User login
-
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]


High obesity rates in Southern states magnify COVID threats
In January, as Mississippi health officials planned for their incoming shipments of COVID-19 vaccine, they assessed the state’s most vulnerable: health care workers, of course, and elderly people in nursing homes. But among those who needed urgent protection from the virus ripping across the Magnolia State were 1 million Mississippians with obesity.
Obesity and weight-related illnesses have been deadly liabilities in the COVID era. A report released this month by the World Obesity Federation found that increased body weight is the second-greatest predictor of COVID-related hospitalization and death across the globe, trailing only old age as a risk factor.
As a fixture of life in the American South – home to 9 of the nation’s 12 heaviest states – obesity is playing a role not only in COVID outcomes, but in the calculus of the vaccination rollout. Mississippi was one of the first states to add a body mass index of 30 or more (a rough gauge of obesity tied to height and weight) to the list of qualifying medical conditions for a shot. About 40% of the state’s adults meet that definition, according to federal health survey data, and combined with the risk group already eligible for vaccination – residents 65 and older – that means fully half of Mississippi’s adults are entitled to vie for a restricted allotment of shots.
At least 29 states have green-lighted obesity for inclusion in the first phases of the vaccine rollout, according to KFF – a vast widening of eligibility that has the potential to overwhelm government efforts and heighten competition for scarce doses.
“We have a lifesaving intervention, and we don’t have enough of it,” said Jen Kates, PhD, director of global health and HIV policy for Kaiser Family Foundation. “Hard choices are being made about who should go first, and there is no right answer.”
The sheer prevalence of obesity in the nation – two in three Americans exceed what is considered a healthy weight – was a public health concern well before the pandemic. But COVID-19 dramatically fast-tracked the discussion from warnings about the long-term damage excess fat tissue can pose to heart, lung and metabolic functions to far more immediate threats.
In the United Kingdom, for example, overweight COVID patients were 67% more likely to require intensive care, and obese patients three times likelier, according to the World Obesity Federation report. A Centers for Disease Control and Prevention study released Monday found a similar trend among U.S. patients and noted that the risk of COVID-related hospitalization, ventilation and death increased with patients’ obesity level.
The counties that hug the southern Mississippi River are home to some of the most concentrated pockets of extreme obesity in the United States. Coronavirus infections began surging in Southern states early last summer, and hospitalizations rose in step.
Deaths in rural stretches of Arkansas, Louisiana, Mississippi, and Tennessee have been overshadowed by the sheer number of deaths in metropolitan areas like New York, Los Angeles, and Essex County, N.J. But as a share of the population, the coronavirus has been similarly unsparing in many Southern communities. In sparsely populated Claiborne County, Miss., on the floodplains of the Mississippi River, 30 residents – about 1 in 300 – had died as of early March. In East Feliciana Parish, La., north of Baton Rouge, with 106 deaths, about 1 in 180 had died by then.
“It’s just math. If the population is more obese and obesity clearly contributes to worse outcomes, then neighborhoods, cities, states and countries that are more obese will have a greater toll from COVID,” said Dr. James de Lemos, MD, a professor of internal medicine at UT Southwestern Medical Center in Dallas who led a study of hospitalized COVID patients published in the medical journal Circulation.
And, because in the U.S. obesity rates tend to be relatively high among African Americans and Latinos who are poor, with diminished access to health care, “it’s a triple whammy,” Dr. de Lemos said. “All these things intersect.”
Poverty and limited access to medical care are common features in the South, where residents like Michelle Antonyshyn, a former registered nurse and mother of seven in Salem, Ark., say they are afraid of the virus. Ms. Antonyshyn, 49, has obesity and debilitating pain in her knees and back, though she does not have high blood pressure or diabetes, two underlying conditions that federal health officials have determined are added risk factors for severe cases of COVID-19.
Still, she said, she “was very concerned just knowing that being obese puts you more at risk for bad outcomes such as being on a ventilator and death.” As a precaution, Ms. Antonyshyn said, she and her large brood locked down early and stopped attending church services in person, watching online instead.
“It’s not the same as having fellowship, but the risk for me was enough,” said Ms. Antonyshyn.
Governors throughout the South seem to recognize that weight can contribute to COVID-19 complications and have pushed for vaccine eligibility rules that prioritize obesity. But on the ground, local health officials are girding for having to tell newly eligible people who qualify as obese that there aren’t enough shots to go around.
In Port Gibson, Miss., Mheja Williams, MD, medical director of the Claiborne County Family Health Center, has been receiving barely enough doses to inoculate the health workers and oldest seniors in her county of 9,600. One week in early February, she received 100 doses.
Obesity and extreme obesity are endemic in Claiborne County, and health officials say the “normalization” of obesity means people often don’t register their weight as a risk factor, whether for COVID or other health issues. The risks are exacerbated by a general flouting of pandemic etiquette: Dr. Williams said that middle-aged and younger residents are not especially vigilant about physical distancing and that mask use is rare.
The rise of obesity in the United States is well documented over the past half-century, as the nation turned from a diet of fruits, vegetables and limited meats to one laden with ultra-processed foods and rich with salt, fat, sugar, and flavorings, along with copious amounts of meat, fast food, and soda. The U.S. has generally led the global obesity race, setting records as even toddlers and young children grew implausibly, dangerously overweight.
Well before COVID, obesity was a leading cause of preventable death in the United States. The National Institutes of Health declared it a disease in 1998, one that fosters heart disease, stroke, type 2 diabetes, and breast, colon, and other cancers.
Researchers say it is no coincidence that nations like the United States, the United Kingdom, and Italy, with relatively high obesity rates, have proved particularly vulnerable to the novel coronavirus.
They believe the virus may exploit underlying metabolic and physiological impairments that often exist in concert with obesity. Extra fat can lead to a cascade of metabolic disruptions, chronic systemic inflammation, and hormonal dysregulation that may thwart the body’s response to infection.
Other respiratory viruses, like influenza and SARS, which appeared in China in 2002, rely on cholesterol to spread enveloped RNA virus to neighboring cells, and researchers have proposed that a similar mechanism may play a role in the spread of the novel coronavirus.
There are also practical problems for coronavirus patients with obesity admitted to the hospital. They can be more difficult to intubate because of excess central weight pressing down on the diaphragm, making breathing with infected lungs even more difficult.
Physicians who specialize in treating patients with obesity say public health officials need to be more forthright and urgent in their messaging, telegraphing the risks of this COVID era.
“It should be explicit and direct,” said Fatima Stanford, MD, an obesity medicine specialist at Massachusetts General Hospital, Boston, and a Harvard Medical School instructor.
Dr. Stanford denounces the fat-shaming and bullying that people with obesity often experience. But telling patients – and the public – that obesity increases the risk of hospitalization and death is crucial, she said.
“I don’t think it’s stigmatizing,” she said. “If you tell them in that way, it’s not to scare you, it’s just giving information. Sometimes people are just unaware.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
In January, as Mississippi health officials planned for their incoming shipments of COVID-19 vaccine, they assessed the state’s most vulnerable: health care workers, of course, and elderly people in nursing homes. But among those who needed urgent protection from the virus ripping across the Magnolia State were 1 million Mississippians with obesity.
Obesity and weight-related illnesses have been deadly liabilities in the COVID era. A report released this month by the World Obesity Federation found that increased body weight is the second-greatest predictor of COVID-related hospitalization and death across the globe, trailing only old age as a risk factor.
As a fixture of life in the American South – home to 9 of the nation’s 12 heaviest states – obesity is playing a role not only in COVID outcomes, but in the calculus of the vaccination rollout. Mississippi was one of the first states to add a body mass index of 30 or more (a rough gauge of obesity tied to height and weight) to the list of qualifying medical conditions for a shot. About 40% of the state’s adults meet that definition, according to federal health survey data, and combined with the risk group already eligible for vaccination – residents 65 and older – that means fully half of Mississippi’s adults are entitled to vie for a restricted allotment of shots.
At least 29 states have green-lighted obesity for inclusion in the first phases of the vaccine rollout, according to KFF – a vast widening of eligibility that has the potential to overwhelm government efforts and heighten competition for scarce doses.
“We have a lifesaving intervention, and we don’t have enough of it,” said Jen Kates, PhD, director of global health and HIV policy for Kaiser Family Foundation. “Hard choices are being made about who should go first, and there is no right answer.”
The sheer prevalence of obesity in the nation – two in three Americans exceed what is considered a healthy weight – was a public health concern well before the pandemic. But COVID-19 dramatically fast-tracked the discussion from warnings about the long-term damage excess fat tissue can pose to heart, lung and metabolic functions to far more immediate threats.
In the United Kingdom, for example, overweight COVID patients were 67% more likely to require intensive care, and obese patients three times likelier, according to the World Obesity Federation report. A Centers for Disease Control and Prevention study released Monday found a similar trend among U.S. patients and noted that the risk of COVID-related hospitalization, ventilation and death increased with patients’ obesity level.
The counties that hug the southern Mississippi River are home to some of the most concentrated pockets of extreme obesity in the United States. Coronavirus infections began surging in Southern states early last summer, and hospitalizations rose in step.
Deaths in rural stretches of Arkansas, Louisiana, Mississippi, and Tennessee have been overshadowed by the sheer number of deaths in metropolitan areas like New York, Los Angeles, and Essex County, N.J. But as a share of the population, the coronavirus has been similarly unsparing in many Southern communities. In sparsely populated Claiborne County, Miss., on the floodplains of the Mississippi River, 30 residents – about 1 in 300 – had died as of early March. In East Feliciana Parish, La., north of Baton Rouge, with 106 deaths, about 1 in 180 had died by then.
“It’s just math. If the population is more obese and obesity clearly contributes to worse outcomes, then neighborhoods, cities, states and countries that are more obese will have a greater toll from COVID,” said Dr. James de Lemos, MD, a professor of internal medicine at UT Southwestern Medical Center in Dallas who led a study of hospitalized COVID patients published in the medical journal Circulation.
And, because in the U.S. obesity rates tend to be relatively high among African Americans and Latinos who are poor, with diminished access to health care, “it’s a triple whammy,” Dr. de Lemos said. “All these things intersect.”
Poverty and limited access to medical care are common features in the South, where residents like Michelle Antonyshyn, a former registered nurse and mother of seven in Salem, Ark., say they are afraid of the virus. Ms. Antonyshyn, 49, has obesity and debilitating pain in her knees and back, though she does not have high blood pressure or diabetes, two underlying conditions that federal health officials have determined are added risk factors for severe cases of COVID-19.
Still, she said, she “was very concerned just knowing that being obese puts you more at risk for bad outcomes such as being on a ventilator and death.” As a precaution, Ms. Antonyshyn said, she and her large brood locked down early and stopped attending church services in person, watching online instead.
“It’s not the same as having fellowship, but the risk for me was enough,” said Ms. Antonyshyn.
Governors throughout the South seem to recognize that weight can contribute to COVID-19 complications and have pushed for vaccine eligibility rules that prioritize obesity. But on the ground, local health officials are girding for having to tell newly eligible people who qualify as obese that there aren’t enough shots to go around.
In Port Gibson, Miss., Mheja Williams, MD, medical director of the Claiborne County Family Health Center, has been receiving barely enough doses to inoculate the health workers and oldest seniors in her county of 9,600. One week in early February, she received 100 doses.
Obesity and extreme obesity are endemic in Claiborne County, and health officials say the “normalization” of obesity means people often don’t register their weight as a risk factor, whether for COVID or other health issues. The risks are exacerbated by a general flouting of pandemic etiquette: Dr. Williams said that middle-aged and younger residents are not especially vigilant about physical distancing and that mask use is rare.
The rise of obesity in the United States is well documented over the past half-century, as the nation turned from a diet of fruits, vegetables and limited meats to one laden with ultra-processed foods and rich with salt, fat, sugar, and flavorings, along with copious amounts of meat, fast food, and soda. The U.S. has generally led the global obesity race, setting records as even toddlers and young children grew implausibly, dangerously overweight.
Well before COVID, obesity was a leading cause of preventable death in the United States. The National Institutes of Health declared it a disease in 1998, one that fosters heart disease, stroke, type 2 diabetes, and breast, colon, and other cancers.
Researchers say it is no coincidence that nations like the United States, the United Kingdom, and Italy, with relatively high obesity rates, have proved particularly vulnerable to the novel coronavirus.
They believe the virus may exploit underlying metabolic and physiological impairments that often exist in concert with obesity. Extra fat can lead to a cascade of metabolic disruptions, chronic systemic inflammation, and hormonal dysregulation that may thwart the body’s response to infection.
Other respiratory viruses, like influenza and SARS, which appeared in China in 2002, rely on cholesterol to spread enveloped RNA virus to neighboring cells, and researchers have proposed that a similar mechanism may play a role in the spread of the novel coronavirus.
There are also practical problems for coronavirus patients with obesity admitted to the hospital. They can be more difficult to intubate because of excess central weight pressing down on the diaphragm, making breathing with infected lungs even more difficult.
Physicians who specialize in treating patients with obesity say public health officials need to be more forthright and urgent in their messaging, telegraphing the risks of this COVID era.
“It should be explicit and direct,” said Fatima Stanford, MD, an obesity medicine specialist at Massachusetts General Hospital, Boston, and a Harvard Medical School instructor.
Dr. Stanford denounces the fat-shaming and bullying that people with obesity often experience. But telling patients – and the public – that obesity increases the risk of hospitalization and death is crucial, she said.
“I don’t think it’s stigmatizing,” she said. “If you tell them in that way, it’s not to scare you, it’s just giving information. Sometimes people are just unaware.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
In January, as Mississippi health officials planned for their incoming shipments of COVID-19 vaccine, they assessed the state’s most vulnerable: health care workers, of course, and elderly people in nursing homes. But among those who needed urgent protection from the virus ripping across the Magnolia State were 1 million Mississippians with obesity.
Obesity and weight-related illnesses have been deadly liabilities in the COVID era. A report released this month by the World Obesity Federation found that increased body weight is the second-greatest predictor of COVID-related hospitalization and death across the globe, trailing only old age as a risk factor.
As a fixture of life in the American South – home to 9 of the nation’s 12 heaviest states – obesity is playing a role not only in COVID outcomes, but in the calculus of the vaccination rollout. Mississippi was one of the first states to add a body mass index of 30 or more (a rough gauge of obesity tied to height and weight) to the list of qualifying medical conditions for a shot. About 40% of the state’s adults meet that definition, according to federal health survey data, and combined with the risk group already eligible for vaccination – residents 65 and older – that means fully half of Mississippi’s adults are entitled to vie for a restricted allotment of shots.
At least 29 states have green-lighted obesity for inclusion in the first phases of the vaccine rollout, according to KFF – a vast widening of eligibility that has the potential to overwhelm government efforts and heighten competition for scarce doses.
“We have a lifesaving intervention, and we don’t have enough of it,” said Jen Kates, PhD, director of global health and HIV policy for Kaiser Family Foundation. “Hard choices are being made about who should go first, and there is no right answer.”
The sheer prevalence of obesity in the nation – two in three Americans exceed what is considered a healthy weight – was a public health concern well before the pandemic. But COVID-19 dramatically fast-tracked the discussion from warnings about the long-term damage excess fat tissue can pose to heart, lung and metabolic functions to far more immediate threats.
In the United Kingdom, for example, overweight COVID patients were 67% more likely to require intensive care, and obese patients three times likelier, according to the World Obesity Federation report. A Centers for Disease Control and Prevention study released Monday found a similar trend among U.S. patients and noted that the risk of COVID-related hospitalization, ventilation and death increased with patients’ obesity level.
The counties that hug the southern Mississippi River are home to some of the most concentrated pockets of extreme obesity in the United States. Coronavirus infections began surging in Southern states early last summer, and hospitalizations rose in step.
Deaths in rural stretches of Arkansas, Louisiana, Mississippi, and Tennessee have been overshadowed by the sheer number of deaths in metropolitan areas like New York, Los Angeles, and Essex County, N.J. But as a share of the population, the coronavirus has been similarly unsparing in many Southern communities. In sparsely populated Claiborne County, Miss., on the floodplains of the Mississippi River, 30 residents – about 1 in 300 – had died as of early March. In East Feliciana Parish, La., north of Baton Rouge, with 106 deaths, about 1 in 180 had died by then.
“It’s just math. If the population is more obese and obesity clearly contributes to worse outcomes, then neighborhoods, cities, states and countries that are more obese will have a greater toll from COVID,” said Dr. James de Lemos, MD, a professor of internal medicine at UT Southwestern Medical Center in Dallas who led a study of hospitalized COVID patients published in the medical journal Circulation.
And, because in the U.S. obesity rates tend to be relatively high among African Americans and Latinos who are poor, with diminished access to health care, “it’s a triple whammy,” Dr. de Lemos said. “All these things intersect.”
Poverty and limited access to medical care are common features in the South, where residents like Michelle Antonyshyn, a former registered nurse and mother of seven in Salem, Ark., say they are afraid of the virus. Ms. Antonyshyn, 49, has obesity and debilitating pain in her knees and back, though she does not have high blood pressure or diabetes, two underlying conditions that federal health officials have determined are added risk factors for severe cases of COVID-19.
Still, she said, she “was very concerned just knowing that being obese puts you more at risk for bad outcomes such as being on a ventilator and death.” As a precaution, Ms. Antonyshyn said, she and her large brood locked down early and stopped attending church services in person, watching online instead.
“It’s not the same as having fellowship, but the risk for me was enough,” said Ms. Antonyshyn.
Governors throughout the South seem to recognize that weight can contribute to COVID-19 complications and have pushed for vaccine eligibility rules that prioritize obesity. But on the ground, local health officials are girding for having to tell newly eligible people who qualify as obese that there aren’t enough shots to go around.
In Port Gibson, Miss., Mheja Williams, MD, medical director of the Claiborne County Family Health Center, has been receiving barely enough doses to inoculate the health workers and oldest seniors in her county of 9,600. One week in early February, she received 100 doses.
Obesity and extreme obesity are endemic in Claiborne County, and health officials say the “normalization” of obesity means people often don’t register their weight as a risk factor, whether for COVID or other health issues. The risks are exacerbated by a general flouting of pandemic etiquette: Dr. Williams said that middle-aged and younger residents are not especially vigilant about physical distancing and that mask use is rare.
