User login
Antipsychotics for migraines, cluster headaches, and nausea
Most evidence supporting antipsychotics as a treatment for migraine headaches and cluster headaches is based on small studies and chart reviews. Some research suggests antipsychotics may effectively treat nausea but side effects such as akathisia may limit their use.
Migraine headaches
Antipsychotic treatment of migraines is supported by the theory that dopaminergic hyperactivity leads to migraine headaches (Table 1). Antipsychotics have been used off-label in migraine patients who do not tolerate triptans or have status migrainosus—intense, debilitating migraine lasting >72 hours.1 Primarily a result of D2 receptor blockade, the serotonergic effects of some second-generation antipsychotics (SGAs) may prevent migraine recurrence. The first-generation antipsychotics (FGAs) prochlorperazine, droperidol, haloperidol, and chlorpromazine have been used for migraine headaches (Table 2).1-27
Prochlorperazine may be an effective treatment of acute headaches9 and refractory chronic daily headache.10 Studies show that buccal prochlorperazine is more effective than oral ergotamine tartrate11 and IV prochlorperazine is more effective than IV ketorolac12 or valproate28 for treating acute headache.
Evidence suggests that chlorpromazine administered IM2 or IV3 is better than placebo for managing migraine pain. In a study comparing IV chlorpromazine, lidocaine, and dihydroergotamine, patients treated with chlorpromazine showed more persistent headache relief 12 to 24 hours post-dose.4 In another study, IV chlorpromazine, 25 mg, was as effective as IM ketorolac, 60 mg.5
Droperidol has been shown to be effective for managing headache, specifically status migrainosus.6 Patients with “benign headache”—headache not caused by an underlying medical disorder—who received droperidol reported greater reduction in visual analog pain scores within 1 hour of dosing compared with those taking prochlorperazine.7 In a randomized trial comparing IM droperidol and IM meperidine, patients with an acute migraine who received droperidol had improved scores on the visual pain analog scale and required less “rescue medication” for breakthrough pain.8 The FDA has issued a “black-box” warning of QTc prolongation with droperidol.
In a double blind, placebo-controlled trial, IV haloperidol, 5 mg, effectively treated migraine headache in 80% of patients compared with 15% of those who received placebo. However, 16% of patients considered the side effects—mainly sedation and akathisia—intolerable and 7% had symptom relapse.13 In an open-label trial of 6 patients with migraine headache, all patients achieved complete or substantial headache relief 25 to 65 minutes after receiving IV haloperidol, 5 mg.14
SGAs often antagonize 5-HT1D receptors and theoretically can render triptan therapy—which stimulates pre-synaptic 5-HT1D receptors—ineffective. This has not been seen clinically and instead, dose-related, non-specific headaches are a common adverse event with SGAs.29,30 A retrospective chart review found olanzapine provided relief for refractory headaches in patients who had failed ≥4 preventive medications. Olanzapine significantly decreased headache days, from 27.5±4.9 before treatment to 21.1±10.7 after treatment. Olanzapine also improved headache severity (measured on a 0 to 10 scale) from 8.7±1.6 before treatment to 2.2±2.1 after treatment.16 Researchers found that 2.5 or 5 mg of olanzapine relieved acute migraines for most patients, with repeat dosing as needed up to 20 mg/d. For prophylactic treatment, 5 or 10 mg of olanzapine was used. Olanzapine’s antinociceptive effect may be related to its action on α-2 adrenoreceptors and to a lesser extent on involvement of opioid and serotonergic receptors.17
In a case series, 3 migraine patients who met criteria for chronic daily headache and migraines but did not have a psychiatric disorder reported significant and sustained headache improvement when treated with risperidone.19 In a case series of 3 migraine patients with co-occurring psychiatric disorders, aripiprazole decreased migraine frequency and severity.15 Although limited data support quetiapine’s efficacy in treating acute migraines, in an open-label, pilot study, patients taking quetiapine, 25 to 75 mg/d, demonstrated a decrease in mean frequency of migraine days from 10.2 to 6.2 and decreased use of rescue medications from 2.3 to 1.2 days per week.18
Table 1
Possible rationale for antipsychotic use for headaches and nausea
| Condition | Possible rationale |
|---|---|
| Migraine | Patients are hypersensitive to dopamine agonists or dopamine transporter dysfunction. Some evidence that the dopamine D2 (DRD2) gene is involved |
| Cluster headache | Pain alleviation possibly related to dopamine receptor antagonism |
| Nausea | D2 and H1 receptor blockage |
Table 2
Antipsychotics for headache and nausea: Strength of the evidence
| Condition | Strength of evidencea |
|---|---|
| Migraine | Intermediate: Chlorpromazine,2-5 droperidol,6-8 prochlorperazine1,10-12 |
| Weak: Haloperidol13,14 | |
| Very weak: Aripiprazole,15 olanzapine,16,17 quetiapine,18 ziprasidone19 | |
| Cluster headache | Weak: Chlorpromazine20 |
| Very weak: Clozapine,21 olanzapine22 | |
| Nausea/vomiting | Intermediate: Droperidol,23 metoclopramide,24 prochlorperazine,25 promethazine25 |
| Weak: Olanzapine26,27 | |
| aStrong: Multiple, well-designed RCTs directly relevant to the recommendation, yielding consistent findings Intermediate: Some evidence from RCTs that support the recommendation, but the scientific support was not optimal Weak: Consensus recommendation in the absence of relevant randomized controlled trials and better evidence than case report or series Very weak: Case reports or case series or preliminary studies RCTs: randomized controlled trials | |
Cluster headaches
Subcutaneous sumatriptan and inhaled oxygen are first-line treatments for cluster headaches.31 A single, small study20 reported that chlorpromazine may prevent cluster headaches, which suggests that D2 receptor blockade may treat such headaches. However, limited supporting evidence relegates its use to a second- or third-line therapy.
In an open-label study (N = 5), olanzapine provided some relief of pain associated with cluster headache within 20 minutes of administration.22 In another study, patients with schizophrenia and comorbid cluster headaches improved with olanzapine.21
Because evidence is limited to small prospective studies, antipsychotic treatment of cluster headache is not well established.20-22 However, olanzapine may benefit patients with comorbid cluster headaches and schizophrenia.
Nausea
The signaling pathways that mediate emesis involve 5-HT3, D2, muscarinic, and histamine receptors.32 Before 5-HT3 antagonists were available, the FGAs metoclopramide, droperidol, prochlorperazine, and promethazine were used to manage acute emesis in emergency departments.23 A double-blind, placebo-controlled trial found IV droperidol, 1.25 mg, was more effective than metoclopramide, 10 mg, or prochlorperazine, 10 mg, for relieving moderate to severe nausea in adult patients.23 However, droperidol and prochlorperazine were associated with akathisia. In addition, this trial did not find a clinically significant difference between groups—including placebo—in anxiety, sedation, or need for rescue medications.23 Use of droperidol to treat nausea decreased after the drug received a “black-box” warning for QT prolongation and torsades de pointes.
Metoclopramide is effective for treating acute migraine and associated nausea24 and has been used to treat gastroparesis because of its effect on upper GI motility. Phenothiazines have been used to treat nausea and studies have shown prochlorperazine to be more effective than promethazine.25 Some studies of prochlorperazine have reported a 44% incidence of akathisia, which limits the drug’s use in patients who may be sensitive to such effects.33 Promethazine can cause sedation and risk of tissue necrosis at the injection site.34
Among SGAs, olanzapine effectively prevented acute and delayed chemotherapy-induced nausea and vomiting in a proof-of-concept study of patients receiving high and moderate emetogenic therapies.26,27 National Comprehensive Cancer Network guidelines cite olanzapine as a potential option for treating refractory and breakthrough emesis.35 In a small study (N = 50), olanzapine showed comparable anti-nausea effect to aprepitant—a neurokinin 1 receptor antagonist—and effectively prevented chemotherapy-induced nausea and vomiting in highly emetogenic chemotherapy.36
Related Resources
- Kelley NE, Tepper DE. Rescue therapy for acute migraine, part 2: neuroleptics, antihistamines, and others. Headache. 2012;52(2):292-306.
- Dusitanond P, Young WB. Neuroleptics and migraine. Cent Nerv Syst Agents Med Chem. 2009;9(1):63-70.
Drug Brand Names
- Aprepitant • Emend
- Aripiprazole • Abilify
- Chlorpromazine • Thorazine
- Dihydroergotamine • D.H.E 45
- Droperidol • Inapsine
- Ergotamine tartrate • Ergostat
- Haloperidol • Haldol
- Ketorolac • Toradol
- Lidocaine • Xylocaine, Lidoderm
- Meperidine • Demerol
- Metoclopramide • Reglan
- Olanzapine • Zyprexa
- Prochlorperazine • Compazine
- Promethazine • Phenergan
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sumatriptan • Imitrex
- Valproate • Depakote
Disclosures
Dr. Macaluso has received grant or research support from EnVivo Pharmaceuticals, Janssen L.P., and Pfizer, Inc.
Dr. Tripathi reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Dusitanond P, Young WB. Neuroleptics and migraine. Cent Nerv Syst Agents Med Chem. 2009;9(1):63-70.
2. McEwen JI, O’Connor HM, Dinsdale HB. Treatment of migraine with intramuscular chlorpromazine. Ann Emerg Med. 1987;16(7):758-763.
3. Bigal M, Bordini CA, Speciali JG. Intravenous chlorpromazine in the emergency department treatment of migraines: a randomized controlled trial. J Emerg Med. 2002;23(2):141-148.
4. Bell R, Montoya D, Shuaib A, et al. A comparative trial of three agents in the treatment of acute migraine headache. Ann Emerg Med. 1990;19(10):1079-1082.
5. Shrestha M, Singh R, Moreden J, et al. Ketorolac vs chlorpromazine in the treatment of acute migraine without aura. A prospective, randomized, double-blind trial. Arch Intern Med. 1996;156(15):1725-1728.
6. Wang SJ, Silberstein SD, Young WB. Droperidol treatment of status migrainosus and refractory migraine. Headache. 1997;37(6):377-382.
7. Miner JR, Fish SJ, Smith SW, et al. Droperidol vs. prochlorperazine for benign headaches in the emergency department. Acad Emerg Med. 2001;8(9):873-879.
8. Richman PB, Allegra J, Eskin B, et al. A randomized clinical trial to assess the efficacy of intramuscular droperidol for the treatment of acute migraine headache. Am J Emerg Med. 2002;20(1):39-42.
9. Jones J, Sklar D, Dougherty J, et al. Randomized double blind trial of intravenous prochlorperazine for the treatment of acute headache. JAMA. 1989;261(8):1174-1176.
10. Lu SR, Fuh JL, Juang KD, et al. Repetitive intravenous prochlorperazine treatment of patients with refractory chronic daily headache. Headache. 2000;40(9):724-729.
11. Sharma S, Prasad A, Nehru R, et al. Efficacy and tolerability of prochlorperazine buccal tablets in treatment of acute migraine. Headache. 2002;42(9):896-902.
12. Seim MB, March JA, Dunn KA. Intravenous ketorolac vs intravenous prochlorperazine for the treatment of migraine headaches. Acad Emerg Med. 1998;5(6):573-576.
13. Honkaniemi J, Liimatainen S, Rainesalo S, et al. Haloperidol in the acute treatment of migraine: a randomized, double-blind, placebo-controlled study. Headache. 2006;46(5):781-787.
14. Fisher H. A new approach to emergency department therapy of migraine headache with intravenous haloperidol: a case series. J Emerg Med. 1995;13(1):119-122.
15. LaPorta LD. Relief from migraine headache with aripiprazole treatment. Headache. 2007;47(6):922-926.
16. Silberstein SD, Peres MF, Hopkins MM, et al. Olanzapine in the treatment of refractory migraine and chronic daily headache. Headache. 2002;42(6):515-518.
17. Schreiber S, Getslev V, Backer MM, et al. The atypical neuroleptics clozapine and olanzapine differ regarding their antinociceptive mechanisms and potency. Pharmacol Biochem Behav. 1999;64(1):75-80.
18. Krymchantowski AV, Jevoux C. Quetiapine for the prevention of migraine refractory to the combination of atenolol + nortriptyline + flunarizine: an open pilot study. Arq Neuropsiquiatr. 2008;66(3B):615-618.
19. Cahill CM, Hardiman O, Murphy KC. Treatment of refractory chronic daily headache with the atypical antipsychotic ziprasidone-a case series. Cephalalgia. 2005;25(10):822-826.
20. Caviness VS, Jr, O’Brien P. Cluster headache: response to chlorpromazine. Headache. 1980;20(3):128-131.
21. Datta SS, Kumar S. Clozapine-responsive cluster headache. Neurol India. 2006;54(2):200-201.
22. Rozen TD. Olanzapine as an abortive agent for cluster headache. Headache. 2001;41(8):813-816.
23. Braude D, Soliz T, Crandall C, et al. Antiemetics in the ED: a randomized controlled trial comparing 3 common agents. Am J Emerg Med. 2006;24(2):177-182.
24. Colman I, Brown MD, Innes GD, et al. Parenteral metoclopramide for acute migraine: meta-analysis of randomised controlled trials. BMJ. 2004;329(7479):1369-1373.
25. Ernst AA, Weiss SJ, Park S, et al. Prochlorperazine versus promethazine for uncomplicated nausea and vomiting in the emergency department: a randomized, double-blind clinical trial. Ann Emerg Med. 2000;36(2):89-94.
26. Navari RM, Einhorn LH, Loehrer PJ Sr, et al. A phase II trial of olanzapine, dexamethasone, and palonosetron for the prevention of chemotherapy-induced nausea and vomiting: a Hoosier oncology group study. Support Care Cancer. 2007;15(11):1285-1291.
27. Passik SD, Navari RM, Jung SH, et al. A phase I trial of olanzapine (Zyprexa) for the prevention of delayed emesis in cancer patients: a Hoosier Oncology Group study. Cancer Invest. 2004;22(3):383-388.
28. Tanen DA, Miller S, French T, et al. Intravenous sodium valproate versus prochlorperazine for the emergency department treatment of acute migraine headaches: a prospective, randomized, double-blind trial. Ann Emerg Med. 2003;41(6):847-853.
29. Caley CF, Cooper CK. Ziprasidone: the fifth atypical antipsychotic. Ann Pharmacother. 2002;36(5):839-851.
30. Geodon [package insert]. New York NY. Pfizer Inc.; 2012.
31. Kudrow L. Response of cluster headache attacks to oxygen inhalation. Headache. 1981;21(1):1-4.
32. Scuderi PE. Pharmacology of antiemetics. Int Anesthesiol Clin. 2003;41(4):41-66.
33. Drotts DL, Vinson DR. Prochlorperazine induces akathisia in emergency patients. Ann Emerg Med. 1999;34(4):469-475.
34. Institute for Safe Medication Practices. Action needed to prevent serious tissue injury with IV promethazine. http://www.ismp.org/newsletters/acutecare/articles/20060810.asp?ptr_y. Published August 10 2006. Accessed November 28, 2012.
35. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. 2010. http://www.nccn.org/professionals/physician_gls/pdf/antiemesis.pdf. Accessed November 29 2012.
36. Navari R, Gray SE, Carr AC. Olanzapine versus aprepitant for the prevention of chemotherapy induced nausea and vomiting (CINV): a randomized phase III trial. J Clin Oncol. 2010;28(15 suppl):9020.-
Most evidence supporting antipsychotics as a treatment for migraine headaches and cluster headaches is based on small studies and chart reviews. Some research suggests antipsychotics may effectively treat nausea but side effects such as akathisia may limit their use.
Migraine headaches
Antipsychotic treatment of migraines is supported by the theory that dopaminergic hyperactivity leads to migraine headaches (Table 1). Antipsychotics have been used off-label in migraine patients who do not tolerate triptans or have status migrainosus—intense, debilitating migraine lasting >72 hours.1 Primarily a result of D2 receptor blockade, the serotonergic effects of some second-generation antipsychotics (SGAs) may prevent migraine recurrence. The first-generation antipsychotics (FGAs) prochlorperazine, droperidol, haloperidol, and chlorpromazine have been used for migraine headaches (Table 2).1-27
Prochlorperazine may be an effective treatment of acute headaches9 and refractory chronic daily headache.10 Studies show that buccal prochlorperazine is more effective than oral ergotamine tartrate11 and IV prochlorperazine is more effective than IV ketorolac12 or valproate28 for treating acute headache.
Evidence suggests that chlorpromazine administered IM2 or IV3 is better than placebo for managing migraine pain. In a study comparing IV chlorpromazine, lidocaine, and dihydroergotamine, patients treated with chlorpromazine showed more persistent headache relief 12 to 24 hours post-dose.4 In another study, IV chlorpromazine, 25 mg, was as effective as IM ketorolac, 60 mg.5
Droperidol has been shown to be effective for managing headache, specifically status migrainosus.6 Patients with “benign headache”—headache not caused by an underlying medical disorder—who received droperidol reported greater reduction in visual analog pain scores within 1 hour of dosing compared with those taking prochlorperazine.7 In a randomized trial comparing IM droperidol and IM meperidine, patients with an acute migraine who received droperidol had improved scores on the visual pain analog scale and required less “rescue medication” for breakthrough pain.8 The FDA has issued a “black-box” warning of QTc prolongation with droperidol.
In a double blind, placebo-controlled trial, IV haloperidol, 5 mg, effectively treated migraine headache in 80% of patients compared with 15% of those who received placebo. However, 16% of patients considered the side effects—mainly sedation and akathisia—intolerable and 7% had symptom relapse.13 In an open-label trial of 6 patients with migraine headache, all patients achieved complete or substantial headache relief 25 to 65 minutes after receiving IV haloperidol, 5 mg.14
SGAs often antagonize 5-HT1D receptors and theoretically can render triptan therapy—which stimulates pre-synaptic 5-HT1D receptors—ineffective. This has not been seen clinically and instead, dose-related, non-specific headaches are a common adverse event with SGAs.29,30 A retrospective chart review found olanzapine provided relief for refractory headaches in patients who had failed ≥4 preventive medications. Olanzapine significantly decreased headache days, from 27.5±4.9 before treatment to 21.1±10.7 after treatment. Olanzapine also improved headache severity (measured on a 0 to 10 scale) from 8.7±1.6 before treatment to 2.2±2.1 after treatment.16 Researchers found that 2.5 or 5 mg of olanzapine relieved acute migraines for most patients, with repeat dosing as needed up to 20 mg/d. For prophylactic treatment, 5 or 10 mg of olanzapine was used. Olanzapine’s antinociceptive effect may be related to its action on α-2 adrenoreceptors and to a lesser extent on involvement of opioid and serotonergic receptors.17
In a case series, 3 migraine patients who met criteria for chronic daily headache and migraines but did not have a psychiatric disorder reported significant and sustained headache improvement when treated with risperidone.19 In a case series of 3 migraine patients with co-occurring psychiatric disorders, aripiprazole decreased migraine frequency and severity.15 Although limited data support quetiapine’s efficacy in treating acute migraines, in an open-label, pilot study, patients taking quetiapine, 25 to 75 mg/d, demonstrated a decrease in mean frequency of migraine days from 10.2 to 6.2 and decreased use of rescue medications from 2.3 to 1.2 days per week.18
Table 1
Possible rationale for antipsychotic use for headaches and nausea
| Condition | Possible rationale |
|---|---|
| Migraine | Patients are hypersensitive to dopamine agonists or dopamine transporter dysfunction. Some evidence that the dopamine D2 (DRD2) gene is involved |
| Cluster headache | Pain alleviation possibly related to dopamine receptor antagonism |
| Nausea | D2 and H1 receptor blockage |
Table 2
Antipsychotics for headache and nausea: Strength of the evidence
| Condition | Strength of evidencea |
|---|---|
| Migraine | Intermediate: Chlorpromazine,2-5 droperidol,6-8 prochlorperazine1,10-12 |
| Weak: Haloperidol13,14 | |
| Very weak: Aripiprazole,15 olanzapine,16,17 quetiapine,18 ziprasidone19 | |
| Cluster headache | Weak: Chlorpromazine20 |
| Very weak: Clozapine,21 olanzapine22 | |
| Nausea/vomiting | Intermediate: Droperidol,23 metoclopramide,24 prochlorperazine,25 promethazine25 |
| Weak: Olanzapine26,27 | |
| aStrong: Multiple, well-designed RCTs directly relevant to the recommendation, yielding consistent findings Intermediate: Some evidence from RCTs that support the recommendation, but the scientific support was not optimal Weak: Consensus recommendation in the absence of relevant randomized controlled trials and better evidence than case report or series Very weak: Case reports or case series or preliminary studies RCTs: randomized controlled trials | |
Cluster headaches
Subcutaneous sumatriptan and inhaled oxygen are first-line treatments for cluster headaches.31 A single, small study20 reported that chlorpromazine may prevent cluster headaches, which suggests that D2 receptor blockade may treat such headaches. However, limited supporting evidence relegates its use to a second- or third-line therapy.
In an open-label study (N = 5), olanzapine provided some relief of pain associated with cluster headache within 20 minutes of administration.22 In another study, patients with schizophrenia and comorbid cluster headaches improved with olanzapine.21
Because evidence is limited to small prospective studies, antipsychotic treatment of cluster headache is not well established.20-22 However, olanzapine may benefit patients with comorbid cluster headaches and schizophrenia.
Nausea
The signaling pathways that mediate emesis involve 5-HT3, D2, muscarinic, and histamine receptors.32 Before 5-HT3 antagonists were available, the FGAs metoclopramide, droperidol, prochlorperazine, and promethazine were used to manage acute emesis in emergency departments.23 A double-blind, placebo-controlled trial found IV droperidol, 1.25 mg, was more effective than metoclopramide, 10 mg, or prochlorperazine, 10 mg, for relieving moderate to severe nausea in adult patients.23 However, droperidol and prochlorperazine were associated with akathisia. In addition, this trial did not find a clinically significant difference between groups—including placebo—in anxiety, sedation, or need for rescue medications.23 Use of droperidol to treat nausea decreased after the drug received a “black-box” warning for QT prolongation and torsades de pointes.
Metoclopramide is effective for treating acute migraine and associated nausea24 and has been used to treat gastroparesis because of its effect on upper GI motility. Phenothiazines have been used to treat nausea and studies have shown prochlorperazine to be more effective than promethazine.25 Some studies of prochlorperazine have reported a 44% incidence of akathisia, which limits the drug’s use in patients who may be sensitive to such effects.33 Promethazine can cause sedation and risk of tissue necrosis at the injection site.34
Among SGAs, olanzapine effectively prevented acute and delayed chemotherapy-induced nausea and vomiting in a proof-of-concept study of patients receiving high and moderate emetogenic therapies.26,27 National Comprehensive Cancer Network guidelines cite olanzapine as a potential option for treating refractory and breakthrough emesis.35 In a small study (N = 50), olanzapine showed comparable anti-nausea effect to aprepitant—a neurokinin 1 receptor antagonist—and effectively prevented chemotherapy-induced nausea and vomiting in highly emetogenic chemotherapy.36
Related Resources
- Kelley NE, Tepper DE. Rescue therapy for acute migraine, part 2: neuroleptics, antihistamines, and others. Headache. 2012;52(2):292-306.
- Dusitanond P, Young WB. Neuroleptics and migraine. Cent Nerv Syst Agents Med Chem. 2009;9(1):63-70.
Drug Brand Names
- Aprepitant • Emend
- Aripiprazole • Abilify
- Chlorpromazine • Thorazine
- Dihydroergotamine • D.H.E 45
- Droperidol • Inapsine
- Ergotamine tartrate • Ergostat
- Haloperidol • Haldol
- Ketorolac • Toradol
- Lidocaine • Xylocaine, Lidoderm
- Meperidine • Demerol
- Metoclopramide • Reglan
- Olanzapine • Zyprexa
- Prochlorperazine • Compazine
- Promethazine • Phenergan
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sumatriptan • Imitrex
- Valproate • Depakote
Disclosures
Dr. Macaluso has received grant or research support from EnVivo Pharmaceuticals, Janssen L.P., and Pfizer, Inc.
Dr. Tripathi reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Most evidence supporting antipsychotics as a treatment for migraine headaches and cluster headaches is based on small studies and chart reviews. Some research suggests antipsychotics may effectively treat nausea but side effects such as akathisia may limit their use.
Migraine headaches
Antipsychotic treatment of migraines is supported by the theory that dopaminergic hyperactivity leads to migraine headaches (Table 1). Antipsychotics have been used off-label in migraine patients who do not tolerate triptans or have status migrainosus—intense, debilitating migraine lasting >72 hours.1 Primarily a result of D2 receptor blockade, the serotonergic effects of some second-generation antipsychotics (SGAs) may prevent migraine recurrence. The first-generation antipsychotics (FGAs) prochlorperazine, droperidol, haloperidol, and chlorpromazine have been used for migraine headaches (Table 2).1-27
Prochlorperazine may be an effective treatment of acute headaches9 and refractory chronic daily headache.10 Studies show that buccal prochlorperazine is more effective than oral ergotamine tartrate11 and IV prochlorperazine is more effective than IV ketorolac12 or valproate28 for treating acute headache.
Evidence suggests that chlorpromazine administered IM2 or IV3 is better than placebo for managing migraine pain. In a study comparing IV chlorpromazine, lidocaine, and dihydroergotamine, patients treated with chlorpromazine showed more persistent headache relief 12 to 24 hours post-dose.4 In another study, IV chlorpromazine, 25 mg, was as effective as IM ketorolac, 60 mg.5
Droperidol has been shown to be effective for managing headache, specifically status migrainosus.6 Patients with “benign headache”—headache not caused by an underlying medical disorder—who received droperidol reported greater reduction in visual analog pain scores within 1 hour of dosing compared with those taking prochlorperazine.7 In a randomized trial comparing IM droperidol and IM meperidine, patients with an acute migraine who received droperidol had improved scores on the visual pain analog scale and required less “rescue medication” for breakthrough pain.8 The FDA has issued a “black-box” warning of QTc prolongation with droperidol.
In a double blind, placebo-controlled trial, IV haloperidol, 5 mg, effectively treated migraine headache in 80% of patients compared with 15% of those who received placebo. However, 16% of patients considered the side effects—mainly sedation and akathisia—intolerable and 7% had symptom relapse.13 In an open-label trial of 6 patients with migraine headache, all patients achieved complete or substantial headache relief 25 to 65 minutes after receiving IV haloperidol, 5 mg.14
SGAs often antagonize 5-HT1D receptors and theoretically can render triptan therapy—which stimulates pre-synaptic 5-HT1D receptors—ineffective. This has not been seen clinically and instead, dose-related, non-specific headaches are a common adverse event with SGAs.29,30 A retrospective chart review found olanzapine provided relief for refractory headaches in patients who had failed ≥4 preventive medications. Olanzapine significantly decreased headache days, from 27.5±4.9 before treatment to 21.1±10.7 after treatment. Olanzapine also improved headache severity (measured on a 0 to 10 scale) from 8.7±1.6 before treatment to 2.2±2.1 after treatment.16 Researchers found that 2.5 or 5 mg of olanzapine relieved acute migraines for most patients, with repeat dosing as needed up to 20 mg/d. For prophylactic treatment, 5 or 10 mg of olanzapine was used. Olanzapine’s antinociceptive effect may be related to its action on α-2 adrenoreceptors and to a lesser extent on involvement of opioid and serotonergic receptors.17
In a case series, 3 migraine patients who met criteria for chronic daily headache and migraines but did not have a psychiatric disorder reported significant and sustained headache improvement when treated with risperidone.19 In a case series of 3 migraine patients with co-occurring psychiatric disorders, aripiprazole decreased migraine frequency and severity.15 Although limited data support quetiapine’s efficacy in treating acute migraines, in an open-label, pilot study, patients taking quetiapine, 25 to 75 mg/d, demonstrated a decrease in mean frequency of migraine days from 10.2 to 6.2 and decreased use of rescue medications from 2.3 to 1.2 days per week.18
Table 1
Possible rationale for antipsychotic use for headaches and nausea
| Condition | Possible rationale |
|---|---|
| Migraine | Patients are hypersensitive to dopamine agonists or dopamine transporter dysfunction. Some evidence that the dopamine D2 (DRD2) gene is involved |
| Cluster headache | Pain alleviation possibly related to dopamine receptor antagonism |
| Nausea | D2 and H1 receptor blockage |
Table 2
Antipsychotics for headache and nausea: Strength of the evidence
| Condition | Strength of evidencea |
|---|---|
| Migraine | Intermediate: Chlorpromazine,2-5 droperidol,6-8 prochlorperazine1,10-12 |
| Weak: Haloperidol13,14 | |
| Very weak: Aripiprazole,15 olanzapine,16,17 quetiapine,18 ziprasidone19 | |
| Cluster headache | Weak: Chlorpromazine20 |
| Very weak: Clozapine,21 olanzapine22 | |
| Nausea/vomiting | Intermediate: Droperidol,23 metoclopramide,24 prochlorperazine,25 promethazine25 |
| Weak: Olanzapine26,27 | |
| aStrong: Multiple, well-designed RCTs directly relevant to the recommendation, yielding consistent findings Intermediate: Some evidence from RCTs that support the recommendation, but the scientific support was not optimal Weak: Consensus recommendation in the absence of relevant randomized controlled trials and better evidence than case report or series Very weak: Case reports or case series or preliminary studies RCTs: randomized controlled trials | |
Cluster headaches
Subcutaneous sumatriptan and inhaled oxygen are first-line treatments for cluster headaches.31 A single, small study20 reported that chlorpromazine may prevent cluster headaches, which suggests that D2 receptor blockade may treat such headaches. However, limited supporting evidence relegates its use to a second- or third-line therapy.
In an open-label study (N = 5), olanzapine provided some relief of pain associated with cluster headache within 20 minutes of administration.22 In another study, patients with schizophrenia and comorbid cluster headaches improved with olanzapine.21
Because evidence is limited to small prospective studies, antipsychotic treatment of cluster headache is not well established.20-22 However, olanzapine may benefit patients with comorbid cluster headaches and schizophrenia.
Nausea
The signaling pathways that mediate emesis involve 5-HT3, D2, muscarinic, and histamine receptors.32 Before 5-HT3 antagonists were available, the FGAs metoclopramide, droperidol, prochlorperazine, and promethazine were used to manage acute emesis in emergency departments.23 A double-blind, placebo-controlled trial found IV droperidol, 1.25 mg, was more effective than metoclopramide, 10 mg, or prochlorperazine, 10 mg, for relieving moderate to severe nausea in adult patients.23 However, droperidol and prochlorperazine were associated with akathisia. In addition, this trial did not find a clinically significant difference between groups—including placebo—in anxiety, sedation, or need for rescue medications.23 Use of droperidol to treat nausea decreased after the drug received a “black-box” warning for QT prolongation and torsades de pointes.
Metoclopramide is effective for treating acute migraine and associated nausea24 and has been used to treat gastroparesis because of its effect on upper GI motility. Phenothiazines have been used to treat nausea and studies have shown prochlorperazine to be more effective than promethazine.25 Some studies of prochlorperazine have reported a 44% incidence of akathisia, which limits the drug’s use in patients who may be sensitive to such effects.33 Promethazine can cause sedation and risk of tissue necrosis at the injection site.34
Among SGAs, olanzapine effectively prevented acute and delayed chemotherapy-induced nausea and vomiting in a proof-of-concept study of patients receiving high and moderate emetogenic therapies.26,27 National Comprehensive Cancer Network guidelines cite olanzapine as a potential option for treating refractory and breakthrough emesis.35 In a small study (N = 50), olanzapine showed comparable anti-nausea effect to aprepitant—a neurokinin 1 receptor antagonist—and effectively prevented chemotherapy-induced nausea and vomiting in highly emetogenic chemotherapy.36
Related Resources
- Kelley NE, Tepper DE. Rescue therapy for acute migraine, part 2: neuroleptics, antihistamines, and others. Headache. 2012;52(2):292-306.
- Dusitanond P, Young WB. Neuroleptics and migraine. Cent Nerv Syst Agents Med Chem. 2009;9(1):63-70.
Drug Brand Names
- Aprepitant • Emend
- Aripiprazole • Abilify
- Chlorpromazine • Thorazine
- Dihydroergotamine • D.H.E 45
- Droperidol • Inapsine
- Ergotamine tartrate • Ergostat
- Haloperidol • Haldol
- Ketorolac • Toradol
- Lidocaine • Xylocaine, Lidoderm
- Meperidine • Demerol
- Metoclopramide • Reglan
- Olanzapine • Zyprexa
- Prochlorperazine • Compazine
- Promethazine • Phenergan
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sumatriptan • Imitrex
- Valproate • Depakote
Disclosures
Dr. Macaluso has received grant or research support from EnVivo Pharmaceuticals, Janssen L.P., and Pfizer, Inc.
Dr. Tripathi reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Dusitanond P, Young WB. Neuroleptics and migraine. Cent Nerv Syst Agents Med Chem. 2009;9(1):63-70.
2. McEwen JI, O’Connor HM, Dinsdale HB. Treatment of migraine with intramuscular chlorpromazine. Ann Emerg Med. 1987;16(7):758-763.
3. Bigal M, Bordini CA, Speciali JG. Intravenous chlorpromazine in the emergency department treatment of migraines: a randomized controlled trial. J Emerg Med. 2002;23(2):141-148.
4. Bell R, Montoya D, Shuaib A, et al. A comparative trial of three agents in the treatment of acute migraine headache. Ann Emerg Med. 1990;19(10):1079-1082.
5. Shrestha M, Singh R, Moreden J, et al. Ketorolac vs chlorpromazine in the treatment of acute migraine without aura. A prospective, randomized, double-blind trial. Arch Intern Med. 1996;156(15):1725-1728.
6. Wang SJ, Silberstein SD, Young WB. Droperidol treatment of status migrainosus and refractory migraine. Headache. 1997;37(6):377-382.
7. Miner JR, Fish SJ, Smith SW, et al. Droperidol vs. prochlorperazine for benign headaches in the emergency department. Acad Emerg Med. 2001;8(9):873-879.
8. Richman PB, Allegra J, Eskin B, et al. A randomized clinical trial to assess the efficacy of intramuscular droperidol for the treatment of acute migraine headache. Am J Emerg Med. 2002;20(1):39-42.
9. Jones J, Sklar D, Dougherty J, et al. Randomized double blind trial of intravenous prochlorperazine for the treatment of acute headache. JAMA. 1989;261(8):1174-1176.
10. Lu SR, Fuh JL, Juang KD, et al. Repetitive intravenous prochlorperazine treatment of patients with refractory chronic daily headache. Headache. 2000;40(9):724-729.
11. Sharma S, Prasad A, Nehru R, et al. Efficacy and tolerability of prochlorperazine buccal tablets in treatment of acute migraine. Headache. 2002;42(9):896-902.
12. Seim MB, March JA, Dunn KA. Intravenous ketorolac vs intravenous prochlorperazine for the treatment of migraine headaches. Acad Emerg Med. 1998;5(6):573-576.
13. Honkaniemi J, Liimatainen S, Rainesalo S, et al. Haloperidol in the acute treatment of migraine: a randomized, double-blind, placebo-controlled study. Headache. 2006;46(5):781-787.
14. Fisher H. A new approach to emergency department therapy of migraine headache with intravenous haloperidol: a case series. J Emerg Med. 1995;13(1):119-122.
15. LaPorta LD. Relief from migraine headache with aripiprazole treatment. Headache. 2007;47(6):922-926.
16. Silberstein SD, Peres MF, Hopkins MM, et al. Olanzapine in the treatment of refractory migraine and chronic daily headache. Headache. 2002;42(6):515-518.
17. Schreiber S, Getslev V, Backer MM, et al. The atypical neuroleptics clozapine and olanzapine differ regarding their antinociceptive mechanisms and potency. Pharmacol Biochem Behav. 1999;64(1):75-80.
18. Krymchantowski AV, Jevoux C. Quetiapine for the prevention of migraine refractory to the combination of atenolol + nortriptyline + flunarizine: an open pilot study. Arq Neuropsiquiatr. 2008;66(3B):615-618.
19. Cahill CM, Hardiman O, Murphy KC. Treatment of refractory chronic daily headache with the atypical antipsychotic ziprasidone-a case series. Cephalalgia. 2005;25(10):822-826.
20. Caviness VS, Jr, O’Brien P. Cluster headache: response to chlorpromazine. Headache. 1980;20(3):128-131.
21. Datta SS, Kumar S. Clozapine-responsive cluster headache. Neurol India. 2006;54(2):200-201.
22. Rozen TD. Olanzapine as an abortive agent for cluster headache. Headache. 2001;41(8):813-816.
23. Braude D, Soliz T, Crandall C, et al. Antiemetics in the ED: a randomized controlled trial comparing 3 common agents. Am J Emerg Med. 2006;24(2):177-182.
24. Colman I, Brown MD, Innes GD, et al. Parenteral metoclopramide for acute migraine: meta-analysis of randomised controlled trials. BMJ. 2004;329(7479):1369-1373.
25. Ernst AA, Weiss SJ, Park S, et al. Prochlorperazine versus promethazine for uncomplicated nausea and vomiting in the emergency department: a randomized, double-blind clinical trial. Ann Emerg Med. 2000;36(2):89-94.
26. Navari RM, Einhorn LH, Loehrer PJ Sr, et al. A phase II trial of olanzapine, dexamethasone, and palonosetron for the prevention of chemotherapy-induced nausea and vomiting: a Hoosier oncology group study. Support Care Cancer. 2007;15(11):1285-1291.
27. Passik SD, Navari RM, Jung SH, et al. A phase I trial of olanzapine (Zyprexa) for the prevention of delayed emesis in cancer patients: a Hoosier Oncology Group study. Cancer Invest. 2004;22(3):383-388.
28. Tanen DA, Miller S, French T, et al. Intravenous sodium valproate versus prochlorperazine for the emergency department treatment of acute migraine headaches: a prospective, randomized, double-blind trial. Ann Emerg Med. 2003;41(6):847-853.
29. Caley CF, Cooper CK. Ziprasidone: the fifth atypical antipsychotic. Ann Pharmacother. 2002;36(5):839-851.
30. Geodon [package insert]. New York NY. Pfizer Inc.; 2012.
31. Kudrow L. Response of cluster headache attacks to oxygen inhalation. Headache. 1981;21(1):1-4.
32. Scuderi PE. Pharmacology of antiemetics. Int Anesthesiol Clin. 2003;41(4):41-66.
33. Drotts DL, Vinson DR. Prochlorperazine induces akathisia in emergency patients. Ann Emerg Med. 1999;34(4):469-475.
34. Institute for Safe Medication Practices. Action needed to prevent serious tissue injury with IV promethazine. http://www.ismp.org/newsletters/acutecare/articles/20060810.asp?ptr_y. Published August 10 2006. Accessed November 28, 2012.
35. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. 2010. http://www.nccn.org/professionals/physician_gls/pdf/antiemesis.pdf. Accessed November 29 2012.
36. Navari R, Gray SE, Carr AC. Olanzapine versus aprepitant for the prevention of chemotherapy induced nausea and vomiting (CINV): a randomized phase III trial. J Clin Oncol. 2010;28(15 suppl):9020.-
1. Dusitanond P, Young WB. Neuroleptics and migraine. Cent Nerv Syst Agents Med Chem. 2009;9(1):63-70.
2. McEwen JI, O’Connor HM, Dinsdale HB. Treatment of migraine with intramuscular chlorpromazine. Ann Emerg Med. 1987;16(7):758-763.
3. Bigal M, Bordini CA, Speciali JG. Intravenous chlorpromazine in the emergency department treatment of migraines: a randomized controlled trial. J Emerg Med. 2002;23(2):141-148.
4. Bell R, Montoya D, Shuaib A, et al. A comparative trial of three agents in the treatment of acute migraine headache. Ann Emerg Med. 1990;19(10):1079-1082.
5. Shrestha M, Singh R, Moreden J, et al. Ketorolac vs chlorpromazine in the treatment of acute migraine without aura. A prospective, randomized, double-blind trial. Arch Intern Med. 1996;156(15):1725-1728.
6. Wang SJ, Silberstein SD, Young WB. Droperidol treatment of status migrainosus and refractory migraine. Headache. 1997;37(6):377-382.
7. Miner JR, Fish SJ, Smith SW, et al. Droperidol vs. prochlorperazine for benign headaches in the emergency department. Acad Emerg Med. 2001;8(9):873-879.
8. Richman PB, Allegra J, Eskin B, et al. A randomized clinical trial to assess the efficacy of intramuscular droperidol for the treatment of acute migraine headache. Am J Emerg Med. 2002;20(1):39-42.
9. Jones J, Sklar D, Dougherty J, et al. Randomized double blind trial of intravenous prochlorperazine for the treatment of acute headache. JAMA. 1989;261(8):1174-1176.
10. Lu SR, Fuh JL, Juang KD, et al. Repetitive intravenous prochlorperazine treatment of patients with refractory chronic daily headache. Headache. 2000;40(9):724-729.
11. Sharma S, Prasad A, Nehru R, et al. Efficacy and tolerability of prochlorperazine buccal tablets in treatment of acute migraine. Headache. 2002;42(9):896-902.
12. Seim MB, March JA, Dunn KA. Intravenous ketorolac vs intravenous prochlorperazine for the treatment of migraine headaches. Acad Emerg Med. 1998;5(6):573-576.
13. Honkaniemi J, Liimatainen S, Rainesalo S, et al. Haloperidol in the acute treatment of migraine: a randomized, double-blind, placebo-controlled study. Headache. 2006;46(5):781-787.
14. Fisher H. A new approach to emergency department therapy of migraine headache with intravenous haloperidol: a case series. J Emerg Med. 1995;13(1):119-122.
15. LaPorta LD. Relief from migraine headache with aripiprazole treatment. Headache. 2007;47(6):922-926.
16. Silberstein SD, Peres MF, Hopkins MM, et al. Olanzapine in the treatment of refractory migraine and chronic daily headache. Headache. 2002;42(6):515-518.
17. Schreiber S, Getslev V, Backer MM, et al. The atypical neuroleptics clozapine and olanzapine differ regarding their antinociceptive mechanisms and potency. Pharmacol Biochem Behav. 1999;64(1):75-80.
18. Krymchantowski AV, Jevoux C. Quetiapine for the prevention of migraine refractory to the combination of atenolol + nortriptyline + flunarizine: an open pilot study. Arq Neuropsiquiatr. 2008;66(3B):615-618.
19. Cahill CM, Hardiman O, Murphy KC. Treatment of refractory chronic daily headache with the atypical antipsychotic ziprasidone-a case series. Cephalalgia. 2005;25(10):822-826.
20. Caviness VS, Jr, O’Brien P. Cluster headache: response to chlorpromazine. Headache. 1980;20(3):128-131.
21. Datta SS, Kumar S. Clozapine-responsive cluster headache. Neurol India. 2006;54(2):200-201.
22. Rozen TD. Olanzapine as an abortive agent for cluster headache. Headache. 2001;41(8):813-816.
23. Braude D, Soliz T, Crandall C, et al. Antiemetics in the ED: a randomized controlled trial comparing 3 common agents. Am J Emerg Med. 2006;24(2):177-182.
24. Colman I, Brown MD, Innes GD, et al. Parenteral metoclopramide for acute migraine: meta-analysis of randomised controlled trials. BMJ. 2004;329(7479):1369-1373.
25. Ernst AA, Weiss SJ, Park S, et al. Prochlorperazine versus promethazine for uncomplicated nausea and vomiting in the emergency department: a randomized, double-blind clinical trial. Ann Emerg Med. 2000;36(2):89-94.
