User login
Nodular Sclerosing Hodgkin Lymphoma With Paraneoplastic Cerebellar Degeneration
Paraneoplastic syndrome is a rare disorder involving manifestations of immune dysregulation triggered by malignancy. The immune system develops antibodies to the malignancy, which can cause cross reactivation with various tissues in the body, resulting in an autoimmune response. Paraneoplastic cerebellar degeneration (PCD) is a rare condition caused by immune-mediated damage to the Purkinje cells of the cerebellar tract. Symptoms may include gait instability, double vision, decreased fine motor skills, and ataxia, with progression to brainstem-associated symptoms, such as nystagmus, dysarthria, and dysphagia. Early detection and treatment of the underlying malignancy is critical to halt the progression of autoimmune-mediated destruction. We present a case of a young adult female patient with PCD caused by Purkinje cell cytoplasmic–Tr (PCA-Tr) antibody with Hodgkin lymphoma.
Case Presentation
A 20-year-old previously healthy active-duty female patient presented to the emergency department with acute worsening of chronic intermittent, recurrent episodes of lightheadedness and vertigo. Symptoms persisted for 9 months until acutely worsening over the 2 weeks prior to presentation. She reported left eye double vision but did not report seeing spots, photophobia, tinnitus, or headache. She felt off-balance, leaning on nearby objects to remain standing. Symptoms primarily occurred during ambulation; however, occasionally they happened at rest. Episodes lasted up to several minutes and occurred up to 15 times a day. The patient reported no fever, night sweats, unexplained weight loss, muscle aches, weakness, numbness or tingling, loss of bowel or bladder function, or rash. She had no recent illnesses, changes to medications, or recent travel. Oral intake to include food and water was adequate and unchanged. The patient had a remote history of mild concussions without loss of consciousness while playing sports 4 years previously. She reported no recent trauma. Nine months before, she received treatment for benign paroxysmal positional vertigo (BPPV) with the Epley maneuver with full resolution of symptoms lasting several days. She reported no prescription or over-the-counter medications, herbal remedies, or supplements. She reported no other medical or surgical history and no pertinent social or family history.
Physical examination revealed a nontoxic-appearing female patient with intermittent conversational dysarthria, saccadic pursuits, horizontal nystagmus with lateral gaze, and vertical nystagmus with vertical gaze. The patient exhibited dysdiadochokinesia, or impaired ability to perform rapid alternating hand movements with repetition. Finger-to-nose testing was impaired and heel-to-shin motion remained intact. A Romberg test was positive, and the patient had tandem gait instability. Strength testing, sensation, reflexes, and cranial nerves were otherwise intact. Initial laboratory testing was unremarkable except for mild normocytic anemia. Her infectious workup, including testing for venereal disease, HIV, COVID-19, and Coccidioidies was negative. Heavy metals analysis and urine drug screen were negative. Ophthalmology was consulted and workup revealed small amplitude downbeat nystagmus in primary gaze, sustained gaze evoked lateral beating jerk nystagmus with rebound nystagmus R>L gaze, but there was no evidence of afferent package defect and optic nerve function remained intact. Magnetic resonance imaging of the brain demonstrated cerebellar vermis hypoplasia with prominence of the superior cerebellar folia. Due to concerns for autoimmune encephalitis, a lumbar puncture was performed. Antibody testing revealed PCA-Tr antibodies, which is commonly associated with Hodgkin lymphoma, prompting further evaluation for malignancy.
Computed tomography (CT) of the chest with contrast demonstrated multiple mediastinal masses with a conglomeration of lymph nodes along the right paratracheal region. Further evaluation was performed with a positron emission tomography (PET)–CT, revealing a large conglomeration of hypermetabolic pretracheal, mediastinal, and right supraclavicular lymph that were suggestive of lymphoma. Mediastinoscopy with excisional lymph node biopsy was performed with immunohistochemical staining confirming diagnosis of a nodular sclerosing variant of Hodgkin lymphoma. The patient was treated with IV immunoglobulin at 0.4g/kg daily for 5 days. A central venous catheter was placed into the patient’s right internal jugular vein and a chemotherapy regimen of doxorubicin 46 mg, vinblastine 11 mg, bleomycin 19 units, and dacarbazine 700 mg was initiated. The patient’s symptoms improved with resolution of dysarthria; however, her visual impairment and gait instability persisted. Repeat PET-CT imaging 2 months later revealed interval improvement with decreased intensity and extent of the hypermetabolic lymph nodes and no new hypermetabolic foci.
Discussion
PCA-Tr antibodies affect the delta/notchlike epidermal growth factor–related receptor, expressed on the dendrites of cerebellar Purkinje cells.1 These fibers are the only output neurons of the cerebellar cortex and are critical to the coordination of motor movements, accounting for the ataxia experienced by patients with this subtype of PCD.2 The link between Hodgkin lymphoma and PCA-Tr antibodies has been established; however, most reports involve men with a median age of 61 years with lymphoma-associated symptoms (such as lymphadenopathy) or systemic symptoms (fever, night sweats, or weight loss) preceding neurologic manifestations in 80% of cases.3
Our patient was a young, previously healthy adult female who initially presented with vertigo, a common concern with frequently benign origins. Although there was temporary resolution of symptoms after Epley maneuvers, symptoms recurred and progressed over several months to include brainstem manifestations of nystagmus, diplopia, and dysarthria. Previous reports indicate that after remission of the Hodgkin lymphoma, PCA-Tr antibodies disappear and symptoms can improve or resolve.4,5 Treatment has just begun for our patient and although there has been initial clinical improvement, given the chronicity of symptoms, it is unclear if complete resolution will be achieved.
Conclusions
PCD can result in debilitating neurologic dysfunction and may be associated with malignancy such as Hodgkin lymphoma. This case offers unique insight due to the patient’s demographics and presentation, which involved brainstem pathology typically associated with late-onset disease and preceded by constitutional symptoms. Clinical suspicion of this rare disorder should be considered in all ages, especially if symptoms are progressive or neurologic manifestations arise, as early detection and treatment of the underlying malignancy are paramount to the prevention of significant disability.
1. de Graaff E, Maat P, Hulsenboom E, et al. Identification of delta/notch-like epidermal growth factor-related receptor as the Tr antigen in paraneoplastic cerebellar degeneration. Ann Neurol. 2012;71(6):815-824. doi:10.1002/ana.23550
2. MacKenzie-Graham A, Tiwari-Woodruff SK, Sharma G, et al. Purkinje cell loss in experimental autoimmune encephalomyelitis. Neuroimage. 2009;48(4):637-651. doi:10.1016/j.neuroimage.2009.06.073
3. Bernal F, Shams’ili S, Rojas I, et al. Anti-Tr antibodies as markers of paraneoplastic cerebellar degeneration and Hodgkin’s disease. Neurology. 2003;60(2):230-234. doi:10.1212/01.wnl.0000041495.87539.98
4. Graus F, Ariño H, Dalmau J. Paraneoplastic neurological syndromes in Hodgkin and non-Hodgkin lymphomas. Blood. 2014;123(21):3230-3238. doi:10.1182/blood-2014-03-537506
5. Aly R, Emmady PD. Paraneoplastic cerebellar degeneration. Updated May 8, 2022. Accessed March 30, 2022. https://www.ncbi.nlm.nih.gov/books/NBK560638
Paraneoplastic syndrome is a rare disorder involving manifestations of immune dysregulation triggered by malignancy. The immune system develops antibodies to the malignancy, which can cause cross reactivation with various tissues in the body, resulting in an autoimmune response. Paraneoplastic cerebellar degeneration (PCD) is a rare condition caused by immune-mediated damage to the Purkinje cells of the cerebellar tract. Symptoms may include gait instability, double vision, decreased fine motor skills, and ataxia, with progression to brainstem-associated symptoms, such as nystagmus, dysarthria, and dysphagia. Early detection and treatment of the underlying malignancy is critical to halt the progression of autoimmune-mediated destruction. We present a case of a young adult female patient with PCD caused by Purkinje cell cytoplasmic–Tr (PCA-Tr) antibody with Hodgkin lymphoma.
Case Presentation
A 20-year-old previously healthy active-duty female patient presented to the emergency department with acute worsening of chronic intermittent, recurrent episodes of lightheadedness and vertigo. Symptoms persisted for 9 months until acutely worsening over the 2 weeks prior to presentation. She reported left eye double vision but did not report seeing spots, photophobia, tinnitus, or headache. She felt off-balance, leaning on nearby objects to remain standing. Symptoms primarily occurred during ambulation; however, occasionally they happened at rest. Episodes lasted up to several minutes and occurred up to 15 times a day. The patient reported no fever, night sweats, unexplained weight loss, muscle aches, weakness, numbness or tingling, loss of bowel or bladder function, or rash. She had no recent illnesses, changes to medications, or recent travel. Oral intake to include food and water was adequate and unchanged. The patient had a remote history of mild concussions without loss of consciousness while playing sports 4 years previously. She reported no recent trauma. Nine months before, she received treatment for benign paroxysmal positional vertigo (BPPV) with the Epley maneuver with full resolution of symptoms lasting several days. She reported no prescription or over-the-counter medications, herbal remedies, or supplements. She reported no other medical or surgical history and no pertinent social or family history.
Physical examination revealed a nontoxic-appearing female patient with intermittent conversational dysarthria, saccadic pursuits, horizontal nystagmus with lateral gaze, and vertical nystagmus with vertical gaze. The patient exhibited dysdiadochokinesia, or impaired ability to perform rapid alternating hand movements with repetition. Finger-to-nose testing was impaired and heel-to-shin motion remained intact. A Romberg test was positive, and the patient had tandem gait instability. Strength testing, sensation, reflexes, and cranial nerves were otherwise intact. Initial laboratory testing was unremarkable except for mild normocytic anemia. Her infectious workup, including testing for venereal disease, HIV, COVID-19, and Coccidioidies was negative. Heavy metals analysis and urine drug screen were negative. Ophthalmology was consulted and workup revealed small amplitude downbeat nystagmus in primary gaze, sustained gaze evoked lateral beating jerk nystagmus with rebound nystagmus R>L gaze, but there was no evidence of afferent package defect and optic nerve function remained intact. Magnetic resonance imaging of the brain demonstrated cerebellar vermis hypoplasia with prominence of the superior cerebellar folia. Due to concerns for autoimmune encephalitis, a lumbar puncture was performed. Antibody testing revealed PCA-Tr antibodies, which is commonly associated with Hodgkin lymphoma, prompting further evaluation for malignancy.
Computed tomography (CT) of the chest with contrast demonstrated multiple mediastinal masses with a conglomeration of lymph nodes along the right paratracheal region. Further evaluation was performed with a positron emission tomography (PET)–CT, revealing a large conglomeration of hypermetabolic pretracheal, mediastinal, and right supraclavicular lymph that were suggestive of lymphoma. Mediastinoscopy with excisional lymph node biopsy was performed with immunohistochemical staining confirming diagnosis of a nodular sclerosing variant of Hodgkin lymphoma. The patient was treated with IV immunoglobulin at 0.4g/kg daily for 5 days. A central venous catheter was placed into the patient’s right internal jugular vein and a chemotherapy regimen of doxorubicin 46 mg, vinblastine 11 mg, bleomycin 19 units, and dacarbazine 700 mg was initiated. The patient’s symptoms improved with resolution of dysarthria; however, her visual impairment and gait instability persisted. Repeat PET-CT imaging 2 months later revealed interval improvement with decreased intensity and extent of the hypermetabolic lymph nodes and no new hypermetabolic foci.
Discussion
PCA-Tr antibodies affect the delta/notchlike epidermal growth factor–related receptor, expressed on the dendrites of cerebellar Purkinje cells.1 These fibers are the only output neurons of the cerebellar cortex and are critical to the coordination of motor movements, accounting for the ataxia experienced by patients with this subtype of PCD.2 The link between Hodgkin lymphoma and PCA-Tr antibodies has been established; however, most reports involve men with a median age of 61 years with lymphoma-associated symptoms (such as lymphadenopathy) or systemic symptoms (fever, night sweats, or weight loss) preceding neurologic manifestations in 80% of cases.3
Our patient was a young, previously healthy adult female who initially presented with vertigo, a common concern with frequently benign origins. Although there was temporary resolution of symptoms after Epley maneuvers, symptoms recurred and progressed over several months to include brainstem manifestations of nystagmus, diplopia, and dysarthria. Previous reports indicate that after remission of the Hodgkin lymphoma, PCA-Tr antibodies disappear and symptoms can improve or resolve.4,5 Treatment has just begun for our patient and although there has been initial clinical improvement, given the chronicity of symptoms, it is unclear if complete resolution will be achieved.
Conclusions
PCD can result in debilitating neurologic dysfunction and may be associated with malignancy such as Hodgkin lymphoma. This case offers unique insight due to the patient’s demographics and presentation, which involved brainstem pathology typically associated with late-onset disease and preceded by constitutional symptoms. Clinical suspicion of this rare disorder should be considered in all ages, especially if symptoms are progressive or neurologic manifestations arise, as early detection and treatment of the underlying malignancy are paramount to the prevention of significant disability.
Paraneoplastic syndrome is a rare disorder involving manifestations of immune dysregulation triggered by malignancy. The immune system develops antibodies to the malignancy, which can cause cross reactivation with various tissues in the body, resulting in an autoimmune response. Paraneoplastic cerebellar degeneration (PCD) is a rare condition caused by immune-mediated damage to the Purkinje cells of the cerebellar tract. Symptoms may include gait instability, double vision, decreased fine motor skills, and ataxia, with progression to brainstem-associated symptoms, such as nystagmus, dysarthria, and dysphagia. Early detection and treatment of the underlying malignancy is critical to halt the progression of autoimmune-mediated destruction. We present a case of a young adult female patient with PCD caused by Purkinje cell cytoplasmic–Tr (PCA-Tr) antibody with Hodgkin lymphoma.
Case Presentation
A 20-year-old previously healthy active-duty female patient presented to the emergency department with acute worsening of chronic intermittent, recurrent episodes of lightheadedness and vertigo. Symptoms persisted for 9 months until acutely worsening over the 2 weeks prior to presentation. She reported left eye double vision but did not report seeing spots, photophobia, tinnitus, or headache. She felt off-balance, leaning on nearby objects to remain standing. Symptoms primarily occurred during ambulation; however, occasionally they happened at rest. Episodes lasted up to several minutes and occurred up to 15 times a day. The patient reported no fever, night sweats, unexplained weight loss, muscle aches, weakness, numbness or tingling, loss of bowel or bladder function, or rash. She had no recent illnesses, changes to medications, or recent travel. Oral intake to include food and water was adequate and unchanged. The patient had a remote history of mild concussions without loss of consciousness while playing sports 4 years previously. She reported no recent trauma. Nine months before, she received treatment for benign paroxysmal positional vertigo (BPPV) with the Epley maneuver with full resolution of symptoms lasting several days. She reported no prescription or over-the-counter medications, herbal remedies, or supplements. She reported no other medical or surgical history and no pertinent social or family history.
Physical examination revealed a nontoxic-appearing female patient with intermittent conversational dysarthria, saccadic pursuits, horizontal nystagmus with lateral gaze, and vertical nystagmus with vertical gaze. The patient exhibited dysdiadochokinesia, or impaired ability to perform rapid alternating hand movements with repetition. Finger-to-nose testing was impaired and heel-to-shin motion remained intact. A Romberg test was positive, and the patient had tandem gait instability. Strength testing, sensation, reflexes, and cranial nerves were otherwise intact. Initial laboratory testing was unremarkable except for mild normocytic anemia. Her infectious workup, including testing for venereal disease, HIV, COVID-19, and Coccidioidies was negative. Heavy metals analysis and urine drug screen were negative. Ophthalmology was consulted and workup revealed small amplitude downbeat nystagmus in primary gaze, sustained gaze evoked lateral beating jerk nystagmus with rebound nystagmus R>L gaze, but there was no evidence of afferent package defect and optic nerve function remained intact. Magnetic resonance imaging of the brain demonstrated cerebellar vermis hypoplasia with prominence of the superior cerebellar folia. Due to concerns for autoimmune encephalitis, a lumbar puncture was performed. Antibody testing revealed PCA-Tr antibodies, which is commonly associated with Hodgkin lymphoma, prompting further evaluation for malignancy.
Computed tomography (CT) of the chest with contrast demonstrated multiple mediastinal masses with a conglomeration of lymph nodes along the right paratracheal region. Further evaluation was performed with a positron emission tomography (PET)–CT, revealing a large conglomeration of hypermetabolic pretracheal, mediastinal, and right supraclavicular lymph that were suggestive of lymphoma. Mediastinoscopy with excisional lymph node biopsy was performed with immunohistochemical staining confirming diagnosis of a nodular sclerosing variant of Hodgkin lymphoma. The patient was treated with IV immunoglobulin at 0.4g/kg daily for 5 days. A central venous catheter was placed into the patient’s right internal jugular vein and a chemotherapy regimen of doxorubicin 46 mg, vinblastine 11 mg, bleomycin 19 units, and dacarbazine 700 mg was initiated. The patient’s symptoms improved with resolution of dysarthria; however, her visual impairment and gait instability persisted. Repeat PET-CT imaging 2 months later revealed interval improvement with decreased intensity and extent of the hypermetabolic lymph nodes and no new hypermetabolic foci.
Discussion
PCA-Tr antibodies affect the delta/notchlike epidermal growth factor–related receptor, expressed on the dendrites of cerebellar Purkinje cells.1 These fibers are the only output neurons of the cerebellar cortex and are critical to the coordination of motor movements, accounting for the ataxia experienced by patients with this subtype of PCD.2 The link between Hodgkin lymphoma and PCA-Tr antibodies has been established; however, most reports involve men with a median age of 61 years with lymphoma-associated symptoms (such as lymphadenopathy) or systemic symptoms (fever, night sweats, or weight loss) preceding neurologic manifestations in 80% of cases.3
Our patient was a young, previously healthy adult female who initially presented with vertigo, a common concern with frequently benign origins. Although there was temporary resolution of symptoms after Epley maneuvers, symptoms recurred and progressed over several months to include brainstem manifestations of nystagmus, diplopia, and dysarthria. Previous reports indicate that after remission of the Hodgkin lymphoma, PCA-Tr antibodies disappear and symptoms can improve or resolve.4,5 Treatment has just begun for our patient and although there has been initial clinical improvement, given the chronicity of symptoms, it is unclear if complete resolution will be achieved.
Conclusions
PCD can result in debilitating neurologic dysfunction and may be associated with malignancy such as Hodgkin lymphoma. This case offers unique insight due to the patient’s demographics and presentation, which involved brainstem pathology typically associated with late-onset disease and preceded by constitutional symptoms. Clinical suspicion of this rare disorder should be considered in all ages, especially if symptoms are progressive or neurologic manifestations arise, as early detection and treatment of the underlying malignancy are paramount to the prevention of significant disability.
