User login
Lithium-induced diabetes insipidus: Prevention and management
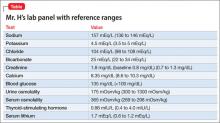
Mr. H, age 33, was diagnosed with bipolar I disorder 9 years ago. For the past year, his mood symptoms have been well controlled with lithium 300 mg, 3 times a day, and olanzapine, 20 mg/d. He presents to the outpatient clinic for a routine visit complaining of insomnia, daytime sleepiness, and increased thirst. He also notes that his tremor has become more prominent over the last few weeks. Concerned about his symptoms, Mr. H’s clinician orders a comprehensive laboratory panel (Table).
Upon further questioning, Mr. H’s physician determines that his insomnia is caused by nocturnal urination, which is consistent with fluid and electrolyte imbalances seen in Mr. H’s laboratory panel. Mr. H is diagnosed with lithium-induced diabetes insipidus.
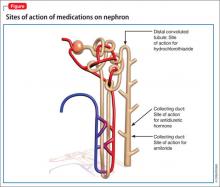
Although lithium’s exact mechanism of action is unknown, it is known that lithium can negatively affect the kidneys.1,2 Typically, antidiuretic hormone (ADH) regulates water permeability in the collecting duct of the nephron, allowing water to be reabsorbed through simple diffusion in the kidney’s collecting duct (Figure).3 Chronic lithium use reduces or desensitizes the kidney’s ability to respond to ADH. Resistance to ADH occurs when lithium accumulates in the cells of the collecting duct and inhibits ADH’s ability to increase water permeability. This inhibition can cause some of Mr. H’s symptoms, such as polydipsia and polyuria, and is estimated to occur in approximately 40% of patients receiving long-term lithium therapy.4,5
Diagnosis
Diagnosing lithium-induced nephrogenic diabetes insipidus (NDI) begins with a history of the patient’s symptoms and ordering lab tests.5 The next step involves a water restriction test, also known as a thirst test, to measure the patient’s ability to concentrate his or her urine. Baseline serum osmolality and electrolytes are compared with new values obtained after completing the water restriction test. Healthy people will have a 2-to-4-fold increase in urine osmolality compared with patients who have NDI. The last step includes administering desmopressin and differentiates between central diabetes insipidus and NDI.6
After desmopressin use, patients who have central diabetes insipidus will have a >50% increase in urine osmolality, whereas patients who have NDI will have <10% increase in urine osmolality. This distinction is important because patients with central diabetes insipidus might have more severe disease and might not benefit from measures commonly used for lithium-induced NDI.7
Prevention and management
Lithium-induced NDI is thought to be dose-dependent and may be prevented by using the lowest effective dose of lithium for an individual patient. It is important that patients taking lithium receive basic electrolyte, hematologic, liver function, renal function, and thyroid function tests at baseline and every 6 to 12 months after the lithium regimen is stable. Additionally, lithium levels should be monitored frequently. The frequency of these tests may range from twice weekly to every 3 to 4 months or longer, depending on the patient’s condition. This monitoring allows the prescriber to quickly identify emerging adverse effects.
Patients with impaired renal function and those with a urine output >3 liters a day are more susceptible to NDI and require monitoring every 3 months. Also, instruct patients to monitor their urine output and educate them about the dangers of fluid and electrolyte imbalances and the signs and symptoms of NDI, such as excessive thirst and urination.1,2
When a patient experiences lithium-induced NDI, re-evaluate treatment and dosage, including simplifying the dosing regimen or switching to once-daily dosing, usually at bedtime. Once-daily dosing results in a lower overall lithium trough, which might allow the kidneys more “drug-free” time.4,5 Additionally, 12-hour lithium levels are approximately 20% higher with once-daily monitoring; continued monitoring is needed during this switch. Patients who have a moderate or severe form of lithium-induced NDI may need to discontinue lithium altogether. There are several options for treating lithium-induced NDI in patients who need to take lithium. Closely monitor kidney function and lithium routinely with these strategies.
Amiloride. This potassium-sparing diuretic minimizes accumulation of lithium by inhibiting collecting duct sodium channels. Studies have shown that amiloride can decrease mean urine volume, increase urine osmolality, and improve the kidneys’ ability to respond to exogenous arginine vasopressin.8
Thiazide diuretics produce mild sodium depletion, which decreases the distal tubule delivery of sodium, therefore increasing water reabsorption in the collecting duct. Hydrochlorothiazide has been shown to reduce urine output by >50% in patients with NDI on a sodium-restricted diet. Hydrochlorothiazide use requires careful monitoring of potassium and lithium levels. Use of a thiazide diuretic also might warrant decreasing the lithium dose by as much as 50% to prevent toxicity.9,10
Low-sodium diet plus hydrochlorothiazide. This route provides another option to decrease urine output during lithium-induced NDI. A reduction in urine output has been shown to be directly proportional to a decrease in salt intake and excretion. Restricting sodium to <2.3 g/d is an appropriate goal for many patients to prevent reoccurring symptoms, which is more than the 3 g/d average that most Americans consume. Potassium and lithium levels must be monitored closely.9
Nonsteroidal anti-inflammatory drugs (NSAIDs). These drugs’ ability to inhibit prostaglandin synthesis prevents prostaglandins from antagonizing actions of ADH in the kidney. The result is increased urine concentration via the actions of ADH. Indomethacin has a greater effect than ibuprofen in increasing ADH’s actions on the kidney. Use of concomitant NSAIDs with lithium requires close monitoring of renal function tests.11
1. Ecelbarger CA. Lithium treatment and remodeling of the collecting duct. Am J Physiol Renal Physiol. 2006;291(1):F37-38.
2. Christensen BM, Kim YH, Kwon TH, et al. Lithium treatment induces a marked proliferation of primarily principal cells in rat kidney inner medullary collecting duct. Am J Physiol Renal Physiol. 2006;291(1):F39-48.
3. Francis SG, Gardner DG. Basic and clinical endocrinology. 7th ed. New York, NY: McGraw Hill; 2003:154-158.
4. Stone KA. Lithium-induced nephrogenic diabetes insipidus. J Am Board Fam Pract. 1999;12(1):43-47.
5. Grünfeld JP, Rossier BC. Lithium nephrotoxicity revisited. Nat Rev Nephrol. 2009;5(5):270-276.
6. Wesche D, Deen PM, Knoers NV. Congenital nephrogenic diabetes insipidus: the current state of affairs. Pediatr Nephrol. 2012;27(12):2183-2204.
7. Rose BD, Post TW. Clinical physiology of acid-base and electrolyte disorders. 5th ed. New York, NY: McGraw-Hill; 2001:754-759,782-783.
8. Batlle DC, von Riotte AB, Gaviria M, et al. Amelioration of polyuria by amiloride in patients receiving long-term lithium therapy. N Engl J Med. 1985;312(7):408-414.
9. Earley LE, Orloff J. The mechanism of antidiuresis associated with the administration of hydrochlorothiazide to patients with vasopressin-resistant diabetes insipidus. J Clin Invest. 1962;41(11):1988-1997.
10. Kim GH, Lee JW, Oh YK, et al. Antidiuretic effect of hydrochlorothiazide in lithium-induced nephrogenic diabetes insipidus is associated with upregulation of aquaporin-2, Na-Cl co-transporter, and epithelial sodium channel. J Am Soc Nephrol. 2004;15(11):2836-2843.
11. Libber S, Harrison H, Spector D. Treatment of nephrogenic diabetes insipidus with prostaglandin synthesis inhibitors. J Pediatr. 1986;108(2):305-311.
Mr. H, age 33, was diagnosed with bipolar I disorder 9 years ago. For the past year, his mood symptoms have been well controlled with lithium 300 mg, 3 times a day, and olanzapine, 20 mg/d. He presents to the outpatient clinic for a routine visit complaining of insomnia, daytime sleepiness, and increased thirst. He also notes that his tremor has become more prominent over the last few weeks. Concerned about his symptoms, Mr. H’s clinician orders a comprehensive laboratory panel (Table).
Upon further questioning, Mr. H’s physician determines that his insomnia is caused by nocturnal urination, which is consistent with fluid and electrolyte imbalances seen in Mr. H’s laboratory panel. Mr. H is diagnosed with lithium-induced diabetes insipidus.
Although lithium’s exact mechanism of action is unknown, it is known that lithium can negatively affect the kidneys.1,2 Typically, antidiuretic hormone (ADH) regulates water permeability in the collecting duct of the nephron, allowing water to be reabsorbed through simple diffusion in the kidney’s collecting duct (Figure).3 Chronic lithium use reduces or desensitizes the kidney’s ability to respond to ADH. Resistance to ADH occurs when lithium accumulates in the cells of the collecting duct and inhibits ADH’s ability to increase water permeability. This inhibition can cause some of Mr. H’s symptoms, such as polydipsia and polyuria, and is estimated to occur in approximately 40% of patients receiving long-term lithium therapy.4,5
Diagnosis
Diagnosing lithium-induced nephrogenic diabetes insipidus (NDI) begins with a history of the patient’s symptoms and ordering lab tests.5 The next step involves a water restriction test, also known as a thirst test, to measure the patient’s ability to concentrate his or her urine. Baseline serum osmolality and electrolytes are compared with new values obtained after completing the water restriction test. Healthy people will have a 2-to-4-fold increase in urine osmolality compared with patients who have NDI. The last step includes administering desmopressin and differentiates between central diabetes insipidus and NDI.6
After desmopressin use, patients who have central diabetes insipidus will have a >50% increase in urine osmolality, whereas patients who have NDI will have <10% increase in urine osmolality. This distinction is important because patients with central diabetes insipidus might have more severe disease and might not benefit from measures commonly used for lithium-induced NDI.7
Prevention and management
Lithium-induced NDI is thought to be dose-dependent and may be prevented by using the lowest effective dose of lithium for an individual patient. It is important that patients taking lithium receive basic electrolyte, hematologic, liver function, renal function, and thyroid function tests at baseline and every 6 to 12 months after the lithium regimen is stable. Additionally, lithium levels should be monitored frequently. The frequency of these tests may range from twice weekly to every 3 to 4 months or longer, depending on the patient’s condition. This monitoring allows the prescriber to quickly identify emerging adverse effects.
Patients with impaired renal function and those with a urine output >3 liters a day are more susceptible to NDI and require monitoring every 3 months. Also, instruct patients to monitor their urine output and educate them about the dangers of fluid and electrolyte imbalances and the signs and symptoms of NDI, such as excessive thirst and urination.1,2
When a patient experiences lithium-induced NDI, re-evaluate treatment and dosage, including simplifying the dosing regimen or switching to once-daily dosing, usually at bedtime. Once-daily dosing results in a lower overall lithium trough, which might allow the kidneys more “drug-free” time.4,5 Additionally, 12-hour lithium levels are approximately 20% higher with once-daily monitoring; continued monitoring is needed during this switch. Patients who have a moderate or severe form of lithium-induced NDI may need to discontinue lithium altogether. There are several options for treating lithium-induced NDI in patients who need to take lithium. Closely monitor kidney function and lithium routinely with these strategies.
Amiloride. This potassium-sparing diuretic minimizes accumulation of lithium by inhibiting collecting duct sodium channels. Studies have shown that amiloride can decrease mean urine volume, increase urine osmolality, and improve the kidneys’ ability to respond to exogenous arginine vasopressin.8
Thiazide diuretics produce mild sodium depletion, which decreases the distal tubule delivery of sodium, therefore increasing water reabsorption in the collecting duct. Hydrochlorothiazide has been shown to reduce urine output by >50% in patients with NDI on a sodium-restricted diet. Hydrochlorothiazide use requires careful monitoring of potassium and lithium levels. Use of a thiazide diuretic also might warrant decreasing the lithium dose by as much as 50% to prevent toxicity.9,10
Low-sodium diet plus hydrochlorothiazide. This route provides another option to decrease urine output during lithium-induced NDI. A reduction in urine output has been shown to be directly proportional to a decrease in salt intake and excretion. Restricting sodium to <2.3 g/d is an appropriate goal for many patients to prevent reoccurring symptoms, which is more than the 3 g/d average that most Americans consume. Potassium and lithium levels must be monitored closely.9
Nonsteroidal anti-inflammatory drugs (NSAIDs). These drugs’ ability to inhibit prostaglandin synthesis prevents prostaglandins from antagonizing actions of ADH in the kidney. The result is increased urine concentration via the actions of ADH. Indomethacin has a greater effect than ibuprofen in increasing ADH’s actions on the kidney. Use of concomitant NSAIDs with lithium requires close monitoring of renal function tests.11
Mr. H, age 33, was diagnosed with bipolar I disorder 9 years ago. For the past year, his mood symptoms have been well controlled with lithium 300 mg, 3 times a day, and olanzapine, 20 mg/d. He presents to the outpatient clinic for a routine visit complaining of insomnia, daytime sleepiness, and increased thirst. He also notes that his tremor has become more prominent over the last few weeks. Concerned about his symptoms, Mr. H’s clinician orders a comprehensive laboratory panel (Table).
Upon further questioning, Mr. H’s physician determines that his insomnia is caused by nocturnal urination, which is consistent with fluid and electrolyte imbalances seen in Mr. H’s laboratory panel. Mr. H is diagnosed with lithium-induced diabetes insipidus.
Although lithium’s exact mechanism of action is unknown, it is known that lithium can negatively affect the kidneys.1,2 Typically, antidiuretic hormone (ADH) regulates water permeability in the collecting duct of the nephron, allowing water to be reabsorbed through simple diffusion in the kidney’s collecting duct (Figure).3 Chronic lithium use reduces or desensitizes the kidney’s ability to respond to ADH. Resistance to ADH occurs when lithium accumulates in the cells of the collecting duct and inhibits ADH’s ability to increase water permeability. This inhibition can cause some of Mr. H’s symptoms, such as polydipsia and polyuria, and is estimated to occur in approximately 40% of patients receiving long-term lithium therapy.4,5
Diagnosis
Diagnosing lithium-induced nephrogenic diabetes insipidus (NDI) begins with a history of the patient’s symptoms and ordering lab tests.5 The next step involves a water restriction test, also known as a thirst test, to measure the patient’s ability to concentrate his or her urine. Baseline serum osmolality and electrolytes are compared with new values obtained after completing the water restriction test. Healthy people will have a 2-to-4-fold increase in urine osmolality compared with patients who have NDI. The last step includes administering desmopressin and differentiates between central diabetes insipidus and NDI.6
After desmopressin use, patients who have central diabetes insipidus will have a >50% increase in urine osmolality, whereas patients who have NDI will have <10% increase in urine osmolality. This distinction is important because patients with central diabetes insipidus might have more severe disease and might not benefit from measures commonly used for lithium-induced NDI.7
Prevention and management
Lithium-induced NDI is thought to be dose-dependent and may be prevented by using the lowest effective dose of lithium for an individual patient. It is important that patients taking lithium receive basic electrolyte, hematologic, liver function, renal function, and thyroid function tests at baseline and every 6 to 12 months after the lithium regimen is stable. Additionally, lithium levels should be monitored frequently. The frequency of these tests may range from twice weekly to every 3 to 4 months or longer, depending on the patient’s condition. This monitoring allows the prescriber to quickly identify emerging adverse effects.
Patients with impaired renal function and those with a urine output >3 liters a day are more susceptible to NDI and require monitoring every 3 months. Also, instruct patients to monitor their urine output and educate them about the dangers of fluid and electrolyte imbalances and the signs and symptoms of NDI, such as excessive thirst and urination.1,2
When a patient experiences lithium-induced NDI, re-evaluate treatment and dosage, including simplifying the dosing regimen or switching to once-daily dosing, usually at bedtime. Once-daily dosing results in a lower overall lithium trough, which might allow the kidneys more “drug-free” time.4,5 Additionally, 12-hour lithium levels are approximately 20% higher with once-daily monitoring; continued monitoring is needed during this switch. Patients who have a moderate or severe form of lithium-induced NDI may need to discontinue lithium altogether. There are several options for treating lithium-induced NDI in patients who need to take lithium. Closely monitor kidney function and lithium routinely with these strategies.
Amiloride. This potassium-sparing diuretic minimizes accumulation of lithium by inhibiting collecting duct sodium channels. Studies have shown that amiloride can decrease mean urine volume, increase urine osmolality, and improve the kidneys’ ability to respond to exogenous arginine vasopressin.8
Thiazide diuretics produce mild sodium depletion, which decreases the distal tubule delivery of sodium, therefore increasing water reabsorption in the collecting duct. Hydrochlorothiazide has been shown to reduce urine output by >50% in patients with NDI on a sodium-restricted diet. Hydrochlorothiazide use requires careful monitoring of potassium and lithium levels. Use of a thiazide diuretic also might warrant decreasing the lithium dose by as much as 50% to prevent toxicity.9,10
Low-sodium diet plus hydrochlorothiazide. This route provides another option to decrease urine output during lithium-induced NDI. A reduction in urine output has been shown to be directly proportional to a decrease in salt intake and excretion. Restricting sodium to <2.3 g/d is an appropriate goal for many patients to prevent reoccurring symptoms, which is more than the 3 g/d average that most Americans consume. Potassium and lithium levels must be monitored closely.9
Nonsteroidal anti-inflammatory drugs (NSAIDs). These drugs’ ability to inhibit prostaglandin synthesis prevents prostaglandins from antagonizing actions of ADH in the kidney. The result is increased urine concentration via the actions of ADH. Indomethacin has a greater effect than ibuprofen in increasing ADH’s actions on the kidney. Use of concomitant NSAIDs with lithium requires close monitoring of renal function tests.11
1. Ecelbarger CA. Lithium treatment and remodeling of the collecting duct. Am J Physiol Renal Physiol. 2006;291(1):F37-38.
2. Christensen BM, Kim YH, Kwon TH, et al. Lithium treatment induces a marked proliferation of primarily principal cells in rat kidney inner medullary collecting duct. Am J Physiol Renal Physiol. 2006;291(1):F39-48.
3. Francis SG, Gardner DG. Basic and clinical endocrinology. 7th ed. New York, NY: McGraw Hill; 2003:154-158.
4. Stone KA. Lithium-induced nephrogenic diabetes insipidus. J Am Board Fam Pract. 1999;12(1):43-47.
5. Grünfeld JP, Rossier BC. Lithium nephrotoxicity revisited. Nat Rev Nephrol. 2009;5(5):270-276.
6. Wesche D, Deen PM, Knoers NV. Congenital nephrogenic diabetes insipidus: the current state of affairs. Pediatr Nephrol. 2012;27(12):2183-2204.
7. Rose BD, Post TW. Clinical physiology of acid-base and electrolyte disorders. 5th ed. New York, NY: McGraw-Hill; 2001:754-759,782-783.
8. Batlle DC, von Riotte AB, Gaviria M, et al. Amelioration of polyuria by amiloride in patients receiving long-term lithium therapy. N Engl J Med. 1985;312(7):408-414.
9. Earley LE, Orloff J. The mechanism of antidiuresis associated with the administration of hydrochlorothiazide to patients with vasopressin-resistant diabetes insipidus. J Clin Invest. 1962;41(11):1988-1997.
10. Kim GH, Lee JW, Oh YK, et al. Antidiuretic effect of hydrochlorothiazide in lithium-induced nephrogenic diabetes insipidus is associated with upregulation of aquaporin-2, Na-Cl co-transporter, and epithelial sodium channel. J Am Soc Nephrol. 2004;15(11):2836-2843.
11. Libber S, Harrison H, Spector D. Treatment of nephrogenic diabetes insipidus with prostaglandin synthesis inhibitors. J Pediatr. 1986;108(2):305-311.
1. Ecelbarger CA. Lithium treatment and remodeling of the collecting duct. Am J Physiol Renal Physiol. 2006;291(1):F37-38.
2. Christensen BM, Kim YH, Kwon TH, et al. Lithium treatment induces a marked proliferation of primarily principal cells in rat kidney inner medullary collecting duct. Am J Physiol Renal Physiol. 2006;291(1):F39-48.
3. Francis SG, Gardner DG. Basic and clinical endocrinology. 7th ed. New York, NY: McGraw Hill; 2003:154-158.
4. Stone KA. Lithium-induced nephrogenic diabetes insipidus. J Am Board Fam Pract. 1999;12(1):43-47.
5. Grünfeld JP, Rossier BC. Lithium nephrotoxicity revisited. Nat Rev Nephrol. 2009;5(5):270-276.
6. Wesche D, Deen PM, Knoers NV. Congenital nephrogenic diabetes insipidus: the current state of affairs. Pediatr Nephrol. 2012;27(12):2183-2204.
7. Rose BD, Post TW. Clinical physiology of acid-base and electrolyte disorders. 5th ed. New York, NY: McGraw-Hill; 2001:754-759,782-783.
8. Batlle DC, von Riotte AB, Gaviria M, et al. Amelioration of polyuria by amiloride in patients receiving long-term lithium therapy. N Engl J Med. 1985;312(7):408-414.
9. Earley LE, Orloff J. The mechanism of antidiuresis associated with the administration of hydrochlorothiazide to patients with vasopressin-resistant diabetes insipidus. J Clin Invest. 1962;41(11):1988-1997.
10. Kim GH, Lee JW, Oh YK, et al. Antidiuretic effect of hydrochlorothiazide in lithium-induced nephrogenic diabetes insipidus is associated with upregulation of aquaporin-2, Na-Cl co-transporter, and epithelial sodium channel. J Am Soc Nephrol. 2004;15(11):2836-2843.
11. Libber S, Harrison H, Spector D. Treatment of nephrogenic diabetes insipidus with prostaglandin synthesis inhibitors. J Pediatr. 1986;108(2):305-311.
A teen who is wasting away
CASE: Weak and passive
Cassandra, age 17, recently was discharged from a medical rehabilitation facility with a diagnosis of conversion disorder. Her school performance and attendance had been steadily declining for the last 6 months as she lost strength and motivation to take care of herself. Cassandra lives with her father, who is her primary caretaker. Her parents are separated and her mother has fibromyalgia and chronic fatigue syndrome, which leaves her unable to care for her daughter or participate in appointments.
Now lethargic and wasting away physically, Cassandra is pushed in a wheelchair by her weary father into a child psychiatrist’s office. She does not look up or make eye contact. Her father says “the doctors didn’t know what they were doing. That needle test, a nerve conduction study they did, is what made her worse.” Although Cassandra moves her arms to adjust herself in the wheelchair, she does not move her legs or try to move the wheelchair.
Cassandra’s father states that she has “congenital neuromyopathy. Her mother gave it to her in utero. But nobody listens to me or orders the tests that will prove I am right.” He insists on obscure and specialized blood tests and immune function panels to prove that a congenital condition is causing his daughter’s deterioration and physical debility. He is unwilling to accept that there is any other cause of her condition.
Cassandra’s father is unemployed and has no social contacts or supports. He asserts that “the medical system” is against him, and he believes medical interventions are harming his daughter. He keeps Cassandra isolated from friends and other family members.
How would you proceed?
a) separate Cassandra from her father during the interview
b) contact Cassandra’s mother for collateral information
c) assure Cassandra that there is no medical cause for her physical condition
d) order the testing her father requests
EVALUATION: Demoralized, hopeless
Cassandra is uncooperative with the interview and answers questions with one-word answers. Her affect is irritable and her demeanor is frustrated. She does not seem concerned that she needs assistance with eating and toileting.
When outpatient treatment with her primary care physician did not stop her physical deterioration, she was referred to a tertiary care academic medical center for a complete medical and neurologic workup. The workup, including an MRI, electroencephalogram, nerve conduction studies, and full immunologic panels, was negative for any physical illness, including neuromuscular degenerative disease. A muscle biopsy was considered, but not ordered because Cassandra and her father resisted.
During this hospitalization, she was diagnosed with conversion disorder by the psychiatry consultation service, and transferred to a physical rehabilitation facility for further care. At the rehab facility, Cassandra’s father interfered with her care, arguing constantly with the medical team. Cassandra demonstrated no effort to work with physical or occupational therapy and was discharged after 2 weeks because of noncompliance with treatment. Cassandra and her father are resentful that no physical cause was found and feel that the medical workup and time at the rehabilitation facility made her condition worse. The rehabilitation hospital referred Cassandra to an outpatient child psychiatrist for follow-up.
During the intake evaluation and follow-up appointments with the child psychiatrist, her affect is negativistic and restrictive. She is resistant to talking about her condition and accepting psychotherapeutic interventions. She is quick to blame others for her lack of progress and unable to take responsibility for working on her treatment plan. Cassandra feels demoralized, depressed, and hopeless about her situation and prospects for recovery. She feels that no one is listening to her father and if “they did just the tests he wants, we will know what is wrong with me and that he is right.”
The author’s observations
Table 1 lists conditions to consider in the differential diagnosis of conversion disorder. Although Cassandra’s conversion disorder diagnosis appears to be appropriate, it is important to consider 2 other possibilities: delusional disorder, somatic type with familial features, and Munchausen syndrome by proxy. An underlying depressive or anxiety disorder also should be considered and treated appropriately.
Conversion disorder has a challenging and often complex presentation in children and adolescents. Conversion disorders in children commonly are associated with stressful family situations including divorce, marital conflict, or loss of a close family member.1 An overbearing and conflict-prone parenting style also is associated with childhood conversion disorders.2 Common physical symptoms in conversion disorder are functional abdominal pain, partial paralysis, numbness, or seizures. Individuals such as Cassandra who are unable to express or verbalize their emotional distress are vulnerable to expressing their distress in somatic symptoms. Cassandra demonstrates La belle indifference, the characteristic attitude of not being overly concerned about what others would consider an alarming functional impairment.
Delusional disorder. A diagnosis of delusional disorder, somatic type with familial features was considered because Cassandra and her father shared persecutory and paranoid beliefs that her condition was brought on by some hidden, unrecognized medical condition. A delusional disorder with shared or “familial” features develops when a parent has strongly held delusional beliefs that are transferred to the child. Typically, it develops within the context of a close relationship with the parent, is similar in content to the parent’s belief, and is not preceded by psychosis or prodromal to schizophrenia.3
Because Cassandra’s father transferred his delusional system to his daughter, she clung to the belief that her physical symptoms and immobility were caused by medical misdiagnosis and failure to recognize her illness. Cassandra’s father strongly resisted and defended against accepting his role in her medical condition.
Munchausen by proxy. Because Cassandra and her father share a delusional system that prevented her from accepting and following treatment recommendations, it is possible that her father created her condition. Munchausen syndrome by proxy is a condition whereby illness-producing behavior in a child is exaggerated, fabricated, or induced by a parent or guardian.4 Separating Cassandra from her father and initiating antipsychotic treatment for him are critical considerations for her recovery.
How would you treat Cassandra?
a) call Child Protective Services (CPS) to remove Cassandra from her father’s custody
b) hospitalize Cassandra for intensive treatment of conversion disorder
c) start Cassandra on an atypical antipsychotic
d) begin cognitive-behavioral therapy (CBT) and an antidepressant
Treatment approach
Treating a patient with a conversion disorder, somatic type starts with validating that the patient’s and parent’s distress is real to them (Table 2).5 The clinician acknowledges that no physical evidence of physiological dysfunction has been found, which can be reassuring to the patient and family. The clinician then states that the patient’s condition and the physical manifestation of the symptoms are real. A patient’s or parent’s resistance to this reassurance may indicate that they have a large investment in the symptoms and perpetuating the dysfunction.
Taking a mind-body approach—explaining that the child’s condition is created and perpetuated by a mind-body connection and is not under their voluntary control—often is well received by patients and parents. The treating clinician emphasizes that the condition is physically disabling and that careful, appropriate, and intensive treatment is necessary.
A rehabilitation model has power for patients with conversion disorder because it acknowledges the patient’s discomfort and loss of function while shifting the focus away from finding what is wrong. The goal is to actively engage patients in their own care to help them return to normal functioning.6
Cassandra was encouraged to participate in physical therapy, go to school, and take care of herself. Actively participating in her care and recovery meant that Cassandra had to leave the sick role behind, which was impossible for her father, who saw her as passive, helpless, and fragile.
TREATMENT: Pharmacotherapy, CBT
During psychiatric evaluation, it becomes clear that in addition to her physical debility, Cassandra has major depressive disorder, moderate without psychotic features. Her depression contributes to her hopelessness and lack of participation in treatment. After discussion with her family about how her depressive symptoms are preventing her recovery, Cassandra is started on escitalopram, 10 mg/d. CBT helps her manage her depressive symptoms, prevent further somatization, and correct misperceptions about her body function and disabilities.
For conversion disorder patients, physical therapy can be combined with incentives tied to improvements in functioning. Cassandra has overwhelming anxiety while attempting physical therapy, which interferes with her participation in the therapy. Lorazepam, 0.5 mg/d, is prescribed for her intense anxiety and panic attacks, which led her to avoid physical therapy.
Staff at the rehabilitation hospital calls CPS because Cassandra’s father interferes with her care and treatment plan. CPS continues to monitor Cassandra’s progress through outpatient care. An individualized education plan and psychoeducational testing help determine a school placement to meet Cassandra’s educational needs.
CPS directs Cassandra to stay with her mother for alternating weeks. While at her mother’s, Cassandra is more interested in taking care of herself. She helps with getting herself into bed and to the toilet. Upon returning to her father’s home, these gains are lost.
The author’s observations
Psychodynamic and unconscious motivators for conversion disorder operate on a deeper, hidden level. The underlying primary conflict in pseudoseizures—a more common conversion disorder—has been described as an inability to express negative emotions such as anger. Social problems, conflict with parents, learning disorders,7 or sexual abuse8 produce the negative emotions caused by the primary conflict. Cassandra yearned for a closer relationship with her mother, yet she remained enmeshed with poor intrapsychic boundaries with her father. The fact that he assisted his 17-year-old daughter with toileting raised the possibility of sexual abuse. Sexual abuse could have led to her depression and physical decline. Cassandra’s physical debility also may have been her way to foster dependency on her father and protect him from perceived persecution.
Conversion disorder may have been a result of Cassandra’s defense mechanisms against admitting abuse and protecting against abandonment. Establishing a therapeutic alliance with Cassandra is essential to allow a graceful exit from the conversion disorder symptoms and her father’s hold on her thinking about her illness. However, this alliance may seem to threaten the child’s special connection with the parent. A therapeutic alliance was elusive in Cassandra’s case and likely nearly impossible.
Both parents underwent court-ordered psychological testing as part of the CPS evaluation. Testing on Cassandra’s father indicated a rigid personality structure with long-standing paranoia and mistrust of authority. Because Cassandra endorsed his delusional system completely, it is likely that her father inculcated her into believing his beliefs and transmitted his delusions to her by their close proximity and time together. Based upon this delusional belief system, Cassandra gave up trying to move her legs and her muscles atrophied. Her legs were so weak that she stopped trying to walk or move, illustrating the power of the mind-body connection to produce functional and physiological changes.
Children who live with a mother with chronic illness are at risk of developing psychosomatic disorders.9 Cassandra’s mother had fibromyalgia and chronic pain with symptoms of headache, weakness, and muscle pain and frequent medical office visits and tests without definitive results or symptom relief. Although Cassandra did not live with her mother, Cassandra’s somatization symptoms may be a result of modeling or observational learning within her family.9 Cassandra may have unconsciously adopted her mother’s symptoms and behaviors as a way to cope with stress and gain attention to her needs.
Cassandra’s negative affect, sensitivity to change, and lack of resiliency were further risk factors for developing a somatoform illness.10 She resisted and would not follow through with physical therapy. Krisnakumar10 also reported that an inability to persist in completing tasks is a risk factor for somatoform disorder. Family dynamics of problematic parental interactions also played a role in her somatoform disorder (Table 3).11
OUTCOME: Foster care, improvement
Cassandra receives weekly CBT and biweekly medication monitoring and demonstrates a moderate improvement in mood with less negativity and irritability. Her anxiety symptoms gradually respond to treatment. However, her emotional gains are not matched with improvement in her physical functioning or participation in physical therapy. Cassandra does not recover her muscular strength or control and shows little improvement in her physical capacity and independence.
After 3 months of treatment, Cassandra does not make sufficient progress or actively participate in treatment. Because her father continues to interfere with the treatment plan and does not receive treatment himself, CPS obtains a court order to prevent her father from directing her medical care and telling her treating physicians which tests to order.
Because these interventions do not improve her treatment response, Cassandra is removed from her parents’ care and placed in a therapeutic foster care home, thereby improving her independence and chances for recovery. After 3 months in foster care, she more actively participates in her physical rehabilitation. Water therapy, with the buoyancy and support in water, helps her regain muscle strength and control of her lower extremities.
Bottom Line
Patients with conversion disorder present with functional impairment and physical symptoms without clear physiological causes. Parents have a strong influence on the presentation and course of conversion disorder in children and adolescents. Parents’ mental and physical illnesses are independent risk factors for childhood somatoform disorders. Evaluation of parents’ psychological and psychiatric state is essential to determine intervention.
Related Resource
- Seltzer WJ. Conversion disorder in childhood and adolescence: a familial/cultural approach. Family Systems Medicine. 1985;3(3):261-280.
Drug Brand Names
Escitalopram • Lexapro
Lorazepam • Ativan
Disclosure
Dr. Leipsic reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Wyllie E, Glazer JP, Benbadis S, et al. Psychiatric features of children and adolescents with pseudoseizures. Arch Pediatr Adolesc Med. 1999;153(3):244-248.
2. Salmon P, Al-Marzooqi SM, Baker G, et al. Childhood family dysfunction and associated abuse in patients with nonepileptic seizures: towards a casual model. Psychosom Med. 2003;65(4):695-700.
3. Manschreck T. Delusional disorder and shared psychotic disorder. In: Sadock BJ, Sadock VA, eds. Kaplan & Sadock’s comprehensive textbook of psychiatry. 7th ed.
Philadelphia, PA: Lippincott Williams & Wilkins; 2000: 1243-1264.
4. Meadow R. Munchausen syndrome by proxy. The hinterland of child abuse. Lancet. 1977;2(8033):343-345.
5. Campo JV, Fritsch SL. Somatization in children and adolescents. J Am Acad Child Adolesc Psychiatry. 1994; 33(9):1223-1235.
6. Campo JV, Fritz G. A management model for pediatric somatization. Psychosomatics. 2001;42(6):467-476.
7. Silver LB. Conversion disorder with pseudoseizures in adolescence: a stress reaction to unrecognized and untreated learning disabilities. J Am Accad Child Psychiatry. 1982; 21(5):508-512.
8. AlperK,DevinskyO,PerrineK,etal.Nonepilepticseizures and childhood sexual and physical abuse. Neurology. 1993; 43(10):1950-1953.
9. Jamison RN, Walker LS. Illness behavior in children of chronic pain patients. Int J Psychiatry Med. 1992;22(4): 329-342.
10. Krisnakumar P, Sumesh P, Mathews L. Tempermental traits associated with conversion disorder. Indian Pediatr. 2006;43(10):895-899.
11. Minuchin S, Rosman BL, Baker L. Psychosomatic families: anorexia nervosa in context. Cambridge, MA: Harvard University Press; 1978.
CASE: Weak and passive
Cassandra, age 17, recently was discharged from a medical rehabilitation facility with a diagnosis of conversion disorder. Her school performance and attendance had been steadily declining for the last 6 months as she lost strength and motivation to take care of herself. Cassandra lives with her father, who is her primary caretaker. Her parents are separated and her mother has fibromyalgia and chronic fatigue syndrome, which leaves her unable to care for her daughter or participate in appointments.
Now lethargic and wasting away physically, Cassandra is pushed in a wheelchair by her weary father into a child psychiatrist’s office. She does not look up or make eye contact. Her father says “the doctors didn’t know what they were doing. That needle test, a nerve conduction study they did, is what made her worse.” Although Cassandra moves her arms to adjust herself in the wheelchair, she does not move her legs or try to move the wheelchair.
Cassandra’s father states that she has “congenital neuromyopathy. Her mother gave it to her in utero. But nobody listens to me or orders the tests that will prove I am right.” He insists on obscure and specialized blood tests and immune function panels to prove that a congenital condition is causing his daughter’s deterioration and physical debility. He is unwilling to accept that there is any other cause of her condition.
Cassandra’s father is unemployed and has no social contacts or supports. He asserts that “the medical system” is against him, and he believes medical interventions are harming his daughter. He keeps Cassandra isolated from friends and other family members.
How would you proceed?
a) separate Cassandra from her father during the interview
b) contact Cassandra’s mother for collateral information
c) assure Cassandra that there is no medical cause for her physical condition
d) order the testing her father requests
EVALUATION: Demoralized, hopeless
Cassandra is uncooperative with the interview and answers questions with one-word answers. Her affect is irritable and her demeanor is frustrated. She does not seem concerned that she needs assistance with eating and toileting.
When outpatient treatment with her primary care physician did not stop her physical deterioration, she was referred to a tertiary care academic medical center for a complete medical and neurologic workup. The workup, including an MRI, electroencephalogram, nerve conduction studies, and full immunologic panels, was negative for any physical illness, including neuromuscular degenerative disease. A muscle biopsy was considered, but not ordered because Cassandra and her father resisted.
During this hospitalization, she was diagnosed with conversion disorder by the psychiatry consultation service, and transferred to a physical rehabilitation facility for further care. At the rehab facility, Cassandra’s father interfered with her care, arguing constantly with the medical team. Cassandra demonstrated no effort to work with physical or occupational therapy and was discharged after 2 weeks because of noncompliance with treatment. Cassandra and her father are resentful that no physical cause was found and feel that the medical workup and time at the rehabilitation facility made her condition worse. The rehabilitation hospital referred Cassandra to an outpatient child psychiatrist for follow-up.
During the intake evaluation and follow-up appointments with the child psychiatrist, her affect is negativistic and restrictive. She is resistant to talking about her condition and accepting psychotherapeutic interventions. She is quick to blame others for her lack of progress and unable to take responsibility for working on her treatment plan. Cassandra feels demoralized, depressed, and hopeless about her situation and prospects for recovery. She feels that no one is listening to her father and if “they did just the tests he wants, we will know what is wrong with me and that he is right.”
The author’s observations
Table 1 lists conditions to consider in the differential diagnosis of conversion disorder. Although Cassandra’s conversion disorder diagnosis appears to be appropriate, it is important to consider 2 other possibilities: delusional disorder, somatic type with familial features, and Munchausen syndrome by proxy. An underlying depressive or anxiety disorder also should be considered and treated appropriately.
Conversion disorder has a challenging and often complex presentation in children and adolescents. Conversion disorders in children commonly are associated with stressful family situations including divorce, marital conflict, or loss of a close family member.1 An overbearing and conflict-prone parenting style also is associated with childhood conversion disorders.2 Common physical symptoms in conversion disorder are functional abdominal pain, partial paralysis, numbness, or seizures. Individuals such as Cassandra who are unable to express or verbalize their emotional distress are vulnerable to expressing their distress in somatic symptoms. Cassandra demonstrates La belle indifference, the characteristic attitude of not being overly concerned about what others would consider an alarming functional impairment.