26. Navari RM, Einhorn LH, Loehrer PJ Sr, et al. A phase II trial of olanzapine, dexamethasone, and palonosetron for the prevention of chemotherapy-induced nausea and vomiting: a Hoosier oncology group study. Support Care Cancer. 2007;15(11):1285-1291.
27. Passik SD, Navari RM, Jung SH, et al. A phase I trial of olanzapine (Zyprexa) for the prevention of delayed emesis in cancer patients: a Hoosier Oncology Group study. Cancer Invest. 2004;22(3):383-388.
28. Tanen DA, Miller S, French T, et al. Intravenous sodium valproate versus prochlorperazine for the emergency department treatment of acute migraine headaches: a prospective, randomized, double-blind trial. Ann Emerg Med. 2003;41(6):847-853.
29. Caley CF, Cooper CK. Ziprasidone: the fifth atypical antipsychotic. Ann Pharmacother. 2002;36(5):839-851.
30. Geodon [package insert]. New York NY. Pfizer Inc.; 2012.
31. Kudrow L. Response of cluster headache attacks to oxygen inhalation. Headache. 1981;21(1):1-4.
32. Scuderi PE. Pharmacology of antiemetics. Int Anesthesiol Clin. 2003;41(4):41-66.
33. Drotts DL, Vinson DR. Prochlorperazine induces akathisia in emergency patients. Ann Emerg Med. 1999;34(4):469-475.
34. Institute for Safe Medication Practices. Action needed to prevent serious tissue injury with IV promethazine. http://www.ismp.org/newsletters/acutecare/articles/20060810.asp?ptr_y. Published August 10 2006. Accessed November 28, 2012.
35. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. 2010. http://www.nccn.org/professionals/physician_gls/pdf/antiemesis.pdf. Accessed November 29 2012.
36. Navari R, Gray SE, Carr AC. Olanzapine versus aprepitant for the prevention of chemotherapy induced nausea and vomiting (CINV): a randomized phase III trial. J Clin Oncol. 2010;28(15 suppl):9020.-
SSRIs for PTSD bring cardiometabolic benefits
LOS ANGELES – The use of selective serotonin reuptake inhibitors in treating combat posttraumatic stress disorder in middle-aged veterans was associated with sharply reduced cardiovascular mortality in a large observational study, according to Dr. Naser Ahmadi of the VA Greater Los Angeles Healthcare System.
Treatment with a selective serotonin reuptake inhibitor also appeared to protect against development of metabolic syndrome in this patient population, he added at the annual scientific sessions of the American Heart Association.
Dr. Ahmadi reported on 1,142 veterans with combat-induced PTSD, 433 of whom had received SSRI therapy for the disorder. Fifty-three percent of subjects met criteria for metabolic syndrome.
During a median 5 years of follow-up through the Veterans Affairs system’s electronic medical record system, the cardiovascular mortality was 21% in patients with PTSD and metabolic syndrome, compared with 14% in those without metabolic syndrome. Thus, comorbid metabolic syndrome in patients with combat PTSD was associated with a 68% increase in the risk of cardiovascular mortality.
The prevalence of metabolic syndrome was 32% among SSRI recipients, compared with 45% in those whose PTSD was not treated SSRIs. After adjusting for age, gender, and conventional cardiovascular risk factors in a multivariate analysis, the relative risk of metabolic syndrome in patients with PTSD was 71% lower with SSRI therapy than without it.
Moreover, the adjusted risk of cardiovascular mortality over the course of a median 5 years of follow-up in this hypothesis-generating observational study was 36% lower in SSRI-treated patients with PTSD and metabolic syndrome than in those not on an SSRI, and 64% lower in SSRI-treated patients with PTSD without metabolic syndrome.
This study received funding from the American Heart Association. Dr. Ahmadi reported having no financial conflicts.
LOS ANGELES – The use of selective serotonin reuptake inhibitors in treating combat posttraumatic stress disorder in middle-aged veterans was associated with sharply reduced cardiovascular mortality in a large observational study, according to Dr. Naser Ahmadi of the VA Greater Los Angeles Healthcare System.
Treatment with a selective serotonin reuptake inhibitor also appeared to protect against development of metabolic syndrome in this patient population, he added at the annual scientific sessions of the American Heart Association.
Dr. Ahmadi reported on 1,142 veterans with combat-induced PTSD, 433 of whom had received SSRI therapy for the disorder. Fifty-three percent of subjects met criteria for metabolic syndrome.
During a median 5 years of follow-up through the Veterans Affairs system’s electronic medical record system, the cardiovascular mortality was 21% in patients with PTSD and metabolic syndrome, compared with 14% in those without metabolic syndrome. Thus, comorbid metabolic syndrome in patients with combat PTSD was associated with a 68% increase in the risk of cardiovascular mortality.
The prevalence of metabolic syndrome was 32% among SSRI recipients, compared with 45% in those whose PTSD was not treated SSRIs. After adjusting for age, gender, and conventional cardiovascular risk factors in a multivariate analysis, the relative risk of metabolic syndrome in patients with PTSD was 71% lower with SSRI therapy than without it.
Moreover, the adjusted risk of cardiovascular mortality over the course of a median 5 years of follow-up in this hypothesis-generating observational study was 36% lower in SSRI-treated patients with PTSD and metabolic syndrome than in those not on an SSRI, and 64% lower in SSRI-treated patients with PTSD without metabolic syndrome.
This study received funding from the American Heart Association. Dr. Ahmadi reported having no financial conflicts.
LOS ANGELES – The use of selective serotonin reuptake inhibitors in treating combat posttraumatic stress disorder in middle-aged veterans was associated with sharply reduced cardiovascular mortality in a large observational study, according to Dr. Naser Ahmadi of the VA Greater Los Angeles Healthcare System.
Treatment with a selective serotonin reuptake inhibitor also appeared to protect against development of metabolic syndrome in this patient population, he added at the annual scientific sessions of the American Heart Association.
Dr. Ahmadi reported on 1,142 veterans with combat-induced PTSD, 433 of whom had received SSRI therapy for the disorder. Fifty-three percent of subjects met criteria for metabolic syndrome.
During a median 5 years of follow-up through the Veterans Affairs system’s electronic medical record system, the cardiovascular mortality was 21% in patients with PTSD and metabolic syndrome, compared with 14% in those without metabolic syndrome. Thus, comorbid metabolic syndrome in patients with combat PTSD was associated with a 68% increase in the risk of cardiovascular mortality.
The prevalence of metabolic syndrome was 32% among SSRI recipients, compared with 45% in those whose PTSD was not treated SSRIs. After adjusting for age, gender, and conventional cardiovascular risk factors in a multivariate analysis, the relative risk of metabolic syndrome in patients with PTSD was 71% lower with SSRI therapy than without it.
Moreover, the adjusted risk of cardiovascular mortality over the course of a median 5 years of follow-up in this hypothesis-generating observational study was 36% lower in SSRI-treated patients with PTSD and metabolic syndrome than in those not on an SSRI, and 64% lower in SSRI-treated patients with PTSD without metabolic syndrome.
This study received funding from the American Heart Association. Dr. Ahmadi reported having no financial conflicts.
AT THE ANNUAL SCIENTIFIC SESSIONS OF THE AMERICAN HEART ASSOCIATION
Major Finding: The relative risk of metabolic syndrome in middle-aged veterans with PTSD was 71% lower with SSRI therapy than without it. Cardiovascular mortality also was substantially lower in patients on an SSRI.
Data Source: Observational study involving 1,142 veterans who were followed for a median of 5 years.
Disclosures: This study received funding from the American Heart Association. Dr. Ahmadi reported having no financial conflicts.
Vitamin deficiencies and mental health: How are they linked?
Discuss this article at www.facebook.com/CurrentPsychiatry
Patients today often are overfed but undernourished. A growing body of literature links dietary choices to brain health and the risk of psychiatric illness. Vitamin deficiencies can affect psychiatric patients in several ways:
- deficiencies may play a causative role in mental illness and exacerbate symptoms
- psychiatric symptoms can result in poor nutrition
- vitamin insufficiency—defined as subclinical deficiency—may compromise patient recovery.
Additionally, genetic differences may compromise vitamin and essential nutrient pathways.
Vitamins are dietary components other than carbohydrates, fats, minerals, and proteins that are necessary for life. B vitamins are required for proper functioning of the methylation cycle, monoamine production, DNA synthesis, and maintenance of phospholipids such as myelin (Figure). Fat-soluble vitamins A, D, and E play important roles in genetic transcription, antioxidant recycling, and inflammatory regulation in the brain.
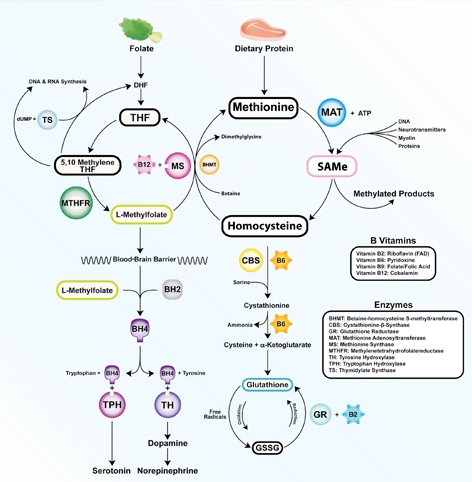
Figure: The methylation cycle
Vitamins B2, B6, B9, and B12 directly impact the functioning of the methylation cycle. Deficiencies pertain to brain function, as neurotransmitters, myelin, and active glutathione are dependent on one-carbon metabolism
Illustration: Mala Nimalasuriya with permission from DrewRamseyMD.com
To help clinicians recognize and treat vitamin deficiencies among psychiatric patients, this article reviews the role of the 6 essential water-soluble vitamins (B1, B2, B6, B9, B12, and C; Table 1,1) and 3 fat-soluble vitamins (A, D, and E; Table 2,1) in brain metabolism and psychiatric pathology. Because numerous sources address using supplements to treat vitamin deficiencies, this article emphasizes food sources, which for many patients are adequate to sustain nutrient status.
Table 1
Water-soluble vitamins: Deficiency, insufficiency, symptoms, and dietary sources
| Deficiency | Insufficiency | Symptoms | At-risk patients | Dietary sources |
|---|---|---|---|---|
| B1 (thiamine): Glycolysis, tricarboxylic acid cycle | ||||
| Rare; 7% in heart failure patients | 5% total, 12% of older women | Wernicke-Korsakoff syndrome, memory impairment, confusion, lack of coordination, paralysis | Older adults, malabsorptive conditions, heavy alcohol use. Those with diabetes are at risk because of increased clearance | Pork, fish, beans, lentils, nuts, rice, and wheat germ. Raw fish, tea, and betel nuts impair absorption |
| B2 (riboflavin): FMN, FAD cofactors in glycolysis and oxidative pathways. B6, folate, and glutathione synthesis | ||||
| 10% to 27% of older adults | <3%; 95% of adolescent girls (measured by EGRAC) | Fatigue, cracked lips, sore throat, bloodshot eyes | Older adults, low intake of animal and dairy products, heavy alcohol use | Dairy, meat and fish, eggs, mushrooms, almonds, leafy greens, and legumes |
| B6 (pyridoxal): Methylation cycle | ||||
| 11% to 24% (<5 ng/mL); 38% of heart failure patients | 14% total, 26% of adults | Dermatitis, glossitis, convulsions, migraine, chronic pain, depression | Older adults, women who use oral contraceptives, alcoholism. 33% to 49% of women age >51 have inadequate intake | Bananas, beans, potatoes, navy beans, salmon, steak, and whole grains |
| B9 (folate): Methylation cycle | ||||
| 0.5% total; up to 50% of depressed patients | 16% of adults, 19% of adolescent girls | Loss of appetite, weight loss, weakness, heart palpitations, behavioral disorders | Depression, pregnancy and lactation, alcoholism, dialysis, liver disease. Deficiency during pregnancy is linked to neural tube defects | Leafy green vegetables, fruits, dried beans, and peas |
| B12 (cobalamin): Methylation cycle (cofactor methionine synthase) | ||||
| 10% to 15% of older adults | <3% to 9% | Depression, irritability, anemia, fatigue, shortness of breath, high blood pressure | Vegetarian or vegan diet, achlorhydria, older adults. Deficiency more often due to poor absorption than low consumption | Meat, seafood, eggs, and dairy |
| C (ascorbic acid): Antioxidant | ||||
| 7.1% | 31% | Scurvy, fatigue, anemia, joint pain, petechia. Symptoms develop after 1 to 3 months of no dietary intake | Smokers, infants fed boiled or evaporated milk, limited dietary variation, patients with malabsorption, chronic illnesses | Citrus fruits, tomatoes and tomato juice, and potatoes |
| EGRAC: erythrocyte glutathione reductase activation coefficient; FAD: flavin adenine dinucleotide; FMN: flavin mononucleotide Source: Reference 1 | ||||
Table 2
Fat-soluble vitamins: Deficiency, insufficiency, symptoms, and dietary sources
| Deficiency | Insufficiency | Symptoms | At-risk patients | Dietary sources |
|---|---|---|---|---|
| A (retinol): Transcription regulation, vision | ||||
| <5% of U.S. population | 44% | Blindness, decreased immunity, corneal and retinal damage | Pregnant women, individuals with strict dietary restrictions, heavy alcohol use, chronic diarrhea, fat malabsorptive conditions | Beef liver, dairy products. Convertible beta-carotene sources: sweet potatoes, carrots, spinach, butternut squash, greens, broccoli, cantaloupe |
| D (cholecalciferol): Hormone, transcriptional regulation | ||||
| ≥50%, 90% of adults age >50 | 69% | Rickets, osteoporosis, muscle twitching | Breast-fed infants, older adults, limited sun exposure, pigmented skin, fat malabsorption, obesity. Older adults have an impaired ability to make vitamin D from the sun. SPF 15 reduces production by 99% | Fatty fish and fish liver oils, sun-dried mushrooms |
| E (tocopherols and tocotrienols): Antioxidant, PUFA protectant, gene regulation | ||||
| Rare | 93% | Anemia, neuropathy, myopathy, abnormal eye movements, weakness, retinal damage | Malabsorptive conditions, HIV, depression | Sunflower, wheat germ, and safflower oils; meats; fish; dairy; green vegetables |
| HIV: human immunodeficiency virus; PUFA: polyunsaturated fatty acids; SPF: sun protection factor Source: Reference 1 | ||||
Water-soluble vitamins
Vitamin B1 (thiamine) is essential for glucose metabolism. Pregnancy, lactation, and fever increase the need for thiamine, and tea, coffee, and shellfish can impair its absorption. Although rare, severe B1 deficiency can lead to beriberi, Wernicke’s encephalopathy (confusion, ataxia, nystagmus), and Korsakoff’s psychosis (confabulation, lack of insight, retrograde and anterograde amnesia, and apathy). Confusion and disorientation stem from the brain’s inability to oxidize glucose for energy because B1 is a critical cofactor in glycolysis and the tricarboxylic acid cycle. Deficiency leads to an increase in reactive oxygen species, proinflammatory cytokines, and blood-brain barrier dysfunction.2 Wernicke’s encephalopathy is most frequently encountered in patients with chronic alcoholism, diabetes, or eating disorders, and after bariatric surgery.3 Iatrogenic Wernicke’s encephalopathy may occur when depleted patients receive IV saline with dextrose without receiving thiamine. Top dietary sources of B1 include pork, fish, beans, lentils, nuts, rice, and wheat germ.
Vitamin B2 (riboflavin) is essential for oxidative pathways, monoamine synthesis, and the methylation cycle. B2 is needed to create the essential flavoprotein coenzymes for synthesis of L-methylfolate—the active form of folate—and for proper utilization of B6. Deficiency can occur after 4 months of inadequate intake.
Although generally B2 deficiency is rare, surveys in the United States have found that 10% to 27% of older adults (age ≥65) are deficient.4 Low intake of dairy products and meat and chronic, excessive alcohol intake are associated with deficiency. Marginal B2 levels are more prevalent in depressed patients, possibly because of B2’s role in the function of glutathione, an endogenous antioxidant.5 Top dietary sources of B2 are dairy products, meat and fish, eggs, mushrooms, almonds, leafy greens, and legumes.
Vitamin B6 refers to 3 distinct compounds: pyridoxine, pyridoxal, and pyridoxamine. B6 is essential to glycolysis, the methylation cycle, and recharging glutathione, an innate antioxidant in the brain. Higher levels of vitamin B6 are associated with a lower prevalence of depression in adolescents,6 and low dietary and plasma B6 increases the risk and severity of depression in geriatric patients7 and predicts depression in prospective trials.8 Deficiency is common (24% to 56%) among patients receiving hemodialysis.9 Women who take oral contraceptives are at increased risk of vitamin B6 deficiency.10 Top dietary sources are fish, beef, poultry, potatoes, legumes, and spinach.
Vitamin B9 (folate) is needed for proper one-carbon metabolism and thus requisite in synthesis of serotonin, norepinephrine, dopamine, and DNA and in phospholipid production. Low maternal folate status increases the risk of neural tube defects in newborns. Folate deficiency and insufficiency are common among patients with mood disorders and correlate with illness severity.11 In a study of 2,682 Finnish men, those in the lowest one-third of folate consumption had a 67% increased relative risk of depression.12 A meta-analysis of 11 studies of 15,315 persons found those who had low folate levels had a significant risk of depression.13 Patients without deficiency but with folate levels near the low end of the normal range also report low mood.14 Compared with controls, patients experiencing a first episode of psychosis have lower levels of folate, B12, and docosahexaenoic acid.15
Dietary folate must be converted to L-methylfolate for use in the brain. Patients with a methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism produce a less active form of the enzyme. The TT genotype is associated with major depression and bipolar disorder.16 Clinical trials have shown that several forms of folate can enhance antidepressant treatment.17 Augmentation with L-methylfolate, which bypasses the MTHFR enzyme, can be an effective strategy for treating depression in these patients.18
Leafy greens and legumes such as lentils are top dietary sources of folate; supplemental folic acid has been linked to an increased risk of cancer and overall mortality.19,20
Vitamin B12 (cobalamin). An essential cofactor in one-carbon metabolism, B12 is needed to produce monoamine neurotransmitters and maintain myelin. Deficiency is found in up to one-third of depressed patients11 and compromises antidepressant response,21 whereas higher vitamin B12 levels are associated with better treatment outcomes.22 B12 deficiency can cause depression, irritability, agitation, psychosis, and obsessive symptoms.23,24 Low B12 levels and elevated homocysteine increase the risk of cognitive decline and Alzheimer’s disease and are linked to a 5-fold increase in the rate of brain atrophy.26
B12 deficiencies may be seen in patients with gastrointestinal illness, older adults with achlorhydria, and vegans and vegetarians, in whom B12 intake can be low. Proton pump inhibitors such as omeprazole interfere with B12 absorption from food.
Psychiatric symptoms of B12 deficiency may present before hematologic findings.23 Folic acid supplementation may mask a B12 deficiency by delaying anemia but will not delay psychiatric symptoms. Ten percent of patients with an insufficiency (low normal levels of 200 to 400 pg/mL) have elevated homocysteine, which increases the risk of psychiatric disorders as well as comorbid illnesses such as cardiovascular disease. Top dietary sources include fish, mollusks (oysters, mussels, and clams), meat, and dairy products.
Vitamin C is vital for the synthesis of monoamines such as serotonin and norepinephrine. Vitamin C’s primary role in the brain is as an antioxidant. As a necessary cofactor, it keeps the copper and iron in metalloenzymes reduced, and also recycles vitamin E. Proper function of the methylation cycle depends on vitamin C, as does collagen synthesis and metabolism of xenobiotics by the liver. It is concentrated in cerebrospinal fluid.
Humans cannot manufacture vitamin C. Although the need for vitamin C (90 mg/d) is thought to be met by diet, studies have found that up to 13.7% of healthy, middle class patients in the United States are depleted.27 Older adults and patients with a poor diet due to drug or alcohol abuse, eating disorders, or affective symptoms are at risk.
Scurvy is caused by vitamin C deficiency and leads to bleeding gums and petechiae. Patients with insufficiency report irritability, loss of appetite, weight loss, and hypochondriasis. Vitamin C intake is significantly lower in older adults (age ≥60) with depression.28 Some research indicates patients with schizophrenia have decreased vitamin C levels and dysfunction of antioxidant defenses.29 Citrus, potatoes, and tomatoes are top dietary sources of vitamin C.
Fat-soluble vitamins
Vitamin A. Although vitamin A activity in the brain is poorly understood, retinol—the active form of vitamin A—is crucial for formation of opsins, which are the basis for vision. Childhood vitamin A deficiency may lead to blindness. Vitamin A also plays an important role in maintaining bone growth, reproduction, cell division, and immune system integrity.30 Animal sources such as beef liver, dairy products, and eggs provide retinol, and plant sources such as carrots, sweet potatoes, and leafy greens provide provitamin A carotenoids that humans convert into retinol.
Deficiency rarely is observed in the United States but remains a common problem for developing nations. In the United States, vitamin A deficiency is most often seen with excessive alcohol use, rigorous dietary restrictions, and gastrointestinal diseases accompanied by poor fat absorption.
Excess vitamin A ingestion may result in bone abnormalities, liver damage, birth defects, and depression. Isotretinoin—a form of vitamin A used to treat severe acne—carries an FDA “black-box” warning for psychiatric adverse effects, including aggression, depression, psychosis, and suicide.
Vitamin D is produced from cholesterol in the epidermis through exposure to sunlight, namely ultraviolet B radiation. After dermal synthesis or ingestion, vitamin D is converted through a series of steps into the active form of vitamin D, calcitriol, which also is known as 25(OH)D3.
Although vitamin D is known for its role in bone growth and mineralization,31 increasing evidence reveals vitamin D’s role in brain function and development.32 Both glial and neuronal cells possess vitamin D receptors in the hippocampus, prefrontal cortex, hypothalamus, thalamus, and substantia nigra—all regions theorized to be linked to depression pathophysiology.33 A review of the association of vitamin D deficiency and psychiatric illnesses will be published in a future issue of Current Psychiatry.
Vitamin D exists in food as either D2 or D3, from plant and animal sources, respectively. Concentrated sources include oily fish, sun-dried or “UVB-irradiated” mushrooms, and milk.
Vitamin E. There are 8 isoforms of vitamin E—4 tocopherols and 4 tocotrienols—that function as fat-soluble antioxidants and also promote innate antioxidant enzymes. Because vitamin E protects neuronal membranes from oxidation, low levels may affect the brain via increased inflammation. Alpha-tocopherol is the most common form of vitamin E in humans, but emerging evidence suggests tocotrienols mediate disease by modifying transcription factors in the brain, such as glutathione reductase, superoxide dismutase, and nuclear factor-kappaB.34 Low plasma vitamin E levels are found in depressed patients, although some data suggest this may be caused by factors other than dietary intake.35 Low vitamin status has been found in up to 70% of older adults.36 Although deficiency is rare, most of the U.S. population (93%) has inadequate dietary intake of vitamin E.1 The reasons for this discrepancy are unclear. Foods rich in vitamin E include almonds, sunflower seeds, leafy greens, and wheat germ.
Recommendations
Patients with depression, alcohol abuse, eating disorders, obsessive-compulsive disorder, or schizophrenia may neglect to care for themselves or adopt particular eating patterns. Deficiencies are more common among geriatric patients and those who are medically ill. Because dietary patterns are linked to the risk of psychiatric disorders, nutritional inquiry often identifies multiple modifiable risk factors, such as folate, vitamin B12, and vitamin D intake.37,38 Nutritional counseling offers clinicians an intervention with minimal side effect risks and the opportunity to modify a behavior that patients engage in 3 times a day.
Psychiatrists should assess patients’ dietary patterns and vitamin status, particularly older adults and those with:
- lower socioeconomic status or food insecurity
- a history of treatment resistance
- restrictive dietary patterns such as veganism
- alcohol abuse.
On initial assessment, test or obtain from other health care providers your patient’s blood levels of folate and vitamins D and B12. In some patients, assessing B2 and B6 levels may provide etiological guidance regarding onset of psychiatric symptoms or failure to respond to pharmacologic treatment. Because treating vitamin deficiencies often includes using supplements, evaluate recent reviews of specific deficiencies and consider consulting with the patient’s primary care provider.
Conduct a simple assessment of dietary patterns by asking patients about a typical breakfast, lunch, and dinner, their favorite snacks and foods, and specific dietary habits or restrictions (eg, not consuming seafood, dairy, meat, etc.). Rudimentary nutritional recommendations can be effective in changing a patient’s eating habits, particularly when provided by a physician. Encourage patients to eat nutrient-dense foods such as leafy greens, beans and legumes, seafood, whole grains, and a variety of vegetables and fruits. For more complex patients, consult with a clinical nutritionist.
Related Resources
- Institute of Medicine. Dietary Reference Intakes: Recommended intakes for individuals. SummaryDRIs/~/media/Files
/Activity%20Files/Nutrition
/DRIs/5_Summary%20Table%20Tables%201-4.pdf" target="_blank">www.iom.edu/Activities/Nutrition/
SummaryDRIs/~/media/Files
/Activity%20Files/Nutrition
/DRIs/5_Summary%20Table%20Tables%201-4.pdf. - The Farmacy: Vitamins. http://drewramseymd.com/index.php/resources/farmacy/category/vitamins.
- Office of Dietary Supplements. National Institutes of Health. Dietary supplements fact sheets. http://ods.od.nih.gov/factsheets/list-all.
- Oregon State University. Linus Pauling Institute. Micronutrient information center. http://lpi.oregonstate.edu/infocenter/vitamins.html.
Drug Brand Names
- Isotretinoin • Accutane
- L-methylfolate • Deplin
- Omeprazole • Prilosec
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Moshfegh A, Goldman J, Cleveland L. United States Department of Agriculture, Agricultural Research Service. What we eat in America NHANES 2001-2002: Usual nutrient intakes from food compared to dietary reference intakes. http://www.ars.usda.gov/SP2UserFiles/Place/12355000/pdf/0102/usualintaketables2001-02.pdf. Published September 2005. Accessed November 27, 2012.
2. Page GL, Laight D, Cummings MH. Thiamine deficiency in diabetes mellitus and the impact of thiamine replacement on glucose metabolism and vascular disease. Int J Clin Pract. 2011;65(6):684-690.
3. McCormick LM, Buchanan JR, Onwuameze OE, et al. Beyond alcoholism: Wernicke-Korsakoff syndrome in patients with psychiatric disorders. Cogn Behav Neurol. 2011;24(4):209-216.
4. Powers HJ. Riboflavin (vitamin B-2) and health. Am J Clin Nutr. 2003;77(6):1352-1360.
5. Naghashpour M, Amani R, Nutr R, et al. Riboflavin status and its association with serum hs-CRP levels among clinical nurses with depression. J Am Coll Nutr. 2011;30(5):340-347.
6. Murakami K, Miyake Y, Sasaki S, et al. Dietary folate, riboflavin, vitamin B-6, and vitamin B-12 and depressive symptoms in early adolescence: the Ryukyus Child Health Study. Psychosom Med. 2010;72(8):763-768.
7. Merete C, Falcon LM, Tucker KL. Vitamin B6 is associated with depressive symptomatology in Massachusetts elders. J Am Coll Nutr. 2008;27(3):421-427.
8. Skarupski KA, Tangney C, Li H, et al. Longitudinal association of vitamin B-6, folate, and vitamin B-12 with depressive symptoms among older adults over time. Am J Clin Nutr. 2010;92(2):330-335.
9. Corken M, Porter J. Is vitamin B(6) deficiency an under-recognized risk in patients receiving haemodialysis? A systematic review: 2000-2010. Nephrology (Carlton). 2011;16(7):619-625.
10. Wilson SM, Bivins BN, Russell KA, et al. Oral contraceptive use: impact on folate, vitamin B6, and vitamin B12 status. Nutr Rev. 2011;69(10):572-583.
11. Coppen A, Bolander-Gouaille C. Treatment of depression: time to consider folic acid and vitamin B12. J Psychopharmacol. 2005;19(1):59-65.
12. Tolmunen T, Voutilainen S, Hintikka J, et al. Dietary folate and depressive symptoms are associated in middle-aged Finnish men. J Nutr. 2003;133(10):3233-3236.
13. Gilbody S, Lightfoot T, Sheldon T. Is low folate a risk factor for depression? A meta-analysis and exploration of heterogeneity. J Epidemiol Community Health. 2007;61(7):631-637.
14. Rösche J, Uhlmann C, Fröscher W. Low serum folate levels as a risk factor for depressive mood in patients with chronic epilepsy. J Neuropsychiatry Clin Neurosci. 2003;15(1):64-66.
15. Kale A, Naphade N, Sapkale S, et al. Reduced folic acid, vitamin B12 and docosahexaenoic acid and increased homocysteine and cortisol in never-medicated schizophrenia patients: implications for altered one-carbon metabolism. Psychiatry Res. 2010;175(1-2):47-53.
16. Gilbody S, Lewis S, Lightfoot T. Methylenetetrahydrofolate reductase (MTHFR) genetic polymorphisms and psychiatric disorders: a HuGE review. Am J Epidemiol. 2007;165(1):1-13.
17. Di Palma C, Urani R, Agricola R, et al. Is methylfolate effective in relieving major depression in chronic alcoholics? A hypothesis of treatment. Curr Ther Res Clin Exp. 1994;55(5):559-568.
18. Papakostas GI, Shelton RC, Zajecka JM, et al. l-Methylfolate as adjunctive therapy for ssri-resistant major depression: results of two randomized, double-blind, parallel-sequential trials. Am J Psychiatry. 2012;169(12):1267-1274.
19. Baggott JE, Oster RA, Tamura T. Meta-analysis of cancer risk in folic acid supplementation trials. Cancer Epidemiol. 2012;36(1):78-81.
20. Figueiredo JC, Grau MV, Haile RW, et al. Folic acid and risk of prostate cancer: results from a randomized clinical trial. J Natl Cancer Inst. 2009;101(6):432-435.
21. Kate N, Grover S, Agarwal M. Does B12 deficiency lead to lack of treatment response to conventional antidepressants? Psychiatry (Edgmont). 2010;7(11):42-44.
22. Hintikka J, Tolmunen T, Tanskanen A, et al. High vitamin B12 level and good treatment outcome may be associated in major depressive disorder. BMC Psychiatry. 2003;3:17.-
23. Lindenbaum J, Healton EB, Savage DG, et al. Neuropsychiatric disorders caused by cobalamin deficiency in the absence of anemia or macrocytosis. N Engl J Med. 1988;318(26):1720-1728.
24. Bar-Shai M, Gott D, Marmor S. Acute psychotic depression as a sole manifestation of vitamin B12 deficiency. Psychosomatics. 2011;52(4):384-386.
25. Sharma V, Biswas D. Cobalamin deficiency presenting as obsessive compulsive disorder: case report. Gen Hosp Psychiatry. 2012;34(5):578.e7-e8.
26. Vogiatzoglou A, Refsum H, Johnston C, et al. Vitamin B12 status and rate of brain volume loss in community-dwelling elderly. Neurology. 2008;71(11):826-832.
27. Smith A, Di Primio G, Humphrey-Murto S. Scurvy in the developed world. CMAJ. 2011;183(11):E752-E725.
28. Payne ME, Steck SE, George RR, et al. Fruit, vegetable, and antioxidant intakes are lower in older adults with depression. J Acad Nutr Diet. 2012;112(12):2022-2027.
29. Dadheech G, Mishra S, Gautam S, et al. Oxidative stress, α-tocopherol, ascorbic acid and reduced glutathione status in schizophrenics. Indian J Clin Biochem. 2006;21(2):34-38.
30. Hinds TS, West WL, Knight EM. Carotenoids and retinoids: a review of research clinical, and public health applications. J Clin Pharmacol. 1997;37(7):551-558.
31. Thacher TD, Clarke BL. Vitamin D insufficiency. Mayo Clin Proc. 2011;86(1):50-60.
32. Berk M, Sanders KM, Pasco JA, et al. Vitamin D deficiency may play a role in depression. Med Hypotheses. 2007;69(6):1316-1319.
33. Eyles DW, Smith S, Kinobe R, et al. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J Chem Neuroanat. 2005;29(1):21-30.
34. Sen CK, Khanna S, Roy S. Tocotrienol: the natural vitamin E to defend the nervous system? Ann N Y Acad Sci. 2004;1031:127-142.
35. Owen AJ, Batterham MJ, Probst YC, et al. Low plasma vitamin E levels in major depression: diet or disease? Eur J Clin Nutr. 2005;59(2):304-306.
36. Panemangalore M, Lee CJ. Evaluation of the indices of retinol and alpha-tocopherol status in free-living elderly. J Gerontol. 1992;47(3):B98-B104.
37. Sánchez-Villegas A, Delgado-Rodríguez M, Alonso A, et al. Association of the Mediterranean dietary pattern with the incidence of depression: the Seguimiento Universidad de Navarra/University of Navarra follow-up (SUN) cohort. Arch Gen Psychiatry. 2009;66(10):1090-1098.
38. Jacka FN, Pasco JA, Mykletun A, et al. Association of Western and traditional diets with depression and anxiety in women. Am J Psychiatry. 2010;167(3):305-311.
Discuss this article at www.facebook.com/CurrentPsychiatry
Patients today often are overfed but undernourished. A growing body of literature links dietary choices to brain health and the risk of psychiatric illness. Vitamin deficiencies can affect psychiatric patients in several ways:
- deficiencies may play a causative role in mental illness and exacerbate symptoms
- psychiatric symptoms can result in poor nutrition
- vitamin insufficiency—defined as subclinical deficiency—may compromise patient recovery.
Additionally, genetic differences may compromise vitamin and essential nutrient pathways.
Vitamins are dietary components other than carbohydrates, fats, minerals, and proteins that are necessary for life. B vitamins are required for proper functioning of the methylation cycle, monoamine production, DNA synthesis, and maintenance of phospholipids such as myelin (Figure). Fat-soluble vitamins A, D, and E play important roles in genetic transcription, antioxidant recycling, and inflammatory regulation in the brain.

Figure: The methylation cycle
Vitamins B2, B6, B9, and B12 directly impact the functioning of the methylation cycle. Deficiencies pertain to brain function, as neurotransmitters, myelin, and active glutathione are dependent on one-carbon metabolism
Illustration: Mala Nimalasuriya with permission from DrewRamseyMD.com
To help clinicians recognize and treat vitamin deficiencies among psychiatric patients, this article reviews the role of the 6 essential water-soluble vitamins (B1, B2, B6, B9, B12, and C; Table 1,1) and 3 fat-soluble vitamins (A, D, and E; Table 2,1) in brain metabolism and psychiatric pathology. Because numerous sources address using supplements to treat vitamin deficiencies, this article emphasizes food sources, which for many patients are adequate to sustain nutrient status.
Table 1
Water-soluble vitamins: Deficiency, insufficiency, symptoms, and dietary sources
| Deficiency | Insufficiency | Symptoms | At-risk patients | Dietary sources |
|---|---|---|---|---|
| B1 (thiamine): Glycolysis, tricarboxylic acid cycle | ||||
| Rare; 7% in heart failure patients | 5% total, 12% of older women | Wernicke-Korsakoff syndrome, memory impairment, confusion, lack of coordination, paralysis | Older adults, malabsorptive conditions, heavy alcohol use. Those with diabetes are at risk because of increased clearance | Pork, fish, beans, lentils, nuts, rice, and wheat germ. Raw fish, tea, and betel nuts impair absorption |
| B2 (riboflavin): FMN, FAD cofactors in glycolysis and oxidative pathways. B6, folate, and glutathione synthesis | ||||
| 10% to 27% of older adults | <3%; 95% of adolescent girls (measured by EGRAC) | Fatigue, cracked lips, sore throat, bloodshot eyes | Older adults, low intake of animal and dairy products, heavy alcohol use | Dairy, meat and fish, eggs, mushrooms, almonds, leafy greens, and legumes |
| B6 (pyridoxal): Methylation cycle | ||||
| 11% to 24% (<5 ng/mL); 38% of heart failure patients | 14% total, 26% of adults | Dermatitis, glossitis, convulsions, migraine, chronic pain, depression | Older adults, women who use oral contraceptives, alcoholism. 33% to 49% of women age >51 have inadequate intake | Bananas, beans, potatoes, navy beans, salmon, steak, and whole grains |
| B9 (folate): Methylation cycle | ||||
| 0.5% total; up to 50% of depressed patients | 16% of adults, 19% of adolescent girls | Loss of appetite, weight loss, weakness, heart palpitations, behavioral disorders | Depression, pregnancy and lactation, alcoholism, dialysis, liver disease. Deficiency during pregnancy is linked to neural tube defects | Leafy green vegetables, fruits, dried beans, and peas |
| B12 (cobalamin): Methylation cycle (cofactor methionine synthase) | ||||
| 10% to 15% of older adults | <3% to 9% | Depression, irritability, anemia, fatigue, shortness of breath, high blood pressure | Vegetarian or vegan diet, achlorhydria, older adults. Deficiency more often due to poor absorption than low consumption | Meat, seafood, eggs, and dairy |
| C (ascorbic acid): Antioxidant | ||||
| 7.1% | 31% | Scurvy, fatigue, anemia, joint pain, petechia. Symptoms develop after 1 to 3 months of no dietary intake | Smokers, infants fed boiled or evaporated milk, limited dietary variation, patients with malabsorption, chronic illnesses | Citrus fruits, tomatoes and tomato juice, and potatoes |
| EGRAC: erythrocyte glutathione reductase activation coefficient; FAD: flavin adenine dinucleotide; FMN: flavin mononucleotide Source: Reference 1 | ||||
Table 2
Fat-soluble vitamins: Deficiency, insufficiency, symptoms, and dietary sources
| Deficiency | Insufficiency | Symptoms | At-risk patients | Dietary sources |
|---|---|---|---|---|
| A (retinol): Transcription regulation, vision | ||||
| <5% of U.S. population | 44% | Blindness, decreased immunity, corneal and retinal damage | Pregnant women, individuals with strict dietary restrictions, heavy alcohol use, chronic diarrhea, fat malabsorptive conditions | Beef liver, dairy products. Convertible beta-carotene sources: sweet potatoes, carrots, spinach, butternut squash, greens, broccoli, cantaloupe |
| D (cholecalciferol): Hormone, transcriptional regulation | ||||
| ≥50%, 90% of adults age >50 | 69% | Rickets, osteoporosis, muscle twitching | Breast-fed infants, older adults, limited sun exposure, pigmented skin, fat malabsorption, obesity. Older adults have an impaired ability to make vitamin D from the sun. SPF 15 reduces production by 99% | Fatty fish and fish liver oils, sun-dried mushrooms |
| E (tocopherols and tocotrienols): Antioxidant, PUFA protectant, gene regulation | ||||
| Rare | 93% | Anemia, neuropathy, myopathy, abnormal eye movements, weakness, retinal damage | Malabsorptive conditions, HIV, depression | Sunflower, wheat germ, and safflower oils; meats; fish; dairy; green vegetables |
| HIV: human immunodeficiency virus; PUFA: polyunsaturated fatty acids; SPF: sun protection factor Source: Reference 1 | ||||
Water-soluble vitamins
Vitamin B1 (thiamine) is essential for glucose metabolism. Pregnancy, lactation, and fever increase the need for thiamine, and tea, coffee, and shellfish can impair its absorption. Although rare, severe B1 deficiency can lead to beriberi, Wernicke’s encephalopathy (confusion, ataxia, nystagmus), and Korsakoff’s psychosis (confabulation, lack of insight, retrograde and anterograde amnesia, and apathy). Confusion and disorientation stem from the brain’s inability to oxidize glucose for energy because B1 is a critical cofactor in glycolysis and the tricarboxylic acid cycle. Deficiency leads to an increase in reactive oxygen species, proinflammatory cytokines, and blood-brain barrier dysfunction.2 Wernicke’s encephalopathy is most frequently encountered in patients with chronic alcoholism, diabetes, or eating disorders, and after bariatric surgery.3 Iatrogenic Wernicke’s encephalopathy may occur when depleted patients receive IV saline with dextrose without receiving thiamine. Top dietary sources of B1 include pork, fish, beans, lentils, nuts, rice, and wheat germ.
Vitamin B2 (riboflavin) is essential for oxidative pathways, monoamine synthesis, and the methylation cycle. B2 is needed to create the essential flavoprotein coenzymes for synthesis of L-methylfolate—the active form of folate—and for proper utilization of B6. Deficiency can occur after 4 months of inadequate intake.
Although generally B2 deficiency is rare, surveys in the United States have found that 10% to 27% of older adults (age ≥65) are deficient.4 Low intake of dairy products and meat and chronic, excessive alcohol intake are associated with deficiency. Marginal B2 levels are more prevalent in depressed patients, possibly because of B2’s role in the function of glutathione, an endogenous antioxidant.5 Top dietary sources of B2 are dairy products, meat and fish, eggs, mushrooms, almonds, leafy greens, and legumes.
Vitamin B6 refers to 3 distinct compounds: pyridoxine, pyridoxal, and pyridoxamine. B6 is essential to glycolysis, the methylation cycle, and recharging glutathione, an innate antioxidant in the brain. Higher levels of vitamin B6 are associated with a lower prevalence of depression in adolescents,6 and low dietary and plasma B6 increases the risk and severity of depression in geriatric patients7 and predicts depression in prospective trials.8 Deficiency is common (24% to 56%) among patients receiving hemodialysis.9 Women who take oral contraceptives are at increased risk of vitamin B6 deficiency.10 Top dietary sources are fish, beef, poultry, potatoes, legumes, and spinach.
Vitamin B9 (folate) is needed for proper one-carbon metabolism and thus requisite in synthesis of serotonin, norepinephrine, dopamine, and DNA and in phospholipid production. Low maternal folate status increases the risk of neural tube defects in newborns. Folate deficiency and insufficiency are common among patients with mood disorders and correlate with illness severity.11 In a study of 2,682 Finnish men, those in the lowest one-third of folate consumption had a 67% increased relative risk of depression.12 A meta-analysis of 11 studies of 15,315 persons found those who had low folate levels had a significant risk of depression.13 Patients without deficiency but with folate levels near the low end of the normal range also report low mood.14 Compared with controls, patients experiencing a first episode of psychosis have lower levels of folate, B12, and docosahexaenoic acid.15
Dietary folate must be converted to L-methylfolate for use in the brain. Patients with a methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism produce a less active form of the enzyme. The TT genotype is associated with major depression and bipolar disorder.16 Clinical trials have shown that several forms of folate can enhance antidepressant treatment.17 Augmentation with L-methylfolate, which bypasses the MTHFR enzyme, can be an effective strategy for treating depression in these patients.18
Leafy greens and legumes such as lentils are top dietary sources of folate; supplemental folic acid has been linked to an increased risk of cancer and overall mortality.19,20
Vitamin B12 (cobalamin). An essential cofactor in one-carbon metabolism, B12 is needed to produce monoamine neurotransmitters and maintain myelin. Deficiency is found in up to one-third of depressed patients11 and compromises antidepressant response,21 whereas higher vitamin B12 levels are associated with better treatment outcomes.22 B12 deficiency can cause depression, irritability, agitation, psychosis, and obsessive symptoms.23,24 Low B12 levels and elevated homocysteine increase the risk of cognitive decline and Alzheimer’s disease and are linked to a 5-fold increase in the rate of brain atrophy.26
B12 deficiencies may be seen in patients with gastrointestinal illness, older adults with achlorhydria, and vegans and vegetarians, in whom B12 intake can be low. Proton pump inhibitors such as omeprazole interfere with B12 absorption from food.
