User login
Reduction in S aureus skin infections may reduce the risk for eczema herpeticum in atopic dermatitis
Key clinical point: Among patients with atopic dermatitis (AD), those with vs without a history of Staphylococcus aureus skin infections have significantly higher odds of having a history of eczema herpeticum (EH).
Major finding: Patients with AD and with vs without a history of S aureus skin infections had a 6.60-fold increased risk of having a history of EH (adjusted odds ratio 6.60; P = .002).
Study details: This multicenter, clinical registry study included 112 patients with AD and with (n = 56) or without (n = 56) a history of EH, matched by age and AD severity.
Disclosures: This study was supported partly by a National Eczema Association Engagement Research Grant. Several authors declared serving as consultants or investigator for or receiving grants, personal fees, or clinical trial support from various organizations.
Source: Moran MC et al. History of S. aureus skin infection significantly associates with history of eczema herpeticum in patients with atopic dermatitis. Dermatol Ther (Heidelb). 2023 (Aug 24). doi: 10.1007/s13555-023-00996-y
Key clinical point: Among patients with atopic dermatitis (AD), those with vs without a history of Staphylococcus aureus skin infections have significantly higher odds of having a history of eczema herpeticum (EH).
Major finding: Patients with AD and with vs without a history of S aureus skin infections had a 6.60-fold increased risk of having a history of EH (adjusted odds ratio 6.60; P = .002).
Study details: This multicenter, clinical registry study included 112 patients with AD and with (n = 56) or without (n = 56) a history of EH, matched by age and AD severity.
Disclosures: This study was supported partly by a National Eczema Association Engagement Research Grant. Several authors declared serving as consultants or investigator for or receiving grants, personal fees, or clinical trial support from various organizations.
Source: Moran MC et al. History of S. aureus skin infection significantly associates with history of eczema herpeticum in patients with atopic dermatitis. Dermatol Ther (Heidelb). 2023 (Aug 24). doi: 10.1007/s13555-023-00996-y
Key clinical point: Among patients with atopic dermatitis (AD), those with vs without a history of Staphylococcus aureus skin infections have significantly higher odds of having a history of eczema herpeticum (EH).
Major finding: Patients with AD and with vs without a history of S aureus skin infections had a 6.60-fold increased risk of having a history of EH (adjusted odds ratio 6.60; P = .002).
Study details: This multicenter, clinical registry study included 112 patients with AD and with (n = 56) or without (n = 56) a history of EH, matched by age and AD severity.
Disclosures: This study was supported partly by a National Eczema Association Engagement Research Grant. Several authors declared serving as consultants or investigator for or receiving grants, personal fees, or clinical trial support from various organizations.
Source: Moran MC et al. History of S. aureus skin infection significantly associates with history of eczema herpeticum in patients with atopic dermatitis. Dermatol Ther (Heidelb). 2023 (Aug 24). doi: 10.1007/s13555-023-00996-y
Dupilumab rapidly controls atopic dermatitis symptoms in children
Key clinical point: Dupilumab rapidly improves the severity of atopic dermatitis (AD) symptoms and shows a favorable safety profile in children with moderate-to-severe AD.
Major finding: Dupilumab significantly reduced the mean Eczema Area and Severity Index (EASI) score at weeks 16, 24, and 52 (all P < .0001) and from weeks 16 to 24 (P < .01) and weeks 16 to 52 (P < .001). By week 52, 86.8% of patients had achieved a ≥ 75% improvement in the EASI score. No serious adverse events were observed, and none of the children discontinued treatment.
Study details: Findings are from a retrospective, observational, real-life study including 96 children (age 6-11 years) with moderate-to-severe AD inadequately controlled with conventional topical therapies who received dupilumab (300 mg on days 1 and 15 and 300 mg every 4 weeks).
Disclosures: This study did not receive any funding. Several authors reported receiving honoraria, travel support, or personal fees from or serving as consultants, investigators, speakers, or advisory board members for or having other ties with various sources.
Source: Patruno C et al. A 52-week multicenter retrospective real-world study on effectiveness and safety of dupilumab in children with atopic dermatitis aged from 6 to 11 years. J Dermatolog Treat. 2023;34:2246602 (Aug 14). doi: 10.1080/09546634.2023.2246602
Key clinical point: Dupilumab rapidly improves the severity of atopic dermatitis (AD) symptoms and shows a favorable safety profile in children with moderate-to-severe AD.
Major finding: Dupilumab significantly reduced the mean Eczema Area and Severity Index (EASI) score at weeks 16, 24, and 52 (all P < .0001) and from weeks 16 to 24 (P < .01) and weeks 16 to 52 (P < .001). By week 52, 86.8% of patients had achieved a ≥ 75% improvement in the EASI score. No serious adverse events were observed, and none of the children discontinued treatment.
Study details: Findings are from a retrospective, observational, real-life study including 96 children (age 6-11 years) with moderate-to-severe AD inadequately controlled with conventional topical therapies who received dupilumab (300 mg on days 1 and 15 and 300 mg every 4 weeks).
Disclosures: This study did not receive any funding. Several authors reported receiving honoraria, travel support, or personal fees from or serving as consultants, investigators, speakers, or advisory board members for or having other ties with various sources.
Source: Patruno C et al. A 52-week multicenter retrospective real-world study on effectiveness and safety of dupilumab in children with atopic dermatitis aged from 6 to 11 years. J Dermatolog Treat. 2023;34:2246602 (Aug 14). doi: 10.1080/09546634.2023.2246602
Key clinical point: Dupilumab rapidly improves the severity of atopic dermatitis (AD) symptoms and shows a favorable safety profile in children with moderate-to-severe AD.
Major finding: Dupilumab significantly reduced the mean Eczema Area and Severity Index (EASI) score at weeks 16, 24, and 52 (all P < .0001) and from weeks 16 to 24 (P < .01) and weeks 16 to 52 (P < .001). By week 52, 86.8% of patients had achieved a ≥ 75% improvement in the EASI score. No serious adverse events were observed, and none of the children discontinued treatment.
Study details: Findings are from a retrospective, observational, real-life study including 96 children (age 6-11 years) with moderate-to-severe AD inadequately controlled with conventional topical therapies who received dupilumab (300 mg on days 1 and 15 and 300 mg every 4 weeks).
Disclosures: This study did not receive any funding. Several authors reported receiving honoraria, travel support, or personal fees from or serving as consultants, investigators, speakers, or advisory board members for or having other ties with various sources.
Source: Patruno C et al. A 52-week multicenter retrospective real-world study on effectiveness and safety of dupilumab in children with atopic dermatitis aged from 6 to 11 years. J Dermatolog Treat. 2023;34:2246602 (Aug 14). doi: 10.1080/09546634.2023.2246602
Implementation of an Automated Phone Call Distribution System in an Inpatient Pharmacy Setting
Pharmacy call centers have been successfully implemented in outpatient and specialty pharmacy settings.1 A centralized pharmacy call center gives patients immediate access to a pharmacist who can view their health records to answer specific questions or fulfill medication renewal requests.2-4 Little literature exists to describe its use in an inpatient setting.
Inpatient pharmacies receive numerous calls from health care professionals and patients. Challenges related to phone calls in the inpatient pharmacy setting may include interruptions, distractions, low accountability, poor efficiency, lack of optimal resources, and staffing.5 An unequal distribution and lack of accountability may exist when answering phone calls for the inpatient pharmacy team, which may contribute to long hold times and call abandonment rates. Phone calls also may be directed inefficiently between clinical pharmacists (CPs) and pharmacy technicians. Team member time related to answering phone calls may not be captured or measured.
The Edward Hines, Jr. Veterans Affairs Hospital (EHJVAH) in Illinois offers primary, extended, and specialty care and is a tertiary care referral center. The facility operates 483 beds and serves 6 community-based outpatient clinics.
Implementation
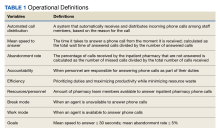
A new inpatient pharmacy service phone line extension was implemented. Data used to report quality metrics were obtained from the Global Navigator (GNAV), an information system that records calls, tracks the performance of agents, and coordinates personnel scheduling. The effectiveness of the ACD system was evaluated by quality metric goals of mean speed to answer ≤ 30 seconds and mean abandonment rate ≤ 5%. This project was determined to be quality improvement and was not reviewed by the EHJVAH Institutional Review Board.
The ACD system was set up in December 2020. After a 1-month implementation period, metrics were reported to the inpatient pharmacy team and leadership. By January 2021, EHJVAH fully implemented an ACD phone system operated by inpatient pharmacy technicians and CPs. EHJVAH inpatient pharmacy includes CPs who practice without a scope of practice and board-certified pharmacy technicians in 3 shifts. The CPs and pharmacy technicians work in the central pharmacy (the main pharmacy and inpatient pharmacy vault) or are decentralized with responsibility for answering phone calls and making deliveries (pharmacy technicians).
The pharmacy leadership team decided to implement 1 phone line with 2 ACD splits. The first split was directed to pharmacy technicians and the second to CPs. The intention was to streamline calls to be directed to proper team members within the inpatient pharmacy. The CP line also was designed to back up the pharmacy technician line. These calls were equally distributed among staff based on a standard algorithm. The pharmacy greeting stated, “Thank you for contacting the inpatient pharmacy at Hines VA Hospital. For missing doses, unit stock requests, or to speak with a pharmacy technician, please press 1. For clinical questions, order verification, or to speak with a pharmacist, please press 2.” Each inpatient pharmacy team member had a unique system login.
Fourteen ACD phone stations were established in the main pharmacy and in decentralized locations for order verification. The stations were distributed across the pharmacy service to optimize workload, space, and resources.
Training and Communication
Before implementing the inpatient pharmacy ACD phone system, the CPs and pharmacy technicians received mandatory ACD training. After the training, pharmacy team members were required to sign off on the training document to indicate that they had completed the course. The pharmacy team was trained on the importance of staffing the phones continuously. As a 24-hour pharmacy service in the acute care setting, any call may be critical for patient care.
A hospital-wide memorandum was distributed via email to all unit managers and hospital staff to educate them on the new ACD phone system, which included a new phone line extension for the inpatient pharmacy. Additionally, the inpatient pharmacy team was trained on the proper way of communicating the ACD phone system process with the hospital staff. The inpatient pharmacy team was notified that there would be an educational period to explain the queue process to hospital staff. Occasionally, hospital staff believed they were speaking to an automated system and hung up before their call was answered. The inpatient pharmacy team was instructed to notify the hospital staff to stay on the line since their call would be answered in the order it was received. Once the inpatient pharmacy team received proper training and felt comfortable with the phone system, it was set up and integrated into the workflow.
Postimplementation Evaluation
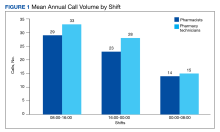
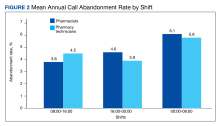
Inpatient pharmacy ACD phone system data were collected for 2021. To evaluate the effectiveness of an ACD system, the pharmacy leadership team set up the following metrics and goals for inpatient CPs and inpatient pharmacy technicians for monthly call volume/abandonment rate, mean speed to answer, mean call volume by shift, and the mean abandonment rate by shift.
Inpatient pharmacy technicians answered 27,655 calls with a mean call abandonment rate of 4.7%. and a mean 15.6 seconds to answer.
Discussion
Since implementing the inpatient pharmacy ACD phone system in January 2021, there have been successes and challenges. The implementation increased accountability and efficiency when answering pharmacy phone calls. An ACD uses an algorithm that ensures equitable distribution of phone calls between CPs and pharmacy technicians. Through this algorithm, the pharmacy team is held more accountable when answering incoming calls. Distributing phone calls equally allows for optimization and balances the workload. The ACD phone system also improved efficiency when answering incoming calls. By incorporating splits when a patient or health care professional calls, ACD routes the question to the appropriate staff member. As a result, CPs spend less time answering questions meant for pharmacy technicians and instead can answer clinical or order verification questions more efficiently.
ACD data also allow pharmacy leadership to assess staffing needs, depending on the call volume. Based on ACD data, the busiest time of day was 8:00 AM to 4:00 PM. Based on this information, pharmacy leadership plans to staff more appropriately to have more pharmacy technicians working during the first shift to attend to phone calls.
The mean call abandonment rate was 4.7% for both CPs and pharmacy technicians, which met the ≤ 5% goal. The highest call abandonment rate was from midnight to 8
Pharmacy technicians handled a higher total call volume, which may be attributed to more phone calls related to missing doses or unit stock requests compared with clinical questions or order verifications. This information may be beneficial to identify opportunities to improve pharmacy operations.
The main challenges encountered in the ACD implementation process were hardware installation and communication with hospital staff about the changes in the inpatient pharmacy phone system. To implement the new inpatient pharmacy ACD phone system, previous telephones and hardware were removed and replaced. Initially, hardware and installation delays made it difficult for the ACD phone system to operate efficiently in the early months of its implementation. The inpatient pharmacy team depends on the telecommunications system and computers for their daily activities. Delays and issues with the hardware and ACD phone system made it more difficult to provide patient care.
Communication is a continuous challenge to ensure that hospital staff are notified of the new inpatient pharmacy ACD phone number. Over time, the understanding and use of the new ACD phone system have increased dramatically, but there are still opportunities to capture any misdirected calls. Informal feedback was obtained at pharmacy huddles and 1-on-1 discussions with pharmacy staff, and the opinions were mixed. Members of the pharmacy staff expressed that the ACD phone system set up an effective way to triage phone calls. Another positive comment was that the system created a means of accountability for pharmacy phone calls. Critical feedback included challenges with triaging phone calls to appropriate pharmacists, because calls are assigned based on an algorithm, whereas clinical coverage is determined by designated unit daily assignments.
Limitations
There are potential limitations to this quality improvement project. This phone system may not apply to all inpatient hospital pharmacy settings. Potential limitations for implementation at other institutions may include but are not limited to, differing pharmacy practice models (centralized vs decentralized), implementation costs, and internal resources.
Future Goals
To improve the quality of service provided to patients and other hospital staff, the pharmacy leadership team can use the data to ensure that inpatient pharmacy technician resources are being used effectively during times of day with the greatest number of incoming ACD calls. The ACD phone system helps determine whether current resources are being used most efficiently and if they are not, can help identify areas of improvement.
The pharmacy leadership team plans on using reports for pharmacy team members to monitor performance. Reports on individual agent activity capture workload; this may be used as a performance-related metric for future performance plans.
Conclusions
The inpatient pharmacy ACD phone system at EHJVAH is a promising application of available technology. The implementation of the ACD system improved accountability, efficiency, work distribution, and the allocation of resources in the inpatient pharmacy service. The ACD phone system has yielded positive performance metrics including mean speed to answer ≤ 30 seconds and abandonment rate ≤ 5% over 12 months after implementation. With time, users of the inpatient pharmacy ACD phone system will become more comfortable with the technology, thus further improving the patient health care quality.
1. Rim MH, Thomas KC, Chandramouli J, Barrus SA, Nickman NA. Implementation and quality assessment of a pharmacy services call center for outpatient pharmacies and specialty pharmacy services in an academic health system. Am J Health Syst Pharm. 2018;75(10):633-641. doi:10.2146/ajhp170319
2. Patterson BJ, Doucette WR, Urmie JM, McDonough RP. Exploring relationships among pharmacy service use, patronage motives, and patient satisfaction. J Am Pharm Assoc (2003). 2013;53(4):382-389. doi:10.1331/JAPhA.2013.12100
3. Walker DM, Sieck CJ, Menser T, Huerta TR, Scheck McAlearney A. Information technology to support patient engagement: where do we stand and where can we go?. J Am Med Inform Assoc. 2017;24(6):1088-1094. doi:10.1093/jamia/ocx043
4. Menichetti J, Libreri C, Lozza E, Graffigna G. Giving patients a starring role in their own care: a bibliometric analysis of the on-going literature debate. Health Expect. 2016;19(3):516-526. doi:10.1111/hex.12299
5. Raimbault M, Guérin A, Caron É, Lebel D, Bussières J-F. Identifying and reducing distractions and interruptions in a pharmacy department. Am J Health Syst Pharm. 2013;70(3):186-190. doi:10.2146/ajhp120344
Pharmacy call centers have been successfully implemented in outpatient and specialty pharmacy settings.1 A centralized pharmacy call center gives patients immediate access to a pharmacist who can view their health records to answer specific questions or fulfill medication renewal requests.2-4 Little literature exists to describe its use in an inpatient setting.