1. de Graaff E, Maat P, Hulsenboom E, et al. Identification of delta/notch-like epidermal growth factor-related receptor as the Tr antigen in paraneoplastic cerebellar degeneration. Ann Neurol. 2012;71(6):815-824. doi:10.1002/ana.23550
2. MacKenzie-Graham A, Tiwari-Woodruff SK, Sharma G, et al. Purkinje cell loss in experimental autoimmune encephalomyelitis. Neuroimage. 2009;48(4):637-651. doi:10.1016/j.neuroimage.2009.06.073
3. Bernal F, Shams’ili S, Rojas I, et al. Anti-Tr antibodies as markers of paraneoplastic cerebellar degeneration and Hodgkin’s disease. Neurology. 2003;60(2):230-234. doi:10.1212/01.wnl.0000041495.87539.98
4. Graus F, Ariño H, Dalmau J. Paraneoplastic neurological syndromes in Hodgkin and non-Hodgkin lymphomas. Blood. 2014;123(21):3230-3238. doi:10.1182/blood-2014-03-537506
5. Aly R, Emmady PD. Paraneoplastic cerebellar degeneration. Updated May 8, 2022. Accessed March 30, 2022. https://www.ncbi.nlm.nih.gov/books/NBK560638
1. de Graaff E, Maat P, Hulsenboom E, et al. Identification of delta/notch-like epidermal growth factor-related receptor as the Tr antigen in paraneoplastic cerebellar degeneration. Ann Neurol. 2012;71(6):815-824. doi:10.1002/ana.23550
2. MacKenzie-Graham A, Tiwari-Woodruff SK, Sharma G, et al. Purkinje cell loss in experimental autoimmune encephalomyelitis. Neuroimage. 2009;48(4):637-651. doi:10.1016/j.neuroimage.2009.06.073
3. Bernal F, Shams’ili S, Rojas I, et al. Anti-Tr antibodies as markers of paraneoplastic cerebellar degeneration and Hodgkin’s disease. Neurology. 2003;60(2):230-234. doi:10.1212/01.wnl.0000041495.87539.98
4. Graus F, Ariño H, Dalmau J. Paraneoplastic neurological syndromes in Hodgkin and non-Hodgkin lymphomas. Blood. 2014;123(21):3230-3238. doi:10.1182/blood-2014-03-537506
5. Aly R, Emmady PD. Paraneoplastic cerebellar degeneration. Updated May 8, 2022. Accessed March 30, 2022. https://www.ncbi.nlm.nih.gov/books/NBK560638
Cancer drug significantly cuts risk for COVID-19 death
, an interim analysis of a phase 3 placebo-controlled trial found.
Sabizabulin treatment consistently and significantly reduced deaths across patient subgroups “regardless of standard of care treatment received, baseline World Health Organization scores, age, comorbidities, vaccination status, COVID-19 variant, or geography,” study investigator Mitchell Steiner, MD, chairman, president, and CEO of Veru, said in a news release.
The company has submitted an emergency use authorization request to the U.S. Food and Drug Administration to use sabizabulin to treat COVID-19.
The analysis was published online in NEJM Evidence.
Sabizabulin, originally developed to treat metastatic castration-resistant prostate cancer, is a novel, investigational, oral microtubule disruptor with dual antiviral and anti-inflammatory activities. Given the drug’s mechanism, researchers at Veru thought that sabizabulin could help treat lung inflammation in patients with COVID-19 as well.
Findings of the interim analysis are based on 150 adults hospitalized with moderate to severe COVID-19 at high risk for acute respiratory distress syndrome and death. The patients were randomly allocated to receive 9 mg oral sabizabulin (n = 98) or placebo (n = 52) once daily for up to 21 days.
Overall, the mortality rate was 20.2% in the sabizabulin group vs. 45.1% in the placebo group. Compared with placebo, treatment with sabizabulin led to a 24.9–percentage point absolute reduction and a 55.2% relative reduction in death (odds ratio, 3.23; P = .0042).
The key secondary endpoint of mortality through day 29 also favored sabizabulin over placebo, with a mortality rate of 17% vs. 35.3%. In this scenario, treatment with sabizabulin resulted in an absolute reduction in deaths of 18.3 percentage points and a relative reduction of 51.8%.
Sabizabulin led to a significant 43% relative reduction in ICU days, a 49% relative reduction in days on mechanical ventilation, and a 26% relative reduction in days in the hospital, compared with placebo.
Adverse and serious adverse events were also lower in the sabizabulin group (61.5%) than the placebo group (78.3%).
The data are “pretty impressive and in a group of patients that we really have limited things to offer,” Aaron Glatt, MD, a spokesperson for the Infectious Diseases Society of America and chief of infectious diseases and hospital epidemiologist at Mount Sinai South Nassau in Oceanside, N.Y., said in an interview. “This is an interim analysis and obviously we’d like to see more data, but it certainly is something that is novel and quite interesting.”
David Boulware, MD, MPH, an infectious disease expert at the University of Minnesota, Minneapolis, told the New York Times that the large number of deaths in the placebo group seemed “rather high” and that the final analysis might reveal a more modest benefit for sabizabulin.
“I would be skeptical” that the reduced risk for death remains 55%, he noted.
The study was funded by Veru Pharmaceuticals. Several authors are employed by the company or have financial relationships with the company.
A version of this article first appeared on Medscape.com.
, an interim analysis of a phase 3 placebo-controlled trial found.
Sabizabulin treatment consistently and significantly reduced deaths across patient subgroups “regardless of standard of care treatment received, baseline World Health Organization scores, age, comorbidities, vaccination status, COVID-19 variant, or geography,” study investigator Mitchell Steiner, MD, chairman, president, and CEO of Veru, said in a news release.
The company has submitted an emergency use authorization request to the U.S. Food and Drug Administration to use sabizabulin to treat COVID-19.
The analysis was published online in NEJM Evidence.
Sabizabulin, originally developed to treat metastatic castration-resistant prostate cancer, is a novel, investigational, oral microtubule disruptor with dual antiviral and anti-inflammatory activities. Given the drug’s mechanism, researchers at Veru thought that sabizabulin could help treat lung inflammation in patients with COVID-19 as well.
Findings of the interim analysis are based on 150 adults hospitalized with moderate to severe COVID-19 at high risk for acute respiratory distress syndrome and death. The patients were randomly allocated to receive 9 mg oral sabizabulin (n = 98) or placebo (n = 52) once daily for up to 21 days.
Overall, the mortality rate was 20.2% in the sabizabulin group vs. 45.1% in the placebo group. Compared with placebo, treatment with sabizabulin led to a 24.9–percentage point absolute reduction and a 55.2% relative reduction in death (odds ratio, 3.23; P = .0042).
The key secondary endpoint of mortality through day 29 also favored sabizabulin over placebo, with a mortality rate of 17% vs. 35.3%. In this scenario, treatment with sabizabulin resulted in an absolute reduction in deaths of 18.3 percentage points and a relative reduction of 51.8%.
Sabizabulin led to a significant 43% relative reduction in ICU days, a 49% relative reduction in days on mechanical ventilation, and a 26% relative reduction in days in the hospital, compared with placebo.
Adverse and serious adverse events were also lower in the sabizabulin group (61.5%) than the placebo group (78.3%).
The data are “pretty impressive and in a group of patients that we really have limited things to offer,” Aaron Glatt, MD, a spokesperson for the Infectious Diseases Society of America and chief of infectious diseases and hospital epidemiologist at Mount Sinai South Nassau in Oceanside, N.Y., said in an interview. “This is an interim analysis and obviously we’d like to see more data, but it certainly is something that is novel and quite interesting.”
David Boulware, MD, MPH, an infectious disease expert at the University of Minnesota, Minneapolis, told the New York Times that the large number of deaths in the placebo group seemed “rather high” and that the final analysis might reveal a more modest benefit for sabizabulin.
“I would be skeptical” that the reduced risk for death remains 55%, he noted.
The study was funded by Veru Pharmaceuticals. Several authors are employed by the company or have financial relationships with the company.
A version of this article first appeared on Medscape.com.
, an interim analysis of a phase 3 placebo-controlled trial found.
Sabizabulin treatment consistently and significantly reduced deaths across patient subgroups “regardless of standard of care treatment received, baseline World Health Organization scores, age, comorbidities, vaccination status, COVID-19 variant, or geography,” study investigator Mitchell Steiner, MD, chairman, president, and CEO of Veru, said in a news release.
The company has submitted an emergency use authorization request to the U.S. Food and Drug Administration to use sabizabulin to treat COVID-19.
The analysis was published online in NEJM Evidence.
Sabizabulin, originally developed to treat metastatic castration-resistant prostate cancer, is a novel, investigational, oral microtubule disruptor with dual antiviral and anti-inflammatory activities. Given the drug’s mechanism, researchers at Veru thought that sabizabulin could help treat lung inflammation in patients with COVID-19 as well.
Findings of the interim analysis are based on 150 adults hospitalized with moderate to severe COVID-19 at high risk for acute respiratory distress syndrome and death. The patients were randomly allocated to receive 9 mg oral sabizabulin (n = 98) or placebo (n = 52) once daily for up to 21 days.
Overall, the mortality rate was 20.2% in the sabizabulin group vs. 45.1% in the placebo group. Compared with placebo, treatment with sabizabulin led to a 24.9–percentage point absolute reduction and a 55.2% relative reduction in death (odds ratio, 3.23; P = .0042).
The key secondary endpoint of mortality through day 29 also favored sabizabulin over placebo, with a mortality rate of 17% vs. 35.3%. In this scenario, treatment with sabizabulin resulted in an absolute reduction in deaths of 18.3 percentage points and a relative reduction of 51.8%.
Sabizabulin led to a significant 43% relative reduction in ICU days, a 49% relative reduction in days on mechanical ventilation, and a 26% relative reduction in days in the hospital, compared with placebo.
Adverse and serious adverse events were also lower in the sabizabulin group (61.5%) than the placebo group (78.3%).
The data are “pretty impressive and in a group of patients that we really have limited things to offer,” Aaron Glatt, MD, a spokesperson for the Infectious Diseases Society of America and chief of infectious diseases and hospital epidemiologist at Mount Sinai South Nassau in Oceanside, N.Y., said in an interview. “This is an interim analysis and obviously we’d like to see more data, but it certainly is something that is novel and quite interesting.”
David Boulware, MD, MPH, an infectious disease expert at the University of Minnesota, Minneapolis, told the New York Times that the large number of deaths in the placebo group seemed “rather high” and that the final analysis might reveal a more modest benefit for sabizabulin.
“I would be skeptical” that the reduced risk for death remains 55%, he noted.
The study was funded by Veru Pharmaceuticals. Several authors are employed by the company or have financial relationships with the company.
A version of this article first appeared on Medscape.com.
FROM NEJM EVIDENCE
Select patients with breast cancer may skip RT after lumpectomy
The women in this trial who skipped radiotherapy, and were treated with breast-conserving surgery followed by endocrine therapy, had an overall survival rate of 97.2%. The local recurrence rate was 2.3%, which was the study’s primary endpoint.
“Women 55 and over, with low-grade luminal A-type breast cancer, following breast conserving surgery and treated with endocrine therapy alone, had a very low rate of local recurrence at 5 years,” commented lead author Timothy Joseph Whelan, MD.
“The prospective and multicenter nature of this study supports that these patients are candidates for the omission of radiotherapy,” said Dr. Whelan, oncology professor and Canada Research Chair in Breast Cancer Research at McMaster University and a radiation oncologist at the Juravinski Cancer Centre, both in Hamilton, Ont.
“Over 300,000 [people] are diagnosed with invasive breast cancer in North America annually, the majority in the United States,” said Dr. Whelan. “We estimate that these results could apply to 10%-15% of them, so about 30,000-40,000 women per year who could avoid the morbidity, the cost, and inconvenience of radiotherapy.”
The results were presented at the annual meeting of the American Society of Clinical Oncology.
Dr. Whelan explained that adjuvant radiation therapy is generally prescribed following breast conservation therapy to lower the risk of local recurrence, but the treatment is also associated with acute and late toxicity. In addition, it can incur high costs and inconvenience for the patient.
Previous studies have found that among women older than 60 with low-grade, luminal A-type breast cancer who received only breast-conserving surgery, there was a low rate of local recurrence. In women aged older than 70 years, the risk of local recurrence was about 4%-5%.
This latest study focused on patients with breast cancer with a luminal A subtype combined with clinical pathological factors (defined as estrogen receptor ≥ 1%, progesterone receptor > 20%, HER2 negative, and Ki67 ≤ 13.25%).
This was a prospective, multicenter cohort study that included 501 patients aged 55 years and older who had undergone breast-conserving surgery for grade 1-2 T1N0 cancer.
The median patient age was 67, with 442 (88%) older than 75 years. The median tumor size was 1.1 cm.
Median follow-up was 5 years. The cohort was followed every 6 months for the first 2 years and then annually.
The primary outcome was local recurrence defined as time from enrollment to any invasive or noninvasive cancer in the ipsilateral breast, and secondary endpoints included contralateral breast cancer, relapse-free survival based on any recurrence, disease free survival, second cancer or death, and overall survival.
At five years, there were 10 events of local recurrence, for a rate of 2.3%. For secondary outcomes, there were eight events of contralateral breast cancer (1.9%); 12 relapses for a recurrence-free survival rate of 97.3%; 47 disease progression (23 second nonbreast cancers) for a disease-free survival rate of 89.9%; and 13 deaths, including 1 from breast cancer, for an overall survival of 97.2%.
Confirms earlier data
Penny R. Anderson, MD, professor in the department of radiation oncology at Fox Chase Cancer Center, Philadelphia, commented that this was an “extremely well-designed and important study.
“It has identified a specific subset of patients to be appropriate candidates for consideration of omission of adjuvant breast radiation therapy after breast-conserving surgery,” she added.
Although previously published trials have helped identify certain patient groups who have a low risk of local recurrence – and therefore, for whom it may be appropriate to omit radiation – they have been based on the traditional clinical and pathologic factors of tumor size, margin status, receptor status, and patient age.
“This LUMINA trial utilizes the molecular-defined intrinsic subtype of luminal A breast cancer to provide additional prognostic information,” she said. “This finding certainly suggests that this group of patients are ideal candidates for the omission of radiation, and that this should be discussed with these patients as a potential option in their treatment management.”
Overall, this trial is a “significant addition and a very relevant contribution to the literature demonstrating that adjuvant breast radiation may safely be omitted in this particular subgroup of breast cancer patients,” she said.
Unanswered questions
Commenting on the study, Julie Gralow, MD, chief medical officer and executive vice president of ASCO, told this news organization that she thinks the take-home message is that there is “clearly a population of early-stage breast cancer [patients] who after lumpectomy do not benefit from radiation.”
“I think where there will be discussion will be what is the optimal way of identifying that group,” she said, noting that in this study the patients were screened for Ki67, a marker of proliferation.
Testing for Ki67 is not the standard of care, Dr. Gralow pointed out, and there is also a problem with reproducibility since “every lab does it somewhat differently, because it is not a standard pathology approach.”
There are now many unanswered questions, she noted. “Do we need that central testing of Ki67? Do we need to develop guidelines for how to do this? Is this better than if you’ve already run an Oncotype or a MammaPrint test to see if the patient needs chemo, then would that suffice? That is where the discussion will be. We can reduce the number of patients who need radiation without an increase in local regional recurrence.”
In terms of clinical practice, Dr. Gralow explained that there are already some data supporting the omission of radiation therapy in an older population with ER-positive small low-grade tumors, and this has become a standard clinical practice. “It’s not based on solid data, but based on an accumulation of retrospective analyses,” she said. “So we have already been doing it for an older population. This would bring down the age group, and it would better define it, and test it prospectively.”
Limitations to note
Also commenting on the study, Deborah Axelrod, MD, director of clinical breast surgery at New York University Langone’s Perlmutter Cancer Center, explained that, in the last decade, knowledge about the behavior of breast cancers based on molecular subtyping has greatly increased. “Results of studies such as this have given us information on which cancers need more treatment and for which cancers we can de-escalate treatment,” she said. “Refining this more, it’s about reducing the morbidity and improving quality of life without compromising the oncological outcome.”
She noted that a big strength of this LUMINA study is that it is prospective and multicenter. “It has been supported by other past studies as well and will define for which patients with newly treated breast cancers can we omit radiation, which has been the standard of care,” said Dr. Axelrod. “It is based on the age and biology of breast cancer in defining which patient can forgo radiation and showed a low risk of recurrence in a specific population of women with a favorable breast cancer profile”
There were limitations to the study. “There is a 5-year follow-up and local recurrence for ER-positive cancers continues to rise after 5 years, so longer-term follow-up will be important,” she said. Also, she pointed out that it is a single-arm study so there is no radiation therapy comparison arm.
Other limitations were that the patients were older with smaller tumors, and all were committed to 5 years of endocrine therapy, although compliance with that has not been reported. There may be some older patients who prefer radiation therapy, especially a week of accelerated partial breast irradiation, rather than commit to 5 years of endocrine therapy as mandated in this study.
“Overall, the takeaway message for patients is that the omission of radiation therapy should be considered an option for older women with localized breast cancer with favorable features who receive endocrine therapies,” said Dr. Axelrod.
LUMINA was sponsored by the Canadian Breast Cancer Foundation and the Canadian Cancer Society. Dr. Whelan has reported research funding from Exact Sciences (Inst). Dr. Axelrod and Dr. Anderson reported no disclosures. Dr. Gralow reported relationships with Genentech, AstraZeneca, Hexal, Puma BioTechnology, Roche, Novartis, Seagen, and Genomic Health.
A version of this article first appeared on Medscape.com.
The women in this trial who skipped radiotherapy, and were treated with breast-conserving surgery followed by endocrine therapy, had an overall survival rate of 97.2%. The local recurrence rate was 2.3%, which was the study’s primary endpoint.
“Women 55 and over, with low-grade luminal A-type breast cancer, following breast conserving surgery and treated with endocrine therapy alone, had a very low rate of local recurrence at 5 years,” commented lead author Timothy Joseph Whelan, MD.
“The prospective and multicenter nature of this study supports that these patients are candidates for the omission of radiotherapy,” said Dr. Whelan, oncology professor and Canada Research Chair in Breast Cancer Research at McMaster University and a radiation oncologist at the Juravinski Cancer Centre, both in Hamilton, Ont.
“Over 300,000 [people] are diagnosed with invasive breast cancer in North America annually, the majority in the United States,” said Dr. Whelan. “We estimate that these results could apply to 10%-15% of them, so about 30,000-40,000 women per year who could avoid the morbidity, the cost, and inconvenience of radiotherapy.”
The results were presented at the annual meeting of the American Society of Clinical Oncology.
Dr. Whelan explained that adjuvant radiation therapy is generally prescribed following breast conservation therapy to lower the risk of local recurrence, but the treatment is also associated with acute and late toxicity. In addition, it can incur high costs and inconvenience for the patient.
Previous studies have found that among women older than 60 with low-grade, luminal A-type breast cancer who received only breast-conserving surgery, there was a low rate of local recurrence. In women aged older than 70 years, the risk of local recurrence was about 4%-5%.
This latest study focused on patients with breast cancer with a luminal A subtype combined with clinical pathological factors (defined as estrogen receptor ≥ 1%, progesterone receptor > 20%, HER2 negative, and Ki67 ≤ 13.25%).
This was a prospective, multicenter cohort study that included 501 patients aged 55 years and older who had undergone breast-conserving surgery for grade 1-2 T1N0 cancer.
The median patient age was 67, with 442 (88%) older than 75 years. The median tumor size was 1.1 cm.
Median follow-up was 5 years. The cohort was followed every 6 months for the first 2 years and then annually.
The primary outcome was local recurrence defined as time from enrollment to any invasive or noninvasive cancer in the ipsilateral breast, and secondary endpoints included contralateral breast cancer, relapse-free survival based on any recurrence, disease free survival, second cancer or death, and overall survival.
At five years, there were 10 events of local recurrence, for a rate of 2.3%. For secondary outcomes, there were eight events of contralateral breast cancer (1.9%); 12 relapses for a recurrence-free survival rate of 97.3%; 47 disease progression (23 second nonbreast cancers) for a disease-free survival rate of 89.9%; and 13 deaths, including 1 from breast cancer, for an overall survival of 97.2%.
Confirms earlier data
Penny R. Anderson, MD, professor in the department of radiation oncology at Fox Chase Cancer Center, Philadelphia, commented that this was an “extremely well-designed and important study.
“It has identified a specific subset of patients to be appropriate candidates for consideration of omission of adjuvant breast radiation therapy after breast-conserving surgery,” she added.