The rise of obesity in the United States is well documented over the past half-century, as the nation turned from a diet of fruits, vegetables and limited meats to one laden with ultra-processed foods and rich with salt, fat, sugar, and flavorings, along with copious amounts of meat, fast food, and soda. The U.S. has generally led the global obesity race, setting records as even toddlers and young children grew implausibly, dangerously overweight.
Well before COVID, obesity was a leading cause of preventable death in the United States. The National Institutes of Health declared it a disease in 1998, one that fosters heart disease, stroke, type 2 diabetes, and breast, colon, and other cancers.
Researchers say it is no coincidence that nations like the United States, the United Kingdom, and Italy, with relatively high obesity rates, have proved particularly vulnerable to the novel coronavirus.
They believe the virus may exploit underlying metabolic and physiological impairments that often exist in concert with obesity. Extra fat can lead to a cascade of metabolic disruptions, chronic systemic inflammation, and hormonal dysregulation that may thwart the body’s response to infection.
Other respiratory viruses, like influenza and SARS, which appeared in China in 2002, rely on cholesterol to spread enveloped RNA virus to neighboring cells, and researchers have proposed that a similar mechanism may play a role in the spread of the novel coronavirus.
There are also practical problems for coronavirus patients with obesity admitted to the hospital. They can be more difficult to intubate because of excess central weight pressing down on the diaphragm, making breathing with infected lungs even more difficult.
Physicians who specialize in treating patients with obesity say public health officials need to be more forthright and urgent in their messaging, telegraphing the risks of this COVID era.
“It should be explicit and direct,” said Fatima Stanford, MD, an obesity medicine specialist at Massachusetts General Hospital, Boston, and a Harvard Medical School instructor.
Dr. Stanford denounces the fat-shaming and bullying that people with obesity often experience. But telling patients – and the public – that obesity increases the risk of hospitalization and death is crucial, she said.
“I don’t think it’s stigmatizing,” she said. “If you tell them in that way, it’s not to scare you, it’s just giving information. Sometimes people are just unaware.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
COVID-19 virus reinfections rare; riskiest after age 65
When researchers analyzed test results of 4 million people in Denmark, they found that less than 1% of those who tested positive experienced reinfection.
Initial infection was associated with about 80% protection overall against getting SARS-CoV-2 again. However, among those older than 65, the protection plummeted to 47%.
“Not everybody is protected against reinfection after a first infection. Older people are at higher risk of catching it again,” co–lead author Daniela Michlmayr, PhD, said in an interview. “Our findings emphasize the importance of policies to protect the elderly and of adhering to infection control measures and restrictions, even if previously infected with COVID-19.”
Verifying the need for vaccination
“The findings also highlight the need to vaccinate people who had COVID-19 before, as natural immunity to infection – especially among the elderly 65 and older – cannot be relied upon,” added Dr. Michlmayr, a researcher in the department of bacteria, parasites, and fungi at the Staten Serums Institut, Copenhagen.
The population-based observational study was published online March 17 in The Lancet.
“The findings make sense, as patients who are immunocompromised or of advanced age may not mount an immune response that is as long-lasting,” David Hirschwerk, MD, said in an interview. “It does underscore the importance of vaccination for people of more advanced age, even if they previously were infected with COVID.
“For those who were infected last spring and have not yet been vaccinated, this helps to support the value of still pursuing the vaccine,” added Dr. Hirschwerk, an infectious disease specialist at Northwell Health in Manhasset, N.Y.
Evidence on reinfection risk was limited prior to this study. “Little is known about protection against SARS-CoV-2 repeat infections, but two studies in the UK have found that immunity could last at least 5 to 6 months after infection,” the authors noted.
Along with co–lead author Christian Holm Hansen, PhD, Dr. Michlmayr and colleagues found that 2.11% of 525,339 individuals tested positive for SARS-CoV-2 during the first surge in Denmark from March to May 2020. Within this group, 0.65% tested positive during a second surge from September to December.
By the end of 2020, more than 10 million people had undergone free polymerase chain reaction testing by the Danish government or through the national TestDenmark program.
“My overall take is that it is great to have such a big dataset looking at this question,” E. John Wherry, PhD, said in an interview. The findings support “what we’ve seen in previous, smaller studies.”
Natural protection against reinfection of approximately 80% “is not as good as the vaccines, but not bad,” added Dr. Wherry, director of the Institute for Immunology at the University of Pennsylvania, Philadelphia.
Age alters immunity?
“Our finding that older people were more likely than younger people to test positive again if they had already tested positive could be explained by natural age-related changes in the immune system of older adults, also referred to as immune senescence,” the authors noted.
The investigators found no significant differences in reinfection rates between women and men.
As with the previous research, this study also indicates that an initial bout with SARS-CoV-2 infection appears to confer protection for at least 6 months. The researchers found no significant differences between people who were followed for 3-6 months and those followed for 7 months or longer.
Variants not included
To account for possible bias among people who got tested repeatedly, Dr. Michlmayr and colleagues performed a sensitivity analysis in a subgroup. They assessed reinfection rates among people who underwent testing frequently and routinely – nurses, doctors, social workers, and health care assistants – and found that 1.2% tested positive a second time during the second surge.
A limitation of the study was the inability to correlate symptoms with risk for reinfection. Also, the researchers did not account for SARS-CoV-2 variants, noting that “during the study period, such variants were not yet established in Denmark; although into 2021 this pattern is changing.”
Asked to speculate whether the results would be different had the study accounted for variants, Dr. Hirschwerk said, “It depends upon the variant, but certainly for the B.1.351 variant, there already has been data clearly demonstrating risk of reinfection with SARS-CoV-2 despite prior infection with the original strain of virus.”
The emergence of SARS-CoV-2 variants of concern that could escape natural and vaccine-related immunity “complicates matters further,” Rosemary J. Boyton, MBBS, and Daniel M. Altmann, PhD, both of Imperial College London, wrote in an accompanying comment in The Lancet.
“Emerging variants of concern might shift immunity below a protective margin, prompting the need for updated vaccines. Interestingly, vaccine responses even after single dose are substantially enhanced in individuals with a history of infection with SARS-CoV-2,” they added.
The current study confirms that “the hope of protective immunity through natural infections might not be within our reach, and a global vaccination program with high efficacy vaccines is the enduring solution,” Dr. Boyton and Dr. Altmann noted.
Cause for alarm?
Despite evidence that reinfection is relatively rare, “many will find the data reported by Hansen and colleagues about protection through natural infection relatively alarming,” Dr. Boyton and Dr. Altmann wrote in their commentary. The 80% protection rate from reinfection in general and the 47% rate among people aged 65 and older “are more concerning figures than offered by previous studies.”
Vaccines appear to provide better quality, quantity, and durability of protection against repeated infection – measured in terms of neutralizing antibodies and T cells – compared with previous infection with SARS-CoV-2, Dr. Boyton and Dr. Altmann wrote.
More research needed
The duration of natural protection against reinfection remains an unanswered question, the researchers noted, “because too little time has elapsed since the beginning of the pandemic.”
Future prospective and longitudinal cohort studies coupled with molecular surveillance are needed to characterize antibody titers and waning of protection against repeat infections, the authors noted. Furthermore, more answers are needed regarding how some virus variants might contribute to reinfection risk.
No funding for the study has been reported. Dr. Michlmayr, Dr. Hirschwerk, Dr. Wherry, Dr. Boyton, and Dr. Altmann have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
When researchers analyzed test results of 4 million people in Denmark, they found that less than 1% of those who tested positive experienced reinfection.
Initial infection was associated with about 80% protection overall against getting SARS-CoV-2 again. However, among those older than 65, the protection plummeted to 47%.
“Not everybody is protected against reinfection after a first infection. Older people are at higher risk of catching it again,” co–lead author Daniela Michlmayr, PhD, said in an interview. “Our findings emphasize the importance of policies to protect the elderly and of adhering to infection control measures and restrictions, even if previously infected with COVID-19.”
Verifying the need for vaccination
“The findings also highlight the need to vaccinate people who had COVID-19 before, as natural immunity to infection – especially among the elderly 65 and older – cannot be relied upon,” added Dr. Michlmayr, a researcher in the department of bacteria, parasites, and fungi at the Staten Serums Institut, Copenhagen.
The population-based observational study was published online March 17 in The Lancet.
“The findings make sense, as patients who are immunocompromised or of advanced age may not mount an immune response that is as long-lasting,” David Hirschwerk, MD, said in an interview. “It does underscore the importance of vaccination for people of more advanced age, even if they previously were infected with COVID.
“For those who were infected last spring and have not yet been vaccinated, this helps to support the value of still pursuing the vaccine,” added Dr. Hirschwerk, an infectious disease specialist at Northwell Health in Manhasset, N.Y.
Evidence on reinfection risk was limited prior to this study. “Little is known about protection against SARS-CoV-2 repeat infections, but two studies in the UK have found that immunity could last at least 5 to 6 months after infection,” the authors noted.
Along with co–lead author Christian Holm Hansen, PhD, Dr. Michlmayr and colleagues found that 2.11% of 525,339 individuals tested positive for SARS-CoV-2 during the first surge in Denmark from March to May 2020. Within this group, 0.65% tested positive during a second surge from September to December.
By the end of 2020, more than 10 million people had undergone free polymerase chain reaction testing by the Danish government or through the national TestDenmark program.
“My overall take is that it is great to have such a big dataset looking at this question,” E. John Wherry, PhD, said in an interview. The findings support “what we’ve seen in previous, smaller studies.”
Natural protection against reinfection of approximately 80% “is not as good as the vaccines, but not bad,” added Dr. Wherry, director of the Institute for Immunology at the University of Pennsylvania, Philadelphia.
Age alters immunity?
“Our finding that older people were more likely than younger people to test positive again if they had already tested positive could be explained by natural age-related changes in the immune system of older adults, also referred to as immune senescence,” the authors noted.
The investigators found no significant differences in reinfection rates between women and men.
As with the previous research, this study also indicates that an initial bout with SARS-CoV-2 infection appears to confer protection for at least 6 months. The researchers found no significant differences between people who were followed for 3-6 months and those followed for 7 months or longer.
Variants not included
To account for possible bias among people who got tested repeatedly, Dr. Michlmayr and colleagues performed a sensitivity analysis in a subgroup. They assessed reinfection rates among people who underwent testing frequently and routinely – nurses, doctors, social workers, and health care assistants – and found that 1.2% tested positive a second time during the second surge.
A limitation of the study was the inability to correlate symptoms with risk for reinfection. Also, the researchers did not account for SARS-CoV-2 variants, noting that “during the study period, such variants were not yet established in Denmark; although into 2021 this pattern is changing.”
Asked to speculate whether the results would be different had the study accounted for variants, Dr. Hirschwerk said, “It depends upon the variant, but certainly for the B.1.351 variant, there already has been data clearly demonstrating risk of reinfection with SARS-CoV-2 despite prior infection with the original strain of virus.”
The emergence of SARS-CoV-2 variants of concern that could escape natural and vaccine-related immunity “complicates matters further,” Rosemary J. Boyton, MBBS, and Daniel M. Altmann, PhD, both of Imperial College London, wrote in an accompanying comment in The Lancet.
“Emerging variants of concern might shift immunity below a protective margin, prompting the need for updated vaccines. Interestingly, vaccine responses even after single dose are substantially enhanced in individuals with a history of infection with SARS-CoV-2,” they added.
The current study confirms that “the hope of protective immunity through natural infections might not be within our reach, and a global vaccination program with high efficacy vaccines is the enduring solution,” Dr. Boyton and Dr. Altmann noted.
Cause for alarm?
Despite evidence that reinfection is relatively rare, “many will find the data reported by Hansen and colleagues about protection through natural infection relatively alarming,” Dr. Boyton and Dr. Altmann wrote in their commentary. The 80% protection rate from reinfection in general and the 47% rate among people aged 65 and older “are more concerning figures than offered by previous studies.”
Vaccines appear to provide better quality, quantity, and durability of protection against repeated infection – measured in terms of neutralizing antibodies and T cells – compared with previous infection with SARS-CoV-2, Dr. Boyton and Dr. Altmann wrote.
More research needed
The duration of natural protection against reinfection remains an unanswered question, the researchers noted, “because too little time has elapsed since the beginning of the pandemic.”
Future prospective and longitudinal cohort studies coupled with molecular surveillance are needed to characterize antibody titers and waning of protection against repeat infections, the authors noted. Furthermore, more answers are needed regarding how some virus variants might contribute to reinfection risk.
No funding for the study has been reported. Dr. Michlmayr, Dr. Hirschwerk, Dr. Wherry, Dr. Boyton, and Dr. Altmann have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
When researchers analyzed test results of 4 million people in Denmark, they found that less than 1% of those who tested positive experienced reinfection.
Initial infection was associated with about 80% protection overall against getting SARS-CoV-2 again. However, among those older than 65, the protection plummeted to 47%.
“Not everybody is protected against reinfection after a first infection. Older people are at higher risk of catching it again,” co–lead author Daniela Michlmayr, PhD, said in an interview. “Our findings emphasize the importance of policies to protect the elderly and of adhering to infection control measures and restrictions, even if previously infected with COVID-19.”
Verifying the need for vaccination
“The findings also highlight the need to vaccinate people who had COVID-19 before, as natural immunity to infection – especially among the elderly 65 and older – cannot be relied upon,” added Dr. Michlmayr, a researcher in the department of bacteria, parasites, and fungi at the Staten Serums Institut, Copenhagen.
The population-based observational study was published online March 17 in The Lancet.
“The findings make sense, as patients who are immunocompromised or of advanced age may not mount an immune response that is as long-lasting,” David Hirschwerk, MD, said in an interview. “It does underscore the importance of vaccination for people of more advanced age, even if they previously were infected with COVID.
“For those who were infected last spring and have not yet been vaccinated, this helps to support the value of still pursuing the vaccine,” added Dr. Hirschwerk, an infectious disease specialist at Northwell Health in Manhasset, N.Y.
Evidence on reinfection risk was limited prior to this study. “Little is known about protection against SARS-CoV-2 repeat infections, but two studies in the UK have found that immunity could last at least 5 to 6 months after infection,” the authors noted.
Along with co–lead author Christian Holm Hansen, PhD, Dr. Michlmayr and colleagues found that 2.11% of 525,339 individuals tested positive for SARS-CoV-2 during the first surge in Denmark from March to May 2020. Within this group, 0.65% tested positive during a second surge from September to December.
By the end of 2020, more than 10 million people had undergone free polymerase chain reaction testing by the Danish government or through the national TestDenmark program.
“My overall take is that it is great to have such a big dataset looking at this question,” E. John Wherry, PhD, said in an interview. The findings support “what we’ve seen in previous, smaller studies.”
Natural protection against reinfection of approximately 80% “is not as good as the vaccines, but not bad,” added Dr. Wherry, director of the Institute for Immunology at the University of Pennsylvania, Philadelphia.
Age alters immunity?
“Our finding that older people were more likely than younger people to test positive again if they had already tested positive could be explained by natural age-related changes in the immune system of older adults, also referred to as immune senescence,” the authors noted.
The investigators found no significant differences in reinfection rates between women and men.
As with the previous research, this study also indicates that an initial bout with SARS-CoV-2 infection appears to confer protection for at least 6 months. The researchers found no significant differences between people who were followed for 3-6 months and those followed for 7 months or longer.
Variants not included
To account for possible bias among people who got tested repeatedly, Dr. Michlmayr and colleagues performed a sensitivity analysis in a subgroup. They assessed reinfection rates among people who underwent testing frequently and routinely – nurses, doctors, social workers, and health care assistants – and found that 1.2% tested positive a second time during the second surge.
A limitation of the study was the inability to correlate symptoms with risk for reinfection. Also, the researchers did not account for SARS-CoV-2 variants, noting that “during the study period, such variants were not yet established in Denmark; although into 2021 this pattern is changing.”
Asked to speculate whether the results would be different had the study accounted for variants, Dr. Hirschwerk said, “It depends upon the variant, but certainly for the B.1.351 variant, there already has been data clearly demonstrating risk of reinfection with SARS-CoV-2 despite prior infection with the original strain of virus.”
The emergence of SARS-CoV-2 variants of concern that could escape natural and vaccine-related immunity “complicates matters further,” Rosemary J. Boyton, MBBS, and Daniel M. Altmann, PhD, both of Imperial College London, wrote in an accompanying comment in The Lancet.
“Emerging variants of concern might shift immunity below a protective margin, prompting the need for updated vaccines. Interestingly, vaccine responses even after single dose are substantially enhanced in individuals with a history of infection with SARS-CoV-2,” they added.
The current study confirms that “the hope of protective immunity through natural infections might not be within our reach, and a global vaccination program with high efficacy vaccines is the enduring solution,” Dr. Boyton and Dr. Altmann noted.
Cause for alarm?
Despite evidence that reinfection is relatively rare, “many will find the data reported by Hansen and colleagues about protection through natural infection relatively alarming,” Dr. Boyton and Dr. Altmann wrote in their commentary. The 80% protection rate from reinfection in general and the 47% rate among people aged 65 and older “are more concerning figures than offered by previous studies.”
Vaccines appear to provide better quality, quantity, and durability of protection against repeated infection – measured in terms of neutralizing antibodies and T cells – compared with previous infection with SARS-CoV-2, Dr. Boyton and Dr. Altmann wrote.
More research needed
The duration of natural protection against reinfection remains an unanswered question, the researchers noted, “because too little time has elapsed since the beginning of the pandemic.”
Future prospective and longitudinal cohort studies coupled with molecular surveillance are needed to characterize antibody titers and waning of protection against repeat infections, the authors noted. Furthermore, more answers are needed regarding how some virus variants might contribute to reinfection risk.
No funding for the study has been reported. Dr. Michlmayr, Dr. Hirschwerk, Dr. Wherry, Dr. Boyton, and Dr. Altmann have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New guidelines dispel myths about COVID-19 treatment
Recommendations, as well as conspiracy theories about COVID-19, have changed at distressing rates over the past year. No disease has ever been more politicized, or more polarizing.
Experts, as well as the least educated, take a stand on what they believe is the most important way to prevent and treat this virus.
