User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Lichen Planus Pemphigoides Treated With Ustekinumab
Case Report
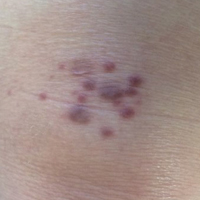
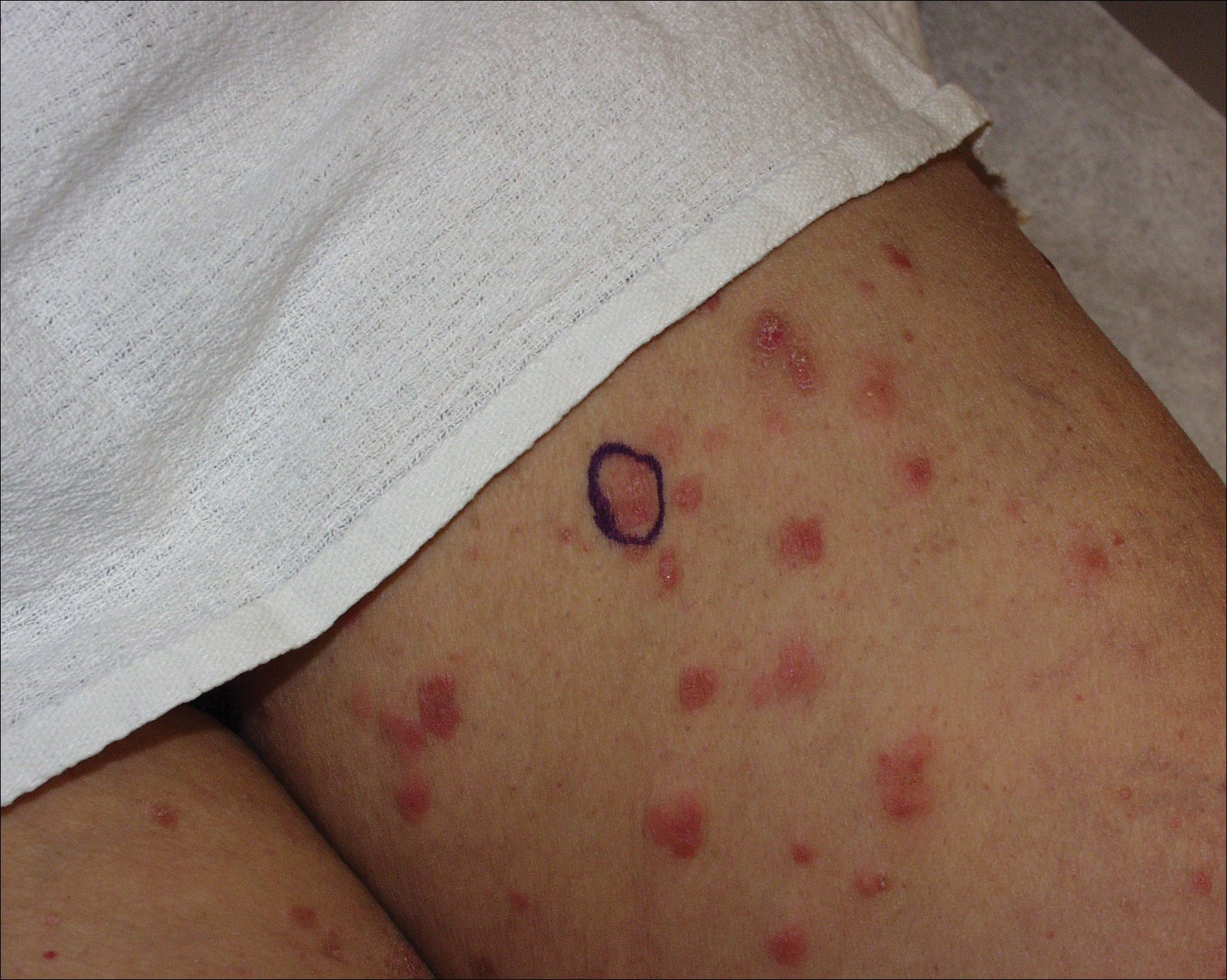
A 71-year-old woman presented with pink to violaceous, flat-topped, polygonal papules consistent with lichen planus (LP) on the volar wrists, extensor elbows, and bilateral lower legs of 3 years’ duration. She also had erythematous, violaceous, infiltrated plaques with microvesiculation on the bilateral thighs of several months’ duration (Figure 1). She reported pruritus, burning, and discomfort. Her medical history included type 2 diabetes mellitus, hypertension, and asthma with no history of skin rashes. A complete physical examination was performed. Age-appropriate screening for malignancy was negative. Hepatitis B and C antibody serologies were negative. Her medications at the time included risedronate and atenolol, which she had been taking for several years.
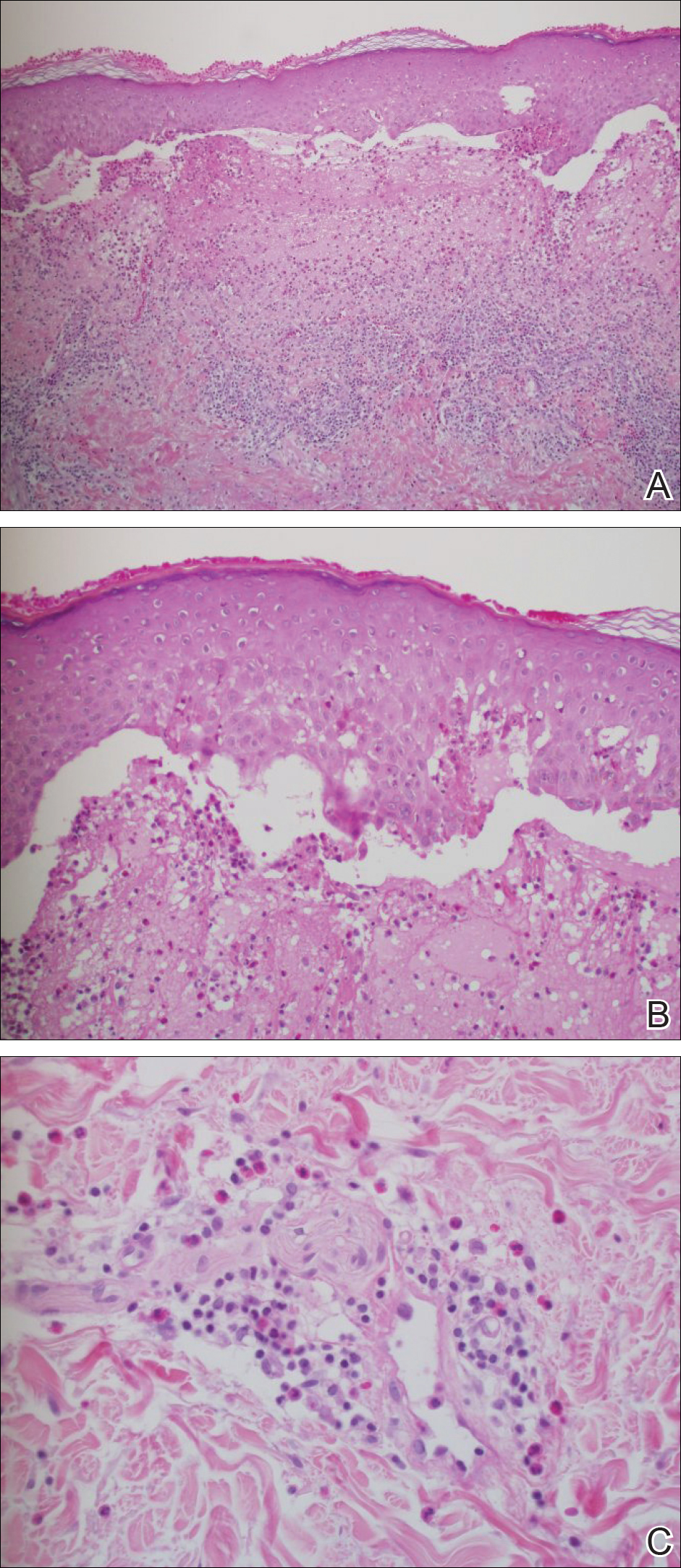
Punch biopsies from perilesional skin were submitted for hematoxylin and eosin staining and direct immunofluorescence (DIF). Histopathology showed a subepidermal blistering disease with tissue eosinophilia consistent with lichen planus pemphigoides (LPP)(Figure 2); direct immunofluorescence was positive for IgG, C3, and type IV collagen at the dermoepidermal junction. Serum BP180 was positive at 51 U/mL (reference range, <14 U/mL) and BP230 was negative. She was then started on tetracycline (500 mg twice daily), nicotinamide (500 mg twice daily), prednisone (5 mg daily), and dapsone (100 mg daily).
After 3 months without improvement, tetracycline and nicotinamide were discontinued, prednisone was increased to 10 mg daily, and dapsone was continued. A repeat biopsy was taken from a new area of involvement on the left lower leg, which revealed a psoriasiform dermatitis with interface changes. The DIF was positive for IgG and C3 along the basement membrane. A serum indirect immunofluorescence for BP180 also was positive.
The patient developed mild hemolytic anemia on dapsone; the medication was eventually discontinued. Subsequent treatments included adequate trials of azathioprine, mycophenolate mofetil, and hydroxychloroquine. Azathioprine (150 mg daily) and hydroxychloroquine (400 mg daily) treatment failed. She initially improved on mycophenolate mofetil (500 mg in the morning and 1000 mg in the evening) with flattening of the papules on the arms and legs and decreased erythema. However, mycophenolate mofetil eventually lost its efficacy and was discontinued.
Because several medications failed (ie, tetracycline, nicotinamide, prednisone, dapsone, azathioprine, mycophenolate mofetil, hydroxychloroquine), she was started on ustekinumab (45 mg) initial loading dose by subcutaneous injection (patient’s weight, 63 kg). At 4 weeks, the patient was given the second subcutaneous injection of ustekinumab (45 mg). She experienced marked improvement with no new lesions. The prior lesions also had decreased in size and were only slightly pink. The prednisone dose was tapered to 5 mg daily.
She had near-complete resolution of the skin lesions 12 weeks after the second dose of ustekinumab. Since then, she has had some recrudescence of the papulosquamous lesions but no vesicles or bullae. With the exception of occasional scattered pink papules on the forearms, her condition greatly improved on ustekinumab. She is no longer taking any of the other medications with the exception of prednisone (down to 1 mg daily) with a plan to gradually taper completely off of it.
Comment
Clinical Presentation
Lichen planus pemphigoides is a rare autoimmune subepidermal blistering disease with few cases reported in the literature. It is considered a clinical variation of bullous pemphigoid (BP) or a coexistence of LP and BP.1,2 It is characterized by bullous lesions developing on LP papules as well as on clinically uninvolved areas of the skin. It has been reported that LPP is provoked by several medications including cinnarizine, captopril, ramipril, simvastatin, psoralen plus UVA, and antituberculous medications (eg, isoniazid, rifampin, ethambutol, pyrazinamide).1 Risedronate or atenolol have not been reported to cause LPP, LP, or BP; however, according to Litt,3 a lichenoid drug eruption has been associated with atenolol. Furthermore, some cases of LPP demonstrate overlapping characteristics with paraneoplastic pemphigus and have been associated with internal malignancy. Hamada et al4 described a case of LPP coupled with colon adenocarcinoma and numerous keratoacanthomas. The earliest depiction of the coexistence of a case of mainstream LP complicated by an extensive bullous eruption was by Kaposi5 in 1892. He coined the term lichen ruber pemphigoides.5
Compared to BP, LPP is believed to affect a younger age group and have a less serious clinical course. The mean age of onset of LPP is in the third to fourth decades of life, while BP typically presents in the sixth decade. When comparing the location of bullae in LPP versus BP, the lesions of LPP tend to occur on the limbs, while BP tends to occur on the trunk.6
Clinically, LPP is distinguished by the existence of bullous lesions developing atop of the lesions of LP as well as on normal skin, with the latter being more commonplace. A classic example of LPP is characterized by an initial episode of traditional LP lesions often having severe pruritus, with or without patches of erythema, with the sudden eruption of tense bullae. These bullae commonly appear on the extremities and can appear over the normal skin, erythematous patches, or preexisting papules.7 In the atypical clinical presentations of this dubious skin condition, the bullae may only be seen on the lesions of LP.8 There also could be a lichenoid erythrodermic manifestation of a bullous eruption.9
Oral lesions of LPP have been described but had not been studied immunopathologically until Allen et al10 portrayed a 59-year-old man with cutaneous and oral lesions of LPP. They performed biopsies on the oral lesions and examined them by routine light microscopy and immunofluorescent techniques. The fine keratotic striae on the anterior buccal mucosal lesions were clinically consistent with oral LP. Perilesional tissue in conjunction with ulceration of the posterior buccal mucosa demonstrated histologic and immunopathologic alterations consistent with BP.10
Histopathology
Histopathologically, the lesions of LP show a bandlike lymphohistiocytic infiltrate, colloid bodies in the dermis, irregular acanthosis with saw-toothed rete ridges, orthokeratosis, wedge-shaped hypergranulosis, and liquefaction degeneration of the basal layer. Direct immunofluorescence shows mainly IgM and C3 deposited on colloid bodies, fibrin, and fibrinogen.11 The histopathology of the bullous lesion of LPP depicts a subepidermal bulla with variable diffuse or sparse lymphohistiocytic infiltrate and frequent eosinophils with or without neutrophils in the upper dermis. The existence of C3 alone or with IgG along the dermoepidermal junction gives confirmation on DIF.7
Autoantibodies
The expression of IgG autoantibodies directed against the basement membrane zone distinguishes LPP from bullous LP.2 IgG autoantibodies to either one or both the 230-kDa and 180-kDa BP (type XVII collagen) antigens has been demonstrated with LPP.4,12-14 Hamada et al4 described a histologic pattern more consistent with paraneoplastic pemphigus. It has been suggested that injury to the basal cells in LP or damage due to other courses of therapy such as psoralen plus UVA unveil suppressed antigenic determinants or produce new antigens, leading to antibody development and production of BP.12,15
Zillikens et al2 performed a study to identify the target antigen of LPP autoantibodies. They used sera from patients with LPP (n=4) and stained the epidermal side of salt-split human skin in a configuration identical to BP sera. In BP, the autoimmune response is directed against BP180, a hemidesmosomal transmembrane collagenous glycoprotein. They demonstrated that sera from BP patients largely reacted with a set of 4 epitopes (MCW-0 through MCW-3) grouped within a 45 amino acid stretch of the major noncollagenous extracellular domain (NC16A) of BP180. By immunoblotting and enzyme-linked immunosorbent assay, LPP sera also were compellingly reactive with recombinant BP180 NC16A. Lichen planus pemphigoides epitopes were additionally mapped using a series of overlapping recombinant segments of the NC16A domain. The authors demonstrated that all LPP sera reacted with amino acids 46 through 59 of domain NC16A, a protein portion that was previously shown to be unreactive with BP sera. In addition, they showed that 2 LPP sera reacted with the immunodominant antigenic region related to BP. Furthermore, they identified a unique epitope within the BP180 NC16A domain—MCW-4—which was distinctively recognized by sera from patients with LPP.2
Pathogenesis
The pathogenesis of both LP and BP has been linked to multiple cytokines that induce apoptosis in basal keratinocytes. Implicated cytokines include IFN-γ, tumor necrosis factor α (TNF-α), IL-1, IL-6, and IL-8, as well as other apoptosis-related molecules, such as Fas/Apo-1 and Bcl-2 in LP.16-18 Soluble E-selectin, vascular endothelial growth factor, IL-1β, IL-8, IL-5, transforming growth factor β1, and TNF-α were found to be elevated in either blister fluid or sera of BP patients.15-17
Management
Lichen planus pemphigoides usually responds well to traditional therapies, with systemic steroids being the most efficacious treatment of extensive disease.12,13 Other options include tetracycline and nicotinamide, isotretinoin, dapsone, and immunosuppressive drugs such as systemic cortico-steroids.12 Demirçay et al12 described a patient with skin lesions that rapidly cleared after the administration of oral methylprednisolone (48 mg/d) and oral dapsone (100 mg/d). The methylprednisolone and dapsone were withdrawn after 12 and 16 weeks, respectively. There was no recurrence during the 1-year follow-up period.12 et al19 described a patient who was treated with pulsed intravenous corticosteroids and continued to develop new papular and vesicular skin lesions. However, when oral acitretin was added to the patient’s regimen, the skin lesions cleared.19 There are several case reports of the successful use of hydroxychloroquine in LP.20,21
Cutaneous, nail, and oral LP also can be treated with TNF-α inhibitors (eg, adalimumab, etanercept) with resolution of lesions.22-25 However, we have not been able to find any reports of treating LPP with biologic medications in a search of PubMed articles indexed for MEDLINE using the terms lichen planus pemphigoides and biologic treatments/therapies. Given the fact that TNF-α and other inflammatory cytokines are involved in the pathogenesis of BP and LP, it is feasible that they also may be involved in the pathogenesis of LPP.
In our patient with cutaneous LPP, we chose to use ustekinumab instead of a primary TNF-α inhibitor because ustekinumab indirectly blocks TNF-α, as well as other proinflammatory cytokines such as IFN-γ, IL-17, and IL-22, which also could have played a role in the patient’s disease. Our goal was to use ustekinumab as a potential corticosteroid-sparing agent. Ustekinumab greatly improved her skin condition and allowed us to discontinue other medications.
- Harting MS, Hsu S. Lichen planus pemphigoides: a case report and review of the literature. Dermatol Online J. 2006;12:10.
- Zillikens D, Caux F, Mascaro JM, et al. Autoantibodies in lichen planus pemphigoides react with a novel epitope within the C-terminal NC16A domain of BP180. J Invest Dermatol. 1999;113:117-121.
- Litt J. Litt’s Drug Eruptions and Reactions Manual. 18th Ed. London, England: Informa Healthcare; 2011.
- Hamada T, Fujimoto W, Okazaki F, et al. Lichen planus pemphigoides and multiple keratoacanthomas associated with colon adenocarcinoma. Br J Dermatol. 2004;151:252-254.
- Kaposi M. Lichen ruber pemphigoides. Arch Derm Syph. 1892;343-346.
- Swale VJ, Black MM, Bhogal BS. Lichen planus pemphigoides: two case reports. Clin Exp Dermatol. 1998;23:132-135.
- Okochi H, Nashiro K, Tsuchida T, et al. Lichen planus pemphigoides: case reports and results of immunofluorescence and immunoelectron microscopic study. J Am Acad Dermatol. 1990;22:626-631.
- Mendiratta V, Asati DP, Koranne RV. Lichen planus pemphigoides in an Indian female. Indian J Dermatol. 2005;50:224-226.
- Joly P, Tanasescu S, Wolkenstein P, et al. Lichenoid erythrodermic bullous pemphigoid of the African patient. J Am Acad Dermatol. 1998;39:691-697.
- Allen , , R. Lichen planus pemphigoides: report of a case with oral lesions. Oral Surg Oral Med Oral Pathol. 1987;63:184-188.
- Rapini RP. Practical Dermatopathology. Philadelphia, PA: Mosby Elsevier; 2005.
- Demirçay Z, Baykal C, Demirkesen C. Lichen planus pemphigoides: report of two cases. Int J Dermatol. 2001;40:757-759.
- Sakuma-Oyama Y, Powell AM, Albert S, et al. Lichen planus pemphigoides evolving into pemphigoid nodularis. Clin Exp Dermatol. 2004;28:613-616.
- Hsu S, Ghohestani RF, Uitto J. Lichen planus pemphigoides with IgG autoantibodies to the 180kd bullous pemphigoid antigen (type XVII collagen). J Am Acad Dermatol. 2000;42:136-141.
- Kuramoto N, Kishimoto S, Shibagaki R, et al. PUVA-induced lichen planus pemphigoides. Br J Dermatol. 2000;142:509-512.
- Ameglio F, D’Auria L, Cordiali-Fei P, et al. Bullous pemphigoid and pemphigus vulgaris: correlated behaviour of serum VEGF, sE-selectin and TNF-alpha levels. J Biol Regul Homeost Agents. 1997;11:148-153.
- Ameglio F, D’auria L, Bonifati C, et al. Cytokine pattern in blister fluid and serum of patients with bullous pemphigoid: relationships with disease intensity. Br J Dermatol. 1998;138:611-614.
- D’Auria L, Mussi A, Bonifati C, et al. Increased serum IL-6, TNF-alpha and IL-10 levels in patients with bullous pemphigoid: relationships with disease activity. J Eur Acad Dermatol Venereol. 1999;12:11-15.
- , ,, . Treatment of lichen planus pemphigoides with acitretin and pulsed corticosteroids. Hautarzt. 2003;54:268-273.
- Eisen D. Hydroxychloroquine sulfate (Plaquenil) improves oral lichen planus: an open trial. J Am Acad Dermatol. 1993;28:609-612.
- James WD, Berger T, Elston D. Andrews’ Diseases of the Skin. 11th ed. Philadelphia, PA: Mosby Elsevier; 2011.
- Holló P, Szakonyi J, Kiss D, et al. Successful treatment of lichen planus with adalimumab. Acta Derm Venereol. 2012;92:385-386.
- Yarom N. Etanercept for the management of oral lichen planus. Am J Clin Dermatol. 2007;8:121.
- Chao TJ. Adalimumab in the management of cutaneous and oral lichen planus. Cutis. 2009;84:325-328.
- Irla N, Schneiter T, Haneke E, et al. Nail lichen planus: successful treatment with etanercept. Case Rep Dermatol. 2010;2:173-176.
Case Report
A 71-year-old woman presented with pink to violaceous, flat-topped, polygonal papules consistent with lichen planus (LP) on the volar wrists, extensor elbows, and bilateral lower legs of 3 years’ duration. She also had erythematous, violaceous, infiltrated plaques with microvesiculation on the bilateral thighs of several months’ duration (Figure 1). She reported pruritus, burning, and discomfort. Her medical history included type 2 diabetes mellitus, hypertension, and asthma with no history of skin rashes. A complete physical examination was performed. Age-appropriate screening for malignancy was negative. Hepatitis B and C antibody serologies were negative. Her medications at the time included risedronate and atenolol, which she had been taking for several years.

Punch biopsies from perilesional skin were submitted for hematoxylin and eosin staining and direct immunofluorescence (DIF). Histopathology showed a subepidermal blistering disease with tissue eosinophilia consistent with lichen planus pemphigoides (LPP)(Figure 2); direct immunofluorescence was positive for IgG, C3, and type IV collagen at the dermoepidermal junction. Serum BP180 was positive at 51 U/mL (reference range, <14 U/mL) and BP230 was negative. She was then started on tetracycline (500 mg twice daily), nicotinamide (500 mg twice daily), prednisone (5 mg daily), and dapsone (100 mg daily).
After 3 months without improvement, tetracycline and nicotinamide were discontinued, prednisone was increased to 10 mg daily, and dapsone was continued. A repeat biopsy was taken from a new area of involvement on the left lower leg, which revealed a psoriasiform dermatitis with interface changes. The DIF was positive for IgG and C3 along the basement membrane. A serum indirect immunofluorescence for BP180 also was positive.

The patient developed mild hemolytic anemia on dapsone; the medication was eventually discontinued. Subsequent treatments included adequate trials of azathioprine, mycophenolate mofetil, and hydroxychloroquine. Azathioprine (150 mg daily) and hydroxychloroquine (400 mg daily) treatment failed. She initially improved on mycophenolate mofetil (500 mg in the morning and 1000 mg in the evening) with flattening of the papules on the arms and legs and decreased erythema. However, mycophenolate mofetil eventually lost its efficacy and was discontinued.
Because several medications failed (ie, tetracycline, nicotinamide, prednisone, dapsone, azathioprine, mycophenolate mofetil, hydroxychloroquine), she was started on ustekinumab (45 mg) initial loading dose by subcutaneous injection (patient’s weight, 63 kg). At 4 weeks, the patient was given the second subcutaneous injection of ustekinumab (45 mg). She experienced marked improvement with no new lesions. The prior lesions also had decreased in size and were only slightly pink. The prednisone dose was tapered to 5 mg daily.
She had near-complete resolution of the skin lesions 12 weeks after the second dose of ustekinumab. Since then, she has had some recrudescence of the papulosquamous lesions but no vesicles or bullae. With the exception of occasional scattered pink papules on the forearms, her condition greatly improved on ustekinumab. She is no longer taking any of the other medications with the exception of prednisone (down to 1 mg daily) with a plan to gradually taper completely off of it.
Comment
Clinical Presentation
Lichen planus pemphigoides is a rare autoimmune subepidermal blistering disease with few cases reported in the literature. It is considered a clinical variation of bullous pemphigoid (BP) or a coexistence of LP and BP.1,2 It is characterized by bullous lesions developing on LP papules as well as on clinically uninvolved areas of the skin. It has been reported that LPP is provoked by several medications including cinnarizine, captopril, ramipril, simvastatin, psoralen plus UVA, and antituberculous medications (eg, isoniazid, rifampin, ethambutol, pyrazinamide).1 Risedronate or atenolol have not been reported to cause LPP, LP, or BP; however, according to Litt,3 a lichenoid drug eruption has been associated with atenolol. Furthermore, some cases of LPP demonstrate overlapping characteristics with paraneoplastic pemphigus and have been associated with internal malignancy. Hamada et al4 described a case of LPP coupled with colon adenocarcinoma and numerous keratoacanthomas. The earliest depiction of the coexistence of a case of mainstream LP complicated by an extensive bullous eruption was by Kaposi5 in 1892. He coined the term lichen ruber pemphigoides.5
Compared to BP, LPP is believed to affect a younger age group and have a less serious clinical course. The mean age of onset of LPP is in the third to fourth decades of life, while BP typically presents in the sixth decade. When comparing the location of bullae in LPP versus BP, the lesions of LPP tend to occur on the limbs, while BP tends to occur on the trunk.6
Clinically, LPP is distinguished by the existence of bullous lesions developing atop of the lesions of LP as well as on normal skin, with the latter being more commonplace. A classic example of LPP is characterized by an initial episode of traditional LP lesions often having severe pruritus, with or without patches of erythema, with the sudden eruption of tense bullae. These bullae commonly appear on the extremities and can appear over the normal skin, erythematous patches, or preexisting papules.7 In the atypical clinical presentations of this dubious skin condition, the bullae may only be seen on the lesions of LP.8 There also could be a lichenoid erythrodermic manifestation of a bullous eruption.9
Oral lesions of LPP have been described but had not been studied immunopathologically until Allen et al10 portrayed a 59-year-old man with cutaneous and oral lesions of LPP. They performed biopsies on the oral lesions and examined them by routine light microscopy and immunofluorescent techniques. The fine keratotic striae on the anterior buccal mucosal lesions were clinically consistent with oral LP. Perilesional tissue in conjunction with ulceration of the posterior buccal mucosa demonstrated histologic and immunopathologic alterations consistent with BP.10
Histopathology
Histopathologically, the lesions of LP show a bandlike lymphohistiocytic infiltrate, colloid bodies in the dermis, irregular acanthosis with saw-toothed rete ridges, orthokeratosis, wedge-shaped hypergranulosis, and liquefaction degeneration of the basal layer. Direct immunofluorescence shows mainly IgM and C3 deposited on colloid bodies, fibrin, and fibrinogen.11 The histopathology of the bullous lesion of LPP depicts a subepidermal bulla with variable diffuse or sparse lymphohistiocytic infiltrate and frequent eosinophils with or without neutrophils in the upper dermis. The existence of C3 alone or with IgG along the dermoepidermal junction gives confirmation on DIF.7
Autoantibodies
The expression of IgG autoantibodies directed against the basement membrane zone distinguishes LPP from bullous LP.2 IgG autoantibodies to either one or both the 230-kDa and 180-kDa BP (type XVII collagen) antigens has been demonstrated with LPP.4,12-14 Hamada et al4 described a histologic pattern more consistent with paraneoplastic pemphigus. It has been suggested that injury to the basal cells in LP or damage due to other courses of therapy such as psoralen plus UVA unveil suppressed antigenic determinants or produce new antigens, leading to antibody development and production of BP.12,15
Zillikens et al2 performed a study to identify the target antigen of LPP autoantibodies. They used sera from patients with LPP (n=4) and stained the epidermal side of salt-split human skin in a configuration identical to BP sera. In BP, the autoimmune response is directed against BP180, a hemidesmosomal transmembrane collagenous glycoprotein. They demonstrated that sera from BP patients largely reacted with a set of 4 epitopes (MCW-0 through MCW-3) grouped within a 45 amino acid stretch of the major noncollagenous extracellular domain (NC16A) of BP180. By immunoblotting and enzyme-linked immunosorbent assay, LPP sera also were compellingly reactive with recombinant BP180 NC16A. Lichen planus pemphigoides epitopes were additionally mapped using a series of overlapping recombinant segments of the NC16A domain. The authors demonstrated that all LPP sera reacted with amino acids 46 through 59 of domain NC16A, a protein portion that was previously shown to be unreactive with BP sera. In addition, they showed that 2 LPP sera reacted with the immunodominant antigenic region related to BP. Furthermore, they identified a unique epitope within the BP180 NC16A domain—MCW-4—which was distinctively recognized by sera from patients with LPP.2
Pathogenesis
The pathogenesis of both LP and BP has been linked to multiple cytokines that induce apoptosis in basal keratinocytes. Implicated cytokines include IFN-γ, tumor necrosis factor α (TNF-α), IL-1, IL-6, and IL-8, as well as other apoptosis-related molecules, such as Fas/Apo-1 and Bcl-2 in LP.16-18 Soluble E-selectin, vascular endothelial growth factor, IL-1β, IL-8, IL-5, transforming growth factor β1, and TNF-α were found to be elevated in either blister fluid or sera of BP patients.15-17
Management
Lichen planus pemphigoides usually responds well to traditional therapies, with systemic steroids being the most efficacious treatment of extensive disease.12,13 Other options include tetracycline and nicotinamide, isotretinoin, dapsone, and immunosuppressive drugs such as systemic cortico-steroids.12 Demirçay et al12 described a patient with skin lesions that rapidly cleared after the administration of oral methylprednisolone (48 mg/d) and oral dapsone (100 mg/d). The methylprednisolone and dapsone were withdrawn after 12 and 16 weeks, respectively. There was no recurrence during the 1-year follow-up period.12 et al19 described a patient who was treated with pulsed intravenous corticosteroids and continued to develop new papular and vesicular skin lesions. However, when oral acitretin was added to the patient’s regimen, the skin lesions cleared.19 There are several case reports of the successful use of hydroxychloroquine in LP.20,21
Cutaneous, nail, and oral LP also can be treated with TNF-α inhibitors (eg, adalimumab, etanercept) with resolution of lesions.22-25 However, we have not been able to find any reports of treating LPP with biologic medications in a search of PubMed articles indexed for MEDLINE using the terms lichen planus pemphigoides and biologic treatments/therapies. Given the fact that TNF-α and other inflammatory cytokines are involved in the pathogenesis of BP and LP, it is feasible that they also may be involved in the pathogenesis of LPP.
In our patient with cutaneous LPP, we chose to use ustekinumab instead of a primary TNF-α inhibitor because ustekinumab indirectly blocks TNF-α, as well as other proinflammatory cytokines such as IFN-γ, IL-17, and IL-22, which also could have played a role in the patient’s disease. Our goal was to use ustekinumab as a potential corticosteroid-sparing agent. Ustekinumab greatly improved her skin condition and allowed us to discontinue other medications.
Case Report
A 71-year-old woman presented with pink to violaceous, flat-topped, polygonal papules consistent with lichen planus (LP) on the volar wrists, extensor elbows, and bilateral lower legs of 3 years’ duration. She also had erythematous, violaceous, infiltrated plaques with microvesiculation on the bilateral thighs of several months’ duration (Figure 1). She reported pruritus, burning, and discomfort. Her medical history included type 2 diabetes mellitus, hypertension, and asthma with no history of skin rashes. A complete physical examination was performed. Age-appropriate screening for malignancy was negative. Hepatitis B and C antibody serologies were negative. Her medications at the time included risedronate and atenolol, which she had been taking for several years.

Punch biopsies from perilesional skin were submitted for hematoxylin and eosin staining and direct immunofluorescence (DIF). Histopathology showed a subepidermal blistering disease with tissue eosinophilia consistent with lichen planus pemphigoides (LPP)(Figure 2); direct immunofluorescence was positive for IgG, C3, and type IV collagen at the dermoepidermal junction. Serum BP180 was positive at 51 U/mL (reference range, <14 U/mL) and BP230 was negative. She was then started on tetracycline (500 mg twice daily), nicotinamide (500 mg twice daily), prednisone (5 mg daily), and dapsone (100 mg daily).
After 3 months without improvement, tetracycline and nicotinamide were discontinued, prednisone was increased to 10 mg daily, and dapsone was continued. A repeat biopsy was taken from a new area of involvement on the left lower leg, which revealed a psoriasiform dermatitis with interface changes. The DIF was positive for IgG and C3 along the basement membrane. A serum indirect immunofluorescence for BP180 also was positive.

