User login
-
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]


Postintubation tracheal injury in the COVID-19 era
Postintubation laryngeal and tracheal injuries may be yet another part of recovery from severe COVID-19 infection for some patients.
Evidence has been accumulating on the link between prolonged intubation and lingering breathing and speaking difficulties, a concern that has become more germane in the wake of the COVID-19 pandemic. Now, researchers in Italy led by Giacomo Fiacchini, MD, and Luca Bruschini, MD, of the University of Pisa have published new research suggesting tracheal complications were particularly common in COVID-19 patients intubated for prolonged periods during the pandemic.
The study may be revealing effects of the pandemic itself, as resources and staff were at times overwhelmed by critical care patients. Of the 98 patients admitted from March 1 to May 31, 47% intubated for longer than 14 days developed full-thickness tracheal lesions, compared with 2.2% of a control group treated during the same time frame in 2019. The difference is eye-popping, but may not be generalizable. “I have not observed an increased rate of tracheal injury, but we haven’t carefully studied that outcome as far as I know,” said Daniel Ouellette, MD, FCCP, who is a senior staff physician and director of the pulmonary inpatient unit at Henry Ford Health System, and an associate professor at Wayne State University, Detroit.
He expressed concern about the retrospective nature of the study, and wondered if the different outcomes might be because of disruptions caused by the pandemic. “It’s not hard to imagine that these patients were seen [during] a great rush of patients, whereas the control group was looked at during a period where that kind of volume didn’t exist. There might have been a tendency for more inexperienced practitioners to be intubating patients because they were in the middle of the epidemic. There might have been less supervision of trainees. Individuals, physicians, teams may have been more rushed. Protocols may not have been followed as closely. It may all be an effect of the epidemic itself,” said Dr. Ouellette.
The investigators suggested that implementation of pronation maneuvers may have increased cuff pressure on the tracheal walls leading to some injuries. In addition, the prothrombotic and antifibrinolytic state of patients with COVID-19 may have contributed, along with the impact of systemic steroids that may have altered normal healing of tracheal wall microwounds caused by intubation, cuff pressure, or tracheostomy.
Other research has suggested increased complications from intubation among COVID-19 patients, including a case series that found heightened frequency of pneumomediastinum. The authors of that study suggested that aggressive disease pathophysiology and accompanying risk of alveolar damage and tracheobronchial injury may be to blame, along with larger-bore tracheal tubes and higher ventilation pressures. That study may also be reflecting the conditions of intubation during the pandemic.
Not all institutions saw an uptick in tracheal injury or pneumomediastinum. Mary Jo S. Farmer, MD, PhD, FCCP, of the department of medicine at University of Massachusetts, Springfield, asked one of the institute’s statisticians to examine pneumothorax frequency from March 15, 2020, to March 1, 2021, comparing the rates between patients who tested positive for SARS-CoV-2 within 14 days of admission, and those who tested negative. The rate was 0.5% in patients who tested positive versus 0.4% in those who tested negative. “My division chief’s gut sense is it’s just the same. The prevalence [of pneumomediastinum] is what we were seeing before,” said Dr. Farmer.
Shortly before the COVID-19 pandemic, researchers at Vanderbilt University Medical Center found that more than half of patients undergoing prolonged intubation experience breathing and speaking difficulties at 10 weeks post intubation. The group has followed up that study with another study looking at treatment timing and outcomes.
The researchers reviewed the experiences of 29 patients with laryngeal injury from endotracheal intubation between May 1, 2014,- and June 1, 2018. Ten patients with posterior glottis injury received early treatment, at a median of 34.7 days to presentation (interquartile range, 1.5-44.8 days). Nineteen patients with posterior glottis stenosis received treatment at a median of 341.9 days (absolute difference, 307.2 days; 95% confidence interval, 124.4-523.3 days). Demographic characteristics and comorbidities were similar between the two groups. At last follow-up, 90% of the early-treatment group were decannulated, compared with 58% of the late group (absolute difference, 32%; 95% CI, –3% to 68%). The early group required a mean of 2.2 interventions, compared with 11.5 in the late group (absolute difference, 9.3; 95% CI, 6.4-12.1). No patients in the early group required an open procedure, compared with 90% of the late-treatment group.
Although early treatment seems promising, the timing of laryngeal injury repair would be a key consideration. “You would worry about patient stability, [making] sure they’re clinically stable and didn’t have any acute ill effects from the injury itself or the underlying illness that led to intubation,” said Dr. Ouellette. For COVID-19 patients, that would mean recovery from pneumonia or any other lung problems, he added.
Together, the studies raise concerns and questions over tracheal and laryngeal injury in the context of COVID-19, but fall short of providing clinical guidance. “It raises the awareness in the mind of the critical care physician about these potential injuries to the larynx surrounding intubation,” said Dr. Farmer.
The studies received no funding. Dr. Ouellette and Dr. Farmer reported no relevant financial disclosures.
Postintubation laryngeal and tracheal injuries may be yet another part of recovery from severe COVID-19 infection for some patients.
Evidence has been accumulating on the link between prolonged intubation and lingering breathing and speaking difficulties, a concern that has become more germane in the wake of the COVID-19 pandemic. Now, researchers in Italy led by Giacomo Fiacchini, MD, and Luca Bruschini, MD, of the University of Pisa have published new research suggesting tracheal complications were particularly common in COVID-19 patients intubated for prolonged periods during the pandemic.
The study may be revealing effects of the pandemic itself, as resources and staff were at times overwhelmed by critical care patients. Of the 98 patients admitted from March 1 to May 31, 47% intubated for longer than 14 days developed full-thickness tracheal lesions, compared with 2.2% of a control group treated during the same time frame in 2019. The difference is eye-popping, but may not be generalizable. “I have not observed an increased rate of tracheal injury, but we haven’t carefully studied that outcome as far as I know,” said Daniel Ouellette, MD, FCCP, who is a senior staff physician and director of the pulmonary inpatient unit at Henry Ford Health System, and an associate professor at Wayne State University, Detroit.
He expressed concern about the retrospective nature of the study, and wondered if the different outcomes might be because of disruptions caused by the pandemic. “It’s not hard to imagine that these patients were seen [during] a great rush of patients, whereas the control group was looked at during a period where that kind of volume didn’t exist. There might have been a tendency for more inexperienced practitioners to be intubating patients because they were in the middle of the epidemic. There might have been less supervision of trainees. Individuals, physicians, teams may have been more rushed. Protocols may not have been followed as closely. It may all be an effect of the epidemic itself,” said Dr. Ouellette.
The investigators suggested that implementation of pronation maneuvers may have increased cuff pressure on the tracheal walls leading to some injuries. In addition, the prothrombotic and antifibrinolytic state of patients with COVID-19 may have contributed, along with the impact of systemic steroids that may have altered normal healing of tracheal wall microwounds caused by intubation, cuff pressure, or tracheostomy.
Other research has suggested increased complications from intubation among COVID-19 patients, including a case series that found heightened frequency of pneumomediastinum. The authors of that study suggested that aggressive disease pathophysiology and accompanying risk of alveolar damage and tracheobronchial injury may be to blame, along with larger-bore tracheal tubes and higher ventilation pressures. That study may also be reflecting the conditions of intubation during the pandemic.
Not all institutions saw an uptick in tracheal injury or pneumomediastinum. Mary Jo S. Farmer, MD, PhD, FCCP, of the department of medicine at University of Massachusetts, Springfield, asked one of the institute’s statisticians to examine pneumothorax frequency from March 15, 2020, to March 1, 2021, comparing the rates between patients who tested positive for SARS-CoV-2 within 14 days of admission, and those who tested negative. The rate was 0.5% in patients who tested positive versus 0.4% in those who tested negative. “My division chief’s gut sense is it’s just the same. The prevalence [of pneumomediastinum] is what we were seeing before,” said Dr. Farmer.
Shortly before the COVID-19 pandemic, researchers at Vanderbilt University Medical Center found that more than half of patients undergoing prolonged intubation experience breathing and speaking difficulties at 10 weeks post intubation. The group has followed up that study with another study looking at treatment timing and outcomes.
The researchers reviewed the experiences of 29 patients with laryngeal injury from endotracheal intubation between May 1, 2014,- and June 1, 2018. Ten patients with posterior glottis injury received early treatment, at a median of 34.7 days to presentation (interquartile range, 1.5-44.8 days). Nineteen patients with posterior glottis stenosis received treatment at a median of 341.9 days (absolute difference, 307.2 days; 95% confidence interval, 124.4-523.3 days). Demographic characteristics and comorbidities were similar between the two groups. At last follow-up, 90% of the early-treatment group were decannulated, compared with 58% of the late group (absolute difference, 32%; 95% CI, –3% to 68%). The early group required a mean of 2.2 interventions, compared with 11.5 in the late group (absolute difference, 9.3; 95% CI, 6.4-12.1). No patients in the early group required an open procedure, compared with 90% of the late-treatment group.
Although early treatment seems promising, the timing of laryngeal injury repair would be a key consideration. “You would worry about patient stability, [making] sure they’re clinically stable and didn’t have any acute ill effects from the injury itself or the underlying illness that led to intubation,” said Dr. Ouellette. For COVID-19 patients, that would mean recovery from pneumonia or any other lung problems, he added.
Together, the studies raise concerns and questions over tracheal and laryngeal injury in the context of COVID-19, but fall short of providing clinical guidance. “It raises the awareness in the mind of the critical care physician about these potential injuries to the larynx surrounding intubation,” said Dr. Farmer.
The studies received no funding. Dr. Ouellette and Dr. Farmer reported no relevant financial disclosures.
Postintubation laryngeal and tracheal injuries may be yet another part of recovery from severe COVID-19 infection for some patients.
Evidence has been accumulating on the link between prolonged intubation and lingering breathing and speaking difficulties, a concern that has become more germane in the wake of the COVID-19 pandemic. Now, researchers in Italy led by Giacomo Fiacchini, MD, and Luca Bruschini, MD, of the University of Pisa have published new research suggesting tracheal complications were particularly common in COVID-19 patients intubated for prolonged periods during the pandemic.
The study may be revealing effects of the pandemic itself, as resources and staff were at times overwhelmed by critical care patients. Of the 98 patients admitted from March 1 to May 31, 47% intubated for longer than 14 days developed full-thickness tracheal lesions, compared with 2.2% of a control group treated during the same time frame in 2019. The difference is eye-popping, but may not be generalizable. “I have not observed an increased rate of tracheal injury, but we haven’t carefully studied that outcome as far as I know,” said Daniel Ouellette, MD, FCCP, who is a senior staff physician and director of the pulmonary inpatient unit at Henry Ford Health System, and an associate professor at Wayne State University, Detroit.
He expressed concern about the retrospective nature of the study, and wondered if the different outcomes might be because of disruptions caused by the pandemic. “It’s not hard to imagine that these patients were seen [during] a great rush of patients, whereas the control group was looked at during a period where that kind of volume didn’t exist. There might have been a tendency for more inexperienced practitioners to be intubating patients because they were in the middle of the epidemic. There might have been less supervision of trainees. Individuals, physicians, teams may have been more rushed. Protocols may not have been followed as closely. It may all be an effect of the epidemic itself,” said Dr. Ouellette.
The investigators suggested that implementation of pronation maneuvers may have increased cuff pressure on the tracheal walls leading to some injuries. In addition, the prothrombotic and antifibrinolytic state of patients with COVID-19 may have contributed, along with the impact of systemic steroids that may have altered normal healing of tracheal wall microwounds caused by intubation, cuff pressure, or tracheostomy.
Other research has suggested increased complications from intubation among COVID-19 patients, including a case series that found heightened frequency of pneumomediastinum. The authors of that study suggested that aggressive disease pathophysiology and accompanying risk of alveolar damage and tracheobronchial injury may be to blame, along with larger-bore tracheal tubes and higher ventilation pressures. That study may also be reflecting the conditions of intubation during the pandemic.
Not all institutions saw an uptick in tracheal injury or pneumomediastinum. Mary Jo S. Farmer, MD, PhD, FCCP, of the department of medicine at University of Massachusetts, Springfield, asked one of the institute’s statisticians to examine pneumothorax frequency from March 15, 2020, to March 1, 2021, comparing the rates between patients who tested positive for SARS-CoV-2 within 14 days of admission, and those who tested negative. The rate was 0.5% in patients who tested positive versus 0.4% in those who tested negative. “My division chief’s gut sense is it’s just the same. The prevalence [of pneumomediastinum] is what we were seeing before,” said Dr. Farmer.
Shortly before the COVID-19 pandemic, researchers at Vanderbilt University Medical Center found that more than half of patients undergoing prolonged intubation experience breathing and speaking difficulties at 10 weeks post intubation. The group has followed up that study with another study looking at treatment timing and outcomes.
The researchers reviewed the experiences of 29 patients with laryngeal injury from endotracheal intubation between May 1, 2014,- and June 1, 2018. Ten patients with posterior glottis injury received early treatment, at a median of 34.7 days to presentation (interquartile range, 1.5-44.8 days). Nineteen patients with posterior glottis stenosis received treatment at a median of 341.9 days (absolute difference, 307.2 days; 95% confidence interval, 124.4-523.3 days). Demographic characteristics and comorbidities were similar between the two groups. At last follow-up, 90% of the early-treatment group were decannulated, compared with 58% of the late group (absolute difference, 32%; 95% CI, –3% to 68%). The early group required a mean of 2.2 interventions, compared with 11.5 in the late group (absolute difference, 9.3; 95% CI, 6.4-12.1). No patients in the early group required an open procedure, compared with 90% of the late-treatment group.
Although early treatment seems promising, the timing of laryngeal injury repair would be a key consideration. “You would worry about patient stability, [making] sure they’re clinically stable and didn’t have any acute ill effects from the injury itself or the underlying illness that led to intubation,” said Dr. Ouellette. For COVID-19 patients, that would mean recovery from pneumonia or any other lung problems, he added.
Together, the studies raise concerns and questions over tracheal and laryngeal injury in the context of COVID-19, but fall short of providing clinical guidance. “It raises the awareness in the mind of the critical care physician about these potential injuries to the larynx surrounding intubation,” said Dr. Farmer.
The studies received no funding. Dr. Ouellette and Dr. Farmer reported no relevant financial disclosures.
FROM JAMA OTOLARYNGOLOGY–HEAD & NECK SURGERY
Time is of the essence: DST up for debate again
Seasonal time change is now up for consideration in the U.S. Congress, prompting sleep medicine specialists to weigh in on the health impact of a major policy change.
As lawmakers in Washington propose an end to seasonal time changes by permanently establishing daylight saving time (DST), the American Academy of Sleep Medicine (AASM) is pushing for a Congressional hearing so scientists can present evidence in favor of converse legislation – to make standard time the new norm.
According to the AASM, ; however, the switch from standard time to DST incurs more risk.
“Current evidence best supports the adoption of year-round standard time, which aligns best with human circadian biology and provides distinct benefits for public health and safety,” the AASM noted in a 2020 position statement on DST.
The statement cites a number of studies that have reported associations between the switch to DST and acute, negative health outcomes, including higher rates of hospital admission, cardiovascular morbidity, atrial fibrillation, and stroke. The time shift has been associated with a spectrum of cellular, metabolic, and circadian derangements, from increased production of inflammatory markers, to higher blood pressure, and loss of sleep. These biological effects may have far-reaching consequences, including increased rates of fatal motor accidents in the days following the time change, and even increased volatility in the stock market, which may stem from cognitive deficits.
U.S. Senator Marco Rubio (R-Fla.) and others in the U.S. Congress have reintroduced the 2019 Sunshine Protection Act, legislation that would make DST permanent across the country. According to a statement on Sen. Rubio’s website, “The bill reflects the Florida legislature’s 2018 enactment of year-round DST; however, for Florida’s change to apply, a change in the federal statute is required. Fifteen other states – Arkansas, Alabama, California, Delaware, Georgia, Idaho, Louisiana, Maine, Ohio, Oregon, South Carolina, Tennessee, Utah, Washington, and Wyoming – have passed similar laws, resolutions, or voter initiatives, and dozens more are looking. The legislation, if enacted, would apply to those states [that] currently participate in DST, which most states observe for eight months out of the year.”
A stitch in time
“The sudden change in clock time disrupts sleep/wake patterns, decreasing total sleep time and sleep quality, leading to decrements in daytime cognition,” said Kannan Ramar, MBBS, MD, president of the AASM and a sleep medicine specialist at Mayo Clinic, Rochester, Minn.
Emphasizing this point, Dr. Ramar noted a recent study that reported an 18% increase in “patient safety-related incidents associated with human error” among health care workers within a week of the spring time change.
“Irregular bedtimes and wake times disrupt the timing of our circadian rhythms, which can lead to symptoms of insomnia or long-term, excessive daytime sleepiness. Lack of sleep can lead to numerous adverse effects on our minds, including decreased cognitive function, trouble concentrating, and general moodiness,” Dr. Ramar said.
He noted that these impacts may be more significant among certain individuals.
“The daylight saving time changes can be especially problematic for any populations that already experience chronic insufficient sleep or other sleep difficulties,” Dr. Ramar said. “Populations at greatest risk include teenagers, who tend to experience chronic sleep restriction during the school week, and night shift workers, who often struggle to sleep well during daytime hours.”
While fewer studies have evaluated the long-term effects of seasonal time changes, the AASM position statement cited evidence that “the body clock does not adjust to daylight saving time after several months,” possibly because “daylight saving time is less well-aligned with intrinsic human circadian physiology, and it disrupts the natural seasonal adjustment of the human clock due to the effect of late-evening light on the circadian rhythm.”
