User login
-
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]


Cutaneous clues linked to COVID-19 coagulation risk
, new evidence suggests.
Researchers at Weill Cornell Medicine NewYork–Presbyterian Medical Center in New York linked livedoid and purpuric skin eruptions to a greater likelihood for occlusive vascular disease associated with SARS-CoV-2 infection in a small case series.
These skin signs could augment coagulation assays in this patient population. “Physicians should consider a hematology consult for potential anticoagulation in patients with these skin presentations and severe COVID-19,” senior author Joanna Harp, MD, said in an interview.
“Physicians should also consider D-dimer, fibrinogen, coagulation studies, and a skin biopsy given that there are other diagnoses on the differential as well.”
The research letter was published online on Aug. 5 in JAMA Dermatology.
The findings build on multiple previous reports of skin manifestations associated with COVID-19, including a study of 375 patients in Spain. Among people with suspected or confirmed SARS-CoV-2 infection, senior author of the Spanish research, Ignacio Garcia-Doval, MD, PhD, also observed livedoid and necrotic skin eruptions more commonly in severe disease.
“I think that this case series [from Harp and colleagues] confirms the findings of our previous paper – that patients with livedoid or necrotic lesions have a worse prognosis, as these are markers of vascular occlusion,” he said in an interview.
Dr. Harp and colleagues reported their observations with four patients aged 40-80 years. Each had severe COVID-19 with acute respiratory distress syndrome and required intubation. Treating clinicians requested a dermatology consult to assess acral fixed livedo racemosa and retiform purpura presentations.
D-dimer levels exceeded 3 mcg/mL in each case. All four patients had a suspected pulmonary embolism within 1-5 days of the dermatologic findings. Prophylactic anticoagulation at admission was changed to therapeutic anticoagulation because of increasing D-dimer levels and the suspected thrombotic events.
“I think that the paper is interesting because it shows the associated histopathological findings and has important clinical implications due to the association with pulmonary embolism,” said Dr. Garcia-Doval, a researcher at the Spanish Academy of Dermatology in Madrid. “These patients should probably be anticoagulated.”
Skin biopsy results
Punch biopsies revealed pauci-inflammatory thrombogenic vasculopathy involving capillaries, venules, arterioles, or small arteries.
Livedo racemosa skin findings point to partial occlusion of cutaneous blood vessels, whereas retiform purpura indicate full occlusion of cutaneous blood vessels.
An inability to confirm the exact timing of the onset of the skin rash was a limitation of the study.
“The findings suggest that clinicians caring for patients with COVID-19 should be aware of livedoid and purpuric rashes as potential manifestations of an underlying hypercoagulable state,” the authors noted. “If these skin findings are identified, a skin biopsy should be considered because the result may guide anticoagulation management.”
Observations during an outbreak
The researchers observed these cases between March 13 and April 3, during the peak of the COVID-19 outbreak in New York.
“We did see additional cases since our study period. However, it has decreased significantly with the falling number of COVID-19 cases in the city,” said Dr. Harp, a dermatologist at NewYork–Presbyterian.
Another contributing factor in the drop in cases was “implementation of earlier, more aggressive anticoagulation in many of these patients at our institution,” she added.
The investigators plan to continue the research. “We are working on a more formalized study,” lead author Caren Droesch, MD, said in an interview.
“But given very low patient numbers in our area we have not started recruiting patients,” said Dr. Droesch, a resident at Weill Cornell Medicine and NewYork–Presbyterian at the time of the study. She is now a dermatologist at Mass General Brigham in Wellesley, Mass.
Consider a dermatology consult
“This is a small case series of four patients, but mirrors what we have seen at our institution and what others have reported about individual patients around the world,” Anthony Fernandez, MD, PhD, a dermatologist at Cleveland Clinic, said in an interview. “The skin, like many other organ systems, can be affected by thrombotic events within the setting of COVID-19 disease.”
As in the current study, Dr. Fernandez observed skin manifestations in people with severe COVID-19 with elevated D-dimer levels. These patients typically require mechanical ventilation in the intensive care unit, he added.
“As these authors point out, it is important for all clinicians caring for COVID-19 patients to look for these rashes,” said Dr. Fernandez, who coauthored a report on skin manifestations in this patient population. “We also agree that clinicians should have a low threshold for consulting dermatology. A skin biopsy is minimally invasive and can be important in confirming or refuting that such rashes are truly reflective of thrombotic vasculopathy.”
Dr. Harp, Dr. Droesch and Dr. Garcia-Doval have disclosed no relevant financial relationships. Dr. Fernandez received funding from the Clinical and Translational Science Collaborative at Case Western Reserve University to study skin manifestations of COVID-19.
A version of this article originally appeared on Medscape.com.
, new evidence suggests.
Researchers at Weill Cornell Medicine NewYork–Presbyterian Medical Center in New York linked livedoid and purpuric skin eruptions to a greater likelihood for occlusive vascular disease associated with SARS-CoV-2 infection in a small case series.
These skin signs could augment coagulation assays in this patient population. “Physicians should consider a hematology consult for potential anticoagulation in patients with these skin presentations and severe COVID-19,” senior author Joanna Harp, MD, said in an interview.
“Physicians should also consider D-dimer, fibrinogen, coagulation studies, and a skin biopsy given that there are other diagnoses on the differential as well.”
The research letter was published online on Aug. 5 in JAMA Dermatology.
The findings build on multiple previous reports of skin manifestations associated with COVID-19, including a study of 375 patients in Spain. Among people with suspected or confirmed SARS-CoV-2 infection, senior author of the Spanish research, Ignacio Garcia-Doval, MD, PhD, also observed livedoid and necrotic skin eruptions more commonly in severe disease.
“I think that this case series [from Harp and colleagues] confirms the findings of our previous paper – that patients with livedoid or necrotic lesions have a worse prognosis, as these are markers of vascular occlusion,” he said in an interview.
Dr. Harp and colleagues reported their observations with four patients aged 40-80 years. Each had severe COVID-19 with acute respiratory distress syndrome and required intubation. Treating clinicians requested a dermatology consult to assess acral fixed livedo racemosa and retiform purpura presentations.
D-dimer levels exceeded 3 mcg/mL in each case. All four patients had a suspected pulmonary embolism within 1-5 days of the dermatologic findings. Prophylactic anticoagulation at admission was changed to therapeutic anticoagulation because of increasing D-dimer levels and the suspected thrombotic events.
“I think that the paper is interesting because it shows the associated histopathological findings and has important clinical implications due to the association with pulmonary embolism,” said Dr. Garcia-Doval, a researcher at the Spanish Academy of Dermatology in Madrid. “These patients should probably be anticoagulated.”
Skin biopsy results
Punch biopsies revealed pauci-inflammatory thrombogenic vasculopathy involving capillaries, venules, arterioles, or small arteries.
Livedo racemosa skin findings point to partial occlusion of cutaneous blood vessels, whereas retiform purpura indicate full occlusion of cutaneous blood vessels.
An inability to confirm the exact timing of the onset of the skin rash was a limitation of the study.
“The findings suggest that clinicians caring for patients with COVID-19 should be aware of livedoid and purpuric rashes as potential manifestations of an underlying hypercoagulable state,” the authors noted. “If these skin findings are identified, a skin biopsy should be considered because the result may guide anticoagulation management.”
Observations during an outbreak
The researchers observed these cases between March 13 and April 3, during the peak of the COVID-19 outbreak in New York.
“We did see additional cases since our study period. However, it has decreased significantly with the falling number of COVID-19 cases in the city,” said Dr. Harp, a dermatologist at NewYork–Presbyterian.
Another contributing factor in the drop in cases was “implementation of earlier, more aggressive anticoagulation in many of these patients at our institution,” she added.
The investigators plan to continue the research. “We are working on a more formalized study,” lead author Caren Droesch, MD, said in an interview.
“But given very low patient numbers in our area we have not started recruiting patients,” said Dr. Droesch, a resident at Weill Cornell Medicine and NewYork–Presbyterian at the time of the study. She is now a dermatologist at Mass General Brigham in Wellesley, Mass.
Consider a dermatology consult
“This is a small case series of four patients, but mirrors what we have seen at our institution and what others have reported about individual patients around the world,” Anthony Fernandez, MD, PhD, a dermatologist at Cleveland Clinic, said in an interview. “The skin, like many other organ systems, can be affected by thrombotic events within the setting of COVID-19 disease.”
As in the current study, Dr. Fernandez observed skin manifestations in people with severe COVID-19 with elevated D-dimer levels. These patients typically require mechanical ventilation in the intensive care unit, he added.
“As these authors point out, it is important for all clinicians caring for COVID-19 patients to look for these rashes,” said Dr. Fernandez, who coauthored a report on skin manifestations in this patient population. “We also agree that clinicians should have a low threshold for consulting dermatology. A skin biopsy is minimally invasive and can be important in confirming or refuting that such rashes are truly reflective of thrombotic vasculopathy.”
Dr. Harp, Dr. Droesch and Dr. Garcia-Doval have disclosed no relevant financial relationships. Dr. Fernandez received funding from the Clinical and Translational Science Collaborative at Case Western Reserve University to study skin manifestations of COVID-19.
A version of this article originally appeared on Medscape.com.
, new evidence suggests.
Researchers at Weill Cornell Medicine NewYork–Presbyterian Medical Center in New York linked livedoid and purpuric skin eruptions to a greater likelihood for occlusive vascular disease associated with SARS-CoV-2 infection in a small case series.
These skin signs could augment coagulation assays in this patient population. “Physicians should consider a hematology consult for potential anticoagulation in patients with these skin presentations and severe COVID-19,” senior author Joanna Harp, MD, said in an interview.
“Physicians should also consider D-dimer, fibrinogen, coagulation studies, and a skin biopsy given that there are other diagnoses on the differential as well.”
The research letter was published online on Aug. 5 in JAMA Dermatology.
The findings build on multiple previous reports of skin manifestations associated with COVID-19, including a study of 375 patients in Spain. Among people with suspected or confirmed SARS-CoV-2 infection, senior author of the Spanish research, Ignacio Garcia-Doval, MD, PhD, also observed livedoid and necrotic skin eruptions more commonly in severe disease.
“I think that this case series [from Harp and colleagues] confirms the findings of our previous paper – that patients with livedoid or necrotic lesions have a worse prognosis, as these are markers of vascular occlusion,” he said in an interview.
Dr. Harp and colleagues reported their observations with four patients aged 40-80 years. Each had severe COVID-19 with acute respiratory distress syndrome and required intubation. Treating clinicians requested a dermatology consult to assess acral fixed livedo racemosa and retiform purpura presentations.
D-dimer levels exceeded 3 mcg/mL in each case. All four patients had a suspected pulmonary embolism within 1-5 days of the dermatologic findings. Prophylactic anticoagulation at admission was changed to therapeutic anticoagulation because of increasing D-dimer levels and the suspected thrombotic events.
“I think that the paper is interesting because it shows the associated histopathological findings and has important clinical implications due to the association with pulmonary embolism,” said Dr. Garcia-Doval, a researcher at the Spanish Academy of Dermatology in Madrid. “These patients should probably be anticoagulated.”
Skin biopsy results
Punch biopsies revealed pauci-inflammatory thrombogenic vasculopathy involving capillaries, venules, arterioles, or small arteries.
Livedo racemosa skin findings point to partial occlusion of cutaneous blood vessels, whereas retiform purpura indicate full occlusion of cutaneous blood vessels.
An inability to confirm the exact timing of the onset of the skin rash was a limitation of the study.
“The findings suggest that clinicians caring for patients with COVID-19 should be aware of livedoid and purpuric rashes as potential manifestations of an underlying hypercoagulable state,” the authors noted. “If these skin findings are identified, a skin biopsy should be considered because the result may guide anticoagulation management.”
Observations during an outbreak
The researchers observed these cases between March 13 and April 3, during the peak of the COVID-19 outbreak in New York.
“We did see additional cases since our study period. However, it has decreased significantly with the falling number of COVID-19 cases in the city,” said Dr. Harp, a dermatologist at NewYork–Presbyterian.
Another contributing factor in the drop in cases was “implementation of earlier, more aggressive anticoagulation in many of these patients at our institution,” she added.
The investigators plan to continue the research. “We are working on a more formalized study,” lead author Caren Droesch, MD, said in an interview.
“But given very low patient numbers in our area we have not started recruiting patients,” said Dr. Droesch, a resident at Weill Cornell Medicine and NewYork–Presbyterian at the time of the study. She is now a dermatologist at Mass General Brigham in Wellesley, Mass.
Consider a dermatology consult
“This is a small case series of four patients, but mirrors what we have seen at our institution and what others have reported about individual patients around the world,” Anthony Fernandez, MD, PhD, a dermatologist at Cleveland Clinic, said in an interview. “The skin, like many other organ systems, can be affected by thrombotic events within the setting of COVID-19 disease.”
As in the current study, Dr. Fernandez observed skin manifestations in people with severe COVID-19 with elevated D-dimer levels. These patients typically require mechanical ventilation in the intensive care unit, he added.
“As these authors point out, it is important for all clinicians caring for COVID-19 patients to look for these rashes,” said Dr. Fernandez, who coauthored a report on skin manifestations in this patient population. “We also agree that clinicians should have a low threshold for consulting dermatology. A skin biopsy is minimally invasive and can be important in confirming or refuting that such rashes are truly reflective of thrombotic vasculopathy.”
Dr. Harp, Dr. Droesch and Dr. Garcia-Doval have disclosed no relevant financial relationships. Dr. Fernandez received funding from the Clinical and Translational Science Collaborative at Case Western Reserve University to study skin manifestations of COVID-19.
A version of this article originally appeared on Medscape.com.
FROM JAMA DERMATOLOGY
When you see something ...
Over the last several decades science has fallen off this country’s radar screen. Yes, STEM (science, technology, engineering, and mathematics) has recently had a brief moment in the spotlight as a buzzword de jour. But the critical importance of careful and systematic investigation into the world around us using observation and trial and error is a tough sell to a large segment of our population.
The COVID-19 pandemic is providing an excellent opportunity for science and medicine to showcase their star qualities. Of course some people in leadership positions persist in disregarding the value of scientific investigation. But I get the feeling that the fear generated by the pandemic is creating some converts among many previous science skeptics. This gathering enthusiasm among the general population is a predictably slow process because that’s the way science works. It often doesn’t provide quick answers. And it is difficult for the nonscientist to see the beauty in the reality that the things we thought were true 2 months ago are likely to be proven wrong today as more observations accumulate.
A recent New York Times article examines the career of one such unscrupulous physician/scientist whose recent exploits threaten to undo much of the positive image the pandemic has cast on science (“The Doctor Behind the Disputed Covid Data,” by Ellen Gabler and Roni Caryn Rabin, The New York Times, July 27, 2020). The subject of the article is the physician who was responsible for providing some of the large data sets on which several papers were published about the apparent ineffectiveness and danger of using hydroxychloroquine in COVID-19 patients. The authenticity of the data sets recently has been seriously questioned, and the articles have been retracted by the journals in which they had appeared.
Based on numerous interviews with coworkers, the Times reporters present a strong case that this individual’s long history of unreliability make his association with allegedly fraudulent data set not surprising but maybe even predictable. At one point in his training, there appears to have been serious questions about advancing the physician to the next level. Despite these concerns, he was allowed to continue and complete his specialty training. It is of note that in his last year of clinical practice, the physician became the subject of three serious malpractice claims that question his competence.
I suspect that some of you have crossed paths with physicians whose competence and/or moral character you found concerning. Were they peers? Were you the individual’s supervisor or was he or she your mentor? How did you respond? Did anyone respond at all?
There has been a lot written and said in recent months about how and when to respond to respond to sexual harassment in the workplace. But I don’t recall reading any articles that discuss how one should respond to incompetence. Of course competency can be a relative term, but in most cases significant incompetence is hard to miss because it tends to be repeated.
It is easy for the airports and subway systems to post signs that say “If you see something say something.” It’s a different story for hospitals and medical schools that may have systems in place for reporting and following up on poor practice. But my sense is that there are too many cases that slip through the cracks.
This is another example of a problem for which I don’t have a solution. However, if this column prompts just one of you who sees something to say something then I have had a good day.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Over the last several decades science has fallen off this country’s radar screen. Yes, STEM (science, technology, engineering, and mathematics) has recently had a brief moment in the spotlight as a buzzword de jour. But the critical importance of careful and systematic investigation into the world around us using observation and trial and error is a tough sell to a large segment of our population.
The COVID-19 pandemic is providing an excellent opportunity for science and medicine to showcase their star qualities. Of course some people in leadership positions persist in disregarding the value of scientific investigation. But I get the feeling that the fear generated by the pandemic is creating some converts among many previous science skeptics. This gathering enthusiasm among the general population is a predictably slow process because that’s the way science works. It often doesn’t provide quick answers. And it is difficult for the nonscientist to see the beauty in the reality that the things we thought were true 2 months ago are likely to be proven wrong today as more observations accumulate.
A recent New York Times article examines the career of one such unscrupulous physician/scientist whose recent exploits threaten to undo much of the positive image the pandemic has cast on science (“The Doctor Behind the Disputed Covid Data,” by Ellen Gabler and Roni Caryn Rabin, The New York Times, July 27, 2020). The subject of the article is the physician who was responsible for providing some of the large data sets on which several papers were published about the apparent ineffectiveness and danger of using hydroxychloroquine in COVID-19 patients. The authenticity of the data sets recently has been seriously questioned, and the articles have been retracted by the journals in which they had appeared.
Based on numerous interviews with coworkers, the Times reporters present a strong case that this individual’s long history of unreliability make his association with allegedly fraudulent data set not surprising but maybe even predictable. At one point in his training, there appears to have been serious questions about advancing the physician to the next level. Despite these concerns, he was allowed to continue and complete his specialty training. It is of note that in his last year of clinical practice, the physician became the subject of three serious malpractice claims that question his competence.
I suspect that some of you have crossed paths with physicians whose competence and/or moral character you found concerning. Were they peers? Were you the individual’s supervisor or was he or she your mentor? How did you respond? Did anyone respond at all?
There has been a lot written and said in recent months about how and when to respond to respond to sexual harassment in the workplace. But I don’t recall reading any articles that discuss how one should respond to incompetence. Of course competency can be a relative term, but in most cases significant incompetence is hard to miss because it tends to be repeated.
It is easy for the airports and subway systems to post signs that say “If you see something say something.” It’s a different story for hospitals and medical schools that may have systems in place for reporting and following up on poor practice. But my sense is that there are too many cases that slip through the cracks.
This is another example of a problem for which I don’t have a solution. However, if this column prompts just one of you who sees something to say something then I have had a good day.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Over the last several decades science has fallen off this country’s radar screen. Yes, STEM (science, technology, engineering, and mathematics) has recently had a brief moment in the spotlight as a buzzword de jour. But the critical importance of careful and systematic investigation into the world around us using observation and trial and error is a tough sell to a large segment of our population.
The COVID-19 pandemic is providing an excellent opportunity for science and medicine to showcase their star qualities. Of course some people in leadership positions persist in disregarding the value of scientific investigation. But I get the feeling that the fear generated by the pandemic is creating some converts among many previous science skeptics. This gathering enthusiasm among the general population is a predictably slow process because that’s the way science works. It often doesn’t provide quick answers. And it is difficult for the nonscientist to see the beauty in the reality that the things we thought were true 2 months ago are likely to be proven wrong today as more observations accumulate.
A recent New York Times article examines the career of one such unscrupulous physician/scientist whose recent exploits threaten to undo much of the positive image the pandemic has cast on science (“The Doctor Behind the Disputed Covid Data,” by Ellen Gabler and Roni Caryn Rabin, The New York Times, July 27, 2020). The subject of the article is the physician who was responsible for providing some of the large data sets on which several papers were published about the apparent ineffectiveness and danger of using hydroxychloroquine in COVID-19 patients. The authenticity of the data sets recently has been seriously questioned, and the articles have been retracted by the journals in which they had appeared.
Based on numerous interviews with coworkers, the Times reporters present a strong case that this individual’s long history of unreliability make his association with allegedly fraudulent data set not surprising but maybe even predictable. At one point in his training, there appears to have been serious questions about advancing the physician to the next level. Despite these concerns, he was allowed to continue and complete his specialty training. It is of note that in his last year of clinical practice, the physician became the subject of three serious malpractice claims that question his competence.
I suspect that some of you have crossed paths with physicians whose competence and/or moral character you found concerning. Were they peers? Were you the individual’s supervisor or was he or she your mentor? How did you respond? Did anyone respond at all?
There has been a lot written and said in recent months about how and when to respond to respond to sexual harassment in the workplace. But I don’t recall reading any articles that discuss how one should respond to incompetence. Of course competency can be a relative term, but in most cases significant incompetence is hard to miss because it tends to be repeated.
It is easy for the airports and subway systems to post signs that say “If you see something say something.” It’s a different story for hospitals and medical schools that may have systems in place for reporting and following up on poor practice. But my sense is that there are too many cases that slip through the cracks.
This is another example of a problem for which I don’t have a solution. However, if this column prompts just one of you who sees something to say something then I have had a good day.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Medicare sticks with E/M pay plan over some groups’ objections
The Trump administration is sticking with a plan to boost certain Medicare pay for many primary care and other specialties focused heavily on office visits while lowering that for other groups to balance these increased costs.
On Aug. 4, the Centers for Medicare & Medicaid Services posted on the Federal Register draft versions of two of its major annual payment measures: the physician fee schedule and the payment rule for hospital outpatient services. On Aug. 3, the CMS informally posted a copy of the physician fee schedule on its own website, allowing medical groups to begin reading the more than 1,300-page rule.
Federal officials normally use annual Medicare payment rules to make many revisions to policies as well as adjust reimbursement.
The draft 2021 physician fee schedule, for example, calls for broadening the authority of clinicians other than physicians to authorize testing of people enrolled in Medicare.
The CMS intends to allow nurse practitioners, physician assistants, and certain other health care professionals to more widely supervise diagnostic psychological and neuropsychological tests, in keeping with applicable state laws.
