User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Preventing brain damage in psychosis
I read with great interest Dr. Nasrallah’s editorial, “FAST and RAPID: Acronyms to prevent brain damage in stroke and psychosis” (From the Editor,
Mitchell L. Glaser, MD
Board-Certified Child/Adolescent and General Psychiatrist
Assistant Professor of Psychiatry
Rush University Medical Center
Chairman
Department of Psychiatry
Medical Director of Child/Adolescent Psychiatry
St. Mary/Elizabeth Medical Center
Clinical Assistant Professor of Psychiatry
Rosalind Franklin University
Chicago, Illinois
Thank you, Dr. Nasrallah, for your incisive thinking and for bringing our attention as psychiatrists to the crucial issues of our clinical practice. I’d like to offer some nuance on the RAPID acronym. First, I’d like to counterpropose DASH: Delusions, Auditory hallucinations, Strange behavior, Hospital now. This is more in line with getting physicians to tune in to the symptoms that should alarm them and bring them to action. I agree that neurodegeneration and illness recurrence are the problems to address. One unsettled issue remains: With early intervention, can we eventually taper patients off antipsychotics to spare them the metabolic and immune morbidity associated with these medications? There is some evidence that this is possible, but it is difficult to collect data. One of the factors delaying treatment, other than lack of recognition, is the general public’s belief that the treatment is sometimes worse than the disease. If we can address this issue in a nuanced fashion, we may get more “early adopters” of these neuron-sparing treatments.
Michael S. Diamond, MD
Private psychiatric practice
Chevy Chase, Maryland
Dr. Nasrallah is right to focus on brain injury patterns, including inflammation and de-myelination, during psychotic episodes. He and Dr. Roque note that starting a patient on a long-acting injectable antipsychotic as soon as possible may prevent subsequent relapse and further brain damage. However, their editorial omits 2 treatments—minocycline and clemastine—that can help stop CNS inflammation, reduce brain damage, and promote remyelination.
Minocycline has been shown to reduce stroke infarct penumbra size and improve outcomes in functional recovery from stroke.1,2 Minocycline’s effects as a potent CNS anti-inflammatory and antiapoptotic agent are well established.
Clemastine has been shown to improve function in multiple sclerosis by activating oligodendrocyte precursor cells into active agents of myelination and fiber bundle stabilization.3 Clemastine reverses acute leukoencephalopathy.4
If we are to treat acute psychosis as a neurologic emergency, we cannot rely on long-acting injectable antipsychotics as the sole treatment. Psychiatric medication alone is not sufficient across every neuropsychiatric condition in which inflammation and white matter damage are part of the etiology, destruction, and pattern of relapse.
The adverse effects risk of adjunctive minocycline and clemastine is low compared with the potential benefits of stopping inflammation, reducing apoptosis, and jump-starting white matter repair. Doses of oral minocycline in the 50- to 100-mg/d range and oral clemastine in the 1.34- to 2.68-mg/d range together can lead to reduced cranial heat, improved cranial suture mobility, and improved elasticity of white matter bundle tracts palpable on physical examination. Both medications show clinical results in improved emotional self-regulation, according to family reports and clinical observations in the outpatient setting. There is no reason to delay neurologic-based adjunctive treatment when our goal is to prevent and reverse brain damage.
Daniel Kerlinsky, MD
Child Psychiatrist
Clinical Assistant Professor
Burrell College of Osteopathic Medicine
Albuquerque, New Mexico
References
1. Hess DH, Fagan SC. Repurposing an old drug to improve the use and safety of tissue plasminogen activator for acute ischemic stroke: minocycline. Rev Neurol Dis. 2010;30(7 pt 2):55S-61S.
2. Vedantam S, Moller AR. Minocycline: a novel stroke therapy. J Neurol Stroke.
3. Green AJ, Gelfand JM, Cree BA, et al. Clemastine fumarate as a remyelinating therapy for multiple sclerosis (ReBUILD): a randomised, controlled, double-blind, crossover trial. Lancet. 2017;390(10111):2481-2489.
4. Cree BAC, Niu J, Hoi KK, et al. Clemastine rescues myelination defects and promotes functional recovery in hypoxic brain injury. Brain. 2018;141(1):85-98.
Continue to: Dr. Nasrallah responds
Dr. Nasrallah responds
Thanks to my colleagues, Drs. Diamond, Glaser, and Kerlinsky, for their cogent letters about my editorial.
To Dr. Glaser: The “ethics” of conducting placebo-controlled studies when developing a new antipsychotic has been raging for some time. For decades, the FDA has insisted on using a placebo group because around 25% to 30% of research participants respond to placebo, and because participants receiving placebo also complain of many adverse effects. So a new drug has to demonstrate a statistically higher efficacy than a placebo, and the adverse effect profile of the placebo group will put the safety and tolerability profile of a new drug in proper perspective. However, in Europe, they do not conduct placebo-controlled studies; instead, they conduct what is called a “non-inferiority” trial of a new antipsychotic compared with a well-established antipsychotic.
Interestingly, even though the discovery of the neurodegenerative effects of untreated psychosis was only 20 years ago (in 1997 after serial MRI scans revealed progressive atrophy), in the 1960s, the first antipsychotic, chlorpromazine, was compared with placebo in a large national study for 6 months. This study showed without a doubt that chlorpromazine has a higher efficacy than placebo. After the study was done, Dr. Philip May at University of California, Los Angeles looked at what happened to the psychotic patients who received placebo for 6 months and found that they became less responsive to treatment, were re-hospitalized more often, and had more negative symptoms and a poorer overall outcome. That was a clue that untreated psychosis can be harmful, and it supports your point about the ethics of using placebo. In contemporary studies, a trial of oral antipsychotics is 6 weeks, not 6 months. In the year-long, placebo-controlled studies of injectable antipsychotics in stable patients, those who show the slightest increase in delusions, hallucinations, or suicidal/homicidal behavior were promptly taken out of the study and treated. This reduced the “harm,” although not completely. Perhaps the FDA will change its policies and adopt the non-inferiority model. That’s what is done with nonpsychiatric disorders such as pneumonia, stroke, or diabetes. But one last fact has to be stated: The placebo response in anxiety, depression, or psychosis is much higher (25% to 35%) than the 1% placebo response in pneumonia.
To Dr. Diamond: I really like DASH, and it is an acronym for quick symptomatic diagnosis. Speedy treatment then follows with the acronym RAPID to prevent brain damage that gets worse with delay.
As for the second issue of tapering off the antipsychotic medication, the evidence is overwhelming in favor of continuous pharmacotherapy. Just as hypertension and diabetes will return if medications are tapered or stopped, so will psychosis, and vengefully so because treatment resistance increases with each relapse.1 This is also true for bipolar disorder recurrences.2 A recent 20-year follow-up study showed that stopping antipsychotic treatment is associated with a much higher mortality rate than continuation therapy.3 Another 7-year study showed the same thing.4 It is literally deadly, and not just neurodegenerative, for persons with schizophrenia to stop their medications.
To Dr. Kerlinsky: I agree with you about using certain adjunctive pharmacotherapies for acute psychosis, which is associated with neuroinflammation, oxidative stress, and neuropil and myelin damage. I support using agents with anti-inflammatory effects (such as minocycline and omega-3 fatty acid), antioxidant effects (such as N-acetylcysteine), and neuroprotective effects (such as minocycline, clemastine, lithium, vitamin D, erythropoietin, etc.). I refer you to my past editorial, “Are you neuroprotecting your patients? 10 Adjunctive therapies to consider,”5 in which I mentioned all the above. I also pointed out the many neuroprotective effects of atypical antipsychotics in another editorial.6 Although off-label, those supplements can be useful interventions that can ameliorate the gray and white matter damage associated with acute psychotic relapses in patients with schizophrenia.
Henry A. Nasrallah, MD
Editor-in-Chief
The Sydney W. Souers Endowed Chair
Professor and Chairman
Department of Psychiatry and Behavioral Neuroscience
Saint Louis University School of Medicine
St. Louis, Missouri
References
1. Emsley R, Oosthuizen P, Koen L, et al. Comparison of treatment response in second-episode versus first-episode schizophrenia. J Clin Psychopharmacol. 2013;33(1):80-83.
2. Post RM. Preventing the maligna
3. Tiihonen J, Tanskanen A, Taipale H. 20-year nationwide follow-up study on discontinuation of antipsychotic treatment in first-episode schizophrenia. Am J Psychiatry. 2018;175(8):765-773.
4. Taipale H, Mittendorfer-Rutz E, Alexanderson K, et al. Antipsychotics and mortality in a nationwide cohort of 29,823 patients with schizophrenia. Schizophr Res. 2018;197:274-280.
5. Nasrallah HA. Are you neuroprotecting your patients? 10 Adjunctive therapies to consider. Current Psychiatry. 2016;15(12):12-14.
6. Nasrallah HA. A decade after the CATIE study, the focus has shifted from effectiveness to neuroprotection. Current Psychiatry. 2015;14(2):19-21.
I read with great interest Dr. Nasrallah’s editorial, “FAST and RAPID: Acronyms to prevent brain damage in stroke and psychosis” (From the Editor,
Mitchell L. Glaser, MD
Board-Certified Child/Adolescent and General Psychiatrist
Assistant Professor of Psychiatry
Rush University Medical Center
Chairman
Department of Psychiatry
Medical Director of Child/Adolescent Psychiatry
St. Mary/Elizabeth Medical Center
Clinical Assistant Professor of Psychiatry
Rosalind Franklin University
Chicago, Illinois
Thank you, Dr. Nasrallah, for your incisive thinking and for bringing our attention as psychiatrists to the crucial issues of our clinical practice. I’d like to offer some nuance on the RAPID acronym. First, I’d like to counterpropose DASH: Delusions, Auditory hallucinations, Strange behavior, Hospital now. This is more in line with getting physicians to tune in to the symptoms that should alarm them and bring them to action. I agree that neurodegeneration and illness recurrence are the problems to address. One unsettled issue remains: With early intervention, can we eventually taper patients off antipsychotics to spare them the metabolic and immune morbidity associated with these medications? There is some evidence that this is possible, but it is difficult to collect data. One of the factors delaying treatment, other than lack of recognition, is the general public’s belief that the treatment is sometimes worse than the disease. If we can address this issue in a nuanced fashion, we may get more “early adopters” of these neuron-sparing treatments.
Michael S. Diamond, MD
Private psychiatric practice
Chevy Chase, Maryland
Dr. Nasrallah is right to focus on brain injury patterns, including inflammation and de-myelination, during psychotic episodes. He and Dr. Roque note that starting a patient on a long-acting injectable antipsychotic as soon as possible may prevent subsequent relapse and further brain damage. However, their editorial omits 2 treatments—minocycline and clemastine—that can help stop CNS inflammation, reduce brain damage, and promote remyelination.
Minocycline has been shown to reduce stroke infarct penumbra size and improve outcomes in functional recovery from stroke.1,2 Minocycline’s effects as a potent CNS anti-inflammatory and antiapoptotic agent are well established.
Clemastine has been shown to improve function in multiple sclerosis by activating oligodendrocyte precursor cells into active agents of myelination and fiber bundle stabilization.3 Clemastine reverses acute leukoencephalopathy.4
If we are to treat acute psychosis as a neurologic emergency, we cannot rely on long-acting injectable antipsychotics as the sole treatment. Psychiatric medication alone is not sufficient across every neuropsychiatric condition in which inflammation and white matter damage are part of the etiology, destruction, and pattern of relapse.
The adverse effects risk of adjunctive minocycline and clemastine is low compared with the potential benefits of stopping inflammation, reducing apoptosis, and jump-starting white matter repair. Doses of oral minocycline in the 50- to 100-mg/d range and oral clemastine in the 1.34- to 2.68-mg/d range together can lead to reduced cranial heat, improved cranial suture mobility, and improved elasticity of white matter bundle tracts palpable on physical examination. Both medications show clinical results in improved emotional self-regulation, according to family reports and clinical observations in the outpatient setting. There is no reason to delay neurologic-based adjunctive treatment when our goal is to prevent and reverse brain damage.
Daniel Kerlinsky, MD
Child Psychiatrist
Clinical Assistant Professor
Burrell College of Osteopathic Medicine
Albuquerque, New Mexico
References
1. Hess DH, Fagan SC. Repurposing an old drug to improve the use and safety of tissue plasminogen activator for acute ischemic stroke: minocycline. Rev Neurol Dis. 2010;30(7 pt 2):55S-61S.
2. Vedantam S, Moller AR. Minocycline: a novel stroke therapy. J Neurol Stroke.
3. Green AJ, Gelfand JM, Cree BA, et al. Clemastine fumarate as a remyelinating therapy for multiple sclerosis (ReBUILD): a randomised, controlled, double-blind, crossover trial. Lancet. 2017;390(10111):2481-2489.
4. Cree BAC, Niu J, Hoi KK, et al. Clemastine rescues myelination defects and promotes functional recovery in hypoxic brain injury. Brain. 2018;141(1):85-98.
Continue to: Dr. Nasrallah responds
Dr. Nasrallah responds
Thanks to my colleagues, Drs. Diamond, Glaser, and Kerlinsky, for their cogent letters about my editorial.
To Dr. Glaser: The “ethics” of conducting placebo-controlled studies when developing a new antipsychotic has been raging for some time. For decades, the FDA has insisted on using a placebo group because around 25% to 30% of research participants respond to placebo, and because participants receiving placebo also complain of many adverse effects. So a new drug has to demonstrate a statistically higher efficacy than a placebo, and the adverse effect profile of the placebo group will put the safety and tolerability profile of a new drug in proper perspective. However, in Europe, they do not conduct placebo-controlled studies; instead, they conduct what is called a “non-inferiority” trial of a new antipsychotic compared with a well-established antipsychotic.
Interestingly, even though the discovery of the neurodegenerative effects of untreated psychosis was only 20 years ago (in 1997 after serial MRI scans revealed progressive atrophy), in the 1960s, the first antipsychotic, chlorpromazine, was compared with placebo in a large national study for 6 months. This study showed without a doubt that chlorpromazine has a higher efficacy than placebo. After the study was done, Dr. Philip May at University of California, Los Angeles looked at what happened to the psychotic patients who received placebo for 6 months and found that they became less responsive to treatment, were re-hospitalized more often, and had more negative symptoms and a poorer overall outcome. That was a clue that untreated psychosis can be harmful, and it supports your point about the ethics of using placebo. In contemporary studies, a trial of oral antipsychotics is 6 weeks, not 6 months. In the year-long, placebo-controlled studies of injectable antipsychotics in stable patients, those who show the slightest increase in delusions, hallucinations, or suicidal/homicidal behavior were promptly taken out of the study and treated. This reduced the “harm,” although not completely. Perhaps the FDA will change its policies and adopt the non-inferiority model. That’s what is done with nonpsychiatric disorders such as pneumonia, stroke, or diabetes. But one last fact has to be stated: The placebo response in anxiety, depression, or psychosis is much higher (25% to 35%) than the 1% placebo response in pneumonia.
To Dr. Diamond: I really like DASH, and it is an acronym for quick symptomatic diagnosis. Speedy treatment then follows with the acronym RAPID to prevent brain damage that gets worse with delay.
As for the second issue of tapering off the antipsychotic medication, the evidence is overwhelming in favor of continuous pharmacotherapy. Just as hypertension and diabetes will return if medications are tapered or stopped, so will psychosis, and vengefully so because treatment resistance increases with each relapse.1 This is also true for bipolar disorder recurrences.2 A recent 20-year follow-up study showed that stopping antipsychotic treatment is associated with a much higher mortality rate than continuation therapy.3 Another 7-year study showed the same thing.4 It is literally deadly, and not just neurodegenerative, for persons with schizophrenia to stop their medications.
To Dr. Kerlinsky: I agree with you about using certain adjunctive pharmacotherapies for acute psychosis, which is associated with neuroinflammation, oxidative stress, and neuropil and myelin damage. I support using agents with anti-inflammatory effects (such as minocycline and omega-3 fatty acid), antioxidant effects (such as N-acetylcysteine), and neuroprotective effects (such as minocycline, clemastine, lithium, vitamin D, erythropoietin, etc.). I refer you to my past editorial, “Are you neuroprotecting your patients? 10 Adjunctive therapies to consider,”5 in which I mentioned all the above. I also pointed out the many neuroprotective effects of atypical antipsychotics in another editorial.6 Although off-label, those supplements can be useful interventions that can ameliorate the gray and white matter damage associated with acute psychotic relapses in patients with schizophrenia.
Henry A. Nasrallah, MD
Editor-in-Chief
The Sydney W. Souers Endowed Chair
Professor and Chairman
Department of Psychiatry and Behavioral Neuroscience
Saint Louis University School of Medicine
St. Louis, Missouri
References
1. Emsley R, Oosthuizen P, Koen L, et al. Comparison of treatment response in second-episode versus first-episode schizophrenia. J Clin Psychopharmacol. 2013;33(1):80-83.
2. Post RM. Preventing the maligna
3. Tiihonen J, Tanskanen A, Taipale H. 20-year nationwide follow-up study on discontinuation of antipsychotic treatment in first-episode schizophrenia. Am J Psychiatry. 2018;175(8):765-773.
4. Taipale H, Mittendorfer-Rutz E, Alexanderson K, et al. Antipsychotics and mortality in a nationwide cohort of 29,823 patients with schizophrenia. Schizophr Res. 2018;197:274-280.
5. Nasrallah HA. Are you neuroprotecting your patients? 10 Adjunctive therapies to consider. Current Psychiatry. 2016;15(12):12-14.
6. Nasrallah HA. A decade after the CATIE study, the focus has shifted from effectiveness to neuroprotection. Current Psychiatry. 2015;14(2):19-21.
I read with great interest Dr. Nasrallah’s editorial, “FAST and RAPID: Acronyms to prevent brain damage in stroke and psychosis” (From the Editor,
Mitchell L. Glaser, MD
Board-Certified Child/Adolescent and General Psychiatrist
Assistant Professor of Psychiatry
Rush University Medical Center
Chairman
Department of Psychiatry
Medical Director of Child/Adolescent Psychiatry
St. Mary/Elizabeth Medical Center
Clinical Assistant Professor of Psychiatry
Rosalind Franklin University
Chicago, Illinois
Thank you, Dr. Nasrallah, for your incisive thinking and for bringing our attention as psychiatrists to the crucial issues of our clinical practice. I’d like to offer some nuance on the RAPID acronym. First, I’d like to counterpropose DASH: Delusions, Auditory hallucinations, Strange behavior, Hospital now. This is more in line with getting physicians to tune in to the symptoms that should alarm them and bring them to action. I agree that neurodegeneration and illness recurrence are the problems to address. One unsettled issue remains: With early intervention, can we eventually taper patients off antipsychotics to spare them the metabolic and immune morbidity associated with these medications? There is some evidence that this is possible, but it is difficult to collect data. One of the factors delaying treatment, other than lack of recognition, is the general public’s belief that the treatment is sometimes worse than the disease. If we can address this issue in a nuanced fashion, we may get more “early adopters” of these neuron-sparing treatments.
Michael S. Diamond, MD
Private psychiatric practice
Chevy Chase, Maryland
Dr. Nasrallah is right to focus on brain injury patterns, including inflammation and de-myelination, during psychotic episodes. He and Dr. Roque note that starting a patient on a long-acting injectable antipsychotic as soon as possible may prevent subsequent relapse and further brain damage. However, their editorial omits 2 treatments—minocycline and clemastine—that can help stop CNS inflammation, reduce brain damage, and promote remyelination.
Minocycline has been shown to reduce stroke infarct penumbra size and improve outcomes in functional recovery from stroke.1,2 Minocycline’s effects as a potent CNS anti-inflammatory and antiapoptotic agent are well established.
Clemastine has been shown to improve function in multiple sclerosis by activating oligodendrocyte precursor cells into active agents of myelination and fiber bundle stabilization.3 Clemastine reverses acute leukoencephalopathy.4
If we are to treat acute psychosis as a neurologic emergency, we cannot rely on long-acting injectable antipsychotics as the sole treatment. Psychiatric medication alone is not sufficient across every neuropsychiatric condition in which inflammation and white matter damage are part of the etiology, destruction, and pattern of relapse.
The adverse effects risk of adjunctive minocycline and clemastine is low compared with the potential benefits of stopping inflammation, reducing apoptosis, and jump-starting white matter repair. Doses of oral minocycline in the 50- to 100-mg/d range and oral clemastine in the 1.34- to 2.68-mg/d range together can lead to reduced cranial heat, improved cranial suture mobility, and improved elasticity of white matter bundle tracts palpable on physical examination. Both medications show clinical results in improved emotional self-regulation, according to family reports and clinical observations in the outpatient setting. There is no reason to delay neurologic-based adjunctive treatment when our goal is to prevent and reverse brain damage.
Daniel Kerlinsky, MD
Child Psychiatrist
Clinical Assistant Professor
Burrell College of Osteopathic Medicine
Albuquerque, New Mexico
References
1. Hess DH, Fagan SC. Repurposing an old drug to improve the use and safety of tissue plasminogen activator for acute ischemic stroke: minocycline. Rev Neurol Dis. 2010;30(7 pt 2):55S-61S.
2. Vedantam S, Moller AR. Minocycline: a novel stroke therapy. J Neurol Stroke.
3. Green AJ, Gelfand JM, Cree BA, et al. Clemastine fumarate as a remyelinating therapy for multiple sclerosis (ReBUILD): a randomised, controlled, double-blind, crossover trial. Lancet. 2017;390(10111):2481-2489.
4. Cree BAC, Niu J, Hoi KK, et al. Clemastine rescues myelination defects and promotes functional recovery in hypoxic brain injury. Brain. 2018;141(1):85-98.
Continue to: Dr. Nasrallah responds
Dr. Nasrallah responds
Thanks to my colleagues, Drs. Diamond, Glaser, and Kerlinsky, for their cogent letters about my editorial.
To Dr. Glaser: The “ethics” of conducting placebo-controlled studies when developing a new antipsychotic has been raging for some time. For decades, the FDA has insisted on using a placebo group because around 25% to 30% of research participants respond to placebo, and because participants receiving placebo also complain of many adverse effects. So a new drug has to demonstrate a statistically higher efficacy than a placebo, and the adverse effect profile of the placebo group will put the safety and tolerability profile of a new drug in proper perspective. However, in Europe, they do not conduct placebo-controlled studies; instead, they conduct what is called a “non-inferiority” trial of a new antipsychotic compared with a well-established antipsychotic.
Interestingly, even though the discovery of the neurodegenerative effects of untreated psychosis was only 20 years ago (in 1997 after serial MRI scans revealed progressive atrophy), in the 1960s, the first antipsychotic, chlorpromazine, was compared with placebo in a large national study for 6 months. This study showed without a doubt that chlorpromazine has a higher efficacy than placebo. After the study was done, Dr. Philip May at University of California, Los Angeles looked at what happened to the psychotic patients who received placebo for 6 months and found that they became less responsive to treatment, were re-hospitalized more often, and had more negative symptoms and a poorer overall outcome. That was a clue that untreated psychosis can be harmful, and it supports your point about the ethics of using placebo. In contemporary studies, a trial of oral antipsychotics is 6 weeks, not 6 months. In the year-long, placebo-controlled studies of injectable antipsychotics in stable patients, those who show the slightest increase in delusions, hallucinations, or suicidal/homicidal behavior were promptly taken out of the study and treated. This reduced the “harm,” although not completely. Perhaps the FDA will change its policies and adopt the non-inferiority model. That’s what is done with nonpsychiatric disorders such as pneumonia, stroke, or diabetes. But one last fact has to be stated: The placebo response in anxiety, depression, or psychosis is much higher (25% to 35%) than the 1% placebo response in pneumonia.
To Dr. Diamond: I really like DASH, and it is an acronym for quick symptomatic diagnosis. Speedy treatment then follows with the acronym RAPID to prevent brain damage that gets worse with delay.
As for the second issue of tapering off the antipsychotic medication, the evidence is overwhelming in favor of continuous pharmacotherapy. Just as hypertension and diabetes will return if medications are tapered or stopped, so will psychosis, and vengefully so because treatment resistance increases with each relapse.1 This is also true for bipolar disorder recurrences.2 A recent 20-year follow-up study showed that stopping antipsychotic treatment is associated with a much higher mortality rate than continuation therapy.3 Another 7-year study showed the same thing.4 It is literally deadly, and not just neurodegenerative, for persons with schizophrenia to stop their medications.
To Dr. Kerlinsky: I agree with you about using certain adjunctive pharmacotherapies for acute psychosis, which is associated with neuroinflammation, oxidative stress, and neuropil and myelin damage. I support using agents with anti-inflammatory effects (such as minocycline and omega-3 fatty acid), antioxidant effects (such as N-acetylcysteine), and neuroprotective effects (such as minocycline, clemastine, lithium, vitamin D, erythropoietin, etc.). I refer you to my past editorial, “Are you neuroprotecting your patients? 10 Adjunctive therapies to consider,”5 in which I mentioned all the above. I also pointed out the many neuroprotective effects of atypical antipsychotics in another editorial.6 Although off-label, those supplements can be useful interventions that can ameliorate the gray and white matter damage associated with acute psychotic relapses in patients with schizophrenia.
Henry A. Nasrallah, MD
Editor-in-Chief
The Sydney W. Souers Endowed Chair
Professor and Chairman
Department of Psychiatry and Behavioral Neuroscience
Saint Louis University School of Medicine
St. Louis, Missouri
References
1. Emsley R, Oosthuizen P, Koen L, et al. Comparison of treatment response in second-episode versus first-episode schizophrenia. J Clin Psychopharmacol. 2013;33(1):80-83.
2. Post RM. Preventing the maligna
3. Tiihonen J, Tanskanen A, Taipale H. 20-year nationwide follow-up study on discontinuation of antipsychotic treatment in first-episode schizophrenia. Am J Psychiatry. 2018;175(8):765-773.
4. Taipale H, Mittendorfer-Rutz E, Alexanderson K, et al. Antipsychotics and mortality in a nationwide cohort of 29,823 patients with schizophrenia. Schizophr Res. 2018;197:274-280.
5. Nasrallah HA. Are you neuroprotecting your patients? 10 Adjunctive therapies to consider. Current Psychiatry. 2016;15(12):12-14.
6. Nasrallah HA. A decade after the CATIE study, the focus has shifted from effectiveness to neuroprotection. Current Psychiatry. 2015;14(2):19-21.
Protein binding changes and drug interactions: What do we know?
Mr. S, age 47, weighs 209 lb and has a history of seizure disorder, bipolar disorder not otherwise specified, hypertension, and type 2 diabetes mellitus. He presents to the emergency department after not taking his medications for 2 days while on vacation. He has increased energy, decreased sleep, and pressured speech, and insists on walking for up to 10 hours per day “in preparation for a marathon,” even though he has a 4-cm foot ulcer. His family reports that he had been compliant with his medications until the present incident.
Mr. S has no known drug allergies. His medications include oral divalproex sodium delayed release (valproic acid [VPA]), 1,000 mg twice a day, oral lisinopril, 20 mg every morning, and insulin glargine, 22 units subcutaneously every evening.
A complete blood count, basic metabolic panel, creatine kinase level, VPA level, and urine drug screen are ordered. Relevant results include a serum creatinine level of 1.4 mg/dL (normal range: 0.6 to 1.2 mg/dL), a glucose serum level of 188 mg/dL (normal range: 70 to 100 mg/dL), and a VPA level of 23 mcg/mL (therapeutic range: 50 to 125 mcg/mL). A liver function panel is within normal limits: albumin level of 3.9 g/dL, aspartate aminotransferase level of 18 IU/L, and alanine aminotransferase level of 14 IU/L. In light of Mr. S’s seizure history, neurology is consulted and the decision is made to continue treating him with VPA because he has been seizure-free for 4.5 years and this medication has also helped with his bipolar disorder.
Mr. S is admitted to the hospital and his home medications are resumed at the current doses. On hospital Day 3, Mr. S’s VPA level is 62 mcg/mL, his obsession with a marathon has remitted, and his sleep pattern has normalized. Infectious disease and podiatry services are consulted for his diabetic foot infection, which has ulcerated down to the bone. IV ertapenem, 1,000 mg/d, is initiated with plans for debridement the following week. Two days later, Mr. S has a witnessed seizure; his VPA level is 9 mcg/mL.
