related to ambulation and other acts of daily living.4-6,8 Unfortunately, studies have shown that the best standard of care (SOC) only heals 30% of DFUs after 20 weeks of therapy.9 With the estimated cost per episode of care approaching $40,000, DFUs remain a costly and important problem.10
The altered extracellular matrix (ECM) in DFUs has been a target for the development of new therapeutic devices that provide a new matrix that is either devoid of cells or can be enriched with fibroblasts.8,11 These bioengineered skin substitutes stimulate the growth of new vessels and generate cytokines essential for tissue repair. In 2013, Lev-Tov et al12 published this study protocol (Dermagraft Oasis Longitudinal Comparative Efficacy [DOLCE] trial) to compare the effectiveness of 2 advanced wound care devices, specifically to evaluate the clinical efficacy of a cellular matrix versus an acellular matrix, which we have amended. The cellular matrix used in the study is a dermal substitute composed of viable newborn foreskin fibroblasts seeded onto a bioabsorbable polyglactin mesh on which fibroblasts generate an ECM.13,14 It is supplied frozen and requires specific thawing steps prior to application. The recommended regimen for treatment of DFUs for this cellular matrix is 8 weekly applications.13,14 In 2016, the cost of the product was reported as $1411 per 5.0×7.5-cm sheet.15 The acellular matrix product used in the study is a bioabsorbable ECM that is derived from porcine small intestinal submucosa.16,17 It is stored at room temperature and has a long shelf life, with a current price of $112.6 for a 3.0×3.5-cm single-layer fenestrated sheet ($1126.60 per box of 10 sheets). The industry-supported randomized controlled trials for each of these devices have reported a 20% added benefit in the rate of wound closure at week 12 compared to SOC.14,17
This article provides the interim report of the trial (registered at www.clinicaltrials.gov with the identifier NCT01450943) described in the published protocol and initiated in 2011,12 focusing on elements that required modification during the trial’s duration.
Methods
Study Protocol
The clinical trial was approved by the Veterans’ Affairs Institutional Research and Development Committee and their institutional review board. This study was funded by the Veteran’s Administration Merit Award (#10554640), which was awarded to 2 of the investigators (S.E.D. and R.R.I.). Eligible veterans were recruited from all 7 sites of the VA Northern California Healthcare System. This trial is a randomized, single-blinded, 3-armed, controlled clinical equivalence trial comparing the effectiveness of an SOC treatment, cellular ECM, and acellular ECM.
Study Products
The SOC dressing applied in the clinical trial included a sterile antimicrobial gel, a nonadherent dressing, and gauze.12 The SOC dressing also was used as a secondary dressing for the active treatment arms. Bacitracin antibiotic ointment was used as an alternative for patients with allergy to iodine.12
Randomization
The inclusion and exclusion criteria were previously outlined.12 After a 2-week screening phase to exclude rapid healers, patients were randomized into a treatment arm and entered the active phase for 12 weeks.
Primary and Secondary Outcomes
The primary outcome was complete wound closure by week 12.12 Complete healing was defined as full reepithelialization with no drainage or dressing requirement. The secondary outcomes included healing at 28 weeks, rate of healing, ulcer recurrence at week 20, association of wound healing with ulcer characteristics or patients’ characteristic, incidence of adverse events, and cost-effectiveness of each treatment compared to the SOC arm.12
Statistical Analysis
To detect a 25% difference in the incidence of ulcer closure between the 2 study groups and the SOC group, the estimation of the sample size was based on 80% power with a significance level of 0.05. Specifically, it was expected that 50% of the cellular and acellular matrix groups and 25% of the SOC group would reach complete wound closure. The protocol indicated that 57 participants would be enrolled in each arm (total of 171 participants). Lev-Tov et al12 discussed the statistical analysis in more detail.
Results
Study Protocol Amendments
Given the number of diabetic patients in the US veteran population, we anticipated that there would be enough participants meeting the inclusion and exclusion criteria; however, because of the difficulty with recruitment, the initial study criteria were modified. The study was initially designed to incorporate DFUs with a minimum size of 1.0 cm2.12
Another limiting criterion was the percentage of total hemoglobin level for hemoglobin A1C (HbA1C). The study was originally established to include participants with an HbA1C level of 10% of total hemoglobin or below.12 Unfortunately, the majority of the potential participants had values substantially higher, and thus could not be enrolled in the trial, requiring another amendment to the study protocol in 2014, which was approved to include patients with an HbA1C level less than 12% of total hemoglobin. This change contributed considerably to the noted increase in enrollment rates in 2015, which almost doubled relative to enrollment under the original exclusion criteria (Figure).

The study has screened more than 600 patients. Among them, 137 were assessed for eligibility; 71 were excluded for various reasons, including screen failure (eg, decrease in wound size by >40% during the 2-week screening phase), loss to follow-up, and adverse events. Sixty-six participants reached the primary outcome at week 12, while 55 participants completed the study (19 in the SOC group; 18 in the cellular matrix group; 18 in the acellular matrix group).
We have stopped enrolling patients from all sites and the community, as we have reached our target enrollment.
Comment
One of the challenges of clinical trials is the recruitment of an adequate number of participants within an appropriate time frame, which is explained by Lasagna’s Law,18 a well-described phenomenon whereby the investigator overestimates the number of potential participants available to meet the inclusion criteria. This so-called funnel-effect was partly encountered in our selection process. A review of the veteran population with DFUs seemed to be more than adequate to fulfill the sample size; however, some important participant-related factors also played a substantial role.
In addition, the Veterans’ Affairs network centralizes health information, making it readily available to all providers participating in their care. As a result, patients with diabetes mellitus typically are seen by a primary care physician along with an endocrinologist, a diabetic nurse, and/or a dietician. Despite the collaboration with an interdisciplinary team, the glycemic control of the participants remains an issue along with other psychosocial factors that are deterrents in patient compliance. As a result, patients with poorly controlled diabetes and an HbA1C level above 10% (and less than 12%) of total hemoglobin who were initially excluded from the study were reincluded after modifying the inclusion criteria. Some patients were interested in joining the study, but physical limitations (eg, impaired mobility) prompted their decision not to join the trial, even though they met all the inclusion criteria.
As far as research-related factors that could affect participation, it is notable that most of the patients were retired; thus, the interventions did not cause additional burden of taking time off from work or loss of productivity. Although randomization could be a deterrent in many clinical trials, the majority of patients were willing to participate without demanding to be assigned to a particular treatment group.
There are many factors that are intertwined and can lead to enrollment and/or attrition rates. It was critical for our team to make some adjustment without compromising the controlled nature of a randomized trial.
Acknowledgment
The authors wish to acknowledge Huong Le, DPM, MPH, who was the coauthor of the study protocol.
- Sen CK, Gordillo GM, Roy S, et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen. 2009;17:763-771.
- Gurtner GC, Werner S, Barrandon Y, et al. Wound repair and regeneration. Nature. 2008;453:314-321.
- Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366:1736-1743.
- Boulton AJ. The diabetic foot: grand overview, epidemiology and pathogenesis. Diabetes Metab Res Rev. 2008;24(suppl 1):S3-S6.
- Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA. 2005;293:217-228.
- Vuorisalo S, Venermo M, Lepäntalo M. Treatment of diabetic foot ulcers. J Cardiovasc Surg (Torino). 2009;50:275-291.
- Meijer JW, Trip J, Jaegers SM, et al. Quality of life in patients with diabetic foot ulcers. Disabil Rehabil. 2001;23:336-340.
- Santema TB, Poyck PP, Ubbink DT. Skin grafting and tissue replacement for treating foot ulcers in people with diabetes. Cochrane Database Syst Rev. 2016;2:CD011255.
- Margolis DJ, Kantor J, Berlin JA. Healing of diabetic neuropathic foot ulcers receiving standard treatment. a meta-analysis. Diabetes Care. 1999;22:692-695.
- Cavanagh P, Attinger C, Abbas Z, et al. Cost of treating diabetic foot ulcers in five different countries. Diabetes Metab Res Rev. 2012;2(suppl 1):107-111.
- Panuncialman J, Falanga V. The science of wound bed preparation. Surg Clin North Am. 2009;89:611-626.
- Lev-Tov H, Li CS, Dahle S, et al. Cellular versus acellular matrix devices in treatment of diabetic foot ulcers: study protocol for a comparative efficacy randomized controlled trial. Trials. 2013;14:8.
- Gentzkow GD, Iwasaki SD, Hershon KS, et al. Use of dermagraft, a cultured human dermis, to treat diabetic foot ulcers. Diabetes Care. 1996;19:350-354.
- Marston WA, Hanft J, Norwood P, et al; Dermagraft Diabetic Foot Ulcer Study Group. The efficacy and safety of Dermagraft in improving the healing of chronic diabetic foot ulcers: results of a prospective randomized trial. Diabetes Care. 2003;26:1701-1705.
- 2016 Dermagraft® Medicare Product and Related Procedure Payment. http://www.dermagraft.com/wp-content/uploads/sites/1/Dermagraft_Hotsheet%202016%20Q1%20HOSPITAL_FINAL.pdf. Accessed November 23, 2017.
- Oasis® Wound Matrix. http://www.oasiswoundmatrix.com/aboutowm. Accessed November 23, 2017.
- Niezgoda JA, Van Gils CC, Frykberg RG, et al. Randomized clinical trial comparing OASIS Wound Matrix to Regranex Gel for diabetic ulcers. Adv Skin Wound Care. 2005;18(5, pt 1):258-266.
- Torgerson JS, Arlinger K, Käppi M, et al. Principles for enhanced recruitment of subjects in a large clinical trial. the XENDOS (XENical in the prevention of Diabetes in Obese Subjects) study experience. Controlled Clin Trials. 2001;22:515-525.
- Sen CK, Gordillo GM, Roy S, et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen. 2009;17:763-771.
- Gurtner GC, Werner S, Barrandon Y, et al. Wound repair and regeneration. Nature. 2008;453:314-321.
- Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366:1736-1743.
- Boulton AJ. The diabetic foot: grand overview, epidemiology and pathogenesis. Diabetes Metab Res Rev. 2008;24(suppl 1):S3-S6.
- Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA. 2005;293:217-228.
- Vuorisalo S, Venermo M, Lepäntalo M. Treatment of diabetic foot ulcers. J Cardiovasc Surg (Torino). 2009;50:275-291.
- Meijer JW, Trip J, Jaegers SM, et al. Quality of life in patients with diabetic foot ulcers. Disabil Rehabil. 2001;23:336-340.
- Santema TB, Poyck PP, Ubbink DT. Skin grafting and tissue replacement for treating foot ulcers in people with diabetes. Cochrane Database Syst Rev. 2016;2:CD011255.
- Margolis DJ, Kantor J, Berlin JA. Healing of diabetic neuropathic foot ulcers receiving standard treatment. a meta-analysis. Diabetes Care. 1999;22:692-695.
- Cavanagh P, Attinger C, Abbas Z, et al. Cost of treating diabetic foot ulcers in five different countries. Diabetes Metab Res Rev. 2012;2(suppl 1):107-111.
- Panuncialman J, Falanga V. The science of wound bed preparation. Surg Clin North Am. 2009;89:611-626.
- Lev-Tov H, Li CS, Dahle S, et al. Cellular versus acellular matrix devices in treatment of diabetic foot ulcers: study protocol for a comparative efficacy randomized controlled trial. Trials. 2013;14:8.
- Gentzkow GD, Iwasaki SD, Hershon KS, et al. Use of dermagraft, a cultured human dermis, to treat diabetic foot ulcers. Diabetes Care. 1996;19:350-354.
- Marston WA, Hanft J, Norwood P, et al; Dermagraft Diabetic Foot Ulcer Study Group. The efficacy and safety of Dermagraft in improving the healing of chronic diabetic foot ulcers: results of a prospective randomized trial. Diabetes Care. 2003;26:1701-1705.
- 2016 Dermagraft® Medicare Product and Related Procedure Payment. http://www.dermagraft.com/wp-content/uploads/sites/1/Dermagraft_Hotsheet%202016%20Q1%20HOSPITAL_FINAL.pdf. Accessed November 23, 2017.
- Oasis® Wound Matrix. http://www.oasiswoundmatrix.com/aboutowm. Accessed November 23, 2017.