Just recently, a study was published revealing that ivermectin is not effective as a COVID-19 treatment while people continue to claim it works. It has never been more important for doctors, and especially family physicians, to have accurate and updated guidelines.
The NIH and CDC have been publishing recommendations and guidelines for the prevention and treatment of COVID-19 since the start of the pandemic. Like any new disease, these have been changing to keep up as new knowledge related to the disease becomes available.
NIH updates treatment guidelines
A recent update to the NIH COVID-19 treatment guidelines was published on March 5, 2021. While the complete guidelines are quite extensive, spanning over 200 pages, it’s most important to understand the most recent updates in them.
Since preventative medicine is an integral part of primary care, it is important to note that no medications have been advised to prevent infection with COVID-19. In fact, taking drugs for pre-exposure prophylaxis (PrEp) is not recommended even in the highest-risk patients, such as health care workers.
In the updated guidelines, tocilizumab in a single IV dose of 8 mg/kg up to a maximum of 800 mg can be given only in combination with dexamethasone (or equivalent corticosteroid) in certain hospitalized patients exhibiting rapid respiratory decompensation. These patients include recently hospitalized patients who have been admitted to the ICU within the previous 24 hours and now require mechanical ventilation or high-flow oxygen via nasal cannula. Those not in the ICU who require rapidly increasing oxygen levels and have significantly increased levels of inflammatory markers should also receive this therapy. In the new guidance, the NIH recommends treating other hospitalized patients who require oxygen with remdesivir, remdesivir + dexamethasone, or dexamethasone alone.
In outpatients, those who have mild to moderate infection and are at increased risk of developing severe disease and/or hospitalization can be treated with bamlanivimab 700 mg + etesevimab 1,400 mg. This should be started as soon as possible after a confirmed diagnosis and within 10 days of symptom onset, according to the NIH recommendations. There is no evidence to support its use in patients hospitalized because of infection. However, it can be used in patients hospitalized for other reasons who have mild to moderate infection, but should be reserved – because of limited supply – for those with the highest risk of complications.
Hydroxychloroquine and casirivimab + imdevimab
One medication that has been touted in the media as a tool to treat COVID-19 has been hydroxychloroquine. Past guidelines recommended against this medication as a treatment because it lacked efficacy and posed risks for no therapeutic benefit. The most recent guidelines also recommend against the use of hydroxychloroquine for pre- and postexposure prophylaxis.
Casirivimab + imdevimab has been another talked about therapy. However, current guidelines recommend against its use in hospitalized patients. In addition, it is advised that hospitalized patients be enrolled in a clinical trial to receive it.
Since the pandemic began, the world has seen more than 120 million infections and more than 2 million deaths. Family physicians have a vital role to play as we are often the first ones patients turn to for treatment and advice. It is imperative we stay current with the guidelines and follow the most recent updates as research data are published.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, N.J. You can contact her at [email protected].
Recommendations, as well as conspiracy theories about COVID-19, have changed at distressing rates over the past year. No disease has ever been more politicized, or more polarizing.
Experts, as well as the least educated, take a stand on what they believe is the most important way to prevent and treat this virus.
Just recently, a study was published revealing that ivermectin is not effective as a COVID-19 treatment while people continue to claim it works. It has never been more important for doctors, and especially family physicians, to have accurate and updated guidelines.
The NIH and CDC have been publishing recommendations and guidelines for the prevention and treatment of COVID-19 since the start of the pandemic. Like any new disease, these have been changing to keep up as new knowledge related to the disease becomes available.
NIH updates treatment guidelines
A recent update to the NIH COVID-19 treatment guidelines was published on March 5, 2021. While the complete guidelines are quite extensive, spanning over 200 pages, it’s most important to understand the most recent updates in them.
Since preventative medicine is an integral part of primary care, it is important to note that no medications have been advised to prevent infection with COVID-19. In fact, taking drugs for pre-exposure prophylaxis (PrEp) is not recommended even in the highest-risk patients, such as health care workers.
In the updated guidelines, tocilizumab in a single IV dose of 8 mg/kg up to a maximum of 800 mg can be given only in combination with dexamethasone (or equivalent corticosteroid) in certain hospitalized patients exhibiting rapid respiratory decompensation. These patients include recently hospitalized patients who have been admitted to the ICU within the previous 24 hours and now require mechanical ventilation or high-flow oxygen via nasal cannula. Those not in the ICU who require rapidly increasing oxygen levels and have significantly increased levels of inflammatory markers should also receive this therapy. In the new guidance, the NIH recommends treating other hospitalized patients who require oxygen with remdesivir, remdesivir + dexamethasone, or dexamethasone alone.
In outpatients, those who have mild to moderate infection and are at increased risk of developing severe disease and/or hospitalization can be treated with bamlanivimab 700 mg + etesevimab 1,400 mg. This should be started as soon as possible after a confirmed diagnosis and within 10 days of symptom onset, according to the NIH recommendations. There is no evidence to support its use in patients hospitalized because of infection. However, it can be used in patients hospitalized for other reasons who have mild to moderate infection, but should be reserved – because of limited supply – for those with the highest risk of complications.
Hydroxychloroquine and casirivimab + imdevimab
One medication that has been touted in the media as a tool to treat COVID-19 has been hydroxychloroquine. Past guidelines recommended against this medication as a treatment because it lacked efficacy and posed risks for no therapeutic benefit. The most recent guidelines also recommend against the use of hydroxychloroquine for pre- and postexposure prophylaxis.
Casirivimab + imdevimab has been another talked about therapy. However, current guidelines recommend against its use in hospitalized patients. In addition, it is advised that hospitalized patients be enrolled in a clinical trial to receive it.
Since the pandemic began, the world has seen more than 120 million infections and more than 2 million deaths. Family physicians have a vital role to play as we are often the first ones patients turn to for treatment and advice. It is imperative we stay current with the guidelines and follow the most recent updates as research data are published.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, N.J. You can contact her at [email protected].
Recommendations, as well as conspiracy theories about COVID-19, have changed at distressing rates over the past year. No disease has ever been more politicized, or more polarizing.
Experts, as well as the least educated, take a stand on what they believe is the most important way to prevent and treat this virus.
Just recently, a study was published revealing that ivermectin is not effective as a COVID-19 treatment while people continue to claim it works. It has never been more important for doctors, and especially family physicians, to have accurate and updated guidelines.
The NIH and CDC have been publishing recommendations and guidelines for the prevention and treatment of COVID-19 since the start of the pandemic. Like any new disease, these have been changing to keep up as new knowledge related to the disease becomes available.
NIH updates treatment guidelines
A recent update to the NIH COVID-19 treatment guidelines was published on March 5, 2021. While the complete guidelines are quite extensive, spanning over 200 pages, it’s most important to understand the most recent updates in them.
Since preventative medicine is an integral part of primary care, it is important to note that no medications have been advised to prevent infection with COVID-19. In fact, taking drugs for pre-exposure prophylaxis (PrEp) is not recommended even in the highest-risk patients, such as health care workers.
In the updated guidelines, tocilizumab in a single IV dose of 8 mg/kg up to a maximum of 800 mg can be given only in combination with dexamethasone (or equivalent corticosteroid) in certain hospitalized patients exhibiting rapid respiratory decompensation. These patients include recently hospitalized patients who have been admitted to the ICU within the previous 24 hours and now require mechanical ventilation or high-flow oxygen via nasal cannula. Those not in the ICU who require rapidly increasing oxygen levels and have significantly increased levels of inflammatory markers should also receive this therapy. In the new guidance, the NIH recommends treating other hospitalized patients who require oxygen with remdesivir, remdesivir + dexamethasone, or dexamethasone alone.
In outpatients, those who have mild to moderate infection and are at increased risk of developing severe disease and/or hospitalization can be treated with bamlanivimab 700 mg + etesevimab 1,400 mg. This should be started as soon as possible after a confirmed diagnosis and within 10 days of symptom onset, according to the NIH recommendations. There is no evidence to support its use in patients hospitalized because of infection. However, it can be used in patients hospitalized for other reasons who have mild to moderate infection, but should be reserved – because of limited supply – for those with the highest risk of complications.
Hydroxychloroquine and casirivimab + imdevimab
One medication that has been touted in the media as a tool to treat COVID-19 has been hydroxychloroquine. Past guidelines recommended against this medication as a treatment because it lacked efficacy and posed risks for no therapeutic benefit. The most recent guidelines also recommend against the use of hydroxychloroquine for pre- and postexposure prophylaxis.
Casirivimab + imdevimab has been another talked about therapy. However, current guidelines recommend against its use in hospitalized patients. In addition, it is advised that hospitalized patients be enrolled in a clinical trial to receive it.
Since the pandemic began, the world has seen more than 120 million infections and more than 2 million deaths. Family physicians have a vital role to play as we are often the first ones patients turn to for treatment and advice. It is imperative we stay current with the guidelines and follow the most recent updates as research data are published.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, N.J. You can contact her at [email protected].
Some with long COVID see relief after vaccination
Several weeks after getting his second dose of an mRNA vaccine, Aaron Goyang thinks his long bout with COVID-19 has finally come to an end.
Mr. Goyang, who is 33 and is a radiology technician in Austin, Tex., thinks he got COVID-19 from some of the coughing, gasping patients he treated last spring.
At the time, testing was scarce, and by the time he was tested – several weeks into his illness – it came back negative. He fought off the initial symptoms but experienced relapse a week later.
Mr. Goyang says that, for the next 8 or 9 months, he was on a roller coaster with extreme shortness of breath and chest tightness that could be so severe it would send him to the emergency department. He had to use an inhaler to get through his workdays.
“Even if I was just sitting around, it would come and take me,” he says. “It almost felt like someone was bear-hugging me constantly, and I just couldn’t get in a good enough breath.”
On his best days, he would walk around his neighborhood, being careful not to overdo it. He tried running once, and it nearly sent him to the hospital.
“Very honestly, I didn’t know if I would ever be able to do it again,” he says.
But Mr. Goyang says that, several weeks after getting the Pfizer vaccine, he was able to run a mile again with no problems. “I was very thankful for that,” he says.
Mr. Goyang is not alone. Some social media groups are dedicated to patients who are living with a condition that’s been known as long COVID and that was recently termed postacute sequelae of SARS-CoV-2 infection (PASC). These patients are sometimes referred to as long haulers.
On social media, patients with PASC are eagerly and anxiously quizzing each other about the vaccines and their effects.
Survivor Corps, which has a public Facebook group with 159,000 members, recently took a poll to see whether there was any substance to rumors that those with long COVID were feeling better after being vaccinated.
“Out of 400 people, 36% showed an improvement in symptoms, anywhere between a mild improvement to complete resolution of symptoms,” said Diana Berrent, a long-COVID patient who founded the group. Survivor Corps has become active in patient advocacy and is a resource for researchers studying the new condition.
Ms. Berrent has become such a trusted voice during the pandemic. She interviewed Anthony Fauci, MD, head of the National Institutes of Allergy and Infectious Diseases, last October.
“The implications are huge,” she says.
“Some of this damage is permanent damage. It’s not going to cure the scarring of your heart tissue, it’s not going to cure the irreparable damage to your lungs, but if it’s making people feel better, then that’s an indication there’s viral persistence going on,” says Ms. Berrent.
“I’ve been saying for months and months, we shouldn’t be calling this postacute anything,” she adds.
Patients report improvement
Daniel Griffin, MD, PhD, is equally excited. He’s an infectious disease specialist at Columbia University, New York. He says about one in five patients he treated for COVID-19 last year never got better. Many of them, such as Mr. Goyang, were health care workers.
“I don’t know if people actually catch this, but a lot of our coworkers are either permanently disabled or died,” Dr. Griffin says.
Health care workers were also among the first to be vaccinated. Dr. Griffin says many of his patients began reaching out to him about a week or two after being vaccinated “and saying, ‘You know, I actually feel better.’ And some of them were saying, ‘I feel all better,’ after being sick – a lot of them – for a year.”
Then he was getting calls and texts from other doctors, asking, “Hey, are you seeing this?”
The benefits of vaccination for some long-haulers came as a surprise. Dr. Griffin says that, before the vaccines came out, many of his patients were worried that getting vaccinated might overstimulate their immune systems and cause symptoms to get worse.
Indeed, a small percentage of people – about 3%-5%, based on informal polls on social media – report that they do experience worsening of symptoms after getting the shot. It’s not clear why.
Dr. Griffin estimates that between 30% and 50% of patients’ symptoms improve after they receive the mRNA vaccines. “I’m seeing this chunk of people – they tell me their brain fog has improved, their fatigue is gone, the fevers that wouldn’t resolve have now gone,” he says. “I’m seeing that personally, and I’m hearing it from my colleagues.”
Dr. Griffin says the observation has launched several studies and that there are several theories about how the vaccines might be affecting long COVID.
An immune system boost?
One possibility is that the virus continues to stimulate the immune system, which continues to fight the virus for months. If that is the case, Dr. Griffin says, the vaccine may be giving the immune system the boost it needs to finally clear the virus away.
Donna Farber, PhD, a professor of microbiology and immunology at Columbia University, has heard the stories, too.
“It is possible that the persisting virus in long COVID-19 may be at a low level – not enough to stimulate a potent immune response to clear the virus, but enough to cause symptoms. Activating the immune response therefore is therapeutic in directing viral clearance,” she says.
Dr. Farber explains that long COVID may be a bit like Lyme disease. Some patients with Lyme disease must take antibiotics for months before their symptoms disappear.
Dr. Griffin says there’s another possibility. Several studies have now shown that people with lingering COVID-19 symptoms develop autoantibodies. There’s a theory that SARS-CoV-2 may create an autoimmune condition that leads to long-term symptoms.
If that is the case, Dr. Griffin says, the vaccine may be helping the body to reset its tolerance to itself, “so maybe now you’re getting a healthy immune response.”
More studies are needed to know for sure.
Either way, the vaccines are a much-needed bit of hope for the long-COVID community, and Dr. Griffin tells his patients who are still worried that, at the very least, they’ll be protected from another SARS-CoV-2 infection.
A version of this article first appeared on Medscape.com.
Several weeks after getting his second dose of an mRNA vaccine, Aaron Goyang thinks his long bout with COVID-19 has finally come to an end.
Mr. Goyang, who is 33 and is a radiology technician in Austin, Tex., thinks he got COVID-19 from some of the coughing, gasping patients he treated last spring.
At the time, testing was scarce, and by the time he was tested – several weeks into his illness – it came back negative. He fought off the initial symptoms but experienced relapse a week later.
Mr. Goyang says that, for the next 8 or 9 months, he was on a roller coaster with extreme shortness of breath and chest tightness that could be so severe it would send him to the emergency department. He had to use an inhaler to get through his workdays.
“Even if I was just sitting around, it would come and take me,” he says. “It almost felt like someone was bear-hugging me constantly, and I just couldn’t get in a good enough breath.”
On his best days, he would walk around his neighborhood, being careful not to overdo it. He tried running once, and it nearly sent him to the hospital.
“Very honestly, I didn’t know if I would ever be able to do it again,” he says.
But Mr. Goyang says that, several weeks after getting the Pfizer vaccine, he was able to run a mile again with no problems. “I was very thankful for that,” he says.
Mr. Goyang is not alone. Some social media groups are dedicated to patients who are living with a condition that’s been known as long COVID and that was recently termed postacute sequelae of SARS-CoV-2 infection (PASC). These patients are sometimes referred to as long haulers.
On social media, patients with PASC are eagerly and anxiously quizzing each other about the vaccines and their effects.
Survivor Corps, which has a public Facebook group with 159,000 members, recently took a poll to see whether there was any substance to rumors that those with long COVID were feeling better after being vaccinated.
“Out of 400 people, 36% showed an improvement in symptoms, anywhere between a mild improvement to complete resolution of symptoms,” said Diana Berrent, a long-COVID patient who founded the group. Survivor Corps has become active in patient advocacy and is a resource for researchers studying the new condition.
Ms. Berrent has become such a trusted voice during the pandemic. She interviewed Anthony Fauci, MD, head of the National Institutes of Allergy and Infectious Diseases, last October.
“The implications are huge,” she says.
“Some of this damage is permanent damage. It’s not going to cure the scarring of your heart tissue, it’s not going to cure the irreparable damage to your lungs, but if it’s making people feel better, then that’s an indication there’s viral persistence going on,” says Ms. Berrent.
“I’ve been saying for months and months, we shouldn’t be calling this postacute anything,” she adds.
Patients report improvement
Daniel Griffin, MD, PhD, is equally excited. He’s an infectious disease specialist at Columbia University, New York. He says about one in five patients he treated for COVID-19 last year never got better. Many of them, such as Mr. Goyang, were health care workers.
“I don’t know if people actually catch this, but a lot of our coworkers are either permanently disabled or died,” Dr. Griffin says.
Health care workers were also among the first to be vaccinated. Dr. Griffin says many of his patients began reaching out to him about a week or two after being vaccinated “and saying, ‘You know, I actually feel better.’ And some of them were saying, ‘I feel all better,’ after being sick – a lot of them – for a year.”
Then he was getting calls and texts from other doctors, asking, “Hey, are you seeing this?”
The benefits of vaccination for some long-haulers came as a surprise. Dr. Griffin says that, before the vaccines came out, many of his patients were worried that getting vaccinated might overstimulate their immune systems and cause symptoms to get worse.
Indeed, a small percentage of people – about 3%-5%, based on informal polls on social media – report that they do experience worsening of symptoms after getting the shot. It’s not clear why.
Dr. Griffin estimates that between 30% and 50% of patients’ symptoms improve after they receive the mRNA vaccines. “I’m seeing this chunk of people – they tell me their brain fog has improved, their fatigue is gone, the fevers that wouldn’t resolve have now gone,” he says. “I’m seeing that personally, and I’m hearing it from my colleagues.”
Dr. Griffin says the observation has launched several studies and that there are several theories about how the vaccines might be affecting long COVID.
An immune system boost?
One possibility is that the virus continues to stimulate the immune system, which continues to fight the virus for months. If that is the case, Dr. Griffin says, the vaccine may be giving the immune system the boost it needs to finally clear the virus away.
Donna Farber, PhD, a professor of microbiology and immunology at Columbia University, has heard the stories, too.
