User login
A Systemic Lupus Erythematosus Incidence Surveillance Report Among DoD Beneficiaries During the COVID-19 Pandemic
A Systemic Lupus Erythematosus Incidence Surveillance Report Among DoD Beneficiaries During the COVID-19 Pandemic
Systemic lupus erythematosus (SLE), or lupus, is a rare autoimmune disease estimated to occur in about 5.1 cases per 100,000 person-years in the United States in 2018.1 The disease predominantly affects females, with an incidence of 8.7 cases per 100,000 person-years vs 1.2 cases per 100,000 person-years in males, and is most common in patients aged 15 to 44 years.1,2
Lupus presents with a constellation of clinical signs and symptoms that evolve, along with hallmark laboratory findings indicative of immune dysregulation and polyclonal B-cell activation. Consequently, a wide array of autoantibodies may be produced, although the combination of epitope specificity can vary from patient to patient.3 Nevertheless, > 98% of individuals diagnosed with lupus produce antinuclear antibodies (ANA), making ANA positivity a near-universal serologic feature at the time of diagnosis.
The pathogenesis of lupus is complex. Research from the past 5 decades supports the role of certain viral infections—such as Epstein-Barr virus (EBV) and cytomegalovirus—as potential triggers.4 These viruses are thought to initiate disease through mechanisms including activation of interferon pathways, exposure of cryptic intracellular antigens, molecular mimicry, and epitope spreading. Subsequent clonal expansion and autoantibody production occur to varying degrees, influenced by viral load and host susceptibility factors.
During the COVID-19 pandemic, it became evident that SARS-CoV-2 exerts profound effects on immune regulation, influencing infection outcomes through mechanisms such as hyperactivation of innate immunity, especially in the lungs, leading to acute respiratory distress syndrome. Additionally, SARS-CoV-2 has been associated with polyclonal B-cell activation and the generation of autoantibodies. This association gained attention after Bastard et al identified anti–type I interferon antibodies in patients with severe COVID-19, predominantly among males with a genetic predisposition. These autoantibodies were shown to impair antiviral defenses and contribute to life-threatening pneumonia.5
Subsequent studies demonstrated the production of a wide spectrum of functional autoantibodies, including ANA, in patients with COVID-19.6,7 These findings were attributed to the acute expansion of autoreactive clones among naïve-derived immunoglobulin G1 antibody-secreting cells during the early stages of infection, with the degree of expansion correlating with disease severity.8,9 Although longitudinal data up to 15 months postinfection suggest this serologic abnormality resolves in more than two-thirds of patients, the number of individuals infected globally has raised serious public health concerns regarding the potential long-term sequelae, including the onset of lupus or other autoimmune diseases in COVID-19 survivors.6-9 A limited number of case reports describing the onset of lupus following SARS-CoV-2 infection support this hypothesis.10
This surveillance analysis investigates lupus incidence among patients within the Military Health System (MHS), encompassing all TRICARE beneficiaries, from January 2018 to December 2022. The objective of this analysis was to examine lupus incidence trends throughout the COVID-19 pandemic, stratified by sex, age, and active-duty status.
Methods
The MHS provides health care services to about 9.5 million US Department of Defense (DoD) beneficiaries. Outpatient health records and laboratory results for individuals receiving care at military treatment facilities (MTFs) between January 1, 2018, and December 31, 2022, were obtained from the Comprehensive Ambulatory/ Professional Encounter Record and MHS GENESIS. For beneficiaries receiving care in the private sector, data were sourced from the TRICARE Encounter Data—Non-Institutional database.
Laboratory test results, including ANA testing, were available only for individuals receiving care at MTFs. These laboratory data were extracted from the Composite Health Care System Chemistry database and MHS GENESIS laboratory systems for the same time frame. Inpatient data were not included in this analysis. Data from 2017 were used solely as a look-back (or washout) period to identify and exclude prevalent lupus cases diagnosed before 2018 and were not included in the final results.
Lupus cases were identified by the presence of a positive ANA test and appropriate International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) codes. A positive ANA result was defined as either a qualitative result marked positive or a titer ≥ 1:80. The ICD-10-CM codes considered indicative of lupus included variations of M32, L93, or H01.12.
M32, L93, or H01.12. For cases with a positive ANA test, a lupus diagnosis required the presence of ≥ 2 lupus related ICD-10-CM codes. In the absence of ANA test results, a stricter criterion was applied: ≥ 4 lupus ICD-10-CM diagnosis codes recorded on separate days were required for inclusion.
Beneficiaries were excluded if they had a negative ANA result, only 1 lupus ICD- 10-CM diagnosis code, 1 positive ANA with only 1 corresponding ICD-10-CM code, or if their diagnosis occurred outside the defined study period. Patients and members of the public were not involved in the design, conduct, reporting, or dissemination of this study.
Results
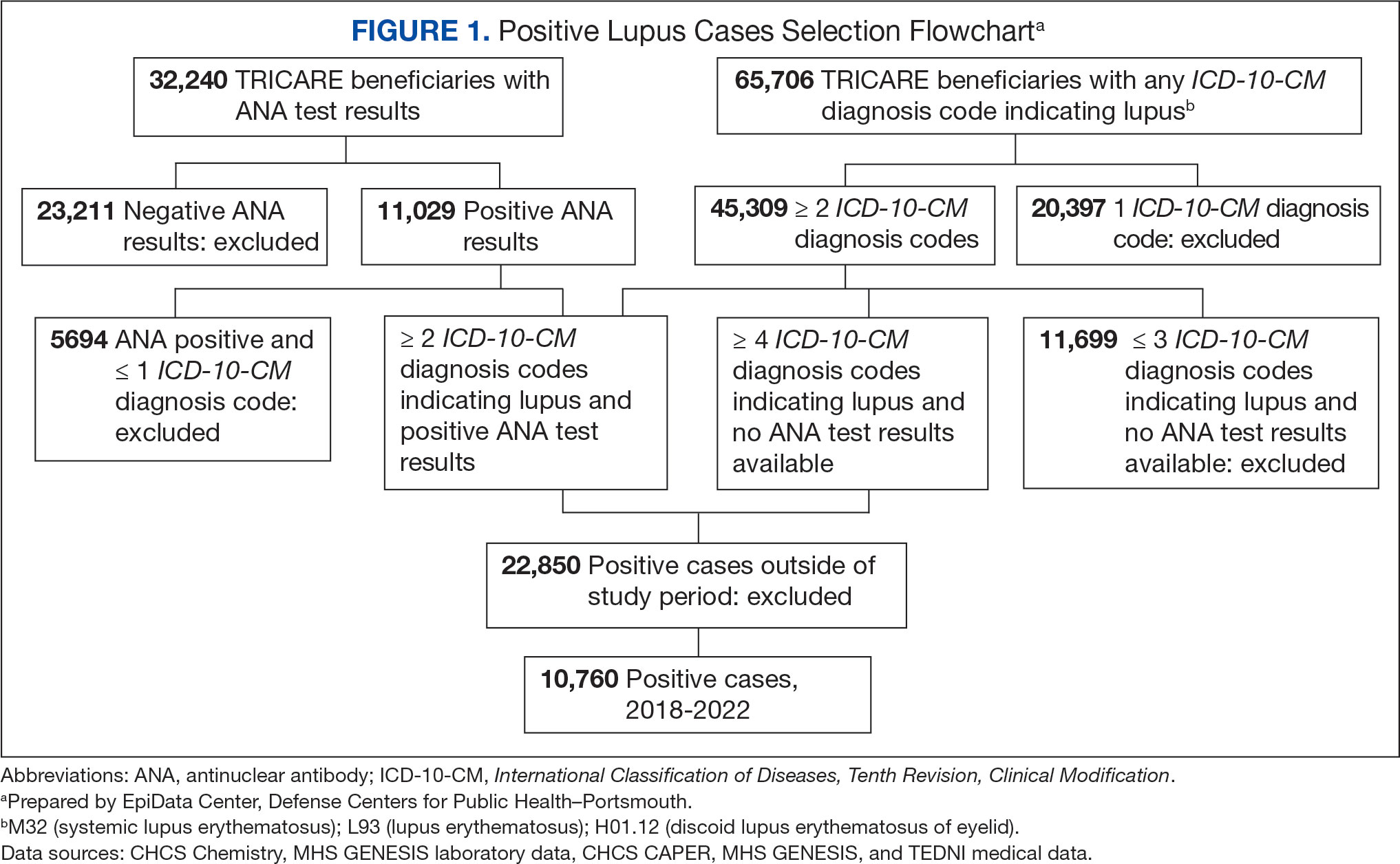
Between January 1, 2017, and December 31, 2022, 99,946 TRICARE beneficiaries had some indication of lupus testing or diagnosis in their health records (Figure 1). Of these beneficiaries, 5335 had a positive ANA result and ≥ 2 ICD-10-CM lupus diagnosis codes. An additional 28,275 beneficiaries had ≥ 4 ICD-10-CM lupus diagnosis codes but no ANA test results. From these groups, the final sample included 10,760 beneficiaries who met the incident case definitions for SLE during the study period (2018 through 2022).
