User login
Body-brain neuroinflammation loop may cause chronic ME/CFS, long COVID symptoms
ME/CFS has been established as resulting from infections, environmental exposures, stressors, and surgery. Similarities have been drawn during the COVID-19 pandemic between ME/CFS and a large subgroup of patients with post-acute sequelae of SARS-CoV-2 infection – also known as post-COVID conditions, or long COVID – who continue to have viral fatigue and other lingering symptoms after their infection resolves.
What has been less clearly understood, the researchers said, is the reason behind why ME/CFS and other postviral fatigue tends to be chronic and can sometime develop into a lifelong condition.
“These diseases are very closely related, and it is clear the biological basis of long COVID is unequivocally connected to the original COVID infection – so there should no longer be any debate and doubt about the fact that postviral fatigue syndromes like ME/CFS are biologically based and involve much disturbed physiology,” Warren Tate, MSc, PhD, emeritus professor in the department of biochemistry at the University of Otago in Dunedin, New Zealand, stated in a press release.
Their hypothesis, set forth in a study published in Frontiers of Neurology, proposes that the systemic immune/inflammatory response that occurs after an infection or stressful event does not revolve, which results in a “fluctuating chronic neuroinflammation that sustains and controls the complex neurological symptoms of ME/CFS and long COVID and facilitates frequent more serious relapses in response to life stress, as evidenced from a comprehensive disruption to the cellular molecular biology and body’s physiological pathways.”
Dr. Tate and colleagues said that it is still unclear how the neuroinflammation occurs, why it’s persistent in ME/CFS, and how it causes symptoms associated with ME/CFS. In their hypothesis, “abnormal signaling or transport of molecules/cells occurs through one or both of neurovascular pathways and/or a dysfunctional blood brain barrier,” they said, noting “the normally separate and contained brain/CNS compartment in the healthy person becomes more porous.” The neurological symptoms associated with ME/CFS occur due to strong signals sent because of persistent “inflammatory signals or immune cells/molecules migrating into the brain,” they explained.
This results in a continuous loop where the central nervous system sends signals back to the body through the hypothalamus/paraventricular nucleus and the brain stem. “The resulting symptoms and the neurologically driven ‘sickness response’ for the ME/CFS patient would persist, preventing healing and a return to the preinfectious/stress-related state,” Dr. Tate and colleagues said.
Lingering inflammation may be the culprit
Commenting on the study, Achillefs Ntranos, MD, a board-certified neurologist in private practice in Scarsdale, N.Y., who was not involved with the research, said previous studies have shown that long COVID is linked to chronic activation of microglia in the brain, which has also been seen to activate in patients with ME/CFS.
“The hypothesis that lingering inflammation in the brain is the culprit behind the neurological symptoms of long COVID and ME/CFS is valid,” he said. “If these cells remain activated in the brain, they can cause a state of increased and lingering inflammation, which can interfere with the function of neurons, thus producing neurological symptoms. Since the neurological symptoms are similar between these entities, the mechanisms that produce them might also be similar.”
While the exact cause of ME/CFS is still unclear, it is often tied to the aftereffects of a flu-like illness, Dr. Ntranos said. “This has led researchers to propose that it arises after a viral infection, with many different types of viruses being associated with it. Other ways researchers think ME/CFS is being brought on after a viral illness is via changes in the immune system, such as chronic production of cytokines, neuroinflammation, and disruption of the hypothalamic-pituitary-adrenal axis, which regulates the body’s response to stress,” he explained.
While a newer condition, long COVID is not all that different from ME/CFS, Dr. Ntranos noted, sharing the catalyst of a viral infection and core neurological symptoms such as fatigue, postexertional malaise, a “brain fog” that makes thinking or concentrating difficult, sleep problems, and lightheadedness, but there are differences that set it apart from ME/CFS.
“Long COVID is unique in having additional symptoms that are specific to the SARS-CoV-2 virus, such as respiratory and cardiovascular symptoms and loss of smell and taste. However most central nervous system effects are the same between these two entities,” he said.
Dr. Ntranos said long COVID’s neurological symptoms are similar to that of multiple sclerosis (MS), such as “brain fog” and postexertional malaise. “Since MS only affects the brain and spinal cord, there are no symptoms from other organ systems, such as the lungs, heart, or digestive system, contrary to long COVID. Furthermore, MS rarely affects smell and taste, making these symptoms unique to COVID,” he said.
However, he pointed out that brain fog and fatigue symptoms on their own can be nonspecific and attributed to many different conditions, such as obstructive sleep apnea, migraines, depression, anxiety, thyroid problems, vitamin deficiencies, dehydration, sleep disorders, and side effects of medications.
“More research needs to be done to understand how these cells are being activated, how they interfere with neuronal function, and why they remain in that state in some people, who then go on to develop fatigue and brain fog,” he said.
This study was funded by the Healthcare Otago Charitable Trust, the Associated New Zealand Myalgic Encephalomyelitis Society, and donations from families of patients with ME/CFS. The authors and Dr. Ntranos report no relevant financial disclosures.
ME/CFS has been established as resulting from infections, environmental exposures, stressors, and surgery. Similarities have been drawn during the COVID-19 pandemic between ME/CFS and a large subgroup of patients with post-acute sequelae of SARS-CoV-2 infection – also known as post-COVID conditions, or long COVID – who continue to have viral fatigue and other lingering symptoms after their infection resolves.
What has been less clearly understood, the researchers said, is the reason behind why ME/CFS and other postviral fatigue tends to be chronic and can sometime develop into a lifelong condition.
“These diseases are very closely related, and it is clear the biological basis of long COVID is unequivocally connected to the original COVID infection – so there should no longer be any debate and doubt about the fact that postviral fatigue syndromes like ME/CFS are biologically based and involve much disturbed physiology,” Warren Tate, MSc, PhD, emeritus professor in the department of biochemistry at the University of Otago in Dunedin, New Zealand, stated in a press release.
Their hypothesis, set forth in a study published in Frontiers of Neurology, proposes that the systemic immune/inflammatory response that occurs after an infection or stressful event does not revolve, which results in a “fluctuating chronic neuroinflammation that sustains and controls the complex neurological symptoms of ME/CFS and long COVID and facilitates frequent more serious relapses in response to life stress, as evidenced from a comprehensive disruption to the cellular molecular biology and body’s physiological pathways.”
Dr. Tate and colleagues said that it is still unclear how the neuroinflammation occurs, why it’s persistent in ME/CFS, and how it causes symptoms associated with ME/CFS. In their hypothesis, “abnormal signaling or transport of molecules/cells occurs through one or both of neurovascular pathways and/or a dysfunctional blood brain barrier,” they said, noting “the normally separate and contained brain/CNS compartment in the healthy person becomes more porous.” The neurological symptoms associated with ME/CFS occur due to strong signals sent because of persistent “inflammatory signals or immune cells/molecules migrating into the brain,” they explained.
This results in a continuous loop where the central nervous system sends signals back to the body through the hypothalamus/paraventricular nucleus and the brain stem. “The resulting symptoms and the neurologically driven ‘sickness response’ for the ME/CFS patient would persist, preventing healing and a return to the preinfectious/stress-related state,” Dr. Tate and colleagues said.
Lingering inflammation may be the culprit
Commenting on the study, Achillefs Ntranos, MD, a board-certified neurologist in private practice in Scarsdale, N.Y., who was not involved with the research, said previous studies have shown that long COVID is linked to chronic activation of microglia in the brain, which has also been seen to activate in patients with ME/CFS.
“The hypothesis that lingering inflammation in the brain is the culprit behind the neurological symptoms of long COVID and ME/CFS is valid,” he said. “If these cells remain activated in the brain, they can cause a state of increased and lingering inflammation, which can interfere with the function of neurons, thus producing neurological symptoms. Since the neurological symptoms are similar between these entities, the mechanisms that produce them might also be similar.”
While the exact cause of ME/CFS is still unclear, it is often tied to the aftereffects of a flu-like illness, Dr. Ntranos said. “This has led researchers to propose that it arises after a viral infection, with many different types of viruses being associated with it. Other ways researchers think ME/CFS is being brought on after a viral illness is via changes in the immune system, such as chronic production of cytokines, neuroinflammation, and disruption of the hypothalamic-pituitary-adrenal axis, which regulates the body’s response to stress,” he explained.
While a newer condition, long COVID is not all that different from ME/CFS, Dr. Ntranos noted, sharing the catalyst of a viral infection and core neurological symptoms such as fatigue, postexertional malaise, a “brain fog” that makes thinking or concentrating difficult, sleep problems, and lightheadedness, but there are differences that set it apart from ME/CFS.
“Long COVID is unique in having additional symptoms that are specific to the SARS-CoV-2 virus, such as respiratory and cardiovascular symptoms and loss of smell and taste. However most central nervous system effects are the same between these two entities,” he said.
Dr. Ntranos said long COVID’s neurological symptoms are similar to that of multiple sclerosis (MS), such as “brain fog” and postexertional malaise. “Since MS only affects the brain and spinal cord, there are no symptoms from other organ systems, such as the lungs, heart, or digestive system, contrary to long COVID. Furthermore, MS rarely affects smell and taste, making these symptoms unique to COVID,” he said.
However, he pointed out that brain fog and fatigue symptoms on their own can be nonspecific and attributed to many different conditions, such as obstructive sleep apnea, migraines, depression, anxiety, thyroid problems, vitamin deficiencies, dehydration, sleep disorders, and side effects of medications.
“More research needs to be done to understand how these cells are being activated, how they interfere with neuronal function, and why they remain in that state in some people, who then go on to develop fatigue and brain fog,” he said.
This study was funded by the Healthcare Otago Charitable Trust, the Associated New Zealand Myalgic Encephalomyelitis Society, and donations from families of patients with ME/CFS. The authors and Dr. Ntranos report no relevant financial disclosures.
ME/CFS has been established as resulting from infections, environmental exposures, stressors, and surgery. Similarities have been drawn during the COVID-19 pandemic between ME/CFS and a large subgroup of patients with post-acute sequelae of SARS-CoV-2 infection – also known as post-COVID conditions, or long COVID – who continue to have viral fatigue and other lingering symptoms after their infection resolves.
What has been less clearly understood, the researchers said, is the reason behind why ME/CFS and other postviral fatigue tends to be chronic and can sometime develop into a lifelong condition.
“These diseases are very closely related, and it is clear the biological basis of long COVID is unequivocally connected to the original COVID infection – so there should no longer be any debate and doubt about the fact that postviral fatigue syndromes like ME/CFS are biologically based and involve much disturbed physiology,” Warren Tate, MSc, PhD, emeritus professor in the department of biochemistry at the University of Otago in Dunedin, New Zealand, stated in a press release.
Their hypothesis, set forth in a study published in Frontiers of Neurology, proposes that the systemic immune/inflammatory response that occurs after an infection or stressful event does not revolve, which results in a “fluctuating chronic neuroinflammation that sustains and controls the complex neurological symptoms of ME/CFS and long COVID and facilitates frequent more serious relapses in response to life stress, as evidenced from a comprehensive disruption to the cellular molecular biology and body’s physiological pathways.”
Dr. Tate and colleagues said that it is still unclear how the neuroinflammation occurs, why it’s persistent in ME/CFS, and how it causes symptoms associated with ME/CFS. In their hypothesis, “abnormal signaling or transport of molecules/cells occurs through one or both of neurovascular pathways and/or a dysfunctional blood brain barrier,” they said, noting “the normally separate and contained brain/CNS compartment in the healthy person becomes more porous.” The neurological symptoms associated with ME/CFS occur due to strong signals sent because of persistent “inflammatory signals or immune cells/molecules migrating into the brain,” they explained.
This results in a continuous loop where the central nervous system sends signals back to the body through the hypothalamus/paraventricular nucleus and the brain stem. “The resulting symptoms and the neurologically driven ‘sickness response’ for the ME/CFS patient would persist, preventing healing and a return to the preinfectious/stress-related state,” Dr. Tate and colleagues said.
Lingering inflammation may be the culprit
Commenting on the study, Achillefs Ntranos, MD, a board-certified neurologist in private practice in Scarsdale, N.Y., who was not involved with the research, said previous studies have shown that long COVID is linked to chronic activation of microglia in the brain, which has also been seen to activate in patients with ME/CFS.
“The hypothesis that lingering inflammation in the brain is the culprit behind the neurological symptoms of long COVID and ME/CFS is valid,” he said. “If these cells remain activated in the brain, they can cause a state of increased and lingering inflammation, which can interfere with the function of neurons, thus producing neurological symptoms. Since the neurological symptoms are similar between these entities, the mechanisms that produce them might also be similar.”
While the exact cause of ME/CFS is still unclear, it is often tied to the aftereffects of a flu-like illness, Dr. Ntranos said. “This has led researchers to propose that it arises after a viral infection, with many different types of viruses being associated with it. Other ways researchers think ME/CFS is being brought on after a viral illness is via changes in the immune system, such as chronic production of cytokines, neuroinflammation, and disruption of the hypothalamic-pituitary-adrenal axis, which regulates the body’s response to stress,” he explained.
While a newer condition, long COVID is not all that different from ME/CFS, Dr. Ntranos noted, sharing the catalyst of a viral infection and core neurological symptoms such as fatigue, postexertional malaise, a “brain fog” that makes thinking or concentrating difficult, sleep problems, and lightheadedness, but there are differences that set it apart from ME/CFS.
“Long COVID is unique in having additional symptoms that are specific to the SARS-CoV-2 virus, such as respiratory and cardiovascular symptoms and loss of smell and taste. However most central nervous system effects are the same between these two entities,” he said.
Dr. Ntranos said long COVID’s neurological symptoms are similar to that of multiple sclerosis (MS), such as “brain fog” and postexertional malaise. “Since MS only affects the brain and spinal cord, there are no symptoms from other organ systems, such as the lungs, heart, or digestive system, contrary to long COVID. Furthermore, MS rarely affects smell and taste, making these symptoms unique to COVID,” he said.
However, he pointed out that brain fog and fatigue symptoms on their own can be nonspecific and attributed to many different conditions, such as obstructive sleep apnea, migraines, depression, anxiety, thyroid problems, vitamin deficiencies, dehydration, sleep disorders, and side effects of medications.
“More research needs to be done to understand how these cells are being activated, how they interfere with neuronal function, and why they remain in that state in some people, who then go on to develop fatigue and brain fog,” he said.
This study was funded by the Healthcare Otago Charitable Trust, the Associated New Zealand Myalgic Encephalomyelitis Society, and donations from families of patients with ME/CFS. The authors and Dr. Ntranos report no relevant financial disclosures.
FROM FRONTIERS IN NEUROLOGY
Hormone therapy didn’t increase recurrence or mortality in women treated for breast cancer
Hormone therapy did not increase mortality in postmenopausal women treated for early-stage estrogen receptor–positive breast cancer, but, in longitudinal data from Denmark, there was a recurrence risk with vaginal estrogen therapy among those treated with aromatase inhibitors.
Genitourinary syndrome of menopause (GSM) – including vaginal dryness, burning, and urinary incontinence – is common in women treated for breast cancer. Adjuvant endocrine therapy, particularly aromatase inhibitors, can aggravate these symptoms. Both local and systemic estrogen therapy are recommended for alleviating GSM symptoms in healthy women, but concerns have been raised about their use in women with breast cancer. Previous studies examining this have suggested possible risks for breast cancer recurrence, but those studies have had several limitations including small samples and short follow-up, particularly for vaginal estrogen therapy.
In the new study, from a national Danish cohort of 8,461 postmenopausal women diagnosed between 1997 and 2004 and treated for early-stage invasive estrogen receptor–positive nonmetastatic breast cancer, neither systemic menopausal hormone therapy (MHT) nor local vaginal estrogen therapy (VET) were associated with an overall increased risk for either breast cancer recurrence or mortality. However, in the subset who had received an aromatase inhibitor – with or without tamoxifen – there was a statistically significant increased risk for breast cancer recurrence, but not mortality.
The results were published in the Journal of the National Cancer Institute.
“The data are reassuring for the majority of women with no adjuvant therapy or tamoxifen. But for those using adjuvant aromatase inhibitors, there might be a small risk,” study lead author Søren Cold, MD, PhD, senior oncologist in the department of oncology at Odense (Denmark) University Hospital, Odense, said in an interview.
Moreover, Dr. Cold noted, while this study didn’t find an increased recurrence risk with MHT for women taking aromatase inhibitors, other studies have. One in particular was stopped because of harm. The reason for the difference here is likely that the previous sample was small – just 133 women.
“Our study is mainly focusing on the use of vaginal estrogen. We had so few patients using systemic menopausal hormone therapy, those data don’t mean much. ... The risk with systemic therapy has been established. The vaginal use hasn’t been thoroughly studied before,” he noted.
Breast cancer recurrence elevated with VET and aromatase inhibitors
The study pool was 9,710 women who underwent complete resection for estrogen-positive breast cancer and were all allocated to 5 years of adjuvant endocrine treatment or no adjuvant treatment, according to guidelines. Overall, 3,112 received no adjuvant endocrine treatment, 2,007 were treated with tamoxifen only, 403 with an aromatase inhibitor, and 2,939 with a sequence of tamoxifen and an aromatase inhibitor.
After exclusion of 1,249 who had received VET or MHT prior to breast cancer diagnosis, there were 6,391 not prescribed any estrogen hormonal treatment, 1,957 prescribed VET, and 133 prescribed MHT with or without VET.
During an estimated median 9.8 years’ follow-up, 1,333 women (16%) had a breast cancer recurrence. Of those, 111 had received VET, 16 MHT, and 1,206 neither. Compared with those receiving no hormonal treatment, the adjusted risk of recurrence was similar for the VET users (hazard ratio, 1.08; 95% confidence interval, 0.89-1.32).
However, there was an increased risk for recurrence associated with initiating VET during aromatase inhibitor treatment (HR, 1.39, 95% CI, 1.04-1.85). For women receiving MHT, the adjusted relative risk of recurrence with aromatase inhibitors wasn’t significant (HR, 1.05; 95% CI, 0.62-1.78).
Overall, compared with women who never used hormonal treatment, the absolute 10-year breast cancer recurrence risk was 19.2% for never-users of VET or MHT, 15.4% in VET users, and 17.1% in MHT users.
No differences found for mortality
Of the 8,461 women in the study, 40% (3,370) died during an estimated median follow-up of 15.2 years. Of those, 497 had received VET, 47 MHT, and 2,826 neither. Compared with the never-users of estrogen therapy, the adjusted HR for overall survival in VET users was 0.78 (95% CI, 0.71-0.87). The analysis stratified by adjuvant endocrine therapy didn’t show an increase in VET users by use of aromatase inhibitors (aHR, 0.94, 95% CI, 0.70-1.26). The same was found for women prescribed MHT, compared with never-users (aHR, 0.94; 95% CI, 0.70-1.26).
Never-users of VET or MHT had an absolute 10-year overall survival of 73.8% versus 79.5% and 80.5% among the women who used VET or MHT, respectively.
Asked to comment, Nanette Santoro, MD, professor and E. Stewart Taylor Chair of Obstetrics & Gynecology at the University of Colorado at Denver, Aurora, said in an interview: “It is important to look at this issue. These findings raise but don’t answer the question that vaginal estradiol may not be as safe as we hope it is for women with breast cancer using an aromatase inhibitor.”
However, she also pointed out that “the overall increase in risk is not enormous; mortality risk was not increased. Women need to consider that there may be some risk associated with this option in their decision making about taking it. Having a satisfying sex life is also important for many women! It is really compassionate use for quality of life, so there is always that unknown element of risk in the discussion. That unknown risk has to be balanced against the benefit that the estrogen provides.”
And, Dr. Santoro also noted that the use of prescription data poses limitations. “It cannot tell us what was going on in the minds of the patient and the prescriber. There may be differences in the prescriber’s impression of the patient’s risk of recurrence that influenced the decision to provide a prescription. ... Women using AIs [aromatase inhibitors] often get pretty severe vaginal dryness symptoms and may need more estrogen to be comfortable with intercourse, but we really cannot tell this from what is in this paper.”
Indeed, Dr. Cold said: “We admit it’s not a randomized study, but we’ve done what was possible to take [confounding] factors into account, including age, tumor size, nodal status, histology, and comorbidities.”
He suggested that a potential therapeutic approach to reducing the recurrence risk might be to switch VET-treated women to tamoxifen after 2-3 years of aromatase inhibitors.
This work was supported by Breast Friends, a part of the Danish Cancer Society. Dr. Cold received support from Breast Friends for the current study. Some of the other coauthors have pharmaceutical company disclosures. Dr. Santoro is a member of the scientific advisory boards for Astellas, Menogenix, Que Oncology, and Amazon Ember, and is a consultant for Ansh Labs.
Hormone therapy did not increase mortality in postmenopausal women treated for early-stage estrogen receptor–positive breast cancer, but, in longitudinal data from Denmark, there was a recurrence risk with vaginal estrogen therapy among those treated with aromatase inhibitors.
