User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Basal Cell Carcinoma Masquerading as a Dermoid Cyst and Bursitis of the Knee
Basal cell carcinoma (BCC) is the most frequently diagnosed skin cancer in the United States. It develops most often on sun-exposed skin, including the face and neck. Although BCCs are slow-growing tumors that rarely metastasize, they can cause notable local destruction with disfigurement if neglected or inadequately treated. Basal cell carcinoma arising on the legs is relatively uncommon.1,2 We present an interesting case of delayed diagnosis of BCC on the left knee due to earlier misdiagnoses of a dermoid cyst and bursitis.
Case Report
A 67-year-old man with no history of skin cancer presented with a painful growing tumor on the left knee of approximately 2 years’ duration. The patient’s primary care physician as well as a general surgeon initially diagnosed it as a dermoid cyst and bursitis. The nodule failed to respond to conservative therapy with nonsteroidal anti-inflammatory drugs and continued to grow until it began to ulcerate. Concerned about the possibility of septic arthritis, the patient’s primary care physician referred him to the emergency department. He was subsequently sent to the dermatology clinic.
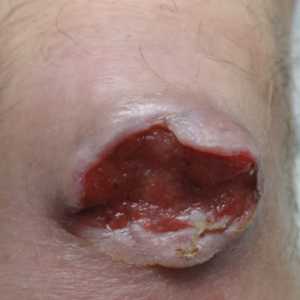
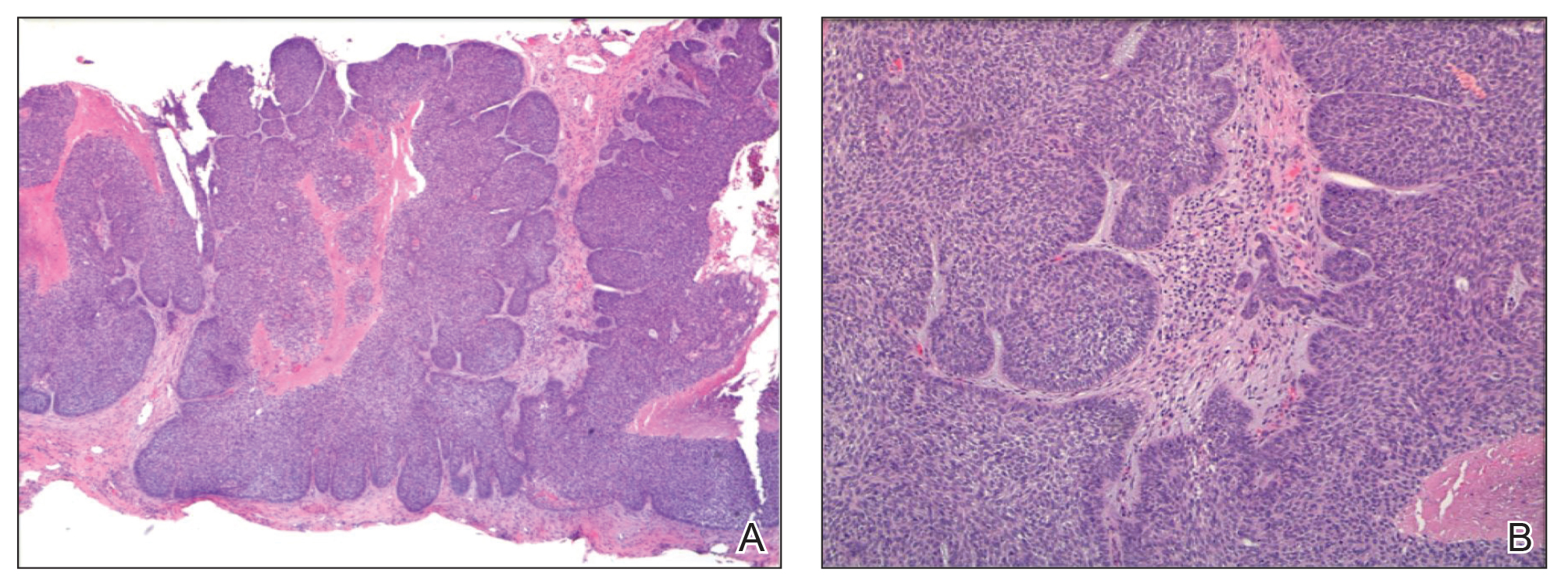
On examination by dermatology, a 6.3×4.4-cm, tender, mobile, ulcerated nodule was noted on the left knee (Figure 1A). No popliteal or inguinal lymph nodes were palpable. Basal cell carcinoma, squamous cell carcinoma, or atypical infection (eg, Leishmania, deep fungal, mycobacterial) was suspected clinically. The patient underwent a diagnostic skin biopsy; hematoxylin and eosin–stained sections revealed lobular proliferation of basaloid cells with peripheral palisading and central tumoral necrosis, consistent with primary BCC (Figure 2).
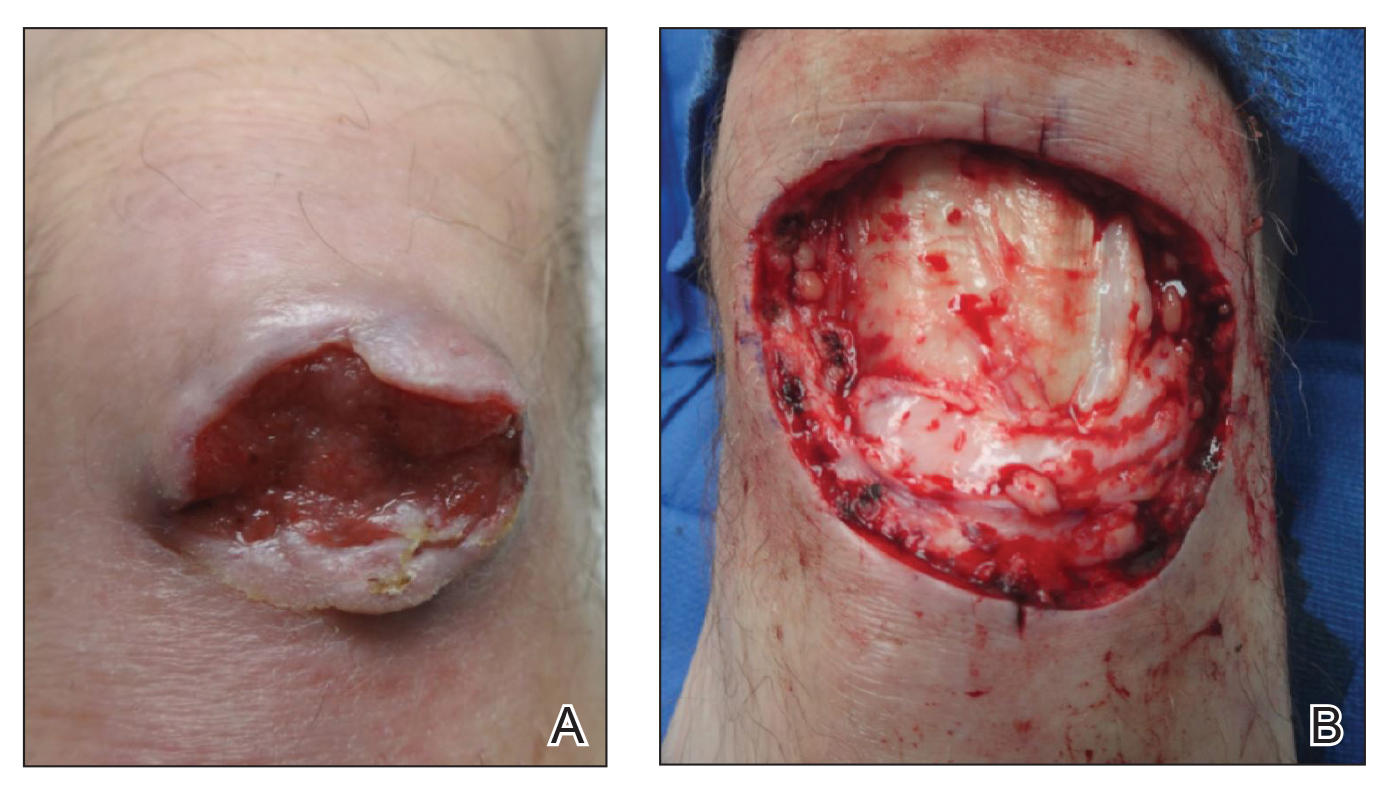
Given the size of the tumor, the patient was referred for Mohs micrographic surgery and eventual reconstruction by a plastic surgeon. The tumor was cleared after 2 stages of Mohs surgery, with a final wound size of 7.7×5.4 cm (Figure 1B). Plastic surgery later performed a gastrocnemius muscle flap with a split-thickness skin graft (175 cm2) to repair the wound.
Comment
Exposure to UV radiation is the primary causative agent of most BCCs, accounting for the preferential distribution of these tumors on sun-exposed areas of the body. Approximately 80% of BCCs are located on the head and neck, 10% occur on the trunk, and only 8% are found on the lower extremities.1
Giant BCC, the finding in this case, is defined by the American Joint Committee on Cancer as a tumor larger than 5 cm in diameter. Fewer than 1% of all BCCs achieve this size; they appear more commonly on the back where they can go unnoticed.2 Neglect and inadequate treatment of the primary tumor are the most important contributing factors to the size of giant BCCs. Giant BCCs also have more aggressive biologic behavior, with an increased risk for local invasion and metastasis.3 In this case, the lesion was larger than 5 cm in diameter and occurred on the lower extremity rather than on the trunk.
This case is unusual because delayed diagnosis of BCC was the result of misdiagnoses of a dermoid cyst and bursitis, with a diagnostic skin biopsy demonstrating BCC almost 2 years later. It should be emphasized that early diagnosis and treatment could prevent tumor expansion. Physicians should have a high degree of suspicion for BCC, especially when a dermoid cyst and knee bursitis fail to respond to conservative management.
- Pearson G, King LE, Boyd AS. Basal cell carcinoma of the lower extremities. Int J Dermatol. 1999;38:852-854.
- Arnaiz J, Gallardo E, Piedra T, et al. Giant basal cell carcinoma on the lower leg: MRI findings. J Plast Reconstr Aesthet Surg. 2007;60:1167-1168.
- Randle HW. Giant basal cell carcinoma [letter]. Int J Dermatol. 1996;35:222-223.
Basal cell carcinoma (BCC) is the most frequently diagnosed skin cancer in the United States. It develops most often on sun-exposed skin, including the face and neck. Although BCCs are slow-growing tumors that rarely metastasize, they can cause notable local destruction with disfigurement if neglected or inadequately treated. Basal cell carcinoma arising on the legs is relatively uncommon.1,2 We present an interesting case of delayed diagnosis of BCC on the left knee due to earlier misdiagnoses of a dermoid cyst and bursitis.
Case Report
A 67-year-old man with no history of skin cancer presented with a painful growing tumor on the left knee of approximately 2 years’ duration. The patient’s primary care physician as well as a general surgeon initially diagnosed it as a dermoid cyst and bursitis. The nodule failed to respond to conservative therapy with nonsteroidal anti-inflammatory drugs and continued to grow until it began to ulcerate. Concerned about the possibility of septic arthritis, the patient’s primary care physician referred him to the emergency department. He was subsequently sent to the dermatology clinic.
On examination by dermatology, a 6.3×4.4-cm, tender, mobile, ulcerated nodule was noted on the left knee (Figure 1A). No popliteal or inguinal lymph nodes were palpable. Basal cell carcinoma, squamous cell carcinoma, or atypical infection (eg, Leishmania, deep fungal, mycobacterial) was suspected clinically. The patient underwent a diagnostic skin biopsy; hematoxylin and eosin–stained sections revealed lobular proliferation of basaloid cells with peripheral palisading and central tumoral necrosis, consistent with primary BCC (Figure 2).


Given the size of the tumor, the patient was referred for Mohs micrographic surgery and eventual reconstruction by a plastic surgeon. The tumor was cleared after 2 stages of Mohs surgery, with a final wound size of 7.7×5.4 cm (Figure 1B). Plastic surgery later performed a gastrocnemius muscle flap with a split-thickness skin graft (175 cm2) to repair the wound.
Comment
Exposure to UV radiation is the primary causative agent of most BCCs, accounting for the preferential distribution of these tumors on sun-exposed areas of the body. Approximately 80% of BCCs are located on the head and neck, 10% occur on the trunk, and only 8% are found on the lower extremities.1
Giant BCC, the finding in this case, is defined by the American Joint Committee on Cancer as a tumor larger than 5 cm in diameter. Fewer than 1% of all BCCs achieve this size; they appear more commonly on the back where they can go unnoticed.2 Neglect and inadequate treatment of the primary tumor are the most important contributing factors to the size of giant BCCs. Giant BCCs also have more aggressive biologic behavior, with an increased risk for local invasion and metastasis.3 In this case, the lesion was larger than 5 cm in diameter and occurred on the lower extremity rather than on the trunk.
This case is unusual because delayed diagnosis of BCC was the result of misdiagnoses of a dermoid cyst and bursitis, with a diagnostic skin biopsy demonstrating BCC almost 2 years later. It should be emphasized that early diagnosis and treatment could prevent tumor expansion. Physicians should have a high degree of suspicion for BCC, especially when a dermoid cyst and knee bursitis fail to respond to conservative management.
Basal cell carcinoma (BCC) is the most frequently diagnosed skin cancer in the United States. It develops most often on sun-exposed skin, including the face and neck. Although BCCs are slow-growing tumors that rarely metastasize, they can cause notable local destruction with disfigurement if neglected or inadequately treated. Basal cell carcinoma arising on the legs is relatively uncommon.1,2 We present an interesting case of delayed diagnosis of BCC on the left knee due to earlier misdiagnoses of a dermoid cyst and bursitis.
Case Report
A 67-year-old man with no history of skin cancer presented with a painful growing tumor on the left knee of approximately 2 years’ duration. The patient’s primary care physician as well as a general surgeon initially diagnosed it as a dermoid cyst and bursitis. The nodule failed to respond to conservative therapy with nonsteroidal anti-inflammatory drugs and continued to grow until it began to ulcerate. Concerned about the possibility of septic arthritis, the patient’s primary care physician referred him to the emergency department. He was subsequently sent to the dermatology clinic.
On examination by dermatology, a 6.3×4.4-cm, tender, mobile, ulcerated nodule was noted on the left knee (Figure 1A). No popliteal or inguinal lymph nodes were palpable. Basal cell carcinoma, squamous cell carcinoma, or atypical infection (eg, Leishmania, deep fungal, mycobacterial) was suspected clinically. The patient underwent a diagnostic skin biopsy; hematoxylin and eosin–stained sections revealed lobular proliferation of basaloid cells with peripheral palisading and central tumoral necrosis, consistent with primary BCC (Figure 2).


Given the size of the tumor, the patient was referred for Mohs micrographic surgery and eventual reconstruction by a plastic surgeon. The tumor was cleared after 2 stages of Mohs surgery, with a final wound size of 7.7×5.4 cm (Figure 1B). Plastic surgery later performed a gastrocnemius muscle flap with a split-thickness skin graft (175 cm2) to repair the wound.
Comment
Exposure to UV radiation is the primary causative agent of most BCCs, accounting for the preferential distribution of these tumors on sun-exposed areas of the body. Approximately 80% of BCCs are located on the head and neck, 10% occur on the trunk, and only 8% are found on the lower extremities.1
Giant BCC, the finding in this case, is defined by the American Joint Committee on Cancer as a tumor larger than 5 cm in diameter. Fewer than 1% of all BCCs achieve this size; they appear more commonly on the back where they can go unnoticed.2 Neglect and inadequate treatment of the primary tumor are the most important contributing factors to the size of giant BCCs. Giant BCCs also have more aggressive biologic behavior, with an increased risk for local invasion and metastasis.3 In this case, the lesion was larger than 5 cm in diameter and occurred on the lower extremity rather than on the trunk.
This case is unusual because delayed diagnosis of BCC was the result of misdiagnoses of a dermoid cyst and bursitis, with a diagnostic skin biopsy demonstrating BCC almost 2 years later. It should be emphasized that early diagnosis and treatment could prevent tumor expansion. Physicians should have a high degree of suspicion for BCC, especially when a dermoid cyst and knee bursitis fail to respond to conservative management.
- Pearson G, King LE, Boyd AS. Basal cell carcinoma of the lower extremities. Int J Dermatol. 1999;38:852-854.
- Arnaiz J, Gallardo E, Piedra T, et al. Giant basal cell carcinoma on the lower leg: MRI findings. J Plast Reconstr Aesthet Surg. 2007;60:1167-1168.
- Randle HW. Giant basal cell carcinoma [letter]. Int J Dermatol. 1996;35:222-223.
- Pearson G, King LE, Boyd AS. Basal cell carcinoma of the lower extremities. Int J Dermatol. 1999;38:852-854.
- Arnaiz J, Gallardo E, Piedra T, et al. Giant basal cell carcinoma on the lower leg: MRI findings. J Plast Reconstr Aesthet Surg. 2007;60:1167-1168.
- Randle HW. Giant basal cell carcinoma [letter]. Int J Dermatol. 1996;35:222-223.
Practice Points
- This case highlights an unusual presentation of basal cell carcinoma masquerading as bursitis.
- Clinicians should be aware of confirmation bias, especially when multiple physicians and specialists are involved in a case.
- When the initial clinical impression is not corroborated by objective data or the condition is not responding to conventional therapy, it is important for clinicians to revisit the possibility of an inaccurate diagnosis.
Quantity and Characteristics of Flap or Graft Repairs for Skin Cancer on the Nose or Ears: A Comparison Between Mohs Micrographic Surgery and Plastic Surgery
The incidence of nonmelanoma skin cancer (NMSC) is steadily increasing, and it accounts for more annual cancer diagnoses than all other malignancies combined.1,2 For NMSCs of the head and neck, Mohs micrographic surgery (MMS) has become a preferred technique because of its high cure rates, intraprocedural margin control, and improved tissue preservation in cosmetically sensitive areas.3 The nose and ears are especially sensitive anatomic locations given their prominent positions and relative lack of skin reservoir and laxity compared to other areas of the head and neck. For the nose and ears, both patients and referring providers may question who is best suited to surgically remove a malignancy and repair the defect with positive functional and cosmetic results, as a large portion of the defects following tumor extirpation will require a flap or graft for repair.
The notion of plastic surgery is strongly associated with supreme cosmesis for many patients and providers, as the specialty trains in several surgical and nonsurgical elective techniques to preserve and improve appearance. Consequently, patients commonly ask dermatologists if they should be referred to a plastic surgeon for skin cancer removal in cosmetically sensitive areas, especially areas that may require more complex surgical repairs. However, recent Medicare data indicate that dermatologists perform the vast majority of reconstructive skin surgeries, with more than 15 times the number of intermediate and complex closures and more than 4 times the number of flaps and grafts as the next closest specialty.4 Earlier studies using Medicare data revealed similar findings, with dermatologic surgeons performing more reconstructions of head and neck skin than both plastic surgeons and otorhinolaryngologists.5 However, these studies did not address the characteristics of the tumor, defects, or repairs performed by the specialties for comparison.
We sought to compare the quantity and characteristics of flaps or grafts performed for skin cancer on the nose or ears by fellowship-trained Mohs surgeons and plastic surgeons at 1 academic institution.
Methods
We performed a retrospective chart review of all skin cancer surgeries requiring a flap or graft on the nose or ears at Baylor Scott & White Health (Temple, Texas) from October 1, 2016, to October 1, 2017. This study was approved by the Baylor Scott & White Health institutional review board.
Data Collection
The analysis included full-time, fellowship-trained Mohs surgeons and all full-time plastic surgeons who accepted skin cancer surgery patient referrals as part of their practice and performed all procedures within our hospital system. We reviewed individual provider schedules for both outpatient consultation and operating room notes to capture each procedure performed. To ensure we captured all procedures for both Mohs and plastic surgeons, we used billing codes for any flap or graft repair done on the nose or ears to cross-reference and confirm the cases found by chart review. The total number of flaps or grafts on the nose or ears were collected. Data also were collected regarding the anatomic location of the skin cancer, final defect size prior to the repair, skin tumor type, repair type (flap or graft), and flap (transposition vs advancement) or graft (full thickness vs partial thickness) type. All surgical data were collected from operative notes. Demographic data, including age, race, and sex, also were collected. We also collected data on the specialty of the physicians who referred patients for surgical management of biopsy-proven skin malignancy.
Statistical Analysis
Sample characteristics were described using descriptive statistics. Frequencies and percentages were used to describe categorical variables. Medians and ranges were used to describe continuous variables due to nonsymmetrically distributed data. χ2 tests (or Fisher exact tests when low cell counts were present) for categorical variables and Wilcoxon signed rank tests for continuous variables were used to test for associations in bivariate comparisons between MMS and plastic surgery.
Results
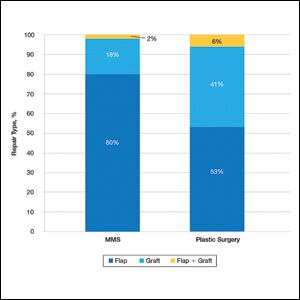
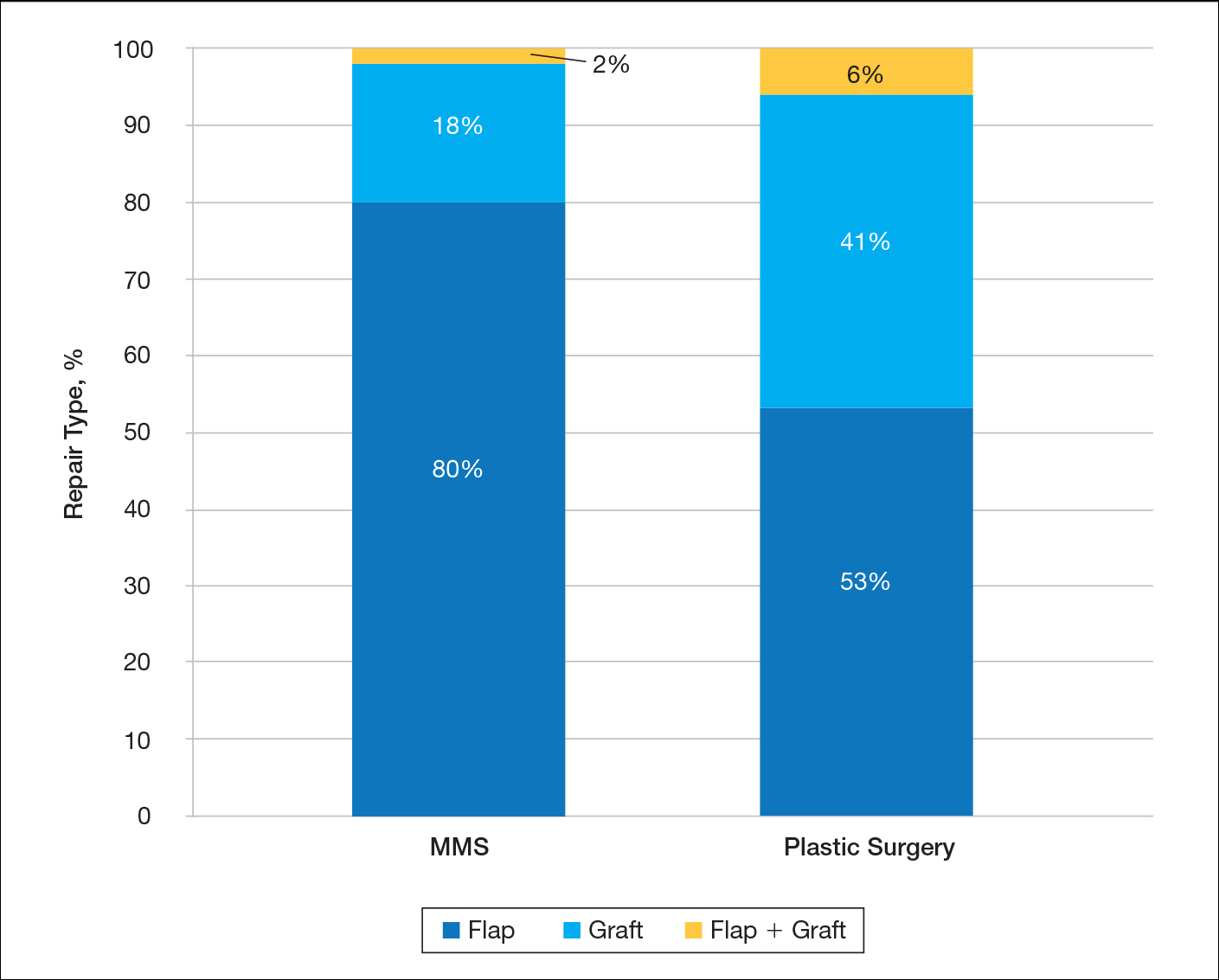
A total of 7 physicians (1 fellowship-trained Mohs surgeon and 6 plastic surgeons) at our institution met the inclusion criteria. The Mohs surgeon performed a significantly higher number of flaps and grafts (n=276) than the plastic surgeons (n=17 combined; average per plastic surgeon, 2.83) on the nose or ears in a 12-month period (P<.05)(Table). The median final defect size was not significantly different between MMS (1.5 cm) and plastic surgery (1.8 cm)(P=.306). Flap repairs were more common in patients undergoing MMS (80%) vs plastic surgery (53%)(P=.022)(Figure). For flap repair, advancement flaps were used more commonly (MMS, 53%; plastic surgery, 35%) than transposition flaps (MMS, 27%; plastic surgery, 12%) by both specialties.
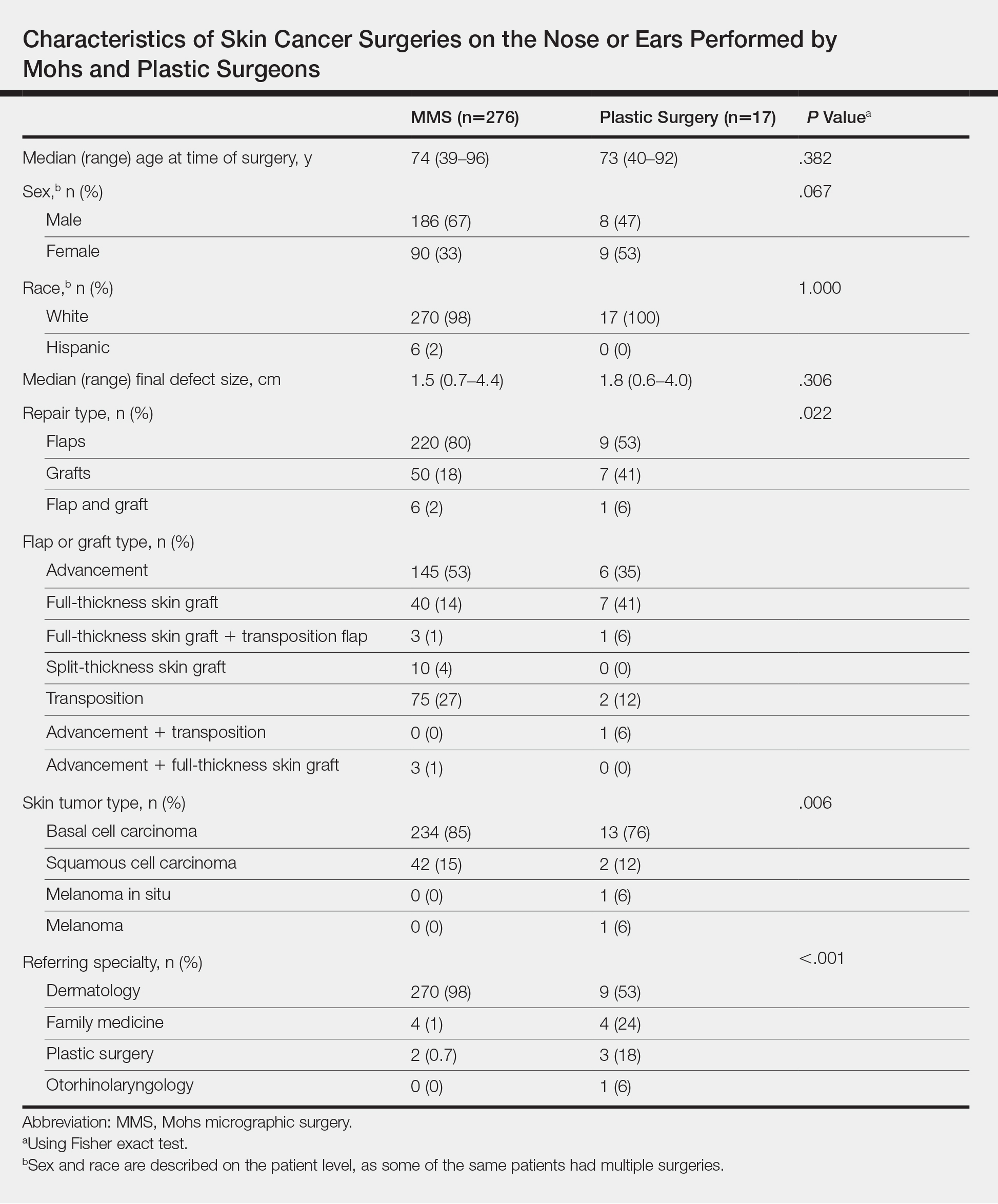
Patient age was similar between MMS (median, 74 years) and plastic surgery (median, 73 years) patients (P=.382), but a greater percentage of women were treated by plastic surgeons (53%) compared with Mohs surgeons (33%). The predominant skin tumor type for both specialties was basal cell carcinoma (MMS, 85%; plastic surgery, 76%). Dermatology was the largest referring specialty to both MMS (98%) and plastic surgery (53%). Family medicine referrals comprised a much larger percentage of cases for plastic surgery (24%) compared to MMS (1%).
Comment
This study supports and adds to recent studies and data regarding the utilization of MMS for the treatment of NMSCs. Although the percentage of all skin cancer surgery is increasing for dermatology, little has been reported on more complex repairs. This study highlights the volume and complexity of skin surgery performed by Mohs surgeons compared to our colleagues in plastic surgery.
Defect Size
The defect sizes prior to repair were not statistically different between the 2 types of surgeries, though the median size was slightly larger for plastic surgery (1.8 cm) compared to MMS (1.5 cm). These non–statistically significant differences may be explained by potentially larger tumors requiring repair by plastic surgeons in an operating room. Plastic surgeons, however, may be more likely to take a larger margin of clinically unaffected tissue as part of the initial layer. Plastic surgeons also may be less likely to curette the lesion prior to excision to obtain more clear tumor margins, possibly leading to more stages and a subsequently larger defect. Knowing the clinical sizes of these NMSCs prior to biopsy would have been beneficial to our study, but these data often were not available from the referring providers.