The patient developed mild hemolytic anemia on dapsone; the medication was eventually discontinued. Subsequent treatments included adequate trials of azathioprine, mycophenolate mofetil, and hydroxychloroquine. Azathioprine (150 mg daily) and hydroxychloroquine (400 mg daily) treatment failed. She initially improved on mycophenolate mofetil (500 mg in the morning and 1000 mg in the evening) with flattening of the papules on the arms and legs and decreased erythema. However, mycophenolate mofetil eventually lost its efficacy and was discontinued.
Because several medications failed (ie, tetracycline, nicotinamide, prednisone, dapsone, azathioprine, mycophenolate mofetil, hydroxychloroquine), she was started on ustekinumab (45 mg) initial loading dose by subcutaneous injection (patient’s weight, 63 kg). At 4 weeks, the patient was given the second subcutaneous injection of ustekinumab (45 mg). She experienced marked improvement with no new lesions. The prior lesions also had decreased in size and were only slightly pink. The prednisone dose was tapered to 5 mg daily.
She had near-complete resolution of the skin lesions 12 weeks after the second dose of ustekinumab. Since then, she has had some recrudescence of the papulosquamous lesions but no vesicles or bullae. With the exception of occasional scattered pink papules on the forearms, her condition greatly improved on ustekinumab. She is no longer taking any of the other medications with the exception of prednisone (down to 1 mg daily) with a plan to gradually taper completely off of it.
Comment
Clinical Presentation
Lichen planus pemphigoides is a rare autoimmune subepidermal blistering disease with few cases reported in the literature. It is considered a clinical variation of bullous pemphigoid (BP) or a coexistence of LP and BP.1,2 It is characterized by bullous lesions developing on LP papules as well as on clinically uninvolved areas of the skin. It has been reported that LPP is provoked by several medications including cinnarizine, captopril, ramipril, simvastatin, psoralen plus UVA, and antituberculous medications (eg, isoniazid, rifampin, ethambutol, pyrazinamide).1 Risedronate or atenolol have not been reported to cause LPP, LP, or BP; however, according to Litt,3 a lichenoid drug eruption has been associated with atenolol. Furthermore, some cases of LPP demonstrate overlapping characteristics with paraneoplastic pemphigus and have been associated with internal malignancy. Hamada et al4 described a case of LPP coupled with colon adenocarcinoma and numerous keratoacanthomas. The earliest depiction of the coexistence of a case of mainstream LP complicated by an extensive bullous eruption was by Kaposi5 in 1892. He coined the term lichen ruber pemphigoides.5
Compared to BP, LPP is believed to affect a younger age group and have a less serious clinical course. The mean age of onset of LPP is in the third to fourth decades of life, while BP typically presents in the sixth decade. When comparing the location of bullae in LPP versus BP, the lesions of LPP tend to occur on the limbs, while BP tends to occur on the trunk.6
Clinically, LPP is distinguished by the existence of bullous lesions developing atop of the lesions of LP as well as on normal skin, with the latter being more commonplace. A classic example of LPP is characterized by an initial episode of traditional LP lesions often having severe pruritus, with or without patches of erythema, with the sudden eruption of tense bullae. These bullae commonly appear on the extremities and can appear over the normal skin, erythematous patches, or preexisting papules.7 In the atypical clinical presentations of this dubious skin condition, the bullae may only be seen on the lesions of LP.8 There also could be a lichenoid erythrodermic manifestation of a bullous eruption.9
Oral lesions of LPP have been described but had not been studied immunopathologically until Allen et al10 portrayed a 59-year-old man with cutaneous and oral lesions of LPP. They performed biopsies on the oral lesions and examined them by routine light microscopy and immunofluorescent techniques. The fine keratotic striae on the anterior buccal mucosal lesions were clinically consistent with oral LP. Perilesional tissue in conjunction with ulceration of the posterior buccal mucosa demonstrated histologic and immunopathologic alterations consistent with BP.10
Histopathology
Histopathologically, the lesions of LP show a bandlike lymphohistiocytic infiltrate, colloid bodies in the dermis, irregular acanthosis with saw-toothed rete ridges, orthokeratosis, wedge-shaped hypergranulosis, and liquefaction degeneration of the basal layer. Direct immunofluorescence shows mainly IgM and C3 deposited on colloid bodies, fibrin, and fibrinogen.11 The histopathology of the bullous lesion of LPP depicts a subepidermal bulla with variable diffuse or sparse lymphohistiocytic infiltrate and frequent eosinophils with or without neutrophils in the upper dermis. The existence of C3 alone or with IgG along the dermoepidermal junction gives confirmation on DIF.7
Autoantibodies
The expression of IgG autoantibodies directed against the basement membrane zone distinguishes LPP from bullous LP.2 IgG autoantibodies to either one or both the 230-kDa and 180-kDa BP (type XVII collagen) antigens has been demonstrated with LPP.4,12-14 Hamada et al4 described a histologic pattern more consistent with paraneoplastic pemphigus. It has been suggested that injury to the basal cells in LP or damage due to other courses of therapy such as psoralen plus UVA unveil suppressed antigenic determinants or produce new antigens, leading to antibody development and production of BP.12,15
Zillikens et al2 performed a study to identify the target antigen of LPP autoantibodies. They used sera from patients with LPP (n=4) and stained the epidermal side of salt-split human skin in a configuration identical to BP sera. In BP, the autoimmune response is directed against BP180, a hemidesmosomal transmembrane collagenous glycoprotein. They demonstrated that sera from BP patients largely reacted with a set of 4 epitopes (MCW-0 through MCW-3) grouped within a 45 amino acid stretch of the major noncollagenous extracellular domain (NC16A) of BP180. By immunoblotting and enzyme-linked immunosorbent assay, LPP sera also were compellingly reactive with recombinant BP180 NC16A. Lichen planus pemphigoides epitopes were additionally mapped using a series of overlapping recombinant segments of the NC16A domain. The authors demonstrated that all LPP sera reacted with amino acids 46 through 59 of domain NC16A, a protein portion that was previously shown to be unreactive with BP sera. In addition, they showed that 2 LPP sera reacted with the immunodominant antigenic region related to BP. Furthermore, they identified a unique epitope within the BP180 NC16A domain—MCW-4—which was distinctively recognized by sera from patients with LPP.2
Pathogenesis
The pathogenesis of both LP and BP has been linked to multiple cytokines that induce apoptosis in basal keratinocytes. Implicated cytokines include IFN-γ, tumor necrosis factor α (TNF-α), IL-1, IL-6, and IL-8, as well as other apoptosis-related molecules, such as Fas/Apo-1 and Bcl-2 in LP.16-18 Soluble E-selectin, vascular endothelial growth factor, IL-1β, IL-8, IL-5, transforming growth factor β1, and TNF-α were found to be elevated in either blister fluid or sera of BP patients.15-17
Management
Lichen planus pemphigoides usually responds well to traditional therapies, with systemic steroids being the most efficacious treatment of extensive disease.12,13 Other options include tetracycline and nicotinamide, isotretinoin, dapsone, and immunosuppressive drugs such as systemic cortico-steroids.12 Demirçay et al12 described a patient with skin lesions that rapidly cleared after the administration of oral methylprednisolone (48 mg/d) and oral dapsone (100 mg/d). The methylprednisolone and dapsone were withdrawn after 12 and 16 weeks, respectively. There was no recurrence during the 1-year follow-up period.12 et al19 described a patient who was treated with pulsed intravenous corticosteroids and continued to develop new papular and vesicular skin lesions. However, when oral acitretin was added to the patient’s regimen, the skin lesions cleared.19 There are several case reports of the successful use of hydroxychloroquine in LP.20,21
Cutaneous, nail, and oral LP also can be treated with TNF-α inhibitors (eg, adalimumab, etanercept) with resolution of lesions.22-25 However, we have not been able to find any reports of treating LPP with biologic medications in a search of PubMed articles indexed for MEDLINE using the terms lichen planus pemphigoides and biologic treatments/therapies. Given the fact that TNF-α and other inflammatory cytokines are involved in the pathogenesis of BP and LP, it is feasible that they also may be involved in the pathogenesis of LPP.
In our patient with cutaneous LPP, we chose to use ustekinumab instead of a primary TNF-α inhibitor because ustekinumab indirectly blocks TNF-α, as well as other proinflammatory cytokines such as IFN-γ, IL-17, and IL-22, which also could have played a role in the patient’s disease. Our goal was to use ustekinumab as a potential corticosteroid-sparing agent. Ustekinumab greatly improved her skin condition and allowed us to discontinue other medications.
- Harting MS, Hsu S. Lichen planus pemphigoides: a case report and review of the literature. Dermatol Online J. 2006;12:10.
- Zillikens D, Caux F, Mascaro JM, et al. Autoantibodies in lichen planus pemphigoides react with a novel epitope within the C-terminal NC16A domain of BP180. J Invest Dermatol. 1999;113:117-121.
- Litt J. Litt’s Drug Eruptions and Reactions Manual. 18th Ed. London, England: Informa Healthcare; 2011.
- Hamada T, Fujimoto W, Okazaki F, et al. Lichen planus pemphigoides and multiple keratoacanthomas associated with colon adenocarcinoma. Br J Dermatol. 2004;151:252-254.
- Kaposi M. Lichen ruber pemphigoides. Arch Derm Syph. 1892;343-346.
- Swale VJ, Black MM, Bhogal BS. Lichen planus pemphigoides: two case reports. Clin Exp Dermatol. 1998;23:132-135.
- Okochi H, Nashiro K, Tsuchida T, et al. Lichen planus pemphigoides: case reports and results of immunofluorescence and immunoelectron microscopic study. J Am Acad Dermatol. 1990;22:626-631.
- Mendiratta V, Asati DP, Koranne RV. Lichen planus pemphigoides in an Indian female. Indian J Dermatol. 2005;50:224-226.
- Joly P, Tanasescu S, Wolkenstein P, et al. Lichenoid erythrodermic bullous pemphigoid of the African patient. J Am Acad Dermatol. 1998;39:691-697.
- Allen , , R. Lichen planus pemphigoides: report of a case with oral lesions. Oral Surg Oral Med Oral Pathol. 1987;63:184-188.
- Rapini RP. Practical Dermatopathology. Philadelphia, PA: Mosby Elsevier; 2005.
- Demirçay Z, Baykal C, Demirkesen C. Lichen planus pemphigoides: report of two cases. Int J Dermatol. 2001;40:757-759.
- Sakuma-Oyama Y, Powell AM, Albert S, et al. Lichen planus pemphigoides evolving into pemphigoid nodularis. Clin Exp Dermatol. 2004;28:613-616.
- Hsu S, Ghohestani RF, Uitto J. Lichen planus pemphigoides with IgG autoantibodies to the 180kd bullous pemphigoid antigen (type XVII collagen). J Am Acad Dermatol. 2000;42:136-141.
- Kuramoto N, Kishimoto S, Shibagaki R, et al. PUVA-induced lichen planus pemphigoides. Br J Dermatol. 2000;142:509-512.
- Ameglio F, D’Auria L, Cordiali-Fei P, et al. Bullous pemphigoid and pemphigus vulgaris: correlated behaviour of serum VEGF, sE-selectin and TNF-alpha levels. J Biol Regul Homeost Agents. 1997;11:148-153.
- Ameglio F, D’auria L, Bonifati C, et al. Cytokine pattern in blister fluid and serum of patients with bullous pemphigoid: relationships with disease intensity. Br J Dermatol. 1998;138:611-614.
- D’Auria L, Mussi A, Bonifati C, et al. Increased serum IL-6, TNF-alpha and IL-10 levels in patients with bullous pemphigoid: relationships with disease activity. J Eur Acad Dermatol Venereol. 1999;12:11-15.
- , ,, . Treatment of lichen planus pemphigoides with acitretin and pulsed corticosteroids. Hautarzt. 2003;54:268-273.
- Eisen D. Hydroxychloroquine sulfate (Plaquenil) improves oral lichen planus: an open trial. J Am Acad Dermatol. 1993;28:609-612.
- James WD, Berger T, Elston D. Andrews’ Diseases of the Skin. 11th ed. Philadelphia, PA: Mosby Elsevier; 2011.
- Holló P, Szakonyi J, Kiss D, et al. Successful treatment of lichen planus with adalimumab. Acta Derm Venereol. 2012;92:385-386.
- Yarom N. Etanercept for the management of oral lichen planus. Am J Clin Dermatol. 2007;8:121.
- Chao TJ. Adalimumab in the management of cutaneous and oral lichen planus. Cutis. 2009;84:325-328.
- Irla N, Schneiter T, Haneke E, et al. Nail lichen planus: successful treatment with etanercept. Case Rep Dermatol. 2010;2:173-176.
- Harting MS, Hsu S. Lichen planus pemphigoides: a case report and review of the literature. Dermatol Online J. 2006;12:10.
- Zillikens D, Caux F, Mascaro JM, et al. Autoantibodies in lichen planus pemphigoides react with a novel epitope within the C-terminal NC16A domain of BP180. J Invest Dermatol. 1999;113:117-121.
- Litt J. Litt’s Drug Eruptions and Reactions Manual. 18th Ed. London, England: Informa Healthcare; 2011.
- Hamada T, Fujimoto W, Okazaki F, et al. Lichen planus pemphigoides and multiple keratoacanthomas associated with colon adenocarcinoma. Br J Dermatol. 2004;151:252-254.
- Kaposi M. Lichen ruber pemphigoides. Arch Derm Syph. 1892;343-346.
- Swale VJ, Black MM, Bhogal BS. Lichen planus pemphigoides: two case reports. Clin Exp Dermatol. 1998;23:132-135.
- Okochi H, Nashiro K, Tsuchida T, et al. Lichen planus pemphigoides: case reports and results of immunofluorescence and immunoelectron microscopic study. J Am Acad Dermatol. 1990;22:626-631.
- Mendiratta V, Asati DP, Koranne RV. Lichen planus pemphigoides in an Indian female. Indian J Dermatol. 2005;50:224-226.
- Joly P, Tanasescu S, Wolkenstein P, et al. Lichenoid erythrodermic bullous pemphigoid of the African patient. J Am Acad Dermatol. 1998;39:691-697.
- Allen , , R. Lichen planus pemphigoides: report of a case with oral lesions. Oral Surg Oral Med Oral Pathol. 1987;63:184-188.
- Rapini RP. Practical Dermatopathology. Philadelphia, PA: Mosby Elsevier; 2005.
- Demirçay Z, Baykal C, Demirkesen C. Lichen planus pemphigoides: report of two cases. Int J Dermatol. 2001;40:757-759.
- Sakuma-Oyama Y, Powell AM, Albert S, et al. Lichen planus pemphigoides evolving into pemphigoid nodularis. Clin Exp Dermatol. 2004;28:613-616.
- Hsu S, Ghohestani RF, Uitto J. Lichen planus pemphigoides with IgG autoantibodies to the 180kd bullous pemphigoid antigen (type XVII collagen). J Am Acad Dermatol. 2000;42:136-141.
- Kuramoto N, Kishimoto S, Shibagaki R, et al. PUVA-induced lichen planus pemphigoides. Br J Dermatol. 2000;142:509-512.
- Ameglio F, D’Auria L, Cordiali-Fei P, et al. Bullous pemphigoid and pemphigus vulgaris: correlated behaviour of serum VEGF, sE-selectin and TNF-alpha levels. J Biol Regul Homeost Agents. 1997;11:148-153.
- Ameglio F, D’auria L, Bonifati C, et al. Cytokine pattern in blister fluid and serum of patients with bullous pemphigoid: relationships with disease intensity. Br J Dermatol. 1998;138:611-614.
- D’Auria L, Mussi A, Bonifati C, et al. Increased serum IL-6, TNF-alpha and IL-10 levels in patients with bullous pemphigoid: relationships with disease activity. J Eur Acad Dermatol Venereol. 1999;12:11-15.
- , ,, . Treatment of lichen planus pemphigoides with acitretin and pulsed corticosteroids. Hautarzt. 2003;54:268-273.
- Eisen D. Hydroxychloroquine sulfate (Plaquenil) improves oral lichen planus: an open trial. J Am Acad Dermatol. 1993;28:609-612.
- James WD, Berger T, Elston D. Andrews’ Diseases of the Skin. 11th ed. Philadelphia, PA: Mosby Elsevier; 2011.
- Holló P, Szakonyi J, Kiss D, et al. Successful treatment of lichen planus with adalimumab. Acta Derm Venereol. 2012;92:385-386.
- Yarom N. Etanercept for the management of oral lichen planus. Am J Clin Dermatol. 2007;8:121.
- Chao TJ. Adalimumab in the management of cutaneous and oral lichen planus. Cutis. 2009;84:325-328.
- Irla N, Schneiter T, Haneke E, et al. Nail lichen planus: successful treatment with etanercept. Case Rep Dermatol. 2010;2:173-176.
- Lichen planus pemphigoides (LPP) is a rare autoimmune subepidermal blistering disease with few cases reported in the literature.
- Because tumor necrosis factor 11α (TNF-11α) and other inflammatory cytokines are involved in the pathogenesis of bullous pemphigoid and lichen planus, it is feasible that they also may be involved in the pathogenesis of LPP.
- Ustekinumab may be used to treat LPP as a potential corticosteroid-sparing agent because it indirectly blocks TNF-α, as well as other proinflammatory cytokines such as IFN-γ, IL-17, and IL-22.
Student Loan Burden and Its Impact on Career Decisions in Dermatology
Dermatology departments in the United States have been facing challenges in recruiting and retaining dermatologists for academic positions. Accordingly, a survey study reported that academic dermatologists were more likely than those in private practice to state that their institutions were recruiting new associates.1 Several factors could explain this phenomenon. Salary differences between jobs in academic and nonacademic settings may contribute to difficulty in recruiting dermatologists into academia, which is exacerbated by a theoretical shortage of dermatologists, leading to graduates who receive and accept private practice job offers.1,2 Furthermore, a large survey study reported that challenges unique to academic dermatologists include longer patient wait times in addition to responsibilities such as research, hospital consultations, medical writing, and teaching. These patterns raise concerns for the future of teaching institutions because academic dermatologists not only train future physicians but also conduct clinical and basic science research necessary to advance the field and improve patient care.2 Thus, it is important to evaluate the factors that affect career decisions in dermatology and to determine if these factors can be addressed. We hypothesized that student loan burden influences career plans in dermatology and that physicians are not fully educated on loan repayment options. The aims of this preliminary study were to explore the influence of student loan burden on career plans in dermatology and to determine if the Public Service Loan Forgiveness (PSLF) program could potentially encourage more dermatologists to consider academic careers.
Methods
The study aimed to investigate the factors that influence career decisions in dermatology and to assess attitudes toward the PSLF program as an option for student loan repayment. The target population included dermatology residents and attending physicians in the United States. Survey questions were adapted from a previously published study3 and were modified based on feedback from reviewers in the University of California (UC) Irvine department of dermatology. The survey was voluntary and did not collect identifying information. This study was granted exemption from oversight by the UC Irvine institutional review board.
Recruitment materials informed potential participants of the nature of the study and provided a hyperlink to the electronic survey. The UC Irvine department of dermatology emailed US dermatology residency program coordinators, requesting that they forward this study to residents and attending physicians in their programs.
Results
Demographics
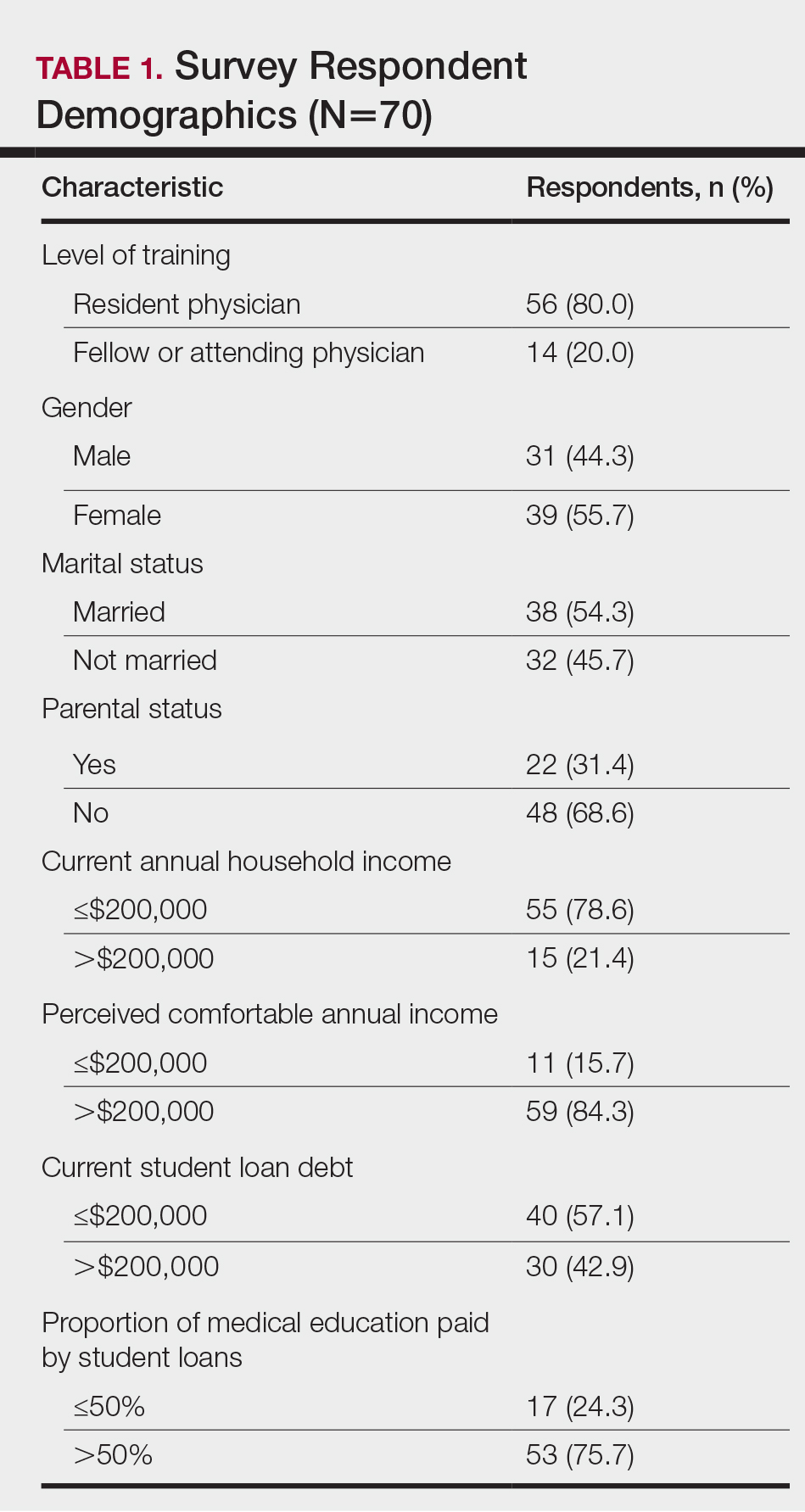
The survey had 70 respondents including residents (56 [80.0%]) and attending physicians (14 [20.0%]). The mean age (SD) of the respondents was 32.4 (6.1) years, with 31 (44.3%) men and 39 (55.7%) women. The majority were married (38 [54.3%]) and did not have children (48 [68.6%]). Most respondents reported an annual household income of $200,000 or less (55 [78.6%]) and perceived a comfortable annual household income as greater than $200,000 (59 [84.3%])(Table 1).
Financing Medical Education
Most respondents currently had $200,000 or less in student loan debt (40 [57.1%]) and financed more than half of their medical education with student loans (53 [75.7%]). A large majority (61 [87.1%]) indicated that some portion of their medical education was funded by student loans.
Career Goals in Dermatology and the Influence of Student Loans
Respondents were asked to specify their career plans before versus after starting dermatology residency training (ie, current career plan). Prior to starting residency, 36 (51.4%) and 34 (48.6%) respondents indicated they were interested in private practice and academia, respectively. After starting residency, the number of respondents interested in private practice increased to 45 (64.3%), and the number of respondents interested in academia decreased to 25 (35.7%). Fifteen (21.4%) respondents changed career trajectories from academia to private practice, 6 (8.6%) changed from private practice to academia, and 49 (70.0%) did not change career goals.
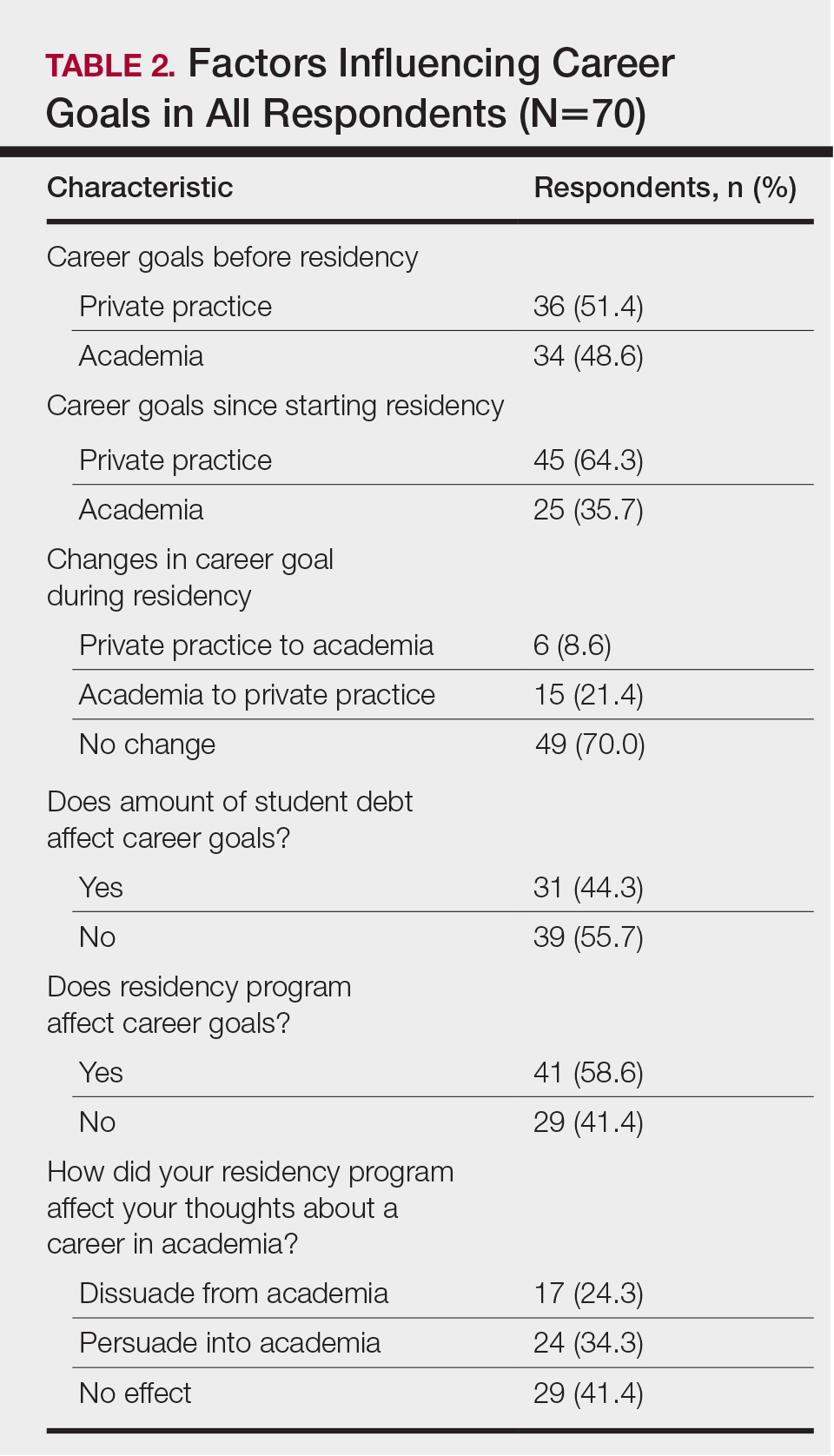
The majority of respondents (39 [55.7%]) indicated that the amount of their student loan debt did not influence their career goals (Table 2); however, those with more than $200,000 in debt were more likely to state that student loans impacted their career goals compared to those with $200,000 or less in debt (70.0% [21/30] vs 25.0% [10/40]; P<.001).
Comparison of Respondents Interested in Careers in Academia vs Private Practice
There were differences in financial circumstances between respondents interested in academia versus those interested in private practice. Compared to respondents interested in academia, those interested in private practice were more likely to have more than $200,000 in student loan debt (24 [53.3%] vs 6 [24.0%]; P<.05), have more than half of their education paid with student loans (38 [84.4%] vs 15 [60.0%]; P<.05), and state that student debt affected their career goals (28 [62.2%] vs 3 [12.0%]; P<.001)(Table 3). Demographic characteristics including gender, marital status, parental status, and current annual household income were not associated with a specific career goal.
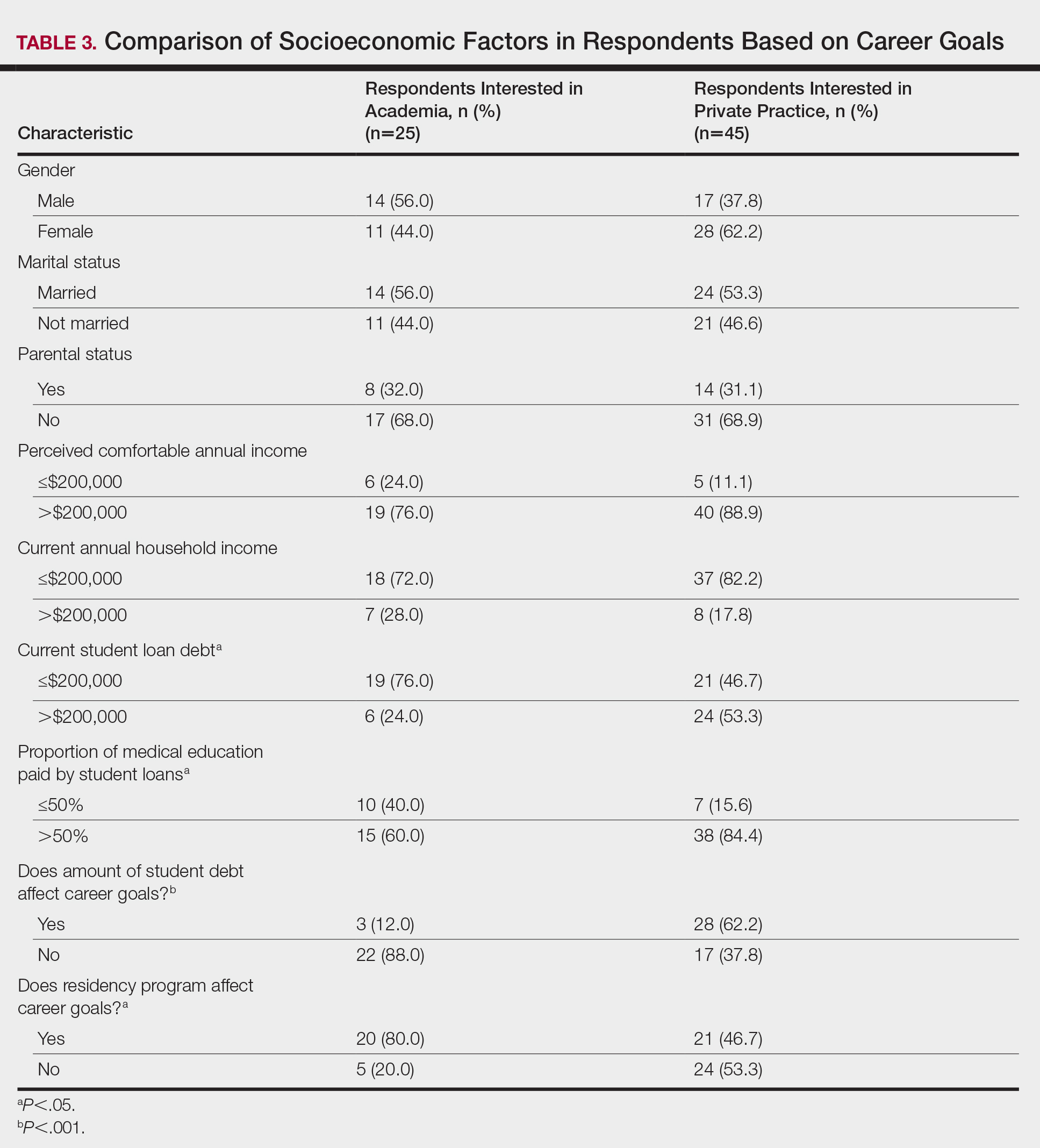
Subgroup analysis was performed on respondents who were initially interested in academic careers but subsequently decided to pursue private practice (n=15).
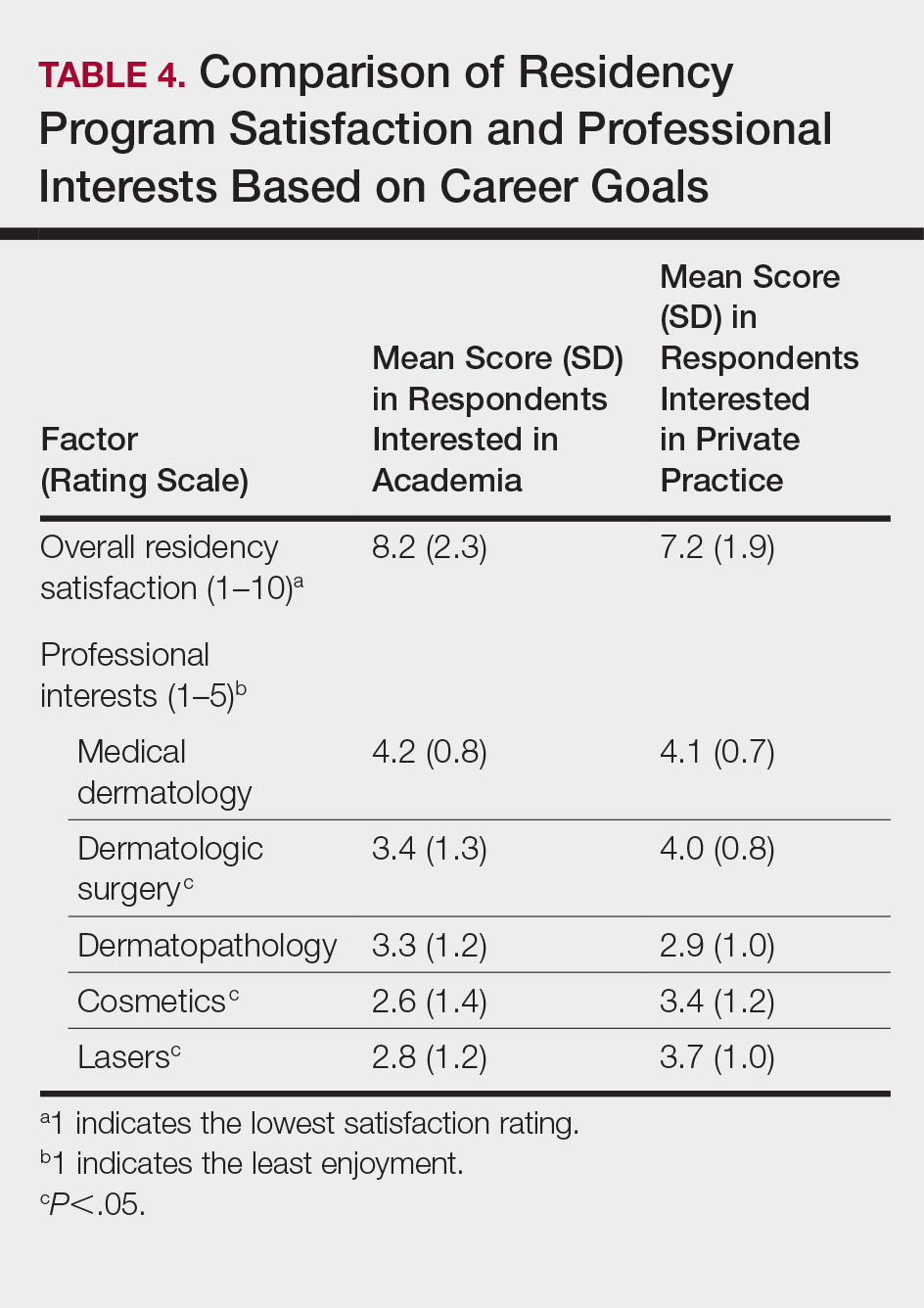
Residency program experience also may influence career trajectory. The majority (n=41 [58.6%]) of respondents indicated that their residency program experience affected their dermatology career goals. Of those, 41.7% and 58.5% stated that their residency program experiences dissuaded and persuaded them into academic positions, respectively. Those interested in academic dermatology were more likely to state that their residency program experience influenced their career goals (80.0% [20/25] vs 46.7% [21/45]; P<.05). Furthermore, those interested in academic positions responded with higher overall residency program satisfaction ratings on a scale of 1 to 10 (1 indicated the lowest satisfaction) than those interested in private practice, but the difference was not significant (mean [SD] score, 8.2 [2.3] vs 7.2 [1.9]; P=.07).
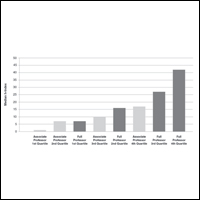
Respondents were asked to rate their interest in the following dermatology-related professional interests on a scale of 1 to 5 (1 indicated the least enjoyment): medical dermatology, dermatologic surgery, dermatopathology, cosmetics, and lasers. Those interested in private practice versus those interested in academic dermatology found more enjoyment in dermatologic surgery (mean [SD] score, 4.0 [0.8] vs 3.4 [1.3]; P<.05), cosmetics (3.4 [1.2] vs 2.6 [1.4]; P<.05), and lasers (3.7 [1.0] vs 2.8 [1.2]; P<.05)(Table 4).
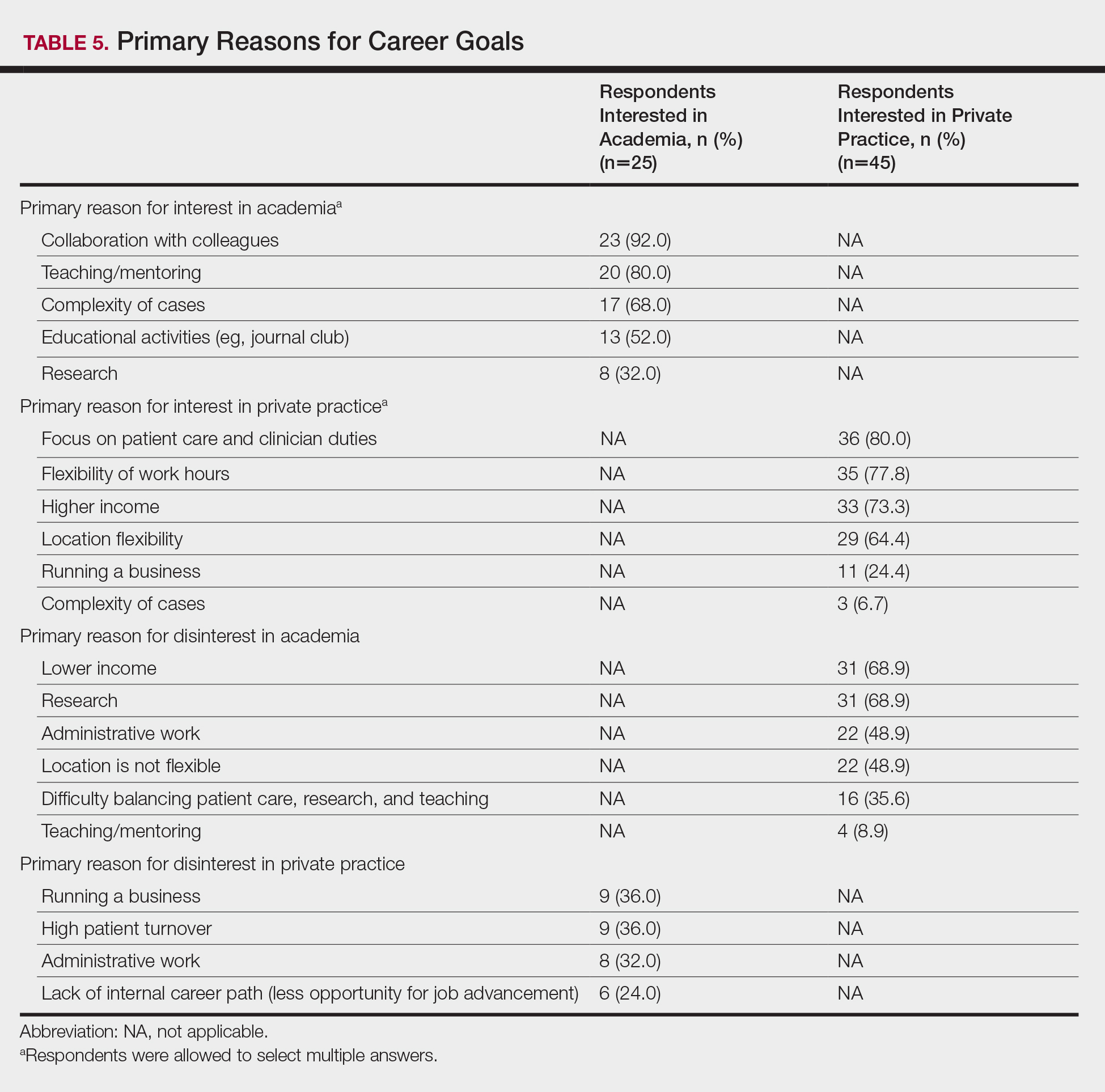
Respondents also were asked to select primary motivating factors for their career goals (ie, academia or private practice) and to indicate reasons for not choosing the alternative. The majority of those pursuing academia were motivated by opportunities to collaborate with colleagues (23 [92.0%]), teach and mentor (20 [80%]), and manage complex cases (17 [68.0%]). Most of the respondents who were pursing private practice were motivated by focus on patient care and clinician duties (36 [80.0%]), flexible work hours (35 [77.8%]), higher income (33 [73.3%]), and location flexibility (29 [64.4%]). Among those interested in academic dermatology, the top factors for disinterest in private practice were running a business (9 [36.0%]) and high patient turnover (9 [36.0%]). Most of those interested in private practice indicated that they were not interested in an academic position because of lower income (31 [68.9%]) and research duties (31 [68.9%])(Table 5).
Awareness of and Attitudes Toward the PSLF Program
The majority of respondents were aware of PSLF (53 [75.7%]); however, only 1 respondent endorsed current plans to use PSLF for loan repayment. Respondents were asked how likely they would be to pursue an academic position if given the option to have their student loans forgiven by the PSLF program. Overall, 44.6% (n=25) of respondents indicated that this option would have no effect or would unlikely convince them to pursue an academic position, and 55.4% (n=31) of respondents indicated that they were somewhat likely, likely, or very likely to pursue academia if PSLF was an option. Of those who stated that they would consider enrolling in PSLF, 64.5% (20/31) of individuals were pursuing careers in private practice. Neither current student loan burden nor career goal was associated with likelihood of enrolling in the PSLF.
Comment
In 2015, 76% of medical school graduates in the United States accrued educational debt, with an average of $189,165, a number that has continued to increase over the years.4 In addition to the increasing cost of medical education, higher interest rates on federal student loans contribute to debt burden. Over the last 2 decades, some research has posited that debt may influence medical specialty selection, with most studies focusing on primary care.5-9 However, there is limited information on the effect of student loan debt on career decisions within dermatology.
The results of our study suggest that financial factors including income and amount of educational debt may influence career decisions in dermatology. There is a known income gap between academic and nonacademic settings.
The PSLF can potentially address this issue and be used as a recruiting tool for dermatology positions in academia. Under PSLF, borrowers can have the remainder of their loan balances forgiven after making 120 monthly payments while employed full time by public service employers, including some academic medical institutions. In our study, a large majority of respondents indicated that they are aware of the PSLF, and more than half said they would consider pursuing positions in academia if their loans could be forgiven through the program; however, when asked about plans for loan repayment, only 1 respondent endorsed current plans to enroll in PSLF. Thus, despite high interest in PSLF among the survey respondents, few had actual plans to use the service, suggesting that perhaps dermatologists are not provided enough information about PSLF to motivate enrollment. In the same way, almost a quarter of respondents were not familiar with the PSLF as a repayment option, further signifying that distribution of information about financial planning may be inadequate. If student loan burden is a notable factor in career decisions in dermatology, it is important that academic institutions provide sufficient information about repayment to encourage informed decisions. As such, it is possible that educating physicians about options such as PSLF can potentially recruit more dermatologists to academic positions.
Aside from financial reasons, residency program experience and differences in practices in academic and nonacademic settings may impact career trajectories. The majority of respondents stated their residency program experience influenced their career decisions; however, the majority of respondents did not change their minds about career goals since starting residency, suggesting that residency program experience may reinforce but not necessarily alter these choices. Interests in specific focuses within dermatology also may influence career decisions. This study suggests that those pursuing private practice positions are more interested in dermatologic surgery, lasers, and cosmetics.
In this study, we did not find an association between gender and career plans in dermatology. In 2013, more than 60% of dermatology resident physicians were female.12 However, a recent study suggested that women face challenges in academic dermatology, including a downtrend in the number of female investigators with grants from the National Institutes of Health.13
This preliminary study has several limitations. First, the small sample size limited generalizability to all dermatologists. Second, responder bias was possible, as those who have stronger opinions about this topic may have been more inclined to participate in this voluntary survey. Future studies with larger sample sizes are needed to further explore the factors that influence career decisions within dermatology and to determine if there are additional means to increase recruitment into academia.
Conclusion
It is recognized that there are challenges in recruiting dermatologists into academic positions. This study suggests that student loan burden influences career decisions in dermatology. Dermatologists may not be fully educated on options for student loan repayment. With increased awareness, the PSLF can potentially be used as a recruitment tool for positions in academic dermatology.
- Resneck JS Jr, Kimball AB. The dermatology workforce shortage. J Am Acad Dermatol. 2004;50:50-54.
- Resneck JS Jr, Tierney EP, Kimball AB. Challenges facing academic dermatology: survey data on the faculty workforce. J Am Acad Dermatol. 2006;54:211-216.
- Lanzon J, Edwards SP, Inglehart MR. Choosing academia versus private practice: factors affecting oral maxillofacial surgery residents’ career choices. J Oral Maxillofac Surg. 2012;70:1751-1761.
- AAMC Medical Student Education: Debt, Costs, and Loan Repayment Fact Card. https://members.aamc.org/eweb/upload/2016_Debt_Fact_Card.pdf. Published October 2016. Accessed November 18, 2017.
- Rosenblatt RA, Andrilla CH. The impact of U.S. medical students’ debt on their choice of primary care careers: an analysis of data from the 2002 medical school graduation questionnaire. Acad Med. 2005;80:815-819.
- Woodworth PA, Chang FC, Helmer SD. Debt and other influences on career choices among surgical and primary care residents in a community-based hospital system. Am J Surg. 2000;180:570-575; discussion 575-576.
- Phillips RL Jr, Dodoo MS, Petterson S, et al. Specialty and geographic distribution of the physician workforce: what influences medical student and resident choices? Robert Graham Center website. http://www.graham-center.org/dam/rgc/documents/publications-reports/monographs-books/Specialty-geography-compressed.pdf. Published March 2, 2009. Accessed November 17, 2017.
- Rosenthal MP, Marquette PA, Diamond JJ. Trends along the debt-income axis: implications for medical students’ selections of family practice careers. Acad Med. 1996;71:675-677.
- McDonald FS, West CP, Popkave C, et al. Educational debt and reported career plans among internal medicine residents. Ann Intern Med. 2008;149:416-420.
- Careers in Medicine. Association of American Medical Colleges website. https://www.aamc.org/cim/specialty/exploreoptions/list/us/336836/dermatology.html. Accessed November 18, 2017.
- Youngclaus JA, Koehler PA, Kotlikoff LJ, et al. Can medical students afford to choose primary care? an economic analysis of physician education debt repayment. Acad Med. 2013;88:16-25.
- Physician specialty data book 2014. Association of American Medical Colleges website. https://members.aamc.org/eweb/upload/Physician Specialty Databook 2014.pdf. Published November 2014. Updated June 3, 2015. Accessed November 17, 2017.
- Cheng MY, Sukhov A, Sultani H, et al. Trends in National Institutes of Health funding of principal investigators in dermatology research by academic degree and sex. JAMA Dermatol. 2016;152:883-888.
Dermatology departments in the United States have been facing challenges in recruiting and retaining dermatologists for academic positions. Accordingly, a survey study reported that academic dermatologists were more likely than those in private practice to state that their institutions were recruiting new associates.1 Several factors could explain this phenomenon. Salary differences between jobs in academic and nonacademic settings may contribute to difficulty in recruiting dermatologists into academia, which is exacerbated by a theoretical shortage of dermatologists, leading to graduates who receive and accept private practice job offers.1,2 Furthermore, a large survey study reported that challenges unique to academic dermatologists include longer patient wait times in addition to responsibilities such as research, hospital consultations, medical writing, and teaching. These patterns raise concerns for the future of teaching institutions because academic dermatologists not only train future physicians but also conduct clinical and basic science research necessary to advance the field and improve patient care.2 Thus, it is important to evaluate the factors that affect career decisions in dermatology and to determine if these factors can be addressed. We hypothesized that student loan burden influences career plans in dermatology and that physicians are not fully educated on loan repayment options. The aims of this preliminary study were to explore the influence of student loan burden on career plans in dermatology and to determine if the Public Service Loan Forgiveness (PSLF) program could potentially encourage more dermatologists to consider academic careers.
Methods
The study aimed to investigate the factors that influence career decisions in dermatology and to assess attitudes toward the PSLF program as an option for student loan repayment. The target population included dermatology residents and attending physicians in the United States. Survey questions were adapted from a previously published study3 and were modified based on feedback from reviewers in the University of California (UC) Irvine department of dermatology. The survey was voluntary and did not collect identifying information. This study was granted exemption from oversight by the UC Irvine institutional review board.
Recruitment materials informed potential participants of the nature of the study and provided a hyperlink to the electronic survey. The UC Irvine department of dermatology emailed US dermatology residency program coordinators, requesting that they forward this study to residents and attending physicians in their programs.
Results
Demographics
The survey had 70 respondents including residents (56 [80.0%]) and attending physicians (14 [20.0%]). The mean age (SD) of the respondents was 32.4 (6.1) years, with 31 (44.3%) men and 39 (55.7%) women. The majority were married (38 [54.3%]) and did not have children (48 [68.6%]). Most respondents reported an annual household income of $200,000 or less (55 [78.6%]) and perceived a comfortable annual household income as greater than $200,000 (59 [84.3%])(Table 1).

Financing Medical Education
Most respondents currently had $200,000 or less in student loan debt (40 [57.1%]) and financed more than half of their medical education with student loans (53 [75.7%]). A large majority (61 [87.1%]) indicated that some portion of their medical education was funded by student loans.
Career Goals in Dermatology and the Influence of Student Loans
Respondents were asked to specify their career plans before versus after starting dermatology residency training (ie, current career plan). Prior to starting residency, 36 (51.4%) and 34 (48.6%) respondents indicated they were interested in private practice and academia, respectively. After starting residency, the number of respondents interested in private practice increased to 45 (64.3%), and the number of respondents interested in academia decreased to 25 (35.7%). Fifteen (21.4%) respondents changed career trajectories from academia to private practice, 6 (8.6%) changed from private practice to academia, and 49 (70.0%) did not change career goals.
The majority of respondents (39 [55.7%]) indicated that the amount of their student loan debt did not influence their career goals (Table 2); however, those with more than $200,000 in debt were more likely to state that student loans impacted their career goals compared to those with $200,000 or less in debt (70.0% [21/30] vs 25.0% [10/40]; P<.001).

Comparison of Respondents Interested in Careers in Academia vs Private Practice
There were differences in financial circumstances between respondents interested in academia versus those interested in private practice. Compared to respondents interested in academia, those interested in private practice were more likely to have more than $200,000 in student loan debt (24 [53.3%] vs 6 [24.0%]; P<.05), have more than half of their education paid with student loans (38 [84.4%] vs 15 [60.0%]; P<.05), and state that student debt affected their career goals (28 [62.2%] vs 3 [12.0%]; P<.001)(Table 3). Demographic characteristics including gender, marital status, parental status, and current annual household income were not associated with a specific career goal.

Subgroup analysis was performed on respondents who were initially interested in academic careers but subsequently decided to pursue private practice (n=15).
Residency program experience also may influence career trajectory. The majority (n=41 [58.6%]) of respondents indicated that their residency program experience affected their dermatology career goals. Of those, 41.7% and 58.5% stated that their residency program experiences dissuaded and persuaded them into academic positions, respectively. Those interested in academic dermatology were more likely to state that their residency program experience influenced their career goals (80.0% [20/25] vs 46.7% [21/45]; P<.05). Furthermore, those interested in academic positions responded with higher overall residency program satisfaction ratings on a scale of 1 to 10 (1 indicated the lowest satisfaction) than those interested in private practice, but the difference was not significant (mean [SD] score, 8.2 [2.3] vs 7.2 [1.9]; P=.07).
Respondents were asked to rate their interest in the following dermatology-related professional interests on a scale of 1 to 5 (1 indicated the least enjoyment): medical dermatology, dermatologic surgery, dermatopathology, cosmetics, and lasers. Those interested in private practice versus those interested in academic dermatology found more enjoyment in dermatologic surgery (mean [SD] score, 4.0 [0.8] vs 3.4 [1.3]; P<.05), cosmetics (3.4 [1.2] vs 2.6 [1.4]; P<.05), and lasers (3.7 [1.0] vs 2.8 [1.2]; P<.05)(Table 4).

Respondents also were asked to select primary motivating factors for their career goals (ie, academia or private practice) and to indicate reasons for not choosing the alternative. The majority of those pursuing academia were motivated by opportunities to collaborate with colleagues (23 [92.0%]), teach and mentor (20 [80%]), and manage complex cases (17 [68.0%]). Most of the respondents who were pursing private practice were motivated by focus on patient care and clinician duties (36 [80.0%]), flexible work hours (35 [77.8%]), higher income (33 [73.3%]), and location flexibility (29 [64.4%]). Among those interested in academic dermatology, the top factors for disinterest in private practice were running a business (9 [36.0%]) and high patient turnover (9 [36.0%]). Most of those interested in private practice indicated that they were not interested in an academic position because of lower income (31 [68.9%]) and research duties (31 [68.9%])(Table 5).

Awareness of and Attitudes Toward the PSLF Program
The majority of respondents were aware of PSLF (53 [75.7%]); however, only 1 respondent endorsed current plans to use PSLF for loan repayment. Respondents were asked how likely they would be to pursue an academic position if given the option to have their student loans forgiven by the PSLF program. Overall, 44.6% (n=25) of respondents indicated that this option would have no effect or would unlikely convince them to pursue an academic position, and 55.4% (n=31) of respondents indicated that they were somewhat likely, likely, or very likely to pursue academia if PSLF was an option. Of those who stated that they would consider enrolling in PSLF, 64.5% (20/31) of individuals were pursuing careers in private practice. Neither current student loan burden nor career goal was associated with likelihood of enrolling in the PSLF.
Comment
In 2015, 76% of medical school graduates in the United States accrued educational debt, with an average of $189,165, a number that has continued to increase over the years.4 In addition to the increasing cost of medical education, higher interest rates on federal student loans contribute to debt burden. Over the last 2 decades, some research has posited that debt may influence medical specialty selection, with most studies focusing on primary care.5-9 However, there is limited information on the effect of student loan debt on career decisions within dermatology.
The results of our study suggest that financial factors including income and amount of educational debt may influence career decisions in dermatology. There is a known income gap between academic and nonacademic settings.
The PSLF can potentially address this issue and be used as a recruiting tool for dermatology positions in academia. Under PSLF, borrowers can have the remainder of their loan balances forgiven after making 120 monthly payments while employed full time by public service employers, including some academic medical institutions. In our study, a large majority of respondents indicated that they are aware of the PSLF, and more than half said they would consider pursuing positions in academia if their loans could be forgiven through the program; however, when asked about plans for loan repayment, only 1 respondent endorsed current plans to enroll in PSLF. Thus, despite high interest in PSLF among the survey respondents, few had actual plans to use the service, suggesting that perhaps dermatologists are not provided enough information about PSLF to motivate enrollment. In the same way, almost a quarter of respondents were not familiar with the PSLF as a repayment option, further signifying that distribution of information about financial planning may be inadequate. If student loan burden is a notable factor in career decisions in dermatology, it is important that academic institutions provide sufficient information about repayment to encourage informed decisions. As such, it is possible that educating physicians about options such as PSLF can potentially recruit more dermatologists to academic positions.
Aside from financial reasons, residency program experience and differences in practices in academic and nonacademic settings may impact career trajectories. The majority of respondents stated their residency program experience influenced their career decisions; however, the majority of respondents did not change their minds about career goals since starting residency, suggesting that residency program experience may reinforce but not necessarily alter these choices. Interests in specific focuses within dermatology also may influence career decisions. This study suggests that those pursuing private practice positions are more interested in dermatologic surgery, lasers, and cosmetics.
In this study, we did not find an association between gender and career plans in dermatology. In 2013, more than 60% of dermatology resident physicians were female.12 However, a recent study suggested that women face challenges in academic dermatology, including a downtrend in the number of female investigators with grants from the National Institutes of Health.13
This preliminary study has several limitations. First, the small sample size limited generalizability to all dermatologists. Second, responder bias was possible, as those who have stronger opinions about this topic may have been more inclined to participate in this voluntary survey. Future studies with larger sample sizes are needed to further explore the factors that influence career decisions within dermatology and to determine if there are additional means to increase recruitment into academia.
Conclusion
It is recognized that there are challenges in recruiting dermatologists into academic positions. This study suggests that student loan burden influences career decisions in dermatology. Dermatologists may not be fully educated on options for student loan repayment. With increased awareness, the PSLF can potentially be used as a recruitment tool for positions in academic dermatology.
Dermatology departments in the United States have been facing challenges in recruiting and retaining dermatologists for academic positions. Accordingly, a survey study reported that academic dermatologists were more likely than those in private practice to state that their institutions were recruiting new associates.1 Several factors could explain this phenomenon. Salary differences between jobs in academic and nonacademic settings may contribute to difficulty in recruiting dermatologists into academia, which is exacerbated by a theoretical shortage of dermatologists, leading to graduates who receive and accept private practice job offers.1,2 Furthermore, a large survey study reported that challenges unique to academic dermatologists include longer patient wait times in addition to responsibilities such as research, hospital consultations, medical writing, and teaching. These patterns raise concerns for the future of teaching institutions because academic dermatologists not only train future physicians but also conduct clinical and basic science research necessary to advance the field and improve patient care.2 Thus, it is important to evaluate the factors that affect career decisions in dermatology and to determine if these factors can be addressed. We hypothesized that student loan burden influences career plans in dermatology and that physicians are not fully educated on loan repayment options. The aims of this preliminary study were to explore the influence of student loan burden on career plans in dermatology and to determine if the Public Service Loan Forgiveness (PSLF) program could potentially encourage more dermatologists to consider academic careers.
Methods
The study aimed to investigate the factors that influence career decisions in dermatology and to assess attitudes toward the PSLF program as an option for student loan repayment. The target population included dermatology residents and attending physicians in the United States. Survey questions were adapted from a previously published study3 and were modified based on feedback from reviewers in the University of California (UC) Irvine department of dermatology. The survey was voluntary and did not collect identifying information. This study was granted exemption from oversight by the UC Irvine institutional review board.
Recruitment materials informed potential participants of the nature of the study and provided a hyperlink to the electronic survey. The UC Irvine department of dermatology emailed US dermatology residency program coordinators, requesting that they forward this study to residents and attending physicians in their programs.
Results
Demographics
The survey had 70 respondents including residents (56 [80.0%]) and attending physicians (14 [20.0%]). The mean age (SD) of the respondents was 32.4 (6.1) years, with 31 (44.3%) men and 39 (55.7%) women. The majority were married (38 [54.3%]) and did not have children (48 [68.6%]). Most respondents reported an annual household income of $200,000 or less (55 [78.6%]) and perceived a comfortable annual household income as greater than $200,000 (59 [84.3%])(Table 1).

Financing Medical Education
Most respondents currently had $200,000 or less in student loan debt (40 [57.1%]) and financed more than half of their medical education with student loans (53 [75.7%]). A large majority (61 [87.1%]) indicated that some portion of their medical education was funded by student loans.
Career Goals in Dermatology and the Influence of Student Loans
Respondents were asked to specify their career plans before versus after starting dermatology residency training (ie, current career plan). Prior to starting residency, 36 (51.4%) and 34 (48.6%) respondents indicated they were interested in private practice and academia, respectively. After starting residency, the number of respondents interested in private practice increased to 45 (64.3%), and the number of respondents interested in academia decreased to 25 (35.7%). Fifteen (21.4%) respondents changed career trajectories from academia to private practice, 6 (8.6%) changed from private practice to academia, and 49 (70.0%) did not change career goals.
The majority of respondents (39 [55.7%]) indicated that the amount of their student loan debt did not influence their career goals (Table 2); however, those with more than $200,000 in debt were more likely to state that student loans impacted their career goals compared to those with $200,000 or less in debt (70.0% [21/30] vs 25.0% [10/40]; P<.001).

Comparison of Respondents Interested in Careers in Academia vs Private Practice
There were differences in financial circumstances between respondents interested in academia versus those interested in private practice. Compared to respondents interested in academia, those interested in private practice were more likely to have more than $200,000 in student loan debt (24 [53.3%] vs 6 [24.0%]; P<.05), have more than half of their education paid with student loans (38 [84.4%] vs 15 [60.0%]; P<.05), and state that student debt affected their career goals (28 [62.2%] vs 3 [12.0%]; P<.001)(Table 3). Demographic characteristics including gender, marital status, parental status, and current annual household income were not associated with a specific career goal.

Subgroup analysis was performed on respondents who were initially interested in academic careers but subsequently decided to pursue private practice (n=15).
Residency program experience also may influence career trajectory. The majority (n=41 [58.6%]) of respondents indicated that their residency program experience affected their dermatology career goals. Of those, 41.7% and 58.5% stated that their residency program experiences dissuaded and persuaded them into academic positions, respectively. Those interested in academic dermatology were more likely to state that their residency program experience influenced their career goals (80.0% [20/25] vs 46.7% [21/45]; P<.05). Furthermore, those interested in academic positions responded with higher overall residency program satisfaction ratings on a scale of 1 to 10 (1 indicated the lowest satisfaction) than those interested in private practice, but the difference was not significant (mean [SD] score, 8.2 [2.3] vs 7.2 [1.9]; P=.07).
Respondents were asked to rate their interest in the following dermatology-related professional interests on a scale of 1 to 5 (1 indicated the least enjoyment): medical dermatology, dermatologic surgery, dermatopathology, cosmetics, and lasers. Those interested in private practice versus those interested in academic dermatology found more enjoyment in dermatologic surgery (mean [SD] score, 4.0 [0.8] vs 3.4 [1.3]; P<.05), cosmetics (3.4 [1.2] vs 2.6 [1.4]; P<.05), and lasers (3.7 [1.0] vs 2.8 [1.2]; P<.05)(Table 4).

Respondents also were asked to select primary motivating factors for their career goals (ie, academia or private practice) and to indicate reasons for not choosing the alternative. The majority of those pursuing academia were motivated by opportunities to collaborate with colleagues (23 [92.0%]), teach and mentor (20 [80%]), and manage complex cases (17 [68.0%]). Most of the respondents who were pursing private practice were motivated by focus on patient care and clinician duties (36 [80.0%]), flexible work hours (35 [77.8%]), higher income (33 [73.3%]), and location flexibility (29 [64.4%]). Among those interested in academic dermatology, the top factors for disinterest in private practice were running a business (9 [36.0%]) and high patient turnover (9 [36.0%]). Most of those interested in private practice indicated that they were not interested in an academic position because of lower income (31 [68.9%]) and research duties (31 [68.9%])(Table 5).

Awareness of and Attitudes Toward the PSLF Program
The majority of respondents were aware of PSLF (53 [75.7%]); however, only 1 respondent endorsed current plans to use PSLF for loan repayment. Respondents were asked how likely they would be to pursue an academic position if given the option to have their student loans forgiven by the PSLF program. Overall, 44.6% (n=25) of respondents indicated that this option would have no effect or would unlikely convince them to pursue an academic position, and 55.4% (n=31) of respondents indicated that they were somewhat likely, likely, or very likely to pursue academia if PSLF was an option. Of those who stated that they would consider enrolling in PSLF, 64.5% (20/31) of individuals were pursuing careers in private practice. Neither current student loan burden nor career goal was associated with likelihood of enrolling in the PSLF.
Comment
In 2015, 76% of medical school graduates in the United States accrued educational debt, with an average of $189,165, a number that has continued to increase over the years.4 In addition to the increasing cost of medical education, higher interest rates on federal student loans contribute to debt burden. Over the last 2 decades, some research has posited that debt may influence medical specialty selection, with most studies focusing on primary care.5-9 However, there is limited information on the effect of student loan debt on career decisions within dermatology.
The results of our study suggest that financial factors including income and amount of educational debt may influence career decisions in dermatology. There is a known income gap between academic and nonacademic settings.
The PSLF can potentially address this issue and be used as a recruiting tool for dermatology positions in academia. Under PSLF, borrowers can have the remainder of their loan balances forgiven after making 120 monthly payments while employed full time by public service employers, including some academic medical institutions. In our study, a large majority of respondents indicated that they are aware of the PSLF, and more than half said they would consider pursuing positions in academia if their loans could be forgiven through the program; however, when asked about plans for loan repayment, only 1 respondent endorsed current plans to enroll in PSLF. Thus, despite high interest in PSLF among the survey respondents, few had actual plans to use the service, suggesting that perhaps dermatologists are not provided enough information about PSLF to motivate enrollment. In the same way, almost a quarter of respondents were not familiar with the PSLF as a repayment option, further signifying that distribution of information about financial planning may be inadequate. If student loan burden is a notable factor in career decisions in dermatology, it is important that academic institutions provide sufficient information about repayment to encourage informed decisions. As such, it is possible that educating physicians about options such as PSLF can potentially recruit more dermatologists to academic positions.
Aside from financial reasons, residency program experience and differences in practices in academic and nonacademic settings may impact career trajectories. The majority of respondents stated their residency program experience influenced their career decisions; however, the majority of respondents did not change their minds about career goals since starting residency, suggesting that residency program experience may reinforce but not necessarily alter these choices. Interests in specific focuses within dermatology also may influence career decisions. This study suggests that those pursuing private practice positions are more interested in dermatologic surgery, lasers, and cosmetics.
In this study, we did not find an association between gender and career plans in dermatology. In 2013, more than 60% of dermatology resident physicians were female.12 However, a recent study suggested that women face challenges in academic dermatology, including a downtrend in the number of female investigators with grants from the National Institutes of Health.13
This preliminary study has several limitations. First, the small sample size limited generalizability to all dermatologists. Second, responder bias was possible, as those who have stronger opinions about this topic may have been more inclined to participate in this voluntary survey. Future studies with larger sample sizes are needed to further explore the factors that influence career decisions within dermatology and to determine if there are additional means to increase recruitment into academia.
Conclusion
It is recognized that there are challenges in recruiting dermatologists into academic positions. This study suggests that student loan burden influences career decisions in dermatology. Dermatologists may not be fully educated on options for student loan repayment. With increased awareness, the PSLF can potentially be used as a recruitment tool for positions in academic dermatology.
- Resneck JS Jr, Kimball AB. The dermatology workforce shortage. J Am Acad Dermatol. 2004;50:50-54.
- Resneck JS Jr, Tierney EP, Kimball AB. Challenges facing academic dermatology: survey data on the faculty workforce. J Am Acad Dermatol. 2006;54:211-216.
- Lanzon J, Edwards SP, Inglehart MR. Choosing academia versus private practice: factors affecting oral maxillofacial surgery residents’ career choices. J Oral Maxillofac Surg. 2012;70:1751-1761.
- AAMC Medical Student Education: Debt, Costs, and Loan Repayment Fact Card. https://members.aamc.org/eweb/upload/2016_Debt_Fact_Card.pdf. Published October 2016. Accessed November 18, 2017.
- Rosenblatt RA, Andrilla CH. The impact of U.S. medical students’ debt on their choice of primary care careers: an analysis of data from the 2002 medical school graduation questionnaire. Acad Med. 2005;80:815-819.
- Woodworth PA, Chang FC, Helmer SD. Debt and other influences on career choices among surgical and primary care residents in a community-based hospital system. Am J Surg. 2000;180:570-575; discussion 575-576.
- Phillips RL Jr, Dodoo MS, Petterson S, et al. Specialty and geographic distribution of the physician workforce: what influences medical student and resident choices? Robert Graham Center website. http://www.graham-center.org/dam/rgc/documents/publications-reports/monographs-books/Specialty-geography-compressed.pdf. Published March 2, 2009. Accessed November 17, 2017.
- Rosenthal MP, Marquette PA, Diamond JJ. Trends along the debt-income axis: implications for medical students’ selections of family practice careers. Acad Med. 1996;71:675-677.
- McDonald FS, West CP, Popkave C, et al. Educational debt and reported career plans among internal medicine residents. Ann Intern Med. 2008;149:416-420.
- Careers in Medicine. Association of American Medical Colleges website. https://www.aamc.org/cim/specialty/exploreoptions/list/us/336836/dermatology.html. Accessed November 18, 2017.
- Youngclaus JA, Koehler PA, Kotlikoff LJ, et al. Can medical students afford to choose primary care? an economic analysis of physician education debt repayment. Acad Med. 2013;88:16-25.
- Physician specialty data book 2014. Association of American Medical Colleges website. https://members.aamc.org/eweb/upload/Physician Specialty Databook 2014.pdf. Published November 2014. Updated June 3, 2015. Accessed November 17, 2017.
- Cheng MY, Sukhov A, Sultani H, et al. Trends in National Institutes of Health funding of principal investigators in dermatology research by academic degree and sex. JAMA Dermatol. 2016;152:883-888.
- Resneck JS Jr, Kimball AB. The dermatology workforce shortage. J Am Acad Dermatol. 2004;50:50-54.
- Resneck JS Jr, Tierney EP, Kimball AB. Challenges facing academic dermatology: survey data on the faculty workforce. J Am Acad Dermatol. 2006;54:211-216.
- Lanzon J, Edwards SP, Inglehart MR. Choosing academia versus private practice: factors affecting oral maxillofacial surgery residents’ career choices. J Oral Maxillofac Surg. 2012;70:1751-1761.
- AAMC Medical Student Education: Debt, Costs, and Loan Repayment Fact Card. https://members.aamc.org/eweb/upload/2016_Debt_Fact_Card.pdf. Published October 2016. Accessed November 18, 2017.
- Rosenblatt RA, Andrilla CH. The impact of U.S. medical students’ debt on their choice of primary care careers: an analysis of data from the 2002 medical school graduation questionnaire. Acad Med. 2005;80:815-819.
- Woodworth PA, Chang FC, Helmer SD. Debt and other influences on career choices among surgical and primary care residents in a community-based hospital system. Am J Surg. 2000;180:570-575; discussion 575-576.
- Phillips RL Jr, Dodoo MS, Petterson S, et al. Specialty and geographic distribution of the physician workforce: what influences medical student and resident choices? Robert Graham Center website. http://www.graham-center.org/dam/rgc/documents/publications-reports/monographs-books/Specialty-geography-compressed.pdf. Published March 2, 2009. Accessed November 17, 2017.
- Rosenthal MP, Marquette PA, Diamond JJ. Trends along the debt-income axis: implications for medical students’ selections of family practice careers. Acad Med. 1996;71:675-677.
- McDonald FS, West CP, Popkave C, et al. Educational debt and reported career plans among internal medicine residents. Ann Intern Med. 2008;149:416-420.
- Careers in Medicine. Association of American Medical Colleges website. https://www.aamc.org/cim/specialty/exploreoptions/list/us/336836/dermatology.html. Accessed November 18, 2017.
- Youngclaus JA, Koehler PA, Kotlikoff LJ, et al. Can medical students afford to choose primary care? an economic analysis of physician education debt repayment. Acad Med. 2013;88:16-25.
- Physician specialty data book 2014. Association of American Medical Colleges website. https://members.aamc.org/eweb/upload/Physician Specialty Databook 2014.pdf. Published November 2014. Updated June 3, 2015. Accessed November 17, 2017.
- Cheng MY, Sukhov A, Sultani H, et al. Trends in National Institutes of Health funding of principal investigators in dermatology research by academic degree and sex. JAMA Dermatol. 2016;152:883-888.
Practice Points
- Academic dermatology departments are facing challenges in recruiting physicians, raising concerns for the future of dermatology education and research.
- Large amounts of student loan burden may influence career plans in dermatology.
- Dermatologists may not be fully knowledgeable of loan repayment options; thus, education on this topic should be prioritized by dermatology training programs.
Multinucleate Cell Angiohistiocytoma
Multinucleate cell angiohistiocytoma (MCAH) is a rare benign, soft-tissue tumor first described in 1985 by Smith and Jones1 that presents clinically as erythematous to violaceous papules most commonly affecting females on the dorsal aspect of the hands and face.2 Multinucleate cell angiohistiocytoma is histologically characterized by vascular and histiocytic proliferations with dermal fibrosis. Few cases have been reported of lesions affecting the lower extremities. We report a case of MCAH affecting the legs.
Case Report
An 83-year-old white man with a history of basal cell carcinoma presented for evaluation of grouped, well-circumscribed, soft, red-violet, painless papules on the right anterior thigh that had been present for 8 months (Figure 1A). A review of symptoms was negative for immunologic, respiratory, and hematologic changes. The patient’s medical history also was remarkable for prostate cancer treated with radiation 18 years prior as well as right hip and left knee implants. The initial clinical impression was Kaposi sarcoma or a granulomatous disorder.
Histopathologic evaluation of a deep shave biopsy initially determined the lesion to be scar tissue without other pathologic findings. The patient returned to the clinic 12 months later for a complete skin examination given his history of skin cancer. Compared to clinical photographs taken a year prior, new violaceous papules were noted on the right thigh (Figure 1B) and left calf. Furthermore, there was no recurrence of the lesion at the prior biopsy site. Shave biopsies of the papules on the right thigh and left calf demonstrated similar histologic findings to each other. There was a mild increase in the number of small blood vessels in the superficial dermis (Figure 2A). A mild perivascular lymphocytic infiltrate surrounded some of these blood vessels. The endothelial cells had small nuclei with no evidence of nuclear pleomorphism. Careful examination of the interstitial dermis revealed scattered multinucleate cells with angulated cytoplasm (Figure 2B). Immunostaining for CD31 and human herpesvirus 8 were negative, excluding an infiltrative vascular tumor and Kaposi sarcoma, respectively. The diagnosis of MCAH was made based on the histopathologic findings.
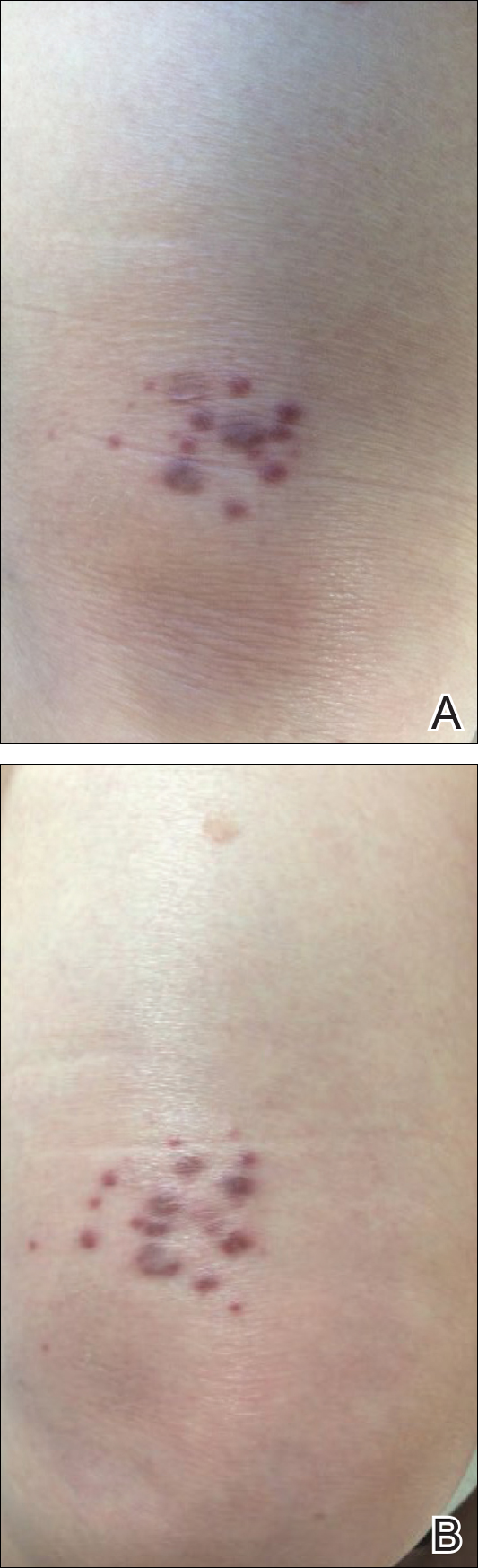
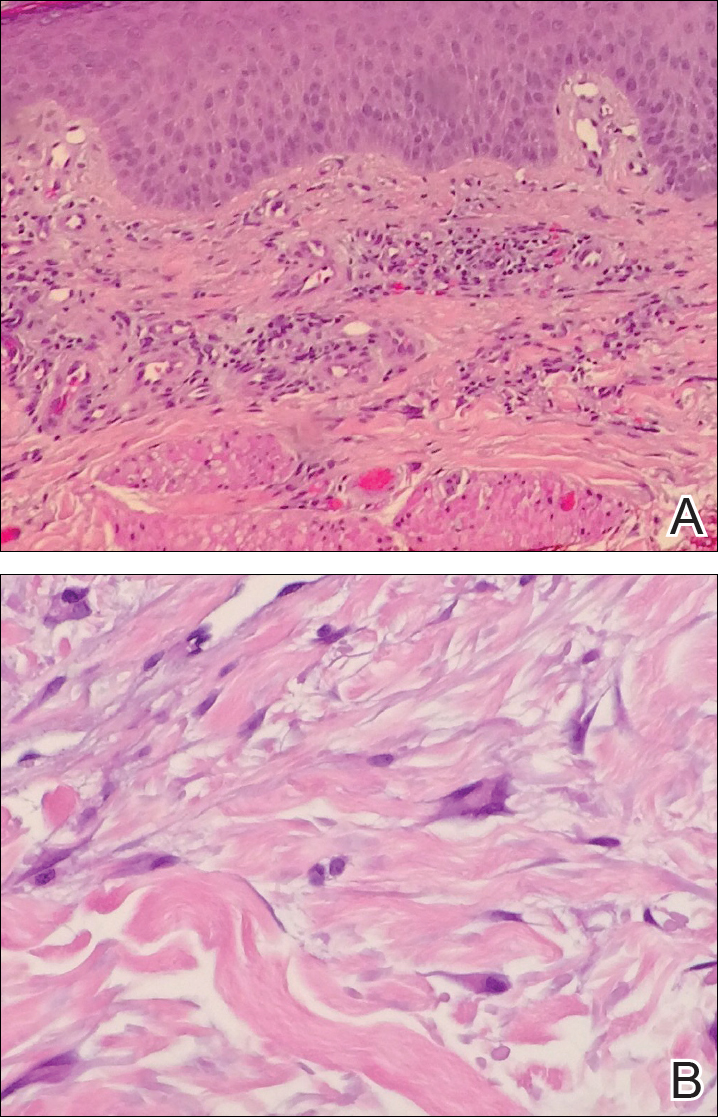
At 1-year follow-up, the condition was stable with no gross changes in the lesions based on prior photographs. Once again, there was no recurrence of the excised lesions at both biopsy sites.
Comment
Presentation
A systematic review of published reports determined that 79% of MCAH cases occur in females, with an average age of onset of 50.1 years.2 However, MCAH likely is underreported due to the overall lack of knowledge regarding this condition by physicians and pathologists. The hands and face are the most commonly affected areas, though other sites of involvement have been reported, including the lower extremities,3,4 oral mucosa and upper lip,5,6 and trunk,7,8 as well as generalized distribution.9-12 Additionally, 1 case presented as a single plaque on the trunk rather than having papular or nodular morphology.8 Multinucleate cell angiohistiocytoma lesions generally are asymptomatic, though pruritus may be present.13 The condition is regarded as benign, though a minority of cases have exhibited spontaneous resolution.14-16
Histopathology
Multinucleate cell angiohistiocytoma histology demonstrates full-thickness dermal microvessel proliferation and fibrosis with characteristic multinucleate giant cells.2,3 Vascular endothelial cells stained positive for CD68 in 60% of cases2 as well as the normal endothelial markers (ie, factor VIII, CD31, CD34). The multinucleate giant cells exhibit immunoreactivity for macrophage/histiocytic markers factor XIIIa and CD68.
Etiology
The pathogenesis of MCAH is not yet fully understood, but it is considered to be a benign vascular or fibrohistiocytic neoplasm.17 Calderaro et al18 described a series of 8 patients who developed MCAH either within a cutaneous neoplastic process or in conjunction with various cutaneous reactive conditions, including hidradenitis suppurativa and chronic radiation dermatitis, as well as overlying a bone prosthesis placed due to degenerative arthritis. These cases suggest that MCAH, or possibly a subset of the disease, is a reactive process.
Differential Diagnosis
The differential diagnosis for MCAH includes Kaposi sarcoma clinically and dermatofibroma and fibrous papules histologically. Sass et al21 determined the in vitro behavior of cultured MCAH cells to contrast markedly with Kaposi sarcoma–derived cells. Although Kaposi sarcoma–derived cells exhibited invasive behavior, cells isolated from MCAH lesions were less elongated and were unable to traverse basement membranes.
Treatment
Surgical excision or cryotherapy appear to be definitive treatments of MCAH; however, a number of cases have reported light and laser modalities as successful alternatives to excision. One case of MCAH affecting the face was treated with pulsed dye laser monotherapy.22 This modality allowed selective coagulation of the vascular structures in MCAH. At 8-month follow-up, the initial lesion was noted to be completely cleared, though similar lesions had recently appeared elsewhere on the face.22 Another case of MCAH affecting the leg was treated with pulsed dye laser and both topical and intralesional corticosteroid combination therapy. In this case, the lesion failed to respond to treatment, which may suggest that facial localization could influence response in pulsed dye laser treatment.3
Intense pulsed light also has been reported as a definitive treatment in 2 cases.2,13 Slight erythema and transient pruritus have been reported immediately following treatment. In this case, complete resolution with only residual hyperpigmentation was reported at 2-month follow-up, with no recurrence during 12 months of follow-up.13
Argon laser therapy has been used in 2 cases. After a single session, lesions were no longer palpable, with no scarring noted at 8 weeks follow-up.23 Lastly, 2 cases of MCAH have been successfully treated with the CO2 laser, with no relapse noted at 2.5- or 5-month follow-up, respectively.24
Conclusion
Multinucleate cell angiohistiocytoma is a rare and likely underdiagnosed dermatologic condition that is believed to be a reactive process. Characteristic histology of MCAH demonstrates microvascular proliferations of the dermis with multinucleate giant cells amidst a fibrous background. Although surgical excision is curative, there are reports in which laser and light therapies were used to effectively treat MCAH.
- Smith NP, Jones EW. Multinucleate cell angiohistiocytoma—a new entity. Br J Dermatol. 1985;113:15.
- Frew JW. Multinucleate cell angiohistiocytoma: clinicopathological correlation of 142 cases with insights into etiology and pathogenesis. Am J Dermatopathol. 2015;37:222-228.
- Applebaum DS, Shuja F, Hicks L, et al. Multinucleate cell angiohistiocytoma: a case report and review of the literature. Dermatol Online J. 2014;20:22610.
- Sagdeo A, Chu EY, Elenitsas R, et al. Multiple asymptomatic violaceous macules on the thigh. Multinucleate cell angiohistiocytoma (MCAH). JAMA Dermatol. 2013;149:357-363.
- Rawal YB, Anderson KM, Rawal SY. Multinucleate cell angiohistiocytoma: an uncommon mucosal tumour. Clin Exp Dermatol. 2009;34:333-336.
- Jones AC, Mullins D, Jimenez F. Multinucleate cell angiohistiocytoma of the upper lip. Oral Surg Oral Med Oral Pathol. 1994;78:743-747.
- Doshi-Chougule BN, Gust A, Mentzel T, et al. Multinucleate cell angiohistiocytoma with hypertrophic nerves. J Cutan Pathol. 2013;40:1048-1053.
- Issa AA, Lui H, Shapiro J, et al. Plaque-type multinucleate cell angiohistiocytoma. J Cutan Med Surg. 1998;3:112-114.
- Doane JA, Purdy K, Pasternak S. Generalized multinucleate cell angiohistiocytoma. J Cutan Med Surg. 2015;19:323-325.
- Marti N, Monteagudo C, Revert A, et al. Multiple papules on the trunk and extremities. generalized multinucleate cell angiohistiocytoma. Int J Dermatol. 2013;52:544-546.
- O’Blenes CA, Walsh NM, Green PJ, et al. Novel case of generalized multinucleate cell angiohistiocytoma. J Cutan Med Surg. 2010;14:178-180.
- Chang SN, Kim HS, Kim SC, et al. Generalized multinucleate cell angiohistiocytoma. J Am Acad Dermatol. 1996;35:320-322.
- Fernández-Jorge B, Del Pozo J, García-Silva J, et al. Multinucleate cell angiohistiocytoma: treatment using intense pulsed light. Dermatol Surg. 2009;35:1141-1143.
- Perez LP, Zulaica A, Rodriguez L, et al. Multinucleate cell angiohistiocytoma. report of five cases. J Cutan Pathol. 2006;33:349-352.
- Shapiro PE, Nova MP, Rosmarin LA, et al. Multinucleate cell angiohistiocytoma: a distinct entity diagnosable by clinical and histologic features. J Am Acad Dermatol. 1994;30:417-422.
- Jaconelli L, Kanitakis J, Ktiouet S, et al. Multinucleate cell angiohistiocytoma: report of three new cases and literature review. Dermatol Online J. 2009;15:4.
- Jones WE, Cerio R, Smith NP. Multinucleate cell angiohistiocytoma: an acquired vascular anomaly to be distinguished from Kaposi’s sarcoma. Br J Dermatol. 1990;122:651-663.
- Calderaro J, Rethers L, Ortonne N. Multinucleated cells angiohistiocytoma: a reactive lesion? Am J Dermatopathol. 2010;32:415-417.
- Cesinaro AM, Roncati L, Maiorana A. Estrogen receptor alpha overexpression in multinucleate cell angiohistiocytoma: new insights into the pathogenesis of a reactive process. Am J Dermatopathol. 2010;32:655-659.
- Losordo DW, Isner JM. Estrogen and angiogenesis: a review. Arterioscler Thromb Vasc Biol. 2001;21:6-12.
- Sass U, Noel JC, Andre J, et al. Multinucleate cell angiohistiocytoma: report of two cases with no evidence of human herpesvirus-8 infection. J Cutan Pathol. 2000;27:258-261.
- Richer V, Lui H. Facial multinucleate cell angiohistiocytoma: long-term remission with 585 nm pulsed dye laser. Clin Exp Dermatol. 2016;41:312-313.
- Kopera D, Smolle J, Kerl H. Multinucleate cell angiohistiocytoma: treatment with argon laser. Br J Dermatol. 1995;133:308-310.
- Väkevä L, Saksela O, Kariniemi AL. Multinucleate cell angiohistiocytoma: a report of four cases in Finland. Acta Derm Venereol. 2003;83:222-223.
Multinucleate cell angiohistiocytoma (MCAH) is a rare benign, soft-tissue tumor first described in 1985 by Smith and Jones1 that presents clinically as erythematous to violaceous papules most commonly affecting females on the dorsal aspect of the hands and face.2 Multinucleate cell angiohistiocytoma is histologically characterized by vascular and histiocytic proliferations with dermal fibrosis. Few cases have been reported of lesions affecting the lower extremities. We report a case of MCAH affecting the legs.
Case Report
An 83-year-old white man with a history of basal cell carcinoma presented for evaluation of grouped, well-circumscribed, soft, red-violet, painless papules on the right anterior thigh that had been present for 8 months (Figure 1A). A review of symptoms was negative for immunologic, respiratory, and hematologic changes. The patient’s medical history also was remarkable for prostate cancer treated with radiation 18 years prior as well as right hip and left knee implants. The initial clinical impression was Kaposi sarcoma or a granulomatous disorder.
Histopathologic evaluation of a deep shave biopsy initially determined the lesion to be scar tissue without other pathologic findings. The patient returned to the clinic 12 months later for a complete skin examination given his history of skin cancer. Compared to clinical photographs taken a year prior, new violaceous papules were noted on the right thigh (Figure 1B) and left calf. Furthermore, there was no recurrence of the lesion at the prior biopsy site. Shave biopsies of the papules on the right thigh and left calf demonstrated similar histologic findings to each other. There was a mild increase in the number of small blood vessels in the superficial dermis (Figure 2A). A mild perivascular lymphocytic infiltrate surrounded some of these blood vessels. The endothelial cells had small nuclei with no evidence of nuclear pleomorphism. Careful examination of the interstitial dermis revealed scattered multinucleate cells with angulated cytoplasm (Figure 2B). Immunostaining for CD31 and human herpesvirus 8 were negative, excluding an infiltrative vascular tumor and Kaposi sarcoma, respectively. The diagnosis of MCAH was made based on the histopathologic findings.