Genitourinary syndrome of menopause (GSM) – including vaginal dryness, burning, and urinary incontinence – is common in women treated for breast cancer. Adjuvant endocrine therapy, particularly aromatase inhibitors, can aggravate these symptoms. Both local and systemic estrogen therapy are recommended for alleviating GSM symptoms in healthy women, but concerns have been raised about their use in women with breast cancer. Previous studies examining this have suggested possible risks for breast cancer recurrence, but those studies have had several limitations including small samples and short follow-up, particularly for vaginal estrogen therapy.
In the new study, from a national Danish cohort of 8,461 postmenopausal women diagnosed between 1997 and 2004 and treated for early-stage invasive estrogen receptor–positive nonmetastatic breast cancer, neither systemic menopausal hormone therapy (MHT) nor local vaginal estrogen therapy (VET) were associated with an overall increased risk for either breast cancer recurrence or mortality. However, in the subset who had received an aromatase inhibitor – with or without tamoxifen – there was a statistically significant increased risk for breast cancer recurrence, but not mortality.
The results were published in the Journal of the National Cancer Institute.
“The data are reassuring for the majority of women with no adjuvant therapy or tamoxifen. But for those using adjuvant aromatase inhibitors, there might be a small risk,” study lead author Søren Cold, MD, PhD, senior oncologist in the department of oncology at Odense (Denmark) University Hospital, Odense, said in an interview.
Moreover, Dr. Cold noted, while this study didn’t find an increased recurrence risk with MHT for women taking aromatase inhibitors, other studies have. One in particular was stopped because of harm. The reason for the difference here is likely that the previous sample was small – just 133 women.
“Our study is mainly focusing on the use of vaginal estrogen. We had so few patients using systemic menopausal hormone therapy, those data don’t mean much. ... The risk with systemic therapy has been established. The vaginal use hasn’t been thoroughly studied before,” he noted.
Breast cancer recurrence elevated with VET and aromatase inhibitors
The study pool was 9,710 women who underwent complete resection for estrogen-positive breast cancer and were all allocated to 5 years of adjuvant endocrine treatment or no adjuvant treatment, according to guidelines. Overall, 3,112 received no adjuvant endocrine treatment, 2,007 were treated with tamoxifen only, 403 with an aromatase inhibitor, and 2,939 with a sequence of tamoxifen and an aromatase inhibitor.
After exclusion of 1,249 who had received VET or MHT prior to breast cancer diagnosis, there were 6,391 not prescribed any estrogen hormonal treatment, 1,957 prescribed VET, and 133 prescribed MHT with or without VET.
During an estimated median 9.8 years’ follow-up, 1,333 women (16%) had a breast cancer recurrence. Of those, 111 had received VET, 16 MHT, and 1,206 neither. Compared with those receiving no hormonal treatment, the adjusted risk of recurrence was similar for the VET users (hazard ratio, 1.08; 95% confidence interval, 0.89-1.32).
However, there was an increased risk for recurrence associated with initiating VET during aromatase inhibitor treatment (HR, 1.39, 95% CI, 1.04-1.85). For women receiving MHT, the adjusted relative risk of recurrence with aromatase inhibitors wasn’t significant (HR, 1.05; 95% CI, 0.62-1.78).
Overall, compared with women who never used hormonal treatment, the absolute 10-year breast cancer recurrence risk was 19.2% for never-users of VET or MHT, 15.4% in VET users, and 17.1% in MHT users.
No differences found for mortality
Of the 8,461 women in the study, 40% (3,370) died during an estimated median follow-up of 15.2 years. Of those, 497 had received VET, 47 MHT, and 2,826 neither. Compared with the never-users of estrogen therapy, the adjusted HR for overall survival in VET users was 0.78 (95% CI, 0.71-0.87). The analysis stratified by adjuvant endocrine therapy didn’t show an increase in VET users by use of aromatase inhibitors (aHR, 0.94, 95% CI, 0.70-1.26). The same was found for women prescribed MHT, compared with never-users (aHR, 0.94; 95% CI, 0.70-1.26).
Never-users of VET or MHT had an absolute 10-year overall survival of 73.8% versus 79.5% and 80.5% among the women who used VET or MHT, respectively.
Asked to comment, Nanette Santoro, MD, professor and E. Stewart Taylor Chair of Obstetrics & Gynecology at the University of Colorado at Denver, Aurora, said in an interview: “It is important to look at this issue. These findings raise but don’t answer the question that vaginal estradiol may not be as safe as we hope it is for women with breast cancer using an aromatase inhibitor.”
However, she also pointed out that “the overall increase in risk is not enormous; mortality risk was not increased. Women need to consider that there may be some risk associated with this option in their decision making about taking it. Having a satisfying sex life is also important for many women! It is really compassionate use for quality of life, so there is always that unknown element of risk in the discussion. That unknown risk has to be balanced against the benefit that the estrogen provides.”
And, Dr. Santoro also noted that the use of prescription data poses limitations. “It cannot tell us what was going on in the minds of the patient and the prescriber. There may be differences in the prescriber’s impression of the patient’s risk of recurrence that influenced the decision to provide a prescription. ... Women using AIs [aromatase inhibitors] often get pretty severe vaginal dryness symptoms and may need more estrogen to be comfortable with intercourse, but we really cannot tell this from what is in this paper.”
Indeed, Dr. Cold said: “We admit it’s not a randomized study, but we’ve done what was possible to take [confounding] factors into account, including age, tumor size, nodal status, histology, and comorbidities.”
He suggested that a potential therapeutic approach to reducing the recurrence risk might be to switch VET-treated women to tamoxifen after 2-3 years of aromatase inhibitors.
This work was supported by Breast Friends, a part of the Danish Cancer Society. Dr. Cold received support from Breast Friends for the current study. Some of the other coauthors have pharmaceutical company disclosures. Dr. Santoro is a member of the scientific advisory boards for Astellas, Menogenix, Que Oncology, and Amazon Ember, and is a consultant for Ansh Labs.
Hormone therapy did not increase mortality in postmenopausal women treated for early-stage estrogen receptor–positive breast cancer, but, in longitudinal data from Denmark, there was a recurrence risk with vaginal estrogen therapy among those treated with aromatase inhibitors.
Genitourinary syndrome of menopause (GSM) – including vaginal dryness, burning, and urinary incontinence – is common in women treated for breast cancer. Adjuvant endocrine therapy, particularly aromatase inhibitors, can aggravate these symptoms. Both local and systemic estrogen therapy are recommended for alleviating GSM symptoms in healthy women, but concerns have been raised about their use in women with breast cancer. Previous studies examining this have suggested possible risks for breast cancer recurrence, but those studies have had several limitations including small samples and short follow-up, particularly for vaginal estrogen therapy.
In the new study, from a national Danish cohort of 8,461 postmenopausal women diagnosed between 1997 and 2004 and treated for early-stage invasive estrogen receptor–positive nonmetastatic breast cancer, neither systemic menopausal hormone therapy (MHT) nor local vaginal estrogen therapy (VET) were associated with an overall increased risk for either breast cancer recurrence or mortality. However, in the subset who had received an aromatase inhibitor – with or without tamoxifen – there was a statistically significant increased risk for breast cancer recurrence, but not mortality.
The results were published in the Journal of the National Cancer Institute.
“The data are reassuring for the majority of women with no adjuvant therapy or tamoxifen. But for those using adjuvant aromatase inhibitors, there might be a small risk,” study lead author Søren Cold, MD, PhD, senior oncologist in the department of oncology at Odense (Denmark) University Hospital, Odense, said in an interview.
Moreover, Dr. Cold noted, while this study didn’t find an increased recurrence risk with MHT for women taking aromatase inhibitors, other studies have. One in particular was stopped because of harm. The reason for the difference here is likely that the previous sample was small – just 133 women.
“Our study is mainly focusing on the use of vaginal estrogen. We had so few patients using systemic menopausal hormone therapy, those data don’t mean much. ... The risk with systemic therapy has been established. The vaginal use hasn’t been thoroughly studied before,” he noted.
Breast cancer recurrence elevated with VET and aromatase inhibitors
The study pool was 9,710 women who underwent complete resection for estrogen-positive breast cancer and were all allocated to 5 years of adjuvant endocrine treatment or no adjuvant treatment, according to guidelines. Overall, 3,112 received no adjuvant endocrine treatment, 2,007 were treated with tamoxifen only, 403 with an aromatase inhibitor, and 2,939 with a sequence of tamoxifen and an aromatase inhibitor.
After exclusion of 1,249 who had received VET or MHT prior to breast cancer diagnosis, there were 6,391 not prescribed any estrogen hormonal treatment, 1,957 prescribed VET, and 133 prescribed MHT with or without VET.
During an estimated median 9.8 years’ follow-up, 1,333 women (16%) had a breast cancer recurrence. Of those, 111 had received VET, 16 MHT, and 1,206 neither. Compared with those receiving no hormonal treatment, the adjusted risk of recurrence was similar for the VET users (hazard ratio, 1.08; 95% confidence interval, 0.89-1.32).
However, there was an increased risk for recurrence associated with initiating VET during aromatase inhibitor treatment (HR, 1.39, 95% CI, 1.04-1.85). For women receiving MHT, the adjusted relative risk of recurrence with aromatase inhibitors wasn’t significant (HR, 1.05; 95% CI, 0.62-1.78).
Overall, compared with women who never used hormonal treatment, the absolute 10-year breast cancer recurrence risk was 19.2% for never-users of VET or MHT, 15.4% in VET users, and 17.1% in MHT users.
No differences found for mortality
Of the 8,461 women in the study, 40% (3,370) died during an estimated median follow-up of 15.2 years. Of those, 497 had received VET, 47 MHT, and 2,826 neither. Compared with the never-users of estrogen therapy, the adjusted HR for overall survival in VET users was 0.78 (95% CI, 0.71-0.87). The analysis stratified by adjuvant endocrine therapy didn’t show an increase in VET users by use of aromatase inhibitors (aHR, 0.94, 95% CI, 0.70-1.26). The same was found for women prescribed MHT, compared with never-users (aHR, 0.94; 95% CI, 0.70-1.26).
Never-users of VET or MHT had an absolute 10-year overall survival of 73.8% versus 79.5% and 80.5% among the women who used VET or MHT, respectively.
Asked to comment, Nanette Santoro, MD, professor and E. Stewart Taylor Chair of Obstetrics & Gynecology at the University of Colorado at Denver, Aurora, said in an interview: “It is important to look at this issue. These findings raise but don’t answer the question that vaginal estradiol may not be as safe as we hope it is for women with breast cancer using an aromatase inhibitor.”
However, she also pointed out that “the overall increase in risk is not enormous; mortality risk was not increased. Women need to consider that there may be some risk associated with this option in their decision making about taking it. Having a satisfying sex life is also important for many women! It is really compassionate use for quality of life, so there is always that unknown element of risk in the discussion. That unknown risk has to be balanced against the benefit that the estrogen provides.”
And, Dr. Santoro also noted that the use of prescription data poses limitations. “It cannot tell us what was going on in the minds of the patient and the prescriber. There may be differences in the prescriber’s impression of the patient’s risk of recurrence that influenced the decision to provide a prescription. ... Women using AIs [aromatase inhibitors] often get pretty severe vaginal dryness symptoms and may need more estrogen to be comfortable with intercourse, but we really cannot tell this from what is in this paper.”
Indeed, Dr. Cold said: “We admit it’s not a randomized study, but we’ve done what was possible to take [confounding] factors into account, including age, tumor size, nodal status, histology, and comorbidities.”
He suggested that a potential therapeutic approach to reducing the recurrence risk might be to switch VET-treated women to tamoxifen after 2-3 years of aromatase inhibitors.
This work was supported by Breast Friends, a part of the Danish Cancer Society. Dr. Cold received support from Breast Friends for the current study. Some of the other coauthors have pharmaceutical company disclosures. Dr. Santoro is a member of the scientific advisory boards for Astellas, Menogenix, Que Oncology, and Amazon Ember, and is a consultant for Ansh Labs.
FROM THE JOURNAL OF THE NATIONAL CANCER INSTITUTE
Parkinson’s disease: Is copper culpable?
, according to investigators. The techniques used in this research also may enable rapid identification of blood-borne cofactors driving abnormal protein development in a range of other neurodegenerative diseases, reported lead author Olena Synhaivska, MSc, of the Swiss Federal Laboratories for Materials Science and Technology, Dübendorf, Switzerland.

“While alpha‑synuclein oligomers are the known neurotoxic species in Parkinson’s disease, the development of effective anti–Parkinson’s disease drugs requires targeting of specific structures arising in the early stages of alpha‑synuclein phase transitions or the nucleation-dependent elongation of oligomers into protofibrils,” the investigators wrote in ACS Chemical Neuroscience. “In parallel, advanced methods are required to routinely characterize the size and morphology of intermediary nano- and microstructures formed during self-assembly and aggregation in the presence of aqueous metal ions to track disease progression in, for example, a blood test, to provide effective personalized patient care.”
Pathologic aggregation of alpha‑synuclein
To better understand the relationship between copper and alpha‑synuclein, the investigators used liquid-based atomic force microscopy to observe the protein in solution over 10 days as it transitioned from a simple monomer to a complex, three-dimensional aggregate. Protein aggregation occurred in the absence or presence of copper; however, when incubated in solution with Cu2+ ions, alpha‑synuclein aggregated faster, predominantly forming annular (ring-shaped) structures that were not observed in the absence of copper.
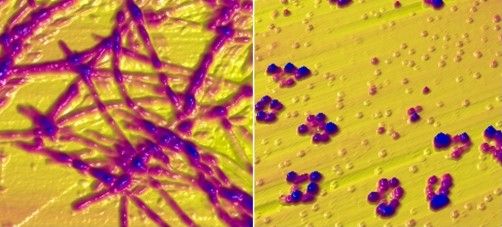
These annular oligomers are noteworthy because they are cytotoxic, and they nucleate formation of alpha‑synuclein filaments, meaning they could serve as early therapeutic targets, according to the investigators.
The above experiments were supported by Raman spectroscopy, which confirmed the various superstructures of alpha‑synuclein formed with or without copper. In addition, the investigators used molecular dynamics computer simulations to map “the dimensions, supramolecular packing interactions, and thermodynamic stabilities” involved in aggregation.
These findings “could potentially serve as guidelines for better understanding protein aggregated states in body fluids from individuals who have been exposed to environmental metals over their lifetime,” the investigators wrote. “The nanoscale imaging, chemical spectroscopy, and integrated modeling-measurement methodologies presented here may inform rapid screening of other potential blood-borne cofactors, for example, other biometals, heavy metals, physiological amino acids, and metabolites, in directing and potentially rerouting intrinsically disordered protein aggregation in the initiation and pathology of neurodegenerative diseases.”
What is copper’s role in Parkinson’s disease pathogenesis?
In a joint written comment, Vikram Khurana MD, PhD, and Richard Krolewski MD, PhD, of Brigham and Women’s Hospital and Harvard Medical School, Boston, said, “This study is important in that it demonstrates that the presence of copper can accelerate and alter the aggregation of wild type alpha‑synuclein. We know that pathologic aggregation of alpha‑synuclein is critical for diseases like Parkinson’s disease known as synucleinopathies – so any insight into how this is happening at the biophysical level has potential implications for altering that process.”
While Dr. Khurana and Dr. Krolewski praised the elegance of the study, including the techniques used to observe alpha‑synuclein aggregation in near real-time, they suggested that more work is needed to determine relevance for patients with Parkinson’s disease.
“It is not clear whether this process is happening in cells, how alpha‑synuclein fibrils might be directly exposed to copper intracellularly (with most of the copper being bound to proteins), and the relevance of the copper concentrations used here are in question,” they said. “Substantially more cell biology and in vivo modeling would be needed to further evaluate the connection of copper specifically to synucleinopathy. All this notwithstanding, the findings are exciting and intriguing and definitely warrant follow-up.”
In the meantime, an increasing number of studies, including a recent preprint by Dr. Khurana and Dr. Krolewski, are strengthening the case for a link between copper exposure and Parkinson’s disease pathogenesis. This body of evidence, they noted, “now spans epidemiology, cell biology, and biophysics.”
Their study, which tested 53 pesticides associated with Parkinson’s disease in patient-derived pluripotent stem cells, found that 2 out of 10 pesticides causing cell death were copper compounds.
“Ongoing work will explore the mechanism of this cell death and investigate ways to mitigate it,” said Dr. Khurana and Dr. Krolewski. “Our hope is that this line of research will raise public awareness about these and other pesticides to reduce potential harm from their use and highlight protective approaches. The study by Dr. Synhaivska and colleagues now raises the possibility of new mechanisms.”
The study by Dr. Synhaivska and colleagues was supported by grants from the Swiss National Science Foundation and the Science Foundation Ireland. The investigators disclosed no conflicts of interest. Dr. Krolewski has been retained as an expert consultant for plaintiffs in a lawsuit on the role of pesticides in Parkinson’s disease causation.
, according to investigators. The techniques used in this research also may enable rapid identification of blood-borne cofactors driving abnormal protein development in a range of other neurodegenerative diseases, reported lead author Olena Synhaivska, MSc, of the Swiss Federal Laboratories for Materials Science and Technology, Dübendorf, Switzerland.

“While alpha‑synuclein oligomers are the known neurotoxic species in Parkinson’s disease, the development of effective anti–Parkinson’s disease drugs requires targeting of specific structures arising in the early stages of alpha‑synuclein phase transitions or the nucleation-dependent elongation of oligomers into protofibrils,” the investigators wrote in ACS Chemical Neuroscience. “In parallel, advanced methods are required to routinely characterize the size and morphology of intermediary nano- and microstructures formed during self-assembly and aggregation in the presence of aqueous metal ions to track disease progression in, for example, a blood test, to provide effective personalized patient care.”
Pathologic aggregation of alpha‑synuclein
To better understand the relationship between copper and alpha‑synuclein, the investigators used liquid-based atomic force microscopy to observe the protein in solution over 10 days as it transitioned from a simple monomer to a complex, three-dimensional aggregate. Protein aggregation occurred in the absence or presence of copper; however, when incubated in solution with Cu2+ ions, alpha‑synuclein aggregated faster, predominantly forming annular (ring-shaped) structures that were not observed in the absence of copper.

These annular oligomers are noteworthy because they are cytotoxic, and they nucleate formation of alpha‑synuclein filaments, meaning they could serve as early therapeutic targets, according to the investigators.
The above experiments were supported by Raman spectroscopy, which confirmed the various superstructures of alpha‑synuclein formed with or without copper. In addition, the investigators used molecular dynamics computer simulations to map “the dimensions, supramolecular packing interactions, and thermodynamic stabilities” involved in aggregation.
These findings “could potentially serve as guidelines for better understanding protein aggregated states in body fluids from individuals who have been exposed to environmental metals over their lifetime,” the investigators wrote. “The nanoscale imaging, chemical spectroscopy, and integrated modeling-measurement methodologies presented here may inform rapid screening of other potential blood-borne cofactors, for example, other biometals, heavy metals, physiological amino acids, and metabolites, in directing and potentially rerouting intrinsically disordered protein aggregation in the initiation and pathology of neurodegenerative diseases.”
What is copper’s role in Parkinson’s disease pathogenesis?
In a joint written comment, Vikram Khurana MD, PhD, and Richard Krolewski MD, PhD, of Brigham and Women’s Hospital and Harvard Medical School, Boston, said, “This study is important in that it demonstrates that the presence of copper can accelerate and alter the aggregation of wild type alpha‑synuclein. We know that pathologic aggregation of alpha‑synuclein is critical for diseases like Parkinson’s disease known as synucleinopathies – so any insight into how this is happening at the biophysical level has potential implications for altering that process.”
While Dr. Khurana and Dr. Krolewski praised the elegance of the study, including the techniques used to observe alpha‑synuclein aggregation in near real-time, they suggested that more work is needed to determine relevance for patients with Parkinson’s disease.
“It is not clear whether this process is happening in cells, how alpha‑synuclein fibrils might be directly exposed to copper intracellularly (with most of the copper being bound to proteins), and the relevance of the copper concentrations used here are in question,” they said. “Substantially more cell biology and in vivo modeling would be needed to further evaluate the connection of copper specifically to synucleinopathy. All this notwithstanding, the findings are exciting and intriguing and definitely warrant follow-up.”
In the meantime, an increasing number of studies, including a recent preprint by Dr. Khurana and Dr. Krolewski, are strengthening the case for a link between copper exposure and Parkinson’s disease pathogenesis. This body of evidence, they noted, “now spans epidemiology, cell biology, and biophysics.”
Their study, which tested 53 pesticides associated with Parkinson’s disease in patient-derived pluripotent stem cells, found that 2 out of 10 pesticides causing cell death were copper compounds.
“Ongoing work will explore the mechanism of this cell death and investigate ways to mitigate it,” said Dr. Khurana and Dr. Krolewski. “Our hope is that this line of research will raise public awareness about these and other pesticides to reduce potential harm from their use and highlight protective approaches. The study by Dr. Synhaivska and colleagues now raises the possibility of new mechanisms.”
The study by Dr. Synhaivska and colleagues was supported by grants from the Swiss National Science Foundation and the Science Foundation Ireland. The investigators disclosed no conflicts of interest. Dr. Krolewski has been retained as an expert consultant for plaintiffs in a lawsuit on the role of pesticides in Parkinson’s disease causation.
