User login
-
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]


Encephalopathy common, often lethal in hospitalized patients with COVID-19
, new research shows. Results of a retrospective study show that of almost 4,500 patients with COVID-19, 12% were diagnosed with TME. Of these, 78% developed encephalopathy immediately prior to hospital admission. Septic encephalopathy, hypoxic-ischemic encephalopathy (HIE), and uremia were the most common causes, although multiple causes were present in close to 80% of patients. TME was also associated with a 24% higher risk of in-hospital death.
“We found that close to one in eight patients who were hospitalized with COVID-19 had TME that was not attributed to the effects of sedatives, and that this is incredibly common among these patients who are critically ill” said lead author Jennifer A. Frontera, MD, New York University.
“The general principle of our findings is to be more aggressive in TME; and from a neurologist perspective, the way to do this is to eliminate the effects of sedation, which is a confounder,” she said.
The study was published online March 16 in Neurocritical Care.
Drilling down
“Many neurological complications of COVID-19 are sequelae of severe illness or secondary effects of multisystem organ failure, but our previous work identified TME as the most common neurological complication,” Dr. Frontera said.
Previous research investigating encephalopathy among patients with COVID-19 included patients who may have been sedated or have had a positive Confusion Assessment Method (CAM) result.
“A lot of the delirium literature is effectively heterogeneous because there are a number of patients who are on sedative medication that, if you could turn it off, these patients would return to normal. Some may have underlying neurological issues that can be addressed, but you can›t get to the bottom of this unless you turn off the sedation,” Dr. Frontera noted.
“We wanted to be specific and try to drill down to see what the underlying cause of the encephalopathy was,” she said.
The researchers retrospectively analyzed data on 4,491 patients (≥ 18 years old) with COVID-19 who were admitted to four New York City hospitals between March 1, 2020, and May 20, 2020. Of these, 559 (12%) with TME were compared with 3,932 patients without TME.
The researchers looked at index admissions and included patients who had:
- New changes in mental status or significant worsening of mental status (in patients with baseline abnormal mental status).
- Hyperglycemia or with transient focal neurologic deficits that resolved with glucose correction.
- An adequate washout of sedating medications (when relevant) prior to mental status assessment.
Potential etiologies included electrolyte abnormalities, organ failure, hypertensive encephalopathy, sepsis or active infection, fever, nutritional deficiency, and environmental injury.
Foreign environment
Most (78%) of the 559 patients diagnosed with TME had already developed encephalopathy immediately prior to hospital admission, the authors report. The most common etiologies of TME among hospitalized patients with COVID-19 are listed below.
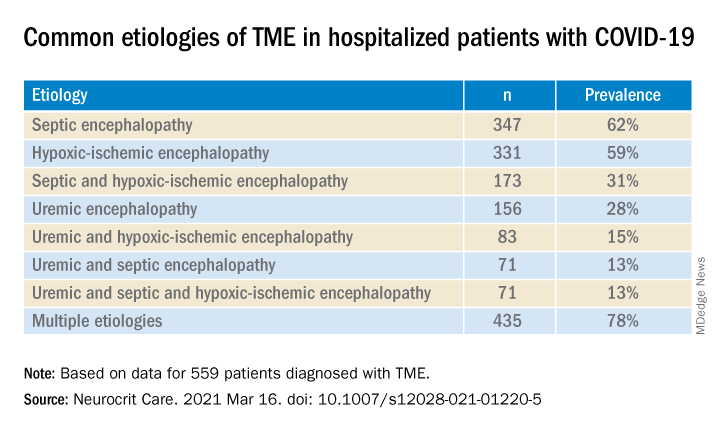
Compared with patients without TME, those with TME – (all Ps < .001):
- Were older (76 vs. 62 years).
- Had higher rates of dementia (27% vs. 3%).
- Had higher rates of psychiatric history (20% vs. 10%).
- Were more often intubated (37% vs. 20%).
- Had a longer length of hospital stay (7.9 vs. 6.0 days).
- Were less often discharged home (25% vs. 66%).
“It’s no surprise that older patients and people with dementia or psychiatric illness are predisposed to becoming encephalopathic,” said Dr. Frontera. “Being in a foreign environment, such as a hospital, or being sleep-deprived in the ICU is likely to make them more confused during their hospital stay.”
Delirium as a symptom
In-hospital mortality or discharge to hospice was considerably higher in the TME versus non-TME patients (44% vs. 18%, respectively).
When the researchers adjusted for confounders (age, sex, race, worse Sequential Organ Failure Assessment score during hospitalization, ventilator status, study week, hospital location, and ICU care level) and excluded patients receiving only comfort care, they found that TME was associated with a 24% increased risk of in-hospital death (30% in patients with TME vs. 16% in those without TME).
The highest mortality risk was associated with hypoxemia, with 42% of patients with HIE dying during hospitalization, compared with 16% of patients without HIE (adjusted hazard ratio 1.56; 95% confidence interval, 1.21-2.00; P = .001).
“Not all patients who are intubated require sedation, but there’s generally a lot of hesitation in reducing or stopping sedation in some patients,” Dr. Frontera observed.
She acknowledged there are “many extremely sick patients whom you can’t ventilate without sedation.”
Nevertheless, “delirium in and of itself does not cause death. It’s a symptom, not a disease, and we have to figure out what causes it. Delirium might not need to be sedated, and it’s more important to see what the causal problem is.”
Independent predictor of death
Commenting on the study, Panayiotis N. Varelas, MD, PhD, vice president of the Neurocritical Care Society, said the study “approached the TME issue better than previously, namely allowing time for sedatives to wear off to have a better sample of patients with this syndrome.”
Dr. Varelas, who is chairman of the department of neurology and professor of neurology at Albany (N.Y.) Medical College, emphasized that TME “is not benign and, in patients with COVID-19, it is an independent predictor of in-hospital mortality.”
“One should take all possible measures … to avoid desaturation and hypotensive episodes and also aggressively treat SAE and uremic encephalopathy in hopes of improving the outcomes,” added Dr. Varelas, who was not involved with the study.
Also commenting on the study, Mitchell Elkind, MD, professor of neurology and epidemiology at Columbia University in New York, who was not associated with the research, said it “nicely distinguishes among the different causes of encephalopathy, including sepsis, hypoxia, and kidney failure … emphasizing just how sick these patients are.”
The study received no direct funding. Individual investigators were supported by grants from the National Institute on Aging and the National Institute of Neurological Disorders and Stroke. The investigators, Dr. Varelas, and Dr. Elkind have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research shows. Results of a retrospective study show that of almost 4,500 patients with COVID-19, 12% were diagnosed with TME. Of these, 78% developed encephalopathy immediately prior to hospital admission. Septic encephalopathy, hypoxic-ischemic encephalopathy (HIE), and uremia were the most common causes, although multiple causes were present in close to 80% of patients. TME was also associated with a 24% higher risk of in-hospital death.
“We found that close to one in eight patients who were hospitalized with COVID-19 had TME that was not attributed to the effects of sedatives, and that this is incredibly common among these patients who are critically ill” said lead author Jennifer A. Frontera, MD, New York University.
“The general principle of our findings is to be more aggressive in TME; and from a neurologist perspective, the way to do this is to eliminate the effects of sedation, which is a confounder,” she said.
The study was published online March 16 in Neurocritical Care.
Drilling down
“Many neurological complications of COVID-19 are sequelae of severe illness or secondary effects of multisystem organ failure, but our previous work identified TME as the most common neurological complication,” Dr. Frontera said.
Previous research investigating encephalopathy among patients with COVID-19 included patients who may have been sedated or have had a positive Confusion Assessment Method (CAM) result.
“A lot of the delirium literature is effectively heterogeneous because there are a number of patients who are on sedative medication that, if you could turn it off, these patients would return to normal. Some may have underlying neurological issues that can be addressed, but you can›t get to the bottom of this unless you turn off the sedation,” Dr. Frontera noted.
“We wanted to be specific and try to drill down to see what the underlying cause of the encephalopathy was,” she said.
The researchers retrospectively analyzed data on 4,491 patients (≥ 18 years old) with COVID-19 who were admitted to four New York City hospitals between March 1, 2020, and May 20, 2020. Of these, 559 (12%) with TME were compared with 3,932 patients without TME.
The researchers looked at index admissions and included patients who had:
- New changes in mental status or significant worsening of mental status (in patients with baseline abnormal mental status).
- Hyperglycemia or with transient focal neurologic deficits that resolved with glucose correction.
- An adequate washout of sedating medications (when relevant) prior to mental status assessment.
Potential etiologies included electrolyte abnormalities, organ failure, hypertensive encephalopathy, sepsis or active infection, fever, nutritional deficiency, and environmental injury.
Foreign environment
Most (78%) of the 559 patients diagnosed with TME had already developed encephalopathy immediately prior to hospital admission, the authors report. The most common etiologies of TME among hospitalized patients with COVID-19 are listed below.

Compared with patients without TME, those with TME – (all Ps < .001):
- Were older (76 vs. 62 years).
- Had higher rates of dementia (27% vs. 3%).
- Had higher rates of psychiatric history (20% vs. 10%).
- Were more often intubated (37% vs. 20%).
- Had a longer length of hospital stay (7.9 vs. 6.0 days).
- Were less often discharged home (25% vs. 66%).
“It’s no surprise that older patients and people with dementia or psychiatric illness are predisposed to becoming encephalopathic,” said Dr. Frontera. “Being in a foreign environment, such as a hospital, or being sleep-deprived in the ICU is likely to make them more confused during their hospital stay.”
Delirium as a symptom
In-hospital mortality or discharge to hospice was considerably higher in the TME versus non-TME patients (44% vs. 18%, respectively).
When the researchers adjusted for confounders (age, sex, race, worse Sequential Organ Failure Assessment score during hospitalization, ventilator status, study week, hospital location, and ICU care level) and excluded patients receiving only comfort care, they found that TME was associated with a 24% increased risk of in-hospital death (30% in patients with TME vs. 16% in those without TME).
The highest mortality risk was associated with hypoxemia, with 42% of patients with HIE dying during hospitalization, compared with 16% of patients without HIE (adjusted hazard ratio 1.56; 95% confidence interval, 1.21-2.00; P = .001).
“Not all patients who are intubated require sedation, but there’s generally a lot of hesitation in reducing or stopping sedation in some patients,” Dr. Frontera observed.
She acknowledged there are “many extremely sick patients whom you can’t ventilate without sedation.”
Nevertheless, “delirium in and of itself does not cause death. It’s a symptom, not a disease, and we have to figure out what causes it. Delirium might not need to be sedated, and it’s more important to see what the causal problem is.”
Independent predictor of death
Commenting on the study, Panayiotis N. Varelas, MD, PhD, vice president of the Neurocritical Care Society, said the study “approached the TME issue better than previously, namely allowing time for sedatives to wear off to have a better sample of patients with this syndrome.”
Dr. Varelas, who is chairman of the department of neurology and professor of neurology at Albany (N.Y.) Medical College, emphasized that TME “is not benign and, in patients with COVID-19, it is an independent predictor of in-hospital mortality.”
“One should take all possible measures … to avoid desaturation and hypotensive episodes and also aggressively treat SAE and uremic encephalopathy in hopes of improving the outcomes,” added Dr. Varelas, who was not involved with the study.
Also commenting on the study, Mitchell Elkind, MD, professor of neurology and epidemiology at Columbia University in New York, who was not associated with the research, said it “nicely distinguishes among the different causes of encephalopathy, including sepsis, hypoxia, and kidney failure … emphasizing just how sick these patients are.”
The study received no direct funding. Individual investigators were supported by grants from the National Institute on Aging and the National Institute of Neurological Disorders and Stroke. The investigators, Dr. Varelas, and Dr. Elkind have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research shows. Results of a retrospective study show that of almost 4,500 patients with COVID-19, 12% were diagnosed with TME. Of these, 78% developed encephalopathy immediately prior to hospital admission. Septic encephalopathy, hypoxic-ischemic encephalopathy (HIE), and uremia were the most common causes, although multiple causes were present in close to 80% of patients. TME was also associated with a 24% higher risk of in-hospital death.
“We found that close to one in eight patients who were hospitalized with COVID-19 had TME that was not attributed to the effects of sedatives, and that this is incredibly common among these patients who are critically ill” said lead author Jennifer A. Frontera, MD, New York University.
“The general principle of our findings is to be more aggressive in TME; and from a neurologist perspective, the way to do this is to eliminate the effects of sedation, which is a confounder,” she said.
The study was published online March 16 in Neurocritical Care.
Drilling down
“Many neurological complications of COVID-19 are sequelae of severe illness or secondary effects of multisystem organ failure, but our previous work identified TME as the most common neurological complication,” Dr. Frontera said.
Previous research investigating encephalopathy among patients with COVID-19 included patients who may have been sedated or have had a positive Confusion Assessment Method (CAM) result.
“A lot of the delirium literature is effectively heterogeneous because there are a number of patients who are on sedative medication that, if you could turn it off, these patients would return to normal. Some may have underlying neurological issues that can be addressed, but you can›t get to the bottom of this unless you turn off the sedation,” Dr. Frontera noted.
“We wanted to be specific and try to drill down to see what the underlying cause of the encephalopathy was,” she said.
The researchers retrospectively analyzed data on 4,491 patients (≥ 18 years old) with COVID-19 who were admitted to four New York City hospitals between March 1, 2020, and May 20, 2020. Of these, 559 (12%) with TME were compared with 3,932 patients without TME.