The draft 2021 hospital outpatient rule proposes a gradual changeover to allow more procedures to be performed on an outpatient basis. This shift could save money for Medicare as well as for the people enrolled in the giant federal health program who need these services, the CMS explained.
Medicare would begin with a change in status for almost 300 musculoskeletal-related services, making them eligible for payment in the hospital outpatient setting when appropriate, CMS wrote in a fact sheet.
The initial reaction to Medicare’s proposed 2021 rules centered on its planned redistribution of funds among medical specialties. The CMS had outlined this plan last year. It is part of longstanding efforts to boost pay for primary care specialists and other physicians whose practice centers more around office visits than procedures.
There is broad support in health policy circles for raising pay for these specialties, but there also are strong objections to the cuts the CMS plans to offset the cost of rising pay for some fields.
Susan R. Bailey, MD, president of the American Medical Association, addressed both of these ideas in an AMA news release on the proposed 2021 physician fee schedule. The increase in pay for office visits, covered under evaluation and management services (E/M), stems from recommendations on resource costs from the AMA/Specialty Society RVS Update Committee, Dr. Bailey said.
“Unfortunately, these office visit payment increases, and a multitude of other new CMS proposed payment increases, are required by statute to be offset by payment reductions to other services, through an unsustainable reduction of nearly 11% to the Medicare conversion factor,” Dr. Bailey explained.
In the news release, Dr. Bailey asked Congress to waive Medicare’s budget-neutrality requirements to allow increases without the cuts.
“Physicians are already experiencing substantial economic hardships due to COVID-19, so these pay cuts could not come at a worse time,” she said.
Winners and losers
The CMS details the possible winners and losers in its payment reshuffle in Table 90 of the proposed 2021 physician fee schedule. In the proposed rule, CMS notes in the draft that these figures are based upon estimates of aggregate allowed charges across all services furnished by physicians and other clinicians.
“Therefore, they are averages, and may not necessarily be representative of what is happening to the particular services furnished by a single practitioner within any given specialty,” the CMS said.
Specialties in line for increases under the 2021 draft rule include allergy/immunology (9%), endocrinology (17%), family practice (13%), general practice (8%), geriatrics (4%), hematology/oncology (14%), internal medicine (4%), nephrology (6%), physician assistants (8%), psychiatry (8%), rheumatology (16%), and urology (8%).
In line for cuts would be anesthesiology (–8%), cardiac surgery (–9%), emergency medicine (–6%), general surgery (–7%), infectious disease (–4%), neurosurgery (–7%), physical/occupational therapy (–9%), plastic surgery (–7%), radiology (–11%), and thoracic surgery (–8%).
An umbrella group, the Surgical Care Coalition, on Aug. 3 had a quick statement ready about the CMS proposal. Writing on behalf of the group was David B. Hoyt, MD, executive director of the American College of Surgeons.
“Today’s proposed rule ignores both patients and the surgeons who care for them. The middle of a pandemic is no time for cuts to any form of health care, but today’s announcement moves ahead as if nothing has changed,” Hoyt said in the statement. “The Surgical Care Coalition believes no physician should see payment cuts that will reduce patients’ access to care.”
The Surgical Care Coalition already has been asking Congress to waive budget-neutrality requirements. Making a similar request Aug. 4 in a unified statement were the American Physical Therapy Association (APTA), the American Occupational Therapy Association (AOTA), and the American Speech-Language-Hearing Association (ASHA).
“Our organizations call on Congress and CMS to advance well-reasoned fee schedule payment policies and waive budget neutrality,” the groups said. “While APTA, AOTA, and ASHA do not oppose payment increases for primary care physicians, we believe these increases can be implemented without imposing payment reductions on other providers.”
A version of this article originally appeared on Medscape.com.
The Trump administration is sticking with a plan to boost certain Medicare pay for many primary care and other specialties focused heavily on office visits while lowering that for other groups to balance these increased costs.
On Aug. 4, the Centers for Medicare & Medicaid Services posted on the Federal Register draft versions of two of its major annual payment measures: the physician fee schedule and the payment rule for hospital outpatient services. On Aug. 3, the CMS informally posted a copy of the physician fee schedule on its own website, allowing medical groups to begin reading the more than 1,300-page rule.
Federal officials normally use annual Medicare payment rules to make many revisions to policies as well as adjust reimbursement.
The draft 2021 physician fee schedule, for example, calls for broadening the authority of clinicians other than physicians to authorize testing of people enrolled in Medicare.
The CMS intends to allow nurse practitioners, physician assistants, and certain other health care professionals to more widely supervise diagnostic psychological and neuropsychological tests, in keeping with applicable state laws.
The draft 2021 hospital outpatient rule proposes a gradual changeover to allow more procedures to be performed on an outpatient basis. This shift could save money for Medicare as well as for the people enrolled in the giant federal health program who need these services, the CMS explained.
Medicare would begin with a change in status for almost 300 musculoskeletal-related services, making them eligible for payment in the hospital outpatient setting when appropriate, CMS wrote in a fact sheet.
The initial reaction to Medicare’s proposed 2021 rules centered on its planned redistribution of funds among medical specialties. The CMS had outlined this plan last year. It is part of longstanding efforts to boost pay for primary care specialists and other physicians whose practice centers more around office visits than procedures.
There is broad support in health policy circles for raising pay for these specialties, but there also are strong objections to the cuts the CMS plans to offset the cost of rising pay for some fields.
Susan R. Bailey, MD, president of the American Medical Association, addressed both of these ideas in an AMA news release on the proposed 2021 physician fee schedule. The increase in pay for office visits, covered under evaluation and management services (E/M), stems from recommendations on resource costs from the AMA/Specialty Society RVS Update Committee, Dr. Bailey said.
“Unfortunately, these office visit payment increases, and a multitude of other new CMS proposed payment increases, are required by statute to be offset by payment reductions to other services, through an unsustainable reduction of nearly 11% to the Medicare conversion factor,” Dr. Bailey explained.
In the news release, Dr. Bailey asked Congress to waive Medicare’s budget-neutrality requirements to allow increases without the cuts.
“Physicians are already experiencing substantial economic hardships due to COVID-19, so these pay cuts could not come at a worse time,” she said.
Winners and losers
The CMS details the possible winners and losers in its payment reshuffle in Table 90 of the proposed 2021 physician fee schedule. In the proposed rule, CMS notes in the draft that these figures are based upon estimates of aggregate allowed charges across all services furnished by physicians and other clinicians.
“Therefore, they are averages, and may not necessarily be representative of what is happening to the particular services furnished by a single practitioner within any given specialty,” the CMS said.
Specialties in line for increases under the 2021 draft rule include allergy/immunology (9%), endocrinology (17%), family practice (13%), general practice (8%), geriatrics (4%), hematology/oncology (14%), internal medicine (4%), nephrology (6%), physician assistants (8%), psychiatry (8%), rheumatology (16%), and urology (8%).
In line for cuts would be anesthesiology (–8%), cardiac surgery (–9%), emergency medicine (–6%), general surgery (–7%), infectious disease (–4%), neurosurgery (–7%), physical/occupational therapy (–9%), plastic surgery (–7%), radiology (–11%), and thoracic surgery (–8%).
An umbrella group, the Surgical Care Coalition, on Aug. 3 had a quick statement ready about the CMS proposal. Writing on behalf of the group was David B. Hoyt, MD, executive director of the American College of Surgeons.
“Today’s proposed rule ignores both patients and the surgeons who care for them. The middle of a pandemic is no time for cuts to any form of health care, but today’s announcement moves ahead as if nothing has changed,” Hoyt said in the statement. “The Surgical Care Coalition believes no physician should see payment cuts that will reduce patients’ access to care.”
The Surgical Care Coalition already has been asking Congress to waive budget-neutrality requirements. Making a similar request Aug. 4 in a unified statement were the American Physical Therapy Association (APTA), the American Occupational Therapy Association (AOTA), and the American Speech-Language-Hearing Association (ASHA).
“Our organizations call on Congress and CMS to advance well-reasoned fee schedule payment policies and waive budget neutrality,” the groups said. “While APTA, AOTA, and ASHA do not oppose payment increases for primary care physicians, we believe these increases can be implemented without imposing payment reductions on other providers.”
A version of this article originally appeared on Medscape.com.
The Trump administration is sticking with a plan to boost certain Medicare pay for many primary care and other specialties focused heavily on office visits while lowering that for other groups to balance these increased costs.
On Aug. 4, the Centers for Medicare & Medicaid Services posted on the Federal Register draft versions of two of its major annual payment measures: the physician fee schedule and the payment rule for hospital outpatient services. On Aug. 3, the CMS informally posted a copy of the physician fee schedule on its own website, allowing medical groups to begin reading the more than 1,300-page rule.
Federal officials normally use annual Medicare payment rules to make many revisions to policies as well as adjust reimbursement.
The draft 2021 physician fee schedule, for example, calls for broadening the authority of clinicians other than physicians to authorize testing of people enrolled in Medicare.
The CMS intends to allow nurse practitioners, physician assistants, and certain other health care professionals to more widely supervise diagnostic psychological and neuropsychological tests, in keeping with applicable state laws.
The draft 2021 hospital outpatient rule proposes a gradual changeover to allow more procedures to be performed on an outpatient basis. This shift could save money for Medicare as well as for the people enrolled in the giant federal health program who need these services, the CMS explained.
Medicare would begin with a change in status for almost 300 musculoskeletal-related services, making them eligible for payment in the hospital outpatient setting when appropriate, CMS wrote in a fact sheet.
The initial reaction to Medicare’s proposed 2021 rules centered on its planned redistribution of funds among medical specialties. The CMS had outlined this plan last year. It is part of longstanding efforts to boost pay for primary care specialists and other physicians whose practice centers more around office visits than procedures.
There is broad support in health policy circles for raising pay for these specialties, but there also are strong objections to the cuts the CMS plans to offset the cost of rising pay for some fields.
Susan R. Bailey, MD, president of the American Medical Association, addressed both of these ideas in an AMA news release on the proposed 2021 physician fee schedule. The increase in pay for office visits, covered under evaluation and management services (E/M), stems from recommendations on resource costs from the AMA/Specialty Society RVS Update Committee, Dr. Bailey said.
“Unfortunately, these office visit payment increases, and a multitude of other new CMS proposed payment increases, are required by statute to be offset by payment reductions to other services, through an unsustainable reduction of nearly 11% to the Medicare conversion factor,” Dr. Bailey explained.
In the news release, Dr. Bailey asked Congress to waive Medicare’s budget-neutrality requirements to allow increases without the cuts.
“Physicians are already experiencing substantial economic hardships due to COVID-19, so these pay cuts could not come at a worse time,” she said.
Winners and losers
The CMS details the possible winners and losers in its payment reshuffle in Table 90 of the proposed 2021 physician fee schedule. In the proposed rule, CMS notes in the draft that these figures are based upon estimates of aggregate allowed charges across all services furnished by physicians and other clinicians.
“Therefore, they are averages, and may not necessarily be representative of what is happening to the particular services furnished by a single practitioner within any given specialty,” the CMS said.
Specialties in line for increases under the 2021 draft rule include allergy/immunology (9%), endocrinology (17%), family practice (13%), general practice (8%), geriatrics (4%), hematology/oncology (14%), internal medicine (4%), nephrology (6%), physician assistants (8%), psychiatry (8%), rheumatology (16%), and urology (8%).
In line for cuts would be anesthesiology (–8%), cardiac surgery (–9%), emergency medicine (–6%), general surgery (–7%), infectious disease (–4%), neurosurgery (–7%), physical/occupational therapy (–9%), plastic surgery (–7%), radiology (–11%), and thoracic surgery (–8%).
An umbrella group, the Surgical Care Coalition, on Aug. 3 had a quick statement ready about the CMS proposal. Writing on behalf of the group was David B. Hoyt, MD, executive director of the American College of Surgeons.
“Today’s proposed rule ignores both patients and the surgeons who care for them. The middle of a pandemic is no time for cuts to any form of health care, but today’s announcement moves ahead as if nothing has changed,” Hoyt said in the statement. “The Surgical Care Coalition believes no physician should see payment cuts that will reduce patients’ access to care.”
The Surgical Care Coalition already has been asking Congress to waive budget-neutrality requirements. Making a similar request Aug. 4 in a unified statement were the American Physical Therapy Association (APTA), the American Occupational Therapy Association (AOTA), and the American Speech-Language-Hearing Association (ASHA).
“Our organizations call on Congress and CMS to advance well-reasoned fee schedule payment policies and waive budget neutrality,” the groups said. “While APTA, AOTA, and ASHA do not oppose payment increases for primary care physicians, we believe these increases can be implemented without imposing payment reductions on other providers.”
A version of this article originally appeared on Medscape.com.
Septicemia first among hospital inpatient costs
according to a recent analysis from the Agency for Healthcare Research and Quality.
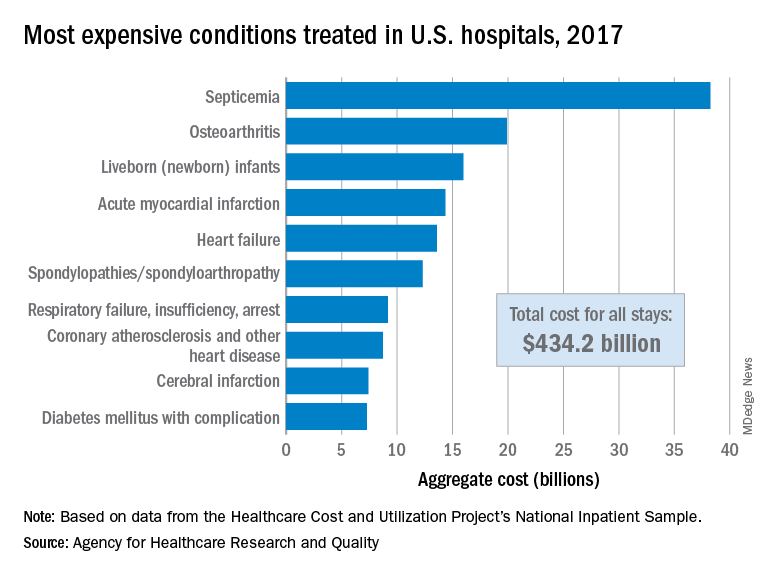
The single most expensive inpatient condition that year, representing about 8.8% of all hospital costs, was septicemia at $38.2 billion, nearly double the $19.9 billion spent on the next most expensive condition, osteoarthritis, Lan Liang, PhD, of the AHRQ, and associates said in a statistical brief.
These figures “represent the hospital’s costs to produce the services – not the amount paid for services by payers – and they do not include separately billed physician fees associated with the hospitalization,” they noted.
Third in overall cost for 2017 but first in total number of stays were live-born infants, with 3.7 million admissions costing just under $16 billion. Hospital costs for acute myocardial infarction ($14.3 billion) made it the fourth most expensive condition, with heart failure fifth at $13.6 billion, based on data from the Healthcare Cost and Utilization Project’s National Inpatient Sample.
The 20 most expensive conditions, which also included coronary atherosclerosis, pneumonia, renal failure, and lower-limb fracture, accounted for close to 47% of all hospital costs and over 43% of all stays in 2017. The total amount spent by hospitals that year, $1.1 trillion, constituted nearly a third of all health care expenditures and was 4.7% higher than in 2016, Dr. Liang and associates reported.
“Although this growth represented deceleration, compared with the 5.8% increase between 2014 and 2015, the consistent year-to-year rise in hospital-related expenses remains a central concern among policymakers,” they wrote.
according to a recent analysis from the Agency for Healthcare Research and Quality.

The single most expensive inpatient condition that year, representing about 8.8% of all hospital costs, was septicemia at $38.2 billion, nearly double the $19.9 billion spent on the next most expensive condition, osteoarthritis, Lan Liang, PhD, of the AHRQ, and associates said in a statistical brief.
These figures “represent the hospital’s costs to produce the services – not the amount paid for services by payers – and they do not include separately billed physician fees associated with the hospitalization,” they noted.
Third in overall cost for 2017 but first in total number of stays were live-born infants, with 3.7 million admissions costing just under $16 billion. Hospital costs for acute myocardial infarction ($14.3 billion) made it the fourth most expensive condition, with heart failure fifth at $13.6 billion, based on data from the Healthcare Cost and Utilization Project’s National Inpatient Sample.
The 20 most expensive conditions, which also included coronary atherosclerosis, pneumonia, renal failure, and lower-limb fracture, accounted for close to 47% of all hospital costs and over 43% of all stays in 2017. The total amount spent by hospitals that year, $1.1 trillion, constituted nearly a third of all health care expenditures and was 4.7% higher than in 2016, Dr. Liang and associates reported.
“Although this growth represented deceleration, compared with the 5.8% increase between 2014 and 2015, the consistent year-to-year rise in hospital-related expenses remains a central concern among policymakers,” they wrote.
according to a recent analysis from the Agency for Healthcare Research and Quality.

The single most expensive inpatient condition that year, representing about 8.8% of all hospital costs, was septicemia at $38.2 billion, nearly double the $19.9 billion spent on the next most expensive condition, osteoarthritis, Lan Liang, PhD, of the AHRQ, and associates said in a statistical brief.
These figures “represent the hospital’s costs to produce the services – not the amount paid for services by payers – and they do not include separately billed physician fees associated with the hospitalization,” they noted.
Third in overall cost for 2017 but first in total number of stays were live-born infants, with 3.7 million admissions costing just under $16 billion. Hospital costs for acute myocardial infarction ($14.3 billion) made it the fourth most expensive condition, with heart failure fifth at $13.6 billion, based on data from the Healthcare Cost and Utilization Project’s National Inpatient Sample.
The 20 most expensive conditions, which also included coronary atherosclerosis, pneumonia, renal failure, and lower-limb fracture, accounted for close to 47% of all hospital costs and over 43% of all stays in 2017. The total amount spent by hospitals that year, $1.1 trillion, constituted nearly a third of all health care expenditures and was 4.7% higher than in 2016, Dr. Liang and associates reported.
“Although this growth represented deceleration, compared with the 5.8% increase between 2014 and 2015, the consistent year-to-year rise in hospital-related expenses remains a central concern among policymakers,” they wrote.
Hepatitis screening now for all patients with cancer on therapy
All patients with cancer who are candidates for systemic anticancer therapy should be screened for hepatitis B virus (HBV) infection prior to or at the start of therapy, according to an updated provisional clinical opinion (PCO) from the American Society of Clinical Oncology.
“This is a new approach [that] will actively take system changes ... but it will ultimately be safer for patients – and that is crucial,” commented Jessica P. Hwang, MD, MPH, cochair of the American Society of Clinical Oncology HBV Screening Expert Panel and the first author of the PCO.
Uptake of this universal screening approach would streamline testing protocols and identify more patients at risk for HBV reactivation who should receive prophylactic antiviral therapy, Dr. Hwang said in an interview.
The PCO calls for antiviral prophylaxis during and for at least 12 months after therapy for those with chronic HBV infection who are receiving any systemic anticancer treatment and for those with have had HBV in the past and are receiving any therapies that pose a risk for HBV reactivation.
“Hepatitis B reactivation can cause really terrible outcomes, like organ failure and even death,” Dr. Hwang, who is also a professor at the University of Texas MD Anderson Cancer Center, Houston, commented in an interview.
“This whole [issue of] reactivation and adverse outcomes with anticancer therapies is completely preventable with good planning, good communication, comanagement with specialists, and antiviral therapy and monitoring,” she added.
The updated opinion was published online July 27 in the Journal of Clinical Oncology.
It was developed in response to new data that call into question the previously recommended risk-adaptive approach to HBV screening of cancer patients, say the authors.
ASCO PCOs are developed “to provide timely clinical guidance” on the basis of emerging practice-changing information. This is the second update to follow the initial HBV screening PCO, published in 2010. In the absence of clear consensus because of limited data, the original PCO called for a risk-based approach to screening. A 2015 update extended the recommendation for screening to patients starting anti-CD20 therapy or who are to undergo stem cell transplant and to those with risk factors for HBV exposure.
The current update provides “a clinically pragmatic approach to HBV screening and management” that is based on the latest findings, say the authors. These include findings from a multicenter prospective cohort study of more than 3000 patients. In that study, 21% of patients with chronic HBV had no known risk factors for the infection. In another large prospective observational cohort study, led by Dr. Hwang, which included more than 2100 patients with cancer, 90% had one or more significant risk factors for HBV infection, making selective screening “inefficient and impractical,” she said.
“The results of these two studies suggest that a universal screening approach, its potential harms (e.g., patient and clinician anxiety about management, financial burden associated with antiviral therapy) notwithstanding, is the most efficient, clinically pragmatic approach to HBV screening in persons anticipating systemic anticancer treatment,” the authors comment.
The screening recommended in the PCO requires three tests: hepatitis B surface antigen (HBsAg), core antibody total immunoglobulin or IgG, and antibody to HBsAg tests.
Anticancer therapy should not be delayed pending the results, they write.
Planning for monitoring and long-term prophylaxis for chronic HBV infection should involve a clinician experienced in HBV management, the authors write. Management of those with past infection should be individualized. Alternatively, patients with past infection can be carefully monitored rather than given prophylactic treatment, as long as frequent and consistent follow-up is possible to allow for rapid initiation of antiviral therapy in the event of reactivation, they say.
Hormonal therapy without systemic anticancer therapy is not likely to lead to HBV reactivation in patients with chronic or past infection; antiviral therapy and management of these patients should follow relevant national HBV guidelines, they note.
Challenges in implementing universal HBV screening
The expert panel acknowledges the challenges associated with implementation of universal HBV screening as recommended in their report and notes that electronic health record–based approaches that use alerts to prompt screening have demonstrated success. In one study of high-risk primary care patients, an EHR alert system significantly increased testing rates (odds ratio, 2.64 in comparison with a control group without alerts), and another study that used a simple “sticky-note” alert system to promote referral of HBsAg patients to hepatologists increased referrals from 28% to 73%.
In a cancer population, a “comprehensive set of multimodal interventions,” including pharmacy staff checks for screening prior to anti-CD20 therapy administration and electronic medication order reviews to assess for appropriate testing and treatment before anti-CD20 therapy, increased testing rates to greater than 90% and antiviral prophylaxis rates to more than 80%.