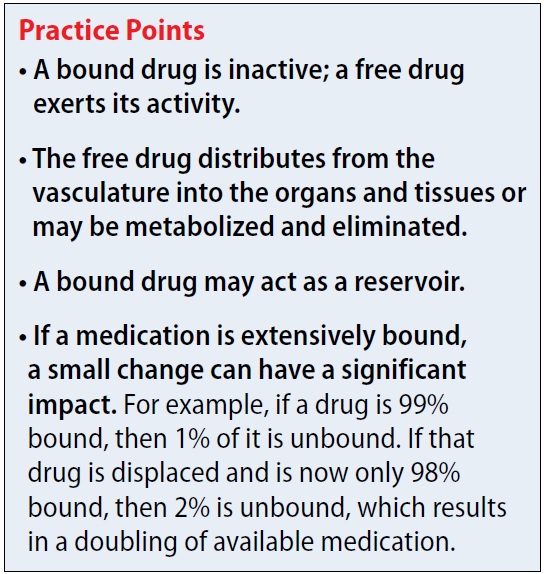
A common question asked of pharmacists is, “Will protein binding changes affect drug dosages?” In this article, I describe how protein binding changes may occur, and the complexity of the dynamic. Being highly bound to a protein typically does not mean all medications will interact, but some interactions can be important. This article does not cover medications that bind to hormones.
Why is protein binding important? When a medication is bound to plasma protein, it is not free to act. There can be a delay in therapeutic effect (because no drug is available to react), delayed elimination, or possibly displacement of another protein-bound medication. Additionally, medications tend not to cross the blood-brain barrier or be eliminated when bound. For example, if a drug is 99% bound (leaving 1% free) and displacement now leaves 2% of the drug free, this event has doubled the amount of free drug. As the unbound medication is eliminated, the drug that is bound to the protein can act as a reservoir. A dynamic relationship exists between bound drug, unbound drug, and rate of elimination.
Which proteins do drugs commonly bind to? The proteins often associated with binding include albumin, alpha-1-acid glycoprotein (AAG), and lipoproteins. Albumin comprises 60% of total plasma protein in the plasma. Lipoproteins include very high-density lipoprotein (VHDL), high-density lipoprotein (HDL), very low-density lipoprotein (VLDL), and low-density lipoprotein (LDL).1 Medications that bind to lipoproteins include cyclosporine, tacrolimus, and propofol.2
Continued to: What common disease states can cause hypoalbuminemia?
What common disease states can cause hypoalbuminemia? Many disease states can result in low albumin levels. The most common ones are malnutrition, malignancies, stress, injury, burns, pregnancy, and diabetes.3 When there is less albumin to bind to, free drug levels may be increased.
Can AAG levels change with disease states as well? Because AAG accounts for a lower percentage of total plasma protein than albumin, there may be less clinical concern regarding AAG. AAG levels usually do not drop, but instead can become elevated during times of trauma, inflammation, and acute myocardial infarction. This could result in increased binding of the free drug.4Which medications bind to red blood cells (RBCs)? There are several locations for drugs to bind to RBCs, including to hemoglobin and the plasma membrane. Medications that commonly bind to RBCs include barbiturates, chlorpromazine, imipramine, and phenytoin.5
What are common highly-bound medications? The Table1 provides examples of medications that are >90% protein-bound. However, this information may be misleading because many medications are highly bound. Zhang et al1 compiled binding data for 222 drugs, half of which bind 90% to 100%. However, the literature does not indicate that they all have clinically significant interactions. Benet and Hoener6 discuss how factors other than protein binding affect potential drug interactions, and the complexity of the body’s ability to compensate for increased free drug. Medication characteristics that may contribute to producing a significant interaction include, but are not limited to:
- free vs protein-bound drug in the plasma or tissue
- volume of distribution
- organs affected
- hepatic bioavailability
- drug clearance.
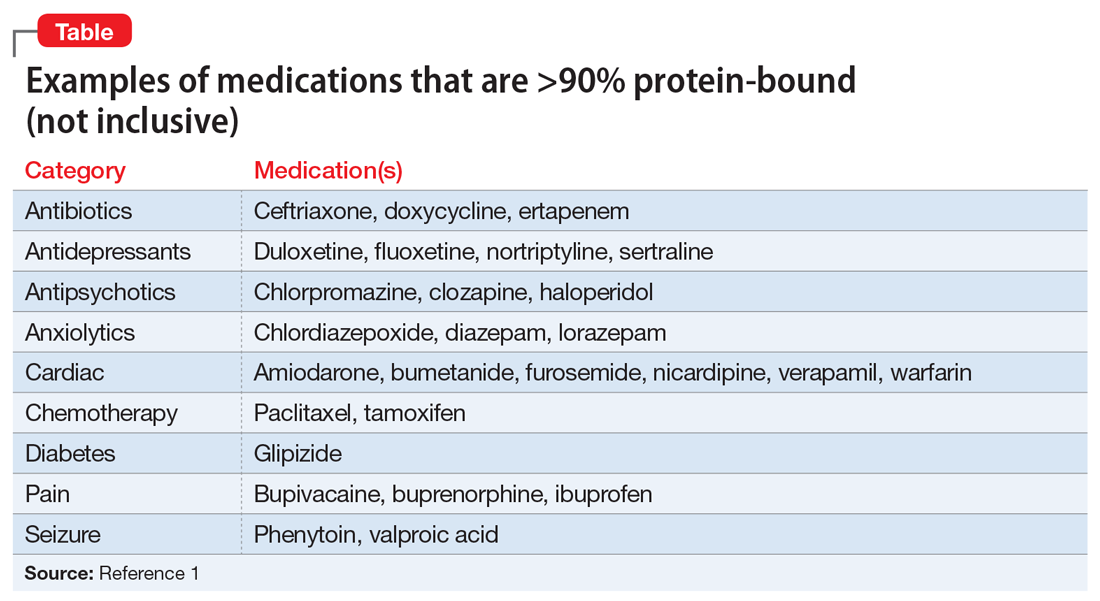
For example, VPA is 93% protein-bound and phenytoin is 91% protein-bound.1 However, this interaction is affected by more than just protein binding. VPA not only displaces the protein-bound phenytoin, but also inhibits its metabolism, which together result in increased free phenytoin levels.
Continued to: Another area of concern is a critically ill patient...
Another area of concern is a critically ill patient who has a change in his or her pH. Medications that are highly bound and have high clearance rates may be affected. This is of particular concern when prescribing antibiotics that are time-dependent, such as beta-lactams.3
What happened to Mr. S? Mr. S likely experienced a drug–drug interaction that resulted in a subtherapeutic VPA level and subsequent seizure. Case reports have shown evidence that the carbapenem class of antibiotics, which includes ertapenem, interacts with VPA.7 Proposed mechanisms include a lowering of VPA serum levels due to a redistribution of the VPA onto the RBCs due to carbapenem. Other theories include the possibility that carbapenems may limit oral VPA absorption, decrease VPA enterohepatic recirculation, and increase VPA metabolism.7 Using VPA and ertapenem together is discouraged because seizures have been reported among patients receiving this combination. If it is medically necessary to administer VPA and ertapenem, closely monitor VPA levels. In Mr. S’s case, another broad-spectrum antibiotic, such as piperacillin-tazobactam, could have been used, for his diabetic foot infection.
While many medications may have high protein binding, there are few clinically important known interactions. However, our understanding of the relationship between protein binding and drug interactions may improve with additional research.
CASE CONTINUED
Under neurology’s care, lacosamide is added for treatment of Mr. S’s seizures. No more seizures are noted during the remainder of his hospitalization. Infectious disease services change his antibiotic to piperacillin-tazobactam. Mr. S continues to progress well and is discharged to a rehabilitation center 2 days later.
Related Resource
- DrugBank. www.drugbank.ca. Canadian Institutes of Health Research.
Drug Brand Names
Amiodarone • Cordarone, Pacerone
Bumetanide • Bumex
Bupivacaine • Marcaine, Sensorcaine
Buprenorphine • Belbuca, Subutex
Ceftriaxone • Rocephin
Chlordiazepoxide • Librium
Chlorpromazine • Thorazine
Clozapine • Clozaril
Cyclosporine • Gengraf, Neoral
Diazepam • Valium
Doxycycline • Acticlate, Doryx
Duloxetine • Cymbalta
Ertapenem • Invanz
Fluoxetine • Prozac, Sarafem
Furosemide • Lasix
Glargine (Insulin) • Lantus, Toujeo
Glipizide • Glucotrol
Haloperidol • Haldol
Ibuprofen • Advil, Motrin
Imipramine • Tofranil
Lacosamide • Vimpat
Lisinopril • Prinivil, Zestril
Lorazepam • Ativan
Nicardipine • Cardene
Nortriptyline • Pamelor
Paclitaxel • Abraxane, Taxol
Phenytoin • Dilantin, Phenytek
Piperacillin-tazobactam • Zosyn
Propofol • Diprivan
Sertraline • Zoloft
Tacrolimus • Prograf
Tamoxifen • Soltamox
Valproic acid • Depakene, Depakote
Verapamil • Calan, Verelan
Warfarin • Coumadin, Jantoven
1. Zhang F, Xue J, Shao J, et al. Compilation of 222 drugs’ plasma protein binding data and guidance for study designs. Drug Discov Today. 2012;17(9-10):475-485.
2. Mehvar R. Role of protein binding in pharmacokinetics. Am J Pharm Edu. 2005;69(5): Article 103;1-8.
3. Roberts JA, Pea F, Lipman J. The clinical relevance of plasma protein binding changes. Clin Pharmacokinet. 2013;52(1):1-8.
4. Schmidt S, Gonzalez D, Derendork H. Significance of protein binding in pharmacokinetics and pharmacodynamics. J Pharm Sci. 2010;99(3):1107-1122.
5. Hinderling P. Red blood cells: a neglected compartment in pharmacokinetics and pharmacodynamics. Pharmacol Rev. 1997;49(3):279-295.
6. Benet LZ, Hoener B. Changes in plasma protein binding have little clinical relevance. Clin Pharmacol Ther. 2002;71(3):115-121.
7. Park MK, Lim KS, Kim T, et al. Reduced valproic acid serum concentrations due to drug interactions with carbapenem antibiotics: overview of 6 cases. Ther Drug Monit. 2012;34(5):599-603.
Mr. S, age 47, weighs 209 lb and has a history of seizure disorder, bipolar disorder not otherwise specified, hypertension, and type 2 diabetes mellitus. He presents to the emergency department after not taking his medications for 2 days while on vacation. He has increased energy, decreased sleep, and pressured speech, and insists on walking for up to 10 hours per day “in preparation for a marathon,” even though he has a 4-cm foot ulcer. His family reports that he had been compliant with his medications until the present incident.
Mr. S has no known drug allergies. His medications include oral divalproex sodium delayed release (valproic acid [VPA]), 1,000 mg twice a day, oral lisinopril, 20 mg every morning, and insulin glargine, 22 units subcutaneously every evening.
A complete blood count, basic metabolic panel, creatine kinase level, VPA level, and urine drug screen are ordered. Relevant results include a serum creatinine level of 1.4 mg/dL (normal range: 0.6 to 1.2 mg/dL), a glucose serum level of 188 mg/dL (normal range: 70 to 100 mg/dL), and a VPA level of 23 mcg/mL (therapeutic range: 50 to 125 mcg/mL). A liver function panel is within normal limits: albumin level of 3.9 g/dL, aspartate aminotransferase level of 18 IU/L, and alanine aminotransferase level of 14 IU/L. In light of Mr. S’s seizure history, neurology is consulted and the decision is made to continue treating him with VPA because he has been seizure-free for 4.5 years and this medication has also helped with his bipolar disorder.
Mr. S is admitted to the hospital and his home medications are resumed at the current doses. On hospital Day 3, Mr. S’s VPA level is 62 mcg/mL, his obsession with a marathon has remitted, and his sleep pattern has normalized. Infectious disease and podiatry services are consulted for his diabetic foot infection, which has ulcerated down to the bone. IV ertapenem, 1,000 mg/d, is initiated with plans for debridement the following week. Two days later, Mr. S has a witnessed seizure; his VPA level is 9 mcg/mL.

A common question asked of pharmacists is, “Will protein binding changes affect drug dosages?” In this article, I describe how protein binding changes may occur, and the complexity of the dynamic. Being highly bound to a protein typically does not mean all medications will interact, but some interactions can be important. This article does not cover medications that bind to hormones.
Why is protein binding important? When a medication is bound to plasma protein, it is not free to act. There can be a delay in therapeutic effect (because no drug is available to react), delayed elimination, or possibly displacement of another protein-bound medication. Additionally, medications tend not to cross the blood-brain barrier or be eliminated when bound. For example, if a drug is 99% bound (leaving 1% free) and displacement now leaves 2% of the drug free, this event has doubled the amount of free drug. As the unbound medication is eliminated, the drug that is bound to the protein can act as a reservoir. A dynamic relationship exists between bound drug, unbound drug, and rate of elimination.
Which proteins do drugs commonly bind to? The proteins often associated with binding include albumin, alpha-1-acid glycoprotein (AAG), and lipoproteins. Albumin comprises 60% of total plasma protein in the plasma. Lipoproteins include very high-density lipoprotein (VHDL), high-density lipoprotein (HDL), very low-density lipoprotein (VLDL), and low-density lipoprotein (LDL).1 Medications that bind to lipoproteins include cyclosporine, tacrolimus, and propofol.2
Continued to: What common disease states can cause hypoalbuminemia?
What common disease states can cause hypoalbuminemia? Many disease states can result in low albumin levels. The most common ones are malnutrition, malignancies, stress, injury, burns, pregnancy, and diabetes.3 When there is less albumin to bind to, free drug levels may be increased.
Can AAG levels change with disease states as well? Because AAG accounts for a lower percentage of total plasma protein than albumin, there may be less clinical concern regarding AAG. AAG levels usually do not drop, but instead can become elevated during times of trauma, inflammation, and acute myocardial infarction. This could result in increased binding of the free drug.4Which medications bind to red blood cells (RBCs)? There are several locations for drugs to bind to RBCs, including to hemoglobin and the plasma membrane. Medications that commonly bind to RBCs include barbiturates, chlorpromazine, imipramine, and phenytoin.5
What are common highly-bound medications? The Table1 provides examples of medications that are >90% protein-bound. However, this information may be misleading because many medications are highly bound. Zhang et al1 compiled binding data for 222 drugs, half of which bind 90% to 100%. However, the literature does not indicate that they all have clinically significant interactions. Benet and Hoener6 discuss how factors other than protein binding affect potential drug interactions, and the complexity of the body’s ability to compensate for increased free drug. Medication characteristics that may contribute to producing a significant interaction include, but are not limited to:
- free vs protein-bound drug in the plasma or tissue
- volume of distribution
- organs affected
- hepatic bioavailability
- drug clearance.

For example, VPA is 93% protein-bound and phenytoin is 91% protein-bound.1 However, this interaction is affected by more than just protein binding. VPA not only displaces the protein-bound phenytoin, but also inhibits its metabolism, which together result in increased free phenytoin levels.
Continued to: Another area of concern is a critically ill patient...
Another area of concern is a critically ill patient who has a change in his or her pH. Medications that are highly bound and have high clearance rates may be affected. This is of particular concern when prescribing antibiotics that are time-dependent, such as beta-lactams.3
What happened to Mr. S? Mr. S likely experienced a drug–drug interaction that resulted in a subtherapeutic VPA level and subsequent seizure. Case reports have shown evidence that the carbapenem class of antibiotics, which includes ertapenem, interacts with VPA.7 Proposed mechanisms include a lowering of VPA serum levels due to a redistribution of the VPA onto the RBCs due to carbapenem. Other theories include the possibility that carbapenems may limit oral VPA absorption, decrease VPA enterohepatic recirculation, and increase VPA metabolism.7 Using VPA and ertapenem together is discouraged because seizures have been reported among patients receiving this combination. If it is medically necessary to administer VPA and ertapenem, closely monitor VPA levels. In Mr. S’s case, another broad-spectrum antibiotic, such as piperacillin-tazobactam, could have been used, for his diabetic foot infection.
While many medications may have high protein binding, there are few clinically important known interactions. However, our understanding of the relationship between protein binding and drug interactions may improve with additional research.
CASE CONTINUED
Under neurology’s care, lacosamide is added for treatment of Mr. S’s seizures. No more seizures are noted during the remainder of his hospitalization. Infectious disease services change his antibiotic to piperacillin-tazobactam. Mr. S continues to progress well and is discharged to a rehabilitation center 2 days later.
Related Resource
- DrugBank. www.drugbank.ca. Canadian Institutes of Health Research.
Drug Brand Names
Amiodarone • Cordarone, Pacerone
Bumetanide • Bumex
Bupivacaine • Marcaine, Sensorcaine
Buprenorphine • Belbuca, Subutex
Ceftriaxone • Rocephin
Chlordiazepoxide • Librium
Chlorpromazine • Thorazine
Clozapine • Clozaril
Cyclosporine • Gengraf, Neoral
Diazepam • Valium
Doxycycline • Acticlate, Doryx
Duloxetine • Cymbalta
Ertapenem • Invanz
Fluoxetine • Prozac, Sarafem
Furosemide • Lasix
Glargine (Insulin) • Lantus, Toujeo
Glipizide • Glucotrol
Haloperidol • Haldol
Ibuprofen • Advil, Motrin
Imipramine • Tofranil
Lacosamide • Vimpat
Lisinopril • Prinivil, Zestril
Lorazepam • Ativan
Nicardipine • Cardene
Nortriptyline • Pamelor
Paclitaxel • Abraxane, Taxol
Phenytoin • Dilantin, Phenytek
Piperacillin-tazobactam • Zosyn
Propofol • Diprivan
Sertraline • Zoloft
Tacrolimus • Prograf
Tamoxifen • Soltamox
Valproic acid • Depakene, Depakote
Verapamil • Calan, Verelan
Warfarin • Coumadin, Jantoven
Mr. S, age 47, weighs 209 lb and has a history of seizure disorder, bipolar disorder not otherwise specified, hypertension, and type 2 diabetes mellitus. He presents to the emergency department after not taking his medications for 2 days while on vacation. He has increased energy, decreased sleep, and pressured speech, and insists on walking for up to 10 hours per day “in preparation for a marathon,” even though he has a 4-cm foot ulcer. His family reports that he had been compliant with his medications until the present incident.
Mr. S has no known drug allergies. His medications include oral divalproex sodium delayed release (valproic acid [VPA]), 1,000 mg twice a day, oral lisinopril, 20 mg every morning, and insulin glargine, 22 units subcutaneously every evening.
A complete blood count, basic metabolic panel, creatine kinase level, VPA level, and urine drug screen are ordered. Relevant results include a serum creatinine level of 1.4 mg/dL (normal range: 0.6 to 1.2 mg/dL), a glucose serum level of 188 mg/dL (normal range: 70 to 100 mg/dL), and a VPA level of 23 mcg/mL (therapeutic range: 50 to 125 mcg/mL). A liver function panel is within normal limits: albumin level of 3.9 g/dL, aspartate aminotransferase level of 18 IU/L, and alanine aminotransferase level of 14 IU/L. In light of Mr. S’s seizure history, neurology is consulted and the decision is made to continue treating him with VPA because he has been seizure-free for 4.5 years and this medication has also helped with his bipolar disorder.
Mr. S is admitted to the hospital and his home medications are resumed at the current doses. On hospital Day 3, Mr. S’s VPA level is 62 mcg/mL, his obsession with a marathon has remitted, and his sleep pattern has normalized. Infectious disease and podiatry services are consulted for his diabetic foot infection, which has ulcerated down to the bone. IV ertapenem, 1,000 mg/d, is initiated with plans for debridement the following week. Two days later, Mr. S has a witnessed seizure; his VPA level is 9 mcg/mL.

A common question asked of pharmacists is, “Will protein binding changes affect drug dosages?” In this article, I describe how protein binding changes may occur, and the complexity of the dynamic. Being highly bound to a protein typically does not mean all medications will interact, but some interactions can be important. This article does not cover medications that bind to hormones.
Why is protein binding important? When a medication is bound to plasma protein, it is not free to act. There can be a delay in therapeutic effect (because no drug is available to react), delayed elimination, or possibly displacement of another protein-bound medication. Additionally, medications tend not to cross the blood-brain barrier or be eliminated when bound. For example, if a drug is 99% bound (leaving 1% free) and displacement now leaves 2% of the drug free, this event has doubled the amount of free drug. As the unbound medication is eliminated, the drug that is bound to the protein can act as a reservoir. A dynamic relationship exists between bound drug, unbound drug, and rate of elimination.
Which proteins do drugs commonly bind to? The proteins often associated with binding include albumin, alpha-1-acid glycoprotein (AAG), and lipoproteins. Albumin comprises 60% of total plasma protein in the plasma. Lipoproteins include very high-density lipoprotein (VHDL), high-density lipoprotein (HDL), very low-density lipoprotein (VLDL), and low-density lipoprotein (LDL).1 Medications that bind to lipoproteins include cyclosporine, tacrolimus, and propofol.2
Continued to: What common disease states can cause hypoalbuminemia?
What common disease states can cause hypoalbuminemia? Many disease states can result in low albumin levels. The most common ones are malnutrition, malignancies, stress, injury, burns, pregnancy, and diabetes.3 When there is less albumin to bind to, free drug levels may be increased.
Can AAG levels change with disease states as well? Because AAG accounts for a lower percentage of total plasma protein than albumin, there may be less clinical concern regarding AAG. AAG levels usually do not drop, but instead can become elevated during times of trauma, inflammation, and acute myocardial infarction. This could result in increased binding of the free drug.4Which medications bind to red blood cells (RBCs)? There are several locations for drugs to bind to RBCs, including to hemoglobin and the plasma membrane. Medications that commonly bind to RBCs include barbiturates, chlorpromazine, imipramine, and phenytoin.5
What are common highly-bound medications? The Table1 provides examples of medications that are >90% protein-bound. However, this information may be misleading because many medications are highly bound. Zhang et al1 compiled binding data for 222 drugs, half of which bind 90% to 100%. However, the literature does not indicate that they all have clinically significant interactions. Benet and Hoener6 discuss how factors other than protein binding affect potential drug interactions, and the complexity of the body’s ability to compensate for increased free drug. Medication characteristics that may contribute to producing a significant interaction include, but are not limited to:
- free vs protein-bound drug in the plasma or tissue
- volume of distribution
- organs affected
- hepatic bioavailability
- drug clearance.

For example, VPA is 93% protein-bound and phenytoin is 91% protein-bound.1 However, this interaction is affected by more than just protein binding. VPA not only displaces the protein-bound phenytoin, but also inhibits its metabolism, which together result in increased free phenytoin levels.
Continued to: Another area of concern is a critically ill patient...
Another area of concern is a critically ill patient who has a change in his or her pH. Medications that are highly bound and have high clearance rates may be affected. This is of particular concern when prescribing antibiotics that are time-dependent, such as beta-lactams.3
What happened to Mr. S? Mr. S likely experienced a drug–drug interaction that resulted in a subtherapeutic VPA level and subsequent seizure. Case reports have shown evidence that the carbapenem class of antibiotics, which includes ertapenem, interacts with VPA.7 Proposed mechanisms include a lowering of VPA serum levels due to a redistribution of the VPA onto the RBCs due to carbapenem. Other theories include the possibility that carbapenems may limit oral VPA absorption, decrease VPA enterohepatic recirculation, and increase VPA metabolism.7 Using VPA and ertapenem together is discouraged because seizures have been reported among patients receiving this combination. If it is medically necessary to administer VPA and ertapenem, closely monitor VPA levels. In Mr. S’s case, another broad-spectrum antibiotic, such as piperacillin-tazobactam, could have been used, for his diabetic foot infection.
While many medications may have high protein binding, there are few clinically important known interactions. However, our understanding of the relationship between protein binding and drug interactions may improve with additional research.
CASE CONTINUED
Under neurology’s care, lacosamide is added for treatment of Mr. S’s seizures. No more seizures are noted during the remainder of his hospitalization. Infectious disease services change his antibiotic to piperacillin-tazobactam. Mr. S continues to progress well and is discharged to a rehabilitation center 2 days later.
Related Resource
- DrugBank. www.drugbank.ca. Canadian Institutes of Health Research.
Drug Brand Names
Amiodarone • Cordarone, Pacerone
Bumetanide • Bumex
Bupivacaine • Marcaine, Sensorcaine
Buprenorphine • Belbuca, Subutex
Ceftriaxone • Rocephin
Chlordiazepoxide • Librium
Chlorpromazine • Thorazine
Clozapine • Clozaril
Cyclosporine • Gengraf, Neoral
Diazepam • Valium
Doxycycline • Acticlate, Doryx
Duloxetine • Cymbalta
Ertapenem • Invanz
Fluoxetine • Prozac, Sarafem
Furosemide • Lasix
Glargine (Insulin) • Lantus, Toujeo
Glipizide • Glucotrol
Haloperidol • Haldol
Ibuprofen • Advil, Motrin
Imipramine • Tofranil
Lacosamide • Vimpat
Lisinopril • Prinivil, Zestril
Lorazepam • Ativan
Nicardipine • Cardene
Nortriptyline • Pamelor
Paclitaxel • Abraxane, Taxol
Phenytoin • Dilantin, Phenytek
Piperacillin-tazobactam • Zosyn
Propofol • Diprivan
Sertraline • Zoloft
Tacrolimus • Prograf
Tamoxifen • Soltamox
Valproic acid • Depakene, Depakote
Verapamil • Calan, Verelan
Warfarin • Coumadin, Jantoven
1. Zhang F, Xue J, Shao J, et al. Compilation of 222 drugs’ plasma protein binding data and guidance for study designs. Drug Discov Today. 2012;17(9-10):475-485.
2. Mehvar R. Role of protein binding in pharmacokinetics. Am J Pharm Edu. 2005;69(5): Article 103;1-8.
3. Roberts JA, Pea F, Lipman J. The clinical relevance of plasma protein binding changes. Clin Pharmacokinet. 2013;52(1):1-8.
4. Schmidt S, Gonzalez D, Derendork H. Significance of protein binding in pharmacokinetics and pharmacodynamics. J Pharm Sci. 2010;99(3):1107-1122.
5. Hinderling P. Red blood cells: a neglected compartment in pharmacokinetics and pharmacodynamics. Pharmacol Rev. 1997;49(3):279-295.
6. Benet LZ, Hoener B. Changes in plasma protein binding have little clinical relevance. Clin Pharmacol Ther. 2002;71(3):115-121.
7. Park MK, Lim KS, Kim T, et al. Reduced valproic acid serum concentrations due to drug interactions with carbapenem antibiotics: overview of 6 cases. Ther Drug Monit. 2012;34(5):599-603.
1. Zhang F, Xue J, Shao J, et al. Compilation of 222 drugs’ plasma protein binding data and guidance for study designs. Drug Discov Today. 2012;17(9-10):475-485.
2. Mehvar R. Role of protein binding in pharmacokinetics. Am J Pharm Edu. 2005;69(5): Article 103;1-8.
3. Roberts JA, Pea F, Lipman J. The clinical relevance of plasma protein binding changes. Clin Pharmacokinet. 2013;52(1):1-8.
4. Schmidt S, Gonzalez D, Derendork H. Significance of protein binding in pharmacokinetics and pharmacodynamics. J Pharm Sci. 2010;99(3):1107-1122.
5. Hinderling P. Red blood cells: a neglected compartment in pharmacokinetics and pharmacodynamics. Pharmacol Rev. 1997;49(3):279-295.
6. Benet LZ, Hoener B. Changes in plasma protein binding have little clinical relevance. Clin Pharmacol Ther. 2002;71(3):115-121.
7. Park MK, Lim KS, Kim T, et al. Reduced valproic acid serum concentrations due to drug interactions with carbapenem antibiotics: overview of 6 cases. Ther Drug Monit. 2012;34(5):599-603.