Inpatient pharmacies receive numerous calls from health care professionals and patients. Challenges related to phone calls in the inpatient pharmacy setting may include interruptions, distractions, low accountability, poor efficiency, lack of optimal resources, and staffing.5 An unequal distribution and lack of accountability may exist when answering phone calls for the inpatient pharmacy team, which may contribute to long hold times and call abandonment rates. Phone calls also may be directed inefficiently between clinical pharmacists (CPs) and pharmacy technicians. Team member time related to answering phone calls may not be captured or measured.
The Edward Hines, Jr. Veterans Affairs Hospital (EHJVAH) in Illinois offers primary, extended, and specialty care and is a tertiary care referral center. The facility operates 483 beds and serves 6 community-based outpatient clinics.
Implementation
A new inpatient pharmacy service phone line extension was implemented. Data used to report quality metrics were obtained from the Global Navigator (GNAV), an information system that records calls, tracks the performance of agents, and coordinates personnel scheduling. The effectiveness of the ACD system was evaluated by quality metric goals of mean speed to answer ≤ 30 seconds and mean abandonment rate ≤ 5%. This project was determined to be quality improvement and was not reviewed by the EHJVAH Institutional Review Board.
The ACD system was set up in December 2020. After a 1-month implementation period, metrics were reported to the inpatient pharmacy team and leadership. By January 2021, EHJVAH fully implemented an ACD phone system operated by inpatient pharmacy technicians and CPs. EHJVAH inpatient pharmacy includes CPs who practice without a scope of practice and board-certified pharmacy technicians in 3 shifts. The CPs and pharmacy technicians work in the central pharmacy (the main pharmacy and inpatient pharmacy vault) or are decentralized with responsibility for answering phone calls and making deliveries (pharmacy technicians).
The pharmacy leadership team decided to implement 1 phone line with 2 ACD splits. The first split was directed to pharmacy technicians and the second to CPs. The intention was to streamline calls to be directed to proper team members within the inpatient pharmacy. The CP line also was designed to back up the pharmacy technician line. These calls were equally distributed among staff based on a standard algorithm. The pharmacy greeting stated, “Thank you for contacting the inpatient pharmacy at Hines VA Hospital. For missing doses, unit stock requests, or to speak with a pharmacy technician, please press 1. For clinical questions, order verification, or to speak with a pharmacist, please press 2.” Each inpatient pharmacy team member had a unique system login.
Fourteen ACD phone stations were established in the main pharmacy and in decentralized locations for order verification. The stations were distributed across the pharmacy service to optimize workload, space, and resources.
Training and Communication
Before implementing the inpatient pharmacy ACD phone system, the CPs and pharmacy technicians received mandatory ACD training. After the training, pharmacy team members were required to sign off on the training document to indicate that they had completed the course. The pharmacy team was trained on the importance of staffing the phones continuously. As a 24-hour pharmacy service in the acute care setting, any call may be critical for patient care.
A hospital-wide memorandum was distributed via email to all unit managers and hospital staff to educate them on the new ACD phone system, which included a new phone line extension for the inpatient pharmacy. Additionally, the inpatient pharmacy team was trained on the proper way of communicating the ACD phone system process with the hospital staff. The inpatient pharmacy team was notified that there would be an educational period to explain the queue process to hospital staff. Occasionally, hospital staff believed they were speaking to an automated system and hung up before their call was answered. The inpatient pharmacy team was instructed to notify the hospital staff to stay on the line since their call would be answered in the order it was received. Once the inpatient pharmacy team received proper training and felt comfortable with the phone system, it was set up and integrated into the workflow.
Postimplementation Evaluation
Inpatient pharmacy ACD phone system data were collected for 2021. To evaluate the effectiveness of an ACD system, the pharmacy leadership team set up the following metrics and goals for inpatient CPs and inpatient pharmacy technicians for monthly call volume/abandonment rate, mean speed to answer, mean call volume by shift, and the mean abandonment rate by shift.
Inpatient pharmacy technicians answered 27,655 calls with a mean call abandonment rate of 4.7%. and a mean 15.6 seconds to answer.
Discussion
Since implementing the inpatient pharmacy ACD phone system in January 2021, there have been successes and challenges. The implementation increased accountability and efficiency when answering pharmacy phone calls. An ACD uses an algorithm that ensures equitable distribution of phone calls between CPs and pharmacy technicians. Through this algorithm, the pharmacy team is held more accountable when answering incoming calls. Distributing phone calls equally allows for optimization and balances the workload. The ACD phone system also improved efficiency when answering incoming calls. By incorporating splits when a patient or health care professional calls, ACD routes the question to the appropriate staff member. As a result, CPs spend less time answering questions meant for pharmacy technicians and instead can answer clinical or order verification questions more efficiently.
ACD data also allow pharmacy leadership to assess staffing needs, depending on the call volume. Based on ACD data, the busiest time of day was 8:00 AM to 4:00 PM. Based on this information, pharmacy leadership plans to staff more appropriately to have more pharmacy technicians working during the first shift to attend to phone calls.
The mean call abandonment rate was 4.7% for both CPs and pharmacy technicians, which met the ≤ 5% goal. The highest call abandonment rate was from midnight to 8
Pharmacy technicians handled a higher total call volume, which may be attributed to more phone calls related to missing doses or unit stock requests compared with clinical questions or order verifications. This information may be beneficial to identify opportunities to improve pharmacy operations.
The main challenges encountered in the ACD implementation process were hardware installation and communication with hospital staff about the changes in the inpatient pharmacy phone system. To implement the new inpatient pharmacy ACD phone system, previous telephones and hardware were removed and replaced. Initially, hardware and installation delays made it difficult for the ACD phone system to operate efficiently in the early months of its implementation. The inpatient pharmacy team depends on the telecommunications system and computers for their daily activities. Delays and issues with the hardware and ACD phone system made it more difficult to provide patient care.
Communication is a continuous challenge to ensure that hospital staff are notified of the new inpatient pharmacy ACD phone number. Over time, the understanding and use of the new ACD phone system have increased dramatically, but there are still opportunities to capture any misdirected calls. Informal feedback was obtained at pharmacy huddles and 1-on-1 discussions with pharmacy staff, and the opinions were mixed. Members of the pharmacy staff expressed that the ACD phone system set up an effective way to triage phone calls. Another positive comment was that the system created a means of accountability for pharmacy phone calls. Critical feedback included challenges with triaging phone calls to appropriate pharmacists, because calls are assigned based on an algorithm, whereas clinical coverage is determined by designated unit daily assignments.
Limitations
There are potential limitations to this quality improvement project. This phone system may not apply to all inpatient hospital pharmacy settings. Potential limitations for implementation at other institutions may include but are not limited to, differing pharmacy practice models (centralized vs decentralized), implementation costs, and internal resources.
Future Goals
To improve the quality of service provided to patients and other hospital staff, the pharmacy leadership team can use the data to ensure that inpatient pharmacy technician resources are being used effectively during times of day with the greatest number of incoming ACD calls. The ACD phone system helps determine whether current resources are being used most efficiently and if they are not, can help identify areas of improvement.
The pharmacy leadership team plans on using reports for pharmacy team members to monitor performance. Reports on individual agent activity capture workload; this may be used as a performance-related metric for future performance plans.
Conclusions
The inpatient pharmacy ACD phone system at EHJVAH is a promising application of available technology. The implementation of the ACD system improved accountability, efficiency, work distribution, and the allocation of resources in the inpatient pharmacy service. The ACD phone system has yielded positive performance metrics including mean speed to answer ≤ 30 seconds and abandonment rate ≤ 5% over 12 months after implementation. With time, users of the inpatient pharmacy ACD phone system will become more comfortable with the technology, thus further improving the patient health care quality.
Pharmacy call centers have been successfully implemented in outpatient and specialty pharmacy settings.1 A centralized pharmacy call center gives patients immediate access to a pharmacist who can view their health records to answer specific questions or fulfill medication renewal requests.2-4 Little literature exists to describe its use in an inpatient setting.
Inpatient pharmacies receive numerous calls from health care professionals and patients. Challenges related to phone calls in the inpatient pharmacy setting may include interruptions, distractions, low accountability, poor efficiency, lack of optimal resources, and staffing.5 An unequal distribution and lack of accountability may exist when answering phone calls for the inpatient pharmacy team, which may contribute to long hold times and call abandonment rates. Phone calls also may be directed inefficiently between clinical pharmacists (CPs) and pharmacy technicians. Team member time related to answering phone calls may not be captured or measured.
The Edward Hines, Jr. Veterans Affairs Hospital (EHJVAH) in Illinois offers primary, extended, and specialty care and is a tertiary care referral center. The facility operates 483 beds and serves 6 community-based outpatient clinics.
Implementation
A new inpatient pharmacy service phone line extension was implemented. Data used to report quality metrics were obtained from the Global Navigator (GNAV), an information system that records calls, tracks the performance of agents, and coordinates personnel scheduling. The effectiveness of the ACD system was evaluated by quality metric goals of mean speed to answer ≤ 30 seconds and mean abandonment rate ≤ 5%. This project was determined to be quality improvement and was not reviewed by the EHJVAH Institutional Review Board.
The ACD system was set up in December 2020. After a 1-month implementation period, metrics were reported to the inpatient pharmacy team and leadership. By January 2021, EHJVAH fully implemented an ACD phone system operated by inpatient pharmacy technicians and CPs. EHJVAH inpatient pharmacy includes CPs who practice without a scope of practice and board-certified pharmacy technicians in 3 shifts. The CPs and pharmacy technicians work in the central pharmacy (the main pharmacy and inpatient pharmacy vault) or are decentralized with responsibility for answering phone calls and making deliveries (pharmacy technicians).
The pharmacy leadership team decided to implement 1 phone line with 2 ACD splits. The first split was directed to pharmacy technicians and the second to CPs. The intention was to streamline calls to be directed to proper team members within the inpatient pharmacy. The CP line also was designed to back up the pharmacy technician line. These calls were equally distributed among staff based on a standard algorithm. The pharmacy greeting stated, “Thank you for contacting the inpatient pharmacy at Hines VA Hospital. For missing doses, unit stock requests, or to speak with a pharmacy technician, please press 1. For clinical questions, order verification, or to speak with a pharmacist, please press 2.” Each inpatient pharmacy team member had a unique system login.
Fourteen ACD phone stations were established in the main pharmacy and in decentralized locations for order verification. The stations were distributed across the pharmacy service to optimize workload, space, and resources.
Training and Communication
Before implementing the inpatient pharmacy ACD phone system, the CPs and pharmacy technicians received mandatory ACD training. After the training, pharmacy team members were required to sign off on the training document to indicate that they had completed the course. The pharmacy team was trained on the importance of staffing the phones continuously. As a 24-hour pharmacy service in the acute care setting, any call may be critical for patient care.
A hospital-wide memorandum was distributed via email to all unit managers and hospital staff to educate them on the new ACD phone system, which included a new phone line extension for the inpatient pharmacy. Additionally, the inpatient pharmacy team was trained on the proper way of communicating the ACD phone system process with the hospital staff. The inpatient pharmacy team was notified that there would be an educational period to explain the queue process to hospital staff. Occasionally, hospital staff believed they were speaking to an automated system and hung up before their call was answered. The inpatient pharmacy team was instructed to notify the hospital staff to stay on the line since their call would be answered in the order it was received. Once the inpatient pharmacy team received proper training and felt comfortable with the phone system, it was set up and integrated into the workflow.
Postimplementation Evaluation
Inpatient pharmacy ACD phone system data were collected for 2021. To evaluate the effectiveness of an ACD system, the pharmacy leadership team set up the following metrics and goals for inpatient CPs and inpatient pharmacy technicians for monthly call volume/abandonment rate, mean speed to answer, mean call volume by shift, and the mean abandonment rate by shift.
Inpatient pharmacy technicians answered 27,655 calls with a mean call abandonment rate of 4.7%. and a mean 15.6 seconds to answer.
Discussion
Since implementing the inpatient pharmacy ACD phone system in January 2021, there have been successes and challenges. The implementation increased accountability and efficiency when answering pharmacy phone calls. An ACD uses an algorithm that ensures equitable distribution of phone calls between CPs and pharmacy technicians. Through this algorithm, the pharmacy team is held more accountable when answering incoming calls. Distributing phone calls equally allows for optimization and balances the workload. The ACD phone system also improved efficiency when answering incoming calls. By incorporating splits when a patient or health care professional calls, ACD routes the question to the appropriate staff member. As a result, CPs spend less time answering questions meant for pharmacy technicians and instead can answer clinical or order verification questions more efficiently.
ACD data also allow pharmacy leadership to assess staffing needs, depending on the call volume. Based on ACD data, the busiest time of day was 8:00 AM to 4:00 PM. Based on this information, pharmacy leadership plans to staff more appropriately to have more pharmacy technicians working during the first shift to attend to phone calls.
The mean call abandonment rate was 4.7% for both CPs and pharmacy technicians, which met the ≤ 5% goal. The highest call abandonment rate was from midnight to 8
Pharmacy technicians handled a higher total call volume, which may be attributed to more phone calls related to missing doses or unit stock requests compared with clinical questions or order verifications. This information may be beneficial to identify opportunities to improve pharmacy operations.
The main challenges encountered in the ACD implementation process were hardware installation and communication with hospital staff about the changes in the inpatient pharmacy phone system. To implement the new inpatient pharmacy ACD phone system, previous telephones and hardware were removed and replaced. Initially, hardware and installation delays made it difficult for the ACD phone system to operate efficiently in the early months of its implementation. The inpatient pharmacy team depends on the telecommunications system and computers for their daily activities. Delays and issues with the hardware and ACD phone system made it more difficult to provide patient care.
Communication is a continuous challenge to ensure that hospital staff are notified of the new inpatient pharmacy ACD phone number. Over time, the understanding and use of the new ACD phone system have increased dramatically, but there are still opportunities to capture any misdirected calls. Informal feedback was obtained at pharmacy huddles and 1-on-1 discussions with pharmacy staff, and the opinions were mixed. Members of the pharmacy staff expressed that the ACD phone system set up an effective way to triage phone calls. Another positive comment was that the system created a means of accountability for pharmacy phone calls. Critical feedback included challenges with triaging phone calls to appropriate pharmacists, because calls are assigned based on an algorithm, whereas clinical coverage is determined by designated unit daily assignments.
Limitations
There are potential limitations to this quality improvement project. This phone system may not apply to all inpatient hospital pharmacy settings. Potential limitations for implementation at other institutions may include but are not limited to, differing pharmacy practice models (centralized vs decentralized), implementation costs, and internal resources.
Future Goals
To improve the quality of service provided to patients and other hospital staff, the pharmacy leadership team can use the data to ensure that inpatient pharmacy technician resources are being used effectively during times of day with the greatest number of incoming ACD calls. The ACD phone system helps determine whether current resources are being used most efficiently and if they are not, can help identify areas of improvement.
The pharmacy leadership team plans on using reports for pharmacy team members to monitor performance. Reports on individual agent activity capture workload; this may be used as a performance-related metric for future performance plans.
Conclusions
The inpatient pharmacy ACD phone system at EHJVAH is a promising application of available technology. The implementation of the ACD system improved accountability, efficiency, work distribution, and the allocation of resources in the inpatient pharmacy service. The ACD phone system has yielded positive performance metrics including mean speed to answer ≤ 30 seconds and abandonment rate ≤ 5% over 12 months after implementation. With time, users of the inpatient pharmacy ACD phone system will become more comfortable with the technology, thus further improving the patient health care quality.