The researchers looked at index admissions and included patients who had:
- New changes in mental status or significant worsening of mental status (in patients with baseline abnormal mental status).
- Hyperglycemia or with transient focal neurologic deficits that resolved with glucose correction.
- An adequate washout of sedating medications (when relevant) prior to mental status assessment.
Potential etiologies included electrolyte abnormalities, organ failure, hypertensive encephalopathy, sepsis or active infection, fever, nutritional deficiency, and environmental injury.
Foreign environment
Most (78%) of the 559 patients diagnosed with TME had already developed encephalopathy immediately prior to hospital admission, the authors report. The most common etiologies of TME among hospitalized patients with COVID-19 are listed below.

Compared with patients without TME, those with TME – (all Ps < .001):
- Were older (76 vs. 62 years).
- Had higher rates of dementia (27% vs. 3%).
- Had higher rates of psychiatric history (20% vs. 10%).
- Were more often intubated (37% vs. 20%).
- Had a longer length of hospital stay (7.9 vs. 6.0 days).
- Were less often discharged home (25% vs. 66%).
“It’s no surprise that older patients and people with dementia or psychiatric illness are predisposed to becoming encephalopathic,” said Dr. Frontera. “Being in a foreign environment, such as a hospital, or being sleep-deprived in the ICU is likely to make them more confused during their hospital stay.”
Delirium as a symptom
In-hospital mortality or discharge to hospice was considerably higher in the TME versus non-TME patients (44% vs. 18%, respectively).
When the researchers adjusted for confounders (age, sex, race, worse Sequential Organ Failure Assessment score during hospitalization, ventilator status, study week, hospital location, and ICU care level) and excluded patients receiving only comfort care, they found that TME was associated with a 24% increased risk of in-hospital death (30% in patients with TME vs. 16% in those without TME).
The highest mortality risk was associated with hypoxemia, with 42% of patients with HIE dying during hospitalization, compared with 16% of patients without HIE (adjusted hazard ratio 1.56; 95% confidence interval, 1.21-2.00; P = .001).
“Not all patients who are intubated require sedation, but there’s generally a lot of hesitation in reducing or stopping sedation in some patients,” Dr. Frontera observed.
She acknowledged there are “many extremely sick patients whom you can’t ventilate without sedation.”
Nevertheless, “delirium in and of itself does not cause death. It’s a symptom, not a disease, and we have to figure out what causes it. Delirium might not need to be sedated, and it’s more important to see what the causal problem is.”
Independent predictor of death
Commenting on the study, Panayiotis N. Varelas, MD, PhD, vice president of the Neurocritical Care Society, said the study “approached the TME issue better than previously, namely allowing time for sedatives to wear off to have a better sample of patients with this syndrome.”
Dr. Varelas, who is chairman of the department of neurology and professor of neurology at Albany (N.Y.) Medical College, emphasized that TME “is not benign and, in patients with COVID-19, it is an independent predictor of in-hospital mortality.”
“One should take all possible measures … to avoid desaturation and hypotensive episodes and also aggressively treat SAE and uremic encephalopathy in hopes of improving the outcomes,” added Dr. Varelas, who was not involved with the study.
Also commenting on the study, Mitchell Elkind, MD, professor of neurology and epidemiology at Columbia University in New York, who was not associated with the research, said it “nicely distinguishes among the different causes of encephalopathy, including sepsis, hypoxia, and kidney failure … emphasizing just how sick these patients are.”
The study received no direct funding. Individual investigators were supported by grants from the National Institute on Aging and the National Institute of Neurological Disorders and Stroke. The investigators, Dr. Varelas, and Dr. Elkind have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NEUROCRITICAL CARE
KRYSTAL-1: Clear activity of adagrasib in KRAS-mutated NSCLC
An objective response rate was seen in 45% of patients, with a further 51% achieving stable disease, for a disease control rate of 96%.
“The vast majority of patients had significant tumor shrinkage,” said study investigator Gregory J. Riely, MD, PhD, when presenting the results at the European Lung Cancer Virtual Congress 2021 (Abstract 990_PR).
Dr. Riely, vice chair of clinical research in the department of medicine at Memorial Sloan Kettering Cancer Center in New York, noted that just 6 of the 70 patients in this phase 1/2 trial showed evidence of measurable tumor growth.
“This new way of targeting an oncogene may very well represent an evolutionary step forward in the management of lung cancer patients, akin to when we first had EGFR inhibitors,” Alastair Greystoke, MBChB, PhD, said in his discussion of the trial.
Dr. Greystoke, a clinical senior lecturer and honorary consultant in medical oncology at Newcastle (England) University, observed that the availability of KRAS-targeting agents could have a large potential impact on clinical practice. They could add another 14% of patients with NSCLC to the list of those who are eligible for molecularly-targeted therapy.
“It may be that soon, almost half our patients with lung adenocarcinoma will have a potential targetable abnormality,” Dr. Greystoke said.
Data confirm KRAS as a therapeutic target
Adagrasib is now the second drug to show promise as an inhibitor of KRAS G12C. In a phase 2 trial, the KRAS inhibitor sotorasib produced a response rate of 37%, a median response duration of 10 months, and a median progression-free survival of 6.8 months in patients with NSCLC.
Data on response duration and progression-free survival are not yet available for adagrasib. However, the duration of response extended past 11 months in four of the six patients who achieved a partial response to adagrasib in the phase 1/1b portion of the KRYSTAL-1 trial.
“What we’ve seen from this data, and data with other agents, is that responses are very heterogeneous,” Dr. Greystoke observed. “A small number of patients do not respond at all. In some patients, responses are short-lived, whilst in other patients, responses are long and still ongoing.”
KRYSTAL-1 study design and safety
KRYSTAL-1 is an ongoing phase 1/2 study designed to assess the safety and clinical activity of adagrasib in patients with advanced solid tumors that have a KRAS G12C mutation, including NSCLC.
Dr. Riely reported data on 79 patients with advanced or metastatic NSCLC who had progressed despite being treated with chemotherapy and immunotherapy. Of these, 18 patients had participated in the phase 1/1b dose-escalation and dose-expansion phase of the study, and 61 had participated in the phase 2 portion. Adagrasib was given at a twice-daily dose of 600 mg.
The patients’ median age was 65 years, 85% were White, and 57% were women. Almost all (95%) were current or former smokers, which is unsurprising since the KRAS G12C mutation is rarely seen in never-smokers. Almost all patients had nonsquamous histology (96%) and had received PD-1 or PD-L1 inhibitors (92%).
Treatment-related adverse events of any grade occurred in 85% of patients, and 30% of patients had grade 3-4 events. The most frequent treatment-related grade 3-4 adverse events were fatigue (6%), increased ALT or AST (each 5%), QT prolongation (3%), anemia (2%), nausea (2%), and vomiting (2%).
Two grade 5 adverse events were recorded – a case of pneumonitis in a patient with recurrent pneumonitis and one case of cardiac failure. Adverse events led to discontinuation in 4.5% of patients.
Greater effect seen with co-mutation
KRAS is commonly co-mutated, so the investigators performed an exploratory analysis to see if the presence of other mutations – STK11, KEAP1, and TP53 – might affect the results of adagrasib.
A greater objective response rate was seen in patients with the STK11 mutation than in those without it (64% and 33%, respectively). STK11 is associated with poorer responses to immune checkpoint inhibitors.
“We hypothesized that adagrasib treatment recruits T cells into the tumor and that T-cell infiltration may reverse STK11-mediated immune suppression,” Dr. Riely said. This theory seemed to be borne out with further analyses, though Dr. Greystoke raised doubts. There was no sign of STK11 mutations having any effect on response rates with adagrasib in preclinical studies.
Patients with KEAP1 as a co-mutation had a lower response rate than that of those without it (36% and 48%, respectively), which is in keeping with what might be expected. KEAP1 is known to be associated to a poor response to chemotherapy and immunotherapy.
“I think this data is very provocative but needs to be confirmed in larger cohorts,” Dr. Greystoke said. It could mean that adagrasib has the potential to turn a “cold tumor, hot,” enabling the use of immunotherapies.
A new cohort has been included in the KRYSTAL-1 study to further evaluate how having both the KRAS G12C and STK11 mutations may affect treatment with adagrasib.
Data could support drug combination
The adagrasib data lend support to the combination of KRAS G12C inhibitors with other molecularly-targeted treatments for NSCLC, Dr. Greystoke said, such as with tyrosine kinase inhibitors or immunotherapies. He noted that high steady-state levels of adagrasib were detected in the blood, and these levels were well above those needed for potential efficacy.
“This gives us confidence that if we do need to drop the dose below the recommended phase 2 dose to allow potential combinations with a small-molecule inhibitor due to overlapping toxicity or overlapping pharmacokinetics, that it is safe to do and shouldn’t [have an] impact on efficacy,” Dr. Greystoke said. “Overall, all this information will help us drive forward the next round of clinical trials of probably a combination of treatments.”
The KRYSTAL-1 study is supported by Mirati Therapeutics, Inc. Dr. Riely disclosed relationships with Mirati Therapeutics, Merck, Novartis, Pfizer, Takeda, and Roche. Dr. Greystoke was not involved in the study but disclosed relationships with Amgen, AstraZeneca, Boehringer-Ingelheim, Bristol-Myers Squibb, Merck, Novartis, Pfizer, Lilly, Takeda, and Roche.
An objective response rate was seen in 45% of patients, with a further 51% achieving stable disease, for a disease control rate of 96%.
“The vast majority of patients had significant tumor shrinkage,” said study investigator Gregory J. Riely, MD, PhD, when presenting the results at the European Lung Cancer Virtual Congress 2021 (Abstract 990_PR).
Dr. Riely, vice chair of clinical research in the department of medicine at Memorial Sloan Kettering Cancer Center in New York, noted that just 6 of the 70 patients in this phase 1/2 trial showed evidence of measurable tumor growth.
“This new way of targeting an oncogene may very well represent an evolutionary step forward in the management of lung cancer patients, akin to when we first had EGFR inhibitors,” Alastair Greystoke, MBChB, PhD, said in his discussion of the trial.
Dr. Greystoke, a clinical senior lecturer and honorary consultant in medical oncology at Newcastle (England) University, observed that the availability of KRAS-targeting agents could have a large potential impact on clinical practice. They could add another 14% of patients with NSCLC to the list of those who are eligible for molecularly-targeted therapy.
“It may be that soon, almost half our patients with lung adenocarcinoma will have a potential targetable abnormality,” Dr. Greystoke said.
Data confirm KRAS as a therapeutic target
Adagrasib is now the second drug to show promise as an inhibitor of KRAS G12C. In a phase 2 trial, the KRAS inhibitor sotorasib produced a response rate of 37%, a median response duration of 10 months, and a median progression-free survival of 6.8 months in patients with NSCLC.
Data on response duration and progression-free survival are not yet available for adagrasib. However, the duration of response extended past 11 months in four of the six patients who achieved a partial response to adagrasib in the phase 1/1b portion of the KRYSTAL-1 trial.
“What we’ve seen from this data, and data with other agents, is that responses are very heterogeneous,” Dr. Greystoke observed. “A small number of patients do not respond at all. In some patients, responses are short-lived, whilst in other patients, responses are long and still ongoing.”
KRYSTAL-1 study design and safety
KRYSTAL-1 is an ongoing phase 1/2 study designed to assess the safety and clinical activity of adagrasib in patients with advanced solid tumors that have a KRAS G12C mutation, including NSCLC.
Dr. Riely reported data on 79 patients with advanced or metastatic NSCLC who had progressed despite being treated with chemotherapy and immunotherapy. Of these, 18 patients had participated in the phase 1/1b dose-escalation and dose-expansion phase of the study, and 61 had participated in the phase 2 portion. Adagrasib was given at a twice-daily dose of 600 mg.
The patients’ median age was 65 years, 85% were White, and 57% were women. Almost all (95%) were current or former smokers, which is unsurprising since the KRAS G12C mutation is rarely seen in never-smokers. Almost all patients had nonsquamous histology (96%) and had received PD-1 or PD-L1 inhibitors (92%).
Treatment-related adverse events of any grade occurred in 85% of patients, and 30% of patients had grade 3-4 events. The most frequent treatment-related grade 3-4 adverse events were fatigue (6%), increased ALT or AST (each 5%), QT prolongation (3%), anemia (2%), nausea (2%), and vomiting (2%).
Two grade 5 adverse events were recorded – a case of pneumonitis in a patient with recurrent pneumonitis and one case of cardiac failure. Adverse events led to discontinuation in 4.5% of patients.
Greater effect seen with co-mutation
KRAS is commonly co-mutated, so the investigators performed an exploratory analysis to see if the presence of other mutations – STK11, KEAP1, and TP53 – might affect the results of adagrasib.
A greater objective response rate was seen in patients with the STK11 mutation than in those without it (64% and 33%, respectively). STK11 is associated with poorer responses to immune checkpoint inhibitors.
“We hypothesized that adagrasib treatment recruits T cells into the tumor and that T-cell infiltration may reverse STK11-mediated immune suppression,” Dr. Riely said. This theory seemed to be borne out with further analyses, though Dr. Greystoke raised doubts. There was no sign of STK11 mutations having any effect on response rates with adagrasib in preclinical studies.
Patients with KEAP1 as a co-mutation had a lower response rate than that of those without it (36% and 48%, respectively), which is in keeping with what might be expected. KEAP1 is known to be associated to a poor response to chemotherapy and immunotherapy.