Unrelenting depression: ‘I would rather be dead than feel this way’
CASE Suicidal ideation, flare-up of ulcerative colitis
Mr. J, age 56, who has a history of major depressive disorder (MDD), generalized anxiety disorder (GAD), and ulcerative colitis (UC), presents to the emergency department (ED) with suicidal ideation and a plan to overdose on his medications. He reports no current emotional or financial stressors in his personal life. Home medications documented at the time of his arrival to the ED include sertraline, 100 mg/d, bupropion, 150 mg/d, buspirone, 10 mg 3 times daily, diazepam 10 mg 3 times daily, as needed, adalimumab, 40 mg IM every 2 weeks, and diphenhydramine, 50 mg every night.
A recent flare-up of UC resulted in Mr. J being placed on a 15-week prednisone taper, beginning at 80 mg/d and decreasing by 5 mg weekly, which was completed 2 weeks before he presented to the ED. After completing the prednisone taper, Mr. J went to his primary care physician (PCP) on 3 separate occasions due to episodes of severe depression. Although the PCP prescribed multiple medications to target Mr. J’s depressive symptoms, he continued to decline.
Subsequently, Mr. J came to the ED and is admitted to the psychiatric unit for safety and stabilization. Upon admission, Mr. J becomes bedridden, and reports that his current depressive episode is the most severe that he has ever experienced in his more than 30 years of having MDD. He says that neither bupropion nor buspirone are helping with his depression, anxiety, or any related symptom.
[polldaddy:10120537]
The authors’ observations
At admission, all of Mr. J’s home medications, except sertraline and adalimumab, which had been prescribed to treat UC (Box1,2), were discontinued. His diazepam was discontinued because the clinician felt it may have been contributing to Mr. J’s inability to walk or get out of bed. Diazepam was not tapered because it was initiated 7 days prior to admission and was thought to be exacerbating his depression and suicidal ideation. Bupropion and buspirone, which were initiated 2 weeks prior, were discontinued because Mr. J reported that neither medication was helping with his depression, anxiety, or any related symptom.
Box
Ulcerative colitis and depressive episodes
Ulcerative colitis (UC) is a chronic condition associated with inflammation in the colon causing extreme abdominal discomfort during acute flare-ups. Moderate to severe UC flare-ups are commonly treated with corticosteroids due to these medications’ anti-inflammatory properties. Although rare, corticosteroid withdrawal has been documented to induce episodes of depression. The pathophysiology of corticosteroid withdrawal inducing neuropsychiatric sequelae remains unclear; however, it is thought to be due to hypothalamic-pituitary-adrenocortical suppression.1 Fardet et al2 concluded that incident rates per 100 person-years at risk during the withdrawal period were 11.1 (95% confidence interval, 10.0, 12.3) for depression.
EVALUATION Poor appetite, anxiety, and continued suicidality
During evaluation, vital signs, laboratory findings, and diagnostic testing are found to be unremarkable. Mr. J’s presentation and complaints are entirely subjective, and include poor appetite, fatigue, difficulty sleeping, sorrow, anxiety, and continued suicidality. Mr. J reports that he feels miserable, which is reflected by his poor eye contact, soft speech, and body language.
Continued to: The authors' observations
The authors’ observations
MDD is a mood disorder characterized by depressed mood and/or loss of interest or pleasure for more than 2 weeks.3 First-line pharmacotherapy for MDD includes monotherapy with a selective serotonin reuptake inhibitor (SSRI), serotonin-norepinephrine reuptake inhibitor (SNRI), mirtazapine, or bupropion.4 Medication selection is typically based on patient-specific factors, adverse effect profile, drug–drug interactions, and cost. Other treatments include electroconvulsive therapy (ECT) or cognitive-behavioral therapy (CBT).4,5 Augmentation agents, such as second-generation antipsychotics, lithium, thyroid hormone supplementation, buspirone, anticonvulsants, and combinations of antidepressants, may also be considered.4
TREATMENT Condition worsens
On Day 2 of hospitalization, Mr. J is started on aripiprazole, 5 mg/d, clonazepam, 1 mg twice daily, and melatonin, 5 mg, each night for sleep. Aripiprazole, 5 mg/d, is initiated as an adjunct to sertraline for MDD because Mr. J reports feeling much worse and continues to report that he would “rather die than feel this way.” Mr. J begins to believe that his current state is his new baseline, and that feeling better is no longer possible.
On Day 3 of hospitalization, records are obtained from a clinician at an outside facility who previously treated Mr
By Day 8 of hospitalization, there is no notable change in Mr. J’s depressive symptoms. On Day 9, sertraline is increased to 200 mg/d, with little improvement from Mr. J’s perspective. The multidisciplinary team evaluates him, and when directly asked, Mr. J cites his 4 greatest complaints to be poor sleep, fatigue, no appetite, and depressed mood. Once again, he states, “I would rather be dead than go on feeling this way.”
[polldaddy:10120587]
The authors’ observations
Due to Mr. J’s severe, unrelenting depressive episode, the treatment team obtained his informed consent to undergo ECT. On Day 9, before initiating ECT, the pharmacist recommended mirtazapine, even though the patient weighed almost 89 kg (196.21 lb) and had a body mass index of 27.8 kg/m2. The treatment team thought that mirtazapine augmentation could potentially help the sertraline work more quickly while targeting Mr. J’s 4 greatest complaints.
Mirtazapine is a central alpha-2 antagonist or noradrenergic and specific serotonergic antidepressant (NaSSA) that works through antagonism of the presynaptic alpha-2 adrenergic receptors to indirectly regulate release of monoamines and increase the release of serotonin and norepinephrine.6 Additionally, mirtazapine has antagonist actions at 5HT2A, 5HT2C, 5HT3, and histamine-1 receptors.6 Potential adverse effects include drowsiness and increased appetite leading to weight gain.7 Mirtazapine’s therapeutic efficacy is similar to SSRIs for treating depression.4 Mirtazapine in combination with an SNRI has been referred to as “California rocket fuel” due to the theoretical pharmacologic synergy and resulting strong antidepressant action.6 It was hypothesized that similar effects could be seen by augmenting the SSRI sertraline with mirtazapine.
Continued to: The time to efficacy with mirtazapine...
The time to efficacy with mirtazapine is approximately 2 to 4 weeks, but anxiety symptoms and poor sleep or insomnia may improve in the first week.8 Studies have suggested the possibility of a more rapid onset of efficacy with mirtazapine than with SSRIs, as well as potential response acceleration in MDD and other psychiatric illnesses such as anxiety disorders or obsessive-compulsive disorder (OCD).9,10 A review that included several double-blind studies and compared mirtazapine with SSRIs found the amount of responders with persistent improvement with onset in Week 1 was more pronounced with mirtazapine.9
Augmenting an SSRI with mirtazapine is a potential therapeutic option because it can help boost the efficacy of the prescribed SSRI while enhancing appetite and blunting the activating or anxiety-like effects of some SSRIs, which may help with relaxation and sleep.4 The combination of an SSRI plus mirtazapine has been studied in patients with MDD, posttraumatic stress disorder, and OCD; it was found to improve symptoms of those conditions due to the medications’ complementary mechanisms of action.4,11-13 Also, mirtazapine has been shown to decrease the rates of relapse after an acute phase of depression.4,14
OUTCOME Rapid improvement
On Day 9, Mr. J receives the first dose of mirtazapine, 7.5 mg at bedtime. On Day 10, when Mr. J wakes, his mood is notably improved. He is more interactive (sitting up in bed reading and making eye contact with the staff during an interview), and he reports improved sleep and eats most of his breakfast.
After receiving 3 doses of mirtazapine, Mr. J reports that he feels back to his normal self; he is interactive, alert, and eating well. Due to the rapid improvement in mood, ECT is discontinued, and he does not receive any ECT treatment during the remainder of his hospitalization.
On Day 11, divalproex is discontinued. Because Mr. J receives only 5 days of therapy with this agent, his divalproex level is not checked. At this point, the treatment team feels confident in ruling out bipolar disorder.
On Day 15, Mr. J is discharged with sertraline, 200 mg/d, mirtazapine, 7.5 mg/d at 7
Ten months after his depressive episode, Mr. J has had no further admissions at the hospital where he received the treatment described here.
Bottom Line
Evidence for the treatment of major depressive disorder induced by corticosteroid withdrawal is limited. Despite trials of agents from multiple medication classes, the depressive episode may not improve. Adding mirtazapine to a selective serotonin reuptake inhibitor or serotonin-norepinephrine reuptake inhibitor may prove successful.
Related Resources
- Watanabe N, Omori IM, Nakagawa A, et al. Mirtazapine versus other antidepressive agents for depression. Cochrane Database Syst Rev. 2011;(12):CD006528.
- Kenna HA, Poon AW, de los Angeles CP, et al. Psychiatric complications of treatment with corticosteroids: review with case report. Psychiatry Clin Neurosci. 2011;65(6):549-560.
Drug Brand Names
Adalimumab • Humira
Aripiprazole • Abilify
Bupropion • Wellbutrin, Zyban
Buspirone • Buspar
Clonazepam • Klonopin
Diazepam • Valium
Diphenhydramine • Benadryl
Divalproex • Depakote, Depakote ER
Lithium • Eskalith, Lithobid
Mirtazapine • Remeron
Prednisone • Deltasone
Sertraline • Zoloft
1. Dixon R, Christy N. On the various forms of corticosteroid withdrawal syndrome. Am J Med. 1980;68(2):224-30.
2. Fardet L, Petersen I, Nazareth I. Suicidal behavior and severe neuropsychiatric disorders following glucocorticoid therapy in primary care. Am J Psychiatry. 2012;169(5):491-497.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder, 3rd ed. Arlington Virginia: American Psychiatric Association. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf. Published October 2010. Accessed March 15, 2017.
5. National Institute for Health and Clinical Excellence (NICE) Clinical Guideline 90. Depression in adults: recognition and management. https://www.nice.org.uk/guidance/cg90. Accessed March 15, 2017.
6. Stahl SM. Stahl’s essential psychopharmacology: neuroscientific basis and practical applications, 4th ed. Cambridge, United Kingdom: Cambridge University Press; 2013;317-322; 363-364.
7. Remeron [package insert]. Whitehouse Station, NJ: Merck & Co., Inc.; 2018.
8. Gorman JM. Mirtazapine: clinical overview. J Clin Psychiatry. 1999;60(suppl 17):9-13; discussion 46-48.
9. Quitkin FM, Taylor BP, Kremer C. Does mirtazapine have a more rapid onset than SSRIs? J Clin Psychiatry. 2001;62(5):358-361.
10. Pallanti S, Quercioli L, Bruscoli M. Response acceleration with mirtazapine augmentation of citalopram in obsessive-compulsive disorder patients without comorbid depression: a pilot study. J Clin Psychiatry. 2004;65(10):1394-1399.
11. Blier P, Gobbi G, Turcotte JE, et al. Mirtazapine and paroxetine in major depression: a comparison of monotherapy versus their combination from treatment initiation. Eur Neuropsychopharmacol. 2009;19(7):457-465.
12. Blier P, Ward HE, Tremblay P, et al. Combination of antidepressant medications from treatment initiation for major depressive disorder: a double-blind randomized study. Am J Psychiatry. 2010;167(3):281-288.
13. Carpenter LL, Yasmin S, Price LH. A double-blind, placebo-controlled study of antidepressant augmentation with mirtazapine. Biol Psychiatry. 2002;51(2):183-188.
14. Schneier FR, Campeas R, Carcamo J, et al. Combined mirtazapine and SSRI treatment of PTSD: a placebo-controlled trial. Depress Anxiety. 2015;32(8):570-579.
CASE Suicidal ideation, flare-up of ulcerative colitis
Mr. J, age 56, who has a history of major depressive disorder (MDD), generalized anxiety disorder (GAD), and ulcerative colitis (UC), presents to the emergency department (ED) with suicidal ideation and a plan to overdose on his medications. He reports no current emotional or financial stressors in his personal life. Home medications documented at the time of his arrival to the ED include sertraline, 100 mg/d, bupropion, 150 mg/d, buspirone, 10 mg 3 times daily, diazepam 10 mg 3 times daily, as needed, adalimumab, 40 mg IM every 2 weeks, and diphenhydramine, 50 mg every night.
A recent flare-up of UC resulted in Mr. J being placed on a 15-week prednisone taper, beginning at 80 mg/d and decreasing by 5 mg weekly, which was completed 2 weeks before he presented to the ED. After completing the prednisone taper, Mr. J went to his primary care physician (PCP) on 3 separate occasions due to episodes of severe depression. Although the PCP prescribed multiple medications to target Mr. J’s depressive symptoms, he continued to decline.
Subsequently, Mr. J came to the ED and is admitted to the psychiatric unit for safety and stabilization. Upon admission, Mr. J becomes bedridden, and reports that his current depressive episode is the most severe that he has ever experienced in his more than 30 years of having MDD. He says that neither bupropion nor buspirone are helping with his depression, anxiety, or any related symptom.
[polldaddy:10120537]
The authors’ observations
At admission, all of Mr. J’s home medications, except sertraline and adalimumab, which had been prescribed to treat UC (Box1,2), were discontinued. His diazepam was discontinued because the clinician felt it may have been contributing to Mr. J’s inability to walk or get out of bed. Diazepam was not tapered because it was initiated 7 days prior to admission and was thought to be exacerbating his depression and suicidal ideation. Bupropion and buspirone, which were initiated 2 weeks prior, were discontinued because Mr. J reported that neither medication was helping with his depression, anxiety, or any related symptom.
Box
Ulcerative colitis and depressive episodes
Ulcerative colitis (UC) is a chronic condition associated with inflammation in the colon causing extreme abdominal discomfort during acute flare-ups. Moderate to severe UC flare-ups are commonly treated with corticosteroids due to these medications’ anti-inflammatory properties. Although rare, corticosteroid withdrawal has been documented to induce episodes of depression. The pathophysiology of corticosteroid withdrawal inducing neuropsychiatric sequelae remains unclear; however, it is thought to be due to hypothalamic-pituitary-adrenocortical suppression.1 Fardet et al2 concluded that incident rates per 100 person-years at risk during the withdrawal period were 11.1 (95% confidence interval, 10.0, 12.3) for depression.
EVALUATION Poor appetite, anxiety, and continued suicidality
During evaluation, vital signs, laboratory findings, and diagnostic testing are found to be unremarkable. Mr. J’s presentation and complaints are entirely subjective, and include poor appetite, fatigue, difficulty sleeping, sorrow, anxiety, and continued suicidality. Mr. J reports that he feels miserable, which is reflected by his poor eye contact, soft speech, and body language.
Continued to: The authors' observations
The authors’ observations
MDD is a mood disorder characterized by depressed mood and/or loss of interest or pleasure for more than 2 weeks.3 First-line pharmacotherapy for MDD includes monotherapy with a selective serotonin reuptake inhibitor (SSRI), serotonin-norepinephrine reuptake inhibitor (SNRI), mirtazapine, or bupropion.4 Medication selection is typically based on patient-specific factors, adverse effect profile, drug–drug interactions, and cost. Other treatments include electroconvulsive therapy (ECT) or cognitive-behavioral therapy (CBT).4,5 Augmentation agents, such as second-generation antipsychotics, lithium, thyroid hormone supplementation, buspirone, anticonvulsants, and combinations of antidepressants, may also be considered.4
TREATMENT Condition worsens
On Day 2 of hospitalization, Mr. J is started on aripiprazole, 5 mg/d, clonazepam, 1 mg twice daily, and melatonin, 5 mg, each night for sleep. Aripiprazole, 5 mg/d, is initiated as an adjunct to sertraline for MDD because Mr. J reports feeling much worse and continues to report that he would “rather die than feel this way.” Mr. J begins to believe that his current state is his new baseline, and that feeling better is no longer possible.
On Day 3 of hospitalization, records are obtained from a clinician at an outside facility who previously treated Mr
By Day 8 of hospitalization, there is no notable change in Mr. J’s depressive symptoms. On Day 9, sertraline is increased to 200 mg/d, with little improvement from Mr. J’s perspective. The multidisciplinary team evaluates him, and when directly asked, Mr. J cites his 4 greatest complaints to be poor sleep, fatigue, no appetite, and depressed mood. Once again, he states, “I would rather be dead than go on feeling this way.”
[polldaddy:10120587]
The authors’ observations
Due to Mr. J’s severe, unrelenting depressive episode, the treatment team obtained his informed consent to undergo ECT. On Day 9, before initiating ECT, the pharmacist recommended mirtazapine, even though the patient weighed almost 89 kg (196.21 lb) and had a body mass index of 27.8 kg/m2. The treatment team thought that mirtazapine augmentation could potentially help the sertraline work more quickly while targeting Mr. J’s 4 greatest complaints.
Mirtazapine is a central alpha-2 antagonist or noradrenergic and specific serotonergic antidepressant (NaSSA) that works through antagonism of the presynaptic alpha-2 adrenergic receptors to indirectly regulate release of monoamines and increase the release of serotonin and norepinephrine.6 Additionally, mirtazapine has antagonist actions at 5HT2A, 5HT2C, 5HT3, and histamine-1 receptors.6 Potential adverse effects include drowsiness and increased appetite leading to weight gain.7 Mirtazapine’s therapeutic efficacy is similar to SSRIs for treating depression.4 Mirtazapine in combination with an SNRI has been referred to as “California rocket fuel” due to the theoretical pharmacologic synergy and resulting strong antidepressant action.6 It was hypothesized that similar effects could be seen by augmenting the SSRI sertraline with mirtazapine.
Continued to: The time to efficacy with mirtazapine...
The time to efficacy with mirtazapine is approximately 2 to 4 weeks, but anxiety symptoms and poor sleep or insomnia may improve in the first week.8 Studies have suggested the possibility of a more rapid onset of efficacy with mirtazapine than with SSRIs, as well as potential response acceleration in MDD and other psychiatric illnesses such as anxiety disorders or obsessive-compulsive disorder (OCD).9,10 A review that included several double-blind studies and compared mirtazapine with SSRIs found the amount of responders with persistent improvement with onset in Week 1 was more pronounced with mirtazapine.9
Augmenting an SSRI with mirtazapine is a potential therapeutic option because it can help boost the efficacy of the prescribed SSRI while enhancing appetite and blunting the activating or anxiety-like effects of some SSRIs, which may help with relaxation and sleep.4 The combination of an SSRI plus mirtazapine has been studied in patients with MDD, posttraumatic stress disorder, and OCD; it was found to improve symptoms of those conditions due to the medications’ complementary mechanisms of action.4,11-13 Also, mirtazapine has been shown to decrease the rates of relapse after an acute phase of depression.4,14
OUTCOME Rapid improvement
On Day 9, Mr. J receives the first dose of mirtazapine, 7.5 mg at bedtime. On Day 10, when Mr. J wakes, his mood is notably improved. He is more interactive (sitting up in bed reading and making eye contact with the staff during an interview), and he reports improved sleep and eats most of his breakfast.
After receiving 3 doses of mirtazapine, Mr. J reports that he feels back to his normal self; he is interactive, alert, and eating well. Due to the rapid improvement in mood, ECT is discontinued, and he does not receive any ECT treatment during the remainder of his hospitalization.
On Day 11, divalproex is discontinued. Because Mr. J receives only 5 days of therapy with this agent, his divalproex level is not checked. At this point, the treatment team feels confident in ruling out bipolar disorder.
On Day 15, Mr. J is discharged with sertraline, 200 mg/d, mirtazapine, 7.5 mg/d at 7
Ten months after his depressive episode, Mr. J has had no further admissions at the hospital where he received the treatment described here.
Bottom Line
Evidence for the treatment of major depressive disorder induced by corticosteroid withdrawal is limited. Despite trials of agents from multiple medication classes, the depressive episode may not improve. Adding mirtazapine to a selective serotonin reuptake inhibitor or serotonin-norepinephrine reuptake inhibitor may prove successful.
Related Resources
- Watanabe N, Omori IM, Nakagawa A, et al. Mirtazapine versus other antidepressive agents for depression. Cochrane Database Syst Rev. 2011;(12):CD006528.
- Kenna HA, Poon AW, de los Angeles CP, et al. Psychiatric complications of treatment with corticosteroids: review with case report. Psychiatry Clin Neurosci. 2011;65(6):549-560.
Drug Brand Names
Adalimumab • Humira
Aripiprazole • Abilify
Bupropion • Wellbutrin, Zyban
Buspirone • Buspar
Clonazepam • Klonopin
Diazepam • Valium
Diphenhydramine • Benadryl
Divalproex • Depakote, Depakote ER
Lithium • Eskalith, Lithobid
Mirtazapine • Remeron
Prednisone • Deltasone
Sertraline • Zoloft
CASE Suicidal ideation, flare-up of ulcerative colitis
Mr. J, age 56, who has a history of major depressive disorder (MDD), generalized anxiety disorder (GAD), and ulcerative colitis (UC), presents to the emergency department (ED) with suicidal ideation and a plan to overdose on his medications. He reports no current emotional or financial stressors in his personal life. Home medications documented at the time of his arrival to the ED include sertraline, 100 mg/d, bupropion, 150 mg/d, buspirone, 10 mg 3 times daily, diazepam 10 mg 3 times daily, as needed, adalimumab, 40 mg IM every 2 weeks, and diphenhydramine, 50 mg every night.
A recent flare-up of UC resulted in Mr. J being placed on a 15-week prednisone taper, beginning at 80 mg/d and decreasing by 5 mg weekly, which was completed 2 weeks before he presented to the ED. After completing the prednisone taper, Mr. J went to his primary care physician (PCP) on 3 separate occasions due to episodes of severe depression. Although the PCP prescribed multiple medications to target Mr. J’s depressive symptoms, he continued to decline.
Subsequently, Mr. J came to the ED and is admitted to the psychiatric unit for safety and stabilization. Upon admission, Mr. J becomes bedridden, and reports that his current depressive episode is the most severe that he has ever experienced in his more than 30 years of having MDD. He says that neither bupropion nor buspirone are helping with his depression, anxiety, or any related symptom.
[polldaddy:10120537]
The authors’ observations
At admission, all of Mr. J’s home medications, except sertraline and adalimumab, which had been prescribed to treat UC (Box1,2), were discontinued. His diazepam was discontinued because the clinician felt it may have been contributing to Mr. J’s inability to walk or get out of bed. Diazepam was not tapered because it was initiated 7 days prior to admission and was thought to be exacerbating his depression and suicidal ideation. Bupropion and buspirone, which were initiated 2 weeks prior, were discontinued because Mr. J reported that neither medication was helping with his depression, anxiety, or any related symptom.
Box
Ulcerative colitis and depressive episodes
Ulcerative colitis (UC) is a chronic condition associated with inflammation in the colon causing extreme abdominal discomfort during acute flare-ups. Moderate to severe UC flare-ups are commonly treated with corticosteroids due to these medications’ anti-inflammatory properties. Although rare, corticosteroid withdrawal has been documented to induce episodes of depression. The pathophysiology of corticosteroid withdrawal inducing neuropsychiatric sequelae remains unclear; however, it is thought to be due to hypothalamic-pituitary-adrenocortical suppression.1 Fardet et al2 concluded that incident rates per 100 person-years at risk during the withdrawal period were 11.1 (95% confidence interval, 10.0, 12.3) for depression.
EVALUATION Poor appetite, anxiety, and continued suicidality
During evaluation, vital signs, laboratory findings, and diagnostic testing are found to be unremarkable. Mr. J’s presentation and complaints are entirely subjective, and include poor appetite, fatigue, difficulty sleeping, sorrow, anxiety, and continued suicidality. Mr. J reports that he feels miserable, which is reflected by his poor eye contact, soft speech, and body language.
Continued to: The authors' observations
The authors’ observations
MDD is a mood disorder characterized by depressed mood and/or loss of interest or pleasure for more than 2 weeks.3 First-line pharmacotherapy for MDD includes monotherapy with a selective serotonin reuptake inhibitor (SSRI), serotonin-norepinephrine reuptake inhibitor (SNRI), mirtazapine, or bupropion.4 Medication selection is typically based on patient-specific factors, adverse effect profile, drug–drug interactions, and cost. Other treatments include electroconvulsive therapy (ECT) or cognitive-behavioral therapy (CBT).4,5 Augmentation agents, such as second-generation antipsychotics, lithium, thyroid hormone supplementation, buspirone, anticonvulsants, and combinations of antidepressants, may also be considered.4
TREATMENT Condition worsens
On Day 2 of hospitalization, Mr. J is started on aripiprazole, 5 mg/d, clonazepam, 1 mg twice daily, and melatonin, 5 mg, each night for sleep. Aripiprazole, 5 mg/d, is initiated as an adjunct to sertraline for MDD because Mr. J reports feeling much worse and continues to report that he would “rather die than feel this way.” Mr. J begins to believe that his current state is his new baseline, and that feeling better is no longer possible.
On Day 3 of hospitalization, records are obtained from a clinician at an outside facility who previously treated Mr
By Day 8 of hospitalization, there is no notable change in Mr. J’s depressive symptoms. On Day 9, sertraline is increased to 200 mg/d, with little improvement from Mr. J’s perspective. The multidisciplinary team evaluates him, and when directly asked, Mr. J cites his 4 greatest complaints to be poor sleep, fatigue, no appetite, and depressed mood. Once again, he states, “I would rather be dead than go on feeling this way.”
[polldaddy:10120587]
The authors’ observations
Due to Mr. J’s severe, unrelenting depressive episode, the treatment team obtained his informed consent to undergo ECT. On Day 9, before initiating ECT, the pharmacist recommended mirtazapine, even though the patient weighed almost 89 kg (196.21 lb) and had a body mass index of 27.8 kg/m2. The treatment team thought that mirtazapine augmentation could potentially help the sertraline work more quickly while targeting Mr. J’s 4 greatest complaints.
Mirtazapine is a central alpha-2 antagonist or noradrenergic and specific serotonergic antidepressant (NaSSA) that works through antagonism of the presynaptic alpha-2 adrenergic receptors to indirectly regulate release of monoamines and increase the release of serotonin and norepinephrine.6 Additionally, mirtazapine has antagonist actions at 5HT2A, 5HT2C, 5HT3, and histamine-1 receptors.6 Potential adverse effects include drowsiness and increased appetite leading to weight gain.7 Mirtazapine’s therapeutic efficacy is similar to SSRIs for treating depression.4 Mirtazapine in combination with an SNRI has been referred to as “California rocket fuel” due to the theoretical pharmacologic synergy and resulting strong antidepressant action.6 It was hypothesized that similar effects could be seen by augmenting the SSRI sertraline with mirtazapine.
Continued to: The time to efficacy with mirtazapine...
The time to efficacy with mirtazapine is approximately 2 to 4 weeks, but anxiety symptoms and poor sleep or insomnia may improve in the first week.8 Studies have suggested the possibility of a more rapid onset of efficacy with mirtazapine than with SSRIs, as well as potential response acceleration in MDD and other psychiatric illnesses such as anxiety disorders or obsessive-compulsive disorder (OCD).9,10 A review that included several double-blind studies and compared mirtazapine with SSRIs found the amount of responders with persistent improvement with onset in Week 1 was more pronounced with mirtazapine.9
Augmenting an SSRI with mirtazapine is a potential therapeutic option because it can help boost the efficacy of the prescribed SSRI while enhancing appetite and blunting the activating or anxiety-like effects of some SSRIs, which may help with relaxation and sleep.4 The combination of an SSRI plus mirtazapine has been studied in patients with MDD, posttraumatic stress disorder, and OCD; it was found to improve symptoms of those conditions due to the medications’ complementary mechanisms of action.4,11-13 Also, mirtazapine has been shown to decrease the rates of relapse after an acute phase of depression.4,14
OUTCOME Rapid improvement
On Day 9, Mr. J receives the first dose of mirtazapine, 7.5 mg at bedtime. On Day 10, when Mr. J wakes, his mood is notably improved. He is more interactive (sitting up in bed reading and making eye contact with the staff during an interview), and he reports improved sleep and eats most of his breakfast.
After receiving 3 doses of mirtazapine, Mr. J reports that he feels back to his normal self; he is interactive, alert, and eating well. Due to the rapid improvement in mood, ECT is discontinued, and he does not receive any ECT treatment during the remainder of his hospitalization.
On Day 11, divalproex is discontinued. Because Mr. J receives only 5 days of therapy with this agent, his divalproex level is not checked. At this point, the treatment team feels confident in ruling out bipolar disorder.
