User login
MASLD: Promising treatments for a newly renamed disease
In the week leading up to this, Intercept Pharmaceuticals announced that their program to develop obeticholic acid (OCA) as a first-to-market, long-awaited potential drug therapy for metabolic dysfunction–associated steatohepatitis (MASH), the more pathogenic form of this disease, was being abandoned after the Food and Drug Administration Gastrointestinal Drugs Advisory Committee voted against accelerated approval of the drug. This decision was made in large part owing to data showing modest efficacy coupled with multiple drug-related side effects, including worsening metabolic dysfunction.
The juxtaposition of these two events highlights what could be a fundamental change in perspective when it comes to the management of patients with MASLD and the development of therapies for this disease.
A more precise nomenclature for a broad-spectrum disease
The updating of the NAFLD nomenclature to MASLD represents an important change in the way that the medical community is being asked to view and approach the disease of “fatty liver.”
The previous nomenclature clarified that pathogenic liver steatosis can exist in the absence of alcohol. However, it failed to define the primary drivers of the most common form of NAFLD: that is, metabolic syndrome diseases. The new nomenclature not only better defines the broad spectrum of steatotic liver diseases (alcohol, metabolic, drug/genetic, cryptogenic) but also refocuses the attention of providers on the fundamental basis of MASLD as a critical member of the metabolic syndrome spectrum of diseases.
This is in line with the recently updated American Association for the Study of Liver Disease Practice Guidelines for NAFLD (now MASLD), which focus on active screening to identify patients at risk for advanced MASLD, in particular those with medically complicated obesity or high-risk metabolic characteristics such as diabetes. This is a change from previous versions of the guidelines that were cautious to recommend broad screening guidelines in part owing to the lack of “available therapies.”
The increasing clinical burden of MASH has led to the recognition that patients do not have the luxury of waiting for “anticipated therapies” that have frequently shown only marginal or insignificant efficacy. As a result, some providers have refocused their efforts on the development of care pathways that can efficiently provide comprehensive lifestyle interventions to treat MASH and related metabolic comorbidities within hepatology or other subspecialty clinics.
Learning from OCA’s limitations
The approach to drug development for MASH seems to be shifting in a similar, metabolic syndrome–focused way.
The risk-benefit analysis from the FDA advisory committee evaluating OCA noted that the drug provided only modest (albeit statistically significant) benefits over placebo in achieving one of two primary clinical endpoints (improvement in fibrosis by one stage without worsening of NASH), but was associated with toxicity as well as significant drug-related side effects, including new or worsening dyslipidemia, accelerated progression to prediabetes or diabetes, and worsening glycemic control in patients with diabetes.
Worsening clinical markers of metabolic health were an important factor in the advisory committee’s decision to not provide accelerated approval of OCA. This informs the criteria upon which future MASH therapies will be evaluated for approval: that an ideal agent for MASH not only will have a significant impact on MASH pathophysiology but will also provide benefit for (or at least not worsen) the metabolic comorbidities associated with MASH.
New treatments on the horizon
Fortunately, several new MASH therapeutics in development seem to have promising metabolic profiles.
In the phase 3 MAESTRO-NASH biopsy trial, treatment with resmetirom, a thyroid hormone receptor–beta selective agonist versus placebo resulted in a clinically significant improvement in liver fibrosis by one stage or more (26% vs. 14%) and NASH resolution (30% vs. 10%). Unlike OCA, resmetirom was associated with a clinically significant reduction in LDL cholesterol (–16% compared with placebo).
Resmetirom has received a breakthrough therapy designation, and Madrigal Pharmaceuticals recently completed submission of a new drug application for accelerated approval of this agent to the FDA.
Semaglutide is a glucagonlike peptide–1 receptor agonist that is already FDA approved for treatment of diabetes and obesity and is being studied in a phase 3 analysis for NASH.
In a phase 2 trial, daily dosing of semaglutide vs placebo led to clinically significant improvement in NASH resolution (59% vs. 17%) but not significant improvement in fibrosis (43% vs 33%). Although it is impossible to make a meaningful comparison in outcomes between clinical trials, it should be noted that the absolute rate of response in this trial was higher than is routinely seen in other NASH trials and that the placebo rate for improvement in fibrosis was higher than expected, a common problem with NASH trials.
As expected, treatment with semaglutide was associated with dose-dependent reduction in body weight and hemoglobin A1c, highlighting the global metabolic benefit of this agent.
Another major class of agents being studied for NASH treatment are the fibroblast growth factor (FGF) 21 analogs.
In the recently published phase 2b ENLIVEN trial, treatment with the FGF21 analog pegozafermin versus placebo led to a statistically significant improvement in liver fibrosis by one stage (27% vs. 7%) and a numerical improvement in NASH resolution (26% vs. 2%).
In addition to its effects directly in the liver, FGF21 has been associated with promoting positive global metabolic effects, including improved peripheral insulin sensitivity and amelioration of dyslipidemia. Consistent with this, treatment with pegozafermin resulted in a decrease in serum triglycerides and an increase in serum HDL cholesterol and adiponectin levels.
Ultimately, the best therapeutic strategy for MASH may be one that uses combination therapy to maximize liver-directed effects and also ameliorate concomitant or contributing metabolic dysfunction. This approach is already being investigated. What seems clear, however, is that MASH therapy without concurrent treatment of metabolic comorbidities is probably destined to be ineffective, if not counterproductive.
So, what’s in a name? When it comes to MASLD, it seems quite a bit.
Dr. Janardhan is an assistant professor, department of internal medicine, at Rush University Medical Center, Chicago. Dr. Reau is a professor, department of internal medicine, Rush University. Dr. Janardhan reported conflicts of interest with Target RWE, Novo Nordisk, Intercept, and Enanta. Dr. Reau reported conflicts of interest with AbbVie, Gilead, and AASLD.
A version of this article appeared on Medscape.com.
In the week leading up to this, Intercept Pharmaceuticals announced that their program to develop obeticholic acid (OCA) as a first-to-market, long-awaited potential drug therapy for metabolic dysfunction–associated steatohepatitis (MASH), the more pathogenic form of this disease, was being abandoned after the Food and Drug Administration Gastrointestinal Drugs Advisory Committee voted against accelerated approval of the drug. This decision was made in large part owing to data showing modest efficacy coupled with multiple drug-related side effects, including worsening metabolic dysfunction.
The juxtaposition of these two events highlights what could be a fundamental change in perspective when it comes to the management of patients with MASLD and the development of therapies for this disease.
A more precise nomenclature for a broad-spectrum disease
The updating of the NAFLD nomenclature to MASLD represents an important change in the way that the medical community is being asked to view and approach the disease of “fatty liver.”
The previous nomenclature clarified that pathogenic liver steatosis can exist in the absence of alcohol. However, it failed to define the primary drivers of the most common form of NAFLD: that is, metabolic syndrome diseases. The new nomenclature not only better defines the broad spectrum of steatotic liver diseases (alcohol, metabolic, drug/genetic, cryptogenic) but also refocuses the attention of providers on the fundamental basis of MASLD as a critical member of the metabolic syndrome spectrum of diseases.
This is in line with the recently updated American Association for the Study of Liver Disease Practice Guidelines for NAFLD (now MASLD), which focus on active screening to identify patients at risk for advanced MASLD, in particular those with medically complicated obesity or high-risk metabolic characteristics such as diabetes. This is a change from previous versions of the guidelines that were cautious to recommend broad screening guidelines in part owing to the lack of “available therapies.”
The increasing clinical burden of MASH has led to the recognition that patients do not have the luxury of waiting for “anticipated therapies” that have frequently shown only marginal or insignificant efficacy. As a result, some providers have refocused their efforts on the development of care pathways that can efficiently provide comprehensive lifestyle interventions to treat MASH and related metabolic comorbidities within hepatology or other subspecialty clinics.
Learning from OCA’s limitations
The approach to drug development for MASH seems to be shifting in a similar, metabolic syndrome–focused way.
The risk-benefit analysis from the FDA advisory committee evaluating OCA noted that the drug provided only modest (albeit statistically significant) benefits over placebo in achieving one of two primary clinical endpoints (improvement in fibrosis by one stage without worsening of NASH), but was associated with toxicity as well as significant drug-related side effects, including new or worsening dyslipidemia, accelerated progression to prediabetes or diabetes, and worsening glycemic control in patients with diabetes.
Worsening clinical markers of metabolic health were an important factor in the advisory committee’s decision to not provide accelerated approval of OCA. This informs the criteria upon which future MASH therapies will be evaluated for approval: that an ideal agent for MASH not only will have a significant impact on MASH pathophysiology but will also provide benefit for (or at least not worsen) the metabolic comorbidities associated with MASH.
New treatments on the horizon
Fortunately, several new MASH therapeutics in development seem to have promising metabolic profiles.
In the phase 3 MAESTRO-NASH biopsy trial, treatment with resmetirom, a thyroid hormone receptor–beta selective agonist versus placebo resulted in a clinically significant improvement in liver fibrosis by one stage or more (26% vs. 14%) and NASH resolution (30% vs. 10%). Unlike OCA, resmetirom was associated with a clinically significant reduction in LDL cholesterol (–16% compared with placebo).
Resmetirom has received a breakthrough therapy designation, and Madrigal Pharmaceuticals recently completed submission of a new drug application for accelerated approval of this agent to the FDA.
Semaglutide is a glucagonlike peptide–1 receptor agonist that is already FDA approved for treatment of diabetes and obesity and is being studied in a phase 3 analysis for NASH.
In a phase 2 trial, daily dosing of semaglutide vs placebo led to clinically significant improvement in NASH resolution (59% vs. 17%) but not significant improvement in fibrosis (43% vs 33%). Although it is impossible to make a meaningful comparison in outcomes between clinical trials, it should be noted that the absolute rate of response in this trial was higher than is routinely seen in other NASH trials and that the placebo rate for improvement in fibrosis was higher than expected, a common problem with NASH trials.
As expected, treatment with semaglutide was associated with dose-dependent reduction in body weight and hemoglobin A1c, highlighting the global metabolic benefit of this agent.
Another major class of agents being studied for NASH treatment are the fibroblast growth factor (FGF) 21 analogs.
In the recently published phase 2b ENLIVEN trial, treatment with the FGF21 analog pegozafermin versus placebo led to a statistically significant improvement in liver fibrosis by one stage (27% vs. 7%) and a numerical improvement in NASH resolution (26% vs. 2%).
In addition to its effects directly in the liver, FGF21 has been associated with promoting positive global metabolic effects, including improved peripheral insulin sensitivity and amelioration of dyslipidemia. Consistent with this, treatment with pegozafermin resulted in a decrease in serum triglycerides and an increase in serum HDL cholesterol and adiponectin levels.
Ultimately, the best therapeutic strategy for MASH may be one that uses combination therapy to maximize liver-directed effects and also ameliorate concomitant or contributing metabolic dysfunction. This approach is already being investigated. What seems clear, however, is that MASH therapy without concurrent treatment of metabolic comorbidities is probably destined to be ineffective, if not counterproductive.
So, what’s in a name? When it comes to MASLD, it seems quite a bit.
Dr. Janardhan is an assistant professor, department of internal medicine, at Rush University Medical Center, Chicago. Dr. Reau is a professor, department of internal medicine, Rush University. Dr. Janardhan reported conflicts of interest with Target RWE, Novo Nordisk, Intercept, and Enanta. Dr. Reau reported conflicts of interest with AbbVie, Gilead, and AASLD.
A version of this article appeared on Medscape.com.
In the week leading up to this, Intercept Pharmaceuticals announced that their program to develop obeticholic acid (OCA) as a first-to-market, long-awaited potential drug therapy for metabolic dysfunction–associated steatohepatitis (MASH), the more pathogenic form of this disease, was being abandoned after the Food and Drug Administration Gastrointestinal Drugs Advisory Committee voted against accelerated approval of the drug. This decision was made in large part owing to data showing modest efficacy coupled with multiple drug-related side effects, including worsening metabolic dysfunction.
The juxtaposition of these two events highlights what could be a fundamental change in perspective when it comes to the management of patients with MASLD and the development of therapies for this disease.
A more precise nomenclature for a broad-spectrum disease
The updating of the NAFLD nomenclature to MASLD represents an important change in the way that the medical community is being asked to view and approach the disease of “fatty liver.”
The previous nomenclature clarified that pathogenic liver steatosis can exist in the absence of alcohol. However, it failed to define the primary drivers of the most common form of NAFLD: that is, metabolic syndrome diseases. The new nomenclature not only better defines the broad spectrum of steatotic liver diseases (alcohol, metabolic, drug/genetic, cryptogenic) but also refocuses the attention of providers on the fundamental basis of MASLD as a critical member of the metabolic syndrome spectrum of diseases.
This is in line with the recently updated American Association for the Study of Liver Disease Practice Guidelines for NAFLD (now MASLD), which focus on active screening to identify patients at risk for advanced MASLD, in particular those with medically complicated obesity or high-risk metabolic characteristics such as diabetes. This is a change from previous versions of the guidelines that were cautious to recommend broad screening guidelines in part owing to the lack of “available therapies.”
The increasing clinical burden of MASH has led to the recognition that patients do not have the luxury of waiting for “anticipated therapies” that have frequently shown only marginal or insignificant efficacy. As a result, some providers have refocused their efforts on the development of care pathways that can efficiently provide comprehensive lifestyle interventions to treat MASH and related metabolic comorbidities within hepatology or other subspecialty clinics.
Learning from OCA’s limitations
The approach to drug development for MASH seems to be shifting in a similar, metabolic syndrome–focused way.
The risk-benefit analysis from the FDA advisory committee evaluating OCA noted that the drug provided only modest (albeit statistically significant) benefits over placebo in achieving one of two primary clinical endpoints (improvement in fibrosis by one stage without worsening of NASH), but was associated with toxicity as well as significant drug-related side effects, including new or worsening dyslipidemia, accelerated progression to prediabetes or diabetes, and worsening glycemic control in patients with diabetes.
Worsening clinical markers of metabolic health were an important factor in the advisory committee’s decision to not provide accelerated approval of OCA. This informs the criteria upon which future MASH therapies will be evaluated for approval: that an ideal agent for MASH not only will have a significant impact on MASH pathophysiology but will also provide benefit for (or at least not worsen) the metabolic comorbidities associated with MASH.
New treatments on the horizon
Fortunately, several new MASH therapeutics in development seem to have promising metabolic profiles.
In the phase 3 MAESTRO-NASH biopsy trial, treatment with resmetirom, a thyroid hormone receptor–beta selective agonist versus placebo resulted in a clinically significant improvement in liver fibrosis by one stage or more (26% vs. 14%) and NASH resolution (30% vs. 10%). Unlike OCA, resmetirom was associated with a clinically significant reduction in LDL cholesterol (–16% compared with placebo).
Resmetirom has received a breakthrough therapy designation, and Madrigal Pharmaceuticals recently completed submission of a new drug application for accelerated approval of this agent to the FDA.
Semaglutide is a glucagonlike peptide–1 receptor agonist that is already FDA approved for treatment of diabetes and obesity and is being studied in a phase 3 analysis for NASH.
In a phase 2 trial, daily dosing of semaglutide vs placebo led to clinically significant improvement in NASH resolution (59% vs. 17%) but not significant improvement in fibrosis (43% vs 33%). Although it is impossible to make a meaningful comparison in outcomes between clinical trials, it should be noted that the absolute rate of response in this trial was higher than is routinely seen in other NASH trials and that the placebo rate for improvement in fibrosis was higher than expected, a common problem with NASH trials.
As expected, treatment with semaglutide was associated with dose-dependent reduction in body weight and hemoglobin A1c, highlighting the global metabolic benefit of this agent.
Another major class of agents being studied for NASH treatment are the fibroblast growth factor (FGF) 21 analogs.
In the recently published phase 2b ENLIVEN trial, treatment with the FGF21 analog pegozafermin versus placebo led to a statistically significant improvement in liver fibrosis by one stage (27% vs. 7%) and a numerical improvement in NASH resolution (26% vs. 2%).
In addition to its effects directly in the liver, FGF21 has been associated with promoting positive global metabolic effects, including improved peripheral insulin sensitivity and amelioration of dyslipidemia. Consistent with this, treatment with pegozafermin resulted in a decrease in serum triglycerides and an increase in serum HDL cholesterol and adiponectin levels.
Ultimately, the best therapeutic strategy for MASH may be one that uses combination therapy to maximize liver-directed effects and also ameliorate concomitant or contributing metabolic dysfunction. This approach is already being investigated. What seems clear, however, is that MASH therapy without concurrent treatment of metabolic comorbidities is probably destined to be ineffective, if not counterproductive.
So, what’s in a name? When it comes to MASLD, it seems quite a bit.
Dr. Janardhan is an assistant professor, department of internal medicine, at Rush University Medical Center, Chicago. Dr. Reau is a professor, department of internal medicine, Rush University. Dr. Janardhan reported conflicts of interest with Target RWE, Novo Nordisk, Intercept, and Enanta. Dr. Reau reported conflicts of interest with AbbVie, Gilead, and AASLD.
A version of this article appeared on Medscape.com.
Prostate cancer screening guidelines: To PSA or not to PSA
In the United States this year, approximately 288,300 men will be newly diagnosed with prostate cancer and about 34,700 men will die from this disease. It is the second leading cause of cancer in men, and one out of every eight men will be diagnosed with this cancer at some point in their lives.
As primary care physicians, a large part of our role is to prevent or detect cancers early. Patients look to us for this guidance. However, prostate cancer screening has long been a controversial issue. Earlier this year, the American Urological Association along with the Society of Urologic Oncology published updated guidelines.
Clear recommendations that come from this set of guidelines that are relevant to primary care physicians include:
- using PSA as the screening test of choice.
- repeating PSA in patients with newly elevated results before moving on to other test.
- offering PSA screening every 2-4 years in patients aged 50-69 years.
- offering baseline screening in those between 45-50 years of age.
In high-risk patients, screening can be initiated at 40-45 years of age. All of these recommendations come with the caveat that we give the patient all the pros and cons and leave it up to their “values and preferences.”
The guidelines make recommendations regarding PSA screening and biopsy standards. These guidelines are very specific in their recommendations; however, the question about whether to do PSA screening in the first place is left open to debate. While shared decision-making is important with any testing, it is more difficult with prostate cancer screening. Patients need to understand that there are possible adverse events that can result because of an elevated PSA, such as unneeded biopsies that may come with complications.