A study of 965 patients in Taiwan showed that a computer-assisted reminder system that prompted for testing prior to ordering anticancer therapy increased screening from 8% to 86% but was less effective for improving the rates of antiviral prophylaxis for those who tested positive for HBV, particularly among physicians treating patients with nonhematologic malignancies.
“Future studies will be needed to make universal HBV screening and linkage to care efficient and systematic, likely based in EHR systems,” the panel says. The authors note that “[o]ngoing studies of HBV tests such as ultrasensitive HBsAg, HBV RNA, and hepatitis B core antigen are being studied and may be useful in predicting risk of HBV reactivation.”
The panel also identified a research gap related to HBV reactivation risks “for the growing list of agents that deplete or modulate B cells.” It notes a need for additional research on the cost-effectiveness of HBV screening. The results of prior cost analyses have been inconsistent and vary with respect to the population studied. For example, universal screening and antiviral prophylaxis approaches have been shown to be cost-effective for patients with hematologic malignancies and high HBV reactivation risk but are less so for patients with solid tumors and lower reactivation risk, they explain.
Dr. Hwang said that not one of the more than 2100 patients in her HBV screening cohort study encountered problems with receiving insurance payment for their HBV screening.
“That’s a really strong statement that insurance payers are accepting of this kind of preventative service,” she said.
Expert panel cochair Andrew Artz, MD, commented that there is now greater acceptance of the need for HBV screening across medical specialties.
“There’s growing consensus among hepatologists, infectious disease specialists, oncologists, and HBV specialists that we need to do a better job of finding patients with hepatitis B [who are] about to receive immunocompromising treatment,” Dr. Artz said in an interview.
Dr. Artz is director of the Program for Aging and Blood Cancers and deputy director of the Center for Cancer and Aging at City of Hope Comprehensive Cancer Center, Duarte, California.
He suggested that the growing acceptance is due in part to the increasing number of anticancer therapies available and the resulting increase in the likelihood of patients receiving therapies that could cause reactivation.
More therapies – and more lines of therapy – could mean greater risk, he explained. He said that testing is easy and that universal screening is the simplest approach to determining who needs it. “There’s no question we will have to change practice,” Dr. Artz said in an interview. “But this is easier than the previous approach that essentially wasn’t being followed because it was too difficult to follow and patients were being missed.”
Most clinicians will appreciate having an approach that’s easier to follow, Dr. Artz predicted.
If there’s a challenge it will be in developing partnerships with HBV specialists, particularly in rural areas. In areas where there is a paucity of subspecialists, oncologists will have to “take some ownership of the issue,” as they often do in such settings, he said.
However, with support from pharmacists, administrators, and others in embracing this guidance, implementation can take place at a systems level rather than an individual clinician level, he added.
The recommendations in this updated PCO were all rated as “strong,” with the exception of the recommendation on hormonal therapy in the absence of systemic anticancer therapy, which was rated as “moderate.” All were based on “informal consensus,” with the exception of the key recommendation for universal HBV screening – use of three specific tests – which was “evidence based.”
The expert panel agreed that the benefits outweigh the harms for each recommendation in the update.
Dr. Hwang received research funding to her institution from Gilead Sciences and Merck Sharp & Dohme. She also has a relationship with the Asian Health Foundation. Dr. Artz received research funding from Miltenyi Biotec. All expert panel members’ disclosures are available in the PCO update.
This article first appeared on Medscape.com.
All patients with cancer who are candidates for systemic anticancer therapy should be screened for hepatitis B virus (HBV) infection prior to or at the start of therapy, according to an updated provisional clinical opinion (PCO) from the American Society of Clinical Oncology.
“This is a new approach [that] will actively take system changes ... but it will ultimately be safer for patients – and that is crucial,” commented Jessica P. Hwang, MD, MPH, cochair of the American Society of Clinical Oncology HBV Screening Expert Panel and the first author of the PCO.
Uptake of this universal screening approach would streamline testing protocols and identify more patients at risk for HBV reactivation who should receive prophylactic antiviral therapy, Dr. Hwang said in an interview.
The PCO calls for antiviral prophylaxis during and for at least 12 months after therapy for those with chronic HBV infection who are receiving any systemic anticancer treatment and for those with have had HBV in the past and are receiving any therapies that pose a risk for HBV reactivation.
“Hepatitis B reactivation can cause really terrible outcomes, like organ failure and even death,” Dr. Hwang, who is also a professor at the University of Texas MD Anderson Cancer Center, Houston, commented in an interview.
“This whole [issue of] reactivation and adverse outcomes with anticancer therapies is completely preventable with good planning, good communication, comanagement with specialists, and antiviral therapy and monitoring,” she added.
The updated opinion was published online July 27 in the Journal of Clinical Oncology.
It was developed in response to new data that call into question the previously recommended risk-adaptive approach to HBV screening of cancer patients, say the authors.
ASCO PCOs are developed “to provide timely clinical guidance” on the basis of emerging practice-changing information. This is the second update to follow the initial HBV screening PCO, published in 2010. In the absence of clear consensus because of limited data, the original PCO called for a risk-based approach to screening. A 2015 update extended the recommendation for screening to patients starting anti-CD20 therapy or who are to undergo stem cell transplant and to those with risk factors for HBV exposure.
The current update provides “a clinically pragmatic approach to HBV screening and management” that is based on the latest findings, say the authors. These include findings from a multicenter prospective cohort study of more than 3000 patients. In that study, 21% of patients with chronic HBV had no known risk factors for the infection. In another large prospective observational cohort study, led by Dr. Hwang, which included more than 2100 patients with cancer, 90% had one or more significant risk factors for HBV infection, making selective screening “inefficient and impractical,” she said.
“The results of these two studies suggest that a universal screening approach, its potential harms (e.g., patient and clinician anxiety about management, financial burden associated with antiviral therapy) notwithstanding, is the most efficient, clinically pragmatic approach to HBV screening in persons anticipating systemic anticancer treatment,” the authors comment.
The screening recommended in the PCO requires three tests: hepatitis B surface antigen (HBsAg), core antibody total immunoglobulin or IgG, and antibody to HBsAg tests.
Anticancer therapy should not be delayed pending the results, they write.
Planning for monitoring and long-term prophylaxis for chronic HBV infection should involve a clinician experienced in HBV management, the authors write. Management of those with past infection should be individualized. Alternatively, patients with past infection can be carefully monitored rather than given prophylactic treatment, as long as frequent and consistent follow-up is possible to allow for rapid initiation of antiviral therapy in the event of reactivation, they say.
Hormonal therapy without systemic anticancer therapy is not likely to lead to HBV reactivation in patients with chronic or past infection; antiviral therapy and management of these patients should follow relevant national HBV guidelines, they note.
Challenges in implementing universal HBV screening
The expert panel acknowledges the challenges associated with implementation of universal HBV screening as recommended in their report and notes that electronic health record–based approaches that use alerts to prompt screening have demonstrated success. In one study of high-risk primary care patients, an EHR alert system significantly increased testing rates (odds ratio, 2.64 in comparison with a control group without alerts), and another study that used a simple “sticky-note” alert system to promote referral of HBsAg patients to hepatologists increased referrals from 28% to 73%.
In a cancer population, a “comprehensive set of multimodal interventions,” including pharmacy staff checks for screening prior to anti-CD20 therapy administration and electronic medication order reviews to assess for appropriate testing and treatment before anti-CD20 therapy, increased testing rates to greater than 90% and antiviral prophylaxis rates to more than 80%.
A study of 965 patients in Taiwan showed that a computer-assisted reminder system that prompted for testing prior to ordering anticancer therapy increased screening from 8% to 86% but was less effective for improving the rates of antiviral prophylaxis for those who tested positive for HBV, particularly among physicians treating patients with nonhematologic malignancies.
“Future studies will be needed to make universal HBV screening and linkage to care efficient and systematic, likely based in EHR systems,” the panel says. The authors note that “[o]ngoing studies of HBV tests such as ultrasensitive HBsAg, HBV RNA, and hepatitis B core antigen are being studied and may be useful in predicting risk of HBV reactivation.”
The panel also identified a research gap related to HBV reactivation risks “for the growing list of agents that deplete or modulate B cells.” It notes a need for additional research on the cost-effectiveness of HBV screening. The results of prior cost analyses have been inconsistent and vary with respect to the population studied. For example, universal screening and antiviral prophylaxis approaches have been shown to be cost-effective for patients with hematologic malignancies and high HBV reactivation risk but are less so for patients with solid tumors and lower reactivation risk, they explain.
Dr. Hwang said that not one of the more than 2100 patients in her HBV screening cohort study encountered problems with receiving insurance payment for their HBV screening.
“That’s a really strong statement that insurance payers are accepting of this kind of preventative service,” she said.
Expert panel cochair Andrew Artz, MD, commented that there is now greater acceptance of the need for HBV screening across medical specialties.
“There’s growing consensus among hepatologists, infectious disease specialists, oncologists, and HBV specialists that we need to do a better job of finding patients with hepatitis B [who are] about to receive immunocompromising treatment,” Dr. Artz said in an interview.
Dr. Artz is director of the Program for Aging and Blood Cancers and deputy director of the Center for Cancer and Aging at City of Hope Comprehensive Cancer Center, Duarte, California.
He suggested that the growing acceptance is due in part to the increasing number of anticancer therapies available and the resulting increase in the likelihood of patients receiving therapies that could cause reactivation.
More therapies – and more lines of therapy – could mean greater risk, he explained. He said that testing is easy and that universal screening is the simplest approach to determining who needs it. “There’s no question we will have to change practice,” Dr. Artz said in an interview. “But this is easier than the previous approach that essentially wasn’t being followed because it was too difficult to follow and patients were being missed.”
Most clinicians will appreciate having an approach that’s easier to follow, Dr. Artz predicted.
If there’s a challenge it will be in developing partnerships with HBV specialists, particularly in rural areas. In areas where there is a paucity of subspecialists, oncologists will have to “take some ownership of the issue,” as they often do in such settings, he said.
However, with support from pharmacists, administrators, and others in embracing this guidance, implementation can take place at a systems level rather than an individual clinician level, he added.
The recommendations in this updated PCO were all rated as “strong,” with the exception of the recommendation on hormonal therapy in the absence of systemic anticancer therapy, which was rated as “moderate.” All were based on “informal consensus,” with the exception of the key recommendation for universal HBV screening – use of three specific tests – which was “evidence based.”
The expert panel agreed that the benefits outweigh the harms for each recommendation in the update.
Dr. Hwang received research funding to her institution from Gilead Sciences and Merck Sharp & Dohme. She also has a relationship with the Asian Health Foundation. Dr. Artz received research funding from Miltenyi Biotec. All expert panel members’ disclosures are available in the PCO update.
This article first appeared on Medscape.com.
All patients with cancer who are candidates for systemic anticancer therapy should be screened for hepatitis B virus (HBV) infection prior to or at the start of therapy, according to an updated provisional clinical opinion (PCO) from the American Society of Clinical Oncology.
“This is a new approach [that] will actively take system changes ... but it will ultimately be safer for patients – and that is crucial,” commented Jessica P. Hwang, MD, MPH, cochair of the American Society of Clinical Oncology HBV Screening Expert Panel and the first author of the PCO.
Uptake of this universal screening approach would streamline testing protocols and identify more patients at risk for HBV reactivation who should receive prophylactic antiviral therapy, Dr. Hwang said in an interview.
The PCO calls for antiviral prophylaxis during and for at least 12 months after therapy for those with chronic HBV infection who are receiving any systemic anticancer treatment and for those with have had HBV in the past and are receiving any therapies that pose a risk for HBV reactivation.
“Hepatitis B reactivation can cause really terrible outcomes, like organ failure and even death,” Dr. Hwang, who is also a professor at the University of Texas MD Anderson Cancer Center, Houston, commented in an interview.
“This whole [issue of] reactivation and adverse outcomes with anticancer therapies is completely preventable with good planning, good communication, comanagement with specialists, and antiviral therapy and monitoring,” she added.
The updated opinion was published online July 27 in the Journal of Clinical Oncology.
It was developed in response to new data that call into question the previously recommended risk-adaptive approach to HBV screening of cancer patients, say the authors.
ASCO PCOs are developed “to provide timely clinical guidance” on the basis of emerging practice-changing information. This is the second update to follow the initial HBV screening PCO, published in 2010. In the absence of clear consensus because of limited data, the original PCO called for a risk-based approach to screening. A 2015 update extended the recommendation for screening to patients starting anti-CD20 therapy or who are to undergo stem cell transplant and to those with risk factors for HBV exposure.
The current update provides “a clinically pragmatic approach to HBV screening and management” that is based on the latest findings, say the authors. These include findings from a multicenter prospective cohort study of more than 3000 patients. In that study, 21% of patients with chronic HBV had no known risk factors for the infection. In another large prospective observational cohort study, led by Dr. Hwang, which included more than 2100 patients with cancer, 90% had one or more significant risk factors for HBV infection, making selective screening “inefficient and impractical,” she said.
“The results of these two studies suggest that a universal screening approach, its potential harms (e.g., patient and clinician anxiety about management, financial burden associated with antiviral therapy) notwithstanding, is the most efficient, clinically pragmatic approach to HBV screening in persons anticipating systemic anticancer treatment,” the authors comment.
The screening recommended in the PCO requires three tests: hepatitis B surface antigen (HBsAg), core antibody total immunoglobulin or IgG, and antibody to HBsAg tests.
Anticancer therapy should not be delayed pending the results, they write.
Planning for monitoring and long-term prophylaxis for chronic HBV infection should involve a clinician experienced in HBV management, the authors write. Management of those with past infection should be individualized. Alternatively, patients with past infection can be carefully monitored rather than given prophylactic treatment, as long as frequent and consistent follow-up is possible to allow for rapid initiation of antiviral therapy in the event of reactivation, they say.
Hormonal therapy without systemic anticancer therapy is not likely to lead to HBV reactivation in patients with chronic or past infection; antiviral therapy and management of these patients should follow relevant national HBV guidelines, they note.
Challenges in implementing universal HBV screening
The expert panel acknowledges the challenges associated with implementation of universal HBV screening as recommended in their report and notes that electronic health record–based approaches that use alerts to prompt screening have demonstrated success. In one study of high-risk primary care patients, an EHR alert system significantly increased testing rates (odds ratio, 2.64 in comparison with a control group without alerts), and another study that used a simple “sticky-note” alert system to promote referral of HBsAg patients to hepatologists increased referrals from 28% to 73%.
In a cancer population, a “comprehensive set of multimodal interventions,” including pharmacy staff checks for screening prior to anti-CD20 therapy administration and electronic medication order reviews to assess for appropriate testing and treatment before anti-CD20 therapy, increased testing rates to greater than 90% and antiviral prophylaxis rates to more than 80%.
A study of 965 patients in Taiwan showed that a computer-assisted reminder system that prompted for testing prior to ordering anticancer therapy increased screening from 8% to 86% but was less effective for improving the rates of antiviral prophylaxis for those who tested positive for HBV, particularly among physicians treating patients with nonhematologic malignancies.
“Future studies will be needed to make universal HBV screening and linkage to care efficient and systematic, likely based in EHR systems,” the panel says. The authors note that “[o]ngoing studies of HBV tests such as ultrasensitive HBsAg, HBV RNA, and hepatitis B core antigen are being studied and may be useful in predicting risk of HBV reactivation.”
The panel also identified a research gap related to HBV reactivation risks “for the growing list of agents that deplete or modulate B cells.” It notes a need for additional research on the cost-effectiveness of HBV screening. The results of prior cost analyses have been inconsistent and vary with respect to the population studied. For example, universal screening and antiviral prophylaxis approaches have been shown to be cost-effective for patients with hematologic malignancies and high HBV reactivation risk but are less so for patients with solid tumors and lower reactivation risk, they explain.
Dr. Hwang said that not one of the more than 2100 patients in her HBV screening cohort study encountered problems with receiving insurance payment for their HBV screening.
“That’s a really strong statement that insurance payers are accepting of this kind of preventative service,” she said.
Expert panel cochair Andrew Artz, MD, commented that there is now greater acceptance of the need for HBV screening across medical specialties.
“There’s growing consensus among hepatologists, infectious disease specialists, oncologists, and HBV specialists that we need to do a better job of finding patients with hepatitis B [who are] about to receive immunocompromising treatment,” Dr. Artz said in an interview.
Dr. Artz is director of the Program for Aging and Blood Cancers and deputy director of the Center for Cancer and Aging at City of Hope Comprehensive Cancer Center, Duarte, California.
He suggested that the growing acceptance is due in part to the increasing number of anticancer therapies available and the resulting increase in the likelihood of patients receiving therapies that could cause reactivation.
More therapies – and more lines of therapy – could mean greater risk, he explained. He said that testing is easy and that universal screening is the simplest approach to determining who needs it. “There’s no question we will have to change practice,” Dr. Artz said in an interview. “But this is easier than the previous approach that essentially wasn’t being followed because it was too difficult to follow and patients were being missed.”
Most clinicians will appreciate having an approach that’s easier to follow, Dr. Artz predicted.
If there’s a challenge it will be in developing partnerships with HBV specialists, particularly in rural areas. In areas where there is a paucity of subspecialists, oncologists will have to “take some ownership of the issue,” as they often do in such settings, he said.
However, with support from pharmacists, administrators, and others in embracing this guidance, implementation can take place at a systems level rather than an individual clinician level, he added.
The recommendations in this updated PCO were all rated as “strong,” with the exception of the recommendation on hormonal therapy in the absence of systemic anticancer therapy, which was rated as “moderate.” All were based on “informal consensus,” with the exception of the key recommendation for universal HBV screening – use of three specific tests – which was “evidence based.”
The expert panel agreed that the benefits outweigh the harms for each recommendation in the update.
Dr. Hwang received research funding to her institution from Gilead Sciences and Merck Sharp & Dohme. She also has a relationship with the Asian Health Foundation. Dr. Artz received research funding from Miltenyi Biotec. All expert panel members’ disclosures are available in the PCO update.
This article first appeared on Medscape.com.
SGLT2 inhibitors have a breakout year
The benefits from sodium-glucose cotransporter 2 inhibitor drugs proven during the past year for cutting heart failure hospitalization rates substantially in patients with heart failure with reduced ejection fraction and slowing progression of chronic kidney disease, all regardless of diabetes status, have thrust this drug class into the top tier of agents for potentially treating millions of patients with cardiac or renal disease.
The sodium-glucose cotransporter 2 (SGLT2) inhibitors, first licensed for U.S. marketing in 2013 purely for glycemic control, have, during the 5 years since the first cardiovascular outcome trial results for the class came out, shown benefits in a range of patients reminiscent of what’s been established for ACE inhibitors and angiotensin receptor blockers (ARBs).
The wide-reaching benefits of SGLT2 inhibitors have recently become even more relevant by showing clinically meaningful effects in patients without type 2 diabetes (T2D). And in an uncanny coincidence, the SGLT2 inhibitors appear to act in complementary harmony with the ACE inhibitors and ARBs for preserving heart and renal function. These properties have made the SGLT2 inhibitors especially attractive as a new weapon for controlling the ascendant disorder of cardiorenal syndrome.
“SGLT2 inhibitors have a relatively greater impact on cardiovascular outcomes, compared with ACE inhibitors and ARBs, but the effects [of the two classes] are synergistic and ideally patients receive both,” said Peter McCullough, MD, a specialist in treating cardiorenal syndrome and other cardiovascular and renal disorders at Baylor, Scott, and White Heart and Vascular Hospital in Dallas. The SGLT2 inhibitors are among the drugs best suited to both treating and preventing cardiorenal syndrome by targeting both ends of the disorder, said Dr. McCullough, who chaired an American Heart Association panel that last year issued a scientific statement on cardiorenal syndrome (Circulation. 2019 Apr 16;139[16]:e840-78).
Although data on the SGLT2 inhibitors “are evolving,” the drug class is “going in the direction” of being “reasonably compared” with the ACE inhibitors and ARBs, said Javed Butler, MD, professor and chair of medicine at the University of Mississippi Medical Center, Jackson. “There are certainly complementary benefits that we see for both cardiovascular and renal outcomes.”
“We’ll think more and more about the SGLT2 inhibitors like renin-angiotensin system [RAS] inhibitors,” said David Z. Cherney, MD, referring to the drug class that includes ACE inhibitors and ARBs. “We should start to approach SGLT2 inhibitors like RAS inhibitors, with pleiotropic effects that go beyond glucose,” said Dr. Cherney, a nephrologist and professor of medicine at the University of Toronto, during the virtual annual scientific sessions of the American Diabetes Association in June 2020.
Working together in the nephron
One of the clearest complementary interactions between the SGLT2 inhibitors and the RAS inhibitors is their ability to reduce intraglomerular pressure, a key mechanism that slows nephron loss and progression of chronic kidney disease. SGLT2 inhibitors reduce sodium absorption in the proximal tubule that causes, through tubuloglomerular feedback, afferent arteriole constriction that lowers intraglomerular pressure, while the RAS inhibitors inhibit efferent arteriole constriction mediated by angiotensin II, also cutting intraglomerular pressure. Together, “they almost work in tandem,” explained Janani Rangaswami, MD, a nephrologist at Einstein Medical Center in Philadelphia, vice chair of the Kidney Council of the AHA, and first author of the 2019 cardiorenal syndrome AHA statement.
“Many had worried that if we target both the afferent and efferent arterioles simultaneously, it might increase the risk for acute kidney injury. What has been reassuring in both the recent data from the DAPA-HF trial and in recent meta-analysis was no evidence of increased risk for acute kidney injury with use of the SGLT2 inhibitor,” Dr. Rangaswami said in an interview. For example, a recent report on more than 39,000 Canadian patients with T2D who were at least 66 years old and newly begun on either an SGLT2 inhibitor or a different oral diabetes drug (a dipeptidyl peptidase–4 inhibitor), found a statistically significant 21% lower rate of acute kidney injury during the first 90 days on treatment with an SGLT2 inhibitor in a propensity score–matched analysis (CMAJ. 2020 Apr 6;192: e351-60).