According to the AASM, permanent DST, as proposed by Sen. Rubio and colleagues, could “result in permanent phase delay, a condition that can also lead to a perpetual discrepancy between the innate biological clock and the extrinsic environmental clock, as well as chronic sleep loss due to early morning social demands that truncate the opportunity to sleep.” This mismatch between sleep/wake cycles and social demands, known as “social jet lag,” has been associated with chronic health risks, including metabolic syndrome, obesity, depression, and cardiovascular disease.
Cardiac impacts of seasonal time change
Muhammad Adeel Rishi, MD, a sleep specialist at Mayo Clinic, Eau Claire, Wis., and lead author of the AASM position statement, highlighted cardiovascular risks in a written statement for this article, noting increased rates of heart attack following the spring time change, and a higher risk of atrial fibrillation.
“Mayo Clinic has not taken a position on this issue,” Dr. Rishi noted. Still, he advocated for permanent standard time as the author of the AASM position statement and vice chair of the AASM public safety committee.
Jay Chudow, MD, and Andrew K. Krumerman, MD, of Montefiore Medical Center, New York, lead author and principal author, respectively, of a recent study that reported increased rates of atrial fibrillation admissions after DST transitions, had the same stance.
“We support elimination of seasonal time changes from a health perspective,” they wrote in a joint comment. “There is mounting evidence of a negative health impact with these seasonal time changes related to effects on sleep and circadian rhythm. Our work found the spring change was associated with more admissions for atrial fibrillation. This added to prior evidence of increased cardiovascular events related to these time changes. If physicians counsel patients on reducing risk factors for disease, shouldn’t we do the same as a society?”
Pros and cons
Not all sleep experts are convinced. Mary Jo Farmer, MD, PhD, FCCP, a sleep specialist and director of pulmonary hypertension services at Baystate Medical Center, and assistant professor of medicine at the University of Massachusetts, Springfield, considers perspectives from both sides of the issue.
“Daylight saving time promotes active lifestyles as people engage in more outdoor activities after work and school, [and] daylight saving time produces economic and safety benefits to society as retail revenues are higher and crimes are lower,” Dr. Farmer said. “Alternatively, moving the clocks forward is a cost burden to the U.S. economy when health issues, decreased productivity, and workplace injuries are considered.”
If one time system is permanently established, Dr. Farmer anticipates divided opinions from patients with sleep issues, regardless of which system is chosen.
“I can tell you, I have a cohort of sleep patients who prefer more evening light and look forward to the spring time change to daylight saving time,” she said. “However, they would not want the sun coming up at 9:00 a.m. in the winter months if we stayed on daylight saving time year-round. Similarly, patients would not want the sun coming up at 4:00 a.m. on the longest day of the year if we stayed on standard time all year round.”
Dr. Farmer called for more research before a decision is made.
“I suggest we need more information about the dangers of staying on daylight saving or standard time year-round because perhaps the current strategy of keeping morning light consistent is not so bad,” she said.
Time for a Congressional hearing?
According to Dr. Ramar, the time is now for a Congressional hearing, as lawmakers and the public need to be adequately informed when considering new legislation.
“There are public misconceptions about daylight saving time and standard time,” Dr. Ramar said. “People often like the idea of daylight saving time because they think it provides more light, and they dislike the concept of standard time because they think it provides more darkness. The reality is that neither time system provides more light or darkness than the other; it is only the timing that changes.”
Until new legislation is introduced, Dr. Ramar offered some practical advice for navigating seasonal time shifts.
“Beginning 2-3 days before the time change, it can be helpful to gradually adjust sleep and wake times, as well as other daily routines such as meal times,” he said. “After the time change, going outside for some morning light can help adjust the timing of your internal body clock.”
The investigators reported no conflicts of interest.
Seasonal time change is now up for consideration in the U.S. Congress, prompting sleep medicine specialists to weigh in on the health impact of a major policy change.
As lawmakers in Washington propose an end to seasonal time changes by permanently establishing daylight saving time (DST), the American Academy of Sleep Medicine (AASM) is pushing for a Congressional hearing so scientists can present evidence in favor of converse legislation – to make standard time the new norm.
According to the AASM, ; however, the switch from standard time to DST incurs more risk.
“Current evidence best supports the adoption of year-round standard time, which aligns best with human circadian biology and provides distinct benefits for public health and safety,” the AASM noted in a 2020 position statement on DST.
The statement cites a number of studies that have reported associations between the switch to DST and acute, negative health outcomes, including higher rates of hospital admission, cardiovascular morbidity, atrial fibrillation, and stroke. The time shift has been associated with a spectrum of cellular, metabolic, and circadian derangements, from increased production of inflammatory markers, to higher blood pressure, and loss of sleep. These biological effects may have far-reaching consequences, including increased rates of fatal motor accidents in the days following the time change, and even increased volatility in the stock market, which may stem from cognitive deficits.
U.S. Senator Marco Rubio (R-Fla.) and others in the U.S. Congress have reintroduced the 2019 Sunshine Protection Act, legislation that would make DST permanent across the country. According to a statement on Sen. Rubio’s website, “The bill reflects the Florida legislature’s 2018 enactment of year-round DST; however, for Florida’s change to apply, a change in the federal statute is required. Fifteen other states – Arkansas, Alabama, California, Delaware, Georgia, Idaho, Louisiana, Maine, Ohio, Oregon, South Carolina, Tennessee, Utah, Washington, and Wyoming – have passed similar laws, resolutions, or voter initiatives, and dozens more are looking. The legislation, if enacted, would apply to those states [that] currently participate in DST, which most states observe for eight months out of the year.”
A stitch in time
“The sudden change in clock time disrupts sleep/wake patterns, decreasing total sleep time and sleep quality, leading to decrements in daytime cognition,” said Kannan Ramar, MBBS, MD, president of the AASM and a sleep medicine specialist at Mayo Clinic, Rochester, Minn.
Emphasizing this point, Dr. Ramar noted a recent study that reported an 18% increase in “patient safety-related incidents associated with human error” among health care workers within a week of the spring time change.
“Irregular bedtimes and wake times disrupt the timing of our circadian rhythms, which can lead to symptoms of insomnia or long-term, excessive daytime sleepiness. Lack of sleep can lead to numerous adverse effects on our minds, including decreased cognitive function, trouble concentrating, and general moodiness,” Dr. Ramar said.
He noted that these impacts may be more significant among certain individuals.
“The daylight saving time changes can be especially problematic for any populations that already experience chronic insufficient sleep or other sleep difficulties,” Dr. Ramar said. “Populations at greatest risk include teenagers, who tend to experience chronic sleep restriction during the school week, and night shift workers, who often struggle to sleep well during daytime hours.”
While fewer studies have evaluated the long-term effects of seasonal time changes, the AASM position statement cited evidence that “the body clock does not adjust to daylight saving time after several months,” possibly because “daylight saving time is less well-aligned with intrinsic human circadian physiology, and it disrupts the natural seasonal adjustment of the human clock due to the effect of late-evening light on the circadian rhythm.”
According to the AASM, permanent DST, as proposed by Sen. Rubio and colleagues, could “result in permanent phase delay, a condition that can also lead to a perpetual discrepancy between the innate biological clock and the extrinsic environmental clock, as well as chronic sleep loss due to early morning social demands that truncate the opportunity to sleep.” This mismatch between sleep/wake cycles and social demands, known as “social jet lag,” has been associated with chronic health risks, including metabolic syndrome, obesity, depression, and cardiovascular disease.
Cardiac impacts of seasonal time change
Muhammad Adeel Rishi, MD, a sleep specialist at Mayo Clinic, Eau Claire, Wis., and lead author of the AASM position statement, highlighted cardiovascular risks in a written statement for this article, noting increased rates of heart attack following the spring time change, and a higher risk of atrial fibrillation.
“Mayo Clinic has not taken a position on this issue,” Dr. Rishi noted. Still, he advocated for permanent standard time as the author of the AASM position statement and vice chair of the AASM public safety committee.
Jay Chudow, MD, and Andrew K. Krumerman, MD, of Montefiore Medical Center, New York, lead author and principal author, respectively, of a recent study that reported increased rates of atrial fibrillation admissions after DST transitions, had the same stance.
“We support elimination of seasonal time changes from a health perspective,” they wrote in a joint comment. “There is mounting evidence of a negative health impact with these seasonal time changes related to effects on sleep and circadian rhythm. Our work found the spring change was associated with more admissions for atrial fibrillation. This added to prior evidence of increased cardiovascular events related to these time changes. If physicians counsel patients on reducing risk factors for disease, shouldn’t we do the same as a society?”
Pros and cons
Not all sleep experts are convinced. Mary Jo Farmer, MD, PhD, FCCP, a sleep specialist and director of pulmonary hypertension services at Baystate Medical Center, and assistant professor of medicine at the University of Massachusetts, Springfield, considers perspectives from both sides of the issue.
“Daylight saving time promotes active lifestyles as people engage in more outdoor activities after work and school, [and] daylight saving time produces economic and safety benefits to society as retail revenues are higher and crimes are lower,” Dr. Farmer said. “Alternatively, moving the clocks forward is a cost burden to the U.S. economy when health issues, decreased productivity, and workplace injuries are considered.”
If one time system is permanently established, Dr. Farmer anticipates divided opinions from patients with sleep issues, regardless of which system is chosen.
“I can tell you, I have a cohort of sleep patients who prefer more evening light and look forward to the spring time change to daylight saving time,” she said. “However, they would not want the sun coming up at 9:00 a.m. in the winter months if we stayed on daylight saving time year-round. Similarly, patients would not want the sun coming up at 4:00 a.m. on the longest day of the year if we stayed on standard time all year round.”
Dr. Farmer called for more research before a decision is made.
“I suggest we need more information about the dangers of staying on daylight saving or standard time year-round because perhaps the current strategy of keeping morning light consistent is not so bad,” she said.
Time for a Congressional hearing?
According to Dr. Ramar, the time is now for a Congressional hearing, as lawmakers and the public need to be adequately informed when considering new legislation.
“There are public misconceptions about daylight saving time and standard time,” Dr. Ramar said. “People often like the idea of daylight saving time because they think it provides more light, and they dislike the concept of standard time because they think it provides more darkness. The reality is that neither time system provides more light or darkness than the other; it is only the timing that changes.”
Until new legislation is introduced, Dr. Ramar offered some practical advice for navigating seasonal time shifts.
“Beginning 2-3 days before the time change, it can be helpful to gradually adjust sleep and wake times, as well as other daily routines such as meal times,” he said. “After the time change, going outside for some morning light can help adjust the timing of your internal body clock.”
The investigators reported no conflicts of interest.
Seasonal time change is now up for consideration in the U.S. Congress, prompting sleep medicine specialists to weigh in on the health impact of a major policy change.
As lawmakers in Washington propose an end to seasonal time changes by permanently establishing daylight saving time (DST), the American Academy of Sleep Medicine (AASM) is pushing for a Congressional hearing so scientists can present evidence in favor of converse legislation – to make standard time the new norm.
According to the AASM, ; however, the switch from standard time to DST incurs more risk.
“Current evidence best supports the adoption of year-round standard time, which aligns best with human circadian biology and provides distinct benefits for public health and safety,” the AASM noted in a 2020 position statement on DST.
The statement cites a number of studies that have reported associations between the switch to DST and acute, negative health outcomes, including higher rates of hospital admission, cardiovascular morbidity, atrial fibrillation, and stroke. The time shift has been associated with a spectrum of cellular, metabolic, and circadian derangements, from increased production of inflammatory markers, to higher blood pressure, and loss of sleep. These biological effects may have far-reaching consequences, including increased rates of fatal motor accidents in the days following the time change, and even increased volatility in the stock market, which may stem from cognitive deficits.
U.S. Senator Marco Rubio (R-Fla.) and others in the U.S. Congress have reintroduced the 2019 Sunshine Protection Act, legislation that would make DST permanent across the country. According to a statement on Sen. Rubio’s website, “The bill reflects the Florida legislature’s 2018 enactment of year-round DST; however, for Florida’s change to apply, a change in the federal statute is required. Fifteen other states – Arkansas, Alabama, California, Delaware, Georgia, Idaho, Louisiana, Maine, Ohio, Oregon, South Carolina, Tennessee, Utah, Washington, and Wyoming – have passed similar laws, resolutions, or voter initiatives, and dozens more are looking. The legislation, if enacted, would apply to those states [that] currently participate in DST, which most states observe for eight months out of the year.”
A stitch in time
“The sudden change in clock time disrupts sleep/wake patterns, decreasing total sleep time and sleep quality, leading to decrements in daytime cognition,” said Kannan Ramar, MBBS, MD, president of the AASM and a sleep medicine specialist at Mayo Clinic, Rochester, Minn.
Emphasizing this point, Dr. Ramar noted a recent study that reported an 18% increase in “patient safety-related incidents associated with human error” among health care workers within a week of the spring time change.
“Irregular bedtimes and wake times disrupt the timing of our circadian rhythms, which can lead to symptoms of insomnia or long-term, excessive daytime sleepiness. Lack of sleep can lead to numerous adverse effects on our minds, including decreased cognitive function, trouble concentrating, and general moodiness,” Dr. Ramar said.
He noted that these impacts may be more significant among certain individuals.
“The daylight saving time changes can be especially problematic for any populations that already experience chronic insufficient sleep or other sleep difficulties,” Dr. Ramar said. “Populations at greatest risk include teenagers, who tend to experience chronic sleep restriction during the school week, and night shift workers, who often struggle to sleep well during daytime hours.”
While fewer studies have evaluated the long-term effects of seasonal time changes, the AASM position statement cited evidence that “the body clock does not adjust to daylight saving time after several months,” possibly because “daylight saving time is less well-aligned with intrinsic human circadian physiology, and it disrupts the natural seasonal adjustment of the human clock due to the effect of late-evening light on the circadian rhythm.”
According to the AASM, permanent DST, as proposed by Sen. Rubio and colleagues, could “result in permanent phase delay, a condition that can also lead to a perpetual discrepancy between the innate biological clock and the extrinsic environmental clock, as well as chronic sleep loss due to early morning social demands that truncate the opportunity to sleep.” This mismatch between sleep/wake cycles and social demands, known as “social jet lag,” has been associated with chronic health risks, including metabolic syndrome, obesity, depression, and cardiovascular disease.
Cardiac impacts of seasonal time change
Muhammad Adeel Rishi, MD, a sleep specialist at Mayo Clinic, Eau Claire, Wis., and lead author of the AASM position statement, highlighted cardiovascular risks in a written statement for this article, noting increased rates of heart attack following the spring time change, and a higher risk of atrial fibrillation.
“Mayo Clinic has not taken a position on this issue,” Dr. Rishi noted. Still, he advocated for permanent standard time as the author of the AASM position statement and vice chair of the AASM public safety committee.
Jay Chudow, MD, and Andrew K. Krumerman, MD, of Montefiore Medical Center, New York, lead author and principal author, respectively, of a recent study that reported increased rates of atrial fibrillation admissions after DST transitions, had the same stance.
“We support elimination of seasonal time changes from a health perspective,” they wrote in a joint comment. “There is mounting evidence of a negative health impact with these seasonal time changes related to effects on sleep and circadian rhythm. Our work found the spring change was associated with more admissions for atrial fibrillation. This added to prior evidence of increased cardiovascular events related to these time changes. If physicians counsel patients on reducing risk factors for disease, shouldn’t we do the same as a society?”
Pros and cons
Not all sleep experts are convinced. Mary Jo Farmer, MD, PhD, FCCP, a sleep specialist and director of pulmonary hypertension services at Baystate Medical Center, and assistant professor of medicine at the University of Massachusetts, Springfield, considers perspectives from both sides of the issue.
“Daylight saving time promotes active lifestyles as people engage in more outdoor activities after work and school, [and] daylight saving time produces economic and safety benefits to society as retail revenues are higher and crimes are lower,” Dr. Farmer said. “Alternatively, moving the clocks forward is a cost burden to the U.S. economy when health issues, decreased productivity, and workplace injuries are considered.”
If one time system is permanently established, Dr. Farmer anticipates divided opinions from patients with sleep issues, regardless of which system is chosen.
“I can tell you, I have a cohort of sleep patients who prefer more evening light and look forward to the spring time change to daylight saving time,” she said. “However, they would not want the sun coming up at 9:00 a.m. in the winter months if we stayed on daylight saving time year-round. Similarly, patients would not want the sun coming up at 4:00 a.m. on the longest day of the year if we stayed on standard time all year round.”
Dr. Farmer called for more research before a decision is made.
“I suggest we need more information about the dangers of staying on daylight saving or standard time year-round because perhaps the current strategy of keeping morning light consistent is not so bad,” she said.
Time for a Congressional hearing?
According to Dr. Ramar, the time is now for a Congressional hearing, as lawmakers and the public need to be adequately informed when considering new legislation.
“There are public misconceptions about daylight saving time and standard time,” Dr. Ramar said. “People often like the idea of daylight saving time because they think it provides more light, and they dislike the concept of standard time because they think it provides more darkness. The reality is that neither time system provides more light or darkness than the other; it is only the timing that changes.”
Until new legislation is introduced, Dr. Ramar offered some practical advice for navigating seasonal time shifts.
“Beginning 2-3 days before the time change, it can be helpful to gradually adjust sleep and wake times, as well as other daily routines such as meal times,” he said. “After the time change, going outside for some morning light can help adjust the timing of your internal body clock.”
The investigators reported no conflicts of interest.
COVID-19 can cause atypical thyroid inflammation
Individuals who experience inflammation of the thyroid gland during acute COVID-19 illness may still have subacute thyroiditis months later, even if thyroid function has normalized, new research suggests.
Furthermore, the thyroiditis seems to be different from thyroid inflammation caused by other viruses, said Ilaria Muller, MD, PhD, when presenting her findings March 21 at the virtual ENDO 2021 meeting.
“SARS-CoV-2 seems to have multifactorial action on thyroid function,” said Dr. Muller, of the University of Milan, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Italy.
