User login
Lung adverse effects in patients taking trastuzumab deruxtecan
although the benefit-to-risk relationship with use of the drug is still positive, say researchers who report a review of early clinical trials with the drug.
T-DXd is a monoclonal antibody that targets HER2. It is approved for use in HER2-positive breast, gastric, and lung cancers.
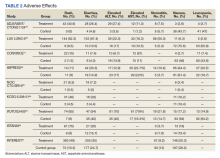
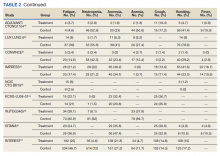
In the new study, investigators analyzed data from early clinical trials that involved patients with advanced cancers who had been heavily pretreated. They found an incidence of just over 15% for interstitial lung disease (ILD)/pneumonitis associated with the drug. Most patients (77.4%) had grade 1 or 2 ILD, but 2.2% of patients had grade 5 ILD.
“Interstitial lung disease is a known risk factor in patients treated with antibody conjugates for cancer,” commented lead author Charles Powell, MD, Icahn School of Medicine at Mount Sinai, New York. This adverse effect can lead to lung fibrosis and can become severe, life threatening, and even fatal, the authors warned.
The authors also discussed management of the event, which involves corticosteroids, and recommended that any patient who develops ILD of grade 3 or higher be hospitalized.
Close monitoring and proactive management may reduce the risk of ILD, they suggested.
Indeed, the incidence of this adverse effect was lower in a later phase 3 trial of the drug (10.5% in the DESTINY-Breast03 trial) and that the adverse events were less severe in this patient population (none of these events were of grade 4 or 5).
“Increased knowledge ... and implementation of ILD/pneumonitis monitoring, diagnosis, and management guidelines” may have resulted in this adverse effect being identified early and treated before it progressed, they commented.
ILD is highlighted in a boxed warning on the product label.
The study was published online in ESMO Open.
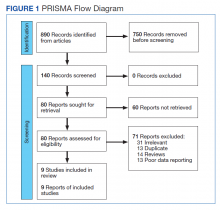
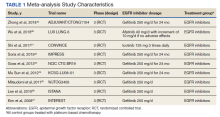
In their review, the investigators evaluated nine early-stage monotherapy clinical trials (phases 1 and 2) involving a total of 1,150 patients (breast cancer, 44.3%; gastric cancer, 25.6%; lung cancer, 17.7%; colorectal cancer, 9.3%, other cancers, 3.0%).
These patients had advanced cancer and had been heavily pretreated with a median of four prior lines of therapy. They received one or more doses of at least 5.4 mg/kg of T-DXd.
Nearly half of the cohort were treated for more than 6 months. A total of 276 potential ILD/pneumonitis events were sent for adjudication; of those, 85% were adjudicated as ILD/pneumonitis.
The overall incidence of adjudicated ILD/pneumonitis events was 15.4%; most were low-grade events. Some 87% of patients experienced their first ILD event within 12 months of treatment. The median time to experiencing an ILD/pneumonitis event was 5.4 months.
Some of the patients who developed grade 1 ILD/pneumonitis were treated and the adverse event resolved. These patients were then rechallenged with the drug. Only 3 of the 47 rechallenged patients experienced recurrence of ILD/pneumonitis, the authors noted.
“Rechallenge with T-DXd after complete resolution of grade 1 events is possible and warrants further investigation,” they commented. They cautioned, however, that rechallenge is not recommended for all patients, at least not for those with grade 2 or higher ILD/pneumonitis.
Overall, the authors concluded that the “benefit-risk of T-DXd treatment is positive,” but they warned that some patients may be at increased risk of developing ILD/pneumonitis
Baseline factors that increase the risk of developing an ILD/pneumonitis event include the following: being younger than 65 years, receiving a T-DXd dose of more than6.4 mg/kg, having a baseline oxygen saturation level of less than 95%, having moderate to severe renal impairment, and having lung comorbidities. In addition, patients who had initially been diagnosed with cancer more than 4 years before receiving the drug were at higher risk of developing ILD/pneumonitis.
“Using learnings from the early clinical trials experience, physician education and patient management protocols were revised and disseminated by the study sponsors [and] more recent trial data in earlier lines of therapy has demonstrated lower rates of ILD events, suggesting close monitoring and proactive management of ILD/pneumonitis is warranted for all patients,” Dr. Powell said in a statement.
The T-DXd clinical trials were sponsored by AstraZeneca and Daiichi Sankyo. Dr. Powell has received fees from Daiichi Sankyo, AstraZeneca, and Voluntis.
A version of this article first appeared on Medscape.com.
although the benefit-to-risk relationship with use of the drug is still positive, say researchers who report a review of early clinical trials with the drug.
T-DXd is a monoclonal antibody that targets HER2. It is approved for use in HER2-positive breast, gastric, and lung cancers.
In the new study, investigators analyzed data from early clinical trials that involved patients with advanced cancers who had been heavily pretreated. They found an incidence of just over 15% for interstitial lung disease (ILD)/pneumonitis associated with the drug. Most patients (77.4%) had grade 1 or 2 ILD, but 2.2% of patients had grade 5 ILD.
“Interstitial lung disease is a known risk factor in patients treated with antibody conjugates for cancer,” commented lead author Charles Powell, MD, Icahn School of Medicine at Mount Sinai, New York. This adverse effect can lead to lung fibrosis and can become severe, life threatening, and even fatal, the authors warned.
The authors also discussed management of the event, which involves corticosteroids, and recommended that any patient who develops ILD of grade 3 or higher be hospitalized.
Close monitoring and proactive management may reduce the risk of ILD, they suggested.
Indeed, the incidence of this adverse effect was lower in a later phase 3 trial of the drug (10.5% in the DESTINY-Breast03 trial) and that the adverse events were less severe in this patient population (none of these events were of grade 4 or 5).
“Increased knowledge ... and implementation of ILD/pneumonitis monitoring, diagnosis, and management guidelines” may have resulted in this adverse effect being identified early and treated before it progressed, they commented.
ILD is highlighted in a boxed warning on the product label.
The study was published online in ESMO Open.
In their review, the investigators evaluated nine early-stage monotherapy clinical trials (phases 1 and 2) involving a total of 1,150 patients (breast cancer, 44.3%; gastric cancer, 25.6%; lung cancer, 17.7%; colorectal cancer, 9.3%, other cancers, 3.0%).
These patients had advanced cancer and had been heavily pretreated with a median of four prior lines of therapy. They received one or more doses of at least 5.4 mg/kg of T-DXd.
Nearly half of the cohort were treated for more than 6 months. A total of 276 potential ILD/pneumonitis events were sent for adjudication; of those, 85% were adjudicated as ILD/pneumonitis.
The overall incidence of adjudicated ILD/pneumonitis events was 15.4%; most were low-grade events. Some 87% of patients experienced their first ILD event within 12 months of treatment. The median time to experiencing an ILD/pneumonitis event was 5.4 months.
Some of the patients who developed grade 1 ILD/pneumonitis were treated and the adverse event resolved. These patients were then rechallenged with the drug. Only 3 of the 47 rechallenged patients experienced recurrence of ILD/pneumonitis, the authors noted.
“Rechallenge with T-DXd after complete resolution of grade 1 events is possible and warrants further investigation,” they commented. They cautioned, however, that rechallenge is not recommended for all patients, at least not for those with grade 2 or higher ILD/pneumonitis.
Overall, the authors concluded that the “benefit-risk of T-DXd treatment is positive,” but they warned that some patients may be at increased risk of developing ILD/pneumonitis
Baseline factors that increase the risk of developing an ILD/pneumonitis event include the following: being younger than 65 years, receiving a T-DXd dose of more than6.4 mg/kg, having a baseline oxygen saturation level of less than 95%, having moderate to severe renal impairment, and having lung comorbidities. In addition, patients who had initially been diagnosed with cancer more than 4 years before receiving the drug were at higher risk of developing ILD/pneumonitis.
“Using learnings from the early clinical trials experience, physician education and patient management protocols were revised and disseminated by the study sponsors [and] more recent trial data in earlier lines of therapy has demonstrated lower rates of ILD events, suggesting close monitoring and proactive management of ILD/pneumonitis is warranted for all patients,” Dr. Powell said in a statement.
The T-DXd clinical trials were sponsored by AstraZeneca and Daiichi Sankyo. Dr. Powell has received fees from Daiichi Sankyo, AstraZeneca, and Voluntis.
A version of this article first appeared on Medscape.com.
although the benefit-to-risk relationship with use of the drug is still positive, say researchers who report a review of early clinical trials with the drug.
T-DXd is a monoclonal antibody that targets HER2. It is approved for use in HER2-positive breast, gastric, and lung cancers.
In the new study, investigators analyzed data from early clinical trials that involved patients with advanced cancers who had been heavily pretreated. They found an incidence of just over 15% for interstitial lung disease (ILD)/pneumonitis associated with the drug. Most patients (77.4%) had grade 1 or 2 ILD, but 2.2% of patients had grade 5 ILD.
“Interstitial lung disease is a known risk factor in patients treated with antibody conjugates for cancer,” commented lead author Charles Powell, MD, Icahn School of Medicine at Mount Sinai, New York. This adverse effect can lead to lung fibrosis and can become severe, life threatening, and even fatal, the authors warned.
The authors also discussed management of the event, which involves corticosteroids, and recommended that any patient who develops ILD of grade 3 or higher be hospitalized.
Close monitoring and proactive management may reduce the risk of ILD, they suggested.
Indeed, the incidence of this adverse effect was lower in a later phase 3 trial of the drug (10.5% in the DESTINY-Breast03 trial) and that the adverse events were less severe in this patient population (none of these events were of grade 4 or 5).
“Increased knowledge ... and implementation of ILD/pneumonitis monitoring, diagnosis, and management guidelines” may have resulted in this adverse effect being identified early and treated before it progressed, they commented.
ILD is highlighted in a boxed warning on the product label.
The study was published online in ESMO Open.
In their review, the investigators evaluated nine early-stage monotherapy clinical trials (phases 1 and 2) involving a total of 1,150 patients (breast cancer, 44.3%; gastric cancer, 25.6%; lung cancer, 17.7%; colorectal cancer, 9.3%, other cancers, 3.0%).
These patients had advanced cancer and had been heavily pretreated with a median of four prior lines of therapy. They received one or more doses of at least 5.4 mg/kg of T-DXd.
Nearly half of the cohort were treated for more than 6 months. A total of 276 potential ILD/pneumonitis events were sent for adjudication; of those, 85% were adjudicated as ILD/pneumonitis.
The overall incidence of adjudicated ILD/pneumonitis events was 15.4%; most were low-grade events. Some 87% of patients experienced their first ILD event within 12 months of treatment. The median time to experiencing an ILD/pneumonitis event was 5.4 months.
Some of the patients who developed grade 1 ILD/pneumonitis were treated and the adverse event resolved. These patients were then rechallenged with the drug. Only 3 of the 47 rechallenged patients experienced recurrence of ILD/pneumonitis, the authors noted.
“Rechallenge with T-DXd after complete resolution of grade 1 events is possible and warrants further investigation,” they commented. They cautioned, however, that rechallenge is not recommended for all patients, at least not for those with grade 2 or higher ILD/pneumonitis.
Overall, the authors concluded that the “benefit-risk of T-DXd treatment is positive,” but they warned that some patients may be at increased risk of developing ILD/pneumonitis
Baseline factors that increase the risk of developing an ILD/pneumonitis event include the following: being younger than 65 years, receiving a T-DXd dose of more than6.4 mg/kg, having a baseline oxygen saturation level of less than 95%, having moderate to severe renal impairment, and having lung comorbidities. In addition, patients who had initially been diagnosed with cancer more than 4 years before receiving the drug were at higher risk of developing ILD/pneumonitis.
“Using learnings from the early clinical trials experience, physician education and patient management protocols were revised and disseminated by the study sponsors [and] more recent trial data in earlier lines of therapy has demonstrated lower rates of ILD events, suggesting close monitoring and proactive management of ILD/pneumonitis is warranted for all patients,” Dr. Powell said in a statement.
The T-DXd clinical trials were sponsored by AstraZeneca and Daiichi Sankyo. Dr. Powell has received fees from Daiichi Sankyo, AstraZeneca, and Voluntis.
A version of this article first appeared on Medscape.com.
FROM ESMO OPEN
The ‘great dynamism’ of radiation oncology
The field of radiation oncology has rapidly evolved in recent years, thanks in large part to findings from randomized clinical trials (RCTs) that have helped shift therapeutic standards, a review of the literature shows.
Highlights from this research reveal how high-tech radiotherapy, such as hypofractionation and stereotactic body radiotherapy, has improved care for many patients, how personalized radiotherapy using image-based guidance has helped tailor treatments, and how endpoints that focus on quality of life and patient satisfaction are emerging.
For instance, Charles B. Simone II, MD, FACRO, who was not involved in the current work, pointed to “a proliferation of trials assessing hypofractionation in the curative setting and stereotactic body radiation therapy in the curative and poly- and oligometastatic settings that have allowed for increased patient convenience and dose intensification, respectively.”
Dr. Simone, chief medical officer, New York Proton Center, Memorial Sloan Kettering Cancer Center, also noted that the first personalized radiotherapy trials using imaging and biological markers have “the profound potential to individualize treatment and improve patient outcomes.”
The review was published in the European Journal of Cancer.
An evolving field
Given the fast-changing landscape for cancer therapeutics and a deluge of research studies, the authors wanted to understand the most notable advances established in recent trials as well as caveats to some approaches and emerging areas to watch.
In the review, Sophie Espenel, MD, from the department of radiation oncology, Gustave Roussy Cancer Campus, Villejuif, France, and colleagues identified 1,347 radiotherapy RCTs that were conducted from January 2018 to December 2021. Of these, the authors selected 110 large phase 2 or 3 RCTs that contained data showing practice-changing or emerging concepts.
Overall, the studies showed “great dynamism” in radiation oncology research and covered a wide range of radiotherapy practices, according to Dr. Espenel and coauthors.
A central area of research has focused on radioimmunotherapy, an approach that aims to enhance the antitumor immune response. One RCT in the preoperative setting showed, for instance, that concurrent stereotactic body radiotherapy delivered at 24 Gy over eight fractions, along with the anti–PD-L1 agent durvalumab, increased major pathologic complete response rates almost eightfold in comparison with durvalumab alone for patients with early-stage lung cancer (53.3% vs. 6.7%).
Although promising, not all trials that evaluated a concurrent chemoradiotherapy-immunotherapy strategy showed positive results. One RCT of locally advanced head and neck squamous cell carcinoma, for instance, found that median progression-free survival was not reached when adding the anti–PD-L1 avelumab to chemoradiotherapy. In addition, trials in the metastatic setting have shown conflicting results, the authors note.
Another topic of interest is that of newer radiosensitizers. A trial that evaluated high-risk locoregionally advanced head and neck squamous cell carcinoma highlighted the efficacy of xevinapant, a pro-apoptotic agent that inhibits apoptosis proteins. Xevinapant was used for the first time in conjunction with a standard high-dose cisplatin chemoradiotherapy. In this study, locoregional control at 18 months was achieved for 54% of patients who received xevinapant vs. 33% of those who received standard care. The toxicity profiles were similar.
The use of high-tech radiotherapy is gaining ground. It allows patients to receive more targeted treatments at lower doses and in shorter time frames. One trial found, for instance, that a more hypofractionated adjuvant whole breast approach, using 26 Gy in five fractions over a week, is as effective and safe as 40 Gy in 15 fractions over 3 weeks. The researchers found that there was no difference in the incidence of locoregional relapses, disease-free survival, and overall survival between the regimens.
Dr. Simone also noted that advanced treatment modalities, such as intensity-modulated radiotherapy, stereotactic radiosurgery, and proton therapy, have the potential to improve patient-reported adverse events and clinical outcomes. “I have seen this both in my clinical practice and in several recent publications,” he says.
Personalization of radiotherapy is also an emerging area that may allow for more tailored treatments with improved outcomes. The authors highlighted a study that found that PMSA PET-CT was better than conventional CT for accurately staging prostate cancer. This approach was also less expensive and led to less radiation exposure.
On the basis of this research, “PMSA PET-CT has since become the [standard of care] for prostate cancer staging,” the authors explain.
Dr. Espenel and colleagues note that as patients survive longer, quality of life and patient satisfaction are increasingly becoming endpoints in RCTs. Experts are focusing more attention on sequelae of treatments and advances in technology that can spare critical organs from radiation and reduce overall treatment time.
Shared decision-making is becoming increasingly possible in many cases as well. For example, with some clinical trials that involved different treatment modalities, outcomes were equivalent, but toxicity profiles differed, allowing patients to choose therapeutic options tailored to their preferences.
Overall, these data demonstrate “a great dynamism of radiation oncology research in most primary tumor types,” the researchers write.
The study received no outside financial support. The authors have disclosed no relevant financial relationships. Dr. Simone is chair of the American Society for Radiation Oncology Lung Resource Panel and the American Society for Radiation Oncology Veteran Affairs Radiation Oncology Quality Surveillance Blue Ribbon Lung Panel and has received honorarium from Varian Medical Systems.
A version of this article first appeared on Medscape.com.
The field of radiation oncology has rapidly evolved in recent years, thanks in large part to findings from randomized clinical trials (RCTs) that have helped shift therapeutic standards, a review of the literature shows.
Highlights from this research reveal how high-tech radiotherapy, such as hypofractionation and stereotactic body radiotherapy, has improved care for many patients, how personalized radiotherapy using image-based guidance has helped tailor treatments, and how endpoints that focus on quality of life and patient satisfaction are emerging.
For instance, Charles B. Simone II, MD, FACRO, who was not involved in the current work, pointed to “a proliferation of trials assessing hypofractionation in the curative setting and stereotactic body radiation therapy in the curative and poly- and oligometastatic settings that have allowed for increased patient convenience and dose intensification, respectively.”
Dr. Simone, chief medical officer, New York Proton Center, Memorial Sloan Kettering Cancer Center, also noted that the first personalized radiotherapy trials using imaging and biological markers have “the profound potential to individualize treatment and improve patient outcomes.”
The review was published in the European Journal of Cancer.
An evolving field
Given the fast-changing landscape for cancer therapeutics and a deluge of research studies, the authors wanted to understand the most notable advances established in recent trials as well as caveats to some approaches and emerging areas to watch.
In the review, Sophie Espenel, MD, from the department of radiation oncology, Gustave Roussy Cancer Campus, Villejuif, France, and colleagues identified 1,347 radiotherapy RCTs that were conducted from January 2018 to December 2021. Of these, the authors selected 110 large phase 2 or 3 RCTs that contained data showing practice-changing or emerging concepts.
Overall, the studies showed “great dynamism” in radiation oncology research and covered a wide range of radiotherapy practices, according to Dr. Espenel and coauthors.
A central area of research has focused on radioimmunotherapy, an approach that aims to enhance the antitumor immune response. One RCT in the preoperative setting showed, for instance, that concurrent stereotactic body radiotherapy delivered at 24 Gy over eight fractions, along with the anti–PD-L1 agent durvalumab, increased major pathologic complete response rates almost eightfold in comparison with durvalumab alone for patients with early-stage lung cancer (53.3% vs. 6.7%).
Although promising, not all trials that evaluated a concurrent chemoradiotherapy-immunotherapy strategy showed positive results. One RCT of locally advanced head and neck squamous cell carcinoma, for instance, found that median progression-free survival was not reached when adding the anti–PD-L1 avelumab to chemoradiotherapy. In addition, trials in the metastatic setting have shown conflicting results, the authors note.
Another topic of interest is that of newer radiosensitizers. A trial that evaluated high-risk locoregionally advanced head and neck squamous cell carcinoma highlighted the efficacy of xevinapant, a pro-apoptotic agent that inhibits apoptosis proteins. Xevinapant was used for the first time in conjunction with a standard high-dose cisplatin chemoradiotherapy. In this study, locoregional control at 18 months was achieved for 54% of patients who received xevinapant vs. 33% of those who received standard care. The toxicity profiles were similar.
The use of high-tech radiotherapy is gaining ground. It allows patients to receive more targeted treatments at lower doses and in shorter time frames. One trial found, for instance, that a more hypofractionated adjuvant whole breast approach, using 26 Gy in five fractions over a week, is as effective and safe as 40 Gy in 15 fractions over 3 weeks. The researchers found that there was no difference in the incidence of locoregional relapses, disease-free survival, and overall survival between the regimens.
Dr. Simone also noted that advanced treatment modalities, such as intensity-modulated radiotherapy, stereotactic radiosurgery, and proton therapy, have the potential to improve patient-reported adverse events and clinical outcomes. “I have seen this both in my clinical practice and in several recent publications,” he says.
Personalization of radiotherapy is also an emerging area that may allow for more tailored treatments with improved outcomes. The authors highlighted a study that found that PMSA PET-CT was better than conventional CT for accurately staging prostate cancer. This approach was also less expensive and led to less radiation exposure.
On the basis of this research, “PMSA PET-CT has since become the [standard of care] for prostate cancer staging,” the authors explain.
Dr. Espenel and colleagues note that as patients survive longer, quality of life and patient satisfaction are increasingly becoming endpoints in RCTs. Experts are focusing more attention on sequelae of treatments and advances in technology that can spare critical organs from radiation and reduce overall treatment time.
Shared decision-making is becoming increasingly possible in many cases as well. For example, with some clinical trials that involved different treatment modalities, outcomes were equivalent, but toxicity profiles differed, allowing patients to choose therapeutic options tailored to their preferences.
Overall, these data demonstrate “a great dynamism of radiation oncology research in most primary tumor types,” the researchers write.
The study received no outside financial support. The authors have disclosed no relevant financial relationships. Dr. Simone is chair of the American Society for Radiation Oncology Lung Resource Panel and the American Society for Radiation Oncology Veteran Affairs Radiation Oncology Quality Surveillance Blue Ribbon Lung Panel and has received honorarium from Varian Medical Systems.
A version of this article first appeared on Medscape.com.
The field of radiation oncology has rapidly evolved in recent years, thanks in large part to findings from randomized clinical trials (RCTs) that have helped shift therapeutic standards, a review of the literature shows.
Highlights from this research reveal how high-tech radiotherapy, such as hypofractionation and stereotactic body radiotherapy, has improved care for many patients, how personalized radiotherapy using image-based guidance has helped tailor treatments, and how endpoints that focus on quality of life and patient satisfaction are emerging.
For instance, Charles B. Simone II, MD, FACRO, who was not involved in the current work, pointed to “a proliferation of trials assessing hypofractionation in the curative setting and stereotactic body radiation therapy in the curative and poly- and oligometastatic settings that have allowed for increased patient convenience and dose intensification, respectively.”
Dr. Simone, chief medical officer, New York Proton Center, Memorial Sloan Kettering Cancer Center, also noted that the first personalized radiotherapy trials using imaging and biological markers have “the profound potential to individualize treatment and improve patient outcomes.”
The review was published in the European Journal of Cancer.
An evolving field
Given the fast-changing landscape for cancer therapeutics and a deluge of research studies, the authors wanted to understand the most notable advances established in recent trials as well as caveats to some approaches and emerging areas to watch.
In the review, Sophie Espenel, MD, from the department of radiation oncology, Gustave Roussy Cancer Campus, Villejuif, France, and colleagues identified 1,347 radiotherapy RCTs that were conducted from January 2018 to December 2021. Of these, the authors selected 110 large phase 2 or 3 RCTs that contained data showing practice-changing or emerging concepts.
Overall, the studies showed “great dynamism” in radiation oncology research and covered a wide range of radiotherapy practices, according to Dr. Espenel and coauthors.