Repair Type
Most patients who underwent MMS had surgical defects repaired with a flap vs a graft, and a much higher percentage of patients who had undergone MMS vs surgical excision with plastic surgery had their defects repaired with flaps. Using a visual analog scale score and Hollander Wound Evaluation Scale, Jacobs et al6 found flaps to be cosmetically superior to grafts following tumor extirpation on the nose. The more frequent use of grafts by plastic surgeons could be at least partially explained by larger defect size or by a few outlier larger lesions among an otherwise small sample size. Larger studies may be needed to see if a true discrepancy in repair preferences exists between the specialties.
Referring Specialty
Primary care physician referral comprised a much larger percentage of cases sent for treatment with plastic surgery (24%) compared to MMS (1%). This statistic may represent a practice gap in the perception of MMS and its benefits among our primary care colleagues, particularly among female patients, as a much higher percentage of women were treated with plastic surgery. Important potential benefits of MMS, particularly tissue conservation, cure rates for skin cancer, and the volume of repairs performed by Mohs surgeons, may need to be emphasized.
Scope of Practice
Our colleagues in plastic surgery are extremely gifted and perform numerous repairs outside the scope of most Mohs surgeons. They are vital to multidisciplinary approaches to patients with skin cancer. Although Mohs surgeons focus on treating skin cancers that arise in a narrower range of anatomic locations, the breadth and variety of surgical procedures performed by plastic surgeons is more diverse. Skin cancer surgery may account for a smaller portion of procedures in a plastic surgery practice.
Limitations
There are several limitations to this study. We did not compare cosmesis or wound healing in patients treated by MMS or plastic surgery. The sample size, particularly with plastic surgery, was small and did not allow for a larger, more powerful comparison of data between the 2 specialties. Finally, our study only represents 1 institution over the course of 1 year.
Conclusion
To provide the best care possible, it is imperative for referring physicians to possess an accurate understanding of the volume of cases and the types of repairs that treating specialties perform on a regular basis for NMSCs. This knowledge is particularly important when there is a treatment overlap among specialties. Our data show Mohs surgeons are performing more complex repairs and reconstructions on even the most cosmetically sensitive areas; therefore, primary care physicians and other specialists may be more likely to involve dermatology in the care of skin cancer.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Rogers HW, Weinstock MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the united states, 2006. Arch Dermatol. 2010;146:283-287.
- Mansouri B, Bicknell LM, Hill D, et al. Mohs micrographic surgery for the management of cutaneous malignancies. Facial Plast Surg Clin North Am. 2017;25:291-301.
- Kantor J. Dermatologists perform more reconstructive surgery in the Medicare population than any other specialist group: a cross-sectional individual-level analysis of Medicare volume and specialist type in cutaneous and reconstructive surgery. J Am Acad Dermatol. 2018;78:171-173.e1.
- Donaldson MR, Coldiron BM. Dermatologists perform the majority of cutaneous reconstructions in the Medicare population: numbers and trends from 2004 to 2009. J Am Acad Dermatol. 2013;68:803-808.
- Jacobs MA, Christenson LJ, Weaver AL, et al. Clinical outcome of cutaneous flaps versus full-thickness skin grafts after Mohs surgery on the nose. Dermatol Surg. 2010;36:23-30.
The incidence of nonmelanoma skin cancer (NMSC) is steadily increasing, and it accounts for more annual cancer diagnoses than all other malignancies combined.1,2 For NMSCs of the head and neck, Mohs micrographic surgery (MMS) has become a preferred technique because of its high cure rates, intraprocedural margin control, and improved tissue preservation in cosmetically sensitive areas.3 The nose and ears are especially sensitive anatomic locations given their prominent positions and relative lack of skin reservoir and laxity compared to other areas of the head and neck. For the nose and ears, both patients and referring providers may question who is best suited to surgically remove a malignancy and repair the defect with positive functional and cosmetic results, as a large portion of the defects following tumor extirpation will require a flap or graft for repair.
The notion of plastic surgery is strongly associated with supreme cosmesis for many patients and providers, as the specialty trains in several surgical and nonsurgical elective techniques to preserve and improve appearance. Consequently, patients commonly ask dermatologists if they should be referred to a plastic surgeon for skin cancer removal in cosmetically sensitive areas, especially areas that may require more complex surgical repairs. However, recent Medicare data indicate that dermatologists perform the vast majority of reconstructive skin surgeries, with more than 15 times the number of intermediate and complex closures and more than 4 times the number of flaps and grafts as the next closest specialty.4 Earlier studies using Medicare data revealed similar findings, with dermatologic surgeons performing more reconstructions of head and neck skin than both plastic surgeons and otorhinolaryngologists.5 However, these studies did not address the characteristics of the tumor, defects, or repairs performed by the specialties for comparison.
We sought to compare the quantity and characteristics of flaps or grafts performed for skin cancer on the nose or ears by fellowship-trained Mohs surgeons and plastic surgeons at 1 academic institution.
Methods
We performed a retrospective chart review of all skin cancer surgeries requiring a flap or graft on the nose or ears at Baylor Scott & White Health (Temple, Texas) from October 1, 2016, to October 1, 2017. This study was approved by the Baylor Scott & White Health institutional review board.
Data Collection
The analysis included full-time, fellowship-trained Mohs surgeons and all full-time plastic surgeons who accepted skin cancer surgery patient referrals as part of their practice and performed all procedures within our hospital system. We reviewed individual provider schedules for both outpatient consultation and operating room notes to capture each procedure performed. To ensure we captured all procedures for both Mohs and plastic surgeons, we used billing codes for any flap or graft repair done on the nose or ears to cross-reference and confirm the cases found by chart review. The total number of flaps or grafts on the nose or ears were collected. Data also were collected regarding the anatomic location of the skin cancer, final defect size prior to the repair, skin tumor type, repair type (flap or graft), and flap (transposition vs advancement) or graft (full thickness vs partial thickness) type. All surgical data were collected from operative notes. Demographic data, including age, race, and sex, also were collected. We also collected data on the specialty of the physicians who referred patients for surgical management of biopsy-proven skin malignancy.
Statistical Analysis
Sample characteristics were described using descriptive statistics. Frequencies and percentages were used to describe categorical variables. Medians and ranges were used to describe continuous variables due to nonsymmetrically distributed data. χ2 tests (or Fisher exact tests when low cell counts were present) for categorical variables and Wilcoxon signed rank tests for continuous variables were used to test for associations in bivariate comparisons between MMS and plastic surgery.
Results
A total of 7 physicians (1 fellowship-trained Mohs surgeon and 6 plastic surgeons) at our institution met the inclusion criteria. The Mohs surgeon performed a significantly higher number of flaps and grafts (n=276) than the plastic surgeons (n=17 combined; average per plastic surgeon, 2.83) on the nose or ears in a 12-month period (P<.05)(Table). The median final defect size was not significantly different between MMS (1.5 cm) and plastic surgery (1.8 cm)(P=.306). Flap repairs were more common in patients undergoing MMS (80%) vs plastic surgery (53%)(P=.022)(Figure). For flap repair, advancement flaps were used more commonly (MMS, 53%; plastic surgery, 35%) than transposition flaps (MMS, 27%; plastic surgery, 12%) by both specialties.


Patient age was similar between MMS (median, 74 years) and plastic surgery (median, 73 years) patients (P=.382), but a greater percentage of women were treated by plastic surgeons (53%) compared with Mohs surgeons (33%). The predominant skin tumor type for both specialties was basal cell carcinoma (MMS, 85%; plastic surgery, 76%). Dermatology was the largest referring specialty to both MMS (98%) and plastic surgery (53%). Family medicine referrals comprised a much larger percentage of cases for plastic surgery (24%) compared to MMS (1%).
Comment
This study supports and adds to recent studies and data regarding the utilization of MMS for the treatment of NMSCs. Although the percentage of all skin cancer surgery is increasing for dermatology, little has been reported on more complex repairs. This study highlights the volume and complexity of skin surgery performed by Mohs surgeons compared to our colleagues in plastic surgery.
Defect Size
The defect sizes prior to repair were not statistically different between the 2 types of surgeries, though the median size was slightly larger for plastic surgery (1.8 cm) compared to MMS (1.5 cm). These non–statistically significant differences may be explained by potentially larger tumors requiring repair by plastic surgeons in an operating room. Plastic surgeons, however, may be more likely to take a larger margin of clinically unaffected tissue as part of the initial layer. Plastic surgeons also may be less likely to curette the lesion prior to excision to obtain more clear tumor margins, possibly leading to more stages and a subsequently larger defect. Knowing the clinical sizes of these NMSCs prior to biopsy would have been beneficial to our study, but these data often were not available from the referring providers.
Repair Type
Most patients who underwent MMS had surgical defects repaired with a flap vs a graft, and a much higher percentage of patients who had undergone MMS vs surgical excision with plastic surgery had their defects repaired with flaps. Using a visual analog scale score and Hollander Wound Evaluation Scale, Jacobs et al6 found flaps to be cosmetically superior to grafts following tumor extirpation on the nose. The more frequent use of grafts by plastic surgeons could be at least partially explained by larger defect size or by a few outlier larger lesions among an otherwise small sample size. Larger studies may be needed to see if a true discrepancy in repair preferences exists between the specialties.
Referring Specialty
Primary care physician referral comprised a much larger percentage of cases sent for treatment with plastic surgery (24%) compared to MMS (1%). This statistic may represent a practice gap in the perception of MMS and its benefits among our primary care colleagues, particularly among female patients, as a much higher percentage of women were treated with plastic surgery. Important potential benefits of MMS, particularly tissue conservation, cure rates for skin cancer, and the volume of repairs performed by Mohs surgeons, may need to be emphasized.
Scope of Practice
Our colleagues in plastic surgery are extremely gifted and perform numerous repairs outside the scope of most Mohs surgeons. They are vital to multidisciplinary approaches to patients with skin cancer. Although Mohs surgeons focus on treating skin cancers that arise in a narrower range of anatomic locations, the breadth and variety of surgical procedures performed by plastic surgeons is more diverse. Skin cancer surgery may account for a smaller portion of procedures in a plastic surgery practice.
Limitations
There are several limitations to this study. We did not compare cosmesis or wound healing in patients treated by MMS or plastic surgery. The sample size, particularly with plastic surgery, was small and did not allow for a larger, more powerful comparison of data between the 2 specialties. Finally, our study only represents 1 institution over the course of 1 year.
Conclusion
To provide the best care possible, it is imperative for referring physicians to possess an accurate understanding of the volume of cases and the types of repairs that treating specialties perform on a regular basis for NMSCs. This knowledge is particularly important when there is a treatment overlap among specialties. Our data show Mohs surgeons are performing more complex repairs and reconstructions on even the most cosmetically sensitive areas; therefore, primary care physicians and other specialists may be more likely to involve dermatology in the care of skin cancer.
The incidence of nonmelanoma skin cancer (NMSC) is steadily increasing, and it accounts for more annual cancer diagnoses than all other malignancies combined.1,2 For NMSCs of the head and neck, Mohs micrographic surgery (MMS) has become a preferred technique because of its high cure rates, intraprocedural margin control, and improved tissue preservation in cosmetically sensitive areas.3 The nose and ears are especially sensitive anatomic locations given their prominent positions and relative lack of skin reservoir and laxity compared to other areas of the head and neck. For the nose and ears, both patients and referring providers may question who is best suited to surgically remove a malignancy and repair the defect with positive functional and cosmetic results, as a large portion of the defects following tumor extirpation will require a flap or graft for repair.
The notion of plastic surgery is strongly associated with supreme cosmesis for many patients and providers, as the specialty trains in several surgical and nonsurgical elective techniques to preserve and improve appearance. Consequently, patients commonly ask dermatologists if they should be referred to a plastic surgeon for skin cancer removal in cosmetically sensitive areas, especially areas that may require more complex surgical repairs. However, recent Medicare data indicate that dermatologists perform the vast majority of reconstructive skin surgeries, with more than 15 times the number of intermediate and complex closures and more than 4 times the number of flaps and grafts as the next closest specialty.4 Earlier studies using Medicare data revealed similar findings, with dermatologic surgeons performing more reconstructions of head and neck skin than both plastic surgeons and otorhinolaryngologists.5 However, these studies did not address the characteristics of the tumor, defects, or repairs performed by the specialties for comparison.
We sought to compare the quantity and characteristics of flaps or grafts performed for skin cancer on the nose or ears by fellowship-trained Mohs surgeons and plastic surgeons at 1 academic institution.
Methods
We performed a retrospective chart review of all skin cancer surgeries requiring a flap or graft on the nose or ears at Baylor Scott & White Health (Temple, Texas) from October 1, 2016, to October 1, 2017. This study was approved by the Baylor Scott & White Health institutional review board.
Data Collection
The analysis included full-time, fellowship-trained Mohs surgeons and all full-time plastic surgeons who accepted skin cancer surgery patient referrals as part of their practice and performed all procedures within our hospital system. We reviewed individual provider schedules for both outpatient consultation and operating room notes to capture each procedure performed. To ensure we captured all procedures for both Mohs and plastic surgeons, we used billing codes for any flap or graft repair done on the nose or ears to cross-reference and confirm the cases found by chart review. The total number of flaps or grafts on the nose or ears were collected. Data also were collected regarding the anatomic location of the skin cancer, final defect size prior to the repair, skin tumor type, repair type (flap or graft), and flap (transposition vs advancement) or graft (full thickness vs partial thickness) type. All surgical data were collected from operative notes. Demographic data, including age, race, and sex, also were collected. We also collected data on the specialty of the physicians who referred patients for surgical management of biopsy-proven skin malignancy.
Statistical Analysis
Sample characteristics were described using descriptive statistics. Frequencies and percentages were used to describe categorical variables. Medians and ranges were used to describe continuous variables due to nonsymmetrically distributed data. χ2 tests (or Fisher exact tests when low cell counts were present) for categorical variables and Wilcoxon signed rank tests for continuous variables were used to test for associations in bivariate comparisons between MMS and plastic surgery.
Results
A total of 7 physicians (1 fellowship-trained Mohs surgeon and 6 plastic surgeons) at our institution met the inclusion criteria. The Mohs surgeon performed a significantly higher number of flaps and grafts (n=276) than the plastic surgeons (n=17 combined; average per plastic surgeon, 2.83) on the nose or ears in a 12-month period (P<.05)(Table). The median final defect size was not significantly different between MMS (1.5 cm) and plastic surgery (1.8 cm)(P=.306). Flap repairs were more common in patients undergoing MMS (80%) vs plastic surgery (53%)(P=.022)(Figure). For flap repair, advancement flaps were used more commonly (MMS, 53%; plastic surgery, 35%) than transposition flaps (MMS, 27%; plastic surgery, 12%) by both specialties.


Patient age was similar between MMS (median, 74 years) and plastic surgery (median, 73 years) patients (P=.382), but a greater percentage of women were treated by plastic surgeons (53%) compared with Mohs surgeons (33%). The predominant skin tumor type for both specialties was basal cell carcinoma (MMS, 85%; plastic surgery, 76%). Dermatology was the largest referring specialty to both MMS (98%) and plastic surgery (53%). Family medicine referrals comprised a much larger percentage of cases for plastic surgery (24%) compared to MMS (1%).
Comment
This study supports and adds to recent studies and data regarding the utilization of MMS for the treatment of NMSCs. Although the percentage of all skin cancer surgery is increasing for dermatology, little has been reported on more complex repairs. This study highlights the volume and complexity of skin surgery performed by Mohs surgeons compared to our colleagues in plastic surgery.
Defect Size
The defect sizes prior to repair were not statistically different between the 2 types of surgeries, though the median size was slightly larger for plastic surgery (1.8 cm) compared to MMS (1.5 cm). These non–statistically significant differences may be explained by potentially larger tumors requiring repair by plastic surgeons in an operating room. Plastic surgeons, however, may be more likely to take a larger margin of clinically unaffected tissue as part of the initial layer. Plastic surgeons also may be less likely to curette the lesion prior to excision to obtain more clear tumor margins, possibly leading to more stages and a subsequently larger defect. Knowing the clinical sizes of these NMSCs prior to biopsy would have been beneficial to our study, but these data often were not available from the referring providers.
Repair Type
Most patients who underwent MMS had surgical defects repaired with a flap vs a graft, and a much higher percentage of patients who had undergone MMS vs surgical excision with plastic surgery had their defects repaired with flaps. Using a visual analog scale score and Hollander Wound Evaluation Scale, Jacobs et al6 found flaps to be cosmetically superior to grafts following tumor extirpation on the nose. The more frequent use of grafts by plastic surgeons could be at least partially explained by larger defect size or by a few outlier larger lesions among an otherwise small sample size. Larger studies may be needed to see if a true discrepancy in repair preferences exists between the specialties.
Referring Specialty
Primary care physician referral comprised a much larger percentage of cases sent for treatment with plastic surgery (24%) compared to MMS (1%). This statistic may represent a practice gap in the perception of MMS and its benefits among our primary care colleagues, particularly among female patients, as a much higher percentage of women were treated with plastic surgery. Important potential benefits of MMS, particularly tissue conservation, cure rates for skin cancer, and the volume of repairs performed by Mohs surgeons, may need to be emphasized.
Scope of Practice
Our colleagues in plastic surgery are extremely gifted and perform numerous repairs outside the scope of most Mohs surgeons. They are vital to multidisciplinary approaches to patients with skin cancer. Although Mohs surgeons focus on treating skin cancers that arise in a narrower range of anatomic locations, the breadth and variety of surgical procedures performed by plastic surgeons is more diverse. Skin cancer surgery may account for a smaller portion of procedures in a plastic surgery practice.
Limitations
There are several limitations to this study. We did not compare cosmesis or wound healing in patients treated by MMS or plastic surgery. The sample size, particularly with plastic surgery, was small and did not allow for a larger, more powerful comparison of data between the 2 specialties. Finally, our study only represents 1 institution over the course of 1 year.
Conclusion
To provide the best care possible, it is imperative for referring physicians to possess an accurate understanding of the volume of cases and the types of repairs that treating specialties perform on a regular basis for NMSCs. This knowledge is particularly important when there is a treatment overlap among specialties. Our data show Mohs surgeons are performing more complex repairs and reconstructions on even the most cosmetically sensitive areas; therefore, primary care physicians and other specialists may be more likely to involve dermatology in the care of skin cancer.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Rogers HW, Weinstock MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the united states, 2006. Arch Dermatol. 2010;146:283-287.
- Mansouri B, Bicknell LM, Hill D, et al. Mohs micrographic surgery for the management of cutaneous malignancies. Facial Plast Surg Clin North Am. 2017;25:291-301.
- Kantor J. Dermatologists perform more reconstructive surgery in the Medicare population than any other specialist group: a cross-sectional individual-level analysis of Medicare volume and specialist type in cutaneous and reconstructive surgery. J Am Acad Dermatol. 2018;78:171-173.e1.
- Donaldson MR, Coldiron BM. Dermatologists perform the majority of cutaneous reconstructions in the Medicare population: numbers and trends from 2004 to 2009. J Am Acad Dermatol. 2013;68:803-808.
- Jacobs MA, Christenson LJ, Weaver AL, et al. Clinical outcome of cutaneous flaps versus full-thickness skin grafts after Mohs surgery on the nose. Dermatol Surg. 2010;36:23-30.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Rogers HW, Weinstock MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the united states, 2006. Arch Dermatol. 2010;146:283-287.
- Mansouri B, Bicknell LM, Hill D, et al. Mohs micrographic surgery for the management of cutaneous malignancies. Facial Plast Surg Clin North Am. 2017;25:291-301.
- Kantor J. Dermatologists perform more reconstructive surgery in the Medicare population than any other specialist group: a cross-sectional individual-level analysis of Medicare volume and specialist type in cutaneous and reconstructive surgery. J Am Acad Dermatol. 2018;78:171-173.e1.
- Donaldson MR, Coldiron BM. Dermatologists perform the majority of cutaneous reconstructions in the Medicare population: numbers and trends from 2004 to 2009. J Am Acad Dermatol. 2013;68:803-808.
- Jacobs MA, Christenson LJ, Weaver AL, et al. Clinical outcome of cutaneous flaps versus full-thickness skin grafts after Mohs surgery on the nose. Dermatol Surg. 2010;36:23-30.
Practice Points
- Patients and nondermatologist physicians may be unaware of how frequently Mohs surgeons perform complex surgical repairs compared to other specialists.
- Compared to plastic surgeons, Mohs surgeons performed a larger number of complex skin cancer repairs on the nose or ears with similar-sized defects.
- Primary care physicians and other specialists may be more likely to involve dermatology in the care of skin cancer through awareness of this type of data.
Dermoscopic Patterns of Acral Melanocytic Lesions in Skin of Color
Acral lentiginous melanoma (ALM) is a rare subtype of melanoma that occurs on the palms, soles, and nail apparatus. Unlike more common types of melanoma, ALM occurs on sun-protected areas of the skin and has distinct clinical, histologic, and genetic features. Acral lentiginous melanoma accounts for a larger proportion of melanomas in individuals with skin of color and has a worse prognosis and recurrence rate than other forms of melanoma.
Population Trends in Skin of Color
Much of the literature on malignant melanoma historically has involved non-Hispanic white patients, but the incidence in lighter-skinned populations has been increasing steadily over the last few decades.1 Although ALM can occur in any race, it disproportionately affects skin of color populations; ALM accounts for only 0.8% to 1% of all melanomas in white populations, but it constitutes 4% to 58% of melanomas in ethnic populations and is the most common melanoma subtype among black Americans.2-5 Acral lentiginous melanoma also is associated with a worse prognosis compared to other subtypes, which may indicate a more aggressive biological nature6 but also may point toward socioeconomic and cultural barriers (eg, low income or education levels, lack of insurance, lower health literacy), leading to disparities in access to care and diagnosis at advanced stages.5
Similarly, the distribution of acral melanocytic nevi appears to demonstrate an association with ethnicity and skin pigmentation. Although skin of color patients have fewer nevi than non-Hispanic whites, the proportion of acral melanocytic nevi tends to be greater.6,7 Given its grim prognosis, accurately differentiating ALM from acral nevi is of utmost importance.
Diagnostic Challenges of Acral Lesions
Due to the unique nature of the surfaces of acral sites, melanocytic lesions on the palms, soles, and nail apparatus present many diagnostic challenges. It can be difficult to distinguish acral melanoma from benign lesions using the naked eye alone. Volar surfaces are characterized by the presence of dermatoglyphics, and pigment deposition along ridges and furrows create particular dermoscopic patterns exclusive to these sites.8 Thus, dermoscopy can be useful on acral surfaces, but the dermoscopic features are different from those on the rest of the body and must be learned separately.
In addition, nearly half of patients are unaware of their acral lesions.6 Acral surfaces may not always be examined by clinicians during total-body skin examinations, leading to further possibility of overlooking a lesion. Obtaining biopsies on glabrous skin or nails also is challenging because they can be more painful and hemostasis can be more difficult, especially in the nail. Acral melanomas also may be amelanotic, including those at subungual sites.
Dermoscopic Patterns of Acral Volar Skin
Dermoscopy is a useful noninvasive tool for distinguishing between benign and malignant acral melanocytic lesions, and its efficacy in improving diagnostic accuracy and decreasing unnecessary biopsies is well-established in the literature.13,14 Acral dermoscopy allows for visualization of pigment along the dermatoglyphics that constitute the characteristic dermoscopic patterns.
Acral Lentiginous Melanoma
The hallmark dermoscopic pattern and most important finding of ALM is the parallel ridge pattern, characterized by parallel linear pigmentation along the ridges of dermatoglyphics. In the early phases of malignancy, the pattern appears light brown and involves most of the lesion; as the tumor develops, increasing melanin production results in focal areas of the parallel ridge pattern with darker bands.15,16 The sensitivity and specificity of a parallel ridge pattern for diagnosing early ALM has been shown to be 86% and 99%, respectively.15,16
A pattern of irregular diffuse pigmentation also can be observed in more advanced ALM. Dermoscopy may reveal a structureless pattern (ie, lack of identifiable structures or patterns) in a background of tan-black coloration due to more exuberant melanocyte proliferation along the epidermis.15 Sensitivity and specificity of this dermoscopic finding for invasive lesions is high at 94% and 97%, respectively.16,17 Interestingly, once ALM lesions have advanced even further, conventional melanoma-associated structures (ie, blue-white veil, polymorphous blood vessels, ulceration, irregular dots/globules or streaks) or atypical forms of typically benign acral dermoscopic patterns may be observed.15
Per a 3-step diagnostic algorithm created by Koga and Saida,18 a suspected acral lesion should first be evaluated for a parallel ridge pattern to determine the need for biopsy, as it is seen in approximately two-thirds of ALMs.19 If no parallel ridge pattern is observed, the lesion should then be checked for any of the typical dermoscopic patterns seen in benign acral nevi (eg, parallel furrow, latticelike, or fibrillar patterns).18 The maximum diameter should be measured only if the lesion does not exhibit any of the typical dermoscopic patterns. If the lesion’s diameter is greater than 7 mm in diameter, it should be biopsied; if the diameter is less than 7 mm, it should have regular clinical and dermoscopic follow-up.18
In 2015, Lallas et al20 developed the BRAAFF checklist, a scoring system of 6 variables: blotches, ridge pattern, asymmetry of structures, asymmetry of colors, parallel furrow pattern, and fibrillar pattern. The checklist also was shown to substantially improve diagnostic accuracy of dermoscopy for ALM, with sensitivity and specificity at 93.1% and 86.7%, respectively.20
Acquired Acral Nevi
Three classic dermoscopic patterns are associated with acquired acral nevi: parallel furrow pattern, latticelike pattern, and fibrillar pattern.15,21 Approximately three-quarters of all acquired acral nevi exhibit one of these patterns, roughly half exhibiting parallel furrow with tan-brown bandlike pigmentation along dermatoglyphic grooves.16,17
Latticelike patterns also are characterized by brown parallel lines along the sulci of dermatoglyphics but additionally have multiple intersecting lines. Thus, this pattern can be considered a variant of the parallel furrow pattern.15 The crisscross markings can be predominantly found in the plantar arch.22 This dermoscopic pattern comprises 15% to 25% of all acral nevi.21
Fibrillar pattern accounts for 10% to 20% of all acral melanocytic nevi.21 Dermoscopically, these lesions demonstrate parallel filamentous streaks that cross dermatoglyphics obliquely. The fibrillar pattern is predominantly found on weight-bearing areas of the sole,22 which likely is explained by pressure causing slanting of melanin columns in the horny layer.23 The fibrillar pattern has been shown to be the benign acral dermoscopic pattern that is most commonly misdiagnosed, with higher reported rates of biopsy.24
Acral Congenital Melanocytic Nevi
Congenital melanocytic nevi (CMN) present at birth or appear during the first few weeks of life. Congenital melanocytic nevi can vary widely in size, shape, and color, and they are occasionally biopsied in cases of larger diameter or dermoscopic atypia to differentiate from melanoma.25 Congenital melanocytic nevi also can occur on acral volar surfaces. Possible dermoscopic patterns include parallel furrow or fibrillar patterns as well as a crista dotted pattern, defined as evenly spaced dots/globules on the ridges near the openings of eccrine ducts.26 A more commonly observed dermoscopic pattern in acral CMN is a combination of the crista dotted and parallel furrow patterns, known as the peas-in-a-pod pattern. Changes in the clinical appearance and dermoscopic features of an acral CMN are possible over time; some lesions also may fade with age.26
Final Thoughts
Acral lentiginous melanoma is a rare but potentially aggressive melanoma subtype that accounts for a larger proportion of melanomas in patients with skin of color than in white patients. Dermoscopy of acral volar skin provides invaluable diagnostic information and allows for better management of acral melanocytic lesions. Dermoscopic patterns such as the parallel ridge, parallel furrow, latticelike, fibrillar, and peas-in-a-pod patterns are unique to acral sites and can be used to differentiate between ALMs, acquired nevi, or CMNs.
- Whiteman DC, Green AC, Olsen CM. The growing burden of invasive melanoma: projections of incidence rates and numbers of new cases in six susceptible populations through 2031. J Invest Dermatol. 2016;136:1161-1171.
- Bradford PT, Goldstein AM, McMaster ML, et al. Acral lentiginous melanoma: incidence and survival patterns in the United States, 1986-2005. Arch Dermatol. 2009;145:427-434.
- Nakamura Y, Fujisawa Y. Diagnosis and management of acral lentiginous melanoma. Curr Treat Options Oncol. 2018;19:42.
- Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907-1914.
- Wang Y, Zhao Y, Ma S. Racial differences in six major subtypes of melanoma: descriptive epidemiology. BMC Cancer. 2016;16:691.
- Madankumar R, Gumaste PV, Martires K, et al. Acral melanocytic lesions in the United States: prevalence, awareness, and dermoscopic patterns in skin-of-color and non-Hispanic white patients. J Am Acad Dermatol. 2016;74:724.e1-730.e1.
- Palicka GA, Rhodes AR. Acral melanocytic nevi: prevalence and distribution of gross morphologic features in white and black adults. Arch Dermatol. 2010;146:1085-1094.
- Thomas L, Phan A, Pralong P, et al. Special locations dermoscopy: facial, acral, and nail. Dermatol Clin. 2013;31:615-624.
- Gong HZ, Zheng HY, Li J. Amelanotic melanoma [published online January 21, 2019]. Melanoma Res. doi:10.1097/CMR.0000000000000571.
- Ise M, Yasuda F, Konohana I, et al. Acral melanoma with hyperkeratosis mimicking a pigmented wart. Dermatol Pract Concept. 2013;3:37-39.
- Serarslan G, Akçaly CM, Atik E. Acral lentiginous melanoma misdiagnosed as tinea pedis: a case report. Int J Dermatol. 2004;43:37-38.
- Gumaste P, Penn L, Cohen N, et al. Acral lentiginous melanoma of the foot misdiagnosed as a traumatic ulcer. a cautionary case. J Am Podiatr Med Assoc. 2015;105:189-194.
- Carli P, de Giorgi V, Chiarugi A, et al. Addition of dermoscopy to conventional naked-eye examination in melanoma screening: a randomized study. J Am Acad Dermatol. 2004;50:683-689.
- Carli P, de Giorgi V, Crocetti E, et al. Improvement of malignant/benign ratio in excised melanocytic lesions in the ‘dermoscopy era’: a retrospective study 1997-2001. Br J Dermatol. 2004;150:687-692.