“I think this data is very provocative but needs to be confirmed in larger cohorts,” Dr. Greystoke said. It could mean that adagrasib has the potential to turn a “cold tumor, hot,” enabling the use of immunotherapies.
A new cohort has been included in the KRYSTAL-1 study to further evaluate how having both the KRAS G12C and STK11 mutations may affect treatment with adagrasib.
Data could support drug combination
The adagrasib data lend support to the combination of KRAS G12C inhibitors with other molecularly-targeted treatments for NSCLC, Dr. Greystoke said, such as with tyrosine kinase inhibitors or immunotherapies. He noted that high steady-state levels of adagrasib were detected in the blood, and these levels were well above those needed for potential efficacy.
“This gives us confidence that if we do need to drop the dose below the recommended phase 2 dose to allow potential combinations with a small-molecule inhibitor due to overlapping toxicity or overlapping pharmacokinetics, that it is safe to do and shouldn’t [have an] impact on efficacy,” Dr. Greystoke said. “Overall, all this information will help us drive forward the next round of clinical trials of probably a combination of treatments.”
The KRYSTAL-1 study is supported by Mirati Therapeutics, Inc. Dr. Riely disclosed relationships with Mirati Therapeutics, Merck, Novartis, Pfizer, Takeda, and Roche. Dr. Greystoke was not involved in the study but disclosed relationships with Amgen, AstraZeneca, Boehringer-Ingelheim, Bristol-Myers Squibb, Merck, Novartis, Pfizer, Lilly, Takeda, and Roche.
An objective response rate was seen in 45% of patients, with a further 51% achieving stable disease, for a disease control rate of 96%.
“The vast majority of patients had significant tumor shrinkage,” said study investigator Gregory J. Riely, MD, PhD, when presenting the results at the European Lung Cancer Virtual Congress 2021 (Abstract 990_PR).
Dr. Riely, vice chair of clinical research in the department of medicine at Memorial Sloan Kettering Cancer Center in New York, noted that just 6 of the 70 patients in this phase 1/2 trial showed evidence of measurable tumor growth.
“This new way of targeting an oncogene may very well represent an evolutionary step forward in the management of lung cancer patients, akin to when we first had EGFR inhibitors,” Alastair Greystoke, MBChB, PhD, said in his discussion of the trial.
Dr. Greystoke, a clinical senior lecturer and honorary consultant in medical oncology at Newcastle (England) University, observed that the availability of KRAS-targeting agents could have a large potential impact on clinical practice. They could add another 14% of patients with NSCLC to the list of those who are eligible for molecularly-targeted therapy.
“It may be that soon, almost half our patients with lung adenocarcinoma will have a potential targetable abnormality,” Dr. Greystoke said.
Data confirm KRAS as a therapeutic target
Adagrasib is now the second drug to show promise as an inhibitor of KRAS G12C. In a phase 2 trial, the KRAS inhibitor sotorasib produced a response rate of 37%, a median response duration of 10 months, and a median progression-free survival of 6.8 months in patients with NSCLC.
Data on response duration and progression-free survival are not yet available for adagrasib. However, the duration of response extended past 11 months in four of the six patients who achieved a partial response to adagrasib in the phase 1/1b portion of the KRYSTAL-1 trial.
“What we’ve seen from this data, and data with other agents, is that responses are very heterogeneous,” Dr. Greystoke observed. “A small number of patients do not respond at all. In some patients, responses are short-lived, whilst in other patients, responses are long and still ongoing.”
KRYSTAL-1 study design and safety
KRYSTAL-1 is an ongoing phase 1/2 study designed to assess the safety and clinical activity of adagrasib in patients with advanced solid tumors that have a KRAS G12C mutation, including NSCLC.
Dr. Riely reported data on 79 patients with advanced or metastatic NSCLC who had progressed despite being treated with chemotherapy and immunotherapy. Of these, 18 patients had participated in the phase 1/1b dose-escalation and dose-expansion phase of the study, and 61 had participated in the phase 2 portion. Adagrasib was given at a twice-daily dose of 600 mg.
The patients’ median age was 65 years, 85% were White, and 57% were women. Almost all (95%) were current or former smokers, which is unsurprising since the KRAS G12C mutation is rarely seen in never-smokers. Almost all patients had nonsquamous histology (96%) and had received PD-1 or PD-L1 inhibitors (92%).
Treatment-related adverse events of any grade occurred in 85% of patients, and 30% of patients had grade 3-4 events. The most frequent treatment-related grade 3-4 adverse events were fatigue (6%), increased ALT or AST (each 5%), QT prolongation (3%), anemia (2%), nausea (2%), and vomiting (2%).
Two grade 5 adverse events were recorded – a case of pneumonitis in a patient with recurrent pneumonitis and one case of cardiac failure. Adverse events led to discontinuation in 4.5% of patients.
Greater effect seen with co-mutation
KRAS is commonly co-mutated, so the investigators performed an exploratory analysis to see if the presence of other mutations – STK11, KEAP1, and TP53 – might affect the results of adagrasib.
A greater objective response rate was seen in patients with the STK11 mutation than in those without it (64% and 33%, respectively). STK11 is associated with poorer responses to immune checkpoint inhibitors.
“We hypothesized that adagrasib treatment recruits T cells into the tumor and that T-cell infiltration may reverse STK11-mediated immune suppression,” Dr. Riely said. This theory seemed to be borne out with further analyses, though Dr. Greystoke raised doubts. There was no sign of STK11 mutations having any effect on response rates with adagrasib in preclinical studies.
Patients with KEAP1 as a co-mutation had a lower response rate than that of those without it (36% and 48%, respectively), which is in keeping with what might be expected. KEAP1 is known to be associated to a poor response to chemotherapy and immunotherapy.
“I think this data is very provocative but needs to be confirmed in larger cohorts,” Dr. Greystoke said. It could mean that adagrasib has the potential to turn a “cold tumor, hot,” enabling the use of immunotherapies.
A new cohort has been included in the KRYSTAL-1 study to further evaluate how having both the KRAS G12C and STK11 mutations may affect treatment with adagrasib.
Data could support drug combination
The adagrasib data lend support to the combination of KRAS G12C inhibitors with other molecularly-targeted treatments for NSCLC, Dr. Greystoke said, such as with tyrosine kinase inhibitors or immunotherapies. He noted that high steady-state levels of adagrasib were detected in the blood, and these levels were well above those needed for potential efficacy.
“This gives us confidence that if we do need to drop the dose below the recommended phase 2 dose to allow potential combinations with a small-molecule inhibitor due to overlapping toxicity or overlapping pharmacokinetics, that it is safe to do and shouldn’t [have an] impact on efficacy,” Dr. Greystoke said. “Overall, all this information will help us drive forward the next round of clinical trials of probably a combination of treatments.”
The KRYSTAL-1 study is supported by Mirati Therapeutics, Inc. Dr. Riely disclosed relationships with Mirati Therapeutics, Merck, Novartis, Pfizer, Takeda, and Roche. Dr. Greystoke was not involved in the study but disclosed relationships with Amgen, AstraZeneca, Boehringer-Ingelheim, Bristol-Myers Squibb, Merck, Novartis, Pfizer, Lilly, Takeda, and Roche.
FROM ELCC 2021
Arcalyst gets FDA nod as first therapy for recurrent pericarditis
The Food and Drug Administration has approved rilonacept (Arcalyst) to treat recurrent pericarditis and reduce the risk for recurrence in adults and children 12 years and older.
Approval of the weekly subcutaneous injection offers patients the first and only FDA-approved therapy for recurrent pericarditis, the agency said in a release.
Recurrent pericarditis is characterized by a remitting relapsing inflammation of the pericardium, and therapeutic options have been limited to NSAIDs, colchicine, and corticosteroids.
Rilonacept is a recombinant fusion protein that blocks interleukin-1 alpha and interleukin-1 beta signaling. It is already approved by the FDA to treat a group of rare inherited inflammatory diseases called cryopyrin-associated periodic syndromes.
The new indication is based on the pivotal phase 3 RHAPSODY trial in 86 patients with acute symptoms of recurrent pericarditis and systemic inflammation. After randomization, pericarditis recurred in 2 of 30 patients (7%) treated with rilonacept and in 23 of 31 patients (74%) treated with placebo, representing a 96% reduction in the relative risk for recurrence with rilonacept.
Patients who received rilonacept were also pain free or had minimal pain on 98% of trial days, whereas those who received placebo had minimal or no pain on 46% of trial days.
The most common adverse effects of rilonacept are injection-site reactions and upper-respiratory tract infections.
Serious, life-threatening infections have been reported in patients taking rilonacept, according to the FDA. Patients with active or chronic infections should not take the drug.
The FDA label also advises that patients should avoid live vaccines while taking rilonacept and that it should be discontinued if a hypersensitivity reaction occurs.
The commercial launch is expected in April, according to the company.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved rilonacept (Arcalyst) to treat recurrent pericarditis and reduce the risk for recurrence in adults and children 12 years and older.
Approval of the weekly subcutaneous injection offers patients the first and only FDA-approved therapy for recurrent pericarditis, the agency said in a release.
Recurrent pericarditis is characterized by a remitting relapsing inflammation of the pericardium, and therapeutic options have been limited to NSAIDs, colchicine, and corticosteroids.
Rilonacept is a recombinant fusion protein that blocks interleukin-1 alpha and interleukin-1 beta signaling. It is already approved by the FDA to treat a group of rare inherited inflammatory diseases called cryopyrin-associated periodic syndromes.
The new indication is based on the pivotal phase 3 RHAPSODY trial in 86 patients with acute symptoms of recurrent pericarditis and systemic inflammation. After randomization, pericarditis recurred in 2 of 30 patients (7%) treated with rilonacept and in 23 of 31 patients (74%) treated with placebo, representing a 96% reduction in the relative risk for recurrence with rilonacept.
Patients who received rilonacept were also pain free or had minimal pain on 98% of trial days, whereas those who received placebo had minimal or no pain on 46% of trial days.
The most common adverse effects of rilonacept are injection-site reactions and upper-respiratory tract infections.
Serious, life-threatening infections have been reported in patients taking rilonacept, according to the FDA. Patients with active or chronic infections should not take the drug.
The FDA label also advises that patients should avoid live vaccines while taking rilonacept and that it should be discontinued if a hypersensitivity reaction occurs.
The commercial launch is expected in April, according to the company.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved rilonacept (Arcalyst) to treat recurrent pericarditis and reduce the risk for recurrence in adults and children 12 years and older.
Approval of the weekly subcutaneous injection offers patients the first and only FDA-approved therapy for recurrent pericarditis, the agency said in a release.
Recurrent pericarditis is characterized by a remitting relapsing inflammation of the pericardium, and therapeutic options have been limited to NSAIDs, colchicine, and corticosteroids.
Rilonacept is a recombinant fusion protein that blocks interleukin-1 alpha and interleukin-1 beta signaling. It is already approved by the FDA to treat a group of rare inherited inflammatory diseases called cryopyrin-associated periodic syndromes.
The new indication is based on the pivotal phase 3 RHAPSODY trial in 86 patients with acute symptoms of recurrent pericarditis and systemic inflammation. After randomization, pericarditis recurred in 2 of 30 patients (7%) treated with rilonacept and in 23 of 31 patients (74%) treated with placebo, representing a 96% reduction in the relative risk for recurrence with rilonacept.
Patients who received rilonacept were also pain free or had minimal pain on 98% of trial days, whereas those who received placebo had minimal or no pain on 46% of trial days.
The most common adverse effects of rilonacept are injection-site reactions and upper-respiratory tract infections.
Serious, life-threatening infections have been reported in patients taking rilonacept, according to the FDA. Patients with active or chronic infections should not take the drug.
The FDA label also advises that patients should avoid live vaccines while taking rilonacept and that it should be discontinued if a hypersensitivity reaction occurs.
The commercial launch is expected in April, according to the company.
A version of this article first appeared on Medscape.com.
In U.S., lockdowns added 2 pounds per month
Americans gained nearly 2 pounds per month under COVID-19 shelter-in-place orders in 2020, according to a new study published March 22, 2021, in JAMA Network Open.
Those who kept the same lockdown habits could have gained 20 pounds during the past year, the study authors said.
“We know that weight gain is a public health problem in the U.S. already, so anything making it worse is definitely concerning, and shelter-in-place orders are so ubiquitous that the sheer number of people affected by this makes it extremely relevant,” Gregory Marcus, MD, the senior author and a cardiologist at the University of California, San Francisco, told the New York Times.
Dr. Marcus and colleagues analyzed more than 7,000 weight measurements from 269 people in 37 states who used Bluetooth-connected scales from Feb. 1 to June 1, 2020. Among the participants, about 52% were women, 77% were White, and they had an average age of 52 years.
The research team found that participants had a steady weight gain of more than half a pound every 10 days. That equals about 1.5-2 pounds per month.
Many of the participants were losing weight before the shelter-in-place orders went into effect, Dr. Marcus said. The lockdown effects could be even greater for those who weren’t losing weight before.
“It’s reasonable to assume these individuals are more engaged with their health in general, and more disciplined and on top of things,” he said. “That suggests we could be underestimating – that this is the tip of the iceberg.”
The small study doesn’t represent all of the nation and can’t be generalized to the U.S. population, the study authors noted, but it’s an indicator of what happened during the pandemic. The participants’ weight increased regardless of their location and chronic medical conditions.