Delusional disorder. A diagnosis of delusional disorder, somatic type with familial features was considered because Cassandra and her father shared persecutory and paranoid beliefs that her condition was brought on by some hidden, unrecognized medical condition. A delusional disorder with shared or “familial” features develops when a parent has strongly held delusional beliefs that are transferred to the child. Typically, it develops within the context of a close relationship with the parent, is similar in content to the parent’s belief, and is not preceded by psychosis or prodromal to schizophrenia.3
Because Cassandra’s father transferred his delusional system to his daughter, she clung to the belief that her physical symptoms and immobility were caused by medical misdiagnosis and failure to recognize her illness. Cassandra’s father strongly resisted and defended against accepting his role in her medical condition.
Munchausen by proxy. Because Cassandra and her father share a delusional system that prevented her from accepting and following treatment recommendations, it is possible that her father created her condition. Munchausen syndrome by proxy is a condition whereby illness-producing behavior in a child is exaggerated, fabricated, or induced by a parent or guardian.4 Separating Cassandra from her father and initiating antipsychotic treatment for him are critical considerations for her recovery.
How would you treat Cassandra?
a) call Child Protective Services (CPS) to remove Cassandra from her father’s custody
b) hospitalize Cassandra for intensive treatment of conversion disorder
c) start Cassandra on an atypical antipsychotic
d) begin cognitive-behavioral therapy (CBT) and an antidepressant
Treatment approach
Treating a patient with a conversion disorder, somatic type starts with validating that the patient’s and parent’s distress is real to them (Table 2).5 The clinician acknowledges that no physical evidence of physiological dysfunction has been found, which can be reassuring to the patient and family. The clinician then states that the patient’s condition and the physical manifestation of the symptoms are real. A patient’s or parent’s resistance to this reassurance may indicate that they have a large investment in the symptoms and perpetuating the dysfunction.
Taking a mind-body approach—explaining that the child’s condition is created and perpetuated by a mind-body connection and is not under their voluntary control—often is well received by patients and parents. The treating clinician emphasizes that the condition is physically disabling and that careful, appropriate, and intensive treatment is necessary.
A rehabilitation model has power for patients with conversion disorder because it acknowledges the patient’s discomfort and loss of function while shifting the focus away from finding what is wrong. The goal is to actively engage patients in their own care to help them return to normal functioning.6
Cassandra was encouraged to participate in physical therapy, go to school, and take care of herself. Actively participating in her care and recovery meant that Cassandra had to leave the sick role behind, which was impossible for her father, who saw her as passive, helpless, and fragile.
TREATMENT: Pharmacotherapy, CBT
During psychiatric evaluation, it becomes clear that in addition to her physical debility, Cassandra has major depressive disorder, moderate without psychotic features. Her depression contributes to her hopelessness and lack of participation in treatment. After discussion with her family about how her depressive symptoms are preventing her recovery, Cassandra is started on escitalopram, 10 mg/d. CBT helps her manage her depressive symptoms, prevent further somatization, and correct misperceptions about her body function and disabilities.
For conversion disorder patients, physical therapy can be combined with incentives tied to improvements in functioning. Cassandra has overwhelming anxiety while attempting physical therapy, which interferes with her participation in the therapy. Lorazepam, 0.5 mg/d, is prescribed for her intense anxiety and panic attacks, which led her to avoid physical therapy.
Staff at the rehabilitation hospital calls CPS because Cassandra’s father interferes with her care and treatment plan. CPS continues to monitor Cassandra’s progress through outpatient care. An individualized education plan and psychoeducational testing help determine a school placement to meet Cassandra’s educational needs.
CPS directs Cassandra to stay with her mother for alternating weeks. While at her mother’s, Cassandra is more interested in taking care of herself. She helps with getting herself into bed and to the toilet. Upon returning to her father’s home, these gains are lost.
The author’s observations
Psychodynamic and unconscious motivators for conversion disorder operate on a deeper, hidden level. The underlying primary conflict in pseudoseizures—a more common conversion disorder—has been described as an inability to express negative emotions such as anger. Social problems, conflict with parents, learning disorders,7 or sexual abuse8 produce the negative emotions caused by the primary conflict. Cassandra yearned for a closer relationship with her mother, yet she remained enmeshed with poor intrapsychic boundaries with her father. The fact that he assisted his 17-year-old daughter with toileting raised the possibility of sexual abuse. Sexual abuse could have led to her depression and physical decline. Cassandra’s physical debility also may have been her way to foster dependency on her father and protect him from perceived persecution.
Conversion disorder may have been a result of Cassandra’s defense mechanisms against admitting abuse and protecting against abandonment. Establishing a therapeutic alliance with Cassandra is essential to allow a graceful exit from the conversion disorder symptoms and her father’s hold on her thinking about her illness. However, this alliance may seem to threaten the child’s special connection with the parent. A therapeutic alliance was elusive in Cassandra’s case and likely nearly impossible.
Both parents underwent court-ordered psychological testing as part of the CPS evaluation. Testing on Cassandra’s father indicated a rigid personality structure with long-standing paranoia and mistrust of authority. Because Cassandra endorsed his delusional system completely, it is likely that her father inculcated her into believing his beliefs and transmitted his delusions to her by their close proximity and time together. Based upon this delusional belief system, Cassandra gave up trying to move her legs and her muscles atrophied. Her legs were so weak that she stopped trying to walk or move, illustrating the power of the mind-body connection to produce functional and physiological changes.
Children who live with a mother with chronic illness are at risk of developing psychosomatic disorders.9 Cassandra’s mother had fibromyalgia and chronic pain with symptoms of headache, weakness, and muscle pain and frequent medical office visits and tests without definitive results or symptom relief. Although Cassandra did not live with her mother, Cassandra’s somatization symptoms may be a result of modeling or observational learning within her family.9 Cassandra may have unconsciously adopted her mother’s symptoms and behaviors as a way to cope with stress and gain attention to her needs.
Cassandra’s negative affect, sensitivity to change, and lack of resiliency were further risk factors for developing a somatoform illness.10 She resisted and would not follow through with physical therapy. Krisnakumar10 also reported that an inability to persist in completing tasks is a risk factor for somatoform disorder. Family dynamics of problematic parental interactions also played a role in her somatoform disorder (Table 3).11
OUTCOME: Foster care, improvement
Cassandra receives weekly CBT and biweekly medication monitoring and demonstrates a moderate improvement in mood with less negativity and irritability. Her anxiety symptoms gradually respond to treatment. However, her emotional gains are not matched with improvement in her physical functioning or participation in physical therapy. Cassandra does not recover her muscular strength or control and shows little improvement in her physical capacity and independence.
After 3 months of treatment, Cassandra does not make sufficient progress or actively participate in treatment. Because her father continues to interfere with the treatment plan and does not receive treatment himself, CPS obtains a court order to prevent her father from directing her medical care and telling her treating physicians which tests to order.
Because these interventions do not improve her treatment response, Cassandra is removed from her parents’ care and placed in a therapeutic foster care home, thereby improving her independence and chances for recovery. After 3 months in foster care, she more actively participates in her physical rehabilitation. Water therapy, with the buoyancy and support in water, helps her regain muscle strength and control of her lower extremities.
Bottom Line
Patients with conversion disorder present with functional impairment and physical symptoms without clear physiological causes. Parents have a strong influence on the presentation and course of conversion disorder in children and adolescents. Parents’ mental and physical illnesses are independent risk factors for childhood somatoform disorders. Evaluation of parents’ psychological and psychiatric state is essential to determine intervention.
Related Resource
- Seltzer WJ. Conversion disorder in childhood and adolescence: a familial/cultural approach. Family Systems Medicine. 1985;3(3):261-280.
Drug Brand Names
Escitalopram • Lexapro
Lorazepam • Ativan
Disclosure
Dr. Leipsic reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE: Weak and passive
Cassandra, age 17, recently was discharged from a medical rehabilitation facility with a diagnosis of conversion disorder. Her school performance and attendance had been steadily declining for the last 6 months as she lost strength and motivation to take care of herself. Cassandra lives with her father, who is her primary caretaker. Her parents are separated and her mother has fibromyalgia and chronic fatigue syndrome, which leaves her unable to care for her daughter or participate in appointments.
Now lethargic and wasting away physically, Cassandra is pushed in a wheelchair by her weary father into a child psychiatrist’s office. She does not look up or make eye contact. Her father says “the doctors didn’t know what they were doing. That needle test, a nerve conduction study they did, is what made her worse.” Although Cassandra moves her arms to adjust herself in the wheelchair, she does not move her legs or try to move the wheelchair.
Cassandra’s father states that she has “congenital neuromyopathy. Her mother gave it to her in utero. But nobody listens to me or orders the tests that will prove I am right.” He insists on obscure and specialized blood tests and immune function panels to prove that a congenital condition is causing his daughter’s deterioration and physical debility. He is unwilling to accept that there is any other cause of her condition.
Cassandra’s father is unemployed and has no social contacts or supports. He asserts that “the medical system” is against him, and he believes medical interventions are harming his daughter. He keeps Cassandra isolated from friends and other family members.
How would you proceed?
a) separate Cassandra from her father during the interview
b) contact Cassandra’s mother for collateral information
c) assure Cassandra that there is no medical cause for her physical condition
d) order the testing her father requests
EVALUATION: Demoralized, hopeless
Cassandra is uncooperative with the interview and answers questions with one-word answers. Her affect is irritable and her demeanor is frustrated. She does not seem concerned that she needs assistance with eating and toileting.
When outpatient treatment with her primary care physician did not stop her physical deterioration, she was referred to a tertiary care academic medical center for a complete medical and neurologic workup. The workup, including an MRI, electroencephalogram, nerve conduction studies, and full immunologic panels, was negative for any physical illness, including neuromuscular degenerative disease. A muscle biopsy was considered, but not ordered because Cassandra and her father resisted.
During this hospitalization, she was diagnosed with conversion disorder by the psychiatry consultation service, and transferred to a physical rehabilitation facility for further care. At the rehab facility, Cassandra’s father interfered with her care, arguing constantly with the medical team. Cassandra demonstrated no effort to work with physical or occupational therapy and was discharged after 2 weeks because of noncompliance with treatment. Cassandra and her father are resentful that no physical cause was found and feel that the medical workup and time at the rehabilitation facility made her condition worse. The rehabilitation hospital referred Cassandra to an outpatient child psychiatrist for follow-up.
During the intake evaluation and follow-up appointments with the child psychiatrist, her affect is negativistic and restrictive. She is resistant to talking about her condition and accepting psychotherapeutic interventions. She is quick to blame others for her lack of progress and unable to take responsibility for working on her treatment plan. Cassandra feels demoralized, depressed, and hopeless about her situation and prospects for recovery. She feels that no one is listening to her father and if “they did just the tests he wants, we will know what is wrong with me and that he is right.”
The author’s observations
Table 1 lists conditions to consider in the differential diagnosis of conversion disorder. Although Cassandra’s conversion disorder diagnosis appears to be appropriate, it is important to consider 2 other possibilities: delusional disorder, somatic type with familial features, and Munchausen syndrome by proxy. An underlying depressive or anxiety disorder also should be considered and treated appropriately.
Conversion disorder has a challenging and often complex presentation in children and adolescents. Conversion disorders in children commonly are associated with stressful family situations including divorce, marital conflict, or loss of a close family member.1 An overbearing and conflict-prone parenting style also is associated with childhood conversion disorders.2 Common physical symptoms in conversion disorder are functional abdominal pain, partial paralysis, numbness, or seizures. Individuals such as Cassandra who are unable to express or verbalize their emotional distress are vulnerable to expressing their distress in somatic symptoms. Cassandra demonstrates La belle indifference, the characteristic attitude of not being overly concerned about what others would consider an alarming functional impairment.
Delusional disorder. A diagnosis of delusional disorder, somatic type with familial features was considered because Cassandra and her father shared persecutory and paranoid beliefs that her condition was brought on by some hidden, unrecognized medical condition. A delusional disorder with shared or “familial” features develops when a parent has strongly held delusional beliefs that are transferred to the child. Typically, it develops within the context of a close relationship with the parent, is similar in content to the parent’s belief, and is not preceded by psychosis or prodromal to schizophrenia.3
Because Cassandra’s father transferred his delusional system to his daughter, she clung to the belief that her physical symptoms and immobility were caused by medical misdiagnosis and failure to recognize her illness. Cassandra’s father strongly resisted and defended against accepting his role in her medical condition.
Munchausen by proxy. Because Cassandra and her father share a delusional system that prevented her from accepting and following treatment recommendations, it is possible that her father created her condition. Munchausen syndrome by proxy is a condition whereby illness-producing behavior in a child is exaggerated, fabricated, or induced by a parent or guardian.4 Separating Cassandra from her father and initiating antipsychotic treatment for him are critical considerations for her recovery.
How would you treat Cassandra?
a) call Child Protective Services (CPS) to remove Cassandra from her father’s custody
b) hospitalize Cassandra for intensive treatment of conversion disorder
c) start Cassandra on an atypical antipsychotic
d) begin cognitive-behavioral therapy (CBT) and an antidepressant
Treatment approach
Treating a patient with a conversion disorder, somatic type starts with validating that the patient’s and parent’s distress is real to them (Table 2).5 The clinician acknowledges that no physical evidence of physiological dysfunction has been found, which can be reassuring to the patient and family. The clinician then states that the patient’s condition and the physical manifestation of the symptoms are real. A patient’s or parent’s resistance to this reassurance may indicate that they have a large investment in the symptoms and perpetuating the dysfunction.
Taking a mind-body approach—explaining that the child’s condition is created and perpetuated by a mind-body connection and is not under their voluntary control—often is well received by patients and parents. The treating clinician emphasizes that the condition is physically disabling and that careful, appropriate, and intensive treatment is necessary.
A rehabilitation model has power for patients with conversion disorder because it acknowledges the patient’s discomfort and loss of function while shifting the focus away from finding what is wrong. The goal is to actively engage patients in their own care to help them return to normal functioning.6
Cassandra was encouraged to participate in physical therapy, go to school, and take care of herself. Actively participating in her care and recovery meant that Cassandra had to leave the sick role behind, which was impossible for her father, who saw her as passive, helpless, and fragile.
TREATMENT: Pharmacotherapy, CBT
During psychiatric evaluation, it becomes clear that in addition to her physical debility, Cassandra has major depressive disorder, moderate without psychotic features. Her depression contributes to her hopelessness and lack of participation in treatment. After discussion with her family about how her depressive symptoms are preventing her recovery, Cassandra is started on escitalopram, 10 mg/d. CBT helps her manage her depressive symptoms, prevent further somatization, and correct misperceptions about her body function and disabilities.
For conversion disorder patients, physical therapy can be combined with incentives tied to improvements in functioning. Cassandra has overwhelming anxiety while attempting physical therapy, which interferes with her participation in the therapy. Lorazepam, 0.5 mg/d, is prescribed for her intense anxiety and panic attacks, which led her to avoid physical therapy.
Staff at the rehabilitation hospital calls CPS because Cassandra’s father interferes with her care and treatment plan. CPS continues to monitor Cassandra’s progress through outpatient care. An individualized education plan and psychoeducational testing help determine a school placement to meet Cassandra’s educational needs.
CPS directs Cassandra to stay with her mother for alternating weeks. While at her mother’s, Cassandra is more interested in taking care of herself. She helps with getting herself into bed and to the toilet. Upon returning to her father’s home, these gains are lost.
The author’s observations
Psychodynamic and unconscious motivators for conversion disorder operate on a deeper, hidden level. The underlying primary conflict in pseudoseizures—a more common conversion disorder—has been described as an inability to express negative emotions such as anger. Social problems, conflict with parents, learning disorders,7 or sexual abuse8 produce the negative emotions caused by the primary conflict. Cassandra yearned for a closer relationship with her mother, yet she remained enmeshed with poor intrapsychic boundaries with her father. The fact that he assisted his 17-year-old daughter with toileting raised the possibility of sexual abuse. Sexual abuse could have led to her depression and physical decline. Cassandra’s physical debility also may have been her way to foster dependency on her father and protect him from perceived persecution.
Conversion disorder may have been a result of Cassandra’s defense mechanisms against admitting abuse and protecting against abandonment. Establishing a therapeutic alliance with Cassandra is essential to allow a graceful exit from the conversion disorder symptoms and her father’s hold on her thinking about her illness. However, this alliance may seem to threaten the child’s special connection with the parent. A therapeutic alliance was elusive in Cassandra’s case and likely nearly impossible.
Both parents underwent court-ordered psychological testing as part of the CPS evaluation. Testing on Cassandra’s father indicated a rigid personality structure with long-standing paranoia and mistrust of authority. Because Cassandra endorsed his delusional system completely, it is likely that her father inculcated her into believing his beliefs and transmitted his delusions to her by their close proximity and time together. Based upon this delusional belief system, Cassandra gave up trying to move her legs and her muscles atrophied. Her legs were so weak that she stopped trying to walk or move, illustrating the power of the mind-body connection to produce functional and physiological changes.
Children who live with a mother with chronic illness are at risk of developing psychosomatic disorders.9 Cassandra’s mother had fibromyalgia and chronic pain with symptoms of headache, weakness, and muscle pain and frequent medical office visits and tests without definitive results or symptom relief. Although Cassandra did not live with her mother, Cassandra’s somatization symptoms may be a result of modeling or observational learning within her family.9 Cassandra may have unconsciously adopted her mother’s symptoms and behaviors as a way to cope with stress and gain attention to her needs.
Cassandra’s negative affect, sensitivity to change, and lack of resiliency were further risk factors for developing a somatoform illness.10 She resisted and would not follow through with physical therapy. Krisnakumar10 also reported that an inability to persist in completing tasks is a risk factor for somatoform disorder. Family dynamics of problematic parental interactions also played a role in her somatoform disorder (Table 3).11
OUTCOME: Foster care, improvement
Cassandra receives weekly CBT and biweekly medication monitoring and demonstrates a moderate improvement in mood with less negativity and irritability. Her anxiety symptoms gradually respond to treatment. However, her emotional gains are not matched with improvement in her physical functioning or participation in physical therapy. Cassandra does not recover her muscular strength or control and shows little improvement in her physical capacity and independence.
After 3 months of treatment, Cassandra does not make sufficient progress or actively participate in treatment. Because her father continues to interfere with the treatment plan and does not receive treatment himself, CPS obtains a court order to prevent her father from directing her medical care and telling her treating physicians which tests to order.
Because these interventions do not improve her treatment response, Cassandra is removed from her parents’ care and placed in a therapeutic foster care home, thereby improving her independence and chances for recovery. After 3 months in foster care, she more actively participates in her physical rehabilitation. Water therapy, with the buoyancy and support in water, helps her regain muscle strength and control of her lower extremities.
Bottom Line
Patients with conversion disorder present with functional impairment and physical symptoms without clear physiological causes. Parents have a strong influence on the presentation and course of conversion disorder in children and adolescents. Parents’ mental and physical illnesses are independent risk factors for childhood somatoform disorders. Evaluation of parents’ psychological and psychiatric state is essential to determine intervention.
Related Resource
- Seltzer WJ. Conversion disorder in childhood and adolescence: a familial/cultural approach. Family Systems Medicine. 1985;3(3):261-280.
Drug Brand Names
Escitalopram • Lexapro
Lorazepam • Ativan
Disclosure
Dr. Leipsic reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Wyllie E, Glazer JP, Benbadis S, et al. Psychiatric features of children and adolescents with pseudoseizures. Arch Pediatr Adolesc Med. 1999;153(3):244-248.
2. Salmon P, Al-Marzooqi SM, Baker G, et al. Childhood family dysfunction and associated abuse in patients with nonepileptic seizures: towards a casual model. Psychosom Med. 2003;65(4):695-700.
3. Manschreck T. Delusional disorder and shared psychotic disorder. In: Sadock BJ, Sadock VA, eds. Kaplan & Sadock’s comprehensive textbook of psychiatry. 7th ed.
Philadelphia, PA: Lippincott Williams & Wilkins; 2000: 1243-1264.
4. Meadow R. Munchausen syndrome by proxy. The hinterland of child abuse. Lancet. 1977;2(8033):343-345.
5. Campo JV, Fritsch SL. Somatization in children and adolescents. J Am Acad Child Adolesc Psychiatry. 1994; 33(9):1223-1235.
6. Campo JV, Fritz G. A management model for pediatric somatization. Psychosomatics. 2001;42(6):467-476.
7. Silver LB. Conversion disorder with pseudoseizures in adolescence: a stress reaction to unrecognized and untreated learning disabilities. J Am Accad Child Psychiatry. 1982; 21(5):508-512.
8. AlperK,DevinskyO,PerrineK,etal.Nonepilepticseizures and childhood sexual and physical abuse. Neurology. 1993; 43(10):1950-1953.
9. Jamison RN, Walker LS. Illness behavior in children of chronic pain patients. Int J Psychiatry Med. 1992;22(4): 329-342.
10. Krisnakumar P, Sumesh P, Mathews L. Tempermental traits associated with conversion disorder. Indian Pediatr. 2006;43(10):895-899.
11. Minuchin S, Rosman BL, Baker L. Psychosomatic families: anorexia nervosa in context. Cambridge, MA: Harvard University Press; 1978.
1. Wyllie E, Glazer JP, Benbadis S, et al. Psychiatric features of children and adolescents with pseudoseizures. Arch Pediatr Adolesc Med. 1999;153(3):244-248.
2. Salmon P, Al-Marzooqi SM, Baker G, et al. Childhood family dysfunction and associated abuse in patients with nonepileptic seizures: towards a casual model. Psychosom Med. 2003;65(4):695-700.
3. Manschreck T. Delusional disorder and shared psychotic disorder. In: Sadock BJ, Sadock VA, eds. Kaplan & Sadock’s comprehensive textbook of psychiatry. 7th ed.
Philadelphia, PA: Lippincott Williams & Wilkins; 2000: 1243-1264.
4. Meadow R. Munchausen syndrome by proxy. The hinterland of child abuse. Lancet. 1977;2(8033):343-345.
5. Campo JV, Fritsch SL. Somatization in children and adolescents. J Am Acad Child Adolesc Psychiatry. 1994; 33(9):1223-1235.
6. Campo JV, Fritz G. A management model for pediatric somatization. Psychosomatics. 2001;42(6):467-476.
7. Silver LB. Conversion disorder with pseudoseizures in adolescence: a stress reaction to unrecognized and untreated learning disabilities. J Am Accad Child Psychiatry. 1982; 21(5):508-512.
8. AlperK,DevinskyO,PerrineK,etal.Nonepilepticseizures and childhood sexual and physical abuse. Neurology. 1993; 43(10):1950-1953.
9. Jamison RN, Walker LS. Illness behavior in children of chronic pain patients. Int J Psychiatry Med. 1992;22(4): 329-342.
10. Krisnakumar P, Sumesh P, Mathews L. Tempermental traits associated with conversion disorder. Indian Pediatr. 2006;43(10):895-899.
11. Minuchin S, Rosman BL, Baker L. Psychosomatic families: anorexia nervosa in context. Cambridge, MA: Harvard University Press; 1978.
HIV: How to provide compassionate care
The prevalence of HIV in persons with untreated psychiatric illness may be 10 to 20 times that of the general population.1 The U.S. Preventive Services Task Force has recommended HIV screening of all persons age 15 to 65 because 20% to 25% of individuals with HIV infection are unaware that they are HIV-positive.2 Because >20% of new HIV infections in the United States are undiagnosed,3 it is crucial to educate patients with mental illness about HIV prevention, make condoms available, and offer HIV testing.
As psychiatrists, we have a unique role in caring for patients at risk for or infected with HIV because in addition to comprehensive medical and psychiatric histories, we routinely take histories of substance use, sexual activities, relationships, and trauma, including childhood neglect and emotional, physical, and sexual abuse. We develop long-term, trusting relationships and work with individuals to change behaviors and maximize life potential.
Increasing awareness of stigma, discrimination, and psychiatric factors involved with the HIV pandemic can lead to decreased transmission of HIV infection and early diagnosis and treatment. Compassionate medical and psychiatric care can mitigate suffering in persons at risk for, infected with, or affected by HIV.
Preventing HIV transmission
AIDS differs from other complex, severe illnesses in 2 ways that are relevant to psychiatrists:
• it is almost entirely preventable
• HIV and AIDS are associated with sex, drugs, and AIDS-associated stigma and discrimination (“AIDSism”).4-6
Unsafe exposure of mucosal surfaces to the virus—primarily from exchanging body fluids in unprotected sexual encounters—accounts for 80% of new HIV infections.7 HIV transmission via sexual encounters is preventable with condoms. Percutaneous or intravenous infection with HIV—primarily from sharing needles in injection drug use—accounts for 20% of new infections.7 Use of alcohol or other substances can lead to sexual coercion, unprotected sex, and exchange of sex for drugs or money. Hence, treating substance use disorders can prevent HIV transmission.
Early diagnosis of HIV can lead to appropriate medical care, quicker onset of antiretroviral (ARV) treatment, and better outcomes. Recent research has shown that pre-exposure prophylaxis with ARV treatment can prevent transmission of HIV8; therefore, becoming aware of risk behaviors and prevention can be lifesaving for serodiscordant couples.
One of the most important ways to prevent HIV’s impact on the brain and CNS is to diagnose HIV shortly after transmission at onset of acute infection. If HIV is diagnosed very early—preferably as soon as possible after inoculation with HIV or at onset of the first flu-like symptoms—and treated with ARVs, the brain has less of an opportunity to act as an independent reservoir for HIV-infected cells and therefore to develop HIV-associated neurocognitive disorders.9,10Table 1 outlines steps psychiatrists can take to help prevent HIV transmission.
Psychiatric disorders and HIV
Psychiatric disorders and distress play a significant role in transmission of, exposure to, and infection with HIV (Table 2).4-6,11 They are relevant for prevention, clinical care, and adherence throughout every aspect of illness.
Comprehensive, compassionate, nonjudgmental care of persons at risk for or infected with HIV begins with a thorough psychiatric evaluation designed to provide an ego-supportive, sensitive, and comprehensive assessment that can guide other clinicians in providing care.12 Setting the tone and demonstrating compassion and respect includes shaking hands, which takes on special relevance in the context of AIDSism and stigma. Assessing the impact of HIV seropositivity or AIDS is best done by asking about the individual’s understanding of his or her diagnosis or illness and its impact. For some persons with HIV, verbalizing this understanding can be relieving as well as revealing. It is a chance for the patient to reveal painful experiences encountered in the home, school, camp, workplace, or community and the anguish of AIDSism and stigma.
Pay attention to sensitive and sometimes painful issues related to sexual history and sexuality. Questions related to sexual history and sexuality in heterosexual men and women as well as gay, lesbian, bisexual, and transgender individuals—such as “What is your sexual function like since you have been ill?” “Do feelings about your sexual identity play a role in your current level of distress?” and “What kind of barrier contraception are you using?”—are included in the comprehensive assessment described by Cohen et al.12
Comprehensive psychiatric evaluations can provide diagnoses, inform treatment, and mitigate anguish, distress, depression, anxiety, and substance use in persons with HIV and AIDS.12 A thorough and comprehensive assessment is crucial because HIV has an affinity for brain and neural tissue and can cause CNS complications such as HIV-associated neurocognitive disorders (HAND), even in otherwise healthy HIV-seropositive individuals. See this article at CurrentPsychiatry.com for a discussion of HAND and delirium in patients with HIV.
Some persons with HIV and AIDS do not have a psychiatric disorder, while others have multiple complex psychiatric disorders that are responses to illness or treatments or are associated with HIV/AIDS (such as HAND) or other medical illnesses and treatments (such as hepatitis C, cirrhosis, end-stage liver disease, HIV nephropathy, end-stage renal disease, anemia, coronary artery disease, and cancer). See this article at CurrentPsychiatry.com for case studies of HIV patients with delirium, depression, posttraumatic stress disorder (PTSD), and substance dependence.
Mood disorders. Depression is common among persons with HIV. Demoralization and bereavement may masquerade as depression and can complicate diagnosis and treatment. Depression and other mood disorders may be related to stigma and AIDSism as well as to biologic, psychological, social, and genetic factors. Because suicide is prevalent among persons with HIV and AIDS,13 every patient with HIV should be evaluated for depression and suicidal ideation.
PTSD is prevalent among persons with HIV. It is a risky diagnosis because it is associated with a sense of a foreshortened future, which leads to a lack of adequate self-care, poor adherence to medical care, risky behaviors, and comorbid substance dependence to help numb the pain of trauma.14,15 Persons with PTSD may have difficulty trusting clinicians and other authority figures if their trauma was a high-betrayal trauma, such as incest or military trauma.14,15
In patients with HIV, PTSD often is overlooked because it may be overshadowed by other psychiatric diagnoses. Intimate partner violence, history of childhood trauma, and childhood sexual abuse are risk factors for HIV infection and PTSD. Increased severity of HIV-related PTSD symptoms is associated with having a greater number of HIV-related physical symptoms, history of pre-HIV trauma, decreased social support, increased perception of stigma, and negative life events.
PTSD also is associated with nonadherence to risk reduction strategies and medical care.14,15 Diagnosis is further complicated by repression or retrograde amnesia of traumatic events and difficulties forming trusting relationships and disclosing HIV status to sexual partners or potential sexual partners because of fear of rejection.
Substance use disorders. Dependence on alcohol and other drugs complicates and perpetuates the HIV pandemic. Sharing needles and other drug paraphernalia is instrumental in HIV transmission. The indirect effects of alcohol and substance abuse include:
• the impact of intimate partner violence, child abuse, neglect, and/or abandonment
• development of PTSD in adults, with early childhood trauma leading to repeating their own history
• lack of self-care
• unhealthy partner choices
• use of drugs and alcohol to numb the pain associated with trauma.
Persons who are using alcohol or other drugs may have difficulty attending to their health, and substance dependence may prevent persons at risk from seeking HIV testing.
Intoxication from alcohol and drug use frequently leads to inappropriate partner choice, violent and coercive sexual behaviors, and lack of condom use. Substance dependence also may lead individuals to exchange sex for drugs and to fail to adhere to safer sexual practices or use sterile drug paraphernalia.
Treating persons with HIV/AIDS
Several organizations publish evidence-based clinical guidelines for treating depression, anxiety, substance abuse, and other psychiatric disorders in patients with HIV/AIDS. One such set of guidelines is available from the New York State Department of Health AIDS Institute at www.hivguidelines.org. As is the case with patients who do not have HIV, psychotherapy and pharmacotherapy are common first-line treatments.
Psychotherapy. Patients with HIV/AIDS with psychiatric comorbidities generally respond well to psychotherapeutic treatments.16,17 The choice of therapy needs to be tailored to the needs of individuals, couples, and families coping with AIDS. Options include:
• individual, couple, family, and group psychotherapy
• crisis intervention
• 12-step programs (Alcohol Anonymous, Narcotics Anonymous, etc.)
• adult survivors of child abuse programs (www.ascasupport.org), groups, and workbooks
• palliative psychiatry
• bereavement therapy
• spiritual support
• relaxation response
• wellness interventions such as exercise, yoga, keeping a journal, writing a life narrative, reading, artwork, movement therapy, listening to music or books on tape, and working on crossword puzzles and jigsaw puzzles.
Psychopharmacotherapy. Accurate diagnosis and awareness of drug-drug and drug-illness interactions are important when treating patients with HIV/AIDS; consult resources in the literature18 and online resources that are updated regularly (see Related Resources). Because persons with AIDS are particularly vulnerable to extrapyramidal and anticholinergic side effects of psychotropics, the principle start very low and go very slow is critical. For patients who are opioid-dependent, be cautious when prescribing medications that are cytochrome P450 3A4 inducers—such as carbamazepine, efavirenz, nevirapine, and ritonavir—because these medications can lower methadone levels in persons receiving agonist treatment and might lead to opioid withdrawal symptoms, discontinuation of ARVs, or relapse to opioids.18 When a person with AIDS is experiencing pain and is on a maintenance dose of methadone for heroin withdrawal, pain should be treated as a separate problem with additional opioids. Methadone for relapse prevention will target opioid tolerance needs and prevent withdrawal but will not provide analgesia for pain.
HIV through the life cycle
From prevention of prenatal transmission to the care of children with HIV to reproductive issues in serodiscordant couples, HIV complicates patients’ development. Table 3 outlines concerns regarding HIV transmission and treatment at different stages of a patient’s life.
Bottom Line
HIV transmission and effective treatment are complicated by a high prevalence of psychiatric comorbidities, including depression and other mood disorders, posttraumatic stress disorder, substance use disorders, and cognitive disorders. With an increased understanding of the issues faced by patients at risk for or infected with HIV, psychiatrists can help prevent HIV transmission, improve adherence to medical care, and diminish suffering, morbidity, and mortality.
Related Resources
- Academy of Psychosomatic Medicine HIV/AIDS Psychiatry Special Interest Group. www.apm.org/sigs/oap.
- New York State Department of Health AIDS Institute. HIV Clinical Resource. www.hivguidelines.org.
- University of Liverpool. HIV drug interactions list. www.hiv-druginteractions.org.
- Toronto General Hospital Immunodeficiency Clinic. Drug interactions tables. www.hivclinic.ca/main/drugs_interact.html.
Drug Brand Names
Bupropion • Wellbutrin, Zyban
Nevirapine • Viramune
Carbamazepine • Carbatrol, Tegretol, others
Olanzapine • Zyprexa
Quetiapine • Seroquel
Clonazepam • Klonopin
Ritonavir • Norvir
Efavirenz • Sustiva
Venlafaxine • Effexor
Escitalopram • Lexapro
Disclosure
Dr. Cohen reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
References
1. Blank MB, Mandell DS, Aiken L, et al. Co-occurrence of HIV and serious mental illness among Medicaid recipients. Psychiatr Serv. 2002;53(7):868-873.
2.Moyer VA, on behalf of the U.S. Preventive Services Task Force. Screening for HIV: U.S. Preventive Services Task Force recommendation statement [published online April 30, 2013]. Ann Intern Med. doi:10.7326/0003-4819-159-1-201307020-00645.
3. Hall HI, Song R, Rhodes P, et al. Estimation of HIV incidence in the United States. JAMA. 2008;300(5):520-529.
4. Cohen MA. AIDSism, a new form of discrimination. Am Med News. 1989;32:43.
5. Cohen MA, Gorman JM. Comprehensive textbook of AIDS psychiatry. New York, NY: Oxford University Press; 2008.
6. Cohen MA, Goforth HW, Lux JZ, et al, eds. Handbook of AIDS psychiatry. New York, NY: Oxford University Press; 2010.
7. World Health Organization, United Nations Children’s Fund, Joint United Nations Programme on HIV/AIDS. Global HIV/AIDS response. Epidemic update and health sector progress towards universal access. Progress report 2011. http://www.unaids.org/en/media/unaids/
contentassets/documents/unaidspublication/2011/
20111130_UA_Report_en.pdf. Accessed April 25, 2013.
8. Centers for Disease Control and Prevention (CDC). Interim guidance for clinicians considering the use of preexposure prophylaxis for the prevention of HIV infection in heterosexually active adults. MMWR Morb Mortal Wkly Rep. 2012;61(31):586-589.
9. Cysique LA, Murray JM, Dunbar M, et al. A screening algorithm for HIV-associated neurocognitive disorders. HIV Med. 2010;11(10):642-649.
10. Simioni S, Cavassini M, Annoni JM, et al. Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS. 2010;24(9):1243-1250.
11. Cohen M, Hoffman RG, Cromwell C, et al. The prevalence of distress in persons with human immunodeficiency virus infection. Psychosomatics. 2002;43(1):10-15.
12. Cohen MA, Batista SM, Lux JZ. A biopsychosocial approach to psychiatric consultation in persons with HIV and AIDS. In: Cohen MA, Goforth HW, Lux JZ, et al, eds. Handbook of AIDS psychiatry. New York, NY: Oxford University Press; 2010:33-60.
13. Carrico AW. Elevated suicide rate among HIV-positive persons despite benefits of antiretroviral therapy: implications for a stress and coping model of suicide. Am J Psychiatry. 2010;167(2):117-119.
14. Cohen MA, Alfonso CA, Hoffman RG, et al. The impact of PTSD on treatment adherence in persons with HIV infection. Gen Hosp Psychiatry. 2001;23(5):294-296.
15. Boarts JM, Sledjeski EM, Bogart LM, et al. The differential impact of PTSD and depression on HIV disease markers and adherence to HAART in people living with HIV. AIDS Behav. 2006;10(3):253-261.
16. Sikkema KJ, Hansen NB, Ghebremichael M, et al. A randomized controlled trial of a coping group intervention for adults with HIV who are AIDS bereaved: longitudinal effects on grief. Health Psychol. 2006;25(5):563-570.
17. Cohen MA. Psychodynamic psychotherapy in an AIDS nursing home. J Am Acad Psychoanal. 1999;27(1):121-133.
18. Cozza KL, Goforth HW, Batista SM. Psychopharmacologic treatment issues in AIDS psychiatry. In: Cohen MA, Goforth HW, Lux JZ, et al, eds. Handbook of AIDS psychiatry. New York, NY: Oxford University Press; 2010:147-199.
The prevalence of HIV in persons with untreated psychiatric illness may be 10 to 20 times that of the general population.1 The U.S. Preventive Services Task Force has recommended HIV screening of all persons age 15 to 65 because 20% to 25% of individuals with HIV infection are unaware that they are HIV-positive.2 Because >20% of new HIV infections in the United States are undiagnosed,3 it is crucial to educate patients with mental illness about HIV prevention, make condoms available, and offer HIV testing.
As psychiatrists, we have a unique role in caring for patients at risk for or infected with HIV because in addition to comprehensive medical and psychiatric histories, we routinely take histories of substance use, sexual activities, relationships, and trauma, including childhood neglect and emotional, physical, and sexual abuse. We develop long-term, trusting relationships and work with individuals to change behaviors and maximize life potential.
Increasing awareness of stigma, discrimination, and psychiatric factors involved with the HIV pandemic can lead to decreased transmission of HIV infection and early diagnosis and treatment. Compassionate medical and psychiatric care can mitigate suffering in persons at risk for, infected with, or affected by HIV.
Preventing HIV transmission
AIDS differs from other complex, severe illnesses in 2 ways that are relevant to psychiatrists:
• it is almost entirely preventable
• HIV and AIDS are associated with sex, drugs, and AIDS-associated stigma and discrimination (“AIDSism”).4-6
Unsafe exposure of mucosal surfaces to the virus—primarily from exchanging body fluids in unprotected sexual encounters—accounts for 80% of new HIV infections.7 HIV transmission via sexual encounters is preventable with condoms. Percutaneous or intravenous infection with HIV—primarily from sharing needles in injection drug use—accounts for 20% of new infections.7 Use of alcohol or other substances can lead to sexual coercion, unprotected sex, and exchange of sex for drugs or money. Hence, treating substance use disorders can prevent HIV transmission.