In July 2020, Dr. Muller and colleagues described patients hospitalized at their institution with severe COVID-19, 15% of whom had thyrotoxicosis due to atypical subacute thyroiditis, compared with just 1% of a comparison group hospitalized in the same subintensive care units during the spring of 2019, as reported by this news organization.
The “atypical” thyroiditis that occurred in the patients with COVID-19 was not associated with neck pain and affected more men than women. Moreover, it was associated with low TSH and free-triiodothyronine (T3) levels, and normal or elevated levels of free-thyroxine (T4), which is a very different presentation to classic nonthyroidal illness syndrome (NTIS) usually seen in critically ill patients, she explained.
Although transient T4 elevations can occur in acute illness, that phenomenon is not associated with low TSH. This newly described scenario appears to be a combination of thyrotoxicosis and NTIS, Dr. Muller and colleagues had speculated last summer.
Follow patients with COVID-19 and thyroid dysfunction for a year
Now, in an assessment of 51 patients 3 months after hospitalization for moderate-to-severe COVID-19 reported by Dr. Muller at ENDO 2021, both inflammatory markers and thyroid function had normalized, yet on imaging, a third of patients still exhibited focal hypoechoic areas suggestive of thyroiditis.
Of those, two-thirds had reduced uptake on thyroid scintigraphy, but few had antithyroid autoantibodies.
“The thyroid dysfunction induced by COVID-19 seems not mediated by autoimmunity. It is important to continue to follow these patients since they might develop thyroid dysfunction during the following months,” Dr. Muller emphasized.
Asked to comment, session moderator Robert W. Lash, MD, the Endocrine Society’s chief professional & clinical affairs officer, told this news organization: “When you’re ICU-level sick, it’s not unusual to have weird thyroid tests. Some viruses cause thyroid problems as well ... What makes this different is that while a lot of thyroid inflammation is caused by antibodies, this was not.”
“It looks like this was [SARS-CoV-2] causing damage to the thyroid gland, which is interesting,” he noted, adding that the thyroid gland expresses high levels of angiotensin-converting enzyme 2 (ACE2) and transmembrane protease serine 2 (TMPRSS2), which allow SARS-CoV-2 to infect human cells.
“This is probably part of that same story,” Dr. Lash said.
For patients who had thyroid abnormalities during acute COVID-19 illness or develop symptoms that might be thyroid-related afterward, he advises: “You should keep an eye on thyroid tests. It just raises your awareness ... You might check their thyroid tests every 6 months for a year.”
Signs of focal thyroiditis despite normalized thyroid function
The 51 patients (33 men and 18 women) hospitalized with moderate-to-severe COVID-19 had no history of thyroid disease and had not been taking thyroid medications, amiodarone, or steroids before baseline TSH was measured.
From baseline to 3 months, TSH rose from 1.2 to 1.6 mIU/L, while serum concentrations of T4, T3, C-reactive protein, and full blood counts had all normalized (all P < 0.01 vs. baseline).
Thyroid ultrasound at 3 months in 49 patients showed signs of focal thyroiditis in 16 (33%).
Among 14 patients of those who further underwent thyroid 99mTc or I123 uptake scans, four (29%) were normal, eight (57%) had focally reduced uptake, and two (14%) had diffusely reduced uptake.
Of the 16 patients with focal thyroiditis, only three were positive for autoantibodies to thyroglobulin (TgAb) or thyroid peroxidase (TPOAb). All were negative for autoantibodies to the TSH receptor.
“Importantly, of the two with diffusely reduced uptake, only one was positive for TPOAb or TgAb,” Dr. Muller noted, adding, “SARS-CoV-2 disease seems to trigger some dysfunction which very likely has complex and multifactorial mechanisms.”
In response to a question about a possible role for biopsies and thyroid cytology, Dr. Muller replied: “That’s definitely the key ... So far we’re just making guesses, so the key will be cytological or histological studies to see what is really going on in the thyroid.”
“What we know is that [unlike] classical thyroiditis that has been described after viral diseases including SARS-CoV-2, these patients have a different scenario ... Probably something is going on within the thyroid with a different mechanism, so surely cytology and histology studies are what we need,” she concluded.
The study was funded by Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, and by a COVID-19 research grant from the European Society of Endocrinology. Dr. Muller and Dr. Lash have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Individuals who experience inflammation of the thyroid gland during acute COVID-19 illness may still have subacute thyroiditis months later, even if thyroid function has normalized, new research suggests.
Furthermore, the thyroiditis seems to be different from thyroid inflammation caused by other viruses, said Ilaria Muller, MD, PhD, when presenting her findings March 21 at the virtual ENDO 2021 meeting.
“SARS-CoV-2 seems to have multifactorial action on thyroid function,” said Dr. Muller, of the University of Milan, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Italy.
In July 2020, Dr. Muller and colleagues described patients hospitalized at their institution with severe COVID-19, 15% of whom had thyrotoxicosis due to atypical subacute thyroiditis, compared with just 1% of a comparison group hospitalized in the same subintensive care units during the spring of 2019, as reported by this news organization.
The “atypical” thyroiditis that occurred in the patients with COVID-19 was not associated with neck pain and affected more men than women. Moreover, it was associated with low TSH and free-triiodothyronine (T3) levels, and normal or elevated levels of free-thyroxine (T4), which is a very different presentation to classic nonthyroidal illness syndrome (NTIS) usually seen in critically ill patients, she explained.
Although transient T4 elevations can occur in acute illness, that phenomenon is not associated with low TSH. This newly described scenario appears to be a combination of thyrotoxicosis and NTIS, Dr. Muller and colleagues had speculated last summer.
Follow patients with COVID-19 and thyroid dysfunction for a year
Now, in an assessment of 51 patients 3 months after hospitalization for moderate-to-severe COVID-19 reported by Dr. Muller at ENDO 2021, both inflammatory markers and thyroid function had normalized, yet on imaging, a third of patients still exhibited focal hypoechoic areas suggestive of thyroiditis.
Of those, two-thirds had reduced uptake on thyroid scintigraphy, but few had antithyroid autoantibodies.
“The thyroid dysfunction induced by COVID-19 seems not mediated by autoimmunity. It is important to continue to follow these patients since they might develop thyroid dysfunction during the following months,” Dr. Muller emphasized.
Asked to comment, session moderator Robert W. Lash, MD, the Endocrine Society’s chief professional & clinical affairs officer, told this news organization: “When you’re ICU-level sick, it’s not unusual to have weird thyroid tests. Some viruses cause thyroid problems as well ... What makes this different is that while a lot of thyroid inflammation is caused by antibodies, this was not.”
“It looks like this was [SARS-CoV-2] causing damage to the thyroid gland, which is interesting,” he noted, adding that the thyroid gland expresses high levels of angiotensin-converting enzyme 2 (ACE2) and transmembrane protease serine 2 (TMPRSS2), which allow SARS-CoV-2 to infect human cells.
“This is probably part of that same story,” Dr. Lash said.
For patients who had thyroid abnormalities during acute COVID-19 illness or develop symptoms that might be thyroid-related afterward, he advises: “You should keep an eye on thyroid tests. It just raises your awareness ... You might check their thyroid tests every 6 months for a year.”
Signs of focal thyroiditis despite normalized thyroid function
The 51 patients (33 men and 18 women) hospitalized with moderate-to-severe COVID-19 had no history of thyroid disease and had not been taking thyroid medications, amiodarone, or steroids before baseline TSH was measured.
From baseline to 3 months, TSH rose from 1.2 to 1.6 mIU/L, while serum concentrations of T4, T3, C-reactive protein, and full blood counts had all normalized (all P < 0.01 vs. baseline).
Thyroid ultrasound at 3 months in 49 patients showed signs of focal thyroiditis in 16 (33%).
Among 14 patients of those who further underwent thyroid 99mTc or I123 uptake scans, four (29%) were normal, eight (57%) had focally reduced uptake, and two (14%) had diffusely reduced uptake.
Of the 16 patients with focal thyroiditis, only three were positive for autoantibodies to thyroglobulin (TgAb) or thyroid peroxidase (TPOAb). All were negative for autoantibodies to the TSH receptor.
“Importantly, of the two with diffusely reduced uptake, only one was positive for TPOAb or TgAb,” Dr. Muller noted, adding, “SARS-CoV-2 disease seems to trigger some dysfunction which very likely has complex and multifactorial mechanisms.”
In response to a question about a possible role for biopsies and thyroid cytology, Dr. Muller replied: “That’s definitely the key ... So far we’re just making guesses, so the key will be cytological or histological studies to see what is really going on in the thyroid.”
“What we know is that [unlike] classical thyroiditis that has been described after viral diseases including SARS-CoV-2, these patients have a different scenario ... Probably something is going on within the thyroid with a different mechanism, so surely cytology and histology studies are what we need,” she concluded.
The study was funded by Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, and by a COVID-19 research grant from the European Society of Endocrinology. Dr. Muller and Dr. Lash have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Individuals who experience inflammation of the thyroid gland during acute COVID-19 illness may still have subacute thyroiditis months later, even if thyroid function has normalized, new research suggests.
Furthermore, the thyroiditis seems to be different from thyroid inflammation caused by other viruses, said Ilaria Muller, MD, PhD, when presenting her findings March 21 at the virtual ENDO 2021 meeting.
“SARS-CoV-2 seems to have multifactorial action on thyroid function,” said Dr. Muller, of the University of Milan, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Italy.
In July 2020, Dr. Muller and colleagues described patients hospitalized at their institution with severe COVID-19, 15% of whom had thyrotoxicosis due to atypical subacute thyroiditis, compared with just 1% of a comparison group hospitalized in the same subintensive care units during the spring of 2019, as reported by this news organization.
The “atypical” thyroiditis that occurred in the patients with COVID-19 was not associated with neck pain and affected more men than women. Moreover, it was associated with low TSH and free-triiodothyronine (T3) levels, and normal or elevated levels of free-thyroxine (T4), which is a very different presentation to classic nonthyroidal illness syndrome (NTIS) usually seen in critically ill patients, she explained.
Although transient T4 elevations can occur in acute illness, that phenomenon is not associated with low TSH. This newly described scenario appears to be a combination of thyrotoxicosis and NTIS, Dr. Muller and colleagues had speculated last summer.
Follow patients with COVID-19 and thyroid dysfunction for a year
Now, in an assessment of 51 patients 3 months after hospitalization for moderate-to-severe COVID-19 reported by Dr. Muller at ENDO 2021, both inflammatory markers and thyroid function had normalized, yet on imaging, a third of patients still exhibited focal hypoechoic areas suggestive of thyroiditis.
Of those, two-thirds had reduced uptake on thyroid scintigraphy, but few had antithyroid autoantibodies.
“The thyroid dysfunction induced by COVID-19 seems not mediated by autoimmunity. It is important to continue to follow these patients since they might develop thyroid dysfunction during the following months,” Dr. Muller emphasized.
Asked to comment, session moderator Robert W. Lash, MD, the Endocrine Society’s chief professional & clinical affairs officer, told this news organization: “When you’re ICU-level sick, it’s not unusual to have weird thyroid tests. Some viruses cause thyroid problems as well ... What makes this different is that while a lot of thyroid inflammation is caused by antibodies, this was not.”
“It looks like this was [SARS-CoV-2] causing damage to the thyroid gland, which is interesting,” he noted, adding that the thyroid gland expresses high levels of angiotensin-converting enzyme 2 (ACE2) and transmembrane protease serine 2 (TMPRSS2), which allow SARS-CoV-2 to infect human cells.
“This is probably part of that same story,” Dr. Lash said.
For patients who had thyroid abnormalities during acute COVID-19 illness or develop symptoms that might be thyroid-related afterward, he advises: “You should keep an eye on thyroid tests. It just raises your awareness ... You might check their thyroid tests every 6 months for a year.”
Signs of focal thyroiditis despite normalized thyroid function
The 51 patients (33 men and 18 women) hospitalized with moderate-to-severe COVID-19 had no history of thyroid disease and had not been taking thyroid medications, amiodarone, or steroids before baseline TSH was measured.
From baseline to 3 months, TSH rose from 1.2 to 1.6 mIU/L, while serum concentrations of T4, T3, C-reactive protein, and full blood counts had all normalized (all P < 0.01 vs. baseline).
Thyroid ultrasound at 3 months in 49 patients showed signs of focal thyroiditis in 16 (33%).
Among 14 patients of those who further underwent thyroid 99mTc or I123 uptake scans, four (29%) were normal, eight (57%) had focally reduced uptake, and two (14%) had diffusely reduced uptake.
Of the 16 patients with focal thyroiditis, only three were positive for autoantibodies to thyroglobulin (TgAb) or thyroid peroxidase (TPOAb). All were negative for autoantibodies to the TSH receptor.
“Importantly, of the two with diffusely reduced uptake, only one was positive for TPOAb or TgAb,” Dr. Muller noted, adding, “SARS-CoV-2 disease seems to trigger some dysfunction which very likely has complex and multifactorial mechanisms.”
In response to a question about a possible role for biopsies and thyroid cytology, Dr. Muller replied: “That’s definitely the key ... So far we’re just making guesses, so the key will be cytological or histological studies to see what is really going on in the thyroid.”
“What we know is that [unlike] classical thyroiditis that has been described after viral diseases including SARS-CoV-2, these patients have a different scenario ... Probably something is going on within the thyroid with a different mechanism, so surely cytology and histology studies are what we need,” she concluded.
The study was funded by Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, and by a COVID-19 research grant from the European Society of Endocrinology. Dr. Muller and Dr. Lash have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Less sleep, more burnout linked to higher COVID-19 risk, study shows
among health care workers considered to be at high risk for exposure to patients with COVID-19, new evidence reveals.
For each additional hour of sleep at night, for example, risk for COVID-19 dropped by 12% in a study of 2844 frontline health care workers.
Furthermore, those who reported experiencing work-related burnout every day were 2.6 times more likely to report having COVID-19, to report having COVID-19 for a longer time, and to experience COVID-19 of more severity.
“This study underscores the importance of non–hygiene-related risk factors for COVID-19 and supports a holistic approach to health – including optimal sleep and job stress reduction to protect our health care workers from this and future pandemics,” senior author Sara B. Seidelmann, MD, said in an interview.
“Our findings add to the literature that sleep duration at night, sleep problems, and burnout may be risk factors for viral illnesses like COVID-19,” wrote Dr. Seidelmann and colleagues.
This is the first study to link COVID-19 risk to sleep habits – including number of hours of sleep at night, daytime napping hours, and severe sleep problems – among health care workers across multiple countries.
The study was published online March 22 in BMJ Nutrition, Prevention, and Health.
The researchers surveyed health care professionals in specialties considered to place personnel at high risk for exposure to SARS-CoV-2: critical care, emergency care, and internal medicine.
The association between sleep and burnout risk factors and COVID-19 did not vary significantly by specialty. “We didn’t detect any significant interactions between age, sex, specialty, or country,” said Dr. Seidelmann, assistant professor of clinical medicine at Columbia University College of Physicians and Surgeons, New York, and an internist at Stamford (Conn.) Hospital.
In addition to the 12% lower risk associated with each additional hour of sleep at night, each 1 additional hour of daytime napping was linked with a 6% increased risk for COVID-19 in an adjusted analysis (odds ratio [OR], 1.06; 95% confidence interval [CI], 1.01-1.12).
Daytime napping slightly increased risk for COVID-19 in five of the six countries included in the study: France, Germany, Italy, the United Kingdom, and the United States. In contrast, in Spain, napping had a nonsignificant protective effect.
The survey asked health care workers to recall nighttime sleep duration, sleep disorders, and burnout in the year prior to onset of the COVID-19 pandemic.
‘Significant, close contact’ with COVID-19?
Lead author Hyunju Kim, NP, Dr. Seidelmann, and colleagues conducted the population-based, case-control study from July 17 to Sept. 25, 2020. They identified health care workers from the SurveyHealthcareGlobus (SHG) network.
Of the respondents, 72% were men. The mean age of the participants was 48 years, and the study population was 77% White, 12% Asian, 6% mixed background, 2% Black, and 1% other. (The remainder preferred not to say).
The 568 health care workers considered to have COVID-19 were classified on the basis of self-reported symptoms. Control participants had no symptoms associated with COVID-19.
All 2,844 participants answered yes to a question about having “significant close contact” with COVID-19 patients in their workplace.
Compared to reporting no sleep problems, having three such problems – difficulty sleeping at night, poor sleep continuity, and frequent use of sleeping pills – was associated with 88% greater odds of COVID-19 (OR, 1.88; 95% CI, 1.17–3.01).
Having one sleep problem was not associated with COVID-19.
More burnout, greater risk
The health care workers reported the severity of any work-related burnout. “There was a significant dose-response relationship between frequency of burnout and COVID-19,” the researchers noted.
Those who reported having burnout rarely or weekly had a 1.3-1.4 greater chance of reporting COVID-19 compared to those who reported having no burnout, for example.
In addition, reporting a high level of burnout was linked to about three times the risk for having COVID-19 of longer duration and of greater severity.
What drives the association between sleep problems, burnout, and higher risk for COVID-19 and severe COVID-19 remains unknown.
“The mechanism underlying these associations isn’t clear, but suboptimal sleep, sleep disorders, and stress may result in immune system dysregulation, increased inflammation, and alterations in hormones such as cortisol and melatonin that may increase vulnerability to viral infections,” Dr. Seidelmann said.
Strengths and limitations
Using a large network of health care workers in the early phase of the pandemic is a strength of the study. How generalizable the findings are outside the SHG database of 1.5 million health care workers remains unknown.
Another limitation was reliance on self-reporting of COVID-19 patient exposure, outcomes, and covariates, which could have introduced bias.
“However,” the researchers noted, “health care workers are likely a reliable source of information.”