Overall, people don’t move around as much during lockdowns, the UCSF researchers reported in another study published in Annals of Internal Medicine in November 2020. According to smartphone data, daily step counts decreased by 27% in March 2020. The step counts increased again throughout the summer but still remained lower than before the COVID-19 pandemic.
“The detrimental health outcomes suggested by these data demonstrate a need to identify concurrent strategies to mitigate weight gain,” the authors wrote in the JAMA Network Open study, “such as encouraging healthy diets and exploring ways to enhance physical activity, as local governments consider new constraints in response to SARS-CoV-2 and potential future pandemics.”
A version of this article first appeared on WebMD.com.
Americans gained nearly 2 pounds per month under COVID-19 shelter-in-place orders in 2020, according to a new study published March 22, 2021, in JAMA Network Open.
Those who kept the same lockdown habits could have gained 20 pounds during the past year, the study authors said.
“We know that weight gain is a public health problem in the U.S. already, so anything making it worse is definitely concerning, and shelter-in-place orders are so ubiquitous that the sheer number of people affected by this makes it extremely relevant,” Gregory Marcus, MD, the senior author and a cardiologist at the University of California, San Francisco, told the New York Times.
Dr. Marcus and colleagues analyzed more than 7,000 weight measurements from 269 people in 37 states who used Bluetooth-connected scales from Feb. 1 to June 1, 2020. Among the participants, about 52% were women, 77% were White, and they had an average age of 52 years.
The research team found that participants had a steady weight gain of more than half a pound every 10 days. That equals about 1.5-2 pounds per month.
Many of the participants were losing weight before the shelter-in-place orders went into effect, Dr. Marcus said. The lockdown effects could be even greater for those who weren’t losing weight before.
“It’s reasonable to assume these individuals are more engaged with their health in general, and more disciplined and on top of things,” he said. “That suggests we could be underestimating – that this is the tip of the iceberg.”
The small study doesn’t represent all of the nation and can’t be generalized to the U.S. population, the study authors noted, but it’s an indicator of what happened during the pandemic. The participants’ weight increased regardless of their location and chronic medical conditions.
Overall, people don’t move around as much during lockdowns, the UCSF researchers reported in another study published in Annals of Internal Medicine in November 2020. According to smartphone data, daily step counts decreased by 27% in March 2020. The step counts increased again throughout the summer but still remained lower than before the COVID-19 pandemic.
“The detrimental health outcomes suggested by these data demonstrate a need to identify concurrent strategies to mitigate weight gain,” the authors wrote in the JAMA Network Open study, “such as encouraging healthy diets and exploring ways to enhance physical activity, as local governments consider new constraints in response to SARS-CoV-2 and potential future pandemics.”
A version of this article first appeared on WebMD.com.
Americans gained nearly 2 pounds per month under COVID-19 shelter-in-place orders in 2020, according to a new study published March 22, 2021, in JAMA Network Open.
Those who kept the same lockdown habits could have gained 20 pounds during the past year, the study authors said.
“We know that weight gain is a public health problem in the U.S. already, so anything making it worse is definitely concerning, and shelter-in-place orders are so ubiquitous that the sheer number of people affected by this makes it extremely relevant,” Gregory Marcus, MD, the senior author and a cardiologist at the University of California, San Francisco, told the New York Times.
Dr. Marcus and colleagues analyzed more than 7,000 weight measurements from 269 people in 37 states who used Bluetooth-connected scales from Feb. 1 to June 1, 2020. Among the participants, about 52% were women, 77% were White, and they had an average age of 52 years.
The research team found that participants had a steady weight gain of more than half a pound every 10 days. That equals about 1.5-2 pounds per month.
Many of the participants were losing weight before the shelter-in-place orders went into effect, Dr. Marcus said. The lockdown effects could be even greater for those who weren’t losing weight before.
“It’s reasonable to assume these individuals are more engaged with their health in general, and more disciplined and on top of things,” he said. “That suggests we could be underestimating – that this is the tip of the iceberg.”
The small study doesn’t represent all of the nation and can’t be generalized to the U.S. population, the study authors noted, but it’s an indicator of what happened during the pandemic. The participants’ weight increased regardless of their location and chronic medical conditions.
Overall, people don’t move around as much during lockdowns, the UCSF researchers reported in another study published in Annals of Internal Medicine in November 2020. According to smartphone data, daily step counts decreased by 27% in March 2020. The step counts increased again throughout the summer but still remained lower than before the COVID-19 pandemic.
“The detrimental health outcomes suggested by these data demonstrate a need to identify concurrent strategies to mitigate weight gain,” the authors wrote in the JAMA Network Open study, “such as encouraging healthy diets and exploring ways to enhance physical activity, as local governments consider new constraints in response to SARS-CoV-2 and potential future pandemics.”
A version of this article first appeared on WebMD.com.
Drug-resistant TB trial stopped early after successful results
Médecins Sans Frontières (MSF/Doctors Without Borders) announced early closure of its phase 2/3 trial of a 6-month multidrug regimen for multidrug-resistant tuberculosis (MDR-TB) because an independent data safety and monitoring board (DSMB) determined that the drug combination in the study regimen was superior to current therapy, according to a press release.
The trial, called TB PRACTECAL, compared the current local standard of care with a 6-month regimen of bedaquiline, pretomanid, linezolid, and moxifloxacin. The interim analysis included 242 patients and the randomized, controlled trial was conducted in sites in Belarus, South Africa, and Uzbekistan.
The preliminary data will be shared with the World Health Organization soon and will also be submitted to a peer-reviewed journal. If it withstands further reviews, as is anticipated, the trial would support the first solely oral regimen for MDR-TB.
In 2019, an estimated 465,000 people developed MDR-TB and 182,000 died. The global burden of TB at that time was about 10 million new cases, many with coexisting HIV.
Current treatment for MDR-TB lasts 9-20 months and is complicated by the need for painful shots and toxic antibiotics. Side effects can include psychiatric problems from quinolones, isoniazid, ethambutol, or cycloserine; deafness from aminoglycosides; and bone marrow suppression from linezolid, among other toxicities.
It’s hoped that the shorter regimen will reduce toxicity and improve patient compliance. Poor adherence to treatment is a major driver of further drug resistance. Current regimens require up to 20 pills per day as well as daily injections.
In a prepared statement from MSF, David Moore, MD, MSc, London School of Hygiene and Tropical Medicine, a member of the TB-PRACTECAL trial’s steering committee, concluded: “The findings could transform the way we treat patients with drug-resistant forms of TB worldwide, who have been neglected for too long.”
This good news is particularly welcome as, in the time of COVID-19, “an estimated 1.4 million fewer people received care for tuberculosis in 2020 than in 2019,” according to the WHO. The drop, an overall 21% reduction in patients beginning treatment, ranged as high as 42% in Indonesia.
Although awaiting complete data, Madhukar Pai, MD, PhD, associate director of the McGill International TB Centre, McGill University, Montreal, shares Dr. Moore’s enthusiasm. In an interview, Dr. Pai compared MDR-TB with extensively drug-resistant TB (XDR-TB).
“I’m excited about the possibility that these trial results might help shorten MDR-TB treatment to 6 months,” said Dr. Pai. “That will be a huge relief to all patients battling drug-resistant disease. The 6-month BPaL regimen (bedaquiline, pretomanid, and linezolid) regimen works well in XDR-TB. So, I would expect the TB PRACTECAL regimen with one added drug (moxifloxacin) to work well in MDR-TB, which is less severe than XDR-TB. Between these two regimens, if we can bring down MDR and XDR treatment to 6 months, all oral, that would be a huge advance.”
The expense of bedaquiline has been a long-standing concern in the global health community. Janssen, a subsidiary of Johnson & Johnson, has reduced the price to $340 per 6-month treatment course for more than 135 eligible low- and middle-income countries.
Previously, the tiered pricing structure was different for low-, middle-, and high-income countries (U.S. $900, $3,000, and $30,000, respectively). “The global TB community has asked Janssen to drop the price of bedaquiline to a level no higher than $32 per month – double the price at which researchers estimated bedaquiline could be sold for a profit,” according to the Treatment Action Group A major source of contention over pricing has been that there has been considerable public investment in the drug›s development.
Dr. Pai concluded: “Bedaquiline is likely the most important drug in both 6-month regimens. We need to work harder to make bedaquiline, an excellent drug, more affordable and accessible.”
While the full data is not yet publicly available, TB PRACTECAL was a randomized, controlled, multicenter study. The fact that enrollment was discontinued early by the DSMB suggests the efficacy data was compelling and that this completely oral regimen will become the standard of care.
Dr. Stone is an infectious disease specialist and author of Resilience: One Family’s Story of Hope and Triumph Over Evil and of Conducting Clinical Research, the essential guide to the topic. A version of this article first appeared on Medscape.com.
Médecins Sans Frontières (MSF/Doctors Without Borders) announced early closure of its phase 2/3 trial of a 6-month multidrug regimen for multidrug-resistant tuberculosis (MDR-TB) because an independent data safety and monitoring board (DSMB) determined that the drug combination in the study regimen was superior to current therapy, according to a press release.
The trial, called TB PRACTECAL, compared the current local standard of care with a 6-month regimen of bedaquiline, pretomanid, linezolid, and moxifloxacin. The interim analysis included 242 patients and the randomized, controlled trial was conducted in sites in Belarus, South Africa, and Uzbekistan.
The preliminary data will be shared with the World Health Organization soon and will also be submitted to a peer-reviewed journal. If it withstands further reviews, as is anticipated, the trial would support the first solely oral regimen for MDR-TB.
In 2019, an estimated 465,000 people developed MDR-TB and 182,000 died. The global burden of TB at that time was about 10 million new cases, many with coexisting HIV.
Current treatment for MDR-TB lasts 9-20 months and is complicated by the need for painful shots and toxic antibiotics. Side effects can include psychiatric problems from quinolones, isoniazid, ethambutol, or cycloserine; deafness from aminoglycosides; and bone marrow suppression from linezolid, among other toxicities.
It’s hoped that the shorter regimen will reduce toxicity and improve patient compliance. Poor adherence to treatment is a major driver of further drug resistance. Current regimens require up to 20 pills per day as well as daily injections.
In a prepared statement from MSF, David Moore, MD, MSc, London School of Hygiene and Tropical Medicine, a member of the TB-PRACTECAL trial’s steering committee, concluded: “The findings could transform the way we treat patients with drug-resistant forms of TB worldwide, who have been neglected for too long.”
This good news is particularly welcome as, in the time of COVID-19, “an estimated 1.4 million fewer people received care for tuberculosis in 2020 than in 2019,” according to the WHO. The drop, an overall 21% reduction in patients beginning treatment, ranged as high as 42% in Indonesia.
Although awaiting complete data, Madhukar Pai, MD, PhD, associate director of the McGill International TB Centre, McGill University, Montreal, shares Dr. Moore’s enthusiasm. In an interview, Dr. Pai compared MDR-TB with extensively drug-resistant TB (XDR-TB).
“I’m excited about the possibility that these trial results might help shorten MDR-TB treatment to 6 months,” said Dr. Pai. “That will be a huge relief to all patients battling drug-resistant disease. The 6-month BPaL regimen (bedaquiline, pretomanid, and linezolid) regimen works well in XDR-TB. So, I would expect the TB PRACTECAL regimen with one added drug (moxifloxacin) to work well in MDR-TB, which is less severe than XDR-TB. Between these two regimens, if we can bring down MDR and XDR treatment to 6 months, all oral, that would be a huge advance.”
The expense of bedaquiline has been a long-standing concern in the global health community. Janssen, a subsidiary of Johnson & Johnson, has reduced the price to $340 per 6-month treatment course for more than 135 eligible low- and middle-income countries.
Previously, the tiered pricing structure was different for low-, middle-, and high-income countries (U.S. $900, $3,000, and $30,000, respectively). “The global TB community has asked Janssen to drop the price of bedaquiline to a level no higher than $32 per month – double the price at which researchers estimated bedaquiline could be sold for a profit,” according to the Treatment Action Group A major source of contention over pricing has been that there has been considerable public investment in the drug›s development.
Dr. Pai concluded: “Bedaquiline is likely the most important drug in both 6-month regimens. We need to work harder to make bedaquiline, an excellent drug, more affordable and accessible.”
While the full data is not yet publicly available, TB PRACTECAL was a randomized, controlled, multicenter study. The fact that enrollment was discontinued early by the DSMB suggests the efficacy data was compelling and that this completely oral regimen will become the standard of care.
Dr. Stone is an infectious disease specialist and author of Resilience: One Family’s Story of Hope and Triumph Over Evil and of Conducting Clinical Research, the essential guide to the topic. A version of this article first appeared on Medscape.com.
Médecins Sans Frontières (MSF/Doctors Without Borders) announced early closure of its phase 2/3 trial of a 6-month multidrug regimen for multidrug-resistant tuberculosis (MDR-TB) because an independent data safety and monitoring board (DSMB) determined that the drug combination in the study regimen was superior to current therapy, according to a press release.
The trial, called TB PRACTECAL, compared the current local standard of care with a 6-month regimen of bedaquiline, pretomanid, linezolid, and moxifloxacin. The interim analysis included 242 patients and the randomized, controlled trial was conducted in sites in Belarus, South Africa, and Uzbekistan.
The preliminary data will be shared with the World Health Organization soon and will also be submitted to a peer-reviewed journal. If it withstands further reviews, as is anticipated, the trial would support the first solely oral regimen for MDR-TB.
In 2019, an estimated 465,000 people developed MDR-TB and 182,000 died. The global burden of TB at that time was about 10 million new cases, many with coexisting HIV.