Early diagnosis of HIV can lead to appropriate medical care, quicker onset of antiretroviral (ARV) treatment, and better outcomes. Recent research has shown that pre-exposure prophylaxis with ARV treatment can prevent transmission of HIV8; therefore, becoming aware of risk behaviors and prevention can be lifesaving for serodiscordant couples.
One of the most important ways to prevent HIV’s impact on the brain and CNS is to diagnose HIV shortly after transmission at onset of acute infection. If HIV is diagnosed very early—preferably as soon as possible after inoculation with HIV or at onset of the first flu-like symptoms—and treated with ARVs, the brain has less of an opportunity to act as an independent reservoir for HIV-infected cells and therefore to develop HIV-associated neurocognitive disorders.9,10Table 1 outlines steps psychiatrists can take to help prevent HIV transmission.
Psychiatric disorders and HIV
Psychiatric disorders and distress play a significant role in transmission of, exposure to, and infection with HIV (Table 2).4-6,11 They are relevant for prevention, clinical care, and adherence throughout every aspect of illness.
Comprehensive, compassionate, nonjudgmental care of persons at risk for or infected with HIV begins with a thorough psychiatric evaluation designed to provide an ego-supportive, sensitive, and comprehensive assessment that can guide other clinicians in providing care.12 Setting the tone and demonstrating compassion and respect includes shaking hands, which takes on special relevance in the context of AIDSism and stigma. Assessing the impact of HIV seropositivity or AIDS is best done by asking about the individual’s understanding of his or her diagnosis or illness and its impact. For some persons with HIV, verbalizing this understanding can be relieving as well as revealing. It is a chance for the patient to reveal painful experiences encountered in the home, school, camp, workplace, or community and the anguish of AIDSism and stigma.
Pay attention to sensitive and sometimes painful issues related to sexual history and sexuality. Questions related to sexual history and sexuality in heterosexual men and women as well as gay, lesbian, bisexual, and transgender individuals—such as “What is your sexual function like since you have been ill?” “Do feelings about your sexual identity play a role in your current level of distress?” and “What kind of barrier contraception are you using?”—are included in the comprehensive assessment described by Cohen et al.12
Comprehensive psychiatric evaluations can provide diagnoses, inform treatment, and mitigate anguish, distress, depression, anxiety, and substance use in persons with HIV and AIDS.12 A thorough and comprehensive assessment is crucial because HIV has an affinity for brain and neural tissue and can cause CNS complications such as HIV-associated neurocognitive disorders (HAND), even in otherwise healthy HIV-seropositive individuals. See this article at CurrentPsychiatry.com for a discussion of HAND and delirium in patients with HIV.
Some persons with HIV and AIDS do not have a psychiatric disorder, while others have multiple complex psychiatric disorders that are responses to illness or treatments or are associated with HIV/AIDS (such as HAND) or other medical illnesses and treatments (such as hepatitis C, cirrhosis, end-stage liver disease, HIV nephropathy, end-stage renal disease, anemia, coronary artery disease, and cancer). See this article at CurrentPsychiatry.com for case studies of HIV patients with delirium, depression, posttraumatic stress disorder (PTSD), and substance dependence.
Mood disorders. Depression is common among persons with HIV. Demoralization and bereavement may masquerade as depression and can complicate diagnosis and treatment. Depression and other mood disorders may be related to stigma and AIDSism as well as to biologic, psychological, social, and genetic factors. Because suicide is prevalent among persons with HIV and AIDS,13 every patient with HIV should be evaluated for depression and suicidal ideation.
PTSD is prevalent among persons with HIV. It is a risky diagnosis because it is associated with a sense of a foreshortened future, which leads to a lack of adequate self-care, poor adherence to medical care, risky behaviors, and comorbid substance dependence to help numb the pain of trauma.14,15 Persons with PTSD may have difficulty trusting clinicians and other authority figures if their trauma was a high-betrayal trauma, such as incest or military trauma.14,15
In patients with HIV, PTSD often is overlooked because it may be overshadowed by other psychiatric diagnoses. Intimate partner violence, history of childhood trauma, and childhood sexual abuse are risk factors for HIV infection and PTSD. Increased severity of HIV-related PTSD symptoms is associated with having a greater number of HIV-related physical symptoms, history of pre-HIV trauma, decreased social support, increased perception of stigma, and negative life events.
PTSD also is associated with nonadherence to risk reduction strategies and medical care.14,15 Diagnosis is further complicated by repression or retrograde amnesia of traumatic events and difficulties forming trusting relationships and disclosing HIV status to sexual partners or potential sexual partners because of fear of rejection.
Substance use disorders. Dependence on alcohol and other drugs complicates and perpetuates the HIV pandemic. Sharing needles and other drug paraphernalia is instrumental in HIV transmission. The indirect effects of alcohol and substance abuse include:
• the impact of intimate partner violence, child abuse, neglect, and/or abandonment
• development of PTSD in adults, with early childhood trauma leading to repeating their own history
• lack of self-care
• unhealthy partner choices
• use of drugs and alcohol to numb the pain associated with trauma.
Persons who are using alcohol or other drugs may have difficulty attending to their health, and substance dependence may prevent persons at risk from seeking HIV testing.
Intoxication from alcohol and drug use frequently leads to inappropriate partner choice, violent and coercive sexual behaviors, and lack of condom use. Substance dependence also may lead individuals to exchange sex for drugs and to fail to adhere to safer sexual practices or use sterile drug paraphernalia.
Treating persons with HIV/AIDS
Several organizations publish evidence-based clinical guidelines for treating depression, anxiety, substance abuse, and other psychiatric disorders in patients with HIV/AIDS. One such set of guidelines is available from the New York State Department of Health AIDS Institute at www.hivguidelines.org. As is the case with patients who do not have HIV, psychotherapy and pharmacotherapy are common first-line treatments.
Psychotherapy. Patients with HIV/AIDS with psychiatric comorbidities generally respond well to psychotherapeutic treatments.16,17 The choice of therapy needs to be tailored to the needs of individuals, couples, and families coping with AIDS. Options include:
• individual, couple, family, and group psychotherapy
• crisis intervention
• 12-step programs (Alcohol Anonymous, Narcotics Anonymous, etc.)
• adult survivors of child abuse programs (www.ascasupport.org), groups, and workbooks
• palliative psychiatry
• bereavement therapy
• spiritual support
• relaxation response
• wellness interventions such as exercise, yoga, keeping a journal, writing a life narrative, reading, artwork, movement therapy, listening to music or books on tape, and working on crossword puzzles and jigsaw puzzles.
Psychopharmacotherapy. Accurate diagnosis and awareness of drug-drug and drug-illness interactions are important when treating patients with HIV/AIDS; consult resources in the literature18 and online resources that are updated regularly (see Related Resources). Because persons with AIDS are particularly vulnerable to extrapyramidal and anticholinergic side effects of psychotropics, the principle start very low and go very slow is critical. For patients who are opioid-dependent, be cautious when prescribing medications that are cytochrome P450 3A4 inducers—such as carbamazepine, efavirenz, nevirapine, and ritonavir—because these medications can lower methadone levels in persons receiving agonist treatment and might lead to opioid withdrawal symptoms, discontinuation of ARVs, or relapse to opioids.18 When a person with AIDS is experiencing pain and is on a maintenance dose of methadone for heroin withdrawal, pain should be treated as a separate problem with additional opioids. Methadone for relapse prevention will target opioid tolerance needs and prevent withdrawal but will not provide analgesia for pain.
HIV through the life cycle
From prevention of prenatal transmission to the care of children with HIV to reproductive issues in serodiscordant couples, HIV complicates patients’ development. Table 3 outlines concerns regarding HIV transmission and treatment at different stages of a patient’s life.
Bottom Line
HIV transmission and effective treatment are complicated by a high prevalence of psychiatric comorbidities, including depression and other mood disorders, posttraumatic stress disorder, substance use disorders, and cognitive disorders. With an increased understanding of the issues faced by patients at risk for or infected with HIV, psychiatrists can help prevent HIV transmission, improve adherence to medical care, and diminish suffering, morbidity, and mortality.
Related Resources
- Academy of Psychosomatic Medicine HIV/AIDS Psychiatry Special Interest Group. www.apm.org/sigs/oap.
- New York State Department of Health AIDS Institute. HIV Clinical Resource. www.hivguidelines.org.
- University of Liverpool. HIV drug interactions list. www.hiv-druginteractions.org.
- Toronto General Hospital Immunodeficiency Clinic. Drug interactions tables. www.hivclinic.ca/main/drugs_interact.html.
Drug Brand Names
Bupropion • Wellbutrin, Zyban
Nevirapine • Viramune
Carbamazepine • Carbatrol, Tegretol, others
Olanzapine • Zyprexa
Quetiapine • Seroquel
Clonazepam • Klonopin
Ritonavir • Norvir
Efavirenz • Sustiva
Venlafaxine • Effexor
Escitalopram • Lexapro
Disclosure
Dr. Cohen reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
References
1. Blank MB, Mandell DS, Aiken L, et al. Co-occurrence of HIV and serious mental illness among Medicaid recipients. Psychiatr Serv. 2002;53(7):868-873.
2.Moyer VA, on behalf of the U.S. Preventive Services Task Force. Screening for HIV: U.S. Preventive Services Task Force recommendation statement [published online April 30, 2013]. Ann Intern Med. doi:10.7326/0003-4819-159-1-201307020-00645.
3. Hall HI, Song R, Rhodes P, et al. Estimation of HIV incidence in the United States. JAMA. 2008;300(5):520-529.
4. Cohen MA. AIDSism, a new form of discrimination. Am Med News. 1989;32:43.
5. Cohen MA, Gorman JM. Comprehensive textbook of AIDS psychiatry. New York, NY: Oxford University Press; 2008.
6. Cohen MA, Goforth HW, Lux JZ, et al, eds. Handbook of AIDS psychiatry. New York, NY: Oxford University Press; 2010.
7. World Health Organization, United Nations Children’s Fund, Joint United Nations Programme on HIV/AIDS. Global HIV/AIDS response. Epidemic update and health sector progress towards universal access. Progress report 2011. http://www.unaids.org/en/media/unaids/
contentassets/documents/unaidspublication/2011/
20111130_UA_Report_en.pdf. Accessed April 25, 2013.
8. Centers for Disease Control and Prevention (CDC). Interim guidance for clinicians considering the use of preexposure prophylaxis for the prevention of HIV infection in heterosexually active adults. MMWR Morb Mortal Wkly Rep. 2012;61(31):586-589.
9. Cysique LA, Murray JM, Dunbar M, et al. A screening algorithm for HIV-associated neurocognitive disorders. HIV Med. 2010;11(10):642-649.
10. Simioni S, Cavassini M, Annoni JM, et al. Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS. 2010;24(9):1243-1250.
11. Cohen M, Hoffman RG, Cromwell C, et al. The prevalence of distress in persons with human immunodeficiency virus infection. Psychosomatics. 2002;43(1):10-15.
12. Cohen MA, Batista SM, Lux JZ. A biopsychosocial approach to psychiatric consultation in persons with HIV and AIDS. In: Cohen MA, Goforth HW, Lux JZ, et al, eds. Handbook of AIDS psychiatry. New York, NY: Oxford University Press; 2010:33-60.
13. Carrico AW. Elevated suicide rate among HIV-positive persons despite benefits of antiretroviral therapy: implications for a stress and coping model of suicide. Am J Psychiatry. 2010;167(2):117-119.
14. Cohen MA, Alfonso CA, Hoffman RG, et al. The impact of PTSD on treatment adherence in persons with HIV infection. Gen Hosp Psychiatry. 2001;23(5):294-296.
15. Boarts JM, Sledjeski EM, Bogart LM, et al. The differential impact of PTSD and depression on HIV disease markers and adherence to HAART in people living with HIV. AIDS Behav. 2006;10(3):253-261.
16. Sikkema KJ, Hansen NB, Ghebremichael M, et al. A randomized controlled trial of a coping group intervention for adults with HIV who are AIDS bereaved: longitudinal effects on grief. Health Psychol. 2006;25(5):563-570.
17. Cohen MA. Psychodynamic psychotherapy in an AIDS nursing home. J Am Acad Psychoanal. 1999;27(1):121-133.
18. Cozza KL, Goforth HW, Batista SM. Psychopharmacologic treatment issues in AIDS psychiatry. In: Cohen MA, Goforth HW, Lux JZ, et al, eds. Handbook of AIDS psychiatry. New York, NY: Oxford University Press; 2010:147-199.
The prevalence of HIV in persons with untreated psychiatric illness may be 10 to 20 times that of the general population.1 The U.S. Preventive Services Task Force has recommended HIV screening of all persons age 15 to 65 because 20% to 25% of individuals with HIV infection are unaware that they are HIV-positive.2 Because >20% of new HIV infections in the United States are undiagnosed,3 it is crucial to educate patients with mental illness about HIV prevention, make condoms available, and offer HIV testing.
As psychiatrists, we have a unique role in caring for patients at risk for or infected with HIV because in addition to comprehensive medical and psychiatric histories, we routinely take histories of substance use, sexual activities, relationships, and trauma, including childhood neglect and emotional, physical, and sexual abuse. We develop long-term, trusting relationships and work with individuals to change behaviors and maximize life potential.
Increasing awareness of stigma, discrimination, and psychiatric factors involved with the HIV pandemic can lead to decreased transmission of HIV infection and early diagnosis and treatment. Compassionate medical and psychiatric care can mitigate suffering in persons at risk for, infected with, or affected by HIV.
Preventing HIV transmission
AIDS differs from other complex, severe illnesses in 2 ways that are relevant to psychiatrists:
• it is almost entirely preventable
• HIV and AIDS are associated with sex, drugs, and AIDS-associated stigma and discrimination (“AIDSism”).4-6
Unsafe exposure of mucosal surfaces to the virus—primarily from exchanging body fluids in unprotected sexual encounters—accounts for 80% of new HIV infections.7 HIV transmission via sexual encounters is preventable with condoms. Percutaneous or intravenous infection with HIV—primarily from sharing needles in injection drug use—accounts for 20% of new infections.7 Use of alcohol or other substances can lead to sexual coercion, unprotected sex, and exchange of sex for drugs or money. Hence, treating substance use disorders can prevent HIV transmission.
Early diagnosis of HIV can lead to appropriate medical care, quicker onset of antiretroviral (ARV) treatment, and better outcomes. Recent research has shown that pre-exposure prophylaxis with ARV treatment can prevent transmission of HIV8; therefore, becoming aware of risk behaviors and prevention can be lifesaving for serodiscordant couples.
One of the most important ways to prevent HIV’s impact on the brain and CNS is to diagnose HIV shortly after transmission at onset of acute infection. If HIV is diagnosed very early—preferably as soon as possible after inoculation with HIV or at onset of the first flu-like symptoms—and treated with ARVs, the brain has less of an opportunity to act as an independent reservoir for HIV-infected cells and therefore to develop HIV-associated neurocognitive disorders.9,10Table 1 outlines steps psychiatrists can take to help prevent HIV transmission.
Psychiatric disorders and HIV
Psychiatric disorders and distress play a significant role in transmission of, exposure to, and infection with HIV (Table 2).4-6,11 They are relevant for prevention, clinical care, and adherence throughout every aspect of illness.
Comprehensive, compassionate, nonjudgmental care of persons at risk for or infected with HIV begins with a thorough psychiatric evaluation designed to provide an ego-supportive, sensitive, and comprehensive assessment that can guide other clinicians in providing care.12 Setting the tone and demonstrating compassion and respect includes shaking hands, which takes on special relevance in the context of AIDSism and stigma. Assessing the impact of HIV seropositivity or AIDS is best done by asking about the individual’s understanding of his or her diagnosis or illness and its impact. For some persons with HIV, verbalizing this understanding can be relieving as well as revealing. It is a chance for the patient to reveal painful experiences encountered in the home, school, camp, workplace, or community and the anguish of AIDSism and stigma.
Pay attention to sensitive and sometimes painful issues related to sexual history and sexuality. Questions related to sexual history and sexuality in heterosexual men and women as well as gay, lesbian, bisexual, and transgender individuals—such as “What is your sexual function like since you have been ill?” “Do feelings about your sexual identity play a role in your current level of distress?” and “What kind of barrier contraception are you using?”—are included in the comprehensive assessment described by Cohen et al.12
Comprehensive psychiatric evaluations can provide diagnoses, inform treatment, and mitigate anguish, distress, depression, anxiety, and substance use in persons with HIV and AIDS.12 A thorough and comprehensive assessment is crucial because HIV has an affinity for brain and neural tissue and can cause CNS complications such as HIV-associated neurocognitive disorders (HAND), even in otherwise healthy HIV-seropositive individuals. See this article at CurrentPsychiatry.com for a discussion of HAND and delirium in patients with HIV.
Some persons with HIV and AIDS do not have a psychiatric disorder, while others have multiple complex psychiatric disorders that are responses to illness or treatments or are associated with HIV/AIDS (such as HAND) or other medical illnesses and treatments (such as hepatitis C, cirrhosis, end-stage liver disease, HIV nephropathy, end-stage renal disease, anemia, coronary artery disease, and cancer). See this article at CurrentPsychiatry.com for case studies of HIV patients with delirium, depression, posttraumatic stress disorder (PTSD), and substance dependence.
Mood disorders. Depression is common among persons with HIV. Demoralization and bereavement may masquerade as depression and can complicate diagnosis and treatment. Depression and other mood disorders may be related to stigma and AIDSism as well as to biologic, psychological, social, and genetic factors. Because suicide is prevalent among persons with HIV and AIDS,13 every patient with HIV should be evaluated for depression and suicidal ideation.
PTSD is prevalent among persons with HIV. It is a risky diagnosis because it is associated with a sense of a foreshortened future, which leads to a lack of adequate self-care, poor adherence to medical care, risky behaviors, and comorbid substance dependence to help numb the pain of trauma.14,15 Persons with PTSD may have difficulty trusting clinicians and other authority figures if their trauma was a high-betrayal trauma, such as incest or military trauma.14,15
In patients with HIV, PTSD often is overlooked because it may be overshadowed by other psychiatric diagnoses. Intimate partner violence, history of childhood trauma, and childhood sexual abuse are risk factors for HIV infection and PTSD. Increased severity of HIV-related PTSD symptoms is associated with having a greater number of HIV-related physical symptoms, history of pre-HIV trauma, decreased social support, increased perception of stigma, and negative life events.
PTSD also is associated with nonadherence to risk reduction strategies and medical care.14,15 Diagnosis is further complicated by repression or retrograde amnesia of traumatic events and difficulties forming trusting relationships and disclosing HIV status to sexual partners or potential sexual partners because of fear of rejection.
Substance use disorders. Dependence on alcohol and other drugs complicates and perpetuates the HIV pandemic. Sharing needles and other drug paraphernalia is instrumental in HIV transmission. The indirect effects of alcohol and substance abuse include:
• the impact of intimate partner violence, child abuse, neglect, and/or abandonment
• development of PTSD in adults, with early childhood trauma leading to repeating their own history
• lack of self-care
• unhealthy partner choices
• use of drugs and alcohol to numb the pain associated with trauma.
Persons who are using alcohol or other drugs may have difficulty attending to their health, and substance dependence may prevent persons at risk from seeking HIV testing.
Intoxication from alcohol and drug use frequently leads to inappropriate partner choice, violent and coercive sexual behaviors, and lack of condom use. Substance dependence also may lead individuals to exchange sex for drugs and to fail to adhere to safer sexual practices or use sterile drug paraphernalia.
Treating persons with HIV/AIDS
Several organizations publish evidence-based clinical guidelines for treating depression, anxiety, substance abuse, and other psychiatric disorders in patients with HIV/AIDS. One such set of guidelines is available from the New York State Department of Health AIDS Institute at www.hivguidelines.org. As is the case with patients who do not have HIV, psychotherapy and pharmacotherapy are common first-line treatments.
Psychotherapy. Patients with HIV/AIDS with psychiatric comorbidities generally respond well to psychotherapeutic treatments.16,17 The choice of therapy needs to be tailored to the needs of individuals, couples, and families coping with AIDS. Options include:
• individual, couple, family, and group psychotherapy
• crisis intervention
• 12-step programs (Alcohol Anonymous, Narcotics Anonymous, etc.)
• adult survivors of child abuse programs (www.ascasupport.org), groups, and workbooks
• palliative psychiatry
• bereavement therapy
• spiritual support
• relaxation response
• wellness interventions such as exercise, yoga, keeping a journal, writing a life narrative, reading, artwork, movement therapy, listening to music or books on tape, and working on crossword puzzles and jigsaw puzzles.
Psychopharmacotherapy. Accurate diagnosis and awareness of drug-drug and drug-illness interactions are important when treating patients with HIV/AIDS; consult resources in the literature18 and online resources that are updated regularly (see Related Resources). Because persons with AIDS are particularly vulnerable to extrapyramidal and anticholinergic side effects of psychotropics, the principle start very low and go very slow is critical. For patients who are opioid-dependent, be cautious when prescribing medications that are cytochrome P450 3A4 inducers—such as carbamazepine, efavirenz, nevirapine, and ritonavir—because these medications can lower methadone levels in persons receiving agonist treatment and might lead to opioid withdrawal symptoms, discontinuation of ARVs, or relapse to opioids.18 When a person with AIDS is experiencing pain and is on a maintenance dose of methadone for heroin withdrawal, pain should be treated as a separate problem with additional opioids. Methadone for relapse prevention will target opioid tolerance needs and prevent withdrawal but will not provide analgesia for pain.
HIV through the life cycle
From prevention of prenatal transmission to the care of children with HIV to reproductive issues in serodiscordant couples, HIV complicates patients’ development. Table 3 outlines concerns regarding HIV transmission and treatment at different stages of a patient’s life.
Bottom Line
HIV transmission and effective treatment are complicated by a high prevalence of psychiatric comorbidities, including depression and other mood disorders, posttraumatic stress disorder, substance use disorders, and cognitive disorders. With an increased understanding of the issues faced by patients at risk for or infected with HIV, psychiatrists can help prevent HIV transmission, improve adherence to medical care, and diminish suffering, morbidity, and mortality.
Related Resources
- Academy of Psychosomatic Medicine HIV/AIDS Psychiatry Special Interest Group. www.apm.org/sigs/oap.
- New York State Department of Health AIDS Institute. HIV Clinical Resource. www.hivguidelines.org.
- University of Liverpool. HIV drug interactions list. www.hiv-druginteractions.org.
- Toronto General Hospital Immunodeficiency Clinic. Drug interactions tables. www.hivclinic.ca/main/drugs_interact.html.
Drug Brand Names
Bupropion • Wellbutrin, Zyban
Nevirapine • Viramune
Carbamazepine • Carbatrol, Tegretol, others
Olanzapine • Zyprexa
Quetiapine • Seroquel
Clonazepam • Klonopin
Ritonavir • Norvir
Efavirenz • Sustiva
Venlafaxine • Effexor
Escitalopram • Lexapro
Disclosure
Dr. Cohen reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
References
1. Blank MB, Mandell DS, Aiken L, et al. Co-occurrence of HIV and serious mental illness among Medicaid recipients. Psychiatr Serv. 2002;53(7):868-873.
2.Moyer VA, on behalf of the U.S. Preventive Services Task Force. Screening for HIV: U.S. Preventive Services Task Force recommendation statement [published online April 30, 2013]. Ann Intern Med. doi:10.7326/0003-4819-159-1-201307020-00645.
3. Hall HI, Song R, Rhodes P, et al. Estimation of HIV incidence in the United States. JAMA. 2008;300(5):520-529.
4. Cohen MA. AIDSism, a new form of discrimination. Am Med News. 1989;32:43.
5. Cohen MA, Gorman JM. Comprehensive textbook of AIDS psychiatry. New York, NY: Oxford University Press; 2008.
6. Cohen MA, Goforth HW, Lux JZ, et al, eds. Handbook of AIDS psychiatry. New York, NY: Oxford University Press; 2010.
7. World Health Organization, United Nations Children’s Fund, Joint United Nations Programme on HIV/AIDS. Global HIV/AIDS response. Epidemic update and health sector progress towards universal access. Progress report 2011. http://www.unaids.org/en/media/unaids/
contentassets/documents/unaidspublication/2011/
20111130_UA_Report_en.pdf. Accessed April 25, 2013.
8. Centers for Disease Control and Prevention (CDC). Interim guidance for clinicians considering the use of preexposure prophylaxis for the prevention of HIV infection in heterosexually active adults. MMWR Morb Mortal Wkly Rep. 2012;61(31):586-589.
9. Cysique LA, Murray JM, Dunbar M, et al. A screening algorithm for HIV-associated neurocognitive disorders. HIV Med. 2010;11(10):642-649.
10. Simioni S, Cavassini M, Annoni JM, et al. Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS. 2010;24(9):1243-1250.
11. Cohen M, Hoffman RG, Cromwell C, et al. The prevalence of distress in persons with human immunodeficiency virus infection. Psychosomatics. 2002;43(1):10-15.
12. Cohen MA, Batista SM, Lux JZ. A biopsychosocial approach to psychiatric consultation in persons with HIV and AIDS. In: Cohen MA, Goforth HW, Lux JZ, et al, eds. Handbook of AIDS psychiatry. New York, NY: Oxford University Press; 2010:33-60.
13. Carrico AW. Elevated suicide rate among HIV-positive persons despite benefits of antiretroviral therapy: implications for a stress and coping model of suicide. Am J Psychiatry. 2010;167(2):117-119.
14. Cohen MA, Alfonso CA, Hoffman RG, et al. The impact of PTSD on treatment adherence in persons with HIV infection. Gen Hosp Psychiatry. 2001;23(5):294-296.
15. Boarts JM, Sledjeski EM, Bogart LM, et al. The differential impact of PTSD and depression on HIV disease markers and adherence to HAART in people living with HIV. AIDS Behav. 2006;10(3):253-261.
16. Sikkema KJ, Hansen NB, Ghebremichael M, et al. A randomized controlled trial of a coping group intervention for adults with HIV who are AIDS bereaved: longitudinal effects on grief. Health Psychol. 2006;25(5):563-570.
17. Cohen MA. Psychodynamic psychotherapy in an AIDS nursing home. J Am Acad Psychoanal. 1999;27(1):121-133.
18. Cozza KL, Goforth HW, Batista SM. Psychopharmacologic treatment issues in AIDS psychiatry. In: Cohen MA, Goforth HW, Lux JZ, et al, eds. Handbook of AIDS psychiatry. New York, NY: Oxford University Press; 2010:147-199.
Antipsychotics linked to sudden cardiac death risk
DENVER – Both the second-generation as well as the first-generation antipsychotic agents proved independently associated with greater than threefold increased risks of sudden cardiac death, according to results from a large, population-based study.
However, schizophrenia per se was not linked to an increased risk, Dr. Audrey Uy-Evanado reported at the annual meeting of the Heart Rhythm Society.
She presented data from the ongoing landmark Oregon Sudden Unexplained Death Study involving 1,544 documented sudden cardiac deaths (SCDs) and 774 matched controls.
The prevalence of diagnosed schizophrenia among patients with SCD was 2.5%, significantly greater than the 0.5% figure in controls. At the time of SCD, 8.2% of subjects were on an antipsychotic agent, compared with 1.9% of controls.
Second-generation, or "atypical," antipsychotic agents such as risperidone (Risperdal) and olanzapine (Zyprexa) were being used by 6.3% of the SCD cohort and 1.3% of controls. First-generation antipsychotic agents such as haloperidol, fluphenazine, and chlorpromazine were used by 2.6% of the SCD group, compared with 0.5% of controls. All of these between-group differences were highly significant.
Third-generation antipsychotic agents such as aripiprazole (Abilify) were so rarely used – only 0.1% of patients were on a third-generation agent – that it wasn’t possible to draw any conclusions about SCD risk, according to Dr. Uy-Evanado of Cedars-Sinai Medical Center in Los Angeles.
In terms of cardiovascular risk factors, hypertension and obesity were significantly more prevalent in the control group – but the SCD cohort had increased rates of diabetes, chronic kidney disease, and severe left ventricular dysfunction.
Aside from schizophrenia, rates of other psychiatric disorders didn’t differ between the two groups.
In a multivariate logistic regression analysis adjusted for age, gender, and comorbidities, having schizophrenia was not associated with an increased risk of SCD. However, patients on a first-generation antipsychotic agent had a 3.76-fold increased risk of SCD (P = .0002), and those on a second-generation antipsychotic drug had a 3.3-fold increased risk.
This study was undertaken as a follow-up to an earlier report from the Oregon Sudden Unexplained Death Study, which broadly identified antipsychotic drug therapy as being associated with SCD. That observation raised the question as to whether the risk is limited to specific classes of antipsychotic agents. The answer appears to be no.
The Oregon Sudden Unexplained Death Study is funded by Oregon Health and Science University and the Centers for Disease Control and Prevention. Dr. Uy-Evanado reported having no conflicts of interest.
DENVER – Both the second-generation as well as the first-generation antipsychotic agents proved independently associated with greater than threefold increased risks of sudden cardiac death, according to results from a large, population-based study.
However, schizophrenia per se was not linked to an increased risk, Dr. Audrey Uy-Evanado reported at the annual meeting of the Heart Rhythm Society.
She presented data from the ongoing landmark Oregon Sudden Unexplained Death Study involving 1,544 documented sudden cardiac deaths (SCDs) and 774 matched controls.
The prevalence of diagnosed schizophrenia among patients with SCD was 2.5%, significantly greater than the 0.5% figure in controls. At the time of SCD, 8.2% of subjects were on an antipsychotic agent, compared with 1.9% of controls.
Second-generation, or "atypical," antipsychotic agents such as risperidone (Risperdal) and olanzapine (Zyprexa) were being used by 6.3% of the SCD cohort and 1.3% of controls. First-generation antipsychotic agents such as haloperidol, fluphenazine, and chlorpromazine were used by 2.6% of the SCD group, compared with 0.5% of controls. All of these between-group differences were highly significant.
Third-generation antipsychotic agents such as aripiprazole (Abilify) were so rarely used – only 0.1% of patients were on a third-generation agent – that it wasn’t possible to draw any conclusions about SCD risk, according to Dr. Uy-Evanado of Cedars-Sinai Medical Center in Los Angeles.
In terms of cardiovascular risk factors, hypertension and obesity were significantly more prevalent in the control group – but the SCD cohort had increased rates of diabetes, chronic kidney disease, and severe left ventricular dysfunction.
Aside from schizophrenia, rates of other psychiatric disorders didn’t differ between the two groups.
In a multivariate logistic regression analysis adjusted for age, gender, and comorbidities, having schizophrenia was not associated with an increased risk of SCD. However, patients on a first-generation antipsychotic agent had a 3.76-fold increased risk of SCD (P = .0002), and those on a second-generation antipsychotic drug had a 3.3-fold increased risk.
This study was undertaken as a follow-up to an earlier report from the Oregon Sudden Unexplained Death Study, which broadly identified antipsychotic drug therapy as being associated with SCD. That observation raised the question as to whether the risk is limited to specific classes of antipsychotic agents. The answer appears to be no.
The Oregon Sudden Unexplained Death Study is funded by Oregon Health and Science University and the Centers for Disease Control and Prevention. Dr. Uy-Evanado reported having no conflicts of interest.
DENVER – Both the second-generation as well as the first-generation antipsychotic agents proved independently associated with greater than threefold increased risks of sudden cardiac death, according to results from a large, population-based study.
However, schizophrenia per se was not linked to an increased risk, Dr. Audrey Uy-Evanado reported at the annual meeting of the Heart Rhythm Society.
She presented data from the ongoing landmark Oregon Sudden Unexplained Death Study involving 1,544 documented sudden cardiac deaths (SCDs) and 774 matched controls.
The prevalence of diagnosed schizophrenia among patients with SCD was 2.5%, significantly greater than the 0.5% figure in controls. At the time of SCD, 8.2% of subjects were on an antipsychotic agent, compared with 1.9% of controls.
Second-generation, or "atypical," antipsychotic agents such as risperidone (Risperdal) and olanzapine (Zyprexa) were being used by 6.3% of the SCD cohort and 1.3% of controls. First-generation antipsychotic agents such as haloperidol, fluphenazine, and chlorpromazine were used by 2.6% of the SCD group, compared with 0.5% of controls. All of these between-group differences were highly significant.
Third-generation antipsychotic agents such as aripiprazole (Abilify) were so rarely used – only 0.1% of patients were on a third-generation agent – that it wasn’t possible to draw any conclusions about SCD risk, according to Dr. Uy-Evanado of Cedars-Sinai Medical Center in Los Angeles.
In terms of cardiovascular risk factors, hypertension and obesity were significantly more prevalent in the control group – but the SCD cohort had increased rates of diabetes, chronic kidney disease, and severe left ventricular dysfunction.
Aside from schizophrenia, rates of other psychiatric disorders didn’t differ between the two groups.
In a multivariate logistic regression analysis adjusted for age, gender, and comorbidities, having schizophrenia was not associated with an increased risk of SCD. However, patients on a first-generation antipsychotic agent had a 3.76-fold increased risk of SCD (P = .0002), and those on a second-generation antipsychotic drug had a 3.3-fold increased risk.
This study was undertaken as a follow-up to an earlier report from the Oregon Sudden Unexplained Death Study, which broadly identified antipsychotic drug therapy as being associated with SCD. That observation raised the question as to whether the risk is limited to specific classes of antipsychotic agents. The answer appears to be no.
The Oregon Sudden Unexplained Death Study is funded by Oregon Health and Science University and the Centers for Disease Control and Prevention. Dr. Uy-Evanado reported having no conflicts of interest.
AT HEART RHYTHM 2013
Major finding: Use of a first-generation antipsychotic agent was independently associated with a 3.76-fold increased risk of sudden cardiac death in a large, ongoing community-based study. Use of a second-generation antipsychotic drug was associated with a 3.31-fold increased risk.
Data source: This analysis from the Oregon Sudden Unexplained Death Study involved 1,544 documented cases of sudden cardiac death and 774 controls.
Disclosures: The Oregon Sudden Unexplained Death Study is sponsored by Oregon Health and Science University and the Centers for Disease Control and Prevention. The presenter reported having no conflicts of interest.
Manage the mental side of skin disorders
Nonpharmacologic interventions such as psychotherapy, hypnosis, and meditation can offer significant benefits to patients with dermatologic conditions including psoriasis, eczema, and urticaria, according to Dr. Richard G. Fried.
While the use of nonpharmacologic interventions may seem counterintuitive, these therapies have been shown to improve psychosocial function and reduce the negative emotional states that can exacerbate or even elicit skin disease, said Dr. Fried of Yardley (Pa.) Dermatology Associates. Dr. Fried detailed several strategies and options for managing the mental aspect of skin disorders in an article in the June issue of Seminars in Cutaneous Medicine and Surgery.
"Clinical studies have demonstrated that psychological stress disrupts skin barrier function and increases the severity of cutaneous infections, and down-regulates antimicrobial peptide expression, resulting in more severe skin infections in mice," Dr. Fried wrote.
"This self-perpetuating negative interaction between stress and impaired skin function has been well-described and often underlies the so-called ‘vicious cycle’ that exists between skin and negative emotional states," he said.
Dr. Fried proposed three broad categories of ‘psychocutaneous’ patients. First, there are individuals with skin manifestations associated a psychiatric diagnosis such as depression, anxiety, or body dysmorphic disorder.
The second category includes patients with psych-derm conditions such as acne excoriée, neurotic excoriations, dermatitis artefacta, and trichotillomania. The third category covers patients with common skin conditions such as acne, rosacea, psoriasis, eczema, and urticaria that are known to be impacted by emotional factors.
Dr. Fried suggested that even patients dealing with the dermatologic effects of aging may be at risk for negative emotional sequelae and may benefit from nonpharmacologic interventions.
Hypnosis is one intervention that has been shown to benefit some patients. For example, long-lasting effects of hypnosis (particularly in highly hypnotizable patients) may include reduced scratching in eczema, and can aid resolution of acne excoriée.
"Recent studies in patients with alopecia areata (including several with ophiasis distribution) demonstrated that hypnosis promotes excellent regrowth in approximately 50% of treated patients and improvements in depression and anxiety in almost all patients," Dr. Fried reported.
Cognitive-behavioral psychotherapy is one of several common psychotherapy interventions used to treat the psychological aspects of skin disorders. Data from a 6-week study of cognitive-behavioral therapy in psoriasis patients showed improvements in anxiety, depression, and psoriasis-related stress, compared with patients who didn’t receive the therapy. In addition, the psychotherapy intervention group showed three times the clinical improvement, compared those undergoing conventional treatment without therapy.
Group psychotherapy has also been associated with symptom reduction, decreased pruritus, fewer eczema relapses, and decreased steroid use in patients with eczema who used it to supplement to their regular medication.
But nonpharmacologic interventions can also be practiced in a less formal manner by dermatologists themselves, Dr. Fried said.
"The power to heal by words, manner, and touch cannot be overstated. Gentle, compassionate, and optimistic comments and gestures can affect the physiology, emotional well-being, and compliance of our patients," he said.
Dr. Fried said referral to a psychodermatologist or mental health professional with an interest in dermatologic manifestations may help some patients, but sometimes treatment can be as simple as a patient’s regular dermatologist offering reassuring words to, "assuage some of the ‘terror of chronicity and progression’ that haunts our patients," he said.
Dr. Fried stated that he has received compensation for the development of educational papers from Ranbaxy, Bayer, Valeant, and Promius.
Nonpharmacologic interventions such as psychotherapy, hypnosis, and meditation can offer significant benefits to patients with dermatologic conditions including psoriasis, eczema, and urticaria, according to Dr. Richard G. Fried.
While the use of nonpharmacologic interventions may seem counterintuitive, these therapies have been shown to improve psychosocial function and reduce the negative emotional states that can exacerbate or even elicit skin disease, said Dr. Fried of Yardley (Pa.) Dermatology Associates. Dr. Fried detailed several strategies and options for managing the mental aspect of skin disorders in an article in the June issue of Seminars in Cutaneous Medicine and Surgery.
"Clinical studies have demonstrated that psychological stress disrupts skin barrier function and increases the severity of cutaneous infections, and down-regulates antimicrobial peptide expression, resulting in more severe skin infections in mice," Dr. Fried wrote.
"This self-perpetuating negative interaction between stress and impaired skin function has been well-described and often underlies the so-called ‘vicious cycle’ that exists between skin and negative emotional states," he said.
Dr. Fried proposed three broad categories of ‘psychocutaneous’ patients. First, there are individuals with skin manifestations associated a psychiatric diagnosis such as depression, anxiety, or body dysmorphic disorder.
The second category includes patients with psych-derm conditions such as acne excoriée, neurotic excoriations, dermatitis artefacta, and trichotillomania. The third category covers patients with common skin conditions such as acne, rosacea, psoriasis, eczema, and urticaria that are known to be impacted by emotional factors.
Dr. Fried suggested that even patients dealing with the dermatologic effects of aging may be at risk for negative emotional sequelae and may benefit from nonpharmacologic interventions.