The authors of this set of guidelines suggest that physicians talk to patients more often about the benefits of the screening than they do about the negative consequences. This assumes that a negative biopsy result is an unnecessary test, which is not a fair assessment. Negative test results can provide useful clinical information. While a PSA result may lead to a biopsy that could have possibly been avoided, we don’t have any better screening tests available. Missing a prostate cancer that could have been detected by PSA screening is also very harmful. Deciding whether to do PSA screening for any given patient then becomes a difficult question.
More research into biomarkers to detect prostate cancer is needed, as suggested by the guideline authors. As primary care doctors, we’re the first ones to order these tests and make decisions regarding the results. While we may not be the ones to do the biopsies, we do need to know when to refer the patients to specialists or when we can just repeat the test.
Population health is often the benchmark used when looking at screening guidelines. But in the primary care setting, we are responsible for individual patients. Applying guidelines that take whole populations into consideration often doesn’t translate well to single patients. We do need to make them responsible for their own health care decisions but, at the same time, we need to offer them some guidance. If the guidelines are clear, this is easy. When they suggest giving patients all the pros and cons and letting them make their own decision, this is hard. Some of them want us to tell them what to do.
Additionally, patients in the primary care setting develop close relationships with their physicians. They are not an elevated PSA test or a negative biopsy result. They have concerns and fears. When they are high risk, the advice is easy. Keeping in mind that prostate cancer is the second leading cause of cancer in men in the United States, we should have clear screening guidelines, such as we do with mammograms in women. Yes, shared decision-making is important, but we also need to know the answer when our patients ask us whether or not they should have a PSA test done.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, N.J.
In the United States this year, approximately 288,300 men will be newly diagnosed with prostate cancer and about 34,700 men will die from this disease. It is the second leading cause of cancer in men, and one out of every eight men will be diagnosed with this cancer at some point in their lives.
As primary care physicians, a large part of our role is to prevent or detect cancers early. Patients look to us for this guidance. However, prostate cancer screening has long been a controversial issue. Earlier this year, the American Urological Association along with the Society of Urologic Oncology published updated guidelines.
Clear recommendations that come from this set of guidelines that are relevant to primary care physicians include:
- using PSA as the screening test of choice.
- repeating PSA in patients with newly elevated results before moving on to other test.
- offering PSA screening every 2-4 years in patients aged 50-69 years.
- offering baseline screening in those between 45-50 years of age.
In high-risk patients, screening can be initiated at 40-45 years of age. All of these recommendations come with the caveat that we give the patient all the pros and cons and leave it up to their “values and preferences.”
The guidelines make recommendations regarding PSA screening and biopsy standards. These guidelines are very specific in their recommendations; however, the question about whether to do PSA screening in the first place is left open to debate. While shared decision-making is important with any testing, it is more difficult with prostate cancer screening. Patients need to understand that there are possible adverse events that can result because of an elevated PSA, such as unneeded biopsies that may come with complications.
The authors of this set of guidelines suggest that physicians talk to patients more often about the benefits of the screening than they do about the negative consequences. This assumes that a negative biopsy result is an unnecessary test, which is not a fair assessment. Negative test results can provide useful clinical information. While a PSA result may lead to a biopsy that could have possibly been avoided, we don’t have any better screening tests available. Missing a prostate cancer that could have been detected by PSA screening is also very harmful. Deciding whether to do PSA screening for any given patient then becomes a difficult question.
More research into biomarkers to detect prostate cancer is needed, as suggested by the guideline authors. As primary care doctors, we’re the first ones to order these tests and make decisions regarding the results. While we may not be the ones to do the biopsies, we do need to know when to refer the patients to specialists or when we can just repeat the test.
Population health is often the benchmark used when looking at screening guidelines. But in the primary care setting, we are responsible for individual patients. Applying guidelines that take whole populations into consideration often doesn’t translate well to single patients. We do need to make them responsible for their own health care decisions but, at the same time, we need to offer them some guidance. If the guidelines are clear, this is easy. When they suggest giving patients all the pros and cons and letting them make their own decision, this is hard. Some of them want us to tell them what to do.
Additionally, patients in the primary care setting develop close relationships with their physicians. They are not an elevated PSA test or a negative biopsy result. They have concerns and fears. When they are high risk, the advice is easy. Keeping in mind that prostate cancer is the second leading cause of cancer in men in the United States, we should have clear screening guidelines, such as we do with mammograms in women. Yes, shared decision-making is important, but we also need to know the answer when our patients ask us whether or not they should have a PSA test done.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, N.J.
In the United States this year, approximately 288,300 men will be newly diagnosed with prostate cancer and about 34,700 men will die from this disease. It is the second leading cause of cancer in men, and one out of every eight men will be diagnosed with this cancer at some point in their lives.
As primary care physicians, a large part of our role is to prevent or detect cancers early. Patients look to us for this guidance. However, prostate cancer screening has long been a controversial issue. Earlier this year, the American Urological Association along with the Society of Urologic Oncology published updated guidelines.
Clear recommendations that come from this set of guidelines that are relevant to primary care physicians include:
- using PSA as the screening test of choice.
- repeating PSA in patients with newly elevated results before moving on to other test.
- offering PSA screening every 2-4 years in patients aged 50-69 years.
- offering baseline screening in those between 45-50 years of age.
In high-risk patients, screening can be initiated at 40-45 years of age. All of these recommendations come with the caveat that we give the patient all the pros and cons and leave it up to their “values and preferences.”
The guidelines make recommendations regarding PSA screening and biopsy standards. These guidelines are very specific in their recommendations; however, the question about whether to do PSA screening in the first place is left open to debate. While shared decision-making is important with any testing, it is more difficult with prostate cancer screening. Patients need to understand that there are possible adverse events that can result because of an elevated PSA, such as unneeded biopsies that may come with complications.
The authors of this set of guidelines suggest that physicians talk to patients more often about the benefits of the screening than they do about the negative consequences. This assumes that a negative biopsy result is an unnecessary test, which is not a fair assessment. Negative test results can provide useful clinical information. While a PSA result may lead to a biopsy that could have possibly been avoided, we don’t have any better screening tests available. Missing a prostate cancer that could have been detected by PSA screening is also very harmful. Deciding whether to do PSA screening for any given patient then becomes a difficult question.
More research into biomarkers to detect prostate cancer is needed, as suggested by the guideline authors. As primary care doctors, we’re the first ones to order these tests and make decisions regarding the results. While we may not be the ones to do the biopsies, we do need to know when to refer the patients to specialists or when we can just repeat the test.
Population health is often the benchmark used when looking at screening guidelines. But in the primary care setting, we are responsible for individual patients. Applying guidelines that take whole populations into consideration often doesn’t translate well to single patients. We do need to make them responsible for their own health care decisions but, at the same time, we need to offer them some guidance. If the guidelines are clear, this is easy. When they suggest giving patients all the pros and cons and letting them make their own decision, this is hard. Some of them want us to tell them what to do.
Additionally, patients in the primary care setting develop close relationships with their physicians. They are not an elevated PSA test or a negative biopsy result. They have concerns and fears. When they are high risk, the advice is easy. Keeping in mind that prostate cancer is the second leading cause of cancer in men in the United States, we should have clear screening guidelines, such as we do with mammograms in women. Yes, shared decision-making is important, but we also need to know the answer when our patients ask us whether or not they should have a PSA test done.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, N.J.
Artificial intelligence in your office
It is difficult to go through any publication or website these days without finding an article about artificial intelligence (AI). Many discuss its current status, while others speculate on potential future applications. Often, AI is described as an “existential threat to human health” by commentators who aren’t even aware of the definition of that term as Kierkegaard conceived it, the role of the individual to breathe meaning into life. Others characterize such cataclysmic predictions as “overblown and misdirected”.
The long-term potential for abuse of AI requires discussion, and should be addressed by policy makers, but that is beyond the scope of this column.
Meanwhile, with no “existential” threat to anybody.
The most popular current AI-based medical applications are automated scribes. They transcribe live consultations between physician and patient automatically and create a searchable report, plus notes for charts and billing.
I’ve written about AI scribes before, but the quality and user-friendliness of these products have improved dramatically in recent years. Language processing capabilities now permit you to speak naturally, without having to memorize specific commands. Some scribes can mimic your writing style based on sample notes that you enter into the system. Others allow you to integrate your own knowledge base, or a bibliography of research studies. With some systems, you can dictate notes directly into most EHR software, ask questions regarding medication dosages, or access a patient’s medical history from hospitals or other offices.
Current popular medical scribe products include DeepCura, DeepScribe, Nuance, Suki, Augmedix, Tali AI, Iodine Software, and ScribeLink. Amazon Web Services recently launched its own product, HealthScribe, as well. (As always, I have no financial interest in any product or service mentioned in this column.)
AI scribes aren’t entirely autonomous, of course; you need to read the output and check for potential inaccuracies. Still, users claim that they substantially reduce documentation and charting time, permitting more patient visits and less after-hours work.
AI can also be used to provide useful content for your patients. If you are not particularly good at writing, or don’t have the time for it, generative algorithms like the much-vaunted ChatGPT can generate posts, FAQs, and other informational content for your website, blog, or social media pages. You can ask for ideas about timely health topics and write general information articles, or create content specific to your location or specialty. You can use it to write emails informing your patients about upcoming office events or educate them on a range of topics, from getting their annual flu shots to scheduling regular screening skin exams.
With some of the same techniques and additional software, you can create entire videos for your website at a fraction of the cost of hiring a video production team. After using ChatGPT to write the content – for example, a 5-minute script on the importance of sunscreen in preventing skin cancer – you can employ a text-to-speech algorithm such as Revoicer to transform the script into audio content, and then a preproduction algorithm like Yepic or Synthesia to generate a video with a synthetic human.
If you are unhappy with your current online presence, you can use AI to create an entire website. Through a series of questions, AI website builders such as Wix ADI, Jimdo, Hostinger, and 10Web gather all the information needed to set up a website draft that is already personalized with medical-specific content. Most offer the option to connect to Instagram, Facebook, Google My Business, and similar sites, to which they can import your office’s logo, images, and descriptive texts.
Some of them are capable of pulling up responsive site pages that automatically adjust to the device – mobile or computer – that the visitor is using. This is important, as I’ve written before, because more than half of all searches for doctors are now made on smartphones, so the more “mobile friendly” your site is, the higher it will be ranked. You can test how easily a visitor can use your website on a mobile device with Google’s free Mobile-Friendly Test.
If you give talks at medical meetings, you know how cumbersome and time-consuming it can be to create Powerpoint presentations. Once again, AI can save you time and trouble. Presentation designers such as Presentations.AI, Deck Robot, iA Presenter, and Beautiful.AI can assemble very acceptable presentations from your primary inputs. You typically choose a template, input your basic data, and AI will format the slides and offer you visuals, animations, voice-overs, and other fancy features. You will also have flexibility in changing segments or images or sizes you don’t like.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
It is difficult to go through any publication or website these days without finding an article about artificial intelligence (AI). Many discuss its current status, while others speculate on potential future applications. Often, AI is described as an “existential threat to human health” by commentators who aren’t even aware of the definition of that term as Kierkegaard conceived it, the role of the individual to breathe meaning into life. Others characterize such cataclysmic predictions as “overblown and misdirected”.
The long-term potential for abuse of AI requires discussion, and should be addressed by policy makers, but that is beyond the scope of this column.
Meanwhile, with no “existential” threat to anybody.
The most popular current AI-based medical applications are automated scribes. They transcribe live consultations between physician and patient automatically and create a searchable report, plus notes for charts and billing.
I’ve written about AI scribes before, but the quality and user-friendliness of these products have improved dramatically in recent years. Language processing capabilities now permit you to speak naturally, without having to memorize specific commands. Some scribes can mimic your writing style based on sample notes that you enter into the system. Others allow you to integrate your own knowledge base, or a bibliography of research studies. With some systems, you can dictate notes directly into most EHR software, ask questions regarding medication dosages, or access a patient’s medical history from hospitals or other offices.
Current popular medical scribe products include DeepCura, DeepScribe, Nuance, Suki, Augmedix, Tali AI, Iodine Software, and ScribeLink. Amazon Web Services recently launched its own product, HealthScribe, as well. (As always, I have no financial interest in any product or service mentioned in this column.)
AI scribes aren’t entirely autonomous, of course; you need to read the output and check for potential inaccuracies. Still, users claim that they substantially reduce documentation and charting time, permitting more patient visits and less after-hours work.
AI can also be used to provide useful content for your patients. If you are not particularly good at writing, or don’t have the time for it, generative algorithms like the much-vaunted ChatGPT can generate posts, FAQs, and other informational content for your website, blog, or social media pages. You can ask for ideas about timely health topics and write general information articles, or create content specific to your location or specialty. You can use it to write emails informing your patients about upcoming office events or educate them on a range of topics, from getting their annual flu shots to scheduling regular screening skin exams.
With some of the same techniques and additional software, you can create entire videos for your website at a fraction of the cost of hiring a video production team. After using ChatGPT to write the content – for example, a 5-minute script on the importance of sunscreen in preventing skin cancer – you can employ a text-to-speech algorithm such as Revoicer to transform the script into audio content, and then a preproduction algorithm like Yepic or Synthesia to generate a video with a synthetic human.
If you are unhappy with your current online presence, you can use AI to create an entire website. Through a series of questions, AI website builders such as Wix ADI, Jimdo, Hostinger, and 10Web gather all the information needed to set up a website draft that is already personalized with medical-specific content. Most offer the option to connect to Instagram, Facebook, Google My Business, and similar sites, to which they can import your office’s logo, images, and descriptive texts.
Some of them are capable of pulling up responsive site pages that automatically adjust to the device – mobile or computer – that the visitor is using. This is important, as I’ve written before, because more than half of all searches for doctors are now made on smartphones, so the more “mobile friendly” your site is, the higher it will be ranked. You can test how easily a visitor can use your website on a mobile device with Google’s free Mobile-Friendly Test.
If you give talks at medical meetings, you know how cumbersome and time-consuming it can be to create Powerpoint presentations. Once again, AI can save you time and trouble. Presentation designers such as Presentations.AI, Deck Robot, iA Presenter, and Beautiful.AI can assemble very acceptable presentations from your primary inputs. You typically choose a template, input your basic data, and AI will format the slides and offer you visuals, animations, voice-overs, and other fancy features. You will also have flexibility in changing segments or images or sizes you don’t like.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
It is difficult to go through any publication or website these days without finding an article about artificial intelligence (AI). Many discuss its current status, while others speculate on potential future applications. Often, AI is described as an “existential threat to human health” by commentators who aren’t even aware of the definition of that term as Kierkegaard conceived it, the role of the individual to breathe meaning into life. Others characterize such cataclysmic predictions as “overblown and misdirected”.
The long-term potential for abuse of AI requires discussion, and should be addressed by policy makers, but that is beyond the scope of this column.
Meanwhile, with no “existential” threat to anybody.
The most popular current AI-based medical applications are automated scribes. They transcribe live consultations between physician and patient automatically and create a searchable report, plus notes for charts and billing.
I’ve written about AI scribes before, but the quality and user-friendliness of these products have improved dramatically in recent years. Language processing capabilities now permit you to speak naturally, without having to memorize specific commands. Some scribes can mimic your writing style based on sample notes that you enter into the system. Others allow you to integrate your own knowledge base, or a bibliography of research studies. With some systems, you can dictate notes directly into most EHR software, ask questions regarding medication dosages, or access a patient’s medical history from hospitals or other offices.
Current popular medical scribe products include DeepCura, DeepScribe, Nuance, Suki, Augmedix, Tali AI, Iodine Software, and ScribeLink. Amazon Web Services recently launched its own product, HealthScribe, as well. (As always, I have no financial interest in any product or service mentioned in this column.)
AI scribes aren’t entirely autonomous, of course; you need to read the output and check for potential inaccuracies. Still, users claim that they substantially reduce documentation and charting time, permitting more patient visits and less after-hours work.
AI can also be used to provide useful content for your patients. If you are not particularly good at writing, or don’t have the time for it, generative algorithms like the much-vaunted ChatGPT can generate posts, FAQs, and other informational content for your website, blog, or social media pages. You can ask for ideas about timely health topics and write general information articles, or create content specific to your location or specialty. You can use it to write emails informing your patients about upcoming office events or educate them on a range of topics, from getting their annual flu shots to scheduling regular screening skin exams.
With some of the same techniques and additional software, you can create entire videos for your website at a fraction of the cost of hiring a video production team. After using ChatGPT to write the content – for example, a 5-minute script on the importance of sunscreen in preventing skin cancer – you can employ a text-to-speech algorithm such as Revoicer to transform the script into audio content, and then a preproduction algorithm like Yepic or Synthesia to generate a video with a synthetic human.
If you are unhappy with your current online presence, you can use AI to create an entire website. Through a series of questions, AI website builders such as Wix ADI, Jimdo, Hostinger, and 10Web gather all the information needed to set up a website draft that is already personalized with medical-specific content. Most offer the option to connect to Instagram, Facebook, Google My Business, and similar sites, to which they can import your office’s logo, images, and descriptive texts.
Some of them are capable of pulling up responsive site pages that automatically adjust to the device – mobile or computer – that the visitor is using. This is important, as I’ve written before, because more than half of all searches for doctors are now made on smartphones, so the more “mobile friendly” your site is, the higher it will be ranked. You can test how easily a visitor can use your website on a mobile device with Google’s free Mobile-Friendly Test.
If you give talks at medical meetings, you know how cumbersome and time-consuming it can be to create Powerpoint presentations. Once again, AI can save you time and trouble. Presentation designers such as Presentations.AI, Deck Robot, iA Presenter, and Beautiful.AI can assemble very acceptable presentations from your primary inputs. You typically choose a template, input your basic data, and AI will format the slides and offer you visuals, animations, voice-overs, and other fancy features. You will also have flexibility in changing segments or images or sizes you don’t like.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
Don’t call them ‘private parts’
This transcript has been edited for clarity.
Today, I’d like to talk about private parts. You know: the genitals, down there.
I hate all of that. I really wish that we can get to a place where we can talk about genitals and sexual health the same way we do about high blood pressure and diabetes. In fact, when a new patient comes in and they get a new diagnosis of diabetes, you spend time explaining to them how their pancreas works. I don’t remember all the details because I’m a urologist. But you explain the details of diabetes, how it works, why therapy is important, and how it’s very important for quality of life.