On Day 15, Mr. J is discharged with sertraline, 200 mg/d, mirtazapine, 7.5 mg/d at 7
Ten months after his depressive episode, Mr. J has had no further admissions at the hospital where he received the treatment described here.
Bottom Line
Evidence for the treatment of major depressive disorder induced by corticosteroid withdrawal is limited. Despite trials of agents from multiple medication classes, the depressive episode may not improve. Adding mirtazapine to a selective serotonin reuptake inhibitor or serotonin-norepinephrine reuptake inhibitor may prove successful.
Related Resources
- Watanabe N, Omori IM, Nakagawa A, et al. Mirtazapine versus other antidepressive agents for depression. Cochrane Database Syst Rev. 2011;(12):CD006528.
- Kenna HA, Poon AW, de los Angeles CP, et al. Psychiatric complications of treatment with corticosteroids: review with case report. Psychiatry Clin Neurosci. 2011;65(6):549-560.
Drug Brand Names
Adalimumab • Humira
Aripiprazole • Abilify
Bupropion • Wellbutrin, Zyban
Buspirone • Buspar
Clonazepam • Klonopin
Diazepam • Valium
Diphenhydramine • Benadryl
Divalproex • Depakote, Depakote ER
Lithium • Eskalith, Lithobid
Mirtazapine • Remeron
Prednisone • Deltasone
Sertraline • Zoloft
1. Dixon R, Christy N. On the various forms of corticosteroid withdrawal syndrome. Am J Med. 1980;68(2):224-30.
2. Fardet L, Petersen I, Nazareth I. Suicidal behavior and severe neuropsychiatric disorders following glucocorticoid therapy in primary care. Am J Psychiatry. 2012;169(5):491-497.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder, 3rd ed. Arlington Virginia: American Psychiatric Association. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf. Published October 2010. Accessed March 15, 2017.
5. National Institute for Health and Clinical Excellence (NICE) Clinical Guideline 90. Depression in adults: recognition and management. https://www.nice.org.uk/guidance/cg90. Accessed March 15, 2017.
6. Stahl SM. Stahl’s essential psychopharmacology: neuroscientific basis and practical applications, 4th ed. Cambridge, United Kingdom: Cambridge University Press; 2013;317-322; 363-364.
7. Remeron [package insert]. Whitehouse Station, NJ: Merck & Co., Inc.; 2018.
8. Gorman JM. Mirtazapine: clinical overview. J Clin Psychiatry. 1999;60(suppl 17):9-13; discussion 46-48.
9. Quitkin FM, Taylor BP, Kremer C. Does mirtazapine have a more rapid onset than SSRIs? J Clin Psychiatry. 2001;62(5):358-361.
10. Pallanti S, Quercioli L, Bruscoli M. Response acceleration with mirtazapine augmentation of citalopram in obsessive-compulsive disorder patients without comorbid depression: a pilot study. J Clin Psychiatry. 2004;65(10):1394-1399.
11. Blier P, Gobbi G, Turcotte JE, et al. Mirtazapine and paroxetine in major depression: a comparison of monotherapy versus their combination from treatment initiation. Eur Neuropsychopharmacol. 2009;19(7):457-465.
12. Blier P, Ward HE, Tremblay P, et al. Combination of antidepressant medications from treatment initiation for major depressive disorder: a double-blind randomized study. Am J Psychiatry. 2010;167(3):281-288.
13. Carpenter LL, Yasmin S, Price LH. A double-blind, placebo-controlled study of antidepressant augmentation with mirtazapine. Biol Psychiatry. 2002;51(2):183-188.
14. Schneier FR, Campeas R, Carcamo J, et al. Combined mirtazapine and SSRI treatment of PTSD: a placebo-controlled trial. Depress Anxiety. 2015;32(8):570-579.
1. Dixon R, Christy N. On the various forms of corticosteroid withdrawal syndrome. Am J Med. 1980;68(2):224-30.
2. Fardet L, Petersen I, Nazareth I. Suicidal behavior and severe neuropsychiatric disorders following glucocorticoid therapy in primary care. Am J Psychiatry. 2012;169(5):491-497.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder, 3rd ed. Arlington Virginia: American Psychiatric Association. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf. Published October 2010. Accessed March 15, 2017.
5. National Institute for Health and Clinical Excellence (NICE) Clinical Guideline 90. Depression in adults: recognition and management. https://www.nice.org.uk/guidance/cg90. Accessed March 15, 2017.
6. Stahl SM. Stahl’s essential psychopharmacology: neuroscientific basis and practical applications, 4th ed. Cambridge, United Kingdom: Cambridge University Press; 2013;317-322; 363-364.
7. Remeron [package insert]. Whitehouse Station, NJ: Merck & Co., Inc.; 2018.
8. Gorman JM. Mirtazapine: clinical overview. J Clin Psychiatry. 1999;60(suppl 17):9-13; discussion 46-48.
9. Quitkin FM, Taylor BP, Kremer C. Does mirtazapine have a more rapid onset than SSRIs? J Clin Psychiatry. 2001;62(5):358-361.
10. Pallanti S, Quercioli L, Bruscoli M. Response acceleration with mirtazapine augmentation of citalopram in obsessive-compulsive disorder patients without comorbid depression: a pilot study. J Clin Psychiatry. 2004;65(10):1394-1399.
11. Blier P, Gobbi G, Turcotte JE, et al. Mirtazapine and paroxetine in major depression: a comparison of monotherapy versus their combination from treatment initiation. Eur Neuropsychopharmacol. 2009;19(7):457-465.
12. Blier P, Ward HE, Tremblay P, et al. Combination of antidepressant medications from treatment initiation for major depressive disorder: a double-blind randomized study. Am J Psychiatry. 2010;167(3):281-288.
13. Carpenter LL, Yasmin S, Price LH. A double-blind, placebo-controlled study of antidepressant augmentation with mirtazapine. Biol Psychiatry. 2002;51(2):183-188.
14. Schneier FR, Campeas R, Carcamo J, et al. Combined mirtazapine and SSRI treatment of PTSD: a placebo-controlled trial. Depress Anxiety. 2015;32(8):570-579.
Vitamin B6 for tardive dyskinesia?
Although antipsychotics have revolutionized the treatment of severe mental illnesses, adverse effects often present a substantial obstacle to adherence. One of the most tenacious and difficult-to-treat adverse effects is tardive dyskinesia (TD), a neuromotor syndrome with characteristic involuntary repetitive movements, typically of the muscles of the jaw, lips, and tongue. In addition to spasms and grimacing, patients can have choreoathetoid movements of the neck. In more extreme presentations, some patients can have difficulty breathing. TD is a largely irreversible condition. It is often a disfiguring lifelong disability that can further stigmatize patients who already suffer scorn and derision. TD usually has a delayed onset after a patient is started on an antipsychotic.1 The syndrome is more commonly associated with first-generation antipsychotics, but affects up to 20% of patients who are treated with second-generation antipsychotics.1 In the United States, TD affects as many as 500,000 patients.1
There are several palliative interventions for TD, but the evidence for a consistently reliable treatment is weak. Branched-chain amino acids, ginkgo biloba, melatonin, and vitamin E have been investigated as interventions. Other approaches include switching to an alternate antipsychotic such as clozapine, adjusting the antipsychotic dose, using anticholinergic medications, adjunctive amantadine, gamma aminobutyric acid agonists, or adding tetrabenazine.
The FDA recently approved two vesicular monoamine transporter 2 (VMAT2) inhibitors, deutetrabenazine and valbenazine, for addressing symptoms of TD. However, these medications can cost tens of thousands of dollars per year, and also carry the risk of adverse effects such as sedation, akathisia, urinary retention, constipation, and muscle pain.2 When treating a patient who develops TD, one might consider other potentially effective therapies with low adverse effect profiles that may be more cost-effective than existing treatments. The bioactive form of vitamin B6 (pyridoxine), pyridoxal-5-phosphate, has been used to treat various antipsychotic-induced movement disorders. Preliminary evidence suggests that vitamin B6 may help reduce the symptoms of TD.
A recent Cochrane Database Review (2015)3 of pyridoxal-5-phosphate treatment for TD found a significant improvement in symptoms compared with placebo. Although the studies included in this review were limited by modest sample sizes and short follow-up periods, 2 of the investigations revealed improvements of >40% in extrapyramidal symptoms with vitamin B6 compared with placebo. Lerner et al (2001)4 conducted a randomized, double-blind, placebo-controlled crossover trial in which 15 inpatients with schizophrenia who met the criteria for TD were assigned to vitamin B6, 400 mg/d, or placebo for 4 weeks. After a 2-week washout period, the placebo group was given vitamin B6 and vice versa. Compared with placebo, mean scores on the parkinsonism and dyskinetic movement subscales of the Extrapyramidal Symptom Rating Scale were significantly better in the third week of treatment with vitamin B6.
Lerner et al (2007)5 later conducted a separate crossover study using the same design with a washout period. This trial included a larger sample size (50 inpatients with DSM-IV diagnoses of schizophrenia or schizoaffective disorder and TD) and the dosage of vitamin B6 was increased to 1,200 mg/d over 26 weeks. Patients who received vitamin B6 experienced a significantly greater decrease in Extrapyramidal Symptom Rating Scale scores compared with those in the placebo group.
Continued to: A 29-year-old woman with treatment-resistant schizophrenia...
Umar et al (2016)6 published a case review of a 29-year-old woman with treatment-resistant schizophrenia with TD who was treated with clozapine, 400 mg/d. She was started on vitamin B6, 450 mg/d, for 4 weeks, and then her dose was increased to 600 mg/d. At 6 months, she experienced a 78% reduction in the severity of her TD symptoms, as measured by the Abnormal Involuntary Movement Scale. The authors reported that this improvement was maintained for 1 year after vitamin B6 was stopped.
Miodownik et al (2008)7 reported in a study of 89 patients with schizophrenia that those with TD (n = 40) had diminished amounts of vitamin B6 in their plasma compared with patients without symptoms of motor disturbances (n = 49).
Vitamin B6 has been known to improve other psychotropic-induced movement disorders. In a study of lithium-induced tremors, treatment with pyridoxine, 900 to 1,200 mg/d, resulted in “impressive improvement until total disappearance of tremor.”8 Lerner et al (2004)9 also reported significant improvement for patients with neuroleptic-induced akathisia who were treated with vitamin B6.
Some proposed mechanisms of action
Pyridoxal-5-phosphate is a coenzyme in the synthesis of dopamine and other neurotransmitters. This might explain in part the biochemical mechanism of vitamin B6 in attenuating motor symptoms following long-term dopamine blockade. Chronic neurotransmitter antagonism may result in an upregulation of dopamine receptors in response. This compensatory reaction might create a dopamine receptor super-sensitivity in the nigrostriatal pathways.10
Another potential mechanism of action might be vitamin B6’s potent antioxidant properties and its scavenging of free radicals. The neurotoxicity of oxidative stress has been implicated in various movement disorders and psychiatric conditions.
In all of the studies described here, patients continued to receive daily antipsychotic treatment. In these trials, the adverse effects of vitamin B6 were minimal or negligible. In one study, vitamin B6 was reported to have had a better adverse effect profile than placebo.4
1. Carbon M, Hsieh CH, Kane JM, et al. Tardive dyskinesia prevalence in the period of second-generation antipsychotic use: a meta-analysis. J Clin Psychiatry. 2017;78(3):e264-e278.
2. Smith Mosley LL, Mosely II JF, Fleischfresser JR, et al. Vesicular monoamine transporter type 2 (VMAT2) inhibitors in the management of tardive dyskinesia. Clin Med Rev Case Rep. 2017;4(12):1-5.
3. Adelufosi AO, Abayomi O, Ojo M. Pyridoxal 5 phosphate for neuroleptic-induced tardive dyskinesia. Cochrane Database Syst Rev. 2015;(4):CD010501.
4. Lerner V, Miodownik C, Kapstan A, et al. Vitamin B(6) in the treatment of tardive dyskinesia: a double-blind, placebo-controlled, crossover study. Am J Psychiatry. 2001;158(9):1511-1514.
5. Lerner V, Miodownik C, Kapstan A, et al. Vitamin B6 treatment for tardive dyskinesia: a randomized, double-blind, placebo-controlled, crossover study. J Clin Psychiatry. 2007;68(11):1648-1654.
6. Umar MU, Isa AA, Abba AH. High dose pyridoxine for the treatment of tardive dyskinesia: clinical case and review of literature. Ther Adv Psychopharmacol. 2016;6(2):152-156.
7. Miodownik C, Meoded A, Libov I, et al. Pyridoxal plasma level in schizophrenic and schizoaffective patients with and without tardive dyskinesia. Clin Neuropharmacol. 2008;31(4):197-203.
8. Miodownik C, Witztum E, Lerner V. Lithium-induced tremor treated with vitamin B6: a preliminary case series. Int J Psychiatry Med. 2002;32(1):103-108.
9. Lerner V, Bergman J, Statsenko N, et al. Vitamin B6 treatment in acute neuroleptic-induced akathisia: a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2004;65(11):1550-1554.
10. Miller, BJ. Tardive dyskinesia: a review of the literature. Psychiatric Times. http://www.psychiatrictimes.com/articles/tardive-dyskinesia-review-literature. Published June 27, 2017. Accessed July 31, 2018.
Although antipsychotics have revolutionized the treatment of severe mental illnesses, adverse effects often present a substantial obstacle to adherence. One of the most tenacious and difficult-to-treat adverse effects is tardive dyskinesia (TD), a neuromotor syndrome with characteristic involuntary repetitive movements, typically of the muscles of the jaw, lips, and tongue. In addition to spasms and grimacing, patients can have choreoathetoid movements of the neck. In more extreme presentations, some patients can have difficulty breathing. TD is a largely irreversible condition. It is often a disfiguring lifelong disability that can further stigmatize patients who already suffer scorn and derision. TD usually has a delayed onset after a patient is started on an antipsychotic.1 The syndrome is more commonly associated with first-generation antipsychotics, but affects up to 20% of patients who are treated with second-generation antipsychotics.1 In the United States, TD affects as many as 500,000 patients.1
There are several palliative interventions for TD, but the evidence for a consistently reliable treatment is weak. Branched-chain amino acids, ginkgo biloba, melatonin, and vitamin E have been investigated as interventions. Other approaches include switching to an alternate antipsychotic such as clozapine, adjusting the antipsychotic dose, using anticholinergic medications, adjunctive amantadine, gamma aminobutyric acid agonists, or adding tetrabenazine.
The FDA recently approved two vesicular monoamine transporter 2 (VMAT2) inhibitors, deutetrabenazine and valbenazine, for addressing symptoms of TD. However, these medications can cost tens of thousands of dollars per year, and also carry the risk of adverse effects such as sedation, akathisia, urinary retention, constipation, and muscle pain.2 When treating a patient who develops TD, one might consider other potentially effective therapies with low adverse effect profiles that may be more cost-effective than existing treatments. The bioactive form of vitamin B6 (pyridoxine), pyridoxal-5-phosphate, has been used to treat various antipsychotic-induced movement disorders. Preliminary evidence suggests that vitamin B6 may help reduce the symptoms of TD.
A recent Cochrane Database Review (2015)3 of pyridoxal-5-phosphate treatment for TD found a significant improvement in symptoms compared with placebo. Although the studies included in this review were limited by modest sample sizes and short follow-up periods, 2 of the investigations revealed improvements of >40% in extrapyramidal symptoms with vitamin B6 compared with placebo. Lerner et al (2001)4 conducted a randomized, double-blind, placebo-controlled crossover trial in which 15 inpatients with schizophrenia who met the criteria for TD were assigned to vitamin B6, 400 mg/d, or placebo for 4 weeks. After a 2-week washout period, the placebo group was given vitamin B6 and vice versa. Compared with placebo, mean scores on the parkinsonism and dyskinetic movement subscales of the Extrapyramidal Symptom Rating Scale were significantly better in the third week of treatment with vitamin B6.
Lerner et al (2007)5 later conducted a separate crossover study using the same design with a washout period. This trial included a larger sample size (50 inpatients with DSM-IV diagnoses of schizophrenia or schizoaffective disorder and TD) and the dosage of vitamin B6 was increased to 1,200 mg/d over 26 weeks. Patients who received vitamin B6 experienced a significantly greater decrease in Extrapyramidal Symptom Rating Scale scores compared with those in the placebo group.
Continued to: A 29-year-old woman with treatment-resistant schizophrenia...
Umar et al (2016)6 published a case review of a 29-year-old woman with treatment-resistant schizophrenia with TD who was treated with clozapine, 400 mg/d. She was started on vitamin B6, 450 mg/d, for 4 weeks, and then her dose was increased to 600 mg/d. At 6 months, she experienced a 78% reduction in the severity of her TD symptoms, as measured by the Abnormal Involuntary Movement Scale. The authors reported that this improvement was maintained for 1 year after vitamin B6 was stopped.
Miodownik et al (2008)7 reported in a study of 89 patients with schizophrenia that those with TD (n = 40) had diminished amounts of vitamin B6 in their plasma compared with patients without symptoms of motor disturbances (n = 49).
Vitamin B6 has been known to improve other psychotropic-induced movement disorders. In a study of lithium-induced tremors, treatment with pyridoxine, 900 to 1,200 mg/d, resulted in “impressive improvement until total disappearance of tremor.”8 Lerner et al (2004)9 also reported significant improvement for patients with neuroleptic-induced akathisia who were treated with vitamin B6.
Some proposed mechanisms of action
Pyridoxal-5-phosphate is a coenzyme in the synthesis of dopamine and other neurotransmitters. This might explain in part the biochemical mechanism of vitamin B6 in attenuating motor symptoms following long-term dopamine blockade. Chronic neurotransmitter antagonism may result in an upregulation of dopamine receptors in response. This compensatory reaction might create a dopamine receptor super-sensitivity in the nigrostriatal pathways.10
Another potential mechanism of action might be vitamin B6’s potent antioxidant properties and its scavenging of free radicals. The neurotoxicity of oxidative stress has been implicated in various movement disorders and psychiatric conditions.
In all of the studies described here, patients continued to receive daily antipsychotic treatment. In these trials, the adverse effects of vitamin B6 were minimal or negligible. In one study, vitamin B6 was reported to have had a better adverse effect profile than placebo.4
Although antipsychotics have revolutionized the treatment of severe mental illnesses, adverse effects often present a substantial obstacle to adherence. One of the most tenacious and difficult-to-treat adverse effects is tardive dyskinesia (TD), a neuromotor syndrome with characteristic involuntary repetitive movements, typically of the muscles of the jaw, lips, and tongue. In addition to spasms and grimacing, patients can have choreoathetoid movements of the neck. In more extreme presentations, some patients can have difficulty breathing. TD is a largely irreversible condition. It is often a disfiguring lifelong disability that can further stigmatize patients who already suffer scorn and derision. TD usually has a delayed onset after a patient is started on an antipsychotic.1 The syndrome is more commonly associated with first-generation antipsychotics, but affects up to 20% of patients who are treated with second-generation antipsychotics.1 In the United States, TD affects as many as 500,000 patients.1
There are several palliative interventions for TD, but the evidence for a consistently reliable treatment is weak. Branched-chain amino acids, ginkgo biloba, melatonin, and vitamin E have been investigated as interventions. Other approaches include switching to an alternate antipsychotic such as clozapine, adjusting the antipsychotic dose, using anticholinergic medications, adjunctive amantadine, gamma aminobutyric acid agonists, or adding tetrabenazine.
The FDA recently approved two vesicular monoamine transporter 2 (VMAT2) inhibitors, deutetrabenazine and valbenazine, for addressing symptoms of TD. However, these medications can cost tens of thousands of dollars per year, and also carry the risk of adverse effects such as sedation, akathisia, urinary retention, constipation, and muscle pain.2 When treating a patient who develops TD, one might consider other potentially effective therapies with low adverse effect profiles that may be more cost-effective than existing treatments. The bioactive form of vitamin B6 (pyridoxine), pyridoxal-5-phosphate, has been used to treat various antipsychotic-induced movement disorders. Preliminary evidence suggests that vitamin B6 may help reduce the symptoms of TD.
A recent Cochrane Database Review (2015)3 of pyridoxal-5-phosphate treatment for TD found a significant improvement in symptoms compared with placebo. Although the studies included in this review were limited by modest sample sizes and short follow-up periods, 2 of the investigations revealed improvements of >40% in extrapyramidal symptoms with vitamin B6 compared with placebo. Lerner et al (2001)4 conducted a randomized, double-blind, placebo-controlled crossover trial in which 15 inpatients with schizophrenia who met the criteria for TD were assigned to vitamin B6, 400 mg/d, or placebo for 4 weeks. After a 2-week washout period, the placebo group was given vitamin B6 and vice versa. Compared with placebo, mean scores on the parkinsonism and dyskinetic movement subscales of the Extrapyramidal Symptom Rating Scale were significantly better in the third week of treatment with vitamin B6.
Lerner et al (2007)5 later conducted a separate crossover study using the same design with a washout period. This trial included a larger sample size (50 inpatients with DSM-IV diagnoses of schizophrenia or schizoaffective disorder and TD) and the dosage of vitamin B6 was increased to 1,200 mg/d over 26 weeks. Patients who received vitamin B6 experienced a significantly greater decrease in Extrapyramidal Symptom Rating Scale scores compared with those in the placebo group.
Continued to: A 29-year-old woman with treatment-resistant schizophrenia...
Umar et al (2016)6 published a case review of a 29-year-old woman with treatment-resistant schizophrenia with TD who was treated with clozapine, 400 mg/d. She was started on vitamin B6, 450 mg/d, for 4 weeks, and then her dose was increased to 600 mg/d. At 6 months, she experienced a 78% reduction in the severity of her TD symptoms, as measured by the Abnormal Involuntary Movement Scale. The authors reported that this improvement was maintained for 1 year after vitamin B6 was stopped.
Miodownik et al (2008)7 reported in a study of 89 patients with schizophrenia that those with TD (n = 40) had diminished amounts of vitamin B6 in their plasma compared with patients without symptoms of motor disturbances (n = 49).
Vitamin B6 has been known to improve other psychotropic-induced movement disorders. In a study of lithium-induced tremors, treatment with pyridoxine, 900 to 1,200 mg/d, resulted in “impressive improvement until total disappearance of tremor.”8 Lerner et al (2004)9 also reported significant improvement for patients with neuroleptic-induced akathisia who were treated with vitamin B6.
Some proposed mechanisms of action
Pyridoxal-5-phosphate is a coenzyme in the synthesis of dopamine and other neurotransmitters. This might explain in part the biochemical mechanism of vitamin B6 in attenuating motor symptoms following long-term dopamine blockade. Chronic neurotransmitter antagonism may result in an upregulation of dopamine receptors in response. This compensatory reaction might create a dopamine receptor super-sensitivity in the nigrostriatal pathways.10
Another potential mechanism of action might be vitamin B6’s potent antioxidant properties and its scavenging of free radicals. The neurotoxicity of oxidative stress has been implicated in various movement disorders and psychiatric conditions.
In all of the studies described here, patients continued to receive daily antipsychotic treatment. In these trials, the adverse effects of vitamin B6 were minimal or negligible. In one study, vitamin B6 was reported to have had a better adverse effect profile than placebo.4
1. Carbon M, Hsieh CH, Kane JM, et al. Tardive dyskinesia prevalence in the period of second-generation antipsychotic use: a meta-analysis. J Clin Psychiatry. 2017;78(3):e264-e278.
2. Smith Mosley LL, Mosely II JF, Fleischfresser JR, et al. Vesicular monoamine transporter type 2 (VMAT2) inhibitors in the management of tardive dyskinesia. Clin Med Rev Case Rep. 2017;4(12):1-5.
3. Adelufosi AO, Abayomi O, Ojo M. Pyridoxal 5 phosphate for neuroleptic-induced tardive dyskinesia. Cochrane Database Syst Rev. 2015;(4):CD010501.
4. Lerner V, Miodownik C, Kapstan A, et al. Vitamin B(6) in the treatment of tardive dyskinesia: a double-blind, placebo-controlled, crossover study. Am J Psychiatry. 2001;158(9):1511-1514.
5. Lerner V, Miodownik C, Kapstan A, et al. Vitamin B6 treatment for tardive dyskinesia: a randomized, double-blind, placebo-controlled, crossover study. J Clin Psychiatry. 2007;68(11):1648-1654.
6. Umar MU, Isa AA, Abba AH. High dose pyridoxine for the treatment of tardive dyskinesia: clinical case and review of literature. Ther Adv Psychopharmacol. 2016;6(2):152-156.
7. Miodownik C, Meoded A, Libov I, et al. Pyridoxal plasma level in schizophrenic and schizoaffective patients with and without tardive dyskinesia. Clin Neuropharmacol. 2008;31(4):197-203.
8. Miodownik C, Witztum E, Lerner V. Lithium-induced tremor treated with vitamin B6: a preliminary case series. Int J Psychiatry Med. 2002;32(1):103-108.
9. Lerner V, Bergman J, Statsenko N, et al. Vitamin B6 treatment in acute neuroleptic-induced akathisia: a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2004;65(11):1550-1554.
10. Miller, BJ. Tardive dyskinesia: a review of the literature. Psychiatric Times. http://www.psychiatrictimes.com/articles/tardive-dyskinesia-review-literature. Published June 27, 2017. Accessed July 31, 2018.
1. Carbon M, Hsieh CH, Kane JM, et al. Tardive dyskinesia prevalence in the period of second-generation antipsychotic use: a meta-analysis. J Clin Psychiatry. 2017;78(3):e264-e278.
2. Smith Mosley LL, Mosely II JF, Fleischfresser JR, et al. Vesicular monoamine transporter type 2 (VMAT2) inhibitors in the management of tardive dyskinesia. Clin Med Rev Case Rep. 2017;4(12):1-5.
3. Adelufosi AO, Abayomi O, Ojo M. Pyridoxal 5 phosphate for neuroleptic-induced tardive dyskinesia. Cochrane Database Syst Rev. 2015;(4):CD010501.
4. Lerner V, Miodownik C, Kapstan A, et al. Vitamin B(6) in the treatment of tardive dyskinesia: a double-blind, placebo-controlled, crossover study. Am J Psychiatry. 2001;158(9):1511-1514.
5. Lerner V, Miodownik C, Kapstan A, et al. Vitamin B6 treatment for tardive dyskinesia: a randomized, double-blind, placebo-controlled, crossover study. J Clin Psychiatry. 2007;68(11):1648-1654.
6. Umar MU, Isa AA, Abba AH. High dose pyridoxine for the treatment of tardive dyskinesia: clinical case and review of literature. Ther Adv Psychopharmacol. 2016;6(2):152-156.
7. Miodownik C, Meoded A, Libov I, et al. Pyridoxal plasma level in schizophrenic and schizoaffective patients with and without tardive dyskinesia. Clin Neuropharmacol. 2008;31(4):197-203.
8. Miodownik C, Witztum E, Lerner V. Lithium-induced tremor treated with vitamin B6: a preliminary case series. Int J Psychiatry Med. 2002;32(1):103-108.
9. Lerner V, Bergman J, Statsenko N, et al. Vitamin B6 treatment in acute neuroleptic-induced akathisia: a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2004;65(11):1550-1554.
10. Miller, BJ. Tardive dyskinesia: a review of the literature. Psychiatric Times. http://www.psychiatrictimes.com/articles/tardive-dyskinesia-review-literature. Published June 27, 2017. Accessed July 31, 2018.
Proactive consultation: A new model of care in consultation-liaison psychiatry
During my residency training, I was trained in the standard “reactive” psychiatric consultation model. In this system, I would see consults placed by the primary team after they identified a behavioral issue in a patient. As a trainee, I experienced frequent frustrations working in this model: Consults that are discharge-dependent (“Can you see the patient before he is discharged this morning?”), consults for acute behavioral dysregulation (“The patient is near the elevator, can you come see him ASAP?”), or consults for consequences of poor management of alcohol/benzodiazepine withdrawal (“The patient is confused and trying to leave”).
As a fellow in consultation-liaison (C-L) psychiatry, I was introduced to the “proactive” consultation model, which avoids some of these issues. In this article, which is intended for residents who have not been exposed to this new approach, I explain how the proactive model changes our experience as C-L clinicians.