A central area of research has focused on radioimmunotherapy, an approach that aims to enhance the antitumor immune response. One RCT in the preoperative setting showed, for instance, that concurrent stereotactic body radiotherapy delivered at 24 Gy over eight fractions, along with the anti–PD-L1 agent durvalumab, increased major pathologic complete response rates almost eightfold in comparison with durvalumab alone for patients with early-stage lung cancer (53.3% vs. 6.7%).
Although promising, not all trials that evaluated a concurrent chemoradiotherapy-immunotherapy strategy showed positive results. One RCT of locally advanced head and neck squamous cell carcinoma, for instance, found that median progression-free survival was not reached when adding the anti–PD-L1 avelumab to chemoradiotherapy. In addition, trials in the metastatic setting have shown conflicting results, the authors note.
Another topic of interest is that of newer radiosensitizers. A trial that evaluated high-risk locoregionally advanced head and neck squamous cell carcinoma highlighted the efficacy of xevinapant, a pro-apoptotic agent that inhibits apoptosis proteins. Xevinapant was used for the first time in conjunction with a standard high-dose cisplatin chemoradiotherapy. In this study, locoregional control at 18 months was achieved for 54% of patients who received xevinapant vs. 33% of those who received standard care. The toxicity profiles were similar.
The use of high-tech radiotherapy is gaining ground. It allows patients to receive more targeted treatments at lower doses and in shorter time frames. One trial found, for instance, that a more hypofractionated adjuvant whole breast approach, using 26 Gy in five fractions over a week, is as effective and safe as 40 Gy in 15 fractions over 3 weeks. The researchers found that there was no difference in the incidence of locoregional relapses, disease-free survival, and overall survival between the regimens.
Dr. Simone also noted that advanced treatment modalities, such as intensity-modulated radiotherapy, stereotactic radiosurgery, and proton therapy, have the potential to improve patient-reported adverse events and clinical outcomes. “I have seen this both in my clinical practice and in several recent publications,” he says.
Personalization of radiotherapy is also an emerging area that may allow for more tailored treatments with improved outcomes. The authors highlighted a study that found that PMSA PET-CT was better than conventional CT for accurately staging prostate cancer. This approach was also less expensive and led to less radiation exposure.
On the basis of this research, “PMSA PET-CT has since become the [standard of care] for prostate cancer staging,” the authors explain.
Dr. Espenel and colleagues note that as patients survive longer, quality of life and patient satisfaction are increasingly becoming endpoints in RCTs. Experts are focusing more attention on sequelae of treatments and advances in technology that can spare critical organs from radiation and reduce overall treatment time.
Shared decision-making is becoming increasingly possible in many cases as well. For example, with some clinical trials that involved different treatment modalities, outcomes were equivalent, but toxicity profiles differed, allowing patients to choose therapeutic options tailored to their preferences.
Overall, these data demonstrate “a great dynamism of radiation oncology research in most primary tumor types,” the researchers write.
The study received no outside financial support. The authors have disclosed no relevant financial relationships. Dr. Simone is chair of the American Society for Radiation Oncology Lung Resource Panel and the American Society for Radiation Oncology Veteran Affairs Radiation Oncology Quality Surveillance Blue Ribbon Lung Panel and has received honorarium from Varian Medical Systems.
A version of this article first appeared on Medscape.com.
FROM THE EUROPEAN JOURNAL OF CANCER
AML’s seasonal peak suggests viral or environmental etiology
Most diagnoses of acute myeloid leukemia (AML) are made during January. This finding strongly implies that seasonal factors, such as infectious agents or environmental triggers, influence the development or proliferation of the disease, which points to prevention opportunities. This was the conclusion of an international study led by a team from the Jiménez Díaz Foundation University Hospital Health Research Institute (IIS-FJD) in Madrid, in collaboration with colleagues from the University of Bristol, England. Their work was published in the British Journal of Haematology.
The study’s aim was to investigate the potential seasonal and long-term trends in AML diagnosis in an overall population and in subgroups according to sex and age. To do so, the researchers examined 26,472 cases of AML diagnosed in Spain between 2004 and 2015. They found seasonality in the diagnosis of this type of leukemia. This “could point to there being an underlying seasonal etiology at play,” noted one of the main authors of the study, Juan Manuel Alonso, MD, a physician in the IIS-FJD’s department of hematology and hemotherapy.
“The environmental triggers involved could be radiation, pollution, allergens, or infectious agents like viruses. We’re leaning toward viruses, because there are already distinct solid tumor and hematologic cancers that are caused by them and because, in the winter months, there’s an increased incidence of cancers due to viral infections,” Dr. Alonso said in an interview. “The etiological mechanism should be different from that exerted by chronic viral pressure, because here we’re dealing with an acute and aggressive disease that probably needs a short incubation period.”
Various hypotheses
In an interview, David Martínez, MD, a hematologist at La Fe University Hospital in Valencia, Spain, described the research as “an extremely well done and much-discussed study on AML, a disease that appears to be diagnosed more frequently at a certain time of year – namely, January.
“There’s no clear explanation for this finding,” Dr. Martínez said. “Several possible reasons have been put forward and are being talked about. The one that seems to hold the most water is the hypothesis that infectious agents and environmental factors may have a greater influence. This is because the idea that they’re involved in neoplastic diseases is nothing new. In fact, there are a lot of publications and a good amount of scientific evidence that link viral infections and environmental factors with the development of oncologic diseases.”
AML is a rare disease yet is responsible for many cancer-related deaths. Mutations that cause AML can occur due to an inherited mutant gene or exposure to certain carcinogens, such as chemotherapy, radiotherapy, ionizing radiation, tobacco, and benzene. These findings are broadly similar to those of a large U.S.-based study by Calip et al., who found a peak of adult AML diagnoses during December and January from 1992 to 2008. Previous smaller studies have provided conflicting evidence, likely due to lower power or to the use of less advanced statistical approaches.
Seasonal factors involved?
Demonstration of seasonal variation in the occurrence of AML would, firstly, provide supportive evidence of etiology by seasonal factors, such as infectious agents or environmental factors, and, secondly, focus research onto the etiologic role of such factors.
The current study used population-based data on cases of AML occurring in Spain from a nationwide hospital discharge registry for the years 2004 to 2015. “This is, to our knowledge, the largest study aimed at investigating the potential seasonal and long-term trends in AML incidence in an overall population and in subgroups according to sex and age while employing novel statistical models with serial dependence for discrete-valued time series,” wrote the researchers.
They extracted information from the register of each case about the date of admission, discharge date, the anonymous identifier for each patient, International Classification of Diseases (ICD)–9 codes, sex, and date of birth, from which they derived age groups as described for the at-risk population. For patients hospitalized on more than one occasion, only the record corresponding to their first diagnosis of AML was selected.
AML cases per month were standardized to months of equal length.
Age/sex-standardized monthly incidence rates of AML were calculated using the census of Spanish population in 2010 as a “standard” population. Age-standardized and sex-standardized monthly incidence rates of AML were calculated.
Nine separate time-series decompositions were performed as an initial exploratory analysis on the monthly incidence rates of AML using data for all cases and data for each sex and age group. Nine separate Poisson generalized linear autoregressive moving average (GLARMA) models were fitted to evaluate the temporal dynamics in AML incidence using data for all cases, and data for each sex and age group.
Long-term trend
A total of 26,472 patients with a first diagnosis of active AML were hospitalized in Spain and registered at the country’s Minimum Basic Data Set (CMBD) during 2004-2015. In the end, there were 26,475 patients in the study population; a greater proportion of cases were male (56.0%), and the median age at diagnosis was 67 years.
Seasonal and trend decomposition using Loess decomposition of the incidence rates observed in the overall population exhibited seasonal fluctuation with a peak in January. A slight upward trend was apparent from visual inspection with an upturn in early 2005 and a downturn at the end of 2013. As for the differences by sex groups and age groups, Dr. Alonso said, “For both sexes and in age groups 5-19, 20-49, and 50-64 years, we found that the results were identical to those found in the overall population.”
The final model included an upward linear long-term trend, as well as the variables monthly seasonality and December 2015. The estimated monthly long-term trend implies that the monthly incidence rates of AML diagnoses annually increased by 0.4% (95% confidence interval [CI], 0.2%-0.6%; P = .0011), given that the other covariates are held constant.
January displayed the highest incidence rate of AML, with a minimum average difference of 7%, when compared with February (95% CI, 2%-12%; P = .0143) and a maximum average difference of 16%, compared with November (95% CI, 11%-21%; P < .0001) and August (95% CI, 10%-21%; P < .0001).
The incidence rate of AML for December 2015 was 0.43 (95% CI, 0.34-0.54; P < .0001) times the average incidence rate for the rest of the study period.
Potential role of viruses
“We have to keep in mind that infectious agents (viral infections) and environmental factors (allergens) don’t disappear in the warmer months,” Dr. Martínez added. “There are just other viruses and different factors. We don’t know the role or the weight that each one of the factors has, either individually or specifically, in the development of AML. In addition, we know that AML is a very heterogeneous disease and that various factors, including genetic ones, can be involved in its etiopathogenesis.”
With respect to the stem cell theory in this leukemia, Dr. Alonso emphasized that, “in theory, the virus could fit into it with no problem. That said, any other environmental agent could also produce the described phenomenon where the rapid proliferation of quiescent leukemic stem cells is stimulated, thereby hastening the diagnosis.”
“Should the etiological factor be found,” Dr. Martínez noted, “we can try to reduce exposure and thereby decrease the incidence of AML. On the other hand, discovering how the environmental factor stimulates the proliferation of quiescent leukemic [stem] cells could enhance our knowledge about the regulation of that.”
As to whether there is evidence for the involvement of infections in other hematologic malignancies, Dr. Martínez reported, “This has already been seen. And this study shows other examples (Epstein-Barr virus and human T-cell lymphotropic virus type 1 with lymphomas), and there could also be Helicobacter pylori and lymphomas.”
Outside of hematology, human papillomavirus has been associated with cervical cancer, tobacco with lung cancer, sun with skin cancer, and diet with the development of some solid neoplasms.
“The study speaks about the concept of a latency period. To accept the idea that a factor or virus that’s more prevalent in winter produces, on its own, AML in a few weeks or months means accepting the idea of a very short latency period – something that’s not usually the case. For that, another explanation is given: An abnormal immune response or that a seasonal infectious agent can be capable of promoting leukemogenesis. These are also hypotheses to be explored in the future,” suggested Dr. Martínez.
New research network
Several potential limitations of this study should be considered. One limitation is that AML cases were obtained from the CMBD registry as defined by ICD-9, and no other AML classifications were available. Another limitation is that information on the date of onset of clinical symptoms was not available for analysis. In addition, a further limitation related to the source of their data may have led the researchers to underestimate the incidence rates of AML in older patients, as only hospitalized patients were captured in their study.
As for continuing the research, the results make it necessary to carry out complementary epidemiologic studies that will examine the association between seasonal risk factors and the increased diagnosis of AML during winter months.
To go forward, the first step would be to secure funding. For this purpose, a network is being put together featuring collaborators from other world-renowned research groups that are at the top of their respective disciplines. Through this network, they hope to be able to apply together for public research grants from countries in Europe and elsewhere as well as to establish collaborations with various companies in the private sector.
“This could open up new therapeutic avenues in the future, as we could try to force leukemic stem cells to divide, thereby reducing the resistance that the standard treatments usually demonstrate,” Dr. Alonso concluded.
Dr. Alonso received research funding from Incyte, Pfizer International, and Astellas Pharma outside the present work. Dr. Martínez disclosed no relevant financial relationships.
This article was translated from the Medscape Spanish edition. A version of the article appeared on Medscape.com.
Most diagnoses of acute myeloid leukemia (AML) are made during January. This finding strongly implies that seasonal factors, such as infectious agents or environmental triggers, influence the development or proliferation of the disease, which points to prevention opportunities. This was the conclusion of an international study led by a team from the Jiménez Díaz Foundation University Hospital Health Research Institute (IIS-FJD) in Madrid, in collaboration with colleagues from the University of Bristol, England. Their work was published in the British Journal of Haematology.
The study’s aim was to investigate the potential seasonal and long-term trends in AML diagnosis in an overall population and in subgroups according to sex and age. To do so, the researchers examined 26,472 cases of AML diagnosed in Spain between 2004 and 2015. They found seasonality in the diagnosis of this type of leukemia. This “could point to there being an underlying seasonal etiology at play,” noted one of the main authors of the study, Juan Manuel Alonso, MD, a physician in the IIS-FJD’s department of hematology and hemotherapy.
“The environmental triggers involved could be radiation, pollution, allergens, or infectious agents like viruses. We’re leaning toward viruses, because there are already distinct solid tumor and hematologic cancers that are caused by them and because, in the winter months, there’s an increased incidence of cancers due to viral infections,” Dr. Alonso said in an interview. “The etiological mechanism should be different from that exerted by chronic viral pressure, because here we’re dealing with an acute and aggressive disease that probably needs a short incubation period.”
Various hypotheses
In an interview, David Martínez, MD, a hematologist at La Fe University Hospital in Valencia, Spain, described the research as “an extremely well done and much-discussed study on AML, a disease that appears to be diagnosed more frequently at a certain time of year – namely, January.
“There’s no clear explanation for this finding,” Dr. Martínez said. “Several possible reasons have been put forward and are being talked about. The one that seems to hold the most water is the hypothesis that infectious agents and environmental factors may have a greater influence. This is because the idea that they’re involved in neoplastic diseases is nothing new. In fact, there are a lot of publications and a good amount of scientific evidence that link viral infections and environmental factors with the development of oncologic diseases.”
AML is a rare disease yet is responsible for many cancer-related deaths. Mutations that cause AML can occur due to an inherited mutant gene or exposure to certain carcinogens, such as chemotherapy, radiotherapy, ionizing radiation, tobacco, and benzene. These findings are broadly similar to those of a large U.S.-based study by Calip et al., who found a peak of adult AML diagnoses during December and January from 1992 to 2008. Previous smaller studies have provided conflicting evidence, likely due to lower power or to the use of less advanced statistical approaches.
Seasonal factors involved?
Demonstration of seasonal variation in the occurrence of AML would, firstly, provide supportive evidence of etiology by seasonal factors, such as infectious agents or environmental factors, and, secondly, focus research onto the etiologic role of such factors.
The current study used population-based data on cases of AML occurring in Spain from a nationwide hospital discharge registry for the years 2004 to 2015. “This is, to our knowledge, the largest study aimed at investigating the potential seasonal and long-term trends in AML incidence in an overall population and in subgroups according to sex and age while employing novel statistical models with serial dependence for discrete-valued time series,” wrote the researchers.
They extracted information from the register of each case about the date of admission, discharge date, the anonymous identifier for each patient, International Classification of Diseases (ICD)–9 codes, sex, and date of birth, from which they derived age groups as described for the at-risk population. For patients hospitalized on more than one occasion, only the record corresponding to their first diagnosis of AML was selected.
AML cases per month were standardized to months of equal length.
Age/sex-standardized monthly incidence rates of AML were calculated using the census of Spanish population in 2010 as a “standard” population. Age-standardized and sex-standardized monthly incidence rates of AML were calculated.
Nine separate time-series decompositions were performed as an initial exploratory analysis on the monthly incidence rates of AML using data for all cases and data for each sex and age group. Nine separate Poisson generalized linear autoregressive moving average (GLARMA) models were fitted to evaluate the temporal dynamics in AML incidence using data for all cases, and data for each sex and age group.
Long-term trend
A total of 26,472 patients with a first diagnosis of active AML were hospitalized in Spain and registered at the country’s Minimum Basic Data Set (CMBD) during 2004-2015. In the end, there were 26,475 patients in the study population; a greater proportion of cases were male (56.0%), and the median age at diagnosis was 67 years.
Seasonal and trend decomposition using Loess decomposition of the incidence rates observed in the overall population exhibited seasonal fluctuation with a peak in January. A slight upward trend was apparent from visual inspection with an upturn in early 2005 and a downturn at the end of 2013. As for the differences by sex groups and age groups, Dr. Alonso said, “For both sexes and in age groups 5-19, 20-49, and 50-64 years, we found that the results were identical to those found in the overall population.”
The final model included an upward linear long-term trend, as well as the variables monthly seasonality and December 2015. The estimated monthly long-term trend implies that the monthly incidence rates of AML diagnoses annually increased by 0.4% (95% confidence interval [CI], 0.2%-0.6%; P = .0011), given that the other covariates are held constant.
January displayed the highest incidence rate of AML, with a minimum average difference of 7%, when compared with February (95% CI, 2%-12%; P = .0143) and a maximum average difference of 16%, compared with November (95% CI, 11%-21%; P < .0001) and August (95% CI, 10%-21%; P < .0001).
The incidence rate of AML for December 2015 was 0.43 (95% CI, 0.34-0.54; P < .0001) times the average incidence rate for the rest of the study period.
Potential role of viruses
“We have to keep in mind that infectious agents (viral infections) and environmental factors (allergens) don’t disappear in the warmer months,” Dr. Martínez added. “There are just other viruses and different factors. We don’t know the role or the weight that each one of the factors has, either individually or specifically, in the development of AML. In addition, we know that AML is a very heterogeneous disease and that various factors, including genetic ones, can be involved in its etiopathogenesis.”
With respect to the stem cell theory in this leukemia, Dr. Alonso emphasized that, “in theory, the virus could fit into it with no problem. That said, any other environmental agent could also produce the described phenomenon where the rapid proliferation of quiescent leukemic stem cells is stimulated, thereby hastening the diagnosis.”
“Should the etiological factor be found,” Dr. Martínez noted, “we can try to reduce exposure and thereby decrease the incidence of AML. On the other hand, discovering how the environmental factor stimulates the proliferation of quiescent leukemic [stem] cells could enhance our knowledge about the regulation of that.”
As to whether there is evidence for the involvement of infections in other hematologic malignancies, Dr. Martínez reported, “This has already been seen. And this study shows other examples (Epstein-Barr virus and human T-cell lymphotropic virus type 1 with lymphomas), and there could also be Helicobacter pylori and lymphomas.”
Outside of hematology, human papillomavirus has been associated with cervical cancer, tobacco with lung cancer, sun with skin cancer, and diet with the development of some solid neoplasms.
“The study speaks about the concept of a latency period. To accept the idea that a factor or virus that’s more prevalent in winter produces, on its own, AML in a few weeks or months means accepting the idea of a very short latency period – something that’s not usually the case. For that, another explanation is given: An abnormal immune response or that a seasonal infectious agent can be capable of promoting leukemogenesis. These are also hypotheses to be explored in the future,” suggested Dr. Martínez.
New research network
Several potential limitations of this study should be considered. One limitation is that AML cases were obtained from the CMBD registry as defined by ICD-9, and no other AML classifications were available. Another limitation is that information on the date of onset of clinical symptoms was not available for analysis. In addition, a further limitation related to the source of their data may have led the researchers to underestimate the incidence rates of AML in older patients, as only hospitalized patients were captured in their study.
As for continuing the research, the results make it necessary to carry out complementary epidemiologic studies that will examine the association between seasonal risk factors and the increased diagnosis of AML during winter months.
To go forward, the first step would be to secure funding. For this purpose, a network is being put together featuring collaborators from other world-renowned research groups that are at the top of their respective disciplines. Through this network, they hope to be able to apply together for public research grants from countries in Europe and elsewhere as well as to establish collaborations with various companies in the private sector.
“This could open up new therapeutic avenues in the future, as we could try to force leukemic stem cells to divide, thereby reducing the resistance that the standard treatments usually demonstrate,” Dr. Alonso concluded.
Dr. Alonso received research funding from Incyte, Pfizer International, and Astellas Pharma outside the present work. Dr. Martínez disclosed no relevant financial relationships.
This article was translated from the Medscape Spanish edition. A version of the article appeared on Medscape.com.
Most diagnoses of acute myeloid leukemia (AML) are made during January. This finding strongly implies that seasonal factors, such as infectious agents or environmental triggers, influence the development or proliferation of the disease, which points to prevention opportunities. This was the conclusion of an international study led by a team from the Jiménez Díaz Foundation University Hospital Health Research Institute (IIS-FJD) in Madrid, in collaboration with colleagues from the University of Bristol, England. Their work was published in the British Journal of Haematology.
The study’s aim was to investigate the potential seasonal and long-term trends in AML diagnosis in an overall population and in subgroups according to sex and age. To do so, the researchers examined 26,472 cases of AML diagnosed in Spain between 2004 and 2015. They found seasonality in the diagnosis of this type of leukemia. This “could point to there being an underlying seasonal etiology at play,” noted one of the main authors of the study, Juan Manuel Alonso, MD, a physician in the IIS-FJD’s department of hematology and hemotherapy.
“The environmental triggers involved could be radiation, pollution, allergens, or infectious agents like viruses. We’re leaning toward viruses, because there are already distinct solid tumor and hematologic cancers that are caused by them and because, in the winter months, there’s an increased incidence of cancers due to viral infections,” Dr. Alonso said in an interview. “The etiological mechanism should be different from that exerted by chronic viral pressure, because here we’re dealing with an acute and aggressive disease that probably needs a short incubation period.”
Various hypotheses
In an interview, David Martínez, MD, a hematologist at La Fe University Hospital in Valencia, Spain, described the research as “an extremely well done and much-discussed study on AML, a disease that appears to be diagnosed more frequently at a certain time of year – namely, January.
“There’s no clear explanation for this finding,” Dr. Martínez said. “Several possible reasons have been put forward and are being talked about. The one that seems to hold the most water is the hypothesis that infectious agents and environmental factors may have a greater influence. This is because the idea that they’re involved in neoplastic diseases is nothing new. In fact, there are a lot of publications and a good amount of scientific evidence that link viral infections and environmental factors with the development of oncologic diseases.”
AML is a rare disease yet is responsible for many cancer-related deaths. Mutations that cause AML can occur due to an inherited mutant gene or exposure to certain carcinogens, such as chemotherapy, radiotherapy, ionizing radiation, tobacco, and benzene. These findings are broadly similar to those of a large U.S.-based study by Calip et al., who found a peak of adult AML diagnoses during December and January from 1992 to 2008. Previous smaller studies have provided conflicting evidence, likely due to lower power or to the use of less advanced statistical approaches.
Seasonal factors involved?
Demonstration of seasonal variation in the occurrence of AML would, firstly, provide supportive evidence of etiology by seasonal factors, such as infectious agents or environmental factors, and, secondly, focus research onto the etiologic role of such factors.
The current study used population-based data on cases of AML occurring in Spain from a nationwide hospital discharge registry for the years 2004 to 2015. “This is, to our knowledge, the largest study aimed at investigating the potential seasonal and long-term trends in AML incidence in an overall population and in subgroups according to sex and age while employing novel statistical models with serial dependence for discrete-valued time series,” wrote the researchers.
They extracted information from the register of each case about the date of admission, discharge date, the anonymous identifier for each patient, International Classification of Diseases (ICD)–9 codes, sex, and date of birth, from which they derived age groups as described for the at-risk population. For patients hospitalized on more than one occasion, only the record corresponding to their first diagnosis of AML was selected.
AML cases per month were standardized to months of equal length.
Age/sex-standardized monthly incidence rates of AML were calculated using the census of Spanish population in 2010 as a “standard” population. Age-standardized and sex-standardized monthly incidence rates of AML were calculated.
Nine separate time-series decompositions were performed as an initial exploratory analysis on the monthly incidence rates of AML using data for all cases and data for each sex and age group. Nine separate Poisson generalized linear autoregressive moving average (GLARMA) models were fitted to evaluate the temporal dynamics in AML incidence using data for all cases, and data for each sex and age group.
Long-term trend
A total of 26,472 patients with a first diagnosis of active AML were hospitalized in Spain and registered at the country’s Minimum Basic Data Set (CMBD) during 2004-2015. In the end, there were 26,475 patients in the study population; a greater proportion of cases were male (56.0%), and the median age at diagnosis was 67 years.