Hypnosis is one intervention that has been shown to benefit some patients. For example, long-lasting effects of hypnosis (particularly in highly hypnotizable patients) may include reduced scratching in eczema, and can aid resolution of acne excoriée.
"Recent studies in patients with alopecia areata (including several with ophiasis distribution) demonstrated that hypnosis promotes excellent regrowth in approximately 50% of treated patients and improvements in depression and anxiety in almost all patients," Dr. Fried reported.
Cognitive-behavioral psychotherapy is one of several common psychotherapy interventions used to treat the psychological aspects of skin disorders. Data from a 6-week study of cognitive-behavioral therapy in psoriasis patients showed improvements in anxiety, depression, and psoriasis-related stress, compared with patients who didn’t receive the therapy. In addition, the psychotherapy intervention group showed three times the clinical improvement, compared those undergoing conventional treatment without therapy.
Group psychotherapy has also been associated with symptom reduction, decreased pruritus, fewer eczema relapses, and decreased steroid use in patients with eczema who used it to supplement to their regular medication.
But nonpharmacologic interventions can also be practiced in a less formal manner by dermatologists themselves, Dr. Fried said.
"The power to heal by words, manner, and touch cannot be overstated. Gentle, compassionate, and optimistic comments and gestures can affect the physiology, emotional well-being, and compliance of our patients," he said.
Dr. Fried said referral to a psychodermatologist or mental health professional with an interest in dermatologic manifestations may help some patients, but sometimes treatment can be as simple as a patient’s regular dermatologist offering reassuring words to, "assuage some of the ‘terror of chronicity and progression’ that haunts our patients," he said.
Dr. Fried stated that he has received compensation for the development of educational papers from Ranbaxy, Bayer, Valeant, and Promius.
Nonpharmacologic interventions such as psychotherapy, hypnosis, and meditation can offer significant benefits to patients with dermatologic conditions including psoriasis, eczema, and urticaria, according to Dr. Richard G. Fried.
While the use of nonpharmacologic interventions may seem counterintuitive, these therapies have been shown to improve psychosocial function and reduce the negative emotional states that can exacerbate or even elicit skin disease, said Dr. Fried of Yardley (Pa.) Dermatology Associates. Dr. Fried detailed several strategies and options for managing the mental aspect of skin disorders in an article in the June issue of Seminars in Cutaneous Medicine and Surgery.
"Clinical studies have demonstrated that psychological stress disrupts skin barrier function and increases the severity of cutaneous infections, and down-regulates antimicrobial peptide expression, resulting in more severe skin infections in mice," Dr. Fried wrote.
"This self-perpetuating negative interaction between stress and impaired skin function has been well-described and often underlies the so-called ‘vicious cycle’ that exists between skin and negative emotional states," he said.
Dr. Fried proposed three broad categories of ‘psychocutaneous’ patients. First, there are individuals with skin manifestations associated a psychiatric diagnosis such as depression, anxiety, or body dysmorphic disorder.
The second category includes patients with psych-derm conditions such as acne excoriée, neurotic excoriations, dermatitis artefacta, and trichotillomania. The third category covers patients with common skin conditions such as acne, rosacea, psoriasis, eczema, and urticaria that are known to be impacted by emotional factors.
Dr. Fried suggested that even patients dealing with the dermatologic effects of aging may be at risk for negative emotional sequelae and may benefit from nonpharmacologic interventions.
Hypnosis is one intervention that has been shown to benefit some patients. For example, long-lasting effects of hypnosis (particularly in highly hypnotizable patients) may include reduced scratching in eczema, and can aid resolution of acne excoriée.
"Recent studies in patients with alopecia areata (including several with ophiasis distribution) demonstrated that hypnosis promotes excellent regrowth in approximately 50% of treated patients and improvements in depression and anxiety in almost all patients," Dr. Fried reported.
Cognitive-behavioral psychotherapy is one of several common psychotherapy interventions used to treat the psychological aspects of skin disorders. Data from a 6-week study of cognitive-behavioral therapy in psoriasis patients showed improvements in anxiety, depression, and psoriasis-related stress, compared with patients who didn’t receive the therapy. In addition, the psychotherapy intervention group showed three times the clinical improvement, compared those undergoing conventional treatment without therapy.
Group psychotherapy has also been associated with symptom reduction, decreased pruritus, fewer eczema relapses, and decreased steroid use in patients with eczema who used it to supplement to their regular medication.
But nonpharmacologic interventions can also be practiced in a less formal manner by dermatologists themselves, Dr. Fried said.
"The power to heal by words, manner, and touch cannot be overstated. Gentle, compassionate, and optimistic comments and gestures can affect the physiology, emotional well-being, and compliance of our patients," he said.
Dr. Fried said referral to a psychodermatologist or mental health professional with an interest in dermatologic manifestations may help some patients, but sometimes treatment can be as simple as a patient’s regular dermatologist offering reassuring words to, "assuage some of the ‘terror of chronicity and progression’ that haunts our patients," he said.
Dr. Fried stated that he has received compensation for the development of educational papers from Ranbaxy, Bayer, Valeant, and Promius.
FROM SEMINARS IN CUTANEOUS MEDICINE AND SURGERY
PSYCHIATRY UPDATE 2014
Current Psychiatry and the American Academy of Clinical Psychiatrists welcomed more than 550 psychiatric practitioners from across the United States and abroad to this annual conference, which was headed by Meeting Chair Richard Balon, MD, and Co-chairs Donald W. Black, MD, and Nagy Youssef, MD, March 27-29, 2014 at the Hilton Chicago in Chicago, Illinois. Attendees earned as many as 10 AMA PRA Category 1 Credits™.
Thursday, March 27, 2014
MORNING SESSION
Obsessive-compulsive disorder can be misdiagnosed as psychosis, anxiety, or a sexual disorder. In addition to contamination, patients can present with pathologic doubt, somatic obsessions, or obsessions about taboo or symmetry. Among FDA-approved medications, clomipramine might be more effective than selective serotonin reuptake inhibitors (SSRIs). Exposure response prevention therapy shows better response than pharmacotherapy, but best outcomes are seen with combination therapy. Jon E. Grant, JD, MD, MPH, University of Chicago, also discussed obsessive-compulsive personality disorder, body dysmorphic disorder, hoarding, trichotillomania, and excoriation disorder—as well as changes in DSM-5 that cover this group of disorders.
Patients with schizophrenia are at higher risk of death from cardiac and pulmonary disease than the general population. The quality of care of patients with psychosis generally is poor, because of lack of recognition, time, and resources, as well as systematic barriers to accessing health care. Questions about weight gain, lethargy, infections, and sexual functioning can help the practitioner assess a patient’s general health. When appropriate, Henry A. Nasrallah, MD, St. Louis University School of Medicine, recommends, consider switching antipsychotics, which might reverse adverse metabolic events.
Nonpharmacologic treatment goals include improving sleep, educating patients, providing them with tools for improving sleep, and creating an opportunity for patient-practitioner discussion. Stimulus control and sleep restriction are primary therapeutic techniques to improve sleep quality and reduce non-sleeping time in bed. Thomas Roth, PhD, Henry Ford Hospital, also discussed how to modify sleep hygiene techniques for pediatric, adolescent, and geriatric patients.
Donald W. Black, MD, University of Iowa, says that work groups for DSM-5 were asked to consider dimensionality and culture and gender issues. New diagnostic categories include obsessive-compulsive and related disorders and trauma and stressor-related disorders. Some diagnoses were reformulated or introduced, including autism spectrum disorder and disruptive mood dysregulation disorder. The multi-axial system was discontinued in DSM-5. He also reviewed coding issues.
In a sponsored symposium, Prakash S. Masand, MD, Global Medical Education, Inc., looked at the clinical challenges of addressing all 3 symptom domains that characterize depression (emotional, physical, and cognitive) as an introduction to reviewing the efficacy, mechanism of action, and side effects of vortioxetine (Brintellix), a new serotonergic agent for treating major depressive disorder (MDD). In all studies submitted to the FDA, vortioxetine was found to be superior to placebo, in at least 1 dosage group, for alleviating depressive symptoms and for reducing the risk of depressive recurrence.
AFTERNOON SESSION
Oppositional defiant disorder is more common in boys (onset at age 6 to 10) and is associated with inconsistent and neglectful parenting. Treatment modalities, including educational training, anticonvulsants, and lithium, do not have a strong evidence base. Intermittent explosive disorder is characterized by short-lived but frequent behavioral outbursts and often begins in adolescence. Dr. Grant also reviewed the evidence on conduct disorder, pyromania, and kleptomania.
Cognitive symptoms of schizophrenia often appear before psychotic symptoms and remain stable across the lifespan. There are no pharmacologic treatments for cognitive deficits in schizophrenia; however, Dr. Nasrallah listed tactics to improve cognitive function, including regular aerobic exercise. These cognitive deficits can be categorized as neurocognitive (memory, learning, executive function) and social (social skills, theory of mind, social cues) and contribute to functional decline and often prevent patients from working and going to school. Dr. Nasrallah described how bipolar disorder (BD) overlaps with schizophrenia in terms of cognitive dysfunction.
Henry Nasrallah, MD
Psychiatric disorders exhibit specific sleep/ wake impairments. Sleep disorders can mimic psychiatric symptoms, such as fatigue, cognitive problems, and depression. Sleep disturbances, including insomnia, obstructive sleep apnea, and decreased need for sleep, often coexist with depression, generalized anxiety disorder, posttraumatic stress disorder, and BD, and insomnia is associated with a greater risk of suicide. With antidepressant treatment, sleep in depressed patients improves but does not normalize. Dr. Roth also reviewed pharmacotherapeutic options and non-drug modalities to improve patients’ sleep.
Antidepressants have no efficacy in treating acute episodes of bipolar depression, and using such agents might yield a poor long-term outcome in BD, according to Robert M. Post, MD, George Washington University School of Medicine, Michael J. Ostacher, MD, MPH, MMSc, Stanford University, and Vivek Singh, MD, University of Texas Health Science Center at San Antonio, in an interactive faculty discussion. For patients with bipolar I disorder, lithium monotherapy or the combination of lithium and valproate is more effective than valproate alone; evidence does not support valproate as a maintenance treatment. When a patient with BD shows partial response, attendees at this sponsored symposium were advised, consider adding psychotherapy and psycho-education. Combining a mood stabilizer and an antipsychotic might be more effective than monotherapy and safer, by allowing lower dosages. The only 3 treatments FDA-approved for bipolar depression are the olanzapine-fluoxetine combination, quetiapine, and lurasidone.
Boaz Levy, PhD, (left) receives the 2014 George Winokur Research Award from Carol S. North, MD, for his article on recovery of cognitive function in patients with co-occuring bipolar disorder and alcohol dependence.
Friday, March 28, 2014
MORNING SESSION
Carmen Pinto, MD, at a sponsored symposium, reviewed the utility and safety of
long-acting injectable (LAI) antipsychotics for treating schizophrenia, with a focus on LAI aripiprazole, a partial HT-receptor agonist/partial HT-receptor antagonist. Four monthly injections (400 mg/injection) of the drug are needed to reach steady state; each injection reaches peak level in 5 to 7 days. LAI aripiprazole has been shown to delay time to relapse due to nonadherence and onset of nonresponse to the drug, and has high patient acceptance—even in those who already stable. Safety and side effects with LAI aripiprazole are the same as seen with the oral formulation.
In multimodal therapy for chronic pain, psychiatrists have a role in assessing
psychiatric comorbidities, coping ability, social functioning, and other life functions, including work and personal relationships. Cognitive-behavioral therapy can be particularly useful for chronic pain by helping patients reframe their pain experiences. Raphael J. Leo, MA, MD, FAPM, University at Buffalo, reviewed non-opioid co-analgesics that can be used for patients with comorbid pain and a substance use disorder. If opioids are necessary, consider “weak” or long-acting opioids. Monitor patients for aberrant, drug-seeking behavior.
In the second part of his overview, Dr. Black highlighted specific changes to DSM-5 of particular concern to clinicians. New chapters were created and disorders were consolidated, he explained, such as autism spectrum disorder, somatic symptom disorder, and major neurocognitive disorder. New diagnoses include hoarding disorder and binge eating disorder. Subtypes of schizophrenia were dropped. Pathologic gambling was renamed gambling disorder and gender dysphoria is now called gender identity disorder. The bereavement exclusion of a major depressive episode was dropped.
Antidepressants are effective in mitigating pain in neuropathy, headache, fibromyalgia, and chronic musculoskeletal pain, and have been advocated for other pain syndromes. Selection of an antidepressant depends on the type of pain condition, comorbid depression or anxiety, tolerability, and medical comorbidities. Dr. Leo presented prescribing strategies for tricyclics, serotoninnorepinephrine
reuptake inhibitors, SSRIs, and other antidepressants.
Treating of BD in geriatric patients becomes complicated because therapeutic choices are narrowed and response to therapy is less successful with age, according to George T. Grossberg, MD, St. Louis University. Rapid cycling tends to be the norm in geriatric BD patients. Look for agitation and irritability, rather than full-blown mania; grandiose delusions; psychiatric comorbidity, especially anxiety disorder; and sexually inappropriate behavior. Pharmacotherapeutic options include: mood stabilizers, atypical antipsychotics, and antidepressants (specifically, bupropion and SSRIs—not TCAs, venlafaxine, or duloxetine—and over the short term only). Consider divalproex for mania and hypomania, used cautiously because of its adverse side-effect potential.
George T. Grossberg, MD
AFTERNOON SESSION
Often, BD is misdiagnosed as unipolar depression, or the correct diagnosis of BD is delayed, according to Gustavo Alva, MD, ATP Clinical Research. Comorbid substance use disorder or an anxiety disorder is common. Comorbid cardiovascular disease brings a greater risk of mortality in patients with BD than suicide. Approximately two-thirds of patients with BD are taking adjunctive medications; however, antidepressants are no more effective than placebo in treating bipolar depression. At this sponsored symposium, Vladimir Maletic, MD, University of South Carolina, described a 6-week trial in which lurasidone plus lithium or divalporex was more effective in reducing depression, as measured by MADRS, than placebo plus lithium or akathisia, somnolence, and extrapyramidal symptoms.
When assessing an older patient with psychosis, first establish the cause of the symptoms, such as Alzheimer’s disease, affective disorder, substance use, or hallucinations associated with grief. Older patients with schizophrenia who have been taking typical antipsychotics for years might benefit from a switch to an atypical or a dosage reduction. Dr. Grossberg recommends considering antipsychotics for older patients when symptoms cause severe emotional distress that does not respond to other interventions or an acute episode that poses a safety risk for patients or others. Choose an antipsychotic based on side effects, and “start low and go slow,” when possible. The goal is to reduce agitation and distress—not necessarily to resolve psychotic symptoms.
Anita H. Clayton, MD, University of Virginia Health System, provided a review of sexual function from puberty through midlife and older years. Social factors play a role in sexual satisfaction, such as gender expectations, religious beliefs, and the influence of reporting in the media. Sexual dysfunction becomes worse in men after age 29; in women, the rate of sexual dysfunction appears to be consistent across the lifespan. Cardiovascular disease is a significant risk factor for sexual dysfunction in men, but not in women. Sexual function and depression have a bidirectional relationship; sexual dysfunction may be a symptom or cause of depression and antidepressants may affect desire and function. Medications, including psychotropics, oral contraceptives, and opioids, can cause sexual dysfunction.
Providers often are reluctant to bring up sexual issues with their patients, Dr. Clayton says, but patients often want to talk about their sexual problems. In reproductive-age women, look for hypoactive sexual desire disorder and pain. In men, assess for erectile dysfunction or premature ejaculation. Inquire about every phase of the sexual response cycle. When managing sexual dysfunction, aim to minimize contributing factors such as illness or medication, consider FDA-approved medications, encourage a healthy lifestyle, and employ psychological interventions when appropriate. In patients with antidepressant-associated sexual dysfunction, consider switching medications or adding an antidote, such as bupropion, buspirone, or sildenafil.
Saturday, March 29, 2014
MORNING SESSION
Because of the lack of double-blind, placebo-controlled trials, the risks of untreated depression vs the risks of antidepressant use in pregnancy are unclear. Marlene P. Freeman, MD, Massachusetts General Hospital, described the limited, long-term data on tricyclics and fluoxetine. Some studies have shown a small risk of birth defects with SSRIs; others did not find an association. For moderate or severe depression, use antidepressants at the lowest dosage and try non-medication options, such as psychotherapy and complementary and alternative medicine. During the third trimester, women may need a higher dosage to maintain therapeutic drug levels. Data indicates that folic acid use during pregnancy is associated with a decreased risk of autism and schizophrenia.
James W. Jefferson, MD, University of Wisconsin School of Medicine and Public Health, recommends ruling out medical conditions, such as cancer, that might be causing your patients’ fatigue or depression. Many medications, including over-the-counter agents and supplements, can cause fatigue. Bupropion was more effective than placebo and SSRIs in treating depressed patients with sleepiness and fatigue. Adding a psychostimulant to an SSRI does not have a significantly better effect than placebo on depressive symptoms. Adjunctive modafanil may improve depression and fatigue. Data for dopamine agonists are limited.
Lithium should be used with caution in pregnant women because of the risk of congenital malformations. Dr. Freeman also discussed the potential risks to the fetus with the mother’s use of valproate and lamotrigine (with the latter, a small increase in oral clefting). High-potency typical antipsychotics are considered safe; low-potency drugs have a higher risk of major malformations. For atypicals, the risk of malformations appears minimal; newborns might display extrapyramidal effects and withdrawal symptoms. Infants exposed to psychostimulants may have lower birth weight, but are not at increased risk of birth defects.
Dr. Jefferson reviewed the efficacy, pharmacokinetics, and adverse effects of vilazodone, levomilnacipran, and vortioxetine, which are antidepressants new to the market. Dr. Jefferson recommends reading package inserts to become familiar with new drugs. He also described studies of medications that were not FDA-approved, including edivoxetine, quetiapine XR monotherapy for MDD, and agomelatine. Agents under investigation include onabotulinumtoxin A injections, ketamine, and lanicemine.
Katherine E. Burdick, PhD, Mount Sinai School of Medicine, defined cognitive domains. First-episode MDD patients perform worse in psychomotor speed and attention than healthy controls. Late-onset depression (after age 60) is associated with worse performance on processing speed and verbal memory. Cognitive deficits in depressed patients range from mild to moderate and are influenced by symptom status and duration of illness. Treating cognitive deficits begins with prevention. Cholinesterase inhibitors are not effective for improving cognition in MDD. Antidepressants, including SSRIs, do not adequately treat cognitive deficits, Roger S. McIntyre, MD, FRCPC, University of Toronto, explained.
Current Psychiatry and the American Academy of Clinical Psychiatrists welcomed more than 550 psychiatric practitioners from across the United States and abroad to this annual conference, which was headed by Meeting Chair Richard Balon, MD, and Co-chairs Donald W. Black, MD, and Nagy Youssef, MD, March 27-29, 2014 at the Hilton Chicago in Chicago, Illinois. Attendees earned as many as 10 AMA PRA Category 1 Credits™.
Thursday, March 27, 2014
MORNING SESSION
Obsessive-compulsive disorder can be misdiagnosed as psychosis, anxiety, or a sexual disorder. In addition to contamination, patients can present with pathologic doubt, somatic obsessions, or obsessions about taboo or symmetry. Among FDA-approved medications, clomipramine might be more effective than selective serotonin reuptake inhibitors (SSRIs). Exposure response prevention therapy shows better response than pharmacotherapy, but best outcomes are seen with combination therapy. Jon E. Grant, JD, MD, MPH, University of Chicago, also discussed obsessive-compulsive personality disorder, body dysmorphic disorder, hoarding, trichotillomania, and excoriation disorder—as well as changes in DSM-5 that cover this group of disorders.
Patients with schizophrenia are at higher risk of death from cardiac and pulmonary disease than the general population. The quality of care of patients with psychosis generally is poor, because of lack of recognition, time, and resources, as well as systematic barriers to accessing health care. Questions about weight gain, lethargy, infections, and sexual functioning can help the practitioner assess a patient’s general health. When appropriate, Henry A. Nasrallah, MD, St. Louis University School of Medicine, recommends, consider switching antipsychotics, which might reverse adverse metabolic events.
Nonpharmacologic treatment goals include improving sleep, educating patients, providing them with tools for improving sleep, and creating an opportunity for patient-practitioner discussion. Stimulus control and sleep restriction are primary therapeutic techniques to improve sleep quality and reduce non-sleeping time in bed. Thomas Roth, PhD, Henry Ford Hospital, also discussed how to modify sleep hygiene techniques for pediatric, adolescent, and geriatric patients.
Donald W. Black, MD, University of Iowa, says that work groups for DSM-5 were asked to consider dimensionality and culture and gender issues. New diagnostic categories include obsessive-compulsive and related disorders and trauma and stressor-related disorders. Some diagnoses were reformulated or introduced, including autism spectrum disorder and disruptive mood dysregulation disorder. The multi-axial system was discontinued in DSM-5. He also reviewed coding issues.
In a sponsored symposium, Prakash S. Masand, MD, Global Medical Education, Inc., looked at the clinical challenges of addressing all 3 symptom domains that characterize depression (emotional, physical, and cognitive) as an introduction to reviewing the efficacy, mechanism of action, and side effects of vortioxetine (Brintellix), a new serotonergic agent for treating major depressive disorder (MDD). In all studies submitted to the FDA, vortioxetine was found to be superior to placebo, in at least 1 dosage group, for alleviating depressive symptoms and for reducing the risk of depressive recurrence.
AFTERNOON SESSION
Oppositional defiant disorder is more common in boys (onset at age 6 to 10) and is associated with inconsistent and neglectful parenting. Treatment modalities, including educational training, anticonvulsants, and lithium, do not have a strong evidence base. Intermittent explosive disorder is characterized by short-lived but frequent behavioral outbursts and often begins in adolescence. Dr. Grant also reviewed the evidence on conduct disorder, pyromania, and kleptomania.
Cognitive symptoms of schizophrenia often appear before psychotic symptoms and remain stable across the lifespan. There are no pharmacologic treatments for cognitive deficits in schizophrenia; however, Dr. Nasrallah listed tactics to improve cognitive function, including regular aerobic exercise. These cognitive deficits can be categorized as neurocognitive (memory, learning, executive function) and social (social skills, theory of mind, social cues) and contribute to functional decline and often prevent patients from working and going to school. Dr. Nasrallah described how bipolar disorder (BD) overlaps with schizophrenia in terms of cognitive dysfunction.
Henry Nasrallah, MD
Psychiatric disorders exhibit specific sleep/ wake impairments. Sleep disorders can mimic psychiatric symptoms, such as fatigue, cognitive problems, and depression. Sleep disturbances, including insomnia, obstructive sleep apnea, and decreased need for sleep, often coexist with depression, generalized anxiety disorder, posttraumatic stress disorder, and BD, and insomnia is associated with a greater risk of suicide. With antidepressant treatment, sleep in depressed patients improves but does not normalize. Dr. Roth also reviewed pharmacotherapeutic options and non-drug modalities to improve patients’ sleep.
Antidepressants have no efficacy in treating acute episodes of bipolar depression, and using such agents might yield a poor long-term outcome in BD, according to Robert M. Post, MD, George Washington University School of Medicine, Michael J. Ostacher, MD, MPH, MMSc, Stanford University, and Vivek Singh, MD, University of Texas Health Science Center at San Antonio, in an interactive faculty discussion. For patients with bipolar I disorder, lithium monotherapy or the combination of lithium and valproate is more effective than valproate alone; evidence does not support valproate as a maintenance treatment. When a patient with BD shows partial response, attendees at this sponsored symposium were advised, consider adding psychotherapy and psycho-education. Combining a mood stabilizer and an antipsychotic might be more effective than monotherapy and safer, by allowing lower dosages. The only 3 treatments FDA-approved for bipolar depression are the olanzapine-fluoxetine combination, quetiapine, and lurasidone.
Boaz Levy, PhD, (left) receives the 2014 George Winokur Research Award from Carol S. North, MD, for his article on recovery of cognitive function in patients with co-occuring bipolar disorder and alcohol dependence.
Friday, March 28, 2014
MORNING SESSION
Carmen Pinto, MD, at a sponsored symposium, reviewed the utility and safety of
long-acting injectable (LAI) antipsychotics for treating schizophrenia, with a focus on LAI aripiprazole, a partial HT-receptor agonist/partial HT-receptor antagonist. Four monthly injections (400 mg/injection) of the drug are needed to reach steady state; each injection reaches peak level in 5 to 7 days. LAI aripiprazole has been shown to delay time to relapse due to nonadherence and onset of nonresponse to the drug, and has high patient acceptance—even in those who already stable. Safety and side effects with LAI aripiprazole are the same as seen with the oral formulation.
In multimodal therapy for chronic pain, psychiatrists have a role in assessing
psychiatric comorbidities, coping ability, social functioning, and other life functions, including work and personal relationships. Cognitive-behavioral therapy can be particularly useful for chronic pain by helping patients reframe their pain experiences. Raphael J. Leo, MA, MD, FAPM, University at Buffalo, reviewed non-opioid co-analgesics that can be used for patients with comorbid pain and a substance use disorder. If opioids are necessary, consider “weak” or long-acting opioids. Monitor patients for aberrant, drug-seeking behavior.
In the second part of his overview, Dr. Black highlighted specific changes to DSM-5 of particular concern to clinicians. New chapters were created and disorders were consolidated, he explained, such as autism spectrum disorder, somatic symptom disorder, and major neurocognitive disorder. New diagnoses include hoarding disorder and binge eating disorder. Subtypes of schizophrenia were dropped. Pathologic gambling was renamed gambling disorder and gender dysphoria is now called gender identity disorder. The bereavement exclusion of a major depressive episode was dropped.
Antidepressants are effective in mitigating pain in neuropathy, headache, fibromyalgia, and chronic musculoskeletal pain, and have been advocated for other pain syndromes. Selection of an antidepressant depends on the type of pain condition, comorbid depression or anxiety, tolerability, and medical comorbidities. Dr. Leo presented prescribing strategies for tricyclics, serotoninnorepinephrine
reuptake inhibitors, SSRIs, and other antidepressants.
Treating of BD in geriatric patients becomes complicated because therapeutic choices are narrowed and response to therapy is less successful with age, according to George T. Grossberg, MD, St. Louis University. Rapid cycling tends to be the norm in geriatric BD patients. Look for agitation and irritability, rather than full-blown mania; grandiose delusions; psychiatric comorbidity, especially anxiety disorder; and sexually inappropriate behavior. Pharmacotherapeutic options include: mood stabilizers, atypical antipsychotics, and antidepressants (specifically, bupropion and SSRIs—not TCAs, venlafaxine, or duloxetine—and over the short term only). Consider divalproex for mania and hypomania, used cautiously because of its adverse side-effect potential.
George T. Grossberg, MD
AFTERNOON SESSION
Often, BD is misdiagnosed as unipolar depression, or the correct diagnosis of BD is delayed, according to Gustavo Alva, MD, ATP Clinical Research. Comorbid substance use disorder or an anxiety disorder is common. Comorbid cardiovascular disease brings a greater risk of mortality in patients with BD than suicide. Approximately two-thirds of patients with BD are taking adjunctive medications; however, antidepressants are no more effective than placebo in treating bipolar depression. At this sponsored symposium, Vladimir Maletic, MD, University of South Carolina, described a 6-week trial in which lurasidone plus lithium or divalporex was more effective in reducing depression, as measured by MADRS, than placebo plus lithium or akathisia, somnolence, and extrapyramidal symptoms.
When assessing an older patient with psychosis, first establish the cause of the symptoms, such as Alzheimer’s disease, affective disorder, substance use, or hallucinations associated with grief. Older patients with schizophrenia who have been taking typical antipsychotics for years might benefit from a switch to an atypical or a dosage reduction. Dr. Grossberg recommends considering antipsychotics for older patients when symptoms cause severe emotional distress that does not respond to other interventions or an acute episode that poses a safety risk for patients or others. Choose an antipsychotic based on side effects, and “start low and go slow,” when possible. The goal is to reduce agitation and distress—not necessarily to resolve psychotic symptoms.
Anita H. Clayton, MD, University of Virginia Health System, provided a review of sexual function from puberty through midlife and older years. Social factors play a role in sexual satisfaction, such as gender expectations, religious beliefs, and the influence of reporting in the media. Sexual dysfunction becomes worse in men after age 29; in women, the rate of sexual dysfunction appears to be consistent across the lifespan. Cardiovascular disease is a significant risk factor for sexual dysfunction in men, but not in women. Sexual function and depression have a bidirectional relationship; sexual dysfunction may be a symptom or cause of depression and antidepressants may affect desire and function. Medications, including psychotropics, oral contraceptives, and opioids, can cause sexual dysfunction.
Providers often are reluctant to bring up sexual issues with their patients, Dr. Clayton says, but patients often want to talk about their sexual problems. In reproductive-age women, look for hypoactive sexual desire disorder and pain. In men, assess for erectile dysfunction or premature ejaculation. Inquire about every phase of the sexual response cycle. When managing sexual dysfunction, aim to minimize contributing factors such as illness or medication, consider FDA-approved medications, encourage a healthy lifestyle, and employ psychological interventions when appropriate. In patients with antidepressant-associated sexual dysfunction, consider switching medications or adding an antidote, such as bupropion, buspirone, or sildenafil.
Saturday, March 29, 2014
MORNING SESSION
Because of the lack of double-blind, placebo-controlled trials, the risks of untreated depression vs the risks of antidepressant use in pregnancy are unclear. Marlene P. Freeman, MD, Massachusetts General Hospital, described the limited, long-term data on tricyclics and fluoxetine. Some studies have shown a small risk of birth defects with SSRIs; others did not find an association. For moderate or severe depression, use antidepressants at the lowest dosage and try non-medication options, such as psychotherapy and complementary and alternative medicine. During the third trimester, women may need a higher dosage to maintain therapeutic drug levels. Data indicates that folic acid use during pregnancy is associated with a decreased risk of autism and schizophrenia.
James W. Jefferson, MD, University of Wisconsin School of Medicine and Public Health, recommends ruling out medical conditions, such as cancer, that might be causing your patients’ fatigue or depression. Many medications, including over-the-counter agents and supplements, can cause fatigue. Bupropion was more effective than placebo and SSRIs in treating depressed patients with sleepiness and fatigue. Adding a psychostimulant to an SSRI does not have a significantly better effect than placebo on depressive symptoms. Adjunctive modafanil may improve depression and fatigue. Data for dopamine agonists are limited.
Lithium should be used with caution in pregnant women because of the risk of congenital malformations. Dr. Freeman also discussed the potential risks to the fetus with the mother’s use of valproate and lamotrigine (with the latter, a small increase in oral clefting). High-potency typical antipsychotics are considered safe; low-potency drugs have a higher risk of major malformations. For atypicals, the risk of malformations appears minimal; newborns might display extrapyramidal effects and withdrawal symptoms. Infants exposed to psychostimulants may have lower birth weight, but are not at increased risk of birth defects.
Dr. Jefferson reviewed the efficacy, pharmacokinetics, and adverse effects of vilazodone, levomilnacipran, and vortioxetine, which are antidepressants new to the market. Dr. Jefferson recommends reading package inserts to become familiar with new drugs. He also described studies of medications that were not FDA-approved, including edivoxetine, quetiapine XR monotherapy for MDD, and agomelatine. Agents under investigation include onabotulinumtoxin A injections, ketamine, and lanicemine.
Katherine E. Burdick, PhD, Mount Sinai School of Medicine, defined cognitive domains. First-episode MDD patients perform worse in psychomotor speed and attention than healthy controls. Late-onset depression (after age 60) is associated with worse performance on processing speed and verbal memory. Cognitive deficits in depressed patients range from mild to moderate and are influenced by symptom status and duration of illness. Treating cognitive deficits begins with prevention. Cholinesterase inhibitors are not effective for improving cognition in MDD. Antidepressants, including SSRIs, do not adequately treat cognitive deficits, Roger S. McIntyre, MD, FRCPC, University of Toronto, explained.
Current Psychiatry and the American Academy of Clinical Psychiatrists welcomed more than 550 psychiatric practitioners from across the United States and abroad to this annual conference, which was headed by Meeting Chair Richard Balon, MD, and Co-chairs Donald W. Black, MD, and Nagy Youssef, MD, March 27-29, 2014 at the Hilton Chicago in Chicago, Illinois. Attendees earned as many as 10 AMA PRA Category 1 Credits™.
Thursday, March 27, 2014
MORNING SESSION
Obsessive-compulsive disorder can be misdiagnosed as psychosis, anxiety, or a sexual disorder. In addition to contamination, patients can present with pathologic doubt, somatic obsessions, or obsessions about taboo or symmetry. Among FDA-approved medications, clomipramine might be more effective than selective serotonin reuptake inhibitors (SSRIs). Exposure response prevention therapy shows better response than pharmacotherapy, but best outcomes are seen with combination therapy. Jon E. Grant, JD, MD, MPH, University of Chicago, also discussed obsessive-compulsive personality disorder, body dysmorphic disorder, hoarding, trichotillomania, and excoriation disorder—as well as changes in DSM-5 that cover this group of disorders.
Patients with schizophrenia are at higher risk of death from cardiac and pulmonary disease than the general population. The quality of care of patients with psychosis generally is poor, because of lack of recognition, time, and resources, as well as systematic barriers to accessing health care. Questions about weight gain, lethargy, infections, and sexual functioning can help the practitioner assess a patient’s general health. When appropriate, Henry A. Nasrallah, MD, St. Louis University School of Medicine, recommends, consider switching antipsychotics, which might reverse adverse metabolic events.
Nonpharmacologic treatment goals include improving sleep, educating patients, providing them with tools for improving sleep, and creating an opportunity for patient-practitioner discussion. Stimulus control and sleep restriction are primary therapeutic techniques to improve sleep quality and reduce non-sleeping time in bed. Thomas Roth, PhD, Henry Ford Hospital, also discussed how to modify sleep hygiene techniques for pediatric, adolescent, and geriatric patients.
Donald W. Black, MD, University of Iowa, says that work groups for DSM-5 were asked to consider dimensionality and culture and gender issues. New diagnostic categories include obsessive-compulsive and related disorders and trauma and stressor-related disorders. Some diagnoses were reformulated or introduced, including autism spectrum disorder and disruptive mood dysregulation disorder. The multi-axial system was discontinued in DSM-5. He also reviewed coding issues.
In a sponsored symposium, Prakash S. Masand, MD, Global Medical Education, Inc., looked at the clinical challenges of addressing all 3 symptom domains that characterize depression (emotional, physical, and cognitive) as an introduction to reviewing the efficacy, mechanism of action, and side effects of vortioxetine (Brintellix), a new serotonergic agent for treating major depressive disorder (MDD). In all studies submitted to the FDA, vortioxetine was found to be superior to placebo, in at least 1 dosage group, for alleviating depressive symptoms and for reducing the risk of depressive recurrence.
AFTERNOON SESSION
Oppositional defiant disorder is more common in boys (onset at age 6 to 10) and is associated with inconsistent and neglectful parenting. Treatment modalities, including educational training, anticonvulsants, and lithium, do not have a strong evidence base. Intermittent explosive disorder is characterized by short-lived but frequent behavioral outbursts and often begins in adolescence. Dr. Grant also reviewed the evidence on conduct disorder, pyromania, and kleptomania.
Cognitive symptoms of schizophrenia often appear before psychotic symptoms and remain stable across the lifespan. There are no pharmacologic treatments for cognitive deficits in schizophrenia; however, Dr. Nasrallah listed tactics to improve cognitive function, including regular aerobic exercise. These cognitive deficits can be categorized as neurocognitive (memory, learning, executive function) and social (social skills, theory of mind, social cues) and contribute to functional decline and often prevent patients from working and going to school. Dr. Nasrallah described how bipolar disorder (BD) overlaps with schizophrenia in terms of cognitive dysfunction.
Henry Nasrallah, MD
Psychiatric disorders exhibit specific sleep/ wake impairments. Sleep disorders can mimic psychiatric symptoms, such as fatigue, cognitive problems, and depression. Sleep disturbances, including insomnia, obstructive sleep apnea, and decreased need for sleep, often coexist with depression, generalized anxiety disorder, posttraumatic stress disorder, and BD, and insomnia is associated with a greater risk of suicide. With antidepressant treatment, sleep in depressed patients improves but does not normalize. Dr. Roth also reviewed pharmacotherapeutic options and non-drug modalities to improve patients’ sleep.
Antidepressants have no efficacy in treating acute episodes of bipolar depression, and using such agents might yield a poor long-term outcome in BD, according to Robert M. Post, MD, George Washington University School of Medicine, Michael J. Ostacher, MD, MPH, MMSc, Stanford University, and Vivek Singh, MD, University of Texas Health Science Center at San Antonio, in an interactive faculty discussion. For patients with bipolar I disorder, lithium monotherapy or the combination of lithium and valproate is more effective than valproate alone; evidence does not support valproate as a maintenance treatment. When a patient with BD shows partial response, attendees at this sponsored symposium were advised, consider adding psychotherapy and psycho-education. Combining a mood stabilizer and an antipsychotic might be more effective than monotherapy and safer, by allowing lower dosages. The only 3 treatments FDA-approved for bipolar depression are the olanzapine-fluoxetine combination, quetiapine, and lurasidone.
Boaz Levy, PhD, (left) receives the 2014 George Winokur Research Award from Carol S. North, MD, for his article on recovery of cognitive function in patients with co-occuring bipolar disorder and alcohol dependence.
Friday, March 28, 2014
MORNING SESSION
Carmen Pinto, MD, at a sponsored symposium, reviewed the utility and safety of
long-acting injectable (LAI) antipsychotics for treating schizophrenia, with a focus on LAI aripiprazole, a partial HT-receptor agonist/partial HT-receptor antagonist. Four monthly injections (400 mg/injection) of the drug are needed to reach steady state; each injection reaches peak level in 5 to 7 days. LAI aripiprazole has been shown to delay time to relapse due to nonadherence and onset of nonresponse to the drug, and has high patient acceptance—even in those who already stable. Safety and side effects with LAI aripiprazole are the same as seen with the oral formulation.
In multimodal therapy for chronic pain, psychiatrists have a role in assessing
psychiatric comorbidities, coping ability, social functioning, and other life functions, including work and personal relationships. Cognitive-behavioral therapy can be particularly useful for chronic pain by helping patients reframe their pain experiences. Raphael J. Leo, MA, MD, FAPM, University at Buffalo, reviewed non-opioid co-analgesics that can be used for patients with comorbid pain and a substance use disorder. If opioids are necessary, consider “weak” or long-acting opioids. Monitor patients for aberrant, drug-seeking behavior.
In the second part of his overview, Dr. Black highlighted specific changes to DSM-5 of particular concern to clinicians. New chapters were created and disorders were consolidated, he explained, such as autism spectrum disorder, somatic symptom disorder, and major neurocognitive disorder. New diagnoses include hoarding disorder and binge eating disorder. Subtypes of schizophrenia were dropped. Pathologic gambling was renamed gambling disorder and gender dysphoria is now called gender identity disorder. The bereavement exclusion of a major depressive episode was dropped.