- Niezgoda JA, Van Gils CC, Frykberg RG, et al. Randomized clinical trial comparing OASIS Wound Matrix to Regranex Gel for diabetic ulcers. Adv Skin Wound Care. 2005;18(5, pt 1):258-266.
- Torgerson JS, Arlinger K, Käppi M, et al. Principles for enhanced recruitment of subjects in a large clinical trial. the XENDOS (XENical in the prevention of Diabetes in Obese Subjects) study experience. Controlled Clin Trials. 2001;22:515-525.
Resident Pearl
- Deciding on the appropriate wound care regimen for diabetic foot ulcers is difficult given the vast amount of wound products on the market. This head-to-head clinical trial compared the use of an expensive cellular matrix and an inexpensive acellular matrix relative to the standard of care. We hope that this study will help to guide therapy based on cost-effectiveness of wound adjuncts without compromising patient care.
Asymptomatic Pink Plaque on the Scapula
The Diagnosis: Primary Cutaneous Follicle Center Lymphoma
Immunohistochemistry revealed a nodular infiltrate consisting of small to large atypical lymphocytes forming an irregular germinal center with notably thinned mantle zones and lack of polarization (Figure, A). Atypical cells stained positively with Bcl-6, and CD20 was diffusely positive (Figure, B-D). Bcl-2 and CD3 colocalized to the reactive T-cell infiltrate, and CD10 was largely negative. Further workup with bone marrow biopsy and full-body positron emission tomography-computed tomography was unremarkable. Given these findings, a diagnosis of primary cutaneous follicle center lymphoma (FCL) was made. At 1 month following radiation therapy, complete clinical clearance of the lymphoma was achieved.
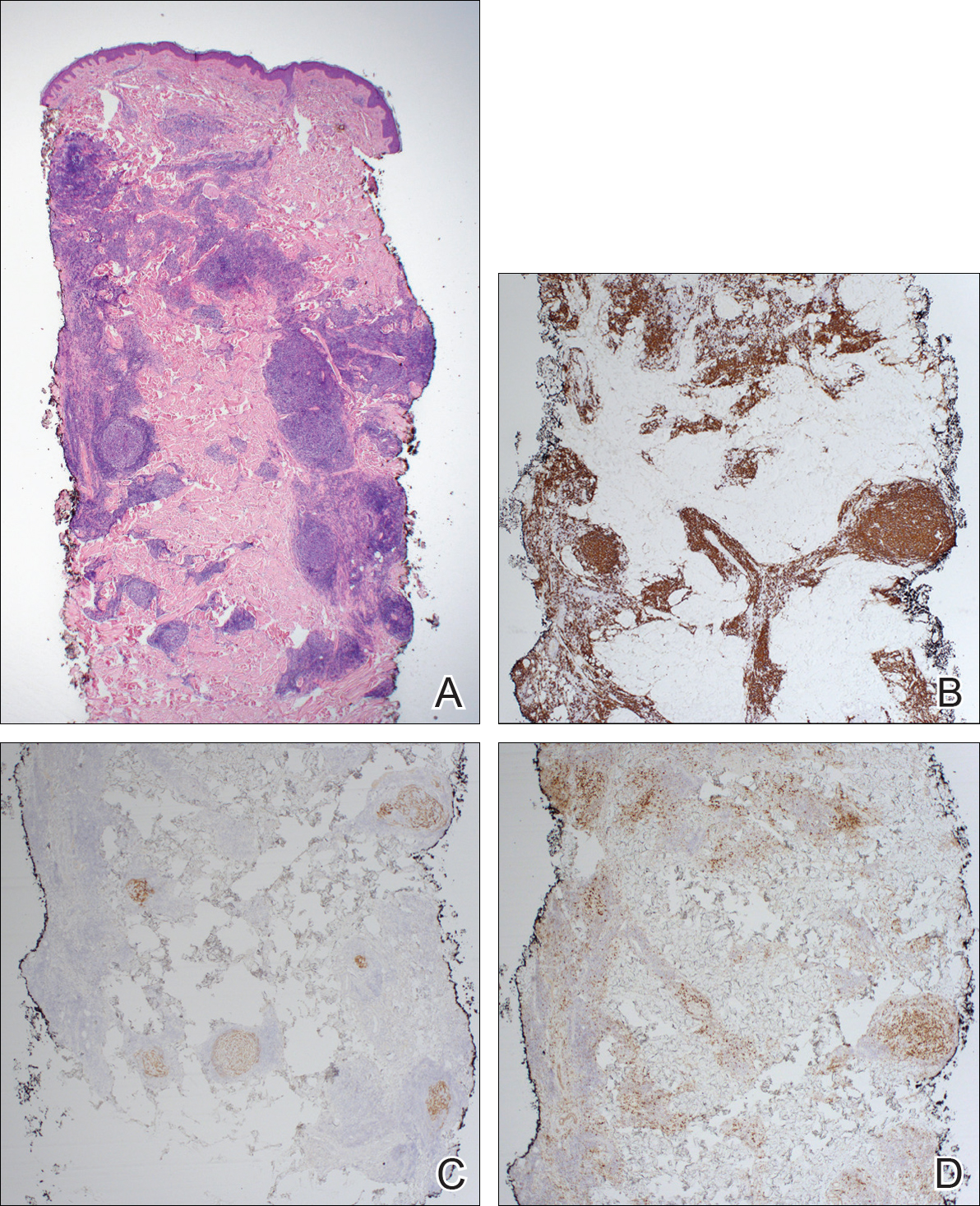
Follicle center lymphoma, also known as cutaneous follicular lymphoma, is the most common subtype of primary cutaneous B-cell lymphomas, representing approximately 57% of cases.1 Follicle center lymphoma typically affects older, non-Hispanic white adults with a median age of onset of 60 years. It has a predilection for the head, neck, and trunk.2 Lesions present as solitary erythematous to violaceous papules, plaques, or nodules, but they can more rarely be multifocal.3 Clinical diagnosis of FCL can be difficult, with papular lesions resembling acne, rosacea, folliculitis, or arthropod assault.4,5 As such, diagnosis of FCL typically relies on histopathologic analysis.
Histologically, FCL can present in several different patterns including follicular, nodular, diffuse, or a pleomorphic mix of these.2,6 The cells are comprised of germinal center B cells, staining positively for Bcl-6, CD20, and CD79a.7 Tumor cells do not exhibit the t(14;18) translocation seen in nodal follicular lymphomas.2,8 Unlike marginal zone lymphoma, FCL stains negatively for Bcl-2 and multiple myeloma 1/interferon regulatory factor 4 (MUM1/IRF-4).2,9 Forkhead box P1 (FOXP1) also is usually negative, but its presence can indicate a poorer prognosis.2 It is important to distinguish primary cutaneous B-cell lymphomas from systemic B-cell lymphoma with secondary cutaneous involvement, as they have a different clinical prognosis and management course. Further workup includes bone marrow biopsy, serum analysis for clonal involvement, and positron emission tomography-computed tomography imaging. Follicle center lymphoma generally has an indolent disease course with a favorable 5-year survival rate of approximately 95%.6,8
Untreated lesions may enlarge slowly or even spontaneously involute.10 The histologic growth pattern and number of lesions do not affect prognosis, but presence on the legs has a 5-year survival rate of 41%.2 Extracutaneous dissemination can occur in 5% to 10% of cases.2 Given the slow progression of FCL, conservative management with observation is an option. However, curative treatment can be reasonably attempted for solitary lesions by excision or radiation. Treatment of FCL often can be complicated by its predilection for the head and neck. Other treatment modalities include topical steroids, imiquimod, nitrogen mustard, and bexarotene.10 More generalized involvement may require systemic therapy with rituximab or chemotherapy. Recurrence after therapy is common, reported in 46.5% of patients, but does not affect prognosis.2
- Zinzani PL, Quaglino P, Pimpinelli N, et al. Prognostic factors in primary cutaneous B-cell lymphoma: The Italian Study Group for Cutaneous Lymphomas. J Clin Oncol. 2006;24:1376-1382.
- Suárez AL, Pulitzer M, Horwitz S, et al. Primary cutaneous B-cell lymphomas: part I. clinical features, diagnosis, and classification. J Am Acad Dermatol. 2013;69:1-13.
- Grange F, Bekkenk MW, Wechsler J, et al. Prognostic factors in primary cutaneous large B-cell lymphomas: a European multicenter study. J Clin Oncol. 2001;19:3602-3610.
- Soon CW, Pincus LB, Ai WZ, et al. Acneiform presentation of primary cutaneous follicle center lymphoma. J Am Acad Dermatol. 2011;65:887-889.
- Massone C, Fink-Puches R, Laimer M, et al. Miliary and agminated-type primary cutaneous follicle center lymphoma: a report of 18 cases. J Am Acad Dermatol. 2011;65:749-755.
- Wilcox RA. CME information: cutaneous B-cell lymphomas: 2015 update on diagnosis, risk-stratification, and management. Am J Hematol. 2015;90:73-76.
- Franco R, Fernandez-Vazquez A, Rodriguez-Peralto JL, et al. Cutaneous follicular B-cell lymphoma: description of a series of 18 cases. Am J Surg Pathol. 2001;25:875-883.
- Kempf W, Denisjuk N, Kerl K, et al. Primary cutaneous B-cell lymphomas. J Dtsch Dermatol Ges. 2012;10:12-22; quiz 23.
- de Leval L HN, Longtine J, Ferry JA, et al. Cutaneous B-cell lymphomas of follicular and marginal zone types: use of Bcl-6, CD10, Bcl-2, and CD21 in differential diagnosis and classification. Am J Surg Pathol. 2001;25:732-741.
- Suárez AL, Querfeld C, Horwitz S, et al. Primary cutaneous B-cell lymphomas: part II. therapy and future directions. J Am Acad Dermatol. 2013;69:1-11.
The Diagnosis: Primary Cutaneous Follicle Center Lymphoma
Immunohistochemistry revealed a nodular infiltrate consisting of small to large atypical lymphocytes forming an irregular germinal center with notably thinned mantle zones and lack of polarization (Figure, A). Atypical cells stained positively with Bcl-6, and CD20 was diffusely positive (Figure, B-D). Bcl-2 and CD3 colocalized to the reactive T-cell infiltrate, and CD10 was largely negative. Further workup with bone marrow biopsy and full-body positron emission tomography-computed tomography was unremarkable. Given these findings, a diagnosis of primary cutaneous follicle center lymphoma (FCL) was made. At 1 month following radiation therapy, complete clinical clearance of the lymphoma was achieved.

Follicle center lymphoma, also known as cutaneous follicular lymphoma, is the most common subtype of primary cutaneous B-cell lymphomas, representing approximately 57% of cases.1 Follicle center lymphoma typically affects older, non-Hispanic white adults with a median age of onset of 60 years. It has a predilection for the head, neck, and trunk.2 Lesions present as solitary erythematous to violaceous papules, plaques, or nodules, but they can more rarely be multifocal.3 Clinical diagnosis of FCL can be difficult, with papular lesions resembling acne, rosacea, folliculitis, or arthropod assault.4,5 As such, diagnosis of FCL typically relies on histopathologic analysis.