, according to investigators. The techniques used in this research also may enable rapid identification of blood-borne cofactors driving abnormal protein development in a range of other neurodegenerative diseases, reported lead author Olena Synhaivska, MSc, of the Swiss Federal Laboratories for Materials Science and Technology, Dübendorf, Switzerland.

“While alpha‑synuclein oligomers are the known neurotoxic species in Parkinson’s disease, the development of effective anti–Parkinson’s disease drugs requires targeting of specific structures arising in the early stages of alpha‑synuclein phase transitions or the nucleation-dependent elongation of oligomers into protofibrils,” the investigators wrote in ACS Chemical Neuroscience. “In parallel, advanced methods are required to routinely characterize the size and morphology of intermediary nano- and microstructures formed during self-assembly and aggregation in the presence of aqueous metal ions to track disease progression in, for example, a blood test, to provide effective personalized patient care.”
Pathologic aggregation of alpha‑synuclein
To better understand the relationship between copper and alpha‑synuclein, the investigators used liquid-based atomic force microscopy to observe the protein in solution over 10 days as it transitioned from a simple monomer to a complex, three-dimensional aggregate. Protein aggregation occurred in the absence or presence of copper; however, when incubated in solution with Cu2+ ions, alpha‑synuclein aggregated faster, predominantly forming annular (ring-shaped) structures that were not observed in the absence of copper.

These annular oligomers are noteworthy because they are cytotoxic, and they nucleate formation of alpha‑synuclein filaments, meaning they could serve as early therapeutic targets, according to the investigators.
The above experiments were supported by Raman spectroscopy, which confirmed the various superstructures of alpha‑synuclein formed with or without copper. In addition, the investigators used molecular dynamics computer simulations to map “the dimensions, supramolecular packing interactions, and thermodynamic stabilities” involved in aggregation.
These findings “could potentially serve as guidelines for better understanding protein aggregated states in body fluids from individuals who have been exposed to environmental metals over their lifetime,” the investigators wrote. “The nanoscale imaging, chemical spectroscopy, and integrated modeling-measurement methodologies presented here may inform rapid screening of other potential blood-borne cofactors, for example, other biometals, heavy metals, physiological amino acids, and metabolites, in directing and potentially rerouting intrinsically disordered protein aggregation in the initiation and pathology of neurodegenerative diseases.”
What is copper’s role in Parkinson’s disease pathogenesis?
In a joint written comment, Vikram Khurana MD, PhD, and Richard Krolewski MD, PhD, of Brigham and Women’s Hospital and Harvard Medical School, Boston, said, “This study is important in that it demonstrates that the presence of copper can accelerate and alter the aggregation of wild type alpha‑synuclein. We know that pathologic aggregation of alpha‑synuclein is critical for diseases like Parkinson’s disease known as synucleinopathies – so any insight into how this is happening at the biophysical level has potential implications for altering that process.”
While Dr. Khurana and Dr. Krolewski praised the elegance of the study, including the techniques used to observe alpha‑synuclein aggregation in near real-time, they suggested that more work is needed to determine relevance for patients with Parkinson’s disease.
“It is not clear whether this process is happening in cells, how alpha‑synuclein fibrils might be directly exposed to copper intracellularly (with most of the copper being bound to proteins), and the relevance of the copper concentrations used here are in question,” they said. “Substantially more cell biology and in vivo modeling would be needed to further evaluate the connection of copper specifically to synucleinopathy. All this notwithstanding, the findings are exciting and intriguing and definitely warrant follow-up.”
In the meantime, an increasing number of studies, including a recent preprint by Dr. Khurana and Dr. Krolewski, are strengthening the case for a link between copper exposure and Parkinson’s disease pathogenesis. This body of evidence, they noted, “now spans epidemiology, cell biology, and biophysics.”
Their study, which tested 53 pesticides associated with Parkinson’s disease in patient-derived pluripotent stem cells, found that 2 out of 10 pesticides causing cell death were copper compounds.
“Ongoing work will explore the mechanism of this cell death and investigate ways to mitigate it,” said Dr. Khurana and Dr. Krolewski. “Our hope is that this line of research will raise public awareness about these and other pesticides to reduce potential harm from their use and highlight protective approaches. The study by Dr. Synhaivska and colleagues now raises the possibility of new mechanisms.”
The study by Dr. Synhaivska and colleagues was supported by grants from the Swiss National Science Foundation and the Science Foundation Ireland. The investigators disclosed no conflicts of interest. Dr. Krolewski has been retained as an expert consultant for plaintiffs in a lawsuit on the role of pesticides in Parkinson’s disease causation.
FROM ACS CHEMICAL NEUROSCIENCE
Safest, most effective medications for spine-related pain in older adults?
, a new comprehensive literature review suggests.
Investigators assessed the evidence for medications used for this indication in older adults by reviewing 138 double-blind, placebo-controlled trials.
Among their key findings and recommendations: Acetaminophen has a favorable safety profile for spine-related pain but nonsteroidal anti-inflammatory drugs (NSAIDs) have greater efficacy.
However, NSAIDs should be used in lower doses in the short term, with gastrointestinal precaution, the researchers note.
Corticosteroids have the least evidence for treating nonspecific back pain, they add.
“Most older people experience neck or low back pain at some point, bothersome enough to see their doctor,” coinvestigator Michael Perloff, MD, PhD, department of neurology, Boston University, said in a news release.
“Our findings provide a helpful medication guide for physicians to use for spine pain in an older population that can have a complex medical history,” Dr. Perloff added.
The results were published online in Drugs and Aging.
Recommendations, warnings
With the graying of the U.S. population, spine-related pain is increasingly common, the investigators note.
Medications play an important role in pain management, but their use has limitations in the elderly, owing to reduced liver and renal function, comorbid medical problems, and polypharmacy.
Other key findings from the literature review include that, although the nerve pain medications gabapentin and pregabalin may cause dizziness or difficulty walking, they also have some demonstrated benefit for neck and back nerve pain, such as sciatica, in older adults.
These agents should be used in lower doses with smaller dose adjustments, the researchers note.
They caution that the muscle relaxants carisoprodol, chlorzoxazone, cyclobenzaprine, metaxalone, methocarbamol, and orphenadrine should be avoided in older adults because of their association with risk for sedation and falls.
‘Rational therapeutic choices’
Three other muscle relaxants – tizanidine, baclofen, and dantrolene – may be helpful for neck and back pain. The most evidence favors tizanidine and baclofen. These should be used in reduced doses. Tizanidine should be avoided in patients with liver disease, and for patients with kidney disease, the dosing of baclofen should be reduced, the investigators write.
Other findings include the following:
- Older tricyclic antidepressants should typically be avoided in this population because of their side effects, but nortriptyline and desipramine may be better tolerated for neck and back nerve pain at lower doses.
- Newer antidepressants, particularly the selective serotonin-norepinephrine reuptake inhibitor duloxetine, have a better safety profile and good efficacy for spine-related nerve pain.
- Traditional opioids are typically avoided in the treatment of spine-related pain in older adults, owing to their associated risks.
However, low-dose opioid therapy may be helpful for severe refractory pain, with close monitoring of patients, the researchers note.
Weaker opioids, such as tramadol, may be better tolerated by older patients. They work well when combined with acetaminophen, but they carry the risk for sedation, upset stomach, and constipation.
“Medications used at the correct dose, for the correct diagnosis, adjusting for preexisting medical problems can result in better use of treatments for spine pain,” coinvestigator Jonathan Fu, MD, also with the department of neurology, Boston University, said in the release.
“Rational therapeutic choices should be targeted to spine pain diagnosis, such as NSAIDs and acetaminophen for arthritic and myofascial-based complaints, gabapentinoids or duloxetine for neuropathic and radicular symptoms, antispastic agents for myofascial-based pain, and combination therapy for mixed etiologies,” the investigators write.
They also emphasize that medications should be coupled with physical therapy and exercise programs, as well as treatment of the underlying degenerative disease process and medical illness, while keeping in mind the need for possible interventions and/or corrective surgery.
The research had no specific funding. The investigators have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a new comprehensive literature review suggests.
Investigators assessed the evidence for medications used for this indication in older adults by reviewing 138 double-blind, placebo-controlled trials.
Among their key findings and recommendations: Acetaminophen has a favorable safety profile for spine-related pain but nonsteroidal anti-inflammatory drugs (NSAIDs) have greater efficacy.
However, NSAIDs should be used in lower doses in the short term, with gastrointestinal precaution, the researchers note.
Corticosteroids have the least evidence for treating nonspecific back pain, they add.
“Most older people experience neck or low back pain at some point, bothersome enough to see their doctor,” coinvestigator Michael Perloff, MD, PhD, department of neurology, Boston University, said in a news release.
“Our findings provide a helpful medication guide for physicians to use for spine pain in an older population that can have a complex medical history,” Dr. Perloff added.
The results were published online in Drugs and Aging.
Recommendations, warnings
With the graying of the U.S. population, spine-related pain is increasingly common, the investigators note.
Medications play an important role in pain management, but their use has limitations in the elderly, owing to reduced liver and renal function, comorbid medical problems, and polypharmacy.
Other key findings from the literature review include that, although the nerve pain medications gabapentin and pregabalin may cause dizziness or difficulty walking, they also have some demonstrated benefit for neck and back nerve pain, such as sciatica, in older adults.
These agents should be used in lower doses with smaller dose adjustments, the researchers note.
They caution that the muscle relaxants carisoprodol, chlorzoxazone, cyclobenzaprine, metaxalone, methocarbamol, and orphenadrine should be avoided in older adults because of their association with risk for sedation and falls.
‘Rational therapeutic choices’
Three other muscle relaxants – tizanidine, baclofen, and dantrolene – may be helpful for neck and back pain. The most evidence favors tizanidine and baclofen. These should be used in reduced doses. Tizanidine should be avoided in patients with liver disease, and for patients with kidney disease, the dosing of baclofen should be reduced, the investigators write.
Other findings include the following:
- Older tricyclic antidepressants should typically be avoided in this population because of their side effects, but nortriptyline and desipramine may be better tolerated for neck and back nerve pain at lower doses.
- Newer antidepressants, particularly the selective serotonin-norepinephrine reuptake inhibitor duloxetine, have a better safety profile and good efficacy for spine-related nerve pain.
- Traditional opioids are typically avoided in the treatment of spine-related pain in older adults, owing to their associated risks.
However, low-dose opioid therapy may be helpful for severe refractory pain, with close monitoring of patients, the researchers note.
Weaker opioids, such as tramadol, may be better tolerated by older patients. They work well when combined with acetaminophen, but they carry the risk for sedation, upset stomach, and constipation.
“Medications used at the correct dose, for the correct diagnosis, adjusting for preexisting medical problems can result in better use of treatments for spine pain,” coinvestigator Jonathan Fu, MD, also with the department of neurology, Boston University, said in the release.
“Rational therapeutic choices should be targeted to spine pain diagnosis, such as NSAIDs and acetaminophen for arthritic and myofascial-based complaints, gabapentinoids or duloxetine for neuropathic and radicular symptoms, antispastic agents for myofascial-based pain, and combination therapy for mixed etiologies,” the investigators write.
They also emphasize that medications should be coupled with physical therapy and exercise programs, as well as treatment of the underlying degenerative disease process and medical illness, while keeping in mind the need for possible interventions and/or corrective surgery.
The research had no specific funding. The investigators have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a new comprehensive literature review suggests.
Investigators assessed the evidence for medications used for this indication in older adults by reviewing 138 double-blind, placebo-controlled trials.
Among their key findings and recommendations: Acetaminophen has a favorable safety profile for spine-related pain but nonsteroidal anti-inflammatory drugs (NSAIDs) have greater efficacy.
However, NSAIDs should be used in lower doses in the short term, with gastrointestinal precaution, the researchers note.
Corticosteroids have the least evidence for treating nonspecific back pain, they add.
“Most older people experience neck or low back pain at some point, bothersome enough to see their doctor,” coinvestigator Michael Perloff, MD, PhD, department of neurology, Boston University, said in a news release.
“Our findings provide a helpful medication guide for physicians to use for spine pain in an older population that can have a complex medical history,” Dr. Perloff added.
The results were published online in Drugs and Aging.
Recommendations, warnings
With the graying of the U.S. population, spine-related pain is increasingly common, the investigators note.
Medications play an important role in pain management, but their use has limitations in the elderly, owing to reduced liver and renal function, comorbid medical problems, and polypharmacy.
Other key findings from the literature review include that, although the nerve pain medications gabapentin and pregabalin may cause dizziness or difficulty walking, they also have some demonstrated benefit for neck and back nerve pain, such as sciatica, in older adults.
These agents should be used in lower doses with smaller dose adjustments, the researchers note.
They caution that the muscle relaxants carisoprodol, chlorzoxazone, cyclobenzaprine, metaxalone, methocarbamol, and orphenadrine should be avoided in older adults because of their association with risk for sedation and falls.
‘Rational therapeutic choices’
Three other muscle relaxants – tizanidine, baclofen, and dantrolene – may be helpful for neck and back pain. The most evidence favors tizanidine and baclofen. These should be used in reduced doses. Tizanidine should be avoided in patients with liver disease, and for patients with kidney disease, the dosing of baclofen should be reduced, the investigators write.
Other findings include the following:
- Older tricyclic antidepressants should typically be avoided in this population because of their side effects, but nortriptyline and desipramine may be better tolerated for neck and back nerve pain at lower doses.
- Newer antidepressants, particularly the selective serotonin-norepinephrine reuptake inhibitor duloxetine, have a better safety profile and good efficacy for spine-related nerve pain.
- Traditional opioids are typically avoided in the treatment of spine-related pain in older adults, owing to their associated risks.
However, low-dose opioid therapy may be helpful for severe refractory pain, with close monitoring of patients, the researchers note.
Weaker opioids, such as tramadol, may be better tolerated by older patients. They work well when combined with acetaminophen, but they carry the risk for sedation, upset stomach, and constipation.
“Medications used at the correct dose, for the correct diagnosis, adjusting for preexisting medical problems can result in better use of treatments for spine pain,” coinvestigator Jonathan Fu, MD, also with the department of neurology, Boston University, said in the release.
“Rational therapeutic choices should be targeted to spine pain diagnosis, such as NSAIDs and acetaminophen for arthritic and myofascial-based complaints, gabapentinoids or duloxetine for neuropathic and radicular symptoms, antispastic agents for myofascial-based pain, and combination therapy for mixed etiologies,” the investigators write.
They also emphasize that medications should be coupled with physical therapy and exercise programs, as well as treatment of the underlying degenerative disease process and medical illness, while keeping in mind the need for possible interventions and/or corrective surgery.
The research had no specific funding. The investigators have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM DRUGS AND AGING
Number of steps per day needed to prevent death in diabetes
Walking 10,000 steps per day may reduce the risk of death for those who have trouble regulating their blood sugar, according to the findings from a study of almost 1,700 American adults with prediabetes or diabetes.
Researchers from the University of Seville, Spain, evaluated U.S. adults with prediabetes and diabetes using data from the Centers for Disease Control and Prevention’s National Health and Nutrition Examination Survey, collected between 2005 and 2006.
The findings were published this month in Diabetes Care.
Of the total, 1,194 adults had prediabetes, and 493 had diabetes. People with diabetes in the study were diagnosed by a doctor or had a fasting blood glucose level higher than 126 mg/dL. People with prediabetes in the study were also diagnosed by a doctor or had a fasting glucose level from 100 to 125 mg/dL.
Over half (56%) of prediabetic adults were male (average age 55 years), and they took an average of 8,500 steps per day. Half (51%) of the diabetic adults were also male (average age 61 years), and they took fewer steps per day – about 6,300.
The people in the study wore an accelerometer on their waist to count their steps for 7 consecutive days. The researchers adjusted for age, sex, ethnicity, smoking, alcohol use, diet, and use of diabetes medications.
Over 9 years, 200 people with prediabetes and 138 with diabetes died. Based on those who survived after follow-up, walking nearly 10,000 steps per day was best for reducing the risk of death from any cause for people with prediabetes and diabetes.
But about 20% of people in the study were removed from the analysis because they had invalid accelerometry data. Adults who are healthy enough to walk 10,000 steps may have different rates of death from those who aren’t, according to the study authors, who called for more research to compare these two groups.
If 10,000 steps seem like a daunting task, talking to a doctor about finding a routine that works for your physical ability could be helpful, the study authors suggest.
A version of this article first appeared on Medscape.com.
Walking 10,000 steps per day may reduce the risk of death for those who have trouble regulating their blood sugar, according to the findings from a study of almost 1,700 American adults with prediabetes or diabetes.
Researchers from the University of Seville, Spain, evaluated U.S. adults with prediabetes and diabetes using data from the Centers for Disease Control and Prevention’s National Health and Nutrition Examination Survey, collected between 2005 and 2006.
The findings were published this month in Diabetes Care.
Of the total, 1,194 adults had prediabetes, and 493 had diabetes. People with diabetes in the study were diagnosed by a doctor or had a fasting blood glucose level higher than 126 mg/dL. People with prediabetes in the study were also diagnosed by a doctor or had a fasting glucose level from 100 to 125 mg/dL.
Over half (56%) of prediabetic adults were male (average age 55 years), and they took an average of 8,500 steps per day. Half (51%) of the diabetic adults were also male (average age 61 years), and they took fewer steps per day – about 6,300.
The people in the study wore an accelerometer on their waist to count their steps for 7 consecutive days. The researchers adjusted for age, sex, ethnicity, smoking, alcohol use, diet, and use of diabetes medications.
Over 9 years, 200 people with prediabetes and 138 with diabetes died. Based on those who survived after follow-up, walking nearly 10,000 steps per day was best for reducing the risk of death from any cause for people with prediabetes and diabetes.
But about 20% of people in the study were removed from the analysis because they had invalid accelerometry data. Adults who are healthy enough to walk 10,000 steps may have different rates of death from those who aren’t, according to the study authors, who called for more research to compare these two groups.
If 10,000 steps seem like a daunting task, talking to a doctor about finding a routine that works for your physical ability could be helpful, the study authors suggest.
A version of this article first appeared on Medscape.com.
Walking 10,000 steps per day may reduce the risk of death for those who have trouble regulating their blood sugar, according to the findings from a study of almost 1,700 American adults with prediabetes or diabetes.
Researchers from the University of Seville, Spain, evaluated U.S. adults with prediabetes and diabetes using data from the Centers for Disease Control and Prevention’s National Health and Nutrition Examination Survey, collected between 2005 and 2006.
The findings were published this month in Diabetes Care.
Of the total, 1,194 adults had prediabetes, and 493 had diabetes. People with diabetes in the study were diagnosed by a doctor or had a fasting blood glucose level higher than 126 mg/dL. People with prediabetes in the study were also diagnosed by a doctor or had a fasting glucose level from 100 to 125 mg/dL.
Over half (56%) of prediabetic adults were male (average age 55 years), and they took an average of 8,500 steps per day. Half (51%) of the diabetic adults were also male (average age 61 years), and they took fewer steps per day – about 6,300.
The people in the study wore an accelerometer on their waist to count their steps for 7 consecutive days. The researchers adjusted for age, sex, ethnicity, smoking, alcohol use, diet, and use of diabetes medications.
Over 9 years, 200 people with prediabetes and 138 with diabetes died. Based on those who survived after follow-up, walking nearly 10,000 steps per day was best for reducing the risk of death from any cause for people with prediabetes and diabetes.
But about 20% of people in the study were removed from the analysis because they had invalid accelerometry data. Adults who are healthy enough to walk 10,000 steps may have different rates of death from those who aren’t, according to the study authors, who called for more research to compare these two groups.
If 10,000 steps seem like a daunting task, talking to a doctor about finding a routine that works for your physical ability could be helpful, the study authors suggest.
A version of this article first appeared on Medscape.com.
Alcohol’s detrimental impact on the brain explained?
Results of a large observational study suggest brain iron accumulation is a “plausible pathway” through which alcohol negatively affects cognition, study Anya Topiwala, MD, PhD, senior clinical researcher, Nuffield Department of Population Health, University of Oxford, England, said in an interview.
Study participants who drank 56 grams of alcohol a week had higher brain iron levels. The U.K. guideline for “low risk” alcohol consumption is less than 14 units weekly, or 112 grams.
“We are finding harmful associations with iron within those low-risk alcohol intake guidelines,” said Dr. Topiwala.
The study was published online in PLOS Medicine.
Early intervention opportunity?
Previous research suggests higher brain iron may be involved in the pathophysiology of Alzheimer’s and Parkinson’s diseases. However, it’s unclear whether deposition plays a role in alcohol’s effect on the brain and if it does, whether this could present an opportunity for early intervention with, for example, chelating agents.
The study included 20,729 participants in the UK Biobank study, which recruited volunteers from 2006 to 2010. Participants had a mean age of 54.8 years, and 48.6% were female.
Participants self-identified as current, never, or previous alcohol consumers. For current drinkers, researchers calculated the total weekly number of U.K. units of alcohol consumed. One unit is 8 grams. A standard drink in the United States is 14 grams. They categorized weekly consumption into quintiles and used the lowest quintile as the reference category.