Much of the concern about possible acute kidney injury stemmed from a property that the SGLT2 inhibitors share with RAS inhibitors: They cause an initial, reversible decline in glomerular filtration rate (GFR), followed by longer-term nephron preservation, a pattern attributable to reduced intraglomerular pressure. The question early on was: “ ‘Does this harm the kidney?’ But what we’ve seen is that patients do better over time, even with this initial hit. Whenever you offload the glomerulus you cut barotrauma and protect renal function,” explained Silvio E. Inzucchi, MD, professor of medicine at Yale University, New Haven, Conn., and director of the Yale Medicine Diabetes Center.
Dr. Inzucchi cautioned, however, that a small number of patients starting treatment with an SGLT2 inhibitor may have their GFR drop too sharply, especially if their GFR was low to start with. “You need to be careful, especially at the lower end of the GFR range. I recheck renal function after 1 month” after a patient starts an SGLT2. Patients whose level falls too low may need to discontinue. He added that it’s hard to set a uniform threshold for alarm, and instead assess patients on a case-by-case basis, but “you need some threshold in mind, where you will stop” treatment.
A smarter diuretic
One of the most intriguing renal effects of SGLT2 inhibitors is their diuretic action. During a talk at the virtual ADA scientific sessions, cardiologist Jeffrey Testani, MD, called them “smart” diuretics, because their effect on diuresis is relatively modest but comes without the neurohormonal price paid when patients take conventional loop diuretics.
”Loop diuretics are particularly bad,” causing neurohormonal activation that includes norepinephrine, renin, and vasopressin, said Dr. Testani, director of heart failure research at Yale. They also fail to produce a meaningful drop in blood volume despite causing substantial natriuresis.
In contrast, SGLT2 inhibitors cause “moderate” natriuresis while producing a significant cut in blood volume. “The body seems content with this lower plasma volume without activating catecholamines or renin, and that’s how the SGLT2 inhibitors differ from other diuretics,” said Dr. Inzucchi.
The class also maintains serum levels of potassium and magnesium, produces significant improvements in serum uric acid levels, and avoids the electrolyte abnormalities, volume depletion, and acute kidney injury that can occur with conventional distal diuretics, Dr. Testani said.
In short, the SGLT2 inhibitors “are safe and easy-to-use diuretics,” which allows them to fill a “huge unmet need for patients with heart failure.” As evidence accumulates for the benefits of the drug class in patients with heart failure and renal disease, “uptake will be extensive,” Dr. Testani predicted, driven in part by how easy it is to add the class to existing cardiorenal drug regimens.
Other standard therapies for patients with heart failure with reduced ejection fraction (HFrEF) risk electrolyte abnormalities, renal dysfunction, significantly lower blood pressure, often make patients feel worse, and involve a slow and laborious titration process, Dr. Testani noted. The SGLT2 inhibitor agents avoid these issues, a property that has played out in quality of life assessments of patients with HFrEF who received a drug from this class.
Outcomes met in trial after trial
In the DAPA-HF trial, with 4,443 patients with HFrEF and divided roughly equally between those with or without T2D, treatment with dapagliflozin (Farxiga) linked with significant improvements in health status and quality of life measured by the Kansas City Cardiomyopathy Questionnaire (Circulation. 2020 Jan 14;141[2]:90-9). “Not all treatments for HFrEF improve symptoms,” but in this study the SGTL2 inhibitor dapagliflozin did, boosting the Kansas City Cardiomyopathy Questionnaire score by about the same magnitude as treatment with a cardiac resynchronization device in patients with HFrEF, said Mikhail N. Kosiborod, MD, director of Cardiometabolic Research at Saint Luke’s Mid America Heart Institute in Kansas City, Mo., speaking at the virtual ADA scientific sessions.
Two more recent renal observations have further solidified the growing role of these drugs for kidney protection. Results from the CREDENCE trial that looked at canagliflozin (Invokana) treatment in 4,401 patients with T2D and albuminuria and chronic kidney disease showed canagliflozin treatment cut the primary, composite renal endpoint by a statistically significant 30%, compared with placebo (N Engl J Med. 2019 Jun 13;380[24]:2295-306). The study stopped earlier than planned because of how effective canagliflozin appeared.
“Never before has a renal protection clinical trial stopped for overwhelming efficacy,” noted nephrologist Katherine R. Tuttle, MD, executive director for research at Providence Health Care in Spokane, Wash. “It’s very exciting to have a treatment that works on both the heart and kidney, given their interrelationship,” she said during the ADA sessions. Dr. Tuttle called the cardiorenal effects from the SGLT2 inhibitors “amazing.”
Just as the DAPA-HF trial’s primary outcome showed the ability of at least one drug from the class, dapagliflozin, to improve outcomes in HFrEF patients without T2D, topline results recently reported from the DAPA-CDK trial showed for the first time renal protection by an SGLT2 inhibitor in patients with chronic kidney disease but no T2D, in a study with about 4,300 patients.
Although detailed results from DAPA-CKD are not yet available, so far the outcomes seem consistent with the CREDENCE findings, and the cumulative renal findings for the class show the SGLT2 inhibitors have “potential for a profound impact on the patients we see in every nephrology clinic, and with dual cardiorenal disease,” said Dr. Rangaswami. The class is now established as “standard of care for patients with chronic kidney disease. The CREDENCE results made that clear.”
The DAPA-CKD findings in patients with chronic kidney disease regardless of their diabetes status “are very important. We really have not had any advances in this space for some time, and chronic kidney disease patients have very poor outcomes, both cardiovascular and renal,” commented Dr. Butler. The advantage from using this drug class in these patients “is huge.”
The DAPA-CKD findings are a “major advance,” agreed Dr. McCullough.
SGLT2 inhibitor use needs to grow
Experts lament that although the evidence favoring the class has been very bullish, prescribing uptake has been slow, perhaps partly explained by the retail U.S. cost for most of these agents, generally about $17/day.
Cost is, unfortunately, an issue right now for these drugs, said Dr. Butler. Generic formulations are imminent, “but we cannot accept waiting. Providing this therapy when insurance coverage is available,” is essential.
The FDA has already granted tentative approval to some generic formulations, although resolution of patent issues can delay generics actually reaching the market. “Generic dapagliflozin will have a major impact; the marketplace for these drugs will shift very quickly,” predicted Dr. McCullough.
But price may not be the sole barrier, cautioned Dr. Rangaswami. “I don’t think it’s just a cost issue. Several factors explain the slow uptake,” of the SGLT2 inhibitors. “The biggest barrier is that this is a new drug class, and understanding how to use the class is not yet where it needs to be in the physician community.” One of the biggest problems is that the SGLT2 inhibitors are still primarily regarded as drugs to treat hyperglycemia.
Physicians who treat patients with heart or renal disease “need to wrap their head around the idea that a drug with antihyperglycemic effects is now in their practice territory, and something they need to prescribe,” she noted. Currently “there is a reluctance to prescribe these drugs given the perception that they are antihyperglycemic agents, and usually get deferred to primary care physicians or endocrinologists. This results in huge missed opportunities by cardiologists and nephrologists in initiating these agents that have major cardiorenal risk reduction effects.”
The key role that cardiologists need to play in prescribing the SGLT2 inhibitors was brought home in a recent study of two representative U.S. health systems that showed patients with T2D were far more likely to see a cardiologist than an endocrinologist (Cardiovasc Endocrinol Metab. 2020 Jun;9[2]:56-9).
“The SGLT2 inhibitors are definitely a game-changing drug class,” summed up Dr. Rangaswami. “We’re going to see a lot of use in patients with heart and kidney disease.”
Dr. Cherney has been a consultant to or has received honoraria from AstraZeneca, Boehringer Ingelheim, Janssen, Lilly, Merck, Mitsubishi Tanabe Pharma, and Sanofi. Dr. Butler has had financial relationships with numerous pharmaceutical companies. Dr. McCullough and Dr. Rangaswami had no disclosures. Dr. Inzucchi has been a consultant to or helped run trials for Abbott, AstraZeneca, Boehringer Ingelheim, Merck, Novo Nordisk, Sanofi/Lexicon, and vTv Therapeutics. Dr. Testani has been a consultant to AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, cardionomic, FIRE1 Magenta Med, Novartis, Reprieve, Sanofi, and W.L. Gore. Dr. Kosiborod has been a consultant to or led trials for Amarin, Amgen, Applied Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, Glytec, Janssen, Eli Lilly, Merck, Novartis, Novo Nordisk, Sanofi, and Vifor. Dr. Tuttle has been a consultant to AstraZeneca, Boehringer Ingelheim, Gilead, Goldfinch Bio, Eli Lilly, and Novo Nordisk.
The benefits from sodium-glucose cotransporter 2 inhibitor drugs proven during the past year for cutting heart failure hospitalization rates substantially in patients with heart failure with reduced ejection fraction and slowing progression of chronic kidney disease, all regardless of diabetes status, have thrust this drug class into the top tier of agents for potentially treating millions of patients with cardiac or renal disease.
The sodium-glucose cotransporter 2 (SGLT2) inhibitors, first licensed for U.S. marketing in 2013 purely for glycemic control, have, during the 5 years since the first cardiovascular outcome trial results for the class came out, shown benefits in a range of patients reminiscent of what’s been established for ACE inhibitors and angiotensin receptor blockers (ARBs).
The wide-reaching benefits of SGLT2 inhibitors have recently become even more relevant by showing clinically meaningful effects in patients without type 2 diabetes (T2D). And in an uncanny coincidence, the SGLT2 inhibitors appear to act in complementary harmony with the ACE inhibitors and ARBs for preserving heart and renal function. These properties have made the SGLT2 inhibitors especially attractive as a new weapon for controlling the ascendant disorder of cardiorenal syndrome.
“SGLT2 inhibitors have a relatively greater impact on cardiovascular outcomes, compared with ACE inhibitors and ARBs, but the effects [of the two classes] are synergistic and ideally patients receive both,” said Peter McCullough, MD, a specialist in treating cardiorenal syndrome and other cardiovascular and renal disorders at Baylor, Scott, and White Heart and Vascular Hospital in Dallas. The SGLT2 inhibitors are among the drugs best suited to both treating and preventing cardiorenal syndrome by targeting both ends of the disorder, said Dr. McCullough, who chaired an American Heart Association panel that last year issued a scientific statement on cardiorenal syndrome (Circulation. 2019 Apr 16;139[16]:e840-78).
Although data on the SGLT2 inhibitors “are evolving,” the drug class is “going in the direction” of being “reasonably compared” with the ACE inhibitors and ARBs, said Javed Butler, MD, professor and chair of medicine at the University of Mississippi Medical Center, Jackson. “There are certainly complementary benefits that we see for both cardiovascular and renal outcomes.”
“We’ll think more and more about the SGLT2 inhibitors like renin-angiotensin system [RAS] inhibitors,” said David Z. Cherney, MD, referring to the drug class that includes ACE inhibitors and ARBs. “We should start to approach SGLT2 inhibitors like RAS inhibitors, with pleiotropic effects that go beyond glucose,” said Dr. Cherney, a nephrologist and professor of medicine at the University of Toronto, during the virtual annual scientific sessions of the American Diabetes Association in June 2020.
Working together in the nephron
One of the clearest complementary interactions between the SGLT2 inhibitors and the RAS inhibitors is their ability to reduce intraglomerular pressure, a key mechanism that slows nephron loss and progression of chronic kidney disease. SGLT2 inhibitors reduce sodium absorption in the proximal tubule that causes, through tubuloglomerular feedback, afferent arteriole constriction that lowers intraglomerular pressure, while the RAS inhibitors inhibit efferent arteriole constriction mediated by angiotensin II, also cutting intraglomerular pressure. Together, “they almost work in tandem,” explained Janani Rangaswami, MD, a nephrologist at Einstein Medical Center in Philadelphia, vice chair of the Kidney Council of the AHA, and first author of the 2019 cardiorenal syndrome AHA statement.
“Many had worried that if we target both the afferent and efferent arterioles simultaneously, it might increase the risk for acute kidney injury. What has been reassuring in both the recent data from the DAPA-HF trial and in recent meta-analysis was no evidence of increased risk for acute kidney injury with use of the SGLT2 inhibitor,” Dr. Rangaswami said in an interview. For example, a recent report on more than 39,000 Canadian patients with T2D who were at least 66 years old and newly begun on either an SGLT2 inhibitor or a different oral diabetes drug (a dipeptidyl peptidase–4 inhibitor), found a statistically significant 21% lower rate of acute kidney injury during the first 90 days on treatment with an SGLT2 inhibitor in a propensity score–matched analysis (CMAJ. 2020 Apr 6;192: e351-60).
Much of the concern about possible acute kidney injury stemmed from a property that the SGLT2 inhibitors share with RAS inhibitors: They cause an initial, reversible decline in glomerular filtration rate (GFR), followed by longer-term nephron preservation, a pattern attributable to reduced intraglomerular pressure. The question early on was: “ ‘Does this harm the kidney?’ But what we’ve seen is that patients do better over time, even with this initial hit. Whenever you offload the glomerulus you cut barotrauma and protect renal function,” explained Silvio E. Inzucchi, MD, professor of medicine at Yale University, New Haven, Conn., and director of the Yale Medicine Diabetes Center.
Dr. Inzucchi cautioned, however, that a small number of patients starting treatment with an SGLT2 inhibitor may have their GFR drop too sharply, especially if their GFR was low to start with. “You need to be careful, especially at the lower end of the GFR range. I recheck renal function after 1 month” after a patient starts an SGLT2. Patients whose level falls too low may need to discontinue. He added that it’s hard to set a uniform threshold for alarm, and instead assess patients on a case-by-case basis, but “you need some threshold in mind, where you will stop” treatment.
A smarter diuretic
One of the most intriguing renal effects of SGLT2 inhibitors is their diuretic action. During a talk at the virtual ADA scientific sessions, cardiologist Jeffrey Testani, MD, called them “smart” diuretics, because their effect on diuresis is relatively modest but comes without the neurohormonal price paid when patients take conventional loop diuretics.
”Loop diuretics are particularly bad,” causing neurohormonal activation that includes norepinephrine, renin, and vasopressin, said Dr. Testani, director of heart failure research at Yale. They also fail to produce a meaningful drop in blood volume despite causing substantial natriuresis.
In contrast, SGLT2 inhibitors cause “moderate” natriuresis while producing a significant cut in blood volume. “The body seems content with this lower plasma volume without activating catecholamines or renin, and that’s how the SGLT2 inhibitors differ from other diuretics,” said Dr. Inzucchi.
The class also maintains serum levels of potassium and magnesium, produces significant improvements in serum uric acid levels, and avoids the electrolyte abnormalities, volume depletion, and acute kidney injury that can occur with conventional distal diuretics, Dr. Testani said.
In short, the SGLT2 inhibitors “are safe and easy-to-use diuretics,” which allows them to fill a “huge unmet need for patients with heart failure.” As evidence accumulates for the benefits of the drug class in patients with heart failure and renal disease, “uptake will be extensive,” Dr. Testani predicted, driven in part by how easy it is to add the class to existing cardiorenal drug regimens.
Other standard therapies for patients with heart failure with reduced ejection fraction (HFrEF) risk electrolyte abnormalities, renal dysfunction, significantly lower blood pressure, often make patients feel worse, and involve a slow and laborious titration process, Dr. Testani noted. The SGLT2 inhibitor agents avoid these issues, a property that has played out in quality of life assessments of patients with HFrEF who received a drug from this class.
Outcomes met in trial after trial
In the DAPA-HF trial, with 4,443 patients with HFrEF and divided roughly equally between those with or without T2D, treatment with dapagliflozin (Farxiga) linked with significant improvements in health status and quality of life measured by the Kansas City Cardiomyopathy Questionnaire (Circulation. 2020 Jan 14;141[2]:90-9). “Not all treatments for HFrEF improve symptoms,” but in this study the SGTL2 inhibitor dapagliflozin did, boosting the Kansas City Cardiomyopathy Questionnaire score by about the same magnitude as treatment with a cardiac resynchronization device in patients with HFrEF, said Mikhail N. Kosiborod, MD, director of Cardiometabolic Research at Saint Luke’s Mid America Heart Institute in Kansas City, Mo., speaking at the virtual ADA scientific sessions.
Two more recent renal observations have further solidified the growing role of these drugs for kidney protection. Results from the CREDENCE trial that looked at canagliflozin (Invokana) treatment in 4,401 patients with T2D and albuminuria and chronic kidney disease showed canagliflozin treatment cut the primary, composite renal endpoint by a statistically significant 30%, compared with placebo (N Engl J Med. 2019 Jun 13;380[24]:2295-306). The study stopped earlier than planned because of how effective canagliflozin appeared.
“Never before has a renal protection clinical trial stopped for overwhelming efficacy,” noted nephrologist Katherine R. Tuttle, MD, executive director for research at Providence Health Care in Spokane, Wash. “It’s very exciting to have a treatment that works on both the heart and kidney, given their interrelationship,” she said during the ADA sessions. Dr. Tuttle called the cardiorenal effects from the SGLT2 inhibitors “amazing.”
Just as the DAPA-HF trial’s primary outcome showed the ability of at least one drug from the class, dapagliflozin, to improve outcomes in HFrEF patients without T2D, topline results recently reported from the DAPA-CDK trial showed for the first time renal protection by an SGLT2 inhibitor in patients with chronic kidney disease but no T2D, in a study with about 4,300 patients.
Although detailed results from DAPA-CKD are not yet available, so far the outcomes seem consistent with the CREDENCE findings, and the cumulative renal findings for the class show the SGLT2 inhibitors have “potential for a profound impact on the patients we see in every nephrology clinic, and with dual cardiorenal disease,” said Dr. Rangaswami. The class is now established as “standard of care for patients with chronic kidney disease. The CREDENCE results made that clear.”
The DAPA-CKD findings in patients with chronic kidney disease regardless of their diabetes status “are very important. We really have not had any advances in this space for some time, and chronic kidney disease patients have very poor outcomes, both cardiovascular and renal,” commented Dr. Butler. The advantage from using this drug class in these patients “is huge.”
The DAPA-CKD findings are a “major advance,” agreed Dr. McCullough.
SGLT2 inhibitor use needs to grow
Experts lament that although the evidence favoring the class has been very bullish, prescribing uptake has been slow, perhaps partly explained by the retail U.S. cost for most of these agents, generally about $17/day.
Cost is, unfortunately, an issue right now for these drugs, said Dr. Butler. Generic formulations are imminent, “but we cannot accept waiting. Providing this therapy when insurance coverage is available,” is essential.
The FDA has already granted tentative approval to some generic formulations, although resolution of patent issues can delay generics actually reaching the market. “Generic dapagliflozin will have a major impact; the marketplace for these drugs will shift very quickly,” predicted Dr. McCullough.
But price may not be the sole barrier, cautioned Dr. Rangaswami. “I don’t think it’s just a cost issue. Several factors explain the slow uptake,” of the SGLT2 inhibitors. “The biggest barrier is that this is a new drug class, and understanding how to use the class is not yet where it needs to be in the physician community.” One of the biggest problems is that the SGLT2 inhibitors are still primarily regarded as drugs to treat hyperglycemia.
Physicians who treat patients with heart or renal disease “need to wrap their head around the idea that a drug with antihyperglycemic effects is now in their practice territory, and something they need to prescribe,” she noted. Currently “there is a reluctance to prescribe these drugs given the perception that they are antihyperglycemic agents, and usually get deferred to primary care physicians or endocrinologists. This results in huge missed opportunities by cardiologists and nephrologists in initiating these agents that have major cardiorenal risk reduction effects.”
The key role that cardiologists need to play in prescribing the SGLT2 inhibitors was brought home in a recent study of two representative U.S. health systems that showed patients with T2D were far more likely to see a cardiologist than an endocrinologist (Cardiovasc Endocrinol Metab. 2020 Jun;9[2]:56-9).
“The SGLT2 inhibitors are definitely a game-changing drug class,” summed up Dr. Rangaswami. “We’re going to see a lot of use in patients with heart and kidney disease.”
Dr. Cherney has been a consultant to or has received honoraria from AstraZeneca, Boehringer Ingelheim, Janssen, Lilly, Merck, Mitsubishi Tanabe Pharma, and Sanofi. Dr. Butler has had financial relationships with numerous pharmaceutical companies. Dr. McCullough and Dr. Rangaswami had no disclosures. Dr. Inzucchi has been a consultant to or helped run trials for Abbott, AstraZeneca, Boehringer Ingelheim, Merck, Novo Nordisk, Sanofi/Lexicon, and vTv Therapeutics. Dr. Testani has been a consultant to AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, cardionomic, FIRE1 Magenta Med, Novartis, Reprieve, Sanofi, and W.L. Gore. Dr. Kosiborod has been a consultant to or led trials for Amarin, Amgen, Applied Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, Glytec, Janssen, Eli Lilly, Merck, Novartis, Novo Nordisk, Sanofi, and Vifor. Dr. Tuttle has been a consultant to AstraZeneca, Boehringer Ingelheim, Gilead, Goldfinch Bio, Eli Lilly, and Novo Nordisk.
The benefits from sodium-glucose cotransporter 2 inhibitor drugs proven during the past year for cutting heart failure hospitalization rates substantially in patients with heart failure with reduced ejection fraction and slowing progression of chronic kidney disease, all regardless of diabetes status, have thrust this drug class into the top tier of agents for potentially treating millions of patients with cardiac or renal disease.
The sodium-glucose cotransporter 2 (SGLT2) inhibitors, first licensed for U.S. marketing in 2013 purely for glycemic control, have, during the 5 years since the first cardiovascular outcome trial results for the class came out, shown benefits in a range of patients reminiscent of what’s been established for ACE inhibitors and angiotensin receptor blockers (ARBs).
The wide-reaching benefits of SGLT2 inhibitors have recently become even more relevant by showing clinically meaningful effects in patients without type 2 diabetes (T2D). And in an uncanny coincidence, the SGLT2 inhibitors appear to act in complementary harmony with the ACE inhibitors and ARBs for preserving heart and renal function. These properties have made the SGLT2 inhibitors especially attractive as a new weapon for controlling the ascendant disorder of cardiorenal syndrome.