Antidepressants are effective in mitigating pain in neuropathy, headache, fibromyalgia, and chronic musculoskeletal pain, and have been advocated for other pain syndromes. Selection of an antidepressant depends on the type of pain condition, comorbid depression or anxiety, tolerability, and medical comorbidities. Dr. Leo presented prescribing strategies for tricyclics, serotoninnorepinephrine
reuptake inhibitors, SSRIs, and other antidepressants.
Treating of BD in geriatric patients becomes complicated because therapeutic choices are narrowed and response to therapy is less successful with age, according to George T. Grossberg, MD, St. Louis University. Rapid cycling tends to be the norm in geriatric BD patients. Look for agitation and irritability, rather than full-blown mania; grandiose delusions; psychiatric comorbidity, especially anxiety disorder; and sexually inappropriate behavior. Pharmacotherapeutic options include: mood stabilizers, atypical antipsychotics, and antidepressants (specifically, bupropion and SSRIs—not TCAs, venlafaxine, or duloxetine—and over the short term only). Consider divalproex for mania and hypomania, used cautiously because of its adverse side-effect potential.
George T. Grossberg, MD
AFTERNOON SESSION
Often, BD is misdiagnosed as unipolar depression, or the correct diagnosis of BD is delayed, according to Gustavo Alva, MD, ATP Clinical Research. Comorbid substance use disorder or an anxiety disorder is common. Comorbid cardiovascular disease brings a greater risk of mortality in patients with BD than suicide. Approximately two-thirds of patients with BD are taking adjunctive medications; however, antidepressants are no more effective than placebo in treating bipolar depression. At this sponsored symposium, Vladimir Maletic, MD, University of South Carolina, described a 6-week trial in which lurasidone plus lithium or divalporex was more effective in reducing depression, as measured by MADRS, than placebo plus lithium or akathisia, somnolence, and extrapyramidal symptoms.
When assessing an older patient with psychosis, first establish the cause of the symptoms, such as Alzheimer’s disease, affective disorder, substance use, or hallucinations associated with grief. Older patients with schizophrenia who have been taking typical antipsychotics for years might benefit from a switch to an atypical or a dosage reduction. Dr. Grossberg recommends considering antipsychotics for older patients when symptoms cause severe emotional distress that does not respond to other interventions or an acute episode that poses a safety risk for patients or others. Choose an antipsychotic based on side effects, and “start low and go slow,” when possible. The goal is to reduce agitation and distress—not necessarily to resolve psychotic symptoms.
Anita H. Clayton, MD, University of Virginia Health System, provided a review of sexual function from puberty through midlife and older years. Social factors play a role in sexual satisfaction, such as gender expectations, religious beliefs, and the influence of reporting in the media. Sexual dysfunction becomes worse in men after age 29; in women, the rate of sexual dysfunction appears to be consistent across the lifespan. Cardiovascular disease is a significant risk factor for sexual dysfunction in men, but not in women. Sexual function and depression have a bidirectional relationship; sexual dysfunction may be a symptom or cause of depression and antidepressants may affect desire and function. Medications, including psychotropics, oral contraceptives, and opioids, can cause sexual dysfunction.
Providers often are reluctant to bring up sexual issues with their patients, Dr. Clayton says, but patients often want to talk about their sexual problems. In reproductive-age women, look for hypoactive sexual desire disorder and pain. In men, assess for erectile dysfunction or premature ejaculation. Inquire about every phase of the sexual response cycle. When managing sexual dysfunction, aim to minimize contributing factors such as illness or medication, consider FDA-approved medications, encourage a healthy lifestyle, and employ psychological interventions when appropriate. In patients with antidepressant-associated sexual dysfunction, consider switching medications or adding an antidote, such as bupropion, buspirone, or sildenafil.
Saturday, March 29, 2014
MORNING SESSION
Because of the lack of double-blind, placebo-controlled trials, the risks of untreated depression vs the risks of antidepressant use in pregnancy are unclear. Marlene P. Freeman, MD, Massachusetts General Hospital, described the limited, long-term data on tricyclics and fluoxetine. Some studies have shown a small risk of birth defects with SSRIs; others did not find an association. For moderate or severe depression, use antidepressants at the lowest dosage and try non-medication options, such as psychotherapy and complementary and alternative medicine. During the third trimester, women may need a higher dosage to maintain therapeutic drug levels. Data indicates that folic acid use during pregnancy is associated with a decreased risk of autism and schizophrenia.
James W. Jefferson, MD, University of Wisconsin School of Medicine and Public Health, recommends ruling out medical conditions, such as cancer, that might be causing your patients’ fatigue or depression. Many medications, including over-the-counter agents and supplements, can cause fatigue. Bupropion was more effective than placebo and SSRIs in treating depressed patients with sleepiness and fatigue. Adding a psychostimulant to an SSRI does not have a significantly better effect than placebo on depressive symptoms. Adjunctive modafanil may improve depression and fatigue. Data for dopamine agonists are limited.
Lithium should be used with caution in pregnant women because of the risk of congenital malformations. Dr. Freeman also discussed the potential risks to the fetus with the mother’s use of valproate and lamotrigine (with the latter, a small increase in oral clefting). High-potency typical antipsychotics are considered safe; low-potency drugs have a higher risk of major malformations. For atypicals, the risk of malformations appears minimal; newborns might display extrapyramidal effects and withdrawal symptoms. Infants exposed to psychostimulants may have lower birth weight, but are not at increased risk of birth defects.
Dr. Jefferson reviewed the efficacy, pharmacokinetics, and adverse effects of vilazodone, levomilnacipran, and vortioxetine, which are antidepressants new to the market. Dr. Jefferson recommends reading package inserts to become familiar with new drugs. He also described studies of medications that were not FDA-approved, including edivoxetine, quetiapine XR monotherapy for MDD, and agomelatine. Agents under investigation include onabotulinumtoxin A injections, ketamine, and lanicemine.
Katherine E. Burdick, PhD, Mount Sinai School of Medicine, defined cognitive domains. First-episode MDD patients perform worse in psychomotor speed and attention than healthy controls. Late-onset depression (after age 60) is associated with worse performance on processing speed and verbal memory. Cognitive deficits in depressed patients range from mild to moderate and are influenced by symptom status and duration of illness. Treating cognitive deficits begins with prevention. Cholinesterase inhibitors are not effective for improving cognition in MDD. Antidepressants, including SSRIs, do not adequately treat cognitive deficits, Roger S. McIntyre, MD, FRCPC, University of Toronto, explained.
How we can improve end-of-life care
My mother and I were in the hospital lobby waiting for my father. My normally upbeat, sharp, energetic mom looked sick, weak, and confused. She had just been discharged from the hospital after experiencing a seizure caused by brain metastasis from long-standing breast cancer. Although we had discussed hospice care during my mother’s admission, we left the hospital without setting up the service because we wanted more time to see if she could recover. Two days later, we arranged hospice because of my mother’s rapidly deteriorating condition and our inability to manage her illness on our own. Five days later, she passed away at home surrounded by loved ones.
I am bewildered that my family did not transition my mother to end-of-life care earlier. Several family members and I work in the medical field. Being resourceful, we chose treatment at a large medical center that was a 3-hour drive from our home. Also, we were familiar with palliative care because other family members had died with hospice. Yet, in those last precious moments, we did not initiate end-of-life care for my mother until it became an emergency. This experience cultivated my interest in palliative care and hospice, specifically, in barriers to access and better ways to transition from life-saving treatment to end-of-life care. As a psychiatry resident, I feel it is important to expand opportunities for psychiatrists to participate in palliative care teams.
Barriers to end-of-life care
Poor physician-patient communication near the end of life can be a major barrier to care. Studies suggest that patients and physicians are ambivalent about end-of-life discussions and tend to avoid them.1 Communication is crucial to helping patients prepare to die well. Researchers have found that end-of-life discussions between patients and physicians can result in fewer aggressive interventions and better quality of life near death.2 These discussions did not increase emotional distress and were associated with lower rates of ventilation, resuscitation, and intensive care unit admissions.2 End-of-life discussions between physicians and patients also lead to earlier hospice enrollment and improve the quality of end-of-life care.2
Approximately one-third of patients spend <1 week in hospice.3 Studies have suggested that patients who receive >1 week of hospice care have improved quality of life compared with patients who do not receive hospice care.1 Addressing end-of-life issues early in treatment of terminally ill patients allows them to become familiar with their options as their disease progresses.
Lack of integration between hospice and the medical community is another barrier. According to Medicare guidelines,4 to be eligible for hospice a patient must have a terminal illness with a prognosis of ≤6 months. The patient must agree to give up curative treatment. If a patient lives >6 months, hospice benefits can be renewed as long as the patient has shown a persistent decline. Patients must desire and accept a palliative approach rather than a curative focus and agree to have all care provided by Medicare-certified hospices.5 These eligibility requirements may give patients and their families a sense that they are losing the forms of health care they had been using, physicians they know, and treatments they had become accustomed to, and are transferring their medical care to a unknown entity. This transition occurs when diseases are progressing and patients may feel vulnerable. In this way, end-of-life care is segregated from the rest of the medical system and can feel foreign and frightening to patients.6
Lack of palliative care specialists. The U.S. population is aging, and more people are living longer with chronic illness. Although the palliative care field is growing, only 60% of U.S. hospitals have palliative care programs and there is a shortage of palliative care clinicians.7
Psychiatry and end-of-life care
Psychiatry and psycho-oncology can help address these barriers by providing resources and expertise to palliative care teams. Psychiatrists can facilitate communication between families and primary clinicians and help patients mentally prepare for end-of-life options.
The dying process may uncover psychological, psychosocial, and existential suffering in patients and their families that often is underdiagnosed and undertreated.8 Psychiatrists can:
- diagnose and treat psychiatric disorders that surface under the stress of a new diagnosis
- aid in the psychodynamics of coping with terminal illness
- assess decision-making capacity
- recognize and treat staff stress
- provide bereavement care.
Because many clinicians believe that mood or anxiety symptoms are “normal reactions” in individuals struggling with end-of-life issues, these patients may not receive psychiatric treatment. However, treating mood and anxiety disorders can improve quality of life in palliative care patients.9
In terminally ill patients, psychological pain often manifests as symptoms of depression and anxiety. Diagnostic criteria for depression may need to be reconsidered because in patients with terminal illness depressive symptoms commonly are associated with functional decline. For example, restrictions on a patient’s ability to participate in activities and disengagement from some areas of interest are common among individuals facing the end of life, but if a patient is unable to find pleasure in any event or activity, he or she may meet criteria for depression. Endicott10 proposed a list of substitute symptoms of depression in terminally ill patients:
- weight loss or gain is substituted with depressed appearance
- loss of energy is substituted with brooding and self-pity
- insomnia or hypersomnia is substituted with social withdrawal
- loss of concentration is substituted with lack of reactivity or inability to be cheered up.
These substitutions are not in DSM-IV-TR but should be considered when assessing a terminally ill patient.
Most recommendations for pharmacologic treatment of depression in terminally ill patients are based on depression treatments for the general population. Selective serotonin reuptake inhibitors are commonly prescribed for terminally ill patients. Mirtazapine, a noradrenergic and serotonergic antidepressant, has been shown to effectively treat adjustment, mood, and anxiety disorders in patients with breast or gynecological cancer.11 Its major side effects—sedation and weight gain—might be beneficial in patients with terminal illness. Psychostimulants also have been shown to elevate mood in patients with advanced malignancies.12
Potential triggers for anxiety in terminally ill patients include chemotherapy, radiation therapy, or acute pain. Also consider anxiety related to death and dying. Certain drugs commonly used in palliative care—such as corticosteroids and psychostimulants—may contribute to anxiety and restlessness. Cancer patients may develop posttraumatic stress disorder symptoms, including re-experiencing frightening aspects of their diagnosis and treatment, nightmares, hypervigilance, and autonomic hyperactivity.13 Pharmacologic treatment of anxiety in dying patients is similar to that in the general population; benzodiazepines and antidepressants are first-line agents.
Several forms of psychotherapy can help terminally ill patients address existential issues (Box).14-16
Dignity therapy is a form of brief individual therapy developed by Chochinov et al14 that focuses on existential distress to help patients feel that their lives have been worthwhile. The goal is to have patients describe what they are most proud of, what they want to be remembered for, and what is most meaningful to them.
Viederman et al15 characterized a type of therapy known as the psychodynamic life narrative. Palliative care psychiatrists use this technique to examine patients’ lives and take stock of successes and failures. The narrative attempts to create a new perspective within terminally ill patients that increases self-esteem by emphasizing past strengths and coping mechanisms that have been successful.
Meaning-centered group therapy, developed by Breitbart,16 emphasizes group didactics, discussion, and experiential exercises, with a focus on themes related to advanced cancer.
A need for training
In a survey of psychiatry residents, 97% of respondents believed psychiatrists should be trained in end-of-life care and 94% felt there should be formal education on palliative care during residency.17 A study evaluating psychiatry residents’ attitudes, perceived preparedness, experiences, and needs in end-of-life care education found that residents felt least prepared when dealing with cultural and spiritual aspects of dying and helping patients with reconciliation and saying goodbye.18 These residents also expressed a desire for more longitudinal exposure to palliative care.
A dedicated palliative care rotation during psychiatry residency training would help build a foundation of knowledge in this field. Residency programs should make a greater effort to incorporate end-of-life issues into consultation-liaison and geriatric rotations. Education on psychosocial, existential, and spiritual distress should be highlighted, with an emphasis on integrating specific psychotherapy techniques into training. These opportunities would provide residents with necessary skills to help patients cope with end-of-life issues.
As a psychiatry resident, I believe training in this field is one way to decrease barriers for patients to access end-of-life care. End-of-life psychiatric training could help build a culture where end-of-life care is integrated into the medical care system, with the goal of helping terminally ill patients die well.
Related Resources
- Chochinov H, Breitbart W. Handbook of psychiatry in palliative medicine. New York, NY: Oxford University Press; 2009.
- Harvard Medical School Center for Palliative Care. www.hms.harvard.edu/pallcare.
Drug Brand Name
- Mirtazapine • Remeron
Disclosure
Dr. Kester reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Leydon GM, Boulton M, Moynihan C, et al. Cancer patients’ information needs and information seeking behaviour: in depth interview study. BMJ. 2000;320(7239):909-913.
2. Wright AA, Zhang B, Ray A, et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300(14):1665-1673.
3. Harrington S, Smith T. The role of chemotherapy at the end of life: when is enough enough? JAMA. 2008;299(22):2667-2678.
4. U.S. Department of Health and Human Services. Centers for Medicare and Medicaid Services. Medicare hospice benefits. http://www.medicare.gov/Pubs/pdf/02154.pdf. Revised January 2013. Accessed March 1, 2013.
5. Meier DE, Isaacs SL, Hughes RG. eds. Palliative care: transforming the care of serious illness. San Francisco, CA: Jossey-Bass; 2010.
6. Rhymes JA. Barriers to effective palliative care of terminal patients. An international perspective. Clin Geriatr Med. 1996;12(2):407-416.
7. Center to Advance Palliative Care. America’s care of serious illness: a state-by-state report card on access to palliative care in our nation’s hospitals. 2011. http://reportcard-live.capc.stackop.com/pdf/state-by-state-report-card.pdf. Accessed March 1, 2013.
8. Söllner W, DeVries A, Steixner E, et al. How successful are oncologists in identifying patient distress, perceived social support, and need for psychosocial counseling? Br J Cancer. 2001;84(2):179-185.
9. Billings JA, Block S. Integrating psychiatry and palliative medicine: the challenges and opportunities. In: Chochinov HM Breitbart W, eds. Handbook of psychiatry in palliative medicine. New York, NY: Oxford University Press; 2009:13–19.
10. Endicott J. Measurement of depression in patients with cancer. Cancer. 1984;53(10 suppl):2243-2249.
11. Thompson DS. Mirtazapine for the treatment of depression and nausea in breast and gynecological oncology. Psychosomatics. 2000;41(4):356-359.
12. Macleod AD. Methylphenidate in terminal depression. J Pain Symptom Manage. 1998;16(3):193-198.
13. Alter CL, Pelcovitz D, Axelrod A, et al. Identification of PTSD in cancer survivors. Psychosomatics. 1996;37(2):137-143.
14. Chochinov HM, Hack T, Hassard T, et al. Dignity therapy: a novel psychotherapeutic intervention for patients near the end of life. J Clin Oncol. 2005;23(24):5520-5525.
15. Viederman M, Perry SW, 3rd. Use of a psychodynamic life narrative in the treatment of depression in the physically ill. Gen Hosp Psychiatry. 1980;2(3):177-185.
16. Breitbart W. Spirituality and meaning in supportive care: spirituality- and meaning-centered group psychotherapy interventions in advanced cancer. Support Care Cancer. 2002;10(4):272-280.
17. Irwin SA, Montross LP, Bhat RG, et al. Psychiatry resident education in palliative care: opportunities, desired training, and outcomes of a targeted educational intervention. Psychosomatics. 2011;52(6):530-536.
18. Tait GR, Hodges BD. End-of-life care education for psychiatric residents: attitudes preparedness, and conceptualizations of dignity. Acad Psychiatry. 2009;33(6):451-456.
My mother and I were in the hospital lobby waiting for my father. My normally upbeat, sharp, energetic mom looked sick, weak, and confused. She had just been discharged from the hospital after experiencing a seizure caused by brain metastasis from long-standing breast cancer. Although we had discussed hospice care during my mother’s admission, we left the hospital without setting up the service because we wanted more time to see if she could recover. Two days later, we arranged hospice because of my mother’s rapidly deteriorating condition and our inability to manage her illness on our own. Five days later, she passed away at home surrounded by loved ones.
I am bewildered that my family did not transition my mother to end-of-life care earlier. Several family members and I work in the medical field. Being resourceful, we chose treatment at a large medical center that was a 3-hour drive from our home. Also, we were familiar with palliative care because other family members had died with hospice. Yet, in those last precious moments, we did not initiate end-of-life care for my mother until it became an emergency. This experience cultivated my interest in palliative care and hospice, specifically, in barriers to access and better ways to transition from life-saving treatment to end-of-life care. As a psychiatry resident, I feel it is important to expand opportunities for psychiatrists to participate in palliative care teams.
Barriers to end-of-life care
Poor physician-patient communication near the end of life can be a major barrier to care. Studies suggest that patients and physicians are ambivalent about end-of-life discussions and tend to avoid them.1 Communication is crucial to helping patients prepare to die well. Researchers have found that end-of-life discussions between patients and physicians can result in fewer aggressive interventions and better quality of life near death.2 These discussions did not increase emotional distress and were associated with lower rates of ventilation, resuscitation, and intensive care unit admissions.2 End-of-life discussions between physicians and patients also lead to earlier hospice enrollment and improve the quality of end-of-life care.2
Approximately one-third of patients spend <1 week in hospice.3 Studies have suggested that patients who receive >1 week of hospice care have improved quality of life compared with patients who do not receive hospice care.1 Addressing end-of-life issues early in treatment of terminally ill patients allows them to become familiar with their options as their disease progresses.
Lack of integration between hospice and the medical community is another barrier. According to Medicare guidelines,4 to be eligible for hospice a patient must have a terminal illness with a prognosis of ≤6 months. The patient must agree to give up curative treatment. If a patient lives >6 months, hospice benefits can be renewed as long as the patient has shown a persistent decline. Patients must desire and accept a palliative approach rather than a curative focus and agree to have all care provided by Medicare-certified hospices.5 These eligibility requirements may give patients and their families a sense that they are losing the forms of health care they had been using, physicians they know, and treatments they had become accustomed to, and are transferring their medical care to a unknown entity. This transition occurs when diseases are progressing and patients may feel vulnerable. In this way, end-of-life care is segregated from the rest of the medical system and can feel foreign and frightening to patients.6
Lack of palliative care specialists. The U.S. population is aging, and more people are living longer with chronic illness. Although the palliative care field is growing, only 60% of U.S. hospitals have palliative care programs and there is a shortage of palliative care clinicians.7
Psychiatry and end-of-life care
Psychiatry and psycho-oncology can help address these barriers by providing resources and expertise to palliative care teams. Psychiatrists can facilitate communication between families and primary clinicians and help patients mentally prepare for end-of-life options.
The dying process may uncover psychological, psychosocial, and existential suffering in patients and their families that often is underdiagnosed and undertreated.8 Psychiatrists can:
- diagnose and treat psychiatric disorders that surface under the stress of a new diagnosis
- aid in the psychodynamics of coping with terminal illness
- assess decision-making capacity
- recognize and treat staff stress
- provide bereavement care.
Because many clinicians believe that mood or anxiety symptoms are “normal reactions” in individuals struggling with end-of-life issues, these patients may not receive psychiatric treatment. However, treating mood and anxiety disorders can improve quality of life in palliative care patients.9
In terminally ill patients, psychological pain often manifests as symptoms of depression and anxiety. Diagnostic criteria for depression may need to be reconsidered because in patients with terminal illness depressive symptoms commonly are associated with functional decline. For example, restrictions on a patient’s ability to participate in activities and disengagement from some areas of interest are common among individuals facing the end of life, but if a patient is unable to find pleasure in any event or activity, he or she may meet criteria for depression. Endicott10 proposed a list of substitute symptoms of depression in terminally ill patients:
- weight loss or gain is substituted with depressed appearance
- loss of energy is substituted with brooding and self-pity
- insomnia or hypersomnia is substituted with social withdrawal
- loss of concentration is substituted with lack of reactivity or inability to be cheered up.
These substitutions are not in DSM-IV-TR but should be considered when assessing a terminally ill patient.
Most recommendations for pharmacologic treatment of depression in terminally ill patients are based on depression treatments for the general population. Selective serotonin reuptake inhibitors are commonly prescribed for terminally ill patients. Mirtazapine, a noradrenergic and serotonergic antidepressant, has been shown to effectively treat adjustment, mood, and anxiety disorders in patients with breast or gynecological cancer.11 Its major side effects—sedation and weight gain—might be beneficial in patients with terminal illness. Psychostimulants also have been shown to elevate mood in patients with advanced malignancies.12
Potential triggers for anxiety in terminally ill patients include chemotherapy, radiation therapy, or acute pain. Also consider anxiety related to death and dying. Certain drugs commonly used in palliative care—such as corticosteroids and psychostimulants—may contribute to anxiety and restlessness. Cancer patients may develop posttraumatic stress disorder symptoms, including re-experiencing frightening aspects of their diagnosis and treatment, nightmares, hypervigilance, and autonomic hyperactivity.13 Pharmacologic treatment of anxiety in dying patients is similar to that in the general population; benzodiazepines and antidepressants are first-line agents.
Several forms of psychotherapy can help terminally ill patients address existential issues (Box).14-16
Dignity therapy is a form of brief individual therapy developed by Chochinov et al14 that focuses on existential distress to help patients feel that their lives have been worthwhile. The goal is to have patients describe what they are most proud of, what they want to be remembered for, and what is most meaningful to them.
Viederman et al15 characterized a type of therapy known as the psychodynamic life narrative. Palliative care psychiatrists use this technique to examine patients’ lives and take stock of successes and failures. The narrative attempts to create a new perspective within terminally ill patients that increases self-esteem by emphasizing past strengths and coping mechanisms that have been successful.
Meaning-centered group therapy, developed by Breitbart,16 emphasizes group didactics, discussion, and experiential exercises, with a focus on themes related to advanced cancer.
A need for training
In a survey of psychiatry residents, 97% of respondents believed psychiatrists should be trained in end-of-life care and 94% felt there should be formal education on palliative care during residency.17 A study evaluating psychiatry residents’ attitudes, perceived preparedness, experiences, and needs in end-of-life care education found that residents felt least prepared when dealing with cultural and spiritual aspects of dying and helping patients with reconciliation and saying goodbye.18 These residents also expressed a desire for more longitudinal exposure to palliative care.
A dedicated palliative care rotation during psychiatry residency training would help build a foundation of knowledge in this field. Residency programs should make a greater effort to incorporate end-of-life issues into consultation-liaison and geriatric rotations. Education on psychosocial, existential, and spiritual distress should be highlighted, with an emphasis on integrating specific psychotherapy techniques into training. These opportunities would provide residents with necessary skills to help patients cope with end-of-life issues.
As a psychiatry resident, I believe training in this field is one way to decrease barriers for patients to access end-of-life care. End-of-life psychiatric training could help build a culture where end-of-life care is integrated into the medical care system, with the goal of helping terminally ill patients die well.
Related Resources
- Chochinov H, Breitbart W. Handbook of psychiatry in palliative medicine. New York, NY: Oxford University Press; 2009.
- Harvard Medical School Center for Palliative Care. www.hms.harvard.edu/pallcare.
Drug Brand Name
- Mirtazapine • Remeron
Disclosure
Dr. Kester reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
My mother and I were in the hospital lobby waiting for my father. My normally upbeat, sharp, energetic mom looked sick, weak, and confused. She had just been discharged from the hospital after experiencing a seizure caused by brain metastasis from long-standing breast cancer. Although we had discussed hospice care during my mother’s admission, we left the hospital without setting up the service because we wanted more time to see if she could recover. Two days later, we arranged hospice because of my mother’s rapidly deteriorating condition and our inability to manage her illness on our own. Five days later, she passed away at home surrounded by loved ones.
I am bewildered that my family did not transition my mother to end-of-life care earlier. Several family members and I work in the medical field. Being resourceful, we chose treatment at a large medical center that was a 3-hour drive from our home. Also, we were familiar with palliative care because other family members had died with hospice. Yet, in those last precious moments, we did not initiate end-of-life care for my mother until it became an emergency. This experience cultivated my interest in palliative care and hospice, specifically, in barriers to access and better ways to transition from life-saving treatment to end-of-life care. As a psychiatry resident, I feel it is important to expand opportunities for psychiatrists to participate in palliative care teams.
Barriers to end-of-life care
Poor physician-patient communication near the end of life can be a major barrier to care. Studies suggest that patients and physicians are ambivalent about end-of-life discussions and tend to avoid them.1 Communication is crucial to helping patients prepare to die well. Researchers have found that end-of-life discussions between patients and physicians can result in fewer aggressive interventions and better quality of life near death.2 These discussions did not increase emotional distress and were associated with lower rates of ventilation, resuscitation, and intensive care unit admissions.2 End-of-life discussions between physicians and patients also lead to earlier hospice enrollment and improve the quality of end-of-life care.2
Approximately one-third of patients spend <1 week in hospice.3 Studies have suggested that patients who receive >1 week of hospice care have improved quality of life compared with patients who do not receive hospice care.1 Addressing end-of-life issues early in treatment of terminally ill patients allows them to become familiar with their options as their disease progresses.
Lack of integration between hospice and the medical community is another barrier. According to Medicare guidelines,4 to be eligible for hospice a patient must have a terminal illness with a prognosis of ≤6 months. The patient must agree to give up curative treatment. If a patient lives >6 months, hospice benefits can be renewed as long as the patient has shown a persistent decline. Patients must desire and accept a palliative approach rather than a curative focus and agree to have all care provided by Medicare-certified hospices.5 These eligibility requirements may give patients and their families a sense that they are losing the forms of health care they had been using, physicians they know, and treatments they had become accustomed to, and are transferring their medical care to a unknown entity. This transition occurs when diseases are progressing and patients may feel vulnerable. In this way, end-of-life care is segregated from the rest of the medical system and can feel foreign and frightening to patients.6
Lack of palliative care specialists. The U.S. population is aging, and more people are living longer with chronic illness. Although the palliative care field is growing, only 60% of U.S. hospitals have palliative care programs and there is a shortage of palliative care clinicians.7
Psychiatry and end-of-life care
Psychiatry and psycho-oncology can help address these barriers by providing resources and expertise to palliative care teams. Psychiatrists can facilitate communication between families and primary clinicians and help patients mentally prepare for end-of-life options.
The dying process may uncover psychological, psychosocial, and existential suffering in patients and their families that often is underdiagnosed and undertreated.8 Psychiatrists can:
- diagnose and treat psychiatric disorders that surface under the stress of a new diagnosis
- aid in the psychodynamics of coping with terminal illness
- assess decision-making capacity
- recognize and treat staff stress
- provide bereavement care.
Because many clinicians believe that mood or anxiety symptoms are “normal reactions” in individuals struggling with end-of-life issues, these patients may not receive psychiatric treatment. However, treating mood and anxiety disorders can improve quality of life in palliative care patients.9
In terminally ill patients, psychological pain often manifests as symptoms of depression and anxiety. Diagnostic criteria for depression may need to be reconsidered because in patients with terminal illness depressive symptoms commonly are associated with functional decline. For example, restrictions on a patient’s ability to participate in activities and disengagement from some areas of interest are common among individuals facing the end of life, but if a patient is unable to find pleasure in any event or activity, he or she may meet criteria for depression. Endicott10 proposed a list of substitute symptoms of depression in terminally ill patients:
- weight loss or gain is substituted with depressed appearance
- loss of energy is substituted with brooding and self-pity
- insomnia or hypersomnia is substituted with social withdrawal
- loss of concentration is substituted with lack of reactivity or inability to be cheered up.
These substitutions are not in DSM-IV-TR but should be considered when assessing a terminally ill patient.
Most recommendations for pharmacologic treatment of depression in terminally ill patients are based on depression treatments for the general population. Selective serotonin reuptake inhibitors are commonly prescribed for terminally ill patients. Mirtazapine, a noradrenergic and serotonergic antidepressant, has been shown to effectively treat adjustment, mood, and anxiety disorders in patients with breast or gynecological cancer.11 Its major side effects—sedation and weight gain—might be beneficial in patients with terminal illness. Psychostimulants also have been shown to elevate mood in patients with advanced malignancies.12
Potential triggers for anxiety in terminally ill patients include chemotherapy, radiation therapy, or acute pain. Also consider anxiety related to death and dying. Certain drugs commonly used in palliative care—such as corticosteroids and psychostimulants—may contribute to anxiety and restlessness. Cancer patients may develop posttraumatic stress disorder symptoms, including re-experiencing frightening aspects of their diagnosis and treatment, nightmares, hypervigilance, and autonomic hyperactivity.13 Pharmacologic treatment of anxiety in dying patients is similar to that in the general population; benzodiazepines and antidepressants are first-line agents.
Several forms of psychotherapy can help terminally ill patients address existential issues (Box).14-16
Dignity therapy is a form of brief individual therapy developed by Chochinov et al14 that focuses on existential distress to help patients feel that their lives have been worthwhile. The goal is to have patients describe what they are most proud of, what they want to be remembered for, and what is most meaningful to them.
Viederman et al15 characterized a type of therapy known as the psychodynamic life narrative. Palliative care psychiatrists use this technique to examine patients’ lives and take stock of successes and failures. The narrative attempts to create a new perspective within terminally ill patients that increases self-esteem by emphasizing past strengths and coping mechanisms that have been successful.
Meaning-centered group therapy, developed by Breitbart,16 emphasizes group didactics, discussion, and experiential exercises, with a focus on themes related to advanced cancer.
A need for training
In a survey of psychiatry residents, 97% of respondents believed psychiatrists should be trained in end-of-life care and 94% felt there should be formal education on palliative care during residency.17 A study evaluating psychiatry residents’ attitudes, perceived preparedness, experiences, and needs in end-of-life care education found that residents felt least prepared when dealing with cultural and spiritual aspects of dying and helping patients with reconciliation and saying goodbye.18 These residents also expressed a desire for more longitudinal exposure to palliative care.
A dedicated palliative care rotation during psychiatry residency training would help build a foundation of knowledge in this field. Residency programs should make a greater effort to incorporate end-of-life issues into consultation-liaison and geriatric rotations. Education on psychosocial, existential, and spiritual distress should be highlighted, with an emphasis on integrating specific psychotherapy techniques into training. These opportunities would provide residents with necessary skills to help patients cope with end-of-life issues.
As a psychiatry resident, I believe training in this field is one way to decrease barriers for patients to access end-of-life care. End-of-life psychiatric training could help build a culture where end-of-life care is integrated into the medical care system, with the goal of helping terminally ill patients die well.
Related Resources
- Chochinov H, Breitbart W. Handbook of psychiatry in palliative medicine. New York, NY: Oxford University Press; 2009.
- Harvard Medical School Center for Palliative Care. www.hms.harvard.edu/pallcare.
Drug Brand Name
- Mirtazapine • Remeron
Disclosure
Dr. Kester reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Leydon GM, Boulton M, Moynihan C, et al. Cancer patients’ information needs and information seeking behaviour: in depth interview study. BMJ. 2000;320(7239):909-913.
2. Wright AA, Zhang B, Ray A, et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300(14):1665-1673.
3. Harrington S, Smith T. The role of chemotherapy at the end of life: when is enough enough? JAMA. 2008;299(22):2667-2678.
4. U.S. Department of Health and Human Services. Centers for Medicare and Medicaid Services. Medicare hospice benefits. http://www.medicare.gov/Pubs/pdf/02154.pdf. Revised January 2013. Accessed March 1, 2013.
5. Meier DE, Isaacs SL, Hughes RG. eds. Palliative care: transforming the care of serious illness. San Francisco, CA: Jossey-Bass; 2010.
6. Rhymes JA. Barriers to effective palliative care of terminal patients. An international perspective. Clin Geriatr Med. 1996;12(2):407-416.
7. Center to Advance Palliative Care. America’s care of serious illness: a state-by-state report card on access to palliative care in our nation’s hospitals. 2011. http://reportcard-live.capc.stackop.com/pdf/state-by-state-report-card.pdf. Accessed March 1, 2013.
8. Söllner W, DeVries A, Steixner E, et al. How successful are oncologists in identifying patient distress, perceived social support, and need for psychosocial counseling? Br J Cancer. 2001;84(2):179-185.
9. Billings JA, Block S. Integrating psychiatry and palliative medicine: the challenges and opportunities. In: Chochinov HM Breitbart W, eds. Handbook of psychiatry in palliative medicine. New York, NY: Oxford University Press; 2009:13–19.
10. Endicott J. Measurement of depression in patients with cancer. Cancer. 1984;53(10 suppl):2243-2249.
11. Thompson DS. Mirtazapine for the treatment of depression and nausea in breast and gynecological oncology. Psychosomatics. 2000;41(4):356-359.
12. Macleod AD. Methylphenidate in terminal depression. J Pain Symptom Manage. 1998;16(3):193-198.
13. Alter CL, Pelcovitz D, Axelrod A, et al. Identification of PTSD in cancer survivors. Psychosomatics. 1996;37(2):137-143.
14. Chochinov HM, Hack T, Hassard T, et al. Dignity therapy: a novel psychotherapeutic intervention for patients near the end of life. J Clin Oncol. 2005;23(24):5520-5525.
15. Viederman M, Perry SW, 3rd. Use of a psychodynamic life narrative in the treatment of depression in the physically ill. Gen Hosp Psychiatry. 1980;2(3):177-185.
16. Breitbart W. Spirituality and meaning in supportive care: spirituality- and meaning-centered group psychotherapy interventions in advanced cancer. Support Care Cancer. 2002;10(4):272-280.
17. Irwin SA, Montross LP, Bhat RG, et al. Psychiatry resident education in palliative care: opportunities, desired training, and outcomes of a targeted educational intervention. Psychosomatics. 2011;52(6):530-536.
18. Tait GR, Hodges BD. End-of-life care education for psychiatric residents: attitudes preparedness, and conceptualizations of dignity. Acad Psychiatry. 2009;33(6):451-456.
1. Leydon GM, Boulton M, Moynihan C, et al. Cancer patients’ information needs and information seeking behaviour: in depth interview study. BMJ. 2000;320(7239):909-913.
2. Wright AA, Zhang B, Ray A, et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300(14):1665-1673.
3. Harrington S, Smith T. The role of chemotherapy at the end of life: when is enough enough? JAMA. 2008;299(22):2667-2678.
4. U.S. Department of Health and Human Services. Centers for Medicare and Medicaid Services. Medicare hospice benefits. http://www.medicare.gov/Pubs/pdf/02154.pdf. Revised January 2013. Accessed March 1, 2013.
5. Meier DE, Isaacs SL, Hughes RG. eds. Palliative care: transforming the care of serious illness. San Francisco, CA: Jossey-Bass; 2010.
6. Rhymes JA. Barriers to effective palliative care of terminal patients. An international perspective. Clin Geriatr Med. 1996;12(2):407-416.
7. Center to Advance Palliative Care. America’s care of serious illness: a state-by-state report card on access to palliative care in our nation’s hospitals. 2011. http://reportcard-live.capc.stackop.com/pdf/state-by-state-report-card.pdf. Accessed March 1, 2013.
8. Söllner W, DeVries A, Steixner E, et al. How successful are oncologists in identifying patient distress, perceived social support, and need for psychosocial counseling? Br J Cancer. 2001;84(2):179-185.
9. Billings JA, Block S. Integrating psychiatry and palliative medicine: the challenges and opportunities. In: Chochinov HM Breitbart W, eds. Handbook of psychiatry in palliative medicine. New York, NY: Oxford University Press; 2009:13–19.
10. Endicott J. Measurement of depression in patients with cancer. Cancer. 1984;53(10 suppl):2243-2249.
11. Thompson DS. Mirtazapine for the treatment of depression and nausea in breast and gynecological oncology. Psychosomatics. 2000;41(4):356-359.
12. Macleod AD. Methylphenidate in terminal depression. J Pain Symptom Manage. 1998;16(3):193-198.
13. Alter CL, Pelcovitz D, Axelrod A, et al. Identification of PTSD in cancer survivors. Psychosomatics. 1996;37(2):137-143.
14. Chochinov HM, Hack T, Hassard T, et al. Dignity therapy: a novel psychotherapeutic intervention for patients near the end of life. J Clin Oncol. 2005;23(24):5520-5525.
15. Viederman M, Perry SW, 3rd. Use of a psychodynamic life narrative in the treatment of depression in the physically ill. Gen Hosp Psychiatry. 1980;2(3):177-185.
16. Breitbart W. Spirituality and meaning in supportive care: spirituality- and meaning-centered group psychotherapy interventions in advanced cancer. Support Care Cancer. 2002;10(4):272-280.
17. Irwin SA, Montross LP, Bhat RG, et al. Psychiatry resident education in palliative care: opportunities, desired training, and outcomes of a targeted educational intervention. Psychosomatics. 2011;52(6):530-536.
18. Tait GR, Hodges BD. End-of-life care education for psychiatric residents: attitudes preparedness, and conceptualizations of dignity. Acad Psychiatry. 2009;33(6):451-456.
PSYCHIATRY UPDATE 2013
Current Psychiatry and the American Academy of Clinical Psychiatrists were pleased to host more than 550 psychiatric practitioners for this conference, led by Meeting Chair Richard Balon, MD, and Meeting Co-Chairs Donald W. Black, MD, and Nagy Youssef, MD, April 4-6, 2013 at the Swissôtel in Chicago, IL. Attendees could earn up to 18 AMA PRA Category 1 Credits™.