- Saida T, Koga H, Uhara H. Key points in dermoscopic differentiation between early acral melanoma and acral nevus. J Dermatol. 2011;38:25-34.
- Ishihara Y, Saida T, Miyazaki A, et al. Early acral melanoma in situ: correlation between the parallel ridge pattern on dermoscopy and microscopic features. Am J Dermatopathol. 2006;28:21-27.
- Saida T, Miyazaki A, Oguchi S, et al. Significance of dermoscopic patterns in detecting malignant melanoma on acral volar skin: results of a multicenter study in Japan. Arch Dermatol. 2004;140:1233-1238.
- Koga H, Saida T. Revised 3-step dermoscopic algorithm for the management of acral melanocytic lesions. Arch Dermatol. 2011;147:741-743.
- Lallas A, Sgouros D, Zalaudek I, et al. Palmar and plantar melanomas differ for sex prevalence and tumor thickness but not for dermoscopic patterns. Melanoma Res. 2014;24:83-87.
- Lallas A, Kyrgidis A, Koga H, et al. The BRAAFF checklist: a new dermoscopic algorithm for diagnosing acral melanoma. Br J Dermatol. 2015;173:1041-1049.
- Saida T, Koga H. Dermoscopic patterns of acral melanocytic nevi: their variations, changes, and significance. Arch Dermatol. 2007;143:1423-1426.
- Miyazaki A, Saida T, Koga H, et al. Anatomical and histopathological correlates of the dermoscopic patterns seen in melanocytic nevi on the sole: a retrospective study. J Am Acad Dermatol. 2005;53:230-236.
- Watanabe S, Sawada M, Ishizaki S, et al. Comparison of dermatoscopic images of acral lentiginous melanoma and acral melanocytic nevus occurring on body weight-bearing areas. Dermatol Pract Concept. 2014;4:47-50.
- Costello CM, Ghanavatian S, Temkit M, et al. Educational and practice gaps in the management of volar melanocytic lesions. J Eur Acad Dermatol Venereol. 2018;32:1450-1455.
- Alikhan A, Ibrahimi OA, Eisen DB. Congenital melanocytic nevi: where are we now? part I. clinical presentation, epidemiology, pathogenesis, histology, malignant transformation, and neurocutaneous melanosis. J Am Acad Dermatol. 2012;67:495.e1-495.e17; quiz 512-514.
- Minagawa A, Koga H, Saida T. Dermoscopic characteristics of congenital melanocytic nevi affecting acral volar skin. Arch Dermatol. 2011;147:809-813.
Acral lentiginous melanoma (ALM) is a rare subtype of melanoma that occurs on the palms, soles, and nail apparatus. Unlike more common types of melanoma, ALM occurs on sun-protected areas of the skin and has distinct clinical, histologic, and genetic features. Acral lentiginous melanoma accounts for a larger proportion of melanomas in individuals with skin of color and has a worse prognosis and recurrence rate than other forms of melanoma.
Population Trends in Skin of Color
Much of the literature on malignant melanoma historically has involved non-Hispanic white patients, but the incidence in lighter-skinned populations has been increasing steadily over the last few decades.1 Although ALM can occur in any race, it disproportionately affects skin of color populations; ALM accounts for only 0.8% to 1% of all melanomas in white populations, but it constitutes 4% to 58% of melanomas in ethnic populations and is the most common melanoma subtype among black Americans.2-5 Acral lentiginous melanoma also is associated with a worse prognosis compared to other subtypes, which may indicate a more aggressive biological nature6 but also may point toward socioeconomic and cultural barriers (eg, low income or education levels, lack of insurance, lower health literacy), leading to disparities in access to care and diagnosis at advanced stages.5
Similarly, the distribution of acral melanocytic nevi appears to demonstrate an association with ethnicity and skin pigmentation. Although skin of color patients have fewer nevi than non-Hispanic whites, the proportion of acral melanocytic nevi tends to be greater.6,7 Given its grim prognosis, accurately differentiating ALM from acral nevi is of utmost importance.
Diagnostic Challenges of Acral Lesions
Due to the unique nature of the surfaces of acral sites, melanocytic lesions on the palms, soles, and nail apparatus present many diagnostic challenges. It can be difficult to distinguish acral melanoma from benign lesions using the naked eye alone. Volar surfaces are characterized by the presence of dermatoglyphics, and pigment deposition along ridges and furrows create particular dermoscopic patterns exclusive to these sites.8 Thus, dermoscopy can be useful on acral surfaces, but the dermoscopic features are different from those on the rest of the body and must be learned separately.
In addition, nearly half of patients are unaware of their acral lesions.6 Acral surfaces may not always be examined by clinicians during total-body skin examinations, leading to further possibility of overlooking a lesion. Obtaining biopsies on glabrous skin or nails also is challenging because they can be more painful and hemostasis can be more difficult, especially in the nail. Acral melanomas also may be amelanotic, including those at subungual sites.
Dermoscopic Patterns of Acral Volar Skin
Dermoscopy is a useful noninvasive tool for distinguishing between benign and malignant acral melanocytic lesions, and its efficacy in improving diagnostic accuracy and decreasing unnecessary biopsies is well-established in the literature.13,14 Acral dermoscopy allows for visualization of pigment along the dermatoglyphics that constitute the characteristic dermoscopic patterns.
Acral Lentiginous Melanoma
The hallmark dermoscopic pattern and most important finding of ALM is the parallel ridge pattern, characterized by parallel linear pigmentation along the ridges of dermatoglyphics. In the early phases of malignancy, the pattern appears light brown and involves most of the lesion; as the tumor develops, increasing melanin production results in focal areas of the parallel ridge pattern with darker bands.15,16 The sensitivity and specificity of a parallel ridge pattern for diagnosing early ALM has been shown to be 86% and 99%, respectively.15,16
A pattern of irregular diffuse pigmentation also can be observed in more advanced ALM. Dermoscopy may reveal a structureless pattern (ie, lack of identifiable structures or patterns) in a background of tan-black coloration due to more exuberant melanocyte proliferation along the epidermis.15 Sensitivity and specificity of this dermoscopic finding for invasive lesions is high at 94% and 97%, respectively.16,17 Interestingly, once ALM lesions have advanced even further, conventional melanoma-associated structures (ie, blue-white veil, polymorphous blood vessels, ulceration, irregular dots/globules or streaks) or atypical forms of typically benign acral dermoscopic patterns may be observed.15
Per a 3-step diagnostic algorithm created by Koga and Saida,18 a suspected acral lesion should first be evaluated for a parallel ridge pattern to determine the need for biopsy, as it is seen in approximately two-thirds of ALMs.19 If no parallel ridge pattern is observed, the lesion should then be checked for any of the typical dermoscopic patterns seen in benign acral nevi (eg, parallel furrow, latticelike, or fibrillar patterns).18 The maximum diameter should be measured only if the lesion does not exhibit any of the typical dermoscopic patterns. If the lesion’s diameter is greater than 7 mm in diameter, it should be biopsied; if the diameter is less than 7 mm, it should have regular clinical and dermoscopic follow-up.18
In 2015, Lallas et al20 developed the BRAAFF checklist, a scoring system of 6 variables: blotches, ridge pattern, asymmetry of structures, asymmetry of colors, parallel furrow pattern, and fibrillar pattern. The checklist also was shown to substantially improve diagnostic accuracy of dermoscopy for ALM, with sensitivity and specificity at 93.1% and 86.7%, respectively.20
Acquired Acral Nevi
Three classic dermoscopic patterns are associated with acquired acral nevi: parallel furrow pattern, latticelike pattern, and fibrillar pattern.15,21 Approximately three-quarters of all acquired acral nevi exhibit one of these patterns, roughly half exhibiting parallel furrow with tan-brown bandlike pigmentation along dermatoglyphic grooves.16,17
Latticelike patterns also are characterized by brown parallel lines along the sulci of dermatoglyphics but additionally have multiple intersecting lines. Thus, this pattern can be considered a variant of the parallel furrow pattern.15 The crisscross markings can be predominantly found in the plantar arch.22 This dermoscopic pattern comprises 15% to 25% of all acral nevi.21
Fibrillar pattern accounts for 10% to 20% of all acral melanocytic nevi.21 Dermoscopically, these lesions demonstrate parallel filamentous streaks that cross dermatoglyphics obliquely. The fibrillar pattern is predominantly found on weight-bearing areas of the sole,22 which likely is explained by pressure causing slanting of melanin columns in the horny layer.23 The fibrillar pattern has been shown to be the benign acral dermoscopic pattern that is most commonly misdiagnosed, with higher reported rates of biopsy.24
Acral Congenital Melanocytic Nevi
Congenital melanocytic nevi (CMN) present at birth or appear during the first few weeks of life. Congenital melanocytic nevi can vary widely in size, shape, and color, and they are occasionally biopsied in cases of larger diameter or dermoscopic atypia to differentiate from melanoma.25 Congenital melanocytic nevi also can occur on acral volar surfaces. Possible dermoscopic patterns include parallel furrow or fibrillar patterns as well as a crista dotted pattern, defined as evenly spaced dots/globules on the ridges near the openings of eccrine ducts.26 A more commonly observed dermoscopic pattern in acral CMN is a combination of the crista dotted and parallel furrow patterns, known as the peas-in-a-pod pattern. Changes in the clinical appearance and dermoscopic features of an acral CMN are possible over time; some lesions also may fade with age.26
Final Thoughts
Acral lentiginous melanoma is a rare but potentially aggressive melanoma subtype that accounts for a larger proportion of melanomas in patients with skin of color than in white patients. Dermoscopy of acral volar skin provides invaluable diagnostic information and allows for better management of acral melanocytic lesions. Dermoscopic patterns such as the parallel ridge, parallel furrow, latticelike, fibrillar, and peas-in-a-pod patterns are unique to acral sites and can be used to differentiate between ALMs, acquired nevi, or CMNs.
Acral lentiginous melanoma (ALM) is a rare subtype of melanoma that occurs on the palms, soles, and nail apparatus. Unlike more common types of melanoma, ALM occurs on sun-protected areas of the skin and has distinct clinical, histologic, and genetic features. Acral lentiginous melanoma accounts for a larger proportion of melanomas in individuals with skin of color and has a worse prognosis and recurrence rate than other forms of melanoma.
Population Trends in Skin of Color
Much of the literature on malignant melanoma historically has involved non-Hispanic white patients, but the incidence in lighter-skinned populations has been increasing steadily over the last few decades.1 Although ALM can occur in any race, it disproportionately affects skin of color populations; ALM accounts for only 0.8% to 1% of all melanomas in white populations, but it constitutes 4% to 58% of melanomas in ethnic populations and is the most common melanoma subtype among black Americans.2-5 Acral lentiginous melanoma also is associated with a worse prognosis compared to other subtypes, which may indicate a more aggressive biological nature6 but also may point toward socioeconomic and cultural barriers (eg, low income or education levels, lack of insurance, lower health literacy), leading to disparities in access to care and diagnosis at advanced stages.5
Similarly, the distribution of acral melanocytic nevi appears to demonstrate an association with ethnicity and skin pigmentation. Although skin of color patients have fewer nevi than non-Hispanic whites, the proportion of acral melanocytic nevi tends to be greater.6,7 Given its grim prognosis, accurately differentiating ALM from acral nevi is of utmost importance.
Diagnostic Challenges of Acral Lesions
Due to the unique nature of the surfaces of acral sites, melanocytic lesions on the palms, soles, and nail apparatus present many diagnostic challenges. It can be difficult to distinguish acral melanoma from benign lesions using the naked eye alone. Volar surfaces are characterized by the presence of dermatoglyphics, and pigment deposition along ridges and furrows create particular dermoscopic patterns exclusive to these sites.8 Thus, dermoscopy can be useful on acral surfaces, but the dermoscopic features are different from those on the rest of the body and must be learned separately.
In addition, nearly half of patients are unaware of their acral lesions.6 Acral surfaces may not always be examined by clinicians during total-body skin examinations, leading to further possibility of overlooking a lesion. Obtaining biopsies on glabrous skin or nails also is challenging because they can be more painful and hemostasis can be more difficult, especially in the nail. Acral melanomas also may be amelanotic, including those at subungual sites.
Dermoscopic Patterns of Acral Volar Skin
Dermoscopy is a useful noninvasive tool for distinguishing between benign and malignant acral melanocytic lesions, and its efficacy in improving diagnostic accuracy and decreasing unnecessary biopsies is well-established in the literature.13,14 Acral dermoscopy allows for visualization of pigment along the dermatoglyphics that constitute the characteristic dermoscopic patterns.
Acral Lentiginous Melanoma
The hallmark dermoscopic pattern and most important finding of ALM is the parallel ridge pattern, characterized by parallel linear pigmentation along the ridges of dermatoglyphics. In the early phases of malignancy, the pattern appears light brown and involves most of the lesion; as the tumor develops, increasing melanin production results in focal areas of the parallel ridge pattern with darker bands.15,16 The sensitivity and specificity of a parallel ridge pattern for diagnosing early ALM has been shown to be 86% and 99%, respectively.15,16
A pattern of irregular diffuse pigmentation also can be observed in more advanced ALM. Dermoscopy may reveal a structureless pattern (ie, lack of identifiable structures or patterns) in a background of tan-black coloration due to more exuberant melanocyte proliferation along the epidermis.15 Sensitivity and specificity of this dermoscopic finding for invasive lesions is high at 94% and 97%, respectively.16,17 Interestingly, once ALM lesions have advanced even further, conventional melanoma-associated structures (ie, blue-white veil, polymorphous blood vessels, ulceration, irregular dots/globules or streaks) or atypical forms of typically benign acral dermoscopic patterns may be observed.15
Per a 3-step diagnostic algorithm created by Koga and Saida,18 a suspected acral lesion should first be evaluated for a parallel ridge pattern to determine the need for biopsy, as it is seen in approximately two-thirds of ALMs.19 If no parallel ridge pattern is observed, the lesion should then be checked for any of the typical dermoscopic patterns seen in benign acral nevi (eg, parallel furrow, latticelike, or fibrillar patterns).18 The maximum diameter should be measured only if the lesion does not exhibit any of the typical dermoscopic patterns. If the lesion’s diameter is greater than 7 mm in diameter, it should be biopsied; if the diameter is less than 7 mm, it should have regular clinical and dermoscopic follow-up.18
In 2015, Lallas et al20 developed the BRAAFF checklist, a scoring system of 6 variables: blotches, ridge pattern, asymmetry of structures, asymmetry of colors, parallel furrow pattern, and fibrillar pattern. The checklist also was shown to substantially improve diagnostic accuracy of dermoscopy for ALM, with sensitivity and specificity at 93.1% and 86.7%, respectively.20
Acquired Acral Nevi
Three classic dermoscopic patterns are associated with acquired acral nevi: parallel furrow pattern, latticelike pattern, and fibrillar pattern.15,21 Approximately three-quarters of all acquired acral nevi exhibit one of these patterns, roughly half exhibiting parallel furrow with tan-brown bandlike pigmentation along dermatoglyphic grooves.16,17
Latticelike patterns also are characterized by brown parallel lines along the sulci of dermatoglyphics but additionally have multiple intersecting lines. Thus, this pattern can be considered a variant of the parallel furrow pattern.15 The crisscross markings can be predominantly found in the plantar arch.22 This dermoscopic pattern comprises 15% to 25% of all acral nevi.21
Fibrillar pattern accounts for 10% to 20% of all acral melanocytic nevi.21 Dermoscopically, these lesions demonstrate parallel filamentous streaks that cross dermatoglyphics obliquely. The fibrillar pattern is predominantly found on weight-bearing areas of the sole,22 which likely is explained by pressure causing slanting of melanin columns in the horny layer.23 The fibrillar pattern has been shown to be the benign acral dermoscopic pattern that is most commonly misdiagnosed, with higher reported rates of biopsy.24
Acral Congenital Melanocytic Nevi
Congenital melanocytic nevi (CMN) present at birth or appear during the first few weeks of life. Congenital melanocytic nevi can vary widely in size, shape, and color, and they are occasionally biopsied in cases of larger diameter or dermoscopic atypia to differentiate from melanoma.25 Congenital melanocytic nevi also can occur on acral volar surfaces. Possible dermoscopic patterns include parallel furrow or fibrillar patterns as well as a crista dotted pattern, defined as evenly spaced dots/globules on the ridges near the openings of eccrine ducts.26 A more commonly observed dermoscopic pattern in acral CMN is a combination of the crista dotted and parallel furrow patterns, known as the peas-in-a-pod pattern. Changes in the clinical appearance and dermoscopic features of an acral CMN are possible over time; some lesions also may fade with age.26
Final Thoughts
Acral lentiginous melanoma is a rare but potentially aggressive melanoma subtype that accounts for a larger proportion of melanomas in patients with skin of color than in white patients. Dermoscopy of acral volar skin provides invaluable diagnostic information and allows for better management of acral melanocytic lesions. Dermoscopic patterns such as the parallel ridge, parallel furrow, latticelike, fibrillar, and peas-in-a-pod patterns are unique to acral sites and can be used to differentiate between ALMs, acquired nevi, or CMNs.
- Whiteman DC, Green AC, Olsen CM. The growing burden of invasive melanoma: projections of incidence rates and numbers of new cases in six susceptible populations through 2031. J Invest Dermatol. 2016;136:1161-1171.
- Bradford PT, Goldstein AM, McMaster ML, et al. Acral lentiginous melanoma: incidence and survival patterns in the United States, 1986-2005. Arch Dermatol. 2009;145:427-434.
- Nakamura Y, Fujisawa Y. Diagnosis and management of acral lentiginous melanoma. Curr Treat Options Oncol. 2018;19:42.
- Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907-1914.
- Wang Y, Zhao Y, Ma S. Racial differences in six major subtypes of melanoma: descriptive epidemiology. BMC Cancer. 2016;16:691.
- Madankumar R, Gumaste PV, Martires K, et al. Acral melanocytic lesions in the United States: prevalence, awareness, and dermoscopic patterns in skin-of-color and non-Hispanic white patients. J Am Acad Dermatol. 2016;74:724.e1-730.e1.
- Palicka GA, Rhodes AR. Acral melanocytic nevi: prevalence and distribution of gross morphologic features in white and black adults. Arch Dermatol. 2010;146:1085-1094.
- Thomas L, Phan A, Pralong P, et al. Special locations dermoscopy: facial, acral, and nail. Dermatol Clin. 2013;31:615-624.
- Gong HZ, Zheng HY, Li J. Amelanotic melanoma [published online January 21, 2019]. Melanoma Res. doi:10.1097/CMR.0000000000000571.
- Ise M, Yasuda F, Konohana I, et al. Acral melanoma with hyperkeratosis mimicking a pigmented wart. Dermatol Pract Concept. 2013;3:37-39.
- Serarslan G, Akçaly CM, Atik E. Acral lentiginous melanoma misdiagnosed as tinea pedis: a case report. Int J Dermatol. 2004;43:37-38.
- Gumaste P, Penn L, Cohen N, et al. Acral lentiginous melanoma of the foot misdiagnosed as a traumatic ulcer. a cautionary case. J Am Podiatr Med Assoc. 2015;105:189-194.
- Carli P, de Giorgi V, Chiarugi A, et al. Addition of dermoscopy to conventional naked-eye examination in melanoma screening: a randomized study. J Am Acad Dermatol. 2004;50:683-689.
- Carli P, de Giorgi V, Crocetti E, et al. Improvement of malignant/benign ratio in excised melanocytic lesions in the ‘dermoscopy era’: a retrospective study 1997-2001. Br J Dermatol. 2004;150:687-692.
- Saida T, Koga H, Uhara H. Key points in dermoscopic differentiation between early acral melanoma and acral nevus. J Dermatol. 2011;38:25-34.
- Ishihara Y, Saida T, Miyazaki A, et al. Early acral melanoma in situ: correlation between the parallel ridge pattern on dermoscopy and microscopic features. Am J Dermatopathol. 2006;28:21-27.
- Saida T, Miyazaki A, Oguchi S, et al. Significance of dermoscopic patterns in detecting malignant melanoma on acral volar skin: results of a multicenter study in Japan. Arch Dermatol. 2004;140:1233-1238.
- Koga H, Saida T. Revised 3-step dermoscopic algorithm for the management of acral melanocytic lesions. Arch Dermatol. 2011;147:741-743.
- Lallas A, Sgouros D, Zalaudek I, et al. Palmar and plantar melanomas differ for sex prevalence and tumor thickness but not for dermoscopic patterns. Melanoma Res. 2014;24:83-87.
- Lallas A, Kyrgidis A, Koga H, et al. The BRAAFF checklist: a new dermoscopic algorithm for diagnosing acral melanoma. Br J Dermatol. 2015;173:1041-1049.
- Saida T, Koga H. Dermoscopic patterns of acral melanocytic nevi: their variations, changes, and significance. Arch Dermatol. 2007;143:1423-1426.
- Miyazaki A, Saida T, Koga H, et al. Anatomical and histopathological correlates of the dermoscopic patterns seen in melanocytic nevi on the sole: a retrospective study. J Am Acad Dermatol. 2005;53:230-236.
- Watanabe S, Sawada M, Ishizaki S, et al. Comparison of dermatoscopic images of acral lentiginous melanoma and acral melanocytic nevus occurring on body weight-bearing areas. Dermatol Pract Concept. 2014;4:47-50.
- Costello CM, Ghanavatian S, Temkit M, et al. Educational and practice gaps in the management of volar melanocytic lesions. J Eur Acad Dermatol Venereol. 2018;32:1450-1455.
- Alikhan A, Ibrahimi OA, Eisen DB. Congenital melanocytic nevi: where are we now? part I. clinical presentation, epidemiology, pathogenesis, histology, malignant transformation, and neurocutaneous melanosis. J Am Acad Dermatol. 2012;67:495.e1-495.e17; quiz 512-514.
- Minagawa A, Koga H, Saida T. Dermoscopic characteristics of congenital melanocytic nevi affecting acral volar skin. Arch Dermatol. 2011;147:809-813.
- Whiteman DC, Green AC, Olsen CM. The growing burden of invasive melanoma: projections of incidence rates and numbers of new cases in six susceptible populations through 2031. J Invest Dermatol. 2016;136:1161-1171.
- Bradford PT, Goldstein AM, McMaster ML, et al. Acral lentiginous melanoma: incidence and survival patterns in the United States, 1986-2005. Arch Dermatol. 2009;145:427-434.
- Nakamura Y, Fujisawa Y. Diagnosis and management of acral lentiginous melanoma. Curr Treat Options Oncol. 2018;19:42.
- Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907-1914.
- Wang Y, Zhao Y, Ma S. Racial differences in six major subtypes of melanoma: descriptive epidemiology. BMC Cancer. 2016;16:691.
- Madankumar R, Gumaste PV, Martires K, et al. Acral melanocytic lesions in the United States: prevalence, awareness, and dermoscopic patterns in skin-of-color and non-Hispanic white patients. J Am Acad Dermatol. 2016;74:724.e1-730.e1.
- Palicka GA, Rhodes AR. Acral melanocytic nevi: prevalence and distribution of gross morphologic features in white and black adults. Arch Dermatol. 2010;146:1085-1094.
- Thomas L, Phan A, Pralong P, et al. Special locations dermoscopy: facial, acral, and nail. Dermatol Clin. 2013;31:615-624.
- Gong HZ, Zheng HY, Li J. Amelanotic melanoma [published online January 21, 2019]. Melanoma Res. doi:10.1097/CMR.0000000000000571.
- Ise M, Yasuda F, Konohana I, et al. Acral melanoma with hyperkeratosis mimicking a pigmented wart. Dermatol Pract Concept. 2013;3:37-39.
- Serarslan G, Akçaly CM, Atik E. Acral lentiginous melanoma misdiagnosed as tinea pedis: a case report. Int J Dermatol. 2004;43:37-38.
- Gumaste P, Penn L, Cohen N, et al. Acral lentiginous melanoma of the foot misdiagnosed as a traumatic ulcer. a cautionary case. J Am Podiatr Med Assoc. 2015;105:189-194.
- Carli P, de Giorgi V, Chiarugi A, et al. Addition of dermoscopy to conventional naked-eye examination in melanoma screening: a randomized study. J Am Acad Dermatol. 2004;50:683-689.
- Carli P, de Giorgi V, Crocetti E, et al. Improvement of malignant/benign ratio in excised melanocytic lesions in the ‘dermoscopy era’: a retrospective study 1997-2001. Br J Dermatol. 2004;150:687-692.
- Saida T, Koga H, Uhara H. Key points in dermoscopic differentiation between early acral melanoma and acral nevus. J Dermatol. 2011;38:25-34.
- Ishihara Y, Saida T, Miyazaki A, et al. Early acral melanoma in situ: correlation between the parallel ridge pattern on dermoscopy and microscopic features. Am J Dermatopathol. 2006;28:21-27.
- Saida T, Miyazaki A, Oguchi S, et al. Significance of dermoscopic patterns in detecting malignant melanoma on acral volar skin: results of a multicenter study in Japan. Arch Dermatol. 2004;140:1233-1238.
- Koga H, Saida T. Revised 3-step dermoscopic algorithm for the management of acral melanocytic lesions. Arch Dermatol. 2011;147:741-743.
- Lallas A, Sgouros D, Zalaudek I, et al. Palmar and plantar melanomas differ for sex prevalence and tumor thickness but not for dermoscopic patterns. Melanoma Res. 2014;24:83-87.
- Lallas A, Kyrgidis A, Koga H, et al. The BRAAFF checklist: a new dermoscopic algorithm for diagnosing acral melanoma. Br J Dermatol. 2015;173:1041-1049.
- Saida T, Koga H. Dermoscopic patterns of acral melanocytic nevi: their variations, changes, and significance. Arch Dermatol. 2007;143:1423-1426.
- Miyazaki A, Saida T, Koga H, et al. Anatomical and histopathological correlates of the dermoscopic patterns seen in melanocytic nevi on the sole: a retrospective study. J Am Acad Dermatol. 2005;53:230-236.
- Watanabe S, Sawada M, Ishizaki S, et al. Comparison of dermatoscopic images of acral lentiginous melanoma and acral melanocytic nevus occurring on body weight-bearing areas. Dermatol Pract Concept. 2014;4:47-50.
- Costello CM, Ghanavatian S, Temkit M, et al. Educational and practice gaps in the management of volar melanocytic lesions. J Eur Acad Dermatol Venereol. 2018;32:1450-1455.
- Alikhan A, Ibrahimi OA, Eisen DB. Congenital melanocytic nevi: where are we now? part I. clinical presentation, epidemiology, pathogenesis, histology, malignant transformation, and neurocutaneous melanosis. J Am Acad Dermatol. 2012;67:495.e1-495.e17; quiz 512-514.
- Minagawa A, Koga H, Saida T. Dermoscopic characteristics of congenital melanocytic nevi affecting acral volar skin. Arch Dermatol. 2011;147:809-813.
Practice Points
- Dermatologists should be familiar with common dermoscopic patterns seen at acral sites in patients with skin of color as well as the most up-to-date diagnostic algorithms.
- Acral lentiginous melanoma should be strongly suspected if dermoscopy reveals a parallel ridge pattern or if dermoscopy of volar skin reveals a lack of typical dermoscopic patterns in lesions with a diameter greater than 7 mm.
The Dayanara Effect: Increasing Skin Cancer Awareness in the Hispanic Community
In February 2019, Dayanara Torres announced that she had been diagnosed with metastatic melanoma. Ms. Torres, a Puerto Rican–born former Miss Universe who has more than 1 million followers on Instagram (@dayanarapr), seemed an unlikely candidate for skin cancer, which often is associated with fair-skinned and light-eyed individuals. She shared the news of her diagnosis in an Instagram video that has now received more than 850,000 views. In the video, Ms. Torres described a new mole with uneven surface that had developed on her leg and noted that she had ignored it, even though it had been growing for years. Ultimately, she was diagnosed with melanoma that had already metastasized to regional lymph nodes in her leg. Ms. Torres concluded the video by urging fans and viewers to be mindful of new or changing skin lesions and to be aware of the seriousness of skin cancer. In March 2019, Ms. Torres posted a follow-up educational video on Instagram highlighting the features of melanoma that has now received more than 300,000 views.
Since her announcement, we have noticed that more Hispanic patients with concerns about skin cancer are presenting to our dermatology clinic, which is located in a highly diverse city (New Brunswick, New Jersey) with approximately 50% of residents identifying as Hispanic.1 Most Hispanic patients typically present to our dermatology clinic for non–skin cancer–related concerns, such as acne, rash, and dyschromia; however, following Ms. Torres’ announcement, many have cited her diagnosis of metastatic melanoma as a cause for concern and a motivating factor in having their skin examined. The diagnosis in a prominent celebrity and Hispanic woman has given a new face to metastatic melanoma.
Although melanoma most commonly occurs in white patients, Hispanic patients experience disproportionately greater morbidity and mortality when diagnosed with melanoma.2 Poor prognosis in patients with skin of color is multifactorial and may be due to poor use of sun protection, misconceptions about melanoma risk, atypical clinical presentation, impaired access to care, and delay in diagnosis. The Hispanic community encompasses a wide variety of individuals with varying levels of skin pigmentation and sun sensitivity.3 However, Hispanics report low levels of sun-protective behaviors. They also may have misconceptions that sunscreen is ineffective in preventing skin cancer and that little can be done to decrease the risk for developing skin cancer.4,5 Additionally, Hispanic patients often have lower perceptions of their personal risk for melanoma and report low rates of clinical and self-examinations compared to non-Hispanic white patients.6-8 Many Hispanic patients have reported that they were not instructed to perform self-examinations of their skin regularly by dermatologists or other providers and did not know the signs of skin cancer.7 Furthermore, a language barrier also may impede communication and education regarding melanoma risk.9
Similar to white patients, superficial spreading melanoma is the most common histologic subtype in Hispanic patients, followed by acral lentiginous melanoma, which is the most common subtype in black and Asian patients.2,4 Compared to non-Hispanic white patients, who most commonly present with truncal melanomas, Hispanic patients (particularly those from Puerto Rico, such as Ms. Torres) are more likely to present with melanoma on the lower extremities.4,10 Additionally, Hispanic patients have high rates of head, neck, and mucosal melanomas compared to all other racial and ethnic groups.2
Hispanic patients diagnosed with melanoma are more likely to present with thicker primary tumors, later stages of disease, and distant metastases compared to non-Hispanic white patients, all of which are associated with poor prognosis.2,4,11 Five-year survival rates for melanoma are lower in Hispanic patients compared to non-Hispanic white patients.12 Although the Hispanic community is diverse in socioeconomic and immigration status as well as occupation, lack of insurance also may contribute to decreased access to care, delayed diagnosis, and ultimately worse survival.
