User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Providing culturally competent postpartum care for South Asian women
As do women from a wide range of cultures, South Asian (SA) women frequently report feelings of shame associated with receiving a psychiatric diagnosis during the postpartum period because they fear it may reflect poorly on their ability to be good mothers or negatively impact their family’s
Be aware of psychosomatic presentations. SA mothers who develop postpartum psychiatric symptoms might not present with complaints of dysphoria, crying, low energy, or suicidal thoughts. They may instead describe psychosomatic symptoms such as headaches and body pains.
Consider their hesitation to use psychiatric terms. SA women may not be comfortable using psychiatric terms such as depression or anxiety. Instead, they might respond more positively when their preferred descriptive terms (ie, sadness, worry, stress) are used by the clinicians who treat them.
Engage the partner and/or family. SA women may emphasize that they are part of a family unit, rather than regarding themselves as individuals. Thus, including family members in the treatment plan may help improve adherence.
Screen for suicide risk. Evidence suggests that young SA women have a higher rate of suicide and suicide attempts than young SA men or non-SA women.1,2 Further, they may be less willing to speak openly about it.
Ask questions about cultural or traditional forms of treatment. SA mothers, particularly those who are breastfeeding, might be wary of Western medicine and may be familiar with traditional Indian medicine practices such as herbal, homeopathic, or Ayurvedic approaches.3 These interventions may include a specified diet, use of herbal treatments, exercise, and lifestyle recommendations.When taking the patient’s history, find out which treatments she is currently using, and discuss whether she can safely continue to use them.
Do not mistake poor eye contact for lack of engagement. Because SA patients may view a physician as a source of authority, they might regard direct eye contact with a physician as being somewhat disrespectful, and they may avoid eye contact altogether.
Continue to: Maintain an active approach
Maintain an active approach. SA women may prefer to view the physician as an expert, rather than a partner with whom to develop a collaborative relationship. Thus, they may feel more comfortable with direct feedback rather than a passive or reflective approach.
Suggest a postpartum support group. In a U.K. study of 17 SA postpartum women, age 20 to 45, group therapy improved health outcomes and overall satisfaction.4 It may be particularly helpful to SA patients if group therapy is facilitated by a culturally sensitive moderator.
Help patients overcome logistical barriers. Lack of transportation, childcare difficulties, and financial limitations are common deterrents to treatment. These barriers may be particularly challenging for SA women of lower socioeconomic status. Postpartum mothers who feel overtasked with caring for their children and undertaking household duties may feel less able to complete therapy.
Screen for adherence. Although SA patients may view clinicians as authority figures, adherence with medications or treatment plans should not be assumed. Many patients may quietly avoid treatments or recommendations instead of discussing their ambivalence with their clinicians.
1. Anand AS, Cochrane R. The mental health status of South Asian women in Britain. A review of the UK literature. Psychol Dev Soc J. 2005;17(2):195-214.
2. Bhugra D, Desai M. Attempted suicide in South Asian women. Advances in Psychiatric Treatment. 2002;8(6):418-423.
3. Chopra A, Doiphode VV. Ayurvedic medicine. Core concept, therapeutic principles, and current relevance. Med Clin North Am. 2002;86(1):75-89,vii.
4. Masood Y, Lovell K, Lunat F, et al. Group psychological intervention for postnatal depression: a nested qualitative study with British South Asian women. BMC Womens Health. 2015;25(15):109.
As do women from a wide range of cultures, South Asian (SA) women frequently report feelings of shame associated with receiving a psychiatric diagnosis during the postpartum period because they fear it may reflect poorly on their ability to be good mothers or negatively impact their family’s
Be aware of psychosomatic presentations. SA mothers who develop postpartum psychiatric symptoms might not present with complaints of dysphoria, crying, low energy, or suicidal thoughts. They may instead describe psychosomatic symptoms such as headaches and body pains.
Consider their hesitation to use psychiatric terms. SA women may not be comfortable using psychiatric terms such as depression or anxiety. Instead, they might respond more positively when their preferred descriptive terms (ie, sadness, worry, stress) are used by the clinicians who treat them.
Engage the partner and/or family. SA women may emphasize that they are part of a family unit, rather than regarding themselves as individuals. Thus, including family members in the treatment plan may help improve adherence.
Screen for suicide risk. Evidence suggests that young SA women have a higher rate of suicide and suicide attempts than young SA men or non-SA women.1,2 Further, they may be less willing to speak openly about it.
Ask questions about cultural or traditional forms of treatment. SA mothers, particularly those who are breastfeeding, might be wary of Western medicine and may be familiar with traditional Indian medicine practices such as herbal, homeopathic, or Ayurvedic approaches.3 These interventions may include a specified diet, use of herbal treatments, exercise, and lifestyle recommendations.When taking the patient’s history, find out which treatments she is currently using, and discuss whether she can safely continue to use them.
Do not mistake poor eye contact for lack of engagement. Because SA patients may view a physician as a source of authority, they might regard direct eye contact with a physician as being somewhat disrespectful, and they may avoid eye contact altogether.
Continue to: Maintain an active approach
Maintain an active approach. SA women may prefer to view the physician as an expert, rather than a partner with whom to develop a collaborative relationship. Thus, they may feel more comfortable with direct feedback rather than a passive or reflective approach.
Suggest a postpartum support group. In a U.K. study of 17 SA postpartum women, age 20 to 45, group therapy improved health outcomes and overall satisfaction.4 It may be particularly helpful to SA patients if group therapy is facilitated by a culturally sensitive moderator.
Help patients overcome logistical barriers. Lack of transportation, childcare difficulties, and financial limitations are common deterrents to treatment. These barriers may be particularly challenging for SA women of lower socioeconomic status. Postpartum mothers who feel overtasked with caring for their children and undertaking household duties may feel less able to complete therapy.
Screen for adherence. Although SA patients may view clinicians as authority figures, adherence with medications or treatment plans should not be assumed. Many patients may quietly avoid treatments or recommendations instead of discussing their ambivalence with their clinicians.
As do women from a wide range of cultures, South Asian (SA) women frequently report feelings of shame associated with receiving a psychiatric diagnosis during the postpartum period because they fear it may reflect poorly on their ability to be good mothers or negatively impact their family’s
Be aware of psychosomatic presentations. SA mothers who develop postpartum psychiatric symptoms might not present with complaints of dysphoria, crying, low energy, or suicidal thoughts. They may instead describe psychosomatic symptoms such as headaches and body pains.
Consider their hesitation to use psychiatric terms. SA women may not be comfortable using psychiatric terms such as depression or anxiety. Instead, they might respond more positively when their preferred descriptive terms (ie, sadness, worry, stress) are used by the clinicians who treat them.
Engage the partner and/or family. SA women may emphasize that they are part of a family unit, rather than regarding themselves as individuals. Thus, including family members in the treatment plan may help improve adherence.
Screen for suicide risk. Evidence suggests that young SA women have a higher rate of suicide and suicide attempts than young SA men or non-SA women.1,2 Further, they may be less willing to speak openly about it.
Ask questions about cultural or traditional forms of treatment. SA mothers, particularly those who are breastfeeding, might be wary of Western medicine and may be familiar with traditional Indian medicine practices such as herbal, homeopathic, or Ayurvedic approaches.3 These interventions may include a specified diet, use of herbal treatments, exercise, and lifestyle recommendations.When taking the patient’s history, find out which treatments she is currently using, and discuss whether she can safely continue to use them.
Do not mistake poor eye contact for lack of engagement. Because SA patients may view a physician as a source of authority, they might regard direct eye contact with a physician as being somewhat disrespectful, and they may avoid eye contact altogether.
Continue to: Maintain an active approach
Maintain an active approach. SA women may prefer to view the physician as an expert, rather than a partner with whom to develop a collaborative relationship. Thus, they may feel more comfortable with direct feedback rather than a passive or reflective approach.
Suggest a postpartum support group. In a U.K. study of 17 SA postpartum women, age 20 to 45, group therapy improved health outcomes and overall satisfaction.4 It may be particularly helpful to SA patients if group therapy is facilitated by a culturally sensitive moderator.
Help patients overcome logistical barriers. Lack of transportation, childcare difficulties, and financial limitations are common deterrents to treatment. These barriers may be particularly challenging for SA women of lower socioeconomic status. Postpartum mothers who feel overtasked with caring for their children and undertaking household duties may feel less able to complete therapy.
Screen for adherence. Although SA patients may view clinicians as authority figures, adherence with medications or treatment plans should not be assumed. Many patients may quietly avoid treatments or recommendations instead of discussing their ambivalence with their clinicians.
1. Anand AS, Cochrane R. The mental health status of South Asian women in Britain. A review of the UK literature. Psychol Dev Soc J. 2005;17(2):195-214.
2. Bhugra D, Desai M. Attempted suicide in South Asian women. Advances in Psychiatric Treatment. 2002;8(6):418-423.
3. Chopra A, Doiphode VV. Ayurvedic medicine. Core concept, therapeutic principles, and current relevance. Med Clin North Am. 2002;86(1):75-89,vii.
4. Masood Y, Lovell K, Lunat F, et al. Group psychological intervention for postnatal depression: a nested qualitative study with British South Asian women. BMC Womens Health. 2015;25(15):109.
1. Anand AS, Cochrane R. The mental health status of South Asian women in Britain. A review of the UK literature. Psychol Dev Soc J. 2005;17(2):195-214.
2. Bhugra D, Desai M. Attempted suicide in South Asian women. Advances in Psychiatric Treatment. 2002;8(6):418-423.
3. Chopra A, Doiphode VV. Ayurvedic medicine. Core concept, therapeutic principles, and current relevance. Med Clin North Am. 2002;86(1):75-89,vii.
4. Masood Y, Lovell K, Lunat F, et al. Group psychological intervention for postnatal depression: a nested qualitative study with British South Asian women. BMC Womens Health. 2015;25(15):109.
Paliperidone palmitate: Adjusting dosing intervals and measuring serum concentrations
Mr. B, age 27, has a 10-year history of schizophrenia. Last year, he was doing well and working 4 hours/day 3 days/week while taking oral risperidone, 6 mg, at bedtime. However, during the past 2 weeks Mr. B began to have a return of auditory hallucinations and reports that he stopped taking his medication again 6 weeks ago.
As a result, he is started on paliperidone palmitate following the product label’s initiation dosing recommendation. On the first day he is given the first dose of the initiation regimen, 234 mg IM. One week later, the second dose of the initiation regimen, 156 mg IM, is given. One month later, the first maintenance dose of 117 mg IM every 28 days is given. All injections are in his deltoid muscle at his request.
After 3 weeks on the first maintenance dose of 117 mg, the voices begin to bother him again. Subsequently, Mr. B’s maintenance dose is increased first to 156 mg, and for the same problem with breakthrough hallucinations the following month to 234 mg, the maximum dose in the product label. After 6 months of receiving 234 mg IM every 28 days, the auditory hallucinations continue to bother him, but only for a few days prior to his next injection. He misses work 1 or 2 times before each injection.
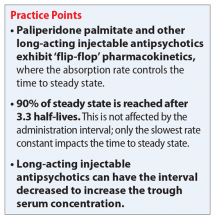
Can the injection frequency for Mr. B’s paliperidone palmitate, 234 mg IM, in the deltoid muscle be increased to every 21 days to prevent the monthly exacerbations? Yes, the injection frequency can be increased, and doing so will increase the concentrations of paliperidone. The use of long-acting injectable antipsychotics (LAIs) is complicated by the lengthy time needed to reach steady state. In the case of paliperidone palmitate, an initiation regimen was developed that achieves therapeutic concentrations that are close to steady state before the oral antipsychotic’s effects are lost. This initiation strategy avoids the need for oral supplementation to maintain clinical efficacy. However, even using an initiation regimen or a loading dose does not decrease the time to final steady state after a dose adjustment due to the slow absorption of the medication from the injection site. The time to steady state is controlled by “flip-flop” pharmacokinetics. In this kind of pharmacokinetics, which is observed with all LAIs, the absorption rate from the injection site is lower than the elimination rate.1
Cleton et al2 reported the pharmacokinetics of paliperidone palmitate for deltoid and gluteal injection sites. By combining the median data for the deltoid injection route from the article by Cleton et al2 and the dosing from Mr. B’s case, I created a model using superposition of a sixth-degree polynomial fitted to the single dose data. Gluteal injections were not included because their increased complexity is beyond the scope of this article, but the time to maximum concentration (gluteal > deltoid) and peak concentration (deltoid > gluteal) are different for each route. The polynomial was a good fit with the adjusted r2 = 0.976, P < .0001. This model illustrates the paliperidone serum concentrations for Mr. B and is shown in the Figure. As you can see, by Day 9, the serum concentrations had reached the lower limit of the expected range of 20 to 60 ng/mL, shown in the shaded region of the Figure.3
Steady state at the routine maintenance dose of 117 mg every 28 days was never reached as the medication was not sufficient to suppress Mr. B’s hallucinations, and his doses needed to be increased each month. First, Mr. B’s dose was increased to 156 mg and then to the maximum recommended dose of 234 mg every 28 days. Steady state can be considered to have been achieved when 90% of the final steady state is reached after 3.3 half-lives. Because of the flip-flop pharmacokinetics, the important half-life is the absorption half-life of approximately 40 days or 132 days at the same dose. In Mr. B’s case, this was Day 221, where the trough concentration was 35 ng/mL. However, this regimen was still inadequate because he had breakthrough symptoms prior to the next injection.
By decreasing the injection interval from 28 days to 21 days, the concentrations will increase to a new steady state. This will take the same 132 days. With the reduced injection frequency of 21 days, 7 injections will have been given prior to reaching the new steady state. Steady state is not dependent on the number of injections, but only on the absorption half-life. This new steady state trough is substantially higher at 52 ng/mL, but still in the expected range for commonly used doses. Because Mr. B’s hallucinations only appeared at the end of the dosing interval, it is reasonable to expect that his new regimen would be successful in suppressing his hallucinations. However, monitoring for peak-related adverse effects is essential. Based upon controlled clinical trials, the potential dose-related adverse effects of paliperidone include akathisia, other extrapyramidal symptoms, weight gain, and QTc prolongation.
Continue to: Would monitoring a patient's paliperidone serum concentrations be useful?
Would monitoring a patient’s paliperidone serum concentrations be useful? Currently, measuring an individual’s paliperidone serum concentration is generally considered unwarranted.3,4 One of the major reasons is a lack of appropriately designed studies to determine a therapeutic range.5 Flexible dose designs, commonly used in registration studies, cloud the relationships between concentration, time, response, and adverse effects. There are additional problems that are the result of diagnostic heterogeneity and placebo responders. A well-designed study to determine the therapeutic range would have ≥1 fixed dose groups and be diagnostically homogeneous. There are currently only a limited number of clinical laboratories that have implemented suitable assays.
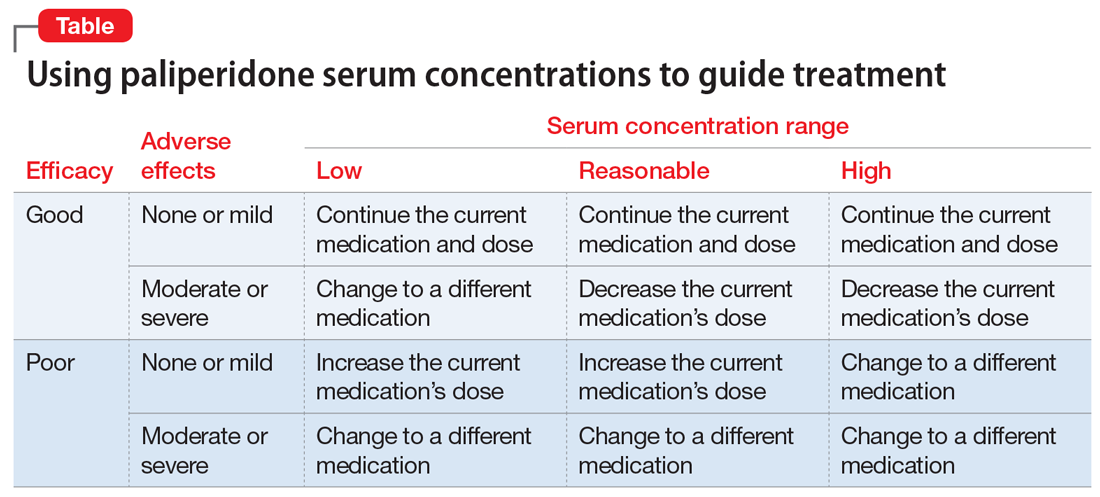
Given the lack of knowledge of a therapeutic range, assured knowledge of nonadherence to LAIs, and the absence of significant drug interactions for paliperidone, there remain a few reasonable justifications for obtaining a patient’s paliperidone serum concentration (Table). If the patient had a good response with mild adverse effects, there is no reason to obtain a paliperidone serum concentration or make any change in the medication or dose. However, if the patient had a good response accompanied by moderate or severe adverse effects, or the patient has a poor response, then obtaining the paliperidone serum concentration could help determine an appropriate course of action.
CASE CONTINUED
After the second dose at the increased frequency on Day 252, the paliperidone serum concentration was maintained above 40 ng/mL. Mr. B continued to tolerate the LAI well and no longer reported any breakthrough hallucinations.
Related Resources
- ARUP Laboratories. Paliperidone, serum or plasma. http://ltd.aruplab.com/tests/pub/2007949.
- LabCorp. Paliperidone Paliperidone (as 9-hydroxyrisperidone), serum or plasma. https://www.labcorp.com/test-menu/38351/paliperidone-as-9-hydroxyrisperidone-serum-or-plasma.
- Janssen Scientific Affairs. Educational dose illustrator. http://www.educationaldoseillustrator.com.
Drug Brand Names
Paliperidone palmitate • Invega Sustenna
Risperidone • Risperdal
1. Jann MW, Ereshefsky L, Saklad SR. Clinical pharmacokinetics of the depot antipsychotics. Clin Pharmacokinet. 1985;10(4):315-333.
2. Cleton A, Rossenu S, Crauwels H, et al. A single-dose, open-label, parallel, randomized, dose-proportionality study of paliperidone after intramuscular injections of paliperidone palmitate in the deltoid or gluteal muscle in patients with schizophrenia. J Clin Pharmacol. 2014;54(9):1048-1057.
3. Taylor D, Paton C, Kapur S. The Maudsley prescribing guidelines in psychiatry. 12th ed. Oxford, UK: John Wiley & Sons, Ltd.; 2015:1-10.
4. Hiemke C, Baumann P, Bergemann N, et al. AGNP consensus guidelines for therapeutic drug monitoring in psychiatry: update 2011. Pharmacopsychiatry. 2011;44(6):195-235.
5. Lopez LV, Kane JM. Plasma levels of second-generation antipsychotics and clinical response in acute psychosis: a review of the literature. Schizophr Res. 2013;147(2-3):368-374.
Mr. B, age 27, has a 10-year history of schizophrenia. Last year, he was doing well and working 4 hours/day 3 days/week while taking oral risperidone, 6 mg, at bedtime. However, during the past 2 weeks Mr. B began to have a return of auditory hallucinations and reports that he stopped taking his medication again 6 weeks ago.
As a result, he is started on paliperidone palmitate following the product label’s initiation dosing recommendation. On the first day he is given the first dose of the initiation regimen, 234 mg IM. One week later, the second dose of the initiation regimen, 156 mg IM, is given. One month later, the first maintenance dose of 117 mg IM every 28 days is given. All injections are in his deltoid muscle at his request.
After 3 weeks on the first maintenance dose of 117 mg, the voices begin to bother him again. Subsequently, Mr. B’s maintenance dose is increased first to 156 mg, and for the same problem with breakthrough hallucinations the following month to 234 mg, the maximum dose in the product label. After 6 months of receiving 234 mg IM every 28 days, the auditory hallucinations continue to bother him, but only for a few days prior to his next injection. He misses work 1 or 2 times before each injection.
Can the injection frequency for Mr. B’s paliperidone palmitate, 234 mg IM, in the deltoid muscle be increased to every 21 days to prevent the monthly exacerbations? Yes, the injection frequency can be increased, and doing so will increase the concentrations of paliperidone. The use of long-acting injectable antipsychotics (LAIs) is complicated by the lengthy time needed to reach steady state. In the case of paliperidone palmitate, an initiation regimen was developed that achieves therapeutic concentrations that are close to steady state before the oral antipsychotic’s effects are lost. This initiation strategy avoids the need for oral supplementation to maintain clinical efficacy. However, even using an initiation regimen or a loading dose does not decrease the time to final steady state after a dose adjustment due to the slow absorption of the medication from the injection site. The time to steady state is controlled by “flip-flop” pharmacokinetics. In this kind of pharmacokinetics, which is observed with all LAIs, the absorption rate from the injection site is lower than the elimination rate.1
Cleton et al2 reported the pharmacokinetics of paliperidone palmitate for deltoid and gluteal injection sites. By combining the median data for the deltoid injection route from the article by Cleton et al2 and the dosing from Mr. B’s case, I created a model using superposition of a sixth-degree polynomial fitted to the single dose data. Gluteal injections were not included because their increased complexity is beyond the scope of this article, but the time to maximum concentration (gluteal > deltoid) and peak concentration (deltoid > gluteal) are different for each route. The polynomial was a good fit with the adjusted r2 = 0.976, P < .0001. This model illustrates the paliperidone serum concentrations for Mr. B and is shown in the Figure. As you can see, by Day 9, the serum concentrations had reached the lower limit of the expected range of 20 to 60 ng/mL, shown in the shaded region of the Figure.3
Steady state at the routine maintenance dose of 117 mg every 28 days was never reached as the medication was not sufficient to suppress Mr. B’s hallucinations, and his doses needed to be increased each month. First, Mr. B’s dose was increased to 156 mg and then to the maximum recommended dose of 234 mg every 28 days. Steady state can be considered to have been achieved when 90% of the final steady state is reached after 3.3 half-lives. Because of the flip-flop pharmacokinetics, the important half-life is the absorption half-life of approximately 40 days or 132 days at the same dose. In Mr. B’s case, this was Day 221, where the trough concentration was 35 ng/mL. However, this regimen was still inadequate because he had breakthrough symptoms prior to the next injection.
By decreasing the injection interval from 28 days to 21 days, the concentrations will increase to a new steady state. This will take the same 132 days. With the reduced injection frequency of 21 days, 7 injections will have been given prior to reaching the new steady state. Steady state is not dependent on the number of injections, but only on the absorption half-life. This new steady state trough is substantially higher at 52 ng/mL, but still in the expected range for commonly used doses. Because Mr. B’s hallucinations only appeared at the end of the dosing interval, it is reasonable to expect that his new regimen would be successful in suppressing his hallucinations. However, monitoring for peak-related adverse effects is essential. Based upon controlled clinical trials, the potential dose-related adverse effects of paliperidone include akathisia, other extrapyramidal symptoms, weight gain, and QTc prolongation.
Continue to: Would monitoring a patient's paliperidone serum concentrations be useful?
Would monitoring a patient’s paliperidone serum concentrations be useful? Currently, measuring an individual’s paliperidone serum concentration is generally considered unwarranted.3,4 One of the major reasons is a lack of appropriately designed studies to determine a therapeutic range.5 Flexible dose designs, commonly used in registration studies, cloud the relationships between concentration, time, response, and adverse effects. There are additional problems that are the result of diagnostic heterogeneity and placebo responders. A well-designed study to determine the therapeutic range would have ≥1 fixed dose groups and be diagnostically homogeneous. There are currently only a limited number of clinical laboratories that have implemented suitable assays.
Given the lack of knowledge of a therapeutic range, assured knowledge of nonadherence to LAIs, and the absence of significant drug interactions for paliperidone, there remain a few reasonable justifications for obtaining a patient’s paliperidone serum concentration (Table). If the patient had a good response with mild adverse effects, there is no reason to obtain a paliperidone serum concentration or make any change in the medication or dose. However, if the patient had a good response accompanied by moderate or severe adverse effects, or the patient has a poor response, then obtaining the paliperidone serum concentration could help determine an appropriate course of action.
CASE CONTINUED
After the second dose at the increased frequency on Day 252, the paliperidone serum concentration was maintained above 40 ng/mL. Mr. B continued to tolerate the LAI well and no longer reported any breakthrough hallucinations.
Related Resources
- ARUP Laboratories. Paliperidone, serum or plasma. http://ltd.aruplab.com/tests/pub/2007949.
- LabCorp. Paliperidone Paliperidone (as 9-hydroxyrisperidone), serum or plasma. https://www.labcorp.com/test-menu/38351/paliperidone-as-9-hydroxyrisperidone-serum-or-plasma.
- Janssen Scientific Affairs. Educational dose illustrator. http://www.educationaldoseillustrator.com.
Drug Brand Names
Paliperidone palmitate • Invega Sustenna
Risperidone • Risperdal
Mr. B, age 27, has a 10-year history of schizophrenia. Last year, he was doing well and working 4 hours/day 3 days/week while taking oral risperidone, 6 mg, at bedtime. However, during the past 2 weeks Mr. B began to have a return of auditory hallucinations and reports that he stopped taking his medication again 6 weeks ago.
As a result, he is started on paliperidone palmitate following the product label’s initiation dosing recommendation. On the first day he is given the first dose of the initiation regimen, 234 mg IM. One week later, the second dose of the initiation regimen, 156 mg IM, is given. One month later, the first maintenance dose of 117 mg IM every 28 days is given. All injections are in his deltoid muscle at his request.
After 3 weeks on the first maintenance dose of 117 mg, the voices begin to bother him again. Subsequently, Mr. B’s maintenance dose is increased first to 156 mg, and for the same problem with breakthrough hallucinations the following month to 234 mg, the maximum dose in the product label. After 6 months of receiving 234 mg IM every 28 days, the auditory hallucinations continue to bother him, but only for a few days prior to his next injection. He misses work 1 or 2 times before each injection.
Can the injection frequency for Mr. B’s paliperidone palmitate, 234 mg IM, in the deltoid muscle be increased to every 21 days to prevent the monthly exacerbations? Yes, the injection frequency can be increased, and doing so will increase the concentrations of paliperidone. The use of long-acting injectable antipsychotics (LAIs) is complicated by the lengthy time needed to reach steady state. In the case of paliperidone palmitate, an initiation regimen was developed that achieves therapeutic concentrations that are close to steady state before the oral antipsychotic’s effects are lost. This initiation strategy avoids the need for oral supplementation to maintain clinical efficacy. However, even using an initiation regimen or a loading dose does not decrease the time to final steady state after a dose adjustment due to the slow absorption of the medication from the injection site. The time to steady state is controlled by “flip-flop” pharmacokinetics. In this kind of pharmacokinetics, which is observed with all LAIs, the absorption rate from the injection site is lower than the elimination rate.1
Cleton et al2 reported the pharmacokinetics of paliperidone palmitate for deltoid and gluteal injection sites. By combining the median data for the deltoid injection route from the article by Cleton et al2 and the dosing from Mr. B’s case, I created a model using superposition of a sixth-degree polynomial fitted to the single dose data. Gluteal injections were not included because their increased complexity is beyond the scope of this article, but the time to maximum concentration (gluteal > deltoid) and peak concentration (deltoid > gluteal) are different for each route. The polynomial was a good fit with the adjusted r2 = 0.976, P < .0001. This model illustrates the paliperidone serum concentrations for Mr. B and is shown in the Figure. As you can see, by Day 9, the serum concentrations had reached the lower limit of the expected range of 20 to 60 ng/mL, shown in the shaded region of the Figure.3
Steady state at the routine maintenance dose of 117 mg every 28 days was never reached as the medication was not sufficient to suppress Mr. B’s hallucinations, and his doses needed to be increased each month. First, Mr. B’s dose was increased to 156 mg and then to the maximum recommended dose of 234 mg every 28 days. Steady state can be considered to have been achieved when 90% of the final steady state is reached after 3.3 half-lives. Because of the flip-flop pharmacokinetics, the important half-life is the absorption half-life of approximately 40 days or 132 days at the same dose. In Mr. B’s case, this was Day 221, where the trough concentration was 35 ng/mL. However, this regimen was still inadequate because he had breakthrough symptoms prior to the next injection.
By decreasing the injection interval from 28 days to 21 days, the concentrations will increase to a new steady state. This will take the same 132 days. With the reduced injection frequency of 21 days, 7 injections will have been given prior to reaching the new steady state. Steady state is not dependent on the number of injections, but only on the absorption half-life. This new steady state trough is substantially higher at 52 ng/mL, but still in the expected range for commonly used doses. Because Mr. B’s hallucinations only appeared at the end of the dosing interval, it is reasonable to expect that his new regimen would be successful in suppressing his hallucinations. However, monitoring for peak-related adverse effects is essential. Based upon controlled clinical trials, the potential dose-related adverse effects of paliperidone include akathisia, other extrapyramidal symptoms, weight gain, and QTc prolongation.
Continue to: Would monitoring a patient's paliperidone serum concentrations be useful?
Would monitoring a patient’s paliperidone serum concentrations be useful? Currently, measuring an individual’s paliperidone serum concentration is generally considered unwarranted.3,4 One of the major reasons is a lack of appropriately designed studies to determine a therapeutic range.5 Flexible dose designs, commonly used in registration studies, cloud the relationships between concentration, time, response, and adverse effects. There are additional problems that are the result of diagnostic heterogeneity and placebo responders. A well-designed study to determine the therapeutic range would have ≥1 fixed dose groups and be diagnostically homogeneous. There are currently only a limited number of clinical laboratories that have implemented suitable assays.
Given the lack of knowledge of a therapeutic range, assured knowledge of nonadherence to LAIs, and the absence of significant drug interactions for paliperidone, there remain a few reasonable justifications for obtaining a patient’s paliperidone serum concentration (Table). If the patient had a good response with mild adverse effects, there is no reason to obtain a paliperidone serum concentration or make any change in the medication or dose. However, if the patient had a good response accompanied by moderate or severe adverse effects, or the patient has a poor response, then obtaining the paliperidone serum concentration could help determine an appropriate course of action.
CASE CONTINUED
After the second dose at the increased frequency on Day 252, the paliperidone serum concentration was maintained above 40 ng/mL. Mr. B continued to tolerate the LAI well and no longer reported any breakthrough hallucinations.
Related Resources
- ARUP Laboratories. Paliperidone, serum or plasma. http://ltd.aruplab.com/tests/pub/2007949.
- LabCorp. Paliperidone Paliperidone (as 9-hydroxyrisperidone), serum or plasma. https://www.labcorp.com/test-menu/38351/paliperidone-as-9-hydroxyrisperidone-serum-or-plasma.
- Janssen Scientific Affairs. Educational dose illustrator. http://www.educationaldoseillustrator.com.
Drug Brand Names
Paliperidone palmitate • Invega Sustenna
Risperidone • Risperdal
1. Jann MW, Ereshefsky L, Saklad SR. Clinical pharmacokinetics of the depot antipsychotics. Clin Pharmacokinet. 1985;10(4):315-333.
2. Cleton A, Rossenu S, Crauwels H, et al. A single-dose, open-label, parallel, randomized, dose-proportionality study of paliperidone after intramuscular injections of paliperidone palmitate in the deltoid or gluteal muscle in patients with schizophrenia. J Clin Pharmacol. 2014;54(9):1048-1057.
3. Taylor D, Paton C, Kapur S. The Maudsley prescribing guidelines in psychiatry. 12th ed. Oxford, UK: John Wiley & Sons, Ltd.; 2015:1-10.
4. Hiemke C, Baumann P, Bergemann N, et al. AGNP consensus guidelines for therapeutic drug monitoring in psychiatry: update 2011. Pharmacopsychiatry. 2011;44(6):195-235.
5. Lopez LV, Kane JM. Plasma levels of second-generation antipsychotics and clinical response in acute psychosis: a review of the literature. Schizophr Res. 2013;147(2-3):368-374.
1. Jann MW, Ereshefsky L, Saklad SR. Clinical pharmacokinetics of the depot antipsychotics. Clin Pharmacokinet. 1985;10(4):315-333.
2. Cleton A, Rossenu S, Crauwels H, et al. A single-dose, open-label, parallel, randomized, dose-proportionality study of paliperidone after intramuscular injections of paliperidone palmitate in the deltoid or gluteal muscle in patients with schizophrenia. J Clin Pharmacol. 2014;54(9):1048-1057.
3. Taylor D, Paton C, Kapur S. The Maudsley prescribing guidelines in psychiatry. 12th ed. Oxford, UK: John Wiley & Sons, Ltd.; 2015:1-10.
4. Hiemke C, Baumann P, Bergemann N, et al. AGNP consensus guidelines for therapeutic drug monitoring in psychiatry: update 2011. Pharmacopsychiatry. 2011;44(6):195-235.
5. Lopez LV, Kane JM. Plasma levels of second-generation antipsychotics and clinical response in acute psychosis: a review of the literature. Schizophr Res. 2013;147(2-3):368-374.
Helping survivors in the aftermath of suicide loss
The loss of a loved one to suicide is often experienced as “devastating.”1 While survivors of suicide loss may be able to move through the grief process without clinical support,2 the traumatic and stigmatizing nature of suicide is likely to make its aftermath more challenging to navigate than other types of loss. Sanford et al3 found that more than two-thirds of suicide loss survivors sought therapy after their loss. Further, when individuals facing these challenges present for treatment, clinicians often face challenges of their own.
Very few clinicians are trained in general grief processes,4 and even those specifically trained in grief and loss have been shown to “miss” several of the common clinical features that are unique to suicide loss.3 In my professional experience, the intensity and duration of suicide grief are often greater than they are for other losses, and many survivors of suicide loss have reported that others, including clinicians, have “pathologized” this, rather than having understood it as normative under the circumstances.
Although there is extensive literature on the aftermath of suicide for surviving loved ones, very few controlled studies have assessed interventions specifically for this population. Yet the U.S. Guidelines for Suicide Postvention5 explicitly call for improved training for those who work with suicide loss survivors, as well as research on these interventions. Jordan and McGann6 noted, “Without a full knowledge of suicide and its aftermath, it is very possible to make clinical errors which can hamper treatment.” This article summarizes what is currently known about the general experience of suicide bereavement and optimal interventions in treatment.
What makes suicide loss unique?
Suicide bereavement is distinct from other types of loss in 3 significant ways7:
- the thematic content of the grief
- the social processes surrounding the survivor
- the impact that suicide has on family systems.
Additionally, the perceived intentionality and preventability of a suicide death, as well as its stigmatized and traumatic nature, differentiate it from other types of traumatic loss.7 These elements are all likely to affect the nature, intensity, and duration of the grief.
Stigma and suicide. Stigma associated with suicide is well documented.8 Former U.S. Surgeon General David Satcher9 specifically described stigma toward suicide as one of the biggest barriers to prevention. In addition, researchers have found that the stigma associated with suicide “spills over” to the bereaved family members. Doka10,11 refers to “disenfranchised grief,” in which bereaved individuals receive the message that their grief is not legitimate, and as a result, they are likely to internalize this view. Studies have shown that individuals bereaved by suicide are also stigmatized, and are believed to be more psychologically disturbed, less likable, more blameworthy, more ashamed, and more in need of professional help than other bereaved individuals.8,12-20
These judgments often mirror suicide loss survivors’ self-punitive assessments, which then become exacerbated by and intertwined with both externally imposed and internalized stigma. Thus, it is not uncommon for survivors of suicide loss to question their own right to grieve, to report low expectations of social support, and to feel compelled to deny or hide the mode of death. To the extent that they are actively grieving, survivors of suicide loss often feel that they must do so in isolation. Thus, the perception of stigma, whether external or internalized, can have a profound effect on decisions about disclosure, requesting support, and ultimately on one’s ability to integrate the loss. Indeed, Feigelman et al21 found that stigmatization after suicide was specifically associated with ongoing grief difficulties, depression, and suicidal ideation.
Continue to: Traumatic nature of suicide
Traumatic nature of suicide. Suicide loss is also quite traumatic, and posttraumatic stress disorder (PTSD) symptoms such as shock, horror, disbelief, and intrusive/perseverative thoughts and questions, particularly in the earlier stages of grief, are common. Sanford et al3 found that the higher the level of “perceived closeness” to the deceased, the more likely that survivors of suicide loss would experience PTSD symptoms. In addition, the dramatic loss of social support following a suicide loss may itself be traumatic, which can serve to compound these difficulties. Notably, Sanford et al3 found that even for those survivors of suicide loss in treatment who endorsed PTSD symptoms, many of their treating clinicians did not assess or diagnose this disorder, thus missing an important component for treatment.
Increased risk for suicidality. Studies have shown that individuals who have lost a loved one to suicide are themselves at heightened risk for suicidal ideation and behaviors.22-27 Therefore, an assessment for suicide risk is always advisable. However, it is important to note that suicidal ideation is not uncommon and can serve different functions for survivors of suicide loss without necessarily progressing to a plan for acting on such ideations. Survivors of suicide loss may wish to “join” their loved one; to understand or identify with the mental state of the deceased; to punish themselves for failing to prevent the suicide; or to end their own pain through death. Therefore, it is crucial to assess the nature and function of expressed ideation (in addition to the presence or absence of plans) before assigning the level of risk.
Elements of suicide grief
After the loss of a loved one to suicide, the path to healing is often complex, with survivors of suicide loss enduring the following challenges:
Existential assumptions are shattered. Several authors28-30 have found that suicide loss is also likely to shatter survivors’ existential assumptions regarding their worldviews, roles, and identities, as well as religious and spiritual beliefs. As one survivor of suicide loss in my practice noted, “The world is gone, nothing is predictable anymore, and it’s no longer safe to assume anything.” Others have described feeling “fragmented” in ways they had never before experienced, and many have reported difficulties in “trusting” their own judgment, the stability of the world, and relationships. “Why?” becomes an emergent and insistent question in the survivor’s efforts to understand the suicide and (ideally) reassemble a coherent narrative around the loss.
Increased duration and intensity of grief. The duration of the grief process is likely to be affected by the traumatic nature of suicide loss, the differential social support accorded to its survivors, and the difficulty in finding systems that can validate and normalize the unique elements in suicide bereavement. The stigmatized reactions of others, particularly when internalized, can present barriers to the processing of grief. In addition, the intensity of the trauma and existential impact, as well as the perseverative nature of several of the unique themes (Box 1), can also prolong the processing and increase the intensity of suicide grief. Clinicians would do well to recognize the relatively “normative” nature of the increased duration and intensity, rather than seeing it as immediately indicative of a DSM diagnosis of complicated/prolonged grief disorder.
Box 1
Common themes in the suicide grief process
Several common themes are likely to emerge during the suicide grief process. Guilt and a sense of failure—around what one did and did not do—can be pervasive and persistent, and are often present even when not objectively warranted.
Anger and blame directed towards the deceased, other family members, and clinicians who had been treating the deceased may also be present, and may be used in efforts to deflect guilt. Any of these themes may be enlisted to create a deceptively simple narrative for understanding the reasons for the suicide.
Shame is often present, and certainly exacerbated by both external and internalized stigma. Feelings of rejection, betrayal, and abandonment by the deceased are also common, as well as fear/hypervigilance regarding the possibility of losing others to suicide. Given the intensity of suicide grief, it has been my observation that there may also be fear in relation to one's own mental status, as many otherwise healthy survivors of suicide loss have described feeling like they're "going crazy." Finally, there may also be relief, particularly if the deceased had been suffering from chronic psychiatric distress or had been cruel or abusive.
Continue to: Family disruption
Family disruption. It is not uncommon for a suicide loss to result in family disruption.6,31-32 This may manifest in the blaming of family members for “sins of omission or commission,”6 conflicts around the disclosure of the suicide both within and outside of the family, discordant grieving styles, and difficulties in understanding and attending to the needs of one’s children while grieving oneself.
Despite the common elements often seen in suicide grief, it is crucial to recognize that this process is not “one size fits all.” Not only are there individual variants, but Grad et al33 found gender-based differences in grieving styles, and cultural issues such as the “meanings” assigned to suicide, and culturally sanctioned grief rituals and behaviors that are also likely to affect how grief is experienced and expressed. In addition, personal variants such as closeness/conflicts with the deceased, histories of previous trauma or loss, pre-existing psychiatric disorders, and attachment orientation34 are likely to impact the grief process.
Losing close friends and colleagues may be similarly traumatic, but these survivors of suicide loss often receive even less social support than those who have kinship losses. Finally, when a suicide loss occurs in a professional capacity (such as the loss of a patient), this is likely to have many additional implications for one’s professional functions and identity.35
Interventions to help survivors
Several goals and “tasks” are involved in the suicide bereavement process (Box 21,6,30,36-40). These can be achieved through the following interventions: Support groups. Many survivors find that support groups that focus on suicide loss are extremely helpful, and research has supported this.1,4,41-44 Interactions with other suicide loss survivors provide hope, connection, and an “antidote” to stigma and shame. Optimally, group facilitators provide education, validation and normalization of the grief trajectory, and facilitate the sharing of both loss experiences and current functioning between group members. As a result, group participants often report renewed connections, increased efficacy in giving and accepting support, and decreased distress (including reductions in PTSD and depressive symptoms). The American Association of Suicidology (www.suicidology.org) and American Foundation of Suicide Prevention (www.afsp.org) provide contact information for suicide loss survivor groups (by geographical area) as well as information about online support groups.
Box 2
Goals and 'tasks' in suicide bereavement
The following goals and "tasks" should be part of the process of suicide bereavement:
- Reduce symptoms of posttraumatic stress disorder and other psychiatric disorders. Given the traumatic nature of the loss, an important goal is to understand and reduce posttraumatic stress disorder and other psychiatric symptoms, and incrementally improving functionality in relation to these.
- Integrate the loss. Recent authors36-38 have highlighted the need for survivors of suicide loss to "bear" and integrate the loss, as opposed to the concept of "getting over it." In these paradigms, the loss becomes an important part of one's identity, and eventually ceases to define it. Optimally, the "whole person" is remembered, not just the suicide. Part of this involves a reinvestment in life, with the acceptance of a "new normal" that takes the loss into account. It is not unusual for survivors of suicide loss to report some guilt in "moving on" and/or experiencing pleasure; often this is felt as a "betrayal" of the deceased.
- Create meaning from the loss. A major goal for those who have lost a loved one to suicide is the ability to find and/or create meaning from the loss. This would include the creation of a loss narrative39 that incorporates both ambiguity and complexity, as well as a regained/renewed sense of purpose in ongoing life.
- Develop ambiguity tolerance. A related "task" in suicide grief is the development of ambiguity tolerance, which generally includes an understanding of the complexity underlying suicide, the ability to offer oneself a "fair trial"30 in relation to one's realistic degree of responsibility, and the acceptance that many questions may remain unanswerable. In addition, in concert with the current understanding of "continuing bonds,"40 survivors should attempt to attend to the ongoing relationship with the deceased, including any "unfinished business."6
- Develop skills to manage stigmatized social responses and/or changes in family and social relationships.