1. Rim MH, Thomas KC, Chandramouli J, Barrus SA, Nickman NA. Implementation and quality assessment of a pharmacy services call center for outpatient pharmacies and specialty pharmacy services in an academic health system. Am J Health Syst Pharm. 2018;75(10):633-641. doi:10.2146/ajhp170319
2. Patterson BJ, Doucette WR, Urmie JM, McDonough RP. Exploring relationships among pharmacy service use, patronage motives, and patient satisfaction. J Am Pharm Assoc (2003). 2013;53(4):382-389. doi:10.1331/JAPhA.2013.12100
3. Walker DM, Sieck CJ, Menser T, Huerta TR, Scheck McAlearney A. Information technology to support patient engagement: where do we stand and where can we go?. J Am Med Inform Assoc. 2017;24(6):1088-1094. doi:10.1093/jamia/ocx043
4. Menichetti J, Libreri C, Lozza E, Graffigna G. Giving patients a starring role in their own care: a bibliometric analysis of the on-going literature debate. Health Expect. 2016;19(3):516-526. doi:10.1111/hex.12299
5. Raimbault M, Guérin A, Caron É, Lebel D, Bussières J-F. Identifying and reducing distractions and interruptions in a pharmacy department. Am J Health Syst Pharm. 2013;70(3):186-190. doi:10.2146/ajhp120344
1. Rim MH, Thomas KC, Chandramouli J, Barrus SA, Nickman NA. Implementation and quality assessment of a pharmacy services call center for outpatient pharmacies and specialty pharmacy services in an academic health system. Am J Health Syst Pharm. 2018;75(10):633-641. doi:10.2146/ajhp170319
2. Patterson BJ, Doucette WR, Urmie JM, McDonough RP. Exploring relationships among pharmacy service use, patronage motives, and patient satisfaction. J Am Pharm Assoc (2003). 2013;53(4):382-389. doi:10.1331/JAPhA.2013.12100
3. Walker DM, Sieck CJ, Menser T, Huerta TR, Scheck McAlearney A. Information technology to support patient engagement: where do we stand and where can we go?. J Am Med Inform Assoc. 2017;24(6):1088-1094. doi:10.1093/jamia/ocx043
4. Menichetti J, Libreri C, Lozza E, Graffigna G. Giving patients a starring role in their own care: a bibliometric analysis of the on-going literature debate. Health Expect. 2016;19(3):516-526. doi:10.1111/hex.12299
5. Raimbault M, Guérin A, Caron É, Lebel D, Bussières J-F. Identifying and reducing distractions and interruptions in a pharmacy department. Am J Health Syst Pharm. 2013;70(3):186-190. doi:10.2146/ajhp120344
Severe atopic dermatitis raises risks for cardiovascular disease and venous thromboembolism
Key clinical point: Severe atopic dermatitis (AD) is associated with higher risks for venous thromboembolism and cardiovascular diseases in both children and adults.
Major finding: Children with severe AD vs those without AD had a significantly increased risk (adjusted hazard ratio; 95% CI) for cerebrovascular accidents (2.43; 1.13-5.22), diabetes (1.46; 1.06-2.01), and deep vein thrombosis (DVT; 2.13; 1.17-3.87). Among adults, the severe AD vs non-AD group had a significantly higher risk for cerebrovascular accidents (1.21; 1.13-1.30), diabetes (1.15; 1.09-1.22), dyslipidemia (1.11; 1.06-1.17), myocardial infarction (1.27; 1.15-1.39), DVT (1.64; 1.49-1.82), and pulmonary embolism (1.39; 1.21-1.60).
Study details: This population-based cohort study included 409,431 children (age < 18 years) and 625,083 adults with AD who were matched with 1,809,029 children and 2,678,888 adults without AD, respectively.
Disclosures: This study was supported by a contract from Pfizer, Inc. Some authors declared serving as consultants for or receiving research grants, honoraria, or consulting fees from various sources, including Pfizer. AR Lemeshow declared being an employee of Pfizer, Inc.
Source: Wan J, Chiesa Fuxench ZC, et al. Incidence of cardiovascular disease and venous thromboembolism in patients with atopic dermatitis. J Allergy Clin Immunol Pract. 2023 (Aug 10). doi: 10.1016/j.jaip.2023.08.007
Key clinical point: Severe atopic dermatitis (AD) is associated with higher risks for venous thromboembolism and cardiovascular diseases in both children and adults.
Major finding: Children with severe AD vs those without AD had a significantly increased risk (adjusted hazard ratio; 95% CI) for cerebrovascular accidents (2.43; 1.13-5.22), diabetes (1.46; 1.06-2.01), and deep vein thrombosis (DVT; 2.13; 1.17-3.87). Among adults, the severe AD vs non-AD group had a significantly higher risk for cerebrovascular accidents (1.21; 1.13-1.30), diabetes (1.15; 1.09-1.22), dyslipidemia (1.11; 1.06-1.17), myocardial infarction (1.27; 1.15-1.39), DVT (1.64; 1.49-1.82), and pulmonary embolism (1.39; 1.21-1.60).
Study details: This population-based cohort study included 409,431 children (age < 18 years) and 625,083 adults with AD who were matched with 1,809,029 children and 2,678,888 adults without AD, respectively.
Disclosures: This study was supported by a contract from Pfizer, Inc. Some authors declared serving as consultants for or receiving research grants, honoraria, or consulting fees from various sources, including Pfizer. AR Lemeshow declared being an employee of Pfizer, Inc.
Source: Wan J, Chiesa Fuxench ZC, et al. Incidence of cardiovascular disease and venous thromboembolism in patients with atopic dermatitis. J Allergy Clin Immunol Pract. 2023 (Aug 10). doi: 10.1016/j.jaip.2023.08.007
Key clinical point: Severe atopic dermatitis (AD) is associated with higher risks for venous thromboembolism and cardiovascular diseases in both children and adults.
Major finding: Children with severe AD vs those without AD had a significantly increased risk (adjusted hazard ratio; 95% CI) for cerebrovascular accidents (2.43; 1.13-5.22), diabetes (1.46; 1.06-2.01), and deep vein thrombosis (DVT; 2.13; 1.17-3.87). Among adults, the severe AD vs non-AD group had a significantly higher risk for cerebrovascular accidents (1.21; 1.13-1.30), diabetes (1.15; 1.09-1.22), dyslipidemia (1.11; 1.06-1.17), myocardial infarction (1.27; 1.15-1.39), DVT (1.64; 1.49-1.82), and pulmonary embolism (1.39; 1.21-1.60).
Study details: This population-based cohort study included 409,431 children (age < 18 years) and 625,083 adults with AD who were matched with 1,809,029 children and 2,678,888 adults without AD, respectively.
Disclosures: This study was supported by a contract from Pfizer, Inc. Some authors declared serving as consultants for or receiving research grants, honoraria, or consulting fees from various sources, including Pfizer. AR Lemeshow declared being an employee of Pfizer, Inc.
Source: Wan J, Chiesa Fuxench ZC, et al. Incidence of cardiovascular disease and venous thromboembolism in patients with atopic dermatitis. J Allergy Clin Immunol Pract. 2023 (Aug 10). doi: 10.1016/j.jaip.2023.08.007
Atopic dermatitis increases the risk for type 2 diabetes mellitus in adults
Key clinical point: Adults with newly diagnosed atopic dermatitis (AD) have a 44% increased risk of subsequently developing type 2 diabetes (T2D).
Major finding: The risk for new-onset T2D was significantly higher in adults with newly diagnosed AD vs control individuals without AD (adjusted hazard ratio 1.44; P < .001), with the risk being significantly greater in both men and women with AD (both P < .001).
Study details: Findings are from a retrospective cohort study including 36,692 adult patients with AD and 36,692 matched control individuals who had never been diagnosed with AD.
Disclosures: This study was supported by the National Research Foundation of Korea funded by the Ministry of Education, Science, and Technology and others. The authors declared no conflicts of interest.
Source: Won Lee S et al. Risk of type 2 diabetes mellitus in adult patients with atopic dermatitis. Diabetes Res Clin Pract. 2023;110883 (Aug 16). doi: 10.1016/j.diabres.2023.110883
Key clinical point: Adults with newly diagnosed atopic dermatitis (AD) have a 44% increased risk of subsequently developing type 2 diabetes (T2D).
Major finding: The risk for new-onset T2D was significantly higher in adults with newly diagnosed AD vs control individuals without AD (adjusted hazard ratio 1.44; P < .001), with the risk being significantly greater in both men and women with AD (both P < .001).
Study details: Findings are from a retrospective cohort study including 36,692 adult patients with AD and 36,692 matched control individuals who had never been diagnosed with AD.
Disclosures: This study was supported by the National Research Foundation of Korea funded by the Ministry of Education, Science, and Technology and others. The authors declared no conflicts of interest.
Source: Won Lee S et al. Risk of type 2 diabetes mellitus in adult patients with atopic dermatitis. Diabetes Res Clin Pract. 2023;110883 (Aug 16). doi: 10.1016/j.diabres.2023.110883
Key clinical point: Adults with newly diagnosed atopic dermatitis (AD) have a 44% increased risk of subsequently developing type 2 diabetes (T2D).
Major finding: The risk for new-onset T2D was significantly higher in adults with newly diagnosed AD vs control individuals without AD (adjusted hazard ratio 1.44; P < .001), with the risk being significantly greater in both men and women with AD (both P < .001).
Study details: Findings are from a retrospective cohort study including 36,692 adult patients with AD and 36,692 matched control individuals who had never been diagnosed with AD.
Disclosures: This study was supported by the National Research Foundation of Korea funded by the Ministry of Education, Science, and Technology and others. The authors declared no conflicts of interest.
Source: Won Lee S et al. Risk of type 2 diabetes mellitus in adult patients with atopic dermatitis. Diabetes Res Clin Pract. 2023;110883 (Aug 16). doi: 10.1016/j.diabres.2023.110883
Tralokinumab is safe and effective in older patients with atopic dermatitis
Key clinical point: Tralokinumab is well tolerated and effective in older adults with moderate-to-severe atopic dermatitis (AD).
Major finding: Compared with the placebo group, a significantly higher proportion of patients in ECZTRA 1 and 2 achieved ≥ 75% improvement in the Eczema Area and Severity Index score (33.9% vs 4.76%; P < .001) and an Investigator’s Global Assessment 0 or 1 score (16.95% vs 0%; P < .001) in the tralokinumab group at week 16. The adverse event (AE) rate was comparable between the groups; however, fewer patients discontinued treatment due to AE in the tralokinumab vs placebo group (5.3% vs 6.9%).
Study details: Findings are from a post hoc analysis of ECZTRA 1, 2, and 3 trials and included 104 older patients (≥65 years) with AD who received tralokinumab (n = 75) or placebo (n = 29).
Disclosures: This study was supported by LEO Pharma. Two authors declared being employees of LEO Pharma. Some authors declared receiving grants, personal fees, or consulting fees from LEO Pharma and other sources.
Source: Merola JF et al. Safety and efficacy of tralokinumab in older adults with moderate-to-severe atopic dermatitis: A secondary analysis. JAMA Dermatol. 2023 (Aug 23). doi: 10.1001/jamadermatol.2023.2626
Key clinical point: Tralokinumab is well tolerated and effective in older adults with moderate-to-severe atopic dermatitis (AD).
Major finding: Compared with the placebo group, a significantly higher proportion of patients in ECZTRA 1 and 2 achieved ≥ 75% improvement in the Eczema Area and Severity Index score (33.9% vs 4.76%; P < .001) and an Investigator’s Global Assessment 0 or 1 score (16.95% vs 0%; P < .001) in the tralokinumab group at week 16. The adverse event (AE) rate was comparable between the groups; however, fewer patients discontinued treatment due to AE in the tralokinumab vs placebo group (5.3% vs 6.9%).
Study details: Findings are from a post hoc analysis of ECZTRA 1, 2, and 3 trials and included 104 older patients (≥65 years) with AD who received tralokinumab (n = 75) or placebo (n = 29).
Disclosures: This study was supported by LEO Pharma. Two authors declared being employees of LEO Pharma. Some authors declared receiving grants, personal fees, or consulting fees from LEO Pharma and other sources.
Source: Merola JF et al. Safety and efficacy of tralokinumab in older adults with moderate-to-severe atopic dermatitis: A secondary analysis. JAMA Dermatol. 2023 (Aug 23). doi: 10.1001/jamadermatol.2023.2626
Key clinical point: Tralokinumab is well tolerated and effective in older adults with moderate-to-severe atopic dermatitis (AD).
Major finding: Compared with the placebo group, a significantly higher proportion of patients in ECZTRA 1 and 2 achieved ≥ 75% improvement in the Eczema Area and Severity Index score (33.9% vs 4.76%; P < .001) and an Investigator’s Global Assessment 0 or 1 score (16.95% vs 0%; P < .001) in the tralokinumab group at week 16. The adverse event (AE) rate was comparable between the groups; however, fewer patients discontinued treatment due to AE in the tralokinumab vs placebo group (5.3% vs 6.9%).
Study details: Findings are from a post hoc analysis of ECZTRA 1, 2, and 3 trials and included 104 older patients (≥65 years) with AD who received tralokinumab (n = 75) or placebo (n = 29).
Disclosures: This study was supported by LEO Pharma. Two authors declared being employees of LEO Pharma. Some authors declared receiving grants, personal fees, or consulting fees from LEO Pharma and other sources.
Source: Merola JF et al. Safety and efficacy of tralokinumab in older adults with moderate-to-severe atopic dermatitis: A secondary analysis. JAMA Dermatol. 2023 (Aug 23). doi: 10.1001/jamadermatol.2023.2626
Using Active Surveillance to Identify Monoclonal Antibody Candidates Among COVID-19–Positive Veterans in the Atlanta VA Health Care System
Early in the COVID-19 pandemic, monoclonal antibody (Mab) therapy was the only outpatient therapy for patients with COVID-19 experiencing mild-to-moderate symptoms. The Blocking Viral Attachment and Cell Entry with SARS-CoV-2 Neutralizing Antibodies (BLAZE-1) and the REGN-COV2 (Regeneron) clinical trials found participants treated with Mab had a shorter duration of symptoms and fewer hospitalizations compared with those receiving placebo.1,2 Mab therapy was most efficacious early in the disease course, and the initial US Food and Drug Administration (FDA) Emergency Use Authorization (EUA) of Mab therapies required use within 10 days of symptom onset.3
The impact of the COVID-19 pandemic has been felt disproportionately among marginalized racial and ethnic groups in the US. The COVID-19 Associated Hospitalization Surveillance Network found that non-Hispanic Black persons have significantly higher rates of hospitalization and death by COVID-19 compared with White persons.4-7 However, marginalized groups are underrepresented in the receipt of therapeutic agents for COVID-19. From March 2020 through August 2021, the mean monthly Mab use among Black patients (2.8%) was lower compared with White patients (4.0%), and Black patients received Mab 22.4% less often than White patients.7
The Mab clinical trials BLAZE-1 and REGN-COV2 study populations consisted of > 80% White participants.1,2 Receipt of COVID-19 outpatient treatments may not align with the disease burden in marginalized racial and ethnic groups, leading to health disparities. Although not exhaustive, reasons for these disparities include patient, health care practitioner, and systems-level issues: patient awareness, trust, and engagement with the health care system; health care practitioner awareness and advocacy to pursue COVID-19 treatment for the patient; and health care capacity to provide the medication and service.7
Here, we describe a novel, quality improvement initiative at the Atlanta Veterans Affairs Health Care System (AVAHCS) in Georgia that paired a proactive laboratory-based surveillance strategy to identify and engage veterans for Mab. By centralizing the surveillance and outreach process, we sought to reduce barriers to the Mab referral process and optimize access to life-saving medication.
Implementation
AVAHCS serves a diverse population of more than 129,000 (50.8% non-Hispanic Black veterans, 37.5% White veterans, and 11.7% of other races) at a main medical campus and 18 surrounding community-based outpatient clinics. From December 28, 2020, to August 31, 2021, veterans with a positive COVID-19 nasopharyngeal polymerase chain reaction (PCR) test at AVAHCS were screened daily. A central Mab team consisting of infectious disease (ID) clinical pharmacists and physicians reviewed daily lists of positive laboratory results and identified high-risk individuals for Mab eligibility, using the FDA EUA inclusion criteria. Eligible patients were called by a Mab team member to discuss Mab treatment, provide anticipatory guidance, obtain verbal consent, and schedule the infusion. Conventional referrals from non-Mab team members (eg, primary care physicians) were also accepted into the screening process and underwent the same procedures and risk prioritization strategy as those identified by the Mab team.
Clinic resources allowed for 1 to 2 patients per day to be given Mab, increasing to a maximum of 5 patients per day during the COVID-19 Delta variant surge. We followed our best clinical judgment in prioritizing patient selection, and we aligned our practice with the standards of our affiliated partner, Emory University. In circumstances where patients who were Mab-eligible outnumbered infusion availability, patients were prioritized using the Veterans Health Administration (VHA) COVID-19 (VACO) Index for 30-day COVID-19 mortality.8 As COVID-19 variants developed resistance to the recommended Mab infusions, bamlanivimab, bamlanivimab-etesevimab, or casirivimab-imdevimab, local protocols adapted to EUA revisions. The Mab team also adopted FDA eligibility criteria revisions as they were available.9,10
We describe the outcomes of our centralized screening process for Mab therapy, as measured by screening, uptake, and time to receipt of Mab from screening. We also describe the demographic and clinical characteristics of Mab recipients. Clinical outcomes include postinfusion adverse events (AEs) at day 1 and day 7, emergency department (ED) visits, inpatient hospitalization, and death.
