User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Why Hidradenitis Suppurativa Should Be on Your Radar
Hidden Risks of Formaldehyde in Hair-Straightening Products
Hidden Risks of Formaldehyde in Hair-Straightening Products
Formaldehyde (FA) is a colorless, flammable, highly pungent gas that remains ubiquitous in the environment despite being a known carcinogen and allergen.1 In the cosmetic industry, FA commonly is used as both a preservative and active ingredient in hairstraightening products. Due to its toxicity and the thermal instability of FA releasers (ie, the release of FA at high temperatures), the US Food and Drug Administration has proposed a ban on formaldehyde and other FA-releasing chemicals (eg, methylene glycol) as an ingredient in hairsmoothing or hair-straightening products marketed in the United States.2 However, the implementation of this ban is not yet in effect.
Hair-straightening products that are referred to as chemical relaxers typically contain alkaline derivatives. Alkaline hair straighteners—which include lye relaxers (active ingredient: sodium hydroxide), nolye relaxers (active ingredients: potassium hydroxide, lithium hydroxide, calcium hydroxide, guanidine hydroxide, or ammonium thioglycolate), and the Japanese hair straightening process (active ingredient: ammonium thioglycolate)—do not contain FA or FA-derivatives as active ingredients.3 Alternatively, acidic hair straighteners—popularly known as keratin treatments—contain either FA or FA-releasers and will be the primary focus of this discussion. As many patients are exposed to these products, we aim to highlight the cutaneous and systemic manifestations of acute and chronic exposure.
How Hair-Straightening Products Work
Hair straighteners that include FA or its derivatives generally contain high and low molecular weights of keratin peptides. The keratin peptides with high molecular weights diffuse into the cuticle while the low-molecular-weight peptides can penetrate further into the cortex of the hair shaft.4 Formaldehyde forms cross-links with the keratin amino acids (eg, tyrosine, arginine), and the application of heat via blow-drying enhances its ability to cross-link the hydrolyzed keratin from the straightening product to the natural keratin in the hair fibers; the use of a heated flat iron further enhances the cross-linking and seals the cuticle.5 The same mechanism of action applies for “safe keratin” (marketing terminology used for FA releasers) treatments, whereby the hydrogen and salt bonds of the hair are weakened, allowing for interconversion of the cysteine bonds of the hair fibers. This chemical conversion allows for the hair shafts to have a stable straight configuration. Of note, this mechanism of action differs from the action of chemical relaxers, which have a high pH and straighten the hair by opening the cuticles and permanently breaking the disulfide bonds in the cortex of the hair shaft—a process that restructures the keratin bonds without requiring heat application.5
The outcome of a keratin treatment, as seen on light microscopy, is the replenishment of gaps in the hair’s cuticle, therefore increasing its mechanical and thermal properties.6 This can give the appearance of increased shine, softness, and tensile strength. However, Sanad et al6 report that, as viewed on transmission electron microscopy, these keratin treatments do not repair lost cuticles, cuticle splitting, or detached cuticle layers from damaged strands.
Lastly, some patients notice lightening of their hair color after a hair-straightening treatment, which is possibly due to inhibition of the enzymatic synthesis of melanin, decomposition of melanin granules, or a direct reaction from chemical neutralizers with a high pH.6 Knowledge of the mechanism of action of hair-straightening treatments will aid dermatologists in educating patients about their immediate and long-term effects. This education subsequently will help patients avoid inappropriate hair care techniques that further damage the hair.
Environmental Distribution and Systemic Absorption of Formaldehyde
Atmospheric FA is absorbed via cutaneous and mucosal surfaces. Atmospheric FA concentrations produced when hair-straightening products are used cannot routinely be predicted because the amount generated depends on factors such as the pH of the preparation, the temperature to which the product is heated during straightening, duration of storage, and aeration and size of the environment in which the product is being used, among others.7
Peteffi et al7 and Aglan et al8 detected a moderate positive correlation between environmental FA concentrations and those in cosmetic products, particularly after blow-drying the hair or using other heat applications; however, the products examined by Peteffi et al7 contained exceedingly high concentrations of FA (up to 5.9%, which is higher than the legal limit of 0.1% in the United States).9 Of note, some products in this study were labelled as “formaldehyde free” but still contained high concentrations of FA.7 This is consistent with data published by the Occupational Health and Safety Administration, which citied salons with exposure limits outside the national recommendations (2.0 FA ppm/air).10 These findings highlight the inadvertent exposure that consumers face from products that are not regulated consistently.
Interestingly, Henault et al11 observed that products with a high concentration of FA dispersed more airborne particles during hair brushing than hair straightening/ironing.11 Further studies are needed to clarify the different routes and methods contributing to FA dispersion and the molecular instability of FA-releasers.
Clinical Correlation
Products that contain low (ie, less than the legal limit) levels of FA are not mandated to declare its presence on the product label; however, many products are contaminated with FA or inappropriately omit FA from the ingredient list, even at elevated concentrations. Consumers therefore may be inadvertently exposed to FA particles. Additionally, occupations with frequent exposure to FA include hairdressers, barbers, beauticians and related workers (33.6% exposure rate); sewers and embroiderers (26.1%); and cooks (19.1%).12
Adverse health effects associated with acute FA exposure include but are not limited to headache, eye irritation, allergic/irritant contact dermatitis, psoriasiform reactions, and acute kidney and respiratory tract injuries. Frontal fibrosing alopecia; non-Hodgkin lymphoma; and cancers of the upper digestive tract, lungs, and bladder also have been associated with chronic FA exposure.7,13 In a cohort of female hairdressers, a longer duration of FA exposure (>8 years) as well as cumulative exposure were associated with an increase in ovarian cancer (OR, 1.48 [0.88 to 2.51]).12 Formalin, the aqueous derivative of FA, also contains phenolic products that can mediate inflammatory response, DNA methylation, and carcinogenesis even with chronic low-level exposure.14 However, evidence supporting a direct correlation of FA exposure with breast carcinoma in both hairstylists and consumers remains controversial.7
Sanchez-Duenas et al15 described a case series of patients who were found to have psoriasiform scalp reactions after exposure to keratin treatments containing FA. The time to development of the lesions was inversely correlated with the number of treatments received, although the mean time to development was 12 months postprocedure.15 These researchers also identified no allergies to the substance on contact testing, which suggests an alternate pathogenesis as a consequence of FA exposure, resulting in the development of a psoriasiform reaction.15
Following adjustment for sex, age, menopause status, and skin color, frontal fibrosing alopecia also has been associated with the use of formalin and FA in hair straighteners.14 This is possibly related to the ability of FA and many phenolic products to induce chronic inflammation; however, a cumulative effect has not been noted consistently across the literature.
Future Directives
Continuous industry regulation is needed to ensure that use of FA is reduced and it is eventually eliminated from consumer products. Additionally, strict regulations are required to ensure products containing FA and FA-releasers are accurately labeled. Physicians and consumers should be aware of the potential health hazards associated with FA and advocate for effective legislation. While there is controversy regarding the level of absorption from environmental exposure and the subsequent biologic effects of absorption, both consumers and workers in industries such as hairdressing and barbering should reduce exposure time to FA and limit the application of heat and contact with products containing FA and FA releasers.
- González-Muñoz P, Conde-Salazar L, Vañó-Galván S. Allergic contact dermatitis caused by cosmetic products. Actas Dermosifiliogr. 2014;105:822-832. doi:10.1016/j.ad.2013.12.018
- Department of Health and Human Services. Use of formaldehyde and formaldehyde-releasing chemicals as an ingredient in hair smoothing products or hair straightening products (RIN: 0910-AI83). Spring 2023. Accessed November 11, 2024. https://www.reginfo.gov/public/do/eAgendaViewRule?pubId=202304&RIN=0910-AI83
- Velasco MVR, de Sá-Dias TC, Dario MF, et al. Impact of acid (“progressive brush”) and alkaline straightening on the hair fiber: differential effects on the cuticle and cortex properties. Int J Trichology. 2022;14:197-203. doi:10.4103/ijt.ijt_158_20
- Malinauskyte E, Shrestha R, Cornwell P, et al. Penetration of different molecular weight hydrolysed keratins into hair fibres and their effects on the physical properties of textured hair. Int J Cosmet Sci. 2021;43:26-37. doi:10.1111/ics.12663
- Weathersby C, McMichael A. Brazilian keratin hair treatment: a review. J Cosmet Dermatol. 2013;12:144-148. doi:10.1111/jocd.12030
- Sanad EM, El]Esawy FM, Mustafa AI, et al. Structural changes of hair shaft after application of chemical hair straighteners: clinical and histopathological study. J Cosmet Dermatol. 2019;18:929-935. doi:10.1111/jocd.12752
- Peteffi GP, Antunes MV, Carrer C, et al. Environmental and biological monitoring of occupational formaldehyde exposure resulting from the use of products for hair straightening. Environ Sci Pollut Res Int. 2016;23:908-917. doi:10.1007/s11356-015-5343-4
- Aglan MA, Mansour GN. Hair straightening products and the risk of occupational formaldehyde exposure in hairstylists. Drug Chem Toxicol. 2020;43:488-495. doi: 10.1080/01480545.2018 .1508215
- Occupational Safety and Health Administration. Hair smoothing products that could release formaldehyde. Hazard Alert Update. September 2011. Accessed November 11, 2024. https://www.osha.gov/sites/default/files/hazard_alert.pdf
- US Department of Labor. US Department of Labor continues to cite beauty salons and manufacturers for formaldehyde exposure from hair smoothing products. December 8, 2011. Accessed November 11, 2024. https://www.dol.gov/newsroom/releases/osha/osha20111208
- Henault P, Lemaire R, Salzedo A, et al. A methodological approach for quantifying aerial formaldehyde released by some hair treatmentsmodeling a hair-salon environment. J Air Waste Manage. 2021;71: 754-760. doi:10.1080/10962247.2021.1893238
- Leung L, Lavoué J, Siemiatycki J, et al. Occupational environment and ovarian cancer risk. Occup Environ Med. 2023;80:489-497. doi:10.1136/oemed-2022-108557
- Bnaya A, Abu-Amer N, Beckerman P, et al. Acute kidney injury and hair-straightening products: a case series. Am J Kidney Dis. 2023;82:43-52.E1. doi:10.1053/j.ajkd.2022.11.016
- Ramos PM, Anzai A, Duque-Estrada B, et al. Risk factors for frontal fibrosing alopecia: a case-control study in a multiracial population. J Am Acad Dermatol. 2021;84:712-718. doi:10.1016/j.jaad.2020.08.076
- Sanchez-Duenas LE, Ruiz-Dueñas A, Guevara-Gutiérrez E, et al. Psoriasiform skin reaction due to Brazilian keratin treatment: a clinicaldermatoscopic study of 43 patients. Int J Trichology. 2022;14:103-108. doi:10.4103/ijt.ijt_62_21
Formaldehyde (FA) is a colorless, flammable, highly pungent gas that remains ubiquitous in the environment despite being a known carcinogen and allergen.1 In the cosmetic industry, FA commonly is used as both a preservative and active ingredient in hairstraightening products. Due to its toxicity and the thermal instability of FA releasers (ie, the release of FA at high temperatures), the US Food and Drug Administration has proposed a ban on formaldehyde and other FA-releasing chemicals (eg, methylene glycol) as an ingredient in hairsmoothing or hair-straightening products marketed in the United States.2 However, the implementation of this ban is not yet in effect.
Hair-straightening products that are referred to as chemical relaxers typically contain alkaline derivatives. Alkaline hair straighteners—which include lye relaxers (active ingredient: sodium hydroxide), nolye relaxers (active ingredients: potassium hydroxide, lithium hydroxide, calcium hydroxide, guanidine hydroxide, or ammonium thioglycolate), and the Japanese hair straightening process (active ingredient: ammonium thioglycolate)—do not contain FA or FA-derivatives as active ingredients.3 Alternatively, acidic hair straighteners—popularly known as keratin treatments—contain either FA or FA-releasers and will be the primary focus of this discussion. As many patients are exposed to these products, we aim to highlight the cutaneous and systemic manifestations of acute and chronic exposure.
How Hair-Straightening Products Work
Hair straighteners that include FA or its derivatives generally contain high and low molecular weights of keratin peptides. The keratin peptides with high molecular weights diffuse into the cuticle while the low-molecular-weight peptides can penetrate further into the cortex of the hair shaft.4 Formaldehyde forms cross-links with the keratin amino acids (eg, tyrosine, arginine), and the application of heat via blow-drying enhances its ability to cross-link the hydrolyzed keratin from the straightening product to the natural keratin in the hair fibers; the use of a heated flat iron further enhances the cross-linking and seals the cuticle.5 The same mechanism of action applies for “safe keratin” (marketing terminology used for FA releasers) treatments, whereby the hydrogen and salt bonds of the hair are weakened, allowing for interconversion of the cysteine bonds of the hair fibers. This chemical conversion allows for the hair shafts to have a stable straight configuration. Of note, this mechanism of action differs from the action of chemical relaxers, which have a high pH and straighten the hair by opening the cuticles and permanently breaking the disulfide bonds in the cortex of the hair shaft—a process that restructures the keratin bonds without requiring heat application.5
The outcome of a keratin treatment, as seen on light microscopy, is the replenishment of gaps in the hair’s cuticle, therefore increasing its mechanical and thermal properties.6 This can give the appearance of increased shine, softness, and tensile strength. However, Sanad et al6 report that, as viewed on transmission electron microscopy, these keratin treatments do not repair lost cuticles, cuticle splitting, or detached cuticle layers from damaged strands.
Lastly, some patients notice lightening of their hair color after a hair-straightening treatment, which is possibly due to inhibition of the enzymatic synthesis of melanin, decomposition of melanin granules, or a direct reaction from chemical neutralizers with a high pH.6 Knowledge of the mechanism of action of hair-straightening treatments will aid dermatologists in educating patients about their immediate and long-term effects. This education subsequently will help patients avoid inappropriate hair care techniques that further damage the hair.
Environmental Distribution and Systemic Absorption of Formaldehyde
Atmospheric FA is absorbed via cutaneous and mucosal surfaces. Atmospheric FA concentrations produced when hair-straightening products are used cannot routinely be predicted because the amount generated depends on factors such as the pH of the preparation, the temperature to which the product is heated during straightening, duration of storage, and aeration and size of the environment in which the product is being used, among others.7
Peteffi et al7 and Aglan et al8 detected a moderate positive correlation between environmental FA concentrations and those in cosmetic products, particularly after blow-drying the hair or using other heat applications; however, the products examined by Peteffi et al7 contained exceedingly high concentrations of FA (up to 5.9%, which is higher than the legal limit of 0.1% in the United States).9 Of note, some products in this study were labelled as “formaldehyde free” but still contained high concentrations of FA.7 This is consistent with data published by the Occupational Health and Safety Administration, which citied salons with exposure limits outside the national recommendations (2.0 FA ppm/air).10 These findings highlight the inadvertent exposure that consumers face from products that are not regulated consistently.
Interestingly, Henault et al11 observed that products with a high concentration of FA dispersed more airborne particles during hair brushing than hair straightening/ironing.11 Further studies are needed to clarify the different routes and methods contributing to FA dispersion and the molecular instability of FA-releasers.
Clinical Correlation
Products that contain low (ie, less than the legal limit) levels of FA are not mandated to declare its presence on the product label; however, many products are contaminated with FA or inappropriately omit FA from the ingredient list, even at elevated concentrations. Consumers therefore may be inadvertently exposed to FA particles. Additionally, occupations with frequent exposure to FA include hairdressers, barbers, beauticians and related workers (33.6% exposure rate); sewers and embroiderers (26.1%); and cooks (19.1%).12
Adverse health effects associated with acute FA exposure include but are not limited to headache, eye irritation, allergic/irritant contact dermatitis, psoriasiform reactions, and acute kidney and respiratory tract injuries. Frontal fibrosing alopecia; non-Hodgkin lymphoma; and cancers of the upper digestive tract, lungs, and bladder also have been associated with chronic FA exposure.7,13 In a cohort of female hairdressers, a longer duration of FA exposure (>8 years) as well as cumulative exposure were associated with an increase in ovarian cancer (OR, 1.48 [0.88 to 2.51]).12 Formalin, the aqueous derivative of FA, also contains phenolic products that can mediate inflammatory response, DNA methylation, and carcinogenesis even with chronic low-level exposure.14 However, evidence supporting a direct correlation of FA exposure with breast carcinoma in both hairstylists and consumers remains controversial.7
Sanchez-Duenas et al15 described a case series of patients who were found to have psoriasiform scalp reactions after exposure to keratin treatments containing FA. The time to development of the lesions was inversely correlated with the number of treatments received, although the mean time to development was 12 months postprocedure.15 These researchers also identified no allergies to the substance on contact testing, which suggests an alternate pathogenesis as a consequence of FA exposure, resulting in the development of a psoriasiform reaction.15
Following adjustment for sex, age, menopause status, and skin color, frontal fibrosing alopecia also has been associated with the use of formalin and FA in hair straighteners.14 This is possibly related to the ability of FA and many phenolic products to induce chronic inflammation; however, a cumulative effect has not been noted consistently across the literature.
Future Directives
Continuous industry regulation is needed to ensure that use of FA is reduced and it is eventually eliminated from consumer products. Additionally, strict regulations are required to ensure products containing FA and FA-releasers are accurately labeled. Physicians and consumers should be aware of the potential health hazards associated with FA and advocate for effective legislation. While there is controversy regarding the level of absorption from environmental exposure and the subsequent biologic effects of absorption, both consumers and workers in industries such as hairdressing and barbering should reduce exposure time to FA and limit the application of heat and contact with products containing FA and FA releasers.
Formaldehyde (FA) is a colorless, flammable, highly pungent gas that remains ubiquitous in the environment despite being a known carcinogen and allergen.1 In the cosmetic industry, FA commonly is used as both a preservative and active ingredient in hairstraightening products. Due to its toxicity and the thermal instability of FA releasers (ie, the release of FA at high temperatures), the US Food and Drug Administration has proposed a ban on formaldehyde and other FA-releasing chemicals (eg, methylene glycol) as an ingredient in hairsmoothing or hair-straightening products marketed in the United States.2 However, the implementation of this ban is not yet in effect.
Hair-straightening products that are referred to as chemical relaxers typically contain alkaline derivatives. Alkaline hair straighteners—which include lye relaxers (active ingredient: sodium hydroxide), nolye relaxers (active ingredients: potassium hydroxide, lithium hydroxide, calcium hydroxide, guanidine hydroxide, or ammonium thioglycolate), and the Japanese hair straightening process (active ingredient: ammonium thioglycolate)—do not contain FA or FA-derivatives as active ingredients.3 Alternatively, acidic hair straighteners—popularly known as keratin treatments—contain either FA or FA-releasers and will be the primary focus of this discussion. As many patients are exposed to these products, we aim to highlight the cutaneous and systemic manifestations of acute and chronic exposure.
How Hair-Straightening Products Work
Hair straighteners that include FA or its derivatives generally contain high and low molecular weights of keratin peptides. The keratin peptides with high molecular weights diffuse into the cuticle while the low-molecular-weight peptides can penetrate further into the cortex of the hair shaft.4 Formaldehyde forms cross-links with the keratin amino acids (eg, tyrosine, arginine), and the application of heat via blow-drying enhances its ability to cross-link the hydrolyzed keratin from the straightening product to the natural keratin in the hair fibers; the use of a heated flat iron further enhances the cross-linking and seals the cuticle.5 The same mechanism of action applies for “safe keratin” (marketing terminology used for FA releasers) treatments, whereby the hydrogen and salt bonds of the hair are weakened, allowing for interconversion of the cysteine bonds of the hair fibers. This chemical conversion allows for the hair shafts to have a stable straight configuration. Of note, this mechanism of action differs from the action of chemical relaxers, which have a high pH and straighten the hair by opening the cuticles and permanently breaking the disulfide bonds in the cortex of the hair shaft—a process that restructures the keratin bonds without requiring heat application.5
The outcome of a keratin treatment, as seen on light microscopy, is the replenishment of gaps in the hair’s cuticle, therefore increasing its mechanical and thermal properties.6 This can give the appearance of increased shine, softness, and tensile strength. However, Sanad et al6 report that, as viewed on transmission electron microscopy, these keratin treatments do not repair lost cuticles, cuticle splitting, or detached cuticle layers from damaged strands.
Lastly, some patients notice lightening of their hair color after a hair-straightening treatment, which is possibly due to inhibition of the enzymatic synthesis of melanin, decomposition of melanin granules, or a direct reaction from chemical neutralizers with a high pH.6 Knowledge of the mechanism of action of hair-straightening treatments will aid dermatologists in educating patients about their immediate and long-term effects. This education subsequently will help patients avoid inappropriate hair care techniques that further damage the hair.
Environmental Distribution and Systemic Absorption of Formaldehyde
Atmospheric FA is absorbed via cutaneous and mucosal surfaces. Atmospheric FA concentrations produced when hair-straightening products are used cannot routinely be predicted because the amount generated depends on factors such as the pH of the preparation, the temperature to which the product is heated during straightening, duration of storage, and aeration and size of the environment in which the product is being used, among others.7
Peteffi et al7 and Aglan et al8 detected a moderate positive correlation between environmental FA concentrations and those in cosmetic products, particularly after blow-drying the hair or using other heat applications; however, the products examined by Peteffi et al7 contained exceedingly high concentrations of FA (up to 5.9%, which is higher than the legal limit of 0.1% in the United States).9 Of note, some products in this study were labelled as “formaldehyde free” but still contained high concentrations of FA.7 This is consistent with data published by the Occupational Health and Safety Administration, which citied salons with exposure limits outside the national recommendations (2.0 FA ppm/air).10 These findings highlight the inadvertent exposure that consumers face from products that are not regulated consistently.
Interestingly, Henault et al11 observed that products with a high concentration of FA dispersed more airborne particles during hair brushing than hair straightening/ironing.11 Further studies are needed to clarify the different routes and methods contributing to FA dispersion and the molecular instability of FA-releasers.
Clinical Correlation
Products that contain low (ie, less than the legal limit) levels of FA are not mandated to declare its presence on the product label; however, many products are contaminated with FA or inappropriately omit FA from the ingredient list, even at elevated concentrations. Consumers therefore may be inadvertently exposed to FA particles. Additionally, occupations with frequent exposure to FA include hairdressers, barbers, beauticians and related workers (33.6% exposure rate); sewers and embroiderers (26.1%); and cooks (19.1%).12
Adverse health effects associated with acute FA exposure include but are not limited to headache, eye irritation, allergic/irritant contact dermatitis, psoriasiform reactions, and acute kidney and respiratory tract injuries. Frontal fibrosing alopecia; non-Hodgkin lymphoma; and cancers of the upper digestive tract, lungs, and bladder also have been associated with chronic FA exposure.7,13 In a cohort of female hairdressers, a longer duration of FA exposure (>8 years) as well as cumulative exposure were associated with an increase in ovarian cancer (OR, 1.48 [0.88 to 2.51]).12 Formalin, the aqueous derivative of FA, also contains phenolic products that can mediate inflammatory response, DNA methylation, and carcinogenesis even with chronic low-level exposure.14 However, evidence supporting a direct correlation of FA exposure with breast carcinoma in both hairstylists and consumers remains controversial.7
Sanchez-Duenas et al15 described a case series of patients who were found to have psoriasiform scalp reactions after exposure to keratin treatments containing FA. The time to development of the lesions was inversely correlated with the number of treatments received, although the mean time to development was 12 months postprocedure.15 These researchers also identified no allergies to the substance on contact testing, which suggests an alternate pathogenesis as a consequence of FA exposure, resulting in the development of a psoriasiform reaction.15
Following adjustment for sex, age, menopause status, and skin color, frontal fibrosing alopecia also has been associated with the use of formalin and FA in hair straighteners.14 This is possibly related to the ability of FA and many phenolic products to induce chronic inflammation; however, a cumulative effect has not been noted consistently across the literature.
Future Directives
Continuous industry regulation is needed to ensure that use of FA is reduced and it is eventually eliminated from consumer products. Additionally, strict regulations are required to ensure products containing FA and FA-releasers are accurately labeled. Physicians and consumers should be aware of the potential health hazards associated with FA and advocate for effective legislation. While there is controversy regarding the level of absorption from environmental exposure and the subsequent biologic effects of absorption, both consumers and workers in industries such as hairdressing and barbering should reduce exposure time to FA and limit the application of heat and contact with products containing FA and FA releasers.
- González-Muñoz P, Conde-Salazar L, Vañó-Galván S. Allergic contact dermatitis caused by cosmetic products. Actas Dermosifiliogr. 2014;105:822-832. doi:10.1016/j.ad.2013.12.018
- Department of Health and Human Services. Use of formaldehyde and formaldehyde-releasing chemicals as an ingredient in hair smoothing products or hair straightening products (RIN: 0910-AI83). Spring 2023. Accessed November 11, 2024. https://www.reginfo.gov/public/do/eAgendaViewRule?pubId=202304&RIN=0910-AI83
- Velasco MVR, de Sá-Dias TC, Dario MF, et al. Impact of acid (“progressive brush”) and alkaline straightening on the hair fiber: differential effects on the cuticle and cortex properties. Int J Trichology. 2022;14:197-203. doi:10.4103/ijt.ijt_158_20
- Malinauskyte E, Shrestha R, Cornwell P, et al. Penetration of different molecular weight hydrolysed keratins into hair fibres and their effects on the physical properties of textured hair. Int J Cosmet Sci. 2021;43:26-37. doi:10.1111/ics.12663
- Weathersby C, McMichael A. Brazilian keratin hair treatment: a review. J Cosmet Dermatol. 2013;12:144-148. doi:10.1111/jocd.12030
- Sanad EM, El]Esawy FM, Mustafa AI, et al. Structural changes of hair shaft after application of chemical hair straighteners: clinical and histopathological study. J Cosmet Dermatol. 2019;18:929-935. doi:10.1111/jocd.12752
- Peteffi GP, Antunes MV, Carrer C, et al. Environmental and biological monitoring of occupational formaldehyde exposure resulting from the use of products for hair straightening. Environ Sci Pollut Res Int. 2016;23:908-917. doi:10.1007/s11356-015-5343-4
- Aglan MA, Mansour GN. Hair straightening products and the risk of occupational formaldehyde exposure in hairstylists. Drug Chem Toxicol. 2020;43:488-495. doi: 10.1080/01480545.2018 .1508215
- Occupational Safety and Health Administration. Hair smoothing products that could release formaldehyde. Hazard Alert Update. September 2011. Accessed November 11, 2024. https://www.osha.gov/sites/default/files/hazard_alert.pdf
- US Department of Labor. US Department of Labor continues to cite beauty salons and manufacturers for formaldehyde exposure from hair smoothing products. December 8, 2011. Accessed November 11, 2024. https://www.dol.gov/newsroom/releases/osha/osha20111208
- Henault P, Lemaire R, Salzedo A, et al. A methodological approach for quantifying aerial formaldehyde released by some hair treatmentsmodeling a hair-salon environment. J Air Waste Manage. 2021;71: 754-760. doi:10.1080/10962247.2021.1893238
- Leung L, Lavoué J, Siemiatycki J, et al. Occupational environment and ovarian cancer risk. Occup Environ Med. 2023;80:489-497. doi:10.1136/oemed-2022-108557
- Bnaya A, Abu-Amer N, Beckerman P, et al. Acute kidney injury and hair-straightening products: a case series. Am J Kidney Dis. 2023;82:43-52.E1. doi:10.1053/j.ajkd.2022.11.016
- Ramos PM, Anzai A, Duque-Estrada B, et al. Risk factors for frontal fibrosing alopecia: a case-control study in a multiracial population. J Am Acad Dermatol. 2021;84:712-718. doi:10.1016/j.jaad.2020.08.076
- Sanchez-Duenas LE, Ruiz-Dueñas A, Guevara-Gutiérrez E, et al. Psoriasiform skin reaction due to Brazilian keratin treatment: a clinicaldermatoscopic study of 43 patients. Int J Trichology. 2022;14:103-108. doi:10.4103/ijt.ijt_62_21
- González-Muñoz P, Conde-Salazar L, Vañó-Galván S. Allergic contact dermatitis caused by cosmetic products. Actas Dermosifiliogr. 2014;105:822-832. doi:10.1016/j.ad.2013.12.018
- Department of Health and Human Services. Use of formaldehyde and formaldehyde-releasing chemicals as an ingredient in hair smoothing products or hair straightening products (RIN: 0910-AI83). Spring 2023. Accessed November 11, 2024. https://www.reginfo.gov/public/do/eAgendaViewRule?pubId=202304&RIN=0910-AI83
- Velasco MVR, de Sá-Dias TC, Dario MF, et al. Impact of acid (“progressive brush”) and alkaline straightening on the hair fiber: differential effects on the cuticle and cortex properties. Int J Trichology. 2022;14:197-203. doi:10.4103/ijt.ijt_158_20
- Malinauskyte E, Shrestha R, Cornwell P, et al. Penetration of different molecular weight hydrolysed keratins into hair fibres and their effects on the physical properties of textured hair. Int J Cosmet Sci. 2021;43:26-37. doi:10.1111/ics.12663
- Weathersby C, McMichael A. Brazilian keratin hair treatment: a review. J Cosmet Dermatol. 2013;12:144-148. doi:10.1111/jocd.12030
- Sanad EM, El]Esawy FM, Mustafa AI, et al. Structural changes of hair shaft after application of chemical hair straighteners: clinical and histopathological study. J Cosmet Dermatol. 2019;18:929-935. doi:10.1111/jocd.12752
- Peteffi GP, Antunes MV, Carrer C, et al. Environmental and biological monitoring of occupational formaldehyde exposure resulting from the use of products for hair straightening. Environ Sci Pollut Res Int. 2016;23:908-917. doi:10.1007/s11356-015-5343-4
- Aglan MA, Mansour GN. Hair straightening products and the risk of occupational formaldehyde exposure in hairstylists. Drug Chem Toxicol. 2020;43:488-495. doi: 10.1080/01480545.2018 .1508215
- Occupational Safety and Health Administration. Hair smoothing products that could release formaldehyde. Hazard Alert Update. September 2011. Accessed November 11, 2024. https://www.osha.gov/sites/default/files/hazard_alert.pdf
- US Department of Labor. US Department of Labor continues to cite beauty salons and manufacturers for formaldehyde exposure from hair smoothing products. December 8, 2011. Accessed November 11, 2024. https://www.dol.gov/newsroom/releases/osha/osha20111208
- Henault P, Lemaire R, Salzedo A, et al. A methodological approach for quantifying aerial formaldehyde released by some hair treatmentsmodeling a hair-salon environment. J Air Waste Manage. 2021;71: 754-760. doi:10.1080/10962247.2021.1893238
- Leung L, Lavoué J, Siemiatycki J, et al. Occupational environment and ovarian cancer risk. Occup Environ Med. 2023;80:489-497. doi:10.1136/oemed-2022-108557
- Bnaya A, Abu-Amer N, Beckerman P, et al. Acute kidney injury and hair-straightening products: a case series. Am J Kidney Dis. 2023;82:43-52.E1. doi:10.1053/j.ajkd.2022.11.016
- Ramos PM, Anzai A, Duque-Estrada B, et al. Risk factors for frontal fibrosing alopecia: a case-control study in a multiracial population. J Am Acad Dermatol. 2021;84:712-718. doi:10.1016/j.jaad.2020.08.076
- Sanchez-Duenas LE, Ruiz-Dueñas A, Guevara-Gutiérrez E, et al. Psoriasiform skin reaction due to Brazilian keratin treatment: a clinicaldermatoscopic study of 43 patients. Int J Trichology. 2022;14:103-108. doi:10.4103/ijt.ijt_62_21
Hidden Risks of Formaldehyde in Hair-Straightening Products
Hidden Risks of Formaldehyde in Hair-Straightening Products

Clinical, Laboratory, and Trichoscopic Features of Pediatric Androgenetic Alopecia
Clinical, Laboratory, and Trichoscopic Features of Pediatric Androgenetic Alopecia
Androgenetic alopecia (AGA) is the most common type of hair loss after adolescence, with a high prevalence of 21.3% among males and 6.0% among females in China.1 In men, AGA manifests as diffuse hair loss in the frontal and temporal areas of the scalp; in women, it is characterized by thinning of the hair on the top of the head with a wide part and less recession of the frontal line. Although the specific pathogenesis of AGA still is unclear, it is believed to be related mainly to genetics and androgen levels.1 Androgenetic alopecia is not considered a life-threatening medical condition, but it can have a major impact on patients’ self-esteem and quality of life.
