User login
ERCC2, KDM6A, and TERT as Key Prognostic Factors in Bladder Cancer: Insights from the AACR Project GENIE Database
Background
Urothelial carcinoma (UC) is among the top 10 frequently diagnosed cancers in the world. Mutations in FGFR3, ARID1A, and TP53 are well documented as being some of the most frequent mutations found in UC. Despite advances in treatment, survival outcomes remain poor, especially in advanced stages. To promote future pharmacotherapeutic development, the molecular understanding of UC needs to be continually updated using more recently available databases.
Methods
This study utilizes the AACR Project GENIE database from the American Association for Cancer Research to explore the mutational profiles of patients with UC. Gene mutation frequencies were calculated, and two Kaplan-Meier curves were drawn for each gene, showing one curve for patients with the mutation and one for those without. Log-Rank tests were calculated with subsequent FDR (Benjamini–Hochberg) correction applied to account for multiple hypothesis testing. Data was analyzed using R 4.4.2 and statistical significance was set at α = 0.05.
Results
In this study, 4525 patients had histology consistent with UC. The 5 most common mutations were TERT (n = 1714, 37.9%), TP53 (n = 1689, 37.3%), KDM6A (n = 1091, 24.1%), ARID1A (n = 872, 19.3%), and FGFR3 (n = 762, 16.8%). Mutations associated with differential survival outcomes included ERCC2 (mutated n = 387, wild type n = 3751, p < 0.0001), KDM6A (mutated n = 1091, wild type n = 3047, p < 0.0001), TERT (mutated n = 1714, wild type n = 2424), and TP53 (mutated n = 1689, wild type n = 2449, p < 0.0001).
Conclusions
Interestingly, while mutations in TP53 and ERCC2 were associated with shorter median survival, mutations in KDM6A and TERT were associated with longer median survival.
Background
Urothelial carcinoma (UC) is among the top 10 frequently diagnosed cancers in the world. Mutations in FGFR3, ARID1A, and TP53 are well documented as being some of the most frequent mutations found in UC. Despite advances in treatment, survival outcomes remain poor, especially in advanced stages. To promote future pharmacotherapeutic development, the molecular understanding of UC needs to be continually updated using more recently available databases.
Methods
This study utilizes the AACR Project GENIE database from the American Association for Cancer Research to explore the mutational profiles of patients with UC. Gene mutation frequencies were calculated, and two Kaplan-Meier curves were drawn for each gene, showing one curve for patients with the mutation and one for those without. Log-Rank tests were calculated with subsequent FDR (Benjamini–Hochberg) correction applied to account for multiple hypothesis testing. Data was analyzed using R 4.4.2 and statistical significance was set at α = 0.05.
Results
In this study, 4525 patients had histology consistent with UC. The 5 most common mutations were TERT (n = 1714, 37.9%), TP53 (n = 1689, 37.3%), KDM6A (n = 1091, 24.1%), ARID1A (n = 872, 19.3%), and FGFR3 (n = 762, 16.8%). Mutations associated with differential survival outcomes included ERCC2 (mutated n = 387, wild type n = 3751, p < 0.0001), KDM6A (mutated n = 1091, wild type n = 3047, p < 0.0001), TERT (mutated n = 1714, wild type n = 2424), and TP53 (mutated n = 1689, wild type n = 2449, p < 0.0001).
Conclusions
Interestingly, while mutations in TP53 and ERCC2 were associated with shorter median survival, mutations in KDM6A and TERT were associated with longer median survival.
Background
Urothelial carcinoma (UC) is among the top 10 frequently diagnosed cancers in the world. Mutations in FGFR3, ARID1A, and TP53 are well documented as being some of the most frequent mutations found in UC. Despite advances in treatment, survival outcomes remain poor, especially in advanced stages. To promote future pharmacotherapeutic development, the molecular understanding of UC needs to be continually updated using more recently available databases.
Methods
This study utilizes the AACR Project GENIE database from the American Association for Cancer Research to explore the mutational profiles of patients with UC. Gene mutation frequencies were calculated, and two Kaplan-Meier curves were drawn for each gene, showing one curve for patients with the mutation and one for those without. Log-Rank tests were calculated with subsequent FDR (Benjamini–Hochberg) correction applied to account for multiple hypothesis testing. Data was analyzed using R 4.4.2 and statistical significance was set at α = 0.05.
Results
In this study, 4525 patients had histology consistent with UC. The 5 most common mutations were TERT (n = 1714, 37.9%), TP53 (n = 1689, 37.3%), KDM6A (n = 1091, 24.1%), ARID1A (n = 872, 19.3%), and FGFR3 (n = 762, 16.8%). Mutations associated with differential survival outcomes included ERCC2 (mutated n = 387, wild type n = 3751, p < 0.0001), KDM6A (mutated n = 1091, wild type n = 3047, p < 0.0001), TERT (mutated n = 1714, wild type n = 2424), and TP53 (mutated n = 1689, wild type n = 2449, p < 0.0001).
Conclusions
Interestingly, while mutations in TP53 and ERCC2 were associated with shorter median survival, mutations in KDM6A and TERT were associated with longer median survival.
Organs of Metastasis Predominate with Age in Non-Small Cell Lung Cancer Subtypes: National Cancer Database Analysis
Background
Patients diagnosed with lung cancer are predominantly non-small cell lung cancer (NSCLC), a leading cause of cancer-related deaths. Thus, it is imperative to investigate and distinguish the differences present at diagnosis to possibly improve survival outcomes. NSCLC commonly metastasizes within older patients near the mean age of 71 years, but also in early onset patients which represents the patients younger than the earliest lung cancer screening age of 50.
Objective
To reveal differences in ratios of metastasis locations in squamous cell carcinoma (SCC), adenocarcinoma (ACC), and adenosquamous carcinoma (ASC).
Methods
The National Cancer Database (NCDB) was utilized to identify patients diagnosed with SCC, ACC, and ASC using the histology codes 8070, 8140, and 8560 from the ICD-O-3.2 from 2004 to 2022. Age groups were 70 years. Metastases located to the brain, liver, bone, and lung were included. Chi-Square tests were performed. The data was analyzed using R version 4.4.2 and statistical significance was set to α = 0.05.
Results
In this study, 1,445,119 patients were analyzed. Chi-Square tests identified significant differences in the ratios of organ metastasis locations between age groups in each subtype (p < 0.001). SCC in each age group similarly metastasized most to bone (36.3%, 34.7%, 34.5%), but notably more local lung metastasis was observed in the oldest group (33.6%). In ACC and ASC, the oldest group also had greater ratios of spread within the lungs (28.0%, 27.2%). Overall, the younger the age group, distant spread to the brain increased (ex. 29.0%, 24.4%, 17.5%). This suggests a widely heterogenous distribution of metastases at diagnosis of NSCLC subtypes and patient age.
Conclusions
This study demonstrated that patients with SCC, ACC, or ASC subtypes of NSCLC share similar predominant locations based in part on patient age, irrespective of cancer origin. NSCLC may more distantly metastasize in younger patients to the brain, while older patients may have locally metastatic cancer. Further analysis of key demographic variables as well as common undertaken treatment options may prove informative and reveal existing differences in survival outcomes.
Background
Patients diagnosed with lung cancer are predominantly non-small cell lung cancer (NSCLC), a leading cause of cancer-related deaths. Thus, it is imperative to investigate and distinguish the differences present at diagnosis to possibly improve survival outcomes. NSCLC commonly metastasizes within older patients near the mean age of 71 years, but also in early onset patients which represents the patients younger than the earliest lung cancer screening age of 50.
Objective
To reveal differences in ratios of metastasis locations in squamous cell carcinoma (SCC), adenocarcinoma (ACC), and adenosquamous carcinoma (ASC).
Methods
The National Cancer Database (NCDB) was utilized to identify patients diagnosed with SCC, ACC, and ASC using the histology codes 8070, 8140, and 8560 from the ICD-O-3.2 from 2004 to 2022. Age groups were 70 years. Metastases located to the brain, liver, bone, and lung were included. Chi-Square tests were performed. The data was analyzed using R version 4.4.2 and statistical significance was set to α = 0.05.
Results
In this study, 1,445,119 patients were analyzed. Chi-Square tests identified significant differences in the ratios of organ metastasis locations between age groups in each subtype (p < 0.001). SCC in each age group similarly metastasized most to bone (36.3%, 34.7%, 34.5%), but notably more local lung metastasis was observed in the oldest group (33.6%). In ACC and ASC, the oldest group also had greater ratios of spread within the lungs (28.0%, 27.2%). Overall, the younger the age group, distant spread to the brain increased (ex. 29.0%, 24.4%, 17.5%). This suggests a widely heterogenous distribution of metastases at diagnosis of NSCLC subtypes and patient age.
Conclusions
This study demonstrated that patients with SCC, ACC, or ASC subtypes of NSCLC share similar predominant locations based in part on patient age, irrespective of cancer origin. NSCLC may more distantly metastasize in younger patients to the brain, while older patients may have locally metastatic cancer. Further analysis of key demographic variables as well as common undertaken treatment options may prove informative and reveal existing differences in survival outcomes.
Background
Patients diagnosed with lung cancer are predominantly non-small cell lung cancer (NSCLC), a leading cause of cancer-related deaths. Thus, it is imperative to investigate and distinguish the differences present at diagnosis to possibly improve survival outcomes. NSCLC commonly metastasizes within older patients near the mean age of 71 years, but also in early onset patients which represents the patients younger than the earliest lung cancer screening age of 50.
Objective
To reveal differences in ratios of metastasis locations in squamous cell carcinoma (SCC), adenocarcinoma (ACC), and adenosquamous carcinoma (ASC).
Methods
The National Cancer Database (NCDB) was utilized to identify patients diagnosed with SCC, ACC, and ASC using the histology codes 8070, 8140, and 8560 from the ICD-O-3.2 from 2004 to 2022. Age groups were 70 years. Metastases located to the brain, liver, bone, and lung were included. Chi-Square tests were performed. The data was analyzed using R version 4.4.2 and statistical significance was set to α = 0.05.
Results
In this study, 1,445,119 patients were analyzed. Chi-Square tests identified significant differences in the ratios of organ metastasis locations between age groups in each subtype (p < 0.001). SCC in each age group similarly metastasized most to bone (36.3%, 34.7%, 34.5%), but notably more local lung metastasis was observed in the oldest group (33.6%). In ACC and ASC, the oldest group also had greater ratios of spread within the lungs (28.0%, 27.2%). Overall, the younger the age group, distant spread to the brain increased (ex. 29.0%, 24.4%, 17.5%). This suggests a widely heterogenous distribution of metastases at diagnosis of NSCLC subtypes and patient age.
Conclusions
This study demonstrated that patients with SCC, ACC, or ASC subtypes of NSCLC share similar predominant locations based in part on patient age, irrespective of cancer origin. NSCLC may more distantly metastasize in younger patients to the brain, while older patients may have locally metastatic cancer. Further analysis of key demographic variables as well as common undertaken treatment options may prove informative and reveal existing differences in survival outcomes.
Shifting Demographics: A Temporal Analysis of the Alarming Rise in Rectal Adenocarcinoma Among Young Adults
Background
Rectal adenocarcinoma has long been associated with older adults, with routine screening typically beginning at age 45 or older. However, recent data reveal a concerning rise in rectal cancer incidence among adults under 40. These early-onset cases often present at later stages and may have distinct biological features. While some research attributes this trend to genetic or environmental factors, the contribution of socioeconomic disparities and healthcare access has not been fully explored. Identifying these influences is essential to shaping targeted prevention and early detection strategies for younger populations.
Objective
To evaluate temporal trends in rectal adenocarcinoma among young adults and assess demographic and socioeconomic predictors of early-onset diagnosis.
Methods
Data were drawn from the National Cancer Database (NCDB) for patients diagnosed with rectal adenocarcinoma from 2004 to 2022. Among 440,316 cases, 17,842 (4.1%) occurred in individuals under 40. Linear regression assessed temporal trends, while logistic regression evaluated associations between early-onset diagnosis and variables including sex, race, insurance status, income level, Charlson-Deyo comorbidity score, and tumor stage. Statistical significance was defined as α = 0.05.
Results
The number of young adults diagnosed rose from 424 in 2004 to 937 in 2022—an increase of over 120%. Each year was associated with a 1.7% rise in odds of early diagnosis (OR = 1.017, p < 0.001). Male patients had 24.7% higher odds (OR = 1.247, p < 0.001), and Black patients had 59.3% higher odds compared to White patients (OR = 1.593, p < 0.001). Non-private insurance was linked to a 41.6% decrease in early diagnosis (OR = 0.584, p < 0.001). Income level was not significant (p = 0.426). Lower Charlson-Deyo scores and higher tumor stages were also associated with early-onset cases.
Conclusions
Rectal adenocarcinoma is increasingly affecting younger adults, with significant associations across demographic and insurance variables. These findings call for improved awareness, early diagnostic strategies, and further research into underlying causes to mitigate this growing public health concern.
Background
Rectal adenocarcinoma has long been associated with older adults, with routine screening typically beginning at age 45 or older. However, recent data reveal a concerning rise in rectal cancer incidence among adults under 40. These early-onset cases often present at later stages and may have distinct biological features. While some research attributes this trend to genetic or environmental factors, the contribution of socioeconomic disparities and healthcare access has not been fully explored. Identifying these influences is essential to shaping targeted prevention and early detection strategies for younger populations.
Objective
To evaluate temporal trends in rectal adenocarcinoma among young adults and assess demographic and socioeconomic predictors of early-onset diagnosis.
Methods
Data were drawn from the National Cancer Database (NCDB) for patients diagnosed with rectal adenocarcinoma from 2004 to 2022. Among 440,316 cases, 17,842 (4.1%) occurred in individuals under 40. Linear regression assessed temporal trends, while logistic regression evaluated associations between early-onset diagnosis and variables including sex, race, insurance status, income level, Charlson-Deyo comorbidity score, and tumor stage. Statistical significance was defined as α = 0.05.
Results
The number of young adults diagnosed rose from 424 in 2004 to 937 in 2022—an increase of over 120%. Each year was associated with a 1.7% rise in odds of early diagnosis (OR = 1.017, p < 0.001). Male patients had 24.7% higher odds (OR = 1.247, p < 0.001), and Black patients had 59.3% higher odds compared to White patients (OR = 1.593, p < 0.001). Non-private insurance was linked to a 41.6% decrease in early diagnosis (OR = 0.584, p < 0.001). Income level was not significant (p = 0.426). Lower Charlson-Deyo scores and higher tumor stages were also associated with early-onset cases.