- Memorialize and honor the deceased. Healing for survivors is facilitated by memorializations, which both validate the mourning process itself while also paying tribute to the richness of the deceased person's life.
- Post-traumatic growth. The relatively new understanding of "post-traumatic" growth is certainly applicable to the "unexpected gifts" many survivors of suicide loss report after they have moved through suicide grief. This includes greater understanding toward oneself, other survivors of suicide loss, and suicidal individuals; gratitude toward those who have provided support; and a desire to "use" their newfound understanding of suicide and suicide grief in ways to honor the deceased and benefit others. Feigelman et al1 found that many longer-term survivors of suicide loss engaged in both direct service and social activism around suicide pre- and postvention.
Individual treatment. The limited research on individual treatment for suicide loss survivors suggests that while most participants find it generally helpful, a significant number of others report that their therapists lack knowledge of suicide grief and endorse stigmatizing attitudes toward suicide and suicide loss survivors.45-46 In addition, Sanford et al3 found that survivors of suicide loss who endorsed PTSD symptoms were not assessed, diagnosed, or treated for these symptoms.
Continue to: This speaks to the importance of understanding what is...
This speaks to the importance of understanding what is “normative” for survivors of suicide loss. In general, “normalization” and psychoeducation about the suicide grief trajectory can play an important role in work with survivors of suicide loss, even in the presence of diagnosable disorders. While PTSD, depressive symptoms, and suicidal ideation are not uncommon in suicide loss survivors, and certainly may warrant clinical assessment and treatment, it can be helpful (and less stigmatizing) for your patients to know that these diagnoses are relatively common and understandable in the context of this devastating experience. For instance, survivors of suicide loss often report feeling relieved when clinicians explain the connections between traumatic loss and PTSD and/or depressive symptoms, and this can also help to relieve secondary anxiety about “going crazy.” Many survivors of suicide loss also describe feeling as though they are functioning on “autopilot” in the earlier stages of grief; it can help them understand the “function” of compartmentalization as potentially adaptive in the short run.
Suicide loss survivors may also find it helpful to learn about suicidal states of mind and their relationships to any types of mental illness their loved ones had struggled with.47
Your role: Help survivors integrate the loss
Before beginning treatment with an individual who has lost a loved one to suicide, clinicians should thoroughly explore their own understanding of and experience with suicide, including assumptions around causation, internalized stigma about suicidal individuals and survivors of suicide loss, their own history of suicide loss or suicidality, cultural/religious attitudes, and anxiety/defenses associated with the topic of suicide. These factors, particularly when unexamined, are likely to impact the treatment relationship and one’s clinical efficacy.
In concert with the existing literature, consider the potential goals and tasks involved in the integration of the individual’s suicide loss, along with any individual factors/variants that may impact the grief trajectory. Kosminsky and Jordon34 described the role of the clinician in this situation as a “transitional attachment figure” who facilitates the management and integration of the loss into the creation of what survivors of suicide loss have termed a “new normal.”
Because suicide loss is often associated with PTSD and other psychiatric illnesses (eg, depression, suicidality, substance abuse), it is essential to balance the assessment and treatment of these issues with attention to grief issues as needed. Again, to the extent that such issues have arisen primarily in the wake of the suicide loss, it can be helpful for patients to understand their connection to the context of the loss.
Continue to: Ideally, the clinician should...
Ideally, the clinician should be “present” with the patient’s pain, normative guilt, and rumination, without attempting to quickly eliminate or “fix” it or provide premature reassurance that the survivor of suicide loss “did nothing wrong.” Rather, as Jordan6 suggests, the clinician should act to promote a “fair trial” with respect to the patient’s guilt and blame, with an understanding of the “tyranny of hindsight.” The promotion of ambiguity tolerance should also play a role in coming to terms with many questions that may remain unanswered.
Optimally, clinicians should encourage patients to attend to their ongoing relationship with the deceased, particularly in the service of resolving “unfinished business,” ultimately integrating the loss into memories of the whole person. In line with this, survivors of suicide loss may be encouraged to create a narrative of the loss that incorporates both complexity and ambiguity. In the service of supporting the suicide loss survivor’s reinvestment in life, it is often helpful to facilitate their ability to anticipate and cope with triggers, such as anniversaries, birthdays, or holidays, as well as to develop and use skills for managing difficult or stigmatizing social or cultural reactions.
Any disruptions in family functioning should also be addressed. Psychoeducation about discordant grieving styles (particularly around gender) and the support of children’s grief may be helpful, and referrals to family or couples therapists should be considered as needed. Finally, the facilitation of suicide loss survivors’ creation of memorializations or rituals can help promote healing and make their loss meaningful.
Bottom Line
Losing a loved one to suicide is often a devastating and traumatic experience, but with optimal support, most survivors are ultimately able to integrate the loss and grow as a result. Understanding the suicide grief trajectory, as well as general guidelines for treatment, will facilitate healing and growth in the aftermath of suicide loss.
Related Resources
- Jordan JR, McIntosh JL. Grief after suicide: understanding the consequences and caring for the survivors. New York, NY: Routledge/Taylor & Francis Group; 2011.
- American Association of Suicidology. http://www.suicidology.org/.
- American Foundation for Suicide Prevention. https://afsp.org/.
1. Feigelman W, Jordan JR, McIntosh JL, et al. Devastating losses: how parents cope with the death of a child to suicide or drugs. New York, NY: Springer; 2012.
2. McIntosh JL. Research on survivors of suicide. In: Stimming MT, Stimming M, eds. Before their time: adult children’s experiences of parental suicide. Philadelphia, PA: Temple University Press; 1999:157-180.
3. Sanford RL, Cerel J, McGann VL, et al. Suicide loss survivors’ experiences with therapy: Implications for clinical practice. Community Ment Health J. 2016;5(2):551-558.
4. Jordan JR, McMenamy J. Interventions for suicide survivors: a review of the literature. Suicide Life Threat Behav. 2004;34(4):337-349.
5. Survivors of Suicide Loss Task Force. Responding to grief, trauma, & distress after a suicide: U.S. national guidelines. Washington, DC: National Action Alliance for Suicide Prevention; 2015.
6. Jordan JR, McGann V. Clinical work with suicide loss survivors: implications of the U.S. postvention guidelines. Death Stud. 2017;41(10):659-672.
7. Jordan JR. Is suicide bereavement different? A reassessment of the literature. Suicide Life Threat Behav. 2001;31(1):91-102.
8. Cvinar JG. Do suicide survivors suffer social stigma: a review of the literature. Perspect Psychiatr Care. 2005;41(1):14-21.
9. U.S. Public Health Service. The Surgeon General’s call to action to prevent suicide. Washington, DC: Department of Health and Human Services; 1999.
10. Doka KJ. Disenfranchised grief: recognizing hidden sorrow. Lexington, MA: Lexington; 1989.
11. Doka KJ. Disenfranchised grief: new directions, challenges, and strategies for practice. Champaign, IL: Research Press; 2002.
12. McIntosh JL. Suicide survivors: the aftermath of suicide and suicidal behavior. In: Bryant CD, ed. Handbook of death & dying. Vol. 1. Thousand Oaks, CA: SAGE Publications; 2003:339-350.
13. Jordan, JR, McIntosh, JL. Is suicide bereavement different? A framework for rethinking the question. In: Jordan JR, McIntosh JL, eds. Grief after suicide: understanding the consequences and caring for the survivors. New York, NY: Routledge/Taylor & Francis Group; 2011:19-42.
14. Dunne EJ, McIntosh JL, Dunne-Maxim K, eds. Suicide and its aftermath: understanding and counseling the survivors. New York, NY: W.W. Norton & Co.; 1987.
15. Harwood D, Hawton K, Hope T, et al. The grief experiences and needs of bereaved relatives and friends of older people dying through suicide: a descriptive and case-control study. J Affect Disord. 2002;72(2):185-194.
16. Armour, M. Violent death: understanding the context of traumatic and stigmatized grief. J Hum Behav Soc Environ. 2006;14(4):53-90.
17. Van Dongen CJ. Social context of postsuicide bereavement. Death Stud. 1993;17(2):125-141.
18. Calhoun LG, Allen BG. Social reactions to the survivor of a suicide in the family: A review of the literature. Omega – Journal of Death and Dying. 1991;23(2):95-107.
19. Range LM. When a loss is due to suicide: unique aspects of bereavement. In: Harvey JH, ed. Perspectives on loss: a sourcebook. Philadelphia, PA: Brunner/Mazel; 1998:213-220.
20. Sveen CA, Walby FA. Suicide survivors’ mental health and grief reactions: a systematic review of controlled studies. Suicide Life Threat Behav. 2008;38(1):13-29.
21. Feigelman W, Gorman BS, Jordan JR. Stigmatization and suicide bereavement. Death Stud. 2009;33(7):591-608.
22. Shneidman ES. Foreword. In: Cain AC, ed. Survivors of suicide. Springfield, IL: Charles C. Thomas; 1972:ix-xi.
23. Jordan JR, McIntosh, JL. Suicide bereavement: Why study survivors of suicide loss? In: Jordan JR, McIntosh JL, eds. Grief after suicide: understanding the consequences and caring for the survivors. New York, NY: Routledge/Taylor & Francis Group; 2011:3-18.
24. Agerbo E. Midlife suicide risk, partner’s psychiatric illness, spouse and child bereavement by suicide or other modes of death: a gender specific study. J Epidemiol Community Health. 2005;59(5):407-412.
25. Hedström P, Liu KY, Nordvik MK. Interaction domains and suicide: a population-based panel study of suicides in Stockholm, 1991-1999. Soc Forces. 2008;87(2):713-740.
26. Qin P, Agerbo E, Mortensen PB. Suicide risk in relation to family history of completed suicide and psychiatric disorders: a nested case-control study based on longitudinal registers. Lancet. 2002;360(9340):1126-1130.
27. Qin P, Mortensen PB. The impact of parental status on the risk of completed suicide. Arch Gen Psychiatry. 2003;60(8):797-802.
28. Neimeyer RA, Sands D. Suicide loss and the quest for meaning. In: Andriessen K, Krysinska K, Grad OT, eds. Postvention in action: the international handbook of suicide bereavement support. Cambridge, MA: Hogrefe; 2017:71-84.
29. Sands DC, Jordan JR, Neimeyer RA. The meanings of suicide: A narrative approach to healing. In: Jordan JR, McIntosh JL, eds. Grief after suicide: understanding the consequences and caring for the survivors. New York, NY: Routledge/Taylor & Francis Group; 2011:249-282.
30. Jordan JR. Principles of grief counseling with adult survivors. In: Jordan JR, McIntosh JL, eds. Grief after suicide: understanding the consequences and caring for the survivors. New York, NY: Routledge/Taylor & Francis Group; 2011:179-224.
31. Cerel J, Jordan JR, Duberstein PR. The impact of suicide on the family. Crisis. 2008;29:38-44.
32. Kaslow NJ, Samples TC, Rhodes M, et al. A family-oriented and culturally sensitive postvention approach with suicide survivors. In: Jordan JR, McIntosh JL, eds. Grief after suicide: understanding the consequences and caring for the survivors. New York, NY: Routledge/Taylor & Francis Group; 2011:301-323.
33. Grad OT, Treven M, Krysinska K. Suicide bereavement and gender. In: Andriessen K, Krysinska K, Grad OT, eds. Postvention in action: the international handbook of suicide bereavement support. Cambridge, MA: Hogrefe; 2017:39-49.
34. Kosminsky PS, Jordan JR. Attachment-informed grief therapy: the clinician’s guide to foundations and applications. New York, NY: Routledge; 2016.
35. Gutin N, McGann VL, Jordan JR. The impact of suicide on professional caregivers. In: Jordan JR, McIntosh JL, eds. Grief after suicide: understanding the consequences and caring for the survivors. New York, NY: Routledge/Taylor & Francis Group; 2011:93-111.
36. Jordan JR. Bereavement after suicide. Psychiatr Ann. 2008;38(10):679-685.
37. Jordan JR. After suicide: clinical work with survivors. Grief Matters. 2009;12(1):4-9.
38. Neimeyer, RA. Traumatic loss and the reconstruction of meaning. J Palliat Med. 2002;5(6):935-942; discussion 942-943.
39. Neimeyer R, ed. Meaning reconstruction & the experience of loss. Washington, DC: American Psychological Association; 2001.
40. Klass, D. Sorrow and solace: Neglected areas in bereavement research. Death Stud. 2013;37(7):597-616.
41. Farberow NL. The Los Angeles Survivors-After-Suicide program: an evaluation. Crisis. 1992;13(1):23-34.
42. McDaid C, Trowman R, Golder S, et al. Interventions for people bereaved through suicide: systematic review. Br J Psychiatry. 2008;193(6):438-443.
43. Groos AD, Shakespeare-Finch J. Positive experiences for participants in suicide bereavement groups: a grounded theory model. Death Stud. 2013;37(1):1-24.
44. Jordan JR. Group work with suicide survivors. In: Jordan JR, McIntosh JL, eds. Grief after suicide: understanding the consequences and caring for the survivors. New York, NY: Routledge/Taylor & Francis Group; 2011:283-300.
45. Wilson A, Marshall A. The support needs and experiences of suicidally bereaved family and friends. Death Stud. 2010;34(7):625-640.
46. McKinnon JM, Chonody J. Exploring the formal supports used by people bereaved through suicide: a qualitative study. Soc Work Ment Health. 2014;12(3):231-248.
47. Myers MF, Fine C. Touched by suicide: hope and healing after loss. New York, NY: Gotham Books; 2006.
The loss of a loved one to suicide is often experienced as “devastating.”1 While survivors of suicide loss may be able to move through the grief process without clinical support,2 the traumatic and stigmatizing nature of suicide is likely to make its aftermath more challenging to navigate than other types of loss. Sanford et al3 found that more than two-thirds of suicide loss survivors sought therapy after their loss. Further, when individuals facing these challenges present for treatment, clinicians often face challenges of their own.
Very few clinicians are trained in general grief processes,4 and even those specifically trained in grief and loss have been shown to “miss” several of the common clinical features that are unique to suicide loss.3 In my professional experience, the intensity and duration of suicide grief are often greater than they are for other losses, and many survivors of suicide loss have reported that others, including clinicians, have “pathologized” this, rather than having understood it as normative under the circumstances.
Although there is extensive literature on the aftermath of suicide for surviving loved ones, very few controlled studies have assessed interventions specifically for this population. Yet the U.S. Guidelines for Suicide Postvention5 explicitly call for improved training for those who work with suicide loss survivors, as well as research on these interventions. Jordan and McGann6 noted, “Without a full knowledge of suicide and its aftermath, it is very possible to make clinical errors which can hamper treatment.” This article summarizes what is currently known about the general experience of suicide bereavement and optimal interventions in treatment.
What makes suicide loss unique?
Suicide bereavement is distinct from other types of loss in 3 significant ways7:
- the thematic content of the grief
- the social processes surrounding the survivor
- the impact that suicide has on family systems.
Additionally, the perceived intentionality and preventability of a suicide death, as well as its stigmatized and traumatic nature, differentiate it from other types of traumatic loss.7 These elements are all likely to affect the nature, intensity, and duration of the grief.
Stigma and suicide. Stigma associated with suicide is well documented.8 Former U.S. Surgeon General David Satcher9 specifically described stigma toward suicide as one of the biggest barriers to prevention. In addition, researchers have found that the stigma associated with suicide “spills over” to the bereaved family members. Doka10,11 refers to “disenfranchised grief,” in which bereaved individuals receive the message that their grief is not legitimate, and as a result, they are likely to internalize this view. Studies have shown that individuals bereaved by suicide are also stigmatized, and are believed to be more psychologically disturbed, less likable, more blameworthy, more ashamed, and more in need of professional help than other bereaved individuals.8,12-20
These judgments often mirror suicide loss survivors’ self-punitive assessments, which then become exacerbated by and intertwined with both externally imposed and internalized stigma. Thus, it is not uncommon for survivors of suicide loss to question their own right to grieve, to report low expectations of social support, and to feel compelled to deny or hide the mode of death. To the extent that they are actively grieving, survivors of suicide loss often feel that they must do so in isolation. Thus, the perception of stigma, whether external or internalized, can have a profound effect on decisions about disclosure, requesting support, and ultimately on one’s ability to integrate the loss. Indeed, Feigelman et al21 found that stigmatization after suicide was specifically associated with ongoing grief difficulties, depression, and suicidal ideation.
Continue to: Traumatic nature of suicide
Traumatic nature of suicide. Suicide loss is also quite traumatic, and posttraumatic stress disorder (PTSD) symptoms such as shock, horror, disbelief, and intrusive/perseverative thoughts and questions, particularly in the earlier stages of grief, are common. Sanford et al3 found that the higher the level of “perceived closeness” to the deceased, the more likely that survivors of suicide loss would experience PTSD symptoms. In addition, the dramatic loss of social support following a suicide loss may itself be traumatic, which can serve to compound these difficulties. Notably, Sanford et al3 found that even for those survivors of suicide loss in treatment who endorsed PTSD symptoms, many of their treating clinicians did not assess or diagnose this disorder, thus missing an important component for treatment.
Increased risk for suicidality. Studies have shown that individuals who have lost a loved one to suicide are themselves at heightened risk for suicidal ideation and behaviors.22-27 Therefore, an assessment for suicide risk is always advisable. However, it is important to note that suicidal ideation is not uncommon and can serve different functions for survivors of suicide loss without necessarily progressing to a plan for acting on such ideations. Survivors of suicide loss may wish to “join” their loved one; to understand or identify with the mental state of the deceased; to punish themselves for failing to prevent the suicide; or to end their own pain through death. Therefore, it is crucial to assess the nature and function of expressed ideation (in addition to the presence or absence of plans) before assigning the level of risk.
Elements of suicide grief
After the loss of a loved one to suicide, the path to healing is often complex, with survivors of suicide loss enduring the following challenges:
Existential assumptions are shattered. Several authors28-30 have found that suicide loss is also likely to shatter survivors’ existential assumptions regarding their worldviews, roles, and identities, as well as religious and spiritual beliefs. As one survivor of suicide loss in my practice noted, “The world is gone, nothing is predictable anymore, and it’s no longer safe to assume anything.” Others have described feeling “fragmented” in ways they had never before experienced, and many have reported difficulties in “trusting” their own judgment, the stability of the world, and relationships. “Why?” becomes an emergent and insistent question in the survivor’s efforts to understand the suicide and (ideally) reassemble a coherent narrative around the loss.
Increased duration and intensity of grief. The duration of the grief process is likely to be affected by the traumatic nature of suicide loss, the differential social support accorded to its survivors, and the difficulty in finding systems that can validate and normalize the unique elements in suicide bereavement. The stigmatized reactions of others, particularly when internalized, can present barriers to the processing of grief. In addition, the intensity of the trauma and existential impact, as well as the perseverative nature of several of the unique themes (Box 1), can also prolong the processing and increase the intensity of suicide grief. Clinicians would do well to recognize the relatively “normative” nature of the increased duration and intensity, rather than seeing it as immediately indicative of a DSM diagnosis of complicated/prolonged grief disorder.
Box 1
Common themes in the suicide grief process
Several common themes are likely to emerge during the suicide grief process. Guilt and a sense of failure—around what one did and did not do—can be pervasive and persistent, and are often present even when not objectively warranted.
Anger and blame directed towards the deceased, other family members, and clinicians who had been treating the deceased may also be present, and may be used in efforts to deflect guilt. Any of these themes may be enlisted to create a deceptively simple narrative for understanding the reasons for the suicide.
Shame is often present, and certainly exacerbated by both external and internalized stigma. Feelings of rejection, betrayal, and abandonment by the deceased are also common, as well as fear/hypervigilance regarding the possibility of losing others to suicide. Given the intensity of suicide grief, it has been my observation that there may also be fear in relation to one's own mental status, as many otherwise healthy survivors of suicide loss have described feeling like they're "going crazy." Finally, there may also be relief, particularly if the deceased had been suffering from chronic psychiatric distress or had been cruel or abusive.
Continue to: Family disruption
Family disruption. It is not uncommon for a suicide loss to result in family disruption.6,31-32 This may manifest in the blaming of family members for “sins of omission or commission,”6 conflicts around the disclosure of the suicide both within and outside of the family, discordant grieving styles, and difficulties in understanding and attending to the needs of one’s children while grieving oneself.
Despite the common elements often seen in suicide grief, it is crucial to recognize that this process is not “one size fits all.” Not only are there individual variants, but Grad et al33 found gender-based differences in grieving styles, and cultural issues such as the “meanings” assigned to suicide, and culturally sanctioned grief rituals and behaviors that are also likely to affect how grief is experienced and expressed. In addition, personal variants such as closeness/conflicts with the deceased, histories of previous trauma or loss, pre-existing psychiatric disorders, and attachment orientation34 are likely to impact the grief process.
Losing close friends and colleagues may be similarly traumatic, but these survivors of suicide loss often receive even less social support than those who have kinship losses. Finally, when a suicide loss occurs in a professional capacity (such as the loss of a patient), this is likely to have many additional implications for one’s professional functions and identity.35
Interventions to help survivors
Several goals and “tasks” are involved in the suicide bereavement process (Box 21,6,30,36-40). These can be achieved through the following interventions: Support groups. Many survivors find that support groups that focus on suicide loss are extremely helpful, and research has supported this.1,4,41-44 Interactions with other suicide loss survivors provide hope, connection, and an “antidote” to stigma and shame. Optimally, group facilitators provide education, validation and normalization of the grief trajectory, and facilitate the sharing of both loss experiences and current functioning between group members. As a result, group participants often report renewed connections, increased efficacy in giving and accepting support, and decreased distress (including reductions in PTSD and depressive symptoms). The American Association of Suicidology (www.suicidology.org) and American Foundation of Suicide Prevention (www.afsp.org) provide contact information for suicide loss survivor groups (by geographical area) as well as information about online support groups.
Box 2
Goals and 'tasks' in suicide bereavement
The following goals and "tasks" should be part of the process of suicide bereavement:
- Reduce symptoms of posttraumatic stress disorder and other psychiatric disorders. Given the traumatic nature of the loss, an important goal is to understand and reduce posttraumatic stress disorder and other psychiatric symptoms, and incrementally improving functionality in relation to these.
- Integrate the loss. Recent authors36-38 have highlighted the need for survivors of suicide loss to "bear" and integrate the loss, as opposed to the concept of "getting over it." In these paradigms, the loss becomes an important part of one's identity, and eventually ceases to define it. Optimally, the "whole person" is remembered, not just the suicide. Part of this involves a reinvestment in life, with the acceptance of a "new normal" that takes the loss into account. It is not unusual for survivors of suicide loss to report some guilt in "moving on" and/or experiencing pleasure; often this is felt as a "betrayal" of the deceased.
- Create meaning from the loss. A major goal for those who have lost a loved one to suicide is the ability to find and/or create meaning from the loss. This would include the creation of a loss narrative39 that incorporates both ambiguity and complexity, as well as a regained/renewed sense of purpose in ongoing life.
- Develop ambiguity tolerance. A related "task" in suicide grief is the development of ambiguity tolerance, which generally includes an understanding of the complexity underlying suicide, the ability to offer oneself a "fair trial"30 in relation to one's realistic degree of responsibility, and the acceptance that many questions may remain unanswerable. In addition, in concert with the current understanding of "continuing bonds,"40 survivors should attempt to attend to the ongoing relationship with the deceased, including any "unfinished business."6
- Develop skills to manage stigmatized social responses and/or changes in family and social relationships.
- Memorialize and honor the deceased. Healing for survivors is facilitated by memorializations, which both validate the mourning process itself while also paying tribute to the richness of the deceased person's life.
- Post-traumatic growth. The relatively new understanding of "post-traumatic" growth is certainly applicable to the "unexpected gifts" many survivors of suicide loss report after they have moved through suicide grief. This includes greater understanding toward oneself, other survivors of suicide loss, and suicidal individuals; gratitude toward those who have provided support; and a desire to "use" their newfound understanding of suicide and suicide grief in ways to honor the deceased and benefit others. Feigelman et al1 found that many longer-term survivors of suicide loss engaged in both direct service and social activism around suicide pre- and postvention.
Individual treatment. The limited research on individual treatment for suicide loss survivors suggests that while most participants find it generally helpful, a significant number of others report that their therapists lack knowledge of suicide grief and endorse stigmatizing attitudes toward suicide and suicide loss survivors.45-46 In addition, Sanford et al3 found that survivors of suicide loss who endorsed PTSD symptoms were not assessed, diagnosed, or treated for these symptoms.
Continue to: This speaks to the importance of understanding what is...
This speaks to the importance of understanding what is “normative” for survivors of suicide loss. In general, “normalization” and psychoeducation about the suicide grief trajectory can play an important role in work with survivors of suicide loss, even in the presence of diagnosable disorders. While PTSD, depressive symptoms, and suicidal ideation are not uncommon in suicide loss survivors, and certainly may warrant clinical assessment and treatment, it can be helpful (and less stigmatizing) for your patients to know that these diagnoses are relatively common and understandable in the context of this devastating experience. For instance, survivors of suicide loss often report feeling relieved when clinicians explain the connections between traumatic loss and PTSD and/or depressive symptoms, and this can also help to relieve secondary anxiety about “going crazy.” Many survivors of suicide loss also describe feeling as though they are functioning on “autopilot” in the earlier stages of grief; it can help them understand the “function” of compartmentalization as potentially adaptive in the short run.
Suicide loss survivors may also find it helpful to learn about suicidal states of mind and their relationships to any types of mental illness their loved ones had struggled with.47
Your role: Help survivors integrate the loss
Before beginning treatment with an individual who has lost a loved one to suicide, clinicians should thoroughly explore their own understanding of and experience with suicide, including assumptions around causation, internalized stigma about suicidal individuals and survivors of suicide loss, their own history of suicide loss or suicidality, cultural/religious attitudes, and anxiety/defenses associated with the topic of suicide. These factors, particularly when unexamined, are likely to impact the treatment relationship and one’s clinical efficacy.
In concert with the existing literature, consider the potential goals and tasks involved in the integration of the individual’s suicide loss, along with any individual factors/variants that may impact the grief trajectory. Kosminsky and Jordon34 described the role of the clinician in this situation as a “transitional attachment figure” who facilitates the management and integration of the loss into the creation of what survivors of suicide loss have termed a “new normal.”
Because suicide loss is often associated with PTSD and other psychiatric illnesses (eg, depression, suicidality, substance abuse), it is essential to balance the assessment and treatment of these issues with attention to grief issues as needed. Again, to the extent that such issues have arisen primarily in the wake of the suicide loss, it can be helpful for patients to understand their connection to the context of the loss.
Continue to: Ideally, the clinician should...
Ideally, the clinician should be “present” with the patient’s pain, normative guilt, and rumination, without attempting to quickly eliminate or “fix” it or provide premature reassurance that the survivor of suicide loss “did nothing wrong.” Rather, as Jordan6 suggests, the clinician should act to promote a “fair trial” with respect to the patient’s guilt and blame, with an understanding of the “tyranny of hindsight.” The promotion of ambiguity tolerance should also play a role in coming to terms with many questions that may remain unanswered.
Optimally, clinicians should encourage patients to attend to their ongoing relationship with the deceased, particularly in the service of resolving “unfinished business,” ultimately integrating the loss into memories of the whole person. In line with this, survivors of suicide loss may be encouraged to create a narrative of the loss that incorporates both complexity and ambiguity. In the service of supporting the suicide loss survivor’s reinvestment in life, it is often helpful to facilitate their ability to anticipate and cope with triggers, such as anniversaries, birthdays, or holidays, as well as to develop and use skills for managing difficult or stigmatizing social or cultural reactions.
Any disruptions in family functioning should also be addressed. Psychoeducation about discordant grieving styles (particularly around gender) and the support of children’s grief may be helpful, and referrals to family or couples therapists should be considered as needed. Finally, the facilitation of suicide loss survivors’ creation of memorializations or rituals can help promote healing and make their loss meaningful.
Bottom Line
Losing a loved one to suicide is often a devastating and traumatic experience, but with optimal support, most survivors are ultimately able to integrate the loss and grow as a result. Understanding the suicide grief trajectory, as well as general guidelines for treatment, will facilitate healing and growth in the aftermath of suicide loss.
Related Resources
- Jordan JR, McIntosh JL. Grief after suicide: understanding the consequences and caring for the survivors. New York, NY: Routledge/Taylor & Francis Group; 2011.
- American Association of Suicidology. http://www.suicidology.org/.
- American Foundation for Suicide Prevention. https://afsp.org/.
The loss of a loved one to suicide is often experienced as “devastating.”1 While survivors of suicide loss may be able to move through the grief process without clinical support,2 the traumatic and stigmatizing nature of suicide is likely to make its aftermath more challenging to navigate than other types of loss. Sanford et al3 found that more than two-thirds of suicide loss survivors sought therapy after their loss. Further, when individuals facing these challenges present for treatment, clinicians often face challenges of their own.
Very few clinicians are trained in general grief processes,4 and even those specifically trained in grief and loss have been shown to “miss” several of the common clinical features that are unique to suicide loss.3 In my professional experience, the intensity and duration of suicide grief are often greater than they are for other losses, and many survivors of suicide loss have reported that others, including clinicians, have “pathologized” this, rather than having understood it as normative under the circumstances.
Although there is extensive literature on the aftermath of suicide for surviving loved ones, very few controlled studies have assessed interventions specifically for this population. Yet the U.S. Guidelines for Suicide Postvention5 explicitly call for improved training for those who work with suicide loss survivors, as well as research on these interventions. Jordan and McGann6 noted, “Without a full knowledge of suicide and its aftermath, it is very possible to make clinical errors which can hamper treatment.” This article summarizes what is currently known about the general experience of suicide bereavement and optimal interventions in treatment.
What makes suicide loss unique?
Suicide bereavement is distinct from other types of loss in 3 significant ways7:
- the thematic content of the grief
- the social processes surrounding the survivor
- the impact that suicide has on family systems.
Additionally, the perceived intentionality and preventability of a suicide death, as well as its stigmatized and traumatic nature, differentiate it from other types of traumatic loss.7 These elements are all likely to affect the nature, intensity, and duration of the grief.
Stigma and suicide. Stigma associated with suicide is well documented.8 Former U.S. Surgeon General David Satcher9 specifically described stigma toward suicide as one of the biggest barriers to prevention. In addition, researchers have found that the stigma associated with suicide “spills over” to the bereaved family members. Doka10,11 refers to “disenfranchised grief,” in which bereaved individuals receive the message that their grief is not legitimate, and as a result, they are likely to internalize this view. Studies have shown that individuals bereaved by suicide are also stigmatized, and are believed to be more psychologically disturbed, less likable, more blameworthy, more ashamed, and more in need of professional help than other bereaved individuals.8,12-20
These judgments often mirror suicide loss survivors’ self-punitive assessments, which then become exacerbated by and intertwined with both externally imposed and internalized stigma. Thus, it is not uncommon for survivors of suicide loss to question their own right to grieve, to report low expectations of social support, and to feel compelled to deny or hide the mode of death. To the extent that they are actively grieving, survivors of suicide loss often feel that they must do so in isolation. Thus, the perception of stigma, whether external or internalized, can have a profound effect on decisions about disclosure, requesting support, and ultimately on one’s ability to integrate the loss. Indeed, Feigelman et al21 found that stigmatization after suicide was specifically associated with ongoing grief difficulties, depression, and suicidal ideation.
Continue to: Traumatic nature of suicide
Traumatic nature of suicide. Suicide loss is also quite traumatic, and posttraumatic stress disorder (PTSD) symptoms such as shock, horror, disbelief, and intrusive/perseverative thoughts and questions, particularly in the earlier stages of grief, are common. Sanford et al3 found that the higher the level of “perceived closeness” to the deceased, the more likely that survivors of suicide loss would experience PTSD symptoms. In addition, the dramatic loss of social support following a suicide loss may itself be traumatic, which can serve to compound these difficulties. Notably, Sanford et al3 found that even for those survivors of suicide loss in treatment who endorsed PTSD symptoms, many of their treating clinicians did not assess or diagnose this disorder, thus missing an important component for treatment.
Increased risk for suicidality. Studies have shown that individuals who have lost a loved one to suicide are themselves at heightened risk for suicidal ideation and behaviors.22-27 Therefore, an assessment for suicide risk is always advisable. However, it is important to note that suicidal ideation is not uncommon and can serve different functions for survivors of suicide loss without necessarily progressing to a plan for acting on such ideations. Survivors of suicide loss may wish to “join” their loved one; to understand or identify with the mental state of the deceased; to punish themselves for failing to prevent the suicide; or to end their own pain through death. Therefore, it is crucial to assess the nature and function of expressed ideation (in addition to the presence or absence of plans) before assigning the level of risk.
Elements of suicide grief
After the loss of a loved one to suicide, the path to healing is often complex, with survivors of suicide loss enduring the following challenges:
Existential assumptions are shattered. Several authors28-30 have found that suicide loss is also likely to shatter survivors’ existential assumptions regarding their worldviews, roles, and identities, as well as religious and spiritual beliefs. As one survivor of suicide loss in my practice noted, “The world is gone, nothing is predictable anymore, and it’s no longer safe to assume anything.” Others have described feeling “fragmented” in ways they had never before experienced, and many have reported difficulties in “trusting” their own judgment, the stability of the world, and relationships. “Why?” becomes an emergent and insistent question in the survivor’s efforts to understand the suicide and (ideally) reassemble a coherent narrative around the loss.
Increased duration and intensity of grief. The duration of the grief process is likely to be affected by the traumatic nature of suicide loss, the differential social support accorded to its survivors, and the difficulty in finding systems that can validate and normalize the unique elements in suicide bereavement. The stigmatized reactions of others, particularly when internalized, can present barriers to the processing of grief. In addition, the intensity of the trauma and existential impact, as well as the perseverative nature of several of the unique themes (Box 1), can also prolong the processing and increase the intensity of suicide grief. Clinicians would do well to recognize the relatively “normative” nature of the increased duration and intensity, rather than seeing it as immediately indicative of a DSM diagnosis of complicated/prolonged grief disorder.
Box 1
Common themes in the suicide grief process
Several common themes are likely to emerge during the suicide grief process. Guilt and a sense of failure—around what one did and did not do—can be pervasive and persistent, and are often present even when not objectively warranted.
Anger and blame directed towards the deceased, other family members, and clinicians who had been treating the deceased may also be present, and may be used in efforts to deflect guilt. Any of these themes may be enlisted to create a deceptively simple narrative for understanding the reasons for the suicide.
Shame is often present, and certainly exacerbated by both external and internalized stigma. Feelings of rejection, betrayal, and abandonment by the deceased are also common, as well as fear/hypervigilance regarding the possibility of losing others to suicide. Given the intensity of suicide grief, it has been my observation that there may also be fear in relation to one's own mental status, as many otherwise healthy survivors of suicide loss have described feeling like they're "going crazy." Finally, there may also be relief, particularly if the deceased had been suffering from chronic psychiatric distress or had been cruel or abusive.
Continue to: Family disruption
Family disruption. It is not uncommon for a suicide loss to result in family disruption.6,31-32 This may manifest in the blaming of family members for “sins of omission or commission,”6 conflicts around the disclosure of the suicide both within and outside of the family, discordant grieving styles, and difficulties in understanding and attending to the needs of one’s children while grieving oneself.
Despite the common elements often seen in suicide grief, it is crucial to recognize that this process is not “one size fits all.” Not only are there individual variants, but Grad et al33 found gender-based differences in grieving styles, and cultural issues such as the “meanings” assigned to suicide, and culturally sanctioned grief rituals and behaviors that are also likely to affect how grief is experienced and expressed. In addition, personal variants such as closeness/conflicts with the deceased, histories of previous trauma or loss, pre-existing psychiatric disorders, and attachment orientation34 are likely to impact the grief process.
Losing close friends and colleagues may be similarly traumatic, but these survivors of suicide loss often receive even less social support than those who have kinship losses. Finally, when a suicide loss occurs in a professional capacity (such as the loss of a patient), this is likely to have many additional implications for one’s professional functions and identity.35
Interventions to help survivors
Several goals and “tasks” are involved in the suicide bereavement process (Box 21,6,30,36-40). These can be achieved through the following interventions: Support groups. Many survivors find that support groups that focus on suicide loss are extremely helpful, and research has supported this.1,4,41-44 Interactions with other suicide loss survivors provide hope, connection, and an “antidote” to stigma and shame. Optimally, group facilitators provide education, validation and normalization of the grief trajectory, and facilitate the sharing of both loss experiences and current functioning between group members. As a result, group participants often report renewed connections, increased efficacy in giving and accepting support, and decreased distress (including reductions in PTSD and depressive symptoms). The American Association of Suicidology (www.suicidology.org) and American Foundation of Suicide Prevention (www.afsp.org) provide contact information for suicide loss survivor groups (by geographical area) as well as information about online support groups.
Box 2
Goals and 'tasks' in suicide bereavement
The following goals and "tasks" should be part of the process of suicide bereavement:
- Reduce symptoms of posttraumatic stress disorder and other psychiatric disorders. Given the traumatic nature of the loss, an important goal is to understand and reduce posttraumatic stress disorder and other psychiatric symptoms, and incrementally improving functionality in relation to these.
- Integrate the loss. Recent authors36-38 have highlighted the need for survivors of suicide loss to "bear" and integrate the loss, as opposed to the concept of "getting over it." In these paradigms, the loss becomes an important part of one's identity, and eventually ceases to define it. Optimally, the "whole person" is remembered, not just the suicide. Part of this involves a reinvestment in life, with the acceptance of a "new normal" that takes the loss into account. It is not unusual for survivors of suicide loss to report some guilt in "moving on" and/or experiencing pleasure; often this is felt as a "betrayal" of the deceased.
- Create meaning from the loss. A major goal for those who have lost a loved one to suicide is the ability to find and/or create meaning from the loss. This would include the creation of a loss narrative39 that incorporates both ambiguity and complexity, as well as a regained/renewed sense of purpose in ongoing life.
- Develop ambiguity tolerance. A related "task" in suicide grief is the development of ambiguity tolerance, which generally includes an understanding of the complexity underlying suicide, the ability to offer oneself a "fair trial"30 in relation to one's realistic degree of responsibility, and the acceptance that many questions may remain unanswerable. In addition, in concert with the current understanding of "continuing bonds,"40 survivors should attempt to attend to the ongoing relationship with the deceased, including any "unfinished business."6
- Develop skills to manage stigmatized social responses and/or changes in family and social relationships.
- Memorialize and honor the deceased. Healing for survivors is facilitated by memorializations, which both validate the mourning process itself while also paying tribute to the richness of the deceased person's life.
- Post-traumatic growth. The relatively new understanding of "post-traumatic" growth is certainly applicable to the "unexpected gifts" many survivors of suicide loss report after they have moved through suicide grief. This includes greater understanding toward oneself, other survivors of suicide loss, and suicidal individuals; gratitude toward those who have provided support; and a desire to "use" their newfound understanding of suicide and suicide grief in ways to honor the deceased and benefit others. Feigelman et al1 found that many longer-term survivors of suicide loss engaged in both direct service and social activism around suicide pre- and postvention.
Individual treatment. The limited research on individual treatment for suicide loss survivors suggests that while most participants find it generally helpful, a significant number of others report that their therapists lack knowledge of suicide grief and endorse stigmatizing attitudes toward suicide and suicide loss survivors.45-46 In addition, Sanford et al3 found that survivors of suicide loss who endorsed PTSD symptoms were not assessed, diagnosed, or treated for these symptoms.
Continue to: This speaks to the importance of understanding what is...
This speaks to the importance of understanding what is “normative” for survivors of suicide loss. In general, “normalization” and psychoeducation about the suicide grief trajectory can play an important role in work with survivors of suicide loss, even in the presence of diagnosable disorders. While PTSD, depressive symptoms, and suicidal ideation are not uncommon in suicide loss survivors, and certainly may warrant clinical assessment and treatment, it can be helpful (and less stigmatizing) for your patients to know that these diagnoses are relatively common and understandable in the context of this devastating experience. For instance, survivors of suicide loss often report feeling relieved when clinicians explain the connections between traumatic loss and PTSD and/or depressive symptoms, and this can also help to relieve secondary anxiety about “going crazy.” Many survivors of suicide loss also describe feeling as though they are functioning on “autopilot” in the earlier stages of grief; it can help them understand the “function” of compartmentalization as potentially adaptive in the short run.
Suicide loss survivors may also find it helpful to learn about suicidal states of mind and their relationships to any types of mental illness their loved ones had struggled with.47
Your role: Help survivors integrate the loss
Before beginning treatment with an individual who has lost a loved one to suicide, clinicians should thoroughly explore their own understanding of and experience with suicide, including assumptions around causation, internalized stigma about suicidal individuals and survivors of suicide loss, their own history of suicide loss or suicidality, cultural/religious attitudes, and anxiety/defenses associated with the topic of suicide. These factors, particularly when unexamined, are likely to impact the treatment relationship and one’s clinical efficacy.
In concert with the existing literature, consider the potential goals and tasks involved in the integration of the individual’s suicide loss, along with any individual factors/variants that may impact the grief trajectory. Kosminsky and Jordon34 described the role of the clinician in this situation as a “transitional attachment figure” who facilitates the management and integration of the loss into the creation of what survivors of suicide loss have termed a “new normal.”
Because suicide loss is often associated with PTSD and other psychiatric illnesses (eg, depression, suicidality, substance abuse), it is essential to balance the assessment and treatment of these issues with attention to grief issues as needed. Again, to the extent that such issues have arisen primarily in the wake of the suicide loss, it can be helpful for patients to understand their connection to the context of the loss.
Continue to: Ideally, the clinician should...
Ideally, the clinician should be “present” with the patient’s pain, normative guilt, and rumination, without attempting to quickly eliminate or “fix” it or provide premature reassurance that the survivor of suicide loss “did nothing wrong.” Rather, as Jordan6 suggests, the clinician should act to promote a “fair trial” with respect to the patient’s guilt and blame, with an understanding of the “tyranny of hindsight.” The promotion of ambiguity tolerance should also play a role in coming to terms with many questions that may remain unanswered.
Optimally, clinicians should encourage patients to attend to their ongoing relationship with the deceased, particularly in the service of resolving “unfinished business,” ultimately integrating the loss into memories of the whole person. In line with this, survivors of suicide loss may be encouraged to create a narrative of the loss that incorporates both complexity and ambiguity. In the service of supporting the suicide loss survivor’s reinvestment in life, it is often helpful to facilitate their ability to anticipate and cope with triggers, such as anniversaries, birthdays, or holidays, as well as to develop and use skills for managing difficult or stigmatizing social or cultural reactions.