Results
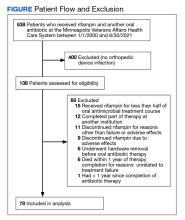
The Mab team screened 2028 veterans who were COVID-19 positive between December 28, 2020, and August 31, 2021, and identified 289 veterans (14%) who met the EUA criteria. One hundred thirty-two veterans (46%) completed Mab infusion, and of the remaining 145 veterans, 124 (86%) declined treatment, and 21 (14%) veterans did not complete Mab infusion largely due to not keeping the appointment. The Mab team active surveillance strategy identified 101 of 132 infusion candidates (77%); 82% had outpatient Mab infusion.
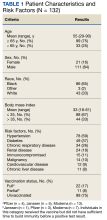
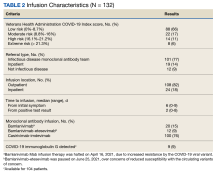
The mean age of veterans who received Mab was 55 years (range, 29-90), and 75% of veterans were aged ≥ 65 years; most were male (84%) and 86 (65%) identified as non-Hispanic Black individuals (Table 1).
Postinfusion AEs reported at day 1 and day 7 occurred for 38 veterans (29%) and 11 veterans (8%), respectively. Sixteen patients (12%) had postinfusion ED visit, and 12 patients (9%) required hospitalization. Eleven of the 12 hospitalized patients (92%) had worsening respiratory symptoms. No deaths occurred in the 132 patients who received Mab.
Discussion
This novel initiative to optimize access to outpatient COVID-19 treatment demonstrated how the Mab team proactively screened and reached out to eligible veterans with COVID-19 promptly. This approach removed layers in the traditional referral process that could be barriers to accessing care. More than three-quarters of patients who received Mab were identified through this strategy, and the uptake was high at 46%. Conventional passive referrals were suboptimal for identifying candidates, which was also the case at a neighboring institution.
In an Emory University study, referrals to the Mab clinic were made through a traditional, decentralized referral system and resulted in a lower uptake of Mab treatment (4.6%).11 One of the key advantages of the AVAHCS program was that we were able to provide individual education about COVID-19 and counsel on the benefits and risks of therapy. Having a structured, telehealth follow-up plan provided additional reassurance and support to the patient. These personalized patient connections likely helped increase acceptance of the Mab therapy.
Our surveillance and outreach strategy had high uptake among Black patients (65%), which exceeded the proportion of AVAHCS Black veterans (54%).12 In the Emory study, just 30% of the participants were Black patients.11 In a study of bamlanivimab use in Chicago, Black individuals represented just 11% of the study population. White patients were more likely to receive bamlanivimab compared with others races, and the likelihood of receiving bamlanivimab was significantly worse for Black patients (odds ratio, 0.28) compared with White patients.13 These studies highlight the disparity in COVID-19 outpatient treatment that does not reflect the racial and minority group representation of the community at large.
Limitations
The VHA medication allocation system at times created a significant mismatch in supply and demand, which significantly limited the AVAHCS Mab program. VHA facilities nationwide with Mab programs received discrete allocations through the US Department of Health and Human Services via VHA pharmacy benefits management services. Despite our large catchment, AVAHCS was allocated 6 or fewer doses of Mab per week during the evaluated period.
Without formal national guidance in the early period of Mab, the AVAHCS Mab team conferred with Emory University Mab clinicians as well as at other VHA facilities in the country to develop an optimal approach to resource allocation. The Mab team considered all EUA criteria to be as inclusive as possible. However, during times of high demand, our utilitarian approach tried to identify the highest-risk patients who would benefit the most from Mab. The VACO index was validated in early 2021, which facilitated decision making when demand was greater than supply. One limitation of the VACO index is its exclusion of several original Mab EUA criteria, including weight, hypertension, and nonmalignancy-related immunosuppression, into its algorithm.3,8
Conclusions
Through proactive screening and direct outreach to patients, the AVAHCS was able to achieve timely administration of Mab infusion that was well within the initial EUA time frame of 10 days and comparable with the time frame in the REGN-COV2 and BLAZE-1 trials. Improving access to resources by changing the referral structure helped engage veterans who may have otherwise missed the time frame for Mab therapy. The experience of the Mab infusion program at the AVAHCS provided valuable insight into how a health care system could effectively screen a large population and distribute the limited resource of Mab therapy in a timely and proportionate fashion among its represented demographic groups.
Acknowledgments
The authors acknowledge the Veterans Health Administration VISN 7 Clinical Resource Hub and Tele Primary Care group for their support.
1. Chen P, Nirula A, Heller B, et al; BLAZE-1 Investigators. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with COVID-19. N Engl J Med. 2021;384(3):229-237. doi:10.1056/NEJMoa2029849
2. Weinreich DM, Sivapalasingam S, Norton T, et al; Trial Investigators. REGN-COV2, a neutralizing antibody cocktail, in outpatients with COVID-19. N Engl J Med. 2021;384(3):238-251. doi:10.1056/NEJMoa2035002
3. US Food and Drug Administration. Fact sheet for health care providers, emergency use authorization (EUA) of bamlanivimab and etesevimab. Accessed August 6, 2023. https://www.fda.gov/media/145802/download
4. Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19)-associated hospitalization surveillance network (COVID-net). Updated March 24, 2023. Accessed August 6, 2023. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/covid-net/purpose-methods.html
5. Centers for Disease Control and Prevention, National Center for Health Statistics. Provisional COVID-19 deaths: distribution of deaths by race and Hispanic origin. Updated July 26, 2023. Accessed August 8, 2023. https://data.cdc.gov/NCHS/Provisional-COVID-19-Deaths-Distribution-of-Deaths/pj7m-y5uh
6. Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among Black patients and White patients with COVID-19. N Engl J Med. 2020;382(26):2534-2543. doi:10.1056NEJMsa2011686
7. Wiltz JL, Feehan AK, Mollinari AM, et al. Racial and ethnic disparities in receipt of medications for treatment of COVID-19 - United States, March 2020-August 2021. MMWR Morb Mortal Wkly Rep. 2022;71(3):96-102. doi:10.15585/mmwr.mm7103e1
8. King JT Jr, Yoon JS, Rentsch CT, et al. Development and validation of a 30-day mortality index based on pre-existing medical administrative data from 13,323 COVID-19 patients: the Veterans Health Administration COVID-19 (VACO) Index. PLoS One. 2020;15(11):e0241825. doi:10.1371/journal.pone.0241825
9. US Food and Drug Administration, Office of Media Affairs. Coronavirus (COVID-19) update: FDA revokes emergency use authorization for monoclonal antibody bamlanivimab. Accessed August 8, 2023. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-monoclonal-antibody-bamlanivimab
10. National Institutes of Health. Information on COVID-19 treatment, prevention and research. Accessed August 8, 2023. https://www.covid19treatmentguidelines.nih.gov
11. Anderson B, Smith Z, Edupuganti S, Yan X, Masi CM, Wu HM. Effect of monoclonal antibody treatment on clinical outcomes in ambulatory patients with coronavirus disease 2019. Open Forum Infect Dis. 2021;8(7):ofab315. Published 2021 Jun 12. doi:10.1093/ofid/ofab315
12. United States Census Bureau. Quick facts: DeKalb County, Georgia. Updated July 1, 2022. Accessed August 8, 2023. www.census.gov/quickfacts/dekalbcountygeorgia
13. Kumar R, Wu EL, Stosor V, et al. Real-world experience of bamlanivimab for coronavirus disease 2019 (COVID-19): a case-control study. Clin Infect Dis. 2022;74(1):24-31. doi:10.1093/cid/ciab305
Early in the COVID-19 pandemic, monoclonal antibody (Mab) therapy was the only outpatient therapy for patients with COVID-19 experiencing mild-to-moderate symptoms. The Blocking Viral Attachment and Cell Entry with SARS-CoV-2 Neutralizing Antibodies (BLAZE-1) and the REGN-COV2 (Regeneron) clinical trials found participants treated with Mab had a shorter duration of symptoms and fewer hospitalizations compared with those receiving placebo.1,2 Mab therapy was most efficacious early in the disease course, and the initial US Food and Drug Administration (FDA) Emergency Use Authorization (EUA) of Mab therapies required use within 10 days of symptom onset.3
The impact of the COVID-19 pandemic has been felt disproportionately among marginalized racial and ethnic groups in the US. The COVID-19 Associated Hospitalization Surveillance Network found that non-Hispanic Black persons have significantly higher rates of hospitalization and death by COVID-19 compared with White persons.4-7 However, marginalized groups are underrepresented in the receipt of therapeutic agents for COVID-19. From March 2020 through August 2021, the mean monthly Mab use among Black patients (2.8%) was lower compared with White patients (4.0%), and Black patients received Mab 22.4% less often than White patients.7
The Mab clinical trials BLAZE-1 and REGN-COV2 study populations consisted of > 80% White participants.1,2 Receipt of COVID-19 outpatient treatments may not align with the disease burden in marginalized racial and ethnic groups, leading to health disparities. Although not exhaustive, reasons for these disparities include patient, health care practitioner, and systems-level issues: patient awareness, trust, and engagement with the health care system; health care practitioner awareness and advocacy to pursue COVID-19 treatment for the patient; and health care capacity to provide the medication and service.7
Here, we describe a novel, quality improvement initiative at the Atlanta Veterans Affairs Health Care System (AVAHCS) in Georgia that paired a proactive laboratory-based surveillance strategy to identify and engage veterans for Mab. By centralizing the surveillance and outreach process, we sought to reduce barriers to the Mab referral process and optimize access to life-saving medication.
Implementation
AVAHCS serves a diverse population of more than 129,000 (50.8% non-Hispanic Black veterans, 37.5% White veterans, and 11.7% of other races) at a main medical campus and 18 surrounding community-based outpatient clinics. From December 28, 2020, to August 31, 2021, veterans with a positive COVID-19 nasopharyngeal polymerase chain reaction (PCR) test at AVAHCS were screened daily. A central Mab team consisting of infectious disease (ID) clinical pharmacists and physicians reviewed daily lists of positive laboratory results and identified high-risk individuals for Mab eligibility, using the FDA EUA inclusion criteria. Eligible patients were called by a Mab team member to discuss Mab treatment, provide anticipatory guidance, obtain verbal consent, and schedule the infusion. Conventional referrals from non-Mab team members (eg, primary care physicians) were also accepted into the screening process and underwent the same procedures and risk prioritization strategy as those identified by the Mab team.
Clinic resources allowed for 1 to 2 patients per day to be given Mab, increasing to a maximum of 5 patients per day during the COVID-19 Delta variant surge. We followed our best clinical judgment in prioritizing patient selection, and we aligned our practice with the standards of our affiliated partner, Emory University. In circumstances where patients who were Mab-eligible outnumbered infusion availability, patients were prioritized using the Veterans Health Administration (VHA) COVID-19 (VACO) Index for 30-day COVID-19 mortality.8 As COVID-19 variants developed resistance to the recommended Mab infusions, bamlanivimab, bamlanivimab-etesevimab, or casirivimab-imdevimab, local protocols adapted to EUA revisions. The Mab team also adopted FDA eligibility criteria revisions as they were available.9,10
We describe the outcomes of our centralized screening process for Mab therapy, as measured by screening, uptake, and time to receipt of Mab from screening. We also describe the demographic and clinical characteristics of Mab recipients. Clinical outcomes include postinfusion adverse events (AEs) at day 1 and day 7, emergency department (ED) visits, inpatient hospitalization, and death.
Results
The Mab team screened 2028 veterans who were COVID-19 positive between December 28, 2020, and August 31, 2021, and identified 289 veterans (14%) who met the EUA criteria. One hundred thirty-two veterans (46%) completed Mab infusion, and of the remaining 145 veterans, 124 (86%) declined treatment, and 21 (14%) veterans did not complete Mab infusion largely due to not keeping the appointment. The Mab team active surveillance strategy identified 101 of 132 infusion candidates (77%); 82% had outpatient Mab infusion.
The mean age of veterans who received Mab was 55 years (range, 29-90), and 75% of veterans were aged ≥ 65 years; most were male (84%) and 86 (65%) identified as non-Hispanic Black individuals (Table 1).
Postinfusion AEs reported at day 1 and day 7 occurred for 38 veterans (29%) and 11 veterans (8%), respectively. Sixteen patients (12%) had postinfusion ED visit, and 12 patients (9%) required hospitalization. Eleven of the 12 hospitalized patients (92%) had worsening respiratory symptoms. No deaths occurred in the 132 patients who received Mab.
Discussion
This novel initiative to optimize access to outpatient COVID-19 treatment demonstrated how the Mab team proactively screened and reached out to eligible veterans with COVID-19 promptly. This approach removed layers in the traditional referral process that could be barriers to accessing care. More than three-quarters of patients who received Mab were identified through this strategy, and the uptake was high at 46%. Conventional passive referrals were suboptimal for identifying candidates, which was also the case at a neighboring institution.
In an Emory University study, referrals to the Mab clinic were made through a traditional, decentralized referral system and resulted in a lower uptake of Mab treatment (4.6%).11 One of the key advantages of the AVAHCS program was that we were able to provide individual education about COVID-19 and counsel on the benefits and risks of therapy. Having a structured, telehealth follow-up plan provided additional reassurance and support to the patient. These personalized patient connections likely helped increase acceptance of the Mab therapy.
Our surveillance and outreach strategy had high uptake among Black patients (65%), which exceeded the proportion of AVAHCS Black veterans (54%).12 In the Emory study, just 30% of the participants were Black patients.11 In a study of bamlanivimab use in Chicago, Black individuals represented just 11% of the study population. White patients were more likely to receive bamlanivimab compared with others races, and the likelihood of receiving bamlanivimab was significantly worse for Black patients (odds ratio, 0.28) compared with White patients.13 These studies highlight the disparity in COVID-19 outpatient treatment that does not reflect the racial and minority group representation of the community at large.
Limitations
The VHA medication allocation system at times created a significant mismatch in supply and demand, which significantly limited the AVAHCS Mab program. VHA facilities nationwide with Mab programs received discrete allocations through the US Department of Health and Human Services via VHA pharmacy benefits management services. Despite our large catchment, AVAHCS was allocated 6 or fewer doses of Mab per week during the evaluated period.
Without formal national guidance in the early period of Mab, the AVAHCS Mab team conferred with Emory University Mab clinicians as well as at other VHA facilities in the country to develop an optimal approach to resource allocation. The Mab team considered all EUA criteria to be as inclusive as possible. However, during times of high demand, our utilitarian approach tried to identify the highest-risk patients who would benefit the most from Mab. The VACO index was validated in early 2021, which facilitated decision making when demand was greater than supply. One limitation of the VACO index is its exclusion of several original Mab EUA criteria, including weight, hypertension, and nonmalignancy-related immunosuppression, into its algorithm.3,8
Conclusions
Through proactive screening and direct outreach to patients, the AVAHCS was able to achieve timely administration of Mab infusion that was well within the initial EUA time frame of 10 days and comparable with the time frame in the REGN-COV2 and BLAZE-1 trials. Improving access to resources by changing the referral structure helped engage veterans who may have otherwise missed the time frame for Mab therapy. The experience of the Mab infusion program at the AVAHCS provided valuable insight into how a health care system could effectively screen a large population and distribute the limited resource of Mab therapy in a timely and proportionate fashion among its represented demographic groups.
Acknowledgments
The authors acknowledge the Veterans Health Administration VISN 7 Clinical Resource Hub and Tele Primary Care group for their support.