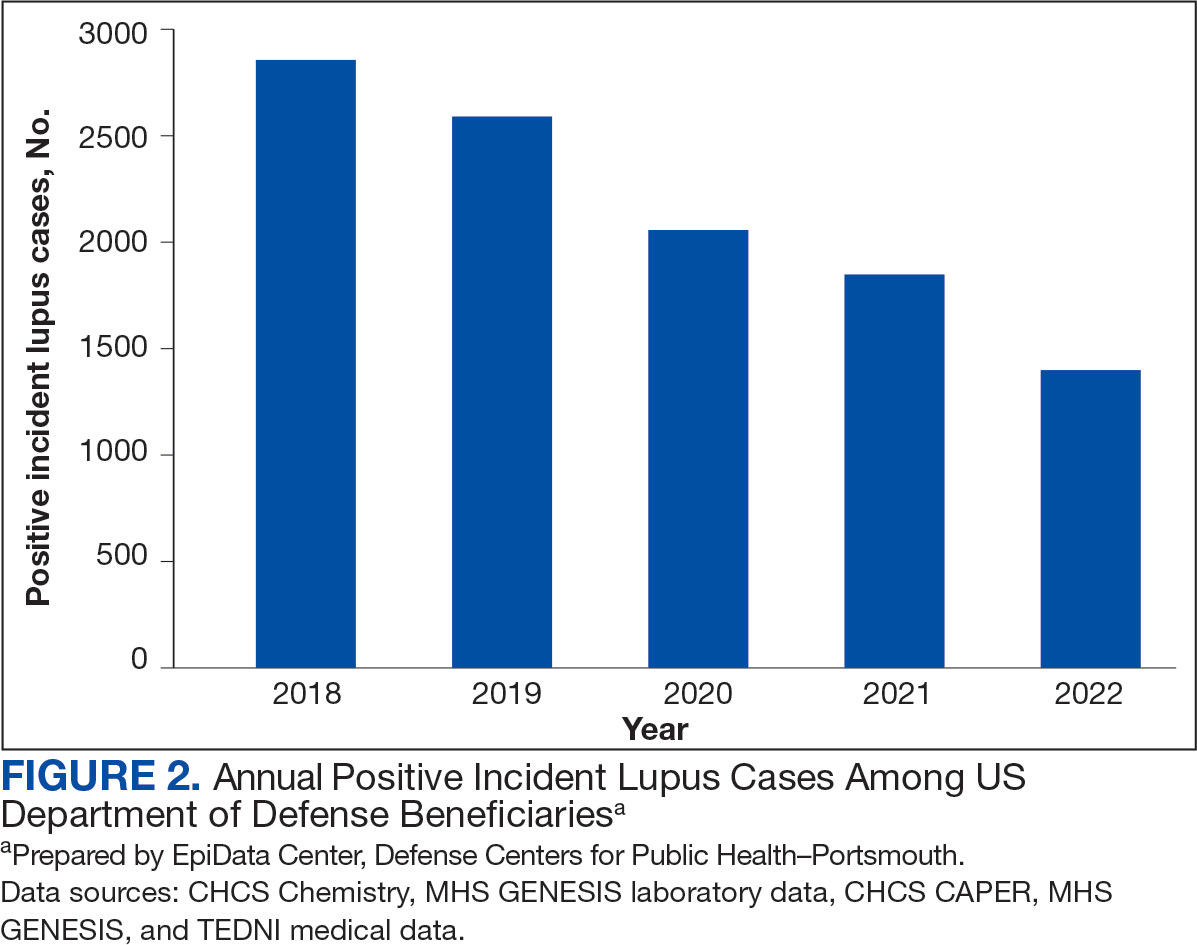
Most cases (85.1%, n = 9157) were diagnosed through TRICARE claims, while 1205 (11.2%) were diagnosed within the MHS. Another 398 (3.7%) had documentation of care both within and outside the MHS. Incident SLE cases declined by an average of 16% annually during the study period (Figure 2). This trend amounted to an overall reduction of 48.2%, from 2866 cases in 2018 to 1399 cases in 2022. This decline occurred despite total medical encounters among DoD beneficiaries remaining relatively stable during the pandemic years, with only a 3.5% change between 2018 and 2022.
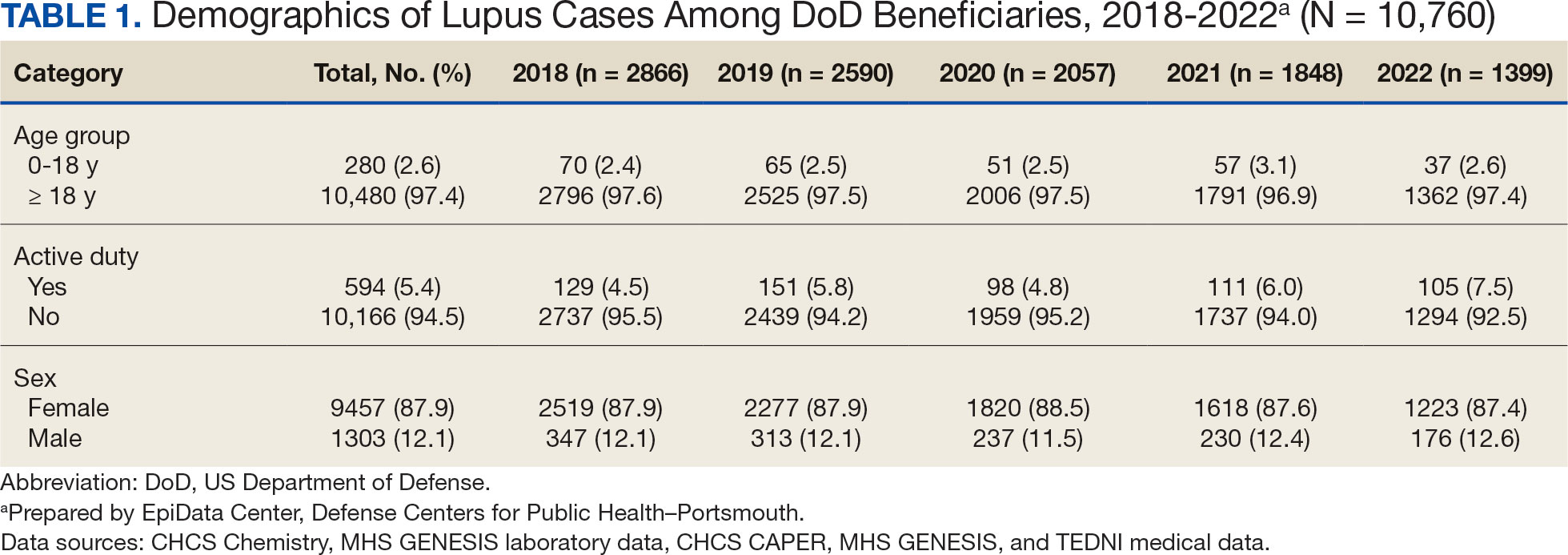
The disease was more prevalent among female beneficiaries, with a female to- male ratio of 7:1 (Table 1). Among women, the number of new cases declined from 2519 in 2018 to 1223 in 2022, while the number of cases among men remained consistently < 350 annually. Similar trends were observed across other strata. Incident SLE cases were more common among nonactive-duty beneficiaries than active-duty service members, with a ratio of 18:1. New cases among active-duty members remained < 155 per year. Age-stratified data revealed that SLE was diagnosed predominantly in individuals aged ≥ 18 years, with a ratio of 37:1 compared with individuals aged < 18 years. Among children, the number of new cases remained < 75 per year throughout the study period.