“It is possible that the persisting virus in long COVID-19 may be at a low level – not enough to stimulate a potent immune response to clear the virus, but enough to cause symptoms. Activating the immune response therefore is therapeutic in directing viral clearance,” she says.
Dr. Farber explains that long COVID may be a bit like Lyme disease. Some patients with Lyme disease must take antibiotics for months before their symptoms disappear.
Dr. Griffin says there’s another possibility. Several studies have now shown that people with lingering COVID-19 symptoms develop autoantibodies. There’s a theory that SARS-CoV-2 may create an autoimmune condition that leads to long-term symptoms.
If that is the case, Dr. Griffin says, the vaccine may be helping the body to reset its tolerance to itself, “so maybe now you’re getting a healthy immune response.”
More studies are needed to know for sure.
Either way, the vaccines are a much-needed bit of hope for the long-COVID community, and Dr. Griffin tells his patients who are still worried that, at the very least, they’ll be protected from another SARS-CoV-2 infection.
A version of this article first appeared on Medscape.com.
Several weeks after getting his second dose of an mRNA vaccine, Aaron Goyang thinks his long bout with COVID-19 has finally come to an end.
Mr. Goyang, who is 33 and is a radiology technician in Austin, Tex., thinks he got COVID-19 from some of the coughing, gasping patients he treated last spring.
At the time, testing was scarce, and by the time he was tested – several weeks into his illness – it came back negative. He fought off the initial symptoms but experienced relapse a week later.
Mr. Goyang says that, for the next 8 or 9 months, he was on a roller coaster with extreme shortness of breath and chest tightness that could be so severe it would send him to the emergency department. He had to use an inhaler to get through his workdays.
“Even if I was just sitting around, it would come and take me,” he says. “It almost felt like someone was bear-hugging me constantly, and I just couldn’t get in a good enough breath.”
On his best days, he would walk around his neighborhood, being careful not to overdo it. He tried running once, and it nearly sent him to the hospital.
“Very honestly, I didn’t know if I would ever be able to do it again,” he says.
But Mr. Goyang says that, several weeks after getting the Pfizer vaccine, he was able to run a mile again with no problems. “I was very thankful for that,” he says.
Mr. Goyang is not alone. Some social media groups are dedicated to patients who are living with a condition that’s been known as long COVID and that was recently termed postacute sequelae of SARS-CoV-2 infection (PASC). These patients are sometimes referred to as long haulers.
On social media, patients with PASC are eagerly and anxiously quizzing each other about the vaccines and their effects.
Survivor Corps, which has a public Facebook group with 159,000 members, recently took a poll to see whether there was any substance to rumors that those with long COVID were feeling better after being vaccinated.
“Out of 400 people, 36% showed an improvement in symptoms, anywhere between a mild improvement to complete resolution of symptoms,” said Diana Berrent, a long-COVID patient who founded the group. Survivor Corps has become active in patient advocacy and is a resource for researchers studying the new condition.
Ms. Berrent has become such a trusted voice during the pandemic. She interviewed Anthony Fauci, MD, head of the National Institutes of Allergy and Infectious Diseases, last October.
“The implications are huge,” she says.
“Some of this damage is permanent damage. It’s not going to cure the scarring of your heart tissue, it’s not going to cure the irreparable damage to your lungs, but if it’s making people feel better, then that’s an indication there’s viral persistence going on,” says Ms. Berrent.
“I’ve been saying for months and months, we shouldn’t be calling this postacute anything,” she adds.
Patients report improvement
Daniel Griffin, MD, PhD, is equally excited. He’s an infectious disease specialist at Columbia University, New York. He says about one in five patients he treated for COVID-19 last year never got better. Many of them, such as Mr. Goyang, were health care workers.
“I don’t know if people actually catch this, but a lot of our coworkers are either permanently disabled or died,” Dr. Griffin says.
Health care workers were also among the first to be vaccinated. Dr. Griffin says many of his patients began reaching out to him about a week or two after being vaccinated “and saying, ‘You know, I actually feel better.’ And some of them were saying, ‘I feel all better,’ after being sick – a lot of them – for a year.”
Then he was getting calls and texts from other doctors, asking, “Hey, are you seeing this?”
The benefits of vaccination for some long-haulers came as a surprise. Dr. Griffin says that, before the vaccines came out, many of his patients were worried that getting vaccinated might overstimulate their immune systems and cause symptoms to get worse.
Indeed, a small percentage of people – about 3%-5%, based on informal polls on social media – report that they do experience worsening of symptoms after getting the shot. It’s not clear why.
Dr. Griffin estimates that between 30% and 50% of patients’ symptoms improve after they receive the mRNA vaccines. “I’m seeing this chunk of people – they tell me their brain fog has improved, their fatigue is gone, the fevers that wouldn’t resolve have now gone,” he says. “I’m seeing that personally, and I’m hearing it from my colleagues.”
Dr. Griffin says the observation has launched several studies and that there are several theories about how the vaccines might be affecting long COVID.
An immune system boost?
One possibility is that the virus continues to stimulate the immune system, which continues to fight the virus for months. If that is the case, Dr. Griffin says, the vaccine may be giving the immune system the boost it needs to finally clear the virus away.
Donna Farber, PhD, a professor of microbiology and immunology at Columbia University, has heard the stories, too.
“It is possible that the persisting virus in long COVID-19 may be at a low level – not enough to stimulate a potent immune response to clear the virus, but enough to cause symptoms. Activating the immune response therefore is therapeutic in directing viral clearance,” she says.
Dr. Farber explains that long COVID may be a bit like Lyme disease. Some patients with Lyme disease must take antibiotics for months before their symptoms disappear.
Dr. Griffin says there’s another possibility. Several studies have now shown that people with lingering COVID-19 symptoms develop autoantibodies. There’s a theory that SARS-CoV-2 may create an autoimmune condition that leads to long-term symptoms.
If that is the case, Dr. Griffin says, the vaccine may be helping the body to reset its tolerance to itself, “so maybe now you’re getting a healthy immune response.”
More studies are needed to know for sure.
Either way, the vaccines are a much-needed bit of hope for the long-COVID community, and Dr. Griffin tells his patients who are still worried that, at the very least, they’ll be protected from another SARS-CoV-2 infection.
A version of this article first appeared on Medscape.com.
Could pollen be driving COVID-19 infections?
Some scientists say they’ve noticed a pattern to the recurring waves of SARS-CoV-2 infections around the globe: As pollen levels increased in outdoor air in 31 countries, COVID-19 cases accelerated.
Yet other recent studies point in the opposite direction, suggesting that peaks in pollen seasons coincide with a fall-off in the spread of some respiratory viruses, like COVID-19 and influenza. There’s even some evidence that pollen may compete with the virus that causes COVID-19 and may even help prevent infection.
So which is it? The answer may still be up in the air.
Doctors don’t fully understand what makes some viruses – like the ones that cause the flu – circulate in seasonal patterns.
There are, of course, many theories. These revolve around things like temperature and humidity – viruses tend to prefer colder, drier air – something that’s thought to help them spread more easily in the winter months. People are exposed to less sunlight during the winter, as they spend more time indoors, and the earth points away from the sun, providing some natural shielding. That may play a role because ultraviolet light from the sun acts like a natural disinfectant and may help keep circulating viral levels down.
In addition, exposure to sunlight helps the body make vitamin D, which may help keep our immune responses strong. Extreme temperatures – both cold and hot – also change our behavior, so that we spend more time cloistered indoors, where we can more easily cough and sneeze on each other and generally swap more germs.
Spike in pollen, jump in infections
The new study, published in the Proceedings of the National Academy of Sciences, adds a new variable to this mix – pollen. It relies on data from 248 airborne pollen–monitoring sites in 31 countries. The study also took into account other effects, such as population density, temperature, humidity, and lockdown orders. The study authors found that, when pollen in an area spiked, so did infections, after an average lag of about 4 days. The study authors say pollen seemed to account for, on average, 44% of the infection rate variability between countries.
The study authors say pollen could be a culprit in respiratory infections, not because the viruses hitch a ride on pollen grains and travel into our mouth, eyes, and nose, but because pollen seems to perturb our immune defenses, even if a person isn’t allergic to it.
“When we inhale pollen, they end up on our nasal mucosa, and here they diminish the expression of genes that are important for the defense against airborne viruses,” study author Stefanie Gilles, PhD, chair of environmental medicine at the Technical University of Munich, said in a press conference.
In a study published last year, Dr. Gilles found that mice exposed to pollen made less interferon and other protective chemical signals to the immune system. Those then infected with respiratory syncytial virus had more virus in their bodies, compared with mice not exposed to pollen. She seemed to see the same effect in human volunteers.
The study authors think pollen may cause the body to drop its defenses against the airborne virus that causes COVID-19, too.
“If you’re in a crowded room, and other people are there that are asymptomatic, and you’ve just been breathing in pollen all day long, chances are that you’re going to be more susceptible to the virus,” says Lewis Ziska, PhD, a plant physiologist who studies pollen, climate change, and health at Columbia University’s Mailman School of Public Health in New York. “Having a mask is obviously really critical in that regard.”
Masks do a great job of blocking pollen, so wearing one is even more important when pollen and viruses are floating around, he says.
Other researchers, however, say that, while the study raises some interesting questions, it can’t prove that pollen is increasing COVID-19 infections.
“Just because two things happen at the same time doesn’t mean that one causes the other,” says Martijn Hoogeveen, PhD, a professor of technical sciences and environment at the Open University in the Netherlands.
Dr. Hoogeveen’s recent study, published in Science of the Total Environment, found that the arrival of pollen season in the Netherlands coincides with the end of flu season, and that COVID-19 infection peaks tend to follow a similar pattern – exactly the opposite of the PNAS study.
Another preprint study, which focused on the Chicago area, found the same thing – as pollen climbs, flu cases drop. The researchers behind that study think pollen may actually compete with viruses in our airways, helping to block them from infecting our cells.
Patterns may be hard to nail down
Why did these studies reach such different conclusions?
Dr. Hoogeveen’s paper focused on a single country and looked at the incidence of flu infections over four seasons, from 2016 to 2020, while the PNAS study collected data on pollen from January through the first week of April 2020.
He thinks that a single season, or really part of a season, may not be long enough to see meaningful patterns, especially considering that this new-to-humans virus was spreading quickly at nearly the same time. He says it will be interesting to follow what happens with COVID-19 infections and pollen in the coming months and years.
Dr. Hoogeveen says that in a large study spanning so many countries it would have been nearly impossible to account for differences in pandemic control strategies. Some countries embraced the use of masks, stay-at-home orders, and social distancing, for example, while others took less stringent measures in order to let the virus run its course in pursuit of herd immunity.
Limiting the study area to a single country or city, he says, helps researchers better understand all the variables that might have been in play along with pollen.
“There is no scientific consensus yet, about what it is driving, and that’s what makes it such an interesting field,” he says.
A version of this article first appeared on Medscape.com.
Some scientists say they’ve noticed a pattern to the recurring waves of SARS-CoV-2 infections around the globe: As pollen levels increased in outdoor air in 31 countries, COVID-19 cases accelerated.
Yet other recent studies point in the opposite direction, suggesting that peaks in pollen seasons coincide with a fall-off in the spread of some respiratory viruses, like COVID-19 and influenza. There’s even some evidence that pollen may compete with the virus that causes COVID-19 and may even help prevent infection.
So which is it? The answer may still be up in the air.
Doctors don’t fully understand what makes some viruses – like the ones that cause the flu – circulate in seasonal patterns.
There are, of course, many theories. These revolve around things like temperature and humidity – viruses tend to prefer colder, drier air – something that’s thought to help them spread more easily in the winter months. People are exposed to less sunlight during the winter, as they spend more time indoors, and the earth points away from the sun, providing some natural shielding. That may play a role because ultraviolet light from the sun acts like a natural disinfectant and may help keep circulating viral levels down.
In addition, exposure to sunlight helps the body make vitamin D, which may help keep our immune responses strong. Extreme temperatures – both cold and hot – also change our behavior, so that we spend more time cloistered indoors, where we can more easily cough and sneeze on each other and generally swap more germs.
Spike in pollen, jump in infections
The new study, published in the Proceedings of the National Academy of Sciences, adds a new variable to this mix – pollen. It relies on data from 248 airborne pollen–monitoring sites in 31 countries. The study also took into account other effects, such as population density, temperature, humidity, and lockdown orders. The study authors found that, when pollen in an area spiked, so did infections, after an average lag of about 4 days. The study authors say pollen seemed to account for, on average, 44% of the infection rate variability between countries.
The study authors say pollen could be a culprit in respiratory infections, not because the viruses hitch a ride on pollen grains and travel into our mouth, eyes, and nose, but because pollen seems to perturb our immune defenses, even if a person isn’t allergic to it.
“When we inhale pollen, they end up on our nasal mucosa, and here they diminish the expression of genes that are important for the defense against airborne viruses,” study author Stefanie Gilles, PhD, chair of environmental medicine at the Technical University of Munich, said in a press conference.
In a study published last year, Dr. Gilles found that mice exposed to pollen made less interferon and other protective chemical signals to the immune system. Those then infected with respiratory syncytial virus had more virus in their bodies, compared with mice not exposed to pollen. She seemed to see the same effect in human volunteers.
The study authors think pollen may cause the body to drop its defenses against the airborne virus that causes COVID-19, too.
“If you’re in a crowded room, and other people are there that are asymptomatic, and you’ve just been breathing in pollen all day long, chances are that you’re going to be more susceptible to the virus,” says Lewis Ziska, PhD, a plant physiologist who studies pollen, climate change, and health at Columbia University’s Mailman School of Public Health in New York. “Having a mask is obviously really critical in that regard.”
Masks do a great job of blocking pollen, so wearing one is even more important when pollen and viruses are floating around, he says.
Other researchers, however, say that, while the study raises some interesting questions, it can’t prove that pollen is increasing COVID-19 infections.
“Just because two things happen at the same time doesn’t mean that one causes the other,” says Martijn Hoogeveen, PhD, a professor of technical sciences and environment at the Open University in the Netherlands.
Dr. Hoogeveen’s recent study, published in Science of the Total Environment, found that the arrival of pollen season in the Netherlands coincides with the end of flu season, and that COVID-19 infection peaks tend to follow a similar pattern – exactly the opposite of the PNAS study.
Another preprint study, which focused on the Chicago area, found the same thing – as pollen climbs, flu cases drop. The researchers behind that study think pollen may actually compete with viruses in our airways, helping to block them from infecting our cells.
Patterns may be hard to nail down
Why did these studies reach such different conclusions?
Dr. Hoogeveen’s paper focused on a single country and looked at the incidence of flu infections over four seasons, from 2016 to 2020, while the PNAS study collected data on pollen from January through the first week of April 2020.
He thinks that a single season, or really part of a season, may not be long enough to see meaningful patterns, especially considering that this new-to-humans virus was spreading quickly at nearly the same time. He says it will be interesting to follow what happens with COVID-19 infections and pollen in the coming months and years.
Dr. Hoogeveen says that in a large study spanning so many countries it would have been nearly impossible to account for differences in pandemic control strategies. Some countries embraced the use of masks, stay-at-home orders, and social distancing, for example, while others took less stringent measures in order to let the virus run its course in pursuit of herd immunity.
Limiting the study area to a single country or city, he says, helps researchers better understand all the variables that might have been in play along with pollen.
“There is no scientific consensus yet, about what it is driving, and that’s what makes it such an interesting field,” he says.
A version of this article first appeared on Medscape.com.
Some scientists say they’ve noticed a pattern to the recurring waves of SARS-CoV-2 infections around the globe: As pollen levels increased in outdoor air in 31 countries, COVID-19 cases accelerated.
Yet other recent studies point in the opposite direction, suggesting that peaks in pollen seasons coincide with a fall-off in the spread of some respiratory viruses, like COVID-19 and influenza. There’s even some evidence that pollen may compete with the virus that causes COVID-19 and may even help prevent infection.
So which is it? The answer may still be up in the air.
Doctors don’t fully understand what makes some viruses – like the ones that cause the flu – circulate in seasonal patterns.
There are, of course, many theories. These revolve around things like temperature and humidity – viruses tend to prefer colder, drier air – something that’s thought to help them spread more easily in the winter months. People are exposed to less sunlight during the winter, as they spend more time indoors, and the earth points away from the sun, providing some natural shielding. That may play a role because ultraviolet light from the sun acts like a natural disinfectant and may help keep circulating viral levels down.
In addition, exposure to sunlight helps the body make vitamin D, which may help keep our immune responses strong. Extreme temperatures – both cold and hot – also change our behavior, so that we spend more time cloistered indoors, where we can more easily cough and sneeze on each other and generally swap more germs.
Spike in pollen, jump in infections
The new study, published in the Proceedings of the National Academy of Sciences, adds a new variable to this mix – pollen. It relies on data from 248 airborne pollen–monitoring sites in 31 countries. The study also took into account other effects, such as population density, temperature, humidity, and lockdown orders. The study authors found that, when pollen in an area spiked, so did infections, after an average lag of about 4 days. The study authors say pollen seemed to account for, on average, 44% of the infection rate variability between countries.
The study authors say pollen could be a culprit in respiratory infections, not because the viruses hitch a ride on pollen grains and travel into our mouth, eyes, and nose, but because pollen seems to perturb our immune defenses, even if a person isn’t allergic to it.
“When we inhale pollen, they end up on our nasal mucosa, and here they diminish the expression of genes that are important for the defense against airborne viruses,” study author Stefanie Gilles, PhD, chair of environmental medicine at the Technical University of Munich, said in a press conference.
In a study published last year, Dr. Gilles found that mice exposed to pollen made less interferon and other protective chemical signals to the immune system. Those then infected with respiratory syncytial virus had more virus in their bodies, compared with mice not exposed to pollen. She seemed to see the same effect in human volunteers.
The study authors think pollen may cause the body to drop its defenses against the airborne virus that causes COVID-19, too.
“If you’re in a crowded room, and other people are there that are asymptomatic, and you’ve just been breathing in pollen all day long, chances are that you’re going to be more susceptible to the virus,” says Lewis Ziska, PhD, a plant physiologist who studies pollen, climate change, and health at Columbia University’s Mailman School of Public Health in New York. “Having a mask is obviously really critical in that regard.”