Early in the COVID-19 pandemic, monoclonal antibody (Mab) therapy was the only outpatient therapy for patients with COVID-19 experiencing mild-to-moderate symptoms. The Blocking Viral Attachment and Cell Entry with SARS-CoV-2 Neutralizing Antibodies (BLAZE-1) and the REGN-COV2 (Regeneron) clinical trials found participants treated with Mab had a shorter duration of symptoms and fewer hospitalizations compared with those receiving placebo.1,2 Mab therapy was most efficacious early in the disease course, and the initial US Food and Drug Administration (FDA) Emergency Use Authorization (EUA) of Mab therapies required use within 10 days of symptom onset.3
The impact of the COVID-19 pandemic has been felt disproportionately among marginalized racial and ethnic groups in the US. The COVID-19 Associated Hospitalization Surveillance Network found that non-Hispanic Black persons have significantly higher rates of hospitalization and death by COVID-19 compared with White persons.4-7 However, marginalized groups are underrepresented in the receipt of therapeutic agents for COVID-19. From March 2020 through August 2021, the mean monthly Mab use among Black patients (2.8%) was lower compared with White patients (4.0%), and Black patients received Mab 22.4% less often than White patients.7
The Mab clinical trials BLAZE-1 and REGN-COV2 study populations consisted of > 80% White participants.1,2 Receipt of COVID-19 outpatient treatments may not align with the disease burden in marginalized racial and ethnic groups, leading to health disparities. Although not exhaustive, reasons for these disparities include patient, health care practitioner, and systems-level issues: patient awareness, trust, and engagement with the health care system; health care practitioner awareness and advocacy to pursue COVID-19 treatment for the patient; and health care capacity to provide the medication and service.7
Here, we describe a novel, quality improvement initiative at the Atlanta Veterans Affairs Health Care System (AVAHCS) in Georgia that paired a proactive laboratory-based surveillance strategy to identify and engage veterans for Mab. By centralizing the surveillance and outreach process, we sought to reduce barriers to the Mab referral process and optimize access to life-saving medication.
Implementation
AVAHCS serves a diverse population of more than 129,000 (50.8% non-Hispanic Black veterans, 37.5% White veterans, and 11.7% of other races) at a main medical campus and 18 surrounding community-based outpatient clinics. From December 28, 2020, to August 31, 2021, veterans with a positive COVID-19 nasopharyngeal polymerase chain reaction (PCR) test at AVAHCS were screened daily. A central Mab team consisting of infectious disease (ID) clinical pharmacists and physicians reviewed daily lists of positive laboratory results and identified high-risk individuals for Mab eligibility, using the FDA EUA inclusion criteria. Eligible patients were called by a Mab team member to discuss Mab treatment, provide anticipatory guidance, obtain verbal consent, and schedule the infusion. Conventional referrals from non-Mab team members (eg, primary care physicians) were also accepted into the screening process and underwent the same procedures and risk prioritization strategy as those identified by the Mab team.
Clinic resources allowed for 1 to 2 patients per day to be given Mab, increasing to a maximum of 5 patients per day during the COVID-19 Delta variant surge. We followed our best clinical judgment in prioritizing patient selection, and we aligned our practice with the standards of our affiliated partner, Emory University. In circumstances where patients who were Mab-eligible outnumbered infusion availability, patients were prioritized using the Veterans Health Administration (VHA) COVID-19 (VACO) Index for 30-day COVID-19 mortality.8 As COVID-19 variants developed resistance to the recommended Mab infusions, bamlanivimab, bamlanivimab-etesevimab, or casirivimab-imdevimab, local protocols adapted to EUA revisions. The Mab team also adopted FDA eligibility criteria revisions as they were available.9,10
We describe the outcomes of our centralized screening process for Mab therapy, as measured by screening, uptake, and time to receipt of Mab from screening. We also describe the demographic and clinical characteristics of Mab recipients. Clinical outcomes include postinfusion adverse events (AEs) at day 1 and day 7, emergency department (ED) visits, inpatient hospitalization, and death.
Results
The Mab team screened 2028 veterans who were COVID-19 positive between December 28, 2020, and August 31, 2021, and identified 289 veterans (14%) who met the EUA criteria. One hundred thirty-two veterans (46%) completed Mab infusion, and of the remaining 145 veterans, 124 (86%) declined treatment, and 21 (14%) veterans did not complete Mab infusion largely due to not keeping the appointment. The Mab team active surveillance strategy identified 101 of 132 infusion candidates (77%); 82% had outpatient Mab infusion.
The mean age of veterans who received Mab was 55 years (range, 29-90), and 75% of veterans were aged ≥ 65 years; most were male (84%) and 86 (65%) identified as non-Hispanic Black individuals (Table 1).
Postinfusion AEs reported at day 1 and day 7 occurred for 38 veterans (29%) and 11 veterans (8%), respectively. Sixteen patients (12%) had postinfusion ED visit, and 12 patients (9%) required hospitalization. Eleven of the 12 hospitalized patients (92%) had worsening respiratory symptoms. No deaths occurred in the 132 patients who received Mab.
Discussion
This novel initiative to optimize access to outpatient COVID-19 treatment demonstrated how the Mab team proactively screened and reached out to eligible veterans with COVID-19 promptly. This approach removed layers in the traditional referral process that could be barriers to accessing care. More than three-quarters of patients who received Mab were identified through this strategy, and the uptake was high at 46%. Conventional passive referrals were suboptimal for identifying candidates, which was also the case at a neighboring institution.
In an Emory University study, referrals to the Mab clinic were made through a traditional, decentralized referral system and resulted in a lower uptake of Mab treatment (4.6%).11 One of the key advantages of the AVAHCS program was that we were able to provide individual education about COVID-19 and counsel on the benefits and risks of therapy. Having a structured, telehealth follow-up plan provided additional reassurance and support to the patient. These personalized patient connections likely helped increase acceptance of the Mab therapy.
Our surveillance and outreach strategy had high uptake among Black patients (65%), which exceeded the proportion of AVAHCS Black veterans (54%).12 In the Emory study, just 30% of the participants were Black patients.11 In a study of bamlanivimab use in Chicago, Black individuals represented just 11% of the study population. White patients were more likely to receive bamlanivimab compared with others races, and the likelihood of receiving bamlanivimab was significantly worse for Black patients (odds ratio, 0.28) compared with White patients.13 These studies highlight the disparity in COVID-19 outpatient treatment that does not reflect the racial and minority group representation of the community at large.
Limitations
The VHA medication allocation system at times created a significant mismatch in supply and demand, which significantly limited the AVAHCS Mab program. VHA facilities nationwide with Mab programs received discrete allocations through the US Department of Health and Human Services via VHA pharmacy benefits management services. Despite our large catchment, AVAHCS was allocated 6 or fewer doses of Mab per week during the evaluated period.
Without formal national guidance in the early period of Mab, the AVAHCS Mab team conferred with Emory University Mab clinicians as well as at other VHA facilities in the country to develop an optimal approach to resource allocation. The Mab team considered all EUA criteria to be as inclusive as possible. However, during times of high demand, our utilitarian approach tried to identify the highest-risk patients who would benefit the most from Mab. The VACO index was validated in early 2021, which facilitated decision making when demand was greater than supply. One limitation of the VACO index is its exclusion of several original Mab EUA criteria, including weight, hypertension, and nonmalignancy-related immunosuppression, into its algorithm.3,8
Conclusions
Through proactive screening and direct outreach to patients, the AVAHCS was able to achieve timely administration of Mab infusion that was well within the initial EUA time frame of 10 days and comparable with the time frame in the REGN-COV2 and BLAZE-1 trials. Improving access to resources by changing the referral structure helped engage veterans who may have otherwise missed the time frame for Mab therapy. The experience of the Mab infusion program at the AVAHCS provided valuable insight into how a health care system could effectively screen a large population and distribute the limited resource of Mab therapy in a timely and proportionate fashion among its represented demographic groups.
Acknowledgments
The authors acknowledge the Veterans Health Administration VISN 7 Clinical Resource Hub and Tele Primary Care group for their support.
1. Chen P, Nirula A, Heller B, et al; BLAZE-1 Investigators. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with COVID-19. N Engl J Med. 2021;384(3):229-237. doi:10.1056/NEJMoa2029849
2. Weinreich DM, Sivapalasingam S, Norton T, et al; Trial Investigators. REGN-COV2, a neutralizing antibody cocktail, in outpatients with COVID-19. N Engl J Med. 2021;384(3):238-251. doi:10.1056/NEJMoa2035002
3. US Food and Drug Administration. Fact sheet for health care providers, emergency use authorization (EUA) of bamlanivimab and etesevimab. Accessed August 6, 2023. https://www.fda.gov/media/145802/download
4. Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19)-associated hospitalization surveillance network (COVID-net). Updated March 24, 2023. Accessed August 6, 2023. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/covid-net/purpose-methods.html
5. Centers for Disease Control and Prevention, National Center for Health Statistics. Provisional COVID-19 deaths: distribution of deaths by race and Hispanic origin. Updated July 26, 2023. Accessed August 8, 2023. https://data.cdc.gov/NCHS/Provisional-COVID-19-Deaths-Distribution-of-Deaths/pj7m-y5uh
6. Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among Black patients and White patients with COVID-19. N Engl J Med. 2020;382(26):2534-2543. doi:10.1056NEJMsa2011686
7. Wiltz JL, Feehan AK, Mollinari AM, et al. Racial and ethnic disparities in receipt of medications for treatment of COVID-19 - United States, March 2020-August 2021. MMWR Morb Mortal Wkly Rep. 2022;71(3):96-102. doi:10.15585/mmwr.mm7103e1
8. King JT Jr, Yoon JS, Rentsch CT, et al. Development and validation of a 30-day mortality index based on pre-existing medical administrative data from 13,323 COVID-19 patients: the Veterans Health Administration COVID-19 (VACO) Index. PLoS One. 2020;15(11):e0241825. doi:10.1371/journal.pone.0241825
9. US Food and Drug Administration, Office of Media Affairs. Coronavirus (COVID-19) update: FDA revokes emergency use authorization for monoclonal antibody bamlanivimab. Accessed August 8, 2023. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-monoclonal-antibody-bamlanivimab
10. National Institutes of Health. Information on COVID-19 treatment, prevention and research. Accessed August 8, 2023. https://www.covid19treatmentguidelines.nih.gov
11. Anderson B, Smith Z, Edupuganti S, Yan X, Masi CM, Wu HM. Effect of monoclonal antibody treatment on clinical outcomes in ambulatory patients with coronavirus disease 2019. Open Forum Infect Dis. 2021;8(7):ofab315. Published 2021 Jun 12. doi:10.1093/ofid/ofab315
12. United States Census Bureau. Quick facts: DeKalb County, Georgia. Updated July 1, 2022. Accessed August 8, 2023. www.census.gov/quickfacts/dekalbcountygeorgia
13. Kumar R, Wu EL, Stosor V, et al. Real-world experience of bamlanivimab for coronavirus disease 2019 (COVID-19): a case-control study. Clin Infect Dis. 2022;74(1):24-31. doi:10.1093/cid/ciab305
1. Chen P, Nirula A, Heller B, et al; BLAZE-1 Investigators. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with COVID-19. N Engl J Med. 2021;384(3):229-237. doi:10.1056/NEJMoa2029849
2. Weinreich DM, Sivapalasingam S, Norton T, et al; Trial Investigators. REGN-COV2, a neutralizing antibody cocktail, in outpatients with COVID-19. N Engl J Med. 2021;384(3):238-251. doi:10.1056/NEJMoa2035002
3. US Food and Drug Administration. Fact sheet for health care providers, emergency use authorization (EUA) of bamlanivimab and etesevimab. Accessed August 6, 2023. https://www.fda.gov/media/145802/download
4. Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19)-associated hospitalization surveillance network (COVID-net). Updated March 24, 2023. Accessed August 6, 2023. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/covid-net/purpose-methods.html
5. Centers for Disease Control and Prevention, National Center for Health Statistics. Provisional COVID-19 deaths: distribution of deaths by race and Hispanic origin. Updated July 26, 2023. Accessed August 8, 2023. https://data.cdc.gov/NCHS/Provisional-COVID-19-Deaths-Distribution-of-Deaths/pj7m-y5uh
6. Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among Black patients and White patients with COVID-19. N Engl J Med. 2020;382(26):2534-2543. doi:10.1056NEJMsa2011686
7. Wiltz JL, Feehan AK, Mollinari AM, et al. Racial and ethnic disparities in receipt of medications for treatment of COVID-19 - United States, March 2020-August 2021. MMWR Morb Mortal Wkly Rep. 2022;71(3):96-102. doi:10.15585/mmwr.mm7103e1
8. King JT Jr, Yoon JS, Rentsch CT, et al. Development and validation of a 30-day mortality index based on pre-existing medical administrative data from 13,323 COVID-19 patients: the Veterans Health Administration COVID-19 (VACO) Index. PLoS One. 2020;15(11):e0241825. doi:10.1371/journal.pone.0241825
9. US Food and Drug Administration, Office of Media Affairs. Coronavirus (COVID-19) update: FDA revokes emergency use authorization for monoclonal antibody bamlanivimab. Accessed August 8, 2023. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-monoclonal-antibody-bamlanivimab
10. National Institutes of Health. Information on COVID-19 treatment, prevention and research. Accessed August 8, 2023. https://www.covid19treatmentguidelines.nih.gov
11. Anderson B, Smith Z, Edupuganti S, Yan X, Masi CM, Wu HM. Effect of monoclonal antibody treatment on clinical outcomes in ambulatory patients with coronavirus disease 2019. Open Forum Infect Dis. 2021;8(7):ofab315. Published 2021 Jun 12. doi:10.1093/ofid/ofab315
12. United States Census Bureau. Quick facts: DeKalb County, Georgia. Updated July 1, 2022. Accessed August 8, 2023. www.census.gov/quickfacts/dekalbcountygeorgia
13. Kumar R, Wu EL, Stosor V, et al. Real-world experience of bamlanivimab for coronavirus disease 2019 (COVID-19): a case-control study. Clin Infect Dis. 2022;74(1):24-31. doi:10.1093/cid/ciab305
Atopic dermatitis tied to a higher risk for inflammatory bowel disease in children and adults
Key clinical point: Children and adults with atopic dermatitis (AD) have a significantly increased risk of developing inflammatory bowel disease (IBD), including Crohn’s disease (CD).
Major finding: Children with vs without AD had a significantly higher risk for IBD (adjusted HR [aHR] 1.44; 95% CI 1.31-1.58) and CD (aHR 1.74; 95% CI 1.54-1.97), but the risk for ulcerative colitis (UC; aHR 1.65; 95% CI 1.02-2.67) was higher only in children with severe AD. Adults with vs without AD had a significantly increased risk for IBD (aHR 1.34; 95% CI 1.27-1.40), CD (aHR 1.36; 95% CI 1.26-1.47), and UC (aHR 1.32; 95% CI 1.24-1.41).
Study details: This population-based cohort study matched children (n = 409,431; age < 18 years) and adults (n = 625,083) with AD with control children (n = 1,809,029) and adults (n = 2,678,888) without AD, respectively.
Disclosures: This study was supported by a contract from Pfizer Inc. Five authors declared receiving grants, personal fees, and fellowship funding from various sources, including Pfizer Inc.
Source: Chiesa Fuxench ZC et al. Risk of inflammatory bowel disease in patients with atopic dermatitis. JAMA Dermatol. 2023 (Aug 30). doi: 10.1001/jamadermatol.2023.2875
Key clinical point: Children and adults with atopic dermatitis (AD) have a significantly increased risk of developing inflammatory bowel disease (IBD), including Crohn’s disease (CD).
Major finding: Children with vs without AD had a significantly higher risk for IBD (adjusted HR [aHR] 1.44; 95% CI 1.31-1.58) and CD (aHR 1.74; 95% CI 1.54-1.97), but the risk for ulcerative colitis (UC; aHR 1.65; 95% CI 1.02-2.67) was higher only in children with severe AD. Adults with vs without AD had a significantly increased risk for IBD (aHR 1.34; 95% CI 1.27-1.40), CD (aHR 1.36; 95% CI 1.26-1.47), and UC (aHR 1.32; 95% CI 1.24-1.41).
Study details: This population-based cohort study matched children (n = 409,431; age < 18 years) and adults (n = 625,083) with AD with control children (n = 1,809,029) and adults (n = 2,678,888) without AD, respectively.
Disclosures: This study was supported by a contract from Pfizer Inc. Five authors declared receiving grants, personal fees, and fellowship funding from various sources, including Pfizer Inc.
Source: Chiesa Fuxench ZC et al. Risk of inflammatory bowel disease in patients with atopic dermatitis. JAMA Dermatol. 2023 (Aug 30). doi: 10.1001/jamadermatol.2023.2875
Key clinical point: Children and adults with atopic dermatitis (AD) have a significantly increased risk of developing inflammatory bowel disease (IBD), including Crohn’s disease (CD).