Conclusions
Rectal adenocarcinoma is increasingly affecting younger adults, with significant associations across demographic and insurance variables. These findings call for improved awareness, early diagnostic strategies, and further research into underlying causes to mitigate this growing public health concern.
Background
Rectal adenocarcinoma has long been associated with older adults, with routine screening typically beginning at age 45 or older. However, recent data reveal a concerning rise in rectal cancer incidence among adults under 40. These early-onset cases often present at later stages and may have distinct biological features. While some research attributes this trend to genetic or environmental factors, the contribution of socioeconomic disparities and healthcare access has not been fully explored. Identifying these influences is essential to shaping targeted prevention and early detection strategies for younger populations.
Objective
To evaluate temporal trends in rectal adenocarcinoma among young adults and assess demographic and socioeconomic predictors of early-onset diagnosis.
Methods
Data were drawn from the National Cancer Database (NCDB) for patients diagnosed with rectal adenocarcinoma from 2004 to 2022. Among 440,316 cases, 17,842 (4.1%) occurred in individuals under 40. Linear regression assessed temporal trends, while logistic regression evaluated associations between early-onset diagnosis and variables including sex, race, insurance status, income level, Charlson-Deyo comorbidity score, and tumor stage. Statistical significance was defined as α = 0.05.
Results
The number of young adults diagnosed rose from 424 in 2004 to 937 in 2022—an increase of over 120%. Each year was associated with a 1.7% rise in odds of early diagnosis (OR = 1.017, p < 0.001). Male patients had 24.7% higher odds (OR = 1.247, p < 0.001), and Black patients had 59.3% higher odds compared to White patients (OR = 1.593, p < 0.001). Non-private insurance was linked to a 41.6% decrease in early diagnosis (OR = 0.584, p < 0.001). Income level was not significant (p = 0.426). Lower Charlson-Deyo scores and higher tumor stages were also associated with early-onset cases.
Conclusions
Rectal adenocarcinoma is increasingly affecting younger adults, with significant associations across demographic and insurance variables. These findings call for improved awareness, early diagnostic strategies, and further research into underlying causes to mitigate this growing public health concern.
Survival Outcomes of Skin Adnexal Tumors: A National Cancer Database Analysis
Purpose
Skin adnexal tumors (SAT) include a group of benign and malignant appendageal tumors that arise from hair follicles, sebaceous glands, or sweat glands. They typically appear as small, painless bumps or nodules on the skin, and are more common in men compared to women. The 5-year overall SAT survival rate ranges from 74-90%. To better understand the differences in survival outcomes based on subtypes of SAT, the National Cancer Database (NCDB) was analyzed.
Methods
A retrospective cohort study of 11,627 patients with histologically confirmed SAT between 2004 and 2021 was conducted across 1,500 Commission on Cancer facilities located in the US and Puerto Rico. Demographic factors such as sex, age, and race were analyzed using Pearson Chi-squared tests, and survival outcomes were analyzed by Kaplan- Meier survival analysis. P value < 0.05 was considered statistically significant.
Results
Most patients with SAT were male (57.3%). The average age at diagnosis was 65.9 (SD=14.4, range 0-90). Of the patient sample, 87.2% were White, 7.6% Black, 2.5% Asian, and 2.7% other. Several subtypes disproportionately affected Black individuals, including apocrine adenocarcinoma (15.7%) and hidradenocarcinoma (13.6%). The estimated 5-year survival of SAT was 74.9% with an overall survival of 135.8 months (SE=1.1). Sebaceous carcinoma (which accounts for 41.8% of all cases) had the lowest average survival time of 119.6 months (SE=1.8), while digital papillary adenocarcinoma had the highest survival at around 183.5 months (SE=4.6).
Conclusions
This study supports a higher frequency of SAT among men. While White patients were more likely to get SAT overall, including the most common sebaceous carcinoma, Black race were associated with higher frequency of rarer subtypes. The average age of diagnosis of SAT mimics other non-melanoma skin cancers, but has a lower overall survival rate. Future studies should consider other risk factors that may be impacting the differences in survival outcomes to guide treatment and address health disparities among the various subtypes.
Purpose
Skin adnexal tumors (SAT) include a group of benign and malignant appendageal tumors that arise from hair follicles, sebaceous glands, or sweat glands. They typically appear as small, painless bumps or nodules on the skin, and are more common in men compared to women. The 5-year overall SAT survival rate ranges from 74-90%. To better understand the differences in survival outcomes based on subtypes of SAT, the National Cancer Database (NCDB) was analyzed.
Methods
A retrospective cohort study of 11,627 patients with histologically confirmed SAT between 2004 and 2021 was conducted across 1,500 Commission on Cancer facilities located in the US and Puerto Rico. Demographic factors such as sex, age, and race were analyzed using Pearson Chi-squared tests, and survival outcomes were analyzed by Kaplan- Meier survival analysis. P value < 0.05 was considered statistically significant.
Results
Most patients with SAT were male (57.3%). The average age at diagnosis was 65.9 (SD=14.4, range 0-90). Of the patient sample, 87.2% were White, 7.6% Black, 2.5% Asian, and 2.7% other. Several subtypes disproportionately affected Black individuals, including apocrine adenocarcinoma (15.7%) and hidradenocarcinoma (13.6%). The estimated 5-year survival of SAT was 74.9% with an overall survival of 135.8 months (SE=1.1). Sebaceous carcinoma (which accounts for 41.8% of all cases) had the lowest average survival time of 119.6 months (SE=1.8), while digital papillary adenocarcinoma had the highest survival at around 183.5 months (SE=4.6).
Conclusions
This study supports a higher frequency of SAT among men. While White patients were more likely to get SAT overall, including the most common sebaceous carcinoma, Black race were associated with higher frequency of rarer subtypes. The average age of diagnosis of SAT mimics other non-melanoma skin cancers, but has a lower overall survival rate. Future studies should consider other risk factors that may be impacting the differences in survival outcomes to guide treatment and address health disparities among the various subtypes.
Purpose
Skin adnexal tumors (SAT) include a group of benign and malignant appendageal tumors that arise from hair follicles, sebaceous glands, or sweat glands. They typically appear as small, painless bumps or nodules on the skin, and are more common in men compared to women. The 5-year overall SAT survival rate ranges from 74-90%. To better understand the differences in survival outcomes based on subtypes of SAT, the National Cancer Database (NCDB) was analyzed.
Methods
A retrospective cohort study of 11,627 patients with histologically confirmed SAT between 2004 and 2021 was conducted across 1,500 Commission on Cancer facilities located in the US and Puerto Rico. Demographic factors such as sex, age, and race were analyzed using Pearson Chi-squared tests, and survival outcomes were analyzed by Kaplan- Meier survival analysis. P value < 0.05 was considered statistically significant.
Results
Most patients with SAT were male (57.3%). The average age at diagnosis was 65.9 (SD=14.4, range 0-90). Of the patient sample, 87.2% were White, 7.6% Black, 2.5% Asian, and 2.7% other. Several subtypes disproportionately affected Black individuals, including apocrine adenocarcinoma (15.7%) and hidradenocarcinoma (13.6%). The estimated 5-year survival of SAT was 74.9% with an overall survival of 135.8 months (SE=1.1). Sebaceous carcinoma (which accounts for 41.8% of all cases) had the lowest average survival time of 119.6 months (SE=1.8), while digital papillary adenocarcinoma had the highest survival at around 183.5 months (SE=4.6).
Conclusions
This study supports a higher frequency of SAT among men. While White patients were more likely to get SAT overall, including the most common sebaceous carcinoma, Black race were associated with higher frequency of rarer subtypes. The average age of diagnosis of SAT mimics other non-melanoma skin cancers, but has a lower overall survival rate. Future studies should consider other risk factors that may be impacting the differences in survival outcomes to guide treatment and address health disparities among the various subtypes.
Clinically Significant Transition Zone Prostate Cancer Detected by UroNav MRI/TRUS Fusion Biopsy in Active Surveillance Prostate Cancer Patients
OBJECTIVE
UroNav MRI/TRUS biopsy offers a more accurate test result regarding prostate cancer. The goal of the UroNav is to find more transitional zone prostate cancers that a standard mapping biopsy is unable to see. This paper aims to evaluate the utility of UroNav MRI/TRUS biopsy to detect clinically significant transition zone cancers in patients on active surveillance with low volume, low grade cancer.
METHODS
We retrospectively analyzed 268 prostate cancer patients from Minnesota Urology over a threeyear period who underwent a UroNav (MRI/TRUS) biopsy as part of standardized follow up in an active surveillance protocol. All patients underwent both biopsy of MRI PiRAD lesions and a standard mapping biopsy at the time of procedure. Patients with positive PiRAD transition zone and negative mapping biopsies were identified. Kaplan-Meier, Cox Proportional Hazards test, ANOVA and Chi-Square tests were performed. Data was analyzed using IBM SPSS version 27 and statistical significance was set at α=0.05.
RESULTS
Of the 268 patients, 68 (25%) of the patients had a normal standard mapping prostate biopsies. Using UroNav technology cancer was found showing a statistically significant amount of prostate cancer in the transitional zone missed by standard mapping biopsy (P value <0.05) Out of these 68 patients 35 (51.5%) were reported to have a Gleason score ≥7 indicating clinically significant prostate cancer.
CONCLUSIONS
The use of UroNav MRI/TRUS fusion biopsy allowed detection of clinically significant transition zone cancer missed by concurrent standard mapping biopsies in an active surveillance population. This should be continually explored to get a larger sample size to see if the UroNav can also detect missed clinically significant prostate cancer at a high rate.
OBJECTIVE
UroNav MRI/TRUS biopsy offers a more accurate test result regarding prostate cancer. The goal of the UroNav is to find more transitional zone prostate cancers that a standard mapping biopsy is unable to see. This paper aims to evaluate the utility of UroNav MRI/TRUS biopsy to detect clinically significant transition zone cancers in patients on active surveillance with low volume, low grade cancer.
METHODS
We retrospectively analyzed 268 prostate cancer patients from Minnesota Urology over a threeyear period who underwent a UroNav (MRI/TRUS) biopsy as part of standardized follow up in an active surveillance protocol. All patients underwent both biopsy of MRI PiRAD lesions and a standard mapping biopsy at the time of procedure. Patients with positive PiRAD transition zone and negative mapping biopsies were identified. Kaplan-Meier, Cox Proportional Hazards test, ANOVA and Chi-Square tests were performed. Data was analyzed using IBM SPSS version 27 and statistical significance was set at α=0.05.
RESULTS
Of the 268 patients, 68 (25%) of the patients had a normal standard mapping prostate biopsies. Using UroNav technology cancer was found showing a statistically significant amount of prostate cancer in the transitional zone missed by standard mapping biopsy (P value <0.05) Out of these 68 patients 35 (51.5%) were reported to have a Gleason score ≥7 indicating clinically significant prostate cancer.
CONCLUSIONS
The use of UroNav MRI/TRUS fusion biopsy allowed detection of clinically significant transition zone cancer missed by concurrent standard mapping biopsies in an active surveillance population. This should be continually explored to get a larger sample size to see if the UroNav can also detect missed clinically significant prostate cancer at a high rate.
OBJECTIVE
UroNav MRI/TRUS biopsy offers a more accurate test result regarding prostate cancer. The goal of the UroNav is to find more transitional zone prostate cancers that a standard mapping biopsy is unable to see. This paper aims to evaluate the utility of UroNav MRI/TRUS biopsy to detect clinically significant transition zone cancers in patients on active surveillance with low volume, low grade cancer.
METHODS
We retrospectively analyzed 268 prostate cancer patients from Minnesota Urology over a threeyear period who underwent a UroNav (MRI/TRUS) biopsy as part of standardized follow up in an active surveillance protocol. All patients underwent both biopsy of MRI PiRAD lesions and a standard mapping biopsy at the time of procedure. Patients with positive PiRAD transition zone and negative mapping biopsies were identified. Kaplan-Meier, Cox Proportional Hazards test, ANOVA and Chi-Square tests were performed. Data was analyzed using IBM SPSS version 27 and statistical significance was set at α=0.05.
RESULTS
Of the 268 patients, 68 (25%) of the patients had a normal standard mapping prostate biopsies. Using UroNav technology cancer was found showing a statistically significant amount of prostate cancer in the transitional zone missed by standard mapping biopsy (P value <0.05) Out of these 68 patients 35 (51.5%) were reported to have a Gleason score ≥7 indicating clinically significant prostate cancer.
CONCLUSIONS
The use of UroNav MRI/TRUS fusion biopsy allowed detection of clinically significant transition zone cancer missed by concurrent standard mapping biopsies in an active surveillance population. This should be continually explored to get a larger sample size to see if the UroNav can also detect missed clinically significant prostate cancer at a high rate.
Detection of Prostate Cancer in the Transitional Zone by Using a UroNav Biopsy
OBJECTIVE
Transitional zone cancers are not accounted for when using standard prostate biopsy techniques. Using MRI/Transrectal ultrasound fusion biopsy (UroNav) can more accurately diagnose transitional zone prostate cancer. The goal of this study is to evaluate 375 patients with transitional zone only cancer found on a UroNav biopsy MRI/Transrectal ultrasound fusion biopsy over a three-year period to evaluate the clinical significance of their cancer.
METHOD
We retrospectively analyzed 1500 patients that underwent a UroNav biopsy over a 3 year period. 375 of these patients had transitional zone only cancers. The patients with transitional and peripheral zone cancer were analyzed. The PIRAD scores were evaluated and the percent cancer determined for each zone. Clinically significant cancer for each zone was also determined.
RESULTS
Of the 1500 patients with a PIRAD lesion, 25% were located in the transitional zone, 36% in the peripheral zone and 39% in both transitional and peripheral zone. Cancer was detected in 40% of transitional zone only lesions, 44% of peripheral zone only lesions and 38% combined zone lesion. Clinically significant cancer was noted in 26%, 27% and 20%, respectively, for the TZ, PZ and combined zones. Kaplan- Meier, Cox Proportional Hazards test, ANOVA and Chi- Square tests were performed. Data was analyzed using IBM SPSS version 27 and statistical significance was set at α=0.05. PIRAD breakdown for transitional zone only cancers are as follows, PIRAD 3 (52% of patients): 24% cancer, 10% clinically significant PIRAD 4 (34% of patients): 43% cancer, 30% clinically significant PIRAD 5 (14% of patients): 75% cancer, 60% clinically significant
CONCLUSIONS
The use of a UroNav biopsy has been instrumental in detecting clinically significant cancers in the transitional zone that otherwise would have been missed on a standard mapping biopsy.
