User login
Organs of Metastasis Predominate with Age in Non-Small Cell Lung Cancer Subtypes: National Cancer Database Analysis
Background
Patients diagnosed with lung cancer are predominantly non-small cell lung cancer (NSCLC), a leading cause of cancer-related deaths. Thus, it is imperative to investigate and distinguish the differences present at diagnosis to possibly improve survival outcomes. NSCLC commonly metastasizes within older patients near the mean age of 71 years, but also in early onset patients which represents the patients younger than the earliest lung cancer screening age of 50.
Objective
To reveal differences in ratios of metastasis locations in squamous cell carcinoma (SCC), adenocarcinoma (ACC), and adenosquamous carcinoma (ASC).
Methods
The National Cancer Database (NCDB) was utilized to identify patients diagnosed with SCC, ACC, and ASC using the histology codes 8070, 8140, and 8560 from the ICD-O-3.2 from 2004 to 2022. Age groups were 70 years. Metastases located to the brain, liver, bone, and lung were included. Chi-Square tests were performed. The data was analyzed using R version 4.4.2 and statistical significance was set to α = 0.05.
Results
In this study, 1,445,119 patients were analyzed. Chi-Square tests identified significant differences in the ratios of organ metastasis locations between age groups in each subtype (p < 0.001). SCC in each age group similarly metastasized most to bone (36.3%, 34.7%, 34.5%), but notably more local lung metastasis was observed in the oldest group (33.6%). In ACC and ASC, the oldest group also had greater ratios of spread within the lungs (28.0%, 27.2%). Overall, the younger the age group, distant spread to the brain increased (ex. 29.0%, 24.4%, 17.5%). This suggests a widely heterogenous distribution of metastases at diagnosis of NSCLC subtypes and patient age.
Conclusions
This study demonstrated that patients with SCC, ACC, or ASC subtypes of NSCLC share similar predominant locations based in part on patient age, irrespective of cancer origin. NSCLC may more distantly metastasize in younger patients to the brain, while older patients may have locally metastatic cancer. Further analysis of key demographic variables as well as common undertaken treatment options may prove informative and reveal existing differences in survival outcomes.
Background
Patients diagnosed with lung cancer are predominantly non-small cell lung cancer (NSCLC), a leading cause of cancer-related deaths. Thus, it is imperative to investigate and distinguish the differences present at diagnosis to possibly improve survival outcomes. NSCLC commonly metastasizes within older patients near the mean age of 71 years, but also in early onset patients which represents the patients younger than the earliest lung cancer screening age of 50.
Objective
To reveal differences in ratios of metastasis locations in squamous cell carcinoma (SCC), adenocarcinoma (ACC), and adenosquamous carcinoma (ASC).
Methods
The National Cancer Database (NCDB) was utilized to identify patients diagnosed with SCC, ACC, and ASC using the histology codes 8070, 8140, and 8560 from the ICD-O-3.2 from 2004 to 2022. Age groups were 70 years. Metastases located to the brain, liver, bone, and lung were included. Chi-Square tests were performed. The data was analyzed using R version 4.4.2 and statistical significance was set to α = 0.05.
Results
In this study, 1,445,119 patients were analyzed. Chi-Square tests identified significant differences in the ratios of organ metastasis locations between age groups in each subtype (p < 0.001). SCC in each age group similarly metastasized most to bone (36.3%, 34.7%, 34.5%), but notably more local lung metastasis was observed in the oldest group (33.6%). In ACC and ASC, the oldest group also had greater ratios of spread within the lungs (28.0%, 27.2%). Overall, the younger the age group, distant spread to the brain increased (ex. 29.0%, 24.4%, 17.5%). This suggests a widely heterogenous distribution of metastases at diagnosis of NSCLC subtypes and patient age.
Conclusions
This study demonstrated that patients with SCC, ACC, or ASC subtypes of NSCLC share similar predominant locations based in part on patient age, irrespective of cancer origin. NSCLC may more distantly metastasize in younger patients to the brain, while older patients may have locally metastatic cancer. Further analysis of key demographic variables as well as common undertaken treatment options may prove informative and reveal existing differences in survival outcomes.
Background
Patients diagnosed with lung cancer are predominantly non-small cell lung cancer (NSCLC), a leading cause of cancer-related deaths. Thus, it is imperative to investigate and distinguish the differences present at diagnosis to possibly improve survival outcomes. NSCLC commonly metastasizes within older patients near the mean age of 71 years, but also in early onset patients which represents the patients younger than the earliest lung cancer screening age of 50.
Objective
To reveal differences in ratios of metastasis locations in squamous cell carcinoma (SCC), adenocarcinoma (ACC), and adenosquamous carcinoma (ASC).
Methods
The National Cancer Database (NCDB) was utilized to identify patients diagnosed with SCC, ACC, and ASC using the histology codes 8070, 8140, and 8560 from the ICD-O-3.2 from 2004 to 2022. Age groups were 70 years. Metastases located to the brain, liver, bone, and lung were included. Chi-Square tests were performed. The data was analyzed using R version 4.4.2 and statistical significance was set to α = 0.05.
Results
In this study, 1,445,119 patients were analyzed. Chi-Square tests identified significant differences in the ratios of organ metastasis locations between age groups in each subtype (p < 0.001). SCC in each age group similarly metastasized most to bone (36.3%, 34.7%, 34.5%), but notably more local lung metastasis was observed in the oldest group (33.6%). In ACC and ASC, the oldest group also had greater ratios of spread within the lungs (28.0%, 27.2%). Overall, the younger the age group, distant spread to the brain increased (ex. 29.0%, 24.4%, 17.5%). This suggests a widely heterogenous distribution of metastases at diagnosis of NSCLC subtypes and patient age.
Conclusions
This study demonstrated that patients with SCC, ACC, or ASC subtypes of NSCLC share similar predominant locations based in part on patient age, irrespective of cancer origin. NSCLC may more distantly metastasize in younger patients to the brain, while older patients may have locally metastatic cancer. Further analysis of key demographic variables as well as common undertaken treatment options may prove informative and reveal existing differences in survival outcomes.
Shifting Demographics: A Temporal Analysis of the Alarming Rise in Rectal Adenocarcinoma Among Young Adults
Background
Rectal adenocarcinoma has long been associated with older adults, with routine screening typically beginning at age 45 or older. However, recent data reveal a concerning rise in rectal cancer incidence among adults under 40. These early-onset cases often present at later stages and may have distinct biological features. While some research attributes this trend to genetic or environmental factors, the contribution of socioeconomic disparities and healthcare access has not been fully explored. Identifying these influences is essential to shaping targeted prevention and early detection strategies for younger populations.
Objective
To evaluate temporal trends in rectal adenocarcinoma among young adults and assess demographic and socioeconomic predictors of early-onset diagnosis.
Methods
Data were drawn from the National Cancer Database (NCDB) for patients diagnosed with rectal adenocarcinoma from 2004 to 2022. Among 440,316 cases, 17,842 (4.1%) occurred in individuals under 40. Linear regression assessed temporal trends, while logistic regression evaluated associations between early-onset diagnosis and variables including sex, race, insurance status, income level, Charlson-Deyo comorbidity score, and tumor stage. Statistical significance was defined as α = 0.05.
Results
The number of young adults diagnosed rose from 424 in 2004 to 937 in 2022—an increase of over 120%. Each year was associated with a 1.7% rise in odds of early diagnosis (OR = 1.017, p < 0.001). Male patients had 24.7% higher odds (OR = 1.247, p < 0.001), and Black patients had 59.3% higher odds compared to White patients (OR = 1.593, p < 0.001). Non-private insurance was linked to a 41.6% decrease in early diagnosis (OR = 0.584, p < 0.001). Income level was not significant (p = 0.426). Lower Charlson-Deyo scores and higher tumor stages were also associated with early-onset cases.
Conclusions
Rectal adenocarcinoma is increasingly affecting younger adults, with significant associations across demographic and insurance variables. These findings call for improved awareness, early diagnostic strategies, and further research into underlying causes to mitigate this growing public health concern.
Background
Rectal adenocarcinoma has long been associated with older adults, with routine screening typically beginning at age 45 or older. However, recent data reveal a concerning rise in rectal cancer incidence among adults under 40. These early-onset cases often present at later stages and may have distinct biological features. While some research attributes this trend to genetic or environmental factors, the contribution of socioeconomic disparities and healthcare access has not been fully explored. Identifying these influences is essential to shaping targeted prevention and early detection strategies for younger populations.
Objective
To evaluate temporal trends in rectal adenocarcinoma among young adults and assess demographic and socioeconomic predictors of early-onset diagnosis.
Methods
Data were drawn from the National Cancer Database (NCDB) for patients diagnosed with rectal adenocarcinoma from 2004 to 2022. Among 440,316 cases, 17,842 (4.1%) occurred in individuals under 40. Linear regression assessed temporal trends, while logistic regression evaluated associations between early-onset diagnosis and variables including sex, race, insurance status, income level, Charlson-Deyo comorbidity score, and tumor stage. Statistical significance was defined as α = 0.05.
Results
The number of young adults diagnosed rose from 424 in 2004 to 937 in 2022—an increase of over 120%. Each year was associated with a 1.7% rise in odds of early diagnosis (OR = 1.017, p < 0.001). Male patients had 24.7% higher odds (OR = 1.247, p < 0.001), and Black patients had 59.3% higher odds compared to White patients (OR = 1.593, p < 0.001). Non-private insurance was linked to a 41.6% decrease in early diagnosis (OR = 0.584, p < 0.001). Income level was not significant (p = 0.426). Lower Charlson-Deyo scores and higher tumor stages were also associated with early-onset cases.
Conclusions
Rectal adenocarcinoma is increasingly affecting younger adults, with significant associations across demographic and insurance variables. These findings call for improved awareness, early diagnostic strategies, and further research into underlying causes to mitigate this growing public health concern.
Background
Rectal adenocarcinoma has long been associated with older adults, with routine screening typically beginning at age 45 or older. However, recent data reveal a concerning rise in rectal cancer incidence among adults under 40. These early-onset cases often present at later stages and may have distinct biological features. While some research attributes this trend to genetic or environmental factors, the contribution of socioeconomic disparities and healthcare access has not been fully explored. Identifying these influences is essential to shaping targeted prevention and early detection strategies for younger populations.
Objective
To evaluate temporal trends in rectal adenocarcinoma among young adults and assess demographic and socioeconomic predictors of early-onset diagnosis.
Methods
Data were drawn from the National Cancer Database (NCDB) for patients diagnosed with rectal adenocarcinoma from 2004 to 2022. Among 440,316 cases, 17,842 (4.1%) occurred in individuals under 40. Linear regression assessed temporal trends, while logistic regression evaluated associations between early-onset diagnosis and variables including sex, race, insurance status, income level, Charlson-Deyo comorbidity score, and tumor stage. Statistical significance was defined as α = 0.05.
Results
The number of young adults diagnosed rose from 424 in 2004 to 937 in 2022—an increase of over 120%. Each year was associated with a 1.7% rise in odds of early diagnosis (OR = 1.017, p < 0.001). Male patients had 24.7% higher odds (OR = 1.247, p < 0.001), and Black patients had 59.3% higher odds compared to White patients (OR = 1.593, p < 0.001). Non-private insurance was linked to a 41.6% decrease in early diagnosis (OR = 0.584, p < 0.001). Income level was not significant (p = 0.426). Lower Charlson-Deyo scores and higher tumor stages were also associated with early-onset cases.
Conclusions
Rectal adenocarcinoma is increasingly affecting younger adults, with significant associations across demographic and insurance variables. These findings call for improved awareness, early diagnostic strategies, and further research into underlying causes to mitigate this growing public health concern.
Epidemiology and Survival of Parotid Gland Malignancies With Brain Metastases: A Population- Based Study
Background
Parotid gland malignancies are a rare subset of salivary gland tumors, comprising approximately 1–3% of all head and neck cancers. While distant metastases commonly involve the lungs, brain metastases are exceedingly rare and remain poorly characterized. Management typically includes stereotactic radiosurgery or whole-brain radiation. This study evaluates the incidence, clinicopathologic features, and survival outcomes of patients with parotid gland tumors and brain metastases using data from Surveillance, Epidemiology, and End Results (SEER) database.
Methods
SEER database (2010–2022) was queried for patients diagnosed with primary malignant neoplasms of the parotid gland (ICD-O-3 site code C07.9). Cases of brain metastases were identified using SEER metastatic site variables. Age-adjusted incidence rates (IR) per 100,000 population were calculated using SEER*Stat 8.4.5. Kaplan-Meier survival analyses were conducted using GraphPad Prism, and survival differences were assessed using the log-rank test.
Results
Among 12,951 patients diagnosed with parotid malignancy, 47 (0.36%) had brain metastases. The median age at diagnosis was 67 years, and 77.5% were male. The overall incidence rate (IR) of brain metastases was 0.00235 per 100,000 population, with a significantly higher rate observed in males compared to females (p < 0.0001). The most common histologic subtype associated with brain involvement was squamous cell carcinoma (SCC, n=10), followed by adenocarcinoma. Median overall survival (mOS) for patients with brain metastases was 2 months (hazard ratio [HR] 6.28; 95% CI: 2.71–14.55), compared to 131 months for those without brain involvement (p < 0.001). 1-year cancer-specific survival for patients with brain metastases was 38%. Among patients with parotid SCC and brain metastases, mOS was 3 months, compared to 39 months in those without brain involvement (HR 5.70; 95% CI: 1.09–29.68; p < 0.0001).
Conclusions
Brain metastases from parotid gland cancers, though rare, are associated with markedly poor outcomes. This highlights the importance of early neurologic assessment and brain imaging in high-risk patients, particularly with SCC histology. Prior studies have shown that TP53 mutations are common in parotid SCC, but their role in CNS spread remains unclear. Future research should explore molecular pathways underlying neurotropism in parotid cancers and investigate targeted systemic therapies with CNS penetration to improve outcomes.