Current treatment for MDR-TB lasts 9-20 months and is complicated by the need for painful shots and toxic antibiotics. Side effects can include psychiatric problems from quinolones, isoniazid, ethambutol, or cycloserine; deafness from aminoglycosides; and bone marrow suppression from linezolid, among other toxicities.
It’s hoped that the shorter regimen will reduce toxicity and improve patient compliance. Poor adherence to treatment is a major driver of further drug resistance. Current regimens require up to 20 pills per day as well as daily injections.
In a prepared statement from MSF, David Moore, MD, MSc, London School of Hygiene and Tropical Medicine, a member of the TB-PRACTECAL trial’s steering committee, concluded: “The findings could transform the way we treat patients with drug-resistant forms of TB worldwide, who have been neglected for too long.”
This good news is particularly welcome as, in the time of COVID-19, “an estimated 1.4 million fewer people received care for tuberculosis in 2020 than in 2019,” according to the WHO. The drop, an overall 21% reduction in patients beginning treatment, ranged as high as 42% in Indonesia.
Although awaiting complete data, Madhukar Pai, MD, PhD, associate director of the McGill International TB Centre, McGill University, Montreal, shares Dr. Moore’s enthusiasm. In an interview, Dr. Pai compared MDR-TB with extensively drug-resistant TB (XDR-TB).
“I’m excited about the possibility that these trial results might help shorten MDR-TB treatment to 6 months,” said Dr. Pai. “That will be a huge relief to all patients battling drug-resistant disease. The 6-month BPaL regimen (bedaquiline, pretomanid, and linezolid) regimen works well in XDR-TB. So, I would expect the TB PRACTECAL regimen with one added drug (moxifloxacin) to work well in MDR-TB, which is less severe than XDR-TB. Between these two regimens, if we can bring down MDR and XDR treatment to 6 months, all oral, that would be a huge advance.”
The expense of bedaquiline has been a long-standing concern in the global health community. Janssen, a subsidiary of Johnson & Johnson, has reduced the price to $340 per 6-month treatment course for more than 135 eligible low- and middle-income countries.
Previously, the tiered pricing structure was different for low-, middle-, and high-income countries (U.S. $900, $3,000, and $30,000, respectively). “The global TB community has asked Janssen to drop the price of bedaquiline to a level no higher than $32 per month – double the price at which researchers estimated bedaquiline could be sold for a profit,” according to the Treatment Action Group A major source of contention over pricing has been that there has been considerable public investment in the drug›s development.
Dr. Pai concluded: “Bedaquiline is likely the most important drug in both 6-month regimens. We need to work harder to make bedaquiline, an excellent drug, more affordable and accessible.”
While the full data is not yet publicly available, TB PRACTECAL was a randomized, controlled, multicenter study. The fact that enrollment was discontinued early by the DSMB suggests the efficacy data was compelling and that this completely oral regimen will become the standard of care.
Dr. Stone is an infectious disease specialist and author of Resilience: One Family’s Story of Hope and Triumph Over Evil and of Conducting Clinical Research, the essential guide to the topic. A version of this article first appeared on Medscape.com.
Vitamin D may protect against COVID-19, especially in Black patients
Higher levels of vitamin D than traditionally considered sufficient may help prevent COVID-19 infection – particularly in Black patients, shows a new single-center, retrospective study looking at the role of vitamin D in prevention of infection.
The study, published recently in JAMA Network Open, noted that expert opinion varies as to what “sufficient” levels of vitamin D are, some define this as 30 ng/mL, while others cite 40 ng/mL or greater.
In their discussion, the authors also noted that their results showed the “risk of positive COVID-19 test results decreased significantly with increased vitamin D level of 30 ng/mL or greater when measured as a continuous variable.”
“These new results tell us that having vitamin D levels above those normally considered sufficient is associated with decreased risk of testing positive for COVID-19, at least in Black individuals,” lead author, David Meltzer, MD, chief of hospital medicine at the University of Chicago, said in a press release from his institution.
“These findings suggest that randomized clinical trials to determine whether increasing vitamin D levels to greater than 30-40 ng/mL affect COVID-19 risk are warranted, especially in Black individuals,” he and his coauthors said.
Vitamin D at time of testing most strongly associated with COVID risk
An earlier study by the same researchers found that vitamin D deficiency (less than 20 ng/mL) may raise the risk of testing positive for COVID-19 in people from various ethnicities, as reported by this news organization.
Data for this latest study were drawn from electronic health records for 4,638 individuals at the University of Chicago Medicine and were used to examine whether the likelihood of a positive COVID-19 test was associated with a person’s most recent vitamin D level (within the previous year), and whether there was any effect of ethnicity on this outcome.
Mean age was 52.8 years, 69% were women, 49% were Black, 43% White, and 8% were another race/ethnicity. A total of 27% of the individuals were deficient in vitamin D (less than 20 ng/mL), 27% had insufficient levels (20-30 ng/mL), 22% had sufficient levels (30-40 ng/mL), and the remaining 24% had levels of 40 ng/mL or greater.
In total, 333 (7%) of people tested positive for COVID-19, including 102 (5%) Whites and 211 (9%) Blacks. And 36% of Black individuals who tested positive for COVID-19 were classified as vitamin D deficient, compared with 16% of Whites.
A positive test result for COVID-19 was not significantly associated with vitamin D levels in white individuals but was in Black individuals.
In Black people, compared with levels of at least 40 ng/mL, vitamin D levels of 30-40 ng/mL were associated with an incidence rate ratio of 2.64 for COVID-19 positivity (P = .01). For levels of 20-30 ng/mL, the IRR was 1.69 (P = 0.21); and for less than 20 ng/mL the IRR was 2.55 (P = .009).
The researchers also found that the risk of positive test results with lower vitamin D levels increased when those levels were lower just prior to the positive COVID-19 test, lending “support [to] the idea that vitamin D level at the time of testing is most strongly associated with COVID-19 risk,” they wrote.
Try upping vitamin D levels to 40 ng/mL or greater to prevent COVID?
In their discussion, the authors noted that significant association of vitamin D levels with COVID-19 risk in Blacks but not in Whites, “could reflect their higher COVID-19 risk, to which socioeconomic factors and structural inequities clearly contribute.
“Biological susceptibility to vitamin D deficiency may also be less frequent in White than Black individuals, since lighter skin increases vitamin D production in response to sunlight, and vitamin D binding proteins may vary by race and affect vitamin D bioavailability.”
Given less than 10% of U.S. adults have a vitamin D level greater than 40 ng/mL, the study findings increase the urgency to consider whether increased sun exposure or supplementation could reduce COVID-19 risk, according to the authors.
“When increased sun exposure is impractical, achieving vitamin D levels of 40 ng/mL or greater typically requires greater supplementation than currently recommended for most individuals of 600-800 IU/d vitamin D3,” they added.
However, Dr. Meltzer also acknowledged that “this is an observational study. We can see that there’s an association between vitamin D levels and likelihood of a COVID-19 diagnosis, but we don’t know exactly why that is, or whether these results are due to the vitamin D directly or other related biological factors.”
All in all, the authors suggested that randomized clinical trials are needed to understand if vitamin D can reduce COVID-19 risk, and as such they should include doses of supplements likely to increase vitamin D to at least 40 ng/mL, and perhaps even higher, although they pointed out that the latter must be achieved safely.
“Studies should also consider the role of vitamin D testing, loading doses, dose adjustments for individuals who are obese or overweight, risks for hypercalcemia, and strategies to monitor for and mitigate hypercalcemia, and that non-White populations, such as Black individuals, may have greater needs for supplementation,” they outlined.
They are now recruiting participants for two separate clinical trials testing the efficacy of vitamin D supplements for preventing COVID-19.
The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Higher levels of vitamin D than traditionally considered sufficient may help prevent COVID-19 infection – particularly in Black patients, shows a new single-center, retrospective study looking at the role of vitamin D in prevention of infection.
The study, published recently in JAMA Network Open, noted that expert opinion varies as to what “sufficient” levels of vitamin D are, some define this as 30 ng/mL, while others cite 40 ng/mL or greater.
In their discussion, the authors also noted that their results showed the “risk of positive COVID-19 test results decreased significantly with increased vitamin D level of 30 ng/mL or greater when measured as a continuous variable.”
“These new results tell us that having vitamin D levels above those normally considered sufficient is associated with decreased risk of testing positive for COVID-19, at least in Black individuals,” lead author, David Meltzer, MD, chief of hospital medicine at the University of Chicago, said in a press release from his institution.
“These findings suggest that randomized clinical trials to determine whether increasing vitamin D levels to greater than 30-40 ng/mL affect COVID-19 risk are warranted, especially in Black individuals,” he and his coauthors said.
Vitamin D at time of testing most strongly associated with COVID risk
An earlier study by the same researchers found that vitamin D deficiency (less than 20 ng/mL) may raise the risk of testing positive for COVID-19 in people from various ethnicities, as reported by this news organization.
Data for this latest study were drawn from electronic health records for 4,638 individuals at the University of Chicago Medicine and were used to examine whether the likelihood of a positive COVID-19 test was associated with a person’s most recent vitamin D level (within the previous year), and whether there was any effect of ethnicity on this outcome.
Mean age was 52.8 years, 69% were women, 49% were Black, 43% White, and 8% were another race/ethnicity. A total of 27% of the individuals were deficient in vitamin D (less than 20 ng/mL), 27% had insufficient levels (20-30 ng/mL), 22% had sufficient levels (30-40 ng/mL), and the remaining 24% had levels of 40 ng/mL or greater.
In total, 333 (7%) of people tested positive for COVID-19, including 102 (5%) Whites and 211 (9%) Blacks. And 36% of Black individuals who tested positive for COVID-19 were classified as vitamin D deficient, compared with 16% of Whites.
A positive test result for COVID-19 was not significantly associated with vitamin D levels in white individuals but was in Black individuals.
In Black people, compared with levels of at least 40 ng/mL, vitamin D levels of 30-40 ng/mL were associated with an incidence rate ratio of 2.64 for COVID-19 positivity (P = .01). For levels of 20-30 ng/mL, the IRR was 1.69 (P = 0.21); and for less than 20 ng/mL the IRR was 2.55 (P = .009).
The researchers also found that the risk of positive test results with lower vitamin D levels increased when those levels were lower just prior to the positive COVID-19 test, lending “support [to] the idea that vitamin D level at the time of testing is most strongly associated with COVID-19 risk,” they wrote.
Try upping vitamin D levels to 40 ng/mL or greater to prevent COVID?
In their discussion, the authors noted that significant association of vitamin D levels with COVID-19 risk in Blacks but not in Whites, “could reflect their higher COVID-19 risk, to which socioeconomic factors and structural inequities clearly contribute.
“Biological susceptibility to vitamin D deficiency may also be less frequent in White than Black individuals, since lighter skin increases vitamin D production in response to sunlight, and vitamin D binding proteins may vary by race and affect vitamin D bioavailability.”
Given less than 10% of U.S. adults have a vitamin D level greater than 40 ng/mL, the study findings increase the urgency to consider whether increased sun exposure or supplementation could reduce COVID-19 risk, according to the authors.
“When increased sun exposure is impractical, achieving vitamin D levels of 40 ng/mL or greater typically requires greater supplementation than currently recommended for most individuals of 600-800 IU/d vitamin D3,” they added.
However, Dr. Meltzer also acknowledged that “this is an observational study. We can see that there’s an association between vitamin D levels and likelihood of a COVID-19 diagnosis, but we don’t know exactly why that is, or whether these results are due to the vitamin D directly or other related biological factors.”
All in all, the authors suggested that randomized clinical trials are needed to understand if vitamin D can reduce COVID-19 risk, and as such they should include doses of supplements likely to increase vitamin D to at least 40 ng/mL, and perhaps even higher, although they pointed out that the latter must be achieved safely.
“Studies should also consider the role of vitamin D testing, loading doses, dose adjustments for individuals who are obese or overweight, risks for hypercalcemia, and strategies to monitor for and mitigate hypercalcemia, and that non-White populations, such as Black individuals, may have greater needs for supplementation,” they outlined.
They are now recruiting participants for two separate clinical trials testing the efficacy of vitamin D supplements for preventing COVID-19.
The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Higher levels of vitamin D than traditionally considered sufficient may help prevent COVID-19 infection – particularly in Black patients, shows a new single-center, retrospective study looking at the role of vitamin D in prevention of infection.
The study, published recently in JAMA Network Open, noted that expert opinion varies as to what “sufficient” levels of vitamin D are, some define this as 30 ng/mL, while others cite 40 ng/mL or greater.
In their discussion, the authors also noted that their results showed the “risk of positive COVID-19 test results decreased significantly with increased vitamin D level of 30 ng/mL or greater when measured as a continuous variable.”
“These new results tell us that having vitamin D levels above those normally considered sufficient is associated with decreased risk of testing positive for COVID-19, at least in Black individuals,” lead author, David Meltzer, MD, chief of hospital medicine at the University of Chicago, said in a press release from his institution.
“These findings suggest that randomized clinical trials to determine whether increasing vitamin D levels to greater than 30-40 ng/mL affect COVID-19 risk are warranted, especially in Black individuals,” he and his coauthors said.
Vitamin D at time of testing most strongly associated with COVID risk
An earlier study by the same researchers found that vitamin D deficiency (less than 20 ng/mL) may raise the risk of testing positive for COVID-19 in people from various ethnicities, as reported by this news organization.