Histologically, FCL can present in several different patterns including follicular, nodular, diffuse, or a pleomorphic mix of these.2,6 The cells are comprised of germinal center B cells, staining positively for Bcl-6, CD20, and CD79a.7 Tumor cells do not exhibit the t(14;18) translocation seen in nodal follicular lymphomas.2,8 Unlike marginal zone lymphoma, FCL stains negatively for Bcl-2 and multiple myeloma 1/interferon regulatory factor 4 (MUM1/IRF-4).2,9 Forkhead box P1 (FOXP1) also is usually negative, but its presence can indicate a poorer prognosis.2 It is important to distinguish primary cutaneous B-cell lymphomas from systemic B-cell lymphoma with secondary cutaneous involvement, as they have a different clinical prognosis and management course. Further workup includes bone marrow biopsy, serum analysis for clonal involvement, and positron emission tomography-computed tomography imaging. Follicle center lymphoma generally has an indolent disease course with a favorable 5-year survival rate of approximately 95%.6,8
Untreated lesions may enlarge slowly or even spontaneously involute.10 The histologic growth pattern and number of lesions do not affect prognosis, but presence on the legs has a 5-year survival rate of 41%.2 Extracutaneous dissemination can occur in 5% to 10% of cases.2 Given the slow progression of FCL, conservative management with observation is an option. However, curative treatment can be reasonably attempted for solitary lesions by excision or radiation. Treatment of FCL often can be complicated by its predilection for the head and neck. Other treatment modalities include topical steroids, imiquimod, nitrogen mustard, and bexarotene.10 More generalized involvement may require systemic therapy with rituximab or chemotherapy. Recurrence after therapy is common, reported in 46.5% of patients, but does not affect prognosis.2
The Diagnosis: Primary Cutaneous Follicle Center Lymphoma
Immunohistochemistry revealed a nodular infiltrate consisting of small to large atypical lymphocytes forming an irregular germinal center with notably thinned mantle zones and lack of polarization (Figure, A). Atypical cells stained positively with Bcl-6, and CD20 was diffusely positive (Figure, B-D). Bcl-2 and CD3 colocalized to the reactive T-cell infiltrate, and CD10 was largely negative. Further workup with bone marrow biopsy and full-body positron emission tomography-computed tomography was unremarkable. Given these findings, a diagnosis of primary cutaneous follicle center lymphoma (FCL) was made. At 1 month following radiation therapy, complete clinical clearance of the lymphoma was achieved.

Follicle center lymphoma, also known as cutaneous follicular lymphoma, is the most common subtype of primary cutaneous B-cell lymphomas, representing approximately 57% of cases.1 Follicle center lymphoma typically affects older, non-Hispanic white adults with a median age of onset of 60 years. It has a predilection for the head, neck, and trunk.2 Lesions present as solitary erythematous to violaceous papules, plaques, or nodules, but they can more rarely be multifocal.3 Clinical diagnosis of FCL can be difficult, with papular lesions resembling acne, rosacea, folliculitis, or arthropod assault.4,5 As such, diagnosis of FCL typically relies on histopathologic analysis.
Histologically, FCL can present in several different patterns including follicular, nodular, diffuse, or a pleomorphic mix of these.2,6 The cells are comprised of germinal center B cells, staining positively for Bcl-6, CD20, and CD79a.7 Tumor cells do not exhibit the t(14;18) translocation seen in nodal follicular lymphomas.2,8 Unlike marginal zone lymphoma, FCL stains negatively for Bcl-2 and multiple myeloma 1/interferon regulatory factor 4 (MUM1/IRF-4).2,9 Forkhead box P1 (FOXP1) also is usually negative, but its presence can indicate a poorer prognosis.2 It is important to distinguish primary cutaneous B-cell lymphomas from systemic B-cell lymphoma with secondary cutaneous involvement, as they have a different clinical prognosis and management course. Further workup includes bone marrow biopsy, serum analysis for clonal involvement, and positron emission tomography-computed tomography imaging. Follicle center lymphoma generally has an indolent disease course with a favorable 5-year survival rate of approximately 95%.6,8
Untreated lesions may enlarge slowly or even spontaneously involute.10 The histologic growth pattern and number of lesions do not affect prognosis, but presence on the legs has a 5-year survival rate of 41%.2 Extracutaneous dissemination can occur in 5% to 10% of cases.2 Given the slow progression of FCL, conservative management with observation is an option. However, curative treatment can be reasonably attempted for solitary lesions by excision or radiation. Treatment of FCL often can be complicated by its predilection for the head and neck. Other treatment modalities include topical steroids, imiquimod, nitrogen mustard, and bexarotene.10 More generalized involvement may require systemic therapy with rituximab or chemotherapy. Recurrence after therapy is common, reported in 46.5% of patients, but does not affect prognosis.2
- Zinzani PL, Quaglino P, Pimpinelli N, et al. Prognostic factors in primary cutaneous B-cell lymphoma: The Italian Study Group for Cutaneous Lymphomas. J Clin Oncol. 2006;24:1376-1382.
- Suárez AL, Pulitzer M, Horwitz S, et al. Primary cutaneous B-cell lymphomas: part I. clinical features, diagnosis, and classification. J Am Acad Dermatol. 2013;69:1-13.
- Grange F, Bekkenk MW, Wechsler J, et al. Prognostic factors in primary cutaneous large B-cell lymphomas: a European multicenter study. J Clin Oncol. 2001;19:3602-3610.
- Soon CW, Pincus LB, Ai WZ, et al. Acneiform presentation of primary cutaneous follicle center lymphoma. J Am Acad Dermatol. 2011;65:887-889.
- Massone C, Fink-Puches R, Laimer M, et al. Miliary and agminated-type primary cutaneous follicle center lymphoma: a report of 18 cases. J Am Acad Dermatol. 2011;65:749-755.
- Wilcox RA. CME information: cutaneous B-cell lymphomas: 2015 update on diagnosis, risk-stratification, and management. Am J Hematol. 2015;90:73-76.
- Franco R, Fernandez-Vazquez A, Rodriguez-Peralto JL, et al. Cutaneous follicular B-cell lymphoma: description of a series of 18 cases. Am J Surg Pathol. 2001;25:875-883.
- Kempf W, Denisjuk N, Kerl K, et al. Primary cutaneous B-cell lymphomas. J Dtsch Dermatol Ges. 2012;10:12-22; quiz 23.
- de Leval L HN, Longtine J, Ferry JA, et al. Cutaneous B-cell lymphomas of follicular and marginal zone types: use of Bcl-6, CD10, Bcl-2, and CD21 in differential diagnosis and classification. Am J Surg Pathol. 2001;25:732-741.
- Suárez AL, Querfeld C, Horwitz S, et al. Primary cutaneous B-cell lymphomas: part II. therapy and future directions. J Am Acad Dermatol. 2013;69:1-11.
- Zinzani PL, Quaglino P, Pimpinelli N, et al. Prognostic factors in primary cutaneous B-cell lymphoma: The Italian Study Group for Cutaneous Lymphomas. J Clin Oncol. 2006;24:1376-1382.
- Suárez AL, Pulitzer M, Horwitz S, et al. Primary cutaneous B-cell lymphomas: part I. clinical features, diagnosis, and classification. J Am Acad Dermatol. 2013;69:1-13.
- Grange F, Bekkenk MW, Wechsler J, et al. Prognostic factors in primary cutaneous large B-cell lymphomas: a European multicenter study. J Clin Oncol. 2001;19:3602-3610.
- Soon CW, Pincus LB, Ai WZ, et al. Acneiform presentation of primary cutaneous follicle center lymphoma. J Am Acad Dermatol. 2011;65:887-889.
- Massone C, Fink-Puches R, Laimer M, et al. Miliary and agminated-type primary cutaneous follicle center lymphoma: a report of 18 cases. J Am Acad Dermatol. 2011;65:749-755.
- Wilcox RA. CME information: cutaneous B-cell lymphomas: 2015 update on diagnosis, risk-stratification, and management. Am J Hematol. 2015;90:73-76.
- Franco R, Fernandez-Vazquez A, Rodriguez-Peralto JL, et al. Cutaneous follicular B-cell lymphoma: description of a series of 18 cases. Am J Surg Pathol. 2001;25:875-883.
- Kempf W, Denisjuk N, Kerl K, et al. Primary cutaneous B-cell lymphomas. J Dtsch Dermatol Ges. 2012;10:12-22; quiz 23.
- de Leval L HN, Longtine J, Ferry JA, et al. Cutaneous B-cell lymphomas of follicular and marginal zone types: use of Bcl-6, CD10, Bcl-2, and CD21 in differential diagnosis and classification. Am J Surg Pathol. 2001;25:732-741.
- Suárez AL, Querfeld C, Horwitz S, et al. Primary cutaneous B-cell lymphomas: part II. therapy and future directions. J Am Acad Dermatol. 2013;69:1-11.
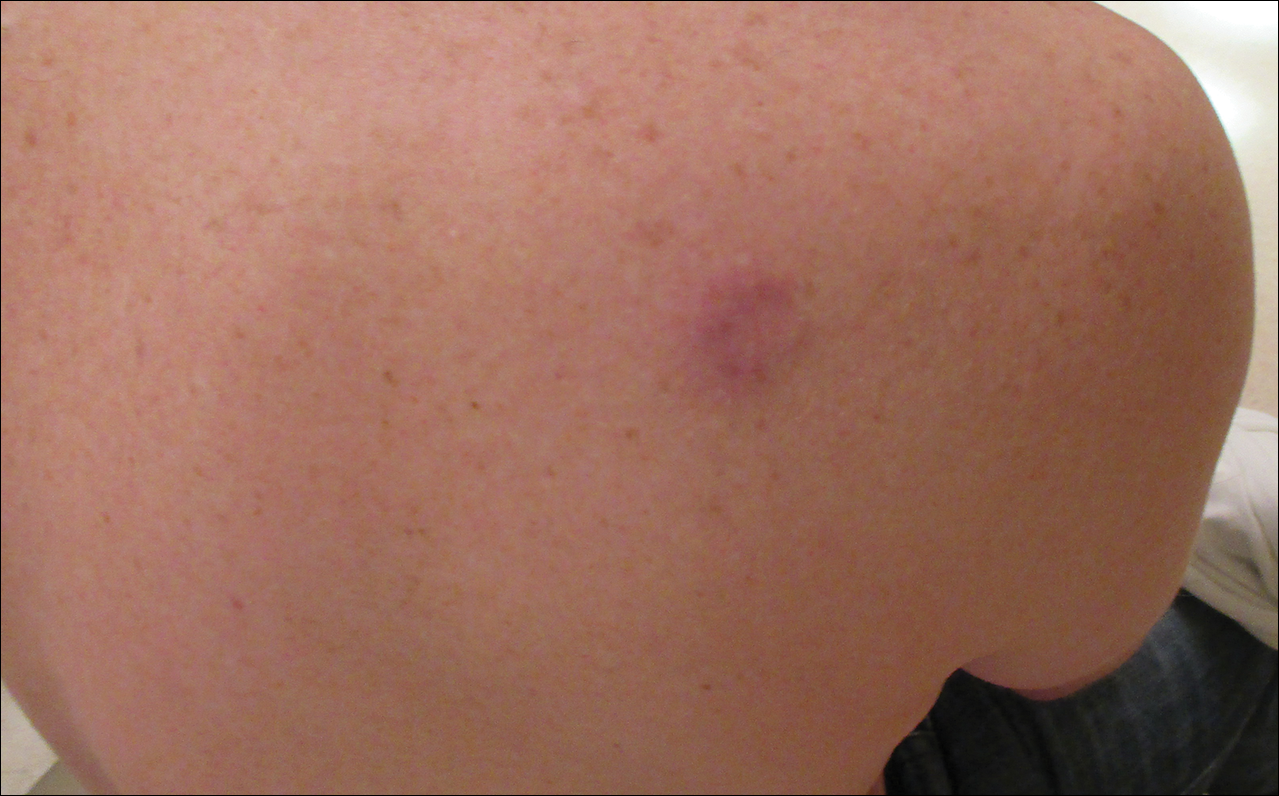
A 36-year-old man presented with a pink plaque on the right side of the scapula of 1 year's duration. The plaque had not grown and was completely asymptomatic. Physical examination revealed a violaceous, pink, 2-cm nodule with overlying telangiectasia. No other concerning lesions were identified on total-body skin examination. A punch biopsy was obtained.