Masks do a great job of blocking pollen, so wearing one is even more important when pollen and viruses are floating around, he says.
Other researchers, however, say that, while the study raises some interesting questions, it can’t prove that pollen is increasing COVID-19 infections.
“Just because two things happen at the same time doesn’t mean that one causes the other,” says Martijn Hoogeveen, PhD, a professor of technical sciences and environment at the Open University in the Netherlands.
Dr. Hoogeveen’s recent study, published in Science of the Total Environment, found that the arrival of pollen season in the Netherlands coincides with the end of flu season, and that COVID-19 infection peaks tend to follow a similar pattern – exactly the opposite of the PNAS study.
Another preprint study, which focused on the Chicago area, found the same thing – as pollen climbs, flu cases drop. The researchers behind that study think pollen may actually compete with viruses in our airways, helping to block them from infecting our cells.
Patterns may be hard to nail down
Why did these studies reach such different conclusions?
Dr. Hoogeveen’s paper focused on a single country and looked at the incidence of flu infections over four seasons, from 2016 to 2020, while the PNAS study collected data on pollen from January through the first week of April 2020.
He thinks that a single season, or really part of a season, may not be long enough to see meaningful patterns, especially considering that this new-to-humans virus was spreading quickly at nearly the same time. He says it will be interesting to follow what happens with COVID-19 infections and pollen in the coming months and years.
Dr. Hoogeveen says that in a large study spanning so many countries it would have been nearly impossible to account for differences in pandemic control strategies. Some countries embraced the use of masks, stay-at-home orders, and social distancing, for example, while others took less stringent measures in order to let the virus run its course in pursuit of herd immunity.
Limiting the study area to a single country or city, he says, helps researchers better understand all the variables that might have been in play along with pollen.
“There is no scientific consensus yet, about what it is driving, and that’s what makes it such an interesting field,” he says.
A version of this article first appeared on Medscape.com.
Decline in child COVID-19 cases picks up after 2-week slowdown
, according to data gathered by the American Academy of Pediatrics and the Children’s Hospital Association.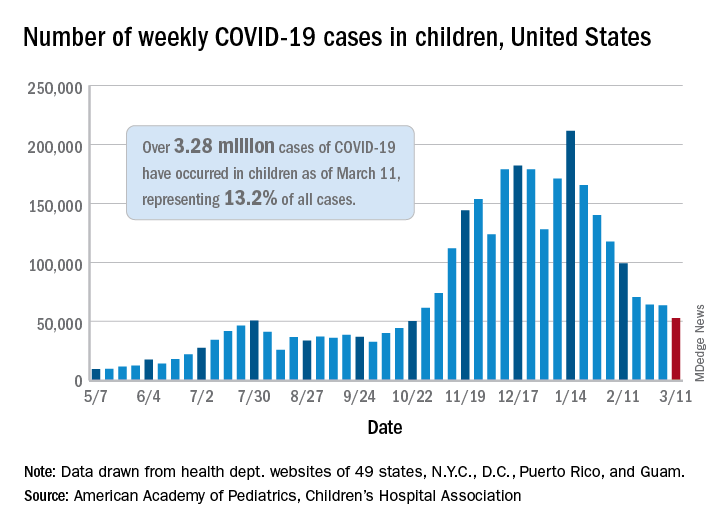
From Feb. 19 to March 4, the drop in new cases averaged just 5% each week, compared with 13.3% per week over the 5-week period from Jan. 15 to Feb. 18. For the week of March 5-11, a total of 52,695 COVID-19 cases were reported in children, down from 63,562 the previous week and the lowest number since late October, based on data from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
In those jurisdictions, 3.28 million children have been infected with SARS-CoV-2, representing 13.2% of all cases since the beginning of the pandemic. The cumulative rate of COVID-19 has now risen to 4,364 cases per 100,000 children nationally, with state rates ranging from 1,062 per 100,000 in Hawaii to 8,692 per 100,000 in North Dakota, the AAP and CHA said in their weekly COVID-19 report.
Hospitalization data are more limited – 24 states and New York City – but continue to show that serious illness is much less common in younger individuals: Children represent just 1.9% of all hospitalizations, and only 0.8% of the children who have been infected were hospitalized. Neither rate has changed since early February, the AAP and CHA said.
The number of deaths in children, however, rose from 253 to 266, the largest 1-week increase since early February in the 43 states (along with New York City, Puerto Rico, and Guam) that are tracking mortality data by age, the AAP and CHA reported.
Among those 46 jurisdictions, there are 10 (9 states and the District of Columbia) that have not yet reported a COVID-19–related child death, while Texas has almost twice as many deaths, 47, as the next state, Arizona, which has 24. Meanwhile, California’s total of 452,000 cases is almost 2½ times higher than the 183,000 recorded by Illinois, according to the report.
, according to data gathered by the American Academy of Pediatrics and the Children’s Hospital Association.
From Feb. 19 to March 4, the drop in new cases averaged just 5% each week, compared with 13.3% per week over the 5-week period from Jan. 15 to Feb. 18. For the week of March 5-11, a total of 52,695 COVID-19 cases were reported in children, down from 63,562 the previous week and the lowest number since late October, based on data from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
In those jurisdictions, 3.28 million children have been infected with SARS-CoV-2, representing 13.2% of all cases since the beginning of the pandemic. The cumulative rate of COVID-19 has now risen to 4,364 cases per 100,000 children nationally, with state rates ranging from 1,062 per 100,000 in Hawaii to 8,692 per 100,000 in North Dakota, the AAP and CHA said in their weekly COVID-19 report.
Hospitalization data are more limited – 24 states and New York City – but continue to show that serious illness is much less common in younger individuals: Children represent just 1.9% of all hospitalizations, and only 0.8% of the children who have been infected were hospitalized. Neither rate has changed since early February, the AAP and CHA said.
The number of deaths in children, however, rose from 253 to 266, the largest 1-week increase since early February in the 43 states (along with New York City, Puerto Rico, and Guam) that are tracking mortality data by age, the AAP and CHA reported.
Among those 46 jurisdictions, there are 10 (9 states and the District of Columbia) that have not yet reported a COVID-19–related child death, while Texas has almost twice as many deaths, 47, as the next state, Arizona, which has 24. Meanwhile, California’s total of 452,000 cases is almost 2½ times higher than the 183,000 recorded by Illinois, according to the report.
, according to data gathered by the American Academy of Pediatrics and the Children’s Hospital Association.
From Feb. 19 to March 4, the drop in new cases averaged just 5% each week, compared with 13.3% per week over the 5-week period from Jan. 15 to Feb. 18. For the week of March 5-11, a total of 52,695 COVID-19 cases were reported in children, down from 63,562 the previous week and the lowest number since late October, based on data from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
In those jurisdictions, 3.28 million children have been infected with SARS-CoV-2, representing 13.2% of all cases since the beginning of the pandemic. The cumulative rate of COVID-19 has now risen to 4,364 cases per 100,000 children nationally, with state rates ranging from 1,062 per 100,000 in Hawaii to 8,692 per 100,000 in North Dakota, the AAP and CHA said in their weekly COVID-19 report.
Hospitalization data are more limited – 24 states and New York City – but continue to show that serious illness is much less common in younger individuals: Children represent just 1.9% of all hospitalizations, and only 0.8% of the children who have been infected were hospitalized. Neither rate has changed since early February, the AAP and CHA said.
The number of deaths in children, however, rose from 253 to 266, the largest 1-week increase since early February in the 43 states (along with New York City, Puerto Rico, and Guam) that are tracking mortality data by age, the AAP and CHA reported.
Among those 46 jurisdictions, there are 10 (9 states and the District of Columbia) that have not yet reported a COVID-19–related child death, while Texas has almost twice as many deaths, 47, as the next state, Arizona, which has 24. Meanwhile, California’s total of 452,000 cases is almost 2½ times higher than the 183,000 recorded by Illinois, according to the report.
‘Major update’ of BP guidance for kidney disease; treat to 120 mm Hg
The new 2021 Kidney Disease: Improving Global Outcomes (KDIGO) clinical practice guideline for blood pressure management for adults with chronic kidney disease (CKD) who are not receiving dialysis advises treating to a target systolic blood pressure of less than 120 mm Hg, provided measurements are “standardized” and that blood pressure is “measured properly.”
This blood pressure target – largely based on evidence from the Systolic Blood Pressure Intervention Trial (SPRINT) – represents “a major update” from the 2012 KDIGO guideline, which advised clinicians to treat to a target blood pressure of less than or equal to 130/80 mm Hg for patients with albuminuria or less than or equal to 140/90 mm Hg for patients without albuminuria.
The new goal is also lower than the less than 130/80 mm Hg target in the 2017 American College of Cardiology/American Heart Association guideline.
In a study of the public health implications of the guideline, Kathryn Foti, PhD, and colleagues determined that 70% of U.S. adults with CKD would now be eligible for treatment to lower blood pressure, as opposed to 50% under the previous KDIGO guideline and 56% under the ACC/AHA guideline.
“This is a major update of an influential set of guidelines for chronic kidney disease patients” at a time when blood pressure control is worsening in the United States, Dr. Foti, a postdoctoral researcher in the department of epidemiology at Johns Hopkins Bloomberg School of Public Health, Baltimore, said in a statement from her institution.
The 2021 KDIGO blood pressure guideline and executive summary and the public health implications study are published online in Kidney International.
First, ‘take blood pressure well’
The cochair of the new KDIGO guidelines, Alfred K. Cheung, MD, from the University of Utah, Salt Lake City, said in an interview that the guideline has “two important points.”
First, “take that blood pressure well,” he said. “That has a lot to do with patient preparation rather than any fancy instrument,” he emphasized.
Second, the guideline proposes a systolic blood pressure target of less than 120 mm Hg for most people with CKD not receiving dialysis, except for children and kidney transplant recipients. This target is “contingent on ‘standardized’ blood pressure measurement.”
The document provides a checklist for obtaining a standardized blood pressure measurement, adapted from the 2017 ACC/AHA blood pressure guidelines. It starts with the patient relaxed and sitting on a chair for more than 5 minutes.
In contrast to this measurement, a “routine” or “casual” office blood pressure measurement could be off by plus or minus 10 mm Hg, Dr. Cheung noted.
In a typical scenario, he continued, a patient cannot find a place to park, rushes into the clinic, and has his or her blood pressure checked right away, which would provide a “totally unreliable” reading. Adding a “fudge factor” (correction factor) would not provide an accurate reading.
Clinicians “would not settle for a potassium measurement that is 5.0 mmol/L plus or minus a few decimal points” to guide treatment, he pointed out.
Second, target 120, properly measured
“The very first chapter of the guidelines is devoted to blood pressure measurement, because we recognize if we’re going to do 120 [mm Hg] – the emphasis is on 120 measured properly – so we try to drive that point home,” Tara I. Chang, MD, guideline second author and a coauthor of the public health implications study, pointed out in an interview.
“There are a lot of other things that we base clinical decisions on where we really require some degree of precision, and blood pressure is important enough that to us it’s kind of in the same boat,” said Dr. Chang, from Stanford (Calif.) University.
“In SPRINT, people were randomized to less than less than 120 vs. less than 140 (they weren’t randomized to <130),” she noted.
“The recommendation should be widely adopted in clinical practice,” the guideline authors write, “since accurate measurements will ensure that proper guidance is being applied to the management of BP, as it is to the management of other risk factors.”
Still need individual treatment
Nevertheless, patients still need individualized treatment, the document stresses. “Not every patient with CKD will be appropriate to target to less than 120,” Dr. Chang said. However, “we want people to at least consider less than 120,” she added, to avoid therapeutic inertia.
“If you take the blood pressure in a standardized manner – such as in the ACCORD trial and in the SPRINT trial – even patients over 75 years old, or people over 80 years old, they have very little side effects,” Dr. Cheung noted.
“In the overall cohort,” he continued, “they do not have a significant increase in serious adverse events, do not have adverse events of postural hypotension, syncope, bradycardia, injurious falls – so people are worried about it, but it’s not borne out by the data.
“That said, I have two cautions,” Dr. Cheung noted. “One. If you drop somebody’s blood pressure rapidly over a week, you may be more likely to get in trouble. If you drop the blood pressure gradually over several weeks, several months, you’re much less likely to get into trouble.”
“Two. If the patient is old, you know the patient has carotid stenosis and already has postural dizziness, you may not want to try on that patient – but just because the patient is old is not the reason not to target 120.”
ACE inhibitors and ARBs beneficial in albuminuria, underused
“How do you get to less than 120? The short answer is, use whatever medications you need to – there is no necessarily right cocktail,” Dr. Chang said.
“We’ve known that angiotensin-converting enzyme (ACE) inhibitors and ARBs [angiotensin II receptor blockers] are beneficial in patients with CKD and in particular those with heavier albuminuria,” she continued. “We’ve known this for over 20 years.”
Yet, the study identified underutilization – “a persistent gap, just like blood pressure control and awareness,” she noted. “We’re just not making much headway.
“We are not recommending ACE inhibitors or ARBs for all the patients,” Dr. Cheung clarified. “If you are diabetic and have heavy proteinuria, that’s when the use of ACE inhibitors and ARBs are most indicated.”
Public health implications
SPRINT showed that treating to a systolic blood pressure of less than 120 mm Hg vs. less than 140 mm Hg reduced the risk for cardiovascular disease by 25% and all-cause mortality by 27% for participants with and those without CKD, Dr. Foti and colleagues stress.
They aimed to estimate how the new guideline would affect (1) the number of U.S. patients with CKD who would be eligible for blood pressure lowering treatment, and (2) the proportion of those with albuminuria who would be eligible for an ACE inhibitor or an ARB.
The researchers analyzed data from 1,699 adults with CKD (estimated glomerular filtration rate, 15-59 mL/min/1.73 m2 or a urinary albumin-to-creatinine ratio of ≥30 mg/g) who participated in the 2015-2018 National Health and Nutrition Examination Survey.
Both the 2021 and 2012 KDIGO guidelines recommend that patients with albuminuria and blood pressure higher than the target value who are not kidney transplant recipients should be treated with an ACE inhibitor or an ARB.
On the basis of the new target, 78% of patients with CKD and albuminuria were eligible for ACE inhibitor/ARB treatment by the 2021 KDIGO guideline, compared with 71% by the 2012 KDIGO guideline. However, only 39% were taking one of these drugs.
These findings show that “with the new guideline and with the lower blood pressure target, you potentially have an even larger pool of people who have blood pressure that’s not under control, and a potential larger group of people who may benefit from ACE inhibitors and ARBs,” Dr. Chang said.
“Our paper is not the only one to show that we haven’t made a whole lot of progress,” she said, “and now that the bar has been lowered, there [have] to be some renewed efforts on controlling blood pressure, because we know that blood pressure control is such an important risk factor for cardiovascular outcomes.”
Dr. Foti is supported by an NIH/National Heart, Lung, and Blood Institute grant. Dr. Cheung has received consultancy fees from Amgen, Bard, Boehringer Ingelheim, Calliditas, Tricida, and UpToDate, and grant/research support from the National Institutes of Health for SPRINT (monies paid to institution). Dr. Chang has received consultancy fees from Bayer, Gilead, Janssen Research and Development, Novo Nordisk, Tricida, and Vascular Dynamics; grant/research support from AstraZeneca and Satellite Healthcare (monies paid to institution), the NIH, and the American Heart Association; is on advisory boards for AstraZeneca and Fresenius Medical Care Renal Therapies Group; and has received workshop honoraria from Fresenius. Disclosures of relevant financial relationships of the other authors are listed in the original articles.
A version of this article first appeared on Medscape.com.
The new 2021 Kidney Disease: Improving Global Outcomes (KDIGO) clinical practice guideline for blood pressure management for adults with chronic kidney disease (CKD) who are not receiving dialysis advises treating to a target systolic blood pressure of less than 120 mm Hg, provided measurements are “standardized” and that blood pressure is “measured properly.”
This blood pressure target – largely based on evidence from the Systolic Blood Pressure Intervention Trial (SPRINT) – represents “a major update” from the 2012 KDIGO guideline, which advised clinicians to treat to a target blood pressure of less than or equal to 130/80 mm Hg for patients with albuminuria or less than or equal to 140/90 mm Hg for patients without albuminuria.
The new goal is also lower than the less than 130/80 mm Hg target in the 2017 American College of Cardiology/American Heart Association guideline.
In a study of the public health implications of the guideline, Kathryn Foti, PhD, and colleagues determined that 70% of U.S. adults with CKD would now be eligible for treatment to lower blood pressure, as opposed to 50% under the previous KDIGO guideline and 56% under the ACC/AHA guideline.
“This is a major update of an influential set of guidelines for chronic kidney disease patients” at a time when blood pressure control is worsening in the United States, Dr. Foti, a postdoctoral researcher in the department of epidemiology at Johns Hopkins Bloomberg School of Public Health, Baltimore, said in a statement from her institution.
The 2021 KDIGO blood pressure guideline and executive summary and the public health implications study are published online in Kidney International.
First, ‘take blood pressure well’
The cochair of the new KDIGO guidelines, Alfred K. Cheung, MD, from the University of Utah, Salt Lake City, said in an interview that the guideline has “two important points.”
First, “take that blood pressure well,” he said. “That has a lot to do with patient preparation rather than any fancy instrument,” he emphasized.
Second, the guideline proposes a systolic blood pressure target of less than 120 mm Hg for most people with CKD not receiving dialysis, except for children and kidney transplant recipients. This target is “contingent on ‘standardized’ blood pressure measurement.”
The document provides a checklist for obtaining a standardized blood pressure measurement, adapted from the 2017 ACC/AHA blood pressure guidelines. It starts with the patient relaxed and sitting on a chair for more than 5 minutes.
In contrast to this measurement, a “routine” or “casual” office blood pressure measurement could be off by plus or minus 10 mm Hg, Dr. Cheung noted.
In a typical scenario, he continued, a patient cannot find a place to park, rushes into the clinic, and has his or her blood pressure checked right away, which would provide a “totally unreliable” reading. Adding a “fudge factor” (correction factor) would not provide an accurate reading.