Participants underwent MRI to determine brain iron levels. Areas of interest were deep brain structures in the basal ganglia.
Mean weekly alcohol consumption was 17.7 units, which is higher than U.K. guidelines for low-risk consumption. “Half of the sample were drinking above what is recommended,” said Dr. Topiwala.
Alcohol consumption was associated with markers of higher iron in the bilateral putamen (beta, 0.08 standard deviation; 95% confidence interval, 0.06-0.09; P < .001), caudate (beta, 0.05; 95% CI, 0.04-0.07; P < .001), and substantia nigra (beta, 0.03; 95% CI; 0.02-0.05; P < .001).
Poorer performance
Drinking more than 7 units (56 grams) weekly was associated with higher susceptibility for all brain regions, except the thalamus.
Controlling for menopause status did not alter associations between alcohol and susceptibility for any brain region. This was also the case when excluding blood pressure and cholesterol as covariates.
There were significant interactions with age in the bilateral putamen and caudate but not with sex, smoking, or Townsend Deprivation Index, which includes such factors as unemployment and living conditions.
To gather data on liver iron levels, participants underwent abdominal imaging at the same time as brain imaging. Dr. Topiwala explained that the liver is a primary storage center for iron, so it was used as “a kind of surrogate marker” of iron in the body.
The researchers showed an indirect effect of alcohol through systemic iron. A 1 SD increase in weekly alcohol consumption was associated with a 0.05 mg/g (95% CI, 0.02-0.07; P < .001) increase in liver iron. In addition, a 1 mg/g increase in liver iron was associated with a 0.44 (95% CI, 0.35-0.52; P < .001) SD increase in left putamen susceptibility.
In this sample, 32% (95% CI, 22-49; P < .001) of alcohol’s total effect on left putamen susceptibility was mediated via higher systemic iron levels.
To minimize the impact of other factors influencing the association between alcohol consumption and brain iron – and the possibility that people with more brain iron drink more – researchers used Mendelian randomization that considers genetically predicted alcohol intake. This analysis supported findings of associations between alcohol consumption and brain iron.
Participants completed a cognitive battery, which included trail-making tests that reflect executive function, puzzle tests that assess fluid intelligence or logic and reasoning, and task-based tests using the “Snap” card game to measure reaction time.
Investigators found the more iron that was present in certain brain regions, the poorer participants’ cognitive performance.
Patients should know about the risks of moderate alcohol intake so they can make decisions about drinking, said Dr. Topiwala. “They should be aware that 14 units of alcohol per week is not a zero risk.”
Novel research
Commenting for this news organization, Heather Snyder, PhD, vice president of medical and scientific relations, Alzheimer’s Association, noted the study’s large size as a strength of the research.
She noted previous research has shown an association between higher iron levels and alcohol dependence and worse cognitive function, but the potential connection of brain iron levels, moderate alcohol consumption, and cognition has not been studied to date.
“This paper aims to look at whether there is a potential biological link between moderate alcohol consumption and cognition through iron-related pathways.”
The authors suggest more work is needed to understand whether alcohol consumption impacts iron-related biologies to affect downstream cognition, said Dr. Snyder. “Although this study does not answer that question, it does highlight some important questions.”
Study authors received funding from Wellcome Trust, UK Medical Research Council, National Institute for Health Research (NIHR) Oxford Biomedical Research Centre, BHF Centre of Research Excellence, British Heart Foundation, NIHR Cambridge Biomedical Research Centre, U.S. Department of Veterans Affairs, China Scholarship Council, and Li Ka Shing Centre for Health Information and Discovery. Dr. Topiwala has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Results of a large observational study suggest brain iron accumulation is a “plausible pathway” through which alcohol negatively affects cognition, study Anya Topiwala, MD, PhD, senior clinical researcher, Nuffield Department of Population Health, University of Oxford, England, said in an interview.
Study participants who drank 56 grams of alcohol a week had higher brain iron levels. The U.K. guideline for “low risk” alcohol consumption is less than 14 units weekly, or 112 grams.
“We are finding harmful associations with iron within those low-risk alcohol intake guidelines,” said Dr. Topiwala.
The study was published online in PLOS Medicine.
Early intervention opportunity?
Previous research suggests higher brain iron may be involved in the pathophysiology of Alzheimer’s and Parkinson’s diseases. However, it’s unclear whether deposition plays a role in alcohol’s effect on the brain and if it does, whether this could present an opportunity for early intervention with, for example, chelating agents.
The study included 20,729 participants in the UK Biobank study, which recruited volunteers from 2006 to 2010. Participants had a mean age of 54.8 years, and 48.6% were female.
Participants self-identified as current, never, or previous alcohol consumers. For current drinkers, researchers calculated the total weekly number of U.K. units of alcohol consumed. One unit is 8 grams. A standard drink in the United States is 14 grams. They categorized weekly consumption into quintiles and used the lowest quintile as the reference category.
Participants underwent MRI to determine brain iron levels. Areas of interest were deep brain structures in the basal ganglia.
Mean weekly alcohol consumption was 17.7 units, which is higher than U.K. guidelines for low-risk consumption. “Half of the sample were drinking above what is recommended,” said Dr. Topiwala.
Alcohol consumption was associated with markers of higher iron in the bilateral putamen (beta, 0.08 standard deviation; 95% confidence interval, 0.06-0.09; P < .001), caudate (beta, 0.05; 95% CI, 0.04-0.07; P < .001), and substantia nigra (beta, 0.03; 95% CI; 0.02-0.05; P < .001).
Poorer performance
Drinking more than 7 units (56 grams) weekly was associated with higher susceptibility for all brain regions, except the thalamus.
Controlling for menopause status did not alter associations between alcohol and susceptibility for any brain region. This was also the case when excluding blood pressure and cholesterol as covariates.
There were significant interactions with age in the bilateral putamen and caudate but not with sex, smoking, or Townsend Deprivation Index, which includes such factors as unemployment and living conditions.
To gather data on liver iron levels, participants underwent abdominal imaging at the same time as brain imaging. Dr. Topiwala explained that the liver is a primary storage center for iron, so it was used as “a kind of surrogate marker” of iron in the body.
The researchers showed an indirect effect of alcohol through systemic iron. A 1 SD increase in weekly alcohol consumption was associated with a 0.05 mg/g (95% CI, 0.02-0.07; P < .001) increase in liver iron. In addition, a 1 mg/g increase in liver iron was associated with a 0.44 (95% CI, 0.35-0.52; P < .001) SD increase in left putamen susceptibility.
In this sample, 32% (95% CI, 22-49; P < .001) of alcohol’s total effect on left putamen susceptibility was mediated via higher systemic iron levels.
To minimize the impact of other factors influencing the association between alcohol consumption and brain iron – and the possibility that people with more brain iron drink more – researchers used Mendelian randomization that considers genetically predicted alcohol intake. This analysis supported findings of associations between alcohol consumption and brain iron.
Participants completed a cognitive battery, which included trail-making tests that reflect executive function, puzzle tests that assess fluid intelligence or logic and reasoning, and task-based tests using the “Snap” card game to measure reaction time.
Investigators found the more iron that was present in certain brain regions, the poorer participants’ cognitive performance.
Patients should know about the risks of moderate alcohol intake so they can make decisions about drinking, said Dr. Topiwala. “They should be aware that 14 units of alcohol per week is not a zero risk.”
Novel research
Commenting for this news organization, Heather Snyder, PhD, vice president of medical and scientific relations, Alzheimer’s Association, noted the study’s large size as a strength of the research.
She noted previous research has shown an association between higher iron levels and alcohol dependence and worse cognitive function, but the potential connection of brain iron levels, moderate alcohol consumption, and cognition has not been studied to date.
“This paper aims to look at whether there is a potential biological link between moderate alcohol consumption and cognition through iron-related pathways.”
The authors suggest more work is needed to understand whether alcohol consumption impacts iron-related biologies to affect downstream cognition, said Dr. Snyder. “Although this study does not answer that question, it does highlight some important questions.”
Study authors received funding from Wellcome Trust, UK Medical Research Council, National Institute for Health Research (NIHR) Oxford Biomedical Research Centre, BHF Centre of Research Excellence, British Heart Foundation, NIHR Cambridge Biomedical Research Centre, U.S. Department of Veterans Affairs, China Scholarship Council, and Li Ka Shing Centre for Health Information and Discovery. Dr. Topiwala has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Results of a large observational study suggest brain iron accumulation is a “plausible pathway” through which alcohol negatively affects cognition, study Anya Topiwala, MD, PhD, senior clinical researcher, Nuffield Department of Population Health, University of Oxford, England, said in an interview.
Study participants who drank 56 grams of alcohol a week had higher brain iron levels. The U.K. guideline for “low risk” alcohol consumption is less than 14 units weekly, or 112 grams.
“We are finding harmful associations with iron within those low-risk alcohol intake guidelines,” said Dr. Topiwala.
The study was published online in PLOS Medicine.
Early intervention opportunity?
Previous research suggests higher brain iron may be involved in the pathophysiology of Alzheimer’s and Parkinson’s diseases. However, it’s unclear whether deposition plays a role in alcohol’s effect on the brain and if it does, whether this could present an opportunity for early intervention with, for example, chelating agents.
The study included 20,729 participants in the UK Biobank study, which recruited volunteers from 2006 to 2010. Participants had a mean age of 54.8 years, and 48.6% were female.
Participants self-identified as current, never, or previous alcohol consumers. For current drinkers, researchers calculated the total weekly number of U.K. units of alcohol consumed. One unit is 8 grams. A standard drink in the United States is 14 grams. They categorized weekly consumption into quintiles and used the lowest quintile as the reference category.
Participants underwent MRI to determine brain iron levels. Areas of interest were deep brain structures in the basal ganglia.
Mean weekly alcohol consumption was 17.7 units, which is higher than U.K. guidelines for low-risk consumption. “Half of the sample were drinking above what is recommended,” said Dr. Topiwala.
Alcohol consumption was associated with markers of higher iron in the bilateral putamen (beta, 0.08 standard deviation; 95% confidence interval, 0.06-0.09; P < .001), caudate (beta, 0.05; 95% CI, 0.04-0.07; P < .001), and substantia nigra (beta, 0.03; 95% CI; 0.02-0.05; P < .001).
Poorer performance
Drinking more than 7 units (56 grams) weekly was associated with higher susceptibility for all brain regions, except the thalamus.
Controlling for menopause status did not alter associations between alcohol and susceptibility for any brain region. This was also the case when excluding blood pressure and cholesterol as covariates.
There were significant interactions with age in the bilateral putamen and caudate but not with sex, smoking, or Townsend Deprivation Index, which includes such factors as unemployment and living conditions.
To gather data on liver iron levels, participants underwent abdominal imaging at the same time as brain imaging. Dr. Topiwala explained that the liver is a primary storage center for iron, so it was used as “a kind of surrogate marker” of iron in the body.
The researchers showed an indirect effect of alcohol through systemic iron. A 1 SD increase in weekly alcohol consumption was associated with a 0.05 mg/g (95% CI, 0.02-0.07; P < .001) increase in liver iron. In addition, a 1 mg/g increase in liver iron was associated with a 0.44 (95% CI, 0.35-0.52; P < .001) SD increase in left putamen susceptibility.
In this sample, 32% (95% CI, 22-49; P < .001) of alcohol’s total effect on left putamen susceptibility was mediated via higher systemic iron levels.
To minimize the impact of other factors influencing the association between alcohol consumption and brain iron – and the possibility that people with more brain iron drink more – researchers used Mendelian randomization that considers genetically predicted alcohol intake. This analysis supported findings of associations between alcohol consumption and brain iron.
Participants completed a cognitive battery, which included trail-making tests that reflect executive function, puzzle tests that assess fluid intelligence or logic and reasoning, and task-based tests using the “Snap” card game to measure reaction time.
Investigators found the more iron that was present in certain brain regions, the poorer participants’ cognitive performance.
Patients should know about the risks of moderate alcohol intake so they can make decisions about drinking, said Dr. Topiwala. “They should be aware that 14 units of alcohol per week is not a zero risk.”
Novel research
Commenting for this news organization, Heather Snyder, PhD, vice president of medical and scientific relations, Alzheimer’s Association, noted the study’s large size as a strength of the research.
She noted previous research has shown an association between higher iron levels and alcohol dependence and worse cognitive function, but the potential connection of brain iron levels, moderate alcohol consumption, and cognition has not been studied to date.
“This paper aims to look at whether there is a potential biological link between moderate alcohol consumption and cognition through iron-related pathways.”
The authors suggest more work is needed to understand whether alcohol consumption impacts iron-related biologies to affect downstream cognition, said Dr. Snyder. “Although this study does not answer that question, it does highlight some important questions.”
Study authors received funding from Wellcome Trust, UK Medical Research Council, National Institute for Health Research (NIHR) Oxford Biomedical Research Centre, BHF Centre of Research Excellence, British Heart Foundation, NIHR Cambridge Biomedical Research Centre, U.S. Department of Veterans Affairs, China Scholarship Council, and Li Ka Shing Centre for Health Information and Discovery. Dr. Topiwala has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM PLOS MEDICINE
Marital status plays modest role in gastric cancer overall survival
, according to research published in the Journal of Investigative Medicine.
Tumor size remained the largest contributor to overall survival, but marital status was among several other significant factors, such as age, race, gender, treatment style, and pathologic stage, that can provide insight into a patient’s likelihood of overall survival, as it does with several other cancers.
“Married patients had the best prognosis, followed by single patients, and the prognosis of separated patients was the worst,” write Lixiang Zhang and colleagues at the First Affiliated Hospital of Anhui Medical University, Hefei, China. “We speculate that this might be due to the fact that married patients had better financial conditions and emotional encouragement, while separated patients might be more likely to experience financial difficulties [and] emotional loss.”
The results were not necessarily surprising to Richard M. Peek, Jr., MD, director of the division of gastroenterology and a professor of medicine at Vanderbilt University Medical Center, who was not involved in the research.
“Marital status is a reflection of support systems, and a strong support system is a prognosticator for increased compliance with medical appointments and medical therapies,” Dr. Peek told this news organization. “It is something to consider when somebody is being treated for gastric cancer, because if they don’t have a strong support system – and marital status can be a proxy for that – then they may need more intensive follow-up and surveillance, for example, than somebody who does not have that support system.”
Exploring the marital status–cancer survival connection
Gastric cancer is the third leading cause of cancer deaths across the world, causing 780,000 deaths in 2018, the authors note. Yet it’s difficult to accurately predict the prognosis in patients who undergo treatment for early stage gastric cancer. Previous research has found marital status to be associated with survival in prostate, cervical, and rectal cancers.
Mark A. Lewis, MD, director of gastrointestinal oncology at Intermountain Healthcare Cancer Center, Utah, told this news organization that the connection between marital status and cancer outcomes has been described previously, including in an even larger analysis using the U.S. Surveillance, Epidemiology, and End Results (SEER) database from 2013. That study found that “unmarried patients are at significantly higher risk of presentation with metastatic cancer, undertreatment, and death resulting from their cancer.”
In this study, the researchers compared marital status and survival rates among 3,647 patients with early-stage gastric cancer, using data from the SEER database. The study only included patients with tumors in the lamina propria, mucosa, and submucosa and excluded those with distant metastasis or distant lymph node metastases, a second cancer, no data on chemotherapy received, or unknown survival time.
Because they were using a nomogram and building a new predictive nomogram that would include marital status, the researchers divided the patient population into a training set of 2,719 patients and a testing set of 928 patients. Using overall survival as the primary endpoint, the analysis included the variables of “age at diagnosis, race, gender, tumor location, histology, grade, stage_T and stage_N, surgery in the primary site, lymph node dissection, chemotherapy, radiation, tumor size, insurance, and marital status,” the authors report.
Among the study population, 53.7% were married, 17.3% were widowed, 14% were single and never married, 7.5% were divorced, 1.1% were separated, and the status of 6.4% was unknown. Age at diagnosis, race, gender, histology, tumor grade, stage T, stage N, surgery type, tumor size, and insurance status were all significantly different between the marital status subgroups.
Married patients had the best prognosis, with an average overall survival of 72 months, compared with an average 60 months in widowed persons, the group with the poorest overall survival. Overall survival was higher in married women (76 months) than in married men (69 months). The same pattern held for women (62 months) and men (52 months) who had been widowed.
“It is worthy to note that survival was significantly better in divorced female patients than in divorced male patients,” the authors report. “Survival was better in female patients than in male patients” across all marital groups.
What long-term relationships reveal
These findings do not mean that simply getting married changes one’s likelihood of survival, however. Rather, a long-term relationship is revealing about other aspects in a person’s life.
“I think it represents more stability in the supportive relationship that you need to really deal with a serious disease like cancer,” Dr. Peek said.
If a patient does not have a long-term partner, their care team can ask other questions to get a sense of what their support network is like, Dr. Peek added. “We want to know, does anybody else live in the house with them? Do they have adequate transportation? Can they make medical appointments? Do they have somebody who can help with the medical issues that are going to come up? Do they have family in the area?”
Cancer treatment requires a multidisciplinary approach, and having someone other than just the patient around to help bring together the different aspects of care from different care teams can make a difference in how the patient fares, Dr. Peek explained. Patients without a strong support system may need closer follow-up and other accommodations, he said.
Providers “may schedule their clinical appointments closer together if they don’t have a support system, or they may be able to reach out and offer transportation assistance and those kinds of things that somebody living alone may need,” Dr. Peek said. Outside resources may be a higher priority for those who lack a support system at home, he added.
Dr. Peek also noted other factors that may play a role in a patient’s survival that these researchers did not have the data to address, such as socioeconomic status, employment, alcohol use, smoking, and infection with Helicobacter pylori, the strongest known risk factor for gastric cancer.
A potentially relevant limitation of the study is that it probably has some selection bias, because the patients who were included probably had the means to have received an earlier diagnosis, said Dr. Lewis, who was not involved in the research.
“Furthermore, just in terms of the group sizes, the baseline characteristics section makes it clear that the preponderance of patients were married, lending that group more statistical weight,” Dr. Lewis said.
“Of the seven attributes in the nomogram, the impact of the marital status seems comparatively meager relative to conventional clinicopathology risk factors like T stage,” he added.
“All in all, I think this study reinforces our awareness that socioeconomic status and social determinants of health play a huge role in cancer outcomes, but it’s not entirely clear that’s modifiable just by getting married,” Dr. Lewis said. “There is a saying in oncology that ‘expensive liquor causes less cancer than cheap liquor,’ which is not negating the carcinogenicity of alcohol but rather identifying different outcomes by socioeconomic status.”
The research was funded by the Natural Science Foundation of Anhui Province. The authors report no relevant financial relationships. Dr. Peek reports no relevant financial relationships. Dr. Lewis reports receiving speaking fees for AstraZeneca/Daiichi Sankyo and having done educational videos for Astellas.
A version of this article first appeared on Medscape.com.
, according to research published in the Journal of Investigative Medicine.
Tumor size remained the largest contributor to overall survival, but marital status was among several other significant factors, such as age, race, gender, treatment style, and pathologic stage, that can provide insight into a patient’s likelihood of overall survival, as it does with several other cancers.
“Married patients had the best prognosis, followed by single patients, and the prognosis of separated patients was the worst,” write Lixiang Zhang and colleagues at the First Affiliated Hospital of Anhui Medical University, Hefei, China. “We speculate that this might be due to the fact that married patients had better financial conditions and emotional encouragement, while separated patients might be more likely to experience financial difficulties [and] emotional loss.”
The results were not necessarily surprising to Richard M. Peek, Jr., MD, director of the division of gastroenterology and a professor of medicine at Vanderbilt University Medical Center, who was not involved in the research.
“Marital status is a reflection of support systems, and a strong support system is a prognosticator for increased compliance with medical appointments and medical therapies,” Dr. Peek told this news organization. “It is something to consider when somebody is being treated for gastric cancer, because if they don’t have a strong support system – and marital status can be a proxy for that – then they may need more intensive follow-up and surveillance, for example, than somebody who does not have that support system.”
Exploring the marital status–cancer survival connection
Gastric cancer is the third leading cause of cancer deaths across the world, causing 780,000 deaths in 2018, the authors note. Yet it’s difficult to accurately predict the prognosis in patients who undergo treatment for early stage gastric cancer. Previous research has found marital status to be associated with survival in prostate, cervical, and rectal cancers.
Mark A. Lewis, MD, director of gastrointestinal oncology at Intermountain Healthcare Cancer Center, Utah, told this news organization that the connection between marital status and cancer outcomes has been described previously, including in an even larger analysis using the U.S. Surveillance, Epidemiology, and End Results (SEER) database from 2013. That study found that “unmarried patients are at significantly higher risk of presentation with metastatic cancer, undertreatment, and death resulting from their cancer.”
In this study, the researchers compared marital status and survival rates among 3,647 patients with early-stage gastric cancer, using data from the SEER database. The study only included patients with tumors in the lamina propria, mucosa, and submucosa and excluded those with distant metastasis or distant lymph node metastases, a second cancer, no data on chemotherapy received, or unknown survival time.