Seasonal and trend decomposition using Loess decomposition of the incidence rates observed in the overall population exhibited seasonal fluctuation with a peak in January. A slight upward trend was apparent from visual inspection with an upturn in early 2005 and a downturn at the end of 2013. As for the differences by sex groups and age groups, Dr. Alonso said, “For both sexes and in age groups 5-19, 20-49, and 50-64 years, we found that the results were identical to those found in the overall population.”
The final model included an upward linear long-term trend, as well as the variables monthly seasonality and December 2015. The estimated monthly long-term trend implies that the monthly incidence rates of AML diagnoses annually increased by 0.4% (95% confidence interval [CI], 0.2%-0.6%; P = .0011), given that the other covariates are held constant.
January displayed the highest incidence rate of AML, with a minimum average difference of 7%, when compared with February (95% CI, 2%-12%; P = .0143) and a maximum average difference of 16%, compared with November (95% CI, 11%-21%; P < .0001) and August (95% CI, 10%-21%; P < .0001).
The incidence rate of AML for December 2015 was 0.43 (95% CI, 0.34-0.54; P < .0001) times the average incidence rate for the rest of the study period.
Potential role of viruses
“We have to keep in mind that infectious agents (viral infections) and environmental factors (allergens) don’t disappear in the warmer months,” Dr. Martínez added. “There are just other viruses and different factors. We don’t know the role or the weight that each one of the factors has, either individually or specifically, in the development of AML. In addition, we know that AML is a very heterogeneous disease and that various factors, including genetic ones, can be involved in its etiopathogenesis.”
With respect to the stem cell theory in this leukemia, Dr. Alonso emphasized that, “in theory, the virus could fit into it with no problem. That said, any other environmental agent could also produce the described phenomenon where the rapid proliferation of quiescent leukemic stem cells is stimulated, thereby hastening the diagnosis.”
“Should the etiological factor be found,” Dr. Martínez noted, “we can try to reduce exposure and thereby decrease the incidence of AML. On the other hand, discovering how the environmental factor stimulates the proliferation of quiescent leukemic [stem] cells could enhance our knowledge about the regulation of that.”
As to whether there is evidence for the involvement of infections in other hematologic malignancies, Dr. Martínez reported, “This has already been seen. And this study shows other examples (Epstein-Barr virus and human T-cell lymphotropic virus type 1 with lymphomas), and there could also be Helicobacter pylori and lymphomas.”
Outside of hematology, human papillomavirus has been associated with cervical cancer, tobacco with lung cancer, sun with skin cancer, and diet with the development of some solid neoplasms.
“The study speaks about the concept of a latency period. To accept the idea that a factor or virus that’s more prevalent in winter produces, on its own, AML in a few weeks or months means accepting the idea of a very short latency period – something that’s not usually the case. For that, another explanation is given: An abnormal immune response or that a seasonal infectious agent can be capable of promoting leukemogenesis. These are also hypotheses to be explored in the future,” suggested Dr. Martínez.
New research network
Several potential limitations of this study should be considered. One limitation is that AML cases were obtained from the CMBD registry as defined by ICD-9, and no other AML classifications were available. Another limitation is that information on the date of onset of clinical symptoms was not available for analysis. In addition, a further limitation related to the source of their data may have led the researchers to underestimate the incidence rates of AML in older patients, as only hospitalized patients were captured in their study.
As for continuing the research, the results make it necessary to carry out complementary epidemiologic studies that will examine the association between seasonal risk factors and the increased diagnosis of AML during winter months.
To go forward, the first step would be to secure funding. For this purpose, a network is being put together featuring collaborators from other world-renowned research groups that are at the top of their respective disciplines. Through this network, they hope to be able to apply together for public research grants from countries in Europe and elsewhere as well as to establish collaborations with various companies in the private sector.
“This could open up new therapeutic avenues in the future, as we could try to force leukemic stem cells to divide, thereby reducing the resistance that the standard treatments usually demonstrate,” Dr. Alonso concluded.
Dr. Alonso received research funding from Incyte, Pfizer International, and Astellas Pharma outside the present work. Dr. Martínez disclosed no relevant financial relationships.
This article was translated from the Medscape Spanish edition. A version of the article appeared on Medscape.com.
FROM THE BRITISH JOURNAL OF HEMATOLOGY
Annual PSA screening important for Black men
, new data suggest.
The data come from a review of 45,834 veterans (aged 55-69 years) who had been diagnosed with prostate cancer. About one-third of these men self-identified as non-Hispanic Black, and the rest were White.
During the study period (2004-2017), 2,465 men (5.4%) died of the disease.
The review found that annual prostate-specific antigen (PSA) screening significantly reduced the risk of dying from prostate cancer among Black men but not White men.
The study was published online in JAMA Oncology.
“These results may be biologically plausible because a shorter screening interval may be valuable for detecting aggressive disease, which is more common in Black men,” say investigators, led by University of California, San Diego, radiation oncology resident Michael Sherer, MD.
“Given that Black men are younger at diagnosis and have worse prostate cancer survival compared with White men,” more intensive screening recommendations “may benefit Black patients,” they write.
The study “conclusions are reasonable,” said Christopher Wallis, MD, PhD, a urologic oncologist at Mount Sinai Hospital in Toronto, when asked for comment.
Annual screening may well have “a greater potential to benefit” Black men, he said. “While we would ideally see randomized data supporting this, those data are unlikely to ever be forthcoming. Thus, this study provides a strong rationale to support the recommendations from many guideline panels (including those from the American Urological Association) that Black men, in the context of shared decision-making, may benefit more from PSA-based prostate cancer screening than the population at large,” he added.
Overall, the findings could help inform screening discussions with Black men, the investigators comments. In its most recent guidance, the U.S. Preventive Services Task Force recommends shared decision-making regarding PSA screening for men aged 55-69 years.
Similar screening frequency
For their study, the team reviewed Veterans Health Administration data to assess PSA screening patterns – which they categorized as no screening, less than annual screening, or annual screening – in the 5 years leading up to diagnosis.
They then correlated screening behaviors with the subsequent risk of dying from prostate cancer.
Overall, the reduction in risk of prostate cancer–specific mortality (PCSM) associated with screening was similar among Black men (subdistribution hazard ratio, 0.56; P = .001) and White men (sHR, 0.58; P = .001).
However, on multivariable regression, annual screening, in comparison with some screening, was associated with a significant reduction in the risk of dying from prostate cancer only among Black men (sHR, 0.65; P = .02), not among White men (sHR, 0.91; P = .35).
The cumulative incidence of PCSM among Black men was 4.7% with annual screening but 7.3% with only some screening.
Among White men, the cumulative incidence of PCSM with annual screening was 5.9% vs. 6.9% with less than annual screening.
Screening frequency was similar between Black men and White men. Black men were younger on average (61.8 vs. 63.1 years) and had slightly higher PSA levels at diagnosis but were not more likely to have regional or metastatic disease.
No funding was reported for this study. The investigators have disclosed no relevant financial relationships. Dr. Wallis has received personal fees from Janssen Canada.
A version of this article first appeared on Medscape.com.
, new data suggest.
The data come from a review of 45,834 veterans (aged 55-69 years) who had been diagnosed with prostate cancer. About one-third of these men self-identified as non-Hispanic Black, and the rest were White.
During the study period (2004-2017), 2,465 men (5.4%) died of the disease.
The review found that annual prostate-specific antigen (PSA) screening significantly reduced the risk of dying from prostate cancer among Black men but not White men.
The study was published online in JAMA Oncology.
“These results may be biologically plausible because a shorter screening interval may be valuable for detecting aggressive disease, which is more common in Black men,” say investigators, led by University of California, San Diego, radiation oncology resident Michael Sherer, MD.
“Given that Black men are younger at diagnosis and have worse prostate cancer survival compared with White men,” more intensive screening recommendations “may benefit Black patients,” they write.
The study “conclusions are reasonable,” said Christopher Wallis, MD, PhD, a urologic oncologist at Mount Sinai Hospital in Toronto, when asked for comment.
Annual screening may well have “a greater potential to benefit” Black men, he said. “While we would ideally see randomized data supporting this, those data are unlikely to ever be forthcoming. Thus, this study provides a strong rationale to support the recommendations from many guideline panels (including those from the American Urological Association) that Black men, in the context of shared decision-making, may benefit more from PSA-based prostate cancer screening than the population at large,” he added.
Overall, the findings could help inform screening discussions with Black men, the investigators comments. In its most recent guidance, the U.S. Preventive Services Task Force recommends shared decision-making regarding PSA screening for men aged 55-69 years.
Similar screening frequency
For their study, the team reviewed Veterans Health Administration data to assess PSA screening patterns – which they categorized as no screening, less than annual screening, or annual screening – in the 5 years leading up to diagnosis.
They then correlated screening behaviors with the subsequent risk of dying from prostate cancer.
Overall, the reduction in risk of prostate cancer–specific mortality (PCSM) associated with screening was similar among Black men (subdistribution hazard ratio, 0.56; P = .001) and White men (sHR, 0.58; P = .001).
However, on multivariable regression, annual screening, in comparison with some screening, was associated with a significant reduction in the risk of dying from prostate cancer only among Black men (sHR, 0.65; P = .02), not among White men (sHR, 0.91; P = .35).
The cumulative incidence of PCSM among Black men was 4.7% with annual screening but 7.3% with only some screening.
Among White men, the cumulative incidence of PCSM with annual screening was 5.9% vs. 6.9% with less than annual screening.
Screening frequency was similar between Black men and White men. Black men were younger on average (61.8 vs. 63.1 years) and had slightly higher PSA levels at diagnosis but were not more likely to have regional or metastatic disease.
No funding was reported for this study. The investigators have disclosed no relevant financial relationships. Dr. Wallis has received personal fees from Janssen Canada.
A version of this article first appeared on Medscape.com.
, new data suggest.
The data come from a review of 45,834 veterans (aged 55-69 years) who had been diagnosed with prostate cancer. About one-third of these men self-identified as non-Hispanic Black, and the rest were White.
During the study period (2004-2017), 2,465 men (5.4%) died of the disease.
The review found that annual prostate-specific antigen (PSA) screening significantly reduced the risk of dying from prostate cancer among Black men but not White men.
The study was published online in JAMA Oncology.
“These results may be biologically plausible because a shorter screening interval may be valuable for detecting aggressive disease, which is more common in Black men,” say investigators, led by University of California, San Diego, radiation oncology resident Michael Sherer, MD.
“Given that Black men are younger at diagnosis and have worse prostate cancer survival compared with White men,” more intensive screening recommendations “may benefit Black patients,” they write.
The study “conclusions are reasonable,” said Christopher Wallis, MD, PhD, a urologic oncologist at Mount Sinai Hospital in Toronto, when asked for comment.
Annual screening may well have “a greater potential to benefit” Black men, he said. “While we would ideally see randomized data supporting this, those data are unlikely to ever be forthcoming. Thus, this study provides a strong rationale to support the recommendations from many guideline panels (including those from the American Urological Association) that Black men, in the context of shared decision-making, may benefit more from PSA-based prostate cancer screening than the population at large,” he added.
Overall, the findings could help inform screening discussions with Black men, the investigators comments. In its most recent guidance, the U.S. Preventive Services Task Force recommends shared decision-making regarding PSA screening for men aged 55-69 years.
Similar screening frequency
For their study, the team reviewed Veterans Health Administration data to assess PSA screening patterns – which they categorized as no screening, less than annual screening, or annual screening – in the 5 years leading up to diagnosis.
They then correlated screening behaviors with the subsequent risk of dying from prostate cancer.
Overall, the reduction in risk of prostate cancer–specific mortality (PCSM) associated with screening was similar among Black men (subdistribution hazard ratio, 0.56; P = .001) and White men (sHR, 0.58; P = .001).
However, on multivariable regression, annual screening, in comparison with some screening, was associated with a significant reduction in the risk of dying from prostate cancer only among Black men (sHR, 0.65; P = .02), not among White men (sHR, 0.91; P = .35).
The cumulative incidence of PCSM among Black men was 4.7% with annual screening but 7.3% with only some screening.
Among White men, the cumulative incidence of PCSM with annual screening was 5.9% vs. 6.9% with less than annual screening.
Screening frequency was similar between Black men and White men. Black men were younger on average (61.8 vs. 63.1 years) and had slightly higher PSA levels at diagnosis but were not more likely to have regional or metastatic disease.
No funding was reported for this study. The investigators have disclosed no relevant financial relationships. Dr. Wallis has received personal fees from Janssen Canada.
A version of this article first appeared on Medscape.com.
FROM JAMA ONCOLOGY
Mechanistic link between herpes virus, Alzheimer’s revealed?
, new research suggests.
“Our results suggest one pathway to Alzheimer’s disease, caused by a VZV infection which creates inflammatory triggers that awaken HSV in the brain,” lead author Dana Cairns, PhD, research associate, department of biomedical engineering at Tufts University, Boston, said in a news release.
The findings were published online in Journal of Alzheimer’s Disease.
‘One-two punch’
Previous research has suggested a correlation between HSV-1 and AD and involvement of VZV. However, the sequence of events that the viruses create to set the disease in motion has been unclear.
“We think we now have evidence of those events,” co–senior author David Kaplan, PhD, chair of the department of biomedical engineering at Tufts, said in the release.
Working with co–senior author Ruth Itzhaki, PhD, University of Oxford, United Kingdom, the researchers infected human-induced neural stem cells (hiNSCs) and 3D brain tissue models with HSV-1 and/or VZV. Dr. Itzhaki was one of the first to hypothesize a connection between herpes virus and AD.
The investigators found that HSV-1 infection of hiNSCs induces amyloid-beta and P-tau accumulation: the main components of AD plaques and neurofibrillary tangles, respectively.
On the other hand, VZV infection of cultured hiNSCs did not lead to amyloid-beta and P-tau accumulation but instead resulted in gliosis and increased levels of proinflammatory cytokines.
“Strikingly,” VZV infection of cells quiescently infected with HSV-1 caused reactivation of HSV-1, leading to AD-like changes, including amyloid-beta and P-tau accumulation, the investigators report.
This suggests that VZV is unlikely to be a direct cause of AD but rather acts indirectly via reactivation of HSV-1, they add.
Similar findings emerged in similar experiments using 3D human brain tissue models.
“It’s a one-two punch of two viruses that are very common and usually harmless, but the lab studies suggest that if a new exposure to VZV wakes up dormant HSV-1, they could cause trouble,” Dr. Cairns said.
The researchers note that vaccination against VZV has been shown previously to reduce risk for dementia. It is possible, they add, that the vaccine is helping to stop the cycle of viral reactivation, inflammation, and neuronal damage.
‘A first step’
Heather M. Snyder, PhD, vice president of Medical & Scientific Relations at the Alzheimer’s Association, said that the study “is using artificial systems with the goal of more clearly and more deeply understanding” the assessed associations.
She added that although it is a first step, it may provide valuable direction for follow-up research.
“This is preliminary work that first needs replication, validation, and further development to understand if any association that is uncovered between viruses and Alzheimer’s/dementia has a mechanistic link,” said Dr. Snyder.
She noted that several past studies have sought to help the research field better understand the links between different viruses and Alzheimer’s and other forms of dementia.
“There have been some challenges in evaluating these associations in our current model systems or in individuals for a number of reasons,” said Dr. Snyder.
However, “the COVID-19 pandemic has created an opportunity to examine and investigate the relationships between different viruses and Alzheimer’s and other dementias by following individuals in more common and well-established ways,” she added.
She reported that her organization is “leading and working with a large global network of studies and investigators to address some of these questions” from during and after the COVID pandemic.
“The lessons we learn and share may inform our understanding of how other viruses are, or are not, connected to Alzheimer’s and other dementia,” Dr. Snyder said.
More information on the Alzheimer’s Association International Cohort Study of Chronic Neurological Sequelae of SARS-CoV-2 is available online.
The study was funded by the National Institutes of Health. Dr. Cairns, Dr. Kaplan, Dr. Itzhaki, and Dr. Snyder have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
“Our results suggest one pathway to Alzheimer’s disease, caused by a VZV infection which creates inflammatory triggers that awaken HSV in the brain,” lead author Dana Cairns, PhD, research associate, department of biomedical engineering at Tufts University, Boston, said in a news release.
The findings were published online in Journal of Alzheimer’s Disease.
‘One-two punch’
Previous research has suggested a correlation between HSV-1 and AD and involvement of VZV. However, the sequence of events that the viruses create to set the disease in motion has been unclear.
“We think we now have evidence of those events,” co–senior author David Kaplan, PhD, chair of the department of biomedical engineering at Tufts, said in the release.
Working with co–senior author Ruth Itzhaki, PhD, University of Oxford, United Kingdom, the researchers infected human-induced neural stem cells (hiNSCs) and 3D brain tissue models with HSV-1 and/or VZV. Dr. Itzhaki was one of the first to hypothesize a connection between herpes virus and AD.
The investigators found that HSV-1 infection of hiNSCs induces amyloid-beta and P-tau accumulation: the main components of AD plaques and neurofibrillary tangles, respectively.
On the other hand, VZV infection of cultured hiNSCs did not lead to amyloid-beta and P-tau accumulation but instead resulted in gliosis and increased levels of proinflammatory cytokines.
“Strikingly,” VZV infection of cells quiescently infected with HSV-1 caused reactivation of HSV-1, leading to AD-like changes, including amyloid-beta and P-tau accumulation, the investigators report.
This suggests that VZV is unlikely to be a direct cause of AD but rather acts indirectly via reactivation of HSV-1, they add.
Similar findings emerged in similar experiments using 3D human brain tissue models.
“It’s a one-two punch of two viruses that are very common and usually harmless, but the lab studies suggest that if a new exposure to VZV wakes up dormant HSV-1, they could cause trouble,” Dr. Cairns said.
The researchers note that vaccination against VZV has been shown previously to reduce risk for dementia. It is possible, they add, that the vaccine is helping to stop the cycle of viral reactivation, inflammation, and neuronal damage.
‘A first step’
Heather M. Snyder, PhD, vice president of Medical & Scientific Relations at the Alzheimer’s Association, said that the study “is using artificial systems with the goal of more clearly and more deeply understanding” the assessed associations.
She added that although it is a first step, it may provide valuable direction for follow-up research.
“This is preliminary work that first needs replication, validation, and further development to understand if any association that is uncovered between viruses and Alzheimer’s/dementia has a mechanistic link,” said Dr. Snyder.
She noted that several past studies have sought to help the research field better understand the links between different viruses and Alzheimer’s and other forms of dementia.
“There have been some challenges in evaluating these associations in our current model systems or in individuals for a number of reasons,” said Dr. Snyder.
However, “the COVID-19 pandemic has created an opportunity to examine and investigate the relationships between different viruses and Alzheimer’s and other dementias by following individuals in more common and well-established ways,” she added.
She reported that her organization is “leading and working with a large global network of studies and investigators to address some of these questions” from during and after the COVID pandemic.
“The lessons we learn and share may inform our understanding of how other viruses are, or are not, connected to Alzheimer’s and other dementia,” Dr. Snyder said.
More information on the Alzheimer’s Association International Cohort Study of Chronic Neurological Sequelae of SARS-CoV-2 is available online.
The study was funded by the National Institutes of Health. Dr. Cairns, Dr. Kaplan, Dr. Itzhaki, and Dr. Snyder have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
“Our results suggest one pathway to Alzheimer’s disease, caused by a VZV infection which creates inflammatory triggers that awaken HSV in the brain,” lead author Dana Cairns, PhD, research associate, department of biomedical engineering at Tufts University, Boston, said in a news release.
The findings were published online in Journal of Alzheimer’s Disease.
‘One-two punch’
Previous research has suggested a correlation between HSV-1 and AD and involvement of VZV. However, the sequence of events that the viruses create to set the disease in motion has been unclear.
“We think we now have evidence of those events,” co–senior author David Kaplan, PhD, chair of the department of biomedical engineering at Tufts, said in the release.
Working with co–senior author Ruth Itzhaki, PhD, University of Oxford, United Kingdom, the researchers infected human-induced neural stem cells (hiNSCs) and 3D brain tissue models with HSV-1 and/or VZV. Dr. Itzhaki was one of the first to hypothesize a connection between herpes virus and AD.
The investigators found that HSV-1 infection of hiNSCs induces amyloid-beta and P-tau accumulation: the main components of AD plaques and neurofibrillary tangles, respectively.
On the other hand, VZV infection of cultured hiNSCs did not lead to amyloid-beta and P-tau accumulation but instead resulted in gliosis and increased levels of proinflammatory cytokines.
“Strikingly,” VZV infection of cells quiescently infected with HSV-1 caused reactivation of HSV-1, leading to AD-like changes, including amyloid-beta and P-tau accumulation, the investigators report.
This suggests that VZV is unlikely to be a direct cause of AD but rather acts indirectly via reactivation of HSV-1, they add.
Similar findings emerged in similar experiments using 3D human brain tissue models.
“It’s a one-two punch of two viruses that are very common and usually harmless, but the lab studies suggest that if a new exposure to VZV wakes up dormant HSV-1, they could cause trouble,” Dr. Cairns said.
The researchers note that vaccination against VZV has been shown previously to reduce risk for dementia. It is possible, they add, that the vaccine is helping to stop the cycle of viral reactivation, inflammation, and neuronal damage.
‘A first step’
Heather M. Snyder, PhD, vice president of Medical & Scientific Relations at the Alzheimer’s Association, said that the study “is using artificial systems with the goal of more clearly and more deeply understanding” the assessed associations.
She added that although it is a first step, it may provide valuable direction for follow-up research.
“This is preliminary work that first needs replication, validation, and further development to understand if any association that is uncovered between viruses and Alzheimer’s/dementia has a mechanistic link,” said Dr. Snyder.
She noted that several past studies have sought to help the research field better understand the links between different viruses and Alzheimer’s and other forms of dementia.
“There have been some challenges in evaluating these associations in our current model systems or in individuals for a number of reasons,” said Dr. Snyder.
However, “the COVID-19 pandemic has created an opportunity to examine and investigate the relationships between different viruses and Alzheimer’s and other dementias by following individuals in more common and well-established ways,” she added.
She reported that her organization is “leading and working with a large global network of studies and investigators to address some of these questions” from during and after the COVID pandemic.
“The lessons we learn and share may inform our understanding of how other viruses are, or are not, connected to Alzheimer’s and other dementia,” Dr. Snyder said.
More information on the Alzheimer’s Association International Cohort Study of Chronic Neurological Sequelae of SARS-CoV-2 is available online.
The study was funded by the National Institutes of Health. Dr. Cairns, Dr. Kaplan, Dr. Itzhaki, and Dr. Snyder have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF ALZHEIMER’S DISEASE
Does PREDICT accurately estimate breast cancer survival?
The PREDICT score does not seem to be particularly accurate when it comes to estimating overall survival (OS) in patients with HER2-positive early breast cancer who are treated with modern chemotherapy and anti-HER2 targeted therapies. This is the conclusion of an international study published in the journal npj Breast Cancer. The work was supervised by Matteo Lambertini, MD, PhD, an oncologist at the IRCCS San Martino Polyclinic Hospital in Genoa, Italy.
As the authors explain, “PREDICT is a publicly available online tool that helps to predict the individual prognosis of patients with early breast cancer and to show the impact of adjuvant treatments administered after breast cancer surgery.” The tool uses traditional clinical-pathological factors. The authors also point out that the original version of this tool was validated in several datasets of patients with breast cancer. In 2011, it was updated to include HER2 status.
The investigators noted that, although the use of PREDICT is recommended to aid decision-making in the adjuvant setting, its prognostic role in patients with HER2-positive early breast cancer who are treated with modern chemotherapy and anti-HER2 therapies – even trastuzumab-based ones – remains unclear.