Psychiatric symptoms of B12 deficiency may present before hematologic findings.23 Folic acid supplementation may mask a B12 deficiency by delaying anemia but will not delay psychiatric symptoms. Ten percent of patients with an insufficiency (low normal levels of 200 to 400 pg/mL) have elevated homocysteine, which increases the risk of psychiatric disorders as well as comorbid illnesses such as cardiovascular disease. Top dietary sources include fish, mollusks (oysters, mussels, and clams), meat, and dairy products.
Vitamin C is vital for the synthesis of monoamines such as serotonin and norepinephrine. Vitamin C’s primary role in the brain is as an antioxidant. As a necessary cofactor, it keeps the copper and iron in metalloenzymes reduced, and also recycles vitamin E. Proper function of the methylation cycle depends on vitamin C, as does collagen synthesis and metabolism of xenobiotics by the liver. It is concentrated in cerebrospinal fluid.
Humans cannot manufacture vitamin C. Although the need for vitamin C (90 mg/d) is thought to be met by diet, studies have found that up to 13.7% of healthy, middle class patients in the United States are depleted.27 Older adults and patients with a poor diet due to drug or alcohol abuse, eating disorders, or affective symptoms are at risk.
Scurvy is caused by vitamin C deficiency and leads to bleeding gums and petechiae. Patients with insufficiency report irritability, loss of appetite, weight loss, and hypochondriasis. Vitamin C intake is significantly lower in older adults (age ≥60) with depression.28 Some research indicates patients with schizophrenia have decreased vitamin C levels and dysfunction of antioxidant defenses.29 Citrus, potatoes, and tomatoes are top dietary sources of vitamin C.
Fat-soluble vitamins
Vitamin A. Although vitamin A activity in the brain is poorly understood, retinol—the active form of vitamin A—is crucial for formation of opsins, which are the basis for vision. Childhood vitamin A deficiency may lead to blindness. Vitamin A also plays an important role in maintaining bone growth, reproduction, cell division, and immune system integrity.30 Animal sources such as beef liver, dairy products, and eggs provide retinol, and plant sources such as carrots, sweet potatoes, and leafy greens provide provitamin A carotenoids that humans convert into retinol.
Deficiency rarely is observed in the United States but remains a common problem for developing nations. In the United States, vitamin A deficiency is most often seen with excessive alcohol use, rigorous dietary restrictions, and gastrointestinal diseases accompanied by poor fat absorption.
Excess vitamin A ingestion may result in bone abnormalities, liver damage, birth defects, and depression. Isotretinoin—a form of vitamin A used to treat severe acne—carries an FDA “black-box” warning for psychiatric adverse effects, including aggression, depression, psychosis, and suicide.
Vitamin D is produced from cholesterol in the epidermis through exposure to sunlight, namely ultraviolet B radiation. After dermal synthesis or ingestion, vitamin D is converted through a series of steps into the active form of vitamin D, calcitriol, which also is known as 25(OH)D3.
Although vitamin D is known for its role in bone growth and mineralization,31 increasing evidence reveals vitamin D’s role in brain function and development.32 Both glial and neuronal cells possess vitamin D receptors in the hippocampus, prefrontal cortex, hypothalamus, thalamus, and substantia nigra—all regions theorized to be linked to depression pathophysiology.33 A review of the association of vitamin D deficiency and psychiatric illnesses will be published in a future issue of Current Psychiatry.
Vitamin D exists in food as either D2 or D3, from plant and animal sources, respectively. Concentrated sources include oily fish, sun-dried or “UVB-irradiated” mushrooms, and milk.
Vitamin E. There are 8 isoforms of vitamin E—4 tocopherols and 4 tocotrienols—that function as fat-soluble antioxidants and also promote innate antioxidant enzymes. Because vitamin E protects neuronal membranes from oxidation, low levels may affect the brain via increased inflammation. Alpha-tocopherol is the most common form of vitamin E in humans, but emerging evidence suggests tocotrienols mediate disease by modifying transcription factors in the brain, such as glutathione reductase, superoxide dismutase, and nuclear factor-kappaB.34 Low plasma vitamin E levels are found in depressed patients, although some data suggest this may be caused by factors other than dietary intake.35 Low vitamin status has been found in up to 70% of older adults.36 Although deficiency is rare, most of the U.S. population (93%) has inadequate dietary intake of vitamin E.1 The reasons for this discrepancy are unclear. Foods rich in vitamin E include almonds, sunflower seeds, leafy greens, and wheat germ.
Recommendations
Patients with depression, alcohol abuse, eating disorders, obsessive-compulsive disorder, or schizophrenia may neglect to care for themselves or adopt particular eating patterns. Deficiencies are more common among geriatric patients and those who are medically ill. Because dietary patterns are linked to the risk of psychiatric disorders, nutritional inquiry often identifies multiple modifiable risk factors, such as folate, vitamin B12, and vitamin D intake.37,38 Nutritional counseling offers clinicians an intervention with minimal side effect risks and the opportunity to modify a behavior that patients engage in 3 times a day.
Psychiatrists should assess patients’ dietary patterns and vitamin status, particularly older adults and those with:
- lower socioeconomic status or food insecurity
- a history of treatment resistance
- restrictive dietary patterns such as veganism
- alcohol abuse.
On initial assessment, test or obtain from other health care providers your patient’s blood levels of folate and vitamins D and B12. In some patients, assessing B2 and B6 levels may provide etiological guidance regarding onset of psychiatric symptoms or failure to respond to pharmacologic treatment. Because treating vitamin deficiencies often includes using supplements, evaluate recent reviews of specific deficiencies and consider consulting with the patient’s primary care provider.
Conduct a simple assessment of dietary patterns by asking patients about a typical breakfast, lunch, and dinner, their favorite snacks and foods, and specific dietary habits or restrictions (eg, not consuming seafood, dairy, meat, etc.). Rudimentary nutritional recommendations can be effective in changing a patient’s eating habits, particularly when provided by a physician. Encourage patients to eat nutrient-dense foods such as leafy greens, beans and legumes, seafood, whole grains, and a variety of vegetables and fruits. For more complex patients, consult with a clinical nutritionist.
Related Resources
- Institute of Medicine. Dietary Reference Intakes: Recommended intakes for individuals. SummaryDRIs/~/media/Files
/Activity%20Files/Nutrition
/DRIs/5_Summary%20Table%20Tables%201-4.pdf" target="_blank">www.iom.edu/Activities/Nutrition/
SummaryDRIs/~/media/Files
/Activity%20Files/Nutrition
/DRIs/5_Summary%20Table%20Tables%201-4.pdf. - The Farmacy: Vitamins. http://drewramseymd.com/index.php/resources/farmacy/category/vitamins.
- Office of Dietary Supplements. National Institutes of Health. Dietary supplements fact sheets. http://ods.od.nih.gov/factsheets/list-all.
- Oregon State University. Linus Pauling Institute. Micronutrient information center. http://lpi.oregonstate.edu/infocenter/vitamins.html.
Drug Brand Names
- Isotretinoin • Accutane
- L-methylfolate • Deplin
- Omeprazole • Prilosec
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Discuss this article at www.facebook.com/CurrentPsychiatry
Patients today often are overfed but undernourished. A growing body of literature links dietary choices to brain health and the risk of psychiatric illness. Vitamin deficiencies can affect psychiatric patients in several ways:
- deficiencies may play a causative role in mental illness and exacerbate symptoms
- psychiatric symptoms can result in poor nutrition
- vitamin insufficiency—defined as subclinical deficiency—may compromise patient recovery.
Additionally, genetic differences may compromise vitamin and essential nutrient pathways.
Vitamins are dietary components other than carbohydrates, fats, minerals, and proteins that are necessary for life. B vitamins are required for proper functioning of the methylation cycle, monoamine production, DNA synthesis, and maintenance of phospholipids such as myelin (Figure). Fat-soluble vitamins A, D, and E play important roles in genetic transcription, antioxidant recycling, and inflammatory regulation in the brain.

Figure: The methylation cycle
Vitamins B2, B6, B9, and B12 directly impact the functioning of the methylation cycle. Deficiencies pertain to brain function, as neurotransmitters, myelin, and active glutathione are dependent on one-carbon metabolism
Illustration: Mala Nimalasuriya with permission from DrewRamseyMD.com
To help clinicians recognize and treat vitamin deficiencies among psychiatric patients, this article reviews the role of the 6 essential water-soluble vitamins (B1, B2, B6, B9, B12, and C; Table 1,1) and 3 fat-soluble vitamins (A, D, and E; Table 2,1) in brain metabolism and psychiatric pathology. Because numerous sources address using supplements to treat vitamin deficiencies, this article emphasizes food sources, which for many patients are adequate to sustain nutrient status.
Table 1
Water-soluble vitamins: Deficiency, insufficiency, symptoms, and dietary sources
| Deficiency | Insufficiency | Symptoms | At-risk patients | Dietary sources |
|---|---|---|---|---|
| B1 (thiamine): Glycolysis, tricarboxylic acid cycle | ||||
| Rare; 7% in heart failure patients | 5% total, 12% of older women | Wernicke-Korsakoff syndrome, memory impairment, confusion, lack of coordination, paralysis | Older adults, malabsorptive conditions, heavy alcohol use. Those with diabetes are at risk because of increased clearance | Pork, fish, beans, lentils, nuts, rice, and wheat germ. Raw fish, tea, and betel nuts impair absorption |
| B2 (riboflavin): FMN, FAD cofactors in glycolysis and oxidative pathways. B6, folate, and glutathione synthesis | ||||
| 10% to 27% of older adults | <3%; 95% of adolescent girls (measured by EGRAC) | Fatigue, cracked lips, sore throat, bloodshot eyes | Older adults, low intake of animal and dairy products, heavy alcohol use | Dairy, meat and fish, eggs, mushrooms, almonds, leafy greens, and legumes |
| B6 (pyridoxal): Methylation cycle | ||||
| 11% to 24% (<5 ng/mL); 38% of heart failure patients | 14% total, 26% of adults | Dermatitis, glossitis, convulsions, migraine, chronic pain, depression | Older adults, women who use oral contraceptives, alcoholism. 33% to 49% of women age >51 have inadequate intake | Bananas, beans, potatoes, navy beans, salmon, steak, and whole grains |
| B9 (folate): Methylation cycle | ||||
| 0.5% total; up to 50% of depressed patients | 16% of adults, 19% of adolescent girls | Loss of appetite, weight loss, weakness, heart palpitations, behavioral disorders | Depression, pregnancy and lactation, alcoholism, dialysis, liver disease. Deficiency during pregnancy is linked to neural tube defects | Leafy green vegetables, fruits, dried beans, and peas |
| B12 (cobalamin): Methylation cycle (cofactor methionine synthase) | ||||
| 10% to 15% of older adults | <3% to 9% | Depression, irritability, anemia, fatigue, shortness of breath, high blood pressure | Vegetarian or vegan diet, achlorhydria, older adults. Deficiency more often due to poor absorption than low consumption | Meat, seafood, eggs, and dairy |
| C (ascorbic acid): Antioxidant | ||||
| 7.1% | 31% | Scurvy, fatigue, anemia, joint pain, petechia. Symptoms develop after 1 to 3 months of no dietary intake | Smokers, infants fed boiled or evaporated milk, limited dietary variation, patients with malabsorption, chronic illnesses | Citrus fruits, tomatoes and tomato juice, and potatoes |
| EGRAC: erythrocyte glutathione reductase activation coefficient; FAD: flavin adenine dinucleotide; FMN: flavin mononucleotide Source: Reference 1 | ||||
Table 2
Fat-soluble vitamins: Deficiency, insufficiency, symptoms, and dietary sources
| Deficiency | Insufficiency | Symptoms | At-risk patients | Dietary sources |
|---|---|---|---|---|
| A (retinol): Transcription regulation, vision | ||||
| <5% of U.S. population | 44% | Blindness, decreased immunity, corneal and retinal damage | Pregnant women, individuals with strict dietary restrictions, heavy alcohol use, chronic diarrhea, fat malabsorptive conditions | Beef liver, dairy products. Convertible beta-carotene sources: sweet potatoes, carrots, spinach, butternut squash, greens, broccoli, cantaloupe |
| D (cholecalciferol): Hormone, transcriptional regulation | ||||
| ≥50%, 90% of adults age >50 | 69% | Rickets, osteoporosis, muscle twitching | Breast-fed infants, older adults, limited sun exposure, pigmented skin, fat malabsorption, obesity. Older adults have an impaired ability to make vitamin D from the sun. SPF 15 reduces production by 99% | Fatty fish and fish liver oils, sun-dried mushrooms |
| E (tocopherols and tocotrienols): Antioxidant, PUFA protectant, gene regulation | ||||
| Rare | 93% | Anemia, neuropathy, myopathy, abnormal eye movements, weakness, retinal damage | Malabsorptive conditions, HIV, depression | Sunflower, wheat germ, and safflower oils; meats; fish; dairy; green vegetables |
| HIV: human immunodeficiency virus; PUFA: polyunsaturated fatty acids; SPF: sun protection factor Source: Reference 1 | ||||
Water-soluble vitamins
Vitamin B1 (thiamine) is essential for glucose metabolism. Pregnancy, lactation, and fever increase the need for thiamine, and tea, coffee, and shellfish can impair its absorption. Although rare, severe B1 deficiency can lead to beriberi, Wernicke’s encephalopathy (confusion, ataxia, nystagmus), and Korsakoff’s psychosis (confabulation, lack of insight, retrograde and anterograde amnesia, and apathy). Confusion and disorientation stem from the brain’s inability to oxidize glucose for energy because B1 is a critical cofactor in glycolysis and the tricarboxylic acid cycle. Deficiency leads to an increase in reactive oxygen species, proinflammatory cytokines, and blood-brain barrier dysfunction.2 Wernicke’s encephalopathy is most frequently encountered in patients with chronic alcoholism, diabetes, or eating disorders, and after bariatric surgery.3 Iatrogenic Wernicke’s encephalopathy may occur when depleted patients receive IV saline with dextrose without receiving thiamine. Top dietary sources of B1 include pork, fish, beans, lentils, nuts, rice, and wheat germ.
Vitamin B2 (riboflavin) is essential for oxidative pathways, monoamine synthesis, and the methylation cycle. B2 is needed to create the essential flavoprotein coenzymes for synthesis of L-methylfolate—the active form of folate—and for proper utilization of B6. Deficiency can occur after 4 months of inadequate intake.
Although generally B2 deficiency is rare, surveys in the United States have found that 10% to 27% of older adults (age ≥65) are deficient.4 Low intake of dairy products and meat and chronic, excessive alcohol intake are associated with deficiency. Marginal B2 levels are more prevalent in depressed patients, possibly because of B2’s role in the function of glutathione, an endogenous antioxidant.5 Top dietary sources of B2 are dairy products, meat and fish, eggs, mushrooms, almonds, leafy greens, and legumes.
Vitamin B6 refers to 3 distinct compounds: pyridoxine, pyridoxal, and pyridoxamine. B6 is essential to glycolysis, the methylation cycle, and recharging glutathione, an innate antioxidant in the brain. Higher levels of vitamin B6 are associated with a lower prevalence of depression in adolescents,6 and low dietary and plasma B6 increases the risk and severity of depression in geriatric patients7 and predicts depression in prospective trials.8 Deficiency is common (24% to 56%) among patients receiving hemodialysis.9 Women who take oral contraceptives are at increased risk of vitamin B6 deficiency.10 Top dietary sources are fish, beef, poultry, potatoes, legumes, and spinach.
Vitamin B9 (folate) is needed for proper one-carbon metabolism and thus requisite in synthesis of serotonin, norepinephrine, dopamine, and DNA and in phospholipid production. Low maternal folate status increases the risk of neural tube defects in newborns. Folate deficiency and insufficiency are common among patients with mood disorders and correlate with illness severity.11 In a study of 2,682 Finnish men, those in the lowest one-third of folate consumption had a 67% increased relative risk of depression.12 A meta-analysis of 11 studies of 15,315 persons found those who had low folate levels had a significant risk of depression.13 Patients without deficiency but with folate levels near the low end of the normal range also report low mood.14 Compared with controls, patients experiencing a first episode of psychosis have lower levels of folate, B12, and docosahexaenoic acid.15
Dietary folate must be converted to L-methylfolate for use in the brain. Patients with a methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism produce a less active form of the enzyme. The TT genotype is associated with major depression and bipolar disorder.16 Clinical trials have shown that several forms of folate can enhance antidepressant treatment.17 Augmentation with L-methylfolate, which bypasses the MTHFR enzyme, can be an effective strategy for treating depression in these patients.18
Leafy greens and legumes such as lentils are top dietary sources of folate; supplemental folic acid has been linked to an increased risk of cancer and overall mortality.19,20
Vitamin B12 (cobalamin). An essential cofactor in one-carbon metabolism, B12 is needed to produce monoamine neurotransmitters and maintain myelin. Deficiency is found in up to one-third of depressed patients11 and compromises antidepressant response,21 whereas higher vitamin B12 levels are associated with better treatment outcomes.22 B12 deficiency can cause depression, irritability, agitation, psychosis, and obsessive symptoms.23,24 Low B12 levels and elevated homocysteine increase the risk of cognitive decline and Alzheimer’s disease and are linked to a 5-fold increase in the rate of brain atrophy.26
B12 deficiencies may be seen in patients with gastrointestinal illness, older adults with achlorhydria, and vegans and vegetarians, in whom B12 intake can be low. Proton pump inhibitors such as omeprazole interfere with B12 absorption from food.
Psychiatric symptoms of B12 deficiency may present before hematologic findings.23 Folic acid supplementation may mask a B12 deficiency by delaying anemia but will not delay psychiatric symptoms. Ten percent of patients with an insufficiency (low normal levels of 200 to 400 pg/mL) have elevated homocysteine, which increases the risk of psychiatric disorders as well as comorbid illnesses such as cardiovascular disease. Top dietary sources include fish, mollusks (oysters, mussels, and clams), meat, and dairy products.
Vitamin C is vital for the synthesis of monoamines such as serotonin and norepinephrine. Vitamin C’s primary role in the brain is as an antioxidant. As a necessary cofactor, it keeps the copper and iron in metalloenzymes reduced, and also recycles vitamin E. Proper function of the methylation cycle depends on vitamin C, as does collagen synthesis and metabolism of xenobiotics by the liver. It is concentrated in cerebrospinal fluid.
Humans cannot manufacture vitamin C. Although the need for vitamin C (90 mg/d) is thought to be met by diet, studies have found that up to 13.7% of healthy, middle class patients in the United States are depleted.27 Older adults and patients with a poor diet due to drug or alcohol abuse, eating disorders, or affective symptoms are at risk.
Scurvy is caused by vitamin C deficiency and leads to bleeding gums and petechiae. Patients with insufficiency report irritability, loss of appetite, weight loss, and hypochondriasis. Vitamin C intake is significantly lower in older adults (age ≥60) with depression.28 Some research indicates patients with schizophrenia have decreased vitamin C levels and dysfunction of antioxidant defenses.29 Citrus, potatoes, and tomatoes are top dietary sources of vitamin C.
Fat-soluble vitamins
Vitamin A. Although vitamin A activity in the brain is poorly understood, retinol—the active form of vitamin A—is crucial for formation of opsins, which are the basis for vision. Childhood vitamin A deficiency may lead to blindness. Vitamin A also plays an important role in maintaining bone growth, reproduction, cell division, and immune system integrity.30 Animal sources such as beef liver, dairy products, and eggs provide retinol, and plant sources such as carrots, sweet potatoes, and leafy greens provide provitamin A carotenoids that humans convert into retinol.
Deficiency rarely is observed in the United States but remains a common problem for developing nations. In the United States, vitamin A deficiency is most often seen with excessive alcohol use, rigorous dietary restrictions, and gastrointestinal diseases accompanied by poor fat absorption.
Excess vitamin A ingestion may result in bone abnormalities, liver damage, birth defects, and depression. Isotretinoin—a form of vitamin A used to treat severe acne—carries an FDA “black-box” warning for psychiatric adverse effects, including aggression, depression, psychosis, and suicide.
Vitamin D is produced from cholesterol in the epidermis through exposure to sunlight, namely ultraviolet B radiation. After dermal synthesis or ingestion, vitamin D is converted through a series of steps into the active form of vitamin D, calcitriol, which also is known as 25(OH)D3.
Although vitamin D is known for its role in bone growth and mineralization,31 increasing evidence reveals vitamin D’s role in brain function and development.32 Both glial and neuronal cells possess vitamin D receptors in the hippocampus, prefrontal cortex, hypothalamus, thalamus, and substantia nigra—all regions theorized to be linked to depression pathophysiology.33 A review of the association of vitamin D deficiency and psychiatric illnesses will be published in a future issue of Current Psychiatry.
Vitamin D exists in food as either D2 or D3, from plant and animal sources, respectively. Concentrated sources include oily fish, sun-dried or “UVB-irradiated” mushrooms, and milk.
Vitamin E. There are 8 isoforms of vitamin E—4 tocopherols and 4 tocotrienols—that function as fat-soluble antioxidants and also promote innate antioxidant enzymes. Because vitamin E protects neuronal membranes from oxidation, low levels may affect the brain via increased inflammation. Alpha-tocopherol is the most common form of vitamin E in humans, but emerging evidence suggests tocotrienols mediate disease by modifying transcription factors in the brain, such as glutathione reductase, superoxide dismutase, and nuclear factor-kappaB.34 Low plasma vitamin E levels are found in depressed patients, although some data suggest this may be caused by factors other than dietary intake.35 Low vitamin status has been found in up to 70% of older adults.36 Although deficiency is rare, most of the U.S. population (93%) has inadequate dietary intake of vitamin E.1 The reasons for this discrepancy are unclear. Foods rich in vitamin E include almonds, sunflower seeds, leafy greens, and wheat germ.
Recommendations
Patients with depression, alcohol abuse, eating disorders, obsessive-compulsive disorder, or schizophrenia may neglect to care for themselves or adopt particular eating patterns. Deficiencies are more common among geriatric patients and those who are medically ill. Because dietary patterns are linked to the risk of psychiatric disorders, nutritional inquiry often identifies multiple modifiable risk factors, such as folate, vitamin B12, and vitamin D intake.37,38 Nutritional counseling offers clinicians an intervention with minimal side effect risks and the opportunity to modify a behavior that patients engage in 3 times a day.
Psychiatrists should assess patients’ dietary patterns and vitamin status, particularly older adults and those with:
- lower socioeconomic status or food insecurity
- a history of treatment resistance
- restrictive dietary patterns such as veganism
- alcohol abuse.
On initial assessment, test or obtain from other health care providers your patient’s blood levels of folate and vitamins D and B12. In some patients, assessing B2 and B6 levels may provide etiological guidance regarding onset of psychiatric symptoms or failure to respond to pharmacologic treatment. Because treating vitamin deficiencies often includes using supplements, evaluate recent reviews of specific deficiencies and consider consulting with the patient’s primary care provider.
Conduct a simple assessment of dietary patterns by asking patients about a typical breakfast, lunch, and dinner, their favorite snacks and foods, and specific dietary habits or restrictions (eg, not consuming seafood, dairy, meat, etc.). Rudimentary nutritional recommendations can be effective in changing a patient’s eating habits, particularly when provided by a physician. Encourage patients to eat nutrient-dense foods such as leafy greens, beans and legumes, seafood, whole grains, and a variety of vegetables and fruits. For more complex patients, consult with a clinical nutritionist.
Related Resources
- Institute of Medicine. Dietary Reference Intakes: Recommended intakes for individuals. SummaryDRIs/~/media/Files
/Activity%20Files/Nutrition
/DRIs/5_Summary%20Table%20Tables%201-4.pdf" target="_blank">www.iom.edu/Activities/Nutrition/
SummaryDRIs/~/media/Files
/Activity%20Files/Nutrition
/DRIs/5_Summary%20Table%20Tables%201-4.pdf. - The Farmacy: Vitamins. http://drewramseymd.com/index.php/resources/farmacy/category/vitamins.
- Office of Dietary Supplements. National Institutes of Health. Dietary supplements fact sheets. http://ods.od.nih.gov/factsheets/list-all.
- Oregon State University. Linus Pauling Institute. Micronutrient information center. http://lpi.oregonstate.edu/infocenter/vitamins.html.
Drug Brand Names
- Isotretinoin • Accutane
- L-methylfolate • Deplin
- Omeprazole • Prilosec
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Moshfegh A, Goldman J, Cleveland L. United States Department of Agriculture, Agricultural Research Service. What we eat in America NHANES 2001-2002: Usual nutrient intakes from food compared to dietary reference intakes. http://www.ars.usda.gov/SP2UserFiles/Place/12355000/pdf/0102/usualintaketables2001-02.pdf. Published September 2005. Accessed November 27, 2012.
2. Page GL, Laight D, Cummings MH. Thiamine deficiency in diabetes mellitus and the impact of thiamine replacement on glucose metabolism and vascular disease. Int J Clin Pract. 2011;65(6):684-690.
3. McCormick LM, Buchanan JR, Onwuameze OE, et al. Beyond alcoholism: Wernicke-Korsakoff syndrome in patients with psychiatric disorders. Cogn Behav Neurol. 2011;24(4):209-216.
4. Powers HJ. Riboflavin (vitamin B-2) and health. Am J Clin Nutr. 2003;77(6):1352-1360.
5. Naghashpour M, Amani R, Nutr R, et al. Riboflavin status and its association with serum hs-CRP levels among clinical nurses with depression. J Am Coll Nutr. 2011;30(5):340-347.
6. Murakami K, Miyake Y, Sasaki S, et al. Dietary folate, riboflavin, vitamin B-6, and vitamin B-12 and depressive symptoms in early adolescence: the Ryukyus Child Health Study. Psychosom Med. 2010;72(8):763-768.
7. Merete C, Falcon LM, Tucker KL. Vitamin B6 is associated with depressive symptomatology in Massachusetts elders. J Am Coll Nutr. 2008;27(3):421-427.
8. Skarupski KA, Tangney C, Li H, et al. Longitudinal association of vitamin B-6, folate, and vitamin B-12 with depressive symptoms among older adults over time. Am J Clin Nutr. 2010;92(2):330-335.
9. Corken M, Porter J. Is vitamin B(6) deficiency an under-recognized risk in patients receiving haemodialysis? A systematic review: 2000-2010. Nephrology (Carlton). 2011;16(7):619-625.
10. Wilson SM, Bivins BN, Russell KA, et al. Oral contraceptive use: impact on folate, vitamin B6, and vitamin B12 status. Nutr Rev. 2011;69(10):572-583.
11. Coppen A, Bolander-Gouaille C. Treatment of depression: time to consider folic acid and vitamin B12. J Psychopharmacol. 2005;19(1):59-65.
12. Tolmunen T, Voutilainen S, Hintikka J, et al. Dietary folate and depressive symptoms are associated in middle-aged Finnish men. J Nutr. 2003;133(10):3233-3236.
13. Gilbody S, Lightfoot T, Sheldon T. Is low folate a risk factor for depression? A meta-analysis and exploration of heterogeneity. J Epidemiol Community Health. 2007;61(7):631-637.
14. Rösche J, Uhlmann C, Fröscher W. Low serum folate levels as a risk factor for depressive mood in patients with chronic epilepsy. J Neuropsychiatry Clin Neurosci. 2003;15(1):64-66.
15. Kale A, Naphade N, Sapkale S, et al. Reduced folic acid, vitamin B12 and docosahexaenoic acid and increased homocysteine and cortisol in never-medicated schizophrenia patients: implications for altered one-carbon metabolism. Psychiatry Res. 2010;175(1-2):47-53.
16. Gilbody S, Lewis S, Lightfoot T. Methylenetetrahydrofolate reductase (MTHFR) genetic polymorphisms and psychiatric disorders: a HuGE review. Am J Epidemiol. 2007;165(1):1-13.
17. Di Palma C, Urani R, Agricola R, et al. Is methylfolate effective in relieving major depression in chronic alcoholics? A hypothesis of treatment. Curr Ther Res Clin Exp. 1994;55(5):559-568.
18. Papakostas GI, Shelton RC, Zajecka JM, et al. l-Methylfolate as adjunctive therapy for ssri-resistant major depression: results of two randomized, double-blind, parallel-sequential trials. Am J Psychiatry. 2012;169(12):1267-1274.
19. Baggott JE, Oster RA, Tamura T. Meta-analysis of cancer risk in folic acid supplementation trials. Cancer Epidemiol. 2012;36(1):78-81.
20. Figueiredo JC, Grau MV, Haile RW, et al. Folic acid and risk of prostate cancer: results from a randomized clinical trial. J Natl Cancer Inst. 2009;101(6):432-435.
21. Kate N, Grover S, Agarwal M. Does B12 deficiency lead to lack of treatment response to conventional antidepressants? Psychiatry (Edgmont). 2010;7(11):42-44.
22. Hintikka J, Tolmunen T, Tanskanen A, et al. High vitamin B12 level and good treatment outcome may be associated in major depressive disorder. BMC Psychiatry. 2003;3:17.-
23. Lindenbaum J, Healton EB, Savage DG, et al. Neuropsychiatric disorders caused by cobalamin deficiency in the absence of anemia or macrocytosis. N Engl J Med. 1988;318(26):1720-1728.
24. Bar-Shai M, Gott D, Marmor S. Acute psychotic depression as a sole manifestation of vitamin B12 deficiency. Psychosomatics. 2011;52(4):384-386.
25. Sharma V, Biswas D. Cobalamin deficiency presenting as obsessive compulsive disorder: case report. Gen Hosp Psychiatry. 2012;34(5):578.e7-e8.
26. Vogiatzoglou A, Refsum H, Johnston C, et al. Vitamin B12 status and rate of brain volume loss in community-dwelling elderly. Neurology. 2008;71(11):826-832.
27. Smith A, Di Primio G, Humphrey-Murto S. Scurvy in the developed world. CMAJ. 2011;183(11):E752-E725.
28. Payne ME, Steck SE, George RR, et al. Fruit, vegetable, and antioxidant intakes are lower in older adults with depression. J Acad Nutr Diet. 2012;112(12):2022-2027.
29. Dadheech G, Mishra S, Gautam S, et al. Oxidative stress, α-tocopherol, ascorbic acid and reduced glutathione status in schizophrenics. Indian J Clin Biochem. 2006;21(2):34-38.
30. Hinds TS, West WL, Knight EM. Carotenoids and retinoids: a review of research clinical, and public health applications. J Clin Pharmacol. 1997;37(7):551-558.
31. Thacher TD, Clarke BL. Vitamin D insufficiency. Mayo Clin Proc. 2011;86(1):50-60.
32. Berk M, Sanders KM, Pasco JA, et al. Vitamin D deficiency may play a role in depression. Med Hypotheses. 2007;69(6):1316-1319.
33. Eyles DW, Smith S, Kinobe R, et al. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J Chem Neuroanat. 2005;29(1):21-30.
34. Sen CK, Khanna S, Roy S. Tocotrienol: the natural vitamin E to defend the nervous system? Ann N Y Acad Sci. 2004;1031:127-142.
35. Owen AJ, Batterham MJ, Probst YC, et al. Low plasma vitamin E levels in major depression: diet or disease? Eur J Clin Nutr. 2005;59(2):304-306.
36. Panemangalore M, Lee CJ. Evaluation of the indices of retinol and alpha-tocopherol status in free-living elderly. J Gerontol. 1992;47(3):B98-B104.
37. Sánchez-Villegas A, Delgado-Rodríguez M, Alonso A, et al. Association of the Mediterranean dietary pattern with the incidence of depression: the Seguimiento Universidad de Navarra/University of Navarra follow-up (SUN) cohort. Arch Gen Psychiatry. 2009;66(10):1090-1098.
38. Jacka FN, Pasco JA, Mykletun A, et al. Association of Western and traditional diets with depression and anxiety in women. Am J Psychiatry. 2010;167(3):305-311.
1. Moshfegh A, Goldman J, Cleveland L. United States Department of Agriculture, Agricultural Research Service. What we eat in America NHANES 2001-2002: Usual nutrient intakes from food compared to dietary reference intakes. http://www.ars.usda.gov/SP2UserFiles/Place/12355000/pdf/0102/usualintaketables2001-02.pdf. Published September 2005. Accessed November 27, 2012.
2. Page GL, Laight D, Cummings MH. Thiamine deficiency in diabetes mellitus and the impact of thiamine replacement on glucose metabolism and vascular disease. Int J Clin Pract. 2011;65(6):684-690.
3. McCormick LM, Buchanan JR, Onwuameze OE, et al. Beyond alcoholism: Wernicke-Korsakoff syndrome in patients with psychiatric disorders. Cogn Behav Neurol. 2011;24(4):209-216.
4. Powers HJ. Riboflavin (vitamin B-2) and health. Am J Clin Nutr. 2003;77(6):1352-1360.
5. Naghashpour M, Amani R, Nutr R, et al. Riboflavin status and its association with serum hs-CRP levels among clinical nurses with depression. J Am Coll Nutr. 2011;30(5):340-347.
6. Murakami K, Miyake Y, Sasaki S, et al. Dietary folate, riboflavin, vitamin B-6, and vitamin B-12 and depressive symptoms in early adolescence: the Ryukyus Child Health Study. Psychosom Med. 2010;72(8):763-768.
7. Merete C, Falcon LM, Tucker KL. Vitamin B6 is associated with depressive symptomatology in Massachusetts elders. J Am Coll Nutr. 2008;27(3):421-427.
8. Skarupski KA, Tangney C, Li H, et al. Longitudinal association of vitamin B-6, folate, and vitamin B-12 with depressive symptoms among older adults over time. Am J Clin Nutr. 2010;92(2):330-335.
9. Corken M, Porter J. Is vitamin B(6) deficiency an under-recognized risk in patients receiving haemodialysis? A systematic review: 2000-2010. Nephrology (Carlton). 2011;16(7):619-625.
10. Wilson SM, Bivins BN, Russell KA, et al. Oral contraceptive use: impact on folate, vitamin B6, and vitamin B12 status. Nutr Rev. 2011;69(10):572-583.
11. Coppen A, Bolander-Gouaille C. Treatment of depression: time to consider folic acid and vitamin B12. J Psychopharmacol. 2005;19(1):59-65.
12. Tolmunen T, Voutilainen S, Hintikka J, et al. Dietary folate and depressive symptoms are associated in middle-aged Finnish men. J Nutr. 2003;133(10):3233-3236.
13. Gilbody S, Lightfoot T, Sheldon T. Is low folate a risk factor for depression? A meta-analysis and exploration of heterogeneity. J Epidemiol Community Health. 2007;61(7):631-637.
14. Rösche J, Uhlmann C, Fröscher W. Low serum folate levels as a risk factor for depressive mood in patients with chronic epilepsy. J Neuropsychiatry Clin Neurosci. 2003;15(1):64-66.
15. Kale A, Naphade N, Sapkale S, et al. Reduced folic acid, vitamin B12 and docosahexaenoic acid and increased homocysteine and cortisol in never-medicated schizophrenia patients: implications for altered one-carbon metabolism. Psychiatry Res. 2010;175(1-2):47-53.
16. Gilbody S, Lewis S, Lightfoot T. Methylenetetrahydrofolate reductase (MTHFR) genetic polymorphisms and psychiatric disorders: a HuGE review. Am J Epidemiol. 2007;165(1):1-13.
17. Di Palma C, Urani R, Agricola R, et al. Is methylfolate effective in relieving major depression in chronic alcoholics? A hypothesis of treatment. Curr Ther Res Clin Exp. 1994;55(5):559-568.
18. Papakostas GI, Shelton RC, Zajecka JM, et al. l-Methylfolate as adjunctive therapy for ssri-resistant major depression: results of two randomized, double-blind, parallel-sequential trials. Am J Psychiatry. 2012;169(12):1267-1274.
19. Baggott JE, Oster RA, Tamura T. Meta-analysis of cancer risk in folic acid supplementation trials. Cancer Epidemiol. 2012;36(1):78-81.
20. Figueiredo JC, Grau MV, Haile RW, et al. Folic acid and risk of prostate cancer: results from a randomized clinical trial. J Natl Cancer Inst. 2009;101(6):432-435.
21. Kate N, Grover S, Agarwal M. Does B12 deficiency lead to lack of treatment response to conventional antidepressants? Psychiatry (Edgmont). 2010;7(11):42-44.
22. Hintikka J, Tolmunen T, Tanskanen A, et al. High vitamin B12 level and good treatment outcome may be associated in major depressive disorder. BMC Psychiatry. 2003;3:17.-
23. Lindenbaum J, Healton EB, Savage DG, et al. Neuropsychiatric disorders caused by cobalamin deficiency in the absence of anemia or macrocytosis. N Engl J Med. 1988;318(26):1720-1728.
24. Bar-Shai M, Gott D, Marmor S. Acute psychotic depression as a sole manifestation of vitamin B12 deficiency. Psychosomatics. 2011;52(4):384-386.
25. Sharma V, Biswas D. Cobalamin deficiency presenting as obsessive compulsive disorder: case report. Gen Hosp Psychiatry. 2012;34(5):578.e7-e8.
26. Vogiatzoglou A, Refsum H, Johnston C, et al. Vitamin B12 status and rate of brain volume loss in community-dwelling elderly. Neurology. 2008;71(11):826-832.
27. Smith A, Di Primio G, Humphrey-Murto S. Scurvy in the developed world. CMAJ. 2011;183(11):E752-E725.
28. Payne ME, Steck SE, George RR, et al. Fruit, vegetable, and antioxidant intakes are lower in older adults with depression. J Acad Nutr Diet. 2012;112(12):2022-2027.
29. Dadheech G, Mishra S, Gautam S, et al. Oxidative stress, α-tocopherol, ascorbic acid and reduced glutathione status in schizophrenics. Indian J Clin Biochem. 2006;21(2):34-38.
30. Hinds TS, West WL, Knight EM. Carotenoids and retinoids: a review of research clinical, and public health applications. J Clin Pharmacol. 1997;37(7):551-558.
31. Thacher TD, Clarke BL. Vitamin D insufficiency. Mayo Clin Proc. 2011;86(1):50-60.
32. Berk M, Sanders KM, Pasco JA, et al. Vitamin D deficiency may play a role in depression. Med Hypotheses. 2007;69(6):1316-1319.
33. Eyles DW, Smith S, Kinobe R, et al. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J Chem Neuroanat. 2005;29(1):21-30.
34. Sen CK, Khanna S, Roy S. Tocotrienol: the natural vitamin E to defend the nervous system? Ann N Y Acad Sci. 2004;1031:127-142.
35. Owen AJ, Batterham MJ, Probst YC, et al. Low plasma vitamin E levels in major depression: diet or disease? Eur J Clin Nutr. 2005;59(2):304-306.
36. Panemangalore M, Lee CJ. Evaluation of the indices of retinol and alpha-tocopherol status in free-living elderly. J Gerontol. 1992;47(3):B98-B104.
37. Sánchez-Villegas A, Delgado-Rodríguez M, Alonso A, et al. Association of the Mediterranean dietary pattern with the incidence of depression: the Seguimiento Universidad de Navarra/University of Navarra follow-up (SUN) cohort. Arch Gen Psychiatry. 2009;66(10):1090-1098.
38. Jacka FN, Pasco JA, Mykletun A, et al. Association of Western and traditional diets with depression and anxiety in women. Am J Psychiatry. 2010;167(3):305-311.
Stiff person syndrome: What psychiatrists need to know
Stiff person syndrome (SPS) is a rare autoimmune condition characterized by stiffness and rigidity in the lower limb muscles. Because SPS often is misdiagnosed as a psychiatric illness and psychiatric comorbidities are common in patients with this disorder,1 awareness and recognition of this unique condition is essential.
An insidious presentation
Patients with SPS present with:2
- axial muscle stiffness slowly progressing to proximal muscles
- unremarkable motor, sensory, and cranial nerve examinations with normal intellectual functioning
- normal muscle strength, although electromyography shows continuous motor activity
- spasms evoked by sudden movements, jarring noise, and emotional distress
- slow and cautious gait to avoid triggering spasms and falls.
Symptoms start slowly and insidiously. Axial muscle stiffness can result in spinal deformity. Involvement is asymmetrical, with a predilection for proximal lower limb and lumbar paraspinal muscles. Affected muscles reveal tight, hard, board-like rigidity. In later stages of SPS, mild atrophy and muscle weakness are likely.
Frequent misdiagnosis
Because facial muscle spasticity is prominent, SPS patients may be misdiagnosed with Parkinson’s disease, primary lateral sclerosis, or multiple sclerosis. Spasms affecting respiratory and thoracic paraspinal muscles (status spasticus) may be misdiagnosed as an anxiety-related condition. These spasms can be life-threatening and require IV diazepam and supportive measures.
More than 60% of SPS patients have a comorbid psychiatric disorder.3 Anxiety disorders—generalized anxiety disorder, agoraphobia, and panic disorder—major depression, and alcohol abuse are the most frequent psychiatric comorbidities seen in SPS patients.3
SPS patients who panic when in public may be misdiagnosed with agoraphobia.3 Emotional stimuli may cause muscle spasms leading to falls. Treating muscle spasticity with γ-aminobutyric acid (GABA) agonists and narcotics can lead to drug abuse and dependence. Muscle spasticity can fluctuate from hour to hour, abate with sleep, and get worse with emotional distress. These findings are why approximately 70% of SPS patients are initially misdiagnosed; conversion disorder is a frequent misdiagnosis.4 Mood disorder in SPS patients may be resistant to antidepressants until these patients are treated with immunotherapy.4
Treating SPS patients
Although early intervention can reduce long-term disability, approximately 50% of SPS patients eventually have to use a wheelchair as a result of pain and immobility.5
Antibodies to glutamic acid decarboxylase, which is the rate-limiting enzyme for GABA synthesis, are present in 85% of SPS patients.5 Therefore, treatment usually includes GABA-enhancing drugs, including sedative anxiolytics (clonazepam and diazepam), antiepileptics (gabapentin, levetiracetam, tiagabine, and vigabatrin), antispasticity drugs (baclofen, dantrolene, and tizanidine), and immunotherapy (corticosteroids, IV immunoglobulins, and rituximab).5 Antidepressants, biofeedback, and relaxation training also can offer relief. Psychotherapy and substance dependency interventions may be needed.
To achieve optimum outcomes in SPS patients, a close collaborative relationship among all treating clinicians—including primary care physicians, neurologists, anesthesiologists, and psychiatrists—is necessary.
Disclosure
Dr. Jain reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Tinsley JA, Barth EM, Black JL, et al. Psychiatric consultations in stiff-man syndrome. J Clin Psychiatry. 1997;58(10):444-449.
2. Egwuonwu S, Chedebeau F. Stiff-person syndrome: a case report and review of the literature. J Natl Med Assoc. 2010;102(12):1261-1263.
3. Black JL, Barth EM, Williams DE, et al. Stiff-man syndrome. Results of interviews and psychologic testing. Psychosomatics. 1998;39(1):38-44.
4. Culav-Sumić J, Bosnjak I, Pastar Z, et al. Anxious depression and the stiff-person plus syndrome. Cogn Behav Neurol. 2008;21(4):242-245.
5. Hadavi S, Noyce AJ, Leslie RD, et al. Stiff person syndrome. Pract Neurol. 2011;11(5):272-282.
Stiff person syndrome (SPS) is a rare autoimmune condition characterized by stiffness and rigidity in the lower limb muscles. Because SPS often is misdiagnosed as a psychiatric illness and psychiatric comorbidities are common in patients with this disorder,1 awareness and recognition of this unique condition is essential.