Because they were using a nomogram and building a new predictive nomogram that would include marital status, the researchers divided the patient population into a training set of 2,719 patients and a testing set of 928 patients. Using overall survival as the primary endpoint, the analysis included the variables of “age at diagnosis, race, gender, tumor location, histology, grade, stage_T and stage_N, surgery in the primary site, lymph node dissection, chemotherapy, radiation, tumor size, insurance, and marital status,” the authors report.
Among the study population, 53.7% were married, 17.3% were widowed, 14% were single and never married, 7.5% were divorced, 1.1% were separated, and the status of 6.4% was unknown. Age at diagnosis, race, gender, histology, tumor grade, stage T, stage N, surgery type, tumor size, and insurance status were all significantly different between the marital status subgroups.
Married patients had the best prognosis, with an average overall survival of 72 months, compared with an average 60 months in widowed persons, the group with the poorest overall survival. Overall survival was higher in married women (76 months) than in married men (69 months). The same pattern held for women (62 months) and men (52 months) who had been widowed.
“It is worthy to note that survival was significantly better in divorced female patients than in divorced male patients,” the authors report. “Survival was better in female patients than in male patients” across all marital groups.
What long-term relationships reveal
These findings do not mean that simply getting married changes one’s likelihood of survival, however. Rather, a long-term relationship is revealing about other aspects in a person’s life.
“I think it represents more stability in the supportive relationship that you need to really deal with a serious disease like cancer,” Dr. Peek said.
If a patient does not have a long-term partner, their care team can ask other questions to get a sense of what their support network is like, Dr. Peek added. “We want to know, does anybody else live in the house with them? Do they have adequate transportation? Can they make medical appointments? Do they have somebody who can help with the medical issues that are going to come up? Do they have family in the area?”
Cancer treatment requires a multidisciplinary approach, and having someone other than just the patient around to help bring together the different aspects of care from different care teams can make a difference in how the patient fares, Dr. Peek explained. Patients without a strong support system may need closer follow-up and other accommodations, he said.
Providers “may schedule their clinical appointments closer together if they don’t have a support system, or they may be able to reach out and offer transportation assistance and those kinds of things that somebody living alone may need,” Dr. Peek said. Outside resources may be a higher priority for those who lack a support system at home, he added.
Dr. Peek also noted other factors that may play a role in a patient’s survival that these researchers did not have the data to address, such as socioeconomic status, employment, alcohol use, smoking, and infection with Helicobacter pylori, the strongest known risk factor for gastric cancer.
A potentially relevant limitation of the study is that it probably has some selection bias, because the patients who were included probably had the means to have received an earlier diagnosis, said Dr. Lewis, who was not involved in the research.
“Furthermore, just in terms of the group sizes, the baseline characteristics section makes it clear that the preponderance of patients were married, lending that group more statistical weight,” Dr. Lewis said.
“Of the seven attributes in the nomogram, the impact of the marital status seems comparatively meager relative to conventional clinicopathology risk factors like T stage,” he added.
“All in all, I think this study reinforces our awareness that socioeconomic status and social determinants of health play a huge role in cancer outcomes, but it’s not entirely clear that’s modifiable just by getting married,” Dr. Lewis said. “There is a saying in oncology that ‘expensive liquor causes less cancer than cheap liquor,’ which is not negating the carcinogenicity of alcohol but rather identifying different outcomes by socioeconomic status.”
The research was funded by the Natural Science Foundation of Anhui Province. The authors report no relevant financial relationships. Dr. Peek reports no relevant financial relationships. Dr. Lewis reports receiving speaking fees for AstraZeneca/Daiichi Sankyo and having done educational videos for Astellas.
A version of this article first appeared on Medscape.com.
, according to research published in the Journal of Investigative Medicine.
Tumor size remained the largest contributor to overall survival, but marital status was among several other significant factors, such as age, race, gender, treatment style, and pathologic stage, that can provide insight into a patient’s likelihood of overall survival, as it does with several other cancers.
“Married patients had the best prognosis, followed by single patients, and the prognosis of separated patients was the worst,” write Lixiang Zhang and colleagues at the First Affiliated Hospital of Anhui Medical University, Hefei, China. “We speculate that this might be due to the fact that married patients had better financial conditions and emotional encouragement, while separated patients might be more likely to experience financial difficulties [and] emotional loss.”
The results were not necessarily surprising to Richard M. Peek, Jr., MD, director of the division of gastroenterology and a professor of medicine at Vanderbilt University Medical Center, who was not involved in the research.
“Marital status is a reflection of support systems, and a strong support system is a prognosticator for increased compliance with medical appointments and medical therapies,” Dr. Peek told this news organization. “It is something to consider when somebody is being treated for gastric cancer, because if they don’t have a strong support system – and marital status can be a proxy for that – then they may need more intensive follow-up and surveillance, for example, than somebody who does not have that support system.”
Exploring the marital status–cancer survival connection
Gastric cancer is the third leading cause of cancer deaths across the world, causing 780,000 deaths in 2018, the authors note. Yet it’s difficult to accurately predict the prognosis in patients who undergo treatment for early stage gastric cancer. Previous research has found marital status to be associated with survival in prostate, cervical, and rectal cancers.
Mark A. Lewis, MD, director of gastrointestinal oncology at Intermountain Healthcare Cancer Center, Utah, told this news organization that the connection between marital status and cancer outcomes has been described previously, including in an even larger analysis using the U.S. Surveillance, Epidemiology, and End Results (SEER) database from 2013. That study found that “unmarried patients are at significantly higher risk of presentation with metastatic cancer, undertreatment, and death resulting from their cancer.”
In this study, the researchers compared marital status and survival rates among 3,647 patients with early-stage gastric cancer, using data from the SEER database. The study only included patients with tumors in the lamina propria, mucosa, and submucosa and excluded those with distant metastasis or distant lymph node metastases, a second cancer, no data on chemotherapy received, or unknown survival time.
Because they were using a nomogram and building a new predictive nomogram that would include marital status, the researchers divided the patient population into a training set of 2,719 patients and a testing set of 928 patients. Using overall survival as the primary endpoint, the analysis included the variables of “age at diagnosis, race, gender, tumor location, histology, grade, stage_T and stage_N, surgery in the primary site, lymph node dissection, chemotherapy, radiation, tumor size, insurance, and marital status,” the authors report.
Among the study population, 53.7% were married, 17.3% were widowed, 14% were single and never married, 7.5% were divorced, 1.1% were separated, and the status of 6.4% was unknown. Age at diagnosis, race, gender, histology, tumor grade, stage T, stage N, surgery type, tumor size, and insurance status were all significantly different between the marital status subgroups.
Married patients had the best prognosis, with an average overall survival of 72 months, compared with an average 60 months in widowed persons, the group with the poorest overall survival. Overall survival was higher in married women (76 months) than in married men (69 months). The same pattern held for women (62 months) and men (52 months) who had been widowed.
“It is worthy to note that survival was significantly better in divorced female patients than in divorced male patients,” the authors report. “Survival was better in female patients than in male patients” across all marital groups.
What long-term relationships reveal
These findings do not mean that simply getting married changes one’s likelihood of survival, however. Rather, a long-term relationship is revealing about other aspects in a person’s life.
“I think it represents more stability in the supportive relationship that you need to really deal with a serious disease like cancer,” Dr. Peek said.
If a patient does not have a long-term partner, their care team can ask other questions to get a sense of what their support network is like, Dr. Peek added. “We want to know, does anybody else live in the house with them? Do they have adequate transportation? Can they make medical appointments? Do they have somebody who can help with the medical issues that are going to come up? Do they have family in the area?”
Cancer treatment requires a multidisciplinary approach, and having someone other than just the patient around to help bring together the different aspects of care from different care teams can make a difference in how the patient fares, Dr. Peek explained. Patients without a strong support system may need closer follow-up and other accommodations, he said.
Providers “may schedule their clinical appointments closer together if they don’t have a support system, or they may be able to reach out and offer transportation assistance and those kinds of things that somebody living alone may need,” Dr. Peek said. Outside resources may be a higher priority for those who lack a support system at home, he added.
Dr. Peek also noted other factors that may play a role in a patient’s survival that these researchers did not have the data to address, such as socioeconomic status, employment, alcohol use, smoking, and infection with Helicobacter pylori, the strongest known risk factor for gastric cancer.
A potentially relevant limitation of the study is that it probably has some selection bias, because the patients who were included probably had the means to have received an earlier diagnosis, said Dr. Lewis, who was not involved in the research.
“Furthermore, just in terms of the group sizes, the baseline characteristics section makes it clear that the preponderance of patients were married, lending that group more statistical weight,” Dr. Lewis said.
“Of the seven attributes in the nomogram, the impact of the marital status seems comparatively meager relative to conventional clinicopathology risk factors like T stage,” he added.
“All in all, I think this study reinforces our awareness that socioeconomic status and social determinants of health play a huge role in cancer outcomes, but it’s not entirely clear that’s modifiable just by getting married,” Dr. Lewis said. “There is a saying in oncology that ‘expensive liquor causes less cancer than cheap liquor,’ which is not negating the carcinogenicity of alcohol but rather identifying different outcomes by socioeconomic status.”
The research was funded by the Natural Science Foundation of Anhui Province. The authors report no relevant financial relationships. Dr. Peek reports no relevant financial relationships. Dr. Lewis reports receiving speaking fees for AstraZeneca/Daiichi Sankyo and having done educational videos for Astellas.
A version of this article first appeared on Medscape.com.
FROM JOURNAL OF INVESTIGATIVE MEDICINE
Moderate drinking shows more benefit for older vs. younger adults
The health risks and benefits of moderate alcohol consumption are complex and remain a hot topic of debate. The data suggest that small amounts of alcohol may reduce the risk of certain health outcomes over time, but increase the risk of others, wrote Dana Bryazka, MS, a researcher at the Institute for Health Metrics and Evaluation (IHME) at the University of Washington, Seattle, and colleagues, in a paper published in the Lancet.
“The amount of alcohol that minimizes health loss is likely to depend on the distribution of underlying causes of disease burden in a given population. Since this distribution varies widely by geography, age, sex, and time, the level of alcohol consumption associated with the lowest risk to health would depend on the age structure and disease composition of that population,” the researchers wrote.
“We estimate that 1.78 million people worldwide died due to alcohol use in 2020,” Ms. Bryazka said in an interview. “It is important that alcohol consumption guidelines and policies are updated to minimize this harm, particularly in the populations at greatest risk,” she said.
“Existing alcohol consumption guidelines frequently vary by sex, with higher consumption thresholds set for males compared to females. Interestingly, with the currently available data we do not see evidence that risk of alcohol use varies by sex,” she noted.
Methods and results
In the study, the researchers conducted a systematic analysis of burden-weighted dose-response relative risk curves across 22 health outcomes. They used disease rates from the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2020 for the years 1990-2020 for 21 regions, including 204 countries and territories. The data were analyzed by 5-year age group, sex, and year for individuals aged 15-95 years and older. The researchers estimated the theoretical minimum risk exposure level (TMREL) and nondrinker equivalent (NDE), meaning the amount of alcohol at which the health risk equals that of a nondrinker.
One standard drink was defined as 10 g of pure alcohol, equivalent to a small glass of red wine (100 mL or 3.4 fluid ounces) at 13% alcohol by volume, a can or bottle of beer (375 mL or 12 fluid ounces) at 3.5% alcohol by volume, or a shot of whiskey or other spirits (30 mL or 1.0 fluid ounces) at 40% alcohol by volume.
Overall, the TMREL was low regardless of age, sex, time, or geography, and varied from 0 to 1.87 standard drinks per day. However, it was lowest for males aged 15-39 years (0.136 drinks per day) and only slightly higher for females aged 15-39 (0.273), representing 1-2 tenths of a standard drink.
For adults aged 40 and older without any underlying health conditions, drinking a small amount of alcohol may provide some benefits, such as reducing the risk of ischemic heart disease, stroke, and diabetes, the researchers noted. In general, for individuals aged 40-64 years, TMRELs ranged from about half a standard drink per day (0.527 drinks for males and 0.562 standard drinks per day for females) to almost two standard drinks (1.69 standard drinks per day for males and 1.82 for females). For those older than 65 years, the TMRELs represented just over 3 standard drinks per day (3.19 for males and 3.51 for females). For individuals aged 40 years and older, the distribution of disease burden varied by region, but was J-shaped across all regions, the researchers noted.
The researchers also found that those individuals consuming harmful amounts of alcohol were most likely to be aged 15-39 (59.1%) and male (76.9%).
The study findings were limited by several factors including the observational design and lack of data on drinking patterns, such as binge drinking, the researchers noted. Other limitations include the lack of data reflecting patterns of alcohol consumption during the COVID-19 pandemic, and exclusion of outcomes often associated with alcohol use, such as depression, anxiety, and dementia, that might reduce estimates of TMREL and NDE.
However, the results add to the ongoing discussion of the relationship between moderate alcohol consumption and health, the researchers said.
“The findings of this study support the development of tailored guidelines and recommendations on alcohol consumption by age and across regions and highlight that existing low consumption thresholds are too high for younger populations in all regions,” they concluded.
Consider individual factors when counseling patients
The takeaway message for primary care is that alcohol consumed in moderation can reduce the risk of ischemic heart disease, stroke, and diabetes, Ms. Bryazka noted. “However, it also increases the risk of many cancers, intentional and unintentional injuries, and infectious diseases like tuberculosis,” she said. “Of these health outcomes, young people are most likely to experience injuries, and as a result, we find that there are significant health risks associated with consuming alcohol for young people. Among older individuals, the relative proportions of these outcomes vary by geography, and so do the risks associated with consuming alcohol,” she explained.
“Importantly, our analysis was conducted at the population level; when evaluating risk at the individual level, it is also important to consider other factors such as the presence of comorbidities and interactions between alcohol and medications,” she emphasized.
Health and alcohol interaction is complicated
“These findings seemingly contradict a previous [Global Burden of Diseases, Injuries, and Risk Factors Study] estimate published in The Lancet, which emphasized that any alcohol use, regardless of amount, leads to health loss across populations,” wrote Robyn Burton, PhD, and Nick Sheron, MD, both of King’s College, London, in an accompanying comment.
However, the novel methods of weighting relative risk curves according to levels of underlying disease drive the difference in results, along with disaggregated estimates by age, sex, and region, they said.
“Across most geographical regions in this latest analysis, injuries accounted for most alcohol-related harm in younger age groups. This led to a minimum risk level of zero, or very close to zero, among individuals aged 15-39 years across all geographical regions,” which is lower than the level for older adults because of the shift in alcohol-related disease burden towards cardiovascular disease and cancers, they said. “This highlights the need to consider existing rates of disease in a population when trying to determine the total harm posed by alcohol,” the commentators wrote.
In an additional commentary, Tony Rao, MD, a visiting clinical research fellow in psychiatry at King’s College, London, noted that “the elephant in the room with this study is the interpretation of risk based on outcomes for cardiovascular disease – particularly in older people. We know that any purported health benefits from alcohol on the heart and circulation are balanced out by the increased risk from other conditions such as cancer, liver disease, and mental disorders such as depression and dementia,” Dr. Rao said. “If we are to simply draw the conclusion that older people should continue or start drinking small amounts because it protects against diseases affecting heart and circulation – which still remains controversial – other lifestyle changes or the use of drugs targeted at individual cardiovascular disorders seem like a less harmful way of improving health and wellbeing.”
Data can guide clinical practice
No previous study has examined the effect of the theoretical minimum risk of alcohol consumption by geography, age, sex, and time in the context of background disease rates, said Noel Deep, MD, in an interview.
“This study enabled the researchers to quantify the proportion of the population that consumed alcohol in amounts that exceeded the thresholds by location, age, sex, and year, and this can serve as a guide in our efforts to target the control of alcohol intake by individuals,” said Dr. Deep, a general internist in private practice in Antigo, Wisc. He also serves as chief medical officer and a staff physician at Aspirus Langlade Hospital in Antigo.
The first take-home message for clinicians is that even low levels of alcohol consumption can have deleterious effects on the health of patients, and patients should be advised accordingly based on the prevalence of diseases in that community and geographic area, Dr. Deep said. “Secondly, clinicians should also consider the risk of alcohol consumption on all forms of health impacts in a given population rather than just focusing on alcohol-related health conditions,” he added.
“This study provides us with the data to tailor our efforts in educating the clinicians and the public about the relationship between alcohol consumption and disease outcomes based on the observed disease rates in each population,” Dr. Deep explained. “The data should provide another reason for physicians to advise their younger patients, especially the younger males, to avoid or minimize alcohol use,” he said. The data also can help clinicians formulate public health messaging and community education to reduce harmful alcohol use, he added.
As for additional research, Dr. Deep said he would like to see data on the difference in the health-related effects of alcohol in binge-drinkers vs. those who regularly consume alcohol on a daily basis. “It would probably also be helpful to figure out what type of alcohol is being studied and the quality of the alcohol,” he said.
The study was supported by the Bill and Melinda Gates Foundation. Ms. Bryazka and colleagues had no financial conflicts to disclose. Dr. Burton disclosed serving as a consultant to the World Health Organization European Office for the Prevention and Control of Noncommunicable Diseases. Dr. Sheron had no financial conflicts to disclose. Dr. Deep had no financial conflicts to disclose, but serves on the Editorial Advisory Board of Internal Medicine News.
The study was supported by the Bill and Melinda Gates Foundation.
The health risks and benefits of moderate alcohol consumption are complex and remain a hot topic of debate. The data suggest that small amounts of alcohol may reduce the risk of certain health outcomes over time, but increase the risk of others, wrote Dana Bryazka, MS, a researcher at the Institute for Health Metrics and Evaluation (IHME) at the University of Washington, Seattle, and colleagues, in a paper published in the Lancet.
“The amount of alcohol that minimizes health loss is likely to depend on the distribution of underlying causes of disease burden in a given population. Since this distribution varies widely by geography, age, sex, and time, the level of alcohol consumption associated with the lowest risk to health would depend on the age structure and disease composition of that population,” the researchers wrote.
“We estimate that 1.78 million people worldwide died due to alcohol use in 2020,” Ms. Bryazka said in an interview. “It is important that alcohol consumption guidelines and policies are updated to minimize this harm, particularly in the populations at greatest risk,” she said.
“Existing alcohol consumption guidelines frequently vary by sex, with higher consumption thresholds set for males compared to females. Interestingly, with the currently available data we do not see evidence that risk of alcohol use varies by sex,” she noted.
Methods and results
In the study, the researchers conducted a systematic analysis of burden-weighted dose-response relative risk curves across 22 health outcomes. They used disease rates from the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2020 for the years 1990-2020 for 21 regions, including 204 countries and territories. The data were analyzed by 5-year age group, sex, and year for individuals aged 15-95 years and older. The researchers estimated the theoretical minimum risk exposure level (TMREL) and nondrinker equivalent (NDE), meaning the amount of alcohol at which the health risk equals that of a nondrinker.
One standard drink was defined as 10 g of pure alcohol, equivalent to a small glass of red wine (100 mL or 3.4 fluid ounces) at 13% alcohol by volume, a can or bottle of beer (375 mL or 12 fluid ounces) at 3.5% alcohol by volume, or a shot of whiskey or other spirits (30 mL or 1.0 fluid ounces) at 40% alcohol by volume.
Overall, the TMREL was low regardless of age, sex, time, or geography, and varied from 0 to 1.87 standard drinks per day. However, it was lowest for males aged 15-39 years (0.136 drinks per day) and only slightly higher for females aged 15-39 (0.273), representing 1-2 tenths of a standard drink.
For adults aged 40 and older without any underlying health conditions, drinking a small amount of alcohol may provide some benefits, such as reducing the risk of ischemic heart disease, stroke, and diabetes, the researchers noted. In general, for individuals aged 40-64 years, TMRELs ranged from about half a standard drink per day (0.527 drinks for males and 0.562 standard drinks per day for females) to almost two standard drinks (1.69 standard drinks per day for males and 1.82 for females). For those older than 65 years, the TMRELs represented just over 3 standard drinks per day (3.19 for males and 3.51 for females). For individuals aged 40 years and older, the distribution of disease burden varied by region, but was J-shaped across all regions, the researchers noted.
The researchers also found that those individuals consuming harmful amounts of alcohol were most likely to be aged 15-39 (59.1%) and male (76.9%).
The study findings were limited by several factors including the observational design and lack of data on drinking patterns, such as binge drinking, the researchers noted. Other limitations include the lack of data reflecting patterns of alcohol consumption during the COVID-19 pandemic, and exclusion of outcomes often associated with alcohol use, such as depression, anxiety, and dementia, that might reduce estimates of TMREL and NDE.
However, the results add to the ongoing discussion of the relationship between moderate alcohol consumption and health, the researchers said.
“The findings of this study support the development of tailored guidelines and recommendations on alcohol consumption by age and across regions and highlight that existing low consumption thresholds are too high for younger populations in all regions,” they concluded.
Consider individual factors when counseling patients
The takeaway message for primary care is that alcohol consumed in moderation can reduce the risk of ischemic heart disease, stroke, and diabetes, Ms. Bryazka noted. “However, it also increases the risk of many cancers, intentional and unintentional injuries, and infectious diseases like tuberculosis,” she said. “Of these health outcomes, young people are most likely to experience injuries, and as a result, we find that there are significant health risks associated with consuming alcohol for young people. Among older individuals, the relative proportions of these outcomes vary by geography, and so do the risks associated with consuming alcohol,” she explained.