Clinicians “would not settle for a potassium measurement that is 5.0 mmol/L plus or minus a few decimal points” to guide treatment, he pointed out.
Second, target 120, properly measured
“The very first chapter of the guidelines is devoted to blood pressure measurement, because we recognize if we’re going to do 120 [mm Hg] – the emphasis is on 120 measured properly – so we try to drive that point home,” Tara I. Chang, MD, guideline second author and a coauthor of the public health implications study, pointed out in an interview.
“There are a lot of other things that we base clinical decisions on where we really require some degree of precision, and blood pressure is important enough that to us it’s kind of in the same boat,” said Dr. Chang, from Stanford (Calif.) University.
“In SPRINT, people were randomized to less than less than 120 vs. less than 140 (they weren’t randomized to <130),” she noted.
“The recommendation should be widely adopted in clinical practice,” the guideline authors write, “since accurate measurements will ensure that proper guidance is being applied to the management of BP, as it is to the management of other risk factors.”
Still need individual treatment
Nevertheless, patients still need individualized treatment, the document stresses. “Not every patient with CKD will be appropriate to target to less than 120,” Dr. Chang said. However, “we want people to at least consider less than 120,” she added, to avoid therapeutic inertia.
“If you take the blood pressure in a standardized manner – such as in the ACCORD trial and in the SPRINT trial – even patients over 75 years old, or people over 80 years old, they have very little side effects,” Dr. Cheung noted.
“In the overall cohort,” he continued, “they do not have a significant increase in serious adverse events, do not have adverse events of postural hypotension, syncope, bradycardia, injurious falls – so people are worried about it, but it’s not borne out by the data.
“That said, I have two cautions,” Dr. Cheung noted. “One. If you drop somebody’s blood pressure rapidly over a week, you may be more likely to get in trouble. If you drop the blood pressure gradually over several weeks, several months, you’re much less likely to get into trouble.”
“Two. If the patient is old, you know the patient has carotid stenosis and already has postural dizziness, you may not want to try on that patient – but just because the patient is old is not the reason not to target 120.”
ACE inhibitors and ARBs beneficial in albuminuria, underused
“How do you get to less than 120? The short answer is, use whatever medications you need to – there is no necessarily right cocktail,” Dr. Chang said.
“We’ve known that angiotensin-converting enzyme (ACE) inhibitors and ARBs [angiotensin II receptor blockers] are beneficial in patients with CKD and in particular those with heavier albuminuria,” she continued. “We’ve known this for over 20 years.”
Yet, the study identified underutilization – “a persistent gap, just like blood pressure control and awareness,” she noted. “We’re just not making much headway.
“We are not recommending ACE inhibitors or ARBs for all the patients,” Dr. Cheung clarified. “If you are diabetic and have heavy proteinuria, that’s when the use of ACE inhibitors and ARBs are most indicated.”
Public health implications
SPRINT showed that treating to a systolic blood pressure of less than 120 mm Hg vs. less than 140 mm Hg reduced the risk for cardiovascular disease by 25% and all-cause mortality by 27% for participants with and those without CKD, Dr. Foti and colleagues stress.
They aimed to estimate how the new guideline would affect (1) the number of U.S. patients with CKD who would be eligible for blood pressure lowering treatment, and (2) the proportion of those with albuminuria who would be eligible for an ACE inhibitor or an ARB.
The researchers analyzed data from 1,699 adults with CKD (estimated glomerular filtration rate, 15-59 mL/min/1.73 m2 or a urinary albumin-to-creatinine ratio of ≥30 mg/g) who participated in the 2015-2018 National Health and Nutrition Examination Survey.
Both the 2021 and 2012 KDIGO guidelines recommend that patients with albuminuria and blood pressure higher than the target value who are not kidney transplant recipients should be treated with an ACE inhibitor or an ARB.
On the basis of the new target, 78% of patients with CKD and albuminuria were eligible for ACE inhibitor/ARB treatment by the 2021 KDIGO guideline, compared with 71% by the 2012 KDIGO guideline. However, only 39% were taking one of these drugs.
These findings show that “with the new guideline and with the lower blood pressure target, you potentially have an even larger pool of people who have blood pressure that’s not under control, and a potential larger group of people who may benefit from ACE inhibitors and ARBs,” Dr. Chang said.
“Our paper is not the only one to show that we haven’t made a whole lot of progress,” she said, “and now that the bar has been lowered, there [have] to be some renewed efforts on controlling blood pressure, because we know that blood pressure control is such an important risk factor for cardiovascular outcomes.”
Dr. Foti is supported by an NIH/National Heart, Lung, and Blood Institute grant. Dr. Cheung has received consultancy fees from Amgen, Bard, Boehringer Ingelheim, Calliditas, Tricida, and UpToDate, and grant/research support from the National Institutes of Health for SPRINT (monies paid to institution). Dr. Chang has received consultancy fees from Bayer, Gilead, Janssen Research and Development, Novo Nordisk, Tricida, and Vascular Dynamics; grant/research support from AstraZeneca and Satellite Healthcare (monies paid to institution), the NIH, and the American Heart Association; is on advisory boards for AstraZeneca and Fresenius Medical Care Renal Therapies Group; and has received workshop honoraria from Fresenius. Disclosures of relevant financial relationships of the other authors are listed in the original articles.
A version of this article first appeared on Medscape.com.
The new 2021 Kidney Disease: Improving Global Outcomes (KDIGO) clinical practice guideline for blood pressure management for adults with chronic kidney disease (CKD) who are not receiving dialysis advises treating to a target systolic blood pressure of less than 120 mm Hg, provided measurements are “standardized” and that blood pressure is “measured properly.”
This blood pressure target – largely based on evidence from the Systolic Blood Pressure Intervention Trial (SPRINT) – represents “a major update” from the 2012 KDIGO guideline, which advised clinicians to treat to a target blood pressure of less than or equal to 130/80 mm Hg for patients with albuminuria or less than or equal to 140/90 mm Hg for patients without albuminuria.
The new goal is also lower than the less than 130/80 mm Hg target in the 2017 American College of Cardiology/American Heart Association guideline.
In a study of the public health implications of the guideline, Kathryn Foti, PhD, and colleagues determined that 70% of U.S. adults with CKD would now be eligible for treatment to lower blood pressure, as opposed to 50% under the previous KDIGO guideline and 56% under the ACC/AHA guideline.
“This is a major update of an influential set of guidelines for chronic kidney disease patients” at a time when blood pressure control is worsening in the United States, Dr. Foti, a postdoctoral researcher in the department of epidemiology at Johns Hopkins Bloomberg School of Public Health, Baltimore, said in a statement from her institution.
The 2021 KDIGO blood pressure guideline and executive summary and the public health implications study are published online in Kidney International.
First, ‘take blood pressure well’
The cochair of the new KDIGO guidelines, Alfred K. Cheung, MD, from the University of Utah, Salt Lake City, said in an interview that the guideline has “two important points.”
First, “take that blood pressure well,” he said. “That has a lot to do with patient preparation rather than any fancy instrument,” he emphasized.
Second, the guideline proposes a systolic blood pressure target of less than 120 mm Hg for most people with CKD not receiving dialysis, except for children and kidney transplant recipients. This target is “contingent on ‘standardized’ blood pressure measurement.”
The document provides a checklist for obtaining a standardized blood pressure measurement, adapted from the 2017 ACC/AHA blood pressure guidelines. It starts with the patient relaxed and sitting on a chair for more than 5 minutes.
In contrast to this measurement, a “routine” or “casual” office blood pressure measurement could be off by plus or minus 10 mm Hg, Dr. Cheung noted.
In a typical scenario, he continued, a patient cannot find a place to park, rushes into the clinic, and has his or her blood pressure checked right away, which would provide a “totally unreliable” reading. Adding a “fudge factor” (correction factor) would not provide an accurate reading.
Clinicians “would not settle for a potassium measurement that is 5.0 mmol/L plus or minus a few decimal points” to guide treatment, he pointed out.
Second, target 120, properly measured
“The very first chapter of the guidelines is devoted to blood pressure measurement, because we recognize if we’re going to do 120 [mm Hg] – the emphasis is on 120 measured properly – so we try to drive that point home,” Tara I. Chang, MD, guideline second author and a coauthor of the public health implications study, pointed out in an interview.
“There are a lot of other things that we base clinical decisions on where we really require some degree of precision, and blood pressure is important enough that to us it’s kind of in the same boat,” said Dr. Chang, from Stanford (Calif.) University.
“In SPRINT, people were randomized to less than less than 120 vs. less than 140 (they weren’t randomized to <130),” she noted.
“The recommendation should be widely adopted in clinical practice,” the guideline authors write, “since accurate measurements will ensure that proper guidance is being applied to the management of BP, as it is to the management of other risk factors.”
Still need individual treatment
Nevertheless, patients still need individualized treatment, the document stresses. “Not every patient with CKD will be appropriate to target to less than 120,” Dr. Chang said. However, “we want people to at least consider less than 120,” she added, to avoid therapeutic inertia.
“If you take the blood pressure in a standardized manner – such as in the ACCORD trial and in the SPRINT trial – even patients over 75 years old, or people over 80 years old, they have very little side effects,” Dr. Cheung noted.
“In the overall cohort,” he continued, “they do not have a significant increase in serious adverse events, do not have adverse events of postural hypotension, syncope, bradycardia, injurious falls – so people are worried about it, but it’s not borne out by the data.
“That said, I have two cautions,” Dr. Cheung noted. “One. If you drop somebody’s blood pressure rapidly over a week, you may be more likely to get in trouble. If you drop the blood pressure gradually over several weeks, several months, you’re much less likely to get into trouble.”
“Two. If the patient is old, you know the patient has carotid stenosis and already has postural dizziness, you may not want to try on that patient – but just because the patient is old is not the reason not to target 120.”
ACE inhibitors and ARBs beneficial in albuminuria, underused
“How do you get to less than 120? The short answer is, use whatever medications you need to – there is no necessarily right cocktail,” Dr. Chang said.
“We’ve known that angiotensin-converting enzyme (ACE) inhibitors and ARBs [angiotensin II receptor blockers] are beneficial in patients with CKD and in particular those with heavier albuminuria,” she continued. “We’ve known this for over 20 years.”
Yet, the study identified underutilization – “a persistent gap, just like blood pressure control and awareness,” she noted. “We’re just not making much headway.
“We are not recommending ACE inhibitors or ARBs for all the patients,” Dr. Cheung clarified. “If you are diabetic and have heavy proteinuria, that’s when the use of ACE inhibitors and ARBs are most indicated.”
Public health implications
SPRINT showed that treating to a systolic blood pressure of less than 120 mm Hg vs. less than 140 mm Hg reduced the risk for cardiovascular disease by 25% and all-cause mortality by 27% for participants with and those without CKD, Dr. Foti and colleagues stress.
They aimed to estimate how the new guideline would affect (1) the number of U.S. patients with CKD who would be eligible for blood pressure lowering treatment, and (2) the proportion of those with albuminuria who would be eligible for an ACE inhibitor or an ARB.
The researchers analyzed data from 1,699 adults with CKD (estimated glomerular filtration rate, 15-59 mL/min/1.73 m2 or a urinary albumin-to-creatinine ratio of ≥30 mg/g) who participated in the 2015-2018 National Health and Nutrition Examination Survey.
Both the 2021 and 2012 KDIGO guidelines recommend that patients with albuminuria and blood pressure higher than the target value who are not kidney transplant recipients should be treated with an ACE inhibitor or an ARB.
On the basis of the new target, 78% of patients with CKD and albuminuria were eligible for ACE inhibitor/ARB treatment by the 2021 KDIGO guideline, compared with 71% by the 2012 KDIGO guideline. However, only 39% were taking one of these drugs.
These findings show that “with the new guideline and with the lower blood pressure target, you potentially have an even larger pool of people who have blood pressure that’s not under control, and a potential larger group of people who may benefit from ACE inhibitors and ARBs,” Dr. Chang said.
“Our paper is not the only one to show that we haven’t made a whole lot of progress,” she said, “and now that the bar has been lowered, there [have] to be some renewed efforts on controlling blood pressure, because we know that blood pressure control is such an important risk factor for cardiovascular outcomes.”
Dr. Foti is supported by an NIH/National Heart, Lung, and Blood Institute grant. Dr. Cheung has received consultancy fees from Amgen, Bard, Boehringer Ingelheim, Calliditas, Tricida, and UpToDate, and grant/research support from the National Institutes of Health for SPRINT (monies paid to institution). Dr. Chang has received consultancy fees from Bayer, Gilead, Janssen Research and Development, Novo Nordisk, Tricida, and Vascular Dynamics; grant/research support from AstraZeneca and Satellite Healthcare (monies paid to institution), the NIH, and the American Heart Association; is on advisory boards for AstraZeneca and Fresenius Medical Care Renal Therapies Group; and has received workshop honoraria from Fresenius. Disclosures of relevant financial relationships of the other authors are listed in the original articles.
A version of this article first appeared on Medscape.com.
Cannabinoids may pose death risk for older patients with COPD
, compared with nonusers, findings from a large study have shown.
Synthetic cannabinoids drugs, such as nabilone and dronabinol, have been approved by the Food and Drug Administration for nausea and vomiting caused by chemotherapy. But their off-label use by adults with COPD to help manage chronic musculoskeletal pain, insomnia, and refractory dyspnea is on the rise, wrote Nicholas T. Vozoris, MD, of the University of Toronto and colleagues.
Cannabinoids may actually contribute to negative respiratory outcomes among individuals with COPD through several possible mechanisms including causing sedation, inducing anxiety, and provoking respiratory muscle weakness, they said.
“Possible adverse respiratory effects of cannabinoids may occur with greater likelihood among older adults (in whom COPD is more prevalent), as this group is known to less efficiently metabolise drugs,” they noted.
In a retrospective, population-based cohort study published in Thorax the researchers identified 185,876 adults aged 66 years and older with COPD using health administrative database information from 2006 to 2016. New cannabinoid users (those starting nabilone or dronabinol) were matched with control nonusers (defined as new users of noncannabinoid drugs). Individuals receiving palliative care, or having a diagnosis of cancer or HIV, were excluded because these are settings where synthetic cannabinoids may be prescribed for nausea or vomiting, and these patients are more likely to be in a poorer state of health.
Overall, new cannabinoid users had significantly higher all-cause mortality rates, compared with nonusers (hazard ratio, 1.64). The effects was greater in high-dose users.
Daniel R. Ouellette, MD, associate professor of medicine at Wayne State University and a senior staff physician at Henry Ford Hospital, both in Detroit, commented that this study has value for clinicians. “Many states are liberalizing cannabinoid use, and it is important to know the health effects of this type of drug on patients with chronic respiratory disease,” he noted. “The study is somewhat surprising. While one might have expected adverse consequences in patients with COPD who inhaled smoke from cannabinoids, it is somewhat unexpected that oral use would be associated with adverse consequences.” He added, “Pain in older adults is a complex problem. Cannabinoids are often recommended for pain in the general community, but pain per se is not a primary symptom for most patients with COPD from their respiratory problems. Physicians treating patients with COPD should diagnose the cause of the pain and provide appropriate treatment.”
Dose makes a difference
All-cause mortality increased by 231% and hospitalization for COPD or pneumonia increased by 178% among new users of higher-dose cannabinoids, compared with nonusers. Higher dose was defined in this study as more than 1.5mg/day of nabilone. No significant differences appeared in new users vs. nonusers in hospitalization for COPD or pneumonia at lower doses, and no significant differences appeared overall in outpatient respiratory exacerbations, emergency department visits for COPD or pneumonia, or COPD- or pneumonia-related mortality.
Potential limitations and implications
“The fact that COPD- or pneumonia-related mortality was not observed to occur with significantly greater rates among cannabinoid users with COPD may suggest that the increased all-cause mortality finding was not being driven by adverse respiratory-related drug effects, as we hypothesized, and instead was possibly a result of unresolved confounding,” the researchers noted.
The study findings were limited by several factors including the inability to prove causation in an observational study, and the potential for confounding based on unmeasured differences between cannabinoid users and nonusers, the researchers said. “Our findings may not be generalizable to all individuals with COPD, as our study included only those aged 66 years and older, and our COPD identification algorithm, while highly specific, had modest sensitivity,” they added. However, the results were strengthened by the large study population and suggest that cannabinoids are not contraindicated for older adults with COPD, the researchers said. “There can be legitimate reasons for using cannabinoids in this population, such as to help treat chemotherapy-related nausea and vomiting, and possibly for end-of-life care,” they emphasized.
The study findings serve to inform clinicians of the significantly increased mortality risk when older adults with COPD initiate cannabinoids, and “this information should be discussed with patients and incorporated in prescribing decision-making and management plans,” along with consideration of using lower doses when possible to minimize adverse events, they concluded.
The study was supported by The Lung Association – Ontario Grant Review/Grant-In-Aid. The researchers had no financial conflicts to disclose.
, compared with nonusers, findings from a large study have shown.
Synthetic cannabinoids drugs, such as nabilone and dronabinol, have been approved by the Food and Drug Administration for nausea and vomiting caused by chemotherapy. But their off-label use by adults with COPD to help manage chronic musculoskeletal pain, insomnia, and refractory dyspnea is on the rise, wrote Nicholas T. Vozoris, MD, of the University of Toronto and colleagues.
Cannabinoids may actually contribute to negative respiratory outcomes among individuals with COPD through several possible mechanisms including causing sedation, inducing anxiety, and provoking respiratory muscle weakness, they said.
“Possible adverse respiratory effects of cannabinoids may occur with greater likelihood among older adults (in whom COPD is more prevalent), as this group is known to less efficiently metabolise drugs,” they noted.
In a retrospective, population-based cohort study published in Thorax the researchers identified 185,876 adults aged 66 years and older with COPD using health administrative database information from 2006 to 2016. New cannabinoid users (those starting nabilone or dronabinol) were matched with control nonusers (defined as new users of noncannabinoid drugs). Individuals receiving palliative care, or having a diagnosis of cancer or HIV, were excluded because these are settings where synthetic cannabinoids may be prescribed for nausea or vomiting, and these patients are more likely to be in a poorer state of health.
Overall, new cannabinoid users had significantly higher all-cause mortality rates, compared with nonusers (hazard ratio, 1.64). The effects was greater in high-dose users.