Insomnia a common challenge
A 2020 meta-analysis examined the effect of insomnia and psychological factors on COVID-19 risk among health care workers. Lead author Kavita Batra, PhD, of the University of Nevada, Las Vegas (UNLV), and colleagues found that the pooled prevalence of insomnia was almost 28%.
“The recent six-country study by Kim and colleagues also underscores this relationship between lack of sleep and having higher odds of COVID-19 infection,” Manoj Sharma, MBBS, PhD, professor of social and behavioral health in the UNLV department of environmental and occupational health, and one of the study authors, said in an interview.
More research is warranted to learn the direction of the association, he said. Does reduced sleep lower immunity and make a health care worker more susceptible to SARS-CoV-2 infection, or does the anxiety associated with COVID-19 contribute to insomnia?
“Practicing sleep hygiene is a must not only for health workers but also for everyone,” Dr. Sharma added. Recommendations include having fixed hours of going to bed, fixed hours of waking up, not overdoing naps, having at least 30 minutes of winding down before sleeping, having a dark bedroom devoid of all electronics and other disturbances, avoiding smoking, alcohol, and stimulants (such as caffeine) before sleeping, and practicing relaxation right before sleeping, he said.
“It is hard for some health care workers, especially those who work night shifts, but it must be a priority to follow as many sleep hygiene measures as possible,” Dr. Sharma said. “After all, if you do not take care of yourself how can you take care of others?”
Dr. Seidelmann, Dr. Batra, and Dr. Sharma have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
among health care workers considered to be at high risk for exposure to patients with COVID-19, new evidence reveals.
For each additional hour of sleep at night, for example, risk for COVID-19 dropped by 12% in a study of 2844 frontline health care workers.
Furthermore, those who reported experiencing work-related burnout every day were 2.6 times more likely to report having COVID-19, to report having COVID-19 for a longer time, and to experience COVID-19 of more severity.
“This study underscores the importance of non–hygiene-related risk factors for COVID-19 and supports a holistic approach to health – including optimal sleep and job stress reduction to protect our health care workers from this and future pandemics,” senior author Sara B. Seidelmann, MD, said in an interview.
“Our findings add to the literature that sleep duration at night, sleep problems, and burnout may be risk factors for viral illnesses like COVID-19,” wrote Dr. Seidelmann and colleagues.
This is the first study to link COVID-19 risk to sleep habits – including number of hours of sleep at night, daytime napping hours, and severe sleep problems – among health care workers across multiple countries.
The study was published online March 22 in BMJ Nutrition, Prevention, and Health.
The researchers surveyed health care professionals in specialties considered to place personnel at high risk for exposure to SARS-CoV-2: critical care, emergency care, and internal medicine.
The association between sleep and burnout risk factors and COVID-19 did not vary significantly by specialty. “We didn’t detect any significant interactions between age, sex, specialty, or country,” said Dr. Seidelmann, assistant professor of clinical medicine at Columbia University College of Physicians and Surgeons, New York, and an internist at Stamford (Conn.) Hospital.
In addition to the 12% lower risk associated with each additional hour of sleep at night, each 1 additional hour of daytime napping was linked with a 6% increased risk for COVID-19 in an adjusted analysis (odds ratio [OR], 1.06; 95% confidence interval [CI], 1.01-1.12).
Daytime napping slightly increased risk for COVID-19 in five of the six countries included in the study: France, Germany, Italy, the United Kingdom, and the United States. In contrast, in Spain, napping had a nonsignificant protective effect.
The survey asked health care workers to recall nighttime sleep duration, sleep disorders, and burnout in the year prior to onset of the COVID-19 pandemic.
‘Significant, close contact’ with COVID-19?
Lead author Hyunju Kim, NP, Dr. Seidelmann, and colleagues conducted the population-based, case-control study from July 17 to Sept. 25, 2020. They identified health care workers from the SurveyHealthcareGlobus (SHG) network.
Of the respondents, 72% were men. The mean age of the participants was 48 years, and the study population was 77% White, 12% Asian, 6% mixed background, 2% Black, and 1% other. (The remainder preferred not to say).
The 568 health care workers considered to have COVID-19 were classified on the basis of self-reported symptoms. Control participants had no symptoms associated with COVID-19.
All 2,844 participants answered yes to a question about having “significant close contact” with COVID-19 patients in their workplace.
Compared to reporting no sleep problems, having three such problems – difficulty sleeping at night, poor sleep continuity, and frequent use of sleeping pills – was associated with 88% greater odds of COVID-19 (OR, 1.88; 95% CI, 1.17–3.01).
Having one sleep problem was not associated with COVID-19.
More burnout, greater risk
The health care workers reported the severity of any work-related burnout. “There was a significant dose-response relationship between frequency of burnout and COVID-19,” the researchers noted.
Those who reported having burnout rarely or weekly had a 1.3-1.4 greater chance of reporting COVID-19 compared to those who reported having no burnout, for example.
In addition, reporting a high level of burnout was linked to about three times the risk for having COVID-19 of longer duration and of greater severity.
What drives the association between sleep problems, burnout, and higher risk for COVID-19 and severe COVID-19 remains unknown.
“The mechanism underlying these associations isn’t clear, but suboptimal sleep, sleep disorders, and stress may result in immune system dysregulation, increased inflammation, and alterations in hormones such as cortisol and melatonin that may increase vulnerability to viral infections,” Dr. Seidelmann said.
Strengths and limitations
Using a large network of health care workers in the early phase of the pandemic is a strength of the study. How generalizable the findings are outside the SHG database of 1.5 million health care workers remains unknown.
Another limitation was reliance on self-reporting of COVID-19 patient exposure, outcomes, and covariates, which could have introduced bias.
“However,” the researchers noted, “health care workers are likely a reliable source of information.”
Insomnia a common challenge
A 2020 meta-analysis examined the effect of insomnia and psychological factors on COVID-19 risk among health care workers. Lead author Kavita Batra, PhD, of the University of Nevada, Las Vegas (UNLV), and colleagues found that the pooled prevalence of insomnia was almost 28%.
“The recent six-country study by Kim and colleagues also underscores this relationship between lack of sleep and having higher odds of COVID-19 infection,” Manoj Sharma, MBBS, PhD, professor of social and behavioral health in the UNLV department of environmental and occupational health, and one of the study authors, said in an interview.
More research is warranted to learn the direction of the association, he said. Does reduced sleep lower immunity and make a health care worker more susceptible to SARS-CoV-2 infection, or does the anxiety associated with COVID-19 contribute to insomnia?
“Practicing sleep hygiene is a must not only for health workers but also for everyone,” Dr. Sharma added. Recommendations include having fixed hours of going to bed, fixed hours of waking up, not overdoing naps, having at least 30 minutes of winding down before sleeping, having a dark bedroom devoid of all electronics and other disturbances, avoiding smoking, alcohol, and stimulants (such as caffeine) before sleeping, and practicing relaxation right before sleeping, he said.
“It is hard for some health care workers, especially those who work night shifts, but it must be a priority to follow as many sleep hygiene measures as possible,” Dr. Sharma said. “After all, if you do not take care of yourself how can you take care of others?”
Dr. Seidelmann, Dr. Batra, and Dr. Sharma have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
among health care workers considered to be at high risk for exposure to patients with COVID-19, new evidence reveals.
For each additional hour of sleep at night, for example, risk for COVID-19 dropped by 12% in a study of 2844 frontline health care workers.
Furthermore, those who reported experiencing work-related burnout every day were 2.6 times more likely to report having COVID-19, to report having COVID-19 for a longer time, and to experience COVID-19 of more severity.
“This study underscores the importance of non–hygiene-related risk factors for COVID-19 and supports a holistic approach to health – including optimal sleep and job stress reduction to protect our health care workers from this and future pandemics,” senior author Sara B. Seidelmann, MD, said in an interview.
“Our findings add to the literature that sleep duration at night, sleep problems, and burnout may be risk factors for viral illnesses like COVID-19,” wrote Dr. Seidelmann and colleagues.
This is the first study to link COVID-19 risk to sleep habits – including number of hours of sleep at night, daytime napping hours, and severe sleep problems – among health care workers across multiple countries.
The study was published online March 22 in BMJ Nutrition, Prevention, and Health.
The researchers surveyed health care professionals in specialties considered to place personnel at high risk for exposure to SARS-CoV-2: critical care, emergency care, and internal medicine.
The association between sleep and burnout risk factors and COVID-19 did not vary significantly by specialty. “We didn’t detect any significant interactions between age, sex, specialty, or country,” said Dr. Seidelmann, assistant professor of clinical medicine at Columbia University College of Physicians and Surgeons, New York, and an internist at Stamford (Conn.) Hospital.
In addition to the 12% lower risk associated with each additional hour of sleep at night, each 1 additional hour of daytime napping was linked with a 6% increased risk for COVID-19 in an adjusted analysis (odds ratio [OR], 1.06; 95% confidence interval [CI], 1.01-1.12).
Daytime napping slightly increased risk for COVID-19 in five of the six countries included in the study: France, Germany, Italy, the United Kingdom, and the United States. In contrast, in Spain, napping had a nonsignificant protective effect.
The survey asked health care workers to recall nighttime sleep duration, sleep disorders, and burnout in the year prior to onset of the COVID-19 pandemic.
‘Significant, close contact’ with COVID-19?
Lead author Hyunju Kim, NP, Dr. Seidelmann, and colleagues conducted the population-based, case-control study from July 17 to Sept. 25, 2020. They identified health care workers from the SurveyHealthcareGlobus (SHG) network.
Of the respondents, 72% were men. The mean age of the participants was 48 years, and the study population was 77% White, 12% Asian, 6% mixed background, 2% Black, and 1% other. (The remainder preferred not to say).
The 568 health care workers considered to have COVID-19 were classified on the basis of self-reported symptoms. Control participants had no symptoms associated with COVID-19.
All 2,844 participants answered yes to a question about having “significant close contact” with COVID-19 patients in their workplace.
Compared to reporting no sleep problems, having three such problems – difficulty sleeping at night, poor sleep continuity, and frequent use of sleeping pills – was associated with 88% greater odds of COVID-19 (OR, 1.88; 95% CI, 1.17–3.01).
Having one sleep problem was not associated with COVID-19.
More burnout, greater risk
The health care workers reported the severity of any work-related burnout. “There was a significant dose-response relationship between frequency of burnout and COVID-19,” the researchers noted.
Those who reported having burnout rarely or weekly had a 1.3-1.4 greater chance of reporting COVID-19 compared to those who reported having no burnout, for example.
In addition, reporting a high level of burnout was linked to about three times the risk for having COVID-19 of longer duration and of greater severity.
What drives the association between sleep problems, burnout, and higher risk for COVID-19 and severe COVID-19 remains unknown.
“The mechanism underlying these associations isn’t clear, but suboptimal sleep, sleep disorders, and stress may result in immune system dysregulation, increased inflammation, and alterations in hormones such as cortisol and melatonin that may increase vulnerability to viral infections,” Dr. Seidelmann said.
Strengths and limitations
Using a large network of health care workers in the early phase of the pandemic is a strength of the study. How generalizable the findings are outside the SHG database of 1.5 million health care workers remains unknown.
Another limitation was reliance on self-reporting of COVID-19 patient exposure, outcomes, and covariates, which could have introduced bias.
“However,” the researchers noted, “health care workers are likely a reliable source of information.”
Insomnia a common challenge
A 2020 meta-analysis examined the effect of insomnia and psychological factors on COVID-19 risk among health care workers. Lead author Kavita Batra, PhD, of the University of Nevada, Las Vegas (UNLV), and colleagues found that the pooled prevalence of insomnia was almost 28%.
“The recent six-country study by Kim and colleagues also underscores this relationship between lack of sleep and having higher odds of COVID-19 infection,” Manoj Sharma, MBBS, PhD, professor of social and behavioral health in the UNLV department of environmental and occupational health, and one of the study authors, said in an interview.
More research is warranted to learn the direction of the association, he said. Does reduced sleep lower immunity and make a health care worker more susceptible to SARS-CoV-2 infection, or does the anxiety associated with COVID-19 contribute to insomnia?
“Practicing sleep hygiene is a must not only for health workers but also for everyone,” Dr. Sharma added. Recommendations include having fixed hours of going to bed, fixed hours of waking up, not overdoing naps, having at least 30 minutes of winding down before sleeping, having a dark bedroom devoid of all electronics and other disturbances, avoiding smoking, alcohol, and stimulants (such as caffeine) before sleeping, and practicing relaxation right before sleeping, he said.
“It is hard for some health care workers, especially those who work night shifts, but it must be a priority to follow as many sleep hygiene measures as possible,” Dr. Sharma said. “After all, if you do not take care of yourself how can you take care of others?”
Dr. Seidelmann, Dr. Batra, and Dr. Sharma have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Here we go again? Rate of COVID-19 in children takes a turn for the worse
After declining for 8 consecutive weeks, new cases of COVID-19 rose among children in the United States, according to the American Academy of Pediatrics and the Children’s Hospital Association.

, ending a streak of declines going back to mid-January, the AAP and CHA said in their weekly COVID-19 report.
Also up for the week was the proportion of all cases occurring in children. The 57,000-plus cases represented 18.7% of the total (304,610) for all ages, and that is the largest share of the new-case burden for the entire pandemic. The previous high, 18.0%, came just 2 weeks earlier, based on data collected from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
Speaking of the entire pandemic, the total number of COVID-19 cases in children is over 3.34 million, and that represents 13.3% of cases among all ages in the United States. The cumulative rate of infection as of March 18 was 4,440 cases per 100,000 children, up from 4,364 per 100,000 a week earlier, the AAP and CHA said.
At the state level, Vermont has now passed the 20% mark (20.1%, to be exact) for children’s proportion of cases and is higher in that measure than any other state. The highest rate of infection (8,763 cases per 100,000) can be found in North Dakota, the AAP/CHA data show.
There were only two new coronavirus-related deaths during the week of March 12-18 after Kansas revised its mortality data, bringing the total to 268 in the 46 jurisdictions (43 states, New York City, Puerto Rico, and Guam) that are reporting deaths by age, the AAP and CHA said.
After declining for 8 consecutive weeks, new cases of COVID-19 rose among children in the United States, according to the American Academy of Pediatrics and the Children’s Hospital Association.

, ending a streak of declines going back to mid-January, the AAP and CHA said in their weekly COVID-19 report.
Also up for the week was the proportion of all cases occurring in children. The 57,000-plus cases represented 18.7% of the total (304,610) for all ages, and that is the largest share of the new-case burden for the entire pandemic. The previous high, 18.0%, came just 2 weeks earlier, based on data collected from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
Speaking of the entire pandemic, the total number of COVID-19 cases in children is over 3.34 million, and that represents 13.3% of cases among all ages in the United States. The cumulative rate of infection as of March 18 was 4,440 cases per 100,000 children, up from 4,364 per 100,000 a week earlier, the AAP and CHA said.
At the state level, Vermont has now passed the 20% mark (20.1%, to be exact) for children’s proportion of cases and is higher in that measure than any other state. The highest rate of infection (8,763 cases per 100,000) can be found in North Dakota, the AAP/CHA data show.
There were only two new coronavirus-related deaths during the week of March 12-18 after Kansas revised its mortality data, bringing the total to 268 in the 46 jurisdictions (43 states, New York City, Puerto Rico, and Guam) that are reporting deaths by age, the AAP and CHA said.
After declining for 8 consecutive weeks, new cases of COVID-19 rose among children in the United States, according to the American Academy of Pediatrics and the Children’s Hospital Association.

, ending a streak of declines going back to mid-January, the AAP and CHA said in their weekly COVID-19 report.
Also up for the week was the proportion of all cases occurring in children. The 57,000-plus cases represented 18.7% of the total (304,610) for all ages, and that is the largest share of the new-case burden for the entire pandemic. The previous high, 18.0%, came just 2 weeks earlier, based on data collected from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
Speaking of the entire pandemic, the total number of COVID-19 cases in children is over 3.34 million, and that represents 13.3% of cases among all ages in the United States. The cumulative rate of infection as of March 18 was 4,440 cases per 100,000 children, up from 4,364 per 100,000 a week earlier, the AAP and CHA said.
At the state level, Vermont has now passed the 20% mark (20.1%, to be exact) for children’s proportion of cases and is higher in that measure than any other state. The highest rate of infection (8,763 cases per 100,000) can be found in North Dakota, the AAP/CHA data show.
There were only two new coronavirus-related deaths during the week of March 12-18 after Kansas revised its mortality data, bringing the total to 268 in the 46 jurisdictions (43 states, New York City, Puerto Rico, and Guam) that are reporting deaths by age, the AAP and CHA said.
Black nonsmokers still at high risk for secondhand smoke exposure
Despite 30+ years of antismoking public policies and dramatic overall decline in secondhand smoke (SHS) exposure, .
No risk-free SHS exposure
Surendranath S. Shastri, MD, of MD Anderson Cancer Center, Houston, and colleagues underscored the U.S. Surgeon General’s determination that there is no risk-free level of SHS exposure in a recent JAMA Internal Medicine Research Letter.
“With the outbreak of the coronavirus disease 2019, which affects lung function, improving smoke-free policies to enhance air quality should be a growing priority,”they wrote.
Dr. Shastri and colleagues looked at 2011-2018 data from the National Health and Nutrition Examination Survey (NHANES), which detailed prevalence of SHS exposure in the U.S. population aged 3 years and older using interviews and biological specimens to test for cotinine levels. For the survey, nonsmokers having serum cotinine levels of 0.05 to 10 ng/mL were considered to have SHS exposure.