OBJECTIVE
Transitional zone cancers are not accounted for when using standard prostate biopsy techniques. Using MRI/Transrectal ultrasound fusion biopsy (UroNav) can more accurately diagnose transitional zone prostate cancer. The goal of this study is to evaluate 375 patients with transitional zone only cancer found on a UroNav biopsy MRI/Transrectal ultrasound fusion biopsy over a three-year period to evaluate the clinical significance of their cancer.
METHOD
We retrospectively analyzed 1500 patients that underwent a UroNav biopsy over a 3 year period. 375 of these patients had transitional zone only cancers. The patients with transitional and peripheral zone cancer were analyzed. The PIRAD scores were evaluated and the percent cancer determined for each zone. Clinically significant cancer for each zone was also determined.
RESULTS
Of the 1500 patients with a PIRAD lesion, 25% were located in the transitional zone, 36% in the peripheral zone and 39% in both transitional and peripheral zone. Cancer was detected in 40% of transitional zone only lesions, 44% of peripheral zone only lesions and 38% combined zone lesion. Clinically significant cancer was noted in 26%, 27% and 20%, respectively, for the TZ, PZ and combined zones. Kaplan- Meier, Cox Proportional Hazards test, ANOVA and Chi- Square tests were performed. Data was analyzed using IBM SPSS version 27 and statistical significance was set at α=0.05. PIRAD breakdown for transitional zone only cancers are as follows, PIRAD 3 (52% of patients): 24% cancer, 10% clinically significant PIRAD 4 (34% of patients): 43% cancer, 30% clinically significant PIRAD 5 (14% of patients): 75% cancer, 60% clinically significant
CONCLUSIONS
The use of a UroNav biopsy has been instrumental in detecting clinically significant cancers in the transitional zone that otherwise would have been missed on a standard mapping biopsy.
OBJECTIVE
Transitional zone cancers are not accounted for when using standard prostate biopsy techniques. Using MRI/Transrectal ultrasound fusion biopsy (UroNav) can more accurately diagnose transitional zone prostate cancer. The goal of this study is to evaluate 375 patients with transitional zone only cancer found on a UroNav biopsy MRI/Transrectal ultrasound fusion biopsy over a three-year period to evaluate the clinical significance of their cancer.
METHOD
We retrospectively analyzed 1500 patients that underwent a UroNav biopsy over a 3 year period. 375 of these patients had transitional zone only cancers. The patients with transitional and peripheral zone cancer were analyzed. The PIRAD scores were evaluated and the percent cancer determined for each zone. Clinically significant cancer for each zone was also determined.
RESULTS
Of the 1500 patients with a PIRAD lesion, 25% were located in the transitional zone, 36% in the peripheral zone and 39% in both transitional and peripheral zone. Cancer was detected in 40% of transitional zone only lesions, 44% of peripheral zone only lesions and 38% combined zone lesion. Clinically significant cancer was noted in 26%, 27% and 20%, respectively, for the TZ, PZ and combined zones. Kaplan- Meier, Cox Proportional Hazards test, ANOVA and Chi- Square tests were performed. Data was analyzed using IBM SPSS version 27 and statistical significance was set at α=0.05. PIRAD breakdown for transitional zone only cancers are as follows, PIRAD 3 (52% of patients): 24% cancer, 10% clinically significant PIRAD 4 (34% of patients): 43% cancer, 30% clinically significant PIRAD 5 (14% of patients): 75% cancer, 60% clinically significant
CONCLUSIONS
The use of a UroNav biopsy has been instrumental in detecting clinically significant cancers in the transitional zone that otherwise would have been missed on a standard mapping biopsy.
Differential Overall Survival and Treatment in Patients With Small Intestine Adenocarcinoma Based on Insurance Status: A National Perspective
BACKGROUND
The incidence of adenocarcinoma, the most common type of small intestine cancer, is increasing. Prior studies found a 5-year survival of about 25% even with surgical resection and lymph node dissection. A recent study found higher survival in insured versus uninsured patients, yet differential outcomes and treatments between private insurance and Medicare, along with Medicaid and no insurance, are unknown. This study aims to determine differential survival and treatment of patients with small intestine adenocarcinoma based on insurance status.
METHODS
The National Cancer Database was used to identify patients diagnosed with small intestine adenocarcinoma from 2004-2019 using the histology code 8140 as assigned by the Commission on Cancer Accreditation program. Kaplan-Meier, Chi-Square, ANOVA, and Cox Proportional Hazards tests were performed. Data was analyzed using IBM SPSS version 28 and statistical significance was set at α=0.05.
RESULTS
Of the 20,933 patients included, 7,629 (32.4%) had private insurance and 13,075 (55.5%) had Medicare. Patients with private insurance had a longer median survival (28.8 months) than patients with Medicare, Medicaid, and no insurance (p<.001), while patients with Medicare had a shorter median survival (12.2 months) than other insurance statuses (p<.001). No median survival difference existed between those with Medicaid (18.9 months) and no insurance (18.0 months) (p=.882). After controlling for age, co-morbidity score, grade, tumor size, low-income, academic facility, surgery of primary site, palliative care, and days between diagnosis and treatment, private insurance was associated with an independent decrease in hazard (HR=.874; p<.001). Patients with private insurance received more surgery (67.8%) than those with Medicaid (58.6%), no insurance (54.4%), and Medicare (52.9%) (p<.001). Patients with Medicare received more adjuvant radiation, but patients with private insurance received more adjuvant chemoradiation (p<.001). While patients with Medicare presented with greater co-morbidities and age, patients with private insurance presented with fewer co-morbidities, smaller sized tumors, and shorter time between diagnosis and treatment (p<.001).
CONCLUSIONS
Since patients with private insurance received the most surgery and displayed the highest overall survival, while patients with Medicare displayed the lowest survival, future research should explore ways to alleviate this disparity in surgical resections.
BACKGROUND
The incidence of adenocarcinoma, the most common type of small intestine cancer, is increasing. Prior studies found a 5-year survival of about 25% even with surgical resection and lymph node dissection. A recent study found higher survival in insured versus uninsured patients, yet differential outcomes and treatments between private insurance and Medicare, along with Medicaid and no insurance, are unknown. This study aims to determine differential survival and treatment of patients with small intestine adenocarcinoma based on insurance status.
METHODS
The National Cancer Database was used to identify patients diagnosed with small intestine adenocarcinoma from 2004-2019 using the histology code 8140 as assigned by the Commission on Cancer Accreditation program. Kaplan-Meier, Chi-Square, ANOVA, and Cox Proportional Hazards tests were performed. Data was analyzed using IBM SPSS version 28 and statistical significance was set at α=0.05.
RESULTS
Of the 20,933 patients included, 7,629 (32.4%) had private insurance and 13,075 (55.5%) had Medicare. Patients with private insurance had a longer median survival (28.8 months) than patients with Medicare, Medicaid, and no insurance (p<.001), while patients with Medicare had a shorter median survival (12.2 months) than other insurance statuses (p<.001). No median survival difference existed between those with Medicaid (18.9 months) and no insurance (18.0 months) (p=.882). After controlling for age, co-morbidity score, grade, tumor size, low-income, academic facility, surgery of primary site, palliative care, and days between diagnosis and treatment, private insurance was associated with an independent decrease in hazard (HR=.874; p<.001). Patients with private insurance received more surgery (67.8%) than those with Medicaid (58.6%), no insurance (54.4%), and Medicare (52.9%) (p<.001). Patients with Medicare received more adjuvant radiation, but patients with private insurance received more adjuvant chemoradiation (p<.001). While patients with Medicare presented with greater co-morbidities and age, patients with private insurance presented with fewer co-morbidities, smaller sized tumors, and shorter time between diagnosis and treatment (p<.001).
CONCLUSIONS
Since patients with private insurance received the most surgery and displayed the highest overall survival, while patients with Medicare displayed the lowest survival, future research should explore ways to alleviate this disparity in surgical resections.
BACKGROUND
The incidence of adenocarcinoma, the most common type of small intestine cancer, is increasing. Prior studies found a 5-year survival of about 25% even with surgical resection and lymph node dissection. A recent study found higher survival in insured versus uninsured patients, yet differential outcomes and treatments between private insurance and Medicare, along with Medicaid and no insurance, are unknown. This study aims to determine differential survival and treatment of patients with small intestine adenocarcinoma based on insurance status.
METHODS
The National Cancer Database was used to identify patients diagnosed with small intestine adenocarcinoma from 2004-2019 using the histology code 8140 as assigned by the Commission on Cancer Accreditation program. Kaplan-Meier, Chi-Square, ANOVA, and Cox Proportional Hazards tests were performed. Data was analyzed using IBM SPSS version 28 and statistical significance was set at α=0.05.
RESULTS
Of the 20,933 patients included, 7,629 (32.4%) had private insurance and 13,075 (55.5%) had Medicare. Patients with private insurance had a longer median survival (28.8 months) than patients with Medicare, Medicaid, and no insurance (p<.001), while patients with Medicare had a shorter median survival (12.2 months) than other insurance statuses (p<.001). No median survival difference existed between those with Medicaid (18.9 months) and no insurance (18.0 months) (p=.882). After controlling for age, co-morbidity score, grade, tumor size, low-income, academic facility, surgery of primary site, palliative care, and days between diagnosis and treatment, private insurance was associated with an independent decrease in hazard (HR=.874; p<.001). Patients with private insurance received more surgery (67.8%) than those with Medicaid (58.6%), no insurance (54.4%), and Medicare (52.9%) (p<.001). Patients with Medicare received more adjuvant radiation, but patients with private insurance received more adjuvant chemoradiation (p<.001). While patients with Medicare presented with greater co-morbidities and age, patients with private insurance presented with fewer co-morbidities, smaller sized tumors, and shorter time between diagnosis and treatment (p<.001).
CONCLUSIONS
Since patients with private insurance received the most surgery and displayed the highest overall survival, while patients with Medicare displayed the lowest survival, future research should explore ways to alleviate this disparity in surgical resections.
Survival and Treatment in Older Patients With Ewing Sarcoma
BACKGROUND
Ewing sarcoma (EWS) is a malignancy which primarily arises in adolescence and has been studied extensively in this population. Much less is www.mdedge.com/fedprac/avaho SEPTEMBER 2023 • S23 known about the rare patient cohort over the age of 40 at diagnosis. In this study, we describe the survival outcomes and clinical characteristics of this population.
METHODS
This retrospective cohort study utilized the National Cancer Database (NCDB) to identify 4600 patients diagnosed between 2004 through 2019. Of these patients, 4058 were under the age of 40 and 542 were over 40. Multivariate Cox regression models and Kaplan- Meier curves were used to estimate survival from diagnosis to death between age groups. Chi-square tests were used to compare demographic and socioeconomic patient characteristics. IBM SPSS version 27.0 was used. p<0.05 was used to indicate statistical significance.
RESULTS
EWS patients older than 40 experienced worse survival outcomes compared to patients under the age of 40. 5-year survival was 43.5% for older patients vs. 64.5% for younger patients (p<0.05). A multivariate Cox proportional hazards model showed that age was independently associated with inferior survival. (HR 2.23; p<0.05). EWS patients over the age of 40 were more likely to have tumors originating from the vertebral column (16.2% vs. 9.6%; p<0.05), cranium (5.5% vs. 4.7%; p<0.05), and had a higher rate of axial tumors (43.3% vs. 32.4%; p<0.05) compared to patients under 40. Additionally, patients older than 40 experienced a significantly longer delay between the date of diagnosis and initiation of systemic treatment (29.85 days vs. 19.37 days; p<0.05). Despite presenting with larger tumors , older patients were less likely to undergo a surgical procedure of the primary site (47.6% vs. 52.2%; p<0.05) and had higher rates of micro- and macroscopic residual tumor following surgical resection.
CONCLUSIONS
An age over 40 is associated with decreased survival for patients with EWS. Due to the rarity of EWS in this cohort, the optimal role of systemic treatment remains unknown and has yet to be clearly elucidated. Consequently, our findings suggest that older patients receive disparities in treatment which may be contributing to decreased survival rates.
BACKGROUND
Ewing sarcoma (EWS) is a malignancy which primarily arises in adolescence and has been studied extensively in this population. Much less is www.mdedge.com/fedprac/avaho SEPTEMBER 2023 • S23 known about the rare patient cohort over the age of 40 at diagnosis. In this study, we describe the survival outcomes and clinical characteristics of this population.
METHODS
This retrospective cohort study utilized the National Cancer Database (NCDB) to identify 4600 patients diagnosed between 2004 through 2019. Of these patients, 4058 were under the age of 40 and 542 were over 40. Multivariate Cox regression models and Kaplan- Meier curves were used to estimate survival from diagnosis to death between age groups. Chi-square tests were used to compare demographic and socioeconomic patient characteristics. IBM SPSS version 27.0 was used. p<0.05 was used to indicate statistical significance.
RESULTS
EWS patients older than 40 experienced worse survival outcomes compared to patients under the age of 40. 5-year survival was 43.5% for older patients vs. 64.5% for younger patients (p<0.05). A multivariate Cox proportional hazards model showed that age was independently associated with inferior survival. (HR 2.23; p<0.05). EWS patients over the age of 40 were more likely to have tumors originating from the vertebral column (16.2% vs. 9.6%; p<0.05), cranium (5.5% vs. 4.7%; p<0.05), and had a higher rate of axial tumors (43.3% vs. 32.4%; p<0.05) compared to patients under 40. Additionally, patients older than 40 experienced a significantly longer delay between the date of diagnosis and initiation of systemic treatment (29.85 days vs. 19.37 days; p<0.05). Despite presenting with larger tumors , older patients were less likely to undergo a surgical procedure of the primary site (47.6% vs. 52.2%; p<0.05) and had higher rates of micro- and macroscopic residual tumor following surgical resection.
CONCLUSIONS
An age over 40 is associated with decreased survival for patients with EWS. Due to the rarity of EWS in this cohort, the optimal role of systemic treatment remains unknown and has yet to be clearly elucidated. Consequently, our findings suggest that older patients receive disparities in treatment which may be contributing to decreased survival rates.