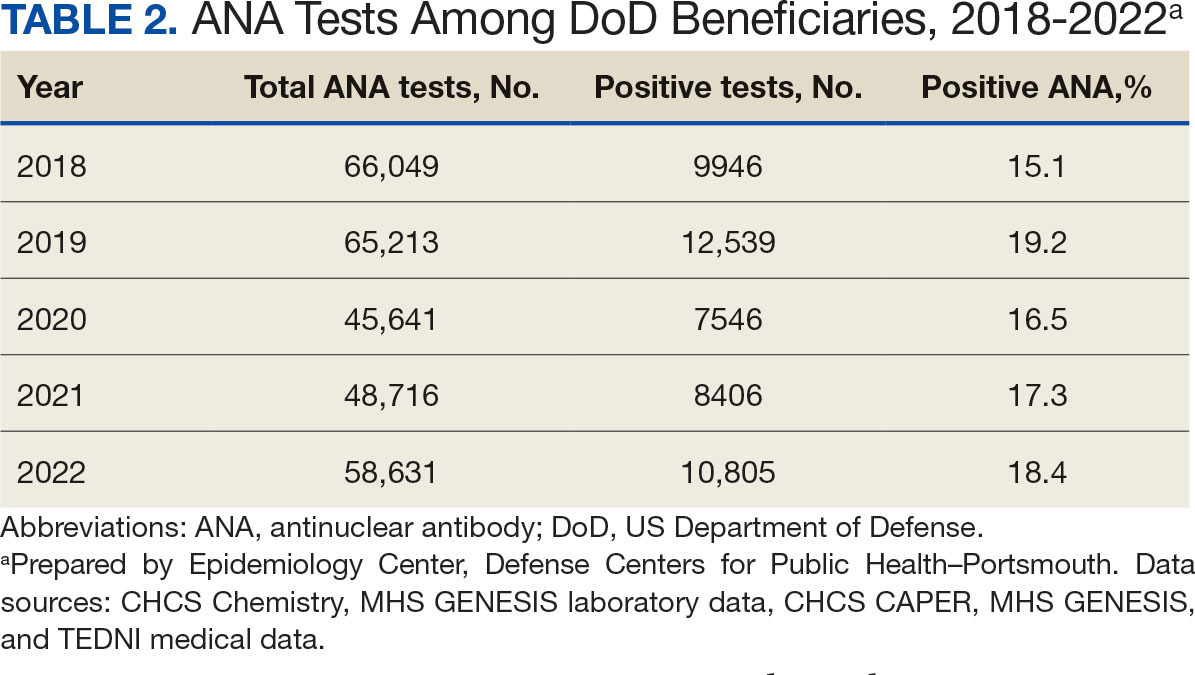
A mean 56,850 ANA tests were conducted annually in centralized laboratories using standardized protocols (Table 2). The mean ANA positivity rate was 17.3%, which remained relatively stable from 2018 through 2022.
Discussion
This study examined the annual incidence of newly diagnosed SLE cases among all TRICARE beneficiaries from January 1, 2018, through December 31, 2022, covering both before and during the peak years of the COVID-19 pandemic. This analysis revealed a steady decline in SLE cases during this period. The reliability of these findings is reinforced by the comprehensiveness of the MHS, one of the largest US health care delivery systems, which maintains near-complete medical data capture for about 9.5 million DoD TRICARE beneficiaries across domestic and international settings.
SLE is a rare autoimmune disorder that presents a diagnostic challenge due to its wide range of nonspecific symptoms, many of which resemble other conditions. To reduce the likelihood of false-positive results and ensure diagnostic accuracy, this study adopted a stringent case definition. Incident cases were identified by the presence of ANA testing in conjunction with lupus-specific ICD-10-CM codes and required ≥ 4 lupus related diagnostic entries. This criterion was necessary due to the absence of ANA test results in data from private sector care settings. Our case definition aligns with established literature. For example, a Vanderbilt University chart review study demonstrated that combining ANA positivity with ≥ 4 lupus related ICD-10-CM codes achieves a positive predictive value of 100%, albeit with a sensitivity of 45%.11 Other studies similarly affirm the diagnostic validity of using recurrent ICD-10-CM codes to improve specificity in identifying lupus cases.12,13
The primary objective of this study was to examine the temporal trend in newly diagnosed lupus cases, rather than derive precise incidence rates. Although the TRICARE system includes about 9.5 million beneficiaries, this number represents a dynamic population with continual inflow and outflow. Accurate incidence rate calculation would require access to detailed denominator data, which were not readily available. In comparison with our findings, a study limited to active-duty service members reported fewer lupus cases. This discrepancy likely reflects differences in case definitions—specifically, the absence of laboratory data, the restricted range of diagnostic codes, and the requirement that diagnoses be rendered by specialists.14 Despite these differences, demographic patterns were consistent, with higher incidence observed in females and individuals aged ≥ 20 years.
A Centers for Disease Control and Prevention (CDC) study of lupus incidence in the general population also reported lower case counts.1 However, the CDC estimates were based on 5 state-level registries, which rely on clinician-reported cases and therefore may underestimate true disease burden. Moreover, the DoD beneficiary population differs markedly from the general population: it includes a large cohort of retirees, ensuring an older demographic; all members have comprehensive health care access; and active-duty personnel are subject to pre-enlistment medical screening. Taken together, these factors suggest this study may offer a more complete and systematically captured profile of lupus incidence.
We observed a marked decline of newly diagnosed SLE cases during the study period, which coincided with the widespread circulation of COVID-19. This decrease is unlikely to be attributable to reduced access to care during the pandemic. The MHS operates under a single-payer model, and the total number of patient encounters remained relatively stable throughout the pandemic.
To our knowledge, this is the only study to monitor lupus incidence in a large US population over the 5-year period encompassing before and during the COVID-19 pandemic. To date, only 4 large-scale surveillance studies have addressed similar questions. 14-17 Our findings are consistent with the most recent of these reports: an analysis limited to active-duty members of the US Armed Forces identified 1127 patients with newly diagnosed lupus between 2000 and 2022 and reported stable incidence trends throughout the pandemic.14 The other 3 studies adopted a different approach, comparing the emergence of autoimmune diseases, including lupus, between individuals with confirmed SARS-CoV-2 infection and those without. Each of these trials concluded that COVID-19 increases the risk of various autoimmune conditions, although the findings specific to lupus were inconsistent.15-17
Chang et al reported a significant increase in new lupus diagnoses (n = 2,926,016), with an adjusted hazard ratio (aHR) of 2.99 (95% CI, 2.68-3.34), spanning all ages and both sexes. The highest incidence was observed in individuals of Asian descent.15 Using German routine health care data from 2020, Tesch et al identified a heightened risk of autoimmune diseases, including lupus, among patients with a history of SARS-CoV-2 infection (n = 641,407; 9.4% children, 57.3% female, 6.4% hospitalized), compared with matched infection-naïve controls (n = 1,560,357).16 Both studies excluded vaccinated individuals.
These 2 studies diverged in their assessment of the relationship between COVID-19 severity and subsequent autoimmune risk. Chang et al found a higher incidence among nonhospitalized ambulatory patients, while Tesch et al reported that increased risk was associated with patients requiring intensive care unit admission.15,16
In contrast, based on a cohort of 4,197,188 individuals, Peng et al found no significant difference in lupus incidence among patients with SARS-CoV-2 infection (aHR, 1.05; 95% CI, 0.79-1.39).17 Notably, within the infected group, the incidence of SLE was significantly lower among vaccinated individuals compared with the unvaccinated group (aHR, 0.29; 95% CI, 0.18-0.47). Similar protective associations were observed for other antibody-mediated autoimmune disorders, including pemphigoid, Graves’ disease, and antiphospholipid antibody syndrome.