An insidious presentation
Patients with SPS present with:2
- axial muscle stiffness slowly progressing to proximal muscles
- unremarkable motor, sensory, and cranial nerve examinations with normal intellectual functioning
- normal muscle strength, although electromyography shows continuous motor activity
- spasms evoked by sudden movements, jarring noise, and emotional distress
- slow and cautious gait to avoid triggering spasms and falls.
Symptoms start slowly and insidiously. Axial muscle stiffness can result in spinal deformity. Involvement is asymmetrical, with a predilection for proximal lower limb and lumbar paraspinal muscles. Affected muscles reveal tight, hard, board-like rigidity. In later stages of SPS, mild atrophy and muscle weakness are likely.
Frequent misdiagnosis
Because facial muscle spasticity is prominent, SPS patients may be misdiagnosed with Parkinson’s disease, primary lateral sclerosis, or multiple sclerosis. Spasms affecting respiratory and thoracic paraspinal muscles (status spasticus) may be misdiagnosed as an anxiety-related condition. These spasms can be life-threatening and require IV diazepam and supportive measures.
More than 60% of SPS patients have a comorbid psychiatric disorder.3 Anxiety disorders—generalized anxiety disorder, agoraphobia, and panic disorder—major depression, and alcohol abuse are the most frequent psychiatric comorbidities seen in SPS patients.3
SPS patients who panic when in public may be misdiagnosed with agoraphobia.3 Emotional stimuli may cause muscle spasms leading to falls. Treating muscle spasticity with γ-aminobutyric acid (GABA) agonists and narcotics can lead to drug abuse and dependence. Muscle spasticity can fluctuate from hour to hour, abate with sleep, and get worse with emotional distress. These findings are why approximately 70% of SPS patients are initially misdiagnosed; conversion disorder is a frequent misdiagnosis.4 Mood disorder in SPS patients may be resistant to antidepressants until these patients are treated with immunotherapy.4
Treating SPS patients
Although early intervention can reduce long-term disability, approximately 50% of SPS patients eventually have to use a wheelchair as a result of pain and immobility.5
Antibodies to glutamic acid decarboxylase, which is the rate-limiting enzyme for GABA synthesis, are present in 85% of SPS patients.5 Therefore, treatment usually includes GABA-enhancing drugs, including sedative anxiolytics (clonazepam and diazepam), antiepileptics (gabapentin, levetiracetam, tiagabine, and vigabatrin), antispasticity drugs (baclofen, dantrolene, and tizanidine), and immunotherapy (corticosteroids, IV immunoglobulins, and rituximab).5 Antidepressants, biofeedback, and relaxation training also can offer relief. Psychotherapy and substance dependency interventions may be needed.
To achieve optimum outcomes in SPS patients, a close collaborative relationship among all treating clinicians—including primary care physicians, neurologists, anesthesiologists, and psychiatrists—is necessary.
Disclosure
Dr. Jain reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Stiff person syndrome (SPS) is a rare autoimmune condition characterized by stiffness and rigidity in the lower limb muscles. Because SPS often is misdiagnosed as a psychiatric illness and psychiatric comorbidities are common in patients with this disorder,1 awareness and recognition of this unique condition is essential.
An insidious presentation
Patients with SPS present with:2
- axial muscle stiffness slowly progressing to proximal muscles
- unremarkable motor, sensory, and cranial nerve examinations with normal intellectual functioning
- normal muscle strength, although electromyography shows continuous motor activity
- spasms evoked by sudden movements, jarring noise, and emotional distress
- slow and cautious gait to avoid triggering spasms and falls.
Symptoms start slowly and insidiously. Axial muscle stiffness can result in spinal deformity. Involvement is asymmetrical, with a predilection for proximal lower limb and lumbar paraspinal muscles. Affected muscles reveal tight, hard, board-like rigidity. In later stages of SPS, mild atrophy and muscle weakness are likely.
Frequent misdiagnosis
Because facial muscle spasticity is prominent, SPS patients may be misdiagnosed with Parkinson’s disease, primary lateral sclerosis, or multiple sclerosis. Spasms affecting respiratory and thoracic paraspinal muscles (status spasticus) may be misdiagnosed as an anxiety-related condition. These spasms can be life-threatening and require IV diazepam and supportive measures.
More than 60% of SPS patients have a comorbid psychiatric disorder.3 Anxiety disorders—generalized anxiety disorder, agoraphobia, and panic disorder—major depression, and alcohol abuse are the most frequent psychiatric comorbidities seen in SPS patients.3
SPS patients who panic when in public may be misdiagnosed with agoraphobia.3 Emotional stimuli may cause muscle spasms leading to falls. Treating muscle spasticity with γ-aminobutyric acid (GABA) agonists and narcotics can lead to drug abuse and dependence. Muscle spasticity can fluctuate from hour to hour, abate with sleep, and get worse with emotional distress. These findings are why approximately 70% of SPS patients are initially misdiagnosed; conversion disorder is a frequent misdiagnosis.4 Mood disorder in SPS patients may be resistant to antidepressants until these patients are treated with immunotherapy.4
Treating SPS patients
Although early intervention can reduce long-term disability, approximately 50% of SPS patients eventually have to use a wheelchair as a result of pain and immobility.5
Antibodies to glutamic acid decarboxylase, which is the rate-limiting enzyme for GABA synthesis, are present in 85% of SPS patients.5 Therefore, treatment usually includes GABA-enhancing drugs, including sedative anxiolytics (clonazepam and diazepam), antiepileptics (gabapentin, levetiracetam, tiagabine, and vigabatrin), antispasticity drugs (baclofen, dantrolene, and tizanidine), and immunotherapy (corticosteroids, IV immunoglobulins, and rituximab).5 Antidepressants, biofeedback, and relaxation training also can offer relief. Psychotherapy and substance dependency interventions may be needed.
To achieve optimum outcomes in SPS patients, a close collaborative relationship among all treating clinicians—including primary care physicians, neurologists, anesthesiologists, and psychiatrists—is necessary.
Disclosure
Dr. Jain reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Tinsley JA, Barth EM, Black JL, et al. Psychiatric consultations in stiff-man syndrome. J Clin Psychiatry. 1997;58(10):444-449.
2. Egwuonwu S, Chedebeau F. Stiff-person syndrome: a case report and review of the literature. J Natl Med Assoc. 2010;102(12):1261-1263.
3. Black JL, Barth EM, Williams DE, et al. Stiff-man syndrome. Results of interviews and psychologic testing. Psychosomatics. 1998;39(1):38-44.
4. Culav-Sumić J, Bosnjak I, Pastar Z, et al. Anxious depression and the stiff-person plus syndrome. Cogn Behav Neurol. 2008;21(4):242-245.
5. Hadavi S, Noyce AJ, Leslie RD, et al. Stiff person syndrome. Pract Neurol. 2011;11(5):272-282.
1. Tinsley JA, Barth EM, Black JL, et al. Psychiatric consultations in stiff-man syndrome. J Clin Psychiatry. 1997;58(10):444-449.
2. Egwuonwu S, Chedebeau F. Stiff-person syndrome: a case report and review of the literature. J Natl Med Assoc. 2010;102(12):1261-1263.
3. Black JL, Barth EM, Williams DE, et al. Stiff-man syndrome. Results of interviews and psychologic testing. Psychosomatics. 1998;39(1):38-44.
4. Culav-Sumić J, Bosnjak I, Pastar Z, et al. Anxious depression and the stiff-person plus syndrome. Cogn Behav Neurol. 2008;21(4):242-245.
5. Hadavi S, Noyce AJ, Leslie RD, et al. Stiff person syndrome. Pract Neurol. 2011;11(5):272-282.
Treating thyroid disorders and depression: 3 case studies
Discuss this article at www.facebook.com/CurrentPsychiatry
Many endocrine disorders can manifest as depression, including relatively rare disorders such as Cushing’s syndrome (hypercortisolism) or Conn’s syndrome (primary hyperaldosteronism) as well as common ones such as diabetes mellitus. Most clinicians do not routinely screen for adrenal disorders when evaluating depressed patients because the yield is low, but do screen for thyroid disease because these disorders often mimic depression. The following 3 cases from my practice illustrate some nuances of screening and treating depressed patients with suspected thyroid abnormalities.
CASE 1: Feeling ‘like an 80-year-old’
Ms. A, age 25, has a gastrointestinal stromal tumor (GIST) and states that she feels “like an 80-year-old woman.” She is sore all over with facial swelling, abdominal cramping, and fatigue. This feeling has worsened since she started chemotherapy with sunitinib for the GIST. Her Patient Health Questionnaire-9 (PHQ-9) score is 14 out of 27, indicating moderate depression. As part of a workup for her depression, what general laboratory tests would be most helpful?
Because Ms. A is of menstruating age, check hemoglobin/hematocrit levels to evaluate for anemia. Monitoring electrolytes would allow you to assess for hypernatremia/hyponatremia, hyperkalemia/hypokalemia, and impaired renal function, all of which could cause depressive symptoms. Depending on Ms. A’s habitus or risk of metabolic syndrome, a fasting blood glucose or hemoglobin A1C test to screen for diabetes mellitus might be valuable because depression may be associated with diabetes.1 A1C is a preferred primary screening test for diabetes (≥6.5% constitutes a positive screen) based on revised clinical practice recommendations of the American Diabetes Association. A1C is available as an office-based test that requires just a drop of blood from a finger prick and does not require a fasting blood sample or a full laboratory analysis.
A popular test for a workup of depression is serum 25-hydroxyvitamin D [25(OH)D] (vitamin D), particularly for patients who live in areas with limited exposure to ultraviolet B radiation from sunlight.2 In a study of older adults, vitamin D levels were 14% lower in patients with minor depression and 14% lower in patients with major depressive disorder compared with controls. This study suggests that depression severity is associated with decreased serum vitamin D levels,3 but the association between depression and vitamin D insufficiency and deficiency is unknown. Checking sex hormones also may be helpful depending on the patient’s symptoms, because testosterone deficiency in men and dehydroepiandrosterone deficiency in women can have a direct impact on a patient’s libido and overall sense of well-being. If repleted, improved levels of sex hormones can lead to a dramatic improvement in mood as well.
Because more than one-half of the estimated 27 million Americans with hyperthyroidism or hypothyroidism are undiagnosed, the American Thyroid Association recommends universal screening for thyroid dysfunction after age 35, with a recheck every 5 years.4 However, checking serum thyroid-stimulating hormone (TSH) levels this often may not be cost-effective. Typically, I do not follow this recommendation when assessing or treating asymptomatic individuals, but Ms. A has symptoms of hypothyroidism (Table 1) and is taking a medication—sunitinib—thought to be associated with hypothyroidism.5 Her serum TSH was very high (110 mIU/L; range 0.28 to 5.00) and her serum free T4 (FT4) was low (0.5 ng/dL; range 0.7 to 1.8). These values were consistent with overt hypothyroidism, defined as low FT4 and elevated TSH levels. This is in contrast to subclinical hypothyroidism (SH), which is defined as having an elevated serum TSH with normal thyroid hormone (T3 and T4) levels. SH presents in 5% of young patients (age <45) and increasingly is being diagnoses in older patients (age >55), who are most likely to suffer adverse effects in mood or cognition.6
Table 1
Hypothyroidism symptoms
| Psychiatric overlap |
| Fatigue |
| Hypersomnolence |
| Cognitive impairment (forgetfulness) |
| Difficulty concentrating or learning |
| Weight gain or fluid retention |
| Somatic signs and symptoms |
| Dry, itchy skin |
| Brittle hair and nails |
| Constipation |
| Myalgias |
| Heavy and/or irregular menstrual cycle |
| Increased rate of miscarriage |
| Sensitivity to cold |
CASE 1 CONTINUED: A classic case
Ms. A is started on a full levothyroxine replacement dose of 1.6 μg/kg/d. For hypothyroid patients who do not have cardiac symptoms, weight-based replacement is thought to be safe and more convenient than starting with a low dose and titrating up.7 Ms. A responds quickly. At 6-week follow-up—the recommended time interval for repeat thyroid lab testing after initiating thyroid replacement—her depressive symptoms are markedly improved and her PHQ-9 score is 6, indicating mild depression.
CASE 2: Chronic pain, low mood, and fatigue
Ms. B, age 62, has fibromyalgia and chronic back pain. She takes cyclobenzaprine, 5 mg 2 to 3 times daily, and oxycodone, 40 mg/d, and describes mild depressive symptoms when she presents for routine follow-up. Most of her complaints are related to chronic pain, but she has a history of low mood and fatigue. She says she was prescribed levothyroxine, but is unable to remember if she stopped taking it because of financial constraints or laboratory/clinical improvement. Her neurologist recently checked her serum TSH, which was elevated at 8.1 mIU/L. Is it best to restart thyroid replacement or wait 6 weeks and recheck her thyroid panel?
Mild SH typically is defined as TSH between 4.5 and 10 mIU/L. In contrast, TSH between 10 and 20 mIU/L is considered severe SH. Because Ms. B did not have prominent new symptoms, I felt it was reasonable to wait the recommended 6 weeks before rechecking her thyroid function. At follow-up, Ms. B’s TSH was 4.64 mIU/L and her FT4 was normal: 0.7 ng/dL. Thyroid replacement was not indicated because she did not have obvious symptoms and treating SH does not impact overall mood and cognition until TSH is ≥10 mIU/L.8,9
CASE 2 CONTINUED: Prominent symptoms emerge
Ms. B returns several months later. Another clinician prescribed duloxetine, titrated from 30 mg to 60 mg, for worsening fibromyalgia. Her depressive symptoms are more prominent at this visit, and her PHQ-9 score has risen from 7 to 14, indicating moderate depression. She says previously she failed or poorly tolerated several antidepressants—fluoxetine, sertraline, and citalopram—but was hoping for a pharmacologic adjustment. Most evidence-based augmentation algorithms for treating major depression start with adding a second “traditional” antidepressant such as bupropion, then move to lithium, second-generation antipsychotics, or lamotrigine.10 But what about thyroid hormone augmentation?
Thyroid hormone often is on the lower rungs of depression treatment algorithms despite Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial data. The data suggest triiodothyronine’s (T3) lower side effect burden and ease of use may offer an advantage over lithium augmentation for depressed patients who have failed several medication trials.11 Liothyronine sodium (triiodothyronine) is a relatively benign medication with potential for augmentation when started at 25 to 50 mcg/d concurrently with antidepressants such as sertraline.12 Unfortunately, most augmentation trials with T3 have been short-term—generally 4 to 8 weeks. In my practice, T3 has limited application; I use it mainly for patients with treatment-resistant depression who have failed several other treatments.
Lithium, the comparison medication to thyroid hormone in the third augmentation arm of the STAR*D trial, requires an annual check of thyroid function (TSH testing) to properly monitor for potential lithium-related hypothyroidism or thyroiditis. Hypothyroidism, for which thyroid replacement is required, with lithium therapy is common, affecting 8% to 27% of patients.13 Patients who rapidly gain weight at the beginning of lithium treatment seem to have a higher risk of developing hypothyroidism.13 However, the risk of developing lithium-induced hypothyroidism is tied to the length of treatment; the longer a patient has been treated with lithium, the greater the risk of developing lithium-induced hypothyroidism.
CASE 3: Unable to slow down
Mr. C, age 45, has a 20-year history of major depression controlled reasonably well with paroxetine, 40 mg. He presents with escalating anxiety, depression, and irritability. His wife is concerned about his overwhelming thoughts of death, especially because Mr. C’s father committed suicide 30 years ago under similar circumstances. Mr. C has been tremulous for the past month and has not been sleeping well. He feels like he is “in constant motion” and unable to slow down. He screens in the “highly likely” range for bipolar disorder on the Bipolar Spectrum Diagnostic Scale14 and is started on divalproex ER, 500 mg/d.
His thyroid function tests returns with a suppressed TSH of 0.03 mIU/L and an elevated FT4 of 3.26 ng/dL. Divalproex is discontinued and he is started on the beta blocker atenolol, 25 mg/d, to target his anxiety, tachycardia, and akathisia. TSH receptor antibody testing was positive, which, along with an abnormal radioactive iodine uptake scan, confirmed a diagnosis of Graves’ disease. He receives methimazole, 20 mg/d, as a temporizing measure. An endocrinologist completes a radioactive iodine (I-131) ablation procedure on Mr. C, which resolves his mood and anxiety symptoms.
Although hypothyroidism commonly is associated with depressive symptoms, hyperthyroidism also may present as depression. Most cases of overt hyperthyroidism are directly referred to an endocrinologist because when treating disorders such as Graves’ disease—the most common cause of hyperthyroidism, especially among women age 20 to 40—many nuclear medicine teams require the expert guidance of an endocrinologist before considering radioiodine ablation. Hyperthyroidism often is accompanied by psychiatric and somatic symptoms of an “overactive” nature (Table 2). However, older patients (age >65) with hyperthyroidism may develop apathetic hyperthyroidism, a subset that comprises approximately 10% to 15% of all hyperthyroidism cases in older adults.15 Rather than becoming nervous, jittery, and restless, patients with apathetic hyperthyroidism are depressed, lethargic, and weak, and may develop proximal myopathy or cardiomyopathy. It is essential to differentiate apathetic hyperthyroidism from typical hyperthyroidism because accurately diagnosing and treating apathetic hyperthyroidism will improve outcomes.15
Table 2
Hyperthyroidism symptoms
| Psychiatric overlap |
| Decrease or increase in appetite |
| Insomnia |
| Fatigue |
| Mood instability |
| Irritability |
| Anxiety, nervousness |
| Somatic signs and symptoms |
| Frequent bowel movement, eg, diarrhea |
| Heart palpitations |
| Heat intolerance |
| Increased sweating |
| Light or missed menstrual periods, fertility problems |
| Muscle weakness |
| Shortness of breath |
| Sudden paralysis |
| Tremor, shakiness, dizziness |
| Vision changes |
| Weight loss or gain |
| Thinning of hair |
| Itching and hives |
| Possible increase in blood sugar |
Using beta blockers to treat hyperthyroidism can help control tachycardia or palpitations, tremulousness, and anxiety that often are inherent in hyperthyroidism. But can beta blockers induce depressive symptoms? A 1-year prospective Dutch study of patients who had survived a myocardial infarction did not find evidence that beta blockers induced depressive symptoms.16 However, the long-term and high-dosage effects of beta blockers still are in question.16 In Mr. C’s case, beta blockers had only positive effects on his symptoms and did not exacerbate his depressive symptoms.
Related Resources
- National Women’s Health Resource Center, Inc. Thyroid disorders. www.healthywomen.org/condition/thyroid-disorders.
- American Thyroid Association. www.thyroid.org.
- American Association of Clinical Endocrinologists. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the evaluation and treatment of hyperthyroidism and hypothyroidism. www.aace.com/files/hypo-hyper.pdf.
Drug Brand Names
- Atenolol • Tenormin
- Bupropion • Wellbutrin, Zyban
- Citalopram • Celexa
- Cyclobenzaprine • Flexeril
- Divalproex ER • Depakote ER
- Duloxetine • Cymbalta
- Fluoxetine • Prozac
- Lamotrigine • Lamictal
- Levothyroxine • Levoxyl, Synthroid
- Liothyronine sodium • Cytomel, Triostat
- Lithium • Eskalith, Lithobid
- Methimazole • Tapazole
- Oxycodone • OxyContin
- Paroxetine • Paxil
- Sertraline • Zoloft
- Sunitinib • Sutent
Disclosure
Dr. Raj is a speaker for AstraZeneca and Merck.
1. Campayo A, de Jonge P, Roy JF, et al. Depressive disorder and incident diabetes mellitus: the effect of characteristics of depression. Am J Psychiatry. 2010;167(5):580-588.
2. Gallagher JC, Sai AJ. Vitamin D insufficiency deficiency, and bone health. J Clin Endocrinol Metab. 2010;95(6):2630-2633.
3. Hoogendijk WJ, Lips P, Dik MG, et al. Depression is associated with decreased 25-hydroxyvitamin D and increased parathyroid hormone levels in older adults. Arch Gen Psychiatry. 2008;65(5):508-512.
4. Ladenson PW, Singer PA, Ain KB, et al. American Thyroid Association guidelines for detection of thyroid dysfunction. Arch Intern Med. 2000;160(11):1573-1575.
5. Wolter P, Dumez H, Schöffski P. Sunitinib and hypothyroidism. N Engl J Med. 2007;356(15):1580; author reply 1580-1581.
6. Biondi B, Cooper DS. The clinical significance of subclinical thyroid dysfunction. Endocr Rev. 2008;29(1):76-131.
7. Roos A, Linn-Rasker SP, van Domburg RT, et al. The starting dose of levothyroxine in primary hypothyroidism treatment: a prospective, randomized, double-blind trial. Arch Intern Med. 2005;165(15):1714-1720.
8. Raj YP. Subclinical hypothyroidism: merely monitor or time to treat? Current Psychiatry. 2009;8(2):47-48.
9. Samuels MH. Cognitive function in subclinical hypothyroidism. J Clin Endocrinol Metab. 2010;95(8):3611-3613.
10. Mann JJ. The medical management of depression. N Engl J Med. 2005;353(17):1819-1834.
11. Nierenberg AA, Fava M, Trivedi MH, et al. A comparison of lithium and T(3) augmentation following two failed medication treatments for depression: a STAR*D report. Am J Psychiatry. 2006;163(9):1519-1530; quiz 1665.
12. Cooper-Kazaz R, Apter JT, Cohen R, et al. Combined treatment with sertraline and liothyronine in major depression: a randomized, double-blind, placebo-controlled trial. Arch Gen Psychiatry. 2007;64(6):679-688.
13. Henry C. Lithium side-effects and predictors of hypothyroidism in patients with bipolar disorder: sex differences. J Psychiatry Neurosci. 2002;27(2):104-107.
14. Ghaemi N, Pies R. The Bipolar Spectrum Diagnostic Scale. http://www.psycheducation.org/depression/BSDS.htm. Published October 2002. Updated June 2003. Accessed October 1 2012.
15. Wu W, Sun Z, Yu J, et al. A clinical retrospective analysis of factors associated with apathetic hyperthyroidism. Pathobiology. 2010;77(1):46-51.
16. van Melle JP, Verbeek DE, van den Berg MP, et al. Beta-blockers and depression after myocardial infarction: a multicenter prospective study. J Am Coll Cardiol. 2006;48(11):2209-2214.
Discuss this article at www.facebook.com/CurrentPsychiatry
Many endocrine disorders can manifest as depression, including relatively rare disorders such as Cushing’s syndrome (hypercortisolism) or Conn’s syndrome (primary hyperaldosteronism) as well as common ones such as diabetes mellitus. Most clinicians do not routinely screen for adrenal disorders when evaluating depressed patients because the yield is low, but do screen for thyroid disease because these disorders often mimic depression. The following 3 cases from my practice illustrate some nuances of screening and treating depressed patients with suspected thyroid abnormalities.
CASE 1: Feeling ‘like an 80-year-old’
Ms. A, age 25, has a gastrointestinal stromal tumor (GIST) and states that she feels “like an 80-year-old woman.” She is sore all over with facial swelling, abdominal cramping, and fatigue. This feeling has worsened since she started chemotherapy with sunitinib for the GIST. Her Patient Health Questionnaire-9 (PHQ-9) score is 14 out of 27, indicating moderate depression. As part of a workup for her depression, what general laboratory tests would be most helpful?
Because Ms. A is of menstruating age, check hemoglobin/hematocrit levels to evaluate for anemia. Monitoring electrolytes would allow you to assess for hypernatremia/hyponatremia, hyperkalemia/hypokalemia, and impaired renal function, all of which could cause depressive symptoms. Depending on Ms. A’s habitus or risk of metabolic syndrome, a fasting blood glucose or hemoglobin A1C test to screen for diabetes mellitus might be valuable because depression may be associated with diabetes.1 A1C is a preferred primary screening test for diabetes (≥6.5% constitutes a positive screen) based on revised clinical practice recommendations of the American Diabetes Association. A1C is available as an office-based test that requires just a drop of blood from a finger prick and does not require a fasting blood sample or a full laboratory analysis.
A popular test for a workup of depression is serum 25-hydroxyvitamin D [25(OH)D] (vitamin D), particularly for patients who live in areas with limited exposure to ultraviolet B radiation from sunlight.2 In a study of older adults, vitamin D levels were 14% lower in patients with minor depression and 14% lower in patients with major depressive disorder compared with controls. This study suggests that depression severity is associated with decreased serum vitamin D levels,3 but the association between depression and vitamin D insufficiency and deficiency is unknown. Checking sex hormones also may be helpful depending on the patient’s symptoms, because testosterone deficiency in men and dehydroepiandrosterone deficiency in women can have a direct impact on a patient’s libido and overall sense of well-being. If repleted, improved levels of sex hormones can lead to a dramatic improvement in mood as well.
Because more than one-half of the estimated 27 million Americans with hyperthyroidism or hypothyroidism are undiagnosed, the American Thyroid Association recommends universal screening for thyroid dysfunction after age 35, with a recheck every 5 years.4 However, checking serum thyroid-stimulating hormone (TSH) levels this often may not be cost-effective. Typically, I do not follow this recommendation when assessing or treating asymptomatic individuals, but Ms. A has symptoms of hypothyroidism (Table 1) and is taking a medication—sunitinib—thought to be associated with hypothyroidism.5 Her serum TSH was very high (110 mIU/L; range 0.28 to 5.00) and her serum free T4 (FT4) was low (0.5 ng/dL; range 0.7 to 1.8). These values were consistent with overt hypothyroidism, defined as low FT4 and elevated TSH levels. This is in contrast to subclinical hypothyroidism (SH), which is defined as having an elevated serum TSH with normal thyroid hormone (T3 and T4) levels. SH presents in 5% of young patients (age <45) and increasingly is being diagnoses in older patients (age >55), who are most likely to suffer adverse effects in mood or cognition.6
Table 1
Hypothyroidism symptoms
| Psychiatric overlap |
| Fatigue |
| Hypersomnolence |
| Cognitive impairment (forgetfulness) |
| Difficulty concentrating or learning |
| Weight gain or fluid retention |
| Somatic signs and symptoms |
| Dry, itchy skin |
| Brittle hair and nails |
| Constipation |
| Myalgias |
| Heavy and/or irregular menstrual cycle |
| Increased rate of miscarriage |
| Sensitivity to cold |
CASE 1 CONTINUED: A classic case
Ms. A is started on a full levothyroxine replacement dose of 1.6 μg/kg/d. For hypothyroid patients who do not have cardiac symptoms, weight-based replacement is thought to be safe and more convenient than starting with a low dose and titrating up.7 Ms. A responds quickly. At 6-week follow-up—the recommended time interval for repeat thyroid lab testing after initiating thyroid replacement—her depressive symptoms are markedly improved and her PHQ-9 score is 6, indicating mild depression.
CASE 2: Chronic pain, low mood, and fatigue
Ms. B, age 62, has fibromyalgia and chronic back pain. She takes cyclobenzaprine, 5 mg 2 to 3 times daily, and oxycodone, 40 mg/d, and describes mild depressive symptoms when she presents for routine follow-up. Most of her complaints are related to chronic pain, but she has a history of low mood and fatigue. She says she was prescribed levothyroxine, but is unable to remember if she stopped taking it because of financial constraints or laboratory/clinical improvement. Her neurologist recently checked her serum TSH, which was elevated at 8.1 mIU/L. Is it best to restart thyroid replacement or wait 6 weeks and recheck her thyroid panel?
Mild SH typically is defined as TSH between 4.5 and 10 mIU/L. In contrast, TSH between 10 and 20 mIU/L is considered severe SH. Because Ms. B did not have prominent new symptoms, I felt it was reasonable to wait the recommended 6 weeks before rechecking her thyroid function. At follow-up, Ms. B’s TSH was 4.64 mIU/L and her FT4 was normal: 0.7 ng/dL. Thyroid replacement was not indicated because she did not have obvious symptoms and treating SH does not impact overall mood and cognition until TSH is ≥10 mIU/L.8,9
CASE 2 CONTINUED: Prominent symptoms emerge
Ms. B returns several months later. Another clinician prescribed duloxetine, titrated from 30 mg to 60 mg, for worsening fibromyalgia. Her depressive symptoms are more prominent at this visit, and her PHQ-9 score has risen from 7 to 14, indicating moderate depression. She says previously she failed or poorly tolerated several antidepressants—fluoxetine, sertraline, and citalopram—but was hoping for a pharmacologic adjustment. Most evidence-based augmentation algorithms for treating major depression start with adding a second “traditional” antidepressant such as bupropion, then move to lithium, second-generation antipsychotics, or lamotrigine.10 But what about thyroid hormone augmentation?
Thyroid hormone often is on the lower rungs of depression treatment algorithms despite Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial data. The data suggest triiodothyronine’s (T3) lower side effect burden and ease of use may offer an advantage over lithium augmentation for depressed patients who have failed several medication trials.11 Liothyronine sodium (triiodothyronine) is a relatively benign medication with potential for augmentation when started at 25 to 50 mcg/d concurrently with antidepressants such as sertraline.12 Unfortunately, most augmentation trials with T3 have been short-term—generally 4 to 8 weeks. In my practice, T3 has limited application; I use it mainly for patients with treatment-resistant depression who have failed several other treatments.
Lithium, the comparison medication to thyroid hormone in the third augmentation arm of the STAR*D trial, requires an annual check of thyroid function (TSH testing) to properly monitor for potential lithium-related hypothyroidism or thyroiditis. Hypothyroidism, for which thyroid replacement is required, with lithium therapy is common, affecting 8% to 27% of patients.13 Patients who rapidly gain weight at the beginning of lithium treatment seem to have a higher risk of developing hypothyroidism.13 However, the risk of developing lithium-induced hypothyroidism is tied to the length of treatment; the longer a patient has been treated with lithium, the greater the risk of developing lithium-induced hypothyroidism.
CASE 3: Unable to slow down
Mr. C, age 45, has a 20-year history of major depression controlled reasonably well with paroxetine, 40 mg. He presents with escalating anxiety, depression, and irritability. His wife is concerned about his overwhelming thoughts of death, especially because Mr. C’s father committed suicide 30 years ago under similar circumstances. Mr. C has been tremulous for the past month and has not been sleeping well. He feels like he is “in constant motion” and unable to slow down. He screens in the “highly likely” range for bipolar disorder on the Bipolar Spectrum Diagnostic Scale14 and is started on divalproex ER, 500 mg/d.
His thyroid function tests returns with a suppressed TSH of 0.03 mIU/L and an elevated FT4 of 3.26 ng/dL. Divalproex is discontinued and he is started on the beta blocker atenolol, 25 mg/d, to target his anxiety, tachycardia, and akathisia. TSH receptor antibody testing was positive, which, along with an abnormal radioactive iodine uptake scan, confirmed a diagnosis of Graves’ disease. He receives methimazole, 20 mg/d, as a temporizing measure. An endocrinologist completes a radioactive iodine (I-131) ablation procedure on Mr. C, which resolves his mood and anxiety symptoms.
Although hypothyroidism commonly is associated with depressive symptoms, hyperthyroidism also may present as depression. Most cases of overt hyperthyroidism are directly referred to an endocrinologist because when treating disorders such as Graves’ disease—the most common cause of hyperthyroidism, especially among women age 20 to 40—many nuclear medicine teams require the expert guidance of an endocrinologist before considering radioiodine ablation. Hyperthyroidism often is accompanied by psychiatric and somatic symptoms of an “overactive” nature (Table 2). However, older patients (age >65) with hyperthyroidism may develop apathetic hyperthyroidism, a subset that comprises approximately 10% to 15% of all hyperthyroidism cases in older adults.15 Rather than becoming nervous, jittery, and restless, patients with apathetic hyperthyroidism are depressed, lethargic, and weak, and may develop proximal myopathy or cardiomyopathy. It is essential to differentiate apathetic hyperthyroidism from typical hyperthyroidism because accurately diagnosing and treating apathetic hyperthyroidism will improve outcomes.15
Table 2
Hyperthyroidism symptoms
| Psychiatric overlap |
| Decrease or increase in appetite |
| Insomnia |
| Fatigue |
| Mood instability |
| Irritability |
| Anxiety, nervousness |
| Somatic signs and symptoms |
| Frequent bowel movement, eg, diarrhea |
| Heart palpitations |
| Heat intolerance |
| Increased sweating |
| Light or missed menstrual periods, fertility problems |
| Muscle weakness |
| Shortness of breath |
| Sudden paralysis |
| Tremor, shakiness, dizziness |
| Vision changes |
| Weight loss or gain |
| Thinning of hair |
| Itching and hives |
| Possible increase in blood sugar |
Using beta blockers to treat hyperthyroidism can help control tachycardia or palpitations, tremulousness, and anxiety that often are inherent in hyperthyroidism. But can beta blockers induce depressive symptoms? A 1-year prospective Dutch study of patients who had survived a myocardial infarction did not find evidence that beta blockers induced depressive symptoms.16 However, the long-term and high-dosage effects of beta blockers still are in question.16 In Mr. C’s case, beta blockers had only positive effects on his symptoms and did not exacerbate his depressive symptoms.
Related Resources
- National Women’s Health Resource Center, Inc. Thyroid disorders. www.healthywomen.org/condition/thyroid-disorders.
- American Thyroid Association. www.thyroid.org.
- American Association of Clinical Endocrinologists. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the evaluation and treatment of hyperthyroidism and hypothyroidism. www.aace.com/files/hypo-hyper.pdf.
Drug Brand Names
- Atenolol • Tenormin
- Bupropion • Wellbutrin, Zyban
- Citalopram • Celexa
- Cyclobenzaprine • Flexeril
- Divalproex ER • Depakote ER
- Duloxetine • Cymbalta
- Fluoxetine • Prozac
- Lamotrigine • Lamictal
- Levothyroxine • Levoxyl, Synthroid
- Liothyronine sodium • Cytomel, Triostat
- Lithium • Eskalith, Lithobid
- Methimazole • Tapazole
- Oxycodone • OxyContin
- Paroxetine • Paxil
- Sertraline • Zoloft
- Sunitinib • Sutent
Disclosure
Dr. Raj is a speaker for AstraZeneca and Merck.
Discuss this article at www.facebook.com/CurrentPsychiatry
Many endocrine disorders can manifest as depression, including relatively rare disorders such as Cushing’s syndrome (hypercortisolism) or Conn’s syndrome (primary hyperaldosteronism) as well as common ones such as diabetes mellitus. Most clinicians do not routinely screen for adrenal disorders when evaluating depressed patients because the yield is low, but do screen for thyroid disease because these disorders often mimic depression. The following 3 cases from my practice illustrate some nuances of screening and treating depressed patients with suspected thyroid abnormalities.
CASE 1: Feeling ‘like an 80-year-old’
Ms. A, age 25, has a gastrointestinal stromal tumor (GIST) and states that she feels “like an 80-year-old woman.” She is sore all over with facial swelling, abdominal cramping, and fatigue. This feeling has worsened since she started chemotherapy with sunitinib for the GIST. Her Patient Health Questionnaire-9 (PHQ-9) score is 14 out of 27, indicating moderate depression. As part of a workup for her depression, what general laboratory tests would be most helpful?
Because Ms. A is of menstruating age, check hemoglobin/hematocrit levels to evaluate for anemia. Monitoring electrolytes would allow you to assess for hypernatremia/hyponatremia, hyperkalemia/hypokalemia, and impaired renal function, all of which could cause depressive symptoms. Depending on Ms. A’s habitus or risk of metabolic syndrome, a fasting blood glucose or hemoglobin A1C test to screen for diabetes mellitus might be valuable because depression may be associated with diabetes.1 A1C is a preferred primary screening test for diabetes (≥6.5% constitutes a positive screen) based on revised clinical practice recommendations of the American Diabetes Association. A1C is available as an office-based test that requires just a drop of blood from a finger prick and does not require a fasting blood sample or a full laboratory analysis.
A popular test for a workup of depression is serum 25-hydroxyvitamin D [25(OH)D] (vitamin D), particularly for patients who live in areas with limited exposure to ultraviolet B radiation from sunlight.2 In a study of older adults, vitamin D levels were 14% lower in patients with minor depression and 14% lower in patients with major depressive disorder compared with controls. This study suggests that depression severity is associated with decreased serum vitamin D levels,3 but the association between depression and vitamin D insufficiency and deficiency is unknown. Checking sex hormones also may be helpful depending on the patient’s symptoms, because testosterone deficiency in men and dehydroepiandrosterone deficiency in women can have a direct impact on a patient’s libido and overall sense of well-being. If repleted, improved levels of sex hormones can lead to a dramatic improvement in mood as well.
Because more than one-half of the estimated 27 million Americans with hyperthyroidism or hypothyroidism are undiagnosed, the American Thyroid Association recommends universal screening for thyroid dysfunction after age 35, with a recheck every 5 years.4 However, checking serum thyroid-stimulating hormone (TSH) levels this often may not be cost-effective. Typically, I do not follow this recommendation when assessing or treating asymptomatic individuals, but Ms. A has symptoms of hypothyroidism (Table 1) and is taking a medication—sunitinib—thought to be associated with hypothyroidism.5 Her serum TSH was very high (110 mIU/L; range 0.28 to 5.00) and her serum free T4 (FT4) was low (0.5 ng/dL; range 0.7 to 1.8). These values were consistent with overt hypothyroidism, defined as low FT4 and elevated TSH levels. This is in contrast to subclinical hypothyroidism (SH), which is defined as having an elevated serum TSH with normal thyroid hormone (T3 and T4) levels. SH presents in 5% of young patients (age <45) and increasingly is being diagnoses in older patients (age >55), who are most likely to suffer adverse effects in mood or cognition.6
Table 1
Hypothyroidism symptoms
| Psychiatric overlap |
| Fatigue |
| Hypersomnolence |
| Cognitive impairment (forgetfulness) |
| Difficulty concentrating or learning |
| Weight gain or fluid retention |
| Somatic signs and symptoms |
| Dry, itchy skin |
| Brittle hair and nails |
| Constipation |
| Myalgias |
| Heavy and/or irregular menstrual cycle |
| Increased rate of miscarriage |
| Sensitivity to cold |
CASE 1 CONTINUED: A classic case
Ms. A is started on a full levothyroxine replacement dose of 1.6 μg/kg/d. For hypothyroid patients who do not have cardiac symptoms, weight-based replacement is thought to be safe and more convenient than starting with a low dose and titrating up.7 Ms. A responds quickly. At 6-week follow-up—the recommended time interval for repeat thyroid lab testing after initiating thyroid replacement—her depressive symptoms are markedly improved and her PHQ-9 score is 6, indicating mild depression.
CASE 2: Chronic pain, low mood, and fatigue
Ms. B, age 62, has fibromyalgia and chronic back pain. She takes cyclobenzaprine, 5 mg 2 to 3 times daily, and oxycodone, 40 mg/d, and describes mild depressive symptoms when she presents for routine follow-up. Most of her complaints are related to chronic pain, but she has a history of low mood and fatigue. She says she was prescribed levothyroxine, but is unable to remember if she stopped taking it because of financial constraints or laboratory/clinical improvement. Her neurologist recently checked her serum TSH, which was elevated at 8.1 mIU/L. Is it best to restart thyroid replacement or wait 6 weeks and recheck her thyroid panel?
Mild SH typically is defined as TSH between 4.5 and 10 mIU/L. In contrast, TSH between 10 and 20 mIU/L is considered severe SH. Because Ms. B did not have prominent new symptoms, I felt it was reasonable to wait the recommended 6 weeks before rechecking her thyroid function. At follow-up, Ms. B’s TSH was 4.64 mIU/L and her FT4 was normal: 0.7 ng/dL. Thyroid replacement was not indicated because she did not have obvious symptoms and treating SH does not impact overall mood and cognition until TSH is ≥10 mIU/L.8,9
CASE 2 CONTINUED: Prominent symptoms emerge
Ms. B returns several months later. Another clinician prescribed duloxetine, titrated from 30 mg to 60 mg, for worsening fibromyalgia. Her depressive symptoms are more prominent at this visit, and her PHQ-9 score has risen from 7 to 14, indicating moderate depression. She says previously she failed or poorly tolerated several antidepressants—fluoxetine, sertraline, and citalopram—but was hoping for a pharmacologic adjustment. Most evidence-based augmentation algorithms for treating major depression start with adding a second “traditional” antidepressant such as bupropion, then move to lithium, second-generation antipsychotics, or lamotrigine.10 But what about thyroid hormone augmentation?
Thyroid hormone often is on the lower rungs of depression treatment algorithms despite Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial data. The data suggest triiodothyronine’s (T3) lower side effect burden and ease of use may offer an advantage over lithium augmentation for depressed patients who have failed several medication trials.11 Liothyronine sodium (triiodothyronine) is a relatively benign medication with potential for augmentation when started at 25 to 50 mcg/d concurrently with antidepressants such as sertraline.12 Unfortunately, most augmentation trials with T3 have been short-term—generally 4 to 8 weeks. In my practice, T3 has limited application; I use it mainly for patients with treatment-resistant depression who have failed several other treatments.
Lithium, the comparison medication to thyroid hormone in the third augmentation arm of the STAR*D trial, requires an annual check of thyroid function (TSH testing) to properly monitor for potential lithium-related hypothyroidism or thyroiditis. Hypothyroidism, for which thyroid replacement is required, with lithium therapy is common, affecting 8% to 27% of patients.13 Patients who rapidly gain weight at the beginning of lithium treatment seem to have a higher risk of developing hypothyroidism.13 However, the risk of developing lithium-induced hypothyroidism is tied to the length of treatment; the longer a patient has been treated with lithium, the greater the risk of developing lithium-induced hypothyroidism.
CASE 3: Unable to slow down
Mr. C, age 45, has a 20-year history of major depression controlled reasonably well with paroxetine, 40 mg. He presents with escalating anxiety, depression, and irritability. His wife is concerned about his overwhelming thoughts of death, especially because Mr. C’s father committed suicide 30 years ago under similar circumstances. Mr. C has been tremulous for the past month and has not been sleeping well. He feels like he is “in constant motion” and unable to slow down. He screens in the “highly likely” range for bipolar disorder on the Bipolar Spectrum Diagnostic Scale14 and is started on divalproex ER, 500 mg/d.
His thyroid function tests returns with a suppressed TSH of 0.03 mIU/L and an elevated FT4 of 3.26 ng/dL. Divalproex is discontinued and he is started on the beta blocker atenolol, 25 mg/d, to target his anxiety, tachycardia, and akathisia. TSH receptor antibody testing was positive, which, along with an abnormal radioactive iodine uptake scan, confirmed a diagnosis of Graves’ disease. He receives methimazole, 20 mg/d, as a temporizing measure. An endocrinologist completes a radioactive iodine (I-131) ablation procedure on Mr. C, which resolves his mood and anxiety symptoms.