These disparities have spurred suggestions for increased education about skin cancer and the signs and symptoms of melanoma, encouragement of self-examinations, and routine clinical skin examinations for Hispanic patients by dermatologists and other providers.8 There is evidence that knowledge-based interventions, especially when presented in Spanish, produce statistically significant improvements in knowledge of skin cancer risk and sun-protective behavior among Hispanic patients.12 Similarly, we have observed that the videos shared by Ms. Torres regarding her melanoma diagnosis and the features of melanoma, in which she spoke in Spanish, have compelled many Hispanic patients to examine their own skin and have led to increased concern for skin cancer in this patient population. In our practice, we refer to the increase in spot checks and skin examinations requested by Hispanic patients as “The Dayanara Effect,” and we hypothesize that this same effect may be taking place throughout the dermatology community.
- New Brunswick, NJ. Data USA website. https://datausa.io/profile/geo/new-brunswick-nj. Accessed April 17, 2019.
- Higgins S, Nazemi A, Feinstein S, et al. Clinical presentations of melanoma in African Americans, Hispanics, and Asians [published online January 4, 2019]. Dermatol Surg. doi:10.1097/dss.0000000000001759.
- Robinson JK, Penedo FJ, Hay JL, et al. Recognizing Latinos’ range of skin pigment and phototypes to enhance skin cancer prevention [published online July 4, 2017]. Pigment Cell Melanoma Res. 2017;30:488-492.
- Garnett E, Townsend J, Steele B, et al. Characteristics, rates, and trends of melanoma incidence among Hispanics in the USA. Cancer Causes Control. 2016;27:647-659.
- Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public. J Am Acad Dermatol. 2014;70:748-762.
- Andreeva VA, Cockburn MG. Cutaneous melanoma and other skin cancer screening among Hispanics in the United States: a review of the evidence, disparities, and need for expanding the intervention and research agendas. Arch Dermatol. 2011;147:743-745.
- Roman C, Lugo-Somolinos A, Thomas N. Skin cancer knowledge and skin self-examinations in the Hispanic population of North Carolina: the patient’s perspective. JAMA Dermatol. 2013;149:103-104.
- Jaimes N, Oliveria S, Halpern A. A cautionary note on melanoma screening in the Hispanic/Latino population. JAMA Dermatol. 2013;149:396-397.
- Wich LG, Ma MW, Price LS, et al. Impact of socioeconomic status and sociodemographic factors on melanoma presentation among ethnic minorities. J Community Health. 2011;36:461-468.
- Rouhani P, Hu S, Kirsner RS. Melanoma in Hispanic and black Americans. Cancer Control. 2008;15:248-253.
- Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival. J Am Acad Dermatol. 2016;75:983-991.
- Kailas A, Botwin AL, Pritchett EN, et al. Assessing the effectiveness of knowledge-based interventions in increasing skin cancer awareness, knowledge, and protective behaviors in skin of color populations. Cutis. 2017;100:235-240.
In February 2019, Dayanara Torres announced that she had been diagnosed with metastatic melanoma. Ms. Torres, a Puerto Rican–born former Miss Universe who has more than 1 million followers on Instagram (@dayanarapr), seemed an unlikely candidate for skin cancer, which often is associated with fair-skinned and light-eyed individuals. She shared the news of her diagnosis in an Instagram video that has now received more than 850,000 views. In the video, Ms. Torres described a new mole with uneven surface that had developed on her leg and noted that she had ignored it, even though it had been growing for years. Ultimately, she was diagnosed with melanoma that had already metastasized to regional lymph nodes in her leg. Ms. Torres concluded the video by urging fans and viewers to be mindful of new or changing skin lesions and to be aware of the seriousness of skin cancer. In March 2019, Ms. Torres posted a follow-up educational video on Instagram highlighting the features of melanoma that has now received more than 300,000 views.
Since her announcement, we have noticed that more Hispanic patients with concerns about skin cancer are presenting to our dermatology clinic, which is located in a highly diverse city (New Brunswick, New Jersey) with approximately 50% of residents identifying as Hispanic.1 Most Hispanic patients typically present to our dermatology clinic for non–skin cancer–related concerns, such as acne, rash, and dyschromia; however, following Ms. Torres’ announcement, many have cited her diagnosis of metastatic melanoma as a cause for concern and a motivating factor in having their skin examined. The diagnosis in a prominent celebrity and Hispanic woman has given a new face to metastatic melanoma.
Although melanoma most commonly occurs in white patients, Hispanic patients experience disproportionately greater morbidity and mortality when diagnosed with melanoma.2 Poor prognosis in patients with skin of color is multifactorial and may be due to poor use of sun protection, misconceptions about melanoma risk, atypical clinical presentation, impaired access to care, and delay in diagnosis. The Hispanic community encompasses a wide variety of individuals with varying levels of skin pigmentation and sun sensitivity.3 However, Hispanics report low levels of sun-protective behaviors. They also may have misconceptions that sunscreen is ineffective in preventing skin cancer and that little can be done to decrease the risk for developing skin cancer.4,5 Additionally, Hispanic patients often have lower perceptions of their personal risk for melanoma and report low rates of clinical and self-examinations compared to non-Hispanic white patients.6-8 Many Hispanic patients have reported that they were not instructed to perform self-examinations of their skin regularly by dermatologists or other providers and did not know the signs of skin cancer.7 Furthermore, a language barrier also may impede communication and education regarding melanoma risk.9
Similar to white patients, superficial spreading melanoma is the most common histologic subtype in Hispanic patients, followed by acral lentiginous melanoma, which is the most common subtype in black and Asian patients.2,4 Compared to non-Hispanic white patients, who most commonly present with truncal melanomas, Hispanic patients (particularly those from Puerto Rico, such as Ms. Torres) are more likely to present with melanoma on the lower extremities.4,10 Additionally, Hispanic patients have high rates of head, neck, and mucosal melanomas compared to all other racial and ethnic groups.2
Hispanic patients diagnosed with melanoma are more likely to present with thicker primary tumors, later stages of disease, and distant metastases compared to non-Hispanic white patients, all of which are associated with poor prognosis.2,4,11 Five-year survival rates for melanoma are lower in Hispanic patients compared to non-Hispanic white patients.12 Although the Hispanic community is diverse in socioeconomic and immigration status as well as occupation, lack of insurance also may contribute to decreased access to care, delayed diagnosis, and ultimately worse survival.
These disparities have spurred suggestions for increased education about skin cancer and the signs and symptoms of melanoma, encouragement of self-examinations, and routine clinical skin examinations for Hispanic patients by dermatologists and other providers.8 There is evidence that knowledge-based interventions, especially when presented in Spanish, produce statistically significant improvements in knowledge of skin cancer risk and sun-protective behavior among Hispanic patients.12 Similarly, we have observed that the videos shared by Ms. Torres regarding her melanoma diagnosis and the features of melanoma, in which she spoke in Spanish, have compelled many Hispanic patients to examine their own skin and have led to increased concern for skin cancer in this patient population. In our practice, we refer to the increase in spot checks and skin examinations requested by Hispanic patients as “The Dayanara Effect,” and we hypothesize that this same effect may be taking place throughout the dermatology community.
In February 2019, Dayanara Torres announced that she had been diagnosed with metastatic melanoma. Ms. Torres, a Puerto Rican–born former Miss Universe who has more than 1 million followers on Instagram (@dayanarapr), seemed an unlikely candidate for skin cancer, which often is associated with fair-skinned and light-eyed individuals. She shared the news of her diagnosis in an Instagram video that has now received more than 850,000 views. In the video, Ms. Torres described a new mole with uneven surface that had developed on her leg and noted that she had ignored it, even though it had been growing for years. Ultimately, she was diagnosed with melanoma that had already metastasized to regional lymph nodes in her leg. Ms. Torres concluded the video by urging fans and viewers to be mindful of new or changing skin lesions and to be aware of the seriousness of skin cancer. In March 2019, Ms. Torres posted a follow-up educational video on Instagram highlighting the features of melanoma that has now received more than 300,000 views.
Since her announcement, we have noticed that more Hispanic patients with concerns about skin cancer are presenting to our dermatology clinic, which is located in a highly diverse city (New Brunswick, New Jersey) with approximately 50% of residents identifying as Hispanic.1 Most Hispanic patients typically present to our dermatology clinic for non–skin cancer–related concerns, such as acne, rash, and dyschromia; however, following Ms. Torres’ announcement, many have cited her diagnosis of metastatic melanoma as a cause for concern and a motivating factor in having their skin examined. The diagnosis in a prominent celebrity and Hispanic woman has given a new face to metastatic melanoma.
Although melanoma most commonly occurs in white patients, Hispanic patients experience disproportionately greater morbidity and mortality when diagnosed with melanoma.2 Poor prognosis in patients with skin of color is multifactorial and may be due to poor use of sun protection, misconceptions about melanoma risk, atypical clinical presentation, impaired access to care, and delay in diagnosis. The Hispanic community encompasses a wide variety of individuals with varying levels of skin pigmentation and sun sensitivity.3 However, Hispanics report low levels of sun-protective behaviors. They also may have misconceptions that sunscreen is ineffective in preventing skin cancer and that little can be done to decrease the risk for developing skin cancer.4,5 Additionally, Hispanic patients often have lower perceptions of their personal risk for melanoma and report low rates of clinical and self-examinations compared to non-Hispanic white patients.6-8 Many Hispanic patients have reported that they were not instructed to perform self-examinations of their skin regularly by dermatologists or other providers and did not know the signs of skin cancer.7 Furthermore, a language barrier also may impede communication and education regarding melanoma risk.9
Similar to white patients, superficial spreading melanoma is the most common histologic subtype in Hispanic patients, followed by acral lentiginous melanoma, which is the most common subtype in black and Asian patients.2,4 Compared to non-Hispanic white patients, who most commonly present with truncal melanomas, Hispanic patients (particularly those from Puerto Rico, such as Ms. Torres) are more likely to present with melanoma on the lower extremities.4,10 Additionally, Hispanic patients have high rates of head, neck, and mucosal melanomas compared to all other racial and ethnic groups.2
Hispanic patients diagnosed with melanoma are more likely to present with thicker primary tumors, later stages of disease, and distant metastases compared to non-Hispanic white patients, all of which are associated with poor prognosis.2,4,11 Five-year survival rates for melanoma are lower in Hispanic patients compared to non-Hispanic white patients.12 Although the Hispanic community is diverse in socioeconomic and immigration status as well as occupation, lack of insurance also may contribute to decreased access to care, delayed diagnosis, and ultimately worse survival.
These disparities have spurred suggestions for increased education about skin cancer and the signs and symptoms of melanoma, encouragement of self-examinations, and routine clinical skin examinations for Hispanic patients by dermatologists and other providers.8 There is evidence that knowledge-based interventions, especially when presented in Spanish, produce statistically significant improvements in knowledge of skin cancer risk and sun-protective behavior among Hispanic patients.12 Similarly, we have observed that the videos shared by Ms. Torres regarding her melanoma diagnosis and the features of melanoma, in which she spoke in Spanish, have compelled many Hispanic patients to examine their own skin and have led to increased concern for skin cancer in this patient population. In our practice, we refer to the increase in spot checks and skin examinations requested by Hispanic patients as “The Dayanara Effect,” and we hypothesize that this same effect may be taking place throughout the dermatology community.
- New Brunswick, NJ. Data USA website. https://datausa.io/profile/geo/new-brunswick-nj. Accessed April 17, 2019.
- Higgins S, Nazemi A, Feinstein S, et al. Clinical presentations of melanoma in African Americans, Hispanics, and Asians [published online January 4, 2019]. Dermatol Surg. doi:10.1097/dss.0000000000001759.
- Robinson JK, Penedo FJ, Hay JL, et al. Recognizing Latinos’ range of skin pigment and phototypes to enhance skin cancer prevention [published online July 4, 2017]. Pigment Cell Melanoma Res. 2017;30:488-492.
- Garnett E, Townsend J, Steele B, et al. Characteristics, rates, and trends of melanoma incidence among Hispanics in the USA. Cancer Causes Control. 2016;27:647-659.
- Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public. J Am Acad Dermatol. 2014;70:748-762.
- Andreeva VA, Cockburn MG. Cutaneous melanoma and other skin cancer screening among Hispanics in the United States: a review of the evidence, disparities, and need for expanding the intervention and research agendas. Arch Dermatol. 2011;147:743-745.
- Roman C, Lugo-Somolinos A, Thomas N. Skin cancer knowledge and skin self-examinations in the Hispanic population of North Carolina: the patient’s perspective. JAMA Dermatol. 2013;149:103-104.
- Jaimes N, Oliveria S, Halpern A. A cautionary note on melanoma screening in the Hispanic/Latino population. JAMA Dermatol. 2013;149:396-397.
- Wich LG, Ma MW, Price LS, et al. Impact of socioeconomic status and sociodemographic factors on melanoma presentation among ethnic minorities. J Community Health. 2011;36:461-468.
- Rouhani P, Hu S, Kirsner RS. Melanoma in Hispanic and black Americans. Cancer Control. 2008;15:248-253.
- Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival. J Am Acad Dermatol. 2016;75:983-991.
- Kailas A, Botwin AL, Pritchett EN, et al. Assessing the effectiveness of knowledge-based interventions in increasing skin cancer awareness, knowledge, and protective behaviors in skin of color populations. Cutis. 2017;100:235-240.
- New Brunswick, NJ. Data USA website. https://datausa.io/profile/geo/new-brunswick-nj. Accessed April 17, 2019.
- Higgins S, Nazemi A, Feinstein S, et al. Clinical presentations of melanoma in African Americans, Hispanics, and Asians [published online January 4, 2019]. Dermatol Surg. doi:10.1097/dss.0000000000001759.
- Robinson JK, Penedo FJ, Hay JL, et al. Recognizing Latinos’ range of skin pigment and phototypes to enhance skin cancer prevention [published online July 4, 2017]. Pigment Cell Melanoma Res. 2017;30:488-492.
- Garnett E, Townsend J, Steele B, et al. Characteristics, rates, and trends of melanoma incidence among Hispanics in the USA. Cancer Causes Control. 2016;27:647-659.
- Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public. J Am Acad Dermatol. 2014;70:748-762.
- Andreeva VA, Cockburn MG. Cutaneous melanoma and other skin cancer screening among Hispanics in the United States: a review of the evidence, disparities, and need for expanding the intervention and research agendas. Arch Dermatol. 2011;147:743-745.
- Roman C, Lugo-Somolinos A, Thomas N. Skin cancer knowledge and skin self-examinations in the Hispanic population of North Carolina: the patient’s perspective. JAMA Dermatol. 2013;149:103-104.
- Jaimes N, Oliveria S, Halpern A. A cautionary note on melanoma screening in the Hispanic/Latino population. JAMA Dermatol. 2013;149:396-397.
- Wich LG, Ma MW, Price LS, et al. Impact of socioeconomic status and sociodemographic factors on melanoma presentation among ethnic minorities. J Community Health. 2011;36:461-468.
- Rouhani P, Hu S, Kirsner RS. Melanoma in Hispanic and black Americans. Cancer Control. 2008;15:248-253.
- Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival. J Am Acad Dermatol. 2016;75:983-991.
- Kailas A, Botwin AL, Pritchett EN, et al. Assessing the effectiveness of knowledge-based interventions in increasing skin cancer awareness, knowledge, and protective behaviors in skin of color populations. Cutis. 2017;100:235-240.
Outpatient Management and Follow-up Recommendations for Adverse Drug Reactions: Guidelines for Posthospitalization Care
It has been estimated that 2 million serious adverse drug reactions (ADRs) occur annually in the United States, resulting in 100,000 deaths.1 Although the acute morbidity and mortality of these ADRs are readily apparent, postdischarge sequalae are critical aspects of a patient’s care. Herein, we present an approach to outpatient dermatologic follow-up of 3 ADRs: acute generalized exanthematous pustulosis (AGEP), drug rash with eosinophilia and systemic symptoms (DRESS) syndrome, and Stevens-Johnson syndrome (SJS)/toxic epidermal necrolysis (TEN). For these ADRs, the first step is prompt diagnosis and discontinuation of any potentially causative medications.
ACUTE GENERALIZED EXANTHEMATOUS PUSTULOSIS
Ninety percent of the time, AGEP is caused by medications, most commonly antibiotics, and less often it is caused by viruses.2-4 It presents as a cutaneous eruption with nonfollicular sterile pustules, fever, and leukocytosis, usually within 5 days after starting a causative medication.5 After stopping the medication, cutaneous findings generally improve within 1 week, and leukocytosis often resolves within 1 week.3
Notable Sequelae
Although AGEP typically is considered benign,2 there have been reports of severe sequelae including death from a systemic inflammatory response and complications such as bacterial superinfection and sepsis.6,7 Visceral involvement can be seen in up to 20% of AGEP patients, with systemic symptoms similar to those seen in DRESS syndrome. Mortality has been reported in up to 5% of cases, mainly in patients with comorbidities and notable mucosal involvement.8 More severe disease can be seen in patients with known dermatologic disease, as AGEP can provoke an isomorphic phenomenon.9 Laboratory alterations typically seen in AGEP include neutrophilia, eosinophilia, and elevated liver enzymes.2
Follow-up Recommendations
Patients should be informed of the expected timeline for resolution and should be counseled on the possibility of rare systemic symptoms. Laboratory abnormalities should be monitored every 2 to 4 weeks until normalized.
DRESS SYNDROME
DRESS syndrome is characterized by a morbilliform eruption that can be accompanied by fever; eosinophilia; purpura; facial edema; lymphadenopathy; and liver, renal, or other organ dysfunction. DRESS syndrome most often presents within 8 weeks of exposure to a causative drug.10,11 The most common causative agents are anticonvulsants, antimicrobials, and allopurinol.12 Treatment includes topical corticosteroids and systemic corticosteroids for internal organ involvement.10
Short-term Sequelae
Several potential sequelae may occur within 6 months of resolution of DRESS syndrome, resulting from both the ADR itself and/or systemic corticosteroids that often are required for treatment.13 Complications secondary to herpesviruses have been reported.14 Cases of cytomegalovirus-induced gastric ulcers can lead to gastrointestinal tract bleeds.15
Infections including Cryptococcus species and herpes zoster also have been reported.16 Patients, particularly those treated with systemic corticosteroids, should be monitored with close follow-up for infectious complications and treatment-related adverse effects.13
Long-term Sequelae
Endocrine
Thyroid gland abnormalities secondary to DRESS syndrome include Graves disease and Hashimoto disease as well as variations in biomarkers including elevated free thyroxine and low and elevated thyroid-stimulating hormone levels.16,17 Type 1 diabetes mellitus also has been seen after DRESS syndrome, developing within the first 10 months after onset with unknown pathogenesis.18
Autoimmune
Other reported sequelae of DRESS syndrome include elevated antinuclear antibodies with possible development into systemic lupus erythematosus, autoimmune hemolytic anemia, vitiligo, and rheumatoid arthritis.11,16 Symptoms may be exacerbated in patients with preexisting autoimmune diseases such as systemic lupus erythematosus and rheumatoid arthritis, and patients with preexisting renal disease are at an increased risk for requiring lifelong hemodialysis after DRESS syndrome.16
Other
Studies have demonstrated that pneumonia, thrombosis, and alopecia can be complications of DRESS syndrome.11,16 Psychiatric disturbances including fear of taking new medications, anxiety, and depression also have been reported.19 Children with DRESS syndrome may develop vitiligo, alopecia, sclerodermatous lesions, photophobia, uveitis, and Vogt-Koyanagi-Harada disease.17
Follow-up Recommendations
It is important to inform patients of both the potential short-term and long-term sequelae of DRESS syndrome, including those associated with treatment. A thorough review of systems should be performed at each visit, along with laboratory evaluation including a complete blood cell count with differential and liver function testing every 1 to 2 weeks after discharge until normalized, with monthly monitoring of glucose, thyroid-stimulating hormone, and free thyroxine levels for 3 months after discharge.
STEVENS-JOHNSON SYNDROME/TOXIC EPIDERMAL NECROLYSIS
Stevens-Johnson syndrome/toxic epidermal necrolysis are severe ADRs that present with dusky violaceous macules. Inciting medications include nonsteroidal anti-inflammatory drugs, allopurinol, antibiotics, and anticonvulsants, and symptoms begin 1 to 3 weeks after medication exposure.12 Initially, the lesions often begin on the trunk and can progress to full-body erythema and exfoliation with a necrotic epidermis and mucosal involvement.12,20
Notable Sequelae
Cutaneous
Chronic eczema can present at any time and can vary in severity in SJS/TEN patients.21 Xerosis and pruritus can be treated with emollients.11 Dyschromia is common. Hypertrophic and keloidal scarring can result from surgical debridement and are best prevented with the use of nonadherent dressings.22 Nail changes such as anonychia, dystrophy, longitudinal ridges, and pterygium also are seen, and topical steroids can be helpful. Other reported dermatologic sequelae include dyschromia and eruption of ectopic sebaceous glands.21,22
Ocular
Ocular sequelae include dry eyes, photophobia, symblepharon, corneal scarring, corneal neovascularization, corneal xerosis, trichiasis, reduced visual acuity, blindness, and subconjunctival fibrosis. The most common sequelae are bilateral conjunctivitis and corneal ulcerations.22,23 Early and regular ophthalmologic follow-up is recommended, as SJS/TEN-induced blindness can result from delayed therapy, destroying corneal stem cells.21 Amniotic membrane transplantation replaces the damaged corneal membrane, which may reduce corneal inflammation.24
Chronic dry eye syndrome can recur for years after SJS/TEN resolves and progresses over time.22 Frequent use of nonpreserved artificial tears and salivary gland transplantation can be helpful.24 Unfortunately, ocular disease may develop months after discharge; therefore, it is recommended that dermatologists ask all SJS/TEN patients about ocular symptoms in follow-up visits. If ocular involvement was present initially, patients should be followed by ophthalmology for at least 1 year after discharge.23
Genitourinary
Genitourinary sequelae in SJS/TEN include adhesions, particularly in the female urethra and vaginal opening; vaginal adenosis; vulvovaginal endometriosis; and persistent genital ulcerations most commonly reported in females.22 Prompt inpatient gynecologic or urologic consultation is critical to reduce these potentially permanent outcomes. Topical corticosteroid therapy is recommended in the acute phase.22
Psychologic
Posttraumatic stress disorder may occur in patients with SJS/TEN. One study showed that 23% (7/30) of patients had posttraumatic stress disorder 6 months after hospitalization for SJS/TEN. The investigators recommended routine psychiatric assessment in the acute disease period and for at least 1 year after discharge.25
Pulmonary, Gastrointestinal, and Renal
Interstitial pneumonia and obliterative bronchitis/bronchiolitis can be caused by SJS/TEN. Interstitial pneumonia tends to occur during the acute course, while obliterative airway disease manifests after resolution of SJS/TEN.21,22 Abnormal pulmonary function testing can be seen in more than half of SJS/TEN patients 2 months after the ADR.22 Gastrointestinal sequelae include esophageal strictures, intestinal ulceration, and cholestasis.22 Renal sequelae include acute kidney injury and glomerulonephritis, which may be secondary to the volume loss seen in SJS/TEN but may be irreversible.21
Special Populations
A correlation with infertility in women has been documented in patients with SJS/TEN; thus, follow-up with obstetrics and gynecology is recommended in women of child-bearing potential. The most considerable risk in pregnant women with SJS/TEN is premature birth, and mucosal necrosis of SJS/TEN can impair vaginal delivery.26 Antiretrovirals can be a cause of SJS/TEN in the human immunodeficiency virus–positive population.27 In those cases, it is best to discontinue the medication and find an alternative.
Risk factors for children can be different and can include viral and febrile illnesses as well as mycoplasma infection.28 Children also can be at an increased risk for poor ocular outcomes, such as permanent deficiency in visual acuity and blindness.29
Follow-up Recommendations
Patients should be counseled regarding sequelae and the multisystem nature of SJS/TEN. Inpatient referrals should be given as needed. It is important to watch for ocular symptoms for 1 year after SJS/TEN resolution. When ocular involvement is present, follow-up with ophthalmology is recommended within 1 month of discharge and then at the discretion of the ophthalmologist. Pulmonary function should be monitored for 1 year after SJS/TEN, starting 1 month after discharge and then at the discretion of the pulmonologist. Patients also should be screened for psychologic sequelae for at least 1 year after discharge.
FINAL THOUGHTS
Adverse drug reactions are notable causes of inpatient hospitalization and may lead to considerable sequelae. These ADRs range in severity from more common and benign maculopapular exanthems to severe multiorgan ADRs such as DRESS syndrome and SJS/TEN.
In AGEP, it is important to monitor patients with preexisting dermatologic diseases and to screen for visceral involvement. DRESS syndrome has the potential to cause immune dysregulation and variable long-term adverse sequelae, both from the disease itself and from corticosteroid therapy. Mucocutaneous sequelae of SJS/TEN can potentially affect a patient’s cutaneous, ocular, genitourinary, mental, pulmonary, gastrointestinal, and renal health.
The baseline recommendations provided here warrant more frequent monitoring if the findings and symptoms are severe. In all of these cases, if a causative medication is identified, it should be added to the patient’s allergy list and the patient should be counseled extensively to avoid this medication and other medications in the same class. If a single agent cannot be identified, referrals for patch testing may be of some utility, particularly in AGEP and DRESS syndrome.30,31
- Preventable adverse drug reactions: a focus on drug interactions. US Food and Drug Administration website. https://www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/DrugInteractionsLabeling/ucm110632.htm. Updated March 6, 2018. Accessed April 12, 2019.
- Thienvibul C, Vachiramon V, Chanprapaph K. Five-year retrospective review of acute generalized exanthematous pustulosis. Dermatol Res Pract. 2015;3:1-8.
- Lee HY, Chou D, Pang SM, et al. Acute generalized exanthematous pustulosis: analysis of cases managed in a tertiary hospital in Singapore. Int J Dermatol. 2010
;49:507-512. - Ropars N, Darrieux L, Tisseau L, et al. Acute generalized exanthematous pustulosis associated with primary Epstein-Barr virus infection. JAAD Case Rep. 2014;1:9-11.
- Hattem S, Beerthuizen G, Kardaun S. Severe flucloxacillin‐induced acute generalized exanthematous pustulosis (AGEP), with toxic epidermal necrolysis (TEN)‐like features: does overlap between AGEP and TEN exist? clinical report and review of the literature. Br J Dermatol. 2014;171:1539-1545.
- Tajmir-Riahi A, Wörl P, Harrer T, et al. Life-threatening atypical case of acute generalized exanthematous pustulosis. Int Arch Allergy Immunol. 2017;174:108-111.
- Feldmeyer L, Heidemeyer K, Yawalkar N. Acute generalized exanthematous pustulosis: pathogenesis, genetic background, clinical variants and therapy. Int J Mol Sci. 2016;17:E1214.
- Szatkowski J, Schwartz RA. Acute generalized exanthematous pustulosis (AGEP). a review and update. J Am Acad Dermatol. 2015;73:843-848.
- Totonchy MB, McNiff JM, Bunick CG. Koebnerization of Hailey-Hailey disease into a cutaneous drug eruption of acute generalized exanthematous pustulosis associated with systemic symptoms. J Cutan Pathol. 2016;43:1031-1035.
- Husain Z, Reddy BY, Schwartz RA. DRESS syndrome: part II. management and therapeutics. J Am Acad Dermatol. 2013;68:709.e1-e9; quiz 718-720.
- Kano Y, Shiohara T. Long-term outcome of patients with severe cutaneous adverse reactions. Dermatologica Sinica. 2013;31:211-216.
- Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. Vol 1. Philadelphia, PA: Elsevier Saunders; 2012.
- Ushigome Y, Kano Y, Ishida T, et al. Short- and long-term outcomes of 34 patients with drug-induced hypersensitivity syndrome in a single institution. J Am Acad Dermatol. 2013;68:721-728.
- Ljungman P, Wang FZ, Clark DA, et al. High levels of human herpesvirus 6 DNA in peripheral blood leucocytes are correlated to platelet engraftment and disease in allogeneic stem cell transplant patients. Br J Haematol. 2000;111:774-781.
- Asano Y, Kagawa H, Kano Y, et al. Cytomegalovirus disease during severe drug eruptions: report of 2 cases and retrospective study of 18 patients with drug-induced hypersensitivity syndrome. Arch Dermatol. 2009;145:1030-1036.
- Kano Y , Tohyama M, Aihara M, et al. Sequelae in 145 patients with drug‐induced hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms: survey conducted by the Asian Research Committee on Severe Cutaneous Adverse Reactions (ASCAR). J Dermatol. 2015;42:276-282.
- Morita C, Yanase T, Shiohara T, et al. Aggressive treatment in paediatric or young patients with drug-induced hypersensitivity syndrome (DiHS)/ drug reaction with eosinophilia and systemic symptoms (DRESS) is associated with future development of type III polyglandular autoimmune syndrome [published online October 27, 2018]. BMJ Case Rep. doi:10.1136/bcr-2018-225528.
- Chiang A, Shiu J, Elsensohn AN, et al. Classic autoimmune type 1 diabetes mellitus after a case of drug reaction with eosinophilia and systemic symptoms (DRESS). JAAD Case Rep. 2018;4:295-297.
- Lew TT, Creamer D, Mackenzie J, et al. Post-traumatic stress disorder following drug reaction with eosinophilia and systemic symptoms. Br J Dermatol. 2015;172:836-837.
- Kumar R, Das A, Das S. Management of Stevens-Johnson syndrome-toxic epidermal necrolysis: looking beyond guidelines! Indian J Dermatol. 2018;63:117-124.
- Yang CW, Cho YT, Chen KL, et al. Long-term sequelae of Stevens-Johnson syndrome/toxic epidermal necrolysis. Acta Derm Venereol. 2016;96:525-529.
- Lee HY, Walsh SA, Creamer D. Long‐term complications of Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN): the spectrum of chronic problems in patients who survive an episode of SJS/TEN necessitates multidisciplinary follow‐up. Br J Dermatol. 2017;177:924-935.
- Hsu M, Jayaram A, Verner R, et al. Indications and outcomes of amniotic membrane transplantation in the management of acute Stevens-Johnson syndrome and toxic epidermal necrolysis: a case-control study. Cornea. 2012;31:1394-1402.