“Importantly, our analysis was conducted at the population level; when evaluating risk at the individual level, it is also important to consider other factors such as the presence of comorbidities and interactions between alcohol and medications,” she emphasized.
Health and alcohol interaction is complicated
“These findings seemingly contradict a previous [Global Burden of Diseases, Injuries, and Risk Factors Study] estimate published in The Lancet, which emphasized that any alcohol use, regardless of amount, leads to health loss across populations,” wrote Robyn Burton, PhD, and Nick Sheron, MD, both of King’s College, London, in an accompanying comment.
However, the novel methods of weighting relative risk curves according to levels of underlying disease drive the difference in results, along with disaggregated estimates by age, sex, and region, they said.
“Across most geographical regions in this latest analysis, injuries accounted for most alcohol-related harm in younger age groups. This led to a minimum risk level of zero, or very close to zero, among individuals aged 15-39 years across all geographical regions,” which is lower than the level for older adults because of the shift in alcohol-related disease burden towards cardiovascular disease and cancers, they said. “This highlights the need to consider existing rates of disease in a population when trying to determine the total harm posed by alcohol,” the commentators wrote.
In an additional commentary, Tony Rao, MD, a visiting clinical research fellow in psychiatry at King’s College, London, noted that “the elephant in the room with this study is the interpretation of risk based on outcomes for cardiovascular disease – particularly in older people. We know that any purported health benefits from alcohol on the heart and circulation are balanced out by the increased risk from other conditions such as cancer, liver disease, and mental disorders such as depression and dementia,” Dr. Rao said. “If we are to simply draw the conclusion that older people should continue or start drinking small amounts because it protects against diseases affecting heart and circulation – which still remains controversial – other lifestyle changes or the use of drugs targeted at individual cardiovascular disorders seem like a less harmful way of improving health and wellbeing.”
Data can guide clinical practice
No previous study has examined the effect of the theoretical minimum risk of alcohol consumption by geography, age, sex, and time in the context of background disease rates, said Noel Deep, MD, in an interview.
“This study enabled the researchers to quantify the proportion of the population that consumed alcohol in amounts that exceeded the thresholds by location, age, sex, and year, and this can serve as a guide in our efforts to target the control of alcohol intake by individuals,” said Dr. Deep, a general internist in private practice in Antigo, Wisc. He also serves as chief medical officer and a staff physician at Aspirus Langlade Hospital in Antigo.
The first take-home message for clinicians is that even low levels of alcohol consumption can have deleterious effects on the health of patients, and patients should be advised accordingly based on the prevalence of diseases in that community and geographic area, Dr. Deep said. “Secondly, clinicians should also consider the risk of alcohol consumption on all forms of health impacts in a given population rather than just focusing on alcohol-related health conditions,” he added.
“This study provides us with the data to tailor our efforts in educating the clinicians and the public about the relationship between alcohol consumption and disease outcomes based on the observed disease rates in each population,” Dr. Deep explained. “The data should provide another reason for physicians to advise their younger patients, especially the younger males, to avoid or minimize alcohol use,” he said. The data also can help clinicians formulate public health messaging and community education to reduce harmful alcohol use, he added.
As for additional research, Dr. Deep said he would like to see data on the difference in the health-related effects of alcohol in binge-drinkers vs. those who regularly consume alcohol on a daily basis. “It would probably also be helpful to figure out what type of alcohol is being studied and the quality of the alcohol,” he said.
The study was supported by the Bill and Melinda Gates Foundation. Ms. Bryazka and colleagues had no financial conflicts to disclose. Dr. Burton disclosed serving as a consultant to the World Health Organization European Office for the Prevention and Control of Noncommunicable Diseases. Dr. Sheron had no financial conflicts to disclose. Dr. Deep had no financial conflicts to disclose, but serves on the Editorial Advisory Board of Internal Medicine News.
The study was supported by the Bill and Melinda Gates Foundation.
The health risks and benefits of moderate alcohol consumption are complex and remain a hot topic of debate. The data suggest that small amounts of alcohol may reduce the risk of certain health outcomes over time, but increase the risk of others, wrote Dana Bryazka, MS, a researcher at the Institute for Health Metrics and Evaluation (IHME) at the University of Washington, Seattle, and colleagues, in a paper published in the Lancet.
“The amount of alcohol that minimizes health loss is likely to depend on the distribution of underlying causes of disease burden in a given population. Since this distribution varies widely by geography, age, sex, and time, the level of alcohol consumption associated with the lowest risk to health would depend on the age structure and disease composition of that population,” the researchers wrote.
“We estimate that 1.78 million people worldwide died due to alcohol use in 2020,” Ms. Bryazka said in an interview. “It is important that alcohol consumption guidelines and policies are updated to minimize this harm, particularly in the populations at greatest risk,” she said.
“Existing alcohol consumption guidelines frequently vary by sex, with higher consumption thresholds set for males compared to females. Interestingly, with the currently available data we do not see evidence that risk of alcohol use varies by sex,” she noted.
Methods and results
In the study, the researchers conducted a systematic analysis of burden-weighted dose-response relative risk curves across 22 health outcomes. They used disease rates from the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2020 for the years 1990-2020 for 21 regions, including 204 countries and territories. The data were analyzed by 5-year age group, sex, and year for individuals aged 15-95 years and older. The researchers estimated the theoretical minimum risk exposure level (TMREL) and nondrinker equivalent (NDE), meaning the amount of alcohol at which the health risk equals that of a nondrinker.
One standard drink was defined as 10 g of pure alcohol, equivalent to a small glass of red wine (100 mL or 3.4 fluid ounces) at 13% alcohol by volume, a can or bottle of beer (375 mL or 12 fluid ounces) at 3.5% alcohol by volume, or a shot of whiskey or other spirits (30 mL or 1.0 fluid ounces) at 40% alcohol by volume.
Overall, the TMREL was low regardless of age, sex, time, or geography, and varied from 0 to 1.87 standard drinks per day. However, it was lowest for males aged 15-39 years (0.136 drinks per day) and only slightly higher for females aged 15-39 (0.273), representing 1-2 tenths of a standard drink.
For adults aged 40 and older without any underlying health conditions, drinking a small amount of alcohol may provide some benefits, such as reducing the risk of ischemic heart disease, stroke, and diabetes, the researchers noted. In general, for individuals aged 40-64 years, TMRELs ranged from about half a standard drink per day (0.527 drinks for males and 0.562 standard drinks per day for females) to almost two standard drinks (1.69 standard drinks per day for males and 1.82 for females). For those older than 65 years, the TMRELs represented just over 3 standard drinks per day (3.19 for males and 3.51 for females). For individuals aged 40 years and older, the distribution of disease burden varied by region, but was J-shaped across all regions, the researchers noted.
The researchers also found that those individuals consuming harmful amounts of alcohol were most likely to be aged 15-39 (59.1%) and male (76.9%).
The study findings were limited by several factors including the observational design and lack of data on drinking patterns, such as binge drinking, the researchers noted. Other limitations include the lack of data reflecting patterns of alcohol consumption during the COVID-19 pandemic, and exclusion of outcomes often associated with alcohol use, such as depression, anxiety, and dementia, that might reduce estimates of TMREL and NDE.
However, the results add to the ongoing discussion of the relationship between moderate alcohol consumption and health, the researchers said.
“The findings of this study support the development of tailored guidelines and recommendations on alcohol consumption by age and across regions and highlight that existing low consumption thresholds are too high for younger populations in all regions,” they concluded.
Consider individual factors when counseling patients
The takeaway message for primary care is that alcohol consumed in moderation can reduce the risk of ischemic heart disease, stroke, and diabetes, Ms. Bryazka noted. “However, it also increases the risk of many cancers, intentional and unintentional injuries, and infectious diseases like tuberculosis,” she said. “Of these health outcomes, young people are most likely to experience injuries, and as a result, we find that there are significant health risks associated with consuming alcohol for young people. Among older individuals, the relative proportions of these outcomes vary by geography, and so do the risks associated with consuming alcohol,” she explained.
“Importantly, our analysis was conducted at the population level; when evaluating risk at the individual level, it is also important to consider other factors such as the presence of comorbidities and interactions between alcohol and medications,” she emphasized.
Health and alcohol interaction is complicated
“These findings seemingly contradict a previous [Global Burden of Diseases, Injuries, and Risk Factors Study] estimate published in The Lancet, which emphasized that any alcohol use, regardless of amount, leads to health loss across populations,” wrote Robyn Burton, PhD, and Nick Sheron, MD, both of King’s College, London, in an accompanying comment.
However, the novel methods of weighting relative risk curves according to levels of underlying disease drive the difference in results, along with disaggregated estimates by age, sex, and region, they said.
“Across most geographical regions in this latest analysis, injuries accounted for most alcohol-related harm in younger age groups. This led to a minimum risk level of zero, or very close to zero, among individuals aged 15-39 years across all geographical regions,” which is lower than the level for older adults because of the shift in alcohol-related disease burden towards cardiovascular disease and cancers, they said. “This highlights the need to consider existing rates of disease in a population when trying to determine the total harm posed by alcohol,” the commentators wrote.
In an additional commentary, Tony Rao, MD, a visiting clinical research fellow in psychiatry at King’s College, London, noted that “the elephant in the room with this study is the interpretation of risk based on outcomes for cardiovascular disease – particularly in older people. We know that any purported health benefits from alcohol on the heart and circulation are balanced out by the increased risk from other conditions such as cancer, liver disease, and mental disorders such as depression and dementia,” Dr. Rao said. “If we are to simply draw the conclusion that older people should continue or start drinking small amounts because it protects against diseases affecting heart and circulation – which still remains controversial – other lifestyle changes or the use of drugs targeted at individual cardiovascular disorders seem like a less harmful way of improving health and wellbeing.”
Data can guide clinical practice
No previous study has examined the effect of the theoretical minimum risk of alcohol consumption by geography, age, sex, and time in the context of background disease rates, said Noel Deep, MD, in an interview.
“This study enabled the researchers to quantify the proportion of the population that consumed alcohol in amounts that exceeded the thresholds by location, age, sex, and year, and this can serve as a guide in our efforts to target the control of alcohol intake by individuals,” said Dr. Deep, a general internist in private practice in Antigo, Wisc. He also serves as chief medical officer and a staff physician at Aspirus Langlade Hospital in Antigo.
The first take-home message for clinicians is that even low levels of alcohol consumption can have deleterious effects on the health of patients, and patients should be advised accordingly based on the prevalence of diseases in that community and geographic area, Dr. Deep said. “Secondly, clinicians should also consider the risk of alcohol consumption on all forms of health impacts in a given population rather than just focusing on alcohol-related health conditions,” he added.
“This study provides us with the data to tailor our efforts in educating the clinicians and the public about the relationship between alcohol consumption and disease outcomes based on the observed disease rates in each population,” Dr. Deep explained. “The data should provide another reason for physicians to advise their younger patients, especially the younger males, to avoid or minimize alcohol use,” he said. The data also can help clinicians formulate public health messaging and community education to reduce harmful alcohol use, he added.
As for additional research, Dr. Deep said he would like to see data on the difference in the health-related effects of alcohol in binge-drinkers vs. those who regularly consume alcohol on a daily basis. “It would probably also be helpful to figure out what type of alcohol is being studied and the quality of the alcohol,” he said.
The study was supported by the Bill and Melinda Gates Foundation. Ms. Bryazka and colleagues had no financial conflicts to disclose. Dr. Burton disclosed serving as a consultant to the World Health Organization European Office for the Prevention and Control of Noncommunicable Diseases. Dr. Sheron had no financial conflicts to disclose. Dr. Deep had no financial conflicts to disclose, but serves on the Editorial Advisory Board of Internal Medicine News.
The study was supported by the Bill and Melinda Gates Foundation.
FROM THE LANCET
Amazon involved with new cancer vaccine clinical trial
The trial is aimed at finding “personalized vaccines” to treat breast cancer and melanoma. The phase 1 trial is recruiting 20 people over the age of 18 to study the safety of the vaccines, according to CNBC.
The Fred Hutchinson Cancer Research Center and University of Washington Cancer Consortium are listed as the researchers of the clinical trial, and Amazon is listed as a collaborator, according to a filing on the ClinicalTrials.gov database.
“Amazon is contributing scientific and machine learning expertise to a partnership with Fred Hutch to explore the development of a personalized treatment for certain forms of cancer,” an Amazon spokesperson told CNBC.
“It’s very early, but Fred Hutch recently received permission from the U.S. Food and Drug Administration to proceed with a phase 1 clinical trial, and it’s unclear whether it will be successful,” the spokesperson said. “This will be a long, multiyear process – should it progress, we would be open to working with other organizations in health care and life sciences that might also be interested in similar efforts.”
In recent years, Amazon has grown its presence in the health care industry, CNBC reported. The company launched an online pharmacy in 2020, developed a telehealth service called Amazon Care, and released its own COVID-19 test during the pandemic.
A research and development group inside Amazon, known as Grand Challenge, oversaw the company’s early cancer vaccine effort, according to Business Insider. It’s now under the purview of a cancer research team that reports to Robert Williams, the company’s vice president of devices.
The study was first posted on ClinicalTrials.gov in October 2021 and began recruiting patients on June 9, according to the filing. The phase 1 trial is expected to run through November 2023.
The phase 1 trial will study the safety of personalized vaccines to treat patients with late-stage melanoma or hormone receptor-positive HER2-negative breast cancer which has either spread to other parts of the body or doesn’t respond to treatment.
More information about the study can be found on ClinicalTrials.gov under the identifier NCT05098210.
A version of this article first appeared on WebMD.com.
The trial is aimed at finding “personalized vaccines” to treat breast cancer and melanoma. The phase 1 trial is recruiting 20 people over the age of 18 to study the safety of the vaccines, according to CNBC.
The Fred Hutchinson Cancer Research Center and University of Washington Cancer Consortium are listed as the researchers of the clinical trial, and Amazon is listed as a collaborator, according to a filing on the ClinicalTrials.gov database.
“Amazon is contributing scientific and machine learning expertise to a partnership with Fred Hutch to explore the development of a personalized treatment for certain forms of cancer,” an Amazon spokesperson told CNBC.
“It’s very early, but Fred Hutch recently received permission from the U.S. Food and Drug Administration to proceed with a phase 1 clinical trial, and it’s unclear whether it will be successful,” the spokesperson said. “This will be a long, multiyear process – should it progress, we would be open to working with other organizations in health care and life sciences that might also be interested in similar efforts.”
In recent years, Amazon has grown its presence in the health care industry, CNBC reported. The company launched an online pharmacy in 2020, developed a telehealth service called Amazon Care, and released its own COVID-19 test during the pandemic.
A research and development group inside Amazon, known as Grand Challenge, oversaw the company’s early cancer vaccine effort, according to Business Insider. It’s now under the purview of a cancer research team that reports to Robert Williams, the company’s vice president of devices.
The study was first posted on ClinicalTrials.gov in October 2021 and began recruiting patients on June 9, according to the filing. The phase 1 trial is expected to run through November 2023.
The phase 1 trial will study the safety of personalized vaccines to treat patients with late-stage melanoma or hormone receptor-positive HER2-negative breast cancer which has either spread to other parts of the body or doesn’t respond to treatment.
More information about the study can be found on ClinicalTrials.gov under the identifier NCT05098210.
A version of this article first appeared on WebMD.com.
The trial is aimed at finding “personalized vaccines” to treat breast cancer and melanoma. The phase 1 trial is recruiting 20 people over the age of 18 to study the safety of the vaccines, according to CNBC.
The Fred Hutchinson Cancer Research Center and University of Washington Cancer Consortium are listed as the researchers of the clinical trial, and Amazon is listed as a collaborator, according to a filing on the ClinicalTrials.gov database.
“Amazon is contributing scientific and machine learning expertise to a partnership with Fred Hutch to explore the development of a personalized treatment for certain forms of cancer,” an Amazon spokesperson told CNBC.
“It’s very early, but Fred Hutch recently received permission from the U.S. Food and Drug Administration to proceed with a phase 1 clinical trial, and it’s unclear whether it will be successful,” the spokesperson said. “This will be a long, multiyear process – should it progress, we would be open to working with other organizations in health care and life sciences that might also be interested in similar efforts.”
In recent years, Amazon has grown its presence in the health care industry, CNBC reported. The company launched an online pharmacy in 2020, developed a telehealth service called Amazon Care, and released its own COVID-19 test during the pandemic.
A research and development group inside Amazon, known as Grand Challenge, oversaw the company’s early cancer vaccine effort, according to Business Insider. It’s now under the purview of a cancer research team that reports to Robert Williams, the company’s vice president of devices.
The study was first posted on ClinicalTrials.gov in October 2021 and began recruiting patients on June 9, according to the filing. The phase 1 trial is expected to run through November 2023.
The phase 1 trial will study the safety of personalized vaccines to treat patients with late-stage melanoma or hormone receptor-positive HER2-negative breast cancer which has either spread to other parts of the body or doesn’t respond to treatment.
More information about the study can be found on ClinicalTrials.gov under the identifier NCT05098210.
A version of this article first appeared on WebMD.com.
Surgical Treatment of Nonmelanoma Skin Cancer in Older Adult Veterans
Skin cancer is the most diagnosed cancer in the United States. Nonmelanoma skin cancers (NMSC), which include basal cell carcinoma and squamous cell carcinoma, are usually cured with removal.1 The incidence of NMSC increases with age and is commonly found in nursing homes and geriatric units. These cancers are not usually metastatic or fatal but can cause local destruction and disfigurement if neglected.2 The current standard of care is to treat diagnosed NMSC; however, the dermatology and geriatric care literature have questioned the logic of treating asymptomatic skin cancers that will not affect a patient’s life expectancy.2-4
Forty-seven percent of the current living veteran population is aged ≥ 65 years.5 Older adult patients are frequently referred to the US Department of Veterans Affairs (VA) surgical service for the treatment of NMSC. The veteran population includes a higher percentage of individuals at an elevated risk of skin cancers (older, White, and male) compared with the general population.6 World War II veterans deployed in regions closer to the equator have been found to have an elevated risk of melanoma and nonmelanoma skin carcinomas.7 A retrospective study of Vietnam veterans exposed to Agent Orange (2,3,7,8-tetrachlorodibenzodioxin) found a significantly higher risk of invasive NMSC in Fitzpatrick skin types I-IV compared with an age-matched subset of the general population.8 Younger veterans who were deployed in Afghanistan and Iraq for Operation Enduring Freedom/Operation Iraqi Freedom worked at more equatorial latitudes than the rest of the US population and may be at increased risk of NMSC. Inadequate sunscreen access, immediate safety concerns, outdoor recreational activities, harsh weather, and insufficient emphasis on sun protection have created a multifactorial challenge for the military population. Riemenschneider and colleagues recommended targeted screening for at-risk veteran patients and prioritizing annual skin cancer screenings during medical mission physical examinations for active military.7
The plastic surgery service regularly receives consults from dermatology, general surgery, and primary care to remove skin cancers on the face, scalp, hands, and forearms. Skin cancer treatment can create serious hardships for older adult patients and their families with multiple appointments for the consult, procedure, and follow-up. Patients are often told to hold their anticoagulant medications when the surgery will be performed on a highly vascular region, such as the scalp or face. This can create wide swings in their laboratory test values and result in life-threatening complications from either bleeding or clotting. The appropriateness of offering surgery to patients with serious comorbidities and a limited life expectancy has been questioned.2-4 The purpose of this study was to measure the morbidity and unrelated 5-year mortality for patients with skin cancer referred to the plastic surgery service to help patients and families make a more informed treatment decision, particularly when the patients are aged > 80 years and have significant life-threatening comorbidities.
Methods
The University of Florida and Malcom Randall VA Medical Center Institutional review board in Gainesville, approved a retrospective review of all consults completed by the plastic surgery service for the treatment of NMSC performed from July 1, 2011 to June 30, 2015. Data collected included age and common life-limiting comorbidities at the time of referral. Morbidities were found on the electronic health record, including coronary artery disease (CAD), congestive heart failure (CHF), cerebral vascular disease (CVD), peripheral vascular disease, dementia, chronic kidney disease (CKD), chronic obstructive pulmonary disease (COPD), tobacco use, diabetes mellitus (DM), liver disease, alcohol use, and obstructive sleep apnea.
Treatment, complications, and 5-year mortality were recorded. A χ2 analysis with P value < .05 was used to determine statistical significance between individual risk factors and 5-year mortality. The relative risk of 5-year mortality was calculated by combining advanced age (aged > 80 years) with the individual comorbidities.