Background
Parotid gland malignancies are a rare subset of salivary gland tumors, comprising approximately 1–3% of all head and neck cancers. While distant metastases commonly involve the lungs, brain metastases are exceedingly rare and remain poorly characterized. Management typically includes stereotactic radiosurgery or whole-brain radiation. This study evaluates the incidence, clinicopathologic features, and survival outcomes of patients with parotid gland tumors and brain metastases using data from Surveillance, Epidemiology, and End Results (SEER) database.
Methods
SEER database (2010–2022) was queried for patients diagnosed with primary malignant neoplasms of the parotid gland (ICD-O-3 site code C07.9). Cases of brain metastases were identified using SEER metastatic site variables. Age-adjusted incidence rates (IR) per 100,000 population were calculated using SEER*Stat 8.4.5. Kaplan-Meier survival analyses were conducted using GraphPad Prism, and survival differences were assessed using the log-rank test.
Results
Among 12,951 patients diagnosed with parotid malignancy, 47 (0.36%) had brain metastases. The median age at diagnosis was 67 years, and 77.5% were male. The overall incidence rate (IR) of brain metastases was 0.00235 per 100,000 population, with a significantly higher rate observed in males compared to females (p < 0.0001). The most common histologic subtype associated with brain involvement was squamous cell carcinoma (SCC, n=10), followed by adenocarcinoma. Median overall survival (mOS) for patients with brain metastases was 2 months (hazard ratio [HR] 6.28; 95% CI: 2.71–14.55), compared to 131 months for those without brain involvement (p < 0.001). 1-year cancer-specific survival for patients with brain metastases was 38%. Among patients with parotid SCC and brain metastases, mOS was 3 months, compared to 39 months in those without brain involvement (HR 5.70; 95% CI: 1.09–29.68; p < 0.0001).
Conclusions
Brain metastases from parotid gland cancers, though rare, are associated with markedly poor outcomes. This highlights the importance of early neurologic assessment and brain imaging in high-risk patients, particularly with SCC histology. Prior studies have shown that TP53 mutations are common in parotid SCC, but their role in CNS spread remains unclear. Future research should explore molecular pathways underlying neurotropism in parotid cancers and investigate targeted systemic therapies with CNS penetration to improve outcomes.
Background
Parotid gland malignancies are a rare subset of salivary gland tumors, comprising approximately 1–3% of all head and neck cancers. While distant metastases commonly involve the lungs, brain metastases are exceedingly rare and remain poorly characterized. Management typically includes stereotactic radiosurgery or whole-brain radiation. This study evaluates the incidence, clinicopathologic features, and survival outcomes of patients with parotid gland tumors and brain metastases using data from Surveillance, Epidemiology, and End Results (SEER) database.
Methods
SEER database (2010–2022) was queried for patients diagnosed with primary malignant neoplasms of the parotid gland (ICD-O-3 site code C07.9). Cases of brain metastases were identified using SEER metastatic site variables. Age-adjusted incidence rates (IR) per 100,000 population were calculated using SEER*Stat 8.4.5. Kaplan-Meier survival analyses were conducted using GraphPad Prism, and survival differences were assessed using the log-rank test.
Results
Among 12,951 patients diagnosed with parotid malignancy, 47 (0.36%) had brain metastases. The median age at diagnosis was 67 years, and 77.5% were male. The overall incidence rate (IR) of brain metastases was 0.00235 per 100,000 population, with a significantly higher rate observed in males compared to females (p < 0.0001). The most common histologic subtype associated with brain involvement was squamous cell carcinoma (SCC, n=10), followed by adenocarcinoma. Median overall survival (mOS) for patients with brain metastases was 2 months (hazard ratio [HR] 6.28; 95% CI: 2.71–14.55), compared to 131 months for those without brain involvement (p < 0.001). 1-year cancer-specific survival for patients with brain metastases was 38%. Among patients with parotid SCC and brain metastases, mOS was 3 months, compared to 39 months in those without brain involvement (HR 5.70; 95% CI: 1.09–29.68; p < 0.0001).
Conclusions
Brain metastases from parotid gland cancers, though rare, are associated with markedly poor outcomes. This highlights the importance of early neurologic assessment and brain imaging in high-risk patients, particularly with SCC histology. Prior studies have shown that TP53 mutations are common in parotid SCC, but their role in CNS spread remains unclear. Future research should explore molecular pathways underlying neurotropism in parotid cancers and investigate targeted systemic therapies with CNS penetration to improve outcomes.
Augmenting DNA Damage by Chemotherapy With CDK7 Inhibition to Disrupt PARP Expression in Cholangiocarcinoma
Papillary Cystadenocarcinoma: NCDB Insights on Outcomes and Socioeconomic Disparities
Background
Papillary cystadenocarcinoma is a rare, aggressive malignancy typically arising in the ovaries, often following malignant transformation of benign precursors. Characterized by local invasion and recurrence, it lacks standardized treatment protocols and comprehensive epidemiological data. Existing literature is limited to case reports and small series, leaving gaps in population-level data to guide clinical decision-making. This study uses the National Cancer Database (NCDB) to assess demographic, socioeconomic, and treatment patterns to identify disparities and inform management.
Methods
A retrospective cohort analysis of 345 patients with histologically confirmed papillary cystadenocarcinoma (ICD-O-3 code 8450) was conducted using the 2004–2020 NCDB. Demographic, treatment, and survival data were described; incidence trends were assessed via linear regression; and survival was analyzed using Kaplan-Meier curves.
Results
The cohort was predominantly female (97.1%), mean age 62.1 years (SD = 14.0), and 87.2% White. Most had private insurance (44.9%) or Medicare (40.9%). Over half (51.9%) resided in metropolitan areas >1 million. Primary tumor sites were ovarian (80.0%) and endometrial (5.2%), with 39.7% presenting at Stage III. Surgery was performed in 90.4% of cases, with 51.9% achieving negative margins. Most were treated at comprehensive community (41.0%) or academic/research programs (28.7%). Primary therapies included chemotherapy (62.3%), radiation (6.4%), and hormone therapy (1.7%). Thirty-day mortality was 1.9%, and 90-day mortality was 5.4%. Survival was 97.7% at 2 years, 94.2% at 5 years, and 88.6% at 10 years. Mean survival was 97.5 months (95% CI: 88.2–106.7).
Conclusions
This is the first NCDB-based analysis of papillary cystadenocarcinoma, offering insight into its clinical characteristics. Ovarian and endometrial origins were most common, reinforcing its gynecologic profile. High surgical rates and margin negativity suggest aggressive local treatment is central to management. Disparities emerged: patients were more likely to live in urban areas, hold private insurance, and receive care at community programs. These findings highlight the need for further investigation into socioeconomic inequities and may inform future guidelines to improve equitable care delivery across health systems, including community-based programs such as the VHA.
Background
Papillary cystadenocarcinoma is a rare, aggressive malignancy typically arising in the ovaries, often following malignant transformation of benign precursors. Characterized by local invasion and recurrence, it lacks standardized treatment protocols and comprehensive epidemiological data. Existing literature is limited to case reports and small series, leaving gaps in population-level data to guide clinical decision-making. This study uses the National Cancer Database (NCDB) to assess demographic, socioeconomic, and treatment patterns to identify disparities and inform management.
Methods
A retrospective cohort analysis of 345 patients with histologically confirmed papillary cystadenocarcinoma (ICD-O-3 code 8450) was conducted using the 2004–2020 NCDB. Demographic, treatment, and survival data were described; incidence trends were assessed via linear regression; and survival was analyzed using Kaplan-Meier curves.
Results
The cohort was predominantly female (97.1%), mean age 62.1 years (SD = 14.0), and 87.2% White. Most had private insurance (44.9%) or Medicare (40.9%). Over half (51.9%) resided in metropolitan areas >1 million. Primary tumor sites were ovarian (80.0%) and endometrial (5.2%), with 39.7% presenting at Stage III. Surgery was performed in 90.4% of cases, with 51.9% achieving negative margins. Most were treated at comprehensive community (41.0%) or academic/research programs (28.7%). Primary therapies included chemotherapy (62.3%), radiation (6.4%), and hormone therapy (1.7%). Thirty-day mortality was 1.9%, and 90-day mortality was 5.4%. Survival was 97.7% at 2 years, 94.2% at 5 years, and 88.6% at 10 years. Mean survival was 97.5 months (95% CI: 88.2–106.7).
Conclusions
This is the first NCDB-based analysis of papillary cystadenocarcinoma, offering insight into its clinical characteristics. Ovarian and endometrial origins were most common, reinforcing its gynecologic profile. High surgical rates and margin negativity suggest aggressive local treatment is central to management. Disparities emerged: patients were more likely to live in urban areas, hold private insurance, and receive care at community programs. These findings highlight the need for further investigation into socioeconomic inequities and may inform future guidelines to improve equitable care delivery across health systems, including community-based programs such as the VHA.
Background
Papillary cystadenocarcinoma is a rare, aggressive malignancy typically arising in the ovaries, often following malignant transformation of benign precursors. Characterized by local invasion and recurrence, it lacks standardized treatment protocols and comprehensive epidemiological data. Existing literature is limited to case reports and small series, leaving gaps in population-level data to guide clinical decision-making. This study uses the National Cancer Database (NCDB) to assess demographic, socioeconomic, and treatment patterns to identify disparities and inform management.
Methods
A retrospective cohort analysis of 345 patients with histologically confirmed papillary cystadenocarcinoma (ICD-O-3 code 8450) was conducted using the 2004–2020 NCDB. Demographic, treatment, and survival data were described; incidence trends were assessed via linear regression; and survival was analyzed using Kaplan-Meier curves.
Results
The cohort was predominantly female (97.1%), mean age 62.1 years (SD = 14.0), and 87.2% White. Most had private insurance (44.9%) or Medicare (40.9%). Over half (51.9%) resided in metropolitan areas >1 million. Primary tumor sites were ovarian (80.0%) and endometrial (5.2%), with 39.7% presenting at Stage III. Surgery was performed in 90.4% of cases, with 51.9% achieving negative margins. Most were treated at comprehensive community (41.0%) or academic/research programs (28.7%). Primary therapies included chemotherapy (62.3%), radiation (6.4%), and hormone therapy (1.7%). Thirty-day mortality was 1.9%, and 90-day mortality was 5.4%. Survival was 97.7% at 2 years, 94.2% at 5 years, and 88.6% at 10 years. Mean survival was 97.5 months (95% CI: 88.2–106.7).
Conclusions
This is the first NCDB-based analysis of papillary cystadenocarcinoma, offering insight into its clinical characteristics. Ovarian and endometrial origins were most common, reinforcing its gynecologic profile. High surgical rates and margin negativity suggest aggressive local treatment is central to management. Disparities emerged: patients were more likely to live in urban areas, hold private insurance, and receive care at community programs. These findings highlight the need for further investigation into socioeconomic inequities and may inform future guidelines to improve equitable care delivery across health systems, including community-based programs such as the VHA.
Assessing Geographical Trends in End-of-Life Cancer Care Using CDC WONDER’s Place of Death Data
Background
19.8% of all deaths in the US in 2023 were due to cancer. Despite its prevalence, there is minimal literature analyzing geographical trends in end-of-life care in cancer patients. This study aims to assess the evolution of end-of-life preferences in cancer patients, particularly during the COVID-19 pandemic, and account for geographical disparities to optimize palliative care delivery.
Methods
The CDC WONDER database was used to collect data on place of death (home, hospice, medical facilities, nursing homes) in patients over 25 years old that died with malignant neoplasms (ICD 10: C00- C97) in the US from 2003-2023. Deaths were stratified by region and urbanization. Proportional mortality was calculated, and statistically significant trends in mortality over time were identified using Joinpoint regression.
Results
There were 13,654,631 total deaths from malignant neoplasms over the study period. Home (40.3%) was the most common place of death followed by medical facilities (30.4%), nursing homes (14.3%), and hospice (8.9%). In 2020, all places experienced a decreased in proportion except for home which rose 7.0% from 41.7% to 48.7%. The South had the highest hospice rates (11.3%); 5.0% greater than the next highest region (Northeast; 8.3%). The West had the highest home rates (47.1%); 6.2% greater than the next closest region (South; 40.9%). The Northeast had the highest medical facility rates (36.0%); 5.5% higher than the next highest region (South, 30.5%). Nonmetro areas (< 50,000 population) had the lowest hospice (4.9%) and highest nursing home rates (15.8%). They also saw a substantial jump (+15.4%) in home deaths from 2019-21. All urbanizations saw a drop in medical facility deaths in 2020 but all have since climbed to surpass their 2019 rates except for nonmetro areas which have dropped 7.3% from 2020-2023.
Conclusion
Hospice and home deaths have increased in frequency with home deaths spiking during the COVID-19 pandemic. Geographical disparities persist in end-of-life care, particularly in nonmetro areas. This highlights the need to increase education and access to palliative care. Further research should aim at why the rural populations have failed to revert to pre-COVID trends like the other urbanization groups.
Background
19.8% of all deaths in the US in 2023 were due to cancer. Despite its prevalence, there is minimal literature analyzing geographical trends in end-of-life care in cancer patients. This study aims to assess the evolution of end-of-life preferences in cancer patients, particularly during the COVID-19 pandemic, and account for geographical disparities to optimize palliative care delivery.
Methods
The CDC WONDER database was used to collect data on place of death (home, hospice, medical facilities, nursing homes) in patients over 25 years old that died with malignant neoplasms (ICD 10: C00- C97) in the US from 2003-2023. Deaths were stratified by region and urbanization. Proportional mortality was calculated, and statistically significant trends in mortality over time were identified using Joinpoint regression.
Results
There were 13,654,631 total deaths from malignant neoplasms over the study period. Home (40.3%) was the most common place of death followed by medical facilities (30.4%), nursing homes (14.3%), and hospice (8.9%). In 2020, all places experienced a decreased in proportion except for home which rose 7.0% from 41.7% to 48.7%. The South had the highest hospice rates (11.3%); 5.0% greater than the next highest region (Northeast; 8.3%). The West had the highest home rates (47.1%); 6.2% greater than the next closest region (South; 40.9%). The Northeast had the highest medical facility rates (36.0%); 5.5% higher than the next highest region (South, 30.5%). Nonmetro areas (< 50,000 population) had the lowest hospice (4.9%) and highest nursing home rates (15.8%). They also saw a substantial jump (+15.4%) in home deaths from 2019-21. All urbanizations saw a drop in medical facility deaths in 2020 but all have since climbed to surpass their 2019 rates except for nonmetro areas which have dropped 7.3% from 2020-2023.