Although hypothyroidism commonly is associated with depressive symptoms, hyperthyroidism also may present as depression. Most cases of overt hyperthyroidism are directly referred to an endocrinologist because when treating disorders such as Graves’ disease—the most common cause of hyperthyroidism, especially among women age 20 to 40—many nuclear medicine teams require the expert guidance of an endocrinologist before considering radioiodine ablation. Hyperthyroidism often is accompanied by psychiatric and somatic symptoms of an “overactive” nature (Table 2). However, older patients (age >65) with hyperthyroidism may develop apathetic hyperthyroidism, a subset that comprises approximately 10% to 15% of all hyperthyroidism cases in older adults.15 Rather than becoming nervous, jittery, and restless, patients with apathetic hyperthyroidism are depressed, lethargic, and weak, and may develop proximal myopathy or cardiomyopathy. It is essential to differentiate apathetic hyperthyroidism from typical hyperthyroidism because accurately diagnosing and treating apathetic hyperthyroidism will improve outcomes.15
Table 2
Hyperthyroidism symptoms
| Psychiatric overlap |
| Decrease or increase in appetite |
| Insomnia |
| Fatigue |
| Mood instability |
| Irritability |
| Anxiety, nervousness |
| Somatic signs and symptoms |
| Frequent bowel movement, eg, diarrhea |
| Heart palpitations |
| Heat intolerance |
| Increased sweating |
| Light or missed menstrual periods, fertility problems |
| Muscle weakness |
| Shortness of breath |
| Sudden paralysis |
| Tremor, shakiness, dizziness |
| Vision changes |
| Weight loss or gain |
| Thinning of hair |
| Itching and hives |
| Possible increase in blood sugar |
Using beta blockers to treat hyperthyroidism can help control tachycardia or palpitations, tremulousness, and anxiety that often are inherent in hyperthyroidism. But can beta blockers induce depressive symptoms? A 1-year prospective Dutch study of patients who had survived a myocardial infarction did not find evidence that beta blockers induced depressive symptoms.16 However, the long-term and high-dosage effects of beta blockers still are in question.16 In Mr. C’s case, beta blockers had only positive effects on his symptoms and did not exacerbate his depressive symptoms.
Related Resources
- National Women’s Health Resource Center, Inc. Thyroid disorders. www.healthywomen.org/condition/thyroid-disorders.
- American Thyroid Association. www.thyroid.org.
- American Association of Clinical Endocrinologists. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the evaluation and treatment of hyperthyroidism and hypothyroidism. www.aace.com/files/hypo-hyper.pdf.
Drug Brand Names
- Atenolol • Tenormin
- Bupropion • Wellbutrin, Zyban
- Citalopram • Celexa
- Cyclobenzaprine • Flexeril
- Divalproex ER • Depakote ER
- Duloxetine • Cymbalta
- Fluoxetine • Prozac
- Lamotrigine • Lamictal
- Levothyroxine • Levoxyl, Synthroid
- Liothyronine sodium • Cytomel, Triostat
- Lithium • Eskalith, Lithobid
- Methimazole • Tapazole
- Oxycodone • OxyContin
- Paroxetine • Paxil
- Sertraline • Zoloft
- Sunitinib • Sutent
Disclosure
Dr. Raj is a speaker for AstraZeneca and Merck.
1. Campayo A, de Jonge P, Roy JF, et al. Depressive disorder and incident diabetes mellitus: the effect of characteristics of depression. Am J Psychiatry. 2010;167(5):580-588.
2. Gallagher JC, Sai AJ. Vitamin D insufficiency deficiency, and bone health. J Clin Endocrinol Metab. 2010;95(6):2630-2633.
3. Hoogendijk WJ, Lips P, Dik MG, et al. Depression is associated with decreased 25-hydroxyvitamin D and increased parathyroid hormone levels in older adults. Arch Gen Psychiatry. 2008;65(5):508-512.
4. Ladenson PW, Singer PA, Ain KB, et al. American Thyroid Association guidelines for detection of thyroid dysfunction. Arch Intern Med. 2000;160(11):1573-1575.
5. Wolter P, Dumez H, Schöffski P. Sunitinib and hypothyroidism. N Engl J Med. 2007;356(15):1580; author reply 1580-1581.
6. Biondi B, Cooper DS. The clinical significance of subclinical thyroid dysfunction. Endocr Rev. 2008;29(1):76-131.
7. Roos A, Linn-Rasker SP, van Domburg RT, et al. The starting dose of levothyroxine in primary hypothyroidism treatment: a prospective, randomized, double-blind trial. Arch Intern Med. 2005;165(15):1714-1720.
8. Raj YP. Subclinical hypothyroidism: merely monitor or time to treat? Current Psychiatry. 2009;8(2):47-48.
9. Samuels MH. Cognitive function in subclinical hypothyroidism. J Clin Endocrinol Metab. 2010;95(8):3611-3613.
10. Mann JJ. The medical management of depression. N Engl J Med. 2005;353(17):1819-1834.
11. Nierenberg AA, Fava M, Trivedi MH, et al. A comparison of lithium and T(3) augmentation following two failed medication treatments for depression: a STAR*D report. Am J Psychiatry. 2006;163(9):1519-1530; quiz 1665.
12. Cooper-Kazaz R, Apter JT, Cohen R, et al. Combined treatment with sertraline and liothyronine in major depression: a randomized, double-blind, placebo-controlled trial. Arch Gen Psychiatry. 2007;64(6):679-688.
13. Henry C. Lithium side-effects and predictors of hypothyroidism in patients with bipolar disorder: sex differences. J Psychiatry Neurosci. 2002;27(2):104-107.
14. Ghaemi N, Pies R. The Bipolar Spectrum Diagnostic Scale. http://www.psycheducation.org/depression/BSDS.htm. Published October 2002. Updated June 2003. Accessed October 1 2012.
15. Wu W, Sun Z, Yu J, et al. A clinical retrospective analysis of factors associated with apathetic hyperthyroidism. Pathobiology. 2010;77(1):46-51.
16. van Melle JP, Verbeek DE, van den Berg MP, et al. Beta-blockers and depression after myocardial infarction: a multicenter prospective study. J Am Coll Cardiol. 2006;48(11):2209-2214.
1. Campayo A, de Jonge P, Roy JF, et al. Depressive disorder and incident diabetes mellitus: the effect of characteristics of depression. Am J Psychiatry. 2010;167(5):580-588.
2. Gallagher JC, Sai AJ. Vitamin D insufficiency deficiency, and bone health. J Clin Endocrinol Metab. 2010;95(6):2630-2633.
3. Hoogendijk WJ, Lips P, Dik MG, et al. Depression is associated with decreased 25-hydroxyvitamin D and increased parathyroid hormone levels in older adults. Arch Gen Psychiatry. 2008;65(5):508-512.
4. Ladenson PW, Singer PA, Ain KB, et al. American Thyroid Association guidelines for detection of thyroid dysfunction. Arch Intern Med. 2000;160(11):1573-1575.
5. Wolter P, Dumez H, Schöffski P. Sunitinib and hypothyroidism. N Engl J Med. 2007;356(15):1580; author reply 1580-1581.
6. Biondi B, Cooper DS. The clinical significance of subclinical thyroid dysfunction. Endocr Rev. 2008;29(1):76-131.
7. Roos A, Linn-Rasker SP, van Domburg RT, et al. The starting dose of levothyroxine in primary hypothyroidism treatment: a prospective, randomized, double-blind trial. Arch Intern Med. 2005;165(15):1714-1720.
8. Raj YP. Subclinical hypothyroidism: merely monitor or time to treat? Current Psychiatry. 2009;8(2):47-48.
9. Samuels MH. Cognitive function in subclinical hypothyroidism. J Clin Endocrinol Metab. 2010;95(8):3611-3613.
10. Mann JJ. The medical management of depression. N Engl J Med. 2005;353(17):1819-1834.
11. Nierenberg AA, Fava M, Trivedi MH, et al. A comparison of lithium and T(3) augmentation following two failed medication treatments for depression: a STAR*D report. Am J Psychiatry. 2006;163(9):1519-1530; quiz 1665.
12. Cooper-Kazaz R, Apter JT, Cohen R, et al. Combined treatment with sertraline and liothyronine in major depression: a randomized, double-blind, placebo-controlled trial. Arch Gen Psychiatry. 2007;64(6):679-688.
13. Henry C. Lithium side-effects and predictors of hypothyroidism in patients with bipolar disorder: sex differences. J Psychiatry Neurosci. 2002;27(2):104-107.
14. Ghaemi N, Pies R. The Bipolar Spectrum Diagnostic Scale. http://www.psycheducation.org/depression/BSDS.htm. Published October 2002. Updated June 2003. Accessed October 1 2012.
15. Wu W, Sun Z, Yu J, et al. A clinical retrospective analysis of factors associated with apathetic hyperthyroidism. Pathobiology. 2010;77(1):46-51.
16. van Melle JP, Verbeek DE, van den Berg MP, et al. Beta-blockers and depression after myocardial infarction: a multicenter prospective study. J Am Coll Cardiol. 2006;48(11):2209-2214.
Group Educational Therapy Helps Psychogenic Seizure Patients
SAN DIEGO – Patients who have been newly diagnosed with psychogenic nonepileptic seizures probably benefit from counseling and group therapy sessions before being referred to psychiatrists for treatment of their underlying mental health problem.
That’s what researchers from the Baylor College of Medicine in Houston found when they compared outcomes for 16 patients immediately referred to mental health services following diagnosis – the standard practice – with 19 others who had three educational group sessions beforehand where they learned about their condition and shared coping strategies.
Following their sessions, support group patients were more likely than were control group patients to agree that "my attacks do not really bother me or affect my life that much anymore" (P less than .001) and that "I have some control over my attacks" (P = .003). Perhaps most tellingly, patients in the treatment group were significantly less likely to return to the emergency department 3 months later (7% vs. 22%, P = .018).
"Clearly what we are seeing is that these patients need significant follow-up, perhaps with a neurologist rather than simply a mental health professional. Psychiatrists don’t feel comfortable addressing this issue; having a team approach with both a neurologist and a psychiatrist probably gives the best outcomes," lead investigator Dr. Atul Maheshwari of the department of neurology at Baylor said at the annual meeting of the American Epilepsy Society.
The patients in the randomized study were adults, mostly male, and had at least one seizure per week. They were diagnosed with psychogenic nonepileptic events (PNEE) in the epilepsy monitoring unit of Houston Veterans Affairs Medical Center, where the study was conducted. Psychiatric problems included depression, posttraumatic stress disorder, and childhood abuse.
The diagnosis of PNEE is often a hard blow to patients convinced that they have epilepsy or a brain tumor and feel like doctors aren’t taking them seriously; accepting that their seizures are caused by underlying psychiatric stress is difficult, Dr. Maheshwari said.
A nurse practitioner led the group sessions with talking points and instructions from a neurologist. "The first session helped [patients] understand [that] PNEE are nonpathologic, don’t cause the brain to be fried or cause long-term brain damage, but still require treatment. They were also shown videos of what PNEE look like and what epileptic seizures look like so they could better understand what’s going on. [Ultimately,] the goal was to take away the negative associations people have with the diagnosis," Dr. Maheshwari said.
"Subsequent sessions focused on finding constructive channels for stress release, focusing on the idea that [PNEE] are manifestations of inner-stress. Patients were allowed to discuss what strategies they found helpful. Peer-to-peer acceptance and evaluation helps," Dr. Maheshwari said.
Identification of triggers, including those for PTSD, was key, Dr. Maheshwari said. "For some people, [that means] identifying the aura so they can use stress-release strategies to avoid the seizure. It’s helpful to share those things with other people who have the same symptoms so they can appreciate they are not alone."
He cautioned that research is ongoing and the trial’s results are preliminary. Also, more people in the treatment group were married. Dr. Maheshwari noted that "we didn’t help out with the frequency and intensity of events, but there were significant improvements in patients’ perceptions of the problem" and quality of life.
Dr. Maheshwari said that he had no relevant disclosures.
SAN DIEGO – Patients who have been newly diagnosed with psychogenic nonepileptic seizures probably benefit from counseling and group therapy sessions before being referred to psychiatrists for treatment of their underlying mental health problem.
That’s what researchers from the Baylor College of Medicine in Houston found when they compared outcomes for 16 patients immediately referred to mental health services following diagnosis – the standard practice – with 19 others who had three educational group sessions beforehand where they learned about their condition and shared coping strategies.
Following their sessions, support group patients were more likely than were control group patients to agree that "my attacks do not really bother me or affect my life that much anymore" (P less than .001) and that "I have some control over my attacks" (P = .003). Perhaps most tellingly, patients in the treatment group were significantly less likely to return to the emergency department 3 months later (7% vs. 22%, P = .018).
"Clearly what we are seeing is that these patients need significant follow-up, perhaps with a neurologist rather than simply a mental health professional. Psychiatrists don’t feel comfortable addressing this issue; having a team approach with both a neurologist and a psychiatrist probably gives the best outcomes," lead investigator Dr. Atul Maheshwari of the department of neurology at Baylor said at the annual meeting of the American Epilepsy Society.
The patients in the randomized study were adults, mostly male, and had at least one seizure per week. They were diagnosed with psychogenic nonepileptic events (PNEE) in the epilepsy monitoring unit of Houston Veterans Affairs Medical Center, where the study was conducted. Psychiatric problems included depression, posttraumatic stress disorder, and childhood abuse.
The diagnosis of PNEE is often a hard blow to patients convinced that they have epilepsy or a brain tumor and feel like doctors aren’t taking them seriously; accepting that their seizures are caused by underlying psychiatric stress is difficult, Dr. Maheshwari said.
A nurse practitioner led the group sessions with talking points and instructions from a neurologist. "The first session helped [patients] understand [that] PNEE are nonpathologic, don’t cause the brain to be fried or cause long-term brain damage, but still require treatment. They were also shown videos of what PNEE look like and what epileptic seizures look like so they could better understand what’s going on. [Ultimately,] the goal was to take away the negative associations people have with the diagnosis," Dr. Maheshwari said.
"Subsequent sessions focused on finding constructive channels for stress release, focusing on the idea that [PNEE] are manifestations of inner-stress. Patients were allowed to discuss what strategies they found helpful. Peer-to-peer acceptance and evaluation helps," Dr. Maheshwari said.
Identification of triggers, including those for PTSD, was key, Dr. Maheshwari said. "For some people, [that means] identifying the aura so they can use stress-release strategies to avoid the seizure. It’s helpful to share those things with other people who have the same symptoms so they can appreciate they are not alone."
He cautioned that research is ongoing and the trial’s results are preliminary. Also, more people in the treatment group were married. Dr. Maheshwari noted that "we didn’t help out with the frequency and intensity of events, but there were significant improvements in patients’ perceptions of the problem" and quality of life.
Dr. Maheshwari said that he had no relevant disclosures.
SAN DIEGO – Patients who have been newly diagnosed with psychogenic nonepileptic seizures probably benefit from counseling and group therapy sessions before being referred to psychiatrists for treatment of their underlying mental health problem.
That’s what researchers from the Baylor College of Medicine in Houston found when they compared outcomes for 16 patients immediately referred to mental health services following diagnosis – the standard practice – with 19 others who had three educational group sessions beforehand where they learned about their condition and shared coping strategies.
Following their sessions, support group patients were more likely than were control group patients to agree that "my attacks do not really bother me or affect my life that much anymore" (P less than .001) and that "I have some control over my attacks" (P = .003). Perhaps most tellingly, patients in the treatment group were significantly less likely to return to the emergency department 3 months later (7% vs. 22%, P = .018).
"Clearly what we are seeing is that these patients need significant follow-up, perhaps with a neurologist rather than simply a mental health professional. Psychiatrists don’t feel comfortable addressing this issue; having a team approach with both a neurologist and a psychiatrist probably gives the best outcomes," lead investigator Dr. Atul Maheshwari of the department of neurology at Baylor said at the annual meeting of the American Epilepsy Society.
The patients in the randomized study were adults, mostly male, and had at least one seizure per week. They were diagnosed with psychogenic nonepileptic events (PNEE) in the epilepsy monitoring unit of Houston Veterans Affairs Medical Center, where the study was conducted. Psychiatric problems included depression, posttraumatic stress disorder, and childhood abuse.
The diagnosis of PNEE is often a hard blow to patients convinced that they have epilepsy or a brain tumor and feel like doctors aren’t taking them seriously; accepting that their seizures are caused by underlying psychiatric stress is difficult, Dr. Maheshwari said.
A nurse practitioner led the group sessions with talking points and instructions from a neurologist. "The first session helped [patients] understand [that] PNEE are nonpathologic, don’t cause the brain to be fried or cause long-term brain damage, but still require treatment. They were also shown videos of what PNEE look like and what epileptic seizures look like so they could better understand what’s going on. [Ultimately,] the goal was to take away the negative associations people have with the diagnosis," Dr. Maheshwari said.
"Subsequent sessions focused on finding constructive channels for stress release, focusing on the idea that [PNEE] are manifestations of inner-stress. Patients were allowed to discuss what strategies they found helpful. Peer-to-peer acceptance and evaluation helps," Dr. Maheshwari said.
Identification of triggers, including those for PTSD, was key, Dr. Maheshwari said. "For some people, [that means] identifying the aura so they can use stress-release strategies to avoid the seizure. It’s helpful to share those things with other people who have the same symptoms so they can appreciate they are not alone."
He cautioned that research is ongoing and the trial’s results are preliminary. Also, more people in the treatment group were married. Dr. Maheshwari noted that "we didn’t help out with the frequency and intensity of events, but there were significant improvements in patients’ perceptions of the problem" and quality of life.
Dr. Maheshwari said that he had no relevant disclosures.
AT THE ANNUAL MEETING OF THE AMERICAN EPILEPSY SOCIETY
Major Finding: Following diagnosis of psychogenic nonepileptic seizures, patients who received three educational group therapy sessions were significantly less likely to return to the emergency department 3 months later than were those who were immediately referred to mental health services (7% vs. 22%, P = .018).
Data Source: This was a randomized study of 35 patients with newly diagnosed psychogenic nonepileptic seizures.
Disclosures: Dr. Maheshwari said that he had no relevant disclosures.
How to provide culturally sensitive care to Arab American patients
Since September 11, 2001, many Arab Americans have faced increased discrimination, which puts them at greater risk for depression and low self-esteem.1 Children and adolescents in particular have been the victims of teasing and taunts. Many Muslim Arab Americans turned to their imams—a mosque’s spiritual leader—rather than a mental health clinician to help them deal with the national tragedy and the fallout that followed.2
Arab Americans may struggle to bridge their personal identity with their cultural one. Traditional Arab values stress the importance of family—both immediate and extended—loyalty to parents, religious adherence, and respect for elders and authority. Adapting those values to typical American values can cause dissonance as Arab Americans grapple to find a balance between renouncing their Arab culture in hopes of fitting in and feeling like outcasts in the country they call home.
Understanding cultural nuances
Be aware of the stigma of mental illness within Arab American communities. Unlike diabetes or heart disease, psychiatric disorders can carry a negative connotation for many Arab Americans.3 They may view mental illness as a personal shortcoming or ascribe their symptoms to supernatural spirits. The fear of being discriminated against for being culturally different and mentally ill may delay or prevent individuals from seeking care.
Understanding these dynamics, as well as Arab American culture, is the first step to evaluating these patients. Being aware of cultural nuances also is important. Patients may say they don’t smoke, but some prodding may reveal that they use a tobacco water pipe, or hookah.
Be cognizant of any preconceived notions that can seep into an assessment. It’s easy to assume that Arab American patients fall into stereotypical gender roles or are unhappy with what may be perceived as inadequate assimilation. Conversely, a patient’s appearance, devotion to cultural and religious values, and family support may lead to an assumption that the patient does not abuse substances or engage in high-risk behavior.
In addition, note that Arab Americans tend to present their mental illness as somatic complaints, which may make them more comfortable seeing a primary care physician than a psychiatrist.
Adjusting treatment
Many Arab Americans’ first choice is to seek support from family, friends, and religious leaders.4 A patient may need to be convinced to take psychotropics the same as they would other medications. Therefore, it may be necessary to involve family members to ensure treatment compliance. Clinicians may need to spend more time with Arab American patients, which can help the clinician grasp the complexity of their issues and allow patients to feel that they’re being cared for by a clinician who respects their cultural and religious beliefs. In conjunction, these steps will help you provide culturally sensitive care that best addresses Arab Americans’ mental health needs.
1. Amer MM, Hovey JD. Socio-demographic differences in acculturation and mental health for a sample of 2nd generation/early immigrant Arab Americans. J Immigr Minor Health. 2007;9(4):335-347.
2. Abu-Ras W, Gheith A, Cournos F. The imam’s role in mental health promotion: a study at 22 mosques in New York City’s Muslim community. J Muslim Ment Health. 2008;3(2):155-176.
3. Carolan MT, Bagherinia G, Juhari R, et al. Contemporary Muslim families: research and practice. Contemp Fam Ther. 2000;22(1):67-79.
4. Moradi B, Hasan NT. Arab American persons’ reported experiences of discrimination and mental health: the mediating role of personal control. J Couns Psychol. 2004;51(4):418-428.
Since September 11, 2001, many Arab Americans have faced increased discrimination, which puts them at greater risk for depression and low self-esteem.1 Children and adolescents in particular have been the victims of teasing and taunts. Many Muslim Arab Americans turned to their imams—a mosque’s spiritual leader—rather than a mental health clinician to help them deal with the national tragedy and the fallout that followed.2
Arab Americans may struggle to bridge their personal identity with their cultural one. Traditional Arab values stress the importance of family—both immediate and extended—loyalty to parents, religious adherence, and respect for elders and authority. Adapting those values to typical American values can cause dissonance as Arab Americans grapple to find a balance between renouncing their Arab culture in hopes of fitting in and feeling like outcasts in the country they call home.
Understanding cultural nuances
Be aware of the stigma of mental illness within Arab American communities. Unlike diabetes or heart disease, psychiatric disorders can carry a negative connotation for many Arab Americans.3 They may view mental illness as a personal shortcoming or ascribe their symptoms to supernatural spirits. The fear of being discriminated against for being culturally different and mentally ill may delay or prevent individuals from seeking care.
Understanding these dynamics, as well as Arab American culture, is the first step to evaluating these patients. Being aware of cultural nuances also is important. Patients may say they don’t smoke, but some prodding may reveal that they use a tobacco water pipe, or hookah.
Be cognizant of any preconceived notions that can seep into an assessment. It’s easy to assume that Arab American patients fall into stereotypical gender roles or are unhappy with what may be perceived as inadequate assimilation. Conversely, a patient’s appearance, devotion to cultural and religious values, and family support may lead to an assumption that the patient does not abuse substances or engage in high-risk behavior.
In addition, note that Arab Americans tend to present their mental illness as somatic complaints, which may make them more comfortable seeing a primary care physician than a psychiatrist.
Adjusting treatment
Many Arab Americans’ first choice is to seek support from family, friends, and religious leaders.4 A patient may need to be convinced to take psychotropics the same as they would other medications. Therefore, it may be necessary to involve family members to ensure treatment compliance. Clinicians may need to spend more time with Arab American patients, which can help the clinician grasp the complexity of their issues and allow patients to feel that they’re being cared for by a clinician who respects their cultural and religious beliefs. In conjunction, these steps will help you provide culturally sensitive care that best addresses Arab Americans’ mental health needs.
Since September 11, 2001, many Arab Americans have faced increased discrimination, which puts them at greater risk for depression and low self-esteem.1 Children and adolescents in particular have been the victims of teasing and taunts. Many Muslim Arab Americans turned to their imams—a mosque’s spiritual leader—rather than a mental health clinician to help them deal with the national tragedy and the fallout that followed.2
Arab Americans may struggle to bridge their personal identity with their cultural one. Traditional Arab values stress the importance of family—both immediate and extended—loyalty to parents, religious adherence, and respect for elders and authority. Adapting those values to typical American values can cause dissonance as Arab Americans grapple to find a balance between renouncing their Arab culture in hopes of fitting in and feeling like outcasts in the country they call home.
Understanding cultural nuances
Be aware of the stigma of mental illness within Arab American communities. Unlike diabetes or heart disease, psychiatric disorders can carry a negative connotation for many Arab Americans.3 They may view mental illness as a personal shortcoming or ascribe their symptoms to supernatural spirits. The fear of being discriminated against for being culturally different and mentally ill may delay or prevent individuals from seeking care.
Understanding these dynamics, as well as Arab American culture, is the first step to evaluating these patients. Being aware of cultural nuances also is important. Patients may say they don’t smoke, but some prodding may reveal that they use a tobacco water pipe, or hookah.
Be cognizant of any preconceived notions that can seep into an assessment. It’s easy to assume that Arab American patients fall into stereotypical gender roles or are unhappy with what may be perceived as inadequate assimilation. Conversely, a patient’s appearance, devotion to cultural and religious values, and family support may lead to an assumption that the patient does not abuse substances or engage in high-risk behavior.
In addition, note that Arab Americans tend to present their mental illness as somatic complaints, which may make them more comfortable seeing a primary care physician than a psychiatrist.
Adjusting treatment
Many Arab Americans’ first choice is to seek support from family, friends, and religious leaders.4 A patient may need to be convinced to take psychotropics the same as they would other medications. Therefore, it may be necessary to involve family members to ensure treatment compliance. Clinicians may need to spend more time with Arab American patients, which can help the clinician grasp the complexity of their issues and allow patients to feel that they’re being cared for by a clinician who respects their cultural and religious beliefs. In conjunction, these steps will help you provide culturally sensitive care that best addresses Arab Americans’ mental health needs.
1. Amer MM, Hovey JD. Socio-demographic differences in acculturation and mental health for a sample of 2nd generation/early immigrant Arab Americans. J Immigr Minor Health. 2007;9(4):335-347.
2. Abu-Ras W, Gheith A, Cournos F. The imam’s role in mental health promotion: a study at 22 mosques in New York City’s Muslim community. J Muslim Ment Health. 2008;3(2):155-176.
3. Carolan MT, Bagherinia G, Juhari R, et al. Contemporary Muslim families: research and practice. Contemp Fam Ther. 2000;22(1):67-79.
4. Moradi B, Hasan NT. Arab American persons’ reported experiences of discrimination and mental health: the mediating role of personal control. J Couns Psychol. 2004;51(4):418-428.
1. Amer MM, Hovey JD. Socio-demographic differences in acculturation and mental health for a sample of 2nd generation/early immigrant Arab Americans. J Immigr Minor Health. 2007;9(4):335-347.
2. Abu-Ras W, Gheith A, Cournos F. The imam’s role in mental health promotion: a study at 22 mosques in New York City’s Muslim community. J Muslim Ment Health. 2008;3(2):155-176.
3. Carolan MT, Bagherinia G, Juhari R, et al. Contemporary Muslim families: research and practice. Contemp Fam Ther. 2000;22(1):67-79.
4. Moradi B, Hasan NT. Arab American persons’ reported experiences of discrimination and mental health: the mediating role of personal control. J Couns Psychol. 2004;51(4):418-428.
Something smells different
CASE: Depressed and hopeless
Ms. D, age 69, has a 20-year history of bipolar II disorder, for which she is taking citalopram, 30 mg/d. She presents to her outpatient psychotherapist with a chief complaint of depressed mood. The therapist refers her for psychiatric hospitalization and electroconvulsive therapy consultation. Upon admission, Ms. D reports that her depressed mood has worsened over the past 5 weeks after a trip to the Dominican Republic. Ms. D had a negative encounter with airport security that she attributed to her 2 artificial knees and caused her to miss her flight. She endorses poor appetite, loss of energy, anhedonia, difficulty concentrating, poor memory, and feelings of hopelessness.
Ms. D reports increasingly frequent panic attacks as well as intermittent right-sided discomfort, unusual noxious smells, and increased falls. She says the falls likely are a result of new bilateral lower extremity weakness coupled with long-standing imbalance. Ms. D says she has experienced brief occasions of foul-smelling odors while showering without evidence of an offending substance. She also reports a mild, occipitally located headache.
Four years ago, Ms. D was hospitalized for a depressive episode without psychotic features and diagnosed with generalized anxiety disorder, for which she is taking clonazepam, 1.5 mg/d. Her last hypomanic episode was several years ago, and was characterized by increased energy with decreased need for sleep, flight of ideas, increased productivity, and impulsivity. Her medical history includes non-insulin dependent diabetes mellitus, chronic low back pain, hyperlipidemia, arthritis, and gastroesophageal reflux disease; her medications include pioglitazone, 30 mg/d, oxybutynin, 15 mg/d, rosuvastatin, 20 mg/d, losartan, 50 mg/d, and omeprazole, 20 mg/d. She also had bilateral knee replacements 9 years ago and an L4-S1 spinal fusion 11 years ago. She has no history of head injuries or seizures. Ms. D’s father had major depressive disorder, her mother died of a cerebrovascular accident at an unknown age, and her brother died of a myocardial infarction at age 52.
The authors’ observations
A striking aspect of Ms. D’s presenting complaints was her intermittent experience of foul smells. Although olfactory hallucinations can occur with psychotic and affective states, they also may be harbingers of an organic etiology involving the temporal lobe.1 Olfactory hallucinations associated with a psychiatric disorder often have an accompanying delusional belief regarding the cause of the smell.2
Olfactory hallucinations have been associated with migraines, epilepsy, and Parkinson’s disease.1-3 Neoplasms, cerebrovascular events, or traumatic brain injuries that result in focal mesial temporal lobe lesions can present as a partial complex seizure with olfactory or gustatory hallucinations and progress to automatisms.4 Characteristic odors in these hallucinations are unpleasant; patients with temporal lobe epilepsy describe the smells as “bad,” “rotten,” “sickening,” and “like burning food.”2 Ms. D’s report of unusual smells warranted consideration of an organic etiology for her mood change and a thorough neurologic examination.
EVALUATION: Neurologic signs
At the time of admission, Ms. D has a blood pressure of 127/68 mm Hg, heart rate of 74 beats per minute, respiratory rate of 16 breaths per minute, and temperature of 36.5°C. Neurologic examination reveals a left facial droop of unknown duration. Motor strength is weak throughout with left-sided focal weakness. Ms. D’s daughter notes that her mother’s smile appears “funny” in her admission photograph but is unsure when the asymmetry in her facial appearance began. Ms. D had been ambulatory before admission. Nursing staff observes Ms. D leans toward her left side and exhibits possible left-sided neglect during the first 12 hours of hospitalization.
When asked about her facial droop, Ms. D replies that she had not noticed any change in her appearance lately. She does not appear to be concerned about her worsening ambulation. On hospital day 2, Ms. D seems to have difficulty using utensils to eat breakfast. Ms. D is dismissive of her worsening motor function and asks to be left alone to finish her meal.
The authors’ observations
Ms. D’s focal neurologic deficits and complaint of a headache on admission were concerning because they could be caused by a cerebrovascular event or space-occupying brain lesion with potential for increased intracranial pressure. Neurologic examination with evaluation for papilledema is indicated, followed by medical transport to the closest medical center for emergent brain imaging. Neither Ms. D nor her daughter could pinpoint the onset of Ms. D’s left-sided facial droop, which precluded administering tissue plasminogen activator for a potential acute ischemic stroke.5
Ms. D’s case prompted us to consider what constitutes timely brain imaging in a patient who presents with psychiatric symptoms. Several neurologic conditions may present first with neurobehavioral symptoms before findings on physical exam. Two series of autopsies conducted >70 years ago at psychiatric hospitals found incidences of brain tumors of 3.45%6 and 13.5%.7 In a 5-year retrospective study, 21% of meningioma cases presented with psychiatric symptoms alone.8 These historical cases suggest that affective, behavioral, and psychotic symptoms may be the only clinical indicators of brain lesions that merit surgery.9-11
Imaging and radiation exposure
With the advent of CT scans in the 1970s, psychiatrists gained a new method of investigating potential structural CNS pathology in patients presenting with psychiatric symptoms. The dramatic increase in CT scan use in recent years and resulting radiation exposure is responsible for 1.5% to 2% of all cancers in the United States.12,13 Certainly, physicians must balance the advantage of early detection of brain lesions with cost-effectiveness and exposure to radiation.14
There is no consensus regarding use of brain imaging in a patient who presents with new-onset psychiatric symptoms. Certainly, patients with localizing neurologic deficits or symptoms of increased intracranial pressure should undergo brain imaging. As for psychiatric patients without neurologic findings, Filley and Kleinschmidt-DeMasters15 provide recommendations based on their 1995 case series, and other authors have recommended imaging for patients age ≥4016 vs ≥5017,18 who present with atypical mental status changes.
OUTCOME: Scan, then surgery
Ms. D’s head CT reveals a large right-sided temporoparietal low-density lesion with 8-mm left lower midline shift (Figure). She undergoes a right temporal craniotomy with resection of the mass, which is confirmed by surgical pathology to be a glioblastoma multiforme World Health Organization grade 4 tumor. Postoperative MRI shows evidence of infarction in the right posterior cerebral artery distribution and residual tumor is identified on follow-up imaging. Ms. D is referred to radiation oncology, where she receives a prognostic median life expectancy of 14 months with radiation and temozolomide treatment.19

Figure: Ms. D’s MRI results
MRI with contrast shows a large right temporal heterogeneous mass consistent with glioblastoma multiforme
The authors’ observations
Glioblastoma is a rare cancer that comprises 25% of all malignant nervous system tumors.20 It is associated with a poor prognosis, with a <30% relative survival rate for adults at 1 year and 3% at 5 years.20 Headaches, seizures, motor weakness, and progressive neurologic deficits are common symptoms of glioblastoma at diagnosis.20 Ms. D was offered the standard of care treatment for a high-grade glioma, including surgical resection followed by concomitant external-beam radiotherapy and chemotherapy.21
Consider structural brain lesions in patients who present with neurobehavioral symptoms, although most of these patients will be diagnosed with a primary psychiatric disorder. Ms. D had a known psychiatric disorder that predated the onset of neurologic symptoms and diagnosis of a rare brain cancer. Before she developed neurologic signs, Ms. D experienced symptoms uncharacteristic of her previous depressive episodes, including olfactory hallucinations, that provided an early indicator of a CNS lesion. Consider brain imaging in patients of any age who do not respond to medications targeting the presumed psychiatric diagnosis to ensure that insidious brain tumors are not missed (Table 1).15
Table 1
When to order neuroimaging for psychiatric patients
| Patient’s age | Most common types of brain tumor | MRI vs CT | Indications to image |
|---|---|---|---|
| ≥40 years | Metastases High-grade gliomas Meningiomas | Roughly equivalent for imaging common tumor types. Base on cost, availability, and relative patient contraindications | New-onset cognitive or emotional dysfunction. Patient is not responding to appropriate pharmacotherapy for psychiatric diagnosis |
| <40 years | Low-grade astrocytomas Oligodendrogliomas | MRI preferred | New-onset cognitive or emotional dysfunction with associated somatic symptoms (headache, nausea, vomiting, papilledema, seizures, or focal deficits). Patient is not responding to appropriate pharmacotherapy for the psychiatric diagnosis |
| Source: Reference 15 | |||
Compared with cerebrovascular lesions, neoplasms are more difficult to clinically correlate with their anatomic location. Neurobehavioral symptoms are more frequently associated with tumors originating in the frontal lobe or temporolimbic regions of the brain. The 3 types of frontal lobe syndromes are dorsolateral, orbitofrontal, and medial-frontal (Table 2).15 Temporolimbic tumors may present with hallucinations, mania, panic attacks, or amnesia. A meta-analysis found a statistically significant association between anorexia and hypothalamic tumors.22 Reports of neuropsychiatric symptoms that respond to pharmacologic treatment further confound the clinical picture.16
Table 2
Frontal lobe syndromes
| Syndrome | Characteristics |
|---|---|
| Dorsolateral | Deficits in executive functioning, including organization and behavior planning |
| Orbitofrontal | Prominent disinhibition |
| Medial-frontal | Apathy, abulia |
| Source: Reference 15 | |
It is uncommon for a patient with a long-standing mood disorder to develop a primary brain cancer. However, Ms. D’s case serves as an important reminder to consider medical comorbidities in our aging psychiatric population. In particular, a patient who develops unusual symptoms or does not respond to previously effective treatments should be more closely examined and the differential diagnosis broadened.
Related Resources
- MD Anderson Cancer Center. Brain tumor videos and podcasts. www.mdanderson.org/patient-and-cancer-information/cancer-information/cancer-types/brain-tumor/videos-and-podcasts/index.html.
- Braun CM, Dumont M, Duval J, et al. Brain modules of hallucination: an analysis of multiple patients with brain lesions. J Psychiatry Neurosci. 2003;28(6):432-449.
Drug Brand Names
- Citalopram • Celexa
- Clonazepam • Klonopin
- Losartan • Cozaar
- Omeprazole • Prilosec
- Oxybutynin • Ditropan
- Pioglitazone • Actos
- Rosuvastatin • Crestor
- Temozolomide • Temodar
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Assad G, Shapiro B. Hallucinations: theoretical and clinical overview. Am J Psychiatry. 1986;143(9):1088-1097.
2. Carter JL. Visual somatosensory, olfactory, and gustatory hallucinations. Psychiatr Clin North Am. 1992;15(2):347-358.
3. Fuller GN, Guiloff RJ. Migrainous olfactory hallucinations. J Neurol Neurosurg Psychiatry. 1987;50(12):1688-1690.
4. Chang BS, Lowenstein DH. Mechanisms of disease: epilepsy. N Engl J Med. 2003;349(13):1257-1266.
5. Lansberg MG, Bluhmki E, Thijs VN. Efficacy and safety of tissue plasminogen activator 3 to 4.5 hours after acute ischemic stroke: a metaanalysis. Stroke. 2009;40(7):2438-2441.
6. Hoffman JL. Intracranial neoplasms: their incidence and mental manifestations. Psychiatr Q. 1937;11(4):561-575.
7. Larson CP. Intracranial tumors in mental hospital patients. Am J Psychiatry. 1940;97(1):49-58.
8. Gupta RK, Kumar R. Benign brain tumours and psychiatric morbidity: a 5-years retrospective data analysis. Aust N Z J Psychiatry. 2004;38(5):316-319.
9. Chambers WR. Neurosurgical conditions masquerading as psychiatric diseases. Am J Psychiatry. 1955;112(5):387-389.
10. Trimble MR, Mendez MF, Cummings JL. Neuropsychiatric symptoms from the temporolimbic lobes. J Neuropsychiatry Clin Neurosci. 1997;9(3):429-438.
11. Uribe VM. Psychiatric symptoms and brain tumor. Am Fam Physician. 1986;34(2):95-98.
12. Brenner DJ, Hall EJ. Computed tomography—an increasing source of radiation exposure. N Engl J Med. 2007;357(2):2277-2284.
13. Berrington de González A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169(22):2071-2077.
14. Weinberger DR. Brain disease and psychiatric illness: when should a psychiatrist order a CAT scan? Am J Psychiatry. 1984;141(12):1521-1526.
15. Filley CM, Kleinschmidt-DeMasters BK. Neurobehavioral presentations of brain neoplasms. West J Med. 1995;163(1):19-25.
16. Moise D, Madhusoodanan S. Psychiatric symptoms associated with brain tumors: a clinical engima. CNS Spectr. 2006;11(1):28-31.
17. Bunevicius A, Deltuva VP, Deltuviene D, et al. Brain lesions manifesting as psychiatric disorders: eight cases. CNS Spectr. 2008;13(11):950-958.
18. Hollister LE, Boutros N. Clinical use of CT and MR scans in psychiatric patients. J Psychiatr Neurosci. 1991;16(4):194-198.
19. Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987-996.
20. Brandes AA, Tosoni A, Franceschi E, et al. Glioblastoma in adults. Crit Rev Oncol Hematol. 2008;67(2):139-152.
21. Chandana SR, Movva S, Arora M, et al. Primary brain tumors in adults. Am Fam Physician. 2008;77(10):1423-1430.
22. Madhusoodanan S, Opler MG, Moise D, et al. Brain tumor location and psychiatric symptoms: is there any association? A meta-analysis of published case studies. Expert Rev Neurother. 2010;10(10):1529-1536.
CASE: Depressed and hopeless
Ms. D, age 69, has a 20-year history of bipolar II disorder, for which she is taking citalopram, 30 mg/d. She presents to her outpatient psychotherapist with a chief complaint of depressed mood. The therapist refers her for psychiatric hospitalization and electroconvulsive therapy consultation. Upon admission, Ms. D reports that her depressed mood has worsened over the past 5 weeks after a trip to the Dominican Republic. Ms. D had a negative encounter with airport security that she attributed to her 2 artificial knees and caused her to miss her flight. She endorses poor appetite, loss of energy, anhedonia, difficulty concentrating, poor memory, and feelings of hopelessness.
Ms. D reports increasingly frequent panic attacks as well as intermittent right-sided discomfort, unusual noxious smells, and increased falls. She says the falls likely are a result of new bilateral lower extremity weakness coupled with long-standing imbalance. Ms. D says she has experienced brief occasions of foul-smelling odors while showering without evidence of an offending substance. She also reports a mild, occipitally located headache.
Four years ago, Ms. D was hospitalized for a depressive episode without psychotic features and diagnosed with generalized anxiety disorder, for which she is taking clonazepam, 1.5 mg/d. Her last hypomanic episode was several years ago, and was characterized by increased energy with decreased need for sleep, flight of ideas, increased productivity, and impulsivity. Her medical history includes non-insulin dependent diabetes mellitus, chronic low back pain, hyperlipidemia, arthritis, and gastroesophageal reflux disease; her medications include pioglitazone, 30 mg/d, oxybutynin, 15 mg/d, rosuvastatin, 20 mg/d, losartan, 50 mg/d, and omeprazole, 20 mg/d. She also had bilateral knee replacements 9 years ago and an L4-S1 spinal fusion 11 years ago. She has no history of head injuries or seizures. Ms. D’s father had major depressive disorder, her mother died of a cerebrovascular accident at an unknown age, and her brother died of a myocardial infarction at age 52.
The authors’ observations
A striking aspect of Ms. D’s presenting complaints was her intermittent experience of foul smells. Although olfactory hallucinations can occur with psychotic and affective states, they also may be harbingers of an organic etiology involving the temporal lobe.1 Olfactory hallucinations associated with a psychiatric disorder often have an accompanying delusional belief regarding the cause of the smell.2
Olfactory hallucinations have been associated with migraines, epilepsy, and Parkinson’s disease.1-3 Neoplasms, cerebrovascular events, or traumatic brain injuries that result in focal mesial temporal lobe lesions can present as a partial complex seizure with olfactory or gustatory hallucinations and progress to automatisms.4 Characteristic odors in these hallucinations are unpleasant; patients with temporal lobe epilepsy describe the smells as “bad,” “rotten,” “sickening,” and “like burning food.”2 Ms. D’s report of unusual smells warranted consideration of an organic etiology for her mood change and a thorough neurologic examination.
EVALUATION: Neurologic signs
At the time of admission, Ms. D has a blood pressure of 127/68 mm Hg, heart rate of 74 beats per minute, respiratory rate of 16 breaths per minute, and temperature of 36.5°C. Neurologic examination reveals a left facial droop of unknown duration. Motor strength is weak throughout with left-sided focal weakness. Ms. D’s daughter notes that her mother’s smile appears “funny” in her admission photograph but is unsure when the asymmetry in her facial appearance began. Ms. D had been ambulatory before admission. Nursing staff observes Ms. D leans toward her left side and exhibits possible left-sided neglect during the first 12 hours of hospitalization.
When asked about her facial droop, Ms. D replies that she had not noticed any change in her appearance lately. She does not appear to be concerned about her worsening ambulation. On hospital day 2, Ms. D seems to have difficulty using utensils to eat breakfast. Ms. D is dismissive of her worsening motor function and asks to be left alone to finish her meal.