At 1-year follow-up, the condition was stable with no gross changes in the lesions based on prior photographs. Once again, there was no recurrence of the excised lesions at both biopsy sites.
Comment
Presentation
A systematic review of published reports determined that 79% of MCAH cases occur in females, with an average age of onset of 50.1 years.2 However, MCAH likely is underreported due to the overall lack of knowledge regarding this condition by physicians and pathologists. The hands and face are the most commonly affected areas, though other sites of involvement have been reported, including the lower extremities,3,4 oral mucosa and upper lip,5,6 and trunk,7,8 as well as generalized distribution.9-12 Additionally, 1 case presented as a single plaque on the trunk rather than having papular or nodular morphology.8 Multinucleate cell angiohistiocytoma lesions generally are asymptomatic, though pruritus may be present.13 The condition is regarded as benign, though a minority of cases have exhibited spontaneous resolution.14-16
Histopathology
Multinucleate cell angiohistiocytoma histology demonstrates full-thickness dermal microvessel proliferation and fibrosis with characteristic multinucleate giant cells.2,3 Vascular endothelial cells stained positive for CD68 in 60% of cases2 as well as the normal endothelial markers (ie, factor VIII, CD31, CD34). The multinucleate giant cells exhibit immunoreactivity for macrophage/histiocytic markers factor XIIIa and CD68.
Etiology
The pathogenesis of MCAH is not yet fully understood, but it is considered to be a benign vascular or fibrohistiocytic neoplasm.17 Calderaro et al18 described a series of 8 patients who developed MCAH either within a cutaneous neoplastic process or in conjunction with various cutaneous reactive conditions, including hidradenitis suppurativa and chronic radiation dermatitis, as well as overlying a bone prosthesis placed due to degenerative arthritis. These cases suggest that MCAH, or possibly a subset of the disease, is a reactive process.
Differential Diagnosis
The differential diagnosis for MCAH includes Kaposi sarcoma clinically and dermatofibroma and fibrous papules histologically. Sass et al21 determined the in vitro behavior of cultured MCAH cells to contrast markedly with Kaposi sarcoma–derived cells. Although Kaposi sarcoma–derived cells exhibited invasive behavior, cells isolated from MCAH lesions were less elongated and were unable to traverse basement membranes.
Treatment
Surgical excision or cryotherapy appear to be definitive treatments of MCAH; however, a number of cases have reported light and laser modalities as successful alternatives to excision. One case of MCAH affecting the face was treated with pulsed dye laser monotherapy.22 This modality allowed selective coagulation of the vascular structures in MCAH. At 8-month follow-up, the initial lesion was noted to be completely cleared, though similar lesions had recently appeared elsewhere on the face.22 Another case of MCAH affecting the leg was treated with pulsed dye laser and both topical and intralesional corticosteroid combination therapy. In this case, the lesion failed to respond to treatment, which may suggest that facial localization could influence response in pulsed dye laser treatment.3
Intense pulsed light also has been reported as a definitive treatment in 2 cases.2,13 Slight erythema and transient pruritus have been reported immediately following treatment. In this case, complete resolution with only residual hyperpigmentation was reported at 2-month follow-up, with no recurrence during 12 months of follow-up.13
Argon laser therapy has been used in 2 cases. After a single session, lesions were no longer palpable, with no scarring noted at 8 weeks follow-up.23 Lastly, 2 cases of MCAH have been successfully treated with the CO2 laser, with no relapse noted at 2.5- or 5-month follow-up, respectively.24
Conclusion
Multinucleate cell angiohistiocytoma is a rare and likely underdiagnosed dermatologic condition that is believed to be a reactive process. Characteristic histology of MCAH demonstrates microvascular proliferations of the dermis with multinucleate giant cells amidst a fibrous background. Although surgical excision is curative, there are reports in which laser and light therapies were used to effectively treat MCAH.
Multinucleate cell angiohistiocytoma (MCAH) is a rare benign, soft-tissue tumor first described in 1985 by Smith and Jones1 that presents clinically as erythematous to violaceous papules most commonly affecting females on the dorsal aspect of the hands and face.2 Multinucleate cell angiohistiocytoma is histologically characterized by vascular and histiocytic proliferations with dermal fibrosis. Few cases have been reported of lesions affecting the lower extremities. We report a case of MCAH affecting the legs.
Case Report
An 83-year-old white man with a history of basal cell carcinoma presented for evaluation of grouped, well-circumscribed, soft, red-violet, painless papules on the right anterior thigh that had been present for 8 months (Figure 1A). A review of symptoms was negative for immunologic, respiratory, and hematologic changes. The patient’s medical history also was remarkable for prostate cancer treated with radiation 18 years prior as well as right hip and left knee implants. The initial clinical impression was Kaposi sarcoma or a granulomatous disorder.
Histopathologic evaluation of a deep shave biopsy initially determined the lesion to be scar tissue without other pathologic findings. The patient returned to the clinic 12 months later for a complete skin examination given his history of skin cancer. Compared to clinical photographs taken a year prior, new violaceous papules were noted on the right thigh (Figure 1B) and left calf. Furthermore, there was no recurrence of the lesion at the prior biopsy site. Shave biopsies of the papules on the right thigh and left calf demonstrated similar histologic findings to each other. There was a mild increase in the number of small blood vessels in the superficial dermis (Figure 2A). A mild perivascular lymphocytic infiltrate surrounded some of these blood vessels. The endothelial cells had small nuclei with no evidence of nuclear pleomorphism. Careful examination of the interstitial dermis revealed scattered multinucleate cells with angulated cytoplasm (Figure 2B). Immunostaining for CD31 and human herpesvirus 8 were negative, excluding an infiltrative vascular tumor and Kaposi sarcoma, respectively. The diagnosis of MCAH was made based on the histopathologic findings.


At 1-year follow-up, the condition was stable with no gross changes in the lesions based on prior photographs. Once again, there was no recurrence of the excised lesions at both biopsy sites.
Comment
Presentation
A systematic review of published reports determined that 79% of MCAH cases occur in females, with an average age of onset of 50.1 years.2 However, MCAH likely is underreported due to the overall lack of knowledge regarding this condition by physicians and pathologists. The hands and face are the most commonly affected areas, though other sites of involvement have been reported, including the lower extremities,3,4 oral mucosa and upper lip,5,6 and trunk,7,8 as well as generalized distribution.9-12 Additionally, 1 case presented as a single plaque on the trunk rather than having papular or nodular morphology.8 Multinucleate cell angiohistiocytoma lesions generally are asymptomatic, though pruritus may be present.13 The condition is regarded as benign, though a minority of cases have exhibited spontaneous resolution.14-16
Histopathology
Multinucleate cell angiohistiocytoma histology demonstrates full-thickness dermal microvessel proliferation and fibrosis with characteristic multinucleate giant cells.2,3 Vascular endothelial cells stained positive for CD68 in 60% of cases2 as well as the normal endothelial markers (ie, factor VIII, CD31, CD34). The multinucleate giant cells exhibit immunoreactivity for macrophage/histiocytic markers factor XIIIa and CD68.
Etiology
The pathogenesis of MCAH is not yet fully understood, but it is considered to be a benign vascular or fibrohistiocytic neoplasm.17 Calderaro et al18 described a series of 8 patients who developed MCAH either within a cutaneous neoplastic process or in conjunction with various cutaneous reactive conditions, including hidradenitis suppurativa and chronic radiation dermatitis, as well as overlying a bone prosthesis placed due to degenerative arthritis. These cases suggest that MCAH, or possibly a subset of the disease, is a reactive process.
Differential Diagnosis
The differential diagnosis for MCAH includes Kaposi sarcoma clinically and dermatofibroma and fibrous papules histologically. Sass et al21 determined the in vitro behavior of cultured MCAH cells to contrast markedly with Kaposi sarcoma–derived cells. Although Kaposi sarcoma–derived cells exhibited invasive behavior, cells isolated from MCAH lesions were less elongated and were unable to traverse basement membranes.
Treatment
Surgical excision or cryotherapy appear to be definitive treatments of MCAH; however, a number of cases have reported light and laser modalities as successful alternatives to excision. One case of MCAH affecting the face was treated with pulsed dye laser monotherapy.22 This modality allowed selective coagulation of the vascular structures in MCAH. At 8-month follow-up, the initial lesion was noted to be completely cleared, though similar lesions had recently appeared elsewhere on the face.22 Another case of MCAH affecting the leg was treated with pulsed dye laser and both topical and intralesional corticosteroid combination therapy. In this case, the lesion failed to respond to treatment, which may suggest that facial localization could influence response in pulsed dye laser treatment.3
Intense pulsed light also has been reported as a definitive treatment in 2 cases.2,13 Slight erythema and transient pruritus have been reported immediately following treatment. In this case, complete resolution with only residual hyperpigmentation was reported at 2-month follow-up, with no recurrence during 12 months of follow-up.13
Argon laser therapy has been used in 2 cases. After a single session, lesions were no longer palpable, with no scarring noted at 8 weeks follow-up.23 Lastly, 2 cases of MCAH have been successfully treated with the CO2 laser, with no relapse noted at 2.5- or 5-month follow-up, respectively.24
Conclusion
Multinucleate cell angiohistiocytoma is a rare and likely underdiagnosed dermatologic condition that is believed to be a reactive process. Characteristic histology of MCAH demonstrates microvascular proliferations of the dermis with multinucleate giant cells amidst a fibrous background. Although surgical excision is curative, there are reports in which laser and light therapies were used to effectively treat MCAH.
- Smith NP, Jones EW. Multinucleate cell angiohistiocytoma—a new entity. Br J Dermatol. 1985;113:15.
- Frew JW. Multinucleate cell angiohistiocytoma: clinicopathological correlation of 142 cases with insights into etiology and pathogenesis. Am J Dermatopathol. 2015;37:222-228.
- Applebaum DS, Shuja F, Hicks L, et al. Multinucleate cell angiohistiocytoma: a case report and review of the literature. Dermatol Online J. 2014;20:22610.
- Sagdeo A, Chu EY, Elenitsas R, et al. Multiple asymptomatic violaceous macules on the thigh. Multinucleate cell angiohistiocytoma (MCAH). JAMA Dermatol. 2013;149:357-363.
- Rawal YB, Anderson KM, Rawal SY. Multinucleate cell angiohistiocytoma: an uncommon mucosal tumour. Clin Exp Dermatol. 2009;34:333-336.
- Jones AC, Mullins D, Jimenez F. Multinucleate cell angiohistiocytoma of the upper lip. Oral Surg Oral Med Oral Pathol. 1994;78:743-747.
- Doshi-Chougule BN, Gust A, Mentzel T, et al. Multinucleate cell angiohistiocytoma with hypertrophic nerves. J Cutan Pathol. 2013;40:1048-1053.
- Issa AA, Lui H, Shapiro J, et al. Plaque-type multinucleate cell angiohistiocytoma. J Cutan Med Surg. 1998;3:112-114.
- Doane JA, Purdy K, Pasternak S. Generalized multinucleate cell angiohistiocytoma. J Cutan Med Surg. 2015;19:323-325.
- Marti N, Monteagudo C, Revert A, et al. Multiple papules on the trunk and extremities. generalized multinucleate cell angiohistiocytoma. Int J Dermatol. 2013;52:544-546.
- O’Blenes CA, Walsh NM, Green PJ, et al. Novel case of generalized multinucleate cell angiohistiocytoma. J Cutan Med Surg. 2010;14:178-180.
- Chang SN, Kim HS, Kim SC, et al. Generalized multinucleate cell angiohistiocytoma. J Am Acad Dermatol. 1996;35:320-322.
- Fernández-Jorge B, Del Pozo J, García-Silva J, et al. Multinucleate cell angiohistiocytoma: treatment using intense pulsed light. Dermatol Surg. 2009;35:1141-1143.
- Perez LP, Zulaica A, Rodriguez L, et al. Multinucleate cell angiohistiocytoma. report of five cases. J Cutan Pathol. 2006;33:349-352.
- Shapiro PE, Nova MP, Rosmarin LA, et al. Multinucleate cell angiohistiocytoma: a distinct entity diagnosable by clinical and histologic features. J Am Acad Dermatol. 1994;30:417-422.
- Jaconelli L, Kanitakis J, Ktiouet S, et al. Multinucleate cell angiohistiocytoma: report of three new cases and literature review. Dermatol Online J. 2009;15:4.
- Jones WE, Cerio R, Smith NP. Multinucleate cell angiohistiocytoma: an acquired vascular anomaly to be distinguished from Kaposi’s sarcoma. Br J Dermatol. 1990;122:651-663.
- Calderaro J, Rethers L, Ortonne N. Multinucleated cells angiohistiocytoma: a reactive lesion? Am J Dermatopathol. 2010;32:415-417.
- Cesinaro AM, Roncati L, Maiorana A. Estrogen receptor alpha overexpression in multinucleate cell angiohistiocytoma: new insights into the pathogenesis of a reactive process. Am J Dermatopathol. 2010;32:655-659.
- Losordo DW, Isner JM. Estrogen and angiogenesis: a review. Arterioscler Thromb Vasc Biol. 2001;21:6-12.
- Sass U, Noel JC, Andre J, et al. Multinucleate cell angiohistiocytoma: report of two cases with no evidence of human herpesvirus-8 infection. J Cutan Pathol. 2000;27:258-261.
- Richer V, Lui H. Facial multinucleate cell angiohistiocytoma: long-term remission with 585 nm pulsed dye laser. Clin Exp Dermatol. 2016;41:312-313.
- Kopera D, Smolle J, Kerl H. Multinucleate cell angiohistiocytoma: treatment with argon laser. Br J Dermatol. 1995;133:308-310.
- Väkevä L, Saksela O, Kariniemi AL. Multinucleate cell angiohistiocytoma: a report of four cases in Finland. Acta Derm Venereol. 2003;83:222-223.
- Smith NP, Jones EW. Multinucleate cell angiohistiocytoma—a new entity. Br J Dermatol. 1985;113:15.
- Frew JW. Multinucleate cell angiohistiocytoma: clinicopathological correlation of 142 cases with insights into etiology and pathogenesis. Am J Dermatopathol. 2015;37:222-228.
- Applebaum DS, Shuja F, Hicks L, et al. Multinucleate cell angiohistiocytoma: a case report and review of the literature. Dermatol Online J. 2014;20:22610.
- Sagdeo A, Chu EY, Elenitsas R, et al. Multiple asymptomatic violaceous macules on the thigh. Multinucleate cell angiohistiocytoma (MCAH). JAMA Dermatol. 2013;149:357-363.
- Rawal YB, Anderson KM, Rawal SY. Multinucleate cell angiohistiocytoma: an uncommon mucosal tumour. Clin Exp Dermatol. 2009;34:333-336.
- Jones AC, Mullins D, Jimenez F. Multinucleate cell angiohistiocytoma of the upper lip. Oral Surg Oral Med Oral Pathol. 1994;78:743-747.
- Doshi-Chougule BN, Gust A, Mentzel T, et al. Multinucleate cell angiohistiocytoma with hypertrophic nerves. J Cutan Pathol. 2013;40:1048-1053.
- Issa AA, Lui H, Shapiro J, et al. Plaque-type multinucleate cell angiohistiocytoma. J Cutan Med Surg. 1998;3:112-114.
- Doane JA, Purdy K, Pasternak S. Generalized multinucleate cell angiohistiocytoma. J Cutan Med Surg. 2015;19:323-325.
- Marti N, Monteagudo C, Revert A, et al. Multiple papules on the trunk and extremities. generalized multinucleate cell angiohistiocytoma. Int J Dermatol. 2013;52:544-546.
- O’Blenes CA, Walsh NM, Green PJ, et al. Novel case of generalized multinucleate cell angiohistiocytoma. J Cutan Med Surg. 2010;14:178-180.
- Chang SN, Kim HS, Kim SC, et al. Generalized multinucleate cell angiohistiocytoma. J Am Acad Dermatol. 1996;35:320-322.
- Fernández-Jorge B, Del Pozo J, García-Silva J, et al. Multinucleate cell angiohistiocytoma: treatment using intense pulsed light. Dermatol Surg. 2009;35:1141-1143.
- Perez LP, Zulaica A, Rodriguez L, et al. Multinucleate cell angiohistiocytoma. report of five cases. J Cutan Pathol. 2006;33:349-352.
- Shapiro PE, Nova MP, Rosmarin LA, et al. Multinucleate cell angiohistiocytoma: a distinct entity diagnosable by clinical and histologic features. J Am Acad Dermatol. 1994;30:417-422.
- Jaconelli L, Kanitakis J, Ktiouet S, et al. Multinucleate cell angiohistiocytoma: report of three new cases and literature review. Dermatol Online J. 2009;15:4.
- Jones WE, Cerio R, Smith NP. Multinucleate cell angiohistiocytoma: an acquired vascular anomaly to be distinguished from Kaposi’s sarcoma. Br J Dermatol. 1990;122:651-663.
- Calderaro J, Rethers L, Ortonne N. Multinucleated cells angiohistiocytoma: a reactive lesion? Am J Dermatopathol. 2010;32:415-417.
- Cesinaro AM, Roncati L, Maiorana A. Estrogen receptor alpha overexpression in multinucleate cell angiohistiocytoma: new insights into the pathogenesis of a reactive process. Am J Dermatopathol. 2010;32:655-659.
- Losordo DW, Isner JM. Estrogen and angiogenesis: a review. Arterioscler Thromb Vasc Biol. 2001;21:6-12.
- Sass U, Noel JC, Andre J, et al. Multinucleate cell angiohistiocytoma: report of two cases with no evidence of human herpesvirus-8 infection. J Cutan Pathol. 2000;27:258-261.
- Richer V, Lui H. Facial multinucleate cell angiohistiocytoma: long-term remission with 585 nm pulsed dye laser. Clin Exp Dermatol. 2016;41:312-313.
- Kopera D, Smolle J, Kerl H. Multinucleate cell angiohistiocytoma: treatment with argon laser. Br J Dermatol. 1995;133:308-310.
- Väkevä L, Saksela O, Kariniemi AL. Multinucleate cell angiohistiocytoma: a report of four cases in Finland. Acta Derm Venereol. 2003;83:222-223.
Practice Points
- Multinucleate cell angiohistiocytoma (MCAH) is a rare underrecognized cutaneous tumor presenting as erythematous to violaceous papules.
- Although it clinically mimics Kaposi sarcoma, MCAH may be distinguished histopathologically by negative immunostaining for human herpesvirus 8.
- Surgical excision and laser therapies are definitive treatments for MCAH, which is a benign lesion.
Direct and Indirect Patient Costs of Dermatology Clinic Visits and Their Impact on Access to Care and Provider Preference
Access to outpatient specialty care is notably limited due to time and out-of-pocket costs to patients, leading to patient dissatisfaction and worsened clinical outcomes. Lost time and earnings pose considerable opportunity costs for patients, with the total opportunity cost for all physician visits per year estimated at $52 billion in 2010 in the United States.1
The field of dermatology exemplifies the access issues patients may face when seeking specialty care given the ongoing national shortage of dermatologists and notably long wait times exceeding 60 days in major cities.2-4 With the high demand and limited number of providers, patients may have longer wait times to see dermatologists in their communities or have to travel further to see dermatologists in distant locations who have available appointments; therefore, patients may be subject to higher associated time, travel, and monetary costs. According to the 2013 Medical Expenditure Panel Survey, dermatology visits in the United States cost an average of $221 per visit compared to $166 for primary care. Dermatology visits had the highest median cost per office visit ($124) and were more often associated with out-of-pocket expenses (60.7%) compared to other specialties.5 Despite these high costs, the number of dermatology visits is increasing each year, with more than 38 million dermatology visits in 2012.6
In light of these factors that limit patient access to dermatologists compared to other specialists, we performed an evaluation of the direct and indirect costs to patients visiting an outpatient dermatology clinic in Boston, Massachusetts, to better understand obstacles to receiving dermatologic care. The impact that time and money have on how patients prefer to receive their care also was evaluated. Conducting this study in Boston may best reflect patient barriers to obtaining dermatologic treatment, as nationwide surveys have found that Boston has the highest cumulative average wait times for physician appointments compared to other US metropolitan cities, with an average wait time of 72 days to see a dermatologist.4 New studies of patient costs associated with dermatology clinic visits are lacking, and existing economic analyses rarely include time costs. Understanding time burden and opportunity costs from the patient perspective may motivate patients and physicians to alter how they receive and provide health care, respectively, to minimize these expenses. Advances in health care technology such as telecommunication may facilitate these changes.
Methods
Study Design
This survey study took place from October 1, 2015, to March 4, 2016, at the department of dermatology outpatient clinic of Tufts Medical Center, an academic university hospital located in downtown Boston, Massachusetts, with no satellite clinics. Five general dermatologists, 2 dermatologic surgeons, and 9 dermatology residents comprised the dermatology department. The study protocol and questionnaire received exemption status from the Tufts University Health Science’s institutional review board.
All adult patients (aged ≥18 years) attending a scheduled dermatology clinic visit within the designated time frame were invited to complete a questionnaire available in English, Spanish, or Chinese. Patients completed the questionnaire on paper or electronically using handheld tablets. Data were then compiled into the REDCap (Research Electronic Data Capture) online database. The questionnaire surveyed patient age; gender; ethnicity; language spoken; highest level of education; employment status; reason for visit (ie, skin condition); duration, cost, and mode of transportation; duration of visit including wait time; companion accompaniment; profession; hourly wage; and number of work hours requested off to attend the visit. Lastly, patients were surveyed on whether they prefer to receive dermatologic care at Tufts, to receive in-person care elsewhere, to use teledermatology, or none of the above.
Statistical Analysis
Total time attributed to the visit was the sum of time for round-trip travel to and from the clinic, wait time, and face-to-face time with care providers. Out-of-pocket patient expenses included round-trip travel expenses, child care expenses, and direct payments such as deductibles and co-pays. Opportunity cost for employed patients was calculated as the patient’s average hourly wage multiplied by either the number of hours taken off from work or the number of hours the patient attributed to the visit, whichever value was higher at the individual level. For the purpose of calculating opportunity costs, travel time, wait time, and face-to-face time were imputed using average values for these variables when not reported. Patients could provide exact hourly wage and annual income or select the closest approximation from 10 wage ranges. For patients who selected a wage range, the midpoint of the range was used as the hourly wage. Total costs were the sum of reported out-of-pocket expenses and calculated opportunity costs. For unemployed patients and those who did not report employment status, hourly wage was assumed to be $0, resulting in opportunity costs of $0. Costs are tabulated for individual patients and analyzed in aggregate.
Differences in patient characteristics between those who preferred their current care provider versus those who preferred to seek care elsewhere or via teledermatology were compared using the χ2 and Student t test. A multivariate logistic regression was then performed to identify predictors of patient preference for their current provider. Potential predictors for regression model were time and cost variables as well as factors selected based on results from bivariate analysis. Data analysis was performed using statistical software.
Results
Demographics
Demographic data for respondents are outlined in Table 1. Of 145 patients who completed the survey, the majority had already seen a dermatologist for their presenting condition (87.4%), and were English speaking (96.5%), white (76.6%), employed (59.4%), and male (50.3%), with a mean age (SD) of 52.3 (18.1) years and education level of 4-year university or higher (64.7%). The most common reasons for dermatology clinic attendance were general skin checks (30.8%) and psoriasis (26.6%). A smaller proportion of patients (16.1%) presented for surgical visits. Other less common conditions that brought patients into the clinic included acne (6.3%), eczema (4.9%), and skin rash (2.8%).
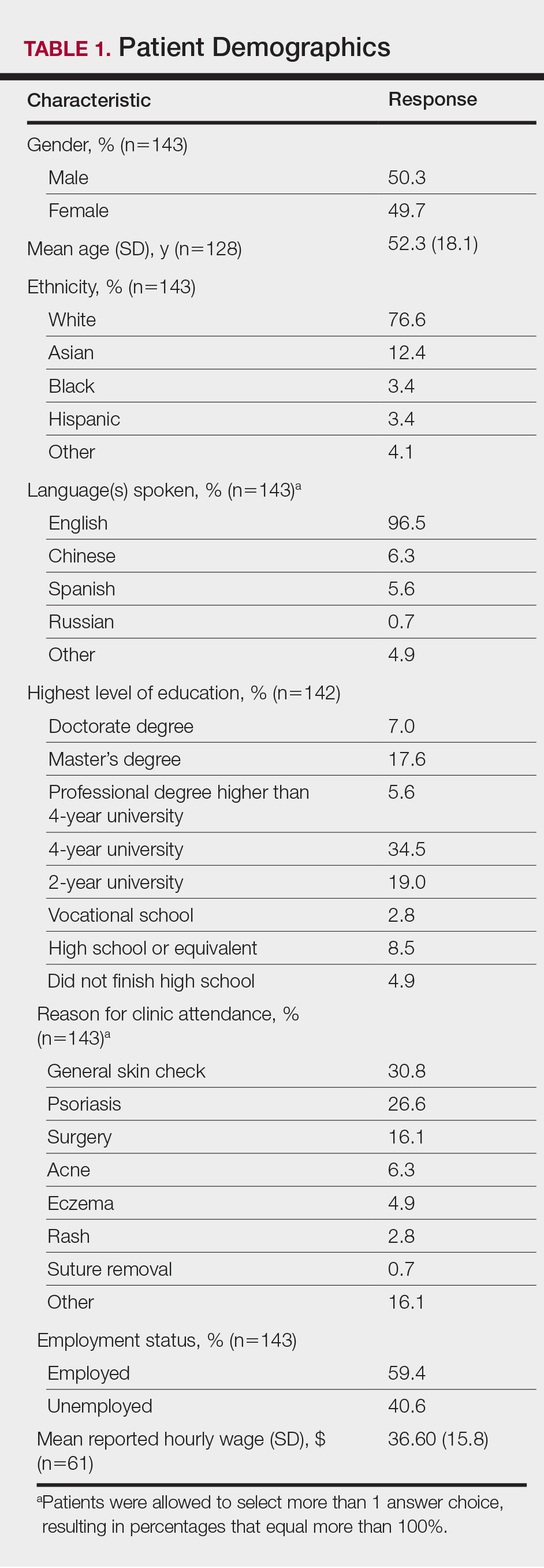
The mean (SD) reported hourly wage of employed patients was $36.60 (15.8). The most common reasons for unemployment were retirement (65.5% [38/58]), disability (10.3% [6/58]), and schooling (10.3% [6/58]).
Time Attributed to Attending Dermatology Clinic Visits
Time costs are reported in Table 2. Patients traveled to the clinic mainly by car (56.5% [78/138]) or train/subway (25.3% [35/138]). One in approximately 5 patients (21.3%) spent more than 1 hour traveling one-way to the clinic. Most patients waited less than 20 minutes to see their care providers. Face-to-face time with providers (ie, residents and attending physicians) ranged from less than 21 minutes to more than 1 hour, with a mean (SD) time of 36.8 (18.9) minutes.
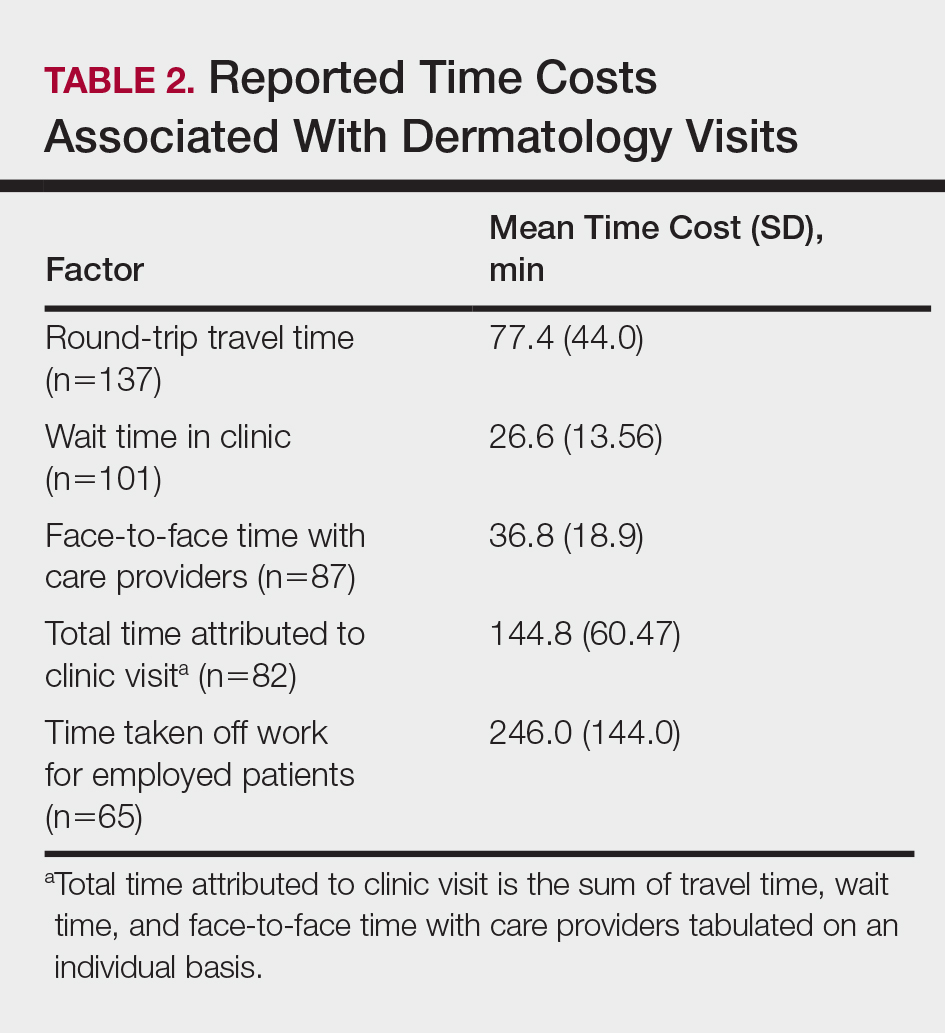
Of the employed respondents, 76.5% (65/85) took off time from work for the appointment. Patients took a mean (SD) of 4.1 (2.4) hours off from work, which was considered sick pay (35%), paid time off (36.6%), or unpaid time (28.3%). The total mean (SD) time dedicated to attending the clinic appointment averaged 144.8 (60.47) minutes. On average, the time spent traveling for the clinic visit was double the amount of time spent with the care provider (77.4 vs 36.8 minutes).
Monetary and Opportunity Costs
The mean (SD) monetary cost associated with clinic attendance for employed patients who reported their wages was $187.50 (103.2)(range, $37.50–$489), most of which was opportunity cost from loss of potential work income (mean [SD], $144.30 [93.6]; range, $27–$432)(Table 3). Similar total and opportunity costs were found for employed patients using the imputed average wage. The mean (SD) total cost per visit for unemployed patients or those who did not report employment status was $38.65 (103.6)(range, $0–$800), which was 4-times less than the cost per visit for employed patients. Mean (SD) and median one-way travel expenses were $16.60 (40.5) and $10, respectively. Mean (SD) and median reported costs for deductibles/co-pays were $44.20 (66.1) and $25, respectively. Only 2 patients reported child care costs, which were valued at $65 and $75.
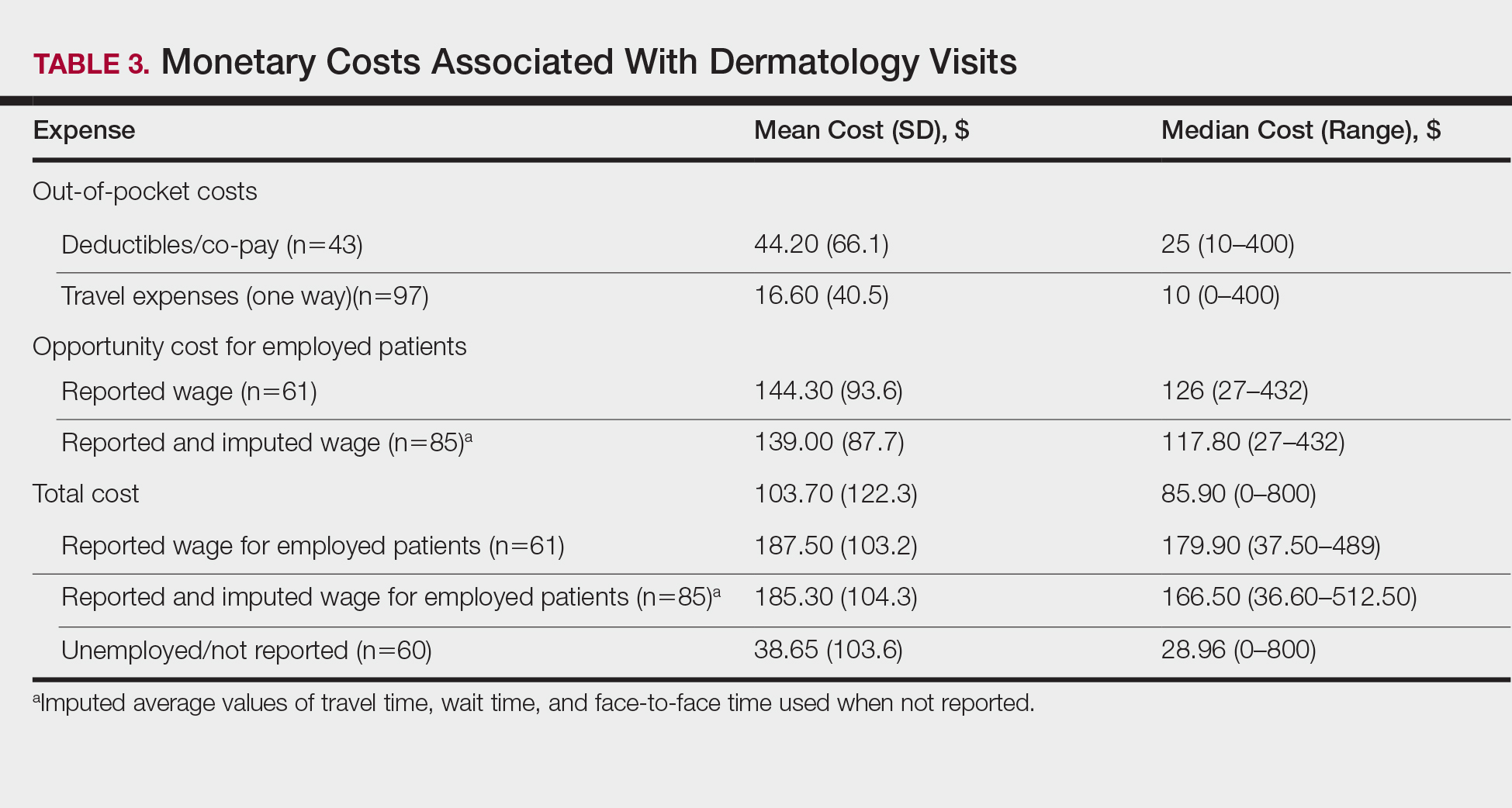
Patient Provider Preference
The majority (59.3% [67/113]) of patients preferred their current care providers, whereas 33.6% (38/113) preferred providers closer to work, home, or in a different unspecified setting. Only 7.0% (8/113) of patients who answered this survey question would choose teledermatology over their current providers.
On multivariate logistic regression (Table 4), patients who had additional out-of-pocket costs were significantly less likely to prefer their current care provider compared to patients with no out-of-pocket costs (odds ratio [OR], 0.27; 95% confidence interval [CI], 0.10-0.71; P<.05). Opportunity costs were not a significant predictor of provider preference. For every minute the travel time increased, the likelihood of preference for the current care provider decreased by 2% (OR, 0.98; 95% CI, 0.95–0.99), and patients who traveled 60 minutes or more round-trip were 71% less likely to choose current provider care than those who traveled less than 60 minutes (OR, 0.29; 95% CI, 0.09-0.96; P<.05). Patients with higher education (≥4 years of college) were 3.29-times more likely to stay with their current care provider than those with lower education (≤2 years of college). Those presenting for skin checks also preferred the current provider more than those with noninflammatory skin conditions such as alopecia and warts (OR, 9.01; 95% CI, 2.28-35.59). Age and gender were not statistically significant predictors of patient provider preference.
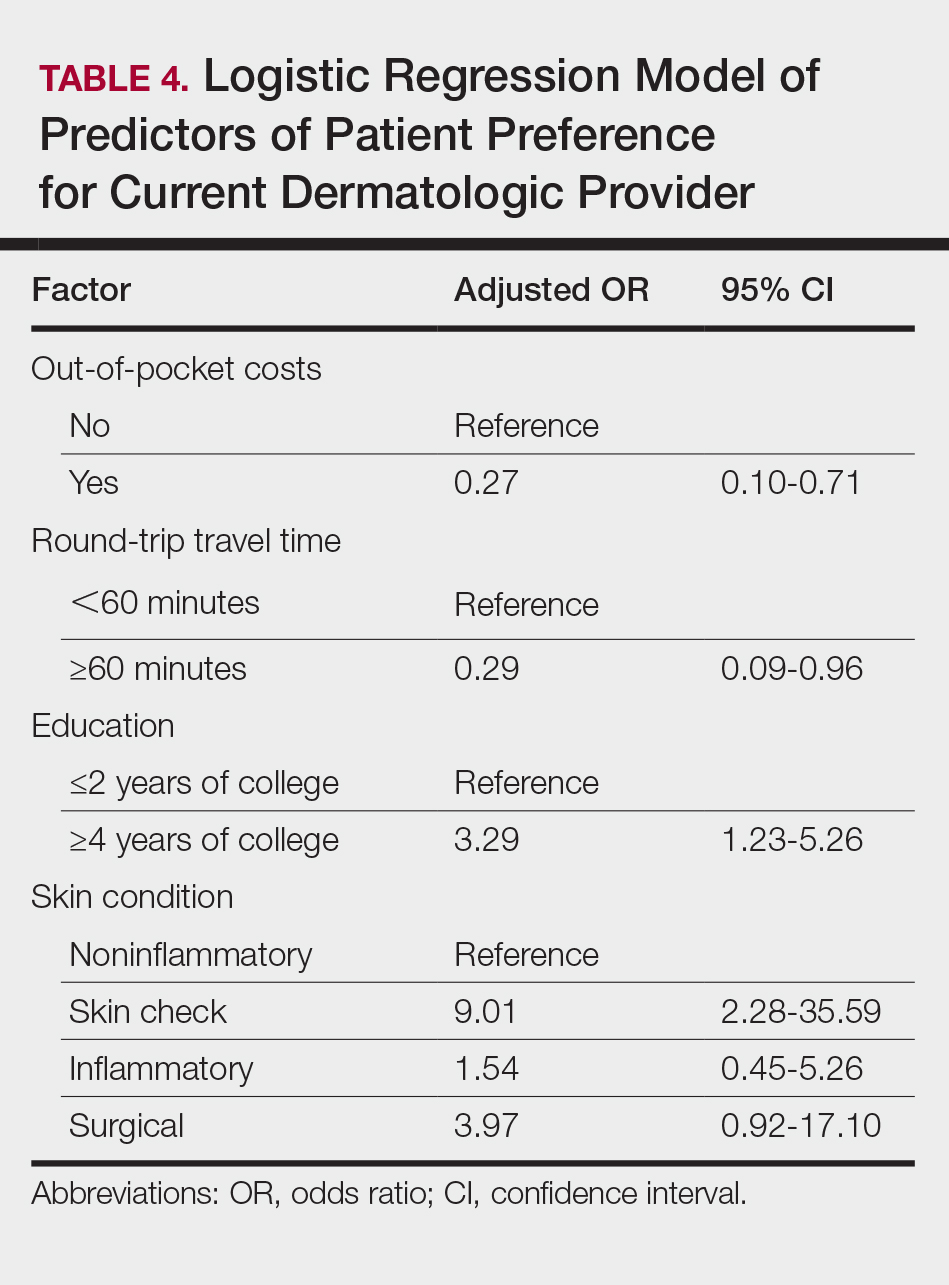
Comment
Our study revealed that patients spend a substantial amount of time and money attending dermatology clinic appointments. Round-trip travel time exceeded 2 hours for 20% of patients and accounted for the majority of the total time attributed to the visit. Patients who were employed typically requested an average of 4 hours off from work, resulting in a mean (SD) opportunity cost of $144.30 (93.6) due to lost wages. Direct costs such as co-pays, deductibles, travel expenses, and child care accounted for a smaller proportion of total costs. The study assumed a wage of $0 for unemployed patients, thus underestimating the true costs of the visit for these patients whose time may otherwise have been spent on leisure, education, volunteerism, or other activities that contribute to individual and societal productivity. The total costs for unemployed patients reflected only direct costs, and thus were notably lower than those for employed patients.
Direct out-of-pocket costs and travel time negatively impacted provider preference. Patients with out-of-pocket costs were much less likely to stay with their current care provider (OR, 0.27; 95% CI, 0.10-0.71), preferring to seek care closer to home/work or teledermatology services. Similarly, for each minute that travel time increased, preference for current care provider decreased by 2%. Those who traveled 60 minutes or more were 71% less likely than those who traveled less than 60 minutes to stay with their current provider when given other options for care. Opportunity costs did not affect provider preference, even though they far exceeded direct costs for employed patients. Perhaps opportunity costs are not as immediately apparent to patients as out-of-pocket costs and travel time, and thus they do not factor as heavily in provider preference.
Despite high time and monetary costs, the majority of patients (60%) still preferred their current care provider, especially those with 4-year university degrees or higher education level (OR, 3.29; 95% CI, 1.23-5.26) and those presenting for skin checks (OR, 9.01; 95% CI, 2.28-35.59). Patients with higher levels of education likely have higher incomes and thus may not be as adversely affected by direct and/or indirect visit costs. Patients presenting for skin checks may value continuity and prefer providers with whom they already have an established therapeutic relationship. Future studies are needed to analyze the impact of these nonmonetary factors on provider preference.
Seeking Alternative Care
Tufts Medical Center does not have satellite dermatology clinics, making it the only option for patients who wish to receive care within the Tufts hospital network. However, patients do have the option of visiting non–Tufts-affiliated dermatology clinics outside of the city. To our knowledge, no formal studies have been performed comparing wait times for dermatology appointments in suburban versus urban Boston areas; however, it has been reported that rural practitioners have longer wait times than urban dermatologists, possibly due to the fact that physicians tend to aggregate in metropolitan areas.2 Thus, the potential for shorter wait times in the Boston metropolitan area may make it a more desirable location to receive care compared to more suburban or even rural areas of Massachusetts, but additional data are needed to substantiate this hypothesis. Additionally, health insurance restrictions, refractory or complex dermatologic conditions, and referring providers’ preference may affect patients’ decisions to seek care at a particular clinic. However, these factors do not alter our finding that those who travel long distances to our dermatology clinic are less likely to stay with their current provider if given the choice to seek care closer to home/work or utilize teledermatology services.
Prior studies have demonstrated patient preference and willingness to accept alternative modes of care delivery to reduce time and monetary costs associated with in-person medical visits.7,8 Dermatology patients at a clinic in Ontario, Canada, considered the time they spent attending the clinic to be even more burdensome than the monetary cost.7 Patients with nondermatologic chronic diseases and high out-of-pocket costs would prefer email rather than a clinic visit as the first method of contact with care providers.8 The explosive growth of direct-to-consumer (DTC) teledermatology services in the last 10 years speaks to patient demand for alternative care delivery that saves time and money. Although telemedicine has been implemented in various specialties, including ophthalmology and neurology, one of the most common applications is teledermatology. With DTC teledermatology, patients can take photographs or videos using personal smartphones and communicate directly with care providers using mobile or online applications. More recent review articles have identified 22 to 29 DTC mobile and web-based teledermatology services, with costs varying from $0 to $250.9-11 The median consultation fee of $59 for DTC teledermatology services is substantially less than total visit costs for employed patients in our study.9 Teledermatology has become an accessible and affordable modality of care, though perhaps not yet fully optimized for quality of care.
With increasingly higher co-pays and high-deductible insurance plans, time and monetary factors play increasingly important roles in patient preference for specialty care providers,12 as demonstrated by our study. Dermatologists can work with patients to reduce the costs of medical visits. Perhaps monitoring of chronic but stable conditions can be accomplished through telecommunication to reduce the number of follow-up visits. For instance, psoriasis patients enrolled in telemonitoring perceived savings of time and expenses through reduction of clinic visits, resulting in high patient satisfaction levels.13 Telephone calls and secure email messaging are other feasible alternatives shown to aid in clinical management and decrease the need for in-person care.8,14 Fewer unnecessary follow-up visits also means more availability for new patients and those with acute needs.
Barriers to obtaining care are not limited to dermatology and are pervasive across most medical specialties. Issues of patient time burden and out-of-pocket expenses are reflected in recent reports focused on quantifying these costs throughout ambulatory care visits and services such as colorectal, cervical, and breast cancer screenings.1,15-18 Similar to our findings, many of these studies also show high time and opportunity costs from the patient perspective. Expansion of telemedicine to reduce patient costs is becoming a viable option for many specialists, though low reimbursement rates restrict its widespread application.9,19 However, this obstacle is not impossible to surmount. One study found that offering teledermatology to Medicaid patients through their primary care providers significantly improved access, allowing for a 63.8% increase in the number of patients visiting a dermatologist (P<.01).20 Currently, a total of 48 state Medicaid programs now cover telemedicine, and a growing number of states are requiring private insurers to cover telehealth services.21 As more dermatologists adopt telemedicine practices, it may allow for better access as well as expanded insurance coverage.
Limitations
The results of our study are limited by the single-institution survey design. Patients were asked to complete the survey while still at the clinic visit to minimize recall bias. Because these patients actually attended their appointments, they might perceive the time and monetary costs associated with the visit to be less problematic than those who canceled their appointments or transferred care elsewhere; however, we were still able to detect a significant impact of time and monetary costs on provider preference in this cohort (P<.05). Larger studies in different geographic settings and other specialty clinics are needed to confirm our findings and to determine if nonmonetary factors such as specific diagnoses, length of time with a certain care provider, or patient socioeconomic status can modulate the impact of time and monetary costs on provider preference.
Conclusion
This study showed that patients expend a substantial amount of time and monetary costs to attend dermatology clinic visits. Data from the current and prior studies suggest that these costs affect patient provider preference for dermatologic care and may pose barriers to necessary medical care. Recognizing direct and indirect patient costs may drive critical changes in health care delivery, such as increased telecommunication utilization, the more cost-saving alternative. Telemedicine, when integrated appropriately, can help minimize expenses for patients while continuing to maintain a high level of care.
- Ray KN, Chari AV, Engberg J, et al. Opportunity costs of ambulatory medical care in the United States. Am J Manag Care. 2015;21:567-574.
- Kimball AB, Resneck JS Jr. The US dermatology workforce: a specialty remains in shortage. J Am Acad Dermatol. 2008;59:741-745.
- Resneck JS Jr, Lipton S, Pletcher MJ. Short wait times for patients seeking cosmetic botulinum toxin appointments with dermatologists. J Am Acad Dermatol. 2007;57:985-989.
- Physician appointment wait times & Medicaid and Medicare acceptance rates. Merritt Hawkins website. https://www.merritthawkins.com/2014-survey/patientwaittime.aspx. Accessed February 15, 2017.
- Machlin SR, Adams SA. Expenses for office-based physician visits by specialty, 2013. Agency for Healthcare Research and Quality website. https://meps.ahrq.gov/data_files/publications/st484/stat484.pdf. Published November 2015. Accessed February 15, 2017.
- National ambulatory medical care survey: 2012 state and national summary tables. CDC website. www.cdc.gov/nchs/data/ahcd/namcs_summary/2012_namcs_web_tables.pdf. Accessed February 15, 2017.
- Vignjevic PM, Hux JE, Fisher BK, et al. Monetary and nonmonetary costs to patients attending an ambulatory dermatology clinic. J Cutan Med Surg. 1999;3:188-192.
- Reed M, Graetz I, Gordon N, Fung V. Patient-initiated e-mails to providers: associations with out-of-pocket visit costs, and impact on care-seeking and health. Am J Manag Care. 2015;21:E632-E639.
- Peart JM, Kovarik C. Direct-to-patient teledermatology practices. J Am Acad Dermatol. 2015;72:907-909.
- Fogel AL, Sarin KY. A survey of direct-to-consumer teledermatology services available to US patients: explosive growth, opportunities and controversy. J Telemed Telecare. 2017;23:19-25.
- Kochmann M, Locatis C. Direct to consumer mobile teledermatology apps: an exploratory study. Telemed J E Health. 2016;22:689-693.
- Helms AD. High-deductible health plans can ruin finances. Kaiser Health News website. https://khn.org/news/high-deductible-health-plans-can-ruin-finances/. Published April 6, 2015. Accessed February 15, 2017.
- Fruhauf J, Schwantzer G, Ambros-Rudolph CM, et al. Pilot study on the acceptance of mobile teledermatology for the home monitoring of high-need patients with psoriasis. Australas J Dermatol. 2012;53:41-46.
- Eisenberg D, Hwa K, Wren SM. Telephone follow-up by a midlevel provider after laparoscopic inguinal hernia repair instead of face-to-face clinic visit. JSLS. 2015;19:e2014.00205.
- Yabroff KR, Guy GP Jr, Ekwueme DU, et al. Annual patient time costs associated with medical care among cancer survivors in the United States. Med Care. 2014;52:594-601.
- Yabroff KR, Davis WW, Lamont EB, et al. Patient time costs associated with cancer care. J Natl Cancer Inst. 2007;99:14-23.
- Jonas DE, Russell LB, Sandler RS, et al. Value of patient time invested in the colonoscopy screening process: time requirements for colonoscopy study. Med Decis Making. 2008;28:56-65.
- Shireman TI, Tsevat J, Goldie SJ. Time costs associated with cervical cancer screening. Int J Technol Assess Health Care. 2001;17:146-152.
- Dorsey ER, Topol EJ. State of telehealth. N Engl J Med. 2016;375:154-161.
- Uscher-Pines L, Malsberger R, Burgette L, et al. Effect of teledermatology on access to dermatology care among Medicaid enrollees. JAMA Dermatol. 2016;152:905-912.
- Thomas L, Capistrant G. State telemedicine gaps analysis: coverage & reimbursement. Telehealth website. http://www.mtelehealth.com/state-telemedicine-gaps-analysis-coverage-reimbursement/. Published January 19, 2016. Accessed February 15, 2017.
Access to outpatient specialty care is notably limited due to time and out-of-pocket costs to patients, leading to patient dissatisfaction and worsened clinical outcomes. Lost time and earnings pose considerable opportunity costs for patients, with the total opportunity cost for all physician visits per year estimated at $52 billion in 2010 in the United States.1
The field of dermatology exemplifies the access issues patients may face when seeking specialty care given the ongoing national shortage of dermatologists and notably long wait times exceeding 60 days in major cities.2-4 With the high demand and limited number of providers, patients may have longer wait times to see dermatologists in their communities or have to travel further to see dermatologists in distant locations who have available appointments; therefore, patients may be subject to higher associated time, travel, and monetary costs. According to the 2013 Medical Expenditure Panel Survey, dermatology visits in the United States cost an average of $221 per visit compared to $166 for primary care. Dermatology visits had the highest median cost per office visit ($124) and were more often associated with out-of-pocket expenses (60.7%) compared to other specialties.5 Despite these high costs, the number of dermatology visits is increasing each year, with more than 38 million dermatology visits in 2012.6
In light of these factors that limit patient access to dermatologists compared to other specialists, we performed an evaluation of the direct and indirect costs to patients visiting an outpatient dermatology clinic in Boston, Massachusetts, to better understand obstacles to receiving dermatologic care. The impact that time and money have on how patients prefer to receive their care also was evaluated. Conducting this study in Boston may best reflect patient barriers to obtaining dermatologic treatment, as nationwide surveys have found that Boston has the highest cumulative average wait times for physician appointments compared to other US metropolitan cities, with an average wait time of 72 days to see a dermatologist.4 New studies of patient costs associated with dermatology clinic visits are lacking, and existing economic analyses rarely include time costs. Understanding time burden and opportunity costs from the patient perspective may motivate patients and physicians to alter how they receive and provide health care, respectively, to minimize these expenses. Advances in health care technology such as telecommunication may facilitate these changes.
Methods
Study Design
This survey study took place from October 1, 2015, to March 4, 2016, at the department of dermatology outpatient clinic of Tufts Medical Center, an academic university hospital located in downtown Boston, Massachusetts, with no satellite clinics. Five general dermatologists, 2 dermatologic surgeons, and 9 dermatology residents comprised the dermatology department. The study protocol and questionnaire received exemption status from the Tufts University Health Science’s institutional review board.
All adult patients (aged ≥18 years) attending a scheduled dermatology clinic visit within the designated time frame were invited to complete a questionnaire available in English, Spanish, or Chinese. Patients completed the questionnaire on paper or electronically using handheld tablets. Data were then compiled into the REDCap (Research Electronic Data Capture) online database. The questionnaire surveyed patient age; gender; ethnicity; language spoken; highest level of education; employment status; reason for visit (ie, skin condition); duration, cost, and mode of transportation; duration of visit including wait time; companion accompaniment; profession; hourly wage; and number of work hours requested off to attend the visit. Lastly, patients were surveyed on whether they prefer to receive dermatologic care at Tufts, to receive in-person care elsewhere, to use teledermatology, or none of the above.
Statistical Analysis
Total time attributed to the visit was the sum of time for round-trip travel to and from the clinic, wait time, and face-to-face time with care providers. Out-of-pocket patient expenses included round-trip travel expenses, child care expenses, and direct payments such as deductibles and co-pays. Opportunity cost for employed patients was calculated as the patient’s average hourly wage multiplied by either the number of hours taken off from work or the number of hours the patient attributed to the visit, whichever value was higher at the individual level. For the purpose of calculating opportunity costs, travel time, wait time, and face-to-face time were imputed using average values for these variables when not reported. Patients could provide exact hourly wage and annual income or select the closest approximation from 10 wage ranges. For patients who selected a wage range, the midpoint of the range was used as the hourly wage. Total costs were the sum of reported out-of-pocket expenses and calculated opportunity costs. For unemployed patients and those who did not report employment status, hourly wage was assumed to be $0, resulting in opportunity costs of $0. Costs are tabulated for individual patients and analyzed in aggregate.
Differences in patient characteristics between those who preferred their current care provider versus those who preferred to seek care elsewhere or via teledermatology were compared using the χ2 and Student t test. A multivariate logistic regression was then performed to identify predictors of patient preference for their current provider. Potential predictors for regression model were time and cost variables as well as factors selected based on results from bivariate analysis. Data analysis was performed using statistical software.
Results
Demographics
Demographic data for respondents are outlined in Table 1. Of 145 patients who completed the survey, the majority had already seen a dermatologist for their presenting condition (87.4%), and were English speaking (96.5%), white (76.6%), employed (59.4%), and male (50.3%), with a mean age (SD) of 52.3 (18.1) years and education level of 4-year university or higher (64.7%). The most common reasons for dermatology clinic attendance were general skin checks (30.8%) and psoriasis (26.6%). A smaller proportion of patients (16.1%) presented for surgical visits. Other less common conditions that brought patients into the clinic included acne (6.3%), eczema (4.9%), and skin rash (2.8%).

