User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Manic after having found a ‘cure’ for Alzheimer’s disease
CASE Reckless driving, impulse buying
Mr. A, age 73, is admitted to the inpatient psychiatric unit at a community hospital for evaluation of a psychotic episode. His admission to the unit was initiated by his primary care physician, who noted that Mr. A was “not making sense” during a routine visit. Mr. A was speaking rapidly about how he had discovered that high-dose omega-3 fatty acid supplements were a “cure” for Alzheimer’s disease. He also believes that he was recently appointed as CEO of Microsoft and Apple for his discoveries.
Three months earlier, Mr. A had started taking high doses of omega-3 fatty acid supplements (10 to 15 g/d) because he believed they were the cure for memory problems, pain, and depression. At that time, he discontinued taking nortriptyline, 25 mg/d, and citalopram, 40 mg/d, which his outpatient psychiatrist had prescribed for major depressive disorder (MDD). Mr. A also had stopped taking buprenorphine, 2 mg, sublingual, 4 times a day, which he had been prescribed for chronic pain.
Mr. A’s wife reports that during the last 2 months, her husband had become irritable, impulsive, grandiose, and was sleeping very little. She added that although her husband’s ophthalmologist had advised him to not drive due to impaired vision, he had been driving recklessly across the metropolitan area. He had also spent nearly $15,000 buying furniture and other items for their home.
In addition to MDD, Mr. A has a history of chronic kidney disease, Leber’s hereditary optic neuropathy, and chronic pain. He has been taking vitamin D3, 2,000 U/d, as a nutritional supplement.
[polldaddy:10091672]
The authors’ observations
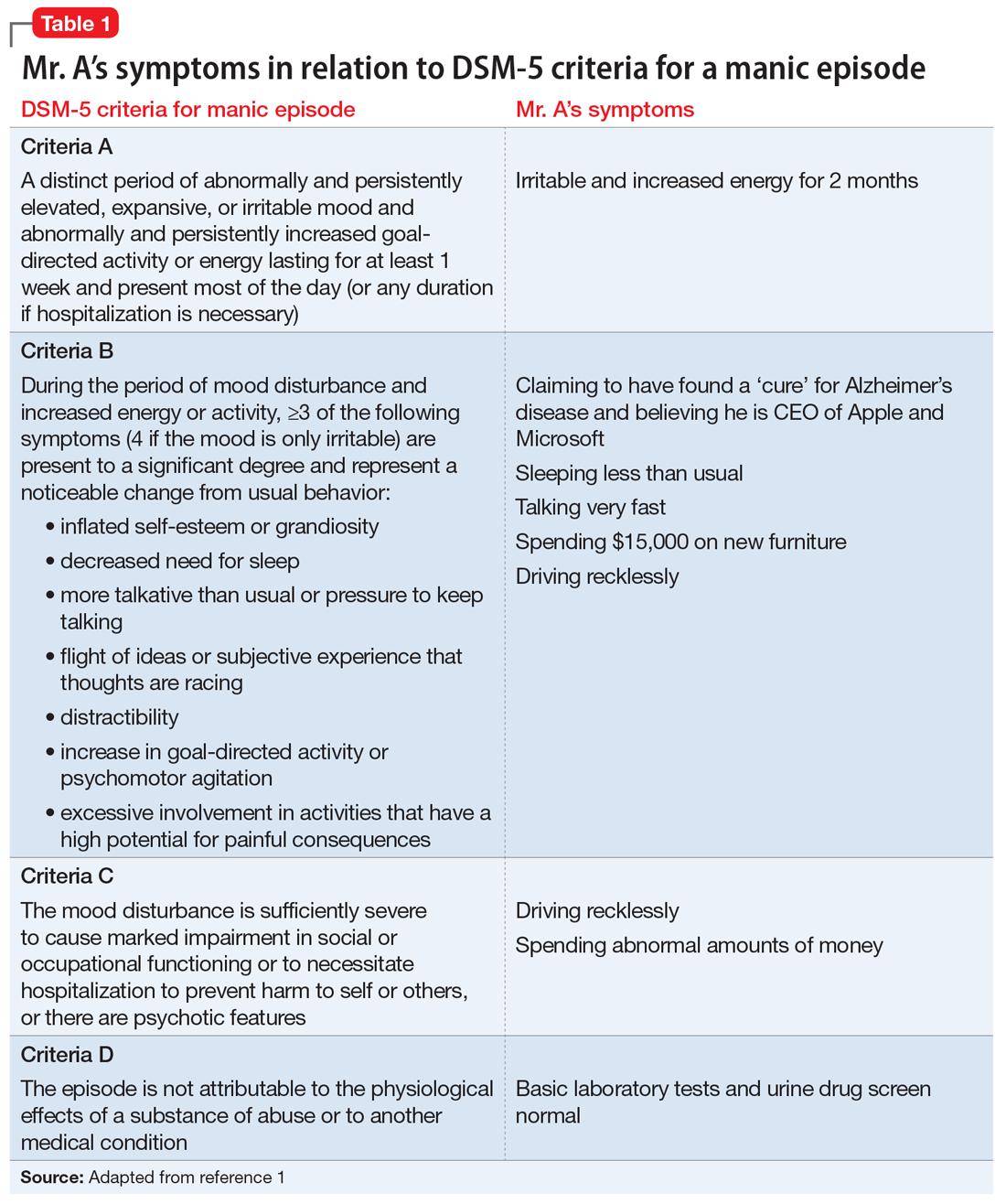
Mr. A met the DSM-5 criteria for a manic episode (Table 11). His manic and delusional symptoms are new. He has a long-standing diagnosis of MDD, which for many years had been successfully treated with antidepressants without a manic switch. The absence of a manic switch when treated with antidepressants without a mood stabilizer suggested that Mr. A did not have bipolarity in terms of a mood disorder diathesis.2 In addition, it would be unusual for an individual to develop a new-onset or primary bipolar disorder after age 60. Individuals in this age group who present with manic symptoms for the first time are preponderantly found to have a general medical or iatrogenic cause for the emergence of these symptoms.3 Mr. A has a history of chronic kidney disease, Leber’s hereditary optic neuropathy, and chronic pain.
Typically a sedentary man, Mr. A had been exhibiting disinhibited behavior, grandiosity, insomnia, and psychosis. These symptoms began 3 months before he was admitted to the psychiatric unit, when he had started taking high doses of omega-3 fatty acid supplements.
Continue to: EVALUATION Persistent mania
EVALUATION Persistent mania
On initial examination, Mr. A is upset and irritable. He is casually dressed and well-groomed. He lacks insight and says he was brought to the hospital against his will, and it is his wife “who is the one who is crazy.” He is oriented to person, place, and time. At times he is found roaming the hallways, being intrusive, hyperverbal, and tangential with pressured speech. He is very difficult to redirect, and regularly interrupts the interview. His vital signs are stable. He walks well, with slow and steady gait, and displays no tremor or bradykinesia.
[polldaddy:10091674]
The authors’ observations
In order to rule out organic causes, a complete blood count, comprehensive metabolic panel, thyroid profile, urine drug screen, and brain MRI were ordered. No abnormalities were found. DHA and EPA levels were not measured because such testing was not available at the laboratory at the hospital.
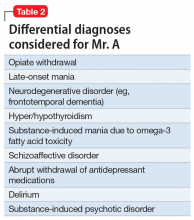
Mania emerging after the sixth decade of life is a rare occurrence. Therefore, we made a substantial effort to try to find another cause that might explain Mr. A’s unusual presentation (Table 2).
Omega-3 fatty acid–induced mania. The major types of omega-3 polyunsaturated fatty acids are EPA and DHA and their precursor, alpha-linolenic acid (ALA). EPA and DHA are found primarily in fatty fish, such as salmon, and in fish oil supplements. Omega-3 fatty acids have beneficial anti-inflammatory, antioxidative, and neuroplastic effects.4 Having properties similar to selective serotonin reuptake inhibitors, omega-3 fatty acids are thought to help prevent depression, have few interactions with other medications, and have a lower adverse-effect burden than antidepressants. They have been found to be beneficial as a maintenance treatment and for prevention of depressive episodes in bipolar depression, but no positive association has been found for bipolar mania.5
Continue to: However, very limited evidence suggests...
However, very limited evidence suggests that omega-3 fatty acid supplements, particularly those with flaxseed oil, can induce hypomania or mania. This association was first reported by Rudin6 in 1981, and later reported in other studies.7 How omega-3 fatty acids might induce mania is unclear.
Mr. A was reportedly taking high doses of an omega-3 fatty acid supplement. We hypothesized that the antidepressant effect of this supplement may have precipitated a manic episode. Mr. A had no history of manic episodes in the past and was stable during the treatment with the outpatient psychiatrist. A first episode mania in the seventh decade of life would be highly unusual without an organic etiology. After laboratory tests found no abnormalities that would point to an organic etiology, iatrogenic causes were considered. After a review of the literature, there was anecdotal evidence for the induction of mania in clinical trials studying the effects of omega-3 supplements on affective disorders.
This led us to ask: How much omega-3 fatty acid supplements, if any, can a patient with a depressive or bipolar disorder safely take? Currently, omega-3 fatty acid supplements are not FDA-approved for the treatment of depression or bipolar disorder. However, patients may take 1.5 to 2 g/d for MDD. Further research is needed to determine the optimal dose. It is unclear at this time if omega-3 fatty acid supplementation has any benefit in the acute or maintenance treatment of bipolar disorder.
Alternative nutritional supplements for mood disorders. Traditionally, mood disorders, such as MDD and bipolar disorder, have been treated with psychotropic medications. However, through the years, sporadic studies have examined the efficacy of nutritional interventions as a cost-effective approach to preventing and treating these conditions.5 Proponents of this approach believe such supplements can increase efficacy, as well as decrease the required dose of psychotropic medications, thus potentially minimizing adverse effects. However, their overuse can pose a potential threat of toxicity or unexpected adverse effects, such as precipitation of mania. Table 38 lists over-the-counter nutritional and/or herbal agents that may cause mania.
Continue to: TREATMENT Nonadherence leads to a court order
TREATMENT Nonadherence leads to a court order
On admission, Mr. A receives a dose of
[polldaddy:10091676]
The authors’ observations
During an acute manic episode, the goal of treatment is urgent mood stabilization. Monotherapy can be used; however, in emergent settings, a combination is often used for a rapid response. The most commonly used agents are lithium, anticonvulsants such as valproic acid, and antipsychotics.9 In addition, benzodiazepines can be used for insomnia, agitation, or anxiety. The decision to use lithium, an anticonvulsant, or an antipsychotic depends upon the specific medication’s adverse effects, the patient’s medical history, previous medication trials, drug–drug interactions, patient preference, and cost.
Because Mr. A has a history of chronic kidney disease, lithium was contraindicated.
[polldaddy:10091678]
Continue to: The authors' observations
The authors’ observations
After the acute episode of mania resolves, maintenance pharmacotherapy typically involves continuing the same regimen that achieved mood stabilization. Monotherapy is typically preferred to combination therapy, but it is not always possible after a manic episode.10 A reasonable approach is to slowly taper the antipsychotic after several months of dual therapy if symptoms continue to be well-controlled. Further adjustments may be necessary, depending on the medications’ adverse effects. Moreover, further acute episodes of mania or depression will also determine future treatment.
OUTCOME Resolution of delusions
Mr. A is discharged 30 days after admission. At this point, his acute manic episode has resolved with non-tangential, non-pressured speech, improved sleep, and decreased impulsivity. His grandiose delusions also have resolved. He is prescribed valproic acid, 1,000 mg/d, and risperidone, 6 mg/d at bedtime, under the care of his outpatient psychiatrist.
Bottom Line
Initial presentation of a manic episode in an older patient is rare. It is important to rule out organic causes. Weak evidence suggests omega-3 fatty acid supplements may have the potential to induce mania in certain patients.
Related Resource
- Ramaswamy S, Driscoll D, Rodriguez A, et al. Nutraceuticals for traumatic brain injury: Should you recommend their use? Current Psychiatry. 2017;16(7):34-38,40,41-45.
Drug Brand Names
Buprenorphine • Suboxone, Subutex
Citalopram • Celexa
Hydrocodone/acetaminophen • Vicodin
Lithium • Eskalith, Lithobid
Lorazepam• Ativan
Nortriptyline • Pamelor
Risperidone • Risperdal
Valproic acid • Depakote
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Goodwin GM, Haddad PM, Ferrier IN, et al. Evidence-based guidelines for treating bipolar disorder: revised third edition recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2016;30(6):495-553.
3. Sami M, Khan H, Nilforooshan R. Late onset mania as an organic syndrome: a review of case reports in the literature. J Affect Disord. 2015:188:226-231.
4. Su KP, Matsuoka Y, Pae CU. Omega-3 polyunsaturated fatty acids in prevention of mood and anxiety disorders. Clin Psychopharmacol Neurosci. 2015;13(2):129-137.
5. Sarris J, Mischoulon D, Schweitzer I. Omega-3 for bipolar disorder: meta-analyses of use in mania and bipolar depression. J Clin Psychiatry. 2012;73(1):81-86.
6. Rudin DO. The major psychoses and neuroses as omega-3 essential fatty acid deficiency syndrome: substrate pellagra. Biol Psychiatry. 1981;16(9):837-850.
7. Su KP, Shen WW, Huang SY. Are omega3 fatty acids beneficial in depression but not mania? Arch Gen Psychiatry. 2000;57(7):716-717.
8. Joshi K, Faubion M. Mania and psychosis associated with St. John’s wort and ginseng. Psychiatry (Edgmont). 2005;2(9):56-61.
9. Grunze H, Vieta E, Goodwin GM, et al. The world federation of societies of biological psychiatry (WFSBP) guidelines for the biological treatment of bipolar disorders: update 2009 on the treatment of acute mania. World J Biol Psychiatry. 2009;10(2):85-116.
10. Suppes T, Vieta E, Liu S, et al; Trial 127 Investigators. Maintenance treatment for patients with bipolar I disorder: results from a North American study of quetiapine in combination with lithium or divalproex (trial 127). Am J Psychiatry. 2009;166(4):476-488.
CASE Reckless driving, impulse buying
Mr. A, age 73, is admitted to the inpatient psychiatric unit at a community hospital for evaluation of a psychotic episode. His admission to the unit was initiated by his primary care physician, who noted that Mr. A was “not making sense” during a routine visit. Mr. A was speaking rapidly about how he had discovered that high-dose omega-3 fatty acid supplements were a “cure” for Alzheimer’s disease. He also believes that he was recently appointed as CEO of Microsoft and Apple for his discoveries.
Three months earlier, Mr. A had started taking high doses of omega-3 fatty acid supplements (10 to 15 g/d) because he believed they were the cure for memory problems, pain, and depression. At that time, he discontinued taking nortriptyline, 25 mg/d, and citalopram, 40 mg/d, which his outpatient psychiatrist had prescribed for major depressive disorder (MDD). Mr. A also had stopped taking buprenorphine, 2 mg, sublingual, 4 times a day, which he had been prescribed for chronic pain.
Mr. A’s wife reports that during the last 2 months, her husband had become irritable, impulsive, grandiose, and was sleeping very little. She added that although her husband’s ophthalmologist had advised him to not drive due to impaired vision, he had been driving recklessly across the metropolitan area. He had also spent nearly $15,000 buying furniture and other items for their home.
In addition to MDD, Mr. A has a history of chronic kidney disease, Leber’s hereditary optic neuropathy, and chronic pain. He has been taking vitamin D3, 2,000 U/d, as a nutritional supplement.
[polldaddy:10091672]
The authors’ observations
Mr. A met the DSM-5 criteria for a manic episode (Table 11). His manic and delusional symptoms are new. He has a long-standing diagnosis of MDD, which for many years had been successfully treated with antidepressants without a manic switch. The absence of a manic switch when treated with antidepressants without a mood stabilizer suggested that Mr. A did not have bipolarity in terms of a mood disorder diathesis.2 In addition, it would be unusual for an individual to develop a new-onset or primary bipolar disorder after age 60. Individuals in this age group who present with manic symptoms for the first time are preponderantly found to have a general medical or iatrogenic cause for the emergence of these symptoms.3 Mr. A has a history of chronic kidney disease, Leber’s hereditary optic neuropathy, and chronic pain.

Typically a sedentary man, Mr. A had been exhibiting disinhibited behavior, grandiosity, insomnia, and psychosis. These symptoms began 3 months before he was admitted to the psychiatric unit, when he had started taking high doses of omega-3 fatty acid supplements.
Continue to: EVALUATION Persistent mania
EVALUATION Persistent mania
On initial examination, Mr. A is upset and irritable. He is casually dressed and well-groomed. He lacks insight and says he was brought to the hospital against his will, and it is his wife “who is the one who is crazy.” He is oriented to person, place, and time. At times he is found roaming the hallways, being intrusive, hyperverbal, and tangential with pressured speech. He is very difficult to redirect, and regularly interrupts the interview. His vital signs are stable. He walks well, with slow and steady gait, and displays no tremor or bradykinesia.
[polldaddy:10091674]
The authors’ observations
In order to rule out organic causes, a complete blood count, comprehensive metabolic panel, thyroid profile, urine drug screen, and brain MRI were ordered. No abnormalities were found. DHA and EPA levels were not measured because such testing was not available at the laboratory at the hospital.
Mania emerging after the sixth decade of life is a rare occurrence. Therefore, we made a substantial effort to try to find another cause that might explain Mr. A’s unusual presentation (Table 2).
Omega-3 fatty acid–induced mania. The major types of omega-3 polyunsaturated fatty acids are EPA and DHA and their precursor, alpha-linolenic acid (ALA). EPA and DHA are found primarily in fatty fish, such as salmon, and in fish oil supplements. Omega-3 fatty acids have beneficial anti-inflammatory, antioxidative, and neuroplastic effects.4 Having properties similar to selective serotonin reuptake inhibitors, omega-3 fatty acids are thought to help prevent depression, have few interactions with other medications, and have a lower adverse-effect burden than antidepressants. They have been found to be beneficial as a maintenance treatment and for prevention of depressive episodes in bipolar depression, but no positive association has been found for bipolar mania.5
Continue to: However, very limited evidence suggests...
However, very limited evidence suggests that omega-3 fatty acid supplements, particularly those with flaxseed oil, can induce hypomania or mania. This association was first reported by Rudin6 in 1981, and later reported in other studies.7 How omega-3 fatty acids might induce mania is unclear.
Mr. A was reportedly taking high doses of an omega-3 fatty acid supplement. We hypothesized that the antidepressant effect of this supplement may have precipitated a manic episode. Mr. A had no history of manic episodes in the past and was stable during the treatment with the outpatient psychiatrist. A first episode mania in the seventh decade of life would be highly unusual without an organic etiology. After laboratory tests found no abnormalities that would point to an organic etiology, iatrogenic causes were considered. After a review of the literature, there was anecdotal evidence for the induction of mania in clinical trials studying the effects of omega-3 supplements on affective disorders.
This led us to ask: How much omega-3 fatty acid supplements, if any, can a patient with a depressive or bipolar disorder safely take? Currently, omega-3 fatty acid supplements are not FDA-approved for the treatment of depression or bipolar disorder. However, patients may take 1.5 to 2 g/d for MDD. Further research is needed to determine the optimal dose. It is unclear at this time if omega-3 fatty acid supplementation has any benefit in the acute or maintenance treatment of bipolar disorder.
Alternative nutritional supplements for mood disorders. Traditionally, mood disorders, such as MDD and bipolar disorder, have been treated with psychotropic medications. However, through the years, sporadic studies have examined the efficacy of nutritional interventions as a cost-effective approach to preventing and treating these conditions.5 Proponents of this approach believe such supplements can increase efficacy, as well as decrease the required dose of psychotropic medications, thus potentially minimizing adverse effects. However, their overuse can pose a potential threat of toxicity or unexpected adverse effects, such as precipitation of mania. Table 38 lists over-the-counter nutritional and/or herbal agents that may cause mania.
Continue to: TREATMENT Nonadherence leads to a court order
TREATMENT Nonadherence leads to a court order
On admission, Mr. A receives a dose of
[polldaddy:10091676]
The authors’ observations
During an acute manic episode, the goal of treatment is urgent mood stabilization. Monotherapy can be used; however, in emergent settings, a combination is often used for a rapid response. The most commonly used agents are lithium, anticonvulsants such as valproic acid, and antipsychotics.9 In addition, benzodiazepines can be used for insomnia, agitation, or anxiety. The decision to use lithium, an anticonvulsant, or an antipsychotic depends upon the specific medication’s adverse effects, the patient’s medical history, previous medication trials, drug–drug interactions, patient preference, and cost.
Because Mr. A has a history of chronic kidney disease, lithium was contraindicated.
[polldaddy:10091678]
Continue to: The authors' observations
The authors’ observations
After the acute episode of mania resolves, maintenance pharmacotherapy typically involves continuing the same regimen that achieved mood stabilization. Monotherapy is typically preferred to combination therapy, but it is not always possible after a manic episode.10 A reasonable approach is to slowly taper the antipsychotic after several months of dual therapy if symptoms continue to be well-controlled. Further adjustments may be necessary, depending on the medications’ adverse effects. Moreover, further acute episodes of mania or depression will also determine future treatment.
OUTCOME Resolution of delusions
Mr. A is discharged 30 days after admission. At this point, his acute manic episode has resolved with non-tangential, non-pressured speech, improved sleep, and decreased impulsivity. His grandiose delusions also have resolved. He is prescribed valproic acid, 1,000 mg/d, and risperidone, 6 mg/d at bedtime, under the care of his outpatient psychiatrist.
Bottom Line
Initial presentation of a manic episode in an older patient is rare. It is important to rule out organic causes. Weak evidence suggests omega-3 fatty acid supplements may have the potential to induce mania in certain patients.
Related Resource
- Ramaswamy S, Driscoll D, Rodriguez A, et al. Nutraceuticals for traumatic brain injury: Should you recommend their use? Current Psychiatry. 2017;16(7):34-38,40,41-45.
Drug Brand Names
Buprenorphine • Suboxone, Subutex
Citalopram • Celexa
Hydrocodone/acetaminophen • Vicodin
Lithium • Eskalith, Lithobid
Lorazepam• Ativan
Nortriptyline • Pamelor
Risperidone • Risperdal
Valproic acid • Depakote
CASE Reckless driving, impulse buying
Mr. A, age 73, is admitted to the inpatient psychiatric unit at a community hospital for evaluation of a psychotic episode. His admission to the unit was initiated by his primary care physician, who noted that Mr. A was “not making sense” during a routine visit. Mr. A was speaking rapidly about how he had discovered that high-dose omega-3 fatty acid supplements were a “cure” for Alzheimer’s disease. He also believes that he was recently appointed as CEO of Microsoft and Apple for his discoveries.
Three months earlier, Mr. A had started taking high doses of omega-3 fatty acid supplements (10 to 15 g/d) because he believed they were the cure for memory problems, pain, and depression. At that time, he discontinued taking nortriptyline, 25 mg/d, and citalopram, 40 mg/d, which his outpatient psychiatrist had prescribed for major depressive disorder (MDD). Mr. A also had stopped taking buprenorphine, 2 mg, sublingual, 4 times a day, which he had been prescribed for chronic pain.
Mr. A’s wife reports that during the last 2 months, her husband had become irritable, impulsive, grandiose, and was sleeping very little. She added that although her husband’s ophthalmologist had advised him to not drive due to impaired vision, he had been driving recklessly across the metropolitan area. He had also spent nearly $15,000 buying furniture and other items for their home.
In addition to MDD, Mr. A has a history of chronic kidney disease, Leber’s hereditary optic neuropathy, and chronic pain. He has been taking vitamin D3, 2,000 U/d, as a nutritional supplement.
[polldaddy:10091672]
The authors’ observations
Mr. A met the DSM-5 criteria for a manic episode (Table 11). His manic and delusional symptoms are new. He has a long-standing diagnosis of MDD, which for many years had been successfully treated with antidepressants without a manic switch. The absence of a manic switch when treated with antidepressants without a mood stabilizer suggested that Mr. A did not have bipolarity in terms of a mood disorder diathesis.2 In addition, it would be unusual for an individual to develop a new-onset or primary bipolar disorder after age 60. Individuals in this age group who present with manic symptoms for the first time are preponderantly found to have a general medical or iatrogenic cause for the emergence of these symptoms.3 Mr. A has a history of chronic kidney disease, Leber’s hereditary optic neuropathy, and chronic pain.

Typically a sedentary man, Mr. A had been exhibiting disinhibited behavior, grandiosity, insomnia, and psychosis. These symptoms began 3 months before he was admitted to the psychiatric unit, when he had started taking high doses of omega-3 fatty acid supplements.
Continue to: EVALUATION Persistent mania
EVALUATION Persistent mania
On initial examination, Mr. A is upset and irritable. He is casually dressed and well-groomed. He lacks insight and says he was brought to the hospital against his will, and it is his wife “who is the one who is crazy.” He is oriented to person, place, and time. At times he is found roaming the hallways, being intrusive, hyperverbal, and tangential with pressured speech. He is very difficult to redirect, and regularly interrupts the interview. His vital signs are stable. He walks well, with slow and steady gait, and displays no tremor or bradykinesia.
[polldaddy:10091674]
The authors’ observations
In order to rule out organic causes, a complete blood count, comprehensive metabolic panel, thyroid profile, urine drug screen, and brain MRI were ordered. No abnormalities were found. DHA and EPA levels were not measured because such testing was not available at the laboratory at the hospital.
Mania emerging after the sixth decade of life is a rare occurrence. Therefore, we made a substantial effort to try to find another cause that might explain Mr. A’s unusual presentation (Table 2).
Omega-3 fatty acid–induced mania. The major types of omega-3 polyunsaturated fatty acids are EPA and DHA and their precursor, alpha-linolenic acid (ALA). EPA and DHA are found primarily in fatty fish, such as salmon, and in fish oil supplements. Omega-3 fatty acids have beneficial anti-inflammatory, antioxidative, and neuroplastic effects.4 Having properties similar to selective serotonin reuptake inhibitors, omega-3 fatty acids are thought to help prevent depression, have few interactions with other medications, and have a lower adverse-effect burden than antidepressants. They have been found to be beneficial as a maintenance treatment and for prevention of depressive episodes in bipolar depression, but no positive association has been found for bipolar mania.5
Continue to: However, very limited evidence suggests...
However, very limited evidence suggests that omega-3 fatty acid supplements, particularly those with flaxseed oil, can induce hypomania or mania. This association was first reported by Rudin6 in 1981, and later reported in other studies.7 How omega-3 fatty acids might induce mania is unclear.
Mr. A was reportedly taking high doses of an omega-3 fatty acid supplement. We hypothesized that the antidepressant effect of this supplement may have precipitated a manic episode. Mr. A had no history of manic episodes in the past and was stable during the treatment with the outpatient psychiatrist. A first episode mania in the seventh decade of life would be highly unusual without an organic etiology. After laboratory tests found no abnormalities that would point to an organic etiology, iatrogenic causes were considered. After a review of the literature, there was anecdotal evidence for the induction of mania in clinical trials studying the effects of omega-3 supplements on affective disorders.
This led us to ask: How much omega-3 fatty acid supplements, if any, can a patient with a depressive or bipolar disorder safely take? Currently, omega-3 fatty acid supplements are not FDA-approved for the treatment of depression or bipolar disorder. However, patients may take 1.5 to 2 g/d for MDD. Further research is needed to determine the optimal dose. It is unclear at this time if omega-3 fatty acid supplementation has any benefit in the acute or maintenance treatment of bipolar disorder.
Alternative nutritional supplements for mood disorders. Traditionally, mood disorders, such as MDD and bipolar disorder, have been treated with psychotropic medications. However, through the years, sporadic studies have examined the efficacy of nutritional interventions as a cost-effective approach to preventing and treating these conditions.5 Proponents of this approach believe such supplements can increase efficacy, as well as decrease the required dose of psychotropic medications, thus potentially minimizing adverse effects. However, their overuse can pose a potential threat of toxicity or unexpected adverse effects, such as precipitation of mania. Table 38 lists over-the-counter nutritional and/or herbal agents that may cause mania.
Continue to: TREATMENT Nonadherence leads to a court order
TREATMENT Nonadherence leads to a court order
On admission, Mr. A receives a dose of
[polldaddy:10091676]
The authors’ observations
During an acute manic episode, the goal of treatment is urgent mood stabilization. Monotherapy can be used; however, in emergent settings, a combination is often used for a rapid response. The most commonly used agents are lithium, anticonvulsants such as valproic acid, and antipsychotics.9 In addition, benzodiazepines can be used for insomnia, agitation, or anxiety. The decision to use lithium, an anticonvulsant, or an antipsychotic depends upon the specific medication’s adverse effects, the patient’s medical history, previous medication trials, drug–drug interactions, patient preference, and cost.
Because Mr. A has a history of chronic kidney disease, lithium was contraindicated.
[polldaddy:10091678]
Continue to: The authors' observations
The authors’ observations
After the acute episode of mania resolves, maintenance pharmacotherapy typically involves continuing the same regimen that achieved mood stabilization. Monotherapy is typically preferred to combination therapy, but it is not always possible after a manic episode.10 A reasonable approach is to slowly taper the antipsychotic after several months of dual therapy if symptoms continue to be well-controlled. Further adjustments may be necessary, depending on the medications’ adverse effects. Moreover, further acute episodes of mania or depression will also determine future treatment.
OUTCOME Resolution of delusions
Mr. A is discharged 30 days after admission. At this point, his acute manic episode has resolved with non-tangential, non-pressured speech, improved sleep, and decreased impulsivity. His grandiose delusions also have resolved. He is prescribed valproic acid, 1,000 mg/d, and risperidone, 6 mg/d at bedtime, under the care of his outpatient psychiatrist.
Bottom Line
Initial presentation of a manic episode in an older patient is rare. It is important to rule out organic causes. Weak evidence suggests omega-3 fatty acid supplements may have the potential to induce mania in certain patients.
Related Resource
- Ramaswamy S, Driscoll D, Rodriguez A, et al. Nutraceuticals for traumatic brain injury: Should you recommend their use? Current Psychiatry. 2017;16(7):34-38,40,41-45.
Drug Brand Names
Buprenorphine • Suboxone, Subutex
Citalopram • Celexa
Hydrocodone/acetaminophen • Vicodin
Lithium • Eskalith, Lithobid
Lorazepam• Ativan
Nortriptyline • Pamelor
Risperidone • Risperdal
Valproic acid • Depakote
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Goodwin GM, Haddad PM, Ferrier IN, et al. Evidence-based guidelines for treating bipolar disorder: revised third edition recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2016;30(6):495-553.
3. Sami M, Khan H, Nilforooshan R. Late onset mania as an organic syndrome: a review of case reports in the literature. J Affect Disord. 2015:188:226-231.
4. Su KP, Matsuoka Y, Pae CU. Omega-3 polyunsaturated fatty acids in prevention of mood and anxiety disorders. Clin Psychopharmacol Neurosci. 2015;13(2):129-137.
5. Sarris J, Mischoulon D, Schweitzer I. Omega-3 for bipolar disorder: meta-analyses of use in mania and bipolar depression. J Clin Psychiatry. 2012;73(1):81-86.
6. Rudin DO. The major psychoses and neuroses as omega-3 essential fatty acid deficiency syndrome: substrate pellagra. Biol Psychiatry. 1981;16(9):837-850.
7. Su KP, Shen WW, Huang SY. Are omega3 fatty acids beneficial in depression but not mania? Arch Gen Psychiatry. 2000;57(7):716-717.
8. Joshi K, Faubion M. Mania and psychosis associated with St. John’s wort and ginseng. Psychiatry (Edgmont). 2005;2(9):56-61.
9. Grunze H, Vieta E, Goodwin GM, et al. The world federation of societies of biological psychiatry (WFSBP) guidelines for the biological treatment of bipolar disorders: update 2009 on the treatment of acute mania. World J Biol Psychiatry. 2009;10(2):85-116.
10. Suppes T, Vieta E, Liu S, et al; Trial 127 Investigators. Maintenance treatment for patients with bipolar I disorder: results from a North American study of quetiapine in combination with lithium or divalproex (trial 127). Am J Psychiatry. 2009;166(4):476-488.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Goodwin GM, Haddad PM, Ferrier IN, et al. Evidence-based guidelines for treating bipolar disorder: revised third edition recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2016;30(6):495-553.
3. Sami M, Khan H, Nilforooshan R. Late onset mania as an organic syndrome: a review of case reports in the literature. J Affect Disord. 2015:188:226-231.
4. Su KP, Matsuoka Y, Pae CU. Omega-3 polyunsaturated fatty acids in prevention of mood and anxiety disorders. Clin Psychopharmacol Neurosci. 2015;13(2):129-137.
5. Sarris J, Mischoulon D, Schweitzer I. Omega-3 for bipolar disorder: meta-analyses of use in mania and bipolar depression. J Clin Psychiatry. 2012;73(1):81-86.
6. Rudin DO. The major psychoses and neuroses as omega-3 essential fatty acid deficiency syndrome: substrate pellagra. Biol Psychiatry. 1981;16(9):837-850.
7. Su KP, Shen WW, Huang SY. Are omega3 fatty acids beneficial in depression but not mania? Arch Gen Psychiatry. 2000;57(7):716-717.
8. Joshi K, Faubion M. Mania and psychosis associated with St. John’s wort and ginseng. Psychiatry (Edgmont). 2005;2(9):56-61.
9. Grunze H, Vieta E, Goodwin GM, et al. The world federation of societies of biological psychiatry (WFSBP) guidelines for the biological treatment of bipolar disorders: update 2009 on the treatment of acute mania. World J Biol Psychiatry. 2009;10(2):85-116.
10. Suppes T, Vieta E, Liu S, et al; Trial 127 Investigators. Maintenance treatment for patients with bipolar I disorder: results from a North American study of quetiapine in combination with lithium or divalproex (trial 127). Am J Psychiatry. 2009;166(4):476-488.
Receptor occupancy and drug response: Understanding the relationship
Most clinicians do not think about receptor occupancy when they prescribe a medication. Most simply assume that if they use a low dose of a medication, they will get a small effect, and if they use a higher dose, they will get a larger effect. However, this is frequently not accurate. Clinicians need to understand the relationship between receptor occupancy and drug response.
In general, when an antagonist of a neurotransmitter receptor is used, it must occupy a minimum of 65% to 70% of the target receptor to be effective. This is clearly the case when the target is a postsynaptic receptor, such as the dopamine D2 receptor.1-3 Similarly, despite significant variability in antidepressant response,4 blockade of 65% to 80% of presynaptic transport proteins—such as the serotonin reuptake pumps when considering serotoninergic antidepressants,5,6 or the norepinephrine reuptake pumps when considering noradrenergic agents such as nortriptyline7—is necessary for these medications to be effective.
It is reasonable to think of the drug response of such agents as following a “threshold” model (Figure 1). This model makes 2 predictions. The first prediction is that a low dose of the drug might result in <50% receptor occupancy, but is not associated with a smaller response; it is simply ineffective. The second prediction is that a very high dose of the drug (eg, one that may exceed 90% receptor occupancy) does not result in any additional benefit, but may cause additional adverse consequences.8