Conclusion
Hospice and home deaths have increased in frequency with home deaths spiking during the COVID-19 pandemic. Geographical disparities persist in end-of-life care, particularly in nonmetro areas. This highlights the need to increase education and access to palliative care. Further research should aim at why the rural populations have failed to revert to pre-COVID trends like the other urbanization groups.
Background
19.8% of all deaths in the US in 2023 were due to cancer. Despite its prevalence, there is minimal literature analyzing geographical trends in end-of-life care in cancer patients. This study aims to assess the evolution of end-of-life preferences in cancer patients, particularly during the COVID-19 pandemic, and account for geographical disparities to optimize palliative care delivery.
Methods
The CDC WONDER database was used to collect data on place of death (home, hospice, medical facilities, nursing homes) in patients over 25 years old that died with malignant neoplasms (ICD 10: C00- C97) in the US from 2003-2023. Deaths were stratified by region and urbanization. Proportional mortality was calculated, and statistically significant trends in mortality over time were identified using Joinpoint regression.
Results
There were 13,654,631 total deaths from malignant neoplasms over the study period. Home (40.3%) was the most common place of death followed by medical facilities (30.4%), nursing homes (14.3%), and hospice (8.9%). In 2020, all places experienced a decreased in proportion except for home which rose 7.0% from 41.7% to 48.7%. The South had the highest hospice rates (11.3%); 5.0% greater than the next highest region (Northeast; 8.3%). The West had the highest home rates (47.1%); 6.2% greater than the next closest region (South; 40.9%). The Northeast had the highest medical facility rates (36.0%); 5.5% higher than the next highest region (South, 30.5%). Nonmetro areas (< 50,000 population) had the lowest hospice (4.9%) and highest nursing home rates (15.8%). They also saw a substantial jump (+15.4%) in home deaths from 2019-21. All urbanizations saw a drop in medical facility deaths in 2020 but all have since climbed to surpass their 2019 rates except for nonmetro areas which have dropped 7.3% from 2020-2023.
Conclusion
Hospice and home deaths have increased in frequency with home deaths spiking during the COVID-19 pandemic. Geographical disparities persist in end-of-life care, particularly in nonmetro areas. This highlights the need to increase education and access to palliative care. Further research should aim at why the rural populations have failed to revert to pre-COVID trends like the other urbanization groups.
Demographical Trends in End-of-Life Care in Malignant Neoplasms: A CDC Wonder Analysis Using Place of Death
Background
In 2024, it was estimated that 2,001,140 new cases of cancer were diagnosed in the United States with 611,720 people succumbing to the disease. There is scant literature analyzing how the place of death in cancer patients has evolved over time, particularly during the COVID-19 pandemic, and how it varies demographically. This study aims to analyze the evolution of end-of-life preferences in cancer patients and assess for racial or sexual disparities to optimize palliative care and ensure it aligns with the patient’s wishes.
Methods
The CDC Wonder database was used to collect data on place of death (home, hospice, medical facilities, nursing homes) in patients over 25 years old who died with malignant neoplasms (ICD-10: C00-C97) in the US from 2003-2023. Deaths were stratified by sex and race. Proportional mortality was calculated, and statistically significant temporal trends in mortality were identified using Joinpoint regression.
Results
From 2003 to 2023, there were 13,654,631 total deaths from malignant cancer. Home deaths were the most common (40.3%) followed by medical facilities (30.4%), nursing homes (14.3%), and hospice (8.9%). In 2020, all places experienced a decrease in proportion except for home which rose 7.1%. From 2003-2023, home (+4.0%) and hospice (+10.0%) rose in frequency while medical facility (-10.9%) and nursing home (-6.8%) declined. Females died in nursing homes at a greater proportion than males (15.8% vs. 13.1%) while males died in medical facilities more frequently (32.4% vs. 28.8%). Black patients were the least likely to die at home (33.1%), 5.9% less than the next lowest (Asian/ Pacific Islander; 39.0%), while Hispanic patients were most likely (46.9%); 5.7% more than the next highest (White, 41.7%). White patients were the least likely to die in medical facilities (28.4%) but were also most likely to die in nursing homes (15.3%).
Conclusions
Hospice and home deaths have increased in frequency with home deaths spiking during the COVID-19 pandemic. Disparities persist in end-of-life care across both sex and racial groups. This highlights the need to increase education and access to palliative care. Further research should elucidate cultural and racial discrepancies surrounding end-of-life treatment and preferences to provide context for these differences.
Background
In 2024, it was estimated that 2,001,140 new cases of cancer were diagnosed in the United States with 611,720 people succumbing to the disease. There is scant literature analyzing how the place of death in cancer patients has evolved over time, particularly during the COVID-19 pandemic, and how it varies demographically. This study aims to analyze the evolution of end-of-life preferences in cancer patients and assess for racial or sexual disparities to optimize palliative care and ensure it aligns with the patient’s wishes.
Methods
The CDC Wonder database was used to collect data on place of death (home, hospice, medical facilities, nursing homes) in patients over 25 years old who died with malignant neoplasms (ICD-10: C00-C97) in the US from 2003-2023. Deaths were stratified by sex and race. Proportional mortality was calculated, and statistically significant temporal trends in mortality were identified using Joinpoint regression.
Results
From 2003 to 2023, there were 13,654,631 total deaths from malignant cancer. Home deaths were the most common (40.3%) followed by medical facilities (30.4%), nursing homes (14.3%), and hospice (8.9%). In 2020, all places experienced a decrease in proportion except for home which rose 7.1%. From 2003-2023, home (+4.0%) and hospice (+10.0%) rose in frequency while medical facility (-10.9%) and nursing home (-6.8%) declined. Females died in nursing homes at a greater proportion than males (15.8% vs. 13.1%) while males died in medical facilities more frequently (32.4% vs. 28.8%). Black patients were the least likely to die at home (33.1%), 5.9% less than the next lowest (Asian/ Pacific Islander; 39.0%), while Hispanic patients were most likely (46.9%); 5.7% more than the next highest (White, 41.7%). White patients were the least likely to die in medical facilities (28.4%) but were also most likely to die in nursing homes (15.3%).
Conclusions
Hospice and home deaths have increased in frequency with home deaths spiking during the COVID-19 pandemic. Disparities persist in end-of-life care across both sex and racial groups. This highlights the need to increase education and access to palliative care. Further research should elucidate cultural and racial discrepancies surrounding end-of-life treatment and preferences to provide context for these differences.
Background
In 2024, it was estimated that 2,001,140 new cases of cancer were diagnosed in the United States with 611,720 people succumbing to the disease. There is scant literature analyzing how the place of death in cancer patients has evolved over time, particularly during the COVID-19 pandemic, and how it varies demographically. This study aims to analyze the evolution of end-of-life preferences in cancer patients and assess for racial or sexual disparities to optimize palliative care and ensure it aligns with the patient’s wishes.
Methods
The CDC Wonder database was used to collect data on place of death (home, hospice, medical facilities, nursing homes) in patients over 25 years old who died with malignant neoplasms (ICD-10: C00-C97) in the US from 2003-2023. Deaths were stratified by sex and race. Proportional mortality was calculated, and statistically significant temporal trends in mortality were identified using Joinpoint regression.
Results
From 2003 to 2023, there were 13,654,631 total deaths from malignant cancer. Home deaths were the most common (40.3%) followed by medical facilities (30.4%), nursing homes (14.3%), and hospice (8.9%). In 2020, all places experienced a decrease in proportion except for home which rose 7.1%. From 2003-2023, home (+4.0%) and hospice (+10.0%) rose in frequency while medical facility (-10.9%) and nursing home (-6.8%) declined. Females died in nursing homes at a greater proportion than males (15.8% vs. 13.1%) while males died in medical facilities more frequently (32.4% vs. 28.8%). Black patients were the least likely to die at home (33.1%), 5.9% less than the next lowest (Asian/ Pacific Islander; 39.0%), while Hispanic patients were most likely (46.9%); 5.7% more than the next highest (White, 41.7%). White patients were the least likely to die in medical facilities (28.4%) but were also most likely to die in nursing homes (15.3%).
Conclusions
Hospice and home deaths have increased in frequency with home deaths spiking during the COVID-19 pandemic. Disparities persist in end-of-life care across both sex and racial groups. This highlights the need to increase education and access to palliative care. Further research should elucidate cultural and racial discrepancies surrounding end-of-life treatment and preferences to provide context for these differences.
Findings from (ImPaCT): Improving Patients With Prostate Cancer’s Access to Germline Testing
Background
With the onset of precision oncology, findings from germline mutational analysis have been helpful in treating patients with cancer and aids in cancer prevention, early detection, and improved overall outcomes. Germline genetic testing is now part of the standard of care for certain types of patients with prostate cancer. There is a very limited body of work that investigated demographic, disease- related and social factors that may be influencing Veterans’ participation in germline genetic testing. This study helps to identify whether certain factors may be influencing decisions on participation in prostate germline testing among Veterans with prostate malignancy.
Methods
The study was conducted using retrospective chart review. Data was collected from the periods of August 1, 2022 to December 31, 2023 among Veterans with prostate cancer who met criteria for germline genetic testing. Demographic and clinical information were collected including age, race, extent of disease (high risk, very high-risk or metastatic disease), significant co-morbidities, educational level, family and personal history of cancer, travel time, germline genetic test findings, impact on treatment approaches, referral for genetic counseling, and whether Veterans agreed or declined germline genetic testing. Data was analyzed using descriptive statistics. A total of 180 charts were reviewed, with 171 meeting the criteria for inclusion. The mean age of the participants is 73, with the youngest being 55 and the oldest being 101 years old. Majority of the participants were African American (77%).
Results
Only about two percent of those who met the inclusion criteria declined to undergo testing with the one living the farthest away from the testing hospital residing 18 miles away. Those who declined testing ranged in age from 67 to 88, majority had high risk prostate cancer and no family history of malignancy, and had 0-1 serious co-morbidity. None of their educational informational was available for review.
Conclusions
Participation in germline genetic testing can be enhanced with adequate patient education and availability of accessible resources, even among patient populations that are not always well-represented in clinical research. The presence of multiple serious co-morbidities and distance from a testing facility do not seem to contribute to hesitancy in germline genetic testing participation.
Background
With the onset of precision oncology, findings from germline mutational analysis have been helpful in treating patients with cancer and aids in cancer prevention, early detection, and improved overall outcomes. Germline genetic testing is now part of the standard of care for certain types of patients with prostate cancer. There is a very limited body of work that investigated demographic, disease- related and social factors that may be influencing Veterans’ participation in germline genetic testing. This study helps to identify whether certain factors may be influencing decisions on participation in prostate germline testing among Veterans with prostate malignancy.
Methods
The study was conducted using retrospective chart review. Data was collected from the periods of August 1, 2022 to December 31, 2023 among Veterans with prostate cancer who met criteria for germline genetic testing. Demographic and clinical information were collected including age, race, extent of disease (high risk, very high-risk or metastatic disease), significant co-morbidities, educational level, family and personal history of cancer, travel time, germline genetic test findings, impact on treatment approaches, referral for genetic counseling, and whether Veterans agreed or declined germline genetic testing. Data was analyzed using descriptive statistics. A total of 180 charts were reviewed, with 171 meeting the criteria for inclusion. The mean age of the participants is 73, with the youngest being 55 and the oldest being 101 years old. Majority of the participants were African American (77%).
Results
Only about two percent of those who met the inclusion criteria declined to undergo testing with the one living the farthest away from the testing hospital residing 18 miles away. Those who declined testing ranged in age from 67 to 88, majority had high risk prostate cancer and no family history of malignancy, and had 0-1 serious co-morbidity. None of their educational informational was available for review.
Conclusions
Participation in germline genetic testing can be enhanced with adequate patient education and availability of accessible resources, even among patient populations that are not always well-represented in clinical research. The presence of multiple serious co-morbidities and distance from a testing facility do not seem to contribute to hesitancy in germline genetic testing participation.
Background
With the onset of precision oncology, findings from germline mutational analysis have been helpful in treating patients with cancer and aids in cancer prevention, early detection, and improved overall outcomes. Germline genetic testing is now part of the standard of care for certain types of patients with prostate cancer. There is a very limited body of work that investigated demographic, disease- related and social factors that may be influencing Veterans’ participation in germline genetic testing. This study helps to identify whether certain factors may be influencing decisions on participation in prostate germline testing among Veterans with prostate malignancy.
Methods
The study was conducted using retrospective chart review. Data was collected from the periods of August 1, 2022 to December 31, 2023 among Veterans with prostate cancer who met criteria for germline genetic testing. Demographic and clinical information were collected including age, race, extent of disease (high risk, very high-risk or metastatic disease), significant co-morbidities, educational level, family and personal history of cancer, travel time, germline genetic test findings, impact on treatment approaches, referral for genetic counseling, and whether Veterans agreed or declined germline genetic testing. Data was analyzed using descriptive statistics. A total of 180 charts were reviewed, with 171 meeting the criteria for inclusion. The mean age of the participants is 73, with the youngest being 55 and the oldest being 101 years old. Majority of the participants were African American (77%).
Results
Only about two percent of those who met the inclusion criteria declined to undergo testing with the one living the farthest away from the testing hospital residing 18 miles away. Those who declined testing ranged in age from 67 to 88, majority had high risk prostate cancer and no family history of malignancy, and had 0-1 serious co-morbidity. None of their educational informational was available for review.
Conclusions
Participation in germline genetic testing can be enhanced with adequate patient education and availability of accessible resources, even among patient populations that are not always well-represented in clinical research. The presence of multiple serious co-morbidities and distance from a testing facility do not seem to contribute to hesitancy in germline genetic testing participation.