Major finding: Children with vs without AD had a significantly higher risk for IBD (adjusted HR [aHR] 1.44; 95% CI 1.31-1.58) and CD (aHR 1.74; 95% CI 1.54-1.97), but the risk for ulcerative colitis (UC; aHR 1.65; 95% CI 1.02-2.67) was higher only in children with severe AD. Adults with vs without AD had a significantly increased risk for IBD (aHR 1.34; 95% CI 1.27-1.40), CD (aHR 1.36; 95% CI 1.26-1.47), and UC (aHR 1.32; 95% CI 1.24-1.41).
Study details: This population-based cohort study matched children (n = 409,431; age < 18 years) and adults (n = 625,083) with AD with control children (n = 1,809,029) and adults (n = 2,678,888) without AD, respectively.
Disclosures: This study was supported by a contract from Pfizer Inc. Five authors declared receiving grants, personal fees, and fellowship funding from various sources, including Pfizer Inc.
Source: Chiesa Fuxench ZC et al. Risk of inflammatory bowel disease in patients with atopic dermatitis. JAMA Dermatol. 2023 (Aug 30). doi: 10.1001/jamadermatol.2023.2875
Dupilumab improves sleep outcomes in atopic dermatitis
Key clinical point: Dupilumab significantly improved the overall sleep continuity and quality, itch, and other signs and symptoms of atopic dermatitis (AD) in patients with moderate-to-severe AD.
Major finding: At week 12, dupilumab vs placebo led to a significant improvement in the sleep Numeric Rating Scale (NRS), peak pruritus NRS, SCORing Atopic Dermatitis (SCORAD), SCORAD sleep visual analog scale, Eczema Area and Severity Index, Epworth Sleepiness Scale, and sleep-related impairment T-scores (all P < .001) and a lower overall treatment-emergent adverse event rate (56.7% vs 67.2%).
Study details: Findings are from the prospective phase 4 DUPISTAD study including patients with moderate-to-severe AD who were randomly assigned to receive 300 mg dupilumab every 2 weeks (n = 127) or placebo (n = 61).
Disclosures: This study was sponsored by Sanofi and Regeneron Pharmaceuticals Inc. Three authors declared being employees of and holding stocks or stock options in Sanofi or Regeneron. Other authors declared receiving grant support, travel grants, or speaker fees from or serving as principal investigators, advisory board members, or consultants for various sources.
Source: Merola JF et al. Dupilumab significantly improves sleep in adults with atopic dermatitis: Results from the 12-week placebo-controlled period of the 24-week phase 4 randomized double-blinded placebo-controlled DUPISTAD study. Br J Dermatol. 2023 (Aug 10). doi: 10.1093/bjd/ljad284
Key clinical point: Dupilumab significantly improved the overall sleep continuity and quality, itch, and other signs and symptoms of atopic dermatitis (AD) in patients with moderate-to-severe AD.
Major finding: At week 12, dupilumab vs placebo led to a significant improvement in the sleep Numeric Rating Scale (NRS), peak pruritus NRS, SCORing Atopic Dermatitis (SCORAD), SCORAD sleep visual analog scale, Eczema Area and Severity Index, Epworth Sleepiness Scale, and sleep-related impairment T-scores (all P < .001) and a lower overall treatment-emergent adverse event rate (56.7% vs 67.2%).
Study details: Findings are from the prospective phase 4 DUPISTAD study including patients with moderate-to-severe AD who were randomly assigned to receive 300 mg dupilumab every 2 weeks (n = 127) or placebo (n = 61).
Disclosures: This study was sponsored by Sanofi and Regeneron Pharmaceuticals Inc. Three authors declared being employees of and holding stocks or stock options in Sanofi or Regeneron. Other authors declared receiving grant support, travel grants, or speaker fees from or serving as principal investigators, advisory board members, or consultants for various sources.
Source: Merola JF et al. Dupilumab significantly improves sleep in adults with atopic dermatitis: Results from the 12-week placebo-controlled period of the 24-week phase 4 randomized double-blinded placebo-controlled DUPISTAD study. Br J Dermatol. 2023 (Aug 10). doi: 10.1093/bjd/ljad284
Key clinical point: Dupilumab significantly improved the overall sleep continuity and quality, itch, and other signs and symptoms of atopic dermatitis (AD) in patients with moderate-to-severe AD.
Major finding: At week 12, dupilumab vs placebo led to a significant improvement in the sleep Numeric Rating Scale (NRS), peak pruritus NRS, SCORing Atopic Dermatitis (SCORAD), SCORAD sleep visual analog scale, Eczema Area and Severity Index, Epworth Sleepiness Scale, and sleep-related impairment T-scores (all P < .001) and a lower overall treatment-emergent adverse event rate (56.7% vs 67.2%).
Study details: Findings are from the prospective phase 4 DUPISTAD study including patients with moderate-to-severe AD who were randomly assigned to receive 300 mg dupilumab every 2 weeks (n = 127) or placebo (n = 61).
Disclosures: This study was sponsored by Sanofi and Regeneron Pharmaceuticals Inc. Three authors declared being employees of and holding stocks or stock options in Sanofi or Regeneron. Other authors declared receiving grant support, travel grants, or speaker fees from or serving as principal investigators, advisory board members, or consultants for various sources.
Source: Merola JF et al. Dupilumab significantly improves sleep in adults with atopic dermatitis: Results from the 12-week placebo-controlled period of the 24-week phase 4 randomized double-blinded placebo-controlled DUPISTAD study. Br J Dermatol. 2023 (Aug 10). doi: 10.1093/bjd/ljad284
Rifampin for Prosthetic Joint Infections: Lessons Learned Over 20 Years at a VA Medical Center
Orthopedic implants are frequently used to repair fractures and replace joints. The number of total joint replacements is high, with > 1 million total hip (THA) and total knee (TKA) arthroplasties performed in the United States each year.1 While most joint arthroplasties are successful and significantly improve patient quality of life, a small proportion become infected.2 Prosthetic joint infection (PJI) causes substantial morbidity and mortality, particularly among older patients, and is difficult and costly to treat.3
The historic gold standard treatment for PJI is a 2-stage replacement, wherein the prosthesis is removed in one procedure and a new prosthesis is implanted in a second procedure after an extended course of antibiotics. This approach requires the patient to undergo 2 major procedures and spend considerable time without a functioning prosthesis, contributing to immobility and deconditioning. This option is difficult for frail or older patients and is associated with high medical costs.4
In 1998, a novel method of treatment known as debridement, antibiotics, and implant retention (DAIR) was evaluated in a small, randomized controlled trial.5 This study used a unique antimicrobial approach: the administration of ciprofloxacin plus either rifampin or placebo for 3 to 6 months, combined with a single surgical debridement. Eliminating a second surgical procedure and largely relying on oral antimicrobials reduces surgical risks and decreases costs.4 Current guidelines endorse DAIR with rifampin and a second antibiotic for patients diagnosed with PJI within about 30 days of prosthesis implantation who have a well-fixed implant without evidence of a sinus tract.6 Clinical trial data demonstrate that this approach is > 90% effective in patients with a well-fixed prosthesis and acute staphylococcal PJI.3,7
Thus far, clinical trials examining this approach have been small and did not include veterans who are typically older and have more comorbidities.8 The Minneapolis Veterans Affairs Health Care System (MVAHCS) infectious disease section has implemented the rifampin-based DAIR approach for orthopedic device-related infections since this approach was first described in 1998 but has not systematically evaluated its effectiveness or whether there are areas for improvement.
METHODS
We conducted a retrospective analysis of patients who underwent DAIR combined with a rifampin-containing regimen at the MVAHCS from January 1, 2001, through June 30, 2021. Inclusion required a diagnosis of orthopedic device-related infection and treatment with DAIR followed by antimicrobial therapy that included rifampin for 1 to 6 months. PJI was defined by meeting ≥ 1 of the following criteria: (1) isolation of the same microorganism from ≥ 2 cultures from joint aspirates or intraoperative tissue specimens; (2) purulence surrounding the prosthesis at the time of surgery; (3) acute inflammation consistent with infection on histopathological examination or periprosthetic tissue; or (4) presence of a sinus tract communicating with the prosthesis.
All cases of orthopedic device infection managed with DAIR and rifampin were included, regardless of implant stability, age of the implant at the time of symptom onset, presence of a sinus tract, or infecting microorganism. Exclusion criteria included patients who started or finished PJI treatment at another facility, were lost to follow-up, discontinued rifampin, died within 1 year of completing antibiotic therapy due to reasons unrelated to treatment failure, received rifampin for < 50% of their antimicrobial treatment course, had complete hardware removal, or had < 1 year between the completion of antimicrobial therapy and the time of data collection.
Management of DAIR procedures at the MVAHCS involves evaluating the fixation of the prosthesis, tissue sampling for microbiological analysis, and thorough debridement of infected tissue. Following debridement, a course of IV antibiotics is administered before initiating oral antibiotic therapy. To protect against resistance, rifampin is combined with another antibiotic typically from the fluoroquinolone, tetracycline, or cephalosporin class. Current guidelines suggest 3 and 6 months of oral antibiotics for prosthetic hip and knee infections, respectively.6
Treatment Outcomes
The primary outcome was treatment success, defined as meeting all of the following: (1) lack of clinical signs and symptoms of infection; (2) absence of radiological signs of loosening or infection within 1 year after the conclusion of treatment; and (3) absence of additional PJI treatment interventions for the prosthesis of concern within 1 year after completing the original antibiotic treatment.
Treatment failure was defined as meeting any of the following: (1) recurrence of PJI (original strain or different microorganism) within 1 year after the completion of antibiotic therapy; (2) death attributed to PJI anytime after the initial debridement; (3) removal of the prosthetic joint within 1 year after the completion of antibiotic therapy; or (4) long-term antibiotic use to suppress the PJI after the completion of the initial antibiotic therapy.
Statistical Analysis
Descriptive statistics were used to define the baseline characteristics of patients receiving rifampin therapy for orthopedic implant infections at the MVAHCS. Variables analyzed were age, sex, race and ethnicity, type of implant, age of implant, duration of symptoms, comorbidities (diabetes and rheumatoid arthritis), and presence of chronic infection. Patients were classified as having a chronic infection if they received previous infection treatment (antibiotics or surgery) for the orthopedic device in question. We created this category because patients with persistent infection after a medical or surgical attempt at treatment are likely to have a higher probability of treatment failure compared with those with no prior therapy. Charlson Comorbidity Index was calculated using clinical information present at the onset of infection.9 Fisher exact test was used to assess differences between categorical variables, and an independent t test was used to assess differences in continuous variables. P < .05 indicated statistical significance.
To assess the ability of a rifampin-based regimen to achieve a cure of PJI, we grouped participants into 2 categories: those with an intent to cure strategy and those without intent to cure based on documentation in the electronic health record (EHR). Participants who were prescribed rifampin with the documented goal of prosthesis retention with no further suppressive antibiotics were included in the intent-to-cure group, the primary focus of this study. Those excluded from the intent-to-cure group were given rifampin and another antibiotic, but there was a documented plan of either ongoing chronic suppression or eventual explantation; these participants were placed in the without-intent-to-cure group. Analysis of treatment success and failure was limited to the intent-to-cure group, whereas both groups were included for assessment of adverse effects (AEs) and treatment duration. This project was reviewed by the MVAHCS Institutional Review Board and determined to be a quality improvement initiative and to not meet the definition of research, and as such did not require review; it was reviewed and approved by the MVAHCS Research and Development Committee.
RESULTS
A total of 538 patients were identified who simultaneously received rifampin and another oral antibiotic between January 1, 2000, and June 30, 2021.
Forty-two participants (54%) had Staphylococcus aureus and 31 participants (40%) had coagulase-negative staphylococci infections, while 11 gram-negative organisms (14%) and 6 gram-positive anaerobic cocci (8%) infections were noted. Cutibacterium acnes and Streptococcus agalactiae were each found in 3 participants (4% of), and diphtheroids (not further identified) was found on 2 participants (3%). Candida albicans was identified in a single participant (1%), along with coagulase-negative staphylococci, and 2 participants (3%) had no identified organisms. There were multiple organisms isolated from 20 patients (26%).
Fifty participants had clear documentation in their EHR that cure of infection was the goal, meeting the criteria for the intent-to-cure group. The remaining 28 participants were placed in the without-intent-to-cure group. Success and failure rates were only measured in the intent-to-cure group, as by definition the without-intent-to-cure group patients would meet the criteria for failure (removal of prosthesis or long-term antibiotic use). The without-intent-to-cure group had a higher median age than the intent-to-cure group (69 years vs 64 years, P = .24) and a higher proportion of male participants (96% vs 80%, P = .09). The median (IQR) implant age of 11 months (1.0-50.5) in the without-intent-to-cure group was also higher than the median implant age of 1 month (0.6-22.0) in the primary group (P = .22). In the without-intent-to-cure group, 19 participants (68%) had a chronic infection, compared with 11 (22%) in the intent-to-cure group (P < .001).
The mean (SD) Charlson Comorbidity Index in the without-intent-to-cure group was 2.5 (1.3) compared with 1.9 (1.4) in the intent-to-cure group (P = .09). There was no significant difference in the type of implant or microbiology of the infecting organism between the 2 groups, although it should be noted that in the intent-to-cure group, 48 patients (96%) had Staphylococcus aureus or coagulase-negative staphylococci isolated.
The median (IQR) dosage of rifampin was 600 mg (300-900). The secondary oral antibiotics used most often were 36 fluoroquinolones (46%) followed by 20 tetracyclines (26%), 6 cephalosporins (8%), and 6 penicillins (8%). Additionally, 6 participants (8%) received IV vancomycin, and 1 participant (1%) was given an oral antifungal in addition to a fluoroquinolone because cultures revealed bacterial and fungal growth. The median (IQR) duration of antimicrobial therapy was 3 months (1.4-3.0). The mean (SD) duration of antimicrobial therapy was 3.6 (2.4) months for TKA infections and 2.4 (0.9) months for THA infections.
Clinical Outcome
Forty-one intent-to-cure group participants (82%) experienced treatment success. We further subdivided the intent-to-cure group by implant age. Participants whose implant was < 2 months old had a success rate of 93%, whereas patients whose implant was older had a success rate of 65% (P = .02).
Secondary Outcomes
The median (IQR) duration of antimicrobial treatment was 3 months (1.4-3.0) for the 38 patients with TKA-related infections and 3 months (1.4-6.0) for the 29 patients with THA infections. AEs were recorded in 24 (31%) of all study participants. Of those with AEs, the average number reported per patient was 1.6. Diarrhea, gastric upset, and nausea were each reported 7 times, accounting for 87% of all recorded AEs. Five participants reported having a rash while on antibiotics, and 2 experienced dysgeusia. One participant reported developing a yeast infection and another experienced vaginitis.
DISCUSSION
Among patients with orthopedic implant infections treated with intent to cure using a rifampin-containing antibiotic regimen at the MVAHCS, 82% had clinical success. Although this is lower than the success rates reported in clinical trials, this is not entirely unexpected.5,7 In most clinical trials studying DAIR and rifampin for PJI, patients are excluded if they do not have an acute staphylococcal infection in the setting of a well-fixed prosthesis without evidence of a sinus tract. Such exclusion criteria were not present in our retrospective study, which was designed to evaluate the real-world practice patterns at this facility. The population at the US Department of Veterans Affairs (VA) is older, more frail, and with more comorbid conditions than populations in prior studies. It is possible that patients with characteristics that would have caused them to be excluded from a clinical trial would be less likely to receive rifampin therapy with the intent to cure. This is suggested by the significantly higher prevalence of chronic infections (68%) in the without-intent-to-cure group compared with 22% in the intent-to-cure group. However, there were reasonably high proportions of participants included in the intent-to-cure group who did have conditions that would have led to their exclusion from prior trials, such as chronic infection (22%) and implant age ≥ 2 months (40%).
When evaluating participants by the age of their implant, treatment success rose to 93% for patients with implants < 2 months old compared with 65% for patients with older implants. This suggests that participants with a newer implant or more recent infection have a greater likelihood of successful treatment, which is consistent with the results of previous clinical trials.5,10 Considering how difficult multiple surgeries can be for older adult patients with comorbidities, we suggest that DAIR with a rifampin-containing regimen be considered as the primary treatment option for early PJIs at the MVAHCS. We also note inconsistent adherence to IDSA treatment guidelines on rifampin therapy, in that patients without intent to cure were prescribed a regimen including rifampin. This may reflect appropriate variability in the care of individual patients but may also offer an opportunity to change processes to improve care.