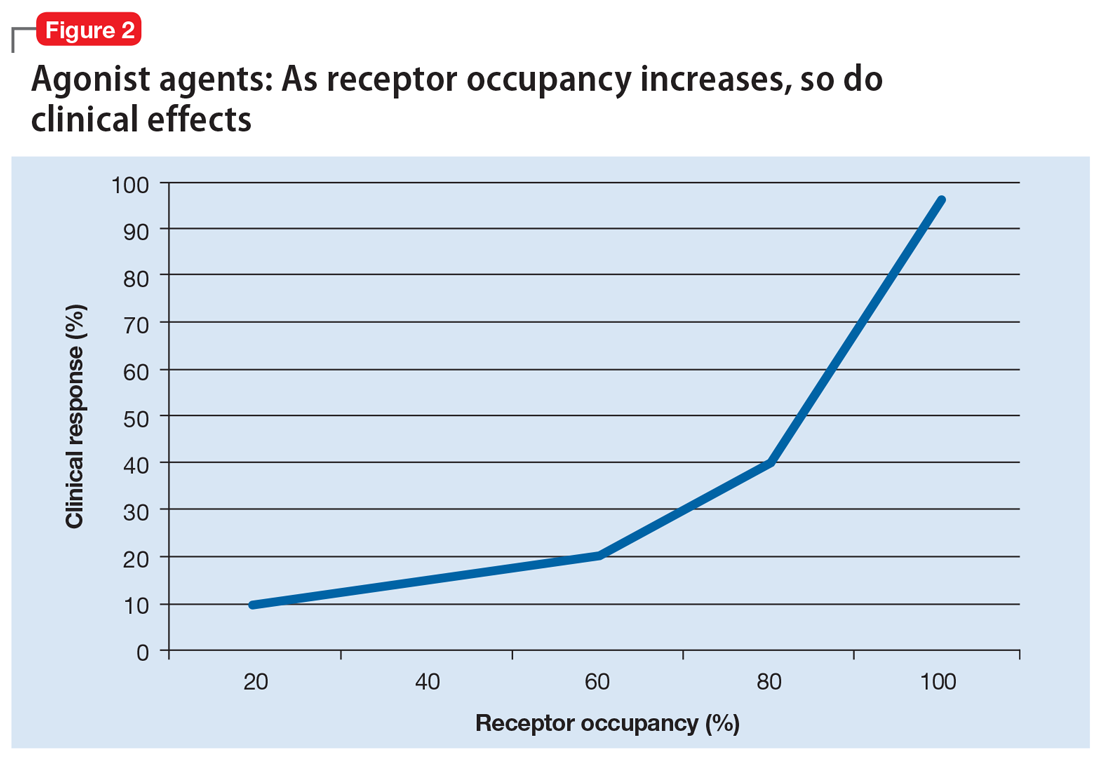
Alternatively, agonist medications, such as benzodiazepines or opiates, have their efficacy in a continuous dose-dependent fashion (Figure 2). Titrating these medications for clinical response is necessary, and minimal effective doses are highly individual. Agonist medications will not be addressed further in this article.
In this article, the term “response” is used to denote the average (population) symptom change in a study population. This term is not used as clinicians often use it to mean that their specific patient’s illness has improved, or that the patient has gone into remission. Furthermore, the information described in this article does not optimize clinical outcome, but instead is intended to help clinicians optimize the use of their pharmacologic tools.
Minimal effective dose
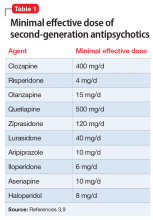
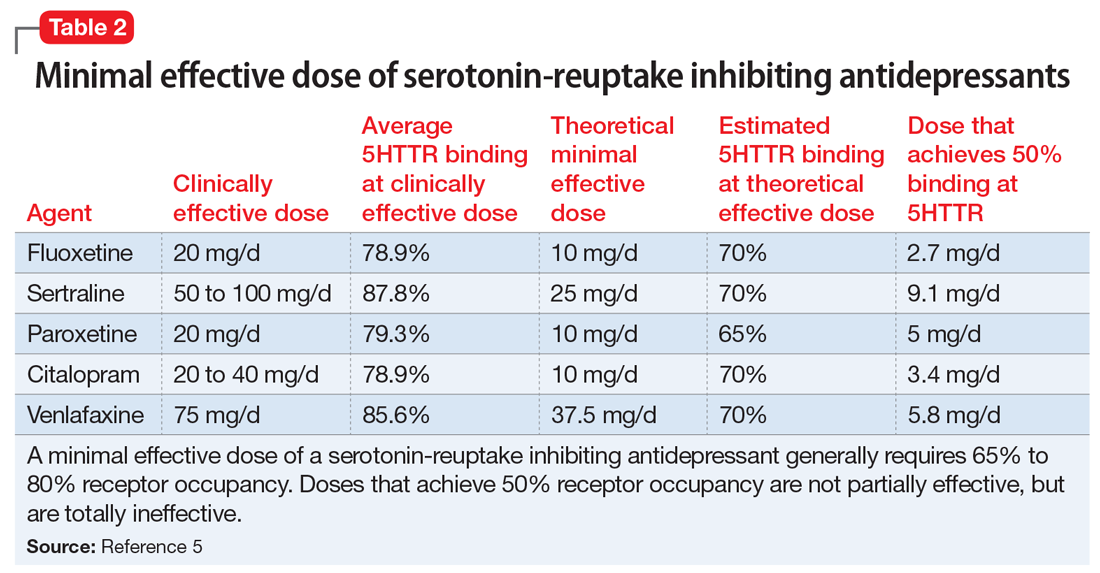
Medications that have a threshold for activity will display that clinically in a minimal effective dose (Table 13,9 and Table 25). The minimal effective dose of medications that act by blocking a neurotransmitter receptor is usually the dose that achieves 65% to 80% receptor occupancy in typical individuals (Table 25). The minimal effective doses for antipsychotics are listed in Table 1.3,9 These doses are known to occupy approximately 65% to 70% of postsynaptic D2 receptors in living humans as confirmed by positron emission tomography (PET) scans.10 Similar minimal effective doses can be determined for serotonin-reuptake inhibiting (SRI) antidepressants (Table 25). In placebo-controlled trials, doses that were smaller than the minimal effective dose did not provide any benefit.
There are important caveats to this. First is the use of partial agonists. Depending on the level of intrinsic activity of a partial agonist and clinical goal, the clinician may aim for a different level of receptor occupancy. For example, aripiprazole will act as a dopamine agonist at lower concentrations, but blocks the receptor at higher concentrations.11 Unlike antagonist antipsychotics, which require only 65% to 70% D2 receptor occupancy to be effective, aripiprazole receptor binding at effective antipsychotic doses is 90% to 95%.12-14 Since aripiprazole has an intrinsic activity of approximately 30% (ie, when it binds, it stimulates the D2 receptor to about 30% of the effect of dopamine binding to the receptor15), binding to 90% of the receptors, and displacing endogenous dopamine, allows aripiprazole to replace the background or tonic tone of dopamine, which has been measured at 19% in people with schizophrenia and 9% in controls.16 Clinically, this still appears as the minimal effective dose achieving maximal response17-19 without significant parkinsonism despite >90% receptor occupancy.12
Continue to: The second caveat is...
The second caveat is the action of low D2 receptor affinity antipsychotics, such as clozapine and quetiapine. These agents generally achieve adequate D2 receptor occupancy for only a brief period of time.20 It has been suggested that continuous receptor occupancy at ≥65% may not be necessary to obtain antipsychotic control.21,22 There may also be specific limbic and cortical (vs striatal) D2 receptor selectivity by clozapine23 compared with other second-generation antipsychotics such as risperidone and olanzapine,24,25 although this point remains debatable.26 Furthermore, the antipsychotic efficacy of low D2 receptor affinity drugs is unreliable, even in controlled, blinded studies (eg, a failed large quetiapine study27). Thus far, the actual antipsychotic mechanism of these agents is yet to be fully understood.
Minimal effective dose achieves maximal response
An interesting aspect of the threshold phenomenon of drug response is that once the minimal effective dose is reached, maximal response is achieved. In other words, there is no additional efficacy with additional dose increases. This is readily demonstrated in some studies in which patients were randomly assigned to different fixed doses or dose ranges. In these studies, there was generally no difference in response rates of different doses, so that once 65% to 80% receptor occupancy is achieved, minimal and maximal clinical response is simultaneously reached.18,28,29
For example, in the original risperidone studies, 6 mg/d was essentially equivalent to 16 mg/d.28 Similarly, lurasidone, 40 mg/d, achieves approximately 65% D2 occupancy.30 When the daily dose is increased to 120 mg, there is no additional benefit in controlling psychosis in schizophrenia.29 This pattern is also seen in partial agonists, where there are no differences between lower and higher doses in terms of response.18
Upon reading this, many clinicians may think “I don’t care what the studies say, I have seen additional benefits with additional doses.” There are several explanations for this. One is that individual patients have genetic variants that may prevent them from responding in a typical fashion. Hints of this are seen in an apparent disconnect between dosage and drug levels, so that it is not surprising that drug levels are a much better predictor of receptor occupancy than dosage.31 Nonetheless, as previously pointed out, for a population, dosage does predict receptor occupancy and outcome. However, for individuals, genetic variations make dosages less reliable. For example, ultrarapid metabolizers of cytochrome P450 (CYP) 2D6 may discontinue risperidone due to nonresponse, or require a higher dose or longer time period to respond.32,33 Similarly, patients who smoke may require an increase in doses of CYP1A2 substrates such as clozapine and olanzapine.34
Alternatively, the clinician may note improvement in mood, sleep, appetite, or other symptoms at lower doses, and then note additional improvements in psychosis or mania at higher doses.3 This occurs due to the varying affinity of different receptors. For example, in bipolar depression trials that used quetiapine in a fixed-dose design, patients who received 300 or 600 mg/d responded in the same fashion, with no additional benefit in improving depression with the higher dose.35 Similarly, in a flexible dose range study that evaluated lurasidone in bipolar depression, an average dose of 34 mg/d (range 20 to 60 mg/d) and an average dose of 83 mg/d (range 80 to 120 mg/d) both resulted in the same response (a 15.4-point reduction in depression ratings and an effect size of 0.51).36 For both quetiapine and lurasidone, higher doses are generally required to control psychosis.29,37 Note that for lurasidone, agitation, but not psychosis, improves with higher doses, which suggests that recruitment of additional receptors results in improvement in a different set of symptoms.9
Continue to: Clinical implications
Clinical implications
The implications for clinicians are relatively clear. Knowing the minimal effective doses for depression, psychosis, or mania informs the target dose. If improvement is seen at lower doses, the clinician needs to assess the profile of symptoms that improved, potential drug–drug interactions, or potential irregularities in the patient’s metabolic pathways. Clinicians need to increase doses above the minimally effective dose carefully, and expend additional effort in analyzing changes in their patient’s symptoms and adverse effects; this analysis should be performed with skepticism and willingness to reduce a dosage if no additional benefit is seen. Attention to these receptor-symptom interactions will improve response and reduce adverse consequences in the majority of patients.
Related Resource
- Lako IM, van den Heuvel ER, Knegtering H, et al. Estimating dopamine D2 receptor occupancy for doses of 8 antipsychotics: a meta-analysis. J Clin Psychopharmacol. 2013;33(5):675-681.
Drug Brand Names
Aripiprazole • Abilify
Asenapine • Saphris
Citalopram • Celexa
Clozapine • Clozaril
Fluoxetine • Prozac
Haloperidol • Haldol
Iloperidone • Fanapt
Lurasidone • Latuda
Nortriptyline • Pamelor
Olanzapine • Zyprexa
Paroxetine • Paxil
Quetiapine • Seroquel
Risperidone • Risperdal
Sertraline • Zoloft
Venlafaxine • Effexor
Ziprasidone • Geodon
1. Farde L, Nordström AL, Wiesel FA, et al. Positron emission tomographic analysis of central D1 and D2 dopamine receptor occupancy in patients treated with classical neuroleptics and clozapine. Relation to extrapyramidal side effects. Arch Gen Psychiatry. 1992;49(7):538-544.
2. Kapur S, Zipursky R, Jones C, et al. Relationship between dopamine D(2) occupancy, clinical response, and side effects: a double-blind PET study of first-episode schizophrenia. Am J Psychiatry. 2000;157(4):514-520.
3. Roberts RJ, Lohano KK, El-Mallakh RS. Antipsychotics as antidepressants. Asia Pacific Psychiatry. 2016;8(3):179-188.
4. Quitkin FM, Rabkin JG, Gerald J, et al. Validity of clinical trials of antidepressants. Am J Psychiatry. 2000;157(3):327-337.
5. Meyer JH, Wilson AA, Sagrati S, et al. Serotonin transporter occupancy of five selective serotonin reuptake inhibitors at different doses: an [11C]DASB positron emission tomography study. Am J Psychiatry. 2004;161(5):826-835.
6. Lundberg J, Tiger M, Landén M, et al. Serotonin transporter occupancy with TCAs and SSRIs: a PET study in patients with major depressive disorder. Int J Neuropsychopharmacol. 2012;15(8):1167-1172.
7. Takano H, Arakawa R, Nogami T, et al. Norepinephrine transporter occupancy by nortriptyline in patients with depression: a positron emission tomography study with (S,S)-[¹8F]FMeNER-D2. Int J Neuropsychopharmacol. 2014;17(4):553-560.
8. Johnson M, Kozielska M, Pilla Reddy V, et al. Dopamine D2 receptor occupancy as a predictor of catalepsy in rats: a pharmacokinetic-pharmacodynamic modeling approach. Pharm Res. 2014;31(10):2605-2617.
9. Allen MH, Citrome L, Pikalov A, et al. Efficacy of lurasidone in the treatment of agitation: a post hoc analysis of five short-term studies in acutely ill patients with schizophrenia. Gen Hosp Psychiatry. 2017;47:75-82.
10. Sekine M, Maeda J, Shimada H, et al. Central nervous system drug evaluation using positron emission tomography. Clin Psychopharmacol Neurosci. 2011;9(1):9-16.
11. Ma GF, Raivio N, Sabrià J, et al. Agonist and antagonist effects of aripiprazole on D2-like receptors controlling rat brain dopamine synthesis depend on the dopaminergic tone. Int J Neuropsychopharmacol. 2014;18(4):pii: pyu046. doi: 10.1093/ijnp/pyu046.
12. Yokoi F, Gründer G, Biziere K, et al. Dopamine D2 and D3 receptor occupancy in normal humans treated with the antipsychotic drug aripiprazole (OPC 14597): a study using positron emission tomography and [11C]raclopride. Neuropsychopharmacology. 2002;27(2):248-259.
13. Gründer G, Carlsson A, Wong DF. Mechanism of new antipsychotic medications: occupancy is not just antagonism. Arch Gen Psychiatry. 2003;60(10):974-977.
14. Mamo D, Graff A, Mizrahi R, et al. Differential effects of aripiprazole on D(2), 5-HT(2), and 5-HT(1A)receptor occupancy in patients with schizophrenia: a triple tracer PET study. Am J Psychiatry. 2007;164(9):1411-1417.
15. Weiden PJ, Preskorn SH, Fahnestock PA, et al. Translating the psychopharmacology of antipsychotics to individualized treatment for severe mental illness: a roadmap. J Clin Psychiatry. 2007;68(suppl 7):1-48.
16. Abi-Dargham A, Rodenhiser J, Printz D, et al. Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proc Natl Acad Sci U S A. 2000;97(14):8104-8109.
17. Kane JM, Carson WH, Saha AR, et al. Efficacy and safety of aripiprazole and haloperidol versus placebo in patients with schizophrenia and schizoaffective disorder. J Clin Psychiatry. 2002;63(9):763-771.
18. Potkin SG, Saha AR, Kujawa MJ, et al. Aripiprazole, an antipsychotic with a novel mechanism of action, and risperidone vs placebo in patients with schizophrenia and schizoaffective disorder. Arch Gen Psychiatry. 2003;60(7):681-690.
19. Cutler AJ, Marcus RN, Hardy SA, et al. The efficacy and safety of lower doses of aripiprazole for the treatment of patients with acute exacerbation of schizophrenia. CNS Spectr. 2006;11(9):691-702; quiz 719.
20. Gründer G, Landvogt C, Vernaleken I, et al. The striatal and extrastriatal D2/D3 receptor-binding profile of clozapine in patients with schizophrenia. Neuropsychopharmacology. 2006;31(5):1027-1035.
21. Mizuno Y, Bies RR, Remington G, et al. Dopamine D2 receptor occupancy with risperidone or olanzapine during maintenance treatment of schizophrenia: a cross-sectional study. Prog Neuropsychopharmacol Biol Psychiatry. 2012;37(1):182-187.
22. Moriguchi S, Bies RR, Remington G, et al. Estimated dopamine D2 receptor occupancy and remission in schizophrenia: analysis of the CATIE data. J Clin Psychopharmacol. 2013;33(5):682-685.
23. Pilowsky LS, Mulligan RS, Acton PD, et al. Limbic selectivity of clozapine. Lancet. 1997;350(9076):490-491.
24. Ito H, Arakawa R, Takahashi H, et al. No regional difference in dopamine D2 receptor occupancy by the second-generation antipsychotic drug risperidone in humans: a positron emission tomography study. Int J Neuropsychopharmacol. 2009;12(5):667-675.
25. Arakawa R, Ito H, Okumura M, et al. Extrastriatal dopamine D(2) receptor occupancy in olanzapine-treated patients with schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2010;260(4):345-350.
26. Xiberas X, Martinot JL, Mallet L, et al. Extrastriatal and striatal D(2) dopamine receptor blockade with haloperidol or new antipsychotic drugs in patients with schizophrenia. Br J Psychiatry. 2001;179:503-508.
27. Cutler AJ, Tran-Johnson T, Kalali A, et al. A failed 6-week, randomized, double-blind, placebo-controlled study of once-daily extended release quetiapine fumarate in patients with acute schizophrenia: lessons learned. Psychopharmacol Bull. 2010;43(4):37-69.
28. Marder SR, Meibach RC. Risperidone in the treatment of schizophrenia. Am J Psychiatry. 1994;151(6):825-835.
29. Meltzer HY, Cucchiaro J, Silva R, et al. Lurasidone in the treatment of schizophrenia: a randomized, double-blind, placebo- and olanzapine-controlled study. Am J Psychiatry. 2011;168(9):957-967.
30. Wong DF, Kuwabara H, Brašic JR, et al. Determination of dopamine D2 receptor occupancy by lurasidone using positron emission tomography in healthy male subjects. Psychopharmacology (Berl). 2013;229(2):245-252.
31. Potkin SG, Keator DB, Kesler-West ML, et al. D2 receptor occupancy following lurasidone treatment in patients with schizophrenia or schizoaffective disorder. CNS Spectr. 2014;19(2):176-181.
32. de Leon J, Susce MT, Pan RM, et al. The CYP2D6 poor metabolizer phenotype may be associated with risperidone adverse drug reactions and discontinuation. J Clin Psychiatry. 2005;66(1):15-27.
33. de Leon J, Susce MT, Pan RM, et al. A study of genetic (CYP2D6 and ABCB1) and environmental (drug inhibitors and inducers) variables that may influence plasma risperidone levels. Pharmacopsychiatry. 2007;40(3):93-102.
34. Narahari A, El-Mallakh RS, Kolikonda MK, et al. How coffee and cigarettes can affect the response to psychopharmacotherapy. Current Psychiatry. 2015;14(10):79-80.
35. Calabrese JR, Keck PE Jr, Macfadden W, et al. A randomized, double-blind, placebo-controlled trial of quetiapine in the treatment of bipolar I or II depression. Am J Psychiatry. 2005;162(7):1351-1360.
36. Loebel A, Cucchiaro J, Silva R, et al. Lurasidone monotherapy in the treatment of bipolar I depression: a randomized, double-blind, placebo-controlled study. Am J Psychiatry. 2014;171(2):160-168.
37. Lindenmayer JP, Brown D, Liu S, et al. The efficacy and tolerability of once-daily extended release quetiapine fumarate in hospitalized patients with acute schizophrenia: a 6-week randomized, double-blind, placebo-controlled study. Psychopharmacol Bull. 2008;41(3):11-35.
Most clinicians do not think about receptor occupancy when they prescribe a medication. Most simply assume that if they use a low dose of a medication, they will get a small effect, and if they use a higher dose, they will get a larger effect. However, this is frequently not accurate. Clinicians need to understand the relationship between receptor occupancy and drug response.
In general, when an antagonist of a neurotransmitter receptor is used, it must occupy a minimum of 65% to 70% of the target receptor to be effective. This is clearly the case when the target is a postsynaptic receptor, such as the dopamine D2 receptor.1-3 Similarly, despite significant variability in antidepressant response,4 blockade of 65% to 80% of presynaptic transport proteins—such as the serotonin reuptake pumps when considering serotoninergic antidepressants,5,6 or the norepinephrine reuptake pumps when considering noradrenergic agents such as nortriptyline7—is necessary for these medications to be effective.
It is reasonable to think of the drug response of such agents as following a “threshold” model (Figure 1). This model makes 2 predictions. The first prediction is that a low dose of the drug might result in <50% receptor occupancy, but is not associated with a smaller response; it is simply ineffective. The second prediction is that a very high dose of the drug (eg, one that may exceed 90% receptor occupancy) does not result in any additional benefit, but may cause additional adverse consequences.8

Alternatively, agonist medications, such as benzodiazepines or opiates, have their efficacy in a continuous dose-dependent fashion (Figure 2). Titrating these medications for clinical response is necessary, and minimal effective doses are highly individual. Agonist medications will not be addressed further in this article.

In this article, the term “response” is used to denote the average (population) symptom change in a study population. This term is not used as clinicians often use it to mean that their specific patient’s illness has improved, or that the patient has gone into remission. Furthermore, the information described in this article does not optimize clinical outcome, but instead is intended to help clinicians optimize the use of their pharmacologic tools.
Minimal effective dose
Medications that have a threshold for activity will display that clinically in a minimal effective dose (Table 13,9 and Table 25). The minimal effective dose of medications that act by blocking a neurotransmitter receptor is usually the dose that achieves 65% to 80% receptor occupancy in typical individuals (Table 25). The minimal effective doses for antipsychotics are listed in Table 1.3,9 These doses are known to occupy approximately 65% to 70% of postsynaptic D2 receptors in living humans as confirmed by positron emission tomography (PET) scans.10 Similar minimal effective doses can be determined for serotonin-reuptake inhibiting (SRI) antidepressants (Table 25). In placebo-controlled trials, doses that were smaller than the minimal effective dose did not provide any benefit.

There are important caveats to this. First is the use of partial agonists. Depending on the level of intrinsic activity of a partial agonist and clinical goal, the clinician may aim for a different level of receptor occupancy. For example, aripiprazole will act as a dopamine agonist at lower concentrations, but blocks the receptor at higher concentrations.11 Unlike antagonist antipsychotics, which require only 65% to 70% D2 receptor occupancy to be effective, aripiprazole receptor binding at effective antipsychotic doses is 90% to 95%.12-14 Since aripiprazole has an intrinsic activity of approximately 30% (ie, when it binds, it stimulates the D2 receptor to about 30% of the effect of dopamine binding to the receptor15), binding to 90% of the receptors, and displacing endogenous dopamine, allows aripiprazole to replace the background or tonic tone of dopamine, which has been measured at 19% in people with schizophrenia and 9% in controls.16 Clinically, this still appears as the minimal effective dose achieving maximal response17-19 without significant parkinsonism despite >90% receptor occupancy.12
Continue to: The second caveat is...
The second caveat is the action of low D2 receptor affinity antipsychotics, such as clozapine and quetiapine. These agents generally achieve adequate D2 receptor occupancy for only a brief period of time.20 It has been suggested that continuous receptor occupancy at ≥65% may not be necessary to obtain antipsychotic control.21,22 There may also be specific limbic and cortical (vs striatal) D2 receptor selectivity by clozapine23 compared with other second-generation antipsychotics such as risperidone and olanzapine,24,25 although this point remains debatable.26 Furthermore, the antipsychotic efficacy of low D2 receptor affinity drugs is unreliable, even in controlled, blinded studies (eg, a failed large quetiapine study27). Thus far, the actual antipsychotic mechanism of these agents is yet to be fully understood.
Minimal effective dose achieves maximal response
An interesting aspect of the threshold phenomenon of drug response is that once the minimal effective dose is reached, maximal response is achieved. In other words, there is no additional efficacy with additional dose increases. This is readily demonstrated in some studies in which patients were randomly assigned to different fixed doses or dose ranges. In these studies, there was generally no difference in response rates of different doses, so that once 65% to 80% receptor occupancy is achieved, minimal and maximal clinical response is simultaneously reached.18,28,29
For example, in the original risperidone studies, 6 mg/d was essentially equivalent to 16 mg/d.28 Similarly, lurasidone, 40 mg/d, achieves approximately 65% D2 occupancy.30 When the daily dose is increased to 120 mg, there is no additional benefit in controlling psychosis in schizophrenia.29 This pattern is also seen in partial agonists, where there are no differences between lower and higher doses in terms of response.18
Upon reading this, many clinicians may think “I don’t care what the studies say, I have seen additional benefits with additional doses.” There are several explanations for this. One is that individual patients have genetic variants that may prevent them from responding in a typical fashion. Hints of this are seen in an apparent disconnect between dosage and drug levels, so that it is not surprising that drug levels are a much better predictor of receptor occupancy than dosage.31 Nonetheless, as previously pointed out, for a population, dosage does predict receptor occupancy and outcome. However, for individuals, genetic variations make dosages less reliable. For example, ultrarapid metabolizers of cytochrome P450 (CYP) 2D6 may discontinue risperidone due to nonresponse, or require a higher dose or longer time period to respond.32,33 Similarly, patients who smoke may require an increase in doses of CYP1A2 substrates such as clozapine and olanzapine.34
Alternatively, the clinician may note improvement in mood, sleep, appetite, or other symptoms at lower doses, and then note additional improvements in psychosis or mania at higher doses.3 This occurs due to the varying affinity of different receptors. For example, in bipolar depression trials that used quetiapine in a fixed-dose design, patients who received 300 or 600 mg/d responded in the same fashion, with no additional benefit in improving depression with the higher dose.35 Similarly, in a flexible dose range study that evaluated lurasidone in bipolar depression, an average dose of 34 mg/d (range 20 to 60 mg/d) and an average dose of 83 mg/d (range 80 to 120 mg/d) both resulted in the same response (a 15.4-point reduction in depression ratings and an effect size of 0.51).36 For both quetiapine and lurasidone, higher doses are generally required to control psychosis.29,37 Note that for lurasidone, agitation, but not psychosis, improves with higher doses, which suggests that recruitment of additional receptors results in improvement in a different set of symptoms.9
Continue to: Clinical implications
Clinical implications
The implications for clinicians are relatively clear. Knowing the minimal effective doses for depression, psychosis, or mania informs the target dose. If improvement is seen at lower doses, the clinician needs to assess the profile of symptoms that improved, potential drug–drug interactions, or potential irregularities in the patient’s metabolic pathways. Clinicians need to increase doses above the minimally effective dose carefully, and expend additional effort in analyzing changes in their patient’s symptoms and adverse effects; this analysis should be performed with skepticism and willingness to reduce a dosage if no additional benefit is seen. Attention to these receptor-symptom interactions will improve response and reduce adverse consequences in the majority of patients.
Related Resource
- Lako IM, van den Heuvel ER, Knegtering H, et al. Estimating dopamine D2 receptor occupancy for doses of 8 antipsychotics: a meta-analysis. J Clin Psychopharmacol. 2013;33(5):675-681.
Drug Brand Names
Aripiprazole • Abilify
Asenapine • Saphris
Citalopram • Celexa
Clozapine • Clozaril
Fluoxetine • Prozac
Haloperidol • Haldol
Iloperidone • Fanapt
Lurasidone • Latuda
Nortriptyline • Pamelor
Olanzapine • Zyprexa
Paroxetine • Paxil
Quetiapine • Seroquel
Risperidone • Risperdal
Sertraline • Zoloft
Venlafaxine • Effexor
Ziprasidone • Geodon
Most clinicians do not think about receptor occupancy when they prescribe a medication. Most simply assume that if they use a low dose of a medication, they will get a small effect, and if they use a higher dose, they will get a larger effect. However, this is frequently not accurate. Clinicians need to understand the relationship between receptor occupancy and drug response.
In general, when an antagonist of a neurotransmitter receptor is used, it must occupy a minimum of 65% to 70% of the target receptor to be effective. This is clearly the case when the target is a postsynaptic receptor, such as the dopamine D2 receptor.1-3 Similarly, despite significant variability in antidepressant response,4 blockade of 65% to 80% of presynaptic transport proteins—such as the serotonin reuptake pumps when considering serotoninergic antidepressants,5,6 or the norepinephrine reuptake pumps when considering noradrenergic agents such as nortriptyline7—is necessary for these medications to be effective.
It is reasonable to think of the drug response of such agents as following a “threshold” model (Figure 1). This model makes 2 predictions. The first prediction is that a low dose of the drug might result in <50% receptor occupancy, but is not associated with a smaller response; it is simply ineffective. The second prediction is that a very high dose of the drug (eg, one that may exceed 90% receptor occupancy) does not result in any additional benefit, but may cause additional adverse consequences.8

Alternatively, agonist medications, such as benzodiazepines or opiates, have their efficacy in a continuous dose-dependent fashion (Figure 2). Titrating these medications for clinical response is necessary, and minimal effective doses are highly individual. Agonist medications will not be addressed further in this article.

In this article, the term “response” is used to denote the average (population) symptom change in a study population. This term is not used as clinicians often use it to mean that their specific patient’s illness has improved, or that the patient has gone into remission. Furthermore, the information described in this article does not optimize clinical outcome, but instead is intended to help clinicians optimize the use of their pharmacologic tools.
Minimal effective dose
Medications that have a threshold for activity will display that clinically in a minimal effective dose (Table 13,9 and Table 25). The minimal effective dose of medications that act by blocking a neurotransmitter receptor is usually the dose that achieves 65% to 80% receptor occupancy in typical individuals (Table 25). The minimal effective doses for antipsychotics are listed in Table 1.3,9 These doses are known to occupy approximately 65% to 70% of postsynaptic D2 receptors in living humans as confirmed by positron emission tomography (PET) scans.10 Similar minimal effective doses can be determined for serotonin-reuptake inhibiting (SRI) antidepressants (Table 25). In placebo-controlled trials, doses that were smaller than the minimal effective dose did not provide any benefit.