BACKGROUND
Ewing sarcoma (EWS) is a malignancy which primarily arises in adolescence and has been studied extensively in this population. Much less is www.mdedge.com/fedprac/avaho SEPTEMBER 2023 • S23 known about the rare patient cohort over the age of 40 at diagnosis. In this study, we describe the survival outcomes and clinical characteristics of this population.
METHODS
This retrospective cohort study utilized the National Cancer Database (NCDB) to identify 4600 patients diagnosed between 2004 through 2019. Of these patients, 4058 were under the age of 40 and 542 were over 40. Multivariate Cox regression models and Kaplan- Meier curves were used to estimate survival from diagnosis to death between age groups. Chi-square tests were used to compare demographic and socioeconomic patient characteristics. IBM SPSS version 27.0 was used. p<0.05 was used to indicate statistical significance.
RESULTS
EWS patients older than 40 experienced worse survival outcomes compared to patients under the age of 40. 5-year survival was 43.5% for older patients vs. 64.5% for younger patients (p<0.05). A multivariate Cox proportional hazards model showed that age was independently associated with inferior survival. (HR 2.23; p<0.05). EWS patients over the age of 40 were more likely to have tumors originating from the vertebral column (16.2% vs. 9.6%; p<0.05), cranium (5.5% vs. 4.7%; p<0.05), and had a higher rate of axial tumors (43.3% vs. 32.4%; p<0.05) compared to patients under 40. Additionally, patients older than 40 experienced a significantly longer delay between the date of diagnosis and initiation of systemic treatment (29.85 days vs. 19.37 days; p<0.05). Despite presenting with larger tumors , older patients were less likely to undergo a surgical procedure of the primary site (47.6% vs. 52.2%; p<0.05) and had higher rates of micro- and macroscopic residual tumor following surgical resection.
CONCLUSIONS
An age over 40 is associated with decreased survival for patients with EWS. Due to the rarity of EWS in this cohort, the optimal role of systemic treatment remains unknown and has yet to be clearly elucidated. Consequently, our findings suggest that older patients receive disparities in treatment which may be contributing to decreased survival rates.
Disparities Affecting Survival Outcomes of Small Intestine Leiomyosarcoma, an NCDB Analysis
BACKGROUND
Leiomyosarcoma is a rare neoplasm of smooth muscle that can originate from various organ systems. Of the gastrointestinal tract, the rarity and the difficulty of diagnosing small intestine leiomyosarcoma affect its poor prognosis. With an average age of diagnosis of 64 years and a median life expectancy of 45 months, there exists a lack of information on the disparities that exist in these patients and how patient demographics contribute to differences in survival outcomes.
METHODS
We used the National Cancer Database to identify patients diagnosed with small intestine leiomyosarcoma (ICD-O-3 histology code 8890) between 2004-2019 (N=406). General patient characteristics were assessed using descriptive statistics. Survival was evaluated using Kaplan-Meier curves and log-rank tests. Significance was set at p<0.05.
RESULTS
When analyzing race, patients diagnosed with small intestine leiomyosarcoma were predominantly White (81.8%) and African American (14.3%); however, White patients had statistically worse survival outcomes than African Americans (67 vs 97 months) (p=0.004). Patients with private insurance had statistically better outcomes when compared to Medicare (p<0.001). When compared to White patients, African Americans had a higher proportion of private insurance (53.4% vs 37.2%) and lower proportion of Medicare coverage (5.2% and 48.2%), a lower average age of diagnosis (60.5 vs 64.7 years), shorter travel distances (14.7 vs 31.1 miles) and fewer days between staging procedure and surgical diagnostics from initial diagnosis (4.54 vs 12.5 days). Patients who received surgical intervention had a statistically significant improved survival outcome than those who did not (78 vs 15 months) (p<0.001) with the majority of these procedures being partial gastrectomies (53.6%). More patients of the cohort were treated at comprehensive community cancer programs (36.2%), followed by academic research programs (32.0%), integrated network cancer programs (18.5%) and community cancer programs (8.6%).
CONCLUSIONS
Factors associated with increased survival outcomes include race, average age of diagnosis, travel distance, fewer days between diagnostic procedure and initial diagnosis, insurance status and surgical treatment. These findings make a valuable contribution to the ongoing research on disparities affecting survival in patients with small intestine leiomyosarcoma.
BACKGROUND
Leiomyosarcoma is a rare neoplasm of smooth muscle that can originate from various organ systems. Of the gastrointestinal tract, the rarity and the difficulty of diagnosing small intestine leiomyosarcoma affect its poor prognosis. With an average age of diagnosis of 64 years and a median life expectancy of 45 months, there exists a lack of information on the disparities that exist in these patients and how patient demographics contribute to differences in survival outcomes.
METHODS
We used the National Cancer Database to identify patients diagnosed with small intestine leiomyosarcoma (ICD-O-3 histology code 8890) between 2004-2019 (N=406). General patient characteristics were assessed using descriptive statistics. Survival was evaluated using Kaplan-Meier curves and log-rank tests. Significance was set at p<0.05.
RESULTS
When analyzing race, patients diagnosed with small intestine leiomyosarcoma were predominantly White (81.8%) and African American (14.3%); however, White patients had statistically worse survival outcomes than African Americans (67 vs 97 months) (p=0.004). Patients with private insurance had statistically better outcomes when compared to Medicare (p<0.001). When compared to White patients, African Americans had a higher proportion of private insurance (53.4% vs 37.2%) and lower proportion of Medicare coverage (5.2% and 48.2%), a lower average age of diagnosis (60.5 vs 64.7 years), shorter travel distances (14.7 vs 31.1 miles) and fewer days between staging procedure and surgical diagnostics from initial diagnosis (4.54 vs 12.5 days). Patients who received surgical intervention had a statistically significant improved survival outcome than those who did not (78 vs 15 months) (p<0.001) with the majority of these procedures being partial gastrectomies (53.6%). More patients of the cohort were treated at comprehensive community cancer programs (36.2%), followed by academic research programs (32.0%), integrated network cancer programs (18.5%) and community cancer programs (8.6%).
CONCLUSIONS
Factors associated with increased survival outcomes include race, average age of diagnosis, travel distance, fewer days between diagnostic procedure and initial diagnosis, insurance status and surgical treatment. These findings make a valuable contribution to the ongoing research on disparities affecting survival in patients with small intestine leiomyosarcoma.
BACKGROUND
Leiomyosarcoma is a rare neoplasm of smooth muscle that can originate from various organ systems. Of the gastrointestinal tract, the rarity and the difficulty of diagnosing small intestine leiomyosarcoma affect its poor prognosis. With an average age of diagnosis of 64 years and a median life expectancy of 45 months, there exists a lack of information on the disparities that exist in these patients and how patient demographics contribute to differences in survival outcomes.
METHODS
We used the National Cancer Database to identify patients diagnosed with small intestine leiomyosarcoma (ICD-O-3 histology code 8890) between 2004-2019 (N=406). General patient characteristics were assessed using descriptive statistics. Survival was evaluated using Kaplan-Meier curves and log-rank tests. Significance was set at p<0.05.
RESULTS
When analyzing race, patients diagnosed with small intestine leiomyosarcoma were predominantly White (81.8%) and African American (14.3%); however, White patients had statistically worse survival outcomes than African Americans (67 vs 97 months) (p=0.004). Patients with private insurance had statistically better outcomes when compared to Medicare (p<0.001). When compared to White patients, African Americans had a higher proportion of private insurance (53.4% vs 37.2%) and lower proportion of Medicare coverage (5.2% and 48.2%), a lower average age of diagnosis (60.5 vs 64.7 years), shorter travel distances (14.7 vs 31.1 miles) and fewer days between staging procedure and surgical diagnostics from initial diagnosis (4.54 vs 12.5 days). Patients who received surgical intervention had a statistically significant improved survival outcome than those who did not (78 vs 15 months) (p<0.001) with the majority of these procedures being partial gastrectomies (53.6%). More patients of the cohort were treated at comprehensive community cancer programs (36.2%), followed by academic research programs (32.0%), integrated network cancer programs (18.5%) and community cancer programs (8.6%).
CONCLUSIONS
Factors associated with increased survival outcomes include race, average age of diagnosis, travel distance, fewer days between diagnostic procedure and initial diagnosis, insurance status and surgical treatment. These findings make a valuable contribution to the ongoing research on disparities affecting survival in patients with small intestine leiomyosarcoma.
Safety Profile of Mutant EGFR-TK Inhibitors in Advanced Non–Small Cell Lung Cancer: A Meta-analysis
Lung cancer has been the leading cause of cancer-related mortality for decades. It is also predicted to remain as the leading cause of cancer-related mortality through 2030.1 Platinum-based chemotherapy, including carboplatin and paclitaxel, was introduced 3 decades ago and revolutionized the management of advanced non–small cell lung cancer (NSCLC). A more recent advancement has been mutant epidermal growth factor receptor–tyrosine kinase (EGFR-TK) inhibitors.1 EGFR is a transmembrane protein that functions by transducing essential growth factor signaling from the extracellular milieu to the cell. As 60% of the advanced NSCLC expresses this receptor, blocking the mutant EGFR receptor was a groundbreaking development in the management of advanced NSCLC.2 Development of mutant EGFR-TK inhibitors has revolutionized the management of advanced NSCLC. This study was conducted to determine the safety profile of mutant EGFR-TK inhibitors in the management of advanced NSCLC.
Methods
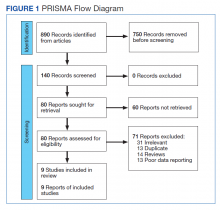
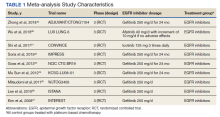
This meta-analysis was conducted according to Cochrane Collaboration guidelines and reported as per Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The findings are summarized in the PRISMA flow diagram (Figure 1). Two authors (MZ and MM) performed a systematic literature search using databases such as MEDLINE (via PubMed), Embase, and Cochrane Library using the medical search terms and their respective entry words with the following search strategy: safety, “mutant EGFR-TK inhibitors,” advanced, “non–small cell,” “lung cancer,” “adverse effect,” and literature. Additionally, unpublished trials were identified from clinicaltrials.gov, and references of all pertinent articles were also scrutinized to ensure the inclusion of all relevant studies. The search was completed on June 1, 2021, and we only included studies available in English. Two authors (MM and MZ) independently screened the search results in a 2-step process based on predetermined inclusion/exclusion criteria. First, 890 articles were evaluated for relevance on title and abstract level, followed by full-text screening of the final list of 140 articles. Any disagreements were resolved by discussion or third-party review, and a total of 9 articles were included in the study.
The following eligibility criteria were used: original articles reporting adverse effects (AEs) of mutant EGFR-TK inhibitors in patients with advanced NSCLC compared with control groups receiving platinum-based chemotherapy. All the patients included in the study had an EGFR mutation but randomly assigned to either treatment or control group. All articles with subjective data on mutant EGFR-TK inhibitors AEs in patients with advanced NSCLC compared with control groups receiving platinum-based chemotherapy were included in the analysis. Only 9 articles qualified the aforementioned selection criteria for eligibility. All qualifying studies were nationwide inpatient or pooled clinical trials data. The reasons for exclusion of the other 71 articles were irrelevant (n = 31), duplicate (n = 13), reviews (n = 14), and poor data reporting (n = 12). Out of the 9 included studies, 9 studies showed correlation of AEs, including rash, diarrhea, nausea, and fatigue. Seven studies showed correlation of AEs including neutropenia, anorexia, and vomiting. Six studies showed correlation of anemia, cough, and stomatitis. Five studies showed correlation of elevated aspartate aminotransferase (AST), alanine aminotransferase (ALT), and leucopenia. Four studies showed correlation of fever between mutant EGFR-TK inhibitors and platinum-based chemotherapy.
The primary endpoints were reported AEs including rash, diarrhea, elevated ALT, elevated AST, stomatitis, nausea, leucopenia, fatigue, neutropenia, anorexia, anemia, cough, vomiting, and fever, respectively. Data on baseline characteristics and clinical outcomes were then extracted, and summary tables were created. Summary estimates of the clinical endpoints were then calculated with risk ratio (RR) and 95% confidence intervals (CIs) using the random-effects model. Heterogeneity between studies was examined with the Cochran Q I2 statistic which can be defined as low (25% to 50%), moderate (50% to 75%), or high (> 75%). Statistical analysis was performed using Comprehensive Meta-Analysis Software CMA Version 3.0.
Results
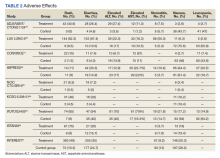
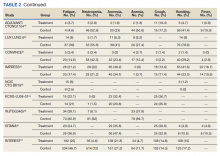
A total of 9 studies including 3415 patients (1775 in EGFR-TK inhibitor treatment group while 1640 patients in platinum-based chemotherapy control group) were included in the study. All 9 studies were phase III randomized control clinical trials conducted to compare the safety profile of mutant EGFR-TK inhibitors in patients with advanced NSCLC. Mean age was 61 years in both treatment and control groups. Further details on study and participant characteristics and safety profile including AEs are summarized in Tables 1 and 2. No evidence of publication bias was found.
Rash developed in 45.8% of patients in the treatment group receiving mutant EGFR-TK inhibitors vs only 5.6% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 7.38 with the 95% CI noted, which was statistically significant, confirming higher rash event rates in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 2).
Diarrhea occurred in 33.6% of patients in the mutant EGFR-TK inhibitors treatment group vs 13.5% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 2.63 and 95% CI was noted, which was statistically significant, confirming higher diarrheal rates in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 3).
Elevated ALT levels developed in 27.9% of patients in the treatment group receiving mutant EGFR-TK inhibitors compared with 15.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 1.37 and 95% CI was noted, which was statistically significant, confirming higher ALT levels in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 4).
Elevated AST levels occurred in 40.7% of patients in the mutant EGFR-TK inhibitors treatment group vs 12.8% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 1.77 and 95% CI was noted, which was statistically significant, confirming elevated AST levels in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 5).
Stomatitis developed in 17.2% of patients in the treatment group receiving mutant EGFR-TK inhibitors compared with 7.9% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 1.53 and 95% CI was noted, which was statistically significant, confirming higher stomatitis event rates in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 6).