Limitations
Due to fundamental differences in study design, we were unable to directly reconcile our findings with those reported in the literature. This study lacked access to reliable documentation of COVID-19 infection status, primarily due to the widespread use of home testing among TRICARE beneficiaries. Additionally, the dataset did not include inpatient records and therefore did not permit evaluation of disease severity. Despite these constraints, it is plausible that the overall burden of COVID-19 infection within the study population was lower than that observed in the general US population.
As of December 2022, the DoD had reported about 750,000 confirmed COVID-19 cases among military personnel, civilian employees, dependents, and DoD contractors.18 Given that TRICARE beneficiaries represent about 2.8% of the total US population—and that > 90 million US individuals were infected between 2020 and 2022—the implied infection rate in our cohort appears to be about one-third of what might be expected.19 This discrepancy may be due to higher adherence to mitigation measures, such as social distancing and mask usage, among DoD-affiliated populations. COVID-19 vaccination was mandated for all active-duty service members, who constitute 5.4% of the study population. The remaining TRICARE beneficiaries also had access to guaranteed health care and vaccination coverage, likely contributing to high overall vaccination rates.
Because > 80% of the study population was composed of individuals from diverse civilian backgrounds, we expect the distribution of infection severity within the DoD beneficiary population to approximate that of the general US population.
Future Directions
The findings of this study offer circumstantial yet real-time evidence of the complexity underlying immune dysregulation at the intersection of host susceptibility and environmental exposures. The stability in ANA positivity rates during the study period mitigates concerns regarding undiagnosed subclinical lupus and may suggest that, overall, immune homeostasis was preserved among DoD beneficiaries.
It is noteworthy that during the COVID-19 pandemic, the incidence of several common infections—such as influenza and EBV—declined markedly, likely as a result of widespread social distancing and other public health interventions.20 Mitigation strategies implemented within the military may have conferred protection not only against COVID-19 but also against other community-acquired pathogens.
In light of these observations, we hypothesize that for COVID-19 to act as a trigger for SLE, a prolonged or repeated disruption of immune equilibrium may be required—potentially mediated by recurrent infections or sustained inflammatory states. The association between viral infections and autoimmunity is well established. Immune dysregulation leading to autoantibody production has been observed not only in the context of SARS-CoV-2 but also following infections with EBV, cytomegalovirus, enteroviruses, hepatitis B and C viruses, HIV, and parvovirus B19.21
This dysregulation is often transient, accompanied by compensatory immune regulatory responses. However, in individuals subjected to successive or overlapping infections, these regulatory mechanisms may become compromised or overwhelmed, due to emergent patterns of immune interference of varying severity. In such cases, a transient immune perturbation may progress into a bona fide autoimmune disease, contingent upon individual risk factors such as genetic predisposition, preexisting immune memory, and regenerative capacity.21
Therefore, we believe the significance of this study is 2-fold. First, lupus is known to develop gradually and may require 3 to 5 years to clinically manifest after the initial break in immunological tolerance.3 Continued public health surveillance represents a more pragmatic strategy than retrospective cohort construction, especially as histories of COVID-19 infection become increasingly complete and definitive. Our findings provide a valuable baseline reference point for future longitudinal studies.
The interpretation of surveillance outcomes—whether indicating an upward trend, a stable baseline, or a downward trend—offers distinct analytical value. Within this study population, we observed neither an upward trajectory that might suggest a direct causal link, nor a flat trend that would imply absence of association between COVID-19 and lupus pathogenesis. Instead, the observation of a downward trend invites consideration of nonlinear or protective influences. From this perspective, we recommend that future investigations adopt a holistic framework when assessing environmental contributions to immune dysregulation—particularly when evaluating the long-term immunopathological consequences of the COVID-19 pandemic on lupus and related autoimmune conditions.
Conclusions
This study identified a declining trend in incident lupus cases during the COVID-19 pandemic among the DoD beneficiary population. Further investigation is warranted to elucidate the underlying factors contributing to this decline. Conducting longitudinal epidemiologic studies and applying multivariable regression analyses will be essential to determine whether incidence rates revert to prepandemic baselines and how these trends may be influenced by evolving environmental factors within the general population.
Systemic lupus erythematosus (SLE), or lupus, is a rare autoimmune disease estimated to occur in about 5.1 cases per 100,000 person-years in the United States in 2018.1 The disease predominantly affects females, with an incidence of 8.7 cases per 100,000 person-years vs 1.2 cases per 100,000 person-years in males, and is most common in patients aged 15 to 44 years.1,2
Lupus presents with a constellation of clinical signs and symptoms that evolve, along with hallmark laboratory findings indicative of immune dysregulation and polyclonal B-cell activation. Consequently, a wide array of autoantibodies may be produced, although the combination of epitope specificity can vary from patient to patient.3 Nevertheless, > 98% of individuals diagnosed with lupus produce antinuclear antibodies (ANA), making ANA positivity a near-universal serologic feature at the time of diagnosis.
The pathogenesis of lupus is complex. Research from the past 5 decades supports the role of certain viral infections—such as Epstein-Barr virus (EBV) and cytomegalovirus—as potential triggers.4 These viruses are thought to initiate disease through mechanisms including activation of interferon pathways, exposure of cryptic intracellular antigens, molecular mimicry, and epitope spreading. Subsequent clonal expansion and autoantibody production occur to varying degrees, influenced by viral load and host susceptibility factors.
During the COVID-19 pandemic, it became evident that SARS-CoV-2 exerts profound effects on immune regulation, influencing infection outcomes through mechanisms such as hyperactivation of innate immunity, especially in the lungs, leading to acute respiratory distress syndrome. Additionally, SARS-CoV-2 has been associated with polyclonal B-cell activation and the generation of autoantibodies. This association gained attention after Bastard et al identified anti–type I interferon antibodies in patients with severe COVID-19, predominantly among males with a genetic predisposition. These autoantibodies were shown to impair antiviral defenses and contribute to life-threatening pneumonia.5
Subsequent studies demonstrated the production of a wide spectrum of functional autoantibodies, including ANA, in patients with COVID-19.6,7 These findings were attributed to the acute expansion of autoreactive clones among naïve-derived immunoglobulin G1 antibody-secreting cells during the early stages of infection, with the degree of expansion correlating with disease severity.8,9 Although longitudinal data up to 15 months postinfection suggest this serologic abnormality resolves in more than two-thirds of patients, the number of individuals infected globally has raised serious public health concerns regarding the potential long-term sequelae, including the onset of lupus or other autoimmune diseases in COVID-19 survivors.6-9 A limited number of case reports describing the onset of lupus following SARS-CoV-2 infection support this hypothesis.10
This surveillance analysis investigates lupus incidence among patients within the Military Health System (MHS), encompassing all TRICARE beneficiaries, from January 2018 to December 2022. The objective of this analysis was to examine lupus incidence trends throughout the COVID-19 pandemic, stratified by sex, age, and active-duty status.
Methods
The MHS provides health care services to about 9.5 million US Department of Defense (DoD) beneficiaries. Outpatient health records and laboratory results for individuals receiving care at military treatment facilities (MTFs) between January 1, 2018, and December 31, 2022, were obtained from the Comprehensive Ambulatory/ Professional Encounter Record and MHS GENESIS. For beneficiaries receiving care in the private sector, data were sourced from the TRICARE Encounter Data—Non-Institutional database.
Laboratory test results, including ANA testing, were available only for individuals receiving care at MTFs. These laboratory data were extracted from the Composite Health Care System Chemistry database and MHS GENESIS laboratory systems for the same time frame. Inpatient data were not included in this analysis. Data from 2017 were used solely as a look-back (or washout) period to identify and exclude prevalent lupus cases diagnosed before 2018 and were not included in the final results.
Lupus cases were identified by the presence of a positive ANA test and appropriate International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) codes. A positive ANA result was defined as either a qualitative result marked positive or a titer ≥ 1:80. The ICD-10-CM codes considered indicative of lupus included variations of M32, L93, or H01.12.