The prevalence of pediatric AGA has been steadily rising over the past few decades and is thought to be correlated to a hyperinsulinemic diet and elevated circulating androgens at younger ages, resulting in early onset in genetically susceptible children and adolescents.2,3 Additionally, studies have shown that early-onset AGA is associated with metabolic syndrome,4-6 which includes conditions such as obesity, insulin resistance, hyperglycemia, and dyslipidemia.7,8 Furthermore, polycystic ovary syndrome (PCOS) is commonly observed in adolescent girls with early-onset AGA. The condition is associated with hormonal imbalances, particularly elevated androgens, which can contribute to the early onset of AGA. In girls, these hormonal changes may accelerate hair thinning and hair loss, making AGA a potential early indicator of underlying PCOS.9,10
Available research on early-onset AGA in pediatric patients is limited, with most studies having a relatively small sample size and generalized findings. Data on pediatric AGA in China is scarce; therefore, the objective of this retrospective study was to analyze the clinical, laboratory, and trichoscopic features of AGA in 133 pediatric patients with AGA who visited the hair disease clinic of the Department of Dermatology at the First Affiliated Hospital of Nanjing Medical University (Nanjing, China), from January 2010 to December 2023.
Methods
Study Population—Pediatric patients with early-onset AGA who were registered for outpatient consultations at the hair disease clinic of the Department of Dermatology at The First Affiliated Hospital of Nanjing Medical University from January 2010 to December 2023 were included. Patients aged 18 years and younger with a definitive diagnosis of AGA were selected for data collection and analysis. Any uncertain information was confirmed through telephone follow-up with patients.
Collection of Demographic Information and Laboratory Tests—Patient demographics and medical history including age, sex, age at disease onset, and duration of AGA were collected from the electronic medical record. Height and weight also were collected to calculate patients’ body mass index (BMI). Detailed laboratory test results were recorded, including assessments of sex hormone—binding globulin (SHBG), vitamin D, testosterone, and ferritin.
Analysis of Comorbidities—Due to the influence of genetic factors on body composition, there are differences in how obesity is defined across racial populations. The World Health Organization international standard defines the term overweight as a BMI greater than 25 and obese as a BMI greater than 30; however, the World Health Organization recommends a lower definition standard for these classifications in the Chinese population. China established specific BMI standards for classification of patients as overweight (24.0.27.9 kg/m2) and obese (≥28 kg/m2).11 During outpatient consultations, a comprehensive medical history was obtained from each patient, including the presence of PCOS, acne, seborrheic dermatitis, hirsutism, and sleeping disorders. During routine outpatient assessments, experienced dermatologists (including W.F.) determined the presence of symptoms and confirmed the diagnosis.
Hair Loss Classification and Trichoscopy—Hair loss patterns for male patients were assessed using the basic and specific classification system, while the Ludwig scale was utilized for female patients.12,13 Trichoscopy was utilized with high-resolution imaging systems and advanced software for image analysis, enabling precise assessment of hair in different scalp regions. Parameters such as hair density, hair diameter, percentage of terminal hairs, and percentage of vellus hair were recorded to monitor changes in hair growth for the patients.
Statistical Analysis—Categorical data were analyzed using the x2 test. A P value less than .05 was considered statistically significant. All statistical analyses were conducted using SPSS software version 26 (IBM).
Results
Patient Characteristics and Hair Loss Patterns—A sample of 133 pediatric patients (60 males, 73 females) who were diagnosed with AGA at the hair disease clinic of the Department of Dermatology at the First Affiliated Hospital of Nanjing Medical University from January 2010 to December 2023 were selected. The mean age of the patients was 15.5 years (range, 10–18 years). The mean age was slightly lower in females compared with males (15.05 vs 16.19 years, respectively). Additionally, females showed earlier onset of the disease, with a mean age at onset of 13.41 years compared to 14.44 years in males. The time between onset of AGA symptoms and first seeking medical care ranged from 4 months to 3 years, with a mean disease duration of 1.72 years. There was no significant difference in the duration of disease between males and females (1.76 and 1.70 years, respectively). Patient characteristics by age group are summarized in eTable 1.
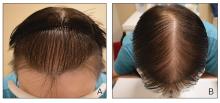
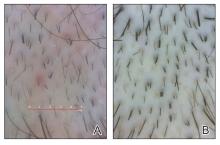
The pediatric patients in our study exhibited hair loss patterns similar to those typically observed in adults. Male patients typically showed diffuse thinning on the crown and varying degrees of temporal thinning, while female patients demonstrated diffuse thinning on the crown with a preserved frontal hairline; however, 5 (8.3%) male patients presented with Christmas tree– like pattern of hair loss with a preserved hairline and a thinning crown (Figures 1 and 2).
Diffuse thinning of the hair on the crown demonstrated a Christmas tree-like pattern with a preserved frontal hairline.
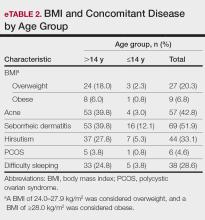
BMI and Comorbidities—Among our study sample, 27.1% (36/133) of patients were identified as overweight or obese. It came to our attention that the prevalence of patients who were overweight and obese was notably higher in patients aged older than 14 but younger than 18 years compared with those aged 14 years or younger (24.1% vs 3.0% [32/133 vs 4/133]). A more detailed analysis of patients who were overweight and obese is outlined in eTable 2.
Seborrheic dermatitis was identified as the most prevalent comorbidity associated with pediatric AGA (51.9% [69/133]), followed by acne (42.8% [57/133]), hirsutism (33.1% [44/133]), and sleep disturbances/insomnia (28.6% [38/133]). The prevalence of these comorbidities varied by age group, with a higher incidence observed among patients aged older than 14 years as compared to those aged 14 years or younger.
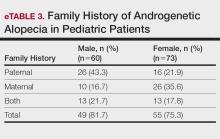
Family History of AGA—Our study results indicated that most (78.2% [104/133]) patients had a family history of AGA. Among males and females, 81.7% and 75.3% (49/60 and 55/73) had a positive family history, respectively. Further analysis showed that 43.3% (26/60) of males and 21.9% (16/73) of females reported AGA in their father, while 16.7% (10/60) of males and 35.6% insert (26/73) of females reported AGA in their mother. Both parents were affected in 21.7% (13/60) of male patients and 17.8% (13/73) of female patients (eTable 3).
Related Laboratory Tests of Pediatric Patients With AGA—The results of laboratory testing for vitamin D deficiency, low SHBG, high testosterone, and low ferritin levels in the study sample are outlined in eTable 4. Among the study participants, 15.9% (10/63) of females exhibited increased levels of both free and total testosterone. Low SHBG was observed in 47.1% (56/119) of patients, with a slightly higher proportion in males (48.2% [27/56] than females (46.0% [29/63]). Vitamin D deficiency was prevalent in 60.5% (72/119) of the study population, with a higher incidence rate in females (71.4%[45/63]) compared to males (48.2%[27/56]). Moreover, 21.8% (26/119) of pediatric patients had low ferritin levels, with a higher incidence rate in females (33.3%[21/63]) compared to males (8.9%[5/56]).
Female Patients With PCOS—In our study, 6 (8.2%) female patients with AGA had been diagnosed with PCOS prior to their referral to the First Affiliated Hospital of Nanjing Medical University. Information regarding their age at treatment, hair loss grade, comorbidities, and laboratory test results is provided in eTable 5.
Degree of Hair Loss at First Visit—In male pediatric patients with AGA, the majority were classified as M type according to the basic and specific classification. Specifically, the main hairloss level in males was concentrated in M1 and M2 (80.0% [48/60]), while specific type F was mainly distributed in F1 and F2 (81.7% [49/60]), and specific type V was mainly distributed in V1 and V2 (80.0% [48/60]). On the other hand, female patients were mainly (87.7% [64/73]) classified as type I or II in the Ludwig scale.
Clinical Features of Trichoscopy Examinations at First Visit—We present the trichoscopic findings of our study regarding hair characteristics, including hair density, hair diameter, terminal hair ratio, and vellus hair ratio, among male and female pediatric participants stratified into 2 age groups: 14 years or younger, and older than 14 but younger than 18 years. In males, those aged 14 years or younger had a lower average hair density than those older than 14 years but thicker hair diameter. Conversely, males aged 14 years and older were more likely to seek treatment of hair loss than those aged 14 years or younger. Among females, those older than 14 years had higher hair density, hair diameter, and terminal hair ratio than those younger than 14 years. Hair trichoscopy characteristics among pediatric patients with AGA in our study population were similar to those of adults with AGA (Figure 3).
Efficacy and Adverse Effects of Topical Minoxidil—There were 56 (42.1%) patients who had used topical minoxidil for more than 6 months: 33 (58.9%) males and 23 (41.1%) females. In terms of efficacy, 51 (91.1%) patients responded positively, demonstrating improved scalp coverage, increased hair density, or greater hair diameter. There were 2 (3.6%) cases of minor adverse reactions: 1 case of scalp itching with increased dandruff that improved with local symptomatic treatment, and 1 case of hirsutism, which improved after discontinuing the drug. Among the 28 (50.0%) pediatric patients who used topical minoxidil for more than 12 months, there were no reported adverse reactions. Overall, topical minoxidil was effective and well tolerated in pediatric patients, with mild adverse reactions.
Comment
In our study, the youngest AGA patient was 10 years old, which is slightly older than a 6-year-old patient reported in the literature.14 Females showed a higher incidence of AGA compared to males, which is consistent with some previous studies14,15 but contradicts the findings of Gonzalez et al16 and Kim et al.17 We speculate that the differences in AGA incidence could be attributed to the diverse genetic background and racial disparities between the populations included in the study by Gonzalez et al16—primarily White patients from Europe and the United States—and our study, which included individuals from East Asia. Furthermore, variations in lifestyle and environment in Europe and the United States vs Asia (eg, dietary habits, stress, environmental pollution) may contribute to the differing sexspecific incidence rates. Additionally, our study showed that female patients tended to experience AGA at a younger age than male patients, as indicated by younger age of disease onset and at the initial visit. These findings are consistent with other studies reporting a slightly younger age of disease onset in female patients.14,16,17 The importance lies in raising awareness among both patients and physicians about early-onset AGA, facilitating earlier detection, diagnosis, and treatment. Furthermore, our study revealed a higher prevalence of a positive family history of AGA in our study population (78.2%) compared to other studies.14 Paternal family history was more commonly observed than maternal history (81.7% and 75.3%, respectively); moreover, 19.5% of patients reported a positive family history of AGA in both parents. Therefore, it is essential to raise awareness among pediatric patients with a positive family history of AGA, as they may experience hair loss at a younger age.
Patients with AGA commonly present with concurrent skin conditions, most notably acne, seborrheic dermatitis, and hirsutism. Therefore, it is important to monitor these associated diseases and adopt appropriate treatments. Moreover, it is worth mentioning that a considerable number of pediatric patients reported experiencing sleep difficulties. It is well known that sleep disturbances can lead to hormonal abnormalities, which are also a risk factor for AGA.18-20 Therefore, further research is needed to investigate whether treating sleep disturbances can delay onset or progression of pediatric AGA. A previous retrospective study reported a PCOS prevalence of 47.4% (9/19) in adolescent females with AGA,16 but our study observed a much lower incidence of 4.5%. This discrepancy may be due to the fact that diagnostic imaging was not required for all female patients suspected of having PCOS in our study, which may have resulted in the exclusion of some undiagnosed PCOS cases from the data analysis.
In our study, a considerable proportion of patients exhibited moderate hair loss at their first visit, and there were differences in hair density and diameter among different age groups, with female patients having finer hair than male patients. Therefore, it is necessary to raise awareness of and perform early diagnosis and treatment of AGA in pediatric patients presenting with hair loss. Upon evaluation of laboratory results, we observed a notable proportion of pediatric patients with AGA who had low levels of vitamin D, SHBG, and ferritin. Notably, female patients were more susceptible to low vitamin D levels compared with males. Screening for these indicators, particularly in female patients, could aid in the diagnosis and treatment of pediatric AGA. Surprisingly, testosterone levels did not show a significant increase in male patients with AGA. Furthermore, only a small percentage of female patients exhibited elevated testosterone levels, indicating that androgens may not play a dominant role in the pathogenesis of male pediatric AGA and that other factors and mechanisms may be involved. Although AGA has been extensively studied in adults, there is limited knowledge about its occurrence and characteristics in children and adolescents. Our study represents one of the few investigations into AGA in this population and is among the largest to explore the clinical features, laboratory testing and results, trichoscopic characteristics, and comorbidities in Chinese pediatric patients with AGA. Our findings offer valuable insights into early clinical characteristics of pediatric AGA in this specific demographic population to inform future research directions and clinical practice guidelines.
Given that we conducted a retrospective study with a relatively small sample size from a single clinic site, the generalizability of our research findings may be limited. In addition, the patients included in our study did not have frequent routine testing for metabolic and hormonal indicators to analyze further correlations between hormonal changes with severity of pediatric AGA. Future research with prospective multicenter designs and larger sample sizes are needed to increase representativeness and generalizability, and comprehensive testing is needed to validate and extend our findings. Furthermore, the psychological impact among pediatric patients with AGA warrants further investigation on early intervention to reduce psychological stress.
Besides enhancing the understanding of AGA in children and adolescents among dermatologists and pediatricians, there is a need for individualized, step-by-step, and comprehensive treatment. Initial assessment generally includes addressing hormonal disorders such as seborrheic dermatitis, folliculitis, PCOS, and acne. Some adult treatments may be effective in pediatric cases. In one study of 15 pediatric patients using minoxidil 5% daily (6 females, 4 males), 4 (66.7%) females had stable alopecia (follow-up, >6 months); 4 (44.4%) males using minoxidil 5% daily and 1 mg finasteride and 5 (55.6%) taking 1 mg of finasteride alone showed hair density gains.16 In another study,21 373 adolescents with AGA (286 boys, 87 girls; age range, 10–17 years) were treated with topical minoxidil solution over an 18-month period, with 95.0% responding positively: 54.0% showed improved scalp coverage, and 41.0% experienced slower hair thinning. Topical minoxidil generally is well tolerated in pediatric patients with no significant impact on blood pressure, pulse rate, or other vital signs.21 The primary adverse reactions to topical minoxidil observed in clinical practice are mild scalp irritation and increased facial hair, which usually resolve upon discontinuation.22 In China, topical minoxidil (available in 2% or 5% concentrations) commonly is used in children and adolescents, with adjustments made based on treatment response and adverse effects. Despite its proven efficacy and tolerability, it is essential that adverse effects be promptly communicated to health care providers for appropriate dosage adjustments, and that concurrent conditions, such as vitamin D and iron deficiencies, be adequately managed. Encouraging patients to adhere to prescribed medications and undergo long-term follow-up typically results in favorable outcomes.
- Jiang W, Yan Q, Tu P, et al. Chinese expert consensus on diagnosis and management of androgenic alopecia in both males and females. Int J Dermatol Venereol. 2019;3:195-202.
- Griggs J, Burroway B, Tosti A. Pediatric androgenetic alopecia: a review. J Am Acad Dermatol. 2021;85:1267-1273.
- Alfredo R, Andrea D, Flavia P. The diagnosis of androgenetic alopecia in children: considerations of pathophysiological plausibility. Australas J Dermatol. 2019;60:279-283.
- Sarkar P, Chakraborti K, Mondal S. Association of metabolic syndrome with early-onset androgenetic alopecia: a case-control study.
Iran J Dermatol. 2022;25:106-110. - Qiu Y, Zhou X, Fu S, et al. Systematic review and meta-analysis of the association between metabolic syndrome and androgenetic alopecia. Acta Derm Venereol. 2022;102:adv000645.
- Memon FH, Rahimoon AG. Androgenetic alopecia as a marker of metabolic syndrome. J Pharm Res Int. 2021;33:146-153.
- Rodríguez-Gutiérrez R, Salcido-Montenegro A, González-González JG. Early clinical expressions of insulin resistance: the real enemy to look for. Diabetes Ther. 2018;9:435-438.
- Wang YX, Chen XW, Wang SB, et al. Association between androgenic alopecia and coronary artery disease: a cross-sectional study of Han Chinese male population. Int J Gen Med. 2021;14:4809-4818.
- Tu YA, Lin SJ, Chen PL, et al. HSD3B1 gene polymorphism and female pattern hair loss in women with polycystic ovary syndrome. J Formos Med Assoc. 2019;118:1225-1231.
- Sanke S, Chander R, Jain A, et al. A comparison of the hormonal profile of early androgenetic alopecia in men with the phenotypic equivalent of polycystic ovarian syndrome in women. JAMA Dermatol. 2016;152:986-991.
- National Health Commission of the People’s Republic of China. (2021). Chinese Guidelines for the Prevention and Control of Overweight and Obesity in Adults.
- Lee WS, Ro BI, Hong SP. A new classification of pattern hair loss that is universal for men and women: basic and specific (BASP) classification. J Am Acad Dermatol. 2007;57:37-46.
- Ludwig, E. Classification of the types of androgenetic alopecia (common baldness) occurring in the female sex. Br J Dermatol. 1977;97:247-254.
- Tosti A, Iorizzo M, Piraccini BM. Androgenetic alopecia in children: report of 20 cases. Br J Dermatol. 2005;152:556-559.
- Özcan D. Pediatric androgenetic alopecia: a retrospective review of clinical characteristics, hormonal assays and metabolic syndrome risk factors in 23 patients. An Bras Dermatol. 2022;97:166-172.
- Gonzalez ME, Cantatore-Francis J, Orlow SJ. Androgenetic alopecia in the paediatric population: a retrospective review of 57 patients. Br J Dermatol. 2010;163:378-385.
- Kim BJ, Kim JY, Eun HC. Androgenetic alopecia in adolescents: a report of 43 cases. J Dermatol. 2006;33:696-699.
- B Liamsombut S, Pomsoong C, Kositkuljorn C. Sleep quality in men with androgenetic alopecia. Sleep Breath. 2023;27:371-378.
- Baik I, Lee S, Thomas RJ. Obstructive sleep apnea, low transferrin saturation levels, and male-pattern baldness. Int J Dermatol. 2019;58:67-74.
- Yi Y, Qiu J, Jia J. Severity of androgenetic alopecia associated with poor sleeping habits and carnivorous eating and junk food consumption—a web-based investigation of male pattern hair loss in China. Dermatol Ther. 2020;33:E13273.
- Price VH. Androgenetic alopecia in adolescents. Cutis. 2003;71:115-121.
- Gomes TF, Soares RO. Pediatric androgenetic alopecia: an updated review. J Dtsch Dermatol Ges. 2023;21:19-25.
Androgenetic alopecia (AGA) is the most common type of hair loss after adolescence, with a high prevalence of 21.3% among males and 6.0% among females in China.1 In men, AGA manifests as diffuse hair loss in the frontal and temporal areas of the scalp; in women, it is characterized by thinning of the hair on the top of the head with a wide part and less recession of the frontal line. Although the specific pathogenesis of AGA still is unclear, it is believed to be related mainly to genetics and androgen levels.1 Androgenetic alopecia is not considered a life-threatening medical condition, but it can have a major impact on patients’ self-esteem and quality of life.
The prevalence of pediatric AGA has been steadily rising over the past few decades and is thought to be correlated to a hyperinsulinemic diet and elevated circulating androgens at younger ages, resulting in early onset in genetically susceptible children and adolescents.2,3 Additionally, studies have shown that early-onset AGA is associated with metabolic syndrome,4-6 which includes conditions such as obesity, insulin resistance, hyperglycemia, and dyslipidemia.7,8 Furthermore, polycystic ovary syndrome (PCOS) is commonly observed in adolescent girls with early-onset AGA. The condition is associated with hormonal imbalances, particularly elevated androgens, which can contribute to the early onset of AGA. In girls, these hormonal changes may accelerate hair thinning and hair loss, making AGA a potential early indicator of underlying PCOS.9,10
Available research on early-onset AGA in pediatric patients is limited, with most studies having a relatively small sample size and generalized findings. Data on pediatric AGA in China is scarce; therefore, the objective of this retrospective study was to analyze the clinical, laboratory, and trichoscopic features of AGA in 133 pediatric patients with AGA who visited the hair disease clinic of the Department of Dermatology at the First Affiliated Hospital of Nanjing Medical University (Nanjing, China), from January 2010 to December 2023.
Methods
Study Population—Pediatric patients with early-onset AGA who were registered for outpatient consultations at the hair disease clinic of the Department of Dermatology at The First Affiliated Hospital of Nanjing Medical University from January 2010 to December 2023 were included. Patients aged 18 years and younger with a definitive diagnosis of AGA were selected for data collection and analysis. Any uncertain information was confirmed through telephone follow-up with patients.
Collection of Demographic Information and Laboratory Tests—Patient demographics and medical history including age, sex, age at disease onset, and duration of AGA were collected from the electronic medical record. Height and weight also were collected to calculate patients’ body mass index (BMI). Detailed laboratory test results were recorded, including assessments of sex hormone—binding globulin (SHBG), vitamin D, testosterone, and ferritin.
Analysis of Comorbidities—Due to the influence of genetic factors on body composition, there are differences in how obesity is defined across racial populations. The World Health Organization international standard defines the term overweight as a BMI greater than 25 and obese as a BMI greater than 30; however, the World Health Organization recommends a lower definition standard for these classifications in the Chinese population. China established specific BMI standards for classification of patients as overweight (24.0.27.9 kg/m2) and obese (≥28 kg/m2).11 During outpatient consultations, a comprehensive medical history was obtained from each patient, including the presence of PCOS, acne, seborrheic dermatitis, hirsutism, and sleeping disorders. During routine outpatient assessments, experienced dermatologists (including W.F.) determined the presence of symptoms and confirmed the diagnosis.
Hair Loss Classification and Trichoscopy—Hair loss patterns for male patients were assessed using the basic and specific classification system, while the Ludwig scale was utilized for female patients.12,13 Trichoscopy was utilized with high-resolution imaging systems and advanced software for image analysis, enabling precise assessment of hair in different scalp regions. Parameters such as hair density, hair diameter, percentage of terminal hairs, and percentage of vellus hair were recorded to monitor changes in hair growth for the patients.
Statistical Analysis—Categorical data were analyzed using the x2 test. A P value less than .05 was considered statistically significant. All statistical analyses were conducted using SPSS software version 26 (IBM).
Results
Patient Characteristics and Hair Loss Patterns—A sample of 133 pediatric patients (60 males, 73 females) who were diagnosed with AGA at the hair disease clinic of the Department of Dermatology at the First Affiliated Hospital of Nanjing Medical University from January 2010 to December 2023 were selected. The mean age of the patients was 15.5 years (range, 10–18 years). The mean age was slightly lower in females compared with males (15.05 vs 16.19 years, respectively). Additionally, females showed earlier onset of the disease, with a mean age at onset of 13.41 years compared to 14.44 years in males. The time between onset of AGA symptoms and first seeking medical care ranged from 4 months to 3 years, with a mean disease duration of 1.72 years. There was no significant difference in the duration of disease between males and females (1.76 and 1.70 years, respectively). Patient characteristics by age group are summarized in eTable 1.
The pediatric patients in our study exhibited hair loss patterns similar to those typically observed in adults. Male patients typically showed diffuse thinning on the crown and varying degrees of temporal thinning, while female patients demonstrated diffuse thinning on the crown with a preserved frontal hairline; however, 5 (8.3%) male patients presented with Christmas tree– like pattern of hair loss with a preserved hairline and a thinning crown (Figures 1 and 2).
Diffuse thinning of the hair on the crown demonstrated a Christmas tree-like pattern with a preserved frontal hairline.
BMI and Comorbidities—Among our study sample, 27.1% (36/133) of patients were identified as overweight or obese. It came to our attention that the prevalence of patients who were overweight and obese was notably higher in patients aged older than 14 but younger than 18 years compared with those aged 14 years or younger (24.1% vs 3.0% [32/133 vs 4/133]). A more detailed analysis of patients who were overweight and obese is outlined in eTable 2.
Seborrheic dermatitis was identified as the most prevalent comorbidity associated with pediatric AGA (51.9% [69/133]), followed by acne (42.8% [57/133]), hirsutism (33.1% [44/133]), and sleep disturbances/insomnia (28.6% [38/133]). The prevalence of these comorbidities varied by age group, with a higher incidence observed among patients aged older than 14 years as compared to those aged 14 years or younger.
Family History of AGA—Our study results indicated that most (78.2% [104/133]) patients had a family history of AGA. Among males and females, 81.7% and 75.3% (49/60 and 55/73) had a positive family history, respectively. Further analysis showed that 43.3% (26/60) of males and 21.9% (16/73) of females reported AGA in their father, while 16.7% (10/60) of males and 35.6% insert (26/73) of females reported AGA in their mother. Both parents were affected in 21.7% (13/60) of male patients and 17.8% (13/73) of female patients (eTable 3).
Related Laboratory Tests of Pediatric Patients With AGA—The results of laboratory testing for vitamin D deficiency, low SHBG, high testosterone, and low ferritin levels in the study sample are outlined in eTable 4. Among the study participants, 15.9% (10/63) of females exhibited increased levels of both free and total testosterone. Low SHBG was observed in 47.1% (56/119) of patients, with a slightly higher proportion in males (48.2% [27/56] than females (46.0% [29/63]). Vitamin D deficiency was prevalent in 60.5% (72/119) of the study population, with a higher incidence rate in females (71.4%[45/63]) compared to males (48.2%[27/56]). Moreover, 21.8% (26/119) of pediatric patients had low ferritin levels, with a higher incidence rate in females (33.3%[21/63]) compared to males (8.9%[5/56]).
Female Patients With PCOS—In our study, 6 (8.2%) female patients with AGA had been diagnosed with PCOS prior to their referral to the First Affiliated Hospital of Nanjing Medical University. Information regarding their age at treatment, hair loss grade, comorbidities, and laboratory test results is provided in eTable 5.
Degree of Hair Loss at First Visit—In male pediatric patients with AGA, the majority were classified as M type according to the basic and specific classification. Specifically, the main hairloss level in males was concentrated in M1 and M2 (80.0% [48/60]), while specific type F was mainly distributed in F1 and F2 (81.7% [49/60]), and specific type V was mainly distributed in V1 and V2 (80.0% [48/60]). On the other hand, female patients were mainly (87.7% [64/73]) classified as type I or II in the Ludwig scale.
Clinical Features of Trichoscopy Examinations at First Visit—We present the trichoscopic findings of our study regarding hair characteristics, including hair density, hair diameter, terminal hair ratio, and vellus hair ratio, among male and female pediatric participants stratified into 2 age groups: 14 years or younger, and older than 14 but younger than 18 years. In males, those aged 14 years or younger had a lower average hair density than those older than 14 years but thicker hair diameter. Conversely, males aged 14 years and older were more likely to seek treatment of hair loss than those aged 14 years or younger. Among females, those older than 14 years had higher hair density, hair diameter, and terminal hair ratio than those younger than 14 years. Hair trichoscopy characteristics among pediatric patients with AGA in our study population were similar to those of adults with AGA (Figure 3).
Efficacy and Adverse Effects of Topical Minoxidil—There were 56 (42.1%) patients who had used topical minoxidil for more than 6 months: 33 (58.9%) males and 23 (41.1%) females. In terms of efficacy, 51 (91.1%) patients responded positively, demonstrating improved scalp coverage, increased hair density, or greater hair diameter. There were 2 (3.6%) cases of minor adverse reactions: 1 case of scalp itching with increased dandruff that improved with local symptomatic treatment, and 1 case of hirsutism, which improved after discontinuing the drug. Among the 28 (50.0%) pediatric patients who used topical minoxidil for more than 12 months, there were no reported adverse reactions. Overall, topical minoxidil was effective and well tolerated in pediatric patients, with mild adverse reactions.