Any disruptions in family functioning should also be addressed. Psychoeducation about discordant grieving styles (particularly around gender) and the support of children’s grief may be helpful, and referrals to family or couples therapists should be considered as needed. Finally, the facilitation of suicide loss survivors’ creation of memorializations or rituals can help promote healing and make their loss meaningful.
Bottom Line
Losing a loved one to suicide is often a devastating and traumatic experience, but with optimal support, most survivors are ultimately able to integrate the loss and grow as a result. Understanding the suicide grief trajectory, as well as general guidelines for treatment, will facilitate healing and growth in the aftermath of suicide loss.
Related Resources
- Jordan JR, McIntosh JL. Grief after suicide: understanding the consequences and caring for the survivors. New York, NY: Routledge/Taylor & Francis Group; 2011.
- American Association of Suicidology. http://www.suicidology.org/.
- American Foundation for Suicide Prevention. https://afsp.org/.
1. Feigelman W, Jordan JR, McIntosh JL, et al. Devastating losses: how parents cope with the death of a child to suicide or drugs. New York, NY: Springer; 2012.
2. McIntosh JL. Research on survivors of suicide. In: Stimming MT, Stimming M, eds. Before their time: adult children’s experiences of parental suicide. Philadelphia, PA: Temple University Press; 1999:157-180.
3. Sanford RL, Cerel J, McGann VL, et al. Suicide loss survivors’ experiences with therapy: Implications for clinical practice. Community Ment Health J. 2016;5(2):551-558.
4. Jordan JR, McMenamy J. Interventions for suicide survivors: a review of the literature. Suicide Life Threat Behav. 2004;34(4):337-349.
5. Survivors of Suicide Loss Task Force. Responding to grief, trauma, & distress after a suicide: U.S. national guidelines. Washington, DC: National Action Alliance for Suicide Prevention; 2015.
6. Jordan JR, McGann V. Clinical work with suicide loss survivors: implications of the U.S. postvention guidelines. Death Stud. 2017;41(10):659-672.
7. Jordan JR. Is suicide bereavement different? A reassessment of the literature. Suicide Life Threat Behav. 2001;31(1):91-102.
8. Cvinar JG. Do suicide survivors suffer social stigma: a review of the literature. Perspect Psychiatr Care. 2005;41(1):14-21.
9. U.S. Public Health Service. The Surgeon General’s call to action to prevent suicide. Washington, DC: Department of Health and Human Services; 1999.
10. Doka KJ. Disenfranchised grief: recognizing hidden sorrow. Lexington, MA: Lexington; 1989.
11. Doka KJ. Disenfranchised grief: new directions, challenges, and strategies for practice. Champaign, IL: Research Press; 2002.
12. McIntosh JL. Suicide survivors: the aftermath of suicide and suicidal behavior. In: Bryant CD, ed. Handbook of death & dying. Vol. 1. Thousand Oaks, CA: SAGE Publications; 2003:339-350.
13. Jordan, JR, McIntosh, JL. Is suicide bereavement different? A framework for rethinking the question. In: Jordan JR, McIntosh JL, eds. Grief after suicide: understanding the consequences and caring for the survivors. New York, NY: Routledge/Taylor & Francis Group; 2011:19-42.
14. Dunne EJ, McIntosh JL, Dunne-Maxim K, eds. Suicide and its aftermath: understanding and counseling the survivors. New York, NY: W.W. Norton & Co.; 1987.
15. Harwood D, Hawton K, Hope T, et al. The grief experiences and needs of bereaved relatives and friends of older people dying through suicide: a descriptive and case-control study. J Affect Disord. 2002;72(2):185-194.
16. Armour, M. Violent death: understanding the context of traumatic and stigmatized grief. J Hum Behav Soc Environ. 2006;14(4):53-90.
17. Van Dongen CJ. Social context of postsuicide bereavement. Death Stud. 1993;17(2):125-141.
18. Calhoun LG, Allen BG. Social reactions to the survivor of a suicide in the family: A review of the literature. Omega – Journal of Death and Dying. 1991;23(2):95-107.
19. Range LM. When a loss is due to suicide: unique aspects of bereavement. In: Harvey JH, ed. Perspectives on loss: a sourcebook. Philadelphia, PA: Brunner/Mazel; 1998:213-220.
20. Sveen CA, Walby FA. Suicide survivors’ mental health and grief reactions: a systematic review of controlled studies. Suicide Life Threat Behav. 2008;38(1):13-29.
21. Feigelman W, Gorman BS, Jordan JR. Stigmatization and suicide bereavement. Death Stud. 2009;33(7):591-608.
22. Shneidman ES. Foreword. In: Cain AC, ed. Survivors of suicide. Springfield, IL: Charles C. Thomas; 1972:ix-xi.
23. Jordan JR, McIntosh, JL. Suicide bereavement: Why study survivors of suicide loss? In: Jordan JR, McIntosh JL, eds. Grief after suicide: understanding the consequences and caring for the survivors. New York, NY: Routledge/Taylor & Francis Group; 2011:3-18.
24. Agerbo E. Midlife suicide risk, partner’s psychiatric illness, spouse and child bereavement by suicide or other modes of death: a gender specific study. J Epidemiol Community Health. 2005;59(5):407-412.
25. Hedström P, Liu KY, Nordvik MK. Interaction domains and suicide: a population-based panel study of suicides in Stockholm, 1991-1999. Soc Forces. 2008;87(2):713-740.
26. Qin P, Agerbo E, Mortensen PB. Suicide risk in relation to family history of completed suicide and psychiatric disorders: a nested case-control study based on longitudinal registers. Lancet. 2002;360(9340):1126-1130.
27. Qin P, Mortensen PB. The impact of parental status on the risk of completed suicide. Arch Gen Psychiatry. 2003;60(8):797-802.
28. Neimeyer RA, Sands D. Suicide loss and the quest for meaning. In: Andriessen K, Krysinska K, Grad OT, eds. Postvention in action: the international handbook of suicide bereavement support. Cambridge, MA: Hogrefe; 2017:71-84.
29. Sands DC, Jordan JR, Neimeyer RA. The meanings of suicide: A narrative approach to healing. In: Jordan JR, McIntosh JL, eds. Grief after suicide: understanding the consequences and caring for the survivors. New York, NY: Routledge/Taylor & Francis Group; 2011:249-282.
30. Jordan JR. Principles of grief counseling with adult survivors. In: Jordan JR, McIntosh JL, eds. Grief after suicide: understanding the consequences and caring for the survivors. New York, NY: Routledge/Taylor & Francis Group; 2011:179-224.
31. Cerel J, Jordan JR, Duberstein PR. The impact of suicide on the family. Crisis. 2008;29:38-44.
32. Kaslow NJ, Samples TC, Rhodes M, et al. A family-oriented and culturally sensitive postvention approach with suicide survivors. In: Jordan JR, McIntosh JL, eds. Grief after suicide: understanding the consequences and caring for the survivors. New York, NY: Routledge/Taylor & Francis Group; 2011:301-323.
33. Grad OT, Treven M, Krysinska K. Suicide bereavement and gender. In: Andriessen K, Krysinska K, Grad OT, eds. Postvention in action: the international handbook of suicide bereavement support. Cambridge, MA: Hogrefe; 2017:39-49.
34. Kosminsky PS, Jordan JR. Attachment-informed grief therapy: the clinician’s guide to foundations and applications. New York, NY: Routledge; 2016.
35. Gutin N, McGann VL, Jordan JR. The impact of suicide on professional caregivers. In: Jordan JR, McIntosh JL, eds. Grief after suicide: understanding the consequences and caring for the survivors. New York, NY: Routledge/Taylor & Francis Group; 2011:93-111.
36. Jordan JR. Bereavement after suicide. Psychiatr Ann. 2008;38(10):679-685.
37. Jordan JR. After suicide: clinical work with survivors. Grief Matters. 2009;12(1):4-9.
38. Neimeyer, RA. Traumatic loss and the reconstruction of meaning. J Palliat Med. 2002;5(6):935-942; discussion 942-943.
39. Neimeyer R, ed. Meaning reconstruction & the experience of loss. Washington, DC: American Psychological Association; 2001.
40. Klass, D. Sorrow and solace: Neglected areas in bereavement research. Death Stud. 2013;37(7):597-616.
41. Farberow NL. The Los Angeles Survivors-After-Suicide program: an evaluation. Crisis. 1992;13(1):23-34.
42. McDaid C, Trowman R, Golder S, et al. Interventions for people bereaved through suicide: systematic review. Br J Psychiatry. 2008;193(6):438-443.
43. Groos AD, Shakespeare-Finch J. Positive experiences for participants in suicide bereavement groups: a grounded theory model. Death Stud. 2013;37(1):1-24.
44. Jordan JR. Group work with suicide survivors. In: Jordan JR, McIntosh JL, eds. Grief after suicide: understanding the consequences and caring for the survivors. New York, NY: Routledge/Taylor & Francis Group; 2011:283-300.
45. Wilson A, Marshall A. The support needs and experiences of suicidally bereaved family and friends. Death Stud. 2010;34(7):625-640.
46. McKinnon JM, Chonody J. Exploring the formal supports used by people bereaved through suicide: a qualitative study. Soc Work Ment Health. 2014;12(3):231-248.
47. Myers MF, Fine C. Touched by suicide: hope and healing after loss. New York, NY: Gotham Books; 2006.
1. Feigelman W, Jordan JR, McIntosh JL, et al. Devastating losses: how parents cope with the death of a child to suicide or drugs. New York, NY: Springer; 2012.
2. McIntosh JL. Research on survivors of suicide. In: Stimming MT, Stimming M, eds. Before their time: adult children’s experiences of parental suicide. Philadelphia, PA: Temple University Press; 1999:157-180.
3. Sanford RL, Cerel J, McGann VL, et al. Suicide loss survivors’ experiences with therapy: Implications for clinical practice. Community Ment Health J. 2016;5(2):551-558.
4. Jordan JR, McMenamy J. Interventions for suicide survivors: a review of the literature. Suicide Life Threat Behav. 2004;34(4):337-349.
5. Survivors of Suicide Loss Task Force. Responding to grief, trauma, & distress after a suicide: U.S. national guidelines. Washington, DC: National Action Alliance for Suicide Prevention; 2015.
6. Jordan JR, McGann V. Clinical work with suicide loss survivors: implications of the U.S. postvention guidelines. Death Stud. 2017;41(10):659-672.
7. Jordan JR. Is suicide bereavement different? A reassessment of the literature. Suicide Life Threat Behav. 2001;31(1):91-102.
8. Cvinar JG. Do suicide survivors suffer social stigma: a review of the literature. Perspect Psychiatr Care. 2005;41(1):14-21.
9. U.S. Public Health Service. The Surgeon General’s call to action to prevent suicide. Washington, DC: Department of Health and Human Services; 1999.
10. Doka KJ. Disenfranchised grief: recognizing hidden sorrow. Lexington, MA: Lexington; 1989.
11. Doka KJ. Disenfranchised grief: new directions, challenges, and strategies for practice. Champaign, IL: Research Press; 2002.
12. McIntosh JL. Suicide survivors: the aftermath of suicide and suicidal behavior. In: Bryant CD, ed. Handbook of death & dying. Vol. 1. Thousand Oaks, CA: SAGE Publications; 2003:339-350.
13. Jordan, JR, McIntosh, JL. Is suicide bereavement different? A framework for rethinking the question. In: Jordan JR, McIntosh JL, eds. Grief after suicide: understanding the consequences and caring for the survivors. New York, NY: Routledge/Taylor & Francis Group; 2011:19-42.
14. Dunne EJ, McIntosh JL, Dunne-Maxim K, eds. Suicide and its aftermath: understanding and counseling the survivors. New York, NY: W.W. Norton & Co.; 1987.
15. Harwood D, Hawton K, Hope T, et al. The grief experiences and needs of bereaved relatives and friends of older people dying through suicide: a descriptive and case-control study. J Affect Disord. 2002;72(2):185-194.
16. Armour, M. Violent death: understanding the context of traumatic and stigmatized grief. J Hum Behav Soc Environ. 2006;14(4):53-90.
17. Van Dongen CJ. Social context of postsuicide bereavement. Death Stud. 1993;17(2):125-141.
18. Calhoun LG, Allen BG. Social reactions to the survivor of a suicide in the family: A review of the literature. Omega – Journal of Death and Dying. 1991;23(2):95-107.
19. Range LM. When a loss is due to suicide: unique aspects of bereavement. In: Harvey JH, ed. Perspectives on loss: a sourcebook. Philadelphia, PA: Brunner/Mazel; 1998:213-220.
20. Sveen CA, Walby FA. Suicide survivors’ mental health and grief reactions: a systematic review of controlled studies. Suicide Life Threat Behav. 2008;38(1):13-29.
21. Feigelman W, Gorman BS, Jordan JR. Stigmatization and suicide bereavement. Death Stud. 2009;33(7):591-608.
22. Shneidman ES. Foreword. In: Cain AC, ed. Survivors of suicide. Springfield, IL: Charles C. Thomas; 1972:ix-xi.
23. Jordan JR, McIntosh, JL. Suicide bereavement: Why study survivors of suicide loss? In: Jordan JR, McIntosh JL, eds. Grief after suicide: understanding the consequences and caring for the survivors. New York, NY: Routledge/Taylor & Francis Group; 2011:3-18.
24. Agerbo E. Midlife suicide risk, partner’s psychiatric illness, spouse and child bereavement by suicide or other modes of death: a gender specific study. J Epidemiol Community Health. 2005;59(5):407-412.
25. Hedström P, Liu KY, Nordvik MK. Interaction domains and suicide: a population-based panel study of suicides in Stockholm, 1991-1999. Soc Forces. 2008;87(2):713-740.
26. Qin P, Agerbo E, Mortensen PB. Suicide risk in relation to family history of completed suicide and psychiatric disorders: a nested case-control study based on longitudinal registers. Lancet. 2002;360(9340):1126-1130.
27. Qin P, Mortensen PB. The impact of parental status on the risk of completed suicide. Arch Gen Psychiatry. 2003;60(8):797-802.
28. Neimeyer RA, Sands D. Suicide loss and the quest for meaning. In: Andriessen K, Krysinska K, Grad OT, eds. Postvention in action: the international handbook of suicide bereavement support. Cambridge, MA: Hogrefe; 2017:71-84.
29. Sands DC, Jordan JR, Neimeyer RA. The meanings of suicide: A narrative approach to healing. In: Jordan JR, McIntosh JL, eds. Grief after suicide: understanding the consequences and caring for the survivors. New York, NY: Routledge/Taylor & Francis Group; 2011:249-282.
30. Jordan JR. Principles of grief counseling with adult survivors. In: Jordan JR, McIntosh JL, eds. Grief after suicide: understanding the consequences and caring for the survivors. New York, NY: Routledge/Taylor & Francis Group; 2011:179-224.
31. Cerel J, Jordan JR, Duberstein PR. The impact of suicide on the family. Crisis. 2008;29:38-44.
32. Kaslow NJ, Samples TC, Rhodes M, et al. A family-oriented and culturally sensitive postvention approach with suicide survivors. In: Jordan JR, McIntosh JL, eds. Grief after suicide: understanding the consequences and caring for the survivors. New York, NY: Routledge/Taylor & Francis Group; 2011:301-323.
33. Grad OT, Treven M, Krysinska K. Suicide bereavement and gender. In: Andriessen K, Krysinska K, Grad OT, eds. Postvention in action: the international handbook of suicide bereavement support. Cambridge, MA: Hogrefe; 2017:39-49.
34. Kosminsky PS, Jordan JR. Attachment-informed grief therapy: the clinician’s guide to foundations and applications. New York, NY: Routledge; 2016.
35. Gutin N, McGann VL, Jordan JR. The impact of suicide on professional caregivers. In: Jordan JR, McIntosh JL, eds. Grief after suicide: understanding the consequences and caring for the survivors. New York, NY: Routledge/Taylor & Francis Group; 2011:93-111.
36. Jordan JR. Bereavement after suicide. Psychiatr Ann. 2008;38(10):679-685.
37. Jordan JR. After suicide: clinical work with survivors. Grief Matters. 2009;12(1):4-9.
38. Neimeyer, RA. Traumatic loss and the reconstruction of meaning. J Palliat Med. 2002;5(6):935-942; discussion 942-943.
39. Neimeyer R, ed. Meaning reconstruction & the experience of loss. Washington, DC: American Psychological Association; 2001.
40. Klass, D. Sorrow and solace: Neglected areas in bereavement research. Death Stud. 2013;37(7):597-616.
41. Farberow NL. The Los Angeles Survivors-After-Suicide program: an evaluation. Crisis. 1992;13(1):23-34.
42. McDaid C, Trowman R, Golder S, et al. Interventions for people bereaved through suicide: systematic review. Br J Psychiatry. 2008;193(6):438-443.
43. Groos AD, Shakespeare-Finch J. Positive experiences for participants in suicide bereavement groups: a grounded theory model. Death Stud. 2013;37(1):1-24.
44. Jordan JR. Group work with suicide survivors. In: Jordan JR, McIntosh JL, eds. Grief after suicide: understanding the consequences and caring for the survivors. New York, NY: Routledge/Taylor & Francis Group; 2011:283-300.
45. Wilson A, Marshall A. The support needs and experiences of suicidally bereaved family and friends. Death Stud. 2010;34(7):625-640.
46. McKinnon JM, Chonody J. Exploring the formal supports used by people bereaved through suicide: a qualitative study. Soc Work Ment Health. 2014;12(3):231-248.
47. Myers MF, Fine C. Touched by suicide: hope and healing after loss. New York, NY: Gotham Books; 2006.
How to avoid denied claims
Unless your practice is cash-only, reimbursements from your patients’ health insurance companies are necessary to ensure its survival. Although the reimbursement process appears straightforward (provide a service, submit a claim, and receive a payment), it is actually quite complex, and, if not properly managed, a claim can be denied at any stage of the process.1 In its 2013 National Health Insurer Report Card, the American Medical Association reported that major payers returned 11% to 29% of claim lines with $0 for payment.1,2 This often is the case because patients are responsible for the balance, but it also occurs as the result of claim edits (up to 7%) and other denials (up to 5%).1,2
Claims can be denied for various reasons, including1:
- missed filing deadlines
- billing for non-covered services
- discrepancies between diagnostic codes, procedures codes, modifiers, and clinician documentation
- missing pre-authorization documentation or a signed Advanced Beneficiary Notice of Non-Coverage.
Strategies for avoiding denials
A psychiatric practice requires a practical system to prevent the occurrence of denials, starting from the point of referral. Working through denials is more costly and timeconsuming than preventing them from occurring in the first place. For every 15 denials prevented each month, your practice can save approximately $4,500 per year in costs associated with correcting those claims; by preventing denials, the practice also receives reimbursement sooner.1 You can be guaranteed to leave significant amounts of money on the table if you are not able to prevent or reduce denials.
The following methods can be used to help reduce the likelihood of having a claim denied.1,3
Obtain the patient’s health insurance information at first contact and confirm his or her coverage benefits, deductibles, copay requirements, and exclusions before scheduling the first appointment. Verify this information at each of the patient’s subsequent visits to reduce the chances of having a claim denied due to invalid subscriber information. Also, keep in mind that Medicaid eligibility can change daily.
Employ a digital record system, such as electronic medical records, to track authorizations.
Know the filing deadlines for each of your payers. If you miss a deadline, there is no recourse.
Continue to: Check each claim
Check each claim for accurate coding, diagnosis, and payment (eg, copay, co-insurance, and/or deductible, depending on the health insurance plan) taken before the claim is submitted. If your practice size permits, assign a staff member to confirm this information and keep track of deadlines for submissions, resubmissions, and appeals of denied claims. Using a single gatekeeper can help decrease the chances that a denial will “slip through the cracks.”
Confirm that diagnostic codes, procedures codes, and modifiers are justified by the clinician’s documentation. Have a medical coder compare notes with the clinician to determine if any critical information needed to justify the codes used has been omitted.
Implement an electronic system that can automatically identify any changes and updates to the Centers for Medicare and Medicaid Services (CMS) regulations and guidance, International Classification of Diseases (ICD) versions and codes, and Current Procedural Terminology (CPT) codes and guidelines. To help reduce denied claims, educate all staff (schedulers, coders, billers, nursing staff, and other clinicians) frequently about these changes, and provide regular feedback to those involved in correcting denials.
1. Marting R. The cure for claims denials. Fam Pract Manag. 2015;22(2):7-10.
2. American Medical Association. 2013 National Health Insurer Report Card. Chicago, IL: American Medical Association; 2013.
3. Tohill M. 8 tips for avoiding denials, improving claims reimbursement . RevCycle Intelligence. https://revcycleintelligence.com/news/8-tips-for-avoiding-denials-improving-claims-reimbursement. Published June 6, 2016. Accessed February 19, 2018.
Unless your practice is cash-only, reimbursements from your patients’ health insurance companies are necessary to ensure its survival. Although the reimbursement process appears straightforward (provide a service, submit a claim, and receive a payment), it is actually quite complex, and, if not properly managed, a claim can be denied at any stage of the process.1 In its 2013 National Health Insurer Report Card, the American Medical Association reported that major payers returned 11% to 29% of claim lines with $0 for payment.1,2 This often is the case because patients are responsible for the balance, but it also occurs as the result of claim edits (up to 7%) and other denials (up to 5%).1,2
Claims can be denied for various reasons, including1:
- missed filing deadlines
- billing for non-covered services
- discrepancies between diagnostic codes, procedures codes, modifiers, and clinician documentation
- missing pre-authorization documentation or a signed Advanced Beneficiary Notice of Non-Coverage.
Strategies for avoiding denials
A psychiatric practice requires a practical system to prevent the occurrence of denials, starting from the point of referral. Working through denials is more costly and timeconsuming than preventing them from occurring in the first place. For every 15 denials prevented each month, your practice can save approximately $4,500 per year in costs associated with correcting those claims; by preventing denials, the practice also receives reimbursement sooner.1 You can be guaranteed to leave significant amounts of money on the table if you are not able to prevent or reduce denials.
The following methods can be used to help reduce the likelihood of having a claim denied.1,3
Obtain the patient’s health insurance information at first contact and confirm his or her coverage benefits, deductibles, copay requirements, and exclusions before scheduling the first appointment. Verify this information at each of the patient’s subsequent visits to reduce the chances of having a claim denied due to invalid subscriber information. Also, keep in mind that Medicaid eligibility can change daily.
Employ a digital record system, such as electronic medical records, to track authorizations.
Know the filing deadlines for each of your payers. If you miss a deadline, there is no recourse.
Continue to: Check each claim
Check each claim for accurate coding, diagnosis, and payment (eg, copay, co-insurance, and/or deductible, depending on the health insurance plan) taken before the claim is submitted. If your practice size permits, assign a staff member to confirm this information and keep track of deadlines for submissions, resubmissions, and appeals of denied claims. Using a single gatekeeper can help decrease the chances that a denial will “slip through the cracks.”
Confirm that diagnostic codes, procedures codes, and modifiers are justified by the clinician’s documentation. Have a medical coder compare notes with the clinician to determine if any critical information needed to justify the codes used has been omitted.
Implement an electronic system that can automatically identify any changes and updates to the Centers for Medicare and Medicaid Services (CMS) regulations and guidance, International Classification of Diseases (ICD) versions and codes, and Current Procedural Terminology (CPT) codes and guidelines. To help reduce denied claims, educate all staff (schedulers, coders, billers, nursing staff, and other clinicians) frequently about these changes, and provide regular feedback to those involved in correcting denials.
Unless your practice is cash-only, reimbursements from your patients’ health insurance companies are necessary to ensure its survival. Although the reimbursement process appears straightforward (provide a service, submit a claim, and receive a payment), it is actually quite complex, and, if not properly managed, a claim can be denied at any stage of the process.1 In its 2013 National Health Insurer Report Card, the American Medical Association reported that major payers returned 11% to 29% of claim lines with $0 for payment.1,2 This often is the case because patients are responsible for the balance, but it also occurs as the result of claim edits (up to 7%) and other denials (up to 5%).1,2
Claims can be denied for various reasons, including1:
- missed filing deadlines
- billing for non-covered services
- discrepancies between diagnostic codes, procedures codes, modifiers, and clinician documentation
- missing pre-authorization documentation or a signed Advanced Beneficiary Notice of Non-Coverage.
Strategies for avoiding denials
A psychiatric practice requires a practical system to prevent the occurrence of denials, starting from the point of referral. Working through denials is more costly and timeconsuming than preventing them from occurring in the first place. For every 15 denials prevented each month, your practice can save approximately $4,500 per year in costs associated with correcting those claims; by preventing denials, the practice also receives reimbursement sooner.1 You can be guaranteed to leave significant amounts of money on the table if you are not able to prevent or reduce denials.
The following methods can be used to help reduce the likelihood of having a claim denied.1,3
Obtain the patient’s health insurance information at first contact and confirm his or her coverage benefits, deductibles, copay requirements, and exclusions before scheduling the first appointment. Verify this information at each of the patient’s subsequent visits to reduce the chances of having a claim denied due to invalid subscriber information. Also, keep in mind that Medicaid eligibility can change daily.
Employ a digital record system, such as electronic medical records, to track authorizations.
Know the filing deadlines for each of your payers. If you miss a deadline, there is no recourse.
Continue to: Check each claim
Check each claim for accurate coding, diagnosis, and payment (eg, copay, co-insurance, and/or deductible, depending on the health insurance plan) taken before the claim is submitted. If your practice size permits, assign a staff member to confirm this information and keep track of deadlines for submissions, resubmissions, and appeals of denied claims. Using a single gatekeeper can help decrease the chances that a denial will “slip through the cracks.”
Confirm that diagnostic codes, procedures codes, and modifiers are justified by the clinician’s documentation. Have a medical coder compare notes with the clinician to determine if any critical information needed to justify the codes used has been omitted.
Implement an electronic system that can automatically identify any changes and updates to the Centers for Medicare and Medicaid Services (CMS) regulations and guidance, International Classification of Diseases (ICD) versions and codes, and Current Procedural Terminology (CPT) codes and guidelines. To help reduce denied claims, educate all staff (schedulers, coders, billers, nursing staff, and other clinicians) frequently about these changes, and provide regular feedback to those involved in correcting denials.
1. Marting R. The cure for claims denials. Fam Pract Manag. 2015;22(2):7-10.
2. American Medical Association. 2013 National Health Insurer Report Card. Chicago, IL: American Medical Association; 2013.
3. Tohill M. 8 tips for avoiding denials, improving claims reimbursement . RevCycle Intelligence. https://revcycleintelligence.com/news/8-tips-for-avoiding-denials-improving-claims-reimbursement. Published June 6, 2016. Accessed February 19, 2018.
1. Marting R. The cure for claims denials. Fam Pract Manag. 2015;22(2):7-10.
2. American Medical Association. 2013 National Health Insurer Report Card. Chicago, IL: American Medical Association; 2013.
3. Tohill M. 8 tips for avoiding denials, improving claims reimbursement . RevCycle Intelligence. https://revcycleintelligence.com/news/8-tips-for-avoiding-denials-improving-claims-reimbursement. Published June 6, 2016. Accessed February 19, 2018.
Clozapine-induced GI hypomotility: From constipation to bowel obstruction
Patients who are treated with clozapine—a second-generation antipsychotic approved for treatment-resistant schizophrenia—require monitoring for serious adverse effects. Many of these adverse effects, such as agranulocytosis or seizures, are familiar to clinicians; however, gastrointestinal (GI) hypomotility is not always recognized as a potentially serious adverse effect, even though it is one of the most common causes for hospital admission.1Its manifestations range from being relatively benign (nausea, vomiting, constipation) to potentially severe (fecal impaction) or even life-threatening (bowel obstruction, ileus, toxic megacolon).2
GI hypomotility is caused by clozapine’s strong anticholinergic properties, which lead to slowed smooth muscle contractions and delayed bowel transit time. It is further compounded by clozapine’s 5-HT3 antagonism, which is also known to slow bowel transit time. To avoid the potentially serious risks associated with GI hypomotility, we offer simple approaches for clinicians to follow when treating patients with clozapine.
Before starting a patient on clozapine, and at all subsequent visits, ask him or her about bowel habits and GI symptoms. Because the onset of GI hypomotility can be subtle, ask patients to pay close attention to their bowel habits and keep a diary to document GI symptoms and bowel movements. Signs of bowel obstruction can include an inability to pass stool, nausea, vomiting, abdominal pain, and a bloated abdomen. Staff who care for patients taking clozapine who live in a supervised setting should be educated about the relevance of a patient’s changing bowel habits or GI complaints. Also, teach patients about simple lifestyle modifications they can make to counteract constipation, including increased physical activity, adequate hydration, and consuming a fiber-rich diet.
If possible, avoid prescribing anticholinergic medications to a patient receiving clozapine because such agents may add to clozapine’s anticholinergic load. Some patients may require medical management of chronic constipation with stool softeners and stimulant laxatives. The Table outlines a typical bowel regimen we use for patients at our clinic. Some clinicians may prefer earlier and more regular use of senna glycoside. Also, patients might need a referral to their primary care physician if prevention has failed and fecal impaction requires enemas or mechanical disimpaction. An urgent referral to the emergency department is needed if a patient has a suspected bowel obstruction.
Acknowledgment
The authors thank Travis Baggett, MD, Department of Medicine, Massachusetts General Hospital, Boston, Massachusetts, for reviewing the medical management of constipation.
1. Stroup TS, Gerhard T, Crystal S, et al. Comparative effectiveness of clozapine and standard antipsychotic treatment in adults with schizophrenia. Am J Psychiatry. 2016;173(2):166-173.
2. Every-Palmer S, Ellis PM. Clozapine-induced gastrointestinal hypomotility: a 22-year bi-national pharmacovigilance study of serious or fatal ‘slow gut’ reactions, and comparison with international drug safety advice. CNS Drugs. 2017;31(8):699-709.
Patients who are treated with clozapine—a second-generation antipsychotic approved for treatment-resistant schizophrenia—require monitoring for serious adverse effects. Many of these adverse effects, such as agranulocytosis or seizures, are familiar to clinicians; however, gastrointestinal (GI) hypomotility is not always recognized as a potentially serious adverse effect, even though it is one of the most common causes for hospital admission.1Its manifestations range from being relatively benign (nausea, vomiting, constipation) to potentially severe (fecal impaction) or even life-threatening (bowel obstruction, ileus, toxic megacolon).2
GI hypomotility is caused by clozapine’s strong anticholinergic properties, which lead to slowed smooth muscle contractions and delayed bowel transit time. It is further compounded by clozapine’s 5-HT3 antagonism, which is also known to slow bowel transit time. To avoid the potentially serious risks associated with GI hypomotility, we offer simple approaches for clinicians to follow when treating patients with clozapine.
Before starting a patient on clozapine, and at all subsequent visits, ask him or her about bowel habits and GI symptoms. Because the onset of GI hypomotility can be subtle, ask patients to pay close attention to their bowel habits and keep a diary to document GI symptoms and bowel movements. Signs of bowel obstruction can include an inability to pass stool, nausea, vomiting, abdominal pain, and a bloated abdomen. Staff who care for patients taking clozapine who live in a supervised setting should be educated about the relevance of a patient’s changing bowel habits or GI complaints. Also, teach patients about simple lifestyle modifications they can make to counteract constipation, including increased physical activity, adequate hydration, and consuming a fiber-rich diet.
If possible, avoid prescribing anticholinergic medications to a patient receiving clozapine because such agents may add to clozapine’s anticholinergic load. Some patients may require medical management of chronic constipation with stool softeners and stimulant laxatives. The Table outlines a typical bowel regimen we use for patients at our clinic. Some clinicians may prefer earlier and more regular use of senna glycoside. Also, patients might need a referral to their primary care physician if prevention has failed and fecal impaction requires enemas or mechanical disimpaction. An urgent referral to the emergency department is needed if a patient has a suspected bowel obstruction.
Acknowledgment
The authors thank Travis Baggett, MD, Department of Medicine, Massachusetts General Hospital, Boston, Massachusetts, for reviewing the medical management of constipation.
Patients who are treated with clozapine—a second-generation antipsychotic approved for treatment-resistant schizophrenia—require monitoring for serious adverse effects. Many of these adverse effects, such as agranulocytosis or seizures, are familiar to clinicians; however, gastrointestinal (GI) hypomotility is not always recognized as a potentially serious adverse effect, even though it is one of the most common causes for hospital admission.1Its manifestations range from being relatively benign (nausea, vomiting, constipation) to potentially severe (fecal impaction) or even life-threatening (bowel obstruction, ileus, toxic megacolon).2
GI hypomotility is caused by clozapine’s strong anticholinergic properties, which lead to slowed smooth muscle contractions and delayed bowel transit time. It is further compounded by clozapine’s 5-HT3 antagonism, which is also known to slow bowel transit time. To avoid the potentially serious risks associated with GI hypomotility, we offer simple approaches for clinicians to follow when treating patients with clozapine.
Before starting a patient on clozapine, and at all subsequent visits, ask him or her about bowel habits and GI symptoms. Because the onset of GI hypomotility can be subtle, ask patients to pay close attention to their bowel habits and keep a diary to document GI symptoms and bowel movements. Signs of bowel obstruction can include an inability to pass stool, nausea, vomiting, abdominal pain, and a bloated abdomen. Staff who care for patients taking clozapine who live in a supervised setting should be educated about the relevance of a patient’s changing bowel habits or GI complaints. Also, teach patients about simple lifestyle modifications they can make to counteract constipation, including increased physical activity, adequate hydration, and consuming a fiber-rich diet.
If possible, avoid prescribing anticholinergic medications to a patient receiving clozapine because such agents may add to clozapine’s anticholinergic load. Some patients may require medical management of chronic constipation with stool softeners and stimulant laxatives. The Table outlines a typical bowel regimen we use for patients at our clinic. Some clinicians may prefer earlier and more regular use of senna glycoside. Also, patients might need a referral to their primary care physician if prevention has failed and fecal impaction requires enemas or mechanical disimpaction. An urgent referral to the emergency department is needed if a patient has a suspected bowel obstruction.
Acknowledgment
The authors thank Travis Baggett, MD, Department of Medicine, Massachusetts General Hospital, Boston, Massachusetts, for reviewing the medical management of constipation.
1. Stroup TS, Gerhard T, Crystal S, et al. Comparative effectiveness of clozapine and standard antipsychotic treatment in adults with schizophrenia. Am J Psychiatry. 2016;173(2):166-173.
2. Every-Palmer S, Ellis PM. Clozapine-induced gastrointestinal hypomotility: a 22-year bi-national pharmacovigilance study of serious or fatal ‘slow gut’ reactions, and comparison with international drug safety advice. CNS Drugs. 2017;31(8):699-709.
1. Stroup TS, Gerhard T, Crystal S, et al. Comparative effectiveness of clozapine and standard antipsychotic treatment in adults with schizophrenia. Am J Psychiatry. 2016;173(2):166-173.
2. Every-Palmer S, Ellis PM. Clozapine-induced gastrointestinal hypomotility: a 22-year bi-national pharmacovigilance study of serious or fatal ‘slow gut’ reactions, and comparison with international drug safety advice. CNS Drugs. 2017;31(8):699-709.
FAST and RAPID: Acronyms to prevent brain damage in stroke and psychosis
Psychosis and stroke are arguably the most serious acute threats to the integrity of the brain, and consequently the mind. Both are unquestionably associated with a grave outcome the longer their treatment is delayed.1,2
While the management of stroke has been elevated to the highest emergent priority because of the progressively deleterious impact of thrombotic ischemia in one of the cerebral arteries, rapid intervention for acute psychosis has never been regarded as an urgent neurologic condition with severe threats to the brain’s structure and function.3,4 There is extensive literature on the serious consequences of a long duration of untreated psychosis (DUP), including treatment resistance, frequent re-hospitalizations, more negative symptoms, and greater disability.5
Physical paralysis from a stroke receives much more attention than “mental paralysis” of psychosis. Both must be rapidly treated, whether for the regional ischemia to brain tissue following a stroke or for the neurotoxicity of neuroinflammation and oxidative stress that lead to widespread neurodegeneration during psychosis.6 While an acronym for the quick recognition of a stroke (FAST: Facial drooping, Arm weakness, Speech difficulties, and Time to call emergency services) is well established, no acronym for the urgency to treat psychosis has been developed. We propose the acronym RAPID (Readily Avoid Psychosis-Induced Damage). The acronym RAPID would hopefully expedite the urgently needed pharmacotherapeutic and psychosocial intervention in psychosis to halt ongoing brain tissue loss.
It is ironic that the legal obstacles for immediate treatment, which do not exist for stroke, often delay administering antipsychotic medication to patients with anosognosia (a neurologic delusional belief that one is not ill, leading to refusal of treatment) for their psychosis and end up harming patients by prolonging their DUP until a court order is obtained to force brain-saving treatment. Frequent psychotic relapses due to nonadherence with medications are also a very common cause for prolonged DUP due to the inexplicable reluctance of some psychiatric practitioners to employ a long-acting injectable antipsychotic (LAI) medication as soon as possible after the onset of psychosis to circumvent subsequent relapses due to the very high risk of poor adherence. Table 1 describes the optimal management of both stroke and acute psychosis once they are rapidly diagnosed, thanks to the FAST and RAPID reminders.
Tragically, the treatments of the mind have become falsely disengaged from the brain, the physical organ whose neurons, electrical impulses, synapses, and neurotransmitters generate the mind with its advanced human functions, such as self-awareness, will, thoughts, mood, speech, executive functions, memories, and social cognition. Recent editorials have challenged psychiatric practitioners to behave like cardiologists7 and oncologists8 by aggressively treating first-episode psychosis to prevent ongoing neurodegeneration due to recurrences. The brain loses 1% of its brain volume (~11 ml) after the first psychotic episode,8 which represents hundreds of millions of cells, billions of synapses, and substantial myelin. A second psychotic episode causes significant additional neuropil and white matter fiber damage and represents a different stage of schizophrenia9 with more severe tissue loss and disruption of neural pathways that trigger the process of treatment resistance and functional disability. Ensuring adherence with LAI antipsychotic formulations immediately after the first psychotic episode may allow many patients with schizophrenia to achieve a relapse-free remission and to return to their baseline functioning.10
In addition to significant brain tissue loss during psychotic episodes, mortality is also a very high risk following discharge from the first hospitalization for psychosis.11 LAI second-generation antipsychotic medications have been shown to be associated with lower mortality and neuroprotective effects,12 compared with oral or injectable first-generation antipsychotics. The highest mortality rate was reported to be associated with the lack of any antipsychotic medication,12 underscoring how untreated psychosis can be fatal.
Bottom line: Rapid treatment of stroke and psychosis is an absolute imperative for minimizing brain damage that respectively leads to physical or mental disability. The acronyms FAST and RAPID are essential reminders of the urgency needed to halt progressive neurodegeneration in those 2 devastating acute threats to the integrity of brain and mind. Intensive physical, psychological, and social rehabilitation must follow the acute treatment of stroke and psychosis, and the prevention of any recurrence is an absolute must. For psychosis, the use of a LAI second-generation antipsychotic before hospital discharge from a first episode of psychosis can be disease-modifying, with a more benign illness trajectory and outcome than the devastating deterioration that follows repetitive psychotic relapse, most often due to nonadherence with oral medications.
Continue to: Psychosis should be conceptualized as...
Psychosis should be conceptualized as a “stroke of the mind,” and it can be prevented in most patients with schizophrenia by adopting injectable antipsychotics as early after the onset of psychosis as possible. Yet, starting a LAI antipsychotic drug in first-episode psychosis before hospital discharge is rarely done, and the few patients who currently receive LAIs (10% of U.S. patients) generally receive them after multiple episodes and a protracted DUP. That’s like calling the fire department when much of the house has turned to ashes, instead of calling them when the first small flame is noticed. It makes so much sense, but the decades-old practice of postponing the use of LAIs continues to ruin the lives of young persons in the prime of life. By changing our practice habits to early use of LAIs, we have nothing to lose and our patients with psychosis may be spared a lifetime of suffering, poverty, stigma, incarceration, and functional disability. Wouldn’t we want to avoid that atrocious outcome for our own family members if they develop schizophrenia?
1. Benjamin EJ, Virani SS, Callaway CW, et al; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2018 Update: a report from the American Heart Association. Circulation. 2018;137(12):e67-e492.
2. Cechnicki A, Cichocki Ł, Kalisz A, et al. Duration of untreated psychosis (DUP) and the course of schizophrenia in a 20-year follow-up study. Psychiatry Res. 2014;219(3):420-425.
3. Davis J, Moylan S, Harvey BH, et al. Neuroprogression in schizophrenia: pathways underpinning clinical staging and therapeutic corollaries. Aust N Z J Psychiatry. 2014;48(6):512-529.
4. Olabi B, Ellison-Wright I, McIntosh AM, et al. Are there progressive brain changes in schizophrenia? A meta-analysis of structural magnetic resonance imaging studies. Biol Psychiatry. 2011;70(1):88-96.
5. Kane JM, Robinson DG, Schooler NR, et al. Comprehensive versus usual community care for first-episode psychosis: 2-year outcomes from the NIMH RAISE Early Treatment Program. Am J Psychiatry. 2016;173(4):362-372.
6. Najjar S, Pearlman DM. Neuroinflammation and white matter pathology in schizophrenia: systematic review. Schizophr Res. 2015;161(1):102-112.
7. Nasrallah HA. For first-episode psychosis, psychiatrists should behave like cardiologists. Current Psychiatry. 2017;16(8):4-7.
8. Cahn W, Hulshoff Pol HE, Lems EB, et al. Brain volume changes in first-episode schizophrenia: a 1-year follow-up study. Arch Gen Psychiatry. 2002;59(11):1002-1010.
9. McGorry P, Nelson B. Why we need a transdiagnostic staging approach to emerging psychopathology, early diagnosis, and treatment. JAMA Psychiatry. 2016;73(3):191-192.
10. Emsley R, Oosthuizen P, Koen L, et al. Remission in patients with first-episode schizophrenia receiving assured antipsychotic medication: a study with risperidone long-acting injection. Int Clin Psychopharmacol. 2008;23(6):325-331.
11. Nasrallah HA. The crisis of poor physical health and early mortality of psychiatric patients. Current Psychiatry. 2018;17(4):7-8,11.
12. Nasrallah HA. Triple advantages of injectable long acting second generation antipsychotics: relapse prevention, neuroprotection, and lower mortality. Schizophr Res. 2018;197:69-70.