Although previously published trials have helped identify certain patient groups who have a low risk of local recurrence – and therefore, for whom it may be appropriate to omit radiation – they have been based on the traditional clinical and pathologic factors of tumor size, margin status, receptor status, and patient age.
“This LUMINA trial utilizes the molecular-defined intrinsic subtype of luminal A breast cancer to provide additional prognostic information,” she said. “This finding certainly suggests that this group of patients are ideal candidates for the omission of radiation, and that this should be discussed with these patients as a potential option in their treatment management.”
Overall, this trial is a “significant addition and a very relevant contribution to the literature demonstrating that adjuvant breast radiation may safely be omitted in this particular subgroup of breast cancer patients,” she said.
Unanswered questions
Commenting on the study, Julie Gralow, MD, chief medical officer and executive vice president of ASCO, told this news organization that she thinks the take-home message is that there is “clearly a population of early-stage breast cancer [patients] who after lumpectomy do not benefit from radiation.”
“I think where there will be discussion will be what is the optimal way of identifying that group,” she said, noting that in this study the patients were screened for Ki67, a marker of proliferation.
Testing for Ki67 is not the standard of care, Dr. Gralow pointed out, and there is also a problem with reproducibility since “every lab does it somewhat differently, because it is not a standard pathology approach.”
There are now many unanswered questions, she noted. “Do we need that central testing of Ki67? Do we need to develop guidelines for how to do this? Is this better than if you’ve already run an Oncotype or a MammaPrint test to see if the patient needs chemo, then would that suffice? That is where the discussion will be. We can reduce the number of patients who need radiation without an increase in local regional recurrence.”
In terms of clinical practice, Dr. Gralow explained that there are already some data supporting the omission of radiation therapy in an older population with ER-positive small low-grade tumors, and this has become a standard clinical practice. “It’s not based on solid data, but based on an accumulation of retrospective analyses,” she said. “So we have already been doing it for an older population. This would bring down the age group, and it would better define it, and test it prospectively.”
Limitations to note
Also commenting on the study, Deborah Axelrod, MD, director of clinical breast surgery at New York University Langone’s Perlmutter Cancer Center, explained that, in the last decade, knowledge about the behavior of breast cancers based on molecular subtyping has greatly increased. “Results of studies such as this have given us information on which cancers need more treatment and for which cancers we can de-escalate treatment,” she said. “Refining this more, it’s about reducing the morbidity and improving quality of life without compromising the oncological outcome.”
She noted that a big strength of this LUMINA study is that it is prospective and multicenter. “It has been supported by other past studies as well and will define for which patients with newly treated breast cancers can we omit radiation, which has been the standard of care,” said Dr. Axelrod. “It is based on the age and biology of breast cancer in defining which patient can forgo radiation and showed a low risk of recurrence in a specific population of women with a favorable breast cancer profile”
There were limitations to the study. “There is a 5-year follow-up and local recurrence for ER-positive cancers continues to rise after 5 years, so longer-term follow-up will be important,” she said. Also, she pointed out that it is a single-arm study so there is no radiation therapy comparison arm.
Other limitations were that the patients were older with smaller tumors, and all were committed to 5 years of endocrine therapy, although compliance with that has not been reported. There may be some older patients who prefer radiation therapy, especially a week of accelerated partial breast irradiation, rather than commit to 5 years of endocrine therapy as mandated in this study.
“Overall, the takeaway message for patients is that the omission of radiation therapy should be considered an option for older women with localized breast cancer with favorable features who receive endocrine therapies,” said Dr. Axelrod.
LUMINA was sponsored by the Canadian Breast Cancer Foundation and the Canadian Cancer Society. Dr. Whelan has reported research funding from Exact Sciences (Inst). Dr. Axelrod and Dr. Anderson reported no disclosures. Dr. Gralow reported relationships with Genentech, AstraZeneca, Hexal, Puma BioTechnology, Roche, Novartis, Seagen, and Genomic Health.
A version of this article first appeared on Medscape.com.
The women in this trial who skipped radiotherapy, and were treated with breast-conserving surgery followed by endocrine therapy, had an overall survival rate of 97.2%. The local recurrence rate was 2.3%, which was the study’s primary endpoint.
“Women 55 and over, with low-grade luminal A-type breast cancer, following breast conserving surgery and treated with endocrine therapy alone, had a very low rate of local recurrence at 5 years,” commented lead author Timothy Joseph Whelan, MD.
“The prospective and multicenter nature of this study supports that these patients are candidates for the omission of radiotherapy,” said Dr. Whelan, oncology professor and Canada Research Chair in Breast Cancer Research at McMaster University and a radiation oncologist at the Juravinski Cancer Centre, both in Hamilton, Ont.
“Over 300,000 [people] are diagnosed with invasive breast cancer in North America annually, the majority in the United States,” said Dr. Whelan. “We estimate that these results could apply to 10%-15% of them, so about 30,000-40,000 women per year who could avoid the morbidity, the cost, and inconvenience of radiotherapy.”
The results were presented at the annual meeting of the American Society of Clinical Oncology.
Dr. Whelan explained that adjuvant radiation therapy is generally prescribed following breast conservation therapy to lower the risk of local recurrence, but the treatment is also associated with acute and late toxicity. In addition, it can incur high costs and inconvenience for the patient.
Previous studies have found that among women older than 60 with low-grade, luminal A-type breast cancer who received only breast-conserving surgery, there was a low rate of local recurrence. In women aged older than 70 years, the risk of local recurrence was about 4%-5%.
This latest study focused on patients with breast cancer with a luminal A subtype combined with clinical pathological factors (defined as estrogen receptor ≥ 1%, progesterone receptor > 20%, HER2 negative, and Ki67 ≤ 13.25%).
This was a prospective, multicenter cohort study that included 501 patients aged 55 years and older who had undergone breast-conserving surgery for grade 1-2 T1N0 cancer.
The median patient age was 67, with 442 (88%) older than 75 years. The median tumor size was 1.1 cm.
Median follow-up was 5 years. The cohort was followed every 6 months for the first 2 years and then annually.
The primary outcome was local recurrence defined as time from enrollment to any invasive or noninvasive cancer in the ipsilateral breast, and secondary endpoints included contralateral breast cancer, relapse-free survival based on any recurrence, disease free survival, second cancer or death, and overall survival.
At five years, there were 10 events of local recurrence, for a rate of 2.3%. For secondary outcomes, there were eight events of contralateral breast cancer (1.9%); 12 relapses for a recurrence-free survival rate of 97.3%; 47 disease progression (23 second nonbreast cancers) for a disease-free survival rate of 89.9%; and 13 deaths, including 1 from breast cancer, for an overall survival of 97.2%.
Confirms earlier data
Penny R. Anderson, MD, professor in the department of radiation oncology at Fox Chase Cancer Center, Philadelphia, commented that this was an “extremely well-designed and important study.
“It has identified a specific subset of patients to be appropriate candidates for consideration of omission of adjuvant breast radiation therapy after breast-conserving surgery,” she added.
Although previously published trials have helped identify certain patient groups who have a low risk of local recurrence – and therefore, for whom it may be appropriate to omit radiation – they have been based on the traditional clinical and pathologic factors of tumor size, margin status, receptor status, and patient age.
“This LUMINA trial utilizes the molecular-defined intrinsic subtype of luminal A breast cancer to provide additional prognostic information,” she said. “This finding certainly suggests that this group of patients are ideal candidates for the omission of radiation, and that this should be discussed with these patients as a potential option in their treatment management.”
Overall, this trial is a “significant addition and a very relevant contribution to the literature demonstrating that adjuvant breast radiation may safely be omitted in this particular subgroup of breast cancer patients,” she said.
Unanswered questions
Commenting on the study, Julie Gralow, MD, chief medical officer and executive vice president of ASCO, told this news organization that she thinks the take-home message is that there is “clearly a population of early-stage breast cancer [patients] who after lumpectomy do not benefit from radiation.”
“I think where there will be discussion will be what is the optimal way of identifying that group,” she said, noting that in this study the patients were screened for Ki67, a marker of proliferation.
Testing for Ki67 is not the standard of care, Dr. Gralow pointed out, and there is also a problem with reproducibility since “every lab does it somewhat differently, because it is not a standard pathology approach.”
There are now many unanswered questions, she noted. “Do we need that central testing of Ki67? Do we need to develop guidelines for how to do this? Is this better than if you’ve already run an Oncotype or a MammaPrint test to see if the patient needs chemo, then would that suffice? That is where the discussion will be. We can reduce the number of patients who need radiation without an increase in local regional recurrence.”
In terms of clinical practice, Dr. Gralow explained that there are already some data supporting the omission of radiation therapy in an older population with ER-positive small low-grade tumors, and this has become a standard clinical practice. “It’s not based on solid data, but based on an accumulation of retrospective analyses,” she said. “So we have already been doing it for an older population. This would bring down the age group, and it would better define it, and test it prospectively.”
Limitations to note
Also commenting on the study, Deborah Axelrod, MD, director of clinical breast surgery at New York University Langone’s Perlmutter Cancer Center, explained that, in the last decade, knowledge about the behavior of breast cancers based on molecular subtyping has greatly increased. “Results of studies such as this have given us information on which cancers need more treatment and for which cancers we can de-escalate treatment,” she said. “Refining this more, it’s about reducing the morbidity and improving quality of life without compromising the oncological outcome.”
She noted that a big strength of this LUMINA study is that it is prospective and multicenter. “It has been supported by other past studies as well and will define for which patients with newly treated breast cancers can we omit radiation, which has been the standard of care,” said Dr. Axelrod. “It is based on the age and biology of breast cancer in defining which patient can forgo radiation and showed a low risk of recurrence in a specific population of women with a favorable breast cancer profile”
There were limitations to the study. “There is a 5-year follow-up and local recurrence for ER-positive cancers continues to rise after 5 years, so longer-term follow-up will be important,” she said. Also, she pointed out that it is a single-arm study so there is no radiation therapy comparison arm.
Other limitations were that the patients were older with smaller tumors, and all were committed to 5 years of endocrine therapy, although compliance with that has not been reported. There may be some older patients who prefer radiation therapy, especially a week of accelerated partial breast irradiation, rather than commit to 5 years of endocrine therapy as mandated in this study.
“Overall, the takeaway message for patients is that the omission of radiation therapy should be considered an option for older women with localized breast cancer with favorable features who receive endocrine therapies,” said Dr. Axelrod.
LUMINA was sponsored by the Canadian Breast Cancer Foundation and the Canadian Cancer Society. Dr. Whelan has reported research funding from Exact Sciences (Inst). Dr. Axelrod and Dr. Anderson reported no disclosures. Dr. Gralow reported relationships with Genentech, AstraZeneca, Hexal, Puma BioTechnology, Roche, Novartis, Seagen, and Genomic Health.
A version of this article first appeared on Medscape.com.
FROM ASCO 2022
Sleep-deprived physicians less empathetic to patient pain?
new research suggests.
In the first of two studies, resident physicians were presented with two hypothetical scenarios involving a patient who complains of pain. They were asked about their likelihood of prescribing pain medication. The test was given to one group of residents who were just starting their day and to another group who were at the end of their night shift after being on call for 26 hours.
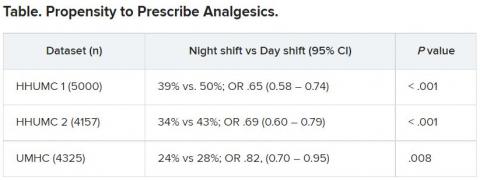
Results showed that the night shift residents were less likely than their daytime counterparts to say they would prescribe pain medication to the patients.
In further analysis of discharge notes from more than 13,000 electronic records of patients presenting with pain complaints at hospitals in Israel and the United States, the likelihood of an analgesic being prescribed during the night shift was 11% lower in Israel and 9% lower in the United States, compared with the day shift.
“Pain management is a major challenge, and a doctor’s perception of a patient’s subjective pain is susceptible to bias,” coinvestigator David Gozal, MD, the Marie M. and Harry L. Smith Endowed Chair of Child Health, University of Missouri–Columbia, said in a press release.
“This study demonstrated that night shift work is an important and previously unrecognized source of bias in pain management, likely stemming from impaired perception of pain,” Dr. Gozal added.
The findings were published online in the Proceedings of the National Academy of Sciences.
‘Directional’ differences
Senior investigator Alex Gileles-Hillel, MD, senior pediatric pulmonologist and sleep researcher at Hadassah University Medical Center, Jerusalem, said in an interview that physicians must make “complex assessments of patients’ subjective pain experience” – and the “subjective nature of pain management decisions can give rise to various biases.”
Dr. Gileles-Hillel has previously researched the cognitive toll of night shift work on physicians.
“It’s pretty established, for example, not to drive when sleep deprived because cognition is impaired,” he said. The current study explored whether sleep deprivation could affect areas other than cognition, including emotions and empathy.
The researchers used “two complementary approaches.” First, they administered tests to measure empathy and pain management decisions in 67 resident physicians at Hadassah Medical Centers either following a 26-hour night shift that began at 8:00 a.m. the day before (n = 36) or immediately before starting the workday (n = 31).
There were no significant differences in demographic, sleep, or burnout measures between the two groups, except that night shift physicians had slept less than those in the daytime group (2.93 vs. 5.96 hours).
Participants completed two tasks. In the empathy-for-pain task, they rated their emotional reactions to pictures of individuals in pain. In the empathy accuracy task, they were asked to assess the feelings of videotaped individuals telling emotional stories.
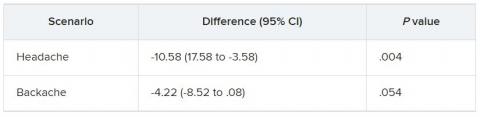
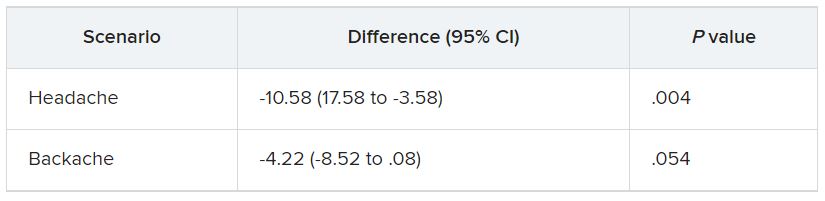
They were then presented with two clinical scenarios: a female patient with a headache and a male patient with a backache. Following that, they were asked to assess the magnitude of the patients’ pain and how likely they would be to prescribe pain medication.
In the empathy-for-pain task, physicians’ empathy scores were significantly lower in the night shift group than in the day group (difference, –0.83; 95% CI, –1.55 to –0.10; P = .026). There were no significant differences between the groups in the empathy accuracy task.
In both scenarios, physicians in the night shift group assessed the patient’s pain as weaker in comparison with physicians in the day group. There was a statistically significant difference in the headache scenario but not the backache scenario.
In the headache scenario, the propensity of the physicians to prescribe analgesics was “directionally lower” but did not reach statistical significance. In the backache scenario, there was no significant difference between the groups’ prescribing propensities.
In both scenarios, pain assessment was positively correlated with the propensity to prescribe analgesics.
Despite the lack of statistical significance, the findings “documented a negative effect of night shift work on physician empathy for pain and a positive association between physician assessment of patient pain and the propensity to prescribe analgesics,” the investigators wrote.
Need for naps?
The researchers then analyzed analgesic prescription patterns drawn from three datasets of discharge notes of patients presenting to the emergency department with pain complaints (n = 13,482) at two branches of Hadassah-Hebrew University Medical Center and the University of Missouri Health Center.
The researchers collected data, including discharge time, medications patients were prescribed upon discharge, and patients’ subjective pain rating on a scale of 0-10 on a visual analogue scale (VAS).
Although patients’ VAS scores did not differ with respect to time or shift, patients were discharged with significantly less prescribed analgesics during the night shift in comparison with the day shift.
No similar differences in prescriptions between night shifts and day shifts were found for nonanalgesic medications, such as for diabetes or blood pressure. This suggests “the effect was specific to pain,” Dr. Gileles-Hillel said.
The pattern remained significant after controlling for potential confounders, including patient and physician variables and emergency department characteristics.
In addition, patients seen during night shifts received fewer analgesics, particularly opioids, than recommended by the World Health Organization for pain management.
“The first study enabled us to measure empathy for pain directly and examine our hypothesis in a controlled environment, while the second enabled us to test the implications by examining real-life pain management decisions,” Dr. Gileles-Hillel said.
“Physicians need to be aware of this,” he noted. “I try to be aware when I’m taking calls [at night] that I’m less empathetic to others and I might be more brief or angry with others.”
On a “house management level, perhaps institutions should try to schedule naps either before or during overnight call. A nap might give a boost and reboot not only to cognitive but also to emotional resources,” Dr. Gileles-Hillel added.
Compromised safety
In a comment, Eti Ben Simon, PhD, a postdoctoral fellow at the Center for Human Sleep Science, University of California, Berkeley, called the study “an important contribution to a growing list of studies that reveal how long night shifts reduce overall safety” for both patients and clinicians.
“It’s time to abandon the notion that the human brain can function as normal after being deprived of sleep for 24 hours,” said Dr. Ben Simon, who was not involved with the research.
“This is especially true in medicine, where we trust others to take care of us and feel our pain. These functions are simply not possible without adequate sleep,” she added.
Also commenting, Kannan Ramar, MD, president of the American Academy of Sleep Medicine, suggested that being cognizant of these findings “may help providers to mitigate this bias” of underprescribing pain medications when treating their patients.
Dr. Ramar, who is also a critical care specialist, pulmonologist, and sleep medicine specialist at Mayo Clinic, Rochester, Minn., was not involved with the research.
He noted that “further studies that systematically evaluate this further in a prospective and blinded way will be important.”
The research was supported in part by grants from the Israel Science Foundation, Joy Ventures, the Recanati Fund at the Jerusalem School of Business at the Hebrew University, and a fellowship from the Azrieli Foundation and received grant support to various investigators from the NIH, the Leda J. Sears Foundation, and the University of Missouri. The investigators, Ramar, and Ben Simon have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests.
In the first of two studies, resident physicians were presented with two hypothetical scenarios involving a patient who complains of pain. They were asked about their likelihood of prescribing pain medication. The test was given to one group of residents who were just starting their day and to another group who were at the end of their night shift after being on call for 26 hours.
Results showed that the night shift residents were less likely than their daytime counterparts to say they would prescribe pain medication to the patients.
In further analysis of discharge notes from more than 13,000 electronic records of patients presenting with pain complaints at hospitals in Israel and the United States, the likelihood of an analgesic being prescribed during the night shift was 11% lower in Israel and 9% lower in the United States, compared with the day shift.
“Pain management is a major challenge, and a doctor’s perception of a patient’s subjective pain is susceptible to bias,” coinvestigator David Gozal, MD, the Marie M. and Harry L. Smith Endowed Chair of Child Health, University of Missouri–Columbia, said in a press release.
“This study demonstrated that night shift work is an important and previously unrecognized source of bias in pain management, likely stemming from impaired perception of pain,” Dr. Gozal added.
The findings were published online in the Proceedings of the National Academy of Sciences.
‘Directional’ differences
Senior investigator Alex Gileles-Hillel, MD, senior pediatric pulmonologist and sleep researcher at Hadassah University Medical Center, Jerusalem, said in an interview that physicians must make “complex assessments of patients’ subjective pain experience” – and the “subjective nature of pain management decisions can give rise to various biases.”
Dr. Gileles-Hillel has previously researched the cognitive toll of night shift work on physicians.
“It’s pretty established, for example, not to drive when sleep deprived because cognition is impaired,” he said. The current study explored whether sleep deprivation could affect areas other than cognition, including emotions and empathy.