- Sant’ Anna AE, Hazarbassanov RM, de Freitas D, et al. Minor salivary glands and labial mucous membrane graft in the treatment of severe symblepharon and dry eye in patients with Stevens-Johnson syndrome. Br J Ophthalmol. 2012;96:234-239.
- Hefez L, Zaghbib K, Sbidian E, et al. Post-traumatic stress disorder in Stevens-Johnson syndrome and toxic epidermal necrolysis: prevalence and risk factors. a prospective study of 31 patients [published online October 3, 2018]. Br J Dermatol. doi:10.1111/bjd.17267.
- Knight L, Todd G, Muloiwa R, et al. Stevens Johnson syndrome and toxic epidermal necrolysis: maternal and foetal outcomes intwenty-two consecutive pregnant HIV infected women [published online August 12, 2015]. PLoS One. doi:10.1371/journal.pone.0135501.
- Tchetnya X, Ngwasiri CA, Munge T, et al. Severe eye complications from toxic epidermal necrolysis following initiation of nevirapine based HAART regimen in a child with HIV infection: a case from Cameroon. BMC Pediatr. 2018;18:108.
- Antoon JW, Goldman JL, Lee B, et al. Incidence, outcomes, and resource use in children with Stevens-Johnson syndrome and toxic epidermal necrolysis. Pediatr Dermatol. 2018;35:182-187.
- Basu S, Shanbhag SS, Gokani A, et al. Chronic ocular sequelae of Stevens-Johnson syndrome in children: long-term impact of appropriate therapy on natural history of disease. Am J Ophthalmol. 2018;189:17-28.
- Pinho A, Marta A, Coutinho I, et al. Long‐term reproducibility of positive patch test reactions in patients with non‐immediate cutaneous adverse drug reactions to antibiotics. Contact Dermatitis. 2017;76:204-209.
- Barbaud A, Collet E, Milpied B, et al. A multicentre study to determine the value and safety of drug patch tests for the three main classes of severe cutaneous adverse drug reactions. Br J Dermatol. 2013;168:555-562.
It has been estimated that 2 million serious adverse drug reactions (ADRs) occur annually in the United States, resulting in 100,000 deaths.1 Although the acute morbidity and mortality of these ADRs are readily apparent, postdischarge sequalae are critical aspects of a patient’s care. Herein, we present an approach to outpatient dermatologic follow-up of 3 ADRs: acute generalized exanthematous pustulosis (AGEP), drug rash with eosinophilia and systemic symptoms (DRESS) syndrome, and Stevens-Johnson syndrome (SJS)/toxic epidermal necrolysis (TEN). For these ADRs, the first step is prompt diagnosis and discontinuation of any potentially causative medications.
ACUTE GENERALIZED EXANTHEMATOUS PUSTULOSIS
Ninety percent of the time, AGEP is caused by medications, most commonly antibiotics, and less often it is caused by viruses.2-4 It presents as a cutaneous eruption with nonfollicular sterile pustules, fever, and leukocytosis, usually within 5 days after starting a causative medication.5 After stopping the medication, cutaneous findings generally improve within 1 week, and leukocytosis often resolves within 1 week.3
Notable Sequelae
Although AGEP typically is considered benign,2 there have been reports of severe sequelae including death from a systemic inflammatory response and complications such as bacterial superinfection and sepsis.6,7 Visceral involvement can be seen in up to 20% of AGEP patients, with systemic symptoms similar to those seen in DRESS syndrome. Mortality has been reported in up to 5% of cases, mainly in patients with comorbidities and notable mucosal involvement.8 More severe disease can be seen in patients with known dermatologic disease, as AGEP can provoke an isomorphic phenomenon.9 Laboratory alterations typically seen in AGEP include neutrophilia, eosinophilia, and elevated liver enzymes.2
Follow-up Recommendations
Patients should be informed of the expected timeline for resolution and should be counseled on the possibility of rare systemic symptoms. Laboratory abnormalities should be monitored every 2 to 4 weeks until normalized.
DRESS SYNDROME
DRESS syndrome is characterized by a morbilliform eruption that can be accompanied by fever; eosinophilia; purpura; facial edema; lymphadenopathy; and liver, renal, or other organ dysfunction. DRESS syndrome most often presents within 8 weeks of exposure to a causative drug.10,11 The most common causative agents are anticonvulsants, antimicrobials, and allopurinol.12 Treatment includes topical corticosteroids and systemic corticosteroids for internal organ involvement.10
Short-term Sequelae
Several potential sequelae may occur within 6 months of resolution of DRESS syndrome, resulting from both the ADR itself and/or systemic corticosteroids that often are required for treatment.13 Complications secondary to herpesviruses have been reported.14 Cases of cytomegalovirus-induced gastric ulcers can lead to gastrointestinal tract bleeds.15
Infections including Cryptococcus species and herpes zoster also have been reported.16 Patients, particularly those treated with systemic corticosteroids, should be monitored with close follow-up for infectious complications and treatment-related adverse effects.13
Long-term Sequelae
Endocrine
Thyroid gland abnormalities secondary to DRESS syndrome include Graves disease and Hashimoto disease as well as variations in biomarkers including elevated free thyroxine and low and elevated thyroid-stimulating hormone levels.16,17 Type 1 diabetes mellitus also has been seen after DRESS syndrome, developing within the first 10 months after onset with unknown pathogenesis.18
Autoimmune
Other reported sequelae of DRESS syndrome include elevated antinuclear antibodies with possible development into systemic lupus erythematosus, autoimmune hemolytic anemia, vitiligo, and rheumatoid arthritis.11,16 Symptoms may be exacerbated in patients with preexisting autoimmune diseases such as systemic lupus erythematosus and rheumatoid arthritis, and patients with preexisting renal disease are at an increased risk for requiring lifelong hemodialysis after DRESS syndrome.16
Other
Studies have demonstrated that pneumonia, thrombosis, and alopecia can be complications of DRESS syndrome.11,16 Psychiatric disturbances including fear of taking new medications, anxiety, and depression also have been reported.19 Children with DRESS syndrome may develop vitiligo, alopecia, sclerodermatous lesions, photophobia, uveitis, and Vogt-Koyanagi-Harada disease.17
Follow-up Recommendations
It is important to inform patients of both the potential short-term and long-term sequelae of DRESS syndrome, including those associated with treatment. A thorough review of systems should be performed at each visit, along with laboratory evaluation including a complete blood cell count with differential and liver function testing every 1 to 2 weeks after discharge until normalized, with monthly monitoring of glucose, thyroid-stimulating hormone, and free thyroxine levels for 3 months after discharge.
STEVENS-JOHNSON SYNDROME/TOXIC EPIDERMAL NECROLYSIS
Stevens-Johnson syndrome/toxic epidermal necrolysis are severe ADRs that present with dusky violaceous macules. Inciting medications include nonsteroidal anti-inflammatory drugs, allopurinol, antibiotics, and anticonvulsants, and symptoms begin 1 to 3 weeks after medication exposure.12 Initially, the lesions often begin on the trunk and can progress to full-body erythema and exfoliation with a necrotic epidermis and mucosal involvement.12,20
Notable Sequelae
Cutaneous
Chronic eczema can present at any time and can vary in severity in SJS/TEN patients.21 Xerosis and pruritus can be treated with emollients.11 Dyschromia is common. Hypertrophic and keloidal scarring can result from surgical debridement and are best prevented with the use of nonadherent dressings.22 Nail changes such as anonychia, dystrophy, longitudinal ridges, and pterygium also are seen, and topical steroids can be helpful. Other reported dermatologic sequelae include dyschromia and eruption of ectopic sebaceous glands.21,22
Ocular
Ocular sequelae include dry eyes, photophobia, symblepharon, corneal scarring, corneal neovascularization, corneal xerosis, trichiasis, reduced visual acuity, blindness, and subconjunctival fibrosis. The most common sequelae are bilateral conjunctivitis and corneal ulcerations.22,23 Early and regular ophthalmologic follow-up is recommended, as SJS/TEN-induced blindness can result from delayed therapy, destroying corneal stem cells.21 Amniotic membrane transplantation replaces the damaged corneal membrane, which may reduce corneal inflammation.24
Chronic dry eye syndrome can recur for years after SJS/TEN resolves and progresses over time.22 Frequent use of nonpreserved artificial tears and salivary gland transplantation can be helpful.24 Unfortunately, ocular disease may develop months after discharge; therefore, it is recommended that dermatologists ask all SJS/TEN patients about ocular symptoms in follow-up visits. If ocular involvement was present initially, patients should be followed by ophthalmology for at least 1 year after discharge.23
Genitourinary
Genitourinary sequelae in SJS/TEN include adhesions, particularly in the female urethra and vaginal opening; vaginal adenosis; vulvovaginal endometriosis; and persistent genital ulcerations most commonly reported in females.22 Prompt inpatient gynecologic or urologic consultation is critical to reduce these potentially permanent outcomes. Topical corticosteroid therapy is recommended in the acute phase.22
Psychologic
Posttraumatic stress disorder may occur in patients with SJS/TEN. One study showed that 23% (7/30) of patients had posttraumatic stress disorder 6 months after hospitalization for SJS/TEN. The investigators recommended routine psychiatric assessment in the acute disease period and for at least 1 year after discharge.25
Pulmonary, Gastrointestinal, and Renal
Interstitial pneumonia and obliterative bronchitis/bronchiolitis can be caused by SJS/TEN. Interstitial pneumonia tends to occur during the acute course, while obliterative airway disease manifests after resolution of SJS/TEN.21,22 Abnormal pulmonary function testing can be seen in more than half of SJS/TEN patients 2 months after the ADR.22 Gastrointestinal sequelae include esophageal strictures, intestinal ulceration, and cholestasis.22 Renal sequelae include acute kidney injury and glomerulonephritis, which may be secondary to the volume loss seen in SJS/TEN but may be irreversible.21
Special Populations
A correlation with infertility in women has been documented in patients with SJS/TEN; thus, follow-up with obstetrics and gynecology is recommended in women of child-bearing potential. The most considerable risk in pregnant women with SJS/TEN is premature birth, and mucosal necrosis of SJS/TEN can impair vaginal delivery.26 Antiretrovirals can be a cause of SJS/TEN in the human immunodeficiency virus–positive population.27 In those cases, it is best to discontinue the medication and find an alternative.
Risk factors for children can be different and can include viral and febrile illnesses as well as mycoplasma infection.28 Children also can be at an increased risk for poor ocular outcomes, such as permanent deficiency in visual acuity and blindness.29
Follow-up Recommendations
Patients should be counseled regarding sequelae and the multisystem nature of SJS/TEN. Inpatient referrals should be given as needed. It is important to watch for ocular symptoms for 1 year after SJS/TEN resolution. When ocular involvement is present, follow-up with ophthalmology is recommended within 1 month of discharge and then at the discretion of the ophthalmologist. Pulmonary function should be monitored for 1 year after SJS/TEN, starting 1 month after discharge and then at the discretion of the pulmonologist. Patients also should be screened for psychologic sequelae for at least 1 year after discharge.
FINAL THOUGHTS
Adverse drug reactions are notable causes of inpatient hospitalization and may lead to considerable sequelae. These ADRs range in severity from more common and benign maculopapular exanthems to severe multiorgan ADRs such as DRESS syndrome and SJS/TEN.
In AGEP, it is important to monitor patients with preexisting dermatologic diseases and to screen for visceral involvement. DRESS syndrome has the potential to cause immune dysregulation and variable long-term adverse sequelae, both from the disease itself and from corticosteroid therapy. Mucocutaneous sequelae of SJS/TEN can potentially affect a patient’s cutaneous, ocular, genitourinary, mental, pulmonary, gastrointestinal, and renal health.
The baseline recommendations provided here warrant more frequent monitoring if the findings and symptoms are severe. In all of these cases, if a causative medication is identified, it should be added to the patient’s allergy list and the patient should be counseled extensively to avoid this medication and other medications in the same class. If a single agent cannot be identified, referrals for patch testing may be of some utility, particularly in AGEP and DRESS syndrome.30,31
It has been estimated that 2 million serious adverse drug reactions (ADRs) occur annually in the United States, resulting in 100,000 deaths.1 Although the acute morbidity and mortality of these ADRs are readily apparent, postdischarge sequalae are critical aspects of a patient’s care. Herein, we present an approach to outpatient dermatologic follow-up of 3 ADRs: acute generalized exanthematous pustulosis (AGEP), drug rash with eosinophilia and systemic symptoms (DRESS) syndrome, and Stevens-Johnson syndrome (SJS)/toxic epidermal necrolysis (TEN). For these ADRs, the first step is prompt diagnosis and discontinuation of any potentially causative medications.
ACUTE GENERALIZED EXANTHEMATOUS PUSTULOSIS
Ninety percent of the time, AGEP is caused by medications, most commonly antibiotics, and less often it is caused by viruses.2-4 It presents as a cutaneous eruption with nonfollicular sterile pustules, fever, and leukocytosis, usually within 5 days after starting a causative medication.5 After stopping the medication, cutaneous findings generally improve within 1 week, and leukocytosis often resolves within 1 week.3
Notable Sequelae
Although AGEP typically is considered benign,2 there have been reports of severe sequelae including death from a systemic inflammatory response and complications such as bacterial superinfection and sepsis.6,7 Visceral involvement can be seen in up to 20% of AGEP patients, with systemic symptoms similar to those seen in DRESS syndrome. Mortality has been reported in up to 5% of cases, mainly in patients with comorbidities and notable mucosal involvement.8 More severe disease can be seen in patients with known dermatologic disease, as AGEP can provoke an isomorphic phenomenon.9 Laboratory alterations typically seen in AGEP include neutrophilia, eosinophilia, and elevated liver enzymes.2
Follow-up Recommendations
Patients should be informed of the expected timeline for resolution and should be counseled on the possibility of rare systemic symptoms. Laboratory abnormalities should be monitored every 2 to 4 weeks until normalized.
DRESS SYNDROME
DRESS syndrome is characterized by a morbilliform eruption that can be accompanied by fever; eosinophilia; purpura; facial edema; lymphadenopathy; and liver, renal, or other organ dysfunction. DRESS syndrome most often presents within 8 weeks of exposure to a causative drug.10,11 The most common causative agents are anticonvulsants, antimicrobials, and allopurinol.12 Treatment includes topical corticosteroids and systemic corticosteroids for internal organ involvement.10
Short-term Sequelae
Several potential sequelae may occur within 6 months of resolution of DRESS syndrome, resulting from both the ADR itself and/or systemic corticosteroids that often are required for treatment.13 Complications secondary to herpesviruses have been reported.14 Cases of cytomegalovirus-induced gastric ulcers can lead to gastrointestinal tract bleeds.15
Infections including Cryptococcus species and herpes zoster also have been reported.16 Patients, particularly those treated with systemic corticosteroids, should be monitored with close follow-up for infectious complications and treatment-related adverse effects.13
Long-term Sequelae
Endocrine
Thyroid gland abnormalities secondary to DRESS syndrome include Graves disease and Hashimoto disease as well as variations in biomarkers including elevated free thyroxine and low and elevated thyroid-stimulating hormone levels.16,17 Type 1 diabetes mellitus also has been seen after DRESS syndrome, developing within the first 10 months after onset with unknown pathogenesis.18
Autoimmune
Other reported sequelae of DRESS syndrome include elevated antinuclear antibodies with possible development into systemic lupus erythematosus, autoimmune hemolytic anemia, vitiligo, and rheumatoid arthritis.11,16 Symptoms may be exacerbated in patients with preexisting autoimmune diseases such as systemic lupus erythematosus and rheumatoid arthritis, and patients with preexisting renal disease are at an increased risk for requiring lifelong hemodialysis after DRESS syndrome.16
Other
Studies have demonstrated that pneumonia, thrombosis, and alopecia can be complications of DRESS syndrome.11,16 Psychiatric disturbances including fear of taking new medications, anxiety, and depression also have been reported.19 Children with DRESS syndrome may develop vitiligo, alopecia, sclerodermatous lesions, photophobia, uveitis, and Vogt-Koyanagi-Harada disease.17
Follow-up Recommendations
It is important to inform patients of both the potential short-term and long-term sequelae of DRESS syndrome, including those associated with treatment. A thorough review of systems should be performed at each visit, along with laboratory evaluation including a complete blood cell count with differential and liver function testing every 1 to 2 weeks after discharge until normalized, with monthly monitoring of glucose, thyroid-stimulating hormone, and free thyroxine levels for 3 months after discharge.
STEVENS-JOHNSON SYNDROME/TOXIC EPIDERMAL NECROLYSIS
Stevens-Johnson syndrome/toxic epidermal necrolysis are severe ADRs that present with dusky violaceous macules. Inciting medications include nonsteroidal anti-inflammatory drugs, allopurinol, antibiotics, and anticonvulsants, and symptoms begin 1 to 3 weeks after medication exposure.12 Initially, the lesions often begin on the trunk and can progress to full-body erythema and exfoliation with a necrotic epidermis and mucosal involvement.12,20
Notable Sequelae
Cutaneous
Chronic eczema can present at any time and can vary in severity in SJS/TEN patients.21 Xerosis and pruritus can be treated with emollients.11 Dyschromia is common. Hypertrophic and keloidal scarring can result from surgical debridement and are best prevented with the use of nonadherent dressings.22 Nail changes such as anonychia, dystrophy, longitudinal ridges, and pterygium also are seen, and topical steroids can be helpful. Other reported dermatologic sequelae include dyschromia and eruption of ectopic sebaceous glands.21,22
Ocular
Ocular sequelae include dry eyes, photophobia, symblepharon, corneal scarring, corneal neovascularization, corneal xerosis, trichiasis, reduced visual acuity, blindness, and subconjunctival fibrosis. The most common sequelae are bilateral conjunctivitis and corneal ulcerations.22,23 Early and regular ophthalmologic follow-up is recommended, as SJS/TEN-induced blindness can result from delayed therapy, destroying corneal stem cells.21 Amniotic membrane transplantation replaces the damaged corneal membrane, which may reduce corneal inflammation.24
Chronic dry eye syndrome can recur for years after SJS/TEN resolves and progresses over time.22 Frequent use of nonpreserved artificial tears and salivary gland transplantation can be helpful.24 Unfortunately, ocular disease may develop months after discharge; therefore, it is recommended that dermatologists ask all SJS/TEN patients about ocular symptoms in follow-up visits. If ocular involvement was present initially, patients should be followed by ophthalmology for at least 1 year after discharge.23
Genitourinary
Genitourinary sequelae in SJS/TEN include adhesions, particularly in the female urethra and vaginal opening; vaginal adenosis; vulvovaginal endometriosis; and persistent genital ulcerations most commonly reported in females.22 Prompt inpatient gynecologic or urologic consultation is critical to reduce these potentially permanent outcomes. Topical corticosteroid therapy is recommended in the acute phase.22
Psychologic
Posttraumatic stress disorder may occur in patients with SJS/TEN. One study showed that 23% (7/30) of patients had posttraumatic stress disorder 6 months after hospitalization for SJS/TEN. The investigators recommended routine psychiatric assessment in the acute disease period and for at least 1 year after discharge.25
Pulmonary, Gastrointestinal, and Renal
Interstitial pneumonia and obliterative bronchitis/bronchiolitis can be caused by SJS/TEN. Interstitial pneumonia tends to occur during the acute course, while obliterative airway disease manifests after resolution of SJS/TEN.21,22 Abnormal pulmonary function testing can be seen in more than half of SJS/TEN patients 2 months after the ADR.22 Gastrointestinal sequelae include esophageal strictures, intestinal ulceration, and cholestasis.22 Renal sequelae include acute kidney injury and glomerulonephritis, which may be secondary to the volume loss seen in SJS/TEN but may be irreversible.21
Special Populations
A correlation with infertility in women has been documented in patients with SJS/TEN; thus, follow-up with obstetrics and gynecology is recommended in women of child-bearing potential. The most considerable risk in pregnant women with SJS/TEN is premature birth, and mucosal necrosis of SJS/TEN can impair vaginal delivery.26 Antiretrovirals can be a cause of SJS/TEN in the human immunodeficiency virus–positive population.27 In those cases, it is best to discontinue the medication and find an alternative.
Risk factors for children can be different and can include viral and febrile illnesses as well as mycoplasma infection.28 Children also can be at an increased risk for poor ocular outcomes, such as permanent deficiency in visual acuity and blindness.29
Follow-up Recommendations
Patients should be counseled regarding sequelae and the multisystem nature of SJS/TEN. Inpatient referrals should be given as needed. It is important to watch for ocular symptoms for 1 year after SJS/TEN resolution. When ocular involvement is present, follow-up with ophthalmology is recommended within 1 month of discharge and then at the discretion of the ophthalmologist. Pulmonary function should be monitored for 1 year after SJS/TEN, starting 1 month after discharge and then at the discretion of the pulmonologist. Patients also should be screened for psychologic sequelae for at least 1 year after discharge.
FINAL THOUGHTS
Adverse drug reactions are notable causes of inpatient hospitalization and may lead to considerable sequelae. These ADRs range in severity from more common and benign maculopapular exanthems to severe multiorgan ADRs such as DRESS syndrome and SJS/TEN.
In AGEP, it is important to monitor patients with preexisting dermatologic diseases and to screen for visceral involvement. DRESS syndrome has the potential to cause immune dysregulation and variable long-term adverse sequelae, both from the disease itself and from corticosteroid therapy. Mucocutaneous sequelae of SJS/TEN can potentially affect a patient’s cutaneous, ocular, genitourinary, mental, pulmonary, gastrointestinal, and renal health.
The baseline recommendations provided here warrant more frequent monitoring if the findings and symptoms are severe. In all of these cases, if a causative medication is identified, it should be added to the patient’s allergy list and the patient should be counseled extensively to avoid this medication and other medications in the same class. If a single agent cannot be identified, referrals for patch testing may be of some utility, particularly in AGEP and DRESS syndrome.30,31
- Preventable adverse drug reactions: a focus on drug interactions. US Food and Drug Administration website. https://www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/DrugInteractionsLabeling/ucm110632.htm. Updated March 6, 2018. Accessed April 12, 2019.
- Thienvibul C, Vachiramon V, Chanprapaph K. Five-year retrospective review of acute generalized exanthematous pustulosis. Dermatol Res Pract. 2015;3:1-8.
- Lee HY, Chou D, Pang SM, et al. Acute generalized exanthematous pustulosis: analysis of cases managed in a tertiary hospital in Singapore. Int J Dermatol. 2010
;49:507-512. - Ropars N, Darrieux L, Tisseau L, et al. Acute generalized exanthematous pustulosis associated with primary Epstein-Barr virus infection. JAAD Case Rep. 2014;1:9-11.
- Hattem S, Beerthuizen G, Kardaun S. Severe flucloxacillin‐induced acute generalized exanthematous pustulosis (AGEP), with toxic epidermal necrolysis (TEN)‐like features: does overlap between AGEP and TEN exist? clinical report and review of the literature. Br J Dermatol. 2014;171:1539-1545.
- Tajmir-Riahi A, Wörl P, Harrer T, et al. Life-threatening atypical case of acute generalized exanthematous pustulosis. Int Arch Allergy Immunol. 2017;174:108-111.
- Feldmeyer L, Heidemeyer K, Yawalkar N. Acute generalized exanthematous pustulosis: pathogenesis, genetic background, clinical variants and therapy. Int J Mol Sci. 2016;17:E1214.
- Szatkowski J, Schwartz RA. Acute generalized exanthematous pustulosis (AGEP). a review and update. J Am Acad Dermatol. 2015;73:843-848.
- Totonchy MB, McNiff JM, Bunick CG. Koebnerization of Hailey-Hailey disease into a cutaneous drug eruption of acute generalized exanthematous pustulosis associated with systemic symptoms. J Cutan Pathol. 2016;43:1031-1035.
- Husain Z, Reddy BY, Schwartz RA. DRESS syndrome: part II. management and therapeutics. J Am Acad Dermatol. 2013;68:709.e1-e9; quiz 718-720.
- Kano Y, Shiohara T. Long-term outcome of patients with severe cutaneous adverse reactions. Dermatologica Sinica. 2013;31:211-216.
- Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. Vol 1. Philadelphia, PA: Elsevier Saunders; 2012.
- Ushigome Y, Kano Y, Ishida T, et al. Short- and long-term outcomes of 34 patients with drug-induced hypersensitivity syndrome in a single institution. J Am Acad Dermatol. 2013;68:721-728.
- Ljungman P, Wang FZ, Clark DA, et al. High levels of human herpesvirus 6 DNA in peripheral blood leucocytes are correlated to platelet engraftment and disease in allogeneic stem cell transplant patients. Br J Haematol. 2000;111:774-781.
- Asano Y, Kagawa H, Kano Y, et al. Cytomegalovirus disease during severe drug eruptions: report of 2 cases and retrospective study of 18 patients with drug-induced hypersensitivity syndrome. Arch Dermatol. 2009;145:1030-1036.
- Kano Y , Tohyama M, Aihara M, et al. Sequelae in 145 patients with drug‐induced hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms: survey conducted by the Asian Research Committee on Severe Cutaneous Adverse Reactions (ASCAR). J Dermatol. 2015;42:276-282.
- Morita C, Yanase T, Shiohara T, et al. Aggressive treatment in paediatric or young patients with drug-induced hypersensitivity syndrome (DiHS)/ drug reaction with eosinophilia and systemic symptoms (DRESS) is associated with future development of type III polyglandular autoimmune syndrome [published online October 27, 2018]. BMJ Case Rep. doi:10.1136/bcr-2018-225528.
- Chiang A, Shiu J, Elsensohn AN, et al. Classic autoimmune type 1 diabetes mellitus after a case of drug reaction with eosinophilia and systemic symptoms (DRESS). JAAD Case Rep. 2018;4:295-297.
- Lew TT, Creamer D, Mackenzie J, et al. Post-traumatic stress disorder following drug reaction with eosinophilia and systemic symptoms. Br J Dermatol. 2015;172:836-837.
- Kumar R, Das A, Das S. Management of Stevens-Johnson syndrome-toxic epidermal necrolysis: looking beyond guidelines! Indian J Dermatol. 2018;63:117-124.
- Yang CW, Cho YT, Chen KL, et al. Long-term sequelae of Stevens-Johnson syndrome/toxic epidermal necrolysis. Acta Derm Venereol. 2016;96:525-529.
- Lee HY, Walsh SA, Creamer D. Long‐term complications of Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN): the spectrum of chronic problems in patients who survive an episode of SJS/TEN necessitates multidisciplinary follow‐up. Br J Dermatol. 2017;177:924-935.
- Hsu M, Jayaram A, Verner R, et al. Indications and outcomes of amniotic membrane transplantation in the management of acute Stevens-Johnson syndrome and toxic epidermal necrolysis: a case-control study. Cornea. 2012;31:1394-1402.
- Sant’ Anna AE, Hazarbassanov RM, de Freitas D, et al. Minor salivary glands and labial mucous membrane graft in the treatment of severe symblepharon and dry eye in patients with Stevens-Johnson syndrome. Br J Ophthalmol. 2012;96:234-239.
- Hefez L, Zaghbib K, Sbidian E, et al. Post-traumatic stress disorder in Stevens-Johnson syndrome and toxic epidermal necrolysis: prevalence and risk factors. a prospective study of 31 patients [published online October 3, 2018]. Br J Dermatol. doi:10.1111/bjd.17267.
- Knight L, Todd G, Muloiwa R, et al. Stevens Johnson syndrome and toxic epidermal necrolysis: maternal and foetal outcomes intwenty-two consecutive pregnant HIV infected women [published online August 12, 2015]. PLoS One. doi:10.1371/journal.pone.0135501.
- Tchetnya X, Ngwasiri CA, Munge T, et al. Severe eye complications from toxic epidermal necrolysis following initiation of nevirapine based HAART regimen in a child with HIV infection: a case from Cameroon. BMC Pediatr. 2018;18:108.
- Antoon JW, Goldman JL, Lee B, et al. Incidence, outcomes, and resource use in children with Stevens-Johnson syndrome and toxic epidermal necrolysis. Pediatr Dermatol. 2018;35:182-187.
- Basu S, Shanbhag SS, Gokani A, et al. Chronic ocular sequelae of Stevens-Johnson syndrome in children: long-term impact of appropriate therapy on natural history of disease. Am J Ophthalmol. 2018;189:17-28.
- Pinho A, Marta A, Coutinho I, et al. Long‐term reproducibility of positive patch test reactions in patients with non‐immediate cutaneous adverse drug reactions to antibiotics. Contact Dermatitis. 2017;76:204-209.
- Barbaud A, Collet E, Milpied B, et al. A multicentre study to determine the value and safety of drug patch tests for the three main classes of severe cutaneous adverse drug reactions. Br J Dermatol. 2013;168:555-562.
- Preventable adverse drug reactions: a focus on drug interactions. US Food and Drug Administration website. https://www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/DrugInteractionsLabeling/ucm110632.htm. Updated March 6, 2018. Accessed April 12, 2019.
- Thienvibul C, Vachiramon V, Chanprapaph K. Five-year retrospective review of acute generalized exanthematous pustulosis. Dermatol Res Pract. 2015;3:1-8.
- Lee HY, Chou D, Pang SM, et al. Acute generalized exanthematous pustulosis: analysis of cases managed in a tertiary hospital in Singapore. Int J Dermatol. 2010
;49:507-512. - Ropars N, Darrieux L, Tisseau L, et al. Acute generalized exanthematous pustulosis associated with primary Epstein-Barr virus infection. JAAD Case Rep. 2014;1:9-11.
- Hattem S, Beerthuizen G, Kardaun S. Severe flucloxacillin‐induced acute generalized exanthematous pustulosis (AGEP), with toxic epidermal necrolysis (TEN)‐like features: does overlap between AGEP and TEN exist? clinical report and review of the literature. Br J Dermatol. 2014;171:1539-1545.
- Tajmir-Riahi A, Wörl P, Harrer T, et al. Life-threatening atypical case of acute generalized exanthematous pustulosis. Int Arch Allergy Immunol. 2017;174:108-111.
- Feldmeyer L, Heidemeyer K, Yawalkar N. Acute generalized exanthematous pustulosis: pathogenesis, genetic background, clinical variants and therapy. Int J Mol Sci. 2016;17:E1214.
- Szatkowski J, Schwartz RA. Acute generalized exanthematous pustulosis (AGEP). a review and update. J Am Acad Dermatol. 2015;73:843-848.
- Totonchy MB, McNiff JM, Bunick CG. Koebnerization of Hailey-Hailey disease into a cutaneous drug eruption of acute generalized exanthematous pustulosis associated with systemic symptoms. J Cutan Pathol. 2016;43:1031-1035.