Comment
In our study, the youngest AGA patient was 10 years old, which is slightly older than a 6-year-old patient reported in the literature.14 Females showed a higher incidence of AGA compared to males, which is consistent with some previous studies14,15 but contradicts the findings of Gonzalez et al16 and Kim et al.17 We speculate that the differences in AGA incidence could be attributed to the diverse genetic background and racial disparities between the populations included in the study by Gonzalez et al16—primarily White patients from Europe and the United States—and our study, which included individuals from East Asia. Furthermore, variations in lifestyle and environment in Europe and the United States vs Asia (eg, dietary habits, stress, environmental pollution) may contribute to the differing sexspecific incidence rates. Additionally, our study showed that female patients tended to experience AGA at a younger age than male patients, as indicated by younger age of disease onset and at the initial visit. These findings are consistent with other studies reporting a slightly younger age of disease onset in female patients.14,16,17 The importance lies in raising awareness among both patients and physicians about early-onset AGA, facilitating earlier detection, diagnosis, and treatment. Furthermore, our study revealed a higher prevalence of a positive family history of AGA in our study population (78.2%) compared to other studies.14 Paternal family history was more commonly observed than maternal history (81.7% and 75.3%, respectively); moreover, 19.5% of patients reported a positive family history of AGA in both parents. Therefore, it is essential to raise awareness among pediatric patients with a positive family history of AGA, as they may experience hair loss at a younger age.
Patients with AGA commonly present with concurrent skin conditions, most notably acne, seborrheic dermatitis, and hirsutism. Therefore, it is important to monitor these associated diseases and adopt appropriate treatments. Moreover, it is worth mentioning that a considerable number of pediatric patients reported experiencing sleep difficulties. It is well known that sleep disturbances can lead to hormonal abnormalities, which are also a risk factor for AGA.18-20 Therefore, further research is needed to investigate whether treating sleep disturbances can delay onset or progression of pediatric AGA. A previous retrospective study reported a PCOS prevalence of 47.4% (9/19) in adolescent females with AGA,16 but our study observed a much lower incidence of 4.5%. This discrepancy may be due to the fact that diagnostic imaging was not required for all female patients suspected of having PCOS in our study, which may have resulted in the exclusion of some undiagnosed PCOS cases from the data analysis.
In our study, a considerable proportion of patients exhibited moderate hair loss at their first visit, and there were differences in hair density and diameter among different age groups, with female patients having finer hair than male patients. Therefore, it is necessary to raise awareness of and perform early diagnosis and treatment of AGA in pediatric patients presenting with hair loss. Upon evaluation of laboratory results, we observed a notable proportion of pediatric patients with AGA who had low levels of vitamin D, SHBG, and ferritin. Notably, female patients were more susceptible to low vitamin D levels compared with males. Screening for these indicators, particularly in female patients, could aid in the diagnosis and treatment of pediatric AGA. Surprisingly, testosterone levels did not show a significant increase in male patients with AGA. Furthermore, only a small percentage of female patients exhibited elevated testosterone levels, indicating that androgens may not play a dominant role in the pathogenesis of male pediatric AGA and that other factors and mechanisms may be involved. Although AGA has been extensively studied in adults, there is limited knowledge about its occurrence and characteristics in children and adolescents. Our study represents one of the few investigations into AGA in this population and is among the largest to explore the clinical features, laboratory testing and results, trichoscopic characteristics, and comorbidities in Chinese pediatric patients with AGA. Our findings offer valuable insights into early clinical characteristics of pediatric AGA in this specific demographic population to inform future research directions and clinical practice guidelines.
Given that we conducted a retrospective study with a relatively small sample size from a single clinic site, the generalizability of our research findings may be limited. In addition, the patients included in our study did not have frequent routine testing for metabolic and hormonal indicators to analyze further correlations between hormonal changes with severity of pediatric AGA. Future research with prospective multicenter designs and larger sample sizes are needed to increase representativeness and generalizability, and comprehensive testing is needed to validate and extend our findings. Furthermore, the psychological impact among pediatric patients with AGA warrants further investigation on early intervention to reduce psychological stress.
Besides enhancing the understanding of AGA in children and adolescents among dermatologists and pediatricians, there is a need for individualized, step-by-step, and comprehensive treatment. Initial assessment generally includes addressing hormonal disorders such as seborrheic dermatitis, folliculitis, PCOS, and acne. Some adult treatments may be effective in pediatric cases. In one study of 15 pediatric patients using minoxidil 5% daily (6 females, 4 males), 4 (66.7%) females had stable alopecia (follow-up, >6 months); 4 (44.4%) males using minoxidil 5% daily and 1 mg finasteride and 5 (55.6%) taking 1 mg of finasteride alone showed hair density gains.16 In another study,21 373 adolescents with AGA (286 boys, 87 girls; age range, 10–17 years) were treated with topical minoxidil solution over an 18-month period, with 95.0% responding positively: 54.0% showed improved scalp coverage, and 41.0% experienced slower hair thinning. Topical minoxidil generally is well tolerated in pediatric patients with no significant impact on blood pressure, pulse rate, or other vital signs.21 The primary adverse reactions to topical minoxidil observed in clinical practice are mild scalp irritation and increased facial hair, which usually resolve upon discontinuation.22 In China, topical minoxidil (available in 2% or 5% concentrations) commonly is used in children and adolescents, with adjustments made based on treatment response and adverse effects. Despite its proven efficacy and tolerability, it is essential that adverse effects be promptly communicated to health care providers for appropriate dosage adjustments, and that concurrent conditions, such as vitamin D and iron deficiencies, be adequately managed. Encouraging patients to adhere to prescribed medications and undergo long-term follow-up typically results in favorable outcomes.
Androgenetic alopecia (AGA) is the most common type of hair loss after adolescence, with a high prevalence of 21.3% among males and 6.0% among females in China.1 In men, AGA manifests as diffuse hair loss in the frontal and temporal areas of the scalp; in women, it is characterized by thinning of the hair on the top of the head with a wide part and less recession of the frontal line. Although the specific pathogenesis of AGA still is unclear, it is believed to be related mainly to genetics and androgen levels.1 Androgenetic alopecia is not considered a life-threatening medical condition, but it can have a major impact on patients’ self-esteem and quality of life.
The prevalence of pediatric AGA has been steadily rising over the past few decades and is thought to be correlated to a hyperinsulinemic diet and elevated circulating androgens at younger ages, resulting in early onset in genetically susceptible children and adolescents.2,3 Additionally, studies have shown that early-onset AGA is associated with metabolic syndrome,4-6 which includes conditions such as obesity, insulin resistance, hyperglycemia, and dyslipidemia.7,8 Furthermore, polycystic ovary syndrome (PCOS) is commonly observed in adolescent girls with early-onset AGA. The condition is associated with hormonal imbalances, particularly elevated androgens, which can contribute to the early onset of AGA. In girls, these hormonal changes may accelerate hair thinning and hair loss, making AGA a potential early indicator of underlying PCOS.9,10
Available research on early-onset AGA in pediatric patients is limited, with most studies having a relatively small sample size and generalized findings. Data on pediatric AGA in China is scarce; therefore, the objective of this retrospective study was to analyze the clinical, laboratory, and trichoscopic features of AGA in 133 pediatric patients with AGA who visited the hair disease clinic of the Department of Dermatology at the First Affiliated Hospital of Nanjing Medical University (Nanjing, China), from January 2010 to December 2023.
Methods
Study Population—Pediatric patients with early-onset AGA who were registered for outpatient consultations at the hair disease clinic of the Department of Dermatology at The First Affiliated Hospital of Nanjing Medical University from January 2010 to December 2023 were included. Patients aged 18 years and younger with a definitive diagnosis of AGA were selected for data collection and analysis. Any uncertain information was confirmed through telephone follow-up with patients.
Collection of Demographic Information and Laboratory Tests—Patient demographics and medical history including age, sex, age at disease onset, and duration of AGA were collected from the electronic medical record. Height and weight also were collected to calculate patients’ body mass index (BMI). Detailed laboratory test results were recorded, including assessments of sex hormone—binding globulin (SHBG), vitamin D, testosterone, and ferritin.
Analysis of Comorbidities—Due to the influence of genetic factors on body composition, there are differences in how obesity is defined across racial populations. The World Health Organization international standard defines the term overweight as a BMI greater than 25 and obese as a BMI greater than 30; however, the World Health Organization recommends a lower definition standard for these classifications in the Chinese population. China established specific BMI standards for classification of patients as overweight (24.0.27.9 kg/m2) and obese (≥28 kg/m2).11 During outpatient consultations, a comprehensive medical history was obtained from each patient, including the presence of PCOS, acne, seborrheic dermatitis, hirsutism, and sleeping disorders. During routine outpatient assessments, experienced dermatologists (including W.F.) determined the presence of symptoms and confirmed the diagnosis.
Hair Loss Classification and Trichoscopy—Hair loss patterns for male patients were assessed using the basic and specific classification system, while the Ludwig scale was utilized for female patients.12,13 Trichoscopy was utilized with high-resolution imaging systems and advanced software for image analysis, enabling precise assessment of hair in different scalp regions. Parameters such as hair density, hair diameter, percentage of terminal hairs, and percentage of vellus hair were recorded to monitor changes in hair growth for the patients.
Statistical Analysis—Categorical data were analyzed using the x2 test. A P value less than .05 was considered statistically significant. All statistical analyses were conducted using SPSS software version 26 (IBM).
Results
Patient Characteristics and Hair Loss Patterns—A sample of 133 pediatric patients (60 males, 73 females) who were diagnosed with AGA at the hair disease clinic of the Department of Dermatology at the First Affiliated Hospital of Nanjing Medical University from January 2010 to December 2023 were selected. The mean age of the patients was 15.5 years (range, 10–18 years). The mean age was slightly lower in females compared with males (15.05 vs 16.19 years, respectively). Additionally, females showed earlier onset of the disease, with a mean age at onset of 13.41 years compared to 14.44 years in males. The time between onset of AGA symptoms and first seeking medical care ranged from 4 months to 3 years, with a mean disease duration of 1.72 years. There was no significant difference in the duration of disease between males and females (1.76 and 1.70 years, respectively). Patient characteristics by age group are summarized in eTable 1.
The pediatric patients in our study exhibited hair loss patterns similar to those typically observed in adults. Male patients typically showed diffuse thinning on the crown and varying degrees of temporal thinning, while female patients demonstrated diffuse thinning on the crown with a preserved frontal hairline; however, 5 (8.3%) male patients presented with Christmas tree– like pattern of hair loss with a preserved hairline and a thinning crown (Figures 1 and 2).
Diffuse thinning of the hair on the crown demonstrated a Christmas tree-like pattern with a preserved frontal hairline.
BMI and Comorbidities—Among our study sample, 27.1% (36/133) of patients were identified as overweight or obese. It came to our attention that the prevalence of patients who were overweight and obese was notably higher in patients aged older than 14 but younger than 18 years compared with those aged 14 years or younger (24.1% vs 3.0% [32/133 vs 4/133]). A more detailed analysis of patients who were overweight and obese is outlined in eTable 2.
Seborrheic dermatitis was identified as the most prevalent comorbidity associated with pediatric AGA (51.9% [69/133]), followed by acne (42.8% [57/133]), hirsutism (33.1% [44/133]), and sleep disturbances/insomnia (28.6% [38/133]). The prevalence of these comorbidities varied by age group, with a higher incidence observed among patients aged older than 14 years as compared to those aged 14 years or younger.
Family History of AGA—Our study results indicated that most (78.2% [104/133]) patients had a family history of AGA. Among males and females, 81.7% and 75.3% (49/60 and 55/73) had a positive family history, respectively. Further analysis showed that 43.3% (26/60) of males and 21.9% (16/73) of females reported AGA in their father, while 16.7% (10/60) of males and 35.6% insert (26/73) of females reported AGA in their mother. Both parents were affected in 21.7% (13/60) of male patients and 17.8% (13/73) of female patients (eTable 3).
Related Laboratory Tests of Pediatric Patients With AGA—The results of laboratory testing for vitamin D deficiency, low SHBG, high testosterone, and low ferritin levels in the study sample are outlined in eTable 4. Among the study participants, 15.9% (10/63) of females exhibited increased levels of both free and total testosterone. Low SHBG was observed in 47.1% (56/119) of patients, with a slightly higher proportion in males (48.2% [27/56] than females (46.0% [29/63]). Vitamin D deficiency was prevalent in 60.5% (72/119) of the study population, with a higher incidence rate in females (71.4%[45/63]) compared to males (48.2%[27/56]). Moreover, 21.8% (26/119) of pediatric patients had low ferritin levels, with a higher incidence rate in females (33.3%[21/63]) compared to males (8.9%[5/56]).
Female Patients With PCOS—In our study, 6 (8.2%) female patients with AGA had been diagnosed with PCOS prior to their referral to the First Affiliated Hospital of Nanjing Medical University. Information regarding their age at treatment, hair loss grade, comorbidities, and laboratory test results is provided in eTable 5.
Degree of Hair Loss at First Visit—In male pediatric patients with AGA, the majority were classified as M type according to the basic and specific classification. Specifically, the main hairloss level in males was concentrated in M1 and M2 (80.0% [48/60]), while specific type F was mainly distributed in F1 and F2 (81.7% [49/60]), and specific type V was mainly distributed in V1 and V2 (80.0% [48/60]). On the other hand, female patients were mainly (87.7% [64/73]) classified as type I or II in the Ludwig scale.
Clinical Features of Trichoscopy Examinations at First Visit—We present the trichoscopic findings of our study regarding hair characteristics, including hair density, hair diameter, terminal hair ratio, and vellus hair ratio, among male and female pediatric participants stratified into 2 age groups: 14 years or younger, and older than 14 but younger than 18 years. In males, those aged 14 years or younger had a lower average hair density than those older than 14 years but thicker hair diameter. Conversely, males aged 14 years and older were more likely to seek treatment of hair loss than those aged 14 years or younger. Among females, those older than 14 years had higher hair density, hair diameter, and terminal hair ratio than those younger than 14 years. Hair trichoscopy characteristics among pediatric patients with AGA in our study population were similar to those of adults with AGA (Figure 3).
Efficacy and Adverse Effects of Topical Minoxidil—There were 56 (42.1%) patients who had used topical minoxidil for more than 6 months: 33 (58.9%) males and 23 (41.1%) females. In terms of efficacy, 51 (91.1%) patients responded positively, demonstrating improved scalp coverage, increased hair density, or greater hair diameter. There were 2 (3.6%) cases of minor adverse reactions: 1 case of scalp itching with increased dandruff that improved with local symptomatic treatment, and 1 case of hirsutism, which improved after discontinuing the drug. Among the 28 (50.0%) pediatric patients who used topical minoxidil for more than 12 months, there were no reported adverse reactions. Overall, topical minoxidil was effective and well tolerated in pediatric patients, with mild adverse reactions.
Comment
In our study, the youngest AGA patient was 10 years old, which is slightly older than a 6-year-old patient reported in the literature.14 Females showed a higher incidence of AGA compared to males, which is consistent with some previous studies14,15 but contradicts the findings of Gonzalez et al16 and Kim et al.17 We speculate that the differences in AGA incidence could be attributed to the diverse genetic background and racial disparities between the populations included in the study by Gonzalez et al16—primarily White patients from Europe and the United States—and our study, which included individuals from East Asia. Furthermore, variations in lifestyle and environment in Europe and the United States vs Asia (eg, dietary habits, stress, environmental pollution) may contribute to the differing sexspecific incidence rates. Additionally, our study showed that female patients tended to experience AGA at a younger age than male patients, as indicated by younger age of disease onset and at the initial visit. These findings are consistent with other studies reporting a slightly younger age of disease onset in female patients.14,16,17 The importance lies in raising awareness among both patients and physicians about early-onset AGA, facilitating earlier detection, diagnosis, and treatment. Furthermore, our study revealed a higher prevalence of a positive family history of AGA in our study population (78.2%) compared to other studies.14 Paternal family history was more commonly observed than maternal history (81.7% and 75.3%, respectively); moreover, 19.5% of patients reported a positive family history of AGA in both parents. Therefore, it is essential to raise awareness among pediatric patients with a positive family history of AGA, as they may experience hair loss at a younger age.
Patients with AGA commonly present with concurrent skin conditions, most notably acne, seborrheic dermatitis, and hirsutism. Therefore, it is important to monitor these associated diseases and adopt appropriate treatments. Moreover, it is worth mentioning that a considerable number of pediatric patients reported experiencing sleep difficulties. It is well known that sleep disturbances can lead to hormonal abnormalities, which are also a risk factor for AGA.18-20 Therefore, further research is needed to investigate whether treating sleep disturbances can delay onset or progression of pediatric AGA. A previous retrospective study reported a PCOS prevalence of 47.4% (9/19) in adolescent females with AGA,16 but our study observed a much lower incidence of 4.5%. This discrepancy may be due to the fact that diagnostic imaging was not required for all female patients suspected of having PCOS in our study, which may have resulted in the exclusion of some undiagnosed PCOS cases from the data analysis.
In our study, a considerable proportion of patients exhibited moderate hair loss at their first visit, and there were differences in hair density and diameter among different age groups, with female patients having finer hair than male patients. Therefore, it is necessary to raise awareness of and perform early diagnosis and treatment of AGA in pediatric patients presenting with hair loss. Upon evaluation of laboratory results, we observed a notable proportion of pediatric patients with AGA who had low levels of vitamin D, SHBG, and ferritin. Notably, female patients were more susceptible to low vitamin D levels compared with males. Screening for these indicators, particularly in female patients, could aid in the diagnosis and treatment of pediatric AGA. Surprisingly, testosterone levels did not show a significant increase in male patients with AGA. Furthermore, only a small percentage of female patients exhibited elevated testosterone levels, indicating that androgens may not play a dominant role in the pathogenesis of male pediatric AGA and that other factors and mechanisms may be involved. Although AGA has been extensively studied in adults, there is limited knowledge about its occurrence and characteristics in children and adolescents. Our study represents one of the few investigations into AGA in this population and is among the largest to explore the clinical features, laboratory testing and results, trichoscopic characteristics, and comorbidities in Chinese pediatric patients with AGA. Our findings offer valuable insights into early clinical characteristics of pediatric AGA in this specific demographic population to inform future research directions and clinical practice guidelines.
Given that we conducted a retrospective study with a relatively small sample size from a single clinic site, the generalizability of our research findings may be limited. In addition, the patients included in our study did not have frequent routine testing for metabolic and hormonal indicators to analyze further correlations between hormonal changes with severity of pediatric AGA. Future research with prospective multicenter designs and larger sample sizes are needed to increase representativeness and generalizability, and comprehensive testing is needed to validate and extend our findings. Furthermore, the psychological impact among pediatric patients with AGA warrants further investigation on early intervention to reduce psychological stress.
Besides enhancing the understanding of AGA in children and adolescents among dermatologists and pediatricians, there is a need for individualized, step-by-step, and comprehensive treatment. Initial assessment generally includes addressing hormonal disorders such as seborrheic dermatitis, folliculitis, PCOS, and acne. Some adult treatments may be effective in pediatric cases. In one study of 15 pediatric patients using minoxidil 5% daily (6 females, 4 males), 4 (66.7%) females had stable alopecia (follow-up, >6 months); 4 (44.4%) males using minoxidil 5% daily and 1 mg finasteride and 5 (55.6%) taking 1 mg of finasteride alone showed hair density gains.16 In another study,21 373 adolescents with AGA (286 boys, 87 girls; age range, 10–17 years) were treated with topical minoxidil solution over an 18-month period, with 95.0% responding positively: 54.0% showed improved scalp coverage, and 41.0% experienced slower hair thinning. Topical minoxidil generally is well tolerated in pediatric patients with no significant impact on blood pressure, pulse rate, or other vital signs.21 The primary adverse reactions to topical minoxidil observed in clinical practice are mild scalp irritation and increased facial hair, which usually resolve upon discontinuation.22 In China, topical minoxidil (available in 2% or 5% concentrations) commonly is used in children and adolescents, with adjustments made based on treatment response and adverse effects. Despite its proven efficacy and tolerability, it is essential that adverse effects be promptly communicated to health care providers for appropriate dosage adjustments, and that concurrent conditions, such as vitamin D and iron deficiencies, be adequately managed. Encouraging patients to adhere to prescribed medications and undergo long-term follow-up typically results in favorable outcomes.
- Jiang W, Yan Q, Tu P, et al. Chinese expert consensus on diagnosis and management of androgenic alopecia in both males and females. Int J Dermatol Venereol. 2019;3:195-202.
- Griggs J, Burroway B, Tosti A. Pediatric androgenetic alopecia: a review. J Am Acad Dermatol. 2021;85:1267-1273.
- Alfredo R, Andrea D, Flavia P. The diagnosis of androgenetic alopecia in children: considerations of pathophysiological plausibility. Australas J Dermatol. 2019;60:279-283.
- Sarkar P, Chakraborti K, Mondal S. Association of metabolic syndrome with early-onset androgenetic alopecia: a case-control study.
Iran J Dermatol. 2022;25:106-110. - Qiu Y, Zhou X, Fu S, et al. Systematic review and meta-analysis of the association between metabolic syndrome and androgenetic alopecia. Acta Derm Venereol. 2022;102:adv000645.
- Memon FH, Rahimoon AG. Androgenetic alopecia as a marker of metabolic syndrome. J Pharm Res Int. 2021;33:146-153.
- Rodríguez-Gutiérrez R, Salcido-Montenegro A, González-González JG. Early clinical expressions of insulin resistance: the real enemy to look for. Diabetes Ther. 2018;9:435-438.
- Wang YX, Chen XW, Wang SB, et al. Association between androgenic alopecia and coronary artery disease: a cross-sectional study of Han Chinese male population. Int J Gen Med. 2021;14:4809-4818.
- Tu YA, Lin SJ, Chen PL, et al. HSD3B1 gene polymorphism and female pattern hair loss in women with polycystic ovary syndrome. J Formos Med Assoc. 2019;118:1225-1231.
- Sanke S, Chander R, Jain A, et al. A comparison of the hormonal profile of early androgenetic alopecia in men with the phenotypic equivalent of polycystic ovarian syndrome in women. JAMA Dermatol. 2016;152:986-991.
- National Health Commission of the People’s Republic of China. (2021). Chinese Guidelines for the Prevention and Control of Overweight and Obesity in Adults.
- Lee WS, Ro BI, Hong SP. A new classification of pattern hair loss that is universal for men and women: basic and specific (BASP) classification. J Am Acad Dermatol. 2007;57:37-46.
- Ludwig, E. Classification of the types of androgenetic alopecia (common baldness) occurring in the female sex. Br J Dermatol. 1977;97:247-254.
- Tosti A, Iorizzo M, Piraccini BM. Androgenetic alopecia in children: report of 20 cases. Br J Dermatol. 2005;152:556-559.
- Özcan D. Pediatric androgenetic alopecia: a retrospective review of clinical characteristics, hormonal assays and metabolic syndrome risk factors in 23 patients. An Bras Dermatol. 2022;97:166-172.
- Gonzalez ME, Cantatore-Francis J, Orlow SJ. Androgenetic alopecia in the paediatric population: a retrospective review of 57 patients. Br J Dermatol. 2010;163:378-385.
- Kim BJ, Kim JY, Eun HC. Androgenetic alopecia in adolescents: a report of 43 cases. J Dermatol. 2006;33:696-699.
- B Liamsombut S, Pomsoong C, Kositkuljorn C. Sleep quality in men with androgenetic alopecia. Sleep Breath. 2023;27:371-378.
- Baik I, Lee S, Thomas RJ. Obstructive sleep apnea, low transferrin saturation levels, and male-pattern baldness. Int J Dermatol. 2019;58:67-74.
- Yi Y, Qiu J, Jia J. Severity of androgenetic alopecia associated with poor sleeping habits and carnivorous eating and junk food consumption—a web-based investigation of male pattern hair loss in China. Dermatol Ther. 2020;33:E13273.
- Price VH. Androgenetic alopecia in adolescents. Cutis. 2003;71:115-121.
- Gomes TF, Soares RO. Pediatric androgenetic alopecia: an updated review. J Dtsch Dermatol Ges. 2023;21:19-25.
- Jiang W, Yan Q, Tu P, et al. Chinese expert consensus on diagnosis and management of androgenic alopecia in both males and females. Int J Dermatol Venereol. 2019;3:195-202.
- Griggs J, Burroway B, Tosti A. Pediatric androgenetic alopecia: a review. J Am Acad Dermatol. 2021;85:1267-1273.
- Alfredo R, Andrea D, Flavia P. The diagnosis of androgenetic alopecia in children: considerations of pathophysiological plausibility. Australas J Dermatol. 2019;60:279-283.
- Sarkar P, Chakraborti K, Mondal S. Association of metabolic syndrome with early-onset androgenetic alopecia: a case-control study.
Iran J Dermatol. 2022;25:106-110. - Qiu Y, Zhou X, Fu S, et al. Systematic review and meta-analysis of the association between metabolic syndrome and androgenetic alopecia. Acta Derm Venereol. 2022;102:adv000645.
- Memon FH, Rahimoon AG. Androgenetic alopecia as a marker of metabolic syndrome. J Pharm Res Int. 2021;33:146-153.
- Rodríguez-Gutiérrez R, Salcido-Montenegro A, González-González JG. Early clinical expressions of insulin resistance: the real enemy to look for. Diabetes Ther. 2018;9:435-438.
- Wang YX, Chen XW, Wang SB, et al. Association between androgenic alopecia and coronary artery disease: a cross-sectional study of Han Chinese male population. Int J Gen Med. 2021;14:4809-4818.
- Tu YA, Lin SJ, Chen PL, et al. HSD3B1 gene polymorphism and female pattern hair loss in women with polycystic ovary syndrome. J Formos Med Assoc. 2019;118:1225-1231.
- Sanke S, Chander R, Jain A, et al. A comparison of the hormonal profile of early androgenetic alopecia in men with the phenotypic equivalent of polycystic ovarian syndrome in women. JAMA Dermatol. 2016;152:986-991.
- National Health Commission of the People’s Republic of China. (2021). Chinese Guidelines for the Prevention and Control of Overweight and Obesity in Adults.
- Lee WS, Ro BI, Hong SP. A new classification of pattern hair loss that is universal for men and women: basic and specific (BASP) classification. J Am Acad Dermatol. 2007;57:37-46.
- Ludwig, E. Classification of the types of androgenetic alopecia (common baldness) occurring in the female sex. Br J Dermatol. 1977;97:247-254.
- Tosti A, Iorizzo M, Piraccini BM. Androgenetic alopecia in children: report of 20 cases. Br J Dermatol. 2005;152:556-559.
- Özcan D. Pediatric androgenetic alopecia: a retrospective review of clinical characteristics, hormonal assays and metabolic syndrome risk factors in 23 patients. An Bras Dermatol. 2022;97:166-172.
- Gonzalez ME, Cantatore-Francis J, Orlow SJ. Androgenetic alopecia in the paediatric population: a retrospective review of 57 patients. Br J Dermatol. 2010;163:378-385.
- Kim BJ, Kim JY, Eun HC. Androgenetic alopecia in adolescents: a report of 43 cases. J Dermatol. 2006;33:696-699.
- B Liamsombut S, Pomsoong C, Kositkuljorn C. Sleep quality in men with androgenetic alopecia. Sleep Breath. 2023;27:371-378.
- Baik I, Lee S, Thomas RJ. Obstructive sleep apnea, low transferrin saturation levels, and male-pattern baldness. Int J Dermatol. 2019;58:67-74.
- Yi Y, Qiu J, Jia J. Severity of androgenetic alopecia associated with poor sleeping habits and carnivorous eating and junk food consumption—a web-based investigation of male pattern hair loss in China. Dermatol Ther. 2020;33:E13273.
- Price VH. Androgenetic alopecia in adolescents. Cutis. 2003;71:115-121.
- Gomes TF, Soares RO. Pediatric androgenetic alopecia: an updated review. J Dtsch Dermatol Ges. 2023;21:19-25.
Clinical, Laboratory, and Trichoscopic Features of Pediatric Androgenetic Alopecia
Clinical, Laboratory, and Trichoscopic Features of Pediatric Androgenetic Alopecia
PRACTICE POINTS
- Early identification of androgenetic alopecia (AGA) is key in pediatric patients, especially in those with a family history of AGA and comorbidities such as seborrheic dermatitis, acne, or sleep disturbances.
- It is important to evaluate pediatric patients with AGA for hormonal imbalances and deficiencies in vitamin D and iron to guide treatment.
- Use targeted therapies such as topical minoxidil to treat pediatric AGA while also monitoring for adverse effects. For optimal outcomes, encourage consistent medication use and regular follow-up.
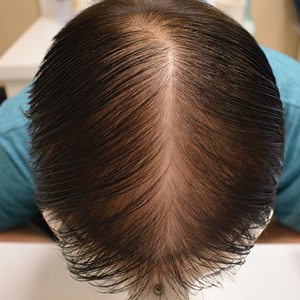
Buruli Ulcer Transmission: Environmental Pathways and Implications for Dermatologic Care
Buruli Ulcer Transmission: Environmental Pathways and Implications for Dermatologic Care
Buruli ulcer (BU) is a potentially disabling necrotizing skin and soft tissue disease caused by Mycobacterium ulcerans infection.1,2 Buruli ulcer is most common in hot and humid climates and has caused considerable morbidity in Western African countries (Côte d’Ivoire, Ghana, and Benin account for 73% of annual cases) and the temperate areas of Australia (283 reported cases in 2017).1-4 In fact, the first recognizable cases of BU were described in 6 Australian individuals living in a riverine area in 1948, although the term Buruli ulcer is derived from the increased number of cases reported from Buruli county in Uganda near the Nile River.1,3,4
From 2002 to 2017, 66,000 cases of BU were reported in 33 countries.1 While the focal distribution has been demonstrated in the tropical areas of Sri Lanka, Malaysia, Papua New Guinea, Peru, and Mexico,4 nontropical nations such as Japan also are affected. Since 1981, 66 cases have been reported in Japan with M ulcerans subspecies—primarily Shinshuense, which has adapted to higher latitudes.1 Herein, we provide an overview of the pathogenesis, clinical presentation, and treatment of BU and highlight aquatic insects and mosquitoes as possible vectors of transmission.