THURSDAY, APRIL 4, 2013
MORNING SESSIONS
Evidence-based medicine and treatment guidelines may not address complex patients with treatment-resistant depression (TRD). Andrew A. Nierenberg, MD, Massachusetts General Hospital, reviewed newer medications for TRD, including olanzapine-fluoxetine combination, ketamine, riluzole, and L-methylfolate; however, use of these medications requires careful consideration of risks and benefits.
Many FDA-approved drugs have a “black-box” warning, but still are widely used. Henry A. Nasrallah, MD, University of Cincinnati, reviewed black-box warnings for antipsychotics, antidepressants, mood stabilizers, benzodiazepines, stimulants, opiates, and hypnotics and offered strategies on how to incorporate these warnings into clinical practice.
Dr. Nierenberg discussed the outcomes of 3 published medication effectiveness studies for bipolar disorder (BD)—STEP-BD, BALANCE, and LiTMUS—and one currently underway, CHOICE. These studies examined monotherapy and combination therapy with antidepressants, anticonvulsants, antipsychotics, and psychosocial interventions.
Although there is an association between psychosis and violence, most psychotic patients are not violent. Rajiv Tandon, MD, University of Florida, reviewed modifiable and nonmodifiable risk factors for violence, key clinical questions to consider, and scales to use when assessing a patient’s risk of violence.
AFTERNOON SESSIONS
Measuring biomarkers can augment other clinical methods to help identify metabolic, structural, and functional brain changes associated with preclinical stages of cognitive disorders. James Ellison, MD, MPH, McLean Hospital, Harvard Medical School, explained how biomarkers can improve the differential diagnosis of memory impairments and aid in identifying different types of dementia.
Donald W. Black, MD, (right) receives the 2013 George Winokur Research
Award from Carol S. North, MD, for his article on pathological gambling
Case-control studies have found a strong association between schizophrenia and type II diabetes, which contributes to higher mortality among schizophrenia patients. Along with vigilant metabolic monitoring, Dr. Tandon recommended a therapeutic approach that includes changing antipsychotics, prescribing metformin, suggesting lifestyle interventions, and treating comorbid conditions.
Depressed older adults may report anxiety, hopelessness, anhedonia, or somatic symptoms, rather than sadness. Depressive symptoms may be associated with vascular disease or cognitive impairment. Dr. Ellison reviewed psychotherapeutic and pharmacologic treatments for older depressed patients.
FRIDAY, APRIL 5, 2013
MORNING SESSIONS
Many strategies exist for treating patients with TRD; adding an atypical antipsychotic has the best evidence, but there are tolerability considerations. Dr. Nierenberg suggested using a combination of treatments.
Pregnancy is inherently risky for women who take antipsychotics. In all patients of childbearing potential, take a thorough reproductive history and ask about contraception use. Marlene P. Freeman, MD, Massachusetts General Hospital, explained that psychotropics with unfavorable FDA pregnancy ratings may be among first-line choices.
George T. Grossberg, MD, (left) speaks with attendees
Clinical symptoms, cognitive deficits, psychiatric comorbidities, genetic factors, neuroimaging features, and pharmacotherapy may overlap considerably between schizophrenia and BD. Dr. Nasrallah described clinical features that differentiate the 2 disorders.
Cognitive enhancers can improve activities of daily living, behavior, and cognition in patients with Alzheimer’s disease. George T. Grossberg, MD, St. Louis University, reviewed the evidence for acetylcholinesterase inhibitors, the NMDA receptor antagonist memantine, combination therapy, and atypical antipsychotics.
Dietary consultation for older patients might help delay or decrease their risk of dementia. Patients should consume omega-3 fatty acids, whole grains, fresh fruits and vegetables, beans, legumes, and certain spices. Dr. Grossberg also suggested patients engage in physical and mental exercises, social and spiritual activities, and stress reduction, and control cardiovascular risk factors.
AFTERNOON SESSIONS
Many women experience anxiety during pregnancy, and the risk is highest during the first trimester. Dr. Freeman reviewed prevalence, diagnosis, and treatment of panic disorder, generalized anxiety disorder, obsessive-compulsive disorder, and posttraumatic stress disorder during pregnancy and postpartum.
Kathleen Brady, MD, PhD, Medical University of South Carolina, explained how methylenedioxypyrovalerone, also known as bath salts, and other designer drugs are not detectable on standard urine drug screens. Agitation, tachycardia, combative behavior, hyperthermia, and hallucinations have been reported.
Kathleen Brady, MD, MPH
Alcohol abuse and depression are highly comorbid and are associated with higher suicidality, more severe symptoms, and poorer treatment response than either disorder alone. Depressive symptoms often are seen during alcohol withdrawal, and may resolve with abstinence. Dr. Brady reviewed the evidence for treating depressed alcoholics with antidepressants, medications targeting alcohol dependence such as disulfiram and naltrexone, and psychotherapy.
Ralph Aquila, MD, Columbia College of Physicians and Surgeons, discussed risk factors for and consequences of treatment nonadherence in patients with schizophrenia. Leslie L. Citrome, MD, MPH, New York Medical College, covered strategies to improve adherence, including identifying and addressing barriers to adherence for individual patients, improving the therapeutic alliance, and considering long-acting injectable antipsychotics.
SATURDAY, APRIL 6, 2013
MORNING SESSIONS
Forty-six percent of depressed patients will stop pharmacotherapy before they have a chance to respond. To minimize short-term side effects, Andrew J. Cutler, MD, Florida Clinical Research Center, suggested educating patients and slowly titrating medications; options for reducing long-term side effects or residual symptoms include switching or augmenting pharmacotherapy.
When treating patients addicted to opioids, outcome measures go beyond general health to obtaining employment and reducing criminal activity. Pharmacotherapy options include methadone maintenance therapy, oral and injectable naltrexone, and oral, sublingual, and implantable buprenorphine. Walter Ling, MD, David Geffen School of Medicine at UCLA, described factors that may improve patient outcomes.
Geriatric BD is relatively common in clinical settings, but there is a lack of evidence-based clinical practice guidelines. James W. Jefferson, MD, University of Wisconsin School of Medicine and Public Health, recommended choosing a treatment based on the illness phase and balancing the benefit of certain pharmacotherapies against short- and long-term risks.
Most medications for treating alcohol dependence work by modulating functions of opioids, glutamate, GABA, and serotonin. Dr. Ling reviewed the evidence base, dosing guidelines, and clinical recommendations for disulfiram, oral and injectable naltrexone, and acamprosate, which are FDA-approved for treating alcohol dependence. He also recommended combining medications with nonpharmacologic treatments, such as 12-step programs.
Most people who die by suicide deny suicide ideation at their last mental health visit. Risk factors for suicide include family history of suicide, childhood or adult trauma, substance abuse, stressful life events, chronic illness, and psychiatric disorders. Dr. Jefferson described suicide rating and tracking scales and encouraged clinicians to document suicide risk evaluations.
AFTERNOON SESSION
Roger S. McIntyre, MD, FRCPCRobert M.A. Hirschfeld, MD, University of Texas Medical Branch, discussed how the concept of allostatic load—bodily “wear and tear” that emerges with sustained allostatic states—may help explain cognitive and physical decline associated with BD. Roger S. McIntyre, MD, FRCPC, University of Toronto, emphasized that BD is a progressive disorder and comorbidities such as metabolic problems may promote this progression. Terence A. Ketter, MD, Stanford School of Medicine, covered new developments in BD treatment, including certain second-generation antipsychotics, dopaminergic neurotransmission enhancers, mood stabilizers, adjunctive antidepressants, and adjunctive psychotherapy.
Current Psychiatry and the American Academy of Clinical Psychiatrists were pleased to host more than 550 psychiatric practitioners for this conference, led by Meeting Chair Richard Balon, MD, and Meeting Co-Chairs Donald W. Black, MD, and Nagy Youssef, MD, April 4-6, 2013 at the Swissôtel in Chicago, IL. Attendees could earn up to 18 AMA PRA Category 1 Credits™.
THURSDAY, APRIL 4, 2013
MORNING SESSIONS
Evidence-based medicine and treatment guidelines may not address complex patients with treatment-resistant depression (TRD). Andrew A. Nierenberg, MD, Massachusetts General Hospital, reviewed newer medications for TRD, including olanzapine-fluoxetine combination, ketamine, riluzole, and L-methylfolate; however, use of these medications requires careful consideration of risks and benefits.
Many FDA-approved drugs have a “black-box” warning, but still are widely used. Henry A. Nasrallah, MD, University of Cincinnati, reviewed black-box warnings for antipsychotics, antidepressants, mood stabilizers, benzodiazepines, stimulants, opiates, and hypnotics and offered strategies on how to incorporate these warnings into clinical practice.
Dr. Nierenberg discussed the outcomes of 3 published medication effectiveness studies for bipolar disorder (BD)—STEP-BD, BALANCE, and LiTMUS—and one currently underway, CHOICE. These studies examined monotherapy and combination therapy with antidepressants, anticonvulsants, antipsychotics, and psychosocial interventions.
Although there is an association between psychosis and violence, most psychotic patients are not violent. Rajiv Tandon, MD, University of Florida, reviewed modifiable and nonmodifiable risk factors for violence, key clinical questions to consider, and scales to use when assessing a patient’s risk of violence.
AFTERNOON SESSIONS
Measuring biomarkers can augment other clinical methods to help identify metabolic, structural, and functional brain changes associated with preclinical stages of cognitive disorders. James Ellison, MD, MPH, McLean Hospital, Harvard Medical School, explained how biomarkers can improve the differential diagnosis of memory impairments and aid in identifying different types of dementia.
Donald W. Black, MD, (right) receives the 2013 George Winokur Research
Award from Carol S. North, MD, for his article on pathological gambling
Case-control studies have found a strong association between schizophrenia and type II diabetes, which contributes to higher mortality among schizophrenia patients. Along with vigilant metabolic monitoring, Dr. Tandon recommended a therapeutic approach that includes changing antipsychotics, prescribing metformin, suggesting lifestyle interventions, and treating comorbid conditions.
Depressed older adults may report anxiety, hopelessness, anhedonia, or somatic symptoms, rather than sadness. Depressive symptoms may be associated with vascular disease or cognitive impairment. Dr. Ellison reviewed psychotherapeutic and pharmacologic treatments for older depressed patients.
FRIDAY, APRIL 5, 2013
MORNING SESSIONS
Many strategies exist for treating patients with TRD; adding an atypical antipsychotic has the best evidence, but there are tolerability considerations. Dr. Nierenberg suggested using a combination of treatments.
Pregnancy is inherently risky for women who take antipsychotics. In all patients of childbearing potential, take a thorough reproductive history and ask about contraception use. Marlene P. Freeman, MD, Massachusetts General Hospital, explained that psychotropics with unfavorable FDA pregnancy ratings may be among first-line choices.
George T. Grossberg, MD, (left) speaks with attendees
Clinical symptoms, cognitive deficits, psychiatric comorbidities, genetic factors, neuroimaging features, and pharmacotherapy may overlap considerably between schizophrenia and BD. Dr. Nasrallah described clinical features that differentiate the 2 disorders.
Cognitive enhancers can improve activities of daily living, behavior, and cognition in patients with Alzheimer’s disease. George T. Grossberg, MD, St. Louis University, reviewed the evidence for acetylcholinesterase inhibitors, the NMDA receptor antagonist memantine, combination therapy, and atypical antipsychotics.
Dietary consultation for older patients might help delay or decrease their risk of dementia. Patients should consume omega-3 fatty acids, whole grains, fresh fruits and vegetables, beans, legumes, and certain spices. Dr. Grossberg also suggested patients engage in physical and mental exercises, social and spiritual activities, and stress reduction, and control cardiovascular risk factors.
AFTERNOON SESSIONS
Many women experience anxiety during pregnancy, and the risk is highest during the first trimester. Dr. Freeman reviewed prevalence, diagnosis, and treatment of panic disorder, generalized anxiety disorder, obsessive-compulsive disorder, and posttraumatic stress disorder during pregnancy and postpartum.
Kathleen Brady, MD, PhD, Medical University of South Carolina, explained how methylenedioxypyrovalerone, also known as bath salts, and other designer drugs are not detectable on standard urine drug screens. Agitation, tachycardia, combative behavior, hyperthermia, and hallucinations have been reported.
Kathleen Brady, MD, MPH
Alcohol abuse and depression are highly comorbid and are associated with higher suicidality, more severe symptoms, and poorer treatment response than either disorder alone. Depressive symptoms often are seen during alcohol withdrawal, and may resolve with abstinence. Dr. Brady reviewed the evidence for treating depressed alcoholics with antidepressants, medications targeting alcohol dependence such as disulfiram and naltrexone, and psychotherapy.
Ralph Aquila, MD, Columbia College of Physicians and Surgeons, discussed risk factors for and consequences of treatment nonadherence in patients with schizophrenia. Leslie L. Citrome, MD, MPH, New York Medical College, covered strategies to improve adherence, including identifying and addressing barriers to adherence for individual patients, improving the therapeutic alliance, and considering long-acting injectable antipsychotics.
SATURDAY, APRIL 6, 2013
MORNING SESSIONS
Forty-six percent of depressed patients will stop pharmacotherapy before they have a chance to respond. To minimize short-term side effects, Andrew J. Cutler, MD, Florida Clinical Research Center, suggested educating patients and slowly titrating medications; options for reducing long-term side effects or residual symptoms include switching or augmenting pharmacotherapy.
When treating patients addicted to opioids, outcome measures go beyond general health to obtaining employment and reducing criminal activity. Pharmacotherapy options include methadone maintenance therapy, oral and injectable naltrexone, and oral, sublingual, and implantable buprenorphine. Walter Ling, MD, David Geffen School of Medicine at UCLA, described factors that may improve patient outcomes.
Geriatric BD is relatively common in clinical settings, but there is a lack of evidence-based clinical practice guidelines. James W. Jefferson, MD, University of Wisconsin School of Medicine and Public Health, recommended choosing a treatment based on the illness phase and balancing the benefit of certain pharmacotherapies against short- and long-term risks.
Most medications for treating alcohol dependence work by modulating functions of opioids, glutamate, GABA, and serotonin. Dr. Ling reviewed the evidence base, dosing guidelines, and clinical recommendations for disulfiram, oral and injectable naltrexone, and acamprosate, which are FDA-approved for treating alcohol dependence. He also recommended combining medications with nonpharmacologic treatments, such as 12-step programs.
Most people who die by suicide deny suicide ideation at their last mental health visit. Risk factors for suicide include family history of suicide, childhood or adult trauma, substance abuse, stressful life events, chronic illness, and psychiatric disorders. Dr. Jefferson described suicide rating and tracking scales and encouraged clinicians to document suicide risk evaluations.
AFTERNOON SESSION
Roger S. McIntyre, MD, FRCPCRobert M.A. Hirschfeld, MD, University of Texas Medical Branch, discussed how the concept of allostatic load—bodily “wear and tear” that emerges with sustained allostatic states—may help explain cognitive and physical decline associated with BD. Roger S. McIntyre, MD, FRCPC, University of Toronto, emphasized that BD is a progressive disorder and comorbidities such as metabolic problems may promote this progression. Terence A. Ketter, MD, Stanford School of Medicine, covered new developments in BD treatment, including certain second-generation antipsychotics, dopaminergic neurotransmission enhancers, mood stabilizers, adjunctive antidepressants, and adjunctive psychotherapy.
Current Psychiatry and the American Academy of Clinical Psychiatrists were pleased to host more than 550 psychiatric practitioners for this conference, led by Meeting Chair Richard Balon, MD, and Meeting Co-Chairs Donald W. Black, MD, and Nagy Youssef, MD, April 4-6, 2013 at the Swissôtel in Chicago, IL. Attendees could earn up to 18 AMA PRA Category 1 Credits™.
THURSDAY, APRIL 4, 2013
MORNING SESSIONS
Evidence-based medicine and treatment guidelines may not address complex patients with treatment-resistant depression (TRD). Andrew A. Nierenberg, MD, Massachusetts General Hospital, reviewed newer medications for TRD, including olanzapine-fluoxetine combination, ketamine, riluzole, and L-methylfolate; however, use of these medications requires careful consideration of risks and benefits.
Many FDA-approved drugs have a “black-box” warning, but still are widely used. Henry A. Nasrallah, MD, University of Cincinnati, reviewed black-box warnings for antipsychotics, antidepressants, mood stabilizers, benzodiazepines, stimulants, opiates, and hypnotics and offered strategies on how to incorporate these warnings into clinical practice.
Dr. Nierenberg discussed the outcomes of 3 published medication effectiveness studies for bipolar disorder (BD)—STEP-BD, BALANCE, and LiTMUS—and one currently underway, CHOICE. These studies examined monotherapy and combination therapy with antidepressants, anticonvulsants, antipsychotics, and psychosocial interventions.
Although there is an association between psychosis and violence, most psychotic patients are not violent. Rajiv Tandon, MD, University of Florida, reviewed modifiable and nonmodifiable risk factors for violence, key clinical questions to consider, and scales to use when assessing a patient’s risk of violence.
AFTERNOON SESSIONS
Measuring biomarkers can augment other clinical methods to help identify metabolic, structural, and functional brain changes associated with preclinical stages of cognitive disorders. James Ellison, MD, MPH, McLean Hospital, Harvard Medical School, explained how biomarkers can improve the differential diagnosis of memory impairments and aid in identifying different types of dementia.
Donald W. Black, MD, (right) receives the 2013 George Winokur Research
Award from Carol S. North, MD, for his article on pathological gambling
Case-control studies have found a strong association between schizophrenia and type II diabetes, which contributes to higher mortality among schizophrenia patients. Along with vigilant metabolic monitoring, Dr. Tandon recommended a therapeutic approach that includes changing antipsychotics, prescribing metformin, suggesting lifestyle interventions, and treating comorbid conditions.
Depressed older adults may report anxiety, hopelessness, anhedonia, or somatic symptoms, rather than sadness. Depressive symptoms may be associated with vascular disease or cognitive impairment. Dr. Ellison reviewed psychotherapeutic and pharmacologic treatments for older depressed patients.
FRIDAY, APRIL 5, 2013
MORNING SESSIONS
Many strategies exist for treating patients with TRD; adding an atypical antipsychotic has the best evidence, but there are tolerability considerations. Dr. Nierenberg suggested using a combination of treatments.
Pregnancy is inherently risky for women who take antipsychotics. In all patients of childbearing potential, take a thorough reproductive history and ask about contraception use. Marlene P. Freeman, MD, Massachusetts General Hospital, explained that psychotropics with unfavorable FDA pregnancy ratings may be among first-line choices.
George T. Grossberg, MD, (left) speaks with attendees
Clinical symptoms, cognitive deficits, psychiatric comorbidities, genetic factors, neuroimaging features, and pharmacotherapy may overlap considerably between schizophrenia and BD. Dr. Nasrallah described clinical features that differentiate the 2 disorders.
Cognitive enhancers can improve activities of daily living, behavior, and cognition in patients with Alzheimer’s disease. George T. Grossberg, MD, St. Louis University, reviewed the evidence for acetylcholinesterase inhibitors, the NMDA receptor antagonist memantine, combination therapy, and atypical antipsychotics.
Dietary consultation for older patients might help delay or decrease their risk of dementia. Patients should consume omega-3 fatty acids, whole grains, fresh fruits and vegetables, beans, legumes, and certain spices. Dr. Grossberg also suggested patients engage in physical and mental exercises, social and spiritual activities, and stress reduction, and control cardiovascular risk factors.
AFTERNOON SESSIONS
Many women experience anxiety during pregnancy, and the risk is highest during the first trimester. Dr. Freeman reviewed prevalence, diagnosis, and treatment of panic disorder, generalized anxiety disorder, obsessive-compulsive disorder, and posttraumatic stress disorder during pregnancy and postpartum.
Kathleen Brady, MD, PhD, Medical University of South Carolina, explained how methylenedioxypyrovalerone, also known as bath salts, and other designer drugs are not detectable on standard urine drug screens. Agitation, tachycardia, combative behavior, hyperthermia, and hallucinations have been reported.
Kathleen Brady, MD, MPH
Alcohol abuse and depression are highly comorbid and are associated with higher suicidality, more severe symptoms, and poorer treatment response than either disorder alone. Depressive symptoms often are seen during alcohol withdrawal, and may resolve with abstinence. Dr. Brady reviewed the evidence for treating depressed alcoholics with antidepressants, medications targeting alcohol dependence such as disulfiram and naltrexone, and psychotherapy.
Ralph Aquila, MD, Columbia College of Physicians and Surgeons, discussed risk factors for and consequences of treatment nonadherence in patients with schizophrenia. Leslie L. Citrome, MD, MPH, New York Medical College, covered strategies to improve adherence, including identifying and addressing barriers to adherence for individual patients, improving the therapeutic alliance, and considering long-acting injectable antipsychotics.
SATURDAY, APRIL 6, 2013
MORNING SESSIONS
Forty-six percent of depressed patients will stop pharmacotherapy before they have a chance to respond. To minimize short-term side effects, Andrew J. Cutler, MD, Florida Clinical Research Center, suggested educating patients and slowly titrating medications; options for reducing long-term side effects or residual symptoms include switching or augmenting pharmacotherapy.
When treating patients addicted to opioids, outcome measures go beyond general health to obtaining employment and reducing criminal activity. Pharmacotherapy options include methadone maintenance therapy, oral and injectable naltrexone, and oral, sublingual, and implantable buprenorphine. Walter Ling, MD, David Geffen School of Medicine at UCLA, described factors that may improve patient outcomes.
Geriatric BD is relatively common in clinical settings, but there is a lack of evidence-based clinical practice guidelines. James W. Jefferson, MD, University of Wisconsin School of Medicine and Public Health, recommended choosing a treatment based on the illness phase and balancing the benefit of certain pharmacotherapies against short- and long-term risks.
Most medications for treating alcohol dependence work by modulating functions of opioids, glutamate, GABA, and serotonin. Dr. Ling reviewed the evidence base, dosing guidelines, and clinical recommendations for disulfiram, oral and injectable naltrexone, and acamprosate, which are FDA-approved for treating alcohol dependence. He also recommended combining medications with nonpharmacologic treatments, such as 12-step programs.
Most people who die by suicide deny suicide ideation at their last mental health visit. Risk factors for suicide include family history of suicide, childhood or adult trauma, substance abuse, stressful life events, chronic illness, and psychiatric disorders. Dr. Jefferson described suicide rating and tracking scales and encouraged clinicians to document suicide risk evaluations.
AFTERNOON SESSION
Roger S. McIntyre, MD, FRCPCRobert M.A. Hirschfeld, MD, University of Texas Medical Branch, discussed how the concept of allostatic load—bodily “wear and tear” that emerges with sustained allostatic states—may help explain cognitive and physical decline associated with BD. Roger S. McIntyre, MD, FRCPC, University of Toronto, emphasized that BD is a progressive disorder and comorbidities such as metabolic problems may promote this progression. Terence A. Ketter, MD, Stanford School of Medicine, covered new developments in BD treatment, including certain second-generation antipsychotics, dopaminergic neurotransmission enhancers, mood stabilizers, adjunctive antidepressants, and adjunctive psychotherapy.
A taste for the unusual
Discuss this article at www.facebook.com/CurrentPsychiatry
CASE: Nauseous and full
Ms. O, age 48, presents to the emergency department reporting a 3-day history of vomiting approximately 5 minutes after consuming solids or liquids. She’s had 10 vomiting episodes, which were associated with “fullness” and an “aching” sensation she rates as 6 on a 10-point scale pain scale that is diffuse over the upper epigastric area, with no palliative factors. Ms. O has not had a bowel movement for 3 days and her last menstrual period was 8 days ago. She is taking lorazepam, 1 mg/d. Her medical and psychiatric history includes anxiety, depression, personality disorder symptoms of affective dysregulation, obesity (270 lbs; medium height), and pica. She was 352 lbs when she underwent a Roux-en-Y gastric bypass 2 years ago. One year earlier, she had a laparoscopic gastric bezoar removal and an incisional hernia repair. Ms. O had no pica-related surgeries before undergoing gastric bypass surgery.
Ms. O denies shortness of breath, chest pain, allergies, smoking, or alcohol abuse, but reports uncontrollable cravings for paper products, specifically cardboard, which she describes as “just so delicious.” This craving led her to consume large amounts of cardboard and newspaper in the days before she began vomiting.
What may be causing Ms. O’s pica symptoms?
- iron deficiency anemia
- complications from gastric bypass surgery
- personality disorder
- generalized anxiety disorder (GAD)
The authors’ observations
DSM-IV-TR diagnostic criteria for pica include the persistent eating of non-nutritive substances for ≥1 month that is inappropriate for the level of a person’s development and not an acceptable part of one’s culture.1 If pica occurs with other mental disorders, it must be severe enough to indicate further clinical assessment to receive a separate diagnosis. Often associated with pregnancy, iron deficiency anemia, early development, and mental retardation, pica has been observed in post-gastric bypass surgery patients, all of whom presented with pagophagia (compulsive ice eating), and in one case was associated with a bezoar causing obstruction of the GI tract.1,2 With the dramatic increase in gastric bypass surgery and the required presurgical mental health evaluation, the consequences of failing to screen patients for pica behaviors can be devastating.
EVALUATION: Low iron
Ms. O’s vital signs on admission are stable, and physical exam is notable for mild abdominal distention with no guarding, tenderness, rigidity, or masses. No rebound tenderness is elicited. CT scan shows evidence of post-surgical changes involving the small bowel consistent with gastric bypass surgery and a hiatal hernia, but no obstruction, focal inflammation, free fluids, or gas. Lab values for amylase, lipase, urinalysis, coagulation studies, cardiac enzymes, and complete metabolic profile are within normal limits. Although not anemic, Ms. O is iron deficient, with ferritin, 10 ng/mL (normal 10 to 120 ng/mL); B12, 299 pg/mL (normal 100 to 700 pg/mL); and iron, 25 μg/dL (normal 50 to 170 μg/dL).
A foreign body is removed endoscopically and the specimen is sent to pathology. It is determined to be a gastric bezoar, yellowish-green in color, measuring 2.5 cm × 1 cm × 0.8 cm. After bezoar removal, Ms. O tolerates food and is discharged home on vitamin B12, 1,000 mcg/d for 2 weeks; folate, 1 mg/d for 1 month; calcium with vitamin D, 1 g/d; and esomeprazole, 40 mg/d for frequent heartburn. She is referred to psychiatry for behavioral modification therapy and medication management.
How would you treat Ms. O?
- start a selective serotonin reuptake inhibitor (SSRI)
- prescribe an atypical antipsychotic
- continue lorazepam
- begin behavioral therapy
HISTORY: Pica during pregnancy
During psychiatric workup, Ms. O admits to having pica urges most of her life, but experienced an uncontrollable exacerbation after gastric bypass surgery. This led to intense, chaotic periods of pica, resulting in a previous bezoar removal. She is particularly attracted to cardboard and newspaper cartoons, but notes she also has felt the urge to eat charcoal, moist soil, clay, chalk, pencils, and new shoes, which she chews on. In the past, her extreme anxiety and preoccupation with these urges had lead to diagnoses of personality disorder not otherwise specified, GAD, and obsessive-compulsive disorder.
Her first experience with pica was during her first pregnancy at age 15, when she had an impulse to eat soil. The urges briefly stopped until she became pregnant again. During each of her 5 pregnancies her pica symptoms returned. At one point during her last pregnancy she reports having felt out of control, eating 2 to 3 pencils with the eraser per day, after which she would feel intense relaxation. Her mother also exhibited symptoms of pica toward charcoal and soil. Ms. O had been taking unknown dosages of lorazepam for anxiety and fluoxetine for depression, both of which she stopped because she feared side effects during her last pregnancy. However, she never experienced any side effects.
The authors’ observations
Although pica is most commonly observed in young children, it sometimes is seen in pregnant women.1 Pica frequently is associated with other mental disorders, such as pervasive developmental disorder and mental retardation,1 and can be associated with premorbid psychosis and anxiety disorders. Occasional vitamin and mineral deficiencies, such as iron or zinc, have been reported, but usually patients’ lab values are normal. Treatment usually is initiated in the context of medical complications, such as iron deficiency anemia. In Ms. O’s case, the precipitating event was mechanical bowel obstruction due to a bezoar.
Several theories about the origins of pica have been proposed, but none truly are explanatory or satisfactory. The nutritional theory—that patients eat non-nutritive substances to compensate for mineral deficiencies—is popular because of pica’s frequent association with mineral deficiencies, but it is unknown whether pica is the cause or the result of the deficiency. An example of this is anemia due to eating clay instead of foods that contain iron. Another theory is that because pica is normal in early childhood development, it may be a manifestation of delayed development or mental retardation. The cultural theory is attractive because pregnant women in several cultures eat starch or clay as a part of their native rituals, and the incidence of pica is relatively high among pregnant African American women who live in rural areas.3 In the Roux-en-Y procedure, bypass of the duodenum and proximal jejunum can significantly decrease a patient’s iron uptake, leading to iron deficiency anemia, and could trigger pica in a susceptible patient.4
Exacerbation after gastric bypass
Kushner et al4 describes re-emergent pica after bariatric surgery in 2 patients with pagophagia associated with concomitant iron deficiency anemia. A 41-year-old white woman presented with pagophagia and a history of childhood consumption of dirt, chalk, and clay. Another patient, a 34-year-old African American woman, suffered from a lifelong desire to eat dirt, which she was able to resist, but experienced pagophagia during pregnancy and later when she developed iron deficiency anemia.4 In another case series, Kushner et al5 describes a 35-year-old woman with iron deficiency anemia with pagophagia presenting 2 years after Roux-en-Y. Her history was significant for eating clay as a child, but this new-onset pagophagia was so intense she purchased 2 snow cone machines, one for home and one for work, to feed her urges. Another patient, a 45-year-old African American woman, had an irresistible craving for calcium carbonate antacids, eating 40 to 50 a day, as well as several 30-ounce cups of ice.5 A third case report details a 33-year-old woman with iron deficiency anemia who presented with nocturnal pagophagia after Roux-en-Y anastomosis. She repeatedly rose during the night to eat the frost off the ice maker in her refrigerator.6 Another case described a female patient who ate cardboard after having a Roux-en-Y.2
Common themes in these case reports are female sex, Roux-en-Y, and dramatic resurgence of previously noted pica behaviors after gastric bypass surgery. Several studies have shown that pagophagia and pica in patients who are iron deficient or have iron deficiency anemia can be rapidly curbed with iron supplements.5 Ms. O, who has low iron, is taking iron supplementation, yet continues to experience pica cravings, albeit less severely. Her pica could be psychiatric in origin, perhaps related to her history of anxiety.
OUTCOME: Combination therapy
We start Ms. O on ziprasidone, 80 mg twice a day, restart lorazepam, 1 mg/d, and schedule monthly follow-up appointments to monitor her pica symptoms. We prescribe ziprasidone because it could treat paranoia and preoccupations and is considered to be weight-neutral. She continues her supplements, including ferrous sulfate, 325 mg 3 times daily. Ms. O attends weekly behavioral therapy sessions, during which the therapist monitors her mood and cravings with response prevention, which entails purposely avoiding behaviors after initiating a distressing stimulus. Ms. O responds well to medication and psychotherapy 1 month after the gastric bezoar removal, and she reports a decreased urge to eat cardboard. She is able to increase the amount of time she can go without eating non-nutritive substances—once daily, rather than repeatedly throughout the day.
The authors’ observations
Each patient with pica likely needs customized care. Children need to be supervised to prevent ingestion of lead-containing substances such as paint chips. Iron supplements are recommended for iron deficiency anemia and prophylaxis for iron deficiency anemia in Roux-en-Y patients.3,4 Pica in pregnant patients should be addressed to maintain adequate nutrition and prevent accidental poisonings.7 Behavioral intervention strategies are based on positive reinforcement and punishment (Table).8 A report of 3 young children with pica noted successful treatment of one with automatic reinforcement, and the other 2 with a combination of social and automatic reinforcement.9 There are no FDA-approved medications for pica. Positive effects have been seen with SSRIs, bupropion, atypical antipsychotics, buprenorphine, and chlorimipramine.10 Olanzapine has shown positive results as a treatment for pica.11 Most pica patients need concurrent psychotherapy.10
Table
Behavioral interventions for pica
| Intervention | Comments |
|---|---|
| Environmental enrichment | Providing additional stimulus to increase neuronal activity and focus behaviors |
| Noncontingent reinforcement | Presenting reinforcers according to a fixed schedule |
| Differential reinforcement | Desired behaviors are reinforced and inappropriate behaviors are ignored |
| Response blocking | Physically block a patient’s attempts to eat nonedible items |
| Source: Reference 8 | |
Related Resources
- Blinder BJ, Salama C. An update on pica: prevalence, contributing causes, and treatment. Psychiatric Times. www.psychiatrictimes.com/display/article/10168/1159376?pageNumber=1. Published May 1, 2008.
- Nurcombe B. Developmental disorders of attachment, feeding, elimination, & sleeping. In: Ebert MH, Loosen PT, Nurcombe B, et al, eds. CURRENT diagnosis & treatment: psychiatry. 2nd ed. New York, NY: McGraw Hill; 2008.
Drug Brand Names
- Buprenorphine • Subutex
- Bupropion • Wellbutrin, Zyban
- Chlorimipramine • Anafranil
- Esomeprazole • Nexium
- Fluoxetine • Prozac
- Lorazepam • Ativan
- Olanzapine • Zyprexa
- Ziprasidone • Geodon
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
2. Patton W, Gibbs K. Cardboard bezoar complicating laparoscopic gastric bypass. Surg Obes Relat Dis. 2010;6(3):313-315.
3. Nurcombe B. Developmental disorders of attachment feeding, elimination, & sleeping. In: Ebert MH, Loosen PT, Nurcombe B, et al, eds. CURRENT diagnosis & treatment: psychiatry. 2nd ed. New York, NY: McGraw Hill; 2008.
4. Kushner F, Gleason B, Shanta-Retelny V. Reemergence of pica following gastric bypass surgery for obesity: a new presentation of an old problem. J Am Diet Assoc. 2004;104(9):1393-1397.
5. Kushner F, Shanta Retelny V. Emergence of pica (ingestion of non-food substances) accompanying iron deficiency anemia after gastric bypass surgery. Obes Surg. 2005;15(10):1491-1495.
6. Marinella MA. Nocturnal pagophagia complicating gastric bypass. Mayo Clin Proc. 2008;83(8):961.-
7. Bernstein B, Weinstein M. Normal pregnancy & prenatal care. In: DeCherney AH Nathan L, Goodwin TM, et al, eds. CURRENT diagnosis & treatment obstetrics & gynecology. 10th ed. New York, NY: McGraw Hill; 2007.
8. Piazza C, Fisher W, Hanley P, et al. Treatment of pica through multiple analyses of its reinforcing functions. J Appl Behav Anal. 1998;31(2):165-189.
9. Williams DE, McAdam D. Assessment behavioral treatment, and prevention of pica: clinical guidelines and recommendations for practitioners. Res Dev Disabil. 2012;33(6):2050-2057.
10. Blinder BJ, Salama C. An update on pica: prevalence contributing causes, and treatment. Psychiatric Times. http://www.psychiatrictimes.com/display/article/10168/1159376?pageNumber=1. Published May 1, 2008. Accessed January 23, 2013.
11. Lerner AJ. Treatment of pica behavior with olanzapine. CNS Spectr. 2008;13(1):19.-
Discuss this article at www.facebook.com/CurrentPsychiatry
CASE: Nauseous and full
Ms. O, age 48, presents to the emergency department reporting a 3-day history of vomiting approximately 5 minutes after consuming solids or liquids. She’s had 10 vomiting episodes, which were associated with “fullness” and an “aching” sensation she rates as 6 on a 10-point scale pain scale that is diffuse over the upper epigastric area, with no palliative factors. Ms. O has not had a bowel movement for 3 days and her last menstrual period was 8 days ago. She is taking lorazepam, 1 mg/d. Her medical and psychiatric history includes anxiety, depression, personality disorder symptoms of affective dysregulation, obesity (270 lbs; medium height), and pica. She was 352 lbs when she underwent a Roux-en-Y gastric bypass 2 years ago. One year earlier, she had a laparoscopic gastric bezoar removal and an incisional hernia repair. Ms. O had no pica-related surgeries before undergoing gastric bypass surgery.
Ms. O denies shortness of breath, chest pain, allergies, smoking, or alcohol abuse, but reports uncontrollable cravings for paper products, specifically cardboard, which she describes as “just so delicious.” This craving led her to consume large amounts of cardboard and newspaper in the days before she began vomiting.
What may be causing Ms. O’s pica symptoms?
- iron deficiency anemia
- complications from gastric bypass surgery
- personality disorder
- generalized anxiety disorder (GAD)
The authors’ observations
DSM-IV-TR diagnostic criteria for pica include the persistent eating of non-nutritive substances for ≥1 month that is inappropriate for the level of a person’s development and not an acceptable part of one’s culture.1 If pica occurs with other mental disorders, it must be severe enough to indicate further clinical assessment to receive a separate diagnosis. Often associated with pregnancy, iron deficiency anemia, early development, and mental retardation, pica has been observed in post-gastric bypass surgery patients, all of whom presented with pagophagia (compulsive ice eating), and in one case was associated with a bezoar causing obstruction of the GI tract.1,2 With the dramatic increase in gastric bypass surgery and the required presurgical mental health evaluation, the consequences of failing to screen patients for pica behaviors can be devastating.
EVALUATION: Low iron
Ms. O’s vital signs on admission are stable, and physical exam is notable for mild abdominal distention with no guarding, tenderness, rigidity, or masses. No rebound tenderness is elicited. CT scan shows evidence of post-surgical changes involving the small bowel consistent with gastric bypass surgery and a hiatal hernia, but no obstruction, focal inflammation, free fluids, or gas. Lab values for amylase, lipase, urinalysis, coagulation studies, cardiac enzymes, and complete metabolic profile are within normal limits. Although not anemic, Ms. O is iron deficient, with ferritin, 10 ng/mL (normal 10 to 120 ng/mL); B12, 299 pg/mL (normal 100 to 700 pg/mL); and iron, 25 μg/dL (normal 50 to 170 μg/dL).
A foreign body is removed endoscopically and the specimen is sent to pathology. It is determined to be a gastric bezoar, yellowish-green in color, measuring 2.5 cm × 1 cm × 0.8 cm. After bezoar removal, Ms. O tolerates food and is discharged home on vitamin B12, 1,000 mcg/d for 2 weeks; folate, 1 mg/d for 1 month; calcium with vitamin D, 1 g/d; and esomeprazole, 40 mg/d for frequent heartburn. She is referred to psychiatry for behavioral modification therapy and medication management.
How would you treat Ms. O?