M32, L93, or H01.12. For cases with a positive ANA test, a lupus diagnosis required the presence of ≥ 2 lupus related ICD-10-CM codes. In the absence of ANA test results, a stricter criterion was applied: ≥ 4 lupus ICD-10-CM diagnosis codes recorded on separate days were required for inclusion.
Beneficiaries were excluded if they had a negative ANA result, only 1 lupus ICD- 10-CM diagnosis code, 1 positive ANA with only 1 corresponding ICD-10-CM code, or if their diagnosis occurred outside the defined study period. Patients and members of the public were not involved in the design, conduct, reporting, or dissemination of this study.
Results
Between January 1, 2017, and December 31, 2022, 99,946 TRICARE beneficiaries had some indication of lupus testing or diagnosis in their health records (Figure 1). Of these beneficiaries, 5335 had a positive ANA result and ≥ 2 ICD-10-CM lupus diagnosis codes. An additional 28,275 beneficiaries had ≥ 4 ICD-10-CM lupus diagnosis codes but no ANA test results. From these groups, the final sample included 10,760 beneficiaries who met the incident case definitions for SLE during the study period (2018 through 2022).

Most cases (85.1%, n = 9157) were diagnosed through TRICARE claims, while 1205 (11.2%) were diagnosed within the MHS. Another 398 (3.7%) had documentation of care both within and outside the MHS. Incident SLE cases declined by an average of 16% annually during the study period (Figure 2). This trend amounted to an overall reduction of 48.2%, from 2866 cases in 2018 to 1399 cases in 2022. This decline occurred despite total medical encounters among DoD beneficiaries remaining relatively stable during the pandemic years, with only a 3.5% change between 2018 and 2022.

The disease was more prevalent among female beneficiaries, with a female to- male ratio of 7:1 (Table 1). Among women, the number of new cases declined from 2519 in 2018 to 1223 in 2022, while the number of cases among men remained consistently < 350 annually. Similar trends were observed across other strata. Incident SLE cases were more common among nonactive-duty beneficiaries than active-duty service members, with a ratio of 18:1. New cases among active-duty members remained < 155 per year. Age-stratified data revealed that SLE was diagnosed predominantly in individuals aged ≥ 18 years, with a ratio of 37:1 compared with individuals aged < 18 years. Among children, the number of new cases remained < 75 per year throughout the study period.

A mean 56,850 ANA tests were conducted annually in centralized laboratories using standardized protocols (Table 2). The mean ANA positivity rate was 17.3%, which remained relatively stable from 2018 through 2022.

Discussion
This study examined the annual incidence of newly diagnosed SLE cases among all TRICARE beneficiaries from January 1, 2018, through December 31, 2022, covering both before and during the peak years of the COVID-19 pandemic. This analysis revealed a steady decline in SLE cases during this period. The reliability of these findings is reinforced by the comprehensiveness of the MHS, one of the largest US health care delivery systems, which maintains near-complete medical data capture for about 9.5 million DoD TRICARE beneficiaries across domestic and international settings.
SLE is a rare autoimmune disorder that presents a diagnostic challenge due to its wide range of nonspecific symptoms, many of which resemble other conditions. To reduce the likelihood of false-positive results and ensure diagnostic accuracy, this study adopted a stringent case definition. Incident cases were identified by the presence of ANA testing in conjunction with lupus-specific ICD-10-CM codes and required ≥ 4 lupus related diagnostic entries. This criterion was necessary due to the absence of ANA test results in data from private sector care settings. Our case definition aligns with established literature. For example, a Vanderbilt University chart review study demonstrated that combining ANA positivity with ≥ 4 lupus related ICD-10-CM codes achieves a positive predictive value of 100%, albeit with a sensitivity of 45%.11 Other studies similarly affirm the diagnostic validity of using recurrent ICD-10-CM codes to improve specificity in identifying lupus cases.12,13
The primary objective of this study was to examine the temporal trend in newly diagnosed lupus cases, rather than derive precise incidence rates. Although the TRICARE system includes about 9.5 million beneficiaries, this number represents a dynamic population with continual inflow and outflow. Accurate incidence rate calculation would require access to detailed denominator data, which were not readily available. In comparison with our findings, a study limited to active-duty service members reported fewer lupus cases. This discrepancy likely reflects differences in case definitions—specifically, the absence of laboratory data, the restricted range of diagnostic codes, and the requirement that diagnoses be rendered by specialists.14 Despite these differences, demographic patterns were consistent, with higher incidence observed in females and individuals aged ≥ 20 years.
A Centers for Disease Control and Prevention (CDC) study of lupus incidence in the general population also reported lower case counts.1 However, the CDC estimates were based on 5 state-level registries, which rely on clinician-reported cases and therefore may underestimate true disease burden. Moreover, the DoD beneficiary population differs markedly from the general population: it includes a large cohort of retirees, ensuring an older demographic; all members have comprehensive health care access; and active-duty personnel are subject to pre-enlistment medical screening. Taken together, these factors suggest this study may offer a more complete and systematically captured profile of lupus incidence.
We observed a marked decline of newly diagnosed SLE cases during the study period, which coincided with the widespread circulation of COVID-19. This decrease is unlikely to be attributable to reduced access to care during the pandemic. The MHS operates under a single-payer model, and the total number of patient encounters remained relatively stable throughout the pandemic.
To our knowledge, this is the only study to monitor lupus incidence in a large US population over the 5-year period encompassing before and during the COVID-19 pandemic. To date, only 4 large-scale surveillance studies have addressed similar questions. 14-17 Our findings are consistent with the most recent of these reports: an analysis limited to active-duty members of the US Armed Forces identified 1127 patients with newly diagnosed lupus between 2000 and 2022 and reported stable incidence trends throughout the pandemic.14 The other 3 studies adopted a different approach, comparing the emergence of autoimmune diseases, including lupus, between individuals with confirmed SARS-CoV-2 infection and those without. Each of these trials concluded that COVID-19 increases the risk of various autoimmune conditions, although the findings specific to lupus were inconsistent.15-17
Chang et al reported a significant increase in new lupus diagnoses (n = 2,926,016), with an adjusted hazard ratio (aHR) of 2.99 (95% CI, 2.68-3.34), spanning all ages and both sexes. The highest incidence was observed in individuals of Asian descent.15 Using German routine health care data from 2020, Tesch et al identified a heightened risk of autoimmune diseases, including lupus, among patients with a history of SARS-CoV-2 infection (n = 641,407; 9.4% children, 57.3% female, 6.4% hospitalized), compared with matched infection-naïve controls (n = 1,560,357).16 Both studies excluded vaccinated individuals.
These 2 studies diverged in their assessment of the relationship between COVID-19 severity and subsequent autoimmune risk. Chang et al found a higher incidence among nonhospitalized ambulatory patients, while Tesch et al reported that increased risk was associated with patients requiring intensive care unit admission.15,16
In contrast, based on a cohort of 4,197,188 individuals, Peng et al found no significant difference in lupus incidence among patients with SARS-CoV-2 infection (aHR, 1.05; 95% CI, 0.79-1.39).17 Notably, within the infected group, the incidence of SLE was significantly lower among vaccinated individuals compared with the unvaccinated group (aHR, 0.29; 95% CI, 0.18-0.47). Similar protective associations were observed for other antibody-mediated autoimmune disorders, including pemphigoid, Graves’ disease, and antiphospholipid antibody syndrome.
Limitations
Due to fundamental differences in study design, we were unable to directly reconcile our findings with those reported in the literature. This study lacked access to reliable documentation of COVID-19 infection status, primarily due to the widespread use of home testing among TRICARE beneficiaries. Additionally, the dataset did not include inpatient records and therefore did not permit evaluation of disease severity. Despite these constraints, it is plausible that the overall burden of COVID-19 infection within the study population was lower than that observed in the general US population.