Linear Porokeratosis Associated With Multiple Squamous Cell Carcinomas
Lesions of porokeratosis are thought to arise from disordered keratinization, though the exact pathogenesis remains uncertain. At least 5 clinical subtypes of porokeratosis have been identified: porokeratosis of Mibelli, disseminated superficial porokeratosis and disseminated superficial actinic porokeratosis (DSAP), linear porokeratosis, punctuate porokeratosis, and porokeratosis palmaris et plantaris disseminata (PPPD).1,2 Linear porokeratosis is a rare subtype with a clinical differential diagnosis that includes lichen striatus, linear lichen planus, linear verrucous epidermal nevus, segmental Darier disease, and incontinentia pigmenti.3 Definitive diagnosis of linear porokeratosis is made by histopathologic examination demonstrating a cornoid lamella, defined as a column of parakeratotic cells that lies at 45°to the surface of the epidermis and contains pyknotic basophilic nuclei.4 Patients with linear porokeratosis typically develop lesions along the lines of Blaschko in infancy or childhood.5,6 Among the different subtypes of porokeratosis, linear porokeratosis demonstrates the highest rate of malignant transformation, therefore requiring close clinical observation.7
Case Report
An 83-year-old woman presented to the outpatient clinic with a large linear plaque on the right leg that had been present since birth. Ten years prior to presentation, a portion of the lesion started to bleed; biopsy of the area was performed by an outside provider demonstrating squamous cell carcinoma (SCC), which was treated with wide local excision. One year prior to presentation, a separate portion of the plaque was biopsied by an outside provider and another diagnosis of SCC was made.
On examination performed during the initial presentation to our clinic, there was a well-demarcated tan to violaceous linear plaque present at the lower buttock and extending along the posterior leg to the skin overlying the Achilles tendon and dorsal aspect of the right foot. Within the plaque, there were areas of atrophy and areas of inflammation, induration, and hyperkeratosis (Figures 1 and 2). Two punch biopsies were performed: one from the edge of the plaque and one from a hyperkeratotic region within the plaque. Histology from the edge of the plaque demonstrated a cornoid lamella, consistent with a porokeratosis (Figure 3), whereas the histology from the hyperkeratotic region demonstrated a lichenoid infiltrate (Figure 4).

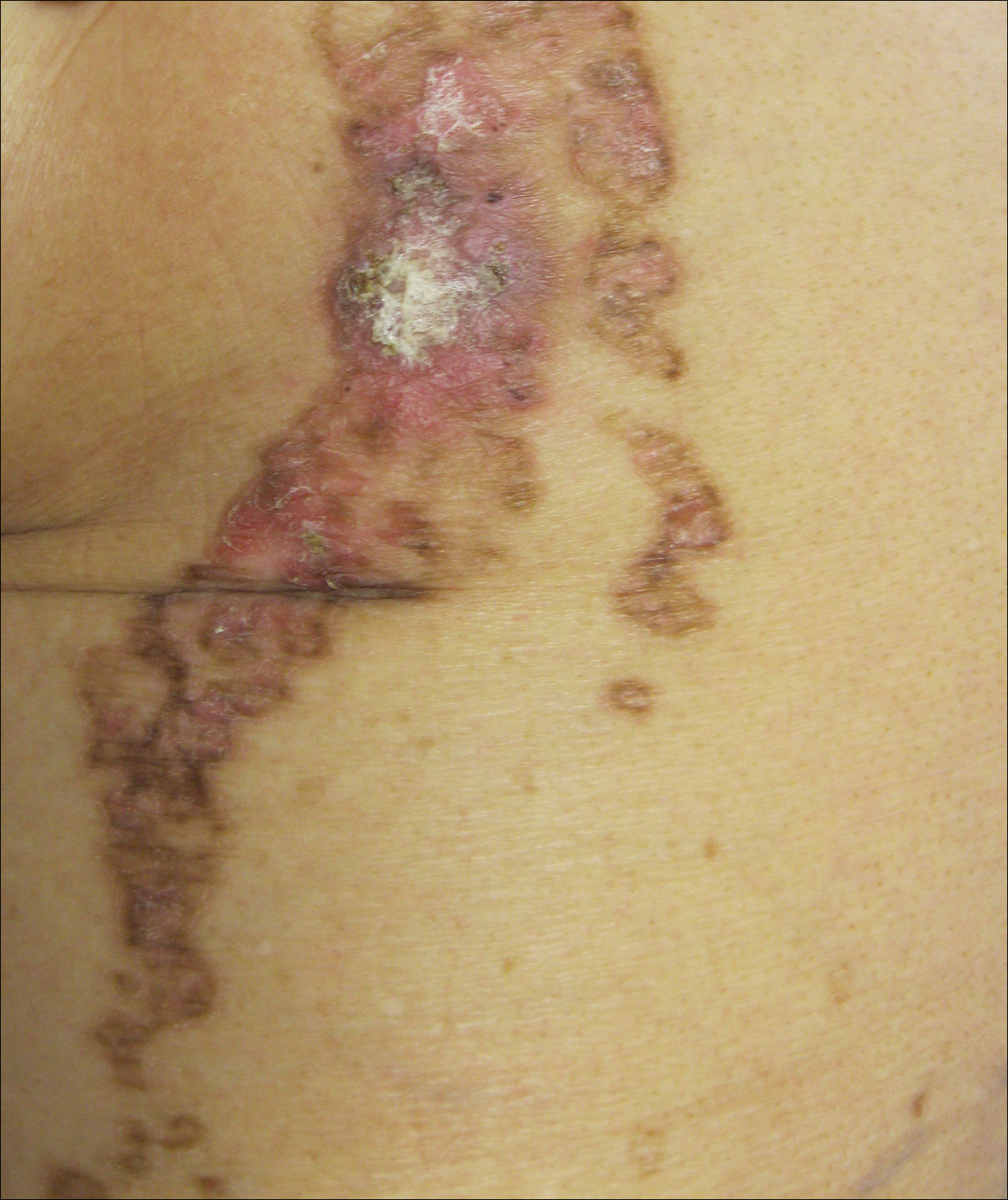
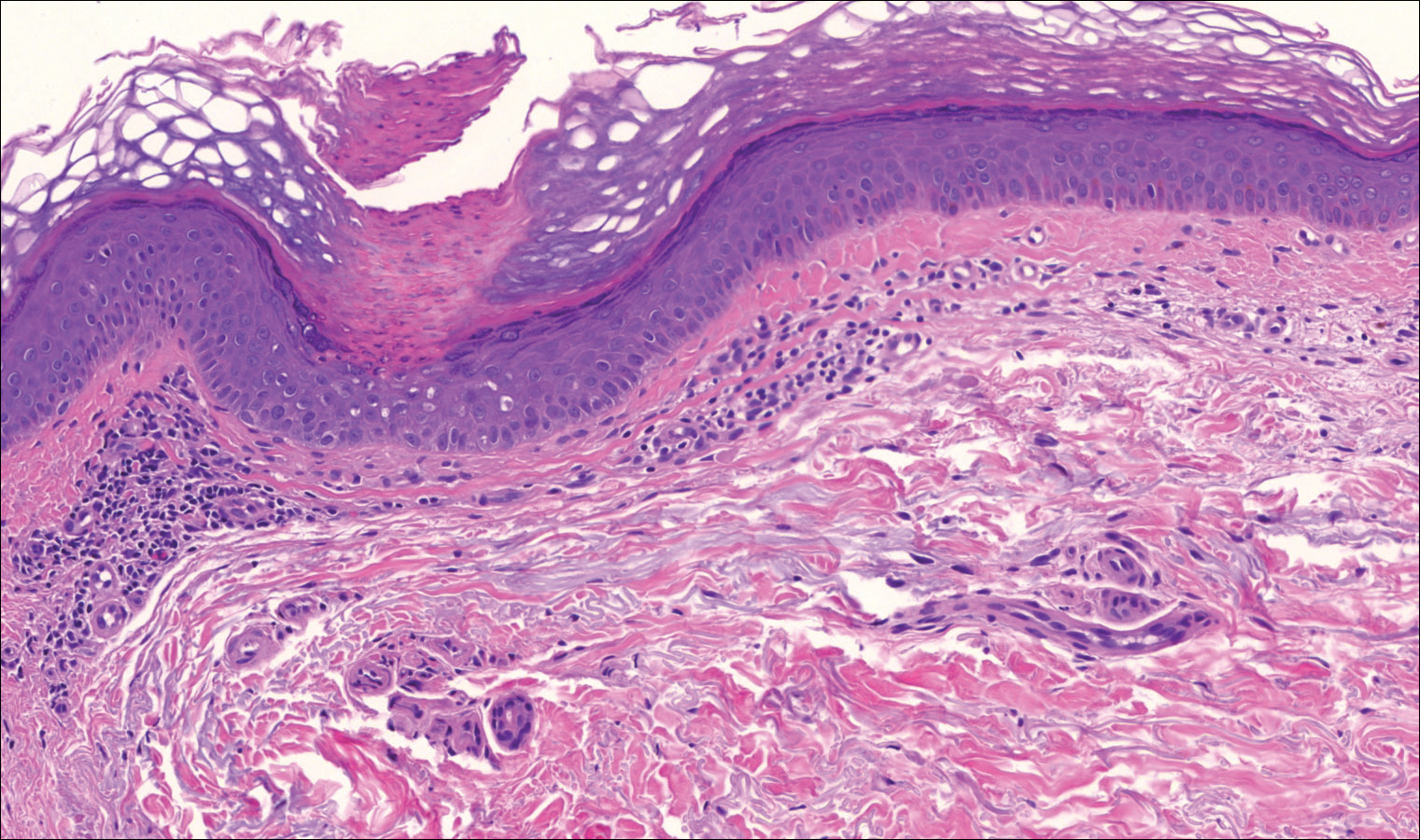
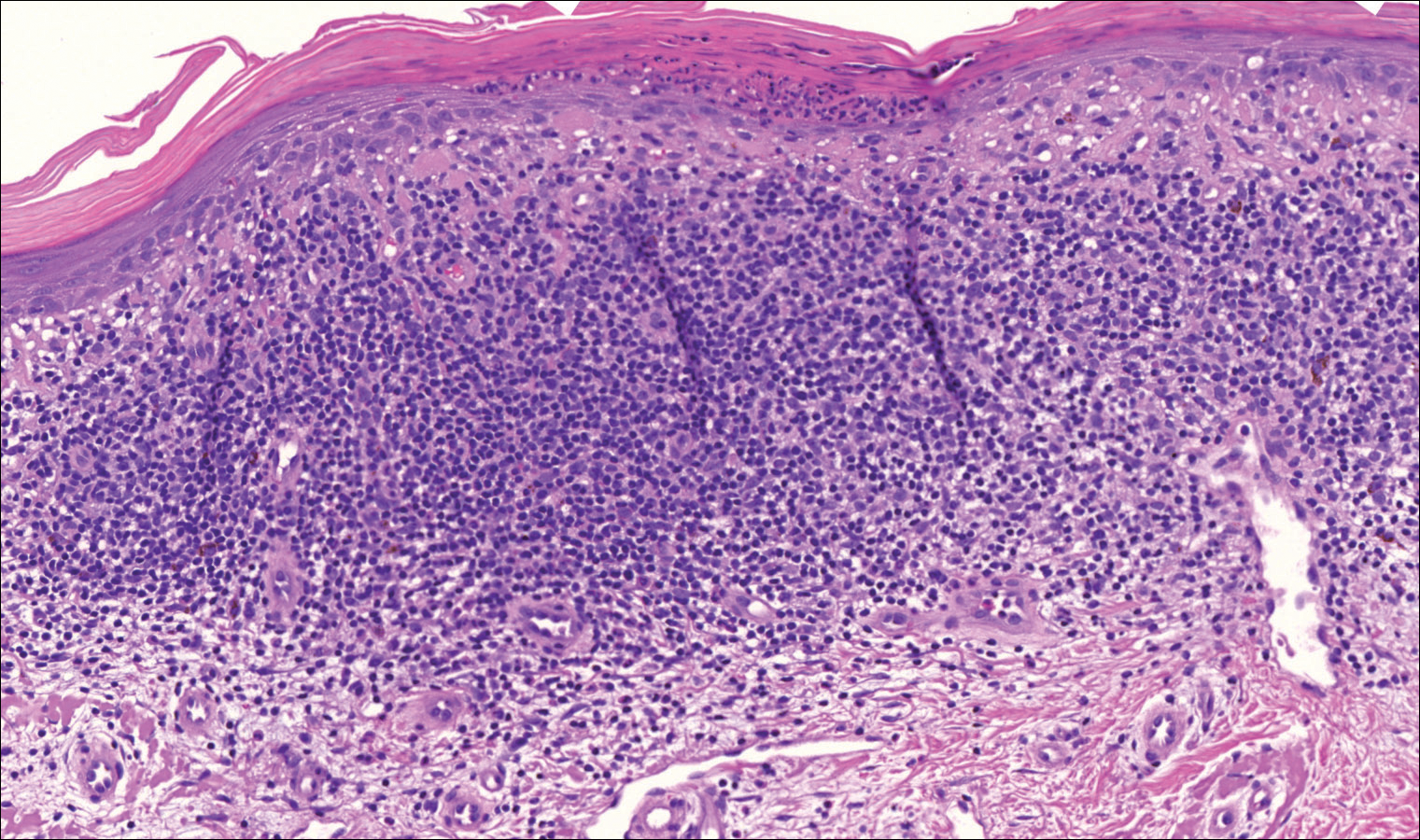
Several treatment options directed at the entire lesion were offered to the patient, but she declined these therapies and opted to address only those areas with clinical features of SCC, such as hyperkeratosis, bleeding, and rapid growth. Although biopsies performed by an outside provider were consistent with SCC, it had not been detected on biopsy performed during her initial visit to our clinic.