- start a selective serotonin reuptake inhibitor (SSRI)
- prescribe an atypical antipsychotic
- continue lorazepam
- begin behavioral therapy
HISTORY: Pica during pregnancy
During psychiatric workup, Ms. O admits to having pica urges most of her life, but experienced an uncontrollable exacerbation after gastric bypass surgery. This led to intense, chaotic periods of pica, resulting in a previous bezoar removal. She is particularly attracted to cardboard and newspaper cartoons, but notes she also has felt the urge to eat charcoal, moist soil, clay, chalk, pencils, and new shoes, which she chews on. In the past, her extreme anxiety and preoccupation with these urges had lead to diagnoses of personality disorder not otherwise specified, GAD, and obsessive-compulsive disorder.
Her first experience with pica was during her first pregnancy at age 15, when she had an impulse to eat soil. The urges briefly stopped until she became pregnant again. During each of her 5 pregnancies her pica symptoms returned. At one point during her last pregnancy she reports having felt out of control, eating 2 to 3 pencils with the eraser per day, after which she would feel intense relaxation. Her mother also exhibited symptoms of pica toward charcoal and soil. Ms. O had been taking unknown dosages of lorazepam for anxiety and fluoxetine for depression, both of which she stopped because she feared side effects during her last pregnancy. However, she never experienced any side effects.
The authors’ observations
Although pica is most commonly observed in young children, it sometimes is seen in pregnant women.1 Pica frequently is associated with other mental disorders, such as pervasive developmental disorder and mental retardation,1 and can be associated with premorbid psychosis and anxiety disorders. Occasional vitamin and mineral deficiencies, such as iron or zinc, have been reported, but usually patients’ lab values are normal. Treatment usually is initiated in the context of medical complications, such as iron deficiency anemia. In Ms. O’s case, the precipitating event was mechanical bowel obstruction due to a bezoar.
Several theories about the origins of pica have been proposed, but none truly are explanatory or satisfactory. The nutritional theory—that patients eat non-nutritive substances to compensate for mineral deficiencies—is popular because of pica’s frequent association with mineral deficiencies, but it is unknown whether pica is the cause or the result of the deficiency. An example of this is anemia due to eating clay instead of foods that contain iron. Another theory is that because pica is normal in early childhood development, it may be a manifestation of delayed development or mental retardation. The cultural theory is attractive because pregnant women in several cultures eat starch or clay as a part of their native rituals, and the incidence of pica is relatively high among pregnant African American women who live in rural areas.3 In the Roux-en-Y procedure, bypass of the duodenum and proximal jejunum can significantly decrease a patient’s iron uptake, leading to iron deficiency anemia, and could trigger pica in a susceptible patient.4
Exacerbation after gastric bypass
Kushner et al4 describes re-emergent pica after bariatric surgery in 2 patients with pagophagia associated with concomitant iron deficiency anemia. A 41-year-old white woman presented with pagophagia and a history of childhood consumption of dirt, chalk, and clay. Another patient, a 34-year-old African American woman, suffered from a lifelong desire to eat dirt, which she was able to resist, but experienced pagophagia during pregnancy and later when she developed iron deficiency anemia.4 In another case series, Kushner et al5 describes a 35-year-old woman with iron deficiency anemia with pagophagia presenting 2 years after Roux-en-Y. Her history was significant for eating clay as a child, but this new-onset pagophagia was so intense she purchased 2 snow cone machines, one for home and one for work, to feed her urges. Another patient, a 45-year-old African American woman, had an irresistible craving for calcium carbonate antacids, eating 40 to 50 a day, as well as several 30-ounce cups of ice.5 A third case report details a 33-year-old woman with iron deficiency anemia who presented with nocturnal pagophagia after Roux-en-Y anastomosis. She repeatedly rose during the night to eat the frost off the ice maker in her refrigerator.6 Another case described a female patient who ate cardboard after having a Roux-en-Y.2
Common themes in these case reports are female sex, Roux-en-Y, and dramatic resurgence of previously noted pica behaviors after gastric bypass surgery. Several studies have shown that pagophagia and pica in patients who are iron deficient or have iron deficiency anemia can be rapidly curbed with iron supplements.5 Ms. O, who has low iron, is taking iron supplementation, yet continues to experience pica cravings, albeit less severely. Her pica could be psychiatric in origin, perhaps related to her history of anxiety.
OUTCOME: Combination therapy
We start Ms. O on ziprasidone, 80 mg twice a day, restart lorazepam, 1 mg/d, and schedule monthly follow-up appointments to monitor her pica symptoms. We prescribe ziprasidone because it could treat paranoia and preoccupations and is considered to be weight-neutral. She continues her supplements, including ferrous sulfate, 325 mg 3 times daily. Ms. O attends weekly behavioral therapy sessions, during which the therapist monitors her mood and cravings with response prevention, which entails purposely avoiding behaviors after initiating a distressing stimulus. Ms. O responds well to medication and psychotherapy 1 month after the gastric bezoar removal, and she reports a decreased urge to eat cardboard. She is able to increase the amount of time she can go without eating non-nutritive substances—once daily, rather than repeatedly throughout the day.
The authors’ observations
Each patient with pica likely needs customized care. Children need to be supervised to prevent ingestion of lead-containing substances such as paint chips. Iron supplements are recommended for iron deficiency anemia and prophylaxis for iron deficiency anemia in Roux-en-Y patients.3,4 Pica in pregnant patients should be addressed to maintain adequate nutrition and prevent accidental poisonings.7 Behavioral intervention strategies are based on positive reinforcement and punishment (Table).8 A report of 3 young children with pica noted successful treatment of one with automatic reinforcement, and the other 2 with a combination of social and automatic reinforcement.9 There are no FDA-approved medications for pica. Positive effects have been seen with SSRIs, bupropion, atypical antipsychotics, buprenorphine, and chlorimipramine.10 Olanzapine has shown positive results as a treatment for pica.11 Most pica patients need concurrent psychotherapy.10
Table
Behavioral interventions for pica
| Intervention | Comments |
|---|---|
| Environmental enrichment | Providing additional stimulus to increase neuronal activity and focus behaviors |
| Noncontingent reinforcement | Presenting reinforcers according to a fixed schedule |
| Differential reinforcement | Desired behaviors are reinforced and inappropriate behaviors are ignored |
| Response blocking | Physically block a patient’s attempts to eat nonedible items |
| Source: Reference 8 | |
Related Resources
- Blinder BJ, Salama C. An update on pica: prevalence, contributing causes, and treatment. Psychiatric Times. www.psychiatrictimes.com/display/article/10168/1159376?pageNumber=1. Published May 1, 2008.
- Nurcombe B. Developmental disorders of attachment, feeding, elimination, & sleeping. In: Ebert MH, Loosen PT, Nurcombe B, et al, eds. CURRENT diagnosis & treatment: psychiatry. 2nd ed. New York, NY: McGraw Hill; 2008.
Drug Brand Names
- Buprenorphine • Subutex
- Bupropion • Wellbutrin, Zyban
- Chlorimipramine • Anafranil
- Esomeprazole • Nexium
- Fluoxetine • Prozac
- Lorazepam • Ativan
- Olanzapine • Zyprexa
- Ziprasidone • Geodon
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Discuss this article at www.facebook.com/CurrentPsychiatry
CASE: Nauseous and full
Ms. O, age 48, presents to the emergency department reporting a 3-day history of vomiting approximately 5 minutes after consuming solids or liquids. She’s had 10 vomiting episodes, which were associated with “fullness” and an “aching” sensation she rates as 6 on a 10-point scale pain scale that is diffuse over the upper epigastric area, with no palliative factors. Ms. O has not had a bowel movement for 3 days and her last menstrual period was 8 days ago. She is taking lorazepam, 1 mg/d. Her medical and psychiatric history includes anxiety, depression, personality disorder symptoms of affective dysregulation, obesity (270 lbs; medium height), and pica. She was 352 lbs when she underwent a Roux-en-Y gastric bypass 2 years ago. One year earlier, she had a laparoscopic gastric bezoar removal and an incisional hernia repair. Ms. O had no pica-related surgeries before undergoing gastric bypass surgery.
Ms. O denies shortness of breath, chest pain, allergies, smoking, or alcohol abuse, but reports uncontrollable cravings for paper products, specifically cardboard, which she describes as “just so delicious.” This craving led her to consume large amounts of cardboard and newspaper in the days before she began vomiting.
What may be causing Ms. O’s pica symptoms?
- iron deficiency anemia
- complications from gastric bypass surgery
- personality disorder
- generalized anxiety disorder (GAD)
The authors’ observations
DSM-IV-TR diagnostic criteria for pica include the persistent eating of non-nutritive substances for ≥1 month that is inappropriate for the level of a person’s development and not an acceptable part of one’s culture.1 If pica occurs with other mental disorders, it must be severe enough to indicate further clinical assessment to receive a separate diagnosis. Often associated with pregnancy, iron deficiency anemia, early development, and mental retardation, pica has been observed in post-gastric bypass surgery patients, all of whom presented with pagophagia (compulsive ice eating), and in one case was associated with a bezoar causing obstruction of the GI tract.1,2 With the dramatic increase in gastric bypass surgery and the required presurgical mental health evaluation, the consequences of failing to screen patients for pica behaviors can be devastating.
EVALUATION: Low iron
Ms. O’s vital signs on admission are stable, and physical exam is notable for mild abdominal distention with no guarding, tenderness, rigidity, or masses. No rebound tenderness is elicited. CT scan shows evidence of post-surgical changes involving the small bowel consistent with gastric bypass surgery and a hiatal hernia, but no obstruction, focal inflammation, free fluids, or gas. Lab values for amylase, lipase, urinalysis, coagulation studies, cardiac enzymes, and complete metabolic profile are within normal limits. Although not anemic, Ms. O is iron deficient, with ferritin, 10 ng/mL (normal 10 to 120 ng/mL); B12, 299 pg/mL (normal 100 to 700 pg/mL); and iron, 25 μg/dL (normal 50 to 170 μg/dL).
A foreign body is removed endoscopically and the specimen is sent to pathology. It is determined to be a gastric bezoar, yellowish-green in color, measuring 2.5 cm × 1 cm × 0.8 cm. After bezoar removal, Ms. O tolerates food and is discharged home on vitamin B12, 1,000 mcg/d for 2 weeks; folate, 1 mg/d for 1 month; calcium with vitamin D, 1 g/d; and esomeprazole, 40 mg/d for frequent heartburn. She is referred to psychiatry for behavioral modification therapy and medication management.
How would you treat Ms. O?
- start a selective serotonin reuptake inhibitor (SSRI)
- prescribe an atypical antipsychotic
- continue lorazepam
- begin behavioral therapy
HISTORY: Pica during pregnancy
During psychiatric workup, Ms. O admits to having pica urges most of her life, but experienced an uncontrollable exacerbation after gastric bypass surgery. This led to intense, chaotic periods of pica, resulting in a previous bezoar removal. She is particularly attracted to cardboard and newspaper cartoons, but notes she also has felt the urge to eat charcoal, moist soil, clay, chalk, pencils, and new shoes, which she chews on. In the past, her extreme anxiety and preoccupation with these urges had lead to diagnoses of personality disorder not otherwise specified, GAD, and obsessive-compulsive disorder.
Her first experience with pica was during her first pregnancy at age 15, when she had an impulse to eat soil. The urges briefly stopped until she became pregnant again. During each of her 5 pregnancies her pica symptoms returned. At one point during her last pregnancy she reports having felt out of control, eating 2 to 3 pencils with the eraser per day, after which she would feel intense relaxation. Her mother also exhibited symptoms of pica toward charcoal and soil. Ms. O had been taking unknown dosages of lorazepam for anxiety and fluoxetine for depression, both of which she stopped because she feared side effects during her last pregnancy. However, she never experienced any side effects.
The authors’ observations
Although pica is most commonly observed in young children, it sometimes is seen in pregnant women.1 Pica frequently is associated with other mental disorders, such as pervasive developmental disorder and mental retardation,1 and can be associated with premorbid psychosis and anxiety disorders. Occasional vitamin and mineral deficiencies, such as iron or zinc, have been reported, but usually patients’ lab values are normal. Treatment usually is initiated in the context of medical complications, such as iron deficiency anemia. In Ms. O’s case, the precipitating event was mechanical bowel obstruction due to a bezoar.
Several theories about the origins of pica have been proposed, but none truly are explanatory or satisfactory. The nutritional theory—that patients eat non-nutritive substances to compensate for mineral deficiencies—is popular because of pica’s frequent association with mineral deficiencies, but it is unknown whether pica is the cause or the result of the deficiency. An example of this is anemia due to eating clay instead of foods that contain iron. Another theory is that because pica is normal in early childhood development, it may be a manifestation of delayed development or mental retardation. The cultural theory is attractive because pregnant women in several cultures eat starch or clay as a part of their native rituals, and the incidence of pica is relatively high among pregnant African American women who live in rural areas.3 In the Roux-en-Y procedure, bypass of the duodenum and proximal jejunum can significantly decrease a patient’s iron uptake, leading to iron deficiency anemia, and could trigger pica in a susceptible patient.4
Exacerbation after gastric bypass
Kushner et al4 describes re-emergent pica after bariatric surgery in 2 patients with pagophagia associated with concomitant iron deficiency anemia. A 41-year-old white woman presented with pagophagia and a history of childhood consumption of dirt, chalk, and clay. Another patient, a 34-year-old African American woman, suffered from a lifelong desire to eat dirt, which she was able to resist, but experienced pagophagia during pregnancy and later when she developed iron deficiency anemia.4 In another case series, Kushner et al5 describes a 35-year-old woman with iron deficiency anemia with pagophagia presenting 2 years after Roux-en-Y. Her history was significant for eating clay as a child, but this new-onset pagophagia was so intense she purchased 2 snow cone machines, one for home and one for work, to feed her urges. Another patient, a 45-year-old African American woman, had an irresistible craving for calcium carbonate antacids, eating 40 to 50 a day, as well as several 30-ounce cups of ice.5 A third case report details a 33-year-old woman with iron deficiency anemia who presented with nocturnal pagophagia after Roux-en-Y anastomosis. She repeatedly rose during the night to eat the frost off the ice maker in her refrigerator.6 Another case described a female patient who ate cardboard after having a Roux-en-Y.2
Common themes in these case reports are female sex, Roux-en-Y, and dramatic resurgence of previously noted pica behaviors after gastric bypass surgery. Several studies have shown that pagophagia and pica in patients who are iron deficient or have iron deficiency anemia can be rapidly curbed with iron supplements.5 Ms. O, who has low iron, is taking iron supplementation, yet continues to experience pica cravings, albeit less severely. Her pica could be psychiatric in origin, perhaps related to her history of anxiety.
OUTCOME: Combination therapy
We start Ms. O on ziprasidone, 80 mg twice a day, restart lorazepam, 1 mg/d, and schedule monthly follow-up appointments to monitor her pica symptoms. We prescribe ziprasidone because it could treat paranoia and preoccupations and is considered to be weight-neutral. She continues her supplements, including ferrous sulfate, 325 mg 3 times daily. Ms. O attends weekly behavioral therapy sessions, during which the therapist monitors her mood and cravings with response prevention, which entails purposely avoiding behaviors after initiating a distressing stimulus. Ms. O responds well to medication and psychotherapy 1 month after the gastric bezoar removal, and she reports a decreased urge to eat cardboard. She is able to increase the amount of time she can go without eating non-nutritive substances—once daily, rather than repeatedly throughout the day.
The authors’ observations
Each patient with pica likely needs customized care. Children need to be supervised to prevent ingestion of lead-containing substances such as paint chips. Iron supplements are recommended for iron deficiency anemia and prophylaxis for iron deficiency anemia in Roux-en-Y patients.3,4 Pica in pregnant patients should be addressed to maintain adequate nutrition and prevent accidental poisonings.7 Behavioral intervention strategies are based on positive reinforcement and punishment (Table).8 A report of 3 young children with pica noted successful treatment of one with automatic reinforcement, and the other 2 with a combination of social and automatic reinforcement.9 There are no FDA-approved medications for pica. Positive effects have been seen with SSRIs, bupropion, atypical antipsychotics, buprenorphine, and chlorimipramine.10 Olanzapine has shown positive results as a treatment for pica.11 Most pica patients need concurrent psychotherapy.10
Table
Behavioral interventions for pica
| Intervention | Comments |
|---|---|
| Environmental enrichment | Providing additional stimulus to increase neuronal activity and focus behaviors |
| Noncontingent reinforcement | Presenting reinforcers according to a fixed schedule |
| Differential reinforcement | Desired behaviors are reinforced and inappropriate behaviors are ignored |
| Response blocking | Physically block a patient’s attempts to eat nonedible items |
| Source: Reference 8 | |
Related Resources
- Blinder BJ, Salama C. An update on pica: prevalence, contributing causes, and treatment. Psychiatric Times. www.psychiatrictimes.com/display/article/10168/1159376?pageNumber=1. Published May 1, 2008.
- Nurcombe B. Developmental disorders of attachment, feeding, elimination, & sleeping. In: Ebert MH, Loosen PT, Nurcombe B, et al, eds. CURRENT diagnosis & treatment: psychiatry. 2nd ed. New York, NY: McGraw Hill; 2008.
Drug Brand Names
- Buprenorphine • Subutex
- Bupropion • Wellbutrin, Zyban
- Chlorimipramine • Anafranil
- Esomeprazole • Nexium
- Fluoxetine • Prozac
- Lorazepam • Ativan
- Olanzapine • Zyprexa
- Ziprasidone • Geodon
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
2. Patton W, Gibbs K. Cardboard bezoar complicating laparoscopic gastric bypass. Surg Obes Relat Dis. 2010;6(3):313-315.
3. Nurcombe B. Developmental disorders of attachment feeding, elimination, & sleeping. In: Ebert MH, Loosen PT, Nurcombe B, et al, eds. CURRENT diagnosis & treatment: psychiatry. 2nd ed. New York, NY: McGraw Hill; 2008.
4. Kushner F, Gleason B, Shanta-Retelny V. Reemergence of pica following gastric bypass surgery for obesity: a new presentation of an old problem. J Am Diet Assoc. 2004;104(9):1393-1397.
5. Kushner F, Shanta Retelny V. Emergence of pica (ingestion of non-food substances) accompanying iron deficiency anemia after gastric bypass surgery. Obes Surg. 2005;15(10):1491-1495.
6. Marinella MA. Nocturnal pagophagia complicating gastric bypass. Mayo Clin Proc. 2008;83(8):961.-
7. Bernstein B, Weinstein M. Normal pregnancy & prenatal care. In: DeCherney AH Nathan L, Goodwin TM, et al, eds. CURRENT diagnosis & treatment obstetrics & gynecology. 10th ed. New York, NY: McGraw Hill; 2007.
8. Piazza C, Fisher W, Hanley P, et al. Treatment of pica through multiple analyses of its reinforcing functions. J Appl Behav Anal. 1998;31(2):165-189.
9. Williams DE, McAdam D. Assessment behavioral treatment, and prevention of pica: clinical guidelines and recommendations for practitioners. Res Dev Disabil. 2012;33(6):2050-2057.
10. Blinder BJ, Salama C. An update on pica: prevalence contributing causes, and treatment. Psychiatric Times. http://www.psychiatrictimes.com/display/article/10168/1159376?pageNumber=1. Published May 1, 2008. Accessed January 23, 2013.
11. Lerner AJ. Treatment of pica behavior with olanzapine. CNS Spectr. 2008;13(1):19.-
1. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
2. Patton W, Gibbs K. Cardboard bezoar complicating laparoscopic gastric bypass. Surg Obes Relat Dis. 2010;6(3):313-315.
3. Nurcombe B. Developmental disorders of attachment feeding, elimination, & sleeping. In: Ebert MH, Loosen PT, Nurcombe B, et al, eds. CURRENT diagnosis & treatment: psychiatry. 2nd ed. New York, NY: McGraw Hill; 2008.
4. Kushner F, Gleason B, Shanta-Retelny V. Reemergence of pica following gastric bypass surgery for obesity: a new presentation of an old problem. J Am Diet Assoc. 2004;104(9):1393-1397.
5. Kushner F, Shanta Retelny V. Emergence of pica (ingestion of non-food substances) accompanying iron deficiency anemia after gastric bypass surgery. Obes Surg. 2005;15(10):1491-1495.
6. Marinella MA. Nocturnal pagophagia complicating gastric bypass. Mayo Clin Proc. 2008;83(8):961.-
7. Bernstein B, Weinstein M. Normal pregnancy & prenatal care. In: DeCherney AH Nathan L, Goodwin TM, et al, eds. CURRENT diagnosis & treatment obstetrics & gynecology. 10th ed. New York, NY: McGraw Hill; 2007.
8. Piazza C, Fisher W, Hanley P, et al. Treatment of pica through multiple analyses of its reinforcing functions. J Appl Behav Anal. 1998;31(2):165-189.
9. Williams DE, McAdam D. Assessment behavioral treatment, and prevention of pica: clinical guidelines and recommendations for practitioners. Res Dev Disabil. 2012;33(6):2050-2057.
10. Blinder BJ, Salama C. An update on pica: prevalence contributing causes, and treatment. Psychiatric Times. http://www.psychiatrictimes.com/display/article/10168/1159376?pageNumber=1. Published May 1, 2008. Accessed January 23, 2013.
11. Lerner AJ. Treatment of pica behavior with olanzapine. CNS Spectr. 2008;13(1):19.-
Inhaled loxapine for agitation
Discuss this article at www.facebook.com/CurrentPsychiatry
Approved by the FDA on December 21, 2012, loxapine inhalation powder is the newest agent commercialized for the acute treatment of agitation associated with schizophrenia or bipolar I disorder in adults (Table 1).1,2 Loxapine is a first-generation antipsychotic that garnered newfound interest because of its potential atypical properties.3 Loxapine’s reformulation allows for direct administration to the lungs, resulting in rapid absorption into systemic circulation. This formulation offers a different method to manage agitation, for which IM formulations of other antipsychotics have been approved.4
Inhaled loxapine is delivered using a handheld device that produces a thermally-generated condensation aerosol.5,6 A single inhalation is sufficient to activate the controlled rapid heating (300 to 500°C in approximately 100 ms) of a thin layer of excipient-free loxapine on a metal substrate. Once vaporized, the medication cools down rapidly and aggregates into particles. The 1- to 3.5-micron aerosol particles of loxapine enter the respiratory track in 7
Table 1
Inhaled loxapine: Fast facts
| Brand name: Adasuve |
| Class: Dibenzoxazepine antipsychotic |
| Indication: Acute treatment of agitation associated with schizophrenia or bipolar I disorder in adults |
| FDA approval date: December 21, 2012 |
| Availability date: Third quarter of 2013 |
| Manufacturer: Alexza Pharmaceuticals |
| Dosing forms: Single-dose inhaler, 10 mg |
| Recommended dose: 10 mg; only a single dose within a 24-hour period is recommended |
| Source: References 1,2 |
How it works
As with all antipsychotics, loxapine is an antagonist at the dopamine D2 receptor. However, loxapine also has clinically relevant serotonin-2A antagonism.3 Pharmacologic effects for loxapine and its metabolites include biogenic amine transporter inhibitor activity, alpha adrenergic blocking effects, and histaminergic and muscarinic receptor affinity.3,8
Clinical pharmacokinetics
In a phase I study of healthy volunteers, inhaled loxapine produced IV administration-type kinetics, with maximum plasma concentration achieved in approximately 2 minutes.6 Plasma exposure to loxapine was dose-proportional. Half-life for the 5- and 10-mg doses was approximately 6 hours. In these patients, exposure to loxapine’s metabolites as a percentage of exposure to the parent compound were 8.79% for 7-OH loxapine, 52.6% for 8-OH loxapine, and 3.96% for amoxapine (all produced as a result of metabolism via liver cytochrome P450 [CYP] enzymes CYP1A2, CYP2D6, and/or CYP3A46). 7-OH loxapine has a 5-fold higher affinity for the dopamine D2 receptor compared with loxapine, and may contribute to the drug’s clinical effect.6
Based on loxapine levels observed in the pharmacokinetic study,6 loxapine is not extensively metabolized in the lungs. Peak plasma concentrations immediately after inhalation are higher than for oral loxapine, but concentration of loxapine and its metabolites after the initial distribution phase is similar to that of oral loxapine.6 Loxapine and its metabolites are excreted through the kidneys.
Efficacy
Three efficacy studies were completed (Table 2)9-11; all were double-blind randomized controlled trials that compared inhaled loxapine, 5 or 10 mg, with placebo. Patients were required to be clinically agitated at baseline, with a score of ≥14 on the Positive and Negative Syndrome Scale Excited Component (PANSS-EC)—which consists of the PANSS items of tension, excitement, hostility, uncooperativeness, and poor impulse control; each item is rated from 1 (absent) to 7 (extreme)—and a score of ≥4 (moderate) on ≥1 item. Patients who were intoxicated or had a positive drug screen for psychostimulants were excluded. Lorazepam was allowed ≥2 hours after the study drug was administered. Change in the PANSS-EC was measured 10 minutes to 24 hours post-dose. The primary endpoint used to statistically test loxapine vs placebo was 2 hours post-dose.
In the initial phase II trial, loxapine 10 mg, but not 5 mg, was superior to placebo on the PANSS-EC at 2 hours.9 The authors described the 5-mg dose effect size as intermediate between placebo and the 10-mg dose, suggesting a possible dose response relationship. The 10-mg dose did separate from placebo as early as 20 minutes post-dose. The small number of patients enrolled is a limitation of this trial, but this was addressed in studies in the phase III program, which were considerably larger. For each of the 2 phase III trials—1 for patients with schizophrenia10 and the other for those with bipolar disorder (BD)11—both doses of loxapine were superior to placebo starting at 10 minutes post-dose. The number needed to treat (NNT) for response—as defined by a Clinical Global Impressions-Improvement score of much improved or very much improved—for loxapine vs placebo is included in Table 2.9-11 NNT for other outcomes, such as reduction on the PANSS-EC by at least 40% from baseline, demonstrated similar results.
12 The lower the NNT, the stronger the effect size.13 See the Box for an explanation of NNT. NNTs in the range of 3 to 5 are comparable to other agents used to treat agitation.4
When examining each individual item on the PANSS-EC in each of the phase III trials, every item improved with treatment, starting 10 to 20 minutes after dosing.14 Each item improved an average of 1 to 2 units from baseline over the first 2 hours post-dose. Moreover, inhaled loxapine appears to reduce agitation equally well in patients with higher or lower levels of agitation at baseline.
Another clinically relevant outcome is whether or not a patient required an additional dose or rescue medication within 24 hours. In the phase III schizophrenia trial,10 60.9% of patients randomized to loxapine, 10 mg, did not require an additional dose or rescue medication, compared with 54.4% and 46.1% for loxapine, 5 mg, and placebo, respectively. This yielded an NNT of 7 when comparing loxapine, 10 mg, with placebo.12 In the BD study,10 61.5%, 41.3%, and 26.7% did not require an additional dose or rescue medication within 24 hours for loxapine, 10 mg, 5 mg, and placebo, respectively. In this study, the NNT for loxapine, 10 mg, vs placebo was 3.12
In general, there appears to be a dose response for efficacy with inhaled loxapine, and therefore the FDA approved the 10-mg dose.2
Table 2
Summary of double-blind RCTs for inhaled loxapine vs inhaled placebo
| Study | Diagnosis | Loxapine | Placebo | Outcomes | Loxapine vs placebo NNT for response at 2 hoursa | ||
|---|---|---|---|---|---|---|---|
| 5 mg | 10 mg | 5 mg | 10 mg | ||||
| Allen et al, 20119 (Phase II) | Agitation associated with schizophrenia | n=45 | n=41 | n=43 | On the PANSS-EC score at 2 hours, loxapine, 10 mg, but not 5 mg, was superior to placebo. Loxapine, 10 mg, separated from placebo at 20 minutes, and control was sustained. On the CGI-I at 2 hours, both doses of loxapine were superior to placebo. Using the BARS, loxapine, 10 mg, was superior to placebo starting at 30 minutes and this effect was sustained. Dysgeusia was observed in 4% and 17% for loxapine, 5 mg and 10 mg, respectively, and 9% for placebo | 4 | 3 |
| Lesem et al, 201110 (Phase III) | Agitation associated with schizophrenia | n=116 | n=113 | n=115 | On the PANSS-EC score and CGI-I at 2 hours, both doses of loxapine were superior to placebo. Loxapine separated from placebo at 10 minutes. Sustained control was observed over 24 hours. Dysgeusia was observed in 9% and 11% for loxapine 5 mg and 10 mg, respectively, and 3% for placebo | 5 | 4 |
| Kwentus et al, 201211 (Phase III) | Agitation associated with bipolar I disorder (manic or mixed episode) | n=104 | n=105 | n=105 | On the PANSS-EC score and CGI-I at 2 hours, both doses of loxapine were superior to placebo. Loxapine separated from placebo at 10 minutes. Sustained control was observed over 24 hours. Dysgeusia was observed in 17% for either loxapine 5 mg or 10 mg, respectively, and 6% for placebo | 3 | 3 |
| aas measured by a CGI-I score of 1 or 2 BARS: Behavioral Activity Rating Scale; CGI-I: Clinical Global Impression Improvement Scale; NNT: number needed to treat; PANSS-EC: Positive and Negative Syndrome Scale Excited Component; RCTs: randomized controlled trials | |||||||
Clinical trials produce a mountain of data that can be difficult to interpret and apply to clinical practice. When reading about studies you may wonder:
- How large is the effect being measured?
- Is it clinically important?
- Are we dealing with a result that may be statistically significant but irrelevant for day-to-day patient care?
Number needed to treat (NNT) and number needed to harm (NNH)—2 tools of evidence-based medicine—can help answer these questions. NNT helps us gauge effect size—or clinical significance. It is different from knowing if a clinical trial result is statistically significant. NNT allows us to place a number on how often we can expect to encounter a difference between 2 interventions. If we see a therapeutic difference once every 100 patients (an NNT of 100), the difference between 2 treatments is not of great concern under most circumstances. But if a difference in outcome is seen once in every 5 patients being treated with 1 intervention vs another (an NNT of 5), the result likely will influence day-to-day practice.
How to calculate NNT (or NNH)
What is the NNT for an outcome for drug A vs drug B?
fA= frequency of outcome for drug A
fB= frequency of outcome for drug B
NNT = 1/[ fA - fB]
By convention, we round up the NNT to the next higher whole number.
For example, let’s say drugs A and B are used to treat depression, and they result in 6-week response rates of 55% and 75%, respectively. The NNT to encounter a difference between drug B and drug A in terms of responders at 6 weeks can be calculated as follows:
- Difference in response rates = 0.75 - 0.55 = 0.20
- NNT = 1 / 0.20 = 5.
Source: Adapted from Citrome L. Dissecting clinical trials with ‘number needed to treat.’ Current Psychiatry. 2007;6(3): 66-71 and Citrome L. Can you interpret confidence intervals? It’s not that difficult. Current Psychiatry. 2007;6(8):77-82
Tolerability and safety
Combined safety results from phase III trials10,11 as well as information about a phase I ECG QT interval study were presented in a poster.15 Among 524 patients receiving loxapine vs 263 receiving placebo, there were no significant differences in the likelihood of experiencing any adverse event, a nervous system adverse event, sedation, sedation or somnolence, or sedation, somnolence or dizziness, when stratified by lorazepam rescue.16 Adverse events that were more frequently encountered with both doses of loxapine (ie, 5 and 10 mg) than placebo are listed in Table 3,15 along with the number needed to harm (NNH). The most commonly encountered adverse event was dysgeusia. The NNH of 10 for dysgeusia for loxapine, 10 mg, vs placebo means that for every 10 patients receiving inhaled loxapine, 10 mg, instead of inhaled placebo, you would encounter 1 additional case of dysgeusia. This contrasts with the NNT for response of 4 and 3 for agitation associated with schizophrenia and BD, respectively. Therefore, one would encounter response more often than dysgeusia when comparing loxapine with placebo.
No important changes in the ECG QT interval after inhaled loxapine, 10 mg, were observed in a phase I study with healthy volunteers.15 Difference from placebo in change from baseline for QTc was
Additional details regarding overall safety and tolerability can be found in a previously published review.17
Table 3
Inhaled loxapine: Incidence of adverse events
| Adverse event | Placebo (n=220) | Loxapine | |||
|---|---|---|---|---|---|
| 5 mg (n=220) | 10 mg (n=218) | ||||
| Rate | Rate | NNH vs placebo | Rate | NNH vs placebo | |
| Dysgeusia | 4% | 13% | 12 | 14% | 10 |
| Sedation or somnolence | 8% | 11% | 34 | 10% | 50 |
| Oral hypoesthesia | 0% | 200 | 2% | 50 | |
| NNH: number needed to harm Source: Reference 15 | |||||
Pulmonary safety
Because this product is inhaled, additional information on pulmonary safety was gathered.18,19 Among 1,095 patients without active airways disease, 1 (0.09%) required treatment for post-treatment airway-related symptoms (bronchospasm). In the agitated patient population, the rate of airway adverse events was 0.4% of loxapine exposures among 524 patients, in which 6.7% had a history of asthma or chronic obstructive pulmonary disease (COPD). Others were likely to have some respiratory impairment because of a history of cigarette smoking, but they did not have active respiratory symptoms that required treatment because such patients were excluded from the trials.12 Phase I spirometry-based studies also were completed in healthy nonsmoking volunteers, in patients with asthma, and in patients with COPD. No clinically relevant effects were observed in healthy volunteers, but in patients with asthma or COPD a reduction in forced expiratory volume was observed. In patients with asthma, rates of bronchospasm as an adverse event were 26.9% for loxapine vs 3.8% for placebo, for a NNH of 5.12 Bronchospasm was not reported for patients with COPD receiving loxapine but was observed in 1 patient who received placebo. All airway adverse events in patients with asthma or COPD were mild or moderate. All respiratory signs or symptoms requiring treatment in the phase I asthma and COPD studies were managed with an inhaled bronchodilator.
Product labeling notes in a warning that inhaled loxapine can cause bronchospasm that has the potential to lead to respiratory distress and respiratory arrest.2 Therefore, inhaled loxapine is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the “ADASUVE REMS.” Enrolled health care facilities are required to have immediate, on-site access to equipment and personnel trained to manage acute bronchospasm, including advanced airway management (intubation and mechanical ventilation). Inhaled loxapine is contraindicated in patients with a current diagnosis or history of asthma, COPD, or other lung diseases associated with bronchospasm; acute respiratory signs or symptoms such as wheezing; current use of medications to treat airway diseases such as asthma or COPD; history of bronchospasm following inhaled loxapine treatment; or known hypersensitivity to loxapine and amoxapine.
Only a single dose within a 24-hour period is recommended. Before administration, patients should be screened for a history of pulmonary disease and examined (including chest auscultation) for respiratory abnormalities (eg, wheezing). After administration, patients require monitoring for signs and symptoms of bronchospasm at least every 15 minutes for ≥1 hour.
Related Resource
- Dinh K, Myers DJ, Glazer M, et al. In vitro aerosol characterization of Staccato(®) Loxapine. Int J Pharm. 2011; 403(1-2):101-108.
Drug Brand Names
- Haloperidol • Haldol
- Lorazepam • Ativan
- Loxapine • Loxitane
- Loxapine inhalation powder • Adasuve
Disclosure
In the past 36 months, Dr. Citrome has engaged in collaborative research with or received consulting or speaking fees from Alexza Pharmaceuticals, Alkermes, AstraZeneca, Avanir Pharmaceuticals, Bristol-Myers Squibb, Eli Lilly and Company, EnVivo Pharmaceuticals, Forest Pharmaceuticals, Genentech, Janssen, L.P., Lundbeck, Merck, Mylan, Novartis, Noven, Otsuka, Pfizer Inc., Shire, Sunovion, and Valeant.
1. Alexza Pharmaceuticals U.S. FDA Approves Alexza’s ADASUVE (loxapine) inhalation powder for the acute treatment of agitation associated with schizophrenia or bipolar I disorder in adults. http://nocache-phx.corporate-ir.net/phoenix.zhtml?c=196151
&p=RssLanding&cat=news&id=1769476. Published December 21, 2012. Accessed January 2, 2013.
2. ADASUVE [package insert]. Mountain View, CA: Alexza Pharmaceuticals; 2012.
3. Ereshefsky L. Pharmacologic and pharmacokinetic considerations in choosing an antipsychotic. J Clin Psychiatry. 1999;60(suppl 10):20-30.
4. Citrome L. Comparison of intramuscular ziprasidone olanzapine, or aripiprazole for agitation: a quantitative review of efficacy and safety. J Clin Psychiatry. 2007;68(12):1876-1885.
5. Noymer P, Myers D, Glazer M, et al. The staccato system: inhaler design characteristics for rapid treatment of CNS disorders. Respiratory Drug Delivery. 2010;1(1):11-20.
6. Spyker DA, Munzar P, Cassella JV. Pharmacokinetics of loxapine following inhalation of a thermally generated aerosol in healthy volunteers. J Clin Pharmacol. 2010;50(2):169-179.
7. Dinh KV, Myers DJ, Noymer PD, et al. In vitro aerosol deposition in the oropharyngeal region for Staccato Loxapine. J Aerosol Med Pulm Drug Deliv. 2010;23(4):253-260.
8. Brunton LL, Lazo JS, Parker KL. eds. Goodman & Gilman’s: the pharmacological basis of therapeutics. 11th ed. New York, NY: McGraw-Hill; 2005:472.
9. Allen MH, Feifel DA, Lesem MD, et al. Efficacy and safety of loxapine for inhalation in the treatment of agitation in patients with schizophrenia: a randomized, double-blind, placebo controlled trial. J Clin Psychiatry. 2011;72(10):1313-1321.
10. Lesem MD, Tran-Johnson TK, Riesenberg RA, et al. Rapid acute treatment of agitation in individuals with schizophrenia: multicentre, randomised, placebo-controlled study of inhaled loxapine. Br J Psychiatry. 2011;198(1):51-58.
11. Kwentus J, Riesenberg RA, Marandi M, et al. Rapid acute treatment of agitation in patients with bipolar I disorder: a multicenter, randomized, placebo-controlled clinical trial with inhaled loxapine. Bipolar Disord. 2012;14(1):31-40.
12. Citrome L. Inhaled loxapine for agitation revisited: focus on effect sizes from 2 Phase III randomised controlled trials in persons with schizophrenia or bipolar disorder. Int J Clin Pract. 2012;66(3):318-325.
13. Citrome L. Compelling or irrelevant? Using number needed to treat can help decide. Acta Psychiatr Scand. 2008;117(6):412-419.
14. Cassella J, Spyker D, Kwentus J, et al. Rapid improvement in the five-item Positive and Negative Syndrome-Excited Component (PANSS-EC) scale for agitation with inhaled loxapine. Poster presented at: 50th meeting of New Research Approaches for Mental Health Interventions; June 14-17, 2010; Boca Raton, FL.
15. Fishman R, Gottwald M, Cassella J. Inhaled loxapine (AZ-004) rapidly and effectively reduces agitation in patients with schizophrenia and bipolar disorder. Poster presented at: 13th annual meeting of the College of Psychiatric and Neurologic Pharmacists; April 18-21 2010; San Antonio, TX.