Nausea occurred in 16.5% of patients in the mutant EGFR-TK inhibitors group vs 42.5% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.37 and 95% CI was noted, which was statistically significant, confirming higher nausea rates in patients receiving platinum-based chemotherapy compared with treatment group for their advanced NSCLC (Figure 7).
Leucopenia developed in 9.7% of patients in the mutant EGFR-TK inhibitors group compared with 51.3% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.18 and 95% CI was noted, which was statistically significant, confirming higher leucopenia incidence in patients receiving platinum-based chemotherapy compared with treatment group for their advanced NSCLC (Figure 8).
Fatigue was reported in 17% of patients in the mutant EGFR-TK inhibitors group compared with 29.5% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.59 and 95% CI was noted, which was statistically significant, confirming higher fatigue rates in patients receiving platinum-based chemotherapy compared with treatment group for their advanced NSCLC (Figure 9).
Neutropenia developed in 6.1% of patients in the mutant EGFR-TK inhibitors group vs 48.2% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.11 and 95% CI was noted, which was statistically significant, confirming higher neutropenia rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 10).
Anorexia developed in 21.3% of patients in the mutant EGFR-TK inhibitors group vs 31.4% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.44 and 95% CI was noted, which was statistically significant, confirming higher anorexia rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 11).
Anemia occurred in 8.7% of patients in the mutant EGFR-TK inhibitors group compared with 32.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.24 and 95% CI was noted, which was statistically significant, confirming higher anorexia rates in patients receiving platinum-based chemotherapy compared with treatment for their advanced NSCLC (Figure 12).
Cough was reported in 17.8% of patients in the mutant EGFR-TK inhibitors group compared with 18.9% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.99 and 95% CI was noted, which was statistically significant, confirming slightly higher cough rates in patients receiving platinum-based chemotherapy compared with treatment for their advanced NSCLC (Figure 13).
Vomiting developed in 11% of patients in the mutant EGFR-TK inhibitors group vs 30.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.35 and 95% CI was noted, which was statistically significant, confirming higher vomiting rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 14).
Fever occurred in 5.6% of patients in the mutant EGFR-TK inhibitors group compared with 30.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.41 and 95% CI was noted, which was statistically significant, confirming higher fever rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 15).
Discussion
Despite the advancement in the treatment of metastatic NSCLC, lung cancer stays as most common cause of cancer-related death in North America and European countries, as patients usually have an advanced disease at the time of diagnosis.3 In the past, platinum-based chemotherapy remained the standard of care for most of the patients affected with advanced NSCLC, but the higher recurrence rate and increase in frequency and intensity of AEs with platinum-based chemotherapy led to the development of targeted therapy for NSCLC, one of which includes
Smoking is the most common reversible risk factor associated with lung cancer. The EURTAC trial was the first perspective study in this regard, which compared safety and efficacy of mutant EGFR-TK inhibitors with platinum-based chemotherapy. Results analyzed in this study were in favor of mutant EGFR-TK inhibitors except in the group of former smokers.5 On the contrary, the OPTIMAL trial showed results in favor of mutant EGFR-TK inhibitors both in active and former smokers; this trial also confirmed the efficacy of mutant EGFR-TK inhibitors in European and Asian populations, confirming the rationale for routine testing of EGFR mutation in all the patients being diagnosed with advanced NSCLC.6 Similarly, osimertinib is one of the most recent mutant EGFR-TK inhibitors developed for the treatment of advanced NSCLC in patients with EGFR-positive receptors.
According to the FLAURA trial, patients receiving osimertinib showed significantly longer progression-free survival compared with platinum-based chemotherapy and early mutant EGFR-TK inhibitors. Median progression-free survival was noted to be 18.9 months, which showed 54% lower risk of disease progression in the treatment group receiving osimertinib.7 The ARCHER study emphasized a significant improvement in overall survival as well as progression-free survival among a patient population receiving dacomitinib compared with platinum-based chemotherapy.8,9
Being a potent targeted therapy, mutant EGFR-TK inhibitors do come with some AEs including diarrhea, which was seen in 33.6% of the patients receiving mutant EGFR-TK inhibitors in our study vs 53% in the chemotherapy group, as was observed in the study conducted by Pless and colleagues.10 Similarly, only 16.5% of patients receiving mutant EGFR-TK inhibitors developed nausea compared with 66% being observed in patients receiving chemotherapy. Correspondingly, only a small fraction of patients (9.7%) receiving mutant EGFR-TK inhibitors developed leucopenia, which was 10 times less reported in mutant EGFR-TK inhibitors compared with patients receiving chemotherapy having a percentage of 100%. A similar trend was reported for neutropenia and anemia in mutant EGFR-TK inhibitors with an incidence of 6.1% and 8.7%, compared with the platinum-based chemotherapy group in which the incidence was found to be 80% and 100%, respectively. It was concluded that platinum-based chemotherapy had played a vital role in the treatment of advanced NSCLC but at an expense of serious and severe AEs which led to discontinuation or withdrawal of treatment, leading to relapse and recurrence of lung cancer.10,11
Zhong and colleagues conducted a phase 2 randomized clinical trial comparing mutant EGFR-TK inhibitors with platinum-based chemotherapy. They concluded that in patients receiving platinum-based chemotherapy, incidence of rash, vomiting, anorexia, neutropenia, and nausea were 29.4%, 47%, 41.2%, 55.8%, and 32.4% compared with 45.8%, 11%, 21.3%, 6.1%, and 16.5%, respectively, reported in patients receiving mutant EGFR-TK inhibitors for their advanced NSCLC.12
Another study was conducted in 2019 by Noronha and colleagues to determine the impact of platinum-based chemotherapy combined with gefitinib on patients with advanced NSCLC.13 They concluded that 70% of the patients receiving combination treatment developed rash, which was significantly higher compared with 45.8% patients receiving the mutant EGFR-TK inhibitors alone in our study. Also, 56% of patients receiving combination therapy developed diarrhea vs 33.6% of patients receiving mutant EGFR-TK inhibitors only. Similarly, 96% of patients in the combination therapy group developed some degree of anemia compared with only 8.7% patients in the mutant EGFR-TK inhibitors group included in our study. In the same way, neutropenia was observed in 55% of patients receiving combination therapy vs 6.1% in patients receiving mutant EGFR-TK inhibitors solely. They concluded that mutant EGFR-TK inhibitors when combined with platinum-based chemotherapy increase the incidence of AEs of chemotherapy by many folds.13,14
Kato and colleagues conducted a study to determine the impact on AEs when erlotinib was combined with anti–vascular endothelial growth factor (VEGF) inhibitors like bevacizumab, they stated that 98.7% of patient in combination therapy developed rash, the incidence of which was only 45.8% in patients receiving mutant EGFR-TK inhibitors as was observed in our study. Similar trends were noticed with other AEs, including diarrhea, fatigue, nausea, and elevated liver enzymes.15
With the latest advancements in the management of advanced NSCLC, nivolumab, a programmed death ligand 1 (PD-L1) inhibitor, was developed and either used as monotherapy in patients with PD-L1 expression or was combined with platinum-based chemotherapy regardless of PD-L1 expression.16,17 Patients expressing lower PD-L1 levels were not omitted from receiving nivolumab as no significant difference was noted in progression-free span and overall survival in patients receiving nivolumab irrespective of PD-L1 levels.15 Rash developed in 17% of patients after receiving nivolumab vs 45.8% patients being observed in our study. A similar trend was observed with diarrhea as only 17% of the population receiving nivolumab developed diarrhea compared with 33.6% of the population receiving mutant EGFR-TK inhibitors in our study. Likewise, only 9.9% of the patients receiving nivolumab developed nausea as an AE compared with 16.5% being observed in mutant EGFR-TK inhibitors in our study. Also, fatigue was observed in 14.4% of the population receiving nivolumab vs 17% observed in patients receiving mutant EGFR-TK inhibitors as was noticed in our study.7,8
Rizvi and colleagues conducted a study on the role of nivolumab when combined with platinum-based chemotherapy in patients with advanced NSCLC and reported that 40% of patients included in the study developed rash compared with 45.8% reported in mutant EGFR-TK inhibitors in our study. Similarly, only 13% of patients in the nivolumab group developed diarrhea vs 33.6% cases reported in the mutant EGFR-TK inhibitors group included in our study. Also, 7% of patients in the nivolumab group developed elevated ALT levels vs 27.9% of patients receiving mutant EGFR-TK inhibitors included in our study, concluding that addition of immune checkpoint inhibitors like nivolumab to platinum-based chemotherapy does not increase the frequency of AEs.18
Conclusions
Our study focused on the safety profile of mutant EGFR-TK inhibitors vs platinum-based chemotherapy in the treatment of advanced NSCLC. Mutant EGFR-TK inhibitors are safer than platinum-based chemotherapy when compared for nausea, leucopenia, fatigue, neutropenia, anorexia, anemia, cough, vomiting, and fever. On the other end, mutant EGFR-TK inhibitors cause slightly higher AEs, including rash, diarrhea, elevated AST and ALT levels, and stomatitis. However, considering that the development of mutant EGFR-TK inhibitors laid a foundation of targeted therapy, we recommend continuing using mutant EGFR-TK inhibitors in patients with advanced NSCLC especially in patients having mutant EGFR receptors. AEs caused by mutant EGFR-TK inhibitors are significant but are usually tolerable and can be avoided by reducing the dosage of it with each cycle or by skipping or delaying the dose until the patient is symptomatic.
1. Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74(11):2913-2921. doi:10.1158/0008-5472.CAN-14-0155
2. da Cunha Santos G, Shepherd FA, Tsao MS. EGFR mutations and lung cancer. Annu Rev Pathol. 2011;6:49-69. doi:10.1146/annurev-pathol-011110-130206
3. Sgambato A, Casaluce F, Maione P, et al. The role of EGFR tyrosine kinase inhibitors in the first-line treatment of advanced non small cell lung cancer patients harboring EGFR mutation. Curr Med Chem. 2012;19(20):3337-3352. doi:10.2174/092986712801215973
4. Rossi A, Di Maio M. Platinum-based chemotherapy in advanced non–small-cell lung cancer: optimal number of treatment cycles. Expert Rev Anticancer Ther. 2016;16(6):653-660. doi:10.1586/14737140.2016.1170596
5. Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non–small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239-246. doi:10.1016/S1470-2045(11)70393-X
6. Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non–small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735-742. doi:10.1016/S1470-2045(11)70184-X
7. Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non–small-cell lung cancer. N Engl J Med. 2018;378(2):113-125. doi:10.1056/NEJMoa1713137
8. Mok TS, Cheng Y, Zhou X, et al. Improvement in overall survival in a randomized study that compared dacomitinib with gefitinib in patients with advanced non–small-cell lung cancer and EGFR-activating mutations. J Clin Oncol. 2018;36(22):2244-2250. doi:10.1200/JCO.2018.78.7994
9. Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947-957. doi:10.1056/NEJMoa0810699
10. Pless M, Stupp R, Ris HB, et al. Induction chemoradiation in stage IIIA/N2 non–small-cell lung cancer: a phase 3 randomised trial. Lancet. 2015;386(9998):1049-1056. doi:10.1016/S0140-6736(15)60294-X
11. Albain KS, Rusch VW, Crowley JJ, et al. Concurrent cisplatin/etoposide plus chest radiotherapy followed by surgery for stages IIIA (N2) and IIIB non–small-cell lung cancer: mature results of Southwest Oncology Group phase II study 8805. J Clin Oncol. 1995;13(8):1880-1892. doi:10.1200/JCO.1995.13.8.1880
12. Zhong WZ, Chen KN, Chen C, et al. Erlotinib versus gemcitabine plus cisplatin as neoadjuvant treatment of Stage IIIA-N2 EGFR-mutant non–small-cell lung cancer (EMERGING-CTONG 1103): a randomized phase II study. J Clin Oncol. 2019;37(25):2235-2245. doi:10.1200/JCO.19.00075
13. Noronha V, Patil VM, Joshi A, et al. Gefitinib versus gefitinib plus pemetrexed and carboplatin chemotherapy in EGFR-mutated lung cancer. J Clin Oncol. 2020;38(2):124-136. doi:10.1200/JCO.19.01154
14. Noronha V, Prabhash K, Thavamani A, et al. EGFR mutations in Indian lung cancer patients: clinical correlation and outcome to EGFR targeted therapy. PLoS One. 2013;8(4):e61561. Published 2013 Apr 19. doi:10.1371/journal.pone.0061561
15. Kato T, Seto T, Nishio M, et al. Erlotinib plus bevacizumab phase ll study in patients with advanced non–small-cell lung cancer (JO25567): updated safety results. Drug Saf. 2018;41(2):229-237. doi:10.1007/s40264-017-0596-0
16. Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus ipilimumab in advanced non–small-cell lung cancer. N Engl J Med. 2019;381(21):2020-2031. doi:10.1056/NEJMoa1910231
17. Hellmann MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378(22):2093-2104. doi:10.1056/NEJMoa1801946
18. Rizvi NA, Hellmann MD, Brahmer JR, et al. Nivolumab in combination with platinum-based doublet chemotherapy for first-line treatment of advanced non–small-cell lung cancer. J Clin Oncol. 2016;34(25):2969-2979. doi:10.1200/JCO.2016.66.9861
19. Zhong WZ, Wang Q, Mao WM, et al. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II-IIIA (N1-N2) EGFR-mutant NSCLC: final overall survival analysis of CTONG1104 Phase III Trial. J Clin Oncol. 2021;39(7):713-722. doi:10.1200/JCO.20.01820
20. Yang JC, Sequist LV, Geater SL, et al. Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: a combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol. 2015;16(7):830-838. doi:10.1016/S1470-2045(15)00026-1
21. Shi YK, Wang L, Han BH, et al. First-line icotinib versus cisplatin/pemetrexed plus pemetrexed maintenance therapy for patients with advanced EGFR mutation-positive lung adenocarcinoma (CONVINCE): a phase 3, open-label, randomized study. Ann Oncol. 2017;28(10):2443-2450. doi:10.1093/annonc/mdx359
22. Soria JC, Wu YL, Nakagawa K, et al. Gefitinib plus chemotherapy versus placebo plus chemotherapy in EGFR-mutation-positive non-small-cell lung cancer after progression on first-line gefitinib (IMPRESS): a phase 3 randomized trial. Lancet Oncol. 2015;16(8):990-998 doi:10.1016/S1470-2045(15)00121-7
23. Goss GD, O’Callaghan C, Lorimer I, et al. Gefitinib versus placebo in completely resected non-small-cell lung cancer: results of the NCIC CTG BR19 study. J Clin Oncol. 2013;31(27):3320-3326. doi:10.1200/JCO.2013.51.1816
24. Sun JM, Lee KH, Kim SW, et al. Gefitinib versus pemetrexed as second-line treatment in patients with non-small cell lung cancer previously treated with platinum-based chemotherapy (KCSG-LU08-01): an open-label, phase 3 trial. Cancer. 2012;118(24):6234-6242. doi:10.1200/JCO.2013.51.1816
25. Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomized phase 3 trial. Lancet Oncol. 2010;11(2):121-128. doi:10.1016/S1470-2045(09)70364-X
26. Lee DH, Park K, Kim JH, Lee JS, et al. Randomized phase III trial of gefitinib versus docetaxel in non-small cell lung cancer patients who have previously received platinum-based chemotherapy. Clin Cancer Res. 2010;16(4):1307-1314. doi:10.1158/1078-0432.CCR-09-1903
27. Kim ES, Hirsh V, Mok T, et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomized phase III trial. Lancet. 2008;22;372(9652):1809-1818. doi:10.1016/S0140-6736(08)61758-4
Lung cancer has been the leading cause of cancer-related mortality for decades. It is also predicted to remain as the leading cause of cancer-related mortality through 2030.1 Platinum-based chemotherapy, including carboplatin and paclitaxel, was introduced 3 decades ago and revolutionized the management of advanced non–small cell lung cancer (NSCLC). A more recent advancement has been mutant epidermal growth factor receptor–tyrosine kinase (EGFR-TK) inhibitors.1 EGFR is a transmembrane protein that functions by transducing essential growth factor signaling from the extracellular milieu to the cell. As 60% of the advanced NSCLC expresses this receptor, blocking the mutant EGFR receptor was a groundbreaking development in the management of advanced NSCLC.2 Development of mutant EGFR-TK inhibitors has revolutionized the management of advanced NSCLC. This study was conducted to determine the safety profile of mutant EGFR-TK inhibitors in the management of advanced NSCLC.