The authors’ observations
Ms. D’s focal neurologic deficits and complaint of a headache on admission were concerning because they could be caused by a cerebrovascular event or space-occupying brain lesion with potential for increased intracranial pressure. Neurologic examination with evaluation for papilledema is indicated, followed by medical transport to the closest medical center for emergent brain imaging. Neither Ms. D nor her daughter could pinpoint the onset of Ms. D’s left-sided facial droop, which precluded administering tissue plasminogen activator for a potential acute ischemic stroke.5
Ms. D’s case prompted us to consider what constitutes timely brain imaging in a patient who presents with psychiatric symptoms. Several neurologic conditions may present first with neurobehavioral symptoms before findings on physical exam. Two series of autopsies conducted >70 years ago at psychiatric hospitals found incidences of brain tumors of 3.45%6 and 13.5%.7 In a 5-year retrospective study, 21% of meningioma cases presented with psychiatric symptoms alone.8 These historical cases suggest that affective, behavioral, and psychotic symptoms may be the only clinical indicators of brain lesions that merit surgery.9-11
Imaging and radiation exposure
With the advent of CT scans in the 1970s, psychiatrists gained a new method of investigating potential structural CNS pathology in patients presenting with psychiatric symptoms. The dramatic increase in CT scan use in recent years and resulting radiation exposure is responsible for 1.5% to 2% of all cancers in the United States.12,13 Certainly, physicians must balance the advantage of early detection of brain lesions with cost-effectiveness and exposure to radiation.14
There is no consensus regarding use of brain imaging in a patient who presents with new-onset psychiatric symptoms. Certainly, patients with localizing neurologic deficits or symptoms of increased intracranial pressure should undergo brain imaging. As for psychiatric patients without neurologic findings, Filley and Kleinschmidt-DeMasters15 provide recommendations based on their 1995 case series, and other authors have recommended imaging for patients age ≥4016 vs ≥5017,18 who present with atypical mental status changes.
OUTCOME: Scan, then surgery
Ms. D’s head CT reveals a large right-sided temporoparietal low-density lesion with 8-mm left lower midline shift (Figure). She undergoes a right temporal craniotomy with resection of the mass, which is confirmed by surgical pathology to be a glioblastoma multiforme World Health Organization grade 4 tumor. Postoperative MRI shows evidence of infarction in the right posterior cerebral artery distribution and residual tumor is identified on follow-up imaging. Ms. D is referred to radiation oncology, where she receives a prognostic median life expectancy of 14 months with radiation and temozolomide treatment.19

Figure: Ms. D’s MRI results
MRI with contrast shows a large right temporal heterogeneous mass consistent with glioblastoma multiforme
The authors’ observations
Glioblastoma is a rare cancer that comprises 25% of all malignant nervous system tumors.20 It is associated with a poor prognosis, with a <30% relative survival rate for adults at 1 year and 3% at 5 years.20 Headaches, seizures, motor weakness, and progressive neurologic deficits are common symptoms of glioblastoma at diagnosis.20 Ms. D was offered the standard of care treatment for a high-grade glioma, including surgical resection followed by concomitant external-beam radiotherapy and chemotherapy.21
Consider structural brain lesions in patients who present with neurobehavioral symptoms, although most of these patients will be diagnosed with a primary psychiatric disorder. Ms. D had a known psychiatric disorder that predated the onset of neurologic symptoms and diagnosis of a rare brain cancer. Before she developed neurologic signs, Ms. D experienced symptoms uncharacteristic of her previous depressive episodes, including olfactory hallucinations, that provided an early indicator of a CNS lesion. Consider brain imaging in patients of any age who do not respond to medications targeting the presumed psychiatric diagnosis to ensure that insidious brain tumors are not missed (Table 1).15
Table 1
When to order neuroimaging for psychiatric patients
| Patient’s age | Most common types of brain tumor | MRI vs CT | Indications to image |
|---|---|---|---|
| ≥40 years | Metastases High-grade gliomas Meningiomas | Roughly equivalent for imaging common tumor types. Base on cost, availability, and relative patient contraindications | New-onset cognitive or emotional dysfunction. Patient is not responding to appropriate pharmacotherapy for psychiatric diagnosis |
| <40 years | Low-grade astrocytomas Oligodendrogliomas | MRI preferred | New-onset cognitive or emotional dysfunction with associated somatic symptoms (headache, nausea, vomiting, papilledema, seizures, or focal deficits). Patient is not responding to appropriate pharmacotherapy for the psychiatric diagnosis |
| Source: Reference 15 | |||
Compared with cerebrovascular lesions, neoplasms are more difficult to clinically correlate with their anatomic location. Neurobehavioral symptoms are more frequently associated with tumors originating in the frontal lobe or temporolimbic regions of the brain. The 3 types of frontal lobe syndromes are dorsolateral, orbitofrontal, and medial-frontal (Table 2).15 Temporolimbic tumors may present with hallucinations, mania, panic attacks, or amnesia. A meta-analysis found a statistically significant association between anorexia and hypothalamic tumors.22 Reports of neuropsychiatric symptoms that respond to pharmacologic treatment further confound the clinical picture.16
Table 2
Frontal lobe syndromes
| Syndrome | Characteristics |
|---|---|
| Dorsolateral | Deficits in executive functioning, including organization and behavior planning |
| Orbitofrontal | Prominent disinhibition |
| Medial-frontal | Apathy, abulia |
| Source: Reference 15 | |
It is uncommon for a patient with a long-standing mood disorder to develop a primary brain cancer. However, Ms. D’s case serves as an important reminder to consider medical comorbidities in our aging psychiatric population. In particular, a patient who develops unusual symptoms or does not respond to previously effective treatments should be more closely examined and the differential diagnosis broadened.
Related Resources
- MD Anderson Cancer Center. Brain tumor videos and podcasts. www.mdanderson.org/patient-and-cancer-information/cancer-information/cancer-types/brain-tumor/videos-and-podcasts/index.html.
- Braun CM, Dumont M, Duval J, et al. Brain modules of hallucination: an analysis of multiple patients with brain lesions. J Psychiatry Neurosci. 2003;28(6):432-449.
Drug Brand Names
- Citalopram • Celexa
- Clonazepam • Klonopin
- Losartan • Cozaar
- Omeprazole • Prilosec
- Oxybutynin • Ditropan
- Pioglitazone • Actos
- Rosuvastatin • Crestor
- Temozolomide • Temodar
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE: Depressed and hopeless
Ms. D, age 69, has a 20-year history of bipolar II disorder, for which she is taking citalopram, 30 mg/d. She presents to her outpatient psychotherapist with a chief complaint of depressed mood. The therapist refers her for psychiatric hospitalization and electroconvulsive therapy consultation. Upon admission, Ms. D reports that her depressed mood has worsened over the past 5 weeks after a trip to the Dominican Republic. Ms. D had a negative encounter with airport security that she attributed to her 2 artificial knees and caused her to miss her flight. She endorses poor appetite, loss of energy, anhedonia, difficulty concentrating, poor memory, and feelings of hopelessness.
Ms. D reports increasingly frequent panic attacks as well as intermittent right-sided discomfort, unusual noxious smells, and increased falls. She says the falls likely are a result of new bilateral lower extremity weakness coupled with long-standing imbalance. Ms. D says she has experienced brief occasions of foul-smelling odors while showering without evidence of an offending substance. She also reports a mild, occipitally located headache.
Four years ago, Ms. D was hospitalized for a depressive episode without psychotic features and diagnosed with generalized anxiety disorder, for which she is taking clonazepam, 1.5 mg/d. Her last hypomanic episode was several years ago, and was characterized by increased energy with decreased need for sleep, flight of ideas, increased productivity, and impulsivity. Her medical history includes non-insulin dependent diabetes mellitus, chronic low back pain, hyperlipidemia, arthritis, and gastroesophageal reflux disease; her medications include pioglitazone, 30 mg/d, oxybutynin, 15 mg/d, rosuvastatin, 20 mg/d, losartan, 50 mg/d, and omeprazole, 20 mg/d. She also had bilateral knee replacements 9 years ago and an L4-S1 spinal fusion 11 years ago. She has no history of head injuries or seizures. Ms. D’s father had major depressive disorder, her mother died of a cerebrovascular accident at an unknown age, and her brother died of a myocardial infarction at age 52.
The authors’ observations
A striking aspect of Ms. D’s presenting complaints was her intermittent experience of foul smells. Although olfactory hallucinations can occur with psychotic and affective states, they also may be harbingers of an organic etiology involving the temporal lobe.1 Olfactory hallucinations associated with a psychiatric disorder often have an accompanying delusional belief regarding the cause of the smell.2
Olfactory hallucinations have been associated with migraines, epilepsy, and Parkinson’s disease.1-3 Neoplasms, cerebrovascular events, or traumatic brain injuries that result in focal mesial temporal lobe lesions can present as a partial complex seizure with olfactory or gustatory hallucinations and progress to automatisms.4 Characteristic odors in these hallucinations are unpleasant; patients with temporal lobe epilepsy describe the smells as “bad,” “rotten,” “sickening,” and “like burning food.”2 Ms. D’s report of unusual smells warranted consideration of an organic etiology for her mood change and a thorough neurologic examination.
EVALUATION: Neurologic signs
At the time of admission, Ms. D has a blood pressure of 127/68 mm Hg, heart rate of 74 beats per minute, respiratory rate of 16 breaths per minute, and temperature of 36.5°C. Neurologic examination reveals a left facial droop of unknown duration. Motor strength is weak throughout with left-sided focal weakness. Ms. D’s daughter notes that her mother’s smile appears “funny” in her admission photograph but is unsure when the asymmetry in her facial appearance began. Ms. D had been ambulatory before admission. Nursing staff observes Ms. D leans toward her left side and exhibits possible left-sided neglect during the first 12 hours of hospitalization.
When asked about her facial droop, Ms. D replies that she had not noticed any change in her appearance lately. She does not appear to be concerned about her worsening ambulation. On hospital day 2, Ms. D seems to have difficulty using utensils to eat breakfast. Ms. D is dismissive of her worsening motor function and asks to be left alone to finish her meal.
The authors’ observations
Ms. D’s focal neurologic deficits and complaint of a headache on admission were concerning because they could be caused by a cerebrovascular event or space-occupying brain lesion with potential for increased intracranial pressure. Neurologic examination with evaluation for papilledema is indicated, followed by medical transport to the closest medical center for emergent brain imaging. Neither Ms. D nor her daughter could pinpoint the onset of Ms. D’s left-sided facial droop, which precluded administering tissue plasminogen activator for a potential acute ischemic stroke.5
Ms. D’s case prompted us to consider what constitutes timely brain imaging in a patient who presents with psychiatric symptoms. Several neurologic conditions may present first with neurobehavioral symptoms before findings on physical exam. Two series of autopsies conducted >70 years ago at psychiatric hospitals found incidences of brain tumors of 3.45%6 and 13.5%.7 In a 5-year retrospective study, 21% of meningioma cases presented with psychiatric symptoms alone.8 These historical cases suggest that affective, behavioral, and psychotic symptoms may be the only clinical indicators of brain lesions that merit surgery.9-11
Imaging and radiation exposure
With the advent of CT scans in the 1970s, psychiatrists gained a new method of investigating potential structural CNS pathology in patients presenting with psychiatric symptoms. The dramatic increase in CT scan use in recent years and resulting radiation exposure is responsible for 1.5% to 2% of all cancers in the United States.12,13 Certainly, physicians must balance the advantage of early detection of brain lesions with cost-effectiveness and exposure to radiation.14
There is no consensus regarding use of brain imaging in a patient who presents with new-onset psychiatric symptoms. Certainly, patients with localizing neurologic deficits or symptoms of increased intracranial pressure should undergo brain imaging. As for psychiatric patients without neurologic findings, Filley and Kleinschmidt-DeMasters15 provide recommendations based on their 1995 case series, and other authors have recommended imaging for patients age ≥4016 vs ≥5017,18 who present with atypical mental status changes.
OUTCOME: Scan, then surgery
Ms. D’s head CT reveals a large right-sided temporoparietal low-density lesion with 8-mm left lower midline shift (Figure). She undergoes a right temporal craniotomy with resection of the mass, which is confirmed by surgical pathology to be a glioblastoma multiforme World Health Organization grade 4 tumor. Postoperative MRI shows evidence of infarction in the right posterior cerebral artery distribution and residual tumor is identified on follow-up imaging. Ms. D is referred to radiation oncology, where she receives a prognostic median life expectancy of 14 months with radiation and temozolomide treatment.19

Figure: Ms. D’s MRI results
MRI with contrast shows a large right temporal heterogeneous mass consistent with glioblastoma multiforme
The authors’ observations
Glioblastoma is a rare cancer that comprises 25% of all malignant nervous system tumors.20 It is associated with a poor prognosis, with a <30% relative survival rate for adults at 1 year and 3% at 5 years.20 Headaches, seizures, motor weakness, and progressive neurologic deficits are common symptoms of glioblastoma at diagnosis.20 Ms. D was offered the standard of care treatment for a high-grade glioma, including surgical resection followed by concomitant external-beam radiotherapy and chemotherapy.21
Consider structural brain lesions in patients who present with neurobehavioral symptoms, although most of these patients will be diagnosed with a primary psychiatric disorder. Ms. D had a known psychiatric disorder that predated the onset of neurologic symptoms and diagnosis of a rare brain cancer. Before she developed neurologic signs, Ms. D experienced symptoms uncharacteristic of her previous depressive episodes, including olfactory hallucinations, that provided an early indicator of a CNS lesion. Consider brain imaging in patients of any age who do not respond to medications targeting the presumed psychiatric diagnosis to ensure that insidious brain tumors are not missed (Table 1).15
Table 1
When to order neuroimaging for psychiatric patients
| Patient’s age | Most common types of brain tumor | MRI vs CT | Indications to image |
|---|---|---|---|
| ≥40 years | Metastases High-grade gliomas Meningiomas | Roughly equivalent for imaging common tumor types. Base on cost, availability, and relative patient contraindications | New-onset cognitive or emotional dysfunction. Patient is not responding to appropriate pharmacotherapy for psychiatric diagnosis |
| <40 years | Low-grade astrocytomas Oligodendrogliomas | MRI preferred | New-onset cognitive or emotional dysfunction with associated somatic symptoms (headache, nausea, vomiting, papilledema, seizures, or focal deficits). Patient is not responding to appropriate pharmacotherapy for the psychiatric diagnosis |
| Source: Reference 15 | |||
Compared with cerebrovascular lesions, neoplasms are more difficult to clinically correlate with their anatomic location. Neurobehavioral symptoms are more frequently associated with tumors originating in the frontal lobe or temporolimbic regions of the brain. The 3 types of frontal lobe syndromes are dorsolateral, orbitofrontal, and medial-frontal (Table 2).15 Temporolimbic tumors may present with hallucinations, mania, panic attacks, or amnesia. A meta-analysis found a statistically significant association between anorexia and hypothalamic tumors.22 Reports of neuropsychiatric symptoms that respond to pharmacologic treatment further confound the clinical picture.16
Table 2
Frontal lobe syndromes
| Syndrome | Characteristics |
|---|---|
| Dorsolateral | Deficits in executive functioning, including organization and behavior planning |
| Orbitofrontal | Prominent disinhibition |
| Medial-frontal | Apathy, abulia |
| Source: Reference 15 | |
It is uncommon for a patient with a long-standing mood disorder to develop a primary brain cancer. However, Ms. D’s case serves as an important reminder to consider medical comorbidities in our aging psychiatric population. In particular, a patient who develops unusual symptoms or does not respond to previously effective treatments should be more closely examined and the differential diagnosis broadened.
Related Resources
- MD Anderson Cancer Center. Brain tumor videos and podcasts. www.mdanderson.org/patient-and-cancer-information/cancer-information/cancer-types/brain-tumor/videos-and-podcasts/index.html.
- Braun CM, Dumont M, Duval J, et al. Brain modules of hallucination: an analysis of multiple patients with brain lesions. J Psychiatry Neurosci. 2003;28(6):432-449.
Drug Brand Names
- Citalopram • Celexa
- Clonazepam • Klonopin
- Losartan • Cozaar
- Omeprazole • Prilosec
- Oxybutynin • Ditropan
- Pioglitazone • Actos
- Rosuvastatin • Crestor
- Temozolomide • Temodar
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Assad G, Shapiro B. Hallucinations: theoretical and clinical overview. Am J Psychiatry. 1986;143(9):1088-1097.
2. Carter JL. Visual somatosensory, olfactory, and gustatory hallucinations. Psychiatr Clin North Am. 1992;15(2):347-358.
3. Fuller GN, Guiloff RJ. Migrainous olfactory hallucinations. J Neurol Neurosurg Psychiatry. 1987;50(12):1688-1690.
4. Chang BS, Lowenstein DH. Mechanisms of disease: epilepsy. N Engl J Med. 2003;349(13):1257-1266.
5. Lansberg MG, Bluhmki E, Thijs VN. Efficacy and safety of tissue plasminogen activator 3 to 4.5 hours after acute ischemic stroke: a metaanalysis. Stroke. 2009;40(7):2438-2441.
6. Hoffman JL. Intracranial neoplasms: their incidence and mental manifestations. Psychiatr Q. 1937;11(4):561-575.
7. Larson CP. Intracranial tumors in mental hospital patients. Am J Psychiatry. 1940;97(1):49-58.
8. Gupta RK, Kumar R. Benign brain tumours and psychiatric morbidity: a 5-years retrospective data analysis. Aust N Z J Psychiatry. 2004;38(5):316-319.
9. Chambers WR. Neurosurgical conditions masquerading as psychiatric diseases. Am J Psychiatry. 1955;112(5):387-389.
10. Trimble MR, Mendez MF, Cummings JL. Neuropsychiatric symptoms from the temporolimbic lobes. J Neuropsychiatry Clin Neurosci. 1997;9(3):429-438.
11. Uribe VM. Psychiatric symptoms and brain tumor. Am Fam Physician. 1986;34(2):95-98.
12. Brenner DJ, Hall EJ. Computed tomography—an increasing source of radiation exposure. N Engl J Med. 2007;357(2):2277-2284.
13. Berrington de González A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169(22):2071-2077.
14. Weinberger DR. Brain disease and psychiatric illness: when should a psychiatrist order a CAT scan? Am J Psychiatry. 1984;141(12):1521-1526.
15. Filley CM, Kleinschmidt-DeMasters BK. Neurobehavioral presentations of brain neoplasms. West J Med. 1995;163(1):19-25.
16. Moise D, Madhusoodanan S. Psychiatric symptoms associated with brain tumors: a clinical engima. CNS Spectr. 2006;11(1):28-31.
17. Bunevicius A, Deltuva VP, Deltuviene D, et al. Brain lesions manifesting as psychiatric disorders: eight cases. CNS Spectr. 2008;13(11):950-958.
18. Hollister LE, Boutros N. Clinical use of CT and MR scans in psychiatric patients. J Psychiatr Neurosci. 1991;16(4):194-198.
19. Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987-996.
20. Brandes AA, Tosoni A, Franceschi E, et al. Glioblastoma in adults. Crit Rev Oncol Hematol. 2008;67(2):139-152.
21. Chandana SR, Movva S, Arora M, et al. Primary brain tumors in adults. Am Fam Physician. 2008;77(10):1423-1430.
22. Madhusoodanan S, Opler MG, Moise D, et al. Brain tumor location and psychiatric symptoms: is there any association? A meta-analysis of published case studies. Expert Rev Neurother. 2010;10(10):1529-1536.
1. Assad G, Shapiro B. Hallucinations: theoretical and clinical overview. Am J Psychiatry. 1986;143(9):1088-1097.
2. Carter JL. Visual somatosensory, olfactory, and gustatory hallucinations. Psychiatr Clin North Am. 1992;15(2):347-358.
3. Fuller GN, Guiloff RJ. Migrainous olfactory hallucinations. J Neurol Neurosurg Psychiatry. 1987;50(12):1688-1690.
4. Chang BS, Lowenstein DH. Mechanisms of disease: epilepsy. N Engl J Med. 2003;349(13):1257-1266.
5. Lansberg MG, Bluhmki E, Thijs VN. Efficacy and safety of tissue plasminogen activator 3 to 4.5 hours after acute ischemic stroke: a metaanalysis. Stroke. 2009;40(7):2438-2441.
6. Hoffman JL. Intracranial neoplasms: their incidence and mental manifestations. Psychiatr Q. 1937;11(4):561-575.
7. Larson CP. Intracranial tumors in mental hospital patients. Am J Psychiatry. 1940;97(1):49-58.
8. Gupta RK, Kumar R. Benign brain tumours and psychiatric morbidity: a 5-years retrospective data analysis. Aust N Z J Psychiatry. 2004;38(5):316-319.
9. Chambers WR. Neurosurgical conditions masquerading as psychiatric diseases. Am J Psychiatry. 1955;112(5):387-389.
10. Trimble MR, Mendez MF, Cummings JL. Neuropsychiatric symptoms from the temporolimbic lobes. J Neuropsychiatry Clin Neurosci. 1997;9(3):429-438.
11. Uribe VM. Psychiatric symptoms and brain tumor. Am Fam Physician. 1986;34(2):95-98.
12. Brenner DJ, Hall EJ. Computed tomography—an increasing source of radiation exposure. N Engl J Med. 2007;357(2):2277-2284.
13. Berrington de González A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169(22):2071-2077.
14. Weinberger DR. Brain disease and psychiatric illness: when should a psychiatrist order a CAT scan? Am J Psychiatry. 1984;141(12):1521-1526.
15. Filley CM, Kleinschmidt-DeMasters BK. Neurobehavioral presentations of brain neoplasms. West J Med. 1995;163(1):19-25.
16. Moise D, Madhusoodanan S. Psychiatric symptoms associated with brain tumors: a clinical engima. CNS Spectr. 2006;11(1):28-31.
17. Bunevicius A, Deltuva VP, Deltuviene D, et al. Brain lesions manifesting as psychiatric disorders: eight cases. CNS Spectr. 2008;13(11):950-958.
18. Hollister LE, Boutros N. Clinical use of CT and MR scans in psychiatric patients. J Psychiatr Neurosci. 1991;16(4):194-198.
19. Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987-996.
20. Brandes AA, Tosoni A, Franceschi E, et al. Glioblastoma in adults. Crit Rev Oncol Hematol. 2008;67(2):139-152.
21. Chandana SR, Movva S, Arora M, et al. Primary brain tumors in adults. Am Fam Physician. 2008;77(10):1423-1430.
22. Madhusoodanan S, Opler MG, Moise D, et al. Brain tumor location and psychiatric symptoms: is there any association? A meta-analysis of published case studies. Expert Rev Neurother. 2010;10(10):1529-1536.
Panic disorder: Break the fear circuit
Ms. K, a 24-year-old waitress who lives with her boyfriend, was referred by her primary care physician for evaluation of panic attacks that began “out of nowhere” at work approximately 6 months ago. The unpredictable attacks occur multiple times per week, causing her to leave work and cancel shifts.
Ms. K reports that before the panic attacks began, she felt happy in her relationship, enjoyed hobbies, and was hopeful about the future. However, she has become concerned that a potentially catastrophic illness is causing her panic attacks. She researches her symptoms on the Internet, and is preoccupied with the possibility of sudden death due to an undiagnosed heart condition. Multiple visits to the emergency room have not identified any physical abnormalities. Her primary care doctor prescribed alprazolam, 0.5 mg as needed for panic attacks, which she reports is helpful, “but only in the moment of the attacks.” Ms. K avoids alcohol and illicit substances and limits her caffeine intake. She is not willing to accept that her life “feels so limited.” Her dream of earning a nursing degree and eventually starting a family now seems unattainable.
Panic disorder (PD) occurs in 3% to 5% of adults, with women affected at roughly twice the rate of men.1 Causing a broad range of distress and varying degrees of impairment, PD commonly occurs with other psychiatric disorders. For most patients, treatment is effective, but those who do not respond to initial approaches require a thoughtful, stepped approach to care. Key considerations include establishing an accurate diagnosis, clarifying comorbid illnesses, ascertaining patient beliefs and expectations, and providing appropriately dosed and maintained treatments.
Panic attacks vs PD
Panic attacks consist of rapid onset of intense anxiety, with prominent somatic symptoms, that peaks within 10 minutes (Figure).2 Attacks in which <4 of the listed symptoms occur are considered limited-symptom panic attacks.
Figure: Body locations of panic attack symptoms
Diagnosis of a panic attack requires the sudden development of intense fear or discomfort characterized by ≥4 of the 13 symptoms listed above that peaks in intensity within 10 minutes of onset
Source: Reference 2
Panic attacks can occur with various disorders, including other anxiety disorders, mood disorders, and substance intoxication or withdrawal. Because serious medical conditions can present with panic-like symptoms, the initial occurrence of such symptoms warrants consideration of physiological causes. For a Box2 that describes the differential diagnosis of panic attacks, see this article at CurrentPsychiatry.com.
To meet diagnostic criteria for panic disorder, panic attacks must initially occur “out of the blue,” meaning no specific object or situation induced the attack. The differential diagnosis of panic attacks includes assessing for other psychiatric disorders that may involve panic attacks. Evaluation requires considering the context in which the panic attacks occur, including their start date, pattern of attacks, instigating situations, and associated thoughts.
Social phobia. Attacks occur only during or immediately before a social interaction in which the patient fears embarrassing himself or herself.
Obsessive-compulsive disorder (OCD). Attacks occur when the patient cannot avoid exposure to an obsessional fear or is prevented from performing a ritual that diffuses obsessional anxiety.
Posttraumatic stress disorder (PTSD). Attacks occur when confronted by a trauma-related memory or trigger.
Specific phobia. Attacks occur only when the patient encounters a specifically feared object, place, or situation, unrelated to social phobia, OCD, or PTSD.
Medical conditions. Conditions to consider include—but are not limited to—hyperthyroidism, pulmonary embolism, myocardial infarction, cardiac dysrhythmias, hypoglycemia, asthma, partial complex seizures, and pheochromocytoma.
Source: Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000
A PD diagnosis requires that repeated panic attacks initially must occur from “out of the blue,” meaning no specific object or situation induced the attack. In addition, the diagnosis requires 1 of 3 types of psychological or behavioral changes as a result of the attacks (Table 1).2 Agoraphobia is diagnosed if 1 of the behavioral changes is avoidance of places or situations from which escape might be embarrassing or difficult should an attack occur. A patient can be diagnosed as having PD with agoraphobia, PD without agoraphobia, or agoraphobia without PD (ie, experiences only limited symptom panic attacks, but avoids situations or stimuli associated with them).
Table 1
Definitions of panic disorder and agoraphobia
| Panic disorder |
|---|
|
| Agoraphobia |
| Anxiety about, or avoidance of, being in places or situations from which escape might be difficult or embarrassing, or in which help may not be available in the event of having an unexpected or situationally predisposed panic attack or panic-like symptoms. Agoraphobic fears typically involve characteristic clusters of situations that include being outside the home alone, being in a crowd, standing in a line, being on a bridge, or traveling in a bus, train, or automobile |
| Source: Reference 2 |
Comorbidities are common in patients with PD and predict greater difficulty achieving remission (Box).1,3-6
The most common psychiatric conditions that co-occur with panic disorder (PD) are other anxiety disorders, mood disorders, personality disorders, and substance use disorders.1 Carefully assess the severity and degree of impairment or distress arising from each condition to prioritize treatment goals. For example, treating panic attacks would be a lower priority in a patient with untreated bipolar disorder.
Assessing comorbid substance abuse is important in selecting PD treatments. Benzodiazepines should almost always be avoided in patients with a history of drug abuse—illicit or prescribed. Although complete abstinence should not be a prerequisite for beginning PD treatment, detoxification and concomitant substance abuse treatment are essential.3
Comorbid mood disorders also affect the course of PD treatment. Antidepressants are effective for treating depression and PD, whereas benzodiazepines are not effective for depression.4 Antidepressants in patients with bipolar disorder are controversial because these medications might induce mixed or elevated mood states or rapid cycling. In these complicated patients, consider antidepressants lower in the treatment algorithm.5
Other conditions to consider before beginning treatment include pregnancy or the possibility of becoming pregnant in the near future and suicidal ideation. PD is associated with increased risk for suicidal ideation and progression to suicide attempts, particularly in patients with a comorbid mood or psychotic disorder.6 In addition, consider the potential impact of medications on comorbid medical conditions.
Treatment begins with education
The goal of treatment is remission of symptoms, ideally including an absence of panic attacks, agoraphobic avoidance, and anticipatory anxiety.1 The Panic Disorder Severity Scale self-report is a validated measure of panic symptoms that may be useful in clinical practice.7
The first step in treatment is educating patients about panic attacks, framing them as an overreactive fear circuit in the brain that produces physical symptoms that are not dangerous. Using a brain model that shows the location of the amygdala, hippocampus, and prefrontal cortex—which play crucial roles in generating and controlling anxiety and fear—can make this discussion more concrete.8 Although highly simplified, such models allow clinicians to demonstrate that excessive reactivity of limbic regions can be reduced by both top-down (cortico-limbic connections via cognitive-behavioral therapy [CBT]) and bottom-up (pharmacotherapy directly acting on limbic structures) approaches. Such discussions lead to treatment recommendations for CBT, pharmacotherapy, or their combination.
No single treatment has emerged as the definitive “best” for PD, and no reliable predictors can guide specific treatment for an individual.3 Combining CBT with pharmacotherapy produces higher short-term response rates than either treatment alone, but in the long term, combination treatment does not appear to be superior to CBT alone.9 Base the initial treatment selection for PD on patient preference, treatment availability and cost, and comorbid medical and psychiatric conditions. For an Algorithm to guide treatment decisions, see this article at CurrentPsychiatry.com.

Algorithm: Treatment for panic disorder: A suggested algorithm
aPoor response to an SSRI should lead to a switch to venlafaxine extended-release, and vice versa
bBenzodiazepines are relatively contraindicated in geriatric patients and patients with a history of substance abuse or dependence
CBT: cognitive-behavioral therapy; MAOI: monoamine oxidase inhibitor; SSRI: selective serotonin reuptake inhibitor; TCA: tricyclic antidepressant; Ven XR: venlafaxine extended-release
First-line treatments
Psychotherapy. CBT is the most efficacious psychotherapy for PD. Twelve to 15 sessions of CBT has demonstrated efficacy for PD, with additional effects on comorbid anxiety and depressive symptoms.10 No large clinical trials of CBT have used cognitive restructuring alone; all have included at least some component of exposure that requires the patient to confront feared physical sensations. Gains during treatment may be steady and gradual or sudden and uneven, with rapid improvement in some but not all symptoms. CBT and pharmacotherapy have demonstrated similar levels of benefit in short-term trials, but CBT has proven superior in most9 but not all11 trials evaluating long-term outcomes, particularly compared with pharmacotherapy that is discontinued during follow-up. Although less studied, group CBT also may be considered if a patient cannot afford individual CBT.
Pharmacotherapy. Evidence supports selective serotonin reuptake inhibitors (SSRIs), venlafaxine extended-release (XR), benzodiazepines, and tricyclic antidepressants (TCAs) as effective treatments for PD.3 No class of medication has demonstrated superiority over others in short-term treatment.3,12 Because of the medical risks associated with benzodiazepines and TCAs, an SSRI or venlafaxine XR should be the first medication option for most patients. Fluoxetine, paroxetine, sertraline, and venlafaxine XR are FDA-approved for PD. Paroxetine is associated with weight gain and may increase the risk for panic recurrence upon discontinuation more than sertraline, making it a less favorable option for many patients.13 Start doses at half the normal starting dose used for treating major depressive disorder and continue for 4 to 7 days, then increase to the minimal effective dose. For a Table3 that lists dosing recommendations for antidepressants to treat PD, see this article at CurrentPsychiatry.com. If there is no improvement by 4 weeks, increase the dose every 2 to 4 weeks until remission is achieved or side effects prevent further dose increases.
Table
Recommended doses for antidepressants used to treat panic disorder
| Medication | Starting dose (mg/d) | Therapeutic range (mg/d) |
|---|---|---|
| SSRIs | ||
| Citalopram | 10 | 20 to 40 |
| Escitalopram | 5 | 10 to 40 |
| Fluoxetine | 5 to 10 | 20 to 80 |
| Fluvoxamine | 25 | 100 to 300 |
| Paroxetine | 10 | 20 to 80 |
| Paroxetine CR | 12.5 | 25 to 50 |
| Sertraline | 25 | 100 to 200 |
| SNRIs | ||
| Duloxetine | 20 to 30 | 60 to 120 |
| Venlafaxine XR | 37.5 | 150 to 225 |
| TCAs | ||
| Clomipramine | 10 to 25 | 100 to 300 |
| Imipramine | 10 | 100 to 300 |
| MAOI | ||
| Phenelzine | 15 | 45 to 90 |
| CR: controlled release; MAOI: monoamine oxidase inhibitor; SNRIs: serotonin-norepinephrine reuptake inhibitors; SSRIs: selective serotonin reuptake inhibitors; TCAs: tricyclic antidepressants; XR: extended release Source: American Psychiatric Association. Practice guideline for the treatment of patients with panic disorder. 2nd ed. Washington, DC: American Psychiatric Association; 2009 | ||
Treatment nonresponse. True non-response needs to be distinguished from poor response caused by inadequate treatment delivery, eg, patients not completing homework assignments in CBT or not adhering to pharmacotherapy. Asking patients about adverse effects or personal and family beliefs about treatment may reveal reasons for nonadherence.
Second-line treatments
Little data are available to guide next-step treatment options in patients who don’t achieve remission from their initial treatment. Patients who benefit from an SSRI, venlafaxine XR, or CBT but still have symptoms should be started on combination treatment. For a patient who experiences complete non-response to the initial treatment, discontinue the first treatment and switch to the other modality. In general, completely ineffective treatments should be discontinued when another treatment is added, but when partial improvement (>30%) occurs, continue the original treatment and augment it with another approach.
For patients pursuing pharmacotherapy, poor response to an adequate SSRI trial usually should lead to a switch to venlafaxine XR, and vice versa. Failure to respond to both of these medication classes should prompt a switch to a benzodiazepine or TCA.
Benzodiazepines are a fast-acting, effective treatment for PD, with efficacy similar to SSRIs in acute and long-term treatment.14 Benzodiazepines may be prescribed with antidepressants at the beginning of treatment to improve response speed.15 Clonazepam and alprazolam are FDA-approved for treating PD. A high-potency, long-acting agent, clonazepam is the preferred initial benzodiazepine, dosed 0.5 to 4 mg/d on a fixed schedule. Although substantial data support using alprazolam for PD, it requires more frequent dosing and has a greater risk of rebound anxiety and abuse potential because of its more rapid onset of action. Compared with immediate-release alprazolam, alprazolam XR has a slower absorption rate and longer steady state in the blood, but this formulation does not have lower abuse potential or greater efficacy. Although not FDA-approved for PD, diazepam and lorazepam also have proven efficacy for PD.3
Benzodiazepines should be considered contraindicated in patients with a history of substance abuse, except in select cases.4 Benzodiazepines generally should be avoided in older patients because of increased risk for falls, cognitive impairment, and motor vehicle accidents. Table 2 lists situations in which benzodiazepines may be used to treat PD.
Table 2
Clinical scenarios in which to consider using benzodiazepines
| Coadministration for 2 to 4 weeks when initiating treatment with an SSRI or venlafaxine XR to achieve more rapid relief and mitigate potential antidepressant-induced anxiety |
| For patients who wish to avoid antidepressants because of concern about sexual dysfunction |
| For patients who need chronic aspirin or an NSAID, which may increase the risk for upper gastrointestinal bleeding when taken in combination with an SSRI |
| For patients with comorbid bipolar disorder or epilepsy |
| Next-step monotherapy or augmentation in patients who respond poorly to an SSRI, venlafaxine XR, TCA, or CBT |
| CBT: cognitive-behavioral therapy; NSAID: nonsteroidal anti-inflammatory drug; SSRI: selective serotonin reuptake inhibitor; TCA: tricyclic antidepressant; XR: extended release |
TCAs are effective as monotherapy for PD. Most support comes from studies of imipramine or clomipramine.12 Similar to SSRIs and venlafaxine XR, use a low initial dose and gradually increase until the patient remits or side effects prevent further increases. SSRI and TCA combinations rarely are used unless the TCA is a relatively specific norepinephrine reuptake inhibitor (eg, desipramine, nortriptyline). Because TCAs are metabolized via the cytochrome P450 2D6 system and some SSRIs—particularly fluoxetine and paroxetine—strongly inhibit 2D6, combinations of TCAs with these agents may lead to dangerously high plasma TCA levels, placing patients at risk for cardiac dysrhythmias and other side effects.16
Monoamine oxidase inhibitors (MAOIs)—particularly phenelzine—are underused for PD. They have the strongest efficacy data for any class of medications outside the first- and second-line agents and have a unique mechanism of action. In patients who can comply with the dietary and medication limitations, an MAOI generally should be the next step after nonresponse to other treatments.3
Alternative treatments
For patients who do not respond to any of the treatments described above, data from uncontrolled studies support mirtazapine, levetiracetam, and the serotonin-norepinephrine reuptake inhibitors duloxetine and milnacipran as monotherapy for PD.17 Pindolol—a beta blocker and 5-HT1A receptor antagonist—proved superior to placebo as an adjunctive agent to SSRIs in treatment-resistant PD in 1 of 2 trials.17 Minimal evidence supports the atypical antipsychotics risperidone and olanzapine in treatment-resistant PD, although a placebo-controlled trial of quetiapine SR coadministered with SSRIs recently was completed (NCT00619892; results pending). Atypical antipsychotics are best reserved for patients with a primary psychotic disorder or bipolar disorder who experience panic attacks.5
Panic-focused psychodynamic psychotherapy, a 12-week (approximately 24 sessions) form of psychotherapy, has demonstrated superiority vs applied relaxation therapy.18 This treatment could be considered for patients who do not respond to standard first-line treatments, but few community therapists are familiar with this method.
For many patients with PD, complementary and alternative medicine (CAM) approaches are appealing. See this article at CurrentPsychiatry.com for a Box that discusses CAM for PD.
Although no complementary and alternative medicine treatments have strong evidence of efficacy as monotherapy for panic disorder (PD), several have data that suggest benefit with little evidence of risk. These include bibliotherapy, yoga, aerobic exercise, and the dietary supplements kava and inositol.a Exercise as a treatment poses a challenge because it can induce symptoms that the patient fears, such as tachycardia and shortness of breath. In addition to any direct physiologic benefit from aerobic exercise, there is also an exposure component that can be harnessed by gradually increasing the exertion level.
Another approach undergoing extensive evaluation is Internet-provided cognitive-behavioral therapy (CBT). Using guided CBT modules with or without therapist support, Internet-provided CBT provides an option for motivated patients unable to complete in-person CBT because of logistical factors.b A helpful resource that reviews Internet self-help and psychotherapy guided programs for PD and other psychiatric conditions is http://beacon.anu.edu.au.
References
a. Antonacci DJ, Davis E, Bloch RM, et al. CAM for your anxious patient: what the evidence says. Current Psychiatry. 2010;9(10):42-52.
b. Johnston L, Titov N, Andrews G, et al. A RCT of a transdiagnostic internet-delivered treatment for three anxiety disorders: examination of support roles and disorder-specific outcomes. PLoS One. 2011;6(11):e28079.
Maintenance treatment
Patients who complete a course of CBT for PD often follow up with several “booster sessions” at monthly or longer intervals that focus on relapse prevention techniques. Few controlled trials have evaluated pharmacotherapy discontinuation in PD. Most guidelines recommend continuing treatment for ≥1 year after achieving remission to minimize the risk of relapse.3 Researchers are focusing on whether medication dosage can be reduced during maintenance without loss of efficacy.
Treatment discontinuation
In the absence of urgent medical need, taper medications for PD gradually over several months. PD patients are highly sensitive to unusual physical sensations, which can occur while discontinuing antidepressants or benzodiazepines. If a benzodiazepine is used in conjunction with an antidepressant, the benzodiazepine should be discontinued first, so that the antidepressant can help ease benzodiazepine-associated discontinuation symptoms. A brief course of CBT during pharmacotherapy discontinuation may increase the likelihood of successful tapering.19
CASE CONTINUED: A successful switch
Ms. K has to discontinue sequential trials of fluoxetine, 40 mg/d, and venlafaxine XR, 225 mg/d because of side effects, and she does not reduce the frequency of her alprazolam use. She agrees to switch from alprazolam to clonazepam, 0.5 mg every morning and 1 mg at bedtime, and to start CBT. Clonazepam reduces her anxiety sufficiently so she can address her symptoms in therapy. Through CBT she becomes motivated to monitor her thoughts and treat them as guesses rather than facts, reviewing the evidence for her thoughts and generating rational responses. She participates in exposure exercises, which she practices between sessions, and grows to tolerate uncomfortable sensations until they no longer signal danger. After 12 CBT sessions, she is panic-free. Despite some trepidation, she agrees to a slow taper off clonazepam, reducing the dose by 0.25 mg every 2 weeks. She continues booster sessions with her therapist to manage any re-emerging anxiety. After an additional 12 weeks, she successfully discontinues clonazepam and remains panic-free.
Related Resources
- American Psychiatric Association. Panic disorder. http://healthyminds.org/Main-Topic/Panic-Disorder.aspx.
- Anxiety and Depression Association of America. Panic disorder & agoraphobia. http://adaa.org/understanding-anxiety/panic-disorder-agoraphobia.
- Mayo Clinic. Panic attacks and panic disorder. www.mayoclinic.com/health/panic-attacks/DS00338.
- National Health Service Self-Help Guides. www.ntw.nhs.uk/pic/selfhelp.
- National Institute of Mental Health. Panic disorder. www.nimh.nih.gov/health/topics/panic-disorder/index.shtml.
Drug Brand Names
- Alprazolam • Xanax
- Alprazolam XR • Xanax XR
- Citalopram • Celexa
- Clomipramine • Anafranil
- Clonazepam • Klonopin
- Desipramine • Norpramin
- Diazepam • Valium
- Duloxetine • Cymbalta
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Imipramine • Tofranil
- Levetiracetam • Keppra
- Lorazepam • Ativan
- Milnacipran • Savella
- Mirtazapine • Remeron
- Nortriptyline • Aventyl, Pamelor
- Olanzapine • Zyprexa
- Paroxetine • Paxil
- Paroxetine CR • Paxil CR
- Phenelzine • Nardil
- Pindolol • Visken
- Quetiapine SR • Seroquel SR
- Risperidone • Risperdal
- Sertraline • Zoloft
- Venlafaxine XR • Effexor XR
Disclosures
Dr. Dunlop receives research support from Bristol-Myers Squibb, GlaxoSmithKline, and the National Institute of Mental Health. He serves as a consultant to MedAvante and Roche.
Ms. Schneider and Dr. Gerardi report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Roy-Byrne PP, Craske MG, Stein MB. Panic disorder. Lancet. 2006;368(9540):1023-1032.
2. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
3. American Psychiatric Association. Practice guideline for the treatment of patients with panic disorder. 2nd ed. Washington DC: American Psychiatric Association; 2009.
4. Dunlop BW, Davis PG. Combination treatment with benzodiazepines and SSRIs for comorbid anxiety and depression: a review. Prim Care Companion J Clin Psychiatry. 2008;10(3):222-228.
5. Rakofsky JJ, Dunlop BW. Treating nonspecific anxiety and anxiety disorders in patients with bipolar disorder: a review. J Clin Psychiatry. 2011;72(1):81-90.
6. Sareen J, Cox BJ, Afifi TO, et al. Anxiety disorders and risk for suicidal ideation and suicide attempts: a population-based longitudinal study of adults. Arch Gen Psychiatry. 2005;62(11):1249-1257.
7. Houck PR, Spiegel DA, Shear MK, et al. Reliability of the self-report version of the panic disorder severity scale. Depress Anxiety. 2002;15(4):183-185.