I say to patients, “You have to know what parts you have in order to figure out how they drive, right?” We want them to drive better.
Let me give you an example. Many men come to see me with complaints of erectile dysfunction. They refuse to take sildenafil and tadalafil (Viagra and Cialis), saying, “Oh my gosh, those are magic pills. I won’t be a man if take them.” We all know that doesn’t make any sense. I explain to them how their penis works: “Your penis is a muscle. The muscle does two things. It contracts and it relaxes, just like your bicep. It’s just that your penis muscle is smooth muscle, which means it responds to fight or flight. It’s on the autonomic nervous system.”
I explain that if the muscle of the penis is relaxed, it fills with blood and expands. It gets big and hard, and it traps the blood. But when the muscles of the penis are contracted, when they are tight, it squeezes out all the blood, like squeezing out a sponge. So the important thing to do if you want to have good erections is to get the muscles to relax. Relaxed muscle increases erections. I get them to understand that sildenafil and tadalafil are phosphodiesterase 5 inhibitors: smooth-muscle relaxants. Instead of saying, “I need to take Viagra or Cialis because I’m broken,” it’s, “Oh hey honey, I need to take my muscle relaxants because my muscles aren’t working the way that they used to.”
In the future, I’ll go into what happens in erectile dysfunction. We’ll go into what can happen with erectile dysfunction and the many reasons why it happens. It’s getting them to understand that if we get the muscles to relax, you will have better erections. This is how the penis works. It’s why the medicine works. The patients will actually try the therapy and they’ll feel so much better about it. They’ll say, “Oh my gosh, this makes so much sense.” They work on their mental muscles to get the muscles of the penis to relax. Understanding anatomy and physiology helps them understand the treatments, which leads to better outcomes.
How about the female side? If a woman comes to see me reporting that she can’t have an orgasm, part of it is education and understanding the anatomy and physiology. The clitoris and the penis are exactly the same thing. The head of the clitoris and the head of the penis are the same. The clitoris has legs that go all the way down to the butt bone. So everyone is sitting on their genitals right now. The butt bones connect to the bottom of the clitoris or the bottom of the penis. They each have legs called crura. When you get patients to understand where their anatomy is and how it functions, they will then understand how to maximize their quality of life.
The clitoris has smooth muscle just like the penis. When that smooth muscle relaxes, it gorges with blood. When you stimulate it, it can lead to orgasm for most people. But, wait a minute. The clitoris is not inside the vagina. It’s outside. It’s behind the labia majora. If you follow the labia minora up, you get to the head of the clitoris. If patients understand that, they then will understand that penetration is not the way the majority of people orgasm.
I love pictures. I show everyone pictures in my office. They help patients to understand why vibration or outside stimulation on the vulva will allow orgasm to happen. And so instead of patients coming in saying, “I’m broken, I can’t orgasm from penetration,” or, “Dr. Rubin, I’m broken because I can’t get erections,” getting them to understand the anatomy and physiology helps them understand the treatment.
As we go forward, I’ll talk more about anatomy and physiology and how to increase the sexual health of our patients. For now though, please stop calling them private parts. Please use your understanding of anatomy and physiology to educate your patients to have better sexual health and higher quality of life. You may be the only clinician to ever do so, and it will make their life so much better.
Dr. Rubin is an assistant clinical professor, department of urology, at Georgetown University, Washington. She reported conflicts of interest with Sprout, Maternal Medical, Absorption Pharmaceuticals, GlaxoSmithKline, and Endo.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Today, I’d like to talk about private parts. You know: the genitals, down there.
I hate all of that. I really wish that we can get to a place where we can talk about genitals and sexual health the same way we do about high blood pressure and diabetes. In fact, when a new patient comes in and they get a new diagnosis of diabetes, you spend time explaining to them how their pancreas works. I don’t remember all the details because I’m a urologist. But you explain the details of diabetes, how it works, why therapy is important, and how it’s very important for quality of life.
I say to patients, “You have to know what parts you have in order to figure out how they drive, right?” We want them to drive better.
Let me give you an example. Many men come to see me with complaints of erectile dysfunction. They refuse to take sildenafil and tadalafil (Viagra and Cialis), saying, “Oh my gosh, those are magic pills. I won’t be a man if take them.” We all know that doesn’t make any sense. I explain to them how their penis works: “Your penis is a muscle. The muscle does two things. It contracts and it relaxes, just like your bicep. It’s just that your penis muscle is smooth muscle, which means it responds to fight or flight. It’s on the autonomic nervous system.”
I explain that if the muscle of the penis is relaxed, it fills with blood and expands. It gets big and hard, and it traps the blood. But when the muscles of the penis are contracted, when they are tight, it squeezes out all the blood, like squeezing out a sponge. So the important thing to do if you want to have good erections is to get the muscles to relax. Relaxed muscle increases erections. I get them to understand that sildenafil and tadalafil are phosphodiesterase 5 inhibitors: smooth-muscle relaxants. Instead of saying, “I need to take Viagra or Cialis because I’m broken,” it’s, “Oh hey honey, I need to take my muscle relaxants because my muscles aren’t working the way that they used to.”
In the future, I’ll go into what happens in erectile dysfunction. We’ll go into what can happen with erectile dysfunction and the many reasons why it happens. It’s getting them to understand that if we get the muscles to relax, you will have better erections. This is how the penis works. It’s why the medicine works. The patients will actually try the therapy and they’ll feel so much better about it. They’ll say, “Oh my gosh, this makes so much sense.” They work on their mental muscles to get the muscles of the penis to relax. Understanding anatomy and physiology helps them understand the treatments, which leads to better outcomes.
How about the female side? If a woman comes to see me reporting that she can’t have an orgasm, part of it is education and understanding the anatomy and physiology. The clitoris and the penis are exactly the same thing. The head of the clitoris and the head of the penis are the same. The clitoris has legs that go all the way down to the butt bone. So everyone is sitting on their genitals right now. The butt bones connect to the bottom of the clitoris or the bottom of the penis. They each have legs called crura. When you get patients to understand where their anatomy is and how it functions, they will then understand how to maximize their quality of life.
The clitoris has smooth muscle just like the penis. When that smooth muscle relaxes, it gorges with blood. When you stimulate it, it can lead to orgasm for most people. But, wait a minute. The clitoris is not inside the vagina. It’s outside. It’s behind the labia majora. If you follow the labia minora up, you get to the head of the clitoris. If patients understand that, they then will understand that penetration is not the way the majority of people orgasm.
I love pictures. I show everyone pictures in my office. They help patients to understand why vibration or outside stimulation on the vulva will allow orgasm to happen. And so instead of patients coming in saying, “I’m broken, I can’t orgasm from penetration,” or, “Dr. Rubin, I’m broken because I can’t get erections,” getting them to understand the anatomy and physiology helps them understand the treatment.
As we go forward, I’ll talk more about anatomy and physiology and how to increase the sexual health of our patients. For now though, please stop calling them private parts. Please use your understanding of anatomy and physiology to educate your patients to have better sexual health and higher quality of life. You may be the only clinician to ever do so, and it will make their life so much better.
Dr. Rubin is an assistant clinical professor, department of urology, at Georgetown University, Washington. She reported conflicts of interest with Sprout, Maternal Medical, Absorption Pharmaceuticals, GlaxoSmithKline, and Endo.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Today, I’d like to talk about private parts. You know: the genitals, down there.
I hate all of that. I really wish that we can get to a place where we can talk about genitals and sexual health the same way we do about high blood pressure and diabetes. In fact, when a new patient comes in and they get a new diagnosis of diabetes, you spend time explaining to them how their pancreas works. I don’t remember all the details because I’m a urologist. But you explain the details of diabetes, how it works, why therapy is important, and how it’s very important for quality of life.
I say to patients, “You have to know what parts you have in order to figure out how they drive, right?” We want them to drive better.
Let me give you an example. Many men come to see me with complaints of erectile dysfunction. They refuse to take sildenafil and tadalafil (Viagra and Cialis), saying, “Oh my gosh, those are magic pills. I won’t be a man if take them.” We all know that doesn’t make any sense. I explain to them how their penis works: “Your penis is a muscle. The muscle does two things. It contracts and it relaxes, just like your bicep. It’s just that your penis muscle is smooth muscle, which means it responds to fight or flight. It’s on the autonomic nervous system.”
I explain that if the muscle of the penis is relaxed, it fills with blood and expands. It gets big and hard, and it traps the blood. But when the muscles of the penis are contracted, when they are tight, it squeezes out all the blood, like squeezing out a sponge. So the important thing to do if you want to have good erections is to get the muscles to relax. Relaxed muscle increases erections. I get them to understand that sildenafil and tadalafil are phosphodiesterase 5 inhibitors: smooth-muscle relaxants. Instead of saying, “I need to take Viagra or Cialis because I’m broken,” it’s, “Oh hey honey, I need to take my muscle relaxants because my muscles aren’t working the way that they used to.”
In the future, I’ll go into what happens in erectile dysfunction. We’ll go into what can happen with erectile dysfunction and the many reasons why it happens. It’s getting them to understand that if we get the muscles to relax, you will have better erections. This is how the penis works. It’s why the medicine works. The patients will actually try the therapy and they’ll feel so much better about it. They’ll say, “Oh my gosh, this makes so much sense.” They work on their mental muscles to get the muscles of the penis to relax. Understanding anatomy and physiology helps them understand the treatments, which leads to better outcomes.
How about the female side? If a woman comes to see me reporting that she can’t have an orgasm, part of it is education and understanding the anatomy and physiology. The clitoris and the penis are exactly the same thing. The head of the clitoris and the head of the penis are the same. The clitoris has legs that go all the way down to the butt bone. So everyone is sitting on their genitals right now. The butt bones connect to the bottom of the clitoris or the bottom of the penis. They each have legs called crura. When you get patients to understand where their anatomy is and how it functions, they will then understand how to maximize their quality of life.
The clitoris has smooth muscle just like the penis. When that smooth muscle relaxes, it gorges with blood. When you stimulate it, it can lead to orgasm for most people. But, wait a minute. The clitoris is not inside the vagina. It’s outside. It’s behind the labia majora. If you follow the labia minora up, you get to the head of the clitoris. If patients understand that, they then will understand that penetration is not the way the majority of people orgasm.
I love pictures. I show everyone pictures in my office. They help patients to understand why vibration or outside stimulation on the vulva will allow orgasm to happen. And so instead of patients coming in saying, “I’m broken, I can’t orgasm from penetration,” or, “Dr. Rubin, I’m broken because I can’t get erections,” getting them to understand the anatomy and physiology helps them understand the treatment.
As we go forward, I’ll talk more about anatomy and physiology and how to increase the sexual health of our patients. For now though, please stop calling them private parts. Please use your understanding of anatomy and physiology to educate your patients to have better sexual health and higher quality of life. You may be the only clinician to ever do so, and it will make their life so much better.
Dr. Rubin is an assistant clinical professor, department of urology, at Georgetown University, Washington. She reported conflicts of interest with Sprout, Maternal Medical, Absorption Pharmaceuticals, GlaxoSmithKline, and Endo.
A version of this article first appeared on Medscape.com.
Psychedelic experience and “oneiroid” state
David Nichols, PhD, a leading expert in the field of psychedelic research and the founding president of the Heffter Research Institute, often answers the question, “What is a psychedelic?” by saying, “The scientific definition I always use is that they are substances that produce changes in thought, mood, and affect, which only occur during dreaming or religious exaltation.”1,2 However, this definition does not account for the experiences of naturally occurring psychoses, such as those seen in schizophrenia.
The phenomenology of psychotic experiences between drug-induced and naturally occurring psychoses differs, and the use of psychedelics does not necessarily replicate or simulate the symptoms of schizophrenia. For example, drug induced hallucinations are often described as more intense and vivid, while those associated with schizophrenia are described as more persistent and distressing.3
An altered state of consciousness is a hallmark of a psychedelic trip, but not a characteristic of schizophrenia. Patients with schizophrenia who have used psychedelics typically report that their experiences under the influence of these drugs are distinct from their usual experiences with the condition. Additionally, according to many experts, the underlying neurobiological mechanisms are different, with psychedelics affecting serotonin receptors and schizophrenia thought to be linked more to dopamine dysregulation, among other factors.
However, there are some instances of naturally occurring psychoses that are difficult to distinguish from the experiences reported by those who take psychedelics. This article explores the similarities between psychedelic experiences induced by serotonergic psychedelics, 5-HT2A agonists, such as LSD, psilocybin, and mescaline, and a rare psychiatric disorder known as the “oneiroid state.”
Origins and definitions
The term “oneiroia” is derived from the Greek words for “sleep” and “similar,” referring to the dreamlike character of the condition. It is widely acknowledged that psychedelics can temporarily induce dreamlike states,4 but this article focuses specifically on the naturally occurring, endogenous dreamlike state.
Oneiroid state was first described by German psychiatrist Wilhelm Mayer-Gross in the early 20th century and was once well-known among European psychiatrists. Some classified it as a part of schizophrenia, others saw it as an unusual manifestation of affective disorder, and still others considered it an atypical psychosis. Nevertheless, this phenomenon has received limited attention in American psychiatric journals.
The key characteristic of this condition is a distinctive state of consciousness marked by vivid and florid hallucinations, and a succession of constantly shifting dreamlike or surrealistic visuals and imagery often similar to mystical or cosmic experiences. Self-awareness and orientation in time and place are often disturbed, and delusions are experienced within this altered state.5
To gain a better understanding of the oneiroid state, it may be helpful to turn to European or other schools of psychiatry with a history of studying this phenomenon, as American psychiatry has limited knowledge in this area.
As described in the “Handbook of Psychiatry” by Russian psychiatrist A.V. Snezhnevsky, published in 1983, oneiroid state, also known as oneiroid syndrome, is a dreamlike and imaginative state characterized by a bizarre combination of reality and vivid phantasmagoric imaginations.6 In this state, individuals are completely detached from their surroundings and experience a change in self-awareness, often displaying either a lack of movement or senseless excitement.
Patients often experience the oneiroid state as active participants, as if they are in a movie theater, not only watching the story, but also being part of it, reacting to it with either “external immobility” or senseless excitement, completely detached from their surroundings. This is similar to the portrayal of the emotions of the characters in Steven Spielberg’s film “Ready Player One.”
The entry in Dr. Snezhnevsky’s “Handbook” states: “Some patients in Oneiroid State experience travels to other worlds, such as interacting with inhabitants on Mars, collecting gems on the moon, exploring invisible cities, participating in conspiracies and insurrections, fighting with pirates, chasing The Flying Dutchman, wandering through ancient Rome, and even visiting heaven or hell. At times, the patient’s imagination reaches a state of mystical contemplation.”6
In contrast to delirium, which never impairs self-awareness, oneiroid state is marked by drastic changes in the sense of self. The memory of the subjective experience during the oneiroia is much more vivid and consistent than in delirium. Patients who have experienced the oneiroid state often have complete recall of their experience, as if they have just woken up from a dream.
It was commonly thought that oneiroid state was part of the group of functional psychoses, rather than organic psychoses, and was not considered to be a result of mind-altering psychedelics. It was not considered a manifestation of epilepsy either. Oneiroid state could last for weeks or even months, making it unlikely to be related to an epileptic seizure. Furthermore, EEG results did not show any seizurelike activity during the state.
An excellent case study on oneiroid state was published in Israel in 2000. The authors described two patients who experienced the oneiroid state for several days or even weeks.7 One of the patients reported that during the illness, he experienced himself aboard a spaceship as a cosmonaut, heading for a different universe. On another occasion, the patient perceived himself as a person living 2,000 years ago and being guilty of Christ’s death.
The second patient reported that everything around her appeared “like in the movies,” and she saw others as characters from comic strips. Both patients alternated between catatonic excitement and sluggishness and would sometimes come back to reality for a few minutes to respond to questions. Physical exams, laboratory tests, neurological tests, and a brain scan were all normal in both cases.
Conclusions
As mentioned earlier, oneiroid state is not widely discussed in American psychiatric journals and is now considered a rare condition globally, despite being a common occurrence in the past century. A diagnosis similar to oneiroid state, known as bouffée délirante, is still in use in some French-speaking countries, with a noticeable decline in frequency.2,8 The widespread acceptance of international classification systems such as the ICD-10, which does not recognize the diagnosis, is likely one of the reasons for this decline.
However, the decrease in the prevalence of the oneiroid state is not unique, as other forms of mental illness, such as the catatonic subtype of schizophrenia, are also becoming less prevalent. The cause of this decline is uncertain. Could changes in the way mental disorders affect 5HT2A receptors be a contributing factor?
In conclusion, as the field of psychedelic research experiences a resurgence, this little-known manifestation of mental illness, oneiroid state, may be worth reexamining.
With the expected approval and regulation of psilocybin and MDMA by the FDA, and classical psychedelics widely regarded as nonaddictive and safer than other recreational drugs, , rather than as a cause for concern.
Dr. Khetsuriani is a supervising psychiatrist at the Bronx Psychiatric Center in Bronx, N.Y., and has a private psychiatric practice located in Manhattan, N.Y.
References
1. Nichols DE. Keynote address, 39th Telluride Mushroom Festival, Aug. 15-18, 2019. https://www.youtube.com/watch?v=RlDCM5JQzRk.
2. Nichols DE. Psychedelics. Pharmacol Rev. 2016 Apr;68(2):264-355. doi: 10.1124/pr.115.011478.
3. Leptourgos P et al. Hallucinations Under Psychedelics and in the Schizophrenia Spectrum: An Interdisciplinary and Multiscale Comparison. Schizophr Bull. 2020 Dec 1;46(6):1396-1408. doi: 10.1093/schbul/sbaa117.
4. Kraehenmann R. Dreams and Psychedelics: Neurophenomenological Comparison and Therapeutic Implications. Curr Neuropharmacol. 2017;15(7):1032-42. doi: 10.2174/1573413713666170619092629.
5. Henri EY et al. “Acute Delusional Psychosis” in Hirsch SR and Shepherd M, eds. Themes and variations in European psychiatry: An anthology. Charlottesville: University Press of Virginia, 1974.
6. Snezhnevsky A, ed. Handbook of Psychiatry. Moscow: Meditsina, 1983.
7. Kaptsan A et al. Oneiroid syndrome: A concept of use for Western psychiatry. Isr J Psychiatry Relat Sci. 2000;37(4):278-85.
8. https://en.wikipedia.org/wiki/Bouff%C3%A9e_d%C3%A9lirante.