The mean (SD) reported hourly wage of employed patients was $36.60 (15.8). The most common reasons for unemployment were retirement (65.5% [38/58]), disability (10.3% [6/58]), and schooling (10.3% [6/58]).
Time Attributed to Attending Dermatology Clinic Visits
Time costs are reported in Table 2. Patients traveled to the clinic mainly by car (56.5% [78/138]) or train/subway (25.3% [35/138]). One in approximately 5 patients (21.3%) spent more than 1 hour traveling one-way to the clinic. Most patients waited less than 20 minutes to see their care providers. Face-to-face time with providers (ie, residents and attending physicians) ranged from less than 21 minutes to more than 1 hour, with a mean (SD) time of 36.8 (18.9) minutes.

Of the employed respondents, 76.5% (65/85) took off time from work for the appointment. Patients took a mean (SD) of 4.1 (2.4) hours off from work, which was considered sick pay (35%), paid time off (36.6%), or unpaid time (28.3%). The total mean (SD) time dedicated to attending the clinic appointment averaged 144.8 (60.47) minutes. On average, the time spent traveling for the clinic visit was double the amount of time spent with the care provider (77.4 vs 36.8 minutes).
Monetary and Opportunity Costs
The mean (SD) monetary cost associated with clinic attendance for employed patients who reported their wages was $187.50 (103.2)(range, $37.50–$489), most of which was opportunity cost from loss of potential work income (mean [SD], $144.30 [93.6]; range, $27–$432)(Table 3). Similar total and opportunity costs were found for employed patients using the imputed average wage. The mean (SD) total cost per visit for unemployed patients or those who did not report employment status was $38.65 (103.6)(range, $0–$800), which was 4-times less than the cost per visit for employed patients. Mean (SD) and median one-way travel expenses were $16.60 (40.5) and $10, respectively. Mean (SD) and median reported costs for deductibles/co-pays were $44.20 (66.1) and $25, respectively. Only 2 patients reported child care costs, which were valued at $65 and $75.

Patient Provider Preference
The majority (59.3% [67/113]) of patients preferred their current care providers, whereas 33.6% (38/113) preferred providers closer to work, home, or in a different unspecified setting. Only 7.0% (8/113) of patients who answered this survey question would choose teledermatology over their current providers.
On multivariate logistic regression (Table 4), patients who had additional out-of-pocket costs were significantly less likely to prefer their current care provider compared to patients with no out-of-pocket costs (odds ratio [OR], 0.27; 95% confidence interval [CI], 0.10-0.71; P<.05). Opportunity costs were not a significant predictor of provider preference. For every minute the travel time increased, the likelihood of preference for the current care provider decreased by 2% (OR, 0.98; 95% CI, 0.95–0.99), and patients who traveled 60 minutes or more round-trip were 71% less likely to choose current provider care than those who traveled less than 60 minutes (OR, 0.29; 95% CI, 0.09-0.96; P<.05). Patients with higher education (≥4 years of college) were 3.29-times more likely to stay with their current care provider than those with lower education (≤2 years of college). Those presenting for skin checks also preferred the current provider more than those with noninflammatory skin conditions such as alopecia and warts (OR, 9.01; 95% CI, 2.28-35.59). Age and gender were not statistically significant predictors of patient provider preference.

Comment
Our study revealed that patients spend a substantial amount of time and money attending dermatology clinic appointments. Round-trip travel time exceeded 2 hours for 20% of patients and accounted for the majority of the total time attributed to the visit. Patients who were employed typically requested an average of 4 hours off from work, resulting in a mean (SD) opportunity cost of $144.30 (93.6) due to lost wages. Direct costs such as co-pays, deductibles, travel expenses, and child care accounted for a smaller proportion of total costs. The study assumed a wage of $0 for unemployed patients, thus underestimating the true costs of the visit for these patients whose time may otherwise have been spent on leisure, education, volunteerism, or other activities that contribute to individual and societal productivity. The total costs for unemployed patients reflected only direct costs, and thus were notably lower than those for employed patients.
Direct out-of-pocket costs and travel time negatively impacted provider preference. Patients with out-of-pocket costs were much less likely to stay with their current care provider (OR, 0.27; 95% CI, 0.10-0.71), preferring to seek care closer to home/work or teledermatology services. Similarly, for each minute that travel time increased, preference for current care provider decreased by 2%. Those who traveled 60 minutes or more were 71% less likely than those who traveled less than 60 minutes to stay with their current provider when given other options for care. Opportunity costs did not affect provider preference, even though they far exceeded direct costs for employed patients. Perhaps opportunity costs are not as immediately apparent to patients as out-of-pocket costs and travel time, and thus they do not factor as heavily in provider preference.
Despite high time and monetary costs, the majority of patients (60%) still preferred their current care provider, especially those with 4-year university degrees or higher education level (OR, 3.29; 95% CI, 1.23-5.26) and those presenting for skin checks (OR, 9.01; 95% CI, 2.28-35.59). Patients with higher levels of education likely have higher incomes and thus may not be as adversely affected by direct and/or indirect visit costs. Patients presenting for skin checks may value continuity and prefer providers with whom they already have an established therapeutic relationship. Future studies are needed to analyze the impact of these nonmonetary factors on provider preference.
Seeking Alternative Care
Tufts Medical Center does not have satellite dermatology clinics, making it the only option for patients who wish to receive care within the Tufts hospital network. However, patients do have the option of visiting non–Tufts-affiliated dermatology clinics outside of the city. To our knowledge, no formal studies have been performed comparing wait times for dermatology appointments in suburban versus urban Boston areas; however, it has been reported that rural practitioners have longer wait times than urban dermatologists, possibly due to the fact that physicians tend to aggregate in metropolitan areas.2 Thus, the potential for shorter wait times in the Boston metropolitan area may make it a more desirable location to receive care compared to more suburban or even rural areas of Massachusetts, but additional data are needed to substantiate this hypothesis. Additionally, health insurance restrictions, refractory or complex dermatologic conditions, and referring providers’ preference may affect patients’ decisions to seek care at a particular clinic. However, these factors do not alter our finding that those who travel long distances to our dermatology clinic are less likely to stay with their current provider if given the choice to seek care closer to home/work or utilize teledermatology services.
Prior studies have demonstrated patient preference and willingness to accept alternative modes of care delivery to reduce time and monetary costs associated with in-person medical visits.7,8 Dermatology patients at a clinic in Ontario, Canada, considered the time they spent attending the clinic to be even more burdensome than the monetary cost.7 Patients with nondermatologic chronic diseases and high out-of-pocket costs would prefer email rather than a clinic visit as the first method of contact with care providers.8 The explosive growth of direct-to-consumer (DTC) teledermatology services in the last 10 years speaks to patient demand for alternative care delivery that saves time and money. Although telemedicine has been implemented in various specialties, including ophthalmology and neurology, one of the most common applications is teledermatology. With DTC teledermatology, patients can take photographs or videos using personal smartphones and communicate directly with care providers using mobile or online applications. More recent review articles have identified 22 to 29 DTC mobile and web-based teledermatology services, with costs varying from $0 to $250.9-11 The median consultation fee of $59 for DTC teledermatology services is substantially less than total visit costs for employed patients in our study.9 Teledermatology has become an accessible and affordable modality of care, though perhaps not yet fully optimized for quality of care.
With increasingly higher co-pays and high-deductible insurance plans, time and monetary factors play increasingly important roles in patient preference for specialty care providers,12 as demonstrated by our study. Dermatologists can work with patients to reduce the costs of medical visits. Perhaps monitoring of chronic but stable conditions can be accomplished through telecommunication to reduce the number of follow-up visits. For instance, psoriasis patients enrolled in telemonitoring perceived savings of time and expenses through reduction of clinic visits, resulting in high patient satisfaction levels.13 Telephone calls and secure email messaging are other feasible alternatives shown to aid in clinical management and decrease the need for in-person care.8,14 Fewer unnecessary follow-up visits also means more availability for new patients and those with acute needs.
Barriers to obtaining care are not limited to dermatology and are pervasive across most medical specialties. Issues of patient time burden and out-of-pocket expenses are reflected in recent reports focused on quantifying these costs throughout ambulatory care visits and services such as colorectal, cervical, and breast cancer screenings.1,15-18 Similar to our findings, many of these studies also show high time and opportunity costs from the patient perspective. Expansion of telemedicine to reduce patient costs is becoming a viable option for many specialists, though low reimbursement rates restrict its widespread application.9,19 However, this obstacle is not impossible to surmount. One study found that offering teledermatology to Medicaid patients through their primary care providers significantly improved access, allowing for a 63.8% increase in the number of patients visiting a dermatologist (P<.01).20 Currently, a total of 48 state Medicaid programs now cover telemedicine, and a growing number of states are requiring private insurers to cover telehealth services.21 As more dermatologists adopt telemedicine practices, it may allow for better access as well as expanded insurance coverage.
Limitations
The results of our study are limited by the single-institution survey design. Patients were asked to complete the survey while still at the clinic visit to minimize recall bias. Because these patients actually attended their appointments, they might perceive the time and monetary costs associated with the visit to be less problematic than those who canceled their appointments or transferred care elsewhere; however, we were still able to detect a significant impact of time and monetary costs on provider preference in this cohort (P<.05). Larger studies in different geographic settings and other specialty clinics are needed to confirm our findings and to determine if nonmonetary factors such as specific diagnoses, length of time with a certain care provider, or patient socioeconomic status can modulate the impact of time and monetary costs on provider preference.
Conclusion
This study showed that patients expend a substantial amount of time and monetary costs to attend dermatology clinic visits. Data from the current and prior studies suggest that these costs affect patient provider preference for dermatologic care and may pose barriers to necessary medical care. Recognizing direct and indirect patient costs may drive critical changes in health care delivery, such as increased telecommunication utilization, the more cost-saving alternative. Telemedicine, when integrated appropriately, can help minimize expenses for patients while continuing to maintain a high level of care.
Access to outpatient specialty care is notably limited due to time and out-of-pocket costs to patients, leading to patient dissatisfaction and worsened clinical outcomes. Lost time and earnings pose considerable opportunity costs for patients, with the total opportunity cost for all physician visits per year estimated at $52 billion in 2010 in the United States.1
The field of dermatology exemplifies the access issues patients may face when seeking specialty care given the ongoing national shortage of dermatologists and notably long wait times exceeding 60 days in major cities.2-4 With the high demand and limited number of providers, patients may have longer wait times to see dermatologists in their communities or have to travel further to see dermatologists in distant locations who have available appointments; therefore, patients may be subject to higher associated time, travel, and monetary costs. According to the 2013 Medical Expenditure Panel Survey, dermatology visits in the United States cost an average of $221 per visit compared to $166 for primary care. Dermatology visits had the highest median cost per office visit ($124) and were more often associated with out-of-pocket expenses (60.7%) compared to other specialties.5 Despite these high costs, the number of dermatology visits is increasing each year, with more than 38 million dermatology visits in 2012.6
In light of these factors that limit patient access to dermatologists compared to other specialists, we performed an evaluation of the direct and indirect costs to patients visiting an outpatient dermatology clinic in Boston, Massachusetts, to better understand obstacles to receiving dermatologic care. The impact that time and money have on how patients prefer to receive their care also was evaluated. Conducting this study in Boston may best reflect patient barriers to obtaining dermatologic treatment, as nationwide surveys have found that Boston has the highest cumulative average wait times for physician appointments compared to other US metropolitan cities, with an average wait time of 72 days to see a dermatologist.4 New studies of patient costs associated with dermatology clinic visits are lacking, and existing economic analyses rarely include time costs. Understanding time burden and opportunity costs from the patient perspective may motivate patients and physicians to alter how they receive and provide health care, respectively, to minimize these expenses. Advances in health care technology such as telecommunication may facilitate these changes.
Methods
Study Design
This survey study took place from October 1, 2015, to March 4, 2016, at the department of dermatology outpatient clinic of Tufts Medical Center, an academic university hospital located in downtown Boston, Massachusetts, with no satellite clinics. Five general dermatologists, 2 dermatologic surgeons, and 9 dermatology residents comprised the dermatology department. The study protocol and questionnaire received exemption status from the Tufts University Health Science’s institutional review board.
All adult patients (aged ≥18 years) attending a scheduled dermatology clinic visit within the designated time frame were invited to complete a questionnaire available in English, Spanish, or Chinese. Patients completed the questionnaire on paper or electronically using handheld tablets. Data were then compiled into the REDCap (Research Electronic Data Capture) online database. The questionnaire surveyed patient age; gender; ethnicity; language spoken; highest level of education; employment status; reason for visit (ie, skin condition); duration, cost, and mode of transportation; duration of visit including wait time; companion accompaniment; profession; hourly wage; and number of work hours requested off to attend the visit. Lastly, patients were surveyed on whether they prefer to receive dermatologic care at Tufts, to receive in-person care elsewhere, to use teledermatology, or none of the above.
Statistical Analysis
Total time attributed to the visit was the sum of time for round-trip travel to and from the clinic, wait time, and face-to-face time with care providers. Out-of-pocket patient expenses included round-trip travel expenses, child care expenses, and direct payments such as deductibles and co-pays. Opportunity cost for employed patients was calculated as the patient’s average hourly wage multiplied by either the number of hours taken off from work or the number of hours the patient attributed to the visit, whichever value was higher at the individual level. For the purpose of calculating opportunity costs, travel time, wait time, and face-to-face time were imputed using average values for these variables when not reported. Patients could provide exact hourly wage and annual income or select the closest approximation from 10 wage ranges. For patients who selected a wage range, the midpoint of the range was used as the hourly wage. Total costs were the sum of reported out-of-pocket expenses and calculated opportunity costs. For unemployed patients and those who did not report employment status, hourly wage was assumed to be $0, resulting in opportunity costs of $0. Costs are tabulated for individual patients and analyzed in aggregate.
Differences in patient characteristics between those who preferred their current care provider versus those who preferred to seek care elsewhere or via teledermatology were compared using the χ2 and Student t test. A multivariate logistic regression was then performed to identify predictors of patient preference for their current provider. Potential predictors for regression model were time and cost variables as well as factors selected based on results from bivariate analysis. Data analysis was performed using statistical software.
Results
Demographics
Demographic data for respondents are outlined in Table 1. Of 145 patients who completed the survey, the majority had already seen a dermatologist for their presenting condition (87.4%), and were English speaking (96.5%), white (76.6%), employed (59.4%), and male (50.3%), with a mean age (SD) of 52.3 (18.1) years and education level of 4-year university or higher (64.7%). The most common reasons for dermatology clinic attendance were general skin checks (30.8%) and psoriasis (26.6%). A smaller proportion of patients (16.1%) presented for surgical visits. Other less common conditions that brought patients into the clinic included acne (6.3%), eczema (4.9%), and skin rash (2.8%).

The mean (SD) reported hourly wage of employed patients was $36.60 (15.8). The most common reasons for unemployment were retirement (65.5% [38/58]), disability (10.3% [6/58]), and schooling (10.3% [6/58]).
Time Attributed to Attending Dermatology Clinic Visits
Time costs are reported in Table 2. Patients traveled to the clinic mainly by car (56.5% [78/138]) or train/subway (25.3% [35/138]). One in approximately 5 patients (21.3%) spent more than 1 hour traveling one-way to the clinic. Most patients waited less than 20 minutes to see their care providers. Face-to-face time with providers (ie, residents and attending physicians) ranged from less than 21 minutes to more than 1 hour, with a mean (SD) time of 36.8 (18.9) minutes.

Of the employed respondents, 76.5% (65/85) took off time from work for the appointment. Patients took a mean (SD) of 4.1 (2.4) hours off from work, which was considered sick pay (35%), paid time off (36.6%), or unpaid time (28.3%). The total mean (SD) time dedicated to attending the clinic appointment averaged 144.8 (60.47) minutes. On average, the time spent traveling for the clinic visit was double the amount of time spent with the care provider (77.4 vs 36.8 minutes).
Monetary and Opportunity Costs
The mean (SD) monetary cost associated with clinic attendance for employed patients who reported their wages was $187.50 (103.2)(range, $37.50–$489), most of which was opportunity cost from loss of potential work income (mean [SD], $144.30 [93.6]; range, $27–$432)(Table 3). Similar total and opportunity costs were found for employed patients using the imputed average wage. The mean (SD) total cost per visit for unemployed patients or those who did not report employment status was $38.65 (103.6)(range, $0–$800), which was 4-times less than the cost per visit for employed patients. Mean (SD) and median one-way travel expenses were $16.60 (40.5) and $10, respectively. Mean (SD) and median reported costs for deductibles/co-pays were $44.20 (66.1) and $25, respectively. Only 2 patients reported child care costs, which were valued at $65 and $75.

Patient Provider Preference
The majority (59.3% [67/113]) of patients preferred their current care providers, whereas 33.6% (38/113) preferred providers closer to work, home, or in a different unspecified setting. Only 7.0% (8/113) of patients who answered this survey question would choose teledermatology over their current providers.
On multivariate logistic regression (Table 4), patients who had additional out-of-pocket costs were significantly less likely to prefer their current care provider compared to patients with no out-of-pocket costs (odds ratio [OR], 0.27; 95% confidence interval [CI], 0.10-0.71; P<.05). Opportunity costs were not a significant predictor of provider preference. For every minute the travel time increased, the likelihood of preference for the current care provider decreased by 2% (OR, 0.98; 95% CI, 0.95–0.99), and patients who traveled 60 minutes or more round-trip were 71% less likely to choose current provider care than those who traveled less than 60 minutes (OR, 0.29; 95% CI, 0.09-0.96; P<.05). Patients with higher education (≥4 years of college) were 3.29-times more likely to stay with their current care provider than those with lower education (≤2 years of college). Those presenting for skin checks also preferred the current provider more than those with noninflammatory skin conditions such as alopecia and warts (OR, 9.01; 95% CI, 2.28-35.59). Age and gender were not statistically significant predictors of patient provider preference.

Comment
Our study revealed that patients spend a substantial amount of time and money attending dermatology clinic appointments. Round-trip travel time exceeded 2 hours for 20% of patients and accounted for the majority of the total time attributed to the visit. Patients who were employed typically requested an average of 4 hours off from work, resulting in a mean (SD) opportunity cost of $144.30 (93.6) due to lost wages. Direct costs such as co-pays, deductibles, travel expenses, and child care accounted for a smaller proportion of total costs. The study assumed a wage of $0 for unemployed patients, thus underestimating the true costs of the visit for these patients whose time may otherwise have been spent on leisure, education, volunteerism, or other activities that contribute to individual and societal productivity. The total costs for unemployed patients reflected only direct costs, and thus were notably lower than those for employed patients.
Direct out-of-pocket costs and travel time negatively impacted provider preference. Patients with out-of-pocket costs were much less likely to stay with their current care provider (OR, 0.27; 95% CI, 0.10-0.71), preferring to seek care closer to home/work or teledermatology services. Similarly, for each minute that travel time increased, preference for current care provider decreased by 2%. Those who traveled 60 minutes or more were 71% less likely than those who traveled less than 60 minutes to stay with their current provider when given other options for care. Opportunity costs did not affect provider preference, even though they far exceeded direct costs for employed patients. Perhaps opportunity costs are not as immediately apparent to patients as out-of-pocket costs and travel time, and thus they do not factor as heavily in provider preference.
Despite high time and monetary costs, the majority of patients (60%) still preferred their current care provider, especially those with 4-year university degrees or higher education level (OR, 3.29; 95% CI, 1.23-5.26) and those presenting for skin checks (OR, 9.01; 95% CI, 2.28-35.59). Patients with higher levels of education likely have higher incomes and thus may not be as adversely affected by direct and/or indirect visit costs. Patients presenting for skin checks may value continuity and prefer providers with whom they already have an established therapeutic relationship. Future studies are needed to analyze the impact of these nonmonetary factors on provider preference.
Seeking Alternative Care
Tufts Medical Center does not have satellite dermatology clinics, making it the only option for patients who wish to receive care within the Tufts hospital network. However, patients do have the option of visiting non–Tufts-affiliated dermatology clinics outside of the city. To our knowledge, no formal studies have been performed comparing wait times for dermatology appointments in suburban versus urban Boston areas; however, it has been reported that rural practitioners have longer wait times than urban dermatologists, possibly due to the fact that physicians tend to aggregate in metropolitan areas.2 Thus, the potential for shorter wait times in the Boston metropolitan area may make it a more desirable location to receive care compared to more suburban or even rural areas of Massachusetts, but additional data are needed to substantiate this hypothesis. Additionally, health insurance restrictions, refractory or complex dermatologic conditions, and referring providers’ preference may affect patients’ decisions to seek care at a particular clinic. However, these factors do not alter our finding that those who travel long distances to our dermatology clinic are less likely to stay with their current provider if given the choice to seek care closer to home/work or utilize teledermatology services.
Prior studies have demonstrated patient preference and willingness to accept alternative modes of care delivery to reduce time and monetary costs associated with in-person medical visits.7,8 Dermatology patients at a clinic in Ontario, Canada, considered the time they spent attending the clinic to be even more burdensome than the monetary cost.7 Patients with nondermatologic chronic diseases and high out-of-pocket costs would prefer email rather than a clinic visit as the first method of contact with care providers.8 The explosive growth of direct-to-consumer (DTC) teledermatology services in the last 10 years speaks to patient demand for alternative care delivery that saves time and money. Although telemedicine has been implemented in various specialties, including ophthalmology and neurology, one of the most common applications is teledermatology. With DTC teledermatology, patients can take photographs or videos using personal smartphones and communicate directly with care providers using mobile or online applications. More recent review articles have identified 22 to 29 DTC mobile and web-based teledermatology services, with costs varying from $0 to $250.9-11 The median consultation fee of $59 for DTC teledermatology services is substantially less than total visit costs for employed patients in our study.9 Teledermatology has become an accessible and affordable modality of care, though perhaps not yet fully optimized for quality of care.
With increasingly higher co-pays and high-deductible insurance plans, time and monetary factors play increasingly important roles in patient preference for specialty care providers,12 as demonstrated by our study. Dermatologists can work with patients to reduce the costs of medical visits. Perhaps monitoring of chronic but stable conditions can be accomplished through telecommunication to reduce the number of follow-up visits. For instance, psoriasis patients enrolled in telemonitoring perceived savings of time and expenses through reduction of clinic visits, resulting in high patient satisfaction levels.13 Telephone calls and secure email messaging are other feasible alternatives shown to aid in clinical management and decrease the need for in-person care.8,14 Fewer unnecessary follow-up visits also means more availability for new patients and those with acute needs.
Barriers to obtaining care are not limited to dermatology and are pervasive across most medical specialties. Issues of patient time burden and out-of-pocket expenses are reflected in recent reports focused on quantifying these costs throughout ambulatory care visits and services such as colorectal, cervical, and breast cancer screenings.1,15-18 Similar to our findings, many of these studies also show high time and opportunity costs from the patient perspective. Expansion of telemedicine to reduce patient costs is becoming a viable option for many specialists, though low reimbursement rates restrict its widespread application.9,19 However, this obstacle is not impossible to surmount. One study found that offering teledermatology to Medicaid patients through their primary care providers significantly improved access, allowing for a 63.8% increase in the number of patients visiting a dermatologist (P<.01).20 Currently, a total of 48 state Medicaid programs now cover telemedicine, and a growing number of states are requiring private insurers to cover telehealth services.21 As more dermatologists adopt telemedicine practices, it may allow for better access as well as expanded insurance coverage.
Limitations
The results of our study are limited by the single-institution survey design. Patients were asked to complete the survey while still at the clinic visit to minimize recall bias. Because these patients actually attended their appointments, they might perceive the time and monetary costs associated with the visit to be less problematic than those who canceled their appointments or transferred care elsewhere; however, we were still able to detect a significant impact of time and monetary costs on provider preference in this cohort (P<.05). Larger studies in different geographic settings and other specialty clinics are needed to confirm our findings and to determine if nonmonetary factors such as specific diagnoses, length of time with a certain care provider, or patient socioeconomic status can modulate the impact of time and monetary costs on provider preference.
Conclusion
This study showed that patients expend a substantial amount of time and monetary costs to attend dermatology clinic visits. Data from the current and prior studies suggest that these costs affect patient provider preference for dermatologic care and may pose barriers to necessary medical care. Recognizing direct and indirect patient costs may drive critical changes in health care delivery, such as increased telecommunication utilization, the more cost-saving alternative. Telemedicine, when integrated appropriately, can help minimize expenses for patients while continuing to maintain a high level of care.
- Ray KN, Chari AV, Engberg J, et al. Opportunity costs of ambulatory medical care in the United States. Am J Manag Care. 2015;21:567-574.
- Kimball AB, Resneck JS Jr. The US dermatology workforce: a specialty remains in shortage. J Am Acad Dermatol. 2008;59:741-745.
- Resneck JS Jr, Lipton S, Pletcher MJ. Short wait times for patients seeking cosmetic botulinum toxin appointments with dermatologists. J Am Acad Dermatol. 2007;57:985-989.
- Physician appointment wait times & Medicaid and Medicare acceptance rates. Merritt Hawkins website. https://www.merritthawkins.com/2014-survey/patientwaittime.aspx. Accessed February 15, 2017.
- Machlin SR, Adams SA. Expenses for office-based physician visits by specialty, 2013. Agency for Healthcare Research and Quality website. https://meps.ahrq.gov/data_files/publications/st484/stat484.pdf. Published November 2015. Accessed February 15, 2017.
- National ambulatory medical care survey: 2012 state and national summary tables. CDC website. www.cdc.gov/nchs/data/ahcd/namcs_summary/2012_namcs_web_tables.pdf. Accessed February 15, 2017.
- Vignjevic PM, Hux JE, Fisher BK, et al. Monetary and nonmonetary costs to patients attending an ambulatory dermatology clinic. J Cutan Med Surg. 1999;3:188-192.
- Reed M, Graetz I, Gordon N, Fung V. Patient-initiated e-mails to providers: associations with out-of-pocket visit costs, and impact on care-seeking and health. Am J Manag Care. 2015;21:E632-E639.
- Peart JM, Kovarik C. Direct-to-patient teledermatology practices. J Am Acad Dermatol. 2015;72:907-909.
- Fogel AL, Sarin KY. A survey of direct-to-consumer teledermatology services available to US patients: explosive growth, opportunities and controversy. J Telemed Telecare. 2017;23:19-25.
- Kochmann M, Locatis C. Direct to consumer mobile teledermatology apps: an exploratory study. Telemed J E Health. 2016;22:689-693.
- Helms AD. High-deductible health plans can ruin finances. Kaiser Health News website. https://khn.org/news/high-deductible-health-plans-can-ruin-finances/. Published April 6, 2015. Accessed February 15, 2017.
- Fruhauf J, Schwantzer G, Ambros-Rudolph CM, et al. Pilot study on the acceptance of mobile teledermatology for the home monitoring of high-need patients with psoriasis. Australas J Dermatol. 2012;53:41-46.
- Eisenberg D, Hwa K, Wren SM. Telephone follow-up by a midlevel provider after laparoscopic inguinal hernia repair instead of face-to-face clinic visit. JSLS. 2015;19:e2014.00205.
- Yabroff KR, Guy GP Jr, Ekwueme DU, et al. Annual patient time costs associated with medical care among cancer survivors in the United States. Med Care. 2014;52:594-601.
- Yabroff KR, Davis WW, Lamont EB, et al. Patient time costs associated with cancer care. J Natl Cancer Inst. 2007;99:14-23.
- Jonas DE, Russell LB, Sandler RS, et al. Value of patient time invested in the colonoscopy screening process: time requirements for colonoscopy study. Med Decis Making. 2008;28:56-65.
- Shireman TI, Tsevat J, Goldie SJ. Time costs associated with cervical cancer screening. Int J Technol Assess Health Care. 2001;17:146-152.
- Dorsey ER, Topol EJ. State of telehealth. N Engl J Med. 2016;375:154-161.
- Uscher-Pines L, Malsberger R, Burgette L, et al. Effect of teledermatology on access to dermatology care among Medicaid enrollees. JAMA Dermatol. 2016;152:905-912.
- Thomas L, Capistrant G. State telemedicine gaps analysis: coverage & reimbursement. Telehealth website. http://www.mtelehealth.com/state-telemedicine-gaps-analysis-coverage-reimbursement/. Published January 19, 2016. Accessed February 15, 2017.
- Ray KN, Chari AV, Engberg J, et al. Opportunity costs of ambulatory medical care in the United States. Am J Manag Care. 2015;21:567-574.
- Kimball AB, Resneck JS Jr. The US dermatology workforce: a specialty remains in shortage. J Am Acad Dermatol. 2008;59:741-745.
- Resneck JS Jr, Lipton S, Pletcher MJ. Short wait times for patients seeking cosmetic botulinum toxin appointments with dermatologists. J Am Acad Dermatol. 2007;57:985-989.
- Physician appointment wait times & Medicaid and Medicare acceptance rates. Merritt Hawkins website. https://www.merritthawkins.com/2014-survey/patientwaittime.aspx. Accessed February 15, 2017.
- Machlin SR, Adams SA. Expenses for office-based physician visits by specialty, 2013. Agency for Healthcare Research and Quality website. https://meps.ahrq.gov/data_files/publications/st484/stat484.pdf. Published November 2015. Accessed February 15, 2017.
- National ambulatory medical care survey: 2012 state and national summary tables. CDC website. www.cdc.gov/nchs/data/ahcd/namcs_summary/2012_namcs_web_tables.pdf. Accessed February 15, 2017.
- Vignjevic PM, Hux JE, Fisher BK, et al. Monetary and nonmonetary costs to patients attending an ambulatory dermatology clinic. J Cutan Med Surg. 1999;3:188-192.
- Reed M, Graetz I, Gordon N, Fung V. Patient-initiated e-mails to providers: associations with out-of-pocket visit costs, and impact on care-seeking and health. Am J Manag Care. 2015;21:E632-E639.
- Peart JM, Kovarik C. Direct-to-patient teledermatology practices. J Am Acad Dermatol. 2015;72:907-909.
- Fogel AL, Sarin KY. A survey of direct-to-consumer teledermatology services available to US patients: explosive growth, opportunities and controversy. J Telemed Telecare. 2017;23:19-25.
- Kochmann M, Locatis C. Direct to consumer mobile teledermatology apps: an exploratory study. Telemed J E Health. 2016;22:689-693.
- Helms AD. High-deductible health plans can ruin finances. Kaiser Health News website. https://khn.org/news/high-deductible-health-plans-can-ruin-finances/. Published April 6, 2015. Accessed February 15, 2017.
- Fruhauf J, Schwantzer G, Ambros-Rudolph CM, et al. Pilot study on the acceptance of mobile teledermatology for the home monitoring of high-need patients with psoriasis. Australas J Dermatol. 2012;53:41-46.
- Eisenberg D, Hwa K, Wren SM. Telephone follow-up by a midlevel provider after laparoscopic inguinal hernia repair instead of face-to-face clinic visit. JSLS. 2015;19:e2014.00205.
- Yabroff KR, Guy GP Jr, Ekwueme DU, et al. Annual patient time costs associated with medical care among cancer survivors in the United States. Med Care. 2014;52:594-601.
- Yabroff KR, Davis WW, Lamont EB, et al. Patient time costs associated with cancer care. J Natl Cancer Inst. 2007;99:14-23.
- Jonas DE, Russell LB, Sandler RS, et al. Value of patient time invested in the colonoscopy screening process: time requirements for colonoscopy study. Med Decis Making. 2008;28:56-65.
- Shireman TI, Tsevat J, Goldie SJ. Time costs associated with cervical cancer screening. Int J Technol Assess Health Care. 2001;17:146-152.
- Dorsey ER, Topol EJ. State of telehealth. N Engl J Med. 2016;375:154-161.
- Uscher-Pines L, Malsberger R, Burgette L, et al. Effect of teledermatology on access to dermatology care among Medicaid enrollees. JAMA Dermatol. 2016;152:905-912.
- Thomas L, Capistrant G. State telemedicine gaps analysis: coverage & reimbursement. Telehealth website. http://www.mtelehealth.com/state-telemedicine-gaps-analysis-coverage-reimbursement/. Published January 19, 2016. Accessed February 15, 2017.
Practice Points
- Physicians should be cognizant of the direct and indirect costs patients are subject to when attending dermatology clinic appointments and implement changes to reduce these costs.
- Telephone calls and secure email messaging are feasible alternatives shown to aid in clinical management and decrease the need for in-person care.
- Telecommunication may be used for the monitoring of chronic but stable conditions to reduce the number of follow-up visits.
The h-Index for Associate and Full Professors of Dermatology in the United States: An Epidemiologic Study of Scholastic Production
Academic promotion requires evidence of scholastic production. The number of publications by a scientist is the most frequently reported metric of scholastic production, but it does not account for the impact of publications. The h-index is a bibliometric measure that combines both volume and impact of scientific contributions. The physicist Jorge E. Hirsch introduced this metric in 2005.1 He defined it as the number of publications (h) by an author that have been cited at least h times. For example, a scientist with 30 publications including 12 that have been cited at least 12 times each has an h-index of 12. h-Index is a superior predictor of future scientific achievement in physics compared with total citation count, total publication count, and citations per publication. Hirsch2 proposed h-index thresholds of 12 and 18 for advancement to associate professor and full professor in physics, respectively.2
h-Index values are not comparable across academic disciplines because they are influenced by the number of journals and authors within the field. Scientists in disciplines with numerous scholars and publications will have higher h-indices. For example, the mean h-index for full professors of cardiothoracic anesthesiology is 12, but the mean h-index for full professors of urology is 22.3,4 Hence, h-index thresholds for professional advancement cannot be generalized but must be calculated on a granular, specialty-specific basis.
In a prior study on h-index among academic dermatologists in the United States, John et al5 reported that fellowship-trained dermatologists had a significantly higher mean h-index than those without fellowship training (13.2 vs 11.7; P<.001). They further found the mean h-index increased with academic rank.5
In our study, we measured mean and median h-indices among associate and full professors of dermatology in academic training programs in the United States with the goal of describing h-index distributions in these 2 academic ranks. We further sought to measure regional differences in h-index between northeastern, southern, central, and western states as defined by the National Resident Matching Program.
Methods
Institutional review board approval was deferred because the study did not require patient information or participation. Using the Association of American Medical Colleges Electronic Residency Application Service website (https://www.aamc.org/services/eras/) we identified dermatology residency training programs accredited by the Accreditation Council for Graduate Medical Education and participating in the Electronic Residency Application Service for the National Resident Matching Program in the United States. We visited the official website of each residency program and identified all associate and full professors of dermatology for further study. We included all faculty members listed as professor, clinical professor, associate professor, or clinical associate professor, and excluded assistant professor, volunteer faculty, research professor, and research associate professor. All faculty held an MD degree or an equivalent degree, such as MBBS or MDCM.
We used the Thomson Reuters (now Clarivate Analytics) Web of Science to calculate h-index and publication counts. The initial search was basic using the professor’s last name and first initial. We then augmented this list by searching for all variations of each professor’s name, with or without middle initial. Each publication in the search results was confirmed as belonging to the author of interest by verifying coauthors, institution information, and subject material. For authors with common names, we additionally consulted their online university profiles for specific names used in their “Selected Publications” lists. In a minority of cases, we also limited Research Domain to “dermatology.” Referring to the verified publication list for each dermatology professor, we used the Web of Science Citation Report function to determine number of publications and h-index for the individual. We tabulated results for associate and full professors and subgrouped those results into 4 geographic regions—northeastern, southern, central, and western states—according to the map used by the National Resident Matching Program. Descriptive statistics were performed with Microsoft Excel.
Results
We identified 300 associate professors and 352 full professors from 81 academic institutions. The number of associate professors per institution ranged from 1 to 25; the number of full professors per institution ranged from 1 to 16. The median and mean h-indices for associate and full professors, including interquartile values, are shown in the Table. There was a broad range of h-index scores among both academic ranks; median and mean h-indices varied more than 5-fold between the bottom and upper quartiles in both associate and full professor cohorts. Median interquartile h-index values for upper-quartile associate professors overlapped with those of lower-quartile full professors (Figure 1). h-Index for associate and full professors was similar across the 4 regions defined by the National Resident Matching Program. Median h-index was highest for full professors in western states and lowest for associate professors in southern states (Figure 2).
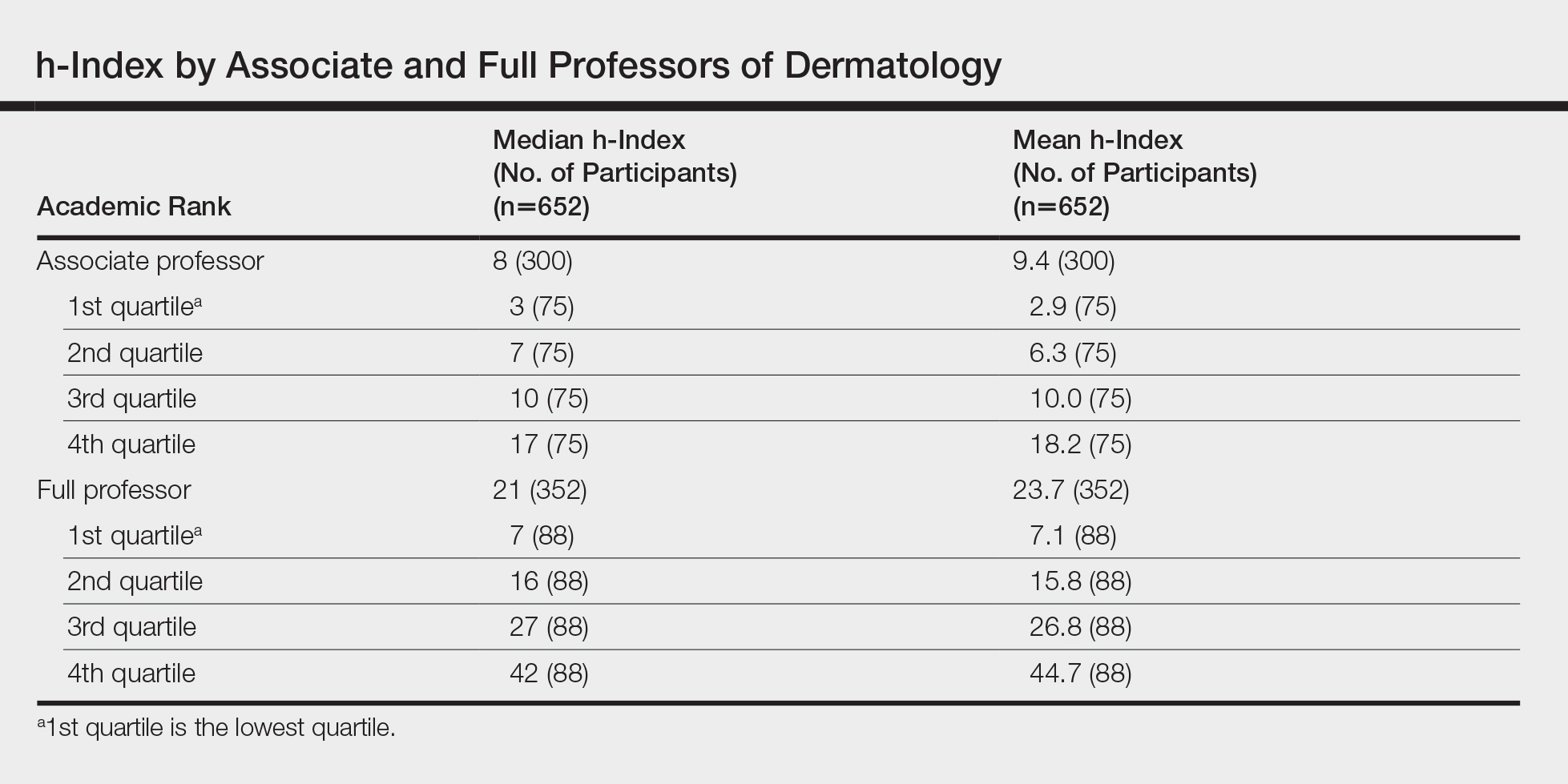
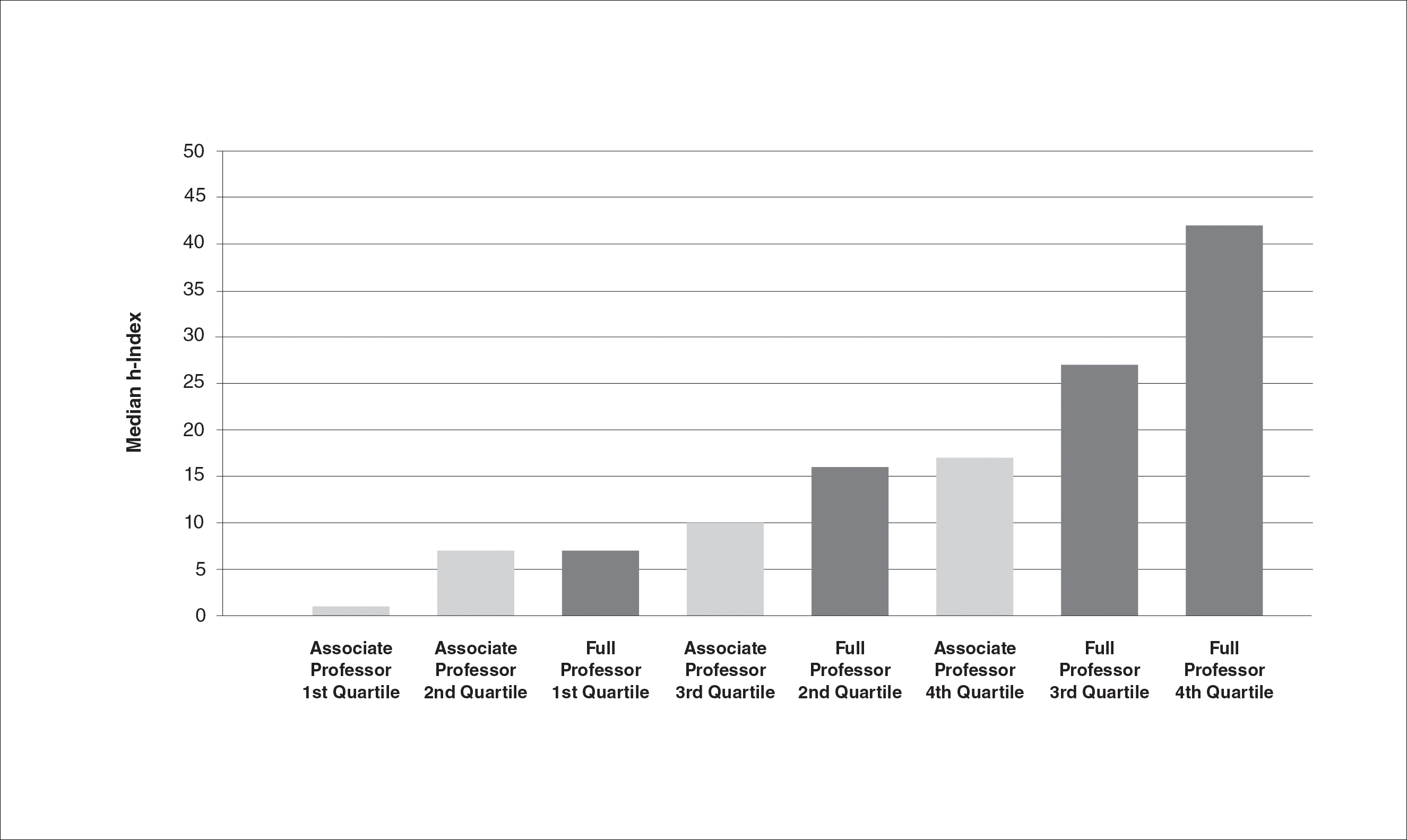

Comment
Professional advancement in academic medicine requires scholastic production. The h-index, defined as the number of publications (h) that have been cited at least h times, is a bibliometric measure that accounts for both volume and impact of an individual’s scientific productivity. The h-index would be a useful tool for determining professional advancement in academic dermatology departments. In this project, we calculated h-index values for 300 associate professors and 352 full professors of dermatology in the United States. We found the median h-index for associate professors was 8 and the median h-index for full professors was 21. There was more than a 5-fold variation in median and mean h-indices between lower and upper quartiles within both the associate and full professor cohorts. The highest median and mean h-indices were found among full professors of dermatology in western states. These results provide the opportunity for academic dermatologists and institutions to compare their research contributions with peers across the United States.
Our results support those of John et al5 who also found academic rank in dermatology was correlated with h-index. Scopus, Web of Science, and Google Scholar can be used to calculate h-index, but they may return different scores for the same individual.6 John et al5 used the Scopus database to calculate h-index. We used Web of Science because Scopus only includes citations since 1996 and Web of Science was used in the original h-index studies by Hirsch.1,2 Institutions that adopt h-index criteria for advancement and resource distribution decisions should be aware that database selection can affect h-index scores.
Caveats With the h-Index
Flaws in the h-index include inflationary effects of self-citation, time bias, and excessive coauthorship. Individuals can increase their h-index by routinely citing their own publications. However, Engqvist and Frommen7 found tripling self-citations increased the h-index by only 1.
Citations tend to increase with time, and authors who have been active for longer periods will have a higher h-index. It is more difficult for junior faculty to distinguish themselves with the h-index, as it takes time for even the most impactful publications to gain citations. Major scientific papers can take years from conception to publication, and an outstanding paper that is 1 year old would have fewer citations than an equally impactful paper that is 10 years old. To adjust for the effect of time bias, Hirsch2 proposed the m-index, in which the h-index is divided by the years between the author’s first and last publication. He proposed that an m-index of 1 would indicate a successful scientist, 2 an outstanding scientist, and 3 a unique individual.2
The literature is increasingly dominated by teams of coauthors, and the number of coauthors within each team has increased over the last 5 decades.8 h-Indices will increase if this trend continues, making it difficult to compare h-indices between different eras. Prosperi et al9 found national differences in kinship-based coauthorship, suggesting nepotism may influence decisions in assigning authorship status. h-Index valuations do not require evidence of meaningful contribution to the work but simply rely on contributors’ self-governance in assigning authorship status.
The h-index also has a bias against highly cited papers. A scientist with a small number of highly influential papers may have a smaller h-index than a scientist with more papers of modest impact. Finally, an author who has changed names (eg, due to marriage) may have an artificially low h-index, as a standard database search would miss publications under a maiden name.
Limitations
This study is limited by possible operator error when compiling each author’s publication list through Web of Science. Our search and refinement methodology took into account that authors may publish with slight variations in name, in various subject areas and fields, and with different institutions and coauthors. Each publication populated through Web of Science was carefully verified by the principal investigator; however, overestimation or underestimation of the number of publications and citations was possible, as the publication lists were not verified by the studied associate and full professors themselves. Our results are consistent with the h-index bar charts published by John et al5 using an alternate citation index, Scopus, which tends to corroborate our findings. This study also is limited by possible time bias because we did not correct the h-index for years of active publication (m-index).
Conclusion
In summary, we found the median h-index for associate professors was 8 and the median h-index for full professors was 21. We found a broad range of h-index values within each academic rank. h-Index for upper-quartile associate professors overlapped with those of lower-quartile full professors. Our results suggest professional advancement occurs over a broad range of scholastic production. Adopting requirements for minimum h-index thresholds for application for promotion might reduce disparities between rank and scientific contributions. We encourage use of the h-index for tracking academic progression and as a parameter to consider in academic promotion.
- Hirsch JE. An index to quantify an individual’s scientific research output. Proc Natl Acad Sci U S A. 2005;102:16569-16572.
- Hirsch JE. Does the H index have predictive power? Proc Natl Acad Sci U S A. 2007;104:19193-19198.
- Pagel PS, Hudetz JA. Scholarly productivity of United States academic cardiothoracic anesthesiologists: influence of fellowship accreditation and transesophageal echocardiographic credentials on h-index and other citation bibliometrics. J Cardiothorac Vasc Anesthesia. 2011;25:761-765.
- Benway BM, Kalidas P, Cabello JM, et al. Does citation analysis reveal association between h-index and academic rank in urology? Urology. 2009;74:30-33.
- John AM, Gupta AB, John ES, et al. The impact of fellowship training on scholarly productivity in academic dermatology. Cutis. 2016;97:353-358.
- Kulkarni AV, Aziz B, Shams I, et al. Comparisons of citations in Web of Science, Scopus, and Google Scholar for articles published in general medical journals. JAMA. 2009;302:1092-1096.
- Engqvist L, Frommen JG. The h-index and self-citations. Trends Ecol Evol. 2008;23:250-252.
- Wuchty S, Jones BF, Uzzi B. The increasing dominance of teams in production of knowledge. Science. 2007;316:1036-1039.
- Prosperi M, Buchan I, Fanti I, et al. Kin of coauthorship in five decades of health science literature. Proc Natl Acad Sci U S A. 2016;113:8957-8962.
Academic promotion requires evidence of scholastic production. The number of publications by a scientist is the most frequently reported metric of scholastic production, but it does not account for the impact of publications. The h-index is a bibliometric measure that combines both volume and impact of scientific contributions. The physicist Jorge E. Hirsch introduced this metric in 2005.1 He defined it as the number of publications (h) by an author that have been cited at least h times. For example, a scientist with 30 publications including 12 that have been cited at least 12 times each has an h-index of 12. h-Index is a superior predictor of future scientific achievement in physics compared with total citation count, total publication count, and citations per publication. Hirsch2 proposed h-index thresholds of 12 and 18 for advancement to associate professor and full professor in physics, respectively.2
h-Index values are not comparable across academic disciplines because they are influenced by the number of journals and authors within the field. Scientists in disciplines with numerous scholars and publications will have higher h-indices. For example, the mean h-index for full professors of cardiothoracic anesthesiology is 12, but the mean h-index for full professors of urology is 22.3,4 Hence, h-index thresholds for professional advancement cannot be generalized but must be calculated on a granular, specialty-specific basis.
In a prior study on h-index among academic dermatologists in the United States, John et al5 reported that fellowship-trained dermatologists had a significantly higher mean h-index than those without fellowship training (13.2 vs 11.7; P<.001). They further found the mean h-index increased with academic rank.5
In our study, we measured mean and median h-indices among associate and full professors of dermatology in academic training programs in the United States with the goal of describing h-index distributions in these 2 academic ranks. We further sought to measure regional differences in h-index between northeastern, southern, central, and western states as defined by the National Resident Matching Program.
Methods
Institutional review board approval was deferred because the study did not require patient information or participation. Using the Association of American Medical Colleges Electronic Residency Application Service website (https://www.aamc.org/services/eras/) we identified dermatology residency training programs accredited by the Accreditation Council for Graduate Medical Education and participating in the Electronic Residency Application Service for the National Resident Matching Program in the United States. We visited the official website of each residency program and identified all associate and full professors of dermatology for further study. We included all faculty members listed as professor, clinical professor, associate professor, or clinical associate professor, and excluded assistant professor, volunteer faculty, research professor, and research associate professor. All faculty held an MD degree or an equivalent degree, such as MBBS or MDCM.
We used the Thomson Reuters (now Clarivate Analytics) Web of Science to calculate h-index and publication counts. The initial search was basic using the professor’s last name and first initial. We then augmented this list by searching for all variations of each professor’s name, with or without middle initial. Each publication in the search results was confirmed as belonging to the author of interest by verifying coauthors, institution information, and subject material. For authors with common names, we additionally consulted their online university profiles for specific names used in their “Selected Publications” lists. In a minority of cases, we also limited Research Domain to “dermatology.” Referring to the verified publication list for each dermatology professor, we used the Web of Science Citation Report function to determine number of publications and h-index for the individual. We tabulated results for associate and full professors and subgrouped those results into 4 geographic regions—northeastern, southern, central, and western states—according to the map used by the National Resident Matching Program. Descriptive statistics were performed with Microsoft Excel.
Results
We identified 300 associate professors and 352 full professors from 81 academic institutions. The number of associate professors per institution ranged from 1 to 25; the number of full professors per institution ranged from 1 to 16. The median and mean h-indices for associate and full professors, including interquartile values, are shown in the Table. There was a broad range of h-index scores among both academic ranks; median and mean h-indices varied more than 5-fold between the bottom and upper quartiles in both associate and full professor cohorts. Median interquartile h-index values for upper-quartile associate professors overlapped with those of lower-quartile full professors (Figure 1). h-Index for associate and full professors was similar across the 4 regions defined by the National Resident Matching Program. Median h-index was highest for full professors in western states and lowest for associate professors in southern states (Figure 2).