Pathogenesis
Mycobacterium ulcerans is a nontuberculous mycobacterium and ubiquitous acid-fast gram-positive bacillus that can be cultured using a Lowenstein-Jensen agar and has a doubling rate of 48 hours.1,5 It produces the small 174-kb plasmid pMUM001-encoded compound mycolactone, a pathogenic toxin that causes immunosuppression, analgesia, and cytotoxic-associated tissue necrosis.1,5-9
Mycolactone is a polyketide macrolide with a 12-membrane lactone with 2 attached acyl side chains.1,7 Mycolactone is synthesized by the giant polyketide synthetases of M ulcerans. Mycolactone post-transcriptionally inhibits the development of lipopolysaccharide-dependent proinflammatory mediators—specifically by blocking protein translocation from the cytosol into the endoplasmic reticulum by targeting the SEC61 translocon.1,6 The lack of translocation of 30% to 50% of proteins leads to cellular stress and apoptosis mediated by Bim/Bcl2. A single point mutation in the SEC61 translocon subunit alpha 1 gene (SEC61A1) is associated with resistance to the cytotoxic effects of mycolactone.1
There are divergent hypotheses regarding the relationship of mycolactone to the Wiskott-Aldrich syndrome protein, with some researchers suggesting that mycolactone can attach to this protein, leading to cell detachment and death.7 However, others have proposed that mycolactone inhibits mTOR, activating the Wiskott-Aldrich syndrome protein and leading to subsequent extensive cytoskeleton remodeling.1 Mycolactone also can cause hypoesthesia, either by activating type 2 angiotensin II receptors and creating downstream neuron hyperpolarization or by killing Schwann cells.1
Transmission
Buruli ulcer caused by M ulcerans has a poorly understood transmission mechanism, and further studies are required to understand the underlying pathophysiology to decrease transmission rates and associated morbidity. Buruli ulcer is widely accepted to be transmitted to humans via predominantly water-rich environments; most cases occur around slow-moving and still bodies of water such as swamps, ponds, and marshes.1 Mycobacterium ulcerans DNA has been found in fish, water insects, and snails.1,4 It also has been present in samples from aquatic insects such as Hemiptera (water strider), Naucoridae (creeping water bugs and saucer bugs), and Belostomatidae (giant water bugs) in West Africa and also from Aulacodes feces and moss.1
Variations in geographic climate may lead to different modes of transmission of BU. For example, mosquitoes have been studied as a potential vector for BU in the temperate climate of Australia.2 However, more data are needed from other countries to support mosquitoes as possible vectors. Wallace et al10 performed a study that showed skin puncture from insect bites or other injuries increases the chance of transmitting M ulcerans in the environment to the skin.
Clinical Presentation
Buruli ulcer most often manifests in healthy children younger than 15 years.1,8 Potential risk factors include residing near a contaminated water source, swimming in a river, and being bitten by an insect in a river during the rainy season. Lack of protective clothing and mosquito nets also have been proposed as considerable risk factors for BU.2 Genetic polymorphism in the solute carrier family 11 member 1 gene (SLC11A1) may increase the risk for BU with M ulcerans transmission. It is essential to understand that infection with M ulcerans does not always lead to the development of BU.1
Buruli ulcer often begins as a painless nodule or papule that patients may confuse with an insect bite. Within a couple of weeks, the induration will grow into ill-defined edematous plaques that gradually turn into necrotic skin, which will eventually slough off to create painless to mildly painful irregular skin ulceration (Figure).11 The surrounding uninvolved skin often is edematous and pigmented. Unfortunately, deep ulcerations can lead to osteomyelitis with exposure of the underlying bone. A secondary bacterial infection may be involved if a foul smell accompanies the ulcer. The vast extension of the ulcer has been known to lead to amputations, contractures, or deformities.1-5 The nodule progression to ulceration varies and can occur within 3 weeks to 1 year of the initial exposure.1,8
skin, which will eventually slough off to create painless to mildly painful
irregular skin ulceration. The image is in the public domain. Ezzedine K,
Pistone T, Cottin J, et al. Buruli ulcer in long-term traveler to Senegal.
Emerg Infect Dis. 2009;15:118-119. doi:10.3201/eid1501.080123
The World Health Organization (WHO) classifies BU into 3 categories: category 1 includes ulcers less than 5 cm in diameter; category 2 involves ulcers that are 5 to 15 cm in diameter; and category 3 involves ulcers that are larger than 15 cm in diameter as well as those involving the breasts, genitals, eyes, bones, or joints.4,8
Diagnosis
Cultures and microscopic examination of M ulcerans acid-fast bacilli can be used to confirm the diagnosis of BU. However, polymerase chain reaction (PCR) is the best confirmatory test, as the WHO reports that 70% of reported cases of BU are confirmed by PCR detection of DNA.1,4 Unfortunately, many BU-endemic areas lack feasible access to perform confirmatory tests such as PCR. Antigen detection assays, loop-mediated isothermal amplification tests, and detection of mycolactone by thinlayer chromatography are being developed to create more rapid and sensitive testing for BU.12
The lack of diagnostic testing for BU means physicians must rely on clinical diagnosis.1 However, the differential diagnosis is extensive and includes ulcers due to diabetes and arterial and venous insufficiency, cutaneous leishmaniasis, and Haemophilus ducreyi ulcers.5 Despite the broad differential, Eddyani et al12 found that BU diagnosed clinically by physicians had a sensitivity of 92%.
Treatment
Surgery was the first-line treatment for BU before the introduction of antibiotics for this condition. Antibiotics have created better outcomes with increased cure rates and decreased amputation.4
Pharmacotherapy—In the early 2000s, the WHO recommended a treatment regimen of once-daily 10 mg/kg rifampin (oral) and 15 mg/kg streptomycin ( intramuscular) for 8 weeks. This treatment protocol is effective for lesions measuring less than 10 cm in diameter and has an average cure rate of 50%.5 Unfortunately, streptomycin is associated with ototoxicity and nephrotoxicity.1,4 Clinicians should be aware that 1.9% to 26% of patients may have paradoxical worsening of BU early during antibiotic use due to increased host inflammatory response, but it subsides with continued treatment.1,4
Researchers in Australia have begun testing and using rifampin plus oral clarithromycin, ciprofloxacin, or moxifloxacin for 3 months. A common combination is oncedaily 10 mg/kg rifampicin and 400 mg/kg moxifloxacin.1 After multiple randomized controlled trials showed the efficacy of rifampicin in combination with clarithromycin, many physicians now recommend 10 mg/kg of rifampicin once daily and 7.5 mg/kg of clarithromycin twice daily.1,5 When BU is severe, intravenous amikacin and oral rifampin can be used for 4 to 8 weeks.5
Wound Management and Surgical Considerations—Since BU can cause extensive widespread ulceration, daily wound care is recommended. Clinicians should note that patients often experience pain during wound dressing, as gauze impairs dermal regeneration and adheres to wounds. A second-line treatment to combat patients’ intolerance to gauze placement—especially for large BU lesions causing mobility issues—includes surgical debridement with wide margins and grafting 4 weeks after antibiotic therapy. This surgical procedure also can treat the releasing contractures that BU is known to cause.1 Severe cases of BU also can be treated with physiotherapy to prevent further disability.5
If histologic analysis of the margins reveals the presence of acid-fast bacilli and granulomas, the probability of future recurrence is high. In those instances, antibiotic therapy is given for prevention. In Australia, the Consensus Council Conference has recommended the removal of not only necrotic tissue but also a small margin of normal tissue to prevent the spread leading to recurrence.1,5,8
Prevention—Multiple prevention techniques have been suggested to combat BU. Long sleeves and pants should be worn outdoors along with insect repellents in BU-endemic areas. Comprehensive—but perhaps impractical—prevention measures include avoidance of swimming and aquatic activities such as boating and fishing in BU-endemic areas. In the event of a skin abrasion, the wound should be cleaned and covered promptly.
There is no vaccine currently available for BU. Bacillus Calmette—Guérin vaccination can provide minimal protection against disseminated BU but with a short-term response.5 Fortunately, M ulcerans–specific vaccines are being developed. Currently, tested vaccines target an enzyme called mycolyl transferase, which is essential for the stability of the mycobacterial cell wall and could have powerful implications in preventing these ulcers. These mycolyl transferase–directed vaccines need to be further explored in the plight against BU.1,5,8
Final Thoughts
Buruli ulcer remains a considerable public health challenge in endemic regions, with substantial morbidity and potential long-term disability. Hence, continued research into its transmission mechanisms, treatment options, and preventive measures is crucial for reducing the impact of this disease on affected populations.
- Yotsu RR, Suzuki K, Simmonds RE, et al. Buruli ulcer: a review of the current knowledge. Curr Trop Med Rep. 2018;5:247-256. doi:10.1007 /s40475-018-0166-2
- Muleta AJ, Lappan R, Stinear TP, et al. Understanding the transmission of Mycobacterium ulcerans: a step towards controlling Buruli ulcer. PLoS Negl Trop Dis. 2021;15:E0009678. doi:10.1371/journal.pntd.0009678
- MacCallum P, Tolhurst JC. A new mycobacterial infection in man. J Pathol Bacteriol. 1948;60:93-122.
- Van der Werf TS, Stienstra Y, Johnson RC, et al. Mycobacterium ulcerans disease. Bull World Health Organ. 2005;83:785-791.
- World Health Organization. Buruli ulcer (Mycobacterium ulcerans infection). January 12, 2023. Accessed November 7, 2024. https://www.who.int/news-room/fact-sheets/detail/buruli-ulcer-(mycobacterium-ulcerans-infection)
- Hall BS, Hill K, McKenna M, et al. The pathogenic mechanism of the Mycobacterium ulcerans virulence factor, mycolactone, depends on blockade of protein translocation into the ER. PLoS Pathog. 2014;10:E1004061. doi:10.1371/journal.ppat.1004061
- Sarfo FS, Phillips R, Wansbrough-Jones M, et al. Recent advances: role of mycolactone in the pathogenesis and monitoring of Mycobacterium ulcerans infection/Buruli ulcer disease. Cell Microbiol. 2016;18:17-29. doi:10.1111/cmi.12547
- Guarner J. Buruli ulcer: review of a neglected skin mycobacterial disease. J Clin Microbiol. 2018;56:E01507- E01517. doi:10.1128 /JCM.01507-17
- Adusumilli S, Mve-Obiang A, Sparer T, et al. Mycobacterium ulcerans toxic macrolide, mycolactone modulates the host immune response and cellular location of M ulcerans in vitro and in vivo. Cell Microbiol. 2005;7:1295-1304. doi:10.1111/j.1462-5822.2005.00557
- Wallace JR, Mangas KM, Porter JL, et al. Mycobacterium ulcerans low infectious dose and mechanical transmission support insect bites and puncturing injuries in the spread of Buruli ulcer. PLoS Negl Trop Dis. 2017;11:E0005553. doi:10.1371/journal.pntd.0005553
- Ezzedine K, Pistone T, Cottin J, et al. Buruli ulcer in long-term traveler to Senegal. Emerg Infect Dis. 2009;15:118-119. doi:10.3201 /eid1501.080123
- Eddyani M, Sopoh GE, Ayelo G, et al. Diagnostic accuracy of clinical and microbiological signs in patients with skin lesions resembling Buruli ulcer in an endemic region. Clin Infect Dis. 2018;67:827-834. doi:10.1093/cid/ciy197
Buruli ulcer (BU) is a potentially disabling necrotizing skin and soft tissue disease caused by Mycobacterium ulcerans infection.1,2 Buruli ulcer is most common in hot and humid climates and has caused considerable morbidity in Western African countries (Côte d’Ivoire, Ghana, and Benin account for 73% of annual cases) and the temperate areas of Australia (283 reported cases in 2017).1-4 In fact, the first recognizable cases of BU were described in 6 Australian individuals living in a riverine area in 1948, although the term Buruli ulcer is derived from the increased number of cases reported from Buruli county in Uganda near the Nile River.1,3,4
From 2002 to 2017, 66,000 cases of BU were reported in 33 countries.1 While the focal distribution has been demonstrated in the tropical areas of Sri Lanka, Malaysia, Papua New Guinea, Peru, and Mexico,4 nontropical nations such as Japan also are affected. Since 1981, 66 cases have been reported in Japan with M ulcerans subspecies—primarily Shinshuense, which has adapted to higher latitudes.1 Herein, we provide an overview of the pathogenesis, clinical presentation, and treatment of BU and highlight aquatic insects and mosquitoes as possible vectors of transmission.
Pathogenesis
Mycobacterium ulcerans is a nontuberculous mycobacterium and ubiquitous acid-fast gram-positive bacillus that can be cultured using a Lowenstein-Jensen agar and has a doubling rate of 48 hours.1,5 It produces the small 174-kb plasmid pMUM001-encoded compound mycolactone, a pathogenic toxin that causes immunosuppression, analgesia, and cytotoxic-associated tissue necrosis.1,5-9
Mycolactone is a polyketide macrolide with a 12-membrane lactone with 2 attached acyl side chains.1,7 Mycolactone is synthesized by the giant polyketide synthetases of M ulcerans. Mycolactone post-transcriptionally inhibits the development of lipopolysaccharide-dependent proinflammatory mediators—specifically by blocking protein translocation from the cytosol into the endoplasmic reticulum by targeting the SEC61 translocon.1,6 The lack of translocation of 30% to 50% of proteins leads to cellular stress and apoptosis mediated by Bim/Bcl2. A single point mutation in the SEC61 translocon subunit alpha 1 gene (SEC61A1) is associated with resistance to the cytotoxic effects of mycolactone.1
There are divergent hypotheses regarding the relationship of mycolactone to the Wiskott-Aldrich syndrome protein, with some researchers suggesting that mycolactone can attach to this protein, leading to cell detachment and death.7 However, others have proposed that mycolactone inhibits mTOR, activating the Wiskott-Aldrich syndrome protein and leading to subsequent extensive cytoskeleton remodeling.1 Mycolactone also can cause hypoesthesia, either by activating type 2 angiotensin II receptors and creating downstream neuron hyperpolarization or by killing Schwann cells.1
Transmission
Buruli ulcer caused by M ulcerans has a poorly understood transmission mechanism, and further studies are required to understand the underlying pathophysiology to decrease transmission rates and associated morbidity. Buruli ulcer is widely accepted to be transmitted to humans via predominantly water-rich environments; most cases occur around slow-moving and still bodies of water such as swamps, ponds, and marshes.1 Mycobacterium ulcerans DNA has been found in fish, water insects, and snails.1,4 It also has been present in samples from aquatic insects such as Hemiptera (water strider), Naucoridae (creeping water bugs and saucer bugs), and Belostomatidae (giant water bugs) in West Africa and also from Aulacodes feces and moss.1
Variations in geographic climate may lead to different modes of transmission of BU. For example, mosquitoes have been studied as a potential vector for BU in the temperate climate of Australia.2 However, more data are needed from other countries to support mosquitoes as possible vectors. Wallace et al10 performed a study that showed skin puncture from insect bites or other injuries increases the chance of transmitting M ulcerans in the environment to the skin.
Clinical Presentation
Buruli ulcer most often manifests in healthy children younger than 15 years.1,8 Potential risk factors include residing near a contaminated water source, swimming in a river, and being bitten by an insect in a river during the rainy season. Lack of protective clothing and mosquito nets also have been proposed as considerable risk factors for BU.2 Genetic polymorphism in the solute carrier family 11 member 1 gene (SLC11A1) may increase the risk for BU with M ulcerans transmission. It is essential to understand that infection with M ulcerans does not always lead to the development of BU.1
Buruli ulcer often begins as a painless nodule or papule that patients may confuse with an insect bite. Within a couple of weeks, the induration will grow into ill-defined edematous plaques that gradually turn into necrotic skin, which will eventually slough off to create painless to mildly painful irregular skin ulceration (Figure).11 The surrounding uninvolved skin often is edematous and pigmented. Unfortunately, deep ulcerations can lead to osteomyelitis with exposure of the underlying bone. A secondary bacterial infection may be involved if a foul smell accompanies the ulcer. The vast extension of the ulcer has been known to lead to amputations, contractures, or deformities.1-5 The nodule progression to ulceration varies and can occur within 3 weeks to 1 year of the initial exposure.1,8
skin, which will eventually slough off to create painless to mildly painful
irregular skin ulceration. The image is in the public domain. Ezzedine K,
Pistone T, Cottin J, et al. Buruli ulcer in long-term traveler to Senegal.
Emerg Infect Dis. 2009;15:118-119. doi:10.3201/eid1501.080123
The World Health Organization (WHO) classifies BU into 3 categories: category 1 includes ulcers less than 5 cm in diameter; category 2 involves ulcers that are 5 to 15 cm in diameter; and category 3 involves ulcers that are larger than 15 cm in diameter as well as those involving the breasts, genitals, eyes, bones, or joints.4,8
Diagnosis
Cultures and microscopic examination of M ulcerans acid-fast bacilli can be used to confirm the diagnosis of BU. However, polymerase chain reaction (PCR) is the best confirmatory test, as the WHO reports that 70% of reported cases of BU are confirmed by PCR detection of DNA.1,4 Unfortunately, many BU-endemic areas lack feasible access to perform confirmatory tests such as PCR. Antigen detection assays, loop-mediated isothermal amplification tests, and detection of mycolactone by thinlayer chromatography are being developed to create more rapid and sensitive testing for BU.12
The lack of diagnostic testing for BU means physicians must rely on clinical diagnosis.1 However, the differential diagnosis is extensive and includes ulcers due to diabetes and arterial and venous insufficiency, cutaneous leishmaniasis, and Haemophilus ducreyi ulcers.5 Despite the broad differential, Eddyani et al12 found that BU diagnosed clinically by physicians had a sensitivity of 92%.
Treatment
Surgery was the first-line treatment for BU before the introduction of antibiotics for this condition. Antibiotics have created better outcomes with increased cure rates and decreased amputation.4
Pharmacotherapy—In the early 2000s, the WHO recommended a treatment regimen of once-daily 10 mg/kg rifampin (oral) and 15 mg/kg streptomycin ( intramuscular) for 8 weeks. This treatment protocol is effective for lesions measuring less than 10 cm in diameter and has an average cure rate of 50%.5 Unfortunately, streptomycin is associated with ototoxicity and nephrotoxicity.1,4 Clinicians should be aware that 1.9% to 26% of patients may have paradoxical worsening of BU early during antibiotic use due to increased host inflammatory response, but it subsides with continued treatment.1,4
Researchers in Australia have begun testing and using rifampin plus oral clarithromycin, ciprofloxacin, or moxifloxacin for 3 months. A common combination is oncedaily 10 mg/kg rifampicin and 400 mg/kg moxifloxacin.1 After multiple randomized controlled trials showed the efficacy of rifampicin in combination with clarithromycin, many physicians now recommend 10 mg/kg of rifampicin once daily and 7.5 mg/kg of clarithromycin twice daily.1,5 When BU is severe, intravenous amikacin and oral rifampin can be used for 4 to 8 weeks.5
Wound Management and Surgical Considerations—Since BU can cause extensive widespread ulceration, daily wound care is recommended. Clinicians should note that patients often experience pain during wound dressing, as gauze impairs dermal regeneration and adheres to wounds. A second-line treatment to combat patients’ intolerance to gauze placement—especially for large BU lesions causing mobility issues—includes surgical debridement with wide margins and grafting 4 weeks after antibiotic therapy. This surgical procedure also can treat the releasing contractures that BU is known to cause.1 Severe cases of BU also can be treated with physiotherapy to prevent further disability.5
If histologic analysis of the margins reveals the presence of acid-fast bacilli and granulomas, the probability of future recurrence is high. In those instances, antibiotic therapy is given for prevention. In Australia, the Consensus Council Conference has recommended the removal of not only necrotic tissue but also a small margin of normal tissue to prevent the spread leading to recurrence.1,5,8
Prevention—Multiple prevention techniques have been suggested to combat BU. Long sleeves and pants should be worn outdoors along with insect repellents in BU-endemic areas. Comprehensive—but perhaps impractical—prevention measures include avoidance of swimming and aquatic activities such as boating and fishing in BU-endemic areas. In the event of a skin abrasion, the wound should be cleaned and covered promptly.
There is no vaccine currently available for BU. Bacillus Calmette—Guérin vaccination can provide minimal protection against disseminated BU but with a short-term response.5 Fortunately, M ulcerans–specific vaccines are being developed. Currently, tested vaccines target an enzyme called mycolyl transferase, which is essential for the stability of the mycobacterial cell wall and could have powerful implications in preventing these ulcers. These mycolyl transferase–directed vaccines need to be further explored in the plight against BU.1,5,8
Final Thoughts
Buruli ulcer remains a considerable public health challenge in endemic regions, with substantial morbidity and potential long-term disability. Hence, continued research into its transmission mechanisms, treatment options, and preventive measures is crucial for reducing the impact of this disease on affected populations.
Buruli ulcer (BU) is a potentially disabling necrotizing skin and soft tissue disease caused by Mycobacterium ulcerans infection.1,2 Buruli ulcer is most common in hot and humid climates and has caused considerable morbidity in Western African countries (Côte d’Ivoire, Ghana, and Benin account for 73% of annual cases) and the temperate areas of Australia (283 reported cases in 2017).1-4 In fact, the first recognizable cases of BU were described in 6 Australian individuals living in a riverine area in 1948, although the term Buruli ulcer is derived from the increased number of cases reported from Buruli county in Uganda near the Nile River.1,3,4
From 2002 to 2017, 66,000 cases of BU were reported in 33 countries.1 While the focal distribution has been demonstrated in the tropical areas of Sri Lanka, Malaysia, Papua New Guinea, Peru, and Mexico,4 nontropical nations such as Japan also are affected. Since 1981, 66 cases have been reported in Japan with M ulcerans subspecies—primarily Shinshuense, which has adapted to higher latitudes.1 Herein, we provide an overview of the pathogenesis, clinical presentation, and treatment of BU and highlight aquatic insects and mosquitoes as possible vectors of transmission.
Pathogenesis
Mycobacterium ulcerans is a nontuberculous mycobacterium and ubiquitous acid-fast gram-positive bacillus that can be cultured using a Lowenstein-Jensen agar and has a doubling rate of 48 hours.1,5 It produces the small 174-kb plasmid pMUM001-encoded compound mycolactone, a pathogenic toxin that causes immunosuppression, analgesia, and cytotoxic-associated tissue necrosis.1,5-9
Mycolactone is a polyketide macrolide with a 12-membrane lactone with 2 attached acyl side chains.1,7 Mycolactone is synthesized by the giant polyketide synthetases of M ulcerans. Mycolactone post-transcriptionally inhibits the development of lipopolysaccharide-dependent proinflammatory mediators—specifically by blocking protein translocation from the cytosol into the endoplasmic reticulum by targeting the SEC61 translocon.1,6 The lack of translocation of 30% to 50% of proteins leads to cellular stress and apoptosis mediated by Bim/Bcl2. A single point mutation in the SEC61 translocon subunit alpha 1 gene (SEC61A1) is associated with resistance to the cytotoxic effects of mycolactone.1
There are divergent hypotheses regarding the relationship of mycolactone to the Wiskott-Aldrich syndrome protein, with some researchers suggesting that mycolactone can attach to this protein, leading to cell detachment and death.7 However, others have proposed that mycolactone inhibits mTOR, activating the Wiskott-Aldrich syndrome protein and leading to subsequent extensive cytoskeleton remodeling.1 Mycolactone also can cause hypoesthesia, either by activating type 2 angiotensin II receptors and creating downstream neuron hyperpolarization or by killing Schwann cells.1
Transmission
Buruli ulcer caused by M ulcerans has a poorly understood transmission mechanism, and further studies are required to understand the underlying pathophysiology to decrease transmission rates and associated morbidity. Buruli ulcer is widely accepted to be transmitted to humans via predominantly water-rich environments; most cases occur around slow-moving and still bodies of water such as swamps, ponds, and marshes.1 Mycobacterium ulcerans DNA has been found in fish, water insects, and snails.1,4 It also has been present in samples from aquatic insects such as Hemiptera (water strider), Naucoridae (creeping water bugs and saucer bugs), and Belostomatidae (giant water bugs) in West Africa and also from Aulacodes feces and moss.1
Variations in geographic climate may lead to different modes of transmission of BU. For example, mosquitoes have been studied as a potential vector for BU in the temperate climate of Australia.2 However, more data are needed from other countries to support mosquitoes as possible vectors. Wallace et al10 performed a study that showed skin puncture from insect bites or other injuries increases the chance of transmitting M ulcerans in the environment to the skin.
Clinical Presentation
Buruli ulcer most often manifests in healthy children younger than 15 years.1,8 Potential risk factors include residing near a contaminated water source, swimming in a river, and being bitten by an insect in a river during the rainy season. Lack of protective clothing and mosquito nets also have been proposed as considerable risk factors for BU.2 Genetic polymorphism in the solute carrier family 11 member 1 gene (SLC11A1) may increase the risk for BU with M ulcerans transmission. It is essential to understand that infection with M ulcerans does not always lead to the development of BU.1
Buruli ulcer often begins as a painless nodule or papule that patients may confuse with an insect bite. Within a couple of weeks, the induration will grow into ill-defined edematous plaques that gradually turn into necrotic skin, which will eventually slough off to create painless to mildly painful irregular skin ulceration (Figure).11 The surrounding uninvolved skin often is edematous and pigmented. Unfortunately, deep ulcerations can lead to osteomyelitis with exposure of the underlying bone. A secondary bacterial infection may be involved if a foul smell accompanies the ulcer. The vast extension of the ulcer has been known to lead to amputations, contractures, or deformities.1-5 The nodule progression to ulceration varies and can occur within 3 weeks to 1 year of the initial exposure.1,8
skin, which will eventually slough off to create painless to mildly painful
irregular skin ulceration. The image is in the public domain. Ezzedine K,
Pistone T, Cottin J, et al. Buruli ulcer in long-term traveler to Senegal.
Emerg Infect Dis. 2009;15:118-119. doi:10.3201/eid1501.080123
The World Health Organization (WHO) classifies BU into 3 categories: category 1 includes ulcers less than 5 cm in diameter; category 2 involves ulcers that are 5 to 15 cm in diameter; and category 3 involves ulcers that are larger than 15 cm in diameter as well as those involving the breasts, genitals, eyes, bones, or joints.4,8
Diagnosis
Cultures and microscopic examination of M ulcerans acid-fast bacilli can be used to confirm the diagnosis of BU. However, polymerase chain reaction (PCR) is the best confirmatory test, as the WHO reports that 70% of reported cases of BU are confirmed by PCR detection of DNA.1,4 Unfortunately, many BU-endemic areas lack feasible access to perform confirmatory tests such as PCR. Antigen detection assays, loop-mediated isothermal amplification tests, and detection of mycolactone by thinlayer chromatography are being developed to create more rapid and sensitive testing for BU.12
The lack of diagnostic testing for BU means physicians must rely on clinical diagnosis.1 However, the differential diagnosis is extensive and includes ulcers due to diabetes and arterial and venous insufficiency, cutaneous leishmaniasis, and Haemophilus ducreyi ulcers.5 Despite the broad differential, Eddyani et al12 found that BU diagnosed clinically by physicians had a sensitivity of 92%.
Treatment
Surgery was the first-line treatment for BU before the introduction of antibiotics for this condition. Antibiotics have created better outcomes with increased cure rates and decreased amputation.4
Pharmacotherapy—In the early 2000s, the WHO recommended a treatment regimen of once-daily 10 mg/kg rifampin (oral) and 15 mg/kg streptomycin ( intramuscular) for 8 weeks. This treatment protocol is effective for lesions measuring less than 10 cm in diameter and has an average cure rate of 50%.5 Unfortunately, streptomycin is associated with ototoxicity and nephrotoxicity.1,4 Clinicians should be aware that 1.9% to 26% of patients may have paradoxical worsening of BU early during antibiotic use due to increased host inflammatory response, but it subsides with continued treatment.1,4
Researchers in Australia have begun testing and using rifampin plus oral clarithromycin, ciprofloxacin, or moxifloxacin for 3 months. A common combination is oncedaily 10 mg/kg rifampicin and 400 mg/kg moxifloxacin.1 After multiple randomized controlled trials showed the efficacy of rifampicin in combination with clarithromycin, many physicians now recommend 10 mg/kg of rifampicin once daily and 7.5 mg/kg of clarithromycin twice daily.1,5 When BU is severe, intravenous amikacin and oral rifampin can be used for 4 to 8 weeks.5
Wound Management and Surgical Considerations—Since BU can cause extensive widespread ulceration, daily wound care is recommended. Clinicians should note that patients often experience pain during wound dressing, as gauze impairs dermal regeneration and adheres to wounds. A second-line treatment to combat patients’ intolerance to gauze placement—especially for large BU lesions causing mobility issues—includes surgical debridement with wide margins and grafting 4 weeks after antibiotic therapy. This surgical procedure also can treat the releasing contractures that BU is known to cause.1 Severe cases of BU also can be treated with physiotherapy to prevent further disability.5
If histologic analysis of the margins reveals the presence of acid-fast bacilli and granulomas, the probability of future recurrence is high. In those instances, antibiotic therapy is given for prevention. In Australia, the Consensus Council Conference has recommended the removal of not only necrotic tissue but also a small margin of normal tissue to prevent the spread leading to recurrence.1,5,8
Prevention—Multiple prevention techniques have been suggested to combat BU. Long sleeves and pants should be worn outdoors along with insect repellents in BU-endemic areas. Comprehensive—but perhaps impractical—prevention measures include avoidance of swimming and aquatic activities such as boating and fishing in BU-endemic areas. In the event of a skin abrasion, the wound should be cleaned and covered promptly.