David Nichols, PhD, a leading expert in the field of psychedelic research and the founding president of the Heffter Research Institute, often answers the question, “What is a psychedelic?” by saying, “The scientific definition I always use is that they are substances that produce changes in thought, mood, and affect, which only occur during dreaming or religious exaltation.”1,2 However, this definition does not account for the experiences of naturally occurring psychoses, such as those seen in schizophrenia.
The phenomenology of psychotic experiences between drug-induced and naturally occurring psychoses differs, and the use of psychedelics does not necessarily replicate or simulate the symptoms of schizophrenia. For example, drug induced hallucinations are often described as more intense and vivid, while those associated with schizophrenia are described as more persistent and distressing.3
An altered state of consciousness is a hallmark of a psychedelic trip, but not a characteristic of schizophrenia. Patients with schizophrenia who have used psychedelics typically report that their experiences under the influence of these drugs are distinct from their usual experiences with the condition. Additionally, according to many experts, the underlying neurobiological mechanisms are different, with psychedelics affecting serotonin receptors and schizophrenia thought to be linked more to dopamine dysregulation, among other factors.
However, there are some instances of naturally occurring psychoses that are difficult to distinguish from the experiences reported by those who take psychedelics. This article explores the similarities between psychedelic experiences induced by serotonergic psychedelics, 5-HT2A agonists, such as LSD, psilocybin, and mescaline, and a rare psychiatric disorder known as the “oneiroid state.”
Origins and definitions
The term “oneiroia” is derived from the Greek words for “sleep” and “similar,” referring to the dreamlike character of the condition. It is widely acknowledged that psychedelics can temporarily induce dreamlike states,4 but this article focuses specifically on the naturally occurring, endogenous dreamlike state.
Oneiroid state was first described by German psychiatrist Wilhelm Mayer-Gross in the early 20th century and was once well-known among European psychiatrists. Some classified it as a part of schizophrenia, others saw it as an unusual manifestation of affective disorder, and still others considered it an atypical psychosis. Nevertheless, this phenomenon has received limited attention in American psychiatric journals.
The key characteristic of this condition is a distinctive state of consciousness marked by vivid and florid hallucinations, and a succession of constantly shifting dreamlike or surrealistic visuals and imagery often similar to mystical or cosmic experiences. Self-awareness and orientation in time and place are often disturbed, and delusions are experienced within this altered state.5
To gain a better understanding of the oneiroid state, it may be helpful to turn to European or other schools of psychiatry with a history of studying this phenomenon, as American psychiatry has limited knowledge in this area.
As described in the “Handbook of Psychiatry” by Russian psychiatrist A.V. Snezhnevsky, published in 1983, oneiroid state, also known as oneiroid syndrome, is a dreamlike and imaginative state characterized by a bizarre combination of reality and vivid phantasmagoric imaginations.6 In this state, individuals are completely detached from their surroundings and experience a change in self-awareness, often displaying either a lack of movement or senseless excitement.
Patients often experience the oneiroid state as active participants, as if they are in a movie theater, not only watching the story, but also being part of it, reacting to it with either “external immobility” or senseless excitement, completely detached from their surroundings. This is similar to the portrayal of the emotions of the characters in Steven Spielberg’s film “Ready Player One.”
The entry in Dr. Snezhnevsky’s “Handbook” states: “Some patients in Oneiroid State experience travels to other worlds, such as interacting with inhabitants on Mars, collecting gems on the moon, exploring invisible cities, participating in conspiracies and insurrections, fighting with pirates, chasing The Flying Dutchman, wandering through ancient Rome, and even visiting heaven or hell. At times, the patient’s imagination reaches a state of mystical contemplation.”6
In contrast to delirium, which never impairs self-awareness, oneiroid state is marked by drastic changes in the sense of self. The memory of the subjective experience during the oneiroia is much more vivid and consistent than in delirium. Patients who have experienced the oneiroid state often have complete recall of their experience, as if they have just woken up from a dream.
It was commonly thought that oneiroid state was part of the group of functional psychoses, rather than organic psychoses, and was not considered to be a result of mind-altering psychedelics. It was not considered a manifestation of epilepsy either. Oneiroid state could last for weeks or even months, making it unlikely to be related to an epileptic seizure. Furthermore, EEG results did not show any seizurelike activity during the state.
An excellent case study on oneiroid state was published in Israel in 2000. The authors described two patients who experienced the oneiroid state for several days or even weeks.7 One of the patients reported that during the illness, he experienced himself aboard a spaceship as a cosmonaut, heading for a different universe. On another occasion, the patient perceived himself as a person living 2,000 years ago and being guilty of Christ’s death.
The second patient reported that everything around her appeared “like in the movies,” and she saw others as characters from comic strips. Both patients alternated between catatonic excitement and sluggishness and would sometimes come back to reality for a few minutes to respond to questions. Physical exams, laboratory tests, neurological tests, and a brain scan were all normal in both cases.
Conclusions
As mentioned earlier, oneiroid state is not widely discussed in American psychiatric journals and is now considered a rare condition globally, despite being a common occurrence in the past century. A diagnosis similar to oneiroid state, known as bouffée délirante, is still in use in some French-speaking countries, with a noticeable decline in frequency.2,8 The widespread acceptance of international classification systems such as the ICD-10, which does not recognize the diagnosis, is likely one of the reasons for this decline.
However, the decrease in the prevalence of the oneiroid state is not unique, as other forms of mental illness, such as the catatonic subtype of schizophrenia, are also becoming less prevalent. The cause of this decline is uncertain. Could changes in the way mental disorders affect 5HT2A receptors be a contributing factor?
In conclusion, as the field of psychedelic research experiences a resurgence, this little-known manifestation of mental illness, oneiroid state, may be worth reexamining.
With the expected approval and regulation of psilocybin and MDMA by the FDA, and classical psychedelics widely regarded as nonaddictive and safer than other recreational drugs, , rather than as a cause for concern.
Dr. Khetsuriani is a supervising psychiatrist at the Bronx Psychiatric Center in Bronx, N.Y., and has a private psychiatric practice located in Manhattan, N.Y.
References
1. Nichols DE. Keynote address, 39th Telluride Mushroom Festival, Aug. 15-18, 2019. https://www.youtube.com/watch?v=RlDCM5JQzRk.
2. Nichols DE. Psychedelics. Pharmacol Rev. 2016 Apr;68(2):264-355. doi: 10.1124/pr.115.011478.
3. Leptourgos P et al. Hallucinations Under Psychedelics and in the Schizophrenia Spectrum: An Interdisciplinary and Multiscale Comparison. Schizophr Bull. 2020 Dec 1;46(6):1396-1408. doi: 10.1093/schbul/sbaa117.
4. Kraehenmann R. Dreams and Psychedelics: Neurophenomenological Comparison and Therapeutic Implications. Curr Neuropharmacol. 2017;15(7):1032-42. doi: 10.2174/1573413713666170619092629.
5. Henri EY et al. “Acute Delusional Psychosis” in Hirsch SR and Shepherd M, eds. Themes and variations in European psychiatry: An anthology. Charlottesville: University Press of Virginia, 1974.
6. Snezhnevsky A, ed. Handbook of Psychiatry. Moscow: Meditsina, 1983.
7. Kaptsan A et al. Oneiroid syndrome: A concept of use for Western psychiatry. Isr J Psychiatry Relat Sci. 2000;37(4):278-85.
8. https://en.wikipedia.org/wiki/Bouff%C3%A9e_d%C3%A9lirante.
David Nichols, PhD, a leading expert in the field of psychedelic research and the founding president of the Heffter Research Institute, often answers the question, “What is a psychedelic?” by saying, “The scientific definition I always use is that they are substances that produce changes in thought, mood, and affect, which only occur during dreaming or religious exaltation.”1,2 However, this definition does not account for the experiences of naturally occurring psychoses, such as those seen in schizophrenia.
The phenomenology of psychotic experiences between drug-induced and naturally occurring psychoses differs, and the use of psychedelics does not necessarily replicate or simulate the symptoms of schizophrenia. For example, drug induced hallucinations are often described as more intense and vivid, while those associated with schizophrenia are described as more persistent and distressing.3
An altered state of consciousness is a hallmark of a psychedelic trip, but not a characteristic of schizophrenia. Patients with schizophrenia who have used psychedelics typically report that their experiences under the influence of these drugs are distinct from their usual experiences with the condition. Additionally, according to many experts, the underlying neurobiological mechanisms are different, with psychedelics affecting serotonin receptors and schizophrenia thought to be linked more to dopamine dysregulation, among other factors.
However, there are some instances of naturally occurring psychoses that are difficult to distinguish from the experiences reported by those who take psychedelics. This article explores the similarities between psychedelic experiences induced by serotonergic psychedelics, 5-HT2A agonists, such as LSD, psilocybin, and mescaline, and a rare psychiatric disorder known as the “oneiroid state.”
Origins and definitions
The term “oneiroia” is derived from the Greek words for “sleep” and “similar,” referring to the dreamlike character of the condition. It is widely acknowledged that psychedelics can temporarily induce dreamlike states,4 but this article focuses specifically on the naturally occurring, endogenous dreamlike state.
Oneiroid state was first described by German psychiatrist Wilhelm Mayer-Gross in the early 20th century and was once well-known among European psychiatrists. Some classified it as a part of schizophrenia, others saw it as an unusual manifestation of affective disorder, and still others considered it an atypical psychosis. Nevertheless, this phenomenon has received limited attention in American psychiatric journals.
The key characteristic of this condition is a distinctive state of consciousness marked by vivid and florid hallucinations, and a succession of constantly shifting dreamlike or surrealistic visuals and imagery often similar to mystical or cosmic experiences. Self-awareness and orientation in time and place are often disturbed, and delusions are experienced within this altered state.5
To gain a better understanding of the oneiroid state, it may be helpful to turn to European or other schools of psychiatry with a history of studying this phenomenon, as American psychiatry has limited knowledge in this area.
As described in the “Handbook of Psychiatry” by Russian psychiatrist A.V. Snezhnevsky, published in 1983, oneiroid state, also known as oneiroid syndrome, is a dreamlike and imaginative state characterized by a bizarre combination of reality and vivid phantasmagoric imaginations.6 In this state, individuals are completely detached from their surroundings and experience a change in self-awareness, often displaying either a lack of movement or senseless excitement.
Patients often experience the oneiroid state as active participants, as if they are in a movie theater, not only watching the story, but also being part of it, reacting to it with either “external immobility” or senseless excitement, completely detached from their surroundings. This is similar to the portrayal of the emotions of the characters in Steven Spielberg’s film “Ready Player One.”
The entry in Dr. Snezhnevsky’s “Handbook” states: “Some patients in Oneiroid State experience travels to other worlds, such as interacting with inhabitants on Mars, collecting gems on the moon, exploring invisible cities, participating in conspiracies and insurrections, fighting with pirates, chasing The Flying Dutchman, wandering through ancient Rome, and even visiting heaven or hell. At times, the patient’s imagination reaches a state of mystical contemplation.”6
In contrast to delirium, which never impairs self-awareness, oneiroid state is marked by drastic changes in the sense of self. The memory of the subjective experience during the oneiroia is much more vivid and consistent than in delirium. Patients who have experienced the oneiroid state often have complete recall of their experience, as if they have just woken up from a dream.
It was commonly thought that oneiroid state was part of the group of functional psychoses, rather than organic psychoses, and was not considered to be a result of mind-altering psychedelics. It was not considered a manifestation of epilepsy either. Oneiroid state could last for weeks or even months, making it unlikely to be related to an epileptic seizure. Furthermore, EEG results did not show any seizurelike activity during the state.
An excellent case study on oneiroid state was published in Israel in 2000. The authors described two patients who experienced the oneiroid state for several days or even weeks.7 One of the patients reported that during the illness, he experienced himself aboard a spaceship as a cosmonaut, heading for a different universe. On another occasion, the patient perceived himself as a person living 2,000 years ago and being guilty of Christ’s death.
The second patient reported that everything around her appeared “like in the movies,” and she saw others as characters from comic strips. Both patients alternated between catatonic excitement and sluggishness and would sometimes come back to reality for a few minutes to respond to questions. Physical exams, laboratory tests, neurological tests, and a brain scan were all normal in both cases.
Conclusions
As mentioned earlier, oneiroid state is not widely discussed in American psychiatric journals and is now considered a rare condition globally, despite being a common occurrence in the past century. A diagnosis similar to oneiroid state, known as bouffée délirante, is still in use in some French-speaking countries, with a noticeable decline in frequency.2,8 The widespread acceptance of international classification systems such as the ICD-10, which does not recognize the diagnosis, is likely one of the reasons for this decline.
However, the decrease in the prevalence of the oneiroid state is not unique, as other forms of mental illness, such as the catatonic subtype of schizophrenia, are also becoming less prevalent. The cause of this decline is uncertain. Could changes in the way mental disorders affect 5HT2A receptors be a contributing factor?
In conclusion, as the field of psychedelic research experiences a resurgence, this little-known manifestation of mental illness, oneiroid state, may be worth reexamining.
With the expected approval and regulation of psilocybin and MDMA by the FDA, and classical psychedelics widely regarded as nonaddictive and safer than other recreational drugs, , rather than as a cause for concern.
Dr. Khetsuriani is a supervising psychiatrist at the Bronx Psychiatric Center in Bronx, N.Y., and has a private psychiatric practice located in Manhattan, N.Y.
References
1. Nichols DE. Keynote address, 39th Telluride Mushroom Festival, Aug. 15-18, 2019. https://www.youtube.com/watch?v=RlDCM5JQzRk.
2. Nichols DE. Psychedelics. Pharmacol Rev. 2016 Apr;68(2):264-355. doi: 10.1124/pr.115.011478.
3. Leptourgos P et al. Hallucinations Under Psychedelics and in the Schizophrenia Spectrum: An Interdisciplinary and Multiscale Comparison. Schizophr Bull. 2020 Dec 1;46(6):1396-1408. doi: 10.1093/schbul/sbaa117.
4. Kraehenmann R. Dreams and Psychedelics: Neurophenomenological Comparison and Therapeutic Implications. Curr Neuropharmacol. 2017;15(7):1032-42. doi: 10.2174/1573413713666170619092629.
5. Henri EY et al. “Acute Delusional Psychosis” in Hirsch SR and Shepherd M, eds. Themes and variations in European psychiatry: An anthology. Charlottesville: University Press of Virginia, 1974.
6. Snezhnevsky A, ed. Handbook of Psychiatry. Moscow: Meditsina, 1983.
7. Kaptsan A et al. Oneiroid syndrome: A concept of use for Western psychiatry. Isr J Psychiatry Relat Sci. 2000;37(4):278-85.
8. https://en.wikipedia.org/wiki/Bouff%C3%A9e_d%C3%A9lirante.
Fighting disparities in palliative and end-of-life care
Palliative care has been shown to improve quality of life, receipt of goal-concordant care, end-of-life decision-making, and improvement in pain and symptoms in individuals with serious illness. However, palliative and end-of-life care remain underutilized in racial and ethnic minorities.1 Health disparities such as access, quality of care, and health outcomes among minority groups exist in delivery and receipt of care within the health care system, and this includes the care of individuals with serious illness and at the end of life.1
Racial and ethnic minorities are less likely to receive goal-concordant care, participate in advance care planning, and have access to palliative care or hospice.2-4 They are more likely to die in a hospital, have inadequate pain and symptom management, and experience poor provider-patient communication.5-7 Other contributing factors include lack of knowledge of hospice and palliative care services, mistrust of the health care system, spiritual and religious beliefs, provider bias, and cultural beliefs.1
Despite these disparities, interventions have had limited success,8 and there are gaps in content, methods, and inclusion of racial and ethnic groups within palliative care research.7
Efforts to improve health equity for people with serious illness have been identified as an “urgent call to action.”1
A few recommended actionable items include delivering culturally competent care by ensuring availability of culturally and linguistically appropriate materials and information, education, and training for providers, and practicing cultural humility; contributing to workforce diversity by hiring and training diverse staff; and partnering with community organizations to build trust and to facilitate dissemination of culturally and linguistically appropriate information to providers in caring for their diverse patient populations.1,9
One of the first steps identified is to recognize that there is a problem and prioritize efforts to understand its “multifaceted nature.”10 This should occur on multiple levels including the individual (patient and caregiver), interpersonal (health care team), organization, and policy levels,10 and be done through clinical, research, and educational platforms.
At the interpersonal level, we as the health care team can start by reflecting, acknowledging biases, seeking educational and training opportunities on cross-cultural interactions, learning about cultural and spiritual beliefs, and developing skills in culturally and linguistically appropriate communication regarding goals of care and advance care planning.1,10
For those seeking resources, organizations such as the Center to Advance Palliative Care’s Project Equity and the American Academy of Hospice and Palliative Medicine have ongoing efforts to educate and train physicians and health care professionals to improve and understand health equity in palliative care by providing resource portals, toolkits, training, and general information.
It is imperative to move forward in actionable ways to address not only racial and ethnic disparities, but advance equity in serious illness care for health care organizations, providers, and policymakers.1
Dr. Kang is in the division of gerontology and geriatric medicine at the University of Washington, Seattle.
References
1. Barrett NJ et al. N C Med J. 2020;81:254-6.
2. Johnson KS et al. J Am Geriatr Soc. 2011;59:732-7.
3. Sharma RK et al. J Clin Oncol. 2015;33:3802-8.
4. Muni S et al. Chest. 2011;139:1025-33.
5. Anderson KO et al. J Pain. 2009;10:1187-204.
6. Mack JW et al. Arch Intern Med. 2010;170:1533-40.
7. Johnson KS. J Palliat Med. 2013;16(11):1329-34.
8. Brown CE et al. J Pain Symptom Manage. 2021;63(5):e465-e71.
9. Chambers B. Center for Advancing Palliative Care. July 9, 2020.
10. Koffman J et al. BMC Palliat Care. 2023;22(64):1-3.
Palliative care has been shown to improve quality of life, receipt of goal-concordant care, end-of-life decision-making, and improvement in pain and symptoms in individuals with serious illness. However, palliative and end-of-life care remain underutilized in racial and ethnic minorities.1 Health disparities such as access, quality of care, and health outcomes among minority groups exist in delivery and receipt of care within the health care system, and this includes the care of individuals with serious illness and at the end of life.1
Racial and ethnic minorities are less likely to receive goal-concordant care, participate in advance care planning, and have access to palliative care or hospice.2-4 They are more likely to die in a hospital, have inadequate pain and symptom management, and experience poor provider-patient communication.5-7 Other contributing factors include lack of knowledge of hospice and palliative care services, mistrust of the health care system, spiritual and religious beliefs, provider bias, and cultural beliefs.1
Despite these disparities, interventions have had limited success,8 and there are gaps in content, methods, and inclusion of racial and ethnic groups within palliative care research.7
Efforts to improve health equity for people with serious illness have been identified as an “urgent call to action.”1
A few recommended actionable items include delivering culturally competent care by ensuring availability of culturally and linguistically appropriate materials and information, education, and training for providers, and practicing cultural humility; contributing to workforce diversity by hiring and training diverse staff; and partnering with community organizations to build trust and to facilitate dissemination of culturally and linguistically appropriate information to providers in caring for their diverse patient populations.1,9
One of the first steps identified is to recognize that there is a problem and prioritize efforts to understand its “multifaceted nature.”10 This should occur on multiple levels including the individual (patient and caregiver), interpersonal (health care team), organization, and policy levels,10 and be done through clinical, research, and educational platforms.