The patient was educated on the risk associated with her condition and instructed to follow up every 6 months to monitor for the development of SCC.
Comment
Porokeratosis is a disorder of keratinization with at least 5 clinical subtypes that share histologic similarities: porokeratosis of Mibelli, disseminated superficial porokeratosis and DSAP, linear porokeratosis, punctate porokeratosis, and PPPD.1,2 Other less common variants of porokeratosis include porokeratosis ptychotropica (a verrucous variant confined to the perianal area) and congenital unilateral linear porokeratosis.8,9
Linear porokeratosis appears in infancy or childhood with plaques that follow the lines of Blaschko.5,6 Most commonly, it presents unilaterally with annular plaques and linear hyperkeratotic papules that preferentially affect the extremities, though it also may present in a more generalized form or appear in a zosteriform pattern.10,11 Linear porokeratosis affects fewer than 20,000 individuals in the United States and accounts for fewer than 13% of all porokeratosis cases.12,13
Despite its relatively low prevalence, early identification of linear porokeratosis is important due to its high oncogenic potential, with malignant transformation to basal cell carcinoma or, more commonly, SCC reported in 19% of reported cases.1,5,7,14 The malignant transformation rate of linear porokeratosis is reported to be higher than rates seen in other porokeratosis subtypes (9.5%, 7.6%, and 3.4% for PPPD, porokeratosis of Mibelli, and DSAP, respectively).7 The risk of malignant transformation from porokeratosis increases with exposure to ionizing radiation, duration of the lesion, larger or coalescing lesions, and advanced age.7,15,16 Histologic studies have provided support for correlation between lesion size and oncogenic potential, with greater numbers of mitotic cells and more abnormal DNA ploidy seen in larger lesions.17
Histopathology
All subtypes of porokeratosis share certain histopathologic features that aid in the diagnosis of the disorder.18 Identification of the clinically observed hyperkeratotic ridged border or cornoid lamella is the primary means of definitively diagnosing porokeratosis; however, cornoid lamellae may be observed in other conditions, including verruca vulgaris and actinic keratosis.4,14
The cornoid lamella appears as a skewed column of densely packed parakeratotic cells with pyknotic basophilic nuclei extending through the stratum corneum from an epidermal invagination.4 Directly beneath the cornoid lamella, the granular layer is markedly diminished or absent, and cells of the stratum spinosum may demonstrate vacuolar changes or dyskeratosis.4,19 The superficial layer of the cornoid lamella may appear to be more centrifugally located and the cornoid lamella may be seen in several locations throughout the lesion.2,20 The degree of epidermal invagination, which is present under the cornoid lamella, varies by porokeratosis subtype; the central portion of the lesion may contain epidermis that ranges from hyperplastic to atrophic.2 Shumack et al21 noted that histologic changes under the cornoid lamella may include a lichenoid tissue reaction, papillary dermal lymphocytic infiltrate, vacuolar changes, dyskeratosis, and liquefaction degeneration of the basal layer. Because many of these histologic features also can be identified in lichen planus, a biopsy of the edge of lesions of porokeratosis is essential for making the correct diagnosis.
Heritability
Although linear porokeratosis has no identified pattern of inheritance and appears sporadic in onset, reports have described concomitant occurrence of linear porokeratosis and DSAP as well as linear porokeratosis arising in children of parents who have a diagnosis of DSAP.5,18,22,23 Based on these findings, it has been hypothesized that linear porokeratosis may represent a mosaic or segmental form of autosomal-dominant inherited subtypes of porokeratosis, such as DSAP.5 According to this hypothesis, loss of heterozygosity in patients with a DSAP mutation during early embryogenesis leads to proliferation of cells that are homozygous or hemizygous for the underlying mutation along lines of Blaschko.24 It has been suggested that the allelic loss implicated in the development of linear porokeratosis is the first step in a multistage process of carcinogenesis, which may help to explain the higher rates of malignant transformation that can be seen in linear porokeratosis.24
Management
Several treatment options exist for porokeratosis, including cryotherapy, topical 5-fluorouracil with or without adjunctive retinoid treatment, topical imiquimod, CO2 laser, shave and linear excision, curettage, dermabrasion, and oral acitretin for widespread lesions.1,25-29 One case report detailed successful treatment of adult-onset linear porokeratosis with tacrolimus ointment 0.1%.30 Treatments for porokeratosis demonstrate variable degrees of success, with the aim of eradicating the clonal population of mutant keratinocytes.2 Additionally, protection from UV radiation should be encouraged, especially in patients who have lesions that occur in areas of high actinic damage.1
Conclusion
We report of a case of linear porokeratosis with associated multiple SCCs that developed within the lesion. Definitive diagnosis of linear porokeratosis is important due to the higher rate of malignant transformation than the rate seen in other porokeratoses. In larger lesions, appropriate sampling and orientation of the pathology specimen is essential for identifying cornoid lamellae, thus allowing for appropriate follow-up and management. Several treatment options are available, though evidence for the effectiveness of any particular therapy is lacking. Research has shed light on possible genetic and molecular abnormalities in linear porokeratosis, but the exact pathogenesis of the disorder remains unclear.
- Curkova AK, Hegyi J, Kozub P, et al. A case of linear porokeratosis treated with photodynamic therapy with confocal microscopy surveillance. Dermatol Ther. 2014;27:144-147.
- Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Saunders; 2012.
- Behera B, Devi B, Nayak BB, et al. Giant inflammatory linear verrucous epidermal nevus: successfully treated with full thickness excision and skin grafting. Indian J Dermatol. 2013;58:461-463.
- Wade TR, Ackerman AB. Cornoid lamellation. a histologic reaction pattern. Am J Dermatopathol. 1980;2:5-15.
- Curnow P, Foley P, Baker C. Multiple squamous cell carcinomas complicating linear porokeratosis. Australas J Dermatol. 2003;44:136-139.
- Rahbari H, Cordero AA, Mehregan AH. Linear porokeratosis. a distinctive clinical variant of porokeratosis of Mibelli. Arch Dermatol. 1974;109:526-528.
- Sasson M, Krain AD. Porokeratosis and cutaneous malignancy. a review. Dermatol Surg. 1996;22:339-342.
- Yeo J, Winhoven S, Tallon B. Porokeratosis ptychotropica: a rare and evolving variant of porokeratosis. J Cutan Pathol. 2013;40:1042-1047.
- Scola N, Skrygan M, Wieland U, et al. Altered gene expression in squamous cell carcinoma arising from congenital unilateral linear porokeratosis. Clin Exp Dermatol. 2012;37:781-785.
- Sertznig P, von Felbert V, Megahed M. Porokeratosis: present concepts. J Eur Acad Dermatol Venereol. 2012;26:404-412.
- Goldner R. Zosteriform porokeratosis of Mibelli. Arch Dermatol. 1971;104:425-426.
- Malhotra SK, Puri KJ, Goyal T, et al. Linear porokeratosis. Dermatol Online J. 2007;13:15.
- Leow YH, Soon YH, Tham SN. A report of 31 cases of porokeratosis at the National Skin Centre. Ann Acad Med Singapore. 1996;25:837-841.
- Vivas AC, Maderal AD, Kirsner RS. Giant ulcerating squamous cell carcinoma arising from linear porokeratosis: a case study. Ostomy Wound Manage. 2012;58:18-20.
- Arranz-Salas I, Sanz-Trelles A, Ojeda DB. p53 alterations in porokeratosis. J Cutan Pathol. 2003;30:455-458.
- Otsuka F, Someya T, Ishibashi Y. Porokeratosis and malignant skin tumors. J Cancer Res Clin Oncol. 1991;117:55-60.
- Otsuka F, Umebayashi Y, Watanabe S, et al. Porokeratosis large skin lesions are susceptible to skin cancer development: histological and cytological explanation for the susceptibility. J Cancer Res Clin Oncol. 1993;119:395-400.
- Lohrer R, Neumann-Acikel A, Eming R, et al. A case of linear porokeratosis superimposed on disseminated superficial actinic porokeratosis. Case Rep Dermatol. 2010;2:130-134.
- Biswas A. Cornoid lamellation revisited: apropos of porokeratosis with emphasis on unusual clinicopathological variants. Am J Dermatopathol. 2015;37:145-155.
- Reed RJ, Leone P. Porokeratosis—a mutant clonal keratosis of the epidermis. I. histogenesis. Arch Dermatol. 1970;101:340-347.
- Shumack S, Commens C, Kossard S. Disseminated superficial actinic porokeratosis. a histological review of 61 cases with particular reference to lymphocytic inflammation. Am J Dermatopathol. 1991;13:26-31.
- Murase J, Gilliam AC. Disseminated superficial actinic porokeratosis co-existing with linear and verrucous porokeratosis in an elderly woman: update on the genetics and clinical expression of porokeratosis. J Am Acad Dermatol. 2010;63:886-891.
- Commens CA, Shumack SP. Linear porokeratosis in two families with disseminated superficial actinic porokeratosis. Pediatr Dermatol. 1987;4:209-214.
- Happle R. Cancer proneness of linear porokeratosis may be explained by allelic loss. Dermatology. 1997;195:20-25.
- Rabbin PE, Baldwin HE. Treatment of porokeratosis of Mibelli with CO2 laser vaporization versus surgical excision with split-thickness skin graft. a comparison. J Dermatol Surg Oncol. 1993;19:199-202.
- Spencer JM, Katz BE. Successful treatment of porokeratosis of Mibelli with diamond fraise dermabrasion. Arch Dermatol. 1992;128:1187-1188.
- Venkatarajan S, LeLeux TM, Yang D, et al. Porokeratosis of Mibelli: successful treatment with 5 percent topical imiquimod and topical 5 percent 5-fluorouracil. Dermatol Online J. 2010;16:10.
- McDonald SG, Peterka ES. Porokeratosis (Mibelli): treatment with topical 5-fluorouracil. J Am Acad Dermatol. 1983;8:107-110.
- Shumack SP, Commens CA. Disseminated superficial actinic porokeratosis: a clinical study. J Am Acad Dermatol. 1989;20:1015-1022.
- Parks AC, Conner KJ, Armstrong CA. Long-term clearance of linear porokeratosis with tacrolimus, 0.1%, ointment. JAMA Dermatol. 2014;150:194-196.
Lesions of porokeratosis are thought to arise from disordered keratinization, though the exact pathogenesis remains uncertain. At least 5 clinical subtypes of porokeratosis have been identified: porokeratosis of Mibelli, disseminated superficial porokeratosis and disseminated superficial actinic porokeratosis (DSAP), linear porokeratosis, punctuate porokeratosis, and porokeratosis palmaris et plantaris disseminata (PPPD).1,2 Linear porokeratosis is a rare subtype with a clinical differential diagnosis that includes lichen striatus, linear lichen planus, linear verrucous epidermal nevus, segmental Darier disease, and incontinentia pigmenti.3 Definitive diagnosis of linear porokeratosis is made by histopathologic examination demonstrating a cornoid lamella, defined as a column of parakeratotic cells that lies at 45°to the surface of the epidermis and contains pyknotic basophilic nuclei.4 Patients with linear porokeratosis typically develop lesions along the lines of Blaschko in infancy or childhood.5,6 Among the different subtypes of porokeratosis, linear porokeratosis demonstrates the highest rate of malignant transformation, therefore requiring close clinical observation.7
Case Report
An 83-year-old woman presented to the outpatient clinic with a large linear plaque on the right leg that had been present since birth. Ten years prior to presentation, a portion of the lesion started to bleed; biopsy of the area was performed by an outside provider demonstrating squamous cell carcinoma (SCC), which was treated with wide local excision. One year prior to presentation, a separate portion of the plaque was biopsied by an outside provider and another diagnosis of SCC was made.