Data for this latest study were drawn from electronic health records for 4,638 individuals at the University of Chicago Medicine and were used to examine whether the likelihood of a positive COVID-19 test was associated with a person’s most recent vitamin D level (within the previous year), and whether there was any effect of ethnicity on this outcome.
Mean age was 52.8 years, 69% were women, 49% were Black, 43% White, and 8% were another race/ethnicity. A total of 27% of the individuals were deficient in vitamin D (less than 20 ng/mL), 27% had insufficient levels (20-30 ng/mL), 22% had sufficient levels (30-40 ng/mL), and the remaining 24% had levels of 40 ng/mL or greater.
In total, 333 (7%) of people tested positive for COVID-19, including 102 (5%) Whites and 211 (9%) Blacks. And 36% of Black individuals who tested positive for COVID-19 were classified as vitamin D deficient, compared with 16% of Whites.
A positive test result for COVID-19 was not significantly associated with vitamin D levels in white individuals but was in Black individuals.
In Black people, compared with levels of at least 40 ng/mL, vitamin D levels of 30-40 ng/mL were associated with an incidence rate ratio of 2.64 for COVID-19 positivity (P = .01). For levels of 20-30 ng/mL, the IRR was 1.69 (P = 0.21); and for less than 20 ng/mL the IRR was 2.55 (P = .009).
The researchers also found that the risk of positive test results with lower vitamin D levels increased when those levels were lower just prior to the positive COVID-19 test, lending “support [to] the idea that vitamin D level at the time of testing is most strongly associated with COVID-19 risk,” they wrote.
Try upping vitamin D levels to 40 ng/mL or greater to prevent COVID?
In their discussion, the authors noted that significant association of vitamin D levels with COVID-19 risk in Blacks but not in Whites, “could reflect their higher COVID-19 risk, to which socioeconomic factors and structural inequities clearly contribute.
“Biological susceptibility to vitamin D deficiency may also be less frequent in White than Black individuals, since lighter skin increases vitamin D production in response to sunlight, and vitamin D binding proteins may vary by race and affect vitamin D bioavailability.”
Given less than 10% of U.S. adults have a vitamin D level greater than 40 ng/mL, the study findings increase the urgency to consider whether increased sun exposure or supplementation could reduce COVID-19 risk, according to the authors.
“When increased sun exposure is impractical, achieving vitamin D levels of 40 ng/mL or greater typically requires greater supplementation than currently recommended for most individuals of 600-800 IU/d vitamin D3,” they added.
However, Dr. Meltzer also acknowledged that “this is an observational study. We can see that there’s an association between vitamin D levels and likelihood of a COVID-19 diagnosis, but we don’t know exactly why that is, or whether these results are due to the vitamin D directly or other related biological factors.”
All in all, the authors suggested that randomized clinical trials are needed to understand if vitamin D can reduce COVID-19 risk, and as such they should include doses of supplements likely to increase vitamin D to at least 40 ng/mL, and perhaps even higher, although they pointed out that the latter must be achieved safely.
“Studies should also consider the role of vitamin D testing, loading doses, dose adjustments for individuals who are obese or overweight, risks for hypercalcemia, and strategies to monitor for and mitigate hypercalcemia, and that non-White populations, such as Black individuals, may have greater needs for supplementation,” they outlined.
They are now recruiting participants for two separate clinical trials testing the efficacy of vitamin D supplements for preventing COVID-19.
The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Senate confirms Murthy as Surgeon General
Seven Republicans – Bill Cassidy (La.), Susan Collins (Maine), Roger Marshall (Kan.), Susan Murkowski (Alaska), Rob Portman (Ohio), Mitt Romney (Utah), and Dan Sullivan (Alaska) – joined all the Democrats and independents in the 57-43 vote approving Dr. Murthy’s nomination.
Dr. Murthy, 43, previously served as the 19th Surgeon General, from December 2014 to April 2017, when he was asked to step down by President Donald J. Trump.
Surgeons General serve 4-year terms.
During his first tenure, Dr. Murthy issued the first-ever Surgeon General’s report on the crisis of addiction and issued a call to action to doctors to help battle the opioid crisis.
When Dr. Murthy was nominated by President-elect Joseph R. Biden Jr. in December, he was acting as cochair of the incoming administration’s COVID-19 transition advisory board.
Early in 2020, before the COVID-19 pandemic hit, Dr. Murthy published a timely book: “Together: The Healing Power of Human Connection in a Sometimes Lonely World”.
He earned his bachelor’s degree from Harvard and his MD and MBA degrees from Yale. He completed his internal medicine residency at Brigham and Women’s Hospital in Boston, where he also served as a hospitalist, and later joined Harvard Medical School as a faculty member in internal medicine.
He is married to Alice Chen, MD. The couple have two children.
A version of this article first appeared on WebMD.com.
Seven Republicans – Bill Cassidy (La.), Susan Collins (Maine), Roger Marshall (Kan.), Susan Murkowski (Alaska), Rob Portman (Ohio), Mitt Romney (Utah), and Dan Sullivan (Alaska) – joined all the Democrats and independents in the 57-43 vote approving Dr. Murthy’s nomination.
Dr. Murthy, 43, previously served as the 19th Surgeon General, from December 2014 to April 2017, when he was asked to step down by President Donald J. Trump.
Surgeons General serve 4-year terms.
During his first tenure, Dr. Murthy issued the first-ever Surgeon General’s report on the crisis of addiction and issued a call to action to doctors to help battle the opioid crisis.
When Dr. Murthy was nominated by President-elect Joseph R. Biden Jr. in December, he was acting as cochair of the incoming administration’s COVID-19 transition advisory board.
Early in 2020, before the COVID-19 pandemic hit, Dr. Murthy published a timely book: “Together: The Healing Power of Human Connection in a Sometimes Lonely World”.
He earned his bachelor’s degree from Harvard and his MD and MBA degrees from Yale. He completed his internal medicine residency at Brigham and Women’s Hospital in Boston, where he also served as a hospitalist, and later joined Harvard Medical School as a faculty member in internal medicine.
He is married to Alice Chen, MD. The couple have two children.
A version of this article first appeared on WebMD.com.
Seven Republicans – Bill Cassidy (La.), Susan Collins (Maine), Roger Marshall (Kan.), Susan Murkowski (Alaska), Rob Portman (Ohio), Mitt Romney (Utah), and Dan Sullivan (Alaska) – joined all the Democrats and independents in the 57-43 vote approving Dr. Murthy’s nomination.
Dr. Murthy, 43, previously served as the 19th Surgeon General, from December 2014 to April 2017, when he was asked to step down by President Donald J. Trump.
Surgeons General serve 4-year terms.
During his first tenure, Dr. Murthy issued the first-ever Surgeon General’s report on the crisis of addiction and issued a call to action to doctors to help battle the opioid crisis.
When Dr. Murthy was nominated by President-elect Joseph R. Biden Jr. in December, he was acting as cochair of the incoming administration’s COVID-19 transition advisory board.
Early in 2020, before the COVID-19 pandemic hit, Dr. Murthy published a timely book: “Together: The Healing Power of Human Connection in a Sometimes Lonely World”.
He earned his bachelor’s degree from Harvard and his MD and MBA degrees from Yale. He completed his internal medicine residency at Brigham and Women’s Hospital in Boston, where he also served as a hospitalist, and later joined Harvard Medical School as a faculty member in internal medicine.
He is married to Alice Chen, MD. The couple have two children.
A version of this article first appeared on WebMD.com.
Change is hard: Lessons from an EHR conversion
During this “go-live,” 5 hospitals and approximately 300 ambulatory service and physician practice locations made the transition, consolidating over 100 disparate electronic systems and dozens of interfaces into one world-class medical record.
If you’ve ever been part of such an event, you know it is anything but simple. On the contrary, it requires an enormous financial investment along with years of planning, hours of meetings, and months of training. No matter how much preparation goes into it, there are sure to be bumps along the way. It is a traumatic and stressful time for all involved, but the end result is well worth the effort. Still, there are lessons to be learned and wisdom to be gleaned, and this month we’d like to share a few that we found most important. We believe that many of these are useful lessons even to those who will never live through a go-live.
Safety always comes first
Patient safety is a term so often used that it has a tendency to be taken for granted. Health systems build processes and procedures to ensure safety – some even win awards and recognition for their efforts. But the best (and safest) health care institutions build patient safety into their cultures. More than just being taught to use checklists or buzzwords, the staff at these institutions are encouraged to put the welfare of patients first, making all other activities secondary to this pursuit. We had the opportunity to witness the benefits of such a culture during this go-live and were incredibly impressed with the results.
To be successful in an EHR transition of any magnitude, an organization needs to hold patient safety as a core value and provide its employees with the tools to execute on that value. This enables staff to prepare adequately and to identify risks and opportunities before the conversion takes place. Once go-live occurs, staff also must feel empowered to speak up when they identify problem areas that might jeopardize patients’ care. They also must be given a clear escalation path to ensure their voices can be heard. Most importantly, everyone must understand that the electronic health record itself is just one piece of a major operational change.
As workflows are modified to adapt to the new technology, unsafe processes should be called out and fixed quickly. While the EHR may offer the latest in decision support and system integration, no advancement in technology can make up for bad outcomes, nor justify processes that lead to patient harm.
Training is no substitute for good support
It takes a long time to train thousands of employees, especially when that training must occur during the era of social distancing in the midst of a pandemic. Still, even in the best of times, education should be married to hands-on experience in order to have a real impact. Unfortunately, this is extremely challenging.
Trainees forget much of what they’ve learned in the weeks or months between education and go-live, so they must be given immediately accessible support to bridge the gap. This is known as “at-the-elbow” (ATE) support, and as the name implies, it consists of individuals who are familiar with the new system and are always available to end users, answering their questions and helping them navigate. Since health care never sleeps, this support needs to be offered 24/7, and it should also be flexible and plentiful.
There are many areas that will require more support than anticipated to accommodate the number of clinical and other staff who will use the system, so support staff must be nimble and available for redeployment. In addition, ensuring high-quality support is essential. As many ATE experts are hired contractors, their knowledge base and communications skills can vary widely. Accountability is key, and end users should feel empowered to identify gaps in coverage and deficits in knowledge base in the ATE.
As employees become more familiar with the new system, the need for ATE will wane, but there will still be questions that arise for many weeks to months, and new EHR users will also be added all the time. A good after–go-live support system should remain available so clinical and clerical employees can get just-in-time assistance whenever they need it.
Users should be given clear expectations
Clinicians going through an EHR conversion may be frustrated to discover that the data transferred from their old system into the new one is not quite what they expected. While structured elements such as allergies and immunizations may transfer, unstructured patient histories may not come over at all.
There may be gaps in data, or the opposite may even be true: an overabundance of useless information may transfer over, leaving doctors with dozens of meaningless data points to sift through and eliminate to clean up the chart. This can be extremely time-consuming and discouraging and may jeopardize the success of the go-live.
Providers deserve clear expectations prior to conversion. They should be told what will and will not transfer and be informed that there will be extra work required for documentation at the outset. They may also want the option to preemptively reduce patient volumes to accommodate the additional effort involved in preparing charts. No matter what, this will be a heavy lift, and physicians should understand the implications long before go-live to prepare accordingly.
Old habits die hard
One of the most common complaints we’ve heard following EHR conversions is that “things just worked better in the old system.” We always respond with a question: “Were things better, or just different?” The truth may lie somewhere in the middle, but there is no question that muscle memory develops over many years, and change is difficult no matter how much better the new system is. Still, appropriate expectations, access to just-in-time support, and a continual focus on safety will ensure that the long-term benefits of a patient-centered and integrated electronic record will far outweigh the initial challenges of go-live.
Dr. Notte is a family physician and chief medical officer of Abington (Pa.) Hospital–Jefferson Health. Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington Hospital–Jefferson Health. They have no conflicts related to the content of this piece.
During this “go-live,” 5 hospitals and approximately 300 ambulatory service and physician practice locations made the transition, consolidating over 100 disparate electronic systems and dozens of interfaces into one world-class medical record.
If you’ve ever been part of such an event, you know it is anything but simple. On the contrary, it requires an enormous financial investment along with years of planning, hours of meetings, and months of training. No matter how much preparation goes into it, there are sure to be bumps along the way. It is a traumatic and stressful time for all involved, but the end result is well worth the effort. Still, there are lessons to be learned and wisdom to be gleaned, and this month we’d like to share a few that we found most important. We believe that many of these are useful lessons even to those who will never live through a go-live.
Safety always comes first
Patient safety is a term so often used that it has a tendency to be taken for granted. Health systems build processes and procedures to ensure safety – some even win awards and recognition for their efforts. But the best (and safest) health care institutions build patient safety into their cultures. More than just being taught to use checklists or buzzwords, the staff at these institutions are encouraged to put the welfare of patients first, making all other activities secondary to this pursuit. We had the opportunity to witness the benefits of such a culture during this go-live and were incredibly impressed with the results.
To be successful in an EHR transition of any magnitude, an organization needs to hold patient safety as a core value and provide its employees with the tools to execute on that value. This enables staff to prepare adequately and to identify risks and opportunities before the conversion takes place. Once go-live occurs, staff also must feel empowered to speak up when they identify problem areas that might jeopardize patients’ care. They also must be given a clear escalation path to ensure their voices can be heard. Most importantly, everyone must understand that the electronic health record itself is just one piece of a major operational change.