Results
Over 4 years, 800 consults for NMSC were completed by the plastic surgery service. Treatment decisions included 210 excisions (with or without reconstruction) in the operating room, 402 excisions (with or without reconstruction) under local anesthesia in clinic, 55 Mohs surgical dermatology referrals, 21 other service or hospital referrals, and 112 patient who were observed, declined intervention, or died prior to intervention. Five-year mortality was 28.6%. No patients died of NMSC. The median age at consult submission for patients deceased 5 years later was 78 years. Complication rate was 5% and included wound infection, dehiscence, bleeding, or graft loss. Two patients, both deceased within 5 years, had unplanned admissions due to bleeding from either a skin graft donor site or recipient bleeding. Aged ≥ 80 years, CAD, CHF, CVD, peripheral vascular disease, dementia, CKD, COPD, and DM were all found individually to be statistically significant predictors of 5-year mortality (Table 1). Combining aged ≥ 80 years plus CAD, CHF, or dementia all increased the 5-year mortality by a relative risk of > 3 (Table 2).
Discussion
The standard of care is to treat NMSC. Most NMSCs are treated surgically without consideration of patient age or life expectancy.2,4,9,10 A prospective cohort study involving a university-based private practice and a VA medical center in San Francisco found a 22.6% overall 5-year mortality and a 43.3% mortality in the group defined as limited life expectancy (LLE) based on age (≥ 85 years) and medical comorbidities. None died due to the NMSC. Leading cause of death was cardiac, cerebrovascular, and respiratory disease, lung and prostate cancer, and Alzheimer disease. The authors suggested the LLE group may be exposed to wound complications without benefiting from the treatment.4
Another study of 440 patients receiving excision for biopsy-proven facial NMSC at the Roudebush VA Medical Center in Indianapolis, Indiana, found no residual carcinoma in 35.3% of excisions, and in patients aged > 90 years, more than half of the excisions had no residual carcinoma. More than half of the patients aged > 90 years died within 1 year, not as a result of the NMSC. The authors argued for watchful waiting in select patients to maximize comfort and outcomes.10
NMSCs are often asymptomatic and not immediately life threatening. Although NMSCs tend to have a favorable prognosis, studies have found that NMSC may be a marker for other poor health outcomes. A significant increased risk for all-cause mortality was found for patients with a history of SCC, which may be attributed to immune status.11 The aging veteran population has more complex health care needs to be considered when developing surgical treatment plans. These medical problems may limit their life expectancy much sooner than the skin cancer will become symptomatic. We found that individuals aged ≥ 80 years who had CAD, CHF, or dementia had a relative risk of 3 or higher for 5-year mortality. The leading cause of death in the United States in years 2011 to 2015 was heart disease. Alzheimer disease was the sixth leading cause of death in those same years.12-14
Skin cancer excisions do not typically require general anesthesia, deep sedation, or large fluid shifts; however, studies have found that when frail patients undergo low-risk procedures, they tend to have a higher mortality rate than their healthier counterparts.15 Frailty is a concept that identifies patients who are at increased risk of dying in 6 to 60 months due to a decline in their physical reserve. Frail patients have increased rates of perioperative mortality and complications. Various tools have been used to assess the components of physical performance, speed, mobility, nutrition status, mental health, and cognition.16 Frailty screening has been initiated in several VA hospitals, including our own in Gainesville, Florida, with the goal of decreasing postoperative morbidity and mortality in older adult patients.17 The patients are given a 1-page screening assessment that asks about their living situation, medical conditions, nutrition status, cognition, and activities of daily living. The results can trigger the clinician to rethink the surgical plan and mobilize more resources to optimize the patient’s health. This study period precedes the initiative at our institution.
The plastic surgery service’s routine practice is to excise skin cancers in the operating room if sedation or general anesthesia will be needed (Figure 1A), for optimal control of bleeding (Figure 1B) in a patient who cannot safely stop blood thinners, or for excision of a highly vascularized area such as the scalp. Surgery is offered in an office-based setting if the area can be closed primarily, left open to close secondarily, or closed with a small skin graft under local anesthesia only (Figure 2). We prefer treating frail patients in the minor procedure clinic, when possible, to avoid the risks of sedation and the additional preoperative visits and transportation requirements. NMSC with unclear margins (Figure 3A) or in cosmetically sensitive areas where tissue needs to be preserved (Figure 3B) are referred to the Mohs dermatologist. The skin cancers in this study were most frequently found on the face, scalp, hands, and forearms based on referral patterns.
Other treatment options for NMSC include curettage and electrodessication, cryotherapy, and radiation; however, ours is a surgical service and patients are typically referred to us by primary care or dermatology when those are not reasonable or desirable options.18 Published complication rates of patients having skin cancer surgery without age restriction have a rate of 3% to 6%, which is consistent with our study of 5%.19-21 Two bleeding complications that needed to be admitted did not require more than a bedside procedure and neither required transfusions. One patient had been instructed to continue taking coumadin during the perioperative office-based procedure due to a recent carotid stent placement in the setting of a rapidly growing basal cell on an easily accessible location.
The most noted comorbidity in patients with wound complications was found to be DM; however, this was not found to be a statistically significant risk factor for wound complications (P = .10). We do not have a set rule for advising for or against NMSC surgery. We do counsel frail patients and their families that not all cancer is immediately life threatening and will work with them to do whatever makes the most sense to achieve their goals, occasionally accepting positive margins in order to debulk a symptomatic growth. The objective of this paper is to contribute to the discussion of performing invasive procedures on older adult veterans with life-limiting comorbidities. Patients and their families will have different thresholds for what they feel needs intervention, especially if other medical problems are consuming much of their time. We also have the community care referral option for patients whose treatment decisions are being dictated by travel hardships.
Strengths and Limitations
A strength of this study is that the data were obtained from a closed system. Patients tend to stay long-term within the VA and their health record is accessible throughout the country as long as they are seen at a VA facility. Complications, therefore, return to the treating service or primary care, who would route the patient back to the surgeon.
One limitation of the study is that this is a retrospective review from 2011. The authors are limited to data that are recorded in the patient record. Multiple health care professionals saw the patients and notes lack consistency in detail. Size of the lesions were not consistently recorded and did not get logged into our database for that reason.
Conclusions
Treatment of NMSC in older adult patients has a low morbidity but needs to be balanced against a patient and family’s goals when the patient presents with life-limiting comorbidities. An elevated 5-year mortality in patients aged > 80 years with serious unrelated medical conditions is intuitive, but this study may help put treatment plans into perspective for families and health care professionals who want to provide an indicated service while maximizing patient quality of life.
Acknowledgments
This manuscript is the result of work supported with resources and the use of facilities at the North Florida/South Georgia Veterans Health System, Gainesville, Florida.
1. American Cancer Society. Cancer Facts & Figures 2021. Accessed May 26, 2022. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2021/cancer-facts-and-figures-2021.pdf
2. Albert A, Knoll MA, Conti JA, Zbar RIS. Non-melanoma skin cancers in the older patient. Curr Oncol Rep. 2019;21(9):79. Published 2019 Jul 29. doi:10.1007/s11912-019-0828-9
3. Linos E, Chren MM, Stijacic Cenzer I, Covinsky KE. Skin cancer in U.S. elderly adults: does life expectancy play a role in treatment decisions? J Am Geriatr Soc. 2016;64(8):1610-1615. doi:10.1111/jgs.14202
4. Linos E, Parvataneni R, Stuart SE, Boscardin WJ, Landefeld CS, Chren MM. Treatment of nonfatal conditions at the end of life: nonmelanoma skin cancer. JAMA Intern Med. 2013;173(11):1006-1012. doi:10.1001/jamainternmed.2013.639
5. O’Malley KA, Vinson L, Kaiser AP, Sager Z, Hinrichs K. Mental health and aging veterans: how the Veterans Health Administration meets the needs of aging veterans. Public Policy Aging Rep. 2020;30(1):19-23. doi:10.1093/ppar/prz027
6. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. Profile of veterans: 2017. Accessed May 26, 2022. https://www.va.gov/vetdata/docs/SpecialReports/Profile_of_Veterans_2017.pdf 7. Riemenschneider K, Liu J, Powers JG. Skin cancer in the military: a systematic review of melanoma and nonmelanoma skin cancer incidence, prevention, and screening among active duty and veteran personnel. J Am Acad Dermatol. 2018;78(6):1185-1192. doi:10.1016/j.jaad.2017.11.062
8. Clemens MW, Kochuba AL, Carter ME, Han K, Liu J, Evans K. Association between Agent Orange exposure and nonmelanotic invasive skin cancer: a pilot study. Plast Reconstr Surg. 2014;133(2):432-437. doi:10.1097/01.prs.0000436859.40151.cf
9. Cameron MC, Lee E, Hibler BP, et al. Basal cell carcinoma: epidemiology; pathophysiology; clinical and histological subtypes; and disease associations. J Am Acad Dermatol. 2019;80(2):303-317. doi:10.1016/j.jaad.2018.03.060
10. Chauhan R, Munger BN, Chu MW, et al. Age at diagnosis as a relative contraindication for intervention in facial nonmelanoma skin cancer. JAMA Surg. 2018;153(4):390-392. doi:10.1001/jamasurg.2017.5073
11. Barton V, Armeson K, Hampras S, et al. Nonmelanoma skin cancer and risk of all-cause and cancer-related mortality: a systematic review. Arch Dermatol Res. 2017;309(4):243-251. doi:10.1007/s00403-017-1724-5
12. Kochanek KD, Murphy SL, Xu JQ, Arias E. Mortality in the United States, 2013. NCHS Data Brief 178. Accessed May 26, 2022. https://www.cdc.gov/nchs/products/databriefs/db178.htm
13. Xu JQ, Kochanek KD, Murphy SL, Arias E. Mortality in the United States, 2012. NCHS Data Brief 168. Accessed May 26, 2022. https://www.cdc.gov/nchs/products/databriefs/db168.htm
14. Xu JQ, Murphy SL, Kochanek KD, Arias E. Mortality in the United States, 2015. NCHS Data Brief 267. Accessed May 26, 2022. https://www.cdc.gov/nchs/products/databriefs/db267.htm
15. Varley PR , Borrebach JD, Arya S, et al. Clinical utility of the risk analysis index as a prospective frailty screening tool within a multi-practice, multi-hospital integrated healthcare system. Ann Surg. 2021;274(6):e1230-e1237. doi:10.1097/SLA.0000000000003808
16. Hall DE, Arya S , Schmid KK, et al. Development and initial validation of the risk analysis index for measuring frailty in surgical populations. JAMA Surg. 2017;152(2):175-182. doi:10.1001/jamasurg.2016.4202
17. US Department of Veterans Affairs, Health Services Research & Development. Improving healthcare for aging veterans. Updated August 30, 2017. Accessed May 26, 2022. https://www.hsrd.research.va.gov/news/feature/aging0917.cfm
18. Leus AJG, Frie M, Haisma MS, et al. Treatment of keratinocyte carcinoma in elderly patients – a review of the current literature. J Eur Acad Dermatol Venereol. 2020;34(9):1932-1943. doi:10.1111/jdv.16268
19. Amici JM, Rogues AM, Lasheras A, et al. A prospective study of the incidence of complications associated with dermatological surgery. Br J Dermatol. 2005;153(5):967-971. doi:10.1111/j.1365-2133.2005.06861.x
20. Arguello-Guerra L, Vargas-Chandomid E, Díaz-González JM, Méndez-Flores S, Ruelas-Villavicencio A, Domínguez-Cherit J. Incidence of complications in dermatological surgery of melanoma and non-melanoma skin cancer in patients with multiple comorbidity and/or antiplatelet-anticoagulants. Five-year experience in our hospital. Cir Cir. 2019;86(1):15-23. doi:10.24875/CIRUE.M18000003
21. Keith DJ, de Berker DA, Bray AP, Cheung ST, Brain A, Mohd Mustapa MF. British Association of Dermatologists’ national audit on nonmelanoma skin cancer excision, 2014. Clin Exp Dermatol. 2017;42(1):46-53. doi:10.1111/ced.12990
Skin cancer is the most diagnosed cancer in the United States. Nonmelanoma skin cancers (NMSC), which include basal cell carcinoma and squamous cell carcinoma, are usually cured with removal.1 The incidence of NMSC increases with age and is commonly found in nursing homes and geriatric units. These cancers are not usually metastatic or fatal but can cause local destruction and disfigurement if neglected.2 The current standard of care is to treat diagnosed NMSC; however, the dermatology and geriatric care literature have questioned the logic of treating asymptomatic skin cancers that will not affect a patient’s life expectancy.2-4
Forty-seven percent of the current living veteran population is aged ≥ 65 years.5 Older adult patients are frequently referred to the US Department of Veterans Affairs (VA) surgical service for the treatment of NMSC. The veteran population includes a higher percentage of individuals at an elevated risk of skin cancers (older, White, and male) compared with the general population.6 World War II veterans deployed in regions closer to the equator have been found to have an elevated risk of melanoma and nonmelanoma skin carcinomas.7 A retrospective study of Vietnam veterans exposed to Agent Orange (2,3,7,8-tetrachlorodibenzodioxin) found a significantly higher risk of invasive NMSC in Fitzpatrick skin types I-IV compared with an age-matched subset of the general population.8 Younger veterans who were deployed in Afghanistan and Iraq for Operation Enduring Freedom/Operation Iraqi Freedom worked at more equatorial latitudes than the rest of the US population and may be at increased risk of NMSC. Inadequate sunscreen access, immediate safety concerns, outdoor recreational activities, harsh weather, and insufficient emphasis on sun protection have created a multifactorial challenge for the military population. Riemenschneider and colleagues recommended targeted screening for at-risk veteran patients and prioritizing annual skin cancer screenings during medical mission physical examinations for active military.7
The plastic surgery service regularly receives consults from dermatology, general surgery, and primary care to remove skin cancers on the face, scalp, hands, and forearms. Skin cancer treatment can create serious hardships for older adult patients and their families with multiple appointments for the consult, procedure, and follow-up. Patients are often told to hold their anticoagulant medications when the surgery will be performed on a highly vascular region, such as the scalp or face. This can create wide swings in their laboratory test values and result in life-threatening complications from either bleeding or clotting. The appropriateness of offering surgery to patients with serious comorbidities and a limited life expectancy has been questioned.2-4 The purpose of this study was to measure the morbidity and unrelated 5-year mortality for patients with skin cancer referred to the plastic surgery service to help patients and families make a more informed treatment decision, particularly when the patients are aged > 80 years and have significant life-threatening comorbidities.
Methods
The University of Florida and Malcom Randall VA Medical Center Institutional review board in Gainesville, approved a retrospective review of all consults completed by the plastic surgery service for the treatment of NMSC performed from July 1, 2011 to June 30, 2015. Data collected included age and common life-limiting comorbidities at the time of referral. Morbidities were found on the electronic health record, including coronary artery disease (CAD), congestive heart failure (CHF), cerebral vascular disease (CVD), peripheral vascular disease, dementia, chronic kidney disease (CKD), chronic obstructive pulmonary disease (COPD), tobacco use, diabetes mellitus (DM), liver disease, alcohol use, and obstructive sleep apnea.
Treatment, complications, and 5-year mortality were recorded. A χ2 analysis with P value < .05 was used to determine statistical significance between individual risk factors and 5-year mortality. The relative risk of 5-year mortality was calculated by combining advanced age (aged > 80 years) with the individual comorbidities.
Results
Over 4 years, 800 consults for NMSC were completed by the plastic surgery service. Treatment decisions included 210 excisions (with or without reconstruction) in the operating room, 402 excisions (with or without reconstruction) under local anesthesia in clinic, 55 Mohs surgical dermatology referrals, 21 other service or hospital referrals, and 112 patient who were observed, declined intervention, or died prior to intervention. Five-year mortality was 28.6%. No patients died of NMSC. The median age at consult submission for patients deceased 5 years later was 78 years. Complication rate was 5% and included wound infection, dehiscence, bleeding, or graft loss. Two patients, both deceased within 5 years, had unplanned admissions due to bleeding from either a skin graft donor site or recipient bleeding. Aged ≥ 80 years, CAD, CHF, CVD, peripheral vascular disease, dementia, CKD, COPD, and DM were all found individually to be statistically significant predictors of 5-year mortality (Table 1). Combining aged ≥ 80 years plus CAD, CHF, or dementia all increased the 5-year mortality by a relative risk of > 3 (Table 2).
Discussion
The standard of care is to treat NMSC. Most NMSCs are treated surgically without consideration of patient age or life expectancy.2,4,9,10 A prospective cohort study involving a university-based private practice and a VA medical center in San Francisco found a 22.6% overall 5-year mortality and a 43.3% mortality in the group defined as limited life expectancy (LLE) based on age (≥ 85 years) and medical comorbidities. None died due to the NMSC. Leading cause of death was cardiac, cerebrovascular, and respiratory disease, lung and prostate cancer, and Alzheimer disease. The authors suggested the LLE group may be exposed to wound complications without benefiting from the treatment.4
Another study of 440 patients receiving excision for biopsy-proven facial NMSC at the Roudebush VA Medical Center in Indianapolis, Indiana, found no residual carcinoma in 35.3% of excisions, and in patients aged > 90 years, more than half of the excisions had no residual carcinoma. More than half of the patients aged > 90 years died within 1 year, not as a result of the NMSC. The authors argued for watchful waiting in select patients to maximize comfort and outcomes.10
NMSCs are often asymptomatic and not immediately life threatening. Although NMSCs tend to have a favorable prognosis, studies have found that NMSC may be a marker for other poor health outcomes. A significant increased risk for all-cause mortality was found for patients with a history of SCC, which may be attributed to immune status.11 The aging veteran population has more complex health care needs to be considered when developing surgical treatment plans. These medical problems may limit their life expectancy much sooner than the skin cancer will become symptomatic. We found that individuals aged ≥ 80 years who had CAD, CHF, or dementia had a relative risk of 3 or higher for 5-year mortality. The leading cause of death in the United States in years 2011 to 2015 was heart disease. Alzheimer disease was the sixth leading cause of death in those same years.12-14
Skin cancer excisions do not typically require general anesthesia, deep sedation, or large fluid shifts; however, studies have found that when frail patients undergo low-risk procedures, they tend to have a higher mortality rate than their healthier counterparts.15 Frailty is a concept that identifies patients who are at increased risk of dying in 6 to 60 months due to a decline in their physical reserve. Frail patients have increased rates of perioperative mortality and complications. Various tools have been used to assess the components of physical performance, speed, mobility, nutrition status, mental health, and cognition.16 Frailty screening has been initiated in several VA hospitals, including our own in Gainesville, Florida, with the goal of decreasing postoperative morbidity and mortality in older adult patients.17 The patients are given a 1-page screening assessment that asks about their living situation, medical conditions, nutrition status, cognition, and activities of daily living. The results can trigger the clinician to rethink the surgical plan and mobilize more resources to optimize the patient’s health. This study period precedes the initiative at our institution.
The plastic surgery service’s routine practice is to excise skin cancers in the operating room if sedation or general anesthesia will be needed (Figure 1A), for optimal control of bleeding (Figure 1B) in a patient who cannot safely stop blood thinners, or for excision of a highly vascularized area such as the scalp. Surgery is offered in an office-based setting if the area can be closed primarily, left open to close secondarily, or closed with a small skin graft under local anesthesia only (Figure 2). We prefer treating frail patients in the minor procedure clinic, when possible, to avoid the risks of sedation and the additional preoperative visits and transportation requirements. NMSC with unclear margins (Figure 3A) or in cosmetically sensitive areas where tissue needs to be preserved (Figure 3B) are referred to the Mohs dermatologist. The skin cancers in this study were most frequently found on the face, scalp, hands, and forearms based on referral patterns.
Other treatment options for NMSC include curettage and electrodessication, cryotherapy, and radiation; however, ours is a surgical service and patients are typically referred to us by primary care or dermatology when those are not reasonable or desirable options.18 Published complication rates of patients having skin cancer surgery without age restriction have a rate of 3% to 6%, which is consistent with our study of 5%.19-21 Two bleeding complications that needed to be admitted did not require more than a bedside procedure and neither required transfusions. One patient had been instructed to continue taking coumadin during the perioperative office-based procedure due to a recent carotid stent placement in the setting of a rapidly growing basal cell on an easily accessible location.
The most noted comorbidity in patients with wound complications was found to be DM; however, this was not found to be a statistically significant risk factor for wound complications (P = .10). We do not have a set rule for advising for or against NMSC surgery. We do counsel frail patients and their families that not all cancer is immediately life threatening and will work with them to do whatever makes the most sense to achieve their goals, occasionally accepting positive margins in order to debulk a symptomatic growth. The objective of this paper is to contribute to the discussion of performing invasive procedures on older adult veterans with life-limiting comorbidities. Patients and their families will have different thresholds for what they feel needs intervention, especially if other medical problems are consuming much of their time. We also have the community care referral option for patients whose treatment decisions are being dictated by travel hardships.
Strengths and Limitations
A strength of this study is that the data were obtained from a closed system. Patients tend to stay long-term within the VA and their health record is accessible throughout the country as long as they are seen at a VA facility. Complications, therefore, return to the treating service or primary care, who would route the patient back to the surgeon.
One limitation of the study is that this is a retrospective review from 2011. The authors are limited to data that are recorded in the patient record. Multiple health care professionals saw the patients and notes lack consistency in detail. Size of the lesions were not consistently recorded and did not get logged into our database for that reason.