There is no vaccine currently available for BU. Bacillus Calmette—Guérin vaccination can provide minimal protection against disseminated BU but with a short-term response.5 Fortunately, M ulcerans–specific vaccines are being developed. Currently, tested vaccines target an enzyme called mycolyl transferase, which is essential for the stability of the mycobacterial cell wall and could have powerful implications in preventing these ulcers. These mycolyl transferase–directed vaccines need to be further explored in the plight against BU.1,5,8
Final Thoughts
Buruli ulcer remains a considerable public health challenge in endemic regions, with substantial morbidity and potential long-term disability. Hence, continued research into its transmission mechanisms, treatment options, and preventive measures is crucial for reducing the impact of this disease on affected populations.
- Yotsu RR, Suzuki K, Simmonds RE, et al. Buruli ulcer: a review of the current knowledge. Curr Trop Med Rep. 2018;5:247-256. doi:10.1007 /s40475-018-0166-2
- Muleta AJ, Lappan R, Stinear TP, et al. Understanding the transmission of Mycobacterium ulcerans: a step towards controlling Buruli ulcer. PLoS Negl Trop Dis. 2021;15:E0009678. doi:10.1371/journal.pntd.0009678
- MacCallum P, Tolhurst JC. A new mycobacterial infection in man. J Pathol Bacteriol. 1948;60:93-122.
- Van der Werf TS, Stienstra Y, Johnson RC, et al. Mycobacterium ulcerans disease. Bull World Health Organ. 2005;83:785-791.
- World Health Organization. Buruli ulcer (Mycobacterium ulcerans infection). January 12, 2023. Accessed November 7, 2024. https://www.who.int/news-room/fact-sheets/detail/buruli-ulcer-(mycobacterium-ulcerans-infection)
- Hall BS, Hill K, McKenna M, et al. The pathogenic mechanism of the Mycobacterium ulcerans virulence factor, mycolactone, depends on blockade of protein translocation into the ER. PLoS Pathog. 2014;10:E1004061. doi:10.1371/journal.ppat.1004061
- Sarfo FS, Phillips R, Wansbrough-Jones M, et al. Recent advances: role of mycolactone in the pathogenesis and monitoring of Mycobacterium ulcerans infection/Buruli ulcer disease. Cell Microbiol. 2016;18:17-29. doi:10.1111/cmi.12547
- Guarner J. Buruli ulcer: review of a neglected skin mycobacterial disease. J Clin Microbiol. 2018;56:E01507- E01517. doi:10.1128 /JCM.01507-17
- Adusumilli S, Mve-Obiang A, Sparer T, et al. Mycobacterium ulcerans toxic macrolide, mycolactone modulates the host immune response and cellular location of M ulcerans in vitro and in vivo. Cell Microbiol. 2005;7:1295-1304. doi:10.1111/j.1462-5822.2005.00557
- Wallace JR, Mangas KM, Porter JL, et al. Mycobacterium ulcerans low infectious dose and mechanical transmission support insect bites and puncturing injuries in the spread of Buruli ulcer. PLoS Negl Trop Dis. 2017;11:E0005553. doi:10.1371/journal.pntd.0005553
- Ezzedine K, Pistone T, Cottin J, et al. Buruli ulcer in long-term traveler to Senegal. Emerg Infect Dis. 2009;15:118-119. doi:10.3201 /eid1501.080123
- Eddyani M, Sopoh GE, Ayelo G, et al. Diagnostic accuracy of clinical and microbiological signs in patients with skin lesions resembling Buruli ulcer in an endemic region. Clin Infect Dis. 2018;67:827-834. doi:10.1093/cid/ciy197
- Yotsu RR, Suzuki K, Simmonds RE, et al. Buruli ulcer: a review of the current knowledge. Curr Trop Med Rep. 2018;5:247-256. doi:10.1007 /s40475-018-0166-2
- Muleta AJ, Lappan R, Stinear TP, et al. Understanding the transmission of Mycobacterium ulcerans: a step towards controlling Buruli ulcer. PLoS Negl Trop Dis. 2021;15:E0009678. doi:10.1371/journal.pntd.0009678
- MacCallum P, Tolhurst JC. A new mycobacterial infection in man. J Pathol Bacteriol. 1948;60:93-122.
- Van der Werf TS, Stienstra Y, Johnson RC, et al. Mycobacterium ulcerans disease. Bull World Health Organ. 2005;83:785-791.
- World Health Organization. Buruli ulcer (Mycobacterium ulcerans infection). January 12, 2023. Accessed November 7, 2024. https://www.who.int/news-room/fact-sheets/detail/buruli-ulcer-(mycobacterium-ulcerans-infection)
- Hall BS, Hill K, McKenna M, et al. The pathogenic mechanism of the Mycobacterium ulcerans virulence factor, mycolactone, depends on blockade of protein translocation into the ER. PLoS Pathog. 2014;10:E1004061. doi:10.1371/journal.ppat.1004061
- Sarfo FS, Phillips R, Wansbrough-Jones M, et al. Recent advances: role of mycolactone in the pathogenesis and monitoring of Mycobacterium ulcerans infection/Buruli ulcer disease. Cell Microbiol. 2016;18:17-29. doi:10.1111/cmi.12547
- Guarner J. Buruli ulcer: review of a neglected skin mycobacterial disease. J Clin Microbiol. 2018;56:E01507- E01517. doi:10.1128 /JCM.01507-17
- Adusumilli S, Mve-Obiang A, Sparer T, et al. Mycobacterium ulcerans toxic macrolide, mycolactone modulates the host immune response and cellular location of M ulcerans in vitro and in vivo. Cell Microbiol. 2005;7:1295-1304. doi:10.1111/j.1462-5822.2005.00557
- Wallace JR, Mangas KM, Porter JL, et al. Mycobacterium ulcerans low infectious dose and mechanical transmission support insect bites and puncturing injuries in the spread of Buruli ulcer. PLoS Negl Trop Dis. 2017;11:E0005553. doi:10.1371/journal.pntd.0005553
- Ezzedine K, Pistone T, Cottin J, et al. Buruli ulcer in long-term traveler to Senegal. Emerg Infect Dis. 2009;15:118-119. doi:10.3201 /eid1501.080123
- Eddyani M, Sopoh GE, Ayelo G, et al. Diagnostic accuracy of clinical and microbiological signs in patients with skin lesions resembling Buruli ulcer in an endemic region. Clin Infect Dis. 2018;67:827-834. doi:10.1093/cid/ciy197
Buruli Ulcer Transmission: Environmental Pathways and Implications for Dermatologic Care
Buruli Ulcer Transmission: Environmental Pathways and Implications for Dermatologic Care
PRACTICE POINTS
- Buruli ulcer (BU) is a necrotizing cutaneous disease caused by Mycobacterium ulcerans with possible transmission from aquatic insects and mosquitoes.
- Buruli ulcer often manifests in children as painless induration that gradually progresses to painless or mildly painful irregular skin ulceration.
- Treatment options for BU include rifampin and streptomycin, but larger lesions may require surgical debridement.
- No vaccine currently exists for M ulcerans, but clinical trials targeting mycolyl transferase are underway.
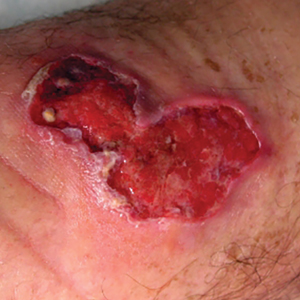
Break the Itch-Scratch Cycle to Treat Prurigo Nodularis
Break the Itch-Scratch Cycle to Treat Prurigo Nodularis
Prurigo nodularis (PN) is a chronic inflammatory skin condition characterized by firm hyperkeratotic nodules that develop when patients persistently scratch or rub intensely itchy areas of the skin. This potent itch-scratch cycle can be traced back to a dysfunctional interplay between cutaneous nerve fibers and the local immune environment.1-3 Pruritis lasting at least 6 weeks is a hallmark symptom of PN and can be accompanied by pain and/or a burning sensation.4 The lesions are symmetrically distributed in areas that are easy to scratch (eg, arms, legs, trunk), typically sparing the face, palms, and soles; however, facial lesions have been reported in pediatric patients with PN, who also are more likely to have back, hand, and foot involvement.5,6
Prurigo nodularis can greatly affect patients’ quality of life, leading to increased rates of depression and anxiety.7-9 Patients with severe symptoms also report increased sleep disturbance, distraction from work, self-consciousness leading to social isolation, and missed days of work/school.9 In one study, patients with PN reported missing at least 1 day of work, school, training, or learning; giving up a leisure activity or sport; or refusing an invitation to dinner or a party in the past 3 months due to the disease.10
Epidemiology
Prurigo nodularis has a prevalence of 72 per 100,000 individuals in the United States,11 most commonly affecting adults aged 51 to 65 years and disproportionately affecting African American and female patients.12,13 Most patients with PN experience a 2-year delay in diagnosis after initial onset of symptoms.10 Adults with PN have an increased likelihood of having other dermatologic conditions, including atopic dermatitis (AD) and psoriasis.11 Nearly two-thirds of pediatric patients with PN present with AD, and those with AD showed more resistance to first-line treatment options.5
Key Clinical Features
Compared to White patients, who typically present with lesions that appear erythematous or pink, patients with darker skin tones may present with hyperpigmented nodules that are larger and darker.12 The pruritic nodules often show signs of scratching or picking (eg, excoriations, lichenification, and angulated erosions).4
Worth Noting
Diagnosis of PN is made clinically, but skin biopsy may be helpful to rule out alternative diseases. Histologically, the hairy palm sign may be present in addition to other histologic features commonly associated with excessive scratching or rubbing of the skin.
Patients with PN have a high risk for HIV, which is not suprising considering HIV is a known systemic cause of generalized chronic pruritus. Other associations include type 2 diabetes mellitus and thyroid, kidney, and liver disease.11,13 Work-up for patients with PN should include a complete blood count with differential; liver and renal function testing; and testing for C-reactive protein, thyroid-stimulating hormone, and lactate dehydrogenase.4,14 Hemoglobin A1c and HIV testing as well as a hepatitis panel also should be considered when appropriate. Because generalized pruritus may be a sign of malignancy, chest radiography and lymph node and abdominal ultrasonography should be performed in patients who have experienced itch for less than 1 year along with B symptoms (fever, night sweats, ≥10% weight loss over 6 months, fatigue).14 Frequent scratching can disrupt the skin barrier, contributing to the increased risk for skin infections.13 All patients with a suspected PN diagnosis also should undergo screening for depression and anxiety, as patients with PN are at an increased risk for these conditions.4
Treatment of PN starts with breaking the itch-scratch cycle by addressing the underlying cause of the pruritus. Therapies are focused on addressing the immunologic and neural components of the disease. Topical treatments include moderate to strong corticosteroids, calcineurin inhibitors (tacrolimus or pimecrolimus), capsaicin, and antipruritic emollients. Systemic agents include phototherapy (narrowband UVB or excimer laser), gabapentin, pregabalin, paroxetine, and amitriptyline to address the neural component of itch. Methotrexate or cyclosporine can be used to address the immunologic component of PN and diminish the itch. That said, methotrexate and cyclosporine often are inadequate to control pruritus.10 Of note, sedating antihistamines are not effective in treating itch in PN but can be used as an adjuvant therapy for sleep disturbances in these patients.15
The only drugs currently approved by the US Food and Drug Administration to treat PN are the biologics dupilumab (targeting the IL-4 receptor) approved in 2022 and nemolizumab (targeting the IL-31 receptor) approved in 2024.16-18 The evidence that these injectable biologics work is heartening in a condition that has historically been very challenging to treat.16,18 It should be noted that the high cost of these 2 medications can restrict access to care for patients who are uninsured or underinsured.
Resolution of a prurigo nodule may result in a hyperpigmented macule taking months to years to fade.
Health Disparity Highlight
Patients with PN have a considerable comorbidity burden, negative impact on quality of life, and increased health care utilization rates.12 Prurigo nodularis is 3.4 times more common in Black patients than White patients.13 Black patients with PN have increased mortality, higher health care utilization rates, and increased systemic inflammation compared to White patients.12,19,20
Social drivers of health (eg, socioeconomic challenges, education, access to high-quality health care) likely contribute to PN. Historically, there has been a paucity of research on PN, as with most conditions that disproportionately affect patients with skin of color. Several PN clinical trials currently are underway to explore additional therapeutic options.11
- Cevikbas F, Wang X, Akiyama T, et al. A sensory neuron–expressed IL-31 receptor mediates T helper cell–dependent itch: involvement of TRPV1 and TRPA1. J Allergy Clin Immunol. 2014;133:448-460.
- Lou H, Lu J, Choi EB, et al. Expression of IL-22 in the skin causes Th2-biased immunity, epidermal barrier dysfunction, and pruritus via stimulating epithelial Th2 cytokines and the GRP pathway. J Immunol. 2017;198:2543-2555.
- Sutaria N, Adawi W, Goldberg R, et al. Itch: pathogenesis and treatment. J Am Acad Dermatol. 2022;86:17-34.
- Elmariah S, Kim B, Berger T, et al. Practical approaches for diagnosis and management of prurigo nodularis: United States expert panel consensus. J Am Acad Dermatol. 2021;84:747-760.
- Kyvayko R, Fachler-Sharp T, Greenberger S, et al. Characterization of paediatric prurigo nodularis: a multicentre retrospective, observational study. Acta Derm Venereol. 2024;104:adv15771.
- Aggarwal P, Choi J, Sutaria N, et al. Clinical characteristics and disease burden in prurigo nodularis. Clin Exp Dermatol. 2021;46:1277-1284.
- Whang KA, Le TK, Khanna R, et al. Health-related quality of life and economic burden of prurigo nodularis. J Am Acad Dermatol. 2022;86:573-580.
- Jørgensen KM, Egeberg A, Gislason GH, et al. Anxiety, depression and suicide in patients with prurigo nodularis. J Eur Acad Dermatol Venereol. 2017;31:E106-E107.
- Rodriguez D, Kwatra SG, Dias-Barbosa C, et al. Patient perspectives on living with severe prurigo nodularis. JAMA Dermatol. 2023;159:1205-1212.
- Misery L, Patras de Campaigno C, Taieb C, et al. Impact of chronic prurigo nodularis on daily life and stigmatization. J Eur Acad Dermatol Venereol. 2023;37:E908-E909.
- Huang AH, Canner JK, Khanna R, et al. Real-world prevalence of prurigo nodularis and burden of associated diseases. J Investigative Dermatol. 2020;140:480-483.e4.
- Sutaria N, Adawi W, Brown I, et al. Racial disparities in mortality among patients with prurigo nodularis: a multi-center cohort study. J Am Acad Dermatol. 2022;82:487-490.
- Boozalis E, Tang O, Patel S, et al. Ethnic differences and comorbidities of 909 prurigo nodularis patients. J Am Acad Dermatol. 2018; 79:714-719.e3.
- Müller S, Zeidler C, Ständer S. Chronic prurigo including prurigo nodularis: new insights and treatments. Am J Clin Dermatol. 2024;25:15-33.
- Williams KA, Roh YS, Brown I, et al. Pathophysiology, diagnosis, and pharmacological treatment of prurigo nodularis. Expert Rev Clin Pharmacol. 2021;14:67-77.
- Kwatra SG, Yosipovitch G, Legat FJ, et al. Phase 3 trial of nemolizumab in patients with prurigo nodularis. N Engl J Med. 2023;389:1579-1589.
- Beck KM, Yang EJ, Sekhon S, et al. Dupilumab treatment for generalized prurigo nodularis. JAMA Dermatol. 2019;155:118-120.
- Yosipovitch G, Mollanazar N, Ständer S, et al. Dupilumab in patients with prurigo nodularis: two randomized, double-blind, placebocontrolled phase 3 trials. Nat Med. 2023;29:1180-1190.
- Wongvibulsin S, Sutaria N, Williams KA, et al. A nationwide study of prurigo nodularis: disease burden and healthcare utilization in the United States. J Invest Dermatol. 2021;141:2530-2533.e1.
- Sutaria N, Alphonse MP, Marani M, et al. Cluster analysis of circulating plasma biomarkers in prurigo nodularis reveals a distinct systemic inflammatory signature in African Americans. J Invest Dermatol. 2022;142:1300-1308.e3.
Prurigo nodularis (PN) is a chronic inflammatory skin condition characterized by firm hyperkeratotic nodules that develop when patients persistently scratch or rub intensely itchy areas of the skin. This potent itch-scratch cycle can be traced back to a dysfunctional interplay between cutaneous nerve fibers and the local immune environment.1-3 Pruritis lasting at least 6 weeks is a hallmark symptom of PN and can be accompanied by pain and/or a burning sensation.4 The lesions are symmetrically distributed in areas that are easy to scratch (eg, arms, legs, trunk), typically sparing the face, palms, and soles; however, facial lesions have been reported in pediatric patients with PN, who also are more likely to have back, hand, and foot involvement.5,6
Prurigo nodularis can greatly affect patients’ quality of life, leading to increased rates of depression and anxiety.7-9 Patients with severe symptoms also report increased sleep disturbance, distraction from work, self-consciousness leading to social isolation, and missed days of work/school.9 In one study, patients with PN reported missing at least 1 day of work, school, training, or learning; giving up a leisure activity or sport; or refusing an invitation to dinner or a party in the past 3 months due to the disease.10
Epidemiology
Prurigo nodularis has a prevalence of 72 per 100,000 individuals in the United States,11 most commonly affecting adults aged 51 to 65 years and disproportionately affecting African American and female patients.12,13 Most patients with PN experience a 2-year delay in diagnosis after initial onset of symptoms.10 Adults with PN have an increased likelihood of having other dermatologic conditions, including atopic dermatitis (AD) and psoriasis.11 Nearly two-thirds of pediatric patients with PN present with AD, and those with AD showed more resistance to first-line treatment options.5
Key Clinical Features
Compared to White patients, who typically present with lesions that appear erythematous or pink, patients with darker skin tones may present with hyperpigmented nodules that are larger and darker.12 The pruritic nodules often show signs of scratching or picking (eg, excoriations, lichenification, and angulated erosions).4
Worth Noting
Diagnosis of PN is made clinically, but skin biopsy may be helpful to rule out alternative diseases. Histologically, the hairy palm sign may be present in addition to other histologic features commonly associated with excessive scratching or rubbing of the skin.
Patients with PN have a high risk for HIV, which is not suprising considering HIV is a known systemic cause of generalized chronic pruritus. Other associations include type 2 diabetes mellitus and thyroid, kidney, and liver disease.11,13 Work-up for patients with PN should include a complete blood count with differential; liver and renal function testing; and testing for C-reactive protein, thyroid-stimulating hormone, and lactate dehydrogenase.4,14 Hemoglobin A1c and HIV testing as well as a hepatitis panel also should be considered when appropriate. Because generalized pruritus may be a sign of malignancy, chest radiography and lymph node and abdominal ultrasonography should be performed in patients who have experienced itch for less than 1 year along with B symptoms (fever, night sweats, ≥10% weight loss over 6 months, fatigue).14 Frequent scratching can disrupt the skin barrier, contributing to the increased risk for skin infections.13 All patients with a suspected PN diagnosis also should undergo screening for depression and anxiety, as patients with PN are at an increased risk for these conditions.4
Treatment of PN starts with breaking the itch-scratch cycle by addressing the underlying cause of the pruritus. Therapies are focused on addressing the immunologic and neural components of the disease. Topical treatments include moderate to strong corticosteroids, calcineurin inhibitors (tacrolimus or pimecrolimus), capsaicin, and antipruritic emollients. Systemic agents include phototherapy (narrowband UVB or excimer laser), gabapentin, pregabalin, paroxetine, and amitriptyline to address the neural component of itch. Methotrexate or cyclosporine can be used to address the immunologic component of PN and diminish the itch. That said, methotrexate and cyclosporine often are inadequate to control pruritus.10 Of note, sedating antihistamines are not effective in treating itch in PN but can be used as an adjuvant therapy for sleep disturbances in these patients.15
The only drugs currently approved by the US Food and Drug Administration to treat PN are the biologics dupilumab (targeting the IL-4 receptor) approved in 2022 and nemolizumab (targeting the IL-31 receptor) approved in 2024.16-18 The evidence that these injectable biologics work is heartening in a condition that has historically been very challenging to treat.16,18 It should be noted that the high cost of these 2 medications can restrict access to care for patients who are uninsured or underinsured.
Resolution of a prurigo nodule may result in a hyperpigmented macule taking months to years to fade.
Health Disparity Highlight
Patients with PN have a considerable comorbidity burden, negative impact on quality of life, and increased health care utilization rates.12 Prurigo nodularis is 3.4 times more common in Black patients than White patients.13 Black patients with PN have increased mortality, higher health care utilization rates, and increased systemic inflammation compared to White patients.12,19,20
Social drivers of health (eg, socioeconomic challenges, education, access to high-quality health care) likely contribute to PN. Historically, there has been a paucity of research on PN, as with most conditions that disproportionately affect patients with skin of color. Several PN clinical trials currently are underway to explore additional therapeutic options.11
Prurigo nodularis (PN) is a chronic inflammatory skin condition characterized by firm hyperkeratotic nodules that develop when patients persistently scratch or rub intensely itchy areas of the skin. This potent itch-scratch cycle can be traced back to a dysfunctional interplay between cutaneous nerve fibers and the local immune environment.1-3 Pruritis lasting at least 6 weeks is a hallmark symptom of PN and can be accompanied by pain and/or a burning sensation.4 The lesions are symmetrically distributed in areas that are easy to scratch (eg, arms, legs, trunk), typically sparing the face, palms, and soles; however, facial lesions have been reported in pediatric patients with PN, who also are more likely to have back, hand, and foot involvement.5,6
Prurigo nodularis can greatly affect patients’ quality of life, leading to increased rates of depression and anxiety.7-9 Patients with severe symptoms also report increased sleep disturbance, distraction from work, self-consciousness leading to social isolation, and missed days of work/school.9 In one study, patients with PN reported missing at least 1 day of work, school, training, or learning; giving up a leisure activity or sport; or refusing an invitation to dinner or a party in the past 3 months due to the disease.10
Epidemiology
Prurigo nodularis has a prevalence of 72 per 100,000 individuals in the United States,11 most commonly affecting adults aged 51 to 65 years and disproportionately affecting African American and female patients.12,13 Most patients with PN experience a 2-year delay in diagnosis after initial onset of symptoms.10 Adults with PN have an increased likelihood of having other dermatologic conditions, including atopic dermatitis (AD) and psoriasis.11 Nearly two-thirds of pediatric patients with PN present with AD, and those with AD showed more resistance to first-line treatment options.5
Key Clinical Features
Compared to White patients, who typically present with lesions that appear erythematous or pink, patients with darker skin tones may present with hyperpigmented nodules that are larger and darker.12 The pruritic nodules often show signs of scratching or picking (eg, excoriations, lichenification, and angulated erosions).4
Worth Noting
Diagnosis of PN is made clinically, but skin biopsy may be helpful to rule out alternative diseases. Histologically, the hairy palm sign may be present in addition to other histologic features commonly associated with excessive scratching or rubbing of the skin.
Patients with PN have a high risk for HIV, which is not suprising considering HIV is a known systemic cause of generalized chronic pruritus. Other associations include type 2 diabetes mellitus and thyroid, kidney, and liver disease.11,13 Work-up for patients with PN should include a complete blood count with differential; liver and renal function testing; and testing for C-reactive protein, thyroid-stimulating hormone, and lactate dehydrogenase.4,14 Hemoglobin A1c and HIV testing as well as a hepatitis panel also should be considered when appropriate. Because generalized pruritus may be a sign of malignancy, chest radiography and lymph node and abdominal ultrasonography should be performed in patients who have experienced itch for less than 1 year along with B symptoms (fever, night sweats, ≥10% weight loss over 6 months, fatigue).14 Frequent scratching can disrupt the skin barrier, contributing to the increased risk for skin infections.13 All patients with a suspected PN diagnosis also should undergo screening for depression and anxiety, as patients with PN are at an increased risk for these conditions.4
Treatment of PN starts with breaking the itch-scratch cycle by addressing the underlying cause of the pruritus. Therapies are focused on addressing the immunologic and neural components of the disease. Topical treatments include moderate to strong corticosteroids, calcineurin inhibitors (tacrolimus or pimecrolimus), capsaicin, and antipruritic emollients. Systemic agents include phototherapy (narrowband UVB or excimer laser), gabapentin, pregabalin, paroxetine, and amitriptyline to address the neural component of itch. Methotrexate or cyclosporine can be used to address the immunologic component of PN and diminish the itch. That said, methotrexate and cyclosporine often are inadequate to control pruritus.10 Of note, sedating antihistamines are not effective in treating itch in PN but can be used as an adjuvant therapy for sleep disturbances in these patients.15
The only drugs currently approved by the US Food and Drug Administration to treat PN are the biologics dupilumab (targeting the IL-4 receptor) approved in 2022 and nemolizumab (targeting the IL-31 receptor) approved in 2024.16-18 The evidence that these injectable biologics work is heartening in a condition that has historically been very challenging to treat.16,18 It should be noted that the high cost of these 2 medications can restrict access to care for patients who are uninsured or underinsured.
Resolution of a prurigo nodule may result in a hyperpigmented macule taking months to years to fade.
Health Disparity Highlight
Patients with PN have a considerable comorbidity burden, negative impact on quality of life, and increased health care utilization rates.12 Prurigo nodularis is 3.4 times more common in Black patients than White patients.13 Black patients with PN have increased mortality, higher health care utilization rates, and increased systemic inflammation compared to White patients.12,19,20
Social drivers of health (eg, socioeconomic challenges, education, access to high-quality health care) likely contribute to PN. Historically, there has been a paucity of research on PN, as with most conditions that disproportionately affect patients with skin of color. Several PN clinical trials currently are underway to explore additional therapeutic options.11
- Cevikbas F, Wang X, Akiyama T, et al. A sensory neuron–expressed IL-31 receptor mediates T helper cell–dependent itch: involvement of TRPV1 and TRPA1. J Allergy Clin Immunol. 2014;133:448-460.
- Lou H, Lu J, Choi EB, et al. Expression of IL-22 in the skin causes Th2-biased immunity, epidermal barrier dysfunction, and pruritus via stimulating epithelial Th2 cytokines and the GRP pathway. J Immunol. 2017;198:2543-2555.
- Sutaria N, Adawi W, Goldberg R, et al. Itch: pathogenesis and treatment. J Am Acad Dermatol. 2022;86:17-34.
- Elmariah S, Kim B, Berger T, et al. Practical approaches for diagnosis and management of prurigo nodularis: United States expert panel consensus. J Am Acad Dermatol. 2021;84:747-760.
- Kyvayko R, Fachler-Sharp T, Greenberger S, et al. Characterization of paediatric prurigo nodularis: a multicentre retrospective, observational study. Acta Derm Venereol. 2024;104:adv15771.
- Aggarwal P, Choi J, Sutaria N, et al. Clinical characteristics and disease burden in prurigo nodularis. Clin Exp Dermatol. 2021;46:1277-1284.
- Whang KA, Le TK, Khanna R, et al. Health-related quality of life and economic burden of prurigo nodularis. J Am Acad Dermatol. 2022;86:573-580.
- Jørgensen KM, Egeberg A, Gislason GH, et al. Anxiety, depression and suicide in patients with prurigo nodularis. J Eur Acad Dermatol Venereol. 2017;31:E106-E107.
- Rodriguez D, Kwatra SG, Dias-Barbosa C, et al. Patient perspectives on living with severe prurigo nodularis. JAMA Dermatol. 2023;159:1205-1212.
- Misery L, Patras de Campaigno C, Taieb C, et al. Impact of chronic prurigo nodularis on daily life and stigmatization. J Eur Acad Dermatol Venereol. 2023;37:E908-E909.
- Huang AH, Canner JK, Khanna R, et al. Real-world prevalence of prurigo nodularis and burden of associated diseases. J Investigative Dermatol. 2020;140:480-483.e4.
- Sutaria N, Adawi W, Brown I, et al. Racial disparities in mortality among patients with prurigo nodularis: a multi-center cohort study. J Am Acad Dermatol. 2022;82:487-490.
- Boozalis E, Tang O, Patel S, et al. Ethnic differences and comorbidities of 909 prurigo nodularis patients. J Am Acad Dermatol. 2018; 79:714-719.e3.
- Müller S, Zeidler C, Ständer S. Chronic prurigo including prurigo nodularis: new insights and treatments. Am J Clin Dermatol. 2024;25:15-33.
- Williams KA, Roh YS, Brown I, et al. Pathophysiology, diagnosis, and pharmacological treatment of prurigo nodularis. Expert Rev Clin Pharmacol. 2021;14:67-77.
- Kwatra SG, Yosipovitch G, Legat FJ, et al. Phase 3 trial of nemolizumab in patients with prurigo nodularis. N Engl J Med. 2023;389:1579-1589.
- Beck KM, Yang EJ, Sekhon S, et al. Dupilumab treatment for generalized prurigo nodularis. JAMA Dermatol. 2019;155:118-120.
- Yosipovitch G, Mollanazar N, Ständer S, et al. Dupilumab in patients with prurigo nodularis: two randomized, double-blind, placebocontrolled phase 3 trials. Nat Med. 2023;29:1180-1190.
- Wongvibulsin S, Sutaria N, Williams KA, et al. A nationwide study of prurigo nodularis: disease burden and healthcare utilization in the United States. J Invest Dermatol. 2021;141:2530-2533.e1.
- Sutaria N, Alphonse MP, Marani M, et al. Cluster analysis of circulating plasma biomarkers in prurigo nodularis reveals a distinct systemic inflammatory signature in African Americans. J Invest Dermatol. 2022;142:1300-1308.e3.
- Cevikbas F, Wang X, Akiyama T, et al. A sensory neuron–expressed IL-31 receptor mediates T helper cell–dependent itch: involvement of TRPV1 and TRPA1. J Allergy Clin Immunol. 2014;133:448-460.
- Lou H, Lu J, Choi EB, et al. Expression of IL-22 in the skin causes Th2-biased immunity, epidermal barrier dysfunction, and pruritus via stimulating epithelial Th2 cytokines and the GRP pathway. J Immunol. 2017;198:2543-2555.
- Sutaria N, Adawi W, Goldberg R, et al. Itch: pathogenesis and treatment. J Am Acad Dermatol. 2022;86:17-34.