The Behavioral Intervention Team
At Yale New Haven Hospital, the Behavioral Intervention Team (BIT) is a proactive, multidisciplinary psychiatric consultation service that serves the internal medicine units at the hospital. The team consists of nurse practitioners, nurse liaison specialists, social workers, and psychiatrists. The team identifies and removes behavioral barriers in the care of hospitalized mentally ill patients.
The BIT collaborates closely with the medical team through formal and informal consultation; co-management of behavioral issues; education of medical, nursing, and social work staff; and direct care of complex patients with behavioral disorders. The BIT assists the medical team with transitions to appropriate outpatient and inpatient psychiatric care. The team also manages the relationship with the insurer when a patient requires a stay in a psychiatric unit.
This model has a critical financial benefit in reducing the length of stay, but it also has many other benefits. It focuses on early recognition and treatment, and helps mitigate the effects of mental or substance use disorders on patients’ recovery. BIT members educate their peers regarding management of a multitude of behavioral issues. This fosters extensive informal collaboration (“curbside consultation”), which helps patients who did not receive a formal consult. The model distributes work more rationally among different professional specialists. It yields a relationship with medical teams that is not only more effective, but also more enjoyable. In the BIT model, psychiatrists pick the cases where they feel they can have the most impact, and avoid the cases they feel they cannot have any.1-3
CASE A better approach to alcohol withdrawal
Mr. X, age 56, has a history of alcohol use disorder, hypertension, and coronary artery disease. He’s had multiple past admissions for complicated alcohol withdrawal. He is transferred from a local community hospital, where he had presented with chest pain. His last drink was 2 days prior to admission, and his blood alcohol level is <10 mg/dL.
During Mr. X’s previous hospitalizations, psychiatric consults were performed in the standard reactive model. The primary team initially prescribed an ineffective dosage of benzodiazepines for his alcohol withdrawal. This escalated his withdrawal into delirium tremens, after which psychiatry was involved. Due to this early ineffective management, the patient had a prolonged medical ICU stay and overall stay, experienced increased medical complications, and required increased staff resources because he was extremely agitated.
Continued to: During this hospitalization...
During this hospitalization, Mr. X arrives with similar medical complaints. The nurse practitioner on the BIT service, who screened all admissions each day, examines the prior notes (she finds the team sign-outs to be particularly useful). She suggests a psychiatric consult on Day 1 of the admission, which the primary medical team orders. The BIT nurse practitioner gives apt recommendations of evidence-based management, including a benzodiazepine taper, high-dose thiamine, and psychopharmacologic approaches to severe agitation. The nurse liaison specialist on the service makes behavioral plans for managing agitation, which she communicates to the nurses caring for Mr. X.
Because his withdrawal is managed more promptly, Mr. X’s length of stay is shorter and he does not experience any medical complications. The BIT social worker helps find appropriate aftercare options, including residential treatment and Alcoholics Anonymous meetings, to which the patient agrees.
Participating in this case was highly educational for me as a trainee. This case is but one example among many where proactive consultation provided prompt care, lowered the rate of complications, reduced length of stay, and resulted in greater provider satisfaction. The Table4 contrasts the proactive and reactive consultation models. The following 5 factors are critical in the proactive consultation model4,5:
1. Standardized and reliable procedure screening of all admissions, involving a mental health professional, through record review and staff contact. This screening should identify patients with issues who will benefit specifically from in-hospital services, rather than just patients with any psychiatric issue. An electronic medical record is essential to efficient screening, team communication, and progress monitoring. Truly integrated consultation would be impossible with a paper chart.
Continued to: 2. Rapid intervention...
2. Rapid intervention that anticipates impending problems before a cascade of complications starts.
3. Collaborative engagement with the primary medical team, sharing the burden of caring for the complex inpatient, and transmitting critical behavioral management skills to all caregivers, including the skill of recognizing patients who can benefit from a psychiatric consultation.
4. Daily and close contact between behavioral and medical teams, ensuring that treatment recommendations are understood, enacted, and reinforced, ineffective treatments are discontinued, and new problems are addressed before complicating consequences arise. Dedicating specific personnel to specific hospital units and placing them in rounds simplifies communication and speeds intervention implementation.
5. A multidisciplinary consultation team, offering a range of responses, including informal curbside consultation, consultation with an advanced practice registered nurse, social work interventions, advice to discharge planning teams, psychological services, and access to specialized providers, such as addiction teams, as well as traditional consultation with an experienced psychiatrist.
Research has shown the effectiveness of proactive, embedded, multidisciplinary approaches.1-3,5 It was a gratifying experience to work in this model. I worked intimately with medical clinicians, and shared the burden of responsibilities leading to optimal patient outcomes. The proactive consultation model truly re-emphasizes the “liaison” component of C-L psychiatry, as it was originally envisioned.
1. Sledge WH, Gueorguieva R, Desan P, et al. Multidisciplinary proactive psychiatric consultation service: impact on length of stay for medical inpatients. Psychother Psychosom. 2015;84(4):208-216.
2. Desan PH, Zimbrean PC, Weinstein AJ, et al. Proactive psychiatric consultation services reduce length of stay for admissions to an inpatient medical team. Psychosomatics. 2011;52(6):513-520.
3. Sledge WH, Bozzo J, White-McCullum BA, et al. The cost-benefit from the perspective of the hospital of a proactive psychiatric consultation service on inpatient general medicine services. Health Econ Outcome Res Open Access. 2016;2(4):122.
4. Sledge WH, Lee HB. Proactive psychiatric consultation for hospitalized patients, a plan for the future. Health Affairs. www.healthaffairs.org/do/10.1377/hblog20150528.048026/full/. Published May 28, 2015. Accessed September 12, 2018.
5. Desan P, Lee H, Zimbrean P, et al. New models of psychiatric consultation in the general medical hospital: liaison psychiatry is back. Psychiatr Ann. 2017;47:355-361.
During my residency training, I was trained in the standard “reactive” psychiatric consultation model. In this system, I would see consults placed by the primary team after they identified a behavioral issue in a patient. As a trainee, I experienced frequent frustrations working in this model: Consults that are discharge-dependent (“Can you see the patient before he is discharged this morning?”), consults for acute behavioral dysregulation (“The patient is near the elevator, can you come see him ASAP?”), or consults for consequences of poor management of alcohol/benzodiazepine withdrawal (“The patient is confused and trying to leave”).
As a fellow in consultation-liaison (C-L) psychiatry, I was introduced to the “proactive” consultation model, which avoids some of these issues. In this article, which is intended for residents who have not been exposed to this new approach, I explain how the proactive model changes our experience as C-L clinicians.
The Behavioral Intervention Team
At Yale New Haven Hospital, the Behavioral Intervention Team (BIT) is a proactive, multidisciplinary psychiatric consultation service that serves the internal medicine units at the hospital. The team consists of nurse practitioners, nurse liaison specialists, social workers, and psychiatrists. The team identifies and removes behavioral barriers in the care of hospitalized mentally ill patients.
The BIT collaborates closely with the medical team through formal and informal consultation; co-management of behavioral issues; education of medical, nursing, and social work staff; and direct care of complex patients with behavioral disorders. The BIT assists the medical team with transitions to appropriate outpatient and inpatient psychiatric care. The team also manages the relationship with the insurer when a patient requires a stay in a psychiatric unit.
This model has a critical financial benefit in reducing the length of stay, but it also has many other benefits. It focuses on early recognition and treatment, and helps mitigate the effects of mental or substance use disorders on patients’ recovery. BIT members educate their peers regarding management of a multitude of behavioral issues. This fosters extensive informal collaboration (“curbside consultation”), which helps patients who did not receive a formal consult. The model distributes work more rationally among different professional specialists. It yields a relationship with medical teams that is not only more effective, but also more enjoyable. In the BIT model, psychiatrists pick the cases where they feel they can have the most impact, and avoid the cases they feel they cannot have any.1-3
CASE A better approach to alcohol withdrawal
Mr. X, age 56, has a history of alcohol use disorder, hypertension, and coronary artery disease. He’s had multiple past admissions for complicated alcohol withdrawal. He is transferred from a local community hospital, where he had presented with chest pain. His last drink was 2 days prior to admission, and his blood alcohol level is <10 mg/dL.
During Mr. X’s previous hospitalizations, psychiatric consults were performed in the standard reactive model. The primary team initially prescribed an ineffective dosage of benzodiazepines for his alcohol withdrawal. This escalated his withdrawal into delirium tremens, after which psychiatry was involved. Due to this early ineffective management, the patient had a prolonged medical ICU stay and overall stay, experienced increased medical complications, and required increased staff resources because he was extremely agitated.
Continued to: During this hospitalization...
During this hospitalization, Mr. X arrives with similar medical complaints. The nurse practitioner on the BIT service, who screened all admissions each day, examines the prior notes (she finds the team sign-outs to be particularly useful). She suggests a psychiatric consult on Day 1 of the admission, which the primary medical team orders. The BIT nurse practitioner gives apt recommendations of evidence-based management, including a benzodiazepine taper, high-dose thiamine, and psychopharmacologic approaches to severe agitation. The nurse liaison specialist on the service makes behavioral plans for managing agitation, which she communicates to the nurses caring for Mr. X.
Because his withdrawal is managed more promptly, Mr. X’s length of stay is shorter and he does not experience any medical complications. The BIT social worker helps find appropriate aftercare options, including residential treatment and Alcoholics Anonymous meetings, to which the patient agrees.
Participating in this case was highly educational for me as a trainee. This case is but one example among many where proactive consultation provided prompt care, lowered the rate of complications, reduced length of stay, and resulted in greater provider satisfaction. The Table4 contrasts the proactive and reactive consultation models. The following 5 factors are critical in the proactive consultation model4,5:
1. Standardized and reliable procedure screening of all admissions, involving a mental health professional, through record review and staff contact. This screening should identify patients with issues who will benefit specifically from in-hospital services, rather than just patients with any psychiatric issue. An electronic medical record is essential to efficient screening, team communication, and progress monitoring. Truly integrated consultation would be impossible with a paper chart.

Continued to: 2. Rapid intervention...
2. Rapid intervention that anticipates impending problems before a cascade of complications starts.
3. Collaborative engagement with the primary medical team, sharing the burden of caring for the complex inpatient, and transmitting critical behavioral management skills to all caregivers, including the skill of recognizing patients who can benefit from a psychiatric consultation.
4. Daily and close contact between behavioral and medical teams, ensuring that treatment recommendations are understood, enacted, and reinforced, ineffective treatments are discontinued, and new problems are addressed before complicating consequences arise. Dedicating specific personnel to specific hospital units and placing them in rounds simplifies communication and speeds intervention implementation.
5. A multidisciplinary consultation team, offering a range of responses, including informal curbside consultation, consultation with an advanced practice registered nurse, social work interventions, advice to discharge planning teams, psychological services, and access to specialized providers, such as addiction teams, as well as traditional consultation with an experienced psychiatrist.
Research has shown the effectiveness of proactive, embedded, multidisciplinary approaches.1-3,5 It was a gratifying experience to work in this model. I worked intimately with medical clinicians, and shared the burden of responsibilities leading to optimal patient outcomes. The proactive consultation model truly re-emphasizes the “liaison” component of C-L psychiatry, as it was originally envisioned.
During my residency training, I was trained in the standard “reactive” psychiatric consultation model. In this system, I would see consults placed by the primary team after they identified a behavioral issue in a patient. As a trainee, I experienced frequent frustrations working in this model: Consults that are discharge-dependent (“Can you see the patient before he is discharged this morning?”), consults for acute behavioral dysregulation (“The patient is near the elevator, can you come see him ASAP?”), or consults for consequences of poor management of alcohol/benzodiazepine withdrawal (“The patient is confused and trying to leave”).
As a fellow in consultation-liaison (C-L) psychiatry, I was introduced to the “proactive” consultation model, which avoids some of these issues. In this article, which is intended for residents who have not been exposed to this new approach, I explain how the proactive model changes our experience as C-L clinicians.
The Behavioral Intervention Team
At Yale New Haven Hospital, the Behavioral Intervention Team (BIT) is a proactive, multidisciplinary psychiatric consultation service that serves the internal medicine units at the hospital. The team consists of nurse practitioners, nurse liaison specialists, social workers, and psychiatrists. The team identifies and removes behavioral barriers in the care of hospitalized mentally ill patients.
The BIT collaborates closely with the medical team through formal and informal consultation; co-management of behavioral issues; education of medical, nursing, and social work staff; and direct care of complex patients with behavioral disorders. The BIT assists the medical team with transitions to appropriate outpatient and inpatient psychiatric care. The team also manages the relationship with the insurer when a patient requires a stay in a psychiatric unit.
This model has a critical financial benefit in reducing the length of stay, but it also has many other benefits. It focuses on early recognition and treatment, and helps mitigate the effects of mental or substance use disorders on patients’ recovery. BIT members educate their peers regarding management of a multitude of behavioral issues. This fosters extensive informal collaboration (“curbside consultation”), which helps patients who did not receive a formal consult. The model distributes work more rationally among different professional specialists. It yields a relationship with medical teams that is not only more effective, but also more enjoyable. In the BIT model, psychiatrists pick the cases where they feel they can have the most impact, and avoid the cases they feel they cannot have any.1-3
CASE A better approach to alcohol withdrawal
Mr. X, age 56, has a history of alcohol use disorder, hypertension, and coronary artery disease. He’s had multiple past admissions for complicated alcohol withdrawal. He is transferred from a local community hospital, where he had presented with chest pain. His last drink was 2 days prior to admission, and his blood alcohol level is <10 mg/dL.
During Mr. X’s previous hospitalizations, psychiatric consults were performed in the standard reactive model. The primary team initially prescribed an ineffective dosage of benzodiazepines for his alcohol withdrawal. This escalated his withdrawal into delirium tremens, after which psychiatry was involved. Due to this early ineffective management, the patient had a prolonged medical ICU stay and overall stay, experienced increased medical complications, and required increased staff resources because he was extremely agitated.
Continued to: During this hospitalization...
During this hospitalization, Mr. X arrives with similar medical complaints. The nurse practitioner on the BIT service, who screened all admissions each day, examines the prior notes (she finds the team sign-outs to be particularly useful). She suggests a psychiatric consult on Day 1 of the admission, which the primary medical team orders. The BIT nurse practitioner gives apt recommendations of evidence-based management, including a benzodiazepine taper, high-dose thiamine, and psychopharmacologic approaches to severe agitation. The nurse liaison specialist on the service makes behavioral plans for managing agitation, which she communicates to the nurses caring for Mr. X.
Because his withdrawal is managed more promptly, Mr. X’s length of stay is shorter and he does not experience any medical complications. The BIT social worker helps find appropriate aftercare options, including residential treatment and Alcoholics Anonymous meetings, to which the patient agrees.
Participating in this case was highly educational for me as a trainee. This case is but one example among many where proactive consultation provided prompt care, lowered the rate of complications, reduced length of stay, and resulted in greater provider satisfaction. The Table4 contrasts the proactive and reactive consultation models. The following 5 factors are critical in the proactive consultation model4,5:
1. Standardized and reliable procedure screening of all admissions, involving a mental health professional, through record review and staff contact. This screening should identify patients with issues who will benefit specifically from in-hospital services, rather than just patients with any psychiatric issue. An electronic medical record is essential to efficient screening, team communication, and progress monitoring. Truly integrated consultation would be impossible with a paper chart.

Continued to: 2. Rapid intervention...
2. Rapid intervention that anticipates impending problems before a cascade of complications starts.
3. Collaborative engagement with the primary medical team, sharing the burden of caring for the complex inpatient, and transmitting critical behavioral management skills to all caregivers, including the skill of recognizing patients who can benefit from a psychiatric consultation.
4. Daily and close contact between behavioral and medical teams, ensuring that treatment recommendations are understood, enacted, and reinforced, ineffective treatments are discontinued, and new problems are addressed before complicating consequences arise. Dedicating specific personnel to specific hospital units and placing them in rounds simplifies communication and speeds intervention implementation.
5. A multidisciplinary consultation team, offering a range of responses, including informal curbside consultation, consultation with an advanced practice registered nurse, social work interventions, advice to discharge planning teams, psychological services, and access to specialized providers, such as addiction teams, as well as traditional consultation with an experienced psychiatrist.
Research has shown the effectiveness of proactive, embedded, multidisciplinary approaches.1-3,5 It was a gratifying experience to work in this model. I worked intimately with medical clinicians, and shared the burden of responsibilities leading to optimal patient outcomes. The proactive consultation model truly re-emphasizes the “liaison” component of C-L psychiatry, as it was originally envisioned.
1. Sledge WH, Gueorguieva R, Desan P, et al. Multidisciplinary proactive psychiatric consultation service: impact on length of stay for medical inpatients. Psychother Psychosom. 2015;84(4):208-216.
2. Desan PH, Zimbrean PC, Weinstein AJ, et al. Proactive psychiatric consultation services reduce length of stay for admissions to an inpatient medical team. Psychosomatics. 2011;52(6):513-520.
3. Sledge WH, Bozzo J, White-McCullum BA, et al. The cost-benefit from the perspective of the hospital of a proactive psychiatric consultation service on inpatient general medicine services. Health Econ Outcome Res Open Access. 2016;2(4):122.
4. Sledge WH, Lee HB. Proactive psychiatric consultation for hospitalized patients, a plan for the future. Health Affairs. www.healthaffairs.org/do/10.1377/hblog20150528.048026/full/. Published May 28, 2015. Accessed September 12, 2018.
5. Desan P, Lee H, Zimbrean P, et al. New models of psychiatric consultation in the general medical hospital: liaison psychiatry is back. Psychiatr Ann. 2017;47:355-361.
1. Sledge WH, Gueorguieva R, Desan P, et al. Multidisciplinary proactive psychiatric consultation service: impact on length of stay for medical inpatients. Psychother Psychosom. 2015;84(4):208-216.
2. Desan PH, Zimbrean PC, Weinstein AJ, et al. Proactive psychiatric consultation services reduce length of stay for admissions to an inpatient medical team. Psychosomatics. 2011;52(6):513-520.
3. Sledge WH, Bozzo J, White-McCullum BA, et al. The cost-benefit from the perspective of the hospital of a proactive psychiatric consultation service on inpatient general medicine services. Health Econ Outcome Res Open Access. 2016;2(4):122.
4. Sledge WH, Lee HB. Proactive psychiatric consultation for hospitalized patients, a plan for the future. Health Affairs. www.healthaffairs.org/do/10.1377/hblog20150528.048026/full/. Published May 28, 2015. Accessed September 12, 2018.
5. Desan P, Lee H, Zimbrean P, et al. New models of psychiatric consultation in the general medical hospital: liaison psychiatry is back. Psychiatr Ann. 2017;47:355-361.
Data-driven prescribing
Computational psychiatry is an emerging field in which artificial intelligence and machine learning are used to find hidden patterns in big data to better understand, predict, and treat mental illness. The field uses various mathematical models to predict the dependent variable y based on the independent variable x. One application of analytics in medicine was the Framingham Heart Study, which used multivariate logistic regression to predict heart disease.1
Analytics could be used to predict the number of bad outcomes associated with different psychiatric medications over time. To demonstrate this, I examined a select data set of 8 psychiatric medications (aripiprazole, ziprasidone, risperidone, olanzapine, sertraline, trazodone, amitriptyline, and lithium) accounting for 59,827 bad outcomes during a 15-year period as reported by U.S. poison control centers,2 and plotted these on the y-axis.
When considering the independent variable to use as a predictor for bad outcomes, I used a composite index derived with the relative lethality (RL) equation, f(x) = 310x /LD50, where x is the daily dose of a medication prescribed for 30 days, and LD50 is the rat oral lethal dose 50.3 I plotted the RL of the 8 medications on the x-axis. Then I attempted to find a mathematical function that would best fit the x and y intersection points (Figure 1). I used the Excel data analysis pack to run a logarithmic regression model (Figure 2).
The model predicts that medications with a lower RL will have fewer serious outcomes, including mortality. The coefficient of determination r2 = 0.968, which indicates that 97% of the variation in serious outcomes is attributed to variation in RL, and 3% may be due to other factors, such as the poor quality of U.S. poison control data. This is a very significant correlation, and the causality is self-evident.
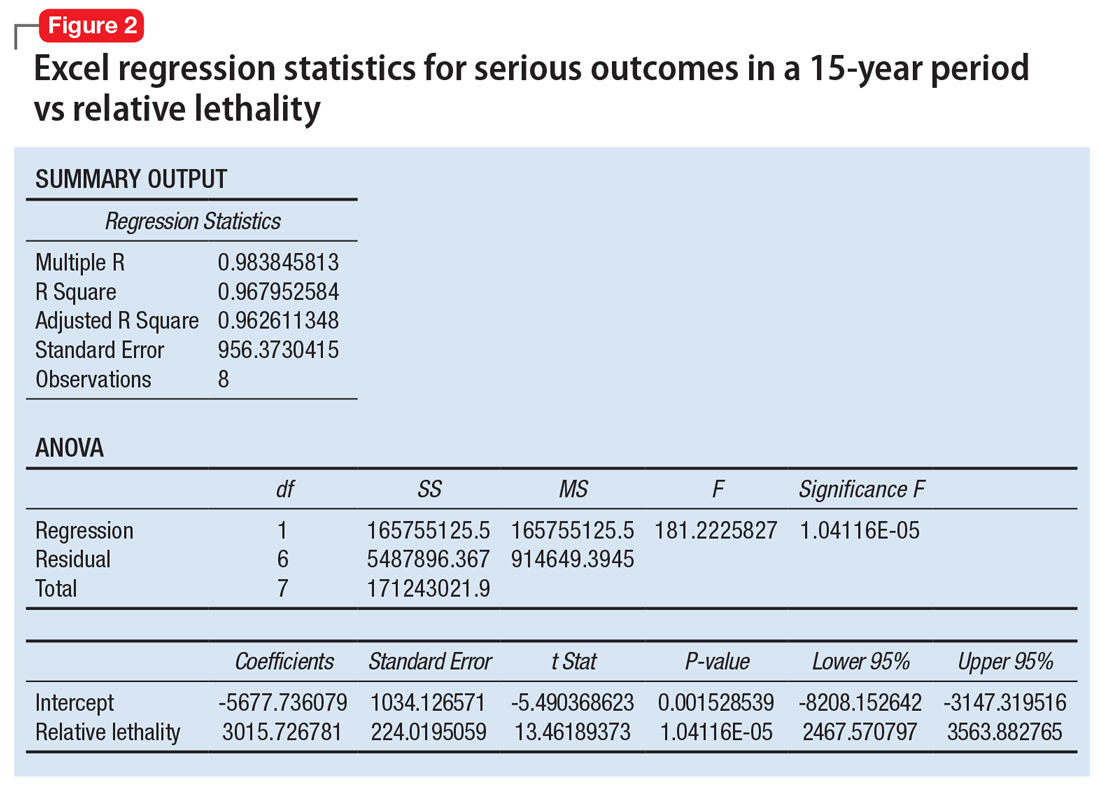
Continued to: The distribution of bad outcomes in the model was...
The distribution of bad outcomes in the model was: 1,446 for aripiprazole (RL = 9.76%), 2,387 for ziprasidone (RL = 24.80%), 5,352 for risperidone (RL = 32.63%), 5,798 for olanzapine (RL = 35.03%), 6,120 for sertraline (RL = 46.72%), 10,343 for trazodone (RL = 269.57%), 13,345 for amitriptyline (RL = 387.50%), and 15,036 for lithium (RL = 1,062.86%). The regression equation is: serious outcomes = –5,677.7 + 3,015.7 × ln (RL).
Some doctors may argue that such a data set is too small to make a meaningful model. However, the number of possible ways of ranking the drugs by bad outcomes is 8! = 40,320, so the probability of guessing the right sequence is P = .000024801. To appreciate how small this probability is, imagine trying to find a person of interest in half a football stadium on Superbowl Sunday.
The RL composite index correctly predicted the ranking order of serious outcomes for the 8 medications and may be useful for finding such outcomes in any drug class. For example, with angiotensin-converting enzyme inhibitors (n = 11) the number of possible combinations is 11! = 39,916,800. The probability of guessing the right sequence is like finding a person of interest in Poland. The model predicts the following decreasing sequence: 1) captopril, 2) fosinopril, 3) quinapril, 4) benazepril, 5) enalapril, 6) lisinopril, 7) moexipril, 8) perindopril, 9) cilazapril, 10) ramipril, 11) trandolapril. The predicted number of bad outcomes is highest for captopril, and lowest for trandolapril. The usefulness of the machine learning algorithm becomes immediately apparent.
Data can inform prescribing
Analytics can expose a critical flaw in the academic psychiatry paradigm for prescribing medications. For example, some doctors may regard lithium as the “gold standard” for treating certain mood disorders, but there is evidence that olanzapine is “significantly more effective than lithium in preventing recurrence of manic and mixed episodes.”4 Olanzapine is also 30 times safer than lithium based on its RL index, and had 9,238 fewer bad outcomes based on the 15-year data from U.S. poison control centers.2 A patient who intends to attempt suicide would easily be able to find the lethal dose of lithium from a “suicide” web site, and would quickly be able to figure out that the monthly amount of lithium his or her psychiatrist prescribed, would exceed the lethal dose.
When academia and reality collide, the use of analytics will have the final word by preventing suicide in the short term and reducing the number of bad outcomes in the long term. The irony of data science is that mathematical models can find optimal solutions to complex problems in a fraction of a second, but it may take years for a paradigm shift.
1. Bertsimas D, O’Hair AK, Pulleyblank WR. The analytics edge. Belmont, MA: Dynamic Ideas LLC; 2016.
2. Nelson JC, Spyker DA. Morbidity and mortality associated with medications used in the treatment of depression: an analysis of cases reported to U.S. poison control centers, 2000-2014. Am J Psychiatry. 2017;174(5):438-450.
3. Giurca D. Decreasing suicide risk with math. Current Psychiatry. 2018;17(2):57-59,A,B.
4. Tohen M, Greil W, Calabrese JR, et al. Olanzapine versus lithium in the maintenance treatment of bipolar disorder: a 12-month, randomized, double-blind, controlled clinical trial. Am J Psychiatry. 2005;162(7):1281-1290.
Computational psychiatry is an emerging field in which artificial intelligence and machine learning are used to find hidden patterns in big data to better understand, predict, and treat mental illness. The field uses various mathematical models to predict the dependent variable y based on the independent variable x. One application of analytics in medicine was the Framingham Heart Study, which used multivariate logistic regression to predict heart disease.1
Analytics could be used to predict the number of bad outcomes associated with different psychiatric medications over time. To demonstrate this, I examined a select data set of 8 psychiatric medications (aripiprazole, ziprasidone, risperidone, olanzapine, sertraline, trazodone, amitriptyline, and lithium) accounting for 59,827 bad outcomes during a 15-year period as reported by U.S. poison control centers,2 and plotted these on the y-axis.
When considering the independent variable to use as a predictor for bad outcomes, I used a composite index derived with the relative lethality (RL) equation, f(x) = 310x /LD50, where x is the daily dose of a medication prescribed for 30 days, and LD50 is the rat oral lethal dose 50.3 I plotted the RL of the 8 medications on the x-axis. Then I attempted to find a mathematical function that would best fit the x and y intersection points (Figure 1). I used the Excel data analysis pack to run a logarithmic regression model (Figure 2).
The model predicts that medications with a lower RL will have fewer serious outcomes, including mortality. The coefficient of determination r2 = 0.968, which indicates that 97% of the variation in serious outcomes is attributed to variation in RL, and 3% may be due to other factors, such as the poor quality of U.S. poison control data. This is a very significant correlation, and the causality is self-evident.

Continued to: The distribution of bad outcomes in the model was...
The distribution of bad outcomes in the model was: 1,446 for aripiprazole (RL = 9.76%), 2,387 for ziprasidone (RL = 24.80%), 5,352 for risperidone (RL = 32.63%), 5,798 for olanzapine (RL = 35.03%), 6,120 for sertraline (RL = 46.72%), 10,343 for trazodone (RL = 269.57%), 13,345 for amitriptyline (RL = 387.50%), and 15,036 for lithium (RL = 1,062.86%). The regression equation is: serious outcomes = –5,677.7 + 3,015.7 × ln (RL).