Therefore, they decided to analyze PREDICT’s prognostic performance using data extracted from the ALTTO trial, the largest adjuvant study ever conducted in the field of HER2-positive early breast cancer. That trial “represented a unique opportunity to investigate the reliability and prognostic performance of PREDICT in women with HER2-positive disease,” according to the investigators. They went on to specify that ALTTO evaluated adjuvant lapatinib plus trastuzumab vs. trastuzumab alone in 8,381 patients – 2,794 of whom were included in their own analysis.
What the analysis found was that, overall, PREDICT underestimated 5-year OS by 6.7%. The observed 5-year OS was 94.7%, and the predicted 5-year OS was 88.0%.
The highest absolute differences were observed for patients with hormone receptor–negative disease, nodal involvement, and large tumor size (13.0%, 15.8%, and 15.3%, respectively),” they wrote. Furthermore, they reported that “the suboptimal performance of this prognostic tool was observed irrespective of type of anti-HER2 treatment, type of chemotherapy regimen, age of the patients at the time of diagnosis, central hormone receptor status, pathological nodal status, and pathological tumor size.”
To potentially explain the reasons for the underestimation of patients’ OS, the authors questioned whether the population used to validate PREDICT accurately mirrored the real-world population of patients with HER2-positive disease treated in the modern era with effective chemotherapy and anti-HER2 targeted therapies. “Moreover, the current standard of care for early breast cancer is even superior to the treatment received by many patients in the ALTTO study. … As such, the discordance between OS estimated by PREDICT and the current real-world OS is expected to be even higher. Therefore,” the researchers concluded, “our results suggest that the current version of PREDICT should be used with caution for prognostication in HER2-positive early breast cancer patients treated in the modern era with effective chemotherapy and anti-HER2 targeted therapies.”
A version of this article first appeared on Medscape.com. This article was translated from Univadis Italy.
The PREDICT score does not seem to be particularly accurate when it comes to estimating overall survival (OS) in patients with HER2-positive early breast cancer who are treated with modern chemotherapy and anti-HER2 targeted therapies. This is the conclusion of an international study published in the journal npj Breast Cancer. The work was supervised by Matteo Lambertini, MD, PhD, an oncologist at the IRCCS San Martino Polyclinic Hospital in Genoa, Italy.
As the authors explain, “PREDICT is a publicly available online tool that helps to predict the individual prognosis of patients with early breast cancer and to show the impact of adjuvant treatments administered after breast cancer surgery.” The tool uses traditional clinical-pathological factors. The authors also point out that the original version of this tool was validated in several datasets of patients with breast cancer. In 2011, it was updated to include HER2 status.
The investigators noted that, although the use of PREDICT is recommended to aid decision-making in the adjuvant setting, its prognostic role in patients with HER2-positive early breast cancer who are treated with modern chemotherapy and anti-HER2 therapies – even trastuzumab-based ones – remains unclear.
Therefore, they decided to analyze PREDICT’s prognostic performance using data extracted from the ALTTO trial, the largest adjuvant study ever conducted in the field of HER2-positive early breast cancer. That trial “represented a unique opportunity to investigate the reliability and prognostic performance of PREDICT in women with HER2-positive disease,” according to the investigators. They went on to specify that ALTTO evaluated adjuvant lapatinib plus trastuzumab vs. trastuzumab alone in 8,381 patients – 2,794 of whom were included in their own analysis.
What the analysis found was that, overall, PREDICT underestimated 5-year OS by 6.7%. The observed 5-year OS was 94.7%, and the predicted 5-year OS was 88.0%.
The highest absolute differences were observed for patients with hormone receptor–negative disease, nodal involvement, and large tumor size (13.0%, 15.8%, and 15.3%, respectively),” they wrote. Furthermore, they reported that “the suboptimal performance of this prognostic tool was observed irrespective of type of anti-HER2 treatment, type of chemotherapy regimen, age of the patients at the time of diagnosis, central hormone receptor status, pathological nodal status, and pathological tumor size.”
To potentially explain the reasons for the underestimation of patients’ OS, the authors questioned whether the population used to validate PREDICT accurately mirrored the real-world population of patients with HER2-positive disease treated in the modern era with effective chemotherapy and anti-HER2 targeted therapies. “Moreover, the current standard of care for early breast cancer is even superior to the treatment received by many patients in the ALTTO study. … As such, the discordance between OS estimated by PREDICT and the current real-world OS is expected to be even higher. Therefore,” the researchers concluded, “our results suggest that the current version of PREDICT should be used with caution for prognostication in HER2-positive early breast cancer patients treated in the modern era with effective chemotherapy and anti-HER2 targeted therapies.”
A version of this article first appeared on Medscape.com. This article was translated from Univadis Italy.
The PREDICT score does not seem to be particularly accurate when it comes to estimating overall survival (OS) in patients with HER2-positive early breast cancer who are treated with modern chemotherapy and anti-HER2 targeted therapies. This is the conclusion of an international study published in the journal npj Breast Cancer. The work was supervised by Matteo Lambertini, MD, PhD, an oncologist at the IRCCS San Martino Polyclinic Hospital in Genoa, Italy.
As the authors explain, “PREDICT is a publicly available online tool that helps to predict the individual prognosis of patients with early breast cancer and to show the impact of adjuvant treatments administered after breast cancer surgery.” The tool uses traditional clinical-pathological factors. The authors also point out that the original version of this tool was validated in several datasets of patients with breast cancer. In 2011, it was updated to include HER2 status.
The investigators noted that, although the use of PREDICT is recommended to aid decision-making in the adjuvant setting, its prognostic role in patients with HER2-positive early breast cancer who are treated with modern chemotherapy and anti-HER2 therapies – even trastuzumab-based ones – remains unclear.
Therefore, they decided to analyze PREDICT’s prognostic performance using data extracted from the ALTTO trial, the largest adjuvant study ever conducted in the field of HER2-positive early breast cancer. That trial “represented a unique opportunity to investigate the reliability and prognostic performance of PREDICT in women with HER2-positive disease,” according to the investigators. They went on to specify that ALTTO evaluated adjuvant lapatinib plus trastuzumab vs. trastuzumab alone in 8,381 patients – 2,794 of whom were included in their own analysis.
What the analysis found was that, overall, PREDICT underestimated 5-year OS by 6.7%. The observed 5-year OS was 94.7%, and the predicted 5-year OS was 88.0%.
The highest absolute differences were observed for patients with hormone receptor–negative disease, nodal involvement, and large tumor size (13.0%, 15.8%, and 15.3%, respectively),” they wrote. Furthermore, they reported that “the suboptimal performance of this prognostic tool was observed irrespective of type of anti-HER2 treatment, type of chemotherapy regimen, age of the patients at the time of diagnosis, central hormone receptor status, pathological nodal status, and pathological tumor size.”
To potentially explain the reasons for the underestimation of patients’ OS, the authors questioned whether the population used to validate PREDICT accurately mirrored the real-world population of patients with HER2-positive disease treated in the modern era with effective chemotherapy and anti-HER2 targeted therapies. “Moreover, the current standard of care for early breast cancer is even superior to the treatment received by many patients in the ALTTO study. … As such, the discordance between OS estimated by PREDICT and the current real-world OS is expected to be even higher. Therefore,” the researchers concluded, “our results suggest that the current version of PREDICT should be used with caution for prognostication in HER2-positive early breast cancer patients treated in the modern era with effective chemotherapy and anti-HER2 targeted therapies.”
A version of this article first appeared on Medscape.com. This article was translated from Univadis Italy.
FROM NPJ BREAST CANCER
‘Obesity paradox’ in AFib challenged as mortality climbs with BMI
The relationship between body mass index (BMI) and all-cause mortality in patients with atrial fibrillation (AFib) is U-shaped, with the risk highest in those who are underweight or severely obese and lowest in patients defined simply as obese, a registry analysis suggests. It also showed a similar relationship between BMI and risk for new or worsening heart failure (HF).
Mortality bottomed out at a BMI of about 30-35 kg/m2, which suggests that mild obesity was protective, compared even with “normal-weight” or “overweight” BMI. Still, mortality went up sharply from there with rising BMI.
But higher BMI, a surrogate for obesity, apparently didn’t worsen outcomes by itself. The risk for death from any cause at higher obesity levels was found to depend a lot on related risk factors and comorbidities when the analysis controlled for conditions such as diabetes and hypertension.
The findings suggest an inverse relationship between BMI and all-cause mortality in AFib only for patients with BMI less than about 30. They therefore argue against any “obesity paradox” in AFib that posits consistently better survival with increasing levels of obesity, say researchers, based on their analysis of patients with new-onset AFib in the GARFIELD-AF registry.
“It’s common practice now for clinicians to discuss weight within a clinic setting when they’re talking to their AFib patients,” observed Christian Fielder Camm, BM, BCh, University of Oxford (England), and Royal Berkshire NHS Foundation Trust, Reading, England. So studies suggesting an inverse association between BMI and AFib-related risk can be a concern.
Such studies “seem to suggest that once you’ve got AFib, maintaining a high or very high BMI may in some way be protective – which is contrary to what would seem to make sense and certainly contrary to what our results have shown,” Dr. Camm told this news organization.
“I think that having further evidence now to suggest, actually, that greater BMI is associated with a greater risk of all-cause mortality and heart failure helps reframe that discussion at the physician-patient interaction level more clearly, and ensures that we’re able to talk to our patients appropriately about risks associated with BMI and atrial fibrillation,” said Dr. Camm, who is lead author on the analysis published in Open Heart.
“Obesity is a cause of most cardiovascular diseases, but [these] data would support that being overweight or having mild obesity does not increase the risk,” observed Carl J. Lavie, MD, of the John Ochsner Heart and Vascular Institute, New Orleans, La., and the Ochsner Clinical School at the University of Queensland, Brisbane, Australia.
“At a BMI of 40, it’s very important for them to lose weight for their long-term prognosis,” Dr. Lavie noted, but “at a BMI of 30, the important thing would be to prevent further weight gain. And if they could keep their BMI of 30, they should have a good prognosis. Their prognosis would be particularly good if they didn’t gain weight and put themselves in a more extreme obesity class that is associated with worse risk.”
The current analysis, Dr. Lavie said, “is way better than the AFFIRM study,” which yielded an obesity-paradox report on its patients with AFib about a dozen years ago. “It’s got more data, more numbers, more statistical power,” and breaks BMI into more categories.
That previous analysis based on the influential AFFIRM randomized trial separated its 4,060 patients with AFib into normal (BMI, 18.5-25), overweight (BMI, 25-30), and obese (BMI, > 30) categories, per the convention at the time. It concluded that “obese patients with atrial fibrillation appear to have better long-term outcomes than nonobese patients.”
Bleeding risk on oral anticoagulants
Also noteworthy in the current analysis, variation in BMI didn’t seem to affect mortality or risk for major bleeding or nonhemorrhagic stroke according to choice of oral anticoagulant – whether a new oral anticoagulant (NOAC) or a vitamin K antagonist (VKA).
“We saw that even in the obese and extremely obese group, all-cause mortality was lower in the group taking NOACs, compared with taking warfarin,” Dr. Camm observed, “which goes against the idea that we would need any kind of dose adjustments for increased BMI.”
Indeed, the report notes, use of NOACs, compared with VKA, was associated with a 23% drop in risk for death among patients who were either normal weight or overweight and also in those who were obese or extremely obese.
Those findings “are basically saying that the NOACs look better than warfarin regardless of weight,” agreed Dr. Lavie. “The problem is that the study is not very powered.”
Whereas the benefits of NOACs, compared to VKA, seem similar for patients with a BMI of 30 or 34, compared with a BMI of 23, for example, “none of the studies has many people with 50 BMI.” Many clinicians “feel uncomfortable giving the same dose of NOAC to somebody who has a 60 BMI,” he said. At least with warfarin, “you can check the INR [international normalized ratio].”
The current analysis included 40,482 patients with recently diagnosed AFib and at least one other stroke risk factor from among the registry’s more than 50,000 patients from 35 countries, enrolled from 2010 to 2016. They were followed for 2 years.
The 703 patients with BMI under 18.5 at AFib diagnosis were classified per World Health Organization definitions as underweight; the 13,095 with BMI 18.5-25 as normal weight; the 15,043 with BMI 25-30 as overweight; the 7,560 with BMI 30-35 as obese; and the 4,081 with BMI above 35 as extremely obese. Their ages averaged 71 years, and 55.6% were men.
BMI effects on different outcomes
Relationships between BMI and all-cause mortality and between BMI and new or worsening HF emerged as U-shaped, the risk climbing with both increasing and decreasing BMI. The nadir BMI for risk was about 30 in the case of mortality and about 25 for new or worsening HF.
The all-cause mortality risk rose by 32% for every 5 BMI points lower than a BMI of 30, and by 16% for every 5 BMI points higher than 30, in a partially adjusted analysis. The risk for new or worsening HF rose significantly with increasing but not decreasing BMI, and the reverse was observed for the endpoint of major bleeding.
The effect of BMI on all-cause mortality was “substantially attenuated” when the analysis was further adjusted with “likely mediators of any association between BMI and outcomes,” including hypertension, diabetes, HF, cerebrovascular events, and history of bleeding, Dr. Camm said.
That blunted BMI-mortality relationship, he said, “suggests that a lot of the effect is mediated through relatively traditional risk factors like hypertension and diabetes.”
The 2010 AFFIRM analysis by BMI, Dr. Lavie noted, “didn’t even look at the underweight; they actually threw them out.” Yet, such patients with AFib, who tend to be extremely frail or have chronic diseases or conditions other than the arrhythmia, are common. A take-home of the current study is that “the underweight with atrial fibrillation have a really bad prognosis.”
That message isn’t heard as much, he observed, “but is as important as saying that BMI 30 has the best prognosis. The worst prognosis is with the underweight or the really extreme obese.”
Dr. Camm discloses research funding from the British Heart Foundation. Disclosures for the other authors are in the report. Dr. Lavie has previously disclosed serving as a speaker and consultant for PAI Health and DSM Nutritional Products and is the author of “The Obesity Paradox: When Thinner Means Sicker and Heavier Means Healthier” (Avery, 2014).
A version of this article first appeared on Medscape.com.
The relationship between body mass index (BMI) and all-cause mortality in patients with atrial fibrillation (AFib) is U-shaped, with the risk highest in those who are underweight or severely obese and lowest in patients defined simply as obese, a registry analysis suggests. It also showed a similar relationship between BMI and risk for new or worsening heart failure (HF).
Mortality bottomed out at a BMI of about 30-35 kg/m2, which suggests that mild obesity was protective, compared even with “normal-weight” or “overweight” BMI. Still, mortality went up sharply from there with rising BMI.
But higher BMI, a surrogate for obesity, apparently didn’t worsen outcomes by itself. The risk for death from any cause at higher obesity levels was found to depend a lot on related risk factors and comorbidities when the analysis controlled for conditions such as diabetes and hypertension.
The findings suggest an inverse relationship between BMI and all-cause mortality in AFib only for patients with BMI less than about 30. They therefore argue against any “obesity paradox” in AFib that posits consistently better survival with increasing levels of obesity, say researchers, based on their analysis of patients with new-onset AFib in the GARFIELD-AF registry.
“It’s common practice now for clinicians to discuss weight within a clinic setting when they’re talking to their AFib patients,” observed Christian Fielder Camm, BM, BCh, University of Oxford (England), and Royal Berkshire NHS Foundation Trust, Reading, England. So studies suggesting an inverse association between BMI and AFib-related risk can be a concern.
Such studies “seem to suggest that once you’ve got AFib, maintaining a high or very high BMI may in some way be protective – which is contrary to what would seem to make sense and certainly contrary to what our results have shown,” Dr. Camm told this news organization.
“I think that having further evidence now to suggest, actually, that greater BMI is associated with a greater risk of all-cause mortality and heart failure helps reframe that discussion at the physician-patient interaction level more clearly, and ensures that we’re able to talk to our patients appropriately about risks associated with BMI and atrial fibrillation,” said Dr. Camm, who is lead author on the analysis published in Open Heart.
“Obesity is a cause of most cardiovascular diseases, but [these] data would support that being overweight or having mild obesity does not increase the risk,” observed Carl J. Lavie, MD, of the John Ochsner Heart and Vascular Institute, New Orleans, La., and the Ochsner Clinical School at the University of Queensland, Brisbane, Australia.
“At a BMI of 40, it’s very important for them to lose weight for their long-term prognosis,” Dr. Lavie noted, but “at a BMI of 30, the important thing would be to prevent further weight gain. And if they could keep their BMI of 30, they should have a good prognosis. Their prognosis would be particularly good if they didn’t gain weight and put themselves in a more extreme obesity class that is associated with worse risk.”
The current analysis, Dr. Lavie said, “is way better than the AFFIRM study,” which yielded an obesity-paradox report on its patients with AFib about a dozen years ago. “It’s got more data, more numbers, more statistical power,” and breaks BMI into more categories.
That previous analysis based on the influential AFFIRM randomized trial separated its 4,060 patients with AFib into normal (BMI, 18.5-25), overweight (BMI, 25-30), and obese (BMI, > 30) categories, per the convention at the time. It concluded that “obese patients with atrial fibrillation appear to have better long-term outcomes than nonobese patients.”
Bleeding risk on oral anticoagulants
Also noteworthy in the current analysis, variation in BMI didn’t seem to affect mortality or risk for major bleeding or nonhemorrhagic stroke according to choice of oral anticoagulant – whether a new oral anticoagulant (NOAC) or a vitamin K antagonist (VKA).
“We saw that even in the obese and extremely obese group, all-cause mortality was lower in the group taking NOACs, compared with taking warfarin,” Dr. Camm observed, “which goes against the idea that we would need any kind of dose adjustments for increased BMI.”
Indeed, the report notes, use of NOACs, compared with VKA, was associated with a 23% drop in risk for death among patients who were either normal weight or overweight and also in those who were obese or extremely obese.
Those findings “are basically saying that the NOACs look better than warfarin regardless of weight,” agreed Dr. Lavie. “The problem is that the study is not very powered.”
Whereas the benefits of NOACs, compared to VKA, seem similar for patients with a BMI of 30 or 34, compared with a BMI of 23, for example, “none of the studies has many people with 50 BMI.” Many clinicians “feel uncomfortable giving the same dose of NOAC to somebody who has a 60 BMI,” he said. At least with warfarin, “you can check the INR [international normalized ratio].”
The current analysis included 40,482 patients with recently diagnosed AFib and at least one other stroke risk factor from among the registry’s more than 50,000 patients from 35 countries, enrolled from 2010 to 2016. They were followed for 2 years.
The 703 patients with BMI under 18.5 at AFib diagnosis were classified per World Health Organization definitions as underweight; the 13,095 with BMI 18.5-25 as normal weight; the 15,043 with BMI 25-30 as overweight; the 7,560 with BMI 30-35 as obese; and the 4,081 with BMI above 35 as extremely obese. Their ages averaged 71 years, and 55.6% were men.
BMI effects on different outcomes
Relationships between BMI and all-cause mortality and between BMI and new or worsening HF emerged as U-shaped, the risk climbing with both increasing and decreasing BMI. The nadir BMI for risk was about 30 in the case of mortality and about 25 for new or worsening HF.
The all-cause mortality risk rose by 32% for every 5 BMI points lower than a BMI of 30, and by 16% for every 5 BMI points higher than 30, in a partially adjusted analysis. The risk for new or worsening HF rose significantly with increasing but not decreasing BMI, and the reverse was observed for the endpoint of major bleeding.
The effect of BMI on all-cause mortality was “substantially attenuated” when the analysis was further adjusted with “likely mediators of any association between BMI and outcomes,” including hypertension, diabetes, HF, cerebrovascular events, and history of bleeding, Dr. Camm said.
That blunted BMI-mortality relationship, he said, “suggests that a lot of the effect is mediated through relatively traditional risk factors like hypertension and diabetes.”
The 2010 AFFIRM analysis by BMI, Dr. Lavie noted, “didn’t even look at the underweight; they actually threw them out.” Yet, such patients with AFib, who tend to be extremely frail or have chronic diseases or conditions other than the arrhythmia, are common. A take-home of the current study is that “the underweight with atrial fibrillation have a really bad prognosis.”
That message isn’t heard as much, he observed, “but is as important as saying that BMI 30 has the best prognosis. The worst prognosis is with the underweight or the really extreme obese.”
Dr. Camm discloses research funding from the British Heart Foundation. Disclosures for the other authors are in the report. Dr. Lavie has previously disclosed serving as a speaker and consultant for PAI Health and DSM Nutritional Products and is the author of “The Obesity Paradox: When Thinner Means Sicker and Heavier Means Healthier” (Avery, 2014).
A version of this article first appeared on Medscape.com.
The relationship between body mass index (BMI) and all-cause mortality in patients with atrial fibrillation (AFib) is U-shaped, with the risk highest in those who are underweight or severely obese and lowest in patients defined simply as obese, a registry analysis suggests. It also showed a similar relationship between BMI and risk for new or worsening heart failure (HF).
Mortality bottomed out at a BMI of about 30-35 kg/m2, which suggests that mild obesity was protective, compared even with “normal-weight” or “overweight” BMI. Still, mortality went up sharply from there with rising BMI.
But higher BMI, a surrogate for obesity, apparently didn’t worsen outcomes by itself. The risk for death from any cause at higher obesity levels was found to depend a lot on related risk factors and comorbidities when the analysis controlled for conditions such as diabetes and hypertension.
The findings suggest an inverse relationship between BMI and all-cause mortality in AFib only for patients with BMI less than about 30. They therefore argue against any “obesity paradox” in AFib that posits consistently better survival with increasing levels of obesity, say researchers, based on their analysis of patients with new-onset AFib in the GARFIELD-AF registry.
“It’s common practice now for clinicians to discuss weight within a clinic setting when they’re talking to their AFib patients,” observed Christian Fielder Camm, BM, BCh, University of Oxford (England), and Royal Berkshire NHS Foundation Trust, Reading, England. So studies suggesting an inverse association between BMI and AFib-related risk can be a concern.
Such studies “seem to suggest that once you’ve got AFib, maintaining a high or very high BMI may in some way be protective – which is contrary to what would seem to make sense and certainly contrary to what our results have shown,” Dr. Camm told this news organization.
“I think that having further evidence now to suggest, actually, that greater BMI is associated with a greater risk of all-cause mortality and heart failure helps reframe that discussion at the physician-patient interaction level more clearly, and ensures that we’re able to talk to our patients appropriately about risks associated with BMI and atrial fibrillation,” said Dr. Camm, who is lead author on the analysis published in Open Heart.
“Obesity is a cause of most cardiovascular diseases, but [these] data would support that being overweight or having mild obesity does not increase the risk,” observed Carl J. Lavie, MD, of the John Ochsner Heart and Vascular Institute, New Orleans, La., and the Ochsner Clinical School at the University of Queensland, Brisbane, Australia.
“At a BMI of 40, it’s very important for them to lose weight for their long-term prognosis,” Dr. Lavie noted, but “at a BMI of 30, the important thing would be to prevent further weight gain. And if they could keep their BMI of 30, they should have a good prognosis. Their prognosis would be particularly good if they didn’t gain weight and put themselves in a more extreme obesity class that is associated with worse risk.”
The current analysis, Dr. Lavie said, “is way better than the AFFIRM study,” which yielded an obesity-paradox report on its patients with AFib about a dozen years ago. “It’s got more data, more numbers, more statistical power,” and breaks BMI into more categories.
That previous analysis based on the influential AFFIRM randomized trial separated its 4,060 patients with AFib into normal (BMI, 18.5-25), overweight (BMI, 25-30), and obese (BMI, > 30) categories, per the convention at the time. It concluded that “obese patients with atrial fibrillation appear to have better long-term outcomes than nonobese patients.”