- Husain Z, Reddy BY, Schwartz RA. DRESS syndrome: part II. management and therapeutics. J Am Acad Dermatol. 2013;68:709.e1-e9; quiz 718-720.
- Kano Y, Shiohara T. Long-term outcome of patients with severe cutaneous adverse reactions. Dermatologica Sinica. 2013;31:211-216.
- Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. Vol 1. Philadelphia, PA: Elsevier Saunders; 2012.
- Ushigome Y, Kano Y, Ishida T, et al. Short- and long-term outcomes of 34 patients with drug-induced hypersensitivity syndrome in a single institution. J Am Acad Dermatol. 2013;68:721-728.
- Ljungman P, Wang FZ, Clark DA, et al. High levels of human herpesvirus 6 DNA in peripheral blood leucocytes are correlated to platelet engraftment and disease in allogeneic stem cell transplant patients. Br J Haematol. 2000;111:774-781.
- Asano Y, Kagawa H, Kano Y, et al. Cytomegalovirus disease during severe drug eruptions: report of 2 cases and retrospective study of 18 patients with drug-induced hypersensitivity syndrome. Arch Dermatol. 2009;145:1030-1036.
- Kano Y , Tohyama M, Aihara M, et al. Sequelae in 145 patients with drug‐induced hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms: survey conducted by the Asian Research Committee on Severe Cutaneous Adverse Reactions (ASCAR). J Dermatol. 2015;42:276-282.
- Morita C, Yanase T, Shiohara T, et al. Aggressive treatment in paediatric or young patients with drug-induced hypersensitivity syndrome (DiHS)/ drug reaction with eosinophilia and systemic symptoms (DRESS) is associated with future development of type III polyglandular autoimmune syndrome [published online October 27, 2018]. BMJ Case Rep. doi:10.1136/bcr-2018-225528.
- Chiang A, Shiu J, Elsensohn AN, et al. Classic autoimmune type 1 diabetes mellitus after a case of drug reaction with eosinophilia and systemic symptoms (DRESS). JAAD Case Rep. 2018;4:295-297.
- Lew TT, Creamer D, Mackenzie J, et al. Post-traumatic stress disorder following drug reaction with eosinophilia and systemic symptoms. Br J Dermatol. 2015;172:836-837.
- Kumar R, Das A, Das S. Management of Stevens-Johnson syndrome-toxic epidermal necrolysis: looking beyond guidelines! Indian J Dermatol. 2018;63:117-124.
- Yang CW, Cho YT, Chen KL, et al. Long-term sequelae of Stevens-Johnson syndrome/toxic epidermal necrolysis. Acta Derm Venereol. 2016;96:525-529.
- Lee HY, Walsh SA, Creamer D. Long‐term complications of Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN): the spectrum of chronic problems in patients who survive an episode of SJS/TEN necessitates multidisciplinary follow‐up. Br J Dermatol. 2017;177:924-935.
- Hsu M, Jayaram A, Verner R, et al. Indications and outcomes of amniotic membrane transplantation in the management of acute Stevens-Johnson syndrome and toxic epidermal necrolysis: a case-control study. Cornea. 2012;31:1394-1402.
- Sant’ Anna AE, Hazarbassanov RM, de Freitas D, et al. Minor salivary glands and labial mucous membrane graft in the treatment of severe symblepharon and dry eye in patients with Stevens-Johnson syndrome. Br J Ophthalmol. 2012;96:234-239.
- Hefez L, Zaghbib K, Sbidian E, et al. Post-traumatic stress disorder in Stevens-Johnson syndrome and toxic epidermal necrolysis: prevalence and risk factors. a prospective study of 31 patients [published online October 3, 2018]. Br J Dermatol. doi:10.1111/bjd.17267.
- Knight L, Todd G, Muloiwa R, et al. Stevens Johnson syndrome and toxic epidermal necrolysis: maternal and foetal outcomes intwenty-two consecutive pregnant HIV infected women [published online August 12, 2015]. PLoS One. doi:10.1371/journal.pone.0135501.
- Tchetnya X, Ngwasiri CA, Munge T, et al. Severe eye complications from toxic epidermal necrolysis following initiation of nevirapine based HAART regimen in a child with HIV infection: a case from Cameroon. BMC Pediatr. 2018;18:108.
- Antoon JW, Goldman JL, Lee B, et al. Incidence, outcomes, and resource use in children with Stevens-Johnson syndrome and toxic epidermal necrolysis. Pediatr Dermatol. 2018;35:182-187.
- Basu S, Shanbhag SS, Gokani A, et al. Chronic ocular sequelae of Stevens-Johnson syndrome in children: long-term impact of appropriate therapy on natural history of disease. Am J Ophthalmol. 2018;189:17-28.
- Pinho A, Marta A, Coutinho I, et al. Long‐term reproducibility of positive patch test reactions in patients with non‐immediate cutaneous adverse drug reactions to antibiotics. Contact Dermatitis. 2017;76:204-209.
- Barbaud A, Collet E, Milpied B, et al. A multicentre study to determine the value and safety of drug patch tests for the three main classes of severe cutaneous adverse drug reactions. Br J Dermatol. 2013;168:555-562.
Practice Points
- In the setting of an adverse drug reaction (ADR), discontinuing the concerning medication is the first and most important step.
- Acute generalized exanthematous pustulosis, drug rash with eosinophilia and systemic symptoms (DRESS) syndrome, and Stevens-Johnson syndrome/toxic epidermal necrolysis all require specific outpatient follow-up after discharge.
Acute-Onset Alopecia
The Diagnosis: Thallium-Induced Alopecia
At the time of presentation, a punch biopsy specimen of the scalp revealed nonscarring alopecia with increased catagen hairs; follicular miniaturization; peribulbar lymphoid infiltrates; and fibrous tract remnants containing melanin, lymphocytes, and occasional mast cells (Figure 1). The differential diagnosis included alopecia areata, syphilis, and toxin-mediated anagen effluvium (AE). Given the abrupt onset affecting multiple individuals in an industrial environment, heavy metal poisoning was suspected. Blood and urine testing was negative, but a few months had elapsed since exposure. Several months after his initial presentation, the patient reported problems with his teeth, thin brittle nails, and resolution of the visual changes. Photographs sent by the patient revealed darkening and degeneration of the gingival margin (Figure 2).
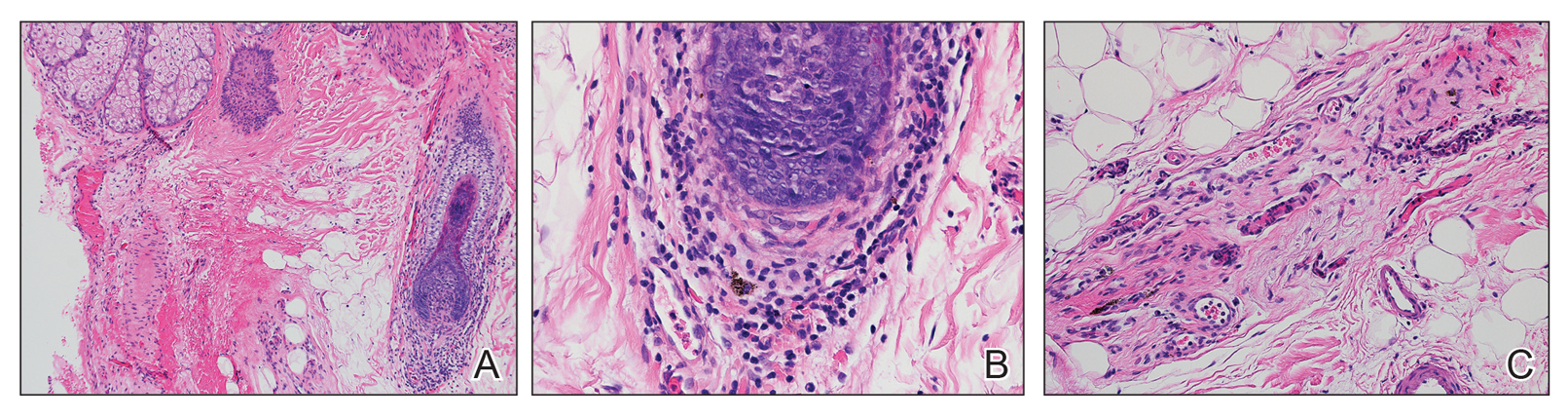
Environmental review revealed the patient was working on a demolition site of a 150-year-old electrical plant near a river. Inundation of rainfall caused a river swell and subsequent flooding of the work site. The patient reported working for more than 2 months in knee-deep muddy water, and he noted that water for consumption and showers was procured on-site from a well-based source that may have been contaminated by the floodwaters.
Acute nonscarring alopecia can be an AE or telogen effluvium (TE), also known as telogen defluvium. The key distinguishing factor is the mode of injury.1 In TE, medications, stress, hormonal shifts, or inflammation induce a synchronized and abrupt transition of hairs from anagen phase to catagen phase, a committed step that then must fully cycle through the telogen phase, culminating in the simultaneous shedding of numerous telogen hairs approximately 3 to 4 months later. Conversely, AE is caused by a sudden insult to the metabolic machinery of the hair matrix. Affected follicles rapidly produce thinner weaker shafts yielding Pohl-Pinkus constrictions or pencil point-shaped fractures that shed approximately 1 to 2 months after injury. The 10% of scalp hairs in the resting telogen phase have no matrix and thus are unaffected. Some etiologies can cause either AE or TE, depending on the dose and intensity of the insult. Common causes of AE include alopecia areata and syphilis, both consisting of abrupt severe bulbar inflammation.1 Other causes include chemotherapy, particularly antimetabolites, alkylating agents, and mitotic inhibitors; radiation; medications (eg, isoniazid); severe protein malnutrition; toxic chemicals (eg, boron/boric acid); and heavy metals (eg, thallium, mercury).
Thallium is one of the most common causes of heavy metal poisoning and is particularly dangerous due to its colorless, tasteless, and odorless characteristics. Although its common use as a rodenticide has dramatically decreased in the United States after it was banned in 1965, it is still used in this fashion in other countries and has a notable industrial presence, particularly in electronics, superconductors, and low-temperature thermometers. Accidental poisoning of a graduate chemistry student during copper research has been reported,2 highlighting that thallium can be inhaled, ingested, or absorbed through the skin. Thallium is even present in mycoplasma agar plates, the ingestion of which has resulted in poisoning.3
Systemic symptoms of thallium poisoning include somnolence, weakness, nausea, vomiting, stomatitis, abdominal pain, diarrhea, tachycardia, hypertension, and polyneuropathy.4-7 Neuropathy often manifests as painful acral dysesthesia and paresthesia, perioral numbness, optic neuropathy causing visual changes, and encephalopathy. Cutaneous findings include diffuse alopecia of the scalp and eyebrows, perioral dermatitis, glossitis, diffuse hyperpigmentation, oral hyperpigmentation (often as a stippled lead line along the gingival margin with subsequent alveolar damage and resorption), melanonychia, palmoplantar keratoderma, acneform or pustular eruption, and nail changes including Mees lines.2,4,5,7-9 Rarely, major organ failure and death may result.10
Toxin panels may not include thallium, and urine and serum tests may be negative if too much time has transpired since the acute exposure. Hair or nail analysis has proved useful in subacute cases11; however, most laboratories require a pencil-thick segment of hair cut at the roots and bundled, weighing at least 500 mg. Thallium poisoning is treated with activated charcoal, Prussian blue, and blood purification therapies (eg, hemodialysis, hemoperfusion, hemofiltration).4,7 Cutaneous findings typically resolve, but neuropathic changes may persist.
- Sperling LC, Cowper SE, Knopp EA. An Atlas of Hair Pathology With Clinical Correlations. 2nd ed. Boca Raton, FL: CRC Press; 2012.
- Campbell C, Bahrami S, Owen C. Anagen effluvium caused by thallium poisoning. JAMA Dermatol. 2016;152:724-726.
- Puschner B, Basso MM. Graham TW. Thallium toxicosis in a dog consequent to ingestion of Mycoplasma agar plates. J Vet Diagn Invest. 2012;24:227-230.
- Sojáková M, Zigrai M, Karaman A, et al. Thallium intoxication: case report. Neuro Endocrinol Lett. 2015;36:311-315.
- Lu Cl, Huang CC, Chang YC, et al. Short-term thallium intoxication: dermatological findings correlated with thallium concentration. Arch Dermatol. 2007;143:93-98.
- Liu EM, Rajagopal R, Grand MG. Optic nerve atrophy and hair loss in a young man. JAMA Ophthalmol. 2015;133:1469-1470.
- Zhang HT, Qiao BP, Liu BP, et al. Study on the treatment of acute thallium poisoning. Am J Med Sci. 2014;347:377-381.
- Misra UK, Kalita J, Yadav RK, et al. Thallium poisoning: emphasis on early diagnosis and response to haemodialysis. Postgrad Med J. 2003;79:103-105.
- Tromme I, Van Neste D, Dobbelaere F, et al. Skin signs in the diagnosis of thallium poisoning. Br J Dermatol. 1998;138:321-325.
- Li S, Huang W, Duan Y, et al. Human fatality due to thallium poisoning: autopsy, microscopy, and mass spectrometry assays. J Forensic Sci. 2015;60:247-251.
- Daniel CR 3rd, Piraccini BM, Tosti A. The nail and hair in forensic science. J Am Acad Dermatol. 2004;50:258-261.
The Diagnosis: Thallium-Induced Alopecia
At the time of presentation, a punch biopsy specimen of the scalp revealed nonscarring alopecia with increased catagen hairs; follicular miniaturization; peribulbar lymphoid infiltrates; and fibrous tract remnants containing melanin, lymphocytes, and occasional mast cells (Figure 1). The differential diagnosis included alopecia areata, syphilis, and toxin-mediated anagen effluvium (AE). Given the abrupt onset affecting multiple individuals in an industrial environment, heavy metal poisoning was suspected. Blood and urine testing was negative, but a few months had elapsed since exposure. Several months after his initial presentation, the patient reported problems with his teeth, thin brittle nails, and resolution of the visual changes. Photographs sent by the patient revealed darkening and degeneration of the gingival margin (Figure 2).


Environmental review revealed the patient was working on a demolition site of a 150-year-old electrical plant near a river. Inundation of rainfall caused a river swell and subsequent flooding of the work site. The patient reported working for more than 2 months in knee-deep muddy water, and he noted that water for consumption and showers was procured on-site from a well-based source that may have been contaminated by the floodwaters.
Acute nonscarring alopecia can be an AE or telogen effluvium (TE), also known as telogen defluvium. The key distinguishing factor is the mode of injury.1 In TE, medications, stress, hormonal shifts, or inflammation induce a synchronized and abrupt transition of hairs from anagen phase to catagen phase, a committed step that then must fully cycle through the telogen phase, culminating in the simultaneous shedding of numerous telogen hairs approximately 3 to 4 months later. Conversely, AE is caused by a sudden insult to the metabolic machinery of the hair matrix. Affected follicles rapidly produce thinner weaker shafts yielding Pohl-Pinkus constrictions or pencil point-shaped fractures that shed approximately 1 to 2 months after injury. The 10% of scalp hairs in the resting telogen phase have no matrix and thus are unaffected. Some etiologies can cause either AE or TE, depending on the dose and intensity of the insult. Common causes of AE include alopecia areata and syphilis, both consisting of abrupt severe bulbar inflammation.1 Other causes include chemotherapy, particularly antimetabolites, alkylating agents, and mitotic inhibitors; radiation; medications (eg, isoniazid); severe protein malnutrition; toxic chemicals (eg, boron/boric acid); and heavy metals (eg, thallium, mercury).
Thallium is one of the most common causes of heavy metal poisoning and is particularly dangerous due to its colorless, tasteless, and odorless characteristics. Although its common use as a rodenticide has dramatically decreased in the United States after it was banned in 1965, it is still used in this fashion in other countries and has a notable industrial presence, particularly in electronics, superconductors, and low-temperature thermometers. Accidental poisoning of a graduate chemistry student during copper research has been reported,2 highlighting that thallium can be inhaled, ingested, or absorbed through the skin. Thallium is even present in mycoplasma agar plates, the ingestion of which has resulted in poisoning.3
Systemic symptoms of thallium poisoning include somnolence, weakness, nausea, vomiting, stomatitis, abdominal pain, diarrhea, tachycardia, hypertension, and polyneuropathy.4-7 Neuropathy often manifests as painful acral dysesthesia and paresthesia, perioral numbness, optic neuropathy causing visual changes, and encephalopathy. Cutaneous findings include diffuse alopecia of the scalp and eyebrows, perioral dermatitis, glossitis, diffuse hyperpigmentation, oral hyperpigmentation (often as a stippled lead line along the gingival margin with subsequent alveolar damage and resorption), melanonychia, palmoplantar keratoderma, acneform or pustular eruption, and nail changes including Mees lines.2,4,5,7-9 Rarely, major organ failure and death may result.10
Toxin panels may not include thallium, and urine and serum tests may be negative if too much time has transpired since the acute exposure. Hair or nail analysis has proved useful in subacute cases11; however, most laboratories require a pencil-thick segment of hair cut at the roots and bundled, weighing at least 500 mg. Thallium poisoning is treated with activated charcoal, Prussian blue, and blood purification therapies (eg, hemodialysis, hemoperfusion, hemofiltration).4,7 Cutaneous findings typically resolve, but neuropathic changes may persist.
The Diagnosis: Thallium-Induced Alopecia
At the time of presentation, a punch biopsy specimen of the scalp revealed nonscarring alopecia with increased catagen hairs; follicular miniaturization; peribulbar lymphoid infiltrates; and fibrous tract remnants containing melanin, lymphocytes, and occasional mast cells (Figure 1). The differential diagnosis included alopecia areata, syphilis, and toxin-mediated anagen effluvium (AE). Given the abrupt onset affecting multiple individuals in an industrial environment, heavy metal poisoning was suspected. Blood and urine testing was negative, but a few months had elapsed since exposure. Several months after his initial presentation, the patient reported problems with his teeth, thin brittle nails, and resolution of the visual changes. Photographs sent by the patient revealed darkening and degeneration of the gingival margin (Figure 2).


Environmental review revealed the patient was working on a demolition site of a 150-year-old electrical plant near a river. Inundation of rainfall caused a river swell and subsequent flooding of the work site. The patient reported working for more than 2 months in knee-deep muddy water, and he noted that water for consumption and showers was procured on-site from a well-based source that may have been contaminated by the floodwaters.
Acute nonscarring alopecia can be an AE or telogen effluvium (TE), also known as telogen defluvium. The key distinguishing factor is the mode of injury.1 In TE, medications, stress, hormonal shifts, or inflammation induce a synchronized and abrupt transition of hairs from anagen phase to catagen phase, a committed step that then must fully cycle through the telogen phase, culminating in the simultaneous shedding of numerous telogen hairs approximately 3 to 4 months later. Conversely, AE is caused by a sudden insult to the metabolic machinery of the hair matrix. Affected follicles rapidly produce thinner weaker shafts yielding Pohl-Pinkus constrictions or pencil point-shaped fractures that shed approximately 1 to 2 months after injury. The 10% of scalp hairs in the resting telogen phase have no matrix and thus are unaffected. Some etiologies can cause either AE or TE, depending on the dose and intensity of the insult. Common causes of AE include alopecia areata and syphilis, both consisting of abrupt severe bulbar inflammation.1 Other causes include chemotherapy, particularly antimetabolites, alkylating agents, and mitotic inhibitors; radiation; medications (eg, isoniazid); severe protein malnutrition; toxic chemicals (eg, boron/boric acid); and heavy metals (eg, thallium, mercury).
Thallium is one of the most common causes of heavy metal poisoning and is particularly dangerous due to its colorless, tasteless, and odorless characteristics. Although its common use as a rodenticide has dramatically decreased in the United States after it was banned in 1965, it is still used in this fashion in other countries and has a notable industrial presence, particularly in electronics, superconductors, and low-temperature thermometers. Accidental poisoning of a graduate chemistry student during copper research has been reported,2 highlighting that thallium can be inhaled, ingested, or absorbed through the skin. Thallium is even present in mycoplasma agar plates, the ingestion of which has resulted in poisoning.3
Systemic symptoms of thallium poisoning include somnolence, weakness, nausea, vomiting, stomatitis, abdominal pain, diarrhea, tachycardia, hypertension, and polyneuropathy.4-7 Neuropathy often manifests as painful acral dysesthesia and paresthesia, perioral numbness, optic neuropathy causing visual changes, and encephalopathy. Cutaneous findings include diffuse alopecia of the scalp and eyebrows, perioral dermatitis, glossitis, diffuse hyperpigmentation, oral hyperpigmentation (often as a stippled lead line along the gingival margin with subsequent alveolar damage and resorption), melanonychia, palmoplantar keratoderma, acneform or pustular eruption, and nail changes including Mees lines.2,4,5,7-9 Rarely, major organ failure and death may result.10
Toxin panels may not include thallium, and urine and serum tests may be negative if too much time has transpired since the acute exposure. Hair or nail analysis has proved useful in subacute cases11; however, most laboratories require a pencil-thick segment of hair cut at the roots and bundled, weighing at least 500 mg. Thallium poisoning is treated with activated charcoal, Prussian blue, and blood purification therapies (eg, hemodialysis, hemoperfusion, hemofiltration).4,7 Cutaneous findings typically resolve, but neuropathic changes may persist.
- Sperling LC, Cowper SE, Knopp EA. An Atlas of Hair Pathology With Clinical Correlations. 2nd ed. Boca Raton, FL: CRC Press; 2012.
- Campbell C, Bahrami S, Owen C. Anagen effluvium caused by thallium poisoning. JAMA Dermatol. 2016;152:724-726.
- Puschner B, Basso MM. Graham TW. Thallium toxicosis in a dog consequent to ingestion of Mycoplasma agar plates. J Vet Diagn Invest. 2012;24:227-230.
- Sojáková M, Zigrai M, Karaman A, et al. Thallium intoxication: case report. Neuro Endocrinol Lett. 2015;36:311-315.
- Lu Cl, Huang CC, Chang YC, et al. Short-term thallium intoxication: dermatological findings correlated with thallium concentration. Arch Dermatol. 2007;143:93-98.
- Liu EM, Rajagopal R, Grand MG. Optic nerve atrophy and hair loss in a young man. JAMA Ophthalmol. 2015;133:1469-1470.
- Zhang HT, Qiao BP, Liu BP, et al. Study on the treatment of acute thallium poisoning. Am J Med Sci. 2014;347:377-381.
- Misra UK, Kalita J, Yadav RK, et al. Thallium poisoning: emphasis on early diagnosis and response to haemodialysis. Postgrad Med J. 2003;79:103-105.
- Tromme I, Van Neste D, Dobbelaere F, et al. Skin signs in the diagnosis of thallium poisoning. Br J Dermatol. 1998;138:321-325.
- Li S, Huang W, Duan Y, et al. Human fatality due to thallium poisoning: autopsy, microscopy, and mass spectrometry assays. J Forensic Sci. 2015;60:247-251.
- Daniel CR 3rd, Piraccini BM, Tosti A. The nail and hair in forensic science. J Am Acad Dermatol. 2004;50:258-261.
- Sperling LC, Cowper SE, Knopp EA. An Atlas of Hair Pathology With Clinical Correlations. 2nd ed. Boca Raton, FL: CRC Press; 2012.
- Campbell C, Bahrami S, Owen C. Anagen effluvium caused by thallium poisoning. JAMA Dermatol. 2016;152:724-726.
- Puschner B, Basso MM. Graham TW. Thallium toxicosis in a dog consequent to ingestion of Mycoplasma agar plates. J Vet Diagn Invest. 2012;24:227-230.
- Sojáková M, Zigrai M, Karaman A, et al. Thallium intoxication: case report. Neuro Endocrinol Lett. 2015;36:311-315.
- Lu Cl, Huang CC, Chang YC, et al. Short-term thallium intoxication: dermatological findings correlated with thallium concentration. Arch Dermatol. 2007;143:93-98.
- Liu EM, Rajagopal R, Grand MG. Optic nerve atrophy and hair loss in a young man. JAMA Ophthalmol. 2015;133:1469-1470.
- Zhang HT, Qiao BP, Liu BP, et al. Study on the treatment of acute thallium poisoning. Am J Med Sci. 2014;347:377-381.
- Misra UK, Kalita J, Yadav RK, et al. Thallium poisoning: emphasis on early diagnosis and response to haemodialysis. Postgrad Med J. 2003;79:103-105.
- Tromme I, Van Neste D, Dobbelaere F, et al. Skin signs in the diagnosis of thallium poisoning. Br J Dermatol. 1998;138:321-325.
- Li S, Huang W, Duan Y, et al. Human fatality due to thallium poisoning: autopsy, microscopy, and mass spectrometry assays. J Forensic Sci. 2015;60:247-251.
- Daniel CR 3rd, Piraccini BM, Tosti A. The nail and hair in forensic science. J Am Acad Dermatol. 2004;50:258-261.

A previously healthy 45-year-old man presented to the dermatology department with abrupt onset of patchy, progressively worsening alopecia of the scalp as well as nausea with emesis and blurry vision of a few weeks' duration. All symptoms were temporally associated with a new demolition job the patient had started at an industrial site. He reported 10 other contractors were similarly affected. The patient denied paresthesia or other skin changes. On physical examination, large patches of smooth alopecia without erythema, scale, scarring, tenderness, or edema that coalesced to involve the majority of the scalp, eyebrows, and eyelashes (inset) were noted.
Hyperhidrosis: Survey of the Cutis Editorial Board
To improve patient care and outcomes, leading dermatologists from the Cutis Editorial Board answered 5 questions on hyperhidrosis. Here’s what we found.
In which areas do patients report hyperhidrosis most frequently?
Nearly 70% of dermatologists see patients with hyperhidrosis of the axillae, followed by the palms and soles (27%). Only 4% of dermatologists indicated that they see hyperhidrosis all over the body.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Hyperhidrosis affects up to 5% of the US population and may remarkably affect quality of life. Primary hyperhidrosis accounts for 93% of cases. Before puberty, hyperhidrosis affects the palms and soles in up to 90% of patients. In adults, the axillae are most commonly affected (51%), followed by plantar (30%), palmar (24%), and facial (10%) areas (Strutton et al).
Next page: Topical treatment
Approximately what percentage of patients are satisfied with topical treatments for hyperhidrosis?
The majority of dermatologists (88%) reported that less than half of their patients are satisfied with topical treatments for hyperhidrosis. Only 12% indicated that 51% to 70% of their patients were satisfied, and none of the respondents indicated that >70% were satisfied.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
There is clearly a need for safe and effective treatments for hyperhidrosis. Treatment of hyperhidrosis should include lifestyle and behavioral modifications. It is helpful to try to avoid hot crowded rooms when feasible, as well as stress, tight clothing, occlusive shoes, alcohol, and spicy foods. Patients should be instructed on proper use of medications, as well as the need to continue therapy for maintenance. Patients should be encouraged to follow up for alternative treatment options in cases of therapy failure.
Next page: Botulinum toxin
On average, how long do the effects of botulinum toxin last in your axillary hyperhidrosis patients?
The effects of botulinum toxin last at least 4 months and up to 6 months in most patients, according to 58% of dermatologists surveyed. Thirty percent reported 2 to 4 months, and 13% reported more than 6 months.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
OnabotulinumtoxinA is approved by the US Food and Drug Administration for severe primary axillary hyperhidrosis. Injections are ideally placed at the dermal-subcutaneous junction, with 1 unit placed every 1 to 2 cm. Dosing is 50 to 100 U per axilla with higher dosing required for the palms and soles (off label). Reported efficacy for axillary hyperhidrosis is 82% to 87%; however, 50% of patients with plantar hyperhidrosis are dissatisfied with the treatment. Sweat reduction is most apparent after 2 weeks and typically persists 6 to 8 months in clinical trials (Botox package insert).
Next page: Systemic anticholinergics
When prescribing systemic anticholinergics for hyperhidrosis, what side effect is most common among your patients?
More than three-quarters of dermatologists (81%) reported that dry mouth is the most common side effect of systemic anticholinergics. Dry eyes is the second most common side effect (15%).
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Systemic anticholinergics are commonly used off label for the treatment of hyperhidrosis. Adverse effects include dry mouth, blurred vision, dry eyes, orthostatic hypotension, gastrointestinal, urinary retention, tachycardia, and drowsiness. Unfortunately, these side effects cause one-third of patients to discontinue treatment (Bajaj and Langtry). A slow escalation of the dose may increase tolerability and reduce these side effects. These anticholinergics should not be taken with other medications with anticholinergic activity to avoid exacerbating these side effects.
Next page: Surgical treatment
What percentage of patients require surgery for treatment of hyperhidrosis after topical, injectable, systemic options and devices have failed?
According to 62% of dermatologists, 10% or less of patients require surgery for treatment of hyperhidrosis after other therapies have failed. Almost one-third indicated that none of their patients require surgical treatment. None of the dermatologists surveyed reported that more than 60% of patients need surgery.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Surgery is an option to treat hyperhidrosis when conservative methods have failed. Surgical therapies include curettage, liposuction, and excision. A last resort is considered sympathectomy. Endoscopic thoracic sympathectomy is employed for palmar, facial, and axillary hyperhidrosis, while endoscopic lumbar sympathectomy is indicated for plantar hyperhidrosis.
Next page: More tips from derms
More Tips From Dermatologists
The dermatologists we polled had the following advice for their peers:
Patients with focal idiopathic hyperhidrosis of the axillae as well as palms/soles report that this condition interferes with the quality of life in major ways, from social interactions to professional interactions. They often don't even know they have a problem and internalize that they must be overly anxious about things. I have patients that buy 3 of the same shirts and change a few times a day, costing a great deal of money (plus cleaning bills for 3 shirts as well) and costing a great deal of wasted time when they could be doing something more productive. It's great that not only botulinum toxins can be helpful for the underarms but also even less-invasive topical anticholinergics (easy to use, no discomfort, predictable, and helping make treatment for axillary hyperhidrosis much more on the radar).—Joel L. Cohen, MD (Denver, Colorado)
More and more patients are presenting to request relief from hyperhidrosis, and increasingly in nontraditional areas (ie, areas other than the axilla and forehead). These include the palms and scalp most commonly, and then the breast, chest, and back. Patients with hyperhidrosis of the feet often present requesting help for their malodorous or smelly feet and shoes.—Fran E. Cook-Bolden, MD (New York, New York)
I have found that systemic hyperhidrosis has usually been responsive to oral glycopyrrolate. But localized hyperhidrosis is more difficult to treat. Glycopyrronium has made life so much easier for my axillary hyperhidrosis patients. Now I am waiting for some game changer for palms and soles.—Lawrence J. Green, MD (Washington, DC)
About This Survey
The survey was fielded electronically to Cutis Editorial Board Members within the United States from March 11, 2019, to April 8, 2019. A total of 26 usable responses were received.