On examination performed during the initial presentation to our clinic, there was a well-demarcated tan to violaceous linear plaque present at the lower buttock and extending along the posterior leg to the skin overlying the Achilles tendon and dorsal aspect of the right foot. Within the plaque, there were areas of atrophy and areas of inflammation, induration, and hyperkeratosis (Figures 1 and 2). Two punch biopsies were performed: one from the edge of the plaque and one from a hyperkeratotic region within the plaque. Histology from the edge of the plaque demonstrated a cornoid lamella, consistent with a porokeratosis (Figure 3), whereas the histology from the hyperkeratotic region demonstrated a lichenoid infiltrate (Figure 4).




Several treatment options directed at the entire lesion were offered to the patient, but she declined these therapies and opted to address only those areas with clinical features of SCC, such as hyperkeratosis, bleeding, and rapid growth. Although biopsies performed by an outside provider were consistent with SCC, it had not been detected on biopsy performed during her initial visit to our clinic.
The patient was educated on the risk associated with her condition and instructed to follow up every 6 months to monitor for the development of SCC.
Comment
Porokeratosis is a disorder of keratinization with at least 5 clinical subtypes that share histologic similarities: porokeratosis of Mibelli, disseminated superficial porokeratosis and DSAP, linear porokeratosis, punctate porokeratosis, and PPPD.1,2 Other less common variants of porokeratosis include porokeratosis ptychotropica (a verrucous variant confined to the perianal area) and congenital unilateral linear porokeratosis.8,9
Linear porokeratosis appears in infancy or childhood with plaques that follow the lines of Blaschko.5,6 Most commonly, it presents unilaterally with annular plaques and linear hyperkeratotic papules that preferentially affect the extremities, though it also may present in a more generalized form or appear in a zosteriform pattern.10,11 Linear porokeratosis affects fewer than 20,000 individuals in the United States and accounts for fewer than 13% of all porokeratosis cases.12,13
Despite its relatively low prevalence, early identification of linear porokeratosis is important due to its high oncogenic potential, with malignant transformation to basal cell carcinoma or, more commonly, SCC reported in 19% of reported cases.1,5,7,14 The malignant transformation rate of linear porokeratosis is reported to be higher than rates seen in other porokeratosis subtypes (9.5%, 7.6%, and 3.4% for PPPD, porokeratosis of Mibelli, and DSAP, respectively).7 The risk of malignant transformation from porokeratosis increases with exposure to ionizing radiation, duration of the lesion, larger or coalescing lesions, and advanced age.7,15,16 Histologic studies have provided support for correlation between lesion size and oncogenic potential, with greater numbers of mitotic cells and more abnormal DNA ploidy seen in larger lesions.17
Histopathology
All subtypes of porokeratosis share certain histopathologic features that aid in the diagnosis of the disorder.18 Identification of the clinically observed hyperkeratotic ridged border or cornoid lamella is the primary means of definitively diagnosing porokeratosis; however, cornoid lamellae may be observed in other conditions, including verruca vulgaris and actinic keratosis.4,14
The cornoid lamella appears as a skewed column of densely packed parakeratotic cells with pyknotic basophilic nuclei extending through the stratum corneum from an epidermal invagination.4 Directly beneath the cornoid lamella, the granular layer is markedly diminished or absent, and cells of the stratum spinosum may demonstrate vacuolar changes or dyskeratosis.4,19 The superficial layer of the cornoid lamella may appear to be more centrifugally located and the cornoid lamella may be seen in several locations throughout the lesion.2,20 The degree of epidermal invagination, which is present under the cornoid lamella, varies by porokeratosis subtype; the central portion of the lesion may contain epidermis that ranges from hyperplastic to atrophic.2 Shumack et al21 noted that histologic changes under the cornoid lamella may include a lichenoid tissue reaction, papillary dermal lymphocytic infiltrate, vacuolar changes, dyskeratosis, and liquefaction degeneration of the basal layer. Because many of these histologic features also can be identified in lichen planus, a biopsy of the edge of lesions of porokeratosis is essential for making the correct diagnosis.
Heritability
Although linear porokeratosis has no identified pattern of inheritance and appears sporadic in onset, reports have described concomitant occurrence of linear porokeratosis and DSAP as well as linear porokeratosis arising in children of parents who have a diagnosis of DSAP.5,18,22,23 Based on these findings, it has been hypothesized that linear porokeratosis may represent a mosaic or segmental form of autosomal-dominant inherited subtypes of porokeratosis, such as DSAP.5 According to this hypothesis, loss of heterozygosity in patients with a DSAP mutation during early embryogenesis leads to proliferation of cells that are homozygous or hemizygous for the underlying mutation along lines of Blaschko.24 It has been suggested that the allelic loss implicated in the development of linear porokeratosis is the first step in a multistage process of carcinogenesis, which may help to explain the higher rates of malignant transformation that can be seen in linear porokeratosis.24
Management
Several treatment options exist for porokeratosis, including cryotherapy, topical 5-fluorouracil with or without adjunctive retinoid treatment, topical imiquimod, CO2 laser, shave and linear excision, curettage, dermabrasion, and oral acitretin for widespread lesions.1,25-29 One case report detailed successful treatment of adult-onset linear porokeratosis with tacrolimus ointment 0.1%.30 Treatments for porokeratosis demonstrate variable degrees of success, with the aim of eradicating the clonal population of mutant keratinocytes.2 Additionally, protection from UV radiation should be encouraged, especially in patients who have lesions that occur in areas of high actinic damage.1
Conclusion
We report of a case of linear porokeratosis with associated multiple SCCs that developed within the lesion. Definitive diagnosis of linear porokeratosis is important due to the higher rate of malignant transformation than the rate seen in other porokeratoses. In larger lesions, appropriate sampling and orientation of the pathology specimen is essential for identifying cornoid lamellae, thus allowing for appropriate follow-up and management. Several treatment options are available, though evidence for the effectiveness of any particular therapy is lacking. Research has shed light on possible genetic and molecular abnormalities in linear porokeratosis, but the exact pathogenesis of the disorder remains unclear.
Lesions of porokeratosis are thought to arise from disordered keratinization, though the exact pathogenesis remains uncertain. At least 5 clinical subtypes of porokeratosis have been identified: porokeratosis of Mibelli, disseminated superficial porokeratosis and disseminated superficial actinic porokeratosis (DSAP), linear porokeratosis, punctuate porokeratosis, and porokeratosis palmaris et plantaris disseminata (PPPD).1,2 Linear porokeratosis is a rare subtype with a clinical differential diagnosis that includes lichen striatus, linear lichen planus, linear verrucous epidermal nevus, segmental Darier disease, and incontinentia pigmenti.3 Definitive diagnosis of linear porokeratosis is made by histopathologic examination demonstrating a cornoid lamella, defined as a column of parakeratotic cells that lies at 45°to the surface of the epidermis and contains pyknotic basophilic nuclei.4 Patients with linear porokeratosis typically develop lesions along the lines of Blaschko in infancy or childhood.5,6 Among the different subtypes of porokeratosis, linear porokeratosis demonstrates the highest rate of malignant transformation, therefore requiring close clinical observation.7
Case Report
An 83-year-old woman presented to the outpatient clinic with a large linear plaque on the right leg that had been present since birth. Ten years prior to presentation, a portion of the lesion started to bleed; biopsy of the area was performed by an outside provider demonstrating squamous cell carcinoma (SCC), which was treated with wide local excision. One year prior to presentation, a separate portion of the plaque was biopsied by an outside provider and another diagnosis of SCC was made.
On examination performed during the initial presentation to our clinic, there was a well-demarcated tan to violaceous linear plaque present at the lower buttock and extending along the posterior leg to the skin overlying the Achilles tendon and dorsal aspect of the right foot. Within the plaque, there were areas of atrophy and areas of inflammation, induration, and hyperkeratosis (Figures 1 and 2). Two punch biopsies were performed: one from the edge of the plaque and one from a hyperkeratotic region within the plaque. Histology from the edge of the plaque demonstrated a cornoid lamella, consistent with a porokeratosis (Figure 3), whereas the histology from the hyperkeratotic region demonstrated a lichenoid infiltrate (Figure 4).




Several treatment options directed at the entire lesion were offered to the patient, but she declined these therapies and opted to address only those areas with clinical features of SCC, such as hyperkeratosis, bleeding, and rapid growth. Although biopsies performed by an outside provider were consistent with SCC, it had not been detected on biopsy performed during her initial visit to our clinic.
The patient was educated on the risk associated with her condition and instructed to follow up every 6 months to monitor for the development of SCC.
Comment
Porokeratosis is a disorder of keratinization with at least 5 clinical subtypes that share histologic similarities: porokeratosis of Mibelli, disseminated superficial porokeratosis and DSAP, linear porokeratosis, punctate porokeratosis, and PPPD.1,2 Other less common variants of porokeratosis include porokeratosis ptychotropica (a verrucous variant confined to the perianal area) and congenital unilateral linear porokeratosis.8,9
Linear porokeratosis appears in infancy or childhood with plaques that follow the lines of Blaschko.5,6 Most commonly, it presents unilaterally with annular plaques and linear hyperkeratotic papules that preferentially affect the extremities, though it also may present in a more generalized form or appear in a zosteriform pattern.10,11 Linear porokeratosis affects fewer than 20,000 individuals in the United States and accounts for fewer than 13% of all porokeratosis cases.12,13
Despite its relatively low prevalence, early identification of linear porokeratosis is important due to its high oncogenic potential, with malignant transformation to basal cell carcinoma or, more commonly, SCC reported in 19% of reported cases.1,5,7,14 The malignant transformation rate of linear porokeratosis is reported to be higher than rates seen in other porokeratosis subtypes (9.5%, 7.6%, and 3.4% for PPPD, porokeratosis of Mibelli, and DSAP, respectively).7 The risk of malignant transformation from porokeratosis increases with exposure to ionizing radiation, duration of the lesion, larger or coalescing lesions, and advanced age.7,15,16 Histologic studies have provided support for correlation between lesion size and oncogenic potential, with greater numbers of mitotic cells and more abnormal DNA ploidy seen in larger lesions.17
Histopathology
All subtypes of porokeratosis share certain histopathologic features that aid in the diagnosis of the disorder.18 Identification of the clinically observed hyperkeratotic ridged border or cornoid lamella is the primary means of definitively diagnosing porokeratosis; however, cornoid lamellae may be observed in other conditions, including verruca vulgaris and actinic keratosis.4,14
The cornoid lamella appears as a skewed column of densely packed parakeratotic cells with pyknotic basophilic nuclei extending through the stratum corneum from an epidermal invagination.4 Directly beneath the cornoid lamella, the granular layer is markedly diminished or absent, and cells of the stratum spinosum may demonstrate vacuolar changes or dyskeratosis.4,19 The superficial layer of the cornoid lamella may appear to be more centrifugally located and the cornoid lamella may be seen in several locations throughout the lesion.2,20 The degree of epidermal invagination, which is present under the cornoid lamella, varies by porokeratosis subtype; the central portion of the lesion may contain epidermis that ranges from hyperplastic to atrophic.2 Shumack et al21 noted that histologic changes under the cornoid lamella may include a lichenoid tissue reaction, papillary dermal lymphocytic infiltrate, vacuolar changes, dyskeratosis, and liquefaction degeneration of the basal layer. Because many of these histologic features also can be identified in lichen planus, a biopsy of the edge of lesions of porokeratosis is essential for making the correct diagnosis.