Bleeding risk on oral anticoagulants
Also noteworthy in the current analysis, variation in BMI didn’t seem to affect mortality or risk for major bleeding or nonhemorrhagic stroke according to choice of oral anticoagulant – whether a new oral anticoagulant (NOAC) or a vitamin K antagonist (VKA).
“We saw that even in the obese and extremely obese group, all-cause mortality was lower in the group taking NOACs, compared with taking warfarin,” Dr. Camm observed, “which goes against the idea that we would need any kind of dose adjustments for increased BMI.”
Indeed, the report notes, use of NOACs, compared with VKA, was associated with a 23% drop in risk for death among patients who were either normal weight or overweight and also in those who were obese or extremely obese.
Those findings “are basically saying that the NOACs look better than warfarin regardless of weight,” agreed Dr. Lavie. “The problem is that the study is not very powered.”
Whereas the benefits of NOACs, compared to VKA, seem similar for patients with a BMI of 30 or 34, compared with a BMI of 23, for example, “none of the studies has many people with 50 BMI.” Many clinicians “feel uncomfortable giving the same dose of NOAC to somebody who has a 60 BMI,” he said. At least with warfarin, “you can check the INR [international normalized ratio].”
The current analysis included 40,482 patients with recently diagnosed AFib and at least one other stroke risk factor from among the registry’s more than 50,000 patients from 35 countries, enrolled from 2010 to 2016. They were followed for 2 years.
The 703 patients with BMI under 18.5 at AFib diagnosis were classified per World Health Organization definitions as underweight; the 13,095 with BMI 18.5-25 as normal weight; the 15,043 with BMI 25-30 as overweight; the 7,560 with BMI 30-35 as obese; and the 4,081 with BMI above 35 as extremely obese. Their ages averaged 71 years, and 55.6% were men.
BMI effects on different outcomes
Relationships between BMI and all-cause mortality and between BMI and new or worsening HF emerged as U-shaped, the risk climbing with both increasing and decreasing BMI. The nadir BMI for risk was about 30 in the case of mortality and about 25 for new or worsening HF.
The all-cause mortality risk rose by 32% for every 5 BMI points lower than a BMI of 30, and by 16% for every 5 BMI points higher than 30, in a partially adjusted analysis. The risk for new or worsening HF rose significantly with increasing but not decreasing BMI, and the reverse was observed for the endpoint of major bleeding.
The effect of BMI on all-cause mortality was “substantially attenuated” when the analysis was further adjusted with “likely mediators of any association between BMI and outcomes,” including hypertension, diabetes, HF, cerebrovascular events, and history of bleeding, Dr. Camm said.
That blunted BMI-mortality relationship, he said, “suggests that a lot of the effect is mediated through relatively traditional risk factors like hypertension and diabetes.”
The 2010 AFFIRM analysis by BMI, Dr. Lavie noted, “didn’t even look at the underweight; they actually threw them out.” Yet, such patients with AFib, who tend to be extremely frail or have chronic diseases or conditions other than the arrhythmia, are common. A take-home of the current study is that “the underweight with atrial fibrillation have a really bad prognosis.”
That message isn’t heard as much, he observed, “but is as important as saying that BMI 30 has the best prognosis. The worst prognosis is with the underweight or the really extreme obese.”
Dr. Camm discloses research funding from the British Heart Foundation. Disclosures for the other authors are in the report. Dr. Lavie has previously disclosed serving as a speaker and consultant for PAI Health and DSM Nutritional Products and is the author of “The Obesity Paradox: When Thinner Means Sicker and Heavier Means Healthier” (Avery, 2014).
A version of this article first appeared on Medscape.com.
FROM OPEN HEART
Safety Profile of Mutant EGFR-TK Inhibitors in Advanced Non–Small Cell Lung Cancer: A Meta-analysis
Lung cancer has been the leading cause of cancer-related mortality for decades. It is also predicted to remain as the leading cause of cancer-related mortality through 2030.1 Platinum-based chemotherapy, including carboplatin and paclitaxel, was introduced 3 decades ago and revolutionized the management of advanced non–small cell lung cancer (NSCLC). A more recent advancement has been mutant epidermal growth factor receptor–tyrosine kinase (EGFR-TK) inhibitors.1 EGFR is a transmembrane protein that functions by transducing essential growth factor signaling from the extracellular milieu to the cell. As 60% of the advanced NSCLC expresses this receptor, blocking the mutant EGFR receptor was a groundbreaking development in the management of advanced NSCLC.2 Development of mutant EGFR-TK inhibitors has revolutionized the management of advanced NSCLC. This study was conducted to determine the safety profile of mutant EGFR-TK inhibitors in the management of advanced NSCLC.
Methods
This meta-analysis was conducted according to Cochrane Collaboration guidelines and reported as per Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The findings are summarized in the PRISMA flow diagram (Figure 1). Two authors (MZ and MM) performed a systematic literature search using databases such as MEDLINE (via PubMed), Embase, and Cochrane Library using the medical search terms and their respective entry words with the following search strategy: safety, “mutant EGFR-TK inhibitors,” advanced, “non–small cell,” “lung cancer,” “adverse effect,” and literature. Additionally, unpublished trials were identified from clinicaltrials.gov, and references of all pertinent articles were also scrutinized to ensure the inclusion of all relevant studies. The search was completed on June 1, 2021, and we only included studies available in English. Two authors (MM and MZ) independently screened the search results in a 2-step process based on predetermined inclusion/exclusion criteria. First, 890 articles were evaluated for relevance on title and abstract level, followed by full-text screening of the final list of 140 articles. Any disagreements were resolved by discussion or third-party review, and a total of 9 articles were included in the study.
The following eligibility criteria were used: original articles reporting adverse effects (AEs) of mutant EGFR-TK inhibitors in patients with advanced NSCLC compared with control groups receiving platinum-based chemotherapy. All the patients included in the study had an EGFR mutation but randomly assigned to either treatment or control group. All articles with subjective data on mutant EGFR-TK inhibitors AEs in patients with advanced NSCLC compared with control groups receiving platinum-based chemotherapy were included in the analysis. Only 9 articles qualified the aforementioned selection criteria for eligibility. All qualifying studies were nationwide inpatient or pooled clinical trials data. The reasons for exclusion of the other 71 articles were irrelevant (n = 31), duplicate (n = 13), reviews (n = 14), and poor data reporting (n = 12). Out of the 9 included studies, 9 studies showed correlation of AEs, including rash, diarrhea, nausea, and fatigue. Seven studies showed correlation of AEs including neutropenia, anorexia, and vomiting. Six studies showed correlation of anemia, cough, and stomatitis. Five studies showed correlation of elevated aspartate aminotransferase (AST), alanine aminotransferase (ALT), and leucopenia. Four studies showed correlation of fever between mutant EGFR-TK inhibitors and platinum-based chemotherapy.
The primary endpoints were reported AEs including rash, diarrhea, elevated ALT, elevated AST, stomatitis, nausea, leucopenia, fatigue, neutropenia, anorexia, anemia, cough, vomiting, and fever, respectively. Data on baseline characteristics and clinical outcomes were then extracted, and summary tables were created. Summary estimates of the clinical endpoints were then calculated with risk ratio (RR) and 95% confidence intervals (CIs) using the random-effects model. Heterogeneity between studies was examined with the Cochran Q I2 statistic which can be defined as low (25% to 50%), moderate (50% to 75%), or high (> 75%). Statistical analysis was performed using Comprehensive Meta-Analysis Software CMA Version 3.0.
Results
A total of 9 studies including 3415 patients (1775 in EGFR-TK inhibitor treatment group while 1640 patients in platinum-based chemotherapy control group) were included in the study. All 9 studies were phase III randomized control clinical trials conducted to compare the safety profile of mutant EGFR-TK inhibitors in patients with advanced NSCLC. Mean age was 61 years in both treatment and control groups. Further details on study and participant characteristics and safety profile including AEs are summarized in Tables 1 and 2. No evidence of publication bias was found.
Rash developed in 45.8% of patients in the treatment group receiving mutant EGFR-TK inhibitors vs only 5.6% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 7.38 with the 95% CI noted, which was statistically significant, confirming higher rash event rates in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 2).
Diarrhea occurred in 33.6% of patients in the mutant EGFR-TK inhibitors treatment group vs 13.5% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 2.63 and 95% CI was noted, which was statistically significant, confirming higher diarrheal rates in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 3).
Elevated ALT levels developed in 27.9% of patients in the treatment group receiving mutant EGFR-TK inhibitors compared with 15.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 1.37 and 95% CI was noted, which was statistically significant, confirming higher ALT levels in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 4).
Elevated AST levels occurred in 40.7% of patients in the mutant EGFR-TK inhibitors treatment group vs 12.8% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 1.77 and 95% CI was noted, which was statistically significant, confirming elevated AST levels in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 5).
Stomatitis developed in 17.2% of patients in the treatment group receiving mutant EGFR-TK inhibitors compared with 7.9% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 1.53 and 95% CI was noted, which was statistically significant, confirming higher stomatitis event rates in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 6).
Nausea occurred in 16.5% of patients in the mutant EGFR-TK inhibitors group vs 42.5% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.37 and 95% CI was noted, which was statistically significant, confirming higher nausea rates in patients receiving platinum-based chemotherapy compared with treatment group for their advanced NSCLC (Figure 7).
Leucopenia developed in 9.7% of patients in the mutant EGFR-TK inhibitors group compared with 51.3% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.18 and 95% CI was noted, which was statistically significant, confirming higher leucopenia incidence in patients receiving platinum-based chemotherapy compared with treatment group for their advanced NSCLC (Figure 8).
Fatigue was reported in 17% of patients in the mutant EGFR-TK inhibitors group compared with 29.5% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.59 and 95% CI was noted, which was statistically significant, confirming higher fatigue rates in patients receiving platinum-based chemotherapy compared with treatment group for their advanced NSCLC (Figure 9).
Neutropenia developed in 6.1% of patients in the mutant EGFR-TK inhibitors group vs 48.2% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.11 and 95% CI was noted, which was statistically significant, confirming higher neutropenia rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 10).
Anorexia developed in 21.3% of patients in the mutant EGFR-TK inhibitors group vs 31.4% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.44 and 95% CI was noted, which was statistically significant, confirming higher anorexia rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 11).
Anemia occurred in 8.7% of patients in the mutant EGFR-TK inhibitors group compared with 32.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.24 and 95% CI was noted, which was statistically significant, confirming higher anorexia rates in patients receiving platinum-based chemotherapy compared with treatment for their advanced NSCLC (Figure 12).
Cough was reported in 17.8% of patients in the mutant EGFR-TK inhibitors group compared with 18.9% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.99 and 95% CI was noted, which was statistically significant, confirming slightly higher cough rates in patients receiving platinum-based chemotherapy compared with treatment for their advanced NSCLC (Figure 13).
Vomiting developed in 11% of patients in the mutant EGFR-TK inhibitors group vs 30.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.35 and 95% CI was noted, which was statistically significant, confirming higher vomiting rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 14).
Fever occurred in 5.6% of patients in the mutant EGFR-TK inhibitors group compared with 30.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.41 and 95% CI was noted, which was statistically significant, confirming higher fever rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 15).
Discussion
Despite the advancement in the treatment of metastatic NSCLC, lung cancer stays as most common cause of cancer-related death in North America and European countries, as patients usually have an advanced disease at the time of diagnosis.3 In the past, platinum-based chemotherapy remained the standard of care for most of the patients affected with advanced NSCLC, but the higher recurrence rate and increase in frequency and intensity of AEs with platinum-based chemotherapy led to the development of targeted therapy for NSCLC, one of which includes
Smoking is the most common reversible risk factor associated with lung cancer. The EURTAC trial was the first perspective study in this regard, which compared safety and efficacy of mutant EGFR-TK inhibitors with platinum-based chemotherapy. Results analyzed in this study were in favor of mutant EGFR-TK inhibitors except in the group of former smokers.5 On the contrary, the OPTIMAL trial showed results in favor of mutant EGFR-TK inhibitors both in active and former smokers; this trial also confirmed the efficacy of mutant EGFR-TK inhibitors in European and Asian populations, confirming the rationale for routine testing of EGFR mutation in all the patients being diagnosed with advanced NSCLC.6 Similarly, osimertinib is one of the most recent mutant EGFR-TK inhibitors developed for the treatment of advanced NSCLC in patients with EGFR-positive receptors.
According to the FLAURA trial, patients receiving osimertinib showed significantly longer progression-free survival compared with platinum-based chemotherapy and early mutant EGFR-TK inhibitors. Median progression-free survival was noted to be 18.9 months, which showed 54% lower risk of disease progression in the treatment group receiving osimertinib.7 The ARCHER study emphasized a significant improvement in overall survival as well as progression-free survival among a patient population receiving dacomitinib compared with platinum-based chemotherapy.8,9
Being a potent targeted therapy, mutant EGFR-TK inhibitors do come with some AEs including diarrhea, which was seen in 33.6% of the patients receiving mutant EGFR-TK inhibitors in our study vs 53% in the chemotherapy group, as was observed in the study conducted by Pless and colleagues.10 Similarly, only 16.5% of patients receiving mutant EGFR-TK inhibitors developed nausea compared with 66% being observed in patients receiving chemotherapy. Correspondingly, only a small fraction of patients (9.7%) receiving mutant EGFR-TK inhibitors developed leucopenia, which was 10 times less reported in mutant EGFR-TK inhibitors compared with patients receiving chemotherapy having a percentage of 100%. A similar trend was reported for neutropenia and anemia in mutant EGFR-TK inhibitors with an incidence of 6.1% and 8.7%, compared with the platinum-based chemotherapy group in which the incidence was found to be 80% and 100%, respectively. It was concluded that platinum-based chemotherapy had played a vital role in the treatment of advanced NSCLC but at an expense of serious and severe AEs which led to discontinuation or withdrawal of treatment, leading to relapse and recurrence of lung cancer.10,11
Zhong and colleagues conducted a phase 2 randomized clinical trial comparing mutant EGFR-TK inhibitors with platinum-based chemotherapy. They concluded that in patients receiving platinum-based chemotherapy, incidence of rash, vomiting, anorexia, neutropenia, and nausea were 29.4%, 47%, 41.2%, 55.8%, and 32.4% compared with 45.8%, 11%, 21.3%, 6.1%, and 16.5%, respectively, reported in patients receiving mutant EGFR-TK inhibitors for their advanced NSCLC.12
Another study was conducted in 2019 by Noronha and colleagues to determine the impact of platinum-based chemotherapy combined with gefitinib on patients with advanced NSCLC.13 They concluded that 70% of the patients receiving combination treatment developed rash, which was significantly higher compared with 45.8% patients receiving the mutant EGFR-TK inhibitors alone in our study. Also, 56% of patients receiving combination therapy developed diarrhea vs 33.6% of patients receiving mutant EGFR-TK inhibitors only. Similarly, 96% of patients in the combination therapy group developed some degree of anemia compared with only 8.7% patients in the mutant EGFR-TK inhibitors group included in our study. In the same way, neutropenia was observed in 55% of patients receiving combination therapy vs 6.1% in patients receiving mutant EGFR-TK inhibitors solely. They concluded that mutant EGFR-TK inhibitors when combined with platinum-based chemotherapy increase the incidence of AEs of chemotherapy by many folds.13,14
Kato and colleagues conducted a study to determine the impact on AEs when erlotinib was combined with anti–vascular endothelial growth factor (VEGF) inhibitors like bevacizumab, they stated that 98.7% of patient in combination therapy developed rash, the incidence of which was only 45.8% in patients receiving mutant EGFR-TK inhibitors as was observed in our study. Similar trends were noticed with other AEs, including diarrhea, fatigue, nausea, and elevated liver enzymes.15
With the latest advancements in the management of advanced NSCLC, nivolumab, a programmed death ligand 1 (PD-L1) inhibitor, was developed and either used as monotherapy in patients with PD-L1 expression or was combined with platinum-based chemotherapy regardless of PD-L1 expression.16,17 Patients expressing lower PD-L1 levels were not omitted from receiving nivolumab as no significant difference was noted in progression-free span and overall survival in patients receiving nivolumab irrespective of PD-L1 levels.15 Rash developed in 17% of patients after receiving nivolumab vs 45.8% patients being observed in our study. A similar trend was observed with diarrhea as only 17% of the population receiving nivolumab developed diarrhea compared with 33.6% of the population receiving mutant EGFR-TK inhibitors in our study. Likewise, only 9.9% of the patients receiving nivolumab developed nausea as an AE compared with 16.5% being observed in mutant EGFR-TK inhibitors in our study. Also, fatigue was observed in 14.4% of the population receiving nivolumab vs 17% observed in patients receiving mutant EGFR-TK inhibitors as was noticed in our study.7,8
Rizvi and colleagues conducted a study on the role of nivolumab when combined with platinum-based chemotherapy in patients with advanced NSCLC and reported that 40% of patients included in the study developed rash compared with 45.8% reported in mutant EGFR-TK inhibitors in our study. Similarly, only 13% of patients in the nivolumab group developed diarrhea vs 33.6% cases reported in the mutant EGFR-TK inhibitors group included in our study. Also, 7% of patients in the nivolumab group developed elevated ALT levels vs 27.9% of patients receiving mutant EGFR-TK inhibitors included in our study, concluding that addition of immune checkpoint inhibitors like nivolumab to platinum-based chemotherapy does not increase the frequency of AEs.18
Conclusions
Our study focused on the safety profile of mutant EGFR-TK inhibitors vs platinum-based chemotherapy in the treatment of advanced NSCLC. Mutant EGFR-TK inhibitors are safer than platinum-based chemotherapy when compared for nausea, leucopenia, fatigue, neutropenia, anorexia, anemia, cough, vomiting, and fever. On the other end, mutant EGFR-TK inhibitors cause slightly higher AEs, including rash, diarrhea, elevated AST and ALT levels, and stomatitis. However, considering that the development of mutant EGFR-TK inhibitors laid a foundation of targeted therapy, we recommend continuing using mutant EGFR-TK inhibitors in patients with advanced NSCLC especially in patients having mutant EGFR receptors. AEs caused by mutant EGFR-TK inhibitors are significant but are usually tolerable and can be avoided by reducing the dosage of it with each cycle or by skipping or delaying the dose until the patient is symptomatic.
1. Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74(11):2913-2921. doi:10.1158/0008-5472.CAN-14-0155
2. da Cunha Santos G, Shepherd FA, Tsao MS. EGFR mutations and lung cancer. Annu Rev Pathol. 2011;6:49-69. doi:10.1146/annurev-pathol-011110-130206
3. Sgambato A, Casaluce F, Maione P, et al. The role of EGFR tyrosine kinase inhibitors in the first-line treatment of advanced non small cell lung cancer patients harboring EGFR mutation. Curr Med Chem. 2012;19(20):3337-3352. doi:10.2174/092986712801215973
4. Rossi A, Di Maio M. Platinum-based chemotherapy in advanced non–small-cell lung cancer: optimal number of treatment cycles. Expert Rev Anticancer Ther. 2016;16(6):653-660. doi:10.1586/14737140.2016.1170596
5. Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non–small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239-246. doi:10.1016/S1470-2045(11)70393-X
6. Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non–small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735-742. doi:10.1016/S1470-2045(11)70184-X
7. Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non–small-cell lung cancer. N Engl J Med. 2018;378(2):113-125. doi:10.1056/NEJMoa1713137
8. Mok TS, Cheng Y, Zhou X, et al. Improvement in overall survival in a randomized study that compared dacomitinib with gefitinib in patients with advanced non–small-cell lung cancer and EGFR-activating mutations. J Clin Oncol. 2018;36(22):2244-2250. doi:10.1200/JCO.2018.78.7994
9. Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947-957. doi:10.1056/NEJMoa0810699
10. Pless M, Stupp R, Ris HB, et al. Induction chemoradiation in stage IIIA/N2 non–small-cell lung cancer: a phase 3 randomised trial. Lancet. 2015;386(9998):1049-1056. doi:10.1016/S0140-6736(15)60294-X
11. Albain KS, Rusch VW, Crowley JJ, et al. Concurrent cisplatin/etoposide plus chest radiotherapy followed by surgery for stages IIIA (N2) and IIIB non–small-cell lung cancer: mature results of Southwest Oncology Group phase II study 8805. J Clin Oncol. 1995;13(8):1880-1892. doi:10.1200/JCO.1995.13.8.1880
12. Zhong WZ, Chen KN, Chen C, et al. Erlotinib versus gemcitabine plus cisplatin as neoadjuvant treatment of Stage IIIA-N2 EGFR-mutant non–small-cell lung cancer (EMERGING-CTONG 1103): a randomized phase II study. J Clin Oncol. 2019;37(25):2235-2245. doi:10.1200/JCO.19.00075
13. Noronha V, Patil VM, Joshi A, et al. Gefitinib versus gefitinib plus pemetrexed and carboplatin chemotherapy in EGFR-mutated lung cancer. J Clin Oncol. 2020;38(2):124-136. doi:10.1200/JCO.19.01154
14. Noronha V, Prabhash K, Thavamani A, et al. EGFR mutations in Indian lung cancer patients: clinical correlation and outcome to EGFR targeted therapy. PLoS One. 2013;8(4):e61561. Published 2013 Apr 19. doi:10.1371/journal.pone.0061561
15. Kato T, Seto T, Nishio M, et al. Erlotinib plus bevacizumab phase ll study in patients with advanced non–small-cell lung cancer (JO25567): updated safety results. Drug Saf. 2018;41(2):229-237. doi:10.1007/s40264-017-0596-0
16. Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus ipilimumab in advanced non–small-cell lung cancer. N Engl J Med. 2019;381(21):2020-2031. doi:10.1056/NEJMoa1910231
17. Hellmann MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378(22):2093-2104. doi:10.1056/NEJMoa1801946
18. Rizvi NA, Hellmann MD, Brahmer JR, et al. Nivolumab in combination with platinum-based doublet chemotherapy for first-line treatment of advanced non–small-cell lung cancer. J Clin Oncol. 2016;34(25):2969-2979. doi:10.1200/JCO.2016.66.9861
19. Zhong WZ, Wang Q, Mao WM, et al. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II-IIIA (N1-N2) EGFR-mutant NSCLC: final overall survival analysis of CTONG1104 Phase III Trial. J Clin Oncol. 2021;39(7):713-722. doi:10.1200/JCO.20.01820
20. Yang JC, Sequist LV, Geater SL, et al. Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: a combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol. 2015;16(7):830-838. doi:10.1016/S1470-2045(15)00026-1
21. Shi YK, Wang L, Han BH, et al. First-line icotinib versus cisplatin/pemetrexed plus pemetrexed maintenance therapy for patients with advanced EGFR mutation-positive lung adenocarcinoma (CONVINCE): a phase 3, open-label, randomized study. Ann Oncol. 2017;28(10):2443-2450. doi:10.1093/annonc/mdx359
22. Soria JC, Wu YL, Nakagawa K, et al. Gefitinib plus chemotherapy versus placebo plus chemotherapy in EGFR-mutation-positive non-small-cell lung cancer after progression on first-line gefitinib (IMPRESS): a phase 3 randomized trial. Lancet Oncol. 2015;16(8):990-998 doi:10.1016/S1470-2045(15)00121-7
23. Goss GD, O’Callaghan C, Lorimer I, et al. Gefitinib versus placebo in completely resected non-small-cell lung cancer: results of the NCIC CTG BR19 study. J Clin Oncol. 2013;31(27):3320-3326. doi:10.1200/JCO.2013.51.1816
24. Sun JM, Lee KH, Kim SW, et al. Gefitinib versus pemetrexed as second-line treatment in patients with non-small cell lung cancer previously treated with platinum-based chemotherapy (KCSG-LU08-01): an open-label, phase 3 trial. Cancer. 2012;118(24):6234-6242. doi:10.1200/JCO.2013.51.1816
25. Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomized phase 3 trial. Lancet Oncol. 2010;11(2):121-128. doi:10.1016/S1470-2045(09)70364-X
26. Lee DH, Park K, Kim JH, Lee JS, et al. Randomized phase III trial of gefitinib versus docetaxel in non-small cell lung cancer patients who have previously received platinum-based chemotherapy. Clin Cancer Res. 2010;16(4):1307-1314. doi:10.1158/1078-0432.CCR-09-1903
27. Kim ES, Hirsh V, Mok T, et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomized phase III trial. Lancet. 2008;22;372(9652):1809-1818. doi:10.1016/S0140-6736(08)61758-4
Lung cancer has been the leading cause of cancer-related mortality for decades. It is also predicted to remain as the leading cause of cancer-related mortality through 2030.1 Platinum-based chemotherapy, including carboplatin and paclitaxel, was introduced 3 decades ago and revolutionized the management of advanced non–small cell lung cancer (NSCLC). A more recent advancement has been mutant epidermal growth factor receptor–tyrosine kinase (EGFR-TK) inhibitors.1 EGFR is a transmembrane protein that functions by transducing essential growth factor signaling from the extracellular milieu to the cell. As 60% of the advanced NSCLC expresses this receptor, blocking the mutant EGFR receptor was a groundbreaking development in the management of advanced NSCLC.2 Development of mutant EGFR-TK inhibitors has revolutionized the management of advanced NSCLC. This study was conducted to determine the safety profile of mutant EGFR-TK inhibitors in the management of advanced NSCLC.