Limitations
Our analysis has limitations. As with any retrospective study evaluating the efficacy of a specific antibiotic, we were not able to attribute specific outcomes to the antibiotic of interest. Since the choice of antibiotics was left to the treating health care practitioner, therapy was not standardized, and because this was a retrospective study, causal relationships could not be inferred. Our analysis was also limited by the lack of intent to cure in 28 participants (36%), which could be an indication of practitioner bias in therapy selection or characteristic differences between the 2 groups. We looked for signs of infection failure 1 year after the completion of antimicrobial therapy, but longer follow-up could have led to higher rates of failure. Also, while participants’ infections were considered cured if they never sought further medical care for the infection at the MVAHCS, it is possible that patients could have sought care at another facility. We note that 9 patients were excluded because they were unable to complete a treatment course due to rifampin AEs, meaning that the success rates reported here reflect the success that may be expected if a patient can tolerate and complete a rifampin-based regimen. This study was conducted in a single VA hospital and may not be generalizable to nonveterans or veterans seeking care at other facilities.
Conclusions
DAIR followed by a short course of IV antibiotics and an oral regimen including rifampin and another antimicrobial is a reasonable option for veterans with acute staphylococcal orthopedic device infections at the MVAHCS. Patients with a well-placed prosthesis and an acute infection seem especially well suited for this treatment, and treatment with intent to cure should be pursued in patients who meet the criteria for rifampin therapy.
Acknowledgments
We thank Erik Stensgard, PharmD, for assistance in compiling the list of patients receiving rifampin and another antimicrobial.
1. Maradit Kremers H, Larson DR, Crowson CS, et al. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surg Am. 2015;97(17):1386-1397. doi:10.2106/JBJS.N.01141
2. Kapadia BH, Berg RA, Daley JA, Fritz J, Bhave A, Mont MA. Periprosthetic joint infection. Lancet. 2016;387(10016):386-394. doi:10.1016/S0140-6736(14)61798-0
3. Zhan C, Kaczmarek R, Loyo-Berrios N, Sangl J, Bright RA. Incidence and short-term outcomes of primary and revision hip replacement in the United States. J Bone Joint Surg Am. 2007;89(3):526-533. doi:10.2106/JBJS.F.00952
4. Fisman DN, Reilly DT, Karchmer AW, Goldie SJ. Clinical effectiveness and cost-effectiveness of 2 management strategies for infected total hip arthroplasty in the elderly. Clin Infect Dis. 2001;32(3):419-430. doi:10.1086/318502
5. Zimmerli W, Widmer AF, Blatter M, Frei R, Ochsner PE. Role of rifampin for treatment of orthopedic implant-related staphylococcal infections: a randomized controlled trial. Foreign-Body Infection (FBI) Study Group. JAMA. 1998;279(19):1537-1541. doi:10.1001/jama.279.19.1537
6. Osmon DR, Berbari EF, Berendt AR, et al. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013;56(1):e1-e25. doi:10.1093/cid/cis803
7. Lora-Tamayo J, Euba G, Cobo J, et al. Short- versus long-duration levofloxacin plus rifampicin for acute staphylococcal prosthetic joint infection managed with implant retention: a randomised clinical trial. Int J Antimicrob Agents. 2016;48(3):310-316. doi:10.1016/j.ijantimicag.2016.05.021
8. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257. doi:10.1001/archinte.160.21.3252
9. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi:10.1016/0021-9681(87)90171-8
10. Vilchez F, Martínez-Pastor JC, García-Ramiro S, et al. Outcome and predictors of treatment failure in early post-surgical prosthetic joint infections due to Staphylococcus aureus treated with debridement. Clin Microbiol Infect. 2011;17(3):439-444. doi:10.1111/j.1469-0691.2010.03244.x
Orthopedic implants are frequently used to repair fractures and replace joints. The number of total joint replacements is high, with > 1 million total hip (THA) and total knee (TKA) arthroplasties performed in the United States each year.1 While most joint arthroplasties are successful and significantly improve patient quality of life, a small proportion become infected.2 Prosthetic joint infection (PJI) causes substantial morbidity and mortality, particularly among older patients, and is difficult and costly to treat.3
The historic gold standard treatment for PJI is a 2-stage replacement, wherein the prosthesis is removed in one procedure and a new prosthesis is implanted in a second procedure after an extended course of antibiotics. This approach requires the patient to undergo 2 major procedures and spend considerable time without a functioning prosthesis, contributing to immobility and deconditioning. This option is difficult for frail or older patients and is associated with high medical costs.4
In 1998, a novel method of treatment known as debridement, antibiotics, and implant retention (DAIR) was evaluated in a small, randomized controlled trial.5 This study used a unique antimicrobial approach: the administration of ciprofloxacin plus either rifampin or placebo for 3 to 6 months, combined with a single surgical debridement. Eliminating a second surgical procedure and largely relying on oral antimicrobials reduces surgical risks and decreases costs.4 Current guidelines endorse DAIR with rifampin and a second antibiotic for patients diagnosed with PJI within about 30 days of prosthesis implantation who have a well-fixed implant without evidence of a sinus tract.6 Clinical trial data demonstrate that this approach is > 90% effective in patients with a well-fixed prosthesis and acute staphylococcal PJI.3,7
Thus far, clinical trials examining this approach have been small and did not include veterans who are typically older and have more comorbidities.8 The Minneapolis Veterans Affairs Health Care System (MVAHCS) infectious disease section has implemented the rifampin-based DAIR approach for orthopedic device-related infections since this approach was first described in 1998 but has not systematically evaluated its effectiveness or whether there are areas for improvement.
METHODS
We conducted a retrospective analysis of patients who underwent DAIR combined with a rifampin-containing regimen at the MVAHCS from January 1, 2001, through June 30, 2021. Inclusion required a diagnosis of orthopedic device-related infection and treatment with DAIR followed by antimicrobial therapy that included rifampin for 1 to 6 months. PJI was defined by meeting ≥ 1 of the following criteria: (1) isolation of the same microorganism from ≥ 2 cultures from joint aspirates or intraoperative tissue specimens; (2) purulence surrounding the prosthesis at the time of surgery; (3) acute inflammation consistent with infection on histopathological examination or periprosthetic tissue; or (4) presence of a sinus tract communicating with the prosthesis.
All cases of orthopedic device infection managed with DAIR and rifampin were included, regardless of implant stability, age of the implant at the time of symptom onset, presence of a sinus tract, or infecting microorganism. Exclusion criteria included patients who started or finished PJI treatment at another facility, were lost to follow-up, discontinued rifampin, died within 1 year of completing antibiotic therapy due to reasons unrelated to treatment failure, received rifampin for < 50% of their antimicrobial treatment course, had complete hardware removal, or had < 1 year between the completion of antimicrobial therapy and the time of data collection.
Management of DAIR procedures at the MVAHCS involves evaluating the fixation of the prosthesis, tissue sampling for microbiological analysis, and thorough debridement of infected tissue. Following debridement, a course of IV antibiotics is administered before initiating oral antibiotic therapy. To protect against resistance, rifampin is combined with another antibiotic typically from the fluoroquinolone, tetracycline, or cephalosporin class. Current guidelines suggest 3 and 6 months of oral antibiotics for prosthetic hip and knee infections, respectively.6
Treatment Outcomes
The primary outcome was treatment success, defined as meeting all of the following: (1) lack of clinical signs and symptoms of infection; (2) absence of radiological signs of loosening or infection within 1 year after the conclusion of treatment; and (3) absence of additional PJI treatment interventions for the prosthesis of concern within 1 year after completing the original antibiotic treatment.
Treatment failure was defined as meeting any of the following: (1) recurrence of PJI (original strain or different microorganism) within 1 year after the completion of antibiotic therapy; (2) death attributed to PJI anytime after the initial debridement; (3) removal of the prosthetic joint within 1 year after the completion of antibiotic therapy; or (4) long-term antibiotic use to suppress the PJI after the completion of the initial antibiotic therapy.
Statistical Analysis
Descriptive statistics were used to define the baseline characteristics of patients receiving rifampin therapy for orthopedic implant infections at the MVAHCS. Variables analyzed were age, sex, race and ethnicity, type of implant, age of implant, duration of symptoms, comorbidities (diabetes and rheumatoid arthritis), and presence of chronic infection. Patients were classified as having a chronic infection if they received previous infection treatment (antibiotics or surgery) for the orthopedic device in question. We created this category because patients with persistent infection after a medical or surgical attempt at treatment are likely to have a higher probability of treatment failure compared with those with no prior therapy. Charlson Comorbidity Index was calculated using clinical information present at the onset of infection.9 Fisher exact test was used to assess differences between categorical variables, and an independent t test was used to assess differences in continuous variables. P < .05 indicated statistical significance.
To assess the ability of a rifampin-based regimen to achieve a cure of PJI, we grouped participants into 2 categories: those with an intent to cure strategy and those without intent to cure based on documentation in the electronic health record (EHR). Participants who were prescribed rifampin with the documented goal of prosthesis retention with no further suppressive antibiotics were included in the intent-to-cure group, the primary focus of this study. Those excluded from the intent-to-cure group were given rifampin and another antibiotic, but there was a documented plan of either ongoing chronic suppression or eventual explantation; these participants were placed in the without-intent-to-cure group. Analysis of treatment success and failure was limited to the intent-to-cure group, whereas both groups were included for assessment of adverse effects (AEs) and treatment duration. This project was reviewed by the MVAHCS Institutional Review Board and determined to be a quality improvement initiative and to not meet the definition of research, and as such did not require review; it was reviewed and approved by the MVAHCS Research and Development Committee.
RESULTS
A total of 538 patients were identified who simultaneously received rifampin and another oral antibiotic between January 1, 2000, and June 30, 2021.
Forty-two participants (54%) had Staphylococcus aureus and 31 participants (40%) had coagulase-negative staphylococci infections, while 11 gram-negative organisms (14%) and 6 gram-positive anaerobic cocci (8%) infections were noted. Cutibacterium acnes and Streptococcus agalactiae were each found in 3 participants (4% of), and diphtheroids (not further identified) was found on 2 participants (3%). Candida albicans was identified in a single participant (1%), along with coagulase-negative staphylococci, and 2 participants (3%) had no identified organisms. There were multiple organisms isolated from 20 patients (26%).
Fifty participants had clear documentation in their EHR that cure of infection was the goal, meeting the criteria for the intent-to-cure group. The remaining 28 participants were placed in the without-intent-to-cure group. Success and failure rates were only measured in the intent-to-cure group, as by definition the without-intent-to-cure group patients would meet the criteria for failure (removal of prosthesis or long-term antibiotic use). The without-intent-to-cure group had a higher median age than the intent-to-cure group (69 years vs 64 years, P = .24) and a higher proportion of male participants (96% vs 80%, P = .09). The median (IQR) implant age of 11 months (1.0-50.5) in the without-intent-to-cure group was also higher than the median implant age of 1 month (0.6-22.0) in the primary group (P = .22). In the without-intent-to-cure group, 19 participants (68%) had a chronic infection, compared with 11 (22%) in the intent-to-cure group (P < .001).
The mean (SD) Charlson Comorbidity Index in the without-intent-to-cure group was 2.5 (1.3) compared with 1.9 (1.4) in the intent-to-cure group (P = .09). There was no significant difference in the type of implant or microbiology of the infecting organism between the 2 groups, although it should be noted that in the intent-to-cure group, 48 patients (96%) had Staphylococcus aureus or coagulase-negative staphylococci isolated.
The median (IQR) dosage of rifampin was 600 mg (300-900). The secondary oral antibiotics used most often were 36 fluoroquinolones (46%) followed by 20 tetracyclines (26%), 6 cephalosporins (8%), and 6 penicillins (8%). Additionally, 6 participants (8%) received IV vancomycin, and 1 participant (1%) was given an oral antifungal in addition to a fluoroquinolone because cultures revealed bacterial and fungal growth. The median (IQR) duration of antimicrobial therapy was 3 months (1.4-3.0). The mean (SD) duration of antimicrobial therapy was 3.6 (2.4) months for TKA infections and 2.4 (0.9) months for THA infections.
Clinical Outcome
Forty-one intent-to-cure group participants (82%) experienced treatment success. We further subdivided the intent-to-cure group by implant age. Participants whose implant was < 2 months old had a success rate of 93%, whereas patients whose implant was older had a success rate of 65% (P = .02).
Secondary Outcomes
The median (IQR) duration of antimicrobial treatment was 3 months (1.4-3.0) for the 38 patients with TKA-related infections and 3 months (1.4-6.0) for the 29 patients with THA infections. AEs were recorded in 24 (31%) of all study participants. Of those with AEs, the average number reported per patient was 1.6. Diarrhea, gastric upset, and nausea were each reported 7 times, accounting for 87% of all recorded AEs. Five participants reported having a rash while on antibiotics, and 2 experienced dysgeusia. One participant reported developing a yeast infection and another experienced vaginitis.
DISCUSSION
Among patients with orthopedic implant infections treated with intent to cure using a rifampin-containing antibiotic regimen at the MVAHCS, 82% had clinical success. Although this is lower than the success rates reported in clinical trials, this is not entirely unexpected.5,7 In most clinical trials studying DAIR and rifampin for PJI, patients are excluded if they do not have an acute staphylococcal infection in the setting of a well-fixed prosthesis without evidence of a sinus tract. Such exclusion criteria were not present in our retrospective study, which was designed to evaluate the real-world practice patterns at this facility. The population at the US Department of Veterans Affairs (VA) is older, more frail, and with more comorbid conditions than populations in prior studies. It is possible that patients with characteristics that would have caused them to be excluded from a clinical trial would be less likely to receive rifampin therapy with the intent to cure. This is suggested by the significantly higher prevalence of chronic infections (68%) in the without-intent-to-cure group compared with 22% in the intent-to-cure group. However, there were reasonably high proportions of participants included in the intent-to-cure group who did have conditions that would have led to their exclusion from prior trials, such as chronic infection (22%) and implant age ≥ 2 months (40%).
When evaluating participants by the age of their implant, treatment success rose to 93% for patients with implants < 2 months old compared with 65% for patients with older implants. This suggests that participants with a newer implant or more recent infection have a greater likelihood of successful treatment, which is consistent with the results of previous clinical trials.5,10 Considering how difficult multiple surgeries can be for older adult patients with comorbidities, we suggest that DAIR with a rifampin-containing regimen be considered as the primary treatment option for early PJIs at the MVAHCS. We also note inconsistent adherence to IDSA treatment guidelines on rifampin therapy, in that patients without intent to cure were prescribed a regimen including rifampin. This may reflect appropriate variability in the care of individual patients but may also offer an opportunity to change processes to improve care.
Limitations
Our analysis has limitations. As with any retrospective study evaluating the efficacy of a specific antibiotic, we were not able to attribute specific outcomes to the antibiotic of interest. Since the choice of antibiotics was left to the treating health care practitioner, therapy was not standardized, and because this was a retrospective study, causal relationships could not be inferred. Our analysis was also limited by the lack of intent to cure in 28 participants (36%), which could be an indication of practitioner bias in therapy selection or characteristic differences between the 2 groups. We looked for signs of infection failure 1 year after the completion of antimicrobial therapy, but longer follow-up could have led to higher rates of failure. Also, while participants’ infections were considered cured if they never sought further medical care for the infection at the MVAHCS, it is possible that patients could have sought care at another facility. We note that 9 patients were excluded because they were unable to complete a treatment course due to rifampin AEs, meaning that the success rates reported here reflect the success that may be expected if a patient can tolerate and complete a rifampin-based regimen. This study was conducted in a single VA hospital and may not be generalizable to nonveterans or veterans seeking care at other facilities.
Conclusions
DAIR followed by a short course of IV antibiotics and an oral regimen including rifampin and another antimicrobial is a reasonable option for veterans with acute staphylococcal orthopedic device infections at the MVAHCS. Patients with a well-placed prosthesis and an acute infection seem especially well suited for this treatment, and treatment with intent to cure should be pursued in patients who meet the criteria for rifampin therapy.
Acknowledgments
We thank Erik Stensgard, PharmD, for assistance in compiling the list of patients receiving rifampin and another antimicrobial.