E-Consults Bridge to Interdisciplinary Team Care for Rural Appalachian Veterans With Chronic Pain and Opioid Use Disorder
E-Consults Bridge to Interdisciplinary Team Care for Rural Appalachian Veterans With Chronic Pain and Opioid Use Disorder
Rural veterans are prescribed long-term opioid therapy for chronic pain at higher rates than urban veterans, increasing their risk of developing opioid use disorder (OUD).1,2 Veterans with co-occurring OUD and chronic pain have more severe health concerns, as well as higher rates of homelessness, psychoactive drug misuse, and mental health disorders, compared to veterans with either chronic pain or OUD alone.3 Interdisciplinary team (IDT) care is recommended for both chronic pain and OUD.4,5 Rural veterans with co-occurring chronic pain and OUD, however, are often unable to access IDTs due to long travel and wait times. As a result, these rural veterans often receive care from primary care practitioners (PCPs) who lack training in pain management and addiction and have low confidence in their ability to provide optimal treatment.6,7
In the Veterans Health Administration, electronic consultations (e-consults) provide support to PCPs by recommending evidence-based approaches such as buprenorphine for OUD and pain IDTs for chronic pain.5,8 However, research on the use of e-consults to connect to IDT care for co-occurring chronic pain and OUD are lacking, as well as studies on IDTs using innovative methods (eg, shared appointments) to overcome treatment barriers (eg, multiple appointments) for rural veterans at higher risk for co-occurring OUD and chronic pain.
This quality improvement study sought to determine the feasibility and impact of a pharmacy e-consult service that provided pain medication recommendations and subsequent referrals to RESTORE, a shared appointment program with an IDT, for assessment and treatment of chronic pain and OUD.
Methods
This retrospective chart review was approved as nonresearch by the Institutional Review Board Chair at the Salem Veterans Affairs Healthcare System (SVAHS), a low-complexity medical center in Virginia that primarily serves a rural and highly rural Central Appalachian veteran population.
This study included veterans whose clinicians placed a pain medication e-consult requesting recommendations for medication adjustments and/or a referral to RESTORE from January 1, 2022, through January 6, 2023. Requests for services that could not be provided through an e-consult were excluded (Figure 1). Veterans who had a pain medication e-consult were identified in the SVAHS electronic medical record (EMR). Data extracted from the EMR included demographics, referral source, reason for referral, RESTORE appointment attendance, OUD diagnosis made during the RESTORE initial evaluation, implementation of medication recommendations by the referrer within 6 months, engagement in ≥ 3 pain education classes, and a shared appointment with a pain IDT within 6 months. Data were entered into a REDCap database, and descriptive statistics summarized the results. Feasibility was assessed by use of the e-consult by PCPs, attendance at the RESTORE appointment, and OUD diagnosis by the RESTORE team.
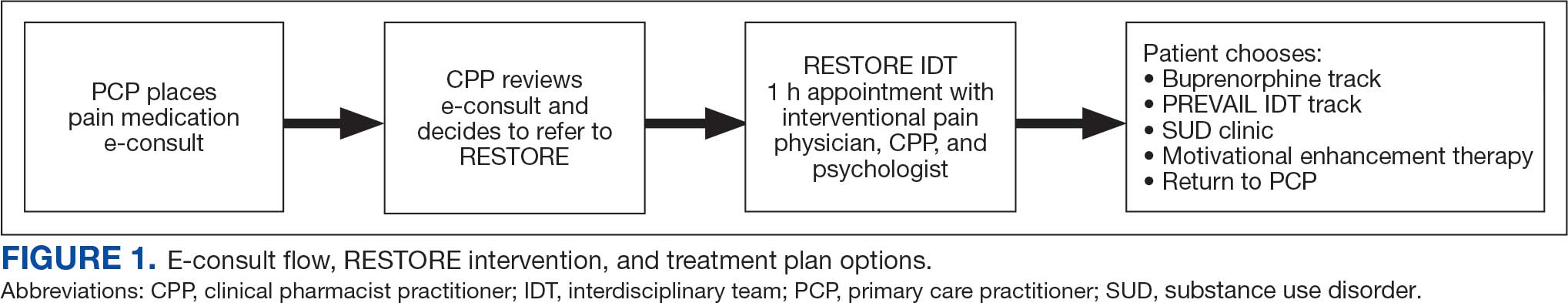
RESTORE Intervention
A pain medication e-consult was followed by referral to a shared appointment with the RESTORE IDT, with subsequent referrals to a pain IDT for chronic pain management if the veteran was amenable.
Pain medication e-consults in the EMR prompted a chart review by a clinical pharmacist practitioner (CPP). Recommendations for changes to medication regimens were documented in the EMR. At completion of the e-consult, the referring clinician received an automated view alert.
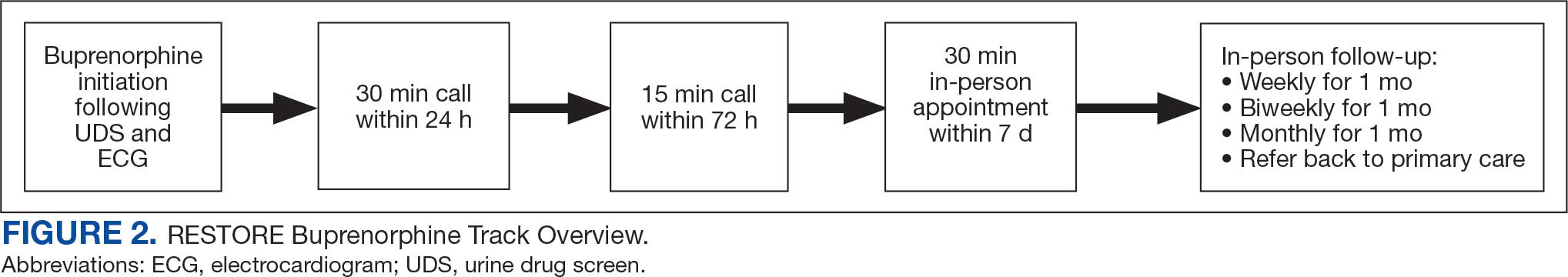
Veterans (and a support person, if preferred) were seen in a 60-minute, face-to-face shared appointment which included a psychologist, CPP, and pain physician. The psychologist conducted an OUD diagnostic interview, provided diagnostic feedback, and used motivational interviewing to provide psychoeducation on the biopsychosocial model of chronic pain, the IDT approach to chronic pain, and an overview of pain IDT care locally available. A CPP and physician then described medication options available to address OUD, if applicable. Together, the IDT and patient used shared decision making to determine a comprehensive treatment plan that may include a referral to the SVAHS PREVAIL Center for Chronic Pain IDT track (PREVAIL IDT track), a referral to substance use care in the case of polysubstance use, or medication initiation.9-11 If medication was prescribed, the patient was subsequently followed by the CPP through phone calls and face-to-face appointments at regularly scheduled intervals in coordination with the prescriber until they were stabilized. After stabilization, the prescription would be managed by their PCP (Figure 2). Veterans whose clinical condition changed significantly or worsened after returning to their PCP were invited to be reevaluated by the RESTORE team and restart care in that program. Individuals who were actively receiving RESTORE team care were discussed in a weekly care coordination meeting with all clinicians from both the PREVAIL and RESTORE teams.
Program Metrics
Pain medication e-consults were placed for 77 patients; 7 were excluded as inappropriate referral requests. Seventy (83%) e-consults were placed by PCPs (Table). Fifty-seven referring PCPs (81%) implemented ≥ 1 medication recommendation and 41 (59%) implemented all recommendations within 6 months. CPPs referred 19 individuals to RESTORE due to concerns related to high risk. All attended the initial evaluation appointment with the RESTORE team, 17 (89%) agreed to be referred to PREVAIL IDT track for nonpharmacologic pain care, and 9 (53%) engaged with that care within 6 months. Of those who attended RESTORE, 7 patients (37%) initiated buprenorphine for OUD with 6 (86%) being prescribed buprenorphine for ≥ 6 months.

Discussion
Most e-consults placed at SVAHS, which primarily serves a rural veteran population in Central Appalachia, resulted in veterans engaging in evidence-based treatment for co-occurring chronic pain and OUD. The use of e-consults and subsequent shared appointments with an IDT appears to be feasible, as the service was most often used by PCPs who often feel unequipped to manage chronic pain.7 The attendance rate for the RESTORE appointments was notable given the typically poor follow-up for patients with OUD. It supports the feasibility of a shared appointment approach which may overcome frequent barriers to care in this vulnerable population (ie, time, transportation). By attending 1 appointment with all clinicians present as opposed to multiple appointments, veterans experience fewer barriers than attending multiple appointments. RESTORE continues to be offered as an active clinical service whose implementation is now supported by changes to SVAHS policies. Since this study was conducted, the number of patients seen weekly has doubled and will soon be tripled based on high demand from PCPs.
While this study was limited to 1 site, had a small sample size, and was limited in scope, its results suggest that future research is warranted. Future studies using a larger sample size utilizing both a randomized control trial design and qualitative methods are needed to answer critical questions such as the role of patient characteristics on treatment effectiveness and the impact of the RESTORE model on long-term OUD medication adherence, patients’ perceptions and satisfaction, barriers to implementation, PCP confidence in providing pain care, and use of evidence-based nonpharmacologic pain management services.12-14
Conclusions
The results of this quality-improvement project suggest that e-consults may facilitate referrals to and patient follow-through with evidence-based treatment for co-occurring chronic pain and OUD among veterans living in rural communities in Central Appalachia who tend to experience significant barriers to traditional care and may require an innovative approach to facilitate effective treatment.
- Lund BC, Ohl ME, Hadlandsmyth K, et al. Regional and rural-urban variation in opioid prescribing in the Veterans Health Administration. Mil Med. 2019;184(11-12):894-900. doi:10.1093/milmed/usz104
- Edlund MJ, Martin BC, Russo JE, et al. The role of opioid prescription in incident opioid abuse and dependence among individuals with chronic noncancer pain: the role of opioid prescription. Clin J Pain. 2014;30(7):557-564. doi:10.1097/AJP.0000000000000021
- MacLean RR, Sofuoglu M, Stefanovics E, et al. Opioid use disorder with chronic pain increases disease burden and service use. Psychol Serv. 2023;20(1):157-165. doi:10.1037/ser0000607
- US Department of Veterans Affairs, US Department of Defense. VA/DoD clinical practice guidelines: use of opioids in the management of chronic pain. Version 4.0. Updated May 2022. Accessed August 4, 2025. https://www.healthquality.va.gov/guidelines/Pain/cot/VADoDOpioidsCPG.pdf
- US Department of Veterans Affairs, US Department of Defense. VA/DoD clinical practice guideline for the diagnosis and treatment of low back pain: the diagnosis and treatment of low back pain. Version 3.0. Updated February 2022. Accessed August 4, 2025. https://www.healthquality.va.gov/guidelines/Pain/lbp/VADoDLBPCPGFinal508.pdf
- Shipton EE, Bate F, Garrick R, et al. Systematic review of pain medicine content, teaching, and assessment in medical school curricula internationally. Pain Ther. 2018;7(2):139-161. doi:10.1007/s40122-018-0103-z
- Jamison RN, Scanlan E, Matthews ML, et al. Attitudes of primary care practitioners in managing chronic pain patients prescribed opioids for pain: a prospective longitudinal controlled trial. Pain Med. 2016;17(1):99-113. doi:10.1111/pme.12871
- Miller DM, Harvey TL. Pharmacist pain e-consults that result in a therapy change. Fed Pract. 2015;32(7):14-19.
- Courtney RE, Schadegg MJ. Chronic, noncancer pain care in the Veterans Administration: current trends and future directions. Anesthesiol Clin. 2023;41(2):519-529. doi:10.1016/j.anclin.2023.02.004
- Courtney RE, Schadegg MJ, Bolton R, et al. Using a whole health approach to build biopsychosocial-spiritual personal health plans for veterans with chronic pain. Pain Manag Nurs. 2024;25(1):69-74. doi:10.1016/j.pmn.2023.09.010
- Darnall BD, Edwards KA, Courtney RE, et al. Innovative treatment formats, technologies, and clinician trainings that improve access to behavioral pain treatment for youth and adults. Front Pain Res. 2023;4. doi:10.3389/fpain.2023.1223172
- Lister JJ, Weaver A, Ellis JD, et al. A systematic review of rural-specific barriers to medication treatment for opioid use disorder in the United States. Am J Drug Alcohol Abuse. 2020;46:273-288. doi:10.1080/00952990.2019.1694536
- Bhatraju EP, Radick AC, Leroux BG, et al. Buprenorphine adherence and illicit opioid use among patients in treatment for opioid use disorder. Am J Drug Alcohol Abuse. 2023;49. doi:10.1080/00952990.2023.2220876
- Courtney RE, Halsey E, Patil T, Mastronardi KV, Browne HS, Darnall BD. Prescription opioid tapering practices and outcomes at a rural VA health care system. Pain Med. 2024;25:480-482. doi:10.1093/pm/pnae013
Rural veterans are prescribed long-term opioid therapy for chronic pain at higher rates than urban veterans, increasing their risk of developing opioid use disorder (OUD).1,2 Veterans with co-occurring OUD and chronic pain have more severe health concerns, as well as higher rates of homelessness, psychoactive drug misuse, and mental health disorders, compared to veterans with either chronic pain or OUD alone.3 Interdisciplinary team (IDT) care is recommended for both chronic pain and OUD.4,5 Rural veterans with co-occurring chronic pain and OUD, however, are often unable to access IDTs due to long travel and wait times. As a result, these rural veterans often receive care from primary care practitioners (PCPs) who lack training in pain management and addiction and have low confidence in their ability to provide optimal treatment.6,7
In the Veterans Health Administration, electronic consultations (e-consults) provide support to PCPs by recommending evidence-based approaches such as buprenorphine for OUD and pain IDTs for chronic pain.5,8 However, research on the use of e-consults to connect to IDT care for co-occurring chronic pain and OUD are lacking, as well as studies on IDTs using innovative methods (eg, shared appointments) to overcome treatment barriers (eg, multiple appointments) for rural veterans at higher risk for co-occurring OUD and chronic pain.