As of December 2022, the DoD had reported about 750,000 confirmed COVID-19 cases among military personnel, civilian employees, dependents, and DoD contractors.18 Given that TRICARE beneficiaries represent about 2.8% of the total US population—and that > 90 million US individuals were infected between 2020 and 2022—the implied infection rate in our cohort appears to be about one-third of what might be expected.19 This discrepancy may be due to higher adherence to mitigation measures, such as social distancing and mask usage, among DoD-affiliated populations. COVID-19 vaccination was mandated for all active-duty service members, who constitute 5.4% of the study population. The remaining TRICARE beneficiaries also had access to guaranteed health care and vaccination coverage, likely contributing to high overall vaccination rates.
Because > 80% of the study population was composed of individuals from diverse civilian backgrounds, we expect the distribution of infection severity within the DoD beneficiary population to approximate that of the general US population.
Future Directions
The findings of this study offer circumstantial yet real-time evidence of the complexity underlying immune dysregulation at the intersection of host susceptibility and environmental exposures. The stability in ANA positivity rates during the study period mitigates concerns regarding undiagnosed subclinical lupus and may suggest that, overall, immune homeostasis was preserved among DoD beneficiaries.
It is noteworthy that during the COVID-19 pandemic, the incidence of several common infections—such as influenza and EBV—declined markedly, likely as a result of widespread social distancing and other public health interventions.20 Mitigation strategies implemented within the military may have conferred protection not only against COVID-19 but also against other community-acquired pathogens.
In light of these observations, we hypothesize that for COVID-19 to act as a trigger for SLE, a prolonged or repeated disruption of immune equilibrium may be required—potentially mediated by recurrent infections or sustained inflammatory states. The association between viral infections and autoimmunity is well established. Immune dysregulation leading to autoantibody production has been observed not only in the context of SARS-CoV-2 but also following infections with EBV, cytomegalovirus, enteroviruses, hepatitis B and C viruses, HIV, and parvovirus B19.21
This dysregulation is often transient, accompanied by compensatory immune regulatory responses. However, in individuals subjected to successive or overlapping infections, these regulatory mechanisms may become compromised or overwhelmed, due to emergent patterns of immune interference of varying severity. In such cases, a transient immune perturbation may progress into a bona fide autoimmune disease, contingent upon individual risk factors such as genetic predisposition, preexisting immune memory, and regenerative capacity.21
Therefore, we believe the significance of this study is 2-fold. First, lupus is known to develop gradually and may require 3 to 5 years to clinically manifest after the initial break in immunological tolerance.3 Continued public health surveillance represents a more pragmatic strategy than retrospective cohort construction, especially as histories of COVID-19 infection become increasingly complete and definitive. Our findings provide a valuable baseline reference point for future longitudinal studies.
The interpretation of surveillance outcomes—whether indicating an upward trend, a stable baseline, or a downward trend—offers distinct analytical value. Within this study population, we observed neither an upward trajectory that might suggest a direct causal link, nor a flat trend that would imply absence of association between COVID-19 and lupus pathogenesis. Instead, the observation of a downward trend invites consideration of nonlinear or protective influences. From this perspective, we recommend that future investigations adopt a holistic framework when assessing environmental contributions to immune dysregulation—particularly when evaluating the long-term immunopathological consequences of the COVID-19 pandemic on lupus and related autoimmune conditions.
Conclusions
This study identified a declining trend in incident lupus cases during the COVID-19 pandemic among the DoD beneficiary population. Further investigation is warranted to elucidate the underlying factors contributing to this decline. Conducting longitudinal epidemiologic studies and applying multivariable regression analyses will be essential to determine whether incidence rates revert to prepandemic baselines and how these trends may be influenced by evolving environmental factors within the general population.
Systemic lupus erythematosus (SLE), or lupus, is a rare autoimmune disease estimated to occur in about 5.1 cases per 100,000 person-years in the United States in 2018.1 The disease predominantly affects females, with an incidence of 8.7 cases per 100,000 person-years vs 1.2 cases per 100,000 person-years in males, and is most common in patients aged 15 to 44 years.1,2
Lupus presents with a constellation of clinical signs and symptoms that evolve, along with hallmark laboratory findings indicative of immune dysregulation and polyclonal B-cell activation. Consequently, a wide array of autoantibodies may be produced, although the combination of epitope specificity can vary from patient to patient.3 Nevertheless, > 98% of individuals diagnosed with lupus produce antinuclear antibodies (ANA), making ANA positivity a near-universal serologic feature at the time of diagnosis.
The pathogenesis of lupus is complex. Research from the past 5 decades supports the role of certain viral infections—such as Epstein-Barr virus (EBV) and cytomegalovirus—as potential triggers.4 These viruses are thought to initiate disease through mechanisms including activation of interferon pathways, exposure of cryptic intracellular antigens, molecular mimicry, and epitope spreading. Subsequent clonal expansion and autoantibody production occur to varying degrees, influenced by viral load and host susceptibility factors.
During the COVID-19 pandemic, it became evident that SARS-CoV-2 exerts profound effects on immune regulation, influencing infection outcomes through mechanisms such as hyperactivation of innate immunity, especially in the lungs, leading to acute respiratory distress syndrome. Additionally, SARS-CoV-2 has been associated with polyclonal B-cell activation and the generation of autoantibodies. This association gained attention after Bastard et al identified anti–type I interferon antibodies in patients with severe COVID-19, predominantly among males with a genetic predisposition. These autoantibodies were shown to impair antiviral defenses and contribute to life-threatening pneumonia.5
Subsequent studies demonstrated the production of a wide spectrum of functional autoantibodies, including ANA, in patients with COVID-19.6,7 These findings were attributed to the acute expansion of autoreactive clones among naïve-derived immunoglobulin G1 antibody-secreting cells during the early stages of infection, with the degree of expansion correlating with disease severity.8,9 Although longitudinal data up to 15 months postinfection suggest this serologic abnormality resolves in more than two-thirds of patients, the number of individuals infected globally has raised serious public health concerns regarding the potential long-term sequelae, including the onset of lupus or other autoimmune diseases in COVID-19 survivors.6-9 A limited number of case reports describing the onset of lupus following SARS-CoV-2 infection support this hypothesis.10
This surveillance analysis investigates lupus incidence among patients within the Military Health System (MHS), encompassing all TRICARE beneficiaries, from January 2018 to December 2022. The objective of this analysis was to examine lupus incidence trends throughout the COVID-19 pandemic, stratified by sex, age, and active-duty status.
Methods
The MHS provides health care services to about 9.5 million US Department of Defense (DoD) beneficiaries. Outpatient health records and laboratory results for individuals receiving care at military treatment facilities (MTFs) between January 1, 2018, and December 31, 2022, were obtained from the Comprehensive Ambulatory/ Professional Encounter Record and MHS GENESIS. For beneficiaries receiving care in the private sector, data were sourced from the TRICARE Encounter Data—Non-Institutional database.
Laboratory test results, including ANA testing, were available only for individuals receiving care at MTFs. These laboratory data were extracted from the Composite Health Care System Chemistry database and MHS GENESIS laboratory systems for the same time frame. Inpatient data were not included in this analysis. Data from 2017 were used solely as a look-back (or washout) period to identify and exclude prevalent lupus cases diagnosed before 2018 and were not included in the final results.
Lupus cases were identified by the presence of a positive ANA test and appropriate International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) codes. A positive ANA result was defined as either a qualitative result marked positive or a titer ≥ 1:80. The ICD-10-CM codes considered indicative of lupus included variations of M32, L93, or H01.12.
M32, L93, or H01.12. For cases with a positive ANA test, a lupus diagnosis required the presence of ≥ 2 lupus related ICD-10-CM codes. In the absence of ANA test results, a stricter criterion was applied: ≥ 4 lupus ICD-10-CM diagnosis codes recorded on separate days were required for inclusion.
Beneficiaries were excluded if they had a negative ANA result, only 1 lupus ICD- 10-CM diagnosis code, 1 positive ANA with only 1 corresponding ICD-10-CM code, or if their diagnosis occurred outside the defined study period. Patients and members of the public were not involved in the design, conduct, reporting, or dissemination of this study.