Orthopedic implants are frequently used to repair fractures and replace joints. The number of total joint replacements is high, with > 1 million total hip (THA) and total knee (TKA) arthroplasties performed in the United States each year.1 While most joint arthroplasties are successful and significantly improve patient quality of life, a small proportion become infected.2 Prosthetic joint infection (PJI) causes substantial morbidity and mortality, particularly among older patients, and is difficult and costly to treat.3
The historic gold standard treatment for PJI is a 2-stage replacement, wherein the prosthesis is removed in one procedure and a new prosthesis is implanted in a second procedure after an extended course of antibiotics. This approach requires the patient to undergo 2 major procedures and spend considerable time without a functioning prosthesis, contributing to immobility and deconditioning. This option is difficult for frail or older patients and is associated with high medical costs.4
In 1998, a novel method of treatment known as debridement, antibiotics, and implant retention (DAIR) was evaluated in a small, randomized controlled trial.5 This study used a unique antimicrobial approach: the administration of ciprofloxacin plus either rifampin or placebo for 3 to 6 months, combined with a single surgical debridement. Eliminating a second surgical procedure and largely relying on oral antimicrobials reduces surgical risks and decreases costs.4 Current guidelines endorse DAIR with rifampin and a second antibiotic for patients diagnosed with PJI within about 30 days of prosthesis implantation who have a well-fixed implant without evidence of a sinus tract.6 Clinical trial data demonstrate that this approach is > 90% effective in patients with a well-fixed prosthesis and acute staphylococcal PJI.3,7
Thus far, clinical trials examining this approach have been small and did not include veterans who are typically older and have more comorbidities.8 The Minneapolis Veterans Affairs Health Care System (MVAHCS) infectious disease section has implemented the rifampin-based DAIR approach for orthopedic device-related infections since this approach was first described in 1998 but has not systematically evaluated its effectiveness or whether there are areas for improvement.
METHODS
We conducted a retrospective analysis of patients who underwent DAIR combined with a rifampin-containing regimen at the MVAHCS from January 1, 2001, through June 30, 2021. Inclusion required a diagnosis of orthopedic device-related infection and treatment with DAIR followed by antimicrobial therapy that included rifampin for 1 to 6 months. PJI was defined by meeting ≥ 1 of the following criteria: (1) isolation of the same microorganism from ≥ 2 cultures from joint aspirates or intraoperative tissue specimens; (2) purulence surrounding the prosthesis at the time of surgery; (3) acute inflammation consistent with infection on histopathological examination or periprosthetic tissue; or (4) presence of a sinus tract communicating with the prosthesis.
All cases of orthopedic device infection managed with DAIR and rifampin were included, regardless of implant stability, age of the implant at the time of symptom onset, presence of a sinus tract, or infecting microorganism. Exclusion criteria included patients who started or finished PJI treatment at another facility, were lost to follow-up, discontinued rifampin, died within 1 year of completing antibiotic therapy due to reasons unrelated to treatment failure, received rifampin for < 50% of their antimicrobial treatment course, had complete hardware removal, or had < 1 year between the completion of antimicrobial therapy and the time of data collection.
Management of DAIR procedures at the MVAHCS involves evaluating the fixation of the prosthesis, tissue sampling for microbiological analysis, and thorough debridement of infected tissue. Following debridement, a course of IV antibiotics is administered before initiating oral antibiotic therapy. To protect against resistance, rifampin is combined with another antibiotic typically from the fluoroquinolone, tetracycline, or cephalosporin class. Current guidelines suggest 3 and 6 months of oral antibiotics for prosthetic hip and knee infections, respectively.6
Treatment Outcomes
The primary outcome was treatment success, defined as meeting all of the following: (1) lack of clinical signs and symptoms of infection; (2) absence of radiological signs of loosening or infection within 1 year after the conclusion of treatment; and (3) absence of additional PJI treatment interventions for the prosthesis of concern within 1 year after completing the original antibiotic treatment.
Treatment failure was defined as meeting any of the following: (1) recurrence of PJI (original strain or different microorganism) within 1 year after the completion of antibiotic therapy; (2) death attributed to PJI anytime after the initial debridement; (3) removal of the prosthetic joint within 1 year after the completion of antibiotic therapy; or (4) long-term antibiotic use to suppress the PJI after the completion of the initial antibiotic therapy.
Statistical Analysis
Descriptive statistics were used to define the baseline characteristics of patients receiving rifampin therapy for orthopedic implant infections at the MVAHCS. Variables analyzed were age, sex, race and ethnicity, type of implant, age of implant, duration of symptoms, comorbidities (diabetes and rheumatoid arthritis), and presence of chronic infection. Patients were classified as having a chronic infection if they received previous infection treatment (antibiotics or surgery) for the orthopedic device in question. We created this category because patients with persistent infection after a medical or surgical attempt at treatment are likely to have a higher probability of treatment failure compared with those with no prior therapy. Charlson Comorbidity Index was calculated using clinical information present at the onset of infection.9 Fisher exact test was used to assess differences between categorical variables, and an independent t test was used to assess differences in continuous variables. P < .05 indicated statistical significance.
To assess the ability of a rifampin-based regimen to achieve a cure of PJI, we grouped participants into 2 categories: those with an intent to cure strategy and those without intent to cure based on documentation in the electronic health record (EHR). Participants who were prescribed rifampin with the documented goal of prosthesis retention with no further suppressive antibiotics were included in the intent-to-cure group, the primary focus of this study. Those excluded from the intent-to-cure group were given rifampin and another antibiotic, but there was a documented plan of either ongoing chronic suppression or eventual explantation; these participants were placed in the without-intent-to-cure group. Analysis of treatment success and failure was limited to the intent-to-cure group, whereas both groups were included for assessment of adverse effects (AEs) and treatment duration. This project was reviewed by the MVAHCS Institutional Review Board and determined to be a quality improvement initiative and to not meet the definition of research, and as such did not require review; it was reviewed and approved by the MVAHCS Research and Development Committee.
RESULTS
A total of 538 patients were identified who simultaneously received rifampin and another oral antibiotic between January 1, 2000, and June 30, 2021.
Forty-two participants (54%) had Staphylococcus aureus and 31 participants (40%) had coagulase-negative staphylococci infections, while 11 gram-negative organisms (14%) and 6 gram-positive anaerobic cocci (8%) infections were noted. Cutibacterium acnes and Streptococcus agalactiae were each found in 3 participants (4% of), and diphtheroids (not further identified) was found on 2 participants (3%). Candida albicans was identified in a single participant (1%), along with coagulase-negative staphylococci, and 2 participants (3%) had no identified organisms. There were multiple organisms isolated from 20 patients (26%).
Fifty participants had clear documentation in their EHR that cure of infection was the goal, meeting the criteria for the intent-to-cure group. The remaining 28 participants were placed in the without-intent-to-cure group. Success and failure rates were only measured in the intent-to-cure group, as by definition the without-intent-to-cure group patients would meet the criteria for failure (removal of prosthesis or long-term antibiotic use). The without-intent-to-cure group had a higher median age than the intent-to-cure group (69 years vs 64 years, P = .24) and a higher proportion of male participants (96% vs 80%, P = .09). The median (IQR) implant age of 11 months (1.0-50.5) in the without-intent-to-cure group was also higher than the median implant age of 1 month (0.6-22.0) in the primary group (P = .22). In the without-intent-to-cure group, 19 participants (68%) had a chronic infection, compared with 11 (22%) in the intent-to-cure group (P < .001).
The mean (SD) Charlson Comorbidity Index in the without-intent-to-cure group was 2.5 (1.3) compared with 1.9 (1.4) in the intent-to-cure group (P = .09). There was no significant difference in the type of implant or microbiology of the infecting organism between the 2 groups, although it should be noted that in the intent-to-cure group, 48 patients (96%) had Staphylococcus aureus or coagulase-negative staphylococci isolated.
The median (IQR) dosage of rifampin was 600 mg (300-900). The secondary oral antibiotics used most often were 36 fluoroquinolones (46%) followed by 20 tetracyclines (26%), 6 cephalosporins (8%), and 6 penicillins (8%). Additionally, 6 participants (8%) received IV vancomycin, and 1 participant (1%) was given an oral antifungal in addition to a fluoroquinolone because cultures revealed bacterial and fungal growth. The median (IQR) duration of antimicrobial therapy was 3 months (1.4-3.0). The mean (SD) duration of antimicrobial therapy was 3.6 (2.4) months for TKA infections and 2.4 (0.9) months for THA infections.
Clinical Outcome
Forty-one intent-to-cure group participants (82%) experienced treatment success. We further subdivided the intent-to-cure group by implant age. Participants whose implant was < 2 months old had a success rate of 93%, whereas patients whose implant was older had a success rate of 65% (P = .02).
Secondary Outcomes
The median (IQR) duration of antimicrobial treatment was 3 months (1.4-3.0) for the 38 patients with TKA-related infections and 3 months (1.4-6.0) for the 29 patients with THA infections. AEs were recorded in 24 (31%) of all study participants. Of those with AEs, the average number reported per patient was 1.6. Diarrhea, gastric upset, and nausea were each reported 7 times, accounting for 87% of all recorded AEs. Five participants reported having a rash while on antibiotics, and 2 experienced dysgeusia. One participant reported developing a yeast infection and another experienced vaginitis.
DISCUSSION
Among patients with orthopedic implant infections treated with intent to cure using a rifampin-containing antibiotic regimen at the MVAHCS, 82% had clinical success. Although this is lower than the success rates reported in clinical trials, this is not entirely unexpected.5,7 In most clinical trials studying DAIR and rifampin for PJI, patients are excluded if they do not have an acute staphylococcal infection in the setting of a well-fixed prosthesis without evidence of a sinus tract. Such exclusion criteria were not present in our retrospective study, which was designed to evaluate the real-world practice patterns at this facility. The population at the US Department of Veterans Affairs (VA) is older, more frail, and with more comorbid conditions than populations in prior studies. It is possible that patients with characteristics that would have caused them to be excluded from a clinical trial would be less likely to receive rifampin therapy with the intent to cure. This is suggested by the significantly higher prevalence of chronic infections (68%) in the without-intent-to-cure group compared with 22% in the intent-to-cure group. However, there were reasonably high proportions of participants included in the intent-to-cure group who did have conditions that would have led to their exclusion from prior trials, such as chronic infection (22%) and implant age ≥ 2 months (40%).
When evaluating participants by the age of their implant, treatment success rose to 93% for patients with implants < 2 months old compared with 65% for patients with older implants. This suggests that participants with a newer implant or more recent infection have a greater likelihood of successful treatment, which is consistent with the results of previous clinical trials.5,10 Considering how difficult multiple surgeries can be for older adult patients with comorbidities, we suggest that DAIR with a rifampin-containing regimen be considered as the primary treatment option for early PJIs at the MVAHCS. We also note inconsistent adherence to IDSA treatment guidelines on rifampin therapy, in that patients without intent to cure were prescribed a regimen including rifampin. This may reflect appropriate variability in the care of individual patients but may also offer an opportunity to change processes to improve care.
Limitations
Our analysis has limitations. As with any retrospective study evaluating the efficacy of a specific antibiotic, we were not able to attribute specific outcomes to the antibiotic of interest. Since the choice of antibiotics was left to the treating health care practitioner, therapy was not standardized, and because this was a retrospective study, causal relationships could not be inferred. Our analysis was also limited by the lack of intent to cure in 28 participants (36%), which could be an indication of practitioner bias in therapy selection or characteristic differences between the 2 groups. We looked for signs of infection failure 1 year after the completion of antimicrobial therapy, but longer follow-up could have led to higher rates of failure. Also, while participants’ infections were considered cured if they never sought further medical care for the infection at the MVAHCS, it is possible that patients could have sought care at another facility. We note that 9 patients were excluded because they were unable to complete a treatment course due to rifampin AEs, meaning that the success rates reported here reflect the success that may be expected if a patient can tolerate and complete a rifampin-based regimen. This study was conducted in a single VA hospital and may not be generalizable to nonveterans or veterans seeking care at other facilities.
Conclusions
DAIR followed by a short course of IV antibiotics and an oral regimen including rifampin and another antimicrobial is a reasonable option for veterans with acute staphylococcal orthopedic device infections at the MVAHCS. Patients with a well-placed prosthesis and an acute infection seem especially well suited for this treatment, and treatment with intent to cure should be pursued in patients who meet the criteria for rifampin therapy.
Acknowledgments
We thank Erik Stensgard, PharmD, for assistance in compiling the list of patients receiving rifampin and another antimicrobial.
1. Maradit Kremers H, Larson DR, Crowson CS, et al. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surg Am. 2015;97(17):1386-1397. doi:10.2106/JBJS.N.01141
2. Kapadia BH, Berg RA, Daley JA, Fritz J, Bhave A, Mont MA. Periprosthetic joint infection. Lancet. 2016;387(10016):386-394. doi:10.1016/S0140-6736(14)61798-0
3. Zhan C, Kaczmarek R, Loyo-Berrios N, Sangl J, Bright RA. Incidence and short-term outcomes of primary and revision hip replacement in the United States. J Bone Joint Surg Am. 2007;89(3):526-533. doi:10.2106/JBJS.F.00952
4. Fisman DN, Reilly DT, Karchmer AW, Goldie SJ. Clinical effectiveness and cost-effectiveness of 2 management strategies for infected total hip arthroplasty in the elderly. Clin Infect Dis. 2001;32(3):419-430. doi:10.1086/318502
5. Zimmerli W, Widmer AF, Blatter M, Frei R, Ochsner PE. Role of rifampin for treatment of orthopedic implant-related staphylococcal infections: a randomized controlled trial. Foreign-Body Infection (FBI) Study Group. JAMA. 1998;279(19):1537-1541. doi:10.1001/jama.279.19.1537
6. Osmon DR, Berbari EF, Berendt AR, et al. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013;56(1):e1-e25. doi:10.1093/cid/cis803
7. Lora-Tamayo J, Euba G, Cobo J, et al. Short- versus long-duration levofloxacin plus rifampicin for acute staphylococcal prosthetic joint infection managed with implant retention: a randomised clinical trial. Int J Antimicrob Agents. 2016;48(3):310-316. doi:10.1016/j.ijantimicag.2016.05.021
8. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257. doi:10.1001/archinte.160.21.3252
9. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi:10.1016/0021-9681(87)90171-8
10. Vilchez F, Martínez-Pastor JC, García-Ramiro S, et al. Outcome and predictors of treatment failure in early post-surgical prosthetic joint infections due to Staphylococcus aureus treated with debridement. Clin Microbiol Infect. 2011;17(3):439-444. doi:10.1111/j.1469-0691.2010.03244.x
1. Maradit Kremers H, Larson DR, Crowson CS, et al. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surg Am. 2015;97(17):1386-1397. doi:10.2106/JBJS.N.01141
2. Kapadia BH, Berg RA, Daley JA, Fritz J, Bhave A, Mont MA. Periprosthetic joint infection. Lancet. 2016;387(10016):386-394. doi:10.1016/S0140-6736(14)61798-0
3. Zhan C, Kaczmarek R, Loyo-Berrios N, Sangl J, Bright RA. Incidence and short-term outcomes of primary and revision hip replacement in the United States. J Bone Joint Surg Am. 2007;89(3):526-533. doi:10.2106/JBJS.F.00952
4. Fisman DN, Reilly DT, Karchmer AW, Goldie SJ. Clinical effectiveness and cost-effectiveness of 2 management strategies for infected total hip arthroplasty in the elderly. Clin Infect Dis. 2001;32(3):419-430. doi:10.1086/318502
5. Zimmerli W, Widmer AF, Blatter M, Frei R, Ochsner PE. Role of rifampin for treatment of orthopedic implant-related staphylococcal infections: a randomized controlled trial. Foreign-Body Infection (FBI) Study Group. JAMA. 1998;279(19):1537-1541. doi:10.1001/jama.279.19.1537
6. Osmon DR, Berbari EF, Berendt AR, et al. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013;56(1):e1-e25. doi:10.1093/cid/cis803
7. Lora-Tamayo J, Euba G, Cobo J, et al. Short- versus long-duration levofloxacin plus rifampicin for acute staphylococcal prosthetic joint infection managed with implant retention: a randomised clinical trial. Int J Antimicrob Agents. 2016;48(3):310-316. doi:10.1016/j.ijantimicag.2016.05.021
8. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257. doi:10.1001/archinte.160.21.3252
9. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi:10.1016/0021-9681(87)90171-8
10. Vilchez F, Martínez-Pastor JC, García-Ramiro S, et al. Outcome and predictors of treatment failure in early post-surgical prosthetic joint infections due to Staphylococcus aureus treated with debridement. Clin Microbiol Infect. 2011;17(3):439-444. doi:10.1111/j.1469-0691.2010.03244.x