- Elmariah S, Kim B, Berger T, et al. Practical approaches for diagnosis and management of prurigo nodularis: United States expert panel consensus. J Am Acad Dermatol. 2021;84:747-760.
- Kyvayko R, Fachler-Sharp T, Greenberger S, et al. Characterization of paediatric prurigo nodularis: a multicentre retrospective, observational study. Acta Derm Venereol. 2024;104:adv15771.
- Aggarwal P, Choi J, Sutaria N, et al. Clinical characteristics and disease burden in prurigo nodularis. Clin Exp Dermatol. 2021;46:1277-1284.
- Whang KA, Le TK, Khanna R, et al. Health-related quality of life and economic burden of prurigo nodularis. J Am Acad Dermatol. 2022;86:573-580.
- Jørgensen KM, Egeberg A, Gislason GH, et al. Anxiety, depression and suicide in patients with prurigo nodularis. J Eur Acad Dermatol Venereol. 2017;31:E106-E107.
- Rodriguez D, Kwatra SG, Dias-Barbosa C, et al. Patient perspectives on living with severe prurigo nodularis. JAMA Dermatol. 2023;159:1205-1212.
- Misery L, Patras de Campaigno C, Taieb C, et al. Impact of chronic prurigo nodularis on daily life and stigmatization. J Eur Acad Dermatol Venereol. 2023;37:E908-E909.
- Huang AH, Canner JK, Khanna R, et al. Real-world prevalence of prurigo nodularis and burden of associated diseases. J Investigative Dermatol. 2020;140:480-483.e4.
- Sutaria N, Adawi W, Brown I, et al. Racial disparities in mortality among patients with prurigo nodularis: a multi-center cohort study. J Am Acad Dermatol. 2022;82:487-490.
- Boozalis E, Tang O, Patel S, et al. Ethnic differences and comorbidities of 909 prurigo nodularis patients. J Am Acad Dermatol. 2018; 79:714-719.e3.
- Müller S, Zeidler C, Ständer S. Chronic prurigo including prurigo nodularis: new insights and treatments. Am J Clin Dermatol. 2024;25:15-33.
- Williams KA, Roh YS, Brown I, et al. Pathophysiology, diagnosis, and pharmacological treatment of prurigo nodularis. Expert Rev Clin Pharmacol. 2021;14:67-77.
- Kwatra SG, Yosipovitch G, Legat FJ, et al. Phase 3 trial of nemolizumab in patients with prurigo nodularis. N Engl J Med. 2023;389:1579-1589.
- Beck KM, Yang EJ, Sekhon S, et al. Dupilumab treatment for generalized prurigo nodularis. JAMA Dermatol. 2019;155:118-120.
- Yosipovitch G, Mollanazar N, Ständer S, et al. Dupilumab in patients with prurigo nodularis: two randomized, double-blind, placebocontrolled phase 3 trials. Nat Med. 2023;29:1180-1190.
- Wongvibulsin S, Sutaria N, Williams KA, et al. A nationwide study of prurigo nodularis: disease burden and healthcare utilization in the United States. J Invest Dermatol. 2021;141:2530-2533.e1.
- Sutaria N, Alphonse MP, Marani M, et al. Cluster analysis of circulating plasma biomarkers in prurigo nodularis reveals a distinct systemic inflammatory signature in African Americans. J Invest Dermatol. 2022;142:1300-1308.e3.
Break the Itch-Scratch Cycle to Treat Prurigo Nodularis
Break the Itch-Scratch Cycle to Treat Prurigo Nodularis
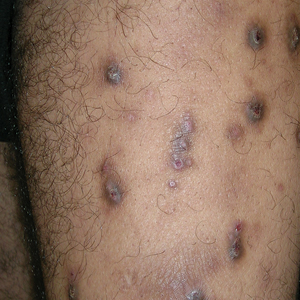
Conservative Approach to Treatment of Cyclosporine-Induced Gingival Hyperplasia With Azithromycin and Chlorhexidine
Conservative Approach to Treatment of Cyclosporine-Induced Gingival Hyperplasia With Azithromycin and Chlorhexidine
Cyclosporine is a calcineurin inhibitor and immunosuppressive medication with several indications, including prevention of parenchymal organ and bone marrow transplant rejection as well as treatment of numerous dermatologic conditions (eg, psoriasis, atopic dermatitis). Although it is an effective medication, there are many known adverse effects including nephrotoxicity, hypertension, and gingival hyperplasia.1 Addressing symptomatic cyclosporine-induced gingival hyperplasia can be challenging, especially if continued use of cyclosporine is necessary for adequate control of the underlying disease. We present a simplified approach for conservative management of cyclosporine-induced gingival hyperplasia that allows for continued use of cyclosporine.
Practice Gap
Cyclosporine-induced gingival hyperplasia is a fibrous overgrowth of the interdental papilla and labial gingiva that may lead to gum pain, difficulty eating, gingivitis, and/ or tooth decay or loss.2 The condition usually occurs 3 to 6 months after starting cyclosporine but may occur as soon as 1 month later.1,3 The pathophysiology of this adverse effect is incompletely understood, but several mechanisms have been implicated, including upregulation of the salivary proinflammatory cytokines IL-1α, IL-8, and IL-6.1 Additionally, patients with cyclosporine-induced gingival hyperplasia have increased bacterial colonization with species such as Porphyromonas gingivalis.4 Risk factors for cyclosporine- induced gingival hyperplasia include higher serum concentrations (>400 ng/mL) of cyclosporine, history of gingival hyperplasia, concomitant use of calcium channel blockers, and insufficient oral hygiene.2,3 A study by Seymour and Smith5 found that proper oral hygiene leads to less severe cases of cyclosporine-induced gingival hyperplasia but does not prevent gingival overgrowth. Treatment of cyclosporine-induced gingival hyperplasia traditionally involves targeting oral bacteria and reducing inflammation. Decreasing dental plaque through regular tooth-brushing and interdental cleaning may reduce symptoms such as bleeding and discomfort of the gums.
The intensity of cyclosporine-induced gingival hyperplasia can be reduced with chlorhexidine or azithromycin. Individually, each therapy has been shown to clinically improve cyclosporine-induced gingival hyperplasia; however, to our knowledge the combination of these treatments has not been reported.1 We present a simplified approach to treating cyclosporine-induced gingival hyperplasia using both azithromycin and chlorhexidine. This conservative approach results in effective and sustained improvement of gingival hyperplasia while allowing patients to continue cyclosporine therapy to control underlying disease with minimal adverse effects.
Technique
Before initiating treatment, it is important to confirm that the etiology of gingival hyperplasia is due to cyclosporine use and rule out nutritional deficiencies and autoimmune conditions as potential causes. Be sure to inquire about nutritional intake, systemic symptoms, and family history of autoimmune conditions. Our approach includes the use of azithromycin 500 mg once daily for 7 days followed by chlorhexidine 0.12% oral solution 15 mL twice daily (swish undiluted for 30 seconds, then spit) for at least 3 months for optimal management of gingival hyperplasia. Chlorhexidine should be continued for at least 6 months to maintain symptom resolution. While cyclosporine therapy may be continued throughout the duration of this regimen, consider switching to other immunosuppressive medications that are not associated with gingival hyperplasia (eg, tacrolimus) if symptoms are severe and/or resistant to therapy.1,6
We applied this technique to treat cyclosporine-induced gingival hyperplasia in a 28-year-old woman with a 3-year history of primary aplastic anemia. The patient initially presented with pain and bleeding of the gums of several months’ duration and reported experiencing gum pain when eating solid foods. Her medications included cyclosporine 225 mg daily for aplastic anemia and dapsone 100 mg daily for pneumocystis pneumonia prophylaxis, both of which were taken for the past 6 months. Oral examination revealed pink to bright red hyperplastic gingivae (Figure). She had no other symptoms associated with aplastic anemia and no signs of vitamin or nutritional deficiencies. She denied pre-existing periodontitis prior to starting cyclosporine and reported that the symptoms started several months after initiating cyclosporine therapy. Thus, the clinical diagnosis of cyclosporine-induced gingival hyperplasia was made, and treatment with azithromycin and chlorhexidine was initiated with marked reduction in symptoms.
Conservative management of gingival hyperplasia with oral hygiene including regular tooth-brushing and flossing and antimicrobial therapies was preferred in this patient to reduce gum pain and minimize the risk for tooth loss while also limiting the use of surgically invasive interventions. Due to limited therapeutic options for aplastic anemia, continued administration of cyclosporine was necessary in our patient to prevent further complications.
Practice Implications
The precise mechanism by which azithromycin treats gingival hyperplasia is unclear but may involve its antimicrobial and anti-inflammatory properties. Small concentrations of azithromycin have been shown to persist in macrophages and fibroblasts of the gingiva even with short-term administration of 3 to 5 days.7 Chlorhexidine is another antimicrobial agent often used in oral rinse solutions to decrease plaque formation and prevent gingivitis. Chlorhexidine can reduce cyclosporine-induced gingival overgrowth when used twice daily.8 After rinsing with chlorhexidine, saliva exhibits antibacterial activity for up to 5 hours; however, tooth and gum discoloration may occur.8
Recurrence of gingival hyperplasia is likely if cyclosporine is not discontinued or maintained with treatment.3 Conventional gingivectomy should be considered for cases in which conservative treatment is ineffective, aesthetic concerns arise, or gingival hyperplasia persists for more than 6 to 12 months after discontinuing cyclosporine.1
We theorize that the microbial properties of azithromycin and chlorhexidine help reduce periodontal inflammation and bacterial overgrowth in patients with cyclosporine-induced gingival hyperplasia, which allows for restoration of gingival health. Our case highlights the efficacy of our treatment approach using a 7-day course of azithromycin followed by twice-daily use of chlorhexidine oral rinse in the treatment of cyclosporine-induced gingival hyperplasia with continued use of cyclosporine.
- Chojnacka-Purpurowicz J, Wygonowska E, Placek W, et al. Cyclosporine-induced gingival overgrowth—review. Dermatol Ther. 2022;35:E15912.
- Greenburg KV, Armitage GC, Shiboski CH. Gingival enlargement among renal transplant recipients in the era of new-generation immunosuppressants. J Periodontol. 2008;79:453-460.
- Cyclosporine (ciclosporin)(systemic): drug information. UpToDate. Accessed December 19, 2023. https://www.uptodate.com/contents/table-of-contents/drug-information/general-drug-information
- Gong Y, Bi W, Cao L, et al. Association of CD14-260 polymorphisms, red-complex periodontopathogens and gingival crevicular fluid cytokine levels with cyclosporine A-induced gingival overgrowth in renal transplant patients. J Periodontal Res. 2013;48:203-212.
- Seymour RA, Smith DG. The effect of a plaque control programme on the incidence and severity of cyclosporin-induced gingival changes. J Clin Periodontol. 1991;18:107-110.
- Nash MM, Zaltzman JS. Efficacy of azithromycin in the treatment of cyclosporine-induced gingival hyperplasia in renal transplant recipients. Transplantation. 1998;65:1611-1615.
- Martín JM, Mateo E, Jordá E. Utilidad de la azitromicina en la hyperplasia gingival inducida por ciclosporina [azithromycin for the treatment of ciclosporin-induced gingival hyperplasia]. Actas Dermosifiliogr. 2016;107:780.
- Gau CH, Tu HS, Chin YT, et al. Can chlorhexidine mouthwash twice daily ameliorate cyclosporine-induced gingival overgrowth? J Formos Med Assoc. 2013;112:131-137.
Cyclosporine is a calcineurin inhibitor and immunosuppressive medication with several indications, including prevention of parenchymal organ and bone marrow transplant rejection as well as treatment of numerous dermatologic conditions (eg, psoriasis, atopic dermatitis). Although it is an effective medication, there are many known adverse effects including nephrotoxicity, hypertension, and gingival hyperplasia.1 Addressing symptomatic cyclosporine-induced gingival hyperplasia can be challenging, especially if continued use of cyclosporine is necessary for adequate control of the underlying disease. We present a simplified approach for conservative management of cyclosporine-induced gingival hyperplasia that allows for continued use of cyclosporine.
Practice Gap
Cyclosporine-induced gingival hyperplasia is a fibrous overgrowth of the interdental papilla and labial gingiva that may lead to gum pain, difficulty eating, gingivitis, and/ or tooth decay or loss.2 The condition usually occurs 3 to 6 months after starting cyclosporine but may occur as soon as 1 month later.1,3 The pathophysiology of this adverse effect is incompletely understood, but several mechanisms have been implicated, including upregulation of the salivary proinflammatory cytokines IL-1α, IL-8, and IL-6.1 Additionally, patients with cyclosporine-induced gingival hyperplasia have increased bacterial colonization with species such as Porphyromonas gingivalis.4 Risk factors for cyclosporine- induced gingival hyperplasia include higher serum concentrations (>400 ng/mL) of cyclosporine, history of gingival hyperplasia, concomitant use of calcium channel blockers, and insufficient oral hygiene.2,3 A study by Seymour and Smith5 found that proper oral hygiene leads to less severe cases of cyclosporine-induced gingival hyperplasia but does not prevent gingival overgrowth. Treatment of cyclosporine-induced gingival hyperplasia traditionally involves targeting oral bacteria and reducing inflammation. Decreasing dental plaque through regular tooth-brushing and interdental cleaning may reduce symptoms such as bleeding and discomfort of the gums.
The intensity of cyclosporine-induced gingival hyperplasia can be reduced with chlorhexidine or azithromycin. Individually, each therapy has been shown to clinically improve cyclosporine-induced gingival hyperplasia; however, to our knowledge the combination of these treatments has not been reported.1 We present a simplified approach to treating cyclosporine-induced gingival hyperplasia using both azithromycin and chlorhexidine. This conservative approach results in effective and sustained improvement of gingival hyperplasia while allowing patients to continue cyclosporine therapy to control underlying disease with minimal adverse effects.
Technique
Before initiating treatment, it is important to confirm that the etiology of gingival hyperplasia is due to cyclosporine use and rule out nutritional deficiencies and autoimmune conditions as potential causes. Be sure to inquire about nutritional intake, systemic symptoms, and family history of autoimmune conditions. Our approach includes the use of azithromycin 500 mg once daily for 7 days followed by chlorhexidine 0.12% oral solution 15 mL twice daily (swish undiluted for 30 seconds, then spit) for at least 3 months for optimal management of gingival hyperplasia. Chlorhexidine should be continued for at least 6 months to maintain symptom resolution. While cyclosporine therapy may be continued throughout the duration of this regimen, consider switching to other immunosuppressive medications that are not associated with gingival hyperplasia (eg, tacrolimus) if symptoms are severe and/or resistant to therapy.1,6
We applied this technique to treat cyclosporine-induced gingival hyperplasia in a 28-year-old woman with a 3-year history of primary aplastic anemia. The patient initially presented with pain and bleeding of the gums of several months’ duration and reported experiencing gum pain when eating solid foods. Her medications included cyclosporine 225 mg daily for aplastic anemia and dapsone 100 mg daily for pneumocystis pneumonia prophylaxis, both of which were taken for the past 6 months. Oral examination revealed pink to bright red hyperplastic gingivae (Figure). She had no other symptoms associated with aplastic anemia and no signs of vitamin or nutritional deficiencies. She denied pre-existing periodontitis prior to starting cyclosporine and reported that the symptoms started several months after initiating cyclosporine therapy. Thus, the clinical diagnosis of cyclosporine-induced gingival hyperplasia was made, and treatment with azithromycin and chlorhexidine was initiated with marked reduction in symptoms.
Conservative management of gingival hyperplasia with oral hygiene including regular tooth-brushing and flossing and antimicrobial therapies was preferred in this patient to reduce gum pain and minimize the risk for tooth loss while also limiting the use of surgically invasive interventions. Due to limited therapeutic options for aplastic anemia, continued administration of cyclosporine was necessary in our patient to prevent further complications.
Practice Implications
The precise mechanism by which azithromycin treats gingival hyperplasia is unclear but may involve its antimicrobial and anti-inflammatory properties. Small concentrations of azithromycin have been shown to persist in macrophages and fibroblasts of the gingiva even with short-term administration of 3 to 5 days.7 Chlorhexidine is another antimicrobial agent often used in oral rinse solutions to decrease plaque formation and prevent gingivitis. Chlorhexidine can reduce cyclosporine-induced gingival overgrowth when used twice daily.8 After rinsing with chlorhexidine, saliva exhibits antibacterial activity for up to 5 hours; however, tooth and gum discoloration may occur.8
Recurrence of gingival hyperplasia is likely if cyclosporine is not discontinued or maintained with treatment.3 Conventional gingivectomy should be considered for cases in which conservative treatment is ineffective, aesthetic concerns arise, or gingival hyperplasia persists for more than 6 to 12 months after discontinuing cyclosporine.1
We theorize that the microbial properties of azithromycin and chlorhexidine help reduce periodontal inflammation and bacterial overgrowth in patients with cyclosporine-induced gingival hyperplasia, which allows for restoration of gingival health. Our case highlights the efficacy of our treatment approach using a 7-day course of azithromycin followed by twice-daily use of chlorhexidine oral rinse in the treatment of cyclosporine-induced gingival hyperplasia with continued use of cyclosporine.
Cyclosporine is a calcineurin inhibitor and immunosuppressive medication with several indications, including prevention of parenchymal organ and bone marrow transplant rejection as well as treatment of numerous dermatologic conditions (eg, psoriasis, atopic dermatitis). Although it is an effective medication, there are many known adverse effects including nephrotoxicity, hypertension, and gingival hyperplasia.1 Addressing symptomatic cyclosporine-induced gingival hyperplasia can be challenging, especially if continued use of cyclosporine is necessary for adequate control of the underlying disease. We present a simplified approach for conservative management of cyclosporine-induced gingival hyperplasia that allows for continued use of cyclosporine.
Practice Gap
Cyclosporine-induced gingival hyperplasia is a fibrous overgrowth of the interdental papilla and labial gingiva that may lead to gum pain, difficulty eating, gingivitis, and/ or tooth decay or loss.2 The condition usually occurs 3 to 6 months after starting cyclosporine but may occur as soon as 1 month later.1,3 The pathophysiology of this adverse effect is incompletely understood, but several mechanisms have been implicated, including upregulation of the salivary proinflammatory cytokines IL-1α, IL-8, and IL-6.1 Additionally, patients with cyclosporine-induced gingival hyperplasia have increased bacterial colonization with species such as Porphyromonas gingivalis.4 Risk factors for cyclosporine- induced gingival hyperplasia include higher serum concentrations (>400 ng/mL) of cyclosporine, history of gingival hyperplasia, concomitant use of calcium channel blockers, and insufficient oral hygiene.2,3 A study by Seymour and Smith5 found that proper oral hygiene leads to less severe cases of cyclosporine-induced gingival hyperplasia but does not prevent gingival overgrowth. Treatment of cyclosporine-induced gingival hyperplasia traditionally involves targeting oral bacteria and reducing inflammation. Decreasing dental plaque through regular tooth-brushing and interdental cleaning may reduce symptoms such as bleeding and discomfort of the gums.
The intensity of cyclosporine-induced gingival hyperplasia can be reduced with chlorhexidine or azithromycin. Individually, each therapy has been shown to clinically improve cyclosporine-induced gingival hyperplasia; however, to our knowledge the combination of these treatments has not been reported.1 We present a simplified approach to treating cyclosporine-induced gingival hyperplasia using both azithromycin and chlorhexidine. This conservative approach results in effective and sustained improvement of gingival hyperplasia while allowing patients to continue cyclosporine therapy to control underlying disease with minimal adverse effects.
Technique
Before initiating treatment, it is important to confirm that the etiology of gingival hyperplasia is due to cyclosporine use and rule out nutritional deficiencies and autoimmune conditions as potential causes. Be sure to inquire about nutritional intake, systemic symptoms, and family history of autoimmune conditions. Our approach includes the use of azithromycin 500 mg once daily for 7 days followed by chlorhexidine 0.12% oral solution 15 mL twice daily (swish undiluted for 30 seconds, then spit) for at least 3 months for optimal management of gingival hyperplasia. Chlorhexidine should be continued for at least 6 months to maintain symptom resolution. While cyclosporine therapy may be continued throughout the duration of this regimen, consider switching to other immunosuppressive medications that are not associated with gingival hyperplasia (eg, tacrolimus) if symptoms are severe and/or resistant to therapy.1,6
We applied this technique to treat cyclosporine-induced gingival hyperplasia in a 28-year-old woman with a 3-year history of primary aplastic anemia. The patient initially presented with pain and bleeding of the gums of several months’ duration and reported experiencing gum pain when eating solid foods. Her medications included cyclosporine 225 mg daily for aplastic anemia and dapsone 100 mg daily for pneumocystis pneumonia prophylaxis, both of which were taken for the past 6 months. Oral examination revealed pink to bright red hyperplastic gingivae (Figure). She had no other symptoms associated with aplastic anemia and no signs of vitamin or nutritional deficiencies. She denied pre-existing periodontitis prior to starting cyclosporine and reported that the symptoms started several months after initiating cyclosporine therapy. Thus, the clinical diagnosis of cyclosporine-induced gingival hyperplasia was made, and treatment with azithromycin and chlorhexidine was initiated with marked reduction in symptoms.
Conservative management of gingival hyperplasia with oral hygiene including regular tooth-brushing and flossing and antimicrobial therapies was preferred in this patient to reduce gum pain and minimize the risk for tooth loss while also limiting the use of surgically invasive interventions. Due to limited therapeutic options for aplastic anemia, continued administration of cyclosporine was necessary in our patient to prevent further complications.
Practice Implications
The precise mechanism by which azithromycin treats gingival hyperplasia is unclear but may involve its antimicrobial and anti-inflammatory properties. Small concentrations of azithromycin have been shown to persist in macrophages and fibroblasts of the gingiva even with short-term administration of 3 to 5 days.7 Chlorhexidine is another antimicrobial agent often used in oral rinse solutions to decrease plaque formation and prevent gingivitis. Chlorhexidine can reduce cyclosporine-induced gingival overgrowth when used twice daily.8 After rinsing with chlorhexidine, saliva exhibits antibacterial activity for up to 5 hours; however, tooth and gum discoloration may occur.8
Recurrence of gingival hyperplasia is likely if cyclosporine is not discontinued or maintained with treatment.3 Conventional gingivectomy should be considered for cases in which conservative treatment is ineffective, aesthetic concerns arise, or gingival hyperplasia persists for more than 6 to 12 months after discontinuing cyclosporine.1
We theorize that the microbial properties of azithromycin and chlorhexidine help reduce periodontal inflammation and bacterial overgrowth in patients with cyclosporine-induced gingival hyperplasia, which allows for restoration of gingival health. Our case highlights the efficacy of our treatment approach using a 7-day course of azithromycin followed by twice-daily use of chlorhexidine oral rinse in the treatment of cyclosporine-induced gingival hyperplasia with continued use of cyclosporine.
- Chojnacka-Purpurowicz J, Wygonowska E, Placek W, et al. Cyclosporine-induced gingival overgrowth—review. Dermatol Ther. 2022;35:E15912.
- Greenburg KV, Armitage GC, Shiboski CH. Gingival enlargement among renal transplant recipients in the era of new-generation immunosuppressants. J Periodontol. 2008;79:453-460.
- Cyclosporine (ciclosporin)(systemic): drug information. UpToDate. Accessed December 19, 2023. https://www.uptodate.com/contents/table-of-contents/drug-information/general-drug-information
- Gong Y, Bi W, Cao L, et al. Association of CD14-260 polymorphisms, red-complex periodontopathogens and gingival crevicular fluid cytokine levels with cyclosporine A-induced gingival overgrowth in renal transplant patients. J Periodontal Res. 2013;48:203-212.
- Seymour RA, Smith DG. The effect of a plaque control programme on the incidence and severity of cyclosporin-induced gingival changes. J Clin Periodontol. 1991;18:107-110.
- Nash MM, Zaltzman JS. Efficacy of azithromycin in the treatment of cyclosporine-induced gingival hyperplasia in renal transplant recipients. Transplantation. 1998;65:1611-1615.
- Martín JM, Mateo E, Jordá E. Utilidad de la azitromicina en la hyperplasia gingival inducida por ciclosporina [azithromycin for the treatment of ciclosporin-induced gingival hyperplasia]. Actas Dermosifiliogr. 2016;107:780.
- Gau CH, Tu HS, Chin YT, et al. Can chlorhexidine mouthwash twice daily ameliorate cyclosporine-induced gingival overgrowth? J Formos Med Assoc. 2013;112:131-137.
- Chojnacka-Purpurowicz J, Wygonowska E, Placek W, et al. Cyclosporine-induced gingival overgrowth—review. Dermatol Ther. 2022;35:E15912.
- Greenburg KV, Armitage GC, Shiboski CH. Gingival enlargement among renal transplant recipients in the era of new-generation immunosuppressants. J Periodontol. 2008;79:453-460.
- Cyclosporine (ciclosporin)(systemic): drug information. UpToDate. Accessed December 19, 2023. https://www.uptodate.com/contents/table-of-contents/drug-information/general-drug-information
- Gong Y, Bi W, Cao L, et al. Association of CD14-260 polymorphisms, red-complex periodontopathogens and gingival crevicular fluid cytokine levels with cyclosporine A-induced gingival overgrowth in renal transplant patients. J Periodontal Res. 2013;48:203-212.
- Seymour RA, Smith DG. The effect of a plaque control programme on the incidence and severity of cyclosporin-induced gingival changes. J Clin Periodontol. 1991;18:107-110.
- Nash MM, Zaltzman JS. Efficacy of azithromycin in the treatment of cyclosporine-induced gingival hyperplasia in renal transplant recipients. Transplantation. 1998;65:1611-1615.
- Martín JM, Mateo E, Jordá E. Utilidad de la azitromicina en la hyperplasia gingival inducida por ciclosporina [azithromycin for the treatment of ciclosporin-induced gingival hyperplasia]. Actas Dermosifiliogr. 2016;107:780.
- Gau CH, Tu HS, Chin YT, et al. Can chlorhexidine mouthwash twice daily ameliorate cyclosporine-induced gingival overgrowth? J Formos Med Assoc. 2013;112:131-137.
Conservative Approach to Treatment of Cyclosporine-Induced Gingival Hyperplasia With Azithromycin and Chlorhexidine
Conservative Approach to Treatment of Cyclosporine-Induced Gingival Hyperplasia With Azithromycin and Chlorhexidine
Program Director Perspectives on DEI Initiatives in the Dermatology Residency Selection Process
Program Director Perspectives on DEI Initiatives in the Dermatology Residency Selection Process
The recent Supreme Court ruling that struck down affirmative action1 has caused many initiatives aimed at promoting diversity, equity, and inclusion (DEI) to fall under scrutiny; however, the American Academy of Dermatology (AAD) published a statement of intent in 2022 recognizing and committing to DEI as a priority in the specialty.2 In this study, we used a formal survey to investigate the perceptions of dermatology program directors (PDs) on DEI programming from the AAD and how DEI is integrated into the resident selection process at varying institutions.
Methods
We conducted a cross-sectional study of dermatology PDs across the United States from April 2024 to July 2024. Program directors were contacted via the Association of Professors of Dermatology PD listserve, which includes all 103 PDs who are members of the organization. Personalized survey links were created and sent individually to each PD’s email address. Thirty responses were received. All survey responses were captured anonymously. The survey consisted of 17 questions focusing on dermatology PD demographics and opinions on DEI initiatives in the AAD and in the dermatology resident selection process. Data were collected using Qualtrics survey tools and analyzed using Qualtrics reports.
Results
Demographics—A total of 30 completed surveys were received. Thirty-three percent (10/30) of respondents were from the Midwest, and 23% (7/30) were from the Northeast. The next most represented region was the West, with 20% (6/30) of respondents. The Southeast and Southwest were the least represented regions captured in our survey, accounting for 13% (4/30) and 10% (3/30) of respondents, respectively. After answering this initial demographic question, 1 respondent stopped the survey, bringing our new total to 29 respondents.
Most (66% [19/29]) of the survey respondents had served as PDs for 5 years or less. Sixty-nine percent (20/29) identified as female, while 31% (9/29) identified as male. Seventy-two percent (21/29) identified as White, 17% (5/29) identified as Asian, 3% (1/29) identified as Black/African American, 3% (1/29) identified as Hispanic or Latinx, and 3% (1/29) identified as mixed race.
Opinions on DEI Initiatives—When asked about their satisfaction level with the current amount of DEI efforts within the AAD, 17% (5/29) of respondents said they were very satisfied, 59% (17/29) said they were satisfied, 17% (5/29) said they were neutral, and 7% (2/29) said they were dissatisfied. Given that none of the questions were mandatory to answer before proceeding with the survey, there were variable response rates to each of the remaining questions, which may have caused respondents to answer only questions they felt strongly about.
Twenty respondents answered when prompted to further classify their level of satisfaction: 70% (14/20) said there should be more DEI efforts through the AAD providing financial support, and 50% (10/20) wanted more nonfinancial support. When given the opportunity to specify which DEI initiatives should be enhanced, the majority (67% [14/21]) of PDs chose the AAD’s health disparities curriculum, followed by the Diversity Mentorship Program (52% [11/21]), AAD Diversity Toolkit (43% [9/21]), and the Skin of Color Curriculum (43% [9/21]). Thiry-three percent (7/30) of PDs wanted enhancement of Medicine Without Barriers: Overcoming Unintended Bias in Practice (an AAD educational resource), and 19% (4/21) of respondents did not think any of the AAD’s DEI initiatives needed to be enhanced. There were 14 responses to a question about choosing which DEI initiatives to reduce with singular votes (7% [1/14] each) to reduce Medicine Without Barriers: Overcoming Unintended Bias in Practice and the Skin of Color Curriculum.
Our survey also invited PDs to introduce ideas for new DEI initiatives or programs. The following were suggestions offered by respondents: education for senior members of the AAD on the importance of DEI in dermatology, professional development resources directed toward academic faculty members to prepare them for interacting with and teaching residents from different backgrounds, and more advertisements and support for the AAD’s Diversity Champion Workshop.