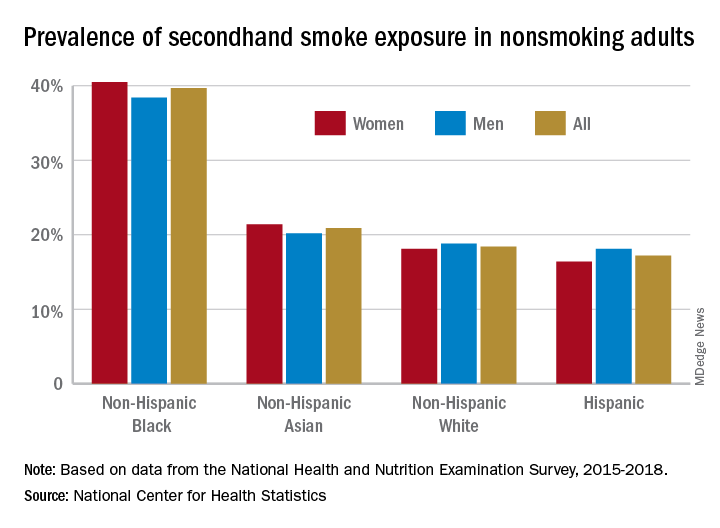
While the prevalence of SHS exposure among nonsmokers declined from 87.5% to 25.3% between 1988 and 2012, levels have stagnated since 2012 and racial and economic disparities are evident. Higher smoking rates, less knowledge about health risks, higher workplace exposure, greater likelihood of living in low-income, multi-unit housing, plus having their communities targeted by tobacco companies, may all help explain higher serum levels of cotinine in populations with lower socioeconomic status.
“Multivariable logistic regression identified younger age (odds ratio [OR], 1.88, for 12-19 years, and OR, 2.29, for 3-11 years), non-Hispanic Black race/ethnicity (OR, 2.75), less than high school education (OR, 1.59), and living below the poverty level (OR, 2.61) as risk factors for SHSe in the 2017-2018 cycle, with little change across all data cycles,” the researchers wrote.
Disparities in SHS exposure
A second report from NHANES data for 2015-2018, published in a National Center for Health Statistics Data Brief (No. 396, February 2021) showed that 20.8% of nonsmoking U.S. adults had SHS exposure, again with greater prevalence among non-Hispanic Black adults (39.7%), than for non-Hispanic White (18.4%), non-Hispanic Asian (20.9%), and Hispanic (17.2%) adults. Exposure was also greater in the younger age groups, with SHS rates for adults aged 18-39 years, 40-59 years, and ≥60 years at 25.6%, 19.1%, and 17.6%, respectively. Lower education (high school or less vs. some college education) and lower income levels were also associated with higher levels of SHS exposure. The investigators noted that among households with smokers, non-Hispanic Black adults are less likely to have complete smoking bans in homes, and among Medicaid or uninsured parents of any race or ethnicity, bans on smoking in family vehicles are less likely.
Overall, the prevalence of SHS exposure declined from 27.7% to 20.7% from 2009 to 2018, but the decreases were mediated by race and income.
SHS exposure in private spaces
A research brief from the Centers for Disease Control and Prevention on SHS exposure in homes and vehicles in the U.S. among middle and high school students also found a general decline in SHS exposure over 2011-2018 in homes (26.8%-20.9%; P < .001) and vehicles (30.2%-19.8%; P < .001). The findings, derived from the National Youth Tobacco Survey for 2011-2019, showed that no reduction occurred in homes among non-Hispanic Black students. Overall, a significant difference in home SHS exposure was observed by race/ethnicity: non-Hispanic Black (28.4%) and non-Hispanic White (27.4%) students both had a higher prevalence compared with Hispanic (20.0%) and non-Hispanic other (20.2%) students (P < .001).
Progress in reducing SHS exposure in public spaces has been made over the last 2 decades, with 27 states and more than 1,000 municipalities implementing comprehensive smoke-free laws that prohibit smoking in indoor public places, including workplaces, restaurants, and bars. While the prevalence of voluntary smoke-free home (83.7%) and vehicle (78.1%) rules has increased over time, private settings remain major sources of SHS exposure for many people, including youths. “Although SHS exposures have declined,” the authors wrote, “more than 6 million young people remain exposed to SHS in these private settings.”
In reviewing the data, Mary Cataletto, MD, FCCP, clinical professor of pediatrics at NYU Long Island School of Medicine, stated that these studies “highlight the need for implementation of smoke-free policies to reduce exposure to secondhand smoke, especially in homes and cars and with focused advocacy efforts in highly affected communities.”
Panagis Galiatsatos, MD, MHS, assistant professor of medicine at Johns Hopkins University, Baltimore, emphasized implementation of smoke-free policies but also treatment for smokers. “I’m not at all surprised by these statistics,” he noted in an interview. “Public health policies have helped us to get to where we are now, but there’s a reason that we have plateaued over the last decade. It’s hard to mitigate secondhand smoke exposure because the ones who are smoking now are the most refractory, challenging cases. ... You need good clinical interventions with counseling supported by pharmacological agents to help them if you want to stop secondhand smoke exposure.” He added, “You have to look at current smokers no differently than you look at patients with stage IV cancer – a group that requires a lot of resources to help them get through. Remember, all of them want to quit, but the promise of well-designed, precision-medicine strategies to help them quit has not been kept. Public health policy isn’t going to do it. We need to manage these patients clinically.”
The investigators had no conflict disclosures.
Despite 30+ years of antismoking public policies and dramatic overall decline in secondhand smoke (SHS) exposure, .
No risk-free SHS exposure
Surendranath S. Shastri, MD, of MD Anderson Cancer Center, Houston, and colleagues underscored the U.S. Surgeon General’s determination that there is no risk-free level of SHS exposure in a recent JAMA Internal Medicine Research Letter.
“With the outbreak of the coronavirus disease 2019, which affects lung function, improving smoke-free policies to enhance air quality should be a growing priority,”they wrote.
Dr. Shastri and colleagues looked at 2011-2018 data from the National Health and Nutrition Examination Survey (NHANES), which detailed prevalence of SHS exposure in the U.S. population aged 3 years and older using interviews and biological specimens to test for cotinine levels. For the survey, nonsmokers having serum cotinine levels of 0.05 to 10 ng/mL were considered to have SHS exposure.

While the prevalence of SHS exposure among nonsmokers declined from 87.5% to 25.3% between 1988 and 2012, levels have stagnated since 2012 and racial and economic disparities are evident. Higher smoking rates, less knowledge about health risks, higher workplace exposure, greater likelihood of living in low-income, multi-unit housing, plus having their communities targeted by tobacco companies, may all help explain higher serum levels of cotinine in populations with lower socioeconomic status.
“Multivariable logistic regression identified younger age (odds ratio [OR], 1.88, for 12-19 years, and OR, 2.29, for 3-11 years), non-Hispanic Black race/ethnicity (OR, 2.75), less than high school education (OR, 1.59), and living below the poverty level (OR, 2.61) as risk factors for SHSe in the 2017-2018 cycle, with little change across all data cycles,” the researchers wrote.
Disparities in SHS exposure
A second report from NHANES data for 2015-2018, published in a National Center for Health Statistics Data Brief (No. 396, February 2021) showed that 20.8% of nonsmoking U.S. adults had SHS exposure, again with greater prevalence among non-Hispanic Black adults (39.7%), than for non-Hispanic White (18.4%), non-Hispanic Asian (20.9%), and Hispanic (17.2%) adults. Exposure was also greater in the younger age groups, with SHS rates for adults aged 18-39 years, 40-59 years, and ≥60 years at 25.6%, 19.1%, and 17.6%, respectively. Lower education (high school or less vs. some college education) and lower income levels were also associated with higher levels of SHS exposure. The investigators noted that among households with smokers, non-Hispanic Black adults are less likely to have complete smoking bans in homes, and among Medicaid or uninsured parents of any race or ethnicity, bans on smoking in family vehicles are less likely.
Overall, the prevalence of SHS exposure declined from 27.7% to 20.7% from 2009 to 2018, but the decreases were mediated by race and income.
SHS exposure in private spaces
A research brief from the Centers for Disease Control and Prevention on SHS exposure in homes and vehicles in the U.S. among middle and high school students also found a general decline in SHS exposure over 2011-2018 in homes (26.8%-20.9%; P < .001) and vehicles (30.2%-19.8%; P < .001). The findings, derived from the National Youth Tobacco Survey for 2011-2019, showed that no reduction occurred in homes among non-Hispanic Black students. Overall, a significant difference in home SHS exposure was observed by race/ethnicity: non-Hispanic Black (28.4%) and non-Hispanic White (27.4%) students both had a higher prevalence compared with Hispanic (20.0%) and non-Hispanic other (20.2%) students (P < .001).
Progress in reducing SHS exposure in public spaces has been made over the last 2 decades, with 27 states and more than 1,000 municipalities implementing comprehensive smoke-free laws that prohibit smoking in indoor public places, including workplaces, restaurants, and bars. While the prevalence of voluntary smoke-free home (83.7%) and vehicle (78.1%) rules has increased over time, private settings remain major sources of SHS exposure for many people, including youths. “Although SHS exposures have declined,” the authors wrote, “more than 6 million young people remain exposed to SHS in these private settings.”
In reviewing the data, Mary Cataletto, MD, FCCP, clinical professor of pediatrics at NYU Long Island School of Medicine, stated that these studies “highlight the need for implementation of smoke-free policies to reduce exposure to secondhand smoke, especially in homes and cars and with focused advocacy efforts in highly affected communities.”
Panagis Galiatsatos, MD, MHS, assistant professor of medicine at Johns Hopkins University, Baltimore, emphasized implementation of smoke-free policies but also treatment for smokers. “I’m not at all surprised by these statistics,” he noted in an interview. “Public health policies have helped us to get to where we are now, but there’s a reason that we have plateaued over the last decade. It’s hard to mitigate secondhand smoke exposure because the ones who are smoking now are the most refractory, challenging cases. ... You need good clinical interventions with counseling supported by pharmacological agents to help them if you want to stop secondhand smoke exposure.” He added, “You have to look at current smokers no differently than you look at patients with stage IV cancer – a group that requires a lot of resources to help them get through. Remember, all of them want to quit, but the promise of well-designed, precision-medicine strategies to help them quit has not been kept. Public health policy isn’t going to do it. We need to manage these patients clinically.”
The investigators had no conflict disclosures.
Despite 30+ years of antismoking public policies and dramatic overall decline in secondhand smoke (SHS) exposure, .
No risk-free SHS exposure
Surendranath S. Shastri, MD, of MD Anderson Cancer Center, Houston, and colleagues underscored the U.S. Surgeon General’s determination that there is no risk-free level of SHS exposure in a recent JAMA Internal Medicine Research Letter.
“With the outbreak of the coronavirus disease 2019, which affects lung function, improving smoke-free policies to enhance air quality should be a growing priority,”they wrote.
Dr. Shastri and colleagues looked at 2011-2018 data from the National Health and Nutrition Examination Survey (NHANES), which detailed prevalence of SHS exposure in the U.S. population aged 3 years and older using interviews and biological specimens to test for cotinine levels. For the survey, nonsmokers having serum cotinine levels of 0.05 to 10 ng/mL were considered to have SHS exposure.

While the prevalence of SHS exposure among nonsmokers declined from 87.5% to 25.3% between 1988 and 2012, levels have stagnated since 2012 and racial and economic disparities are evident. Higher smoking rates, less knowledge about health risks, higher workplace exposure, greater likelihood of living in low-income, multi-unit housing, plus having their communities targeted by tobacco companies, may all help explain higher serum levels of cotinine in populations with lower socioeconomic status.
“Multivariable logistic regression identified younger age (odds ratio [OR], 1.88, for 12-19 years, and OR, 2.29, for 3-11 years), non-Hispanic Black race/ethnicity (OR, 2.75), less than high school education (OR, 1.59), and living below the poverty level (OR, 2.61) as risk factors for SHSe in the 2017-2018 cycle, with little change across all data cycles,” the researchers wrote.
Disparities in SHS exposure
A second report from NHANES data for 2015-2018, published in a National Center for Health Statistics Data Brief (No. 396, February 2021) showed that 20.8% of nonsmoking U.S. adults had SHS exposure, again with greater prevalence among non-Hispanic Black adults (39.7%), than for non-Hispanic White (18.4%), non-Hispanic Asian (20.9%), and Hispanic (17.2%) adults. Exposure was also greater in the younger age groups, with SHS rates for adults aged 18-39 years, 40-59 years, and ≥60 years at 25.6%, 19.1%, and 17.6%, respectively. Lower education (high school or less vs. some college education) and lower income levels were also associated with higher levels of SHS exposure. The investigators noted that among households with smokers, non-Hispanic Black adults are less likely to have complete smoking bans in homes, and among Medicaid or uninsured parents of any race or ethnicity, bans on smoking in family vehicles are less likely.
Overall, the prevalence of SHS exposure declined from 27.7% to 20.7% from 2009 to 2018, but the decreases were mediated by race and income.
SHS exposure in private spaces
A research brief from the Centers for Disease Control and Prevention on SHS exposure in homes and vehicles in the U.S. among middle and high school students also found a general decline in SHS exposure over 2011-2018 in homes (26.8%-20.9%; P < .001) and vehicles (30.2%-19.8%; P < .001). The findings, derived from the National Youth Tobacco Survey for 2011-2019, showed that no reduction occurred in homes among non-Hispanic Black students. Overall, a significant difference in home SHS exposure was observed by race/ethnicity: non-Hispanic Black (28.4%) and non-Hispanic White (27.4%) students both had a higher prevalence compared with Hispanic (20.0%) and non-Hispanic other (20.2%) students (P < .001).
Progress in reducing SHS exposure in public spaces has been made over the last 2 decades, with 27 states and more than 1,000 municipalities implementing comprehensive smoke-free laws that prohibit smoking in indoor public places, including workplaces, restaurants, and bars. While the prevalence of voluntary smoke-free home (83.7%) and vehicle (78.1%) rules has increased over time, private settings remain major sources of SHS exposure for many people, including youths. “Although SHS exposures have declined,” the authors wrote, “more than 6 million young people remain exposed to SHS in these private settings.”
In reviewing the data, Mary Cataletto, MD, FCCP, clinical professor of pediatrics at NYU Long Island School of Medicine, stated that these studies “highlight the need for implementation of smoke-free policies to reduce exposure to secondhand smoke, especially in homes and cars and with focused advocacy efforts in highly affected communities.”
Panagis Galiatsatos, MD, MHS, assistant professor of medicine at Johns Hopkins University, Baltimore, emphasized implementation of smoke-free policies but also treatment for smokers. “I’m not at all surprised by these statistics,” he noted in an interview. “Public health policies have helped us to get to where we are now, but there’s a reason that we have plateaued over the last decade. It’s hard to mitigate secondhand smoke exposure because the ones who are smoking now are the most refractory, challenging cases. ... You need good clinical interventions with counseling supported by pharmacological agents to help them if you want to stop secondhand smoke exposure.” He added, “You have to look at current smokers no differently than you look at patients with stage IV cancer – a group that requires a lot of resources to help them get through. Remember, all of them want to quit, but the promise of well-designed, precision-medicine strategies to help them quit has not been kept. Public health policy isn’t going to do it. We need to manage these patients clinically.”
The investigators had no conflict disclosures.
Melatonin not recommended for early-stage NSCLC
There was a hint of benefit with melatonin among patients with stage III/IV NSCLC. These patients had a hazard reduction of 25% in 5-year DFS. However, the median DFS for patients with advanced disease was the same whether they received melatonin or placebo – 18 months.
In the overall study population, melatonin had no beneficial effects on quality of life, sleep, anxiety, depression, pain, or fatigue, and it did not reduce adverse events from chemotherapy or radiation.
These results were reported in EClinicalMedicine.
“In light of the results, we do not recommend the inclusion of adjuvant melatonin for patients with early-stage NSCLC. Evidence suggests there may be a benefit for those with late-stage disease,” the authors wrote. “However, because of the mixed findings observed, we recommend a follow-up randomized, controlled trial involving a larger population focusing on later-stage resected lung cancer to clarify these results.”
“I would very much like to pursue another controlled study of melatonin specifically in a group of late-stage lung cancer and possibly in other more advanced cancer types,” said lead author Dugald Seely, ND, of the Canadian College of Naturopathic Medicine in Toronto.
Study rationale and design
Melatonin has shown promise for treating patients with lung cancer, Dr. Seely and colleagues noted. Melatonin is often recommended by naturopathic doctors following lung cancer surgery, but until now there was no high-level evidence regarding the practice.
For their study, Dr. Seely and colleagues evaluated 709 patients who had undergone NSCLC resection. The patients were randomized to receive placebo (n = 353) or melatonin (n = 356) 1 hour before bedtime for 1 year. A 20-mg melatonin dose was used, which is common in clinical practice and research.
The study arms were well matched, with no “clinically meaningful” differences in demographics, surgery type, cancer type, stage of cancer, or preoperative comorbidities, according to the researchers.
The mean age in both treatment arms was 67 years. Overall, 134 participants received adjuvant chemotherapy (66 melatonin, 68 placebo), and 43 had adjuvant radiation (22 melatonin, 21 placebo).
Results
For 2-year DFS, melatonin showed an adjusted relative risk of 1.01 (95% confidence interval, 0.83-1.22; P = .94) versus placebo. The adjusted relative risk in the per-protocol analysis was 1.12 (95% CI, 0.96-1.32; P = .14.)
At 5 years, the median DFS was not reached in either treatment arm. Melatonin showed a hazard ratio of 0.97 (95% CI, 0.86-1.09; P = .84) for 5-year DFS.
Among patients with stage I-II NSCLC, the median DFS was not reached at 5 years in either treatment arm. Among patients with stage III-IV NSCLC, the median DFS was 18 months in both arms.