As workflows are modified to adapt to the new technology, unsafe processes should be called out and fixed quickly. While the EHR may offer the latest in decision support and system integration, no advancement in technology can make up for bad outcomes, nor justify processes that lead to patient harm.
Training is no substitute for good support
It takes a long time to train thousands of employees, especially when that training must occur during the era of social distancing in the midst of a pandemic. Still, even in the best of times, education should be married to hands-on experience in order to have a real impact. Unfortunately, this is extremely challenging.
Trainees forget much of what they’ve learned in the weeks or months between education and go-live, so they must be given immediately accessible support to bridge the gap. This is known as “at-the-elbow” (ATE) support, and as the name implies, it consists of individuals who are familiar with the new system and are always available to end users, answering their questions and helping them navigate. Since health care never sleeps, this support needs to be offered 24/7, and it should also be flexible and plentiful.
There are many areas that will require more support than anticipated to accommodate the number of clinical and other staff who will use the system, so support staff must be nimble and available for redeployment. In addition, ensuring high-quality support is essential. As many ATE experts are hired contractors, their knowledge base and communications skills can vary widely. Accountability is key, and end users should feel empowered to identify gaps in coverage and deficits in knowledge base in the ATE.
As employees become more familiar with the new system, the need for ATE will wane, but there will still be questions that arise for many weeks to months, and new EHR users will also be added all the time. A good after–go-live support system should remain available so clinical and clerical employees can get just-in-time assistance whenever they need it.
Users should be given clear expectations
Clinicians going through an EHR conversion may be frustrated to discover that the data transferred from their old system into the new one is not quite what they expected. While structured elements such as allergies and immunizations may transfer, unstructured patient histories may not come over at all.
There may be gaps in data, or the opposite may even be true: an overabundance of useless information may transfer over, leaving doctors with dozens of meaningless data points to sift through and eliminate to clean up the chart. This can be extremely time-consuming and discouraging and may jeopardize the success of the go-live.
Providers deserve clear expectations prior to conversion. They should be told what will and will not transfer and be informed that there will be extra work required for documentation at the outset. They may also want the option to preemptively reduce patient volumes to accommodate the additional effort involved in preparing charts. No matter what, this will be a heavy lift, and physicians should understand the implications long before go-live to prepare accordingly.
Old habits die hard
One of the most common complaints we’ve heard following EHR conversions is that “things just worked better in the old system.” We always respond with a question: “Were things better, or just different?” The truth may lie somewhere in the middle, but there is no question that muscle memory develops over many years, and change is difficult no matter how much better the new system is. Still, appropriate expectations, access to just-in-time support, and a continual focus on safety will ensure that the long-term benefits of a patient-centered and integrated electronic record will far outweigh the initial challenges of go-live.
Dr. Notte is a family physician and chief medical officer of Abington (Pa.) Hospital–Jefferson Health. Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington Hospital–Jefferson Health. They have no conflicts related to the content of this piece.
During this “go-live,” 5 hospitals and approximately 300 ambulatory service and physician practice locations made the transition, consolidating over 100 disparate electronic systems and dozens of interfaces into one world-class medical record.
If you’ve ever been part of such an event, you know it is anything but simple. On the contrary, it requires an enormous financial investment along with years of planning, hours of meetings, and months of training. No matter how much preparation goes into it, there are sure to be bumps along the way. It is a traumatic and stressful time for all involved, but the end result is well worth the effort. Still, there are lessons to be learned and wisdom to be gleaned, and this month we’d like to share a few that we found most important. We believe that many of these are useful lessons even to those who will never live through a go-live.
Safety always comes first
Patient safety is a term so often used that it has a tendency to be taken for granted. Health systems build processes and procedures to ensure safety – some even win awards and recognition for their efforts. But the best (and safest) health care institutions build patient safety into their cultures. More than just being taught to use checklists or buzzwords, the staff at these institutions are encouraged to put the welfare of patients first, making all other activities secondary to this pursuit. We had the opportunity to witness the benefits of such a culture during this go-live and were incredibly impressed with the results.
To be successful in an EHR transition of any magnitude, an organization needs to hold patient safety as a core value and provide its employees with the tools to execute on that value. This enables staff to prepare adequately and to identify risks and opportunities before the conversion takes place. Once go-live occurs, staff also must feel empowered to speak up when they identify problem areas that might jeopardize patients’ care. They also must be given a clear escalation path to ensure their voices can be heard. Most importantly, everyone must understand that the electronic health record itself is just one piece of a major operational change.
As workflows are modified to adapt to the new technology, unsafe processes should be called out and fixed quickly. While the EHR may offer the latest in decision support and system integration, no advancement in technology can make up for bad outcomes, nor justify processes that lead to patient harm.
Training is no substitute for good support
It takes a long time to train thousands of employees, especially when that training must occur during the era of social distancing in the midst of a pandemic. Still, even in the best of times, education should be married to hands-on experience in order to have a real impact. Unfortunately, this is extremely challenging.
Trainees forget much of what they’ve learned in the weeks or months between education and go-live, so they must be given immediately accessible support to bridge the gap. This is known as “at-the-elbow” (ATE) support, and as the name implies, it consists of individuals who are familiar with the new system and are always available to end users, answering their questions and helping them navigate. Since health care never sleeps, this support needs to be offered 24/7, and it should also be flexible and plentiful.
There are many areas that will require more support than anticipated to accommodate the number of clinical and other staff who will use the system, so support staff must be nimble and available for redeployment. In addition, ensuring high-quality support is essential. As many ATE experts are hired contractors, their knowledge base and communications skills can vary widely. Accountability is key, and end users should feel empowered to identify gaps in coverage and deficits in knowledge base in the ATE.
As employees become more familiar with the new system, the need for ATE will wane, but there will still be questions that arise for many weeks to months, and new EHR users will also be added all the time. A good after–go-live support system should remain available so clinical and clerical employees can get just-in-time assistance whenever they need it.
Users should be given clear expectations
Clinicians going through an EHR conversion may be frustrated to discover that the data transferred from their old system into the new one is not quite what they expected. While structured elements such as allergies and immunizations may transfer, unstructured patient histories may not come over at all.
There may be gaps in data, or the opposite may even be true: an overabundance of useless information may transfer over, leaving doctors with dozens of meaningless data points to sift through and eliminate to clean up the chart. This can be extremely time-consuming and discouraging and may jeopardize the success of the go-live.
Providers deserve clear expectations prior to conversion. They should be told what will and will not transfer and be informed that there will be extra work required for documentation at the outset. They may also want the option to preemptively reduce patient volumes to accommodate the additional effort involved in preparing charts. No matter what, this will be a heavy lift, and physicians should understand the implications long before go-live to prepare accordingly.
Old habits die hard
One of the most common complaints we’ve heard following EHR conversions is that “things just worked better in the old system.” We always respond with a question: “Were things better, or just different?” The truth may lie somewhere in the middle, but there is no question that muscle memory develops over many years, and change is difficult no matter how much better the new system is. Still, appropriate expectations, access to just-in-time support, and a continual focus on safety will ensure that the long-term benefits of a patient-centered and integrated electronic record will far outweigh the initial challenges of go-live.
Dr. Notte is a family physician and chief medical officer of Abington (Pa.) Hospital–Jefferson Health. Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington Hospital–Jefferson Health. They have no conflicts related to the content of this piece.
Top JAMA editor on leave amid podcast investigation
The American Medical Association’s Joint Oversight Committee announced that Howard Bauchner, MD, is on leave beginning at the end of the day on March 25. Dr. Bauchner is the top editor at JAMA, the journal of the AMA.
“The decision to place the editor-in-chief on administrative leave neither implicates nor exonerates individuals and is standard operating procedure for such investigations,” the committee said in a statement.
More than 2,000 people signed a petition on Change.org calling for an investigation at JAMA over the February podcast episode, called “Structural Racism for Doctors: What Is It?”
Already, Edward H. Livingston, MD, the host of the podcast, has resigned as deputy editor of the journal.
During the podcast, Dr. Livingston, who is White, said, “Structural racism is an unfortunate term. Personally, I think taking racism out of the conversation will help. Many of us are offended by the concept that we are racist.”
The audio of the podcast has been deleted from JAMA’s website. In its place is audio of a statement from Dr. Bauchner. In his statement, which he released in the week prior to his being on leave, he said the comments in the podcast, which also featured Mitch Katz, MD, were “inaccurate, offensive, hurtful, and inconsistent with the standards of JAMA.”
Also deleted was a JAMA tweet promoting the podcast episode. The tweet said: “No physician is racist, so how can there be structural racism in health care? An explanation of the idea by doctors for doctors in this user-friendly podcast.”
This story will be updated.
A version of this article first appeared on WedMD.com.
The American Medical Association’s Joint Oversight Committee announced that Howard Bauchner, MD, is on leave beginning at the end of the day on March 25. Dr. Bauchner is the top editor at JAMA, the journal of the AMA.
“The decision to place the editor-in-chief on administrative leave neither implicates nor exonerates individuals and is standard operating procedure for such investigations,” the committee said in a statement.
More than 2,000 people signed a petition on Change.org calling for an investigation at JAMA over the February podcast episode, called “Structural Racism for Doctors: What Is It?”
Already, Edward H. Livingston, MD, the host of the podcast, has resigned as deputy editor of the journal.
During the podcast, Dr. Livingston, who is White, said, “Structural racism is an unfortunate term. Personally, I think taking racism out of the conversation will help. Many of us are offended by the concept that we are racist.”
The audio of the podcast has been deleted from JAMA’s website. In its place is audio of a statement from Dr. Bauchner. In his statement, which he released in the week prior to his being on leave, he said the comments in the podcast, which also featured Mitch Katz, MD, were “inaccurate, offensive, hurtful, and inconsistent with the standards of JAMA.”
Also deleted was a JAMA tweet promoting the podcast episode. The tweet said: “No physician is racist, so how can there be structural racism in health care? An explanation of the idea by doctors for doctors in this user-friendly podcast.”
This story will be updated.
A version of this article first appeared on WedMD.com.
The American Medical Association’s Joint Oversight Committee announced that Howard Bauchner, MD, is on leave beginning at the end of the day on March 25. Dr. Bauchner is the top editor at JAMA, the journal of the AMA.
“The decision to place the editor-in-chief on administrative leave neither implicates nor exonerates individuals and is standard operating procedure for such investigations,” the committee said in a statement.
More than 2,000 people signed a petition on Change.org calling for an investigation at JAMA over the February podcast episode, called “Structural Racism for Doctors: What Is It?”
Already, Edward H. Livingston, MD, the host of the podcast, has resigned as deputy editor of the journal.
During the podcast, Dr. Livingston, who is White, said, “Structural racism is an unfortunate term. Personally, I think taking racism out of the conversation will help. Many of us are offended by the concept that we are racist.”
The audio of the podcast has been deleted from JAMA’s website. In its place is audio of a statement from Dr. Bauchner. In his statement, which he released in the week prior to his being on leave, he said the comments in the podcast, which also featured Mitch Katz, MD, were “inaccurate, offensive, hurtful, and inconsistent with the standards of JAMA.”
Also deleted was a JAMA tweet promoting the podcast episode. The tweet said: “No physician is racist, so how can there be structural racism in health care? An explanation of the idea by doctors for doctors in this user-friendly podcast.”
This story will be updated.
A version of this article first appeared on WedMD.com.
COVID-19 variants now detected in more animals, may find hosts in mice
The new SARS-CoV-2 variants are not just problems for humans.
New research shows they can also infect animals, and for the first time, variants have been able to infect mice, a development that may complicate efforts to rein in the global spread of the virus.
In addition, two new studies have implications for pets. Veterinarians in Texas and the United Kingdom have documented infections of B.1.1.7 – the fast-spreading variant first found in the United Kingdom – in dogs and cats. The animals in the U.K. study also had heart damage, but it’s unclear if the damage was caused by the virus or was already there and was found as a result of their infections.
Animal studies of SARS-CoV-2 and its emerging variants are urgent, said Sarah Hamer, DVM, PhD, a veterinarian and epidemiologist at Texas A&M University, College Station.
She’s part of a network of scientists who are swabbing the pets of people who are diagnosed with COVID-19 to find out how often the virus passes from people to animals.
The collaboration is part of the One Health initiative through the Centers for Disease Control and Prevention. One Health aims to tackle infectious diseases by recognizing that people can’t be fully protected from pathogens unless animals and the environment are also safeguarded. “Over 70% of emerging diseases of humans have their origins in animal populations,” Dr. Hamer said. “So if we are only focusing on studying disease as it emerges in humans and ignoring where those pathogens have been transmitted or circulating for years, then we might miss the ability to detect early emergence. We might miss the ability to control these diseases before they become problems for human health.”
Variants move to mice
In new work, researchers at the Institut Pasteur in Paris have shown that the B.1.351 and P.1 variants of concern, which were first identified in South Africa and Brazil, respectively, can infect mice, giving the virus a potential new host. Older versions of the virus couldn’t infect mice because they weren’t able bind to receptors on their cells. These two variants can.
On one hand, that’s a good thing, because it will help scientists more easily conduct experiments in mice. Before, if they wanted to do an experiment with SARS-CoV-2 in mice, they had to use a special strain of mouse that was bred to carry human ACE2 receptors on their lung cells. Now that mice can become naturally infected, any breed will do, making it less costly and time-consuming to study the virus in animals.
On the other hand, the idea that the virus could have more and different ways to spread isn’t good news.