Heritability
Although linear porokeratosis has no identified pattern of inheritance and appears sporadic in onset, reports have described concomitant occurrence of linear porokeratosis and DSAP as well as linear porokeratosis arising in children of parents who have a diagnosis of DSAP.5,18,22,23 Based on these findings, it has been hypothesized that linear porokeratosis may represent a mosaic or segmental form of autosomal-dominant inherited subtypes of porokeratosis, such as DSAP.5 According to this hypothesis, loss of heterozygosity in patients with a DSAP mutation during early embryogenesis leads to proliferation of cells that are homozygous or hemizygous for the underlying mutation along lines of Blaschko.24 It has been suggested that the allelic loss implicated in the development of linear porokeratosis is the first step in a multistage process of carcinogenesis, which may help to explain the higher rates of malignant transformation that can be seen in linear porokeratosis.24
Management
Several treatment options exist for porokeratosis, including cryotherapy, topical 5-fluorouracil with or without adjunctive retinoid treatment, topical imiquimod, CO2 laser, shave and linear excision, curettage, dermabrasion, and oral acitretin for widespread lesions.1,25-29 One case report detailed successful treatment of adult-onset linear porokeratosis with tacrolimus ointment 0.1%.30 Treatments for porokeratosis demonstrate variable degrees of success, with the aim of eradicating the clonal population of mutant keratinocytes.2 Additionally, protection from UV radiation should be encouraged, especially in patients who have lesions that occur in areas of high actinic damage.1
Conclusion
We report of a case of linear porokeratosis with associated multiple SCCs that developed within the lesion. Definitive diagnosis of linear porokeratosis is important due to the higher rate of malignant transformation than the rate seen in other porokeratoses. In larger lesions, appropriate sampling and orientation of the pathology specimen is essential for identifying cornoid lamellae, thus allowing for appropriate follow-up and management. Several treatment options are available, though evidence for the effectiveness of any particular therapy is lacking. Research has shed light on possible genetic and molecular abnormalities in linear porokeratosis, but the exact pathogenesis of the disorder remains unclear.
- Curkova AK, Hegyi J, Kozub P, et al. A case of linear porokeratosis treated with photodynamic therapy with confocal microscopy surveillance. Dermatol Ther. 2014;27:144-147.
- Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Saunders; 2012.
- Behera B, Devi B, Nayak BB, et al. Giant inflammatory linear verrucous epidermal nevus: successfully treated with full thickness excision and skin grafting. Indian J Dermatol. 2013;58:461-463.
- Wade TR, Ackerman AB. Cornoid lamellation. a histologic reaction pattern. Am J Dermatopathol. 1980;2:5-15.
- Curnow P, Foley P, Baker C. Multiple squamous cell carcinomas complicating linear porokeratosis. Australas J Dermatol. 2003;44:136-139.
- Rahbari H, Cordero AA, Mehregan AH. Linear porokeratosis. a distinctive clinical variant of porokeratosis of Mibelli. Arch Dermatol. 1974;109:526-528.
- Sasson M, Krain AD. Porokeratosis and cutaneous malignancy. a review. Dermatol Surg. 1996;22:339-342.
- Yeo J, Winhoven S, Tallon B. Porokeratosis ptychotropica: a rare and evolving variant of porokeratosis. J Cutan Pathol. 2013;40:1042-1047.
- Scola N, Skrygan M, Wieland U, et al. Altered gene expression in squamous cell carcinoma arising from congenital unilateral linear porokeratosis. Clin Exp Dermatol. 2012;37:781-785.
- Sertznig P, von Felbert V, Megahed M. Porokeratosis: present concepts. J Eur Acad Dermatol Venereol. 2012;26:404-412.
- Goldner R. Zosteriform porokeratosis of Mibelli. Arch Dermatol. 1971;104:425-426.
- Malhotra SK, Puri KJ, Goyal T, et al. Linear porokeratosis. Dermatol Online J. 2007;13:15.
- Leow YH, Soon YH, Tham SN. A report of 31 cases of porokeratosis at the National Skin Centre. Ann Acad Med Singapore. 1996;25:837-841.
- Vivas AC, Maderal AD, Kirsner RS. Giant ulcerating squamous cell carcinoma arising from linear porokeratosis: a case study. Ostomy Wound Manage. 2012;58:18-20.
- Arranz-Salas I, Sanz-Trelles A, Ojeda DB. p53 alterations in porokeratosis. J Cutan Pathol. 2003;30:455-458.
- Otsuka F, Someya T, Ishibashi Y. Porokeratosis and malignant skin tumors. J Cancer Res Clin Oncol. 1991;117:55-60.
- Otsuka F, Umebayashi Y, Watanabe S, et al. Porokeratosis large skin lesions are susceptible to skin cancer development: histological and cytological explanation for the susceptibility. J Cancer Res Clin Oncol. 1993;119:395-400.
- Lohrer R, Neumann-Acikel A, Eming R, et al. A case of linear porokeratosis superimposed on disseminated superficial actinic porokeratosis. Case Rep Dermatol. 2010;2:130-134.
- Biswas A. Cornoid lamellation revisited: apropos of porokeratosis with emphasis on unusual clinicopathological variants. Am J Dermatopathol. 2015;37:145-155.
- Reed RJ, Leone P. Porokeratosis—a mutant clonal keratosis of the epidermis. I. histogenesis. Arch Dermatol. 1970;101:340-347.
- Shumack S, Commens C, Kossard S. Disseminated superficial actinic porokeratosis. a histological review of 61 cases with particular reference to lymphocytic inflammation. Am J Dermatopathol. 1991;13:26-31.
- Murase J, Gilliam AC. Disseminated superficial actinic porokeratosis co-existing with linear and verrucous porokeratosis in an elderly woman: update on the genetics and clinical expression of porokeratosis. J Am Acad Dermatol. 2010;63:886-891.
- Commens CA, Shumack SP. Linear porokeratosis in two families with disseminated superficial actinic porokeratosis. Pediatr Dermatol. 1987;4:209-214.
- Happle R. Cancer proneness of linear porokeratosis may be explained by allelic loss. Dermatology. 1997;195:20-25.
- Rabbin PE, Baldwin HE. Treatment of porokeratosis of Mibelli with CO2 laser vaporization versus surgical excision with split-thickness skin graft. a comparison. J Dermatol Surg Oncol. 1993;19:199-202.
- Spencer JM, Katz BE. Successful treatment of porokeratosis of Mibelli with diamond fraise dermabrasion. Arch Dermatol. 1992;128:1187-1188.
- Venkatarajan S, LeLeux TM, Yang D, et al. Porokeratosis of Mibelli: successful treatment with 5 percent topical imiquimod and topical 5 percent 5-fluorouracil. Dermatol Online J. 2010;16:10.
- McDonald SG, Peterka ES. Porokeratosis (Mibelli): treatment with topical 5-fluorouracil. J Am Acad Dermatol. 1983;8:107-110.
- Shumack SP, Commens CA. Disseminated superficial actinic porokeratosis: a clinical study. J Am Acad Dermatol. 1989;20:1015-1022.
- Parks AC, Conner KJ, Armstrong CA. Long-term clearance of linear porokeratosis with tacrolimus, 0.1%, ointment. JAMA Dermatol. 2014;150:194-196.
- Curkova AK, Hegyi J, Kozub P, et al. A case of linear porokeratosis treated with photodynamic therapy with confocal microscopy surveillance. Dermatol Ther. 2014;27:144-147.
- Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Saunders; 2012.
- Behera B, Devi B, Nayak BB, et al. Giant inflammatory linear verrucous epidermal nevus: successfully treated with full thickness excision and skin grafting. Indian J Dermatol. 2013;58:461-463.
- Wade TR, Ackerman AB. Cornoid lamellation. a histologic reaction pattern. Am J Dermatopathol. 1980;2:5-15.
- Curnow P, Foley P, Baker C. Multiple squamous cell carcinomas complicating linear porokeratosis. Australas J Dermatol. 2003;44:136-139.
- Rahbari H, Cordero AA, Mehregan AH. Linear porokeratosis. a distinctive clinical variant of porokeratosis of Mibelli. Arch Dermatol. 1974;109:526-528.
- Sasson M, Krain AD. Porokeratosis and cutaneous malignancy. a review. Dermatol Surg. 1996;22:339-342.
- Yeo J, Winhoven S, Tallon B. Porokeratosis ptychotropica: a rare and evolving variant of porokeratosis. J Cutan Pathol. 2013;40:1042-1047.
- Scola N, Skrygan M, Wieland U, et al. Altered gene expression in squamous cell carcinoma arising from congenital unilateral linear porokeratosis. Clin Exp Dermatol. 2012;37:781-785.
- Sertznig P, von Felbert V, Megahed M. Porokeratosis: present concepts. J Eur Acad Dermatol Venereol. 2012;26:404-412.
- Goldner R. Zosteriform porokeratosis of Mibelli. Arch Dermatol. 1971;104:425-426.
- Malhotra SK, Puri KJ, Goyal T, et al. Linear porokeratosis. Dermatol Online J. 2007;13:15.
- Leow YH, Soon YH, Tham SN. A report of 31 cases of porokeratosis at the National Skin Centre. Ann Acad Med Singapore. 1996;25:837-841.
- Vivas AC, Maderal AD, Kirsner RS. Giant ulcerating squamous cell carcinoma arising from linear porokeratosis: a case study. Ostomy Wound Manage. 2012;58:18-20.
- Arranz-Salas I, Sanz-Trelles A, Ojeda DB. p53 alterations in porokeratosis. J Cutan Pathol. 2003;30:455-458.
- Otsuka F, Someya T, Ishibashi Y. Porokeratosis and malignant skin tumors. J Cancer Res Clin Oncol. 1991;117:55-60.
- Otsuka F, Umebayashi Y, Watanabe S, et al. Porokeratosis large skin lesions are susceptible to skin cancer development: histological and cytological explanation for the susceptibility. J Cancer Res Clin Oncol. 1993;119:395-400.
- Lohrer R, Neumann-Acikel A, Eming R, et al. A case of linear porokeratosis superimposed on disseminated superficial actinic porokeratosis. Case Rep Dermatol. 2010;2:130-134.
- Biswas A. Cornoid lamellation revisited: apropos of porokeratosis with emphasis on unusual clinicopathological variants. Am J Dermatopathol. 2015;37:145-155.
- Reed RJ, Leone P. Porokeratosis—a mutant clonal keratosis of the epidermis. I. histogenesis. Arch Dermatol. 1970;101:340-347.
- Shumack S, Commens C, Kossard S. Disseminated superficial actinic porokeratosis. a histological review of 61 cases with particular reference to lymphocytic inflammation. Am J Dermatopathol. 1991;13:26-31.
- Murase J, Gilliam AC. Disseminated superficial actinic porokeratosis co-existing with linear and verrucous porokeratosis in an elderly woman: update on the genetics and clinical expression of porokeratosis. J Am Acad Dermatol. 2010;63:886-891.
- Commens CA, Shumack SP. Linear porokeratosis in two families with disseminated superficial actinic porokeratosis. Pediatr Dermatol. 1987;4:209-214.
- Happle R. Cancer proneness of linear porokeratosis may be explained by allelic loss. Dermatology. 1997;195:20-25.
- Rabbin PE, Baldwin HE. Treatment of porokeratosis of Mibelli with CO2 laser vaporization versus surgical excision with split-thickness skin graft. a comparison. J Dermatol Surg Oncol. 1993;19:199-202.
- Spencer JM, Katz BE. Successful treatment of porokeratosis of Mibelli with diamond fraise dermabrasion. Arch Dermatol. 1992;128:1187-1188.
- Venkatarajan S, LeLeux TM, Yang D, et al. Porokeratosis of Mibelli: successful treatment with 5 percent topical imiquimod and topical 5 percent 5-fluorouracil. Dermatol Online J. 2010;16:10.
- McDonald SG, Peterka ES. Porokeratosis (Mibelli): treatment with topical 5-fluorouracil. J Am Acad Dermatol. 1983;8:107-110.
- Shumack SP, Commens CA. Disseminated superficial actinic porokeratosis: a clinical study. J Am Acad Dermatol. 1989;20:1015-1022.
- Parks AC, Conner KJ, Armstrong CA. Long-term clearance of linear porokeratosis with tacrolimus, 0.1%, ointment. JAMA Dermatol. 2014;150:194-196.
Practice Points
- Porokeratosis represents a heterogeneous group of skin disorders.
- Porokeratosis can be inherited in an autosomal-dominant pattern, though many patients lack a family history.