Psychosis and stroke are arguably the most serious acute threats to the integrity of the brain, and consequently the mind. Both are unquestionably associated with a grave outcome the longer their treatment is delayed.1,2
While the management of stroke has been elevated to the highest emergent priority because of the progressively deleterious impact of thrombotic ischemia in one of the cerebral arteries, rapid intervention for acute psychosis has never been regarded as an urgent neurologic condition with severe threats to the brain’s structure and function.3,4 There is extensive literature on the serious consequences of a long duration of untreated psychosis (DUP), including treatment resistance, frequent re-hospitalizations, more negative symptoms, and greater disability.5
Physical paralysis from a stroke receives much more attention than “mental paralysis” of psychosis. Both must be rapidly treated, whether for the regional ischemia to brain tissue following a stroke or for the neurotoxicity of neuroinflammation and oxidative stress that lead to widespread neurodegeneration during psychosis.6 While an acronym for the quick recognition of a stroke (FAST: Facial drooping, Arm weakness, Speech difficulties, and Time to call emergency services) is well established, no acronym for the urgency to treat psychosis has been developed. We propose the acronym RAPID (Readily Avoid Psychosis-Induced Damage). The acronym RAPID would hopefully expedite the urgently needed pharmacotherapeutic and psychosocial intervention in psychosis to halt ongoing brain tissue loss.
It is ironic that the legal obstacles for immediate treatment, which do not exist for stroke, often delay administering antipsychotic medication to patients with anosognosia (a neurologic delusional belief that one is not ill, leading to refusal of treatment) for their psychosis and end up harming patients by prolonging their DUP until a court order is obtained to force brain-saving treatment. Frequent psychotic relapses due to nonadherence with medications are also a very common cause for prolonged DUP due to the inexplicable reluctance of some psychiatric practitioners to employ a long-acting injectable antipsychotic (LAI) medication as soon as possible after the onset of psychosis to circumvent subsequent relapses due to the very high risk of poor adherence. Table 1 describes the optimal management of both stroke and acute psychosis once they are rapidly diagnosed, thanks to the FAST and RAPID reminders.
Tragically, the treatments of the mind have become falsely disengaged from the brain, the physical organ whose neurons, electrical impulses, synapses, and neurotransmitters generate the mind with its advanced human functions, such as self-awareness, will, thoughts, mood, speech, executive functions, memories, and social cognition. Recent editorials have challenged psychiatric practitioners to behave like cardiologists7 and oncologists8 by aggressively treating first-episode psychosis to prevent ongoing neurodegeneration due to recurrences. The brain loses 1% of its brain volume (~11 ml) after the first psychotic episode,8 which represents hundreds of millions of cells, billions of synapses, and substantial myelin. A second psychotic episode causes significant additional neuropil and white matter fiber damage and represents a different stage of schizophrenia9 with more severe tissue loss and disruption of neural pathways that trigger the process of treatment resistance and functional disability. Ensuring adherence with LAI antipsychotic formulations immediately after the first psychotic episode may allow many patients with schizophrenia to achieve a relapse-free remission and to return to their baseline functioning.10
In addition to significant brain tissue loss during psychotic episodes, mortality is also a very high risk following discharge from the first hospitalization for psychosis.11 LAI second-generation antipsychotic medications have been shown to be associated with lower mortality and neuroprotective effects,12 compared with oral or injectable first-generation antipsychotics. The highest mortality rate was reported to be associated with the lack of any antipsychotic medication,12 underscoring how untreated psychosis can be fatal.
Bottom line: Rapid treatment of stroke and psychosis is an absolute imperative for minimizing brain damage that respectively leads to physical or mental disability. The acronyms FAST and RAPID are essential reminders of the urgency needed to halt progressive neurodegeneration in those 2 devastating acute threats to the integrity of brain and mind. Intensive physical, psychological, and social rehabilitation must follow the acute treatment of stroke and psychosis, and the prevention of any recurrence is an absolute must. For psychosis, the use of a LAI second-generation antipsychotic before hospital discharge from a first episode of psychosis can be disease-modifying, with a more benign illness trajectory and outcome than the devastating deterioration that follows repetitive psychotic relapse, most often due to nonadherence with oral medications.
Continue to: Psychosis should be conceptualized as...
Psychosis should be conceptualized as a “stroke of the mind,” and it can be prevented in most patients with schizophrenia by adopting injectable antipsychotics as early after the onset of psychosis as possible. Yet, starting a LAI antipsychotic drug in first-episode psychosis before hospital discharge is rarely done, and the few patients who currently receive LAIs (10% of U.S. patients) generally receive them after multiple episodes and a protracted DUP. That’s like calling the fire department when much of the house has turned to ashes, instead of calling them when the first small flame is noticed. It makes so much sense, but the decades-old practice of postponing the use of LAIs continues to ruin the lives of young persons in the prime of life. By changing our practice habits to early use of LAIs, we have nothing to lose and our patients with psychosis may be spared a lifetime of suffering, poverty, stigma, incarceration, and functional disability. Wouldn’t we want to avoid that atrocious outcome for our own family members if they develop schizophrenia?
Psychosis and stroke are arguably the most serious acute threats to the integrity of the brain, and consequently the mind. Both are unquestionably associated with a grave outcome the longer their treatment is delayed.1,2
While the management of stroke has been elevated to the highest emergent priority because of the progressively deleterious impact of thrombotic ischemia in one of the cerebral arteries, rapid intervention for acute psychosis has never been regarded as an urgent neurologic condition with severe threats to the brain’s structure and function.3,4 There is extensive literature on the serious consequences of a long duration of untreated psychosis (DUP), including treatment resistance, frequent re-hospitalizations, more negative symptoms, and greater disability.5
Physical paralysis from a stroke receives much more attention than “mental paralysis” of psychosis. Both must be rapidly treated, whether for the regional ischemia to brain tissue following a stroke or for the neurotoxicity of neuroinflammation and oxidative stress that lead to widespread neurodegeneration during psychosis.6 While an acronym for the quick recognition of a stroke (FAST: Facial drooping, Arm weakness, Speech difficulties, and Time to call emergency services) is well established, no acronym for the urgency to treat psychosis has been developed. We propose the acronym RAPID (Readily Avoid Psychosis-Induced Damage). The acronym RAPID would hopefully expedite the urgently needed pharmacotherapeutic and psychosocial intervention in psychosis to halt ongoing brain tissue loss.
It is ironic that the legal obstacles for immediate treatment, which do not exist for stroke, often delay administering antipsychotic medication to patients with anosognosia (a neurologic delusional belief that one is not ill, leading to refusal of treatment) for their psychosis and end up harming patients by prolonging their DUP until a court order is obtained to force brain-saving treatment. Frequent psychotic relapses due to nonadherence with medications are also a very common cause for prolonged DUP due to the inexplicable reluctance of some psychiatric practitioners to employ a long-acting injectable antipsychotic (LAI) medication as soon as possible after the onset of psychosis to circumvent subsequent relapses due to the very high risk of poor adherence. Table 1 describes the optimal management of both stroke and acute psychosis once they are rapidly diagnosed, thanks to the FAST and RAPID reminders.
Tragically, the treatments of the mind have become falsely disengaged from the brain, the physical organ whose neurons, electrical impulses, synapses, and neurotransmitters generate the mind with its advanced human functions, such as self-awareness, will, thoughts, mood, speech, executive functions, memories, and social cognition. Recent editorials have challenged psychiatric practitioners to behave like cardiologists7 and oncologists8 by aggressively treating first-episode psychosis to prevent ongoing neurodegeneration due to recurrences. The brain loses 1% of its brain volume (~11 ml) after the first psychotic episode,8 which represents hundreds of millions of cells, billions of synapses, and substantial myelin. A second psychotic episode causes significant additional neuropil and white matter fiber damage and represents a different stage of schizophrenia9 with more severe tissue loss and disruption of neural pathways that trigger the process of treatment resistance and functional disability. Ensuring adherence with LAI antipsychotic formulations immediately after the first psychotic episode may allow many patients with schizophrenia to achieve a relapse-free remission and to return to their baseline functioning.10
In addition to significant brain tissue loss during psychotic episodes, mortality is also a very high risk following discharge from the first hospitalization for psychosis.11 LAI second-generation antipsychotic medications have been shown to be associated with lower mortality and neuroprotective effects,12 compared with oral or injectable first-generation antipsychotics. The highest mortality rate was reported to be associated with the lack of any antipsychotic medication,12 underscoring how untreated psychosis can be fatal.
Bottom line: Rapid treatment of stroke and psychosis is an absolute imperative for minimizing brain damage that respectively leads to physical or mental disability. The acronyms FAST and RAPID are essential reminders of the urgency needed to halt progressive neurodegeneration in those 2 devastating acute threats to the integrity of brain and mind. Intensive physical, psychological, and social rehabilitation must follow the acute treatment of stroke and psychosis, and the prevention of any recurrence is an absolute must. For psychosis, the use of a LAI second-generation antipsychotic before hospital discharge from a first episode of psychosis can be disease-modifying, with a more benign illness trajectory and outcome than the devastating deterioration that follows repetitive psychotic relapse, most often due to nonadherence with oral medications.
Continue to: Psychosis should be conceptualized as...
Psychosis should be conceptualized as a “stroke of the mind,” and it can be prevented in most patients with schizophrenia by adopting injectable antipsychotics as early after the onset of psychosis as possible. Yet, starting a LAI antipsychotic drug in first-episode psychosis before hospital discharge is rarely done, and the few patients who currently receive LAIs (10% of U.S. patients) generally receive them after multiple episodes and a protracted DUP. That’s like calling the fire department when much of the house has turned to ashes, instead of calling them when the first small flame is noticed. It makes so much sense, but the decades-old practice of postponing the use of LAIs continues to ruin the lives of young persons in the prime of life. By changing our practice habits to early use of LAIs, we have nothing to lose and our patients with psychosis may be spared a lifetime of suffering, poverty, stigma, incarceration, and functional disability. Wouldn’t we want to avoid that atrocious outcome for our own family members if they develop schizophrenia?
1. Benjamin EJ, Virani SS, Callaway CW, et al; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2018 Update: a report from the American Heart Association. Circulation. 2018;137(12):e67-e492.
2. Cechnicki A, Cichocki Ł, Kalisz A, et al. Duration of untreated psychosis (DUP) and the course of schizophrenia in a 20-year follow-up study. Psychiatry Res. 2014;219(3):420-425.
3. Davis J, Moylan S, Harvey BH, et al. Neuroprogression in schizophrenia: pathways underpinning clinical staging and therapeutic corollaries. Aust N Z J Psychiatry. 2014;48(6):512-529.
4. Olabi B, Ellison-Wright I, McIntosh AM, et al. Are there progressive brain changes in schizophrenia? A meta-analysis of structural magnetic resonance imaging studies. Biol Psychiatry. 2011;70(1):88-96.
5. Kane JM, Robinson DG, Schooler NR, et al. Comprehensive versus usual community care for first-episode psychosis: 2-year outcomes from the NIMH RAISE Early Treatment Program. Am J Psychiatry. 2016;173(4):362-372.
6. Najjar S, Pearlman DM. Neuroinflammation and white matter pathology in schizophrenia: systematic review. Schizophr Res. 2015;161(1):102-112.
7. Nasrallah HA. For first-episode psychosis, psychiatrists should behave like cardiologists. Current Psychiatry. 2017;16(8):4-7.
8. Cahn W, Hulshoff Pol HE, Lems EB, et al. Brain volume changes in first-episode schizophrenia: a 1-year follow-up study. Arch Gen Psychiatry. 2002;59(11):1002-1010.
9. McGorry P, Nelson B. Why we need a transdiagnostic staging approach to emerging psychopathology, early diagnosis, and treatment. JAMA Psychiatry. 2016;73(3):191-192.
10. Emsley R, Oosthuizen P, Koen L, et al. Remission in patients with first-episode schizophrenia receiving assured antipsychotic medication: a study with risperidone long-acting injection. Int Clin Psychopharmacol. 2008;23(6):325-331.
11. Nasrallah HA. The crisis of poor physical health and early mortality of psychiatric patients. Current Psychiatry. 2018;17(4):7-8,11.
12. Nasrallah HA. Triple advantages of injectable long acting second generation antipsychotics: relapse prevention, neuroprotection, and lower mortality. Schizophr Res. 2018;197:69-70.
1. Benjamin EJ, Virani SS, Callaway CW, et al; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2018 Update: a report from the American Heart Association. Circulation. 2018;137(12):e67-e492.
2. Cechnicki A, Cichocki Ł, Kalisz A, et al. Duration of untreated psychosis (DUP) and the course of schizophrenia in a 20-year follow-up study. Psychiatry Res. 2014;219(3):420-425.
3. Davis J, Moylan S, Harvey BH, et al. Neuroprogression in schizophrenia: pathways underpinning clinical staging and therapeutic corollaries. Aust N Z J Psychiatry. 2014;48(6):512-529.
4. Olabi B, Ellison-Wright I, McIntosh AM, et al. Are there progressive brain changes in schizophrenia? A meta-analysis of structural magnetic resonance imaging studies. Biol Psychiatry. 2011;70(1):88-96.
5. Kane JM, Robinson DG, Schooler NR, et al. Comprehensive versus usual community care for first-episode psychosis: 2-year outcomes from the NIMH RAISE Early Treatment Program. Am J Psychiatry. 2016;173(4):362-372.
6. Najjar S, Pearlman DM. Neuroinflammation and white matter pathology in schizophrenia: systematic review. Schizophr Res. 2015;161(1):102-112.
7. Nasrallah HA. For first-episode psychosis, psychiatrists should behave like cardiologists. Current Psychiatry. 2017;16(8):4-7.
8. Cahn W, Hulshoff Pol HE, Lems EB, et al. Brain volume changes in first-episode schizophrenia: a 1-year follow-up study. Arch Gen Psychiatry. 2002;59(11):1002-1010.
9. McGorry P, Nelson B. Why we need a transdiagnostic staging approach to emerging psychopathology, early diagnosis, and treatment. JAMA Psychiatry. 2016;73(3):191-192.
10. Emsley R, Oosthuizen P, Koen L, et al. Remission in patients with first-episode schizophrenia receiving assured antipsychotic medication: a study with risperidone long-acting injection. Int Clin Psychopharmacol. 2008;23(6):325-331.
11. Nasrallah HA. The crisis of poor physical health and early mortality of psychiatric patients. Current Psychiatry. 2018;17(4):7-8,11.
12. Nasrallah HA. Triple advantages of injectable long acting second generation antipsychotics: relapse prevention, neuroprotection, and lower mortality. Schizophr Res. 2018;197:69-70.
Bugs on her skin—but nobody else sees them
CASE Scratching, anxious, and hopeless
Ms. L, age 74, who is paraplegic and uses a wheelchair, presents to our hospital’s emergency department (ED) accompanied by staff from the nursing home where she resides. She reports that she can feel and see bugs crawling all over her skin, biting
Ms. L experiences generalized pruritus with excoriations scattered over her upper and lower extremities and her trunk. She copes with the pruritus by scratching. She reports that the bugs are present throughout the day and are worse at night when she tries to go to bed. Nothing she does provides relief from the infestation. Earlier, at the nursing home, Ms. L had obtained a detergent powder and used it in an attempt to purge the bugs. She now has large swaths of irritated skin, mostly on her lower back and perineal region.
She says the bug infestation became unbearable 3 weeks ago, but she can’t identify any precipitants for her symptoms. Ms. L reports that the impact of the bugs on her daily activity, sleep, and quality of life is enormous. Despite her complaints, neither the nursing home staff nor the ED staff can find any evidence of bugs on Ms. L’s clothes or skin.
Because Ms. L resorted to such drastic measures in her attempt to rid her body of the bugs, she is considered a safety risk and is admitted to the psychiatric unit, although she vehemently denies any intention to harm herself.
On the psychiatric unit, Ms. L states that the infestation began approximately 2 years ago. She began to experience severe worsening of her symptoms a few weeks before presenting to the ED.
During evaluation, Ms. L is alert and oriented to person, place, and situation. She is also quite cooperative but guarded in describing her infestation. There is some degree of suspiciousness and paranoia with regards to her infestation; she is very sensitive to how the clinical staff respond to her condition. She appears worried, and exhibits anxiety, sadness, hopelessness, and tearfulness. Her thought process is goal-directed, but preoccupied by the bugs.
[polldaddy:10064801]
Continue to: The authors' observations
The authors’ observations
Delusional parasitosis is a rare disorder that is defined by an individual having a fixed, false belief that he or she is being infected or grossly invaded by a living organism. Karl A. Ekbom, a Swedish neurologist, was the first practitioner to definitively describe this affliction in 1938.1
Primary delusional parasitosis is a disease defined by this single psychotic symptom without other classic symptoms of schizophrenia; this single symptom cannot be attributed to the effects of substance abuse or a medical condition. Many affected patients remain functional in their daily lives; only a minority of patients experience delusions that interfere with usual activity.2 Secondary delusional parasitosis is a symptom of another psychiatric or medical disease.
Morgellons disease is characterized by symptoms similar to primary delusional parasitosis, but symptoms of this condition also include the delusional belief that inanimate objects, usually fibers, are in the skin as well as the parasites.3
A population-based study among individuals living in Olmsted County, Minnesota from 1976 to 2010 found that the incidence of delusional infestation was 1.9 cases (95% confidence interval, 1.5 to 2.4) per 100,000 person-years.4 In a retrospective study of 147 patients with delusional parasitosis, 33% of these patients described themselves as disabled, 28% were retired, and 26% were employed.5 In this study, the mean age of diagnosis was 57, with a female-to-male ratio of 2.89:1.5
Continue to: HISTORY Prior psychiatric hospitalization
HISTORY Prior psychiatric hospitalization
Ms. L, who is divorced and retired, lives in a nursing home and has no pets, no exposure to scabies, no recent travel, no allergies, and no difficulty with her hygiene except at the peak of her illness. She denies any alcohol or illicit drug use but reports a 6 pack year history of smoking. She has a son, 2 grandchildren, and 2 great grandchildren who all live in town and see her regularly. She reports no history of arrests or legal problems.
Ms. L has a history of depression and anxiety that culminated in a “nervous breakdown” in 1985 with a brief stay in a psychiatric hospital. She reports that she had seen a therapist for 6 years as part of her treatment following that event. During her hospitalization, she was treated with a tricyclic antidepressant and received electroconvulsive therapy. She denies being suicidal during the incident in 1985 or at any point in time before or since then. She now takes venlafaxine, 75 mg/d, for depression and anxiety.
Ms. L’s paraplegia resulted from her sixth corrective surgery for scoliosis, which occurred 6 years ago. She has had chronic pain since this surgery. Her medical history also includes hypertension, atrial fibrillation, mild neurocognitive changes, and gastroesophageal reflux disease.
EVALUATION Skin examination, blood analysis normal
On admission, Ms. L undergoes a skin examination, which yields no evidence consistent with infestation with Pediculus humanus corporis (body louse) or Sarcoptes scabiei (scabies).6 Blood analysis shows no iron deficiency, renal failure, hyperbilirubinemia, or eosinophilia. In the ED, the medical team examines Ms. L and explores other medical and dermatological causes of her condition. Because dermatological causes had been ruled out before Ms. L was admitted to the inpatient psychiatric unit, no dermatology consult is requested.
Continue to: TREATMENT A first-generation antipsychotic
TREATMENT A first-generation antipsychotic
When Ms. L is admitted to the psychiatric unit, she is started
During the week, Ms. L’s perphenazine is titrated up to 24 mg twice daily and venlafaxine is titrated to 150 mg/d. A Montreal Cognitive Assessment (MoCA) is performed within the first 2 days of admission and she scores 16/30, indicating moderate cognitive impairment. On Friday, the attending physician explains that her medications should start to have therapeutic effect. During this time, this clinician engages in cognitive restructuring by providing validation of Ms. L’s suffering, verbal support, and medication compliance counseling. At this time, the treating team also suggests to Ms. L that she should expect the activity and effects of the bugs to dissipate. She is receptive to this suggestion. She also participates in the milieu, including unit activities, but is limited in her ability to engage in group therapy due to the intensity of her illness.
Throughout the weekend, the on-call physician also engages Ms. L and reports minor improvement.
OUTCOME Significant relief
On re-evaluation Monday morning—almost a week after Ms. L had been admitted to the inpatient psychiatric unit—she has achieved significant relief from her delusions. She says that she has no idea where the bugs have gone. Ms. L appears to be a completely different person. She no longer appears guarded. The suspiciousness, paranoia, hopelessness, and negative outlook she previously experienced have significantly diminished. Her MoCA score improves to 25/30, indicating no cognitive impairment (Table). She is discharged after a 7-night stay on the inpatient psychiatric unit.
Continue to: The authors' observations
The authors’ observations
During one of the clinical multidisciplinary treatment team meetings held for Ms. L, it was initially estimated that it would take at least 2 weeks for the delusional parasitosis to significantly respond to antipsychotic therapy. However, it is our professional opinion that the applied cognitive restructuring, with validation of her suffering, verbal support, and medication adherence counseling, expedited her recovery. This coincided with the aggressive titration of her antipsychotic and antidepressant, although the treatment team’s acknowledgment of Ms. L’s misery appeared to lower her guard and make her more susceptible to the power of cognitive restructuring. The efforts to validate the patient’s feelings and decrease hopelessness by telling her that the medication would make the bugs go away appeared to be the tipping point for her recovery. Patients with primary delusional parasitosis often are guarded and may feel alone in their predicament when they are met with perplexed responses from individuals with whom they discuss their symptoms. Compared with patients with schizophrenia, patients with delusional parasitosis maintain normal cognitive functioning, which may give them the insight to understand how their experience may be perceived as incompatible with reality.7 This understanding, coupled with some perceived helplessness, can lead a patient to fear having a severe mental decompensation, which can contribute to a delayed or complicated recovery.
The cognitive process described above might have been responsible for the difference in Ms. L’s MoCA scores because her performance in the initial test was hindered by her constant obsession with the bugs, which made her distracted during the test. By the time she responded to treatment, she gained significant clarity of thought, which enabled her to perform optimally in the test.
The difficulty in treating patients with delusional parasitosis may be further affected by lack of insight, and the fact that they often do not present to a psychiatrist for treatment in a timely manner because their delusion is impregnable and presents them with an alternate reality. These patients are more likely to seek out primary care physicians, dermatologists, infectious disease doctors, and entomologists because of the fervor of their delusion and the intensity of their discomfort. Because of this, a collaboration between these providers would likely lead to improved care and treatment acceptance for patients with delusional parasitosis.
Antipsychotics are the preferred medication for treating delusional parasitosis, and the literature supports their use for this purpose.6,8 The overall response rate is 60% to 100%.6 Previously, in small placebo-controlled trials, the first-generation antipsychotic (FGA) pimozide was considered first-line treatment for this disease.6 However, this antipsychotic is no longer favored because evidence is mounting that other FGAs result in comparable response rates with fewer tolerability issues.8,9
The bulk of data on the use of antipsychotics for treating delusional parasitosis comes from retrospective case reports and case series.6 Multiple antipsychotics have been shown to be effective in treating delusional parasitosis, including both FGAs and second-generation antipsychotics (SGAs).6,10 Published case reports and series have shown the effectiveness of the FGAs
Continue to: The SGAs risperidone, olanzapine, aripiprazole...
The SGAs
When selecting antipsychotic therapy for a patient diagnosed with delusional parasitosis, consider patient-specific factors, such as age, medication history, comorbidities, and the adverse-effect profile of the medication(s). These medications should be started at a low dose and titrated based on efficacy and safety. The optimal duration of therapy varies by patient. Patients should continually be assessed for possible treatment discontinuation, although if therapy is tapered off, patients need to be closely monitored for possible relapse or recurrence of symptoms.
Ms. L received perphenazine titrated up to 24 mg/d for the treatment of delusional parasitosis. The maximum dose used for Ms. L was higher than those used in previous reports, although she appeared to tolerate the medication well and respond rapidly. Her symptoms showed improvement within 1 week. Importantly, in published case reports, patients have been resistant to the use of psychotropic medications without other treatment modalities (eg, psychotherapy, various behavioral approaches). We conclude that Ms. L’s response was attributable to the use of the combination of psychotherapeutic techniques and the effectiveness of perphenazine and venlafaxine.
Bottom Line
Managing patients with primary delusional parasitosis can be challenging due to the fixed nature of the delusion. A combination of antipsychotics and psychotherapeutic techniques can benefit some patients. The optimal duration of treatment varies by patient.
Related Resource
- Trenton A, Pansare N, Tobia A, et al. Delusional parasitosis on the psychiatric consultation service-a longitudinal perspective: case study. BJPsych Open. 2017;3(3):154-158.
Drug Brand Names
Aripiprazole • Abilify
Haloperidol • Haldol
Olanzapine • Zyprexa
Paliperidone • Invega
Paliperidone palmitate • Invega Sustenna
Perphenazine • Trilafon
Pimozide • Orap
Quetiapine • Seroquel
Risperidone • Risperdal
Venlafaxine • Effexor
Ziprasidone • Geodon
1. Ekbom KA. Der präsenile dermatozoenwahn [in Swedish]. Acta Psychiatr Neurol Scand. 1938;13(3):227-259.
2. Lynch PJ. Delusions of parasitosis. Semin Dermatol. 1993;12(1):39-45.
3. Middelveen MJ, Fesler MC, Stricker RB. History of Morgellons disease: from delusion to definition. Clin Cosmet Investig Dermatol. 2018;11:71-90.
4. Bailey CH, Andersen LK, Lowe GC, et al. A population-based study of the incidence of delusional infestation in Olmsted County, Minnesota, 1976–2010. Br J Dermatol. 2014;170(5):1130-1135.
5. Foster AA, Hylwa SA, Bury JE, et al. Delusional infestation: clinical presentation in 147 patients seen at Mayo Clinic. J Am Acad Dermatol. 2012;67(4):673.e1-e10.
6. Lepping P, Russell I, Freudenmann RW. Antipsychotic treatment of primary delusional parasitosis: systematic review. Br J Psychiatry. 2007;191(3):198-205.
7. Freudenmann RW, Lepping P. Delusional infestation. Clin Microbiol Rev. 2009;22(4):690-732.
8. Mercan S, Altunay IK, Taskintuna N, et al. Atypical antipsychotic drugs in the treatment of delusional parasitosis. Intl J Psychiatry Med. 2007:37(1):29-37.
9. Trabert W. 100 years of delusional parasitosis. Meta-analysis of 1,223 case reports. Psychopathology. 1995;28(5):238-246.
10. Freudenmann RW, Lepping P. Second-generation antipsychotics in primary and secondary delusional parasitosis. J Clin Psychopharmacol. 2008;28(5):500-508.
11. Boggild AK, Nicks BA, Yen L, et al. Delusional parasitosis: six-year experience with 23 consecutive cases at an academic medical center. Int J Infect Dis. 2010;14(4):e317-e321.
CASE Scratching, anxious, and hopeless
Ms. L, age 74, who is paraplegic and uses a wheelchair, presents to our hospital’s emergency department (ED) accompanied by staff from the nursing home where she resides. She reports that she can feel and see bugs crawling all over her skin, biting
Ms. L experiences generalized pruritus with excoriations scattered over her upper and lower extremities and her trunk. She copes with the pruritus by scratching. She reports that the bugs are present throughout the day and are worse at night when she tries to go to bed. Nothing she does provides relief from the infestation. Earlier, at the nursing home, Ms. L had obtained a detergent powder and used it in an attempt to purge the bugs. She now has large swaths of irritated skin, mostly on her lower back and perineal region.
She says the bug infestation became unbearable 3 weeks ago, but she can’t identify any precipitants for her symptoms. Ms. L reports that the impact of the bugs on her daily activity, sleep, and quality of life is enormous. Despite her complaints, neither the nursing home staff nor the ED staff can find any evidence of bugs on Ms. L’s clothes or skin.
Because Ms. L resorted to such drastic measures in her attempt to rid her body of the bugs, she is considered a safety risk and is admitted to the psychiatric unit, although she vehemently denies any intention to harm herself.
On the psychiatric unit, Ms. L states that the infestation began approximately 2 years ago. She began to experience severe worsening of her symptoms a few weeks before presenting to the ED.
During evaluation, Ms. L is alert and oriented to person, place, and situation. She is also quite cooperative but guarded in describing her infestation. There is some degree of suspiciousness and paranoia with regards to her infestation; she is very sensitive to how the clinical staff respond to her condition. She appears worried, and exhibits anxiety, sadness, hopelessness, and tearfulness. Her thought process is goal-directed, but preoccupied by the bugs.
[polldaddy:10064801]
Continue to: The authors' observations
The authors’ observations
Delusional parasitosis is a rare disorder that is defined by an individual having a fixed, false belief that he or she is being infected or grossly invaded by a living organism. Karl A. Ekbom, a Swedish neurologist, was the first practitioner to definitively describe this affliction in 1938.1
Primary delusional parasitosis is a disease defined by this single psychotic symptom without other classic symptoms of schizophrenia; this single symptom cannot be attributed to the effects of substance abuse or a medical condition. Many affected patients remain functional in their daily lives; only a minority of patients experience delusions that interfere with usual activity.2 Secondary delusional parasitosis is a symptom of another psychiatric or medical disease.
Morgellons disease is characterized by symptoms similar to primary delusional parasitosis, but symptoms of this condition also include the delusional belief that inanimate objects, usually fibers, are in the skin as well as the parasites.3
A population-based study among individuals living in Olmsted County, Minnesota from 1976 to 2010 found that the incidence of delusional infestation was 1.9 cases (95% confidence interval, 1.5 to 2.4) per 100,000 person-years.4 In a retrospective study of 147 patients with delusional parasitosis, 33% of these patients described themselves as disabled, 28% were retired, and 26% were employed.5 In this study, the mean age of diagnosis was 57, with a female-to-male ratio of 2.89:1.5
Continue to: HISTORY Prior psychiatric hospitalization
HISTORY Prior psychiatric hospitalization
Ms. L, who is divorced and retired, lives in a nursing home and has no pets, no exposure to scabies, no recent travel, no allergies, and no difficulty with her hygiene except at the peak of her illness. She denies any alcohol or illicit drug use but reports a 6 pack year history of smoking. She has a son, 2 grandchildren, and 2 great grandchildren who all live in town and see her regularly. She reports no history of arrests or legal problems.
Ms. L has a history of depression and anxiety that culminated in a “nervous breakdown” in 1985 with a brief stay in a psychiatric hospital. She reports that she had seen a therapist for 6 years as part of her treatment following that event. During her hospitalization, she was treated with a tricyclic antidepressant and received electroconvulsive therapy. She denies being suicidal during the incident in 1985 or at any point in time before or since then. She now takes venlafaxine, 75 mg/d, for depression and anxiety.
Ms. L’s paraplegia resulted from her sixth corrective surgery for scoliosis, which occurred 6 years ago. She has had chronic pain since this surgery. Her medical history also includes hypertension, atrial fibrillation, mild neurocognitive changes, and gastroesophageal reflux disease.
EVALUATION Skin examination, blood analysis normal
On admission, Ms. L undergoes a skin examination, which yields no evidence consistent with infestation with Pediculus humanus corporis (body louse) or Sarcoptes scabiei (scabies).6 Blood analysis shows no iron deficiency, renal failure, hyperbilirubinemia, or eosinophilia. In the ED, the medical team examines Ms. L and explores other medical and dermatological causes of her condition. Because dermatological causes had been ruled out before Ms. L was admitted to the inpatient psychiatric unit, no dermatology consult is requested.
Continue to: TREATMENT A first-generation antipsychotic
TREATMENT A first-generation antipsychotic
When Ms. L is admitted to the psychiatric unit, she is started
During the week, Ms. L’s perphenazine is titrated up to 24 mg twice daily and venlafaxine is titrated to 150 mg/d. A Montreal Cognitive Assessment (MoCA) is performed within the first 2 days of admission and she scores 16/30, indicating moderate cognitive impairment. On Friday, the attending physician explains that her medications should start to have therapeutic effect. During this time, this clinician engages in cognitive restructuring by providing validation of Ms. L’s suffering, verbal support, and medication compliance counseling. At this time, the treating team also suggests to Ms. L that she should expect the activity and effects of the bugs to dissipate. She is receptive to this suggestion. She also participates in the milieu, including unit activities, but is limited in her ability to engage in group therapy due to the intensity of her illness.
Throughout the weekend, the on-call physician also engages Ms. L and reports minor improvement.
OUTCOME Significant relief
On re-evaluation Monday morning—almost a week after Ms. L had been admitted to the inpatient psychiatric unit—she has achieved significant relief from her delusions. She says that she has no idea where the bugs have gone. Ms. L appears to be a completely different person. She no longer appears guarded. The suspiciousness, paranoia, hopelessness, and negative outlook she previously experienced have significantly diminished. Her MoCA score improves to 25/30, indicating no cognitive impairment (Table). She is discharged after a 7-night stay on the inpatient psychiatric unit.
Continue to: The authors' observations
The authors’ observations
During one of the clinical multidisciplinary treatment team meetings held for Ms. L, it was initially estimated that it would take at least 2 weeks for the delusional parasitosis to significantly respond to antipsychotic therapy. However, it is our professional opinion that the applied cognitive restructuring, with validation of her suffering, verbal support, and medication adherence counseling, expedited her recovery. This coincided with the aggressive titration of her antipsychotic and antidepressant, although the treatment team’s acknowledgment of Ms. L’s misery appeared to lower her guard and make her more susceptible to the power of cognitive restructuring. The efforts to validate the patient’s feelings and decrease hopelessness by telling her that the medication would make the bugs go away appeared to be the tipping point for her recovery. Patients with primary delusional parasitosis often are guarded and may feel alone in their predicament when they are met with perplexed responses from individuals with whom they discuss their symptoms. Compared with patients with schizophrenia, patients with delusional parasitosis maintain normal cognitive functioning, which may give them the insight to understand how their experience may be perceived as incompatible with reality.7 This understanding, coupled with some perceived helplessness, can lead a patient to fear having a severe mental decompensation, which can contribute to a delayed or complicated recovery.
The cognitive process described above might have been responsible for the difference in Ms. L’s MoCA scores because her performance in the initial test was hindered by her constant obsession with the bugs, which made her distracted during the test. By the time she responded to treatment, she gained significant clarity of thought, which enabled her to perform optimally in the test.
The difficulty in treating patients with delusional parasitosis may be further affected by lack of insight, and the fact that they often do not present to a psychiatrist for treatment in a timely manner because their delusion is impregnable and presents them with an alternate reality. These patients are more likely to seek out primary care physicians, dermatologists, infectious disease doctors, and entomologists because of the fervor of their delusion and the intensity of their discomfort. Because of this, a collaboration between these providers would likely lead to improved care and treatment acceptance for patients with delusional parasitosis.
Antipsychotics are the preferred medication for treating delusional parasitosis, and the literature supports their use for this purpose.6,8 The overall response rate is 60% to 100%.6 Previously, in small placebo-controlled trials, the first-generation antipsychotic (FGA) pimozide was considered first-line treatment for this disease.6 However, this antipsychotic is no longer favored because evidence is mounting that other FGAs result in comparable response rates with fewer tolerability issues.8,9
The bulk of data on the use of antipsychotics for treating delusional parasitosis comes from retrospective case reports and case series.6 Multiple antipsychotics have been shown to be effective in treating delusional parasitosis, including both FGAs and second-generation antipsychotics (SGAs).6,10 Published case reports and series have shown the effectiveness of the FGAs
Continue to: The SGAs risperidone, olanzapine, aripiprazole...
The SGAs
When selecting antipsychotic therapy for a patient diagnosed with delusional parasitosis, consider patient-specific factors, such as age, medication history, comorbidities, and the adverse-effect profile of the medication(s). These medications should be started at a low dose and titrated based on efficacy and safety. The optimal duration of therapy varies by patient. Patients should continually be assessed for possible treatment discontinuation, although if therapy is tapered off, patients need to be closely monitored for possible relapse or recurrence of symptoms.
Ms. L received perphenazine titrated up to 24 mg/d for the treatment of delusional parasitosis. The maximum dose used for Ms. L was higher than those used in previous reports, although she appeared to tolerate the medication well and respond rapidly. Her symptoms showed improvement within 1 week. Importantly, in published case reports, patients have been resistant to the use of psychotropic medications without other treatment modalities (eg, psychotherapy, various behavioral approaches). We conclude that Ms. L’s response was attributable to the use of the combination of psychotherapeutic techniques and the effectiveness of perphenazine and venlafaxine.
Bottom Line
Managing patients with primary delusional parasitosis can be challenging due to the fixed nature of the delusion. A combination of antipsychotics and psychotherapeutic techniques can benefit some patients. The optimal duration of treatment varies by patient.
Related Resource
- Trenton A, Pansare N, Tobia A, et al. Delusional parasitosis on the psychiatric consultation service-a longitudinal perspective: case study. BJPsych Open. 2017;3(3):154-158.
Drug Brand Names
Aripiprazole • Abilify
Haloperidol • Haldol
Olanzapine • Zyprexa
Paliperidone • Invega
Paliperidone palmitate • Invega Sustenna
Perphenazine • Trilafon
Pimozide • Orap
Quetiapine • Seroquel
Risperidone • Risperdal
Venlafaxine • Effexor
Ziprasidone • Geodon
CASE Scratching, anxious, and hopeless
Ms. L, age 74, who is paraplegic and uses a wheelchair, presents to our hospital’s emergency department (ED) accompanied by staff from the nursing home where she resides. She reports that she can feel and see bugs crawling all over her skin, biting
Ms. L experiences generalized pruritus with excoriations scattered over her upper and lower extremities and her trunk. She copes with the pruritus by scratching. She reports that the bugs are present throughout the day and are worse at night when she tries to go to bed. Nothing she does provides relief from the infestation. Earlier, at the nursing home, Ms. L had obtained a detergent powder and used it in an attempt to purge the bugs. She now has large swaths of irritated skin, mostly on her lower back and perineal region.
She says the bug infestation became unbearable 3 weeks ago, but she can’t identify any precipitants for her symptoms. Ms. L reports that the impact of the bugs on her daily activity, sleep, and quality of life is enormous. Despite her complaints, neither the nursing home staff nor the ED staff can find any evidence of bugs on Ms. L’s clothes or skin.
Because Ms. L resorted to such drastic measures in her attempt to rid her body of the bugs, she is considered a safety risk and is admitted to the psychiatric unit, although she vehemently denies any intention to harm herself.
On the psychiatric unit, Ms. L states that the infestation began approximately 2 years ago. She began to experience severe worsening of her symptoms a few weeks before presenting to the ED.
During evaluation, Ms. L is alert and oriented to person, place, and situation. She is also quite cooperative but guarded in describing her infestation. There is some degree of suspiciousness and paranoia with regards to her infestation; she is very sensitive to how the clinical staff respond to her condition. She appears worried, and exhibits anxiety, sadness, hopelessness, and tearfulness. Her thought process is goal-directed, but preoccupied by the bugs.
[polldaddy:10064801]
Continue to: The authors' observations
The authors’ observations
Delusional parasitosis is a rare disorder that is defined by an individual having a fixed, false belief that he or she is being infected or grossly invaded by a living organism. Karl A. Ekbom, a Swedish neurologist, was the first practitioner to definitively describe this affliction in 1938.1
Primary delusional parasitosis is a disease defined by this single psychotic symptom without other classic symptoms of schizophrenia; this single symptom cannot be attributed to the effects of substance abuse or a medical condition. Many affected patients remain functional in their daily lives; only a minority of patients experience delusions that interfere with usual activity.2 Secondary delusional parasitosis is a symptom of another psychiatric or medical disease.
Morgellons disease is characterized by symptoms similar to primary delusional parasitosis, but symptoms of this condition also include the delusional belief that inanimate objects, usually fibers, are in the skin as well as the parasites.3
A population-based study among individuals living in Olmsted County, Minnesota from 1976 to 2010 found that the incidence of delusional infestation was 1.9 cases (95% confidence interval, 1.5 to 2.4) per 100,000 person-years.4 In a retrospective study of 147 patients with delusional parasitosis, 33% of these patients described themselves as disabled, 28% were retired, and 26% were employed.5 In this study, the mean age of diagnosis was 57, with a female-to-male ratio of 2.89:1.5
Continue to: HISTORY Prior psychiatric hospitalization
HISTORY Prior psychiatric hospitalization
Ms. L, who is divorced and retired, lives in a nursing home and has no pets, no exposure to scabies, no recent travel, no allergies, and no difficulty with her hygiene except at the peak of her illness. She denies any alcohol or illicit drug use but reports a 6 pack year history of smoking. She has a son, 2 grandchildren, and 2 great grandchildren who all live in town and see her regularly. She reports no history of arrests or legal problems.
Ms. L has a history of depression and anxiety that culminated in a “nervous breakdown” in 1985 with a brief stay in a psychiatric hospital. She reports that she had seen a therapist for 6 years as part of her treatment following that event. During her hospitalization, she was treated with a tricyclic antidepressant and received electroconvulsive therapy. She denies being suicidal during the incident in 1985 or at any point in time before or since then. She now takes venlafaxine, 75 mg/d, for depression and anxiety.
Ms. L’s paraplegia resulted from her sixth corrective surgery for scoliosis, which occurred 6 years ago. She has had chronic pain since this surgery. Her medical history also includes hypertension, atrial fibrillation, mild neurocognitive changes, and gastroesophageal reflux disease.
EVALUATION Skin examination, blood analysis normal
On admission, Ms. L undergoes a skin examination, which yields no evidence consistent with infestation with Pediculus humanus corporis (body louse) or Sarcoptes scabiei (scabies).6 Blood analysis shows no iron deficiency, renal failure, hyperbilirubinemia, or eosinophilia. In the ED, the medical team examines Ms. L and explores other medical and dermatological causes of her condition. Because dermatological causes had been ruled out before Ms. L was admitted to the inpatient psychiatric unit, no dermatology consult is requested.
Continue to: TREATMENT A first-generation antipsychotic
TREATMENT A first-generation antipsychotic
When Ms. L is admitted to the psychiatric unit, she is started
During the week, Ms. L’s perphenazine is titrated up to 24 mg twice daily and venlafaxine is titrated to 150 mg/d. A Montreal Cognitive Assessment (MoCA) is performed within the first 2 days of admission and she scores 16/30, indicating moderate cognitive impairment. On Friday, the attending physician explains that her medications should start to have therapeutic effect. During this time, this clinician engages in cognitive restructuring by providing validation of Ms. L’s suffering, verbal support, and medication compliance counseling. At this time, the treating team also suggests to Ms. L that she should expect the activity and effects of the bugs to dissipate. She is receptive to this suggestion. She also participates in the milieu, including unit activities, but is limited in her ability to engage in group therapy due to the intensity of her illness.
Throughout the weekend, the on-call physician also engages Ms. L and reports minor improvement.