Bajaj V, Langtry JA. Use of oral glycopyrronium bromide in hyperhidrosis. Br J Dermatol. 2007;157:118-121.
Botox [package insert]. Madison, NJ: Allergan, Inc; 2018.
Strutton DR, Kowalski JW, Glaser DA, et al. US prevalence of hyperhidrosis and impact on individuals with axillary hyperhidrosis: Results from a national survey. J Am Acad Dermatol. 2004;51:241-248.
To improve patient care and outcomes, leading dermatologists from the Cutis Editorial Board answered 5 questions on hyperhidrosis. Here’s what we found.
In which areas do patients report hyperhidrosis most frequently?
Nearly 70% of dermatologists see patients with hyperhidrosis of the axillae, followed by the palms and soles (27%). Only 4% of dermatologists indicated that they see hyperhidrosis all over the body.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Hyperhidrosis affects up to 5% of the US population and may remarkably affect quality of life. Primary hyperhidrosis accounts for 93% of cases. Before puberty, hyperhidrosis affects the palms and soles in up to 90% of patients. In adults, the axillae are most commonly affected (51%), followed by plantar (30%), palmar (24%), and facial (10%) areas (Strutton et al).
Next page: Topical treatment
Approximately what percentage of patients are satisfied with topical treatments for hyperhidrosis?
The majority of dermatologists (88%) reported that less than half of their patients are satisfied with topical treatments for hyperhidrosis. Only 12% indicated that 51% to 70% of their patients were satisfied, and none of the respondents indicated that >70% were satisfied.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
There is clearly a need for safe and effective treatments for hyperhidrosis. Treatment of hyperhidrosis should include lifestyle and behavioral modifications. It is helpful to try to avoid hot crowded rooms when feasible, as well as stress, tight clothing, occlusive shoes, alcohol, and spicy foods. Patients should be instructed on proper use of medications, as well as the need to continue therapy for maintenance. Patients should be encouraged to follow up for alternative treatment options in cases of therapy failure.
Next page: Botulinum toxin
On average, how long do the effects of botulinum toxin last in your axillary hyperhidrosis patients?
The effects of botulinum toxin last at least 4 months and up to 6 months in most patients, according to 58% of dermatologists surveyed. Thirty percent reported 2 to 4 months, and 13% reported more than 6 months.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
OnabotulinumtoxinA is approved by the US Food and Drug Administration for severe primary axillary hyperhidrosis. Injections are ideally placed at the dermal-subcutaneous junction, with 1 unit placed every 1 to 2 cm. Dosing is 50 to 100 U per axilla with higher dosing required for the palms and soles (off label). Reported efficacy for axillary hyperhidrosis is 82% to 87%; however, 50% of patients with plantar hyperhidrosis are dissatisfied with the treatment. Sweat reduction is most apparent after 2 weeks and typically persists 6 to 8 months in clinical trials (Botox package insert).
Next page: Systemic anticholinergics
When prescribing systemic anticholinergics for hyperhidrosis, what side effect is most common among your patients?
More than three-quarters of dermatologists (81%) reported that dry mouth is the most common side effect of systemic anticholinergics. Dry eyes is the second most common side effect (15%).
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Systemic anticholinergics are commonly used off label for the treatment of hyperhidrosis. Adverse effects include dry mouth, blurred vision, dry eyes, orthostatic hypotension, gastrointestinal, urinary retention, tachycardia, and drowsiness. Unfortunately, these side effects cause one-third of patients to discontinue treatment (Bajaj and Langtry). A slow escalation of the dose may increase tolerability and reduce these side effects. These anticholinergics should not be taken with other medications with anticholinergic activity to avoid exacerbating these side effects.
Next page: Surgical treatment
What percentage of patients require surgery for treatment of hyperhidrosis after topical, injectable, systemic options and devices have failed?
According to 62% of dermatologists, 10% or less of patients require surgery for treatment of hyperhidrosis after other therapies have failed. Almost one-third indicated that none of their patients require surgical treatment. None of the dermatologists surveyed reported that more than 60% of patients need surgery.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Surgery is an option to treat hyperhidrosis when conservative methods have failed. Surgical therapies include curettage, liposuction, and excision. A last resort is considered sympathectomy. Endoscopic thoracic sympathectomy is employed for palmar, facial, and axillary hyperhidrosis, while endoscopic lumbar sympathectomy is indicated for plantar hyperhidrosis.
Next page: More tips from derms
More Tips From Dermatologists
The dermatologists we polled had the following advice for their peers:
Patients with focal idiopathic hyperhidrosis of the axillae as well as palms/soles report that this condition interferes with the quality of life in major ways, from social interactions to professional interactions. They often don't even know they have a problem and internalize that they must be overly anxious about things. I have patients that buy 3 of the same shirts and change a few times a day, costing a great deal of money (plus cleaning bills for 3 shirts as well) and costing a great deal of wasted time when they could be doing something more productive. It's great that not only botulinum toxins can be helpful for the underarms but also even less-invasive topical anticholinergics (easy to use, no discomfort, predictable, and helping make treatment for axillary hyperhidrosis much more on the radar).—Joel L. Cohen, MD (Denver, Colorado)
More and more patients are presenting to request relief from hyperhidrosis, and increasingly in nontraditional areas (ie, areas other than the axilla and forehead). These include the palms and scalp most commonly, and then the breast, chest, and back. Patients with hyperhidrosis of the feet often present requesting help for their malodorous or smelly feet and shoes.—Fran E. Cook-Bolden, MD (New York, New York)
I have found that systemic hyperhidrosis has usually been responsive to oral glycopyrrolate. But localized hyperhidrosis is more difficult to treat. Glycopyrronium has made life so much easier for my axillary hyperhidrosis patients. Now I am waiting for some game changer for palms and soles.—Lawrence J. Green, MD (Washington, DC)
About This Survey
The survey was fielded electronically to Cutis Editorial Board Members within the United States from March 11, 2019, to April 8, 2019. A total of 26 usable responses were received.
To improve patient care and outcomes, leading dermatologists from the Cutis Editorial Board answered 5 questions on hyperhidrosis. Here’s what we found.
In which areas do patients report hyperhidrosis most frequently?
Nearly 70% of dermatologists see patients with hyperhidrosis of the axillae, followed by the palms and soles (27%). Only 4% of dermatologists indicated that they see hyperhidrosis all over the body.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Hyperhidrosis affects up to 5% of the US population and may remarkably affect quality of life. Primary hyperhidrosis accounts for 93% of cases. Before puberty, hyperhidrosis affects the palms and soles in up to 90% of patients. In adults, the axillae are most commonly affected (51%), followed by plantar (30%), palmar (24%), and facial (10%) areas (Strutton et al).
Next page: Topical treatment
Approximately what percentage of patients are satisfied with topical treatments for hyperhidrosis?
The majority of dermatologists (88%) reported that less than half of their patients are satisfied with topical treatments for hyperhidrosis. Only 12% indicated that 51% to 70% of their patients were satisfied, and none of the respondents indicated that >70% were satisfied.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
There is clearly a need for safe and effective treatments for hyperhidrosis. Treatment of hyperhidrosis should include lifestyle and behavioral modifications. It is helpful to try to avoid hot crowded rooms when feasible, as well as stress, tight clothing, occlusive shoes, alcohol, and spicy foods. Patients should be instructed on proper use of medications, as well as the need to continue therapy for maintenance. Patients should be encouraged to follow up for alternative treatment options in cases of therapy failure.
Next page: Botulinum toxin
On average, how long do the effects of botulinum toxin last in your axillary hyperhidrosis patients?
The effects of botulinum toxin last at least 4 months and up to 6 months in most patients, according to 58% of dermatologists surveyed. Thirty percent reported 2 to 4 months, and 13% reported more than 6 months.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
OnabotulinumtoxinA is approved by the US Food and Drug Administration for severe primary axillary hyperhidrosis. Injections are ideally placed at the dermal-subcutaneous junction, with 1 unit placed every 1 to 2 cm. Dosing is 50 to 100 U per axilla with higher dosing required for the palms and soles (off label). Reported efficacy for axillary hyperhidrosis is 82% to 87%; however, 50% of patients with plantar hyperhidrosis are dissatisfied with the treatment. Sweat reduction is most apparent after 2 weeks and typically persists 6 to 8 months in clinical trials (Botox package insert).
Next page: Systemic anticholinergics
When prescribing systemic anticholinergics for hyperhidrosis, what side effect is most common among your patients?
More than three-quarters of dermatologists (81%) reported that dry mouth is the most common side effect of systemic anticholinergics. Dry eyes is the second most common side effect (15%).
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Systemic anticholinergics are commonly used off label for the treatment of hyperhidrosis. Adverse effects include dry mouth, blurred vision, dry eyes, orthostatic hypotension, gastrointestinal, urinary retention, tachycardia, and drowsiness. Unfortunately, these side effects cause one-third of patients to discontinue treatment (Bajaj and Langtry). A slow escalation of the dose may increase tolerability and reduce these side effects. These anticholinergics should not be taken with other medications with anticholinergic activity to avoid exacerbating these side effects.
Next page: Surgical treatment
What percentage of patients require surgery for treatment of hyperhidrosis after topical, injectable, systemic options and devices have failed?
According to 62% of dermatologists, 10% or less of patients require surgery for treatment of hyperhidrosis after other therapies have failed. Almost one-third indicated that none of their patients require surgical treatment. None of the dermatologists surveyed reported that more than 60% of patients need surgery.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Surgery is an option to treat hyperhidrosis when conservative methods have failed. Surgical therapies include curettage, liposuction, and excision. A last resort is considered sympathectomy. Endoscopic thoracic sympathectomy is employed for palmar, facial, and axillary hyperhidrosis, while endoscopic lumbar sympathectomy is indicated for plantar hyperhidrosis.
Next page: More tips from derms
More Tips From Dermatologists
The dermatologists we polled had the following advice for their peers:
Patients with focal idiopathic hyperhidrosis of the axillae as well as palms/soles report that this condition interferes with the quality of life in major ways, from social interactions to professional interactions. They often don't even know they have a problem and internalize that they must be overly anxious about things. I have patients that buy 3 of the same shirts and change a few times a day, costing a great deal of money (plus cleaning bills for 3 shirts as well) and costing a great deal of wasted time when they could be doing something more productive. It's great that not only botulinum toxins can be helpful for the underarms but also even less-invasive topical anticholinergics (easy to use, no discomfort, predictable, and helping make treatment for axillary hyperhidrosis much more on the radar).—Joel L. Cohen, MD (Denver, Colorado)
More and more patients are presenting to request relief from hyperhidrosis, and increasingly in nontraditional areas (ie, areas other than the axilla and forehead). These include the palms and scalp most commonly, and then the breast, chest, and back. Patients with hyperhidrosis of the feet often present requesting help for their malodorous or smelly feet and shoes.—Fran E. Cook-Bolden, MD (New York, New York)
I have found that systemic hyperhidrosis has usually been responsive to oral glycopyrrolate. But localized hyperhidrosis is more difficult to treat. Glycopyrronium has made life so much easier for my axillary hyperhidrosis patients. Now I am waiting for some game changer for palms and soles.—Lawrence J. Green, MD (Washington, DC)
About This Survey
The survey was fielded electronically to Cutis Editorial Board Members within the United States from March 11, 2019, to April 8, 2019. A total of 26 usable responses were received.
Bajaj V, Langtry JA. Use of oral glycopyrronium bromide in hyperhidrosis. Br J Dermatol. 2007;157:118-121.
Botox [package insert]. Madison, NJ: Allergan, Inc; 2018.
Strutton DR, Kowalski JW, Glaser DA, et al. US prevalence of hyperhidrosis and impact on individuals with axillary hyperhidrosis: Results from a national survey. J Am Acad Dermatol. 2004;51:241-248.
Bajaj V, Langtry JA. Use of oral glycopyrronium bromide in hyperhidrosis. Br J Dermatol. 2007;157:118-121.
Botox [package insert]. Madison, NJ: Allergan, Inc; 2018.
Strutton DR, Kowalski JW, Glaser DA, et al. US prevalence of hyperhidrosis and impact on individuals with axillary hyperhidrosis: Results from a national survey. J Am Acad Dermatol. 2004;51:241-248.
Acquired Hypertrichosis of the Periorbital Area and Malar Cheek
The Diagnosis: Bimatoprost-Induced Hypertrichosis
Latanoprost, a prostaglandin analogue, typically is prescribed by ophthalmologists as eye drops to reduce intraocular pressure in open-angle glaucoma.1 Common adverse reactions of latanoprost drops include blurred vision, ocular irritation, darkening of the eyelid skin, and pigmentation of the iris.
In 1997, Johnstone2 reported hypertrichosis and increased pigmentation of the eyelashes of both eyes and adjacent skin after latanoprost drops were used in glaucoma patients. Subsequently, topical latanoprost and bimatoprost, a similar analogue, are now utilized for the cosmetic purpose of thickening and lengthening the eyelashes due to the hypertrichosis effect. Travoprost, another prostaglandin analogue used to treat glaucoma, also has been associated with periocular hypertrichosis.3 Concomitant poliosis of the eyelashes with hypertrichosis from latanoprost also has been reported.4 Our patient specifically purchased the eye drops (marketed as generic bimatoprost) to lengthen her eyelashes and had noticed an increase in length. She denied a family history of increased facial hair in females.
Along with gingival hyperplasia, systemic cyclosporine may cause generalized hypertrichosis consisting of terminal hair growth, particularly on the face and forearms. However, hypertrichosis from cyclosporine ophthalmic emulsion 0.05% rarely has been reported5 but would be more likely to occur in a patient reporting a history of chronic dry eye. Oral acetazolamide, not eye drops, is prescribed for glaucoma and typically is not associated with hypertrichosis. Betamethasone and timolol eye drops may cause burning, stinging, redness, or watering of the eyes, but they do not typically cause hypertrichosis.
Other systemic medications (eg, zidovudine, phenytoin, minoxidil, danazol, anabolic steroids) may cause hypertrichosis but not typically localized to the periocular area. Phenytoin usually causes hair growth on the limbs but not on the face and trunk. Oral minoxidil causes hypertrichosis, predominately on the face, lower legs, and forearms.
Systemic conditions such as endocrine abnormalities or porphyria cutanea tarda also may cause hypertrichosis; however, it typically does not present in small focal areas, and other stigmata often are present such as signs of virilization in hirsutism (ie, deepening of voice, pattern alopecia, acne) or liver disease with photosensitive erosions and bullae that leave scars and milia in porphyria cutanea tarda. Acquired hypertrichosis lanuginosa deserves consideration, in part due to its association with lung and colon cancers; however, it consists of softer, downy, nonterminal hairs (malignant down) and is more generalized on the face. Malnutrition from anorexia nervosa may similarly induce hypertrichosis lanuginose.
The molecular mechanism for latanoprost-induced hypertrichosis is unknown; however, it may promote anagen growth as well as hypertrophic changes in the affected follicles.6 Patients should use extreme caution when purchasing unregulated medications due to the risk for impurities, less stable formulation, or inaccurate concentrations. Comparison between brand name and approved generic latanoprost has found notable differences, including variations in active-ingredient concentration, poor stability in warmer temperatures, and higher levels of particulate matter.7 Some cosmetic eyelash enhancers sold over-the-counter or online may contain prostaglandin analogues, but they may not be listed as ingredients.8 One report noted a bimatoprost product with a concentration level double that of brand-name bimatoprost that was discovered using high-performance liquid chromatography-tandem mass spectrometry.9
Treatment options for eliminating the excess hairs include discontinuing the prostaglandin analogue or applying it only to the eyelid margin with an appropriate applicator. Waxing, manual extraction, laser hair removal, electrolysis, and depilatory creams are alternative treatments.
- Alm A. Latanoprost in the treatment of glaucoma. Clin Ophthalmol. 2014;8:1967-1985.
- Johnstone MA. Hypertrichosis and increased pigmentation of eyelashes and adjacent hair in the region of the ipsilateral eyelids of patients treated with unilateral topical latanoprost. Am J Ophthalmol. 1997;124:544-547.
- Ortiz-Perez S, Olver JM. Hypertrichosis of the upper cheek area associated with travoprost treatment of glaucoma. Ophthalmic Plast Reconstr Surg. 2010;26:376-377.
- Özyurt S, Çetinkaya GS. Hypertrichosis of the malar areas and poliosis of the eyelashes caused by latanoprost. Actas Dermosifiliogr. 2015;106:74-75.
- Lei HL, Ku WC, Sun MH, et al. Cyclosporine A eye drop-induced elongated eyelashes: a case report. Case Rep Ophthalmol. 2011;2:398-400.
- Johnstone MA, Albert DM. Prostaglandin-induced hair growth. Surv Ophthalmol. 2002;47(suppl 1):S185-S202.
- Kahook MY, Fechtner RD, Katz LJ, et al. A comparison of active ingredients and preservatives between brand name and generic topical glaucoma medications using liquid chromatography-tandem mass spectrometry. Curr Eye Res. 2012;37:101-108.
- Swedish Medical Products Agency. Pharmaceutical ingredients in one out of three eyelash serums. https://www.dr-jetskeultee.nl/jetskeultee/download/common/artikel-wimpers-ingredients.pdf. Published April 15, 2013. Accessed April 11, 2019.
- Marchei E, De Orsi D, Guarino C, et al. High performance liquid chromatography tandem mass spectrometry measurement of bimatoprost, latanoprost and travoprost in eyelash enhancing cosmetic serums. Cosmetics. 2016;3:4.
The Diagnosis: Bimatoprost-Induced Hypertrichosis
Latanoprost, a prostaglandin analogue, typically is prescribed by ophthalmologists as eye drops to reduce intraocular pressure in open-angle glaucoma.1 Common adverse reactions of latanoprost drops include blurred vision, ocular irritation, darkening of the eyelid skin, and pigmentation of the iris.
In 1997, Johnstone2 reported hypertrichosis and increased pigmentation of the eyelashes of both eyes and adjacent skin after latanoprost drops were used in glaucoma patients. Subsequently, topical latanoprost and bimatoprost, a similar analogue, are now utilized for the cosmetic purpose of thickening and lengthening the eyelashes due to the hypertrichosis effect. Travoprost, another prostaglandin analogue used to treat glaucoma, also has been associated with periocular hypertrichosis.3 Concomitant poliosis of the eyelashes with hypertrichosis from latanoprost also has been reported.4 Our patient specifically purchased the eye drops (marketed as generic bimatoprost) to lengthen her eyelashes and had noticed an increase in length. She denied a family history of increased facial hair in females.
Along with gingival hyperplasia, systemic cyclosporine may cause generalized hypertrichosis consisting of terminal hair growth, particularly on the face and forearms. However, hypertrichosis from cyclosporine ophthalmic emulsion 0.05% rarely has been reported5 but would be more likely to occur in a patient reporting a history of chronic dry eye. Oral acetazolamide, not eye drops, is prescribed for glaucoma and typically is not associated with hypertrichosis. Betamethasone and timolol eye drops may cause burning, stinging, redness, or watering of the eyes, but they do not typically cause hypertrichosis.
Other systemic medications (eg, zidovudine, phenytoin, minoxidil, danazol, anabolic steroids) may cause hypertrichosis but not typically localized to the periocular area. Phenytoin usually causes hair growth on the limbs but not on the face and trunk. Oral minoxidil causes hypertrichosis, predominately on the face, lower legs, and forearms.
Systemic conditions such as endocrine abnormalities or porphyria cutanea tarda also may cause hypertrichosis; however, it typically does not present in small focal areas, and other stigmata often are present such as signs of virilization in hirsutism (ie, deepening of voice, pattern alopecia, acne) or liver disease with photosensitive erosions and bullae that leave scars and milia in porphyria cutanea tarda. Acquired hypertrichosis lanuginosa deserves consideration, in part due to its association with lung and colon cancers; however, it consists of softer, downy, nonterminal hairs (malignant down) and is more generalized on the face. Malnutrition from anorexia nervosa may similarly induce hypertrichosis lanuginose.
The molecular mechanism for latanoprost-induced hypertrichosis is unknown; however, it may promote anagen growth as well as hypertrophic changes in the affected follicles.6 Patients should use extreme caution when purchasing unregulated medications due to the risk for impurities, less stable formulation, or inaccurate concentrations. Comparison between brand name and approved generic latanoprost has found notable differences, including variations in active-ingredient concentration, poor stability in warmer temperatures, and higher levels of particulate matter.7 Some cosmetic eyelash enhancers sold over-the-counter or online may contain prostaglandin analogues, but they may not be listed as ingredients.8 One report noted a bimatoprost product with a concentration level double that of brand-name bimatoprost that was discovered using high-performance liquid chromatography-tandem mass spectrometry.9
Treatment options for eliminating the excess hairs include discontinuing the prostaglandin analogue or applying it only to the eyelid margin with an appropriate applicator. Waxing, manual extraction, laser hair removal, electrolysis, and depilatory creams are alternative treatments.
The Diagnosis: Bimatoprost-Induced Hypertrichosis
Latanoprost, a prostaglandin analogue, typically is prescribed by ophthalmologists as eye drops to reduce intraocular pressure in open-angle glaucoma.1 Common adverse reactions of latanoprost drops include blurred vision, ocular irritation, darkening of the eyelid skin, and pigmentation of the iris.
In 1997, Johnstone2 reported hypertrichosis and increased pigmentation of the eyelashes of both eyes and adjacent skin after latanoprost drops were used in glaucoma patients. Subsequently, topical latanoprost and bimatoprost, a similar analogue, are now utilized for the cosmetic purpose of thickening and lengthening the eyelashes due to the hypertrichosis effect. Travoprost, another prostaglandin analogue used to treat glaucoma, also has been associated with periocular hypertrichosis.3 Concomitant poliosis of the eyelashes with hypertrichosis from latanoprost also has been reported.4 Our patient specifically purchased the eye drops (marketed as generic bimatoprost) to lengthen her eyelashes and had noticed an increase in length. She denied a family history of increased facial hair in females.
Along with gingival hyperplasia, systemic cyclosporine may cause generalized hypertrichosis consisting of terminal hair growth, particularly on the face and forearms. However, hypertrichosis from cyclosporine ophthalmic emulsion 0.05% rarely has been reported5 but would be more likely to occur in a patient reporting a history of chronic dry eye. Oral acetazolamide, not eye drops, is prescribed for glaucoma and typically is not associated with hypertrichosis. Betamethasone and timolol eye drops may cause burning, stinging, redness, or watering of the eyes, but they do not typically cause hypertrichosis.
Other systemic medications (eg, zidovudine, phenytoin, minoxidil, danazol, anabolic steroids) may cause hypertrichosis but not typically localized to the periocular area. Phenytoin usually causes hair growth on the limbs but not on the face and trunk. Oral minoxidil causes hypertrichosis, predominately on the face, lower legs, and forearms.
Systemic conditions such as endocrine abnormalities or porphyria cutanea tarda also may cause hypertrichosis; however, it typically does not present in small focal areas, and other stigmata often are present such as signs of virilization in hirsutism (ie, deepening of voice, pattern alopecia, acne) or liver disease with photosensitive erosions and bullae that leave scars and milia in porphyria cutanea tarda. Acquired hypertrichosis lanuginosa deserves consideration, in part due to its association with lung and colon cancers; however, it consists of softer, downy, nonterminal hairs (malignant down) and is more generalized on the face. Malnutrition from anorexia nervosa may similarly induce hypertrichosis lanuginose.
The molecular mechanism for latanoprost-induced hypertrichosis is unknown; however, it may promote anagen growth as well as hypertrophic changes in the affected follicles.6 Patients should use extreme caution when purchasing unregulated medications due to the risk for impurities, less stable formulation, or inaccurate concentrations. Comparison between brand name and approved generic latanoprost has found notable differences, including variations in active-ingredient concentration, poor stability in warmer temperatures, and higher levels of particulate matter.7 Some cosmetic eyelash enhancers sold over-the-counter or online may contain prostaglandin analogues, but they may not be listed as ingredients.8 One report noted a bimatoprost product with a concentration level double that of brand-name bimatoprost that was discovered using high-performance liquid chromatography-tandem mass spectrometry.9
Treatment options for eliminating the excess hairs include discontinuing the prostaglandin analogue or applying it only to the eyelid margin with an appropriate applicator. Waxing, manual extraction, laser hair removal, electrolysis, and depilatory creams are alternative treatments.
- Alm A. Latanoprost in the treatment of glaucoma. Clin Ophthalmol. 2014;8:1967-1985.
- Johnstone MA. Hypertrichosis and increased pigmentation of eyelashes and adjacent hair in the region of the ipsilateral eyelids of patients treated with unilateral topical latanoprost. Am J Ophthalmol. 1997;124:544-547.
- Ortiz-Perez S, Olver JM. Hypertrichosis of the upper cheek area associated with travoprost treatment of glaucoma. Ophthalmic Plast Reconstr Surg. 2010;26:376-377.
- Özyurt S, Çetinkaya GS. Hypertrichosis of the malar areas and poliosis of the eyelashes caused by latanoprost. Actas Dermosifiliogr. 2015;106:74-75.
- Lei HL, Ku WC, Sun MH, et al. Cyclosporine A eye drop-induced elongated eyelashes: a case report. Case Rep Ophthalmol. 2011;2:398-400.
- Johnstone MA, Albert DM. Prostaglandin-induced hair growth. Surv Ophthalmol. 2002;47(suppl 1):S185-S202.
- Kahook MY, Fechtner RD, Katz LJ, et al. A comparison of active ingredients and preservatives between brand name and generic topical glaucoma medications using liquid chromatography-tandem mass spectrometry. Curr Eye Res. 2012;37:101-108.
- Swedish Medical Products Agency. Pharmaceutical ingredients in one out of three eyelash serums. https://www.dr-jetskeultee.nl/jetskeultee/download/common/artikel-wimpers-ingredients.pdf. Published April 15, 2013. Accessed April 11, 2019.
- Marchei E, De Orsi D, Guarino C, et al. High performance liquid chromatography tandem mass spectrometry measurement of bimatoprost, latanoprost and travoprost in eyelash enhancing cosmetic serums. Cosmetics. 2016;3:4.
- Alm A. Latanoprost in the treatment of glaucoma. Clin Ophthalmol. 2014;8:1967-1985.
- Johnstone MA. Hypertrichosis and increased pigmentation of eyelashes and adjacent hair in the region of the ipsilateral eyelids of patients treated with unilateral topical latanoprost. Am J Ophthalmol. 1997;124:544-547.
- Ortiz-Perez S, Olver JM. Hypertrichosis of the upper cheek area associated with travoprost treatment of glaucoma. Ophthalmic Plast Reconstr Surg. 2010;26:376-377.
- Özyurt S, Çetinkaya GS. Hypertrichosis of the malar areas and poliosis of the eyelashes caused by latanoprost. Actas Dermosifiliogr. 2015;106:74-75.
- Lei HL, Ku WC, Sun MH, et al. Cyclosporine A eye drop-induced elongated eyelashes: a case report. Case Rep Ophthalmol. 2011;2:398-400.
- Johnstone MA, Albert DM. Prostaglandin-induced hair growth. Surv Ophthalmol. 2002;47(suppl 1):S185-S202.
- Kahook MY, Fechtner RD, Katz LJ, et al. A comparison of active ingredients and preservatives between brand name and generic topical glaucoma medications using liquid chromatography-tandem mass spectrometry. Curr Eye Res. 2012;37:101-108.
- Swedish Medical Products Agency. Pharmaceutical ingredients in one out of three eyelash serums. https://www.dr-jetskeultee.nl/jetskeultee/download/common/artikel-wimpers-ingredients.pdf. Published April 15, 2013. Accessed April 11, 2019.
- Marchei E, De Orsi D, Guarino C, et al. High performance liquid chromatography tandem mass spectrometry measurement of bimatoprost, latanoprost and travoprost in eyelash enhancing cosmetic serums. Cosmetics. 2016;3:4.
An otherwise healthy woman in her late 50s with Fitzpatrick skin type II presented to the dermatology department for a scheduled cosmetic botulinum toxin injection. Her medical history was notable only for periodic nonsurgical cosmetic procedures including botulinum toxin and dermal fillers, and she was not taking any daily systemic medications. During the preoperative assessment, subtle bilateral and symmetric hypertrichosis with darker terminal hair formation was noted on the periorbital skin and zygomatic cheek. Upon inquiry, the patient admitted to purchasing a “special eye drop” from Mexico and using it regularly. After instillation of 2 to 3 drops per eye, she would laterally wipe the resulting excess drops away from the eyes with her hands and then wash her hands. She denied a change in eye color from their natural brown but did report using blue color contact lenses. She denied an increase in hair growth elsewhere including the upper lip, chin, upper chest, forearms, and hands. She denied deepening of her voice, acne, or hair thinning.
Squamous Cell Carcinoma With Perineural Involvement in Nevus Sebaceus
First reported in 1895, nevus sebaceus (NS) is a con genital papillomatous hamartoma most commonly found on the scalp and face. 1 Lesions typically are yellow-orange plaques and often are hairless. Nevus sebaceus is most prominent in the few first months after birth and again at puberty during development of the sebaceous glands. Development of epithelial hyperplasia, cysts, verrucas, and benign or malignant tumors has been reported. 1 The most common benign tumors are syringocystadenoma papilliferum and trichoblastoma. Cases of malignancy are rare, and basal cell carcinoma is the predominant form (approximately 2% of cases). Squamous cell carcinoma (SCC) and adnexal carcinoma are reported at even lower rates. 1 Malignant transformation occurring during childhood is extremely uncommon. According to a PubMed search of articles indexed for MEDLINE using the terms nevus sebaceous, malignancy, and squamous cell carcinoma and narrowing the results to children, there have been only 4 prior reports of SCC developing within an NS in a child. 2-5 We report a case of SCC arising in an NS in a 13-year-old adolescent girl with perineural invasion.
Case Report
A 13-year-old fair-skinned adolescent girl presented with a hairless 2×2.5-cm yellow plaque at the hairline on the anterior central scalp. The plaque had been present since birth and had progressively developed a superiorly located 3×5-mm erythematous verrucous nodule (Figure 1) with an approximate height of 6 mm over the last year. The nodule was subjected to regular trauma and bled with minimal insult. The patient appeared otherwise healthy, with no history of skin cancer or other chronic medical conditions. There was no evidence of lymphadenopathy on examination, and no other skin abnormalities were noted. There was no reported family history of skin cancer or chronic skin conditions suggestive of increased risk for cancer or other pathologic dermatoses. Differential diagnoses for the plaque and nodule complex included verruca, Spitz nevus, or secondary neoplasm within NS.