- The presence of a cornoid lamella is the characteristic finding of porokeratosis on histology.
- The rate of malignant transformation to squamous cell carcinoma is highest in linear porokeratosis, lowest in disseminated superficial actinic porokeratosis, and unreported in the punctate type.
Oral and Injectable Medications for Psoriasis: Benefits and Downsides Requiring Patient Support
Approximately three-quarters of respondents indicated that they have used oral or injectable medications (eg, methotrexate, acitretin, cyclosporine, apremilast, biologics) to control their psoriasis, according to a public meeting hosted by the US Food and Drug Administration (FDA) to hear patient perspectives on psoriasis. Approximately 70 psoriasis patients or patient representatives attended the meeting in person and others attended through a live webcast.
Patients universally spoke about the benefits of their current treatments, especially the biologics, but variable experiences regarding the effectiveness of the therapies were reported, ranging from excellent improvement, to improvement that lasted only a few months, to a near-complete clearance. However, limitations to these therapies also were mentioned, which are areas where dermatologists can provide counseling and alternatives. For example, treatments were reported to be effective in clearing cutaneous psoriasis symptoms such as flaking and scaling, but pruritus, burning, and pain were still problematic and mostly limited to areas where the cutaneous symptoms had been located.
Other treatment downsides that dermatologists should discuss with patients are side effects, including fatigue, nausea, fluctuations in weight, increased facial hair growth, nosebleeds, increased blood pressure, headaches, and palpitations, according to the patients present at the meeting. Patients also expressed concern about immune compromise from the biologics. Others reported concerns that the treatments addressed specific psoriasis symptoms but led to worsening of other symptoms or development of new conditions such as uveitis and psoriatic arthritis. The burden of treatment infusions or required blood work also were discussed. These are areas in which dermatologists may be best suited to provide more patient education or support when prescribing these therapies. The National Psoriasis Foundation’s Patient Navigation Center is a tool for patients to access information and interact with members of the psoriasis patient community.
The psoriasis public meeting in March 2016 was the FDA’s 18th patient-focused drug development meeting. The FDA sought this information to have a greater understanding of the burden of psoriasis on patients and the treatments currently used to treat psoriasis and its symptoms. This information will help guide the FDA as they consider future drug approvals.
Approximately three-quarters of respondents indicated that they have used oral or injectable medications (eg, methotrexate, acitretin, cyclosporine, apremilast, biologics) to control their psoriasis, according to a public meeting hosted by the US Food and Drug Administration (FDA) to hear patient perspectives on psoriasis. Approximately 70 psoriasis patients or patient representatives attended the meeting in person and others attended through a live webcast.
Patients universally spoke about the benefits of their current treatments, especially the biologics, but variable experiences regarding the effectiveness of the therapies were reported, ranging from excellent improvement, to improvement that lasted only a few months, to a near-complete clearance. However, limitations to these therapies also were mentioned, which are areas where dermatologists can provide counseling and alternatives. For example, treatments were reported to be effective in clearing cutaneous psoriasis symptoms such as flaking and scaling, but pruritus, burning, and pain were still problematic and mostly limited to areas where the cutaneous symptoms had been located.
Other treatment downsides that dermatologists should discuss with patients are side effects, including fatigue, nausea, fluctuations in weight, increased facial hair growth, nosebleeds, increased blood pressure, headaches, and palpitations, according to the patients present at the meeting. Patients also expressed concern about immune compromise from the biologics. Others reported concerns that the treatments addressed specific psoriasis symptoms but led to worsening of other symptoms or development of new conditions such as uveitis and psoriatic arthritis. The burden of treatment infusions or required blood work also were discussed. These are areas in which dermatologists may be best suited to provide more patient education or support when prescribing these therapies. The National Psoriasis Foundation’s Patient Navigation Center is a tool for patients to access information and interact with members of the psoriasis patient community.
The psoriasis public meeting in March 2016 was the FDA’s 18th patient-focused drug development meeting. The FDA sought this information to have a greater understanding of the burden of psoriasis on patients and the treatments currently used to treat psoriasis and its symptoms. This information will help guide the FDA as they consider future drug approvals.
Approximately three-quarters of respondents indicated that they have used oral or injectable medications (eg, methotrexate, acitretin, cyclosporine, apremilast, biologics) to control their psoriasis, according to a public meeting hosted by the US Food and Drug Administration (FDA) to hear patient perspectives on psoriasis. Approximately 70 psoriasis patients or patient representatives attended the meeting in person and others attended through a live webcast.
Patients universally spoke about the benefits of their current treatments, especially the biologics, but variable experiences regarding the effectiveness of the therapies were reported, ranging from excellent improvement, to improvement that lasted only a few months, to a near-complete clearance. However, limitations to these therapies also were mentioned, which are areas where dermatologists can provide counseling and alternatives. For example, treatments were reported to be effective in clearing cutaneous psoriasis symptoms such as flaking and scaling, but pruritus, burning, and pain were still problematic and mostly limited to areas where the cutaneous symptoms had been located.
Other treatment downsides that dermatologists should discuss with patients are side effects, including fatigue, nausea, fluctuations in weight, increased facial hair growth, nosebleeds, increased blood pressure, headaches, and palpitations, according to the patients present at the meeting. Patients also expressed concern about immune compromise from the biologics. Others reported concerns that the treatments addressed specific psoriasis symptoms but led to worsening of other symptoms or development of new conditions such as uveitis and psoriatic arthritis. The burden of treatment infusions or required blood work also were discussed. These are areas in which dermatologists may be best suited to provide more patient education or support when prescribing these therapies. The National Psoriasis Foundation’s Patient Navigation Center is a tool for patients to access information and interact with members of the psoriasis patient community.
The psoriasis public meeting in March 2016 was the FDA’s 18th patient-focused drug development meeting. The FDA sought this information to have a greater understanding of the burden of psoriasis on patients and the treatments currently used to treat psoriasis and its symptoms. This information will help guide the FDA as they consider future drug approvals.
The Syphilis Epidemic: Dermatologists on the Frontline of Treatment and Diagnosis
Debunking Actinic Keratosis Myths: Do All Actinic Keratoses Progress to Squamous Cell Carcinoma?
Myth: Hypertrophic actinic keratoses are more likely to progress to squamous cell carcinoma
Actinic keratosis (AK) indicates cumulative UV exposure and is the initial lesion in the majority of invasive cutaneous squamous cell carcinomas (SCCs). However, most AKs do not progress to invasive SCC and it currently is not possible to clinically or histopathologically determine which AK lesions will progress to SCC.
The rates of progression of individual AK lesions to SCC vary. In 2011, Feldman and Fleischer summarized 6 studies of 560 to 6691 patients with AK. The AK progression to SCC was found to range from 0.075% per year per lesion to 14% over 5 years.
Criscione et al found that the risk of progression of AK to primary SCC was 0.60% at 1 year and 2.57% at 4 years. In this study, 187 primary SCCs were diagnosed after enrollment, with 65% arising in previously clinically diagnosed and documented AKs. Therefore, although the authors noted low risks of progression of AK to SCC, they observed that the majority of SCCs arose from AKs.
The risk for progression of AK to invasive SCC with the potential for metastasis warrants treatment of AK with lesion- or field-directed therapy or a combined approach when indicated. Dermatologists also should monitor AK patients closely.
Expert Commentary
It’s a myth that hypertrophic lesions are more likely to turn into SCC. In fact, it’s the lesions with follicular extension that are more likely to progress to SCC. These may be hypertrophic or atrophic or simple AKs. The genetics of AKs that become hypertrophic may be different from those that become invasive. These lesions may be more likely to first grow outward before becoming invasive.
—Gary Goldenberg (New York, New York)
Criscione VD, Weinstock MA, Naylor MF, et al. Actinic keratoses: natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention trial. Cancer. 2009;115:2523-2530.
Feldman SR, Fleischer AB Jr. Progression of actinic keratosis to squamous cell carcinoma revisited: clinical and treatment implications. Cutis. 2011;87:201-207.
Pandey S, Mercer SE, Dallas K, et al. Evaluation of the prognostic significance of follicular extension in actinic keratoses. J Clin Aesthet Dermatol. 2012;5:25-28.
Myth: Hypertrophic actinic keratoses are more likely to progress to squamous cell carcinoma
Actinic keratosis (AK) indicates cumulative UV exposure and is the initial lesion in the majority of invasive cutaneous squamous cell carcinomas (SCCs). However, most AKs do not progress to invasive SCC and it currently is not possible to clinically or histopathologically determine which AK lesions will progress to SCC.
The rates of progression of individual AK lesions to SCC vary. In 2011, Feldman and Fleischer summarized 6 studies of 560 to 6691 patients with AK. The AK progression to SCC was found to range from 0.075% per year per lesion to 14% over 5 years.
Criscione et al found that the risk of progression of AK to primary SCC was 0.60% at 1 year and 2.57% at 4 years. In this study, 187 primary SCCs were diagnosed after enrollment, with 65% arising in previously clinically diagnosed and documented AKs. Therefore, although the authors noted low risks of progression of AK to SCC, they observed that the majority of SCCs arose from AKs.
The risk for progression of AK to invasive SCC with the potential for metastasis warrants treatment of AK with lesion- or field-directed therapy or a combined approach when indicated. Dermatologists also should monitor AK patients closely.
Expert Commentary
It’s a myth that hypertrophic lesions are more likely to turn into SCC. In fact, it’s the lesions with follicular extension that are more likely to progress to SCC. These may be hypertrophic or atrophic or simple AKs. The genetics of AKs that become hypertrophic may be different from those that become invasive. These lesions may be more likely to first grow outward before becoming invasive.
—Gary Goldenberg (New York, New York)
Myth: Hypertrophic actinic keratoses are more likely to progress to squamous cell carcinoma
Actinic keratosis (AK) indicates cumulative UV exposure and is the initial lesion in the majority of invasive cutaneous squamous cell carcinomas (SCCs). However, most AKs do not progress to invasive SCC and it currently is not possible to clinically or histopathologically determine which AK lesions will progress to SCC.
The rates of progression of individual AK lesions to SCC vary. In 2011, Feldman and Fleischer summarized 6 studies of 560 to 6691 patients with AK. The AK progression to SCC was found to range from 0.075% per year per lesion to 14% over 5 years.
Criscione et al found that the risk of progression of AK to primary SCC was 0.60% at 1 year and 2.57% at 4 years. In this study, 187 primary SCCs were diagnosed after enrollment, with 65% arising in previously clinically diagnosed and documented AKs. Therefore, although the authors noted low risks of progression of AK to SCC, they observed that the majority of SCCs arose from AKs.
The risk for progression of AK to invasive SCC with the potential for metastasis warrants treatment of AK with lesion- or field-directed therapy or a combined approach when indicated. Dermatologists also should monitor AK patients closely.
Expert Commentary
It’s a myth that hypertrophic lesions are more likely to turn into SCC. In fact, it’s the lesions with follicular extension that are more likely to progress to SCC. These may be hypertrophic or atrophic or simple AKs. The genetics of AKs that become hypertrophic may be different from those that become invasive. These lesions may be more likely to first grow outward before becoming invasive.
—Gary Goldenberg (New York, New York)
Criscione VD, Weinstock MA, Naylor MF, et al. Actinic keratoses: natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention trial. Cancer. 2009;115:2523-2530.
Feldman SR, Fleischer AB Jr. Progression of actinic keratosis to squamous cell carcinoma revisited: clinical and treatment implications. Cutis. 2011;87:201-207.
Pandey S, Mercer SE, Dallas K, et al. Evaluation of the prognostic significance of follicular extension in actinic keratoses. J Clin Aesthet Dermatol. 2012;5:25-28.
Criscione VD, Weinstock MA, Naylor MF, et al. Actinic keratoses: natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention trial. Cancer. 2009;115:2523-2530.
Feldman SR, Fleischer AB Jr. Progression of actinic keratosis to squamous cell carcinoma revisited: clinical and treatment implications. Cutis. 2011;87:201-207.
Pandey S, Mercer SE, Dallas K, et al. Evaluation of the prognostic significance of follicular extension in actinic keratoses. J Clin Aesthet Dermatol. 2012;5:25-28.