There are important caveats to this. First is the use of partial agonists. Depending on the level of intrinsic activity of a partial agonist and clinical goal, the clinician may aim for a different level of receptor occupancy. For example, aripiprazole will act as a dopamine agonist at lower concentrations, but blocks the receptor at higher concentrations.11 Unlike antagonist antipsychotics, which require only 65% to 70% D2 receptor occupancy to be effective, aripiprazole receptor binding at effective antipsychotic doses is 90% to 95%.12-14 Since aripiprazole has an intrinsic activity of approximately 30% (ie, when it binds, it stimulates the D2 receptor to about 30% of the effect of dopamine binding to the receptor15), binding to 90% of the receptors, and displacing endogenous dopamine, allows aripiprazole to replace the background or tonic tone of dopamine, which has been measured at 19% in people with schizophrenia and 9% in controls.16 Clinically, this still appears as the minimal effective dose achieving maximal response17-19 without significant parkinsonism despite >90% receptor occupancy.12
Continue to: The second caveat is...
The second caveat is the action of low D2 receptor affinity antipsychotics, such as clozapine and quetiapine. These agents generally achieve adequate D2 receptor occupancy for only a brief period of time.20 It has been suggested that continuous receptor occupancy at ≥65% may not be necessary to obtain antipsychotic control.21,22 There may also be specific limbic and cortical (vs striatal) D2 receptor selectivity by clozapine23 compared with other second-generation antipsychotics such as risperidone and olanzapine,24,25 although this point remains debatable.26 Furthermore, the antipsychotic efficacy of low D2 receptor affinity drugs is unreliable, even in controlled, blinded studies (eg, a failed large quetiapine study27). Thus far, the actual antipsychotic mechanism of these agents is yet to be fully understood.
Minimal effective dose achieves maximal response
An interesting aspect of the threshold phenomenon of drug response is that once the minimal effective dose is reached, maximal response is achieved. In other words, there is no additional efficacy with additional dose increases. This is readily demonstrated in some studies in which patients were randomly assigned to different fixed doses or dose ranges. In these studies, there was generally no difference in response rates of different doses, so that once 65% to 80% receptor occupancy is achieved, minimal and maximal clinical response is simultaneously reached.18,28,29
For example, in the original risperidone studies, 6 mg/d was essentially equivalent to 16 mg/d.28 Similarly, lurasidone, 40 mg/d, achieves approximately 65% D2 occupancy.30 When the daily dose is increased to 120 mg, there is no additional benefit in controlling psychosis in schizophrenia.29 This pattern is also seen in partial agonists, where there are no differences between lower and higher doses in terms of response.18
Upon reading this, many clinicians may think “I don’t care what the studies say, I have seen additional benefits with additional doses.” There are several explanations for this. One is that individual patients have genetic variants that may prevent them from responding in a typical fashion. Hints of this are seen in an apparent disconnect between dosage and drug levels, so that it is not surprising that drug levels are a much better predictor of receptor occupancy than dosage.31 Nonetheless, as previously pointed out, for a population, dosage does predict receptor occupancy and outcome. However, for individuals, genetic variations make dosages less reliable. For example, ultrarapid metabolizers of cytochrome P450 (CYP) 2D6 may discontinue risperidone due to nonresponse, or require a higher dose or longer time period to respond.32,33 Similarly, patients who smoke may require an increase in doses of CYP1A2 substrates such as clozapine and olanzapine.34
Alternatively, the clinician may note improvement in mood, sleep, appetite, or other symptoms at lower doses, and then note additional improvements in psychosis or mania at higher doses.3 This occurs due to the varying affinity of different receptors. For example, in bipolar depression trials that used quetiapine in a fixed-dose design, patients who received 300 or 600 mg/d responded in the same fashion, with no additional benefit in improving depression with the higher dose.35 Similarly, in a flexible dose range study that evaluated lurasidone in bipolar depression, an average dose of 34 mg/d (range 20 to 60 mg/d) and an average dose of 83 mg/d (range 80 to 120 mg/d) both resulted in the same response (a 15.4-point reduction in depression ratings and an effect size of 0.51).36 For both quetiapine and lurasidone, higher doses are generally required to control psychosis.29,37 Note that for lurasidone, agitation, but not psychosis, improves with higher doses, which suggests that recruitment of additional receptors results in improvement in a different set of symptoms.9
Continue to: Clinical implications
Clinical implications
The implications for clinicians are relatively clear. Knowing the minimal effective doses for depression, psychosis, or mania informs the target dose. If improvement is seen at lower doses, the clinician needs to assess the profile of symptoms that improved, potential drug–drug interactions, or potential irregularities in the patient’s metabolic pathways. Clinicians need to increase doses above the minimally effective dose carefully, and expend additional effort in analyzing changes in their patient’s symptoms and adverse effects; this analysis should be performed with skepticism and willingness to reduce a dosage if no additional benefit is seen. Attention to these receptor-symptom interactions will improve response and reduce adverse consequences in the majority of patients.
Related Resource
- Lako IM, van den Heuvel ER, Knegtering H, et al. Estimating dopamine D2 receptor occupancy for doses of 8 antipsychotics: a meta-analysis. J Clin Psychopharmacol. 2013;33(5):675-681.
Drug Brand Names
Aripiprazole • Abilify
Asenapine • Saphris
Citalopram • Celexa
Clozapine • Clozaril
Fluoxetine • Prozac
Haloperidol • Haldol
Iloperidone • Fanapt
Lurasidone • Latuda
Nortriptyline • Pamelor
Olanzapine • Zyprexa
Paroxetine • Paxil
Quetiapine • Seroquel
Risperidone • Risperdal
Sertraline • Zoloft
Venlafaxine • Effexor
Ziprasidone • Geodon
1. Farde L, Nordström AL, Wiesel FA, et al. Positron emission tomographic analysis of central D1 and D2 dopamine receptor occupancy in patients treated with classical neuroleptics and clozapine. Relation to extrapyramidal side effects. Arch Gen Psychiatry. 1992;49(7):538-544.
2. Kapur S, Zipursky R, Jones C, et al. Relationship between dopamine D(2) occupancy, clinical response, and side effects: a double-blind PET study of first-episode schizophrenia. Am J Psychiatry. 2000;157(4):514-520.
3. Roberts RJ, Lohano KK, El-Mallakh RS. Antipsychotics as antidepressants. Asia Pacific Psychiatry. 2016;8(3):179-188.
4. Quitkin FM, Rabkin JG, Gerald J, et al. Validity of clinical trials of antidepressants. Am J Psychiatry. 2000;157(3):327-337.
5. Meyer JH, Wilson AA, Sagrati S, et al. Serotonin transporter occupancy of five selective serotonin reuptake inhibitors at different doses: an [11C]DASB positron emission tomography study. Am J Psychiatry. 2004;161(5):826-835.
6. Lundberg J, Tiger M, Landén M, et al. Serotonin transporter occupancy with TCAs and SSRIs: a PET study in patients with major depressive disorder. Int J Neuropsychopharmacol. 2012;15(8):1167-1172.
7. Takano H, Arakawa R, Nogami T, et al. Norepinephrine transporter occupancy by nortriptyline in patients with depression: a positron emission tomography study with (S,S)-[¹8F]FMeNER-D2. Int J Neuropsychopharmacol. 2014;17(4):553-560.
8. Johnson M, Kozielska M, Pilla Reddy V, et al. Dopamine D2 receptor occupancy as a predictor of catalepsy in rats: a pharmacokinetic-pharmacodynamic modeling approach. Pharm Res. 2014;31(10):2605-2617.
9. Allen MH, Citrome L, Pikalov A, et al. Efficacy of lurasidone in the treatment of agitation: a post hoc analysis of five short-term studies in acutely ill patients with schizophrenia. Gen Hosp Psychiatry. 2017;47:75-82.
10. Sekine M, Maeda J, Shimada H, et al. Central nervous system drug evaluation using positron emission tomography. Clin Psychopharmacol Neurosci. 2011;9(1):9-16.
11. Ma GF, Raivio N, Sabrià J, et al. Agonist and antagonist effects of aripiprazole on D2-like receptors controlling rat brain dopamine synthesis depend on the dopaminergic tone. Int J Neuropsychopharmacol. 2014;18(4):pii: pyu046. doi: 10.1093/ijnp/pyu046.
12. Yokoi F, Gründer G, Biziere K, et al. Dopamine D2 and D3 receptor occupancy in normal humans treated with the antipsychotic drug aripiprazole (OPC 14597): a study using positron emission tomography and [11C]raclopride. Neuropsychopharmacology. 2002;27(2):248-259.
13. Gründer G, Carlsson A, Wong DF. Mechanism of new antipsychotic medications: occupancy is not just antagonism. Arch Gen Psychiatry. 2003;60(10):974-977.
14. Mamo D, Graff A, Mizrahi R, et al. Differential effects of aripiprazole on D(2), 5-HT(2), and 5-HT(1A)receptor occupancy in patients with schizophrenia: a triple tracer PET study. Am J Psychiatry. 2007;164(9):1411-1417.
15. Weiden PJ, Preskorn SH, Fahnestock PA, et al. Translating the psychopharmacology of antipsychotics to individualized treatment for severe mental illness: a roadmap. J Clin Psychiatry. 2007;68(suppl 7):1-48.
16. Abi-Dargham A, Rodenhiser J, Printz D, et al. Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proc Natl Acad Sci U S A. 2000;97(14):8104-8109.
17. Kane JM, Carson WH, Saha AR, et al. Efficacy and safety of aripiprazole and haloperidol versus placebo in patients with schizophrenia and schizoaffective disorder. J Clin Psychiatry. 2002;63(9):763-771.
18. Potkin SG, Saha AR, Kujawa MJ, et al. Aripiprazole, an antipsychotic with a novel mechanism of action, and risperidone vs placebo in patients with schizophrenia and schizoaffective disorder. Arch Gen Psychiatry. 2003;60(7):681-690.
19. Cutler AJ, Marcus RN, Hardy SA, et al. The efficacy and safety of lower doses of aripiprazole for the treatment of patients with acute exacerbation of schizophrenia. CNS Spectr. 2006;11(9):691-702; quiz 719.
20. Gründer G, Landvogt C, Vernaleken I, et al. The striatal and extrastriatal D2/D3 receptor-binding profile of clozapine in patients with schizophrenia. Neuropsychopharmacology. 2006;31(5):1027-1035.
21. Mizuno Y, Bies RR, Remington G, et al. Dopamine D2 receptor occupancy with risperidone or olanzapine during maintenance treatment of schizophrenia: a cross-sectional study. Prog Neuropsychopharmacol Biol Psychiatry. 2012;37(1):182-187.
22. Moriguchi S, Bies RR, Remington G, et al. Estimated dopamine D2 receptor occupancy and remission in schizophrenia: analysis of the CATIE data. J Clin Psychopharmacol. 2013;33(5):682-685.
23. Pilowsky LS, Mulligan RS, Acton PD, et al. Limbic selectivity of clozapine. Lancet. 1997;350(9076):490-491.
24. Ito H, Arakawa R, Takahashi H, et al. No regional difference in dopamine D2 receptor occupancy by the second-generation antipsychotic drug risperidone in humans: a positron emission tomography study. Int J Neuropsychopharmacol. 2009;12(5):667-675.
25. Arakawa R, Ito H, Okumura M, et al. Extrastriatal dopamine D(2) receptor occupancy in olanzapine-treated patients with schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2010;260(4):345-350.
26. Xiberas X, Martinot JL, Mallet L, et al. Extrastriatal and striatal D(2) dopamine receptor blockade with haloperidol or new antipsychotic drugs in patients with schizophrenia. Br J Psychiatry. 2001;179:503-508.
27. Cutler AJ, Tran-Johnson T, Kalali A, et al. A failed 6-week, randomized, double-blind, placebo-controlled study of once-daily extended release quetiapine fumarate in patients with acute schizophrenia: lessons learned. Psychopharmacol Bull. 2010;43(4):37-69.
28. Marder SR, Meibach RC. Risperidone in the treatment of schizophrenia. Am J Psychiatry. 1994;151(6):825-835.
29. Meltzer HY, Cucchiaro J, Silva R, et al. Lurasidone in the treatment of schizophrenia: a randomized, double-blind, placebo- and olanzapine-controlled study. Am J Psychiatry. 2011;168(9):957-967.
30. Wong DF, Kuwabara H, Brašic JR, et al. Determination of dopamine D2 receptor occupancy by lurasidone using positron emission tomography in healthy male subjects. Psychopharmacology (Berl). 2013;229(2):245-252.
31. Potkin SG, Keator DB, Kesler-West ML, et al. D2 receptor occupancy following lurasidone treatment in patients with schizophrenia or schizoaffective disorder. CNS Spectr. 2014;19(2):176-181.
32. de Leon J, Susce MT, Pan RM, et al. The CYP2D6 poor metabolizer phenotype may be associated with risperidone adverse drug reactions and discontinuation. J Clin Psychiatry. 2005;66(1):15-27.
33. de Leon J, Susce MT, Pan RM, et al. A study of genetic (CYP2D6 and ABCB1) and environmental (drug inhibitors and inducers) variables that may influence plasma risperidone levels. Pharmacopsychiatry. 2007;40(3):93-102.
34. Narahari A, El-Mallakh RS, Kolikonda MK, et al. How coffee and cigarettes can affect the response to psychopharmacotherapy. Current Psychiatry. 2015;14(10):79-80.
35. Calabrese JR, Keck PE Jr, Macfadden W, et al. A randomized, double-blind, placebo-controlled trial of quetiapine in the treatment of bipolar I or II depression. Am J Psychiatry. 2005;162(7):1351-1360.
36. Loebel A, Cucchiaro J, Silva R, et al. Lurasidone monotherapy in the treatment of bipolar I depression: a randomized, double-blind, placebo-controlled study. Am J Psychiatry. 2014;171(2):160-168.
37. Lindenmayer JP, Brown D, Liu S, et al. The efficacy and tolerability of once-daily extended release quetiapine fumarate in hospitalized patients with acute schizophrenia: a 6-week randomized, double-blind, placebo-controlled study. Psychopharmacol Bull. 2008;41(3):11-35.
1. Farde L, Nordström AL, Wiesel FA, et al. Positron emission tomographic analysis of central D1 and D2 dopamine receptor occupancy in patients treated with classical neuroleptics and clozapine. Relation to extrapyramidal side effects. Arch Gen Psychiatry. 1992;49(7):538-544.
2. Kapur S, Zipursky R, Jones C, et al. Relationship between dopamine D(2) occupancy, clinical response, and side effects: a double-blind PET study of first-episode schizophrenia. Am J Psychiatry. 2000;157(4):514-520.
3. Roberts RJ, Lohano KK, El-Mallakh RS. Antipsychotics as antidepressants. Asia Pacific Psychiatry. 2016;8(3):179-188.
4. Quitkin FM, Rabkin JG, Gerald J, et al. Validity of clinical trials of antidepressants. Am J Psychiatry. 2000;157(3):327-337.
5. Meyer JH, Wilson AA, Sagrati S, et al. Serotonin transporter occupancy of five selective serotonin reuptake inhibitors at different doses: an [11C]DASB positron emission tomography study. Am J Psychiatry. 2004;161(5):826-835.
6. Lundberg J, Tiger M, Landén M, et al. Serotonin transporter occupancy with TCAs and SSRIs: a PET study in patients with major depressive disorder. Int J Neuropsychopharmacol. 2012;15(8):1167-1172.
7. Takano H, Arakawa R, Nogami T, et al. Norepinephrine transporter occupancy by nortriptyline in patients with depression: a positron emission tomography study with (S,S)-[¹8F]FMeNER-D2. Int J Neuropsychopharmacol. 2014;17(4):553-560.
8. Johnson M, Kozielska M, Pilla Reddy V, et al. Dopamine D2 receptor occupancy as a predictor of catalepsy in rats: a pharmacokinetic-pharmacodynamic modeling approach. Pharm Res. 2014;31(10):2605-2617.
9. Allen MH, Citrome L, Pikalov A, et al. Efficacy of lurasidone in the treatment of agitation: a post hoc analysis of five short-term studies in acutely ill patients with schizophrenia. Gen Hosp Psychiatry. 2017;47:75-82.
10. Sekine M, Maeda J, Shimada H, et al. Central nervous system drug evaluation using positron emission tomography. Clin Psychopharmacol Neurosci. 2011;9(1):9-16.
11. Ma GF, Raivio N, Sabrià J, et al. Agonist and antagonist effects of aripiprazole on D2-like receptors controlling rat brain dopamine synthesis depend on the dopaminergic tone. Int J Neuropsychopharmacol. 2014;18(4):pii: pyu046. doi: 10.1093/ijnp/pyu046.
12. Yokoi F, Gründer G, Biziere K, et al. Dopamine D2 and D3 receptor occupancy in normal humans treated with the antipsychotic drug aripiprazole (OPC 14597): a study using positron emission tomography and [11C]raclopride. Neuropsychopharmacology. 2002;27(2):248-259.
13. Gründer G, Carlsson A, Wong DF. Mechanism of new antipsychotic medications: occupancy is not just antagonism. Arch Gen Psychiatry. 2003;60(10):974-977.
14. Mamo D, Graff A, Mizrahi R, et al. Differential effects of aripiprazole on D(2), 5-HT(2), and 5-HT(1A)receptor occupancy in patients with schizophrenia: a triple tracer PET study. Am J Psychiatry. 2007;164(9):1411-1417.
15. Weiden PJ, Preskorn SH, Fahnestock PA, et al. Translating the psychopharmacology of antipsychotics to individualized treatment for severe mental illness: a roadmap. J Clin Psychiatry. 2007;68(suppl 7):1-48.
16. Abi-Dargham A, Rodenhiser J, Printz D, et al. Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proc Natl Acad Sci U S A. 2000;97(14):8104-8109.
17. Kane JM, Carson WH, Saha AR, et al. Efficacy and safety of aripiprazole and haloperidol versus placebo in patients with schizophrenia and schizoaffective disorder. J Clin Psychiatry. 2002;63(9):763-771.
18. Potkin SG, Saha AR, Kujawa MJ, et al. Aripiprazole, an antipsychotic with a novel mechanism of action, and risperidone vs placebo in patients with schizophrenia and schizoaffective disorder. Arch Gen Psychiatry. 2003;60(7):681-690.
19. Cutler AJ, Marcus RN, Hardy SA, et al. The efficacy and safety of lower doses of aripiprazole for the treatment of patients with acute exacerbation of schizophrenia. CNS Spectr. 2006;11(9):691-702; quiz 719.
20. Gründer G, Landvogt C, Vernaleken I, et al. The striatal and extrastriatal D2/D3 receptor-binding profile of clozapine in patients with schizophrenia. Neuropsychopharmacology. 2006;31(5):1027-1035.
21. Mizuno Y, Bies RR, Remington G, et al. Dopamine D2 receptor occupancy with risperidone or olanzapine during maintenance treatment of schizophrenia: a cross-sectional study. Prog Neuropsychopharmacol Biol Psychiatry. 2012;37(1):182-187.
22. Moriguchi S, Bies RR, Remington G, et al. Estimated dopamine D2 receptor occupancy and remission in schizophrenia: analysis of the CATIE data. J Clin Psychopharmacol. 2013;33(5):682-685.
23. Pilowsky LS, Mulligan RS, Acton PD, et al. Limbic selectivity of clozapine. Lancet. 1997;350(9076):490-491.
24. Ito H, Arakawa R, Takahashi H, et al. No regional difference in dopamine D2 receptor occupancy by the second-generation antipsychotic drug risperidone in humans: a positron emission tomography study. Int J Neuropsychopharmacol. 2009;12(5):667-675.
25. Arakawa R, Ito H, Okumura M, et al. Extrastriatal dopamine D(2) receptor occupancy in olanzapine-treated patients with schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2010;260(4):345-350.
26. Xiberas X, Martinot JL, Mallet L, et al. Extrastriatal and striatal D(2) dopamine receptor blockade with haloperidol or new antipsychotic drugs in patients with schizophrenia. Br J Psychiatry. 2001;179:503-508.
27. Cutler AJ, Tran-Johnson T, Kalali A, et al. A failed 6-week, randomized, double-blind, placebo-controlled study of once-daily extended release quetiapine fumarate in patients with acute schizophrenia: lessons learned. Psychopharmacol Bull. 2010;43(4):37-69.
28. Marder SR, Meibach RC. Risperidone in the treatment of schizophrenia. Am J Psychiatry. 1994;151(6):825-835.
29. Meltzer HY, Cucchiaro J, Silva R, et al. Lurasidone in the treatment of schizophrenia: a randomized, double-blind, placebo- and olanzapine-controlled study. Am J Psychiatry. 2011;168(9):957-967.
30. Wong DF, Kuwabara H, Brašic JR, et al. Determination of dopamine D2 receptor occupancy by lurasidone using positron emission tomography in healthy male subjects. Psychopharmacology (Berl). 2013;229(2):245-252.
31. Potkin SG, Keator DB, Kesler-West ML, et al. D2 receptor occupancy following lurasidone treatment in patients with schizophrenia or schizoaffective disorder. CNS Spectr. 2014;19(2):176-181.
32. de Leon J, Susce MT, Pan RM, et al. The CYP2D6 poor metabolizer phenotype may be associated with risperidone adverse drug reactions and discontinuation. J Clin Psychiatry. 2005;66(1):15-27.
33. de Leon J, Susce MT, Pan RM, et al. A study of genetic (CYP2D6 and ABCB1) and environmental (drug inhibitors and inducers) variables that may influence plasma risperidone levels. Pharmacopsychiatry. 2007;40(3):93-102.
34. Narahari A, El-Mallakh RS, Kolikonda MK, et al. How coffee and cigarettes can affect the response to psychopharmacotherapy. Current Psychiatry. 2015;14(10):79-80.
35. Calabrese JR, Keck PE Jr, Macfadden W, et al. A randomized, double-blind, placebo-controlled trial of quetiapine in the treatment of bipolar I or II depression. Am J Psychiatry. 2005;162(7):1351-1360.
36. Loebel A, Cucchiaro J, Silva R, et al. Lurasidone monotherapy in the treatment of bipolar I depression: a randomized, double-blind, placebo-controlled study. Am J Psychiatry. 2014;171(2):160-168.
37. Lindenmayer JP, Brown D, Liu S, et al. The efficacy and tolerability of once-daily extended release quetiapine fumarate in hospitalized patients with acute schizophrenia: a 6-week randomized, double-blind, placebo-controlled study. Psychopharmacol Bull. 2008;41(3):11-35.
It takes guts to be mentally ill: Microbiota and psychopathology
What is the largest endocrine organ in the human body?
Here is a clue: It is also the largest immune organ in humans!
Still scratching your head? Here is another clue: This organ also contains a “second brain,” which is connected to big brain inside the head by the vagus nerve.
Okay, enough guessing: It’s the 30-foot long gastrointestinal (GI) tract, which is generally associated only with eating and digestion. But it is far more than a digestive tract. It is home to about 100 trillion diverse bacteria, including 1,000 known species, which together are known as “microbiota.” Its combined DNA is called the “microbiome” and is 10,000% larger than the human genome. Those trillions of bacteria in our guts are a symbiotic (commensal) organ that is vital for the normal functions of the human body.1
While this vast array of microorganisms is vital to sustaining a healthy human existence, it can also be involved in multiple psychiatric disorders, including depression, psychosis, anxiety, autism, and attention-deficit/hyperactivity disorder (ADHD). Humans acquire their unique sets of microbiota as they pass through the mother’s vagina at birth and while breastfeeding, as well as from exposure to various environmental sources in the first few months of life.2
The microbiota in the GI tract are an intimate neighbor of the “enteric brain,” comprised of 100 million neurons plus glia-like support cell structures. This “second brain” produces over 30 neurotransmitters, several of which (dopamine, serotonin, γ-aminobutyric acid [GABA], acetylcholine) have been implicated in major psychiatric disorders.3
The brain and gut have a dynamic bidirectional communication system, mediated by neural, hormonal, and immunological crosstalk and influences. The GI tract secretes dozens of peptides and other signaling molecules that influence the brain. The microbiota also interact with and are regulated by gut hormones such as oxytocin, ghrelin, neuropeptide Y, cholecystokinin, corticotrophin-releasing factor, and pancreatic polypeptide.4 The microbiota modulate brain development, functions, and behavior, and maintain the intestinal barrier, which, if disrupted, would result in the gut becoming “leaky” and triggering low-grade inflammation such as that associated with depression.5
Continue to: But don't overlook the importance of...
But don’t overlook the importance of diet. It is a major factor in shaping the composition of the microbiota. What we eat can have a preventative or reparative effect on neuroimmune or neuroinflammatory disease. An emerging body of evidence suggests that the diet and its effects on the gut microbiota can modify a person’s genes by epigenetic mechanisms (altering DNA methylation and histone effects). Probiotics can exert epigenetic effects by influencing cytokines, by producing short-chain fatty acids (SCFAs), by vitamin synthesis, and by producing several well-known neurotransmitters.6
The bidirectional trafficking across the microbiome-gut-brain axis includes reciprocal effects. The brain influences the microbiome composition by regulating satiety, the hypothalamic-pituitary axis, and with neuropeptides.7 In return, the microbiome conveys information to the brain about the intestinal status via infectious agents, intestinal neurotransmitters and modulators, cytokines, sensory vagal fibers, and various metabolites. Failure of these normal interactions can lead to a variety of pathological processes, including inflammatory, autoimmune, degenerative, metabolic, cognitive, mood, and behavioral adverse effects. Therapeutic interventions for these adverse consequences can be implemented through microbiome manipulations (such as fecal transplants), nutritional strategies, and reinforcement of the enteric and brain barriers.
Alterations in the microbiota, such as by the intake of antibiotics or by intestinal inflammation, can lead to psychiatric disorders.8 The following findings link gut microbiome disruptions with several psychiatric disorders:
Schizophrenia prodrome. Fecal bacteria show an increase in SCFAs, which can activate microglia (the initial step in triggering psychosis).9 These bacteria have been shown to lead to an increase in choline levels in the anterior cingulate, a known biomarker for membrane dysfunction, which is one of the biological models of schizophrenia.
Schizophrenia—first-episode. A recent study reported abnormalities in the gut microbiota of patients with first-episode psychosis, with a lower number of certain fecal bacteria (including bifidobacterium, E. coli, and lactobacillus) and high levels of Clostridium coccoides. After 6 months of risperidone treatment, the above changes were reversed.10
Continue to: Another study of fecal microbiota...
Another study of fecal microbiota in a first-episode psychosis cohort found significant differences in several bacterial strains compared with a healthy control group, and those with the strongest difference had more severe psychotic symptoms and poorer response after 12 months of antipsychotic treatment.11
Autism has been linked to increased microbiota diversity, and an excess of bacteroides has been associated with a higher diversity of autism. Fecal samples from autistic children were reported to have an increase in SCFAs. Interestingly, a certain strain of lactobacillus can modulate oxytocin or reverse some autistic symptoms.
Depression has been associated with increased diversity of microbiota alpha. Patients with depression have been reported to have low numbers of bifidobacterium
ADHD. Some studies suggest that ADHD may be linked to factors that can alter gut microbiota, including birthing mode, type of infant feeding, maternal health, and early stressors. In addition, dietary influences on gut microbiota can modify ADHD symptoms.14
Alzheimer’s disease. Metabolic dysregulation, such as obesity and diabetes, can inflame the gut microbiota, and are known risk factors for Alzheimer’s disease.15
Continue to: Irritable bowel sydrome...
Irritable bowel syndrome (IBS). Fecal microbiota transplantation has been shown to improve IBS by increasing the diversity of gut microbiota.16 It also improves patients’ mood, not just their IBS symptoms.
Alcohol use. Both alcohol consumption and alcohol withdrawal have been shown to cause immune dysregulation in the brain leading to neuroinflammation. This is attributed to the alteration in the composition of the microbiome (dysbiosis), which has a negative effect on the microbe-host homeostasis.17
The discovery of microbiome-gut-brain interactions and their bidirectional immune, endocrine, and neurotransmitter effects has been a momentous paradigm shift in health, neuroscience, and psychiatry.18 It has opened wide vistas of research for potential innovations in the prevention and treatment of various psychiatric disorders. Radical medical interventions that were previously inconceivable, such as fecal transplantation,19 are an example of the bold insights this new field of microbiome-gut-brain interaction is bringing to the landscape of medicine, including psychiatry. It has also highlighted the previously underappreciated importance of nutrition in health and disease.20
1. Nasrallah HA. Psychoneurogastroenterology: the abdominal brain, the microbiome, and psychiatry. Current Psychiatry. 2015;14(5):10-11.
2. Dinan TG, Borre YE, Cryan JF. Genomics of schizophrenia: time to consider the gut microbiome? Mol Psychiatry. 2014;19(12):1252-1257.
3. Alam R, Abdolmaleky HM, Zhou JR. Microbiome, inflammation, epigenetic alterations, and mental diseases. Am J Med Genet B Neuropsychiatr Genet. 2017;174(6):651-660.
4. Lach G, Schellekens H, Dinan TG, et al. Anxiety, depression, and the microbiome: a role for gut peptides. Neurotherapeutics. 2018;15(1):36-59.
5. Kelly JR, Kennedy PJ, Cryan JF, et al. Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front Cell Neurosci. 2015;9:392.
6. Rodrigues-Amorim D, Rivera-Baltanás T, Regueiro B, et al. The role of the gut microbiota in schizophrenia: current and future perspectives. World J Biol Psychiatry. 2018;21:1-15.
7. Petra AI, Panagiotidou S, Hatziagelaki E, et al. Gut-microbiota-brain axis and its effect on neuropsychiatric disorders with suspected immune dysregulation. Clin Ther. 2015;37(5):984-995.
8. Lurie I, Yang YX, Haynes K, et al. Antibiotic exposure and the risk for depression, anxiety, or psychosis: a nested case-control study. J Clin Psychiatry. 2015;76(11):1522-1528.
9. He Y, Kosciolek T, Tang J, et al. Gut microbiome and magnetic resonance spectroscopy study of subjects at ultra-high risk for psychosis may support the membrane hypothesis. Eur Psychiatry. 2018;53:37-45.
10. Yuan X, Zhang P, Wang Y, et al. Changes in metabolism and microbiota after 24-week risperidone treatment in drug naïve, normal weight patients with first episode schizophrenia. Schizophr Res. 2018;pii: S0920-9964(18)30274-3. [Epub ahead of print]. doi: 10.1016/j.schres.2018.05.017.
11. Dickerson F, Severance E, Yolken R. The microbiome, immunity, and schizophrenia and bipolar disorder. Brain Behav Immun. 2017;62:46-52.
12. Huang R, Wang K, Hu J. Effect of probiotics on depression: a systematic review and meta-analysis of randomized controlled trials. Nutrients. 2016;8(8):pii: E483. doi: 10.3390/nu8080483.
13. Carding S, Verbeke K, Vipond DT, et al. Dysbiosis of the gut microbiota in disease. Microb Ecol Health Dis. 2015;26:26191. doi: 10.3402/mehd.v26.26191.
14. Thapar A, Cooper M, Eyre O, et al. Practitioner review: what have we learnt about the causes of ADHD? J Child Psychol Psychiatry. 2013;54(1):3-16.
15. Jiang C, Li G, Huang P, et al. The gut microbiota and Alzheimer’s disease. J Alzheimers Dis. 2017;58(1):1-15.
16. Kurokawa S, Kishimoto T, Mizuno S, et al. The effect of fecal microbiota transplantation on psychiatric symptoms among patients with irritable bowel syndrome, functional diarrhea and functional constipation: an open-label observational study. J Affect Disord. 2018;235:506-512.
17. Hillemacher T, Bachmann O, Kahl KG, et al. Alcohol, microbiome, and their effect on psychiatric disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2018;85:105-115.
18. Doré J, Multon MC, Béhier JM; participants of Giens XXXII, Round Table No. 2. The human gut microbiome as source of innovation for health: which physiological and therapeutic outcomes could we expect? Therapie. 2017;72(1):21-38.
19. Vemuri RC, Gundamaraju R, Shinde T, et al. Therapeutic interventions for gut dysbiosis and related disorders in the elderly: antibiotics, probiotics or faecal microbiota transplantation? Benef Microbes. 2017;8(2):179-192.
20. Lombardi VC, De Meirleir KL, Subramanian K, et al. Nutritional modulation of the intestinal microbiota; future opportunities for the prevention and treatment of neuroimmune and neuroinflammatory disease. J Nutr Biochem. 2018;61:1-16.
What is the largest endocrine organ in the human body?
Here is a clue: It is also the largest immune organ in humans!
Still scratching your head? Here is another clue: This organ also contains a “second brain,” which is connected to big brain inside the head by the vagus nerve.
Okay, enough guessing: It’s the 30-foot long gastrointestinal (GI) tract, which is generally associated only with eating and digestion. But it is far more than a digestive tract. It is home to about 100 trillion diverse bacteria, including 1,000 known species, which together are known as “microbiota.” Its combined DNA is called the “microbiome” and is 10,000% larger than the human genome. Those trillions of bacteria in our guts are a symbiotic (commensal) organ that is vital for the normal functions of the human body.1
While this vast array of microorganisms is vital to sustaining a healthy human existence, it can also be involved in multiple psychiatric disorders, including depression, psychosis, anxiety, autism, and attention-deficit/hyperactivity disorder (ADHD). Humans acquire their unique sets of microbiota as they pass through the mother’s vagina at birth and while breastfeeding, as well as from exposure to various environmental sources in the first few months of life.2
The microbiota in the GI tract are an intimate neighbor of the “enteric brain,” comprised of 100 million neurons plus glia-like support cell structures. This “second brain” produces over 30 neurotransmitters, several of which (dopamine, serotonin, γ-aminobutyric acid [GABA], acetylcholine) have been implicated in major psychiatric disorders.3
The brain and gut have a dynamic bidirectional communication system, mediated by neural, hormonal, and immunological crosstalk and influences. The GI tract secretes dozens of peptides and other signaling molecules that influence the brain. The microbiota also interact with and are regulated by gut hormones such as oxytocin, ghrelin, neuropeptide Y, cholecystokinin, corticotrophin-releasing factor, and pancreatic polypeptide.4 The microbiota modulate brain development, functions, and behavior, and maintain the intestinal barrier, which, if disrupted, would result in the gut becoming “leaky” and triggering low-grade inflammation such as that associated with depression.5
Continue to: But don't overlook the importance of...
But don’t overlook the importance of diet. It is a major factor in shaping the composition of the microbiota. What we eat can have a preventative or reparative effect on neuroimmune or neuroinflammatory disease. An emerging body of evidence suggests that the diet and its effects on the gut microbiota can modify a person’s genes by epigenetic mechanisms (altering DNA methylation and histone effects). Probiotics can exert epigenetic effects by influencing cytokines, by producing short-chain fatty acids (SCFAs), by vitamin synthesis, and by producing several well-known neurotransmitters.6
The bidirectional trafficking across the microbiome-gut-brain axis includes reciprocal effects. The brain influences the microbiome composition by regulating satiety, the hypothalamic-pituitary axis, and with neuropeptides.7 In return, the microbiome conveys information to the brain about the intestinal status via infectious agents, intestinal neurotransmitters and modulators, cytokines, sensory vagal fibers, and various metabolites. Failure of these normal interactions can lead to a variety of pathological processes, including inflammatory, autoimmune, degenerative, metabolic, cognitive, mood, and behavioral adverse effects. Therapeutic interventions for these adverse consequences can be implemented through microbiome manipulations (such as fecal transplants), nutritional strategies, and reinforcement of the enteric and brain barriers.
Alterations in the microbiota, such as by the intake of antibiotics or by intestinal inflammation, can lead to psychiatric disorders.8 The following findings link gut microbiome disruptions with several psychiatric disorders:
Schizophrenia prodrome. Fecal bacteria show an increase in SCFAs, which can activate microglia (the initial step in triggering psychosis).9 These bacteria have been shown to lead to an increase in choline levels in the anterior cingulate, a known biomarker for membrane dysfunction, which is one of the biological models of schizophrenia.
Schizophrenia—first-episode. A recent study reported abnormalities in the gut microbiota of patients with first-episode psychosis, with a lower number of certain fecal bacteria (including bifidobacterium, E. coli, and lactobacillus) and high levels of Clostridium coccoides. After 6 months of risperidone treatment, the above changes were reversed.10
Continue to: Another study of fecal microbiota...
Another study of fecal microbiota in a first-episode psychosis cohort found significant differences in several bacterial strains compared with a healthy control group, and those with the strongest difference had more severe psychotic symptoms and poorer response after 12 months of antipsychotic treatment.11
Autism has been linked to increased microbiota diversity, and an excess of bacteroides has been associated with a higher diversity of autism. Fecal samples from autistic children were reported to have an increase in SCFAs. Interestingly, a certain strain of lactobacillus can modulate oxytocin or reverse some autistic symptoms.
Depression has been associated with increased diversity of microbiota alpha. Patients with depression have been reported to have low numbers of bifidobacterium
ADHD. Some studies suggest that ADHD may be linked to factors that can alter gut microbiota, including birthing mode, type of infant feeding, maternal health, and early stressors. In addition, dietary influences on gut microbiota can modify ADHD symptoms.14
Alzheimer’s disease. Metabolic dysregulation, such as obesity and diabetes, can inflame the gut microbiota, and are known risk factors for Alzheimer’s disease.15
Continue to: Irritable bowel sydrome...
Irritable bowel syndrome (IBS). Fecal microbiota transplantation has been shown to improve IBS by increasing the diversity of gut microbiota.16 It also improves patients’ mood, not just their IBS symptoms.
Alcohol use. Both alcohol consumption and alcohol withdrawal have been shown to cause immune dysregulation in the brain leading to neuroinflammation. This is attributed to the alteration in the composition of the microbiome (dysbiosis), which has a negative effect on the microbe-host homeostasis.17
The discovery of microbiome-gut-brain interactions and their bidirectional immune, endocrine, and neurotransmitter effects has been a momentous paradigm shift in health, neuroscience, and psychiatry.18 It has opened wide vistas of research for potential innovations in the prevention and treatment of various psychiatric disorders. Radical medical interventions that were previously inconceivable, such as fecal transplantation,19 are an example of the bold insights this new field of microbiome-gut-brain interaction is bringing to the landscape of medicine, including psychiatry. It has also highlighted the previously underappreciated importance of nutrition in health and disease.20
What is the largest endocrine organ in the human body?
Here is a clue: It is also the largest immune organ in humans!
Still scratching your head? Here is another clue: This organ also contains a “second brain,” which is connected to big brain inside the head by the vagus nerve.
Okay, enough guessing: It’s the 30-foot long gastrointestinal (GI) tract, which is generally associated only with eating and digestion. But it is far more than a digestive tract. It is home to about 100 trillion diverse bacteria, including 1,000 known species, which together are known as “microbiota.” Its combined DNA is called the “microbiome” and is 10,000% larger than the human genome. Those trillions of bacteria in our guts are a symbiotic (commensal) organ that is vital for the normal functions of the human body.1
While this vast array of microorganisms is vital to sustaining a healthy human existence, it can also be involved in multiple psychiatric disorders, including depression, psychosis, anxiety, autism, and attention-deficit/hyperactivity disorder (ADHD). Humans acquire their unique sets of microbiota as they pass through the mother’s vagina at birth and while breastfeeding, as well as from exposure to various environmental sources in the first few months of life.2
The microbiota in the GI tract are an intimate neighbor of the “enteric brain,” comprised of 100 million neurons plus glia-like support cell structures. This “second brain” produces over 30 neurotransmitters, several of which (dopamine, serotonin, γ-aminobutyric acid [GABA], acetylcholine) have been implicated in major psychiatric disorders.3
The brain and gut have a dynamic bidirectional communication system, mediated by neural, hormonal, and immunological crosstalk and influences. The GI tract secretes dozens of peptides and other signaling molecules that influence the brain. The microbiota also interact with and are regulated by gut hormones such as oxytocin, ghrelin, neuropeptide Y, cholecystokinin, corticotrophin-releasing factor, and pancreatic polypeptide.4 The microbiota modulate brain development, functions, and behavior, and maintain the intestinal barrier, which, if disrupted, would result in the gut becoming “leaky” and triggering low-grade inflammation such as that associated with depression.5
Continue to: But don't overlook the importance of...
But don’t overlook the importance of diet. It is a major factor in shaping the composition of the microbiota. What we eat can have a preventative or reparative effect on neuroimmune or neuroinflammatory disease. An emerging body of evidence suggests that the diet and its effects on the gut microbiota can modify a person’s genes by epigenetic mechanisms (altering DNA methylation and histone effects). Probiotics can exert epigenetic effects by influencing cytokines, by producing short-chain fatty acids (SCFAs), by vitamin synthesis, and by producing several well-known neurotransmitters.6
The bidirectional trafficking across the microbiome-gut-brain axis includes reciprocal effects. The brain influences the microbiome composition by regulating satiety, the hypothalamic-pituitary axis, and with neuropeptides.7 In return, the microbiome conveys information to the brain about the intestinal status via infectious agents, intestinal neurotransmitters and modulators, cytokines, sensory vagal fibers, and various metabolites. Failure of these normal interactions can lead to a variety of pathological processes, including inflammatory, autoimmune, degenerative, metabolic, cognitive, mood, and behavioral adverse effects. Therapeutic interventions for these adverse consequences can be implemented through microbiome manipulations (such as fecal transplants), nutritional strategies, and reinforcement of the enteric and brain barriers.
Alterations in the microbiota, such as by the intake of antibiotics or by intestinal inflammation, can lead to psychiatric disorders.8 The following findings link gut microbiome disruptions with several psychiatric disorders:
Schizophrenia prodrome. Fecal bacteria show an increase in SCFAs, which can activate microglia (the initial step in triggering psychosis).9 These bacteria have been shown to lead to an increase in choline levels in the anterior cingulate, a known biomarker for membrane dysfunction, which is one of the biological models of schizophrenia.
Schizophrenia—first-episode. A recent study reported abnormalities in the gut microbiota of patients with first-episode psychosis, with a lower number of certain fecal bacteria (including bifidobacterium, E. coli, and lactobacillus) and high levels of Clostridium coccoides. After 6 months of risperidone treatment, the above changes were reversed.10
Continue to: Another study of fecal microbiota...
Another study of fecal microbiota in a first-episode psychosis cohort found significant differences in several bacterial strains compared with a healthy control group, and those with the strongest difference had more severe psychotic symptoms and poorer response after 12 months of antipsychotic treatment.11
Autism has been linked to increased microbiota diversity, and an excess of bacteroides has been associated with a higher diversity of autism. Fecal samples from autistic children were reported to have an increase in SCFAs. Interestingly, a certain strain of lactobacillus can modulate oxytocin or reverse some autistic symptoms.
Depression has been associated with increased diversity of microbiota alpha. Patients with depression have been reported to have low numbers of bifidobacterium
ADHD. Some studies suggest that ADHD may be linked to factors that can alter gut microbiota, including birthing mode, type of infant feeding, maternal health, and early stressors. In addition, dietary influences on gut microbiota can modify ADHD symptoms.14
Alzheimer’s disease. Metabolic dysregulation, such as obesity and diabetes, can inflame the gut microbiota, and are known risk factors for Alzheimer’s disease.15
Continue to: Irritable bowel sydrome...
Irritable bowel syndrome (IBS). Fecal microbiota transplantation has been shown to improve IBS by increasing the diversity of gut microbiota.16 It also improves patients’ mood, not just their IBS symptoms.
Alcohol use. Both alcohol consumption and alcohol withdrawal have been shown to cause immune dysregulation in the brain leading to neuroinflammation. This is attributed to the alteration in the composition of the microbiome (dysbiosis), which has a negative effect on the microbe-host homeostasis.17
The discovery of microbiome-gut-brain interactions and their bidirectional immune, endocrine, and neurotransmitter effects has been a momentous paradigm shift in health, neuroscience, and psychiatry.18 It has opened wide vistas of research for potential innovations in the prevention and treatment of various psychiatric disorders. Radical medical interventions that were previously inconceivable, such as fecal transplantation,19 are an example of the bold insights this new field of microbiome-gut-brain interaction is bringing to the landscape of medicine, including psychiatry. It has also highlighted the previously underappreciated importance of nutrition in health and disease.20
1. Nasrallah HA. Psychoneurogastroenterology: the abdominal brain, the microbiome, and psychiatry. Current Psychiatry. 2015;14(5):10-11.
2. Dinan TG, Borre YE, Cryan JF. Genomics of schizophrenia: time to consider the gut microbiome? Mol Psychiatry. 2014;19(12):1252-1257.
3. Alam R, Abdolmaleky HM, Zhou JR. Microbiome, inflammation, epigenetic alterations, and mental diseases. Am J Med Genet B Neuropsychiatr Genet. 2017;174(6):651-660.
4. Lach G, Schellekens H, Dinan TG, et al. Anxiety, depression, and the microbiome: a role for gut peptides. Neurotherapeutics. 2018;15(1):36-59.
5. Kelly JR, Kennedy PJ, Cryan JF, et al. Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front Cell Neurosci. 2015;9:392.
6. Rodrigues-Amorim D, Rivera-Baltanás T, Regueiro B, et al. The role of the gut microbiota in schizophrenia: current and future perspectives. World J Biol Psychiatry. 2018;21:1-15.
7. Petra AI, Panagiotidou S, Hatziagelaki E, et al. Gut-microbiota-brain axis and its effect on neuropsychiatric disorders with suspected immune dysregulation. Clin Ther. 2015;37(5):984-995.
8. Lurie I, Yang YX, Haynes K, et al. Antibiotic exposure and the risk for depression, anxiety, or psychosis: a nested case-control study. J Clin Psychiatry. 2015;76(11):1522-1528.
9. He Y, Kosciolek T, Tang J, et al. Gut microbiome and magnetic resonance spectroscopy study of subjects at ultra-high risk for psychosis may support the membrane hypothesis. Eur Psychiatry. 2018;53:37-45.
10. Yuan X, Zhang P, Wang Y, et al. Changes in metabolism and microbiota after 24-week risperidone treatment in drug naïve, normal weight patients with first episode schizophrenia. Schizophr Res. 2018;pii: S0920-9964(18)30274-3. [Epub ahead of print]. doi: 10.1016/j.schres.2018.05.017.
11. Dickerson F, Severance E, Yolken R. The microbiome, immunity, and schizophrenia and bipolar disorder. Brain Behav Immun. 2017;62:46-52.
12. Huang R, Wang K, Hu J. Effect of probiotics on depression: a systematic review and meta-analysis of randomized controlled trials. Nutrients. 2016;8(8):pii: E483. doi: 10.3390/nu8080483.
13. Carding S, Verbeke K, Vipond DT, et al. Dysbiosis of the gut microbiota in disease. Microb Ecol Health Dis. 2015;26:26191. doi: 10.3402/mehd.v26.26191.
14. Thapar A, Cooper M, Eyre O, et al. Practitioner review: what have we learnt about the causes of ADHD? J Child Psychol Psychiatry. 2013;54(1):3-16.
15. Jiang C, Li G, Huang P, et al. The gut microbiota and Alzheimer’s disease. J Alzheimers Dis. 2017;58(1):1-15.
16. Kurokawa S, Kishimoto T, Mizuno S, et al. The effect of fecal microbiota transplantation on psychiatric symptoms among patients with irritable bowel syndrome, functional diarrhea and functional constipation: an open-label observational study. J Affect Disord. 2018;235:506-512.
17. Hillemacher T, Bachmann O, Kahl KG, et al. Alcohol, microbiome, and their effect on psychiatric disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2018;85:105-115.
18. Doré J, Multon MC, Béhier JM; participants of Giens XXXII, Round Table No. 2. The human gut microbiome as source of innovation for health: which physiological and therapeutic outcomes could we expect? Therapie. 2017;72(1):21-38.
19. Vemuri RC, Gundamaraju R, Shinde T, et al. Therapeutic interventions for gut dysbiosis and related disorders in the elderly: antibiotics, probiotics or faecal microbiota transplantation? Benef Microbes. 2017;8(2):179-192.
20. Lombardi VC, De Meirleir KL, Subramanian K, et al. Nutritional modulation of the intestinal microbiota; future opportunities for the prevention and treatment of neuroimmune and neuroinflammatory disease. J Nutr Biochem. 2018;61:1-16.
1. Nasrallah HA. Psychoneurogastroenterology: the abdominal brain, the microbiome, and psychiatry. Current Psychiatry. 2015;14(5):10-11.
2. Dinan TG, Borre YE, Cryan JF. Genomics of schizophrenia: time to consider the gut microbiome? Mol Psychiatry. 2014;19(12):1252-1257.
3. Alam R, Abdolmaleky HM, Zhou JR. Microbiome, inflammation, epigenetic alterations, and mental diseases. Am J Med Genet B Neuropsychiatr Genet. 2017;174(6):651-660.
4. Lach G, Schellekens H, Dinan TG, et al. Anxiety, depression, and the microbiome: a role for gut peptides. Neurotherapeutics. 2018;15(1):36-59.
5. Kelly JR, Kennedy PJ, Cryan JF, et al. Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front Cell Neurosci. 2015;9:392.
6. Rodrigues-Amorim D, Rivera-Baltanás T, Regueiro B, et al. The role of the gut microbiota in schizophrenia: current and future perspectives. World J Biol Psychiatry. 2018;21:1-15.
7. Petra AI, Panagiotidou S, Hatziagelaki E, et al. Gut-microbiota-brain axis and its effect on neuropsychiatric disorders with suspected immune dysregulation. Clin Ther. 2015;37(5):984-995.
8. Lurie I, Yang YX, Haynes K, et al. Antibiotic exposure and the risk for depression, anxiety, or psychosis: a nested case-control study. J Clin Psychiatry. 2015;76(11):1522-1528.
9. He Y, Kosciolek T, Tang J, et al. Gut microbiome and magnetic resonance spectroscopy study of subjects at ultra-high risk for psychosis may support the membrane hypothesis. Eur Psychiatry. 2018;53:37-45.
10. Yuan X, Zhang P, Wang Y, et al. Changes in metabolism and microbiota after 24-week risperidone treatment in drug naïve, normal weight patients with first episode schizophrenia. Schizophr Res. 2018;pii: S0920-9964(18)30274-3. [Epub ahead of print]. doi: 10.1016/j.schres.2018.05.017.
11. Dickerson F, Severance E, Yolken R. The microbiome, immunity, and schizophrenia and bipolar disorder. Brain Behav Immun. 2017;62:46-52.
12. Huang R, Wang K, Hu J. Effect of probiotics on depression: a systematic review and meta-analysis of randomized controlled trials. Nutrients. 2016;8(8):pii: E483. doi: 10.3390/nu8080483.
13. Carding S, Verbeke K, Vipond DT, et al. Dysbiosis of the gut microbiota in disease. Microb Ecol Health Dis. 2015;26:26191. doi: 10.3402/mehd.v26.26191.
14. Thapar A, Cooper M, Eyre O, et al. Practitioner review: what have we learnt about the causes of ADHD? J Child Psychol Psychiatry. 2013;54(1):3-16.
15. Jiang C, Li G, Huang P, et al. The gut microbiota and Alzheimer’s disease. J Alzheimers Dis. 2017;58(1):1-15.
16. Kurokawa S, Kishimoto T, Mizuno S, et al. The effect of fecal microbiota transplantation on psychiatric symptoms among patients with irritable bowel syndrome, functional diarrhea and functional constipation: an open-label observational study. J Affect Disord. 2018;235:506-512.
17. Hillemacher T, Bachmann O, Kahl KG, et al. Alcohol, microbiome, and their effect on psychiatric disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2018;85:105-115.
18. Doré J, Multon MC, Béhier JM; participants of Giens XXXII, Round Table No. 2. The human gut microbiome as source of innovation for health: which physiological and therapeutic outcomes could we expect? Therapie. 2017;72(1):21-38.
19. Vemuri RC, Gundamaraju R, Shinde T, et al. Therapeutic interventions for gut dysbiosis and related disorders in the elderly: antibiotics, probiotics or faecal microbiota transplantation? Benef Microbes. 2017;8(2):179-192.
20. Lombardi VC, De Meirleir KL, Subramanian K, et al. Nutritional modulation of the intestinal microbiota; future opportunities for the prevention and treatment of neuroimmune and neuroinflammatory disease. J Nutr Biochem. 2018;61:1-16.
Pharmacogenetic testing in children: What to test and how to use it
The use of pharmacogenetic testing to help drive decisions for medication management of patients with psychiatric illnesses is growing. It’s becoming increasingly common for patients or the parents of pediatric patients to request pharmacogenetic testing or to bring the results of prior testing to their appointment. In these situations, patients may ask clinicians to consider the recommendations from these testing reports, which rarely provide guidance specific to pediatric patients. However, this can be difficult for clinicians who did not receive education in pharmacogenetics and may not be familiar with the evidence or options for pharmacogenetic testing. Many of the pharmacogenetic associations identified thus far have been discovered in adults, but studies in pediatric patients are relatively rare. This article reviews pharmacogenetic testing and the evidence supporting it, and describes implementation of routine pharmacogenetics testing at a children’s hospital.
CASE
Testing leads to dose adjustment, improvement
Ms. R, age 16, presents with treatment-resistant major depressive disorder that is characterized by a significant neurovegetative burden and prominent anhedonia, as well as intermittent suicidal ideation without intent or plan. She reportedly did not improve after multiple medication trials, including
Augmentation strategies included
_
Drug metabolism and genetic variants
It is common for patients with psychiatric disorders to receive trials of multiple psychotropic medications prior to identifying one that reduces symptom burden without producing intolerable adverse effects. Due to the high frequency of toxicity-related adverse effects (observed in 20% to 70% of patients),1 these medications are frequently initiated at low doses and titrated slowly until the patient either experiences an intolerable adverse effect or achieves symptomatic remission.1,2 The practice of slow titration at the start of treatment increases the risk of undertreatment in many patients, and may ultimately lead to a medication change due to the lack of response.
Many of the medications used to treat psychiatric illnesses are primarily metabolized by 2 CYP enzymes expressed in the liver, encoded by the CYP2D6 and CYP2C19 genes3 (Table 13-7 and Table 23,6,7). These drug-metabolizing enzymes affect the pharmacokinetics of many medications. Some medications are converted to an active form by these enzymes, and some are inactivated. The contributions of CYP enzymes to the pharmacokinetics of neuropsychiatric medications have been well-described; however, there is less evidence on whether variants in these genes are associated with treatment efficacy, especially in pediatric patients.8,9 CYP2D6 enzyme activity reaches adult levels soon after birth, but children may have higher CYP2C19 activity than adults.4 CYP3A4 also contributes to the metabolism of many medications; however, there is only weak evidence that genetic variants in CYP3A4 contribute to variability in the pharmacokinetics of these medications, and there are currently no dosing guidelines based on pharmacogenetics available for this gene.10
As is common in the pharmacogenetic field, genotypes are denoted with a “star allele” (eg, *2) rather than positional nomenclature (eg, c.681G>A). The normal allele is usually designated as *1, and this result is given in the absence of the tested alleles. There is no consensus on the minimum set of alleles to be tested for most genes,11 so commercially available tests vary widely in what alleles are tested (and therefore what they exclude before calling a normal allele).12 The metabolizer phenotype for a patient is determined by taking into account the activity of each of the patient’s 2 alleles (eg, *1/*2). A patient is categorized as a poor-, intermediate-, normal- (extensive-), or ultra-rapid metabolizer. Generally, the allele definitions are widely agreed upon (what genetic variant or variants comprise the *2 allele) due to nomenclature committees for each gene; however, because there are no standards for interpretation, the interpretation of the activity of the alleles and conversion to metabolizer phenotype varies among clinics.13
Continue to: Guidelines help with genotype-guided dosing
Guidelines help with genotype-guided dosing