Comment
Professional advancement in academic medicine requires scholastic production. The h-index, defined as the number of publications (h) that have been cited at least h times, is a bibliometric measure that accounts for both volume and impact of an individual’s scientific productivity. The h-index would be a useful tool for determining professional advancement in academic dermatology departments. In this project, we calculated h-index values for 300 associate professors and 352 full professors of dermatology in the United States. We found the median h-index for associate professors was 8 and the median h-index for full professors was 21. There was more than a 5-fold variation in median and mean h-indices between lower and upper quartiles within both the associate and full professor cohorts. The highest median and mean h-indices were found among full professors of dermatology in western states. These results provide the opportunity for academic dermatologists and institutions to compare their research contributions with peers across the United States.
Our results support those of John et al5 who also found academic rank in dermatology was correlated with h-index. Scopus, Web of Science, and Google Scholar can be used to calculate h-index, but they may return different scores for the same individual.6 John et al5 used the Scopus database to calculate h-index. We used Web of Science because Scopus only includes citations since 1996 and Web of Science was used in the original h-index studies by Hirsch.1,2 Institutions that adopt h-index criteria for advancement and resource distribution decisions should be aware that database selection can affect h-index scores.
Caveats With the h-Index
Flaws in the h-index include inflationary effects of self-citation, time bias, and excessive coauthorship. Individuals can increase their h-index by routinely citing their own publications. However, Engqvist and Frommen7 found tripling self-citations increased the h-index by only 1.
Citations tend to increase with time, and authors who have been active for longer periods will have a higher h-index. It is more difficult for junior faculty to distinguish themselves with the h-index, as it takes time for even the most impactful publications to gain citations. Major scientific papers can take years from conception to publication, and an outstanding paper that is 1 year old would have fewer citations than an equally impactful paper that is 10 years old. To adjust for the effect of time bias, Hirsch2 proposed the m-index, in which the h-index is divided by the years between the author’s first and last publication. He proposed that an m-index of 1 would indicate a successful scientist, 2 an outstanding scientist, and 3 a unique individual.2
The literature is increasingly dominated by teams of coauthors, and the number of coauthors within each team has increased over the last 5 decades.8 h-Indices will increase if this trend continues, making it difficult to compare h-indices between different eras. Prosperi et al9 found national differences in kinship-based coauthorship, suggesting nepotism may influence decisions in assigning authorship status. h-Index valuations do not require evidence of meaningful contribution to the work but simply rely on contributors’ self-governance in assigning authorship status.
The h-index also has a bias against highly cited papers. A scientist with a small number of highly influential papers may have a smaller h-index than a scientist with more papers of modest impact. Finally, an author who has changed names (eg, due to marriage) may have an artificially low h-index, as a standard database search would miss publications under a maiden name.
Limitations
This study is limited by possible operator error when compiling each author’s publication list through Web of Science. Our search and refinement methodology took into account that authors may publish with slight variations in name, in various subject areas and fields, and with different institutions and coauthors. Each publication populated through Web of Science was carefully verified by the principal investigator; however, overestimation or underestimation of the number of publications and citations was possible, as the publication lists were not verified by the studied associate and full professors themselves. Our results are consistent with the h-index bar charts published by John et al5 using an alternate citation index, Scopus, which tends to corroborate our findings. This study also is limited by possible time bias because we did not correct the h-index for years of active publication (m-index).
Conclusion
In summary, we found the median h-index for associate professors was 8 and the median h-index for full professors was 21. We found a broad range of h-index values within each academic rank. h-Index for upper-quartile associate professors overlapped with those of lower-quartile full professors. Our results suggest professional advancement occurs over a broad range of scholastic production. Adopting requirements for minimum h-index thresholds for application for promotion might reduce disparities between rank and scientific contributions. We encourage use of the h-index for tracking academic progression and as a parameter to consider in academic promotion.
Academic promotion requires evidence of scholastic production. The number of publications by a scientist is the most frequently reported metric of scholastic production, but it does not account for the impact of publications. The h-index is a bibliometric measure that combines both volume and impact of scientific contributions. The physicist Jorge E. Hirsch introduced this metric in 2005.1 He defined it as the number of publications (h) by an author that have been cited at least h times. For example, a scientist with 30 publications including 12 that have been cited at least 12 times each has an h-index of 12. h-Index is a superior predictor of future scientific achievement in physics compared with total citation count, total publication count, and citations per publication. Hirsch2 proposed h-index thresholds of 12 and 18 for advancement to associate professor and full professor in physics, respectively.2
h-Index values are not comparable across academic disciplines because they are influenced by the number of journals and authors within the field. Scientists in disciplines with numerous scholars and publications will have higher h-indices. For example, the mean h-index for full professors of cardiothoracic anesthesiology is 12, but the mean h-index for full professors of urology is 22.3,4 Hence, h-index thresholds for professional advancement cannot be generalized but must be calculated on a granular, specialty-specific basis.
In a prior study on h-index among academic dermatologists in the United States, John et al5 reported that fellowship-trained dermatologists had a significantly higher mean h-index than those without fellowship training (13.2 vs 11.7; P<.001). They further found the mean h-index increased with academic rank.5
In our study, we measured mean and median h-indices among associate and full professors of dermatology in academic training programs in the United States with the goal of describing h-index distributions in these 2 academic ranks. We further sought to measure regional differences in h-index between northeastern, southern, central, and western states as defined by the National Resident Matching Program.
Methods
Institutional review board approval was deferred because the study did not require patient information or participation. Using the Association of American Medical Colleges Electronic Residency Application Service website (https://www.aamc.org/services/eras/) we identified dermatology residency training programs accredited by the Accreditation Council for Graduate Medical Education and participating in the Electronic Residency Application Service for the National Resident Matching Program in the United States. We visited the official website of each residency program and identified all associate and full professors of dermatology for further study. We included all faculty members listed as professor, clinical professor, associate professor, or clinical associate professor, and excluded assistant professor, volunteer faculty, research professor, and research associate professor. All faculty held an MD degree or an equivalent degree, such as MBBS or MDCM.
We used the Thomson Reuters (now Clarivate Analytics) Web of Science to calculate h-index and publication counts. The initial search was basic using the professor’s last name and first initial. We then augmented this list by searching for all variations of each professor’s name, with or without middle initial. Each publication in the search results was confirmed as belonging to the author of interest by verifying coauthors, institution information, and subject material. For authors with common names, we additionally consulted their online university profiles for specific names used in their “Selected Publications” lists. In a minority of cases, we also limited Research Domain to “dermatology.” Referring to the verified publication list for each dermatology professor, we used the Web of Science Citation Report function to determine number of publications and h-index for the individual. We tabulated results for associate and full professors and subgrouped those results into 4 geographic regions—northeastern, southern, central, and western states—according to the map used by the National Resident Matching Program. Descriptive statistics were performed with Microsoft Excel.
Results
We identified 300 associate professors and 352 full professors from 81 academic institutions. The number of associate professors per institution ranged from 1 to 25; the number of full professors per institution ranged from 1 to 16. The median and mean h-indices for associate and full professors, including interquartile values, are shown in the Table. There was a broad range of h-index scores among both academic ranks; median and mean h-indices varied more than 5-fold between the bottom and upper quartiles in both associate and full professor cohorts. Median interquartile h-index values for upper-quartile associate professors overlapped with those of lower-quartile full professors (Figure 1). h-Index for associate and full professors was similar across the 4 regions defined by the National Resident Matching Program. Median h-index was highest for full professors in western states and lowest for associate professors in southern states (Figure 2).



Comment
Professional advancement in academic medicine requires scholastic production. The h-index, defined as the number of publications (h) that have been cited at least h times, is a bibliometric measure that accounts for both volume and impact of an individual’s scientific productivity. The h-index would be a useful tool for determining professional advancement in academic dermatology departments. In this project, we calculated h-index values for 300 associate professors and 352 full professors of dermatology in the United States. We found the median h-index for associate professors was 8 and the median h-index for full professors was 21. There was more than a 5-fold variation in median and mean h-indices between lower and upper quartiles within both the associate and full professor cohorts. The highest median and mean h-indices were found among full professors of dermatology in western states. These results provide the opportunity for academic dermatologists and institutions to compare their research contributions with peers across the United States.
Our results support those of John et al5 who also found academic rank in dermatology was correlated with h-index. Scopus, Web of Science, and Google Scholar can be used to calculate h-index, but they may return different scores for the same individual.6 John et al5 used the Scopus database to calculate h-index. We used Web of Science because Scopus only includes citations since 1996 and Web of Science was used in the original h-index studies by Hirsch.1,2 Institutions that adopt h-index criteria for advancement and resource distribution decisions should be aware that database selection can affect h-index scores.
Caveats With the h-Index
Flaws in the h-index include inflationary effects of self-citation, time bias, and excessive coauthorship. Individuals can increase their h-index by routinely citing their own publications. However, Engqvist and Frommen7 found tripling self-citations increased the h-index by only 1.
Citations tend to increase with time, and authors who have been active for longer periods will have a higher h-index. It is more difficult for junior faculty to distinguish themselves with the h-index, as it takes time for even the most impactful publications to gain citations. Major scientific papers can take years from conception to publication, and an outstanding paper that is 1 year old would have fewer citations than an equally impactful paper that is 10 years old. To adjust for the effect of time bias, Hirsch2 proposed the m-index, in which the h-index is divided by the years between the author’s first and last publication. He proposed that an m-index of 1 would indicate a successful scientist, 2 an outstanding scientist, and 3 a unique individual.2
The literature is increasingly dominated by teams of coauthors, and the number of coauthors within each team has increased over the last 5 decades.8 h-Indices will increase if this trend continues, making it difficult to compare h-indices between different eras. Prosperi et al9 found national differences in kinship-based coauthorship, suggesting nepotism may influence decisions in assigning authorship status. h-Index valuations do not require evidence of meaningful contribution to the work but simply rely on contributors’ self-governance in assigning authorship status.
The h-index also has a bias against highly cited papers. A scientist with a small number of highly influential papers may have a smaller h-index than a scientist with more papers of modest impact. Finally, an author who has changed names (eg, due to marriage) may have an artificially low h-index, as a standard database search would miss publications under a maiden name.
Limitations
This study is limited by possible operator error when compiling each author’s publication list through Web of Science. Our search and refinement methodology took into account that authors may publish with slight variations in name, in various subject areas and fields, and with different institutions and coauthors. Each publication populated through Web of Science was carefully verified by the principal investigator; however, overestimation or underestimation of the number of publications and citations was possible, as the publication lists were not verified by the studied associate and full professors themselves. Our results are consistent with the h-index bar charts published by John et al5 using an alternate citation index, Scopus, which tends to corroborate our findings. This study also is limited by possible time bias because we did not correct the h-index for years of active publication (m-index).
Conclusion
In summary, we found the median h-index for associate professors was 8 and the median h-index for full professors was 21. We found a broad range of h-index values within each academic rank. h-Index for upper-quartile associate professors overlapped with those of lower-quartile full professors. Our results suggest professional advancement occurs over a broad range of scholastic production. Adopting requirements for minimum h-index thresholds for application for promotion might reduce disparities between rank and scientific contributions. We encourage use of the h-index for tracking academic progression and as a parameter to consider in academic promotion.
- Hirsch JE. An index to quantify an individual’s scientific research output. Proc Natl Acad Sci U S A. 2005;102:16569-16572.
- Hirsch JE. Does the H index have predictive power? Proc Natl Acad Sci U S A. 2007;104:19193-19198.
- Pagel PS, Hudetz JA. Scholarly productivity of United States academic cardiothoracic anesthesiologists: influence of fellowship accreditation and transesophageal echocardiographic credentials on h-index and other citation bibliometrics. J Cardiothorac Vasc Anesthesia. 2011;25:761-765.
- Benway BM, Kalidas P, Cabello JM, et al. Does citation analysis reveal association between h-index and academic rank in urology? Urology. 2009;74:30-33.
- John AM, Gupta AB, John ES, et al. The impact of fellowship training on scholarly productivity in academic dermatology. Cutis. 2016;97:353-358.
- Kulkarni AV, Aziz B, Shams I, et al. Comparisons of citations in Web of Science, Scopus, and Google Scholar for articles published in general medical journals. JAMA. 2009;302:1092-1096.
- Engqvist L, Frommen JG. The h-index and self-citations. Trends Ecol Evol. 2008;23:250-252.
- Wuchty S, Jones BF, Uzzi B. The increasing dominance of teams in production of knowledge. Science. 2007;316:1036-1039.
- Prosperi M, Buchan I, Fanti I, et al. Kin of coauthorship in five decades of health science literature. Proc Natl Acad Sci U S A. 2016;113:8957-8962.
- Hirsch JE. An index to quantify an individual’s scientific research output. Proc Natl Acad Sci U S A. 2005;102:16569-16572.
- Hirsch JE. Does the H index have predictive power? Proc Natl Acad Sci U S A. 2007;104:19193-19198.
- Pagel PS, Hudetz JA. Scholarly productivity of United States academic cardiothoracic anesthesiologists: influence of fellowship accreditation and transesophageal echocardiographic credentials on h-index and other citation bibliometrics. J Cardiothorac Vasc Anesthesia. 2011;25:761-765.
- Benway BM, Kalidas P, Cabello JM, et al. Does citation analysis reveal association between h-index and academic rank in urology? Urology. 2009;74:30-33.
- John AM, Gupta AB, John ES, et al. The impact of fellowship training on scholarly productivity in academic dermatology. Cutis. 2016;97:353-358.
- Kulkarni AV, Aziz B, Shams I, et al. Comparisons of citations in Web of Science, Scopus, and Google Scholar for articles published in general medical journals. JAMA. 2009;302:1092-1096.
- Engqvist L, Frommen JG. The h-index and self-citations. Trends Ecol Evol. 2008;23:250-252.
- Wuchty S, Jones BF, Uzzi B. The increasing dominance of teams in production of knowledge. Science. 2007;316:1036-1039.
- Prosperi M, Buchan I, Fanti I, et al. Kin of coauthorship in five decades of health science literature. Proc Natl Acad Sci U S A. 2016;113:8957-8962.
Practice Points
- Promotion in academic dermatology requires evidence of scholastic production. The h-index is a bibliometric measure that combines both volume and impact of scientific contributions.
- Our study’s findings provide data-driven parameters to consider in academic promotion.
- Institutions that adopt h-index criteria for advancement and resource distribution decisions should be aware that database selection can affect h-index scores.
What’s Eating You? Head Lice (Pediculus humanus capitis)
The head louse (Pediculus humanus capitis) is a blood-sucking arthropod of the suborder Anoplura. Lice are obligate human parasites that have infested humans since antiquity. Pediculosis capitis is an infestation of the scalp by head lice. It is estimated that 6 to 12 million individuals in the United States are affected with head lice per year.1 Resistance to topical chemical pediculicides is widespread, and new agents have been developed to address this gap in care.
Characteristics of Head Lice
The head louse is a tan-gray–colored, wingless insect measuring approximately 2- to 3-mm long with 3 body segments. It has 6 legs with claws used to grasp individual hairs, and it moves by crawling; it does not fly or jump.2,3 The head louse has an elongated abdomen and a small head with short antennae and anterior piercing mouthparts (Figure 1).4 Nits are transparent, flask-shaped, 0.5- to 0.8-mm egg cases found firmly cemented to the hair shafts approximately 1 to 4 mm above the level of the scalp (Figure 2).5 The head louse resides on scalp hair and feeds off the scalp itself. Both lice and nits can be present throughout the scalp but are most commonly found in the postauricular and occipital scalp.3,4
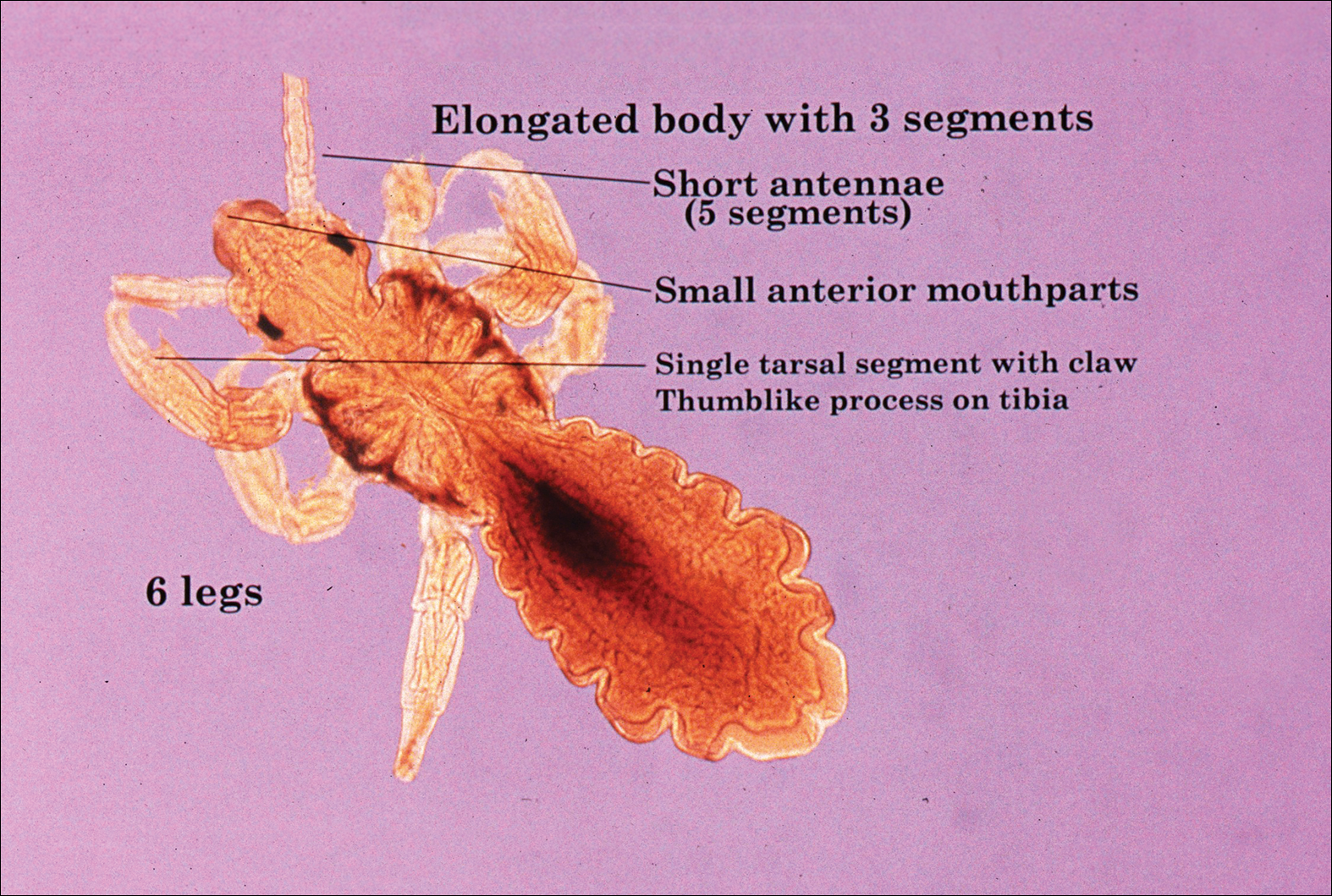
Female lice live approximately 30 days and lay 5 to 10 eggs per day. Eggs incubate individually in nits laid close to the scalp for 8 to 10 days before hatching.1,6 The newly hatched nymphs (also called instars) have multiple exoskeletons that are shed as they grow.7 Nymphs mature into adults in approximately 2 weeks, and the life cycle begins again.8 Head lice are obligate human parasites, feeding approximately every 4 to 6 hours on the blood of the host; however, they can survive up to 4 days without a blood meal on fomites if the climate and conditions are favorable.5,9
Epidemiology and Transmission
Head lice infestations commonly occur in children aged 3 to 11 years and are more prevalent in girls and women.1,10 Infestation rates are not reliably recorded, and few population-based studies have been performed; however, it is estimated that 6 to 12 million individuals are infested annually in the United States.1 Prevalence in some European populations has been estimated to range from 1% to 20%.11 A 2008 literature review found that worldwide prevalence varied across populations from 0.7% to 59%.10
Transmission occurs most frequently from direct head-to-head contact. One study found that transmission is most likely to occur when hairs are arranged in a parallel alignment and move slowly in relation to one another.12 Although controversial and probably less notable, transmission also may occur indirectly via fomites or the sharing of hairbrushes, hats, or other headgear.13,14 Classrooms are a common place for transmission.1 A 2009 study in Germany found an increase in health department consultations for head lice when schools reopened after vacations. The investigators also found that pediculicide sales peaked from mid-September through October, subsequent to schools reopening after the summer holiday.15 There is some evidence that overcrowded housing also can lead to increased incidence and transmission.16,17 There is no consistent correlation of infestation with socioeconomic status.1,17,18
Clinical Manifestations and Diagnosis
Clinically, patients with head lice present with scalp pruritus and sometimes posterior cervical or occipital lymphadenopathy. Pediculosis also can be asymptomatic. With the first exposure, symptoms may not develop for up to 4 to 6 weeks as the immune system develops sensitivity to the louse saliva.6 Bite reactions consisting of papules or wheals are related to immune sensitization.5 Louse feces and excoriations from scratching to relieve itch also may be present on examination. Secondary infection of excoriations also is possible.1
Diagnosis of an active infestation is made by identifying living lice. Because lice move quickly and can be difficult to detect, tightly attached nits on the hair shaft within 4 mm of the scalp are at least indicative of a historic infestation and can be suggestive of active infestation.1,19 Dermoscopy is a helpful tool in differentiating eggs containing nymphs from the empty cases of hatched lice and also from amorphous pseudonits (hair casts)(Figure 3).19,20 Wet combing improves the accuracy of diagnosing an active infection.21

Treatment
Effective treatment of head lice requires eradication of all living lice as well as louse eggs. Topically applied pyrethroids, including pyrethrin shampoos and mousses and permethrin lotion 1%, are considered the first-line therapy.8 Pyrethroids are over-the-counter treatments that act by interfering with sodium transport in the louse, causing depolarization of the neuromembranes and respiratory paralysis.22 Pyrethrins are natural compounds derived from the chrysanthemum plant; permethrin is a synthetic compound. Pyrethrins often are combined with piperonyl butoxide, an insecticide synergist that improves efficacy by inhibiting pyrethrin catabolism.23 Resistance to pyrethroids has become an increasingly important problem in the United States and worldwide.
Malathion lotion 0.5% is another therapeutic option for head lice. Malathion is a prescription organophosphate cholinesterase inhibitor that also causes respiratory paralysis of the louse and is one of the few treatments that is ovicidal.22 It was withdrawn from the market in 1995 due to its flammability and a theoretical risk of respiratory depression if ingested; however, it was reintroduced in 1999 and remains an effective treatment option with little resistance in the United States.24
Lindane 1% (shampoo and lotion), an organochloride compound that acts by causing neuronal hyperstimulation and eventual paralysis of lice, is no longer recommended due to its serious side effects, including central nervous system toxicity and increased risk of seizure.8,24
New US Food and Drug Administration–Approved Therapies
Newer topical treatments include benzyl alcohol lotion 5%, spinosad topical suspension 0.9%, ivermectin lotion 0.5%, and dimethicone-based products. Benzyl alcohol was approved by the US Food and Drug Administration (FDA) in 2009 and is available in the United States by prescription.25 Benzyl alcohol kills lice by asphyxiation. Phase 2 and 3 clinical trials showed significant treatment success 1 day posttreatment (fewer live lice than the vehicle alone; P=.004) and 2 weeks posttreatment (absence of live lice compared to the vehicle alone; P=.001).26
Spinosad was approved by the FDA in 2011 and is available in the United States by prescription.25 It contains the compounds spinosyn A and spinosyn D, which are naturally derived through fermentation by the soil bacterium Saccharopolyspora spinosa. It also contains benzyl alcohol. Spinosad paralyzes lice by disrupting neuronal activity and is at least partially ovicidal.27 Phase 3 clinical trials published in 2009 showed that spinosad was significantly more effective than permethrin in eradicating head lice (P<.001).28
Topical ivermectin was approved by the FDA in 2012 for prescription use.25 It acts on chloride ion channels, causing hyperpolarization of the muscle cells of lice and resulting in paralysis and death. Oral ivermectin (200 μg/kg) given once and repeated in 10 days is not FDA approved for the treatment of head lice but has shown some effectiveness and is sometimes used.8 A comparison study of topical versus oral ivermectin published in 2014 found that eradication was achieved in 88% (n=27) of topical ivermectin users after 1 treatment and 100% (n=31) after 2 treatments. Oral ivermectin produced cure rates of 45% (n=14) after 1 treatment and 97% (n=30) after 2 treatments. Both topical and oral ivermectin treatments are well tolerated.29
Physically Acting Preparations
Products with a physical mode of action are a new attractive option for treatment of pediculosis because the development of resistance is less likely. Studies of silicone-based fluids that physically occlude the respiratory system of the louse, such as dimethicone liquid gel 4%, have shown superiority over treatment with pyrethroids.30,31 Although the safety of dimethicone has been demonstrated, silicone-based treatments have not yet been widely adopted in the United States and are not currently used as a first-line treatment.32 However, use of such physically acting pediculicides may in time surpass traditional neurotoxic treatments due to their low susceptibility to resistance and good safety profile.33,34
Alternative Therapies
Nonchemical treatments for head lice that have shown variable success include wet combing, hot air treatments, and varying occlusive treatments. Physical removal via wet combing requires persistent repeated treatments over several weeks; for example, wet combing may be performed every 3 days for at least 2 weeks or until no head lice are detected on 4 consecutive occasions.35 Cure rates range from 38% to 75% with wet combing as a sole treatment of head lice.36 Because this treatment has minimal risks and no adverse side effects, it can be considered as an alternative treatment for some patients.
Hot air treatments also have been studied. A 2006 study showed that a hot air treatment device had the potential to eradicate head lice, most likely by desiccation. Specifically, 30 minutes of exposure to hot air (at 58.9°F, slightly cooler than a standard hair dryer) using the custom-built device resulted in 98% mortality of eggs and 80% mortality of hatched lice.37 Large randomized controlled trials of hot air treatments have not been performed.
Other alternative treatments include plant-derived oils. A laboratory study of essential oils found that spearmint, cassia, and clove showed pediculicidal activity similar to malathion with improved ovicidal activity.38 However, there is a potential for development of contact dermatitis from essential oils.
Complete Eradication of Head Lice
Removal of nits is an important component of effective lice eradication. Biochemical analysis has revealed that the nit sheath of the head louse is similar in composition to amyloid, rendering it difficult to design products that will unravel the nit sheath while leaving human hair undamaged.39 Because pediculicides are not necessarily ovicidal and complete physical nit removal is difficult to achieve, re-treatment in 7 to 10 days often is advisable to ensure that lice in all stages of the life cycle have been killed.4 Treatment of any secondary bacterial infection also is important. Although transmission of lice via fomites is less likely than from head-to-head contact, the cleaning of hats, hairbrushes, and linens is prudent. Diagnosing and treating infested close contacts also is essential to achieving eradication.4 Coordinated surveillance, education, and treatment efforts in high-risk communities can help detect asymptomatic cases and control local epidemics in a cost-effective manner.40 However, “no nit” policies at schools likely cause a net harm, as nit removal is difficult and children with nonviable nits are then excluded from the classroom.5
Treatment Resistance
Resistance to topical neurotoxic treatments is becoming increasingly common.41-43 Therefore, it is important to identify local patterns of resistance, if possible, when selecting a therapy for head lice. Improper usage, changes in pediculicide formulations and packaging, decreased product efficacy, and natural selection have all contributed to this rise in resistance.7 Additionally, due to protection from multiple exoskeletons and the natural molting process as they mature into adults, nymphs may only receive a sublethal dose when exposed to pediculicides, contributing further to resistance.7 Resistance to synthetic pyrethroids is most predominant, likely due to selection pressure because permethrin historically has been the most widely used insecticide for pediculosis. A 2014 study found that the frequency of sodium-channel insensitivity to pyrethroids, also known as knockdown resistance (or kdr), in US head louse populations collected over a 10-year period was 84.4% and approached 100% in some communities in recent years.44 This evidence strongly supports the use of alternative therapeutic categories to effectively eradicate head lice infestations.
Conclusion
Head lice infestation is common in children, and although it is not harmful to the host, it can be an irritating and symptomatic problem and can lead to notable distress, missed days of school, and secondary infections. Identifying active adult lice is the gold standard for diagnosis. Current recommended treatments include pyrethroids as the first-line therapy; however, resistance to these neurotoxic agents is becoming increasingly common. Alternative therapies such as newer neurotoxic agents or pediculicides with physical mechanisms of action (eg, dimethicone-based products) should be considered, particularly in regions where resistance is known to be high. Education about head lice, proper use of treatment, and coordinated diagnosis are necessary for effective management of this problem.
- Chosidow O. Scabies and pediculosis. Lancet. 2000;355:819-826.
- Centers for Disease Control and Prevention. Head lice. http://www.cdc.gov/parasites/lice/head/index.html. Updated September 24, 2013. Accessed November 9, 2017.
- Hurwitz S. Lice (pediculosis). In: Hurwitz S. Hurwitz Clinical Pediatric Dermatology: A Textbook of Skin Disorders of Childhood and Adolescence. 2nd ed. Philadelphia, PA: WB Saunders Company; 1993:416-419.
- Elston DM. What’s eating you? Pediculus humanus (head louse and body louse). Cutis. 1999;63:259-264.
- Ko CJ, Elston DM. Pediculosis. J Am Acad Dermatol. 2004;50:1-12.
- Frankowski BL, Weiner LB. Head lice. Pediatrics. 2002;110:638-643.
- Meinking TL. Clinical update on resistance and treatment of pediculosis capitis. Am J Manag Care. 2004;10(9 suppl):S264-S268.
- Devore CD, Schutze GE. Head lice. Pediatrics. 2015;135:E1355-E1365.
- Burkhart CN. Fomite transmission with head lice: a continuing controversy. Lancet. 2003;361:99-100.
- Falagas ME, Matthaiou DK, Rafailidis PI, et al. Worldwide prevalence of head lice. Emerg Infect Dis. 2008;14:1493-1494.
- Feldmeier H. Pediculosis capitis: new insights into epidemiology, diagnosis and treatment. Eur J Clin Microbiol Infect Dis. 2012;31:2105-2110.
- Canyon DV, Speare R, Muller R. Spatial and kinetic factors for the transfer of head lice (Pediculus capitis) between hairs. J Invest Dermatol. 2002;119:629-631.
- Burkhart CN, Burkhart CG. Fomite transmission in head lice. J Am Acad Dermatol. 2007;56:1044-1047.
- Canyon DV, Speare R. Indirect transmission of head lice via inanimate objects. Open Dermatol J. 2010;4:72-76.
- Bauer E, Jahnke C, Feldmeier H. Seasonal fluctuations of head lice infestation in Germany. Parasitol Res. 2009;104:677-681.
- Balcioglu IC, Kurt O, Limoncu ME, et al. Rural life, lower socioeconomic status and parasitic infections. Parasitol Int. 2007;56:129-133.
- Lesshafft H, Baier A, Guerra H, et al. Prevalence and risk factors associated with pediculosis capitis in an impoverished urban community in Lima, Peru. J Glob Infect Dis. 2013;5:138-143.
- Tagka A, Lambrou GI, Braoudaki M, et al. Socioeconomical factors associated with pediculosis (Phthiraptera: Pediculidae) in Athens, Greece. J Med Entomol. 2016;53:919-922.
- Di Stefani A, Hofmann-Wellenhof R, Zalaudek I. Dermoscopy for diagnosis and treatment monitoring of pediculosis capitis. J Am Acad Dermatol. 2006;54:909-911.
- Bakos RM, Bakos L. Dermoscopy for diagnosis of pediculosis capitis. J Am Acad Dermatol. 2007;57:727-728.
- Jahnke C, Bauer E, Hengge UR, et al. Accuracy of diagnosis of pediculosis capitis: visual inspection vs wet combing. Arch Dermatol. 2009;145:309-313.
- Elston DM. Drugs used in the treatment of pediculosis. J Drugs Dermatol. 2005;4:207-211.
- National Pesticide Information Center. Piperonyl butoxide (general fact sheet). http://npic.orst.edu/factsheets/pbogen.pdf/. Accessed November 13, 2017.
- Diamantis SA, Morrell DS, Burkhart CN. Treatment of head lice. Dermatol Ther. 2009;22:273-278.
- United States Food and Drug Administration. Treating and preventing head lice. http://www.fda.gov/forconsumers/consumerupdates/ucm171730.htm. Published July 13, 2010. Updated November 8, 2017. Accessed November 13, 2017.
- Meinking TL, Villar ME, Vicaria M, et al. The clinical trials supporting benzyl alcohol lotion 5% (UlesfiaTM): a safe and effective topical treatment for head lice (Pediculosis Humanus Capitis). Pediatr Dermatol. 2010;27:19-24.
- McCormack PL. Spinosad in pediculosis capitis. Am J Clin Dermatol. 2011;12:349-353.
- Stough D, Shellabarger S, Quiring J, et al. Efficacy and safety of spinosad and permethrin creme rinses for pediculosis capitis (head lice). Pediatrics. 2009;124:E389-E395.
- Ahmad HM, Abdel-Azim ES, Abdel-Aziz RT. Assessment of topical versus oral ivermectin as a treatment for head lice. Dermatol Ther. 2014;27:307-310.
- Heukelbach J, Pilger D, Oliveira FA, et al. A highly efficacious pediculicide based on dimethicone: randomized observer blinded comparative trial. BMC Infect Dis. 2008;8:115.
- Burgess IF, Brunton ER, Burgess NA. Single application of 4% dimethicone liquid gel versus two applications of 1% permethrin creme rinse for treatment of head louse infestation: a randomised controlled trial. BMC Dermatol. 2013;13:5.
- Ihde ES, Boscamp JR, Loh JM, et al. Safety and efficacy of a 100% dimethicone pediculocide in school-age children. BMC Pediatr. 2015;15:70.
- Heukelbach J, Oliveira FA, Richter J, et al. Dimethicone-based pediculicides: a physical approach to eradicate head lice. Open Dermatol J. 2010;4:77-81.
- Feldmeier H. Treatment of pediculosis capitis: a critical appraisal of the current literature. Am J Clin Dermatol. 2014;15:401-412.
- Glasziou P, Bennett J, Greenberg P, et al; Handbook Of Non Drug Intervention (HANDI) Project Team. Wet combing for the eradication of head lice. Aust Fam Physician. 2013;42:129-130.
- Tebruegge M, Runnacles J. Is wet combing effective in children with pediculosis capitis infestation? Arch Dis Child. 2007;92:818-820.
- Goates BM, Atkin JS, Wilding KG, et al. An effective nonchemical treatment for head lice: a lot of hot air. Pediatrics. 2006;118:1962-1970.
- Yones DA, Bakir HY, Bayoumi SA. Chemical composition and efficacy of some selected plant oils against Pediculus humanus capitis in vitro. Parasitol Res. 2016;115:3209-3218.
- Burkhart CN, Burkhart CG. Head lice: scientific assessment of the nit sheath with clinical ramifications and therapeutic options. J Am Acad Dermatol. 2005;53:129-133.
- Ibarra J, Fry F, Wickenden C, et al. The impact of well-developed preventative strategies on the eradication of head lice. Perspect Public Health. 2009;129:165-173.
- Mumcuoglu KY, Hemingway J, Miller J, et al. Permethrin resistance in the head louse pediculus humanus capitis from Israel. Med Vet Entomol. 1995;9:427-432.
- Meinking TL, Serrano L, Hard B, et al. Comparative in vitro pediculicidal efficacy of treatments in a resistant head lice population in the United States. Arch Dermatol. 2002;138:220-224.
- Hemingway J, Miller J, Mumcuoglu KY. Pyrethroid resistance mechanisms in the head louse Pediculus capitis from Israel: implications for control. Med Vet Entomol. 1999;13:89-96.
- Yoon KS, Previte DJ, Hodgdon HE, et al. Knockdown resistance allele frequencies in North American head louse (Anoplura: Pediculidae) populations. J Med Entomol. 2014;51:450-457.
The head louse (Pediculus humanus capitis) is a blood-sucking arthropod of the suborder Anoplura. Lice are obligate human parasites that have infested humans since antiquity. Pediculosis capitis is an infestation of the scalp by head lice. It is estimated that 6 to 12 million individuals in the United States are affected with head lice per year.1 Resistance to topical chemical pediculicides is widespread, and new agents have been developed to address this gap in care.
Characteristics of Head Lice
The head louse is a tan-gray–colored, wingless insect measuring approximately 2- to 3-mm long with 3 body segments. It has 6 legs with claws used to grasp individual hairs, and it moves by crawling; it does not fly or jump.2,3 The head louse has an elongated abdomen and a small head with short antennae and anterior piercing mouthparts (Figure 1).4 Nits are transparent, flask-shaped, 0.5- to 0.8-mm egg cases found firmly cemented to the hair shafts approximately 1 to 4 mm above the level of the scalp (Figure 2).5 The head louse resides on scalp hair and feeds off the scalp itself. Both lice and nits can be present throughout the scalp but are most commonly found in the postauricular and occipital scalp.3,4


Female lice live approximately 30 days and lay 5 to 10 eggs per day. Eggs incubate individually in nits laid close to the scalp for 8 to 10 days before hatching.1,6 The newly hatched nymphs (also called instars) have multiple exoskeletons that are shed as they grow.7 Nymphs mature into adults in approximately 2 weeks, and the life cycle begins again.8 Head lice are obligate human parasites, feeding approximately every 4 to 6 hours on the blood of the host; however, they can survive up to 4 days without a blood meal on fomites if the climate and conditions are favorable.5,9
Epidemiology and Transmission
Head lice infestations commonly occur in children aged 3 to 11 years and are more prevalent in girls and women.1,10 Infestation rates are not reliably recorded, and few population-based studies have been performed; however, it is estimated that 6 to 12 million individuals are infested annually in the United States.1 Prevalence in some European populations has been estimated to range from 1% to 20%.11 A 2008 literature review found that worldwide prevalence varied across populations from 0.7% to 59%.10
Transmission occurs most frequently from direct head-to-head contact. One study found that transmission is most likely to occur when hairs are arranged in a parallel alignment and move slowly in relation to one another.12 Although controversial and probably less notable, transmission also may occur indirectly via fomites or the sharing of hairbrushes, hats, or other headgear.13,14 Classrooms are a common place for transmission.1 A 2009 study in Germany found an increase in health department consultations for head lice when schools reopened after vacations. The investigators also found that pediculicide sales peaked from mid-September through October, subsequent to schools reopening after the summer holiday.15 There is some evidence that overcrowded housing also can lead to increased incidence and transmission.16,17 There is no consistent correlation of infestation with socioeconomic status.1,17,18
Clinical Manifestations and Diagnosis
Clinically, patients with head lice present with scalp pruritus and sometimes posterior cervical or occipital lymphadenopathy. Pediculosis also can be asymptomatic. With the first exposure, symptoms may not develop for up to 4 to 6 weeks as the immune system develops sensitivity to the louse saliva.6 Bite reactions consisting of papules or wheals are related to immune sensitization.5 Louse feces and excoriations from scratching to relieve itch also may be present on examination. Secondary infection of excoriations also is possible.1
Diagnosis of an active infestation is made by identifying living lice. Because lice move quickly and can be difficult to detect, tightly attached nits on the hair shaft within 4 mm of the scalp are at least indicative of a historic infestation and can be suggestive of active infestation.1,19 Dermoscopy is a helpful tool in differentiating eggs containing nymphs from the empty cases of hatched lice and also from amorphous pseudonits (hair casts)(Figure 3).19,20 Wet combing improves the accuracy of diagnosing an active infection.21

Treatment
Effective treatment of head lice requires eradication of all living lice as well as louse eggs. Topically applied pyrethroids, including pyrethrin shampoos and mousses and permethrin lotion 1%, are considered the first-line therapy.8 Pyrethroids are over-the-counter treatments that act by interfering with sodium transport in the louse, causing depolarization of the neuromembranes and respiratory paralysis.22 Pyrethrins are natural compounds derived from the chrysanthemum plant; permethrin is a synthetic compound. Pyrethrins often are combined with piperonyl butoxide, an insecticide synergist that improves efficacy by inhibiting pyrethrin catabolism.23 Resistance to pyrethroids has become an increasingly important problem in the United States and worldwide.
Malathion lotion 0.5% is another therapeutic option for head lice. Malathion is a prescription organophosphate cholinesterase inhibitor that also causes respiratory paralysis of the louse and is one of the few treatments that is ovicidal.22 It was withdrawn from the market in 1995 due to its flammability and a theoretical risk of respiratory depression if ingested; however, it was reintroduced in 1999 and remains an effective treatment option with little resistance in the United States.24
Lindane 1% (shampoo and lotion), an organochloride compound that acts by causing neuronal hyperstimulation and eventual paralysis of lice, is no longer recommended due to its serious side effects, including central nervous system toxicity and increased risk of seizure.8,24
New US Food and Drug Administration–Approved Therapies
Newer topical treatments include benzyl alcohol lotion 5%, spinosad topical suspension 0.9%, ivermectin lotion 0.5%, and dimethicone-based products. Benzyl alcohol was approved by the US Food and Drug Administration (FDA) in 2009 and is available in the United States by prescription.25 Benzyl alcohol kills lice by asphyxiation. Phase 2 and 3 clinical trials showed significant treatment success 1 day posttreatment (fewer live lice than the vehicle alone; P=.004) and 2 weeks posttreatment (absence of live lice compared to the vehicle alone; P=.001).26
Spinosad was approved by the FDA in 2011 and is available in the United States by prescription.25 It contains the compounds spinosyn A and spinosyn D, which are naturally derived through fermentation by the soil bacterium Saccharopolyspora spinosa. It also contains benzyl alcohol. Spinosad paralyzes lice by disrupting neuronal activity and is at least partially ovicidal.27 Phase 3 clinical trials published in 2009 showed that spinosad was significantly more effective than permethrin in eradicating head lice (P<.001).28
Topical ivermectin was approved by the FDA in 2012 for prescription use.25 It acts on chloride ion channels, causing hyperpolarization of the muscle cells of lice and resulting in paralysis and death. Oral ivermectin (200 μg/kg) given once and repeated in 10 days is not FDA approved for the treatment of head lice but has shown some effectiveness and is sometimes used.8 A comparison study of topical versus oral ivermectin published in 2014 found that eradication was achieved in 88% (n=27) of topical ivermectin users after 1 treatment and 100% (n=31) after 2 treatments. Oral ivermectin produced cure rates of 45% (n=14) after 1 treatment and 97% (n=30) after 2 treatments. Both topical and oral ivermectin treatments are well tolerated.29
Physically Acting Preparations
Products with a physical mode of action are a new attractive option for treatment of pediculosis because the development of resistance is less likely. Studies of silicone-based fluids that physically occlude the respiratory system of the louse, such as dimethicone liquid gel 4%, have shown superiority over treatment with pyrethroids.30,31 Although the safety of dimethicone has been demonstrated, silicone-based treatments have not yet been widely adopted in the United States and are not currently used as a first-line treatment.32 However, use of such physically acting pediculicides may in time surpass traditional neurotoxic treatments due to their low susceptibility to resistance and good safety profile.33,34
Alternative Therapies
Nonchemical treatments for head lice that have shown variable success include wet combing, hot air treatments, and varying occlusive treatments. Physical removal via wet combing requires persistent repeated treatments over several weeks; for example, wet combing may be performed every 3 days for at least 2 weeks or until no head lice are detected on 4 consecutive occasions.35 Cure rates range from 38% to 75% with wet combing as a sole treatment of head lice.36 Because this treatment has minimal risks and no adverse side effects, it can be considered as an alternative treatment for some patients.
Hot air treatments also have been studied. A 2006 study showed that a hot air treatment device had the potential to eradicate head lice, most likely by desiccation. Specifically, 30 minutes of exposure to hot air (at 58.9°F, slightly cooler than a standard hair dryer) using the custom-built device resulted in 98% mortality of eggs and 80% mortality of hatched lice.37 Large randomized controlled trials of hot air treatments have not been performed.
Other alternative treatments include plant-derived oils. A laboratory study of essential oils found that spearmint, cassia, and clove showed pediculicidal activity similar to malathion with improved ovicidal activity.38 However, there is a potential for development of contact dermatitis from essential oils.
Complete Eradication of Head Lice
Removal of nits is an important component of effective lice eradication. Biochemical analysis has revealed that the nit sheath of the head louse is similar in composition to amyloid, rendering it difficult to design products that will unravel the nit sheath while leaving human hair undamaged.39 Because pediculicides are not necessarily ovicidal and complete physical nit removal is difficult to achieve, re-treatment in 7 to 10 days often is advisable to ensure that lice in all stages of the life cycle have been killed.4 Treatment of any secondary bacterial infection also is important. Although transmission of lice via fomites is less likely than from head-to-head contact, the cleaning of hats, hairbrushes, and linens is prudent. Diagnosing and treating infested close contacts also is essential to achieving eradication.4 Coordinated surveillance, education, and treatment efforts in high-risk communities can help detect asymptomatic cases and control local epidemics in a cost-effective manner.40 However, “no nit” policies at schools likely cause a net harm, as nit removal is difficult and children with nonviable nits are then excluded from the classroom.5
Treatment Resistance
Resistance to topical neurotoxic treatments is becoming increasingly common.41-43 Therefore, it is important to identify local patterns of resistance, if possible, when selecting a therapy for head lice. Improper usage, changes in pediculicide formulations and packaging, decreased product efficacy, and natural selection have all contributed to this rise in resistance.7 Additionally, due to protection from multiple exoskeletons and the natural molting process as they mature into adults, nymphs may only receive a sublethal dose when exposed to pediculicides, contributing further to resistance.7 Resistance to synthetic pyrethroids is most predominant, likely due to selection pressure because permethrin historically has been the most widely used insecticide for pediculosis. A 2014 study found that the frequency of sodium-channel insensitivity to pyrethroids, also known as knockdown resistance (or kdr), in US head louse populations collected over a 10-year period was 84.4% and approached 100% in some communities in recent years.44 This evidence strongly supports the use of alternative therapeutic categories to effectively eradicate head lice infestations.
Conclusion
Head lice infestation is common in children, and although it is not harmful to the host, it can be an irritating and symptomatic problem and can lead to notable distress, missed days of school, and secondary infections. Identifying active adult lice is the gold standard for diagnosis. Current recommended treatments include pyrethroids as the first-line therapy; however, resistance to these neurotoxic agents is becoming increasingly common. Alternative therapies such as newer neurotoxic agents or pediculicides with physical mechanisms of action (eg, dimethicone-based products) should be considered, particularly in regions where resistance is known to be high. Education about head lice, proper use of treatment, and coordinated diagnosis are necessary for effective management of this problem.
The head louse (Pediculus humanus capitis) is a blood-sucking arthropod of the suborder Anoplura. Lice are obligate human parasites that have infested humans since antiquity. Pediculosis capitis is an infestation of the scalp by head lice. It is estimated that 6 to 12 million individuals in the United States are affected with head lice per year.1 Resistance to topical chemical pediculicides is widespread, and new agents have been developed to address this gap in care.
Characteristics of Head Lice
The head louse is a tan-gray–colored, wingless insect measuring approximately 2- to 3-mm long with 3 body segments. It has 6 legs with claws used to grasp individual hairs, and it moves by crawling; it does not fly or jump.2,3 The head louse has an elongated abdomen and a small head with short antennae and anterior piercing mouthparts (Figure 1).4 Nits are transparent, flask-shaped, 0.5- to 0.8-mm egg cases found firmly cemented to the hair shafts approximately 1 to 4 mm above the level of the scalp (Figure 2).5 The head louse resides on scalp hair and feeds off the scalp itself. Both lice and nits can be present throughout the scalp but are most commonly found in the postauricular and occipital scalp.3,4


Female lice live approximately 30 days and lay 5 to 10 eggs per day. Eggs incubate individually in nits laid close to the scalp for 8 to 10 days before hatching.1,6 The newly hatched nymphs (also called instars) have multiple exoskeletons that are shed as they grow.7 Nymphs mature into adults in approximately 2 weeks, and the life cycle begins again.8 Head lice are obligate human parasites, feeding approximately every 4 to 6 hours on the blood of the host; however, they can survive up to 4 days without a blood meal on fomites if the climate and conditions are favorable.5,9
Epidemiology and Transmission
Head lice infestations commonly occur in children aged 3 to 11 years and are more prevalent in girls and women.1,10 Infestation rates are not reliably recorded, and few population-based studies have been performed; however, it is estimated that 6 to 12 million individuals are infested annually in the United States.1 Prevalence in some European populations has been estimated to range from 1% to 20%.11 A 2008 literature review found that worldwide prevalence varied across populations from 0.7% to 59%.10
Transmission occurs most frequently from direct head-to-head contact. One study found that transmission is most likely to occur when hairs are arranged in a parallel alignment and move slowly in relation to one another.12 Although controversial and probably less notable, transmission also may occur indirectly via fomites or the sharing of hairbrushes, hats, or other headgear.13,14 Classrooms are a common place for transmission.1 A 2009 study in Germany found an increase in health department consultations for head lice when schools reopened after vacations. The investigators also found that pediculicide sales peaked from mid-September through October, subsequent to schools reopening after the summer holiday.15 There is some evidence that overcrowded housing also can lead to increased incidence and transmission.16,17 There is no consistent correlation of infestation with socioeconomic status.1,17,18
Clinical Manifestations and Diagnosis
Clinically, patients with head lice present with scalp pruritus and sometimes posterior cervical or occipital lymphadenopathy. Pediculosis also can be asymptomatic. With the first exposure, symptoms may not develop for up to 4 to 6 weeks as the immune system develops sensitivity to the louse saliva.6 Bite reactions consisting of papules or wheals are related to immune sensitization.5 Louse feces and excoriations from scratching to relieve itch also may be present on examination. Secondary infection of excoriations also is possible.1
Diagnosis of an active infestation is made by identifying living lice. Because lice move quickly and can be difficult to detect, tightly attached nits on the hair shaft within 4 mm of the scalp are at least indicative of a historic infestation and can be suggestive of active infestation.1,19 Dermoscopy is a helpful tool in differentiating eggs containing nymphs from the empty cases of hatched lice and also from amorphous pseudonits (hair casts)(Figure 3).19,20 Wet combing improves the accuracy of diagnosing an active infection.21