The researchers used “two complementary approaches.” First, they administered tests to measure empathy and pain management decisions in 67 resident physicians at Hadassah Medical Centers either following a 26-hour night shift that began at 8:00 a.m. the day before (n = 36) or immediately before starting the workday (n = 31).
There were no significant differences in demographic, sleep, or burnout measures between the two groups, except that night shift physicians had slept less than those in the daytime group (2.93 vs. 5.96 hours).
Participants completed two tasks. In the empathy-for-pain task, they rated their emotional reactions to pictures of individuals in pain. In the empathy accuracy task, they were asked to assess the feelings of videotaped individuals telling emotional stories.
They were then presented with two clinical scenarios: a female patient with a headache and a male patient with a backache. Following that, they were asked to assess the magnitude of the patients’ pain and how likely they would be to prescribe pain medication.
In the empathy-for-pain task, physicians’ empathy scores were significantly lower in the night shift group than in the day group (difference, –0.83; 95% CI, –1.55 to –0.10; P = .026). There were no significant differences between the groups in the empathy accuracy task.
In both scenarios, physicians in the night shift group assessed the patient’s pain as weaker in comparison with physicians in the day group. There was a statistically significant difference in the headache scenario but not the backache scenario.
In the headache scenario, the propensity of the physicians to prescribe analgesics was “directionally lower” but did not reach statistical significance. In the backache scenario, there was no significant difference between the groups’ prescribing propensities.
In both scenarios, pain assessment was positively correlated with the propensity to prescribe analgesics.
Despite the lack of statistical significance, the findings “documented a negative effect of night shift work on physician empathy for pain and a positive association between physician assessment of patient pain and the propensity to prescribe analgesics,” the investigators wrote.
Need for naps?
The researchers then analyzed analgesic prescription patterns drawn from three datasets of discharge notes of patients presenting to the emergency department with pain complaints (n = 13,482) at two branches of Hadassah-Hebrew University Medical Center and the University of Missouri Health Center.
The researchers collected data, including discharge time, medications patients were prescribed upon discharge, and patients’ subjective pain rating on a scale of 0-10 on a visual analogue scale (VAS).
Although patients’ VAS scores did not differ with respect to time or shift, patients were discharged with significantly less prescribed analgesics during the night shift in comparison with the day shift.
No similar differences in prescriptions between night shifts and day shifts were found for nonanalgesic medications, such as for diabetes or blood pressure. This suggests “the effect was specific to pain,” Dr. Gileles-Hillel said.
The pattern remained significant after controlling for potential confounders, including patient and physician variables and emergency department characteristics.
In addition, patients seen during night shifts received fewer analgesics, particularly opioids, than recommended by the World Health Organization for pain management.
“The first study enabled us to measure empathy for pain directly and examine our hypothesis in a controlled environment, while the second enabled us to test the implications by examining real-life pain management decisions,” Dr. Gileles-Hillel said.
“Physicians need to be aware of this,” he noted. “I try to be aware when I’m taking calls [at night] that I’m less empathetic to others and I might be more brief or angry with others.”
On a “house management level, perhaps institutions should try to schedule naps either before or during overnight call. A nap might give a boost and reboot not only to cognitive but also to emotional resources,” Dr. Gileles-Hillel added.
Compromised safety
In a comment, Eti Ben Simon, PhD, a postdoctoral fellow at the Center for Human Sleep Science, University of California, Berkeley, called the study “an important contribution to a growing list of studies that reveal how long night shifts reduce overall safety” for both patients and clinicians.
“It’s time to abandon the notion that the human brain can function as normal after being deprived of sleep for 24 hours,” said Dr. Ben Simon, who was not involved with the research.
“This is especially true in medicine, where we trust others to take care of us and feel our pain. These functions are simply not possible without adequate sleep,” she added.
Also commenting, Kannan Ramar, MD, president of the American Academy of Sleep Medicine, suggested that being cognizant of these findings “may help providers to mitigate this bias” of underprescribing pain medications when treating their patients.
Dr. Ramar, who is also a critical care specialist, pulmonologist, and sleep medicine specialist at Mayo Clinic, Rochester, Minn., was not involved with the research.
He noted that “further studies that systematically evaluate this further in a prospective and blinded way will be important.”
The research was supported in part by grants from the Israel Science Foundation, Joy Ventures, the Recanati Fund at the Jerusalem School of Business at the Hebrew University, and a fellowship from the Azrieli Foundation and received grant support to various investigators from the NIH, the Leda J. Sears Foundation, and the University of Missouri. The investigators, Ramar, and Ben Simon have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests.
In the first of two studies, resident physicians were presented with two hypothetical scenarios involving a patient who complains of pain. They were asked about their likelihood of prescribing pain medication. The test was given to one group of residents who were just starting their day and to another group who were at the end of their night shift after being on call for 26 hours.
Results showed that the night shift residents were less likely than their daytime counterparts to say they would prescribe pain medication to the patients.
In further analysis of discharge notes from more than 13,000 electronic records of patients presenting with pain complaints at hospitals in Israel and the United States, the likelihood of an analgesic being prescribed during the night shift was 11% lower in Israel and 9% lower in the United States, compared with the day shift.
“Pain management is a major challenge, and a doctor’s perception of a patient’s subjective pain is susceptible to bias,” coinvestigator David Gozal, MD, the Marie M. and Harry L. Smith Endowed Chair of Child Health, University of Missouri–Columbia, said in a press release.
“This study demonstrated that night shift work is an important and previously unrecognized source of bias in pain management, likely stemming from impaired perception of pain,” Dr. Gozal added.
The findings were published online in the Proceedings of the National Academy of Sciences.
‘Directional’ differences
Senior investigator Alex Gileles-Hillel, MD, senior pediatric pulmonologist and sleep researcher at Hadassah University Medical Center, Jerusalem, said in an interview that physicians must make “complex assessments of patients’ subjective pain experience” – and the “subjective nature of pain management decisions can give rise to various biases.”
Dr. Gileles-Hillel has previously researched the cognitive toll of night shift work on physicians.
“It’s pretty established, for example, not to drive when sleep deprived because cognition is impaired,” he said. The current study explored whether sleep deprivation could affect areas other than cognition, including emotions and empathy.
The researchers used “two complementary approaches.” First, they administered tests to measure empathy and pain management decisions in 67 resident physicians at Hadassah Medical Centers either following a 26-hour night shift that began at 8:00 a.m. the day before (n = 36) or immediately before starting the workday (n = 31).
There were no significant differences in demographic, sleep, or burnout measures between the two groups, except that night shift physicians had slept less than those in the daytime group (2.93 vs. 5.96 hours).
Participants completed two tasks. In the empathy-for-pain task, they rated their emotional reactions to pictures of individuals in pain. In the empathy accuracy task, they were asked to assess the feelings of videotaped individuals telling emotional stories.
They were then presented with two clinical scenarios: a female patient with a headache and a male patient with a backache. Following that, they were asked to assess the magnitude of the patients’ pain and how likely they would be to prescribe pain medication.
In the empathy-for-pain task, physicians’ empathy scores were significantly lower in the night shift group than in the day group (difference, –0.83; 95% CI, –1.55 to –0.10; P = .026). There were no significant differences between the groups in the empathy accuracy task.
In both scenarios, physicians in the night shift group assessed the patient’s pain as weaker in comparison with physicians in the day group. There was a statistically significant difference in the headache scenario but not the backache scenario.
In the headache scenario, the propensity of the physicians to prescribe analgesics was “directionally lower” but did not reach statistical significance. In the backache scenario, there was no significant difference between the groups’ prescribing propensities.
In both scenarios, pain assessment was positively correlated with the propensity to prescribe analgesics.
Despite the lack of statistical significance, the findings “documented a negative effect of night shift work on physician empathy for pain and a positive association between physician assessment of patient pain and the propensity to prescribe analgesics,” the investigators wrote.
Need for naps?
The researchers then analyzed analgesic prescription patterns drawn from three datasets of discharge notes of patients presenting to the emergency department with pain complaints (n = 13,482) at two branches of Hadassah-Hebrew University Medical Center and the University of Missouri Health Center.
The researchers collected data, including discharge time, medications patients were prescribed upon discharge, and patients’ subjective pain rating on a scale of 0-10 on a visual analogue scale (VAS).
Although patients’ VAS scores did not differ with respect to time or shift, patients were discharged with significantly less prescribed analgesics during the night shift in comparison with the day shift.
No similar differences in prescriptions between night shifts and day shifts were found for nonanalgesic medications, such as for diabetes or blood pressure. This suggests “the effect was specific to pain,” Dr. Gileles-Hillel said.
The pattern remained significant after controlling for potential confounders, including patient and physician variables and emergency department characteristics.
In addition, patients seen during night shifts received fewer analgesics, particularly opioids, than recommended by the World Health Organization for pain management.
“The first study enabled us to measure empathy for pain directly and examine our hypothesis in a controlled environment, while the second enabled us to test the implications by examining real-life pain management decisions,” Dr. Gileles-Hillel said.
“Physicians need to be aware of this,” he noted. “I try to be aware when I’m taking calls [at night] that I’m less empathetic to others and I might be more brief or angry with others.”
On a “house management level, perhaps institutions should try to schedule naps either before or during overnight call. A nap might give a boost and reboot not only to cognitive but also to emotional resources,” Dr. Gileles-Hillel added.
Compromised safety
In a comment, Eti Ben Simon, PhD, a postdoctoral fellow at the Center for Human Sleep Science, University of California, Berkeley, called the study “an important contribution to a growing list of studies that reveal how long night shifts reduce overall safety” for both patients and clinicians.
“It’s time to abandon the notion that the human brain can function as normal after being deprived of sleep for 24 hours,” said Dr. Ben Simon, who was not involved with the research.
“This is especially true in medicine, where we trust others to take care of us and feel our pain. These functions are simply not possible without adequate sleep,” she added.
Also commenting, Kannan Ramar, MD, president of the American Academy of Sleep Medicine, suggested that being cognizant of these findings “may help providers to mitigate this bias” of underprescribing pain medications when treating their patients.
Dr. Ramar, who is also a critical care specialist, pulmonologist, and sleep medicine specialist at Mayo Clinic, Rochester, Minn., was not involved with the research.
He noted that “further studies that systematically evaluate this further in a prospective and blinded way will be important.”
The research was supported in part by grants from the Israel Science Foundation, Joy Ventures, the Recanati Fund at the Jerusalem School of Business at the Hebrew University, and a fellowship from the Azrieli Foundation and received grant support to various investigators from the NIH, the Leda J. Sears Foundation, and the University of Missouri. The investigators, Ramar, and Ben Simon have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE PROCEEDINGS OF THE NATIONAL ACADEMY OF SCIENCES
Study confirms BRCA1 and BRCA2 linked to seven cancers
from prior analyses showing associations with breast, ovarian, prostate, and pancreatic cancers. The finding, published in JAMA Oncology suggests a possible broader clinical relevance for BRCA1 and BRCA2 genetic testing.
Pathogenic variants in BRCA1 were found to be associated with biliary tract cancer, in BRCA2 with esophageal cancer, and in BRCA1/2 with gastric cancer.
“The results suggest the range of cancer types associated with pathogenic variants in BRCA1 and BRCA2 is likely broader than that determined from previous analysis of largely European ancestry cohorts,” wrote authors who were led by Yukihide Momozawa, DVM, PhD, RIKEN Center for Integrative Medical Sciences, Japan.
“These risk association findings, together with our analysis of an association with family history of cancer and clinical phenotypes, are relevant for developing and adapting guidelines about genetic testing, treatment options, and treatability with PARP [poly adenosine diphosphate-ribose polymerase] inhibitors for each cancer type,” the authors wrote.
Dr. Momozawa and associates conducted a large-scale sequencing study across 14 common cancer types in 63,828 patients (mean age 64 years, 42% female) and 37,086 controls on data drawn from a Japanese nationwide biobank between April 2003 and March 2018. They estimated the risk of each cancer type and determined clinical characteristics associated with pathogenic variant carrier status, while also investigating the utility of family history in detecting patients with pathogenic variants.
Three hundred fifteen pathogenic variants were identified. An odds ratios of greater than 4.0 (with P < 1 × 10−4 as the threshold of significance) for the pathogenic variants were found for biliary tract cancer (OR, 17.4; 95% confidence interval, 5.8-51.9) in BRCA1, esophageal cancer (OR, 5.6; 95% CI, 2.9-11.0) in BRCA2, and gastric cancer (OR, 5.2; 95% CI, 2.6-10.5) in BRCA1, and (OR, 4.7; 95% CI, 3.1-7.1) in BRCA2. Two other cancer types were found to be associated with BRCA1, and four other cancer types with BRCA2. Enrichment of carrier patients was shown in biliary tract, female breast, ovarian, and prostate cancers in accordance with increased numbers of reported cancer types in relatives.
Male patients with breast cancer had a very high carrier frequency of pathogenic variants in BRCA2 (18.9%), but not BRCA1 (1.89%). Patients with ovarian cancer showed the next highest proportion (BRCA1, 4.86%; BRCA2, 3.42%). Frequency exceeding 1% was seen for several other cancer types (two cancer types for BRCA1, four cancer types for BRCA2). More than one cancer types was identified in 4,128 patients (6.3%). Carrier frequency of pathogenic variants in BRCA1 was 0.44% with one cancer type, 0.85% with two cancer types, and 0.69% with three cancer types. It was 0.97%, 1.40%, and 1.74%, respectively, in BRCA2.
“The results of this large-scale registry-based case-control study suggest that pathogenic variants in BRCA1 and BRCA2 were associated with the risk of seven cancer types. These results indicate broader clinical relevance of BRCA1 and BRCA2 genetic testing,” the authors wrote.
PARP inhibitors were developed based on the mechanism in BRCA1 and BRCA2 of homologous recombination repair defects associated with pathogenic variants. PARP inhibitors have been found to have therapeutic efficacy also in pathogenic variants found to be enriched in prostate and pancreatic cancers. While risk for additional cancer types (for example, biliary tract cancer, cervical cancer, colorectal cancer, endometrial cancer, esophageal cancer, and stomach cancer) has been reported after analyzing family members for the presence of pathogenic variants and performing case-control analyses, evidence for an association with these cancer types has not been considered sufficient for them to be adopted into clinical management guidelines, the authors wrote.
In an interview, Dr. Momozawa said that BRCA1 and BRCA2 genetic testing should be expanded in Japan. “But further studies are needed to reveal how much. If a clinical trial of a PARP inhibitor for these three cancer types reveals its clinical utility, the importance of this expansion will increase.”
Dr. Momozawa and associates state that while their selection of controls without a family history of cancer affects the generalizability of the study results, the estimated cumulative risks were comparable with those based on prospective cohorts, suggesting the study design did not greatly affect the results.
from prior analyses showing associations with breast, ovarian, prostate, and pancreatic cancers. The finding, published in JAMA Oncology suggests a possible broader clinical relevance for BRCA1 and BRCA2 genetic testing.
Pathogenic variants in BRCA1 were found to be associated with biliary tract cancer, in BRCA2 with esophageal cancer, and in BRCA1/2 with gastric cancer.
“The results suggest the range of cancer types associated with pathogenic variants in BRCA1 and BRCA2 is likely broader than that determined from previous analysis of largely European ancestry cohorts,” wrote authors who were led by Yukihide Momozawa, DVM, PhD, RIKEN Center for Integrative Medical Sciences, Japan.
“These risk association findings, together with our analysis of an association with family history of cancer and clinical phenotypes, are relevant for developing and adapting guidelines about genetic testing, treatment options, and treatability with PARP [poly adenosine diphosphate-ribose polymerase] inhibitors for each cancer type,” the authors wrote.
Dr. Momozawa and associates conducted a large-scale sequencing study across 14 common cancer types in 63,828 patients (mean age 64 years, 42% female) and 37,086 controls on data drawn from a Japanese nationwide biobank between April 2003 and March 2018. They estimated the risk of each cancer type and determined clinical characteristics associated with pathogenic variant carrier status, while also investigating the utility of family history in detecting patients with pathogenic variants.
Three hundred fifteen pathogenic variants were identified. An odds ratios of greater than 4.0 (with P < 1 × 10−4 as the threshold of significance) for the pathogenic variants were found for biliary tract cancer (OR, 17.4; 95% confidence interval, 5.8-51.9) in BRCA1, esophageal cancer (OR, 5.6; 95% CI, 2.9-11.0) in BRCA2, and gastric cancer (OR, 5.2; 95% CI, 2.6-10.5) in BRCA1, and (OR, 4.7; 95% CI, 3.1-7.1) in BRCA2. Two other cancer types were found to be associated with BRCA1, and four other cancer types with BRCA2. Enrichment of carrier patients was shown in biliary tract, female breast, ovarian, and prostate cancers in accordance with increased numbers of reported cancer types in relatives.
Male patients with breast cancer had a very high carrier frequency of pathogenic variants in BRCA2 (18.9%), but not BRCA1 (1.89%). Patients with ovarian cancer showed the next highest proportion (BRCA1, 4.86%; BRCA2, 3.42%). Frequency exceeding 1% was seen for several other cancer types (two cancer types for BRCA1, four cancer types for BRCA2). More than one cancer types was identified in 4,128 patients (6.3%). Carrier frequency of pathogenic variants in BRCA1 was 0.44% with one cancer type, 0.85% with two cancer types, and 0.69% with three cancer types. It was 0.97%, 1.40%, and 1.74%, respectively, in BRCA2.
“The results of this large-scale registry-based case-control study suggest that pathogenic variants in BRCA1 and BRCA2 were associated with the risk of seven cancer types. These results indicate broader clinical relevance of BRCA1 and BRCA2 genetic testing,” the authors wrote.
PARP inhibitors were developed based on the mechanism in BRCA1 and BRCA2 of homologous recombination repair defects associated with pathogenic variants. PARP inhibitors have been found to have therapeutic efficacy also in pathogenic variants found to be enriched in prostate and pancreatic cancers. While risk for additional cancer types (for example, biliary tract cancer, cervical cancer, colorectal cancer, endometrial cancer, esophageal cancer, and stomach cancer) has been reported after analyzing family members for the presence of pathogenic variants and performing case-control analyses, evidence for an association with these cancer types has not been considered sufficient for them to be adopted into clinical management guidelines, the authors wrote.
In an interview, Dr. Momozawa said that BRCA1 and BRCA2 genetic testing should be expanded in Japan. “But further studies are needed to reveal how much. If a clinical trial of a PARP inhibitor for these three cancer types reveals its clinical utility, the importance of this expansion will increase.”
Dr. Momozawa and associates state that while their selection of controls without a family history of cancer affects the generalizability of the study results, the estimated cumulative risks were comparable with those based on prospective cohorts, suggesting the study design did not greatly affect the results.
from prior analyses showing associations with breast, ovarian, prostate, and pancreatic cancers. The finding, published in JAMA Oncology suggests a possible broader clinical relevance for BRCA1 and BRCA2 genetic testing.
Pathogenic variants in BRCA1 were found to be associated with biliary tract cancer, in BRCA2 with esophageal cancer, and in BRCA1/2 with gastric cancer.
“The results suggest the range of cancer types associated with pathogenic variants in BRCA1 and BRCA2 is likely broader than that determined from previous analysis of largely European ancestry cohorts,” wrote authors who were led by Yukihide Momozawa, DVM, PhD, RIKEN Center for Integrative Medical Sciences, Japan.
“These risk association findings, together with our analysis of an association with family history of cancer and clinical phenotypes, are relevant for developing and adapting guidelines about genetic testing, treatment options, and treatability with PARP [poly adenosine diphosphate-ribose polymerase] inhibitors for each cancer type,” the authors wrote.