8. Ninan PT, Dunlop BW. Neurobiology and etiology of panic disorder. J Clin Psychiatry. 2005;66(suppl 4):3-7.
9. Furukawa TA, Watanabe N, Churchill R. Psychotherapy plus antidepressant for panic disorder with or without agoraphobia: systematic review. Br J Psychiatry. 2006;188:305-312.
10. Barlow DH, Gorman JM, Shear MK, et al. Cognitive-behavioral therapy, imipramine, or their combination for panic disorder: a randomized controlled trial. JAMA. 2000;283(19):2529-2536.
11. van Apeldoorn FJ, Timmerman ME, Mersch PP, et al. A randomized trial of cognitive-behavioral therapy or selective serotonin reuptake inhibitor or both combined for panic disorder with or without agoraphobia: treatment results through 1-year follow-up. J Clin Psychiatry. 2010;71(5):574-586.
12. Bakker A, van Balkom AJ, Spinhoven P. SSRIs vs. TCAs in the treatment of panic disorder: a meta-analysis. Acta Psychiatr Scand. 2002;106(3):163-167.
13. Bandelow B, Behnke K, Lenoir S, et al. Sertraline versus paroxetine in the treatment of panic disorder: an acute, double-blind noninferiority comparison. J Clin Psychiatry. 2004;65(3):405-413.
14. Nardi AE, Freire RC, Mochcovitch MD, et al. A randomized, naturalistic, parallel-group study for the long-term treatment of panic disorder with clonazepam or paroxetine. J Clin Psychopharmacol. 2012;32(1):120-126.
15. Goddard AW, Brouette T, Almai A, et al. Early coadministration of clonazepam with sertraline for panic disorder. Arch Gen Psychiatry. 2001;58(7):681-686.
16. Preskorn SH, Shah R, Neff M, et al. The potential for clinically significant drug-drug interactions involving the CYP 2D6 system: effects with fluoxetine and paroxetine versus sertraline. J Psychiatr Pract. 2007;13(1):5-12.
17. Perna G, Guerriero G, Caldirola D. Emerging drugs for panic disorder. Expert Opin Emerg Drugs. 2011;16(4):631-645.
18. Milrod B, Leon AC, Busch F, et al. A randomized controlled clinical trial of psychoanalytic psychotherapy for panic disorder. Am J Psychiatry. 2007;164(2):265-272.
19. Otto MW, Pollack MH, Sachs GS, et al. Discontinuation of benzodiazepine treatment: efficacy of cognitive-behavioral therapy for patients with panic disorder. Am J Psychiatry. 1993;150(10):1485-1490.
Ms. K, a 24-year-old waitress who lives with her boyfriend, was referred by her primary care physician for evaluation of panic attacks that began “out of nowhere” at work approximately 6 months ago. The unpredictable attacks occur multiple times per week, causing her to leave work and cancel shifts.
Ms. K reports that before the panic attacks began, she felt happy in her relationship, enjoyed hobbies, and was hopeful about the future. However, she has become concerned that a potentially catastrophic illness is causing her panic attacks. She researches her symptoms on the Internet, and is preoccupied with the possibility of sudden death due to an undiagnosed heart condition. Multiple visits to the emergency room have not identified any physical abnormalities. Her primary care doctor prescribed alprazolam, 0.5 mg as needed for panic attacks, which she reports is helpful, “but only in the moment of the attacks.” Ms. K avoids alcohol and illicit substances and limits her caffeine intake. She is not willing to accept that her life “feels so limited.” Her dream of earning a nursing degree and eventually starting a family now seems unattainable.
Panic disorder (PD) occurs in 3% to 5% of adults, with women affected at roughly twice the rate of men.1 Causing a broad range of distress and varying degrees of impairment, PD commonly occurs with other psychiatric disorders. For most patients, treatment is effective, but those who do not respond to initial approaches require a thoughtful, stepped approach to care. Key considerations include establishing an accurate diagnosis, clarifying comorbid illnesses, ascertaining patient beliefs and expectations, and providing appropriately dosed and maintained treatments.
Panic attacks vs PD
Panic attacks consist of rapid onset of intense anxiety, with prominent somatic symptoms, that peaks within 10 minutes (Figure).2 Attacks in which <4 of the listed symptoms occur are considered limited-symptom panic attacks.

Figure: Body locations of panic attack symptoms
Diagnosis of a panic attack requires the sudden development of intense fear or discomfort characterized by ≥4 of the 13 symptoms listed above that peaks in intensity within 10 minutes of onset
Source: Reference 2
Panic attacks can occur with various disorders, including other anxiety disorders, mood disorders, and substance intoxication or withdrawal. Because serious medical conditions can present with panic-like symptoms, the initial occurrence of such symptoms warrants consideration of physiological causes. For a Box2 that describes the differential diagnosis of panic attacks, see this article at CurrentPsychiatry.com.
To meet diagnostic criteria for panic disorder, panic attacks must initially occur “out of the blue,” meaning no specific object or situation induced the attack. The differential diagnosis of panic attacks includes assessing for other psychiatric disorders that may involve panic attacks. Evaluation requires considering the context in which the panic attacks occur, including their start date, pattern of attacks, instigating situations, and associated thoughts.
Social phobia. Attacks occur only during or immediately before a social interaction in which the patient fears embarrassing himself or herself.
Obsessive-compulsive disorder (OCD). Attacks occur when the patient cannot avoid exposure to an obsessional fear or is prevented from performing a ritual that diffuses obsessional anxiety.
Posttraumatic stress disorder (PTSD). Attacks occur when confronted by a trauma-related memory or trigger.
Specific phobia. Attacks occur only when the patient encounters a specifically feared object, place, or situation, unrelated to social phobia, OCD, or PTSD.
Medical conditions. Conditions to consider include—but are not limited to—hyperthyroidism, pulmonary embolism, myocardial infarction, cardiac dysrhythmias, hypoglycemia, asthma, partial complex seizures, and pheochromocytoma.
Source: Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000
A PD diagnosis requires that repeated panic attacks initially must occur from “out of the blue,” meaning no specific object or situation induced the attack. In addition, the diagnosis requires 1 of 3 types of psychological or behavioral changes as a result of the attacks (Table 1).2 Agoraphobia is diagnosed if 1 of the behavioral changes is avoidance of places or situations from which escape might be embarrassing or difficult should an attack occur. A patient can be diagnosed as having PD with agoraphobia, PD without agoraphobia, or agoraphobia without PD (ie, experiences only limited symptom panic attacks, but avoids situations or stimuli associated with them).
Table 1
Definitions of panic disorder and agoraphobia
| Panic disorder |
|---|
|
| Agoraphobia |
| Anxiety about, or avoidance of, being in places or situations from which escape might be difficult or embarrassing, or in which help may not be available in the event of having an unexpected or situationally predisposed panic attack or panic-like symptoms. Agoraphobic fears typically involve characteristic clusters of situations that include being outside the home alone, being in a crowd, standing in a line, being on a bridge, or traveling in a bus, train, or automobile |
| Source: Reference 2 |
Comorbidities are common in patients with PD and predict greater difficulty achieving remission (Box).1,3-6
The most common psychiatric conditions that co-occur with panic disorder (PD) are other anxiety disorders, mood disorders, personality disorders, and substance use disorders.1 Carefully assess the severity and degree of impairment or distress arising from each condition to prioritize treatment goals. For example, treating panic attacks would be a lower priority in a patient with untreated bipolar disorder.
Assessing comorbid substance abuse is important in selecting PD treatments. Benzodiazepines should almost always be avoided in patients with a history of drug abuse—illicit or prescribed. Although complete abstinence should not be a prerequisite for beginning PD treatment, detoxification and concomitant substance abuse treatment are essential.3
Comorbid mood disorders also affect the course of PD treatment. Antidepressants are effective for treating depression and PD, whereas benzodiazepines are not effective for depression.4 Antidepressants in patients with bipolar disorder are controversial because these medications might induce mixed or elevated mood states or rapid cycling. In these complicated patients, consider antidepressants lower in the treatment algorithm.5
Other conditions to consider before beginning treatment include pregnancy or the possibility of becoming pregnant in the near future and suicidal ideation. PD is associated with increased risk for suicidal ideation and progression to suicide attempts, particularly in patients with a comorbid mood or psychotic disorder.6 In addition, consider the potential impact of medications on comorbid medical conditions.
Treatment begins with education
The goal of treatment is remission of symptoms, ideally including an absence of panic attacks, agoraphobic avoidance, and anticipatory anxiety.1 The Panic Disorder Severity Scale self-report is a validated measure of panic symptoms that may be useful in clinical practice.7
The first step in treatment is educating patients about panic attacks, framing them as an overreactive fear circuit in the brain that produces physical symptoms that are not dangerous. Using a brain model that shows the location of the amygdala, hippocampus, and prefrontal cortex—which play crucial roles in generating and controlling anxiety and fear—can make this discussion more concrete.8 Although highly simplified, such models allow clinicians to demonstrate that excessive reactivity of limbic regions can be reduced by both top-down (cortico-limbic connections via cognitive-behavioral therapy [CBT]) and bottom-up (pharmacotherapy directly acting on limbic structures) approaches. Such discussions lead to treatment recommendations for CBT, pharmacotherapy, or their combination.
No single treatment has emerged as the definitive “best” for PD, and no reliable predictors can guide specific treatment for an individual.3 Combining CBT with pharmacotherapy produces higher short-term response rates than either treatment alone, but in the long term, combination treatment does not appear to be superior to CBT alone.9 Base the initial treatment selection for PD on patient preference, treatment availability and cost, and comorbid medical and psychiatric conditions. For an Algorithm to guide treatment decisions, see this article at CurrentPsychiatry.com.

Algorithm: Treatment for panic disorder: A suggested algorithm
aPoor response to an SSRI should lead to a switch to venlafaxine extended-release, and vice versa
bBenzodiazepines are relatively contraindicated in geriatric patients and patients with a history of substance abuse or dependence
CBT: cognitive-behavioral therapy; MAOI: monoamine oxidase inhibitor; SSRI: selective serotonin reuptake inhibitor; TCA: tricyclic antidepressant; Ven XR: venlafaxine extended-release
First-line treatments
Psychotherapy. CBT is the most efficacious psychotherapy for PD. Twelve to 15 sessions of CBT has demonstrated efficacy for PD, with additional effects on comorbid anxiety and depressive symptoms.10 No large clinical trials of CBT have used cognitive restructuring alone; all have included at least some component of exposure that requires the patient to confront feared physical sensations. Gains during treatment may be steady and gradual or sudden and uneven, with rapid improvement in some but not all symptoms. CBT and pharmacotherapy have demonstrated similar levels of benefit in short-term trials, but CBT has proven superior in most9 but not all11 trials evaluating long-term outcomes, particularly compared with pharmacotherapy that is discontinued during follow-up. Although less studied, group CBT also may be considered if a patient cannot afford individual CBT.
Pharmacotherapy. Evidence supports selective serotonin reuptake inhibitors (SSRIs), venlafaxine extended-release (XR), benzodiazepines, and tricyclic antidepressants (TCAs) as effective treatments for PD.3 No class of medication has demonstrated superiority over others in short-term treatment.3,12 Because of the medical risks associated with benzodiazepines and TCAs, an SSRI or venlafaxine XR should be the first medication option for most patients. Fluoxetine, paroxetine, sertraline, and venlafaxine XR are FDA-approved for PD. Paroxetine is associated with weight gain and may increase the risk for panic recurrence upon discontinuation more than sertraline, making it a less favorable option for many patients.13 Start doses at half the normal starting dose used for treating major depressive disorder and continue for 4 to 7 days, then increase to the minimal effective dose. For a Table3 that lists dosing recommendations for antidepressants to treat PD, see this article at CurrentPsychiatry.com. If there is no improvement by 4 weeks, increase the dose every 2 to 4 weeks until remission is achieved or side effects prevent further dose increases.
Table
Recommended doses for antidepressants used to treat panic disorder
| Medication | Starting dose (mg/d) | Therapeutic range (mg/d) |
|---|---|---|
| SSRIs | ||
| Citalopram | 10 | 20 to 40 |
| Escitalopram | 5 | 10 to 40 |
| Fluoxetine | 5 to 10 | 20 to 80 |
| Fluvoxamine | 25 | 100 to 300 |
| Paroxetine | 10 | 20 to 80 |
| Paroxetine CR | 12.5 | 25 to 50 |
| Sertraline | 25 | 100 to 200 |
| SNRIs | ||
| Duloxetine | 20 to 30 | 60 to 120 |
| Venlafaxine XR | 37.5 | 150 to 225 |
| TCAs | ||
| Clomipramine | 10 to 25 | 100 to 300 |
| Imipramine | 10 | 100 to 300 |
| MAOI | ||
| Phenelzine | 15 | 45 to 90 |
| CR: controlled release; MAOI: monoamine oxidase inhibitor; SNRIs: serotonin-norepinephrine reuptake inhibitors; SSRIs: selective serotonin reuptake inhibitors; TCAs: tricyclic antidepressants; XR: extended release Source: American Psychiatric Association. Practice guideline for the treatment of patients with panic disorder. 2nd ed. Washington, DC: American Psychiatric Association; 2009 | ||
Treatment nonresponse. True non-response needs to be distinguished from poor response caused by inadequate treatment delivery, eg, patients not completing homework assignments in CBT or not adhering to pharmacotherapy. Asking patients about adverse effects or personal and family beliefs about treatment may reveal reasons for nonadherence.
Second-line treatments
Little data are available to guide next-step treatment options in patients who don’t achieve remission from their initial treatment. Patients who benefit from an SSRI, venlafaxine XR, or CBT but still have symptoms should be started on combination treatment. For a patient who experiences complete non-response to the initial treatment, discontinue the first treatment and switch to the other modality. In general, completely ineffective treatments should be discontinued when another treatment is added, but when partial improvement (>30%) occurs, continue the original treatment and augment it with another approach.
For patients pursuing pharmacotherapy, poor response to an adequate SSRI trial usually should lead to a switch to venlafaxine XR, and vice versa. Failure to respond to both of these medication classes should prompt a switch to a benzodiazepine or TCA.
Benzodiazepines are a fast-acting, effective treatment for PD, with efficacy similar to SSRIs in acute and long-term treatment.14 Benzodiazepines may be prescribed with antidepressants at the beginning of treatment to improve response speed.15 Clonazepam and alprazolam are FDA-approved for treating PD. A high-potency, long-acting agent, clonazepam is the preferred initial benzodiazepine, dosed 0.5 to 4 mg/d on a fixed schedule. Although substantial data support using alprazolam for PD, it requires more frequent dosing and has a greater risk of rebound anxiety and abuse potential because of its more rapid onset of action. Compared with immediate-release alprazolam, alprazolam XR has a slower absorption rate and longer steady state in the blood, but this formulation does not have lower abuse potential or greater efficacy. Although not FDA-approved for PD, diazepam and lorazepam also have proven efficacy for PD.3
Benzodiazepines should be considered contraindicated in patients with a history of substance abuse, except in select cases.4 Benzodiazepines generally should be avoided in older patients because of increased risk for falls, cognitive impairment, and motor vehicle accidents. Table 2 lists situations in which benzodiazepines may be used to treat PD.
Table 2
Clinical scenarios in which to consider using benzodiazepines
| Coadministration for 2 to 4 weeks when initiating treatment with an SSRI or venlafaxine XR to achieve more rapid relief and mitigate potential antidepressant-induced anxiety |
| For patients who wish to avoid antidepressants because of concern about sexual dysfunction |
| For patients who need chronic aspirin or an NSAID, which may increase the risk for upper gastrointestinal bleeding when taken in combination with an SSRI |
| For patients with comorbid bipolar disorder or epilepsy |
| Next-step monotherapy or augmentation in patients who respond poorly to an SSRI, venlafaxine XR, TCA, or CBT |
| CBT: cognitive-behavioral therapy; NSAID: nonsteroidal anti-inflammatory drug; SSRI: selective serotonin reuptake inhibitor; TCA: tricyclic antidepressant; XR: extended release |
TCAs are effective as monotherapy for PD. Most support comes from studies of imipramine or clomipramine.12 Similar to SSRIs and venlafaxine XR, use a low initial dose and gradually increase until the patient remits or side effects prevent further increases. SSRI and TCA combinations rarely are used unless the TCA is a relatively specific norepinephrine reuptake inhibitor (eg, desipramine, nortriptyline). Because TCAs are metabolized via the cytochrome P450 2D6 system and some SSRIs—particularly fluoxetine and paroxetine—strongly inhibit 2D6, combinations of TCAs with these agents may lead to dangerously high plasma TCA levels, placing patients at risk for cardiac dysrhythmias and other side effects.16
Monoamine oxidase inhibitors (MAOIs)—particularly phenelzine—are underused for PD. They have the strongest efficacy data for any class of medications outside the first- and second-line agents and have a unique mechanism of action. In patients who can comply with the dietary and medication limitations, an MAOI generally should be the next step after nonresponse to other treatments.3
Alternative treatments
For patients who do not respond to any of the treatments described above, data from uncontrolled studies support mirtazapine, levetiracetam, and the serotonin-norepinephrine reuptake inhibitors duloxetine and milnacipran as monotherapy for PD.17 Pindolol—a beta blocker and 5-HT1A receptor antagonist—proved superior to placebo as an adjunctive agent to SSRIs in treatment-resistant PD in 1 of 2 trials.17 Minimal evidence supports the atypical antipsychotics risperidone and olanzapine in treatment-resistant PD, although a placebo-controlled trial of quetiapine SR coadministered with SSRIs recently was completed (NCT00619892; results pending). Atypical antipsychotics are best reserved for patients with a primary psychotic disorder or bipolar disorder who experience panic attacks.5
Panic-focused psychodynamic psychotherapy, a 12-week (approximately 24 sessions) form of psychotherapy, has demonstrated superiority vs applied relaxation therapy.18 This treatment could be considered for patients who do not respond to standard first-line treatments, but few community therapists are familiar with this method.
For many patients with PD, complementary and alternative medicine (CAM) approaches are appealing. See this article at CurrentPsychiatry.com for a Box that discusses CAM for PD.
Although no complementary and alternative medicine treatments have strong evidence of efficacy as monotherapy for panic disorder (PD), several have data that suggest benefit with little evidence of risk. These include bibliotherapy, yoga, aerobic exercise, and the dietary supplements kava and inositol.a Exercise as a treatment poses a challenge because it can induce symptoms that the patient fears, such as tachycardia and shortness of breath. In addition to any direct physiologic benefit from aerobic exercise, there is also an exposure component that can be harnessed by gradually increasing the exertion level.
Another approach undergoing extensive evaluation is Internet-provided cognitive-behavioral therapy (CBT). Using guided CBT modules with or without therapist support, Internet-provided CBT provides an option for motivated patients unable to complete in-person CBT because of logistical factors.b A helpful resource that reviews Internet self-help and psychotherapy guided programs for PD and other psychiatric conditions is http://beacon.anu.edu.au.
References
a. Antonacci DJ, Davis E, Bloch RM, et al. CAM for your anxious patient: what the evidence says. Current Psychiatry. 2010;9(10):42-52.
b. Johnston L, Titov N, Andrews G, et al. A RCT of a transdiagnostic internet-delivered treatment for three anxiety disorders: examination of support roles and disorder-specific outcomes. PLoS One. 2011;6(11):e28079.
Maintenance treatment
Patients who complete a course of CBT for PD often follow up with several “booster sessions” at monthly or longer intervals that focus on relapse prevention techniques. Few controlled trials have evaluated pharmacotherapy discontinuation in PD. Most guidelines recommend continuing treatment for ≥1 year after achieving remission to minimize the risk of relapse.3 Researchers are focusing on whether medication dosage can be reduced during maintenance without loss of efficacy.
Treatment discontinuation
In the absence of urgent medical need, taper medications for PD gradually over several months. PD patients are highly sensitive to unusual physical sensations, which can occur while discontinuing antidepressants or benzodiazepines. If a benzodiazepine is used in conjunction with an antidepressant, the benzodiazepine should be discontinued first, so that the antidepressant can help ease benzodiazepine-associated discontinuation symptoms. A brief course of CBT during pharmacotherapy discontinuation may increase the likelihood of successful tapering.19
CASE CONTINUED: A successful switch
Ms. K has to discontinue sequential trials of fluoxetine, 40 mg/d, and venlafaxine XR, 225 mg/d because of side effects, and she does not reduce the frequency of her alprazolam use. She agrees to switch from alprazolam to clonazepam, 0.5 mg every morning and 1 mg at bedtime, and to start CBT. Clonazepam reduces her anxiety sufficiently so she can address her symptoms in therapy. Through CBT she becomes motivated to monitor her thoughts and treat them as guesses rather than facts, reviewing the evidence for her thoughts and generating rational responses. She participates in exposure exercises, which she practices between sessions, and grows to tolerate uncomfortable sensations until they no longer signal danger. After 12 CBT sessions, she is panic-free. Despite some trepidation, she agrees to a slow taper off clonazepam, reducing the dose by 0.25 mg every 2 weeks. She continues booster sessions with her therapist to manage any re-emerging anxiety. After an additional 12 weeks, she successfully discontinues clonazepam and remains panic-free.
Related Resources
- American Psychiatric Association. Panic disorder. http://healthyminds.org/Main-Topic/Panic-Disorder.aspx.
- Anxiety and Depression Association of America. Panic disorder & agoraphobia. http://adaa.org/understanding-anxiety/panic-disorder-agoraphobia.
- Mayo Clinic. Panic attacks and panic disorder. www.mayoclinic.com/health/panic-attacks/DS00338.
- National Health Service Self-Help Guides. www.ntw.nhs.uk/pic/selfhelp.
- National Institute of Mental Health. Panic disorder. www.nimh.nih.gov/health/topics/panic-disorder/index.shtml.
Drug Brand Names
- Alprazolam • Xanax
- Alprazolam XR • Xanax XR
- Citalopram • Celexa
- Clomipramine • Anafranil
- Clonazepam • Klonopin
- Desipramine • Norpramin
- Diazepam • Valium
- Duloxetine • Cymbalta
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Imipramine • Tofranil
- Levetiracetam • Keppra
- Lorazepam • Ativan
- Milnacipran • Savella
- Mirtazapine • Remeron
- Nortriptyline • Aventyl, Pamelor
- Olanzapine • Zyprexa
- Paroxetine • Paxil
- Paroxetine CR • Paxil CR
- Phenelzine • Nardil
- Pindolol • Visken
- Quetiapine SR • Seroquel SR
- Risperidone • Risperdal
- Sertraline • Zoloft
- Venlafaxine XR • Effexor XR
Disclosures
Dr. Dunlop receives research support from Bristol-Myers Squibb, GlaxoSmithKline, and the National Institute of Mental Health. He serves as a consultant to MedAvante and Roche.
Ms. Schneider and Dr. Gerardi report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Ms. K, a 24-year-old waitress who lives with her boyfriend, was referred by her primary care physician for evaluation of panic attacks that began “out of nowhere” at work approximately 6 months ago. The unpredictable attacks occur multiple times per week, causing her to leave work and cancel shifts.
Ms. K reports that before the panic attacks began, she felt happy in her relationship, enjoyed hobbies, and was hopeful about the future. However, she has become concerned that a potentially catastrophic illness is causing her panic attacks. She researches her symptoms on the Internet, and is preoccupied with the possibility of sudden death due to an undiagnosed heart condition. Multiple visits to the emergency room have not identified any physical abnormalities. Her primary care doctor prescribed alprazolam, 0.5 mg as needed for panic attacks, which she reports is helpful, “but only in the moment of the attacks.” Ms. K avoids alcohol and illicit substances and limits her caffeine intake. She is not willing to accept that her life “feels so limited.” Her dream of earning a nursing degree and eventually starting a family now seems unattainable.
Panic disorder (PD) occurs in 3% to 5% of adults, with women affected at roughly twice the rate of men.1 Causing a broad range of distress and varying degrees of impairment, PD commonly occurs with other psychiatric disorders. For most patients, treatment is effective, but those who do not respond to initial approaches require a thoughtful, stepped approach to care. Key considerations include establishing an accurate diagnosis, clarifying comorbid illnesses, ascertaining patient beliefs and expectations, and providing appropriately dosed and maintained treatments.
Panic attacks vs PD
Panic attacks consist of rapid onset of intense anxiety, with prominent somatic symptoms, that peaks within 10 minutes (Figure).2 Attacks in which <4 of the listed symptoms occur are considered limited-symptom panic attacks.

Figure: Body locations of panic attack symptoms
Diagnosis of a panic attack requires the sudden development of intense fear or discomfort characterized by ≥4 of the 13 symptoms listed above that peaks in intensity within 10 minutes of onset
Source: Reference 2
Panic attacks can occur with various disorders, including other anxiety disorders, mood disorders, and substance intoxication or withdrawal. Because serious medical conditions can present with panic-like symptoms, the initial occurrence of such symptoms warrants consideration of physiological causes. For a Box2 that describes the differential diagnosis of panic attacks, see this article at CurrentPsychiatry.com.
To meet diagnostic criteria for panic disorder, panic attacks must initially occur “out of the blue,” meaning no specific object or situation induced the attack. The differential diagnosis of panic attacks includes assessing for other psychiatric disorders that may involve panic attacks. Evaluation requires considering the context in which the panic attacks occur, including their start date, pattern of attacks, instigating situations, and associated thoughts.
Social phobia. Attacks occur only during or immediately before a social interaction in which the patient fears embarrassing himself or herself.
Obsessive-compulsive disorder (OCD). Attacks occur when the patient cannot avoid exposure to an obsessional fear or is prevented from performing a ritual that diffuses obsessional anxiety.
Posttraumatic stress disorder (PTSD). Attacks occur when confronted by a trauma-related memory or trigger.
Specific phobia. Attacks occur only when the patient encounters a specifically feared object, place, or situation, unrelated to social phobia, OCD, or PTSD.
Medical conditions. Conditions to consider include—but are not limited to—hyperthyroidism, pulmonary embolism, myocardial infarction, cardiac dysrhythmias, hypoglycemia, asthma, partial complex seizures, and pheochromocytoma.
Source: Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000
A PD diagnosis requires that repeated panic attacks initially must occur from “out of the blue,” meaning no specific object or situation induced the attack. In addition, the diagnosis requires 1 of 3 types of psychological or behavioral changes as a result of the attacks (Table 1).2 Agoraphobia is diagnosed if 1 of the behavioral changes is avoidance of places or situations from which escape might be embarrassing or difficult should an attack occur. A patient can be diagnosed as having PD with agoraphobia, PD without agoraphobia, or agoraphobia without PD (ie, experiences only limited symptom panic attacks, but avoids situations or stimuli associated with them).
Table 1
Definitions of panic disorder and agoraphobia
| Panic disorder |
|---|
|
| Agoraphobia |
| Anxiety about, or avoidance of, being in places or situations from which escape might be difficult or embarrassing, or in which help may not be available in the event of having an unexpected or situationally predisposed panic attack or panic-like symptoms. Agoraphobic fears typically involve characteristic clusters of situations that include being outside the home alone, being in a crowd, standing in a line, being on a bridge, or traveling in a bus, train, or automobile |
| Source: Reference 2 |
Comorbidities are common in patients with PD and predict greater difficulty achieving remission (Box).1,3-6
The most common psychiatric conditions that co-occur with panic disorder (PD) are other anxiety disorders, mood disorders, personality disorders, and substance use disorders.1 Carefully assess the severity and degree of impairment or distress arising from each condition to prioritize treatment goals. For example, treating panic attacks would be a lower priority in a patient with untreated bipolar disorder.
Assessing comorbid substance abuse is important in selecting PD treatments. Benzodiazepines should almost always be avoided in patients with a history of drug abuse—illicit or prescribed. Although complete abstinence should not be a prerequisite for beginning PD treatment, detoxification and concomitant substance abuse treatment are essential.3
Comorbid mood disorders also affect the course of PD treatment. Antidepressants are effective for treating depression and PD, whereas benzodiazepines are not effective for depression.4 Antidepressants in patients with bipolar disorder are controversial because these medications might induce mixed or elevated mood states or rapid cycling. In these complicated patients, consider antidepressants lower in the treatment algorithm.5
Other conditions to consider before beginning treatment include pregnancy or the possibility of becoming pregnant in the near future and suicidal ideation. PD is associated with increased risk for suicidal ideation and progression to suicide attempts, particularly in patients with a comorbid mood or psychotic disorder.6 In addition, consider the potential impact of medications on comorbid medical conditions.
Treatment begins with education
The goal of treatment is remission of symptoms, ideally including an absence of panic attacks, agoraphobic avoidance, and anticipatory anxiety.1 The Panic Disorder Severity Scale self-report is a validated measure of panic symptoms that may be useful in clinical practice.7
The first step in treatment is educating patients about panic attacks, framing them as an overreactive fear circuit in the brain that produces physical symptoms that are not dangerous. Using a brain model that shows the location of the amygdala, hippocampus, and prefrontal cortex—which play crucial roles in generating and controlling anxiety and fear—can make this discussion more concrete.8 Although highly simplified, such models allow clinicians to demonstrate that excessive reactivity of limbic regions can be reduced by both top-down (cortico-limbic connections via cognitive-behavioral therapy [CBT]) and bottom-up (pharmacotherapy directly acting on limbic structures) approaches. Such discussions lead to treatment recommendations for CBT, pharmacotherapy, or their combination.
No single treatment has emerged as the definitive “best” for PD, and no reliable predictors can guide specific treatment for an individual.3 Combining CBT with pharmacotherapy produces higher short-term response rates than either treatment alone, but in the long term, combination treatment does not appear to be superior to CBT alone.9 Base the initial treatment selection for PD on patient preference, treatment availability and cost, and comorbid medical and psychiatric conditions. For an Algorithm to guide treatment decisions, see this article at CurrentPsychiatry.com.

Algorithm: Treatment for panic disorder: A suggested algorithm
aPoor response to an SSRI should lead to a switch to venlafaxine extended-release, and vice versa
bBenzodiazepines are relatively contraindicated in geriatric patients and patients with a history of substance abuse or dependence
CBT: cognitive-behavioral therapy; MAOI: monoamine oxidase inhibitor; SSRI: selective serotonin reuptake inhibitor; TCA: tricyclic antidepressant; Ven XR: venlafaxine extended-release
First-line treatments
Psychotherapy. CBT is the most efficacious psychotherapy for PD. Twelve to 15 sessions of CBT has demonstrated efficacy for PD, with additional effects on comorbid anxiety and depressive symptoms.10 No large clinical trials of CBT have used cognitive restructuring alone; all have included at least some component of exposure that requires the patient to confront feared physical sensations. Gains during treatment may be steady and gradual or sudden and uneven, with rapid improvement in some but not all symptoms. CBT and pharmacotherapy have demonstrated similar levels of benefit in short-term trials, but CBT has proven superior in most9 but not all11 trials evaluating long-term outcomes, particularly compared with pharmacotherapy that is discontinued during follow-up. Although less studied, group CBT also may be considered if a patient cannot afford individual CBT.
Pharmacotherapy. Evidence supports selective serotonin reuptake inhibitors (SSRIs), venlafaxine extended-release (XR), benzodiazepines, and tricyclic antidepressants (TCAs) as effective treatments for PD.3 No class of medication has demonstrated superiority over others in short-term treatment.3,12 Because of the medical risks associated with benzodiazepines and TCAs, an SSRI or venlafaxine XR should be the first medication option for most patients. Fluoxetine, paroxetine, sertraline, and venlafaxine XR are FDA-approved for PD. Paroxetine is associated with weight gain and may increase the risk for panic recurrence upon discontinuation more than sertraline, making it a less favorable option for many patients.13 Start doses at half the normal starting dose used for treating major depressive disorder and continue for 4 to 7 days, then increase to the minimal effective dose. For a Table3 that lists dosing recommendations for antidepressants to treat PD, see this article at CurrentPsychiatry.com. If there is no improvement by 4 weeks, increase the dose every 2 to 4 weeks until remission is achieved or side effects prevent further dose increases.
Table
Recommended doses for antidepressants used to treat panic disorder
| Medication | Starting dose (mg/d) | Therapeutic range (mg/d) |
|---|---|---|
| SSRIs | ||
| Citalopram | 10 | 20 to 40 |
| Escitalopram | 5 | 10 to 40 |
| Fluoxetine | 5 to 10 | 20 to 80 |
| Fluvoxamine | 25 | 100 to 300 |
| Paroxetine | 10 | 20 to 80 |
| Paroxetine CR | 12.5 | 25 to 50 |
| Sertraline | 25 | 100 to 200 |
| SNRIs | ||
| Duloxetine | 20 to 30 | 60 to 120 |
| Venlafaxine XR | 37.5 | 150 to 225 |
| TCAs | ||
| Clomipramine | 10 to 25 | 100 to 300 |
| Imipramine | 10 | 100 to 300 |
| MAOI | ||
| Phenelzine | 15 | 45 to 90 |
| CR: controlled release; MAOI: monoamine oxidase inhibitor; SNRIs: serotonin-norepinephrine reuptake inhibitors; SSRIs: selective serotonin reuptake inhibitors; TCAs: tricyclic antidepressants; XR: extended release Source: American Psychiatric Association. Practice guideline for the treatment of patients with panic disorder. 2nd ed. Washington, DC: American Psychiatric Association; 2009 | ||
Treatment nonresponse. True non-response needs to be distinguished from poor response caused by inadequate treatment delivery, eg, patients not completing homework assignments in CBT or not adhering to pharmacotherapy. Asking patients about adverse effects or personal and family beliefs about treatment may reveal reasons for nonadherence.
Second-line treatments
Little data are available to guide next-step treatment options in patients who don’t achieve remission from their initial treatment. Patients who benefit from an SSRI, venlafaxine XR, or CBT but still have symptoms should be started on combination treatment. For a patient who experiences complete non-response to the initial treatment, discontinue the first treatment and switch to the other modality. In general, completely ineffective treatments should be discontinued when another treatment is added, but when partial improvement (>30%) occurs, continue the original treatment and augment it with another approach.
For patients pursuing pharmacotherapy, poor response to an adequate SSRI trial usually should lead to a switch to venlafaxine XR, and vice versa. Failure to respond to both of these medication classes should prompt a switch to a benzodiazepine or TCA.
Benzodiazepines are a fast-acting, effective treatment for PD, with efficacy similar to SSRIs in acute and long-term treatment.14 Benzodiazepines may be prescribed with antidepressants at the beginning of treatment to improve response speed.15 Clonazepam and alprazolam are FDA-approved for treating PD. A high-potency, long-acting agent, clonazepam is the preferred initial benzodiazepine, dosed 0.5 to 4 mg/d on a fixed schedule. Although substantial data support using alprazolam for PD, it requires more frequent dosing and has a greater risk of rebound anxiety and abuse potential because of its more rapid onset of action. Compared with immediate-release alprazolam, alprazolam XR has a slower absorption rate and longer steady state in the blood, but this formulation does not have lower abuse potential or greater efficacy. Although not FDA-approved for PD, diazepam and lorazepam also have proven efficacy for PD.3
Benzodiazepines should be considered contraindicated in patients with a history of substance abuse, except in select cases.4 Benzodiazepines generally should be avoided in older patients because of increased risk for falls, cognitive impairment, and motor vehicle accidents. Table 2 lists situations in which benzodiazepines may be used to treat PD.
Table 2
Clinical scenarios in which to consider using benzodiazepines
| Coadministration for 2 to 4 weeks when initiating treatment with an SSRI or venlafaxine XR to achieve more rapid relief and mitigate potential antidepressant-induced anxiety |
| For patients who wish to avoid antidepressants because of concern about sexual dysfunction |
| For patients who need chronic aspirin or an NSAID, which may increase the risk for upper gastrointestinal bleeding when taken in combination with an SSRI |
| For patients with comorbid bipolar disorder or epilepsy |
| Next-step monotherapy or augmentation in patients who respond poorly to an SSRI, venlafaxine XR, TCA, or CBT |
| CBT: cognitive-behavioral therapy; NSAID: nonsteroidal anti-inflammatory drug; SSRI: selective serotonin reuptake inhibitor; TCA: tricyclic antidepressant; XR: extended release |
TCAs are effective as monotherapy for PD. Most support comes from studies of imipramine or clomipramine.12 Similar to SSRIs and venlafaxine XR, use a low initial dose and gradually increase until the patient remits or side effects prevent further increases. SSRI and TCA combinations rarely are used unless the TCA is a relatively specific norepinephrine reuptake inhibitor (eg, desipramine, nortriptyline). Because TCAs are metabolized via the cytochrome P450 2D6 system and some SSRIs—particularly fluoxetine and paroxetine—strongly inhibit 2D6, combinations of TCAs with these agents may lead to dangerously high plasma TCA levels, placing patients at risk for cardiac dysrhythmias and other side effects.16
Monoamine oxidase inhibitors (MAOIs)—particularly phenelzine—are underused for PD. They have the strongest efficacy data for any class of medications outside the first- and second-line agents and have a unique mechanism of action. In patients who can comply with the dietary and medication limitations, an MAOI generally should be the next step after nonresponse to other treatments.3
Alternative treatments
For patients who do not respond to any of the treatments described above, data from uncontrolled studies support mirtazapine, levetiracetam, and the serotonin-norepinephrine reuptake inhibitors duloxetine and milnacipran as monotherapy for PD.17 Pindolol—a beta blocker and 5-HT1A receptor antagonist—proved superior to placebo as an adjunctive agent to SSRIs in treatment-resistant PD in 1 of 2 trials.17 Minimal evidence supports the atypical antipsychotics risperidone and olanzapine in treatment-resistant PD, although a placebo-controlled trial of quetiapine SR coadministered with SSRIs recently was completed (NCT00619892; results pending). Atypical antipsychotics are best reserved for patients with a primary psychotic disorder or bipolar disorder who experience panic attacks.5
Panic-focused psychodynamic psychotherapy, a 12-week (approximately 24 sessions) form of psychotherapy, has demonstrated superiority vs applied relaxation therapy.18 This treatment could be considered for patients who do not respond to standard first-line treatments, but few community therapists are familiar with this method.
For many patients with PD, complementary and alternative medicine (CAM) approaches are appealing. See this article at CurrentPsychiatry.com for a Box that discusses CAM for PD.
Although no complementary and alternative medicine treatments have strong evidence of efficacy as monotherapy for panic disorder (PD), several have data that suggest benefit with little evidence of risk. These include bibliotherapy, yoga, aerobic exercise, and the dietary supplements kava and inositol.a Exercise as a treatment poses a challenge because it can induce symptoms that the patient fears, such as tachycardia and shortness of breath. In addition to any direct physiologic benefit from aerobic exercise, there is also an exposure component that can be harnessed by gradually increasing the exertion level.
Another approach undergoing extensive evaluation is Internet-provided cognitive-behavioral therapy (CBT). Using guided CBT modules with or without therapist support, Internet-provided CBT provides an option for motivated patients unable to complete in-person CBT because of logistical factors.b A helpful resource that reviews Internet self-help and psychotherapy guided programs for PD and other psychiatric conditions is http://beacon.anu.edu.au.
References
a. Antonacci DJ, Davis E, Bloch RM, et al. CAM for your anxious patient: what the evidence says. Current Psychiatry. 2010;9(10):42-52.
b. Johnston L, Titov N, Andrews G, et al. A RCT of a transdiagnostic internet-delivered treatment for three anxiety disorders: examination of support roles and disorder-specific outcomes. PLoS One. 2011;6(11):e28079.
Maintenance treatment
Patients who complete a course of CBT for PD often follow up with several “booster sessions” at monthly or longer intervals that focus on relapse prevention techniques. Few controlled trials have evaluated pharmacotherapy discontinuation in PD. Most guidelines recommend continuing treatment for ≥1 year after achieving remission to minimize the risk of relapse.3 Researchers are focusing on whether medication dosage can be reduced during maintenance without loss of efficacy.
Treatment discontinuation
In the absence of urgent medical need, taper medications for PD gradually over several months. PD patients are highly sensitive to unusual physical sensations, which can occur while discontinuing antidepressants or benzodiazepines. If a benzodiazepine is used in conjunction with an antidepressant, the benzodiazepine should be discontinued first, so that the antidepressant can help ease benzodiazepine-associated discontinuation symptoms. A brief course of CBT during pharmacotherapy discontinuation may increase the likelihood of successful tapering.19
CASE CONTINUED: A successful switch
Ms. K has to discontinue sequential trials of fluoxetine, 40 mg/d, and venlafaxine XR, 225 mg/d because of side effects, and she does not reduce the frequency of her alprazolam use. She agrees to switch from alprazolam to clonazepam, 0.5 mg every morning and 1 mg at bedtime, and to start CBT. Clonazepam reduces her anxiety sufficiently so she can address her symptoms in therapy. Through CBT she becomes motivated to monitor her thoughts and treat them as guesses rather than facts, reviewing the evidence for her thoughts and generating rational responses. She participates in exposure exercises, which she practices between sessions, and grows to tolerate uncomfortable sensations until they no longer signal danger. After 12 CBT sessions, she is panic-free. Despite some trepidation, she agrees to a slow taper off clonazepam, reducing the dose by 0.25 mg every 2 weeks. She continues booster sessions with her therapist to manage any re-emerging anxiety. After an additional 12 weeks, she successfully discontinues clonazepam and remains panic-free.
Related Resources
- American Psychiatric Association. Panic disorder. http://healthyminds.org/Main-Topic/Panic-Disorder.aspx.
- Anxiety and Depression Association of America. Panic disorder & agoraphobia. http://adaa.org/understanding-anxiety/panic-disorder-agoraphobia.
- Mayo Clinic. Panic attacks and panic disorder. www.mayoclinic.com/health/panic-attacks/DS00338.
- National Health Service Self-Help Guides. www.ntw.nhs.uk/pic/selfhelp.
- National Institute of Mental Health. Panic disorder. www.nimh.nih.gov/health/topics/panic-disorder/index.shtml.
Drug Brand Names
- Alprazolam • Xanax
- Alprazolam XR • Xanax XR
- Citalopram • Celexa
- Clomipramine • Anafranil
- Clonazepam • Klonopin
- Desipramine • Norpramin
- Diazepam • Valium
- Duloxetine • Cymbalta
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Imipramine • Tofranil
- Levetiracetam • Keppra
- Lorazepam • Ativan
- Milnacipran • Savella
- Mirtazapine • Remeron
- Nortriptyline • Aventyl, Pamelor
- Olanzapine • Zyprexa
- Paroxetine • Paxil
- Paroxetine CR • Paxil CR
- Phenelzine • Nardil
- Pindolol • Visken
- Quetiapine SR • Seroquel SR
- Risperidone • Risperdal
- Sertraline • Zoloft
- Venlafaxine XR • Effexor XR
Disclosures
Dr. Dunlop receives research support from Bristol-Myers Squibb, GlaxoSmithKline, and the National Institute of Mental Health. He serves as a consultant to MedAvante and Roche.
Ms. Schneider and Dr. Gerardi report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Roy-Byrne PP, Craske MG, Stein MB. Panic disorder. Lancet. 2006;368(9540):1023-1032.
2. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
3. American Psychiatric Association. Practice guideline for the treatment of patients with panic disorder. 2nd ed. Washington DC: American Psychiatric Association; 2009.
4. Dunlop BW, Davis PG. Combination treatment with benzodiazepines and SSRIs for comorbid anxiety and depression: a review. Prim Care Companion J Clin Psychiatry. 2008;10(3):222-228.