Some doctors may argue that such a data set is too small to make a meaningful model. However, the number of possible ways of ranking the drugs by bad outcomes is 8! = 40,320, so the probability of guessing the right sequence is P = .000024801. To appreciate how small this probability is, imagine trying to find a person of interest in half a football stadium on Superbowl Sunday.
The RL composite index correctly predicted the ranking order of serious outcomes for the 8 medications and may be useful for finding such outcomes in any drug class. For example, with angiotensin-converting enzyme inhibitors (n = 11) the number of possible combinations is 11! = 39,916,800. The probability of guessing the right sequence is like finding a person of interest in Poland. The model predicts the following decreasing sequence: 1) captopril, 2) fosinopril, 3) quinapril, 4) benazepril, 5) enalapril, 6) lisinopril, 7) moexipril, 8) perindopril, 9) cilazapril, 10) ramipril, 11) trandolapril. The predicted number of bad outcomes is highest for captopril, and lowest for trandolapril. The usefulness of the machine learning algorithm becomes immediately apparent.
Data can inform prescribing
Analytics can expose a critical flaw in the academic psychiatry paradigm for prescribing medications. For example, some doctors may regard lithium as the “gold standard” for treating certain mood disorders, but there is evidence that olanzapine is “significantly more effective than lithium in preventing recurrence of manic and mixed episodes.”4 Olanzapine is also 30 times safer than lithium based on its RL index, and had 9,238 fewer bad outcomes based on the 15-year data from U.S. poison control centers.2 A patient who intends to attempt suicide would easily be able to find the lethal dose of lithium from a “suicide” web site, and would quickly be able to figure out that the monthly amount of lithium his or her psychiatrist prescribed, would exceed the lethal dose.
When academia and reality collide, the use of analytics will have the final word by preventing suicide in the short term and reducing the number of bad outcomes in the long term. The irony of data science is that mathematical models can find optimal solutions to complex problems in a fraction of a second, but it may take years for a paradigm shift.
Computational psychiatry is an emerging field in which artificial intelligence and machine learning are used to find hidden patterns in big data to better understand, predict, and treat mental illness. The field uses various mathematical models to predict the dependent variable y based on the independent variable x. One application of analytics in medicine was the Framingham Heart Study, which used multivariate logistic regression to predict heart disease.1
Analytics could be used to predict the number of bad outcomes associated with different psychiatric medications over time. To demonstrate this, I examined a select data set of 8 psychiatric medications (aripiprazole, ziprasidone, risperidone, olanzapine, sertraline, trazodone, amitriptyline, and lithium) accounting for 59,827 bad outcomes during a 15-year period as reported by U.S. poison control centers,2 and plotted these on the y-axis.
When considering the independent variable to use as a predictor for bad outcomes, I used a composite index derived with the relative lethality (RL) equation, f(x) = 310x /LD50, where x is the daily dose of a medication prescribed for 30 days, and LD50 is the rat oral lethal dose 50.3 I plotted the RL of the 8 medications on the x-axis. Then I attempted to find a mathematical function that would best fit the x and y intersection points (Figure 1). I used the Excel data analysis pack to run a logarithmic regression model (Figure 2).
The model predicts that medications with a lower RL will have fewer serious outcomes, including mortality. The coefficient of determination r2 = 0.968, which indicates that 97% of the variation in serious outcomes is attributed to variation in RL, and 3% may be due to other factors, such as the poor quality of U.S. poison control data. This is a very significant correlation, and the causality is self-evident.

Continued to: The distribution of bad outcomes in the model was...
The distribution of bad outcomes in the model was: 1,446 for aripiprazole (RL = 9.76%), 2,387 for ziprasidone (RL = 24.80%), 5,352 for risperidone (RL = 32.63%), 5,798 for olanzapine (RL = 35.03%), 6,120 for sertraline (RL = 46.72%), 10,343 for trazodone (RL = 269.57%), 13,345 for amitriptyline (RL = 387.50%), and 15,036 for lithium (RL = 1,062.86%). The regression equation is: serious outcomes = –5,677.7 + 3,015.7 × ln (RL).
Some doctors may argue that such a data set is too small to make a meaningful model. However, the number of possible ways of ranking the drugs by bad outcomes is 8! = 40,320, so the probability of guessing the right sequence is P = .000024801. To appreciate how small this probability is, imagine trying to find a person of interest in half a football stadium on Superbowl Sunday.
The RL composite index correctly predicted the ranking order of serious outcomes for the 8 medications and may be useful for finding such outcomes in any drug class. For example, with angiotensin-converting enzyme inhibitors (n = 11) the number of possible combinations is 11! = 39,916,800. The probability of guessing the right sequence is like finding a person of interest in Poland. The model predicts the following decreasing sequence: 1) captopril, 2) fosinopril, 3) quinapril, 4) benazepril, 5) enalapril, 6) lisinopril, 7) moexipril, 8) perindopril, 9) cilazapril, 10) ramipril, 11) trandolapril. The predicted number of bad outcomes is highest for captopril, and lowest for trandolapril. The usefulness of the machine learning algorithm becomes immediately apparent.
Data can inform prescribing
Analytics can expose a critical flaw in the academic psychiatry paradigm for prescribing medications. For example, some doctors may regard lithium as the “gold standard” for treating certain mood disorders, but there is evidence that olanzapine is “significantly more effective than lithium in preventing recurrence of manic and mixed episodes.”4 Olanzapine is also 30 times safer than lithium based on its RL index, and had 9,238 fewer bad outcomes based on the 15-year data from U.S. poison control centers.2 A patient who intends to attempt suicide would easily be able to find the lethal dose of lithium from a “suicide” web site, and would quickly be able to figure out that the monthly amount of lithium his or her psychiatrist prescribed, would exceed the lethal dose.
When academia and reality collide, the use of analytics will have the final word by preventing suicide in the short term and reducing the number of bad outcomes in the long term. The irony of data science is that mathematical models can find optimal solutions to complex problems in a fraction of a second, but it may take years for a paradigm shift.
1. Bertsimas D, O’Hair AK, Pulleyblank WR. The analytics edge. Belmont, MA: Dynamic Ideas LLC; 2016.
2. Nelson JC, Spyker DA. Morbidity and mortality associated with medications used in the treatment of depression: an analysis of cases reported to U.S. poison control centers, 2000-2014. Am J Psychiatry. 2017;174(5):438-450.
3. Giurca D. Decreasing suicide risk with math. Current Psychiatry. 2018;17(2):57-59,A,B.
4. Tohen M, Greil W, Calabrese JR, et al. Olanzapine versus lithium in the maintenance treatment of bipolar disorder: a 12-month, randomized, double-blind, controlled clinical trial. Am J Psychiatry. 2005;162(7):1281-1290.
1. Bertsimas D, O’Hair AK, Pulleyblank WR. The analytics edge. Belmont, MA: Dynamic Ideas LLC; 2016.
2. Nelson JC, Spyker DA. Morbidity and mortality associated with medications used in the treatment of depression: an analysis of cases reported to U.S. poison control centers, 2000-2014. Am J Psychiatry. 2017;174(5):438-450.
3. Giurca D. Decreasing suicide risk with math. Current Psychiatry. 2018;17(2):57-59,A,B.
4. Tohen M, Greil W, Calabrese JR, et al. Olanzapine versus lithium in the maintenance treatment of bipolar disorder: a 12-month, randomized, double-blind, controlled clinical trial. Am J Psychiatry. 2005;162(7):1281-1290.
Managing procedural pain in a patient taking naltrexone
Mr. M, age 55, presents to his primary care physician (PCP) with hematochezia. Mr. M states that for the past week, he has noticed blood upon wiping after a bowel movement and is worried that he might have cancer.
Mr. M has a 10-year history of opioid use disorder as diagnosed by his psychiatrist. He is presently maintained on long-acting injectable naltrexone, 380 mg IM every 4 weeks, and has not used opioids for the past 1.5 years. Mr. M is also taking simvastatin, 40 mg, for dyslipidemia, lisinopril, 5 mg, for hypertension, and cetirizine, 5 mg as needed, for seasonal allergies.
A standard workup including a physical examination and laboratory tests are performed. Mr. M’s PCP would like for him to undergo a colonoscopy to investigate the etiology of the bleeding. In consultation with both the PCP and psychiatrist, the gastroenterologist determines that the colonoscopy can be performed within 48 hours with no changes to Mr. M’s medication regimen. The gastroenterologist utilizes a nonopioid, ketorolac, 30 mg IV, for pain management during the procedure. Diverticula were identified in the lower gastrointestinal tract and are treated endoscopically. Mr. M is successfully withdrawn from sedation with no adverse events or pain and continues to be in opioid remission.
Naltrexone competitively antagonizes opioid receptors with the highest affinity for the µ-opioid receptor. It is approved for treatment of alcohol and opioid dependence following opioid detoxification.1 Its competitive inhibition at the µ-opioid receptor results in the inhibition of exogenous opioid effects. The medication is available as an orally administered tablet as well as a long-acting injection administered intramuscularly (Table 11). The long-acting injection can be useful in patients who have difficulty with adherence, because good adherence to naltrexone is required to maximize efficacy.
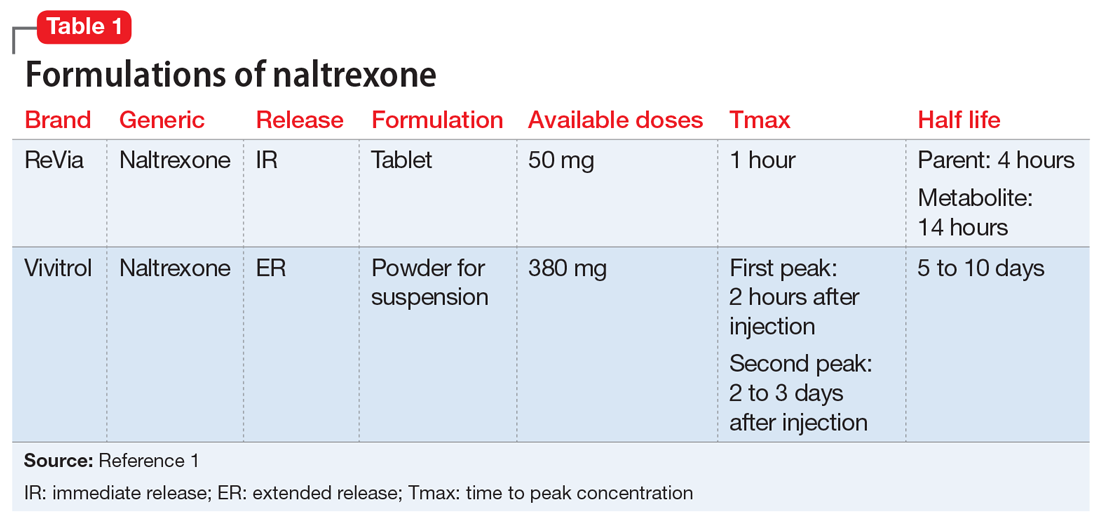
Due to its ability to block opioid analgesic effects, naltrexone presents a unique challenge for patients taking it who need to undergo procedures that require pain control. Pharmacologic regimens used during procedures often contain a sedative agent, such as propofol, and an opioid for analgesia. Alternative strategies are needed for patients taking naltrexone who require an opioid analgesic agent for procedures such as colonoscopies.
One strategy could be to withhold naltrexone before the procedure to ensure that the medication will not compete with the opioid agent to relieve pain. This strategy depends on the urgency of the procedure, the formulation of naltrexone being used, and patient-specific factors that may increase the risk for adverse events. For a non-urgent, elective procedure, it may be acceptable to hold oral naltrexone approximately 72 hours before the procedure. However, this is likely not a favorable approach for patients who may be at high risk for relapse or for patients who are receiving the long-acting formulation. Additionally, the use of an opioid agent intra- or post-operatively for pain may increase the risk of relapse. The use of opioids for such procedures may also be more difficult in a patient with a history of opioid abuse or dependence because he or she may have developed tolerance to opioids. Conversely, if a patient has been treated with naltrexone for an extended period, a lack of tolerance may increase the risk of respiratory depression with opioid administration due to upregulation of the opioid receptor.2
Continue to: Nonopioid analgesic agents
Nonopioid analgesic agents
For a patient receiving naltrexone who needs to undergo a procedure, a multidisciplinary consultation between the patient’s psychiatrist and other clinicians is key for providing a regimen that is safe and effective. A nonopioid analgesic agent may be considered to avoid the problematic interactions possible in these patients (Table 23-5). Nonopioid regimens can be utilized alone or in combination, and may include the following3-5:
Ketamine is a non-competitive antagonist at the N-methyl-
Dexmedetomidine is an alpha-2 agonist that can provide sedative and analgesic effects. It can cause procedural hypotension and bradycardia, so caution is advised in patients with cardiac disease and hepatic and/or renal insufficiencies.
Nonsteroidal anti-inflammatory drugs (NSAIDs), such as ibuprofen or ketorolac, inhibit cyclooxygenase enzymes and can be considered in analgesic regimens. However, for most surgical procedures, the increased risk of bleeding due to platelet inhibition is a concern.
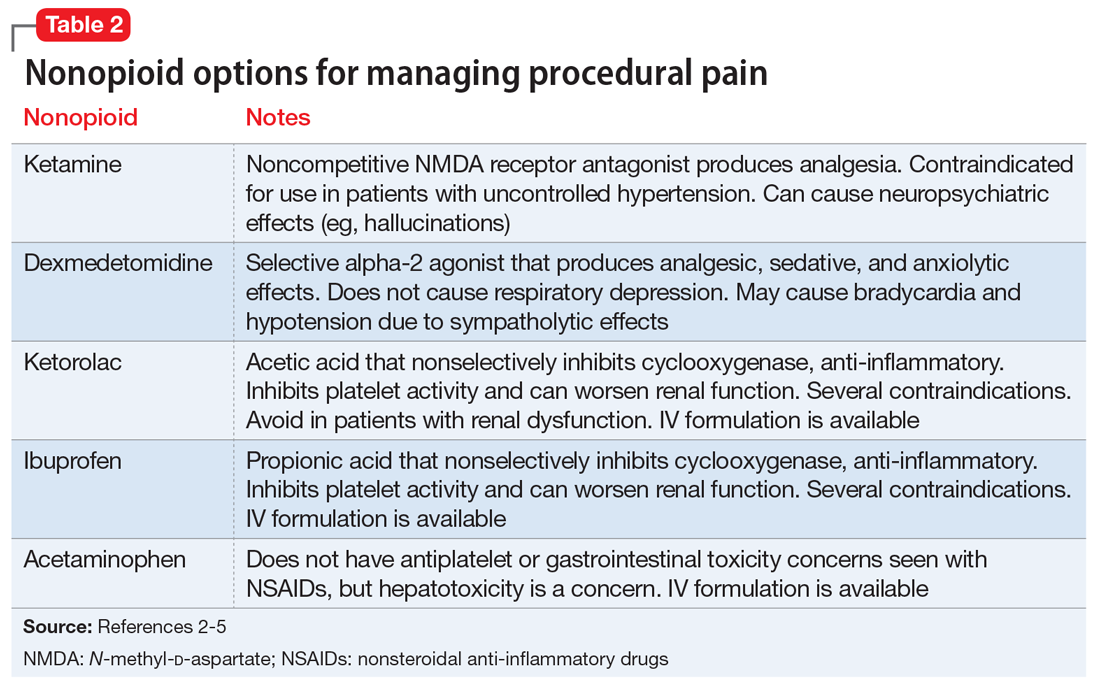
Continue to: Acetaminophen
Acetaminophen. Although its full mechanism of action has not been discovered, acetaminophen may also act on the cyclooxygenase pathway to produce analgesia. Compared with the oral formulation, IV acetaminophen is more expensive but may offer certain advantages, including faster plasma peak levels and lower production of acetaminophen’s toxic metabolite, N-acetyl-p-benzoquinone imine. Nonetheless, hepatotoxicity and overdose remain a concern.
The use of nonopioid analgesics during elective procedures that require pain control will allow continued use of an opioid antagonist such as naltrexone, while minimizing the risk for withdrawal or relapse. Their use must be evaluated on a case-by-case basis to ensure maximum safety and efficacy for each patient from both a medical and psychiatric standpoint. Overall, with the proper expertise and consultation, nonopioid pain regimens represent a reasonable alternative to opiates for patients who take naltrexone.
Related Resources
- American Society of Anesthesiologists. Standards guidelines and related resources. https://www.asahq.org/quality-and-practice-management/standards-guidelines-and-related-resources-search.
- American Society of Addiction Medicine. Clinical resources. https://www.asam.org/resources/guidelines-and-consensus-documents.
Drug Brand Names
Acetaminophen • Tylenol
Cetirizine • Zyrtec
Dexmedetomidine • Precedex
Ibuprofen • Caldolor (IV), Motrin (oral)
Ketamine • Ketalar
Ketorolac • Toradol
Lisinopril • Prinivil, Zestril
Naltrexone • ReVia, Vivitrol
Propofol • Diprivan
Simvastatin • Juvisync, Simcor
1. Vivitrol [package insert]. Waltham, MA: Alkermes, Inc.; 2015.
2. Yoburn BC, Duttaroy A, Shah S, et al. Opioid antagonist-induced receptor upregulation: effects of concurrent agonist administration. Brain Res Bull. 1994;33(2):237-240.
3. Vadivelu N, Chang D, Lumermann L, et al. Management of patients on abuse-deterrent opioids in the ambulatory surgery setting. Curr Pain Headache Rep. 2017;21(2):10.
4. Koh W, Nguyen KP, Jahr JS. Intravenous non-opioid analgesia for peri- and postoperative pain management: a scientific review of intravenous acetaminophen and ibuprofen. Korean J Anesthesiol. 2015;68(1):3-12.
5. Kaye AD, Cornett EM, Helander E, et al. An update on nonopioids: intravenous or oral analgesics for perioperative pain management. Anesthesiol Clin. 2017;35(2):e55-e71.
Mr. M, age 55, presents to his primary care physician (PCP) with hematochezia. Mr. M states that for the past week, he has noticed blood upon wiping after a bowel movement and is worried that he might have cancer.
Mr. M has a 10-year history of opioid use disorder as diagnosed by his psychiatrist. He is presently maintained on long-acting injectable naltrexone, 380 mg IM every 4 weeks, and has not used opioids for the past 1.5 years. Mr. M is also taking simvastatin, 40 mg, for dyslipidemia, lisinopril, 5 mg, for hypertension, and cetirizine, 5 mg as needed, for seasonal allergies.
A standard workup including a physical examination and laboratory tests are performed. Mr. M’s PCP would like for him to undergo a colonoscopy to investigate the etiology of the bleeding. In consultation with both the PCP and psychiatrist, the gastroenterologist determines that the colonoscopy can be performed within 48 hours with no changes to Mr. M’s medication regimen. The gastroenterologist utilizes a nonopioid, ketorolac, 30 mg IV, for pain management during the procedure. Diverticula were identified in the lower gastrointestinal tract and are treated endoscopically. Mr. M is successfully withdrawn from sedation with no adverse events or pain and continues to be in opioid remission.
Naltrexone competitively antagonizes opioid receptors with the highest affinity for the µ-opioid receptor. It is approved for treatment of alcohol and opioid dependence following opioid detoxification.1 Its competitive inhibition at the µ-opioid receptor results in the inhibition of exogenous opioid effects. The medication is available as an orally administered tablet as well as a long-acting injection administered intramuscularly (Table 11). The long-acting injection can be useful in patients who have difficulty with adherence, because good adherence to naltrexone is required to maximize efficacy.

Due to its ability to block opioid analgesic effects, naltrexone presents a unique challenge for patients taking it who need to undergo procedures that require pain control. Pharmacologic regimens used during procedures often contain a sedative agent, such as propofol, and an opioid for analgesia. Alternative strategies are needed for patients taking naltrexone who require an opioid analgesic agent for procedures such as colonoscopies.
One strategy could be to withhold naltrexone before the procedure to ensure that the medication will not compete with the opioid agent to relieve pain. This strategy depends on the urgency of the procedure, the formulation of naltrexone being used, and patient-specific factors that may increase the risk for adverse events. For a non-urgent, elective procedure, it may be acceptable to hold oral naltrexone approximately 72 hours before the procedure. However, this is likely not a favorable approach for patients who may be at high risk for relapse or for patients who are receiving the long-acting formulation. Additionally, the use of an opioid agent intra- or post-operatively for pain may increase the risk of relapse. The use of opioids for such procedures may also be more difficult in a patient with a history of opioid abuse or dependence because he or she may have developed tolerance to opioids. Conversely, if a patient has been treated with naltrexone for an extended period, a lack of tolerance may increase the risk of respiratory depression with opioid administration due to upregulation of the opioid receptor.2
Continue to: Nonopioid analgesic agents
Nonopioid analgesic agents
For a patient receiving naltrexone who needs to undergo a procedure, a multidisciplinary consultation between the patient’s psychiatrist and other clinicians is key for providing a regimen that is safe and effective. A nonopioid analgesic agent may be considered to avoid the problematic interactions possible in these patients (Table 23-5). Nonopioid regimens can be utilized alone or in combination, and may include the following3-5:
Ketamine is a non-competitive antagonist at the N-methyl-
Dexmedetomidine is an alpha-2 agonist that can provide sedative and analgesic effects. It can cause procedural hypotension and bradycardia, so caution is advised in patients with cardiac disease and hepatic and/or renal insufficiencies.
Nonsteroidal anti-inflammatory drugs (NSAIDs), such as ibuprofen or ketorolac, inhibit cyclooxygenase enzymes and can be considered in analgesic regimens. However, for most surgical procedures, the increased risk of bleeding due to platelet inhibition is a concern.

Continue to: Acetaminophen
Acetaminophen. Although its full mechanism of action has not been discovered, acetaminophen may also act on the cyclooxygenase pathway to produce analgesia. Compared with the oral formulation, IV acetaminophen is more expensive but may offer certain advantages, including faster plasma peak levels and lower production of acetaminophen’s toxic metabolite, N-acetyl-p-benzoquinone imine. Nonetheless, hepatotoxicity and overdose remain a concern.
The use of nonopioid analgesics during elective procedures that require pain control will allow continued use of an opioid antagonist such as naltrexone, while minimizing the risk for withdrawal or relapse. Their use must be evaluated on a case-by-case basis to ensure maximum safety and efficacy for each patient from both a medical and psychiatric standpoint. Overall, with the proper expertise and consultation, nonopioid pain regimens represent a reasonable alternative to opiates for patients who take naltrexone.
Related Resources
- American Society of Anesthesiologists. Standards guidelines and related resources. https://www.asahq.org/quality-and-practice-management/standards-guidelines-and-related-resources-search.
- American Society of Addiction Medicine. Clinical resources. https://www.asam.org/resources/guidelines-and-consensus-documents.
Drug Brand Names
Acetaminophen • Tylenol
Cetirizine • Zyrtec
Dexmedetomidine • Precedex
Ibuprofen • Caldolor (IV), Motrin (oral)
Ketamine • Ketalar
Ketorolac • Toradol
Lisinopril • Prinivil, Zestril
Naltrexone • ReVia, Vivitrol
Propofol • Diprivan
Simvastatin • Juvisync, Simcor
Mr. M, age 55, presents to his primary care physician (PCP) with hematochezia. Mr. M states that for the past week, he has noticed blood upon wiping after a bowel movement and is worried that he might have cancer.
Mr. M has a 10-year history of opioid use disorder as diagnosed by his psychiatrist. He is presently maintained on long-acting injectable naltrexone, 380 mg IM every 4 weeks, and has not used opioids for the past 1.5 years. Mr. M is also taking simvastatin, 40 mg, for dyslipidemia, lisinopril, 5 mg, for hypertension, and cetirizine, 5 mg as needed, for seasonal allergies.
A standard workup including a physical examination and laboratory tests are performed. Mr. M’s PCP would like for him to undergo a colonoscopy to investigate the etiology of the bleeding. In consultation with both the PCP and psychiatrist, the gastroenterologist determines that the colonoscopy can be performed within 48 hours with no changes to Mr. M’s medication regimen. The gastroenterologist utilizes a nonopioid, ketorolac, 30 mg IV, for pain management during the procedure. Diverticula were identified in the lower gastrointestinal tract and are treated endoscopically. Mr. M is successfully withdrawn from sedation with no adverse events or pain and continues to be in opioid remission.
Naltrexone competitively antagonizes opioid receptors with the highest affinity for the µ-opioid receptor. It is approved for treatment of alcohol and opioid dependence following opioid detoxification.1 Its competitive inhibition at the µ-opioid receptor results in the inhibition of exogenous opioid effects. The medication is available as an orally administered tablet as well as a long-acting injection administered intramuscularly (Table 11). The long-acting injection can be useful in patients who have difficulty with adherence, because good adherence to naltrexone is required to maximize efficacy.

Due to its ability to block opioid analgesic effects, naltrexone presents a unique challenge for patients taking it who need to undergo procedures that require pain control. Pharmacologic regimens used during procedures often contain a sedative agent, such as propofol, and an opioid for analgesia. Alternative strategies are needed for patients taking naltrexone who require an opioid analgesic agent for procedures such as colonoscopies.
One strategy could be to withhold naltrexone before the procedure to ensure that the medication will not compete with the opioid agent to relieve pain. This strategy depends on the urgency of the procedure, the formulation of naltrexone being used, and patient-specific factors that may increase the risk for adverse events. For a non-urgent, elective procedure, it may be acceptable to hold oral naltrexone approximately 72 hours before the procedure. However, this is likely not a favorable approach for patients who may be at high risk for relapse or for patients who are receiving the long-acting formulation. Additionally, the use of an opioid agent intra- or post-operatively for pain may increase the risk of relapse. The use of opioids for such procedures may also be more difficult in a patient with a history of opioid abuse or dependence because he or she may have developed tolerance to opioids. Conversely, if a patient has been treated with naltrexone for an extended period, a lack of tolerance may increase the risk of respiratory depression with opioid administration due to upregulation of the opioid receptor.2
Continue to: Nonopioid analgesic agents
Nonopioid analgesic agents
For a patient receiving naltrexone who needs to undergo a procedure, a multidisciplinary consultation between the patient’s psychiatrist and other clinicians is key for providing a regimen that is safe and effective. A nonopioid analgesic agent may be considered to avoid the problematic interactions possible in these patients (Table 23-5). Nonopioid regimens can be utilized alone or in combination, and may include the following3-5:
Ketamine is a non-competitive antagonist at the N-methyl-
Dexmedetomidine is an alpha-2 agonist that can provide sedative and analgesic effects. It can cause procedural hypotension and bradycardia, so caution is advised in patients with cardiac disease and hepatic and/or renal insufficiencies.
Nonsteroidal anti-inflammatory drugs (NSAIDs), such as ibuprofen or ketorolac, inhibit cyclooxygenase enzymes and can be considered in analgesic regimens. However, for most surgical procedures, the increased risk of bleeding due to platelet inhibition is a concern.

Continue to: Acetaminophen
Acetaminophen. Although its full mechanism of action has not been discovered, acetaminophen may also act on the cyclooxygenase pathway to produce analgesia. Compared with the oral formulation, IV acetaminophen is more expensive but may offer certain advantages, including faster plasma peak levels and lower production of acetaminophen’s toxic metabolite, N-acetyl-p-benzoquinone imine. Nonetheless, hepatotoxicity and overdose remain a concern.
The use of nonopioid analgesics during elective procedures that require pain control will allow continued use of an opioid antagonist such as naltrexone, while minimizing the risk for withdrawal or relapse. Their use must be evaluated on a case-by-case basis to ensure maximum safety and efficacy for each patient from both a medical and psychiatric standpoint. Overall, with the proper expertise and consultation, nonopioid pain regimens represent a reasonable alternative to opiates for patients who take naltrexone.
Related Resources
- American Society of Anesthesiologists. Standards guidelines and related resources. https://www.asahq.org/quality-and-practice-management/standards-guidelines-and-related-resources-search.
- American Society of Addiction Medicine. Clinical resources. https://www.asam.org/resources/guidelines-and-consensus-documents.