Methods
This meta-analysis was conducted according to Cochrane Collaboration guidelines and reported as per Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The findings are summarized in the PRISMA flow diagram (Figure 1). Two authors (MZ and MM) performed a systematic literature search using databases such as MEDLINE (via PubMed), Embase, and Cochrane Library using the medical search terms and their respective entry words with the following search strategy: safety, “mutant EGFR-TK inhibitors,” advanced, “non–small cell,” “lung cancer,” “adverse effect,” and literature. Additionally, unpublished trials were identified from clinicaltrials.gov, and references of all pertinent articles were also scrutinized to ensure the inclusion of all relevant studies. The search was completed on June 1, 2021, and we only included studies available in English. Two authors (MM and MZ) independently screened the search results in a 2-step process based on predetermined inclusion/exclusion criteria. First, 890 articles were evaluated for relevance on title and abstract level, followed by full-text screening of the final list of 140 articles. Any disagreements were resolved by discussion or third-party review, and a total of 9 articles were included in the study.
The following eligibility criteria were used: original articles reporting adverse effects (AEs) of mutant EGFR-TK inhibitors in patients with advanced NSCLC compared with control groups receiving platinum-based chemotherapy. All the patients included in the study had an EGFR mutation but randomly assigned to either treatment or control group. All articles with subjective data on mutant EGFR-TK inhibitors AEs in patients with advanced NSCLC compared with control groups receiving platinum-based chemotherapy were included in the analysis. Only 9 articles qualified the aforementioned selection criteria for eligibility. All qualifying studies were nationwide inpatient or pooled clinical trials data. The reasons for exclusion of the other 71 articles were irrelevant (n = 31), duplicate (n = 13), reviews (n = 14), and poor data reporting (n = 12). Out of the 9 included studies, 9 studies showed correlation of AEs, including rash, diarrhea, nausea, and fatigue. Seven studies showed correlation of AEs including neutropenia, anorexia, and vomiting. Six studies showed correlation of anemia, cough, and stomatitis. Five studies showed correlation of elevated aspartate aminotransferase (AST), alanine aminotransferase (ALT), and leucopenia. Four studies showed correlation of fever between mutant EGFR-TK inhibitors and platinum-based chemotherapy.
The primary endpoints were reported AEs including rash, diarrhea, elevated ALT, elevated AST, stomatitis, nausea, leucopenia, fatigue, neutropenia, anorexia, anemia, cough, vomiting, and fever, respectively. Data on baseline characteristics and clinical outcomes were then extracted, and summary tables were created. Summary estimates of the clinical endpoints were then calculated with risk ratio (RR) and 95% confidence intervals (CIs) using the random-effects model. Heterogeneity between studies was examined with the Cochran Q I2 statistic which can be defined as low (25% to 50%), moderate (50% to 75%), or high (> 75%). Statistical analysis was performed using Comprehensive Meta-Analysis Software CMA Version 3.0.
Results
A total of 9 studies including 3415 patients (1775 in EGFR-TK inhibitor treatment group while 1640 patients in platinum-based chemotherapy control group) were included in the study. All 9 studies were phase III randomized control clinical trials conducted to compare the safety profile of mutant EGFR-TK inhibitors in patients with advanced NSCLC. Mean age was 61 years in both treatment and control groups. Further details on study and participant characteristics and safety profile including AEs are summarized in Tables 1 and 2. No evidence of publication bias was found.
Rash developed in 45.8% of patients in the treatment group receiving mutant EGFR-TK inhibitors vs only 5.6% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 7.38 with the 95% CI noted, which was statistically significant, confirming higher rash event rates in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 2).
Diarrhea occurred in 33.6% of patients in the mutant EGFR-TK inhibitors treatment group vs 13.5% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 2.63 and 95% CI was noted, which was statistically significant, confirming higher diarrheal rates in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 3).
Elevated ALT levels developed in 27.9% of patients in the treatment group receiving mutant EGFR-TK inhibitors compared with 15.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 1.37 and 95% CI was noted, which was statistically significant, confirming higher ALT levels in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 4).
Elevated AST levels occurred in 40.7% of patients in the mutant EGFR-TK inhibitors treatment group vs 12.8% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 1.77 and 95% CI was noted, which was statistically significant, confirming elevated AST levels in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 5).
Stomatitis developed in 17.2% of patients in the treatment group receiving mutant EGFR-TK inhibitors compared with 7.9% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 1.53 and 95% CI was noted, which was statistically significant, confirming higher stomatitis event rates in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 6).
Nausea occurred in 16.5% of patients in the mutant EGFR-TK inhibitors group vs 42.5% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.37 and 95% CI was noted, which was statistically significant, confirming higher nausea rates in patients receiving platinum-based chemotherapy compared with treatment group for their advanced NSCLC (Figure 7).
Leucopenia developed in 9.7% of patients in the mutant EGFR-TK inhibitors group compared with 51.3% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.18 and 95% CI was noted, which was statistically significant, confirming higher leucopenia incidence in patients receiving platinum-based chemotherapy compared with treatment group for their advanced NSCLC (Figure 8).
Fatigue was reported in 17% of patients in the mutant EGFR-TK inhibitors group compared with 29.5% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.59 and 95% CI was noted, which was statistically significant, confirming higher fatigue rates in patients receiving platinum-based chemotherapy compared with treatment group for their advanced NSCLC (Figure 9).
Neutropenia developed in 6.1% of patients in the mutant EGFR-TK inhibitors group vs 48.2% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.11 and 95% CI was noted, which was statistically significant, confirming higher neutropenia rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 10).
Anorexia developed in 21.3% of patients in the mutant EGFR-TK inhibitors group vs 31.4% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.44 and 95% CI was noted, which was statistically significant, confirming higher anorexia rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 11).
Anemia occurred in 8.7% of patients in the mutant EGFR-TK inhibitors group compared with 32.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.24 and 95% CI was noted, which was statistically significant, confirming higher anorexia rates in patients receiving platinum-based chemotherapy compared with treatment for their advanced NSCLC (Figure 12).
Cough was reported in 17.8% of patients in the mutant EGFR-TK inhibitors group compared with 18.9% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.99 and 95% CI was noted, which was statistically significant, confirming slightly higher cough rates in patients receiving platinum-based chemotherapy compared with treatment for their advanced NSCLC (Figure 13).
Vomiting developed in 11% of patients in the mutant EGFR-TK inhibitors group vs 30.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.35 and 95% CI was noted, which was statistically significant, confirming higher vomiting rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 14).
Fever occurred in 5.6% of patients in the mutant EGFR-TK inhibitors group compared with 30.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.41 and 95% CI was noted, which was statistically significant, confirming higher fever rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 15).
Discussion
Despite the advancement in the treatment of metastatic NSCLC, lung cancer stays as most common cause of cancer-related death in North America and European countries, as patients usually have an advanced disease at the time of diagnosis.3 In the past, platinum-based chemotherapy remained the standard of care for most of the patients affected with advanced NSCLC, but the higher recurrence rate and increase in frequency and intensity of AEs with platinum-based chemotherapy led to the development of targeted therapy for NSCLC, one of which includes
Smoking is the most common reversible risk factor associated with lung cancer. The EURTAC trial was the first perspective study in this regard, which compared safety and efficacy of mutant EGFR-TK inhibitors with platinum-based chemotherapy. Results analyzed in this study were in favor of mutant EGFR-TK inhibitors except in the group of former smokers.5 On the contrary, the OPTIMAL trial showed results in favor of mutant EGFR-TK inhibitors both in active and former smokers; this trial also confirmed the efficacy of mutant EGFR-TK inhibitors in European and Asian populations, confirming the rationale for routine testing of EGFR mutation in all the patients being diagnosed with advanced NSCLC.6 Similarly, osimertinib is one of the most recent mutant EGFR-TK inhibitors developed for the treatment of advanced NSCLC in patients with EGFR-positive receptors.
According to the FLAURA trial, patients receiving osimertinib showed significantly longer progression-free survival compared with platinum-based chemotherapy and early mutant EGFR-TK inhibitors. Median progression-free survival was noted to be 18.9 months, which showed 54% lower risk of disease progression in the treatment group receiving osimertinib.7 The ARCHER study emphasized a significant improvement in overall survival as well as progression-free survival among a patient population receiving dacomitinib compared with platinum-based chemotherapy.8,9
Being a potent targeted therapy, mutant EGFR-TK inhibitors do come with some AEs including diarrhea, which was seen in 33.6% of the patients receiving mutant EGFR-TK inhibitors in our study vs 53% in the chemotherapy group, as was observed in the study conducted by Pless and colleagues.10 Similarly, only 16.5% of patients receiving mutant EGFR-TK inhibitors developed nausea compared with 66% being observed in patients receiving chemotherapy. Correspondingly, only a small fraction of patients (9.7%) receiving mutant EGFR-TK inhibitors developed leucopenia, which was 10 times less reported in mutant EGFR-TK inhibitors compared with patients receiving chemotherapy having a percentage of 100%. A similar trend was reported for neutropenia and anemia in mutant EGFR-TK inhibitors with an incidence of 6.1% and 8.7%, compared with the platinum-based chemotherapy group in which the incidence was found to be 80% and 100%, respectively. It was concluded that platinum-based chemotherapy had played a vital role in the treatment of advanced NSCLC but at an expense of serious and severe AEs which led to discontinuation or withdrawal of treatment, leading to relapse and recurrence of lung cancer.10,11
Zhong and colleagues conducted a phase 2 randomized clinical trial comparing mutant EGFR-TK inhibitors with platinum-based chemotherapy. They concluded that in patients receiving platinum-based chemotherapy, incidence of rash, vomiting, anorexia, neutropenia, and nausea were 29.4%, 47%, 41.2%, 55.8%, and 32.4% compared with 45.8%, 11%, 21.3%, 6.1%, and 16.5%, respectively, reported in patients receiving mutant EGFR-TK inhibitors for their advanced NSCLC.12
Another study was conducted in 2019 by Noronha and colleagues to determine the impact of platinum-based chemotherapy combined with gefitinib on patients with advanced NSCLC.13 They concluded that 70% of the patients receiving combination treatment developed rash, which was significantly higher compared with 45.8% patients receiving the mutant EGFR-TK inhibitors alone in our study. Also, 56% of patients receiving combination therapy developed diarrhea vs 33.6% of patients receiving mutant EGFR-TK inhibitors only. Similarly, 96% of patients in the combination therapy group developed some degree of anemia compared with only 8.7% patients in the mutant EGFR-TK inhibitors group included in our study. In the same way, neutropenia was observed in 55% of patients receiving combination therapy vs 6.1% in patients receiving mutant EGFR-TK inhibitors solely. They concluded that mutant EGFR-TK inhibitors when combined with platinum-based chemotherapy increase the incidence of AEs of chemotherapy by many folds.13,14
Kato and colleagues conducted a study to determine the impact on AEs when erlotinib was combined with anti–vascular endothelial growth factor (VEGF) inhibitors like bevacizumab, they stated that 98.7% of patient in combination therapy developed rash, the incidence of which was only 45.8% in patients receiving mutant EGFR-TK inhibitors as was observed in our study. Similar trends were noticed with other AEs, including diarrhea, fatigue, nausea, and elevated liver enzymes.15
With the latest advancements in the management of advanced NSCLC, nivolumab, a programmed death ligand 1 (PD-L1) inhibitor, was developed and either used as monotherapy in patients with PD-L1 expression or was combined with platinum-based chemotherapy regardless of PD-L1 expression.16,17 Patients expressing lower PD-L1 levels were not omitted from receiving nivolumab as no significant difference was noted in progression-free span and overall survival in patients receiving nivolumab irrespective of PD-L1 levels.15 Rash developed in 17% of patients after receiving nivolumab vs 45.8% patients being observed in our study. A similar trend was observed with diarrhea as only 17% of the population receiving nivolumab developed diarrhea compared with 33.6% of the population receiving mutant EGFR-TK inhibitors in our study. Likewise, only 9.9% of the patients receiving nivolumab developed nausea as an AE compared with 16.5% being observed in mutant EGFR-TK inhibitors in our study. Also, fatigue was observed in 14.4% of the population receiving nivolumab vs 17% observed in patients receiving mutant EGFR-TK inhibitors as was noticed in our study.7,8
Rizvi and colleagues conducted a study on the role of nivolumab when combined with platinum-based chemotherapy in patients with advanced NSCLC and reported that 40% of patients included in the study developed rash compared with 45.8% reported in mutant EGFR-TK inhibitors in our study. Similarly, only 13% of patients in the nivolumab group developed diarrhea vs 33.6% cases reported in the mutant EGFR-TK inhibitors group included in our study. Also, 7% of patients in the nivolumab group developed elevated ALT levels vs 27.9% of patients receiving mutant EGFR-TK inhibitors included in our study, concluding that addition of immune checkpoint inhibitors like nivolumab to platinum-based chemotherapy does not increase the frequency of AEs.18
Conclusions
Our study focused on the safety profile of mutant EGFR-TK inhibitors vs platinum-based chemotherapy in the treatment of advanced NSCLC. Mutant EGFR-TK inhibitors are safer than platinum-based chemotherapy when compared for nausea, leucopenia, fatigue, neutropenia, anorexia, anemia, cough, vomiting, and fever. On the other end, mutant EGFR-TK inhibitors cause slightly higher AEs, including rash, diarrhea, elevated AST and ALT levels, and stomatitis. However, considering that the development of mutant EGFR-TK inhibitors laid a foundation of targeted therapy, we recommend continuing using mutant EGFR-TK inhibitors in patients with advanced NSCLC especially in patients having mutant EGFR receptors. AEs caused by mutant EGFR-TK inhibitors are significant but are usually tolerable and can be avoided by reducing the dosage of it with each cycle or by skipping or delaying the dose until the patient is symptomatic.