Melatonin showed a hazard ratio of 0.97 (95% CI, 0.85-1.11; P = .66) in patients with early-stage NSCLC and a hazard reduction of 25% (HR, 0.75; 95% CI, 0.61-0.92; P = .005) in patients with late-stage NSCLC.
For the entire cohort, there were no significant differences between treatment arms in the number, severity, or seriousness of adverse events. Likewise, there were no significant differences between the treatment arms with regard to fatigue, quality of life, or sleep at 1 or 2 years.
Dr. Seely said the most surprising thing about this study was that melatonin didn’t help with sleep.
“Since initiation of the trial, my thinking on the right dose of melatonin to support sleep has changed. Clinically, I see extended-release and, indeed, lower doses to be more effective than 20 mg nightly,” he noted.
Dr. Seely and colleagues also assessed proposed mechanisms for melatonin’s possible benefit in NSCLC but found no effect on natural killer cell cytotoxicity or phenotype and no effect on blood levels of inflammatory cytokines in a substudy of 92 patients.
This research was funded by the Lotte and John Hecht Memorial Foundation and the Gateway for Cancer Research Foundation. The researchers had no relevant disclosures.
There was a hint of benefit with melatonin among patients with stage III/IV NSCLC. These patients had a hazard reduction of 25% in 5-year DFS. However, the median DFS for patients with advanced disease was the same whether they received melatonin or placebo – 18 months.
In the overall study population, melatonin had no beneficial effects on quality of life, sleep, anxiety, depression, pain, or fatigue, and it did not reduce adverse events from chemotherapy or radiation.
These results were reported in EClinicalMedicine.
“In light of the results, we do not recommend the inclusion of adjuvant melatonin for patients with early-stage NSCLC. Evidence suggests there may be a benefit for those with late-stage disease,” the authors wrote. “However, because of the mixed findings observed, we recommend a follow-up randomized, controlled trial involving a larger population focusing on later-stage resected lung cancer to clarify these results.”
“I would very much like to pursue another controlled study of melatonin specifically in a group of late-stage lung cancer and possibly in other more advanced cancer types,” said lead author Dugald Seely, ND, of the Canadian College of Naturopathic Medicine in Toronto.
Study rationale and design
Melatonin has shown promise for treating patients with lung cancer, Dr. Seely and colleagues noted. Melatonin is often recommended by naturopathic doctors following lung cancer surgery, but until now there was no high-level evidence regarding the practice.
For their study, Dr. Seely and colleagues evaluated 709 patients who had undergone NSCLC resection. The patients were randomized to receive placebo (n = 353) or melatonin (n = 356) 1 hour before bedtime for 1 year. A 20-mg melatonin dose was used, which is common in clinical practice and research.
The study arms were well matched, with no “clinically meaningful” differences in demographics, surgery type, cancer type, stage of cancer, or preoperative comorbidities, according to the researchers.
The mean age in both treatment arms was 67 years. Overall, 134 participants received adjuvant chemotherapy (66 melatonin, 68 placebo), and 43 had adjuvant radiation (22 melatonin, 21 placebo).
Results
For 2-year DFS, melatonin showed an adjusted relative risk of 1.01 (95% confidence interval, 0.83-1.22; P = .94) versus placebo. The adjusted relative risk in the per-protocol analysis was 1.12 (95% CI, 0.96-1.32; P = .14.)
At 5 years, the median DFS was not reached in either treatment arm. Melatonin showed a hazard ratio of 0.97 (95% CI, 0.86-1.09; P = .84) for 5-year DFS.
Among patients with stage I-II NSCLC, the median DFS was not reached at 5 years in either treatment arm. Among patients with stage III-IV NSCLC, the median DFS was 18 months in both arms.
Melatonin showed a hazard ratio of 0.97 (95% CI, 0.85-1.11; P = .66) in patients with early-stage NSCLC and a hazard reduction of 25% (HR, 0.75; 95% CI, 0.61-0.92; P = .005) in patients with late-stage NSCLC.
For the entire cohort, there were no significant differences between treatment arms in the number, severity, or seriousness of adverse events. Likewise, there were no significant differences between the treatment arms with regard to fatigue, quality of life, or sleep at 1 or 2 years.
Dr. Seely said the most surprising thing about this study was that melatonin didn’t help with sleep.
“Since initiation of the trial, my thinking on the right dose of melatonin to support sleep has changed. Clinically, I see extended-release and, indeed, lower doses to be more effective than 20 mg nightly,” he noted.
Dr. Seely and colleagues also assessed proposed mechanisms for melatonin’s possible benefit in NSCLC but found no effect on natural killer cell cytotoxicity or phenotype and no effect on blood levels of inflammatory cytokines in a substudy of 92 patients.
This research was funded by the Lotte and John Hecht Memorial Foundation and the Gateway for Cancer Research Foundation. The researchers had no relevant disclosures.
There was a hint of benefit with melatonin among patients with stage III/IV NSCLC. These patients had a hazard reduction of 25% in 5-year DFS. However, the median DFS for patients with advanced disease was the same whether they received melatonin or placebo – 18 months.
In the overall study population, melatonin had no beneficial effects on quality of life, sleep, anxiety, depression, pain, or fatigue, and it did not reduce adverse events from chemotherapy or radiation.
These results were reported in EClinicalMedicine.
“In light of the results, we do not recommend the inclusion of adjuvant melatonin for patients with early-stage NSCLC. Evidence suggests there may be a benefit for those with late-stage disease,” the authors wrote. “However, because of the mixed findings observed, we recommend a follow-up randomized, controlled trial involving a larger population focusing on later-stage resected lung cancer to clarify these results.”
“I would very much like to pursue another controlled study of melatonin specifically in a group of late-stage lung cancer and possibly in other more advanced cancer types,” said lead author Dugald Seely, ND, of the Canadian College of Naturopathic Medicine in Toronto.
Study rationale and design
Melatonin has shown promise for treating patients with lung cancer, Dr. Seely and colleagues noted. Melatonin is often recommended by naturopathic doctors following lung cancer surgery, but until now there was no high-level evidence regarding the practice.
For their study, Dr. Seely and colleagues evaluated 709 patients who had undergone NSCLC resection. The patients were randomized to receive placebo (n = 353) or melatonin (n = 356) 1 hour before bedtime for 1 year. A 20-mg melatonin dose was used, which is common in clinical practice and research.
The study arms were well matched, with no “clinically meaningful” differences in demographics, surgery type, cancer type, stage of cancer, or preoperative comorbidities, according to the researchers.
The mean age in both treatment arms was 67 years. Overall, 134 participants received adjuvant chemotherapy (66 melatonin, 68 placebo), and 43 had adjuvant radiation (22 melatonin, 21 placebo).
Results
For 2-year DFS, melatonin showed an adjusted relative risk of 1.01 (95% confidence interval, 0.83-1.22; P = .94) versus placebo. The adjusted relative risk in the per-protocol analysis was 1.12 (95% CI, 0.96-1.32; P = .14.)
At 5 years, the median DFS was not reached in either treatment arm. Melatonin showed a hazard ratio of 0.97 (95% CI, 0.86-1.09; P = .84) for 5-year DFS.
Among patients with stage I-II NSCLC, the median DFS was not reached at 5 years in either treatment arm. Among patients with stage III-IV NSCLC, the median DFS was 18 months in both arms.
Melatonin showed a hazard ratio of 0.97 (95% CI, 0.85-1.11; P = .66) in patients with early-stage NSCLC and a hazard reduction of 25% (HR, 0.75; 95% CI, 0.61-0.92; P = .005) in patients with late-stage NSCLC.
For the entire cohort, there were no significant differences between treatment arms in the number, severity, or seriousness of adverse events. Likewise, there were no significant differences between the treatment arms with regard to fatigue, quality of life, or sleep at 1 or 2 years.
Dr. Seely said the most surprising thing about this study was that melatonin didn’t help with sleep.
“Since initiation of the trial, my thinking on the right dose of melatonin to support sleep has changed. Clinically, I see extended-release and, indeed, lower doses to be more effective than 20 mg nightly,” he noted.
Dr. Seely and colleagues also assessed proposed mechanisms for melatonin’s possible benefit in NSCLC but found no effect on natural killer cell cytotoxicity or phenotype and no effect on blood levels of inflammatory cytokines in a substudy of 92 patients.
This research was funded by the Lotte and John Hecht Memorial Foundation and the Gateway for Cancer Research Foundation. The researchers had no relevant disclosures.
FROM ECLINICALMEDICINE
Update: U.S. regulators question AstraZeneca vaccine trial data
Federal regulators on March 23 said they were “concerned” that drug maker AstraZeneca included “outdated information” in its announcement the previous day that the company’s COVID-19 vaccine was effective.
The federal Data and Safety Monitoring Board shared those concerns with the company as well as with the National Institute of Allergy and Infectious Diseases, and the U.S. Biomedical Advanced Research and Development Authority, according to a statement from NIAID issued early March 23.
“We urge the company to work with the DSMB to review the efficacy data and ensure the most accurate, up-to-date efficacy data be made public as quickly as possible,” the agency said.
The NIAID statement does not say what data may have been outdated or how it may have changed the results. The company said March 22 it plans to see U.S. authorization for the vaccine in April.
The statement from NIAID comes a day after AstraZeneca said the interim results of their phase III U.S. study found it was 79% effective against symptomatic COVID-19, 80% effective in people 65 years and older, and 100% effective against severe or critical disease and hospitalization.
Company officials and clinical trial investigators on March 22 also addressed the recent concerns about blood clots, how well the vaccine will perform against variants, and provided a timeline for seeking regulatory approval.
“There are many countries in Europe and throughout the world that have already authorized this. The fact that a United States-run study has confirmed the efficacy and safety of this vaccine, I think is an important contribution to global health in general,” Anthony Fauci, MD, chief medical advisor to President Joe Biden, said during a White House press briefing March 22.
Andy Slavitt, White House senior advisor for the COVID-19 Response Team, had a more tempered reaction.
“It’s important to remind everyone we cannot and will not get ahead of the FDA,” he said. “While we would certainly call today’s news encouraging, it’s the kind of thing we like to see, we have a rigorous process that will come once an EUA is submitted and that will give us more information.”
With 30 million doses at the ready, the company plans to file for FDA emergency use authorization “within weeks,” Menelas Pangalos, executive vice president of biopharmaceuticals research and development at AstraZeneca, said during a media briefing March 22.
Risk of thrombosis addressed
Regarding highly publicized reports of problems with blood clots from the AstraZeneca vaccine, the World Health Organization found the vaccine creates no greater risks, as did the European Medicines Agency
“We’ve had absolute confidence in the efficacy of the vaccine. Seeing this data now I hope gives others increased confidence that this is a very safe and effective vaccine,” Mr. Pangalos said.
“We’re glad this is being investigated really thoroughly,” Magda Sobieszczyk, MD, an infectious disease specialist at Columbia University In New York City, said. “It’s incredibly reassuring that the regulatory agencies have looked at the data thoroughly and there is no enhanced signal above what is seen in the population.”
“There were no concerning signals noted in the U.S. data,” she added.
Regarding the risk of blood clots, “These data are therefore timely in further addressing any safety concerns that could undermine vaccine uptake.” Andrew Garrett, PhD, executive vice president of scientific operations at ICON Clinical Research, agreed.
The vaccine was well-tolerated, the company reported, with no serious adverse events. Temporary pain and tenderness at the injection site, mild-to-moderate headaches, fatigue, chills, fever, muscle aches. and malaise were among the reported reactions.
The phase III interim results show 141 cases of symptomatic COVID-19 in the study of 32,449 adults. “We don’t have the whole breakdown yet . . . these are the high-level results we just got this week,” Mr. Pangalos said. Further information on rates of mild to moderate COVID-19 illness between groups is not yet available, for example.
The company explained that participants were randomly assigned to vaccine or placebo, with twice as many receiving the actual vaccine.
The trial is ongoing, so the FDA will receive information on more than the 141 COVID-19 symptomatic cases when the company submits a full primary analysis to the agency, Mr. Pangalos said.
In the phase III study, patients received two doses 4 weeks apart.
Beyond the U.S. study, the company has additional information, including real-world data from the United Kingdom, that it intends to submit to the FDA. Part of this evidence suggests increased efficacy when a second dose is administered at 3 months
‘Robust’ findings
“This is a large study, so these results can be expected to be robust. They could be expected to be even more so if there were more cases to compare between the groups, but 141 is still a substantial number of cases,” said Peter English, MD, of Horsham, United Kingdom, who is immediate past chair of the British Medical Association Public Health Medicine Committee.
Experts welcomed the 80% efficacy in people 65 and older in particular. “Importantly, the trial provides further support for efficacy in the elderly where previous clinical trial data, other than immunologic data, had been lacking,” Dr. Garrett said.
“It is clear this vaccine has very good efficacy. Remember that 60% was, prior to any trials being started, regarded as a good target,” said Stephen Evans, professor of pharmacoepidemiology at the London School of Hygiene & Tropical Medicine. “This efficacy does not show a notable decline at older ages. This was expected and the speculation that it was ineffective or quasi-ineffective at older ages was totally unjustified.
“This is good news for the global community and one hopes that any political statements around this good news are avoided,” he added.
Efficacy against variants?
Regarding virus variants, Mr. Pangalos noted the study was conducted when several variants of concern were in circulation.
“What I can say is given this study was conducted much later in terms of timing, it’s very encouraging that we’ve got such high efficacy numbers when undoubtedly there are variants of concern in circulation in this study,” Mr. Pangalos said.
“It also highlights why we believe that against severe disease, our vaccine will be effective against all variants of concern,” he added.
Once the company submits its EUA to the FDA, the company is ready to immediately distribute 30 million doses of the vaccine and expects to ship 50 million total within the first month, Ruud Dobber, PhD, AstraZeneca executive vice president and president of the AZ Biopharmaceuticals Business Unit, said during the briefing.
The vaccine can be stored at 2 to 8 degrees Celsius for at least 6 months. Like other COVID-19 vaccines already authorized for emergency use, the duration of protection with the AstraZeneca product remains unknown.
This article was updated March 23, 2021.
A version of this article first appeared on WebMD.com.
Federal regulators on March 23 said they were “concerned” that drug maker AstraZeneca included “outdated information” in its announcement the previous day that the company’s COVID-19 vaccine was effective.
The federal Data and Safety Monitoring Board shared those concerns with the company as well as with the National Institute of Allergy and Infectious Diseases, and the U.S. Biomedical Advanced Research and Development Authority, according to a statement from NIAID issued early March 23.
“We urge the company to work with the DSMB to review the efficacy data and ensure the most accurate, up-to-date efficacy data be made public as quickly as possible,” the agency said.
The NIAID statement does not say what data may have been outdated or how it may have changed the results. The company said March 22 it plans to see U.S. authorization for the vaccine in April.
The statement from NIAID comes a day after AstraZeneca said the interim results of their phase III U.S. study found it was 79% effective against symptomatic COVID-19, 80% effective in people 65 years and older, and 100% effective against severe or critical disease and hospitalization.
Company officials and clinical trial investigators on March 22 also addressed the recent concerns about blood clots, how well the vaccine will perform against variants, and provided a timeline for seeking regulatory approval.
“There are many countries in Europe and throughout the world that have already authorized this. The fact that a United States-run study has confirmed the efficacy and safety of this vaccine, I think is an important contribution to global health in general,” Anthony Fauci, MD, chief medical advisor to President Joe Biden, said during a White House press briefing March 22.
Andy Slavitt, White House senior advisor for the COVID-19 Response Team, had a more tempered reaction.
“It’s important to remind everyone we cannot and will not get ahead of the FDA,” he said. “While we would certainly call today’s news encouraging, it’s the kind of thing we like to see, we have a rigorous process that will come once an EUA is submitted and that will give us more information.”
With 30 million doses at the ready, the company plans to file for FDA emergency use authorization “within weeks,” Menelas Pangalos, executive vice president of biopharmaceuticals research and development at AstraZeneca, said during a media briefing March 22.
Risk of thrombosis addressed
Regarding highly publicized reports of problems with blood clots from the AstraZeneca vaccine, the World Health Organization found the vaccine creates no greater risks, as did the European Medicines Agency
“We’ve had absolute confidence in the efficacy of the vaccine. Seeing this data now I hope gives others increased confidence that this is a very safe and effective vaccine,” Mr. Pangalos said.
“We’re glad this is being investigated really thoroughly,” Magda Sobieszczyk, MD, an infectious disease specialist at Columbia University In New York City, said. “It’s incredibly reassuring that the regulatory agencies have looked at the data thoroughly and there is no enhanced signal above what is seen in the population.”
“There were no concerning signals noted in the U.S. data,” she added.
Regarding the risk of blood clots, “These data are therefore timely in further addressing any safety concerns that could undermine vaccine uptake.” Andrew Garrett, PhD, executive vice president of scientific operations at ICON Clinical Research, agreed.
The vaccine was well-tolerated, the company reported, with no serious adverse events. Temporary pain and tenderness at the injection site, mild-to-moderate headaches, fatigue, chills, fever, muscle aches. and malaise were among the reported reactions.
The phase III interim results show 141 cases of symptomatic COVID-19 in the study of 32,449 adults. “We don’t have the whole breakdown yet . . . these are the high-level results we just got this week,” Mr. Pangalos said. Further information on rates of mild to moderate COVID-19 illness between groups is not yet available, for example.
The company explained that participants were randomly assigned to vaccine or placebo, with twice as many receiving the actual vaccine.
The trial is ongoing, so the FDA will receive information on more than the 141 COVID-19 symptomatic cases when the company submits a full primary analysis to the agency, Mr. Pangalos said.
In the phase III study, patients received two doses 4 weeks apart.