OUTCOME Significant relief
On re-evaluation Monday morning—almost a week after Ms. L had been admitted to the inpatient psychiatric unit—she has achieved significant relief from her delusions. She says that she has no idea where the bugs have gone. Ms. L appears to be a completely different person. She no longer appears guarded. The suspiciousness, paranoia, hopelessness, and negative outlook she previously experienced have significantly diminished. Her MoCA score improves to 25/30, indicating no cognitive impairment (Table). She is discharged after a 7-night stay on the inpatient psychiatric unit.
Continue to: The authors' observations
The authors’ observations
During one of the clinical multidisciplinary treatment team meetings held for Ms. L, it was initially estimated that it would take at least 2 weeks for the delusional parasitosis to significantly respond to antipsychotic therapy. However, it is our professional opinion that the applied cognitive restructuring, with validation of her suffering, verbal support, and medication adherence counseling, expedited her recovery. This coincided with the aggressive titration of her antipsychotic and antidepressant, although the treatment team’s acknowledgment of Ms. L’s misery appeared to lower her guard and make her more susceptible to the power of cognitive restructuring. The efforts to validate the patient’s feelings and decrease hopelessness by telling her that the medication would make the bugs go away appeared to be the tipping point for her recovery. Patients with primary delusional parasitosis often are guarded and may feel alone in their predicament when they are met with perplexed responses from individuals with whom they discuss their symptoms. Compared with patients with schizophrenia, patients with delusional parasitosis maintain normal cognitive functioning, which may give them the insight to understand how their experience may be perceived as incompatible with reality.7 This understanding, coupled with some perceived helplessness, can lead a patient to fear having a severe mental decompensation, which can contribute to a delayed or complicated recovery.
The cognitive process described above might have been responsible for the difference in Ms. L’s MoCA scores because her performance in the initial test was hindered by her constant obsession with the bugs, which made her distracted during the test. By the time she responded to treatment, she gained significant clarity of thought, which enabled her to perform optimally in the test.
The difficulty in treating patients with delusional parasitosis may be further affected by lack of insight, and the fact that they often do not present to a psychiatrist for treatment in a timely manner because their delusion is impregnable and presents them with an alternate reality. These patients are more likely to seek out primary care physicians, dermatologists, infectious disease doctors, and entomologists because of the fervor of their delusion and the intensity of their discomfort. Because of this, a collaboration between these providers would likely lead to improved care and treatment acceptance for patients with delusional parasitosis.
Antipsychotics are the preferred medication for treating delusional parasitosis, and the literature supports their use for this purpose.6,8 The overall response rate is 60% to 100%.6 Previously, in small placebo-controlled trials, the first-generation antipsychotic (FGA) pimozide was considered first-line treatment for this disease.6 However, this antipsychotic is no longer favored because evidence is mounting that other FGAs result in comparable response rates with fewer tolerability issues.8,9
The bulk of data on the use of antipsychotics for treating delusional parasitosis comes from retrospective case reports and case series.6 Multiple antipsychotics have been shown to be effective in treating delusional parasitosis, including both FGAs and second-generation antipsychotics (SGAs).6,10 Published case reports and series have shown the effectiveness of the FGAs
Continue to: The SGAs risperidone, olanzapine, aripiprazole...
The SGAs
When selecting antipsychotic therapy for a patient diagnosed with delusional parasitosis, consider patient-specific factors, such as age, medication history, comorbidities, and the adverse-effect profile of the medication(s). These medications should be started at a low dose and titrated based on efficacy and safety. The optimal duration of therapy varies by patient. Patients should continually be assessed for possible treatment discontinuation, although if therapy is tapered off, patients need to be closely monitored for possible relapse or recurrence of symptoms.
Ms. L received perphenazine titrated up to 24 mg/d for the treatment of delusional parasitosis. The maximum dose used for Ms. L was higher than those used in previous reports, although she appeared to tolerate the medication well and respond rapidly. Her symptoms showed improvement within 1 week. Importantly, in published case reports, patients have been resistant to the use of psychotropic medications without other treatment modalities (eg, psychotherapy, various behavioral approaches). We conclude that Ms. L’s response was attributable to the use of the combination of psychotherapeutic techniques and the effectiveness of perphenazine and venlafaxine.
Bottom Line
Managing patients with primary delusional parasitosis can be challenging due to the fixed nature of the delusion. A combination of antipsychotics and psychotherapeutic techniques can benefit some patients. The optimal duration of treatment varies by patient.
Related Resource
- Trenton A, Pansare N, Tobia A, et al. Delusional parasitosis on the psychiatric consultation service-a longitudinal perspective: case study. BJPsych Open. 2017;3(3):154-158.
Drug Brand Names
Aripiprazole • Abilify
Haloperidol • Haldol
Olanzapine • Zyprexa
Paliperidone • Invega
Paliperidone palmitate • Invega Sustenna
Perphenazine • Trilafon
Pimozide • Orap
Quetiapine • Seroquel
Risperidone • Risperdal
Venlafaxine • Effexor
Ziprasidone • Geodon
1. Ekbom KA. Der präsenile dermatozoenwahn [in Swedish]. Acta Psychiatr Neurol Scand. 1938;13(3):227-259.
2. Lynch PJ. Delusions of parasitosis. Semin Dermatol. 1993;12(1):39-45.
3. Middelveen MJ, Fesler MC, Stricker RB. History of Morgellons disease: from delusion to definition. Clin Cosmet Investig Dermatol. 2018;11:71-90.
4. Bailey CH, Andersen LK, Lowe GC, et al. A population-based study of the incidence of delusional infestation in Olmsted County, Minnesota, 1976–2010. Br J Dermatol. 2014;170(5):1130-1135.
5. Foster AA, Hylwa SA, Bury JE, et al. Delusional infestation: clinical presentation in 147 patients seen at Mayo Clinic. J Am Acad Dermatol. 2012;67(4):673.e1-e10.
6. Lepping P, Russell I, Freudenmann RW. Antipsychotic treatment of primary delusional parasitosis: systematic review. Br J Psychiatry. 2007;191(3):198-205.
7. Freudenmann RW, Lepping P. Delusional infestation. Clin Microbiol Rev. 2009;22(4):690-732.
8. Mercan S, Altunay IK, Taskintuna N, et al. Atypical antipsychotic drugs in the treatment of delusional parasitosis. Intl J Psychiatry Med. 2007:37(1):29-37.
9. Trabert W. 100 years of delusional parasitosis. Meta-analysis of 1,223 case reports. Psychopathology. 1995;28(5):238-246.
10. Freudenmann RW, Lepping P. Second-generation antipsychotics in primary and secondary delusional parasitosis. J Clin Psychopharmacol. 2008;28(5):500-508.
11. Boggild AK, Nicks BA, Yen L, et al. Delusional parasitosis: six-year experience with 23 consecutive cases at an academic medical center. Int J Infect Dis. 2010;14(4):e317-e321.
1. Ekbom KA. Der präsenile dermatozoenwahn [in Swedish]. Acta Psychiatr Neurol Scand. 1938;13(3):227-259.
2. Lynch PJ. Delusions of parasitosis. Semin Dermatol. 1993;12(1):39-45.
3. Middelveen MJ, Fesler MC, Stricker RB. History of Morgellons disease: from delusion to definition. Clin Cosmet Investig Dermatol. 2018;11:71-90.
4. Bailey CH, Andersen LK, Lowe GC, et al. A population-based study of the incidence of delusional infestation in Olmsted County, Minnesota, 1976–2010. Br J Dermatol. 2014;170(5):1130-1135.
5. Foster AA, Hylwa SA, Bury JE, et al. Delusional infestation: clinical presentation in 147 patients seen at Mayo Clinic. J Am Acad Dermatol. 2012;67(4):673.e1-e10.
6. Lepping P, Russell I, Freudenmann RW. Antipsychotic treatment of primary delusional parasitosis: systematic review. Br J Psychiatry. 2007;191(3):198-205.
7. Freudenmann RW, Lepping P. Delusional infestation. Clin Microbiol Rev. 2009;22(4):690-732.
8. Mercan S, Altunay IK, Taskintuna N, et al. Atypical antipsychotic drugs in the treatment of delusional parasitosis. Intl J Psychiatry Med. 2007:37(1):29-37.
9. Trabert W. 100 years of delusional parasitosis. Meta-analysis of 1,223 case reports. Psychopathology. 1995;28(5):238-246.
10. Freudenmann RW, Lepping P. Second-generation antipsychotics in primary and secondary delusional parasitosis. J Clin Psychopharmacol. 2008;28(5):500-508.
11. Boggild AK, Nicks BA, Yen L, et al. Delusional parasitosis: six-year experience with 23 consecutive cases at an academic medical center. Int J Infect Dis. 2010;14(4):e317-e321.
Xenomelia: Profile of a man with intense desire to amputate a healthy limb
Xenomelia, literally meaning “foreign limb,” is a neuropsychiatric condition in which nonpsychotic individuals have an intense, persistent belief that one or more of their limbs does not belong to their body; instead they regard it as an alien appendage that should be discarded.1 This unwavering, fixed belief resembles a delusion and is often debilitating to the point where the affected person strongly desires amputation of the unwanted limb. Traditionally, such requests often are denied by the medical community, which may cause an individual who has xenomelia to attempt risky self-amputation, or to injure the limb in a manner that makes subsequent amputation medically necessary.1
The name for this condition has evolved over the years, depending on the emphasis given to specific characteristics. It was once called apotemnophilia, meaning “love of amputation,” when the condition was believed to be a fetish involving sexual gratification derived from being an amputee.2,3 The term “body integrity identity disorder” (BIID) was introduced several decades later to incorporate the condition into a broader spectrum of accepted psychiatric pathologies, reasoning that it was the cause of a mismatch between objective and subjective body schema, similar to anorexia nervosa or body dysmorphic disorder.4,5 This name also served to draw parallels between this condition and gender identity disorder. However, unlike these other disorders, individuals with this condition have sufficient factual insight to know they appear “normal” to others. The newest term, xenomelia, was established to acknowledge the neurologic component of the condition after neuroimaging studies showed structural changes to the right parietal lobe in individuals who desired amputation of their left lower limb, thus linking the part of the brain that processes sensory input from the affected limb.6
While particular nuances in symptomatology were modified in formulating these older names, certain hallmark features of xenomelia have remained the same.7 The condition starts in early childhood, prior to puberty. Those who have it feel intense distress, and are resigned to the notion that nothing but amputation can alleviate their distress. Xenomelia is overwhelmingly more common in males than females. It is accompanied by nontraditional attitudes about disability, including admiration of amputees and complete apathy and disregard toward the impairment that amputation would cause.
While the data are insufficient to draw a definitive conclusion, the trend in the published literature suggests in xenomelia, the lower left leg is predominantly the limb implicated in the condition, in right-handed individuals.1
Here, we describe the case of a young man, Mr. H, with xenomelia who contacted us after reading about this condition in a review we recently published.1 He agreed to allow us to anonymously describe his history and symptoms so that clinicians can recognize and help other individuals with xenomelia. His history may also help stimulate exploration of etiological factors and novel treatment strategies for xenomelia, other than amputation of a healthy limb.
CASE
‘I have this limb that should not be’
Mr. H, age 31, is a white male of Eastern European descent who was born, raised, and resides in a major metropolitan area in the western United States. He is married, college-educated, and currently works as a computer programmer for a prominent technology company. During our conversation via telephone, he exhibits above-average intelligence, appears to be in euthymic mood, and speaks with broad affect. Mr. H displays no psychotic symptoms such as overt delusions, hallucinations, reality distortion, or response to internal stimuli. His past psychiatric history includes attention-deficit/hyperactivity disorder (ADHD), which was diagnosed at age 6 and treated with appropriate medication under the care of a psychiatrist until age 18, when Mr. H decided to discontinue treatment. He no longer endorses symptoms of ADHD. He has no chronic medical conditions other than season allergies, for which he sometimes takes antihistamines, and occasional exacerbation of sciatica, for which he takes an over-the-counter nonsteroidal anti-inflammatory medication. Mr. H also has episodic insomnia, which he attributes to job-related stress and working odd hours. He was treated for meningitis as an infant, and underwent a bilateral myringotomy as a young child to treat recurrent ear infections. He has no other surgical history. He was raised in a middle-class Christian household that included both parents, who are still alive, still together, and have no significant psychiatric or medical history. He has no siblings.
Although he lives an ostensibly normal life, Mr. H suffers in silence and secrecy with xenomelia. According to him, there was never a time in his life when he didn’t feel that his left leg was “too long” and he was “walking on a stilt.” He says, “It takes a daily toll on my health and well-being.” He can clearly recall being 4 years old and playing games in which he would pretend to injure his left leg. He says, “When we played ‘make believe,’ the game would always end with something ‘happening’ to [my left leg].” He enjoys outdoor sports like snowboarding and mountain biking, and although he denies self-injurious behavior, he says in the event of an accident, he would prefer to land on his left leg, because it is the part of his body that he considers most “expendable.” One of his most vivid memories of childhood was going shopping with his parents and seeing an older man with only one leg standing on crutches in the parking lot outside the entrance. He remembers feeling “jealous” of this man.
Continue to: Although his parents were not particularly wealthy...
Although his parents were not particularly wealthy, they sent him to a private Christian school for most of his childhood. Mr. H admits that while there he didn’t fit in and felt like an outcast, in part because he didn’t come from the level of wealth of his classmates, and because having ADHD left him isolative and avoidant. “I was always the one going away to take medication,” he explains, and he also developed a hostile attitude. He was suspended from school multiple times for fighting. These years left him tremendously anxious and depressed, and he would often find it therapeutic to sit with his left leg bent underneath him, so as to hide its undesired portion. It was common for him to tie his leg up and stare at himself in the mirror for minutes to hours as a form of stress reduction.
Most of Mr. H’s social circle is composed of friends he has known since childhood, none of whom are aware of his condition. He acknowledges that his feelings are “bizarre in nature” and so he has kept this secret on a “need-to-know” basis out of “fear of rejection, mockery, and damage to my reputation.” Through the years, he has sought out and encountered others with this condition, first anonymously on the internet, then in-person once he gets to know and trust them. He claims to know and be friendly with several people with xenomelia in his own city, some of whom have undergone amputation and are extremely happy with the results. According to Mr. H, there is a community aspect to xenomelia in his city, and people with the condition often meet each other socially. He has revealed his secret to 2 women he dated, including his present wife, who he told 3 years into their relationship. “I was prepared for her to leave me,” he recalls. Although he has never connected the desire for amputation with sexuality, he certainly believes that amputating his left leg would enhance his sex life. “Do I find amputees sexy?” he asks, “I would say yes.” On a 10-point scale, he considers his sex life to be a “7 or 8,” and it would reach 10 if he underwent amputation.
Mr. H has a calendar on which he keeps track of the days when he feels “impaired” by his xenomelia. He marks each day as either “red” or “green.” So far, he does not recognize a pattern of exacerbation. “I have my good days, then I have my bad days,” he laments. “On good days, I think about amputation and where my leg should actually end, but it is something I can quickly push off. On my bad days, I am constantly reminded in one way or another that, yes, I have this limb that should not be.” While he has never sought treatment for this condition from a health care professional, he developed his own therapeutic regimen that includes yoga, hiking, and daily use of cannabis, which “helps take the edge off.” He used alcohol in the past as self-medication, but stopped drinking to excess when it started to disrupt other aspects of his life. According to Mr. H, the goal is to distract himself from the condition, which provides temporary relief. “I find if my mind is more engaged, the amputation thoughts are fewer and less in intensity.” He reports that the months leading up to his wedding were particularly therapeutic because wedding planning provided an excellent distraction.
Overall, his current desire for amputation is steadily increasing. “Lately it has become more of a roller coaster,” he says. “If there’s a safe way to do it, I’ll do it.” An amputation would allow him to “feel good, complete, grounded, and content.” If he were to undergo amputation, he would use a prosthetic in order to retain mobility and keep his physique as discreet as possible. He has made initial inquiries into getting an amputation, saying, “I have heard of rumors of surgeons willing to perform the surgery, for a price. However, I have not completed the ‘vetting process’ to actually come into contact with the surgeons themselves.” Similar to others with xenomelia, he is easily able to draw a line on his leg, exactly where the desired amputation should occur.8 For most of his life, that line would have been 2 inches above his knee, but in recent years, the line has drifted lower, to 2 inches below the knee. However, he “wouldn’t mind either” line of amputation. He indicates the area below the desired line is less sensitive to pain than the corresponding part of his right leg, particularly his toes.
Mr. H’s wife is extremely supportive and understanding of her husband’s condition, but is opposed to the possibility of amputation (Box).
Box
Xenomelia: A spouse's perspective
Mr. H's wife is extremely compassionate, empathetic, and supportive of her husband's struggle with xenomelia. She denies noticing any hint of his condition until he informed her. "He expected me to freak out more than I did," she recalls. In her experience, Mr. H can go days at a time without having a "flare-up" of his condition. She believes that the intermittent worsening of her husband's condition might be associated with increased work-related stress and anxiety. She encouraged him to maintain a calendar for tracking the days with exacerbations. On days when Mr. H's xenomelia is worse, she attempts to distract him with hobbies and activities. She has accompanied Mr. H when he meets others with xenomelia, although she finds these meetings quite unremarkable. "They all seem like normal people," she says. "It's usually just an average conversation." While she is committed to helping her husband cope with xenomelia, she is averse to the possibility of amputation. "I'm willing to help in any way I can, but I'm hesitant for him to amputate a healthy limb," she admits. "I'm worried about his mobility."
Continue to: Much left to be learned about xenomelia
Much left to be learned about xenomelia
What remains to be discovered about xenomelia falls into 2 areas:
- the possible usefulness of various neuroimaging modalities (morphological MRI, functional MRI, magnetic resonance spectroscopy, and diffusion tensor imaging) to identify and localize anomalous neural pathways or neuroanatomical foci associated with this condition, such as an aberrantly developed or poorly myelinated right parietal lobe, which houses the limb’s physical proprioception
- a biopsychosocial inquiry into whether there exists a specific combination of a given individual’s organic brain, mind, and environmental interactions that may give rise to this condition, and whether we might detect a prodrome that arises in early childhood. The objective of any research into this condition would be to minimize its effects, if not prevent them altogether.1
As this case illustrates, xenomelia begins in early childhood, with symptoms being reported in children as young as age 3.7 However, no published literature has investigated these early stages. We’ve learned that individuals with xenomelia often can point to key childhood experiences or memories related to seeing people with amputated limbs. They remember feeling a sense of wonder, fascination, or other strong emotion. It may be in this memory that xenomelia is permanently imprinted. This was definitely true for Mr. H, who never knew a time when he didn’t endure some level of debilitation from xenomelia, and distinctly remembers feeling jealous upon seeing a man with the amputated leg standing on crutches in a store parking lot. Although he has come across many amputees in his life, Mr. H says he vividly remembers everything about that particular man in that particular moment, adding “I can still see the clothes he was wearing. I can still see the cars in the parking lot.” That was likely his moment of vivid and powerful imprinting.
Particularly influential changes occur in adolescence, not just in the course of physical development, but in the formulation of self-identity, which involves the inevitable comparison of one’s own appearance to that of others, with heightened awareness of what others might perceive. This phenomenon is known as “the imaginary audience,” and it is often overemphasized in the minds of individuals with xenomelia.7 Mr. H is a textbook example of someone acutely aware of his “audience,” suffering from the embarrassment that came from being less wealthy than others at his school, and having to manage his ADHD in plain sight of his classmates, who knew that he required medication. It is no surprise that he felt like an outcast and got suspended for fighting. He would relieve anxiety by tying his leg up and staring at himself in the mirror, finding refuge in front of an audience of one that understood and sympathized with his suffering.
Among the most notorious aspects of this condition is investigation into the possibility of there being a sexual component to the desire for amputation. The notion that the desire is a fetish employed for the purpose of sexual arousal was first propagated by Penthouse magazine in the 1970s.9 Learning that xenomelia exists in a child long before sexual maturation—and in an older adult long after sexual drive peaks—suggests the condition is independent of sexuality. However, this aspect of xenomelia continues to be investigated. A recent study found that >70% of individuals with xenomelia are at least partially motivated by the perceived enhancement in sexual gratification.10 Individuals with this motivation are predominantly male, homosexual, come from a religious background, and are far more likely to self-amputate.10 Mr. H admitted that he is sexually attracted to amputees, and while he had no complaints about his sex life, he felt it could only reach the highest levels of gratification if he were an amputee.
It is reasonable to posit that there is a genetic mechanism that creates a cortical template of one’s body, and this template connects with the limbic system, encoding a visual preference for and attraction to one’s own idealized and preferred body morphology that includes an amputated limb.11 Therefore, if Mr. H sees himself as an amputee, it would be reasonable for him to identify with and be attracted to other amputees. However, Mr. H is clearly not preoccupied with sexuality, and believes that heightened sexual gratification would be an ancillary bonus, and not the main objective, of amputation.
Continue to: Most individuals who have particpated in research studies about xenomelia tend to...
Most individuals who have participated in research studies about xenomelia tend to be older, mainly in their 60s. This is particularly true of individuals who go through with amputation. At some point, the need for a person to invoke their autonomy, alleviate their debilitation, and fulfill their desire may supersede their aversion to physical disability and social ridicule. At this stage in his life, Mr. H can’t commit to going forward with the amputation. However, he regards the likelihood of undergoing amputation to be quite high. He made initial inquiries to find a surgeon who would be willing to perform the procedure. Given that he has found people with xenomelia who have undergone amputation, he will likely will be able find a surgeon to perform the procedure. Mr. H reports that just about everyone he has ever known with xenomelia who underwent amputation is completely satisfied with their decision, even years later. He has come across only one person who regretted the amputation, and he believes that person was likely suffering from other psychiatric issues, and did not have true xenomelia.
In the mind of an individual with xenomelia, the desire for amputation is separate from a desire to be disabled. Mr. H is mindful of the assumed irrationality of removing a healthy but “alien” limb to replace it with a prosthetic limb that is equally alien. The perceived irony is not lost on him. He values his mobility, and has no desire to use crutches, a wheelchair, or any other ambulatory tool. This is consistent with most individuals with xenomelia, who are neither motived by the desire to flaunt their amputated limb, nor by the sympathy they might receive from others by endorsing impaired mobility. They don’t consider themselves disabled. On the contrary, for them, amputation is a much-desired enhancement to their health and well-being.
Increased opportunities for research
The internet, social media, and even peer-reviewed medical journals offer ever-increasing opportunities for individuals with xenomelia, such as Mr. H, to have their story told, regardless of whether they choose to identify themselves or remain anonymous. There are no published data about the prevalence of xenomelia, but it is almost certainly rare. However, if Mr. H was able to meet multiple people with xenomelia in his own city and form a supportive community with them, then perhaps it isn’t exactly as rare as one might initially assume. People with xenomelia may tend to look for each other, hoping those with the same condition might show them the greatest empathy.
From Mr. H’s experience, it appears that it would be possible to locate a sufficient number of individuals with xenomelia for the purposes of conducting research, which might allow for results with acceptable statistical power. There are plenty of individual patient stories, and by documenting these stories in published literature, it is likely that patterns would emerge and causality might be determined. Such data might be bolstered by a possible strong neurologic corroboration based on what is found via neuroimaging.
Informed research into xenomelia is still in the early stages, and it is clear that there is much left to discover. It is vital that, moving forward, investigation into this condition be thorough and objective, with the goal of alleviating this secretive and debilitating neuropsychiatric condition.
Continue to: Bottom Line
Bottom Line
Individuals with xenomelia have the persistent belief that one or more of their limbs does not belong to their body but is an alien appendage that should be removed. Patients with this condition may resort to self-amputation or self-mutilation that requires subsequent surgical amputation. Xenomelia may be related to anomalous brain development, with a lack of neural representation of a limb in the right parietal lobe.
Related Resources
- Hilti LM, Hänggi J, Vitacco DA, et al. The desire for healthy limb amputation: structural brain correlates and clinical features of xenomelia. Brain. 2013;136(pt 1):318-329.
- Brugger P, Lenggenhager B, Giummarra MJ. Xenomelia: a social neuroscience view of altered bodily self-consciousness. Front Psychol. 2013;4:204. doi:10.3389/fpsyg.2013.00204.
1. Upadhyaya MA, Nasrallah HA. The intense desire for healthy limb amputation: a dis-proprioceptive neuropsychiatric disorder. Ann Clin Psychiatry. 2017;29(2):125-132.
2. Sedda A, Bottini G. Apotemnophilia, body integrity identity disorder or xenomelia? Psychiatric and neurologic etiologies face each other. Neuropsychiatr Dis Treat. 2014;10:1255-1265.
3. Money J, Jobaris R, Furth G. Apotemnophilia: two cases of self-demand amputation as a paraphilia. J Sex Res. 1977;13(2):115-125.
4. Blom RM, Hennekam RC, Denys D. Body integrity identity disorder. PLoS One. 2012;7(4):e34702. doi: 10.1371/journal.pone.0034702.
5. First MB. Desire for amputation of a limb: paraphilia, psychosis, or a new type of identity disorder. Psychol Med. 2005;35(6):919-928.
6. McGeoch PD, Brang D, Song T, et al. Xenomelia: a new right parietal lobe syndrome. J Neurol Neurosurg Psychiatry. 2011;82(12):1314-1319.
7. Nowakowski P, Karczmarczyk A. The rest is not me… An attempt to explain xenomelia--neurodevelopmental hypothesis. Postepy Psychiatrii i Neurologii. 2016;25(3):196-208.
8. Brang D, McGeoch PD, Ramachandran VS. Apotemnophilia: a neurological disorder. Neuroreport. 2008;19(13):1305-1306.
9. Forum. Penthouse. September 1972:128.
10. Blom RM, van der Wal SJ, Vulink NC, et al. Role of sexuality in body integrity identity disorder (BIID): a cross-sectional internet-based survey study. J Sex Med. 2017;14(8):1028-1035.
11. Ramachandran VS, Brang D, McGeoch PD, et al. Sexual and food preference in apotemnophilia and anorexia: interactions between ‘beliefs’ and ‘needs’ regulated by two-way connections between body image and limbic structures. Perception. 2009;38(5):775-777.
Xenomelia, literally meaning “foreign limb,” is a neuropsychiatric condition in which nonpsychotic individuals have an intense, persistent belief that one or more of their limbs does not belong to their body; instead they regard it as an alien appendage that should be discarded.1 This unwavering, fixed belief resembles a delusion and is often debilitating to the point where the affected person strongly desires amputation of the unwanted limb. Traditionally, such requests often are denied by the medical community, which may cause an individual who has xenomelia to attempt risky self-amputation, or to injure the limb in a manner that makes subsequent amputation medically necessary.1
The name for this condition has evolved over the years, depending on the emphasis given to specific characteristics. It was once called apotemnophilia, meaning “love of amputation,” when the condition was believed to be a fetish involving sexual gratification derived from being an amputee.2,3 The term “body integrity identity disorder” (BIID) was introduced several decades later to incorporate the condition into a broader spectrum of accepted psychiatric pathologies, reasoning that it was the cause of a mismatch between objective and subjective body schema, similar to anorexia nervosa or body dysmorphic disorder.4,5 This name also served to draw parallels between this condition and gender identity disorder. However, unlike these other disorders, individuals with this condition have sufficient factual insight to know they appear “normal” to others. The newest term, xenomelia, was established to acknowledge the neurologic component of the condition after neuroimaging studies showed structural changes to the right parietal lobe in individuals who desired amputation of their left lower limb, thus linking the part of the brain that processes sensory input from the affected limb.6
While particular nuances in symptomatology were modified in formulating these older names, certain hallmark features of xenomelia have remained the same.7 The condition starts in early childhood, prior to puberty. Those who have it feel intense distress, and are resigned to the notion that nothing but amputation can alleviate their distress. Xenomelia is overwhelmingly more common in males than females. It is accompanied by nontraditional attitudes about disability, including admiration of amputees and complete apathy and disregard toward the impairment that amputation would cause.
While the data are insufficient to draw a definitive conclusion, the trend in the published literature suggests in xenomelia, the lower left leg is predominantly the limb implicated in the condition, in right-handed individuals.1
Here, we describe the case of a young man, Mr. H, with xenomelia who contacted us after reading about this condition in a review we recently published.1 He agreed to allow us to anonymously describe his history and symptoms so that clinicians can recognize and help other individuals with xenomelia. His history may also help stimulate exploration of etiological factors and novel treatment strategies for xenomelia, other than amputation of a healthy limb.
CASE
‘I have this limb that should not be’
Mr. H, age 31, is a white male of Eastern European descent who was born, raised, and resides in a major metropolitan area in the western United States. He is married, college-educated, and currently works as a computer programmer for a prominent technology company. During our conversation via telephone, he exhibits above-average intelligence, appears to be in euthymic mood, and speaks with broad affect. Mr. H displays no psychotic symptoms such as overt delusions, hallucinations, reality distortion, or response to internal stimuli. His past psychiatric history includes attention-deficit/hyperactivity disorder (ADHD), which was diagnosed at age 6 and treated with appropriate medication under the care of a psychiatrist until age 18, when Mr. H decided to discontinue treatment. He no longer endorses symptoms of ADHD. He has no chronic medical conditions other than season allergies, for which he sometimes takes antihistamines, and occasional exacerbation of sciatica, for which he takes an over-the-counter nonsteroidal anti-inflammatory medication. Mr. H also has episodic insomnia, which he attributes to job-related stress and working odd hours. He was treated for meningitis as an infant, and underwent a bilateral myringotomy as a young child to treat recurrent ear infections. He has no other surgical history. He was raised in a middle-class Christian household that included both parents, who are still alive, still together, and have no significant psychiatric or medical history. He has no siblings.
Although he lives an ostensibly normal life, Mr. H suffers in silence and secrecy with xenomelia. According to him, there was never a time in his life when he didn’t feel that his left leg was “too long” and he was “walking on a stilt.” He says, “It takes a daily toll on my health and well-being.” He can clearly recall being 4 years old and playing games in which he would pretend to injure his left leg. He says, “When we played ‘make believe,’ the game would always end with something ‘happening’ to [my left leg].” He enjoys outdoor sports like snowboarding and mountain biking, and although he denies self-injurious behavior, he says in the event of an accident, he would prefer to land on his left leg, because it is the part of his body that he considers most “expendable.” One of his most vivid memories of childhood was going shopping with his parents and seeing an older man with only one leg standing on crutches in the parking lot outside the entrance. He remembers feeling “jealous” of this man.
Continue to: Although his parents were not particularly wealthy...
Although his parents were not particularly wealthy, they sent him to a private Christian school for most of his childhood. Mr. H admits that while there he didn’t fit in and felt like an outcast, in part because he didn’t come from the level of wealth of his classmates, and because having ADHD left him isolative and avoidant. “I was always the one going away to take medication,” he explains, and he also developed a hostile attitude. He was suspended from school multiple times for fighting. These years left him tremendously anxious and depressed, and he would often find it therapeutic to sit with his left leg bent underneath him, so as to hide its undesired portion. It was common for him to tie his leg up and stare at himself in the mirror for minutes to hours as a form of stress reduction.
Most of Mr. H’s social circle is composed of friends he has known since childhood, none of whom are aware of his condition. He acknowledges that his feelings are “bizarre in nature” and so he has kept this secret on a “need-to-know” basis out of “fear of rejection, mockery, and damage to my reputation.” Through the years, he has sought out and encountered others with this condition, first anonymously on the internet, then in-person once he gets to know and trust them. He claims to know and be friendly with several people with xenomelia in his own city, some of whom have undergone amputation and are extremely happy with the results. According to Mr. H, there is a community aspect to xenomelia in his city, and people with the condition often meet each other socially. He has revealed his secret to 2 women he dated, including his present wife, who he told 3 years into their relationship. “I was prepared for her to leave me,” he recalls. Although he has never connected the desire for amputation with sexuality, he certainly believes that amputating his left leg would enhance his sex life. “Do I find amputees sexy?” he asks, “I would say yes.” On a 10-point scale, he considers his sex life to be a “7 or 8,” and it would reach 10 if he underwent amputation.
Mr. H has a calendar on which he keeps track of the days when he feels “impaired” by his xenomelia. He marks each day as either “red” or “green.” So far, he does not recognize a pattern of exacerbation. “I have my good days, then I have my bad days,” he laments. “On good days, I think about amputation and where my leg should actually end, but it is something I can quickly push off. On my bad days, I am constantly reminded in one way or another that, yes, I have this limb that should not be.” While he has never sought treatment for this condition from a health care professional, he developed his own therapeutic regimen that includes yoga, hiking, and daily use of cannabis, which “helps take the edge off.” He used alcohol in the past as self-medication, but stopped drinking to excess when it started to disrupt other aspects of his life. According to Mr. H, the goal is to distract himself from the condition, which provides temporary relief. “I find if my mind is more engaged, the amputation thoughts are fewer and less in intensity.” He reports that the months leading up to his wedding were particularly therapeutic because wedding planning provided an excellent distraction.
Overall, his current desire for amputation is steadily increasing. “Lately it has become more of a roller coaster,” he says. “If there’s a safe way to do it, I’ll do it.” An amputation would allow him to “feel good, complete, grounded, and content.” If he were to undergo amputation, he would use a prosthetic in order to retain mobility and keep his physique as discreet as possible. He has made initial inquiries into getting an amputation, saying, “I have heard of rumors of surgeons willing to perform the surgery, for a price. However, I have not completed the ‘vetting process’ to actually come into contact with the surgeons themselves.” Similar to others with xenomelia, he is easily able to draw a line on his leg, exactly where the desired amputation should occur.8 For most of his life, that line would have been 2 inches above his knee, but in recent years, the line has drifted lower, to 2 inches below the knee. However, he “wouldn’t mind either” line of amputation. He indicates the area below the desired line is less sensitive to pain than the corresponding part of his right leg, particularly his toes.
Mr. H’s wife is extremely supportive and understanding of her husband’s condition, but is opposed to the possibility of amputation (Box).
Box
Xenomelia: A spouse's perspective
Mr. H's wife is extremely compassionate, empathetic, and supportive of her husband's struggle with xenomelia. She denies noticing any hint of his condition until he informed her. "He expected me to freak out more than I did," she recalls. In her experience, Mr. H can go days at a time without having a "flare-up" of his condition. She believes that the intermittent worsening of her husband's condition might be associated with increased work-related stress and anxiety. She encouraged him to maintain a calendar for tracking the days with exacerbations. On days when Mr. H's xenomelia is worse, she attempts to distract him with hobbies and activities. She has accompanied Mr. H when he meets others with xenomelia, although she finds these meetings quite unremarkable. "They all seem like normal people," she says. "It's usually just an average conversation." While she is committed to helping her husband cope with xenomelia, she is averse to the possibility of amputation. "I'm willing to help in any way I can, but I'm hesitant for him to amputate a healthy limb," she admits. "I'm worried about his mobility."
Continue to: Much left to be learned about xenomelia
Much left to be learned about xenomelia
What remains to be discovered about xenomelia falls into 2 areas:
- the possible usefulness of various neuroimaging modalities (morphological MRI, functional MRI, magnetic resonance spectroscopy, and diffusion tensor imaging) to identify and localize anomalous neural pathways or neuroanatomical foci associated with this condition, such as an aberrantly developed or poorly myelinated right parietal lobe, which houses the limb’s physical proprioception
- a biopsychosocial inquiry into whether there exists a specific combination of a given individual’s organic brain, mind, and environmental interactions that may give rise to this condition, and whether we might detect a prodrome that arises in early childhood. The objective of any research into this condition would be to minimize its effects, if not prevent them altogether.1
As this case illustrates, xenomelia begins in early childhood, with symptoms being reported in children as young as age 3.7 However, no published literature has investigated these early stages. We’ve learned that individuals with xenomelia often can point to key childhood experiences or memories related to seeing people with amputated limbs. They remember feeling a sense of wonder, fascination, or other strong emotion. It may be in this memory that xenomelia is permanently imprinted. This was definitely true for Mr. H, who never knew a time when he didn’t endure some level of debilitation from xenomelia, and distinctly remembers feeling jealous upon seeing a man with the amputated leg standing on crutches in a store parking lot. Although he has come across many amputees in his life, Mr. H says he vividly remembers everything about that particular man in that particular moment, adding “I can still see the clothes he was wearing. I can still see the cars in the parking lot.” That was likely his moment of vivid and powerful imprinting.
Particularly influential changes occur in adolescence, not just in the course of physical development, but in the formulation of self-identity, which involves the inevitable comparison of one’s own appearance to that of others, with heightened awareness of what others might perceive. This phenomenon is known as “the imaginary audience,” and it is often overemphasized in the minds of individuals with xenomelia.7 Mr. H is a textbook example of someone acutely aware of his “audience,” suffering from the embarrassment that came from being less wealthy than others at his school, and having to manage his ADHD in plain sight of his classmates, who knew that he required medication. It is no surprise that he felt like an outcast and got suspended for fighting. He would relieve anxiety by tying his leg up and staring at himself in the mirror, finding refuge in front of an audience of one that understood and sympathized with his suffering.
Among the most notorious aspects of this condition is investigation into the possibility of there being a sexual component to the desire for amputation. The notion that the desire is a fetish employed for the purpose of sexual arousal was first propagated by Penthouse magazine in the 1970s.9 Learning that xenomelia exists in a child long before sexual maturation—and in an older adult long after sexual drive peaks—suggests the condition is independent of sexuality. However, this aspect of xenomelia continues to be investigated. A recent study found that >70% of individuals with xenomelia are at least partially motivated by the perceived enhancement in sexual gratification.10 Individuals with this motivation are predominantly male, homosexual, come from a religious background, and are far more likely to self-amputate.10 Mr. H admitted that he is sexually attracted to amputees, and while he had no complaints about his sex life, he felt it could only reach the highest levels of gratification if he were an amputee.
It is reasonable to posit that there is a genetic mechanism that creates a cortical template of one’s body, and this template connects with the limbic system, encoding a visual preference for and attraction to one’s own idealized and preferred body morphology that includes an amputated limb.11 Therefore, if Mr. H sees himself as an amputee, it would be reasonable for him to identify with and be attracted to other amputees. However, Mr. H is clearly not preoccupied with sexuality, and believes that heightened sexual gratification would be an ancillary bonus, and not the main objective, of amputation.
Continue to: Most individuals who have particpated in research studies about xenomelia tend to...
Most individuals who have participated in research studies about xenomelia tend to be older, mainly in their 60s. This is particularly true of individuals who go through with amputation. At some point, the need for a person to invoke their autonomy, alleviate their debilitation, and fulfill their desire may supersede their aversion to physical disability and social ridicule. At this stage in his life, Mr. H can’t commit to going forward with the amputation. However, he regards the likelihood of undergoing amputation to be quite high. He made initial inquiries to find a surgeon who would be willing to perform the procedure. Given that he has found people with xenomelia who have undergone amputation, he will likely will be able find a surgeon to perform the procedure. Mr. H reports that just about everyone he has ever known with xenomelia who underwent amputation is completely satisfied with their decision, even years later. He has come across only one person who regretted the amputation, and he believes that person was likely suffering from other psychiatric issues, and did not have true xenomelia.
In the mind of an individual with xenomelia, the desire for amputation is separate from a desire to be disabled. Mr. H is mindful of the assumed irrationality of removing a healthy but “alien” limb to replace it with a prosthetic limb that is equally alien. The perceived irony is not lost on him. He values his mobility, and has no desire to use crutches, a wheelchair, or any other ambulatory tool. This is consistent with most individuals with xenomelia, who are neither motived by the desire to flaunt their amputated limb, nor by the sympathy they might receive from others by endorsing impaired mobility. They don’t consider themselves disabled. On the contrary, for them, amputation is a much-desired enhancement to their health and well-being.
Increased opportunities for research
The internet, social media, and even peer-reviewed medical journals offer ever-increasing opportunities for individuals with xenomelia, such as Mr. H, to have their story told, regardless of whether they choose to identify themselves or remain anonymous. There are no published data about the prevalence of xenomelia, but it is almost certainly rare. However, if Mr. H was able to meet multiple people with xenomelia in his own city and form a supportive community with them, then perhaps it isn’t exactly as rare as one might initially assume. People with xenomelia may tend to look for each other, hoping those with the same condition might show them the greatest empathy.
From Mr. H’s experience, it appears that it would be possible to locate a sufficient number of individuals with xenomelia for the purposes of conducting research, which might allow for results with acceptable statistical power. There are plenty of individual patient stories, and by documenting these stories in published literature, it is likely that patterns would emerge and causality might be determined. Such data might be bolstered by a possible strong neurologic corroboration based on what is found via neuroimaging.
Informed research into xenomelia is still in the early stages, and it is clear that there is much left to discover. It is vital that, moving forward, investigation into this condition be thorough and objective, with the goal of alleviating this secretive and debilitating neuropsychiatric condition.
Continue to: Bottom Line
Bottom Line
Individuals with xenomelia have the persistent belief that one or more of their limbs does not belong to their body but is an alien appendage that should be removed. Patients with this condition may resort to self-amputation or self-mutilation that requires subsequent surgical amputation. Xenomelia may be related to anomalous brain development, with a lack of neural representation of a limb in the right parietal lobe.
Related Resources
- Hilti LM, Hänggi J, Vitacco DA, et al. The desire for healthy limb amputation: structural brain correlates and clinical features of xenomelia. Brain. 2013;136(pt 1):318-329.
- Brugger P, Lenggenhager B, Giummarra MJ. Xenomelia: a social neuroscience view of altered bodily self-consciousness. Front Psychol. 2013;4:204. doi:10.3389/fpsyg.2013.00204.
Xenomelia, literally meaning “foreign limb,” is a neuropsychiatric condition in which nonpsychotic individuals have an intense, persistent belief that one or more of their limbs does not belong to their body; instead they regard it as an alien appendage that should be discarded.1 This unwavering, fixed belief resembles a delusion and is often debilitating to the point where the affected person strongly desires amputation of the unwanted limb. Traditionally, such requests often are denied by the medical community, which may cause an individual who has xenomelia to attempt risky self-amputation, or to injure the limb in a manner that makes subsequent amputation medically necessary.1
The name for this condition has evolved over the years, depending on the emphasis given to specific characteristics. It was once called apotemnophilia, meaning “love of amputation,” when the condition was believed to be a fetish involving sexual gratification derived from being an amputee.2,3 The term “body integrity identity disorder” (BIID) was introduced several decades later to incorporate the condition into a broader spectrum of accepted psychiatric pathologies, reasoning that it was the cause of a mismatch between objective and subjective body schema, similar to anorexia nervosa or body dysmorphic disorder.4,5 This name also served to draw parallels between this condition and gender identity disorder. However, unlike these other disorders, individuals with this condition have sufficient factual insight to know they appear “normal” to others. The newest term, xenomelia, was established to acknowledge the neurologic component of the condition after neuroimaging studies showed structural changes to the right parietal lobe in individuals who desired amputation of their left lower limb, thus linking the part of the brain that processes sensory input from the affected limb.6
While particular nuances in symptomatology were modified in formulating these older names, certain hallmark features of xenomelia have remained the same.7 The condition starts in early childhood, prior to puberty. Those who have it feel intense distress, and are resigned to the notion that nothing but amputation can alleviate their distress. Xenomelia is overwhelmingly more common in males than females. It is accompanied by nontraditional attitudes about disability, including admiration of amputees and complete apathy and disregard toward the impairment that amputation would cause.