Results
Between January 1, 2017, and December 31, 2022, 99,946 TRICARE beneficiaries had some indication of lupus testing or diagnosis in their health records (Figure 1). Of these beneficiaries, 5335 had a positive ANA result and ≥ 2 ICD-10-CM lupus diagnosis codes. An additional 28,275 beneficiaries had ≥ 4 ICD-10-CM lupus diagnosis codes but no ANA test results. From these groups, the final sample included 10,760 beneficiaries who met the incident case definitions for SLE during the study period (2018 through 2022).

Most cases (85.1%, n = 9157) were diagnosed through TRICARE claims, while 1205 (11.2%) were diagnosed within the MHS. Another 398 (3.7%) had documentation of care both within and outside the MHS. Incident SLE cases declined by an average of 16% annually during the study period (Figure 2). This trend amounted to an overall reduction of 48.2%, from 2866 cases in 2018 to 1399 cases in 2022. This decline occurred despite total medical encounters among DoD beneficiaries remaining relatively stable during the pandemic years, with only a 3.5% change between 2018 and 2022.

The disease was more prevalent among female beneficiaries, with a female to- male ratio of 7:1 (Table 1). Among women, the number of new cases declined from 2519 in 2018 to 1223 in 2022, while the number of cases among men remained consistently < 350 annually. Similar trends were observed across other strata. Incident SLE cases were more common among nonactive-duty beneficiaries than active-duty service members, with a ratio of 18:1. New cases among active-duty members remained < 155 per year. Age-stratified data revealed that SLE was diagnosed predominantly in individuals aged ≥ 18 years, with a ratio of 37:1 compared with individuals aged < 18 years. Among children, the number of new cases remained < 75 per year throughout the study period.

A mean 56,850 ANA tests were conducted annually in centralized laboratories using standardized protocols (Table 2). The mean ANA positivity rate was 17.3%, which remained relatively stable from 2018 through 2022.

Discussion
This study examined the annual incidence of newly diagnosed SLE cases among all TRICARE beneficiaries from January 1, 2018, through December 31, 2022, covering both before and during the peak years of the COVID-19 pandemic. This analysis revealed a steady decline in SLE cases during this period. The reliability of these findings is reinforced by the comprehensiveness of the MHS, one of the largest US health care delivery systems, which maintains near-complete medical data capture for about 9.5 million DoD TRICARE beneficiaries across domestic and international settings.
SLE is a rare autoimmune disorder that presents a diagnostic challenge due to its wide range of nonspecific symptoms, many of which resemble other conditions. To reduce the likelihood of false-positive results and ensure diagnostic accuracy, this study adopted a stringent case definition. Incident cases were identified by the presence of ANA testing in conjunction with lupus-specific ICD-10-CM codes and required ≥ 4 lupus related diagnostic entries. This criterion was necessary due to the absence of ANA test results in data from private sector care settings. Our case definition aligns with established literature. For example, a Vanderbilt University chart review study demonstrated that combining ANA positivity with ≥ 4 lupus related ICD-10-CM codes achieves a positive predictive value of 100%, albeit with a sensitivity of 45%.11 Other studies similarly affirm the diagnostic validity of using recurrent ICD-10-CM codes to improve specificity in identifying lupus cases.12,13
The primary objective of this study was to examine the temporal trend in newly diagnosed lupus cases, rather than derive precise incidence rates. Although the TRICARE system includes about 9.5 million beneficiaries, this number represents a dynamic population with continual inflow and outflow. Accurate incidence rate calculation would require access to detailed denominator data, which were not readily available. In comparison with our findings, a study limited to active-duty service members reported fewer lupus cases. This discrepancy likely reflects differences in case definitions—specifically, the absence of laboratory data, the restricted range of diagnostic codes, and the requirement that diagnoses be rendered by specialists.14 Despite these differences, demographic patterns were consistent, with higher incidence observed in females and individuals aged ≥ 20 years.
A Centers for Disease Control and Prevention (CDC) study of lupus incidence in the general population also reported lower case counts.1 However, the CDC estimates were based on 5 state-level registries, which rely on clinician-reported cases and therefore may underestimate true disease burden. Moreover, the DoD beneficiary population differs markedly from the general population: it includes a large cohort of retirees, ensuring an older demographic; all members have comprehensive health care access; and active-duty personnel are subject to pre-enlistment medical screening. Taken together, these factors suggest this study may offer a more complete and systematically captured profile of lupus incidence.
We observed a marked decline of newly diagnosed SLE cases during the study period, which coincided with the widespread circulation of COVID-19. This decrease is unlikely to be attributable to reduced access to care during the pandemic. The MHS operates under a single-payer model, and the total number of patient encounters remained relatively stable throughout the pandemic.
To our knowledge, this is the only study to monitor lupus incidence in a large US population over the 5-year period encompassing before and during the COVID-19 pandemic. To date, only 4 large-scale surveillance studies have addressed similar questions. 14-17 Our findings are consistent with the most recent of these reports: an analysis limited to active-duty members of the US Armed Forces identified 1127 patients with newly diagnosed lupus between 2000 and 2022 and reported stable incidence trends throughout the pandemic.14 The other 3 studies adopted a different approach, comparing the emergence of autoimmune diseases, including lupus, between individuals with confirmed SARS-CoV-2 infection and those without. Each of these trials concluded that COVID-19 increases the risk of various autoimmune conditions, although the findings specific to lupus were inconsistent.15-17
Chang et al reported a significant increase in new lupus diagnoses (n = 2,926,016), with an adjusted hazard ratio (aHR) of 2.99 (95% CI, 2.68-3.34), spanning all ages and both sexes. The highest incidence was observed in individuals of Asian descent.15 Using German routine health care data from 2020, Tesch et al identified a heightened risk of autoimmune diseases, including lupus, among patients with a history of SARS-CoV-2 infection (n = 641,407; 9.4% children, 57.3% female, 6.4% hospitalized), compared with matched infection-naïve controls (n = 1,560,357).16 Both studies excluded vaccinated individuals.
These 2 studies diverged in their assessment of the relationship between COVID-19 severity and subsequent autoimmune risk. Chang et al found a higher incidence among nonhospitalized ambulatory patients, while Tesch et al reported that increased risk was associated with patients requiring intensive care unit admission.15,16
In contrast, based on a cohort of 4,197,188 individuals, Peng et al found no significant difference in lupus incidence among patients with SARS-CoV-2 infection (aHR, 1.05; 95% CI, 0.79-1.39).17 Notably, within the infected group, the incidence of SLE was significantly lower among vaccinated individuals compared with the unvaccinated group (aHR, 0.29; 95% CI, 0.18-0.47). Similar protective associations were observed for other antibody-mediated autoimmune disorders, including pemphigoid, Graves’ disease, and antiphospholipid antibody syndrome.
Limitations
Due to fundamental differences in study design, we were unable to directly reconcile our findings with those reported in the literature. This study lacked access to reliable documentation of COVID-19 infection status, primarily due to the widespread use of home testing among TRICARE beneficiaries. Additionally, the dataset did not include inpatient records and therefore did not permit evaluation of disease severity. Despite these constraints, it is plausible that the overall burden of COVID-19 infection within the study population was lower than that observed in the general US population.
As of December 2022, the DoD had reported about 750,000 confirmed COVID-19 cases among military personnel, civilian employees, dependents, and DoD contractors.18 Given that TRICARE beneficiaries represent about 2.8% of the total US population—and that > 90 million US individuals were infected between 2020 and 2022—the implied infection rate in our cohort appears to be about one-third of what might be expected.19 This discrepancy may be due to higher adherence to mitigation measures, such as social distancing and mask usage, among DoD-affiliated populations. COVID-19 vaccination was mandated for all active-duty service members, who constitute 5.4% of the study population. The remaining TRICARE beneficiaries also had access to guaranteed health care and vaccination coverage, likely contributing to high overall vaccination rates.
Because > 80% of the study population was composed of individuals from diverse civilian backgrounds, we expect the distribution of infection severity within the DoD beneficiary population to approximate that of the general US population.