Drug Brand Names
Acetaminophen • Tylenol
Cetirizine • Zyrtec
Dexmedetomidine • Precedex
Ibuprofen • Caldolor (IV), Motrin (oral)
Ketamine • Ketalar
Ketorolac • Toradol
Lisinopril • Prinivil, Zestril
Naltrexone • ReVia, Vivitrol
Propofol • Diprivan
Simvastatin • Juvisync, Simcor
1. Vivitrol [package insert]. Waltham, MA: Alkermes, Inc.; 2015.
2. Yoburn BC, Duttaroy A, Shah S, et al. Opioid antagonist-induced receptor upregulation: effects of concurrent agonist administration. Brain Res Bull. 1994;33(2):237-240.
3. Vadivelu N, Chang D, Lumermann L, et al. Management of patients on abuse-deterrent opioids in the ambulatory surgery setting. Curr Pain Headache Rep. 2017;21(2):10.
4. Koh W, Nguyen KP, Jahr JS. Intravenous non-opioid analgesia for peri- and postoperative pain management: a scientific review of intravenous acetaminophen and ibuprofen. Korean J Anesthesiol. 2015;68(1):3-12.
5. Kaye AD, Cornett EM, Helander E, et al. An update on nonopioids: intravenous or oral analgesics for perioperative pain management. Anesthesiol Clin. 2017;35(2):e55-e71.
1. Vivitrol [package insert]. Waltham, MA: Alkermes, Inc.; 2015.
2. Yoburn BC, Duttaroy A, Shah S, et al. Opioid antagonist-induced receptor upregulation: effects of concurrent agonist administration. Brain Res Bull. 1994;33(2):237-240.
3. Vadivelu N, Chang D, Lumermann L, et al. Management of patients on abuse-deterrent opioids in the ambulatory surgery setting. Curr Pain Headache Rep. 2017;21(2):10.
4. Koh W, Nguyen KP, Jahr JS. Intravenous non-opioid analgesia for peri- and postoperative pain management: a scientific review of intravenous acetaminophen and ibuprofen. Korean J Anesthesiol. 2015;68(1):3-12.
5. Kaye AD, Cornett EM, Helander E, et al. An update on nonopioids: intravenous or oral analgesics for perioperative pain management. Anesthesiol Clin. 2017;35(2):e55-e71.
Physician impairment: A need for prevention
Psychiatry is a field of passion. The reward of experiencing growth and change alongside our patients is what bolsters us through years of difficult training, overnight shifts, endless paperwork, regulatory mandates, and frequent worry about our patients. As physicians, we don’t live for weekends as many other professionals do. To the contrary, we spend them on call, moonlighting, laboring over journal articles, and perfecting lectures.
That passion is what makes us trusted clinicians and experts in our field. It can also make it difficult for us to disconnect from our work, frequently leading to burnout. Physician self-care, support, and professional development are critical topics that modern-day medicine minimizes at the peril of physicians and public health.1
Psychiatry lends itself to a deep and intimate understanding of another human being. The therapist delves into the lives of his or her patients, hears their stories, and holds their secrets. In some cases, we might be the only ones who truly see patients for who they uniquely are, and come to understand them on a deeper level than their closest family and friends. This can be both thrilling and intense. As we delve into the psyche of another individual, contemplate which interpretation we should share, and resonate with our patients, it is easy to become bogged down with our own countertransference, sentiment, and worry, and to become consumed by our work. A professional hazard, some might quip.
Therefore, personal restoration—a tool that keeps our clinical skills sharp—is vitally important to caring for oneself and one’s patient. Surprisingly, this can be neglected until we begin to experience burnout, which over time could transform into impairment, thus endangering ourselves, our patients, and our profession.
Over the past decade, physician impairment has been exhaustively described, researched, and addressed. However, most analyses have focused on identifying impairment, and offering guidance on how to properly report it. How do we shift from managing the crisis to preventing it? To answer this question, this article:
- reviews the dilemma of physician impairment
- explores the duty we have to patients, ourselves, and the profession
- discusses shifting the focus on impairment to prevention through well-being.
Continue to: Dilemma
Dilemma: Vulnerability to impairment
The cornerstone for well-being is a balanced life. No matter how much one loves his or her work, there must be balance between work, relationships, and hobbies. Without that equilibrium, everyone is put at risk.2
Just as our patients, we are not immune to mental illness, cognitive decline, or substance abuse.3 We might even be more susceptible. For many physicians, their identity is intimately tied to their work.4 Dr. Robin Weiss captured that intimate relationship5:
“… [A] therapist may spend hundreds of hours, perhaps more than a thousand, hearing about a patient’s most exalted aspirations and most murderous, hateful fantasies. During this time, the patient may endure excruciating losses, unbearable shame, bitter sadness and great triumphs. You may accompany patients through torturous adolescence into adulthood. Or you may meet them in middle age and be with them as they age and eventually die. You collaborate in a deep process of discovery. Few encounters are this deeply honest, and therefore intimate.”
Given the stories we hear and the resulting intimacy and countertransference that inevitably arise, psychiatrists are even more prone to burnout than other physicians.6 Physician impairment is a public health issue that affects not just physicians but also their families, colleagues, and patients.
“Impairment” for the purpose of this article means a physical, mental, or substance-related disorder that interferes with a physician’s ability to undertake professional activities competently and safely.7 Predisposing factors for physician impairment include an obsessive-compulsive personality type, a family history of mental illness, sensation-seeking behavior, denial of personal problems, perfectionism, and idealism.8,9 Also, work stress becomes a significant factor in already vulnerable physicians, leading to a greater risk for mental illness.10
Continue to: Some warning signs of impairment include...
Some warning signs of impairment include a lack of personal hygiene, emotional lability, sleep deprivation, inattention to our pages or phone calls, and increased professional errors.11 When it comes to addressing such impairment, previous research and literature has focused on how to monitor ourselves and our colleagues; anything less would put the reputation and integrity of the medical profession at risk.3 This has led to a culture of doing nothing but work until things go too far, and then reporting the problems. But what about intervening before things get too far?
Duty: To ourselves, our colleagues, and our patients
There has been much discussion on how to report impaired colleagues, but little on how to help and support ourselves and our colleagues before things escalate into serious problems. And this lack of discussion is at the detriment of individual practitioners, their families, and patients. Physicians-in-training, including psychiatric residents, are at particularly high risk for developing stress-related problems, depression, and substance misuse.12 Occupational demands, self-criticism, and denial of one’s distress are common among physicians, as is self-treatment with drugs and alcohol.13
We all know by now that doctors and physician health programs (PHPs) have a duty to report impaired colleagues who continue to practice despite reasonable offers of assistance. There are an abundance of PHPs that are in place to assist with such situations. The American Medical Association’s official position on reporting impairment is outlined in Policy H-275.952.7 There also is the Federation of State Medical Boards. Its policy states that PHPs have “a primary commitment to [help] state medical boards … protect the public … [These] programs [should] demonstrate an ongoing track of record of ensuring safety to the public and reveal deficiencies if they occur.”14
Legal and ethical issues, however, complicate interventions for colleagues who need assistance.15 Despite the existence of PHPs, it would be much easier—not to mention helpful—to help a colleague by carrying out early interventions.
Discussion: Prevention as a solution
More emphasis should be placed on prevention. That’s where self-care and well-being come into play. Awareness of and sensitivity to physician vulnerability, early detection, and prevention of impairment are important.
Continue to: There has been a paradigm shift in focus...
There has been a paradigm shift in focus across medical boards, professional societies, and medical colleges. They are recognizing that personal well-being can help prevent burnout and, in turn, change the landscape of medicine from endless work to balanced lives that yield more satisfying and joyful work. It is becoming an accepted fact in medicine that well-being is just as important as integrity, professionalism, and patient safety. For example, the American Academy of Medical Colleges (AAMC) issued a statement emphasizing the importance of clinician well-being and dedicated its June 2016 Leadership Forum to a range of topics addressing depression, resilience, burnout, and suicide in academic medicine.16
Anita Everett, MD, put the spotlight on physician well-being during her term as American Psychiatric Association President (2017 to 2018). She formed a specific workgroup on Physician Wellness and Burnout where there is a community focus on prevention and self-care.17 A strong sense of community and purpose is almost always part of the prescription for promoting greater well-being.2
The importance of this issue is also trickling down from policymakers into hospitals and community health centers. Consider an initiative at Minneapolis’s Hennepin County Medical Center. Leaders there created a “reset room” for physicians to quietly decompress. The room is complete with LED lights, flameless candles, a sound machine, comfortable chairs, several plants, and an “in use” sign on the door.18 Other personal strategies to help prevent burnout include making environmental changes, encouraging hobbies, and streamlining burdensome tasks such as paperwork and electronic medical record systems.
As physician health and well-being are finally emerging as a “hot topic,”2 educational and treatment resources are increasingly available for any of us to explore. Consider a simple Google search to look into your State’s PHPs, and get involved in your professional societies to make change.
The culture is starting to shift, and leading by example will be a key to propelling further progress in this area. Model our own self-care for colleagues and patients alike. As Mark Twain said, we might love our work, but we must remember that being solely defined by work comes to the detriment of our health. Maintaining balance is what will allow us to sustain long careers ahead doing what we love.
1. Mahoney, D, Freedy J, Brock C. Improving physician well-being. JAMA Intern Med. 2015;175(4):648-649.
2. Yellowlees P. Addressing physician health and well-being is patient safety issue. Psychiatric News. 2018;53(12):20-21.
3. Mossman D, Farrell HM. Physician impairment: when should you report? Current Psychiatry. 2011;10(9):67-71.
4. Lindeman S, Henriksson M, Isometsä E, et al. Treatment of mental disorders in seven physicians committing suicide. Crisis. 1999;20(2):86-89.
5. Weiss R. How therapists mourn. New York Times. July 4, 2015:SR2.
6. Kumar S. Burnout in psychiatrists. World Psychiatry. 2007;6(3):186-189.
7. American Medical Association. Report 2 of the Council on Science and Public Health (A-11). Physician health programs (Reference Committee D). https://www.ama-assn.org/sites/default/files/media-browser/public/about-ama/councils/Council%20Reports/council-on-science-public-health/a11-csaph-physician-health-programs.pdf. Accessed August 6, 2018.
8. Boisaubin EV, Levine RE. Identifying and assisting the impaired physician. Am J Med Sci. 2001;322(1):31-36.
9. Bissel L, Jones RW. The alcoholic physician: a survey. Am J Psychiatry. 1976;133(10):1142-1146.
10. Vaillant GE, Sobowale NC, McArthur C. Some psychologic vulnerabilities of physicians. N Engl J Med. 1972;287(8):372-375.
11. McGovern MP, Agnes DH, Leon S. Characteristics of physicians presenting for assessment at a behavioral health center. J Addict Dis. 2000;19(2):59-73.
12. Broquet KE, Rockey PH. Teaching residents and program directors about physician impairment. Acad Psychiatry. 2004;28(3):221-225.
13. Meier DE, Back AL, Morrison RS. The inner life of physicians and care of the seriously ill. JAMA. 2001;286(23):3007-3014.
14. Federation of State Medical Boards of the United States. Policy on physician impairment. http://www.csam-asam.org/pdf/misc/FSMB2011.pdf. Published 2011. Accessed July 15, 2018.
15. Bright RP, Krahn L. Impaired physicians: how to recognize, when to report, and where to refer. Current Psychiatry. 2010;9(6):11-20.
16. Academy of American Colleges. Well-being in academic medicine. https://www.aamc.org/initiatives/462280/well-being-academic-medicine.html. Updated July 9, 2018. Accessed July 17, 2018.
17. American Psychiatric Association. Well-being and burnout. https://www.psychiatry.org/psychiatrists/practice/well-being-and-burnout. Updated February 22, 2018. Accessed July 17, 2018.
18. Parks T. Physicians take to “reset room” to battle burnout. AMA Wire. https://wire.ama-assn.org/practice-management/physicians-take-reset-room-battle-burnout. Published June 8, 2016. Accessed July 18, 2018.
Psychiatry is a field of passion. The reward of experiencing growth and change alongside our patients is what bolsters us through years of difficult training, overnight shifts, endless paperwork, regulatory mandates, and frequent worry about our patients. As physicians, we don’t live for weekends as many other professionals do. To the contrary, we spend them on call, moonlighting, laboring over journal articles, and perfecting lectures.
That passion is what makes us trusted clinicians and experts in our field. It can also make it difficult for us to disconnect from our work, frequently leading to burnout. Physician self-care, support, and professional development are critical topics that modern-day medicine minimizes at the peril of physicians and public health.1
Psychiatry lends itself to a deep and intimate understanding of another human being. The therapist delves into the lives of his or her patients, hears their stories, and holds their secrets. In some cases, we might be the only ones who truly see patients for who they uniquely are, and come to understand them on a deeper level than their closest family and friends. This can be both thrilling and intense. As we delve into the psyche of another individual, contemplate which interpretation we should share, and resonate with our patients, it is easy to become bogged down with our own countertransference, sentiment, and worry, and to become consumed by our work. A professional hazard, some might quip.
Therefore, personal restoration—a tool that keeps our clinical skills sharp—is vitally important to caring for oneself and one’s patient. Surprisingly, this can be neglected until we begin to experience burnout, which over time could transform into impairment, thus endangering ourselves, our patients, and our profession.
Over the past decade, physician impairment has been exhaustively described, researched, and addressed. However, most analyses have focused on identifying impairment, and offering guidance on how to properly report it. How do we shift from managing the crisis to preventing it? To answer this question, this article:
- reviews the dilemma of physician impairment
- explores the duty we have to patients, ourselves, and the profession
- discusses shifting the focus on impairment to prevention through well-being.
Continue to: Dilemma
Dilemma: Vulnerability to impairment
The cornerstone for well-being is a balanced life. No matter how much one loves his or her work, there must be balance between work, relationships, and hobbies. Without that equilibrium, everyone is put at risk.2
Just as our patients, we are not immune to mental illness, cognitive decline, or substance abuse.3 We might even be more susceptible. For many physicians, their identity is intimately tied to their work.4 Dr. Robin Weiss captured that intimate relationship5:
“… [A] therapist may spend hundreds of hours, perhaps more than a thousand, hearing about a patient’s most exalted aspirations and most murderous, hateful fantasies. During this time, the patient may endure excruciating losses, unbearable shame, bitter sadness and great triumphs. You may accompany patients through torturous adolescence into adulthood. Or you may meet them in middle age and be with them as they age and eventually die. You collaborate in a deep process of discovery. Few encounters are this deeply honest, and therefore intimate.”
Given the stories we hear and the resulting intimacy and countertransference that inevitably arise, psychiatrists are even more prone to burnout than other physicians.6 Physician impairment is a public health issue that affects not just physicians but also their families, colleagues, and patients.
“Impairment” for the purpose of this article means a physical, mental, or substance-related disorder that interferes with a physician’s ability to undertake professional activities competently and safely.7 Predisposing factors for physician impairment include an obsessive-compulsive personality type, a family history of mental illness, sensation-seeking behavior, denial of personal problems, perfectionism, and idealism.8,9 Also, work stress becomes a significant factor in already vulnerable physicians, leading to a greater risk for mental illness.10
Continue to: Some warning signs of impairment include...
Some warning signs of impairment include a lack of personal hygiene, emotional lability, sleep deprivation, inattention to our pages or phone calls, and increased professional errors.11 When it comes to addressing such impairment, previous research and literature has focused on how to monitor ourselves and our colleagues; anything less would put the reputation and integrity of the medical profession at risk.3 This has led to a culture of doing nothing but work until things go too far, and then reporting the problems. But what about intervening before things get too far?
Duty: To ourselves, our colleagues, and our patients
There has been much discussion on how to report impaired colleagues, but little on how to help and support ourselves and our colleagues before things escalate into serious problems. And this lack of discussion is at the detriment of individual practitioners, their families, and patients. Physicians-in-training, including psychiatric residents, are at particularly high risk for developing stress-related problems, depression, and substance misuse.12 Occupational demands, self-criticism, and denial of one’s distress are common among physicians, as is self-treatment with drugs and alcohol.13
We all know by now that doctors and physician health programs (PHPs) have a duty to report impaired colleagues who continue to practice despite reasonable offers of assistance. There are an abundance of PHPs that are in place to assist with such situations. The American Medical Association’s official position on reporting impairment is outlined in Policy H-275.952.7 There also is the Federation of State Medical Boards. Its policy states that PHPs have “a primary commitment to [help] state medical boards … protect the public … [These] programs [should] demonstrate an ongoing track of record of ensuring safety to the public and reveal deficiencies if they occur.”14
Legal and ethical issues, however, complicate interventions for colleagues who need assistance.15 Despite the existence of PHPs, it would be much easier—not to mention helpful—to help a colleague by carrying out early interventions.
Discussion: Prevention as a solution
More emphasis should be placed on prevention. That’s where self-care and well-being come into play. Awareness of and sensitivity to physician vulnerability, early detection, and prevention of impairment are important.
Continue to: There has been a paradigm shift in focus...
There has been a paradigm shift in focus across medical boards, professional societies, and medical colleges. They are recognizing that personal well-being can help prevent burnout and, in turn, change the landscape of medicine from endless work to balanced lives that yield more satisfying and joyful work. It is becoming an accepted fact in medicine that well-being is just as important as integrity, professionalism, and patient safety. For example, the American Academy of Medical Colleges (AAMC) issued a statement emphasizing the importance of clinician well-being and dedicated its June 2016 Leadership Forum to a range of topics addressing depression, resilience, burnout, and suicide in academic medicine.16
Anita Everett, MD, put the spotlight on physician well-being during her term as American Psychiatric Association President (2017 to 2018). She formed a specific workgroup on Physician Wellness and Burnout where there is a community focus on prevention and self-care.17 A strong sense of community and purpose is almost always part of the prescription for promoting greater well-being.2
The importance of this issue is also trickling down from policymakers into hospitals and community health centers. Consider an initiative at Minneapolis’s Hennepin County Medical Center. Leaders there created a “reset room” for physicians to quietly decompress. The room is complete with LED lights, flameless candles, a sound machine, comfortable chairs, several plants, and an “in use” sign on the door.18 Other personal strategies to help prevent burnout include making environmental changes, encouraging hobbies, and streamlining burdensome tasks such as paperwork and electronic medical record systems.
As physician health and well-being are finally emerging as a “hot topic,”2 educational and treatment resources are increasingly available for any of us to explore. Consider a simple Google search to look into your State’s PHPs, and get involved in your professional societies to make change.
The culture is starting to shift, and leading by example will be a key to propelling further progress in this area. Model our own self-care for colleagues and patients alike. As Mark Twain said, we might love our work, but we must remember that being solely defined by work comes to the detriment of our health. Maintaining balance is what will allow us to sustain long careers ahead doing what we love.
Psychiatry is a field of passion. The reward of experiencing growth and change alongside our patients is what bolsters us through years of difficult training, overnight shifts, endless paperwork, regulatory mandates, and frequent worry about our patients. As physicians, we don’t live for weekends as many other professionals do. To the contrary, we spend them on call, moonlighting, laboring over journal articles, and perfecting lectures.
That passion is what makes us trusted clinicians and experts in our field. It can also make it difficult for us to disconnect from our work, frequently leading to burnout. Physician self-care, support, and professional development are critical topics that modern-day medicine minimizes at the peril of physicians and public health.1
Psychiatry lends itself to a deep and intimate understanding of another human being. The therapist delves into the lives of his or her patients, hears their stories, and holds their secrets. In some cases, we might be the only ones who truly see patients for who they uniquely are, and come to understand them on a deeper level than their closest family and friends. This can be both thrilling and intense. As we delve into the psyche of another individual, contemplate which interpretation we should share, and resonate with our patients, it is easy to become bogged down with our own countertransference, sentiment, and worry, and to become consumed by our work. A professional hazard, some might quip.
Therefore, personal restoration—a tool that keeps our clinical skills sharp—is vitally important to caring for oneself and one’s patient. Surprisingly, this can be neglected until we begin to experience burnout, which over time could transform into impairment, thus endangering ourselves, our patients, and our profession.
Over the past decade, physician impairment has been exhaustively described, researched, and addressed. However, most analyses have focused on identifying impairment, and offering guidance on how to properly report it. How do we shift from managing the crisis to preventing it? To answer this question, this article:
- reviews the dilemma of physician impairment
- explores the duty we have to patients, ourselves, and the profession
- discusses shifting the focus on impairment to prevention through well-being.
Continue to: Dilemma
Dilemma: Vulnerability to impairment
The cornerstone for well-being is a balanced life. No matter how much one loves his or her work, there must be balance between work, relationships, and hobbies. Without that equilibrium, everyone is put at risk.2
Just as our patients, we are not immune to mental illness, cognitive decline, or substance abuse.3 We might even be more susceptible. For many physicians, their identity is intimately tied to their work.4 Dr. Robin Weiss captured that intimate relationship5:
“… [A] therapist may spend hundreds of hours, perhaps more than a thousand, hearing about a patient’s most exalted aspirations and most murderous, hateful fantasies. During this time, the patient may endure excruciating losses, unbearable shame, bitter sadness and great triumphs. You may accompany patients through torturous adolescence into adulthood. Or you may meet them in middle age and be with them as they age and eventually die. You collaborate in a deep process of discovery. Few encounters are this deeply honest, and therefore intimate.”
Given the stories we hear and the resulting intimacy and countertransference that inevitably arise, psychiatrists are even more prone to burnout than other physicians.6 Physician impairment is a public health issue that affects not just physicians but also their families, colleagues, and patients.
“Impairment” for the purpose of this article means a physical, mental, or substance-related disorder that interferes with a physician’s ability to undertake professional activities competently and safely.7 Predisposing factors for physician impairment include an obsessive-compulsive personality type, a family history of mental illness, sensation-seeking behavior, denial of personal problems, perfectionism, and idealism.8,9 Also, work stress becomes a significant factor in already vulnerable physicians, leading to a greater risk for mental illness.10
Continue to: Some warning signs of impairment include...
Some warning signs of impairment include a lack of personal hygiene, emotional lability, sleep deprivation, inattention to our pages or phone calls, and increased professional errors.11 When it comes to addressing such impairment, previous research and literature has focused on how to monitor ourselves and our colleagues; anything less would put the reputation and integrity of the medical profession at risk.3 This has led to a culture of doing nothing but work until things go too far, and then reporting the problems. But what about intervening before things get too far?
Duty: To ourselves, our colleagues, and our patients
There has been much discussion on how to report impaired colleagues, but little on how to help and support ourselves and our colleagues before things escalate into serious problems. And this lack of discussion is at the detriment of individual practitioners, their families, and patients. Physicians-in-training, including psychiatric residents, are at particularly high risk for developing stress-related problems, depression, and substance misuse.12 Occupational demands, self-criticism, and denial of one’s distress are common among physicians, as is self-treatment with drugs and alcohol.13
We all know by now that doctors and physician health programs (PHPs) have a duty to report impaired colleagues who continue to practice despite reasonable offers of assistance. There are an abundance of PHPs that are in place to assist with such situations. The American Medical Association’s official position on reporting impairment is outlined in Policy H-275.952.7 There also is the Federation of State Medical Boards. Its policy states that PHPs have “a primary commitment to [help] state medical boards … protect the public … [These] programs [should] demonstrate an ongoing track of record of ensuring safety to the public and reveal deficiencies if they occur.”14
Legal and ethical issues, however, complicate interventions for colleagues who need assistance.15 Despite the existence of PHPs, it would be much easier—not to mention helpful—to help a colleague by carrying out early interventions.
Discussion: Prevention as a solution
More emphasis should be placed on prevention. That’s where self-care and well-being come into play. Awareness of and sensitivity to physician vulnerability, early detection, and prevention of impairment are important.
Continue to: There has been a paradigm shift in focus...
There has been a paradigm shift in focus across medical boards, professional societies, and medical colleges. They are recognizing that personal well-being can help prevent burnout and, in turn, change the landscape of medicine from endless work to balanced lives that yield more satisfying and joyful work. It is becoming an accepted fact in medicine that well-being is just as important as integrity, professionalism, and patient safety. For example, the American Academy of Medical Colleges (AAMC) issued a statement emphasizing the importance of clinician well-being and dedicated its June 2016 Leadership Forum to a range of topics addressing depression, resilience, burnout, and suicide in academic medicine.16
Anita Everett, MD, put the spotlight on physician well-being during her term as American Psychiatric Association President (2017 to 2018). She formed a specific workgroup on Physician Wellness and Burnout where there is a community focus on prevention and self-care.17 A strong sense of community and purpose is almost always part of the prescription for promoting greater well-being.2
The importance of this issue is also trickling down from policymakers into hospitals and community health centers. Consider an initiative at Minneapolis’s Hennepin County Medical Center. Leaders there created a “reset room” for physicians to quietly decompress. The room is complete with LED lights, flameless candles, a sound machine, comfortable chairs, several plants, and an “in use” sign on the door.18 Other personal strategies to help prevent burnout include making environmental changes, encouraging hobbies, and streamlining burdensome tasks such as paperwork and electronic medical record systems.
As physician health and well-being are finally emerging as a “hot topic,”2 educational and treatment resources are increasingly available for any of us to explore. Consider a simple Google search to look into your State’s PHPs, and get involved in your professional societies to make change.
The culture is starting to shift, and leading by example will be a key to propelling further progress in this area. Model our own self-care for colleagues and patients alike. As Mark Twain said, we might love our work, but we must remember that being solely defined by work comes to the detriment of our health. Maintaining balance is what will allow us to sustain long careers ahead doing what we love.
1. Mahoney, D, Freedy J, Brock C. Improving physician well-being. JAMA Intern Med. 2015;175(4):648-649.
2. Yellowlees P. Addressing physician health and well-being is patient safety issue. Psychiatric News. 2018;53(12):20-21.
3. Mossman D, Farrell HM. Physician impairment: when should you report? Current Psychiatry. 2011;10(9):67-71.
4. Lindeman S, Henriksson M, Isometsä E, et al. Treatment of mental disorders in seven physicians committing suicide. Crisis. 1999;20(2):86-89.
5. Weiss R. How therapists mourn. New York Times. July 4, 2015:SR2.
6. Kumar S. Burnout in psychiatrists. World Psychiatry. 2007;6(3):186-189.
7. American Medical Association. Report 2 of the Council on Science and Public Health (A-11). Physician health programs (Reference Committee D). https://www.ama-assn.org/sites/default/files/media-browser/public/about-ama/councils/Council%20Reports/council-on-science-public-health/a11-csaph-physician-health-programs.pdf. Accessed August 6, 2018.
8. Boisaubin EV, Levine RE. Identifying and assisting the impaired physician. Am J Med Sci. 2001;322(1):31-36.
9. Bissel L, Jones RW. The alcoholic physician: a survey. Am J Psychiatry. 1976;133(10):1142-1146.
10. Vaillant GE, Sobowale NC, McArthur C. Some psychologic vulnerabilities of physicians. N Engl J Med. 1972;287(8):372-375.
11. McGovern MP, Agnes DH, Leon S. Characteristics of physicians presenting for assessment at a behavioral health center. J Addict Dis. 2000;19(2):59-73.
12. Broquet KE, Rockey PH. Teaching residents and program directors about physician impairment. Acad Psychiatry. 2004;28(3):221-225.
13. Meier DE, Back AL, Morrison RS. The inner life of physicians and care of the seriously ill. JAMA. 2001;286(23):3007-3014.
14. Federation of State Medical Boards of the United States. Policy on physician impairment. http://www.csam-asam.org/pdf/misc/FSMB2011.pdf. Published 2011. Accessed July 15, 2018.
15. Bright RP, Krahn L. Impaired physicians: how to recognize, when to report, and where to refer. Current Psychiatry. 2010;9(6):11-20.
16. Academy of American Colleges. Well-being in academic medicine. https://www.aamc.org/initiatives/462280/well-being-academic-medicine.html. Updated July 9, 2018. Accessed July 17, 2018.
17. American Psychiatric Association. Well-being and burnout. https://www.psychiatry.org/psychiatrists/practice/well-being-and-burnout. Updated February 22, 2018. Accessed July 17, 2018.
18. Parks T. Physicians take to “reset room” to battle burnout. AMA Wire. https://wire.ama-assn.org/practice-management/physicians-take-reset-room-battle-burnout. Published June 8, 2016. Accessed July 18, 2018.