Dr. Momozawa and associates conducted a large-scale sequencing study across 14 common cancer types in 63,828 patients (mean age 64 years, 42% female) and 37,086 controls on data drawn from a Japanese nationwide biobank between April 2003 and March 2018. They estimated the risk of each cancer type and determined clinical characteristics associated with pathogenic variant carrier status, while also investigating the utility of family history in detecting patients with pathogenic variants.
Three hundred fifteen pathogenic variants were identified. An odds ratios of greater than 4.0 (with P < 1 × 10−4 as the threshold of significance) for the pathogenic variants were found for biliary tract cancer (OR, 17.4; 95% confidence interval, 5.8-51.9) in BRCA1, esophageal cancer (OR, 5.6; 95% CI, 2.9-11.0) in BRCA2, and gastric cancer (OR, 5.2; 95% CI, 2.6-10.5) in BRCA1, and (OR, 4.7; 95% CI, 3.1-7.1) in BRCA2. Two other cancer types were found to be associated with BRCA1, and four other cancer types with BRCA2. Enrichment of carrier patients was shown in biliary tract, female breast, ovarian, and prostate cancers in accordance with increased numbers of reported cancer types in relatives.
Male patients with breast cancer had a very high carrier frequency of pathogenic variants in BRCA2 (18.9%), but not BRCA1 (1.89%). Patients with ovarian cancer showed the next highest proportion (BRCA1, 4.86%; BRCA2, 3.42%). Frequency exceeding 1% was seen for several other cancer types (two cancer types for BRCA1, four cancer types for BRCA2). More than one cancer types was identified in 4,128 patients (6.3%). Carrier frequency of pathogenic variants in BRCA1 was 0.44% with one cancer type, 0.85% with two cancer types, and 0.69% with three cancer types. It was 0.97%, 1.40%, and 1.74%, respectively, in BRCA2.
“The results of this large-scale registry-based case-control study suggest that pathogenic variants in BRCA1 and BRCA2 were associated with the risk of seven cancer types. These results indicate broader clinical relevance of BRCA1 and BRCA2 genetic testing,” the authors wrote.
PARP inhibitors were developed based on the mechanism in BRCA1 and BRCA2 of homologous recombination repair defects associated with pathogenic variants. PARP inhibitors have been found to have therapeutic efficacy also in pathogenic variants found to be enriched in prostate and pancreatic cancers. While risk for additional cancer types (for example, biliary tract cancer, cervical cancer, colorectal cancer, endometrial cancer, esophageal cancer, and stomach cancer) has been reported after analyzing family members for the presence of pathogenic variants and performing case-control analyses, evidence for an association with these cancer types has not been considered sufficient for them to be adopted into clinical management guidelines, the authors wrote.
In an interview, Dr. Momozawa said that BRCA1 and BRCA2 genetic testing should be expanded in Japan. “But further studies are needed to reveal how much. If a clinical trial of a PARP inhibitor for these three cancer types reveals its clinical utility, the importance of this expansion will increase.”
Dr. Momozawa and associates state that while their selection of controls without a family history of cancer affects the generalizability of the study results, the estimated cumulative risks were comparable with those based on prospective cohorts, suggesting the study design did not greatly affect the results.
FROM JAMA ONCOLOGY
‘Striking’ jump in cost of brand-name epilepsy meds
, a new analysis shows.
After adjustment for inflation, the cost of a 1-year supply of brand-name ASMs grew 277%, while generics became 42% less expensive.
“Our study makes transparent striking trends in brand name prescribing patterns,” the study team wrote.
Since 2010, the costs for brand-name ASMs have “consistently” increased. Costs were particularly boosted by increases in prescriptions for lacosamide (Vimpat), in addition to a “steep increase in the cost per pill, with brand-name drugs costing 10 times more than their generic counterparts,” first author Samuel Waller Terman, MD, of the University of Michigan, Ann Arbor, added in a news release.
The study was published online in Neurology.
Is a 10-fold increase in cost worth it?
To evaluate trends in ASM prescriptions and costs, the researchers used a random sample of 20% of Medicare beneficiaries with coverage from 2008 to 2018. There were 77,000 to 133,000 patients with epilepsy each year.
Over time, likely because of increasing availability of generics, brand-name ASMs made up a smaller proportion of pills prescribed, from 56% in 2008 to 14% in 2018, but still made up 79% of prescription drug costs in 2018.
The annual cost of brand-name ASMs rose from $2,800 in 2008 to $10,700 in 2018, while the cost of generic drugs decreased from $800 to $460 during that time.
An increased number of prescriptions for lacosamide was responsible for 45% of the total increase in brand-name costs.
As of 2018, lacosamide comprised 30% of all brand-name pill supply (followed by pregabalin, at 15%) and 30% of all brand-name costs (followed by clobazam and pregabalin, both at 9%), the investigators reported.
Brand-name antiepileptic drug costs decreased from 2008 to 2010, but after the introduction of lacosamide, total brand-name costs steadily rose from $72 million in 2010 (in 2018 dollars) to $256 million in 2018, they noted.
Because the dataset consists of a 20% random Medicare sample, total Medicare costs for brand-name ASMs for beneficiaries with epilepsy alone likely rose from roughly $360 million in 2010 to $1.3 billion in 2018, they added.
“Clinicians must remain cognizant of this societal cost magnitude when judging whether the 10-fold increased expense per pill for brand name medications is worth the possible benefits,” they wrote.
“While newer-generation drugs have potential advantages such as limited drug interactions and different side effect profiles, there have been conflicting studies on whether they are cost effective,” Dr. Terman noted in a news release.
A barrier to treatment
The authors of an accompanying editorial propose that the problem of prescription drug costs could be solved through a combination of competition and government regulation of prices. Patients and physicians are the most important stakeholders in this issue.
“When something represents 14% of the total use, but contributes 79% of the cost, it would be wise to consider alternatives, assuming that these alternatives are not of lower quality,” wrote Wyatt Bensken, with Case Western Reserve University, Cleveland, and Iván Sánchez Fernández, MD, with Boston Medical Center.
“When there are several ASMs with a similar mechanism of action, similar efficacy, similar safety and tolerability profile, and different costs, it would be unwise to choose the more expensive alternative just because it is newer,” they said.
This study, they added, provides data to “understand, and begin to act, on the challenging problem of the cost of prescription ASMs. After all, what is the point of having a large number of ASMs if their cost severely limits their use?”
A limitation of the study is that only Medicare prescription claims were included, so the results may not apply to younger patients with private insurance.
The study received no direct funding. The authors and editorialists have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a new analysis shows.
After adjustment for inflation, the cost of a 1-year supply of brand-name ASMs grew 277%, while generics became 42% less expensive.
“Our study makes transparent striking trends in brand name prescribing patterns,” the study team wrote.
Since 2010, the costs for brand-name ASMs have “consistently” increased. Costs were particularly boosted by increases in prescriptions for lacosamide (Vimpat), in addition to a “steep increase in the cost per pill, with brand-name drugs costing 10 times more than their generic counterparts,” first author Samuel Waller Terman, MD, of the University of Michigan, Ann Arbor, added in a news release.
The study was published online in Neurology.
Is a 10-fold increase in cost worth it?
To evaluate trends in ASM prescriptions and costs, the researchers used a random sample of 20% of Medicare beneficiaries with coverage from 2008 to 2018. There were 77,000 to 133,000 patients with epilepsy each year.
Over time, likely because of increasing availability of generics, brand-name ASMs made up a smaller proportion of pills prescribed, from 56% in 2008 to 14% in 2018, but still made up 79% of prescription drug costs in 2018.
The annual cost of brand-name ASMs rose from $2,800 in 2008 to $10,700 in 2018, while the cost of generic drugs decreased from $800 to $460 during that time.
An increased number of prescriptions for lacosamide was responsible for 45% of the total increase in brand-name costs.
As of 2018, lacosamide comprised 30% of all brand-name pill supply (followed by pregabalin, at 15%) and 30% of all brand-name costs (followed by clobazam and pregabalin, both at 9%), the investigators reported.
Brand-name antiepileptic drug costs decreased from 2008 to 2010, but after the introduction of lacosamide, total brand-name costs steadily rose from $72 million in 2010 (in 2018 dollars) to $256 million in 2018, they noted.
Because the dataset consists of a 20% random Medicare sample, total Medicare costs for brand-name ASMs for beneficiaries with epilepsy alone likely rose from roughly $360 million in 2010 to $1.3 billion in 2018, they added.
“Clinicians must remain cognizant of this societal cost magnitude when judging whether the 10-fold increased expense per pill for brand name medications is worth the possible benefits,” they wrote.
“While newer-generation drugs have potential advantages such as limited drug interactions and different side effect profiles, there have been conflicting studies on whether they are cost effective,” Dr. Terman noted in a news release.
A barrier to treatment
The authors of an accompanying editorial propose that the problem of prescription drug costs could be solved through a combination of competition and government regulation of prices. Patients and physicians are the most important stakeholders in this issue.
“When something represents 14% of the total use, but contributes 79% of the cost, it would be wise to consider alternatives, assuming that these alternatives are not of lower quality,” wrote Wyatt Bensken, with Case Western Reserve University, Cleveland, and Iván Sánchez Fernández, MD, with Boston Medical Center.
“When there are several ASMs with a similar mechanism of action, similar efficacy, similar safety and tolerability profile, and different costs, it would be unwise to choose the more expensive alternative just because it is newer,” they said.
This study, they added, provides data to “understand, and begin to act, on the challenging problem of the cost of prescription ASMs. After all, what is the point of having a large number of ASMs if their cost severely limits their use?”
A limitation of the study is that only Medicare prescription claims were included, so the results may not apply to younger patients with private insurance.
The study received no direct funding. The authors and editorialists have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a new analysis shows.
After adjustment for inflation, the cost of a 1-year supply of brand-name ASMs grew 277%, while generics became 42% less expensive.
“Our study makes transparent striking trends in brand name prescribing patterns,” the study team wrote.
Since 2010, the costs for brand-name ASMs have “consistently” increased. Costs were particularly boosted by increases in prescriptions for lacosamide (Vimpat), in addition to a “steep increase in the cost per pill, with brand-name drugs costing 10 times more than their generic counterparts,” first author Samuel Waller Terman, MD, of the University of Michigan, Ann Arbor, added in a news release.
The study was published online in Neurology.
Is a 10-fold increase in cost worth it?
To evaluate trends in ASM prescriptions and costs, the researchers used a random sample of 20% of Medicare beneficiaries with coverage from 2008 to 2018. There were 77,000 to 133,000 patients with epilepsy each year.
Over time, likely because of increasing availability of generics, brand-name ASMs made up a smaller proportion of pills prescribed, from 56% in 2008 to 14% in 2018, but still made up 79% of prescription drug costs in 2018.
The annual cost of brand-name ASMs rose from $2,800 in 2008 to $10,700 in 2018, while the cost of generic drugs decreased from $800 to $460 during that time.
An increased number of prescriptions for lacosamide was responsible for 45% of the total increase in brand-name costs.
As of 2018, lacosamide comprised 30% of all brand-name pill supply (followed by pregabalin, at 15%) and 30% of all brand-name costs (followed by clobazam and pregabalin, both at 9%), the investigators reported.
Brand-name antiepileptic drug costs decreased from 2008 to 2010, but after the introduction of lacosamide, total brand-name costs steadily rose from $72 million in 2010 (in 2018 dollars) to $256 million in 2018, they noted.
Because the dataset consists of a 20% random Medicare sample, total Medicare costs for brand-name ASMs for beneficiaries with epilepsy alone likely rose from roughly $360 million in 2010 to $1.3 billion in 2018, they added.
“Clinicians must remain cognizant of this societal cost magnitude when judging whether the 10-fold increased expense per pill for brand name medications is worth the possible benefits,” they wrote.
“While newer-generation drugs have potential advantages such as limited drug interactions and different side effect profiles, there have been conflicting studies on whether they are cost effective,” Dr. Terman noted in a news release.
A barrier to treatment
The authors of an accompanying editorial propose that the problem of prescription drug costs could be solved through a combination of competition and government regulation of prices. Patients and physicians are the most important stakeholders in this issue.
“When something represents 14% of the total use, but contributes 79% of the cost, it would be wise to consider alternatives, assuming that these alternatives are not of lower quality,” wrote Wyatt Bensken, with Case Western Reserve University, Cleveland, and Iván Sánchez Fernández, MD, with Boston Medical Center.
“When there are several ASMs with a similar mechanism of action, similar efficacy, similar safety and tolerability profile, and different costs, it would be unwise to choose the more expensive alternative just because it is newer,” they said.
This study, they added, provides data to “understand, and begin to act, on the challenging problem of the cost of prescription ASMs. After all, what is the point of having a large number of ASMs if their cost severely limits their use?”
A limitation of the study is that only Medicare prescription claims were included, so the results may not apply to younger patients with private insurance.
The study received no direct funding. The authors and editorialists have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
If nuclear disaster strikes, U.S. hematologists stand ready
For many Americans – especially those too young to know much about the Cold War or Hiroshima – Russia’s invasion of Ukraine might mark the first time they’ve truly considered the dangers of nuclear weapons. But dozens of hematologists in the United States already know the drill and have placed themselves on the front lines. These physicians stand prepared to treat patients exposed to radiation caused by nuclear accidents or attacks on U.S. soil.
They work nationwide at 74 medical centers that make up the Radiation Injury Treatment Network, ready to manage cases of acute radiation syndrome (ARS) during disasters. While RITN keeps a low profile, it’s been in the news lately amid anxieties about the Ukraine conflict, nuclear plant accidents, and the potential launching of nuclear weapons by foreign adversaries.
“The Radiation Injury Treatment Network helps plan responses for disaster scenarios where a person’s cells would be damaged after having been exposed to ionizing radiation,” program director Cullen Case Jr., MPA, said in an interview.
A U.S. Army veteran who took part in hurricane response early in his career, Mr. Case now oversees preparedness activities among all RITN hospitals, blood donor centers, and cord blood banks, in readiness for a mass casualty radiological incident. He also serves as a senior manager of the National Marrow Donor Program/Be a Match Marrow Registry.
Intense preparation for nuclear attacks or accidents is necessary, Mr. Case said, despite the doomsday scenarios disseminated on television shows and movies.
“The most frequent misconception we hear is that a nuclear disaster will encompass the whole world and be so complete that preparedness isn’t useful. However, many planning scenarios include smaller-scale incidents where survivors will need prompt and expert care,” he said.
In the wake of 9/11, the National Marrow Donor Program and the American Society for Blood and Marrow Transplantation established the RITN in 2006, with a mission to prepare for nuclear disaster and help manage the response if one occurs.
“The widespread availability of radioactive material has made future exposure events, accidental or intentional, nearly inevitable,” RITN leaders warned in a 2008 report. “Hematologists, oncologists, and HSCT [hematopoietic stem cell transplantation] physicians are uniquely suited to care for victims of radiation exposure, creating a collective responsibility to prepare for a variety of contingencies.”
RITN doesn’t just train physicians, Mr. Case noted. All medical centers within the RITN are required to conduct an annual tabletop exercise where a radiation disaster scenario and a set of discussion questions are presented to the team.
Hematologists specially equipped to treat radiation injuries
Why are hematologists involved in treating people exposed to dangerously high levels of radiation? The answer has to do with how radiation harms the body, said Dr. Ann A. Jakubowski, a hematologist/oncologist and transplant physician at Memorial Sloan Kettering Cancer Center, New York, who serves as a medical director for RITN.
“One of the most common toxicities from radiation exposure and a major player in acute radiation syndrome is hematologic toxicity– damage to the bone marrow by the radiation, with a resultant decrease in peripheral blood counts,” she said in an interview. “This is similar to what is often seen in the treatment of cancers with radiation and/or chemotherapy.”
In cases of severe and nonreversible radiation damage to the bone marrow, Dr. Jakubowski noted, “patients can be considered for a stem cell transplant to provide new healthy cells to repopulate the bone marrow, which provides recovery of peripheral blood counts. Hematologist/oncologists are the physicians who manage stem cell transplants.”
The crucial role of hematologists in radiation injuries is not new. In fact, these physicians have been closely intertwined with nuclear research since the dawn of the atomic age. The work of developing atomic bombs also led investigators to an understanding of the structure and processes of hematopoiesis and helped them to identify hematopoietic stem cells and prove their existence in humans.
Disaster response poses multiple challenges
As noted in a recent article in ASH Clinical News, the challenges of treating radiation injuries would be intense, especially in the event of a nuclear accident or attack that affects a wide area. For starters, how quickly can medical professionals be mobilized, and will there be enough physicians comfortable treating patients? Fortunately, irradiated patients should not pose a direct risk to medical professionals who treat them.
“The expectation is that the patients will all be decontaminated,” said Nelson Chao, MD, MBA, one of the founders of RITN and a hematologist/oncologist and transplant physician at Duke University, Durham, N.C.
Dr. Jakubowski questions whether there will be adequate resources to handle the influx of patients who need more intensive treatment, as well as outpatients who “received lower doses of radiation and may experience a period of low blood counts but are expected to eventually recover blood counts.”
And if many people are injured, Dr. Chao asks, how will physicians “adopt altered standards of care to treat large numbers of patients?”
There will also be a need for physicians who aren’t hematologists, Dr. Jakubowski said. “There may be many victims who have both radiation exposure and traumatic or burn injuries, which need to be addressed first, before the hematologist can start addressing the consequences of ARS. Traumatic and burn injuries will require surgical resources.”
In addition, ARS affects the gastrointestinal track and central nervous system/cardiovascular, and it has multiple stages, she noted.
“Although we have methods of supporting the hematopoietic system – transfusions and growth factors – and even replacing it with a stem cell transplant, this will not necessarily fix the badly damaged other organs, Dr. Jakubowski said. “Also, not all radioactive isotopes are equal in their effects, nor are the various types of radiation exposure.”
Training goes beyond transplants and drugs
RITN offers individual hematologists specialized education about treating radiation injuries through annual exercises, modules, and “just-in-time” training.
For example, the RITN webpage devoted to triage includes guidelines for transferring radiation injury patients, triage guidelines for cytokine administration in cases of ARS, an exposure and symptom triage tool, and more. The treatment page includes details about subjects such as when human leukocyte antigen typing of casualties is appropriate and how to keep yourself safe while treating patients.
Another focus is teaching hematologists to react quickly in disasters, Mr. Case said. “The vast majority of hematologists have little to no experience in responding to disasters and making decisions with imperfect or incomplete information, as emergency medicine practitioners must do regularly.”
“Some of the RITN tabletop exercises present physicians and advanced practitioners with an incomplete set of patient information and ask physicians to then determine and prioritize their care,” Mr. Case said. “The resulting discussions help to lay the groundwork for being able to shift to the crisis standards of care mindset that would be necessary during a radiological disaster.”
Here’s how hematologists can get involved
If you want to help improve the nation’s response to radiation injuries, Mr. Case suggests checking RITN’s list of participating hospitals. If your facility is already part of this network, he said, contact its bone marrow transplant unit for more information.
In such cases, Dr. Jakubowski suggests that you “consider periodically giving a presentation to staff on the basics of radiation injury and the center’s role in RITN.” And if you’re not part of RITN, she said, consider contacting the network about becoming a member.
Hematologists, Mr. Case said, can also take advantage of RITN’s free short overview courses, review the RITN Treatment Guidelines, or watch short videos on the RITN’s YouTube channel.
He highlighted the Radiation Emergency Medical Management website administered by the Department of Health & Human Services, the Center for Disease Control’s radiation emergencies webpage, and the Department of Energy’s Radiation Emergency Assistance Center/Training Site.
For many Americans – especially those too young to know much about the Cold War or Hiroshima – Russia’s invasion of Ukraine might mark the first time they’ve truly considered the dangers of nuclear weapons. But dozens of hematologists in the United States already know the drill and have placed themselves on the front lines. These physicians stand prepared to treat patients exposed to radiation caused by nuclear accidents or attacks on U.S. soil.