“From the beginning of the epidemic and since human coronaviruses emerged from animals, it has been very important to establish in which species the virus can replicate, in particular the species that live close to humans,” said Xavier Montagutelli, DVM, PhD, head of the Mouse Genetics Laboratory at the Institut Pasteur. His study was published as a preprint ahead of peer review on BioRXIV.
Once a virus establishes itself within a population of animals, it will continue to spread and change and may eventually be passed back to humans. It’s the reason that birds and pigs are closely monitored for influenza viruses.
So far, with SARS-CoV-2, only one animal has been found to catch and spread the virus and pass it back to people – farmed mink. Researchers have also documented SARS-CoV-2 antibodies in escaped mink living near mink farms in Utah, suggesting the virus has the potential to be transmitted to wild populations.
And the move of the virus into mice suggests that SARS-CoV-2 could establish itself in a population of wild animals that live close to humans.
“At this point, we have no evidence that wild mice are infected, or can become infected from humans,” Dr. Montagutelli said. He added that his findings emphasize the need to regularly test animals for signs of the infection. He said these surveys will need to be updated as more variants emerge.
“So far, we’ve been lucky that our livestock species aren’t really susceptible to this,” said Scott Weese, DVM, a professor at Ontario Veterinary College at the University of Guelph, who studies emerging infectious diseases that pass between animals and people.
While the outbreaks on mink farms have been bad, imagine what would happen, Dr. Weese said, if the virus moved to pigs.
“If this infects a barn with a few thousand pigs – which is like the mink scenario – but we have a lot more pig farms than mink farms,” he said.
“With these variants, we have to reset,” he said. “We’ve figured all this about animals and how it spreads or how it doesn’t, but now we need to repeat all those studies to make sure it’s the same thing.”
Pets catch variants, too
Pets living with people who are infected with SARS-CoV-2 can catch it from their owners, and cats are particularly susceptible, Dr. Weese said.
Contact tracing studies, which also tested animals for signs of the virus, have found that about half of cats living with infected people have signs of infection, while 20%-30% of dogs were sick.
“It’s quite common,” for pets to get COVID, Dr. Weese said.
Now, two new studies have shown that pets can also be infected by the newer B.1.1.7 variant.
The first study, from researchers at Texas A&M, documented the variant in a dog and a cat from Brazos County, Texas. Neither the older black Lab mix or the older domestic shorthair cat had symptoms of COVID-19. They were tested as part of a project funded by the CDC.
Dr. Weese said pets are at risk by people who are infected, but they don’t seem to play a big role in spreading the disease to humans. So if you have pets, there’s no reason to worry that they could bring the virus home to you. You’re more likely to be a risk to them.
The second study, from a specialty animal hospital in southeast England, documented infection by the B.1.1.7 virus variant in 11 dogs and cats. Most of the pets had unusual symptoms, including inflamed hearts and heart damage.
Dr. Weese called this study interesting and said its findings deserve more investigation, but pointed out that the study can’t determine whether the infection caused the heart damage, or whether it was already there.
“This is a human virus. There’s no doubt about it. It can affect other species, but it likes people a lot better,” he said. “If you think about the big picture and what is the potential role of animals, pets are pretty low risk.”
A version of this article first appeared on Medscape.com.
The new SARS-CoV-2 variants are not just problems for humans.
New research shows they can also infect animals, and for the first time, variants have been able to infect mice, a development that may complicate efforts to rein in the global spread of the virus.
In addition, two new studies have implications for pets. Veterinarians in Texas and the United Kingdom have documented infections of B.1.1.7 – the fast-spreading variant first found in the United Kingdom – in dogs and cats. The animals in the U.K. study also had heart damage, but it’s unclear if the damage was caused by the virus or was already there and was found as a result of their infections.
Animal studies of SARS-CoV-2 and its emerging variants are urgent, said Sarah Hamer, DVM, PhD, a veterinarian and epidemiologist at Texas A&M University, College Station.
She’s part of a network of scientists who are swabbing the pets of people who are diagnosed with COVID-19 to find out how often the virus passes from people to animals.
The collaboration is part of the One Health initiative through the Centers for Disease Control and Prevention. One Health aims to tackle infectious diseases by recognizing that people can’t be fully protected from pathogens unless animals and the environment are also safeguarded. “Over 70% of emerging diseases of humans have their origins in animal populations,” Dr. Hamer said. “So if we are only focusing on studying disease as it emerges in humans and ignoring where those pathogens have been transmitted or circulating for years, then we might miss the ability to detect early emergence. We might miss the ability to control these diseases before they become problems for human health.”
Variants move to mice
In new work, researchers at the Institut Pasteur in Paris have shown that the B.1.351 and P.1 variants of concern, which were first identified in South Africa and Brazil, respectively, can infect mice, giving the virus a potential new host. Older versions of the virus couldn’t infect mice because they weren’t able bind to receptors on their cells. These two variants can.
On one hand, that’s a good thing, because it will help scientists more easily conduct experiments in mice. Before, if they wanted to do an experiment with SARS-CoV-2 in mice, they had to use a special strain of mouse that was bred to carry human ACE2 receptors on their lung cells. Now that mice can become naturally infected, any breed will do, making it less costly and time-consuming to study the virus in animals.
On the other hand, the idea that the virus could have more and different ways to spread isn’t good news.
“From the beginning of the epidemic and since human coronaviruses emerged from animals, it has been very important to establish in which species the virus can replicate, in particular the species that live close to humans,” said Xavier Montagutelli, DVM, PhD, head of the Mouse Genetics Laboratory at the Institut Pasteur. His study was published as a preprint ahead of peer review on BioRXIV.
Once a virus establishes itself within a population of animals, it will continue to spread and change and may eventually be passed back to humans. It’s the reason that birds and pigs are closely monitored for influenza viruses.
So far, with SARS-CoV-2, only one animal has been found to catch and spread the virus and pass it back to people – farmed mink. Researchers have also documented SARS-CoV-2 antibodies in escaped mink living near mink farms in Utah, suggesting the virus has the potential to be transmitted to wild populations.
And the move of the virus into mice suggests that SARS-CoV-2 could establish itself in a population of wild animals that live close to humans.
“At this point, we have no evidence that wild mice are infected, or can become infected from humans,” Dr. Montagutelli said. He added that his findings emphasize the need to regularly test animals for signs of the infection. He said these surveys will need to be updated as more variants emerge.
“So far, we’ve been lucky that our livestock species aren’t really susceptible to this,” said Scott Weese, DVM, a professor at Ontario Veterinary College at the University of Guelph, who studies emerging infectious diseases that pass between animals and people.
While the outbreaks on mink farms have been bad, imagine what would happen, Dr. Weese said, if the virus moved to pigs.
“If this infects a barn with a few thousand pigs – which is like the mink scenario – but we have a lot more pig farms than mink farms,” he said.
“With these variants, we have to reset,” he said. “We’ve figured all this about animals and how it spreads or how it doesn’t, but now we need to repeat all those studies to make sure it’s the same thing.”
Pets catch variants, too
Pets living with people who are infected with SARS-CoV-2 can catch it from their owners, and cats are particularly susceptible, Dr. Weese said.
Contact tracing studies, which also tested animals for signs of the virus, have found that about half of cats living with infected people have signs of infection, while 20%-30% of dogs were sick.
“It’s quite common,” for pets to get COVID, Dr. Weese said.
Now, two new studies have shown that pets can also be infected by the newer B.1.1.7 variant.
The first study, from researchers at Texas A&M, documented the variant in a dog and a cat from Brazos County, Texas. Neither the older black Lab mix or the older domestic shorthair cat had symptoms of COVID-19. They were tested as part of a project funded by the CDC.
Dr. Weese said pets are at risk by people who are infected, but they don’t seem to play a big role in spreading the disease to humans. So if you have pets, there’s no reason to worry that they could bring the virus home to you. You’re more likely to be a risk to them.
The second study, from a specialty animal hospital in southeast England, documented infection by the B.1.1.7 virus variant in 11 dogs and cats. Most of the pets had unusual symptoms, including inflamed hearts and heart damage.
Dr. Weese called this study interesting and said its findings deserve more investigation, but pointed out that the study can’t determine whether the infection caused the heart damage, or whether it was already there.
“This is a human virus. There’s no doubt about it. It can affect other species, but it likes people a lot better,” he said. “If you think about the big picture and what is the potential role of animals, pets are pretty low risk.”
A version of this article first appeared on Medscape.com.
The new SARS-CoV-2 variants are not just problems for humans.
New research shows they can also infect animals, and for the first time, variants have been able to infect mice, a development that may complicate efforts to rein in the global spread of the virus.
In addition, two new studies have implications for pets. Veterinarians in Texas and the United Kingdom have documented infections of B.1.1.7 – the fast-spreading variant first found in the United Kingdom – in dogs and cats. The animals in the U.K. study also had heart damage, but it’s unclear if the damage was caused by the virus or was already there and was found as a result of their infections.
Animal studies of SARS-CoV-2 and its emerging variants are urgent, said Sarah Hamer, DVM, PhD, a veterinarian and epidemiologist at Texas A&M University, College Station.
She’s part of a network of scientists who are swabbing the pets of people who are diagnosed with COVID-19 to find out how often the virus passes from people to animals.
The collaboration is part of the One Health initiative through the Centers for Disease Control and Prevention. One Health aims to tackle infectious diseases by recognizing that people can’t be fully protected from pathogens unless animals and the environment are also safeguarded. “Over 70% of emerging diseases of humans have their origins in animal populations,” Dr. Hamer said. “So if we are only focusing on studying disease as it emerges in humans and ignoring where those pathogens have been transmitted or circulating for years, then we might miss the ability to detect early emergence. We might miss the ability to control these diseases before they become problems for human health.”
Variants move to mice
In new work, researchers at the Institut Pasteur in Paris have shown that the B.1.351 and P.1 variants of concern, which were first identified in South Africa and Brazil, respectively, can infect mice, giving the virus a potential new host. Older versions of the virus couldn’t infect mice because they weren’t able bind to receptors on their cells. These two variants can.
On one hand, that’s a good thing, because it will help scientists more easily conduct experiments in mice. Before, if they wanted to do an experiment with SARS-CoV-2 in mice, they had to use a special strain of mouse that was bred to carry human ACE2 receptors on their lung cells. Now that mice can become naturally infected, any breed will do, making it less costly and time-consuming to study the virus in animals.
On the other hand, the idea that the virus could have more and different ways to spread isn’t good news.
“From the beginning of the epidemic and since human coronaviruses emerged from animals, it has been very important to establish in which species the virus can replicate, in particular the species that live close to humans,” said Xavier Montagutelli, DVM, PhD, head of the Mouse Genetics Laboratory at the Institut Pasteur. His study was published as a preprint ahead of peer review on BioRXIV.
Once a virus establishes itself within a population of animals, it will continue to spread and change and may eventually be passed back to humans. It’s the reason that birds and pigs are closely monitored for influenza viruses.
So far, with SARS-CoV-2, only one animal has been found to catch and spread the virus and pass it back to people – farmed mink. Researchers have also documented SARS-CoV-2 antibodies in escaped mink living near mink farms in Utah, suggesting the virus has the potential to be transmitted to wild populations.
And the move of the virus into mice suggests that SARS-CoV-2 could establish itself in a population of wild animals that live close to humans.
“At this point, we have no evidence that wild mice are infected, or can become infected from humans,” Dr. Montagutelli said. He added that his findings emphasize the need to regularly test animals for signs of the infection. He said these surveys will need to be updated as more variants emerge.
“So far, we’ve been lucky that our livestock species aren’t really susceptible to this,” said Scott Weese, DVM, a professor at Ontario Veterinary College at the University of Guelph, who studies emerging infectious diseases that pass between animals and people.
While the outbreaks on mink farms have been bad, imagine what would happen, Dr. Weese said, if the virus moved to pigs.
“If this infects a barn with a few thousand pigs – which is like the mink scenario – but we have a lot more pig farms than mink farms,” he said.
“With these variants, we have to reset,” he said. “We’ve figured all this about animals and how it spreads or how it doesn’t, but now we need to repeat all those studies to make sure it’s the same thing.”
Pets catch variants, too
Pets living with people who are infected with SARS-CoV-2 can catch it from their owners, and cats are particularly susceptible, Dr. Weese said.
Contact tracing studies, which also tested animals for signs of the virus, have found that about half of cats living with infected people have signs of infection, while 20%-30% of dogs were sick.
“It’s quite common,” for pets to get COVID, Dr. Weese said.
Now, two new studies have shown that pets can also be infected by the newer B.1.1.7 variant.
The first study, from researchers at Texas A&M, documented the variant in a dog and a cat from Brazos County, Texas. Neither the older black Lab mix or the older domestic shorthair cat had symptoms of COVID-19. They were tested as part of a project funded by the CDC.
Dr. Weese said pets are at risk by people who are infected, but they don’t seem to play a big role in spreading the disease to humans. So if you have pets, there’s no reason to worry that they could bring the virus home to you. You’re more likely to be a risk to them.
The second study, from a specialty animal hospital in southeast England, documented infection by the B.1.1.7 virus variant in 11 dogs and cats. Most of the pets had unusual symptoms, including inflamed hearts and heart damage.
Dr. Weese called this study interesting and said its findings deserve more investigation, but pointed out that the study can’t determine whether the infection caused the heart damage, or whether it was already there.
“This is a human virus. There’s no doubt about it. It can affect other species, but it likes people a lot better,” he said. “If you think about the big picture and what is the potential role of animals, pets are pretty low risk.”
A version of this article first appeared on Medscape.com.