DEI in Resident Selection—When asked about the role that DEI plays in how programs develop their match lists for residency, 13% (3/23) of PDs responded that it plays a very large role, 52% (12/23) stated that it plays a large role, 26% (6/23) responded that it plays somewhat of a role, 4% (1/23) stated that it plays a small role, and 4% (1/23) stated that it plays no role. Twenty-four percent (4/17) of respondents were PDs in states that have legislation limiting or defunding DEI initiatives at institutions of higher education. Another 12% (2/17) were from states where such legislation was pending a vote, while 59% (10/17) of respondents indicated that their state had not introduced such legislation. Four percent (1/17) indicated that they were from a state that had introduced legislation to limit or defund DEI initiatives that failed to pass. Only 17 respondents answered this question, which may be due to a lack of awareness among respondents of state-specific legislation on limiting or defunding DEI initiatives.
Resident Selection Factors—Ninety-six percent (22/23) of PDs stated that their residency program uses a holistic review that takes into account factors such as experiences (eg, volunteer work, research endeavors), personal attributes, and metrics in a balanced manner. No PDs offered United States Medical Licensing Examination Step score cutoffs or medical school clerkship cutoff grades. When asked to rank the importance placed on individual factors in the residency application, the following were ranked from most to least important in the process: performance on clerkships/rotations, performance on interviews, letters of recommendation, clerkship grades, United States Medical Licensing Examination Step scores, research content/ quality, race/ethnicity, history of teaching and mentorship, volunteering, and research amount. When asked to indicate the most pertinent factors used to incorporate DEI in resident selection, the most popular factor was lived experience/life, which was chosen by 90% (18/20) of PDs followed by 75% (15/20) of respondents incorporating underrepresented in medicine (URM) status (including Black, Latinx, and Native American applicants) and 70% (4/20) incorporating socioeconomic status. Sexual orientation and geographic ties of the applicant to the region of the residency program was incorporated by 45% (9/20) of respondents, and other characteristics of race and sex each were incorporated by 30% (6/20) of respondents. Religion was the least incorporated, with 10% (2/20) of PDs selecting this classification. In considering URM status when choosing dermatology residents, 100% (11/11) of respondents indicated that their institution promotes diversity as a part of the recruitment process. Eighty-two percent (9/11) of respondents try to recruit URM applicants to reflect their patient population, 82% (9/11) try as part of a belief that a diverse group benefits everyone in their program, and 45% (5/11) try in order to address societal inequities and as a broader mission to diversify the health care workforce. Seventy-three percent (8/11) indicated that they pay attention to URM status throughout the application process.
Comment
Diversity in the US population is steadily increasing. Within the past decade, the diversity index (the probability that 2 people chosen at random will be from different racial and ethnic groups) has grown from 54.9% in 2010 to 61.1% in 2020.3 There was a 24.9% increase in population groups other than non-Hispanic Whites from 2010 to 2020, an increase in diversity that was present in every region of the United States.4 The field of dermatology already does not reflect the racial distribution of the nation,4 with Black individuals accounting for 13.7% of the nation’s population but only 3% of dermatologists; similarly, Hispanic individuals account for 19.5% of the population but only comprise 4.2% of dermatologists.5,6 There is overwhelming evidence that patients prefer to be diagnosed and treated by physicians who reflect their own demographics.7 Furthermore, physicians who prescribe treatment plans that reflect and respect socioeconomic and religious beliefs of the populations they serve enable patients to meet treatment expectations and experience better outcomes.8 Direct action is required to ensure that the specialty more accurately represents the evolving demographics of the country. This can be accomplished in myriad ways, including but not limited to cultural humility training9 for current dermatologists and trainees and recruitment of a more diverse workforce. These measures can ultimately improve treatment approaches and outcomes for dermatologic conditions across various groups.10
There are efforts by various dermatologic organizations, including the AAD, Society for Pediatric Dermatology, Pediatric Dermatology Research Alliance, Skin of Color Society, Women’s Dermatologic Society, and American Society for Dermatologic Surgery, that are focused on promoting DEI through research, education, and mentorship of potential future dermatologists.11 However, the perceptions, opinions, and selection process instituted by PDs are most consequential in determining the diversity of the specialty, as PDs are at the forefront of establishing the next generation of dermatologists. Through this study, we have found that most PDs recognize the importance of diversity in residency education and recruitment without it being the only deciding factor.
The main limitation of this study was the small sample size, which may not adequately represent all dermatology residency programs accredited by the Accreditation Council for Graduate Medical Education as a result of selection bias toward respondents who were more likely to participate in survey-based research on topics of DEI.
Conclusion
This study revealed that, among dermatology residency PDs, there is interest in modifying the resources and initiatives surrounding DEI in the field. It also revealed that DEI remains a consideration in the resident selection process despite the recent Supreme Court ruling. In conclusion, there is an eagerness among dermatology PDs to incorporate DEI into resident selection even though gaps in knowledge and awareness remain.
- Supreme Court of the United States. Students for Fair Admissions, Inc v President and Fellows of Harvard College (No. 20–1199). Argued October 31, 2022. Decided June 29, 2023. https://www.supremecourt.gov/opinions/22pdf/20-1199_hgdj.pdf
- American Academy of Dermatology. AAD’s DEI Statement of Intent. Published March 28, 2022. Accessed November 18, 2024. https://www.aad.org/member/career/diversity/diversity-statement-of-intent
- Jensen E, Jones N, Rabe M, et al. The chance that two people chosen at random are of different race or ethnicity groups has increased since 2010. United States Census Bureau. August 12, 2021. Accessed November 5, 2024. https://www.census.gov/library/stories/2021/08/2020-united-states-population-more-racially-ethnically-diverse-than-2010.html
- Johnson K. New Census reflects growing U.S. population diversity, with children in the forefront. University of New Hampshire Carsey School of Public Policy. October 6, 2021. Accessed November 5, 2024. https://carsey.unh.edu/publication/new-census-reflects-growing-us-population-diversity-children-forefront
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74; 584-587. doi:10.1016/j.jaad.2015.10.044
- United States Census Bureau. QuickFacts: United States. Population estimates, July 1, 2023 (V2023). Accessed November 5, 2024. https://www.census.gov/quickfacts/fact/table/US/PST045222
- Saha S, Beach MC. Impact of physician race on patient decision-making and ratings of physicians: a randomized experiment using video vignettes. J Gen Intern Med. 2020;35:1084-1091. doi:10.1007/s11606-020-05646-z
- Nair L, Adetayo OA. Cultural competence and ethnic diversity in healthcare. Plast Reconstr Surg Glob Open. 2019;7:E2219. doi:10.1097/GOX.0000000000002219
- Yeager KA, Bauer-Wu S. Cultural humility: essential foundation for clinical researchers. Appl Nurs Res. 2013;26:251-256. doi:10.1016/j.apnr.2013.06.008
- Narla S, Heath CR, Alexis A, et al. Racial disparities in dermatology. Arch Dermatol Res. 2023;315:1215-1223. doi:10.1007/s00403-022- 02507-z
- Desai SR, Khanna R, Glass D, et al. Embracing diversity in dermatology: creation of a culture of equity and inclusion in dermatology. Int J Womens Dermatol. 2021;7:378-382. doi:10.1016/j.ijwd.2021.08.002
The recent Supreme Court ruling that struck down affirmative action1 has caused many initiatives aimed at promoting diversity, equity, and inclusion (DEI) to fall under scrutiny; however, the American Academy of Dermatology (AAD) published a statement of intent in 2022 recognizing and committing to DEI as a priority in the specialty.2 In this study, we used a formal survey to investigate the perceptions of dermatology program directors (PDs) on DEI programming from the AAD and how DEI is integrated into the resident selection process at varying institutions.
Methods
We conducted a cross-sectional study of dermatology PDs across the United States from April 2024 to July 2024. Program directors were contacted via the Association of Professors of Dermatology PD listserve, which includes all 103 PDs who are members of the organization. Personalized survey links were created and sent individually to each PD’s email address. Thirty responses were received. All survey responses were captured anonymously. The survey consisted of 17 questions focusing on dermatology PD demographics and opinions on DEI initiatives in the AAD and in the dermatology resident selection process. Data were collected using Qualtrics survey tools and analyzed using Qualtrics reports.
Results
Demographics—A total of 30 completed surveys were received. Thirty-three percent (10/30) of respondents were from the Midwest, and 23% (7/30) were from the Northeast. The next most represented region was the West, with 20% (6/30) of respondents. The Southeast and Southwest were the least represented regions captured in our survey, accounting for 13% (4/30) and 10% (3/30) of respondents, respectively. After answering this initial demographic question, 1 respondent stopped the survey, bringing our new total to 29 respondents.
Most (66% [19/29]) of the survey respondents had served as PDs for 5 years or less. Sixty-nine percent (20/29) identified as female, while 31% (9/29) identified as male. Seventy-two percent (21/29) identified as White, 17% (5/29) identified as Asian, 3% (1/29) identified as Black/African American, 3% (1/29) identified as Hispanic or Latinx, and 3% (1/29) identified as mixed race.
Opinions on DEI Initiatives—When asked about their satisfaction level with the current amount of DEI efforts within the AAD, 17% (5/29) of respondents said they were very satisfied, 59% (17/29) said they were satisfied, 17% (5/29) said they were neutral, and 7% (2/29) said they were dissatisfied. Given that none of the questions were mandatory to answer before proceeding with the survey, there were variable response rates to each of the remaining questions, which may have caused respondents to answer only questions they felt strongly about.
Twenty respondents answered when prompted to further classify their level of satisfaction: 70% (14/20) said there should be more DEI efforts through the AAD providing financial support, and 50% (10/20) wanted more nonfinancial support. When given the opportunity to specify which DEI initiatives should be enhanced, the majority (67% [14/21]) of PDs chose the AAD’s health disparities curriculum, followed by the Diversity Mentorship Program (52% [11/21]), AAD Diversity Toolkit (43% [9/21]), and the Skin of Color Curriculum (43% [9/21]). Thiry-three percent (7/30) of PDs wanted enhancement of Medicine Without Barriers: Overcoming Unintended Bias in Practice (an AAD educational resource), and 19% (4/21) of respondents did not think any of the AAD’s DEI initiatives needed to be enhanced. There were 14 responses to a question about choosing which DEI initiatives to reduce with singular votes (7% [1/14] each) to reduce Medicine Without Barriers: Overcoming Unintended Bias in Practice and the Skin of Color Curriculum.
Our survey also invited PDs to introduce ideas for new DEI initiatives or programs. The following were suggestions offered by respondents: education for senior members of the AAD on the importance of DEI in dermatology, professional development resources directed toward academic faculty members to prepare them for interacting with and teaching residents from different backgrounds, and more advertisements and support for the AAD’s Diversity Champion Workshop.
DEI in Resident Selection—When asked about the role that DEI plays in how programs develop their match lists for residency, 13% (3/23) of PDs responded that it plays a very large role, 52% (12/23) stated that it plays a large role, 26% (6/23) responded that it plays somewhat of a role, 4% (1/23) stated that it plays a small role, and 4% (1/23) stated that it plays no role. Twenty-four percent (4/17) of respondents were PDs in states that have legislation limiting or defunding DEI initiatives at institutions of higher education. Another 12% (2/17) were from states where such legislation was pending a vote, while 59% (10/17) of respondents indicated that their state had not introduced such legislation. Four percent (1/17) indicated that they were from a state that had introduced legislation to limit or defund DEI initiatives that failed to pass. Only 17 respondents answered this question, which may be due to a lack of awareness among respondents of state-specific legislation on limiting or defunding DEI initiatives.
Resident Selection Factors—Ninety-six percent (22/23) of PDs stated that their residency program uses a holistic review that takes into account factors such as experiences (eg, volunteer work, research endeavors), personal attributes, and metrics in a balanced manner. No PDs offered United States Medical Licensing Examination Step score cutoffs or medical school clerkship cutoff grades. When asked to rank the importance placed on individual factors in the residency application, the following were ranked from most to least important in the process: performance on clerkships/rotations, performance on interviews, letters of recommendation, clerkship grades, United States Medical Licensing Examination Step scores, research content/ quality, race/ethnicity, history of teaching and mentorship, volunteering, and research amount. When asked to indicate the most pertinent factors used to incorporate DEI in resident selection, the most popular factor was lived experience/life, which was chosen by 90% (18/20) of PDs followed by 75% (15/20) of respondents incorporating underrepresented in medicine (URM) status (including Black, Latinx, and Native American applicants) and 70% (4/20) incorporating socioeconomic status. Sexual orientation and geographic ties of the applicant to the region of the residency program was incorporated by 45% (9/20) of respondents, and other characteristics of race and sex each were incorporated by 30% (6/20) of respondents. Religion was the least incorporated, with 10% (2/20) of PDs selecting this classification. In considering URM status when choosing dermatology residents, 100% (11/11) of respondents indicated that their institution promotes diversity as a part of the recruitment process. Eighty-two percent (9/11) of respondents try to recruit URM applicants to reflect their patient population, 82% (9/11) try as part of a belief that a diverse group benefits everyone in their program, and 45% (5/11) try in order to address societal inequities and as a broader mission to diversify the health care workforce. Seventy-three percent (8/11) indicated that they pay attention to URM status throughout the application process.
Comment
Diversity in the US population is steadily increasing. Within the past decade, the diversity index (the probability that 2 people chosen at random will be from different racial and ethnic groups) has grown from 54.9% in 2010 to 61.1% in 2020.3 There was a 24.9% increase in population groups other than non-Hispanic Whites from 2010 to 2020, an increase in diversity that was present in every region of the United States.4 The field of dermatology already does not reflect the racial distribution of the nation,4 with Black individuals accounting for 13.7% of the nation’s population but only 3% of dermatologists; similarly, Hispanic individuals account for 19.5% of the population but only comprise 4.2% of dermatologists.5,6 There is overwhelming evidence that patients prefer to be diagnosed and treated by physicians who reflect their own demographics.7 Furthermore, physicians who prescribe treatment plans that reflect and respect socioeconomic and religious beliefs of the populations they serve enable patients to meet treatment expectations and experience better outcomes.8 Direct action is required to ensure that the specialty more accurately represents the evolving demographics of the country. This can be accomplished in myriad ways, including but not limited to cultural humility training9 for current dermatologists and trainees and recruitment of a more diverse workforce. These measures can ultimately improve treatment approaches and outcomes for dermatologic conditions across various groups.10
There are efforts by various dermatologic organizations, including the AAD, Society for Pediatric Dermatology, Pediatric Dermatology Research Alliance, Skin of Color Society, Women’s Dermatologic Society, and American Society for Dermatologic Surgery, that are focused on promoting DEI through research, education, and mentorship of potential future dermatologists.11 However, the perceptions, opinions, and selection process instituted by PDs are most consequential in determining the diversity of the specialty, as PDs are at the forefront of establishing the next generation of dermatologists. Through this study, we have found that most PDs recognize the importance of diversity in residency education and recruitment without it being the only deciding factor.
The main limitation of this study was the small sample size, which may not adequately represent all dermatology residency programs accredited by the Accreditation Council for Graduate Medical Education as a result of selection bias toward respondents who were more likely to participate in survey-based research on topics of DEI.
Conclusion
This study revealed that, among dermatology residency PDs, there is interest in modifying the resources and initiatives surrounding DEI in the field. It also revealed that DEI remains a consideration in the resident selection process despite the recent Supreme Court ruling. In conclusion, there is an eagerness among dermatology PDs to incorporate DEI into resident selection even though gaps in knowledge and awareness remain.
The recent Supreme Court ruling that struck down affirmative action1 has caused many initiatives aimed at promoting diversity, equity, and inclusion (DEI) to fall under scrutiny; however, the American Academy of Dermatology (AAD) published a statement of intent in 2022 recognizing and committing to DEI as a priority in the specialty.2 In this study, we used a formal survey to investigate the perceptions of dermatology program directors (PDs) on DEI programming from the AAD and how DEI is integrated into the resident selection process at varying institutions.
Methods
We conducted a cross-sectional study of dermatology PDs across the United States from April 2024 to July 2024. Program directors were contacted via the Association of Professors of Dermatology PD listserve, which includes all 103 PDs who are members of the organization. Personalized survey links were created and sent individually to each PD’s email address. Thirty responses were received. All survey responses were captured anonymously. The survey consisted of 17 questions focusing on dermatology PD demographics and opinions on DEI initiatives in the AAD and in the dermatology resident selection process. Data were collected using Qualtrics survey tools and analyzed using Qualtrics reports.
Results
Demographics—A total of 30 completed surveys were received. Thirty-three percent (10/30) of respondents were from the Midwest, and 23% (7/30) were from the Northeast. The next most represented region was the West, with 20% (6/30) of respondents. The Southeast and Southwest were the least represented regions captured in our survey, accounting for 13% (4/30) and 10% (3/30) of respondents, respectively. After answering this initial demographic question, 1 respondent stopped the survey, bringing our new total to 29 respondents.
Most (66% [19/29]) of the survey respondents had served as PDs for 5 years or less. Sixty-nine percent (20/29) identified as female, while 31% (9/29) identified as male. Seventy-two percent (21/29) identified as White, 17% (5/29) identified as Asian, 3% (1/29) identified as Black/African American, 3% (1/29) identified as Hispanic or Latinx, and 3% (1/29) identified as mixed race.
Opinions on DEI Initiatives—When asked about their satisfaction level with the current amount of DEI efforts within the AAD, 17% (5/29) of respondents said they were very satisfied, 59% (17/29) said they were satisfied, 17% (5/29) said they were neutral, and 7% (2/29) said they were dissatisfied. Given that none of the questions were mandatory to answer before proceeding with the survey, there were variable response rates to each of the remaining questions, which may have caused respondents to answer only questions they felt strongly about.
Twenty respondents answered when prompted to further classify their level of satisfaction: 70% (14/20) said there should be more DEI efforts through the AAD providing financial support, and 50% (10/20) wanted more nonfinancial support. When given the opportunity to specify which DEI initiatives should be enhanced, the majority (67% [14/21]) of PDs chose the AAD’s health disparities curriculum, followed by the Diversity Mentorship Program (52% [11/21]), AAD Diversity Toolkit (43% [9/21]), and the Skin of Color Curriculum (43% [9/21]). Thiry-three percent (7/30) of PDs wanted enhancement of Medicine Without Barriers: Overcoming Unintended Bias in Practice (an AAD educational resource), and 19% (4/21) of respondents did not think any of the AAD’s DEI initiatives needed to be enhanced. There were 14 responses to a question about choosing which DEI initiatives to reduce with singular votes (7% [1/14] each) to reduce Medicine Without Barriers: Overcoming Unintended Bias in Practice and the Skin of Color Curriculum.
Our survey also invited PDs to introduce ideas for new DEI initiatives or programs. The following were suggestions offered by respondents: education for senior members of the AAD on the importance of DEI in dermatology, professional development resources directed toward academic faculty members to prepare them for interacting with and teaching residents from different backgrounds, and more advertisements and support for the AAD’s Diversity Champion Workshop.
DEI in Resident Selection—When asked about the role that DEI plays in how programs develop their match lists for residency, 13% (3/23) of PDs responded that it plays a very large role, 52% (12/23) stated that it plays a large role, 26% (6/23) responded that it plays somewhat of a role, 4% (1/23) stated that it plays a small role, and 4% (1/23) stated that it plays no role. Twenty-four percent (4/17) of respondents were PDs in states that have legislation limiting or defunding DEI initiatives at institutions of higher education. Another 12% (2/17) were from states where such legislation was pending a vote, while 59% (10/17) of respondents indicated that their state had not introduced such legislation. Four percent (1/17) indicated that they were from a state that had introduced legislation to limit or defund DEI initiatives that failed to pass. Only 17 respondents answered this question, which may be due to a lack of awareness among respondents of state-specific legislation on limiting or defunding DEI initiatives.
Resident Selection Factors—Ninety-six percent (22/23) of PDs stated that their residency program uses a holistic review that takes into account factors such as experiences (eg, volunteer work, research endeavors), personal attributes, and metrics in a balanced manner. No PDs offered United States Medical Licensing Examination Step score cutoffs or medical school clerkship cutoff grades. When asked to rank the importance placed on individual factors in the residency application, the following were ranked from most to least important in the process: performance on clerkships/rotations, performance on interviews, letters of recommendation, clerkship grades, United States Medical Licensing Examination Step scores, research content/ quality, race/ethnicity, history of teaching and mentorship, volunteering, and research amount. When asked to indicate the most pertinent factors used to incorporate DEI in resident selection, the most popular factor was lived experience/life, which was chosen by 90% (18/20) of PDs followed by 75% (15/20) of respondents incorporating underrepresented in medicine (URM) status (including Black, Latinx, and Native American applicants) and 70% (4/20) incorporating socioeconomic status. Sexual orientation and geographic ties of the applicant to the region of the residency program was incorporated by 45% (9/20) of respondents, and other characteristics of race and sex each were incorporated by 30% (6/20) of respondents. Religion was the least incorporated, with 10% (2/20) of PDs selecting this classification. In considering URM status when choosing dermatology residents, 100% (11/11) of respondents indicated that their institution promotes diversity as a part of the recruitment process. Eighty-two percent (9/11) of respondents try to recruit URM applicants to reflect their patient population, 82% (9/11) try as part of a belief that a diverse group benefits everyone in their program, and 45% (5/11) try in order to address societal inequities and as a broader mission to diversify the health care workforce. Seventy-three percent (8/11) indicated that they pay attention to URM status throughout the application process.
Comment
Diversity in the US population is steadily increasing. Within the past decade, the diversity index (the probability that 2 people chosen at random will be from different racial and ethnic groups) has grown from 54.9% in 2010 to 61.1% in 2020.3 There was a 24.9% increase in population groups other than non-Hispanic Whites from 2010 to 2020, an increase in diversity that was present in every region of the United States.4 The field of dermatology already does not reflect the racial distribution of the nation,4 with Black individuals accounting for 13.7% of the nation’s population but only 3% of dermatologists; similarly, Hispanic individuals account for 19.5% of the population but only comprise 4.2% of dermatologists.5,6 There is overwhelming evidence that patients prefer to be diagnosed and treated by physicians who reflect their own demographics.7 Furthermore, physicians who prescribe treatment plans that reflect and respect socioeconomic and religious beliefs of the populations they serve enable patients to meet treatment expectations and experience better outcomes.8 Direct action is required to ensure that the specialty more accurately represents the evolving demographics of the country. This can be accomplished in myriad ways, including but not limited to cultural humility training9 for current dermatologists and trainees and recruitment of a more diverse workforce. These measures can ultimately improve treatment approaches and outcomes for dermatologic conditions across various groups.10
There are efforts by various dermatologic organizations, including the AAD, Society for Pediatric Dermatology, Pediatric Dermatology Research Alliance, Skin of Color Society, Women’s Dermatologic Society, and American Society for Dermatologic Surgery, that are focused on promoting DEI through research, education, and mentorship of potential future dermatologists.11 However, the perceptions, opinions, and selection process instituted by PDs are most consequential in determining the diversity of the specialty, as PDs are at the forefront of establishing the next generation of dermatologists. Through this study, we have found that most PDs recognize the importance of diversity in residency education and recruitment without it being the only deciding factor.
The main limitation of this study was the small sample size, which may not adequately represent all dermatology residency programs accredited by the Accreditation Council for Graduate Medical Education as a result of selection bias toward respondents who were more likely to participate in survey-based research on topics of DEI.
Conclusion
This study revealed that, among dermatology residency PDs, there is interest in modifying the resources and initiatives surrounding DEI in the field. It also revealed that DEI remains a consideration in the resident selection process despite the recent Supreme Court ruling. In conclusion, there is an eagerness among dermatology PDs to incorporate DEI into resident selection even though gaps in knowledge and awareness remain.
- Supreme Court of the United States. Students for Fair Admissions, Inc v President and Fellows of Harvard College (No. 20–1199). Argued October 31, 2022. Decided June 29, 2023. https://www.supremecourt.gov/opinions/22pdf/20-1199_hgdj.pdf
- American Academy of Dermatology. AAD’s DEI Statement of Intent. Published March 28, 2022. Accessed November 18, 2024. https://www.aad.org/member/career/diversity/diversity-statement-of-intent
- Jensen E, Jones N, Rabe M, et al. The chance that two people chosen at random are of different race or ethnicity groups has increased since 2010. United States Census Bureau. August 12, 2021. Accessed November 5, 2024. https://www.census.gov/library/stories/2021/08/2020-united-states-population-more-racially-ethnically-diverse-than-2010.html
- Johnson K. New Census reflects growing U.S. population diversity, with children in the forefront. University of New Hampshire Carsey School of Public Policy. October 6, 2021. Accessed November 5, 2024. https://carsey.unh.edu/publication/new-census-reflects-growing-us-population-diversity-children-forefront
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74; 584-587. doi:10.1016/j.jaad.2015.10.044
- United States Census Bureau. QuickFacts: United States. Population estimates, July 1, 2023 (V2023). Accessed November 5, 2024. https://www.census.gov/quickfacts/fact/table/US/PST045222
- Saha S, Beach MC. Impact of physician race on patient decision-making and ratings of physicians: a randomized experiment using video vignettes. J Gen Intern Med. 2020;35:1084-1091. doi:10.1007/s11606-020-05646-z
- Nair L, Adetayo OA. Cultural competence and ethnic diversity in healthcare. Plast Reconstr Surg Glob Open. 2019;7:E2219. doi:10.1097/GOX.0000000000002219
- Yeager KA, Bauer-Wu S. Cultural humility: essential foundation for clinical researchers. Appl Nurs Res. 2013;26:251-256. doi:10.1016/j.apnr.2013.06.008
- Narla S, Heath CR, Alexis A, et al. Racial disparities in dermatology. Arch Dermatol Res. 2023;315:1215-1223. doi:10.1007/s00403-022- 02507-z
- Desai SR, Khanna R, Glass D, et al. Embracing diversity in dermatology: creation of a culture of equity and inclusion in dermatology. Int J Womens Dermatol. 2021;7:378-382. doi:10.1016/j.ijwd.2021.08.002
- Supreme Court of the United States. Students for Fair Admissions, Inc v President and Fellows of Harvard College (No. 20–1199). Argued October 31, 2022. Decided June 29, 2023. https://www.supremecourt.gov/opinions/22pdf/20-1199_hgdj.pdf
- American Academy of Dermatology. AAD’s DEI Statement of Intent. Published March 28, 2022. Accessed November 18, 2024. https://www.aad.org/member/career/diversity/diversity-statement-of-intent
- Jensen E, Jones N, Rabe M, et al. The chance that two people chosen at random are of different race or ethnicity groups has increased since 2010. United States Census Bureau. August 12, 2021. Accessed November 5, 2024. https://www.census.gov/library/stories/2021/08/2020-united-states-population-more-racially-ethnically-diverse-than-2010.html
- Johnson K. New Census reflects growing U.S. population diversity, with children in the forefront. University of New Hampshire Carsey School of Public Policy. October 6, 2021. Accessed November 5, 2024. https://carsey.unh.edu/publication/new-census-reflects-growing-us-population-diversity-children-forefront
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74; 584-587. doi:10.1016/j.jaad.2015.10.044
- United States Census Bureau. QuickFacts: United States. Population estimates, July 1, 2023 (V2023). Accessed November 5, 2024. https://www.census.gov/quickfacts/fact/table/US/PST045222
- Saha S, Beach MC. Impact of physician race on patient decision-making and ratings of physicians: a randomized experiment using video vignettes. J Gen Intern Med. 2020;35:1084-1091. doi:10.1007/s11606-020-05646-z
- Nair L, Adetayo OA. Cultural competence and ethnic diversity in healthcare. Plast Reconstr Surg Glob Open. 2019;7:E2219. doi:10.1097/GOX.0000000000002219
- Yeager KA, Bauer-Wu S. Cultural humility: essential foundation for clinical researchers. Appl Nurs Res. 2013;26:251-256. doi:10.1016/j.apnr.2013.06.008
- Narla S, Heath CR, Alexis A, et al. Racial disparities in dermatology. Arch Dermatol Res. 2023;315:1215-1223. doi:10.1007/s00403-022- 02507-z
- Desai SR, Khanna R, Glass D, et al. Embracing diversity in dermatology: creation of a culture of equity and inclusion in dermatology. Int J Womens Dermatol. 2021;7:378-382. doi:10.1016/j.ijwd.2021.08.002
Program Director Perspectives on DEI Initiatives in the Dermatology Residency Selection Process
Program Director Perspectives on DEI Initiatives in the Dermatology Residency Selection Process
PRACTICE POINTS
- A majority of dermatology program directors (PDs) express support for increased diversity, equity, and inclusion (DEI) funding through the American Academy of Dermatology, including initiatives centered on education and mentorship.
- Dermatology PDs are invested in recruiting underrepresented in medicine applicants to create residency classes that are representative of their patient populations.
Successful Treatment of Severe Dystrophic Nail Psoriasis With Deucravacitinib
Successful Treatment of Severe Dystrophic Nail Psoriasis With Deucravacitinib
To the Editor:
Psoriasis is a chronic inflammatory skin condition that commonly affects the nail matrix and/or nail bed.1 Nail involvement is present in up to 50% of patients with cutaneous psoriasis and 80% of patients with psoriatic arthritis.1 Approximately 5% to 10% of patients with psoriasis demonstrate isolated nail involvement with no skin or joint manifestations.1 Nail psoriasis can cause severe pain and psychological distress, and extreme cases may cause considerable morbidity and functional impairment.2,3 Treatment often requires a long duration and may not result in complete recovery due to the slow rate of nail growth. Patients can progress to permanent nail loss if not treated properly, making early recognition and treatment crucial.1,2 Despite the availability of various treatment options, many cases remain refractory to standard interventions, which underscores the need for novel therapeutic approaches. Herein, we present a severe case of refractory isolated nail psoriasis that was successfully treated with deucravacitinib, an oral tyrosine kinase 2 (TYK2) inhibitor.
A 59-year-old woman presented with a progressive, yellow, hyperkeratotic lesion on the left thumbnail of 2 years’ duration. The patient noted initial discoloration and peeling at the distal end of the nail. Over time, the discoloration progressed to encompass the entire nail. Previous treatments performed by outside physicians including topical corticosteroids, calcineurin inhibitors, and 2 surgeries to remove the nail plate and nail bed all were unsuccessful. The patient also reported severe left thumbnail pain and pruritus that considerably impaired her ability to work. The rest of the nails were unaffected, and she had no personal or family history of psoriasis. Her medical history was notable for hypertension, gastroesophageal reflux disease, and osteomyelitis of the right thumb without nail involvement. Drug allergies included penicillin G benzathine, sulfonamides, amoxicillin, and ciprofloxacin.