They work nationwide at 74 medical centers that make up the Radiation Injury Treatment Network, ready to manage cases of acute radiation syndrome (ARS) during disasters. While RITN keeps a low profile, it’s been in the news lately amid anxieties about the Ukraine conflict, nuclear plant accidents, and the potential launching of nuclear weapons by foreign adversaries.
“The Radiation Injury Treatment Network helps plan responses for disaster scenarios where a person’s cells would be damaged after having been exposed to ionizing radiation,” program director Cullen Case Jr., MPA, said in an interview.
A U.S. Army veteran who took part in hurricane response early in his career, Mr. Case now oversees preparedness activities among all RITN hospitals, blood donor centers, and cord blood banks, in readiness for a mass casualty radiological incident. He also serves as a senior manager of the National Marrow Donor Program/Be a Match Marrow Registry.
Intense preparation for nuclear attacks or accidents is necessary, Mr. Case said, despite the doomsday scenarios disseminated on television shows and movies.
“The most frequent misconception we hear is that a nuclear disaster will encompass the whole world and be so complete that preparedness isn’t useful. However, many planning scenarios include smaller-scale incidents where survivors will need prompt and expert care,” he said.
In the wake of 9/11, the National Marrow Donor Program and the American Society for Blood and Marrow Transplantation established the RITN in 2006, with a mission to prepare for nuclear disaster and help manage the response if one occurs.

“The widespread availability of radioactive material has made future exposure events, accidental or intentional, nearly inevitable,” RITN leaders warned in a 2008 report. “Hematologists, oncologists, and HSCT [hematopoietic stem cell transplantation] physicians are uniquely suited to care for victims of radiation exposure, creating a collective responsibility to prepare for a variety of contingencies.”
RITN doesn’t just train physicians, Mr. Case noted. All medical centers within the RITN are required to conduct an annual tabletop exercise where a radiation disaster scenario and a set of discussion questions are presented to the team.
Hematologists specially equipped to treat radiation injuries
Why are hematologists involved in treating people exposed to dangerously high levels of radiation? The answer has to do with how radiation harms the body, said Dr. Ann A. Jakubowski, a hematologist/oncologist and transplant physician at Memorial Sloan Kettering Cancer Center, New York, who serves as a medical director for RITN.
“One of the most common toxicities from radiation exposure and a major player in acute radiation syndrome is hematologic toxicity– damage to the bone marrow by the radiation, with a resultant decrease in peripheral blood counts,” she said in an interview. “This is similar to what is often seen in the treatment of cancers with radiation and/or chemotherapy.”
In cases of severe and nonreversible radiation damage to the bone marrow, Dr. Jakubowski noted, “patients can be considered for a stem cell transplant to provide new healthy cells to repopulate the bone marrow, which provides recovery of peripheral blood counts. Hematologist/oncologists are the physicians who manage stem cell transplants.”
The crucial role of hematologists in radiation injuries is not new. In fact, these physicians have been closely intertwined with nuclear research since the dawn of the atomic age. The work of developing atomic bombs also led investigators to an understanding of the structure and processes of hematopoiesis and helped them to identify hematopoietic stem cells and prove their existence in humans.
Disaster response poses multiple challenges
As noted in a recent article in ASH Clinical News, the challenges of treating radiation injuries would be intense, especially in the event of a nuclear accident or attack that affects a wide area. For starters, how quickly can medical professionals be mobilized, and will there be enough physicians comfortable treating patients? Fortunately, irradiated patients should not pose a direct risk to medical professionals who treat them.
“The expectation is that the patients will all be decontaminated,” said Nelson Chao, MD, MBA, one of the founders of RITN and a hematologist/oncologist and transplant physician at Duke University, Durham, N.C.
Dr. Jakubowski questions whether there will be adequate resources to handle the influx of patients who need more intensive treatment, as well as outpatients who “received lower doses of radiation and may experience a period of low blood counts but are expected to eventually recover blood counts.”

And if many people are injured, Dr. Chao asks, how will physicians “adopt altered standards of care to treat large numbers of patients?”
There will also be a need for physicians who aren’t hematologists, Dr. Jakubowski said. “There may be many victims who have both radiation exposure and traumatic or burn injuries, which need to be addressed first, before the hematologist can start addressing the consequences of ARS. Traumatic and burn injuries will require surgical resources.”
In addition, ARS affects the gastrointestinal track and central nervous system/cardiovascular, and it has multiple stages, she noted.
“Although we have methods of supporting the hematopoietic system – transfusions and growth factors – and even replacing it with a stem cell transplant, this will not necessarily fix the badly damaged other organs, Dr. Jakubowski said. “Also, not all radioactive isotopes are equal in their effects, nor are the various types of radiation exposure.”
Training goes beyond transplants and drugs
RITN offers individual hematologists specialized education about treating radiation injuries through annual exercises, modules, and “just-in-time” training.
For example, the RITN webpage devoted to triage includes guidelines for transferring radiation injury patients, triage guidelines for cytokine administration in cases of ARS, an exposure and symptom triage tool, and more. The treatment page includes details about subjects such as when human leukocyte antigen typing of casualties is appropriate and how to keep yourself safe while treating patients.
Another focus is teaching hematologists to react quickly in disasters, Mr. Case said. “The vast majority of hematologists have little to no experience in responding to disasters and making decisions with imperfect or incomplete information, as emergency medicine practitioners must do regularly.”
“Some of the RITN tabletop exercises present physicians and advanced practitioners with an incomplete set of patient information and ask physicians to then determine and prioritize their care,” Mr. Case said. “The resulting discussions help to lay the groundwork for being able to shift to the crisis standards of care mindset that would be necessary during a radiological disaster.”
Here’s how hematologists can get involved
If you want to help improve the nation’s response to radiation injuries, Mr. Case suggests checking RITN’s list of participating hospitals. If your facility is already part of this network, he said, contact its bone marrow transplant unit for more information.
In such cases, Dr. Jakubowski suggests that you “consider periodically giving a presentation to staff on the basics of radiation injury and the center’s role in RITN.” And if you’re not part of RITN, she said, consider contacting the network about becoming a member.
Hematologists, Mr. Case said, can also take advantage of RITN’s free short overview courses, review the RITN Treatment Guidelines, or watch short videos on the RITN’s YouTube channel.
He highlighted the Radiation Emergency Medical Management website administered by the Department of Health & Human Services, the Center for Disease Control’s radiation emergencies webpage, and the Department of Energy’s Radiation Emergency Assistance Center/Training Site.
For many Americans – especially those too young to know much about the Cold War or Hiroshima – Russia’s invasion of Ukraine might mark the first time they’ve truly considered the dangers of nuclear weapons. But dozens of hematologists in the United States already know the drill and have placed themselves on the front lines. These physicians stand prepared to treat patients exposed to radiation caused by nuclear accidents or attacks on U.S. soil.
They work nationwide at 74 medical centers that make up the Radiation Injury Treatment Network, ready to manage cases of acute radiation syndrome (ARS) during disasters. While RITN keeps a low profile, it’s been in the news lately amid anxieties about the Ukraine conflict, nuclear plant accidents, and the potential launching of nuclear weapons by foreign adversaries.
“The Radiation Injury Treatment Network helps plan responses for disaster scenarios where a person’s cells would be damaged after having been exposed to ionizing radiation,” program director Cullen Case Jr., MPA, said in an interview.
A U.S. Army veteran who took part in hurricane response early in his career, Mr. Case now oversees preparedness activities among all RITN hospitals, blood donor centers, and cord blood banks, in readiness for a mass casualty radiological incident. He also serves as a senior manager of the National Marrow Donor Program/Be a Match Marrow Registry.
Intense preparation for nuclear attacks or accidents is necessary, Mr. Case said, despite the doomsday scenarios disseminated on television shows and movies.
“The most frequent misconception we hear is that a nuclear disaster will encompass the whole world and be so complete that preparedness isn’t useful. However, many planning scenarios include smaller-scale incidents where survivors will need prompt and expert care,” he said.
In the wake of 9/11, the National Marrow Donor Program and the American Society for Blood and Marrow Transplantation established the RITN in 2006, with a mission to prepare for nuclear disaster and help manage the response if one occurs.

“The widespread availability of radioactive material has made future exposure events, accidental or intentional, nearly inevitable,” RITN leaders warned in a 2008 report. “Hematologists, oncologists, and HSCT [hematopoietic stem cell transplantation] physicians are uniquely suited to care for victims of radiation exposure, creating a collective responsibility to prepare for a variety of contingencies.”
RITN doesn’t just train physicians, Mr. Case noted. All medical centers within the RITN are required to conduct an annual tabletop exercise where a radiation disaster scenario and a set of discussion questions are presented to the team.
Hematologists specially equipped to treat radiation injuries
Why are hematologists involved in treating people exposed to dangerously high levels of radiation? The answer has to do with how radiation harms the body, said Dr. Ann A. Jakubowski, a hematologist/oncologist and transplant physician at Memorial Sloan Kettering Cancer Center, New York, who serves as a medical director for RITN.
“One of the most common toxicities from radiation exposure and a major player in acute radiation syndrome is hematologic toxicity– damage to the bone marrow by the radiation, with a resultant decrease in peripheral blood counts,” she said in an interview. “This is similar to what is often seen in the treatment of cancers with radiation and/or chemotherapy.”
In cases of severe and nonreversible radiation damage to the bone marrow, Dr. Jakubowski noted, “patients can be considered for a stem cell transplant to provide new healthy cells to repopulate the bone marrow, which provides recovery of peripheral blood counts. Hematologist/oncologists are the physicians who manage stem cell transplants.”
The crucial role of hematologists in radiation injuries is not new. In fact, these physicians have been closely intertwined with nuclear research since the dawn of the atomic age. The work of developing atomic bombs also led investigators to an understanding of the structure and processes of hematopoiesis and helped them to identify hematopoietic stem cells and prove their existence in humans.
Disaster response poses multiple challenges
As noted in a recent article in ASH Clinical News, the challenges of treating radiation injuries would be intense, especially in the event of a nuclear accident or attack that affects a wide area. For starters, how quickly can medical professionals be mobilized, and will there be enough physicians comfortable treating patients? Fortunately, irradiated patients should not pose a direct risk to medical professionals who treat them.
“The expectation is that the patients will all be decontaminated,” said Nelson Chao, MD, MBA, one of the founders of RITN and a hematologist/oncologist and transplant physician at Duke University, Durham, N.C.
Dr. Jakubowski questions whether there will be adequate resources to handle the influx of patients who need more intensive treatment, as well as outpatients who “received lower doses of radiation and may experience a period of low blood counts but are expected to eventually recover blood counts.”

And if many people are injured, Dr. Chao asks, how will physicians “adopt altered standards of care to treat large numbers of patients?”
There will also be a need for physicians who aren’t hematologists, Dr. Jakubowski said. “There may be many victims who have both radiation exposure and traumatic or burn injuries, which need to be addressed first, before the hematologist can start addressing the consequences of ARS. Traumatic and burn injuries will require surgical resources.”
In addition, ARS affects the gastrointestinal track and central nervous system/cardiovascular, and it has multiple stages, she noted.
“Although we have methods of supporting the hematopoietic system – transfusions and growth factors – and even replacing it with a stem cell transplant, this will not necessarily fix the badly damaged other organs, Dr. Jakubowski said. “Also, not all radioactive isotopes are equal in their effects, nor are the various types of radiation exposure.”
Training goes beyond transplants and drugs
RITN offers individual hematologists specialized education about treating radiation injuries through annual exercises, modules, and “just-in-time” training.
For example, the RITN webpage devoted to triage includes guidelines for transferring radiation injury patients, triage guidelines for cytokine administration in cases of ARS, an exposure and symptom triage tool, and more. The treatment page includes details about subjects such as when human leukocyte antigen typing of casualties is appropriate and how to keep yourself safe while treating patients.
Another focus is teaching hematologists to react quickly in disasters, Mr. Case said. “The vast majority of hematologists have little to no experience in responding to disasters and making decisions with imperfect or incomplete information, as emergency medicine practitioners must do regularly.”
“Some of the RITN tabletop exercises present physicians and advanced practitioners with an incomplete set of patient information and ask physicians to then determine and prioritize their care,” Mr. Case said. “The resulting discussions help to lay the groundwork for being able to shift to the crisis standards of care mindset that would be necessary during a radiological disaster.”
Here’s how hematologists can get involved
If you want to help improve the nation’s response to radiation injuries, Mr. Case suggests checking RITN’s list of participating hospitals. If your facility is already part of this network, he said, contact its bone marrow transplant unit for more information.
In such cases, Dr. Jakubowski suggests that you “consider periodically giving a presentation to staff on the basics of radiation injury and the center’s role in RITN.” And if you’re not part of RITN, she said, consider contacting the network about becoming a member.
Hematologists, Mr. Case said, can also take advantage of RITN’s free short overview courses, review the RITN Treatment Guidelines, or watch short videos on the RITN’s YouTube channel.
He highlighted the Radiation Emergency Medical Management website administered by the Department of Health & Human Services, the Center for Disease Control’s radiation emergencies webpage, and the Department of Energy’s Radiation Emergency Assistance Center/Training Site.
Sociogenomics may explain race disparities in breast cancer mortality
Racial differences in cancer outcomes are widespread. Studies indicate that Black people face higher rates of mortality for most cancers than their White counterparts. To bridge this racial gap, researchers need to investigate the biological effects of structural racism and discrimination on cancer outcomes, experts say.
“As a physician, I always like to think that I can influence care in that if I just find the right drugs, help patients understand what their options are, it will help them,” said Ruth Carlos, MD, a radiologist with the University of Michigan Hospital, Ann Arbor. But these things alone are often not enough, because a large proportion of the variation in cancer outcomes is attributable to neighborhood social conditions and the physical environment. “It is incredibly important for us to start to understand just how the neighborhood exerts this effect.”
In a commentary published in the Journal of Clinical Oncology, Dr. Carlos and colleagues highlighted the limitations of previous studies aimed at identifying the causes of racial differences in cancer outcomes. They call upon researchers to turn instead to the long-underexamined biological effects of structural racism and discrimination that contribute to these differences.
In the past, studies on the role of race in health outcomes largely looked at race as a proxy for genetic predisposition. But such an interpretation is flawed, because no genes are specific for a racial or ethnic group, Dr. Carlos and coauthors wrote. Researchers have shown that the vast majority of genetic variation occurs within, rather than between groups.
In an analysis published in Science, researchers reported that within-group differences account for more than 90% of genetic variation.
“Using race in these analyses was not necessarily wrong, but the inferences may have been flawed or incomplete,” Dr. Carlos said. On one hand, looking at genetic predisposition has led to important insights, such as the link between mutations in the BRCA gene and increased risk for breast and ovarian cancer.
However, genetic variation alone is not enough to explain the disparities in cancer outcomes between racial and ethnic groups. The fact that breast cancer can be more aggressive in Black women raises several questions, Dr. Carlos said. Is the cancer worse because Black women have a specific genetic predisposition? Is it worse because Black women exist in a society that marginalizes them and exposes them to increased stress, which in turn produces bad outcomes? Or, could it be both?
Despite progress in the screening, diagnosis and treatment of breast cancer, Black women are 40% more likely to die from the disease than White women. At the time of diagnosis, Black women are more likely to have high-grade, more aggressive breast cancer molecular subtypes, and to have had their cancer spread to the lymph nodes. They also tend to be diagnosed at more advanced stages of breast cancer while at the same time, experience higher rates of false-positive screening results.
Although researchers have hypothesized that genetic differences related to African or European ancestry might contribute, studies have not turned up any differences in cancer susceptibility genes by race. Other factors, such as racial differences in the stage of presentation, molecular subtypes, and disparities in treatment, have also emerged as potential culprits.
In her commentary, Dr. Carlos and colleagues wrote that disparities in breast cancer outcomes previously attributed to race need to be examined from multiple angles. This means looking at both the complex interactions between social conditions and policies, which encompass racism both at the individual and structural level, and stressors such as the experience of discrimination in addition to potential biological and genetic contributions.
Many studies now provide evidence for the harmful effects of racism on health. For breast cancer, specifically, studies also suggest that factors such as racial segregation can influence the stage at which Black women get diagnosed and their likelihood of dying from the disease.
However, an important question that remains is what biological changes occur in women exposed to the kind of persistent low-level stress that is associated with structural racism and discrimination, Dr. Carlos said. “We don’t know what stress pathways actually manifest in the body and how they eventually produce the disease.” Studies to address this issue are important, “especially if you would like to develop interventions to prevent or mitigate disease.”
To address this issue, Dr. Carlos and colleagues called upon the research community to conduct both studies that delineate the underlying biology as well as those that test potential interventions – particularly those associated with breast cancer screening outcomes – to try to shed light on why Black women receive more false positives and diagnoses of more aggressive cancer.
Interventions that can target these specific biological pathways could potentially reduce the negative effects of structural racism and discrimination as well as the effects of other social factors that contribute to breast cancer outcomes, “to ultimately help enhance clinical outcomes and close persistent disparities gaps,” the authors wrote.
Racial differences in cancer outcomes are widespread. Studies indicate that Black people face higher rates of mortality for most cancers than their White counterparts. To bridge this racial gap, researchers need to investigate the biological effects of structural racism and discrimination on cancer outcomes, experts say.
“As a physician, I always like to think that I can influence care in that if I just find the right drugs, help patients understand what their options are, it will help them,” said Ruth Carlos, MD, a radiologist with the University of Michigan Hospital, Ann Arbor. But these things alone are often not enough, because a large proportion of the variation in cancer outcomes is attributable to neighborhood social conditions and the physical environment. “It is incredibly important for us to start to understand just how the neighborhood exerts this effect.”
In a commentary published in the Journal of Clinical Oncology, Dr. Carlos and colleagues highlighted the limitations of previous studies aimed at identifying the causes of racial differences in cancer outcomes. They call upon researchers to turn instead to the long-underexamined biological effects of structural racism and discrimination that contribute to these differences.
In the past, studies on the role of race in health outcomes largely looked at race as a proxy for genetic predisposition. But such an interpretation is flawed, because no genes are specific for a racial or ethnic group, Dr. Carlos and coauthors wrote. Researchers have shown that the vast majority of genetic variation occurs within, rather than between groups.
In an analysis published in Science, researchers reported that within-group differences account for more than 90% of genetic variation.
“Using race in these analyses was not necessarily wrong, but the inferences may have been flawed or incomplete,” Dr. Carlos said. On one hand, looking at genetic predisposition has led to important insights, such as the link between mutations in the BRCA gene and increased risk for breast and ovarian cancer.
However, genetic variation alone is not enough to explain the disparities in cancer outcomes between racial and ethnic groups. The fact that breast cancer can be more aggressive in Black women raises several questions, Dr. Carlos said. Is the cancer worse because Black women have a specific genetic predisposition? Is it worse because Black women exist in a society that marginalizes them and exposes them to increased stress, which in turn produces bad outcomes? Or, could it be both?
Despite progress in the screening, diagnosis and treatment of breast cancer, Black women are 40% more likely to die from the disease than White women. At the time of diagnosis, Black women are more likely to have high-grade, more aggressive breast cancer molecular subtypes, and to have had their cancer spread to the lymph nodes. They also tend to be diagnosed at more advanced stages of breast cancer while at the same time, experience higher rates of false-positive screening results.
Although researchers have hypothesized that genetic differences related to African or European ancestry might contribute, studies have not turned up any differences in cancer susceptibility genes by race. Other factors, such as racial differences in the stage of presentation, molecular subtypes, and disparities in treatment, have also emerged as potential culprits.
In her commentary, Dr. Carlos and colleagues wrote that disparities in breast cancer outcomes previously attributed to race need to be examined from multiple angles. This means looking at both the complex interactions between social conditions and policies, which encompass racism both at the individual and structural level, and stressors such as the experience of discrimination in addition to potential biological and genetic contributions.