While the data are insufficient to draw a definitive conclusion, the trend in the published literature suggests in xenomelia, the lower left leg is predominantly the limb implicated in the condition, in right-handed individuals.1
Here, we describe the case of a young man, Mr. H, with xenomelia who contacted us after reading about this condition in a review we recently published.1 He agreed to allow us to anonymously describe his history and symptoms so that clinicians can recognize and help other individuals with xenomelia. His history may also help stimulate exploration of etiological factors and novel treatment strategies for xenomelia, other than amputation of a healthy limb.
CASE
‘I have this limb that should not be’
Mr. H, age 31, is a white male of Eastern European descent who was born, raised, and resides in a major metropolitan area in the western United States. He is married, college-educated, and currently works as a computer programmer for a prominent technology company. During our conversation via telephone, he exhibits above-average intelligence, appears to be in euthymic mood, and speaks with broad affect. Mr. H displays no psychotic symptoms such as overt delusions, hallucinations, reality distortion, or response to internal stimuli. His past psychiatric history includes attention-deficit/hyperactivity disorder (ADHD), which was diagnosed at age 6 and treated with appropriate medication under the care of a psychiatrist until age 18, when Mr. H decided to discontinue treatment. He no longer endorses symptoms of ADHD. He has no chronic medical conditions other than season allergies, for which he sometimes takes antihistamines, and occasional exacerbation of sciatica, for which he takes an over-the-counter nonsteroidal anti-inflammatory medication. Mr. H also has episodic insomnia, which he attributes to job-related stress and working odd hours. He was treated for meningitis as an infant, and underwent a bilateral myringotomy as a young child to treat recurrent ear infections. He has no other surgical history. He was raised in a middle-class Christian household that included both parents, who are still alive, still together, and have no significant psychiatric or medical history. He has no siblings.
Although he lives an ostensibly normal life, Mr. H suffers in silence and secrecy with xenomelia. According to him, there was never a time in his life when he didn’t feel that his left leg was “too long” and he was “walking on a stilt.” He says, “It takes a daily toll on my health and well-being.” He can clearly recall being 4 years old and playing games in which he would pretend to injure his left leg. He says, “When we played ‘make believe,’ the game would always end with something ‘happening’ to [my left leg].” He enjoys outdoor sports like snowboarding and mountain biking, and although he denies self-injurious behavior, he says in the event of an accident, he would prefer to land on his left leg, because it is the part of his body that he considers most “expendable.” One of his most vivid memories of childhood was going shopping with his parents and seeing an older man with only one leg standing on crutches in the parking lot outside the entrance. He remembers feeling “jealous” of this man.
Continue to: Although his parents were not particularly wealthy...
Although his parents were not particularly wealthy, they sent him to a private Christian school for most of his childhood. Mr. H admits that while there he didn’t fit in and felt like an outcast, in part because he didn’t come from the level of wealth of his classmates, and because having ADHD left him isolative and avoidant. “I was always the one going away to take medication,” he explains, and he also developed a hostile attitude. He was suspended from school multiple times for fighting. These years left him tremendously anxious and depressed, and he would often find it therapeutic to sit with his left leg bent underneath him, so as to hide its undesired portion. It was common for him to tie his leg up and stare at himself in the mirror for minutes to hours as a form of stress reduction.
Most of Mr. H’s social circle is composed of friends he has known since childhood, none of whom are aware of his condition. He acknowledges that his feelings are “bizarre in nature” and so he has kept this secret on a “need-to-know” basis out of “fear of rejection, mockery, and damage to my reputation.” Through the years, he has sought out and encountered others with this condition, first anonymously on the internet, then in-person once he gets to know and trust them. He claims to know and be friendly with several people with xenomelia in his own city, some of whom have undergone amputation and are extremely happy with the results. According to Mr. H, there is a community aspect to xenomelia in his city, and people with the condition often meet each other socially. He has revealed his secret to 2 women he dated, including his present wife, who he told 3 years into their relationship. “I was prepared for her to leave me,” he recalls. Although he has never connected the desire for amputation with sexuality, he certainly believes that amputating his left leg would enhance his sex life. “Do I find amputees sexy?” he asks, “I would say yes.” On a 10-point scale, he considers his sex life to be a “7 or 8,” and it would reach 10 if he underwent amputation.
Mr. H has a calendar on which he keeps track of the days when he feels “impaired” by his xenomelia. He marks each day as either “red” or “green.” So far, he does not recognize a pattern of exacerbation. “I have my good days, then I have my bad days,” he laments. “On good days, I think about amputation and where my leg should actually end, but it is something I can quickly push off. On my bad days, I am constantly reminded in one way or another that, yes, I have this limb that should not be.” While he has never sought treatment for this condition from a health care professional, he developed his own therapeutic regimen that includes yoga, hiking, and daily use of cannabis, which “helps take the edge off.” He used alcohol in the past as self-medication, but stopped drinking to excess when it started to disrupt other aspects of his life. According to Mr. H, the goal is to distract himself from the condition, which provides temporary relief. “I find if my mind is more engaged, the amputation thoughts are fewer and less in intensity.” He reports that the months leading up to his wedding were particularly therapeutic because wedding planning provided an excellent distraction.
Overall, his current desire for amputation is steadily increasing. “Lately it has become more of a roller coaster,” he says. “If there’s a safe way to do it, I’ll do it.” An amputation would allow him to “feel good, complete, grounded, and content.” If he were to undergo amputation, he would use a prosthetic in order to retain mobility and keep his physique as discreet as possible. He has made initial inquiries into getting an amputation, saying, “I have heard of rumors of surgeons willing to perform the surgery, for a price. However, I have not completed the ‘vetting process’ to actually come into contact with the surgeons themselves.” Similar to others with xenomelia, he is easily able to draw a line on his leg, exactly where the desired amputation should occur.8 For most of his life, that line would have been 2 inches above his knee, but in recent years, the line has drifted lower, to 2 inches below the knee. However, he “wouldn’t mind either” line of amputation. He indicates the area below the desired line is less sensitive to pain than the corresponding part of his right leg, particularly his toes.
Mr. H’s wife is extremely supportive and understanding of her husband’s condition, but is opposed to the possibility of amputation (Box).
Box
Xenomelia: A spouse's perspective
Mr. H's wife is extremely compassionate, empathetic, and supportive of her husband's struggle with xenomelia. She denies noticing any hint of his condition until he informed her. "He expected me to freak out more than I did," she recalls. In her experience, Mr. H can go days at a time without having a "flare-up" of his condition. She believes that the intermittent worsening of her husband's condition might be associated with increased work-related stress and anxiety. She encouraged him to maintain a calendar for tracking the days with exacerbations. On days when Mr. H's xenomelia is worse, she attempts to distract him with hobbies and activities. She has accompanied Mr. H when he meets others with xenomelia, although she finds these meetings quite unremarkable. "They all seem like normal people," she says. "It's usually just an average conversation." While she is committed to helping her husband cope with xenomelia, she is averse to the possibility of amputation. "I'm willing to help in any way I can, but I'm hesitant for him to amputate a healthy limb," she admits. "I'm worried about his mobility."
Continue to: Much left to be learned about xenomelia
Much left to be learned about xenomelia
What remains to be discovered about xenomelia falls into 2 areas:
- the possible usefulness of various neuroimaging modalities (morphological MRI, functional MRI, magnetic resonance spectroscopy, and diffusion tensor imaging) to identify and localize anomalous neural pathways or neuroanatomical foci associated with this condition, such as an aberrantly developed or poorly myelinated right parietal lobe, which houses the limb’s physical proprioception
- a biopsychosocial inquiry into whether there exists a specific combination of a given individual’s organic brain, mind, and environmental interactions that may give rise to this condition, and whether we might detect a prodrome that arises in early childhood. The objective of any research into this condition would be to minimize its effects, if not prevent them altogether.1
As this case illustrates, xenomelia begins in early childhood, with symptoms being reported in children as young as age 3.7 However, no published literature has investigated these early stages. We’ve learned that individuals with xenomelia often can point to key childhood experiences or memories related to seeing people with amputated limbs. They remember feeling a sense of wonder, fascination, or other strong emotion. It may be in this memory that xenomelia is permanently imprinted. This was definitely true for Mr. H, who never knew a time when he didn’t endure some level of debilitation from xenomelia, and distinctly remembers feeling jealous upon seeing a man with the amputated leg standing on crutches in a store parking lot. Although he has come across many amputees in his life, Mr. H says he vividly remembers everything about that particular man in that particular moment, adding “I can still see the clothes he was wearing. I can still see the cars in the parking lot.” That was likely his moment of vivid and powerful imprinting.
Particularly influential changes occur in adolescence, not just in the course of physical development, but in the formulation of self-identity, which involves the inevitable comparison of one’s own appearance to that of others, with heightened awareness of what others might perceive. This phenomenon is known as “the imaginary audience,” and it is often overemphasized in the minds of individuals with xenomelia.7 Mr. H is a textbook example of someone acutely aware of his “audience,” suffering from the embarrassment that came from being less wealthy than others at his school, and having to manage his ADHD in plain sight of his classmates, who knew that he required medication. It is no surprise that he felt like an outcast and got suspended for fighting. He would relieve anxiety by tying his leg up and staring at himself in the mirror, finding refuge in front of an audience of one that understood and sympathized with his suffering.
Among the most notorious aspects of this condition is investigation into the possibility of there being a sexual component to the desire for amputation. The notion that the desire is a fetish employed for the purpose of sexual arousal was first propagated by Penthouse magazine in the 1970s.9 Learning that xenomelia exists in a child long before sexual maturation—and in an older adult long after sexual drive peaks—suggests the condition is independent of sexuality. However, this aspect of xenomelia continues to be investigated. A recent study found that >70% of individuals with xenomelia are at least partially motivated by the perceived enhancement in sexual gratification.10 Individuals with this motivation are predominantly male, homosexual, come from a religious background, and are far more likely to self-amputate.10 Mr. H admitted that he is sexually attracted to amputees, and while he had no complaints about his sex life, he felt it could only reach the highest levels of gratification if he were an amputee.
It is reasonable to posit that there is a genetic mechanism that creates a cortical template of one’s body, and this template connects with the limbic system, encoding a visual preference for and attraction to one’s own idealized and preferred body morphology that includes an amputated limb.11 Therefore, if Mr. H sees himself as an amputee, it would be reasonable for him to identify with and be attracted to other amputees. However, Mr. H is clearly not preoccupied with sexuality, and believes that heightened sexual gratification would be an ancillary bonus, and not the main objective, of amputation.
Continue to: Most individuals who have particpated in research studies about xenomelia tend to...
Most individuals who have participated in research studies about xenomelia tend to be older, mainly in their 60s. This is particularly true of individuals who go through with amputation. At some point, the need for a person to invoke their autonomy, alleviate their debilitation, and fulfill their desire may supersede their aversion to physical disability and social ridicule. At this stage in his life, Mr. H can’t commit to going forward with the amputation. However, he regards the likelihood of undergoing amputation to be quite high. He made initial inquiries to find a surgeon who would be willing to perform the procedure. Given that he has found people with xenomelia who have undergone amputation, he will likely will be able find a surgeon to perform the procedure. Mr. H reports that just about everyone he has ever known with xenomelia who underwent amputation is completely satisfied with their decision, even years later. He has come across only one person who regretted the amputation, and he believes that person was likely suffering from other psychiatric issues, and did not have true xenomelia.
In the mind of an individual with xenomelia, the desire for amputation is separate from a desire to be disabled. Mr. H is mindful of the assumed irrationality of removing a healthy but “alien” limb to replace it with a prosthetic limb that is equally alien. The perceived irony is not lost on him. He values his mobility, and has no desire to use crutches, a wheelchair, or any other ambulatory tool. This is consistent with most individuals with xenomelia, who are neither motived by the desire to flaunt their amputated limb, nor by the sympathy they might receive from others by endorsing impaired mobility. They don’t consider themselves disabled. On the contrary, for them, amputation is a much-desired enhancement to their health and well-being.
Increased opportunities for research
The internet, social media, and even peer-reviewed medical journals offer ever-increasing opportunities for individuals with xenomelia, such as Mr. H, to have their story told, regardless of whether they choose to identify themselves or remain anonymous. There are no published data about the prevalence of xenomelia, but it is almost certainly rare. However, if Mr. H was able to meet multiple people with xenomelia in his own city and form a supportive community with them, then perhaps it isn’t exactly as rare as one might initially assume. People with xenomelia may tend to look for each other, hoping those with the same condition might show them the greatest empathy.
From Mr. H’s experience, it appears that it would be possible to locate a sufficient number of individuals with xenomelia for the purposes of conducting research, which might allow for results with acceptable statistical power. There are plenty of individual patient stories, and by documenting these stories in published literature, it is likely that patterns would emerge and causality might be determined. Such data might be bolstered by a possible strong neurologic corroboration based on what is found via neuroimaging.
Informed research into xenomelia is still in the early stages, and it is clear that there is much left to discover. It is vital that, moving forward, investigation into this condition be thorough and objective, with the goal of alleviating this secretive and debilitating neuropsychiatric condition.
Continue to: Bottom Line
Bottom Line
Individuals with xenomelia have the persistent belief that one or more of their limbs does not belong to their body but is an alien appendage that should be removed. Patients with this condition may resort to self-amputation or self-mutilation that requires subsequent surgical amputation. Xenomelia may be related to anomalous brain development, with a lack of neural representation of a limb in the right parietal lobe.
Related Resources
- Hilti LM, Hänggi J, Vitacco DA, et al. The desire for healthy limb amputation: structural brain correlates and clinical features of xenomelia. Brain. 2013;136(pt 1):318-329.
- Brugger P, Lenggenhager B, Giummarra MJ. Xenomelia: a social neuroscience view of altered bodily self-consciousness. Front Psychol. 2013;4:204. doi:10.3389/fpsyg.2013.00204.
1. Upadhyaya MA, Nasrallah HA. The intense desire for healthy limb amputation: a dis-proprioceptive neuropsychiatric disorder. Ann Clin Psychiatry. 2017;29(2):125-132.
2. Sedda A, Bottini G. Apotemnophilia, body integrity identity disorder or xenomelia? Psychiatric and neurologic etiologies face each other. Neuropsychiatr Dis Treat. 2014;10:1255-1265.
3. Money J, Jobaris R, Furth G. Apotemnophilia: two cases of self-demand amputation as a paraphilia. J Sex Res. 1977;13(2):115-125.
4. Blom RM, Hennekam RC, Denys D. Body integrity identity disorder. PLoS One. 2012;7(4):e34702. doi: 10.1371/journal.pone.0034702.
5. First MB. Desire for amputation of a limb: paraphilia, psychosis, or a new type of identity disorder. Psychol Med. 2005;35(6):919-928.
6. McGeoch PD, Brang D, Song T, et al. Xenomelia: a new right parietal lobe syndrome. J Neurol Neurosurg Psychiatry. 2011;82(12):1314-1319.
7. Nowakowski P, Karczmarczyk A. The rest is not me… An attempt to explain xenomelia--neurodevelopmental hypothesis. Postepy Psychiatrii i Neurologii. 2016;25(3):196-208.
8. Brang D, McGeoch PD, Ramachandran VS. Apotemnophilia: a neurological disorder. Neuroreport. 2008;19(13):1305-1306.
9. Forum. Penthouse. September 1972:128.
10. Blom RM, van der Wal SJ, Vulink NC, et al. Role of sexuality in body integrity identity disorder (BIID): a cross-sectional internet-based survey study. J Sex Med. 2017;14(8):1028-1035.
11. Ramachandran VS, Brang D, McGeoch PD, et al. Sexual and food preference in apotemnophilia and anorexia: interactions between ‘beliefs’ and ‘needs’ regulated by two-way connections between body image and limbic structures. Perception. 2009;38(5):775-777.
1. Upadhyaya MA, Nasrallah HA. The intense desire for healthy limb amputation: a dis-proprioceptive neuropsychiatric disorder. Ann Clin Psychiatry. 2017;29(2):125-132.
2. Sedda A, Bottini G. Apotemnophilia, body integrity identity disorder or xenomelia? Psychiatric and neurologic etiologies face each other. Neuropsychiatr Dis Treat. 2014;10:1255-1265.
3. Money J, Jobaris R, Furth G. Apotemnophilia: two cases of self-demand amputation as a paraphilia. J Sex Res. 1977;13(2):115-125.
4. Blom RM, Hennekam RC, Denys D. Body integrity identity disorder. PLoS One. 2012;7(4):e34702. doi: 10.1371/journal.pone.0034702.
5. First MB. Desire for amputation of a limb: paraphilia, psychosis, or a new type of identity disorder. Psychol Med. 2005;35(6):919-928.
6. McGeoch PD, Brang D, Song T, et al. Xenomelia: a new right parietal lobe syndrome. J Neurol Neurosurg Psychiatry. 2011;82(12):1314-1319.
7. Nowakowski P, Karczmarczyk A. The rest is not me… An attempt to explain xenomelia--neurodevelopmental hypothesis. Postepy Psychiatrii i Neurologii. 2016;25(3):196-208.
8. Brang D, McGeoch PD, Ramachandran VS. Apotemnophilia: a neurological disorder. Neuroreport. 2008;19(13):1305-1306.
9. Forum. Penthouse. September 1972:128.
10. Blom RM, van der Wal SJ, Vulink NC, et al. Role of sexuality in body integrity identity disorder (BIID): a cross-sectional internet-based survey study. J Sex Med. 2017;14(8):1028-1035.
11. Ramachandran VS, Brang D, McGeoch PD, et al. Sexual and food preference in apotemnophilia and anorexia: interactions between ‘beliefs’ and ‘needs’ regulated by two-way connections between body image and limbic structures. Perception. 2009;38(5):775-777.
Catatonia: How to identify and treat it
Is catatonia a rare condition that belongs in the history books, or is it more prevalent than we think? If we think we don’t see it often, how will we recognize it? And how do we treat it? This article reviews the evolution of our understanding of the phenomenology and therapy of this interesting and complex condition.
History of the concept
In 1874, Kahlbaum1,2 was the first to propose a syndrome of motor dysfunction characterized by mutism, immobility, staring gaze, negativism, stereotyped behavior, waxy flexibility, and verbal stereotypies that he called catatonia. Kahlbaum conceptualized catatonia as a distinct disorder,3 but Kraepelin reformulated it as a feature of dementia praecox.4 Although Bleuler felt that catatonia could occur in other psychiatric disorders and in normal people,4 he also included catatonia as a marker of schizophrenia, where it remained from DSM-I through DSM-IV.3 As was believed to be true of schizophrenia, Kraepelin considered catatonia to be characterized by poor prognosis, whereas Bleuler eliminated poor prognosis as a criterion for catatonia.3
In DSM-IV, catatonia was still a subtype of schizophrenia, but for the first time it was expanded diagnostically to become both a specifier in mood disorders, and a syndrome resulting from a general medical condition.5,6 In DSM-5, catatonic schizophrenia was deleted, and catatonia became a specifier for 10 disorders, including schizophrenia, mood disorders, and general medical conditions.3,5-9 In ICD-10, however, catatonia is still associated primarily with schizophrenia.10
A wide range of presentations
Catatonia is a cyclical syndrome characterized by alterations in motor, behavioral, and vocal signs occurring in the context of medical, neurologic, and psychiatric disorders.8 The most common features are immobility, waxy flexibility, stupor, mutism, negativism, echolalia, echopraxia, peculiarities of voluntary movement, and rigidity.7,11 Features of catatonia that have been repeatedly described through the years are summarized in Table 1.8,12,13 In general, presentations of catatonia are not specific to any psychiatric or medical etiology.13,14
Catatonia often is described along a continuum from retarded/stuporous to excited,14,15 and from benign to malignant.13 Examples of these ranges of presentation include5,12,13,15-19:
Stuporous/retarded catatonia (Kahlbaum syndrome) is a primarily negative syndrome in which stupor, mutism, negativism, obsessional slowness, and posturing predominate. Akinetic mutism and coma vigil are sometimes considered to be types of stuporous catatonia, as occasionally are locked-in syndrome and abulia caused by anterior cingulate lesions.
Excited catatonia (hyperkinetic variant, Bell’s mania, oneirophrenia, oneroid state/syndrome, catatonia raptus) is characterized by agitation, combativeness, verbigeration, stereotypies, grimacing, and echo phenomena (echopraxia and echolalia).
Continue to: Malignant (lethal) catatonia
Malignant (lethal) catatonia consists of catatonia accompanied by excitement, stupor, altered level of consciousness, catalepsy, hyperthermia, and autonomic instability with tachycardia, tachypnea, hypertension, and labile blood pressure. Autonomic dysregulation, fever, rhabdomyolysis, and acute renal failure can be causes of morbidity and mortality. Neuroleptic malignant syndrome (NMS)—which is associated with dopamine antagonists, especially antipsychotics—is considered a form of malignant catatonia and has a mortality rate of 10% to 20%. Signs of NMS include muscle rigidity, fever, diaphoresis, rigor, altered consciousness, mutism, tachycardia, hypertension, leukocytosis, and laboratory evidence of muscle damage. Serotonin syndrome can be difficult to distinguish from malignant catatonia, but it is usually not associated with waxy flexibility and rigidity.
Several specific subtypes of catatonia that may exist anywhere along dimensions of activity and severity also have been described:
Periodic catatonia. In 1908, Kraepelin described a form of periodic catatonia, with rapid shifts from excitement to stupor.4 Later, Gjessing described periodic catatonia in schizophrenia and reported success treating it with high doses of thyroid hormone.4 Today, periodic catatonia refers to the rapid onset of recurrent, brief hypokinetic or hyperkinetic episodes lasting 4 to 10 days and recurring during the course of weeks to years. Patients often are asymptomatic between episodes except for grimacing, stereotypies, and negativism later in the course.13,15 At least some forms of periodic catatonia are familial,4 with autosomal dominant transmission possibly linked to chromosome 15q15.13
A familial form of catatonia has been described that has a poor response to standard therapies (benzodiazepines and electroconvulsive therapy [ECT]), but in view of the high comorbidity of catatonia and bipolar disorder, it is difficult to determine whether this is a separate condition, or a group of patients with bipolar disorder.5
Late (ie, late-onset) catatonia is well described in the Japanese literature.10 Reported primarily in women without a known medical illness or brain disorder, late catatonia begins with prodromal hypochondriacal or depressive symptoms during a stressful situation, followed by unprovoked anxiety and agitation. Some patients develop hallucinations, delusions, and recurrent excitement, along with anxiety and agitation. The next stage involves typical catatonic features (mainly excitement, retardation, negativism, and autonomic disturbance), progressing to stupor, mutism, verbal stereotypies, and negativism, including refusal of food. Most patients have residual symptoms following improvement. A few cases have been noted to remit with ECT, with relapse when treatment was discontinued. Late catatonia has been thought to be associated with late-onset schizophrenia or bipolar disorder, or to be an independent entity.
Continue to: Untreated catatonia can have...
Untreated catatonia can have serious medical complications, including deep vein thrombosis, pulmonary embolism, aspiration pneumonia, infection, metabolic disorders, decubitus ulcers, malnutrition, dehydration, contractures, thrombosis, urinary retention, rhabdomyolysis, acute renal failure, sepsis, disseminated intravascular coagulation, and cardiac arrest.11,12,16,20,21 Mortality approaches 10%.12 In children and adolescents, catatonia increases the risk of premature death (including by suicide) 60-fold.22
Not as rare as you might think
With the shift from inpatient to outpatient care driven by deinstitutionalization, longitudinal close observation became less common, and clinicians got the impression that the dramatic catatonia that was common in the hospital had become rare.3 The impression that catatonia was unimportant was strengthened by expanding industry promotion of antipsychotic medications while ignoring catatonia, for which the industry had no specific treatment.3 With recent research, however, catatonia has been reported in 7% to 38% of adult psychiatric patients, including 9% to 25% of inpatients, 20% to 25% of patients with mania,3,5 and 20% of patients with major depressive episodes.7 Catatonia has been noted in .6% to 18% of adolescent psychiatric inpatients (especially in communication and social disorders programs),5,8,22 some children,5 and 6% to 18% of adult and juvenile patients with autism spectrum disorder (ASD).23 In the medical setting, catatonia occurs in 12% to 37% of patients with delirium,8,14,17,18,20,24 7% to 45% of medically ill patients, including those with no psychiatric history,12,13 and 4% of ICU patients.12 Several substances have been linked to catatonia; these are discussed later.11 Contrary to earlier impressions, catatonia is more common in mood disorders, particularly mixed bipolar disorder, especially mania,5 than in schizophrenia.7,8,17,25
Pathophysiology/etiology
Conditions associated with catatonia have different features that act through a final common pathway,7 possibly related to the neurobiology of an extreme fear response called tonic immobility that has been conserved through evolution.8 This mechanism may be mediated by decreased dopamine signaling in basal ganglia, orbitofrontal, and limbic systems, including the hypothalamus and basal forebrain.3,17,20 Subcortical reduction of dopaminergic neurotransmission appears to be related to reduced GABAA receptor signaling and dysfunction of N-methyl-
Up to one-quarter of cases of catatonia are secondary to medical (mostly neurologic) factors or substances.15Table 25,13,15 lists common medical and neurological causes. Medications and substances known to cause catatonia are noted in Table 3.5,8,13,16,26
Catatonia can be a specifier, or a separate condition
DSM-5 criteria for catatonia are summarized in Table 4.28 With these features, catatonia can be a specifier for depressive, bipolar, or psychotic disorders; a complication of a medical disorder; or another separate diagnosis.8 The diagnosis of catatonia in DSM-5 is made when the clinical picture is dominated by ≥3 of the following core features8,15:
- motoric immobility as evidenced by catalepsy (including waxy flexibility) or stupor
- excessive purposeless motor activity that is not influenced by external stimuli
- extreme negativism or mutism
- peculiarities of voluntary movement such as posturing, stereotyped movements, prominent mannerisms, or prominent grimacing
- echolalia or echopraxia.
Continue to: DSM-5 criteria for the diagnosis of catatonia are more...
DSM-5 criteria for the diagnosis of catatonia are more restrictive than DSM-IV criteria. As a result, they exclude a significant number of patients who would be considered catatonic in other systems.29 For example, DSM-5 criteria do not include common features noted in Table 1,8,12,13 such as rigidity and staring.14,29 If the diagnosis is not obvious, it might be suspected in the presence of >1 of posturing, automatic obedience, or waxy flexibility, or >2 of echopraxia/echolalia, gegenhalten, negativism, mitgehen, or stereotypy/vergiberation.12 Clues to catatonia that are not included in formal diagnostic systems and are easily confused with features of psychosis include whispered or robotic speech, uncharacteristic foreign accent, tiptoe walking, hopping, rituals, and odd mannerisms.5
There are several catatonia rating scales containing between 14 and 40 items that are useful in diagnosing and following treatment response in catatonia (Table 58,13,15,29). Of these, the Kanner Scale is primarily applied in neuropsychiatric settings, while the Bush-Francis Catatonia Rating Scale (BFCRS) has had the most widespread use. The BFCRS consists of 23 items, the first 14 of which are used as a screening instrument. It requires 2 of its first 14 items to diagnose catatonia, while DSM-5 requires 3 of 12 signs.29 If the diagnosis remains in doubt, a benzodiazepine agonist test can be instructive.9,12 The presence of catatonia is suggested by significant improvement, ideally assessed prospectively by improvement of BFCRS scores, shortly after administration of a single dose of 1 to 2 mg lorazepam or 5 mg diazepam IV, or 10 mg zolpidem orally. Further evaluation generally consists of a careful medical and psychiatric histories of patient and family, review of all medications, history of substance use with toxicology as indicated, physical examination focusing on autonomic dysregulation, examination for delirium, and laboratory tests as suggested by the history and examination that may include complete blood count, creatine kinase, serum iron, blood urea nitrogen, electrolytes, creatinine, prolactin, anti-NMDA antibodies, thyroid function tests, serology, metabolic panel, human immunodeficiency virus testing, EEG, and neuroimaging.8,15,16
A complex differential diagnosis
Manifestations of numerous psychiatric and neurologic disorders can mimic or be identical to those of catatonia. The differential diagnosis is complicated by the fact that some of these disorders can cause catatonia, which is then masked by the primary disorder; some disorders (eg, NMS) are forms of catatonia. Table 65,8,12,19,26,30 lists conditions to consider.
Some of these conditions warrant discussion. ASD may have catatonia-like features such as echolalia, echopraxia, excitement, combativeness, grimacing, mutism, logorrhea, verbigeration, catalepsy, mannerisms, rigidity, staring and withdrawal.8 Catatonia may also be a stage of deterioration of autism, in which case it is characterized by increases in slowness of movement and speech, reliance on physical or verbal prompting from others, passivity, and lack of motivation.23 At the same time, catatonic features such as mutism, stereotypic speech, repetitive behavior, echolalia, posturing, mannerisms, purposeless agitation, and rigidity in catatonia can be misinterpreted as signs of ASD.8 Catatonia should be suspected as a complication of longstanding ASD in the presence of a consistent, marked change in motor behavior, such as immobility, decreased speech, stupor, excitement, or mixtures or alternations of stupor and excitement.8 Freezing while doing something, difficulty crossing lines, or uncharacteristic persistence of a particular behavior may also herald the presence of catatonia with ASD.8
Catatonia caused by a neurologic or metabolic factor or a substance can be difficult to distinguish from delirium complicated by catatonia. Delirium may be identified in patients with catatonia by the presence of a waxing and waning level of consciousness (vs fluctuating behavior in catatonia) and slowing of the EEG.12,15 Antipsychotic medications can improve delirium but worsen catatonia, while benzodiazepines can improve catatonia but worsen delirium.
Continue to: Among other neurologic syndromes...
Among other neurologic syndromes that can be confused with catatonia, locked-in syndrome consists of total immobility except for vertical extraocular movements and blinking. In this state, patients attempt to communicate with their eyes, while catatonic patients do not try to communicate. There is no response to a lorazepam challenge test. Stiff man syndrome is associated with painful spasms precipitated by touch, noise, or emotional stimuli. Baclofen can resolve stiff man syndrome, but it can induce catatonia. Paratonia refers to generalized increased motor tone that is idiopathic, or associated with neurodegeneration, encephalopathy, or medications. The only motor sign is increased tone, and other signs of catatonia are absent. Catatonia is usually associated with some motor behaviors and interaction with the environment, even if it is negative, while the coma vigil patient is completely unresponsive. Frontotemporal dementia is progressive, while catatonia usually improves without residual dementia.30
Benzodiazepines, ECT are the usual treatments
Experience dictates that the general principles of treatment noted in Table 712,15,23,31 apply to all patients with catatonia. Since the first reported improvement of catatonia with amobarbital in 1930,6 there have been no controlled studies of specific treatments of catatonia.13 Meaningful treatment trials are either naturalistic, or have been performed only for NMS and malignant catatonia.5 However, multiple case reports and case series suggest that treatments with agents that have anticonvulsant properties (benzodiazepines, barbiturates) and ECT are effective.5
Benzodiazepines and related compounds. Case series have suggested a 60% to 80% remission rate of catatonia with benzodiazepines, the most commonly utilized of which has been lorazepam.7,13,32 Treatment begins with a lorazepam challenge test of 1 to 2 mg in adults and 0.5 to 1 mg in children and geriatric patients,9,15 administered orally (including via nasogastric tube), IM, or IV. Following a response (≥50% improvement), the dose is increased to 2 mg 3 times per day. The dose is further increased to 6 to 16 mg/d, and sometimes up to 30 mg/d.9,11 Oral is less effective than sublingual or IM administration.11 Diazepam can be helpful at doses 5 times the lorazepam dose.9,17 A zo
One alternative benzodiazepine protocol utilizes an initial IV dose of 2 mg lorazepam, repeated 3 to 5 times per day; the dose is increased to 10 to 12 mg/d if the first doses are partially effective.16 A lorazepam/diazepam approach involves a combination of IM lorazepam and IV diazepam.11 The protocol begins with 2 mg of IM lorazepam. If there is no effect within 2 hours, a second 2 mg dose is administered, followed by an IV infusion of 10 mg diazepam in 500 ml of normal saline at 1.25 mg/hour until catatonia remits.
An Indian study of 107 patients (mean age 26) receiving relatively low doses of lorazepam (3 to 6 mg/d for at least 3 days) found that factors suggesting a robust response include a shorter duration of catatonia and waxy flexibility, while passivity, mutism, and auditory hallucinations describing the patient in the third person were associated with a poorer acute response.31 Catatonia with marked retardation and mutism complicating schizophrenia, especially with chronic negative symptoms, may be associated with a lower response rate to benzodiazepines.20,33 Maintenance lorazepam has been effective in reducing relapse and recurrence.11 There are no controlled studies of maintenance treatment with benzodiazepines, but clinical reports suggest that doses in the range of 4 to 10 mg/d are effective.32
Continue to: ECT was used for catatonia in 1934...
ECT was first used for catatonia in 1934, when Laszlo Meduna used chemically induced seizures in catatonic patients who had been on tube feeding for months and no longer needed it after treatment.6,7 As was true for other disorders, this approach was replaced by ECT.7 In various case series, the effectiveness of ECT in catatonia has been 53% to 100%.7,13,15 Right unilateral ECT has been reported to be effective with 1 treatment.21 However, the best-established approach is with bitemporal ECT with a suprathreshold stimulus,9 usually with an acute course of 6 to 20 treatments.20 ECT has been reported to be equally safe and effective in adolescents and adults.34 Continued ECT is usually necessary until the patient has returned to baseline.9
ECT usually is recommended within 24 hours for treatment-resistant malignant catatonia or refusal to eat or drink, and within 2 to 3 days if medications are not sufficiently effective in other forms of catatonia.12,15,20 If ECT is initiated after a benzodiazepine trial, the benzodiazepine antagonist flumazenil is administered first to reverse the anticonvulsant effect.9 Some experts recommend using a muscle relaxant other than succinylcholine in the presence of evidence of muscle damage.7
Alternatives to benzodiazepines and ECT. Based on case reports, the treatments described in Table 813,15,17,20,25 have been used for patients with catatonia who do not tolerate or respond to standard treatments. The largest number of case reports have been with NMDA antagonists, while the presumed involvement of reduced dopamine signaling suggests that dopaminergic medications should be helpful. Dantrolene, which blocks release of calcium from intracellular stores and has been used to treat malignant hyperthermia, is sometimes used for NMS, often with disappointing results.
Whereas first-generation antipsychotics definitely increase the risk of catatonia and second-generation antipsychotics (SGAs) probably do so, SGAs are sometimes necessary to treat persistent psychosis in patients with schizophrenia who develop catatonia. Of these medications, clozapine may be most desirable because of low potency for dopamine receptor blockade and modulation of glutamatergic signaling. Partial dopamine agonism by aripiprazole, and the potential for increased subcortical prefrontal dopamine release resulting from serotonin 5HT2A antagonism and 5HT1A agonism by other SGAs, could also be helpful or at least not harmful in catatonia. Lorazepam is usually administered along with these medications to ameliorate treatment-emergent exacerbation of catatonia.
There are no controlled studies of any of these treatments. Based on case reports, most experts would recommend initiating treatment of catatonia with lorazepam, followed by ECT if necessary or in the presence of life-threatening catatonia. If ECT is not available, ineffective, or not tolerated, the first alternatives to be considered would be an NMDA antagonist or an anticonvulsant.20
Continue to: Course varies by patient, underlying cause
Course varies by patient, underlying cause
The response to benzodiazepines or ECT can vary from episode to episode11 and is similar in adults and younger patients.22 Many patients recover completely after a single episode, while relapse after remission occurs repeatedly in periodic catatonia, which involves chronic alternating stupor and excitement waxing and waning over years.11 Relapses may occur frequently, or every few years.11 Some cases of catatonia initially have an episodic course and become chronic and deteriorating, possibly paralleling the original descriptions of the natural history of untreated catatonia, while malignant catatonia can be complicated by medical morbidity or death.4 The long-term prognosis generally depends on the underlying cause of catatonia.5
Bottom Line
Much more common than many clinicians realize, catatonia can be overlooked because symptoms can mimic or overlap with features of an underlying medical or neurologic disorder. Suspect catatonia when one of these illnesses has an unexpected course or an inadequate treatment response. Be alert to characteristic changes in behavior and speech. A benzodiazepine challenge can be used to diagnose and begin treatment of catatonia. Consider electroconvulsive therapy sooner rather than later, especially for severely ill patients.
Related Resources
- Gibson RC, Walcott G. Benzodiazepines for catatonia in people with schizophrenia and other serious mental illnesses. Cochrane Database Syst Rev. 2008;(4):CD006570.
- Newcastle University. Catatonia. https://youtu.be/_s1lzxHRO4U.
Drug Brand Names
Amantadine • Symmetrel
Amobarbital • Amytal
Aripiprazole • Abilify
Azithromycin • Zithromax
Baclofen • Lioresal
Benztropine • Cogentin
Carbamazepine • Carbatrol, Tegretol
Carbidopa/levodopa • Sinemet
Ciprofloxacin • Cipro
Clozapine • Clozaril
Dantrolene • Dantrium
Dexamethasone • Decadron
Dextromethorphan/quinidine • Neudexta
Diazepam • Valium
Disulfiram • Antabuse
Flumazenil • Romazicon
Fluoxetine • Prozac
Fluvoxamine • Luvox
Levetiracetam • Keppra
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Memantine • Namenda
Methylphenidate • Ritalin
Minocycline • Minocin
Olanzapine • Zyprexa
Risperidone • Risperdal
Succinylcholine • Anectine
Topiramate • Topamax
Trihexyphenidyl • Artane
Valproate • Depakote
Ziprasidone • Geodon
Zolpidem • Ambien
1. Kahlbaum KL. Catatonia. Baltimore, MD: John Hopkins University Press; 1973.
2. Kahlbaum KL. Die Katatonie oder das Spannungsirresein. Berlin: Hirschwald; 1874.
3. Tang VM, Duffin J. Catatonia in the history of psychiatry: construction and deconstruction of a disease concept. Perspect Biol Med. 2014;57(4):524-537.
4. Carroll BT. Kahlbaum’s catatonia revisited. Psychiatry Clin Neurosci. 2001;55(5):431-436.
5. Taylor MA, Fink M. Catatonia in psychiatric classification: a home of its own. Am J Psychiatry. 2003;160(7):1233-1241.
6. Fink M, Fricchione GL, Rummans T, et al. Catatonia is a systemic medical syndrome. Acta Psychiatr Scand. 2016;133(3):250-251.
7. Medda P, Toni C, Luchini F, et al. Catatonia in 26 patients with bipolar disorder: clinical features and response to electroconvulsive therapy. Bipolar Disord. 2015;17(8):892-901.
8. Mazzone L, Postorino V, Valeri G, et al. Catatonia in patients with autism: prevalence and management. CNS Drugs. 2014;28(3):205-215.
9. Fink M, Kellner CH, McCall WV. Optimizing ECT technique in treating catatonia. J ECT. 2016;32(3):149-150.
10. Kocha H, Moriguchi S, Mimura M. Revisiting the concept of late catatonia. Compr Psychiatry. 2014;55(7):1485-1490.
11. Lin CC, Hung YL, Tsai MC, et al. Relapses and recurrences of catatonia: 30-case analysis and literature review. Compr Psychiatry. 2016;66:157-165.
12. Saddawi-Konefka D, Berg SM, Nejad SH, et al. Catatonia in the ICU: An important and underdiagnosed cause of altered mental status. A case series and review of the literature. Crit Care Med. 2013;42(3):e234-e241.
13. Wijemanne S, Jankovic J. Movement disorders in catatonia. J Neurol Neurosurg Psychiatry. 2015;86(8):825-832.
14. Grover S, Chakrabarti S, Ghormode D, et al. Catatonia in inpatients with psychiatric disorders: a comparison of schizophrenia and mood disorders. Psychiatry Res. 2015;229(3):919-925.
15. Oldham MA, Lee HB. Catatonia vis-à-vis delirium: the significance of recognizing catatonia in altered mental status. Gen Hosp Psychiatry. 2015;37(6):554-559.
16. Tuerlings JH, van Waarde JA, Verwey B. A retrospective study of 34 catatonic patients: analysis of clinical ‘care and treatment. Gen Hosp Psychiatry. 2010;32(6):631-635.
17. Ohi K, Kuwata A, Shimada T, et al. Response to benzodiazepines and the clinical course in malignant catatonia associated with schizophrenia: a case report. Medicine (Baltimore). 2017;96(16):e6566. doi: 10.1097/MD.0000000000006566.
18. Komatsu T, Nomura T, Takami H, et al. Catatonic symptoms appearing before autonomic symptoms help distinguish neuroleptic malignant syndrome from malignant catatonia. Intern Med. 2016;55(19):2893-2897.
19. Lang FU, Lang S, Becker T, et al. Neuroleptic malignant syndrome or catatonia? Trying to solve the catatonic dilemma. Psychopharmacology (Berl). 2015;232(1):1-5.
20. Beach SR, Gomez-Bernal F, Huffman JC, et al. Alternative treatment strategies for catatonia: a systematic review. Gen Hosp Psychiatry. 2017;48:1-19.
21. Kugler JL, Hauptman AJ, Collier SJ, et al. Treatment of catatonia with ultrabrief right unilateral electroconvulsive therapy: a case series. J ECT. 2015;31(3):192-196.
22. Raffin M, Zugaj-Bensaou L, Bodeau N, et al. Treatment use in a prospective naturalistic cohort of children and adolescents with catatonia. Eur Child Adolesc Psychiatry. 2015;24(4):441-449.
23. DeJong H, Bunton P, Hare DJ. A systematic review of interventions used to treat catatonic symptoms in people with autistic spectrum disorders. J Autism Dev Disord. 2014;44(9):2127-2136.
24. Wachtel L, Commins E, Park MH, et al. Neuroleptic malignant syndrome and delirious mania as malignant catatonia in autism: prompt relief with electroconvulsive therapy. Acta Psychiatr Scand. 2015;132(4):319-320.
25. Fink M, Taylor MA. Catatonia: subtype or syndrome in DSM? Am J Psychiatry. 2006;163(11):1875-1876.
26. Khan M, Pace L, Truong A, et al. Catatonia secondary to synthetic cannabinoid use in two patients with no previous psychosis. Am J Addictions. 2016;25(1):25-27.