This quality improvement study sought to determine the feasibility and impact of a pharmacy e-consult service that provided pain medication recommendations and subsequent referrals to RESTORE, a shared appointment program with an IDT, for assessment and treatment of chronic pain and OUD.
Methods
This retrospective chart review was approved as nonresearch by the Institutional Review Board Chair at the Salem Veterans Affairs Healthcare System (SVAHS), a low-complexity medical center in Virginia that primarily serves a rural and highly rural Central Appalachian veteran population.
This study included veterans whose clinicians placed a pain medication e-consult requesting recommendations for medication adjustments and/or a referral to RESTORE from January 1, 2022, through January 6, 2023. Requests for services that could not be provided through an e-consult were excluded (Figure 1). Veterans who had a pain medication e-consult were identified in the SVAHS electronic medical record (EMR). Data extracted from the EMR included demographics, referral source, reason for referral, RESTORE appointment attendance, OUD diagnosis made during the RESTORE initial evaluation, implementation of medication recommendations by the referrer within 6 months, engagement in ≥ 3 pain education classes, and a shared appointment with a pain IDT within 6 months. Data were entered into a REDCap database, and descriptive statistics summarized the results. Feasibility was assessed by use of the e-consult by PCPs, attendance at the RESTORE appointment, and OUD diagnosis by the RESTORE team.

RESTORE Intervention
A pain medication e-consult was followed by referral to a shared appointment with the RESTORE IDT, with subsequent referrals to a pain IDT for chronic pain management if the veteran was amenable.
Pain medication e-consults in the EMR prompted a chart review by a clinical pharmacist practitioner (CPP). Recommendations for changes to medication regimens were documented in the EMR. At completion of the e-consult, the referring clinician received an automated view alert.
Veterans (and a support person, if preferred) were seen in a 60-minute, face-to-face shared appointment which included a psychologist, CPP, and pain physician. The psychologist conducted an OUD diagnostic interview, provided diagnostic feedback, and used motivational interviewing to provide psychoeducation on the biopsychosocial model of chronic pain, the IDT approach to chronic pain, and an overview of pain IDT care locally available. A CPP and physician then described medication options available to address OUD, if applicable. Together, the IDT and patient used shared decision making to determine a comprehensive treatment plan that may include a referral to the SVAHS PREVAIL Center for Chronic Pain IDT track (PREVAIL IDT track), a referral to substance use care in the case of polysubstance use, or medication initiation.9-11 If medication was prescribed, the patient was subsequently followed by the CPP through phone calls and face-to-face appointments at regularly scheduled intervals in coordination with the prescriber until they were stabilized. After stabilization, the prescription would be managed by their PCP (Figure 2). Veterans whose clinical condition changed significantly or worsened after returning to their PCP were invited to be reevaluated by the RESTORE team and restart care in that program. Individuals who were actively receiving RESTORE team care were discussed in a weekly care coordination meeting with all clinicians from both the PREVAIL and RESTORE teams.

Program Metrics
Pain medication e-consults were placed for 77 patients; 7 were excluded as inappropriate referral requests. Seventy (83%) e-consults were placed by PCPs (Table). Fifty-seven referring PCPs (81%) implemented ≥ 1 medication recommendation and 41 (59%) implemented all recommendations within 6 months. CPPs referred 19 individuals to RESTORE due to concerns related to high risk. All attended the initial evaluation appointment with the RESTORE team, 17 (89%) agreed to be referred to PREVAIL IDT track for nonpharmacologic pain care, and 9 (53%) engaged with that care within 6 months. Of those who attended RESTORE, 7 patients (37%) initiated buprenorphine for OUD with 6 (86%) being prescribed buprenorphine for ≥ 6 months.

Discussion
Most e-consults placed at SVAHS, which primarily serves a rural veteran population in Central Appalachia, resulted in veterans engaging in evidence-based treatment for co-occurring chronic pain and OUD. The use of e-consults and subsequent shared appointments with an IDT appears to be feasible, as the service was most often used by PCPs who often feel unequipped to manage chronic pain.7 The attendance rate for the RESTORE appointments was notable given the typically poor follow-up for patients with OUD. It supports the feasibility of a shared appointment approach which may overcome frequent barriers to care in this vulnerable population (ie, time, transportation). By attending 1 appointment with all clinicians present as opposed to multiple appointments, veterans experience fewer barriers than attending multiple appointments. RESTORE continues to be offered as an active clinical service whose implementation is now supported by changes to SVAHS policies. Since this study was conducted, the number of patients seen weekly has doubled and will soon be tripled based on high demand from PCPs.
While this study was limited to 1 site, had a small sample size, and was limited in scope, its results suggest that future research is warranted. Future studies using a larger sample size utilizing both a randomized control trial design and qualitative methods are needed to answer critical questions such as the role of patient characteristics on treatment effectiveness and the impact of the RESTORE model on long-term OUD medication adherence, patients’ perceptions and satisfaction, barriers to implementation, PCP confidence in providing pain care, and use of evidence-based nonpharmacologic pain management services.12-14
Conclusions
The results of this quality-improvement project suggest that e-consults may facilitate referrals to and patient follow-through with evidence-based treatment for co-occurring chronic pain and OUD among veterans living in rural communities in Central Appalachia who tend to experience significant barriers to traditional care and may require an innovative approach to facilitate effective treatment.
Rural veterans are prescribed long-term opioid therapy for chronic pain at higher rates than urban veterans, increasing their risk of developing opioid use disorder (OUD).1,2 Veterans with co-occurring OUD and chronic pain have more severe health concerns, as well as higher rates of homelessness, psychoactive drug misuse, and mental health disorders, compared to veterans with either chronic pain or OUD alone.3 Interdisciplinary team (IDT) care is recommended for both chronic pain and OUD.4,5 Rural veterans with co-occurring chronic pain and OUD, however, are often unable to access IDTs due to long travel and wait times. As a result, these rural veterans often receive care from primary care practitioners (PCPs) who lack training in pain management and addiction and have low confidence in their ability to provide optimal treatment.6,7
In the Veterans Health Administration, electronic consultations (e-consults) provide support to PCPs by recommending evidence-based approaches such as buprenorphine for OUD and pain IDTs for chronic pain.5,8 However, research on the use of e-consults to connect to IDT care for co-occurring chronic pain and OUD are lacking, as well as studies on IDTs using innovative methods (eg, shared appointments) to overcome treatment barriers (eg, multiple appointments) for rural veterans at higher risk for co-occurring OUD and chronic pain.
This quality improvement study sought to determine the feasibility and impact of a pharmacy e-consult service that provided pain medication recommendations and subsequent referrals to RESTORE, a shared appointment program with an IDT, for assessment and treatment of chronic pain and OUD.
Methods
This retrospective chart review was approved as nonresearch by the Institutional Review Board Chair at the Salem Veterans Affairs Healthcare System (SVAHS), a low-complexity medical center in Virginia that primarily serves a rural and highly rural Central Appalachian veteran population.
This study included veterans whose clinicians placed a pain medication e-consult requesting recommendations for medication adjustments and/or a referral to RESTORE from January 1, 2022, through January 6, 2023. Requests for services that could not be provided through an e-consult were excluded (Figure 1). Veterans who had a pain medication e-consult were identified in the SVAHS electronic medical record (EMR). Data extracted from the EMR included demographics, referral source, reason for referral, RESTORE appointment attendance, OUD diagnosis made during the RESTORE initial evaluation, implementation of medication recommendations by the referrer within 6 months, engagement in ≥ 3 pain education classes, and a shared appointment with a pain IDT within 6 months. Data were entered into a REDCap database, and descriptive statistics summarized the results. Feasibility was assessed by use of the e-consult by PCPs, attendance at the RESTORE appointment, and OUD diagnosis by the RESTORE team.

RESTORE Intervention
A pain medication e-consult was followed by referral to a shared appointment with the RESTORE IDT, with subsequent referrals to a pain IDT for chronic pain management if the veteran was amenable.
Pain medication e-consults in the EMR prompted a chart review by a clinical pharmacist practitioner (CPP). Recommendations for changes to medication regimens were documented in the EMR. At completion of the e-consult, the referring clinician received an automated view alert.
Veterans (and a support person, if preferred) were seen in a 60-minute, face-to-face shared appointment which included a psychologist, CPP, and pain physician. The psychologist conducted an OUD diagnostic interview, provided diagnostic feedback, and used motivational interviewing to provide psychoeducation on the biopsychosocial model of chronic pain, the IDT approach to chronic pain, and an overview of pain IDT care locally available. A CPP and physician then described medication options available to address OUD, if applicable. Together, the IDT and patient used shared decision making to determine a comprehensive treatment plan that may include a referral to the SVAHS PREVAIL Center for Chronic Pain IDT track (PREVAIL IDT track), a referral to substance use care in the case of polysubstance use, or medication initiation.9-11 If medication was prescribed, the patient was subsequently followed by the CPP through phone calls and face-to-face appointments at regularly scheduled intervals in coordination with the prescriber until they were stabilized. After stabilization, the prescription would be managed by their PCP (Figure 2). Veterans whose clinical condition changed significantly or worsened after returning to their PCP were invited to be reevaluated by the RESTORE team and restart care in that program. Individuals who were actively receiving RESTORE team care were discussed in a weekly care coordination meeting with all clinicians from both the PREVAIL and RESTORE teams.

Program Metrics
Pain medication e-consults were placed for 77 patients; 7 were excluded as inappropriate referral requests. Seventy (83%) e-consults were placed by PCPs (Table). Fifty-seven referring PCPs (81%) implemented ≥ 1 medication recommendation and 41 (59%) implemented all recommendations within 6 months. CPPs referred 19 individuals to RESTORE due to concerns related to high risk. All attended the initial evaluation appointment with the RESTORE team, 17 (89%) agreed to be referred to PREVAIL IDT track for nonpharmacologic pain care, and 9 (53%) engaged with that care within 6 months. Of those who attended RESTORE, 7 patients (37%) initiated buprenorphine for OUD with 6 (86%) being prescribed buprenorphine for ≥ 6 months.

Discussion
Most e-consults placed at SVAHS, which primarily serves a rural veteran population in Central Appalachia, resulted in veterans engaging in evidence-based treatment for co-occurring chronic pain and OUD. The use of e-consults and subsequent shared appointments with an IDT appears to be feasible, as the service was most often used by PCPs who often feel unequipped to manage chronic pain.7 The attendance rate for the RESTORE appointments was notable given the typically poor follow-up for patients with OUD. It supports the feasibility of a shared appointment approach which may overcome frequent barriers to care in this vulnerable population (ie, time, transportation). By attending 1 appointment with all clinicians present as opposed to multiple appointments, veterans experience fewer barriers than attending multiple appointments. RESTORE continues to be offered as an active clinical service whose implementation is now supported by changes to SVAHS policies. Since this study was conducted, the number of patients seen weekly has doubled and will soon be tripled based on high demand from PCPs.
While this study was limited to 1 site, had a small sample size, and was limited in scope, its results suggest that future research is warranted. Future studies using a larger sample size utilizing both a randomized control trial design and qualitative methods are needed to answer critical questions such as the role of patient characteristics on treatment effectiveness and the impact of the RESTORE model on long-term OUD medication adherence, patients’ perceptions and satisfaction, barriers to implementation, PCP confidence in providing pain care, and use of evidence-based nonpharmacologic pain management services.12-14
Conclusions
The results of this quality-improvement project suggest that e-consults may facilitate referrals to and patient follow-through with evidence-based treatment for co-occurring chronic pain and OUD among veterans living in rural communities in Central Appalachia who tend to experience significant barriers to traditional care and may require an innovative approach to facilitate effective treatment.
- Lund BC, Ohl ME, Hadlandsmyth K, et al. Regional and rural-urban variation in opioid prescribing in the Veterans Health Administration. Mil Med. 2019;184(11-12):894-900. doi:10.1093/milmed/usz104
- Edlund MJ, Martin BC, Russo JE, et al. The role of opioid prescription in incident opioid abuse and dependence among individuals with chronic noncancer pain: the role of opioid prescription. Clin J Pain. 2014;30(7):557-564. doi:10.1097/AJP.0000000000000021
- MacLean RR, Sofuoglu M, Stefanovics E, et al. Opioid use disorder with chronic pain increases disease burden and service use. Psychol Serv. 2023;20(1):157-165. doi:10.1037/ser0000607
- US Department of Veterans Affairs, US Department of Defense. VA/DoD clinical practice guidelines: use of opioids in the management of chronic pain. Version 4.0. Updated May 2022. Accessed August 4, 2025. https://www.healthquality.va.gov/guidelines/Pain/cot/VADoDOpioidsCPG.pdf
- US Department of Veterans Affairs, US Department of Defense. VA/DoD clinical practice guideline for the diagnosis and treatment of low back pain: the diagnosis and treatment of low back pain. Version 3.0. Updated February 2022. Accessed August 4, 2025. https://www.healthquality.va.gov/guidelines/Pain/lbp/VADoDLBPCPGFinal508.pdf
- Shipton EE, Bate F, Garrick R, et al. Systematic review of pain medicine content, teaching, and assessment in medical school curricula internationally. Pain Ther. 2018;7(2):139-161. doi:10.1007/s40122-018-0103-z
- Jamison RN, Scanlan E, Matthews ML, et al. Attitudes of primary care practitioners in managing chronic pain patients prescribed opioids for pain: a prospective longitudinal controlled trial. Pain Med. 2016;17(1):99-113. doi:10.1111/pme.12871
- Miller DM, Harvey TL. Pharmacist pain e-consults that result in a therapy change. Fed Pract. 2015;32(7):14-19.