Treatment
Effective treatment of head lice requires eradication of all living lice as well as louse eggs. Topically applied pyrethroids, including pyrethrin shampoos and mousses and permethrin lotion 1%, are considered the first-line therapy.8 Pyrethroids are over-the-counter treatments that act by interfering with sodium transport in the louse, causing depolarization of the neuromembranes and respiratory paralysis.22 Pyrethrins are natural compounds derived from the chrysanthemum plant; permethrin is a synthetic compound. Pyrethrins often are combined with piperonyl butoxide, an insecticide synergist that improves efficacy by inhibiting pyrethrin catabolism.23 Resistance to pyrethroids has become an increasingly important problem in the United States and worldwide.
Malathion lotion 0.5% is another therapeutic option for head lice. Malathion is a prescription organophosphate cholinesterase inhibitor that also causes respiratory paralysis of the louse and is one of the few treatments that is ovicidal.22 It was withdrawn from the market in 1995 due to its flammability and a theoretical risk of respiratory depression if ingested; however, it was reintroduced in 1999 and remains an effective treatment option with little resistance in the United States.24
Lindane 1% (shampoo and lotion), an organochloride compound that acts by causing neuronal hyperstimulation and eventual paralysis of lice, is no longer recommended due to its serious side effects, including central nervous system toxicity and increased risk of seizure.8,24
New US Food and Drug Administration–Approved Therapies
Newer topical treatments include benzyl alcohol lotion 5%, spinosad topical suspension 0.9%, ivermectin lotion 0.5%, and dimethicone-based products. Benzyl alcohol was approved by the US Food and Drug Administration (FDA) in 2009 and is available in the United States by prescription.25 Benzyl alcohol kills lice by asphyxiation. Phase 2 and 3 clinical trials showed significant treatment success 1 day posttreatment (fewer live lice than the vehicle alone; P=.004) and 2 weeks posttreatment (absence of live lice compared to the vehicle alone; P=.001).26
Spinosad was approved by the FDA in 2011 and is available in the United States by prescription.25 It contains the compounds spinosyn A and spinosyn D, which are naturally derived through fermentation by the soil bacterium Saccharopolyspora spinosa. It also contains benzyl alcohol. Spinosad paralyzes lice by disrupting neuronal activity and is at least partially ovicidal.27 Phase 3 clinical trials published in 2009 showed that spinosad was significantly more effective than permethrin in eradicating head lice (P<.001).28
Topical ivermectin was approved by the FDA in 2012 for prescription use.25 It acts on chloride ion channels, causing hyperpolarization of the muscle cells of lice and resulting in paralysis and death. Oral ivermectin (200 μg/kg) given once and repeated in 10 days is not FDA approved for the treatment of head lice but has shown some effectiveness and is sometimes used.8 A comparison study of topical versus oral ivermectin published in 2014 found that eradication was achieved in 88% (n=27) of topical ivermectin users after 1 treatment and 100% (n=31) after 2 treatments. Oral ivermectin produced cure rates of 45% (n=14) after 1 treatment and 97% (n=30) after 2 treatments. Both topical and oral ivermectin treatments are well tolerated.29
Physically Acting Preparations
Products with a physical mode of action are a new attractive option for treatment of pediculosis because the development of resistance is less likely. Studies of silicone-based fluids that physically occlude the respiratory system of the louse, such as dimethicone liquid gel 4%, have shown superiority over treatment with pyrethroids.30,31 Although the safety of dimethicone has been demonstrated, silicone-based treatments have not yet been widely adopted in the United States and are not currently used as a first-line treatment.32 However, use of such physically acting pediculicides may in time surpass traditional neurotoxic treatments due to their low susceptibility to resistance and good safety profile.33,34
Alternative Therapies
Nonchemical treatments for head lice that have shown variable success include wet combing, hot air treatments, and varying occlusive treatments. Physical removal via wet combing requires persistent repeated treatments over several weeks; for example, wet combing may be performed every 3 days for at least 2 weeks or until no head lice are detected on 4 consecutive occasions.35 Cure rates range from 38% to 75% with wet combing as a sole treatment of head lice.36 Because this treatment has minimal risks and no adverse side effects, it can be considered as an alternative treatment for some patients.
Hot air treatments also have been studied. A 2006 study showed that a hot air treatment device had the potential to eradicate head lice, most likely by desiccation. Specifically, 30 minutes of exposure to hot air (at 58.9°F, slightly cooler than a standard hair dryer) using the custom-built device resulted in 98% mortality of eggs and 80% mortality of hatched lice.37 Large randomized controlled trials of hot air treatments have not been performed.
Other alternative treatments include plant-derived oils. A laboratory study of essential oils found that spearmint, cassia, and clove showed pediculicidal activity similar to malathion with improved ovicidal activity.38 However, there is a potential for development of contact dermatitis from essential oils.
Complete Eradication of Head Lice
Removal of nits is an important component of effective lice eradication. Biochemical analysis has revealed that the nit sheath of the head louse is similar in composition to amyloid, rendering it difficult to design products that will unravel the nit sheath while leaving human hair undamaged.39 Because pediculicides are not necessarily ovicidal and complete physical nit removal is difficult to achieve, re-treatment in 7 to 10 days often is advisable to ensure that lice in all stages of the life cycle have been killed.4 Treatment of any secondary bacterial infection also is important. Although transmission of lice via fomites is less likely than from head-to-head contact, the cleaning of hats, hairbrushes, and linens is prudent. Diagnosing and treating infested close contacts also is essential to achieving eradication.4 Coordinated surveillance, education, and treatment efforts in high-risk communities can help detect asymptomatic cases and control local epidemics in a cost-effective manner.40 However, “no nit” policies at schools likely cause a net harm, as nit removal is difficult and children with nonviable nits are then excluded from the classroom.5
Treatment Resistance
Resistance to topical neurotoxic treatments is becoming increasingly common.41-43 Therefore, it is important to identify local patterns of resistance, if possible, when selecting a therapy for head lice. Improper usage, changes in pediculicide formulations and packaging, decreased product efficacy, and natural selection have all contributed to this rise in resistance.7 Additionally, due to protection from multiple exoskeletons and the natural molting process as they mature into adults, nymphs may only receive a sublethal dose when exposed to pediculicides, contributing further to resistance.7 Resistance to synthetic pyrethroids is most predominant, likely due to selection pressure because permethrin historically has been the most widely used insecticide for pediculosis. A 2014 study found that the frequency of sodium-channel insensitivity to pyrethroids, also known as knockdown resistance (or kdr), in US head louse populations collected over a 10-year period was 84.4% and approached 100% in some communities in recent years.44 This evidence strongly supports the use of alternative therapeutic categories to effectively eradicate head lice infestations.
Conclusion
Head lice infestation is common in children, and although it is not harmful to the host, it can be an irritating and symptomatic problem and can lead to notable distress, missed days of school, and secondary infections. Identifying active adult lice is the gold standard for diagnosis. Current recommended treatments include pyrethroids as the first-line therapy; however, resistance to these neurotoxic agents is becoming increasingly common. Alternative therapies such as newer neurotoxic agents or pediculicides with physical mechanisms of action (eg, dimethicone-based products) should be considered, particularly in regions where resistance is known to be high. Education about head lice, proper use of treatment, and coordinated diagnosis are necessary for effective management of this problem.
- Chosidow O. Scabies and pediculosis. Lancet. 2000;355:819-826.
- Centers for Disease Control and Prevention. Head lice. http://www.cdc.gov/parasites/lice/head/index.html. Updated September 24, 2013. Accessed November 9, 2017.
- Hurwitz S. Lice (pediculosis). In: Hurwitz S. Hurwitz Clinical Pediatric Dermatology: A Textbook of Skin Disorders of Childhood and Adolescence. 2nd ed. Philadelphia, PA: WB Saunders Company; 1993:416-419.
- Elston DM. What’s eating you? Pediculus humanus (head louse and body louse). Cutis. 1999;63:259-264.
- Ko CJ, Elston DM. Pediculosis. J Am Acad Dermatol. 2004;50:1-12.
- Frankowski BL, Weiner LB. Head lice. Pediatrics. 2002;110:638-643.
- Meinking TL. Clinical update on resistance and treatment of pediculosis capitis. Am J Manag Care. 2004;10(9 suppl):S264-S268.
- Devore CD, Schutze GE. Head lice. Pediatrics. 2015;135:E1355-E1365.
- Burkhart CN. Fomite transmission with head lice: a continuing controversy. Lancet. 2003;361:99-100.
- Falagas ME, Matthaiou DK, Rafailidis PI, et al. Worldwide prevalence of head lice. Emerg Infect Dis. 2008;14:1493-1494.
- Feldmeier H. Pediculosis capitis: new insights into epidemiology, diagnosis and treatment. Eur J Clin Microbiol Infect Dis. 2012;31:2105-2110.
- Canyon DV, Speare R, Muller R. Spatial and kinetic factors for the transfer of head lice (Pediculus capitis) between hairs. J Invest Dermatol. 2002;119:629-631.
- Burkhart CN, Burkhart CG. Fomite transmission in head lice. J Am Acad Dermatol. 2007;56:1044-1047.
- Canyon DV, Speare R. Indirect transmission of head lice via inanimate objects. Open Dermatol J. 2010;4:72-76.
- Bauer E, Jahnke C, Feldmeier H. Seasonal fluctuations of head lice infestation in Germany. Parasitol Res. 2009;104:677-681.
- Balcioglu IC, Kurt O, Limoncu ME, et al. Rural life, lower socioeconomic status and parasitic infections. Parasitol Int. 2007;56:129-133.
- Lesshafft H, Baier A, Guerra H, et al. Prevalence and risk factors associated with pediculosis capitis in an impoverished urban community in Lima, Peru. J Glob Infect Dis. 2013;5:138-143.
- Tagka A, Lambrou GI, Braoudaki M, et al. Socioeconomical factors associated with pediculosis (Phthiraptera: Pediculidae) in Athens, Greece. J Med Entomol. 2016;53:919-922.
- Di Stefani A, Hofmann-Wellenhof R, Zalaudek I. Dermoscopy for diagnosis and treatment monitoring of pediculosis capitis. J Am Acad Dermatol. 2006;54:909-911.
- Bakos RM, Bakos L. Dermoscopy for diagnosis of pediculosis capitis. J Am Acad Dermatol. 2007;57:727-728.
- Jahnke C, Bauer E, Hengge UR, et al. Accuracy of diagnosis of pediculosis capitis: visual inspection vs wet combing. Arch Dermatol. 2009;145:309-313.
- Elston DM. Drugs used in the treatment of pediculosis. J Drugs Dermatol. 2005;4:207-211.
- National Pesticide Information Center. Piperonyl butoxide (general fact sheet). http://npic.orst.edu/factsheets/pbogen.pdf/. Accessed November 13, 2017.
- Diamantis SA, Morrell DS, Burkhart CN. Treatment of head lice. Dermatol Ther. 2009;22:273-278.
- United States Food and Drug Administration. Treating and preventing head lice. http://www.fda.gov/forconsumers/consumerupdates/ucm171730.htm. Published July 13, 2010. Updated November 8, 2017. Accessed November 13, 2017.
- Meinking TL, Villar ME, Vicaria M, et al. The clinical trials supporting benzyl alcohol lotion 5% (UlesfiaTM): a safe and effective topical treatment for head lice (Pediculosis Humanus Capitis). Pediatr Dermatol. 2010;27:19-24.
- McCormack PL. Spinosad in pediculosis capitis. Am J Clin Dermatol. 2011;12:349-353.
- Stough D, Shellabarger S, Quiring J, et al. Efficacy and safety of spinosad and permethrin creme rinses for pediculosis capitis (head lice). Pediatrics. 2009;124:E389-E395.
- Ahmad HM, Abdel-Azim ES, Abdel-Aziz RT. Assessment of topical versus oral ivermectin as a treatment for head lice. Dermatol Ther. 2014;27:307-310.
- Heukelbach J, Pilger D, Oliveira FA, et al. A highly efficacious pediculicide based on dimethicone: randomized observer blinded comparative trial. BMC Infect Dis. 2008;8:115.
- Burgess IF, Brunton ER, Burgess NA. Single application of 4% dimethicone liquid gel versus two applications of 1% permethrin creme rinse for treatment of head louse infestation: a randomised controlled trial. BMC Dermatol. 2013;13:5.
- Ihde ES, Boscamp JR, Loh JM, et al. Safety and efficacy of a 100% dimethicone pediculocide in school-age children. BMC Pediatr. 2015;15:70.
- Heukelbach J, Oliveira FA, Richter J, et al. Dimethicone-based pediculicides: a physical approach to eradicate head lice. Open Dermatol J. 2010;4:77-81.
- Feldmeier H. Treatment of pediculosis capitis: a critical appraisal of the current literature. Am J Clin Dermatol. 2014;15:401-412.
- Glasziou P, Bennett J, Greenberg P, et al; Handbook Of Non Drug Intervention (HANDI) Project Team. Wet combing for the eradication of head lice. Aust Fam Physician. 2013;42:129-130.
- Tebruegge M, Runnacles J. Is wet combing effective in children with pediculosis capitis infestation? Arch Dis Child. 2007;92:818-820.
- Goates BM, Atkin JS, Wilding KG, et al. An effective nonchemical treatment for head lice: a lot of hot air. Pediatrics. 2006;118:1962-1970.
- Yones DA, Bakir HY, Bayoumi SA. Chemical composition and efficacy of some selected plant oils against Pediculus humanus capitis in vitro. Parasitol Res. 2016;115:3209-3218.
- Burkhart CN, Burkhart CG. Head lice: scientific assessment of the nit sheath with clinical ramifications and therapeutic options. J Am Acad Dermatol. 2005;53:129-133.
- Ibarra J, Fry F, Wickenden C, et al. The impact of well-developed preventative strategies on the eradication of head lice. Perspect Public Health. 2009;129:165-173.
- Mumcuoglu KY, Hemingway J, Miller J, et al. Permethrin resistance in the head louse pediculus humanus capitis from Israel. Med Vet Entomol. 1995;9:427-432.
- Meinking TL, Serrano L, Hard B, et al. Comparative in vitro pediculicidal efficacy of treatments in a resistant head lice population in the United States. Arch Dermatol. 2002;138:220-224.
- Hemingway J, Miller J, Mumcuoglu KY. Pyrethroid resistance mechanisms in the head louse Pediculus capitis from Israel: implications for control. Med Vet Entomol. 1999;13:89-96.
- Yoon KS, Previte DJ, Hodgdon HE, et al. Knockdown resistance allele frequencies in North American head louse (Anoplura: Pediculidae) populations. J Med Entomol. 2014;51:450-457.
- Chosidow O. Scabies and pediculosis. Lancet. 2000;355:819-826.
- Centers for Disease Control and Prevention. Head lice. http://www.cdc.gov/parasites/lice/head/index.html. Updated September 24, 2013. Accessed November 9, 2017.
- Hurwitz S. Lice (pediculosis). In: Hurwitz S. Hurwitz Clinical Pediatric Dermatology: A Textbook of Skin Disorders of Childhood and Adolescence. 2nd ed. Philadelphia, PA: WB Saunders Company; 1993:416-419.
- Elston DM. What’s eating you? Pediculus humanus (head louse and body louse). Cutis. 1999;63:259-264.
- Ko CJ, Elston DM. Pediculosis. J Am Acad Dermatol. 2004;50:1-12.
- Frankowski BL, Weiner LB. Head lice. Pediatrics. 2002;110:638-643.
- Meinking TL. Clinical update on resistance and treatment of pediculosis capitis. Am J Manag Care. 2004;10(9 suppl):S264-S268.
- Devore CD, Schutze GE. Head lice. Pediatrics. 2015;135:E1355-E1365.
- Burkhart CN. Fomite transmission with head lice: a continuing controversy. Lancet. 2003;361:99-100.
- Falagas ME, Matthaiou DK, Rafailidis PI, et al. Worldwide prevalence of head lice. Emerg Infect Dis. 2008;14:1493-1494.
- Feldmeier H. Pediculosis capitis: new insights into epidemiology, diagnosis and treatment. Eur J Clin Microbiol Infect Dis. 2012;31:2105-2110.
- Canyon DV, Speare R, Muller R. Spatial and kinetic factors for the transfer of head lice (Pediculus capitis) between hairs. J Invest Dermatol. 2002;119:629-631.
- Burkhart CN, Burkhart CG. Fomite transmission in head lice. J Am Acad Dermatol. 2007;56:1044-1047.
- Canyon DV, Speare R. Indirect transmission of head lice via inanimate objects. Open Dermatol J. 2010;4:72-76.
- Bauer E, Jahnke C, Feldmeier H. Seasonal fluctuations of head lice infestation in Germany. Parasitol Res. 2009;104:677-681.
- Balcioglu IC, Kurt O, Limoncu ME, et al. Rural life, lower socioeconomic status and parasitic infections. Parasitol Int. 2007;56:129-133.
- Lesshafft H, Baier A, Guerra H, et al. Prevalence and risk factors associated with pediculosis capitis in an impoverished urban community in Lima, Peru. J Glob Infect Dis. 2013;5:138-143.
- Tagka A, Lambrou GI, Braoudaki M, et al. Socioeconomical factors associated with pediculosis (Phthiraptera: Pediculidae) in Athens, Greece. J Med Entomol. 2016;53:919-922.
- Di Stefani A, Hofmann-Wellenhof R, Zalaudek I. Dermoscopy for diagnosis and treatment monitoring of pediculosis capitis. J Am Acad Dermatol. 2006;54:909-911.
- Bakos RM, Bakos L. Dermoscopy for diagnosis of pediculosis capitis. J Am Acad Dermatol. 2007;57:727-728.
- Jahnke C, Bauer E, Hengge UR, et al. Accuracy of diagnosis of pediculosis capitis: visual inspection vs wet combing. Arch Dermatol. 2009;145:309-313.
- Elston DM. Drugs used in the treatment of pediculosis. J Drugs Dermatol. 2005;4:207-211.
- National Pesticide Information Center. Piperonyl butoxide (general fact sheet). http://npic.orst.edu/factsheets/pbogen.pdf/. Accessed November 13, 2017.
- Diamantis SA, Morrell DS, Burkhart CN. Treatment of head lice. Dermatol Ther. 2009;22:273-278.
- United States Food and Drug Administration. Treating and preventing head lice. http://www.fda.gov/forconsumers/consumerupdates/ucm171730.htm. Published July 13, 2010. Updated November 8, 2017. Accessed November 13, 2017.
- Meinking TL, Villar ME, Vicaria M, et al. The clinical trials supporting benzyl alcohol lotion 5% (UlesfiaTM): a safe and effective topical treatment for head lice (Pediculosis Humanus Capitis). Pediatr Dermatol. 2010;27:19-24.
- McCormack PL. Spinosad in pediculosis capitis. Am J Clin Dermatol. 2011;12:349-353.
- Stough D, Shellabarger S, Quiring J, et al. Efficacy and safety of spinosad and permethrin creme rinses for pediculosis capitis (head lice). Pediatrics. 2009;124:E389-E395.
- Ahmad HM, Abdel-Azim ES, Abdel-Aziz RT. Assessment of topical versus oral ivermectin as a treatment for head lice. Dermatol Ther. 2014;27:307-310.
- Heukelbach J, Pilger D, Oliveira FA, et al. A highly efficacious pediculicide based on dimethicone: randomized observer blinded comparative trial. BMC Infect Dis. 2008;8:115.
- Burgess IF, Brunton ER, Burgess NA. Single application of 4% dimethicone liquid gel versus two applications of 1% permethrin creme rinse for treatment of head louse infestation: a randomised controlled trial. BMC Dermatol. 2013;13:5.
- Ihde ES, Boscamp JR, Loh JM, et al. Safety and efficacy of a 100% dimethicone pediculocide in school-age children. BMC Pediatr. 2015;15:70.
- Heukelbach J, Oliveira FA, Richter J, et al. Dimethicone-based pediculicides: a physical approach to eradicate head lice. Open Dermatol J. 2010;4:77-81.
- Feldmeier H. Treatment of pediculosis capitis: a critical appraisal of the current literature. Am J Clin Dermatol. 2014;15:401-412.
- Glasziou P, Bennett J, Greenberg P, et al; Handbook Of Non Drug Intervention (HANDI) Project Team. Wet combing for the eradication of head lice. Aust Fam Physician. 2013;42:129-130.
- Tebruegge M, Runnacles J. Is wet combing effective in children with pediculosis capitis infestation? Arch Dis Child. 2007;92:818-820.
- Goates BM, Atkin JS, Wilding KG, et al. An effective nonchemical treatment for head lice: a lot of hot air. Pediatrics. 2006;118:1962-1970.
- Yones DA, Bakir HY, Bayoumi SA. Chemical composition and efficacy of some selected plant oils against Pediculus humanus capitis in vitro. Parasitol Res. 2016;115:3209-3218.
- Burkhart CN, Burkhart CG. Head lice: scientific assessment of the nit sheath with clinical ramifications and therapeutic options. J Am Acad Dermatol. 2005;53:129-133.
- Ibarra J, Fry F, Wickenden C, et al. The impact of well-developed preventative strategies on the eradication of head lice. Perspect Public Health. 2009;129:165-173.
- Mumcuoglu KY, Hemingway J, Miller J, et al. Permethrin resistance in the head louse pediculus humanus capitis from Israel. Med Vet Entomol. 1995;9:427-432.
- Meinking TL, Serrano L, Hard B, et al. Comparative in vitro pediculicidal efficacy of treatments in a resistant head lice population in the United States. Arch Dermatol. 2002;138:220-224.
- Hemingway J, Miller J, Mumcuoglu KY. Pyrethroid resistance mechanisms in the head louse Pediculus capitis from Israel: implications for control. Med Vet Entomol. 1999;13:89-96.
- Yoon KS, Previte DJ, Hodgdon HE, et al. Knockdown resistance allele frequencies in North American head louse (Anoplura: Pediculidae) populations. J Med Entomol. 2014;51:450-457.
Practice Points
- Transmission of head lice occurs most frequently from direct head-to-head contact; however, head lice can survive up to 4 days on fomites.
- Patients present with scalp pruritus and bite reactions (papules or wheals), but pediculosis can be asymptomatic, particularly with the first exposure before the immune system has developed sensitivity to the louse saliva.
- Topical pyrethroids are available over-the-counter and are considered first-line therapy; however, resistance to pyrethroids has become an important problem in the United States and worldwide.
- Newer topical treatments such as benzyl alcohol lotion 5%, spinosad topical suspension 0.9%, and ivermectin lotion 0.5% can be prescribed as alternative therapies, particularly if resistance to pyrethroids is a concern.
Approach to Treatment of Medical and Cosmetic Facial Concerns in Skin of Color Patients
The approach to the treatment of common skin disorders and cosmetic concerns in patients with skin of color (SOC) requires the clinician to understand the biological differences, nuances, and special considerations that are unique to patients with darker skin types.1-3 This article addresses 4 common facial concerns in SOC patients—acne, rosacea, facial hyperpigmentation, and cosmetic enhancement—and provides treatment recommendations and management pearls to assist the clinician with optimal outcomes for SOC patients.
Acne in SOC Patients
Acne vulgaris is one of the most common conditions that dermatologists treat and is estimated to affect 40 to 50 million individuals in the United States.1 Many of these acne patients are individuals with SOC.2-4 A study of 2835 females (aged 10–70 years) conducted in 4 different cities—Los Angeles, California; London, United Kingdom; Akita, Japan; and Rome, Italy—demonstrated acne prevalence of 37% in blacks, 32% in Hispanics, 30% in Asians, 24% in whites, and 23% in Continental Indians.5 Blacks, Hispanics, and Continental Indians demonstrated equal prevalence with comedonal and inflammatory acne. Asians displayed more inflammatory acne lesions than comedones. In contrast, whites demonstrated more comedones than inflammatory acne. Dyspigmentation, postinflammatory hyperpigmentation (PIH), and atrophic scars were more common in black and Hispanic females than other ethnicities.5 This study illustrated that acne-induced PIH is a common sequela in SOC patients and is the main reason they seek treatment.6,7
The pathogenesis of acne is the same in all racial and ethnic groups: (1) follicular hyperkeratinization and the formation of a microcomedone caused by abnormal desquamation of the keratinocytes within the sebaceous follicle, (2) production of sebum by circulating androgens, (3) proliferation of Propionibacterium acnes, and (4) inflammation. Subclinical inflammation is present throughout all stages of acne, including normal-appearing skin, inflammatory lesions, comedones, and scarring, and may contribute to PIH in acne patients with SOC (Figure 1).8 A thorough history should be obtained from acne patients, including answers to the following questions7:
- What skin and hair care products do you use?
- Do you use sunscreen daily?
- What cosmetic products or makeup do you use?
- Do you use any ethnic skin care products, including skin lightening creams?
- Do you have a history of keloids?

It is important to ask these questions to assess if the SOC patient has developed pomade acne,9 acne cosmetica,10 or a potential risk of skin irritation from the use of skin care practices. It is best to take total control of the patient’s skin care regimen and discontinue use of toners, astringents, witch hazel, exfoliants, and rubbing alcohol, which may lead to skin dryness and irritation, particularly when combined with topical acne medications.
Treatment
Treatment of acne in SOC patients is similar to generally recommended treatments, with special considerations. Consider the following key points when treating acne in SOC patients:
- Treat acne early and aggressively to prevent or minimize subsequent PIH and acne scarring.
- Balance aggressive treatment with nonirritating topical skin care.
- Most importantly, target PIH in addition to acne and choose a regimen that limits skin irritation that might exacerbate existing PIH.7
Develop a maintenance program to control future breakouts. Topical agents can be used as monotherapy or in fixed combinations and may include benzoyl peroxide, antibiotics, dapsone, azelaic acid (AZA), and retinoids. Similar to white patients, topical retinoids remain a first-line treatment for acne in patients with SOC.11,12
Tolerability must be managed in SOC acne patients. Therapeutic maneuvers that can be instituted should include a discussion on using gentle skin care, initiating therapy with a retinoid applied every other night starting with a low concentration and gradually titrating up, and applying a moisturizer before or after applying acne medication. Oral therapies consist of antibiotics (doxycycline, minocycline), retinoids (isotretinoin), and hormonal modulators (oral contraceptives, spironolactone). Isotretinoin, recommended for patients with nodulocystic acne, may play a possible role in treating acne-induced PIH.13
Two common procedural therapies for acne include comedone extraction and intralesional corticosteroid injection. A 6- to 8-week course of a topical retinoid prior to comedonal extraction may facilitate the procedure and is recommended in SOC patients to help reduce cutaneous trauma and PIH.11 Inflammatory acne lesions can be treated with intralesional injection of triamcinolone acetonide 2.5 or 5.0 mg/mL, which usually reduces inflammation within 2 to 5 days.11
Treatment of acne-induced PIH includes sun protection, topical and oral medications, chemical peels, lasers, and energy devices. Treatment of hypertrophic scarring and keloids involves intralesional injection of triamcinolone acetonide 20, 30, or 40 mg/mL every 4 weeks until the lesion is flat.11
Superficial chemical peels can be used to treat acne and PIH in SOC patients,14 such as salicylic acid (20%–30%), glycolic acid (20%–70%), trichloroacetic acid (15%–30%), and Jessner peels.
Acne Scarring
Surgical approaches to acne scarring in patients with SOC include elliptical excision, punch excision, punch elevation, punch autografting, dermal grafting, dermal planning, subcutaneous incision (subcision), dermabrasion, microneedling, fillers, and laser skin resurfacing. The treatment of choice depends on the size, type, and depth of the scar and the clinician’s preference.
Lasers
Fractional photothermolysis has emerged as a treatment option for acne scars in SOC patients. This procedure produces microscopic columns of thermal injury in the epidermis and dermis, sparing the surrounding tissue and minimizing downtime and adverse events. Because fractional photothermolysis does not target melanin and produces limited epidermal injury, darker Fitzpatrick skin types (IV–VI) can be safely and effectively treated with this procedure.15
Rosacea in SOC Patients
Rosacea is a chronic inflammatory disorder that affects the vasculature and pilosebaceous units of the face. It commonly is seen in Fitzpatrick skin types I and II; however, rosacea can occur in all skin types (Figure 2). Triggers include emotional stress, extreme environmental temperatures, hot and spicy foods, red wine or alcohol, and topical irritants or allergens found in common cosmetic products.16
Data suggest that 4% of rosacea patients in the United States are of African, Latino, or Asian descent.11 National Ambulatory Medical Care Survey data revealed that of 31.5 million rosacea visits, 2% of patients were black, 2.3% were Asian or Pacific Islander, and 3.9% were Hispanic or Latino. In a 5-year longitudinal study of 2587 rosacea patients enrolled in Medicaid in North Carolina who were prescribed at least 1 topical treatment for rosacea, 16.27% were black and 10% were of a race other than white.17
Although the pathogenesis of rosacea is unclear, hypotheses include immune system abnormalities, neurogenic dysregulation, presence of microorganisms (eg, Demodex folliculorum), UV damage, and skin barrier dysfunction.18
The 4 major subtypes of rosacea are erythematotelangiectatic, papulopustular, phymatous, and ocular rosacea.16 Interestingly, rosacea in SOC patients may present with hypopigmentation surrounding the borders of the facial erythema. For phymatous rosacea, isotretinoin may reduce incipient rhinophyma but must be carefully monitored and pregnancy must be excluded. Surgical or laser therapy may be indicated to recontour the nose if severe.
There are several skin conditions that can present with facial erythema in patients with SOC, including seborrheic dermatitis, systemic lupus erythematosus, and contact dermatitis. It is important to note that the detection of facial erythema in darker skin types may be difficult; therefore, laboratory evaluation (antinuclear antibodies), patch testing, and skin biopsy should be considered if the clinical diagnosis is unclear.
Treatment
Treatment of rosacea in SOC patients does not differ from other racial groups. Common strategies include gentle skin care, sun protection (sun protection factor 30+), and barrier repair creams. Topical agents include metronidazole, AZA, sodium sulfacetamide/sulfur, ivermectin, and retinoids.16 Oral treatments include antibiotics in the tetracycline family (eg, subantimicrobial dose doxycycline) and isotretinoin.16 Persistent erythema associated with rosacea can be treated with brimonidine19 and oxymetazoline.20 Vascular lasers and intense pulsed light may be used to address the vascular components of rosacea21; however, the latter is not recommended in Fitzpatrick skin types IV through VI.
Facial Hyperpigmentation in SOC Patients
Hyperpigmentation disorders can be divided into conditions that affect Fitzpatrick skin types I through III and IV though VI. Mottled hyperpigmentation (photodamage) and solar lentigines occur in patients with lighter skin types as compared to melasma, PIH, and age-related (UV-induced) hyperpigmentation, which occur more commonly in patients with darker skin types. Facial hyperpigmentation is a common concern in SOC patients. In a survey of cosmetic concerns of 100 women with SOC, hyperpigmentation or dark spots (86%) and blotchy uneven skin (80%) were the top concerns.22 In addition, facial hyperpigmentation has been shown to negatively impact quality of life.23
Postinflammatory hyperpigmentation occurs from a pathophysiological response to inflammation, cutaneous irritation or injury, and subsequent melanocyte lability. Postinflammatory hyperpigmentation is a common presenting concern in patients with SOC and is seen as a result of many inflammatory skin disorders (eg, acne, eczema) and dermatologic procedures (eg, adverse reaction to electrodesiccation, microdermabrasion, chemical peels, laser surgery).24
Melasma is an acquired idiopathic disorder of hyperpigmentation and often referred to as the mask of pregnancy (Figure 3). It occurs on sun-exposed areas of skin, mainly in women with Fitzpatrick skin types III through V. Associated factors or triggers include pregnancy, hormonal treatments, exposure to UV radiation, and medications.25 Hereditary factors play a role in more than 40% of cases.26
Other not-so-common facial dyschromias include contact dermatitis, acanthosis nigricans, exogenous ochronosis, lichen planus pigmentosus (associated with frontal fibrosing alopecia),27 drug-induced hyperpigmentation (associated with minocycline or diltiazem),28,29 and UV-induced (age-related) hyperpigmentation.
Treatment
The treatment of hyperpigmentation should provide the following: (1) protection from sun exposure; (2) inhibition of tyrosinase, the enzyme responsible for the conversion of tyrosine to melanin; (3) inhibition of melanosome transfer from the melanocyte to the keratinocyte; (4) removal of melanin from the epidermis through exfoliation; and (5) destruction or disruption of melanin in the dermis.30 Therapies for facial hyperpigmentation are listed in Table 1.

Topical therapies include prescription medications and nonprescription cosmeceuticals. Prescription medications include hydroquinone (HQ), topical retinoids, and AZA. Hydroquinone, a tyrosinase inhibitor, is the gold standard for skin lightening and often is used as a first-line therapy. It is used as a monotherapy (HQ 4%) or as a fixed combination with tretinoin 0.05% and fluocinolone 0.01%.31 Use caution with HQ in high concentrations (6% and higher) and low concentrations (2% [over-the-counter strength]) used long-term due to the potential risk of exogenous ochronosis.
Topical retinoids have been shown to be effective therapeutic agents for melasma and PIH. Tretinoin,32 tazarotene,33 and adapalene34 all have demonstrated efficacy for acne and acne-induced PIH in SOC patients. Patients must be monitored for the development of retinoid dermatitis and worsening of hyperpigmentation.
Azelaic acid is a naturally occurring dicarboxylic acid obtained from cultures of Malassezia furfur. Azelaic acid inhibits tyrosinase activity, DNA synthesis, and mitochondrial enzymes, thus blocking direct cytotoxic effects toward melanocytes. Azelaic acid is approved by the US Food and Drug Administration for acne in a 20% cream formulation and rosacea in 15% gel and foam formulations, and it is used off label for melasma and PIH.35
Oral tranexamic acid is currently used as a hemostatic agent due to its ability to inhibit the plasminogen-plasmin pathway. In melasma, it blocks the interaction between melanocytes and keratinocytes in the epidermis and modulates the vascular component of melasma in the dermis. In an open-label study, 561 Asian melasma patients were treated with oral tranexamic acid 250 mg twice daily for 4 months. Results demonstrated improvement in 90% of patients, and 7.1% reported adverse effects (eg, abdominal bloating and pain, nausea, vomiting, headache, tinnitus, numbness, menstrual irregularities).36 Coagulation screening should be monitored monthly, and any patient with a history of clotting abnormalities should be excluded from off-label treatment with oral tranexamic acid.
Nonprescription cosmeceuticals are available over-the-counter or are office dispensed.37 For optimal results, cosmeceutical agents for skin lightening are used in combination. Most of these combinations are HQ free and have additive benefits such as a multimodal skin lightening agent containing key ingredients that correct and prevent skin pigmentation via several pathways affecting melanogenesis.38 It is an excellent alternative to HQ for mottled and diffuse UV-induced hyperpigmentation and can be used for maintenance therapy in patients with melasma.
Photoprotection is an essential component of therapy for melasma and PIH, but there is a paucity of data on the benefits for SOC patients. Halder et al39 performed a randomized prospective study of 89 black and Hispanic patients who applied sunscreen with a sun protection factor of 30 or 60 daily for 8 weeks. Clinical grading, triplicate L*A*B chromameter, and clinical photography were taken at baseline and weeks 4 and 8. The results demonstrated skin lightening in both black and Hispanic patients and support the use of sunscreen in the prevention and management of dyschromia in SOC patients.39 Visible light also may play a role in melasma development, and thus use of sunscreens or makeup containing iron oxides are recommended.40
Procedural treatments for facial hyperpigmentation include microdermabrasion, chemical peels, lasers, energy-based devices, and microneedling. There are many types and formulations of chemical peeling agents available; however, superficial and medium-depth chemical peels are recommended for SOC patients (Table 2). Deep chemical peels are not recommended for SOC patients due to the potential increased risk for PIH and scarring.

Cosmetic Enhancement in SOC Patients
Cosmetic procedures are gaining popularity in the SOC population and account for more than 20% of cosmetic procedures in the United States.41 Facial cosmetic concerns in SOC include dyschromia, benign growths (dermatosis papulosa nigra), hyperkinetic facial lines, volume loss, and skin laxity.42 Key principles to consider when treating SOC patients are the impact of ethnicity on aging and facial structure, the patient’s desired cosmetic outcome, tissue reaction to anticipated treatments, and the patient’s expectations for recommended therapies.
Aging in SOC Patients
Skin aging can be classified as intrinsic aging or extrinsic aging. Intrinsic aging is genetic and involves subsurface changes such as volume loss, muscle atrophy, and resorption of bony structure. Extrinsic aging (or photoaging) involves surface changes of the epidermis/dermis and manifests as mottled pigmentation, textural changes, and fine wrinkling. Due to the photoprotection of melanin (black skin=SPF 13.4), skin aging in SOC patients is delayed by 10 to 20 years.43 In addition, SOC patients have more reactive collagen and can benefit from noninvasive cosmetic procedures such as fillers and skin-tightening procedures.42
Cosmetic Treatments and Procedures
Dermatosis papulosa nigra (benign growths of skin that have a genetic predisposition)44 occur mainly on the face but can involve the entire body. Treatment modalities include electrodesiccation, cryotherapy, scissor excision, and laser surgery.45
Treatment of hyperkinetic facial lines with botulinum toxin type A is a safe and effective procedure in patients with SOC. Grimes and Shabazz46 performed a 4-month, randomized, double-blind study that evaluated the treatment of glabellar lines in women with Fitzpatrick skin types V and VI. The results demonstrated that the duration of effects was the same in the patients who received either 20 or 30 U of botulinum toxin type A.46 Dynamic rhytides (furrows and frown/scowl lines arising from laughing, frowning, or smiling) can be treated safely in patients with SOC using botulinum toxin type A off label for relaxation of the upper and lower hyperkinetic muscles that result in these unwanted signs of aging. Botulinum toxin type A often is used for etched-in crow’s-feet, which rarely are evident in SOC patients.47 Facial shaping also can be accomplished by injecting botulinum toxin type A in combination with soft-tissue dermal fillers.47
Although black individuals do not experience perioral rhytides at the frequency of white individuals, they experience a variety of other cosmetic issues related to skin sagging and sinking. Currently available hyaluronic acid (HA) fillers have been shown to be safe in patients with Fitzpatrick skin types IV through VI.48 Two studies evaluated fillers in patients with SOC, specifically HA49 and calcium hydroxylapatite,50 focused on treatment of the nasolabial folds and the potential risk for dyspigmentation and keloidal scarring. Taylor et al49 noted that the risk of hyperpigmentation was 6% to 9% for large- and small-particle HA, respectively, and was associated with the serial or multiple puncture injection technique. No hypertrophic or keloidal scarring occurred in both studies.49,50
Facial contouring applications with fillers include glabellar lines, temples, nasal bridge, tear troughs, malar and submalar areas, nasolabial folds, radial lines, lips, marionette lines, mental crease, and chin. Hyaluronic acid fillers also can be used for lip enhancement.47 Although white women are looking to increase the size of their lips, black women are seeking augmentation to restore their lip size to that of their youth. Black individuals do not experience the same frequency of perioral rhytides as white patients, but they experience a variety of other issues related to skin sagging and sinking. Unlike white women, enhancement of the vermilion border rarely is performed in black women due to development of rhytides, predominantly in the body of the lip below the vermilion border in response to volume loss in the upper lip while the lower lip usually maintains its same appearance.47
Facial enhancement utilizing poly-L-lactic acid can be used safely in SOC patients.51 Poly-L-lactic acid microparticles induce collagen formation, leading to dermal thickening over 3 to 6 months; however, multiple sessions are required to achieve optimal aesthetic results.
Patients with more reactive collagen can benefit from noninvasive cosmetic procedures such as skin-tightening procedures.52 Radiofrequency and microfocused ultrasound are cosmetic procedures used to provide skin tightening and facial lifting. They are safe and effective treatments for patients with Fitzpatrick skin types IV to VI.53 Histologically, there is less thinning of collagen bundles and elastic tissue in ethnic skin. Due to stimulation of collagen by these procedures, most SOC patients will experience a more enhanced response, requiring fewer treatment sessions than white individuals.
Conclusion
Medical and aesthetic facial concerns in SOC patients vary and can be a source of emotional and psychological distress that can negatively impact quality of life. The approach to the treatment of SOC patients should be a balance between tolerability and efficacy, considering the potential risk for PIH.
- White GM. Recent findings in the epidemiologic evidence, classification, and subtypes of acne vulgaris. J Am Acad Dermatol. 1998;39(2 pt 3):S34-S37.
- Halder RM, Grimes PE, McLaurin CL, et al. Incidence of common dermatoses in a predominantly black dermatologic practice. Cutis. 1983;32:388, 390.
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Perkins AC, Cheng CE, Hillebrand GG, et al. Comparison of the epidemiology of acne vulgaris among Caucasians, Asian, Continental Indian and African American women. J Eur Acad Dermatol Venereol. 2011;25:1054-1060.
- Taylor SC, Cook-Bolden F, Rahman Z, et al. Acne vulgaris in skin of color. J Am Acad Dermatol. 2002;46(2 suppl):S98-S106.
- Davis EC, Callender VD. A review of acne in ethnic skin: pathogenesis, clinical manifestations, and management strategies. J Clin Aesthet Dermatol. 2010;3:24-38.
- Halder RM, Holmes YC, Bridgeman-Shah S, et al. A clinicohistologic study of acne vulgaris in black females (abstract). J Invest Dermatol. 1996;106:888.
- Plewig G, Fulton JE, Kligman AM. Pomade acne. Arch Dermatol. 1970;101:580-584.
- Kligman AM, Mills OH. Acne cosmetica. Arch Dermatol. 1972;106:893-897.
- Halder RM, Brooks HL, Callender VD. Acne in ethnic skin. Dermatol Clin. 2003;21:609-615.
- Callender VD. Acne in ethnic skin: special considerations for therapy. Dermatol Ther. 2004;17:184-195.
- Winhoven SM. Postinflammatory hyperpigmentation in an Asian patient. a dramatic response to oral isotretinoin (13-cis-retinoic acid). Br J Med. 2005;152:368-403.
- Sarkar R, Bansal S, Garg VK. Chemical peels for melasma in dark-skinned patients. J Cutan Aesthet Surg. 2012;5:247-253.
- Alexis AF, Coley MK, Nijhawan RI, et al. Nonablative fractional laser resurfacing for acne scarring in patients with Fitzpatrick skin phototypes IV-VI. Dermatol Surg. 2016;42:392-402.
- Culp B, Scheinfeld N. Rosacea: a review. P T. 2009;34:38-45.
- Al-Dabagh A, Davis SA, McMichael AJ, et al. Rosacea in skin of color: not a rare diagnosis. Dermatol Online J. 2014:20. pii:13030/qt1mv9r0ss.
- Del Rosso JQ. Advances in understanding and managing rosacea: part 1: connecting the dots between pathophysiological mechanisms and common clinical features of rosacea with emphasis on vascular changes and facial erythema. J Clin Aesthet Dermatol. 2012;5:16-25.
- Jackson JM, Knuckles M, Minni JP, et al. The role of brimonidine tartrate gel in the treatment of rosacea. Clin Cosmet Investig Dermatol. 2015;23:529-538.
- Patel NU, Shukla S, Zaki J, et al. Oxymetazoline hydrochloride cream for facial erythema associated with rosacea. Expert Rev Clin Pharmacol. 2017;10:104954.
- Weinkle AP, Doktor V, Emer J. Update on the management of rosacea. Clin Cosmet Investig Dermatol. 2015;8:159-177.
- Grimes PE. Skin and hair cosmetic issues in women of color. Dermatol Clin. 2000;19:659-665.
- Taylor A, Pawaskar M, Taylor SL, et al. Prevalence of pigmentary disorders and their impact on quality of life: a prospective cohort study. J Cosmet Dermatol. 2008;7:164-168.
- Davis EC, Callender VD. Postinflammatory hyperpigmentation: a review of the epidemiology, clinical features, and treatment options in skin of color. J Clin Aesthet Dermatol. 2010;3:20-31.
- Grimes PE. Melasma: etiologic and therapeutic considerations. Arch Dermatol. 1995;131:1453-1457.
- Handel AC, Miot LD, Miot HA. Melasma: a clinical and epidemiological review. An Bras Dermatol. 2014;89:771-782.
- Callender VD, Reid SD, Obayan O, et al. Diagnostic clues to frontal fibrosing alopecia in patients of African descent. J Clin Aesthet Dermatol. 2016;9:45-51.
- Narang T, Sawatkar GU, Kumaran MS, et al. Minocycline for recurrent and/or chronic erythema nodosum leprosum. JAMA Dermatol. 2015;151:1026-1028.
- Boyer M, Katta R, Markus R. Diltiazem-induced photodistributed hyperpigmentation. Dermatol Online J. 2003;9:10.
- Pandya AG, Guevara IL. Disorders of hyperpigmentation. Dermatol Clin. 2000;18:91-98.
- Taylor SC, Torok H, Jones T, et al. Efficacy and safety of a new triple-combination agent for the treatment of facial melasma. Cutis. 2003;72:67-72.
- Bulengo-Ransby SM. Topical tretinoin (retinoic acid) therapy for hyperpigmented lesions caused by inflammation of the skin in black patients. N Engl J Med. 1993;328:1438-1443.
- Grimes P, Callender V. Tazarotene cream for postinflammatory hyperpigmentation and acne vulgaris in darker skin: a double-blind, randomized, vehicle-controlled study. Cutis. 2006;77:45-50.
- Jacyk WK. Adapalene in the treatment of African patients. J Eur Acad Dermatol Venereol. 2001;15(suppl 3):37-42.
- Kircik LH. Efficacy and safety of azelaic acid (AzA) gel 15% in the treatment of postinflammatory hyperpigmentation and acne: a 16-week, baseline-controlled study. J Drugs Dermatol. 2011;10:586-590.
- Lee HC, Thng TG, Goh CL. Oral tranexamic acid (TA) in the treatment of melasma. J Am Acad Dermatol. 2016;75:385-392.
- Kindred C, Okereke U, Callender VD. Skin-lightening agents: an overview of prescription, office-dispensed, and over-the-counter products. Cosmet Dermatol. 2013;26:18-26.
- Makino ET, Kadoya K, Sigler ML, et al. Development and clinical assessment of a comprehensive product for pigmentation control in multiple ethnic populations. J Drugs Dermatol. 2016;15:1562-1570.
- Halder R, Rodney I, Munhutu M, et al. Evaluation and effectiveness of a photoprotection composition (sunscreen) on subjects of skin of color. J Am Acad Dermatol. 2015;72(suppl 1):AB215.
- Castanedo-Cazares JP, Hernandez-Blanco D, Carlos-Ortega B, et al. Near-visible light and UV photoprotection in the treatment of melasma: a double-blind randomized trial. Photodermatol Photoimmunol Photomed. 2014;30:35-42.
- American Society for Aesthetic Plastic Surgery. 2016 Cosmetic Surgery National Data Bank Statistics. https://www.surgery.org/sites/default/files/ASAPS-Stats2016.pdf. Accessed November 15, 2017.
- Burgess CM. Soft tissue augmentation in skin of color: market growth, available fillers and successful techniques. J Drugs Dermatol. 2007;6:51-55.
- Davis EC, Callender VD. Aesthetic dermatology for aging ethnic skin. Dermatol Surg. 2011;37:901-917.
- Grimes PE, Arora S, Minus HR, et al. Dermatosis papulosa nigra. Cutis. 1983;32:385-386.
- Lupo M. Dermatosis papulosa nigra: treatment options. J Drugs Dermatol. 2007;6:29-30.
- Grimes PE, Shabazz D. A four-month randomized, double-blind evaluation of the efficacy of botulinum toxin type A for the treatment of glabellar lines in women with skin types V and VI. Dermatol Surg. 2009;35:429-435.
- Burgess CM, Awosika O. Ethnic and gender considerations in the use of facial injectables: African-American patients. Plast Reconstr Surg. 2015;136(5 suppl):28S-31S.
- Taylor SC, Kelly AP, Lim HW, et al, eds. Taylor and Kelly’s Dermatology for Skin of Color. 2nd ed. New York, NY: McGraw-Hill Education; 2016.
- Taylor SC, Burgess CM, Callender VD. Safety of nonanimal stabilized hyaluronic acid dermal fillers in patients with skin of color: a randomized, evaluator-blinded comparative trial. Dermatol Surg. 2009;35(suppl 2):1653-1660.
- Marmur ES, Taylor SC, Grimes PE, et al. Six-month safety results of calcium hydroxylapatite for treatment of nasolabial folds in Fitzpatrick skin types IV to VI. Dermatol Surg. 2009;35(suppl 2):1641-1645.
- Hamilton TK, Burgess CM. Consideration for the use of injectable poly-L-lactic acid in people of color. J Drugs Dermatol. 2010;9:451-456.
- Fabi SG, Goldman MP. Retrospective evaluation of micro-focused ultrasound for lifting and tightening of the face and neck. Dermatol Surg. 2014;40:569-575.
- Harris MO, Sundaram HA. Safety of microfocused ultrasound with visualization in patients with Fitzpatrick skin phototypes III to VI. JAMA Facial Plast Surg. 2015;17:355-357.
The approach to the treatment of common skin disorders and cosmetic concerns in patients with skin of color (SOC) requires the clinician to understand the biological differences, nuances, and special considerations that are unique to patients with darker skin types.1-3 This article addresses 4 common facial concerns in SOC patients—acne, rosacea, facial hyperpigmentation, and cosmetic enhancement—and provides treatment recommendations and management pearls to assist the clinician with optimal outcomes for SOC patients.
Acne in SOC Patients
Acne vulgaris is one of the most common conditions that dermatologists treat and is estimated to affect 40 to 50 million individuals in the United States.1 Many of these acne patients are individuals with SOC.2-4 A study of 2835 females (aged 10–70 years) conducted in 4 different cities—Los Angeles, California; London, United Kingdom; Akita, Japan; and Rome, Italy—demonstrated acne prevalence of 37% in blacks, 32% in Hispanics, 30% in Asians, 24% in whites, and 23% in Continental Indians.5 Blacks, Hispanics, and Continental Indians demonstrated equal prevalence with comedonal and inflammatory acne. Asians displayed more inflammatory acne lesions than comedones. In contrast, whites demonstrated more comedones than inflammatory acne. Dyspigmentation, postinflammatory hyperpigmentation (PIH), and atrophic scars were more common in black and Hispanic females than other ethnicities.5 This study illustrated that acne-induced PIH is a common sequela in SOC patients and is the main reason they seek treatment.6,7
The pathogenesis of acne is the same in all racial and ethnic groups: (1) follicular hyperkeratinization and the formation of a microcomedone caused by abnormal desquamation of the keratinocytes within the sebaceous follicle, (2) production of sebum by circulating androgens, (3) proliferation of Propionibacterium acnes, and (4) inflammation. Subclinical inflammation is present throughout all stages of acne, including normal-appearing skin, inflammatory lesions, comedones, and scarring, and may contribute to PIH in acne patients with SOC (Figure 1).8 A thorough history should be obtained from acne patients, including answers to the following questions7:
- What skin and hair care products do you use?
- Do you use sunscreen daily?
- What cosmetic products or makeup do you use?
- Do you use any ethnic skin care products, including skin lightening creams?
- Do you have a history of keloids?

It is important to ask these questions to assess if the SOC patient has developed pomade acne,9 acne cosmetica,10 or a potential risk of skin irritation from the use of skin care practices. It is best to take total control of the patient’s skin care regimen and discontinue use of toners, astringents, witch hazel, exfoliants, and rubbing alcohol, which may lead to skin dryness and irritation, particularly when combined with topical acne medications.
Treatment
Treatment of acne in SOC patients is similar to generally recommended treatments, with special considerations. Consider the following key points when treating acne in SOC patients:
- Treat acne early and aggressively to prevent or minimize subsequent PIH and acne scarring.
- Balance aggressive treatment with nonirritating topical skin care.
- Most importantly, target PIH in addition to acne and choose a regimen that limits skin irritation that might exacerbate existing PIH.7
Develop a maintenance program to control future breakouts. Topical agents can be used as monotherapy or in fixed combinations and may include benzoyl peroxide, antibiotics, dapsone, azelaic acid (AZA), and retinoids. Similar to white patients, topical retinoids remain a first-line treatment for acne in patients with SOC.11,12
Tolerability must be managed in SOC acne patients. Therapeutic maneuvers that can be instituted should include a discussion on using gentle skin care, initiating therapy with a retinoid applied every other night starting with a low concentration and gradually titrating up, and applying a moisturizer before or after applying acne medication. Oral therapies consist of antibiotics (doxycycline, minocycline), retinoids (isotretinoin), and hormonal modulators (oral contraceptives, spironolactone). Isotretinoin, recommended for patients with nodulocystic acne, may play a possible role in treating acne-induced PIH.13
Two common procedural therapies for acne include comedone extraction and intralesional corticosteroid injection. A 6- to 8-week course of a topical retinoid prior to comedonal extraction may facilitate the procedure and is recommended in SOC patients to help reduce cutaneous trauma and PIH.11 Inflammatory acne lesions can be treated with intralesional injection of triamcinolone acetonide 2.5 or 5.0 mg/mL, which usually reduces inflammation within 2 to 5 days.11
Treatment of acne-induced PIH includes sun protection, topical and oral medications, chemical peels, lasers, and energy devices. Treatment of hypertrophic scarring and keloids involves intralesional injection of triamcinolone acetonide 20, 30, or 40 mg/mL every 4 weeks until the lesion is flat.11
Superficial chemical peels can be used to treat acne and PIH in SOC patients,14 such as salicylic acid (20%–30%), glycolic acid (20%–70%), trichloroacetic acid (15%–30%), and Jessner peels.
Acne Scarring
Surgical approaches to acne scarring in patients with SOC include elliptical excision, punch excision, punch elevation, punch autografting, dermal grafting, dermal planning, subcutaneous incision (subcision), dermabrasion, microneedling, fillers, and laser skin resurfacing. The treatment of choice depends on the size, type, and depth of the scar and the clinician’s preference.
Lasers
Fractional photothermolysis has emerged as a treatment option for acne scars in SOC patients. This procedure produces microscopic columns of thermal injury in the epidermis and dermis, sparing the surrounding tissue and minimizing downtime and adverse events. Because fractional photothermolysis does not target melanin and produces limited epidermal injury, darker Fitzpatrick skin types (IV–VI) can be safely and effectively treated with this procedure.15
Rosacea in SOC Patients
Rosacea is a chronic inflammatory disorder that affects the vasculature and pilosebaceous units of the face. It commonly is seen in Fitzpatrick skin types I and II; however, rosacea can occur in all skin types (Figure 2). Triggers include emotional stress, extreme environmental temperatures, hot and spicy foods, red wine or alcohol, and topical irritants or allergens found in common cosmetic products.16

Data suggest that 4% of rosacea patients in the United States are of African, Latino, or Asian descent.11 National Ambulatory Medical Care Survey data revealed that of 31.5 million rosacea visits, 2% of patients were black, 2.3% were Asian or Pacific Islander, and 3.9% were Hispanic or Latino. In a 5-year longitudinal study of 2587 rosacea patients enrolled in Medicaid in North Carolina who were prescribed at least 1 topical treatment for rosacea, 16.27% were black and 10% were of a race other than white.17
Although the pathogenesis of rosacea is unclear, hypotheses include immune system abnormalities, neurogenic dysregulation, presence of microorganisms (eg, Demodex folliculorum), UV damage, and skin barrier dysfunction.18
The 4 major subtypes of rosacea are erythematotelangiectatic, papulopustular, phymatous, and ocular rosacea.16 Interestingly, rosacea in SOC patients may present with hypopigmentation surrounding the borders of the facial erythema. For phymatous rosacea, isotretinoin may reduce incipient rhinophyma but must be carefully monitored and pregnancy must be excluded. Surgical or laser therapy may be indicated to recontour the nose if severe.
There are several skin conditions that can present with facial erythema in patients with SOC, including seborrheic dermatitis, systemic lupus erythematosus, and contact dermatitis. It is important to note that the detection of facial erythema in darker skin types may be difficult; therefore, laboratory evaluation (antinuclear antibodies), patch testing, and skin biopsy should be considered if the clinical diagnosis is unclear.
Treatment
Treatment of rosacea in SOC patients does not differ from other racial groups. Common strategies include gentle skin care, sun protection (sun protection factor 30+), and barrier repair creams. Topical agents include metronidazole, AZA, sodium sulfacetamide/sulfur, ivermectin, and retinoids.16 Oral treatments include antibiotics in the tetracycline family (eg, subantimicrobial dose doxycycline) and isotretinoin.16 Persistent erythema associated with rosacea can be treated with brimonidine19 and oxymetazoline.20 Vascular lasers and intense pulsed light may be used to address the vascular components of rosacea21; however, the latter is not recommended in Fitzpatrick skin types IV through VI.
Facial Hyperpigmentation in SOC Patients
Hyperpigmentation disorders can be divided into conditions that affect Fitzpatrick skin types I through III and IV though VI. Mottled hyperpigmentation (photodamage) and solar lentigines occur in patients with lighter skin types as compared to melasma, PIH, and age-related (UV-induced) hyperpigmentation, which occur more commonly in patients with darker skin types. Facial hyperpigmentation is a common concern in SOC patients. In a survey of cosmetic concerns of 100 women with SOC, hyperpigmentation or dark spots (86%) and blotchy uneven skin (80%) were the top concerns.22 In addition, facial hyperpigmentation has been shown to negatively impact quality of life.23
Postinflammatory hyperpigmentation occurs from a pathophysiological response to inflammation, cutaneous irritation or injury, and subsequent melanocyte lability. Postinflammatory hyperpigmentation is a common presenting concern in patients with SOC and is seen as a result of many inflammatory skin disorders (eg, acne, eczema) and dermatologic procedures (eg, adverse reaction to electrodesiccation, microdermabrasion, chemical peels, laser surgery).24
Melasma is an acquired idiopathic disorder of hyperpigmentation and often referred to as the mask of pregnancy (Figure 3). It occurs on sun-exposed areas of skin, mainly in women with Fitzpatrick skin types III through V. Associated factors or triggers include pregnancy, hormonal treatments, exposure to UV radiation, and medications.25 Hereditary factors play a role in more than 40% of cases.26

Other not-so-common facial dyschromias include contact dermatitis, acanthosis nigricans, exogenous ochronosis, lichen planus pigmentosus (associated with frontal fibrosing alopecia),27 drug-induced hyperpigmentation (associated with minocycline or diltiazem),28,29 and UV-induced (age-related) hyperpigmentation.
Treatment
The treatment of hyperpigmentation should provide the following: (1) protection from sun exposure; (2) inhibition of tyrosinase, the enzyme responsible for the conversion of tyrosine to melanin; (3) inhibition of melanosome transfer from the melanocyte to the keratinocyte; (4) removal of melanin from the epidermis through exfoliation; and (5) destruction or disruption of melanin in the dermis.30 Therapies for facial hyperpigmentation are listed in Table 1.

Topical therapies include prescription medications and nonprescription cosmeceuticals. Prescription medications include hydroquinone (HQ), topical retinoids, and AZA. Hydroquinone, a tyrosinase inhibitor, is the gold standard for skin lightening and often is used as a first-line therapy. It is used as a monotherapy (HQ 4%) or as a fixed combination with tretinoin 0.05% and fluocinolone 0.01%.31 Use caution with HQ in high concentrations (6% and higher) and low concentrations (2% [over-the-counter strength]) used long-term due to the potential risk of exogenous ochronosis.
Topical retinoids have been shown to be effective therapeutic agents for melasma and PIH. Tretinoin,32 tazarotene,33 and adapalene34 all have demonstrated efficacy for acne and acne-induced PIH in SOC patients. Patients must be monitored for the development of retinoid dermatitis and worsening of hyperpigmentation.
Azelaic acid is a naturally occurring dicarboxylic acid obtained from cultures of Malassezia furfur. Azelaic acid inhibits tyrosinase activity, DNA synthesis, and mitochondrial enzymes, thus blocking direct cytotoxic effects toward melanocytes. Azelaic acid is approved by the US Food and Drug Administration for acne in a 20% cream formulation and rosacea in 15% gel and foam formulations, and it is used off label for melasma and PIH.35
Oral tranexamic acid is currently used as a hemostatic agent due to its ability to inhibit the plasminogen-plasmin pathway. In melasma, it blocks the interaction between melanocytes and keratinocytes in the epidermis and modulates the vascular component of melasma in the dermis. In an open-label study, 561 Asian melasma patients were treated with oral tranexamic acid 250 mg twice daily for 4 months. Results demonstrated improvement in 90% of patients, and 7.1% reported adverse effects (eg, abdominal bloating and pain, nausea, vomiting, headache, tinnitus, numbness, menstrual irregularities).36 Coagulation screening should be monitored monthly, and any patient with a history of clotting abnormalities should be excluded from off-label treatment with oral tranexamic acid.
Nonprescription cosmeceuticals are available over-the-counter or are office dispensed.37 For optimal results, cosmeceutical agents for skin lightening are used in combination. Most of these combinations are HQ free and have additive benefits such as a multimodal skin lightening agent containing key ingredients that correct and prevent skin pigmentation via several pathways affecting melanogenesis.38 It is an excellent alternative to HQ for mottled and diffuse UV-induced hyperpigmentation and can be used for maintenance therapy in patients with melasma.
Photoprotection is an essential component of therapy for melasma and PIH, but there is a paucity of data on the benefits for SOC patients. Halder et al39 performed a randomized prospective study of 89 black and Hispanic patients who applied sunscreen with a sun protection factor of 30 or 60 daily for 8 weeks. Clinical grading, triplicate L*A*B chromameter, and clinical photography were taken at baseline and weeks 4 and 8. The results demonstrated skin lightening in both black and Hispanic patients and support the use of sunscreen in the prevention and management of dyschromia in SOC patients.39 Visible light also may play a role in melasma development, and thus use of sunscreens or makeup containing iron oxides are recommended.40
Procedural treatments for facial hyperpigmentation include microdermabrasion, chemical peels, lasers, energy-based devices, and microneedling. There are many types and formulations of chemical peeling agents available; however, superficial and medium-depth chemical peels are recommended for SOC patients (Table 2). Deep chemical peels are not recommended for SOC patients due to the potential increased risk for PIH and scarring.