Methods
This meta-analysis was conducted according to Cochrane Collaboration guidelines and reported as per Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The findings are summarized in the PRISMA flow diagram (Figure 1). Two authors (MZ and MM) performed a systematic literature search using databases such as MEDLINE (via PubMed), Embase, and Cochrane Library using the medical search terms and their respective entry words with the following search strategy: safety, “mutant EGFR-TK inhibitors,” advanced, “non–small cell,” “lung cancer,” “adverse effect,” and literature. Additionally, unpublished trials were identified from clinicaltrials.gov, and references of all pertinent articles were also scrutinized to ensure the inclusion of all relevant studies. The search was completed on June 1, 2021, and we only included studies available in English. Two authors (MM and MZ) independently screened the search results in a 2-step process based on predetermined inclusion/exclusion criteria. First, 890 articles were evaluated for relevance on title and abstract level, followed by full-text screening of the final list of 140 articles. Any disagreements were resolved by discussion or third-party review, and a total of 9 articles were included in the study.
The following eligibility criteria were used: original articles reporting adverse effects (AEs) of mutant EGFR-TK inhibitors in patients with advanced NSCLC compared with control groups receiving platinum-based chemotherapy. All the patients included in the study had an EGFR mutation but randomly assigned to either treatment or control group. All articles with subjective data on mutant EGFR-TK inhibitors AEs in patients with advanced NSCLC compared with control groups receiving platinum-based chemotherapy were included in the analysis. Only 9 articles qualified the aforementioned selection criteria for eligibility. All qualifying studies were nationwide inpatient or pooled clinical trials data. The reasons for exclusion of the other 71 articles were irrelevant (n = 31), duplicate (n = 13), reviews (n = 14), and poor data reporting (n = 12). Out of the 9 included studies, 9 studies showed correlation of AEs, including rash, diarrhea, nausea, and fatigue. Seven studies showed correlation of AEs including neutropenia, anorexia, and vomiting. Six studies showed correlation of anemia, cough, and stomatitis. Five studies showed correlation of elevated aspartate aminotransferase (AST), alanine aminotransferase (ALT), and leucopenia. Four studies showed correlation of fever between mutant EGFR-TK inhibitors and platinum-based chemotherapy.
The primary endpoints were reported AEs including rash, diarrhea, elevated ALT, elevated AST, stomatitis, nausea, leucopenia, fatigue, neutropenia, anorexia, anemia, cough, vomiting, and fever, respectively. Data on baseline characteristics and clinical outcomes were then extracted, and summary tables were created. Summary estimates of the clinical endpoints were then calculated with risk ratio (RR) and 95% confidence intervals (CIs) using the random-effects model. Heterogeneity between studies was examined with the Cochran Q I2 statistic which can be defined as low (25% to 50%), moderate (50% to 75%), or high (> 75%). Statistical analysis was performed using Comprehensive Meta-Analysis Software CMA Version 3.0.
Results
A total of 9 studies including 3415 patients (1775 in EGFR-TK inhibitor treatment group while 1640 patients in platinum-based chemotherapy control group) were included in the study. All 9 studies were phase III randomized control clinical trials conducted to compare the safety profile of mutant EGFR-TK inhibitors in patients with advanced NSCLC. Mean age was 61 years in both treatment and control groups. Further details on study and participant characteristics and safety profile including AEs are summarized in Tables 1 and 2. No evidence of publication bias was found.
Rash developed in 45.8% of patients in the treatment group receiving mutant EGFR-TK inhibitors vs only 5.6% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 7.38 with the 95% CI noted, which was statistically significant, confirming higher rash event rates in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 2).
Diarrhea occurred in 33.6% of patients in the mutant EGFR-TK inhibitors treatment group vs 13.5% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 2.63 and 95% CI was noted, which was statistically significant, confirming higher diarrheal rates in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 3).
Elevated ALT levels developed in 27.9% of patients in the treatment group receiving mutant EGFR-TK inhibitors compared with 15.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 1.37 and 95% CI was noted, which was statistically significant, confirming higher ALT levels in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 4).
Elevated AST levels occurred in 40.7% of patients in the mutant EGFR-TK inhibitors treatment group vs 12.8% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 1.77 and 95% CI was noted, which was statistically significant, confirming elevated AST levels in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 5).
Stomatitis developed in 17.2% of patients in the treatment group receiving mutant EGFR-TK inhibitors compared with 7.9% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 1.53 and 95% CI was noted, which was statistically significant, confirming higher stomatitis event rates in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 6).
Nausea occurred in 16.5% of patients in the mutant EGFR-TK inhibitors group vs 42.5% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.37 and 95% CI was noted, which was statistically significant, confirming higher nausea rates in patients receiving platinum-based chemotherapy compared with treatment group for their advanced NSCLC (Figure 7).
Leucopenia developed in 9.7% of patients in the mutant EGFR-TK inhibitors group compared with 51.3% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.18 and 95% CI was noted, which was statistically significant, confirming higher leucopenia incidence in patients receiving platinum-based chemotherapy compared with treatment group for their advanced NSCLC (Figure 8).
Fatigue was reported in 17% of patients in the mutant EGFR-TK inhibitors group compared with 29.5% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.59 and 95% CI was noted, which was statistically significant, confirming higher fatigue rates in patients receiving platinum-based chemotherapy compared with treatment group for their advanced NSCLC (Figure 9).
Neutropenia developed in 6.1% of patients in the mutant EGFR-TK inhibitors group vs 48.2% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.11 and 95% CI was noted, which was statistically significant, confirming higher neutropenia rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 10).
Anorexia developed in 21.3% of patients in the mutant EGFR-TK inhibitors group vs 31.4% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.44 and 95% CI was noted, which was statistically significant, confirming higher anorexia rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 11).
Anemia occurred in 8.7% of patients in the mutant EGFR-TK inhibitors group compared with 32.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.24 and 95% CI was noted, which was statistically significant, confirming higher anorexia rates in patients receiving platinum-based chemotherapy compared with treatment for their advanced NSCLC (Figure 12).
Cough was reported in 17.8% of patients in the mutant EGFR-TK inhibitors group compared with 18.9% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.99 and 95% CI was noted, which was statistically significant, confirming slightly higher cough rates in patients receiving platinum-based chemotherapy compared with treatment for their advanced NSCLC (Figure 13).
Vomiting developed in 11% of patients in the mutant EGFR-TK inhibitors group vs 30.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.35 and 95% CI was noted, which was statistically significant, confirming higher vomiting rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 14).
Fever occurred in 5.6% of patients in the mutant EGFR-TK inhibitors group compared with 30.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.41 and 95% CI was noted, which was statistically significant, confirming higher fever rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 15).
Discussion
Despite the advancement in the treatment of metastatic NSCLC, lung cancer stays as most common cause of cancer-related death in North America and European countries, as patients usually have an advanced disease at the time of diagnosis.3 In the past, platinum-based chemotherapy remained the standard of care for most of the patients affected with advanced NSCLC, but the higher recurrence rate and increase in frequency and intensity of AEs with platinum-based chemotherapy led to the development of targeted therapy for NSCLC, one of which includes
Smoking is the most common reversible risk factor associated with lung cancer. The EURTAC trial was the first perspective study in this regard, which compared safety and efficacy of mutant EGFR-TK inhibitors with platinum-based chemotherapy. Results analyzed in this study were in favor of mutant EGFR-TK inhibitors except in the group of former smokers.5 On the contrary, the OPTIMAL trial showed results in favor of mutant EGFR-TK inhibitors both in active and former smokers; this trial also confirmed the efficacy of mutant EGFR-TK inhibitors in European and Asian populations, confirming the rationale for routine testing of EGFR mutation in all the patients being diagnosed with advanced NSCLC.6 Similarly, osimertinib is one of the most recent mutant EGFR-TK inhibitors developed for the treatment of advanced NSCLC in patients with EGFR-positive receptors.
According to the FLAURA trial, patients receiving osimertinib showed significantly longer progression-free survival compared with platinum-based chemotherapy and early mutant EGFR-TK inhibitors. Median progression-free survival was noted to be 18.9 months, which showed 54% lower risk of disease progression in the treatment group receiving osimertinib.7 The ARCHER study emphasized a significant improvement in overall survival as well as progression-free survival among a patient population receiving dacomitinib compared with platinum-based chemotherapy.8,9
Being a potent targeted therapy, mutant EGFR-TK inhibitors do come with some AEs including diarrhea, which was seen in 33.6% of the patients receiving mutant EGFR-TK inhibitors in our study vs 53% in the chemotherapy group, as was observed in the study conducted by Pless and colleagues.10 Similarly, only 16.5% of patients receiving mutant EGFR-TK inhibitors developed nausea compared with 66% being observed in patients receiving chemotherapy. Correspondingly, only a small fraction of patients (9.7%) receiving mutant EGFR-TK inhibitors developed leucopenia, which was 10 times less reported in mutant EGFR-TK inhibitors compared with patients receiving chemotherapy having a percentage of 100%. A similar trend was reported for neutropenia and anemia in mutant EGFR-TK inhibitors with an incidence of 6.1% and 8.7%, compared with the platinum-based chemotherapy group in which the incidence was found to be 80% and 100%, respectively. It was concluded that platinum-based chemotherapy had played a vital role in the treatment of advanced NSCLC but at an expense of serious and severe AEs which led to discontinuation or withdrawal of treatment, leading to relapse and recurrence of lung cancer.10,11
Zhong and colleagues conducted a phase 2 randomized clinical trial comparing mutant EGFR-TK inhibitors with platinum-based chemotherapy. They concluded that in patients receiving platinum-based chemotherapy, incidence of rash, vomiting, anorexia, neutropenia, and nausea were 29.4%, 47%, 41.2%, 55.8%, and 32.4% compared with 45.8%, 11%, 21.3%, 6.1%, and 16.5%, respectively, reported in patients receiving mutant EGFR-TK inhibitors for their advanced NSCLC.12
Another study was conducted in 2019 by Noronha and colleagues to determine the impact of platinum-based chemotherapy combined with gefitinib on patients with advanced NSCLC.13 They concluded that 70% of the patients receiving combination treatment developed rash, which was significantly higher compared with 45.8% patients receiving the mutant EGFR-TK inhibitors alone in our study. Also, 56% of patients receiving combination therapy developed diarrhea vs 33.6% of patients receiving mutant EGFR-TK inhibitors only. Similarly, 96% of patients in the combination therapy group developed some degree of anemia compared with only 8.7% patients in the mutant EGFR-TK inhibitors group included in our study. In the same way, neutropenia was observed in 55% of patients receiving combination therapy vs 6.1% in patients receiving mutant EGFR-TK inhibitors solely. They concluded that mutant EGFR-TK inhibitors when combined with platinum-based chemotherapy increase the incidence of AEs of chemotherapy by many folds.13,14
Kato and colleagues conducted a study to determine the impact on AEs when erlotinib was combined with anti–vascular endothelial growth factor (VEGF) inhibitors like bevacizumab, they stated that 98.7% of patient in combination therapy developed rash, the incidence of which was only 45.8% in patients receiving mutant EGFR-TK inhibitors as was observed in our study. Similar trends were noticed with other AEs, including diarrhea, fatigue, nausea, and elevated liver enzymes.15
With the latest advancements in the management of advanced NSCLC, nivolumab, a programmed death ligand 1 (PD-L1) inhibitor, was developed and either used as monotherapy in patients with PD-L1 expression or was combined with platinum-based chemotherapy regardless of PD-L1 expression.16,17 Patients expressing lower PD-L1 levels were not omitted from receiving nivolumab as no significant difference was noted in progression-free span and overall survival in patients receiving nivolumab irrespective of PD-L1 levels.15 Rash developed in 17% of patients after receiving nivolumab vs 45.8% patients being observed in our study. A similar trend was observed with diarrhea as only 17% of the population receiving nivolumab developed diarrhea compared with 33.6% of the population receiving mutant EGFR-TK inhibitors in our study. Likewise, only 9.9% of the patients receiving nivolumab developed nausea as an AE compared with 16.5% being observed in mutant EGFR-TK inhibitors in our study. Also, fatigue was observed in 14.4% of the population receiving nivolumab vs 17% observed in patients receiving mutant EGFR-TK inhibitors as was noticed in our study.7,8
Rizvi and colleagues conducted a study on the role of nivolumab when combined with platinum-based chemotherapy in patients with advanced NSCLC and reported that 40% of patients included in the study developed rash compared with 45.8% reported in mutant EGFR-TK inhibitors in our study. Similarly, only 13% of patients in the nivolumab group developed diarrhea vs 33.6% cases reported in the mutant EGFR-TK inhibitors group included in our study. Also, 7% of patients in the nivolumab group developed elevated ALT levels vs 27.9% of patients receiving mutant EGFR-TK inhibitors included in our study, concluding that addition of immune checkpoint inhibitors like nivolumab to platinum-based chemotherapy does not increase the frequency of AEs.18
Conclusions
Our study focused on the safety profile of mutant EGFR-TK inhibitors vs platinum-based chemotherapy in the treatment of advanced NSCLC. Mutant EGFR-TK inhibitors are safer than platinum-based chemotherapy when compared for nausea, leucopenia, fatigue, neutropenia, anorexia, anemia, cough, vomiting, and fever. On the other end, mutant EGFR-TK inhibitors cause slightly higher AEs, including rash, diarrhea, elevated AST and ALT levels, and stomatitis. However, considering that the development of mutant EGFR-TK inhibitors laid a foundation of targeted therapy, we recommend continuing using mutant EGFR-TK inhibitors in patients with advanced NSCLC especially in patients having mutant EGFR receptors. AEs caused by mutant EGFR-TK inhibitors are significant but are usually tolerable and can be avoided by reducing the dosage of it with each cycle or by skipping or delaying the dose until the patient is symptomatic.
Lung cancer has been the leading cause of cancer-related mortality for decades. It is also predicted to remain as the leading cause of cancer-related mortality through 2030.1 Platinum-based chemotherapy, including carboplatin and paclitaxel, was introduced 3 decades ago and revolutionized the management of advanced non–small cell lung cancer (NSCLC). A more recent advancement has been mutant epidermal growth factor receptor–tyrosine kinase (EGFR-TK) inhibitors.1 EGFR is a transmembrane protein that functions by transducing essential growth factor signaling from the extracellular milieu to the cell. As 60% of the advanced NSCLC expresses this receptor, blocking the mutant EGFR receptor was a groundbreaking development in the management of advanced NSCLC.2 Development of mutant EGFR-TK inhibitors has revolutionized the management of advanced NSCLC. This study was conducted to determine the safety profile of mutant EGFR-TK inhibitors in the management of advanced NSCLC.
Methods
This meta-analysis was conducted according to Cochrane Collaboration guidelines and reported as per Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The findings are summarized in the PRISMA flow diagram (Figure 1). Two authors (MZ and MM) performed a systematic literature search using databases such as MEDLINE (via PubMed), Embase, and Cochrane Library using the medical search terms and their respective entry words with the following search strategy: safety, “mutant EGFR-TK inhibitors,” advanced, “non–small cell,” “lung cancer,” “adverse effect,” and literature. Additionally, unpublished trials were identified from clinicaltrials.gov, and references of all pertinent articles were also scrutinized to ensure the inclusion of all relevant studies. The search was completed on June 1, 2021, and we only included studies available in English. Two authors (MM and MZ) independently screened the search results in a 2-step process based on predetermined inclusion/exclusion criteria. First, 890 articles were evaluated for relevance on title and abstract level, followed by full-text screening of the final list of 140 articles. Any disagreements were resolved by discussion or third-party review, and a total of 9 articles were included in the study.
The following eligibility criteria were used: original articles reporting adverse effects (AEs) of mutant EGFR-TK inhibitors in patients with advanced NSCLC compared with control groups receiving platinum-based chemotherapy. All the patients included in the study had an EGFR mutation but randomly assigned to either treatment or control group. All articles with subjective data on mutant EGFR-TK inhibitors AEs in patients with advanced NSCLC compared with control groups receiving platinum-based chemotherapy were included in the analysis. Only 9 articles qualified the aforementioned selection criteria for eligibility. All qualifying studies were nationwide inpatient or pooled clinical trials data. The reasons for exclusion of the other 71 articles were irrelevant (n = 31), duplicate (n = 13), reviews (n = 14), and poor data reporting (n = 12). Out of the 9 included studies, 9 studies showed correlation of AEs, including rash, diarrhea, nausea, and fatigue. Seven studies showed correlation of AEs including neutropenia, anorexia, and vomiting. Six studies showed correlation of anemia, cough, and stomatitis. Five studies showed correlation of elevated aspartate aminotransferase (AST), alanine aminotransferase (ALT), and leucopenia. Four studies showed correlation of fever between mutant EGFR-TK inhibitors and platinum-based chemotherapy.
The primary endpoints were reported AEs including rash, diarrhea, elevated ALT, elevated AST, stomatitis, nausea, leucopenia, fatigue, neutropenia, anorexia, anemia, cough, vomiting, and fever, respectively. Data on baseline characteristics and clinical outcomes were then extracted, and summary tables were created. Summary estimates of the clinical endpoints were then calculated with risk ratio (RR) and 95% confidence intervals (CIs) using the random-effects model. Heterogeneity between studies was examined with the Cochran Q I2 statistic which can be defined as low (25% to 50%), moderate (50% to 75%), or high (> 75%). Statistical analysis was performed using Comprehensive Meta-Analysis Software CMA Version 3.0.
Results
A total of 9 studies including 3415 patients (1775 in EGFR-TK inhibitor treatment group while 1640 patients in platinum-based chemotherapy control group) were included in the study. All 9 studies were phase III randomized control clinical trials conducted to compare the safety profile of mutant EGFR-TK inhibitors in patients with advanced NSCLC. Mean age was 61 years in both treatment and control groups. Further details on study and participant characteristics and safety profile including AEs are summarized in Tables 1 and 2. No evidence of publication bias was found.
Rash developed in 45.8% of patients in the treatment group receiving mutant EGFR-TK inhibitors vs only 5.6% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 7.38 with the 95% CI noted, which was statistically significant, confirming higher rash event rates in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 2).
Diarrhea occurred in 33.6% of patients in the mutant EGFR-TK inhibitors treatment group vs 13.5% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 2.63 and 95% CI was noted, which was statistically significant, confirming higher diarrheal rates in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 3).
Elevated ALT levels developed in 27.9% of patients in the treatment group receiving mutant EGFR-TK inhibitors compared with 15.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 1.37 and 95% CI was noted, which was statistically significant, confirming higher ALT levels in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 4).
Elevated AST levels occurred in 40.7% of patients in the mutant EGFR-TK inhibitors treatment group vs 12.8% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 1.77 and 95% CI was noted, which was statistically significant, confirming elevated AST levels in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 5).
Stomatitis developed in 17.2% of patients in the treatment group receiving mutant EGFR-TK inhibitors compared with 7.9% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 1.53 and 95% CI was noted, which was statistically significant, confirming higher stomatitis event rates in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 6).
Nausea occurred in 16.5% of patients in the mutant EGFR-TK inhibitors group vs 42.5% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.37 and 95% CI was noted, which was statistically significant, confirming higher nausea rates in patients receiving platinum-based chemotherapy compared with treatment group for their advanced NSCLC (Figure 7).
Leucopenia developed in 9.7% of patients in the mutant EGFR-TK inhibitors group compared with 51.3% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.18 and 95% CI was noted, which was statistically significant, confirming higher leucopenia incidence in patients receiving platinum-based chemotherapy compared with treatment group for their advanced NSCLC (Figure 8).
Fatigue was reported in 17% of patients in the mutant EGFR-TK inhibitors group compared with 29.5% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.59 and 95% CI was noted, which was statistically significant, confirming higher fatigue rates in patients receiving platinum-based chemotherapy compared with treatment group for their advanced NSCLC (Figure 9).
Neutropenia developed in 6.1% of patients in the mutant EGFR-TK inhibitors group vs 48.2% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.11 and 95% CI was noted, which was statistically significant, confirming higher neutropenia rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 10).
Anorexia developed in 21.3% of patients in the mutant EGFR-TK inhibitors group vs 31.4% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.44 and 95% CI was noted, which was statistically significant, confirming higher anorexia rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 11).
Anemia occurred in 8.7% of patients in the mutant EGFR-TK inhibitors group compared with 32.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.24 and 95% CI was noted, which was statistically significant, confirming higher anorexia rates in patients receiving platinum-based chemotherapy compared with treatment for their advanced NSCLC (Figure 12).
Cough was reported in 17.8% of patients in the mutant EGFR-TK inhibitors group compared with 18.9% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.99 and 95% CI was noted, which was statistically significant, confirming slightly higher cough rates in patients receiving platinum-based chemotherapy compared with treatment for their advanced NSCLC (Figure 13).
Vomiting developed in 11% of patients in the mutant EGFR-TK inhibitors group vs 30.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.35 and 95% CI was noted, which was statistically significant, confirming higher vomiting rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 14).
Fever occurred in 5.6% of patients in the mutant EGFR-TK inhibitors group compared with 30.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.41 and 95% CI was noted, which was statistically significant, confirming higher fever rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 15).
Discussion
Despite the advancement in the treatment of metastatic NSCLC, lung cancer stays as most common cause of cancer-related death in North America and European countries, as patients usually have an advanced disease at the time of diagnosis.3 In the past, platinum-based chemotherapy remained the standard of care for most of the patients affected with advanced NSCLC, but the higher recurrence rate and increase in frequency and intensity of AEs with platinum-based chemotherapy led to the development of targeted therapy for NSCLC, one of which includes
Smoking is the most common reversible risk factor associated with lung cancer. The EURTAC trial was the first perspective study in this regard, which compared safety and efficacy of mutant EGFR-TK inhibitors with platinum-based chemotherapy. Results analyzed in this study were in favor of mutant EGFR-TK inhibitors except in the group of former smokers.5 On the contrary, the OPTIMAL trial showed results in favor of mutant EGFR-TK inhibitors both in active and former smokers; this trial also confirmed the efficacy of mutant EGFR-TK inhibitors in European and Asian populations, confirming the rationale for routine testing of EGFR mutation in all the patients being diagnosed with advanced NSCLC.6 Similarly, osimertinib is one of the most recent mutant EGFR-TK inhibitors developed for the treatment of advanced NSCLC in patients with EGFR-positive receptors.