Excision was conducted under local anesthesia without complication. An elliptical section of skin measuring 0.8×2.5 cm was excised to a depth of 3 mm. The resulting wound was closed using a complex linear repair. The section was placed in formalin specimen transport medium and sent to Walter Reed National Military Medical Center (Bethesda, Maryland). Microscopic examination of the specimen revealed features typical for NS, including mild verrucous epidermal hyperplasia, sebaceous gland hyperplasia, presence of apocrine glands, and hamartomatous follicular proliferations (Figure 2). An even more papillomatous epidermal proliferation that was comprised of atypical squamous cells was present within the lesion. Similar atypical squamous cells infiltrated the superficial dermis in nests, cords, and single cells (Figure 3A). One focus showed perineural invasion with a small superficial nerve fiber surrounded by SCC (Figure 3B). The tumor was completely excised, with negative surgical margins extending approximately 2 mm. Adjuvant radiation therapy and further specialized Mohs micrographic excision were not performed because of the clear histologic appearance of the carcinoma and strong evidence of complete excision.
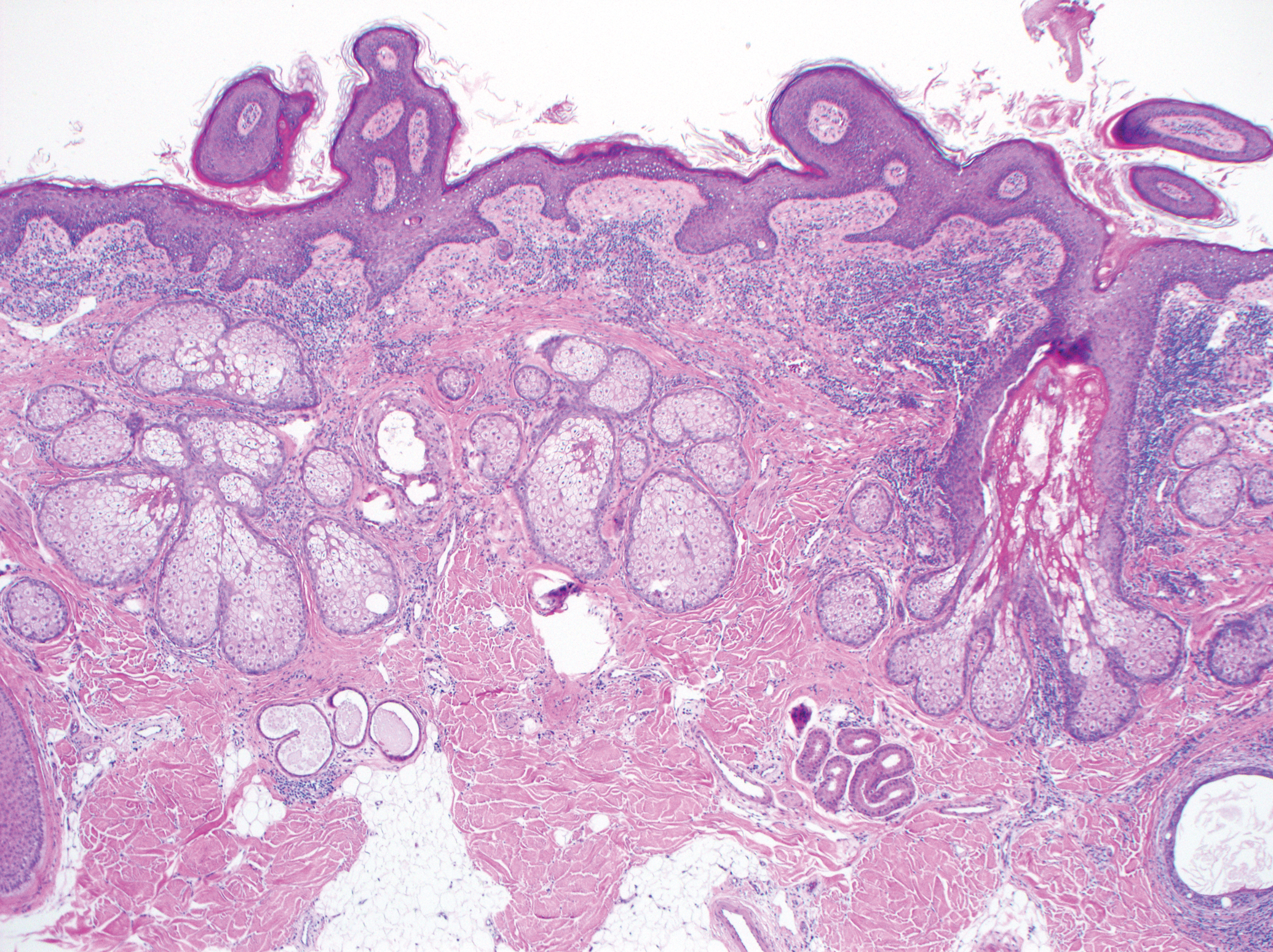
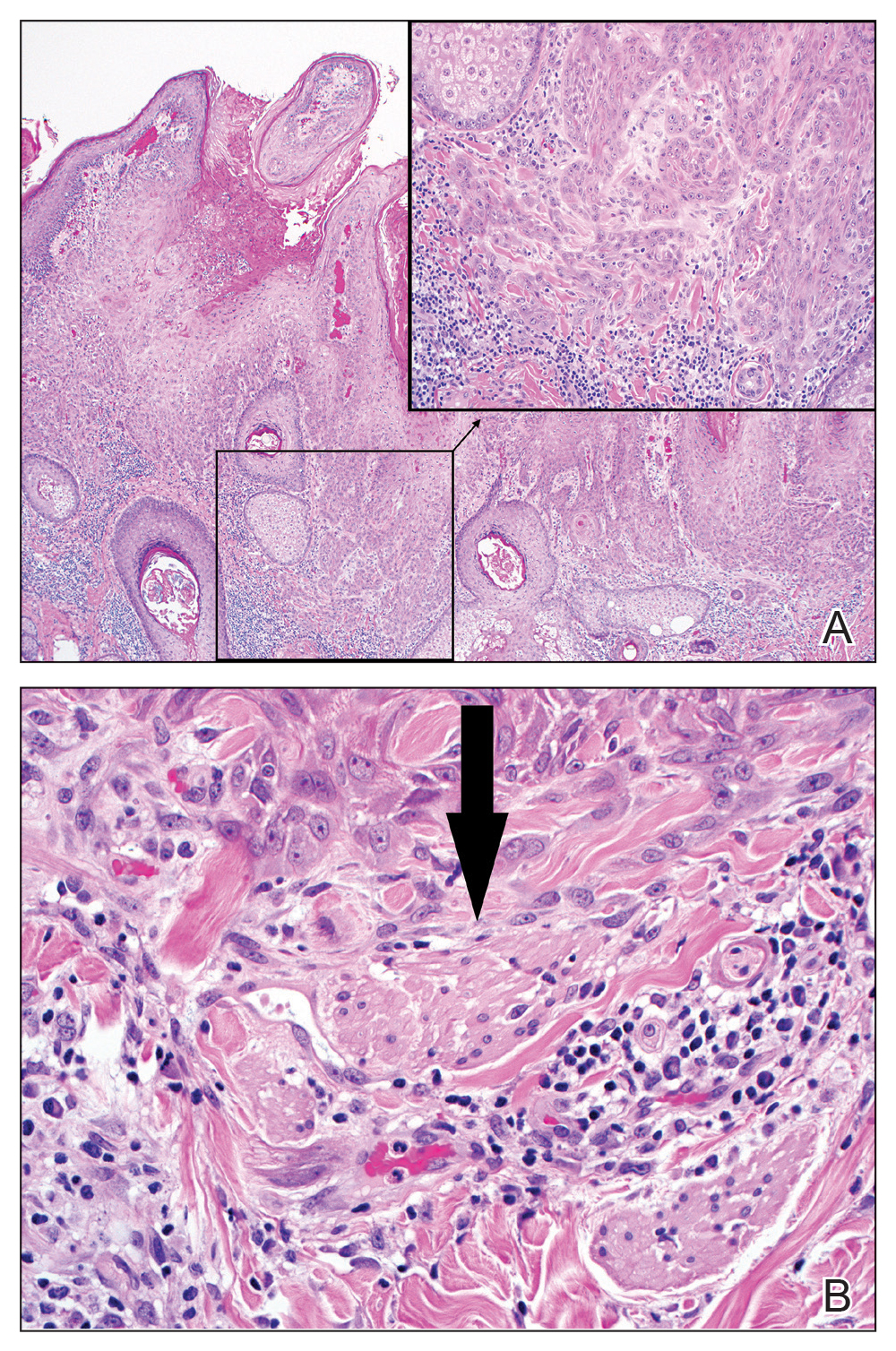
At 2-week follow-up, the surgical scar on the anterior central forehead was well healed without evidence of SCC recurrence. On physical examination there was neither lymphadenopathy nor signs of neurologic deficit, except for superficial cutaneous hypoesthesia in the immediate area surrounding the healed site. Following discussion with the patient and her parents, it was decided that the patient would obtain baseline laboratory tests, chest radiography, and abdominal ultrasonography, and she would undergo serial follow-up examinations every 3 months for the next 2 years. Annual follow-up was recommended after 2 years, with the caveat to return sooner if recurrence or symptoms were to arise.
Comment
Historically, there has been variability in the histopathologic interpretation of SCC in NS in the literature. Retrospective analysis of the histologic evidence of SCC in the 2 earliest possible cases of pediatric SCC in NS have been questioned due to the lack of clinical data presented and the possibility that the diagnosis of SCC was inaccurate.6 Our case was histopathologically interpreted as superficially invasive, well-differentiated SCC arising within an NS; therefore, we classified this case as SCC and took every precaution to ensure the lesion was completely excised, given the potentially invasive nature of SCC.
Our case is unique because it represents SCC in NS with histologic evidence of perineural involvement. Perineural invasion is a major route of tumor spread in SCC and may result in increased occurrence of regional lymph node spread and distant metastases, with path of least resistance or neural cell adhesion as possible spreading methods.7-9 However, there is a notable amount of prognostic variability based on tumor type, the nerve involved, and degree of involvement.9 It is common for cutaneous SCC to occur with invasion of small intradermal nerves, but a poor outcome is less likely in asymptomatic patients who have perineural involvement that was incidentally discovered on histologic examination.10
In our patient, the entire tumor was completely removed with local excision. Recurrence of the SCC or future symptoms of deep neural invasion were not anticipated given the postoperative evidence of clear margins in the excised skin and subdermal structures as well as the lack of preoperative and postoperative symptoms. Close clinical follow-up was warranted to monitor for early signs of recurrence or neural involvement. We have confidence that the planned follow-up regimen in our patient will reveal any early signs of new occurrence or recurrence.
In the case of recurrence, Mohs micrographic surgery would likely be indicated. We elected not to treat with adjuvant radiotherapy because its benefit in cutaneous SCC with perineural invasion is debatable based on the lack of randomized controlled clinical evidence.10,11 The patient obtained postoperative baseline complete blood cell count with differential, posterior/anterior and lateral chest radiographs, as well as abdominal ultrasonography. Each returned negative findings of hematologic or distant organ metastases, with subsequent follow-up visits also negative for any new concerning findings.
- Cribier B, Scrivener Y, Grosshans E. Tumors arising in nevus sebaceus: a study of 596 cases. J Am Acad Dermatol. 2000;42(2, pt 1):263-268.
- Aguayo R, Pallares J, Cassanova JM, et al. Squamous cell carcinoma developing in Jadassohn’s sebaceous nevus: case report and review of the literature. Dermatol Surg. 2010;36:1763-1768.
- Taher M, Feibleman C, Bennet R. Squamous cell carcinoma arising in a nevus sebaceous of Jadassohn in a 9-year-old girl: treatment using Mohs micrographic surgery with literature review. Dermatol Surg. 2010;36:1203-1208.
- Hidvegi NC, Kangesu L, Wolfe KQ. Squamous cell carcinoma complicating naevus sebaceous of Jadassohn in a child. Br J Plast Surg. 2003;56:50-52.
- Belhadjali H, Moussa A, Yahia S, et al. Simultaneous occurrence of squamous cell carcinomas within a nevus sebaceous of Jadassohn in an 11-year-old girl. Pediatr Dermatol. 2009;26:236-237.
- Wilson-Jones EW, Heyl T. Naevus sebaceus: a report of 140 cases with special regard to the development of secondary malignant tumors. Br J Dermatol. 1970;82:99-117.
- Ballantyne AJ, McCarten AB, Ibanez ML. The extension of cancer of the head and neck through perineural peripheral nerves. Am J Surg. 1963;106:651-667.
- Goepfert H, Dichtel WJ, Medina JE, et al. Perineural invasion in squamous cell skin carcinoma of the head and neck. Am J Surg. 1984;148:542-547.
- Feasel AM, Brown TJ, Bogle MA, et al. Perineural invasion of cutaneous malignancies. Dermatol Surg. 2001;27:531-542.
- Cottel WI. Perineural invasion by squamous cell carcinoma. J Dermatol Surg Oncol. 1982;8:589-600.
- Mendenhall WM, Parsons JT, Mendenhall NP, et al. Carcinoma of the skin of the head and neck with perineural invasion. Head Neck. 1989;11:301-308.
First reported in 1895, nevus sebaceus (NS) is a con genital papillomatous hamartoma most commonly found on the scalp and face. 1 Lesions typically are yellow-orange plaques and often are hairless. Nevus sebaceus is most prominent in the few first months after birth and again at puberty during development of the sebaceous glands. Development of epithelial hyperplasia, cysts, verrucas, and benign or malignant tumors has been reported. 1 The most common benign tumors are syringocystadenoma papilliferum and trichoblastoma. Cases of malignancy are rare, and basal cell carcinoma is the predominant form (approximately 2% of cases). Squamous cell carcinoma (SCC) and adnexal carcinoma are reported at even lower rates. 1 Malignant transformation occurring during childhood is extremely uncommon. According to a PubMed search of articles indexed for MEDLINE using the terms nevus sebaceous, malignancy, and squamous cell carcinoma and narrowing the results to children, there have been only 4 prior reports of SCC developing within an NS in a child. 2-5 We report a case of SCC arising in an NS in a 13-year-old adolescent girl with perineural invasion.
Case Report
A 13-year-old fair-skinned adolescent girl presented with a hairless 2×2.5-cm yellow plaque at the hairline on the anterior central scalp. The plaque had been present since birth and had progressively developed a superiorly located 3×5-mm erythematous verrucous nodule (Figure 1) with an approximate height of 6 mm over the last year. The nodule was subjected to regular trauma and bled with minimal insult. The patient appeared otherwise healthy, with no history of skin cancer or other chronic medical conditions. There was no evidence of lymphadenopathy on examination, and no other skin abnormalities were noted. There was no reported family history of skin cancer or chronic skin conditions suggestive of increased risk for cancer or other pathologic dermatoses. Differential diagnoses for the plaque and nodule complex included verruca, Spitz nevus, or secondary neoplasm within NS.

Excision was conducted under local anesthesia without complication. An elliptical section of skin measuring 0.8×2.5 cm was excised to a depth of 3 mm. The resulting wound was closed using a complex linear repair. The section was placed in formalin specimen transport medium and sent to Walter Reed National Military Medical Center (Bethesda, Maryland). Microscopic examination of the specimen revealed features typical for NS, including mild verrucous epidermal hyperplasia, sebaceous gland hyperplasia, presence of apocrine glands, and hamartomatous follicular proliferations (Figure 2). An even more papillomatous epidermal proliferation that was comprised of atypical squamous cells was present within the lesion. Similar atypical squamous cells infiltrated the superficial dermis in nests, cords, and single cells (Figure 3A). One focus showed perineural invasion with a small superficial nerve fiber surrounded by SCC (Figure 3B). The tumor was completely excised, with negative surgical margins extending approximately 2 mm. Adjuvant radiation therapy and further specialized Mohs micrographic excision were not performed because of the clear histologic appearance of the carcinoma and strong evidence of complete excision.


At 2-week follow-up, the surgical scar on the anterior central forehead was well healed without evidence of SCC recurrence. On physical examination there was neither lymphadenopathy nor signs of neurologic deficit, except for superficial cutaneous hypoesthesia in the immediate area surrounding the healed site. Following discussion with the patient and her parents, it was decided that the patient would obtain baseline laboratory tests, chest radiography, and abdominal ultrasonography, and she would undergo serial follow-up examinations every 3 months for the next 2 years. Annual follow-up was recommended after 2 years, with the caveat to return sooner if recurrence or symptoms were to arise.
Comment
Historically, there has been variability in the histopathologic interpretation of SCC in NS in the literature. Retrospective analysis of the histologic evidence of SCC in the 2 earliest possible cases of pediatric SCC in NS have been questioned due to the lack of clinical data presented and the possibility that the diagnosis of SCC was inaccurate.6 Our case was histopathologically interpreted as superficially invasive, well-differentiated SCC arising within an NS; therefore, we classified this case as SCC and took every precaution to ensure the lesion was completely excised, given the potentially invasive nature of SCC.
Our case is unique because it represents SCC in NS with histologic evidence of perineural involvement. Perineural invasion is a major route of tumor spread in SCC and may result in increased occurrence of regional lymph node spread and distant metastases, with path of least resistance or neural cell adhesion as possible spreading methods.7-9 However, there is a notable amount of prognostic variability based on tumor type, the nerve involved, and degree of involvement.9 It is common for cutaneous SCC to occur with invasion of small intradermal nerves, but a poor outcome is less likely in asymptomatic patients who have perineural involvement that was incidentally discovered on histologic examination.10
In our patient, the entire tumor was completely removed with local excision. Recurrence of the SCC or future symptoms of deep neural invasion were not anticipated given the postoperative evidence of clear margins in the excised skin and subdermal structures as well as the lack of preoperative and postoperative symptoms. Close clinical follow-up was warranted to monitor for early signs of recurrence or neural involvement. We have confidence that the planned follow-up regimen in our patient will reveal any early signs of new occurrence or recurrence.
In the case of recurrence, Mohs micrographic surgery would likely be indicated. We elected not to treat with adjuvant radiotherapy because its benefit in cutaneous SCC with perineural invasion is debatable based on the lack of randomized controlled clinical evidence.10,11 The patient obtained postoperative baseline complete blood cell count with differential, posterior/anterior and lateral chest radiographs, as well as abdominal ultrasonography. Each returned negative findings of hematologic or distant organ metastases, with subsequent follow-up visits also negative for any new concerning findings.
First reported in 1895, nevus sebaceus (NS) is a con genital papillomatous hamartoma most commonly found on the scalp and face. 1 Lesions typically are yellow-orange plaques and often are hairless. Nevus sebaceus is most prominent in the few first months after birth and again at puberty during development of the sebaceous glands. Development of epithelial hyperplasia, cysts, verrucas, and benign or malignant tumors has been reported. 1 The most common benign tumors are syringocystadenoma papilliferum and trichoblastoma. Cases of malignancy are rare, and basal cell carcinoma is the predominant form (approximately 2% of cases). Squamous cell carcinoma (SCC) and adnexal carcinoma are reported at even lower rates. 1 Malignant transformation occurring during childhood is extremely uncommon. According to a PubMed search of articles indexed for MEDLINE using the terms nevus sebaceous, malignancy, and squamous cell carcinoma and narrowing the results to children, there have been only 4 prior reports of SCC developing within an NS in a child. 2-5 We report a case of SCC arising in an NS in a 13-year-old adolescent girl with perineural invasion.
Case Report
A 13-year-old fair-skinned adolescent girl presented with a hairless 2×2.5-cm yellow plaque at the hairline on the anterior central scalp. The plaque had been present since birth and had progressively developed a superiorly located 3×5-mm erythematous verrucous nodule (Figure 1) with an approximate height of 6 mm over the last year. The nodule was subjected to regular trauma and bled with minimal insult. The patient appeared otherwise healthy, with no history of skin cancer or other chronic medical conditions. There was no evidence of lymphadenopathy on examination, and no other skin abnormalities were noted. There was no reported family history of skin cancer or chronic skin conditions suggestive of increased risk for cancer or other pathologic dermatoses. Differential diagnoses for the plaque and nodule complex included verruca, Spitz nevus, or secondary neoplasm within NS.

Excision was conducted under local anesthesia without complication. An elliptical section of skin measuring 0.8×2.5 cm was excised to a depth of 3 mm. The resulting wound was closed using a complex linear repair. The section was placed in formalin specimen transport medium and sent to Walter Reed National Military Medical Center (Bethesda, Maryland). Microscopic examination of the specimen revealed features typical for NS, including mild verrucous epidermal hyperplasia, sebaceous gland hyperplasia, presence of apocrine glands, and hamartomatous follicular proliferations (Figure 2). An even more papillomatous epidermal proliferation that was comprised of atypical squamous cells was present within the lesion. Similar atypical squamous cells infiltrated the superficial dermis in nests, cords, and single cells (Figure 3A). One focus showed perineural invasion with a small superficial nerve fiber surrounded by SCC (Figure 3B). The tumor was completely excised, with negative surgical margins extending approximately 2 mm. Adjuvant radiation therapy and further specialized Mohs micrographic excision were not performed because of the clear histologic appearance of the carcinoma and strong evidence of complete excision.


At 2-week follow-up, the surgical scar on the anterior central forehead was well healed without evidence of SCC recurrence. On physical examination there was neither lymphadenopathy nor signs of neurologic deficit, except for superficial cutaneous hypoesthesia in the immediate area surrounding the healed site. Following discussion with the patient and her parents, it was decided that the patient would obtain baseline laboratory tests, chest radiography, and abdominal ultrasonography, and she would undergo serial follow-up examinations every 3 months for the next 2 years. Annual follow-up was recommended after 2 years, with the caveat to return sooner if recurrence or symptoms were to arise.
Comment
Historically, there has been variability in the histopathologic interpretation of SCC in NS in the literature. Retrospective analysis of the histologic evidence of SCC in the 2 earliest possible cases of pediatric SCC in NS have been questioned due to the lack of clinical data presented and the possibility that the diagnosis of SCC was inaccurate.6 Our case was histopathologically interpreted as superficially invasive, well-differentiated SCC arising within an NS; therefore, we classified this case as SCC and took every precaution to ensure the lesion was completely excised, given the potentially invasive nature of SCC.
Our case is unique because it represents SCC in NS with histologic evidence of perineural involvement. Perineural invasion is a major route of tumor spread in SCC and may result in increased occurrence of regional lymph node spread and distant metastases, with path of least resistance or neural cell adhesion as possible spreading methods.7-9 However, there is a notable amount of prognostic variability based on tumor type, the nerve involved, and degree of involvement.9 It is common for cutaneous SCC to occur with invasion of small intradermal nerves, but a poor outcome is less likely in asymptomatic patients who have perineural involvement that was incidentally discovered on histologic examination.10
In our patient, the entire tumor was completely removed with local excision. Recurrence of the SCC or future symptoms of deep neural invasion were not anticipated given the postoperative evidence of clear margins in the excised skin and subdermal structures as well as the lack of preoperative and postoperative symptoms. Close clinical follow-up was warranted to monitor for early signs of recurrence or neural involvement. We have confidence that the planned follow-up regimen in our patient will reveal any early signs of new occurrence or recurrence.
In the case of recurrence, Mohs micrographic surgery would likely be indicated. We elected not to treat with adjuvant radiotherapy because its benefit in cutaneous SCC with perineural invasion is debatable based on the lack of randomized controlled clinical evidence.10,11 The patient obtained postoperative baseline complete blood cell count with differential, posterior/anterior and lateral chest radiographs, as well as abdominal ultrasonography. Each returned negative findings of hematologic or distant organ metastases, with subsequent follow-up visits also negative for any new concerning findings.
- Cribier B, Scrivener Y, Grosshans E. Tumors arising in nevus sebaceus: a study of 596 cases. J Am Acad Dermatol. 2000;42(2, pt 1):263-268.
- Aguayo R, Pallares J, Cassanova JM, et al. Squamous cell carcinoma developing in Jadassohn’s sebaceous nevus: case report and review of the literature. Dermatol Surg. 2010;36:1763-1768.
- Taher M, Feibleman C, Bennet R. Squamous cell carcinoma arising in a nevus sebaceous of Jadassohn in a 9-year-old girl: treatment using Mohs micrographic surgery with literature review. Dermatol Surg. 2010;36:1203-1208.
- Hidvegi NC, Kangesu L, Wolfe KQ. Squamous cell carcinoma complicating naevus sebaceous of Jadassohn in a child. Br J Plast Surg. 2003;56:50-52.
- Belhadjali H, Moussa A, Yahia S, et al. Simultaneous occurrence of squamous cell carcinomas within a nevus sebaceous of Jadassohn in an 11-year-old girl. Pediatr Dermatol. 2009;26:236-237.
- Wilson-Jones EW, Heyl T. Naevus sebaceus: a report of 140 cases with special regard to the development of secondary malignant tumors. Br J Dermatol. 1970;82:99-117.
- Ballantyne AJ, McCarten AB, Ibanez ML. The extension of cancer of the head and neck through perineural peripheral nerves. Am J Surg. 1963;106:651-667.
- Goepfert H, Dichtel WJ, Medina JE, et al. Perineural invasion in squamous cell skin carcinoma of the head and neck. Am J Surg. 1984;148:542-547.
- Feasel AM, Brown TJ, Bogle MA, et al. Perineural invasion of cutaneous malignancies. Dermatol Surg. 2001;27:531-542.
- Cottel WI. Perineural invasion by squamous cell carcinoma. J Dermatol Surg Oncol. 1982;8:589-600.
- Mendenhall WM, Parsons JT, Mendenhall NP, et al. Carcinoma of the skin of the head and neck with perineural invasion. Head Neck. 1989;11:301-308.
- Cribier B, Scrivener Y, Grosshans E. Tumors arising in nevus sebaceus: a study of 596 cases. J Am Acad Dermatol. 2000;42(2, pt 1):263-268.
- Aguayo R, Pallares J, Cassanova JM, et al. Squamous cell carcinoma developing in Jadassohn’s sebaceous nevus: case report and review of the literature. Dermatol Surg. 2010;36:1763-1768.
- Taher M, Feibleman C, Bennet R. Squamous cell carcinoma arising in a nevus sebaceous of Jadassohn in a 9-year-old girl: treatment using Mohs micrographic surgery with literature review. Dermatol Surg. 2010;36:1203-1208.
- Hidvegi NC, Kangesu L, Wolfe KQ. Squamous cell carcinoma complicating naevus sebaceous of Jadassohn in a child. Br J Plast Surg. 2003;56:50-52.
- Belhadjali H, Moussa A, Yahia S, et al. Simultaneous occurrence of squamous cell carcinomas within a nevus sebaceous of Jadassohn in an 11-year-old girl. Pediatr Dermatol. 2009;26:236-237.
- Wilson-Jones EW, Heyl T. Naevus sebaceus: a report of 140 cases with special regard to the development of secondary malignant tumors. Br J Dermatol. 1970;82:99-117.
- Ballantyne AJ, McCarten AB, Ibanez ML. The extension of cancer of the head and neck through perineural peripheral nerves. Am J Surg. 1963;106:651-667.
- Goepfert H, Dichtel WJ, Medina JE, et al. Perineural invasion in squamous cell skin carcinoma of the head and neck. Am J Surg. 1984;148:542-547.
- Feasel AM, Brown TJ, Bogle MA, et al. Perineural invasion of cutaneous malignancies. Dermatol Surg. 2001;27:531-542.
- Cottel WI. Perineural invasion by squamous cell carcinoma. J Dermatol Surg Oncol. 1982;8:589-600.
- Mendenhall WM, Parsons JT, Mendenhall NP, et al. Carcinoma of the skin of the head and neck with perineural invasion. Head Neck. 1989;11:301-308.
Practice Points
- Nevus sebaceus (NS) is frequently found on the scalp and may increase in size during puberty.
- Commonly found additional neoplasms within NS include trichoblastoma and syringocystadenoma papilliferum. Malignancies are possible but rare.
Update in Psoriasis: Optimizing Combination Topical Therapies to Improve Adherence and Patient Outcomes
Click here to download this supplement.
A majority of patients with psoriasis are undertreated. In recent years, however, new formulations of topical medications for psoriasis have been introduced, with the goal of enhancing penetration, efficacy, and patient acceptance. Along with new systemic therapies, these treatments allow for more aggressive goals for disease clearance.
Topical medications can be used as monotherapy but combining topical agents can increase efficacy and may allow for use of lower doses with fewer adverse events. Fixed-dose combination treatments combine active ingredients in one vehicle and may improve patient adherence and acceptance by simplifying the treatment regimen.
This supplement provides physicians with education on evidence-based assessment and management of psoriasis, the combination of systemic and topical medications, and strategies for improving patient adherence to treatment regimens.
Click here to download this supplement.
Click here to download this supplement.
A majority of patients with psoriasis are undertreated. In recent years, however, new formulations of topical medications for psoriasis have been introduced, with the goal of enhancing penetration, efficacy, and patient acceptance. Along with new systemic therapies, these treatments allow for more aggressive goals for disease clearance.
Topical medications can be used as monotherapy but combining topical agents can increase efficacy and may allow for use of lower doses with fewer adverse events. Fixed-dose combination treatments combine active ingredients in one vehicle and may improve patient adherence and acceptance by simplifying the treatment regimen.
This supplement provides physicians with education on evidence-based assessment and management of psoriasis, the combination of systemic and topical medications, and strategies for improving patient adherence to treatment regimens.
Click here to download this supplement.
Click here to download this supplement.
A majority of patients with psoriasis are undertreated. In recent years, however, new formulations of topical medications for psoriasis have been introduced, with the goal of enhancing penetration, efficacy, and patient acceptance. Along with new systemic therapies, these treatments allow for more aggressive goals for disease clearance.
Topical medications can be used as monotherapy but combining topical agents can increase efficacy and may allow for use of lower doses with fewer adverse events. Fixed-dose combination treatments combine active ingredients in one vehicle and may improve patient adherence and acceptance by simplifying the treatment regimen.
This supplement provides physicians with education on evidence-based assessment and management of psoriasis, the combination of systemic and topical medications, and strategies for improving patient adherence to treatment regimens.
Click here to download this supplement.