Each CPIC guideline specifically addresses use in pediatric patients, indicating that there are relatively few studies in pediatrics, but “it may be appropriate to extrapolate these recommendations to adolescents or possibly younger children with close monitoring.”4 The DPWG guidelines do not mention whether or not the recommendations are applicable to children. Neither CPIC nor the DPWG provides guidance on when to test; however, the French National Network of Pharmacogenetics (Réseau national de pharmacogénétique) recommends CYP2D6 and CYP2C19 genotyping before initiating antidepressant treatment, especially in patients with a high risk of toxicity.16
In the case above, Ms. R was determined to be a CYP2D6 ultra-rapid metabolizer. Because she showed some initial response to aripiprazole and venlafaxine ER, which are both metabolized by CYP2D6, these medications were very quickly titrated up, and the increased dosages produced the desired response. Venlafaxine is metabolized to the active metabolite O-desmethylvenlafaxine by CYP2D6. The DPWG recommends increasing the dose of venlafaxine in CYP2D6 ultra-rapid metabolizers to 150% of the normal dose based on the decreased serum concentrations of venlafaxine and O-desmethylvenlafaxine in these patients.6 Aripiprazole is also metabolized by CYP2D6; however, the FDA and DPWG give no recommendations for ultra-rapid metabolizers, but do recommend reducing the dose of aripiprazole in CYP2D6 poor metabolizers.
Multiple studies in adults have analyzed the association between pharmacokinetic (CYP2D6 and CYP2C19) or pharmacodynamic genes (SLC6A4, HTR2A, and GRIK4) and outcomes,17 including some large clinical trials that conducted genome-wide association studies18-20 and meta-analyses across multiple studies.21,22 Most pharmacogenetic studies in psychiatric patients are small, and very few have included pediatric patients. However, with more interest in neuropsychiatric pharmacogenetics, these studies are becoming more common.23-26
Continue to: Limited evidence from studies of commercially available tests
Limited evidence from studies of commercially available tests
Several pharmacogenetic tests are commercially available, including some that focus on providing information that can be used specifically when prescribing psychiatric medications, such as the GeneSight Psychotropic test, CNSdose, Genomind, and Neuropharmagen.
In an industry-sponsored, nonrandomized clinical trial that included patients for whom prescribing decisions were made based on the GeneSight test, outcomes in adults were improved compared with treatment as usual,27 inpatient stays were shorter,28 and pharmacy costs were reduced.29 In one of these studies, the authors noted that the traditional, single-gene analysis was not associated with improved outcomes, whereas the multiple gene combination (pharmacokinetic and pharmacodynamic genes) was associated with improved outcomes among patients with depression.27 However, when GeneSightwas compared with treatment as usual in a small randomized trial, there was not a significant association between use of the test and improved outcomes among patients with treatment-resistant depression.30 The results of a much larger randomized trial (N = 1,167) are available31 and expected to be published, but patients younger than age 18 were excluded from this study.32 A retrospective study conducted in adult psychiatric patients found that patients whose treatment followed recommendations of a pharmacogenetic test including 20 genes were almost 4 times more likely to improve than patients whose treatment did not follow the recommendations.33
Pharmacogenetic testing at our pediatric inpatient unit
The Cincinnati Children’s Division of Child and Adolescent Psychiatry is the largest psychiatric inpatient service in a U.S. pediatric hospital. Starting in 2004, we adopted pharmacogenetically-guided dosing of psychiatric medications.34 CYP2D6 and CYP2C19 were chosen for testing because the enzymes encoded by these genes metabolize many of the antidepressants and antipsychotics that patients admitted to our unit will receive, and the clinicians wanted all available tools to help improve the care of these patients. To date, the Genetic Pharmacology Service (GPS) has performed >25,000 tests for variants in CYP2D6 and CYP2C19 as part of inpatient care. Patients provide a specimen (blood or buccal swab) at the time of admission to inpatient psychiatry, genotyping is performed onsite by the Molecular Genetics Laboratory (certified by the College of American Pathologists [CAP]/Clinical Laboratory Improvement Amendments [CLIA]) and the results are posted to the medical record within 2 business days. The report contains the patient’s alleles for CYP2D6 and CYP2C19, the genotype-predicted metabolizer phenotype, and dosing recommendations for 19 drugs (provided as a percentage of the standard dose). Insurance is billed for the test, and reimbursement is usually received when the test is performed as part of an inpatient stay.
The GPS team performed a retrospective chart review after the first panel was implemented in 2005.23 The study included 279 patients who were receiving a medication metabolized by one of the 2 genes tested. The poor metabolizers had the highest efficacy and highest number of adverse drug reactions, while ultra-rapid metabolizers had the lowest efficacy and lowest number of adverse reactions during their initial inpatient stay. In patients not treated with medications metabolized by CYP2D6 or CYP2C19, there was no association between metabolizer status and efficacy or adverse drug reactions. In this retrospective study, there was no association between metabolizer status and length of stay.
Overcoming the challenges
One challenge with many of the pharmacogenetic tests is interpretation of the results. The reports can span more than 20 pages, and clinicians may not have time to thoroughly read and understand how best to use all of this information. Sometimes the reports can make it seem like the first-line medication for the patient’s condition is not the best choice, but it could work well when dosed appropriately based on the patient’s genotype. Each commercially available test has a different way of presenting results,13 so when choosing a pharmacogenetic test, one should be sure to see a sample report. Vo et al35 recently reviewed factors to consider when choosing a pharmacogenetic test.
Continue to: Because patients and families also have difficulty understanding the reports...
Because patients and families also have difficulty understanding the reports, we created patient education sheets,36 written at an eighth grade level with feedback from parents and modeled on those provided by St. Jude Children’s Research Hospital.37 St. Jude Children’s Research Hospital also has pharmacogenetic competencies that pharmacists and nurses must pass.38,39 The following is a sample explanation that one of our nurses uses to educate parents on what is being tested and what effect the results will have on the treatment plan.
“During your child’s stay we will be completing a genetic test to help us understand how he/she processes the types of medications that we may be likely to start during their hospitalization. This does not tell us which medication will be best—unfortunately within the field of psychiatry there is still some unavoidable trial and error; rather, what it will do is tell us how to make sure that the dosing is at a level that would be safe for the way your child’s body breaks down the medicine, so that he/she can get the intended benefit of the medicine’s effects, while decreasing the risk of uncomfortable side effects, where possible.”
Other challenges in pharmacogenetic testing are the cost, disease risk, and concern about how genetic information will be used. Because these tests are often not covered by health insurance, some commercial pharmacogenetic testing companies offer an out-of-pocket maximum in the $250 to $350 range to reduce the cost to the patient. Some pharmacogenetic testing companies also test for genes associated with disease, so if a clinician orders the test, he or she may be responsible for sharing that information with the patient. For most pharmacogenetic testing companies, the turn-around time is 2 to 10 days. Genetic information is protected by federal laws, including Genetic Information Nondiscrimination Act (GINA) and Health Insurance Portability and Accountability Act (HIPAA).
The choice of psychotropic medication is complex, and although we would like pharmacogenetics to be the only answer to why every patient does or does not respond to a medication, it is not. Response to medication is influenced by age, comorbidities, illness severity, illness duration, compliance, gender, concomitant medications, and potentially more.40 Pharmacogenetics is another tool at the clinician’s disposal to help in choosing a medication and dose. There is a clear association between CYP2D6 and CYP2C19 and exposure to many antidepressants and antipsychotics (reviewed by Stingl et al3); however, the link between exposure and response is much weaker. It may be strengthened by the inclusion of pharmacodynamic information (the level of expression of the drug target), which can be influenced by genetic variants.41 At the present time, the most evidence exists for testing CYP2D6 and CYP2C19, and the CPIC4,5,15 and DWPG6 guidelines provide evidence-based recommendations for how to adjust medication dosages based on the results.
There is clearly much more research that needs to be done in the field of neuropsychiatric pharmacogenetics, especially in pediatric populations. As we see increased utilization of pharmacogenetic tests in psychiatry, there is also a need for pharmacogenetic education of patients, families, nurses, pharmacists, and psychiatrists. Several good pharmacogenetic resources that contain up-to-date summaries of the available evidence linking pharmacogenetic variants to medication response, implementation resources, and educational resources are available. These include CPIC (www.cpicpgx.org), PharmGKB (www.pharmgkb.org), and the IGNITE Spark Toolbox (https://ignite-genomics.org/spark-toolbox/clinicians/).
Acknowledgements
The author thanks Jen Milau, APRN, for the case study and sample explanation, and Jeffrey Strawn, MD, FAACP, Ethan Poweleit, and Stacey Aldrich, MS, for help with preparing this manuscript.
1. Cipriani A, Zhou X, Del Giovane C, et al. Comparative efficacy and tolerability of antidepressants for major depressive disorder in children and adolescents: a network meta-analysis. Lancet. 2016; 388(10047):881-890.
2. Correll CU, Sheridan EM, DelBello MP. Antipsychotic and mood stabilizer efficacy and tolerability in pediatric and adult patients with bipolar I mania: a comparative analysis of acute, randomized, placebo-controlled trials. Bipolar Disord. 2010;12(2):116-141.
3. Stingl JC, Brockmoller J, Viviani R. Genetic variability of drug-metabolizing enzymes: the dual impact on psychiatric therapy and regulation of brain function. Mol Psychiatry. 2013;18(3):273-287.
4. Hicks JK, Bishop JR, Sangkuhl K, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline for CYP2D6 and CYP2C19 genotypes and dosing of selective serotonin reuptake inhibitors. Clin Pharmacol Ther. 2015;98(2):127-134.
5. Hicks JK, Sangkuhl K, Swen JJ, et al. Clinical pharmacogenetics implementation consortium guideline (CPIC) for CYP2D6 and CYP2C19 genotypes and dosing of tricyclic antidepressants: 2016 update. Clin Pharmacol Ther. 2017;102(1):37-44.
6. Swen JJ, Nijenhuis M, de Boer A, et al. Pharmacogenetics: from bench to byte--an update of guidelines. Clin Pharmacol Ther. 2011;89(5):662-673.
7. Swen JJ, Wilting I, de Goede AL, et al. Pharmacogenetics: from bench to byte. Clin Pharmacol Ther. 2008;83(5):781-787.
8. GENDEP Investigators, MARS Investigators, and STAR*D Investigators. Common genetic variation and antidepressant efficacy in major depressive disorder: a meta-analysis of three genome-wide pharmacogenetic studies. Am J Psychiatry. 2013;170(2):207-217.
9. Ji Y, Schaid DJ, Desta Z, et al. Citalopram and escitalopram plasma drug and metabolite concentrations: genome-wide associations. Br J Clin Pharmacol. 2014;78(2):373-383.
10. Werk AN, Cascorbi I. Functionalgene variants of CYP3A4. Clin Pharmacol Ther. 2014:96(3):340-348.
11. Pratt VM, Del Tredici AL, Hachad H, et al. Recommendations for clinical CYP2C19 genotyping allele selection: a report of the Association for Molecular Pathology. J Mol Diagn. 2018;20(3):269-276.
12. Bousman CA, Jaksa P, Pantelis C. Systematic evaluation of commercial pharmacogenetic testing in psychiatry: a focus on CYP2D6 and CYP2C19 allele coverage and results reporting. Pharmacogenet Genomics. 2017;27(11):387-393.
13. Hicks JK, Swen JJ, Gaedigk A. Challenges in CYP2D6 phenotype assignment from genotype data: a critical assessment and call for standardization. Curr Drug Metab. 2014;15(2):218-232.
14. Caudle KE, Klein TE, Hoffman JM, et al. Incorporation of pharmacogenomics into routine clinical practice: the Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline development process. Curr Drug Metab. 2014;15(2):209-217.
15. Hicks JK, Swen JJ, Thorn CF, et al. Clinical Pharmacogenetics Implementation Consortium guideline for CYP2D6 and CYP2C19 genotypes and dosing of tricyclic antidepressants. Clin Pharmacol Ther. 2013;93(5):402-408.
16. Quaranta S, Dupouey J, Colle R, et al. Pharmacogenetics of antidepressant drugs: State of the art and clinical implementation - recommendations from the French National Network of Pharmacogenetics. Therapie. 2017;72(2):311-318.
17. Fabbri C, Minarini A, Nitsu T, et al. Understanding the pharmacogenetics of selective serotonin reuptake inhibitors. Expert Opin Drug Metab Toxicol. 2014;10(8):1093-1118.
18. Mrazek DA, Rush AJ, Biernacka JM, et al. SLC6A4 variation and citalopram response. Am J Med Genet B Neuropsychiatr Genet. 2009;150B(3):341-351.
19. Biernacka JM, Sangkuhl K, Jenkins G, et al. The International SSRI Pharmacogenomics Consortium (ISPC): a genome-wide association study of antidepressant treatment response. Transl Psychiatry. 2015;5:e553. doi: 10.1038/tp.2015.47.
20. Horstmann S, Lucae S, Menke A, et al. Polymorphisms in GRIK4, HTR2A, and FKBP5 show interactive effects in predicting remission to antidepressant treatment. Neuropsychopharmacology. 2010;35(3):727-740.
21. Porcelli S, Fabbri C, Serretti A. Meta-analysis of serotonin transporter gene promoter polymorphism (5-HTTLPR) association with antidepressant efficacy. Eur Neuropsychopharmacol. 2012;22(4):239-258.
22. Niitsu T, Fabbri C, Bentini F, et al. Pharmacogenetics in major depression: a comprehensive meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2013;45:183-194.
23. Prows CA, Nick TG, Saldaña SN, et al. Drug-metabolizing enzyme genotypes and aggressive behavior treatment response in hospitalized pediatric psychiatric patients. J Child Adolesc Psychopharmacol. 2009;19(4):385-394.
24. Rotberg B, Kronenberg S, Carmel M, et al. Additive effects of 5-HTTLPR (serotonin transporter) and tryptophan hydroxylase 2 G-703T gene polymorphisms on the clinical response to citalopram among children and adolescents with depression and anxiety disorders. J Child Adolesc Psychopharmacol. 2013;23(2):117-122.
25. Kronenberg S, Apter A, Brent D, et al. Serotonin transporter polymorphism (5-HTTLPR) and citalopram effectiveness and side effects in children with depression and/or anxiety disorders. J Child Adolesc Psychopharmacol. 2007;17(6):741-750.
26. AlOlaby RR, Sweha SR, Silva M, et al. Molecular biomarkers predictive of sertraline treatment response in young children with fragile X syndrome. Brain Dev. 2017;39(6):483-492.
27. Altar CA, Carhart JM, Allen JD, et al. Clinical validity: Combinatorial pharmacogenomics predicts antidepressant responses and healthcare utilizations better than single gene phenotypes. Pharmacogenomics J. 2015;15(5):443-451.
28. Winner J, Allen JD, Altar CA, et al. Psychiatric pharmacogenomics predicts health resource utilization of outpatients with anxiety and depression. Transl Psychiatry. 2013;3:e242. doi:10.1038/tp.2013.2.
29. Winner JG, Carhart JM, Altar CA, et al. Combinatorial pharmacogenomic guidance for psychiatric medications reduces overall pharmacy costs in a 1 year prospective evaluation. Curr Med Res Opin. 2015;31(9):1633-1643.
30. Winner JG, Carhart JM, Altar CA, et al. A prospective, randomized, double-blind study assessing the clinical impact of integrated pharmacogenomic testing for major depressive disorder. Discov Med. 2013;16(89):219-227.
31. Genesight. GUIDED clinical study. https://genesight.com/greden-study/. Updated May 31, 2018. Accessed August 1, 2018.
32. U.S. National Library of Medicine ClinicalTrials.gov. Genomics used to improve DEpression decisions (GUIDED). https://clinicaltrials.gov/ct2/show/NCT02109939. Accessed July 24, 2018.
33. Espadaler J, Tuson M, Lopez-Ibor JM, et al. Pharmacogenetic testing for the guidance of psychiatric treatment: a multicenter retrospective analysis. CNS Spectrums. 2017;22(4):315-324.
34. Ramsey LB, Prows CA, Zhang K, et al. Implementation of pharmacogenetics at Cincinnati Children’s Hospital Medical Center: lessons learned over 14 years of personalizing medicine. Clin Pharmacol Ther. 2018. doi: 10.1002/cpt.1165. [Epub ahead of print].
35. Vo TT, Bell GC, Owusu Obeng A, et al. Pharmacogenomics implementation: considerations for selecting a reference laboratory. Pharmacotherapy. 2017;37(9):1014-1022.
36. Cincinnati Children’s Hospital. Genetic Pharmacology Service: Education. www.cincinnatichildrens.org/gpsinfo. Accessed August 1, 2018.
37. St. Jude Children’s Research Hospital. Do You Know...Cytochrome P450 2D6 (CYP2D6) and medicines. https://www.stjude.org/treatment/patient-resources/caregiver-resources/patient-family-education-sheets/pharmacy-and-medicines/cytochrome-p450-2d6-cyp2d6-and-medicines.html. Accessed August 1, 2018.
38. St. Jude Children’s Research Hospital. Implementation Resources for Professionals: Clinical Pharmacogenetics at St. Jude. https://www.stjude.org/research/clinical-trials/pg4kds-pharmaceutical-science/implementation-resources-for-professionals.html. Accessed August 1, 2018.
39. Hoffman JM, Haider CE, Wilkinson MR, et al. PG4KDS: a model for the clinical implementation of pre-emptive pharmacogenetics. Am J Med Genet C Semin Med Genet. 2014;166C(1):45-55.
40. Wehry AM, Ramsey LB, Dulemba SE, et al. Pharmacogenomic testing in child and adolescent psychiatry: an evidence-based review. Curr Probl Pediatr Adolesc Health Care. 2018;48(2):40-49.
41. Tomita T, Yasui-Furukori N, Nakagami T, et al. The influence of 5-HTTLPR genotype on the association between the plasma concentration and therapeutic effect of paroxetine in patients with major depressive disorder. PLoS One. 2014;9(5):e98099. doi: 10.1371/journal.pone.0098099.
The use of pharmacogenetic testing to help drive decisions for medication management of patients with psychiatric illnesses is growing. It’s becoming increasingly common for patients or the parents of pediatric patients to request pharmacogenetic testing or to bring the results of prior testing to their appointment. In these situations, patients may ask clinicians to consider the recommendations from these testing reports, which rarely provide guidance specific to pediatric patients. However, this can be difficult for clinicians who did not receive education in pharmacogenetics and may not be familiar with the evidence or options for pharmacogenetic testing. Many of the pharmacogenetic associations identified thus far have been discovered in adults, but studies in pediatric patients are relatively rare. This article reviews pharmacogenetic testing and the evidence supporting it, and describes implementation of routine pharmacogenetics testing at a children’s hospital.
CASE
Testing leads to dose adjustment, improvement
Ms. R, age 16, presents with treatment-resistant major depressive disorder that is characterized by a significant neurovegetative burden and prominent anhedonia, as well as intermittent suicidal ideation without intent or plan. She reportedly did not improve after multiple medication trials, including
Augmentation strategies included
_
Drug metabolism and genetic variants
It is common for patients with psychiatric disorders to receive trials of multiple psychotropic medications prior to identifying one that reduces symptom burden without producing intolerable adverse effects. Due to the high frequency of toxicity-related adverse effects (observed in 20% to 70% of patients),1 these medications are frequently initiated at low doses and titrated slowly until the patient either experiences an intolerable adverse effect or achieves symptomatic remission.1,2 The practice of slow titration at the start of treatment increases the risk of undertreatment in many patients, and may ultimately lead to a medication change due to the lack of response.
Many of the medications used to treat psychiatric illnesses are primarily metabolized by 2 CYP enzymes expressed in the liver, encoded by the CYP2D6 and CYP2C19 genes3 (Table 13-7 and Table 23,6,7). These drug-metabolizing enzymes affect the pharmacokinetics of many medications. Some medications are converted to an active form by these enzymes, and some are inactivated. The contributions of CYP enzymes to the pharmacokinetics of neuropsychiatric medications have been well-described; however, there is less evidence on whether variants in these genes are associated with treatment efficacy, especially in pediatric patients.8,9 CYP2D6 enzyme activity reaches adult levels soon after birth, but children may have higher CYP2C19 activity than adults.4 CYP3A4 also contributes to the metabolism of many medications; however, there is only weak evidence that genetic variants in CYP3A4 contribute to variability in the pharmacokinetics of these medications, and there are currently no dosing guidelines based on pharmacogenetics available for this gene.10

As is common in the pharmacogenetic field, genotypes are denoted with a “star allele” (eg, *2) rather than positional nomenclature (eg, c.681G>A). The normal allele is usually designated as *1, and this result is given in the absence of the tested alleles. There is no consensus on the minimum set of alleles to be tested for most genes,11 so commercially available tests vary widely in what alleles are tested (and therefore what they exclude before calling a normal allele).12 The metabolizer phenotype for a patient is determined by taking into account the activity of each of the patient’s 2 alleles (eg, *1/*2). A patient is categorized as a poor-, intermediate-, normal- (extensive-), or ultra-rapid metabolizer. Generally, the allele definitions are widely agreed upon (what genetic variant or variants comprise the *2 allele) due to nomenclature committees for each gene; however, because there are no standards for interpretation, the interpretation of the activity of the alleles and conversion to metabolizer phenotype varies among clinics.13
Continue to: Guidelines help with genotype-guided dosing
Guidelines help with genotype-guided dosing

Each CPIC guideline specifically addresses use in pediatric patients, indicating that there are relatively few studies in pediatrics, but “it may be appropriate to extrapolate these recommendations to adolescents or possibly younger children with close monitoring.”4 The DPWG guidelines do not mention whether or not the recommendations are applicable to children. Neither CPIC nor the DPWG provides guidance on when to test; however, the French National Network of Pharmacogenetics (Réseau national de pharmacogénétique) recommends CYP2D6 and CYP2C19 genotyping before initiating antidepressant treatment, especially in patients with a high risk of toxicity.16
In the case above, Ms. R was determined to be a CYP2D6 ultra-rapid metabolizer. Because she showed some initial response to aripiprazole and venlafaxine ER, which are both metabolized by CYP2D6, these medications were very quickly titrated up, and the increased dosages produced the desired response. Venlafaxine is metabolized to the active metabolite O-desmethylvenlafaxine by CYP2D6. The DPWG recommends increasing the dose of venlafaxine in CYP2D6 ultra-rapid metabolizers to 150% of the normal dose based on the decreased serum concentrations of venlafaxine and O-desmethylvenlafaxine in these patients.6 Aripiprazole is also metabolized by CYP2D6; however, the FDA and DPWG give no recommendations for ultra-rapid metabolizers, but do recommend reducing the dose of aripiprazole in CYP2D6 poor metabolizers.
Multiple studies in adults have analyzed the association between pharmacokinetic (CYP2D6 and CYP2C19) or pharmacodynamic genes (SLC6A4, HTR2A, and GRIK4) and outcomes,17 including some large clinical trials that conducted genome-wide association studies18-20 and meta-analyses across multiple studies.21,22 Most pharmacogenetic studies in psychiatric patients are small, and very few have included pediatric patients. However, with more interest in neuropsychiatric pharmacogenetics, these studies are becoming more common.23-26
Continue to: Limited evidence from studies of commercially available tests
Limited evidence from studies of commercially available tests
Several pharmacogenetic tests are commercially available, including some that focus on providing information that can be used specifically when prescribing psychiatric medications, such as the GeneSight Psychotropic test, CNSdose, Genomind, and Neuropharmagen.
In an industry-sponsored, nonrandomized clinical trial that included patients for whom prescribing decisions were made based on the GeneSight test, outcomes in adults were improved compared with treatment as usual,27 inpatient stays were shorter,28 and pharmacy costs were reduced.29 In one of these studies, the authors noted that the traditional, single-gene analysis was not associated with improved outcomes, whereas the multiple gene combination (pharmacokinetic and pharmacodynamic genes) was associated with improved outcomes among patients with depression.27 However, when GeneSightwas compared with treatment as usual in a small randomized trial, there was not a significant association between use of the test and improved outcomes among patients with treatment-resistant depression.30 The results of a much larger randomized trial (N = 1,167) are available31 and expected to be published, but patients younger than age 18 were excluded from this study.32 A retrospective study conducted in adult psychiatric patients found that patients whose treatment followed recommendations of a pharmacogenetic test including 20 genes were almost 4 times more likely to improve than patients whose treatment did not follow the recommendations.33
Pharmacogenetic testing at our pediatric inpatient unit
The Cincinnati Children’s Division of Child and Adolescent Psychiatry is the largest psychiatric inpatient service in a U.S. pediatric hospital. Starting in 2004, we adopted pharmacogenetically-guided dosing of psychiatric medications.34 CYP2D6 and CYP2C19 were chosen for testing because the enzymes encoded by these genes metabolize many of the antidepressants and antipsychotics that patients admitted to our unit will receive, and the clinicians wanted all available tools to help improve the care of these patients. To date, the Genetic Pharmacology Service (GPS) has performed >25,000 tests for variants in CYP2D6 and CYP2C19 as part of inpatient care. Patients provide a specimen (blood or buccal swab) at the time of admission to inpatient psychiatry, genotyping is performed onsite by the Molecular Genetics Laboratory (certified by the College of American Pathologists [CAP]/Clinical Laboratory Improvement Amendments [CLIA]) and the results are posted to the medical record within 2 business days. The report contains the patient’s alleles for CYP2D6 and CYP2C19, the genotype-predicted metabolizer phenotype, and dosing recommendations for 19 drugs (provided as a percentage of the standard dose). Insurance is billed for the test, and reimbursement is usually received when the test is performed as part of an inpatient stay.
The GPS team performed a retrospective chart review after the first panel was implemented in 2005.23 The study included 279 patients who were receiving a medication metabolized by one of the 2 genes tested. The poor metabolizers had the highest efficacy and highest number of adverse drug reactions, while ultra-rapid metabolizers had the lowest efficacy and lowest number of adverse reactions during their initial inpatient stay. In patients not treated with medications metabolized by CYP2D6 or CYP2C19, there was no association between metabolizer status and efficacy or adverse drug reactions. In this retrospective study, there was no association between metabolizer status and length of stay.
Overcoming the challenges
One challenge with many of the pharmacogenetic tests is interpretation of the results. The reports can span more than 20 pages, and clinicians may not have time to thoroughly read and understand how best to use all of this information. Sometimes the reports can make it seem like the first-line medication for the patient’s condition is not the best choice, but it could work well when dosed appropriately based on the patient’s genotype. Each commercially available test has a different way of presenting results,13 so when choosing a pharmacogenetic test, one should be sure to see a sample report. Vo et al35 recently reviewed factors to consider when choosing a pharmacogenetic test.
Continue to: Because patients and families also have difficulty understanding the reports...
Because patients and families also have difficulty understanding the reports, we created patient education sheets,36 written at an eighth grade level with feedback from parents and modeled on those provided by St. Jude Children’s Research Hospital.37 St. Jude Children’s Research Hospital also has pharmacogenetic competencies that pharmacists and nurses must pass.38,39 The following is a sample explanation that one of our nurses uses to educate parents on what is being tested and what effect the results will have on the treatment plan.
“During your child’s stay we will be completing a genetic test to help us understand how he/she processes the types of medications that we may be likely to start during their hospitalization. This does not tell us which medication will be best—unfortunately within the field of psychiatry there is still some unavoidable trial and error; rather, what it will do is tell us how to make sure that the dosing is at a level that would be safe for the way your child’s body breaks down the medicine, so that he/she can get the intended benefit of the medicine’s effects, while decreasing the risk of uncomfortable side effects, where possible.”
Other challenges in pharmacogenetic testing are the cost, disease risk, and concern about how genetic information will be used. Because these tests are often not covered by health insurance, some commercial pharmacogenetic testing companies offer an out-of-pocket maximum in the $250 to $350 range to reduce the cost to the patient. Some pharmacogenetic testing companies also test for genes associated with disease, so if a clinician orders the test, he or she may be responsible for sharing that information with the patient. For most pharmacogenetic testing companies, the turn-around time is 2 to 10 days. Genetic information is protected by federal laws, including Genetic Information Nondiscrimination Act (GINA) and Health Insurance Portability and Accountability Act (HIPAA).
The choice of psychotropic medication is complex, and although we would like pharmacogenetics to be the only answer to why every patient does or does not respond to a medication, it is not. Response to medication is influenced by age, comorbidities, illness severity, illness duration, compliance, gender, concomitant medications, and potentially more.40 Pharmacogenetics is another tool at the clinician’s disposal to help in choosing a medication and dose. There is a clear association between CYP2D6 and CYP2C19 and exposure to many antidepressants and antipsychotics (reviewed by Stingl et al3); however, the link between exposure and response is much weaker. It may be strengthened by the inclusion of pharmacodynamic information (the level of expression of the drug target), which can be influenced by genetic variants.41 At the present time, the most evidence exists for testing CYP2D6 and CYP2C19, and the CPIC4,5,15 and DWPG6 guidelines provide evidence-based recommendations for how to adjust medication dosages based on the results.
There is clearly much more research that needs to be done in the field of neuropsychiatric pharmacogenetics, especially in pediatric populations. As we see increased utilization of pharmacogenetic tests in psychiatry, there is also a need for pharmacogenetic education of patients, families, nurses, pharmacists, and psychiatrists. Several good pharmacogenetic resources that contain up-to-date summaries of the available evidence linking pharmacogenetic variants to medication response, implementation resources, and educational resources are available. These include CPIC (www.cpicpgx.org), PharmGKB (www.pharmgkb.org), and the IGNITE Spark Toolbox (https://ignite-genomics.org/spark-toolbox/clinicians/).
Acknowledgements
The author thanks Jen Milau, APRN, for the case study and sample explanation, and Jeffrey Strawn, MD, FAACP, Ethan Poweleit, and Stacey Aldrich, MS, for help with preparing this manuscript.
The use of pharmacogenetic testing to help drive decisions for medication management of patients with psychiatric illnesses is growing. It’s becoming increasingly common for patients or the parents of pediatric patients to request pharmacogenetic testing or to bring the results of prior testing to their appointment. In these situations, patients may ask clinicians to consider the recommendations from these testing reports, which rarely provide guidance specific to pediatric patients. However, this can be difficult for clinicians who did not receive education in pharmacogenetics and may not be familiar with the evidence or options for pharmacogenetic testing. Many of the pharmacogenetic associations identified thus far have been discovered in adults, but studies in pediatric patients are relatively rare. This article reviews pharmacogenetic testing and the evidence supporting it, and describes implementation of routine pharmacogenetics testing at a children’s hospital.
CASE
Testing leads to dose adjustment, improvement
Ms. R, age 16, presents with treatment-resistant major depressive disorder that is characterized by a significant neurovegetative burden and prominent anhedonia, as well as intermittent suicidal ideation without intent or plan. She reportedly did not improve after multiple medication trials, including
Augmentation strategies included
_
Drug metabolism and genetic variants
It is common for patients with psychiatric disorders to receive trials of multiple psychotropic medications prior to identifying one that reduces symptom burden without producing intolerable adverse effects. Due to the high frequency of toxicity-related adverse effects (observed in 20% to 70% of patients),1 these medications are frequently initiated at low doses and titrated slowly until the patient either experiences an intolerable adverse effect or achieves symptomatic remission.1,2 The practice of slow titration at the start of treatment increases the risk of undertreatment in many patients, and may ultimately lead to a medication change due to the lack of response.
Many of the medications used to treat psychiatric illnesses are primarily metabolized by 2 CYP enzymes expressed in the liver, encoded by the CYP2D6 and CYP2C19 genes3 (Table 13-7 and Table 23,6,7). These drug-metabolizing enzymes affect the pharmacokinetics of many medications. Some medications are converted to an active form by these enzymes, and some are inactivated. The contributions of CYP enzymes to the pharmacokinetics of neuropsychiatric medications have been well-described; however, there is less evidence on whether variants in these genes are associated with treatment efficacy, especially in pediatric patients.8,9 CYP2D6 enzyme activity reaches adult levels soon after birth, but children may have higher CYP2C19 activity than adults.4 CYP3A4 also contributes to the metabolism of many medications; however, there is only weak evidence that genetic variants in CYP3A4 contribute to variability in the pharmacokinetics of these medications, and there are currently no dosing guidelines based on pharmacogenetics available for this gene.10

As is common in the pharmacogenetic field, genotypes are denoted with a “star allele” (eg, *2) rather than positional nomenclature (eg, c.681G>A). The normal allele is usually designated as *1, and this result is given in the absence of the tested alleles. There is no consensus on the minimum set of alleles to be tested for most genes,11 so commercially available tests vary widely in what alleles are tested (and therefore what they exclude before calling a normal allele).12 The metabolizer phenotype for a patient is determined by taking into account the activity of each of the patient’s 2 alleles (eg, *1/*2). A patient is categorized as a poor-, intermediate-, normal- (extensive-), or ultra-rapid metabolizer. Generally, the allele definitions are widely agreed upon (what genetic variant or variants comprise the *2 allele) due to nomenclature committees for each gene; however, because there are no standards for interpretation, the interpretation of the activity of the alleles and conversion to metabolizer phenotype varies among clinics.13
Continue to: Guidelines help with genotype-guided dosing
Guidelines help with genotype-guided dosing