Many studies now provide evidence for the harmful effects of racism on health. For breast cancer, specifically, studies also suggest that factors such as racial segregation can influence the stage at which Black women get diagnosed and their likelihood of dying from the disease.
However, an important question that remains is what biological changes occur in women exposed to the kind of persistent low-level stress that is associated with structural racism and discrimination, Dr. Carlos said. “We don’t know what stress pathways actually manifest in the body and how they eventually produce the disease.” Studies to address this issue are important, “especially if you would like to develop interventions to prevent or mitigate disease.”
To address this issue, Dr. Carlos and colleagues called upon the research community to conduct both studies that delineate the underlying biology as well as those that test potential interventions – particularly those associated with breast cancer screening outcomes – to try to shed light on why Black women receive more false positives and diagnoses of more aggressive cancer.
Interventions that can target these specific biological pathways could potentially reduce the negative effects of structural racism and discrimination as well as the effects of other social factors that contribute to breast cancer outcomes, “to ultimately help enhance clinical outcomes and close persistent disparities gaps,” the authors wrote.
Racial differences in cancer outcomes are widespread. Studies indicate that Black people face higher rates of mortality for most cancers than their White counterparts. To bridge this racial gap, researchers need to investigate the biological effects of structural racism and discrimination on cancer outcomes, experts say.
“As a physician, I always like to think that I can influence care in that if I just find the right drugs, help patients understand what their options are, it will help them,” said Ruth Carlos, MD, a radiologist with the University of Michigan Hospital, Ann Arbor. But these things alone are often not enough, because a large proportion of the variation in cancer outcomes is attributable to neighborhood social conditions and the physical environment. “It is incredibly important for us to start to understand just how the neighborhood exerts this effect.”
In a commentary published in the Journal of Clinical Oncology, Dr. Carlos and colleagues highlighted the limitations of previous studies aimed at identifying the causes of racial differences in cancer outcomes. They call upon researchers to turn instead to the long-underexamined biological effects of structural racism and discrimination that contribute to these differences.
In the past, studies on the role of race in health outcomes largely looked at race as a proxy for genetic predisposition. But such an interpretation is flawed, because no genes are specific for a racial or ethnic group, Dr. Carlos and coauthors wrote. Researchers have shown that the vast majority of genetic variation occurs within, rather than between groups.
In an analysis published in Science, researchers reported that within-group differences account for more than 90% of genetic variation.
“Using race in these analyses was not necessarily wrong, but the inferences may have been flawed or incomplete,” Dr. Carlos said. On one hand, looking at genetic predisposition has led to important insights, such as the link between mutations in the BRCA gene and increased risk for breast and ovarian cancer.
However, genetic variation alone is not enough to explain the disparities in cancer outcomes between racial and ethnic groups. The fact that breast cancer can be more aggressive in Black women raises several questions, Dr. Carlos said. Is the cancer worse because Black women have a specific genetic predisposition? Is it worse because Black women exist in a society that marginalizes them and exposes them to increased stress, which in turn produces bad outcomes? Or, could it be both?
Despite progress in the screening, diagnosis and treatment of breast cancer, Black women are 40% more likely to die from the disease than White women. At the time of diagnosis, Black women are more likely to have high-grade, more aggressive breast cancer molecular subtypes, and to have had their cancer spread to the lymph nodes. They also tend to be diagnosed at more advanced stages of breast cancer while at the same time, experience higher rates of false-positive screening results.
Although researchers have hypothesized that genetic differences related to African or European ancestry might contribute, studies have not turned up any differences in cancer susceptibility genes by race. Other factors, such as racial differences in the stage of presentation, molecular subtypes, and disparities in treatment, have also emerged as potential culprits.
In her commentary, Dr. Carlos and colleagues wrote that disparities in breast cancer outcomes previously attributed to race need to be examined from multiple angles. This means looking at both the complex interactions between social conditions and policies, which encompass racism both at the individual and structural level, and stressors such as the experience of discrimination in addition to potential biological and genetic contributions.
Many studies now provide evidence for the harmful effects of racism on health. For breast cancer, specifically, studies also suggest that factors such as racial segregation can influence the stage at which Black women get diagnosed and their likelihood of dying from the disease.
However, an important question that remains is what biological changes occur in women exposed to the kind of persistent low-level stress that is associated with structural racism and discrimination, Dr. Carlos said. “We don’t know what stress pathways actually manifest in the body and how they eventually produce the disease.” Studies to address this issue are important, “especially if you would like to develop interventions to prevent or mitigate disease.”
To address this issue, Dr. Carlos and colleagues called upon the research community to conduct both studies that delineate the underlying biology as well as those that test potential interventions – particularly those associated with breast cancer screening outcomes – to try to shed light on why Black women receive more false positives and diagnoses of more aggressive cancer.
Interventions that can target these specific biological pathways could potentially reduce the negative effects of structural racism and discrimination as well as the effects of other social factors that contribute to breast cancer outcomes, “to ultimately help enhance clinical outcomes and close persistent disparities gaps,” the authors wrote.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Quality of life benefit exaggerated in some cancer studies
, according to a study published in JAMA Oncology.
The study found trials that failed to show improved quality of life often reported their quality of life outcomes more favorably. Non–immunotherapy-targeted drugs were found to lead to worse quality of life outcomes more often than did cytotoxic agents. And, while there is an association between quality of life benefit and overall survival, no such association was found with progression-free survival.
“In this study, we evaluated the outcomes of cancer drug trials with regard to patients’ quality of life and found that only a quarter of phase 3 cancer drug trials in the advanced-disease setting demonstrated improved quality of life,” wrote authors who were led by Bishal Gyawali, MD, PhD, of the Cancer Research Institute, Queen’s University, Kingston, Ont.
“Improved quality of life outcomes were associated with improved overall survival but not with improved progression-free survival. Importantly, almost half of the cancer drugs drug trials that showed improved progression-free survival showed no improved overall survival or quality of life (i.e., PFS-only benefit). Some reports included conclusions regarding quality of life (QOL) findings that were not directly supported by the trial data, particularly for inferior or non–statistically significant QOL outcomes, thereby framing the findings in a favorable light or downplaying detrimental effects of the study intervention on QOL. Furthermore, contrary to common perception, inferior QOL outcomes were more common with targeted drugs than cytotoxic drugs. Taken together, these findings have important policy implications,” the authors wrote.
These findings are based on the results of a cohort study of 45 phase 3 research clinical trials of 24,806 patients. Only a small percentage of patients showed QOL benefits. The study found that industry-funded clinical trial reports often framed QOL findings more favorably than was warranted by the data.
The study found improved QOL with experimental agents in 11 of 45 randomized controlled trials (24.4%). Studies that reported improved QOL were more likely to also show improved overall survival as compared with trials in which quality of life was not improved (7 of 11 [64%] versus 10 of 34 [29%] trials). For improved progression-free survival, however, there was no positive association (6 of 11 [55%] trials versus 17 of 34 [50%] trials without improved QOL). Among six trials reporting worsening QOL, three (50%) were trials of targeted drugs. Among 11 trials reporting improved QOL, 6 (55%) were trials of immunotherapy drugs. Among the 34 trials in which QOL was not improved compared with controls, the findings were framed favorably (versus neutrally or negatively) in the abstract or conclusions in 16 (47%), an observation that was statistically significantly associated with industry funding (chi-squared = 6.35; P = .01).
“It is important to clearly understand and communicate the effects of cancer drugs”
To fulfill the obligation to inform patients about proposed treatments, the authors wrote that it is important to clearly understand and communicate the effects of cancer drugs on patient quality of life alongside their effects on overall survival and intermediate end points such as progression-free survival. “Patients with advanced cancer expect treatment to help them live longer or have better lives,” the authors wrote. In that respect, in clinical trials of cancer medicines, overall survival and quality of life are the most important measures. Toxicity profiles and disease progression delays do not reliably predict quality of life, and studies have shown poor correlations between quality of life, overall survival, and progression-free survival. This raises the question of validity of progression-free survival as a surrogate endpoint. “Progression-free survival is meaningless without overall survival or quality of life gains,” Dr. Gyawali said in an interview.
Writing in The Lancet Oncology in March, Dr. Gyawali stated that, because progression free survival “does not directly measure how a patient feels or functions, or how long a patient lives, progression-free survival was not intended to inform clinical practice or establish whether a new therapy provides clinically meaningful benefits for patients. However, over the past 2 decades, it has become the most common primary endpoint in oncology clinical trials. We are deeply worried about how the term survival in this phrase can influence clinical practice and patient choices. We propose replacing the phrase progression-free survival with a less ambiguous term: progression-free interval.”
In JAMA Oncology, Dr. Gyawali aimed to elucidate relationships between QOL, overall survival, and progression-free survival, and to assess, as well, how QOL results are framed, especially in industry-sponsored research. When drug trials they analyzed showed no change in QOL but reported that QOL did not worsen or QOL was maintained rather than stating that QOL did not improve, or if there was downplaying of worse QOL outcomes, the study had favorable interpretation, Dr. Gyawali and associates wrote. The expectation of patients receiving cancer drugs would be improved QOL rather than “not worse” QOL, Dr. Gyawali said.
Regarding the finding that QOL outcomes were described as favorable in 47% of trials with unimproved QOL outcomes, Dr. Gyawali said, “the bias in reporting should be corrected by the reviewers and editors of journals. Also, quality of life reporting should be made mandatory. Without unbiased quality of life information, informed decision making on whether or not to use a certain drug is impossible. Patients and physicians need to know that information. Regulators can demand that this should be mandatory in all trials in noncurative settings.”
He remarked further on the worsening QOL in some targeted drug trials, “People tout chemo-free regimens as automatically having better quality of life, but that doesn’t seem to be the case. Targeted drugs can have a severe impact on quality of life, probably due to prolonged duration of side effects. Quality of life should be measured and reported for all drugs.”
Dr. Gyawali and associates noted the limitation in that several studies with negative QOL results are not published at all or are published after a considerable delay, so the present observations may understate the issues that have been raised.
Dr. Gyawali declared that he received no funding and disclosed no conflicts of interest for this study.
, according to a study published in JAMA Oncology.
The study found trials that failed to show improved quality of life often reported their quality of life outcomes more favorably. Non–immunotherapy-targeted drugs were found to lead to worse quality of life outcomes more often than did cytotoxic agents. And, while there is an association between quality of life benefit and overall survival, no such association was found with progression-free survival.
“In this study, we evaluated the outcomes of cancer drug trials with regard to patients’ quality of life and found that only a quarter of phase 3 cancer drug trials in the advanced-disease setting demonstrated improved quality of life,” wrote authors who were led by Bishal Gyawali, MD, PhD, of the Cancer Research Institute, Queen’s University, Kingston, Ont.
“Improved quality of life outcomes were associated with improved overall survival but not with improved progression-free survival. Importantly, almost half of the cancer drugs drug trials that showed improved progression-free survival showed no improved overall survival or quality of life (i.e., PFS-only benefit). Some reports included conclusions regarding quality of life (QOL) findings that were not directly supported by the trial data, particularly for inferior or non–statistically significant QOL outcomes, thereby framing the findings in a favorable light or downplaying detrimental effects of the study intervention on QOL. Furthermore, contrary to common perception, inferior QOL outcomes were more common with targeted drugs than cytotoxic drugs. Taken together, these findings have important policy implications,” the authors wrote.
These findings are based on the results of a cohort study of 45 phase 3 research clinical trials of 24,806 patients. Only a small percentage of patients showed QOL benefits. The study found that industry-funded clinical trial reports often framed QOL findings more favorably than was warranted by the data.
The study found improved QOL with experimental agents in 11 of 45 randomized controlled trials (24.4%). Studies that reported improved QOL were more likely to also show improved overall survival as compared with trials in which quality of life was not improved (7 of 11 [64%] versus 10 of 34 [29%] trials). For improved progression-free survival, however, there was no positive association (6 of 11 [55%] trials versus 17 of 34 [50%] trials without improved QOL). Among six trials reporting worsening QOL, three (50%) were trials of targeted drugs. Among 11 trials reporting improved QOL, 6 (55%) were trials of immunotherapy drugs. Among the 34 trials in which QOL was not improved compared with controls, the findings were framed favorably (versus neutrally or negatively) in the abstract or conclusions in 16 (47%), an observation that was statistically significantly associated with industry funding (chi-squared = 6.35; P = .01).
“It is important to clearly understand and communicate the effects of cancer drugs”
To fulfill the obligation to inform patients about proposed treatments, the authors wrote that it is important to clearly understand and communicate the effects of cancer drugs on patient quality of life alongside their effects on overall survival and intermediate end points such as progression-free survival. “Patients with advanced cancer expect treatment to help them live longer or have better lives,” the authors wrote. In that respect, in clinical trials of cancer medicines, overall survival and quality of life are the most important measures. Toxicity profiles and disease progression delays do not reliably predict quality of life, and studies have shown poor correlations between quality of life, overall survival, and progression-free survival. This raises the question of validity of progression-free survival as a surrogate endpoint. “Progression-free survival is meaningless without overall survival or quality of life gains,” Dr. Gyawali said in an interview.
Writing in The Lancet Oncology in March, Dr. Gyawali stated that, because progression free survival “does not directly measure how a patient feels or functions, or how long a patient lives, progression-free survival was not intended to inform clinical practice or establish whether a new therapy provides clinically meaningful benefits for patients. However, over the past 2 decades, it has become the most common primary endpoint in oncology clinical trials. We are deeply worried about how the term survival in this phrase can influence clinical practice and patient choices. We propose replacing the phrase progression-free survival with a less ambiguous term: progression-free interval.”
In JAMA Oncology, Dr. Gyawali aimed to elucidate relationships between QOL, overall survival, and progression-free survival, and to assess, as well, how QOL results are framed, especially in industry-sponsored research. When drug trials they analyzed showed no change in QOL but reported that QOL did not worsen or QOL was maintained rather than stating that QOL did not improve, or if there was downplaying of worse QOL outcomes, the study had favorable interpretation, Dr. Gyawali and associates wrote. The expectation of patients receiving cancer drugs would be improved QOL rather than “not worse” QOL, Dr. Gyawali said.
Regarding the finding that QOL outcomes were described as favorable in 47% of trials with unimproved QOL outcomes, Dr. Gyawali said, “the bias in reporting should be corrected by the reviewers and editors of journals. Also, quality of life reporting should be made mandatory. Without unbiased quality of life information, informed decision making on whether or not to use a certain drug is impossible. Patients and physicians need to know that information. Regulators can demand that this should be mandatory in all trials in noncurative settings.”
He remarked further on the worsening QOL in some targeted drug trials, “People tout chemo-free regimens as automatically having better quality of life, but that doesn’t seem to be the case. Targeted drugs can have a severe impact on quality of life, probably due to prolonged duration of side effects. Quality of life should be measured and reported for all drugs.”
Dr. Gyawali and associates noted the limitation in that several studies with negative QOL results are not published at all or are published after a considerable delay, so the present observations may understate the issues that have been raised.
Dr. Gyawali declared that he received no funding and disclosed no conflicts of interest for this study.
, according to a study published in JAMA Oncology.
The study found trials that failed to show improved quality of life often reported their quality of life outcomes more favorably. Non–immunotherapy-targeted drugs were found to lead to worse quality of life outcomes more often than did cytotoxic agents. And, while there is an association between quality of life benefit and overall survival, no such association was found with progression-free survival.
“In this study, we evaluated the outcomes of cancer drug trials with regard to patients’ quality of life and found that only a quarter of phase 3 cancer drug trials in the advanced-disease setting demonstrated improved quality of life,” wrote authors who were led by Bishal Gyawali, MD, PhD, of the Cancer Research Institute, Queen’s University, Kingston, Ont.
“Improved quality of life outcomes were associated with improved overall survival but not with improved progression-free survival. Importantly, almost half of the cancer drugs drug trials that showed improved progression-free survival showed no improved overall survival or quality of life (i.e., PFS-only benefit). Some reports included conclusions regarding quality of life (QOL) findings that were not directly supported by the trial data, particularly for inferior or non–statistically significant QOL outcomes, thereby framing the findings in a favorable light or downplaying detrimental effects of the study intervention on QOL. Furthermore, contrary to common perception, inferior QOL outcomes were more common with targeted drugs than cytotoxic drugs. Taken together, these findings have important policy implications,” the authors wrote.
These findings are based on the results of a cohort study of 45 phase 3 research clinical trials of 24,806 patients. Only a small percentage of patients showed QOL benefits. The study found that industry-funded clinical trial reports often framed QOL findings more favorably than was warranted by the data.
The study found improved QOL with experimental agents in 11 of 45 randomized controlled trials (24.4%). Studies that reported improved QOL were more likely to also show improved overall survival as compared with trials in which quality of life was not improved (7 of 11 [64%] versus 10 of 34 [29%] trials). For improved progression-free survival, however, there was no positive association (6 of 11 [55%] trials versus 17 of 34 [50%] trials without improved QOL). Among six trials reporting worsening QOL, three (50%) were trials of targeted drugs. Among 11 trials reporting improved QOL, 6 (55%) were trials of immunotherapy drugs. Among the 34 trials in which QOL was not improved compared with controls, the findings were framed favorably (versus neutrally or negatively) in the abstract or conclusions in 16 (47%), an observation that was statistically significantly associated with industry funding (chi-squared = 6.35; P = .01).
“It is important to clearly understand and communicate the effects of cancer drugs”
To fulfill the obligation to inform patients about proposed treatments, the authors wrote that it is important to clearly understand and communicate the effects of cancer drugs on patient quality of life alongside their effects on overall survival and intermediate end points such as progression-free survival. “Patients with advanced cancer expect treatment to help them live longer or have better lives,” the authors wrote. In that respect, in clinical trials of cancer medicines, overall survival and quality of life are the most important measures. Toxicity profiles and disease progression delays do not reliably predict quality of life, and studies have shown poor correlations between quality of life, overall survival, and progression-free survival. This raises the question of validity of progression-free survival as a surrogate endpoint. “Progression-free survival is meaningless without overall survival or quality of life gains,” Dr. Gyawali said in an interview.
Writing in The Lancet Oncology in March, Dr. Gyawali stated that, because progression free survival “does not directly measure how a patient feels or functions, or how long a patient lives, progression-free survival was not intended to inform clinical practice or establish whether a new therapy provides clinically meaningful benefits for patients. However, over the past 2 decades, it has become the most common primary endpoint in oncology clinical trials. We are deeply worried about how the term survival in this phrase can influence clinical practice and patient choices. We propose replacing the phrase progression-free survival with a less ambiguous term: progression-free interval.”
In JAMA Oncology, Dr. Gyawali aimed to elucidate relationships between QOL, overall survival, and progression-free survival, and to assess, as well, how QOL results are framed, especially in industry-sponsored research. When drug trials they analyzed showed no change in QOL but reported that QOL did not worsen or QOL was maintained rather than stating that QOL did not improve, or if there was downplaying of worse QOL outcomes, the study had favorable interpretation, Dr. Gyawali and associates wrote. The expectation of patients receiving cancer drugs would be improved QOL rather than “not worse” QOL, Dr. Gyawali said.
Regarding the finding that QOL outcomes were described as favorable in 47% of trials with unimproved QOL outcomes, Dr. Gyawali said, “the bias in reporting should be corrected by the reviewers and editors of journals. Also, quality of life reporting should be made mandatory. Without unbiased quality of life information, informed decision making on whether or not to use a certain drug is impossible. Patients and physicians need to know that information. Regulators can demand that this should be mandatory in all trials in noncurative settings.”