At the interpersonal level, we as the health care team can start by reflecting, acknowledging biases, seeking educational and training opportunities on cross-cultural interactions, learning about cultural and spiritual beliefs, and developing skills in culturally and linguistically appropriate communication regarding goals of care and advance care planning.1,10
For those seeking resources, organizations such as the Center to Advance Palliative Care’s Project Equity and the American Academy of Hospice and Palliative Medicine have ongoing efforts to educate and train physicians and health care professionals to improve and understand health equity in palliative care by providing resource portals, toolkits, training, and general information.
It is imperative to move forward in actionable ways to address not only racial and ethnic disparities, but advance equity in serious illness care for health care organizations, providers, and policymakers.1
Dr. Kang is in the division of gerontology and geriatric medicine at the University of Washington, Seattle.
References
1. Barrett NJ et al. N C Med J. 2020;81:254-6.
2. Johnson KS et al. J Am Geriatr Soc. 2011;59:732-7.
3. Sharma RK et al. J Clin Oncol. 2015;33:3802-8.
4. Muni S et al. Chest. 2011;139:1025-33.
5. Anderson KO et al. J Pain. 2009;10:1187-204.
6. Mack JW et al. Arch Intern Med. 2010;170:1533-40.
7. Johnson KS. J Palliat Med. 2013;16(11):1329-34.
8. Brown CE et al. J Pain Symptom Manage. 2021;63(5):e465-e71.
9. Chambers B. Center for Advancing Palliative Care. July 9, 2020.
10. Koffman J et al. BMC Palliat Care. 2023;22(64):1-3.
Palliative care has been shown to improve quality of life, receipt of goal-concordant care, end-of-life decision-making, and improvement in pain and symptoms in individuals with serious illness. However, palliative and end-of-life care remain underutilized in racial and ethnic minorities.1 Health disparities such as access, quality of care, and health outcomes among minority groups exist in delivery and receipt of care within the health care system, and this includes the care of individuals with serious illness and at the end of life.1
Racial and ethnic minorities are less likely to receive goal-concordant care, participate in advance care planning, and have access to palliative care or hospice.2-4 They are more likely to die in a hospital, have inadequate pain and symptom management, and experience poor provider-patient communication.5-7 Other contributing factors include lack of knowledge of hospice and palliative care services, mistrust of the health care system, spiritual and religious beliefs, provider bias, and cultural beliefs.1
Despite these disparities, interventions have had limited success,8 and there are gaps in content, methods, and inclusion of racial and ethnic groups within palliative care research.7
Efforts to improve health equity for people with serious illness have been identified as an “urgent call to action.”1
A few recommended actionable items include delivering culturally competent care by ensuring availability of culturally and linguistically appropriate materials and information, education, and training for providers, and practicing cultural humility; contributing to workforce diversity by hiring and training diverse staff; and partnering with community organizations to build trust and to facilitate dissemination of culturally and linguistically appropriate information to providers in caring for their diverse patient populations.1,9
One of the first steps identified is to recognize that there is a problem and prioritize efforts to understand its “multifaceted nature.”10 This should occur on multiple levels including the individual (patient and caregiver), interpersonal (health care team), organization, and policy levels,10 and be done through clinical, research, and educational platforms.
At the interpersonal level, we as the health care team can start by reflecting, acknowledging biases, seeking educational and training opportunities on cross-cultural interactions, learning about cultural and spiritual beliefs, and developing skills in culturally and linguistically appropriate communication regarding goals of care and advance care planning.1,10
For those seeking resources, organizations such as the Center to Advance Palliative Care’s Project Equity and the American Academy of Hospice and Palliative Medicine have ongoing efforts to educate and train physicians and health care professionals to improve and understand health equity in palliative care by providing resource portals, toolkits, training, and general information.
It is imperative to move forward in actionable ways to address not only racial and ethnic disparities, but advance equity in serious illness care for health care organizations, providers, and policymakers.1
Dr. Kang is in the division of gerontology and geriatric medicine at the University of Washington, Seattle.
References
1. Barrett NJ et al. N C Med J. 2020;81:254-6.
2. Johnson KS et al. J Am Geriatr Soc. 2011;59:732-7.
3. Sharma RK et al. J Clin Oncol. 2015;33:3802-8.
4. Muni S et al. Chest. 2011;139:1025-33.
5. Anderson KO et al. J Pain. 2009;10:1187-204.
6. Mack JW et al. Arch Intern Med. 2010;170:1533-40.
7. Johnson KS. J Palliat Med. 2013;16(11):1329-34.
8. Brown CE et al. J Pain Symptom Manage. 2021;63(5):e465-e71.
9. Chambers B. Center for Advancing Palliative Care. July 9, 2020.
10. Koffman J et al. BMC Palliat Care. 2023;22(64):1-3.
Bad blood: Could brain bleeds be contagious?
This transcript has been edited for clarity.
How do you tell if a condition is caused by an infection?
It seems like an obvious question, right? In the post–van Leeuwenhoek era we can look at whatever part of the body is diseased under a microscope and see microbes – you know, the usual suspects.
Except when we can’t. And there are plenty of cases where we can’t: where the microbe is too small to be seen without more advanced imaging techniques, like with viruses; or when the pathogen is sparsely populated or hard to culture, like Mycobacterium.
Finding out that a condition is the result of an infection is not only an exercise for 19th century physicians. After all, it was 2008 when Barry Marshall and Robin Warren won their Nobel Prize for proving that stomach ulcers, long thought to be due to “stress,” were actually caused by a tiny microbe called Helicobacter pylori.
And this week, we are looking at a study which, once again, begins to suggest that a condition thought to be more or less random – cerebral amyloid angiopathy – may actually be the result of an infectious disease.
We’re talking about this paper, appearing in JAMA, which is just a great example of old-fashioned shoe-leather epidemiology. But let’s get up to speed on cerebral amyloid angiopathy (CAA) first.
CAA is characterized by the deposition of amyloid protein in the brain. While there are some genetic causes, they are quite rare, and most cases are thought to be idiopathic. Recent analyses suggest that somewhere between 5% and 7% of cognitively normal older adults have CAA, but the rate is much higher among those with intracerebral hemorrhage – brain bleeds. In fact, CAA is the second-most common cause of bleeding in the brain, second only to severe hypertension.
An article in Nature highlights cases that seemed to develop after the administration of cadaveric pituitary hormone.
Other studies have shown potential transmission via dura mater grafts and neurosurgical instruments. But despite those clues, no infectious organism has been identified. Some have suggested that the long latent period and difficulty of finding a responsible microbe points to a prion-like disease not yet known. But these studies are more or less case series. The new JAMA paper gives us, if not a smoking gun, a pretty decent set of fingerprints.
Here’s the idea: If CAA is caused by some infectious agent, it may be transmitted in the blood. We know that a decent percentage of people who have spontaneous brain bleeds have CAA. If those people donated blood in the past, maybe the people who received that blood would be at risk for brain bleeds too.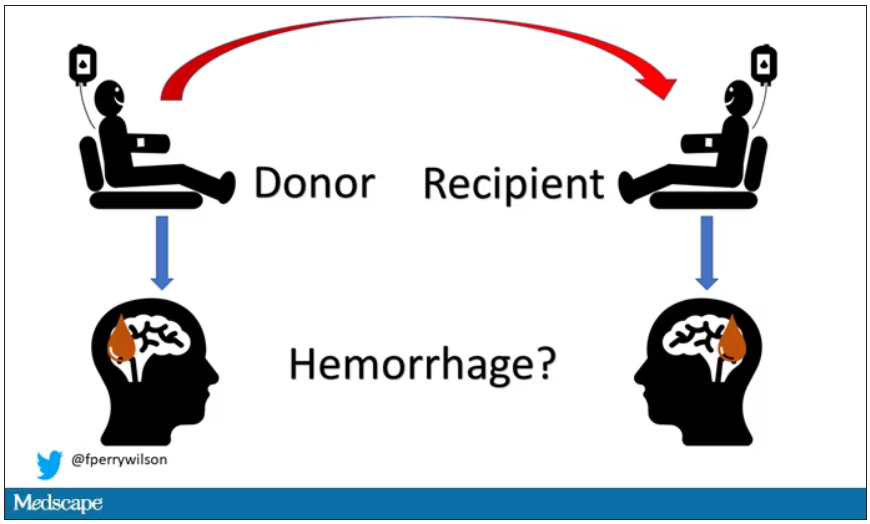
Of course, to really test that hypothesis, you’d need to know who every blood donor in a country was and every person who received that blood and all their subsequent diagnoses for basically their entire lives. No one has that kind of data, right?
Well, if you’ve been watching this space, you’ll know that a few countries do. Enter Sweden and Denmark, with their national electronic health record that captures all of this information, and much more, on every single person who lives or has lived in those countries since before 1970. Unbelievable.
So that’s exactly what the researchers, led by Jingchen Zhao at Karolinska (Sweden) University, did. They identified roughly 760,000 individuals in Sweden and 330,000 people in Denmark who had received a blood transfusion between 1970 and 2017.
Of course, most of those blood donors – 99% of them, actually – never went on to have any bleeding in the brain. It is a rare thing, fortunately.
But some of the donors did, on average within about 5 years of the time they donated blood. The researchers characterized each donor as either never having a brain bleed, having a single bleed, or having multiple bleeds. The latter is most strongly associated with CAA.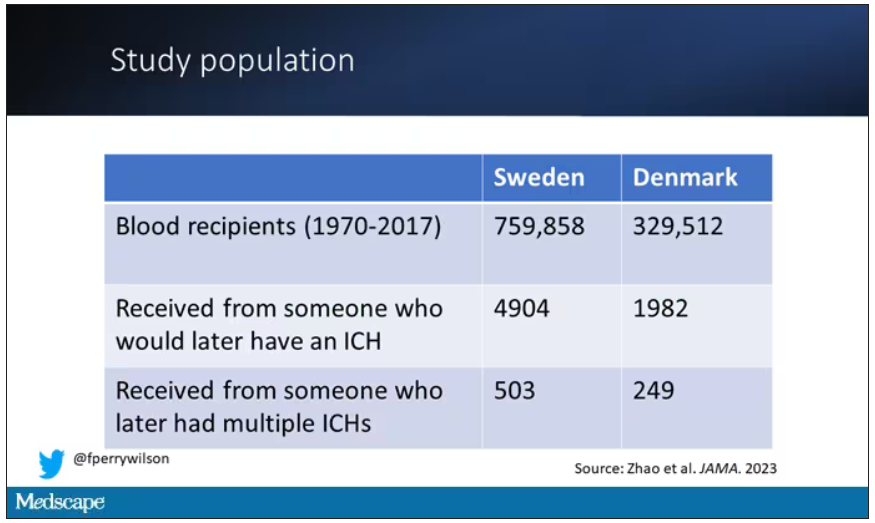
The big question: Would recipients who got blood from individuals who later on had brain bleeds, have brain bleeds themselves?
The answer is yes, though with an asterisk. You can see the results here. The risk of recipients having a brain bleed was lowest if the blood they received was from people who never had a brain bleed, higher if the individual had a single brain bleed, and highest if they got blood from a donor who would go on to have multiple brain bleeds.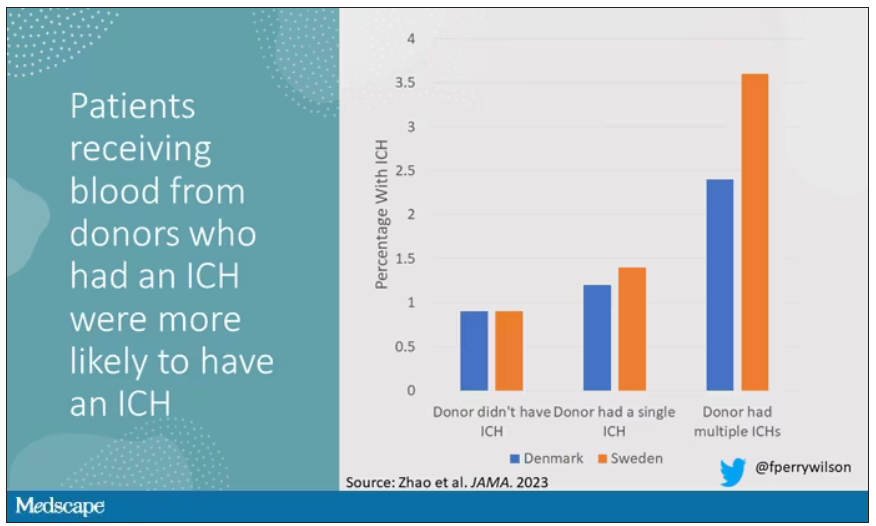
All in all, individuals who received blood from someone who would later have multiple hemorrhages were three times more likely to themselves develop bleeds themselves. It’s fairly compelling evidence of a transmissible agent.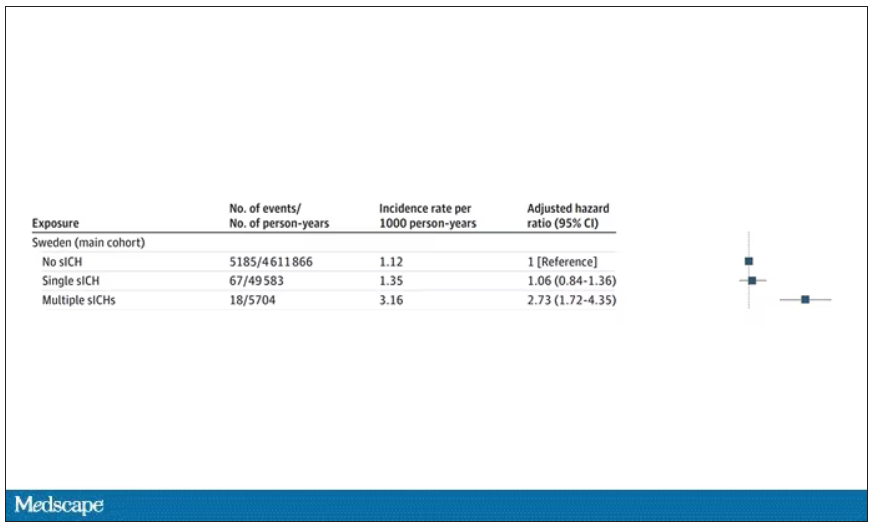
Of course, there are some potential confounders to consider here. Whose blood you get is not totally random. If, for example, people with type O blood are just more likely to have brain bleeds, then you could get results like this, as type O tends to donate to type O and both groups would have higher risk after donation. But the authors adjusted for blood type. They also adjusted for number of transfusions, calendar year, age, sex, and indication for transfusion.
Perhaps most compelling, and most clever, is that they used ischemic stroke as a negative control. Would people who received blood from someone who later had an ischemic stroke themselves be more likely to go on to have an ischemic stroke? No signal at all. It does not appear that there is a transmissible agent associated with ischemic stroke – only the brain bleeds.
I know what you’re thinking. What’s the agent? What’s the microbe, or virus, or prion, or toxin? The study gives us no insight there. These nationwide databases are awesome but they can only do so much. Because of the vagaries of medical coding and the difficulty of making the CAA diagnosis, the authors are using brain bleeds as a proxy here; we don’t even know for sure whether these were CAA-associated brain bleeds.
It’s also worth noting that there’s little we can do about this. None of the blood donors in this study had a brain bleed prior to donation; it’s not like we could screen people out of donating in the future. We have no test for whatever this agent is, if it even exists, nor do we have a potential treatment. Fortunately, whatever it is, it is extremely rare.
Still, this paper feels like a shot across the bow. At this point, the probability has shifted strongly away from CAA being a purely random disease and toward it being an infectious one. It may be time to round up some of the unusual suspects.
Dr. F. Perry Wilson is an associate professor of medicine and public health and director of Yale University’s Clinical and Translational Research Accelerator in New Haven, Conn. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
How do you tell if a condition is caused by an infection?
It seems like an obvious question, right? In the post–van Leeuwenhoek era we can look at whatever part of the body is diseased under a microscope and see microbes – you know, the usual suspects.
Except when we can’t. And there are plenty of cases where we can’t: where the microbe is too small to be seen without more advanced imaging techniques, like with viruses; or when the pathogen is sparsely populated or hard to culture, like Mycobacterium.
Finding out that a condition is the result of an infection is not only an exercise for 19th century physicians. After all, it was 2008 when Barry Marshall and Robin Warren won their Nobel Prize for proving that stomach ulcers, long thought to be due to “stress,” were actually caused by a tiny microbe called Helicobacter pylori.
And this week, we are looking at a study which, once again, begins to suggest that a condition thought to be more or less random – cerebral amyloid angiopathy – may actually be the result of an infectious disease.
We’re talking about this paper, appearing in JAMA, which is just a great example of old-fashioned shoe-leather epidemiology. But let’s get up to speed on cerebral amyloid angiopathy (CAA) first.
CAA is characterized by the deposition of amyloid protein in the brain. While there are some genetic causes, they are quite rare, and most cases are thought to be idiopathic. Recent analyses suggest that somewhere between 5% and 7% of cognitively normal older adults have CAA, but the rate is much higher among those with intracerebral hemorrhage – brain bleeds. In fact, CAA is the second-most common cause of bleeding in the brain, second only to severe hypertension.
An article in Nature highlights cases that seemed to develop after the administration of cadaveric pituitary hormone.