Daniel R. Ouellette, MD, associate professor of medicine at Wayne State University and a senior staff physician at Henry Ford Hospital, both in Detroit, commented that this study has value for clinicians. “Many states are liberalizing cannabinoid use, and it is important to know the health effects of this type of drug on patients with chronic respiratory disease,” he noted. “The study is somewhat surprising. While one might have expected adverse consequences in patients with COPD who inhaled smoke from cannabinoids, it is somewhat unexpected that oral use would be associated with adverse consequences.” He added, “Pain in older adults is a complex problem. Cannabinoids are often recommended for pain in the general community, but pain per se is not a primary symptom for most patients with COPD from their respiratory problems. Physicians treating patients with COPD should diagnose the cause of the pain and provide appropriate treatment.”
Dose makes a difference
All-cause mortality increased by 231% and hospitalization for COPD or pneumonia increased by 178% among new users of higher-dose cannabinoids, compared with nonusers. Higher dose was defined in this study as more than 1.5mg/day of nabilone. No significant differences appeared in new users vs. nonusers in hospitalization for COPD or pneumonia at lower doses, and no significant differences appeared overall in outpatient respiratory exacerbations, emergency department visits for COPD or pneumonia, or COPD- or pneumonia-related mortality.
Potential limitations and implications
“The fact that COPD- or pneumonia-related mortality was not observed to occur with significantly greater rates among cannabinoid users with COPD may suggest that the increased all-cause mortality finding was not being driven by adverse respiratory-related drug effects, as we hypothesized, and instead was possibly a result of unresolved confounding,” the researchers noted.
The study findings were limited by several factors including the inability to prove causation in an observational study, and the potential for confounding based on unmeasured differences between cannabinoid users and nonusers, the researchers said. “Our findings may not be generalizable to all individuals with COPD, as our study included only those aged 66 years and older, and our COPD identification algorithm, while highly specific, had modest sensitivity,” they added. However, the results were strengthened by the large study population and suggest that cannabinoids are not contraindicated for older adults with COPD, the researchers said. “There can be legitimate reasons for using cannabinoids in this population, such as to help treat chemotherapy-related nausea and vomiting, and possibly for end-of-life care,” they emphasized.
The study findings serve to inform clinicians of the significantly increased mortality risk when older adults with COPD initiate cannabinoids, and “this information should be discussed with patients and incorporated in prescribing decision-making and management plans,” along with consideration of using lower doses when possible to minimize adverse events, they concluded.
The study was supported by The Lung Association – Ontario Grant Review/Grant-In-Aid. The researchers had no financial conflicts to disclose.
, compared with nonusers, findings from a large study have shown.
Synthetic cannabinoids drugs, such as nabilone and dronabinol, have been approved by the Food and Drug Administration for nausea and vomiting caused by chemotherapy. But their off-label use by adults with COPD to help manage chronic musculoskeletal pain, insomnia, and refractory dyspnea is on the rise, wrote Nicholas T. Vozoris, MD, of the University of Toronto and colleagues.
Cannabinoids may actually contribute to negative respiratory outcomes among individuals with COPD through several possible mechanisms including causing sedation, inducing anxiety, and provoking respiratory muscle weakness, they said.
“Possible adverse respiratory effects of cannabinoids may occur with greater likelihood among older adults (in whom COPD is more prevalent), as this group is known to less efficiently metabolise drugs,” they noted.
In a retrospective, population-based cohort study published in Thorax the researchers identified 185,876 adults aged 66 years and older with COPD using health administrative database information from 2006 to 2016. New cannabinoid users (those starting nabilone or dronabinol) were matched with control nonusers (defined as new users of noncannabinoid drugs). Individuals receiving palliative care, or having a diagnosis of cancer or HIV, were excluded because these are settings where synthetic cannabinoids may be prescribed for nausea or vomiting, and these patients are more likely to be in a poorer state of health.
Overall, new cannabinoid users had significantly higher all-cause mortality rates, compared with nonusers (hazard ratio, 1.64). The effects was greater in high-dose users.
Daniel R. Ouellette, MD, associate professor of medicine at Wayne State University and a senior staff physician at Henry Ford Hospital, both in Detroit, commented that this study has value for clinicians. “Many states are liberalizing cannabinoid use, and it is important to know the health effects of this type of drug on patients with chronic respiratory disease,” he noted. “The study is somewhat surprising. While one might have expected adverse consequences in patients with COPD who inhaled smoke from cannabinoids, it is somewhat unexpected that oral use would be associated with adverse consequences.” He added, “Pain in older adults is a complex problem. Cannabinoids are often recommended for pain in the general community, but pain per se is not a primary symptom for most patients with COPD from their respiratory problems. Physicians treating patients with COPD should diagnose the cause of the pain and provide appropriate treatment.”
Dose makes a difference
All-cause mortality increased by 231% and hospitalization for COPD or pneumonia increased by 178% among new users of higher-dose cannabinoids, compared with nonusers. Higher dose was defined in this study as more than 1.5mg/day of nabilone. No significant differences appeared in new users vs. nonusers in hospitalization for COPD or pneumonia at lower doses, and no significant differences appeared overall in outpatient respiratory exacerbations, emergency department visits for COPD or pneumonia, or COPD- or pneumonia-related mortality.
Potential limitations and implications
“The fact that COPD- or pneumonia-related mortality was not observed to occur with significantly greater rates among cannabinoid users with COPD may suggest that the increased all-cause mortality finding was not being driven by adverse respiratory-related drug effects, as we hypothesized, and instead was possibly a result of unresolved confounding,” the researchers noted.
The study findings were limited by several factors including the inability to prove causation in an observational study, and the potential for confounding based on unmeasured differences between cannabinoid users and nonusers, the researchers said. “Our findings may not be generalizable to all individuals with COPD, as our study included only those aged 66 years and older, and our COPD identification algorithm, while highly specific, had modest sensitivity,” they added. However, the results were strengthened by the large study population and suggest that cannabinoids are not contraindicated for older adults with COPD, the researchers said. “There can be legitimate reasons for using cannabinoids in this population, such as to help treat chemotherapy-related nausea and vomiting, and possibly for end-of-life care,” they emphasized.
The study findings serve to inform clinicians of the significantly increased mortality risk when older adults with COPD initiate cannabinoids, and “this information should be discussed with patients and incorporated in prescribing decision-making and management plans,” along with consideration of using lower doses when possible to minimize adverse events, they concluded.
The study was supported by The Lung Association – Ontario Grant Review/Grant-In-Aid. The researchers had no financial conflicts to disclose.
FROM THORAX
Expert recommendations for targeted therapies in advanced NSCLC
The guidelines, jointly released by the American Society of Clinical Oncology (ASCO) and Ontario Health (OH), were published in the Journal of Clinical Oncology. The recommendations are based on results from 54 studies published or presented from Dec. 2015 to May 2020.
The new guidelines supplant ASCO’s 2017 guidelines on stage IV NSCLC. Several driver mutations were touched upon in the 2017 document, but their corresponding targeted therapies were not recommended as first-line treatment.
With substantial progress in targeted therapies since 2017, treatment decision-making in 2021 focuses on the molecular signatures of tumors and PD-L1 score, according to the authors of the current guidelines, Nasser Hanna, MD, of Indiana University, Indianapolis, and colleagues.
“All patients with nonsquamous NSCLC should have the results of testing for potentially targetable mutations (alterations) before implementing therapy for advanced lung cancer, regardless of smoking status recommendations,” the authors wrote.
They noted that about a third of patients with NSCLC have known targetable genetic alterations. The Food and Drug Administration has approved therapeutics targeting seven alterations: EGFR and ALK alterations, ROS-1 fusions, BRAF V600e mutations, RET fusions, MET exon 14 skipping mutations, and NTRK fusions.
EGFR-mutant NSCLC
The authors’ recommendation for osimertinib as first-line therapy applies to patients who have EGFR-activating mutations in exon 19 (deletion), exon 21 L858R, or exon 20 T790M.
The authors also said osimertinib is an option for patients with other EGFR mutations. Alternatively, these patients can receive afatinib or treatments outlined in the ASCO/OH nondriver mutation guideline, which was published in the Journal of Clinical Oncology in 2020.
If osimertinib is not available for first-line treatment, other options include gefitinib, erlotinib, icotinib, gefitinib plus chemotherapy, dacomitinib, afatinib, erlotinib plus bevacizumab, or erlotinib plus ramucirumab.
The authors recommend osimertinib in the second-line setting for patients who did not receive osimertinib initially and who have a T790M mutation at the time of progression. For patients who have progressed on EGFR tyrosine kinase inhibitors and have no T790M mutation or if their disease has progressed on osimertinib, second-line treatment should be based on the ASCO/OH nondriver mutation guideline, according to Dr. Hanna and colleagues.
ALK-mutant NSCLC
For patients with ALK alterations, the authors recommend alectinib or brigatinib as first-line treatment. If these agents are not available, ceritinib or crizotinib should be offered.
In the second-line setting, if alectinib or brigatinib were given initially, lorlatinib may be offered. If crizotinib was given as first-line therapy, then alectinib, brigatinib, or ceritinib should be offered.
If crizotinib was given in the first-line setting and alectinib, brigatinib, or ceritinib were given in the second-line setting, third-line treatment should be lorlatinib or standard treatment based on the ASCO/OH nondriver mutation guideline.
Other mutations
For stage IV NSCLC patients with alterations in ROS1, BRAF, RET, MET, or NTRK, the authors recommend either targeted or standard nontargeted therapy upfront, with the approach not given first-line used in the second line.
“It is unknown if improved outcomes would be seen when comparing standard nondriver mutation treatment with using the targeted therapy in the first- or second-line setting,” the authors wrote.
They noted that the recommendations for EGFR-activating mutations and ALK fusions are based on results from phase 3 trials, but recommendations for other targetable mutations are supported by phase 2 single-arm data.
The authors also noted promising reports for agents aimed at other molecular targets, including aberrations in KRAS, HER2, and NRG-1.
“Although there are insufficient data to recommend targeted therapy in these and other subgroups at the time of this guideline update, we anticipate rapid evolution of the evidence and availability of targeted therapies in these subgroups of patients soon,” the authors wrote.
Cost considerations
The authors noted that cost is a consideration when deciding on treatment, and costs can vary widely. According to 2020 Medicare drug prices, the monthly cost of ramucirumab was $61, while the monthly cost of ceritinib was $21,107.
“Increasingly, individuals with cancer are required to pay a larger proportion of their treatment costs through deductibles and coinsurance. Higher patient out-of-pocket costs have been shown to be a barrier to initiating and adhering to recommended cancer treatments,” the authors wrote.
“Discussion of cost can be an important part of shared decision-making. Clinicians should discuss with patients the use of less expensive alternatives when it is practical and feasible for treatment of the patient’s disease,” they added.
The guidelines were funded by ASCO. The authors had numerous disclosures, including Dr. Hanna, who disclosed relationships with UpToDate, Merck KGaA, Bristol-Myers Squibb, AstraZeneca/MedImmune, Genentech, and BeyondSpring Pharmaceuticals.
The guidelines, jointly released by the American Society of Clinical Oncology (ASCO) and Ontario Health (OH), were published in the Journal of Clinical Oncology. The recommendations are based on results from 54 studies published or presented from Dec. 2015 to May 2020.
The new guidelines supplant ASCO’s 2017 guidelines on stage IV NSCLC. Several driver mutations were touched upon in the 2017 document, but their corresponding targeted therapies were not recommended as first-line treatment.
With substantial progress in targeted therapies since 2017, treatment decision-making in 2021 focuses on the molecular signatures of tumors and PD-L1 score, according to the authors of the current guidelines, Nasser Hanna, MD, of Indiana University, Indianapolis, and colleagues.
“All patients with nonsquamous NSCLC should have the results of testing for potentially targetable mutations (alterations) before implementing therapy for advanced lung cancer, regardless of smoking status recommendations,” the authors wrote.
They noted that about a third of patients with NSCLC have known targetable genetic alterations. The Food and Drug Administration has approved therapeutics targeting seven alterations: EGFR and ALK alterations, ROS-1 fusions, BRAF V600e mutations, RET fusions, MET exon 14 skipping mutations, and NTRK fusions.
EGFR-mutant NSCLC
The authors’ recommendation for osimertinib as first-line therapy applies to patients who have EGFR-activating mutations in exon 19 (deletion), exon 21 L858R, or exon 20 T790M.
The authors also said osimertinib is an option for patients with other EGFR mutations. Alternatively, these patients can receive afatinib or treatments outlined in the ASCO/OH nondriver mutation guideline, which was published in the Journal of Clinical Oncology in 2020.
If osimertinib is not available for first-line treatment, other options include gefitinib, erlotinib, icotinib, gefitinib plus chemotherapy, dacomitinib, afatinib, erlotinib plus bevacizumab, or erlotinib plus ramucirumab.
The authors recommend osimertinib in the second-line setting for patients who did not receive osimertinib initially and who have a T790M mutation at the time of progression. For patients who have progressed on EGFR tyrosine kinase inhibitors and have no T790M mutation or if their disease has progressed on osimertinib, second-line treatment should be based on the ASCO/OH nondriver mutation guideline, according to Dr. Hanna and colleagues.
ALK-mutant NSCLC
For patients with ALK alterations, the authors recommend alectinib or brigatinib as first-line treatment. If these agents are not available, ceritinib or crizotinib should be offered.
In the second-line setting, if alectinib or brigatinib were given initially, lorlatinib may be offered. If crizotinib was given as first-line therapy, then alectinib, brigatinib, or ceritinib should be offered.
If crizotinib was given in the first-line setting and alectinib, brigatinib, or ceritinib were given in the second-line setting, third-line treatment should be lorlatinib or standard treatment based on the ASCO/OH nondriver mutation guideline.
Other mutations
For stage IV NSCLC patients with alterations in ROS1, BRAF, RET, MET, or NTRK, the authors recommend either targeted or standard nontargeted therapy upfront, with the approach not given first-line used in the second line.
“It is unknown if improved outcomes would be seen when comparing standard nondriver mutation treatment with using the targeted therapy in the first- or second-line setting,” the authors wrote.
They noted that the recommendations for EGFR-activating mutations and ALK fusions are based on results from phase 3 trials, but recommendations for other targetable mutations are supported by phase 2 single-arm data.
The authors also noted promising reports for agents aimed at other molecular targets, including aberrations in KRAS, HER2, and NRG-1.
“Although there are insufficient data to recommend targeted therapy in these and other subgroups at the time of this guideline update, we anticipate rapid evolution of the evidence and availability of targeted therapies in these subgroups of patients soon,” the authors wrote.
Cost considerations
The authors noted that cost is a consideration when deciding on treatment, and costs can vary widely. According to 2020 Medicare drug prices, the monthly cost of ramucirumab was $61, while the monthly cost of ceritinib was $21,107.
“Increasingly, individuals with cancer are required to pay a larger proportion of their treatment costs through deductibles and coinsurance. Higher patient out-of-pocket costs have been shown to be a barrier to initiating and adhering to recommended cancer treatments,” the authors wrote.
“Discussion of cost can be an important part of shared decision-making. Clinicians should discuss with patients the use of less expensive alternatives when it is practical and feasible for treatment of the patient’s disease,” they added.
The guidelines were funded by ASCO. The authors had numerous disclosures, including Dr. Hanna, who disclosed relationships with UpToDate, Merck KGaA, Bristol-Myers Squibb, AstraZeneca/MedImmune, Genentech, and BeyondSpring Pharmaceuticals.
The guidelines, jointly released by the American Society of Clinical Oncology (ASCO) and Ontario Health (OH), were published in the Journal of Clinical Oncology. The recommendations are based on results from 54 studies published or presented from Dec. 2015 to May 2020.
The new guidelines supplant ASCO’s 2017 guidelines on stage IV NSCLC. Several driver mutations were touched upon in the 2017 document, but their corresponding targeted therapies were not recommended as first-line treatment.
With substantial progress in targeted therapies since 2017, treatment decision-making in 2021 focuses on the molecular signatures of tumors and PD-L1 score, according to the authors of the current guidelines, Nasser Hanna, MD, of Indiana University, Indianapolis, and colleagues.
“All patients with nonsquamous NSCLC should have the results of testing for potentially targetable mutations (alterations) before implementing therapy for advanced lung cancer, regardless of smoking status recommendations,” the authors wrote.
They noted that about a third of patients with NSCLC have known targetable genetic alterations. The Food and Drug Administration has approved therapeutics targeting seven alterations: EGFR and ALK alterations, ROS-1 fusions, BRAF V600e mutations, RET fusions, MET exon 14 skipping mutations, and NTRK fusions.
EGFR-mutant NSCLC
The authors’ recommendation for osimertinib as first-line therapy applies to patients who have EGFR-activating mutations in exon 19 (deletion), exon 21 L858R, or exon 20 T790M.
The authors also said osimertinib is an option for patients with other EGFR mutations. Alternatively, these patients can receive afatinib or treatments outlined in the ASCO/OH nondriver mutation guideline, which was published in the Journal of Clinical Oncology in 2020.
If osimertinib is not available for first-line treatment, other options include gefitinib, erlotinib, icotinib, gefitinib plus chemotherapy, dacomitinib, afatinib, erlotinib plus bevacizumab, or erlotinib plus ramucirumab.
The authors recommend osimertinib in the second-line setting for patients who did not receive osimertinib initially and who have a T790M mutation at the time of progression. For patients who have progressed on EGFR tyrosine kinase inhibitors and have no T790M mutation or if their disease has progressed on osimertinib, second-line treatment should be based on the ASCO/OH nondriver mutation guideline, according to Dr. Hanna and colleagues.
ALK-mutant NSCLC
For patients with ALK alterations, the authors recommend alectinib or brigatinib as first-line treatment. If these agents are not available, ceritinib or crizotinib should be offered.
In the second-line setting, if alectinib or brigatinib were given initially, lorlatinib may be offered. If crizotinib was given as first-line therapy, then alectinib, brigatinib, or ceritinib should be offered.
If crizotinib was given in the first-line setting and alectinib, brigatinib, or ceritinib were given in the second-line setting, third-line treatment should be lorlatinib or standard treatment based on the ASCO/OH nondriver mutation guideline.