“SGLT2 inhibitors have a relatively greater impact on cardiovascular outcomes, compared with ACE inhibitors and ARBs, but the effects [of the two classes] are synergistic and ideally patients receive both,” said Peter McCullough, MD, a specialist in treating cardiorenal syndrome and other cardiovascular and renal disorders at Baylor, Scott, and White Heart and Vascular Hospital in Dallas. The SGLT2 inhibitors are among the drugs best suited to both treating and preventing cardiorenal syndrome by targeting both ends of the disorder, said Dr. McCullough, who chaired an American Heart Association panel that last year issued a scientific statement on cardiorenal syndrome (Circulation. 2019 Apr 16;139[16]:e840-78).
Although data on the SGLT2 inhibitors “are evolving,” the drug class is “going in the direction” of being “reasonably compared” with the ACE inhibitors and ARBs, said Javed Butler, MD, professor and chair of medicine at the University of Mississippi Medical Center, Jackson. “There are certainly complementary benefits that we see for both cardiovascular and renal outcomes.”
“We’ll think more and more about the SGLT2 inhibitors like renin-angiotensin system [RAS] inhibitors,” said David Z. Cherney, MD, referring to the drug class that includes ACE inhibitors and ARBs. “We should start to approach SGLT2 inhibitors like RAS inhibitors, with pleiotropic effects that go beyond glucose,” said Dr. Cherney, a nephrologist and professor of medicine at the University of Toronto, during the virtual annual scientific sessions of the American Diabetes Association in June 2020.
Working together in the nephron
One of the clearest complementary interactions between the SGLT2 inhibitors and the RAS inhibitors is their ability to reduce intraglomerular pressure, a key mechanism that slows nephron loss and progression of chronic kidney disease. SGLT2 inhibitors reduce sodium absorption in the proximal tubule that causes, through tubuloglomerular feedback, afferent arteriole constriction that lowers intraglomerular pressure, while the RAS inhibitors inhibit efferent arteriole constriction mediated by angiotensin II, also cutting intraglomerular pressure. Together, “they almost work in tandem,” explained Janani Rangaswami, MD, a nephrologist at Einstein Medical Center in Philadelphia, vice chair of the Kidney Council of the AHA, and first author of the 2019 cardiorenal syndrome AHA statement.
“Many had worried that if we target both the afferent and efferent arterioles simultaneously, it might increase the risk for acute kidney injury. What has been reassuring in both the recent data from the DAPA-HF trial and in recent meta-analysis was no evidence of increased risk for acute kidney injury with use of the SGLT2 inhibitor,” Dr. Rangaswami said in an interview. For example, a recent report on more than 39,000 Canadian patients with T2D who were at least 66 years old and newly begun on either an SGLT2 inhibitor or a different oral diabetes drug (a dipeptidyl peptidase–4 inhibitor), found a statistically significant 21% lower rate of acute kidney injury during the first 90 days on treatment with an SGLT2 inhibitor in a propensity score–matched analysis (CMAJ. 2020 Apr 6;192: e351-60).
Much of the concern about possible acute kidney injury stemmed from a property that the SGLT2 inhibitors share with RAS inhibitors: They cause an initial, reversible decline in glomerular filtration rate (GFR), followed by longer-term nephron preservation, a pattern attributable to reduced intraglomerular pressure. The question early on was: “ ‘Does this harm the kidney?’ But what we’ve seen is that patients do better over time, even with this initial hit. Whenever you offload the glomerulus you cut barotrauma and protect renal function,” explained Silvio E. Inzucchi, MD, professor of medicine at Yale University, New Haven, Conn., and director of the Yale Medicine Diabetes Center.
Dr. Inzucchi cautioned, however, that a small number of patients starting treatment with an SGLT2 inhibitor may have their GFR drop too sharply, especially if their GFR was low to start with. “You need to be careful, especially at the lower end of the GFR range. I recheck renal function after 1 month” after a patient starts an SGLT2. Patients whose level falls too low may need to discontinue. He added that it’s hard to set a uniform threshold for alarm, and instead assess patients on a case-by-case basis, but “you need some threshold in mind, where you will stop” treatment.
A smarter diuretic
One of the most intriguing renal effects of SGLT2 inhibitors is their diuretic action. During a talk at the virtual ADA scientific sessions, cardiologist Jeffrey Testani, MD, called them “smart” diuretics, because their effect on diuresis is relatively modest but comes without the neurohormonal price paid when patients take conventional loop diuretics.
”Loop diuretics are particularly bad,” causing neurohormonal activation that includes norepinephrine, renin, and vasopressin, said Dr. Testani, director of heart failure research at Yale. They also fail to produce a meaningful drop in blood volume despite causing substantial natriuresis.
In contrast, SGLT2 inhibitors cause “moderate” natriuresis while producing a significant cut in blood volume. “The body seems content with this lower plasma volume without activating catecholamines or renin, and that’s how the SGLT2 inhibitors differ from other diuretics,” said Dr. Inzucchi.
The class also maintains serum levels of potassium and magnesium, produces significant improvements in serum uric acid levels, and avoids the electrolyte abnormalities, volume depletion, and acute kidney injury that can occur with conventional distal diuretics, Dr. Testani said.
In short, the SGLT2 inhibitors “are safe and easy-to-use diuretics,” which allows them to fill a “huge unmet need for patients with heart failure.” As evidence accumulates for the benefits of the drug class in patients with heart failure and renal disease, “uptake will be extensive,” Dr. Testani predicted, driven in part by how easy it is to add the class to existing cardiorenal drug regimens.
Other standard therapies for patients with heart failure with reduced ejection fraction (HFrEF) risk electrolyte abnormalities, renal dysfunction, significantly lower blood pressure, often make patients feel worse, and involve a slow and laborious titration process, Dr. Testani noted. The SGLT2 inhibitor agents avoid these issues, a property that has played out in quality of life assessments of patients with HFrEF who received a drug from this class.
Outcomes met in trial after trial
In the DAPA-HF trial, with 4,443 patients with HFrEF and divided roughly equally between those with or without T2D, treatment with dapagliflozin (Farxiga) linked with significant improvements in health status and quality of life measured by the Kansas City Cardiomyopathy Questionnaire (Circulation. 2020 Jan 14;141[2]:90-9). “Not all treatments for HFrEF improve symptoms,” but in this study the SGTL2 inhibitor dapagliflozin did, boosting the Kansas City Cardiomyopathy Questionnaire score by about the same magnitude as treatment with a cardiac resynchronization device in patients with HFrEF, said Mikhail N. Kosiborod, MD, director of Cardiometabolic Research at Saint Luke’s Mid America Heart Institute in Kansas City, Mo., speaking at the virtual ADA scientific sessions.
Two more recent renal observations have further solidified the growing role of these drugs for kidney protection. Results from the CREDENCE trial that looked at canagliflozin (Invokana) treatment in 4,401 patients with T2D and albuminuria and chronic kidney disease showed canagliflozin treatment cut the primary, composite renal endpoint by a statistically significant 30%, compared with placebo (N Engl J Med. 2019 Jun 13;380[24]:2295-306). The study stopped earlier than planned because of how effective canagliflozin appeared.
“Never before has a renal protection clinical trial stopped for overwhelming efficacy,” noted nephrologist Katherine R. Tuttle, MD, executive director for research at Providence Health Care in Spokane, Wash. “It’s very exciting to have a treatment that works on both the heart and kidney, given their interrelationship,” she said during the ADA sessions. Dr. Tuttle called the cardiorenal effects from the SGLT2 inhibitors “amazing.”
Just as the DAPA-HF trial’s primary outcome showed the ability of at least one drug from the class, dapagliflozin, to improve outcomes in HFrEF patients without T2D, topline results recently reported from the DAPA-CDK trial showed for the first time renal protection by an SGLT2 inhibitor in patients with chronic kidney disease but no T2D, in a study with about 4,300 patients.
Although detailed results from DAPA-CKD are not yet available, so far the outcomes seem consistent with the CREDENCE findings, and the cumulative renal findings for the class show the SGLT2 inhibitors have “potential for a profound impact on the patients we see in every nephrology clinic, and with dual cardiorenal disease,” said Dr. Rangaswami. The class is now established as “standard of care for patients with chronic kidney disease. The CREDENCE results made that clear.”
The DAPA-CKD findings in patients with chronic kidney disease regardless of their diabetes status “are very important. We really have not had any advances in this space for some time, and chronic kidney disease patients have very poor outcomes, both cardiovascular and renal,” commented Dr. Butler. The advantage from using this drug class in these patients “is huge.”
The DAPA-CKD findings are a “major advance,” agreed Dr. McCullough.
SGLT2 inhibitor use needs to grow
Experts lament that although the evidence favoring the class has been very bullish, prescribing uptake has been slow, perhaps partly explained by the retail U.S. cost for most of these agents, generally about $17/day.
Cost is, unfortunately, an issue right now for these drugs, said Dr. Butler. Generic formulations are imminent, “but we cannot accept waiting. Providing this therapy when insurance coverage is available,” is essential.
The FDA has already granted tentative approval to some generic formulations, although resolution of patent issues can delay generics actually reaching the market. “Generic dapagliflozin will have a major impact; the marketplace for these drugs will shift very quickly,” predicted Dr. McCullough.
But price may not be the sole barrier, cautioned Dr. Rangaswami. “I don’t think it’s just a cost issue. Several factors explain the slow uptake,” of the SGLT2 inhibitors. “The biggest barrier is that this is a new drug class, and understanding how to use the class is not yet where it needs to be in the physician community.” One of the biggest problems is that the SGLT2 inhibitors are still primarily regarded as drugs to treat hyperglycemia.
Physicians who treat patients with heart or renal disease “need to wrap their head around the idea that a drug with antihyperglycemic effects is now in their practice territory, and something they need to prescribe,” she noted. Currently “there is a reluctance to prescribe these drugs given the perception that they are antihyperglycemic agents, and usually get deferred to primary care physicians or endocrinologists. This results in huge missed opportunities by cardiologists and nephrologists in initiating these agents that have major cardiorenal risk reduction effects.”
The key role that cardiologists need to play in prescribing the SGLT2 inhibitors was brought home in a recent study of two representative U.S. health systems that showed patients with T2D were far more likely to see a cardiologist than an endocrinologist (Cardiovasc Endocrinol Metab. 2020 Jun;9[2]:56-9).
“The SGLT2 inhibitors are definitely a game-changing drug class,” summed up Dr. Rangaswami. “We’re going to see a lot of use in patients with heart and kidney disease.”
Dr. Cherney has been a consultant to or has received honoraria from AstraZeneca, Boehringer Ingelheim, Janssen, Lilly, Merck, Mitsubishi Tanabe Pharma, and Sanofi. Dr. Butler has had financial relationships with numerous pharmaceutical companies. Dr. McCullough and Dr. Rangaswami had no disclosures. Dr. Inzucchi has been a consultant to or helped run trials for Abbott, AstraZeneca, Boehringer Ingelheim, Merck, Novo Nordisk, Sanofi/Lexicon, and vTv Therapeutics. Dr. Testani has been a consultant to AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, cardionomic, FIRE1 Magenta Med, Novartis, Reprieve, Sanofi, and W.L. Gore. Dr. Kosiborod has been a consultant to or led trials for Amarin, Amgen, Applied Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, Glytec, Janssen, Eli Lilly, Merck, Novartis, Novo Nordisk, Sanofi, and Vifor. Dr. Tuttle has been a consultant to AstraZeneca, Boehringer Ingelheim, Gilead, Goldfinch Bio, Eli Lilly, and Novo Nordisk.
AHA on cannabis: No evidence of heart benefits, but potential harms
Evidence for a link between cannabis use and cardiovascular health remains unsupported, and the potential risks outweigh any potential benefits, according to a scientific statement from the American Heart Association.
The increased legalization of cannabis and cannabis products in the United States has driven medical professionals to evaluate the safety and efficacy of cannabis in relation to health conditions, wrote Robert L. Page II, PharmD, of the University of Colorado, Aurora, and colleagues.
In a statement published in Circulation, the researchers noted that although cannabis has been shown to relieve pain and other symptoms in certain conditions, clinicians in the United States have been limited from studying its health effects because of federal law restrictions. “Cannabis remains a schedule I controlled substance, deeming no accepted medical use, a high potential for abuse, and an unacceptable safety profile,” the researchers wrote.
The statement addresses issues with the use of cannabis by individuals with cardiovascular disease or those at increased risk. Observational studies have shown no cardiovascular benefits associated with cannabis, the writers noted. The most common chemicals in cannabis include THC (tetrahydrocannabinolic acid) and CBD (cannabidiol).
Some research has shown associations between CBD cardiovascular features including lower blood pressure and reduced inflammation, the writers noted. However, THC, the component of cannabis associated with a “high” or intoxication, has been associated with heart rhythm abnormalities. The writers cited data suggesting an increased risk of heart attacks, atrial fibrillation and heart failure, although more research is needed.
The statement outlines common cannabis formulations including plant-based, extracts, crystalline forms, edible products, and tinctures. In addition, the statement notes that synthetic cannabis products are marketed and used in the United States without subject to regulation.
“Over the past 5 years, we have seen a surge in cannabis use, particularly during the COVID-19 pandemic here in Colorado, especially among adolescents and young adults,” Dr. Page said in an interview. Because of the surge, health care practitioners need to familiarize themselves with not only the benefits, but risks associated with cannabis use regardless of the formulation,” he said. As heart disease remains a leading cause of death in the United States, understanding the cardiovascular risks associated with cannabis is crucial at this time.
Dr. Page noted that popular attitudes about cannabis could pose risks to users’ cardiovascular health. “One leading misconception about cannabis is because it is ‘natural’ it must be safe,” Dr. Page said. “As with all medications, cannabis has side effects, some of which can be cardiovascular in nature,” he said. “Significant drug-drug interactions can occur as CBD and THC, both found in cannabis, inhibit CYP3A4, which metabolizes a large number of medications used to treat many cardiovascular conditions,” he noted.
“Unfortunately, much of the published data is observational in nature due to the federal restrictions on cannabis as a schedule I drug,” said Dr. Page. “Nonetheless, safety signals have emerged regarding cannabis use and adverse cardiovascular outcomes, including myocardial infarction, heart failure, and atrial fibrillation. Carefully designed prospective short- and long-term studies regarding cannabis use and cardiovascular safety are needed,” he emphasized.
Areas in particular need of additional research include the cardiovascular effects of cannabis in several vulnerable populations such as adolescents, older adults, pregnant women, transplant recipients, and those with underlying cardiovascular disease, said Dr. Page.
“Nonetheless, based on the safety signals described within this Clinical Science Statement, an open discussion regarding the risks of using cannabis needs to occur between patient and health care providers,” he said. “Furthermore, patients must be transparent regarding their cannabis use with their cardiologist and primary care provider. The cannabis story will continue to evolve and is a rapidly moving/changing target,” he said.
“Whether cannabis use is a definitive risk factor for cardiovascular disease as with tobacco use is still unknown, and both acute and long-term studies are desperately needed to address this issue,” he said.
Dr. Page had no relevant financial conflicts to disclose.
SOURCE: Page et al. Circulation. 2020 Aug 5. doi: 10.1161/CIR.0000000000000883.
Evidence for a link between cannabis use and cardiovascular health remains unsupported, and the potential risks outweigh any potential benefits, according to a scientific statement from the American Heart Association.
The increased legalization of cannabis and cannabis products in the United States has driven medical professionals to evaluate the safety and efficacy of cannabis in relation to health conditions, wrote Robert L. Page II, PharmD, of the University of Colorado, Aurora, and colleagues.
In a statement published in Circulation, the researchers noted that although cannabis has been shown to relieve pain and other symptoms in certain conditions, clinicians in the United States have been limited from studying its health effects because of federal law restrictions. “Cannabis remains a schedule I controlled substance, deeming no accepted medical use, a high potential for abuse, and an unacceptable safety profile,” the researchers wrote.
The statement addresses issues with the use of cannabis by individuals with cardiovascular disease or those at increased risk. Observational studies have shown no cardiovascular benefits associated with cannabis, the writers noted. The most common chemicals in cannabis include THC (tetrahydrocannabinolic acid) and CBD (cannabidiol).
Some research has shown associations between CBD cardiovascular features including lower blood pressure and reduced inflammation, the writers noted. However, THC, the component of cannabis associated with a “high” or intoxication, has been associated with heart rhythm abnormalities. The writers cited data suggesting an increased risk of heart attacks, atrial fibrillation and heart failure, although more research is needed.
The statement outlines common cannabis formulations including plant-based, extracts, crystalline forms, edible products, and tinctures. In addition, the statement notes that synthetic cannabis products are marketed and used in the United States without subject to regulation.
“Over the past 5 years, we have seen a surge in cannabis use, particularly during the COVID-19 pandemic here in Colorado, especially among adolescents and young adults,” Dr. Page said in an interview. Because of the surge, health care practitioners need to familiarize themselves with not only the benefits, but risks associated with cannabis use regardless of the formulation,” he said. As heart disease remains a leading cause of death in the United States, understanding the cardiovascular risks associated with cannabis is crucial at this time.
Dr. Page noted that popular attitudes about cannabis could pose risks to users’ cardiovascular health. “One leading misconception about cannabis is because it is ‘natural’ it must be safe,” Dr. Page said. “As with all medications, cannabis has side effects, some of which can be cardiovascular in nature,” he said. “Significant drug-drug interactions can occur as CBD and THC, both found in cannabis, inhibit CYP3A4, which metabolizes a large number of medications used to treat many cardiovascular conditions,” he noted.
“Unfortunately, much of the published data is observational in nature due to the federal restrictions on cannabis as a schedule I drug,” said Dr. Page. “Nonetheless, safety signals have emerged regarding cannabis use and adverse cardiovascular outcomes, including myocardial infarction, heart failure, and atrial fibrillation. Carefully designed prospective short- and long-term studies regarding cannabis use and cardiovascular safety are needed,” he emphasized.
Areas in particular need of additional research include the cardiovascular effects of cannabis in several vulnerable populations such as adolescents, older adults, pregnant women, transplant recipients, and those with underlying cardiovascular disease, said Dr. Page.
“Nonetheless, based on the safety signals described within this Clinical Science Statement, an open discussion regarding the risks of using cannabis needs to occur between patient and health care providers,” he said. “Furthermore, patients must be transparent regarding their cannabis use with their cardiologist and primary care provider. The cannabis story will continue to evolve and is a rapidly moving/changing target,” he said.
“Whether cannabis use is a definitive risk factor for cardiovascular disease as with tobacco use is still unknown, and both acute and long-term studies are desperately needed to address this issue,” he said.
Dr. Page had no relevant financial conflicts to disclose.
SOURCE: Page et al. Circulation. 2020 Aug 5. doi: 10.1161/CIR.0000000000000883.
Evidence for a link between cannabis use and cardiovascular health remains unsupported, and the potential risks outweigh any potential benefits, according to a scientific statement from the American Heart Association.
The increased legalization of cannabis and cannabis products in the United States has driven medical professionals to evaluate the safety and efficacy of cannabis in relation to health conditions, wrote Robert L. Page II, PharmD, of the University of Colorado, Aurora, and colleagues.
In a statement published in Circulation, the researchers noted that although cannabis has been shown to relieve pain and other symptoms in certain conditions, clinicians in the United States have been limited from studying its health effects because of federal law restrictions. “Cannabis remains a schedule I controlled substance, deeming no accepted medical use, a high potential for abuse, and an unacceptable safety profile,” the researchers wrote.
The statement addresses issues with the use of cannabis by individuals with cardiovascular disease or those at increased risk. Observational studies have shown no cardiovascular benefits associated with cannabis, the writers noted. The most common chemicals in cannabis include THC (tetrahydrocannabinolic acid) and CBD (cannabidiol).
Some research has shown associations between CBD cardiovascular features including lower blood pressure and reduced inflammation, the writers noted. However, THC, the component of cannabis associated with a “high” or intoxication, has been associated with heart rhythm abnormalities. The writers cited data suggesting an increased risk of heart attacks, atrial fibrillation and heart failure, although more research is needed.
The statement outlines common cannabis formulations including plant-based, extracts, crystalline forms, edible products, and tinctures. In addition, the statement notes that synthetic cannabis products are marketed and used in the United States without subject to regulation.
“Over the past 5 years, we have seen a surge in cannabis use, particularly during the COVID-19 pandemic here in Colorado, especially among adolescents and young adults,” Dr. Page said in an interview. Because of the surge, health care practitioners need to familiarize themselves with not only the benefits, but risks associated with cannabis use regardless of the formulation,” he said. As heart disease remains a leading cause of death in the United States, understanding the cardiovascular risks associated with cannabis is crucial at this time.
Dr. Page noted that popular attitudes about cannabis could pose risks to users’ cardiovascular health. “One leading misconception about cannabis is because it is ‘natural’ it must be safe,” Dr. Page said. “As with all medications, cannabis has side effects, some of which can be cardiovascular in nature,” he said. “Significant drug-drug interactions can occur as CBD and THC, both found in cannabis, inhibit CYP3A4, which metabolizes a large number of medications used to treat many cardiovascular conditions,” he noted.
“Unfortunately, much of the published data is observational in nature due to the federal restrictions on cannabis as a schedule I drug,” said Dr. Page. “Nonetheless, safety signals have emerged regarding cannabis use and adverse cardiovascular outcomes, including myocardial infarction, heart failure, and atrial fibrillation. Carefully designed prospective short- and long-term studies regarding cannabis use and cardiovascular safety are needed,” he emphasized.
Areas in particular need of additional research include the cardiovascular effects of cannabis in several vulnerable populations such as adolescents, older adults, pregnant women, transplant recipients, and those with underlying cardiovascular disease, said Dr. Page.
“Nonetheless, based on the safety signals described within this Clinical Science Statement, an open discussion regarding the risks of using cannabis needs to occur between patient and health care providers,” he said. “Furthermore, patients must be transparent regarding their cannabis use with their cardiologist and primary care provider. The cannabis story will continue to evolve and is a rapidly moving/changing target,” he said.
“Whether cannabis use is a definitive risk factor for cardiovascular disease as with tobacco use is still unknown, and both acute and long-term studies are desperately needed to address this issue,” he said.