1. Mahoney, D, Freedy J, Brock C. Improving physician well-being. JAMA Intern Med. 2015;175(4):648-649.
2. Yellowlees P. Addressing physician health and well-being is patient safety issue. Psychiatric News. 2018;53(12):20-21.
3. Mossman D, Farrell HM. Physician impairment: when should you report? Current Psychiatry. 2011;10(9):67-71.
4. Lindeman S, Henriksson M, Isometsä E, et al. Treatment of mental disorders in seven physicians committing suicide. Crisis. 1999;20(2):86-89.
5. Weiss R. How therapists mourn. New York Times. July 4, 2015:SR2.
6. Kumar S. Burnout in psychiatrists. World Psychiatry. 2007;6(3):186-189.
7. American Medical Association. Report 2 of the Council on Science and Public Health (A-11). Physician health programs (Reference Committee D). https://www.ama-assn.org/sites/default/files/media-browser/public/about-ama/councils/Council%20Reports/council-on-science-public-health/a11-csaph-physician-health-programs.pdf. Accessed August 6, 2018.
8. Boisaubin EV, Levine RE. Identifying and assisting the impaired physician. Am J Med Sci. 2001;322(1):31-36.
9. Bissel L, Jones RW. The alcoholic physician: a survey. Am J Psychiatry. 1976;133(10):1142-1146.
10. Vaillant GE, Sobowale NC, McArthur C. Some psychologic vulnerabilities of physicians. N Engl J Med. 1972;287(8):372-375.
11. McGovern MP, Agnes DH, Leon S. Characteristics of physicians presenting for assessment at a behavioral health center. J Addict Dis. 2000;19(2):59-73.
12. Broquet KE, Rockey PH. Teaching residents and program directors about physician impairment. Acad Psychiatry. 2004;28(3):221-225.
13. Meier DE, Back AL, Morrison RS. The inner life of physicians and care of the seriously ill. JAMA. 2001;286(23):3007-3014.
14. Federation of State Medical Boards of the United States. Policy on physician impairment. http://www.csam-asam.org/pdf/misc/FSMB2011.pdf. Published 2011. Accessed July 15, 2018.
15. Bright RP, Krahn L. Impaired physicians: how to recognize, when to report, and where to refer. Current Psychiatry. 2010;9(6):11-20.
16. Academy of American Colleges. Well-being in academic medicine. https://www.aamc.org/initiatives/462280/well-being-academic-medicine.html. Updated July 9, 2018. Accessed July 17, 2018.
17. American Psychiatric Association. Well-being and burnout. https://www.psychiatry.org/psychiatrists/practice/well-being-and-burnout. Updated February 22, 2018. Accessed July 17, 2018.
18. Parks T. Physicians take to “reset room” to battle burnout. AMA Wire. https://wire.ama-assn.org/practice-management/physicians-take-reset-room-battle-burnout. Published June 8, 2016. Accessed July 18, 2018.
Short sleep linked to elevated blood pressure
CHICAGO – Consider 24-hour ambulatory blood pressure monitoring when patients complain about not getting enough sleep. You might catch hypertension early, according to researchers from the University of Pennsylvania, Philadelphia, and elsewhere.
They found a less than 7 hours a night and a mean in the study of 5.5 hours. Every 2-2.5 minutes of lost sleep was associated with an increase of 1 mm Hg in 24-hour mean systolic blood pressure and a increase of 1 beat per minute in heart rate.
The relationship was independent of office BP, nocturnal dipping status, and BP variability. It held in both the obese and nonobese, and in patients with and without obstructive sleep apnea (OSA). However, the relationship was found only among subjects who were not on antihypertensive medications.
“Adults with shorter sleep duration may benefit from screening with 24-hour ambulatory BP monitoring to promote earlier detection of hypertension and potentially mitigate the” the risk of cardiovascular disease. “This may be particularly important in screening for masked hypertension,” meaning normal pressures in the office, but elevated pressures at home, said investigators led by Jordana Cohen, MD, of the department of medicine at the University of Pennsylvania in a presentation at the joint scientific sessions of AHA Council on Hypertension, AHA Council on Kidney in Cardiovascular Disease, and American Society of Hypertension.
Dr. Cohen suggested that perhaps the sympathetic and endothelial derangements that drive hypertension in OSA also affect people with insufficient sleep. It may be that the normal morning surge in blood pressure persists longer into the day, she suggested. The investigative team analyzed data from two studies. The first, LIMBS (Lifestyle Modification in BP Lowering Study), was a phase 2 trial assessing yoga for blood pressure lowering. It was conducted in West Philadelphia and excluded people with diabetes, hypertension, OSA, and kidney or cardiovascular disease. The new analysis included 66 LIMBS subjects who had 24-hour blood pressure monitoring and kept sleep diaries to record their sleep duration (J Clin Hypertens (Greenwich). 2016 Aug;18[8]:809-16).
The team also analyzed 153 subjects from the PISA (Penn Icelandic Sleep Apnea) cohort, an ongoing project assessing continuous positive airway pressure for OSA, among other things. PISA includes patients with OSA, diabetes, hypertension, and kidney or cardiovascular disease. Sleep duration in the 153 subjects was again self-reported, but corroborated by actigraphy (J Sleep Res. 2015 Jun;24[3]:328-38).
The new findings were driven mostly by higher daytime systolic BP among short sleepers in LIMBS, and higher systolic pressures during both day and night among short sleepers in PISA, compared with subjects who slept at least 7 hours, and a mean of 8.5 hours, with napping included in overall sleep duration assessment.
In LIMBS, the mean 24-hour systolic BP was 12.7 mm Hg higher and the average heart rate 8 bpm faster among short sleepers; in PISA, the mean 24-hour systolic BP was 4.7 mm Hg higher and the heart rate 2 bpm faster.
Every 2.57 minutes of sleep lost in LIMBS and every 1.99 minute of lost sleep in PISA was associated with a 1–mm Hg gain in mean systolic BP and about a 1-bpm increase in heart rate. The findings were statistically significant and adjusted for age, race, body mass index, nocturnal dipping status, and office systolic blood pressure.
Baseline characteristics were generally well matched between short and long sleepers in both studies. However, while mean office systolic BP in LIMBS was the same in both sleep groups at about 139 mm Hg, the mean office systolic BP among long sleepers in PISA was 130 mm Hg versus 136 mm Hg among short sleepers, a significant difference.
It’s unclear why some people slept less, Dr. Cohen said, and the use of sleeping pills wasn’t considered in the analysis. Patients were an average of about 50 years old, with a body mass index of about 30 mg/m2. The same model of 24-hour blood pressure monitor was used in both studies.
The work was funded by the National Institutes of Health. The investigators had no disclosures.
CHICAGO – Consider 24-hour ambulatory blood pressure monitoring when patients complain about not getting enough sleep. You might catch hypertension early, according to researchers from the University of Pennsylvania, Philadelphia, and elsewhere.
They found a less than 7 hours a night and a mean in the study of 5.5 hours. Every 2-2.5 minutes of lost sleep was associated with an increase of 1 mm Hg in 24-hour mean systolic blood pressure and a increase of 1 beat per minute in heart rate.
The relationship was independent of office BP, nocturnal dipping status, and BP variability. It held in both the obese and nonobese, and in patients with and without obstructive sleep apnea (OSA). However, the relationship was found only among subjects who were not on antihypertensive medications.
“Adults with shorter sleep duration may benefit from screening with 24-hour ambulatory BP monitoring to promote earlier detection of hypertension and potentially mitigate the” the risk of cardiovascular disease. “This may be particularly important in screening for masked hypertension,” meaning normal pressures in the office, but elevated pressures at home, said investigators led by Jordana Cohen, MD, of the department of medicine at the University of Pennsylvania in a presentation at the joint scientific sessions of AHA Council on Hypertension, AHA Council on Kidney in Cardiovascular Disease, and American Society of Hypertension.
Dr. Cohen suggested that perhaps the sympathetic and endothelial derangements that drive hypertension in OSA also affect people with insufficient sleep. It may be that the normal morning surge in blood pressure persists longer into the day, she suggested. The investigative team analyzed data from two studies. The first, LIMBS (Lifestyle Modification in BP Lowering Study), was a phase 2 trial assessing yoga for blood pressure lowering. It was conducted in West Philadelphia and excluded people with diabetes, hypertension, OSA, and kidney or cardiovascular disease. The new analysis included 66 LIMBS subjects who had 24-hour blood pressure monitoring and kept sleep diaries to record their sleep duration (J Clin Hypertens (Greenwich). 2016 Aug;18[8]:809-16).
The team also analyzed 153 subjects from the PISA (Penn Icelandic Sleep Apnea) cohort, an ongoing project assessing continuous positive airway pressure for OSA, among other things. PISA includes patients with OSA, diabetes, hypertension, and kidney or cardiovascular disease. Sleep duration in the 153 subjects was again self-reported, but corroborated by actigraphy (J Sleep Res. 2015 Jun;24[3]:328-38).
The new findings were driven mostly by higher daytime systolic BP among short sleepers in LIMBS, and higher systolic pressures during both day and night among short sleepers in PISA, compared with subjects who slept at least 7 hours, and a mean of 8.5 hours, with napping included in overall sleep duration assessment.
In LIMBS, the mean 24-hour systolic BP was 12.7 mm Hg higher and the average heart rate 8 bpm faster among short sleepers; in PISA, the mean 24-hour systolic BP was 4.7 mm Hg higher and the heart rate 2 bpm faster.
Every 2.57 minutes of sleep lost in LIMBS and every 1.99 minute of lost sleep in PISA was associated with a 1–mm Hg gain in mean systolic BP and about a 1-bpm increase in heart rate. The findings were statistically significant and adjusted for age, race, body mass index, nocturnal dipping status, and office systolic blood pressure.
Baseline characteristics were generally well matched between short and long sleepers in both studies. However, while mean office systolic BP in LIMBS was the same in both sleep groups at about 139 mm Hg, the mean office systolic BP among long sleepers in PISA was 130 mm Hg versus 136 mm Hg among short sleepers, a significant difference.
It’s unclear why some people slept less, Dr. Cohen said, and the use of sleeping pills wasn’t considered in the analysis. Patients were an average of about 50 years old, with a body mass index of about 30 mg/m2. The same model of 24-hour blood pressure monitor was used in both studies.
The work was funded by the National Institutes of Health. The investigators had no disclosures.
CHICAGO – Consider 24-hour ambulatory blood pressure monitoring when patients complain about not getting enough sleep. You might catch hypertension early, according to researchers from the University of Pennsylvania, Philadelphia, and elsewhere.
They found a less than 7 hours a night and a mean in the study of 5.5 hours. Every 2-2.5 minutes of lost sleep was associated with an increase of 1 mm Hg in 24-hour mean systolic blood pressure and a increase of 1 beat per minute in heart rate.
The relationship was independent of office BP, nocturnal dipping status, and BP variability. It held in both the obese and nonobese, and in patients with and without obstructive sleep apnea (OSA). However, the relationship was found only among subjects who were not on antihypertensive medications.
“Adults with shorter sleep duration may benefit from screening with 24-hour ambulatory BP monitoring to promote earlier detection of hypertension and potentially mitigate the” the risk of cardiovascular disease. “This may be particularly important in screening for masked hypertension,” meaning normal pressures in the office, but elevated pressures at home, said investigators led by Jordana Cohen, MD, of the department of medicine at the University of Pennsylvania in a presentation at the joint scientific sessions of AHA Council on Hypertension, AHA Council on Kidney in Cardiovascular Disease, and American Society of Hypertension.
Dr. Cohen suggested that perhaps the sympathetic and endothelial derangements that drive hypertension in OSA also affect people with insufficient sleep. It may be that the normal morning surge in blood pressure persists longer into the day, she suggested. The investigative team analyzed data from two studies. The first, LIMBS (Lifestyle Modification in BP Lowering Study), was a phase 2 trial assessing yoga for blood pressure lowering. It was conducted in West Philadelphia and excluded people with diabetes, hypertension, OSA, and kidney or cardiovascular disease. The new analysis included 66 LIMBS subjects who had 24-hour blood pressure monitoring and kept sleep diaries to record their sleep duration (J Clin Hypertens (Greenwich). 2016 Aug;18[8]:809-16).
The team also analyzed 153 subjects from the PISA (Penn Icelandic Sleep Apnea) cohort, an ongoing project assessing continuous positive airway pressure for OSA, among other things. PISA includes patients with OSA, diabetes, hypertension, and kidney or cardiovascular disease. Sleep duration in the 153 subjects was again self-reported, but corroborated by actigraphy (J Sleep Res. 2015 Jun;24[3]:328-38).
The new findings were driven mostly by higher daytime systolic BP among short sleepers in LIMBS, and higher systolic pressures during both day and night among short sleepers in PISA, compared with subjects who slept at least 7 hours, and a mean of 8.5 hours, with napping included in overall sleep duration assessment.
In LIMBS, the mean 24-hour systolic BP was 12.7 mm Hg higher and the average heart rate 8 bpm faster among short sleepers; in PISA, the mean 24-hour systolic BP was 4.7 mm Hg higher and the heart rate 2 bpm faster.
Every 2.57 minutes of sleep lost in LIMBS and every 1.99 minute of lost sleep in PISA was associated with a 1–mm Hg gain in mean systolic BP and about a 1-bpm increase in heart rate. The findings were statistically significant and adjusted for age, race, body mass index, nocturnal dipping status, and office systolic blood pressure.
Baseline characteristics were generally well matched between short and long sleepers in both studies. However, while mean office systolic BP in LIMBS was the same in both sleep groups at about 139 mm Hg, the mean office systolic BP among long sleepers in PISA was 130 mm Hg versus 136 mm Hg among short sleepers, a significant difference.
It’s unclear why some people slept less, Dr. Cohen said, and the use of sleeping pills wasn’t considered in the analysis. Patients were an average of about 50 years old, with a body mass index of about 30 mg/m2. The same model of 24-hour blood pressure monitor was used in both studies.
The work was funded by the National Institutes of Health. The investigators had no disclosures.
REPORTING FROM JOINT HYPERTENSION 2018
Key clinical point: Ambulatory blood pressure monitoring in patients complaining about lack of sleep could detect hypertension.
Major finding: Every 2-2.5 minutes of lost sleep was associated with a 1–mm Hg increase in 24-hour mean systolic blood pressure and a 1-bpm increase in heart rate.
Study details: Post-hoc review of 219 patients in two trials
Disclosures: The work was funded by the National Institutes of Health. The investigators didn’t have any disclosures.
The benzodiazepine dilemma
As clinicians, we are faced with a conflict when deciding whether or not to prescribe a benzodiazepine. If we prescribe one of these agents, we might be putting our patients at risk for dependence and abuse. However, if we do not prescribe them, we risk providing inadequate treatment, especially for patients with panic disorder.
Benzodiazepine dependence and abuse can take many forms. Dependence can be psychological as well as physiologic. While many patients will adhere to their prescribing regimen, some may sell their benzodiazepines, falsely claim that they have “panic attacks,” or take a fatal overdose of an opioid and benzodiazepine combination.
Here I discuss the pros and cons of restricting benzodiazepines use to low doses and/or combination therapy with antidepressants.
_
Weighing the benefits of restricted prescribing
Some double-blind studies referenced in the American Psychiatric Association (APA) 2010 Practice Guideline for the Treatment of Patients with Panic Disorder1 suggest that benzodiazepine duration of treatment and dosages should be severely restricted. These studies found that:
- Although the combination of a selective serotonin reuptake inhibitor (SSRI) and a benzodiazepine initially decreased the number of panic attacks more quickly than SSRI monotherapy, the 2 treatments are equally effective after 4 or 5 weeks.2,3
- For the treatment of panic disorder, a low dosage of a benzodiazepine (clonazepam 1 mg/d or alprazolam 2 mg/d) was as effective as a higher dosage (clonazepam 2 mg/d or alprazolam 6 mg/d).4,5
However, these studies could be misleading. They all excluded patients with a comorbid condition, such as bipolar disorder or depression, that was more severe than their panic disorder. Severe comorbidity is associated with more severe panic symptoms,6,7 which might require an SSRI/benzodiazepine combination or a higher benzodiazepine dosage.
The APA Practice Guideline suggests the following possible options:
- benzodiazepine augmentation if there is a partial response to an SSRI
- substitution with a different SSRI or a serotonin-norepinephrine reuptake inhibitor (SNRI) if there is no response to an SSRI
- benzodiazepine augmentation or substitution if there is still no therapeutic response.
Continue to: The APA Practice Guideline also states...
The APA Practice Guideline also states that although the highest “usual therapeutic dose” for panic disorder is clonazepam 2 mg/d or alprazolam 4 mg/d, “higher doses are sometimes used for patients who do not respond to the usual therapeutic dose.”1
Presumably, an SSRI/benzodiazepine combination should be considered if an SSRI alleviates major depressive disorder but does not alleviate a comorbid panic disorder. However, the APA Practice Guideline does not include studies that investigated this clinical scenario.
Monitor carefully for dependency/abuse
Restricting benzodiazepine use to low doses over a short period of time may decrease the risk of dependence and abuse. However, this practice may also limit or prevent effective treatment for adherent patients with panic disorder who do not adequately respond to SSRI or SNRI monotherapy.
Therefore, clinicians need to carefully differentiate between patients who are adherent to their prescribed dosages and those who may be at risk for benzodiazepine dependence and abuse. Consider using prescription drug monitoring programs and drug screens to help detect patients who “doctor shop” for benzodiazepines, or who could be abusing opioids, alcohol, marijuana, or other substances while taking a benzodiazepine.
1. American Psychiatric Association. Practice guideline for the treatment of patients with panic disorder, 2nd edition. Washington DC: American Psychiatric Association. 2010. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/panicdisorder.pdf. Accessed March 7, 2018.
2. Goddard AW, Brouette T, Almai A, et al. Early coadministration of clonazepam with sertraline for panic disorder. Arch Gen Psychiatry. 2001;58(7):681-686.
3. Pollack MH, Simon NM, Worthington JJ, et al. Combined paroxetine and clonazepam treatment strategies compared to paroxetine monotherapy for panic disorder. J Psychopharmacol. 2003;17(3):276-282.
4. Lydiard RB, Lesser IM, Ballenger JC, et al. A fixed-dose study of alprazolam 2 mg, alprazolam 6 mg, and placebo in panic disorder. J Clin Psychopharmacol. 1992;12(2):966-103.
5. Rosenbaum JF, Moroz G, Bowden CL. Clonazepam in the treatment of panic disorder with or without agoraphobia: a dose-response study of efficacy, safety, and discontinuance. Clonazepam Panic Disorder Dose-Response Study Group. J Clin Psychopharmacol. 1997;17(5):390-400.
6. Goodwin RD, Hoven CW. Bipolar-panic comorbidity in the general population: prevalence and associated morbidity. J Affect Disord. 2002;70(1):27-33.
7. Roy-Byrne PP, Stang P, Wittchen HU, et al. Lifetime panic-depression comorbidity in the National Comorbidity Survey. Association with symptoms, impairment, course and help-seeking. Br J Psychiatry. 2000;176:229-235.
As clinicians, we are faced with a conflict when deciding whether or not to prescribe a benzodiazepine. If we prescribe one of these agents, we might be putting our patients at risk for dependence and abuse. However, if we do not prescribe them, we risk providing inadequate treatment, especially for patients with panic disorder.
Benzodiazepine dependence and abuse can take many forms. Dependence can be psychological as well as physiologic. While many patients will adhere to their prescribing regimen, some may sell their benzodiazepines, falsely claim that they have “panic attacks,” or take a fatal overdose of an opioid and benzodiazepine combination.
Here I discuss the pros and cons of restricting benzodiazepines use to low doses and/or combination therapy with antidepressants.
_
Weighing the benefits of restricted prescribing
Some double-blind studies referenced in the American Psychiatric Association (APA) 2010 Practice Guideline for the Treatment of Patients with Panic Disorder1 suggest that benzodiazepine duration of treatment and dosages should be severely restricted. These studies found that:
- Although the combination of a selective serotonin reuptake inhibitor (SSRI) and a benzodiazepine initially decreased the number of panic attacks more quickly than SSRI monotherapy, the 2 treatments are equally effective after 4 or 5 weeks.2,3
- For the treatment of panic disorder, a low dosage of a benzodiazepine (clonazepam 1 mg/d or alprazolam 2 mg/d) was as effective as a higher dosage (clonazepam 2 mg/d or alprazolam 6 mg/d).4,5
However, these studies could be misleading. They all excluded patients with a comorbid condition, such as bipolar disorder or depression, that was more severe than their panic disorder. Severe comorbidity is associated with more severe panic symptoms,6,7 which might require an SSRI/benzodiazepine combination or a higher benzodiazepine dosage.
The APA Practice Guideline suggests the following possible options:
- benzodiazepine augmentation if there is a partial response to an SSRI
- substitution with a different SSRI or a serotonin-norepinephrine reuptake inhibitor (SNRI) if there is no response to an SSRI
- benzodiazepine augmentation or substitution if there is still no therapeutic response.
Continue to: The APA Practice Guideline also states...
The APA Practice Guideline also states that although the highest “usual therapeutic dose” for panic disorder is clonazepam 2 mg/d or alprazolam 4 mg/d, “higher doses are sometimes used for patients who do not respond to the usual therapeutic dose.”1
Presumably, an SSRI/benzodiazepine combination should be considered if an SSRI alleviates major depressive disorder but does not alleviate a comorbid panic disorder. However, the APA Practice Guideline does not include studies that investigated this clinical scenario.
Monitor carefully for dependency/abuse
Restricting benzodiazepine use to low doses over a short period of time may decrease the risk of dependence and abuse. However, this practice may also limit or prevent effective treatment for adherent patients with panic disorder who do not adequately respond to SSRI or SNRI monotherapy.
Therefore, clinicians need to carefully differentiate between patients who are adherent to their prescribed dosages and those who may be at risk for benzodiazepine dependence and abuse. Consider using prescription drug monitoring programs and drug screens to help detect patients who “doctor shop” for benzodiazepines, or who could be abusing opioids, alcohol, marijuana, or other substances while taking a benzodiazepine.
As clinicians, we are faced with a conflict when deciding whether or not to prescribe a benzodiazepine. If we prescribe one of these agents, we might be putting our patients at risk for dependence and abuse. However, if we do not prescribe them, we risk providing inadequate treatment, especially for patients with panic disorder.
Benzodiazepine dependence and abuse can take many forms. Dependence can be psychological as well as physiologic. While many patients will adhere to their prescribing regimen, some may sell their benzodiazepines, falsely claim that they have “panic attacks,” or take a fatal overdose of an opioid and benzodiazepine combination.
Here I discuss the pros and cons of restricting benzodiazepines use to low doses and/or combination therapy with antidepressants.
_
Weighing the benefits of restricted prescribing
Some double-blind studies referenced in the American Psychiatric Association (APA) 2010 Practice Guideline for the Treatment of Patients with Panic Disorder1 suggest that benzodiazepine duration of treatment and dosages should be severely restricted. These studies found that:
- Although the combination of a selective serotonin reuptake inhibitor (SSRI) and a benzodiazepine initially decreased the number of panic attacks more quickly than SSRI monotherapy, the 2 treatments are equally effective after 4 or 5 weeks.2,3
- For the treatment of panic disorder, a low dosage of a benzodiazepine (clonazepam 1 mg/d or alprazolam 2 mg/d) was as effective as a higher dosage (clonazepam 2 mg/d or alprazolam 6 mg/d).4,5
However, these studies could be misleading. They all excluded patients with a comorbid condition, such as bipolar disorder or depression, that was more severe than their panic disorder. Severe comorbidity is associated with more severe panic symptoms,6,7 which might require an SSRI/benzodiazepine combination or a higher benzodiazepine dosage.
The APA Practice Guideline suggests the following possible options:
- benzodiazepine augmentation if there is a partial response to an SSRI
- substitution with a different SSRI or a serotonin-norepinephrine reuptake inhibitor (SNRI) if there is no response to an SSRI
- benzodiazepine augmentation or substitution if there is still no therapeutic response.
Continue to: The APA Practice Guideline also states...
The APA Practice Guideline also states that although the highest “usual therapeutic dose” for panic disorder is clonazepam 2 mg/d or alprazolam 4 mg/d, “higher doses are sometimes used for patients who do not respond to the usual therapeutic dose.”1
Presumably, an SSRI/benzodiazepine combination should be considered if an SSRI alleviates major depressive disorder but does not alleviate a comorbid panic disorder. However, the APA Practice Guideline does not include studies that investigated this clinical scenario.
Monitor carefully for dependency/abuse
Restricting benzodiazepine use to low doses over a short period of time may decrease the risk of dependence and abuse. However, this practice may also limit or prevent effective treatment for adherent patients with panic disorder who do not adequately respond to SSRI or SNRI monotherapy.
Therefore, clinicians need to carefully differentiate between patients who are adherent to their prescribed dosages and those who may be at risk for benzodiazepine dependence and abuse. Consider using prescription drug monitoring programs and drug screens to help detect patients who “doctor shop” for benzodiazepines, or who could be abusing opioids, alcohol, marijuana, or other substances while taking a benzodiazepine.
1. American Psychiatric Association. Practice guideline for the treatment of patients with panic disorder, 2nd edition. Washington DC: American Psychiatric Association. 2010. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/panicdisorder.pdf. Accessed March 7, 2018.
2. Goddard AW, Brouette T, Almai A, et al. Early coadministration of clonazepam with sertraline for panic disorder. Arch Gen Psychiatry. 2001;58(7):681-686.
3. Pollack MH, Simon NM, Worthington JJ, et al. Combined paroxetine and clonazepam treatment strategies compared to paroxetine monotherapy for panic disorder. J Psychopharmacol. 2003;17(3):276-282.
4. Lydiard RB, Lesser IM, Ballenger JC, et al. A fixed-dose study of alprazolam 2 mg, alprazolam 6 mg, and placebo in panic disorder. J Clin Psychopharmacol. 1992;12(2):966-103.
5. Rosenbaum JF, Moroz G, Bowden CL. Clonazepam in the treatment of panic disorder with or without agoraphobia: a dose-response study of efficacy, safety, and discontinuance. Clonazepam Panic Disorder Dose-Response Study Group. J Clin Psychopharmacol. 1997;17(5):390-400.
6. Goodwin RD, Hoven CW. Bipolar-panic comorbidity in the general population: prevalence and associated morbidity. J Affect Disord. 2002;70(1):27-33.
7. Roy-Byrne PP, Stang P, Wittchen HU, et al. Lifetime panic-depression comorbidity in the National Comorbidity Survey. Association with symptoms, impairment, course and help-seeking. Br J Psychiatry. 2000;176:229-235.
1. American Psychiatric Association. Practice guideline for the treatment of patients with panic disorder, 2nd edition. Washington DC: American Psychiatric Association. 2010. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/panicdisorder.pdf. Accessed March 7, 2018.
2. Goddard AW, Brouette T, Almai A, et al. Early coadministration of clonazepam with sertraline for panic disorder. Arch Gen Psychiatry. 2001;58(7):681-686.
3. Pollack MH, Simon NM, Worthington JJ, et al. Combined paroxetine and clonazepam treatment strategies compared to paroxetine monotherapy for panic disorder. J Psychopharmacol. 2003;17(3):276-282.
4. Lydiard RB, Lesser IM, Ballenger JC, et al. A fixed-dose study of alprazolam 2 mg, alprazolam 6 mg, and placebo in panic disorder. J Clin Psychopharmacol. 1992;12(2):966-103.
5. Rosenbaum JF, Moroz G, Bowden CL. Clonazepam in the treatment of panic disorder with or without agoraphobia: a dose-response study of efficacy, safety, and discontinuance. Clonazepam Panic Disorder Dose-Response Study Group. J Clin Psychopharmacol. 1997;17(5):390-400.
6. Goodwin RD, Hoven CW. Bipolar-panic comorbidity in the general population: prevalence and associated morbidity. J Affect Disord. 2002;70(1):27-33.
7. Roy-Byrne PP, Stang P, Wittchen HU, et al. Lifetime panic-depression comorbidity in the National Comorbidity Survey. Association with symptoms, impairment, course and help-seeking. Br J Psychiatry. 2000;176:229-235.