Each CPIC guideline specifically addresses use in pediatric patients, indicating that there are relatively few studies in pediatrics, but “it may be appropriate to extrapolate these recommendations to adolescents or possibly younger children with close monitoring.”4 The DPWG guidelines do not mention whether or not the recommendations are applicable to children. Neither CPIC nor the DPWG provides guidance on when to test; however, the French National Network of Pharmacogenetics (Réseau national de pharmacogénétique) recommends CYP2D6 and CYP2C19 genotyping before initiating antidepressant treatment, especially in patients with a high risk of toxicity.16
In the case above, Ms. R was determined to be a CYP2D6 ultra-rapid metabolizer. Because she showed some initial response to aripiprazole and venlafaxine ER, which are both metabolized by CYP2D6, these medications were very quickly titrated up, and the increased dosages produced the desired response. Venlafaxine is metabolized to the active metabolite O-desmethylvenlafaxine by CYP2D6. The DPWG recommends increasing the dose of venlafaxine in CYP2D6 ultra-rapid metabolizers to 150% of the normal dose based on the decreased serum concentrations of venlafaxine and O-desmethylvenlafaxine in these patients.6 Aripiprazole is also metabolized by CYP2D6; however, the FDA and DPWG give no recommendations for ultra-rapid metabolizers, but do recommend reducing the dose of aripiprazole in CYP2D6 poor metabolizers.
Multiple studies in adults have analyzed the association between pharmacokinetic (CYP2D6 and CYP2C19) or pharmacodynamic genes (SLC6A4, HTR2A, and GRIK4) and outcomes,17 including some large clinical trials that conducted genome-wide association studies18-20 and meta-analyses across multiple studies.21,22 Most pharmacogenetic studies in psychiatric patients are small, and very few have included pediatric patients. However, with more interest in neuropsychiatric pharmacogenetics, these studies are becoming more common.23-26
Continue to: Limited evidence from studies of commercially available tests
Limited evidence from studies of commercially available tests
Several pharmacogenetic tests are commercially available, including some that focus on providing information that can be used specifically when prescribing psychiatric medications, such as the GeneSight Psychotropic test, CNSdose, Genomind, and Neuropharmagen.
In an industry-sponsored, nonrandomized clinical trial that included patients for whom prescribing decisions were made based on the GeneSight test, outcomes in adults were improved compared with treatment as usual,27 inpatient stays were shorter,28 and pharmacy costs were reduced.29 In one of these studies, the authors noted that the traditional, single-gene analysis was not associated with improved outcomes, whereas the multiple gene combination (pharmacokinetic and pharmacodynamic genes) was associated with improved outcomes among patients with depression.27 However, when GeneSightwas compared with treatment as usual in a small randomized trial, there was not a significant association between use of the test and improved outcomes among patients with treatment-resistant depression.30 The results of a much larger randomized trial (N = 1,167) are available31 and expected to be published, but patients younger than age 18 were excluded from this study.32 A retrospective study conducted in adult psychiatric patients found that patients whose treatment followed recommendations of a pharmacogenetic test including 20 genes were almost 4 times more likely to improve than patients whose treatment did not follow the recommendations.33
Pharmacogenetic testing at our pediatric inpatient unit
The Cincinnati Children’s Division of Child and Adolescent Psychiatry is the largest psychiatric inpatient service in a U.S. pediatric hospital. Starting in 2004, we adopted pharmacogenetically-guided dosing of psychiatric medications.34 CYP2D6 and CYP2C19 were chosen for testing because the enzymes encoded by these genes metabolize many of the antidepressants and antipsychotics that patients admitted to our unit will receive, and the clinicians wanted all available tools to help improve the care of these patients. To date, the Genetic Pharmacology Service (GPS) has performed >25,000 tests for variants in CYP2D6 and CYP2C19 as part of inpatient care. Patients provide a specimen (blood or buccal swab) at the time of admission to inpatient psychiatry, genotyping is performed onsite by the Molecular Genetics Laboratory (certified by the College of American Pathologists [CAP]/Clinical Laboratory Improvement Amendments [CLIA]) and the results are posted to the medical record within 2 business days. The report contains the patient’s alleles for CYP2D6 and CYP2C19, the genotype-predicted metabolizer phenotype, and dosing recommendations for 19 drugs (provided as a percentage of the standard dose). Insurance is billed for the test, and reimbursement is usually received when the test is performed as part of an inpatient stay.
The GPS team performed a retrospective chart review after the first panel was implemented in 2005.23 The study included 279 patients who were receiving a medication metabolized by one of the 2 genes tested. The poor metabolizers had the highest efficacy and highest number of adverse drug reactions, while ultra-rapid metabolizers had the lowest efficacy and lowest number of adverse reactions during their initial inpatient stay. In patients not treated with medications metabolized by CYP2D6 or CYP2C19, there was no association between metabolizer status and efficacy or adverse drug reactions. In this retrospective study, there was no association between metabolizer status and length of stay.
Overcoming the challenges
One challenge with many of the pharmacogenetic tests is interpretation of the results. The reports can span more than 20 pages, and clinicians may not have time to thoroughly read and understand how best to use all of this information. Sometimes the reports can make it seem like the first-line medication for the patient’s condition is not the best choice, but it could work well when dosed appropriately based on the patient’s genotype. Each commercially available test has a different way of presenting results,13 so when choosing a pharmacogenetic test, one should be sure to see a sample report. Vo et al35 recently reviewed factors to consider when choosing a pharmacogenetic test.
Continue to: Because patients and families also have difficulty understanding the reports...
Because patients and families also have difficulty understanding the reports, we created patient education sheets,36 written at an eighth grade level with feedback from parents and modeled on those provided by St. Jude Children’s Research Hospital.37 St. Jude Children’s Research Hospital also has pharmacogenetic competencies that pharmacists and nurses must pass.38,39 The following is a sample explanation that one of our nurses uses to educate parents on what is being tested and what effect the results will have on the treatment plan.
“During your child’s stay we will be completing a genetic test to help us understand how he/she processes the types of medications that we may be likely to start during their hospitalization. This does not tell us which medication will be best—unfortunately within the field of psychiatry there is still some unavoidable trial and error; rather, what it will do is tell us how to make sure that the dosing is at a level that would be safe for the way your child’s body breaks down the medicine, so that he/she can get the intended benefit of the medicine’s effects, while decreasing the risk of uncomfortable side effects, where possible.”
Other challenges in pharmacogenetic testing are the cost, disease risk, and concern about how genetic information will be used. Because these tests are often not covered by health insurance, some commercial pharmacogenetic testing companies offer an out-of-pocket maximum in the $250 to $350 range to reduce the cost to the patient. Some pharmacogenetic testing companies also test for genes associated with disease, so if a clinician orders the test, he or she may be responsible for sharing that information with the patient. For most pharmacogenetic testing companies, the turn-around time is 2 to 10 days. Genetic information is protected by federal laws, including Genetic Information Nondiscrimination Act (GINA) and Health Insurance Portability and Accountability Act (HIPAA).
The choice of psychotropic medication is complex, and although we would like pharmacogenetics to be the only answer to why every patient does or does not respond to a medication, it is not. Response to medication is influenced by age, comorbidities, illness severity, illness duration, compliance, gender, concomitant medications, and potentially more.40 Pharmacogenetics is another tool at the clinician’s disposal to help in choosing a medication and dose. There is a clear association between CYP2D6 and CYP2C19 and exposure to many antidepressants and antipsychotics (reviewed by Stingl et al3); however, the link between exposure and response is much weaker. It may be strengthened by the inclusion of pharmacodynamic information (the level of expression of the drug target), which can be influenced by genetic variants.41 At the present time, the most evidence exists for testing CYP2D6 and CYP2C19, and the CPIC4,5,15 and DWPG6 guidelines provide evidence-based recommendations for how to adjust medication dosages based on the results.
There is clearly much more research that needs to be done in the field of neuropsychiatric pharmacogenetics, especially in pediatric populations. As we see increased utilization of pharmacogenetic tests in psychiatry, there is also a need for pharmacogenetic education of patients, families, nurses, pharmacists, and psychiatrists. Several good pharmacogenetic resources that contain up-to-date summaries of the available evidence linking pharmacogenetic variants to medication response, implementation resources, and educational resources are available. These include CPIC (www.cpicpgx.org), PharmGKB (www.pharmgkb.org), and the IGNITE Spark Toolbox (https://ignite-genomics.org/spark-toolbox/clinicians/).
Acknowledgements
The author thanks Jen Milau, APRN, for the case study and sample explanation, and Jeffrey Strawn, MD, FAACP, Ethan Poweleit, and Stacey Aldrich, MS, for help with preparing this manuscript.
1. Cipriani A, Zhou X, Del Giovane C, et al. Comparative efficacy and tolerability of antidepressants for major depressive disorder in children and adolescents: a network meta-analysis. Lancet. 2016; 388(10047):881-890.
2. Correll CU, Sheridan EM, DelBello MP. Antipsychotic and mood stabilizer efficacy and tolerability in pediatric and adult patients with bipolar I mania: a comparative analysis of acute, randomized, placebo-controlled trials. Bipolar Disord. 2010;12(2):116-141.
3. Stingl JC, Brockmoller J, Viviani R. Genetic variability of drug-metabolizing enzymes: the dual impact on psychiatric therapy and regulation of brain function. Mol Psychiatry. 2013;18(3):273-287.
4. Hicks JK, Bishop JR, Sangkuhl K, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline for CYP2D6 and CYP2C19 genotypes and dosing of selective serotonin reuptake inhibitors. Clin Pharmacol Ther. 2015;98(2):127-134.
5. Hicks JK, Sangkuhl K, Swen JJ, et al. Clinical pharmacogenetics implementation consortium guideline (CPIC) for CYP2D6 and CYP2C19 genotypes and dosing of tricyclic antidepressants: 2016 update. Clin Pharmacol Ther. 2017;102(1):37-44.
6. Swen JJ, Nijenhuis M, de Boer A, et al. Pharmacogenetics: from bench to byte--an update of guidelines. Clin Pharmacol Ther. 2011;89(5):662-673.
7. Swen JJ, Wilting I, de Goede AL, et al. Pharmacogenetics: from bench to byte. Clin Pharmacol Ther. 2008;83(5):781-787.
8. GENDEP Investigators, MARS Investigators, and STAR*D Investigators. Common genetic variation and antidepressant efficacy in major depressive disorder: a meta-analysis of three genome-wide pharmacogenetic studies. Am J Psychiatry. 2013;170(2):207-217.
9. Ji Y, Schaid DJ, Desta Z, et al. Citalopram and escitalopram plasma drug and metabolite concentrations: genome-wide associations. Br J Clin Pharmacol. 2014;78(2):373-383.
10. Werk AN, Cascorbi I. Functionalgene variants of CYP3A4. Clin Pharmacol Ther. 2014:96(3):340-348.
11. Pratt VM, Del Tredici AL, Hachad H, et al. Recommendations for clinical CYP2C19 genotyping allele selection: a report of the Association for Molecular Pathology. J Mol Diagn. 2018;20(3):269-276.
12. Bousman CA, Jaksa P, Pantelis C. Systematic evaluation of commercial pharmacogenetic testing in psychiatry: a focus on CYP2D6 and CYP2C19 allele coverage and results reporting. Pharmacogenet Genomics. 2017;27(11):387-393.
13. Hicks JK, Swen JJ, Gaedigk A. Challenges in CYP2D6 phenotype assignment from genotype data: a critical assessment and call for standardization. Curr Drug Metab. 2014;15(2):218-232.
14. Caudle KE, Klein TE, Hoffman JM, et al. Incorporation of pharmacogenomics into routine clinical practice: the Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline development process. Curr Drug Metab. 2014;15(2):209-217.
15. Hicks JK, Swen JJ, Thorn CF, et al. Clinical Pharmacogenetics Implementation Consortium guideline for CYP2D6 and CYP2C19 genotypes and dosing of tricyclic antidepressants. Clin Pharmacol Ther. 2013;93(5):402-408.
16. Quaranta S, Dupouey J, Colle R, et al. Pharmacogenetics of antidepressant drugs: State of the art and clinical implementation - recommendations from the French National Network of Pharmacogenetics. Therapie. 2017;72(2):311-318.
17. Fabbri C, Minarini A, Nitsu T, et al. Understanding the pharmacogenetics of selective serotonin reuptake inhibitors. Expert Opin Drug Metab Toxicol. 2014;10(8):1093-1118.
18. Mrazek DA, Rush AJ, Biernacka JM, et al. SLC6A4 variation and citalopram response. Am J Med Genet B Neuropsychiatr Genet. 2009;150B(3):341-351.
19. Biernacka JM, Sangkuhl K, Jenkins G, et al. The International SSRI Pharmacogenomics Consortium (ISPC): a genome-wide association study of antidepressant treatment response. Transl Psychiatry. 2015;5:e553. doi: 10.1038/tp.2015.47.
20. Horstmann S, Lucae S, Menke A, et al. Polymorphisms in GRIK4, HTR2A, and FKBP5 show interactive effects in predicting remission to antidepressant treatment. Neuropsychopharmacology. 2010;35(3):727-740.
21. Porcelli S, Fabbri C, Serretti A. Meta-analysis of serotonin transporter gene promoter polymorphism (5-HTTLPR) association with antidepressant efficacy. Eur Neuropsychopharmacol. 2012;22(4):239-258.
22. Niitsu T, Fabbri C, Bentini F, et al. Pharmacogenetics in major depression: a comprehensive meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2013;45:183-194.
23. Prows CA, Nick TG, Saldaña SN, et al. Drug-metabolizing enzyme genotypes and aggressive behavior treatment response in hospitalized pediatric psychiatric patients. J Child Adolesc Psychopharmacol. 2009;19(4):385-394.
24. Rotberg B, Kronenberg S, Carmel M, et al. Additive effects of 5-HTTLPR (serotonin transporter) and tryptophan hydroxylase 2 G-703T gene polymorphisms on the clinical response to citalopram among children and adolescents with depression and anxiety disorders. J Child Adolesc Psychopharmacol. 2013;23(2):117-122.
25. Kronenberg S, Apter A, Brent D, et al. Serotonin transporter polymorphism (5-HTTLPR) and citalopram effectiveness and side effects in children with depression and/or anxiety disorders. J Child Adolesc Psychopharmacol. 2007;17(6):741-750.
26. AlOlaby RR, Sweha SR, Silva M, et al. Molecular biomarkers predictive of sertraline treatment response in young children with fragile X syndrome. Brain Dev. 2017;39(6):483-492.
27. Altar CA, Carhart JM, Allen JD, et al. Clinical validity: Combinatorial pharmacogenomics predicts antidepressant responses and healthcare utilizations better than single gene phenotypes. Pharmacogenomics J. 2015;15(5):443-451.
28. Winner J, Allen JD, Altar CA, et al. Psychiatric pharmacogenomics predicts health resource utilization of outpatients with anxiety and depression. Transl Psychiatry. 2013;3:e242. doi:10.1038/tp.2013.2.
29. Winner JG, Carhart JM, Altar CA, et al. Combinatorial pharmacogenomic guidance for psychiatric medications reduces overall pharmacy costs in a 1 year prospective evaluation. Curr Med Res Opin. 2015;31(9):1633-1643.
30. Winner JG, Carhart JM, Altar CA, et al. A prospective, randomized, double-blind study assessing the clinical impact of integrated pharmacogenomic testing for major depressive disorder. Discov Med. 2013;16(89):219-227.
31. Genesight. GUIDED clinical study. https://genesight.com/greden-study/. Updated May 31, 2018. Accessed August 1, 2018.
32. U.S. National Library of Medicine ClinicalTrials.gov. Genomics used to improve DEpression decisions (GUIDED). https://clinicaltrials.gov/ct2/show/NCT02109939. Accessed July 24, 2018.
33. Espadaler J, Tuson M, Lopez-Ibor JM, et al. Pharmacogenetic testing for the guidance of psychiatric treatment: a multicenter retrospective analysis. CNS Spectrums. 2017;22(4):315-324.
34. Ramsey LB, Prows CA, Zhang K, et al. Implementation of pharmacogenetics at Cincinnati Children’s Hospital Medical Center: lessons learned over 14 years of personalizing medicine. Clin Pharmacol Ther. 2018. doi: 10.1002/cpt.1165. [Epub ahead of print].
35. Vo TT, Bell GC, Owusu Obeng A, et al. Pharmacogenomics implementation: considerations for selecting a reference laboratory. Pharmacotherapy. 2017;37(9):1014-1022.
36. Cincinnati Children’s Hospital. Genetic Pharmacology Service: Education. www.cincinnatichildrens.org/gpsinfo. Accessed August 1, 2018.
37. St. Jude Children’s Research Hospital. Do You Know...Cytochrome P450 2D6 (CYP2D6) and medicines. https://www.stjude.org/treatment/patient-resources/caregiver-resources/patient-family-education-sheets/pharmacy-and-medicines/cytochrome-p450-2d6-cyp2d6-and-medicines.html. Accessed August 1, 2018.
38. St. Jude Children’s Research Hospital. Implementation Resources for Professionals: Clinical Pharmacogenetics at St. Jude. https://www.stjude.org/research/clinical-trials/pg4kds-pharmaceutical-science/implementation-resources-for-professionals.html. Accessed August 1, 2018.
39. Hoffman JM, Haider CE, Wilkinson MR, et al. PG4KDS: a model for the clinical implementation of pre-emptive pharmacogenetics. Am J Med Genet C Semin Med Genet. 2014;166C(1):45-55.
40. Wehry AM, Ramsey LB, Dulemba SE, et al. Pharmacogenomic testing in child and adolescent psychiatry: an evidence-based review. Curr Probl Pediatr Adolesc Health Care. 2018;48(2):40-49.
41. Tomita T, Yasui-Furukori N, Nakagami T, et al. The influence of 5-HTTLPR genotype on the association between the plasma concentration and therapeutic effect of paroxetine in patients with major depressive disorder. PLoS One. 2014;9(5):e98099. doi: 10.1371/journal.pone.0098099.
1. Cipriani A, Zhou X, Del Giovane C, et al. Comparative efficacy and tolerability of antidepressants for major depressive disorder in children and adolescents: a network meta-analysis. Lancet. 2016; 388(10047):881-890.
2. Correll CU, Sheridan EM, DelBello MP. Antipsychotic and mood stabilizer efficacy and tolerability in pediatric and adult patients with bipolar I mania: a comparative analysis of acute, randomized, placebo-controlled trials. Bipolar Disord. 2010;12(2):116-141.
3. Stingl JC, Brockmoller J, Viviani R. Genetic variability of drug-metabolizing enzymes: the dual impact on psychiatric therapy and regulation of brain function. Mol Psychiatry. 2013;18(3):273-287.
4. Hicks JK, Bishop JR, Sangkuhl K, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline for CYP2D6 and CYP2C19 genotypes and dosing of selective serotonin reuptake inhibitors. Clin Pharmacol Ther. 2015;98(2):127-134.
5. Hicks JK, Sangkuhl K, Swen JJ, et al. Clinical pharmacogenetics implementation consortium guideline (CPIC) for CYP2D6 and CYP2C19 genotypes and dosing of tricyclic antidepressants: 2016 update. Clin Pharmacol Ther. 2017;102(1):37-44.
6. Swen JJ, Nijenhuis M, de Boer A, et al. Pharmacogenetics: from bench to byte--an update of guidelines. Clin Pharmacol Ther. 2011;89(5):662-673.
7. Swen JJ, Wilting I, de Goede AL, et al. Pharmacogenetics: from bench to byte. Clin Pharmacol Ther. 2008;83(5):781-787.
8. GENDEP Investigators, MARS Investigators, and STAR*D Investigators. Common genetic variation and antidepressant efficacy in major depressive disorder: a meta-analysis of three genome-wide pharmacogenetic studies. Am J Psychiatry. 2013;170(2):207-217.
9. Ji Y, Schaid DJ, Desta Z, et al. Citalopram and escitalopram plasma drug and metabolite concentrations: genome-wide associations. Br J Clin Pharmacol. 2014;78(2):373-383.
10. Werk AN, Cascorbi I. Functionalgene variants of CYP3A4. Clin Pharmacol Ther. 2014:96(3):340-348.
11. Pratt VM, Del Tredici AL, Hachad H, et al. Recommendations for clinical CYP2C19 genotyping allele selection: a report of the Association for Molecular Pathology. J Mol Diagn. 2018;20(3):269-276.
12. Bousman CA, Jaksa P, Pantelis C. Systematic evaluation of commercial pharmacogenetic testing in psychiatry: a focus on CYP2D6 and CYP2C19 allele coverage and results reporting. Pharmacogenet Genomics. 2017;27(11):387-393.
13. Hicks JK, Swen JJ, Gaedigk A. Challenges in CYP2D6 phenotype assignment from genotype data: a critical assessment and call for standardization. Curr Drug Metab. 2014;15(2):218-232.
14. Caudle KE, Klein TE, Hoffman JM, et al. Incorporation of pharmacogenomics into routine clinical practice: the Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline development process. Curr Drug Metab. 2014;15(2):209-217.
15. Hicks JK, Swen JJ, Thorn CF, et al. Clinical Pharmacogenetics Implementation Consortium guideline for CYP2D6 and CYP2C19 genotypes and dosing of tricyclic antidepressants. Clin Pharmacol Ther. 2013;93(5):402-408.
16. Quaranta S, Dupouey J, Colle R, et al. Pharmacogenetics of antidepressant drugs: State of the art and clinical implementation - recommendations from the French National Network of Pharmacogenetics. Therapie. 2017;72(2):311-318.
17. Fabbri C, Minarini A, Nitsu T, et al. Understanding the pharmacogenetics of selective serotonin reuptake inhibitors. Expert Opin Drug Metab Toxicol. 2014;10(8):1093-1118.
18. Mrazek DA, Rush AJ, Biernacka JM, et al. SLC6A4 variation and citalopram response. Am J Med Genet B Neuropsychiatr Genet. 2009;150B(3):341-351.
19. Biernacka JM, Sangkuhl K, Jenkins G, et al. The International SSRI Pharmacogenomics Consortium (ISPC): a genome-wide association study of antidepressant treatment response. Transl Psychiatry. 2015;5:e553. doi: 10.1038/tp.2015.47.
20. Horstmann S, Lucae S, Menke A, et al. Polymorphisms in GRIK4, HTR2A, and FKBP5 show interactive effects in predicting remission to antidepressant treatment. Neuropsychopharmacology. 2010;35(3):727-740.
21. Porcelli S, Fabbri C, Serretti A. Meta-analysis of serotonin transporter gene promoter polymorphism (5-HTTLPR) association with antidepressant efficacy. Eur Neuropsychopharmacol. 2012;22(4):239-258.
22. Niitsu T, Fabbri C, Bentini F, et al. Pharmacogenetics in major depression: a comprehensive meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2013;45:183-194.
23. Prows CA, Nick TG, Saldaña SN, et al. Drug-metabolizing enzyme genotypes and aggressive behavior treatment response in hospitalized pediatric psychiatric patients. J Child Adolesc Psychopharmacol. 2009;19(4):385-394.
24. Rotberg B, Kronenberg S, Carmel M, et al. Additive effects of 5-HTTLPR (serotonin transporter) and tryptophan hydroxylase 2 G-703T gene polymorphisms on the clinical response to citalopram among children and adolescents with depression and anxiety disorders. J Child Adolesc Psychopharmacol. 2013;23(2):117-122.
25. Kronenberg S, Apter A, Brent D, et al. Serotonin transporter polymorphism (5-HTTLPR) and citalopram effectiveness and side effects in children with depression and/or anxiety disorders. J Child Adolesc Psychopharmacol. 2007;17(6):741-750.
26. AlOlaby RR, Sweha SR, Silva M, et al. Molecular biomarkers predictive of sertraline treatment response in young children with fragile X syndrome. Brain Dev. 2017;39(6):483-492.
27. Altar CA, Carhart JM, Allen JD, et al. Clinical validity: Combinatorial pharmacogenomics predicts antidepressant responses and healthcare utilizations better than single gene phenotypes. Pharmacogenomics J. 2015;15(5):443-451.
28. Winner J, Allen JD, Altar CA, et al. Psychiatric pharmacogenomics predicts health resource utilization of outpatients with anxiety and depression. Transl Psychiatry. 2013;3:e242. doi:10.1038/tp.2013.2.
29. Winner JG, Carhart JM, Altar CA, et al. Combinatorial pharmacogenomic guidance for psychiatric medications reduces overall pharmacy costs in a 1 year prospective evaluation. Curr Med Res Opin. 2015;31(9):1633-1643.
30. Winner JG, Carhart JM, Altar CA, et al. A prospective, randomized, double-blind study assessing the clinical impact of integrated pharmacogenomic testing for major depressive disorder. Discov Med. 2013;16(89):219-227.
31. Genesight. GUIDED clinical study. https://genesight.com/greden-study/. Updated May 31, 2018. Accessed August 1, 2018.
32. U.S. National Library of Medicine ClinicalTrials.gov. Genomics used to improve DEpression decisions (GUIDED). https://clinicaltrials.gov/ct2/show/NCT02109939. Accessed July 24, 2018.
33. Espadaler J, Tuson M, Lopez-Ibor JM, et al. Pharmacogenetic testing for the guidance of psychiatric treatment: a multicenter retrospective analysis. CNS Spectrums. 2017;22(4):315-324.
34. Ramsey LB, Prows CA, Zhang K, et al. Implementation of pharmacogenetics at Cincinnati Children’s Hospital Medical Center: lessons learned over 14 years of personalizing medicine. Clin Pharmacol Ther. 2018. doi: 10.1002/cpt.1165. [Epub ahead of print].
35. Vo TT, Bell GC, Owusu Obeng A, et al. Pharmacogenomics implementation: considerations for selecting a reference laboratory. Pharmacotherapy. 2017;37(9):1014-1022.
36. Cincinnati Children’s Hospital. Genetic Pharmacology Service: Education. www.cincinnatichildrens.org/gpsinfo. Accessed August 1, 2018.
37. St. Jude Children’s Research Hospital. Do You Know...Cytochrome P450 2D6 (CYP2D6) and medicines. https://www.stjude.org/treatment/patient-resources/caregiver-resources/patient-family-education-sheets/pharmacy-and-medicines/cytochrome-p450-2d6-cyp2d6-and-medicines.html. Accessed August 1, 2018.
38. St. Jude Children’s Research Hospital. Implementation Resources for Professionals: Clinical Pharmacogenetics at St. Jude. https://www.stjude.org/research/clinical-trials/pg4kds-pharmaceutical-science/implementation-resources-for-professionals.html. Accessed August 1, 2018.
39. Hoffman JM, Haider CE, Wilkinson MR, et al. PG4KDS: a model for the clinical implementation of pre-emptive pharmacogenetics. Am J Med Genet C Semin Med Genet. 2014;166C(1):45-55.
40. Wehry AM, Ramsey LB, Dulemba SE, et al. Pharmacogenomic testing in child and adolescent psychiatry: an evidence-based review. Curr Probl Pediatr Adolesc Health Care. 2018;48(2):40-49.
41. Tomita T, Yasui-Furukori N, Nakagami T, et al. The influence of 5-HTTLPR genotype on the association between the plasma concentration and therapeutic effect of paroxetine in patients with major depressive disorder. PLoS One. 2014;9(5):e98099. doi: 10.1371/journal.pone.0098099.
Real-world challenges in managing ‘dual diagnosis’ patients
The term “dual diagnosis” describes the clinically challenging comorbidity of a substance use disorder (SUD) along with another major mental illness. Based on data from the Epidemiologic Catchment Area study, the lifetime prevalence of SUDs among patients with mental illness is approximately 30%, and is higher among patients with certain mental disorders, such as schizophrenia (47%), bipolar disorder (61%), and antisocial personality disorder (84%).1 These statistics highlight that addiction is often the rule rather than the exception among those with severe mental illness.1 Not surprisingly, the combined effects of having an SUD along with another mental illness are uniformly negative (Table 12-4).
Based on outcomes research, the core tenets of evidence-based dual-diagnosis treatment include the importance of integrated (rather than parallel) and simultaneous (rather than sequential) care, which means an ideal treatment program includes a unified, multidisciplinary team whose coordinated efforts focus on treating both disorders concurrently.2 Evidence-based psychotherapies for addiction, including motivational interviewing, cognitive-behavioral therapy, relapse prevention, contingency management, skills training, and/or case management, are a necessity,3,5 and must be balanced with rational and appropriate pharmacotherapy targeting both the SUD as well as the other disorder (Table 22,3,5-9).
3 ‘Real-world’ clinical challenges
Ideal vs real-world treatment
Treating patients with co-occurring disorders (CODs) within integrated dual-disorder treatment (IDDT) programs sounds straightforward. However, implementing evidence-based “best practice” treatment is a significant challenge in the real world for several reasons. First, individuals with CODs often struggle with poor insight, low motivation to change, and lack of access to health care. According to the Substance Abuse and Mental Health Services Administration (SAMHSA), 52% of individuals with CODs in the U.S. received no treatment at all in 2016.10 For patients with dual disorders who do seek care, most are not given access to specialty SUD treatment10 and may instead find themselves treated by psychiatrists with limited SUD training who fail to provide evidence-based psychotherapies and underutilize pharmacotherapies for SUDs.11 In the setting of CODs, the “harm reduction model” can be conflated with therapeutic nihilism, resulting in the neglect of SUD issues, with clinicians expecting patients to seek SUD treatment on their own, through self-help groups such as Alcoholics Anonymous or in other community treatment programs staffed by nonprofessionals that often are not tailored to the unique needs of patients with dual disorders. Psychiatrists working with other mental health professionals who provide psychotherapy for SUDs often do so in parallel rather than in an evidence-based, integrated fashion.
IDDT programs are not widely available. One study found that fewer than 20% of addiction treatment programs and fewer than 10% of mental health programs in the U.S. met criteria for dual diagnosis–capable services.12 Getting treatment programs to become dual diagnosis–capable is possible, but it is a time-consuming and costly endeavor that, once achieved, requires continuous staff training and programmatic adaptations to interruptions in funding.13-16 With myriad barriers to the establishment and maintenance of IDDTs, many patients with dual disorders are left without access to the most effective and comprehensive care; as few as 4% of individuals with CODs are treated within integrated programs.17
Diagnostic dilemmas
Establishing whether or not a patient with an active SUD has another serious mental illness (SMI) is a crucial first step for optimizing treatment, but diagnostic reliability can prove challenging and requires careful clinical assessment (Table 3). As always in psychiatry, accurate diagnosis is limited to careful clinical assessment18 and, in the case of possible dual disorders, is complicated by the fact that both SUDs as well as non-SUDs can result in the same psychiatric symptoms (eg, insomnia, anxiety, depression, manic behaviors, and psychosis). Clinicians must therefore distinguish between:
- Symptoms of substance intoxication or withdrawal vs independent symptoms of an underlying psychiatric disorder (that persist beyond a month after cessation of intoxication or withdrawal)
- Subclinical symptoms vs threshold mental illness, keeping in mind that some mood and anxiety states can be normal given social situations and stressors (eg, turmoil in relationships, employment difficulties, homelessness, etc.)
- Any mental illness (AMI) vs SMI. The latter is defined by SAMHSA as AMI that substantially interferes with or limits ≥1 major life activities.10
With these distinctions in mind, data from the 2016 National Survey on Drug Use and Health indicate that dual-diagnosis comorbidity was higher when the threshold for mental illness was lower—among the 19 million adults in the U.S. with SUDs, the past-year prevalence was 43% for AMI and 14% for SMI.10 Looking at substance-induced disorders vs “independent” disorders, the 2001-2002 National Epidemiologic Survey on Alcohol and Related Conditions found that for individuals with SUDs, the past-year prevalence of an independent mood or anxiety disorder was 35% and 26%, respectively.19 Taken together, these findings illustrate the substantial rate of dual-diagnosis comorbidity, the diagnostic heterogeneity and range of severity of CODs,20 and the potential for both false negatives (eg, diagnosing a substance-induced syndrome when in fact a patient has an underlying disorder) and false positives (diagnosing a full-blown mental illness when symptoms are subclinical or substance-induced) when performing diagnostic assessments in the setting of known SUDs.
Continue to: False positives are more likely...
False positives are more likely when patients seeking treatment for non-SUDs don’t disclose active drug use, even when asked. Both patients and their treating clinicians may also be prone to underestimating the significant potential for morbidity associated with SUDs, such that substance-induced symptoms may be misattributed to a dual disorder. Diagnostic questioning and thorough chart review that includes careful assessment of whether psychiatric symptoms preceded the onset of substance use, and whether they persisted in the setting of extended sobriety, is therefore paramount for minimizing false positives when assessing for dual diagnoses.18,21 Likewise, random urine toxicology testing can be invaluable in verifying claims regarding sobriety.
Another factor that can complicate diagnosis is that there are often considerable secondary gains (eg, disability income, hospitalization, housing, access to prescription medications, and mitigation of the blame and stigma associated with addiction) associated with having a dual disorder as opposed to having “just” a SUD. As a result, for some patients, obtaining a non-SUD diagnosis can be highly incentivized.22,23 Clinicians must therefore be savvy about the high potential for malingering, embellishment, and mislabeling of symptoms when conducting diagnostic interviews. For example, in assessing for psychosis, the frequent endorsement of “hearing voices” in patients with SUDs often results in a diagnosis of schizophrenia or unspecified psychotic disorder,22 despite the fact that this symptom can occur during substance intoxication and withdrawal, is well documented among people without mental illness as well as those with non-psychotic disorders,24 and can resolve without medications or with non-antipsychotic pharmacotherapy.25
When assessing for dual disorders, diagnostic false positives and false negatives can both contribute to inappropriate treatment and unrealistic expectations for recovery, and therefore underscore the importance of careful diagnostic assessment. Even with diligent assessment, however, diagnostic clarity can prove elusive due to inadequate sobriety, inconsistent reporting, and poor memory.26 Therefore, for patients with known SUDs but diagnostic uncertainty about a dual disorder, the work-up should include a trial of prospective observation, with completion of appropriate detoxification, throughout a 1-month period of sobriety and in the absence of psychiatric medications, to determine if there are persistent symptoms that would justify a dual diagnosis. In research settings, such observations have revealed that most of depressive symptoms among alcoholics who present for substance abuse treatment resolve after a month of abstinence.27 A similar time course for resolution has been noted for anxiety, distress, fatigue, and depressive symptoms among individuals with cocaine dependence.28 These findings support the guideline established in DSM-IV that symptoms persisting beyond a month of sobriety “should be considered to be manifestations of an independent, non-substance-induced mental disorder,”29 while symptoms occurring within that month may well be substance-induced. Unfortunately, in real-world clinical practice, and particularly in outpatient settings, it can be quite difficult to achieve the requisite period of sobriety for reliable diagnosis, and patients are often prematurely prescribed medications (eg, an antidepressant, antipsychotic, or mood stabilizer) that can confound the cause of symptomatic resolution. Such prescriptions are driven by compelling pressures from patients to relieve their acute suffering, as well as the predilection of some clinicians to give patients “the benefit of doubt” in assessing for dual diagnoses. However, whether an inappropriate diagnosis or a prescription for an unnecessary medication represents a benefit is debatable at best.
Pharmacotherapy
A third real-world challenge in managing patients with dual disorders involves optimizing pharmacotherapy. Unfortunately, because patients with SUDs often are excluded from clinical trials, evidence-based guidance for patients with dual disorders is lacking. In addition, medications for both CODs often remain inaccessible to patients with dual disorders for 3 reasons:
- SUDs negatively impact medication adherence among patients with dual disorders, who sometimes point out that “it says right here on the bottle not to take this medication with drugs or alcohol!”
- Some self-help groups still espouse blanket opposition of any “psychotropic” medications, even when clearly indicated for patients with COD. Groups that recognize the importance of pharmacotherapy, such as Dual Diagnosis Anonymous (DDA), have emerged, but are not yet widely available.30
- Although there are increasing options for FDA-approved medications for SUDs, they are limited to the treatment of alcohol, opioid, and nicotine use disorders31; are often restricted due to hospital and health insurance formularies32; and remain underprescribed for patients with dual disorders.11
Continue to: Although underutilization of pharmacotherapy is...
Although underutilization of pharmacotherapy is a pitfall to be avoided in the treatment of patients with dual disorders, medication overutilization can be just as problematic. Patients with dual disorders are sometimes singularly focused on resolving acute anxiety, depression, or psychosis at the expense of working towards sobriety.33 Although the “self-medication hypothesis” is frequently invoked by patients and clinicians alike to suggest that substance use occurs in the service of “treating” underlying disorders,34 this theory has not been well supported in studies.35-37 Some patients may pledge dedication to abstinence, but still pressure physicians for a pharmacologic solution to their suffering. With expanding legalization of cannabis for both recreational and medical purposes, patients are increasingly seeking doctors’ recommendations for “medical marijuana” for a wide range of complaints, despite the fact that data supporting a therapeutic role for cannabis in the treatment of mental illness is sparse,38 whereas the potential harm in terms of either causing or worsening psychosis is well established.39,40 Clinicians must be knowledgeable about the abuse potential of prescribed medications, ranging from sleep aids, analgesics, and muscle relaxants to antidepressants and antipsychotics, while also being mindful of the psychological meaningfulness of seeking, prescribing, and not prescribing medications.41
Although the simultaneous treatment of patients with dual disorders that includes pharmacotherapy for both SUDs and CODs is vital for optimizing clinical outcomes, clinicians should strive for diagnostic accuracy and use medications judiciously. In addition, although pharmacotherapy often is necessary to deliver evidence-based treatment for patients with dual disorders, it is inadequate as standalone treatment and should be administered along with psychosocial interventions within an integrated, multidisciplinary treatment setting.
The keys to optimal outcomes
The treatment of patients with dual disorders can be challenging, to say the least. Ideal, evidence-based therapy in the form of an IDDT program can be difficult for clinicians to implement and for patients to access. Best efforts to perform meticulous clinical assessment to clarify diagnoses, use pharmacotherapy judiciously, work collaboratively in a multidisciplinary setting, and optimize treatment given available resources are keys to clinical success.
Bottom Line
Ideal treatment of patients with dual disorders consists of simultaneous, integrated interventions delivered by a multidisciplinary team. However, in the real world, limited resources, diagnostic challenges, and both over- and underutilization of pharmacotherapy often hamper optimal treatment.
Related Resources
- Substance Abuse and Mental Health Services Administration. Co-occurring disorders. https://www.samhsa.gov/disorders/co-occurring.
- National Alliance on Mental Illness. Dual diagnosis. https://www.nami.org/Learn-More/Mental-Health-Conditions/Related-Conditions/Dual-Diagnosis.
- Foundations Recovery Network. Dual diagnosis support groups. http://www.dualdiagnosis.org/resource/ddrn/self-helpsupport-groups/support-groups.
- Substance Abuse and Mental Health Services Administration. Integrated treatment for co-occurring disorders evidence-based practices (EBP) KIT. https://store.samhsa.gov/product/Integrated-Treatment-for-Co-Occurring-Disorders-Evidence-Based-Practices-EBP-KIT/SMA08-4367.
- Substance Abuse and Mental Health Services Administration. Substance abuse treatment for persons with co-occurring disorders. https://store.samhsa.gov/shin/content/SMA13-3992/SMA13-3992.pdf.
1. Regier DA, Farmer ME, Rae DS, et al. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the epidemiologic catchment area (ECA) study. JAMA. 1990;264(19):2511-2518.
2. Drake RE, Mercer-McFadden C, Muesner KT, et al. Review of integrated mental health and substance abuse treatment for patients with dual disorders. Schizophr Bull. 1998;24(4):589-608.
3. Horsfall J, Cleary M, Hunt GE, et al. Psychosocial treatments for people with co-occurring severe mental illness and substance use disorders (dual diagnosis): a review of empiric evidence. Harv Rev Psychiatry. 2009;17(1):24-34.
4. Krawczyk N, Feder KA, Saloner B, et al. The association of psychiatric comorbidity with treatment completion among clients admitted to substance use treatment programs in a U.S. national sample. Drug Alcohol Depend. 2017;175:157-163.
5. Brunette MF, Muesner KT. Psychosocial interventions for the long-term management of patients with severe mental illness and co-occurring substance use disorder. J Clin Psychiatry. 2006;67(suppl 7):10-17.
6. Tiet QQ, Mausbach B. Treatments for patients with dual diagnosis: a review. Alcohol Clin Exp Res. 2007;31(4):513-536.
7. Kelly TM, Daley DC, Douaihy AB. Treatment of substance abusing patients with comorbid psychiatric disorders. Addict Behav. 2012;37(1):11-24.
8. Tsuang JT, Ho AP, Eckman TA, et al. Dual diagnosis treatment for patients with schizophrenia who are substance dependent. Psychatr Serv. 1997;48(7):887-889.
9. Rosen MI, Rosenheck RA, Shaner A, et al. Veterans who may need a payee to prevent misuse of funds for drugs. Psychiatr Serv. 2002;53(8):995-1000.
10. Substance Abuse and Mental Health Services Administration. Key substance use and mental health indicators in the United States: results from the 2016 National Survey on Drug Use and Health. HHS Publication No. SMA 17-5044, NSDUH Series H-52. Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration. https://www.samhsa.gov/data/sites/default/files/NSDUH-FFR1-2016/NSDUH-FFR1-2016.pdf. Published September 2017. Accessed August 7, 2018.
11. Rubinsky AD, Chen C, Batki SL, et al. Comparative utilization of pharmacotherapy for alcohol use disorder and other psychiatric disorders among U.S. Veterans Health Administration patients with dual diagnoses. J Psychiatr Res. 2015;69:150-157.
12. McGovern MP, Lambert-Harris C, McHugo GJ, et al. Improving the dual diagnosis capability of addiction and mental health treatment services: implementation factors associated with program level changes. J Dual Diag. 2010;6:237-250.
13. Reno R. Maintaining quality of care in a comprehensive dual diagnosis treatment program. Psychiatr Serv. 2001;52(5):673-675.
14. McGovern MP, Lambert-Harris, Gotham HJ, et al. Dual diagnosis capability in mental health and addiction treatment services: an assessment of programs across multiple state systems. Adm Policy Ment Health. 2014;41(2):205-214.
15. Gotham HJ, Claus RE, Selig K, et al. Increasing program capabilities to provide treatment for co-occurring substance use and mental disorders: organizational characteristics. J Subs Abuse Treat. 2010;38(2):160-169.
16. Priester MA, Browne T, Iachini A, et al. Treatment access barriers and disparities among individuals with co-occurring mental health and substance use disorders: an integrative literature review. J Subst Abuse Treat. 2016;61:47-59.
17. Drake RE, Bond GR. Implementing integrated mental health and substance abuse services. J Dual Diagnosis. 2010;6(3-4):251-262.
18. Miele GM, Trautman KD, Hasin DS. Assessing comorbid mental and substance-use disorders: a guide for clinical practice. J Pract Psychiatry Behav Health. 1996;5:272-282.
19. Stinson FS, Grant BF, Dawson DA, et al. Comorbidity between DSM-IV alcohol and specific drug use disorders in the United States: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Drug Alcohol Depend. 2015;80(1):105-116.
20. Flynn PM, Brown BS. Co-occurring disorders in substance abuse treatment: Issues and prospects. J Subt Abuse Treat. 2008;34(1):36-47.
21. Grant BF, Stintson FS, Dawson DA, et al. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders. Arch Gen Psychiatry. 2004;61(8):807-816.
22. Pierre JM, Wirshing DA, Wirshing WC. “Iatrogenic malingering” in VA substance abuse treatment. Psych Services. 2003;54(2):253-254.
23. Pierre JM, Shnayder I, Wirshing DA, et al. Intranasal quetiapine abuse. Am J Psychiatry. 2004;161(9):1718.
24. Pierre JM. Hallucinations in non-psychotic disorders: Toward a differential diagnosis of “hearing voices.” Harv Rev Psychiatry. 2010;18(1):22-35.
25. Pierre JM. Nonantipsychotic therapy for monosymptomatic auditory hallucinations. Biol Psychiatry. 2010;68(7):e33-e34.
26. Shaner A, Roberts LJ, Eckman TA, et al. Sources of diagnostic uncertainty for chronically psychotic cocaine abusers. Psychiatr Serv. 1998;49(5):684-690.
27. Brown SA, Shuckit MA. Changes in depression among abstinent alcoholics. J Stud Alcohol. 1988;49(5):412-417.
28. Weddington WW, Brown BS, Haertzen CA, et al. Changes in mood, craving, and sleep during short-term abstinence reported by male cocaine addicts. A controlled, residential study. Arch Gen Psychiatry. 1990;47(9):861-868.
29. American Psychiatric Association. Diagnostic and statistical manual of mental disorders, 4th edition. Washington, DC: American Psychiatric Association; 1994:210.
30. Roush S, Monica C, Carpenter-Song E, et al. First-person perspectives on Dual Diagnosis Anonymous (DDA): a qualitative study. J Dual Diagnosis. 2015;11(2):136-141.
31. Klein JW. Pharmacotherapy for substance abuse disorders. Med Clin N Am. 2016;100(4):891-910.
32. Horgan CM, Reif S, Hodgkin D, et al. Availability of addiction medications in private health plans. J Subst Abuse Treat. 2008;34(2):147-156.
33. Frances RJ. The wrath of grapes versus the self-medication hypothesis. Harvard Rev Psychiatry. 1997;4(5):287-289.
34. Khantzian EJ. The self-medication hypothesis of substance use disorders: a reconsideration and recent applications. Harvard Rev Psychiatry. 1997;4(5):231-244.
35. Hall DH, Queener JE. Self-medication hypothesis of substance use: testing Khantzian’s updated theory. J Psychoactive Drugs. 2007;39(2):151-158.
36. Henwood B, Padgett DK. Reevaluating the self-medication hypothesis among the dually diagnosed. Am J Addict. 2007;16(3):160-165.
37. Lembke A. Time to abandon the self-medication hypothesis in patients with psychiatric disorders. Am J Drug Alc Abuse. 2012;38(6):524-529.
38. Wilkinson ST, Radhakrishnan R, D’Souza DC. A systematic review of the evidence for medical marijuana in psychiatric indications. J Clin Psychiatry. 2016;77(8):1050-1064.
39. Walsh Z, Gonzalez R, Crosby K, et al. Medical cannabis and mental health: a guided systematic review. Clin Psychol Rev. 2017;51:15-29.
40. Pierre JM. Risks of increasingly potent cannabis: the joint effects of potency and frequency. Current Psychiatry. 2017;16:14-20.
41. Zweben JE, Smith DE. Considerations in using psychotropic medication with dual diagnosis patients in recovery. J Psychoactive Drugs. 1989;21(2):221-228.
The term “dual diagnosis” describes the clinically challenging comorbidity of a substance use disorder (SUD) along with another major mental illness. Based on data from the Epidemiologic Catchment Area study, the lifetime prevalence of SUDs among patients with mental illness is approximately 30%, and is higher among patients with certain mental disorders, such as schizophrenia (47%), bipolar disorder (61%), and antisocial personality disorder (84%).1 These statistics highlight that addiction is often the rule rather than the exception among those with severe mental illness.1 Not surprisingly, the combined effects of having an SUD along with another mental illness are uniformly negative (Table 12-4).
Based on outcomes research, the core tenets of evidence-based dual-diagnosis treatment include the importance of integrated (rather than parallel) and simultaneous (rather than sequential) care, which means an ideal treatment program includes a unified, multidisciplinary team whose coordinated efforts focus on treating both disorders concurrently.2 Evidence-based psychotherapies for addiction, including motivational interviewing, cognitive-behavioral therapy, relapse prevention, contingency management, skills training, and/or case management, are a necessity,3,5 and must be balanced with rational and appropriate pharmacotherapy targeting both the SUD as well as the other disorder (Table 22,3,5-9).
3 ‘Real-world’ clinical challenges
Ideal vs real-world treatment
Treating patients with co-occurring disorders (CODs) within integrated dual-disorder treatment (IDDT) programs sounds straightforward. However, implementing evidence-based “best practice” treatment is a significant challenge in the real world for several reasons. First, individuals with CODs often struggle with poor insight, low motivation to change, and lack of access to health care. According to the Substance Abuse and Mental Health Services Administration (SAMHSA), 52% of individuals with CODs in the U.S. received no treatment at all in 2016.10 For patients with dual disorders who do seek care, most are not given access to specialty SUD treatment10 and may instead find themselves treated by psychiatrists with limited SUD training who fail to provide evidence-based psychotherapies and underutilize pharmacotherapies for SUDs.11 In the setting of CODs, the “harm reduction model” can be conflated with therapeutic nihilism, resulting in the neglect of SUD issues, with clinicians expecting patients to seek SUD treatment on their own, through self-help groups such as Alcoholics Anonymous or in other community treatment programs staffed by nonprofessionals that often are not tailored to the unique needs of patients with dual disorders. Psychiatrists working with other mental health professionals who provide psychotherapy for SUDs often do so in parallel rather than in an evidence-based, integrated fashion.
IDDT programs are not widely available. One study found that fewer than 20% of addiction treatment programs and fewer than 10% of mental health programs in the U.S. met criteria for dual diagnosis–capable services.12 Getting treatment programs to become dual diagnosis–capable is possible, but it is a time-consuming and costly endeavor that, once achieved, requires continuous staff training and programmatic adaptations to interruptions in funding.13-16 With myriad barriers to the establishment and maintenance of IDDTs, many patients with dual disorders are left without access to the most effective and comprehensive care; as few as 4% of individuals with CODs are treated within integrated programs.17
Diagnostic dilemmas
Establishing whether or not a patient with an active SUD has another serious mental illness (SMI) is a crucial first step for optimizing treatment, but diagnostic reliability can prove challenging and requires careful clinical assessment (Table 3). As always in psychiatry, accurate diagnosis is limited to careful clinical assessment18 and, in the case of possible dual disorders, is complicated by the fact that both SUDs as well as non-SUDs can result in the same psychiatric symptoms (eg, insomnia, anxiety, depression, manic behaviors, and psychosis). Clinicians must therefore distinguish between:
- Symptoms of substance intoxication or withdrawal vs independent symptoms of an underlying psychiatric disorder (that persist beyond a month after cessation of intoxication or withdrawal)
- Subclinical symptoms vs threshold mental illness, keeping in mind that some mood and anxiety states can be normal given social situations and stressors (eg, turmoil in relationships, employment difficulties, homelessness, etc.)
- Any mental illness (AMI) vs SMI. The latter is defined by SAMHSA as AMI that substantially interferes with or limits ≥1 major life activities.10
With these distinctions in mind, data from the 2016 National Survey on Drug Use and Health indicate that dual-diagnosis comorbidity was higher when the threshold for mental illness was lower—among the 19 million adults in the U.S. with SUDs, the past-year prevalence was 43% for AMI and 14% for SMI.10 Looking at substance-induced disorders vs “independent” disorders, the 2001-2002 National Epidemiologic Survey on Alcohol and Related Conditions found that for individuals with SUDs, the past-year prevalence of an independent mood or anxiety disorder was 35% and 26%, respectively.19 Taken together, these findings illustrate the substantial rate of dual-diagnosis comorbidity, the diagnostic heterogeneity and range of severity of CODs,20 and the potential for both false negatives (eg, diagnosing a substance-induced syndrome when in fact a patient has an underlying disorder) and false positives (diagnosing a full-blown mental illness when symptoms are subclinical or substance-induced) when performing diagnostic assessments in the setting of known SUDs.
Continue to: False positives are more likely...
False positives are more likely when patients seeking treatment for non-SUDs don’t disclose active drug use, even when asked. Both patients and their treating clinicians may also be prone to underestimating the significant potential for morbidity associated with SUDs, such that substance-induced symptoms may be misattributed to a dual disorder. Diagnostic questioning and thorough chart review that includes careful assessment of whether psychiatric symptoms preceded the onset of substance use, and whether they persisted in the setting of extended sobriety, is therefore paramount for minimizing false positives when assessing for dual diagnoses.18,21 Likewise, random urine toxicology testing can be invaluable in verifying claims regarding sobriety.
Another factor that can complicate diagnosis is that there are often considerable secondary gains (eg, disability income, hospitalization, housing, access to prescription medications, and mitigation of the blame and stigma associated with addiction) associated with having a dual disorder as opposed to having “just” a SUD. As a result, for some patients, obtaining a non-SUD diagnosis can be highly incentivized.22,23 Clinicians must therefore be savvy about the high potential for malingering, embellishment, and mislabeling of symptoms when conducting diagnostic interviews. For example, in assessing for psychosis, the frequent endorsement of “hearing voices” in patients with SUDs often results in a diagnosis of schizophrenia or unspecified psychotic disorder,22 despite the fact that this symptom can occur during substance intoxication and withdrawal, is well documented among people without mental illness as well as those with non-psychotic disorders,24 and can resolve without medications or with non-antipsychotic pharmacotherapy.25
When assessing for dual disorders, diagnostic false positives and false negatives can both contribute to inappropriate treatment and unrealistic expectations for recovery, and therefore underscore the importance of careful diagnostic assessment. Even with diligent assessment, however, diagnostic clarity can prove elusive due to inadequate sobriety, inconsistent reporting, and poor memory.26 Therefore, for patients with known SUDs but diagnostic uncertainty about a dual disorder, the work-up should include a trial of prospective observation, with completion of appropriate detoxification, throughout a 1-month period of sobriety and in the absence of psychiatric medications, to determine if there are persistent symptoms that would justify a dual diagnosis. In research settings, such observations have revealed that most of depressive symptoms among alcoholics who present for substance abuse treatment resolve after a month of abstinence.27 A similar time course for resolution has been noted for anxiety, distress, fatigue, and depressive symptoms among individuals with cocaine dependence.28 These findings support the guideline established in DSM-IV that symptoms persisting beyond a month of sobriety “should be considered to be manifestations of an independent, non-substance-induced mental disorder,”29 while symptoms occurring within that month may well be substance-induced. Unfortunately, in real-world clinical practice, and particularly in outpatient settings, it can be quite difficult to achieve the requisite period of sobriety for reliable diagnosis, and patients are often prematurely prescribed medications (eg, an antidepressant, antipsychotic, or mood stabilizer) that can confound the cause of symptomatic resolution. Such prescriptions are driven by compelling pressures from patients to relieve their acute suffering, as well as the predilection of some clinicians to give patients “the benefit of doubt” in assessing for dual diagnoses. However, whether an inappropriate diagnosis or a prescription for an unnecessary medication represents a benefit is debatable at best.
Pharmacotherapy
A third real-world challenge in managing patients with dual disorders involves optimizing pharmacotherapy. Unfortunately, because patients with SUDs often are excluded from clinical trials, evidence-based guidance for patients with dual disorders is lacking. In addition, medications for both CODs often remain inaccessible to patients with dual disorders for 3 reasons:
- SUDs negatively impact medication adherence among patients with dual disorders, who sometimes point out that “it says right here on the bottle not to take this medication with drugs or alcohol!”
- Some self-help groups still espouse blanket opposition of any “psychotropic” medications, even when clearly indicated for patients with COD. Groups that recognize the importance of pharmacotherapy, such as Dual Diagnosis Anonymous (DDA), have emerged, but are not yet widely available.30
- Although there are increasing options for FDA-approved medications for SUDs, they are limited to the treatment of alcohol, opioid, and nicotine use disorders31; are often restricted due to hospital and health insurance formularies32; and remain underprescribed for patients with dual disorders.11
Continue to: Although underutilization of pharmacotherapy is...
Although underutilization of pharmacotherapy is a pitfall to be avoided in the treatment of patients with dual disorders, medication overutilization can be just as problematic. Patients with dual disorders are sometimes singularly focused on resolving acute anxiety, depression, or psychosis at the expense of working towards sobriety.33 Although the “self-medication hypothesis” is frequently invoked by patients and clinicians alike to suggest that substance use occurs in the service of “treating” underlying disorders,34 this theory has not been well supported in studies.35-37 Some patients may pledge dedication to abstinence, but still pressure physicians for a pharmacologic solution to their suffering. With expanding legalization of cannabis for both recreational and medical purposes, patients are increasingly seeking doctors’ recommendations for “medical marijuana” for a wide range of complaints, despite the fact that data supporting a therapeutic role for cannabis in the treatment of mental illness is sparse,38 whereas the potential harm in terms of either causing or worsening psychosis is well established.39,40 Clinicians must be knowledgeable about the abuse potential of prescribed medications, ranging from sleep aids, analgesics, and muscle relaxants to antidepressants and antipsychotics, while also being mindful of the psychological meaningfulness of seeking, prescribing, and not prescribing medications.41
Although the simultaneous treatment of patients with dual disorders that includes pharmacotherapy for both SUDs and CODs is vital for optimizing clinical outcomes, clinicians should strive for diagnostic accuracy and use medications judiciously. In addition, although pharmacotherapy often is necessary to deliver evidence-based treatment for patients with dual disorders, it is inadequate as standalone treatment and should be administered along with psychosocial interventions within an integrated, multidisciplinary treatment setting.
The keys to optimal outcomes
The treatment of patients with dual disorders can be challenging, to say the least. Ideal, evidence-based therapy in the form of an IDDT program can be difficult for clinicians to implement and for patients to access. Best efforts to perform meticulous clinical assessment to clarify diagnoses, use pharmacotherapy judiciously, work collaboratively in a multidisciplinary setting, and optimize treatment given available resources are keys to clinical success.
Bottom Line
Ideal treatment of patients with dual disorders consists of simultaneous, integrated interventions delivered by a multidisciplinary team. However, in the real world, limited resources, diagnostic challenges, and both over- and underutilization of pharmacotherapy often hamper optimal treatment.
Related Resources
- Substance Abuse and Mental Health Services Administration. Co-occurring disorders. https://www.samhsa.gov/disorders/co-occurring.
- National Alliance on Mental Illness. Dual diagnosis. https://www.nami.org/Learn-More/Mental-Health-Conditions/Related-Conditions/Dual-Diagnosis.
- Foundations Recovery Network. Dual diagnosis support groups. http://www.dualdiagnosis.org/resource/ddrn/self-helpsupport-groups/support-groups.
- Substance Abuse and Mental Health Services Administration. Integrated treatment for co-occurring disorders evidence-based practices (EBP) KIT. https://store.samhsa.gov/product/Integrated-Treatment-for-Co-Occurring-Disorders-Evidence-Based-Practices-EBP-KIT/SMA08-4367.
- Substance Abuse and Mental Health Services Administration. Substance abuse treatment for persons with co-occurring disorders. https://store.samhsa.gov/shin/content/SMA13-3992/SMA13-3992.pdf.
The term “dual diagnosis” describes the clinically challenging comorbidity of a substance use disorder (SUD) along with another major mental illness. Based on data from the Epidemiologic Catchment Area study, the lifetime prevalence of SUDs among patients with mental illness is approximately 30%, and is higher among patients with certain mental disorders, such as schizophrenia (47%), bipolar disorder (61%), and antisocial personality disorder (84%).1 These statistics highlight that addiction is often the rule rather than the exception among those with severe mental illness.1 Not surprisingly, the combined effects of having an SUD along with another mental illness are uniformly negative (Table 12-4).
Based on outcomes research, the core tenets of evidence-based dual-diagnosis treatment include the importance of integrated (rather than parallel) and simultaneous (rather than sequential) care, which means an ideal treatment program includes a unified, multidisciplinary team whose coordinated efforts focus on treating both disorders concurrently.2 Evidence-based psychotherapies for addiction, including motivational interviewing, cognitive-behavioral therapy, relapse prevention, contingency management, skills training, and/or case management, are a necessity,3,5 and must be balanced with rational and appropriate pharmacotherapy targeting both the SUD as well as the other disorder (Table 22,3,5-9).
3 ‘Real-world’ clinical challenges
Ideal vs real-world treatment
Treating patients with co-occurring disorders (CODs) within integrated dual-disorder treatment (IDDT) programs sounds straightforward. However, implementing evidence-based “best practice” treatment is a significant challenge in the real world for several reasons. First, individuals with CODs often struggle with poor insight, low motivation to change, and lack of access to health care. According to the Substance Abuse and Mental Health Services Administration (SAMHSA), 52% of individuals with CODs in the U.S. received no treatment at all in 2016.10 For patients with dual disorders who do seek care, most are not given access to specialty SUD treatment10 and may instead find themselves treated by psychiatrists with limited SUD training who fail to provide evidence-based psychotherapies and underutilize pharmacotherapies for SUDs.11 In the setting of CODs, the “harm reduction model” can be conflated with therapeutic nihilism, resulting in the neglect of SUD issues, with clinicians expecting patients to seek SUD treatment on their own, through self-help groups such as Alcoholics Anonymous or in other community treatment programs staffed by nonprofessionals that often are not tailored to the unique needs of patients with dual disorders. Psychiatrists working with other mental health professionals who provide psychotherapy for SUDs often do so in parallel rather than in an evidence-based, integrated fashion.
IDDT programs are not widely available. One study found that fewer than 20% of addiction treatment programs and fewer than 10% of mental health programs in the U.S. met criteria for dual diagnosis–capable services.12 Getting treatment programs to become dual diagnosis–capable is possible, but it is a time-consuming and costly endeavor that, once achieved, requires continuous staff training and programmatic adaptations to interruptions in funding.13-16 With myriad barriers to the establishment and maintenance of IDDTs, many patients with dual disorders are left without access to the most effective and comprehensive care; as few as 4% of individuals with CODs are treated within integrated programs.17
Diagnostic dilemmas
Establishing whether or not a patient with an active SUD has another serious mental illness (SMI) is a crucial first step for optimizing treatment, but diagnostic reliability can prove challenging and requires careful clinical assessment (Table 3). As always in psychiatry, accurate diagnosis is limited to careful clinical assessment18 and, in the case of possible dual disorders, is complicated by the fact that both SUDs as well as non-SUDs can result in the same psychiatric symptoms (eg, insomnia, anxiety, depression, manic behaviors, and psychosis). Clinicians must therefore distinguish between:
- Symptoms of substance intoxication or withdrawal vs independent symptoms of an underlying psychiatric disorder (that persist beyond a month after cessation of intoxication or withdrawal)
- Subclinical symptoms vs threshold mental illness, keeping in mind that some mood and anxiety states can be normal given social situations and stressors (eg, turmoil in relationships, employment difficulties, homelessness, etc.)
- Any mental illness (AMI) vs SMI. The latter is defined by SAMHSA as AMI that substantially interferes with or limits ≥1 major life activities.10
With these distinctions in mind, data from the 2016 National Survey on Drug Use and Health indicate that dual-diagnosis comorbidity was higher when the threshold for mental illness was lower—among the 19 million adults in the U.S. with SUDs, the past-year prevalence was 43% for AMI and 14% for SMI.10 Looking at substance-induced disorders vs “independent” disorders, the 2001-2002 National Epidemiologic Survey on Alcohol and Related Conditions found that for individuals with SUDs, the past-year prevalence of an independent mood or anxiety disorder was 35% and 26%, respectively.19 Taken together, these findings illustrate the substantial rate of dual-diagnosis comorbidity, the diagnostic heterogeneity and range of severity of CODs,20 and the potential for both false negatives (eg, diagnosing a substance-induced syndrome when in fact a patient has an underlying disorder) and false positives (diagnosing a full-blown mental illness when symptoms are subclinical or substance-induced) when performing diagnostic assessments in the setting of known SUDs.
Continue to: False positives are more likely...
False positives are more likely when patients seeking treatment for non-SUDs don’t disclose active drug use, even when asked. Both patients and their treating clinicians may also be prone to underestimating the significant potential for morbidity associated with SUDs, such that substance-induced symptoms may be misattributed to a dual disorder. Diagnostic questioning and thorough chart review that includes careful assessment of whether psychiatric symptoms preceded the onset of substance use, and whether they persisted in the setting of extended sobriety, is therefore paramount for minimizing false positives when assessing for dual diagnoses.18,21 Likewise, random urine toxicology testing can be invaluable in verifying claims regarding sobriety.
Another factor that can complicate diagnosis is that there are often considerable secondary gains (eg, disability income, hospitalization, housing, access to prescription medications, and mitigation of the blame and stigma associated with addiction) associated with having a dual disorder as opposed to having “just” a SUD. As a result, for some patients, obtaining a non-SUD diagnosis can be highly incentivized.22,23 Clinicians must therefore be savvy about the high potential for malingering, embellishment, and mislabeling of symptoms when conducting diagnostic interviews. For example, in assessing for psychosis, the frequent endorsement of “hearing voices” in patients with SUDs often results in a diagnosis of schizophrenia or unspecified psychotic disorder,22 despite the fact that this symptom can occur during substance intoxication and withdrawal, is well documented among people without mental illness as well as those with non-psychotic disorders,24 and can resolve without medications or with non-antipsychotic pharmacotherapy.25
When assessing for dual disorders, diagnostic false positives and false negatives can both contribute to inappropriate treatment and unrealistic expectations for recovery, and therefore underscore the importance of careful diagnostic assessment. Even with diligent assessment, however, diagnostic clarity can prove elusive due to inadequate sobriety, inconsistent reporting, and poor memory.26 Therefore, for patients with known SUDs but diagnostic uncertainty about a dual disorder, the work-up should include a trial of prospective observation, with completion of appropriate detoxification, throughout a 1-month period of sobriety and in the absence of psychiatric medications, to determine if there are persistent symptoms that would justify a dual diagnosis. In research settings, such observations have revealed that most of depressive symptoms among alcoholics who present for substance abuse treatment resolve after a month of abstinence.27 A similar time course for resolution has been noted for anxiety, distress, fatigue, and depressive symptoms among individuals with cocaine dependence.28 These findings support the guideline established in DSM-IV that symptoms persisting beyond a month of sobriety “should be considered to be manifestations of an independent, non-substance-induced mental disorder,”29 while symptoms occurring within that month may well be substance-induced. Unfortunately, in real-world clinical practice, and particularly in outpatient settings, it can be quite difficult to achieve the requisite period of sobriety for reliable diagnosis, and patients are often prematurely prescribed medications (eg, an antidepressant, antipsychotic, or mood stabilizer) that can confound the cause of symptomatic resolution. Such prescriptions are driven by compelling pressures from patients to relieve their acute suffering, as well as the predilection of some clinicians to give patients “the benefit of doubt” in assessing for dual diagnoses. However, whether an inappropriate diagnosis or a prescription for an unnecessary medication represents a benefit is debatable at best.
Pharmacotherapy
A third real-world challenge in managing patients with dual disorders involves optimizing pharmacotherapy. Unfortunately, because patients with SUDs often are excluded from clinical trials, evidence-based guidance for patients with dual disorders is lacking. In addition, medications for both CODs often remain inaccessible to patients with dual disorders for 3 reasons:
- SUDs negatively impact medication adherence among patients with dual disorders, who sometimes point out that “it says right here on the bottle not to take this medication with drugs or alcohol!”
- Some self-help groups still espouse blanket opposition of any “psychotropic” medications, even when clearly indicated for patients with COD. Groups that recognize the importance of pharmacotherapy, such as Dual Diagnosis Anonymous (DDA), have emerged, but are not yet widely available.30
- Although there are increasing options for FDA-approved medications for SUDs, they are limited to the treatment of alcohol, opioid, and nicotine use disorders31; are often restricted due to hospital and health insurance formularies32; and remain underprescribed for patients with dual disorders.11
Continue to: Although underutilization of pharmacotherapy is...
Although underutilization of pharmacotherapy is a pitfall to be avoided in the treatment of patients with dual disorders, medication overutilization can be just as problematic. Patients with dual disorders are sometimes singularly focused on resolving acute anxiety, depression, or psychosis at the expense of working towards sobriety.33 Although the “self-medication hypothesis” is frequently invoked by patients and clinicians alike to suggest that substance use occurs in the service of “treating” underlying disorders,34 this theory has not been well supported in studies.35-37 Some patients may pledge dedication to abstinence, but still pressure physicians for a pharmacologic solution to their suffering. With expanding legalization of cannabis for both recreational and medical purposes, patients are increasingly seeking doctors’ recommendations for “medical marijuana” for a wide range of complaints, despite the fact that data supporting a therapeutic role for cannabis in the treatment of mental illness is sparse,38 whereas the potential harm in terms of either causing or worsening psychosis is well established.39,40 Clinicians must be knowledgeable about the abuse potential of prescribed medications, ranging from sleep aids, analgesics, and muscle relaxants to antidepressants and antipsychotics, while also being mindful of the psychological meaningfulness of seeking, prescribing, and not prescribing medications.41
Although the simultaneous treatment of patients with dual disorders that includes pharmacotherapy for both SUDs and CODs is vital for optimizing clinical outcomes, clinicians should strive for diagnostic accuracy and use medications judiciously. In addition, although pharmacotherapy often is necessary to deliver evidence-based treatment for patients with dual disorders, it is inadequate as standalone treatment and should be administered along with psychosocial interventions within an integrated, multidisciplinary treatment setting.
The keys to optimal outcomes
The treatment of patients with dual disorders can be challenging, to say the least. Ideal, evidence-based therapy in the form of an IDDT program can be difficult for clinicians to implement and for patients to access. Best efforts to perform meticulous clinical assessment to clarify diagnoses, use pharmacotherapy judiciously, work collaboratively in a multidisciplinary setting, and optimize treatment given available resources are keys to clinical success.
Bottom Line
Ideal treatment of patients with dual disorders consists of simultaneous, integrated interventions delivered by a multidisciplinary team. However, in the real world, limited resources, diagnostic challenges, and both over- and underutilization of pharmacotherapy often hamper optimal treatment.
Related Resources
- Substance Abuse and Mental Health Services Administration. Co-occurring disorders. https://www.samhsa.gov/disorders/co-occurring.
- National Alliance on Mental Illness. Dual diagnosis. https://www.nami.org/Learn-More/Mental-Health-Conditions/Related-Conditions/Dual-Diagnosis.
- Foundations Recovery Network. Dual diagnosis support groups. http://www.dualdiagnosis.org/resource/ddrn/self-helpsupport-groups/support-groups.
- Substance Abuse and Mental Health Services Administration. Integrated treatment for co-occurring disorders evidence-based practices (EBP) KIT. https://store.samhsa.gov/product/Integrated-Treatment-for-Co-Occurring-Disorders-Evidence-Based-Practices-EBP-KIT/SMA08-4367.
- Substance Abuse and Mental Health Services Administration. Substance abuse treatment for persons with co-occurring disorders. https://store.samhsa.gov/shin/content/SMA13-3992/SMA13-3992.pdf.
1. Regier DA, Farmer ME, Rae DS, et al. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the epidemiologic catchment area (ECA) study. JAMA. 1990;264(19):2511-2518.
2. Drake RE, Mercer-McFadden C, Muesner KT, et al. Review of integrated mental health and substance abuse treatment for patients with dual disorders. Schizophr Bull. 1998;24(4):589-608.
3. Horsfall J, Cleary M, Hunt GE, et al. Psychosocial treatments for people with co-occurring severe mental illness and substance use disorders (dual diagnosis): a review of empiric evidence. Harv Rev Psychiatry. 2009;17(1):24-34.
4. Krawczyk N, Feder KA, Saloner B, et al. The association of psychiatric comorbidity with treatment completion among clients admitted to substance use treatment programs in a U.S. national sample. Drug Alcohol Depend. 2017;175:157-163.
5. Brunette MF, Muesner KT. Psychosocial interventions for the long-term management of patients with severe mental illness and co-occurring substance use disorder. J Clin Psychiatry. 2006;67(suppl 7):10-17.
6. Tiet QQ, Mausbach B. Treatments for patients with dual diagnosis: a review. Alcohol Clin Exp Res. 2007;31(4):513-536.
7. Kelly TM, Daley DC, Douaihy AB. Treatment of substance abusing patients with comorbid psychiatric disorders. Addict Behav. 2012;37(1):11-24.
8. Tsuang JT, Ho AP, Eckman TA, et al. Dual diagnosis treatment for patients with schizophrenia who are substance dependent. Psychatr Serv. 1997;48(7):887-889.
9. Rosen MI, Rosenheck RA, Shaner A, et al. Veterans who may need a payee to prevent misuse of funds for drugs. Psychiatr Serv. 2002;53(8):995-1000.
10. Substance Abuse and Mental Health Services Administration. Key substance use and mental health indicators in the United States: results from the 2016 National Survey on Drug Use and Health. HHS Publication No. SMA 17-5044, NSDUH Series H-52. Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration. https://www.samhsa.gov/data/sites/default/files/NSDUH-FFR1-2016/NSDUH-FFR1-2016.pdf. Published September 2017. Accessed August 7, 2018.
11. Rubinsky AD, Chen C, Batki SL, et al. Comparative utilization of pharmacotherapy for alcohol use disorder and other psychiatric disorders among U.S. Veterans Health Administration patients with dual diagnoses. J Psychiatr Res. 2015;69:150-157.
12. McGovern MP, Lambert-Harris C, McHugo GJ, et al. Improving the dual diagnosis capability of addiction and mental health treatment services: implementation factors associated with program level changes. J Dual Diag. 2010;6:237-250.
13. Reno R. Maintaining quality of care in a comprehensive dual diagnosis treatment program. Psychiatr Serv. 2001;52(5):673-675.
14. McGovern MP, Lambert-Harris, Gotham HJ, et al. Dual diagnosis capability in mental health and addiction treatment services: an assessment of programs across multiple state systems. Adm Policy Ment Health. 2014;41(2):205-214.
15. Gotham HJ, Claus RE, Selig K, et al. Increasing program capabilities to provide treatment for co-occurring substance use and mental disorders: organizational characteristics. J Subs Abuse Treat. 2010;38(2):160-169.
16. Priester MA, Browne T, Iachini A, et al. Treatment access barriers and disparities among individuals with co-occurring mental health and substance use disorders: an integrative literature review. J Subst Abuse Treat. 2016;61:47-59.
17. Drake RE, Bond GR. Implementing integrated mental health and substance abuse services. J Dual Diagnosis. 2010;6(3-4):251-262.
18. Miele GM, Trautman KD, Hasin DS. Assessing comorbid mental and substance-use disorders: a guide for clinical practice. J Pract Psychiatry Behav Health. 1996;5:272-282.
19. Stinson FS, Grant BF, Dawson DA, et al. Comorbidity between DSM-IV alcohol and specific drug use disorders in the United States: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Drug Alcohol Depend. 2015;80(1):105-116.
20. Flynn PM, Brown BS. Co-occurring disorders in substance abuse treatment: Issues and prospects. J Subt Abuse Treat. 2008;34(1):36-47.
21. Grant BF, Stintson FS, Dawson DA, et al. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders. Arch Gen Psychiatry. 2004;61(8):807-816.
22. Pierre JM, Wirshing DA, Wirshing WC. “Iatrogenic malingering” in VA substance abuse treatment. Psych Services. 2003;54(2):253-254.
23. Pierre JM, Shnayder I, Wirshing DA, et al. Intranasal quetiapine abuse. Am J Psychiatry. 2004;161(9):1718.
24. Pierre JM. Hallucinations in non-psychotic disorders: Toward a differential diagnosis of “hearing voices.” Harv Rev Psychiatry. 2010;18(1):22-35.
25. Pierre JM. Nonantipsychotic therapy for monosymptomatic auditory hallucinations. Biol Psychiatry. 2010;68(7):e33-e34.
26. Shaner A, Roberts LJ, Eckman TA, et al. Sources of diagnostic uncertainty for chronically psychotic cocaine abusers. Psychiatr Serv. 1998;49(5):684-690.
27. Brown SA, Shuckit MA. Changes in depression among abstinent alcoholics. J Stud Alcohol. 1988;49(5):412-417.
28. Weddington WW, Brown BS, Haertzen CA, et al. Changes in mood, craving, and sleep during short-term abstinence reported by male cocaine addicts. A controlled, residential study. Arch Gen Psychiatry. 1990;47(9):861-868.
29. American Psychiatric Association. Diagnostic and statistical manual of mental disorders, 4th edition. Washington, DC: American Psychiatric Association; 1994:210.
30. Roush S, Monica C, Carpenter-Song E, et al. First-person perspectives on Dual Diagnosis Anonymous (DDA): a qualitative study. J Dual Diagnosis. 2015;11(2):136-141.
31. Klein JW. Pharmacotherapy for substance abuse disorders. Med Clin N Am. 2016;100(4):891-910.
32. Horgan CM, Reif S, Hodgkin D, et al. Availability of addiction medications in private health plans. J Subst Abuse Treat. 2008;34(2):147-156.
33. Frances RJ. The wrath of grapes versus the self-medication hypothesis. Harvard Rev Psychiatry. 1997;4(5):287-289.
34. Khantzian EJ. The self-medication hypothesis of substance use disorders: a reconsideration and recent applications. Harvard Rev Psychiatry. 1997;4(5):231-244.
35. Hall DH, Queener JE. Self-medication hypothesis of substance use: testing Khantzian’s updated theory. J Psychoactive Drugs. 2007;39(2):151-158.
36. Henwood B, Padgett DK. Reevaluating the self-medication hypothesis among the dually diagnosed. Am J Addict. 2007;16(3):160-165.
37. Lembke A. Time to abandon the self-medication hypothesis in patients with psychiatric disorders. Am J Drug Alc Abuse. 2012;38(6):524-529.
38. Wilkinson ST, Radhakrishnan R, D’Souza DC. A systematic review of the evidence for medical marijuana in psychiatric indications. J Clin Psychiatry. 2016;77(8):1050-1064.
39. Walsh Z, Gonzalez R, Crosby K, et al. Medical cannabis and mental health: a guided systematic review. Clin Psychol Rev. 2017;51:15-29.
40. Pierre JM. Risks of increasingly potent cannabis: the joint effects of potency and frequency. Current Psychiatry. 2017;16:14-20.
41. Zweben JE, Smith DE. Considerations in using psychotropic medication with dual diagnosis patients in recovery. J Psychoactive Drugs. 1989;21(2):221-228.
1. Regier DA, Farmer ME, Rae DS, et al. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the epidemiologic catchment area (ECA) study. JAMA. 1990;264(19):2511-2518.
2. Drake RE, Mercer-McFadden C, Muesner KT, et al. Review of integrated mental health and substance abuse treatment for patients with dual disorders. Schizophr Bull. 1998;24(4):589-608.
3. Horsfall J, Cleary M, Hunt GE, et al. Psychosocial treatments for people with co-occurring severe mental illness and substance use disorders (dual diagnosis): a review of empiric evidence. Harv Rev Psychiatry. 2009;17(1):24-34.
4. Krawczyk N, Feder KA, Saloner B, et al. The association of psychiatric comorbidity with treatment completion among clients admitted to substance use treatment programs in a U.S. national sample. Drug Alcohol Depend. 2017;175:157-163.
5. Brunette MF, Muesner KT. Psychosocial interventions for the long-term management of patients with severe mental illness and co-occurring substance use disorder. J Clin Psychiatry. 2006;67(suppl 7):10-17.
6. Tiet QQ, Mausbach B. Treatments for patients with dual diagnosis: a review. Alcohol Clin Exp Res. 2007;31(4):513-536.
7. Kelly TM, Daley DC, Douaihy AB. Treatment of substance abusing patients with comorbid psychiatric disorders. Addict Behav. 2012;37(1):11-24.
8. Tsuang JT, Ho AP, Eckman TA, et al. Dual diagnosis treatment for patients with schizophrenia who are substance dependent. Psychatr Serv. 1997;48(7):887-889.
9. Rosen MI, Rosenheck RA, Shaner A, et al. Veterans who may need a payee to prevent misuse of funds for drugs. Psychiatr Serv. 2002;53(8):995-1000.
10. Substance Abuse and Mental Health Services Administration. Key substance use and mental health indicators in the United States: results from the 2016 National Survey on Drug Use and Health. HHS Publication No. SMA 17-5044, NSDUH Series H-52. Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration. https://www.samhsa.gov/data/sites/default/files/NSDUH-FFR1-2016/NSDUH-FFR1-2016.pdf. Published September 2017. Accessed August 7, 2018.
11. Rubinsky AD, Chen C, Batki SL, et al. Comparative utilization of pharmacotherapy for alcohol use disorder and other psychiatric disorders among U.S. Veterans Health Administration patients with dual diagnoses. J Psychiatr Res. 2015;69:150-157.
12. McGovern MP, Lambert-Harris C, McHugo GJ, et al. Improving the dual diagnosis capability of addiction and mental health treatment services: implementation factors associated with program level changes. J Dual Diag. 2010;6:237-250.
13. Reno R. Maintaining quality of care in a comprehensive dual diagnosis treatment program. Psychiatr Serv. 2001;52(5):673-675.
14. McGovern MP, Lambert-Harris, Gotham HJ, et al. Dual diagnosis capability in mental health and addiction treatment services: an assessment of programs across multiple state systems. Adm Policy Ment Health. 2014;41(2):205-214.
15. Gotham HJ, Claus RE, Selig K, et al. Increasing program capabilities to provide treatment for co-occurring substance use and mental disorders: organizational characteristics. J Subs Abuse Treat. 2010;38(2):160-169.
16. Priester MA, Browne T, Iachini A, et al. Treatment access barriers and disparities among individuals with co-occurring mental health and substance use disorders: an integrative literature review. J Subst Abuse Treat. 2016;61:47-59.
17. Drake RE, Bond GR. Implementing integrated mental health and substance abuse services. J Dual Diagnosis. 2010;6(3-4):251-262.
18. Miele GM, Trautman KD, Hasin DS. Assessing comorbid mental and substance-use disorders: a guide for clinical practice. J Pract Psychiatry Behav Health. 1996;5:272-282.
19. Stinson FS, Grant BF, Dawson DA, et al. Comorbidity between DSM-IV alcohol and specific drug use disorders in the United States: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Drug Alcohol Depend. 2015;80(1):105-116.
20. Flynn PM, Brown BS. Co-occurring disorders in substance abuse treatment: Issues and prospects. J Subt Abuse Treat. 2008;34(1):36-47.
21. Grant BF, Stintson FS, Dawson DA, et al. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders. Arch Gen Psychiatry. 2004;61(8):807-816.
22. Pierre JM, Wirshing DA, Wirshing WC. “Iatrogenic malingering” in VA substance abuse treatment. Psych Services. 2003;54(2):253-254.
23. Pierre JM, Shnayder I, Wirshing DA, et al. Intranasal quetiapine abuse. Am J Psychiatry. 2004;161(9):1718.
24. Pierre JM. Hallucinations in non-psychotic disorders: Toward a differential diagnosis of “hearing voices.” Harv Rev Psychiatry. 2010;18(1):22-35.
25. Pierre JM. Nonantipsychotic therapy for monosymptomatic auditory hallucinations. Biol Psychiatry. 2010;68(7):e33-e34.
26. Shaner A, Roberts LJ, Eckman TA, et al. Sources of diagnostic uncertainty for chronically psychotic cocaine abusers. Psychiatr Serv. 1998;49(5):684-690.
27. Brown SA, Shuckit MA. Changes in depression among abstinent alcoholics. J Stud Alcohol. 1988;49(5):412-417.
28. Weddington WW, Brown BS, Haertzen CA, et al. Changes in mood, craving, and sleep during short-term abstinence reported by male cocaine addicts. A controlled, residential study. Arch Gen Psychiatry. 1990;47(9):861-868.
29. American Psychiatric Association. Diagnostic and statistical manual of mental disorders, 4th edition. Washington, DC: American Psychiatric Association; 1994:210.
30. Roush S, Monica C, Carpenter-Song E, et al. First-person perspectives on Dual Diagnosis Anonymous (DDA): a qualitative study. J Dual Diagnosis. 2015;11(2):136-141.
31. Klein JW. Pharmacotherapy for substance abuse disorders. Med Clin N Am. 2016;100(4):891-910.
32. Horgan CM, Reif S, Hodgkin D, et al. Availability of addiction medications in private health plans. J Subst Abuse Treat. 2008;34(2):147-156.
33. Frances RJ. The wrath of grapes versus the self-medication hypothesis. Harvard Rev Psychiatry. 1997;4(5):287-289.
34. Khantzian EJ. The self-medication hypothesis of substance use disorders: a reconsideration and recent applications. Harvard Rev Psychiatry. 1997;4(5):231-244.
35. Hall DH, Queener JE. Self-medication hypothesis of substance use: testing Khantzian’s updated theory. J Psychoactive Drugs. 2007;39(2):151-158.
36. Henwood B, Padgett DK. Reevaluating the self-medication hypothesis among the dually diagnosed. Am J Addict. 2007;16(3):160-165.
37. Lembke A. Time to abandon the self-medication hypothesis in patients with psychiatric disorders. Am J Drug Alc Abuse. 2012;38(6):524-529.
38. Wilkinson ST, Radhakrishnan R, D’Souza DC. A systematic review of the evidence for medical marijuana in psychiatric indications. J Clin Psychiatry. 2016;77(8):1050-1064.
39. Walsh Z, Gonzalez R, Crosby K, et al. Medical cannabis and mental health: a guided systematic review. Clin Psychol Rev. 2017;51:15-29.
40. Pierre JM. Risks of increasingly potent cannabis: the joint effects of potency and frequency. Current Psychiatry. 2017;16:14-20.
41. Zweben JE, Smith DE. Considerations in using psychotropic medication with dual diagnosis patients in recovery. J Psychoactive Drugs. 1989;21(2):221-228.
Principles for freshly minted psychiatrists
I just finished reading Dr. Nasrallah’s editorial “The DNA of psychiatric practice: A covenant with our patients” (From the Editor,
David W. Goodman, MD, FAPA
Assistant Professor
Department of Psychiatry and Behavioral Sciences
Johns Hopkins School of Medicine
Baltimore, Maryland
Unfortunately, there is no way a physician who uses an electronic medical record can “Maintain total and unimpeachable confidentiality” as the “The medical record is a clinical, billing, legal, and research document.” Since 2003, patients no longer need to give consent for their medical records to be seen by the many staff members who work in treatment, payment, and health care operations, as long as these individuals follow the Health Insurance Portability and Accountability Act of 1996 (HIPAA). Even de-identified data is no longer safe because re-identification is still possible with all the databases available for cross-referencing (ie, Facebook and hospitals as one instance).
So, when a patient finally tells you about a history of sexual abuse, do you make it clear to him or her that although this information is no longer private, it can be expected to be kept confidential by all the business associates, covered entities, government agencies, etc., who see their records?
Maybe there also would be fewer physician suicides if they could be assured of receiving truly private, off-the-grid psychiatric treatment.
Susan Israel, MD
Private psychiatric practice (retired)
Woodbridge, Connecticut
I just read your excellent and exhaustive May editorial, which offered advice for new psychiatrists. I was surprised to see that nowhere on the list was “Please remember to practice what you preach and be vigilant about self-care. We have become increasingly aware of the high rates of burnout among physicians. Know your own limitations so that you can appreciate the work that you do.”
Hal D. Cash, MD
Private psychiatric practice (retired)
Mica Collaborative
Wellesley, Massachusetts
Continue to: Dr. Nasrallah's editorial should have...
Dr. Nasrallah’s editorial should have listed something about the terms of payment for the psychiatrist who “provides” his or her clinical services to patients. This is an ethical issue. As you know, usually a corporation, rather than a patient, pays the psychiatrist. This payment may come from a health insurance company, government program, or (increasingly) a large clinic. When an organization pays the psychiatrist, it calls the tune for both the doctor’s employment and the patient’s access to quality care. Contracts between the hiring organization and psychiatrists are crucial, and therefore, most young doctors must join a hiring organization for financial reasons after completing their psychiatric residency. The young psychiatrists with whom I speak tell me they have no alternative but to be a “corporate dependent” in the world of 2018 psychiatric practice. They are aware of your (and my) noble principles, which should govern their relationships with patients. But the boss often does not agree with such principles.
In my book Passion for Patients1 and as President of the 501(c)3 Minnesota Physician-Patient Alliance think tank (www.physician-patient.org), I argue for empowering patients with the means to direct payments to their physicians. Allowing patients this option is especially important for forming and maintaining strong relationship-based psychiatric and other medical treatments. In 1996, I was fed up with being a psychiatric medical director for 5 years at a large Minnesota Preferred Provider Organization. For me, the saving grace was being able to have an independent, private psychiatric practice. Most of my patients agreed.Therefore, I suggest another principle: “Build and maintain an independent psychiatric practice as an escape option no matter what you do should you decide the ethical practice of psychiatry is not possible if you are employed by a given organization.”
Lee Beecher, MD
Member
Editorial Advisory Board
Clinical Psychiatry News
Adjunct Professor of Psychiatry
University of Minnesota
Minneapolis, Minnesota
Reference
1. Beecher L, Racer D. Passion for patients. St. Paul, MN: Alethos Press; 2017.
I agree with Dr. Nasrallah’s guiding principles of psychiatry, which he proposes to govern the relationships of psychiatrists with their patients. However, there is one glaring omission. The first principle should be “to appropriately diagnose the patient’s condition,” which may or may not be based in psychiatry. Misdiagnoses and inappropriate pharmacologic therapy have ruined the lives of some very good friends of mine, and the need to first do no harm by misdiagnosing the patient, especially in psychiatric emergency rooms and on inpatient units, cannot be overemphasized.
These situations may not rear their head in the everyday practice of psychiatry. However, medical malpractice, especially in the field of psychiatry, is a constant caution that all new physicians need to watch for.
I would like to thank Dr. Nasrallah for his efforts to strengthen the patient–psychiatrist contract.
Rama Kasturi, PhD
Associate Professor (retired)
Department of Pharmacology and Cell Biophysics
Director (1999 to 2013)
Medical Pharmacology Tutorial Program University of Cincinnati, College of Medicine
Cincinnati, Ohio
Continue to: Dr. Nasrallah responds
Dr. Nasrallah responds
I thank my 5 colleagues for their perspectives on my editorial. You all made cogent points.
I agree with Dr. Israel that our patients’ records are now accessible by many entities due to the drastic changes in our health care delivery system. However, while I regard the basic psychiatric signs and symptoms as medical data, like heart disease or cancer, there are personal details that emerge during psychotherapy that should remain confidential and not be included in the written record, and thus are not accessible to billers, health insurance companies, or malpractice lawyers. As for physicians who consider suicide because of fear of the consequences of receiving psychiatric treatment that becomes a matter of record, that is a matter of the unfortunate stigma and ignorance about mental illness and how treatable it can be.
Regarding Dr. Cash’s comments, I agree that psychiatrists should be (and are almost always are) introspective about their vulnerabilities and limitations, and should act accordingly, which includes taking care of their needs to stay healthy and avoid burnout.
As Dr. Beecher pointed out, the employment model for psychiatrists does have many implications and constraints for patient care. I concur that having a small direct-care practice, sometimes called a “cash practice,” provides patients who can afford it the complete privacy they desire, with no one having access to their medical records except for their psychiatrist. Your book is a useful resource in that regard.
Dr. Kasturi is right about the importance of arriving at an accurate diagnosis before embarking on treatment; otherwise, patients will suffer from “therapeutic misadventures.” I have observed this being experienced by some of the patients referred to me because of “treatment resistance.”
Thanks again to my colleagues for their comments and suggestions to the newly minted psychiatrists for whom my editorial was intended.
Henry A. Nasrallah, MD
The Sydney W. Souers Endowed ChairProfessor and Chairman
Department of Psychiatry and Behavioral Neuroscience
Saint Louis University School of Medicine
St. Louis, Missouri
I just finished reading Dr. Nasrallah’s editorial “The DNA of psychiatric practice: A covenant with our patients” (From the Editor,
David W. Goodman, MD, FAPA
Assistant Professor
Department of Psychiatry and Behavioral Sciences
Johns Hopkins School of Medicine
Baltimore, Maryland
Unfortunately, there is no way a physician who uses an electronic medical record can “Maintain total and unimpeachable confidentiality” as the “The medical record is a clinical, billing, legal, and research document.” Since 2003, patients no longer need to give consent for their medical records to be seen by the many staff members who work in treatment, payment, and health care operations, as long as these individuals follow the Health Insurance Portability and Accountability Act of 1996 (HIPAA). Even de-identified data is no longer safe because re-identification is still possible with all the databases available for cross-referencing (ie, Facebook and hospitals as one instance).
So, when a patient finally tells you about a history of sexual abuse, do you make it clear to him or her that although this information is no longer private, it can be expected to be kept confidential by all the business associates, covered entities, government agencies, etc., who see their records?
Maybe there also would be fewer physician suicides if they could be assured of receiving truly private, off-the-grid psychiatric treatment.
Susan Israel, MD
Private psychiatric practice (retired)
Woodbridge, Connecticut
I just read your excellent and exhaustive May editorial, which offered advice for new psychiatrists. I was surprised to see that nowhere on the list was “Please remember to practice what you preach and be vigilant about self-care. We have become increasingly aware of the high rates of burnout among physicians. Know your own limitations so that you can appreciate the work that you do.”
Hal D. Cash, MD
Private psychiatric practice (retired)
Mica Collaborative
Wellesley, Massachusetts
Continue to: Dr. Nasrallah's editorial should have...
Dr. Nasrallah’s editorial should have listed something about the terms of payment for the psychiatrist who “provides” his or her clinical services to patients. This is an ethical issue. As you know, usually a corporation, rather than a patient, pays the psychiatrist. This payment may come from a health insurance company, government program, or (increasingly) a large clinic. When an organization pays the psychiatrist, it calls the tune for both the doctor’s employment and the patient’s access to quality care. Contracts between the hiring organization and psychiatrists are crucial, and therefore, most young doctors must join a hiring organization for financial reasons after completing their psychiatric residency. The young psychiatrists with whom I speak tell me they have no alternative but to be a “corporate dependent” in the world of 2018 psychiatric practice. They are aware of your (and my) noble principles, which should govern their relationships with patients. But the boss often does not agree with such principles.
In my book Passion for Patients1 and as President of the 501(c)3 Minnesota Physician-Patient Alliance think tank (www.physician-patient.org), I argue for empowering patients with the means to direct payments to their physicians. Allowing patients this option is especially important for forming and maintaining strong relationship-based psychiatric and other medical treatments. In 1996, I was fed up with being a psychiatric medical director for 5 years at a large Minnesota Preferred Provider Organization. For me, the saving grace was being able to have an independent, private psychiatric practice. Most of my patients agreed.Therefore, I suggest another principle: “Build and maintain an independent psychiatric practice as an escape option no matter what you do should you decide the ethical practice of psychiatry is not possible if you are employed by a given organization.”
Lee Beecher, MD
Member
Editorial Advisory Board
Clinical Psychiatry News
Adjunct Professor of Psychiatry
University of Minnesota
Minneapolis, Minnesota
Reference
1. Beecher L, Racer D. Passion for patients. St. Paul, MN: Alethos Press; 2017.
I agree with Dr. Nasrallah’s guiding principles of psychiatry, which he proposes to govern the relationships of psychiatrists with their patients. However, there is one glaring omission. The first principle should be “to appropriately diagnose the patient’s condition,” which may or may not be based in psychiatry. Misdiagnoses and inappropriate pharmacologic therapy have ruined the lives of some very good friends of mine, and the need to first do no harm by misdiagnosing the patient, especially in psychiatric emergency rooms and on inpatient units, cannot be overemphasized.
These situations may not rear their head in the everyday practice of psychiatry. However, medical malpractice, especially in the field of psychiatry, is a constant caution that all new physicians need to watch for.
I would like to thank Dr. Nasrallah for his efforts to strengthen the patient–psychiatrist contract.
Rama Kasturi, PhD
Associate Professor (retired)
Department of Pharmacology and Cell Biophysics
Director (1999 to 2013)
Medical Pharmacology Tutorial Program University of Cincinnati, College of Medicine
Cincinnati, Ohio
Continue to: Dr. Nasrallah responds
Dr. Nasrallah responds
I thank my 5 colleagues for their perspectives on my editorial. You all made cogent points.
I agree with Dr. Israel that our patients’ records are now accessible by many entities due to the drastic changes in our health care delivery system. However, while I regard the basic psychiatric signs and symptoms as medical data, like heart disease or cancer, there are personal details that emerge during psychotherapy that should remain confidential and not be included in the written record, and thus are not accessible to billers, health insurance companies, or malpractice lawyers. As for physicians who consider suicide because of fear of the consequences of receiving psychiatric treatment that becomes a matter of record, that is a matter of the unfortunate stigma and ignorance about mental illness and how treatable it can be.
Regarding Dr. Cash’s comments, I agree that psychiatrists should be (and are almost always are) introspective about their vulnerabilities and limitations, and should act accordingly, which includes taking care of their needs to stay healthy and avoid burnout.
As Dr. Beecher pointed out, the employment model for psychiatrists does have many implications and constraints for patient care. I concur that having a small direct-care practice, sometimes called a “cash practice,” provides patients who can afford it the complete privacy they desire, with no one having access to their medical records except for their psychiatrist. Your book is a useful resource in that regard.
Dr. Kasturi is right about the importance of arriving at an accurate diagnosis before embarking on treatment; otherwise, patients will suffer from “therapeutic misadventures.” I have observed this being experienced by some of the patients referred to me because of “treatment resistance.”
Thanks again to my colleagues for their comments and suggestions to the newly minted psychiatrists for whom my editorial was intended.
Henry A. Nasrallah, MD
The Sydney W. Souers Endowed ChairProfessor and Chairman
Department of Psychiatry and Behavioral Neuroscience
Saint Louis University School of Medicine
St. Louis, Missouri
I just finished reading Dr. Nasrallah’s editorial “The DNA of psychiatric practice: A covenant with our patients” (From the Editor,
David W. Goodman, MD, FAPA
Assistant Professor
Department of Psychiatry and Behavioral Sciences
Johns Hopkins School of Medicine
Baltimore, Maryland
Unfortunately, there is no way a physician who uses an electronic medical record can “Maintain total and unimpeachable confidentiality” as the “The medical record is a clinical, billing, legal, and research document.” Since 2003, patients no longer need to give consent for their medical records to be seen by the many staff members who work in treatment, payment, and health care operations, as long as these individuals follow the Health Insurance Portability and Accountability Act of 1996 (HIPAA). Even de-identified data is no longer safe because re-identification is still possible with all the databases available for cross-referencing (ie, Facebook and hospitals as one instance).
So, when a patient finally tells you about a history of sexual abuse, do you make it clear to him or her that although this information is no longer private, it can be expected to be kept confidential by all the business associates, covered entities, government agencies, etc., who see their records?
Maybe there also would be fewer physician suicides if they could be assured of receiving truly private, off-the-grid psychiatric treatment.
Susan Israel, MD
Private psychiatric practice (retired)
Woodbridge, Connecticut
I just read your excellent and exhaustive May editorial, which offered advice for new psychiatrists. I was surprised to see that nowhere on the list was “Please remember to practice what you preach and be vigilant about self-care. We have become increasingly aware of the high rates of burnout among physicians. Know your own limitations so that you can appreciate the work that you do.”
Hal D. Cash, MD
Private psychiatric practice (retired)
Mica Collaborative
Wellesley, Massachusetts
Continue to: Dr. Nasrallah's editorial should have...
Dr. Nasrallah’s editorial should have listed something about the terms of payment for the psychiatrist who “provides” his or her clinical services to patients. This is an ethical issue. As you know, usually a corporation, rather than a patient, pays the psychiatrist. This payment may come from a health insurance company, government program, or (increasingly) a large clinic. When an organization pays the psychiatrist, it calls the tune for both the doctor’s employment and the patient’s access to quality care. Contracts between the hiring organization and psychiatrists are crucial, and therefore, most young doctors must join a hiring organization for financial reasons after completing their psychiatric residency. The young psychiatrists with whom I speak tell me they have no alternative but to be a “corporate dependent” in the world of 2018 psychiatric practice. They are aware of your (and my) noble principles, which should govern their relationships with patients. But the boss often does not agree with such principles.
In my book Passion for Patients1 and as President of the 501(c)3 Minnesota Physician-Patient Alliance think tank (www.physician-patient.org), I argue for empowering patients with the means to direct payments to their physicians. Allowing patients this option is especially important for forming and maintaining strong relationship-based psychiatric and other medical treatments. In 1996, I was fed up with being a psychiatric medical director for 5 years at a large Minnesota Preferred Provider Organization. For me, the saving grace was being able to have an independent, private psychiatric practice. Most of my patients agreed.Therefore, I suggest another principle: “Build and maintain an independent psychiatric practice as an escape option no matter what you do should you decide the ethical practice of psychiatry is not possible if you are employed by a given organization.”
Lee Beecher, MD
Member
Editorial Advisory Board
Clinical Psychiatry News
Adjunct Professor of Psychiatry
University of Minnesota
Minneapolis, Minnesota
Reference
1. Beecher L, Racer D. Passion for patients. St. Paul, MN: Alethos Press; 2017.
I agree with Dr. Nasrallah’s guiding principles of psychiatry, which he proposes to govern the relationships of psychiatrists with their patients. However, there is one glaring omission. The first principle should be “to appropriately diagnose the patient’s condition,” which may or may not be based in psychiatry. Misdiagnoses and inappropriate pharmacologic therapy have ruined the lives of some very good friends of mine, and the need to first do no harm by misdiagnosing the patient, especially in psychiatric emergency rooms and on inpatient units, cannot be overemphasized.
These situations may not rear their head in the everyday practice of psychiatry. However, medical malpractice, especially in the field of psychiatry, is a constant caution that all new physicians need to watch for.
I would like to thank Dr. Nasrallah for his efforts to strengthen the patient–psychiatrist contract.
Rama Kasturi, PhD
Associate Professor (retired)
Department of Pharmacology and Cell Biophysics
Director (1999 to 2013)
Medical Pharmacology Tutorial Program University of Cincinnati, College of Medicine
Cincinnati, Ohio
Continue to: Dr. Nasrallah responds
Dr. Nasrallah responds
I thank my 5 colleagues for their perspectives on my editorial. You all made cogent points.
I agree with Dr. Israel that our patients’ records are now accessible by many entities due to the drastic changes in our health care delivery system. However, while I regard the basic psychiatric signs and symptoms as medical data, like heart disease or cancer, there are personal details that emerge during psychotherapy that should remain confidential and not be included in the written record, and thus are not accessible to billers, health insurance companies, or malpractice lawyers. As for physicians who consider suicide because of fear of the consequences of receiving psychiatric treatment that becomes a matter of record, that is a matter of the unfortunate stigma and ignorance about mental illness and how treatable it can be.
Regarding Dr. Cash’s comments, I agree that psychiatrists should be (and are almost always are) introspective about their vulnerabilities and limitations, and should act accordingly, which includes taking care of their needs to stay healthy and avoid burnout.
As Dr. Beecher pointed out, the employment model for psychiatrists does have many implications and constraints for patient care. I concur that having a small direct-care practice, sometimes called a “cash practice,” provides patients who can afford it the complete privacy they desire, with no one having access to their medical records except for their psychiatrist. Your book is a useful resource in that regard.
Dr. Kasturi is right about the importance of arriving at an accurate diagnosis before embarking on treatment; otherwise, patients will suffer from “therapeutic misadventures.” I have observed this being experienced by some of the patients referred to me because of “treatment resistance.”
Thanks again to my colleagues for their comments and suggestions to the newly minted psychiatrists for whom my editorial was intended.
Henry A. Nasrallah, MD
The Sydney W. Souers Endowed ChairProfessor and Chairman
Department of Psychiatry and Behavioral Neuroscience
Saint Louis University School of Medicine
St. Louis, Missouri
CBT for depression: What the evidence says
Major depressive disorder (MDD) has a devastating impact on individuals and society because of its high prevalence, its recurrent nature, its frequent comorbidity with other disorders, and the functional impairment it causes. Compared with other chronic diseases, such as arthritis, asthma, and diabetes, MDD produces the greatest decrement in health worldwide.1 The goals in treating MDD should be not just to reduce symptom severity but also to achieve continuing remission and lower the risk for relapse.2
Antidepressants are the most common treatment for depression.3 Among psychotherapies used to treat MDD, cognitive-behavioral therapy (CBT) has been identified as an effective treatment.4 Collaborative care models have been reported to manage MDD more effectively.5 In this article, we review the evidence supporting the use of CBT as monotherapy and in combination with antidepressants for acute and long-term treatment of MDD.
Acute treatment: Not too soon for CBT
Mild to moderate depression
Research has indicated that for the treatment of mild MDD, antidepressants are unlikely to be more effective than placebo.6,7 Studies also have reported that response to antidepressants begins to outpace response to placebo only when symptoms are no longer mild. Using antidepressants for patients with mild depression could therefore place them at risk of overtreatment.8 In keeping with these findings, the American Psychiatric Association (APA) has recommended the use of evidence-based psychotherapies, such as CBT, as an initial treatment choice for patients with mild to moderate MDD.9
Two recent studies have suggested that the combination of CBT plus antidepressants could boost improvement in psychosocial functioning for patients with mild MDD.10,11 However, neither study included a group of patients who received only CBT to evaluate if CBT alone could have also produced similar effects. Other limitations include the lack of a control group in one study and small sample sizes in both studies. However, both studies had a long follow-up period and specifically studied the impact on psychosocial functioning.
Moderate to severe depression
Earlier depression treatment guidelines suggested that antidepressants should be used to treat more severe depression, while psychotherapy should be used mainly for mild depression.12 This recommendation was influenced by the well-known National Institute of Mental Health (NIMH) Treatment of Depression Collaborative Research Program, a multicenter randomized controlled trial (RCT) that used a placebo control.13 In this study, CBT was compared with antidepressants and found to be no more effective than placebo for more severely depressed patients.13 However, this finding was not consistent across the 3 sites where the study was conducted; at the site where CBT was provided by more experienced CBT therapists, patients with more severe depression who received CBT fared as well as patients treated with antidepressants.14 A later double-blind RCT that used experienced therapists found that CBT was as effective as antidepressants (monoamine oxidase inhibitors), and both treatments were superior to placebo in reducing symptoms of atypical depression.15
Another placebo-controlled RCT conducted at 2 sites found that CBT was as effective as antidepressants in the treatment of moderately to severely depressed patients. As in the NIMH Treatment of Depression Collaborative Research Program trial,13 in this study, there were indications that the results were dependent on therapist experience.16 These findings suggest that the experience of the therapist is an important factor.
A recent meta-analysis of treatments of the acute phase of MDD compared 11 RCTs of CBT and second-generation antidepressants (selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, and other medications with related mechanisms of action).17 It found that as a first-step treatment, CBT and antidepressants had a similar impact on symptom relief in patients with moderate to severe depression. Patients treated with antidepressants also had a higher risk of experiencing adverse events or discontinuing treatment because of adverse events. However, this meta-analysis included trials that had methodological shortcomings, which reduces the strength of these conclusions.
Continue to: Patients with MDD and comorbid personality disorders have been...
Patients with MDD and comorbid personality disorders have been reported to have poorer outcomes, regardless of the treatment used.18 Fournier et al19 examined the impact of antidepressants and CBT in moderately to severely depressed patients with and without a personality disorder. They found that a combination of antidepressants and CBT was suitable for patients with personality disorders because antidepressants would boost the initial response and CBT would help sustain improvement in the long term.
Presently, the APA suggests that the combination of psychotherapy and antidepressants may be used as an initial treatment for patients with moderate to severe MDD.9 As research brings to light other factors that affect treatment outcomes, these guidelines could change.
Table 110,11,15,16 summarizes the findings of select studies evaluating the use of CBT for the acute treatment of depression.
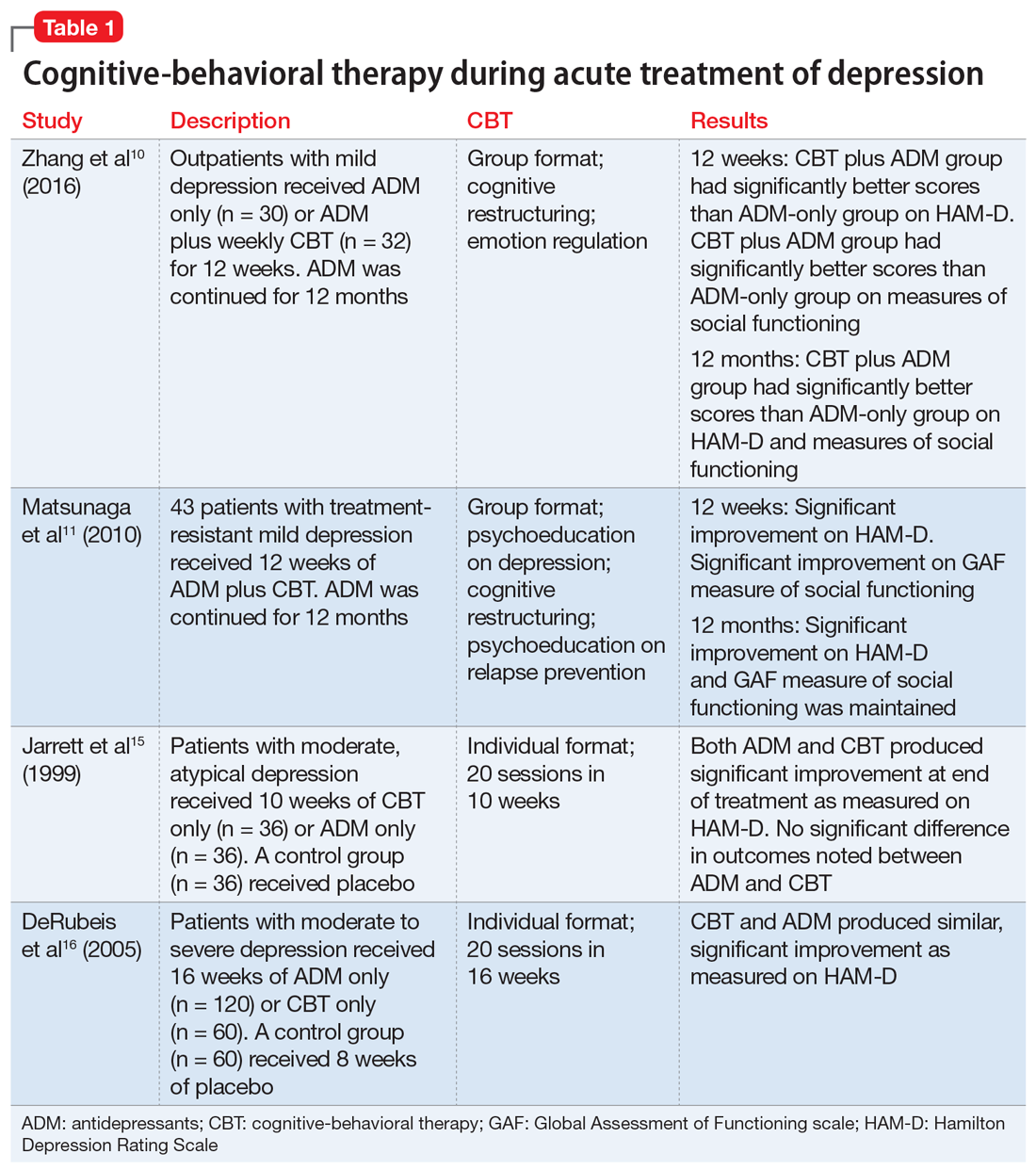
CBT’s role in long-term treatment
Recurrence and relapse are major problems associated with MDD. The large majority of individuals who experience an episode of depression go on to experience more episodes of depression,20 and the risk of recurrence increases after each successive episode.21
To reduce the risk of relapse and the return of symptoms, it is recommended that patients treated with antidepressants continue pharmacotherapy for 4 to 9 months after remission.9 Maintenance pharmacotherapy, which involves keeping patients on antidepressants beyond the point of recovery, is intended to reduce the risk of recurrence, and is standard treatment for patients with chronic or recurrent MDD.22 However, this preventive effect exists only while the patient continues to take the medication. Rates of symptom recurrence following medication withdrawal are often high regardless of how long patients have taken medications.23
Continue to: Studies examining CBT as a maintenance treatment...
Studies examining CBT as a maintenance treatment—provided alone or in combination with or sequentially with antidepressants—have found it has an enduring effect that extends beyond the end of treatment and equals the impact of continuing antidepressants.24-27 A recent meta-analysis of 10 trials where CBT had been provided to patients after acute treatment found that the risk of relapse was reduced by 21% in the first year and by 28% in the first 2 years.28
Studies have compared the prophylactic impact of maintenance CBT and antidepressants. In an early study, 40 patients who had been successfully treated with antidepressants but had residual symptoms were randomly assigned to 20 weeks of CBT or to clinical management.29 By the end of 20 weeks, patients were tapered off their antidepressant. All patients were then followed for 2 years, during which time they received no treatment. At the 2-year follow-up, the CBT group had a relapse rate of 25%, compared with 80% in the antidepressant group.29 Weaknesses of this study include a small sample size, and the fact that a single therapist provided the CBT.
This study was extended to a 6-year follow-up; antidepressants were prescribed only to patients who relapsed. The CBT group continued to have a significantly lower relapse rate (40%) compared with the antidepressant group (90%).30
In another RCT, patients with depression who had recovered with CBT or medication continued with the same treatment during a maintenance phase.26 The CBT group received 3 booster sessions during the next year and antidepressant group received medication. At the end of the second year (without CBT or medication) CBT patients were less likely to relapse compared with patients receiving antidepressants. The adjusted relapse rates were 17.3% for CBT and 53.6% for antidepressants.26
An RCT that included 452 patients with severe depression used a long intervention period (up to 42 weeks) and a flexible treatment algorithm to more closely model the strategies used in clinical practice.31 Patients were randomly assigned to antidepressants only or in combination with CBT. At the end of 12 months, outcome assessment by blinded interviewers indicated that patients with more severe depression were more likely to benefit from the combination of antidepressants and CBT (76.9% vs 60.3%) and those with severe, non-chronic depression received the most benefit (79.5% vs 62.8%). The lack of a CBT-only group limits the generalizability of these findings. Neither patients nor clinicians were blinded to the treatment assignment, which is a common limitation in psychotherapy studies but could have contributed to the finding that combined treatment was more effective.
Continue to: Some evidence suggests...
Some evidence suggests that augmenting treatment as usual (TAU) with CBT can have a resilient protective impact that also intensifies with the number of depressive episodes experienced. In an RCT, 172 patients with depression in remission were randomly assigned to TAU or to TAU augmented with CBT.32 The time to recurrence was assessed over the course of 10 years. Augmenting TAU with CBT had a significant protective impact that was greater for patients who had >3 previous episodes.32
Another long-term study assessed the longitudinal course of 158 patients who received CBT, medication, and clinical management, or medication and clinical management alone.33 Patients were followed 6 years after randomization (4.5 years after completion of CBT). Researchers found the effects of CBT in preventing relapse and recurrence persisted for several years.33
Table 224,26,29-32 summarizes the findings of select studies evaluating the use of CBT for the long-term treatment of depression.
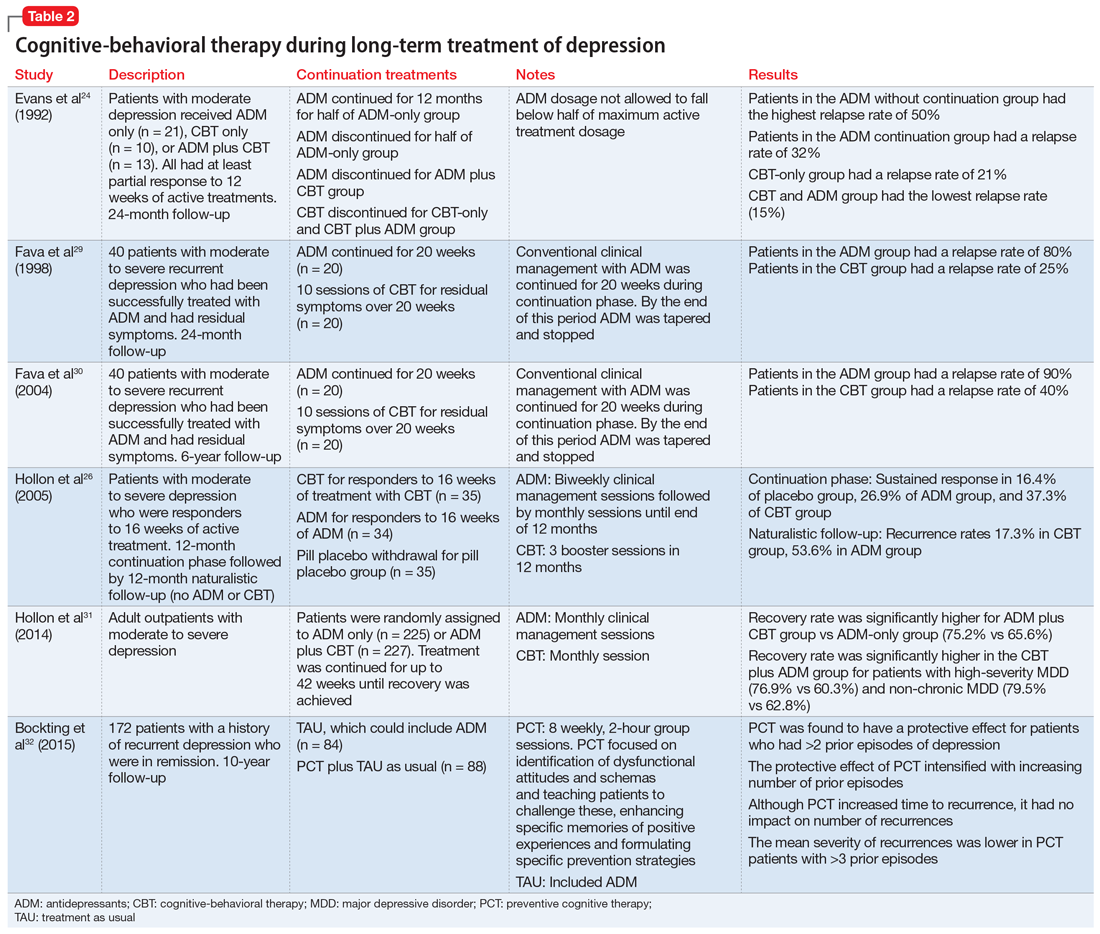
Limitations of long-term studies
Studies that have examined the efficacy of adding CBT to antidepressants in the continuation and maintenance treatment of patients with MDD have had some limitations. The definitions of relapse and recurrence have not always been clearly delineated in all studies. This is important because recurrence rates tend to be lower, and long-term follow-up would be needed to detect multiple recurrences so that their incidence is not underestimated. In addition, the types of CBT interventions utilized has varied across studies. Some studies have employed standard interventions such as cognitive restructuring, while others have added strategies that focus on enhancing memories for positive experiences or interventions to encourage medication adherence. Despite these limitations, research has shown promising results and suggests that adding CBT to the maintenance treatment of patients with depression—with or without antidepressants—is likely to reduce the rate of relapse and recurrence.
Consider CBT for all depressed patients
Research indicates that CBT can be the preferred treatment for patients with mild to moderate MDD. Antidepressants significantly reduce depressive symptoms in patients with moderate to severe MDD. Some research suggests that CBT can be as effective as antidepressants for moderate and severe MDD. However, as the severity and chronicity of depression increase, other moderating factors need to be considered. The expertise of the CBT therapist has an impact on outcomes. Treatment protocols that utilize CBT plus antidepressants are likely to be more effective than CBT or antidepressants alone. Incorporating CBT in the acute phase of depression treatment, with or without antidepressants, can have a long-term impact. For maintenance treatment, CBT alone and CBT plus antidepressants have been found to help sustain remission.
Continue to: Bottom Line
Bottom Line
Cognitive-behavioral therapy (CBT) can be an effective treatment for patients with major depressive disorder, regardless of symptom severity. The expertise of the clinician who provides CBT has a substantial impact on outcomes. Combination treatment with CBT plus antidepressants is more likely to be effective than either treatment alone.
Related Resources
- Flynn HA, Warren R. Using CBT effectively for treating depression and anxiety. Current Psychiatry. 2014;13(6):45-53.
- Ijaz S, Davies P, Williams CJ, et al. Psychological therapies for treatment-resistant depression in adults. Cochrane Database Syst Rev. 2018;5:CD010558.
1. Moussavi S, Chatterji S, Verdes E, et al. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet. 2007;370(9590):851-858.
2. Keller MB. Past, present, and future directions for defining optimal treatment outcome in depression: remission and beyond. JAMA. 2003;289(23):3152-3160.
3. Marcus SC, Olfson M. National trends in the treatment for depression from 1998 to 2007. Arch Gen Psychiatry. 2010;67(12):1265-1273.
4. Chambless DL, Ollendick TH. Empirically supported psychological interventions: controversies and evidence. Annu Rev Psychol. 2001;52:685-716.
5. Oxman TE, Dietrich AJ, Schulberg HC. Evidence-based models of integrated management of depression in primary care. Psychiatr Clin North Am. 2005;28(4):1061-1077.
6. Fournier JC, DeRubeis RJ, Hollon SD, et al. Antidepressant drug effects and depression severity: a patient-level meta-analysis. JAMA. 2010;303(1):47-53.
7. Paykel ES, Hollyman JA, Freeling P, et al. Predictors of therapeutic benefit from amitriptyline in mild depression: a general practice placebo-controlled trial. J Affect Disord. 1988;14(1):83-95
8. Marcus SC, Olfson M. National trends in the treatment for depression from 1998 to 2007. Arch Gen Psychiatry. 2010;67(12):1265-1273.
9. Practice guideline for the treatment of patients with major depressive disorder, 3rd ed. Arlington, VA: American Psychiatric Association; 2010.
10. Zhang B, Ding X, Lu W, et al. Effect of group cognitive-behavioral therapy on the quality of life and social functioning of patients with mild depression. Shanghai Arch Psychiatry. 2016;28(1):18-27.
11. Matsunaga M, Okamoto Y, Suzuki S et.al. Psychosocial functioning in patients with treatment-resistant depression after group cognitive behavioral therapy. BMC Psychiatry. 2010;10:22.
12. American Psychiatric Association. Practice Guideline for Major Depressive Disorder in Adults. Am J Psychiatry. 1993;150(suppl 4):1-26.
13. Elkin I, Shea MT, Watkins JT, et al. National Institute of Mental Health Treatment of Depression Collaborative Research Program. General effectiveness of treatments. Arch Gen Psychiatry. 1989;46(11):971-982; discussion 983.
14. Jacobson NS, Hollon SD. Prospects for future comparisons between drugs and psychotherapy: lessons from the CBT-versus-pharmacotherapy exchange. J Consult Clin Psychol. 1996;64(1):104-108.
15. Jarrett RB, Schaffer M, McIntire D, et al. Treatment of atypical depression with cognitive therapy or phenelzine: a double-blind, placebo-controlled trial. Arch Gen Psychiatry. 1999;56(5):431-437.
16. DeRubeis RJ, Hollon SD, Amsterdam JD, et al. Cognitive therapy vs medications in the treatment of moderate to severe depression. Arch Gen Psychiatry. 2005;62(4):409-416.
17. Amick HR, Gartlehner G, Gaynes BN, et al. Comparative benefits and harms of second generation antidepressants and cognitive behavioral therapies in initial treatment of major depressive disorder: systematic review and meta-analysis. BMJ. 2015;351:h6019. doi: 10.1136/bmj.h6019.
18. Newton-Howes G, Tyrer P, Johnson T. Personality disorder and the outcome of depression: meta-analysis of published studies. Br J Psychiatry. 2006;188(1):13-20.
19. Fournier JC, DeRubeis RJ, Shelton RC, et al. Antidepressant medications v. cognitive therapy in people with depression with or without personality disorder. Br J Psychiatry. 2008;192(2):124-129.
20. Mueller TI, Leon AC, Keller MB, et al. Recurrence after recovery from major depressive disorder during 15 years of observational follow-up. Am J Psychiatry. 1999;156(7):1000-1006.
21. Solomon DA, Keller MB, Leon AC, et al. Multiple recurrences of major depressive disorder. Am J Psychiatry. 2000;157(2):229-233.
22. Frank E, Prien RF, Jarrett RB, et al. Conceptualization and rationale for consensus definitions of terms in major depressive disorder. Remission, recovery, relapse, and recurrence. Arch Gen Psychiatry. 1991;48(9):851-855.
23. Thase ME. Relapse and recurrence of depression: an updated practical approach for prevention. In: Palmer KJ, ed. Drug treatment issues in depression. Auckland, New Zealand: Adis International; 2000:35-52.
24. Evans MD, Hollon, SD, DeRubeis RJ, et al. Differential relapse following cognitive therapy and pharmacotherapy for depression. Arch Gen Psychiatry. 1992;49(10):802-808.
25. Vittengal JR, Clark LA, Dunn TW, et al. Reducing relapse and recurrence in unipolar depression: a comparative meta-analysis of cognitive-behavioral therapy’s effects. J Consult Clin Psychol. 2007;75(3):475-488.
26. Hollon SD, DeRubeis RJ, Shelton RC, et al. Prevention of relapse following cognitive therapy vs medications in moderate to severe depression. Arch Gen Psychiatry. 2005;62(4):417-422.
27. Paykel ES, Scott J, Teasdale JD, et al. Prevention of relapse in residual depression by cognitive therapy: a controlled trial. Arch Gen Psychiatry. 1999;56(9):829-835.
28. Clarke K, Mayo-Wilson E, Kenny J, et al. Can non-pharmacological interventions prevent relapse in adults who have recovered from depression? A systematic review and meta-analysis of randomised controlled trials. Clin Psychol Rev. 2015;39:58-70.
29. Fava GA, Rafanelli C, Grandi, S, et al. Prevention of recurrent depression with cognitive behavioral therapy: preliminary findings. Arch Gen Psychiatry. 1998;55(9):816-820.
30. Fava GA, Ruini C, Rafanelli C, et al. Six-year outcome of cognitive behavior therapy for prevention of recurrent depression. Am J Psychiatry. 2004;161(10):1872-1876.
31. Hollon SD, DeRubeis RJ, Fawcett J, et al. Effect of cognitive therapy with antidepressant medications vs antidepressants alone on the rate of recovery in major depressive disorder: a randomized clinical trial. JAMA Psychiatry. 2014;71(10):1157-1164.
32. Bockting CL, Smid NH, Koeter MW, et al. Enduring effects of preventive cognitive therapy in adults remitted from recurrent depression: a 10 year follow-up of a randomized controlled trial. J Affect Disord. 2015;185:188-194.
33. Paykel ES, Scott J, Cornwall PL, et al. Duration of relapse prevention after cognitive therapy in residual depression: follow-up of controlled trial. Psychol Med. 2005;35(1):59-68.
Major depressive disorder (MDD) has a devastating impact on individuals and society because of its high prevalence, its recurrent nature, its frequent comorbidity with other disorders, and the functional impairment it causes. Compared with other chronic diseases, such as arthritis, asthma, and diabetes, MDD produces the greatest decrement in health worldwide.1 The goals in treating MDD should be not just to reduce symptom severity but also to achieve continuing remission and lower the risk for relapse.2
Antidepressants are the most common treatment for depression.3 Among psychotherapies used to treat MDD, cognitive-behavioral therapy (CBT) has been identified as an effective treatment.4 Collaborative care models have been reported to manage MDD more effectively.5 In this article, we review the evidence supporting the use of CBT as monotherapy and in combination with antidepressants for acute and long-term treatment of MDD.
Acute treatment: Not too soon for CBT
Mild to moderate depression
Research has indicated that for the treatment of mild MDD, antidepressants are unlikely to be more effective than placebo.6,7 Studies also have reported that response to antidepressants begins to outpace response to placebo only when symptoms are no longer mild. Using antidepressants for patients with mild depression could therefore place them at risk of overtreatment.8 In keeping with these findings, the American Psychiatric Association (APA) has recommended the use of evidence-based psychotherapies, such as CBT, as an initial treatment choice for patients with mild to moderate MDD.9
Two recent studies have suggested that the combination of CBT plus antidepressants could boost improvement in psychosocial functioning for patients with mild MDD.10,11 However, neither study included a group of patients who received only CBT to evaluate if CBT alone could have also produced similar effects. Other limitations include the lack of a control group in one study and small sample sizes in both studies. However, both studies had a long follow-up period and specifically studied the impact on psychosocial functioning.
Moderate to severe depression
Earlier depression treatment guidelines suggested that antidepressants should be used to treat more severe depression, while psychotherapy should be used mainly for mild depression.12 This recommendation was influenced by the well-known National Institute of Mental Health (NIMH) Treatment of Depression Collaborative Research Program, a multicenter randomized controlled trial (RCT) that used a placebo control.13 In this study, CBT was compared with antidepressants and found to be no more effective than placebo for more severely depressed patients.13 However, this finding was not consistent across the 3 sites where the study was conducted; at the site where CBT was provided by more experienced CBT therapists, patients with more severe depression who received CBT fared as well as patients treated with antidepressants.14 A later double-blind RCT that used experienced therapists found that CBT was as effective as antidepressants (monoamine oxidase inhibitors), and both treatments were superior to placebo in reducing symptoms of atypical depression.15
Another placebo-controlled RCT conducted at 2 sites found that CBT was as effective as antidepressants in the treatment of moderately to severely depressed patients. As in the NIMH Treatment of Depression Collaborative Research Program trial,13 in this study, there were indications that the results were dependent on therapist experience.16 These findings suggest that the experience of the therapist is an important factor.
A recent meta-analysis of treatments of the acute phase of MDD compared 11 RCTs of CBT and second-generation antidepressants (selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, and other medications with related mechanisms of action).17 It found that as a first-step treatment, CBT and antidepressants had a similar impact on symptom relief in patients with moderate to severe depression. Patients treated with antidepressants also had a higher risk of experiencing adverse events or discontinuing treatment because of adverse events. However, this meta-analysis included trials that had methodological shortcomings, which reduces the strength of these conclusions.
Continue to: Patients with MDD and comorbid personality disorders have been...
Patients with MDD and comorbid personality disorders have been reported to have poorer outcomes, regardless of the treatment used.18 Fournier et al19 examined the impact of antidepressants and CBT in moderately to severely depressed patients with and without a personality disorder. They found that a combination of antidepressants and CBT was suitable for patients with personality disorders because antidepressants would boost the initial response and CBT would help sustain improvement in the long term.
Presently, the APA suggests that the combination of psychotherapy and antidepressants may be used as an initial treatment for patients with moderate to severe MDD.9 As research brings to light other factors that affect treatment outcomes, these guidelines could change.
Table 110,11,15,16 summarizes the findings of select studies evaluating the use of CBT for the acute treatment of depression.