Beyond the U.S. study, the company has additional information, including real-world data from the United Kingdom, that it intends to submit to the FDA. Part of this evidence suggests increased efficacy when a second dose is administered at 3 months
‘Robust’ findings
“This is a large study, so these results can be expected to be robust. They could be expected to be even more so if there were more cases to compare between the groups, but 141 is still a substantial number of cases,” said Peter English, MD, of Horsham, United Kingdom, who is immediate past chair of the British Medical Association Public Health Medicine Committee.
Experts welcomed the 80% efficacy in people 65 and older in particular. “Importantly, the trial provides further support for efficacy in the elderly where previous clinical trial data, other than immunologic data, had been lacking,” Dr. Garrett said.
“It is clear this vaccine has very good efficacy. Remember that 60% was, prior to any trials being started, regarded as a good target,” said Stephen Evans, professor of pharmacoepidemiology at the London School of Hygiene & Tropical Medicine. “This efficacy does not show a notable decline at older ages. This was expected and the speculation that it was ineffective or quasi-ineffective at older ages was totally unjustified.
“This is good news for the global community and one hopes that any political statements around this good news are avoided,” he added.
Efficacy against variants?
Regarding virus variants, Mr. Pangalos noted the study was conducted when several variants of concern were in circulation.
“What I can say is given this study was conducted much later in terms of timing, it’s very encouraging that we’ve got such high efficacy numbers when undoubtedly there are variants of concern in circulation in this study,” Mr. Pangalos said.
“It also highlights why we believe that against severe disease, our vaccine will be effective against all variants of concern,” he added.
Once the company submits its EUA to the FDA, the company is ready to immediately distribute 30 million doses of the vaccine and expects to ship 50 million total within the first month, Ruud Dobber, PhD, AstraZeneca executive vice president and president of the AZ Biopharmaceuticals Business Unit, said during the briefing.
The vaccine can be stored at 2 to 8 degrees Celsius for at least 6 months. Like other COVID-19 vaccines already authorized for emergency use, the duration of protection with the AstraZeneca product remains unknown.
This article was updated March 23, 2021.
A version of this article first appeared on WebMD.com.
Federal regulators on March 23 said they were “concerned” that drug maker AstraZeneca included “outdated information” in its announcement the previous day that the company’s COVID-19 vaccine was effective.
The federal Data and Safety Monitoring Board shared those concerns with the company as well as with the National Institute of Allergy and Infectious Diseases, and the U.S. Biomedical Advanced Research and Development Authority, according to a statement from NIAID issued early March 23.
“We urge the company to work with the DSMB to review the efficacy data and ensure the most accurate, up-to-date efficacy data be made public as quickly as possible,” the agency said.
The NIAID statement does not say what data may have been outdated or how it may have changed the results. The company said March 22 it plans to see U.S. authorization for the vaccine in April.
The statement from NIAID comes a day after AstraZeneca said the interim results of their phase III U.S. study found it was 79% effective against symptomatic COVID-19, 80% effective in people 65 years and older, and 100% effective against severe or critical disease and hospitalization.
Company officials and clinical trial investigators on March 22 also addressed the recent concerns about blood clots, how well the vaccine will perform against variants, and provided a timeline for seeking regulatory approval.
“There are many countries in Europe and throughout the world that have already authorized this. The fact that a United States-run study has confirmed the efficacy and safety of this vaccine, I think is an important contribution to global health in general,” Anthony Fauci, MD, chief medical advisor to President Joe Biden, said during a White House press briefing March 22.
Andy Slavitt, White House senior advisor for the COVID-19 Response Team, had a more tempered reaction.
“It’s important to remind everyone we cannot and will not get ahead of the FDA,” he said. “While we would certainly call today’s news encouraging, it’s the kind of thing we like to see, we have a rigorous process that will come once an EUA is submitted and that will give us more information.”
With 30 million doses at the ready, the company plans to file for FDA emergency use authorization “within weeks,” Menelas Pangalos, executive vice president of biopharmaceuticals research and development at AstraZeneca, said during a media briefing March 22.
Risk of thrombosis addressed
Regarding highly publicized reports of problems with blood clots from the AstraZeneca vaccine, the World Health Organization found the vaccine creates no greater risks, as did the European Medicines Agency
“We’ve had absolute confidence in the efficacy of the vaccine. Seeing this data now I hope gives others increased confidence that this is a very safe and effective vaccine,” Mr. Pangalos said.
“We’re glad this is being investigated really thoroughly,” Magda Sobieszczyk, MD, an infectious disease specialist at Columbia University In New York City, said. “It’s incredibly reassuring that the regulatory agencies have looked at the data thoroughly and there is no enhanced signal above what is seen in the population.”
“There were no concerning signals noted in the U.S. data,” she added.
Regarding the risk of blood clots, “These data are therefore timely in further addressing any safety concerns that could undermine vaccine uptake.” Andrew Garrett, PhD, executive vice president of scientific operations at ICON Clinical Research, agreed.
The vaccine was well-tolerated, the company reported, with no serious adverse events. Temporary pain and tenderness at the injection site, mild-to-moderate headaches, fatigue, chills, fever, muscle aches. and malaise were among the reported reactions.
The phase III interim results show 141 cases of symptomatic COVID-19 in the study of 32,449 adults. “We don’t have the whole breakdown yet . . . these are the high-level results we just got this week,” Mr. Pangalos said. Further information on rates of mild to moderate COVID-19 illness between groups is not yet available, for example.
The company explained that participants were randomly assigned to vaccine or placebo, with twice as many receiving the actual vaccine.
The trial is ongoing, so the FDA will receive information on more than the 141 COVID-19 symptomatic cases when the company submits a full primary analysis to the agency, Mr. Pangalos said.
In the phase III study, patients received two doses 4 weeks apart.
Beyond the U.S. study, the company has additional information, including real-world data from the United Kingdom, that it intends to submit to the FDA. Part of this evidence suggests increased efficacy when a second dose is administered at 3 months
‘Robust’ findings
“This is a large study, so these results can be expected to be robust. They could be expected to be even more so if there were more cases to compare between the groups, but 141 is still a substantial number of cases,” said Peter English, MD, of Horsham, United Kingdom, who is immediate past chair of the British Medical Association Public Health Medicine Committee.
Experts welcomed the 80% efficacy in people 65 and older in particular. “Importantly, the trial provides further support for efficacy in the elderly where previous clinical trial data, other than immunologic data, had been lacking,” Dr. Garrett said.
“It is clear this vaccine has very good efficacy. Remember that 60% was, prior to any trials being started, regarded as a good target,” said Stephen Evans, professor of pharmacoepidemiology at the London School of Hygiene & Tropical Medicine. “This efficacy does not show a notable decline at older ages. This was expected and the speculation that it was ineffective or quasi-ineffective at older ages was totally unjustified.
“This is good news for the global community and one hopes that any political statements around this good news are avoided,” he added.
Efficacy against variants?
Regarding virus variants, Mr. Pangalos noted the study was conducted when several variants of concern were in circulation.
“What I can say is given this study was conducted much later in terms of timing, it’s very encouraging that we’ve got such high efficacy numbers when undoubtedly there are variants of concern in circulation in this study,” Mr. Pangalos said.
“It also highlights why we believe that against severe disease, our vaccine will be effective against all variants of concern,” he added.
Once the company submits its EUA to the FDA, the company is ready to immediately distribute 30 million doses of the vaccine and expects to ship 50 million total within the first month, Ruud Dobber, PhD, AstraZeneca executive vice president and president of the AZ Biopharmaceuticals Business Unit, said during the briefing.
The vaccine can be stored at 2 to 8 degrees Celsius for at least 6 months. Like other COVID-19 vaccines already authorized for emergency use, the duration of protection with the AstraZeneca product remains unknown.
This article was updated March 23, 2021.
A version of this article first appeared on WebMD.com.
How to talk to patients reluctant to get a COVID-19 vaccine
Family physician Mitchell A. Kaminski, MD, MBA, was still awash in feelings of joy and relief at recently being vaccinated against COVID-19 when a patient’s comments stopped him cold. The patient, a middle-aged man with several comorbidities had just declined the pneumonia vaccine – and he added, without prompting, that he wouldn’t be getting the COVID vaccine either. This patient had heard getting vaccinated could kill him.
Dr. Kaminski countered with medical facts, including that the very rare side effects hadn’t killed anyone in the United States but COVID was killing thousands of people every day. “Well then, I’ll just risk getting COVID,” Dr. Kaminski recalled the patient saying. Conversation over.
That experience caused Dr. Kaminski, who is program director for population health at Thomas Jefferson University, Philadelphia, to rethink the way he talks to patients who are uncertain or skeptical about getting a COVID-19 vaccine. Now, if he saw that patient who seemed fearful of dying from a vaccination, Dr. Kaminski said he would be more curious.
Instead of outright contradicting the beliefs of a patient who is reluctant to get vaccinated, Dr. Kaminski now gently asks about the reasons for their discomfort and offers information about the vaccines. But mostly, he listens.
Conversations between physicians and patients about the risks that come with getting a COVID-19 vaccine are becoming more common in general as eligibility for immunizations expands.
About 80% of Americans say that they are most likely to turn to doctors, nurses and other health professionals for help in deciding whether to get the COVID vaccine, according to research by the Kaiser Family Foundation.
Getting beyond the distrust
While patients often feel a strong connection with their health providers, distrust in the medical establishment still exists, especially among some populations. The Kaiser Family Foundation reported that a third of Black respondents are taking a “wait-and-see” approach, while 23% said they will get it only if it’s required – or not at all.
Distrust persists from historical racist events in medicine, such as the infamous Tuskegee experiments in which treatment was withheld from Black men with syphilis. But physicians shouldn’t assume that all Black patients have the same reasons for vaccine hesitancy, said Krys Foster, MD, MPH, a family physician at Thomas Jefferson University.
“In my experience caring for patients who are uncertain or have concerns about receiving the vaccine, I’ve learned that many are just seeking more information, or even my approval to say that it is safe to proceed given their medical history,” she said.
Sources such as the COVID Racial Data Tracker have found that Black Americans have a higher COVID death rate than other racial or ethnic groups, making vaccination even more vital. Yet fear of the vaccine could be triggered by misinformation that can be found in various places online, Dr. Foster said.
To encourage people to get vaccinated and dispel false information, Dr. Foster takes time to discuss how safe it is to get a COVID-19 vaccine and the vaccines’ side effects, then quickly pivots to discussing how to get vaccinated.
It can be difficult for some people to find appointments or access testing sites. The failure to get the vaccine shouldn’t automatically be attributed to “hesitancy,” she said. “The onus is on the medical community to help fix the health injustices inflicted on communities of color by providing equitable information and access and stop placing blame on them for having the ‘wrong’ vaccine attitude.”
Give your testimonial
Jamie Loehr, MD, of Cayuga Family Medicine in Ithaca, N.Y., said he has always had a higher-than-average number of patients who refused or delayed their children’s vaccines. He does not kick them out of his practice but politely continues to educate them about the vaccines.
When patients ask Dr. Loehr if he trusts the vaccine, he responds with confidence: “I not only believe in it, I got it and I recommend it to anyone who can possibly get it.”
He was surprised recently when a mother who has expressed reluctance to vaccinate her young children came for a checkup and told him she had already received a COVID vaccine. “She made the decision on her own that this was important enough that she wanted to get it,” he said.
Health care worker hesitancy
Some health care workers’ unease about being at the front of the line for vaccines may be another source of vaccine hesitancy among members of the general population that physicians need to address. In a survey of almost 3,500 health care workers conducted in October and November 2020 and published in January 2021 in Vaccines, only about a third (36%) said they would get the vaccine as soon as it became available. By mid- to late-February, 54% of health care workers reported having been vaccinated and another 10% planned to get the vaccine as soon as possible, according to the Kaiser Family Foundation COVID-19 Vaccine Monitor.
Resolving doubts about the vaccines requires a thoughtful approach toward health care colleagues, said Eileen Barrett, MD, MPH, an internist and hospitalist who was a coauthor of the Vaccines paper and who serves on the editorial advisory board of Internal Medicine News. “We should meet people where they are and do our best to hear their concerns, listening thoughtfully without condescension. Validate how important their role is in endorsing vaccination and also validate asking questions.”
There’s power in the strong personal testimonial of physicians and other health care workers – not just to influence patients, but as a model for fellow health professionals, as well, noted Dr. Barrett, who cares for COVID-19 patients and is associate professor in the division of hospital medicine, department of internal medicine, at the University of New Mexico, Albuquerque.
‘Do it for your loved ones’
The Reagan-Udall Foundation, a nonprofit organization created by Congress to support the Food and Drug Administration, tested some messaging with focus groups. Participants responded favorably to this statement about why the vaccines were developed so quickly: “Vaccine development moved faster than normal because everyone’s making it their highest priority.”
People did not feel motivated to get the vaccine out of a sense of civic duty, said Susan Winckler, RPh, Esq, who is CEO of the foundation. But they did think the following was a good reason to get vaccinated: “By getting a vaccine, I could protect my children, my parents, and other loved ones.”
Physicians also can work with community influencers, such as faith leaders, to build confidence in vaccines. That’s part of the strategy of Roll Up Your Sleeves, a campaign spearheaded by agilon health, a company that partners with physician practices to develop value-based care for Medicare Advantage patients.
For example, Wilmington Health in North Carolina answered questions about the vaccines in Facebook Live events and created a Spanish-language video to boost vaccine confidence in the Latinx community. Additionally, PriMED Physicians in Dayton, Ohio, reached out to Black churches to provide a vaccine-awareness video and a PriMED doctor participated in a webinar sponsored by the Nigerian Women Cultural Organization to help dispel myths about COVID-19 and the vaccines.
“This is a way to deepen our relationship with our patients,” said Ben Kornitzer, MD, chief medical officer of agilon. “It’s helping to walk them through this door where on one side is the pandemic and social isolation and on the other side is a return to their life and loved ones.”
The messages provided by primary care physicians can be powerful and affirming, said Ms. Winckler.
“The path forward is to make a space for people to ask questions,” she continued, noting that the Reagan-Udall Foundation provides charts that show how the timeline for vaccine development was compressed without skipping any steps.
Strategies and background information on how to reinforce confidence in COVID-19 vaccines are also available on a page of the Centers for Disease Control and Prevention’s website.
None of the experts interviewed reported any relevant conflicts of interest. The Reagan-Udall Foundation has received sponsorships from Johnson & Johnson and AstraZeneca and has had a safety surveillance contract with Pfizer.
Family physician Mitchell A. Kaminski, MD, MBA, was still awash in feelings of joy and relief at recently being vaccinated against COVID-19 when a patient’s comments stopped him cold. The patient, a middle-aged man with several comorbidities had just declined the pneumonia vaccine – and he added, without prompting, that he wouldn’t be getting the COVID vaccine either. This patient had heard getting vaccinated could kill him.
Dr. Kaminski countered with medical facts, including that the very rare side effects hadn’t killed anyone in the United States but COVID was killing thousands of people every day. “Well then, I’ll just risk getting COVID,” Dr. Kaminski recalled the patient saying. Conversation over.
That experience caused Dr. Kaminski, who is program director for population health at Thomas Jefferson University, Philadelphia, to rethink the way he talks to patients who are uncertain or skeptical about getting a COVID-19 vaccine. Now, if he saw that patient who seemed fearful of dying from a vaccination, Dr. Kaminski said he would be more curious.
Instead of outright contradicting the beliefs of a patient who is reluctant to get vaccinated, Dr. Kaminski now gently asks about the reasons for their discomfort and offers information about the vaccines. But mostly, he listens.
Conversations between physicians and patients about the risks that come with getting a COVID-19 vaccine are becoming more common in general as eligibility for immunizations expands.
About 80% of Americans say that they are most likely to turn to doctors, nurses and other health professionals for help in deciding whether to get the COVID vaccine, according to research by the Kaiser Family Foundation.
Getting beyond the distrust
While patients often feel a strong connection with their health providers, distrust in the medical establishment still exists, especially among some populations. The Kaiser Family Foundation reported that a third of Black respondents are taking a “wait-and-see” approach, while 23% said they will get it only if it’s required – or not at all.
Distrust persists from historical racist events in medicine, such as the infamous Tuskegee experiments in which treatment was withheld from Black men with syphilis. But physicians shouldn’t assume that all Black patients have the same reasons for vaccine hesitancy, said Krys Foster, MD, MPH, a family physician at Thomas Jefferson University.
“In my experience caring for patients who are uncertain or have concerns about receiving the vaccine, I’ve learned that many are just seeking more information, or even my approval to say that it is safe to proceed given their medical history,” she said.
Sources such as the COVID Racial Data Tracker have found that Black Americans have a higher COVID death rate than other racial or ethnic groups, making vaccination even more vital. Yet fear of the vaccine could be triggered by misinformation that can be found in various places online, Dr. Foster said.
To encourage people to get vaccinated and dispel false information, Dr. Foster takes time to discuss how safe it is to get a COVID-19 vaccine and the vaccines’ side effects, then quickly pivots to discussing how to get vaccinated.
It can be difficult for some people to find appointments or access testing sites. The failure to get the vaccine shouldn’t automatically be attributed to “hesitancy,” she said. “The onus is on the medical community to help fix the health injustices inflicted on communities of color by providing equitable information and access and stop placing blame on them for having the ‘wrong’ vaccine attitude.”
Give your testimonial
Jamie Loehr, MD, of Cayuga Family Medicine in Ithaca, N.Y., said he has always had a higher-than-average number of patients who refused or delayed their children’s vaccines. He does not kick them out of his practice but politely continues to educate them about the vaccines.
When patients ask Dr. Loehr if he trusts the vaccine, he responds with confidence: “I not only believe in it, I got it and I recommend it to anyone who can possibly get it.”
He was surprised recently when a mother who has expressed reluctance to vaccinate her young children came for a checkup and told him she had already received a COVID vaccine. “She made the decision on her own that this was important enough that she wanted to get it,” he said.