Dr. Page had no relevant financial conflicts to disclose.
SOURCE: Page et al. Circulation. 2020 Aug 5. doi: 10.1161/CIR.0000000000000883.
FROM CIRCULATION
All NSAIDs raise post-MI risk but some are safer than others: Next chapter
Patients on antithrombotics after an acute MI will face a greater risk for bleeding and secondary cardiovascular (CV) events if they start taking any nonaspirin NSAID, confirms a large observational study.
Like other research before it, the new study suggests those risks will be much lower for some nonaspirin NSAIDs than others. But it may also challenge at least some conventional thinking about the safety of these drugs, and is based solely on a large cohort in South Korea, a group for which such NSAID data has been in short supply.
“It was intriguing that our study presented better safety profiles with celecoxib and meloxicam versus other subtypes of NSAIDs,” noted the report, published online July 27 in the Journal of the American College of Cardiology.
Most of the NSAIDs included in the analysis, “including naproxen, conferred a significantly higher risk for cardiovascular and bleeding events, compared with celecoxib and meloxicam,” wrote the authors, led by Dong Oh Kang, MD, Korea University Guro Hospital, Seoul, South Korea.
A main contribution of the study “is the thorough and comprehensive evaluation of the Korean population by use of the nationwide prescription claims database that reflects real-world clinical practice,” senior author Cheol Ung Choi, MD, PhD, of the same institution, said in an interview.
“Because we included the largest number of patients of any comparable clinical studies on NSAID treatment after MI thus far, our study may allow the generalizability of the adverse events of NSAIDs to all patients by constituting global evidence encompassing different population groups,” Dr. Choi said.
The analysis has limitations along with its strengths, the authors acknowledged, including its observational design and potential for confounding not addressed in statistical adjustments.
Observers of the study concurred, but some cited evidence pointing to such confounding that is serious enough to question the entire study’s validity.
Among the cohort of more than 100,000 patients followed for an average of about 2.3 years after their MI, the adjusted risk of thromboembolic CV events went up almost 7 times for those who took any NSAID for at least 4 consecutive weeks, compared with those who didn’t take NSAIDs, based on prescription records.
Their adjusted risk of bleeding events – which included gastrointestinal, intracranial, respiratory, or urinary tract bleeding or posthemorrhagic anemia, the group writes – was increased 300%.
There was wide variance in the adjusted hazard ratios for outcomes by type of NSAID. The risk of CV events climbed from a low of about 3 with meloxicam and almost 5 for celecoxib to more than 10 and 12 for naproxen and dexibuprofen, respectively.
The hazard ratios for bleeding ranged from about 3 for both meloxicam and celecoxib to more than 6 for naproxen.
Of note, celecoxib and meloxicam both preferentially target the cyclooxygenase type 2 (COX-2) pathway, and naproxen among NSAIDs once had a reputation for relative cardiac safety, although subsequent studies have challenged that notion.
“On the basis of the contemporary guidelines, NSAID treatment should be limited as much as possible after MI; however, our data suggest that celecoxib and meloxicam could be considered possible alternative choices in patients with MI when NSAID prescription is unavoidable,” the group wrote.
They acknowledged some limitations of the analysis, including an observational design and the possibility of unidentified confounders; that mortality outcomes were not available from the National Health Insurance Service database used in the study; and that the 2009-2013 span for the data didn’t allow consideration of more contemporary antiplatelet agents and direct oral anticoagulants.
Also, NSAID use was based on prescriptions without regard to over-the-counter usage. Although use of over-the-counter NSAIDs is common in Korea, “most MI patients in Korea are prescribed most medications, including NSAIDs, in the hospital. So I think that usage of over-the-counter NSAIDs did not change the results,” Dr. Choi said.
“This study breaks new ground by demonstrating cardiovascular safety of meloxicam (and not only of celecoxib), probably because of its higher COX-2 selectivity,” wrote the authors of an accompanying editorial, Juan J. Badimon, PhD, and Carlos G. Santos-Gallego, MD, both of the Icahn School of Medicine at Mount Sinai, New York.
Notably, “this paper rejects the cardiovascular safety of naproxen, which had been suggested classically and in the previous Danish data, but that was not evident in this study.” The finding is consistent with the PRECISION trial, in which both bleeding and CV risk were increased with naproxen versus other NSAIDs, observed Dr. Badimon and Dr. Santos-Gallego.
They agreed with the authors in recommending that, “although NSAID treatment should be avoided in patients with MI, if the use of NSAIDs is inevitable due to comorbidities, the prescription of celecoxib and meloxicam could be considered as alternative options.”
But, “as no study is perfect, this article also presents some limitations,” the editorial agreed, citing some of the same issues noted by Dr. Kang and associates, along with potential confounding by indication and the lack of “clinical information to adjust (e.g., angiographic features, left ventricular function).”
“There’s undoubtedly residual confounding,” James M. Brophy, MD, PhD, a pharmacoepidemiologist at McGill University, Montreal, said in an interview.
The 400%-900% relative risks for CV events “are just too far in left field, compared to everything else we know,” he said. “There has never been a class of drugs that have shown this sort of magnitude of effect for adverse events.”
Even in PRECISION with its more than 24,000 high-coronary-risk patients randomized and followed for 5 years, Dr. Brophy observed, relative risks for the different NSAIDs varied by an order of magnitude of only 1-2.
“You should be interpreting things in the context of what is already known,” Dr. Brophy said. “The only conclusion I would draw is the paper is fatally flawed.”
The registry included 108,232 primarily male patients followed from their first diagnosed MI for CV and bleeding events. About 1.9% were prescribed at least one NSAID for 4 or more consecutive weeks during the follow-up period averaging 2.3 years, the group reported.
The most frequently prescribed NSAID was diclofenac, at about 72% of prescribed NSAIDs in the analysis for CV events and about 69% in the bleeding-event analysis.
Adding any NSAID to post-MI antithrombotic therapy led to an adjusted HR of 6.96 (P < .001) for CV events and 4.08 (P < .001) for bleeding events, compared with no NSAID treatment.
The 88% of the cohort who were on dual-antiplatelet therapy with aspirin and clopidogrel showed very nearly the same risk increases for both endpoints.
Further studies are needed to confirm the results “and ensure their generalizability to other populations,” Dr. Choi said. They should be validated especially using the claims data bases of countries near Korea, “such as Japan and Taiwan, to examine the reproducibility of the results in similar ethnic populations.”
That the study focused on a cohort in Korea is a strength, contended the authors as well as Dr. Badimon and Dr. Santos-Gallego, given “that most data about NSAIDs were extracted from Western populations, but the risk of thrombosis/bleeding post-MI varies according to ethnicity,” according to the editorial
Dr. Brophy agreed, but doubted that ethnic differences are responsible for variation in relative risks between the current results and other studies. “There are pharmacogenomic differences between different ethnicities as to how they activate these drugs. But I suspect that sort of difference is really minor. Maybe it leads to a 2% or a 5% difference in risks.”
Dr. Kang and associates, Dr. Badimon, Dr. Santos-Gallego, and Dr. Brophy disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Patients on antithrombotics after an acute MI will face a greater risk for bleeding and secondary cardiovascular (CV) events if they start taking any nonaspirin NSAID, confirms a large observational study.
Like other research before it, the new study suggests those risks will be much lower for some nonaspirin NSAIDs than others. But it may also challenge at least some conventional thinking about the safety of these drugs, and is based solely on a large cohort in South Korea, a group for which such NSAID data has been in short supply.
“It was intriguing that our study presented better safety profiles with celecoxib and meloxicam versus other subtypes of NSAIDs,” noted the report, published online July 27 in the Journal of the American College of Cardiology.
Most of the NSAIDs included in the analysis, “including naproxen, conferred a significantly higher risk for cardiovascular and bleeding events, compared with celecoxib and meloxicam,” wrote the authors, led by Dong Oh Kang, MD, Korea University Guro Hospital, Seoul, South Korea.
A main contribution of the study “is the thorough and comprehensive evaluation of the Korean population by use of the nationwide prescription claims database that reflects real-world clinical practice,” senior author Cheol Ung Choi, MD, PhD, of the same institution, said in an interview.
“Because we included the largest number of patients of any comparable clinical studies on NSAID treatment after MI thus far, our study may allow the generalizability of the adverse events of NSAIDs to all patients by constituting global evidence encompassing different population groups,” Dr. Choi said.
The analysis has limitations along with its strengths, the authors acknowledged, including its observational design and potential for confounding not addressed in statistical adjustments.
Observers of the study concurred, but some cited evidence pointing to such confounding that is serious enough to question the entire study’s validity.
Among the cohort of more than 100,000 patients followed for an average of about 2.3 years after their MI, the adjusted risk of thromboembolic CV events went up almost 7 times for those who took any NSAID for at least 4 consecutive weeks, compared with those who didn’t take NSAIDs, based on prescription records.
Their adjusted risk of bleeding events – which included gastrointestinal, intracranial, respiratory, or urinary tract bleeding or posthemorrhagic anemia, the group writes – was increased 300%.
There was wide variance in the adjusted hazard ratios for outcomes by type of NSAID. The risk of CV events climbed from a low of about 3 with meloxicam and almost 5 for celecoxib to more than 10 and 12 for naproxen and dexibuprofen, respectively.
The hazard ratios for bleeding ranged from about 3 for both meloxicam and celecoxib to more than 6 for naproxen.
Of note, celecoxib and meloxicam both preferentially target the cyclooxygenase type 2 (COX-2) pathway, and naproxen among NSAIDs once had a reputation for relative cardiac safety, although subsequent studies have challenged that notion.
“On the basis of the contemporary guidelines, NSAID treatment should be limited as much as possible after MI; however, our data suggest that celecoxib and meloxicam could be considered possible alternative choices in patients with MI when NSAID prescription is unavoidable,” the group wrote.
They acknowledged some limitations of the analysis, including an observational design and the possibility of unidentified confounders; that mortality outcomes were not available from the National Health Insurance Service database used in the study; and that the 2009-2013 span for the data didn’t allow consideration of more contemporary antiplatelet agents and direct oral anticoagulants.
Also, NSAID use was based on prescriptions without regard to over-the-counter usage. Although use of over-the-counter NSAIDs is common in Korea, “most MI patients in Korea are prescribed most medications, including NSAIDs, in the hospital. So I think that usage of over-the-counter NSAIDs did not change the results,” Dr. Choi said.
“This study breaks new ground by demonstrating cardiovascular safety of meloxicam (and not only of celecoxib), probably because of its higher COX-2 selectivity,” wrote the authors of an accompanying editorial, Juan J. Badimon, PhD, and Carlos G. Santos-Gallego, MD, both of the Icahn School of Medicine at Mount Sinai, New York.
Notably, “this paper rejects the cardiovascular safety of naproxen, which had been suggested classically and in the previous Danish data, but that was not evident in this study.” The finding is consistent with the PRECISION trial, in which both bleeding and CV risk were increased with naproxen versus other NSAIDs, observed Dr. Badimon and Dr. Santos-Gallego.
They agreed with the authors in recommending that, “although NSAID treatment should be avoided in patients with MI, if the use of NSAIDs is inevitable due to comorbidities, the prescription of celecoxib and meloxicam could be considered as alternative options.”
But, “as no study is perfect, this article also presents some limitations,” the editorial agreed, citing some of the same issues noted by Dr. Kang and associates, along with potential confounding by indication and the lack of “clinical information to adjust (e.g., angiographic features, left ventricular function).”
“There’s undoubtedly residual confounding,” James M. Brophy, MD, PhD, a pharmacoepidemiologist at McGill University, Montreal, said in an interview.
The 400%-900% relative risks for CV events “are just too far in left field, compared to everything else we know,” he said. “There has never been a class of drugs that have shown this sort of magnitude of effect for adverse events.”
Even in PRECISION with its more than 24,000 high-coronary-risk patients randomized and followed for 5 years, Dr. Brophy observed, relative risks for the different NSAIDs varied by an order of magnitude of only 1-2.
“You should be interpreting things in the context of what is already known,” Dr. Brophy said. “The only conclusion I would draw is the paper is fatally flawed.”
The registry included 108,232 primarily male patients followed from their first diagnosed MI for CV and bleeding events. About 1.9% were prescribed at least one NSAID for 4 or more consecutive weeks during the follow-up period averaging 2.3 years, the group reported.
The most frequently prescribed NSAID was diclofenac, at about 72% of prescribed NSAIDs in the analysis for CV events and about 69% in the bleeding-event analysis.
Adding any NSAID to post-MI antithrombotic therapy led to an adjusted HR of 6.96 (P < .001) for CV events and 4.08 (P < .001) for bleeding events, compared with no NSAID treatment.
The 88% of the cohort who were on dual-antiplatelet therapy with aspirin and clopidogrel showed very nearly the same risk increases for both endpoints.
Further studies are needed to confirm the results “and ensure their generalizability to other populations,” Dr. Choi said. They should be validated especially using the claims data bases of countries near Korea, “such as Japan and Taiwan, to examine the reproducibility of the results in similar ethnic populations.”
That the study focused on a cohort in Korea is a strength, contended the authors as well as Dr. Badimon and Dr. Santos-Gallego, given “that most data about NSAIDs were extracted from Western populations, but the risk of thrombosis/bleeding post-MI varies according to ethnicity,” according to the editorial
Dr. Brophy agreed, but doubted that ethnic differences are responsible for variation in relative risks between the current results and other studies. “There are pharmacogenomic differences between different ethnicities as to how they activate these drugs. But I suspect that sort of difference is really minor. Maybe it leads to a 2% or a 5% difference in risks.”
Dr. Kang and associates, Dr. Badimon, Dr. Santos-Gallego, and Dr. Brophy disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Patients on antithrombotics after an acute MI will face a greater risk for bleeding and secondary cardiovascular (CV) events if they start taking any nonaspirin NSAID, confirms a large observational study.
Like other research before it, the new study suggests those risks will be much lower for some nonaspirin NSAIDs than others. But it may also challenge at least some conventional thinking about the safety of these drugs, and is based solely on a large cohort in South Korea, a group for which such NSAID data has been in short supply.
“It was intriguing that our study presented better safety profiles with celecoxib and meloxicam versus other subtypes of NSAIDs,” noted the report, published online July 27 in the Journal of the American College of Cardiology.
Most of the NSAIDs included in the analysis, “including naproxen, conferred a significantly higher risk for cardiovascular and bleeding events, compared with celecoxib and meloxicam,” wrote the authors, led by Dong Oh Kang, MD, Korea University Guro Hospital, Seoul, South Korea.
A main contribution of the study “is the thorough and comprehensive evaluation of the Korean population by use of the nationwide prescription claims database that reflects real-world clinical practice,” senior author Cheol Ung Choi, MD, PhD, of the same institution, said in an interview.
“Because we included the largest number of patients of any comparable clinical studies on NSAID treatment after MI thus far, our study may allow the generalizability of the adverse events of NSAIDs to all patients by constituting global evidence encompassing different population groups,” Dr. Choi said.
The analysis has limitations along with its strengths, the authors acknowledged, including its observational design and potential for confounding not addressed in statistical adjustments.
Observers of the study concurred, but some cited evidence pointing to such confounding that is serious enough to question the entire study’s validity.
Among the cohort of more than 100,000 patients followed for an average of about 2.3 years after their MI, the adjusted risk of thromboembolic CV events went up almost 7 times for those who took any NSAID for at least 4 consecutive weeks, compared with those who didn’t take NSAIDs, based on prescription records.
Their adjusted risk of bleeding events – which included gastrointestinal, intracranial, respiratory, or urinary tract bleeding or posthemorrhagic anemia, the group writes – was increased 300%.
There was wide variance in the adjusted hazard ratios for outcomes by type of NSAID. The risk of CV events climbed from a low of about 3 with meloxicam and almost 5 for celecoxib to more than 10 and 12 for naproxen and dexibuprofen, respectively.
The hazard ratios for bleeding ranged from about 3 for both meloxicam and celecoxib to more than 6 for naproxen.
Of note, celecoxib and meloxicam both preferentially target the cyclooxygenase type 2 (COX-2) pathway, and naproxen among NSAIDs once had a reputation for relative cardiac safety, although subsequent studies have challenged that notion.
“On the basis of the contemporary guidelines, NSAID treatment should be limited as much as possible after MI; however, our data suggest that celecoxib and meloxicam could be considered possible alternative choices in patients with MI when NSAID prescription is unavoidable,” the group wrote.
They acknowledged some limitations of the analysis, including an observational design and the possibility of unidentified confounders; that mortality outcomes were not available from the National Health Insurance Service database used in the study; and that the 2009-2013 span for the data didn’t allow consideration of more contemporary antiplatelet agents and direct oral anticoagulants.
Also, NSAID use was based on prescriptions without regard to over-the-counter usage. Although use of over-the-counter NSAIDs is common in Korea, “most MI patients in Korea are prescribed most medications, including NSAIDs, in the hospital. So I think that usage of over-the-counter NSAIDs did not change the results,” Dr. Choi said.
“This study breaks new ground by demonstrating cardiovascular safety of meloxicam (and not only of celecoxib), probably because of its higher COX-2 selectivity,” wrote the authors of an accompanying editorial, Juan J. Badimon, PhD, and Carlos G. Santos-Gallego, MD, both of the Icahn School of Medicine at Mount Sinai, New York.
Notably, “this paper rejects the cardiovascular safety of naproxen, which had been suggested classically and in the previous Danish data, but that was not evident in this study.” The finding is consistent with the PRECISION trial, in which both bleeding and CV risk were increased with naproxen versus other NSAIDs, observed Dr. Badimon and Dr. Santos-Gallego.
They agreed with the authors in recommending that, “although NSAID treatment should be avoided in patients with MI, if the use of NSAIDs is inevitable due to comorbidities, the prescription of celecoxib and meloxicam could be considered as alternative options.”
But, “as no study is perfect, this article also presents some limitations,” the editorial agreed, citing some of the same issues noted by Dr. Kang and associates, along with potential confounding by indication and the lack of “clinical information to adjust (e.g., angiographic features, left ventricular function).”
“There’s undoubtedly residual confounding,” James M. Brophy, MD, PhD, a pharmacoepidemiologist at McGill University, Montreal, said in an interview.
The 400%-900% relative risks for CV events “are just too far in left field, compared to everything else we know,” he said. “There has never been a class of drugs that have shown this sort of magnitude of effect for adverse events.”
Even in PRECISION with its more than 24,000 high-coronary-risk patients randomized and followed for 5 years, Dr. Brophy observed, relative risks for the different NSAIDs varied by an order of magnitude of only 1-2.
“You should be interpreting things in the context of what is already known,” Dr. Brophy said. “The only conclusion I would draw is the paper is fatally flawed.”
The registry included 108,232 primarily male patients followed from their first diagnosed MI for CV and bleeding events. About 1.9% were prescribed at least one NSAID for 4 or more consecutive weeks during the follow-up period averaging 2.3 years, the group reported.
The most frequently prescribed NSAID was diclofenac, at about 72% of prescribed NSAIDs in the analysis for CV events and about 69% in the bleeding-event analysis.
Adding any NSAID to post-MI antithrombotic therapy led to an adjusted HR of 6.96 (P < .001) for CV events and 4.08 (P < .001) for bleeding events, compared with no NSAID treatment.
The 88% of the cohort who were on dual-antiplatelet therapy with aspirin and clopidogrel showed very nearly the same risk increases for both endpoints.
Further studies are needed to confirm the results “and ensure their generalizability to other populations,” Dr. Choi said. They should be validated especially using the claims data bases of countries near Korea, “such as Japan and Taiwan, to examine the reproducibility of the results in similar ethnic populations.”
That the study focused on a cohort in Korea is a strength, contended the authors as well as Dr. Badimon and Dr. Santos-Gallego, given “that most data about NSAIDs were extracted from Western populations, but the risk of thrombosis/bleeding post-MI varies according to ethnicity,” according to the editorial
Dr. Brophy agreed, but doubted that ethnic differences are responsible for variation in relative risks between the current results and other studies. “There are pharmacogenomic differences between different ethnicities as to how they activate these drugs. But I suspect that sort of difference is really minor. Maybe it leads to a 2% or a 5% difference in risks.”
Dr. Kang and associates, Dr. Badimon, Dr. Santos-Gallego, and Dr. Brophy disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Value of palliative care shines clearly in a crisis
Hospitalists have played a key role
For some palliative care professionals, the COVID-19 pandemic, particularly in viral hot spots like New York City, represents a “moment” that could lead to greater awareness of what this service offers to seriously ill patients in a crisis.
They say it has provided an opportunity to show what palliative care teams can contribute to the difficult circumstances of patients with severe symptoms, isolated and alone in quarantined hospitals, with poor survival rates, perhaps sedated for extended stays on scarce ventilators – and for their family members, who are able to visit them only virtually via telephone or tablet.
But it has also highlighted gaps – including insufficient staffing for some palliative care teams. Hospitalists and other clinicians in the hospital need to learn the basics of primary palliative care, such as how to communicate bad news, initiate goals of care conversations, and address common symptoms of serious illness, such as pain. That way, they could shoulder more of the demand for this kind of care when palliative care specialists are in short supply.
Hospitalists, some of whom also have pursued a specialization in palliative care, have played key roles in clarifying and redefining the new role for palliative care, whom it is meant for, and who should provide it. Central to this new role is the greater use of telemedicine – for talking to hospitalized patients without increasing viral exposure, for linking up with family members who can’t visit their loved ones in the hospital, and for helping frontline hospital staff who need a palliative care consultation – or just a chance to debrief on what they are seeing.
A pandemic wake-up call
Elizabeth Gundersen, MD, FHM, FAAHPM, director of the hospice and palliative medicine fellowship program at the Charles E. Schmidt College of Medicine at Florida Atlantic University (FAU) in Boca Raton, practiced hospital medicine for 10 years before pursuing a fellowship in hospice and palliative medicine and working as an academic palliative medicine physician. She calls the pandemic a wake-up call for gaps in care and all the things that weren’t working well in the health care system.