27. Komatsu T, Nomura T, Takami H, et al. Catatonic symptoms appearing before autonomic symptoms help distinguish neuroleptic malignant syndrome from malignant catatonia. Intern Med. 2016;55(19):2893-2897.
28. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
29. Wilson JE, Niu K, Nicolson SE, et al. The diagnostic criteria and structure of catatonia. Schizophr Res. 2015;164(1-3):256-262.
30. Ducharme S, Dickerson BC, Larvie M, et al. Differentiating frontotemporal dementia from catatonia: a complex neuropsychiatric challenge. J Neuropsychiatry Clin Neurosci. 2015;27(2):e174-e176.
31. Narayanaswamy JC, Tibrewal P, Zutshi A, et al. Clinical predictors of response to treatment in catatonia. Gen Hosp Psychiatry. 2012;34(3):312-316.
32. Thamizh JS, Harshini M, Selvakumar N, et al. Maintenance lorazepam for treatment of recurrent catatonic states: a case series and implications. Asian J Psychiatr. 2016;22:147-149
33. Ungvari GS, Chiu HF, Chow LY, et al. Lorazepam for chronic catatonia: a randomized, double-blind, placebo-controlled cross-over study. Psychopharmacology (Berl). 1999;142(4):393-398.
34. Flamarique I, Baeza I, de la Serna E, et al. Long-term effectiveness of electroconvulsive therapy in adolescents with schizophrenia spectrum disorders. Eur Child Adolesc Psychiatry. 2015;24(5):517-524.
Is catatonia a rare condition that belongs in the history books, or is it more prevalent than we think? If we think we don’t see it often, how will we recognize it? And how do we treat it? This article reviews the evolution of our understanding of the phenomenology and therapy of this interesting and complex condition.
History of the concept
In 1874, Kahlbaum1,2 was the first to propose a syndrome of motor dysfunction characterized by mutism, immobility, staring gaze, negativism, stereotyped behavior, waxy flexibility, and verbal stereotypies that he called catatonia. Kahlbaum conceptualized catatonia as a distinct disorder,3 but Kraepelin reformulated it as a feature of dementia praecox.4 Although Bleuler felt that catatonia could occur in other psychiatric disorders and in normal people,4 he also included catatonia as a marker of schizophrenia, where it remained from DSM-I through DSM-IV.3 As was believed to be true of schizophrenia, Kraepelin considered catatonia to be characterized by poor prognosis, whereas Bleuler eliminated poor prognosis as a criterion for catatonia.3
In DSM-IV, catatonia was still a subtype of schizophrenia, but for the first time it was expanded diagnostically to become both a specifier in mood disorders, and a syndrome resulting from a general medical condition.5,6 In DSM-5, catatonic schizophrenia was deleted, and catatonia became a specifier for 10 disorders, including schizophrenia, mood disorders, and general medical conditions.3,5-9 In ICD-10, however, catatonia is still associated primarily with schizophrenia.10
A wide range of presentations
Catatonia is a cyclical syndrome characterized by alterations in motor, behavioral, and vocal signs occurring in the context of medical, neurologic, and psychiatric disorders.8 The most common features are immobility, waxy flexibility, stupor, mutism, negativism, echolalia, echopraxia, peculiarities of voluntary movement, and rigidity.7,11 Features of catatonia that have been repeatedly described through the years are summarized in Table 1.8,12,13 In general, presentations of catatonia are not specific to any psychiatric or medical etiology.13,14
Catatonia often is described along a continuum from retarded/stuporous to excited,14,15 and from benign to malignant.13 Examples of these ranges of presentation include5,12,13,15-19:
Stuporous/retarded catatonia (Kahlbaum syndrome) is a primarily negative syndrome in which stupor, mutism, negativism, obsessional slowness, and posturing predominate. Akinetic mutism and coma vigil are sometimes considered to be types of stuporous catatonia, as occasionally are locked-in syndrome and abulia caused by anterior cingulate lesions.
Excited catatonia (hyperkinetic variant, Bell’s mania, oneirophrenia, oneroid state/syndrome, catatonia raptus) is characterized by agitation, combativeness, verbigeration, stereotypies, grimacing, and echo phenomena (echopraxia and echolalia).
Continue to: Malignant (lethal) catatonia
Malignant (lethal) catatonia consists of catatonia accompanied by excitement, stupor, altered level of consciousness, catalepsy, hyperthermia, and autonomic instability with tachycardia, tachypnea, hypertension, and labile blood pressure. Autonomic dysregulation, fever, rhabdomyolysis, and acute renal failure can be causes of morbidity and mortality. Neuroleptic malignant syndrome (NMS)—which is associated with dopamine antagonists, especially antipsychotics—is considered a form of malignant catatonia and has a mortality rate of 10% to 20%. Signs of NMS include muscle rigidity, fever, diaphoresis, rigor, altered consciousness, mutism, tachycardia, hypertension, leukocytosis, and laboratory evidence of muscle damage. Serotonin syndrome can be difficult to distinguish from malignant catatonia, but it is usually not associated with waxy flexibility and rigidity.
Several specific subtypes of catatonia that may exist anywhere along dimensions of activity and severity also have been described:
Periodic catatonia. In 1908, Kraepelin described a form of periodic catatonia, with rapid shifts from excitement to stupor.4 Later, Gjessing described periodic catatonia in schizophrenia and reported success treating it with high doses of thyroid hormone.4 Today, periodic catatonia refers to the rapid onset of recurrent, brief hypokinetic or hyperkinetic episodes lasting 4 to 10 days and recurring during the course of weeks to years. Patients often are asymptomatic between episodes except for grimacing, stereotypies, and negativism later in the course.13,15 At least some forms of periodic catatonia are familial,4 with autosomal dominant transmission possibly linked to chromosome 15q15.13
A familial form of catatonia has been described that has a poor response to standard therapies (benzodiazepines and electroconvulsive therapy [ECT]), but in view of the high comorbidity of catatonia and bipolar disorder, it is difficult to determine whether this is a separate condition, or a group of patients with bipolar disorder.5
Late (ie, late-onset) catatonia is well described in the Japanese literature.10 Reported primarily in women without a known medical illness or brain disorder, late catatonia begins with prodromal hypochondriacal or depressive symptoms during a stressful situation, followed by unprovoked anxiety and agitation. Some patients develop hallucinations, delusions, and recurrent excitement, along with anxiety and agitation. The next stage involves typical catatonic features (mainly excitement, retardation, negativism, and autonomic disturbance), progressing to stupor, mutism, verbal stereotypies, and negativism, including refusal of food. Most patients have residual symptoms following improvement. A few cases have been noted to remit with ECT, with relapse when treatment was discontinued. Late catatonia has been thought to be associated with late-onset schizophrenia or bipolar disorder, or to be an independent entity.
Continue to: Untreated catatonia can have...
Untreated catatonia can have serious medical complications, including deep vein thrombosis, pulmonary embolism, aspiration pneumonia, infection, metabolic disorders, decubitus ulcers, malnutrition, dehydration, contractures, thrombosis, urinary retention, rhabdomyolysis, acute renal failure, sepsis, disseminated intravascular coagulation, and cardiac arrest.11,12,16,20,21 Mortality approaches 10%.12 In children and adolescents, catatonia increases the risk of premature death (including by suicide) 60-fold.22
Not as rare as you might think
With the shift from inpatient to outpatient care driven by deinstitutionalization, longitudinal close observation became less common, and clinicians got the impression that the dramatic catatonia that was common in the hospital had become rare.3 The impression that catatonia was unimportant was strengthened by expanding industry promotion of antipsychotic medications while ignoring catatonia, for which the industry had no specific treatment.3 With recent research, however, catatonia has been reported in 7% to 38% of adult psychiatric patients, including 9% to 25% of inpatients, 20% to 25% of patients with mania,3,5 and 20% of patients with major depressive episodes.7 Catatonia has been noted in .6% to 18% of adolescent psychiatric inpatients (especially in communication and social disorders programs),5,8,22 some children,5 and 6% to 18% of adult and juvenile patients with autism spectrum disorder (ASD).23 In the medical setting, catatonia occurs in 12% to 37% of patients with delirium,8,14,17,18,20,24 7% to 45% of medically ill patients, including those with no psychiatric history,12,13 and 4% of ICU patients.12 Several substances have been linked to catatonia; these are discussed later.11 Contrary to earlier impressions, catatonia is more common in mood disorders, particularly mixed bipolar disorder, especially mania,5 than in schizophrenia.7,8,17,25
Pathophysiology/etiology
Conditions associated with catatonia have different features that act through a final common pathway,7 possibly related to the neurobiology of an extreme fear response called tonic immobility that has been conserved through evolution.8 This mechanism may be mediated by decreased dopamine signaling in basal ganglia, orbitofrontal, and limbic systems, including the hypothalamus and basal forebrain.3,17,20 Subcortical reduction of dopaminergic neurotransmission appears to be related to reduced GABAA receptor signaling and dysfunction of N-methyl-
Up to one-quarter of cases of catatonia are secondary to medical (mostly neurologic) factors or substances.15Table 25,13,15 lists common medical and neurological causes. Medications and substances known to cause catatonia are noted in Table 3.5,8,13,16,26
Catatonia can be a specifier, or a separate condition
DSM-5 criteria for catatonia are summarized in Table 4.28 With these features, catatonia can be a specifier for depressive, bipolar, or psychotic disorders; a complication of a medical disorder; or another separate diagnosis.8 The diagnosis of catatonia in DSM-5 is made when the clinical picture is dominated by ≥3 of the following core features8,15:
- motoric immobility as evidenced by catalepsy (including waxy flexibility) or stupor
- excessive purposeless motor activity that is not influenced by external stimuli
- extreme negativism or mutism
- peculiarities of voluntary movement such as posturing, stereotyped movements, prominent mannerisms, or prominent grimacing
- echolalia or echopraxia.
Continue to: DSM-5 criteria for the diagnosis of catatonia are more...
DSM-5 criteria for the diagnosis of catatonia are more restrictive than DSM-IV criteria. As a result, they exclude a significant number of patients who would be considered catatonic in other systems.29 For example, DSM-5 criteria do not include common features noted in Table 1,8,12,13 such as rigidity and staring.14,29 If the diagnosis is not obvious, it might be suspected in the presence of >1 of posturing, automatic obedience, or waxy flexibility, or >2 of echopraxia/echolalia, gegenhalten, negativism, mitgehen, or stereotypy/vergiberation.12 Clues to catatonia that are not included in formal diagnostic systems and are easily confused with features of psychosis include whispered or robotic speech, uncharacteristic foreign accent, tiptoe walking, hopping, rituals, and odd mannerisms.5
There are several catatonia rating scales containing between 14 and 40 items that are useful in diagnosing and following treatment response in catatonia (Table 58,13,15,29). Of these, the Kanner Scale is primarily applied in neuropsychiatric settings, while the Bush-Francis Catatonia Rating Scale (BFCRS) has had the most widespread use. The BFCRS consists of 23 items, the first 14 of which are used as a screening instrument. It requires 2 of its first 14 items to diagnose catatonia, while DSM-5 requires 3 of 12 signs.29 If the diagnosis remains in doubt, a benzodiazepine agonist test can be instructive.9,12 The presence of catatonia is suggested by significant improvement, ideally assessed prospectively by improvement of BFCRS scores, shortly after administration of a single dose of 1 to 2 mg lorazepam or 5 mg diazepam IV, or 10 mg zolpidem orally. Further evaluation generally consists of a careful medical and psychiatric histories of patient and family, review of all medications, history of substance use with toxicology as indicated, physical examination focusing on autonomic dysregulation, examination for delirium, and laboratory tests as suggested by the history and examination that may include complete blood count, creatine kinase, serum iron, blood urea nitrogen, electrolytes, creatinine, prolactin, anti-NMDA antibodies, thyroid function tests, serology, metabolic panel, human immunodeficiency virus testing, EEG, and neuroimaging.8,15,16
A complex differential diagnosis
Manifestations of numerous psychiatric and neurologic disorders can mimic or be identical to those of catatonia. The differential diagnosis is complicated by the fact that some of these disorders can cause catatonia, which is then masked by the primary disorder; some disorders (eg, NMS) are forms of catatonia. Table 65,8,12,19,26,30 lists conditions to consider.
Some of these conditions warrant discussion. ASD may have catatonia-like features such as echolalia, echopraxia, excitement, combativeness, grimacing, mutism, logorrhea, verbigeration, catalepsy, mannerisms, rigidity, staring and withdrawal.8 Catatonia may also be a stage of deterioration of autism, in which case it is characterized by increases in slowness of movement and speech, reliance on physical or verbal prompting from others, passivity, and lack of motivation.23 At the same time, catatonic features such as mutism, stereotypic speech, repetitive behavior, echolalia, posturing, mannerisms, purposeless agitation, and rigidity in catatonia can be misinterpreted as signs of ASD.8 Catatonia should be suspected as a complication of longstanding ASD in the presence of a consistent, marked change in motor behavior, such as immobility, decreased speech, stupor, excitement, or mixtures or alternations of stupor and excitement.8 Freezing while doing something, difficulty crossing lines, or uncharacteristic persistence of a particular behavior may also herald the presence of catatonia with ASD.8
Catatonia caused by a neurologic or metabolic factor or a substance can be difficult to distinguish from delirium complicated by catatonia. Delirium may be identified in patients with catatonia by the presence of a waxing and waning level of consciousness (vs fluctuating behavior in catatonia) and slowing of the EEG.12,15 Antipsychotic medications can improve delirium but worsen catatonia, while benzodiazepines can improve catatonia but worsen delirium.
Continue to: Among other neurologic syndromes...
Among other neurologic syndromes that can be confused with catatonia, locked-in syndrome consists of total immobility except for vertical extraocular movements and blinking. In this state, patients attempt to communicate with their eyes, while catatonic patients do not try to communicate. There is no response to a lorazepam challenge test. Stiff man syndrome is associated with painful spasms precipitated by touch, noise, or emotional stimuli. Baclofen can resolve stiff man syndrome, but it can induce catatonia. Paratonia refers to generalized increased motor tone that is idiopathic, or associated with neurodegeneration, encephalopathy, or medications. The only motor sign is increased tone, and other signs of catatonia are absent. Catatonia is usually associated with some motor behaviors and interaction with the environment, even if it is negative, while the coma vigil patient is completely unresponsive. Frontotemporal dementia is progressive, while catatonia usually improves without residual dementia.30
Benzodiazepines, ECT are the usual treatments
Experience dictates that the general principles of treatment noted in Table 712,15,23,31 apply to all patients with catatonia. Since the first reported improvement of catatonia with amobarbital in 1930,6 there have been no controlled studies of specific treatments of catatonia.13 Meaningful treatment trials are either naturalistic, or have been performed only for NMS and malignant catatonia.5 However, multiple case reports and case series suggest that treatments with agents that have anticonvulsant properties (benzodiazepines, barbiturates) and ECT are effective.5
Benzodiazepines and related compounds. Case series have suggested a 60% to 80% remission rate of catatonia with benzodiazepines, the most commonly utilized of which has been lorazepam.7,13,32 Treatment begins with a lorazepam challenge test of 1 to 2 mg in adults and 0.5 to 1 mg in children and geriatric patients,9,15 administered orally (including via nasogastric tube), IM, or IV. Following a response (≥50% improvement), the dose is increased to 2 mg 3 times per day. The dose is further increased to 6 to 16 mg/d, and sometimes up to 30 mg/d.9,11 Oral is less effective than sublingual or IM administration.11 Diazepam can be helpful at doses 5 times the lorazepam dose.9,17 A zo
One alternative benzodiazepine protocol utilizes an initial IV dose of 2 mg lorazepam, repeated 3 to 5 times per day; the dose is increased to 10 to 12 mg/d if the first doses are partially effective.16 A lorazepam/diazepam approach involves a combination of IM lorazepam and IV diazepam.11 The protocol begins with 2 mg of IM lorazepam. If there is no effect within 2 hours, a second 2 mg dose is administered, followed by an IV infusion of 10 mg diazepam in 500 ml of normal saline at 1.25 mg/hour until catatonia remits.
An Indian study of 107 patients (mean age 26) receiving relatively low doses of lorazepam (3 to 6 mg/d for at least 3 days) found that factors suggesting a robust response include a shorter duration of catatonia and waxy flexibility, while passivity, mutism, and auditory hallucinations describing the patient in the third person were associated with a poorer acute response.31 Catatonia with marked retardation and mutism complicating schizophrenia, especially with chronic negative symptoms, may be associated with a lower response rate to benzodiazepines.20,33 Maintenance lorazepam has been effective in reducing relapse and recurrence.11 There are no controlled studies of maintenance treatment with benzodiazepines, but clinical reports suggest that doses in the range of 4 to 10 mg/d are effective.32
Continue to: ECT was used for catatonia in 1934...
ECT was first used for catatonia in 1934, when Laszlo Meduna used chemically induced seizures in catatonic patients who had been on tube feeding for months and no longer needed it after treatment.6,7 As was true for other disorders, this approach was replaced by ECT.7 In various case series, the effectiveness of ECT in catatonia has been 53% to 100%.7,13,15 Right unilateral ECT has been reported to be effective with 1 treatment.21 However, the best-established approach is with bitemporal ECT with a suprathreshold stimulus,9 usually with an acute course of 6 to 20 treatments.20 ECT has been reported to be equally safe and effective in adolescents and adults.34 Continued ECT is usually necessary until the patient has returned to baseline.9
ECT usually is recommended within 24 hours for treatment-resistant malignant catatonia or refusal to eat or drink, and within 2 to 3 days if medications are not sufficiently effective in other forms of catatonia.12,15,20 If ECT is initiated after a benzodiazepine trial, the benzodiazepine antagonist flumazenil is administered first to reverse the anticonvulsant effect.9 Some experts recommend using a muscle relaxant other than succinylcholine in the presence of evidence of muscle damage.7
Alternatives to benzodiazepines and ECT. Based on case reports, the treatments described in Table 813,15,17,20,25 have been used for patients with catatonia who do not tolerate or respond to standard treatments. The largest number of case reports have been with NMDA antagonists, while the presumed involvement of reduced dopamine signaling suggests that dopaminergic medications should be helpful. Dantrolene, which blocks release of calcium from intracellular stores and has been used to treat malignant hyperthermia, is sometimes used for NMS, often with disappointing results.
Whereas first-generation antipsychotics definitely increase the risk of catatonia and second-generation antipsychotics (SGAs) probably do so, SGAs are sometimes necessary to treat persistent psychosis in patients with schizophrenia who develop catatonia. Of these medications, clozapine may be most desirable because of low potency for dopamine receptor blockade and modulation of glutamatergic signaling. Partial dopamine agonism by aripiprazole, and the potential for increased subcortical prefrontal dopamine release resulting from serotonin 5HT2A antagonism and 5HT1A agonism by other SGAs, could also be helpful or at least not harmful in catatonia. Lorazepam is usually administered along with these medications to ameliorate treatment-emergent exacerbation of catatonia.
There are no controlled studies of any of these treatments. Based on case reports, most experts would recommend initiating treatment of catatonia with lorazepam, followed by ECT if necessary or in the presence of life-threatening catatonia. If ECT is not available, ineffective, or not tolerated, the first alternatives to be considered would be an NMDA antagonist or an anticonvulsant.20
Continue to: Course varies by patient, underlying cause
Course varies by patient, underlying cause
The response to benzodiazepines or ECT can vary from episode to episode11 and is similar in adults and younger patients.22 Many patients recover completely after a single episode, while relapse after remission occurs repeatedly in periodic catatonia, which involves chronic alternating stupor and excitement waxing and waning over years.11 Relapses may occur frequently, or every few years.11 Some cases of catatonia initially have an episodic course and become chronic and deteriorating, possibly paralleling the original descriptions of the natural history of untreated catatonia, while malignant catatonia can be complicated by medical morbidity or death.4 The long-term prognosis generally depends on the underlying cause of catatonia.5
Bottom Line
Much more common than many clinicians realize, catatonia can be overlooked because symptoms can mimic or overlap with features of an underlying medical or neurologic disorder. Suspect catatonia when one of these illnesses has an unexpected course or an inadequate treatment response. Be alert to characteristic changes in behavior and speech. A benzodiazepine challenge can be used to diagnose and begin treatment of catatonia. Consider electroconvulsive therapy sooner rather than later, especially for severely ill patients.
Related Resources
- Gibson RC, Walcott G. Benzodiazepines for catatonia in people with schizophrenia and other serious mental illnesses. Cochrane Database Syst Rev. 2008;(4):CD006570.
- Newcastle University. Catatonia. https://youtu.be/_s1lzxHRO4U.
Drug Brand Names
Amantadine • Symmetrel
Amobarbital • Amytal
Aripiprazole • Abilify
Azithromycin • Zithromax
Baclofen • Lioresal
Benztropine • Cogentin
Carbamazepine • Carbatrol, Tegretol
Carbidopa/levodopa • Sinemet
Ciprofloxacin • Cipro
Clozapine • Clozaril
Dantrolene • Dantrium
Dexamethasone • Decadron
Dextromethorphan/quinidine • Neudexta
Diazepam • Valium
Disulfiram • Antabuse
Flumazenil • Romazicon
Fluoxetine • Prozac
Fluvoxamine • Luvox
Levetiracetam • Keppra
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Memantine • Namenda
Methylphenidate • Ritalin
Minocycline • Minocin
Olanzapine • Zyprexa
Risperidone • Risperdal
Succinylcholine • Anectine
Topiramate • Topamax
Trihexyphenidyl • Artane
Valproate • Depakote
Ziprasidone • Geodon
Zolpidem • Ambien
Is catatonia a rare condition that belongs in the history books, or is it more prevalent than we think? If we think we don’t see it often, how will we recognize it? And how do we treat it? This article reviews the evolution of our understanding of the phenomenology and therapy of this interesting and complex condition.
History of the concept
In 1874, Kahlbaum1,2 was the first to propose a syndrome of motor dysfunction characterized by mutism, immobility, staring gaze, negativism, stereotyped behavior, waxy flexibility, and verbal stereotypies that he called catatonia. Kahlbaum conceptualized catatonia as a distinct disorder,3 but Kraepelin reformulated it as a feature of dementia praecox.4 Although Bleuler felt that catatonia could occur in other psychiatric disorders and in normal people,4 he also included catatonia as a marker of schizophrenia, where it remained from DSM-I through DSM-IV.3 As was believed to be true of schizophrenia, Kraepelin considered catatonia to be characterized by poor prognosis, whereas Bleuler eliminated poor prognosis as a criterion for catatonia.3
In DSM-IV, catatonia was still a subtype of schizophrenia, but for the first time it was expanded diagnostically to become both a specifier in mood disorders, and a syndrome resulting from a general medical condition.5,6 In DSM-5, catatonic schizophrenia was deleted, and catatonia became a specifier for 10 disorders, including schizophrenia, mood disorders, and general medical conditions.3,5-9 In ICD-10, however, catatonia is still associated primarily with schizophrenia.10
A wide range of presentations
Catatonia is a cyclical syndrome characterized by alterations in motor, behavioral, and vocal signs occurring in the context of medical, neurologic, and psychiatric disorders.8 The most common features are immobility, waxy flexibility, stupor, mutism, negativism, echolalia, echopraxia, peculiarities of voluntary movement, and rigidity.7,11 Features of catatonia that have been repeatedly described through the years are summarized in Table 1.8,12,13 In general, presentations of catatonia are not specific to any psychiatric or medical etiology.13,14
Catatonia often is described along a continuum from retarded/stuporous to excited,14,15 and from benign to malignant.13 Examples of these ranges of presentation include5,12,13,15-19:
Stuporous/retarded catatonia (Kahlbaum syndrome) is a primarily negative syndrome in which stupor, mutism, negativism, obsessional slowness, and posturing predominate. Akinetic mutism and coma vigil are sometimes considered to be types of stuporous catatonia, as occasionally are locked-in syndrome and abulia caused by anterior cingulate lesions.
Excited catatonia (hyperkinetic variant, Bell’s mania, oneirophrenia, oneroid state/syndrome, catatonia raptus) is characterized by agitation, combativeness, verbigeration, stereotypies, grimacing, and echo phenomena (echopraxia and echolalia).
Continue to: Malignant (lethal) catatonia
Malignant (lethal) catatonia consists of catatonia accompanied by excitement, stupor, altered level of consciousness, catalepsy, hyperthermia, and autonomic instability with tachycardia, tachypnea, hypertension, and labile blood pressure. Autonomic dysregulation, fever, rhabdomyolysis, and acute renal failure can be causes of morbidity and mortality. Neuroleptic malignant syndrome (NMS)—which is associated with dopamine antagonists, especially antipsychotics—is considered a form of malignant catatonia and has a mortality rate of 10% to 20%. Signs of NMS include muscle rigidity, fever, diaphoresis, rigor, altered consciousness, mutism, tachycardia, hypertension, leukocytosis, and laboratory evidence of muscle damage. Serotonin syndrome can be difficult to distinguish from malignant catatonia, but it is usually not associated with waxy flexibility and rigidity.
Several specific subtypes of catatonia that may exist anywhere along dimensions of activity and severity also have been described:
Periodic catatonia. In 1908, Kraepelin described a form of periodic catatonia, with rapid shifts from excitement to stupor.4 Later, Gjessing described periodic catatonia in schizophrenia and reported success treating it with high doses of thyroid hormone.4 Today, periodic catatonia refers to the rapid onset of recurrent, brief hypokinetic or hyperkinetic episodes lasting 4 to 10 days and recurring during the course of weeks to years. Patients often are asymptomatic between episodes except for grimacing, stereotypies, and negativism later in the course.13,15 At least some forms of periodic catatonia are familial,4 with autosomal dominant transmission possibly linked to chromosome 15q15.13
A familial form of catatonia has been described that has a poor response to standard therapies (benzodiazepines and electroconvulsive therapy [ECT]), but in view of the high comorbidity of catatonia and bipolar disorder, it is difficult to determine whether this is a separate condition, or a group of patients with bipolar disorder.5
Late (ie, late-onset) catatonia is well described in the Japanese literature.10 Reported primarily in women without a known medical illness or brain disorder, late catatonia begins with prodromal hypochondriacal or depressive symptoms during a stressful situation, followed by unprovoked anxiety and agitation. Some patients develop hallucinations, delusions, and recurrent excitement, along with anxiety and agitation. The next stage involves typical catatonic features (mainly excitement, retardation, negativism, and autonomic disturbance), progressing to stupor, mutism, verbal stereotypies, and negativism, including refusal of food. Most patients have residual symptoms following improvement. A few cases have been noted to remit with ECT, with relapse when treatment was discontinued. Late catatonia has been thought to be associated with late-onset schizophrenia or bipolar disorder, or to be an independent entity.
Continue to: Untreated catatonia can have...
Untreated catatonia can have serious medical complications, including deep vein thrombosis, pulmonary embolism, aspiration pneumonia, infection, metabolic disorders, decubitus ulcers, malnutrition, dehydration, contractures, thrombosis, urinary retention, rhabdomyolysis, acute renal failure, sepsis, disseminated intravascular coagulation, and cardiac arrest.11,12,16,20,21 Mortality approaches 10%.12 In children and adolescents, catatonia increases the risk of premature death (including by suicide) 60-fold.22
Not as rare as you might think
With the shift from inpatient to outpatient care driven by deinstitutionalization, longitudinal close observation became less common, and clinicians got the impression that the dramatic catatonia that was common in the hospital had become rare.3 The impression that catatonia was unimportant was strengthened by expanding industry promotion of antipsychotic medications while ignoring catatonia, for which the industry had no specific treatment.3 With recent research, however, catatonia has been reported in 7% to 38% of adult psychiatric patients, including 9% to 25% of inpatients, 20% to 25% of patients with mania,3,5 and 20% of patients with major depressive episodes.7 Catatonia has been noted in .6% to 18% of adolescent psychiatric inpatients (especially in communication and social disorders programs),5,8,22 some children,5 and 6% to 18% of adult and juvenile patients with autism spectrum disorder (ASD).23 In the medical setting, catatonia occurs in 12% to 37% of patients with delirium,8,14,17,18,20,24 7% to 45% of medically ill patients, including those with no psychiatric history,12,13 and 4% of ICU patients.12 Several substances have been linked to catatonia; these are discussed later.11 Contrary to earlier impressions, catatonia is more common in mood disorders, particularly mixed bipolar disorder, especially mania,5 than in schizophrenia.7,8,17,25
Pathophysiology/etiology
Conditions associated with catatonia have different features that act through a final common pathway,7 possibly related to the neurobiology of an extreme fear response called tonic immobility that has been conserved through evolution.8 This mechanism may be mediated by decreased dopamine signaling in basal ganglia, orbitofrontal, and limbic systems, including the hypothalamus and basal forebrain.3,17,20 Subcortical reduction of dopaminergic neurotransmission appears to be related to reduced GABAA receptor signaling and dysfunction of N-methyl-
Up to one-quarter of cases of catatonia are secondary to medical (mostly neurologic) factors or substances.15Table 25,13,15 lists common medical and neurological causes. Medications and substances known to cause catatonia are noted in Table 3.5,8,13,16,26
Catatonia can be a specifier, or a separate condition
DSM-5 criteria for catatonia are summarized in Table 4.28 With these features, catatonia can be a specifier for depressive, bipolar, or psychotic disorders; a complication of a medical disorder; or another separate diagnosis.8 The diagnosis of catatonia in DSM-5 is made when the clinical picture is dominated by ≥3 of the following core features8,15:
- motoric immobility as evidenced by catalepsy (including waxy flexibility) or stupor
- excessive purposeless motor activity that is not influenced by external stimuli
- extreme negativism or mutism
- peculiarities of voluntary movement such as posturing, stereotyped movements, prominent mannerisms, or prominent grimacing
- echolalia or echopraxia.
Continue to: DSM-5 criteria for the diagnosis of catatonia are more...
DSM-5 criteria for the diagnosis of catatonia are more restrictive than DSM-IV criteria. As a result, they exclude a significant number of patients who would be considered catatonic in other systems.29 For example, DSM-5 criteria do not include common features noted in Table 1,8,12,13 such as rigidity and staring.14,29 If the diagnosis is not obvious, it might be suspected in the presence of >1 of posturing, automatic obedience, or waxy flexibility, or >2 of echopraxia/echolalia, gegenhalten, negativism, mitgehen, or stereotypy/vergiberation.12 Clues to catatonia that are not included in formal diagnostic systems and are easily confused with features of psychosis include whispered or robotic speech, uncharacteristic foreign accent, tiptoe walking, hopping, rituals, and odd mannerisms.5
There are several catatonia rating scales containing between 14 and 40 items that are useful in diagnosing and following treatment response in catatonia (Table 58,13,15,29). Of these, the Kanner Scale is primarily applied in neuropsychiatric settings, while the Bush-Francis Catatonia Rating Scale (BFCRS) has had the most widespread use. The BFCRS consists of 23 items, the first 14 of which are used as a screening instrument. It requires 2 of its first 14 items to diagnose catatonia, while DSM-5 requires 3 of 12 signs.29 If the diagnosis remains in doubt, a benzodiazepine agonist test can be instructive.9,12 The presence of catatonia is suggested by significant improvement, ideally assessed prospectively by improvement of BFCRS scores, shortly after administration of a single dose of 1 to 2 mg lorazepam or 5 mg diazepam IV, or 10 mg zolpidem orally. Further evaluation generally consists of a careful medical and psychiatric histories of patient and family, review of all medications, history of substance use with toxicology as indicated, physical examination focusing on autonomic dysregulation, examination for delirium, and laboratory tests as suggested by the history and examination that may include complete blood count, creatine kinase, serum iron, blood urea nitrogen, electrolytes, creatinine, prolactin, anti-NMDA antibodies, thyroid function tests, serology, metabolic panel, human immunodeficiency virus testing, EEG, and neuroimaging.8,15,16
A complex differential diagnosis
Manifestations of numerous psychiatric and neurologic disorders can mimic or be identical to those of catatonia. The differential diagnosis is complicated by the fact that some of these disorders can cause catatonia, which is then masked by the primary disorder; some disorders (eg, NMS) are forms of catatonia. Table 65,8,12,19,26,30 lists conditions to consider.
Some of these conditions warrant discussion. ASD may have catatonia-like features such as echolalia, echopraxia, excitement, combativeness, grimacing, mutism, logorrhea, verbigeration, catalepsy, mannerisms, rigidity, staring and withdrawal.8 Catatonia may also be a stage of deterioration of autism, in which case it is characterized by increases in slowness of movement and speech, reliance on physical or verbal prompting from others, passivity, and lack of motivation.23 At the same time, catatonic features such as mutism, stereotypic speech, repetitive behavior, echolalia, posturing, mannerisms, purposeless agitation, and rigidity in catatonia can be misinterpreted as signs of ASD.8 Catatonia should be suspected as a complication of longstanding ASD in the presence of a consistent, marked change in motor behavior, such as immobility, decreased speech, stupor, excitement, or mixtures or alternations of stupor and excitement.8 Freezing while doing something, difficulty crossing lines, or uncharacteristic persistence of a particular behavior may also herald the presence of catatonia with ASD.8
Catatonia caused by a neurologic or metabolic factor or a substance can be difficult to distinguish from delirium complicated by catatonia. Delirium may be identified in patients with catatonia by the presence of a waxing and waning level of consciousness (vs fluctuating behavior in catatonia) and slowing of the EEG.12,15 Antipsychotic medications can improve delirium but worsen catatonia, while benzodiazepines can improve catatonia but worsen delirium.
Continue to: Among other neurologic syndromes...
Among other neurologic syndromes that can be confused with catatonia, locked-in syndrome consists of total immobility except for vertical extraocular movements and blinking. In this state, patients attempt to communicate with their eyes, while catatonic patients do not try to communicate. There is no response to a lorazepam challenge test. Stiff man syndrome is associated with painful spasms precipitated by touch, noise, or emotional stimuli. Baclofen can resolve stiff man syndrome, but it can induce catatonia. Paratonia refers to generalized increased motor tone that is idiopathic, or associated with neurodegeneration, encephalopathy, or medications. The only motor sign is increased tone, and other signs of catatonia are absent. Catatonia is usually associated with some motor behaviors and interaction with the environment, even if it is negative, while the coma vigil patient is completely unresponsive. Frontotemporal dementia is progressive, while catatonia usually improves without residual dementia.30
Benzodiazepines, ECT are the usual treatments
Experience dictates that the general principles of treatment noted in Table 712,15,23,31 apply to all patients with catatonia. Since the first reported improvement of catatonia with amobarbital in 1930,6 there have been no controlled studies of specific treatments of catatonia.13 Meaningful treatment trials are either naturalistic, or have been performed only for NMS and malignant catatonia.5 However, multiple case reports and case series suggest that treatments with agents that have anticonvulsant properties (benzodiazepines, barbiturates) and ECT are effective.5
Benzodiazepines and related compounds. Case series have suggested a 60% to 80% remission rate of catatonia with benzodiazepines, the most commonly utilized of which has been lorazepam.7,13,32 Treatment begins with a lorazepam challenge test of 1 to 2 mg in adults and 0.5 to 1 mg in children and geriatric patients,9,15 administered orally (including via nasogastric tube), IM, or IV. Following a response (≥50% improvement), the dose is increased to 2 mg 3 times per day. The dose is further increased to 6 to 16 mg/d, and sometimes up to 30 mg/d.9,11 Oral is less effective than sublingual or IM administration.11 Diazepam can be helpful at doses 5 times the lorazepam dose.9,17 A zo
One alternative benzodiazepine protocol utilizes an initial IV dose of 2 mg lorazepam, repeated 3 to 5 times per day; the dose is increased to 10 to 12 mg/d if the first doses are partially effective.16 A lorazepam/diazepam approach involves a combination of IM lorazepam and IV diazepam.11 The protocol begins with 2 mg of IM lorazepam. If there is no effect within 2 hours, a second 2 mg dose is administered, followed by an IV infusion of 10 mg diazepam in 500 ml of normal saline at 1.25 mg/hour until catatonia remits.
An Indian study of 107 patients (mean age 26) receiving relatively low doses of lorazepam (3 to 6 mg/d for at least 3 days) found that factors suggesting a robust response include a shorter duration of catatonia and waxy flexibility, while passivity, mutism, and auditory hallucinations describing the patient in the third person were associated with a poorer acute response.31 Catatonia with marked retardation and mutism complicating schizophrenia, especially with chronic negative symptoms, may be associated with a lower response rate to benzodiazepines.20,33 Maintenance lorazepam has been effective in reducing relapse and recurrence.11 There are no controlled studies of maintenance treatment with benzodiazepines, but clinical reports suggest that doses in the range of 4 to 10 mg/d are effective.32
Continue to: ECT was used for catatonia in 1934...
ECT was first used for catatonia in 1934, when Laszlo Meduna used chemically induced seizures in catatonic patients who had been on tube feeding for months and no longer needed it after treatment.6,7 As was true for other disorders, this approach was replaced by ECT.7 In various case series, the effectiveness of ECT in catatonia has been 53% to 100%.7,13,15 Right unilateral ECT has been reported to be effective with 1 treatment.21 However, the best-established approach is with bitemporal ECT with a suprathreshold stimulus,9 usually with an acute course of 6 to 20 treatments.20 ECT has been reported to be equally safe and effective in adolescents and adults.34 Continued ECT is usually necessary until the patient has returned to baseline.9
ECT usually is recommended within 24 hours for treatment-resistant malignant catatonia or refusal to eat or drink, and within 2 to 3 days if medications are not sufficiently effective in other forms of catatonia.12,15,20 If ECT is initiated after a benzodiazepine trial, the benzodiazepine antagonist flumazenil is administered first to reverse the anticonvulsant effect.9 Some experts recommend using a muscle relaxant other than succinylcholine in the presence of evidence of muscle damage.7
Alternatives to benzodiazepines and ECT. Based on case reports, the treatments described in Table 813,15,17,20,25 have been used for patients with catatonia who do not tolerate or respond to standard treatments. The largest number of case reports have been with NMDA antagonists, while the presumed involvement of reduced dopamine signaling suggests that dopaminergic medications should be helpful. Dantrolene, which blocks release of calcium from intracellular stores and has been used to treat malignant hyperthermia, is sometimes used for NMS, often with disappointing results.
Whereas first-generation antipsychotics definitely increase the risk of catatonia and second-generation antipsychotics (SGAs) probably do so, SGAs are sometimes necessary to treat persistent psychosis in patients with schizophrenia who develop catatonia. Of these medications, clozapine may be most desirable because of low potency for dopamine receptor blockade and modulation of glutamatergic signaling. Partial dopamine agonism by aripiprazole, and the potential for increased subcortical prefrontal dopamine release resulting from serotonin 5HT2A antagonism and 5HT1A agonism by other SGAs, could also be helpful or at least not harmful in catatonia. Lorazepam is usually administered along with these medications to ameliorate treatment-emergent exacerbation of catatonia.
There are no controlled studies of any of these treatments. Based on case reports, most experts would recommend initiating treatment of catatonia with lorazepam, followed by ECT if necessary or in the presence of life-threatening catatonia. If ECT is not available, ineffective, or not tolerated, the first alternatives to be considered would be an NMDA antagonist or an anticonvulsant.20
Continue to: Course varies by patient, underlying cause
Course varies by patient, underlying cause
The response to benzodiazepines or ECT can vary from episode to episode11 and is similar in adults and younger patients.22 Many patients recover completely after a single episode, while relapse after remission occurs repeatedly in periodic catatonia, which involves chronic alternating stupor and excitement waxing and waning over years.11 Relapses may occur frequently, or every few years.11 Some cases of catatonia initially have an episodic course and become chronic and deteriorating, possibly paralleling the original descriptions of the natural history of untreated catatonia, while malignant catatonia can be complicated by medical morbidity or death.4 The long-term prognosis generally depends on the underlying cause of catatonia.5
Bottom Line
Much more common than many clinicians realize, catatonia can be overlooked because symptoms can mimic or overlap with features of an underlying medical or neurologic disorder. Suspect catatonia when one of these illnesses has an unexpected course or an inadequate treatment response. Be alert to characteristic changes in behavior and speech. A benzodiazepine challenge can be used to diagnose and begin treatment of catatonia. Consider electroconvulsive therapy sooner rather than later, especially for severely ill patients.
Related Resources
- Gibson RC, Walcott G. Benzodiazepines for catatonia in people with schizophrenia and other serious mental illnesses. Cochrane Database Syst Rev. 2008;(4):CD006570.
- Newcastle University. Catatonia. https://youtu.be/_s1lzxHRO4U.
Drug Brand Names
Amantadine • Symmetrel
Amobarbital • Amytal
Aripiprazole • Abilify
Azithromycin • Zithromax
Baclofen • Lioresal
Benztropine • Cogentin
Carbamazepine • Carbatrol, Tegretol
Carbidopa/levodopa • Sinemet
Ciprofloxacin • Cipro
Clozapine • Clozaril
Dantrolene • Dantrium
Dexamethasone • Decadron
Dextromethorphan/quinidine • Neudexta
Diazepam • Valium
Disulfiram • Antabuse
Flumazenil • Romazicon
Fluoxetine • Prozac
Fluvoxamine • Luvox
Levetiracetam • Keppra
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Memantine • Namenda
Methylphenidate • Ritalin
Minocycline • Minocin
Olanzapine • Zyprexa
Risperidone • Risperdal
Succinylcholine • Anectine
Topiramate • Topamax
Trihexyphenidyl • Artane
Valproate • Depakote
Ziprasidone • Geodon
Zolpidem • Ambien
1. Kahlbaum KL. Catatonia. Baltimore, MD: John Hopkins University Press; 1973.
2. Kahlbaum KL. Die Katatonie oder das Spannungsirresein. Berlin: Hirschwald; 1874.
3. Tang VM, Duffin J. Catatonia in the history of psychiatry: construction and deconstruction of a disease concept. Perspect Biol Med. 2014;57(4):524-537.
4. Carroll BT. Kahlbaum’s catatonia revisited. Psychiatry Clin Neurosci. 2001;55(5):431-436.
5. Taylor MA, Fink M. Catatonia in psychiatric classification: a home of its own. Am J Psychiatry. 2003;160(7):1233-1241.
6. Fink M, Fricchione GL, Rummans T, et al. Catatonia is a systemic medical syndrome. Acta Psychiatr Scand. 2016;133(3):250-251.
7. Medda P, Toni C, Luchini F, et al. Catatonia in 26 patients with bipolar disorder: clinical features and response to electroconvulsive therapy. Bipolar Disord. 2015;17(8):892-901.
8. Mazzone L, Postorino V, Valeri G, et al. Catatonia in patients with autism: prevalence and management. CNS Drugs. 2014;28(3):205-215.