Health care worker hesitancy
Some health care workers’ unease about being at the front of the line for vaccines may be another source of vaccine hesitancy among members of the general population that physicians need to address. In a survey of almost 3,500 health care workers conducted in October and November 2020 and published in January 2021 in Vaccines, only about a third (36%) said they would get the vaccine as soon as it became available. By mid- to late-February, 54% of health care workers reported having been vaccinated and another 10% planned to get the vaccine as soon as possible, according to the Kaiser Family Foundation COVID-19 Vaccine Monitor.
Resolving doubts about the vaccines requires a thoughtful approach toward health care colleagues, said Eileen Barrett, MD, MPH, an internist and hospitalist who was a coauthor of the Vaccines paper and who serves on the editorial advisory board of Internal Medicine News. “We should meet people where they are and do our best to hear their concerns, listening thoughtfully without condescension. Validate how important their role is in endorsing vaccination and also validate asking questions.”
There’s power in the strong personal testimonial of physicians and other health care workers – not just to influence patients, but as a model for fellow health professionals, as well, noted Dr. Barrett, who cares for COVID-19 patients and is associate professor in the division of hospital medicine, department of internal medicine, at the University of New Mexico, Albuquerque.
‘Do it for your loved ones’
The Reagan-Udall Foundation, a nonprofit organization created by Congress to support the Food and Drug Administration, tested some messaging with focus groups. Participants responded favorably to this statement about why the vaccines were developed so quickly: “Vaccine development moved faster than normal because everyone’s making it their highest priority.”
People did not feel motivated to get the vaccine out of a sense of civic duty, said Susan Winckler, RPh, Esq, who is CEO of the foundation. But they did think the following was a good reason to get vaccinated: “By getting a vaccine, I could protect my children, my parents, and other loved ones.”
Physicians also can work with community influencers, such as faith leaders, to build confidence in vaccines. That’s part of the strategy of Roll Up Your Sleeves, a campaign spearheaded by agilon health, a company that partners with physician practices to develop value-based care for Medicare Advantage patients.
For example, Wilmington Health in North Carolina answered questions about the vaccines in Facebook Live events and created a Spanish-language video to boost vaccine confidence in the Latinx community. Additionally, PriMED Physicians in Dayton, Ohio, reached out to Black churches to provide a vaccine-awareness video and a PriMED doctor participated in a webinar sponsored by the Nigerian Women Cultural Organization to help dispel myths about COVID-19 and the vaccines.
“This is a way to deepen our relationship with our patients,” said Ben Kornitzer, MD, chief medical officer of agilon. “It’s helping to walk them through this door where on one side is the pandemic and social isolation and on the other side is a return to their life and loved ones.”
The messages provided by primary care physicians can be powerful and affirming, said Ms. Winckler.
“The path forward is to make a space for people to ask questions,” she continued, noting that the Reagan-Udall Foundation provides charts that show how the timeline for vaccine development was compressed without skipping any steps.
Strategies and background information on how to reinforce confidence in COVID-19 vaccines are also available on a page of the Centers for Disease Control and Prevention’s website.
None of the experts interviewed reported any relevant conflicts of interest. The Reagan-Udall Foundation has received sponsorships from Johnson & Johnson and AstraZeneca and has had a safety surveillance contract with Pfizer.
Family physician Mitchell A. Kaminski, MD, MBA, was still awash in feelings of joy and relief at recently being vaccinated against COVID-19 when a patient’s comments stopped him cold. The patient, a middle-aged man with several comorbidities had just declined the pneumonia vaccine – and he added, without prompting, that he wouldn’t be getting the COVID vaccine either. This patient had heard getting vaccinated could kill him.
Dr. Kaminski countered with medical facts, including that the very rare side effects hadn’t killed anyone in the United States but COVID was killing thousands of people every day. “Well then, I’ll just risk getting COVID,” Dr. Kaminski recalled the patient saying. Conversation over.
That experience caused Dr. Kaminski, who is program director for population health at Thomas Jefferson University, Philadelphia, to rethink the way he talks to patients who are uncertain or skeptical about getting a COVID-19 vaccine. Now, if he saw that patient who seemed fearful of dying from a vaccination, Dr. Kaminski said he would be more curious.
Instead of outright contradicting the beliefs of a patient who is reluctant to get vaccinated, Dr. Kaminski now gently asks about the reasons for their discomfort and offers information about the vaccines. But mostly, he listens.
Conversations between physicians and patients about the risks that come with getting a COVID-19 vaccine are becoming more common in general as eligibility for immunizations expands.
About 80% of Americans say that they are most likely to turn to doctors, nurses and other health professionals for help in deciding whether to get the COVID vaccine, according to research by the Kaiser Family Foundation.
Getting beyond the distrust
While patients often feel a strong connection with their health providers, distrust in the medical establishment still exists, especially among some populations. The Kaiser Family Foundation reported that a third of Black respondents are taking a “wait-and-see” approach, while 23% said they will get it only if it’s required – or not at all.
Distrust persists from historical racist events in medicine, such as the infamous Tuskegee experiments in which treatment was withheld from Black men with syphilis. But physicians shouldn’t assume that all Black patients have the same reasons for vaccine hesitancy, said Krys Foster, MD, MPH, a family physician at Thomas Jefferson University.
“In my experience caring for patients who are uncertain or have concerns about receiving the vaccine, I’ve learned that many are just seeking more information, or even my approval to say that it is safe to proceed given their medical history,” she said.
Sources such as the COVID Racial Data Tracker have found that Black Americans have a higher COVID death rate than other racial or ethnic groups, making vaccination even more vital. Yet fear of the vaccine could be triggered by misinformation that can be found in various places online, Dr. Foster said.
To encourage people to get vaccinated and dispel false information, Dr. Foster takes time to discuss how safe it is to get a COVID-19 vaccine and the vaccines’ side effects, then quickly pivots to discussing how to get vaccinated.
It can be difficult for some people to find appointments or access testing sites. The failure to get the vaccine shouldn’t automatically be attributed to “hesitancy,” she said. “The onus is on the medical community to help fix the health injustices inflicted on communities of color by providing equitable information and access and stop placing blame on them for having the ‘wrong’ vaccine attitude.”
Give your testimonial
Jamie Loehr, MD, of Cayuga Family Medicine in Ithaca, N.Y., said he has always had a higher-than-average number of patients who refused or delayed their children’s vaccines. He does not kick them out of his practice but politely continues to educate them about the vaccines.
When patients ask Dr. Loehr if he trusts the vaccine, he responds with confidence: “I not only believe in it, I got it and I recommend it to anyone who can possibly get it.”
He was surprised recently when a mother who has expressed reluctance to vaccinate her young children came for a checkup and told him she had already received a COVID vaccine. “She made the decision on her own that this was important enough that she wanted to get it,” he said.
Health care worker hesitancy
Some health care workers’ unease about being at the front of the line for vaccines may be another source of vaccine hesitancy among members of the general population that physicians need to address. In a survey of almost 3,500 health care workers conducted in October and November 2020 and published in January 2021 in Vaccines, only about a third (36%) said they would get the vaccine as soon as it became available. By mid- to late-February, 54% of health care workers reported having been vaccinated and another 10% planned to get the vaccine as soon as possible, according to the Kaiser Family Foundation COVID-19 Vaccine Monitor.
Resolving doubts about the vaccines requires a thoughtful approach toward health care colleagues, said Eileen Barrett, MD, MPH, an internist and hospitalist who was a coauthor of the Vaccines paper and who serves on the editorial advisory board of Internal Medicine News. “We should meet people where they are and do our best to hear their concerns, listening thoughtfully without condescension. Validate how important their role is in endorsing vaccination and also validate asking questions.”
There’s power in the strong personal testimonial of physicians and other health care workers – not just to influence patients, but as a model for fellow health professionals, as well, noted Dr. Barrett, who cares for COVID-19 patients and is associate professor in the division of hospital medicine, department of internal medicine, at the University of New Mexico, Albuquerque.
‘Do it for your loved ones’
The Reagan-Udall Foundation, a nonprofit organization created by Congress to support the Food and Drug Administration, tested some messaging with focus groups. Participants responded favorably to this statement about why the vaccines were developed so quickly: “Vaccine development moved faster than normal because everyone’s making it their highest priority.”
People did not feel motivated to get the vaccine out of a sense of civic duty, said Susan Winckler, RPh, Esq, who is CEO of the foundation. But they did think the following was a good reason to get vaccinated: “By getting a vaccine, I could protect my children, my parents, and other loved ones.”
Physicians also can work with community influencers, such as faith leaders, to build confidence in vaccines. That’s part of the strategy of Roll Up Your Sleeves, a campaign spearheaded by agilon health, a company that partners with physician practices to develop value-based care for Medicare Advantage patients.
For example, Wilmington Health in North Carolina answered questions about the vaccines in Facebook Live events and created a Spanish-language video to boost vaccine confidence in the Latinx community. Additionally, PriMED Physicians in Dayton, Ohio, reached out to Black churches to provide a vaccine-awareness video and a PriMED doctor participated in a webinar sponsored by the Nigerian Women Cultural Organization to help dispel myths about COVID-19 and the vaccines.
“This is a way to deepen our relationship with our patients,” said Ben Kornitzer, MD, chief medical officer of agilon. “It’s helping to walk them through this door where on one side is the pandemic and social isolation and on the other side is a return to their life and loved ones.”
The messages provided by primary care physicians can be powerful and affirming, said Ms. Winckler.
“The path forward is to make a space for people to ask questions,” she continued, noting that the Reagan-Udall Foundation provides charts that show how the timeline for vaccine development was compressed without skipping any steps.
Strategies and background information on how to reinforce confidence in COVID-19 vaccines are also available on a page of the Centers for Disease Control and Prevention’s website.
None of the experts interviewed reported any relevant conflicts of interest. The Reagan-Udall Foundation has received sponsorships from Johnson & Johnson and AstraZeneca and has had a safety surveillance contract with Pfizer.
2021 match sets records: Who matched and who didn’t?
A total of 38,106 positions were offered, up 850 spots (2.3%) from 2020. Of those, 35,194 were first-year (PGY-1) positions, which was 928 more than the previous year (2.7%). A record 5,915 programs were part of the Match, 88 more than 2020.
“The application and recruitment cycle was upended as a result of the pandemic, yet the results of the Match continue to demonstrate strong and consistent outcomes for participants,” Donna L. Lamb, DHSc, MBA, BSN, NRMP president and CEO, said in a new release.
The report comes amid a year of Zoom interview fatigue, canceled testing, and virus fears and work-arounds, which the NMRP has never had to wrestle with since it was established in 1952.
Despite challenges, fill rates increased across the board. Of the 38,106 total positions offered, 36,179 were filled, representing a 2.6% increase over 2020. Of the 35,194 first-year positions available, 33,535 were filled, representing a 2.9% increase.
Those rates drove the percentage of all positions filled to 94.9% (up from 94.6%) and the percentage of PGY-1 positions filled to 94.8% (also up from 94.6%). There were 1,927 unfilled positions, a decline of 71 (3.6%) from 2020.
Primary care results strong
Of the first-year positions offered, 17,649 (49.6%) were in family medicine, internal medicine, and pediatrics. That’s an increase of 514 positions (3%) over 2020.
Of first-year positions offered in 2021, 16,860 (95.5%) were filled. U.S. seniors took 11,013 (65.3%) of those slots; that represents a slight decline (0.3%) from 2020. Family medicine saw a gain of 63 U.S. MD seniors who matched, and internal medicine saw a gain of 93 U.S. DO seniors who matched.
Some specialties filled all positions
PGY-1 specialties with 30 positions or more that filled all available positions include dermatology, medicine – emergency medicine, medicine – pediatrics, neurologic surgery, otolaryngology, integrated plastic surgery, and vascular surgery.*
PGY-1 specialties with 30 positions or more that filled more than 90% with U.S. seniors include dermatology (100%), medicine – emergency medicine (93.6%), medicine – pediatrics (93.5%), otolaryngology (93.2%), orthopedic surgery (92.8%), and integrated plastic surgery (90.4%).*
PGY-1 specialties with at least 30 positions that filled less than 50% with U.S. seniors include pathology (41.4 %) and surgery–preliminary (28%).
The number of U.S. citizen international medical graduates who submitted rank-ordered lists was 5,295, an increase of 128 (2.5%) over 2020 and the highest in 6 years; 3,152 of them matched to first-year positions, down two PGY-1 matched applicants over last year.
Full data are available on the NRMP’s website.
Correction, 3/22/21: An earlier version of this article misstated the affected specialties.
A version of this article first appeared on Medscape.com.
A total of 38,106 positions were offered, up 850 spots (2.3%) from 2020. Of those, 35,194 were first-year (PGY-1) positions, which was 928 more than the previous year (2.7%). A record 5,915 programs were part of the Match, 88 more than 2020.
“The application and recruitment cycle was upended as a result of the pandemic, yet the results of the Match continue to demonstrate strong and consistent outcomes for participants,” Donna L. Lamb, DHSc, MBA, BSN, NRMP president and CEO, said in a new release.
The report comes amid a year of Zoom interview fatigue, canceled testing, and virus fears and work-arounds, which the NMRP has never had to wrestle with since it was established in 1952.
Despite challenges, fill rates increased across the board. Of the 38,106 total positions offered, 36,179 were filled, representing a 2.6% increase over 2020. Of the 35,194 first-year positions available, 33,535 were filled, representing a 2.9% increase.
Those rates drove the percentage of all positions filled to 94.9% (up from 94.6%) and the percentage of PGY-1 positions filled to 94.8% (also up from 94.6%). There were 1,927 unfilled positions, a decline of 71 (3.6%) from 2020.
Primary care results strong
Of the first-year positions offered, 17,649 (49.6%) were in family medicine, internal medicine, and pediatrics. That’s an increase of 514 positions (3%) over 2020.
Of first-year positions offered in 2021, 16,860 (95.5%) were filled. U.S. seniors took 11,013 (65.3%) of those slots; that represents a slight decline (0.3%) from 2020. Family medicine saw a gain of 63 U.S. MD seniors who matched, and internal medicine saw a gain of 93 U.S. DO seniors who matched.
Some specialties filled all positions
PGY-1 specialties with 30 positions or more that filled all available positions include dermatology, medicine – emergency medicine, medicine – pediatrics, neurologic surgery, otolaryngology, integrated plastic surgery, and vascular surgery.*
PGY-1 specialties with 30 positions or more that filled more than 90% with U.S. seniors include dermatology (100%), medicine – emergency medicine (93.6%), medicine – pediatrics (93.5%), otolaryngology (93.2%), orthopedic surgery (92.8%), and integrated plastic surgery (90.4%).*
PGY-1 specialties with at least 30 positions that filled less than 50% with U.S. seniors include pathology (41.4 %) and surgery–preliminary (28%).
The number of U.S. citizen international medical graduates who submitted rank-ordered lists was 5,295, an increase of 128 (2.5%) over 2020 and the highest in 6 years; 3,152 of them matched to first-year positions, down two PGY-1 matched applicants over last year.
Full data are available on the NRMP’s website.
Correction, 3/22/21: An earlier version of this article misstated the affected specialties.
A version of this article first appeared on Medscape.com.
A total of 38,106 positions were offered, up 850 spots (2.3%) from 2020. Of those, 35,194 were first-year (PGY-1) positions, which was 928 more than the previous year (2.7%). A record 5,915 programs were part of the Match, 88 more than 2020.
“The application and recruitment cycle was upended as a result of the pandemic, yet the results of the Match continue to demonstrate strong and consistent outcomes for participants,” Donna L. Lamb, DHSc, MBA, BSN, NRMP president and CEO, said in a new release.
The report comes amid a year of Zoom interview fatigue, canceled testing, and virus fears and work-arounds, which the NMRP has never had to wrestle with since it was established in 1952.
Despite challenges, fill rates increased across the board. Of the 38,106 total positions offered, 36,179 were filled, representing a 2.6% increase over 2020. Of the 35,194 first-year positions available, 33,535 were filled, representing a 2.9% increase.
Those rates drove the percentage of all positions filled to 94.9% (up from 94.6%) and the percentage of PGY-1 positions filled to 94.8% (also up from 94.6%). There were 1,927 unfilled positions, a decline of 71 (3.6%) from 2020.
Primary care results strong
Of the first-year positions offered, 17,649 (49.6%) were in family medicine, internal medicine, and pediatrics. That’s an increase of 514 positions (3%) over 2020.
Of first-year positions offered in 2021, 16,860 (95.5%) were filled. U.S. seniors took 11,013 (65.3%) of those slots; that represents a slight decline (0.3%) from 2020. Family medicine saw a gain of 63 U.S. MD seniors who matched, and internal medicine saw a gain of 93 U.S. DO seniors who matched.
Some specialties filled all positions
PGY-1 specialties with 30 positions or more that filled all available positions include dermatology, medicine – emergency medicine, medicine – pediatrics, neurologic surgery, otolaryngology, integrated plastic surgery, and vascular surgery.*
PGY-1 specialties with 30 positions or more that filled more than 90% with U.S. seniors include dermatology (100%), medicine – emergency medicine (93.6%), medicine – pediatrics (93.5%), otolaryngology (93.2%), orthopedic surgery (92.8%), and integrated plastic surgery (90.4%).*
PGY-1 specialties with at least 30 positions that filled less than 50% with U.S. seniors include pathology (41.4 %) and surgery–preliminary (28%).
The number of U.S. citizen international medical graduates who submitted rank-ordered lists was 5,295, an increase of 128 (2.5%) over 2020 and the highest in 6 years; 3,152 of them matched to first-year positions, down two PGY-1 matched applicants over last year.
Full data are available on the NRMP’s website.
Correction, 3/22/21: An earlier version of this article misstated the affected specialties.
A version of this article first appeared on Medscape.com.