Other studies have shown potential transmission via dura mater grafts and neurosurgical instruments. But despite those clues, no infectious organism has been identified. Some have suggested that the long latent period and difficulty of finding a responsible microbe points to a prion-like disease not yet known. But these studies are more or less case series. The new JAMA paper gives us, if not a smoking gun, a pretty decent set of fingerprints.
Here’s the idea: If CAA is caused by some infectious agent, it may be transmitted in the blood. We know that a decent percentage of people who have spontaneous brain bleeds have CAA. If those people donated blood in the past, maybe the people who received that blood would be at risk for brain bleeds too.
Of course, to really test that hypothesis, you’d need to know who every blood donor in a country was and every person who received that blood and all their subsequent diagnoses for basically their entire lives. No one has that kind of data, right?
Well, if you’ve been watching this space, you’ll know that a few countries do. Enter Sweden and Denmark, with their national electronic health record that captures all of this information, and much more, on every single person who lives or has lived in those countries since before 1970. Unbelievable.
So that’s exactly what the researchers, led by Jingchen Zhao at Karolinska (Sweden) University, did. They identified roughly 760,000 individuals in Sweden and 330,000 people in Denmark who had received a blood transfusion between 1970 and 2017.
Of course, most of those blood donors – 99% of them, actually – never went on to have any bleeding in the brain. It is a rare thing, fortunately.
But some of the donors did, on average within about 5 years of the time they donated blood. The researchers characterized each donor as either never having a brain bleed, having a single bleed, or having multiple bleeds. The latter is most strongly associated with CAA.
The big question: Would recipients who got blood from individuals who later on had brain bleeds, have brain bleeds themselves?
The answer is yes, though with an asterisk. You can see the results here. The risk of recipients having a brain bleed was lowest if the blood they received was from people who never had a brain bleed, higher if the individual had a single brain bleed, and highest if they got blood from a donor who would go on to have multiple brain bleeds.
All in all, individuals who received blood from someone who would later have multiple hemorrhages were three times more likely to themselves develop bleeds themselves. It’s fairly compelling evidence of a transmissible agent.
Of course, there are some potential confounders to consider here. Whose blood you get is not totally random. If, for example, people with type O blood are just more likely to have brain bleeds, then you could get results like this, as type O tends to donate to type O and both groups would have higher risk after donation. But the authors adjusted for blood type. They also adjusted for number of transfusions, calendar year, age, sex, and indication for transfusion.
Perhaps most compelling, and most clever, is that they used ischemic stroke as a negative control. Would people who received blood from someone who later had an ischemic stroke themselves be more likely to go on to have an ischemic stroke? No signal at all. It does not appear that there is a transmissible agent associated with ischemic stroke – only the brain bleeds.
I know what you’re thinking. What’s the agent? What’s the microbe, or virus, or prion, or toxin? The study gives us no insight there. These nationwide databases are awesome but they can only do so much. Because of the vagaries of medical coding and the difficulty of making the CAA diagnosis, the authors are using brain bleeds as a proxy here; we don’t even know for sure whether these were CAA-associated brain bleeds.
It’s also worth noting that there’s little we can do about this. None of the blood donors in this study had a brain bleed prior to donation; it’s not like we could screen people out of donating in the future. We have no test for whatever this agent is, if it even exists, nor do we have a potential treatment. Fortunately, whatever it is, it is extremely rare.
Still, this paper feels like a shot across the bow. At this point, the probability has shifted strongly away from CAA being a purely random disease and toward it being an infectious one. It may be time to round up some of the unusual suspects.
Dr. F. Perry Wilson is an associate professor of medicine and public health and director of Yale University’s Clinical and Translational Research Accelerator in New Haven, Conn. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
How do you tell if a condition is caused by an infection?
It seems like an obvious question, right? In the post–van Leeuwenhoek era we can look at whatever part of the body is diseased under a microscope and see microbes – you know, the usual suspects.
Except when we can’t. And there are plenty of cases where we can’t: where the microbe is too small to be seen without more advanced imaging techniques, like with viruses; or when the pathogen is sparsely populated or hard to culture, like Mycobacterium.
Finding out that a condition is the result of an infection is not only an exercise for 19th century physicians. After all, it was 2008 when Barry Marshall and Robin Warren won their Nobel Prize for proving that stomach ulcers, long thought to be due to “stress,” were actually caused by a tiny microbe called Helicobacter pylori.
And this week, we are looking at a study which, once again, begins to suggest that a condition thought to be more or less random – cerebral amyloid angiopathy – may actually be the result of an infectious disease.
We’re talking about this paper, appearing in JAMA, which is just a great example of old-fashioned shoe-leather epidemiology. But let’s get up to speed on cerebral amyloid angiopathy (CAA) first.
CAA is characterized by the deposition of amyloid protein in the brain. While there are some genetic causes, they are quite rare, and most cases are thought to be idiopathic. Recent analyses suggest that somewhere between 5% and 7% of cognitively normal older adults have CAA, but the rate is much higher among those with intracerebral hemorrhage – brain bleeds. In fact, CAA is the second-most common cause of bleeding in the brain, second only to severe hypertension.
An article in Nature highlights cases that seemed to develop after the administration of cadaveric pituitary hormone.
Other studies have shown potential transmission via dura mater grafts and neurosurgical instruments. But despite those clues, no infectious organism has been identified. Some have suggested that the long latent period and difficulty of finding a responsible microbe points to a prion-like disease not yet known. But these studies are more or less case series. The new JAMA paper gives us, if not a smoking gun, a pretty decent set of fingerprints.
Here’s the idea: If CAA is caused by some infectious agent, it may be transmitted in the blood. We know that a decent percentage of people who have spontaneous brain bleeds have CAA. If those people donated blood in the past, maybe the people who received that blood would be at risk for brain bleeds too.
Of course, to really test that hypothesis, you’d need to know who every blood donor in a country was and every person who received that blood and all their subsequent diagnoses for basically their entire lives. No one has that kind of data, right?
Well, if you’ve been watching this space, you’ll know that a few countries do. Enter Sweden and Denmark, with their national electronic health record that captures all of this information, and much more, on every single person who lives or has lived in those countries since before 1970. Unbelievable.
So that’s exactly what the researchers, led by Jingchen Zhao at Karolinska (Sweden) University, did. They identified roughly 760,000 individuals in Sweden and 330,000 people in Denmark who had received a blood transfusion between 1970 and 2017.
Of course, most of those blood donors – 99% of them, actually – never went on to have any bleeding in the brain. It is a rare thing, fortunately.
But some of the donors did, on average within about 5 years of the time they donated blood. The researchers characterized each donor as either never having a brain bleed, having a single bleed, or having multiple bleeds. The latter is most strongly associated with CAA.
The big question: Would recipients who got blood from individuals who later on had brain bleeds, have brain bleeds themselves?
The answer is yes, though with an asterisk. You can see the results here. The risk of recipients having a brain bleed was lowest if the blood they received was from people who never had a brain bleed, higher if the individual had a single brain bleed, and highest if they got blood from a donor who would go on to have multiple brain bleeds.
All in all, individuals who received blood from someone who would later have multiple hemorrhages were three times more likely to themselves develop bleeds themselves. It’s fairly compelling evidence of a transmissible agent.
Of course, there are some potential confounders to consider here. Whose blood you get is not totally random. If, for example, people with type O blood are just more likely to have brain bleeds, then you could get results like this, as type O tends to donate to type O and both groups would have higher risk after donation. But the authors adjusted for blood type. They also adjusted for number of transfusions, calendar year, age, sex, and indication for transfusion.
Perhaps most compelling, and most clever, is that they used ischemic stroke as a negative control. Would people who received blood from someone who later had an ischemic stroke themselves be more likely to go on to have an ischemic stroke? No signal at all. It does not appear that there is a transmissible agent associated with ischemic stroke – only the brain bleeds.
I know what you’re thinking. What’s the agent? What’s the microbe, or virus, or prion, or toxin? The study gives us no insight there. These nationwide databases are awesome but they can only do so much. Because of the vagaries of medical coding and the difficulty of making the CAA diagnosis, the authors are using brain bleeds as a proxy here; we don’t even know for sure whether these were CAA-associated brain bleeds.
It’s also worth noting that there’s little we can do about this. None of the blood donors in this study had a brain bleed prior to donation; it’s not like we could screen people out of donating in the future. We have no test for whatever this agent is, if it even exists, nor do we have a potential treatment. Fortunately, whatever it is, it is extremely rare.
Still, this paper feels like a shot across the bow. At this point, the probability has shifted strongly away from CAA being a purely random disease and toward it being an infectious one. It may be time to round up some of the unusual suspects.
Dr. F. Perry Wilson is an associate professor of medicine and public health and director of Yale University’s Clinical and Translational Research Accelerator in New Haven, Conn. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Disenfranchised grief: What it looks like, where it goes
What happens to grief when those around you don’t understand it? Where does it go? How do you process it?
Disenfranchised grief, when someone or society more generally doesn’t see a loss as worthy of mourning, can deprive people of experiencing or processing their sadness. This grief, which may be triggered by the death of an ex-spouse, a pet, a failed adoption, can be painful and long-lasting.
Suzanne Cole, MD: ‘I didn’t feel the right to grieve’
During the COVID-19 pandemic, my little sister unexpectedly died. Though she was not one of the nearly 7 million people who died of the virus, in 2021 she became another type of statistic: one of the 109,699 people in the United State who died from a drug overdose. Hers was from fentanyl laced with methamphetamines.
Her death unraveled me. I felt deep guilt that I could not pull her from the sweeping current that had wrenched her from mainstream society into the underbelly of sex work and toward the solace of mind-altering drugs.
But I did not feel the right to grieve for her as I have grieved for other loved ones who were not blamed for their exit from this world. My sister was living a sordid life on the fringes of society. My grief felt invalid, undeserved. Yet, in the eyes of other “upstanding citizens,” her life was not as worth grieving – or so I thought. I tucked my sorrow into a small corner of my soul so no one would see, and I carried on.
To this day, the shame I feel robbed me of the ability to freely talk about her or share the searing pain I feel. Tears still prick my eyes when I think of her, but I have become adept at swallowing them, shaking off the waves of grief as though nothing happened. Even now, I cannot shake the pervasive feeling that my silent tears don’t deserve to be wept.
Don S. Dizon, MD: Working through tragedy
As a medical student, I worked with an outpatient physician as part of a third-year rotation. When we met, the first thing that struck me was how disheveled he looked. His clothes were wrinkled, and his pants were baggy. He took cigarette breaks, which I found disturbing.
But I quickly came to admire him. Despite my first impression, he was the type of doctor I aspired to be. He didn’t need to look at a patient’s chart to recall who they were. He just knew them. He greeted patients warmly, asked about their family. He even remembered the special occasions his patients had mentioned since their past visit. He epitomized empathy and connectedness.
Spending one day in clinic brought to light the challenges of forming such bonds with patients. A man came into the cancer clinic reporting chest pain and was triaged to an exam room. Soon after, the patient was found unresponsive on the floor. Nurses were yelling for help, and the doctor ran in and started CPR while minutes ticked by waiting for an ambulance that could take him to the ED.
By the time help arrived, the patient was blue.
He had died in the clinic in the middle of the day, as the waiting room filled. After the body was taken away, the doctor went into the bathroom. About 20 minutes later, he came out, eyes bloodshot, and continued with the rest of his day, ensuring each patient was seen and cared for.
As a medical student, it hit me how hard it must be to see something so tragic like the end of a life and then continue with your day as if nothing had happened. This is an experience of grief I later came to know well after nearly 30 years treating patients with advanced cancers: compartmentalizing it and carrying on.
A space for grieving: The Schwartz Center Rounds
Disenfranchised grief, the grief that is hard to share and often seems wrong to feel in the first place, can be triggered in many situations. Losing a person others don’t believe deserve to be grieved, such as an abusive partner or someone who committed a crime; losing someone you cared for in a professional role; a loss experienced in a breakup or same-sex partnership, if that relationship was not accepted by one’s family; loss from infertility, miscarriage, stillbirth, or failed adoption; loss that may be taboo or stigmatized, such as deaths via suicide or abortion; and loss of a job, home, or possession that you treasure.
Many of us have had similar situations or will, and the feeling that no one understands the need to mourn can be paralyzing and alienating. In the early days, intense, crushing feelings can cause intrusive, distracting thoughts, and over time, that grief can linger and find a permanent place in our minds.
More and more, though, we are being given opportunities to reflect on these sad moments.
The Schwartz Rounds are an example of such an opportunity. In these rounds, we gather to talk about the experience of caring for people, not the science of medicine.
During one particularly powerful rounds, I spoke to my colleagues about my initial meeting with a patient who was very sick. I detailed the experience of telling her children and her at that initial consult how I thought she was dying and that I did not recommend therapy. I remember how they cried. And I remembered how powerless I felt.
As I recalled that memory during Schwartz Rounds, I could not stop from crying. The unfairness of being a physician meeting someone for the first time and having to tell them such bad news overwhelmed me.
Even more poignant, I had the chance to reconnect with this woman’s children, who were present that day, not as audience members but as participants. Their presence may have brought my emotions to the surface more strongly. In that moment, I could show them the feelings I had bottled up for the sake of professionalism. Ultimately, I felt relieved, freer somehow, as if this burden my soul was carrying had been lifted.
Although we are both grateful for forums like this, these opportunities to share and express the grief we may have hidden away are not as common as they should be.
As physicians, we may express grief by shedding tears at the bedside of a patient nearing the end of life or through the anxiety we feel when our patient suffers a severe reaction to treatment. But we tend to put it away, to go on with our day, because there are others to be seen and cared for and more work to be done. Somehow, we move forward, shedding tears in one room and celebrating victories in another.
We need to create more spaces to express and feel grief, so we don’t get lost in it. Because understanding how grief impacts us, as people and as providers, is one of the most important realizations we can make as we go about our time-honored profession as healers.
Dr. Dizon is the director of women’s cancers at Lifespan Cancer Institute, director of medical oncology at Rhode Island Hospital, and a professor of medicine at Brown University, all in Providence. He reported conflicts of interest with Regeneron, AstraZeneca, Clovis, Bristol-Myers Squibb, and Kazia.
A version of this article first appeared on Medscape.com.
What happens to grief when those around you don’t understand it? Where does it go? How do you process it?
Disenfranchised grief, when someone or society more generally doesn’t see a loss as worthy of mourning, can deprive people of experiencing or processing their sadness. This grief, which may be triggered by the death of an ex-spouse, a pet, a failed adoption, can be painful and long-lasting.
Suzanne Cole, MD: ‘I didn’t feel the right to grieve’
During the COVID-19 pandemic, my little sister unexpectedly died. Though she was not one of the nearly 7 million people who died of the virus, in 2021 she became another type of statistic: one of the 109,699 people in the United State who died from a drug overdose. Hers was from fentanyl laced with methamphetamines.
Her death unraveled me. I felt deep guilt that I could not pull her from the sweeping current that had wrenched her from mainstream society into the underbelly of sex work and toward the solace of mind-altering drugs.
But I did not feel the right to grieve for her as I have grieved for other loved ones who were not blamed for their exit from this world. My sister was living a sordid life on the fringes of society. My grief felt invalid, undeserved. Yet, in the eyes of other “upstanding citizens,” her life was not as worth grieving – or so I thought. I tucked my sorrow into a small corner of my soul so no one would see, and I carried on.
To this day, the shame I feel robbed me of the ability to freely talk about her or share the searing pain I feel. Tears still prick my eyes when I think of her, but I have become adept at swallowing them, shaking off the waves of grief as though nothing happened. Even now, I cannot shake the pervasive feeling that my silent tears don’t deserve to be wept.
Don S. Dizon, MD: Working through tragedy
As a medical student, I worked with an outpatient physician as part of a third-year rotation. When we met, the first thing that struck me was how disheveled he looked. His clothes were wrinkled, and his pants were baggy. He took cigarette breaks, which I found disturbing.
But I quickly came to admire him. Despite my first impression, he was the type of doctor I aspired to be. He didn’t need to look at a patient’s chart to recall who they were. He just knew them. He greeted patients warmly, asked about their family. He even remembered the special occasions his patients had mentioned since their past visit. He epitomized empathy and connectedness.
Spending one day in clinic brought to light the challenges of forming such bonds with patients. A man came into the cancer clinic reporting chest pain and was triaged to an exam room. Soon after, the patient was found unresponsive on the floor. Nurses were yelling for help, and the doctor ran in and started CPR while minutes ticked by waiting for an ambulance that could take him to the ED.
By the time help arrived, the patient was blue.
He had died in the clinic in the middle of the day, as the waiting room filled. After the body was taken away, the doctor went into the bathroom. About 20 minutes later, he came out, eyes bloodshot, and continued with the rest of his day, ensuring each patient was seen and cared for.
As a medical student, it hit me how hard it must be to see something so tragic like the end of a life and then continue with your day as if nothing had happened. This is an experience of grief I later came to know well after nearly 30 years treating patients with advanced cancers: compartmentalizing it and carrying on.
A space for grieving: The Schwartz Center Rounds
Disenfranchised grief, the grief that is hard to share and often seems wrong to feel in the first place, can be triggered in many situations. Losing a person others don’t believe deserve to be grieved, such as an abusive partner or someone who committed a crime; losing someone you cared for in a professional role; a loss experienced in a breakup or same-sex partnership, if that relationship was not accepted by one’s family; loss from infertility, miscarriage, stillbirth, or failed adoption; loss that may be taboo or stigmatized, such as deaths via suicide or abortion; and loss of a job, home, or possession that you treasure.
Many of us have had similar situations or will, and the feeling that no one understands the need to mourn can be paralyzing and alienating. In the early days, intense, crushing feelings can cause intrusive, distracting thoughts, and over time, that grief can linger and find a permanent place in our minds.
More and more, though, we are being given opportunities to reflect on these sad moments.
The Schwartz Rounds are an example of such an opportunity. In these rounds, we gather to talk about the experience of caring for people, not the science of medicine.
During one particularly powerful rounds, I spoke to my colleagues about my initial meeting with a patient who was very sick. I detailed the experience of telling her children and her at that initial consult how I thought she was dying and that I did not recommend therapy. I remember how they cried. And I remembered how powerless I felt.
As I recalled that memory during Schwartz Rounds, I could not stop from crying. The unfairness of being a physician meeting someone for the first time and having to tell them such bad news overwhelmed me.