5. Rakofsky JJ, Dunlop BW. Treating nonspecific anxiety and anxiety disorders in patients with bipolar disorder: a review. J Clin Psychiatry. 2011;72(1):81-90.
6. Sareen J, Cox BJ, Afifi TO, et al. Anxiety disorders and risk for suicidal ideation and suicide attempts: a population-based longitudinal study of adults. Arch Gen Psychiatry. 2005;62(11):1249-1257.
7. Houck PR, Spiegel DA, Shear MK, et al. Reliability of the self-report version of the panic disorder severity scale. Depress Anxiety. 2002;15(4):183-185.
8. Ninan PT, Dunlop BW. Neurobiology and etiology of panic disorder. J Clin Psychiatry. 2005;66(suppl 4):3-7.
9. Furukawa TA, Watanabe N, Churchill R. Psychotherapy plus antidepressant for panic disorder with or without agoraphobia: systematic review. Br J Psychiatry. 2006;188:305-312.
10. Barlow DH, Gorman JM, Shear MK, et al. Cognitive-behavioral therapy, imipramine, or their combination for panic disorder: a randomized controlled trial. JAMA. 2000;283(19):2529-2536.
11. van Apeldoorn FJ, Timmerman ME, Mersch PP, et al. A randomized trial of cognitive-behavioral therapy or selective serotonin reuptake inhibitor or both combined for panic disorder with or without agoraphobia: treatment results through 1-year follow-up. J Clin Psychiatry. 2010;71(5):574-586.
12. Bakker A, van Balkom AJ, Spinhoven P. SSRIs vs. TCAs in the treatment of panic disorder: a meta-analysis. Acta Psychiatr Scand. 2002;106(3):163-167.
13. Bandelow B, Behnke K, Lenoir S, et al. Sertraline versus paroxetine in the treatment of panic disorder: an acute, double-blind noninferiority comparison. J Clin Psychiatry. 2004;65(3):405-413.
14. Nardi AE, Freire RC, Mochcovitch MD, et al. A randomized, naturalistic, parallel-group study for the long-term treatment of panic disorder with clonazepam or paroxetine. J Clin Psychopharmacol. 2012;32(1):120-126.
15. Goddard AW, Brouette T, Almai A, et al. Early coadministration of clonazepam with sertraline for panic disorder. Arch Gen Psychiatry. 2001;58(7):681-686.
16. Preskorn SH, Shah R, Neff M, et al. The potential for clinically significant drug-drug interactions involving the CYP 2D6 system: effects with fluoxetine and paroxetine versus sertraline. J Psychiatr Pract. 2007;13(1):5-12.
17. Perna G, Guerriero G, Caldirola D. Emerging drugs for panic disorder. Expert Opin Emerg Drugs. 2011;16(4):631-645.
18. Milrod B, Leon AC, Busch F, et al. A randomized controlled clinical trial of psychoanalytic psychotherapy for panic disorder. Am J Psychiatry. 2007;164(2):265-272.
19. Otto MW, Pollack MH, Sachs GS, et al. Discontinuation of benzodiazepine treatment: efficacy of cognitive-behavioral therapy for patients with panic disorder. Am J Psychiatry. 1993;150(10):1485-1490.
1. Roy-Byrne PP, Craske MG, Stein MB. Panic disorder. Lancet. 2006;368(9540):1023-1032.
2. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
3. American Psychiatric Association. Practice guideline for the treatment of patients with panic disorder. 2nd ed. Washington DC: American Psychiatric Association; 2009.
4. Dunlop BW, Davis PG. Combination treatment with benzodiazepines and SSRIs for comorbid anxiety and depression: a review. Prim Care Companion J Clin Psychiatry. 2008;10(3):222-228.
5. Rakofsky JJ, Dunlop BW. Treating nonspecific anxiety and anxiety disorders in patients with bipolar disorder: a review. J Clin Psychiatry. 2011;72(1):81-90.
6. Sareen J, Cox BJ, Afifi TO, et al. Anxiety disorders and risk for suicidal ideation and suicide attempts: a population-based longitudinal study of adults. Arch Gen Psychiatry. 2005;62(11):1249-1257.
7. Houck PR, Spiegel DA, Shear MK, et al. Reliability of the self-report version of the panic disorder severity scale. Depress Anxiety. 2002;15(4):183-185.
8. Ninan PT, Dunlop BW. Neurobiology and etiology of panic disorder. J Clin Psychiatry. 2005;66(suppl 4):3-7.
9. Furukawa TA, Watanabe N, Churchill R. Psychotherapy plus antidepressant for panic disorder with or without agoraphobia: systematic review. Br J Psychiatry. 2006;188:305-312.
10. Barlow DH, Gorman JM, Shear MK, et al. Cognitive-behavioral therapy, imipramine, or their combination for panic disorder: a randomized controlled trial. JAMA. 2000;283(19):2529-2536.
11. van Apeldoorn FJ, Timmerman ME, Mersch PP, et al. A randomized trial of cognitive-behavioral therapy or selective serotonin reuptake inhibitor or both combined for panic disorder with or without agoraphobia: treatment results through 1-year follow-up. J Clin Psychiatry. 2010;71(5):574-586.
12. Bakker A, van Balkom AJ, Spinhoven P. SSRIs vs. TCAs in the treatment of panic disorder: a meta-analysis. Acta Psychiatr Scand. 2002;106(3):163-167.
13. Bandelow B, Behnke K, Lenoir S, et al. Sertraline versus paroxetine in the treatment of panic disorder: an acute, double-blind noninferiority comparison. J Clin Psychiatry. 2004;65(3):405-413.
14. Nardi AE, Freire RC, Mochcovitch MD, et al. A randomized, naturalistic, parallel-group study for the long-term treatment of panic disorder with clonazepam or paroxetine. J Clin Psychopharmacol. 2012;32(1):120-126.
15. Goddard AW, Brouette T, Almai A, et al. Early coadministration of clonazepam with sertraline for panic disorder. Arch Gen Psychiatry. 2001;58(7):681-686.
16. Preskorn SH, Shah R, Neff M, et al. The potential for clinically significant drug-drug interactions involving the CYP 2D6 system: effects with fluoxetine and paroxetine versus sertraline. J Psychiatr Pract. 2007;13(1):5-12.
17. Perna G, Guerriero G, Caldirola D. Emerging drugs for panic disorder. Expert Opin Emerg Drugs. 2011;16(4):631-645.
18. Milrod B, Leon AC, Busch F, et al. A randomized controlled clinical trial of psychoanalytic psychotherapy for panic disorder. Am J Psychiatry. 2007;164(2):265-272.
19. Otto MW, Pollack MH, Sachs GS, et al. Discontinuation of benzodiazepine treatment: efficacy of cognitive-behavioral therapy for patients with panic disorder. Am J Psychiatry. 1993;150(10):1485-1490.
Epilepsy or something else?
CASE: Seizure-like symptoms
Ms. T, age 20, is brought to the emergency room (ER) by her father because she refuses to eat and drink, is unable to function at home, lies in bed all day, and does not attend to her activities of daily living (ADLs). Ms. T lives with her family, is not enrolled in school, and is unemployed. In the ER she initially is uncooperative and mute and then suddenly becomes agitated and has a seizure-like episode characterized by jerking of her trunk followed by random, asymmetrical movements of her legs and arms, closing both eyes, weeping, foaming at the mouth, moaning, and marked unresponsiveness. The episode lasts for >5 minutes.
The authors’ observations
Based on Ms. T’s presentation, the medical team considered acute epileptic seizures. Asymmetrical jerking of the body may be seen in frontal lobe epilepsy or seizures of the supplementary sensorimotor area. Frontal lobe epilepsy can present with bilateral asynchronous motor activity with consciousness during the event and a lack of postictal confusion.1 Seizures of the supplementary sensorimotor area—also known as the secondary motor area—are particularly problematic because typically they present with bilateral asymmetric tonic posturing followed by a few clonic movements, intact consciousness, and rarely postictal confusion. Adding to the diagnostic uncertainty, some “soft signs” thought to indicate PNES (eg, pelvic thrusting, crying) are common with frontal lobe epilepsy.1,2
PNES are episodes of altered movement, sensation, or experience that may be mistaken for epileptic seizures but are not a consequence of abnormal cortical discharges. Instead they are caused by physiological or psychological factors.3 Behaviors or signs that strongly suggest PNES include:
- gradual onset or termination
- pseudosleep, when the patient appears to be asleep but electroencephalography (EEG) findings indicate he or she is awake
- discontinuous (stop-and-go), irregular, or asynchronous (out-of-phase) activity—including side-to-side head movement, pelvic thrusting, and opisthotonic posturing—stuttering, and weeping4
- eye closure.5
Ms. T’s father said his daughter had been hospitalized several times for episodes characterized by pelvic thrusting, stuttering, and pseudosleep, which raised the possibility of PNES. Definitive diagnosis of PNES comes from video EEG when a patient is observed having typical seizures without accompanying EEG abnormalities.6
EVALUATION: Inconclusive data
Ms. T is admitted to the medical unit to rule out a seizure disorder. Physical examination is unremarkable and laboratory tests are within normal limits. The neurology service requests a head MRI, which is inconclusive. Inpatient video EEG with 24-hour monitoring does not indicate acute epileptic seizures. Ms. T’s father says that she has experienced many paroxysmal motor episodes and all neurologic tests, exams, and labs have failed to find a cause for these episodes. She did not receive any antiepileptic medications. A psychiatric consult is requested to clarify the diagnosis. Ms. T is transferred to an inpatient psychiatric unit for further evaluation and management.
The authors’ observations
Fleisher et al7 suggested that traumatic events may lead to presentations similar to PNES. Because Ms. T was molested by a family friend as a child, we considered posttraumatic stress disorder (PTSD) in the differential diagnosis, although she has not reported symptoms of intrusive recollections, avoidance, numbing, or hyperarousal.
We also considered conversion disorder and dissociative disorder. Patients with conversion disorder have ≥1 symptoms or signs that affect voluntary motor or sensory function that cannot be explained by a neurologic or general medical condition.8 Dissociative disorder is a disruption in usually integrated functions of consciousness, memory, identity, or perception of the environment.8 The presentation of patients with PNES may resemble that of patients with dissociative disorder.8 In a study of 45 adult PNES patients, Bowman et al8 found that PNES often are comorbid with other psychiatric disorders, including somatoform disorders (89%), dissociative disorders (91%), affective disorders (64%), personality disorders (62%), PTSD (49%), and other anxiety disorders (47%).
TREATMENT: Managing aggression
In the psychiatric unit, Ms. T initially is irritable and disorganized with poor oral intake and regressed behavior; she often is found in the fetal position, crying and talking in a childish manner. Throughout her admission, she receives several anxiolytics and antipsychotics—including lorazepam, up to 6 mg/d, clonazepam, up to 3 mg/d, haloperidol, up to 10 mg/d, and quetiapine, up to 200 mg/d—to help manage her aggressive behaviors after her seizure-like episodes. Further evaluation reveals that Ms. T has no psychotic symptoms, overt delusions, or perceptual disturbances and her thought process is coherent and clear. She has no history of substance abuse. Her ability to perform ADLs improves within a few days. She complains of depressed mood and engages in head banging, which requires close observation.
Ms. T has a history of mood and behavioral problems since early childhood characterized by episodic dysphoric mood, anxiety, and agitation. She has had trials of several antidepressants, including sertraline, fluoxetine, venlafaxine, and escitalopram, and anxiolytics, including lorazepam, clonazepam, and alprazolam. Her outpatient psychiatrist describes a history of physical and sexual abuse starting at age 7. At age 9, after her mother died from breast cancer, Ms. T and her siblings were moved to foster care, where she was physically abused by the staff. She remained in foster care until age 18.
The authors’ observations
PNES pose a diagnostic and therapeutic challenge. Many PNES patients seek medical attention for their seizures. PNES patients misdiagnosed as having epilepsy have a worse prognosis because they do not receive appropriate treatment9 and may experience side effects if antiepileptics are prescribed.10 Finally, the financial burden of medical care can be significant. Ms. T had several hospitalizations, including extensive neurologic workup, intensive care unit admissions for intubation, and use of antiepileptics with almost no benefit.
Psychosocial assessments of PNES patients have revealed that sexual abuse, family conflicts, and death of a family member often play an important role.11 It is possible that as a result of childhood trauma, Ms. T exhibited a regressed and primitive defense mechanism to deal with the trauma. PNES usually are considered when a patient presents with:
- absence of therapeutic response to antiepileptics
- loss of response (therapeutic failure) to antiepileptics
- paradoxical response to antiepileptics (worsening or unexpected responses)
- atypical, multiple, or inconsistent seizures
- seizures that occur soon after emotional stress.12
We concluded Ms. T had PNES because of the unusual presentations of her seizures, negative video EEG findings, failure to respond to antiepileptics, lack of risk factors for epilepsy, and aggressive behaviors before or after the seizures ( Table ).4,10,11,13 Diagnosing PNES early allows clinicians to focus on appropriate treatment modalities (eg, psychotherapy, antidepressants), prevents costly neurologic workups and treatments (eg, routine EEGs, trials of several antiepileptics), and provides patients with diagnostic assurance.10
Table
Characteristics of psychogenic nonepileptic seizures
| Characteristic | Comment |
|---|---|
| Duration | May be prolonged |
| Timing | Usually occur only during the day |
| Physical harm | Rare |
| Tongue biting | Rare |
| Urinary incontinence | Rare |
| Motor activity | Prolonged |
| Cyanosis | No |
| Postictal confusion | Rare |
| Related to medication changes | No |
| Interictal EEG | Normal |
| Ictal EEG | Normal |
| Presence of secondary gain | Common |
| EEG: electroencephalography Source: References 4,10,11,13 | |
3 components of treatment
Presenting the PNES diagnosis to the patient. The neurologist and the psychiatrist should convey to the patient that they see the symptoms as “real” and not “all in your head.”14
Withdrawing antiepileptic medications. Antiepileptic medication withdrawal is recommended when a thorough diagnostic workup shows no evidence of epileptic seizures.15 Oto et al16 reported 49% of PNES patients became seizure-free 12 months after discontinuing antiepileptics.
Psychotherapy and pharmacotherapy. Open-label studies of psychological treatments for PNES have demonstrated that a cognitive-behavioral therapy-based approach and brief augmented psychodynamic interpersonal therapy could reduce seizures.17 In a pilot, randomized, placebo-controlled trial, PNES patients who received flexibly dosed sertraline reported a 45% reduction in seizures compared with an 8% increase in the placebo group.18 Similar improvements in seizure frequency have been reported in PNES patients with anxiety or depression treated with venlafaxine.19
OUTCOME: Support, improvement
During the next several days, Ms. T has random episodes of seizures with foaming of the mouth and unresponsiveness. These episodes last from 5 to 30 minutes and require transfer to the ER. After each episode, Ms. T is medically cleared and sent back to the psychiatric unit. The neurologist recommends avoiding antiepileptics. Ms. T responds well to the structured inpatient setting and supportive psychotherapy. Her episodes decrease and her mood becomes more stable. She refrains from self-injurious behaviors and is discharged home with outpatient follow-up.
Related Resource
- Marsh P, Benbadis S, Fernandez F. Psychogenic nonepileptic seizures: ways to win over skeptical patients. Current Psychiatry. 2008;7(1):21-35.
Drug Brand Names
- Alprazolam • Xanax
- Clonazepam • Klonopin
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Haloperidol • Haldol
- Lorazepam • Ativan
- Quetiapine • Seroquel
- Sertraline • Zoloft
- Venlafaxine • Effexor
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Kellinghaus C, Lüders HO. Frontal lobe epilepsy. Epileptic Disord. 2004;6(4):223-239.
2. Kanner AM, Morris HH, Lüders H, et al. Supplementary motor seizures mimicking pseudoseizures: some clinical differences. Neurology. 1990;40(9):1404-1407.
3. Hall-Patch L, Brown R, House A, et al. Acceptability and effectiveness of a strategy for the communication of the diagnosis of psychogenic nonepileptic seizures. Epilepsia. 2010;51(1):70-78.
4. Reuber M, Elger CE. Psychogenic nonepileptic seizures: review and update. Epilepsy Behav. 2003;4(3):205-216.
5. Chung SS, Gerber P, Kirlin KA. Ictal eye closure is a reliable indicator for psychogenic nonepileptic seizures. Neurology. 2006;66(11):1730-1731.
6. Mostacci B, Bisulli F, Alvisi L, et al. Ictal characteristics of psychogenic nonepileptic seizures: what we have learned from video/EEG recordings—a literature review. Epilepsy Behav. 2011;22(2):144-153.
7. Fleisher W, Staley D, Krawetz P, et al. Comparative study of trauma-related phenomena in subjects with pseudoseizures and subjects with epilepsy. Am J Psychiatry. 2002;159(4):660-663.
8. Bowman ES, Markand ON. Psychodynamics and psychiatric diagnoses of pseudoseizure subjects. Am J Psychiatry. 1996;153(1):57-63.
9. Benbadis SR. The EEG in nonepileptic seizures. J Clin Neurophysiol. 2006;23(4):340-352.
10. Brown RJ, Syed TU, Benbadis S, et al. Psychogenic nonepileptic seizures. Epilepsy Behav. 2011;22(1):85-93.
11. Bodde NM, Brooks JL, Baker GA, et al. Psychogenic non-epileptic seizures—definition, etiology, treatment and prognostic issues: a critical review. Seizure. 2009;18(8):543-553.
12. Alsaadi TM, Marquez AV. Psychogenic nonepileptic seizures. Am Fam Physician. 2005;72(5):849-856.
13. Bradley WG, Daroff RB, Fenichel GM, et al. eds. Neurology in clinical practice: principles of diagnosis and management. 4th ed. Philadelphia, PA: Butterworth Heinemann; 2004:19-20, 1971–1972.
14. Harden CL, Ferrando SJ. Delivering the diagnosis of psychogenic pseudoseizures: should the neurologist or the psychiatrist be responsible? Epilepsy Behav. 2001;2(6):519-523.
15. Oto M, Espie CA, Duncan R. An exploratory randomized controlled trial of immediate versus delayed withdrawal of antiepileptic drugs in patients with psychogenic nonepileptic attacks (PNEAs). Epilepsia. 2010;51(10):1994-1999.
16. Oto M, Espie C, Pelosi A, et al. The safety of antiepileptic drug withdrawal in patients with non-epileptic seizures. J Neurol Neurosurg Psychiatry. 2005;76(12):1682-1685.
17. Goldstein LH, Mellers JD. Recent developments in our understanding of the semiology and treatment of psychogenic nonepileptic seizures. Curr Neurol Neurosci Rep. 2012;12(4):436-444.
18. LaFrance WC, Jr, Keitner GI, Papandonatos GD, et al. Pilot pharmacologic randomized controlled trial for psychogenic nonepileptic seizures. Neurology. 2010;75(13):1166-1173.
19. Pintor L, Baillés E, Matrai S, et al. Efficiency of venlafaxine in patients with psychogenic nonepileptic seizures and anxiety and/or depressive disorders. J Neuropsychiatry Clin Neurosci. 2010;22(4):401-408.
CASE: Seizure-like symptoms
Ms. T, age 20, is brought to the emergency room (ER) by her father because she refuses to eat and drink, is unable to function at home, lies in bed all day, and does not attend to her activities of daily living (ADLs). Ms. T lives with her family, is not enrolled in school, and is unemployed. In the ER she initially is uncooperative and mute and then suddenly becomes agitated and has a seizure-like episode characterized by jerking of her trunk followed by random, asymmetrical movements of her legs and arms, closing both eyes, weeping, foaming at the mouth, moaning, and marked unresponsiveness. The episode lasts for >5 minutes.
The authors’ observations
Based on Ms. T’s presentation, the medical team considered acute epileptic seizures. Asymmetrical jerking of the body may be seen in frontal lobe epilepsy or seizures of the supplementary sensorimotor area. Frontal lobe epilepsy can present with bilateral asynchronous motor activity with consciousness during the event and a lack of postictal confusion.1 Seizures of the supplementary sensorimotor area—also known as the secondary motor area—are particularly problematic because typically they present with bilateral asymmetric tonic posturing followed by a few clonic movements, intact consciousness, and rarely postictal confusion. Adding to the diagnostic uncertainty, some “soft signs” thought to indicate PNES (eg, pelvic thrusting, crying) are common with frontal lobe epilepsy.1,2
PNES are episodes of altered movement, sensation, or experience that may be mistaken for epileptic seizures but are not a consequence of abnormal cortical discharges. Instead they are caused by physiological or psychological factors.3 Behaviors or signs that strongly suggest PNES include:
- gradual onset or termination
- pseudosleep, when the patient appears to be asleep but electroencephalography (EEG) findings indicate he or she is awake
- discontinuous (stop-and-go), irregular, or asynchronous (out-of-phase) activity—including side-to-side head movement, pelvic thrusting, and opisthotonic posturing—stuttering, and weeping4
- eye closure.5
Ms. T’s father said his daughter had been hospitalized several times for episodes characterized by pelvic thrusting, stuttering, and pseudosleep, which raised the possibility of PNES. Definitive diagnosis of PNES comes from video EEG when a patient is observed having typical seizures without accompanying EEG abnormalities.6
EVALUATION: Inconclusive data
Ms. T is admitted to the medical unit to rule out a seizure disorder. Physical examination is unremarkable and laboratory tests are within normal limits. The neurology service requests a head MRI, which is inconclusive. Inpatient video EEG with 24-hour monitoring does not indicate acute epileptic seizures. Ms. T’s father says that she has experienced many paroxysmal motor episodes and all neurologic tests, exams, and labs have failed to find a cause for these episodes. She did not receive any antiepileptic medications. A psychiatric consult is requested to clarify the diagnosis. Ms. T is transferred to an inpatient psychiatric unit for further evaluation and management.
The authors’ observations
Fleisher et al7 suggested that traumatic events may lead to presentations similar to PNES. Because Ms. T was molested by a family friend as a child, we considered posttraumatic stress disorder (PTSD) in the differential diagnosis, although she has not reported symptoms of intrusive recollections, avoidance, numbing, or hyperarousal.
We also considered conversion disorder and dissociative disorder. Patients with conversion disorder have ≥1 symptoms or signs that affect voluntary motor or sensory function that cannot be explained by a neurologic or general medical condition.8 Dissociative disorder is a disruption in usually integrated functions of consciousness, memory, identity, or perception of the environment.8 The presentation of patients with PNES may resemble that of patients with dissociative disorder.8 In a study of 45 adult PNES patients, Bowman et al8 found that PNES often are comorbid with other psychiatric disorders, including somatoform disorders (89%), dissociative disorders (91%), affective disorders (64%), personality disorders (62%), PTSD (49%), and other anxiety disorders (47%).
TREATMENT: Managing aggression
In the psychiatric unit, Ms. T initially is irritable and disorganized with poor oral intake and regressed behavior; she often is found in the fetal position, crying and talking in a childish manner. Throughout her admission, she receives several anxiolytics and antipsychotics—including lorazepam, up to 6 mg/d, clonazepam, up to 3 mg/d, haloperidol, up to 10 mg/d, and quetiapine, up to 200 mg/d—to help manage her aggressive behaviors after her seizure-like episodes. Further evaluation reveals that Ms. T has no psychotic symptoms, overt delusions, or perceptual disturbances and her thought process is coherent and clear. She has no history of substance abuse. Her ability to perform ADLs improves within a few days. She complains of depressed mood and engages in head banging, which requires close observation.
Ms. T has a history of mood and behavioral problems since early childhood characterized by episodic dysphoric mood, anxiety, and agitation. She has had trials of several antidepressants, including sertraline, fluoxetine, venlafaxine, and escitalopram, and anxiolytics, including lorazepam, clonazepam, and alprazolam. Her outpatient psychiatrist describes a history of physical and sexual abuse starting at age 7. At age 9, after her mother died from breast cancer, Ms. T and her siblings were moved to foster care, where she was physically abused by the staff. She remained in foster care until age 18.
The authors’ observations
PNES pose a diagnostic and therapeutic challenge. Many PNES patients seek medical attention for their seizures. PNES patients misdiagnosed as having epilepsy have a worse prognosis because they do not receive appropriate treatment9 and may experience side effects if antiepileptics are prescribed.10 Finally, the financial burden of medical care can be significant. Ms. T had several hospitalizations, including extensive neurologic workup, intensive care unit admissions for intubation, and use of antiepileptics with almost no benefit.
Psychosocial assessments of PNES patients have revealed that sexual abuse, family conflicts, and death of a family member often play an important role.11 It is possible that as a result of childhood trauma, Ms. T exhibited a regressed and primitive defense mechanism to deal with the trauma. PNES usually are considered when a patient presents with:
- absence of therapeutic response to antiepileptics
- loss of response (therapeutic failure) to antiepileptics
- paradoxical response to antiepileptics (worsening or unexpected responses)
- atypical, multiple, or inconsistent seizures
- seizures that occur soon after emotional stress.12
We concluded Ms. T had PNES because of the unusual presentations of her seizures, negative video EEG findings, failure to respond to antiepileptics, lack of risk factors for epilepsy, and aggressive behaviors before or after the seizures ( Table ).4,10,11,13 Diagnosing PNES early allows clinicians to focus on appropriate treatment modalities (eg, psychotherapy, antidepressants), prevents costly neurologic workups and treatments (eg, routine EEGs, trials of several antiepileptics), and provides patients with diagnostic assurance.10
Table
Characteristics of psychogenic nonepileptic seizures
| Characteristic | Comment |
|---|---|
| Duration | May be prolonged |
| Timing | Usually occur only during the day |
| Physical harm | Rare |
| Tongue biting | Rare |
| Urinary incontinence | Rare |
| Motor activity | Prolonged |
| Cyanosis | No |
| Postictal confusion | Rare |
| Related to medication changes | No |
| Interictal EEG | Normal |
| Ictal EEG | Normal |
| Presence of secondary gain | Common |
| EEG: electroencephalography Source: References 4,10,11,13 | |
3 components of treatment
Presenting the PNES diagnosis to the patient. The neurologist and the psychiatrist should convey to the patient that they see the symptoms as “real” and not “all in your head.”14
Withdrawing antiepileptic medications. Antiepileptic medication withdrawal is recommended when a thorough diagnostic workup shows no evidence of epileptic seizures.15 Oto et al16 reported 49% of PNES patients became seizure-free 12 months after discontinuing antiepileptics.
Psychotherapy and pharmacotherapy. Open-label studies of psychological treatments for PNES have demonstrated that a cognitive-behavioral therapy-based approach and brief augmented psychodynamic interpersonal therapy could reduce seizures.17 In a pilot, randomized, placebo-controlled trial, PNES patients who received flexibly dosed sertraline reported a 45% reduction in seizures compared with an 8% increase in the placebo group.18 Similar improvements in seizure frequency have been reported in PNES patients with anxiety or depression treated with venlafaxine.19
OUTCOME: Support, improvement
During the next several days, Ms. T has random episodes of seizures with foaming of the mouth and unresponsiveness. These episodes last from 5 to 30 minutes and require transfer to the ER. After each episode, Ms. T is medically cleared and sent back to the psychiatric unit. The neurologist recommends avoiding antiepileptics. Ms. T responds well to the structured inpatient setting and supportive psychotherapy. Her episodes decrease and her mood becomes more stable. She refrains from self-injurious behaviors and is discharged home with outpatient follow-up.
Related Resource
- Marsh P, Benbadis S, Fernandez F. Psychogenic nonepileptic seizures: ways to win over skeptical patients. Current Psychiatry. 2008;7(1):21-35.
Drug Brand Names
- Alprazolam • Xanax
- Clonazepam • Klonopin
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Haloperidol • Haldol
- Lorazepam • Ativan
- Quetiapine • Seroquel
- Sertraline • Zoloft
- Venlafaxine • Effexor
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE: Seizure-like symptoms
Ms. T, age 20, is brought to the emergency room (ER) by her father because she refuses to eat and drink, is unable to function at home, lies in bed all day, and does not attend to her activities of daily living (ADLs). Ms. T lives with her family, is not enrolled in school, and is unemployed. In the ER she initially is uncooperative and mute and then suddenly becomes agitated and has a seizure-like episode characterized by jerking of her trunk followed by random, asymmetrical movements of her legs and arms, closing both eyes, weeping, foaming at the mouth, moaning, and marked unresponsiveness. The episode lasts for >5 minutes.
The authors’ observations
Based on Ms. T’s presentation, the medical team considered acute epileptic seizures. Asymmetrical jerking of the body may be seen in frontal lobe epilepsy or seizures of the supplementary sensorimotor area. Frontal lobe epilepsy can present with bilateral asynchronous motor activity with consciousness during the event and a lack of postictal confusion.1 Seizures of the supplementary sensorimotor area—also known as the secondary motor area—are particularly problematic because typically they present with bilateral asymmetric tonic posturing followed by a few clonic movements, intact consciousness, and rarely postictal confusion. Adding to the diagnostic uncertainty, some “soft signs” thought to indicate PNES (eg, pelvic thrusting, crying) are common with frontal lobe epilepsy.1,2
PNES are episodes of altered movement, sensation, or experience that may be mistaken for epileptic seizures but are not a consequence of abnormal cortical discharges. Instead they are caused by physiological or psychological factors.3 Behaviors or signs that strongly suggest PNES include:
- gradual onset or termination
- pseudosleep, when the patient appears to be asleep but electroencephalography (EEG) findings indicate he or she is awake
- discontinuous (stop-and-go), irregular, or asynchronous (out-of-phase) activity—including side-to-side head movement, pelvic thrusting, and opisthotonic posturing—stuttering, and weeping4
- eye closure.5
Ms. T’s father said his daughter had been hospitalized several times for episodes characterized by pelvic thrusting, stuttering, and pseudosleep, which raised the possibility of PNES. Definitive diagnosis of PNES comes from video EEG when a patient is observed having typical seizures without accompanying EEG abnormalities.6
EVALUATION: Inconclusive data
Ms. T is admitted to the medical unit to rule out a seizure disorder. Physical examination is unremarkable and laboratory tests are within normal limits. The neurology service requests a head MRI, which is inconclusive. Inpatient video EEG with 24-hour monitoring does not indicate acute epileptic seizures. Ms. T’s father says that she has experienced many paroxysmal motor episodes and all neurologic tests, exams, and labs have failed to find a cause for these episodes. She did not receive any antiepileptic medications. A psychiatric consult is requested to clarify the diagnosis. Ms. T is transferred to an inpatient psychiatric unit for further evaluation and management.
The authors’ observations
Fleisher et al7 suggested that traumatic events may lead to presentations similar to PNES. Because Ms. T was molested by a family friend as a child, we considered posttraumatic stress disorder (PTSD) in the differential diagnosis, although she has not reported symptoms of intrusive recollections, avoidance, numbing, or hyperarousal.
We also considered conversion disorder and dissociative disorder. Patients with conversion disorder have ≥1 symptoms or signs that affect voluntary motor or sensory function that cannot be explained by a neurologic or general medical condition.8 Dissociative disorder is a disruption in usually integrated functions of consciousness, memory, identity, or perception of the environment.8 The presentation of patients with PNES may resemble that of patients with dissociative disorder.8 In a study of 45 adult PNES patients, Bowman et al8 found that PNES often are comorbid with other psychiatric disorders, including somatoform disorders (89%), dissociative disorders (91%), affective disorders (64%), personality disorders (62%), PTSD (49%), and other anxiety disorders (47%).
TREATMENT: Managing aggression
In the psychiatric unit, Ms. T initially is irritable and disorganized with poor oral intake and regressed behavior; she often is found in the fetal position, crying and talking in a childish manner. Throughout her admission, she receives several anxiolytics and antipsychotics—including lorazepam, up to 6 mg/d, clonazepam, up to 3 mg/d, haloperidol, up to 10 mg/d, and quetiapine, up to 200 mg/d—to help manage her aggressive behaviors after her seizure-like episodes. Further evaluation reveals that Ms. T has no psychotic symptoms, overt delusions, or perceptual disturbances and her thought process is coherent and clear. She has no history of substance abuse. Her ability to perform ADLs improves within a few days. She complains of depressed mood and engages in head banging, which requires close observation.
Ms. T has a history of mood and behavioral problems since early childhood characterized by episodic dysphoric mood, anxiety, and agitation. She has had trials of several antidepressants, including sertraline, fluoxetine, venlafaxine, and escitalopram, and anxiolytics, including lorazepam, clonazepam, and alprazolam. Her outpatient psychiatrist describes a history of physical and sexual abuse starting at age 7. At age 9, after her mother died from breast cancer, Ms. T and her siblings were moved to foster care, where she was physically abused by the staff. She remained in foster care until age 18.
The authors’ observations
PNES pose a diagnostic and therapeutic challenge. Many PNES patients seek medical attention for their seizures. PNES patients misdiagnosed as having epilepsy have a worse prognosis because they do not receive appropriate treatment9 and may experience side effects if antiepileptics are prescribed.10 Finally, the financial burden of medical care can be significant. Ms. T had several hospitalizations, including extensive neurologic workup, intensive care unit admissions for intubation, and use of antiepileptics with almost no benefit.
Psychosocial assessments of PNES patients have revealed that sexual abuse, family conflicts, and death of a family member often play an important role.11 It is possible that as a result of childhood trauma, Ms. T exhibited a regressed and primitive defense mechanism to deal with the trauma. PNES usually are considered when a patient presents with:
- absence of therapeutic response to antiepileptics
- loss of response (therapeutic failure) to antiepileptics
- paradoxical response to antiepileptics (worsening or unexpected responses)
- atypical, multiple, or inconsistent seizures
- seizures that occur soon after emotional stress.12
We concluded Ms. T had PNES because of the unusual presentations of her seizures, negative video EEG findings, failure to respond to antiepileptics, lack of risk factors for epilepsy, and aggressive behaviors before or after the seizures ( Table ).4,10,11,13 Diagnosing PNES early allows clinicians to focus on appropriate treatment modalities (eg, psychotherapy, antidepressants), prevents costly neurologic workups and treatments (eg, routine EEGs, trials of several antiepileptics), and provides patients with diagnostic assurance.10
Table
Characteristics of psychogenic nonepileptic seizures
| Characteristic | Comment |
|---|---|
| Duration | May be prolonged |
| Timing | Usually occur only during the day |
| Physical harm | Rare |
| Tongue biting | Rare |
| Urinary incontinence | Rare |
| Motor activity | Prolonged |
| Cyanosis | No |
| Postictal confusion | Rare |
| Related to medication changes | No |
| Interictal EEG | Normal |
| Ictal EEG | Normal |
| Presence of secondary gain | Common |
| EEG: electroencephalography Source: References 4,10,11,13 | |
3 components of treatment
Presenting the PNES diagnosis to the patient. The neurologist and the psychiatrist should convey to the patient that they see the symptoms as “real” and not “all in your head.”14
Withdrawing antiepileptic medications. Antiepileptic medication withdrawal is recommended when a thorough diagnostic workup shows no evidence of epileptic seizures.15 Oto et al16 reported 49% of PNES patients became seizure-free 12 months after discontinuing antiepileptics.
Psychotherapy and pharmacotherapy. Open-label studies of psychological treatments for PNES have demonstrated that a cognitive-behavioral therapy-based approach and brief augmented psychodynamic interpersonal therapy could reduce seizures.17 In a pilot, randomized, placebo-controlled trial, PNES patients who received flexibly dosed sertraline reported a 45% reduction in seizures compared with an 8% increase in the placebo group.18 Similar improvements in seizure frequency have been reported in PNES patients with anxiety or depression treated with venlafaxine.19
OUTCOME: Support, improvement
During the next several days, Ms. T has random episodes of seizures with foaming of the mouth and unresponsiveness. These episodes last from 5 to 30 minutes and require transfer to the ER. After each episode, Ms. T is medically cleared and sent back to the psychiatric unit. The neurologist recommends avoiding antiepileptics. Ms. T responds well to the structured inpatient setting and supportive psychotherapy. Her episodes decrease and her mood becomes more stable. She refrains from self-injurious behaviors and is discharged home with outpatient follow-up.
Related Resource
- Marsh P, Benbadis S, Fernandez F. Psychogenic nonepileptic seizures: ways to win over skeptical patients. Current Psychiatry. 2008;7(1):21-35.
Drug Brand Names
- Alprazolam • Xanax
- Clonazepam • Klonopin
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Haloperidol • Haldol
- Lorazepam • Ativan
- Quetiapine • Seroquel
- Sertraline • Zoloft
- Venlafaxine • Effexor
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Kellinghaus C, Lüders HO. Frontal lobe epilepsy. Epileptic Disord. 2004;6(4):223-239.
2. Kanner AM, Morris HH, Lüders H, et al. Supplementary motor seizures mimicking pseudoseizures: some clinical differences. Neurology. 1990;40(9):1404-1407.
3. Hall-Patch L, Brown R, House A, et al. Acceptability and effectiveness of a strategy for the communication of the diagnosis of psychogenic nonepileptic seizures. Epilepsia. 2010;51(1):70-78.
4. Reuber M, Elger CE. Psychogenic nonepileptic seizures: review and update. Epilepsy Behav. 2003;4(3):205-216.
5. Chung SS, Gerber P, Kirlin KA. Ictal eye closure is a reliable indicator for psychogenic nonepileptic seizures. Neurology. 2006;66(11):1730-1731.
6. Mostacci B, Bisulli F, Alvisi L, et al. Ictal characteristics of psychogenic nonepileptic seizures: what we have learned from video/EEG recordings—a literature review. Epilepsy Behav. 2011;22(2):144-153.
7. Fleisher W, Staley D, Krawetz P, et al. Comparative study of trauma-related phenomena in subjects with pseudoseizures and subjects with epilepsy. Am J Psychiatry. 2002;159(4):660-663.
8. Bowman ES, Markand ON. Psychodynamics and psychiatric diagnoses of pseudoseizure subjects. Am J Psychiatry. 1996;153(1):57-63.
9. Benbadis SR. The EEG in nonepileptic seizures. J Clin Neurophysiol. 2006;23(4):340-352.
10. Brown RJ, Syed TU, Benbadis S, et al. Psychogenic nonepileptic seizures. Epilepsy Behav. 2011;22(1):85-93.
11. Bodde NM, Brooks JL, Baker GA, et al. Psychogenic non-epileptic seizures—definition, etiology, treatment and prognostic issues: a critical review. Seizure. 2009;18(8):543-553.
12. Alsaadi TM, Marquez AV. Psychogenic nonepileptic seizures. Am Fam Physician. 2005;72(5):849-856.
13. Bradley WG, Daroff RB, Fenichel GM, et al. eds. Neurology in clinical practice: principles of diagnosis and management. 4th ed. Philadelphia, PA: Butterworth Heinemann; 2004:19-20, 1971–1972.
14. Harden CL, Ferrando SJ. Delivering the diagnosis of psychogenic pseudoseizures: should the neurologist or the psychiatrist be responsible? Epilepsy Behav. 2001;2(6):519-523.
15. Oto M, Espie CA, Duncan R. An exploratory randomized controlled trial of immediate versus delayed withdrawal of antiepileptic drugs in patients with psychogenic nonepileptic attacks (PNEAs). Epilepsia. 2010;51(10):1994-1999.
16. Oto M, Espie C, Pelosi A, et al. The safety of antiepileptic drug withdrawal in patients with non-epileptic seizures. J Neurol Neurosurg Psychiatry. 2005;76(12):1682-1685.
17. Goldstein LH, Mellers JD. Recent developments in our understanding of the semiology and treatment of psychogenic nonepileptic seizures. Curr Neurol Neurosci Rep. 2012;12(4):436-444.
18. LaFrance WC, Jr, Keitner GI, Papandonatos GD, et al. Pilot pharmacologic randomized controlled trial for psychogenic nonepileptic seizures. Neurology. 2010;75(13):1166-1173.
19. Pintor L, Baillés E, Matrai S, et al. Efficiency of venlafaxine in patients with psychogenic nonepileptic seizures and anxiety and/or depressive disorders. J Neuropsychiatry Clin Neurosci. 2010;22(4):401-408.
1. Kellinghaus C, Lüders HO. Frontal lobe epilepsy. Epileptic Disord. 2004;6(4):223-239.
2. Kanner AM, Morris HH, Lüders H, et al. Supplementary motor seizures mimicking pseudoseizures: some clinical differences. Neurology. 1990;40(9):1404-1407.
3. Hall-Patch L, Brown R, House A, et al. Acceptability and effectiveness of a strategy for the communication of the diagnosis of psychogenic nonepileptic seizures. Epilepsia. 2010;51(1):70-78.
4. Reuber M, Elger CE. Psychogenic nonepileptic seizures: review and update. Epilepsy Behav. 2003;4(3):205-216.
5. Chung SS, Gerber P, Kirlin KA. Ictal eye closure is a reliable indicator for psychogenic nonepileptic seizures. Neurology. 2006;66(11):1730-1731.
6. Mostacci B, Bisulli F, Alvisi L, et al. Ictal characteristics of psychogenic nonepileptic seizures: what we have learned from video/EEG recordings—a literature review. Epilepsy Behav. 2011;22(2):144-153.
7. Fleisher W, Staley D, Krawetz P, et al. Comparative study of trauma-related phenomena in subjects with pseudoseizures and subjects with epilepsy. Am J Psychiatry. 2002;159(4):660-663.
8. Bowman ES, Markand ON. Psychodynamics and psychiatric diagnoses of pseudoseizure subjects. Am J Psychiatry. 1996;153(1):57-63.
9. Benbadis SR. The EEG in nonepileptic seizures. J Clin Neurophysiol. 2006;23(4):340-352.
10. Brown RJ, Syed TU, Benbadis S, et al. Psychogenic nonepileptic seizures. Epilepsy Behav. 2011;22(1):85-93.
11. Bodde NM, Brooks JL, Baker GA, et al. Psychogenic non-epileptic seizures—definition, etiology, treatment and prognostic issues: a critical review. Seizure. 2009;18(8):543-553.
12. Alsaadi TM, Marquez AV. Psychogenic nonepileptic seizures. Am Fam Physician. 2005;72(5):849-856.
13. Bradley WG, Daroff RB, Fenichel GM, et al. eds. Neurology in clinical practice: principles of diagnosis and management. 4th ed. Philadelphia, PA: Butterworth Heinemann; 2004:19-20, 1971–1972.
14. Harden CL, Ferrando SJ. Delivering the diagnosis of psychogenic pseudoseizures: should the neurologist or the psychiatrist be responsible? Epilepsy Behav. 2001;2(6):519-523.
15. Oto M, Espie CA, Duncan R. An exploratory randomized controlled trial of immediate versus delayed withdrawal of antiepileptic drugs in patients with psychogenic nonepileptic attacks (PNEAs). Epilepsia. 2010;51(10):1994-1999.
16. Oto M, Espie C, Pelosi A, et al. The safety of antiepileptic drug withdrawal in patients with non-epileptic seizures. J Neurol Neurosurg Psychiatry. 2005;76(12):1682-1685.
17. Goldstein LH, Mellers JD. Recent developments in our understanding of the semiology and treatment of psychogenic nonepileptic seizures. Curr Neurol Neurosci Rep. 2012;12(4):436-444.
18. LaFrance WC, Jr, Keitner GI, Papandonatos GD, et al. Pilot pharmacologic randomized controlled trial for psychogenic nonepileptic seizures. Neurology. 2010;75(13):1166-1173.
19. Pintor L, Baillés E, Matrai S, et al. Efficiency of venlafaxine in patients with psychogenic nonepileptic seizures and anxiety and/or depressive disorders. J Neuropsychiatry Clin Neurosci. 2010;22(4):401-408.