According to the FLAURA trial, patients receiving osimertinib showed significantly longer progression-free survival compared with platinum-based chemotherapy and early mutant EGFR-TK inhibitors. Median progression-free survival was noted to be 18.9 months, which showed 54% lower risk of disease progression in the treatment group receiving osimertinib.7 The ARCHER study emphasized a significant improvement in overall survival as well as progression-free survival among a patient population receiving dacomitinib compared with platinum-based chemotherapy.8,9
Being a potent targeted therapy, mutant EGFR-TK inhibitors do come with some AEs including diarrhea, which was seen in 33.6% of the patients receiving mutant EGFR-TK inhibitors in our study vs 53% in the chemotherapy group, as was observed in the study conducted by Pless and colleagues.10 Similarly, only 16.5% of patients receiving mutant EGFR-TK inhibitors developed nausea compared with 66% being observed in patients receiving chemotherapy. Correspondingly, only a small fraction of patients (9.7%) receiving mutant EGFR-TK inhibitors developed leucopenia, which was 10 times less reported in mutant EGFR-TK inhibitors compared with patients receiving chemotherapy having a percentage of 100%. A similar trend was reported for neutropenia and anemia in mutant EGFR-TK inhibitors with an incidence of 6.1% and 8.7%, compared with the platinum-based chemotherapy group in which the incidence was found to be 80% and 100%, respectively. It was concluded that platinum-based chemotherapy had played a vital role in the treatment of advanced NSCLC but at an expense of serious and severe AEs which led to discontinuation or withdrawal of treatment, leading to relapse and recurrence of lung cancer.10,11
Zhong and colleagues conducted a phase 2 randomized clinical trial comparing mutant EGFR-TK inhibitors with platinum-based chemotherapy. They concluded that in patients receiving platinum-based chemotherapy, incidence of rash, vomiting, anorexia, neutropenia, and nausea were 29.4%, 47%, 41.2%, 55.8%, and 32.4% compared with 45.8%, 11%, 21.3%, 6.1%, and 16.5%, respectively, reported in patients receiving mutant EGFR-TK inhibitors for their advanced NSCLC.12
Another study was conducted in 2019 by Noronha and colleagues to determine the impact of platinum-based chemotherapy combined with gefitinib on patients with advanced NSCLC.13 They concluded that 70% of the patients receiving combination treatment developed rash, which was significantly higher compared with 45.8% patients receiving the mutant EGFR-TK inhibitors alone in our study. Also, 56% of patients receiving combination therapy developed diarrhea vs 33.6% of patients receiving mutant EGFR-TK inhibitors only. Similarly, 96% of patients in the combination therapy group developed some degree of anemia compared with only 8.7% patients in the mutant EGFR-TK inhibitors group included in our study. In the same way, neutropenia was observed in 55% of patients receiving combination therapy vs 6.1% in patients receiving mutant EGFR-TK inhibitors solely. They concluded that mutant EGFR-TK inhibitors when combined with platinum-based chemotherapy increase the incidence of AEs of chemotherapy by many folds.13,14
Kato and colleagues conducted a study to determine the impact on AEs when erlotinib was combined with anti–vascular endothelial growth factor (VEGF) inhibitors like bevacizumab, they stated that 98.7% of patient in combination therapy developed rash, the incidence of which was only 45.8% in patients receiving mutant EGFR-TK inhibitors as was observed in our study. Similar trends were noticed with other AEs, including diarrhea, fatigue, nausea, and elevated liver enzymes.15
With the latest advancements in the management of advanced NSCLC, nivolumab, a programmed death ligand 1 (PD-L1) inhibitor, was developed and either used as monotherapy in patients with PD-L1 expression or was combined with platinum-based chemotherapy regardless of PD-L1 expression.16,17 Patients expressing lower PD-L1 levels were not omitted from receiving nivolumab as no significant difference was noted in progression-free span and overall survival in patients receiving nivolumab irrespective of PD-L1 levels.15 Rash developed in 17% of patients after receiving nivolumab vs 45.8% patients being observed in our study. A similar trend was observed with diarrhea as only 17% of the population receiving nivolumab developed diarrhea compared with 33.6% of the population receiving mutant EGFR-TK inhibitors in our study. Likewise, only 9.9% of the patients receiving nivolumab developed nausea as an AE compared with 16.5% being observed in mutant EGFR-TK inhibitors in our study. Also, fatigue was observed in 14.4% of the population receiving nivolumab vs 17% observed in patients receiving mutant EGFR-TK inhibitors as was noticed in our study.7,8
Rizvi and colleagues conducted a study on the role of nivolumab when combined with platinum-based chemotherapy in patients with advanced NSCLC and reported that 40% of patients included in the study developed rash compared with 45.8% reported in mutant EGFR-TK inhibitors in our study. Similarly, only 13% of patients in the nivolumab group developed diarrhea vs 33.6% cases reported in the mutant EGFR-TK inhibitors group included in our study. Also, 7% of patients in the nivolumab group developed elevated ALT levels vs 27.9% of patients receiving mutant EGFR-TK inhibitors included in our study, concluding that addition of immune checkpoint inhibitors like nivolumab to platinum-based chemotherapy does not increase the frequency of AEs.18
Conclusions
Our study focused on the safety profile of mutant EGFR-TK inhibitors vs platinum-based chemotherapy in the treatment of advanced NSCLC. Mutant EGFR-TK inhibitors are safer than platinum-based chemotherapy when compared for nausea, leucopenia, fatigue, neutropenia, anorexia, anemia, cough, vomiting, and fever. On the other end, mutant EGFR-TK inhibitors cause slightly higher AEs, including rash, diarrhea, elevated AST and ALT levels, and stomatitis. However, considering that the development of mutant EGFR-TK inhibitors laid a foundation of targeted therapy, we recommend continuing using mutant EGFR-TK inhibitors in patients with advanced NSCLC especially in patients having mutant EGFR receptors. AEs caused by mutant EGFR-TK inhibitors are significant but are usually tolerable and can be avoided by reducing the dosage of it with each cycle or by skipping or delaying the dose until the patient is symptomatic.
1. Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74(11):2913-2921. doi:10.1158/0008-5472.CAN-14-0155
2. da Cunha Santos G, Shepherd FA, Tsao MS. EGFR mutations and lung cancer. Annu Rev Pathol. 2011;6:49-69. doi:10.1146/annurev-pathol-011110-130206
3. Sgambato A, Casaluce F, Maione P, et al. The role of EGFR tyrosine kinase inhibitors in the first-line treatment of advanced non small cell lung cancer patients harboring EGFR mutation. Curr Med Chem. 2012;19(20):3337-3352. doi:10.2174/092986712801215973
4. Rossi A, Di Maio M. Platinum-based chemotherapy in advanced non–small-cell lung cancer: optimal number of treatment cycles. Expert Rev Anticancer Ther. 2016;16(6):653-660. doi:10.1586/14737140.2016.1170596
5. Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non–small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239-246. doi:10.1016/S1470-2045(11)70393-X
6. Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non–small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735-742. doi:10.1016/S1470-2045(11)70184-X
7. Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non–small-cell lung cancer. N Engl J Med. 2018;378(2):113-125. doi:10.1056/NEJMoa1713137
8. Mok TS, Cheng Y, Zhou X, et al. Improvement in overall survival in a randomized study that compared dacomitinib with gefitinib in patients with advanced non–small-cell lung cancer and EGFR-activating mutations. J Clin Oncol. 2018;36(22):2244-2250. doi:10.1200/JCO.2018.78.7994
9. Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947-957. doi:10.1056/NEJMoa0810699
10. Pless M, Stupp R, Ris HB, et al. Induction chemoradiation in stage IIIA/N2 non–small-cell lung cancer: a phase 3 randomised trial. Lancet. 2015;386(9998):1049-1056. doi:10.1016/S0140-6736(15)60294-X
11. Albain KS, Rusch VW, Crowley JJ, et al. Concurrent cisplatin/etoposide plus chest radiotherapy followed by surgery for stages IIIA (N2) and IIIB non–small-cell lung cancer: mature results of Southwest Oncology Group phase II study 8805. J Clin Oncol. 1995;13(8):1880-1892. doi:10.1200/JCO.1995.13.8.1880
12. Zhong WZ, Chen KN, Chen C, et al. Erlotinib versus gemcitabine plus cisplatin as neoadjuvant treatment of Stage IIIA-N2 EGFR-mutant non–small-cell lung cancer (EMERGING-CTONG 1103): a randomized phase II study. J Clin Oncol. 2019;37(25):2235-2245. doi:10.1200/JCO.19.00075
13. Noronha V, Patil VM, Joshi A, et al. Gefitinib versus gefitinib plus pemetrexed and carboplatin chemotherapy in EGFR-mutated lung cancer. J Clin Oncol. 2020;38(2):124-136. doi:10.1200/JCO.19.01154
14. Noronha V, Prabhash K, Thavamani A, et al. EGFR mutations in Indian lung cancer patients: clinical correlation and outcome to EGFR targeted therapy. PLoS One. 2013;8(4):e61561. Published 2013 Apr 19. doi:10.1371/journal.pone.0061561
15. Kato T, Seto T, Nishio M, et al. Erlotinib plus bevacizumab phase ll study in patients with advanced non–small-cell lung cancer (JO25567): updated safety results. Drug Saf. 2018;41(2):229-237. doi:10.1007/s40264-017-0596-0
16. Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus ipilimumab in advanced non–small-cell lung cancer. N Engl J Med. 2019;381(21):2020-2031. doi:10.1056/NEJMoa1910231
17. Hellmann MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378(22):2093-2104. doi:10.1056/NEJMoa1801946
18. Rizvi NA, Hellmann MD, Brahmer JR, et al. Nivolumab in combination with platinum-based doublet chemotherapy for first-line treatment of advanced non–small-cell lung cancer. J Clin Oncol. 2016;34(25):2969-2979. doi:10.1200/JCO.2016.66.9861
19. Zhong WZ, Wang Q, Mao WM, et al. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II-IIIA (N1-N2) EGFR-mutant NSCLC: final overall survival analysis of CTONG1104 Phase III Trial. J Clin Oncol. 2021;39(7):713-722. doi:10.1200/JCO.20.01820
20. Yang JC, Sequist LV, Geater SL, et al. Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: a combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol. 2015;16(7):830-838. doi:10.1016/S1470-2045(15)00026-1
21. Shi YK, Wang L, Han BH, et al. First-line icotinib versus cisplatin/pemetrexed plus pemetrexed maintenance therapy for patients with advanced EGFR mutation-positive lung adenocarcinoma (CONVINCE): a phase 3, open-label, randomized study. Ann Oncol. 2017;28(10):2443-2450. doi:10.1093/annonc/mdx359
22. Soria JC, Wu YL, Nakagawa K, et al. Gefitinib plus chemotherapy versus placebo plus chemotherapy in EGFR-mutation-positive non-small-cell lung cancer after progression on first-line gefitinib (IMPRESS): a phase 3 randomized trial. Lancet Oncol. 2015;16(8):990-998 doi:10.1016/S1470-2045(15)00121-7
23. Goss GD, O’Callaghan C, Lorimer I, et al. Gefitinib versus placebo in completely resected non-small-cell lung cancer: results of the NCIC CTG BR19 study. J Clin Oncol. 2013;31(27):3320-3326. doi:10.1200/JCO.2013.51.1816
24. Sun JM, Lee KH, Kim SW, et al. Gefitinib versus pemetrexed as second-line treatment in patients with non-small cell lung cancer previously treated with platinum-based chemotherapy (KCSG-LU08-01): an open-label, phase 3 trial. Cancer. 2012;118(24):6234-6242. doi:10.1200/JCO.2013.51.1816
25. Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomized phase 3 trial. Lancet Oncol. 2010;11(2):121-128. doi:10.1016/S1470-2045(09)70364-X
26. Lee DH, Park K, Kim JH, Lee JS, et al. Randomized phase III trial of gefitinib versus docetaxel in non-small cell lung cancer patients who have previously received platinum-based chemotherapy. Clin Cancer Res. 2010;16(4):1307-1314. doi:10.1158/1078-0432.CCR-09-1903
27. Kim ES, Hirsh V, Mok T, et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomized phase III trial. Lancet. 2008;22;372(9652):1809-1818. doi:10.1016/S0140-6736(08)61758-4
1. Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74(11):2913-2921. doi:10.1158/0008-5472.CAN-14-0155
2. da Cunha Santos G, Shepherd FA, Tsao MS. EGFR mutations and lung cancer. Annu Rev Pathol. 2011;6:49-69. doi:10.1146/annurev-pathol-011110-130206
3. Sgambato A, Casaluce F, Maione P, et al. The role of EGFR tyrosine kinase inhibitors in the first-line treatment of advanced non small cell lung cancer patients harboring EGFR mutation. Curr Med Chem. 2012;19(20):3337-3352. doi:10.2174/092986712801215973
4. Rossi A, Di Maio M. Platinum-based chemotherapy in advanced non–small-cell lung cancer: optimal number of treatment cycles. Expert Rev Anticancer Ther. 2016;16(6):653-660. doi:10.1586/14737140.2016.1170596
5. Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non–small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239-246. doi:10.1016/S1470-2045(11)70393-X
6. Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non–small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735-742. doi:10.1016/S1470-2045(11)70184-X
7. Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non–small-cell lung cancer. N Engl J Med. 2018;378(2):113-125. doi:10.1056/NEJMoa1713137
8. Mok TS, Cheng Y, Zhou X, et al. Improvement in overall survival in a randomized study that compared dacomitinib with gefitinib in patients with advanced non–small-cell lung cancer and EGFR-activating mutations. J Clin Oncol. 2018;36(22):2244-2250. doi:10.1200/JCO.2018.78.7994
9. Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947-957. doi:10.1056/NEJMoa0810699
10. Pless M, Stupp R, Ris HB, et al. Induction chemoradiation in stage IIIA/N2 non–small-cell lung cancer: a phase 3 randomised trial. Lancet. 2015;386(9998):1049-1056. doi:10.1016/S0140-6736(15)60294-X
11. Albain KS, Rusch VW, Crowley JJ, et al. Concurrent cisplatin/etoposide plus chest radiotherapy followed by surgery for stages IIIA (N2) and IIIB non–small-cell lung cancer: mature results of Southwest Oncology Group phase II study 8805. J Clin Oncol. 1995;13(8):1880-1892. doi:10.1200/JCO.1995.13.8.1880
12. Zhong WZ, Chen KN, Chen C, et al. Erlotinib versus gemcitabine plus cisplatin as neoadjuvant treatment of Stage IIIA-N2 EGFR-mutant non–small-cell lung cancer (EMERGING-CTONG 1103): a randomized phase II study. J Clin Oncol. 2019;37(25):2235-2245. doi:10.1200/JCO.19.00075
13. Noronha V, Patil VM, Joshi A, et al. Gefitinib versus gefitinib plus pemetrexed and carboplatin chemotherapy in EGFR-mutated lung cancer. J Clin Oncol. 2020;38(2):124-136. doi:10.1200/JCO.19.01154
14. Noronha V, Prabhash K, Thavamani A, et al. EGFR mutations in Indian lung cancer patients: clinical correlation and outcome to EGFR targeted therapy. PLoS One. 2013;8(4):e61561. Published 2013 Apr 19. doi:10.1371/journal.pone.0061561
15. Kato T, Seto T, Nishio M, et al. Erlotinib plus bevacizumab phase ll study in patients with advanced non–small-cell lung cancer (JO25567): updated safety results. Drug Saf. 2018;41(2):229-237. doi:10.1007/s40264-017-0596-0
16. Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus ipilimumab in advanced non–small-cell lung cancer. N Engl J Med. 2019;381(21):2020-2031. doi:10.1056/NEJMoa1910231
17. Hellmann MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378(22):2093-2104. doi:10.1056/NEJMoa1801946
18. Rizvi NA, Hellmann MD, Brahmer JR, et al. Nivolumab in combination with platinum-based doublet chemotherapy for first-line treatment of advanced non–small-cell lung cancer. J Clin Oncol. 2016;34(25):2969-2979. doi:10.1200/JCO.2016.66.9861
19. Zhong WZ, Wang Q, Mao WM, et al. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II-IIIA (N1-N2) EGFR-mutant NSCLC: final overall survival analysis of CTONG1104 Phase III Trial. J Clin Oncol. 2021;39(7):713-722. doi:10.1200/JCO.20.01820
20. Yang JC, Sequist LV, Geater SL, et al. Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: a combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol. 2015;16(7):830-838. doi:10.1016/S1470-2045(15)00026-1
21. Shi YK, Wang L, Han BH, et al. First-line icotinib versus cisplatin/pemetrexed plus pemetrexed maintenance therapy for patients with advanced EGFR mutation-positive lung adenocarcinoma (CONVINCE): a phase 3, open-label, randomized study. Ann Oncol. 2017;28(10):2443-2450. doi:10.1093/annonc/mdx359
22. Soria JC, Wu YL, Nakagawa K, et al. Gefitinib plus chemotherapy versus placebo plus chemotherapy in EGFR-mutation-positive non-small-cell lung cancer after progression on first-line gefitinib (IMPRESS): a phase 3 randomized trial. Lancet Oncol. 2015;16(8):990-998 doi:10.1016/S1470-2045(15)00121-7
23. Goss GD, O’Callaghan C, Lorimer I, et al. Gefitinib versus placebo in completely resected non-small-cell lung cancer: results of the NCIC CTG BR19 study. J Clin Oncol. 2013;31(27):3320-3326. doi:10.1200/JCO.2013.51.1816
24. Sun JM, Lee KH, Kim SW, et al. Gefitinib versus pemetrexed as second-line treatment in patients with non-small cell lung cancer previously treated with platinum-based chemotherapy (KCSG-LU08-01): an open-label, phase 3 trial. Cancer. 2012;118(24):6234-6242. doi:10.1200/JCO.2013.51.1816
25. Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomized phase 3 trial. Lancet Oncol. 2010;11(2):121-128. doi:10.1016/S1470-2045(09)70364-X
26. Lee DH, Park K, Kim JH, Lee JS, et al. Randomized phase III trial of gefitinib versus docetaxel in non-small cell lung cancer patients who have previously received platinum-based chemotherapy. Clin Cancer Res. 2010;16(4):1307-1314. doi:10.1158/1078-0432.CCR-09-1903
27. Kim ES, Hirsh V, Mok T, et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomized phase III trial. Lancet. 2008;22;372(9652):1809-1818. doi:10.1016/S0140-6736(08)61758-4
Getting cancer research on track again may require a ‘behemoth’ effort
In 2016, as vice president, Joe Biden launched the Cancer Moonshot program just 1 year after his son Beau died from glioblastoma multiforme. His objective, he said, was to “cure” cancer, but to get close to that goal, to get cancer research just back up to pre-COVID-19 pandemic levels.
There has been a significant decrease in the launch of new clinical trials for cancer and biologic therapies since 2020. “That can affect every aspect of our research operation. It really affected our capacity to continue to move forward at a fast pace. It will require a behemoth effort to get back to pre-COVID times,” said Tanios S. Bekaii-Saab, MD, leader of the gastrointestinal cancer program at Mayo Clinic in Phoenix.
Congress passed the 21st Century Cures Act in 2016 authorizing $1.8 billion for Cancer Moonshot over 7 years. More recently, the program received $194 million from the $6.9 billion National Cancer Institute budget in FY 2022.
Joseph Alvarnas, MD, a hematologist oncologist and vice president of government affairs at City of Hope, Duarte, Calif., sees the Moonshot budget as a potential shortcoming.
“The priorities are well founded and based on what we would think are the most important things to cover, but, if we’re going to achieve these extraordinarily ambitious goals of halving cancer mortality and serving communities more equitably, it’s going to need more funding positioned at making these things real,” he said.
Moonshot is being positioned as an opportunity to double down on efforts started in 2016, but treating cancer is complex and goes well beyond funding new research.
“We know that we have amazing research and progress around innovations that will drive us toward the goal of reducing the death rate from cancer. But we also know that we have tools that aren’t reaching all parts of the country, so we have a great opportunity to make sure that we’re doing all we can to prevent, detect and treat cancer,” Dr. Carnival said.
Can cancer be cured?
The Biden administration relaunched Moonshot in 2022 with newly defined goals: Cut the rate of cancer-related deaths in half within 25 years; improve the experience of people with cancer, cancer survivors, and their families; and “end cancer as we know it,” President Biden said in a press conference in February.
Cancer is the second leading cause of death in the United States after heart disease, but it may indeed be possible to cut the total number of cancer-related deaths in half over the next 25 years.
“As a hematologist who’s been involved in both research and clinical care, I think it’s important to realize this is actually doable. Between 1990 and 2020 cancer mortality rates decreased by 31%, and in the last American Cancer Society’s annual report, mortality rates dropped by the largest percentages for 2 consecutive years in a row. The question shifts now from ‘Is this possible? to ‘How do we ensure that it’s possible?’ The spirit of Cancer Moonshot 2.0 is identifying the multiple paths to move this effort forward,” Dr. Alvarnas said.
But without a significant infusion of cash for research, it’s doubtful cancer-related deaths will drop by 50% over the next 25 years.
“There are a lot of big and lofty goals in Cancer Moonshot, and the words ‘ending cancer,’ well those are big words,” Dr. Bekaii-Saab said. “The reality is how do we measure in 25 years the impact of this today? I think it will require significantly more funding over the next few years to achieve the goals set by the Moonshot. Otherwise it will be a 7-year done deal that will accrue a lot of great numbers but won’t make a dent in those goals for the next 25 years. To stop it at some point and not invest more into it, we will probably lose most of the benefit.”
Closing the loop on data sharing
Moonshot has been instrumental in fostering research collaborations by encouraging data sharing among scientists.
“It also brought together a new way for the National Cancer Institute and Department of Energy to drive progress on some of the big data initiatives. The initial Cancer Moonshot infused a sense of urgency and hope into this effort,” said Danielle Carnival, PhD, coordinator of Cancer Moonshot.
Between 2017 and 2022, Cancer Moonshot created more than 70 consortiums or programs, and funded about 240 research projects. Its fundamental goals of improving data sharing and encouraging collaboration are very important, Dr. Bekaii-Saab said.
“Because, historically, what happens with cancer is that researchers compete for resources...and they become very protective of their data. Sharing gets more difficult, collaborations become more onerous, and it becomes counterproductive,” he said.
Dr. Bekaii-Saab highlighted two networks created specifically for data sharing. They include the Human Tumor Atlas for cellular, morphological, and molecular tumor data, and PDXNet, a patient derived xenograft research network.
A shift in funding priorities?
Cancer funding has been stagnant for years. When adjusted for growth, it hasn’t had a significant infusion of funding since at least 2003—at least in relative terms, Dr. Bekaii-Saab said. “This affects a lot of the things we do, including NCI-funded clinical trials. It pushes us to work with the private sector, which is not necessarily a detriment, but it doesn’t advance the academic mission at the same level. So, overall, I wouldn’t call it tragic, but I do think we’re falling behind,” he said.
“I think when we do the process for the budget for FY24 and after we’ve had time to really explore the best ideas and build the foundation for some of these new aspects of the Cancer Moonshot, we hope to have something more concrete going toward these efforts,” Dr. Carnival said.
But in addition to funding, Dr. Alvarnas says, it is equally important to address gaps in care. Not all patients have access to existing cancer treatments.
“The great challenge to us in the 2020s is not only about developing new and more effective technologies, but also in doing a better job of getting existing life-saving treatments into the hands of underserved populations. One of the really positive challenges set forth by the Biden administration is the idea that financing care equity is as important, if not more so, than advancing technologies. If there’s been stagnation, it’s because from a government and resourcing point of view, that priority has been ineffectively supported financially.”