- Courtney RE, Schadegg MJ. Chronic, noncancer pain care in the Veterans Administration: current trends and future directions. Anesthesiol Clin. 2023;41(2):519-529. doi:10.1016/j.anclin.2023.02.004
- Courtney RE, Schadegg MJ, Bolton R, et al. Using a whole health approach to build biopsychosocial-spiritual personal health plans for veterans with chronic pain. Pain Manag Nurs. 2024;25(1):69-74. doi:10.1016/j.pmn.2023.09.010
- Darnall BD, Edwards KA, Courtney RE, et al. Innovative treatment formats, technologies, and clinician trainings that improve access to behavioral pain treatment for youth and adults. Front Pain Res. 2023;4. doi:10.3389/fpain.2023.1223172
- Lister JJ, Weaver A, Ellis JD, et al. A systematic review of rural-specific barriers to medication treatment for opioid use disorder in the United States. Am J Drug Alcohol Abuse. 2020;46:273-288. doi:10.1080/00952990.2019.1694536
- Bhatraju EP, Radick AC, Leroux BG, et al. Buprenorphine adherence and illicit opioid use among patients in treatment for opioid use disorder. Am J Drug Alcohol Abuse. 2023;49. doi:10.1080/00952990.2023.2220876
- Courtney RE, Halsey E, Patil T, Mastronardi KV, Browne HS, Darnall BD. Prescription opioid tapering practices and outcomes at a rural VA health care system. Pain Med. 2024;25:480-482. doi:10.1093/pm/pnae013
- Lund BC, Ohl ME, Hadlandsmyth K, et al. Regional and rural-urban variation in opioid prescribing in the Veterans Health Administration. Mil Med. 2019;184(11-12):894-900. doi:10.1093/milmed/usz104
- Edlund MJ, Martin BC, Russo JE, et al. The role of opioid prescription in incident opioid abuse and dependence among individuals with chronic noncancer pain: the role of opioid prescription. Clin J Pain. 2014;30(7):557-564. doi:10.1097/AJP.0000000000000021
- MacLean RR, Sofuoglu M, Stefanovics E, et al. Opioid use disorder with chronic pain increases disease burden and service use. Psychol Serv. 2023;20(1):157-165. doi:10.1037/ser0000607
- US Department of Veterans Affairs, US Department of Defense. VA/DoD clinical practice guidelines: use of opioids in the management of chronic pain. Version 4.0. Updated May 2022. Accessed August 4, 2025. https://www.healthquality.va.gov/guidelines/Pain/cot/VADoDOpioidsCPG.pdf
- US Department of Veterans Affairs, US Department of Defense. VA/DoD clinical practice guideline for the diagnosis and treatment of low back pain: the diagnosis and treatment of low back pain. Version 3.0. Updated February 2022. Accessed August 4, 2025. https://www.healthquality.va.gov/guidelines/Pain/lbp/VADoDLBPCPGFinal508.pdf
- Shipton EE, Bate F, Garrick R, et al. Systematic review of pain medicine content, teaching, and assessment in medical school curricula internationally. Pain Ther. 2018;7(2):139-161. doi:10.1007/s40122-018-0103-z
- Jamison RN, Scanlan E, Matthews ML, et al. Attitudes of primary care practitioners in managing chronic pain patients prescribed opioids for pain: a prospective longitudinal controlled trial. Pain Med. 2016;17(1):99-113. doi:10.1111/pme.12871
- Miller DM, Harvey TL. Pharmacist pain e-consults that result in a therapy change. Fed Pract. 2015;32(7):14-19.
- Courtney RE, Schadegg MJ. Chronic, noncancer pain care in the Veterans Administration: current trends and future directions. Anesthesiol Clin. 2023;41(2):519-529. doi:10.1016/j.anclin.2023.02.004
- Courtney RE, Schadegg MJ, Bolton R, et al. Using a whole health approach to build biopsychosocial-spiritual personal health plans for veterans with chronic pain. Pain Manag Nurs. 2024;25(1):69-74. doi:10.1016/j.pmn.2023.09.010
- Darnall BD, Edwards KA, Courtney RE, et al. Innovative treatment formats, technologies, and clinician trainings that improve access to behavioral pain treatment for youth and adults. Front Pain Res. 2023;4. doi:10.3389/fpain.2023.1223172
- Lister JJ, Weaver A, Ellis JD, et al. A systematic review of rural-specific barriers to medication treatment for opioid use disorder in the United States. Am J Drug Alcohol Abuse. 2020;46:273-288. doi:10.1080/00952990.2019.1694536
- Bhatraju EP, Radick AC, Leroux BG, et al. Buprenorphine adherence and illicit opioid use among patients in treatment for opioid use disorder. Am J Drug Alcohol Abuse. 2023;49. doi:10.1080/00952990.2023.2220876
- Courtney RE, Halsey E, Patil T, Mastronardi KV, Browne HS, Darnall BD. Prescription opioid tapering practices and outcomes at a rural VA health care system. Pain Med. 2024;25:480-482. doi:10.1093/pm/pnae013
E-Consults Bridge to Interdisciplinary Team Care for Rural Appalachian Veterans With Chronic Pain and Opioid Use Disorder
E-Consults Bridge to Interdisciplinary Team Care for Rural Appalachian Veterans With Chronic Pain and Opioid Use Disorder
Unique Presentation of Postpartum Hypereosinophilic Syndrome With Atypical Features and Therapeutic Challenges
Unique Presentation of Postpartum Hypereosinophilic Syndrome With Atypical Features and Therapeutic Challenges
Hypereosinophilic syndrome (HES) is defined by marked, persistent absolute eosinophil count (AEC) > 1500 cells/μL on ≥ 2 peripheral smears separated by ≥ 1 month with evidence of accompanied end-organ damage, in the absence of other causes of eosinophilia such as malignancy, atopy, or parasitic infections.1-5 Hypereosinophilic infiltration can impact almost every organ system; however, the most profound complications in patients with HES are related to leukemias and cardiac manifestations of the disease.3,4 Although rare, the associated morbidity and mortality of HES are considerable, making prompt recognition and treatment essential. Management involves targeted therapy based on pathologic classification of HES and on decreasing associated inflammation, fibrosis, and end-organ damage.3,5-7
The patient in this case report met the diagnostic criteria for HES. However, this patient had several clinical and laboratory features that made it difficult to characterize a specific HES variant. Moreover, she had additional immunomodulating factors in the setting of pregnancy. This is the first documented case of HES of undetermined etiology diagnosed postpartum and managed in the setting of a new pregnancy.2,8
CASE PRESENTATION
A 32-year-old female active-duty military service member with allergic rhinitis and a history of childhood eczema was referred to allergy/immunology for evaluation of a new, progressive pruritic rash. Symptoms started 3 months after the birth of her first child, with a new diffuse erythematous skin rash sparing her palms, soles, and mucosal surfaces. Given her history of atopy, the rash was initially treated as severe atopic dermatitis with appropriate topical medications. The rash gradually worsened, with the development of intermittent facial swelling, night sweats, dyspnea, recurrent epigastric abdominal pain, and nausea with vomiting, resulting in decreased oral intake and weight loss.
The patient was hospitalized and received an expedited multidisciplinary evaluation by dermatology, hematology/oncology, and gastroenterology. Her AEC of 4787 cells/μL peaked on admission and was markedly elevated from the 1070 cells/μL reported in the third trimester of her pregnancy. She was found to have mature eosinophilia on skin biopsy (Figure 1), endoscopic duodenal biopsy (Figure 2), peripheral blood smear (Figure 3), and bone marrow biopsy (Figure 4).




Radiographic imaging of the chest, abdomen, and pelvis revealed hepatomegaly without detectable neoplasm. There was no clinical evidence of cardiac involvement, and evaluation with electrocardiography and echocardiography did not indicate myocarditis. Extensive laboratory testing revealed no genetic mutations indicative of familial, myeloproliferative, or lymphocytic variants of HES.
The patient received topical emollients, omeprazole 40 mg daily, and ondansetron 8 mg 3 times daily as needed for symptom management, and was started on oral prednisone 40 mg daily with improvement in dyspnea, night sweats, and gastrointestinal complaints. During the patient's 6-day hospitalization and treatment, her AECs gradually decreased to 2110 cells/μL, and decreased to 1600 cells/μL over the course of a month, remaining in the hypereosinophilic range. The patient was discovered to be pregnant while symptoms were improving, resulting in stepwise discontinuation of oral steroids, but she reported continued improvement in symptoms.
DISCUSSION
Peripheral eosinophilia has a broad differential diagnoses, including HES, parasitic infections, atopic hypersensitivity diseases, eosinophilic lung diseases, eosinophilic gastrointestinal diseases, vasculitides such as eosinophilic granulomatosis with polyangiitis, genetic syndromes predisposing to eosinophilia, episodic angioedema with eosinophilia, and chronic metabolic disease with adrenal insufficiency.1-5 HES, although rare, is a disease process with potentially devastating associated morbidity and mortality if not promptly recognized and treated. HES is further delineated by hypereosinophilia with associated eosinophil-mediated organ damage or dysfunction.3-5
Clinical manifestations of HES can differ greatly depending on the HES variant and degree of organ involvement at the time of diagnosis and throughout the disease course. Patients with HES, as well as those with asymptomatic eosinophilia or hypereosinophilia, should be closely monitored for disease progression. In addition to trending peripheral AECs, clinicians should screen for symptoms of organ involvement and perform targeted evaluation of the suspected organs to promptly identify early signs of organ involvement and initiate treatment.1-4 Recommendations regarding screening intervals vary widely from monthly to annually, depending on a patient’s specific clinical picture.
HES has been subdivided into clinically relevant variants, including myeloproliferative (M-HES), T lymphocytic (L-HES), organ-restricted (or overlap) HES, familial HES, idiopathic HES, and specific syndromes with associated hypereosinophilia.3-5,9 Patients with M-HES have elevated circulating leukocyte precursors and clinical manifestations, including but not limited to hepatosplenomegaly, anemia, and thrombocytopenia. The most commonly associated genetic mutations include the FIP1L1-PDGFR-α fusion, BCR-ABL1, PDGFRA/B, JAK2, KIT, and FGFR1.3-6 L-HES usually has predominant skin and soft tissue involvement secondary to immunoglobulin E-mediated actions with clonal expansion of T cells (most commonly CD3-4+ or CD3+CD4-CD8-).3,5,6 Familial HES, a rare variant, follows an autosomal dominant inheritance pattern and is usually present at birth. It involves chromosome 5, which contains genes coding for cytokines that drive eosinophilic proliferation, including interleukin (IL)-3, IL-5, and granulocyte-macrophage colony-stimulating factor.5,9 Hypereosinophilia in the setting of end-organ damage restricted to a single organ is considered organ-restricted HES. There can be significant hepatic and gastrointestinal dysfunction, with or without malabsorption.
HES can also manifest with hematologic malignancy, restrictive obliterative cardiomyopathies, renal injury manifested by hematuria and electrolyte derangements, and neurologic complications including hemiparesis, dysarthria, and even coma.6 Endothelial damage due to eosinophil-driven inflammation can result in thrombus formation and increased risk of thromboembolic events in various organs.3 Idiopathic HES, otherwise known as HES of unknown etiology or significance, is a diagnosis of exclusion and constitutes a cohort of patients who do not fit into the other delineated categories.3-5 These patients often have multisystem involvement, making classification and treatment a challenge.5
The patient described in this case met the diagnostic criteria for HES, but her complicated clinical and laboratory features were challenging to characterize into a specific variant of HES. Organ-restricted HES was ruled out due to skin, marrow, and duodenal infiltration. She also had the potential for lung involvement based on her clinical symptoms, however no biopsy was obtained. Laboratory testing revealed no deletions or mutations indicative of familial, myeloproliferative, or lymphocytic variants. Her multisystem involvement without an underlying associated syndrome suggests idiopathic HES or HES of undetermined significance.1-5
Most patients with HES are diagnosed between the ages of 20 and 50 years.10 While HES has its peak incidence in the fourth decade of life, acute onset of new symptoms 3 months postpartum makes this an unusual presentation. In this unique case, it is important to highlight the role of the physiologic changes of pregnancy in inflammatory mediation. The physiologic changes that occur in pregnancy to ensure fetal tolerance can have profound implications for leukocyte count, AEC, and subsequent inflammatory responses. The phenomenon of inflammatory amelioration during pregnancy is well-documented, but there has only been 1 known published case report discussing decreasing HES symptoms during pregnancy with prepregnancy and postpartum hypereosinophilia.8 It is suggested that this amelioration is secondary to cortisol and progesterone shifts that occur in pregnancy. Physiologic increases in adrenocorticotropic hormone in pregnancy leads to subsequent secretion of endogenous steroids by the adrenal cortex. In turn, pregnancy can lead to leukocytosis and eosinopenia.8 Overall, pregnancy can have beneficial immunomodulating properties in the spectrum of hypereosinophilic syndromes. Even so, this patient with HES diagnosed postpartum remains at risk for the sequelae of hypereosinophilia, regardless of potential for AEC reduction during pregnancy. Therefore, treatment considerations need to be made with the safety of the maternal-fetal dyad as a priority.
Treatment
The treatment of symptomatic HES without acute life-threatening features or associated malignancy is generally determined by clinical variant.2-4 There is insufficient data to support initiation of treatment solely based on persistently elevated AEC. Patients with peripheral eosinophilia and hypereosinophilia should be monitored periodically with appropriate subspecialist evaluation for occult end-organ involvement, and targeted therapies should be deferred until an HES diagnosis.1-4 First-line therapy in most HES variants is systemic glucocorticoids.2,3,7 Since the disease course for this patient did not precisely match an HES variant, it was challenging to ascertain the optimal personalized treatment regimen. The approach to therapy was further complicated by newly identified pregnancy necessitating cessation of systemic glucocorticoids. In addition to glucocorticoids, hydroxyurea and interferon-α are among treatments historically used for HES, with tyrosine kinase inhibitors and monoclonal antibodies targeting IL-5 becoming more common.1-4 Although this patient may ultimately benefit from an IL-5 targeting biologic medication such as mepolizumab, safety in pregnancy is not well-studied and may require close clinical monitoring with treatment deferred until after delivery if possible.3,7,8,11
Military service members with frequent geographic relocation have additional barriers to timely diagnosis with often-limited access to subspecialty care depending on the duty station. While the patient was able to receive care at a large military medical center with many subspecialists, prompt recognition and timely referral to specialists would be even more critical at a smaller treatment facility. Depending on the severity and variant of HES, patients may warrant evaluation and treatment by hematology/oncology, cardiology, pulmonology, and immunology. Although HES can present in young children and older adults, this condition is most often diagnosed during the third and fourth decades of life, putting clinicians on the front line of hypereosinophilia identification and evaluation.10 Military physicians have the additional duty to not only think ahead in their diverse clinical settings to ensure proper care for patients, but also maintain a broad differential inclusive of more rare disease processes such as HES.