“Now we are seeing more clearly what’s lacking – or broken – and what we will carry forward from this experience into the post-COVID world,” she said. Some hospitalists do palliative care very well, and others don’t feel as comfortable in having these difficult conversations with patients. But in the uncertain course of the virus they get thrust into it.
Although FAU’s associated hospitals were not as inundated with COVID-19 patients in the early weeks of the pandemic as were other regions, the volume of other patients plummeted, Dr. Gundersen said, adding that “there’s still been incredible intensity and worry about the virus. For me, the basic role of palliative care hasn’t changed, and the phrase I have always used when introducing myself – ‘we’re an extra layer of support for the patient and family’ – still holds true,” she said.
“I try to make it clear to people that palliative care is not synonymous with end-of-life care. We don’t want people to think that a palliative care referral implies imminent death. The goal is not to get more people to have a do not attempt resuscitation (DNAR) order, but to determine the patient and family’s treatment goals and whether a DNAR order fits those goals.”
The tough conversations
Dr. Gundersen is cochair of SHM’s Palliative Care Special Interest Group, along with Rab Razzak, MD, clinical director of palliative medicine at University Hospitals Cleveland Medical Center, one of the hospitals affiliated with Case Western University in Cleveland. (Connect with them on Twitter: @Top_Gundersen and @rabrazzak.)
Dr. Razzak also transitioned from hospital medicine to palliative medicine 10 years ago. “As a hospitalist, I enjoyed the tough conversations and bringing the human element into my health care interactions,” he explained. “To me, palliative care is a philosophy of care that puts the person we call the patient at the center of the interaction, while we try to figure out how to best care for them as a person.”
When the pandemic hit, University Hospitals made 20 ICU beds available for COVID-19 patients, Dr. Razzak said. This unit has since been full but not overflowing, while overall hospital census went down. The palliative care team at the hospital includes four inpatient doctors, nurse practitioners, and a chaplain, as well as an outpatient team primarily focused on oncology.
“In some settings, palliative care has been at the forefront of difficult conversations, when things aren’t going well for the patient and there’s much uncertainty,” Dr. Razzak said. The interface between hospital medicine and palliative care can be complementary, he added. “We talk about primary palliative care, which we want every discipline to be able to do – lead meaningful conversations, help manage symptoms.”
The take-home message for hospitalists, he said, is to get training in how to have these discussions, using such resources as VitalTalk (https://www.vitaltalk.org/), a nonprofit organization that disseminates education in communication skills for difficult conversations, and the Center to Advance Palliative Care (www.capc.org) at Icahn School of Medicine at Mount Sinai in New York City. “Once you’ve mastered the conversation, it will get easier. But ask for help when you need it, and learn how to know when you need it.”
Dr. Gundersen added that hospital medicine groups and palliative care teams could reach out to each other and talk about what they did in the crisis and how they can work together in the future. She recommends frequent ongoing support and collaboration that could range from formal conferences or training sessions to informal team interactions, perhaps with sandwiches in the doctor’s lounge – provided that there’s room for social distancing. She has recently started giving talks in the community and grand rounds presentations in hospitals about palliative care.
Other approaches and applications
In New York City, the initial epicenter for the pandemic in the United States, the adult palliative care service of Columbia University Medical Center (CUMC) experienced a sevenfold increase in consultation requests at the apex of the crisis, said its director, Craig Blinderman, MD. That demand was impossible to meet with existing staff. So Dr. Blinderman and colleagues established a virtual consultation model, recruiting and deploying volunteer out-of-state palliative care specialists to staff it.
An eight-bed palliative care unit was opened at CUMC for COVID-19 patients whose surrogates had opted not to initiate or continue intubation or life-sustaining treatments. This helped to relieve some of the pressures on the ICUs while making it possible for in-person visits to the hospice unit by families – in full PPE. Palliative care staff were embedded in various units in the hospital.
A palliative care response team composed of a hospice and palliative medicine fellow and four psychiatry residents or fellows, based in the emergency department and with supervision from the palliative care team, provided time-critical goals of care conversations with families using telemedicine – and a forum for listening to their suffering. Dr. Blinderman and colleagues also have found time to write up their experience for medical journals.1,2
There’s no reason to think that hospitalists, with a little basic training, couldn’t be having these same goals of care conversations, Dr. Blinderman said. “But the fact that hospitalists, at the pandemic’s peak, along with ICU doctors, were seeing an unprecedented magnitude of dying on a daily basis generated a lot of moral distress for them.”
Palliative care professionals, because they engage with these issues in a different way, may be somewhat better equipped to deal with the sheer emotional demands when so many are dying, as at the peak of the surge in New York. “We don’t see dying as a failure on our part but an opportunity to relieve suffering,” Dr. Blinderman said. And the palliative care field also emphasizes the importance of self-care for its practitioners.
“How do we meet the incredible palliative care needs in the epicenter of a pandemic? That question also applies to other kinds of crises we could imagine, for example, climate-related disasters,” Dr. Blinderman said. “What lessons have we learned about the value of palliative care and how to start incorporating it more integrally into the delivery of hospital care? Here we showed that we could work collaboratively with our colleagues at other major medical centers, bringing together their expertise to help us when we didn’t have the bandwidth to meet the demand,” he said.
Scripts can help
“Also, it won’t make sense to just go back to normal (after the crisis fades),” Dr. Blinderman said. “We need to take a close look at how our society is functioning in the wake of the pandemic and the ways the health care system has failed us. We have learned that we’re all interconnected and we need to work together to serve our communities – locally and nationally – applying basic distributive justice.”
Could there be, for example, a national infrastructure for mobilizing and deploying palliative care resources to areas of greatest need, similar to what was done in New York?
At Northwestern Medicine in Chicago, a number of palliative care clinicians at the system’s hospitals worked together to develop scripts designed to help other clinicians start goals of care conversations with patients and families, for use in the hospital as well as in outpatient primary care and other settings, with results integrated into the system’s electronic health record.
Front-line clinicians may not have the time to ask for formal consults from palliative care because of high volume and rapidly changing patient status, explained Eytan Szmuilowicz, MD, director of the section of palliative medicine at Northwestern Memorial Hospital. Or they may not have access to specialty-level palliative care in their settings.
The scripts are aimed at primary care, emergency physicians, and hospitalists needing to consider critical care placement or attempted resuscitation and to ICU clinicians helping families make decisions about life-sustaining treatments. They also can help facilitate advance care planning discussions. An example is “CALMER,” a six-step mnemonic guide to promote goals of care discussions with hospitalized patients. For more information on these scripts, contact Dr. Szmuilowicz: [email protected].
Eerily quiet
The COVID-19 crisis has been quite a whirlwind for hospital medicine, said Jeanie Youngwerth, MD, a hospitalist and program director of the palliative care service at the University of Colorado in Denver, which was a significant viral hotspot early on.
“When it first started, things seemed to change almost overnight – starting on Friday, March 13. People had to take action right away to develop work flows and the technology to allow us to see as many patients as possible,” she said. By the time Monday came, it was a whole new ballgame.
Dr. Youngwerth and two colleagues worked quickly to develop inpatient telemedicine capacity where none existed. “We knew we would not be going into patients’ rooms, but most of our team showed up in the hospital to work with the primary care teams. Our job was to see what we could do that actually made a difference,” she said.
“The hospital became a very strange place. You’d walk down the hallway and it was eerily quiet. Everybody you came across was being so nice to each other.” Televisits became a powerful way to bring the human connection back to medical care.
“What we learned from families was that they were thirsting to have some kind of connection with their loved one, and to be able to talk about their loved one and who they were as a person,” she said. “We’d contact the family through video visits and then, when the family meeting ended, the nurse would bring an iPad into the patient’s room so the family could see their loved one on a ventilator. They would immediately start communicating with their loved one, praying aloud, singing, playing music. It would make a huge difference for the family – and for the staff.”
References
1. Nakagawa S et al. Pandemic palliative care consultations spanning state and institutional borders. J Am Geriatr Soc. 2020 May 22. doi: 10.1111/jgs.16643.
2. Lee J Abrukin L, Flores S. Early intervention of palliative care in the emergency department during the COVID-19 pandemic. JAMA Intern Med. 2020 Jun 5. doi: 10.1001/jamainternmed.2020.2713.
Hospitalists have played a key role
Hospitalists have played a key role
For some palliative care professionals, the COVID-19 pandemic, particularly in viral hot spots like New York City, represents a “moment” that could lead to greater awareness of what this service offers to seriously ill patients in a crisis.
They say it has provided an opportunity to show what palliative care teams can contribute to the difficult circumstances of patients with severe symptoms, isolated and alone in quarantined hospitals, with poor survival rates, perhaps sedated for extended stays on scarce ventilators – and for their family members, who are able to visit them only virtually via telephone or tablet.
But it has also highlighted gaps – including insufficient staffing for some palliative care teams. Hospitalists and other clinicians in the hospital need to learn the basics of primary palliative care, such as how to communicate bad news, initiate goals of care conversations, and address common symptoms of serious illness, such as pain. That way, they could shoulder more of the demand for this kind of care when palliative care specialists are in short supply.
Hospitalists, some of whom also have pursued a specialization in palliative care, have played key roles in clarifying and redefining the new role for palliative care, whom it is meant for, and who should provide it. Central to this new role is the greater use of telemedicine – for talking to hospitalized patients without increasing viral exposure, for linking up with family members who can’t visit their loved ones in the hospital, and for helping frontline hospital staff who need a palliative care consultation – or just a chance to debrief on what they are seeing.
A pandemic wake-up call
Elizabeth Gundersen, MD, FHM, FAAHPM, director of the hospice and palliative medicine fellowship program at the Charles E. Schmidt College of Medicine at Florida Atlantic University (FAU) in Boca Raton, practiced hospital medicine for 10 years before pursuing a fellowship in hospice and palliative medicine and working as an academic palliative medicine physician. She calls the pandemic a wake-up call for gaps in care and all the things that weren’t working well in the health care system.
“Now we are seeing more clearly what’s lacking – or broken – and what we will carry forward from this experience into the post-COVID world,” she said. Some hospitalists do palliative care very well, and others don’t feel as comfortable in having these difficult conversations with patients. But in the uncertain course of the virus they get thrust into it.
Although FAU’s associated hospitals were not as inundated with COVID-19 patients in the early weeks of the pandemic as were other regions, the volume of other patients plummeted, Dr. Gundersen said, adding that “there’s still been incredible intensity and worry about the virus. For me, the basic role of palliative care hasn’t changed, and the phrase I have always used when introducing myself – ‘we’re an extra layer of support for the patient and family’ – still holds true,” she said.
“I try to make it clear to people that palliative care is not synonymous with end-of-life care. We don’t want people to think that a palliative care referral implies imminent death. The goal is not to get more people to have a do not attempt resuscitation (DNAR) order, but to determine the patient and family’s treatment goals and whether a DNAR order fits those goals.”
The tough conversations
Dr. Gundersen is cochair of SHM’s Palliative Care Special Interest Group, along with Rab Razzak, MD, clinical director of palliative medicine at University Hospitals Cleveland Medical Center, one of the hospitals affiliated with Case Western University in Cleveland. (Connect with them on Twitter: @Top_Gundersen and @rabrazzak.)
Dr. Razzak also transitioned from hospital medicine to palliative medicine 10 years ago. “As a hospitalist, I enjoyed the tough conversations and bringing the human element into my health care interactions,” he explained. “To me, palliative care is a philosophy of care that puts the person we call the patient at the center of the interaction, while we try to figure out how to best care for them as a person.”
When the pandemic hit, University Hospitals made 20 ICU beds available for COVID-19 patients, Dr. Razzak said. This unit has since been full but not overflowing, while overall hospital census went down. The palliative care team at the hospital includes four inpatient doctors, nurse practitioners, and a chaplain, as well as an outpatient team primarily focused on oncology.
“In some settings, palliative care has been at the forefront of difficult conversations, when things aren’t going well for the patient and there’s much uncertainty,” Dr. Razzak said. The interface between hospital medicine and palliative care can be complementary, he added. “We talk about primary palliative care, which we want every discipline to be able to do – lead meaningful conversations, help manage symptoms.”
The take-home message for hospitalists, he said, is to get training in how to have these discussions, using such resources as VitalTalk (https://www.vitaltalk.org/), a nonprofit organization that disseminates education in communication skills for difficult conversations, and the Center to Advance Palliative Care (www.capc.org) at Icahn School of Medicine at Mount Sinai in New York City. “Once you’ve mastered the conversation, it will get easier. But ask for help when you need it, and learn how to know when you need it.”
Dr. Gundersen added that hospital medicine groups and palliative care teams could reach out to each other and talk about what they did in the crisis and how they can work together in the future. She recommends frequent ongoing support and collaboration that could range from formal conferences or training sessions to informal team interactions, perhaps with sandwiches in the doctor’s lounge – provided that there’s room for social distancing. She has recently started giving talks in the community and grand rounds presentations in hospitals about palliative care.
Other approaches and applications
In New York City, the initial epicenter for the pandemic in the United States, the adult palliative care service of Columbia University Medical Center (CUMC) experienced a sevenfold increase in consultation requests at the apex of the crisis, said its director, Craig Blinderman, MD. That demand was impossible to meet with existing staff. So Dr. Blinderman and colleagues established a virtual consultation model, recruiting and deploying volunteer out-of-state palliative care specialists to staff it.
An eight-bed palliative care unit was opened at CUMC for COVID-19 patients whose surrogates had opted not to initiate or continue intubation or life-sustaining treatments. This helped to relieve some of the pressures on the ICUs while making it possible for in-person visits to the hospice unit by families – in full PPE. Palliative care staff were embedded in various units in the hospital.
A palliative care response team composed of a hospice and palliative medicine fellow and four psychiatry residents or fellows, based in the emergency department and with supervision from the palliative care team, provided time-critical goals of care conversations with families using telemedicine – and a forum for listening to their suffering. Dr. Blinderman and colleagues also have found time to write up their experience for medical journals.1,2
There’s no reason to think that hospitalists, with a little basic training, couldn’t be having these same goals of care conversations, Dr. Blinderman said. “But the fact that hospitalists, at the pandemic’s peak, along with ICU doctors, were seeing an unprecedented magnitude of dying on a daily basis generated a lot of moral distress for them.”
Palliative care professionals, because they engage with these issues in a different way, may be somewhat better equipped to deal with the sheer emotional demands when so many are dying, as at the peak of the surge in New York. “We don’t see dying as a failure on our part but an opportunity to relieve suffering,” Dr. Blinderman said. And the palliative care field also emphasizes the importance of self-care for its practitioners.
“How do we meet the incredible palliative care needs in the epicenter of a pandemic? That question also applies to other kinds of crises we could imagine, for example, climate-related disasters,” Dr. Blinderman said. “What lessons have we learned about the value of palliative care and how to start incorporating it more integrally into the delivery of hospital care? Here we showed that we could work collaboratively with our colleagues at other major medical centers, bringing together their expertise to help us when we didn’t have the bandwidth to meet the demand,” he said.
Scripts can help
“Also, it won’t make sense to just go back to normal (after the crisis fades),” Dr. Blinderman said. “We need to take a close look at how our society is functioning in the wake of the pandemic and the ways the health care system has failed us. We have learned that we’re all interconnected and we need to work together to serve our communities – locally and nationally – applying basic distributive justice.”
Could there be, for example, a national infrastructure for mobilizing and deploying palliative care resources to areas of greatest need, similar to what was done in New York?
At Northwestern Medicine in Chicago, a number of palliative care clinicians at the system’s hospitals worked together to develop scripts designed to help other clinicians start goals of care conversations with patients and families, for use in the hospital as well as in outpatient primary care and other settings, with results integrated into the system’s electronic health record.
Front-line clinicians may not have the time to ask for formal consults from palliative care because of high volume and rapidly changing patient status, explained Eytan Szmuilowicz, MD, director of the section of palliative medicine at Northwestern Memorial Hospital. Or they may not have access to specialty-level palliative care in their settings.
The scripts are aimed at primary care, emergency physicians, and hospitalists needing to consider critical care placement or attempted resuscitation and to ICU clinicians helping families make decisions about life-sustaining treatments. They also can help facilitate advance care planning discussions. An example is “CALMER,” a six-step mnemonic guide to promote goals of care discussions with hospitalized patients. For more information on these scripts, contact Dr. Szmuilowicz: [email protected].
Eerily quiet
The COVID-19 crisis has been quite a whirlwind for hospital medicine, said Jeanie Youngwerth, MD, a hospitalist and program director of the palliative care service at the University of Colorado in Denver, which was a significant viral hotspot early on.
“When it first started, things seemed to change almost overnight – starting on Friday, March 13. People had to take action right away to develop work flows and the technology to allow us to see as many patients as possible,” she said. By the time Monday came, it was a whole new ballgame.
Dr. Youngwerth and two colleagues worked quickly to develop inpatient telemedicine capacity where none existed. “We knew we would not be going into patients’ rooms, but most of our team showed up in the hospital to work with the primary care teams. Our job was to see what we could do that actually made a difference,” she said.
“The hospital became a very strange place. You’d walk down the hallway and it was eerily quiet. Everybody you came across was being so nice to each other.” Televisits became a powerful way to bring the human connection back to medical care.
“What we learned from families was that they were thirsting to have some kind of connection with their loved one, and to be able to talk about their loved one and who they were as a person,” she said. “We’d contact the family through video visits and then, when the family meeting ended, the nurse would bring an iPad into the patient’s room so the family could see their loved one on a ventilator. They would immediately start communicating with their loved one, praying aloud, singing, playing music. It would make a huge difference for the family – and for the staff.”
References
1. Nakagawa S et al. Pandemic palliative care consultations spanning state and institutional borders. J Am Geriatr Soc. 2020 May 22. doi: 10.1111/jgs.16643.
2. Lee J Abrukin L, Flores S. Early intervention of palliative care in the emergency department during the COVID-19 pandemic. JAMA Intern Med. 2020 Jun 5. doi: 10.1001/jamainternmed.2020.2713.
For some palliative care professionals, the COVID-19 pandemic, particularly in viral hot spots like New York City, represents a “moment” that could lead to greater awareness of what this service offers to seriously ill patients in a crisis.
They say it has provided an opportunity to show what palliative care teams can contribute to the difficult circumstances of patients with severe symptoms, isolated and alone in quarantined hospitals, with poor survival rates, perhaps sedated for extended stays on scarce ventilators – and for their family members, who are able to visit them only virtually via telephone or tablet.
But it has also highlighted gaps – including insufficient staffing for some palliative care teams. Hospitalists and other clinicians in the hospital need to learn the basics of primary palliative care, such as how to communicate bad news, initiate goals of care conversations, and address common symptoms of serious illness, such as pain. That way, they could shoulder more of the demand for this kind of care when palliative care specialists are in short supply.
Hospitalists, some of whom also have pursued a specialization in palliative care, have played key roles in clarifying and redefining the new role for palliative care, whom it is meant for, and who should provide it. Central to this new role is the greater use of telemedicine – for talking to hospitalized patients without increasing viral exposure, for linking up with family members who can’t visit their loved ones in the hospital, and for helping frontline hospital staff who need a palliative care consultation – or just a chance to debrief on what they are seeing.
A pandemic wake-up call
Elizabeth Gundersen, MD, FHM, FAAHPM, director of the hospice and palliative medicine fellowship program at the Charles E. Schmidt College of Medicine at Florida Atlantic University (FAU) in Boca Raton, practiced hospital medicine for 10 years before pursuing a fellowship in hospice and palliative medicine and working as an academic palliative medicine physician. She calls the pandemic a wake-up call for gaps in care and all the things that weren’t working well in the health care system.
“Now we are seeing more clearly what’s lacking – or broken – and what we will carry forward from this experience into the post-COVID world,” she said. Some hospitalists do palliative care very well, and others don’t feel as comfortable in having these difficult conversations with patients. But in the uncertain course of the virus they get thrust into it.
Although FAU’s associated hospitals were not as inundated with COVID-19 patients in the early weeks of the pandemic as were other regions, the volume of other patients plummeted, Dr. Gundersen said, adding that “there’s still been incredible intensity and worry about the virus. For me, the basic role of palliative care hasn’t changed, and the phrase I have always used when introducing myself – ‘we’re an extra layer of support for the patient and family’ – still holds true,” she said.
“I try to make it clear to people that palliative care is not synonymous with end-of-life care. We don’t want people to think that a palliative care referral implies imminent death. The goal is not to get more people to have a do not attempt resuscitation (DNAR) order, but to determine the patient and family’s treatment goals and whether a DNAR order fits those goals.”
The tough conversations
Dr. Gundersen is cochair of SHM’s Palliative Care Special Interest Group, along with Rab Razzak, MD, clinical director of palliative medicine at University Hospitals Cleveland Medical Center, one of the hospitals affiliated with Case Western University in Cleveland. (Connect with them on Twitter: @Top_Gundersen and @rabrazzak.)
Dr. Razzak also transitioned from hospital medicine to palliative medicine 10 years ago. “As a hospitalist, I enjoyed the tough conversations and bringing the human element into my health care interactions,” he explained. “To me, palliative care is a philosophy of care that puts the person we call the patient at the center of the interaction, while we try to figure out how to best care for them as a person.”
When the pandemic hit, University Hospitals made 20 ICU beds available for COVID-19 patients, Dr. Razzak said. This unit has since been full but not overflowing, while overall hospital census went down. The palliative care team at the hospital includes four inpatient doctors, nurse practitioners, and a chaplain, as well as an outpatient team primarily focused on oncology.
“In some settings, palliative care has been at the forefront of difficult conversations, when things aren’t going well for the patient and there’s much uncertainty,” Dr. Razzak said. The interface between hospital medicine and palliative care can be complementary, he added. “We talk about primary palliative care, which we want every discipline to be able to do – lead meaningful conversations, help manage symptoms.”
The take-home message for hospitalists, he said, is to get training in how to have these discussions, using such resources as VitalTalk (https://www.vitaltalk.org/), a nonprofit organization that disseminates education in communication skills for difficult conversations, and the Center to Advance Palliative Care (www.capc.org) at Icahn School of Medicine at Mount Sinai in New York City. “Once you’ve mastered the conversation, it will get easier. But ask for help when you need it, and learn how to know when you need it.”