Future Directions
The findings of this study offer circumstantial yet real-time evidence of the complexity underlying immune dysregulation at the intersection of host susceptibility and environmental exposures. The stability in ANA positivity rates during the study period mitigates concerns regarding undiagnosed subclinical lupus and may suggest that, overall, immune homeostasis was preserved among DoD beneficiaries.
It is noteworthy that during the COVID-19 pandemic, the incidence of several common infections—such as influenza and EBV—declined markedly, likely as a result of widespread social distancing and other public health interventions.20 Mitigation strategies implemented within the military may have conferred protection not only against COVID-19 but also against other community-acquired pathogens.
In light of these observations, we hypothesize that for COVID-19 to act as a trigger for SLE, a prolonged or repeated disruption of immune equilibrium may be required—potentially mediated by recurrent infections or sustained inflammatory states. The association between viral infections and autoimmunity is well established. Immune dysregulation leading to autoantibody production has been observed not only in the context of SARS-CoV-2 but also following infections with EBV, cytomegalovirus, enteroviruses, hepatitis B and C viruses, HIV, and parvovirus B19.21
This dysregulation is often transient, accompanied by compensatory immune regulatory responses. However, in individuals subjected to successive or overlapping infections, these regulatory mechanisms may become compromised or overwhelmed, due to emergent patterns of immune interference of varying severity. In such cases, a transient immune perturbation may progress into a bona fide autoimmune disease, contingent upon individual risk factors such as genetic predisposition, preexisting immune memory, and regenerative capacity.21
Therefore, we believe the significance of this study is 2-fold. First, lupus is known to develop gradually and may require 3 to 5 years to clinically manifest after the initial break in immunological tolerance.3 Continued public health surveillance represents a more pragmatic strategy than retrospective cohort construction, especially as histories of COVID-19 infection become increasingly complete and definitive. Our findings provide a valuable baseline reference point for future longitudinal studies.
The interpretation of surveillance outcomes—whether indicating an upward trend, a stable baseline, or a downward trend—offers distinct analytical value. Within this study population, we observed neither an upward trajectory that might suggest a direct causal link, nor a flat trend that would imply absence of association between COVID-19 and lupus pathogenesis. Instead, the observation of a downward trend invites consideration of nonlinear or protective influences. From this perspective, we recommend that future investigations adopt a holistic framework when assessing environmental contributions to immune dysregulation—particularly when evaluating the long-term immunopathological consequences of the COVID-19 pandemic on lupus and related autoimmune conditions.
Conclusions
This study identified a declining trend in incident lupus cases during the COVID-19 pandemic among the DoD beneficiary population. Further investigation is warranted to elucidate the underlying factors contributing to this decline. Conducting longitudinal epidemiologic studies and applying multivariable regression analyses will be essential to determine whether incidence rates revert to prepandemic baselines and how these trends may be influenced by evolving environmental factors within the general population.
A Systemic Lupus Erythematosus Incidence Surveillance Report Among DoD Beneficiaries During the COVID-19 Pandemic
A Systemic Lupus Erythematosus Incidence Surveillance Report Among DoD Beneficiaries During the COVID-19 Pandemic
- Izmirly PM, Ferucci ED, Somers EC, et al. Incidence rates of systemic lupus erythematosus in the USA: estimates from a meta-analysis of the Centers for Disease Control and Prevention national lupus registries. Lupus Sci Med. 2021;8(1):e000614. doi:10.1136/lupus-2021-000614
- Centers for Disease Control and Prevention. People with lupus. May 15, 2024. Accessed May 10, 2025. https:// www.cdc.gov/lupus/data-research/index.html
- Arbuckle MR, McClain MT, Rubertone MV, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003;349(16):1526-1533. doi:10.1056/nejmoa021933
- Li ZX, Zeng S, Wu HX, Zhou Y. The risk of systemic lupus erythematosus associated with Epstein–Barr virus infection: a systematic review and meta-analysis. Clin Exp Med. 2019;19(1):23-36. doi:10.1007/s10238-018-0535-0
- Bastard P, Rosen LB, Zhang Q, et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370(6515):eabd4585. doi:10.1126/science.abd4585
- Chang SE, Feng A, Meng W, et al. New-onset IgG autoantibodies in hospitalized patients with COVID-19. Nat Commun. 2021;12(1):5417. doi:10.1038/s41467-021-25509-3
- Lee SJ, Yoon T, Ha JW, et al. Prevalence, clinical significance, and persistence of autoantibodies in COVID-19. Virol J. 2023;20(1):236. doi:10.1186/s12985-023-02191-z
- Woodruff MC, Ramonell RP, Haddad NS, et al. Dysregulated naive B cells and de novo autoreactivity in severe COVID-19. Nature. 2022;611(7934):139-147. doi:10.1038/s41586-022-05273-0
- Taeschler P, Cervia C, Zurbuchen Y, et al. Autoantibodies in COVID-19 correlate with antiviral humoral responses and distinct immune signatures. Allergy. 2022;77(8):2415-2430. doi:10.1111/all.15302
- Gracia-Ramos AE, Martin-Nares E, Hernández-Molina G. New onset of autoimmune diseases following COVID-19 diagnosis. Cells. 2021;10(12):3592 doi:10.3390/cells10123592
- Barnado A, Carroll R, Denny JC, Crofford L. Using IC-10-CM codes to identify patients with systemic lupus erythematosus in the electronic health record [abstract]. Arthritis Rheumatol. 2018;70(suppl 9):abstract 1692. Accessed May 10, 2025. https://acrabstracts.org/abstract/using-icd-10-cm-codes-to-identify-patients-with-systemic-lupus-erythematosus-in-the-electronic-health-record
- Feldman C, Curtis JR, Oates JC, Yazdany J, Izmirly P. Validating claims-based algorithms for a systemic lupus erythematosus diagnosis in Medicare data for informed use of the Lupus Index: a tool for geospatial research. Lupus Sci Med. 2024;11(2):e001329. doi:10.1136/lupus-2024-001329
- Moe SR, Haukeland H, Brunborg C, et al. POS1472: Accuracy of disease-specific ICD-10 code for incident systemic lupus erythematosus; results from a population-based cohort study set in Norway [abstract]. Ann Rheum Dis. 2023;82(suppl 1):1090-1091. doi:10.1136/annrheumdis-2023-eular.1189
- Denagamage P, Mabila SL, McQuistan AA. Trends and disparities in systemic lupus erythematosus incidence among U.S. active component service members, 2000–2022. MSMR. 2023;30(12):2-5.
- Chang R, Yen-Ting Chen T, Wang SI, Hung YM, Chen HY, Wei CJ. Risk of autoimmune diseases in patients with COVID-19: a retrospective cohort study. EClinicalMedicine. 2023;56:101783. doi:10.1016/j.eclinm.2022.101783
- Tesch F, Ehm F, Vivirito A, et al. Incident autoimmune diseases in association with SARS-CoV-2 infection: a matched cohort study. Clin Rheumatol. 2023;42(10):2905- 2914. doi:10.1007/s10067-023-06670-0
- Peng K, Li X, Yang D, et al. Risk of autoimmune diseases following COVID-19 and the potential protective effect from vaccination: a population-based cohort study. EClinicalMedicine. 2023;63:102154. doi:10.1016/j.eclinm.2023.102154
- US Department of Defense. Coronavirus: DOD response. Updated December 20, 2022. Accessed May 10, 2025. https://www.defense.gov/Spotlights/Coronavirus-DOD-Response/
- Elflein J. Number of cumulative cases of COVID-19 in the United States from January 20, 2020 to November 11, 2022, by week. Statista. https://www.statista.com/statistics/1103185/cumulative-coronavirus-covid19-cases-number-us-by-day
- Ye Z, Chen L, Zhong H, Cao L, Fu P, Xu J. Epidemiology and clinical characteristics of Epstein-Barr virus infection among children in Shanghai, China, 2017- 2022. Front Cell Infect Microbiol. 2023;13:1139068. doi:10.3389/fcimb.2023.1139068
- Johnson D, Jiang W. Infectious diseases, autoantibodies, and autoimmunity. J Autoimmun. 2023;137:102962. doi:10.1016/j.jaut.2022.102962