CBT’s role in long-term treatment
Recurrence and relapse are major problems associated with MDD. The large majority of individuals who experience an episode of depression go on to experience more episodes of depression,20 and the risk of recurrence increases after each successive episode.21
To reduce the risk of relapse and the return of symptoms, it is recommended that patients treated with antidepressants continue pharmacotherapy for 4 to 9 months after remission.9 Maintenance pharmacotherapy, which involves keeping patients on antidepressants beyond the point of recovery, is intended to reduce the risk of recurrence, and is standard treatment for patients with chronic or recurrent MDD.22 However, this preventive effect exists only while the patient continues to take the medication. Rates of symptom recurrence following medication withdrawal are often high regardless of how long patients have taken medications.23
Continue to: Studies examining CBT as a maintenance treatment...
Studies examining CBT as a maintenance treatment—provided alone or in combination with or sequentially with antidepressants—have found it has an enduring effect that extends beyond the end of treatment and equals the impact of continuing antidepressants.24-27 A recent meta-analysis of 10 trials where CBT had been provided to patients after acute treatment found that the risk of relapse was reduced by 21% in the first year and by 28% in the first 2 years.28
Studies have compared the prophylactic impact of maintenance CBT and antidepressants. In an early study, 40 patients who had been successfully treated with antidepressants but had residual symptoms were randomly assigned to 20 weeks of CBT or to clinical management.29 By the end of 20 weeks, patients were tapered off their antidepressant. All patients were then followed for 2 years, during which time they received no treatment. At the 2-year follow-up, the CBT group had a relapse rate of 25%, compared with 80% in the antidepressant group.29 Weaknesses of this study include a small sample size, and the fact that a single therapist provided the CBT.
This study was extended to a 6-year follow-up; antidepressants were prescribed only to patients who relapsed. The CBT group continued to have a significantly lower relapse rate (40%) compared with the antidepressant group (90%).30
In another RCT, patients with depression who had recovered with CBT or medication continued with the same treatment during a maintenance phase.26 The CBT group received 3 booster sessions during the next year and antidepressant group received medication. At the end of the second year (without CBT or medication) CBT patients were less likely to relapse compared with patients receiving antidepressants. The adjusted relapse rates were 17.3% for CBT and 53.6% for antidepressants.26
An RCT that included 452 patients with severe depression used a long intervention period (up to 42 weeks) and a flexible treatment algorithm to more closely model the strategies used in clinical practice.31 Patients were randomly assigned to antidepressants only or in combination with CBT. At the end of 12 months, outcome assessment by blinded interviewers indicated that patients with more severe depression were more likely to benefit from the combination of antidepressants and CBT (76.9% vs 60.3%) and those with severe, non-chronic depression received the most benefit (79.5% vs 62.8%). The lack of a CBT-only group limits the generalizability of these findings. Neither patients nor clinicians were blinded to the treatment assignment, which is a common limitation in psychotherapy studies but could have contributed to the finding that combined treatment was more effective.
Continue to: Some evidence suggests...
Some evidence suggests that augmenting treatment as usual (TAU) with CBT can have a resilient protective impact that also intensifies with the number of depressive episodes experienced. In an RCT, 172 patients with depression in remission were randomly assigned to TAU or to TAU augmented with CBT.32 The time to recurrence was assessed over the course of 10 years. Augmenting TAU with CBT had a significant protective impact that was greater for patients who had >3 previous episodes.32
Another long-term study assessed the longitudinal course of 158 patients who received CBT, medication, and clinical management, or medication and clinical management alone.33 Patients were followed 6 years after randomization (4.5 years after completion of CBT). Researchers found the effects of CBT in preventing relapse and recurrence persisted for several years.33
Table 224,26,29-32 summarizes the findings of select studies evaluating the use of CBT for the long-term treatment of depression.