Even more poignant, I had the chance to reconnect with this woman’s children, who were present that day, not as audience members but as participants. Their presence may have brought my emotions to the surface more strongly. In that moment, I could show them the feelings I had bottled up for the sake of professionalism. Ultimately, I felt relieved, freer somehow, as if this burden my soul was carrying had been lifted.
Although we are both grateful for forums like this, these opportunities to share and express the grief we may have hidden away are not as common as they should be.
As physicians, we may express grief by shedding tears at the bedside of a patient nearing the end of life or through the anxiety we feel when our patient suffers a severe reaction to treatment. But we tend to put it away, to go on with our day, because there are others to be seen and cared for and more work to be done. Somehow, we move forward, shedding tears in one room and celebrating victories in another.
We need to create more spaces to express and feel grief, so we don’t get lost in it. Because understanding how grief impacts us, as people and as providers, is one of the most important realizations we can make as we go about our time-honored profession as healers.
Dr. Dizon is the director of women’s cancers at Lifespan Cancer Institute, director of medical oncology at Rhode Island Hospital, and a professor of medicine at Brown University, all in Providence. He reported conflicts of interest with Regeneron, AstraZeneca, Clovis, Bristol-Myers Squibb, and Kazia.
A version of this article first appeared on Medscape.com.
What happens to grief when those around you don’t understand it? Where does it go? How do you process it?
Disenfranchised grief, when someone or society more generally doesn’t see a loss as worthy of mourning, can deprive people of experiencing or processing their sadness. This grief, which may be triggered by the death of an ex-spouse, a pet, a failed adoption, can be painful and long-lasting.
Suzanne Cole, MD: ‘I didn’t feel the right to grieve’
During the COVID-19 pandemic, my little sister unexpectedly died. Though she was not one of the nearly 7 million people who died of the virus, in 2021 she became another type of statistic: one of the 109,699 people in the United State who died from a drug overdose. Hers was from fentanyl laced with methamphetamines.
Her death unraveled me. I felt deep guilt that I could not pull her from the sweeping current that had wrenched her from mainstream society into the underbelly of sex work and toward the solace of mind-altering drugs.
But I did not feel the right to grieve for her as I have grieved for other loved ones who were not blamed for their exit from this world. My sister was living a sordid life on the fringes of society. My grief felt invalid, undeserved. Yet, in the eyes of other “upstanding citizens,” her life was not as worth grieving – or so I thought. I tucked my sorrow into a small corner of my soul so no one would see, and I carried on.
To this day, the shame I feel robbed me of the ability to freely talk about her or share the searing pain I feel. Tears still prick my eyes when I think of her, but I have become adept at swallowing them, shaking off the waves of grief as though nothing happened. Even now, I cannot shake the pervasive feeling that my silent tears don’t deserve to be wept.
Don S. Dizon, MD: Working through tragedy
As a medical student, I worked with an outpatient physician as part of a third-year rotation. When we met, the first thing that struck me was how disheveled he looked. His clothes were wrinkled, and his pants were baggy. He took cigarette breaks, which I found disturbing.
But I quickly came to admire him. Despite my first impression, he was the type of doctor I aspired to be. He didn’t need to look at a patient’s chart to recall who they were. He just knew them. He greeted patients warmly, asked about their family. He even remembered the special occasions his patients had mentioned since their past visit. He epitomized empathy and connectedness.
Spending one day in clinic brought to light the challenges of forming such bonds with patients. A man came into the cancer clinic reporting chest pain and was triaged to an exam room. Soon after, the patient was found unresponsive on the floor. Nurses were yelling for help, and the doctor ran in and started CPR while minutes ticked by waiting for an ambulance that could take him to the ED.
By the time help arrived, the patient was blue.
He had died in the clinic in the middle of the day, as the waiting room filled. After the body was taken away, the doctor went into the bathroom. About 20 minutes later, he came out, eyes bloodshot, and continued with the rest of his day, ensuring each patient was seen and cared for.
As a medical student, it hit me how hard it must be to see something so tragic like the end of a life and then continue with your day as if nothing had happened. This is an experience of grief I later came to know well after nearly 30 years treating patients with advanced cancers: compartmentalizing it and carrying on.
A space for grieving: The Schwartz Center Rounds
Disenfranchised grief, the grief that is hard to share and often seems wrong to feel in the first place, can be triggered in many situations. Losing a person others don’t believe deserve to be grieved, such as an abusive partner or someone who committed a crime; losing someone you cared for in a professional role; a loss experienced in a breakup or same-sex partnership, if that relationship was not accepted by one’s family; loss from infertility, miscarriage, stillbirth, or failed adoption; loss that may be taboo or stigmatized, such as deaths via suicide or abortion; and loss of a job, home, or possession that you treasure.
Many of us have had similar situations or will, and the feeling that no one understands the need to mourn can be paralyzing and alienating. In the early days, intense, crushing feelings can cause intrusive, distracting thoughts, and over time, that grief can linger and find a permanent place in our minds.
More and more, though, we are being given opportunities to reflect on these sad moments.
The Schwartz Rounds are an example of such an opportunity. In these rounds, we gather to talk about the experience of caring for people, not the science of medicine.
During one particularly powerful rounds, I spoke to my colleagues about my initial meeting with a patient who was very sick. I detailed the experience of telling her children and her at that initial consult how I thought she was dying and that I did not recommend therapy. I remember how they cried. And I remembered how powerless I felt.
As I recalled that memory during Schwartz Rounds, I could not stop from crying. The unfairness of being a physician meeting someone for the first time and having to tell them such bad news overwhelmed me.
Even more poignant, I had the chance to reconnect with this woman’s children, who were present that day, not as audience members but as participants. Their presence may have brought my emotions to the surface more strongly. In that moment, I could show them the feelings I had bottled up for the sake of professionalism. Ultimately, I felt relieved, freer somehow, as if this burden my soul was carrying had been lifted.
Although we are both grateful for forums like this, these opportunities to share and express the grief we may have hidden away are not as common as they should be.
As physicians, we may express grief by shedding tears at the bedside of a patient nearing the end of life or through the anxiety we feel when our patient suffers a severe reaction to treatment. But we tend to put it away, to go on with our day, because there are others to be seen and cared for and more work to be done. Somehow, we move forward, shedding tears in one room and celebrating victories in another.
We need to create more spaces to express and feel grief, so we don’t get lost in it. Because understanding how grief impacts us, as people and as providers, is one of the most important realizations we can make as we go about our time-honored profession as healers.
Dr. Dizon is the director of women’s cancers at Lifespan Cancer Institute, director of medical oncology at Rhode Island Hospital, and a professor of medicine at Brown University, all in Providence. He reported conflicts of interest with Regeneron, AstraZeneca, Clovis, Bristol-Myers Squibb, and Kazia.
A version of this article first appeared on Medscape.com.
Universal anxiety screening recommendation is a good start
A very good thing happened this summer for patients with anxiety and the psychiatrists, psychologists, and other mental health professionals who provide treatment for them. The U.S. Preventive Services Task Force recommended anxiety screening for all adults younger than 65.
On the surface, this is a great recommendation for recognition and caring for those who deal with and suffer from an anxiety disorder or multiple anxiety disorders. Although the USPSTF recommendations are independent of the U.S. government and are not an official position of the Department of Health & Human Services, they are a wonderful start at recognizing the importance of mental health care.
After all, anxiety disorders are the most commonly experienced and diagnosed mental disorders, according to the DSM-5.
They range mainly from generalized anxiety disorder (GAD), to panic attacks and panic disorder, separation anxiety, specific type phobias (bridges, tunnels, insects, snakes, and the list goes on), to other phobias, including agoraphobia, social phobia, and of course, anxiety caused by medical conditions. GAD alone occurs in, at least, more than 3% of the population.
Those of us who have been treating anxiety disorders for decades recognize them as an issue affecting both mental and physical well-being, not only because of the emotional causes but the physical distress and illnesses that anxiety may precipitate or worsen.
For example, blood pressure– and heart-related issues, GI disorders, and musculoskeletal issues are just a few examples of how our bodies and organ systems are affected by anxiety. Just the momentary physical symptoms of tachycardia or the “runs” before an exam are fine examples of how anxiety may affect patients physically, and an ongoing, consistent anxiety is potentially more harmful.
In fact, a first panic attack or episode of generalized anxiety may be so serious that an emergency department or physician visit is necessary to rule out a heart attack, asthma, or breathing issues – even a hormone or thyroid emergency, or a cardiac arrhythmia. Panic attacks alone create a high number of ED visits.
Treatments mainly include medication management and a variety of psychotherapy techniques. Currently, the most preferred, first-choice medications are the SSRI antidepressants, which are Food and Drug Administration approved for anxiety as well. These include Zoloft (sertraline), Prozac/Sarafem (fluoxetine), Celexa (citalopram), and Lexapro (escitalopram).
For many years, benzodiazepines (that is, tranquillizers) such as Valium (diazepam), Ativan (lorazepam), and Klonopin/Rivotril (clonazepam) to name a few, were the mainstay of anxiety treatment, but they have proven addictive and may affect cognition and memory. As the current opioid epidemic has shown, when combined with opioids, benzodiazepines are a potentially lethal combination and when used, they need to be for shorter-term care and monitored very judiciously.
It should be noted that after ongoing long-term use of an SSRI for anxiety or depression, it should not be stopped abruptly, as a variety of physical symptoms (for example, flu-like symptoms) may occur.
Benefits of nonmedicinal therapies
There are a variety of talk therapies, from dynamic psychotherapies to cognitive-behavioral therapies (CBT), plus relaxation techniques and guided imagery that have all had a good amount of success in treating generalized anxiety, panic disorder, as well as various types of phobias.
When medications are stopped, the anxiety symptoms may well return. But when using nonmedicinal therapies, clinicians have discovered that when patients develop a new perspective on the anxiety problem or have a new technique to treat anxiety, it may well be long lasting.
For me, using CBT, relaxation techniques, hypnosis, and guided imagery has been very successful in treating anxiety disorders with long-lasting results. Once a person learns to relax, whether it’s from deep breathing exercises, hypnosis (which is not sleep), mindfulness, or meditation, a strategy of guided imagery can be taught, which allows a person to practice as well as control their anxiety as a lifetime process. For example, I like imagining a large movie screen to desensitize and project anxieties.
In many instances, a combination of a medication and a talk therapy approach works best, but there are an equal number of instances in which just medication or just talk therapy is needed. Once again, knowledge, clinical judgment, and the art of care are required to make these assessments.
In other words, recognizing and treating anxiety requires highly specialized training, which is why I thought the USPSTF recommendations raise a few critical questions.
Questions and concerns
One issue, of course, is the exclusion of those patients over age 65 because of a lack of “data.” Why such an exclusion? Does this mean that data are lacking for this age group?
The concept of using solely evidenced-based data in psychiatry is itself an interesting concept because our profession, like many other medical specialties, requires practitioners to use a combination of art and science. And much can be said either way about the clarity of accuracy in the diversity of issues that arise when treating emotional disorders.
When looking at the over-65 population, has anyone thought of clinical knowledge, judgment, experience, observation, and, of course, common sense?
Just consider the worry (a cardinal feature of anxiety) that besets people over 65 when it comes to issues such as retirement, financial security, “empty nesting,” physical health issues, decreased socialization that resulted from the COVID-19 pandemic, and the perpetual loss of relatives and friends.
In addition, as we age, anxiety can come simply from the loss of identity as active lifestyles decrease and the reality of nearing life’s end becomes more of a reality. It would seem that this population would benefit enormously from anxiety screening and possible treatment.
Another major concern is that the screening and potential treatment of patients is aimed at primary care physicians. Putting the sole responsibility of providing mental health care on these overworked PCPs defies common sense unless we’re okay with 1- to 2-minute assessments of mental health issues and no doubt, a pharmacology-only approach.
If this follows the same route as well-intentioned PCPs treating depression, where 5-minute medication management is far too common, the only proper diagnostic course – the in-depth interview necessary to make a proper diagnosis – is often missing.
For example, in depression alone, it takes psychiatric experience and time to differentiate a major depressive disorder from a bipolar depression and to provide the appropriate medication and treatment plan with careful follow-up. In my experience, this usually does not happen in the exceedingly overworked, time-driven day of a PCP.
Anxiety disorders and depression can prove debilitating, and if a PCP wants the responsibility of treatment, a mandated mental health program should be followed – just as here in New York, prescribers are mandated to take a pain control, opioid, and infection control CME course to keep our licenses up to date.
Short of mandating a mental health program for PCPs, it should be part of training and CME courses that Psychiatry is a super specialty, much like orthopedics and ophthalmology, and primary care physicians should never hesitate to make referrals to the specialist.
The big picture for me, and I hope for us all, is that the USPSTF has started things rolling by making it clear that PCPs and other health care clinicians need to screen for anxiety as a disabling disorder that is quite treatable.
This approach will help to advance the destigmatization of mental health disorders. But as result, with more patients diagnosed, there will be a need for more psychiatrists – and psychologists with PhDs or PsyDs – to fill the gaps in mental health care.
Dr. London is a practicing psychiatrist and has been a newspaper columnist for 35 years, specializing in and writing about short-term therapy, including cognitive-behavioral therapy and guided imagery. He is author of “Find Freedom Fast” (New York: Kettlehole Publishing, 2019). He has no conflicts of interest.
A very good thing happened this summer for patients with anxiety and the psychiatrists, psychologists, and other mental health professionals who provide treatment for them. The U.S. Preventive Services Task Force recommended anxiety screening for all adults younger than 65.
On the surface, this is a great recommendation for recognition and caring for those who deal with and suffer from an anxiety disorder or multiple anxiety disorders. Although the USPSTF recommendations are independent of the U.S. government and are not an official position of the Department of Health & Human Services, they are a wonderful start at recognizing the importance of mental health care.
After all, anxiety disorders are the most commonly experienced and diagnosed mental disorders, according to the DSM-5.
They range mainly from generalized anxiety disorder (GAD), to panic attacks and panic disorder, separation anxiety, specific type phobias (bridges, tunnels, insects, snakes, and the list goes on), to other phobias, including agoraphobia, social phobia, and of course, anxiety caused by medical conditions. GAD alone occurs in, at least, more than 3% of the population.
Those of us who have been treating anxiety disorders for decades recognize them as an issue affecting both mental and physical well-being, not only because of the emotional causes but the physical distress and illnesses that anxiety may precipitate or worsen.
For example, blood pressure– and heart-related issues, GI disorders, and musculoskeletal issues are just a few examples of how our bodies and organ systems are affected by anxiety. Just the momentary physical symptoms of tachycardia or the “runs” before an exam are fine examples of how anxiety may affect patients physically, and an ongoing, consistent anxiety is potentially more harmful.
In fact, a first panic attack or episode of generalized anxiety may be so serious that an emergency department or physician visit is necessary to rule out a heart attack, asthma, or breathing issues – even a hormone or thyroid emergency, or a cardiac arrhythmia. Panic attacks alone create a high number of ED visits.
Treatments mainly include medication management and a variety of psychotherapy techniques. Currently, the most preferred, first-choice medications are the SSRI antidepressants, which are Food and Drug Administration approved for anxiety as well. These include Zoloft (sertraline), Prozac/Sarafem (fluoxetine), Celexa (citalopram), and Lexapro (escitalopram).
For many years, benzodiazepines (that is, tranquillizers) such as Valium (diazepam), Ativan (lorazepam), and Klonopin/Rivotril (clonazepam) to name a few, were the mainstay of anxiety treatment, but they have proven addictive and may affect cognition and memory. As the current opioid epidemic has shown, when combined with opioids, benzodiazepines are a potentially lethal combination and when used, they need to be for shorter-term care and monitored very judiciously.
It should be noted that after ongoing long-term use of an SSRI for anxiety or depression, it should not be stopped abruptly, as a variety of physical symptoms (for example, flu-like symptoms) may occur.
Benefits of nonmedicinal therapies
There are a variety of talk therapies, from dynamic psychotherapies to cognitive-behavioral therapies (CBT), plus relaxation techniques and guided imagery that have all had a good amount of success in treating generalized anxiety, panic disorder, as well as various types of phobias.
When medications are stopped, the anxiety symptoms may well return. But when using nonmedicinal therapies, clinicians have discovered that when patients develop a new perspective on the anxiety problem or have a new technique to treat anxiety, it may well be long lasting.
For me, using CBT, relaxation techniques, hypnosis, and guided imagery has been very successful in treating anxiety disorders with long-lasting results. Once a person learns to relax, whether it’s from deep breathing exercises, hypnosis (which is not sleep), mindfulness, or meditation, a strategy of guided imagery can be taught, which allows a person to practice as well as control their anxiety as a lifetime process. For example, I like imagining a large movie screen to desensitize and project anxieties.
In many instances, a combination of a medication and a talk therapy approach works best, but there are an equal number of instances in which just medication or just talk therapy is needed. Once again, knowledge, clinical judgment, and the art of care are required to make these assessments.
In other words, recognizing and treating anxiety requires highly specialized training, which is why I thought the USPSTF recommendations raise a few critical questions.
Questions and concerns
One issue, of course, is the exclusion of those patients over age 65 because of a lack of “data.” Why such an exclusion? Does this mean that data are lacking for this age group?
The concept of using solely evidenced-based data in psychiatry is itself an interesting concept because our profession, like many other medical specialties, requires practitioners to use a combination of art and science. And much can be said either way about the clarity of accuracy in the diversity of issues that arise when treating emotional disorders.
When looking at the over-65 population, has anyone thought of clinical knowledge, judgment, experience, observation, and, of course, common sense?
Just consider the worry (a cardinal feature of anxiety) that besets people over 65 when it comes to issues such as retirement, financial security, “empty nesting,” physical health issues, decreased socialization that resulted from the COVID-19 pandemic, and the perpetual loss of relatives and friends.
In addition, as we age, anxiety can come simply from the loss of identity as active lifestyles decrease and the reality of nearing life’s end becomes more of a reality. It would seem that this population would benefit enormously from anxiety screening and possible treatment.
Another major concern is that the screening and potential treatment of patients is aimed at primary care physicians. Putting the sole responsibility of providing mental health care on these overworked PCPs defies common sense unless we’re okay with 1- to 2-minute assessments of mental health issues and no doubt, a pharmacology-only approach.
If this follows the same route as well-intentioned PCPs treating depression, where 5-minute medication management is far too common, the only proper diagnostic course – the in-depth interview necessary to make a proper diagnosis – is often missing.