9. Fink M, Kellner CH, McCall WV. Optimizing ECT technique in treating catatonia. J ECT. 2016;32(3):149-150.
10. Kocha H, Moriguchi S, Mimura M. Revisiting the concept of late catatonia. Compr Psychiatry. 2014;55(7):1485-1490.
11. Lin CC, Hung YL, Tsai MC, et al. Relapses and recurrences of catatonia: 30-case analysis and literature review. Compr Psychiatry. 2016;66:157-165.
12. Saddawi-Konefka D, Berg SM, Nejad SH, et al. Catatonia in the ICU: An important and underdiagnosed cause of altered mental status. A case series and review of the literature. Crit Care Med. 2013;42(3):e234-e241.
13. Wijemanne S, Jankovic J. Movement disorders in catatonia. J Neurol Neurosurg Psychiatry. 2015;86(8):825-832.
14. Grover S, Chakrabarti S, Ghormode D, et al. Catatonia in inpatients with psychiatric disorders: a comparison of schizophrenia and mood disorders. Psychiatry Res. 2015;229(3):919-925.
15. Oldham MA, Lee HB. Catatonia vis-à-vis delirium: the significance of recognizing catatonia in altered mental status. Gen Hosp Psychiatry. 2015;37(6):554-559.
16. Tuerlings JH, van Waarde JA, Verwey B. A retrospective study of 34 catatonic patients: analysis of clinical ‘care and treatment. Gen Hosp Psychiatry. 2010;32(6):631-635.
17. Ohi K, Kuwata A, Shimada T, et al. Response to benzodiazepines and the clinical course in malignant catatonia associated with schizophrenia: a case report. Medicine (Baltimore). 2017;96(16):e6566. doi: 10.1097/MD.0000000000006566.
18. Komatsu T, Nomura T, Takami H, et al. Catatonic symptoms appearing before autonomic symptoms help distinguish neuroleptic malignant syndrome from malignant catatonia. Intern Med. 2016;55(19):2893-2897.
19. Lang FU, Lang S, Becker T, et al. Neuroleptic malignant syndrome or catatonia? Trying to solve the catatonic dilemma. Psychopharmacology (Berl). 2015;232(1):1-5.
20. Beach SR, Gomez-Bernal F, Huffman JC, et al. Alternative treatment strategies for catatonia: a systematic review. Gen Hosp Psychiatry. 2017;48:1-19.
21. Kugler JL, Hauptman AJ, Collier SJ, et al. Treatment of catatonia with ultrabrief right unilateral electroconvulsive therapy: a case series. J ECT. 2015;31(3):192-196.
22. Raffin M, Zugaj-Bensaou L, Bodeau N, et al. Treatment use in a prospective naturalistic cohort of children and adolescents with catatonia. Eur Child Adolesc Psychiatry. 2015;24(4):441-449.
23. DeJong H, Bunton P, Hare DJ. A systematic review of interventions used to treat catatonic symptoms in people with autistic spectrum disorders. J Autism Dev Disord. 2014;44(9):2127-2136.
24. Wachtel L, Commins E, Park MH, et al. Neuroleptic malignant syndrome and delirious mania as malignant catatonia in autism: prompt relief with electroconvulsive therapy. Acta Psychiatr Scand. 2015;132(4):319-320.
25. Fink M, Taylor MA. Catatonia: subtype or syndrome in DSM? Am J Psychiatry. 2006;163(11):1875-1876.
26. Khan M, Pace L, Truong A, et al. Catatonia secondary to synthetic cannabinoid use in two patients with no previous psychosis. Am J Addictions. 2016;25(1):25-27.
27. Komatsu T, Nomura T, Takami H, et al. Catatonic symptoms appearing before autonomic symptoms help distinguish neuroleptic malignant syndrome from malignant catatonia. Intern Med. 2016;55(19):2893-2897.
28. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
29. Wilson JE, Niu K, Nicolson SE, et al. The diagnostic criteria and structure of catatonia. Schizophr Res. 2015;164(1-3):256-262.
30. Ducharme S, Dickerson BC, Larvie M, et al. Differentiating frontotemporal dementia from catatonia: a complex neuropsychiatric challenge. J Neuropsychiatry Clin Neurosci. 2015;27(2):e174-e176.
31. Narayanaswamy JC, Tibrewal P, Zutshi A, et al. Clinical predictors of response to treatment in catatonia. Gen Hosp Psychiatry. 2012;34(3):312-316.
32. Thamizh JS, Harshini M, Selvakumar N, et al. Maintenance lorazepam for treatment of recurrent catatonic states: a case series and implications. Asian J Psychiatr. 2016;22:147-149
33. Ungvari GS, Chiu HF, Chow LY, et al. Lorazepam for chronic catatonia: a randomized, double-blind, placebo-controlled cross-over study. Psychopharmacology (Berl). 1999;142(4):393-398.
34. Flamarique I, Baeza I, de la Serna E, et al. Long-term effectiveness of electroconvulsive therapy in adolescents with schizophrenia spectrum disorders. Eur Child Adolesc Psychiatry. 2015;24(5):517-524.
1. Kahlbaum KL. Catatonia. Baltimore, MD: John Hopkins University Press; 1973.
2. Kahlbaum KL. Die Katatonie oder das Spannungsirresein. Berlin: Hirschwald; 1874.
3. Tang VM, Duffin J. Catatonia in the history of psychiatry: construction and deconstruction of a disease concept. Perspect Biol Med. 2014;57(4):524-537.
4. Carroll BT. Kahlbaum’s catatonia revisited. Psychiatry Clin Neurosci. 2001;55(5):431-436.
5. Taylor MA, Fink M. Catatonia in psychiatric classification: a home of its own. Am J Psychiatry. 2003;160(7):1233-1241.
6. Fink M, Fricchione GL, Rummans T, et al. Catatonia is a systemic medical syndrome. Acta Psychiatr Scand. 2016;133(3):250-251.
7. Medda P, Toni C, Luchini F, et al. Catatonia in 26 patients with bipolar disorder: clinical features and response to electroconvulsive therapy. Bipolar Disord. 2015;17(8):892-901.
8. Mazzone L, Postorino V, Valeri G, et al. Catatonia in patients with autism: prevalence and management. CNS Drugs. 2014;28(3):205-215.
9. Fink M, Kellner CH, McCall WV. Optimizing ECT technique in treating catatonia. J ECT. 2016;32(3):149-150.
10. Kocha H, Moriguchi S, Mimura M. Revisiting the concept of late catatonia. Compr Psychiatry. 2014;55(7):1485-1490.
11. Lin CC, Hung YL, Tsai MC, et al. Relapses and recurrences of catatonia: 30-case analysis and literature review. Compr Psychiatry. 2016;66:157-165.
12. Saddawi-Konefka D, Berg SM, Nejad SH, et al. Catatonia in the ICU: An important and underdiagnosed cause of altered mental status. A case series and review of the literature. Crit Care Med. 2013;42(3):e234-e241.
13. Wijemanne S, Jankovic J. Movement disorders in catatonia. J Neurol Neurosurg Psychiatry. 2015;86(8):825-832.
14. Grover S, Chakrabarti S, Ghormode D, et al. Catatonia in inpatients with psychiatric disorders: a comparison of schizophrenia and mood disorders. Psychiatry Res. 2015;229(3):919-925.
15. Oldham MA, Lee HB. Catatonia vis-à-vis delirium: the significance of recognizing catatonia in altered mental status. Gen Hosp Psychiatry. 2015;37(6):554-559.
16. Tuerlings JH, van Waarde JA, Verwey B. A retrospective study of 34 catatonic patients: analysis of clinical ‘care and treatment. Gen Hosp Psychiatry. 2010;32(6):631-635.
17. Ohi K, Kuwata A, Shimada T, et al. Response to benzodiazepines and the clinical course in malignant catatonia associated with schizophrenia: a case report. Medicine (Baltimore). 2017;96(16):e6566. doi: 10.1097/MD.0000000000006566.
18. Komatsu T, Nomura T, Takami H, et al. Catatonic symptoms appearing before autonomic symptoms help distinguish neuroleptic malignant syndrome from malignant catatonia. Intern Med. 2016;55(19):2893-2897.
19. Lang FU, Lang S, Becker T, et al. Neuroleptic malignant syndrome or catatonia? Trying to solve the catatonic dilemma. Psychopharmacology (Berl). 2015;232(1):1-5.
20. Beach SR, Gomez-Bernal F, Huffman JC, et al. Alternative treatment strategies for catatonia: a systematic review. Gen Hosp Psychiatry. 2017;48:1-19.
21. Kugler JL, Hauptman AJ, Collier SJ, et al. Treatment of catatonia with ultrabrief right unilateral electroconvulsive therapy: a case series. J ECT. 2015;31(3):192-196.
22. Raffin M, Zugaj-Bensaou L, Bodeau N, et al. Treatment use in a prospective naturalistic cohort of children and adolescents with catatonia. Eur Child Adolesc Psychiatry. 2015;24(4):441-449.
23. DeJong H, Bunton P, Hare DJ. A systematic review of interventions used to treat catatonic symptoms in people with autistic spectrum disorders. J Autism Dev Disord. 2014;44(9):2127-2136.
24. Wachtel L, Commins E, Park MH, et al. Neuroleptic malignant syndrome and delirious mania as malignant catatonia in autism: prompt relief with electroconvulsive therapy. Acta Psychiatr Scand. 2015;132(4):319-320.
25. Fink M, Taylor MA. Catatonia: subtype or syndrome in DSM? Am J Psychiatry. 2006;163(11):1875-1876.
26. Khan M, Pace L, Truong A, et al. Catatonia secondary to synthetic cannabinoid use in two patients with no previous psychosis. Am J Addictions. 2016;25(1):25-27.
27. Komatsu T, Nomura T, Takami H, et al. Catatonic symptoms appearing before autonomic symptoms help distinguish neuroleptic malignant syndrome from malignant catatonia. Intern Med. 2016;55(19):2893-2897.
28. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
29. Wilson JE, Niu K, Nicolson SE, et al. The diagnostic criteria and structure of catatonia. Schizophr Res. 2015;164(1-3):256-262.
30. Ducharme S, Dickerson BC, Larvie M, et al. Differentiating frontotemporal dementia from catatonia: a complex neuropsychiatric challenge. J Neuropsychiatry Clin Neurosci. 2015;27(2):e174-e176.
31. Narayanaswamy JC, Tibrewal P, Zutshi A, et al. Clinical predictors of response to treatment in catatonia. Gen Hosp Psychiatry. 2012;34(3):312-316.
32. Thamizh JS, Harshini M, Selvakumar N, et al. Maintenance lorazepam for treatment of recurrent catatonic states: a case series and implications. Asian J Psychiatr. 2016;22:147-149
33. Ungvari GS, Chiu HF, Chow LY, et al. Lorazepam for chronic catatonia: a randomized, double-blind, placebo-controlled cross-over study. Psychopharmacology (Berl). 1999;142(4):393-398.
34. Flamarique I, Baeza I, de la Serna E, et al. Long-term effectiveness of electroconvulsive therapy in adolescents with schizophrenia spectrum disorders. Eur Child Adolesc Psychiatry. 2015;24(5):517-524.
Benzodiazepines for anxious depression
Benzodiazepines’ potential antidepressant properties and their role in the treatment of depression were fairly extensively examined during the 1980s and early 1990s. There were various reasons for this investigation—from the adverse effects of available antidepressants (tricyclic antidepressants [TCAs] and monoamine oxidase inhibitors) to the delay of action of the existing antidepressants and treatment resistance of a significant portion of depressed patients. Benzodiazepines had already been used in the treatment of depressive disorders for decades, but not as monotherapy or main treatment agents, but rather in combination with existing antidepressants to alleviate initial or persistent anxiety, and to help with insomnia. Some authors1 felt that specific benzodiazepines, such as alprazolam, were effective in mild and moderate depression, although not as effective as TCAs for patients with endogenous or melancholic depression. Others2 proposed that benzodiazepines, particularly alprazolam, may be a useful treatment option for patients for whom antidepressants are contraindicated, poorly tolerated, or ineffective. Petty et al2 suggested that the antidepressant efficacy of benzodiazepines was consistent with the then-entertained γ-aminobutyric acid theory of depression.
A shift from benzodiazepines to antidepressants
The evidence for using benzodiazepines in anxious depression was based on results of several studies, but it has not been adequately analyzed, summarized, and promoted. Then, after the arrival of the selective serotonin reuptake inhibitors (SSRIs) (fluoxetine arrived in the United States in 1987, and paroxetine and sertraline arrived in 1992), interest in benzodiazepines gradually waned. Within a few years, the SSRIs were also approved for various anxiety disorders. The SSRIs were heavily promoted not only for the treatment of depressive disorders, but also anxiety disorders, and were touted as well-tolerated medications without abuse potential. Benzodiazepines, on the other hand, were frequently described as less effective and having a substantial abuse potential.
Looking back, these claims were not properly substantiated. Berney et al3 concluded in a systematic review that comparative data of a high level of proof for using newer antidepressants in anxiety disorders rather that benzodiazepines were not available. Then, 5 years later, Offidani et al4 demonstrated in a systematic review and meta-analysis that benzodiazepines were more effective and better tolerated in the treatment of various anxiety disorders than TCAs. In addition, in a few studies comparing benzodiazepines with newer antidepressants such as paroxetine and venlafaxine, benzodiazepines were either comparable or showed greater improvement and fewer adverse effects that these antidepressants. Similarly to Berney et al,3 Offidani et al4 concluded that the change in the prescribing pattern favoring newer antidepressants over benzodiazepines for the treatment of anxiety disorders occurred without supporting evidence.
As far as abuse potential, the American Psychiatric Association Task Force on Benzodiazepine Dependency concluded that benzodiazepines do not strongly reinforce their own use and are not widely abused.5 When abuse occurs, it is almost always in the context of abusing other substances. The Task Force also noted that physiological dependence develops when benzodiazepines are used chronically; dependence being defined mostly in terms of symptoms of discontinuance.5 Thus, benzodiazepines need to be used appropriately, not in extremely high doses, and under medical supervision.
Nevertheless, the judgment, right or wrong, was out—benzodiazepines were deemed problematic and to be avoided. This has become, unfortunately, a pattern of many prescribing psychiatrists’ practice.
What about benzodiazepines for anxious depression?
Recently Benasi et al6 filled the void by investigating data from studies using benzodiazepines as monotherapy in depressive disorders (I was one of the co-authors of this study). They conducted a systematic review of 38 published randomized controlled trials that used benzodiazepines as a monotherapy vs placebo, antidepressants, or both. Patients in these trials were primarily diagnosed with depressive disorder or anxious depression. The majority of these studies used alprazolam as the benzodiazepine (other benzodiazepines used were adinazolam, bromazepam, chlordiazepoxide, and lorazepam) and imipramine or amitriptyline as the antidepressant comparator (other antidepressants used were desipramine, dothiepin, doxepin, and only one newer antidepressant, fluvoxamine, in one study). There was a lack of significant differences in response rate between benzodiazepines and placebo, and between benzodiazepines and TCAs.
In more than half of the studies comparing benzodiazepines with TCAs and/or placebo, benzodiazepines were significantly more effective than placebo and as effective as TCAs. In 11 studies, TCAs were better than benzodiazepines, while benzodiazepines were better than TCAs in one study. In 12 studies, benzodiazepines were associated with a faster onset of action than TCAs. Adverse effects occurred more frequently with TCAs, with the exception of drowsiness and cognitive impairment, which occurred more frequently with benzodiazepines. The findings of the meta-analysis (22 studies) confirmed the low response of anxious depression to psychotropic medications, whether TCAs or benzodiazepines. There was no demonstrated superiority of antidepressants over benzodiazepines for anxious depression. Thus, clearly, benzodiazepines are a bona fide therapeutic option for anxious depression and so far, there is no indication that antidepressants are preferable for this indication.
Continue to: However, it is important to note...
However, it is important to note that there are almost no studies comparing benzodiazepines to newer antidepressants for anxious depression. One double-blind 6-week study of 112 patients7 compared fluvoxamine with lorazepam for mixed anxiety and depression in general practice. There were no significant differences between treatments at any point in the study. Lorazepam produced more sedation, while fluvoxamine produced more nausea and vomiting.
We clearly need randomized controlled trials comparing benzodiazepines with newer antidepressants in anxious depression. However, as in the case with anxiety disorders, these types of trials are strikingly missing.
Any clinical wisdom?
Anxiety could be a serious clinical problem in the treatment of patients with depressive disorder(s). We have not always paid enough attention to anxiety and related issues in depressed patients. Interestingly, anxiety has not been listed among symptoms of major depression disorder (MDD) in several editions of the Diagnostic and Statistical Manual of Mental Disorders (DSM). Only and finally did DSM-58 add a specifier “with anxious distress” for both MDD and persistent depressive disorder (dysthymia), although this specifier still avoids the word “anxiety” in the description of its symptomatology.
It is difficult to disentangle whether the anxiety is part of depressive disorder symptomatology or whether it is a comorbid anxiety disorder. As I noted in a previous article,9 psychiatric comorbidity is a confusing phenomenon. Nevertheless, anxiety and depression are highly comorbid or co-symptomatologic. In a study by Kessler et al,10 45.7% of survey responders with lifetime MDD had ≥1 lifetime anxiety disorder. Similarly, in a STAR*D study,11 in Level 1, 53.2% of patients had anxious depression.
Kessler et al10 raised an interesting question about the importance of temporally primary anxiety disorders as risk markers vs causal risk factors for the onset and persistence of subsequent MDD, including the possibility that anxiety disorders might primarily be risk markers for MDD onset and causal risk factors for MDD persistence. As is well-known, mood disorders should be treated as soon as possible after they are diagnosed, and should be treated vigorously, addressing the major symptomatology.
Continue to: These findings emphasize the need to...
These findings emphasize the need to pay more attention to anxiety in depressed patients (especially those newly diagnosed) and for forceful treatment of anxious depression. Importantly, in the STAR*D study,11 remission in anxious Level 1 (treated with citalopram) depressed patients was significantly less likely and took longer to occur than in patients with nonanxious depression. In addition, ratings of adverse effects frequency, intensity, and burden, as well as the number of serious adverse events, were significantly greater in the anxious depression group. Similarly, in Level 2 (either switched to bupropion, sertraline or venlafaxine, or citalopram augmented with bupropion or buspirone), patients with anxious depression fared significantly worse in both the switching and augmentation options. One wonders if Level 1 patients treated with benzodiazepines, and Level 2 patients switched to benzodiazepines or offered augmentation with them would not have fared better, especially in view of the fact that many old and new antidepressants have significant adverse effects and are difficult to discontinue due to withdrawal symptoms such as dizziness, vertigo, and, in case of newer antidepressants, brain “zaps.” Benzodiazepines certainly have serious withdrawal symptoms, including anxiety, rebound insomnia, and withdrawal seizures, especially when discontinued abruptly and when the dose was high. Thus, as is the case for many other medications (eg, steroids, anticoagulants, and some antidepressants), benzodiazepines must be tapered carefully in order to avoid discontinuance signs and symptoms. Because benzodiazepines have been involved in nearly one-third of overdose-related deaths (either separately or in combination with opioids), and the FDA strongly warns against co-prescribing benzodiazepines and opioids, they need to be prescribed appropriately, carefully weighing their risks and benefits.12
Because the analysis by Benasi et al6 demonstrated that benzodiazepines seem comparably effective as antidepressants in anxious depression, we should be considering using benzodiazepines as monotherapy for this indication more frequently and vigorously, considering their similar efficacy, faster onset of action, and better tolerability, while also considering their risks. Clinicians use them in combinations anyway. We also need rigorous trials comparing benzodiazepines with newer antidepressants for anxious depression.
1. Birkenhäger TK, Moleman P, Nolen WA. Benzodiazepines for depression? A review of the literature. Int Clin Psychopharmacol. 1995;10(3):181-195.
2. Petty F, Trivedi MH, Fulton M, et al. Benzodiazepines as antidepressants: does GABA play a role in depression? Biol Psychiatry. 1995;38(9):578-591.
3. Berney P, Halperin D, Tango R, et al. A major change of prescribing pattern in absence of adequate evidence: benzodiazepines versus newer antidepressants in anxiety disorders. Psychopharmacol Bull. 2008;41(3):39-47.
4. Offidani E, Guidi J, Tomba E, et al. Efficacy and tolerability of benzodiazepines versus antidepressants in anxiety disorders: a systematic review and meta-analysis. Psychother Psychosom. 2013;82(6):355-362.
5. The American Psychiatric Association Task Force on Benzodiazepine Dependence. Benzodiazepine dependence, toxicity, and abuse. Washington, DC: American Psychiatric Association; 1990.
6. Benasi G, Guidi J, Offidani E, et al. Benzodiazepines as a monotherapy in depressive disorders: a systematic review. Psychother Psychosom. 2018;87(2):65-74.
7. Laws D, Ashford JJ, Anstee JA. A multicentre double-blind comparative trial of fluvoxamine versus lorazepam in mixed anxiety and depression treated in general practice. Acta Psychiatr Scand. 1990;81(2):185-189.
8. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
9. Balon R. The confusion of psychiatric comorbidity. Ann Clin Psychiatry. 2016;28(3):153-154.
10. Kessler RC, Sampson NA, Berglund P, et al. Anxious and non-anxious major depressive disorder in the World Health Organization World Mental Health Surveys. Epidemiol Psychiatr Sci. 2015;24(3):210-226.
11. Fava M, Rush AJ, Alpert JE, et al. Difference in treatment outcome in outpatients with anxious versus nonanxious depression: a STAR*D report. Am J Psychiatry. 2008;165(3):342-351.
12. Salzman C, Shader RI. Not again: benzodiazepines once more under attack. J Clin Psychopharmacol. 2015;35(5):493-495.
Benzodiazepines’ potential antidepressant properties and their role in the treatment of depression were fairly extensively examined during the 1980s and early 1990s. There were various reasons for this investigation—from the adverse effects of available antidepressants (tricyclic antidepressants [TCAs] and monoamine oxidase inhibitors) to the delay of action of the existing antidepressants and treatment resistance of a significant portion of depressed patients. Benzodiazepines had already been used in the treatment of depressive disorders for decades, but not as monotherapy or main treatment agents, but rather in combination with existing antidepressants to alleviate initial or persistent anxiety, and to help with insomnia. Some authors1 felt that specific benzodiazepines, such as alprazolam, were effective in mild and moderate depression, although not as effective as TCAs for patients with endogenous or melancholic depression. Others2 proposed that benzodiazepines, particularly alprazolam, may be a useful treatment option for patients for whom antidepressants are contraindicated, poorly tolerated, or ineffective. Petty et al2 suggested that the antidepressant efficacy of benzodiazepines was consistent with the then-entertained γ-aminobutyric acid theory of depression.
A shift from benzodiazepines to antidepressants
The evidence for using benzodiazepines in anxious depression was based on results of several studies, but it has not been adequately analyzed, summarized, and promoted. Then, after the arrival of the selective serotonin reuptake inhibitors (SSRIs) (fluoxetine arrived in the United States in 1987, and paroxetine and sertraline arrived in 1992), interest in benzodiazepines gradually waned. Within a few years, the SSRIs were also approved for various anxiety disorders. The SSRIs were heavily promoted not only for the treatment of depressive disorders, but also anxiety disorders, and were touted as well-tolerated medications without abuse potential. Benzodiazepines, on the other hand, were frequently described as less effective and having a substantial abuse potential.
Looking back, these claims were not properly substantiated. Berney et al3 concluded in a systematic review that comparative data of a high level of proof for using newer antidepressants in anxiety disorders rather that benzodiazepines were not available. Then, 5 years later, Offidani et al4 demonstrated in a systematic review and meta-analysis that benzodiazepines were more effective and better tolerated in the treatment of various anxiety disorders than TCAs. In addition, in a few studies comparing benzodiazepines with newer antidepressants such as paroxetine and venlafaxine, benzodiazepines were either comparable or showed greater improvement and fewer adverse effects that these antidepressants. Similarly to Berney et al,3 Offidani et al4 concluded that the change in the prescribing pattern favoring newer antidepressants over benzodiazepines for the treatment of anxiety disorders occurred without supporting evidence.
As far as abuse potential, the American Psychiatric Association Task Force on Benzodiazepine Dependency concluded that benzodiazepines do not strongly reinforce their own use and are not widely abused.5 When abuse occurs, it is almost always in the context of abusing other substances. The Task Force also noted that physiological dependence develops when benzodiazepines are used chronically; dependence being defined mostly in terms of symptoms of discontinuance.5 Thus, benzodiazepines need to be used appropriately, not in extremely high doses, and under medical supervision.
Nevertheless, the judgment, right or wrong, was out—benzodiazepines were deemed problematic and to be avoided. This has become, unfortunately, a pattern of many prescribing psychiatrists’ practice.
What about benzodiazepines for anxious depression?
Recently Benasi et al6 filled the void by investigating data from studies using benzodiazepines as monotherapy in depressive disorders (I was one of the co-authors of this study). They conducted a systematic review of 38 published randomized controlled trials that used benzodiazepines as a monotherapy vs placebo, antidepressants, or both. Patients in these trials were primarily diagnosed with depressive disorder or anxious depression. The majority of these studies used alprazolam as the benzodiazepine (other benzodiazepines used were adinazolam, bromazepam, chlordiazepoxide, and lorazepam) and imipramine or amitriptyline as the antidepressant comparator (other antidepressants used were desipramine, dothiepin, doxepin, and only one newer antidepressant, fluvoxamine, in one study). There was a lack of significant differences in response rate between benzodiazepines and placebo, and between benzodiazepines and TCAs.
In more than half of the studies comparing benzodiazepines with TCAs and/or placebo, benzodiazepines were significantly more effective than placebo and as effective as TCAs. In 11 studies, TCAs were better than benzodiazepines, while benzodiazepines were better than TCAs in one study. In 12 studies, benzodiazepines were associated with a faster onset of action than TCAs. Adverse effects occurred more frequently with TCAs, with the exception of drowsiness and cognitive impairment, which occurred more frequently with benzodiazepines. The findings of the meta-analysis (22 studies) confirmed the low response of anxious depression to psychotropic medications, whether TCAs or benzodiazepines. There was no demonstrated superiority of antidepressants over benzodiazepines for anxious depression. Thus, clearly, benzodiazepines are a bona fide therapeutic option for anxious depression and so far, there is no indication that antidepressants are preferable for this indication.
Continue to: However, it is important to note...
However, it is important to note that there are almost no studies comparing benzodiazepines to newer antidepressants for anxious depression. One double-blind 6-week study of 112 patients7 compared fluvoxamine with lorazepam for mixed anxiety and depression in general practice. There were no significant differences between treatments at any point in the study. Lorazepam produced more sedation, while fluvoxamine produced more nausea and vomiting.
We clearly need randomized controlled trials comparing benzodiazepines with newer antidepressants in anxious depression. However, as in the case with anxiety disorders, these types of trials are strikingly missing.
Any clinical wisdom?
Anxiety could be a serious clinical problem in the treatment of patients with depressive disorder(s). We have not always paid enough attention to anxiety and related issues in depressed patients. Interestingly, anxiety has not been listed among symptoms of major depression disorder (MDD) in several editions of the Diagnostic and Statistical Manual of Mental Disorders (DSM). Only and finally did DSM-58 add a specifier “with anxious distress” for both MDD and persistent depressive disorder (dysthymia), although this specifier still avoids the word “anxiety” in the description of its symptomatology.
It is difficult to disentangle whether the anxiety is part of depressive disorder symptomatology or whether it is a comorbid anxiety disorder. As I noted in a previous article,9 psychiatric comorbidity is a confusing phenomenon. Nevertheless, anxiety and depression are highly comorbid or co-symptomatologic. In a study by Kessler et al,10 45.7% of survey responders with lifetime MDD had ≥1 lifetime anxiety disorder. Similarly, in a STAR*D study,11 in Level 1, 53.2% of patients had anxious depression.
Kessler et al10 raised an interesting question about the importance of temporally primary anxiety disorders as risk markers vs causal risk factors for the onset and persistence of subsequent MDD, including the possibility that anxiety disorders might primarily be risk markers for MDD onset and causal risk factors for MDD persistence. As is well-known, mood disorders should be treated as soon as possible after they are diagnosed, and should be treated vigorously, addressing the major symptomatology.
Continue to: These findings emphasize the need to...
These findings emphasize the need to pay more attention to anxiety in depressed patients (especially those newly diagnosed) and for forceful treatment of anxious depression. Importantly, in the STAR*D study,11 remission in anxious Level 1 (treated with citalopram) depressed patients was significantly less likely and took longer to occur than in patients with nonanxious depression. In addition, ratings of adverse effects frequency, intensity, and burden, as well as the number of serious adverse events, were significantly greater in the anxious depression group. Similarly, in Level 2 (either switched to bupropion, sertraline or venlafaxine, or citalopram augmented with bupropion or buspirone), patients with anxious depression fared significantly worse in both the switching and augmentation options. One wonders if Level 1 patients treated with benzodiazepines, and Level 2 patients switched to benzodiazepines or offered augmentation with them would not have fared better, especially in view of the fact that many old and new antidepressants have significant adverse effects and are difficult to discontinue due to withdrawal symptoms such as dizziness, vertigo, and, in case of newer antidepressants, brain “zaps.” Benzodiazepines certainly have serious withdrawal symptoms, including anxiety, rebound insomnia, and withdrawal seizures, especially when discontinued abruptly and when the dose was high. Thus, as is the case for many other medications (eg, steroids, anticoagulants, and some antidepressants), benzodiazepines must be tapered carefully in order to avoid discontinuance signs and symptoms. Because benzodiazepines have been involved in nearly one-third of overdose-related deaths (either separately or in combination with opioids), and the FDA strongly warns against co-prescribing benzodiazepines and opioids, they need to be prescribed appropriately, carefully weighing their risks and benefits.12
Because the analysis by Benasi et al6 demonstrated that benzodiazepines seem comparably effective as antidepressants in anxious depression, we should be considering using benzodiazepines as monotherapy for this indication more frequently and vigorously, considering their similar efficacy, faster onset of action, and better tolerability, while also considering their risks. Clinicians use them in combinations anyway. We also need rigorous trials comparing benzodiazepines with newer antidepressants for anxious depression.
Benzodiazepines’ potential antidepressant properties and their role in the treatment of depression were fairly extensively examined during the 1980s and early 1990s. There were various reasons for this investigation—from the adverse effects of available antidepressants (tricyclic antidepressants [TCAs] and monoamine oxidase inhibitors) to the delay of action of the existing antidepressants and treatment resistance of a significant portion of depressed patients. Benzodiazepines had already been used in the treatment of depressive disorders for decades, but not as monotherapy or main treatment agents, but rather in combination with existing antidepressants to alleviate initial or persistent anxiety, and to help with insomnia. Some authors1 felt that specific benzodiazepines, such as alprazolam, were effective in mild and moderate depression, although not as effective as TCAs for patients with endogenous or melancholic depression. Others2 proposed that benzodiazepines, particularly alprazolam, may be a useful treatment option for patients for whom antidepressants are contraindicated, poorly tolerated, or ineffective. Petty et al2 suggested that the antidepressant efficacy of benzodiazepines was consistent with the then-entertained γ-aminobutyric acid theory of depression.
A shift from benzodiazepines to antidepressants
The evidence for using benzodiazepines in anxious depression was based on results of several studies, but it has not been adequately analyzed, summarized, and promoted. Then, after the arrival of the selective serotonin reuptake inhibitors (SSRIs) (fluoxetine arrived in the United States in 1987, and paroxetine and sertraline arrived in 1992), interest in benzodiazepines gradually waned. Within a few years, the SSRIs were also approved for various anxiety disorders. The SSRIs were heavily promoted not only for the treatment of depressive disorders, but also anxiety disorders, and were touted as well-tolerated medications without abuse potential. Benzodiazepines, on the other hand, were frequently described as less effective and having a substantial abuse potential.
Looking back, these claims were not properly substantiated. Berney et al3 concluded in a systematic review that comparative data of a high level of proof for using newer antidepressants in anxiety disorders rather that benzodiazepines were not available. Then, 5 years later, Offidani et al4 demonstrated in a systematic review and meta-analysis that benzodiazepines were more effective and better tolerated in the treatment of various anxiety disorders than TCAs. In addition, in a few studies comparing benzodiazepines with newer antidepressants such as paroxetine and venlafaxine, benzodiazepines were either comparable or showed greater improvement and fewer adverse effects that these antidepressants. Similarly to Berney et al,3 Offidani et al4 concluded that the change in the prescribing pattern favoring newer antidepressants over benzodiazepines for the treatment of anxiety disorders occurred without supporting evidence.
As far as abuse potential, the American Psychiatric Association Task Force on Benzodiazepine Dependency concluded that benzodiazepines do not strongly reinforce their own use and are not widely abused.5 When abuse occurs, it is almost always in the context of abusing other substances. The Task Force also noted that physiological dependence develops when benzodiazepines are used chronically; dependence being defined mostly in terms of symptoms of discontinuance.5 Thus, benzodiazepines need to be used appropriately, not in extremely high doses, and under medical supervision.
Nevertheless, the judgment, right or wrong, was out—benzodiazepines were deemed problematic and to be avoided. This has become, unfortunately, a pattern of many prescribing psychiatrists’ practice.
What about benzodiazepines for anxious depression?
Recently Benasi et al6 filled the void by investigating data from studies using benzodiazepines as monotherapy in depressive disorders (I was one of the co-authors of this study). They conducted a systematic review of 38 published randomized controlled trials that used benzodiazepines as a monotherapy vs placebo, antidepressants, or both. Patients in these trials were primarily diagnosed with depressive disorder or anxious depression. The majority of these studies used alprazolam as the benzodiazepine (other benzodiazepines used were adinazolam, bromazepam, chlordiazepoxide, and lorazepam) and imipramine or amitriptyline as the antidepressant comparator (other antidepressants used were desipramine, dothiepin, doxepin, and only one newer antidepressant, fluvoxamine, in one study). There was a lack of significant differences in response rate between benzodiazepines and placebo, and between benzodiazepines and TCAs.
In more than half of the studies comparing benzodiazepines with TCAs and/or placebo, benzodiazepines were significantly more effective than placebo and as effective as TCAs. In 11 studies, TCAs were better than benzodiazepines, while benzodiazepines were better than TCAs in one study. In 12 studies, benzodiazepines were associated with a faster onset of action than TCAs. Adverse effects occurred more frequently with TCAs, with the exception of drowsiness and cognitive impairment, which occurred more frequently with benzodiazepines. The findings of the meta-analysis (22 studies) confirmed the low response of anxious depression to psychotropic medications, whether TCAs or benzodiazepines. There was no demonstrated superiority of antidepressants over benzodiazepines for anxious depression. Thus, clearly, benzodiazepines are a bona fide therapeutic option for anxious depression and so far, there is no indication that antidepressants are preferable for this indication.
Continue to: However, it is important to note...
However, it is important to note that there are almost no studies comparing benzodiazepines to newer antidepressants for anxious depression. One double-blind 6-week study of 112 patients7 compared fluvoxamine with lorazepam for mixed anxiety and depression in general practice. There were no significant differences between treatments at any point in the study. Lorazepam produced more sedation, while fluvoxamine produced more nausea and vomiting.
We clearly need randomized controlled trials comparing benzodiazepines with newer antidepressants in anxious depression. However, as in the case with anxiety disorders, these types of trials are strikingly missing.
Any clinical wisdom?
Anxiety could be a serious clinical problem in the treatment of patients with depressive disorder(s). We have not always paid enough attention to anxiety and related issues in depressed patients. Interestingly, anxiety has not been listed among symptoms of major depression disorder (MDD) in several editions of the Diagnostic and Statistical Manual of Mental Disorders (DSM). Only and finally did DSM-58 add a specifier “with anxious distress” for both MDD and persistent depressive disorder (dysthymia), although this specifier still avoids the word “anxiety” in the description of its symptomatology.
It is difficult to disentangle whether the anxiety is part of depressive disorder symptomatology or whether it is a comorbid anxiety disorder. As I noted in a previous article,9 psychiatric comorbidity is a confusing phenomenon. Nevertheless, anxiety and depression are highly comorbid or co-symptomatologic. In a study by Kessler et al,10 45.7% of survey responders with lifetime MDD had ≥1 lifetime anxiety disorder. Similarly, in a STAR*D study,11 in Level 1, 53.2% of patients had anxious depression.
Kessler et al10 raised an interesting question about the importance of temporally primary anxiety disorders as risk markers vs causal risk factors for the onset and persistence of subsequent MDD, including the possibility that anxiety disorders might primarily be risk markers for MDD onset and causal risk factors for MDD persistence. As is well-known, mood disorders should be treated as soon as possible after they are diagnosed, and should be treated vigorously, addressing the major symptomatology.
Continue to: These findings emphasize the need to...
These findings emphasize the need to pay more attention to anxiety in depressed patients (especially those newly diagnosed) and for forceful treatment of anxious depression. Importantly, in the STAR*D study,11 remission in anxious Level 1 (treated with citalopram) depressed patients was significantly less likely and took longer to occur than in patients with nonanxious depression. In addition, ratings of adverse effects frequency, intensity, and burden, as well as the number of serious adverse events, were significantly greater in the anxious depression group. Similarly, in Level 2 (either switched to bupropion, sertraline or venlafaxine, or citalopram augmented with bupropion or buspirone), patients with anxious depression fared significantly worse in both the switching and augmentation options. One wonders if Level 1 patients treated with benzodiazepines, and Level 2 patients switched to benzodiazepines or offered augmentation with them would not have fared better, especially in view of the fact that many old and new antidepressants have significant adverse effects and are difficult to discontinue due to withdrawal symptoms such as dizziness, vertigo, and, in case of newer antidepressants, brain “zaps.” Benzodiazepines certainly have serious withdrawal symptoms, including anxiety, rebound insomnia, and withdrawal seizures, especially when discontinued abruptly and when the dose was high. Thus, as is the case for many other medications (eg, steroids, anticoagulants, and some antidepressants), benzodiazepines must be tapered carefully in order to avoid discontinuance signs and symptoms. Because benzodiazepines have been involved in nearly one-third of overdose-related deaths (either separately or in combination with opioids), and the FDA strongly warns against co-prescribing benzodiazepines and opioids, they need to be prescribed appropriately, carefully weighing their risks and benefits.12
Because the analysis by Benasi et al6 demonstrated that benzodiazepines seem comparably effective as antidepressants in anxious depression, we should be considering using benzodiazepines as monotherapy for this indication more frequently and vigorously, considering their similar efficacy, faster onset of action, and better tolerability, while also considering their risks. Clinicians use them in combinations anyway. We also need rigorous trials comparing benzodiazepines with newer antidepressants for anxious depression.
1. Birkenhäger TK, Moleman P, Nolen WA. Benzodiazepines for depression? A review of the literature. Int Clin Psychopharmacol. 1995;10(3):181-195.
2. Petty F, Trivedi MH, Fulton M, et al. Benzodiazepines as antidepressants: does GABA play a role in depression? Biol Psychiatry. 1995;38(9):578-591.
3. Berney P, Halperin D, Tango R, et al. A major change of prescribing pattern in absence of adequate evidence: benzodiazepines versus newer antidepressants in anxiety disorders. Psychopharmacol Bull. 2008;41(3):39-47.
4. Offidani E, Guidi J, Tomba E, et al. Efficacy and tolerability of benzodiazepines versus antidepressants in anxiety disorders: a systematic review and meta-analysis. Psychother Psychosom. 2013;82(6):355-362.
5. The American Psychiatric Association Task Force on Benzodiazepine Dependence. Benzodiazepine dependence, toxicity, and abuse. Washington, DC: American Psychiatric Association; 1990.
6. Benasi G, Guidi J, Offidani E, et al. Benzodiazepines as a monotherapy in depressive disorders: a systematic review. Psychother Psychosom. 2018;87(2):65-74.
7. Laws D, Ashford JJ, Anstee JA. A multicentre double-blind comparative trial of fluvoxamine versus lorazepam in mixed anxiety and depression treated in general practice. Acta Psychiatr Scand. 1990;81(2):185-189.
8. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
9. Balon R. The confusion of psychiatric comorbidity. Ann Clin Psychiatry. 2016;28(3):153-154.
10. Kessler RC, Sampson NA, Berglund P, et al. Anxious and non-anxious major depressive disorder in the World Health Organization World Mental Health Surveys. Epidemiol Psychiatr Sci. 2015;24(3):210-226.
11. Fava M, Rush AJ, Alpert JE, et al. Difference in treatment outcome in outpatients with anxious versus nonanxious depression: a STAR*D report. Am J Psychiatry. 2008;165(3):342-351.
12. Salzman C, Shader RI. Not again: benzodiazepines once more under attack. J Clin Psychopharmacol. 2015;35(5):493-495.
1. Birkenhäger TK, Moleman P, Nolen WA. Benzodiazepines for depression? A review of the literature. Int Clin Psychopharmacol. 1995;10(3):181-195.
2. Petty F, Trivedi MH, Fulton M, et al. Benzodiazepines as antidepressants: does GABA play a role in depression? Biol Psychiatry. 1995;38(9):578-591.
3. Berney P, Halperin D, Tango R, et al. A major change of prescribing pattern in absence of adequate evidence: benzodiazepines versus newer antidepressants in anxiety disorders. Psychopharmacol Bull. 2008;41(3):39-47.
4. Offidani E, Guidi J, Tomba E, et al. Efficacy and tolerability of benzodiazepines versus antidepressants in anxiety disorders: a systematic review and meta-analysis. Psychother Psychosom. 2013;82(6):355-362.
5. The American Psychiatric Association Task Force on Benzodiazepine Dependence. Benzodiazepine dependence, toxicity, and abuse. Washington, DC: American Psychiatric Association; 1990.
6. Benasi G, Guidi J, Offidani E, et al. Benzodiazepines as a monotherapy in depressive disorders: a systematic review. Psychother Psychosom. 2018;87(2):65-74.
7. Laws D, Ashford JJ, Anstee JA. A multicentre double-blind comparative trial of fluvoxamine versus lorazepam in mixed anxiety and depression treated in general practice. Acta Psychiatr Scand. 1990;81(2):185-189.
8. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
9. Balon R. The confusion of psychiatric comorbidity. Ann Clin Psychiatry. 2016;28(3):153-154.
10. Kessler RC, Sampson NA, Berglund P, et al. Anxious and non-anxious major depressive disorder in the World Health Organization World Mental Health Surveys. Epidemiol Psychiatr Sci. 2015;24(3):210-226.
11. Fava M, Rush AJ, Alpert JE, et al. Difference in treatment outcome in outpatients with anxious versus nonanxious depression: a STAR*D report. Am J Psychiatry. 2008;165(3):342-351.
12. Salzman C, Shader RI. Not again: benzodiazepines once more under attack. J Clin Psychopharmacol. 2015;35(5):493-495.