Lung cancer has been the leading cause of cancer-related mortality for decades. It is also predicted to remain as the leading cause of cancer-related mortality through 2030.1 Platinum-based chemotherapy, including carboplatin and paclitaxel, was introduced 3 decades ago and revolutionized the management of advanced non–small cell lung cancer (NSCLC). A more recent advancement has been mutant epidermal growth factor receptor–tyrosine kinase (EGFR-TK) inhibitors.1 EGFR is a transmembrane protein that functions by transducing essential growth factor signaling from the extracellular milieu to the cell. As 60% of the advanced NSCLC expresses this receptor, blocking the mutant EGFR receptor was a groundbreaking development in the management of advanced NSCLC.2 Development of mutant EGFR-TK inhibitors has revolutionized the management of advanced NSCLC. This study was conducted to determine the safety profile of mutant EGFR-TK inhibitors in the management of advanced NSCLC.
Methods
This meta-analysis was conducted according to Cochrane Collaboration guidelines and reported as per Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The findings are summarized in the PRISMA flow diagram (Figure 1). Two authors (MZ and MM) performed a systematic literature search using databases such as MEDLINE (via PubMed), Embase, and Cochrane Library using the medical search terms and their respective entry words with the following search strategy: safety, “mutant EGFR-TK inhibitors,” advanced, “non–small cell,” “lung cancer,” “adverse effect,” and literature. Additionally, unpublished trials were identified from clinicaltrials.gov, and references of all pertinent articles were also scrutinized to ensure the inclusion of all relevant studies. The search was completed on June 1, 2021, and we only included studies available in English. Two authors (MM and MZ) independently screened the search results in a 2-step process based on predetermined inclusion/exclusion criteria. First, 890 articles were evaluated for relevance on title and abstract level, followed by full-text screening of the final list of 140 articles. Any disagreements were resolved by discussion or third-party review, and a total of 9 articles were included in the study.
The following eligibility criteria were used: original articles reporting adverse effects (AEs) of mutant EGFR-TK inhibitors in patients with advanced NSCLC compared with control groups receiving platinum-based chemotherapy. All the patients included in the study had an EGFR mutation but randomly assigned to either treatment or control group. All articles with subjective data on mutant EGFR-TK inhibitors AEs in patients with advanced NSCLC compared with control groups receiving platinum-based chemotherapy were included in the analysis. Only 9 articles qualified the aforementioned selection criteria for eligibility. All qualifying studies were nationwide inpatient or pooled clinical trials data. The reasons for exclusion of the other 71 articles were irrelevant (n = 31), duplicate (n = 13), reviews (n = 14), and poor data reporting (n = 12). Out of the 9 included studies, 9 studies showed correlation of AEs, including rash, diarrhea, nausea, and fatigue. Seven studies showed correlation of AEs including neutropenia, anorexia, and vomiting. Six studies showed correlation of anemia, cough, and stomatitis. Five studies showed correlation of elevated aspartate aminotransferase (AST), alanine aminotransferase (ALT), and leucopenia. Four studies showed correlation of fever between mutant EGFR-TK inhibitors and platinum-based chemotherapy.
The primary endpoints were reported AEs including rash, diarrhea, elevated ALT, elevated AST, stomatitis, nausea, leucopenia, fatigue, neutropenia, anorexia, anemia, cough, vomiting, and fever, respectively. Data on baseline characteristics and clinical outcomes were then extracted, and summary tables were created. Summary estimates of the clinical endpoints were then calculated with risk ratio (RR) and 95% confidence intervals (CIs) using the random-effects model. Heterogeneity between studies was examined with the Cochran Q I2 statistic which can be defined as low (25% to 50%), moderate (50% to 75%), or high (> 75%). Statistical analysis was performed using Comprehensive Meta-Analysis Software CMA Version 3.0.
Results
A total of 9 studies including 3415 patients (1775 in EGFR-TK inhibitor treatment group while 1640 patients in platinum-based chemotherapy control group) were included in the study. All 9 studies were phase III randomized control clinical trials conducted to compare the safety profile of mutant EGFR-TK inhibitors in patients with advanced NSCLC. Mean age was 61 years in both treatment and control groups. Further details on study and participant characteristics and safety profile including AEs are summarized in Tables 1 and 2. No evidence of publication bias was found.
Rash developed in 45.8% of patients in the treatment group receiving mutant EGFR-TK inhibitors vs only 5.6% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 7.38 with the 95% CI noted, which was statistically significant, confirming higher rash event rates in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 2).
Diarrhea occurred in 33.6% of patients in the mutant EGFR-TK inhibitors treatment group vs 13.5% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 2.63 and 95% CI was noted, which was statistically significant, confirming higher diarrheal rates in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 3).
Elevated ALT levels developed in 27.9% of patients in the treatment group receiving mutant EGFR-TK inhibitors compared with 15.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 1.37 and 95% CI was noted, which was statistically significant, confirming higher ALT levels in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 4).
Elevated AST levels occurred in 40.7% of patients in the mutant EGFR-TK inhibitors treatment group vs 12.8% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 1.77 and 95% CI was noted, which was statistically significant, confirming elevated AST levels in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 5).
Stomatitis developed in 17.2% of patients in the treatment group receiving mutant EGFR-TK inhibitors compared with 7.9% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 1.53 and 95% CI was noted, which was statistically significant, confirming higher stomatitis event rates in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 6).
Nausea occurred in 16.5% of patients in the mutant EGFR-TK inhibitors group vs 42.5% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.37 and 95% CI was noted, which was statistically significant, confirming higher nausea rates in patients receiving platinum-based chemotherapy compared with treatment group for their advanced NSCLC (Figure 7).
Leucopenia developed in 9.7% of patients in the mutant EGFR-TK inhibitors group compared with 51.3% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.18 and 95% CI was noted, which was statistically significant, confirming higher leucopenia incidence in patients receiving platinum-based chemotherapy compared with treatment group for their advanced NSCLC (Figure 8).
Fatigue was reported in 17% of patients in the mutant EGFR-TK inhibitors group compared with 29.5% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.59 and 95% CI was noted, which was statistically significant, confirming higher fatigue rates in patients receiving platinum-based chemotherapy compared with treatment group for their advanced NSCLC (Figure 9).
Neutropenia developed in 6.1% of patients in the mutant EGFR-TK inhibitors group vs 48.2% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.11 and 95% CI was noted, which was statistically significant, confirming higher neutropenia rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 10).
Anorexia developed in 21.3% of patients in the mutant EGFR-TK inhibitors group vs 31.4% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.44 and 95% CI was noted, which was statistically significant, confirming higher anorexia rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 11).
Anemia occurred in 8.7% of patients in the mutant EGFR-TK inhibitors group compared with 32.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.24 and 95% CI was noted, which was statistically significant, confirming higher anorexia rates in patients receiving platinum-based chemotherapy compared with treatment for their advanced NSCLC (Figure 12).
Cough was reported in 17.8% of patients in the mutant EGFR-TK inhibitors group compared with 18.9% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.99 and 95% CI was noted, which was statistically significant, confirming slightly higher cough rates in patients receiving platinum-based chemotherapy compared with treatment for their advanced NSCLC (Figure 13).
Vomiting developed in 11% of patients in the mutant EGFR-TK inhibitors group vs 30.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.35 and 95% CI was noted, which was statistically significant, confirming higher vomiting rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 14).
Fever occurred in 5.6% of patients in the mutant EGFR-TK inhibitors group compared with 30.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.41 and 95% CI was noted, which was statistically significant, confirming higher fever rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 15).
Discussion
Despite the advancement in the treatment of metastatic NSCLC, lung cancer stays as most common cause of cancer-related death in North America and European countries, as patients usually have an advanced disease at the time of diagnosis.3 In the past, platinum-based chemotherapy remained the standard of care for most of the patients affected with advanced NSCLC, but the higher recurrence rate and increase in frequency and intensity of AEs with platinum-based chemotherapy led to the development of targeted therapy for NSCLC, one of which includes
Smoking is the most common reversible risk factor associated with lung cancer. The EURTAC trial was the first perspective study in this regard, which compared safety and efficacy of mutant EGFR-TK inhibitors with platinum-based chemotherapy. Results analyzed in this study were in favor of mutant EGFR-TK inhibitors except in the group of former smokers.5 On the contrary, the OPTIMAL trial showed results in favor of mutant EGFR-TK inhibitors both in active and former smokers; this trial also confirmed the efficacy of mutant EGFR-TK inhibitors in European and Asian populations, confirming the rationale for routine testing of EGFR mutation in all the patients being diagnosed with advanced NSCLC.6 Similarly, osimertinib is one of the most recent mutant EGFR-TK inhibitors developed for the treatment of advanced NSCLC in patients with EGFR-positive receptors.
According to the FLAURA trial, patients receiving osimertinib showed significantly longer progression-free survival compared with platinum-based chemotherapy and early mutant EGFR-TK inhibitors. Median progression-free survival was noted to be 18.9 months, which showed 54% lower risk of disease progression in the treatment group receiving osimertinib.7 The ARCHER study emphasized a significant improvement in overall survival as well as progression-free survival among a patient population receiving dacomitinib compared with platinum-based chemotherapy.8,9
Being a potent targeted therapy, mutant EGFR-TK inhibitors do come with some AEs including diarrhea, which was seen in 33.6% of the patients receiving mutant EGFR-TK inhibitors in our study vs 53% in the chemotherapy group, as was observed in the study conducted by Pless and colleagues.10 Similarly, only 16.5% of patients receiving mutant EGFR-TK inhibitors developed nausea compared with 66% being observed in patients receiving chemotherapy. Correspondingly, only a small fraction of patients (9.7%) receiving mutant EGFR-TK inhibitors developed leucopenia, which was 10 times less reported in mutant EGFR-TK inhibitors compared with patients receiving chemotherapy having a percentage of 100%. A similar trend was reported for neutropenia and anemia in mutant EGFR-TK inhibitors with an incidence of 6.1% and 8.7%, compared with the platinum-based chemotherapy group in which the incidence was found to be 80% and 100%, respectively. It was concluded that platinum-based chemotherapy had played a vital role in the treatment of advanced NSCLC but at an expense of serious and severe AEs which led to discontinuation or withdrawal of treatment, leading to relapse and recurrence of lung cancer.10,11
Zhong and colleagues conducted a phase 2 randomized clinical trial comparing mutant EGFR-TK inhibitors with platinum-based chemotherapy. They concluded that in patients receiving platinum-based chemotherapy, incidence of rash, vomiting, anorexia, neutropenia, and nausea were 29.4%, 47%, 41.2%, 55.8%, and 32.4% compared with 45.8%, 11%, 21.3%, 6.1%, and 16.5%, respectively, reported in patients receiving mutant EGFR-TK inhibitors for their advanced NSCLC.12
Another study was conducted in 2019 by Noronha and colleagues to determine the impact of platinum-based chemotherapy combined with gefitinib on patients with advanced NSCLC.13 They concluded that 70% of the patients receiving combination treatment developed rash, which was significantly higher compared with 45.8% patients receiving the mutant EGFR-TK inhibitors alone in our study. Also, 56% of patients receiving combination therapy developed diarrhea vs 33.6% of patients receiving mutant EGFR-TK inhibitors only. Similarly, 96% of patients in the combination therapy group developed some degree of anemia compared with only 8.7% patients in the mutant EGFR-TK inhibitors group included in our study. In the same way, neutropenia was observed in 55% of patients receiving combination therapy vs 6.1% in patients receiving mutant EGFR-TK inhibitors solely. They concluded that mutant EGFR-TK inhibitors when combined with platinum-based chemotherapy increase the incidence of AEs of chemotherapy by many folds.13,14
Kato and colleagues conducted a study to determine the impact on AEs when erlotinib was combined with anti–vascular endothelial growth factor (VEGF) inhibitors like bevacizumab, they stated that 98.7% of patient in combination therapy developed rash, the incidence of which was only 45.8% in patients receiving mutant EGFR-TK inhibitors as was observed in our study. Similar trends were noticed with other AEs, including diarrhea, fatigue, nausea, and elevated liver enzymes.15
With the latest advancements in the management of advanced NSCLC, nivolumab, a programmed death ligand 1 (PD-L1) inhibitor, was developed and either used as monotherapy in patients with PD-L1 expression or was combined with platinum-based chemotherapy regardless of PD-L1 expression.16,17 Patients expressing lower PD-L1 levels were not omitted from receiving nivolumab as no significant difference was noted in progression-free span and overall survival in patients receiving nivolumab irrespective of PD-L1 levels.15 Rash developed in 17% of patients after receiving nivolumab vs 45.8% patients being observed in our study. A similar trend was observed with diarrhea as only 17% of the population receiving nivolumab developed diarrhea compared with 33.6% of the population receiving mutant EGFR-TK inhibitors in our study. Likewise, only 9.9% of the patients receiving nivolumab developed nausea as an AE compared with 16.5% being observed in mutant EGFR-TK inhibitors in our study. Also, fatigue was observed in 14.4% of the population receiving nivolumab vs 17% observed in patients receiving mutant EGFR-TK inhibitors as was noticed in our study.7,8
Rizvi and colleagues conducted a study on the role of nivolumab when combined with platinum-based chemotherapy in patients with advanced NSCLC and reported that 40% of patients included in the study developed rash compared with 45.8% reported in mutant EGFR-TK inhibitors in our study. Similarly, only 13% of patients in the nivolumab group developed diarrhea vs 33.6% cases reported in the mutant EGFR-TK inhibitors group included in our study. Also, 7% of patients in the nivolumab group developed elevated ALT levels vs 27.9% of patients receiving mutant EGFR-TK inhibitors included in our study, concluding that addition of immune checkpoint inhibitors like nivolumab to platinum-based chemotherapy does not increase the frequency of AEs.18
Conclusions
Our study focused on the safety profile of mutant EGFR-TK inhibitors vs platinum-based chemotherapy in the treatment of advanced NSCLC. Mutant EGFR-TK inhibitors are safer than platinum-based chemotherapy when compared for nausea, leucopenia, fatigue, neutropenia, anorexia, anemia, cough, vomiting, and fever. On the other end, mutant EGFR-TK inhibitors cause slightly higher AEs, including rash, diarrhea, elevated AST and ALT levels, and stomatitis. However, considering that the development of mutant EGFR-TK inhibitors laid a foundation of targeted therapy, we recommend continuing using mutant EGFR-TK inhibitors in patients with advanced NSCLC especially in patients having mutant EGFR receptors. AEs caused by mutant EGFR-TK inhibitors are significant but are usually tolerable and can be avoided by reducing the dosage of it with each cycle or by skipping or delaying the dose until the patient is symptomatic.
1. Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74(11):2913-2921. doi:10.1158/0008-5472.CAN-14-0155
2. da Cunha Santos G, Shepherd FA, Tsao MS. EGFR mutations and lung cancer. Annu Rev Pathol. 2011;6:49-69. doi:10.1146/annurev-pathol-011110-130206
3. Sgambato A, Casaluce F, Maione P, et al. The role of EGFR tyrosine kinase inhibitors in the first-line treatment of advanced non small cell lung cancer patients harboring EGFR mutation. Curr Med Chem. 2012;19(20):3337-3352. doi:10.2174/092986712801215973
4. Rossi A, Di Maio M. Platinum-based chemotherapy in advanced non–small-cell lung cancer: optimal number of treatment cycles. Expert Rev Anticancer Ther. 2016;16(6):653-660. doi:10.1586/14737140.2016.1170596
5. Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non–small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239-246. doi:10.1016/S1470-2045(11)70393-X
6. Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non–small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735-742. doi:10.1016/S1470-2045(11)70184-X
7. Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non–small-cell lung cancer. N Engl J Med. 2018;378(2):113-125. doi:10.1056/NEJMoa1713137
8. Mok TS, Cheng Y, Zhou X, et al. Improvement in overall survival in a randomized study that compared dacomitinib with gefitinib in patients with advanced non–small-cell lung cancer and EGFR-activating mutations. J Clin Oncol. 2018;36(22):2244-2250. doi:10.1200/JCO.2018.78.7994
9. Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947-957. doi:10.1056/NEJMoa0810699
10. Pless M, Stupp R, Ris HB, et al. Induction chemoradiation in stage IIIA/N2 non–small-cell lung cancer: a phase 3 randomised trial. Lancet. 2015;386(9998):1049-1056. doi:10.1016/S0140-6736(15)60294-X
11. Albain KS, Rusch VW, Crowley JJ, et al. Concurrent cisplatin/etoposide plus chest radiotherapy followed by surgery for stages IIIA (N2) and IIIB non–small-cell lung cancer: mature results of Southwest Oncology Group phase II study 8805. J Clin Oncol. 1995;13(8):1880-1892. doi:10.1200/JCO.1995.13.8.1880
12. Zhong WZ, Chen KN, Chen C, et al. Erlotinib versus gemcitabine plus cisplatin as neoadjuvant treatment of Stage IIIA-N2 EGFR-mutant non–small-cell lung cancer (EMERGING-CTONG 1103): a randomized phase II study. J Clin Oncol. 2019;37(25):2235-2245. doi:10.1200/JCO.19.00075
13. Noronha V, Patil VM, Joshi A, et al. Gefitinib versus gefitinib plus pemetrexed and carboplatin chemotherapy in EGFR-mutated lung cancer. J Clin Oncol. 2020;38(2):124-136. doi:10.1200/JCO.19.01154
14. Noronha V, Prabhash K, Thavamani A, et al. EGFR mutations in Indian lung cancer patients: clinical correlation and outcome to EGFR targeted therapy. PLoS One. 2013;8(4):e61561. Published 2013 Apr 19. doi:10.1371/journal.pone.0061561
15. Kato T, Seto T, Nishio M, et al. Erlotinib plus bevacizumab phase ll study in patients with advanced non–small-cell lung cancer (JO25567): updated safety results. Drug Saf. 2018;41(2):229-237. doi:10.1007/s40264-017-0596-0
16. Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus ipilimumab in advanced non–small-cell lung cancer. N Engl J Med. 2019;381(21):2020-2031. doi:10.1056/NEJMoa1910231
17. Hellmann MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378(22):2093-2104. doi:10.1056/NEJMoa1801946
18. Rizvi NA, Hellmann MD, Brahmer JR, et al. Nivolumab in combination with platinum-based doublet chemotherapy for first-line treatment of advanced non–small-cell lung cancer. J Clin Oncol. 2016;34(25):2969-2979. doi:10.1200/JCO.2016.66.9861
19. Zhong WZ, Wang Q, Mao WM, et al. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II-IIIA (N1-N2) EGFR-mutant NSCLC: final overall survival analysis of CTONG1104 Phase III Trial. J Clin Oncol. 2021;39(7):713-722. doi:10.1200/JCO.20.01820
20. Yang JC, Sequist LV, Geater SL, et al. Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: a combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol. 2015;16(7):830-838. doi:10.1016/S1470-2045(15)00026-1
21. Shi YK, Wang L, Han BH, et al. First-line icotinib versus cisplatin/pemetrexed plus pemetrexed maintenance therapy for patients with advanced EGFR mutation-positive lung adenocarcinoma (CONVINCE): a phase 3, open-label, randomized study. Ann Oncol. 2017;28(10):2443-2450. doi:10.1093/annonc/mdx359
22. Soria JC, Wu YL, Nakagawa K, et al. Gefitinib plus chemotherapy versus placebo plus chemotherapy in EGFR-mutation-positive non-small-cell lung cancer after progression on first-line gefitinib (IMPRESS): a phase 3 randomized trial. Lancet Oncol. 2015;16(8):990-998 doi:10.1016/S1470-2045(15)00121-7
23. Goss GD, O’Callaghan C, Lorimer I, et al. Gefitinib versus placebo in completely resected non-small-cell lung cancer: results of the NCIC CTG BR19 study. J Clin Oncol. 2013;31(27):3320-3326. doi:10.1200/JCO.2013.51.1816
24. Sun JM, Lee KH, Kim SW, et al. Gefitinib versus pemetrexed as second-line treatment in patients with non-small cell lung cancer previously treated with platinum-based chemotherapy (KCSG-LU08-01): an open-label, phase 3 trial. Cancer. 2012;118(24):6234-6242. doi:10.1200/JCO.2013.51.1816
25. Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomized phase 3 trial. Lancet Oncol. 2010;11(2):121-128. doi:10.1016/S1470-2045(09)70364-X
26. Lee DH, Park K, Kim JH, Lee JS, et al. Randomized phase III trial of gefitinib versus docetaxel in non-small cell lung cancer patients who have previously received platinum-based chemotherapy. Clin Cancer Res. 2010;16(4):1307-1314. doi:10.1158/1078-0432.CCR-09-1903
27. Kim ES, Hirsh V, Mok T, et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomized phase III trial. Lancet. 2008;22;372(9652):1809-1818. doi:10.1016/S0140-6736(08)61758-4
1. Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74(11):2913-2921. doi:10.1158/0008-5472.CAN-14-0155
2. da Cunha Santos G, Shepherd FA, Tsao MS. EGFR mutations and lung cancer. Annu Rev Pathol. 2011;6:49-69. doi:10.1146/annurev-pathol-011110-130206
3. Sgambato A, Casaluce F, Maione P, et al. The role of EGFR tyrosine kinase inhibitors in the first-line treatment of advanced non small cell lung cancer patients harboring EGFR mutation. Curr Med Chem. 2012;19(20):3337-3352. doi:10.2174/092986712801215973
4. Rossi A, Di Maio M. Platinum-based chemotherapy in advanced non–small-cell lung cancer: optimal number of treatment cycles. Expert Rev Anticancer Ther. 2016;16(6):653-660. doi:10.1586/14737140.2016.1170596
5. Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non–small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239-246. doi:10.1016/S1470-2045(11)70393-X
6. Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non–small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735-742. doi:10.1016/S1470-2045(11)70184-X
7. Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non–small-cell lung cancer. N Engl J Med. 2018;378(2):113-125. doi:10.1056/NEJMoa1713137
8. Mok TS, Cheng Y, Zhou X, et al. Improvement in overall survival in a randomized study that compared dacomitinib with gefitinib in patients with advanced non–small-cell lung cancer and EGFR-activating mutations. J Clin Oncol. 2018;36(22):2244-2250. doi:10.1200/JCO.2018.78.7994
9. Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947-957. doi:10.1056/NEJMoa0810699
10. Pless M, Stupp R, Ris HB, et al. Induction chemoradiation in stage IIIA/N2 non–small-cell lung cancer: a phase 3 randomised trial. Lancet. 2015;386(9998):1049-1056. doi:10.1016/S0140-6736(15)60294-X
11. Albain KS, Rusch VW, Crowley JJ, et al. Concurrent cisplatin/etoposide plus chest radiotherapy followed by surgery for stages IIIA (N2) and IIIB non–small-cell lung cancer: mature results of Southwest Oncology Group phase II study 8805. J Clin Oncol. 1995;13(8):1880-1892. doi:10.1200/JCO.1995.13.8.1880
12. Zhong WZ, Chen KN, Chen C, et al. Erlotinib versus gemcitabine plus cisplatin as neoadjuvant treatment of Stage IIIA-N2 EGFR-mutant non–small-cell lung cancer (EMERGING-CTONG 1103): a randomized phase II study. J Clin Oncol. 2019;37(25):2235-2245. doi:10.1200/JCO.19.00075
13. Noronha V, Patil VM, Joshi A, et al. Gefitinib versus gefitinib plus pemetrexed and carboplatin chemotherapy in EGFR-mutated lung cancer. J Clin Oncol. 2020;38(2):124-136. doi:10.1200/JCO.19.01154
14. Noronha V, Prabhash K, Thavamani A, et al. EGFR mutations in Indian lung cancer patients: clinical correlation and outcome to EGFR targeted therapy. PLoS One. 2013;8(4):e61561. Published 2013 Apr 19. doi:10.1371/journal.pone.0061561
15. Kato T, Seto T, Nishio M, et al. Erlotinib plus bevacizumab phase ll study in patients with advanced non–small-cell lung cancer (JO25567): updated safety results. Drug Saf. 2018;41(2):229-237. doi:10.1007/s40264-017-0596-0
16. Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus ipilimumab in advanced non–small-cell lung cancer. N Engl J Med. 2019;381(21):2020-2031. doi:10.1056/NEJMoa1910231
17. Hellmann MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378(22):2093-2104. doi:10.1056/NEJMoa1801946
18. Rizvi NA, Hellmann MD, Brahmer JR, et al. Nivolumab in combination with platinum-based doublet chemotherapy for first-line treatment of advanced non–small-cell lung cancer. J Clin Oncol. 2016;34(25):2969-2979. doi:10.1200/JCO.2016.66.9861
19. Zhong WZ, Wang Q, Mao WM, et al. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II-IIIA (N1-N2) EGFR-mutant NSCLC: final overall survival analysis of CTONG1104 Phase III Trial. J Clin Oncol. 2021;39(7):713-722. doi:10.1200/JCO.20.01820
20. Yang JC, Sequist LV, Geater SL, et al. Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: a combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol. 2015;16(7):830-838. doi:10.1016/S1470-2045(15)00026-1
21. Shi YK, Wang L, Han BH, et al. First-line icotinib versus cisplatin/pemetrexed plus pemetrexed maintenance therapy for patients with advanced EGFR mutation-positive lung adenocarcinoma (CONVINCE): a phase 3, open-label, randomized study. Ann Oncol. 2017;28(10):2443-2450. doi:10.1093/annonc/mdx359
22. Soria JC, Wu YL, Nakagawa K, et al. Gefitinib plus chemotherapy versus placebo plus chemotherapy in EGFR-mutation-positive non-small-cell lung cancer after progression on first-line gefitinib (IMPRESS): a phase 3 randomized trial. Lancet Oncol. 2015;16(8):990-998 doi:10.1016/S1470-2045(15)00121-7
23. Goss GD, O’Callaghan C, Lorimer I, et al. Gefitinib versus placebo in completely resected non-small-cell lung cancer: results of the NCIC CTG BR19 study. J Clin Oncol. 2013;31(27):3320-3326. doi:10.1200/JCO.2013.51.1816
24. Sun JM, Lee KH, Kim SW, et al. Gefitinib versus pemetrexed as second-line treatment in patients with non-small cell lung cancer previously treated with platinum-based chemotherapy (KCSG-LU08-01): an open-label, phase 3 trial. Cancer. 2012;118(24):6234-6242. doi:10.1200/JCO.2013.51.1816
25. Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomized phase 3 trial. Lancet Oncol. 2010;11(2):121-128. doi:10.1016/S1470-2045(09)70364-X
26. Lee DH, Park K, Kim JH, Lee JS, et al. Randomized phase III trial of gefitinib versus docetaxel in non-small cell lung cancer patients who have previously received platinum-based chemotherapy. Clin Cancer Res. 2010;16(4):1307-1314. doi:10.1158/1078-0432.CCR-09-1903
27. Kim ES, Hirsh V, Mok T, et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomized phase III trial. Lancet. 2008;22;372(9652):1809-1818. doi:10.1016/S0140-6736(08)61758-4