Cosmetic Enhancement in SOC Patients
Cosmetic procedures are gaining popularity in the SOC population and account for more than 20% of cosmetic procedures in the United States.41 Facial cosmetic concerns in SOC include dyschromia, benign growths (dermatosis papulosa nigra), hyperkinetic facial lines, volume loss, and skin laxity.42 Key principles to consider when treating SOC patients are the impact of ethnicity on aging and facial structure, the patient’s desired cosmetic outcome, tissue reaction to anticipated treatments, and the patient’s expectations for recommended therapies.
Aging in SOC Patients
Skin aging can be classified as intrinsic aging or extrinsic aging. Intrinsic aging is genetic and involves subsurface changes such as volume loss, muscle atrophy, and resorption of bony structure. Extrinsic aging (or photoaging) involves surface changes of the epidermis/dermis and manifests as mottled pigmentation, textural changes, and fine wrinkling. Due to the photoprotection of melanin (black skin=SPF 13.4), skin aging in SOC patients is delayed by 10 to 20 years.43 In addition, SOC patients have more reactive collagen and can benefit from noninvasive cosmetic procedures such as fillers and skin-tightening procedures.42
Cosmetic Treatments and Procedures
Dermatosis papulosa nigra (benign growths of skin that have a genetic predisposition)44 occur mainly on the face but can involve the entire body. Treatment modalities include electrodesiccation, cryotherapy, scissor excision, and laser surgery.45
Treatment of hyperkinetic facial lines with botulinum toxin type A is a safe and effective procedure in patients with SOC. Grimes and Shabazz46 performed a 4-month, randomized, double-blind study that evaluated the treatment of glabellar lines in women with Fitzpatrick skin types V and VI. The results demonstrated that the duration of effects was the same in the patients who received either 20 or 30 U of botulinum toxin type A.46 Dynamic rhytides (furrows and frown/scowl lines arising from laughing, frowning, or smiling) can be treated safely in patients with SOC using botulinum toxin type A off label for relaxation of the upper and lower hyperkinetic muscles that result in these unwanted signs of aging. Botulinum toxin type A often is used for etched-in crow’s-feet, which rarely are evident in SOC patients.47 Facial shaping also can be accomplished by injecting botulinum toxin type A in combination with soft-tissue dermal fillers.47
Although black individuals do not experience perioral rhytides at the frequency of white individuals, they experience a variety of other cosmetic issues related to skin sagging and sinking. Currently available hyaluronic acid (HA) fillers have been shown to be safe in patients with Fitzpatrick skin types IV through VI.48 Two studies evaluated fillers in patients with SOC, specifically HA49 and calcium hydroxylapatite,50 focused on treatment of the nasolabial folds and the potential risk for dyspigmentation and keloidal scarring. Taylor et al49 noted that the risk of hyperpigmentation was 6% to 9% for large- and small-particle HA, respectively, and was associated with the serial or multiple puncture injection technique. No hypertrophic or keloidal scarring occurred in both studies.49,50
Facial contouring applications with fillers include glabellar lines, temples, nasal bridge, tear troughs, malar and submalar areas, nasolabial folds, radial lines, lips, marionette lines, mental crease, and chin. Hyaluronic acid fillers also can be used for lip enhancement.47 Although white women are looking to increase the size of their lips, black women are seeking augmentation to restore their lip size to that of their youth. Black individuals do not experience the same frequency of perioral rhytides as white patients, but they experience a variety of other issues related to skin sagging and sinking. Unlike white women, enhancement of the vermilion border rarely is performed in black women due to development of rhytides, predominantly in the body of the lip below the vermilion border in response to volume loss in the upper lip while the lower lip usually maintains its same appearance.47
Facial enhancement utilizing poly-L-lactic acid can be used safely in SOC patients.51 Poly-L-lactic acid microparticles induce collagen formation, leading to dermal thickening over 3 to 6 months; however, multiple sessions are required to achieve optimal aesthetic results.
Patients with more reactive collagen can benefit from noninvasive cosmetic procedures such as skin-tightening procedures.52 Radiofrequency and microfocused ultrasound are cosmetic procedures used to provide skin tightening and facial lifting. They are safe and effective treatments for patients with Fitzpatrick skin types IV to VI.53 Histologically, there is less thinning of collagen bundles and elastic tissue in ethnic skin. Due to stimulation of collagen by these procedures, most SOC patients will experience a more enhanced response, requiring fewer treatment sessions than white individuals.
Conclusion
Medical and aesthetic facial concerns in SOC patients vary and can be a source of emotional and psychological distress that can negatively impact quality of life. The approach to the treatment of SOC patients should be a balance between tolerability and efficacy, considering the potential risk for PIH.
The approach to the treatment of common skin disorders and cosmetic concerns in patients with skin of color (SOC) requires the clinician to understand the biological differences, nuances, and special considerations that are unique to patients with darker skin types.1-3 This article addresses 4 common facial concerns in SOC patients—acne, rosacea, facial hyperpigmentation, and cosmetic enhancement—and provides treatment recommendations and management pearls to assist the clinician with optimal outcomes for SOC patients.
Acne in SOC Patients
Acne vulgaris is one of the most common conditions that dermatologists treat and is estimated to affect 40 to 50 million individuals in the United States.1 Many of these acne patients are individuals with SOC.2-4 A study of 2835 females (aged 10–70 years) conducted in 4 different cities—Los Angeles, California; London, United Kingdom; Akita, Japan; and Rome, Italy—demonstrated acne prevalence of 37% in blacks, 32% in Hispanics, 30% in Asians, 24% in whites, and 23% in Continental Indians.5 Blacks, Hispanics, and Continental Indians demonstrated equal prevalence with comedonal and inflammatory acne. Asians displayed more inflammatory acne lesions than comedones. In contrast, whites demonstrated more comedones than inflammatory acne. Dyspigmentation, postinflammatory hyperpigmentation (PIH), and atrophic scars were more common in black and Hispanic females than other ethnicities.5 This study illustrated that acne-induced PIH is a common sequela in SOC patients and is the main reason they seek treatment.6,7
The pathogenesis of acne is the same in all racial and ethnic groups: (1) follicular hyperkeratinization and the formation of a microcomedone caused by abnormal desquamation of the keratinocytes within the sebaceous follicle, (2) production of sebum by circulating androgens, (3) proliferation of Propionibacterium acnes, and (4) inflammation. Subclinical inflammation is present throughout all stages of acne, including normal-appearing skin, inflammatory lesions, comedones, and scarring, and may contribute to PIH in acne patients with SOC (Figure 1).8 A thorough history should be obtained from acne patients, including answers to the following questions7:
- What skin and hair care products do you use?
- Do you use sunscreen daily?
- What cosmetic products or makeup do you use?
- Do you use any ethnic skin care products, including skin lightening creams?
- Do you have a history of keloids?

It is important to ask these questions to assess if the SOC patient has developed pomade acne,9 acne cosmetica,10 or a potential risk of skin irritation from the use of skin care practices. It is best to take total control of the patient’s skin care regimen and discontinue use of toners, astringents, witch hazel, exfoliants, and rubbing alcohol, which may lead to skin dryness and irritation, particularly when combined with topical acne medications.
Treatment
Treatment of acne in SOC patients is similar to generally recommended treatments, with special considerations. Consider the following key points when treating acne in SOC patients:
- Treat acne early and aggressively to prevent or minimize subsequent PIH and acne scarring.
- Balance aggressive treatment with nonirritating topical skin care.
- Most importantly, target PIH in addition to acne and choose a regimen that limits skin irritation that might exacerbate existing PIH.7
Develop a maintenance program to control future breakouts. Topical agents can be used as monotherapy or in fixed combinations and may include benzoyl peroxide, antibiotics, dapsone, azelaic acid (AZA), and retinoids. Similar to white patients, topical retinoids remain a first-line treatment for acne in patients with SOC.11,12
Tolerability must be managed in SOC acne patients. Therapeutic maneuvers that can be instituted should include a discussion on using gentle skin care, initiating therapy with a retinoid applied every other night starting with a low concentration and gradually titrating up, and applying a moisturizer before or after applying acne medication. Oral therapies consist of antibiotics (doxycycline, minocycline), retinoids (isotretinoin), and hormonal modulators (oral contraceptives, spironolactone). Isotretinoin, recommended for patients with nodulocystic acne, may play a possible role in treating acne-induced PIH.13
Two common procedural therapies for acne include comedone extraction and intralesional corticosteroid injection. A 6- to 8-week course of a topical retinoid prior to comedonal extraction may facilitate the procedure and is recommended in SOC patients to help reduce cutaneous trauma and PIH.11 Inflammatory acne lesions can be treated with intralesional injection of triamcinolone acetonide 2.5 or 5.0 mg/mL, which usually reduces inflammation within 2 to 5 days.11
Treatment of acne-induced PIH includes sun protection, topical and oral medications, chemical peels, lasers, and energy devices. Treatment of hypertrophic scarring and keloids involves intralesional injection of triamcinolone acetonide 20, 30, or 40 mg/mL every 4 weeks until the lesion is flat.11
Superficial chemical peels can be used to treat acne and PIH in SOC patients,14 such as salicylic acid (20%–30%), glycolic acid (20%–70%), trichloroacetic acid (15%–30%), and Jessner peels.
Acne Scarring
Surgical approaches to acne scarring in patients with SOC include elliptical excision, punch excision, punch elevation, punch autografting, dermal grafting, dermal planning, subcutaneous incision (subcision), dermabrasion, microneedling, fillers, and laser skin resurfacing. The treatment of choice depends on the size, type, and depth of the scar and the clinician’s preference.
Lasers
Fractional photothermolysis has emerged as a treatment option for acne scars in SOC patients. This procedure produces microscopic columns of thermal injury in the epidermis and dermis, sparing the surrounding tissue and minimizing downtime and adverse events. Because fractional photothermolysis does not target melanin and produces limited epidermal injury, darker Fitzpatrick skin types (IV–VI) can be safely and effectively treated with this procedure.15
Rosacea in SOC Patients
Rosacea is a chronic inflammatory disorder that affects the vasculature and pilosebaceous units of the face. It commonly is seen in Fitzpatrick skin types I and II; however, rosacea can occur in all skin types (Figure 2). Triggers include emotional stress, extreme environmental temperatures, hot and spicy foods, red wine or alcohol, and topical irritants or allergens found in common cosmetic products.16

Data suggest that 4% of rosacea patients in the United States are of African, Latino, or Asian descent.11 National Ambulatory Medical Care Survey data revealed that of 31.5 million rosacea visits, 2% of patients were black, 2.3% were Asian or Pacific Islander, and 3.9% were Hispanic or Latino. In a 5-year longitudinal study of 2587 rosacea patients enrolled in Medicaid in North Carolina who were prescribed at least 1 topical treatment for rosacea, 16.27% were black and 10% were of a race other than white.17
Although the pathogenesis of rosacea is unclear, hypotheses include immune system abnormalities, neurogenic dysregulation, presence of microorganisms (eg, Demodex folliculorum), UV damage, and skin barrier dysfunction.18
The 4 major subtypes of rosacea are erythematotelangiectatic, papulopustular, phymatous, and ocular rosacea.16 Interestingly, rosacea in SOC patients may present with hypopigmentation surrounding the borders of the facial erythema. For phymatous rosacea, isotretinoin may reduce incipient rhinophyma but must be carefully monitored and pregnancy must be excluded. Surgical or laser therapy may be indicated to recontour the nose if severe.
There are several skin conditions that can present with facial erythema in patients with SOC, including seborrheic dermatitis, systemic lupus erythematosus, and contact dermatitis. It is important to note that the detection of facial erythema in darker skin types may be difficult; therefore, laboratory evaluation (antinuclear antibodies), patch testing, and skin biopsy should be considered if the clinical diagnosis is unclear.
Treatment
Treatment of rosacea in SOC patients does not differ from other racial groups. Common strategies include gentle skin care, sun protection (sun protection factor 30+), and barrier repair creams. Topical agents include metronidazole, AZA, sodium sulfacetamide/sulfur, ivermectin, and retinoids.16 Oral treatments include antibiotics in the tetracycline family (eg, subantimicrobial dose doxycycline) and isotretinoin.16 Persistent erythema associated with rosacea can be treated with brimonidine19 and oxymetazoline.20 Vascular lasers and intense pulsed light may be used to address the vascular components of rosacea21; however, the latter is not recommended in Fitzpatrick skin types IV through VI.
Facial Hyperpigmentation in SOC Patients
Hyperpigmentation disorders can be divided into conditions that affect Fitzpatrick skin types I through III and IV though VI. Mottled hyperpigmentation (photodamage) and solar lentigines occur in patients with lighter skin types as compared to melasma, PIH, and age-related (UV-induced) hyperpigmentation, which occur more commonly in patients with darker skin types. Facial hyperpigmentation is a common concern in SOC patients. In a survey of cosmetic concerns of 100 women with SOC, hyperpigmentation or dark spots (86%) and blotchy uneven skin (80%) were the top concerns.22 In addition, facial hyperpigmentation has been shown to negatively impact quality of life.23
Postinflammatory hyperpigmentation occurs from a pathophysiological response to inflammation, cutaneous irritation or injury, and subsequent melanocyte lability. Postinflammatory hyperpigmentation is a common presenting concern in patients with SOC and is seen as a result of many inflammatory skin disorders (eg, acne, eczema) and dermatologic procedures (eg, adverse reaction to electrodesiccation, microdermabrasion, chemical peels, laser surgery).24
Melasma is an acquired idiopathic disorder of hyperpigmentation and often referred to as the mask of pregnancy (Figure 3). It occurs on sun-exposed areas of skin, mainly in women with Fitzpatrick skin types III through V. Associated factors or triggers include pregnancy, hormonal treatments, exposure to UV radiation, and medications.25 Hereditary factors play a role in more than 40% of cases.26

Other not-so-common facial dyschromias include contact dermatitis, acanthosis nigricans, exogenous ochronosis, lichen planus pigmentosus (associated with frontal fibrosing alopecia),27 drug-induced hyperpigmentation (associated with minocycline or diltiazem),28,29 and UV-induced (age-related) hyperpigmentation.
Treatment
The treatment of hyperpigmentation should provide the following: (1) protection from sun exposure; (2) inhibition of tyrosinase, the enzyme responsible for the conversion of tyrosine to melanin; (3) inhibition of melanosome transfer from the melanocyte to the keratinocyte; (4) removal of melanin from the epidermis through exfoliation; and (5) destruction or disruption of melanin in the dermis.30 Therapies for facial hyperpigmentation are listed in Table 1.

Topical therapies include prescription medications and nonprescription cosmeceuticals. Prescription medications include hydroquinone (HQ), topical retinoids, and AZA. Hydroquinone, a tyrosinase inhibitor, is the gold standard for skin lightening and often is used as a first-line therapy. It is used as a monotherapy (HQ 4%) or as a fixed combination with tretinoin 0.05% and fluocinolone 0.01%.31 Use caution with HQ in high concentrations (6% and higher) and low concentrations (2% [over-the-counter strength]) used long-term due to the potential risk of exogenous ochronosis.
Topical retinoids have been shown to be effective therapeutic agents for melasma and PIH. Tretinoin,32 tazarotene,33 and adapalene34 all have demonstrated efficacy for acne and acne-induced PIH in SOC patients. Patients must be monitored for the development of retinoid dermatitis and worsening of hyperpigmentation.
Azelaic acid is a naturally occurring dicarboxylic acid obtained from cultures of Malassezia furfur. Azelaic acid inhibits tyrosinase activity, DNA synthesis, and mitochondrial enzymes, thus blocking direct cytotoxic effects toward melanocytes. Azelaic acid is approved by the US Food and Drug Administration for acne in a 20% cream formulation and rosacea in 15% gel and foam formulations, and it is used off label for melasma and PIH.35
Oral tranexamic acid is currently used as a hemostatic agent due to its ability to inhibit the plasminogen-plasmin pathway. In melasma, it blocks the interaction between melanocytes and keratinocytes in the epidermis and modulates the vascular component of melasma in the dermis. In an open-label study, 561 Asian melasma patients were treated with oral tranexamic acid 250 mg twice daily for 4 months. Results demonstrated improvement in 90% of patients, and 7.1% reported adverse effects (eg, abdominal bloating and pain, nausea, vomiting, headache, tinnitus, numbness, menstrual irregularities).36 Coagulation screening should be monitored monthly, and any patient with a history of clotting abnormalities should be excluded from off-label treatment with oral tranexamic acid.
Nonprescription cosmeceuticals are available over-the-counter or are office dispensed.37 For optimal results, cosmeceutical agents for skin lightening are used in combination. Most of these combinations are HQ free and have additive benefits such as a multimodal skin lightening agent containing key ingredients that correct and prevent skin pigmentation via several pathways affecting melanogenesis.38 It is an excellent alternative to HQ for mottled and diffuse UV-induced hyperpigmentation and can be used for maintenance therapy in patients with melasma.
Photoprotection is an essential component of therapy for melasma and PIH, but there is a paucity of data on the benefits for SOC patients. Halder et al39 performed a randomized prospective study of 89 black and Hispanic patients who applied sunscreen with a sun protection factor of 30 or 60 daily for 8 weeks. Clinical grading, triplicate L*A*B chromameter, and clinical photography were taken at baseline and weeks 4 and 8. The results demonstrated skin lightening in both black and Hispanic patients and support the use of sunscreen in the prevention and management of dyschromia in SOC patients.39 Visible light also may play a role in melasma development, and thus use of sunscreens or makeup containing iron oxides are recommended.40
Procedural treatments for facial hyperpigmentation include microdermabrasion, chemical peels, lasers, energy-based devices, and microneedling. There are many types and formulations of chemical peeling agents available; however, superficial and medium-depth chemical peels are recommended for SOC patients (Table 2). Deep chemical peels are not recommended for SOC patients due to the potential increased risk for PIH and scarring.