CONCLUSIONS
This case emphasizes how uncontrolled or untreated HES can lead to significant end-organ damage involving multiple systems and high morbidity. Prompt recognition of hypereosinophilia with potential HES can help expedite coordination of multidisciplinary care across multiple specialties to minimize delays in diagnosis and treatment. Doing so may minimize associated morbidity and mortality, especially in individuals located at more remote duty stations or deployed to austere environments.
- Cogan E, Roufosse F. Clinical management of the hypereosinophilic syndromes. Expert Rev Hematol. 2012;5:275-290. doi: 10.1586/ehm.12.14
- Klion A. Hypereosinophilic syndrome: approach to treatment in the era of precision medicine. Hematology Am Soc Hematol Educ Program. 2018;2018:326-331. doi:10.1182/asheducation-2018.1.326
- Shomali W, Gotlib J. World health organization-defined eosinophilic disorders: 2022 update on diagnosis, risk stratification, and management. Am J Hematol. 2022;97:129-148. doi:10.1002/ajh.26352
- Helbig G, Klion AD. Hypereosinophilic syndromes - an enigmatic group of disorders with an intriguing clinical spectrum and challenging treatment. Blood Rev. 2021;49:100809. doi:10.1016/j.blre.2021.100809
- Valent P, Klion AD, Horny HP, et al. Contemporary consensus proposal on criteria and classification of eosinophilic disorders and related syndromes. J Allergy Clin Immunol. 2012;130:607-612.e9. doi:10.1016/j.jaci.2012.02.019
- Roufosse FE, Goldman M, Cogan E. Hypereosinophilic syndromes. Orphanet J Rare Dis. 2007;2:37. doi:10.1186/1750-1172-2-37
- Pitlick MM, Li JT, Pongdee T. Current and emerging biologic therapies targeting eosinophilic disorders. World Allergy Organ J. 2022;15:100676. doi:10.1016/j.waojou.2022.10067
- Ault P, Cortes J, Lynn A, Keating M, Verstovsek S. Pregnancy in a patient with hypereosinophilic syndrome. Leuk Res. 2009;33:186-187. doi:10.1016/j.leukres.2008.05.013
- Rioux JD, Stone VA, Daly MJ, et al. Familial eosinophilia maps to the cytokine gene cluster on human chromosomal region 5q31-q33. Am J Hum Genet. 1998;63:1086-1094. doi:10.1086/302053
- Williams KW, Ware J, Abiodun A, et al. Hypereosinophilia in children and adults: a retrospective comparison. J Allergy Clin Immunol Pract. 2016;4:941-947.e1. doi:10.1016/j.jaip.2016.03.020
- Pane F, Lefevre G, Kwon N, et al. Characterization of disease flares and impact of mepolizumab in patients with hypereosinophilic syndrome. Front Immunol. 2022;13:935996. doi:10.3389/fimmu.2022.935996
Hypereosinophilic syndrome (HES) is defined by marked, persistent absolute eosinophil count (AEC) > 1500 cells/μL on ≥ 2 peripheral smears separated by ≥ 1 month with evidence of accompanied end-organ damage, in the absence of other causes of eosinophilia such as malignancy, atopy, or parasitic infections.1-5 Hypereosinophilic infiltration can impact almost every organ system; however, the most profound complications in patients with HES are related to leukemias and cardiac manifestations of the disease.3,4 Although rare, the associated morbidity and mortality of HES are considerable, making prompt recognition and treatment essential. Management involves targeted therapy based on pathologic classification of HES and on decreasing associated inflammation, fibrosis, and end-organ damage.3,5-7
The patient in this case report met the diagnostic criteria for HES. However, this patient had several clinical and laboratory features that made it difficult to characterize a specific HES variant. Moreover, she had additional immunomodulating factors in the setting of pregnancy. This is the first documented case of HES of undetermined etiology diagnosed postpartum and managed in the setting of a new pregnancy.2,8
CASE PRESENTATION
A 32-year-old female active-duty military service member with allergic rhinitis and a history of childhood eczema was referred to allergy/immunology for evaluation of a new, progressive pruritic rash. Symptoms started 3 months after the birth of her first child, with a new diffuse erythematous skin rash sparing her palms, soles, and mucosal surfaces. Given her history of atopy, the rash was initially treated as severe atopic dermatitis with appropriate topical medications. The rash gradually worsened, with the development of intermittent facial swelling, night sweats, dyspnea, recurrent epigastric abdominal pain, and nausea with vomiting, resulting in decreased oral intake and weight loss.
The patient was hospitalized and received an expedited multidisciplinary evaluation by dermatology, hematology/oncology, and gastroenterology. Her AEC of 4787 cells/μL peaked on admission and was markedly elevated from the 1070 cells/μL reported in the third trimester of her pregnancy. She was found to have mature eosinophilia on skin biopsy (Figure 1), endoscopic duodenal biopsy (Figure 2), peripheral blood smear (Figure 3), and bone marrow biopsy (Figure 4).




Radiographic imaging of the chest, abdomen, and pelvis revealed hepatomegaly without detectable neoplasm. There was no clinical evidence of cardiac involvement, and evaluation with electrocardiography and echocardiography did not indicate myocarditis. Extensive laboratory testing revealed no genetic mutations indicative of familial, myeloproliferative, or lymphocytic variants of HES.
The patient received topical emollients, omeprazole 40 mg daily, and ondansetron 8 mg 3 times daily as needed for symptom management, and was started on oral prednisone 40 mg daily with improvement in dyspnea, night sweats, and gastrointestinal complaints. During the patient's 6-day hospitalization and treatment, her AECs gradually decreased to 2110 cells/μL, and decreased to 1600 cells/μL over the course of a month, remaining in the hypereosinophilic range. The patient was discovered to be pregnant while symptoms were improving, resulting in stepwise discontinuation of oral steroids, but she reported continued improvement in symptoms.
DISCUSSION
Peripheral eosinophilia has a broad differential diagnoses, including HES, parasitic infections, atopic hypersensitivity diseases, eosinophilic lung diseases, eosinophilic gastrointestinal diseases, vasculitides such as eosinophilic granulomatosis with polyangiitis, genetic syndromes predisposing to eosinophilia, episodic angioedema with eosinophilia, and chronic metabolic disease with adrenal insufficiency.1-5 HES, although rare, is a disease process with potentially devastating associated morbidity and mortality if not promptly recognized and treated. HES is further delineated by hypereosinophilia with associated eosinophil-mediated organ damage or dysfunction.3-5
Clinical manifestations of HES can differ greatly depending on the HES variant and degree of organ involvement at the time of diagnosis and throughout the disease course. Patients with HES, as well as those with asymptomatic eosinophilia or hypereosinophilia, should be closely monitored for disease progression. In addition to trending peripheral AECs, clinicians should screen for symptoms of organ involvement and perform targeted evaluation of the suspected organs to promptly identify early signs of organ involvement and initiate treatment.1-4 Recommendations regarding screening intervals vary widely from monthly to annually, depending on a patient’s specific clinical picture.
HES has been subdivided into clinically relevant variants, including myeloproliferative (M-HES), T lymphocytic (L-HES), organ-restricted (or overlap) HES, familial HES, idiopathic HES, and specific syndromes with associated hypereosinophilia.3-5,9 Patients with M-HES have elevated circulating leukocyte precursors and clinical manifestations, including but not limited to hepatosplenomegaly, anemia, and thrombocytopenia. The most commonly associated genetic mutations include the FIP1L1-PDGFR-α fusion, BCR-ABL1, PDGFRA/B, JAK2, KIT, and FGFR1.3-6 L-HES usually has predominant skin and soft tissue involvement secondary to immunoglobulin E-mediated actions with clonal expansion of T cells (most commonly CD3-4+ or CD3+CD4-CD8-).3,5,6 Familial HES, a rare variant, follows an autosomal dominant inheritance pattern and is usually present at birth. It involves chromosome 5, which contains genes coding for cytokines that drive eosinophilic proliferation, including interleukin (IL)-3, IL-5, and granulocyte-macrophage colony-stimulating factor.5,9 Hypereosinophilia in the setting of end-organ damage restricted to a single organ is considered organ-restricted HES. There can be significant hepatic and gastrointestinal dysfunction, with or without malabsorption.
HES can also manifest with hematologic malignancy, restrictive obliterative cardiomyopathies, renal injury manifested by hematuria and electrolyte derangements, and neurologic complications including hemiparesis, dysarthria, and even coma.6 Endothelial damage due to eosinophil-driven inflammation can result in thrombus formation and increased risk of thromboembolic events in various organs.3 Idiopathic HES, otherwise known as HES of unknown etiology or significance, is a diagnosis of exclusion and constitutes a cohort of patients who do not fit into the other delineated categories.3-5 These patients often have multisystem involvement, making classification and treatment a challenge.5
The patient described in this case met the diagnostic criteria for HES, but her complicated clinical and laboratory features were challenging to characterize into a specific variant of HES. Organ-restricted HES was ruled out due to skin, marrow, and duodenal infiltration. She also had the potential for lung involvement based on her clinical symptoms, however no biopsy was obtained. Laboratory testing revealed no deletions or mutations indicative of familial, myeloproliferative, or lymphocytic variants. Her multisystem involvement without an underlying associated syndrome suggests idiopathic HES or HES of undetermined significance.1-5
Most patients with HES are diagnosed between the ages of 20 and 50 years.10 While HES has its peak incidence in the fourth decade of life, acute onset of new symptoms 3 months postpartum makes this an unusual presentation. In this unique case, it is important to highlight the role of the physiologic changes of pregnancy in inflammatory mediation. The physiologic changes that occur in pregnancy to ensure fetal tolerance can have profound implications for leukocyte count, AEC, and subsequent inflammatory responses. The phenomenon of inflammatory amelioration during pregnancy is well-documented, but there has only been 1 known published case report discussing decreasing HES symptoms during pregnancy with prepregnancy and postpartum hypereosinophilia.8 It is suggested that this amelioration is secondary to cortisol and progesterone shifts that occur in pregnancy. Physiologic increases in adrenocorticotropic hormone in pregnancy leads to subsequent secretion of endogenous steroids by the adrenal cortex. In turn, pregnancy can lead to leukocytosis and eosinopenia.8 Overall, pregnancy can have beneficial immunomodulating properties in the spectrum of hypereosinophilic syndromes. Even so, this patient with HES diagnosed postpartum remains at risk for the sequelae of hypereosinophilia, regardless of potential for AEC reduction during pregnancy. Therefore, treatment considerations need to be made with the safety of the maternal-fetal dyad as a priority.
Treatment
The treatment of symptomatic HES without acute life-threatening features or associated malignancy is generally determined by clinical variant.2-4 There is insufficient data to support initiation of treatment solely based on persistently elevated AEC. Patients with peripheral eosinophilia and hypereosinophilia should be monitored periodically with appropriate subspecialist evaluation for occult end-organ involvement, and targeted therapies should be deferred until an HES diagnosis.1-4 First-line therapy in most HES variants is systemic glucocorticoids.2,3,7 Since the disease course for this patient did not precisely match an HES variant, it was challenging to ascertain the optimal personalized treatment regimen. The approach to therapy was further complicated by newly identified pregnancy necessitating cessation of systemic glucocorticoids. In addition to glucocorticoids, hydroxyurea and interferon-α are among treatments historically used for HES, with tyrosine kinase inhibitors and monoclonal antibodies targeting IL-5 becoming more common.1-4 Although this patient may ultimately benefit from an IL-5 targeting biologic medication such as mepolizumab, safety in pregnancy is not well-studied and may require close clinical monitoring with treatment deferred until after delivery if possible.3,7,8,11
Military service members with frequent geographic relocation have additional barriers to timely diagnosis with often-limited access to subspecialty care depending on the duty station. While the patient was able to receive care at a large military medical center with many subspecialists, prompt recognition and timely referral to specialists would be even more critical at a smaller treatment facility. Depending on the severity and variant of HES, patients may warrant evaluation and treatment by hematology/oncology, cardiology, pulmonology, and immunology. Although HES can present in young children and older adults, this condition is most often diagnosed during the third and fourth decades of life, putting clinicians on the front line of hypereosinophilia identification and evaluation.10 Military physicians have the additional duty to not only think ahead in their diverse clinical settings to ensure proper care for patients, but also maintain a broad differential inclusive of more rare disease processes such as HES.
CONCLUSIONS
This case emphasizes how uncontrolled or untreated HES can lead to significant end-organ damage involving multiple systems and high morbidity. Prompt recognition of hypereosinophilia with potential HES can help expedite coordination of multidisciplinary care across multiple specialties to minimize delays in diagnosis and treatment. Doing so may minimize associated morbidity and mortality, especially in individuals located at more remote duty stations or deployed to austere environments.
Hypereosinophilic syndrome (HES) is defined by marked, persistent absolute eosinophil count (AEC) > 1500 cells/μL on ≥ 2 peripheral smears separated by ≥ 1 month with evidence of accompanied end-organ damage, in the absence of other causes of eosinophilia such as malignancy, atopy, or parasitic infections.1-5 Hypereosinophilic infiltration can impact almost every organ system; however, the most profound complications in patients with HES are related to leukemias and cardiac manifestations of the disease.3,4 Although rare, the associated morbidity and mortality of HES are considerable, making prompt recognition and treatment essential. Management involves targeted therapy based on pathologic classification of HES and on decreasing associated inflammation, fibrosis, and end-organ damage.3,5-7
The patient in this case report met the diagnostic criteria for HES. However, this patient had several clinical and laboratory features that made it difficult to characterize a specific HES variant. Moreover, she had additional immunomodulating factors in the setting of pregnancy. This is the first documented case of HES of undetermined etiology diagnosed postpartum and managed in the setting of a new pregnancy.2,8
CASE PRESENTATION
A 32-year-old female active-duty military service member with allergic rhinitis and a history of childhood eczema was referred to allergy/immunology for evaluation of a new, progressive pruritic rash. Symptoms started 3 months after the birth of her first child, with a new diffuse erythematous skin rash sparing her palms, soles, and mucosal surfaces. Given her history of atopy, the rash was initially treated as severe atopic dermatitis with appropriate topical medications. The rash gradually worsened, with the development of intermittent facial swelling, night sweats, dyspnea, recurrent epigastric abdominal pain, and nausea with vomiting, resulting in decreased oral intake and weight loss.