Other mutations
For stage IV NSCLC patients with alterations in ROS1, BRAF, RET, MET, or NTRK, the authors recommend either targeted or standard nontargeted therapy upfront, with the approach not given first-line used in the second line.
“It is unknown if improved outcomes would be seen when comparing standard nondriver mutation treatment with using the targeted therapy in the first- or second-line setting,” the authors wrote.
They noted that the recommendations for EGFR-activating mutations and ALK fusions are based on results from phase 3 trials, but recommendations for other targetable mutations are supported by phase 2 single-arm data.
The authors also noted promising reports for agents aimed at other molecular targets, including aberrations in KRAS, HER2, and NRG-1.
“Although there are insufficient data to recommend targeted therapy in these and other subgroups at the time of this guideline update, we anticipate rapid evolution of the evidence and availability of targeted therapies in these subgroups of patients soon,” the authors wrote.
Cost considerations
The authors noted that cost is a consideration when deciding on treatment, and costs can vary widely. According to 2020 Medicare drug prices, the monthly cost of ramucirumab was $61, while the monthly cost of ceritinib was $21,107.
“Increasingly, individuals with cancer are required to pay a larger proportion of their treatment costs through deductibles and coinsurance. Higher patient out-of-pocket costs have been shown to be a barrier to initiating and adhering to recommended cancer treatments,” the authors wrote.
“Discussion of cost can be an important part of shared decision-making. Clinicians should discuss with patients the use of less expensive alternatives when it is practical and feasible for treatment of the patient’s disease,” they added.
The guidelines were funded by ASCO. The authors had numerous disclosures, including Dr. Hanna, who disclosed relationships with UpToDate, Merck KGaA, Bristol-Myers Squibb, AstraZeneca/MedImmune, Genentech, and BeyondSpring Pharmaceuticals.
FROM JOURNAL OF CLINICAL ONCOLOGY
First pill for COVID-19 could be ready by year’s end
New pills to treat patients with COVID-19 are currently in midstage clinical trials and, if successful, could be ready by the end of the year.
Only one treatment – remdesivir (Veklury) – has been fully approved by the U.S. Food and Drug Administration for patients in the hospital and it must be administered intravenously.
Hopes for a day when patients with COVID-19 can take a pill to rid their bodies of the virus got a boost when early trial results were presented at a medical conference.
Interim phase 2 results for the oral experimental COVID-19 drug molnupiravir, designed to do for patients with COVID-19 what oseltamivir (Tamiflu) can do for patients with the flu, were presented at the Conference on Retroviruses and Opportunistic Infections 2021 Annual Meeting, as reported by this news organization.
In the small study, the pill significantly reduced infectious virus in patients who were symptomatic and had tested positive for COVID-19 during the previous 4 days but were not hospitalized.
After 5 days of treatment, no participants who received molnupiravir had detectable virus, whereas 24% who received placebo did.
Two other oral agents are being developed by RedHill Biopharma: one for severe COVID-19 infection for hospitalized patients and one for patients at home with mild infection.
The first, opaganib (Yeliva), proceeded to a phase 2/3 global trial for hospitalized patients after the company announced top-line safety and efficacy data in December. In phase 2, the drug was shown to be safe in patients requiring oxygen and effectively reduced the need for oxygen by the end of the treatment period.
A key feature is that it is both an antiviral and an anti-inflammatory, Gilead Raday, RedHill’s chief operating officer, said in an interview. Data are expected midyear on its performance in 464 patients. The drug is being tested on top of remdesivir or in addition to dexamethasone.
The second, upamostat (RHB-107), is currently undergoing a phase 2/3 trial in the United States and is being investigated for use in nonhospitalized COVID-19 patients.
“I would expect data to be available in the second half of this year,” Mr. Raday said.
Upamostat is a novel serine protease inhibitor expected to be effective against emerging variants because it targets human cell factors involved in viral entry, according to the company.
Other drugs are being investigated in trials that are in earlier stages.
Urgent need for oral agents
Infectious disease specialists are watching the move toward a COVID-19 pill enthusiastically.
“We badly need an oral treatment option for COVID,” said Sarah Doernberg, MD, an infectious disease specialist from the University of California, San Francisco.
“It’s a real gap in our armamentarium for COVID in outpatient treatment, which is where most who contract COVID-19 will seek care,” she said in an interview.
Although some studies have shown the benefit of monoclonal antibodies for prevention and early treatment, there are major logistical issues because all the current options require IV administration, she explained.
“If we had a pill to treat early COVID, especially in high-risk patients, it would fill a gap,” she said, noting that a pill could help people get better faster and prevent hospital stays.
Studies of molnupiravir suggest that it decreases viral shedding in the first few days after COVID infection, Dr. Doernberg reported.
There is excitement around the drug, but it will be important to see whether the results translate into fewer people requiring hospital admission and whether people feel better faster.
“I want to see the clinical data,” Dr. Doernberg said.
She will also be watching for the upamostat and opaganib results in the coming weeks.
“If these drugs are successful, I think it’s possible we could use them – maybe under an emergency use authorization – this year,” she said.
Once antiviral pills are a viable option for COVID-19 treatment, questions will arise about their use, she said.
One question is whether patients who are getting remdesivir in the hospital and are ready to leave after 5 days should continue treatment with antiviral pills at home.
Another is whether the pills – if they are shown to be effective – will be helpful for COVID post exposure. That use would be important for people who do not have COVID-19 but who are in close contact with someone who does, such as a member of their household.
“We have that model,” Dr. Doernberg said. “We know that oseltamivir can be used for postexposure prophylaxis and can help to prevent development of clinical disease.”
But she cautioned that a challenge with COVID is that people are contagious very early. A pill would need to come with the ability to test for COVID-19 early and get patients linked to care immediately.
“Those are not small challenges,” she said.
Vaccines alone won’t end the COVID threat
Treatments are part of the “belt-and-suspenders” approach, along with vaccines to combat COVID-19, Dr. Doernberg said.
“We’re not going to eradicate COVID,” she said. “We’re still going to need treatments for people who either don’t respond to the vaccine or haven’t gotten the vaccine or developed disease despite the vaccine.”
Oral formulations are desperately needed, agreed Kenneth Johnson, PhD, professor of molecular biosciences at the University of Texas at Austin.
Right now, remdesivir treatments involve patients being hooked up to an IV for 30-120 minutes each day for 5 days. And the cost of a 5-day course of remdesivir ranges from $2340 to $3120 in the United States.
“We’re hoping we can come up with something that is a little bit easier to administer, and without as many concerns for toxic side effects,” he said.
Dr. Johnson’s team at UT-Austin recently made a key discovery about the way remdesivir stops the replication of viral RNA.
The understanding of where the virus starts to replicate in the infection chain of events and how and where it reacts with remdesivir might lead to the development of better, more concentrated pill forms of antivirals in the future, with fewer toxicities, he said.
The team used a lab dish to recreate the step-by-step process that occurs when a patient who is infected with SARS-CoV-2 receives remdesivir.
The discovery was published online in Molecular Cell in January and will be printed in the April issue of the journal.
The discovery won’t lead to an effective COVID-19 pill for our current crisis, but will be important for the next generation of drugs needed to deal with future coronaviruses, Dr. Johnson explained.
And there will be other coronaviruses, he said, noting that this one is the third in 20 years to jump from animals to humans. “It’s just a matter of time,” he said.
A version of this article first appeared on Medscape.com.
New pills to treat patients with COVID-19 are currently in midstage clinical trials and, if successful, could be ready by the end of the year.
Only one treatment – remdesivir (Veklury) – has been fully approved by the U.S. Food and Drug Administration for patients in the hospital and it must be administered intravenously.
Hopes for a day when patients with COVID-19 can take a pill to rid their bodies of the virus got a boost when early trial results were presented at a medical conference.
Interim phase 2 results for the oral experimental COVID-19 drug molnupiravir, designed to do for patients with COVID-19 what oseltamivir (Tamiflu) can do for patients with the flu, were presented at the Conference on Retroviruses and Opportunistic Infections 2021 Annual Meeting, as reported by this news organization.
In the small study, the pill significantly reduced infectious virus in patients who were symptomatic and had tested positive for COVID-19 during the previous 4 days but were not hospitalized.
After 5 days of treatment, no participants who received molnupiravir had detectable virus, whereas 24% who received placebo did.
Two other oral agents are being developed by RedHill Biopharma: one for severe COVID-19 infection for hospitalized patients and one for patients at home with mild infection.
The first, opaganib (Yeliva), proceeded to a phase 2/3 global trial for hospitalized patients after the company announced top-line safety and efficacy data in December. In phase 2, the drug was shown to be safe in patients requiring oxygen and effectively reduced the need for oxygen by the end of the treatment period.
A key feature is that it is both an antiviral and an anti-inflammatory, Gilead Raday, RedHill’s chief operating officer, said in an interview. Data are expected midyear on its performance in 464 patients. The drug is being tested on top of remdesivir or in addition to dexamethasone.
The second, upamostat (RHB-107), is currently undergoing a phase 2/3 trial in the United States and is being investigated for use in nonhospitalized COVID-19 patients.
“I would expect data to be available in the second half of this year,” Mr. Raday said.
Upamostat is a novel serine protease inhibitor expected to be effective against emerging variants because it targets human cell factors involved in viral entry, according to the company.
Other drugs are being investigated in trials that are in earlier stages.
Urgent need for oral agents
Infectious disease specialists are watching the move toward a COVID-19 pill enthusiastically.
“We badly need an oral treatment option for COVID,” said Sarah Doernberg, MD, an infectious disease specialist from the University of California, San Francisco.
“It’s a real gap in our armamentarium for COVID in outpatient treatment, which is where most who contract COVID-19 will seek care,” she said in an interview.
Although some studies have shown the benefit of monoclonal antibodies for prevention and early treatment, there are major logistical issues because all the current options require IV administration, she explained.
“If we had a pill to treat early COVID, especially in high-risk patients, it would fill a gap,” she said, noting that a pill could help people get better faster and prevent hospital stays.
Studies of molnupiravir suggest that it decreases viral shedding in the first few days after COVID infection, Dr. Doernberg reported.
There is excitement around the drug, but it will be important to see whether the results translate into fewer people requiring hospital admission and whether people feel better faster.
“I want to see the clinical data,” Dr. Doernberg said.
She will also be watching for the upamostat and opaganib results in the coming weeks.
“If these drugs are successful, I think it’s possible we could use them – maybe under an emergency use authorization – this year,” she said.
Once antiviral pills are a viable option for COVID-19 treatment, questions will arise about their use, she said.
One question is whether patients who are getting remdesivir in the hospital and are ready to leave after 5 days should continue treatment with antiviral pills at home.
Another is whether the pills – if they are shown to be effective – will be helpful for COVID post exposure. That use would be important for people who do not have COVID-19 but who are in close contact with someone who does, such as a member of their household.
“We have that model,” Dr. Doernberg said. “We know that oseltamivir can be used for postexposure prophylaxis and can help to prevent development of clinical disease.”
But she cautioned that a challenge with COVID is that people are contagious very early. A pill would need to come with the ability to test for COVID-19 early and get patients linked to care immediately.
“Those are not small challenges,” she said.
Vaccines alone won’t end the COVID threat
Treatments are part of the “belt-and-suspenders” approach, along with vaccines to combat COVID-19, Dr. Doernberg said.
“We’re not going to eradicate COVID,” she said. “We’re still going to need treatments for people who either don’t respond to the vaccine or haven’t gotten the vaccine or developed disease despite the vaccine.”
Oral formulations are desperately needed, agreed Kenneth Johnson, PhD, professor of molecular biosciences at the University of Texas at Austin.
Right now, remdesivir treatments involve patients being hooked up to an IV for 30-120 minutes each day for 5 days. And the cost of a 5-day course of remdesivir ranges from $2340 to $3120 in the United States.
“We’re hoping we can come up with something that is a little bit easier to administer, and without as many concerns for toxic side effects,” he said.
Dr. Johnson’s team at UT-Austin recently made a key discovery about the way remdesivir stops the replication of viral RNA.
The understanding of where the virus starts to replicate in the infection chain of events and how and where it reacts with remdesivir might lead to the development of better, more concentrated pill forms of antivirals in the future, with fewer toxicities, he said.
The team used a lab dish to recreate the step-by-step process that occurs when a patient who is infected with SARS-CoV-2 receives remdesivir.
The discovery was published online in Molecular Cell in January and will be printed in the April issue of the journal.
The discovery won’t lead to an effective COVID-19 pill for our current crisis, but will be important for the next generation of drugs needed to deal with future coronaviruses, Dr. Johnson explained.
And there will be other coronaviruses, he said, noting that this one is the third in 20 years to jump from animals to humans. “It’s just a matter of time,” he said.
A version of this article first appeared on Medscape.com.
New pills to treat patients with COVID-19 are currently in midstage clinical trials and, if successful, could be ready by the end of the year.
Only one treatment – remdesivir (Veklury) – has been fully approved by the U.S. Food and Drug Administration for patients in the hospital and it must be administered intravenously.
Hopes for a day when patients with COVID-19 can take a pill to rid their bodies of the virus got a boost when early trial results were presented at a medical conference.
Interim phase 2 results for the oral experimental COVID-19 drug molnupiravir, designed to do for patients with COVID-19 what oseltamivir (Tamiflu) can do for patients with the flu, were presented at the Conference on Retroviruses and Opportunistic Infections 2021 Annual Meeting, as reported by this news organization.
In the small study, the pill significantly reduced infectious virus in patients who were symptomatic and had tested positive for COVID-19 during the previous 4 days but were not hospitalized.
After 5 days of treatment, no participants who received molnupiravir had detectable virus, whereas 24% who received placebo did.
Two other oral agents are being developed by RedHill Biopharma: one for severe COVID-19 infection for hospitalized patients and one for patients at home with mild infection.
The first, opaganib (Yeliva), proceeded to a phase 2/3 global trial for hospitalized patients after the company announced top-line safety and efficacy data in December. In phase 2, the drug was shown to be safe in patients requiring oxygen and effectively reduced the need for oxygen by the end of the treatment period.
A key feature is that it is both an antiviral and an anti-inflammatory, Gilead Raday, RedHill’s chief operating officer, said in an interview. Data are expected midyear on its performance in 464 patients. The drug is being tested on top of remdesivir or in addition to dexamethasone.
The second, upamostat (RHB-107), is currently undergoing a phase 2/3 trial in the United States and is being investigated for use in nonhospitalized COVID-19 patients.
“I would expect data to be available in the second half of this year,” Mr. Raday said.
Upamostat is a novel serine protease inhibitor expected to be effective against emerging variants because it targets human cell factors involved in viral entry, according to the company.
Other drugs are being investigated in trials that are in earlier stages.
Urgent need for oral agents
Infectious disease specialists are watching the move toward a COVID-19 pill enthusiastically.
“We badly need an oral treatment option for COVID,” said Sarah Doernberg, MD, an infectious disease specialist from the University of California, San Francisco.
“It’s a real gap in our armamentarium for COVID in outpatient treatment, which is where most who contract COVID-19 will seek care,” she said in an interview.
Although some studies have shown the benefit of monoclonal antibodies for prevention and early treatment, there are major logistical issues because all the current options require IV administration, she explained.
“If we had a pill to treat early COVID, especially in high-risk patients, it would fill a gap,” she said, noting that a pill could help people get better faster and prevent hospital stays.
Studies of molnupiravir suggest that it decreases viral shedding in the first few days after COVID infection, Dr. Doernberg reported.
There is excitement around the drug, but it will be important to see whether the results translate into fewer people requiring hospital admission and whether people feel better faster.
“I want to see the clinical data,” Dr. Doernberg said.
She will also be watching for the upamostat and opaganib results in the coming weeks.
“If these drugs are successful, I think it’s possible we could use them – maybe under an emergency use authorization – this year,” she said.
Once antiviral pills are a viable option for COVID-19 treatment, questions will arise about their use, she said.
One question is whether patients who are getting remdesivir in the hospital and are ready to leave after 5 days should continue treatment with antiviral pills at home.
Another is whether the pills – if they are shown to be effective – will be helpful for COVID post exposure. That use would be important for people who do not have COVID-19 but who are in close contact with someone who does, such as a member of their household.
“We have that model,” Dr. Doernberg said. “We know that oseltamivir can be used for postexposure prophylaxis and can help to prevent development of clinical disease.”
But she cautioned that a challenge with COVID is that people are contagious very early. A pill would need to come with the ability to test for COVID-19 early and get patients linked to care immediately.
“Those are not small challenges,” she said.
Vaccines alone won’t end the COVID threat
Treatments are part of the “belt-and-suspenders” approach, along with vaccines to combat COVID-19, Dr. Doernberg said.
“We’re not going to eradicate COVID,” she said. “We’re still going to need treatments for people who either don’t respond to the vaccine or haven’t gotten the vaccine or developed disease despite the vaccine.”
Oral formulations are desperately needed, agreed Kenneth Johnson, PhD, professor of molecular biosciences at the University of Texas at Austin.
Right now, remdesivir treatments involve patients being hooked up to an IV for 30-120 minutes each day for 5 days. And the cost of a 5-day course of remdesivir ranges from $2340 to $3120 in the United States.
“We’re hoping we can come up with something that is a little bit easier to administer, and without as many concerns for toxic side effects,” he said.
Dr. Johnson’s team at UT-Austin recently made a key discovery about the way remdesivir stops the replication of viral RNA.
The understanding of where the virus starts to replicate in the infection chain of events and how and where it reacts with remdesivir might lead to the development of better, more concentrated pill forms of antivirals in the future, with fewer toxicities, he said.
The team used a lab dish to recreate the step-by-step process that occurs when a patient who is infected with SARS-CoV-2 receives remdesivir.
The discovery was published online in Molecular Cell in January and will be printed in the April issue of the journal.
The discovery won’t lead to an effective COVID-19 pill for our current crisis, but will be important for the next generation of drugs needed to deal with future coronaviruses, Dr. Johnson explained.
And there will be other coronaviruses, he said, noting that this one is the third in 20 years to jump from animals to humans. “It’s just a matter of time,” he said.
A version of this article first appeared on Medscape.com.