Limitations of long-term studies
Studies that have examined the efficacy of adding CBT to antidepressants in the continuation and maintenance treatment of patients with MDD have had some limitations. The definitions of relapse and recurrence have not always been clearly delineated in all studies. This is important because recurrence rates tend to be lower, and long-term follow-up would be needed to detect multiple recurrences so that their incidence is not underestimated. In addition, the types of CBT interventions utilized has varied across studies. Some studies have employed standard interventions such as cognitive restructuring, while others have added strategies that focus on enhancing memories for positive experiences or interventions to encourage medication adherence. Despite these limitations, research has shown promising results and suggests that adding CBT to the maintenance treatment of patients with depression—with or without antidepressants—is likely to reduce the rate of relapse and recurrence.
Consider CBT for all depressed patients
Research indicates that CBT can be the preferred treatment for patients with mild to moderate MDD. Antidepressants significantly reduce depressive symptoms in patients with moderate to severe MDD. Some research suggests that CBT can be as effective as antidepressants for moderate and severe MDD. However, as the severity and chronicity of depression increase, other moderating factors need to be considered. The expertise of the CBT therapist has an impact on outcomes. Treatment protocols that utilize CBT plus antidepressants are likely to be more effective than CBT or antidepressants alone. Incorporating CBT in the acute phase of depression treatment, with or without antidepressants, can have a long-term impact. For maintenance treatment, CBT alone and CBT plus antidepressants have been found to help sustain remission.
Continue to: Bottom Line
Bottom Line
Cognitive-behavioral therapy (CBT) can be an effective treatment for patients with major depressive disorder, regardless of symptom severity. The expertise of the clinician who provides CBT has a substantial impact on outcomes. Combination treatment with CBT plus antidepressants is more likely to be effective than either treatment alone.
Related Resources
- Flynn HA, Warren R. Using CBT effectively for treating depression and anxiety. Current Psychiatry. 2014;13(6):45-53.
- Ijaz S, Davies P, Williams CJ, et al. Psychological therapies for treatment-resistant depression in adults. Cochrane Database Syst Rev. 2018;5:CD010558.
Major depressive disorder (MDD) has a devastating impact on individuals and society because of its high prevalence, its recurrent nature, its frequent comorbidity with other disorders, and the functional impairment it causes. Compared with other chronic diseases, such as arthritis, asthma, and diabetes, MDD produces the greatest decrement in health worldwide.1 The goals in treating MDD should be not just to reduce symptom severity but also to achieve continuing remission and lower the risk for relapse.2
Antidepressants are the most common treatment for depression.3 Among psychotherapies used to treat MDD, cognitive-behavioral therapy (CBT) has been identified as an effective treatment.4 Collaborative care models have been reported to manage MDD more effectively.5 In this article, we review the evidence supporting the use of CBT as monotherapy and in combination with antidepressants for acute and long-term treatment of MDD.
Acute treatment: Not too soon for CBT
Mild to moderate depression
Research has indicated that for the treatment of mild MDD, antidepressants are unlikely to be more effective than placebo.6,7 Studies also have reported that response to antidepressants begins to outpace response to placebo only when symptoms are no longer mild. Using antidepressants for patients with mild depression could therefore place them at risk of overtreatment.8 In keeping with these findings, the American Psychiatric Association (APA) has recommended the use of evidence-based psychotherapies, such as CBT, as an initial treatment choice for patients with mild to moderate MDD.9
Two recent studies have suggested that the combination of CBT plus antidepressants could boost improvement in psychosocial functioning for patients with mild MDD.10,11 However, neither study included a group of patients who received only CBT to evaluate if CBT alone could have also produced similar effects. Other limitations include the lack of a control group in one study and small sample sizes in both studies. However, both studies had a long follow-up period and specifically studied the impact on psychosocial functioning.
Moderate to severe depression
Earlier depression treatment guidelines suggested that antidepressants should be used to treat more severe depression, while psychotherapy should be used mainly for mild depression.12 This recommendation was influenced by the well-known National Institute of Mental Health (NIMH) Treatment of Depression Collaborative Research Program, a multicenter randomized controlled trial (RCT) that used a placebo control.13 In this study, CBT was compared with antidepressants and found to be no more effective than placebo for more severely depressed patients.13 However, this finding was not consistent across the 3 sites where the study was conducted; at the site where CBT was provided by more experienced CBT therapists, patients with more severe depression who received CBT fared as well as patients treated with antidepressants.14 A later double-blind RCT that used experienced therapists found that CBT was as effective as antidepressants (monoamine oxidase inhibitors), and both treatments were superior to placebo in reducing symptoms of atypical depression.15
Another placebo-controlled RCT conducted at 2 sites found that CBT was as effective as antidepressants in the treatment of moderately to severely depressed patients. As in the NIMH Treatment of Depression Collaborative Research Program trial,13 in this study, there were indications that the results were dependent on therapist experience.16 These findings suggest that the experience of the therapist is an important factor.
A recent meta-analysis of treatments of the acute phase of MDD compared 11 RCTs of CBT and second-generation antidepressants (selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, and other medications with related mechanisms of action).17 It found that as a first-step treatment, CBT and antidepressants had a similar impact on symptom relief in patients with moderate to severe depression. Patients treated with antidepressants also had a higher risk of experiencing adverse events or discontinuing treatment because of adverse events. However, this meta-analysis included trials that had methodological shortcomings, which reduces the strength of these conclusions.
Continue to: Patients with MDD and comorbid personality disorders have been...
Patients with MDD and comorbid personality disorders have been reported to have poorer outcomes, regardless of the treatment used.18 Fournier et al19 examined the impact of antidepressants and CBT in moderately to severely depressed patients with and without a personality disorder. They found that a combination of antidepressants and CBT was suitable for patients with personality disorders because antidepressants would boost the initial response and CBT would help sustain improvement in the long term.
Presently, the APA suggests that the combination of psychotherapy and antidepressants may be used as an initial treatment for patients with moderate to severe MDD.9 As research brings to light other factors that affect treatment outcomes, these guidelines could change.
Table 110,11,15,16 summarizes the findings of select studies evaluating the use of CBT for the acute treatment of depression.