The patient was hospitalized and received an expedited multidisciplinary evaluation by dermatology, hematology/oncology, and gastroenterology. Her AEC of 4787 cells/μL peaked on admission and was markedly elevated from the 1070 cells/μL reported in the third trimester of her pregnancy. She was found to have mature eosinophilia on skin biopsy (Figure 1), endoscopic duodenal biopsy (Figure 2), peripheral blood smear (Figure 3), and bone marrow biopsy (Figure 4).




Radiographic imaging of the chest, abdomen, and pelvis revealed hepatomegaly without detectable neoplasm. There was no clinical evidence of cardiac involvement, and evaluation with electrocardiography and echocardiography did not indicate myocarditis. Extensive laboratory testing revealed no genetic mutations indicative of familial, myeloproliferative, or lymphocytic variants of HES.
The patient received topical emollients, omeprazole 40 mg daily, and ondansetron 8 mg 3 times daily as needed for symptom management, and was started on oral prednisone 40 mg daily with improvement in dyspnea, night sweats, and gastrointestinal complaints. During the patient's 6-day hospitalization and treatment, her AECs gradually decreased to 2110 cells/μL, and decreased to 1600 cells/μL over the course of a month, remaining in the hypereosinophilic range. The patient was discovered to be pregnant while symptoms were improving, resulting in stepwise discontinuation of oral steroids, but she reported continued improvement in symptoms.
DISCUSSION
Peripheral eosinophilia has a broad differential diagnoses, including HES, parasitic infections, atopic hypersensitivity diseases, eosinophilic lung diseases, eosinophilic gastrointestinal diseases, vasculitides such as eosinophilic granulomatosis with polyangiitis, genetic syndromes predisposing to eosinophilia, episodic angioedema with eosinophilia, and chronic metabolic disease with adrenal insufficiency.1-5 HES, although rare, is a disease process with potentially devastating associated morbidity and mortality if not promptly recognized and treated. HES is further delineated by hypereosinophilia with associated eosinophil-mediated organ damage or dysfunction.3-5
Clinical manifestations of HES can differ greatly depending on the HES variant and degree of organ involvement at the time of diagnosis and throughout the disease course. Patients with HES, as well as those with asymptomatic eosinophilia or hypereosinophilia, should be closely monitored for disease progression. In addition to trending peripheral AECs, clinicians should screen for symptoms of organ involvement and perform targeted evaluation of the suspected organs to promptly identify early signs of organ involvement and initiate treatment.1-4 Recommendations regarding screening intervals vary widely from monthly to annually, depending on a patient’s specific clinical picture.
HES has been subdivided into clinically relevant variants, including myeloproliferative (M-HES), T lymphocytic (L-HES), organ-restricted (or overlap) HES, familial HES, idiopathic HES, and specific syndromes with associated hypereosinophilia.3-5,9 Patients with M-HES have elevated circulating leukocyte precursors and clinical manifestations, including but not limited to hepatosplenomegaly, anemia, and thrombocytopenia. The most commonly associated genetic mutations include the FIP1L1-PDGFR-α fusion, BCR-ABL1, PDGFRA/B, JAK2, KIT, and FGFR1.3-6 L-HES usually has predominant skin and soft tissue involvement secondary to immunoglobulin E-mediated actions with clonal expansion of T cells (most commonly CD3-4+ or CD3+CD4-CD8-).3,5,6 Familial HES, a rare variant, follows an autosomal dominant inheritance pattern and is usually present at birth. It involves chromosome 5, which contains genes coding for cytokines that drive eosinophilic proliferation, including interleukin (IL)-3, IL-5, and granulocyte-macrophage colony-stimulating factor.5,9 Hypereosinophilia in the setting of end-organ damage restricted to a single organ is considered organ-restricted HES. There can be significant hepatic and gastrointestinal dysfunction, with or without malabsorption.
HES can also manifest with hematologic malignancy, restrictive obliterative cardiomyopathies, renal injury manifested by hematuria and electrolyte derangements, and neurologic complications including hemiparesis, dysarthria, and even coma.6 Endothelial damage due to eosinophil-driven inflammation can result in thrombus formation and increased risk of thromboembolic events in various organs.3 Idiopathic HES, otherwise known as HES of unknown etiology or significance, is a diagnosis of exclusion and constitutes a cohort of patients who do not fit into the other delineated categories.3-5 These patients often have multisystem involvement, making classification and treatment a challenge.5
The patient described in this case met the diagnostic criteria for HES, but her complicated clinical and laboratory features were challenging to characterize into a specific variant of HES. Organ-restricted HES was ruled out due to skin, marrow, and duodenal infiltration. She also had the potential for lung involvement based on her clinical symptoms, however no biopsy was obtained. Laboratory testing revealed no deletions or mutations indicative of familial, myeloproliferative, or lymphocytic variants. Her multisystem involvement without an underlying associated syndrome suggests idiopathic HES or HES of undetermined significance.1-5
Most patients with HES are diagnosed between the ages of 20 and 50 years.10 While HES has its peak incidence in the fourth decade of life, acute onset of new symptoms 3 months postpartum makes this an unusual presentation. In this unique case, it is important to highlight the role of the physiologic changes of pregnancy in inflammatory mediation. The physiologic changes that occur in pregnancy to ensure fetal tolerance can have profound implications for leukocyte count, AEC, and subsequent inflammatory responses. The phenomenon of inflammatory amelioration during pregnancy is well-documented, but there has only been 1 known published case report discussing decreasing HES symptoms during pregnancy with prepregnancy and postpartum hypereosinophilia.8 It is suggested that this amelioration is secondary to cortisol and progesterone shifts that occur in pregnancy. Physiologic increases in adrenocorticotropic hormone in pregnancy leads to subsequent secretion of endogenous steroids by the adrenal cortex. In turn, pregnancy can lead to leukocytosis and eosinopenia.8 Overall, pregnancy can have beneficial immunomodulating properties in the spectrum of hypereosinophilic syndromes. Even so, this patient with HES diagnosed postpartum remains at risk for the sequelae of hypereosinophilia, regardless of potential for AEC reduction during pregnancy. Therefore, treatment considerations need to be made with the safety of the maternal-fetal dyad as a priority.
Treatment
The treatment of symptomatic HES without acute life-threatening features or associated malignancy is generally determined by clinical variant.2-4 There is insufficient data to support initiation of treatment solely based on persistently elevated AEC. Patients with peripheral eosinophilia and hypereosinophilia should be monitored periodically with appropriate subspecialist evaluation for occult end-organ involvement, and targeted therapies should be deferred until an HES diagnosis.1-4 First-line therapy in most HES variants is systemic glucocorticoids.2,3,7 Since the disease course for this patient did not precisely match an HES variant, it was challenging to ascertain the optimal personalized treatment regimen. The approach to therapy was further complicated by newly identified pregnancy necessitating cessation of systemic glucocorticoids. In addition to glucocorticoids, hydroxyurea and interferon-α are among treatments historically used for HES, with tyrosine kinase inhibitors and monoclonal antibodies targeting IL-5 becoming more common.1-4 Although this patient may ultimately benefit from an IL-5 targeting biologic medication such as mepolizumab, safety in pregnancy is not well-studied and may require close clinical monitoring with treatment deferred until after delivery if possible.3,7,8,11
Military service members with frequent geographic relocation have additional barriers to timely diagnosis with often-limited access to subspecialty care depending on the duty station. While the patient was able to receive care at a large military medical center with many subspecialists, prompt recognition and timely referral to specialists would be even more critical at a smaller treatment facility. Depending on the severity and variant of HES, patients may warrant evaluation and treatment by hematology/oncology, cardiology, pulmonology, and immunology. Although HES can present in young children and older adults, this condition is most often diagnosed during the third and fourth decades of life, putting clinicians on the front line of hypereosinophilia identification and evaluation.10 Military physicians have the additional duty to not only think ahead in their diverse clinical settings to ensure proper care for patients, but also maintain a broad differential inclusive of more rare disease processes such as HES.
CONCLUSIONS
This case emphasizes how uncontrolled or untreated HES can lead to significant end-organ damage involving multiple systems and high morbidity. Prompt recognition of hypereosinophilia with potential HES can help expedite coordination of multidisciplinary care across multiple specialties to minimize delays in diagnosis and treatment. Doing so may minimize associated morbidity and mortality, especially in individuals located at more remote duty stations or deployed to austere environments.
- Cogan E, Roufosse F. Clinical management of the hypereosinophilic syndromes. Expert Rev Hematol. 2012;5:275-290. doi: 10.1586/ehm.12.14
- Klion A. Hypereosinophilic syndrome: approach to treatment in the era of precision medicine. Hematology Am Soc Hematol Educ Program. 2018;2018:326-331. doi:10.1182/asheducation-2018.1.326
- Shomali W, Gotlib J. World health organization-defined eosinophilic disorders: 2022 update on diagnosis, risk stratification, and management. Am J Hematol. 2022;97:129-148. doi:10.1002/ajh.26352
- Helbig G, Klion AD. Hypereosinophilic syndromes - an enigmatic group of disorders with an intriguing clinical spectrum and challenging treatment. Blood Rev. 2021;49:100809. doi:10.1016/j.blre.2021.100809
- Valent P, Klion AD, Horny HP, et al. Contemporary consensus proposal on criteria and classification of eosinophilic disorders and related syndromes. J Allergy Clin Immunol. 2012;130:607-612.e9. doi:10.1016/j.jaci.2012.02.019
- Roufosse FE, Goldman M, Cogan E. Hypereosinophilic syndromes. Orphanet J Rare Dis. 2007;2:37. doi:10.1186/1750-1172-2-37
- Pitlick MM, Li JT, Pongdee T. Current and emerging biologic therapies targeting eosinophilic disorders. World Allergy Organ J. 2022;15:100676. doi:10.1016/j.waojou.2022.10067
- Ault P, Cortes J, Lynn A, Keating M, Verstovsek S. Pregnancy in a patient with hypereosinophilic syndrome. Leuk Res. 2009;33:186-187. doi:10.1016/j.leukres.2008.05.013
- Rioux JD, Stone VA, Daly MJ, et al. Familial eosinophilia maps to the cytokine gene cluster on human chromosomal region 5q31-q33. Am J Hum Genet. 1998;63:1086-1094. doi:10.1086/302053
- Williams KW, Ware J, Abiodun A, et al. Hypereosinophilia in children and adults: a retrospective comparison. J Allergy Clin Immunol Pract. 2016;4:941-947.e1. doi:10.1016/j.jaip.2016.03.020
- Pane F, Lefevre G, Kwon N, et al. Characterization of disease flares and impact of mepolizumab in patients with hypereosinophilic syndrome. Front Immunol. 2022;13:935996. doi:10.3389/fimmu.2022.935996
- Cogan E, Roufosse F. Clinical management of the hypereosinophilic syndromes. Expert Rev Hematol. 2012;5:275-290. doi: 10.1586/ehm.12.14
- Klion A. Hypereosinophilic syndrome: approach to treatment in the era of precision medicine. Hematology Am Soc Hematol Educ Program. 2018;2018:326-331. doi:10.1182/asheducation-2018.1.326
- Shomali W, Gotlib J. World health organization-defined eosinophilic disorders: 2022 update on diagnosis, risk stratification, and management. Am J Hematol. 2022;97:129-148. doi:10.1002/ajh.26352
- Helbig G, Klion AD. Hypereosinophilic syndromes - an enigmatic group of disorders with an intriguing clinical spectrum and challenging treatment. Blood Rev. 2021;49:100809. doi:10.1016/j.blre.2021.100809
- Valent P, Klion AD, Horny HP, et al. Contemporary consensus proposal on criteria and classification of eosinophilic disorders and related syndromes. J Allergy Clin Immunol. 2012;130:607-612.e9. doi:10.1016/j.jaci.2012.02.019
- Roufosse FE, Goldman M, Cogan E. Hypereosinophilic syndromes. Orphanet J Rare Dis. 2007;2:37. doi:10.1186/1750-1172-2-37
- Pitlick MM, Li JT, Pongdee T. Current and emerging biologic therapies targeting eosinophilic disorders. World Allergy Organ J. 2022;15:100676. doi:10.1016/j.waojou.2022.10067
- Ault P, Cortes J, Lynn A, Keating M, Verstovsek S. Pregnancy in a patient with hypereosinophilic syndrome. Leuk Res. 2009;33:186-187. doi:10.1016/j.leukres.2008.05.013
- Rioux JD, Stone VA, Daly MJ, et al. Familial eosinophilia maps to the cytokine gene cluster on human chromosomal region 5q31-q33. Am J Hum Genet. 1998;63:1086-1094. doi:10.1086/302053
- Williams KW, Ware J, Abiodun A, et al. Hypereosinophilia in children and adults: a retrospective comparison. J Allergy Clin Immunol Pract. 2016;4:941-947.e1. doi:10.1016/j.jaip.2016.03.020
- Pane F, Lefevre G, Kwon N, et al. Characterization of disease flares and impact of mepolizumab in patients with hypereosinophilic syndrome. Front Immunol. 2022;13:935996. doi:10.3389/fimmu.2022.935996
Unique Presentation of Postpartum Hypereosinophilic Syndrome With Atypical Features and Therapeutic Challenges
Unique Presentation of Postpartum Hypereosinophilic Syndrome With Atypical Features and Therapeutic Challenges