Physical examination of the left thumbnail revealed severe yellow, hyperkeratotic, dystrophic changes with a large, yellow, crumbling hyperkeratotic plaque that extended from approximately 1 cm beyond the nail plate to the proximal end of the distal interphalangeal joint, to and along the lateral nail folds, with extensive distal onycholysis. The proximal and lateral nail folds demonstrated erythema as well as maceration that was extremely tender to minimal palpation (Figure 1). No cutaneous lesions were noted elsewhere on the body. The patient had no tenderness, swelling, or stiffness in any of the joints. The differential diagnosis at the time included squamous cell carcinoma of the nail bed and acrodermatitis continua of Hallopeau.
Radiography of the left thumb revealed irregular swelling and nonspecific soft tissue enlargement at the tip of the digit. A nail clipping from the left thumbnail and 3-mm punch biopsies of the lateral and proximal nail folds as well as the horn of the proximal nail fold (Figure 2) were negative for fungus and confirmed psoriasiform dermatitis of the nail.
The patient was started on vinegar soaks (1:1 ratio of vinegar to water) every other day as well as urea cream 10%, ammonium lactate 15%, and petrolatum twice daily for 2 months without considerable improvement. Due to lack of improvement during this 2-month period, the patient subsequently was started on oral deucravacitinib 6 mg/d along with continued use of petrolatum twice daily and vinegar soaks every other day. We selected a trial of deucravacitinib for our patient because of its convenient daily oral dosing and promising clinical evidence.4,5 After 2 months of treatment with deucravacitinib, the patient reported substantial improvement and satisfaction with the treatment results. Physical examination of the left thumbnail after 2 months of deucravacitinib treatment revealed mildly hyperkeratotic, yellow, dystrophic changes of the nail with notable improvement of the yellow hyperkeratotic plaque on the distal thumbnail. Normal-appearing nail growth was noted at the proximal nail fold, demonstrating considerable improvement from the initial presentation (Figure 3). However, the patient had developed multiple oral ulcers, generalized pruritus, and an annular urticarial plaque on the left arm. As such, deucravacitinib was discontinued after 2 months of treatment. These symptoms resolved within a week of discontinuing deucravacitinib.
While the etiology of nail psoriasis remains unclear, it is believed to be due to a combination of immunologic, genetic, and environmental factors.3 Classical clinical features include nail pitting, leukonychia, onycholysis, nail bed hyperkeratosis, and splinter hemorrhages.1,3 Our patient exhibited a severe form of nail psoriasis, encompassing the entire nail matrix and bed and extending to the distal interphalangeal joint and lateral nail folds. Previous surgical interventions may have triggered the Koebner phenomenon—which commonly is associated with psoriasis—and resulted in new skin lesions as a secondary response to the surgical trauma.6 The severity of the condition profoundly impacted her quality of life and considerably hindered her ability to work.
Treatment for nail psoriasis includes topical or systemic therapies such as corticosteroids, vitamin D analogs, tacrolimus, and tumor necrosis factor α inhibitors.1,3 Topical treatment is challenging because it is difficult to deliver medication effectively to the nail bed and nail matrix, and patient adherence may be poor.2 Although it has been shown to be effective, intralesional triamcinolone can be associated with pain as the most common adverse effect.7 Systemic medications such as oral methotrexate also may be effective but are contraindicated in pregnant patients and are associated with potential adverse events (AEs), including hepatotoxicity and acute kidney injury.8 The use of biologics may be challenging due to potential AEs and patient reluctance toward injection-based treatments.9
Deucravacitinib is a TYK2 inhibitor approved for treatment of plaque psoriasis.10 Tyrosine kinase 2 is an intracellular kinase that mediates the signaling of IL-23 and other cytokines involved in psoriasis pathogenesis.10 Deucravacitinib selectively binds to the regulatory domain of TYK2, leading to targeted allosteric inhibition of TYK2-mediated IL-23 and type I interferon signaling.4,5,10 Compared with biologics, deucravacitinib is advantageous because it can be administered as a daily oral pill, encouraging high patient compliance.
In the POETYK PSO-1 and PSO-2 phase 3 randomized controlled trials, 20.9% (n=332) and 20.3% (n=510) of deucravacitinib-treated patients with moderate to severe nail involvement achieved a Physician’s Global Assessment of Fingernail score of 0/1 compared with 8.8% (n=165) and 7.9% (n=254) of patients in the placebo group, respectively. All patients in these trials had a diagnosis of plaque psoriasis with at least 10% body surface area involvement; none of the patients had isolated nail psoriasis.4,5
The phase 3 POETYK PSO-1 and PSO-2 trials demonstrated deucravacitinib to be safe and well tolerated with minimal AEs.4,5 However, the development of AEs in our patient, including oral ulcers and generalized pruritus, underscores the need for close monitoring and consideration of potential risks of treatment. Common AEs associated with deucravacitinib include upper respiratory infections (19.2% [n=840]), increased blood creatine phosphokinase levels (2.7% [n=840]), herpes simplex virus (2.0% [n=840]), and mouth ulcers (1.9% [n=840]).11
Patient education also is a crucial component in the treatment of nail psoriasis. Physicians should emphasize the slow growth of nails and need for prolonged treatment. Clear communication and realistic expectations are essential for ensuring patient adherence to treatment.
Our case highlights the potential efficacy and safety of deucravacitinib for treatment of nail psoriasis, potentially laying the groundwork for future clinical studies. Our patient had a severe case of nail psoriasis that involved the entire nail bed and nail plate, resulting in extreme pain, pruritus, and functional impairment. Her case was unique because involvement was isolated to the nail without any accompanying skin or joint manifestations. She showed a favorable response to deucravacitinib within only 2 months of treatment and exhibited considerable improvement of nail psoriasis, with a reported high level of satisfaction with the treatment. We plan to continue to monitor the patient for long-term results. Future randomized clinical trials with longer follow-up periods are crucial to further establish the efficacy and safety of deucravacitinib for treatment of nail psoriasis.
- Hwang JK, Grover C, Iorizzo M, et al. Nail psoriasis and nail lichen planus: updates on diagnosis and management. J Am Acad Dermatol. 2024;90:585-596. doi:10.1016/j.jaad.2023.11.024
- Ji C, Wang H, Bao C, et al. Challenge of nail psoriasis: an update review. Clin Rev Allergy Immunol. 2021;61:377-402. doi:10.1007/s12016-021-08896-9
- Muneer H, Sathe NC, Masood S. Nail psoriasis. StatPearls [Internet]. StatPearls Publishing; 2024 Jan-. Updated March 1, 2024. Accessed October 24, 2024. https://www.ncbi.nlm.nih.gov/books/NBK559260/
- Armstrong AW, Gooderham M, Warren RB, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO-1 trial. J Am Acad Dermatol. 2023;88:29-39. doi:10.1016/j.jaad.2022.07.002
- Strober B, Thaçi D, Sofen H, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 Program fOr Evaluation of TYK2 inhibitor psoriasis second trial. J Am Acad Dermatol. 2023;88:40-51. doi:10.1016/j.jaad.2022.08.061
- Sanchez DP, Sonthalia S. Koebner phenomenon. StatPearls [Internet]. StatPearls Publishing; 2024 Jan-. Updated November 14, 2022. Accessed April 11, 2024. https://www.ncbi.nlm.nih.gov/books/NBK553108/
- Grover C, Kharghoria G, Bansal S. Triamcinolone acetonide injections in nail psoriasis: a pragmatic analysis. Skin Appendage Disord. 2024;10:50-59. doi:10.1159/000534699
- Hanoodi M, Mittal M. Methotrexate. StatPearls [Internet]. StatPearls Publishing; 2024 Jan-. Updated August 16, 2023. Accessed April 11, 2024. https://www.ncbi.nlm.nih.gov/books/NBK556114/
- Singh JA, Wells GA, Christensen R, et al. Adverse effects of biologics: a network meta-analysis and Cochrane overview. Cochrane Database Syst Rev. 2011;2011:Cd008794. doi:10.1002/14651858.CD008794.pub2
- Thaçi D, Strober B, Gordon KB, et al. Deucravacitinib in moderate to severe psoriasis: clinical and quality-of-life outcomes in a phase 2 trial. Dermatol Ther (Heidelb). 2022;12:495-510. doi:10.1007/s13555-021-00649-y
- Week 0-16: demonstrated safety profile. Bristol-Myers Squibb. 2024. Accessed October 24, 2024. https://www.sotyktuhcp.com/safety-profile?cid=sem_2465603&gclid=CjwKCAiA9ourBhAVEiwA3L5RFnyYqmxbqkz1_zBNPz3dcyHKCSFf1XQ-7acznV0XbR5DDJHYkZcKJxoCWN0QAvD_BwE&gclsrc=aw.ds
To the Editor:
Psoriasis is a chronic inflammatory skin condition that commonly affects the nail matrix and/or nail bed.1 Nail involvement is present in up to 50% of patients with cutaneous psoriasis and 80% of patients with psoriatic arthritis.1 Approximately 5% to 10% of patients with psoriasis demonstrate isolated nail involvement with no skin or joint manifestations.1 Nail psoriasis can cause severe pain and psychological distress, and extreme cases may cause considerable morbidity and functional impairment.2,3 Treatment often requires a long duration and may not result in complete recovery due to the slow rate of nail growth. Patients can progress to permanent nail loss if not treated properly, making early recognition and treatment crucial.1,2 Despite the availability of various treatment options, many cases remain refractory to standard interventions, which underscores the need for novel therapeutic approaches. Herein, we present a severe case of refractory isolated nail psoriasis that was successfully treated with deucravacitinib, an oral tyrosine kinase 2 (TYK2) inhibitor.
A 59-year-old woman presented with a progressive, yellow, hyperkeratotic lesion on the left thumbnail of 2 years’ duration. The patient noted initial discoloration and peeling at the distal end of the nail. Over time, the discoloration progressed to encompass the entire nail. Previous treatments performed by outside physicians including topical corticosteroids, calcineurin inhibitors, and 2 surgeries to remove the nail plate and nail bed all were unsuccessful. The patient also reported severe left thumbnail pain and pruritus that considerably impaired her ability to work. The rest of the nails were unaffected, and she had no personal or family history of psoriasis. Her medical history was notable for hypertension, gastroesophageal reflux disease, and osteomyelitis of the right thumb without nail involvement. Drug allergies included penicillin G benzathine, sulfonamides, amoxicillin, and ciprofloxacin.
Physical examination of the left thumbnail revealed severe yellow, hyperkeratotic, dystrophic changes with a large, yellow, crumbling hyperkeratotic plaque that extended from approximately 1 cm beyond the nail plate to the proximal end of the distal interphalangeal joint, to and along the lateral nail folds, with extensive distal onycholysis. The proximal and lateral nail folds demonstrated erythema as well as maceration that was extremely tender to minimal palpation (Figure 1). No cutaneous lesions were noted elsewhere on the body. The patient had no tenderness, swelling, or stiffness in any of the joints. The differential diagnosis at the time included squamous cell carcinoma of the nail bed and acrodermatitis continua of Hallopeau.
Radiography of the left thumb revealed irregular swelling and nonspecific soft tissue enlargement at the tip of the digit. A nail clipping from the left thumbnail and 3-mm punch biopsies of the lateral and proximal nail folds as well as the horn of the proximal nail fold (Figure 2) were negative for fungus and confirmed psoriasiform dermatitis of the nail.
The patient was started on vinegar soaks (1:1 ratio of vinegar to water) every other day as well as urea cream 10%, ammonium lactate 15%, and petrolatum twice daily for 2 months without considerable improvement. Due to lack of improvement during this 2-month period, the patient subsequently was started on oral deucravacitinib 6 mg/d along with continued use of petrolatum twice daily and vinegar soaks every other day. We selected a trial of deucravacitinib for our patient because of its convenient daily oral dosing and promising clinical evidence.4,5 After 2 months of treatment with deucravacitinib, the patient reported substantial improvement and satisfaction with the treatment results. Physical examination of the left thumbnail after 2 months of deucravacitinib treatment revealed mildly hyperkeratotic, yellow, dystrophic changes of the nail with notable improvement of the yellow hyperkeratotic plaque on the distal thumbnail. Normal-appearing nail growth was noted at the proximal nail fold, demonstrating considerable improvement from the initial presentation (Figure 3). However, the patient had developed multiple oral ulcers, generalized pruritus, and an annular urticarial plaque on the left arm. As such, deucravacitinib was discontinued after 2 months of treatment. These symptoms resolved within a week of discontinuing deucravacitinib.
While the etiology of nail psoriasis remains unclear, it is believed to be due to a combination of immunologic, genetic, and environmental factors.3 Classical clinical features include nail pitting, leukonychia, onycholysis, nail bed hyperkeratosis, and splinter hemorrhages.1,3 Our patient exhibited a severe form of nail psoriasis, encompassing the entire nail matrix and bed and extending to the distal interphalangeal joint and lateral nail folds. Previous surgical interventions may have triggered the Koebner phenomenon—which commonly is associated with psoriasis—and resulted in new skin lesions as a secondary response to the surgical trauma.6 The severity of the condition profoundly impacted her quality of life and considerably hindered her ability to work.
Treatment for nail psoriasis includes topical or systemic therapies such as corticosteroids, vitamin D analogs, tacrolimus, and tumor necrosis factor α inhibitors.1,3 Topical treatment is challenging because it is difficult to deliver medication effectively to the nail bed and nail matrix, and patient adherence may be poor.2 Although it has been shown to be effective, intralesional triamcinolone can be associated with pain as the most common adverse effect.7 Systemic medications such as oral methotrexate also may be effective but are contraindicated in pregnant patients and are associated with potential adverse events (AEs), including hepatotoxicity and acute kidney injury.8 The use of biologics may be challenging due to potential AEs and patient reluctance toward injection-based treatments.9
Deucravacitinib is a TYK2 inhibitor approved for treatment of plaque psoriasis.10 Tyrosine kinase 2 is an intracellular kinase that mediates the signaling of IL-23 and other cytokines involved in psoriasis pathogenesis.10 Deucravacitinib selectively binds to the regulatory domain of TYK2, leading to targeted allosteric inhibition of TYK2-mediated IL-23 and type I interferon signaling.4,5,10 Compared with biologics, deucravacitinib is advantageous because it can be administered as a daily oral pill, encouraging high patient compliance.
In the POETYK PSO-1 and PSO-2 phase 3 randomized controlled trials, 20.9% (n=332) and 20.3% (n=510) of deucravacitinib-treated patients with moderate to severe nail involvement achieved a Physician’s Global Assessment of Fingernail score of 0/1 compared with 8.8% (n=165) and 7.9% (n=254) of patients in the placebo group, respectively. All patients in these trials had a diagnosis of plaque psoriasis with at least 10% body surface area involvement; none of the patients had isolated nail psoriasis.4,5
The phase 3 POETYK PSO-1 and PSO-2 trials demonstrated deucravacitinib to be safe and well tolerated with minimal AEs.4,5 However, the development of AEs in our patient, including oral ulcers and generalized pruritus, underscores the need for close monitoring and consideration of potential risks of treatment. Common AEs associated with deucravacitinib include upper respiratory infections (19.2% [n=840]), increased blood creatine phosphokinase levels (2.7% [n=840]), herpes simplex virus (2.0% [n=840]), and mouth ulcers (1.9% [n=840]).11
Patient education also is a crucial component in the treatment of nail psoriasis. Physicians should emphasize the slow growth of nails and need for prolonged treatment. Clear communication and realistic expectations are essential for ensuring patient adherence to treatment.
Our case highlights the potential efficacy and safety of deucravacitinib for treatment of nail psoriasis, potentially laying the groundwork for future clinical studies. Our patient had a severe case of nail psoriasis that involved the entire nail bed and nail plate, resulting in extreme pain, pruritus, and functional impairment. Her case was unique because involvement was isolated to the nail without any accompanying skin or joint manifestations. She showed a favorable response to deucravacitinib within only 2 months of treatment and exhibited considerable improvement of nail psoriasis, with a reported high level of satisfaction with the treatment. We plan to continue to monitor the patient for long-term results. Future randomized clinical trials with longer follow-up periods are crucial to further establish the efficacy and safety of deucravacitinib for treatment of nail psoriasis.
To the Editor:
Psoriasis is a chronic inflammatory skin condition that commonly affects the nail matrix and/or nail bed.1 Nail involvement is present in up to 50% of patients with cutaneous psoriasis and 80% of patients with psoriatic arthritis.1 Approximately 5% to 10% of patients with psoriasis demonstrate isolated nail involvement with no skin or joint manifestations.1 Nail psoriasis can cause severe pain and psychological distress, and extreme cases may cause considerable morbidity and functional impairment.2,3 Treatment often requires a long duration and may not result in complete recovery due to the slow rate of nail growth. Patients can progress to permanent nail loss if not treated properly, making early recognition and treatment crucial.1,2 Despite the availability of various treatment options, many cases remain refractory to standard interventions, which underscores the need for novel therapeutic approaches. Herein, we present a severe case of refractory isolated nail psoriasis that was successfully treated with deucravacitinib, an oral tyrosine kinase 2 (TYK2) inhibitor.
A 59-year-old woman presented with a progressive, yellow, hyperkeratotic lesion on the left thumbnail of 2 years’ duration. The patient noted initial discoloration and peeling at the distal end of the nail. Over time, the discoloration progressed to encompass the entire nail. Previous treatments performed by outside physicians including topical corticosteroids, calcineurin inhibitors, and 2 surgeries to remove the nail plate and nail bed all were unsuccessful. The patient also reported severe left thumbnail pain and pruritus that considerably impaired her ability to work. The rest of the nails were unaffected, and she had no personal or family history of psoriasis. Her medical history was notable for hypertension, gastroesophageal reflux disease, and osteomyelitis of the right thumb without nail involvement. Drug allergies included penicillin G benzathine, sulfonamides, amoxicillin, and ciprofloxacin.
Physical examination of the left thumbnail revealed severe yellow, hyperkeratotic, dystrophic changes with a large, yellow, crumbling hyperkeratotic plaque that extended from approximately 1 cm beyond the nail plate to the proximal end of the distal interphalangeal joint, to and along the lateral nail folds, with extensive distal onycholysis. The proximal and lateral nail folds demonstrated erythema as well as maceration that was extremely tender to minimal palpation (Figure 1). No cutaneous lesions were noted elsewhere on the body. The patient had no tenderness, swelling, or stiffness in any of the joints. The differential diagnosis at the time included squamous cell carcinoma of the nail bed and acrodermatitis continua of Hallopeau.
Radiography of the left thumb revealed irregular swelling and nonspecific soft tissue enlargement at the tip of the digit. A nail clipping from the left thumbnail and 3-mm punch biopsies of the lateral and proximal nail folds as well as the horn of the proximal nail fold (Figure 2) were negative for fungus and confirmed psoriasiform dermatitis of the nail.
The patient was started on vinegar soaks (1:1 ratio of vinegar to water) every other day as well as urea cream 10%, ammonium lactate 15%, and petrolatum twice daily for 2 months without considerable improvement. Due to lack of improvement during this 2-month period, the patient subsequently was started on oral deucravacitinib 6 mg/d along with continued use of petrolatum twice daily and vinegar soaks every other day. We selected a trial of deucravacitinib for our patient because of its convenient daily oral dosing and promising clinical evidence.4,5 After 2 months of treatment with deucravacitinib, the patient reported substantial improvement and satisfaction with the treatment results. Physical examination of the left thumbnail after 2 months of deucravacitinib treatment revealed mildly hyperkeratotic, yellow, dystrophic changes of the nail with notable improvement of the yellow hyperkeratotic plaque on the distal thumbnail. Normal-appearing nail growth was noted at the proximal nail fold, demonstrating considerable improvement from the initial presentation (Figure 3). However, the patient had developed multiple oral ulcers, generalized pruritus, and an annular urticarial plaque on the left arm. As such, deucravacitinib was discontinued after 2 months of treatment. These symptoms resolved within a week of discontinuing deucravacitinib.
While the etiology of nail psoriasis remains unclear, it is believed to be due to a combination of immunologic, genetic, and environmental factors.3 Classical clinical features include nail pitting, leukonychia, onycholysis, nail bed hyperkeratosis, and splinter hemorrhages.1,3 Our patient exhibited a severe form of nail psoriasis, encompassing the entire nail matrix and bed and extending to the distal interphalangeal joint and lateral nail folds. Previous surgical interventions may have triggered the Koebner phenomenon—which commonly is associated with psoriasis—and resulted in new skin lesions as a secondary response to the surgical trauma.6 The severity of the condition profoundly impacted her quality of life and considerably hindered her ability to work.
Treatment for nail psoriasis includes topical or systemic therapies such as corticosteroids, vitamin D analogs, tacrolimus, and tumor necrosis factor α inhibitors.1,3 Topical treatment is challenging because it is difficult to deliver medication effectively to the nail bed and nail matrix, and patient adherence may be poor.2 Although it has been shown to be effective, intralesional triamcinolone can be associated with pain as the most common adverse effect.7 Systemic medications such as oral methotrexate also may be effective but are contraindicated in pregnant patients and are associated with potential adverse events (AEs), including hepatotoxicity and acute kidney injury.8 The use of biologics may be challenging due to potential AEs and patient reluctance toward injection-based treatments.9
Deucravacitinib is a TYK2 inhibitor approved for treatment of plaque psoriasis.10 Tyrosine kinase 2 is an intracellular kinase that mediates the signaling of IL-23 and other cytokines involved in psoriasis pathogenesis.10 Deucravacitinib selectively binds to the regulatory domain of TYK2, leading to targeted allosteric inhibition of TYK2-mediated IL-23 and type I interferon signaling.4,5,10 Compared with biologics, deucravacitinib is advantageous because it can be administered as a daily oral pill, encouraging high patient compliance.
In the POETYK PSO-1 and PSO-2 phase 3 randomized controlled trials, 20.9% (n=332) and 20.3% (n=510) of deucravacitinib-treated patients with moderate to severe nail involvement achieved a Physician’s Global Assessment of Fingernail score of 0/1 compared with 8.8% (n=165) and 7.9% (n=254) of patients in the placebo group, respectively. All patients in these trials had a diagnosis of plaque psoriasis with at least 10% body surface area involvement; none of the patients had isolated nail psoriasis.4,5
The phase 3 POETYK PSO-1 and PSO-2 trials demonstrated deucravacitinib to be safe and well tolerated with minimal AEs.4,5 However, the development of AEs in our patient, including oral ulcers and generalized pruritus, underscores the need for close monitoring and consideration of potential risks of treatment. Common AEs associated with deucravacitinib include upper respiratory infections (19.2% [n=840]), increased blood creatine phosphokinase levels (2.7% [n=840]), herpes simplex virus (2.0% [n=840]), and mouth ulcers (1.9% [n=840]).11
Patient education also is a crucial component in the treatment of nail psoriasis. Physicians should emphasize the slow growth of nails and need for prolonged treatment. Clear communication and realistic expectations are essential for ensuring patient adherence to treatment.
Our case highlights the potential efficacy and safety of deucravacitinib for treatment of nail psoriasis, potentially laying the groundwork for future clinical studies. Our patient had a severe case of nail psoriasis that involved the entire nail bed and nail plate, resulting in extreme pain, pruritus, and functional impairment. Her case was unique because involvement was isolated to the nail without any accompanying skin or joint manifestations. She showed a favorable response to deucravacitinib within only 2 months of treatment and exhibited considerable improvement of nail psoriasis, with a reported high level of satisfaction with the treatment. We plan to continue to monitor the patient for long-term results. Future randomized clinical trials with longer follow-up periods are crucial to further establish the efficacy and safety of deucravacitinib for treatment of nail psoriasis.
- Hwang JK, Grover C, Iorizzo M, et al. Nail psoriasis and nail lichen planus: updates on diagnosis and management. J Am Acad Dermatol. 2024;90:585-596. doi:10.1016/j.jaad.2023.11.024
- Ji C, Wang H, Bao C, et al. Challenge of nail psoriasis: an update review. Clin Rev Allergy Immunol. 2021;61:377-402. doi:10.1007/s12016-021-08896-9
- Muneer H, Sathe NC, Masood S. Nail psoriasis. StatPearls [Internet]. StatPearls Publishing; 2024 Jan-. Updated March 1, 2024. Accessed October 24, 2024. https://www.ncbi.nlm.nih.gov/books/NBK559260/
- Armstrong AW, Gooderham M, Warren RB, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO-1 trial. J Am Acad Dermatol. 2023;88:29-39. doi:10.1016/j.jaad.2022.07.002
- Strober B, Thaçi D, Sofen H, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 Program fOr Evaluation of TYK2 inhibitor psoriasis second trial. J Am Acad Dermatol. 2023;88:40-51. doi:10.1016/j.jaad.2022.08.061
- Sanchez DP, Sonthalia S. Koebner phenomenon. StatPearls [Internet]. StatPearls Publishing; 2024 Jan-. Updated November 14, 2022. Accessed April 11, 2024. https://www.ncbi.nlm.nih.gov/books/NBK553108/
- Grover C, Kharghoria G, Bansal S. Triamcinolone acetonide injections in nail psoriasis: a pragmatic analysis. Skin Appendage Disord. 2024;10:50-59. doi:10.1159/000534699
- Hanoodi M, Mittal M. Methotrexate. StatPearls [Internet]. StatPearls Publishing; 2024 Jan-. Updated August 16, 2023. Accessed April 11, 2024. https://www.ncbi.nlm.nih.gov/books/NBK556114/
- Singh JA, Wells GA, Christensen R, et al. Adverse effects of biologics: a network meta-analysis and Cochrane overview. Cochrane Database Syst Rev. 2011;2011:Cd008794. doi:10.1002/14651858.CD008794.pub2
- Thaçi D, Strober B, Gordon KB, et al. Deucravacitinib in moderate to severe psoriasis: clinical and quality-of-life outcomes in a phase 2 trial. Dermatol Ther (Heidelb). 2022;12:495-510. doi:10.1007/s13555-021-00649-y
- Week 0-16: demonstrated safety profile. Bristol-Myers Squibb. 2024. Accessed October 24, 2024. https://www.sotyktuhcp.com/safety-profile?cid=sem_2465603&gclid=CjwKCAiA9ourBhAVEiwA3L5RFnyYqmxbqkz1_zBNPz3dcyHKCSFf1XQ-7acznV0XbR5DDJHYkZcKJxoCWN0QAvD_BwE&gclsrc=aw.ds
- Hwang JK, Grover C, Iorizzo M, et al. Nail psoriasis and nail lichen planus: updates on diagnosis and management. J Am Acad Dermatol. 2024;90:585-596. doi:10.1016/j.jaad.2023.11.024
- Ji C, Wang H, Bao C, et al. Challenge of nail psoriasis: an update review. Clin Rev Allergy Immunol. 2021;61:377-402. doi:10.1007/s12016-021-08896-9
- Muneer H, Sathe NC, Masood S. Nail psoriasis. StatPearls [Internet]. StatPearls Publishing; 2024 Jan-. Updated March 1, 2024. Accessed October 24, 2024. https://www.ncbi.nlm.nih.gov/books/NBK559260/
- Armstrong AW, Gooderham M, Warren RB, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO-1 trial. J Am Acad Dermatol. 2023;88:29-39. doi:10.1016/j.jaad.2022.07.002
- Strober B, Thaçi D, Sofen H, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 Program fOr Evaluation of TYK2 inhibitor psoriasis second trial. J Am Acad Dermatol. 2023;88:40-51. doi:10.1016/j.jaad.2022.08.061
- Sanchez DP, Sonthalia S. Koebner phenomenon. StatPearls [Internet]. StatPearls Publishing; 2024 Jan-. Updated November 14, 2022. Accessed April 11, 2024. https://www.ncbi.nlm.nih.gov/books/NBK553108/
- Grover C, Kharghoria G, Bansal S. Triamcinolone acetonide injections in nail psoriasis: a pragmatic analysis. Skin Appendage Disord. 2024;10:50-59. doi:10.1159/000534699
- Hanoodi M, Mittal M. Methotrexate. StatPearls [Internet]. StatPearls Publishing; 2024 Jan-. Updated August 16, 2023. Accessed April 11, 2024. https://www.ncbi.nlm.nih.gov/books/NBK556114/
- Singh JA, Wells GA, Christensen R, et al. Adverse effects of biologics: a network meta-analysis and Cochrane overview. Cochrane Database Syst Rev. 2011;2011:Cd008794. doi:10.1002/14651858.CD008794.pub2
- Thaçi D, Strober B, Gordon KB, et al. Deucravacitinib in moderate to severe psoriasis: clinical and quality-of-life outcomes in a phase 2 trial. Dermatol Ther (Heidelb). 2022;12:495-510. doi:10.1007/s13555-021-00649-y
- Week 0-16: demonstrated safety profile. Bristol-Myers Squibb. 2024. Accessed October 24, 2024. https://www.sotyktuhcp.com/safety-profile?cid=sem_2465603&gclid=CjwKCAiA9ourBhAVEiwA3L5RFnyYqmxbqkz1_zBNPz3dcyHKCSFf1XQ-7acznV0XbR5DDJHYkZcKJxoCWN0QAvD_BwE&gclsrc=aw.ds
Successful Treatment of Severe Dystrophic Nail Psoriasis With Deucravacitinib
Successful Treatment of Severe Dystrophic Nail Psoriasis With Deucravacitinib
PRACTICE POINTS
- Nail psoriasis can masquerade as other dermatologic conditions, including squamous cell carcinoma of the nail bed and acrodermatitis continua of Hallopeau.
- Nail psoriasis can progress to permanent nail loss if not treated properly, making early recognition and treatment crucial.
- Deucravacitinib, an oral tyrosine kinase 2 inhibitor approved for the treatment of plaque psoriasis, has shown promise as an effective treatment for nail psoriasis in cases that are refractory to standard therapies.