CBT’s role in long-term treatment
Recurrence and relapse are major problems associated with MDD. The large majority of individuals who experience an episode of depression go on to experience more episodes of depression,20 and the risk of recurrence increases after each successive episode.21
To reduce the risk of relapse and the return of symptoms, it is recommended that patients treated with antidepressants continue pharmacotherapy for 4 to 9 months after remission.9 Maintenance pharmacotherapy, which involves keeping patients on antidepressants beyond the point of recovery, is intended to reduce the risk of recurrence, and is standard treatment for patients with chronic or recurrent MDD.22 However, this preventive effect exists only while the patient continues to take the medication. Rates of symptom recurrence following medication withdrawal are often high regardless of how long patients have taken medications.23
Continue to: Studies examining CBT as a maintenance treatment...
Studies examining CBT as a maintenance treatment—provided alone or in combination with or sequentially with antidepressants—have found it has an enduring effect that extends beyond the end of treatment and equals the impact of continuing antidepressants.24-27 A recent meta-analysis of 10 trials where CBT had been provided to patients after acute treatment found that the risk of relapse was reduced by 21% in the first year and by 28% in the first 2 years.28
Studies have compared the prophylactic impact of maintenance CBT and antidepressants. In an early study, 40 patients who had been successfully treated with antidepressants but had residual symptoms were randomly assigned to 20 weeks of CBT or to clinical management.29 By the end of 20 weeks, patients were tapered off their antidepressant. All patients were then followed for 2 years, during which time they received no treatment. At the 2-year follow-up, the CBT group had a relapse rate of 25%, compared with 80% in the antidepressant group.29 Weaknesses of this study include a small sample size, and the fact that a single therapist provided the CBT.
This study was extended to a 6-year follow-up; antidepressants were prescribed only to patients who relapsed. The CBT group continued to have a significantly lower relapse rate (40%) compared with the antidepressant group (90%).30
In another RCT, patients with depression who had recovered with CBT or medication continued with the same treatment during a maintenance phase.26 The CBT group received 3 booster sessions during the next year and antidepressant group received medication. At the end of the second year (without CBT or medication) CBT patients were less likely to relapse compared with patients receiving antidepressants. The adjusted relapse rates were 17.3% for CBT and 53.6% for antidepressants.26
An RCT that included 452 patients with severe depression used a long intervention period (up to 42 weeks) and a flexible treatment algorithm to more closely model the strategies used in clinical practice.31 Patients were randomly assigned to antidepressants only or in combination with CBT. At the end of 12 months, outcome assessment by blinded interviewers indicated that patients with more severe depression were more likely to benefit from the combination of antidepressants and CBT (76.9% vs 60.3%) and those with severe, non-chronic depression received the most benefit (79.5% vs 62.8%). The lack of a CBT-only group limits the generalizability of these findings. Neither patients nor clinicians were blinded to the treatment assignment, which is a common limitation in psychotherapy studies but could have contributed to the finding that combined treatment was more effective.
Continue to: Some evidence suggests...
Some evidence suggests that augmenting treatment as usual (TAU) with CBT can have a resilient protective impact that also intensifies with the number of depressive episodes experienced. In an RCT, 172 patients with depression in remission were randomly assigned to TAU or to TAU augmented with CBT.32 The time to recurrence was assessed over the course of 10 years. Augmenting TAU with CBT had a significant protective impact that was greater for patients who had >3 previous episodes.32
Another long-term study assessed the longitudinal course of 158 patients who received CBT, medication, and clinical management, or medication and clinical management alone.33 Patients were followed 6 years after randomization (4.5 years after completion of CBT). Researchers found the effects of CBT in preventing relapse and recurrence persisted for several years.33
Table 224,26,29-32 summarizes the findings of select studies evaluating the use of CBT for the long-term treatment of depression.

Limitations of long-term studies
Studies that have examined the efficacy of adding CBT to antidepressants in the continuation and maintenance treatment of patients with MDD have had some limitations. The definitions of relapse and recurrence have not always been clearly delineated in all studies. This is important because recurrence rates tend to be lower, and long-term follow-up would be needed to detect multiple recurrences so that their incidence is not underestimated. In addition, the types of CBT interventions utilized has varied across studies. Some studies have employed standard interventions such as cognitive restructuring, while others have added strategies that focus on enhancing memories for positive experiences or interventions to encourage medication adherence. Despite these limitations, research has shown promising results and suggests that adding CBT to the maintenance treatment of patients with depression—with or without antidepressants—is likely to reduce the rate of relapse and recurrence.
Consider CBT for all depressed patients
Research indicates that CBT can be the preferred treatment for patients with mild to moderate MDD. Antidepressants significantly reduce depressive symptoms in patients with moderate to severe MDD. Some research suggests that CBT can be as effective as antidepressants for moderate and severe MDD. However, as the severity and chronicity of depression increase, other moderating factors need to be considered. The expertise of the CBT therapist has an impact on outcomes. Treatment protocols that utilize CBT plus antidepressants are likely to be more effective than CBT or antidepressants alone. Incorporating CBT in the acute phase of depression treatment, with or without antidepressants, can have a long-term impact. For maintenance treatment, CBT alone and CBT plus antidepressants have been found to help sustain remission.
Continue to: Bottom Line
Bottom Line
Cognitive-behavioral therapy (CBT) can be an effective treatment for patients with major depressive disorder, regardless of symptom severity. The expertise of the clinician who provides CBT has a substantial impact on outcomes. Combination treatment with CBT plus antidepressants is more likely to be effective than either treatment alone.
Related Resources
- Flynn HA, Warren R. Using CBT effectively for treating depression and anxiety. Current Psychiatry. 2014;13(6):45-53.
- Ijaz S, Davies P, Williams CJ, et al. Psychological therapies for treatment-resistant depression in adults. Cochrane Database Syst Rev. 2018;5:CD010558.
1. Moussavi S, Chatterji S, Verdes E, et al. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet. 2007;370(9590):851-858.
2. Keller MB. Past, present, and future directions for defining optimal treatment outcome in depression: remission and beyond. JAMA. 2003;289(23):3152-3160.
3. Marcus SC, Olfson M. National trends in the treatment for depression from 1998 to 2007. Arch Gen Psychiatry. 2010;67(12):1265-1273.
4. Chambless DL, Ollendick TH. Empirically supported psychological interventions: controversies and evidence. Annu Rev Psychol. 2001;52:685-716.
5. Oxman TE, Dietrich AJ, Schulberg HC. Evidence-based models of integrated management of depression in primary care. Psychiatr Clin North Am. 2005;28(4):1061-1077.
6. Fournier JC, DeRubeis RJ, Hollon SD, et al. Antidepressant drug effects and depression severity: a patient-level meta-analysis. JAMA. 2010;303(1):47-53.
7. Paykel ES, Hollyman JA, Freeling P, et al. Predictors of therapeutic benefit from amitriptyline in mild depression: a general practice placebo-controlled trial. J Affect Disord. 1988;14(1):83-95
8. Marcus SC, Olfson M. National trends in the treatment for depression from 1998 to 2007. Arch Gen Psychiatry. 2010;67(12):1265-1273.
9. Practice guideline for the treatment of patients with major depressive disorder, 3rd ed. Arlington, VA: American Psychiatric Association; 2010.
10. Zhang B, Ding X, Lu W, et al. Effect of group cognitive-behavioral therapy on the quality of life and social functioning of patients with mild depression. Shanghai Arch Psychiatry. 2016;28(1):18-27.
11. Matsunaga M, Okamoto Y, Suzuki S et.al. Psychosocial functioning in patients with treatment-resistant depression after group cognitive behavioral therapy. BMC Psychiatry. 2010;10:22.
12. American Psychiatric Association. Practice Guideline for Major Depressive Disorder in Adults. Am J Psychiatry. 1993;150(suppl 4):1-26.
13. Elkin I, Shea MT, Watkins JT, et al. National Institute of Mental Health Treatment of Depression Collaborative Research Program. General effectiveness of treatments. Arch Gen Psychiatry. 1989;46(11):971-982; discussion 983.
14. Jacobson NS, Hollon SD. Prospects for future comparisons between drugs and psychotherapy: lessons from the CBT-versus-pharmacotherapy exchange. J Consult Clin Psychol. 1996;64(1):104-108.
15. Jarrett RB, Schaffer M, McIntire D, et al. Treatment of atypical depression with cognitive therapy or phenelzine: a double-blind, placebo-controlled trial. Arch Gen Psychiatry. 1999;56(5):431-437.
16. DeRubeis RJ, Hollon SD, Amsterdam JD, et al. Cognitive therapy vs medications in the treatment of moderate to severe depression. Arch Gen Psychiatry. 2005;62(4):409-416.
17. Amick HR, Gartlehner G, Gaynes BN, et al. Comparative benefits and harms of second generation antidepressants and cognitive behavioral therapies in initial treatment of major depressive disorder: systematic review and meta-analysis. BMJ. 2015;351:h6019. doi: 10.1136/bmj.h6019.
18. Newton-Howes G, Tyrer P, Johnson T. Personality disorder and the outcome of depression: meta-analysis of published studies. Br J Psychiatry. 2006;188(1):13-20.
19. Fournier JC, DeRubeis RJ, Shelton RC, et al. Antidepressant medications v. cognitive therapy in people with depression with or without personality disorder. Br J Psychiatry. 2008;192(2):124-129.
20. Mueller TI, Leon AC, Keller MB, et al. Recurrence after recovery from major depressive disorder during 15 years of observational follow-up. Am J Psychiatry. 1999;156(7):1000-1006.
21. Solomon DA, Keller MB, Leon AC, et al. Multiple recurrences of major depressive disorder. Am J Psychiatry. 2000;157(2):229-233.
22. Frank E, Prien RF, Jarrett RB, et al. Conceptualization and rationale for consensus definitions of terms in major depressive disorder. Remission, recovery, relapse, and recurrence. Arch Gen Psychiatry. 1991;48(9):851-855.
23. Thase ME. Relapse and recurrence of depression: an updated practical approach for prevention. In: Palmer KJ, ed. Drug treatment issues in depression. Auckland, New Zealand: Adis International; 2000:35-52.
24. Evans MD, Hollon, SD, DeRubeis RJ, et al. Differential relapse following cognitive therapy and pharmacotherapy for depression. Arch Gen Psychiatry. 1992;49(10):802-808.
25. Vittengal JR, Clark LA, Dunn TW, et al. Reducing relapse and recurrence in unipolar depression: a comparative meta-analysis of cognitive-behavioral therapy’s effects. J Consult Clin Psychol. 2007;75(3):475-488.
26. Hollon SD, DeRubeis RJ, Shelton RC, et al. Prevention of relapse following cognitive therapy vs medications in moderate to severe depression. Arch Gen Psychiatry. 2005;62(4):417-422.
27. Paykel ES, Scott J, Teasdale JD, et al. Prevention of relapse in residual depression by cognitive therapy: a controlled trial. Arch Gen Psychiatry. 1999;56(9):829-835.
28. Clarke K, Mayo-Wilson E, Kenny J, et al. Can non-pharmacological interventions prevent relapse in adults who have recovered from depression? A systematic review and meta-analysis of randomised controlled trials. Clin Psychol Rev. 2015;39:58-70.
29. Fava GA, Rafanelli C, Grandi, S, et al. Prevention of recurrent depression with cognitive behavioral therapy: preliminary findings. Arch Gen Psychiatry. 1998;55(9):816-820.
30. Fava GA, Ruini C, Rafanelli C, et al. Six-year outcome of cognitive behavior therapy for prevention of recurrent depression. Am J Psychiatry. 2004;161(10):1872-1876.
31. Hollon SD, DeRubeis RJ, Fawcett J, et al. Effect of cognitive therapy with antidepressant medications vs antidepressants alone on the rate of recovery in major depressive disorder: a randomized clinical trial. JAMA Psychiatry. 2014;71(10):1157-1164.
32. Bockting CL, Smid NH, Koeter MW, et al. Enduring effects of preventive cognitive therapy in adults remitted from recurrent depression: a 10 year follow-up of a randomized controlled trial. J Affect Disord. 2015;185:188-194.
33. Paykel ES, Scott J, Cornwall PL, et al. Duration of relapse prevention after cognitive therapy in residual depression: follow-up of controlled trial. Psychol Med. 2005;35(1):59-68.
1. Moussavi S, Chatterji S, Verdes E, et al. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet. 2007;370(9590):851-858.
2. Keller MB. Past, present, and future directions for defining optimal treatment outcome in depression: remission and beyond. JAMA. 2003;289(23):3152-3160.
3. Marcus SC, Olfson M. National trends in the treatment for depression from 1998 to 2007. Arch Gen Psychiatry. 2010;67(12):1265-1273.
4. Chambless DL, Ollendick TH. Empirically supported psychological interventions: controversies and evidence. Annu Rev Psychol. 2001;52:685-716.
5. Oxman TE, Dietrich AJ, Schulberg HC. Evidence-based models of integrated management of depression in primary care. Psychiatr Clin North Am. 2005;28(4):1061-1077.
6. Fournier JC, DeRubeis RJ, Hollon SD, et al. Antidepressant drug effects and depression severity: a patient-level meta-analysis. JAMA. 2010;303(1):47-53.
7. Paykel ES, Hollyman JA, Freeling P, et al. Predictors of therapeutic benefit from amitriptyline in mild depression: a general practice placebo-controlled trial. J Affect Disord. 1988;14(1):83-95
8. Marcus SC, Olfson M. National trends in the treatment for depression from 1998 to 2007. Arch Gen Psychiatry. 2010;67(12):1265-1273.
9. Practice guideline for the treatment of patients with major depressive disorder, 3rd ed. Arlington, VA: American Psychiatric Association; 2010.
10. Zhang B, Ding X, Lu W, et al. Effect of group cognitive-behavioral therapy on the quality of life and social functioning of patients with mild depression. Shanghai Arch Psychiatry. 2016;28(1):18-27.
11. Matsunaga M, Okamoto Y, Suzuki S et.al. Psychosocial functioning in patients with treatment-resistant depression after group cognitive behavioral therapy. BMC Psychiatry. 2010;10:22.
12. American Psychiatric Association. Practice Guideline for Major Depressive Disorder in Adults. Am J Psychiatry. 1993;150(suppl 4):1-26.
13. Elkin I, Shea MT, Watkins JT, et al. National Institute of Mental Health Treatment of Depression Collaborative Research Program. General effectiveness of treatments. Arch Gen Psychiatry. 1989;46(11):971-982; discussion 983.
14. Jacobson NS, Hollon SD. Prospects for future comparisons between drugs and psychotherapy: lessons from the CBT-versus-pharmacotherapy exchange. J Consult Clin Psychol. 1996;64(1):104-108.
15. Jarrett RB, Schaffer M, McIntire D, et al. Treatment of atypical depression with cognitive therapy or phenelzine: a double-blind, placebo-controlled trial. Arch Gen Psychiatry. 1999;56(5):431-437.
16. DeRubeis RJ, Hollon SD, Amsterdam JD, et al. Cognitive therapy vs medications in the treatment of moderate to severe depression. Arch Gen Psychiatry. 2005;62(4):409-416.
17. Amick HR, Gartlehner G, Gaynes BN, et al. Comparative benefits and harms of second generation antidepressants and cognitive behavioral therapies in initial treatment of major depressive disorder: systematic review and meta-analysis. BMJ. 2015;351:h6019. doi: 10.1136/bmj.h6019.
18. Newton-Howes G, Tyrer P, Johnson T. Personality disorder and the outcome of depression: meta-analysis of published studies. Br J Psychiatry. 2006;188(1):13-20.
19. Fournier JC, DeRubeis RJ, Shelton RC, et al. Antidepressant medications v. cognitive therapy in people with depression with or without personality disorder. Br J Psychiatry. 2008;192(2):124-129.
20. Mueller TI, Leon AC, Keller MB, et al. Recurrence after recovery from major depressive disorder during 15 years of observational follow-up. Am J Psychiatry. 1999;156(7):1000-1006.
21. Solomon DA, Keller MB, Leon AC, et al. Multiple recurrences of major depressive disorder. Am J Psychiatry. 2000;157(2):229-233.
22. Frank E, Prien RF, Jarrett RB, et al. Conceptualization and rationale for consensus definitions of terms in major depressive disorder. Remission, recovery, relapse, and recurrence. Arch Gen Psychiatry. 1991;48(9):851-855.
23. Thase ME. Relapse and recurrence of depression: an updated practical approach for prevention. In: Palmer KJ, ed. Drug treatment issues in depression. Auckland, New Zealand: Adis International; 2000:35-52.
24. Evans MD, Hollon, SD, DeRubeis RJ, et al. Differential relapse following cognitive therapy and pharmacotherapy for depression. Arch Gen Psychiatry. 1992;49(10):802-808.
25. Vittengal JR, Clark LA, Dunn TW, et al. Reducing relapse and recurrence in unipolar depression: a comparative meta-analysis of cognitive-behavioral therapy’s effects. J Consult Clin Psychol. 2007;75(3):475-488.
26. Hollon SD, DeRubeis RJ, Shelton RC, et al. Prevention of relapse following cognitive therapy vs medications in moderate to severe depression. Arch Gen Psychiatry. 2005;62(4):417-422.
27. Paykel ES, Scott J, Teasdale JD, et al. Prevention of relapse in residual depression by cognitive therapy: a controlled trial. Arch Gen Psychiatry. 1999;56(9):829-835.
28. Clarke K, Mayo-Wilson E, Kenny J, et al. Can non-pharmacological interventions prevent relapse in adults who have recovered from depression? A systematic review and meta-analysis of randomised controlled trials. Clin Psychol Rev. 2015;39:58-70.
29. Fava GA, Rafanelli C, Grandi, S, et al. Prevention of recurrent depression with cognitive behavioral therapy: preliminary findings. Arch Gen Psychiatry. 1998;55(9):816-820.
30. Fava GA, Ruini C, Rafanelli C, et al. Six-year outcome of cognitive behavior therapy for prevention of recurrent depression. Am J Psychiatry. 2004;161(10):1872-1876.
31. Hollon SD, DeRubeis RJ, Fawcett J, et al. Effect of cognitive therapy with antidepressant medications vs antidepressants alone on the rate of recovery in major depressive disorder: a randomized clinical trial. JAMA Psychiatry. 2014;71(10):1157-1164.
32. Bockting CL, Smid NH, Koeter MW, et al. Enduring effects of preventive cognitive therapy in adults remitted from recurrent depression: a 10 year follow-up of a randomized controlled trial. J Affect Disord. 2015;185:188-194.
33. Paykel ES, Scott J, Cornwall PL, et al. Duration of relapse prevention after cognitive therapy in residual depression: follow-up of controlled trial. Psychol Med. 2005;35(1):59-68.
Providing culturally competent postpartum care for South Asian women
As do women from a wide range of cultures, South Asian (SA) women frequently report feelings of shame associated with receiving a psychiatric diagnosis during the postpartum period because they fear it may reflect poorly on their ability to be good mothers or negatively impact their family’s
Be aware of psychosomatic presentations. SA mothers who develop postpartum psychiatric symptoms might not present with complaints of dysphoria, crying, low energy, or suicidal thoughts. They may instead describe psychosomatic symptoms such as headaches and body pains.
Consider their hesitation to use psychiatric terms. SA women may not be comfortable using psychiatric terms such as depression or anxiety. Instead, they might respond more positively when their preferred descriptive terms (ie, sadness, worry, stress) are used by the clinicians who treat them.
Engage the partner and/or family. SA women may emphasize that they are part of a family unit, rather than regarding themselves as individuals. Thus, including family members in the treatment plan may help improve adherence.
Screen for suicide risk. Evidence suggests that young SA women have a higher rate of suicide and suicide attempts than young SA men or non-SA women.1,2 Further, they may be less willing to speak openly about it.
Ask questions about cultural or traditional forms of treatment. SA mothers, particularly those who are breastfeeding, might be wary of Western medicine and may be familiar with traditional Indian medicine practices such as herbal, homeopathic, or Ayurvedic approaches.3 These interventions may include a specified diet, use of herbal treatments, exercise, and lifestyle recommendations.When taking the patient’s history, find out which treatments she is currently using, and discuss whether she can safely continue to use them.
Do not mistake poor eye contact for lack of engagement. Because SA patients may view a physician as a source of authority, they might regard direct eye contact with a physician as being somewhat disrespectful, and they may avoid eye contact altogether.
Continue to: Maintain an active approach
Maintain an active approach. SA women may prefer to view the physician as an expert, rather than a partner with whom to develop a collaborative relationship. Thus, they may feel more comfortable with direct feedback rather than a passive or reflective approach.
Suggest a postpartum support group. In a U.K. study of 17 SA postpartum women, age 20 to 45, group therapy improved health outcomes and overall satisfaction.4 It may be particularly helpful to SA patients if group therapy is facilitated by a culturally sensitive moderator.
Help patients overcome logistical barriers. Lack of transportation, childcare difficulties, and financial limitations are common deterrents to treatment. These barriers may be particularly challenging for SA women of lower socioeconomic status. Postpartum mothers who feel overtasked with caring for their children and undertaking household duties may feel less able to complete therapy.
Screen for adherence. Although SA patients may view clinicians as authority figures, adherence with medications or treatment plans should not be assumed. Many patients may quietly avoid treatments or recommendations instead of discussing their ambivalence with their clinicians.
1. Anand AS, Cochrane R. The mental health status of South Asian women in Britain. A review of the UK literature. Psychol Dev Soc J. 2005;17(2):195-214.
2. Bhugra D, Desai M. Attempted suicide in South Asian women. Advances in Psychiatric Treatment. 2002;8(6):418-423.
3. Chopra A, Doiphode VV. Ayurvedic medicine. Core concept, therapeutic principles, and current relevance. Med Clin North Am. 2002;86(1):75-89,vii.
4. Masood Y, Lovell K, Lunat F, et al. Group psychological intervention for postnatal depression: a nested qualitative study with British South Asian women. BMC Womens Health. 2015;25(15):109.
As do women from a wide range of cultures, South Asian (SA) women frequently report feelings of shame associated with receiving a psychiatric diagnosis during the postpartum period because they fear it may reflect poorly on their ability to be good mothers or negatively impact their family’s
Be aware of psychosomatic presentations. SA mothers who develop postpartum psychiatric symptoms might not present with complaints of dysphoria, crying, low energy, or suicidal thoughts. They may instead describe psychosomatic symptoms such as headaches and body pains.
Consider their hesitation to use psychiatric terms. SA women may not be comfortable using psychiatric terms such as depression or anxiety. Instead, they might respond more positively when their preferred descriptive terms (ie, sadness, worry, stress) are used by the clinicians who treat them.
Engage the partner and/or family. SA women may emphasize that they are part of a family unit, rather than regarding themselves as individuals. Thus, including family members in the treatment plan may help improve adherence.
Screen for suicide risk. Evidence suggests that young SA women have a higher rate of suicide and suicide attempts than young SA men or non-SA women.1,2 Further, they may be less willing to speak openly about it.
Ask questions about cultural or traditional forms of treatment. SA mothers, particularly those who are breastfeeding, might be wary of Western medicine and may be familiar with traditional Indian medicine practices such as herbal, homeopathic, or Ayurvedic approaches.3 These interventions may include a specified diet, use of herbal treatments, exercise, and lifestyle recommendations.When taking the patient’s history, find out which treatments she is currently using, and discuss whether she can safely continue to use them.
Do not mistake poor eye contact for lack of engagement. Because SA patients may view a physician as a source of authority, they might regard direct eye contact with a physician as being somewhat disrespectful, and they may avoid eye contact altogether.
Continue to: Maintain an active approach
Maintain an active approach. SA women may prefer to view the physician as an expert, rather than a partner with whom to develop a collaborative relationship. Thus, they may feel more comfortable with direct feedback rather than a passive or reflective approach.
Suggest a postpartum support group. In a U.K. study of 17 SA postpartum women, age 20 to 45, group therapy improved health outcomes and overall satisfaction.4 It may be particularly helpful to SA patients if group therapy is facilitated by a culturally sensitive moderator.
Help patients overcome logistical barriers. Lack of transportation, childcare difficulties, and financial limitations are common deterrents to treatment. These barriers may be particularly challenging for SA women of lower socioeconomic status. Postpartum mothers who feel overtasked with caring for their children and undertaking household duties may feel less able to complete therapy.
Screen for adherence. Although SA patients may view clinicians as authority figures, adherence with medications or treatment plans should not be assumed. Many patients may quietly avoid treatments or recommendations instead of discussing their ambivalence with their clinicians.
As do women from a wide range of cultures, South Asian (SA) women frequently report feelings of shame associated with receiving a psychiatric diagnosis during the postpartum period because they fear it may reflect poorly on their ability to be good mothers or negatively impact their family’s
Be aware of psychosomatic presentations. SA mothers who develop postpartum psychiatric symptoms might not present with complaints of dysphoria, crying, low energy, or suicidal thoughts. They may instead describe psychosomatic symptoms such as headaches and body pains.
Consider their hesitation to use psychiatric terms. SA women may not be comfortable using psychiatric terms such as depression or anxiety. Instead, they might respond more positively when their preferred descriptive terms (ie, sadness, worry, stress) are used by the clinicians who treat them.
Engage the partner and/or family. SA women may emphasize that they are part of a family unit, rather than regarding themselves as individuals. Thus, including family members in the treatment plan may help improve adherence.
Screen for suicide risk. Evidence suggests that young SA women have a higher rate of suicide and suicide attempts than young SA men or non-SA women.1,2 Further, they may be less willing to speak openly about it.
Ask questions about cultural or traditional forms of treatment. SA mothers, particularly those who are breastfeeding, might be wary of Western medicine and may be familiar with traditional Indian medicine practices such as herbal, homeopathic, or Ayurvedic approaches.3 These interventions may include a specified diet, use of herbal treatments, exercise, and lifestyle recommendations.When taking the patient’s history, find out which treatments she is currently using, and discuss whether she can safely continue to use them.
Do not mistake poor eye contact for lack of engagement. Because SA patients may view a physician as a source of authority, they might regard direct eye contact with a physician as being somewhat disrespectful, and they may avoid eye contact altogether.
Continue to: Maintain an active approach
Maintain an active approach. SA women may prefer to view the physician as an expert, rather than a partner with whom to develop a collaborative relationship. Thus, they may feel more comfortable with direct feedback rather than a passive or reflective approach.
Suggest a postpartum support group. In a U.K. study of 17 SA postpartum women, age 20 to 45, group therapy improved health outcomes and overall satisfaction.4 It may be particularly helpful to SA patients if group therapy is facilitated by a culturally sensitive moderator.
Help patients overcome logistical barriers. Lack of transportation, childcare difficulties, and financial limitations are common deterrents to treatment. These barriers may be particularly challenging for SA women of lower socioeconomic status. Postpartum mothers who feel overtasked with caring for their children and undertaking household duties may feel less able to complete therapy.
Screen for adherence. Although SA patients may view clinicians as authority figures, adherence with medications or treatment plans should not be assumed. Many patients may quietly avoid treatments or recommendations instead of discussing their ambivalence with their clinicians.
1. Anand AS, Cochrane R. The mental health status of South Asian women in Britain. A review of the UK literature. Psychol Dev Soc J. 2005;17(2):195-214.
2. Bhugra D, Desai M. Attempted suicide in South Asian women. Advances in Psychiatric Treatment. 2002;8(6):418-423.
3. Chopra A, Doiphode VV. Ayurvedic medicine. Core concept, therapeutic principles, and current relevance. Med Clin North Am. 2002;86(1):75-89,vii.
4. Masood Y, Lovell K, Lunat F, et al. Group psychological intervention for postnatal depression: a nested qualitative study with British South Asian women. BMC Womens Health. 2015;25(15):109.
1. Anand AS, Cochrane R. The mental health status of South Asian women in Britain. A review of the UK literature. Psychol Dev Soc J. 2005;17(2):195-214.
2. Bhugra D, Desai M. Attempted suicide in South Asian women. Advances in Psychiatric Treatment. 2002;8(6):418-423.
3. Chopra A, Doiphode VV. Ayurvedic medicine. Core concept, therapeutic principles, and current relevance. Med Clin North Am. 2002;86(1):75-89,vii.
4. Masood Y, Lovell K, Lunat F, et al. Group psychological intervention for postnatal depression: a nested qualitative study with British South Asian women. BMC Womens Health. 2015;25(15):109.