Cosmetic Enhancement in SOC Patients
Cosmetic procedures are gaining popularity in the SOC population and account for more than 20% of cosmetic procedures in the United States.41 Facial cosmetic concerns in SOC include dyschromia, benign growths (dermatosis papulosa nigra), hyperkinetic facial lines, volume loss, and skin laxity.42 Key principles to consider when treating SOC patients are the impact of ethnicity on aging and facial structure, the patient’s desired cosmetic outcome, tissue reaction to anticipated treatments, and the patient’s expectations for recommended therapies.
Aging in SOC Patients
Skin aging can be classified as intrinsic aging or extrinsic aging. Intrinsic aging is genetic and involves subsurface changes such as volume loss, muscle atrophy, and resorption of bony structure. Extrinsic aging (or photoaging) involves surface changes of the epidermis/dermis and manifests as mottled pigmentation, textural changes, and fine wrinkling. Due to the photoprotection of melanin (black skin=SPF 13.4), skin aging in SOC patients is delayed by 10 to 20 years.43 In addition, SOC patients have more reactive collagen and can benefit from noninvasive cosmetic procedures such as fillers and skin-tightening procedures.42
Cosmetic Treatments and Procedures
Dermatosis papulosa nigra (benign growths of skin that have a genetic predisposition)44 occur mainly on the face but can involve the entire body. Treatment modalities include electrodesiccation, cryotherapy, scissor excision, and laser surgery.45
Treatment of hyperkinetic facial lines with botulinum toxin type A is a safe and effective procedure in patients with SOC. Grimes and Shabazz46 performed a 4-month, randomized, double-blind study that evaluated the treatment of glabellar lines in women with Fitzpatrick skin types V and VI. The results demonstrated that the duration of effects was the same in the patients who received either 20 or 30 U of botulinum toxin type A.46 Dynamic rhytides (furrows and frown/scowl lines arising from laughing, frowning, or smiling) can be treated safely in patients with SOC using botulinum toxin type A off label for relaxation of the upper and lower hyperkinetic muscles that result in these unwanted signs of aging. Botulinum toxin type A often is used for etched-in crow’s-feet, which rarely are evident in SOC patients.47 Facial shaping also can be accomplished by injecting botulinum toxin type A in combination with soft-tissue dermal fillers.47
Although black individuals do not experience perioral rhytides at the frequency of white individuals, they experience a variety of other cosmetic issues related to skin sagging and sinking. Currently available hyaluronic acid (HA) fillers have been shown to be safe in patients with Fitzpatrick skin types IV through VI.48 Two studies evaluated fillers in patients with SOC, specifically HA49 and calcium hydroxylapatite,50 focused on treatment of the nasolabial folds and the potential risk for dyspigmentation and keloidal scarring. Taylor et al49 noted that the risk of hyperpigmentation was 6% to 9% for large- and small-particle HA, respectively, and was associated with the serial or multiple puncture injection technique. No hypertrophic or keloidal scarring occurred in both studies.49,50
Facial contouring applications with fillers include glabellar lines, temples, nasal bridge, tear troughs, malar and submalar areas, nasolabial folds, radial lines, lips, marionette lines, mental crease, and chin. Hyaluronic acid fillers also can be used for lip enhancement.47 Although white women are looking to increase the size of their lips, black women are seeking augmentation to restore their lip size to that of their youth. Black individuals do not experience the same frequency of perioral rhytides as white patients, but they experience a variety of other issues related to skin sagging and sinking. Unlike white women, enhancement of the vermilion border rarely is performed in black women due to development of rhytides, predominantly in the body of the lip below the vermilion border in response to volume loss in the upper lip while the lower lip usually maintains its same appearance.47
Facial enhancement utilizing poly-L-lactic acid can be used safely in SOC patients.51 Poly-L-lactic acid microparticles induce collagen formation, leading to dermal thickening over 3 to 6 months; however, multiple sessions are required to achieve optimal aesthetic results.
Patients with more reactive collagen can benefit from noninvasive cosmetic procedures such as skin-tightening procedures.52 Radiofrequency and microfocused ultrasound are cosmetic procedures used to provide skin tightening and facial lifting. They are safe and effective treatments for patients with Fitzpatrick skin types IV to VI.53 Histologically, there is less thinning of collagen bundles and elastic tissue in ethnic skin. Due to stimulation of collagen by these procedures, most SOC patients will experience a more enhanced response, requiring fewer treatment sessions than white individuals.
Conclusion
Medical and aesthetic facial concerns in SOC patients vary and can be a source of emotional and psychological distress that can negatively impact quality of life. The approach to the treatment of SOC patients should be a balance between tolerability and efficacy, considering the potential risk for PIH.
- White GM. Recent findings in the epidemiologic evidence, classification, and subtypes of acne vulgaris. J Am Acad Dermatol. 1998;39(2 pt 3):S34-S37.
- Halder RM, Grimes PE, McLaurin CL, et al. Incidence of common dermatoses in a predominantly black dermatologic practice. Cutis. 1983;32:388, 390.
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Perkins AC, Cheng CE, Hillebrand GG, et al. Comparison of the epidemiology of acne vulgaris among Caucasians, Asian, Continental Indian and African American women. J Eur Acad Dermatol Venereol. 2011;25:1054-1060.
- Taylor SC, Cook-Bolden F, Rahman Z, et al. Acne vulgaris in skin of color. J Am Acad Dermatol. 2002;46(2 suppl):S98-S106.
- Davis EC, Callender VD. A review of acne in ethnic skin: pathogenesis, clinical manifestations, and management strategies. J Clin Aesthet Dermatol. 2010;3:24-38.
- Halder RM, Holmes YC, Bridgeman-Shah S, et al. A clinicohistologic study of acne vulgaris in black females (abstract). J Invest Dermatol. 1996;106:888.
- Plewig G, Fulton JE, Kligman AM. Pomade acne. Arch Dermatol. 1970;101:580-584.
- Kligman AM, Mills OH. Acne cosmetica. Arch Dermatol. 1972;106:893-897.
- Halder RM, Brooks HL, Callender VD. Acne in ethnic skin. Dermatol Clin. 2003;21:609-615.
- Callender VD. Acne in ethnic skin: special considerations for therapy. Dermatol Ther. 2004;17:184-195.
- Winhoven SM. Postinflammatory hyperpigmentation in an Asian patient. a dramatic response to oral isotretinoin (13-cis-retinoic acid). Br J Med. 2005;152:368-403.
- Sarkar R, Bansal S, Garg VK. Chemical peels for melasma in dark-skinned patients. J Cutan Aesthet Surg. 2012;5:247-253.
- Alexis AF, Coley MK, Nijhawan RI, et al. Nonablative fractional laser resurfacing for acne scarring in patients with Fitzpatrick skin phototypes IV-VI. Dermatol Surg. 2016;42:392-402.
- Culp B, Scheinfeld N. Rosacea: a review. P T. 2009;34:38-45.
- Al-Dabagh A, Davis SA, McMichael AJ, et al. Rosacea in skin of color: not a rare diagnosis. Dermatol Online J. 2014:20. pii:13030/qt1mv9r0ss.
- Del Rosso JQ. Advances in understanding and managing rosacea: part 1: connecting the dots between pathophysiological mechanisms and common clinical features of rosacea with emphasis on vascular changes and facial erythema. J Clin Aesthet Dermatol. 2012;5:16-25.
- Jackson JM, Knuckles M, Minni JP, et al. The role of brimonidine tartrate gel in the treatment of rosacea. Clin Cosmet Investig Dermatol. 2015;23:529-538.
- Patel NU, Shukla S, Zaki J, et al. Oxymetazoline hydrochloride cream for facial erythema associated with rosacea. Expert Rev Clin Pharmacol. 2017;10:104954.
- Weinkle AP, Doktor V, Emer J. Update on the management of rosacea. Clin Cosmet Investig Dermatol. 2015;8:159-177.
- Grimes PE. Skin and hair cosmetic issues in women of color. Dermatol Clin. 2000;19:659-665.
- Taylor A, Pawaskar M, Taylor SL, et al. Prevalence of pigmentary disorders and their impact on quality of life: a prospective cohort study. J Cosmet Dermatol. 2008;7:164-168.
- Davis EC, Callender VD. Postinflammatory hyperpigmentation: a review of the epidemiology, clinical features, and treatment options in skin of color. J Clin Aesthet Dermatol. 2010;3:20-31.
- Grimes PE. Melasma: etiologic and therapeutic considerations. Arch Dermatol. 1995;131:1453-1457.
- Handel AC, Miot LD, Miot HA. Melasma: a clinical and epidemiological review. An Bras Dermatol. 2014;89:771-782.
- Callender VD, Reid SD, Obayan O, et al. Diagnostic clues to frontal fibrosing alopecia in patients of African descent. J Clin Aesthet Dermatol. 2016;9:45-51.
- Narang T, Sawatkar GU, Kumaran MS, et al. Minocycline for recurrent and/or chronic erythema nodosum leprosum. JAMA Dermatol. 2015;151:1026-1028.
- Boyer M, Katta R, Markus R. Diltiazem-induced photodistributed hyperpigmentation. Dermatol Online J. 2003;9:10.
- Pandya AG, Guevara IL. Disorders of hyperpigmentation. Dermatol Clin. 2000;18:91-98.
- Taylor SC, Torok H, Jones T, et al. Efficacy and safety of a new triple-combination agent for the treatment of facial melasma. Cutis. 2003;72:67-72.
- Bulengo-Ransby SM. Topical tretinoin (retinoic acid) therapy for hyperpigmented lesions caused by inflammation of the skin in black patients. N Engl J Med. 1993;328:1438-1443.
- Grimes P, Callender V. Tazarotene cream for postinflammatory hyperpigmentation and acne vulgaris in darker skin: a double-blind, randomized, vehicle-controlled study. Cutis. 2006;77:45-50.
- Jacyk WK. Adapalene in the treatment of African patients. J Eur Acad Dermatol Venereol. 2001;15(suppl 3):37-42.
- Kircik LH. Efficacy and safety of azelaic acid (AzA) gel 15% in the treatment of postinflammatory hyperpigmentation and acne: a 16-week, baseline-controlled study. J Drugs Dermatol. 2011;10:586-590.
- Lee HC, Thng TG, Goh CL. Oral tranexamic acid (TA) in the treatment of melasma. J Am Acad Dermatol. 2016;75:385-392.
- Kindred C, Okereke U, Callender VD. Skin-lightening agents: an overview of prescription, office-dispensed, and over-the-counter products. Cosmet Dermatol. 2013;26:18-26.
- Makino ET, Kadoya K, Sigler ML, et al. Development and clinical assessment of a comprehensive product for pigmentation control in multiple ethnic populations. J Drugs Dermatol. 2016;15:1562-1570.
- Halder R, Rodney I, Munhutu M, et al. Evaluation and effectiveness of a photoprotection composition (sunscreen) on subjects of skin of color. J Am Acad Dermatol. 2015;72(suppl 1):AB215.
- Castanedo-Cazares JP, Hernandez-Blanco D, Carlos-Ortega B, et al. Near-visible light and UV photoprotection in the treatment of melasma: a double-blind randomized trial. Photodermatol Photoimmunol Photomed. 2014;30:35-42.
- American Society for Aesthetic Plastic Surgery. 2016 Cosmetic Surgery National Data Bank Statistics. https://www.surgery.org/sites/default/files/ASAPS-Stats2016.pdf. Accessed November 15, 2017.
- Burgess CM. Soft tissue augmentation in skin of color: market growth, available fillers and successful techniques. J Drugs Dermatol. 2007;6:51-55.
- Davis EC, Callender VD. Aesthetic dermatology for aging ethnic skin. Dermatol Surg. 2011;37:901-917.
- Grimes PE, Arora S, Minus HR, et al. Dermatosis papulosa nigra. Cutis. 1983;32:385-386.
- Lupo M. Dermatosis papulosa nigra: treatment options. J Drugs Dermatol. 2007;6:29-30.
- Grimes PE, Shabazz D. A four-month randomized, double-blind evaluation of the efficacy of botulinum toxin type A for the treatment of glabellar lines in women with skin types V and VI. Dermatol Surg. 2009;35:429-435.
- Burgess CM, Awosika O. Ethnic and gender considerations in the use of facial injectables: African-American patients. Plast Reconstr Surg. 2015;136(5 suppl):28S-31S.
- Taylor SC, Kelly AP, Lim HW, et al, eds. Taylor and Kelly’s Dermatology for Skin of Color. 2nd ed. New York, NY: McGraw-Hill Education; 2016.
- Taylor SC, Burgess CM, Callender VD. Safety of nonanimal stabilized hyaluronic acid dermal fillers in patients with skin of color: a randomized, evaluator-blinded comparative trial. Dermatol Surg. 2009;35(suppl 2):1653-1660.
- Marmur ES, Taylor SC, Grimes PE, et al. Six-month safety results of calcium hydroxylapatite for treatment of nasolabial folds in Fitzpatrick skin types IV to VI. Dermatol Surg. 2009;35(suppl 2):1641-1645.
- Hamilton TK, Burgess CM. Consideration for the use of injectable poly-L-lactic acid in people of color. J Drugs Dermatol. 2010;9:451-456.
- Fabi SG, Goldman MP. Retrospective evaluation of micro-focused ultrasound for lifting and tightening of the face and neck. Dermatol Surg. 2014;40:569-575.
- Harris MO, Sundaram HA. Safety of microfocused ultrasound with visualization in patients with Fitzpatrick skin phototypes III to VI. JAMA Facial Plast Surg. 2015;17:355-357.
- White GM. Recent findings in the epidemiologic evidence, classification, and subtypes of acne vulgaris. J Am Acad Dermatol. 1998;39(2 pt 3):S34-S37.
- Halder RM, Grimes PE, McLaurin CL, et al. Incidence of common dermatoses in a predominantly black dermatologic practice. Cutis. 1983;32:388, 390.
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Perkins AC, Cheng CE, Hillebrand GG, et al. Comparison of the epidemiology of acne vulgaris among Caucasians, Asian, Continental Indian and African American women. J Eur Acad Dermatol Venereol. 2011;25:1054-1060.
- Taylor SC, Cook-Bolden F, Rahman Z, et al. Acne vulgaris in skin of color. J Am Acad Dermatol. 2002;46(2 suppl):S98-S106.
- Davis EC, Callender VD. A review of acne in ethnic skin: pathogenesis, clinical manifestations, and management strategies. J Clin Aesthet Dermatol. 2010;3:24-38.
- Halder RM, Holmes YC, Bridgeman-Shah S, et al. A clinicohistologic study of acne vulgaris in black females (abstract). J Invest Dermatol. 1996;106:888.
- Plewig G, Fulton JE, Kligman AM. Pomade acne. Arch Dermatol. 1970;101:580-584.
- Kligman AM, Mills OH. Acne cosmetica. Arch Dermatol. 1972;106:893-897.
- Halder RM, Brooks HL, Callender VD. Acne in ethnic skin. Dermatol Clin. 2003;21:609-615.
- Callender VD. Acne in ethnic skin: special considerations for therapy. Dermatol Ther. 2004;17:184-195.
- Winhoven SM. Postinflammatory hyperpigmentation in an Asian patient. a dramatic response to oral isotretinoin (13-cis-retinoic acid). Br J Med. 2005;152:368-403.
- Sarkar R, Bansal S, Garg VK. Chemical peels for melasma in dark-skinned patients. J Cutan Aesthet Surg. 2012;5:247-253.
- Alexis AF, Coley MK, Nijhawan RI, et al. Nonablative fractional laser resurfacing for acne scarring in patients with Fitzpatrick skin phototypes IV-VI. Dermatol Surg. 2016;42:392-402.
- Culp B, Scheinfeld N. Rosacea: a review. P T. 2009;34:38-45.
- Al-Dabagh A, Davis SA, McMichael AJ, et al. Rosacea in skin of color: not a rare diagnosis. Dermatol Online J. 2014:20. pii:13030/qt1mv9r0ss.
- Del Rosso JQ. Advances in understanding and managing rosacea: part 1: connecting the dots between pathophysiological mechanisms and common clinical features of rosacea with emphasis on vascular changes and facial erythema. J Clin Aesthet Dermatol. 2012;5:16-25.
- Jackson JM, Knuckles M, Minni JP, et al. The role of brimonidine tartrate gel in the treatment of rosacea. Clin Cosmet Investig Dermatol. 2015;23:529-538.
- Patel NU, Shukla S, Zaki J, et al. Oxymetazoline hydrochloride cream for facial erythema associated with rosacea. Expert Rev Clin Pharmacol. 2017;10:104954.
- Weinkle AP, Doktor V, Emer J. Update on the management of rosacea. Clin Cosmet Investig Dermatol. 2015;8:159-177.
- Grimes PE. Skin and hair cosmetic issues in women of color. Dermatol Clin. 2000;19:659-665.
- Taylor A, Pawaskar M, Taylor SL, et al. Prevalence of pigmentary disorders and their impact on quality of life: a prospective cohort study. J Cosmet Dermatol. 2008;7:164-168.
- Davis EC, Callender VD. Postinflammatory hyperpigmentation: a review of the epidemiology, clinical features, and treatment options in skin of color. J Clin Aesthet Dermatol. 2010;3:20-31.
- Grimes PE. Melasma: etiologic and therapeutic considerations. Arch Dermatol. 1995;131:1453-1457.
- Handel AC, Miot LD, Miot HA. Melasma: a clinical and epidemiological review. An Bras Dermatol. 2014;89:771-782.
- Callender VD, Reid SD, Obayan O, et al. Diagnostic clues to frontal fibrosing alopecia in patients of African descent. J Clin Aesthet Dermatol. 2016;9:45-51.
- Narang T, Sawatkar GU, Kumaran MS, et al. Minocycline for recurrent and/or chronic erythema nodosum leprosum. JAMA Dermatol. 2015;151:1026-1028.
- Boyer M, Katta R, Markus R. Diltiazem-induced photodistributed hyperpigmentation. Dermatol Online J. 2003;9:10.
- Pandya AG, Guevara IL. Disorders of hyperpigmentation. Dermatol Clin. 2000;18:91-98.
- Taylor SC, Torok H, Jones T, et al. Efficacy and safety of a new triple-combination agent for the treatment of facial melasma. Cutis. 2003;72:67-72.
- Bulengo-Ransby SM. Topical tretinoin (retinoic acid) therapy for hyperpigmented lesions caused by inflammation of the skin in black patients. N Engl J Med. 1993;328:1438-1443.
- Grimes P, Callender V. Tazarotene cream for postinflammatory hyperpigmentation and acne vulgaris in darker skin: a double-blind, randomized, vehicle-controlled study. Cutis. 2006;77:45-50.
- Jacyk WK. Adapalene in the treatment of African patients. J Eur Acad Dermatol Venereol. 2001;15(suppl 3):37-42.
- Kircik LH. Efficacy and safety of azelaic acid (AzA) gel 15% in the treatment of postinflammatory hyperpigmentation and acne: a 16-week, baseline-controlled study. J Drugs Dermatol. 2011;10:586-590.
- Lee HC, Thng TG, Goh CL. Oral tranexamic acid (TA) in the treatment of melasma. J Am Acad Dermatol. 2016;75:385-392.
- Kindred C, Okereke U, Callender VD. Skin-lightening agents: an overview of prescription, office-dispensed, and over-the-counter products. Cosmet Dermatol. 2013;26:18-26.
- Makino ET, Kadoya K, Sigler ML, et al. Development and clinical assessment of a comprehensive product for pigmentation control in multiple ethnic populations. J Drugs Dermatol. 2016;15:1562-1570.
- Halder R, Rodney I, Munhutu M, et al. Evaluation and effectiveness of a photoprotection composition (sunscreen) on subjects of skin of color. J Am Acad Dermatol. 2015;72(suppl 1):AB215.
- Castanedo-Cazares JP, Hernandez-Blanco D, Carlos-Ortega B, et al. Near-visible light and UV photoprotection in the treatment of melasma: a double-blind randomized trial. Photodermatol Photoimmunol Photomed. 2014;30:35-42.
- American Society for Aesthetic Plastic Surgery. 2016 Cosmetic Surgery National Data Bank Statistics. https://www.surgery.org/sites/default/files/ASAPS-Stats2016.pdf. Accessed November 15, 2017.
- Burgess CM. Soft tissue augmentation in skin of color: market growth, available fillers and successful techniques. J Drugs Dermatol. 2007;6:51-55.
- Davis EC, Callender VD. Aesthetic dermatology for aging ethnic skin. Dermatol Surg. 2011;37:901-917.
- Grimes PE, Arora S, Minus HR, et al. Dermatosis papulosa nigra. Cutis. 1983;32:385-386.
- Lupo M. Dermatosis papulosa nigra: treatment options. J Drugs Dermatol. 2007;6:29-30.
- Grimes PE, Shabazz D. A four-month randomized, double-blind evaluation of the efficacy of botulinum toxin type A for the treatment of glabellar lines in women with skin types V and VI. Dermatol Surg. 2009;35:429-435.
- Burgess CM, Awosika O. Ethnic and gender considerations in the use of facial injectables: African-American patients. Plast Reconstr Surg. 2015;136(5 suppl):28S-31S.
- Taylor SC, Kelly AP, Lim HW, et al, eds. Taylor and Kelly’s Dermatology for Skin of Color. 2nd ed. New York, NY: McGraw-Hill Education; 2016.
- Taylor SC, Burgess CM, Callender VD. Safety of nonanimal stabilized hyaluronic acid dermal fillers in patients with skin of color: a randomized, evaluator-blinded comparative trial. Dermatol Surg. 2009;35(suppl 2):1653-1660.
- Marmur ES, Taylor SC, Grimes PE, et al. Six-month safety results of calcium hydroxylapatite for treatment of nasolabial folds in Fitzpatrick skin types IV to VI. Dermatol Surg. 2009;35(suppl 2):1641-1645.
- Hamilton TK, Burgess CM. Consideration for the use of injectable poly-L-lactic acid in people of color. J Drugs Dermatol. 2010;9:451-456.
- Fabi SG, Goldman MP. Retrospective evaluation of micro-focused ultrasound for lifting and tightening of the face and neck. Dermatol Surg. 2014;40:569-575.
- Harris MO, Sundaram HA. Safety of microfocused ultrasound with visualization in patients with Fitzpatrick skin phototypes III to VI. JAMA Facial Plast Surg. 2015;17:355-357.
Practice Points
- Treat acne in skin of color (SOC) patients early and aggressively to prevent or minimize subsequent postinflammatory hyperpigmentation (PIH) and acne scarring.
- Vascular lasers and intense pulsed light may be used to address the vascular components of rosacea; however, the latter is not recommended in Fitzpatrick skin types IV to VI.
- Hydroquinone is the gold standard for skin lightening and is often used as a first-line therapy for melasma and PIH.
- Photoprotection is an essential component of therapy for hyperpigmented skin disorders.
- Cosmetic procedures are gaining popularity in the SOC population. When treating SOC patients, consider the impact of ethnicity on aging and facial structure, the patient's desired cosmetic outcome, tissue reaction to anticipated treatments, and the patient's expectations for recommended therapies.
Pediatric Periorificial Dermatitis
Perioral dermatitis is an acneform eruption presenting with erythematous papules, vesicles, and rarely pustules clustered around the orifices of the face. 1 Lesions may be found near the eyes, mouth, and nose but typically spare the vermilion border of the lips. 2 Nguyen and Eichenfield 3 preferred the term periorificial dermatitis (POD), which has since been adopted by others. 4 Patients may report pruritus, but there generally are no systemic symptoms unless patients have comorbid conditions such as atopic dermatitis. 5 Although this condition has been well examined in the literature on adults, data in the pediatric population are far more limited, consisting of case series and retrospective chart reviews. In 1979, Wilkinson et al 6 published a study of more than 200 patients with perioral dermatitis, but only 15 patients younger than 12 years were included.
Etiology
Although the exact pathogenesis of POD is unknown, a common denominator among many patients is prior exposure to topical corticosteroids.3,7-9 Periorificial dermatitis also has been linked to the use of systemic corticosteroids in pediatric patients.10 The exact relationship between steroid use and dermatitis is unknown; it may be related to a change in the flora of hair follicles and in particular an association with fusiform bacteria–rich conditions.11 Aside from steroid exposure, POD has been associated with the use of physical sunscreen in pediatric patients with dry skin,12 rosin in chewing gum,13 and inhaled corticosteroids in those with asthma.14 In one case, a 15-year-old adolescent girl developed POD and swelling of the lips after 2 years of playing a flute made of cocus wood.15,16
Epidemiology
Comorbidities and Family History
Goel et al17 (N=222) reported the following comorbidities associated with pediatric POD: atopic dermatitis (29.3%), asthma (14.9%), and allergies (9.9%). Steroid exposure was noted in 58.1% of patients.17 Similarly, Nguyen and Eichenfield3 (N=79) found that the most common comorbidities were atopic dermatitis (14%), keratosis pilaris (14%), viral infections (14%), acne (10%), and seborrheic dermatitis (10%). Family history of atopy was noted in 55% of patients and family history of rosacea was noted in 3%. In a case series of 11 pediatric patients, 3 (27%) had keratosis pilaris, 7 (64%) had a family history of atopy, and 2 (18%) had a family history of rosacea.8 Weston and Morelli9 found a much higher incidence of familial rosacea (20%) in 106 children with steroid rosacea.
Clinical Presentation
Periorificial dermatitis generally presents with small, pink- to flesh-colored papules in a perioral, periocular, and perinasal distribution. Although many patients are white, a particularly prominent variant has been noted in black children with papules that may be hyperpigmented.18 In a 2006 chart review in 79 pediatric POD patients aged 6 months to 18 years, Nguyen and Eichenfield3 reported that 92% (73/79) of patients presented for a facial rash with an average duration ranging from 2 weeks to 4 years.
Boeck et al19 described 7 pediatric patients with perioral dermatitis. Six (86%) patients had perioral lesions, and 6 (86%) had previously been treated with moderate- to high-potency topical corticosteroids. Skin prick tests were negative in 6 (86%) patients.19 In one case report, a 6-year-old boy did not present with the classic acneform lesions but rather sharply demarcated eczematous patches around the eyes, nose, and mouth. The rash began to fade after 2 weeks of using metronidazole gel 1%, and after 4 months he was only left with mild hyperpigmentation.4
Periorificial dermatitis was once thought to be a juvenile form of rosacea.5 In 1972, Savin et al8 described 11 pediatric patients with “rosacea-like” facial flushing, papules, pustules, and scaling over the cheeks, forehead, and chin. In some patients, the eyelids also were involved. At least 8 patients had been using potent topical corticosteroids and had noticed exacerbation of their skin lesions after stopping therapy.8
Variants of POD
Several other variants of POD have been described in pediatric patients including childhood granulomatous periorificial dermatitis (CGPD)(also known as facial Afro-Caribbean [childhood] eruption) and lupus miliaris disseminatus faciei. Childhood granulomatous periorificial dermatitis presents in prepubertal children as dome-shaped, red to yellow-brown, monomorphous papules around the eyes, nose, and mouth; there are no systemic findings.20,21 It occurs equally in males and females and is more commonly seen in dark-skinned patients. Childhood granulomatous periorificial dermatitis usually resolves within a few months to years but may be associated with blepharitis or conjunctivitis.20 Urbatsch et al20 analyzed extrafacial lesions in 8 patients (aged 2–12 years) with CGPD. Lesions were found on the trunk (38% [3/8]), neck (25% [2/8]), ears (25% [2/8]), extremities (50% [4/8]), labia majora (38% [3/8]), and abdomen (13% [1/8]). In addition, 2 (25% [2/8]) patients had blepharitis.20
Lupus miliaris disseminatus faciei, which occurs in adolescents and adults, commonly involves the eyelids and central areas of the face such as the nose and upper lips. Patients typically present with erythematous or flesh-colored papules.1
Diagnosis
Diagnosis of POD is made clinically based on the observation of papules (and sometimes pustules) around the orifices of the face, sparing the vermilion border, together with a lack of comedones.17 Laboratory tests are not useful.5 Biopsies rarely are performed, and the results mimic those of rosacea, demonstrating a perifollicular lymphohistiocytic infiltrate, epithelioid cells, and occasionally giant cells.5,22,23 Early papular lesions can show mild acanthosis, epidermal edema, and parakeratosis.23 Biopsies in patients with CGPD reveal noncaseating perifollicular granulomas.20
Treatment and Clinical Outcome
Although topical corticosteroids can improve facial lesions in pediatric POD, the eruption often rebounds when therapy is discontinued.1 One therapy frequently used in adults is oral tetracyclines; however, these agents must not be used in patients younger than 9 years due to potential dental staining.4 The standards are either topical metronidazole twice daily with clearance in 3 to 8 weeks or oral erythromycin.7
In the review conducted by Goel et al,17 treatment included azithromycin (44.6%), topical metronidazole (42.3%), sodium sulfacetamide lotion (35.6%), oral antibiotic monotherapy (15.3%), topical agent monotherapy (44.6%), and combined oral and topical agent therapy (40.1%). Of those patients who presented for a follow-up visit (59%), 72% of cases resolved and 10.7% showed some improvement. For those patients who returned for follow-up, the average duration until symptom resolution was approximately 4 months. The most common side effects were pigmentation changes (1.8%), worsening of symptoms (1.8%), gastrointestinal upset (0.9%), irritant dermatitis (0.9%), and xerosis (0.5%).17
Changes were made to the treatment plans for 16 patients, most often due to inadequate treatment response.17 Five patients treated with sodium sulfacetamide lotion also were started on oral azithromycin. Four patients treated with oral antibiotics were given a topical agent (metronidazole or sodium sulfacetamide lotion). Other modifications included replacing sodium sulfacetamide lotion with topical metronidazole and an oral antibiotic (azithromycin or doxycycline, n=3), adjusting the doses of oral or topical medications (n=2), adding tacrolimus (n=1), and replacing topical metronidazole with sodium sulfacetamide lotion (n=1). Of the patients who underwent a change in treatment plan, 5 experienced symptom recurrence, 4 had mild improvement, and 1 patient had no improvement. Six patients were lost to follow-up.17
In the study conducted by Nguyen and Eichenfield,3 follow-up visits occurred approximately 3 months after the first visit.
In the case series by Boeck et al,19 all patients were started on metronidazole gel 1% applied once daily for the first week, and then twice daily until the lesions resolved. All patients showed improvement after 4 to 6 weeks, and eventually the disease cleared between 3 and 6 months. All patients were still symptom free during a 2-year observation period.19
Manders and Lucky7 described 14 patients with POD (aged 9 months to 6.5 years). Eight patients used only metronidazole gel 0.75%, while 5 used the gel in combination with topical corticosteroids (21% [3/14]), oral erythromycin (7% [1/14]), or topical erythromycin (7% [1/14]); 1 patient remained on hydrocortisone 1% and cleared. Patients responded well within 1 to 8 weeks and were symptom free for up to 16 months. Mid- to high-potency steroids were discontinued in all patients.7
In some pediatric patients with CGPD, recovery occurs faster with the use of oral macrolides or tetracyclines, either alone or in combination with topical antibiotics or sulfur-based lotions.20 Extrafacial lesions associated with CGPD do not appear to negatively impact treatment response or duration of disease. In the review conducted by Urbatsch et al,20 7 of 8 (88%) CGPD patients with extrafacial lesions were treated with oral agents including erythromycin, hydroxychloroquine, cyclosporine, minocycline, and azithromycin. Most of these patients also were using topical agents such as triamcinolone acetonide, desonide, metronidazole, and erythromycin. The time to resolution ranged from several weeks to 6 months.20
Weston and Morelli9 described a treatment regimen for steroid rosacea. The study included data on 106 children (60 females, 46 males) who had been exposed to mostly class 7 low-potency agents. All patients were advised to immediately stop topical steroid therapy without gradual withdrawal and to begin oral erythromycin stearate 30 mg/kg daily in 2 doses per day for 4 weeks. Patients who were unable to tolerate erythromycin were advised to use topical clindamycin phosphate twice daily for 4 weeks (n=6). Eighty-six percent of patients showed resolution within 4 weeks, and 100% showed clearance by 8 weeks. Twenty-two percent of patients had clearance within 3 weeks. There was no difference in the duration until resolution for those who had used oral or topical antibiotics.9 A different study suggested that low-potency topical steroids can be used to control inflammation when weaning patients off of strong steroids.5
Differential Diagnosis
The differential diagnosis should include acne vulgaris, allergic contact dermatitis, irritant contact dermatitis, seborrheic dermatitis, impetigo, dermatophyte infection, rosacea, and angiofibromas.4
Acne vulgaris commonly is found in older adolescents, and unlike POD, it will present with open or closed comedones.2 In patients aged 1 to 7 years, acne is a reason to consider endocrine evaluation. Allergic contact dermatitis is extremely pruritic, and the lesions often are papulovesicular with active weeping or crusting. Patients with irritant contact dermatitis often report burning and pain, and papules and pustules typically are absent. A thorough history can help rule out allergic or irritant contact dermatitis. Seborrheic dermatitis presents with erythema and scaling of the scalp, eyebrows, and nasolabial folds; it tends to spare the perioral regions and also lacks papules.2 The lesions of impetigo typically have a yellow-brown exudate, which forms a honey-colored crust.24 Tinea faciei, unlike the other tinea infections, can have an extremely variable presentation. Lesions usually begin as scaly macules that develop raised borders with central hypopigmentation, but papules, vesicles, and crusts can be seen.25 Potassium hydroxide
Conclusion
Diagnosis of POD is clinical and rests upon the finding of erythematous papules on the face near the eyes, mouth, and nose. Extrafacial lesions also have been described, particularly in pediatric patients with CGPD. Many patients will report a history of atopic dermatitis and asthma. Therapy for POD includes both topical and systemic agents. For those with mild disease, topical metronidazole commonly is used. For patients requiring oral antibiotics, tetracyclines or macrolides can be prescribed based on the age of the patient. Many pediatric patients who begin with both oral and topical agents can later be maintained on topical therapy, sometimes with a low-dose oral antibiotic. Periorificial dermatitis has an excellent prognosis and most pediatric patients show marked improvement within weeks to months.
- Tempark T, Shwayder TA. Perioral dermatitis: a review of the condition with special attention to treatment options. Am J Clin Dermatol. 2014;15:101-113.
- McFarland SL, Polcari IC. Morphology-based diagnosis of acneiform eruptions. Pediatr Ann. 2015;44:E188-E193.
- Nguyen V, Eichenfield LF. Periorificial dermatitis in children and adolescents. J Am Acad Dermatol. 2006;55:781-785.
- Kihiczak GG, Cruz MA, Schwartz RA. Periorificial dermatitis in children: an update and description of a child with striking features. Int J Dermatol. 2009;48:304-306.
- Laude TA, Salvemini JN. Perioral dermatitis in children. Sem Cutan Med Surg. 1999;18:206-209.
- Wilkinson DS, Kirton V, Wilkinson JD. Perioral dermatitis: a 12-year review. Br J Dermatol. 1979;101:245-257.
- Manders SM, Lucky AW. Perioral dermatitis in childhood. J Am Acad Dermatol. 1992;27(5 pt 1):688-692.
- Savin JA, Alexander S, Marks R. A rosacea-like eruption of children. Br J Dermatol. 1972;87:425-429.
- Weston WL, Morelli JG. Steroid rosacea in prepubertal children. Arch Pediatr Adolesc Med. 2000;154:62-64.
- Clementson B, Smidt AC. Periorificial dermatitis due to systemic corticosteroids in children: report of two cases. Pediatr Dermatol. 2012;29:331-332.
- Takiwaki H, Tsuda H, Arase S, et al. Differences between intrafollicular microorganism profiles in perioral and seborrhoeic dermatitis. Clin Exp Dermatol. 2003;28:531-534.
- Abeck D, Geisenfelder B, Brandt O. Physical sunscreens with high sun protection factor may cause perioral dermatitis in children. J Dtsch Dermatol Ges. 2009;7:701-703.
- Satyawan I, Oranje AP, van Joost T. Perioral dermatitis in a child due to rosin in chewing gum. Contact Dermatitis. 1990;22:182-183.
- Dubus JC, Marguet C, Deschildre A, et al. Local side-effects of inhaled corticosteroids in asthmatic children: influence of drug, dose, age, and device. Allergy. 2001;56:944-948.
- Hausen BM, Bruhn G, Koenig WA. New hydroxyisoflavans as contact sensitizers in cocus wood Brya ebenus DC (Fabaceae). Contact Dermatitis. 1991;25:149-155.
- Dirschka T, Weber K, Tronnier H. Topical cosmetics and perioral dermatitis. J Dtsch Dermatol Ges. 2004;2:194-199.
- Goel NS, Burkhart CN, Morrell DS. Pediatric periorificial dermatitis: clinical course and treatment outcomes in 222 patients. Pediatr Dermatol. 2015;32:333-336.
- Cribier B, Lieber-Mbomeyo A, Lipsker D. Clinical and histological study of a case of facial Afro-Caribbean childhood eruption (FACE) [in French][published online July 23, 2008]. Ann Dermatol Venerol. 2008;135:663-667.
- Boeck K, Abeck D, Werfel S, et al. Perioral dermatitis in children—clinical presentation, pathogenesis-related factors and response to topical metronidazole. Dermatology. 1997;195:235-238.
- Urbatsch AJ, Frieden I, Williams ML, et al. Extrafacial and generalized granulomatous periorificial dermatitis. Arch Dermatol. 2002;138:1354-1358.
- Kroshinsky D, Glick SA. Pediatric rosacea. Dermatol Ther. 2006;19:196-201.
- Ramelet AA, Delacrétaz J. Histopathologic study of perioral dermatitis [in French]. Dermatologica. 1981;163:361-369.
- Ljubojevi´c S, Lipozenci´c J, Turci´c P. Perioral dermatitis. Acta Dermatovenerol Croat. 2008;16:96-100.
- Nichols RL, Florman S. Clinical presentations of soft-tissue infections and surgical site infections. Clin Infect Dis. 2001;33(suppl 2):S84-S93.
- Lin RL, Szepietowski JC, Schwartz RA. Tinea faciei, an often deceptive facial eruption. Int J Dermatol. 2004;43:437-440.
Perioral dermatitis is an acneform eruption presenting with erythematous papules, vesicles, and rarely pustules clustered around the orifices of the face. 1 Lesions may be found near the eyes, mouth, and nose but typically spare the vermilion border of the lips. 2 Nguyen and Eichenfield 3 preferred the term periorificial dermatitis (POD), which has since been adopted by others. 4 Patients may report pruritus, but there generally are no systemic symptoms unless patients have comorbid conditions such as atopic dermatitis. 5 Although this condition has been well examined in the literature on adults, data in the pediatric population are far more limited, consisting of case series and retrospective chart reviews. In 1979, Wilkinson et al 6 published a study of more than 200 patients with perioral dermatitis, but only 15 patients younger than 12 years were included.
Etiology
Although the exact pathogenesis of POD is unknown, a common denominator among many patients is prior exposure to topical corticosteroids.3,7-9 Periorificial dermatitis also has been linked to the use of systemic corticosteroids in pediatric patients.10 The exact relationship between steroid use and dermatitis is unknown; it may be related to a change in the flora of hair follicles and in particular an association with fusiform bacteria–rich conditions.11 Aside from steroid exposure, POD has been associated with the use of physical sunscreen in pediatric patients with dry skin,12 rosin in chewing gum,13 and inhaled corticosteroids in those with asthma.14 In one case, a 15-year-old adolescent girl developed POD and swelling of the lips after 2 years of playing a flute made of cocus wood.15,16
Epidemiology
Comorbidities and Family History
Goel et al17 (N=222) reported the following comorbidities associated with pediatric POD: atopic dermatitis (29.3%), asthma (14.9%), and allergies (9.9%). Steroid exposure was noted in 58.1% of patients.17 Similarly, Nguyen and Eichenfield3 (N=79) found that the most common comorbidities were atopic dermatitis (14%), keratosis pilaris (14%), viral infections (14%), acne (10%), and seborrheic dermatitis (10%). Family history of atopy was noted in 55% of patients and family history of rosacea was noted in 3%. In a case series of 11 pediatric patients, 3 (27%) had keratosis pilaris, 7 (64%) had a family history of atopy, and 2 (18%) had a family history of rosacea.8 Weston and Morelli9 found a much higher incidence of familial rosacea (20%) in 106 children with steroid rosacea.
Clinical Presentation
Periorificial dermatitis generally presents with small, pink- to flesh-colored papules in a perioral, periocular, and perinasal distribution. Although many patients are white, a particularly prominent variant has been noted in black children with papules that may be hyperpigmented.18 In a 2006 chart review in 79 pediatric POD patients aged 6 months to 18 years, Nguyen and Eichenfield3 reported that 92% (73/79) of patients presented for a facial rash with an average duration ranging from 2 weeks to 4 years.
Boeck et al19 described 7 pediatric patients with perioral dermatitis. Six (86%) patients had perioral lesions, and 6 (86%) had previously been treated with moderate- to high-potency topical corticosteroids. Skin prick tests were negative in 6 (86%) patients.19 In one case report, a 6-year-old boy did not present with the classic acneform lesions but rather sharply demarcated eczematous patches around the eyes, nose, and mouth. The rash began to fade after 2 weeks of using metronidazole gel 1%, and after 4 months he was only left with mild hyperpigmentation.4
Periorificial dermatitis was once thought to be a juvenile form of rosacea.5 In 1972, Savin et al8 described 11 pediatric patients with “rosacea-like” facial flushing, papules, pustules, and scaling over the cheeks, forehead, and chin. In some patients, the eyelids also were involved. At least 8 patients had been using potent topical corticosteroids and had noticed exacerbation of their skin lesions after stopping therapy.8
Variants of POD
Several other variants of POD have been described in pediatric patients including childhood granulomatous periorificial dermatitis (CGPD)(also known as facial Afro-Caribbean [childhood] eruption) and lupus miliaris disseminatus faciei. Childhood granulomatous periorificial dermatitis presents in prepubertal children as dome-shaped, red to yellow-brown, monomorphous papules around the eyes, nose, and mouth; there are no systemic findings.20,21 It occurs equally in males and females and is more commonly seen in dark-skinned patients. Childhood granulomatous periorificial dermatitis usually resolves within a few months to years but may be associated with blepharitis or conjunctivitis.20 Urbatsch et al20 analyzed extrafacial lesions in 8 patients (aged 2–12 years) with CGPD. Lesions were found on the trunk (38% [3/8]), neck (25% [2/8]), ears (25% [2/8]), extremities (50% [4/8]), labia majora (38% [3/8]), and abdomen (13% [1/8]). In addition, 2 (25% [2/8]) patients had blepharitis.20
Lupus miliaris disseminatus faciei, which occurs in adolescents and adults, commonly involves the eyelids and central areas of the face such as the nose and upper lips. Patients typically present with erythematous or flesh-colored papules.1
Diagnosis
Diagnosis of POD is made clinically based on the observation of papules (and sometimes pustules) around the orifices of the face, sparing the vermilion border, together with a lack of comedones.17 Laboratory tests are not useful.5 Biopsies rarely are performed, and the results mimic those of rosacea, demonstrating a perifollicular lymphohistiocytic infiltrate, epithelioid cells, and occasionally giant cells.5,22,23 Early papular lesions can show mild acanthosis, epidermal edema, and parakeratosis.23 Biopsies in patients with CGPD reveal noncaseating perifollicular granulomas.20
Treatment and Clinical Outcome
Although topical corticosteroids can improve facial lesions in pediatric POD, the eruption often rebounds when therapy is discontinued.1 One therapy frequently used in adults is oral tetracyclines; however, these agents must not be used in patients younger than 9 years due to potential dental staining.4 The standards are either topical metronidazole twice daily with clearance in 3 to 8 weeks or oral erythromycin.7
In the review conducted by Goel et al,17 treatment included azithromycin (44.6%), topical metronidazole (42.3%), sodium sulfacetamide lotion (35.6%), oral antibiotic monotherapy (15.3%), topical agent monotherapy (44.6%), and combined oral and topical agent therapy (40.1%). Of those patients who presented for a follow-up visit (59%), 72% of cases resolved and 10.7% showed some improvement. For those patients who returned for follow-up, the average duration until symptom resolution was approximately 4 months. The most common side effects were pigmentation changes (1.8%), worsening of symptoms (1.8%), gastrointestinal upset (0.9%), irritant dermatitis (0.9%), and xerosis (0.5%).17
Changes were made to the treatment plans for 16 patients, most often due to inadequate treatment response.17 Five patients treated with sodium sulfacetamide lotion also were started on oral azithromycin. Four patients treated with oral antibiotics were given a topical agent (metronidazole or sodium sulfacetamide lotion). Other modifications included replacing sodium sulfacetamide lotion with topical metronidazole and an oral antibiotic (azithromycin or doxycycline, n=3), adjusting the doses of oral or topical medications (n=2), adding tacrolimus (n=1), and replacing topical metronidazole with sodium sulfacetamide lotion (n=1). Of the patients who underwent a change in treatment plan, 5 experienced symptom recurrence, 4 had mild improvement, and 1 patient had no improvement. Six patients were lost to follow-up.17
In the study conducted by Nguyen and Eichenfield,3 follow-up visits occurred approximately 3 months after the first visit.
In the case series by Boeck et al,19 all patients were started on metronidazole gel 1% applied once daily for the first week, and then twice daily until the lesions resolved. All patients showed improvement after 4 to 6 weeks, and eventually the disease cleared between 3 and 6 months. All patients were still symptom free during a 2-year observation period.19
Manders and Lucky7 described 14 patients with POD (aged 9 months to 6.5 years). Eight patients used only metronidazole gel 0.75%, while 5 used the gel in combination with topical corticosteroids (21% [3/14]), oral erythromycin (7% [1/14]), or topical erythromycin (7% [1/14]); 1 patient remained on hydrocortisone 1% and cleared. Patients responded well within 1 to 8 weeks and were symptom free for up to 16 months. Mid- to high-potency steroids were discontinued in all patients.7
In some pediatric patients with CGPD, recovery occurs faster with the use of oral macrolides or tetracyclines, either alone or in combination with topical antibiotics or sulfur-based lotions.20 Extrafacial lesions associated with CGPD do not appear to negatively impact treatment response or duration of disease. In the review conducted by Urbatsch et al,20 7 of 8 (88%) CGPD patients with extrafacial lesions were treated with oral agents including erythromycin, hydroxychloroquine, cyclosporine, minocycline, and azithromycin. Most of these patients also were using topical agents such as triamcinolone acetonide, desonide, metronidazole, and erythromycin. The time to resolution ranged from several weeks to 6 months.20
Weston and Morelli9 described a treatment regimen for steroid rosacea. The study included data on 106 children (60 females, 46 males) who had been exposed to mostly class 7 low-potency agents. All patients were advised to immediately stop topical steroid therapy without gradual withdrawal and to begin oral erythromycin stearate 30 mg/kg daily in 2 doses per day for 4 weeks. Patients who were unable to tolerate erythromycin were advised to use topical clindamycin phosphate twice daily for 4 weeks (n=6). Eighty-six percent of patients showed resolution within 4 weeks, and 100% showed clearance by 8 weeks. Twenty-two percent of patients had clearance within 3 weeks. There was no difference in the duration until resolution for those who had used oral or topical antibiotics.9 A different study suggested that low-potency topical steroids can be used to control inflammation when weaning patients off of strong steroids.5
Differential Diagnosis
The differential diagnosis should include acne vulgaris, allergic contact dermatitis, irritant contact dermatitis, seborrheic dermatitis, impetigo, dermatophyte infection, rosacea, and angiofibromas.4
Acne vulgaris commonly is found in older adolescents, and unlike POD, it will present with open or closed comedones.2 In patients aged 1 to 7 years, acne is a reason to consider endocrine evaluation. Allergic contact dermatitis is extremely pruritic, and the lesions often are papulovesicular with active weeping or crusting. Patients with irritant contact dermatitis often report burning and pain, and papules and pustules typically are absent. A thorough history can help rule out allergic or irritant contact dermatitis. Seborrheic dermatitis presents with erythema and scaling of the scalp, eyebrows, and nasolabial folds; it tends to spare the perioral regions and also lacks papules.2 The lesions of impetigo typically have a yellow-brown exudate, which forms a honey-colored crust.24 Tinea faciei, unlike the other tinea infections, can have an extremely variable presentation. Lesions usually begin as scaly macules that develop raised borders with central hypopigmentation, but papules, vesicles, and crusts can be seen.25 Potassium hydroxide
Conclusion
Diagnosis of POD is clinical and rests upon the finding of erythematous papules on the face near the eyes, mouth, and nose. Extrafacial lesions also have been described, particularly in pediatric patients with CGPD. Many patients will report a history of atopic dermatitis and asthma. Therapy for POD includes both topical and systemic agents. For those with mild disease, topical metronidazole commonly is used. For patients requiring oral antibiotics, tetracyclines or macrolides can be prescribed based on the age of the patient. Many pediatric patients who begin with both oral and topical agents can later be maintained on topical therapy, sometimes with a low-dose oral antibiotic. Periorificial dermatitis has an excellent prognosis and most pediatric patients show marked improvement within weeks to months.
Perioral dermatitis is an acneform eruption presenting with erythematous papules, vesicles, and rarely pustules clustered around the orifices of the face. 1 Lesions may be found near the eyes, mouth, and nose but typically spare the vermilion border of the lips. 2 Nguyen and Eichenfield 3 preferred the term periorificial dermatitis (POD), which has since been adopted by others. 4 Patients may report pruritus, but there generally are no systemic symptoms unless patients have comorbid conditions such as atopic dermatitis. 5 Although this condition has been well examined in the literature on adults, data in the pediatric population are far more limited, consisting of case series and retrospective chart reviews. In 1979, Wilkinson et al 6 published a study of more than 200 patients with perioral dermatitis, but only 15 patients younger than 12 years were included.
Etiology
Although the exact pathogenesis of POD is unknown, a common denominator among many patients is prior exposure to topical corticosteroids.3,7-9 Periorificial dermatitis also has been linked to the use of systemic corticosteroids in pediatric patients.10 The exact relationship between steroid use and dermatitis is unknown; it may be related to a change in the flora of hair follicles and in particular an association with fusiform bacteria–rich conditions.11 Aside from steroid exposure, POD has been associated with the use of physical sunscreen in pediatric patients with dry skin,12 rosin in chewing gum,13 and inhaled corticosteroids in those with asthma.14 In one case, a 15-year-old adolescent girl developed POD and swelling of the lips after 2 years of playing a flute made of cocus wood.15,16
Epidemiology
Comorbidities and Family History
Goel et al17 (N=222) reported the following comorbidities associated with pediatric POD: atopic dermatitis (29.3%), asthma (14.9%), and allergies (9.9%). Steroid exposure was noted in 58.1% of patients.17 Similarly, Nguyen and Eichenfield3 (N=79) found that the most common comorbidities were atopic dermatitis (14%), keratosis pilaris (14%), viral infections (14%), acne (10%), and seborrheic dermatitis (10%). Family history of atopy was noted in 55% of patients and family history of rosacea was noted in 3%. In a case series of 11 pediatric patients, 3 (27%) had keratosis pilaris, 7 (64%) had a family history of atopy, and 2 (18%) had a family history of rosacea.8 Weston and Morelli9 found a much higher incidence of familial rosacea (20%) in 106 children with steroid rosacea.
Clinical Presentation
Periorificial dermatitis generally presents with small, pink- to flesh-colored papules in a perioral, periocular, and perinasal distribution. Although many patients are white, a particularly prominent variant has been noted in black children with papules that may be hyperpigmented.18 In a 2006 chart review in 79 pediatric POD patients aged 6 months to 18 years, Nguyen and Eichenfield3 reported that 92% (73/79) of patients presented for a facial rash with an average duration ranging from 2 weeks to 4 years.
Boeck et al19 described 7 pediatric patients with perioral dermatitis. Six (86%) patients had perioral lesions, and 6 (86%) had previously been treated with moderate- to high-potency topical corticosteroids. Skin prick tests were negative in 6 (86%) patients.19 In one case report, a 6-year-old boy did not present with the classic acneform lesions but rather sharply demarcated eczematous patches around the eyes, nose, and mouth. The rash began to fade after 2 weeks of using metronidazole gel 1%, and after 4 months he was only left with mild hyperpigmentation.4
Periorificial dermatitis was once thought to be a juvenile form of rosacea.5 In 1972, Savin et al8 described 11 pediatric patients with “rosacea-like” facial flushing, papules, pustules, and scaling over the cheeks, forehead, and chin. In some patients, the eyelids also were involved. At least 8 patients had been using potent topical corticosteroids and had noticed exacerbation of their skin lesions after stopping therapy.8
Variants of POD
Several other variants of POD have been described in pediatric patients including childhood granulomatous periorificial dermatitis (CGPD)(also known as facial Afro-Caribbean [childhood] eruption) and lupus miliaris disseminatus faciei. Childhood granulomatous periorificial dermatitis presents in prepubertal children as dome-shaped, red to yellow-brown, monomorphous papules around the eyes, nose, and mouth; there are no systemic findings.20,21 It occurs equally in males and females and is more commonly seen in dark-skinned patients. Childhood granulomatous periorificial dermatitis usually resolves within a few months to years but may be associated with blepharitis or conjunctivitis.20 Urbatsch et al20 analyzed extrafacial lesions in 8 patients (aged 2–12 years) with CGPD. Lesions were found on the trunk (38% [3/8]), neck (25% [2/8]), ears (25% [2/8]), extremities (50% [4/8]), labia majora (38% [3/8]), and abdomen (13% [1/8]). In addition, 2 (25% [2/8]) patients had blepharitis.20
Lupus miliaris disseminatus faciei, which occurs in adolescents and adults, commonly involves the eyelids and central areas of the face such as the nose and upper lips. Patients typically present with erythematous or flesh-colored papules.1
Diagnosis
Diagnosis of POD is made clinically based on the observation of papules (and sometimes pustules) around the orifices of the face, sparing the vermilion border, together with a lack of comedones.17 Laboratory tests are not useful.5 Biopsies rarely are performed, and the results mimic those of rosacea, demonstrating a perifollicular lymphohistiocytic infiltrate, epithelioid cells, and occasionally giant cells.5,22,23 Early papular lesions can show mild acanthosis, epidermal edema, and parakeratosis.23 Biopsies in patients with CGPD reveal noncaseating perifollicular granulomas.20
Treatment and Clinical Outcome
Although topical corticosteroids can improve facial lesions in pediatric POD, the eruption often rebounds when therapy is discontinued.1 One therapy frequently used in adults is oral tetracyclines; however, these agents must not be used in patients younger than 9 years due to potential dental staining.4 The standards are either topical metronidazole twice daily with clearance in 3 to 8 weeks or oral erythromycin.7
In the review conducted by Goel et al,17 treatment included azithromycin (44.6%), topical metronidazole (42.3%), sodium sulfacetamide lotion (35.6%), oral antibiotic monotherapy (15.3%), topical agent monotherapy (44.6%), and combined oral and topical agent therapy (40.1%). Of those patients who presented for a follow-up visit (59%), 72% of cases resolved and 10.7% showed some improvement. For those patients who returned for follow-up, the average duration until symptom resolution was approximately 4 months. The most common side effects were pigmentation changes (1.8%), worsening of symptoms (1.8%), gastrointestinal upset (0.9%), irritant dermatitis (0.9%), and xerosis (0.5%).17
Changes were made to the treatment plans for 16 patients, most often due to inadequate treatment response.17 Five patients treated with sodium sulfacetamide lotion also were started on oral azithromycin. Four patients treated with oral antibiotics were given a topical agent (metronidazole or sodium sulfacetamide lotion). Other modifications included replacing sodium sulfacetamide lotion with topical metronidazole and an oral antibiotic (azithromycin or doxycycline, n=3), adjusting the doses of oral or topical medications (n=2), adding tacrolimus (n=1), and replacing topical metronidazole with sodium sulfacetamide lotion (n=1). Of the patients who underwent a change in treatment plan, 5 experienced symptom recurrence, 4 had mild improvement, and 1 patient had no improvement. Six patients were lost to follow-up.17
In the study conducted by Nguyen and Eichenfield,3 follow-up visits occurred approximately 3 months after the first visit.
In the case series by Boeck et al,19 all patients were started on metronidazole gel 1% applied once daily for the first week, and then twice daily until the lesions resolved. All patients showed improvement after 4 to 6 weeks, and eventually the disease cleared between 3 and 6 months. All patients were still symptom free during a 2-year observation period.19
Manders and Lucky7 described 14 patients with POD (aged 9 months to 6.5 years). Eight patients used only metronidazole gel 0.75%, while 5 used the gel in combination with topical corticosteroids (21% [3/14]), oral erythromycin (7% [1/14]), or topical erythromycin (7% [1/14]); 1 patient remained on hydrocortisone 1% and cleared. Patients responded well within 1 to 8 weeks and were symptom free for up to 16 months. Mid- to high-potency steroids were discontinued in all patients.7
In some pediatric patients with CGPD, recovery occurs faster with the use of oral macrolides or tetracyclines, either alone or in combination with topical antibiotics or sulfur-based lotions.20 Extrafacial lesions associated with CGPD do not appear to negatively impact treatment response or duration of disease. In the review conducted by Urbatsch et al,20 7 of 8 (88%) CGPD patients with extrafacial lesions were treated with oral agents including erythromycin, hydroxychloroquine, cyclosporine, minocycline, and azithromycin. Most of these patients also were using topical agents such as triamcinolone acetonide, desonide, metronidazole, and erythromycin. The time to resolution ranged from several weeks to 6 months.20
Weston and Morelli9 described a treatment regimen for steroid rosacea. The study included data on 106 children (60 females, 46 males) who had been exposed to mostly class 7 low-potency agents. All patients were advised to immediately stop topical steroid therapy without gradual withdrawal and to begin oral erythromycin stearate 30 mg/kg daily in 2 doses per day for 4 weeks. Patients who were unable to tolerate erythromycin were advised to use topical clindamycin phosphate twice daily for 4 weeks (n=6). Eighty-six percent of patients showed resolution within 4 weeks, and 100% showed clearance by 8 weeks. Twenty-two percent of patients had clearance within 3 weeks. There was no difference in the duration until resolution for those who had used oral or topical antibiotics.9 A different study suggested that low-potency topical steroids can be used to control inflammation when weaning patients off of strong steroids.5
Differential Diagnosis
The differential diagnosis should include acne vulgaris, allergic contact dermatitis, irritant contact dermatitis, seborrheic dermatitis, impetigo, dermatophyte infection, rosacea, and angiofibromas.4
Acne vulgaris commonly is found in older adolescents, and unlike POD, it will present with open or closed comedones.2 In patients aged 1 to 7 years, acne is a reason to consider endocrine evaluation. Allergic contact dermatitis is extremely pruritic, and the lesions often are papulovesicular with active weeping or crusting. Patients with irritant contact dermatitis often report burning and pain, and papules and pustules typically are absent. A thorough history can help rule out allergic or irritant contact dermatitis. Seborrheic dermatitis presents with erythema and scaling of the scalp, eyebrows, and nasolabial folds; it tends to spare the perioral regions and also lacks papules.2 The lesions of impetigo typically have a yellow-brown exudate, which forms a honey-colored crust.24 Tinea faciei, unlike the other tinea infections, can have an extremely variable presentation. Lesions usually begin as scaly macules that develop raised borders with central hypopigmentation, but papules, vesicles, and crusts can be seen.25 Potassium hydroxide
Conclusion
Diagnosis of POD is clinical and rests upon the finding of erythematous papules on the face near the eyes, mouth, and nose. Extrafacial lesions also have been described, particularly in pediatric patients with CGPD. Many patients will report a history of atopic dermatitis and asthma. Therapy for POD includes both topical and systemic agents. For those with mild disease, topical metronidazole commonly is used. For patients requiring oral antibiotics, tetracyclines or macrolides can be prescribed based on the age of the patient. Many pediatric patients who begin with both oral and topical agents can later be maintained on topical therapy, sometimes with a low-dose oral antibiotic. Periorificial dermatitis has an excellent prognosis and most pediatric patients show marked improvement within weeks to months.
- Tempark T, Shwayder TA. Perioral dermatitis: a review of the condition with special attention to treatment options. Am J Clin Dermatol. 2014;15:101-113.
- McFarland SL, Polcari IC. Morphology-based diagnosis of acneiform eruptions. Pediatr Ann. 2015;44:E188-E193.
- Nguyen V, Eichenfield LF. Periorificial dermatitis in children and adolescents. J Am Acad Dermatol. 2006;55:781-785.
- Kihiczak GG, Cruz MA, Schwartz RA. Periorificial dermatitis in children: an update and description of a child with striking features. Int J Dermatol. 2009;48:304-306.
- Laude TA, Salvemini JN. Perioral dermatitis in children. Sem Cutan Med Surg. 1999;18:206-209.
- Wilkinson DS, Kirton V, Wilkinson JD. Perioral dermatitis: a 12-year review. Br J Dermatol. 1979;101:245-257.
- Manders SM, Lucky AW. Perioral dermatitis in childhood. J Am Acad Dermatol. 1992;27(5 pt 1):688-692.
- Savin JA, Alexander S, Marks R. A rosacea-like eruption of children. Br J Dermatol. 1972;87:425-429.
- Weston WL, Morelli JG. Steroid rosacea in prepubertal children. Arch Pediatr Adolesc Med. 2000;154:62-64.
- Clementson B, Smidt AC. Periorificial dermatitis due to systemic corticosteroids in children: report of two cases. Pediatr Dermatol. 2012;29:331-332.
- Takiwaki H, Tsuda H, Arase S, et al. Differences between intrafollicular microorganism profiles in perioral and seborrhoeic dermatitis. Clin Exp Dermatol. 2003;28:531-534.
- Abeck D, Geisenfelder B, Brandt O. Physical sunscreens with high sun protection factor may cause perioral dermatitis in children. J Dtsch Dermatol Ges. 2009;7:701-703.
- Satyawan I, Oranje AP, van Joost T. Perioral dermatitis in a child due to rosin in chewing gum. Contact Dermatitis. 1990;22:182-183.
- Dubus JC, Marguet C, Deschildre A, et al. Local side-effects of inhaled corticosteroids in asthmatic children: influence of drug, dose, age, and device. Allergy. 2001;56:944-948.
- Hausen BM, Bruhn G, Koenig WA. New hydroxyisoflavans as contact sensitizers in cocus wood Brya ebenus DC (Fabaceae). Contact Dermatitis. 1991;25:149-155.
- Dirschka T, Weber K, Tronnier H. Topical cosmetics and perioral dermatitis. J Dtsch Dermatol Ges. 2004;2:194-199.
- Goel NS, Burkhart CN, Morrell DS. Pediatric periorificial dermatitis: clinical course and treatment outcomes in 222 patients. Pediatr Dermatol. 2015;32:333-336.
- Cribier B, Lieber-Mbomeyo A, Lipsker D. Clinical and histological study of a case of facial Afro-Caribbean childhood eruption (FACE) [in French][published online July 23, 2008]. Ann Dermatol Venerol. 2008;135:663-667.
- Boeck K, Abeck D, Werfel S, et al. Perioral dermatitis in children—clinical presentation, pathogenesis-related factors and response to topical metronidazole. Dermatology. 1997;195:235-238.
- Urbatsch AJ, Frieden I, Williams ML, et al. Extrafacial and generalized granulomatous periorificial dermatitis. Arch Dermatol. 2002;138:1354-1358.
- Kroshinsky D, Glick SA. Pediatric rosacea. Dermatol Ther. 2006;19:196-201.
- Ramelet AA, Delacrétaz J. Histopathologic study of perioral dermatitis [in French]. Dermatologica. 1981;163:361-369.
- Ljubojevi´c S, Lipozenci´c J, Turci´c P. Perioral dermatitis. Acta Dermatovenerol Croat. 2008;16:96-100.
- Nichols RL, Florman S. Clinical presentations of soft-tissue infections and surgical site infections. Clin Infect Dis. 2001;33(suppl 2):S84-S93.
- Lin RL, Szepietowski JC, Schwartz RA. Tinea faciei, an often deceptive facial eruption. Int J Dermatol. 2004;43:437-440.
- Tempark T, Shwayder TA. Perioral dermatitis: a review of the condition with special attention to treatment options. Am J Clin Dermatol. 2014;15:101-113.
- McFarland SL, Polcari IC. Morphology-based diagnosis of acneiform eruptions. Pediatr Ann. 2015;44:E188-E193.
- Nguyen V, Eichenfield LF. Periorificial dermatitis in children and adolescents. J Am Acad Dermatol. 2006;55:781-785.
- Kihiczak GG, Cruz MA, Schwartz RA. Periorificial dermatitis in children: an update and description of a child with striking features. Int J Dermatol. 2009;48:304-306.
- Laude TA, Salvemini JN. Perioral dermatitis in children. Sem Cutan Med Surg. 1999;18:206-209.
- Wilkinson DS, Kirton V, Wilkinson JD. Perioral dermatitis: a 12-year review. Br J Dermatol. 1979;101:245-257.
- Manders SM, Lucky AW. Perioral dermatitis in childhood. J Am Acad Dermatol. 1992;27(5 pt 1):688-692.
- Savin JA, Alexander S, Marks R. A rosacea-like eruption of children. Br J Dermatol. 1972;87:425-429.
- Weston WL, Morelli JG. Steroid rosacea in prepubertal children. Arch Pediatr Adolesc Med. 2000;154:62-64.
- Clementson B, Smidt AC. Periorificial dermatitis due to systemic corticosteroids in children: report of two cases. Pediatr Dermatol. 2012;29:331-332.
- Takiwaki H, Tsuda H, Arase S, et al. Differences between intrafollicular microorganism profiles in perioral and seborrhoeic dermatitis. Clin Exp Dermatol. 2003;28:531-534.
- Abeck D, Geisenfelder B, Brandt O. Physical sunscreens with high sun protection factor may cause perioral dermatitis in children. J Dtsch Dermatol Ges. 2009;7:701-703.
- Satyawan I, Oranje AP, van Joost T. Perioral dermatitis in a child due to rosin in chewing gum. Contact Dermatitis. 1990;22:182-183.
- Dubus JC, Marguet C, Deschildre A, et al. Local side-effects of inhaled corticosteroids in asthmatic children: influence of drug, dose, age, and device. Allergy. 2001;56:944-948.
- Hausen BM, Bruhn G, Koenig WA. New hydroxyisoflavans as contact sensitizers in cocus wood Brya ebenus DC (Fabaceae). Contact Dermatitis. 1991;25:149-155.
- Dirschka T, Weber K, Tronnier H. Topical cosmetics and perioral dermatitis. J Dtsch Dermatol Ges. 2004;2:194-199.
- Goel NS, Burkhart CN, Morrell DS. Pediatric periorificial dermatitis: clinical course and treatment outcomes in 222 patients. Pediatr Dermatol. 2015;32:333-336.
- Cribier B, Lieber-Mbomeyo A, Lipsker D. Clinical and histological study of a case of facial Afro-Caribbean childhood eruption (FACE) [in French][published online July 23, 2008]. Ann Dermatol Venerol. 2008;135:663-667.
- Boeck K, Abeck D, Werfel S, et al. Perioral dermatitis in children—clinical presentation, pathogenesis-related factors and response to topical metronidazole. Dermatology. 1997;195:235-238.
- Urbatsch AJ, Frieden I, Williams ML, et al. Extrafacial and generalized granulomatous periorificial dermatitis. Arch Dermatol. 2002;138:1354-1358.
- Kroshinsky D, Glick SA. Pediatric rosacea. Dermatol Ther. 2006;19:196-201.
- Ramelet AA, Delacrétaz J. Histopathologic study of perioral dermatitis [in French]. Dermatologica. 1981;163:361-369.
- Ljubojevi´c S, Lipozenci´c J, Turci´c P. Perioral dermatitis. Acta Dermatovenerol Croat. 2008;16:96-100.
- Nichols RL, Florman S. Clinical presentations of soft-tissue infections and surgical site infections. Clin Infect Dis. 2001;33(suppl 2):S84-S93.
- Lin RL, Szepietowski JC, Schwartz RA. Tinea faciei, an often deceptive facial eruption. Int J Dermatol. 2004;43:437-440.
Practice Points
- Periorificial dermatitis (POD) affects young children and presents as flesh-colored papules around the mouth, nose, and even groin.
- Periorificial dermatitis has been associated with prior use of topical or inhaled steroids.
- Children with POD can be treated with oral erythromycin.
Topical 5-Fluorouracil Made Easy?
What is the recent research behind 5-fluorouracil cream 5% combined with calcipotriol ointment 0.005% for actinic keratoses?
Cunningham et al published a randomized double-blind study in which 131 patients with actinic keratoses (AKs) were assigned to either 5-fluorouracil (5-FU) cream 5% combined with calcipotriol (calcipotriene) ointment 0.005% twice daily to the face, scalp, and arms for 4 days, or 5-FU 5% combined with petrolatum applied in the same fashion. There was an 87.8% versus 26.3% mean reduction in the number of AKs and less severe pain, crusting, and ulceration in the study cohort compared to the 5-FU plus petrolatum group.
The same study also investigated immune parameters in these patients and found that the study group preferentially displayed activated thymic stromal lymphopoietin and a CD4 T cell-mediated reaction, among other effects. In prior studies, thymic stromal lymphopoietin has been shown to be upregulated in barrier-defective skin, displays antitumor activity, and is enhanced by topical calcipotriol application based on its original indication for psoriasis.
How do these study results impact patient care?
In a perfect world, every patient could tolerate and afford chemopreventative measures such as 5-FU cream, apply it diffusely to sun-exposed skin, and experience no severe irritant reactions and/or social pariah status. We all know that this product is effective, and we all overprepare patients to use it, knowing that they will call our offices panicked and fearful that they are allergic to or are becoming infected by this cream.
Although further study clearly is needed to determine the optimal application amount, duration of use, and vehicle mix, this new compound utilizing 2 topicals that are familiar to us--5-FU cream approved for AKs and early squamous cell skin cancers and calcipotriol ointment (though available only in cream in the United States currently) for psoriasis--is an encouraging step. Home therapy for AKs and possibly early nonmelanoma skin cancers that is more tolerable, of shorter duration, and in turn more effective than the current options would lessen the burden of treating these lesions surgically or rescheduling 5-FU patients often for irritation reaction education.
How do patients respond to this regimen?
In my own anecdotal experience, this regimen has been well received by patients and often is covered by most insurances when written as 2 separate prescriptions (both in cream vehicle). They still report some irritation, but I prefer to utilize it segmentally instead of treating all sun-exposed areas at once (ie, treat one side of the face/scalp twice daily for 4 days, then the other, or even divide it into smaller segments once the prior segment has healed). This combination, in addition to, for example, adding nicotinamide 500 mg twice daily to a patient's skin cancer chemopreventative sequence, is in my opinion a novel but safe, effective, and well-tolerated field therapy recommendation.
American Academy of Dermatology Actinic Keratosis Overview
American Academy of Family Physicians Actinic Keratoses Information
Suggested Readings
- Cunningham TJ, Tabacchi M, Eliane JP, et al. Randomized trial of calcipotriol combined with 5-fluorouracil for skin cancer precursor immunotherapy. J Clin Invest. 2017;127:106-116.
- Demehri S, Turkoz A, Manivasagam S, et al. Elevated epidermal thymic stromal lymphopoietin levels establish an antitumor environment in the skin. Cancer Cell. 2012;22:494-505.
- Rosamilia LL. Three Cheers for B3? Cutis. July 7, 2015. http://www.mdedge.com/cutis/article/101102/nonmelanoma-skin-cancer/three-cheers-b3. Accessed November 20, 2017.
- Sato-Deguchi E, Imafuku S, Chou B, et al. Topical vitamin D(3) analogues induce thymic stromal lymphopoietin and cathelicidin in psoriatic skin lesions. Br J Dermatol. 2012;167:77-84.
What is the recent research behind 5-fluorouracil cream 5% combined with calcipotriol ointment 0.005% for actinic keratoses?
Cunningham et al published a randomized double-blind study in which 131 patients with actinic keratoses (AKs) were assigned to either 5-fluorouracil (5-FU) cream 5% combined with calcipotriol (calcipotriene) ointment 0.005% twice daily to the face, scalp, and arms for 4 days, or 5-FU 5% combined with petrolatum applied in the same fashion. There was an 87.8% versus 26.3% mean reduction in the number of AKs and less severe pain, crusting, and ulceration in the study cohort compared to the 5-FU plus petrolatum group.
The same study also investigated immune parameters in these patients and found that the study group preferentially displayed activated thymic stromal lymphopoietin and a CD4 T cell-mediated reaction, among other effects. In prior studies, thymic stromal lymphopoietin has been shown to be upregulated in barrier-defective skin, displays antitumor activity, and is enhanced by topical calcipotriol application based on its original indication for psoriasis.
How do these study results impact patient care?
In a perfect world, every patient could tolerate and afford chemopreventative measures such as 5-FU cream, apply it diffusely to sun-exposed skin, and experience no severe irritant reactions and/or social pariah status. We all know that this product is effective, and we all overprepare patients to use it, knowing that they will call our offices panicked and fearful that they are allergic to or are becoming infected by this cream.
Although further study clearly is needed to determine the optimal application amount, duration of use, and vehicle mix, this new compound utilizing 2 topicals that are familiar to us--5-FU cream approved for AKs and early squamous cell skin cancers and calcipotriol ointment (though available only in cream in the United States currently) for psoriasis--is an encouraging step. Home therapy for AKs and possibly early nonmelanoma skin cancers that is more tolerable, of shorter duration, and in turn more effective than the current options would lessen the burden of treating these lesions surgically or rescheduling 5-FU patients often for irritation reaction education.
How do patients respond to this regimen?
In my own anecdotal experience, this regimen has been well received by patients and often is covered by most insurances when written as 2 separate prescriptions (both in cream vehicle). They still report some irritation, but I prefer to utilize it segmentally instead of treating all sun-exposed areas at once (ie, treat one side of the face/scalp twice daily for 4 days, then the other, or even divide it into smaller segments once the prior segment has healed). This combination, in addition to, for example, adding nicotinamide 500 mg twice daily to a patient's skin cancer chemopreventative sequence, is in my opinion a novel but safe, effective, and well-tolerated field therapy recommendation.
American Academy of Dermatology Actinic Keratosis Overview
American Academy of Family Physicians Actinic Keratoses Information
Suggested Readings
- Cunningham TJ, Tabacchi M, Eliane JP, et al. Randomized trial of calcipotriol combined with 5-fluorouracil for skin cancer precursor immunotherapy. J Clin Invest. 2017;127:106-116.
- Demehri S, Turkoz A, Manivasagam S, et al. Elevated epidermal thymic stromal lymphopoietin levels establish an antitumor environment in the skin. Cancer Cell. 2012;22:494-505.
- Rosamilia LL. Three Cheers for B3? Cutis. July 7, 2015. http://www.mdedge.com/cutis/article/101102/nonmelanoma-skin-cancer/three-cheers-b3. Accessed November 20, 2017.
- Sato-Deguchi E, Imafuku S, Chou B, et al. Topical vitamin D(3) analogues induce thymic stromal lymphopoietin and cathelicidin in psoriatic skin lesions. Br J Dermatol. 2012;167:77-84.
What is the recent research behind 5-fluorouracil cream 5% combined with calcipotriol ointment 0.005% for actinic keratoses?
Cunningham et al published a randomized double-blind study in which 131 patients with actinic keratoses (AKs) were assigned to either 5-fluorouracil (5-FU) cream 5% combined with calcipotriol (calcipotriene) ointment 0.005% twice daily to the face, scalp, and arms for 4 days, or 5-FU 5% combined with petrolatum applied in the same fashion. There was an 87.8% versus 26.3% mean reduction in the number of AKs and less severe pain, crusting, and ulceration in the study cohort compared to the 5-FU plus petrolatum group.
The same study also investigated immune parameters in these patients and found that the study group preferentially displayed activated thymic stromal lymphopoietin and a CD4 T cell-mediated reaction, among other effects. In prior studies, thymic stromal lymphopoietin has been shown to be upregulated in barrier-defective skin, displays antitumor activity, and is enhanced by topical calcipotriol application based on its original indication for psoriasis.
How do these study results impact patient care?
In a perfect world, every patient could tolerate and afford chemopreventative measures such as 5-FU cream, apply it diffusely to sun-exposed skin, and experience no severe irritant reactions and/or social pariah status. We all know that this product is effective, and we all overprepare patients to use it, knowing that they will call our offices panicked and fearful that they are allergic to or are becoming infected by this cream.
Although further study clearly is needed to determine the optimal application amount, duration of use, and vehicle mix, this new compound utilizing 2 topicals that are familiar to us--5-FU cream approved for AKs and early squamous cell skin cancers and calcipotriol ointment (though available only in cream in the United States currently) for psoriasis--is an encouraging step. Home therapy for AKs and possibly early nonmelanoma skin cancers that is more tolerable, of shorter duration, and in turn more effective than the current options would lessen the burden of treating these lesions surgically or rescheduling 5-FU patients often for irritation reaction education.
How do patients respond to this regimen?
In my own anecdotal experience, this regimen has been well received by patients and often is covered by most insurances when written as 2 separate prescriptions (both in cream vehicle). They still report some irritation, but I prefer to utilize it segmentally instead of treating all sun-exposed areas at once (ie, treat one side of the face/scalp twice daily for 4 days, then the other, or even divide it into smaller segments once the prior segment has healed). This combination, in addition to, for example, adding nicotinamide 500 mg twice daily to a patient's skin cancer chemopreventative sequence, is in my opinion a novel but safe, effective, and well-tolerated field therapy recommendation.
American Academy of Dermatology Actinic Keratosis Overview
American Academy of Family Physicians Actinic Keratoses Information
Suggested Readings
- Cunningham TJ, Tabacchi M, Eliane JP, et al. Randomized trial of calcipotriol combined with 5-fluorouracil for skin cancer precursor immunotherapy. J Clin Invest. 2017;127:106-116.
- Demehri S, Turkoz A, Manivasagam S, et al. Elevated epidermal thymic stromal lymphopoietin levels establish an antitumor environment in the skin. Cancer Cell. 2012;22:494-505.
- Rosamilia LL. Three Cheers for B3? Cutis. July 7, 2015. http://www.mdedge.com/cutis/article/101102/nonmelanoma-skin-cancer/three-cheers-b3. Accessed November 20, 2017.
- Sato-Deguchi E, Imafuku S, Chou B, et al. Topical vitamin D(3) analogues induce thymic stromal lymphopoietin and cathelicidin in psoriatic skin lesions. Br J Dermatol. 2012;167:77-84.
Diversity in the Dermatology Workforce: 2017 Status Update
Physician diversity benefits patient care: Patients are more satisfied during race-concordant visits, report their physicians as more engaged and responsive to their needs, and experience notably longer visits.1,2 Nonwhite physicians (ie, races and ethnicities that are underrepresented in medicine [URM] with respect to the general population) are more likely to care for underserved communities. Furthermore, increased diversity in the learning environment supports preparedness of all trainees to serve diverse patients.3 For these reasons, a more diverse physician workforce can contribute to better access to care in all communities, thus addressing health disparities.1,4
Increasing diversity in the dermatology workforce has been identified as an emerging priority.5 Dermatology is one of the least diverse specialties,5 and the representation of URM dermatologists is lower compared to other medical specialties and the general US population. The proportion of specialty leaders from underrepresented backgrounds may be even smaller. The lack of diversity in academic dermatology has negative consequences for patients and communities. Increasing the diversity of resident trainees is the only way to improve the diversity gap within the dermatology workforce.6
Recent commentary on this topic has highlighted several priorities for addressing the dermatology diversity gap,6-11 including the following: (1) making diversity an explicit goal in dermatology; (2) ensuring early exposure to dermatology in medical school; (3) supporting mentorship programs for minority medical students; (4) increasing medical student diversity; (5) encouraging that all dermatology program directors and leaders train in implicit bias; and (6) reviewing residency admission criteria to ensure they are objective and equitable, not biased against any applicants.
The process of reviewing residency selection criteria has begun. In 2017, Chen and Shinkai7 called for our specialty to rethink the selection process. The authors argued that emphasis on test scores, grades, and publications systematically disadvantages underrepresented minorities and students from lower socioeconomic statuses. The authors proposed several solutions: (1) make diversity an explicit goal of the selection process, (2) shift away from test scores for all applicants, (3) change the interview format, (4) prioritize other competencies such as observation skills, and (5) recruit and retain faculty who support URM trainees.7
Several dermatology leadership groups have taken action to promote programs that aim to improve diversity within dermatology. The Dermatology Diversity Champions initiative includes 6 US dermatology residency programs that are committed to increasing diversity and collaborate to evaluate pilot approaches. The American Academy of Dermatology President’s Conference on Diversity in Dermatology in Chicago, Illinois, in August 2017, as well as the focus on diversity in residency training programs at the Annual Meeting of the Association of Professors of Dermatology in Chicago, Illinois, in October 2017, are strong indicators that our specialty as a whole is aware and eager to embrace diversity as a priority. The American Academy of Dermatology President’s Conference, which was comprised of representatives from many leadership organizations and interest groups within dermatology, identified 3 action items: (1) increase the pipeline of URM students into medical school, (2) increase interest in dermatology among URM medical students, and (3) increase URM representation in residency training programs.
There are many strengths, weaknesses, opportunities, and threats/barriers (SWOT) to attaining this goal. Current strengths include strong support from dermatology leaders and activities that build on existing mentorship and diversity efforts by leaders within our specialty. SWOT analysis highlights several key opportunities of this mission, including connecting with the House of Medicine in shared efforts to improve diversity, as well as increased understanding of skin of color, health disparities, and implicit bias among physicians. Although faculty development will require time and financial investment, it will lead to tremendous benefits and opportunities for all dermatologists, including URM physicians. Other weaknesses and threats/barriers are outlined in the Figure.
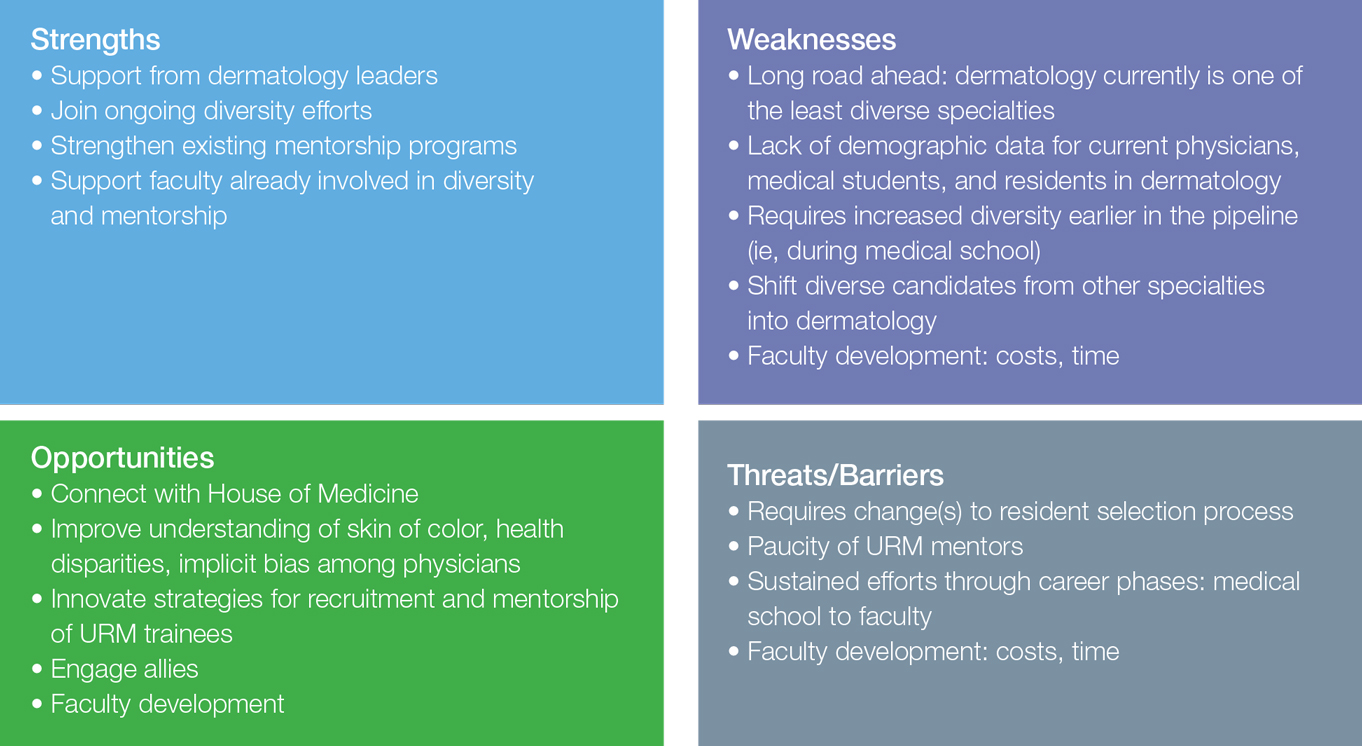
Final Thoughts
We are far from reaching our goal of a diverse dermatology workforce, and the road ahead is long. We have a start and we have momentum. We can move forward by spreading the word that all types of diversity are a priority for our specialty. Making a true difference will require commitment and sustained efforts. Dermatology can lead the way as all of American medicine strives to attain workforce diversity.
- Saha S. Taking diversity seriously: the merits of increasing minority representation in medicine. JAMA Intern Med. 2014;174:291-292.
- Cooper LA, Roter DL, Johnson RL, et al. Patient-centered communication, ratings of care, and concordance of patient and physician race. Ann Intern Med. 2003;139:907-915.
- Saha S, Guiton G, Wimmers PF, et al. Student body racial and ethnic composition and diversity-related outcomes in US medical schools. JAMA. 2008;300:1135-1145.
- Marrast LM, Zallman L, Woolhandler S, et al. Minority physicians’ role in the care of underserved patients: diversifying the physician workforce may be key in addressing health disparities. JAMA Intern Med. 2014;174:289-291.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Lester J, Wintroub B, Linos E. Disparities in academic dermatology. JAMA Dermatol. 2016;152:878-879.
- Chen A, Shinkai K. Rethinking how we select dermatology applicants—turning the tide. JAMA Dermatol. 2017;153:259-260.
- Granstein RD, Cornelius L, Shinkai K. Diversity in dermatology—a call for action. JAMA Dermatol. 2017;153:499-500.
- McKesey J, Berger TG, Lim HW, et al. Cultural competence for the 21st century dermatologist practicing in the United States. J Am Acad Dermatol. 2017;77:1159-1169.
- Van Voorhees AS, Enos CW. Diversity in dermatology residency programs. J Investig Dermatol Symp Proc. 2017;18:S46-S49.
- Imadojemu S, James WD. Increasing African American representation in dermatology. JAMA Dermatol. 2016;152:15-16.
Physician diversity benefits patient care: Patients are more satisfied during race-concordant visits, report their physicians as more engaged and responsive to their needs, and experience notably longer visits.1,2 Nonwhite physicians (ie, races and ethnicities that are underrepresented in medicine [URM] with respect to the general population) are more likely to care for underserved communities. Furthermore, increased diversity in the learning environment supports preparedness of all trainees to serve diverse patients.3 For these reasons, a more diverse physician workforce can contribute to better access to care in all communities, thus addressing health disparities.1,4
Increasing diversity in the dermatology workforce has been identified as an emerging priority.5 Dermatology is one of the least diverse specialties,5 and the representation of URM dermatologists is lower compared to other medical specialties and the general US population. The proportion of specialty leaders from underrepresented backgrounds may be even smaller. The lack of diversity in academic dermatology has negative consequences for patients and communities. Increasing the diversity of resident trainees is the only way to improve the diversity gap within the dermatology workforce.6
Recent commentary on this topic has highlighted several priorities for addressing the dermatology diversity gap,6-11 including the following: (1) making diversity an explicit goal in dermatology; (2) ensuring early exposure to dermatology in medical school; (3) supporting mentorship programs for minority medical students; (4) increasing medical student diversity; (5) encouraging that all dermatology program directors and leaders train in implicit bias; and (6) reviewing residency admission criteria to ensure they are objective and equitable, not biased against any applicants.
The process of reviewing residency selection criteria has begun. In 2017, Chen and Shinkai7 called for our specialty to rethink the selection process. The authors argued that emphasis on test scores, grades, and publications systematically disadvantages underrepresented minorities and students from lower socioeconomic statuses. The authors proposed several solutions: (1) make diversity an explicit goal of the selection process, (2) shift away from test scores for all applicants, (3) change the interview format, (4) prioritize other competencies such as observation skills, and (5) recruit and retain faculty who support URM trainees.7
Several dermatology leadership groups have taken action to promote programs that aim to improve diversity within dermatology. The Dermatology Diversity Champions initiative includes 6 US dermatology residency programs that are committed to increasing diversity and collaborate to evaluate pilot approaches. The American Academy of Dermatology President’s Conference on Diversity in Dermatology in Chicago, Illinois, in August 2017, as well as the focus on diversity in residency training programs at the Annual Meeting of the Association of Professors of Dermatology in Chicago, Illinois, in October 2017, are strong indicators that our specialty as a whole is aware and eager to embrace diversity as a priority. The American Academy of Dermatology President’s Conference, which was comprised of representatives from many leadership organizations and interest groups within dermatology, identified 3 action items: (1) increase the pipeline of URM students into medical school, (2) increase interest in dermatology among URM medical students, and (3) increase URM representation in residency training programs.
There are many strengths, weaknesses, opportunities, and threats/barriers (SWOT) to attaining this goal. Current strengths include strong support from dermatology leaders and activities that build on existing mentorship and diversity efforts by leaders within our specialty. SWOT analysis highlights several key opportunities of this mission, including connecting with the House of Medicine in shared efforts to improve diversity, as well as increased understanding of skin of color, health disparities, and implicit bias among physicians. Although faculty development will require time and financial investment, it will lead to tremendous benefits and opportunities for all dermatologists, including URM physicians. Other weaknesses and threats/barriers are outlined in the Figure.

Final Thoughts
We are far from reaching our goal of a diverse dermatology workforce, and the road ahead is long. We have a start and we have momentum. We can move forward by spreading the word that all types of diversity are a priority for our specialty. Making a true difference will require commitment and sustained efforts. Dermatology can lead the way as all of American medicine strives to attain workforce diversity.
Physician diversity benefits patient care: Patients are more satisfied during race-concordant visits, report their physicians as more engaged and responsive to their needs, and experience notably longer visits.1,2 Nonwhite physicians (ie, races and ethnicities that are underrepresented in medicine [URM] with respect to the general population) are more likely to care for underserved communities. Furthermore, increased diversity in the learning environment supports preparedness of all trainees to serve diverse patients.3 For these reasons, a more diverse physician workforce can contribute to better access to care in all communities, thus addressing health disparities.1,4
Increasing diversity in the dermatology workforce has been identified as an emerging priority.5 Dermatology is one of the least diverse specialties,5 and the representation of URM dermatologists is lower compared to other medical specialties and the general US population. The proportion of specialty leaders from underrepresented backgrounds may be even smaller. The lack of diversity in academic dermatology has negative consequences for patients and communities. Increasing the diversity of resident trainees is the only way to improve the diversity gap within the dermatology workforce.6
Recent commentary on this topic has highlighted several priorities for addressing the dermatology diversity gap,6-11 including the following: (1) making diversity an explicit goal in dermatology; (2) ensuring early exposure to dermatology in medical school; (3) supporting mentorship programs for minority medical students; (4) increasing medical student diversity; (5) encouraging that all dermatology program directors and leaders train in implicit bias; and (6) reviewing residency admission criteria to ensure they are objective and equitable, not biased against any applicants.
The process of reviewing residency selection criteria has begun. In 2017, Chen and Shinkai7 called for our specialty to rethink the selection process. The authors argued that emphasis on test scores, grades, and publications systematically disadvantages underrepresented minorities and students from lower socioeconomic statuses. The authors proposed several solutions: (1) make diversity an explicit goal of the selection process, (2) shift away from test scores for all applicants, (3) change the interview format, (4) prioritize other competencies such as observation skills, and (5) recruit and retain faculty who support URM trainees.7
Several dermatology leadership groups have taken action to promote programs that aim to improve diversity within dermatology. The Dermatology Diversity Champions initiative includes 6 US dermatology residency programs that are committed to increasing diversity and collaborate to evaluate pilot approaches. The American Academy of Dermatology President’s Conference on Diversity in Dermatology in Chicago, Illinois, in August 2017, as well as the focus on diversity in residency training programs at the Annual Meeting of the Association of Professors of Dermatology in Chicago, Illinois, in October 2017, are strong indicators that our specialty as a whole is aware and eager to embrace diversity as a priority. The American Academy of Dermatology President’s Conference, which was comprised of representatives from many leadership organizations and interest groups within dermatology, identified 3 action items: (1) increase the pipeline of URM students into medical school, (2) increase interest in dermatology among URM medical students, and (3) increase URM representation in residency training programs.
There are many strengths, weaknesses, opportunities, and threats/barriers (SWOT) to attaining this goal. Current strengths include strong support from dermatology leaders and activities that build on existing mentorship and diversity efforts by leaders within our specialty. SWOT analysis highlights several key opportunities of this mission, including connecting with the House of Medicine in shared efforts to improve diversity, as well as increased understanding of skin of color, health disparities, and implicit bias among physicians. Although faculty development will require time and financial investment, it will lead to tremendous benefits and opportunities for all dermatologists, including URM physicians. Other weaknesses and threats/barriers are outlined in the Figure.

Final Thoughts
We are far from reaching our goal of a diverse dermatology workforce, and the road ahead is long. We have a start and we have momentum. We can move forward by spreading the word that all types of diversity are a priority for our specialty. Making a true difference will require commitment and sustained efforts. Dermatology can lead the way as all of American medicine strives to attain workforce diversity.
- Saha S. Taking diversity seriously: the merits of increasing minority representation in medicine. JAMA Intern Med. 2014;174:291-292.
- Cooper LA, Roter DL, Johnson RL, et al. Patient-centered communication, ratings of care, and concordance of patient and physician race. Ann Intern Med. 2003;139:907-915.
- Saha S, Guiton G, Wimmers PF, et al. Student body racial and ethnic composition and diversity-related outcomes in US medical schools. JAMA. 2008;300:1135-1145.
- Marrast LM, Zallman L, Woolhandler S, et al. Minority physicians’ role in the care of underserved patients: diversifying the physician workforce may be key in addressing health disparities. JAMA Intern Med. 2014;174:289-291.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Lester J, Wintroub B, Linos E. Disparities in academic dermatology. JAMA Dermatol. 2016;152:878-879.
- Chen A, Shinkai K. Rethinking how we select dermatology applicants—turning the tide. JAMA Dermatol. 2017;153:259-260.
- Granstein RD, Cornelius L, Shinkai K. Diversity in dermatology—a call for action. JAMA Dermatol. 2017;153:499-500.
- McKesey J, Berger TG, Lim HW, et al. Cultural competence for the 21st century dermatologist practicing in the United States. J Am Acad Dermatol. 2017;77:1159-1169.
- Van Voorhees AS, Enos CW. Diversity in dermatology residency programs. J Investig Dermatol Symp Proc. 2017;18:S46-S49.
- Imadojemu S, James WD. Increasing African American representation in dermatology. JAMA Dermatol. 2016;152:15-16.
- Saha S. Taking diversity seriously: the merits of increasing minority representation in medicine. JAMA Intern Med. 2014;174:291-292.
- Cooper LA, Roter DL, Johnson RL, et al. Patient-centered communication, ratings of care, and concordance of patient and physician race. Ann Intern Med. 2003;139:907-915.
- Saha S, Guiton G, Wimmers PF, et al. Student body racial and ethnic composition and diversity-related outcomes in US medical schools. JAMA. 2008;300:1135-1145.
- Marrast LM, Zallman L, Woolhandler S, et al. Minority physicians’ role in the care of underserved patients: diversifying the physician workforce may be key in addressing health disparities. JAMA Intern Med. 2014;174:289-291.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Lester J, Wintroub B, Linos E. Disparities in academic dermatology. JAMA Dermatol. 2016;152:878-879.
- Chen A, Shinkai K. Rethinking how we select dermatology applicants—turning the tide. JAMA Dermatol. 2017;153:259-260.
- Granstein RD, Cornelius L, Shinkai K. Diversity in dermatology—a call for action. JAMA Dermatol. 2017;153:499-500.
- McKesey J, Berger TG, Lim HW, et al. Cultural competence for the 21st century dermatologist practicing in the United States. J Am Acad Dermatol. 2017;77:1159-1169.
- Van Voorhees AS, Enos CW. Diversity in dermatology residency programs. J Investig Dermatol Symp Proc. 2017;18:S46-S49.
- Imadojemu S, James WD. Increasing African American representation in dermatology. JAMA Dermatol. 2016;152:15-16.