Conclusions
Treatment of NMSC in older adult patients has a low morbidity but needs to be balanced against a patient and family’s goals when the patient presents with life-limiting comorbidities. An elevated 5-year mortality in patients aged > 80 years with serious unrelated medical conditions is intuitive, but this study may help put treatment plans into perspective for families and health care professionals who want to provide an indicated service while maximizing patient quality of life.
Acknowledgments
This manuscript is the result of work supported with resources and the use of facilities at the North Florida/South Georgia Veterans Health System, Gainesville, Florida.
Skin cancer is the most diagnosed cancer in the United States. Nonmelanoma skin cancers (NMSC), which include basal cell carcinoma and squamous cell carcinoma, are usually cured with removal.1 The incidence of NMSC increases with age and is commonly found in nursing homes and geriatric units. These cancers are not usually metastatic or fatal but can cause local destruction and disfigurement if neglected.2 The current standard of care is to treat diagnosed NMSC; however, the dermatology and geriatric care literature have questioned the logic of treating asymptomatic skin cancers that will not affect a patient’s life expectancy.2-4
Forty-seven percent of the current living veteran population is aged ≥ 65 years.5 Older adult patients are frequently referred to the US Department of Veterans Affairs (VA) surgical service for the treatment of NMSC. The veteran population includes a higher percentage of individuals at an elevated risk of skin cancers (older, White, and male) compared with the general population.6 World War II veterans deployed in regions closer to the equator have been found to have an elevated risk of melanoma and nonmelanoma skin carcinomas.7 A retrospective study of Vietnam veterans exposed to Agent Orange (2,3,7,8-tetrachlorodibenzodioxin) found a significantly higher risk of invasive NMSC in Fitzpatrick skin types I-IV compared with an age-matched subset of the general population.8 Younger veterans who were deployed in Afghanistan and Iraq for Operation Enduring Freedom/Operation Iraqi Freedom worked at more equatorial latitudes than the rest of the US population and may be at increased risk of NMSC. Inadequate sunscreen access, immediate safety concerns, outdoor recreational activities, harsh weather, and insufficient emphasis on sun protection have created a multifactorial challenge for the military population. Riemenschneider and colleagues recommended targeted screening for at-risk veteran patients and prioritizing annual skin cancer screenings during medical mission physical examinations for active military.7
The plastic surgery service regularly receives consults from dermatology, general surgery, and primary care to remove skin cancers on the face, scalp, hands, and forearms. Skin cancer treatment can create serious hardships for older adult patients and their families with multiple appointments for the consult, procedure, and follow-up. Patients are often told to hold their anticoagulant medications when the surgery will be performed on a highly vascular region, such as the scalp or face. This can create wide swings in their laboratory test values and result in life-threatening complications from either bleeding or clotting. The appropriateness of offering surgery to patients with serious comorbidities and a limited life expectancy has been questioned.2-4 The purpose of this study was to measure the morbidity and unrelated 5-year mortality for patients with skin cancer referred to the plastic surgery service to help patients and families make a more informed treatment decision, particularly when the patients are aged > 80 years and have significant life-threatening comorbidities.
Methods
The University of Florida and Malcom Randall VA Medical Center Institutional review board in Gainesville, approved a retrospective review of all consults completed by the plastic surgery service for the treatment of NMSC performed from July 1, 2011 to June 30, 2015. Data collected included age and common life-limiting comorbidities at the time of referral. Morbidities were found on the electronic health record, including coronary artery disease (CAD), congestive heart failure (CHF), cerebral vascular disease (CVD), peripheral vascular disease, dementia, chronic kidney disease (CKD), chronic obstructive pulmonary disease (COPD), tobacco use, diabetes mellitus (DM), liver disease, alcohol use, and obstructive sleep apnea.
Treatment, complications, and 5-year mortality were recorded. A χ2 analysis with P value < .05 was used to determine statistical significance between individual risk factors and 5-year mortality. The relative risk of 5-year mortality was calculated by combining advanced age (aged > 80 years) with the individual comorbidities.
Results
Over 4 years, 800 consults for NMSC were completed by the plastic surgery service. Treatment decisions included 210 excisions (with or without reconstruction) in the operating room, 402 excisions (with or without reconstruction) under local anesthesia in clinic, 55 Mohs surgical dermatology referrals, 21 other service or hospital referrals, and 112 patient who were observed, declined intervention, or died prior to intervention. Five-year mortality was 28.6%. No patients died of NMSC. The median age at consult submission for patients deceased 5 years later was 78 years. Complication rate was 5% and included wound infection, dehiscence, bleeding, or graft loss. Two patients, both deceased within 5 years, had unplanned admissions due to bleeding from either a skin graft donor site or recipient bleeding. Aged ≥ 80 years, CAD, CHF, CVD, peripheral vascular disease, dementia, CKD, COPD, and DM were all found individually to be statistically significant predictors of 5-year mortality (Table 1). Combining aged ≥ 80 years plus CAD, CHF, or dementia all increased the 5-year mortality by a relative risk of > 3 (Table 2).
Discussion
The standard of care is to treat NMSC. Most NMSCs are treated surgically without consideration of patient age or life expectancy.2,4,9,10 A prospective cohort study involving a university-based private practice and a VA medical center in San Francisco found a 22.6% overall 5-year mortality and a 43.3% mortality in the group defined as limited life expectancy (LLE) based on age (≥ 85 years) and medical comorbidities. None died due to the NMSC. Leading cause of death was cardiac, cerebrovascular, and respiratory disease, lung and prostate cancer, and Alzheimer disease. The authors suggested the LLE group may be exposed to wound complications without benefiting from the treatment.4
Another study of 440 patients receiving excision for biopsy-proven facial NMSC at the Roudebush VA Medical Center in Indianapolis, Indiana, found no residual carcinoma in 35.3% of excisions, and in patients aged > 90 years, more than half of the excisions had no residual carcinoma. More than half of the patients aged > 90 years died within 1 year, not as a result of the NMSC. The authors argued for watchful waiting in select patients to maximize comfort and outcomes.10
NMSCs are often asymptomatic and not immediately life threatening. Although NMSCs tend to have a favorable prognosis, studies have found that NMSC may be a marker for other poor health outcomes. A significant increased risk for all-cause mortality was found for patients with a history of SCC, which may be attributed to immune status.11 The aging veteran population has more complex health care needs to be considered when developing surgical treatment plans. These medical problems may limit their life expectancy much sooner than the skin cancer will become symptomatic. We found that individuals aged ≥ 80 years who had CAD, CHF, or dementia had a relative risk of 3 or higher for 5-year mortality. The leading cause of death in the United States in years 2011 to 2015 was heart disease. Alzheimer disease was the sixth leading cause of death in those same years.12-14
Skin cancer excisions do not typically require general anesthesia, deep sedation, or large fluid shifts; however, studies have found that when frail patients undergo low-risk procedures, they tend to have a higher mortality rate than their healthier counterparts.15 Frailty is a concept that identifies patients who are at increased risk of dying in 6 to 60 months due to a decline in their physical reserve. Frail patients have increased rates of perioperative mortality and complications. Various tools have been used to assess the components of physical performance, speed, mobility, nutrition status, mental health, and cognition.16 Frailty screening has been initiated in several VA hospitals, including our own in Gainesville, Florida, with the goal of decreasing postoperative morbidity and mortality in older adult patients.17 The patients are given a 1-page screening assessment that asks about their living situation, medical conditions, nutrition status, cognition, and activities of daily living. The results can trigger the clinician to rethink the surgical plan and mobilize more resources to optimize the patient’s health. This study period precedes the initiative at our institution.
The plastic surgery service’s routine practice is to excise skin cancers in the operating room if sedation or general anesthesia will be needed (Figure 1A), for optimal control of bleeding (Figure 1B) in a patient who cannot safely stop blood thinners, or for excision of a highly vascularized area such as the scalp. Surgery is offered in an office-based setting if the area can be closed primarily, left open to close secondarily, or closed with a small skin graft under local anesthesia only (Figure 2). We prefer treating frail patients in the minor procedure clinic, when possible, to avoid the risks of sedation and the additional preoperative visits and transportation requirements. NMSC with unclear margins (Figure 3A) or in cosmetically sensitive areas where tissue needs to be preserved (Figure 3B) are referred to the Mohs dermatologist. The skin cancers in this study were most frequently found on the face, scalp, hands, and forearms based on referral patterns.
Other treatment options for NMSC include curettage and electrodessication, cryotherapy, and radiation; however, ours is a surgical service and patients are typically referred to us by primary care or dermatology when those are not reasonable or desirable options.18 Published complication rates of patients having skin cancer surgery without age restriction have a rate of 3% to 6%, which is consistent with our study of 5%.19-21 Two bleeding complications that needed to be admitted did not require more than a bedside procedure and neither required transfusions. One patient had been instructed to continue taking coumadin during the perioperative office-based procedure due to a recent carotid stent placement in the setting of a rapidly growing basal cell on an easily accessible location.
The most noted comorbidity in patients with wound complications was found to be DM; however, this was not found to be a statistically significant risk factor for wound complications (P = .10). We do not have a set rule for advising for or against NMSC surgery. We do counsel frail patients and their families that not all cancer is immediately life threatening and will work with them to do whatever makes the most sense to achieve their goals, occasionally accepting positive margins in order to debulk a symptomatic growth. The objective of this paper is to contribute to the discussion of performing invasive procedures on older adult veterans with life-limiting comorbidities. Patients and their families will have different thresholds for what they feel needs intervention, especially if other medical problems are consuming much of their time. We also have the community care referral option for patients whose treatment decisions are being dictated by travel hardships.
Strengths and Limitations
A strength of this study is that the data were obtained from a closed system. Patients tend to stay long-term within the VA and their health record is accessible throughout the country as long as they are seen at a VA facility. Complications, therefore, return to the treating service or primary care, who would route the patient back to the surgeon.
One limitation of the study is that this is a retrospective review from 2011. The authors are limited to data that are recorded in the patient record. Multiple health care professionals saw the patients and notes lack consistency in detail. Size of the lesions were not consistently recorded and did not get logged into our database for that reason.
Conclusions
Treatment of NMSC in older adult patients has a low morbidity but needs to be balanced against a patient and family’s goals when the patient presents with life-limiting comorbidities. An elevated 5-year mortality in patients aged > 80 years with serious unrelated medical conditions is intuitive, but this study may help put treatment plans into perspective for families and health care professionals who want to provide an indicated service while maximizing patient quality of life.
Acknowledgments
This manuscript is the result of work supported with resources and the use of facilities at the North Florida/South Georgia Veterans Health System, Gainesville, Florida.
1. American Cancer Society. Cancer Facts & Figures 2021. Accessed May 26, 2022. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2021/cancer-facts-and-figures-2021.pdf
2. Albert A, Knoll MA, Conti JA, Zbar RIS. Non-melanoma skin cancers in the older patient. Curr Oncol Rep. 2019;21(9):79. Published 2019 Jul 29. doi:10.1007/s11912-019-0828-9
3. Linos E, Chren MM, Stijacic Cenzer I, Covinsky KE. Skin cancer in U.S. elderly adults: does life expectancy play a role in treatment decisions? J Am Geriatr Soc. 2016;64(8):1610-1615. doi:10.1111/jgs.14202
4. Linos E, Parvataneni R, Stuart SE, Boscardin WJ, Landefeld CS, Chren MM. Treatment of nonfatal conditions at the end of life: nonmelanoma skin cancer. JAMA Intern Med. 2013;173(11):1006-1012. doi:10.1001/jamainternmed.2013.639
5. O’Malley KA, Vinson L, Kaiser AP, Sager Z, Hinrichs K. Mental health and aging veterans: how the Veterans Health Administration meets the needs of aging veterans. Public Policy Aging Rep. 2020;30(1):19-23. doi:10.1093/ppar/prz027
6. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. Profile of veterans: 2017. Accessed May 26, 2022. https://www.va.gov/vetdata/docs/SpecialReports/Profile_of_Veterans_2017.pdf 7. Riemenschneider K, Liu J, Powers JG. Skin cancer in the military: a systematic review of melanoma and nonmelanoma skin cancer incidence, prevention, and screening among active duty and veteran personnel. J Am Acad Dermatol. 2018;78(6):1185-1192. doi:10.1016/j.jaad.2017.11.062
8. Clemens MW, Kochuba AL, Carter ME, Han K, Liu J, Evans K. Association between Agent Orange exposure and nonmelanotic invasive skin cancer: a pilot study. Plast Reconstr Surg. 2014;133(2):432-437. doi:10.1097/01.prs.0000436859.40151.cf
9. Cameron MC, Lee E, Hibler BP, et al. Basal cell carcinoma: epidemiology; pathophysiology; clinical and histological subtypes; and disease associations. J Am Acad Dermatol. 2019;80(2):303-317. doi:10.1016/j.jaad.2018.03.060
10. Chauhan R, Munger BN, Chu MW, et al. Age at diagnosis as a relative contraindication for intervention in facial nonmelanoma skin cancer. JAMA Surg. 2018;153(4):390-392. doi:10.1001/jamasurg.2017.5073
11. Barton V, Armeson K, Hampras S, et al. Nonmelanoma skin cancer and risk of all-cause and cancer-related mortality: a systematic review. Arch Dermatol Res. 2017;309(4):243-251. doi:10.1007/s00403-017-1724-5
12. Kochanek KD, Murphy SL, Xu JQ, Arias E. Mortality in the United States, 2013. NCHS Data Brief 178. Accessed May 26, 2022. https://www.cdc.gov/nchs/products/databriefs/db178.htm
13. Xu JQ, Kochanek KD, Murphy SL, Arias E. Mortality in the United States, 2012. NCHS Data Brief 168. Accessed May 26, 2022. https://www.cdc.gov/nchs/products/databriefs/db168.htm
14. Xu JQ, Murphy SL, Kochanek KD, Arias E. Mortality in the United States, 2015. NCHS Data Brief 267. Accessed May 26, 2022. https://www.cdc.gov/nchs/products/databriefs/db267.htm
15. Varley PR , Borrebach JD, Arya S, et al. Clinical utility of the risk analysis index as a prospective frailty screening tool within a multi-practice, multi-hospital integrated healthcare system. Ann Surg. 2021;274(6):e1230-e1237. doi:10.1097/SLA.0000000000003808
16. Hall DE, Arya S , Schmid KK, et al. Development and initial validation of the risk analysis index for measuring frailty in surgical populations. JAMA Surg. 2017;152(2):175-182. doi:10.1001/jamasurg.2016.4202
17. US Department of Veterans Affairs, Health Services Research & Development. Improving healthcare for aging veterans. Updated August 30, 2017. Accessed May 26, 2022. https://www.hsrd.research.va.gov/news/feature/aging0917.cfm
18. Leus AJG, Frie M, Haisma MS, et al. Treatment of keratinocyte carcinoma in elderly patients – a review of the current literature. J Eur Acad Dermatol Venereol. 2020;34(9):1932-1943. doi:10.1111/jdv.16268
19. Amici JM, Rogues AM, Lasheras A, et al. A prospective study of the incidence of complications associated with dermatological surgery. Br J Dermatol. 2005;153(5):967-971. doi:10.1111/j.1365-2133.2005.06861.x
20. Arguello-Guerra L, Vargas-Chandomid E, Díaz-González JM, Méndez-Flores S, Ruelas-Villavicencio A, Domínguez-Cherit J. Incidence of complications in dermatological surgery of melanoma and non-melanoma skin cancer in patients with multiple comorbidity and/or antiplatelet-anticoagulants. Five-year experience in our hospital. Cir Cir. 2019;86(1):15-23. doi:10.24875/CIRUE.M18000003
21. Keith DJ, de Berker DA, Bray AP, Cheung ST, Brain A, Mohd Mustapa MF. British Association of Dermatologists’ national audit on nonmelanoma skin cancer excision, 2014. Clin Exp Dermatol. 2017;42(1):46-53. doi:10.1111/ced.12990
1. American Cancer Society. Cancer Facts & Figures 2021. Accessed May 26, 2022. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2021/cancer-facts-and-figures-2021.pdf
2. Albert A, Knoll MA, Conti JA, Zbar RIS. Non-melanoma skin cancers in the older patient. Curr Oncol Rep. 2019;21(9):79. Published 2019 Jul 29. doi:10.1007/s11912-019-0828-9
3. Linos E, Chren MM, Stijacic Cenzer I, Covinsky KE. Skin cancer in U.S. elderly adults: does life expectancy play a role in treatment decisions? J Am Geriatr Soc. 2016;64(8):1610-1615. doi:10.1111/jgs.14202
4. Linos E, Parvataneni R, Stuart SE, Boscardin WJ, Landefeld CS, Chren MM. Treatment of nonfatal conditions at the end of life: nonmelanoma skin cancer. JAMA Intern Med. 2013;173(11):1006-1012. doi:10.1001/jamainternmed.2013.639
5. O’Malley KA, Vinson L, Kaiser AP, Sager Z, Hinrichs K. Mental health and aging veterans: how the Veterans Health Administration meets the needs of aging veterans. Public Policy Aging Rep. 2020;30(1):19-23. doi:10.1093/ppar/prz027
6. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. Profile of veterans: 2017. Accessed May 26, 2022. https://www.va.gov/vetdata/docs/SpecialReports/Profile_of_Veterans_2017.pdf 7. Riemenschneider K, Liu J, Powers JG. Skin cancer in the military: a systematic review of melanoma and nonmelanoma skin cancer incidence, prevention, and screening among active duty and veteran personnel. J Am Acad Dermatol. 2018;78(6):1185-1192. doi:10.1016/j.jaad.2017.11.062
8. Clemens MW, Kochuba AL, Carter ME, Han K, Liu J, Evans K. Association between Agent Orange exposure and nonmelanotic invasive skin cancer: a pilot study. Plast Reconstr Surg. 2014;133(2):432-437. doi:10.1097/01.prs.0000436859.40151.cf
9. Cameron MC, Lee E, Hibler BP, et al. Basal cell carcinoma: epidemiology; pathophysiology; clinical and histological subtypes; and disease associations. J Am Acad Dermatol. 2019;80(2):303-317. doi:10.1016/j.jaad.2018.03.060
10. Chauhan R, Munger BN, Chu MW, et al. Age at diagnosis as a relative contraindication for intervention in facial nonmelanoma skin cancer. JAMA Surg. 2018;153(4):390-392. doi:10.1001/jamasurg.2017.5073
11. Barton V, Armeson K, Hampras S, et al. Nonmelanoma skin cancer and risk of all-cause and cancer-related mortality: a systematic review. Arch Dermatol Res. 2017;309(4):243-251. doi:10.1007/s00403-017-1724-5
12. Kochanek KD, Murphy SL, Xu JQ, Arias E. Mortality in the United States, 2013. NCHS Data Brief 178. Accessed May 26, 2022. https://www.cdc.gov/nchs/products/databriefs/db178.htm
13. Xu JQ, Kochanek KD, Murphy SL, Arias E. Mortality in the United States, 2012. NCHS Data Brief 168. Accessed May 26, 2022. https://www.cdc.gov/nchs/products/databriefs/db168.htm
14. Xu JQ, Murphy SL, Kochanek KD, Arias E. Mortality in the United States, 2015. NCHS Data Brief 267. Accessed May 26, 2022. https://www.cdc.gov/nchs/products/databriefs/db267.htm
15. Varley PR , Borrebach JD, Arya S, et al. Clinical utility of the risk analysis index as a prospective frailty screening tool within a multi-practice, multi-hospital integrated healthcare system. Ann Surg. 2021;274(6):e1230-e1237. doi:10.1097/SLA.0000000000003808
16. Hall DE, Arya S , Schmid KK, et al. Development and initial validation of the risk analysis index for measuring frailty in surgical populations. JAMA Surg. 2017;152(2):175-182. doi:10.1001/jamasurg.2016.4202
17. US Department of Veterans Affairs, Health Services Research & Development. Improving healthcare for aging veterans. Updated August 30, 2017. Accessed May 26, 2022. https://www.hsrd.research.va.gov/news/feature/aging0917.cfm
18. Leus AJG, Frie M, Haisma MS, et al. Treatment of keratinocyte carcinoma in elderly patients – a review of the current literature. J Eur Acad Dermatol Venereol. 2020;34(9):1932-1943. doi:10.1111/jdv.16268
19. Amici JM, Rogues AM, Lasheras A, et al. A prospective study of the incidence of complications associated with dermatological surgery. Br J Dermatol. 2005;153(5):967-971. doi:10.1111/j.1365-2133.2005.06861.x
20. Arguello-Guerra L, Vargas-Chandomid E, Díaz-González JM, Méndez-Flores S, Ruelas-Villavicencio A, Domínguez-Cherit J. Incidence of complications in dermatological surgery of melanoma and non-melanoma skin cancer in patients with multiple comorbidity and/or antiplatelet-anticoagulants. Five-year experience in our hospital. Cir Cir. 2019;86(1):15-23. doi:10.24875/CIRUE.M18000003
21. Keith DJ, de Berker DA, Bray AP, Cheung ST, Brain A, Mohd Mustapa MF. British Association of Dermatologists’ national audit on nonmelanoma skin cancer excision, 2014. Clin Exp Dermatol. 2017;42(1):46-53. doi:10.1111/ced.12990