He remarked further on the worsening QOL in some targeted drug trials, “People tout chemo-free regimens as automatically having better quality of life, but that doesn’t seem to be the case. Targeted drugs can have a severe impact on quality of life, probably due to prolonged duration of side effects. Quality of life should be measured and reported for all drugs.”
Dr. Gyawali and associates noted the limitation in that several studies with negative QOL results are not published at all or are published after a considerable delay, so the present observations may understate the issues that have been raised.
Dr. Gyawali declared that he received no funding and disclosed no conflicts of interest for this study.
FROM JAMA ONCOLOGY
Will the headache field embrace rofecoxib?
In June, the Concord, Mass.–based company Tremeau Pharmaceuticals announced that the Food and Drug Administration was letting it proceed with a phase 3 clinical trial to test rofecoxib, the once-bestselling painkiller known as Vioxx, in patients with migraine.
The anti-inflammatory drug, a cyclooxygenase-2 (COX-2) inhibitor, received its first FDA approval in 1999 and became widely prescribed for arthritis and acute pain. In 2004 it was withdrawn by its manufacturer, Merck, after being shown to raise the risk of cardiovascular events.
In clinical trials and in real-world epidemiological studies, rofecoxib was associated with elevated heart attack, stroke, and related deaths; one 2005 study estimated that it had been responsible for some 38,000 excess deaths in the United States before being withdrawn. In 2007 Merck, beset with allegations that it had suppressed and mischaracterized rofecoxib’s safety data, paid out nearly $5 billion to settle thousands of lawsuits filed by patients and their families.
, an indication for which it received an orphan drug designation in 2017 and the agency’s green light for trials in 2020.
Brad Sippy, Tremeau’s chief executive officer, said that his company chose the two indications in part because both patient populations have low cardiovascular risk. Migraine patients are generally younger than the arthritis populations formerly treated with rofecoxib and are unlikely to take the drug for more than a day or 2 at time, avoiding the risks associated with extended exposure.
A crowded market
The past several years have seen the emergence of a cornucopia of new migraine treatments, including monoclonal antibodies such as erenumab (Aimovig, Amgen), which help prevent attacks by blocking the vasodilator calcitonin gene-related peptide, or CGRP. In addition to the standard arsenal of triptans and nonsteroidal anti-inflammatory drugs for acute pain relief, migraine patients can now choose among serotonin-blocking agents such as lasmiditan (Reyvow, Eli Lilly), known as “ditans,” and small-molecule CGRP antagonists such as ubrogepant (Ubrelvy, Abbie), known as “gepants.” Some NSAIDs, including one COX inhibitor, have been formulated into rapidly absorbed powders or liquids for migraine.
Mr. Sippy said he sees a role for rofecoxib even in this crowded space. “Migraine as you know is a multimodal situation – few people say that only one drug works for them,” he said. “We think this is an option that would basically be like a high dose of ibuprofen,” but with less frequent dosing and lower gastrointestinal and platelet effects compared with ibuprofen and other NSAIDs.
An improved formulation
Rofecoxib “crosses the blood brain barrier very readily – better than other COX inhibitors on the market,” Mr. Sippy added. “It was well absorbed in its original formulation, and our product is even better absorbed than the original – we estimate it’s probably an hour quicker to [peak concentration].” In addition, he said, “our formulation is more efficient at delivering the drug so we don’t need as much active ingredient – our 17.5 milligrams gets you the same systemic exposure as 25 milligrams of the old product.”
A different mechanism of action
Neurologist Alan M. Rapoport, MD, editor-in-chief of Neurology Reviews and professor of neurology at the University of California, Los Angeles, said that he was “cautiously optimistic” that “if used correctly and not too frequently, [rofecoxib] will find its niche in migraine treatment.”
“Patients liked Vioxx,” said Dr. Rapoport, past president of the International Headache Society. Even people currently on prevention “need to have an acute care drug handy.” While some patients on monoclonal antibodies have had success with gepants for acute care, “these both target the same pathway. It’s always nice to have options with a different mechanism of action.”
One of the arguments Tremeau has cited for reintroducing rofecoxib has been an urgent need for alternatives to opioid painkillers. Indeed some analysts have linked the demise of Vioxx with a subsequent increase in opioid prescribing.
Dr. Rapoport noted that he never prescribes opioids or butalbital, a barbiturate, for migraine, and that most headache specialists avoid them in clinical practice. But in the emergency setting, he said, patients receive them all too frequently.
Mr. Sippy said that opioid prescribing, while not unknown in migraine, was a bigger problem in hemophilic arthropathy, the first indication his company has pursued for rofecoxib. People with hemophilia “have a kind of arthritis that would respond well to an anti-inflammatory drug but they can’t take NSAIDs due to bleeding risk. This is why so many end up on opioids. Rofecoxib, as a COX-2 inhibitor, doesn’t have any effect on platelet aggregation, which would make it another option.”
No unique risks at prescribed doses
The migraine indication originally started out narrower: Patients with both migraine and bleeding disorders. “But in talking with the FDA, they encouraged us to develop it for migraine,” Mr. Sippy said. The company is considering pursuing a third indication: menstrual pain co-occurring with migraine. Tremeau has not ruled out seeking an indication in patients with arthritis who cannot take other painkillers, whether opioids or NSAIDs.
Five years ago, when Tremeau first announced its plans to bring rofecoxib back – indeed the company was set up for that purpose and has only this and another COX-2 inhibitor in development – some experts warned that there is little to prevent the drug from being used off-label, whether in higher doses or for other diseases.
“That’s something else we’re seeking to solve in addition to going for younger populations,” said Mr. Sippy, who worked at Merck during the Vioxx crisis and later headed neurology at Sunovion before starting his own company.
“We’re going for the former middle dose as our high dose and now we know that you don’t want to take more than the prescribed amount. If it doesn’t work you get off it; you don’t want to dose-creep on it. That’s been a key insight: At the appropriate dose, this product has no unique risk relative to the drug class and potentially some unique benefits,” he said.
Risk versus benefit
Joseph Ross, MD, a health policy researcher at Yale University in New Haven, Conn., who in a 2018 editorial expressed concerns about rofecoxib’s revival, said in an email that he felt its use in migraine could be justified, with caveats.
During Vioxx’s original approval and time on the market, “there was a cardiovascular risk associated with use that was not being transparently and clearly reported to patients and clinicians,” Dr. Ross said.
“In terms of testing the product for use in patients with migraine – a population of generally younger patients at lower risk of cardiovascular disease – my only concern is that the risk is clearly communicated and that there is adequate postmarket safety surveillance,” he said. “If patients are making fully informed decisions, the potential benefit of the drug with respect to pain control may be worth the risks.”
Dr. Rapoport serves as an adviser for AbbVie, Amgen, Biohaven, Cala Health, Collegium Pharmaceutical, Satsuma, Teva, Theranica and Xoc; he is on the speakers bureau of AbbVie, Amgen, Biohaven, Impel, Lundbeck, and Teva. Dr. Ross disclosed research support from Johnson and Johnson, the Medical Device Innovation Consortium, and the Laura and John Arnold Foundation, along with government grants; he is also an expert witness in a lawsuit against Biogen.
In June, the Concord, Mass.–based company Tremeau Pharmaceuticals announced that the Food and Drug Administration was letting it proceed with a phase 3 clinical trial to test rofecoxib, the once-bestselling painkiller known as Vioxx, in patients with migraine.
The anti-inflammatory drug, a cyclooxygenase-2 (COX-2) inhibitor, received its first FDA approval in 1999 and became widely prescribed for arthritis and acute pain. In 2004 it was withdrawn by its manufacturer, Merck, after being shown to raise the risk of cardiovascular events.
In clinical trials and in real-world epidemiological studies, rofecoxib was associated with elevated heart attack, stroke, and related deaths; one 2005 study estimated that it had been responsible for some 38,000 excess deaths in the United States before being withdrawn. In 2007 Merck, beset with allegations that it had suppressed and mischaracterized rofecoxib’s safety data, paid out nearly $5 billion to settle thousands of lawsuits filed by patients and their families.
, an indication for which it received an orphan drug designation in 2017 and the agency’s green light for trials in 2020.
Brad Sippy, Tremeau’s chief executive officer, said that his company chose the two indications in part because both patient populations have low cardiovascular risk. Migraine patients are generally younger than the arthritis populations formerly treated with rofecoxib and are unlikely to take the drug for more than a day or 2 at time, avoiding the risks associated with extended exposure.
A crowded market
The past several years have seen the emergence of a cornucopia of new migraine treatments, including monoclonal antibodies such as erenumab (Aimovig, Amgen), which help prevent attacks by blocking the vasodilator calcitonin gene-related peptide, or CGRP. In addition to the standard arsenal of triptans and nonsteroidal anti-inflammatory drugs for acute pain relief, migraine patients can now choose among serotonin-blocking agents such as lasmiditan (Reyvow, Eli Lilly), known as “ditans,” and small-molecule CGRP antagonists such as ubrogepant (Ubrelvy, Abbie), known as “gepants.” Some NSAIDs, including one COX inhibitor, have been formulated into rapidly absorbed powders or liquids for migraine.
Mr. Sippy said he sees a role for rofecoxib even in this crowded space. “Migraine as you know is a multimodal situation – few people say that only one drug works for them,” he said. “We think this is an option that would basically be like a high dose of ibuprofen,” but with less frequent dosing and lower gastrointestinal and platelet effects compared with ibuprofen and other NSAIDs.
An improved formulation
Rofecoxib “crosses the blood brain barrier very readily – better than other COX inhibitors on the market,” Mr. Sippy added. “It was well absorbed in its original formulation, and our product is even better absorbed than the original – we estimate it’s probably an hour quicker to [peak concentration].” In addition, he said, “our formulation is more efficient at delivering the drug so we don’t need as much active ingredient – our 17.5 milligrams gets you the same systemic exposure as 25 milligrams of the old product.”
A different mechanism of action
Neurologist Alan M. Rapoport, MD, editor-in-chief of Neurology Reviews and professor of neurology at the University of California, Los Angeles, said that he was “cautiously optimistic” that “if used correctly and not too frequently, [rofecoxib] will find its niche in migraine treatment.”
“Patients liked Vioxx,” said Dr. Rapoport, past president of the International Headache Society. Even people currently on prevention “need to have an acute care drug handy.” While some patients on monoclonal antibodies have had success with gepants for acute care, “these both target the same pathway. It’s always nice to have options with a different mechanism of action.”
One of the arguments Tremeau has cited for reintroducing rofecoxib has been an urgent need for alternatives to opioid painkillers. Indeed some analysts have linked the demise of Vioxx with a subsequent increase in opioid prescribing.
Dr. Rapoport noted that he never prescribes opioids or butalbital, a barbiturate, for migraine, and that most headache specialists avoid them in clinical practice. But in the emergency setting, he said, patients receive them all too frequently.
Mr. Sippy said that opioid prescribing, while not unknown in migraine, was a bigger problem in hemophilic arthropathy, the first indication his company has pursued for rofecoxib. People with hemophilia “have a kind of arthritis that would respond well to an anti-inflammatory drug but they can’t take NSAIDs due to bleeding risk. This is why so many end up on opioids. Rofecoxib, as a COX-2 inhibitor, doesn’t have any effect on platelet aggregation, which would make it another option.”
No unique risks at prescribed doses
The migraine indication originally started out narrower: Patients with both migraine and bleeding disorders. “But in talking with the FDA, they encouraged us to develop it for migraine,” Mr. Sippy said. The company is considering pursuing a third indication: menstrual pain co-occurring with migraine. Tremeau has not ruled out seeking an indication in patients with arthritis who cannot take other painkillers, whether opioids or NSAIDs.
Five years ago, when Tremeau first announced its plans to bring rofecoxib back – indeed the company was set up for that purpose and has only this and another COX-2 inhibitor in development – some experts warned that there is little to prevent the drug from being used off-label, whether in higher doses or for other diseases.
“That’s something else we’re seeking to solve in addition to going for younger populations,” said Mr. Sippy, who worked at Merck during the Vioxx crisis and later headed neurology at Sunovion before starting his own company.
“We’re going for the former middle dose as our high dose and now we know that you don’t want to take more than the prescribed amount. If it doesn’t work you get off it; you don’t want to dose-creep on it. That’s been a key insight: At the appropriate dose, this product has no unique risk relative to the drug class and potentially some unique benefits,” he said.
Risk versus benefit
Joseph Ross, MD, a health policy researcher at Yale University in New Haven, Conn., who in a 2018 editorial expressed concerns about rofecoxib’s revival, said in an email that he felt its use in migraine could be justified, with caveats.
During Vioxx’s original approval and time on the market, “there was a cardiovascular risk associated with use that was not being transparently and clearly reported to patients and clinicians,” Dr. Ross said.
“In terms of testing the product for use in patients with migraine – a population of generally younger patients at lower risk of cardiovascular disease – my only concern is that the risk is clearly communicated and that there is adequate postmarket safety surveillance,” he said. “If patients are making fully informed decisions, the potential benefit of the drug with respect to pain control may be worth the risks.”
Dr. Rapoport serves as an adviser for AbbVie, Amgen, Biohaven, Cala Health, Collegium Pharmaceutical, Satsuma, Teva, Theranica and Xoc; he is on the speakers bureau of AbbVie, Amgen, Biohaven, Impel, Lundbeck, and Teva. Dr. Ross disclosed research support from Johnson and Johnson, the Medical Device Innovation Consortium, and the Laura and John Arnold Foundation, along with government grants; he is also an expert witness in a lawsuit against Biogen.
In June, the Concord, Mass.–based company Tremeau Pharmaceuticals announced that the Food and Drug Administration was letting it proceed with a phase 3 clinical trial to test rofecoxib, the once-bestselling painkiller known as Vioxx, in patients with migraine.
The anti-inflammatory drug, a cyclooxygenase-2 (COX-2) inhibitor, received its first FDA approval in 1999 and became widely prescribed for arthritis and acute pain. In 2004 it was withdrawn by its manufacturer, Merck, after being shown to raise the risk of cardiovascular events.
In clinical trials and in real-world epidemiological studies, rofecoxib was associated with elevated heart attack, stroke, and related deaths; one 2005 study estimated that it had been responsible for some 38,000 excess deaths in the United States before being withdrawn. In 2007 Merck, beset with allegations that it had suppressed and mischaracterized rofecoxib’s safety data, paid out nearly $5 billion to settle thousands of lawsuits filed by patients and their families.
, an indication for which it received an orphan drug designation in 2017 and the agency’s green light for trials in 2020.
Brad Sippy, Tremeau’s chief executive officer, said that his company chose the two indications in part because both patient populations have low cardiovascular risk. Migraine patients are generally younger than the arthritis populations formerly treated with rofecoxib and are unlikely to take the drug for more than a day or 2 at time, avoiding the risks associated with extended exposure.
A crowded market
The past several years have seen the emergence of a cornucopia of new migraine treatments, including monoclonal antibodies such as erenumab (Aimovig, Amgen), which help prevent attacks by blocking the vasodilator calcitonin gene-related peptide, or CGRP. In addition to the standard arsenal of triptans and nonsteroidal anti-inflammatory drugs for acute pain relief, migraine patients can now choose among serotonin-blocking agents such as lasmiditan (Reyvow, Eli Lilly), known as “ditans,” and small-molecule CGRP antagonists such as ubrogepant (Ubrelvy, Abbie), known as “gepants.” Some NSAIDs, including one COX inhibitor, have been formulated into rapidly absorbed powders or liquids for migraine.
Mr. Sippy said he sees a role for rofecoxib even in this crowded space. “Migraine as you know is a multimodal situation – few people say that only one drug works for them,” he said. “We think this is an option that would basically be like a high dose of ibuprofen,” but with less frequent dosing and lower gastrointestinal and platelet effects compared with ibuprofen and other NSAIDs.
An improved formulation
Rofecoxib “crosses the blood brain barrier very readily – better than other COX inhibitors on the market,” Mr. Sippy added. “It was well absorbed in its original formulation, and our product is even better absorbed than the original – we estimate it’s probably an hour quicker to [peak concentration].” In addition, he said, “our formulation is more efficient at delivering the drug so we don’t need as much active ingredient – our 17.5 milligrams gets you the same systemic exposure as 25 milligrams of the old product.”
A different mechanism of action
Neurologist Alan M. Rapoport, MD, editor-in-chief of Neurology Reviews and professor of neurology at the University of California, Los Angeles, said that he was “cautiously optimistic” that “if used correctly and not too frequently, [rofecoxib] will find its niche in migraine treatment.”
“Patients liked Vioxx,” said Dr. Rapoport, past president of the International Headache Society. Even people currently on prevention “need to have an acute care drug handy.” While some patients on monoclonal antibodies have had success with gepants for acute care, “these both target the same pathway. It’s always nice to have options with a different mechanism of action.”
One of the arguments Tremeau has cited for reintroducing rofecoxib has been an urgent need for alternatives to opioid painkillers. Indeed some analysts have linked the demise of Vioxx with a subsequent increase in opioid prescribing.
Dr. Rapoport noted that he never prescribes opioids or butalbital, a barbiturate, for migraine, and that most headache specialists avoid them in clinical practice. But in the emergency setting, he said, patients receive them all too frequently.
Mr. Sippy said that opioid prescribing, while not unknown in migraine, was a bigger problem in hemophilic arthropathy, the first indication his company has pursued for rofecoxib. People with hemophilia “have a kind of arthritis that would respond well to an anti-inflammatory drug but they can’t take NSAIDs due to bleeding risk. This is why so many end up on opioids. Rofecoxib, as a COX-2 inhibitor, doesn’t have any effect on platelet aggregation, which would make it another option.”
No unique risks at prescribed doses
The migraine indication originally started out narrower: Patients with both migraine and bleeding disorders. “But in talking with the FDA, they encouraged us to develop it for migraine,” Mr. Sippy said. The company is considering pursuing a third indication: menstrual pain co-occurring with migraine. Tremeau has not ruled out seeking an indication in patients with arthritis who cannot take other painkillers, whether opioids or NSAIDs.
Five years ago, when Tremeau first announced its plans to bring rofecoxib back – indeed the company was set up for that purpose and has only this and another COX-2 inhibitor in development – some experts warned that there is little to prevent the drug from being used off-label, whether in higher doses or for other diseases.
“That’s something else we’re seeking to solve in addition to going for younger populations,” said Mr. Sippy, who worked at Merck during the Vioxx crisis and later headed neurology at Sunovion before starting his own company.
“We’re going for the former middle dose as our high dose and now we know that you don’t want to take more than the prescribed amount. If it doesn’t work you get off it; you don’t want to dose-creep on it. That’s been a key insight: At the appropriate dose, this product has no unique risk relative to the drug class and potentially some unique benefits,” he said.
Risk versus benefit
Joseph Ross, MD, a health policy researcher at Yale University in New Haven, Conn., who in a 2018 editorial expressed concerns about rofecoxib’s revival, said in an email that he felt its use in migraine could be justified, with caveats.
During Vioxx’s original approval and time on the market, “there was a cardiovascular risk associated with use that was not being transparently and clearly reported to patients and clinicians,” Dr. Ross said.
“In terms of testing the product for use in patients with migraine – a population of generally younger patients at lower risk of cardiovascular disease – my only concern is that the risk is clearly communicated and that there is adequate postmarket safety surveillance,” he said. “If patients are making fully informed decisions, the potential benefit of the drug with respect to pain control may be worth the risks.”
Dr. Rapoport serves as an adviser for AbbVie, Amgen, Biohaven, Cala Health, Collegium Pharmaceutical, Satsuma, Teva, Theranica and Xoc; he is on the speakers bureau of AbbVie, Amgen, Biohaven, Impel, Lundbeck, and Teva. Dr. Ross disclosed research support from Johnson and Johnson, the Medical Device Innovation Consortium, and the Laura and John Arnold Foundation, along with government grants; he is also an expert witness in a lawsuit against Biogen.