Dr. Gundersen added that hospital medicine groups and palliative care teams could reach out to each other and talk about what they did in the crisis and how they can work together in the future. She recommends frequent ongoing support and collaboration that could range from formal conferences or training sessions to informal team interactions, perhaps with sandwiches in the doctor’s lounge – provided that there’s room for social distancing. She has recently started giving talks in the community and grand rounds presentations in hospitals about palliative care.
Other approaches and applications
In New York City, the initial epicenter for the pandemic in the United States, the adult palliative care service of Columbia University Medical Center (CUMC) experienced a sevenfold increase in consultation requests at the apex of the crisis, said its director, Craig Blinderman, MD. That demand was impossible to meet with existing staff. So Dr. Blinderman and colleagues established a virtual consultation model, recruiting and deploying volunteer out-of-state palliative care specialists to staff it.
An eight-bed palliative care unit was opened at CUMC for COVID-19 patients whose surrogates had opted not to initiate or continue intubation or life-sustaining treatments. This helped to relieve some of the pressures on the ICUs while making it possible for in-person visits to the hospice unit by families – in full PPE. Palliative care staff were embedded in various units in the hospital.
A palliative care response team composed of a hospice and palliative medicine fellow and four psychiatry residents or fellows, based in the emergency department and with supervision from the palliative care team, provided time-critical goals of care conversations with families using telemedicine – and a forum for listening to their suffering. Dr. Blinderman and colleagues also have found time to write up their experience for medical journals.1,2
There’s no reason to think that hospitalists, with a little basic training, couldn’t be having these same goals of care conversations, Dr. Blinderman said. “But the fact that hospitalists, at the pandemic’s peak, along with ICU doctors, were seeing an unprecedented magnitude of dying on a daily basis generated a lot of moral distress for them.”
Palliative care professionals, because they engage with these issues in a different way, may be somewhat better equipped to deal with the sheer emotional demands when so many are dying, as at the peak of the surge in New York. “We don’t see dying as a failure on our part but an opportunity to relieve suffering,” Dr. Blinderman said. And the palliative care field also emphasizes the importance of self-care for its practitioners.
“How do we meet the incredible palliative care needs in the epicenter of a pandemic? That question also applies to other kinds of crises we could imagine, for example, climate-related disasters,” Dr. Blinderman said. “What lessons have we learned about the value of palliative care and how to start incorporating it more integrally into the delivery of hospital care? Here we showed that we could work collaboratively with our colleagues at other major medical centers, bringing together their expertise to help us when we didn’t have the bandwidth to meet the demand,” he said.
Scripts can help
“Also, it won’t make sense to just go back to normal (after the crisis fades),” Dr. Blinderman said. “We need to take a close look at how our society is functioning in the wake of the pandemic and the ways the health care system has failed us. We have learned that we’re all interconnected and we need to work together to serve our communities – locally and nationally – applying basic distributive justice.”
Could there be, for example, a national infrastructure for mobilizing and deploying palliative care resources to areas of greatest need, similar to what was done in New York?
At Northwestern Medicine in Chicago, a number of palliative care clinicians at the system’s hospitals worked together to develop scripts designed to help other clinicians start goals of care conversations with patients and families, for use in the hospital as well as in outpatient primary care and other settings, with results integrated into the system’s electronic health record.
Front-line clinicians may not have the time to ask for formal consults from palliative care because of high volume and rapidly changing patient status, explained Eytan Szmuilowicz, MD, director of the section of palliative medicine at Northwestern Memorial Hospital. Or they may not have access to specialty-level palliative care in their settings.
The scripts are aimed at primary care, emergency physicians, and hospitalists needing to consider critical care placement or attempted resuscitation and to ICU clinicians helping families make decisions about life-sustaining treatments. They also can help facilitate advance care planning discussions. An example is “CALMER,” a six-step mnemonic guide to promote goals of care discussions with hospitalized patients. For more information on these scripts, contact Dr. Szmuilowicz: [email protected].
Eerily quiet
The COVID-19 crisis has been quite a whirlwind for hospital medicine, said Jeanie Youngwerth, MD, a hospitalist and program director of the palliative care service at the University of Colorado in Denver, which was a significant viral hotspot early on.
“When it first started, things seemed to change almost overnight – starting on Friday, March 13. People had to take action right away to develop work flows and the technology to allow us to see as many patients as possible,” she said. By the time Monday came, it was a whole new ballgame.
Dr. Youngwerth and two colleagues worked quickly to develop inpatient telemedicine capacity where none existed. “We knew we would not be going into patients’ rooms, but most of our team showed up in the hospital to work with the primary care teams. Our job was to see what we could do that actually made a difference,” she said.
“The hospital became a very strange place. You’d walk down the hallway and it was eerily quiet. Everybody you came across was being so nice to each other.” Televisits became a powerful way to bring the human connection back to medical care.
“What we learned from families was that they were thirsting to have some kind of connection with their loved one, and to be able to talk about their loved one and who they were as a person,” she said. “We’d contact the family through video visits and then, when the family meeting ended, the nurse would bring an iPad into the patient’s room so the family could see their loved one on a ventilator. They would immediately start communicating with their loved one, praying aloud, singing, playing music. It would make a huge difference for the family – and for the staff.”
References
1. Nakagawa S et al. Pandemic palliative care consultations spanning state and institutional borders. J Am Geriatr Soc. 2020 May 22. doi: 10.1111/jgs.16643.
2. Lee J Abrukin L, Flores S. Early intervention of palliative care in the emergency department during the COVID-19 pandemic. JAMA Intern Med. 2020 Jun 5. doi: 10.1001/jamainternmed.2020.2713.
ACC panel defines, advises on heart failure with ‘recovered’ EF
Because heart failure patients with recovered ejection fraction are a complex and diverse group, little consensus has emerged on how to define, diagnose, and manage this growing population.
To provide some clarity for identifying and treating these patients, a Journal of the American College of Cardiology scientific expert panel has issued a consensus document. Published Aug. 3 in the Journal of the American College of Cardiology, it provides a working definition of heart failure with recovered ejection fraction (HFrecEF) and recommends approaches for treatment and follow-up.
Defining a new class of HF
“Part of the impetus of this was to bring attention to what we think is a new class of heart failure, and it requires different treatment modalities and different ways of thinking about it,” expert panel member Douglas L. Mann, MD, cardiologist-in-chief at Barnes Jewish Hospital in St. Louis, said in an interview. “It’s a newly discovered HF biology about which we know very little and it’s very confusing to just go on the ejection fraction alone.”
The panel, led by Jane E. Wilcox, MD, of Northwestern University, Chicago, recommends three components for a working definition of HFrecEF: 1) documentation of a decreased left ventricle ejection fraction (LVEF) of less than 40% at baseline; 2) a 10% or greater absolute improvement in LVEF; and 3) a second measurement of LVEF >40%.
“We try to give it a nomenclature that clearly indicates what it is,” Dr. Mann said. “There has been a lot of confusing terminology.” Among the terms the panel calls out in the lexicon of modestly recovered EF, in addition to HFrecEF, are HF improved EF, HF with preserved EF (HFpEF), borderline HFpEF and HF with mid-range EF (HFmrEF).
The panel also recommends that guideline-based medical and device therapies for HFrecEF should continue indefinitely until there’s a better understanding of the biology and clinical epidemiology of HFrecEF, and that these patients should have close clinical follow-up because of the high risk of HF relapse.
Determining EF’s ‘trajectory’
The findings presented in the statement should help cardiologists distinguish HFrecEF from HFpEF, Dr. Mann noted. “Because EF is moving, we also have to emphasize the importance of following the trajectory of EF,” he said. “It’s not enough to know where the EF is; you have to know where it came from – if it had been from a higher number or a lower number – because that will help inform you about the patient’s disease.”
In that regard, the panel states that the level of change in LVEF – the “trajectory” – will provide clues to the nature and extent of myocardial injury, degree and duration of LV remodeling, and the type of therapy that’s indicated. Clinicians should consider HFmrEF, a description the European Society of Cardiology has endorsed, as an entity different from HFrecEF without data on LVEF trajectory.
The statement delves into the biology of HFrecEF, defining reverse LV remodeling as the restoration of some normalization to cardiac myocyte size and LV chamber geometry that results in a leftward shift toward normalization of end-diastolic pressure volume. The panel also noted that cardiac remodeling in reverse LV remodeling and recovery of LV function is bidirectional and involves multiple molecular and cellular changes that contribute to changes in the heart’s size, shape and function, and explains the role gene expression has in HF-related LV changes.
The statement explores the recovery of LV function, noting that spontaneous recovery often occurs when the cause of the myocardial dysfunction resolves. Common causes are chronic tachycardia and thyroid disease.
That panel noted that “super responders” to cardiac resynchronization therapy can provide insight into HFrecEF. Favorable responders include women, patients with nonischemic HF, very wide ECG ventricular depolarization wavelength with left-bundle branch block morphology, and dyssynchrony on ECG.
The panel states that, “regardless of the definition of HFrecEF,” the evidence suggests that younger patients, women, and those with nonischemic disease, shorter disease duration, and relatively few comorbidities are more likely to recover LVEF – and their outcomes are typically better than those of patients with HF reduced EF (HFrEF) and HFpEF.
Clinicians should bear in mind, however, that patients on guideline-directed medical therapy (GDMT) who achieve complete normalization of LV structure and function are prone to recurrent LV dysfunction and HF. The panel explored the role of potential treatment for three different etiologies of HF. Little is known about Takotsubo cardiomyopathy, considered a transient form of LV dysfunction, in terms of how many of these patients will develop HFrEF or if they’ll benefit from GDMT. Alcohol-induced cardiomyopathy patients should continue on medical therapy even if they have HFrecEF, as should patients with fulminant and nonfulminant myocarditis.
Managing HFrecEF
Management should include assessment of jugular vein distention and signs of volume overload – “particularly concerning in HFrecEF” – the panel noted. ECG is cost effective, and signs of left-bundle branch block are predictors of low success with GDMT alone. The panel also recommended a family history going back three generations and consideration of genetic testing to determine the risk for sudden cardiac death. Two-dimensional ECG can help predict GDMT response and cardiovascular magnetic resonance imaging can provide information about myocardial substrate at the time of diagnosis of HFrEF.
The panel suggested four areas for future research: 1) improved phenotyping of HFrEF; 2) use of inception cohorts to better understand the natural history of HFrecEF; 3) clinical trials to better define those clinical care components most effective at maintaining remission; and 4) basic studies to better define the biology of HFrecEF. “The goal,” wrote Dr. Wilcox and colleagues, “is to develop new therapeutic targets that will enable patients with HFrecEF to experience a durable remission from HF.”
Dr. Wilcox reported receiving funding from the National Institutes of Health and the American Heart Association, and financial relationships with Abbott, Medtronic, and Cytokinetics. Dr. Mann has received funding from NIH and reports financial relationships with MyoKardia and Novartis. Coauthors reported funding from NIH and AHA and financial relationships with Novartis, Amgen, AstraZeneca, Thoratec Corporation (Abbott), Sanofi, Pfizer, MyoKardia and American Regent.
SOURCE: Wilcox JE et al. J Am Coll Cardiol. 2020;76:719-34.
Because heart failure patients with recovered ejection fraction are a complex and diverse group, little consensus has emerged on how to define, diagnose, and manage this growing population.
To provide some clarity for identifying and treating these patients, a Journal of the American College of Cardiology scientific expert panel has issued a consensus document. Published Aug. 3 in the Journal of the American College of Cardiology, it provides a working definition of heart failure with recovered ejection fraction (HFrecEF) and recommends approaches for treatment and follow-up.
Defining a new class of HF
“Part of the impetus of this was to bring attention to what we think is a new class of heart failure, and it requires different treatment modalities and different ways of thinking about it,” expert panel member Douglas L. Mann, MD, cardiologist-in-chief at Barnes Jewish Hospital in St. Louis, said in an interview. “It’s a newly discovered HF biology about which we know very little and it’s very confusing to just go on the ejection fraction alone.”
The panel, led by Jane E. Wilcox, MD, of Northwestern University, Chicago, recommends three components for a working definition of HFrecEF: 1) documentation of a decreased left ventricle ejection fraction (LVEF) of less than 40% at baseline; 2) a 10% or greater absolute improvement in LVEF; and 3) a second measurement of LVEF >40%.
“We try to give it a nomenclature that clearly indicates what it is,” Dr. Mann said. “There has been a lot of confusing terminology.” Among the terms the panel calls out in the lexicon of modestly recovered EF, in addition to HFrecEF, are HF improved EF, HF with preserved EF (HFpEF), borderline HFpEF and HF with mid-range EF (HFmrEF).
The panel also recommends that guideline-based medical and device therapies for HFrecEF should continue indefinitely until there’s a better understanding of the biology and clinical epidemiology of HFrecEF, and that these patients should have close clinical follow-up because of the high risk of HF relapse.
Determining EF’s ‘trajectory’
The findings presented in the statement should help cardiologists distinguish HFrecEF from HFpEF, Dr. Mann noted. “Because EF is moving, we also have to emphasize the importance of following the trajectory of EF,” he said. “It’s not enough to know where the EF is; you have to know where it came from – if it had been from a higher number or a lower number – because that will help inform you about the patient’s disease.”
In that regard, the panel states that the level of change in LVEF – the “trajectory” – will provide clues to the nature and extent of myocardial injury, degree and duration of LV remodeling, and the type of therapy that’s indicated. Clinicians should consider HFmrEF, a description the European Society of Cardiology has endorsed, as an entity different from HFrecEF without data on LVEF trajectory.
The statement delves into the biology of HFrecEF, defining reverse LV remodeling as the restoration of some normalization to cardiac myocyte size and LV chamber geometry that results in a leftward shift toward normalization of end-diastolic pressure volume. The panel also noted that cardiac remodeling in reverse LV remodeling and recovery of LV function is bidirectional and involves multiple molecular and cellular changes that contribute to changes in the heart’s size, shape and function, and explains the role gene expression has in HF-related LV changes.
The statement explores the recovery of LV function, noting that spontaneous recovery often occurs when the cause of the myocardial dysfunction resolves. Common causes are chronic tachycardia and thyroid disease.
That panel noted that “super responders” to cardiac resynchronization therapy can provide insight into HFrecEF. Favorable responders include women, patients with nonischemic HF, very wide ECG ventricular depolarization wavelength with left-bundle branch block morphology, and dyssynchrony on ECG.
The panel states that, “regardless of the definition of HFrecEF,” the evidence suggests that younger patients, women, and those with nonischemic disease, shorter disease duration, and relatively few comorbidities are more likely to recover LVEF – and their outcomes are typically better than those of patients with HF reduced EF (HFrEF) and HFpEF.
Clinicians should bear in mind, however, that patients on guideline-directed medical therapy (GDMT) who achieve complete normalization of LV structure and function are prone to recurrent LV dysfunction and HF. The panel explored the role of potential treatment for three different etiologies of HF. Little is known about Takotsubo cardiomyopathy, considered a transient form of LV dysfunction, in terms of how many of these patients will develop HFrEF or if they’ll benefit from GDMT. Alcohol-induced cardiomyopathy patients should continue on medical therapy even if they have HFrecEF, as should patients with fulminant and nonfulminant myocarditis.
Managing HFrecEF
Management should include assessment of jugular vein distention and signs of volume overload – “particularly concerning in HFrecEF” – the panel noted. ECG is cost effective, and signs of left-bundle branch block are predictors of low success with GDMT alone. The panel also recommended a family history going back three generations and consideration of genetic testing to determine the risk for sudden cardiac death. Two-dimensional ECG can help predict GDMT response and cardiovascular magnetic resonance imaging can provide information about myocardial substrate at the time of diagnosis of HFrEF.
The panel suggested four areas for future research: 1) improved phenotyping of HFrEF; 2) use of inception cohorts to better understand the natural history of HFrecEF; 3) clinical trials to better define those clinical care components most effective at maintaining remission; and 4) basic studies to better define the biology of HFrecEF. “The goal,” wrote Dr. Wilcox and colleagues, “is to develop new therapeutic targets that will enable patients with HFrecEF to experience a durable remission from HF.”
Dr. Wilcox reported receiving funding from the National Institutes of Health and the American Heart Association, and financial relationships with Abbott, Medtronic, and Cytokinetics. Dr. Mann has received funding from NIH and reports financial relationships with MyoKardia and Novartis. Coauthors reported funding from NIH and AHA and financial relationships with Novartis, Amgen, AstraZeneca, Thoratec Corporation (Abbott), Sanofi, Pfizer, MyoKardia and American Regent.
SOURCE: Wilcox JE et al. J Am Coll Cardiol. 2020;76:719-34.
Because heart failure patients with recovered ejection fraction are a complex and diverse group, little consensus has emerged on how to define, diagnose, and manage this growing population.
To provide some clarity for identifying and treating these patients, a Journal of the American College of Cardiology scientific expert panel has issued a consensus document. Published Aug. 3 in the Journal of the American College of Cardiology, it provides a working definition of heart failure with recovered ejection fraction (HFrecEF) and recommends approaches for treatment and follow-up.
Defining a new class of HF
“Part of the impetus of this was to bring attention to what we think is a new class of heart failure, and it requires different treatment modalities and different ways of thinking about it,” expert panel member Douglas L. Mann, MD, cardiologist-in-chief at Barnes Jewish Hospital in St. Louis, said in an interview. “It’s a newly discovered HF biology about which we know very little and it’s very confusing to just go on the ejection fraction alone.”
The panel, led by Jane E. Wilcox, MD, of Northwestern University, Chicago, recommends three components for a working definition of HFrecEF: 1) documentation of a decreased left ventricle ejection fraction (LVEF) of less than 40% at baseline; 2) a 10% or greater absolute improvement in LVEF; and 3) a second measurement of LVEF >40%.
“We try to give it a nomenclature that clearly indicates what it is,” Dr. Mann said. “There has been a lot of confusing terminology.” Among the terms the panel calls out in the lexicon of modestly recovered EF, in addition to HFrecEF, are HF improved EF, HF with preserved EF (HFpEF), borderline HFpEF and HF with mid-range EF (HFmrEF).
The panel also recommends that guideline-based medical and device therapies for HFrecEF should continue indefinitely until there’s a better understanding of the biology and clinical epidemiology of HFrecEF, and that these patients should have close clinical follow-up because of the high risk of HF relapse.
Determining EF’s ‘trajectory’
The findings presented in the statement should help cardiologists distinguish HFrecEF from HFpEF, Dr. Mann noted. “Because EF is moving, we also have to emphasize the importance of following the trajectory of EF,” he said. “It’s not enough to know where the EF is; you have to know where it came from – if it had been from a higher number or a lower number – because that will help inform you about the patient’s disease.”
In that regard, the panel states that the level of change in LVEF – the “trajectory” – will provide clues to the nature and extent of myocardial injury, degree and duration of LV remodeling, and the type of therapy that’s indicated. Clinicians should consider HFmrEF, a description the European Society of Cardiology has endorsed, as an entity different from HFrecEF without data on LVEF trajectory.
The statement delves into the biology of HFrecEF, defining reverse LV remodeling as the restoration of some normalization to cardiac myocyte size and LV chamber geometry that results in a leftward shift toward normalization of end-diastolic pressure volume. The panel also noted that cardiac remodeling in reverse LV remodeling and recovery of LV function is bidirectional and involves multiple molecular and cellular changes that contribute to changes in the heart’s size, shape and function, and explains the role gene expression has in HF-related LV changes.
The statement explores the recovery of LV function, noting that spontaneous recovery often occurs when the cause of the myocardial dysfunction resolves. Common causes are chronic tachycardia and thyroid disease.
That panel noted that “super responders” to cardiac resynchronization therapy can provide insight into HFrecEF. Favorable responders include women, patients with nonischemic HF, very wide ECG ventricular depolarization wavelength with left-bundle branch block morphology, and dyssynchrony on ECG.
The panel states that, “regardless of the definition of HFrecEF,” the evidence suggests that younger patients, women, and those with nonischemic disease, shorter disease duration, and relatively few comorbidities are more likely to recover LVEF – and their outcomes are typically better than those of patients with HF reduced EF (HFrEF) and HFpEF.
Clinicians should bear in mind, however, that patients on guideline-directed medical therapy (GDMT) who achieve complete normalization of LV structure and function are prone to recurrent LV dysfunction and HF. The panel explored the role of potential treatment for three different etiologies of HF. Little is known about Takotsubo cardiomyopathy, considered a transient form of LV dysfunction, in terms of how many of these patients will develop HFrEF or if they’ll benefit from GDMT. Alcohol-induced cardiomyopathy patients should continue on medical therapy even if they have HFrecEF, as should patients with fulminant and nonfulminant myocarditis.
Managing HFrecEF
Management should include assessment of jugular vein distention and signs of volume overload – “particularly concerning in HFrecEF” – the panel noted. ECG is cost effective, and signs of left-bundle branch block are predictors of low success with GDMT alone. The panel also recommended a family history going back three generations and consideration of genetic testing to determine the risk for sudden cardiac death. Two-dimensional ECG can help predict GDMT response and cardiovascular magnetic resonance imaging can provide information about myocardial substrate at the time of diagnosis of HFrEF.
The panel suggested four areas for future research: 1) improved phenotyping of HFrEF; 2) use of inception cohorts to better understand the natural history of HFrecEF; 3) clinical trials to better define those clinical care components most effective at maintaining remission; and 4) basic studies to better define the biology of HFrecEF. “The goal,” wrote Dr. Wilcox and colleagues, “is to develop new therapeutic targets that will enable patients with HFrecEF to experience a durable remission from HF.”
Dr. Wilcox reported receiving funding from the National Institutes of Health and the American Heart Association, and financial relationships with Abbott, Medtronic, and Cytokinetics. Dr. Mann has received funding from NIH and reports financial relationships with MyoKardia and Novartis. Coauthors reported funding from NIH and AHA and financial relationships with Novartis, Amgen, AstraZeneca, Thoratec Corporation (Abbott), Sanofi, Pfizer, MyoKardia and American Regent.
SOURCE: Wilcox JE et al. J Am Coll Cardiol. 2020;76:719-34.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY


