16. Fishman R, Spyker D, Cassella J. The safety of concomitant use of lorazepam rescue in treating agitation with inhaled loxapine (AZ-004). Poster presented at: 50th meeting of New Research Approaches for Mental Health Interventions; June 14-17, 2010; Boca Raton, FL.
17. Citrome L. Aerosolised antipsychotic assuages agitation: inhaled loxapine for agitation associated with schizophrenia or bipolar disorder. Int J Clin Pract. 2011;65(3):330-340.
18. Alexza Pharmaceuticals. Adasuve (loxapine) inhalation powder NDA 022549. Psychopharmacologic drug advisory committee briefing document. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Psychopharma
cologicDrugsAdvisoryCommittee/UCM282900.pdf. Published December 12, 2011. Accessed January 2, 2013.
19. Food and Drug Administration Briefing document for NDA 022549. Psychopharmacologic Drug Advisory Committee Briefing Document. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Psychopharma
cologicDrugsAdvisoryCommittee/UCM282897.pdf. Accessed January 2, 2013.
Discuss this article at www.facebook.com/CurrentPsychiatry
Approved by the FDA on December 21, 2012, loxapine inhalation powder is the newest agent commercialized for the acute treatment of agitation associated with schizophrenia or bipolar I disorder in adults (Table 1).1,2 Loxapine is a first-generation antipsychotic that garnered newfound interest because of its potential atypical properties.3 Loxapine’s reformulation allows for direct administration to the lungs, resulting in rapid absorption into systemic circulation. This formulation offers a different method to manage agitation, for which IM formulations of other antipsychotics have been approved.4
Inhaled loxapine is delivered using a handheld device that produces a thermally-generated condensation aerosol.5,6 A single inhalation is sufficient to activate the controlled rapid heating (300 to 500°C in approximately 100 ms) of a thin layer of excipient-free loxapine on a metal substrate. Once vaporized, the medication cools down rapidly and aggregates into particles. The 1- to 3.5-micron aerosol particles of loxapine enter the respiratory track in 7
Table 1
Inhaled loxapine: Fast facts
| Brand name: Adasuve |
| Class: Dibenzoxazepine antipsychotic |
| Indication: Acute treatment of agitation associated with schizophrenia or bipolar I disorder in adults |
| FDA approval date: December 21, 2012 |
| Availability date: Third quarter of 2013 |
| Manufacturer: Alexza Pharmaceuticals |
| Dosing forms: Single-dose inhaler, 10 mg |
| Recommended dose: 10 mg; only a single dose within a 24-hour period is recommended |
| Source: References 1,2 |
How it works
As with all antipsychotics, loxapine is an antagonist at the dopamine D2 receptor. However, loxapine also has clinically relevant serotonin-2A antagonism.3 Pharmacologic effects for loxapine and its metabolites include biogenic amine transporter inhibitor activity, alpha adrenergic blocking effects, and histaminergic and muscarinic receptor affinity.3,8
Clinical pharmacokinetics
In a phase I study of healthy volunteers, inhaled loxapine produced IV administration-type kinetics, with maximum plasma concentration achieved in approximately 2 minutes.6 Plasma exposure to loxapine was dose-proportional. Half-life for the 5- and 10-mg doses was approximately 6 hours. In these patients, exposure to loxapine’s metabolites as a percentage of exposure to the parent compound were 8.79% for 7-OH loxapine, 52.6% for 8-OH loxapine, and 3.96% for amoxapine (all produced as a result of metabolism via liver cytochrome P450 [CYP] enzymes CYP1A2, CYP2D6, and/or CYP3A46). 7-OH loxapine has a 5-fold higher affinity for the dopamine D2 receptor compared with loxapine, and may contribute to the drug’s clinical effect.6
Based on loxapine levels observed in the pharmacokinetic study,6 loxapine is not extensively metabolized in the lungs. Peak plasma concentrations immediately after inhalation are higher than for oral loxapine, but concentration of loxapine and its metabolites after the initial distribution phase is similar to that of oral loxapine.6 Loxapine and its metabolites are excreted through the kidneys.
Efficacy
Three efficacy studies were completed (Table 2)9-11; all were double-blind randomized controlled trials that compared inhaled loxapine, 5 or 10 mg, with placebo. Patients were required to be clinically agitated at baseline, with a score of ≥14 on the Positive and Negative Syndrome Scale Excited Component (PANSS-EC)—which consists of the PANSS items of tension, excitement, hostility, uncooperativeness, and poor impulse control; each item is rated from 1 (absent) to 7 (extreme)—and a score of ≥4 (moderate) on ≥1 item. Patients who were intoxicated or had a positive drug screen for psychostimulants were excluded. Lorazepam was allowed ≥2 hours after the study drug was administered. Change in the PANSS-EC was measured 10 minutes to 24 hours post-dose. The primary endpoint used to statistically test loxapine vs placebo was 2 hours post-dose.
In the initial phase II trial, loxapine 10 mg, but not 5 mg, was superior to placebo on the PANSS-EC at 2 hours.9 The authors described the 5-mg dose effect size as intermediate between placebo and the 10-mg dose, suggesting a possible dose response relationship. The 10-mg dose did separate from placebo as early as 20 minutes post-dose. The small number of patients enrolled is a limitation of this trial, but this was addressed in studies in the phase III program, which were considerably larger. For each of the 2 phase III trials—1 for patients with schizophrenia10 and the other for those with bipolar disorder (BD)11—both doses of loxapine were superior to placebo starting at 10 minutes post-dose. The number needed to treat (NNT) for response—as defined by a Clinical Global Impressions-Improvement score of much improved or very much improved—for loxapine vs placebo is included in Table 2.9-11 NNT for other outcomes, such as reduction on the PANSS-EC by at least 40% from baseline, demonstrated similar results.
12 The lower the NNT, the stronger the effect size.13 See the Box for an explanation of NNT. NNTs in the range of 3 to 5 are comparable to other agents used to treat agitation.4
When examining each individual item on the PANSS-EC in each of the phase III trials, every item improved with treatment, starting 10 to 20 minutes after dosing.14 Each item improved an average of 1 to 2 units from baseline over the first 2 hours post-dose. Moreover, inhaled loxapine appears to reduce agitation equally well in patients with higher or lower levels of agitation at baseline.
Another clinically relevant outcome is whether or not a patient required an additional dose or rescue medication within 24 hours. In the phase III schizophrenia trial,10 60.9% of patients randomized to loxapine, 10 mg, did not require an additional dose or rescue medication, compared with 54.4% and 46.1% for loxapine, 5 mg, and placebo, respectively. This yielded an NNT of 7 when comparing loxapine, 10 mg, with placebo.12 In the BD study,10 61.5%, 41.3%, and 26.7% did not require an additional dose or rescue medication within 24 hours for loxapine, 10 mg, 5 mg, and placebo, respectively. In this study, the NNT for loxapine, 10 mg, vs placebo was 3.12
In general, there appears to be a dose response for efficacy with inhaled loxapine, and therefore the FDA approved the 10-mg dose.2
Table 2
Summary of double-blind RCTs for inhaled loxapine vs inhaled placebo
| Study | Diagnosis | Loxapine | Placebo | Outcomes | Loxapine vs placebo NNT for response at 2 hoursa | ||
|---|---|---|---|---|---|---|---|
| 5 mg | 10 mg | 5 mg | 10 mg | ||||
| Allen et al, 20119 (Phase II) | Agitation associated with schizophrenia | n=45 | n=41 | n=43 | On the PANSS-EC score at 2 hours, loxapine, 10 mg, but not 5 mg, was superior to placebo. Loxapine, 10 mg, separated from placebo at 20 minutes, and control was sustained. On the CGI-I at 2 hours, both doses of loxapine were superior to placebo. Using the BARS, loxapine, 10 mg, was superior to placebo starting at 30 minutes and this effect was sustained. Dysgeusia was observed in 4% and 17% for loxapine, 5 mg and 10 mg, respectively, and 9% for placebo | 4 | 3 |
| Lesem et al, 201110 (Phase III) | Agitation associated with schizophrenia | n=116 | n=113 | n=115 | On the PANSS-EC score and CGI-I at 2 hours, both doses of loxapine were superior to placebo. Loxapine separated from placebo at 10 minutes. Sustained control was observed over 24 hours. Dysgeusia was observed in 9% and 11% for loxapine 5 mg and 10 mg, respectively, and 3% for placebo | 5 | 4 |
| Kwentus et al, 201211 (Phase III) | Agitation associated with bipolar I disorder (manic or mixed episode) | n=104 | n=105 | n=105 | On the PANSS-EC score and CGI-I at 2 hours, both doses of loxapine were superior to placebo. Loxapine separated from placebo at 10 minutes. Sustained control was observed over 24 hours. Dysgeusia was observed in 17% for either loxapine 5 mg or 10 mg, respectively, and 6% for placebo | 3 | 3 |
| aas measured by a CGI-I score of 1 or 2 BARS: Behavioral Activity Rating Scale; CGI-I: Clinical Global Impression Improvement Scale; NNT: number needed to treat; PANSS-EC: Positive and Negative Syndrome Scale Excited Component; RCTs: randomized controlled trials | |||||||
Clinical trials produce a mountain of data that can be difficult to interpret and apply to clinical practice. When reading about studies you may wonder:
- How large is the effect being measured?
- Is it clinically important?
- Are we dealing with a result that may be statistically significant but irrelevant for day-to-day patient care?
Number needed to treat (NNT) and number needed to harm (NNH)—2 tools of evidence-based medicine—can help answer these questions. NNT helps us gauge effect size—or clinical significance. It is different from knowing if a clinical trial result is statistically significant. NNT allows us to place a number on how often we can expect to encounter a difference between 2 interventions. If we see a therapeutic difference once every 100 patients (an NNT of 100), the difference between 2 treatments is not of great concern under most circumstances. But if a difference in outcome is seen once in every 5 patients being treated with 1 intervention vs another (an NNT of 5), the result likely will influence day-to-day practice.
How to calculate NNT (or NNH)
What is the NNT for an outcome for drug A vs drug B?
fA= frequency of outcome for drug A
fB= frequency of outcome for drug B
NNT = 1/[ fA - fB]
By convention, we round up the NNT to the next higher whole number.
For example, let’s say drugs A and B are used to treat depression, and they result in 6-week response rates of 55% and 75%, respectively. The NNT to encounter a difference between drug B and drug A in terms of responders at 6 weeks can be calculated as follows:
- Difference in response rates = 0.75 - 0.55 = 0.20
- NNT = 1 / 0.20 = 5.
Source: Adapted from Citrome L. Dissecting clinical trials with ‘number needed to treat.’ Current Psychiatry. 2007;6(3): 66-71 and Citrome L. Can you interpret confidence intervals? It’s not that difficult. Current Psychiatry. 2007;6(8):77-82
Tolerability and safety
Combined safety results from phase III trials10,11 as well as information about a phase I ECG QT interval study were presented in a poster.15 Among 524 patients receiving loxapine vs 263 receiving placebo, there were no significant differences in the likelihood of experiencing any adverse event, a nervous system adverse event, sedation, sedation or somnolence, or sedation, somnolence or dizziness, when stratified by lorazepam rescue.16 Adverse events that were more frequently encountered with both doses of loxapine (ie, 5 and 10 mg) than placebo are listed in Table 3,15 along with the number needed to harm (NNH). The most commonly encountered adverse event was dysgeusia. The NNH of 10 for dysgeusia for loxapine, 10 mg, vs placebo means that for every 10 patients receiving inhaled loxapine, 10 mg, instead of inhaled placebo, you would encounter 1 additional case of dysgeusia. This contrasts with the NNT for response of 4 and 3 for agitation associated with schizophrenia and BD, respectively. Therefore, one would encounter response more often than dysgeusia when comparing loxapine with placebo.
No important changes in the ECG QT interval after inhaled loxapine, 10 mg, were observed in a phase I study with healthy volunteers.15 Difference from placebo in change from baseline for QTc was
Additional details regarding overall safety and tolerability can be found in a previously published review.17
Table 3
Inhaled loxapine: Incidence of adverse events
| Adverse event | Placebo (n=220) | Loxapine | |||
|---|---|---|---|---|---|
| 5 mg (n=220) | 10 mg (n=218) | ||||
| Rate | Rate | NNH vs placebo | Rate | NNH vs placebo | |
| Dysgeusia | 4% | 13% | 12 | 14% | 10 |
| Sedation or somnolence | 8% | 11% | 34 | 10% | 50 |
| Oral hypoesthesia | 0% | 200 | 2% | 50 | |
| NNH: number needed to harm Source: Reference 15 | |||||
Pulmonary safety
Because this product is inhaled, additional information on pulmonary safety was gathered.18,19 Among 1,095 patients without active airways disease, 1 (0.09%) required treatment for post-treatment airway-related symptoms (bronchospasm). In the agitated patient population, the rate of airway adverse events was 0.4% of loxapine exposures among 524 patients, in which 6.7% had a history of asthma or chronic obstructive pulmonary disease (COPD). Others were likely to have some respiratory impairment because of a history of cigarette smoking, but they did not have active respiratory symptoms that required treatment because such patients were excluded from the trials.12 Phase I spirometry-based studies also were completed in healthy nonsmoking volunteers, in patients with asthma, and in patients with COPD. No clinically relevant effects were observed in healthy volunteers, but in patients with asthma or COPD a reduction in forced expiratory volume was observed. In patients with asthma, rates of bronchospasm as an adverse event were 26.9% for loxapine vs 3.8% for placebo, for a NNH of 5.12 Bronchospasm was not reported for patients with COPD receiving loxapine but was observed in 1 patient who received placebo. All airway adverse events in patients with asthma or COPD were mild or moderate. All respiratory signs or symptoms requiring treatment in the phase I asthma and COPD studies were managed with an inhaled bronchodilator.
Product labeling notes in a warning that inhaled loxapine can cause bronchospasm that has the potential to lead to respiratory distress and respiratory arrest.2 Therefore, inhaled loxapine is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the “ADASUVE REMS.” Enrolled health care facilities are required to have immediate, on-site access to equipment and personnel trained to manage acute bronchospasm, including advanced airway management (intubation and mechanical ventilation). Inhaled loxapine is contraindicated in patients with a current diagnosis or history of asthma, COPD, or other lung diseases associated with bronchospasm; acute respiratory signs or symptoms such as wheezing; current use of medications to treat airway diseases such as asthma or COPD; history of bronchospasm following inhaled loxapine treatment; or known hypersensitivity to loxapine and amoxapine.
Only a single dose within a 24-hour period is recommended. Before administration, patients should be screened for a history of pulmonary disease and examined (including chest auscultation) for respiratory abnormalities (eg, wheezing). After administration, patients require monitoring for signs and symptoms of bronchospasm at least every 15 minutes for ≥1 hour.
Related Resource
- Dinh K, Myers DJ, Glazer M, et al. In vitro aerosol characterization of Staccato(®) Loxapine. Int J Pharm. 2011; 403(1-2):101-108.
Drug Brand Names
- Haloperidol • Haldol
- Lorazepam • Ativan
- Loxapine • Loxitane
- Loxapine inhalation powder • Adasuve
Disclosure
In the past 36 months, Dr. Citrome has engaged in collaborative research with or received consulting or speaking fees from Alexza Pharmaceuticals, Alkermes, AstraZeneca, Avanir Pharmaceuticals, Bristol-Myers Squibb, Eli Lilly and Company, EnVivo Pharmaceuticals, Forest Pharmaceuticals, Genentech, Janssen, L.P., Lundbeck, Merck, Mylan, Novartis, Noven, Otsuka, Pfizer Inc., Shire, Sunovion, and Valeant.
Discuss this article at www.facebook.com/CurrentPsychiatry
Approved by the FDA on December 21, 2012, loxapine inhalation powder is the newest agent commercialized for the acute treatment of agitation associated with schizophrenia or bipolar I disorder in adults (Table 1).1,2 Loxapine is a first-generation antipsychotic that garnered newfound interest because of its potential atypical properties.3 Loxapine’s reformulation allows for direct administration to the lungs, resulting in rapid absorption into systemic circulation. This formulation offers a different method to manage agitation, for which IM formulations of other antipsychotics have been approved.4
Inhaled loxapine is delivered using a handheld device that produces a thermally-generated condensation aerosol.5,6 A single inhalation is sufficient to activate the controlled rapid heating (300 to 500°C in approximately 100 ms) of a thin layer of excipient-free loxapine on a metal substrate. Once vaporized, the medication cools down rapidly and aggregates into particles. The 1- to 3.5-micron aerosol particles of loxapine enter the respiratory track in 7
Table 1
Inhaled loxapine: Fast facts
| Brand name: Adasuve |
| Class: Dibenzoxazepine antipsychotic |
| Indication: Acute treatment of agitation associated with schizophrenia or bipolar I disorder in adults |
| FDA approval date: December 21, 2012 |
| Availability date: Third quarter of 2013 |
| Manufacturer: Alexza Pharmaceuticals |
| Dosing forms: Single-dose inhaler, 10 mg |
| Recommended dose: 10 mg; only a single dose within a 24-hour period is recommended |
| Source: References 1,2 |
How it works
As with all antipsychotics, loxapine is an antagonist at the dopamine D2 receptor. However, loxapine also has clinically relevant serotonin-2A antagonism.3 Pharmacologic effects for loxapine and its metabolites include biogenic amine transporter inhibitor activity, alpha adrenergic blocking effects, and histaminergic and muscarinic receptor affinity.3,8
Clinical pharmacokinetics
In a phase I study of healthy volunteers, inhaled loxapine produced IV administration-type kinetics, with maximum plasma concentration achieved in approximately 2 minutes.6 Plasma exposure to loxapine was dose-proportional. Half-life for the 5- and 10-mg doses was approximately 6 hours. In these patients, exposure to loxapine’s metabolites as a percentage of exposure to the parent compound were 8.79% for 7-OH loxapine, 52.6% for 8-OH loxapine, and 3.96% for amoxapine (all produced as a result of metabolism via liver cytochrome P450 [CYP] enzymes CYP1A2, CYP2D6, and/or CYP3A46). 7-OH loxapine has a 5-fold higher affinity for the dopamine D2 receptor compared with loxapine, and may contribute to the drug’s clinical effect.6
Based on loxapine levels observed in the pharmacokinetic study,6 loxapine is not extensively metabolized in the lungs. Peak plasma concentrations immediately after inhalation are higher than for oral loxapine, but concentration of loxapine and its metabolites after the initial distribution phase is similar to that of oral loxapine.6 Loxapine and its metabolites are excreted through the kidneys.
Efficacy
Three efficacy studies were completed (Table 2)9-11; all were double-blind randomized controlled trials that compared inhaled loxapine, 5 or 10 mg, with placebo. Patients were required to be clinically agitated at baseline, with a score of ≥14 on the Positive and Negative Syndrome Scale Excited Component (PANSS-EC)—which consists of the PANSS items of tension, excitement, hostility, uncooperativeness, and poor impulse control; each item is rated from 1 (absent) to 7 (extreme)—and a score of ≥4 (moderate) on ≥1 item. Patients who were intoxicated or had a positive drug screen for psychostimulants were excluded. Lorazepam was allowed ≥2 hours after the study drug was administered. Change in the PANSS-EC was measured 10 minutes to 24 hours post-dose. The primary endpoint used to statistically test loxapine vs placebo was 2 hours post-dose.
In the initial phase II trial, loxapine 10 mg, but not 5 mg, was superior to placebo on the PANSS-EC at 2 hours.9 The authors described the 5-mg dose effect size as intermediate between placebo and the 10-mg dose, suggesting a possible dose response relationship. The 10-mg dose did separate from placebo as early as 20 minutes post-dose. The small number of patients enrolled is a limitation of this trial, but this was addressed in studies in the phase III program, which were considerably larger. For each of the 2 phase III trials—1 for patients with schizophrenia10 and the other for those with bipolar disorder (BD)11—both doses of loxapine were superior to placebo starting at 10 minutes post-dose. The number needed to treat (NNT) for response—as defined by a Clinical Global Impressions-Improvement score of much improved or very much improved—for loxapine vs placebo is included in Table 2.9-11 NNT for other outcomes, such as reduction on the PANSS-EC by at least 40% from baseline, demonstrated similar results.
12 The lower the NNT, the stronger the effect size.13 See the Box for an explanation of NNT. NNTs in the range of 3 to 5 are comparable to other agents used to treat agitation.4
When examining each individual item on the PANSS-EC in each of the phase III trials, every item improved with treatment, starting 10 to 20 minutes after dosing.14 Each item improved an average of 1 to 2 units from baseline over the first 2 hours post-dose. Moreover, inhaled loxapine appears to reduce agitation equally well in patients with higher or lower levels of agitation at baseline.
Another clinically relevant outcome is whether or not a patient required an additional dose or rescue medication within 24 hours. In the phase III schizophrenia trial,10 60.9% of patients randomized to loxapine, 10 mg, did not require an additional dose or rescue medication, compared with 54.4% and 46.1% for loxapine, 5 mg, and placebo, respectively. This yielded an NNT of 7 when comparing loxapine, 10 mg, with placebo.12 In the BD study,10 61.5%, 41.3%, and 26.7% did not require an additional dose or rescue medication within 24 hours for loxapine, 10 mg, 5 mg, and placebo, respectively. In this study, the NNT for loxapine, 10 mg, vs placebo was 3.12
In general, there appears to be a dose response for efficacy with inhaled loxapine, and therefore the FDA approved the 10-mg dose.2
Table 2
Summary of double-blind RCTs for inhaled loxapine vs inhaled placebo
| Study | Diagnosis | Loxapine | Placebo | Outcomes | Loxapine vs placebo NNT for response at 2 hoursa | ||
|---|---|---|---|---|---|---|---|
| 5 mg | 10 mg | 5 mg | 10 mg | ||||
| Allen et al, 20119 (Phase II) | Agitation associated with schizophrenia | n=45 | n=41 | n=43 | On the PANSS-EC score at 2 hours, loxapine, 10 mg, but not 5 mg, was superior to placebo. Loxapine, 10 mg, separated from placebo at 20 minutes, and control was sustained. On the CGI-I at 2 hours, both doses of loxapine were superior to placebo. Using the BARS, loxapine, 10 mg, was superior to placebo starting at 30 minutes and this effect was sustained. Dysgeusia was observed in 4% and 17% for loxapine, 5 mg and 10 mg, respectively, and 9% for placebo | 4 | 3 |
| Lesem et al, 201110 (Phase III) | Agitation associated with schizophrenia | n=116 | n=113 | n=115 | On the PANSS-EC score and CGI-I at 2 hours, both doses of loxapine were superior to placebo. Loxapine separated from placebo at 10 minutes. Sustained control was observed over 24 hours. Dysgeusia was observed in 9% and 11% for loxapine 5 mg and 10 mg, respectively, and 3% for placebo | 5 | 4 |
| Kwentus et al, 201211 (Phase III) | Agitation associated with bipolar I disorder (manic or mixed episode) | n=104 | n=105 | n=105 | On the PANSS-EC score and CGI-I at 2 hours, both doses of loxapine were superior to placebo. Loxapine separated from placebo at 10 minutes. Sustained control was observed over 24 hours. Dysgeusia was observed in 17% for either loxapine 5 mg or 10 mg, respectively, and 6% for placebo | 3 | 3 |
| aas measured by a CGI-I score of 1 or 2 BARS: Behavioral Activity Rating Scale; CGI-I: Clinical Global Impression Improvement Scale; NNT: number needed to treat; PANSS-EC: Positive and Negative Syndrome Scale Excited Component; RCTs: randomized controlled trials | |||||||
Clinical trials produce a mountain of data that can be difficult to interpret and apply to clinical practice. When reading about studies you may wonder:
- How large is the effect being measured?
- Is it clinically important?
- Are we dealing with a result that may be statistically significant but irrelevant for day-to-day patient care?
Number needed to treat (NNT) and number needed to harm (NNH)—2 tools of evidence-based medicine—can help answer these questions. NNT helps us gauge effect size—or clinical significance. It is different from knowing if a clinical trial result is statistically significant. NNT allows us to place a number on how often we can expect to encounter a difference between 2 interventions. If we see a therapeutic difference once every 100 patients (an NNT of 100), the difference between 2 treatments is not of great concern under most circumstances. But if a difference in outcome is seen once in every 5 patients being treated with 1 intervention vs another (an NNT of 5), the result likely will influence day-to-day practice.
How to calculate NNT (or NNH)
What is the NNT for an outcome for drug A vs drug B?
fA= frequency of outcome for drug A
fB= frequency of outcome for drug B
NNT = 1/[ fA - fB]
By convention, we round up the NNT to the next higher whole number.
For example, let’s say drugs A and B are used to treat depression, and they result in 6-week response rates of 55% and 75%, respectively. The NNT to encounter a difference between drug B and drug A in terms of responders at 6 weeks can be calculated as follows:
- Difference in response rates = 0.75 - 0.55 = 0.20
- NNT = 1 / 0.20 = 5.
Source: Adapted from Citrome L. Dissecting clinical trials with ‘number needed to treat.’ Current Psychiatry. 2007;6(3): 66-71 and Citrome L. Can you interpret confidence intervals? It’s not that difficult. Current Psychiatry. 2007;6(8):77-82
Tolerability and safety
Combined safety results from phase III trials10,11 as well as information about a phase I ECG QT interval study were presented in a poster.15 Among 524 patients receiving loxapine vs 263 receiving placebo, there were no significant differences in the likelihood of experiencing any adverse event, a nervous system adverse event, sedation, sedation or somnolence, or sedation, somnolence or dizziness, when stratified by lorazepam rescue.16 Adverse events that were more frequently encountered with both doses of loxapine (ie, 5 and 10 mg) than placebo are listed in Table 3,15 along with the number needed to harm (NNH). The most commonly encountered adverse event was dysgeusia. The NNH of 10 for dysgeusia for loxapine, 10 mg, vs placebo means that for every 10 patients receiving inhaled loxapine, 10 mg, instead of inhaled placebo, you would encounter 1 additional case of dysgeusia. This contrasts with the NNT for response of 4 and 3 for agitation associated with schizophrenia and BD, respectively. Therefore, one would encounter response more often than dysgeusia when comparing loxapine with placebo.
No important changes in the ECG QT interval after inhaled loxapine, 10 mg, were observed in a phase I study with healthy volunteers.15 Difference from placebo in change from baseline for QTc was
Additional details regarding overall safety and tolerability can be found in a previously published review.17
Table 3
Inhaled loxapine: Incidence of adverse events
| Adverse event | Placebo (n=220) | Loxapine | |||
|---|---|---|---|---|---|
| 5 mg (n=220) | 10 mg (n=218) | ||||
| Rate | Rate | NNH vs placebo | Rate | NNH vs placebo | |
| Dysgeusia | 4% | 13% | 12 | 14% | 10 |
| Sedation or somnolence | 8% | 11% | 34 | 10% | 50 |
| Oral hypoesthesia | 0% | 200 | 2% | 50 | |
| NNH: number needed to harm Source: Reference 15 | |||||
Pulmonary safety
Because this product is inhaled, additional information on pulmonary safety was gathered.18,19 Among 1,095 patients without active airways disease, 1 (0.09%) required treatment for post-treatment airway-related symptoms (bronchospasm). In the agitated patient population, the rate of airway adverse events was 0.4% of loxapine exposures among 524 patients, in which 6.7% had a history of asthma or chronic obstructive pulmonary disease (COPD). Others were likely to have some respiratory impairment because of a history of cigarette smoking, but they did not have active respiratory symptoms that required treatment because such patients were excluded from the trials.12 Phase I spirometry-based studies also were completed in healthy nonsmoking volunteers, in patients with asthma, and in patients with COPD. No clinically relevant effects were observed in healthy volunteers, but in patients with asthma or COPD a reduction in forced expiratory volume was observed. In patients with asthma, rates of bronchospasm as an adverse event were 26.9% for loxapine vs 3.8% for placebo, for a NNH of 5.12 Bronchospasm was not reported for patients with COPD receiving loxapine but was observed in 1 patient who received placebo. All airway adverse events in patients with asthma or COPD were mild or moderate. All respiratory signs or symptoms requiring treatment in the phase I asthma and COPD studies were managed with an inhaled bronchodilator.
Product labeling notes in a warning that inhaled loxapine can cause bronchospasm that has the potential to lead to respiratory distress and respiratory arrest.2 Therefore, inhaled loxapine is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the “ADASUVE REMS.” Enrolled health care facilities are required to have immediate, on-site access to equipment and personnel trained to manage acute bronchospasm, including advanced airway management (intubation and mechanical ventilation). Inhaled loxapine is contraindicated in patients with a current diagnosis or history of asthma, COPD, or other lung diseases associated with bronchospasm; acute respiratory signs or symptoms such as wheezing; current use of medications to treat airway diseases such as asthma or COPD; history of bronchospasm following inhaled loxapine treatment; or known hypersensitivity to loxapine and amoxapine.
Only a single dose within a 24-hour period is recommended. Before administration, patients should be screened for a history of pulmonary disease and examined (including chest auscultation) for respiratory abnormalities (eg, wheezing). After administration, patients require monitoring for signs and symptoms of bronchospasm at least every 15 minutes for ≥1 hour.
Related Resource
- Dinh K, Myers DJ, Glazer M, et al. In vitro aerosol characterization of Staccato(®) Loxapine. Int J Pharm. 2011; 403(1-2):101-108.
Drug Brand Names
- Haloperidol • Haldol
- Lorazepam • Ativan
- Loxapine • Loxitane
- Loxapine inhalation powder • Adasuve
Disclosure
In the past 36 months, Dr. Citrome has engaged in collaborative research with or received consulting or speaking fees from Alexza Pharmaceuticals, Alkermes, AstraZeneca, Avanir Pharmaceuticals, Bristol-Myers Squibb, Eli Lilly and Company, EnVivo Pharmaceuticals, Forest Pharmaceuticals, Genentech, Janssen, L.P., Lundbeck, Merck, Mylan, Novartis, Noven, Otsuka, Pfizer Inc., Shire, Sunovion, and Valeant.
1. Alexza Pharmaceuticals U.S. FDA Approves Alexza’s ADASUVE (loxapine) inhalation powder for the acute treatment of agitation associated with schizophrenia or bipolar I disorder in adults. http://nocache-phx.corporate-ir.net/phoenix.zhtml?c=196151
&p=RssLanding&cat=news&id=1769476. Published December 21, 2012. Accessed January 2, 2013.
2. ADASUVE [package insert]. Mountain View, CA: Alexza Pharmaceuticals; 2012.
3. Ereshefsky L. Pharmacologic and pharmacokinetic considerations in choosing an antipsychotic. J Clin Psychiatry. 1999;60(suppl 10):20-30.
4. Citrome L. Comparison of intramuscular ziprasidone olanzapine, or aripiprazole for agitation: a quantitative review of efficacy and safety. J Clin Psychiatry. 2007;68(12):1876-1885.
5. Noymer P, Myers D, Glazer M, et al. The staccato system: inhaler design characteristics for rapid treatment of CNS disorders. Respiratory Drug Delivery. 2010;1(1):11-20.
6. Spyker DA, Munzar P, Cassella JV. Pharmacokinetics of loxapine following inhalation of a thermally generated aerosol in healthy volunteers. J Clin Pharmacol. 2010;50(2):169-179.
7. Dinh KV, Myers DJ, Noymer PD, et al. In vitro aerosol deposition in the oropharyngeal region for Staccato Loxapine. J Aerosol Med Pulm Drug Deliv. 2010;23(4):253-260.
8. Brunton LL, Lazo JS, Parker KL. eds. Goodman & Gilman’s: the pharmacological basis of therapeutics. 11th ed. New York, NY: McGraw-Hill; 2005:472.
9. Allen MH, Feifel DA, Lesem MD, et al. Efficacy and safety of loxapine for inhalation in the treatment of agitation in patients with schizophrenia: a randomized, double-blind, placebo controlled trial. J Clin Psychiatry. 2011;72(10):1313-1321.
10. Lesem MD, Tran-Johnson TK, Riesenberg RA, et al. Rapid acute treatment of agitation in individuals with schizophrenia: multicentre, randomised, placebo-controlled study of inhaled loxapine. Br J Psychiatry. 2011;198(1):51-58.
11. Kwentus J, Riesenberg RA, Marandi M, et al. Rapid acute treatment of agitation in patients with bipolar I disorder: a multicenter, randomized, placebo-controlled clinical trial with inhaled loxapine. Bipolar Disord. 2012;14(1):31-40.
12. Citrome L. Inhaled loxapine for agitation revisited: focus on effect sizes from 2 Phase III randomised controlled trials in persons with schizophrenia or bipolar disorder. Int J Clin Pract. 2012;66(3):318-325.
13. Citrome L. Compelling or irrelevant? Using number needed to treat can help decide. Acta Psychiatr Scand. 2008;117(6):412-419.
14. Cassella J, Spyker D, Kwentus J, et al. Rapid improvement in the five-item Positive and Negative Syndrome-Excited Component (PANSS-EC) scale for agitation with inhaled loxapine. Poster presented at: 50th meeting of New Research Approaches for Mental Health Interventions; June 14-17, 2010; Boca Raton, FL.
15. Fishman R, Gottwald M, Cassella J. Inhaled loxapine (AZ-004) rapidly and effectively reduces agitation in patients with schizophrenia and bipolar disorder. Poster presented at: 13th annual meeting of the College of Psychiatric and Neurologic Pharmacists; April 18-21 2010; San Antonio, TX.
16. Fishman R, Spyker D, Cassella J. The safety of concomitant use of lorazepam rescue in treating agitation with inhaled loxapine (AZ-004). Poster presented at: 50th meeting of New Research Approaches for Mental Health Interventions; June 14-17, 2010; Boca Raton, FL.
17. Citrome L. Aerosolised antipsychotic assuages agitation: inhaled loxapine for agitation associated with schizophrenia or bipolar disorder. Int J Clin Pract. 2011;65(3):330-340.
18. Alexza Pharmaceuticals. Adasuve (loxapine) inhalation powder NDA 022549. Psychopharmacologic drug advisory committee briefing document. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Psychopharma
cologicDrugsAdvisoryCommittee/UCM282900.pdf. Published December 12, 2011. Accessed January 2, 2013.
19. Food and Drug Administration Briefing document for NDA 022549. Psychopharmacologic Drug Advisory Committee Briefing Document. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Psychopharma
cologicDrugsAdvisoryCommittee/UCM282897.pdf. Accessed January 2, 2013.
1. Alexza Pharmaceuticals U.S. FDA Approves Alexza’s ADASUVE (loxapine) inhalation powder for the acute treatment of agitation associated with schizophrenia or bipolar I disorder in adults. http://nocache-phx.corporate-ir.net/phoenix.zhtml?c=196151
&p=RssLanding&cat=news&id=1769476. Published December 21, 2012. Accessed January 2, 2013.
2. ADASUVE [package insert]. Mountain View, CA: Alexza Pharmaceuticals; 2012.
3. Ereshefsky L. Pharmacologic and pharmacokinetic considerations in choosing an antipsychotic. J Clin Psychiatry. 1999;60(suppl 10):20-30.
4. Citrome L. Comparison of intramuscular ziprasidone olanzapine, or aripiprazole for agitation: a quantitative review of efficacy and safety. J Clin Psychiatry. 2007;68(12):1876-1885.
5. Noymer P, Myers D, Glazer M, et al. The staccato system: inhaler design characteristics for rapid treatment of CNS disorders. Respiratory Drug Delivery. 2010;1(1):11-20.
6. Spyker DA, Munzar P, Cassella JV. Pharmacokinetics of loxapine following inhalation of a thermally generated aerosol in healthy volunteers. J Clin Pharmacol. 2010;50(2):169-179.
7. Dinh KV, Myers DJ, Noymer PD, et al. In vitro aerosol deposition in the oropharyngeal region for Staccato Loxapine. J Aerosol Med Pulm Drug Deliv. 2010;23(4):253-260.
8. Brunton LL, Lazo JS, Parker KL. eds. Goodman & Gilman’s: the pharmacological basis of therapeutics. 11th ed. New York, NY: McGraw-Hill; 2005:472.
9. Allen MH, Feifel DA, Lesem MD, et al. Efficacy and safety of loxapine for inhalation in the treatment of agitation in patients with schizophrenia: a randomized, double-blind, placebo controlled trial. J Clin Psychiatry. 2011;72(10):1313-1321.
10. Lesem MD, Tran-Johnson TK, Riesenberg RA, et al. Rapid acute treatment of agitation in individuals with schizophrenia: multicentre, randomised, placebo-controlled study of inhaled loxapine. Br J Psychiatry. 2011;198(1):51-58.
11. Kwentus J, Riesenberg RA, Marandi M, et al. Rapid acute treatment of agitation in patients with bipolar I disorder: a multicenter, randomized, placebo-controlled clinical trial with inhaled loxapine. Bipolar Disord. 2012;14(1):31-40.
12. Citrome L. Inhaled loxapine for agitation revisited: focus on effect sizes from 2 Phase III randomised controlled trials in persons with schizophrenia or bipolar disorder. Int J Clin Pract. 2012;66(3):318-325.
13. Citrome L. Compelling or irrelevant? Using number needed to treat can help decide. Acta Psychiatr Scand. 2008;117(6):412-419.
14. Cassella J, Spyker D, Kwentus J, et al. Rapid improvement in the five-item Positive and Negative Syndrome-Excited Component (PANSS-EC) scale for agitation with inhaled loxapine. Poster presented at: 50th meeting of New Research Approaches for Mental Health Interventions; June 14-17, 2010; Boca Raton, FL.
15. Fishman R, Gottwald M, Cassella J. Inhaled loxapine (AZ-004) rapidly and effectively reduces agitation in patients with schizophrenia and bipolar disorder. Poster presented at: 13th annual meeting of the College of Psychiatric and Neurologic Pharmacists; April 18-21 2010; San Antonio, TX.
16. Fishman R, Spyker D, Cassella J. The safety of concomitant use of lorazepam rescue in treating agitation with inhaled loxapine (AZ-004). Poster presented at: 50th meeting of New Research Approaches for Mental Health Interventions; June 14-17, 2010; Boca Raton, FL.
17. Citrome L. Aerosolised antipsychotic assuages agitation: inhaled loxapine for agitation associated with schizophrenia or bipolar disorder. Int J Clin Pract. 2011;65(3):330-340.
18. Alexza Pharmaceuticals. Adasuve (loxapine) inhalation powder NDA 022549. Psychopharmacologic drug advisory committee briefing document. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Psychopharma
cologicDrugsAdvisoryCommittee/UCM282900.pdf. Published December 12, 2011. Accessed January 2, 2013.
19. Food and Drug Administration Briefing document for NDA 022549. Psychopharmacologic Drug Advisory Committee Briefing Document. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Psychopharma
cologicDrugsAdvisoryCommittee/UCM282897.pdf. Accessed January 2, 2013.