The pandemic stymies cancer research
The pandemic has had a significant impact on cancer research. As in other fields, it disrupted ongoing research, but it may have also contributed to the loss of employees who resigned in what’s been called the “Great Resignation.” “A lot of employees just decided to change jobs in the middle of the pandemic, which led to a cancer research staffing crisis,” Dr. Bekaii-Saab said.
“We all recognized that turning so much of the attention of the entire biomedical research engine and health system to the COVID-19 pandemic would have an impact across cancer research, screenings and care,” Dr. Carnival said. “There is work to do to get us back to whole, but from a research perspective, we’ve seen a reorientation of the trial networks we were using for COVID-19 research, back to their initial purpose. Some of those are cancer and oncology networks, so we’re excited about that and fully believe that we can catch up.”
But then there’s also the impact the pandemic has had on cancer patients who delayed their care at the primary level. This, Dr. Bekaii-Saab fears, will lead to more patients presenting with more advanced disease in years to come. “One of the biggest problems was that a lot of patients delayed their care at the primary level. My biggest concern is that in the years to come we will see a lot more patients presenting with more advanced cancer.”
In 2016, as vice president, Joe Biden launched the Cancer Moonshot program just 1 year after his son Beau died from glioblastoma multiforme. His objective, he said, was to “cure” cancer, but to get close to that goal, to get cancer research just back up to pre-COVID-19 pandemic levels.
There has been a significant decrease in the launch of new clinical trials for cancer and biologic therapies since 2020. “That can affect every aspect of our research operation. It really affected our capacity to continue to move forward at a fast pace. It will require a behemoth effort to get back to pre-COVID times,” said Tanios S. Bekaii-Saab, MD, leader of the gastrointestinal cancer program at Mayo Clinic in Phoenix.
Congress passed the 21st Century Cures Act in 2016 authorizing $1.8 billion for Cancer Moonshot over 7 years. More recently, the program received $194 million from the $6.9 billion National Cancer Institute budget in FY 2022.
Joseph Alvarnas, MD, a hematologist oncologist and vice president of government affairs at City of Hope, Duarte, Calif., sees the Moonshot budget as a potential shortcoming.
“The priorities are well founded and based on what we would think are the most important things to cover, but, if we’re going to achieve these extraordinarily ambitious goals of halving cancer mortality and serving communities more equitably, it’s going to need more funding positioned at making these things real,” he said.
Moonshot is being positioned as an opportunity to double down on efforts started in 2016, but treating cancer is complex and goes well beyond funding new research.
“We know that we have amazing research and progress around innovations that will drive us toward the goal of reducing the death rate from cancer. But we also know that we have tools that aren’t reaching all parts of the country, so we have a great opportunity to make sure that we’re doing all we can to prevent, detect and treat cancer,” Dr. Carnival said.
Can cancer be cured?
The Biden administration relaunched Moonshot in 2022 with newly defined goals: Cut the rate of cancer-related deaths in half within 25 years; improve the experience of people with cancer, cancer survivors, and their families; and “end cancer as we know it,” President Biden said in a press conference in February.
Cancer is the second leading cause of death in the United States after heart disease, but it may indeed be possible to cut the total number of cancer-related deaths in half over the next 25 years.
“As a hematologist who’s been involved in both research and clinical care, I think it’s important to realize this is actually doable. Between 1990 and 2020 cancer mortality rates decreased by 31%, and in the last American Cancer Society’s annual report, mortality rates dropped by the largest percentages for 2 consecutive years in a row. The question shifts now from ‘Is this possible? to ‘How do we ensure that it’s possible?’ The spirit of Cancer Moonshot 2.0 is identifying the multiple paths to move this effort forward,” Dr. Alvarnas said.
But without a significant infusion of cash for research, it’s doubtful cancer-related deaths will drop by 50% over the next 25 years.
“There are a lot of big and lofty goals in Cancer Moonshot, and the words ‘ending cancer,’ well those are big words,” Dr. Bekaii-Saab said. “The reality is how do we measure in 25 years the impact of this today? I think it will require significantly more funding over the next few years to achieve the goals set by the Moonshot. Otherwise it will be a 7-year done deal that will accrue a lot of great numbers but won’t make a dent in those goals for the next 25 years. To stop it at some point and not invest more into it, we will probably lose most of the benefit.”
Closing the loop on data sharing
Moonshot has been instrumental in fostering research collaborations by encouraging data sharing among scientists.
“It also brought together a new way for the National Cancer Institute and Department of Energy to drive progress on some of the big data initiatives. The initial Cancer Moonshot infused a sense of urgency and hope into this effort,” said Danielle Carnival, PhD, coordinator of Cancer Moonshot.
Between 2017 and 2022, Cancer Moonshot created more than 70 consortiums or programs, and funded about 240 research projects. Its fundamental goals of improving data sharing and encouraging collaboration are very important, Dr. Bekaii-Saab said.
“Because, historically, what happens with cancer is that researchers compete for resources...and they become very protective of their data. Sharing gets more difficult, collaborations become more onerous, and it becomes counterproductive,” he said.
Dr. Bekaii-Saab highlighted two networks created specifically for data sharing. They include the Human Tumor Atlas for cellular, morphological, and molecular tumor data, and PDXNet, a patient derived xenograft research network.
A shift in funding priorities?
Cancer funding has been stagnant for years. When adjusted for growth, it hasn’t had a significant infusion of funding since at least 2003—at least in relative terms, Dr. Bekaii-Saab said. “This affects a lot of the things we do, including NCI-funded clinical trials. It pushes us to work with the private sector, which is not necessarily a detriment, but it doesn’t advance the academic mission at the same level. So, overall, I wouldn’t call it tragic, but I do think we’re falling behind,” he said.
“I think when we do the process for the budget for FY24 and after we’ve had time to really explore the best ideas and build the foundation for some of these new aspects of the Cancer Moonshot, we hope to have something more concrete going toward these efforts,” Dr. Carnival said.
But in addition to funding, Dr. Alvarnas says, it is equally important to address gaps in care. Not all patients have access to existing cancer treatments.
“The great challenge to us in the 2020s is not only about developing new and more effective technologies, but also in doing a better job of getting existing life-saving treatments into the hands of underserved populations. One of the really positive challenges set forth by the Biden administration is the idea that financing care equity is as important, if not more so, than advancing technologies. If there’s been stagnation, it’s because from a government and resourcing point of view, that priority has been ineffectively supported financially.”
The pandemic stymies cancer research
The pandemic has had a significant impact on cancer research. As in other fields, it disrupted ongoing research, but it may have also contributed to the loss of employees who resigned in what’s been called the “Great Resignation.” “A lot of employees just decided to change jobs in the middle of the pandemic, which led to a cancer research staffing crisis,” Dr. Bekaii-Saab said.
“We all recognized that turning so much of the attention of the entire biomedical research engine and health system to the COVID-19 pandemic would have an impact across cancer research, screenings and care,” Dr. Carnival said. “There is work to do to get us back to whole, but from a research perspective, we’ve seen a reorientation of the trial networks we were using for COVID-19 research, back to their initial purpose. Some of those are cancer and oncology networks, so we’re excited about that and fully believe that we can catch up.”
But then there’s also the impact the pandemic has had on cancer patients who delayed their care at the primary level. This, Dr. Bekaii-Saab fears, will lead to more patients presenting with more advanced disease in years to come. “One of the biggest problems was that a lot of patients delayed their care at the primary level. My biggest concern is that in the years to come we will see a lot more patients presenting with more advanced cancer.”
In 2016, as vice president, Joe Biden launched the Cancer Moonshot program just 1 year after his son Beau died from glioblastoma multiforme. His objective, he said, was to “cure” cancer, but to get close to that goal, to get cancer research just back up to pre-COVID-19 pandemic levels.
There has been a significant decrease in the launch of new clinical trials for cancer and biologic therapies since 2020. “That can affect every aspect of our research operation. It really affected our capacity to continue to move forward at a fast pace. It will require a behemoth effort to get back to pre-COVID times,” said Tanios S. Bekaii-Saab, MD, leader of the gastrointestinal cancer program at Mayo Clinic in Phoenix.
Congress passed the 21st Century Cures Act in 2016 authorizing $1.8 billion for Cancer Moonshot over 7 years. More recently, the program received $194 million from the $6.9 billion National Cancer Institute budget in FY 2022.
Joseph Alvarnas, MD, a hematologist oncologist and vice president of government affairs at City of Hope, Duarte, Calif., sees the Moonshot budget as a potential shortcoming.
“The priorities are well founded and based on what we would think are the most important things to cover, but, if we’re going to achieve these extraordinarily ambitious goals of halving cancer mortality and serving communities more equitably, it’s going to need more funding positioned at making these things real,” he said.
Moonshot is being positioned as an opportunity to double down on efforts started in 2016, but treating cancer is complex and goes well beyond funding new research.
“We know that we have amazing research and progress around innovations that will drive us toward the goal of reducing the death rate from cancer. But we also know that we have tools that aren’t reaching all parts of the country, so we have a great opportunity to make sure that we’re doing all we can to prevent, detect and treat cancer,” Dr. Carnival said.
Can cancer be cured?
The Biden administration relaunched Moonshot in 2022 with newly defined goals: Cut the rate of cancer-related deaths in half within 25 years; improve the experience of people with cancer, cancer survivors, and their families; and “end cancer as we know it,” President Biden said in a press conference in February.
Cancer is the second leading cause of death in the United States after heart disease, but it may indeed be possible to cut the total number of cancer-related deaths in half over the next 25 years.
“As a hematologist who’s been involved in both research and clinical care, I think it’s important to realize this is actually doable. Between 1990 and 2020 cancer mortality rates decreased by 31%, and in the last American Cancer Society’s annual report, mortality rates dropped by the largest percentages for 2 consecutive years in a row. The question shifts now from ‘Is this possible? to ‘How do we ensure that it’s possible?’ The spirit of Cancer Moonshot 2.0 is identifying the multiple paths to move this effort forward,” Dr. Alvarnas said.
But without a significant infusion of cash for research, it’s doubtful cancer-related deaths will drop by 50% over the next 25 years.
“There are a lot of big and lofty goals in Cancer Moonshot, and the words ‘ending cancer,’ well those are big words,” Dr. Bekaii-Saab said. “The reality is how do we measure in 25 years the impact of this today? I think it will require significantly more funding over the next few years to achieve the goals set by the Moonshot. Otherwise it will be a 7-year done deal that will accrue a lot of great numbers but won’t make a dent in those goals for the next 25 years. To stop it at some point and not invest more into it, we will probably lose most of the benefit.”
Closing the loop on data sharing
Moonshot has been instrumental in fostering research collaborations by encouraging data sharing among scientists.
“It also brought together a new way for the National Cancer Institute and Department of Energy to drive progress on some of the big data initiatives. The initial Cancer Moonshot infused a sense of urgency and hope into this effort,” said Danielle Carnival, PhD, coordinator of Cancer Moonshot.
Between 2017 and 2022, Cancer Moonshot created more than 70 consortiums or programs, and funded about 240 research projects. Its fundamental goals of improving data sharing and encouraging collaboration are very important, Dr. Bekaii-Saab said.
“Because, historically, what happens with cancer is that researchers compete for resources...and they become very protective of their data. Sharing gets more difficult, collaborations become more onerous, and it becomes counterproductive,” he said.
Dr. Bekaii-Saab highlighted two networks created specifically for data sharing. They include the Human Tumor Atlas for cellular, morphological, and molecular tumor data, and PDXNet, a patient derived xenograft research network.
A shift in funding priorities?
Cancer funding has been stagnant for years. When adjusted for growth, it hasn’t had a significant infusion of funding since at least 2003—at least in relative terms, Dr. Bekaii-Saab said. “This affects a lot of the things we do, including NCI-funded clinical trials. It pushes us to work with the private sector, which is not necessarily a detriment, but it doesn’t advance the academic mission at the same level. So, overall, I wouldn’t call it tragic, but I do think we’re falling behind,” he said.
“I think when we do the process for the budget for FY24 and after we’ve had time to really explore the best ideas and build the foundation for some of these new aspects of the Cancer Moonshot, we hope to have something more concrete going toward these efforts,” Dr. Carnival said.
But in addition to funding, Dr. Alvarnas says, it is equally important to address gaps in care. Not all patients have access to existing cancer treatments.
“The great challenge to us in the 2020s is not only about developing new and more effective technologies, but also in doing a better job of getting existing life-saving treatments into the hands of underserved populations. One of the really positive challenges set forth by the Biden administration is the idea that financing care equity is as important, if not more so, than advancing technologies. If there’s been stagnation, it’s because from a government and resourcing point of view, that priority has been ineffectively supported financially.”
The pandemic stymies cancer research
The pandemic has had a significant impact on cancer research. As in other fields, it disrupted ongoing research, but it may have also contributed to the loss of employees who resigned in what’s been called the “Great Resignation.” “A lot of employees just decided to change jobs in the middle of the pandemic, which led to a cancer research staffing crisis,” Dr. Bekaii-Saab said.
“We all recognized that turning so much of the attention of the entire biomedical research engine and health system to the COVID-19 pandemic would have an impact across cancer research, screenings and care,” Dr. Carnival said. “There is work to do to get us back to whole, but from a research perspective, we’ve seen a reorientation of the trial networks we were using for COVID-19 research, back to their initial purpose. Some of those are cancer and oncology networks, so we’re excited about that and fully believe that we can catch up.”
But then there’s also the impact the pandemic has had on cancer patients who delayed their care at the primary level. This, Dr. Bekaii-Saab fears, will lead to more patients presenting with more advanced disease in years to come. “One of the biggest problems was that a lot of patients delayed their care at the primary level. My biggest concern is that in the years to come we will see a lot more patients presenting with more advanced cancer.”
Genomic Assays in HR-Positive/HER2-Negative Breast Cancer
As both a clinician and investigator in the breast cancer space, how would you describe our current understanding of genomic assays in hormone receptor (HR)-positive/human epidermal growth factor receptor 2 (HER2)-negative disease?
Dr. Kalinsky: There are a number of commercially-available assays for patients with early-stage HR-positive/HER2-negative breast cancer. We have seen the results of 3 large randomized phase 3 trials that have demonstrated and helped establish the clinical utility of assays, including the oncotype DX 21-gene recurrence score. This was evaluated in patients with node-negative breast cancer in the TAILORx trial and in patients with 1 to 3 nodes involved in the RxPONDER trial.
We also have data with the 70-gene MammaPrint assay from the MINDACT study, looking for patients who had a discordance between clinical and genomic risk. MINDACT has been published and was updated recently with data for 8-year distant metastasis-free survival. It is these studies that have helped establish what we do in the clinic and when we consider offering genomic assays in patients with this subtype of breast cancer.
How are you working to bring these genomic assays into practice?
Dr. Kalinsky: In 2020, we reported the initial results from the RxPonder study demonstrating that for patients with HR-positive HER2-negative breast cancer with 1 to 3 nodes involved, two-thirds of the patients were postmenopausal. For patients who had a recurrence score of 25 or less, we did not identify a subgroup of patients who benefited from chemotherapy.
For the premenopausal women, which was one-third of the patients, we saw that all those patients benefited from the addition of chemotherapy if the recurrence score was 25 or less. We did a number of subgroup analyses, which we reported on at the San Antonio Breast Cancer Symposium in 2021.
Several analyses are ongoing. These include some subgroup analyses looking at quality of life as well as a collection of circulating markers. In addition, there is ongoing biomarker work looking at tumor tissue to see if there are differences between the biology of premenopausal versus postmenopausal women.
What value does genomic testing bring to the treatment of HR-positive/HER2-negative breast cancer?
Dr. Kalinsky: These assays have achieved clinical utility, and this has been reflected in the recent update to the ASCO Guidelines for genomic assays. We have also learned that it is not just the assay by itself, but also the clinical features of a patient that help determine risk. In other words, it’s not just dependent on the score, but also involves the context of other important clinical features, including patient and tumor characteristics such as tumor size, patient age, and tumor grade. All of these add value and help us assess a patient’s individualized risk.
Is there a specific profile or qualifications candidates must meet for genomic testing to be done?
Dr. Kalinsky: We offer genomic tests for patients with HR-positive/HER2-negative breast cancer who are node-negative or have 1 to 3 nodes involved. There are other commercially-available tests such as the Breast Cancer Index, which assesses risk of recurrence in years 5 to 10 and looks at whether there is potential utility for continuing anti-estrogen therapy. That assay provides both prognostic and predictive information.
Is there any additional insight on genomic assays in HR-positive/HER2-negative breast cancer you would like to share?
Dr. Kalinsky: We’ve been talking about tumor-based assays. However, the question is, what’s going to be the role for circulating markers, such as circulating tumor DNA (ctDNA) or circulating tumor cells? There is a lot of information that we’re hoping to understand, not just regarding the prognostic significance but also the predictive utility. If you have a patient with a subtype of breast cancer and we know this subgroup can be at risk for late recurrence, if you identify said marker and you switch the therapy, do you see clearance of ctDNA? Does that lead to an improvement in outcome? That is an important question that is going to be answered in current and future trials.
As both a clinician and investigator in the breast cancer space, how would you describe our current understanding of genomic assays in hormone receptor (HR)-positive/human epidermal growth factor receptor 2 (HER2)-negative disease?
Dr. Kalinsky: There are a number of commercially-available assays for patients with early-stage HR-positive/HER2-negative breast cancer. We have seen the results of 3 large randomized phase 3 trials that have demonstrated and helped establish the clinical utility of assays, including the oncotype DX 21-gene recurrence score. This was evaluated in patients with node-negative breast cancer in the TAILORx trial and in patients with 1 to 3 nodes involved in the RxPONDER trial.
We also have data with the 70-gene MammaPrint assay from the MINDACT study, looking for patients who had a discordance between clinical and genomic risk. MINDACT has been published and was updated recently with data for 8-year distant metastasis-free survival. It is these studies that have helped establish what we do in the clinic and when we consider offering genomic assays in patients with this subtype of breast cancer.
How are you working to bring these genomic assays into practice?
Dr. Kalinsky: In 2020, we reported the initial results from the RxPonder study demonstrating that for patients with HR-positive HER2-negative breast cancer with 1 to 3 nodes involved, two-thirds of the patients were postmenopausal. For patients who had a recurrence score of 25 or less, we did not identify a subgroup of patients who benefited from chemotherapy.
For the premenopausal women, which was one-third of the patients, we saw that all those patients benefited from the addition of chemotherapy if the recurrence score was 25 or less. We did a number of subgroup analyses, which we reported on at the San Antonio Breast Cancer Symposium in 2021.
Several analyses are ongoing. These include some subgroup analyses looking at quality of life as well as a collection of circulating markers. In addition, there is ongoing biomarker work looking at tumor tissue to see if there are differences between the biology of premenopausal versus postmenopausal women.
What value does genomic testing bring to the treatment of HR-positive/HER2-negative breast cancer?
Dr. Kalinsky: These assays have achieved clinical utility, and this has been reflected in the recent update to the ASCO Guidelines for genomic assays. We have also learned that it is not just the assay by itself, but also the clinical features of a patient that help determine risk. In other words, it’s not just dependent on the score, but also involves the context of other important clinical features, including patient and tumor characteristics such as tumor size, patient age, and tumor grade. All of these add value and help us assess a patient’s individualized risk.
Is there a specific profile or qualifications candidates must meet for genomic testing to be done?
Dr. Kalinsky: We offer genomic tests for patients with HR-positive/HER2-negative breast cancer who are node-negative or have 1 to 3 nodes involved. There are other commercially-available tests such as the Breast Cancer Index, which assesses risk of recurrence in years 5 to 10 and looks at whether there is potential utility for continuing anti-estrogen therapy. That assay provides both prognostic and predictive information.
Is there any additional insight on genomic assays in HR-positive/HER2-negative breast cancer you would like to share?
Dr. Kalinsky: We’ve been talking about tumor-based assays. However, the question is, what’s going to be the role for circulating markers, such as circulating tumor DNA (ctDNA) or circulating tumor cells? There is a lot of information that we’re hoping to understand, not just regarding the prognostic significance but also the predictive utility. If you have a patient with a subtype of breast cancer and we know this subgroup can be at risk for late recurrence, if you identify said marker and you switch the therapy, do you see clearance of ctDNA? Does that lead to an improvement in outcome? That is an important question that is going to be answered in current and future trials.
As both a clinician and investigator in the breast cancer space, how would you describe our current understanding of genomic assays in hormone receptor (HR)-positive/human epidermal growth factor receptor 2 (HER2)-negative disease?
Dr. Kalinsky: There are a number of commercially-available assays for patients with early-stage HR-positive/HER2-negative breast cancer. We have seen the results of 3 large randomized phase 3 trials that have demonstrated and helped establish the clinical utility of assays, including the oncotype DX 21-gene recurrence score. This was evaluated in patients with node-negative breast cancer in the TAILORx trial and in patients with 1 to 3 nodes involved in the RxPONDER trial.
We also have data with the 70-gene MammaPrint assay from the MINDACT study, looking for patients who had a discordance between clinical and genomic risk. MINDACT has been published and was updated recently with data for 8-year distant metastasis-free survival. It is these studies that have helped establish what we do in the clinic and when we consider offering genomic assays in patients with this subtype of breast cancer.
How are you working to bring these genomic assays into practice?
Dr. Kalinsky: In 2020, we reported the initial results from the RxPonder study demonstrating that for patients with HR-positive HER2-negative breast cancer with 1 to 3 nodes involved, two-thirds of the patients were postmenopausal. For patients who had a recurrence score of 25 or less, we did not identify a subgroup of patients who benefited from chemotherapy.
For the premenopausal women, which was one-third of the patients, we saw that all those patients benefited from the addition of chemotherapy if the recurrence score was 25 or less. We did a number of subgroup analyses, which we reported on at the San Antonio Breast Cancer Symposium in 2021.
Several analyses are ongoing. These include some subgroup analyses looking at quality of life as well as a collection of circulating markers. In addition, there is ongoing biomarker work looking at tumor tissue to see if there are differences between the biology of premenopausal versus postmenopausal women.
What value does genomic testing bring to the treatment of HR-positive/HER2-negative breast cancer?
Dr. Kalinsky: These assays have achieved clinical utility, and this has been reflected in the recent update to the ASCO Guidelines for genomic assays. We have also learned that it is not just the assay by itself, but also the clinical features of a patient that help determine risk. In other words, it’s not just dependent on the score, but also involves the context of other important clinical features, including patient and tumor characteristics such as tumor size, patient age, and tumor grade. All of these add value and help us assess a patient’s individualized risk.
Is there a specific profile or qualifications candidates must meet for genomic testing to be done?
Dr. Kalinsky: We offer genomic tests for patients with HR-positive/HER2-negative breast cancer who are node-negative or have 1 to 3 nodes involved. There are other commercially-available tests such as the Breast Cancer Index, which assesses risk of recurrence in years 5 to 10 and looks at whether there is potential utility for continuing anti-estrogen therapy. That assay provides both prognostic and predictive information.
Is there any additional insight on genomic assays in HR-positive/HER2-negative breast cancer you would like to share?
Dr. Kalinsky: We’ve been talking about tumor-based assays. However, the question is, what’s going to be the role for circulating markers, such as circulating tumor DNA (ctDNA) or circulating tumor cells? There is a lot of information that we’re hoping to understand, not just regarding the prognostic significance but also the predictive utility. If you have a patient with a subtype of breast cancer and we know this subgroup can be at risk for late recurrence, if you identify said marker and you switch the therapy, do you see clearance of ctDNA? Does that lead to an improvement in outcome? That is an important question that is going to be answered in current and future trials.