For example, in depression alone, it takes psychiatric experience and time to differentiate a major depressive disorder from a bipolar depression and to provide the appropriate medication and treatment plan with careful follow-up. In my experience, this usually does not happen in the exceedingly overworked, time-driven day of a PCP.
Anxiety disorders and depression can prove debilitating, and if a PCP wants the responsibility of treatment, a mandated mental health program should be followed – just as here in New York, prescribers are mandated to take a pain control, opioid, and infection control CME course to keep our licenses up to date.
Short of mandating a mental health program for PCPs, it should be part of training and CME courses that Psychiatry is a super specialty, much like orthopedics and ophthalmology, and primary care physicians should never hesitate to make referrals to the specialist.
The big picture for me, and I hope for us all, is that the USPSTF has started things rolling by making it clear that PCPs and other health care clinicians need to screen for anxiety as a disabling disorder that is quite treatable.
This approach will help to advance the destigmatization of mental health disorders. But as result, with more patients diagnosed, there will be a need for more psychiatrists – and psychologists with PhDs or PsyDs – to fill the gaps in mental health care.
Dr. London is a practicing psychiatrist and has been a newspaper columnist for 35 years, specializing in and writing about short-term therapy, including cognitive-behavioral therapy and guided imagery. He is author of “Find Freedom Fast” (New York: Kettlehole Publishing, 2019). He has no conflicts of interest.
A very good thing happened this summer for patients with anxiety and the psychiatrists, psychologists, and other mental health professionals who provide treatment for them. The U.S. Preventive Services Task Force recommended anxiety screening for all adults younger than 65.
On the surface, this is a great recommendation for recognition and caring for those who deal with and suffer from an anxiety disorder or multiple anxiety disorders. Although the USPSTF recommendations are independent of the U.S. government and are not an official position of the Department of Health & Human Services, they are a wonderful start at recognizing the importance of mental health care.
After all, anxiety disorders are the most commonly experienced and diagnosed mental disorders, according to the DSM-5.
They range mainly from generalized anxiety disorder (GAD), to panic attacks and panic disorder, separation anxiety, specific type phobias (bridges, tunnels, insects, snakes, and the list goes on), to other phobias, including agoraphobia, social phobia, and of course, anxiety caused by medical conditions. GAD alone occurs in, at least, more than 3% of the population.
Those of us who have been treating anxiety disorders for decades recognize them as an issue affecting both mental and physical well-being, not only because of the emotional causes but the physical distress and illnesses that anxiety may precipitate or worsen.
For example, blood pressure– and heart-related issues, GI disorders, and musculoskeletal issues are just a few examples of how our bodies and organ systems are affected by anxiety. Just the momentary physical symptoms of tachycardia or the “runs” before an exam are fine examples of how anxiety may affect patients physically, and an ongoing, consistent anxiety is potentially more harmful.
In fact, a first panic attack or episode of generalized anxiety may be so serious that an emergency department or physician visit is necessary to rule out a heart attack, asthma, or breathing issues – even a hormone or thyroid emergency, or a cardiac arrhythmia. Panic attacks alone create a high number of ED visits.
Treatments mainly include medication management and a variety of psychotherapy techniques. Currently, the most preferred, first-choice medications are the SSRI antidepressants, which are Food and Drug Administration approved for anxiety as well. These include Zoloft (sertraline), Prozac/Sarafem (fluoxetine), Celexa (citalopram), and Lexapro (escitalopram).
For many years, benzodiazepines (that is, tranquillizers) such as Valium (diazepam), Ativan (lorazepam), and Klonopin/Rivotril (clonazepam) to name a few, were the mainstay of anxiety treatment, but they have proven addictive and may affect cognition and memory. As the current opioid epidemic has shown, when combined with opioids, benzodiazepines are a potentially lethal combination and when used, they need to be for shorter-term care and monitored very judiciously.
It should be noted that after ongoing long-term use of an SSRI for anxiety or depression, it should not be stopped abruptly, as a variety of physical symptoms (for example, flu-like symptoms) may occur.
Benefits of nonmedicinal therapies
There are a variety of talk therapies, from dynamic psychotherapies to cognitive-behavioral therapies (CBT), plus relaxation techniques and guided imagery that have all had a good amount of success in treating generalized anxiety, panic disorder, as well as various types of phobias.
When medications are stopped, the anxiety symptoms may well return. But when using nonmedicinal therapies, clinicians have discovered that when patients develop a new perspective on the anxiety problem or have a new technique to treat anxiety, it may well be long lasting.
For me, using CBT, relaxation techniques, hypnosis, and guided imagery has been very successful in treating anxiety disorders with long-lasting results. Once a person learns to relax, whether it’s from deep breathing exercises, hypnosis (which is not sleep), mindfulness, or meditation, a strategy of guided imagery can be taught, which allows a person to practice as well as control their anxiety as a lifetime process. For example, I like imagining a large movie screen to desensitize and project anxieties.
In many instances, a combination of a medication and a talk therapy approach works best, but there are an equal number of instances in which just medication or just talk therapy is needed. Once again, knowledge, clinical judgment, and the art of care are required to make these assessments.
In other words, recognizing and treating anxiety requires highly specialized training, which is why I thought the USPSTF recommendations raise a few critical questions.
Questions and concerns
One issue, of course, is the exclusion of those patients over age 65 because of a lack of “data.” Why such an exclusion? Does this mean that data are lacking for this age group?
The concept of using solely evidenced-based data in psychiatry is itself an interesting concept because our profession, like many other medical specialties, requires practitioners to use a combination of art and science. And much can be said either way about the clarity of accuracy in the diversity of issues that arise when treating emotional disorders.
When looking at the over-65 population, has anyone thought of clinical knowledge, judgment, experience, observation, and, of course, common sense?
Just consider the worry (a cardinal feature of anxiety) that besets people over 65 when it comes to issues such as retirement, financial security, “empty nesting,” physical health issues, decreased socialization that resulted from the COVID-19 pandemic, and the perpetual loss of relatives and friends.
In addition, as we age, anxiety can come simply from the loss of identity as active lifestyles decrease and the reality of nearing life’s end becomes more of a reality. It would seem that this population would benefit enormously from anxiety screening and possible treatment.
Another major concern is that the screening and potential treatment of patients is aimed at primary care physicians. Putting the sole responsibility of providing mental health care on these overworked PCPs defies common sense unless we’re okay with 1- to 2-minute assessments of mental health issues and no doubt, a pharmacology-only approach.
If this follows the same route as well-intentioned PCPs treating depression, where 5-minute medication management is far too common, the only proper diagnostic course – the in-depth interview necessary to make a proper diagnosis – is often missing.
For example, in depression alone, it takes psychiatric experience and time to differentiate a major depressive disorder from a bipolar depression and to provide the appropriate medication and treatment plan with careful follow-up. In my experience, this usually does not happen in the exceedingly overworked, time-driven day of a PCP.
Anxiety disorders and depression can prove debilitating, and if a PCP wants the responsibility of treatment, a mandated mental health program should be followed – just as here in New York, prescribers are mandated to take a pain control, opioid, and infection control CME course to keep our licenses up to date.
Short of mandating a mental health program for PCPs, it should be part of training and CME courses that Psychiatry is a super specialty, much like orthopedics and ophthalmology, and primary care physicians should never hesitate to make referrals to the specialist.
The big picture for me, and I hope for us all, is that the USPSTF has started things rolling by making it clear that PCPs and other health care clinicians need to screen for anxiety as a disabling disorder that is quite treatable.
This approach will help to advance the destigmatization of mental health disorders. But as result, with more patients diagnosed, there will be a need for more psychiatrists – and psychologists with PhDs or PsyDs – to fill the gaps in mental health care.
Dr. London is a practicing psychiatrist and has been a newspaper columnist for 35 years, specializing in and writing about short-term therapy, including cognitive-behavioral therapy and guided imagery. He is author of “Find Freedom Fast” (New York: Kettlehole Publishing, 2019). He has no conflicts of interest.
A White male presented with a purulent erythematous edematous plaque with central necrosis and ulceration on his right flank
Lyme disease is the most commonly transmitted tick-borne illness in the United States. This infection is typically transmitted through a bite by the Ixodes tick commonly found in the Midwest, Northeast, and mid-Atlantic regions; however, the geographical distribution continues to expand over time in the United States. Ticks must be attached for 24-48 hours to transmit the pathogen. There are three general stages of the disease: early localized, early disseminated, and late disseminated.
The most common presentation is the early localized disease, which manifests between 3 and 30 days after an infected tick bite. Approximately 70%-80% of cases feature a targetlike lesion that expands centrifugally at the site of the bite. Most commonly, lesions appear on the abdomen, groin, axilla, and popliteal fossa. The diagnosis of ECM requires lesions at least 5 cm in size. Lesions may be asymptomatic, although burning may occur in half of patients. Atypical presentations include bullous, vesicular, hemorrhagic, or necrotic lesions. Up to half of patients may develop multiple ECM lesions. Palms and soles are spared. Differential diagnoses include arthropod reactions, pyoderma gangrenosum, cellulitis, herpes simplex virus and varicella zoster virus, contact dermatitis, or granuloma annulare. The rash is often accompanied by systemic symptoms including fatigue, myalgia, headache, and fever.
The next two stages include early and late disseminated infection. Early disseminated infection often occurs 3-12 weeks after infection and is characterized by muscle pain, dizziness, headache, and cardiac symptoms. CNS involvement occurs in about 20% of patients. Joint involvement may include the knee, ankle, and wrist. If symptoms are only in one joint, septic arthritis is part of the differential diagnosis, so clinical correlation and labs must be considered. Late disseminated infection occurs months or years after initial infection and includes neurologic and rheumatologic symptoms including meningitis, Bell’s palsy, arthritis, and dysesthesia. Knee arthritis is a key feature of this stage. Patients commonly have radicular pain and fibromyalgia-type pain. More severe disease processes include encephalomyelitis, arrhythmias, and heart block.
ECM is often a clinical diagnosis because serologic testing may not be positive during the first 2 weeks of infection. The screening serologic test is the ELISA, and a Western blot confirms the results. Skin histopathology for Lyme disease is often nonspecific and reveals a perivascular infiltrate of histiocytes, plasma cells, and lymphocytes. Silver stain or antibody testing may be used to identify the spirochete. In acrodermatitis chronica atrophicans, late Lyme disease presenting on the distal extremities, lymphocytic and plasma cell infiltrates are present. In borrelial lymphocytoma, a dense dermal lymphocytic infiltrate is present.
The standard for treatment of early localized disease is oral doxycycline in adults. Alternatives may be used if a patient is allergic or for children under 9. Disseminated disease may be treated with IV ceftriaxone and topical steroids are used if ocular symptoms are involved. Early treatment is often curative.
This patient’s antibodies were negative initially, but became positive after 6 weeks. He was treated empirically at the time of his office visit with doxycycline for 1 month.
This case and the photo were submitted by Lucas Shapiro, BS, of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Fla., and Susannah Berke, MD, Three Rivers Dermatology, Coraopolis, Pa. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at MDedge.com/Dermatology. To submit a case for possible publication, send an email to [email protected].
References
Carriveau A et al. Nurs Clin North Am. 2019 Jun;54(2):261-75.
Skar GL and Simonsen KA. Lyme Disease. [Updated 2023 May 31]. In: “StatPearls” [Internet]. Treasure Island, Fla.: StatPearls Publishing; 2023 Jan.
Tiger JB et al. J Am Acad Dermatol. 2014 Oct;71(4):e133-4.
Lyme disease is the most commonly transmitted tick-borne illness in the United States. This infection is typically transmitted through a bite by the Ixodes tick commonly found in the Midwest, Northeast, and mid-Atlantic regions; however, the geographical distribution continues to expand over time in the United States. Ticks must be attached for 24-48 hours to transmit the pathogen. There are three general stages of the disease: early localized, early disseminated, and late disseminated.
The most common presentation is the early localized disease, which manifests between 3 and 30 days after an infected tick bite. Approximately 70%-80% of cases feature a targetlike lesion that expands centrifugally at the site of the bite. Most commonly, lesions appear on the abdomen, groin, axilla, and popliteal fossa. The diagnosis of ECM requires lesions at least 5 cm in size. Lesions may be asymptomatic, although burning may occur in half of patients. Atypical presentations include bullous, vesicular, hemorrhagic, or necrotic lesions. Up to half of patients may develop multiple ECM lesions. Palms and soles are spared. Differential diagnoses include arthropod reactions, pyoderma gangrenosum, cellulitis, herpes simplex virus and varicella zoster virus, contact dermatitis, or granuloma annulare. The rash is often accompanied by systemic symptoms including fatigue, myalgia, headache, and fever.
The next two stages include early and late disseminated infection. Early disseminated infection often occurs 3-12 weeks after infection and is characterized by muscle pain, dizziness, headache, and cardiac symptoms. CNS involvement occurs in about 20% of patients. Joint involvement may include the knee, ankle, and wrist. If symptoms are only in one joint, septic arthritis is part of the differential diagnosis, so clinical correlation and labs must be considered. Late disseminated infection occurs months or years after initial infection and includes neurologic and rheumatologic symptoms including meningitis, Bell’s palsy, arthritis, and dysesthesia. Knee arthritis is a key feature of this stage. Patients commonly have radicular pain and fibromyalgia-type pain. More severe disease processes include encephalomyelitis, arrhythmias, and heart block.
ECM is often a clinical diagnosis because serologic testing may not be positive during the first 2 weeks of infection. The screening serologic test is the ELISA, and a Western blot confirms the results. Skin histopathology for Lyme disease is often nonspecific and reveals a perivascular infiltrate of histiocytes, plasma cells, and lymphocytes. Silver stain or antibody testing may be used to identify the spirochete. In acrodermatitis chronica atrophicans, late Lyme disease presenting on the distal extremities, lymphocytic and plasma cell infiltrates are present. In borrelial lymphocytoma, a dense dermal lymphocytic infiltrate is present.
The standard for treatment of early localized disease is oral doxycycline in adults. Alternatives may be used if a patient is allergic or for children under 9. Disseminated disease may be treated with IV ceftriaxone and topical steroids are used if ocular symptoms are involved. Early treatment is often curative.
This patient’s antibodies were negative initially, but became positive after 6 weeks. He was treated empirically at the time of his office visit with doxycycline for 1 month.
This case and the photo were submitted by Lucas Shapiro, BS, of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Fla., and Susannah Berke, MD, Three Rivers Dermatology, Coraopolis, Pa. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at MDedge.com/Dermatology. To submit a case for possible publication, send an email to [email protected].
References
Carriveau A et al. Nurs Clin North Am. 2019 Jun;54(2):261-75.
Skar GL and Simonsen KA. Lyme Disease. [Updated 2023 May 31]. In: “StatPearls” [Internet]. Treasure Island, Fla.: StatPearls Publishing; 2023 Jan.
Tiger JB et al. J Am Acad Dermatol. 2014 Oct;71(4):e133-4.
Lyme disease is the most commonly transmitted tick-borne illness in the United States. This infection is typically transmitted through a bite by the Ixodes tick commonly found in the Midwest, Northeast, and mid-Atlantic regions; however, the geographical distribution continues to expand over time in the United States. Ticks must be attached for 24-48 hours to transmit the pathogen. There are three general stages of the disease: early localized, early disseminated, and late disseminated.
The most common presentation is the early localized disease, which manifests between 3 and 30 days after an infected tick bite. Approximately 70%-80% of cases feature a targetlike lesion that expands centrifugally at the site of the bite. Most commonly, lesions appear on the abdomen, groin, axilla, and popliteal fossa. The diagnosis of ECM requires lesions at least 5 cm in size. Lesions may be asymptomatic, although burning may occur in half of patients. Atypical presentations include bullous, vesicular, hemorrhagic, or necrotic lesions. Up to half of patients may develop multiple ECM lesions. Palms and soles are spared. Differential diagnoses include arthropod reactions, pyoderma gangrenosum, cellulitis, herpes simplex virus and varicella zoster virus, contact dermatitis, or granuloma annulare. The rash is often accompanied by systemic symptoms including fatigue, myalgia, headache, and fever.
The next two stages include early and late disseminated infection. Early disseminated infection often occurs 3-12 weeks after infection and is characterized by muscle pain, dizziness, headache, and cardiac symptoms. CNS involvement occurs in about 20% of patients. Joint involvement may include the knee, ankle, and wrist. If symptoms are only in one joint, septic arthritis is part of the differential diagnosis, so clinical correlation and labs must be considered. Late disseminated infection occurs months or years after initial infection and includes neurologic and rheumatologic symptoms including meningitis, Bell’s palsy, arthritis, and dysesthesia. Knee arthritis is a key feature of this stage. Patients commonly have radicular pain and fibromyalgia-type pain. More severe disease processes include encephalomyelitis, arrhythmias, and heart block.
ECM is often a clinical diagnosis because serologic testing may not be positive during the first 2 weeks of infection. The screening serologic test is the ELISA, and a Western blot confirms the results. Skin histopathology for Lyme disease is often nonspecific and reveals a perivascular infiltrate of histiocytes, plasma cells, and lymphocytes. Silver stain or antibody testing may be used to identify the spirochete. In acrodermatitis chronica atrophicans, late Lyme disease presenting on the distal extremities, lymphocytic and plasma cell infiltrates are present. In borrelial lymphocytoma, a dense dermal lymphocytic infiltrate is present.
The standard for treatment of early localized disease is oral doxycycline in adults. Alternatives may be used if a patient is allergic or for children under 9. Disseminated disease may be treated with IV ceftriaxone and topical steroids are used if ocular symptoms are involved. Early treatment is often curative.
This patient’s antibodies were negative initially, but became positive after 6 weeks. He was treated empirically at the time of his office visit with doxycycline for 1 month.
This case and the photo were submitted by Lucas Shapiro, BS, of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Fla., and Susannah Berke, MD, Three Rivers Dermatology, Coraopolis, Pa. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at MDedge.com/Dermatology. To submit a case for possible publication, send an email to [email protected].
References
Carriveau A et al. Nurs Clin North Am. 2019 Jun;54(2):261-75.
Skar GL and Simonsen KA. Lyme Disease. [Updated 2023 May 31]. In: “StatPearls” [Internet]. Treasure Island, Fla.: StatPearls Publishing; 2023 Jan.
Tiger JB et al. J Am Acad Dermatol. 2014 Oct;71(4):e133-4.