User login
VIDEO: Treat insomnia in depressed, even suicidal people
LOS ANGELES – The relationship between depression, suicidality, and insomnia is more complex than once thought. Insomnia might contribute to the other problems and make them worse, instead of simply being a symptom of depression.
Dr. Vaughn McCall, professor and chairman of the department of psychiatry and health behavior at the Medical College of Georgia, Augusta, is running a randomized trial to see whether the judicious use of zolpidem (Ambien CR) improves the moods and outlooks of depressed patients on SSRIs who have active suicidal ideation. The results are still a few years away. In the meantime, he explains in this video from the American Association of Suicidology annual meeting that new or worsening insomnia cannot be ignored in depression. He also describes how to safely prescribe sleeping pills when necessary.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LOS ANGELES – The relationship between depression, suicidality, and insomnia is more complex than once thought. Insomnia might contribute to the other problems and make them worse, instead of simply being a symptom of depression.
Dr. Vaughn McCall, professor and chairman of the department of psychiatry and health behavior at the Medical College of Georgia, Augusta, is running a randomized trial to see whether the judicious use of zolpidem (Ambien CR) improves the moods and outlooks of depressed patients on SSRIs who have active suicidal ideation. The results are still a few years away. In the meantime, he explains in this video from the American Association of Suicidology annual meeting that new or worsening insomnia cannot be ignored in depression. He also describes how to safely prescribe sleeping pills when necessary.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LOS ANGELES – The relationship between depression, suicidality, and insomnia is more complex than once thought. Insomnia might contribute to the other problems and make them worse, instead of simply being a symptom of depression.
Dr. Vaughn McCall, professor and chairman of the department of psychiatry and health behavior at the Medical College of Georgia, Augusta, is running a randomized trial to see whether the judicious use of zolpidem (Ambien CR) improves the moods and outlooks of depressed patients on SSRIs who have active suicidal ideation. The results are still a few years away. In the meantime, he explains in this video from the American Association of Suicidology annual meeting that new or worsening insomnia cannot be ignored in depression. He also describes how to safely prescribe sleeping pills when necessary.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
EXPERT ANALYSIS FROM THE AAS ANNUAL CONFERENCE
Studies highlight insomnia-depression link, underscore role for brief CBT
SAN ANTONIO – Brief cognitive-behavioral therapy is particularly helpful for the treatment of insomnia, including insomnia that occurs in association with depression or other psychiatric conditions.
Even cognitive-behavioral therapy (CBT) sessions lasting only 8-10 minutes, when accompanied by informational handouts, can lead to improvements in insomnia, Dr. Donna M. Sudak, professor and director of the psychotherapy training program at Drexel University, Philadelphia, said during a premeeting workshop on high-yield brief CBT at the annual meeting of the American College of Psychiatrists.
"It’s really rapid," she said of the intervention and its effects on insomnia.
Dr. Sudak noted that in patients with depression, it often is assumed that "the insomnia component of depression really has to do with the depression itself," but in fact, treating the insomnia appears to also make a real difference in the depression, suggesting the two conditions are interrelated.
"CBT works really well, and it also may be important in terms of managing other conditions," she said.
In an article that synthesized the relevant empirical evidence related to the efficacy of CBT for insomnia (CBT-I) and the benzodiazepines and benzodiazepine-receptor antagonists often used for the treatment of insomnia, Dr. Sudak notes that chronic insomnia affects an estimated 6% to 10% of the population, and that the highly prevalent sleep disorder is accompanied by significant daytime impairment.
"Insomnia has significant consequences for daytime functioning and health-related quality of life. The disorder conveys serious occupational and economic burdens, including mood disturbance, sleepiness, fatigue, cognitive impairment, and high rates of absenteeism and ‘presenteeism,’ " she wrote.
She also noted that insomnia sufferers are at increased risk of compromised psychological and physical health.
In the article, which has been accepted for publication in the International Journal of Cognitive Psychotherapy, she notes that CBT-I, which typically involves six to eight individual or group sessions that employ strategies such as stimulus control, sleep restriction, relaxation, and cognitive restructuring, is recommended by the American Academy of Sleep Medicine and the National Institutes of Health based on the substantial support in the literature for its efficacy and effectiveness in treating primary insomnia. She cites, for example, a meta-analysis of randomized controlled trials that suggests that CBT-I has moderate to large effects with respect to improving sleep (Sleep and Biol. Rhythms 2011;9:24-34).
Other studies have found a high rate of treatment response and clinically significant remissions with CBT-I, she noted.
CBT-I for comorbid insomnia, psychiatric illness
CBT-I also is beneficial in patients with coexisting insomnia and psychiatric illness.
"An estimated 30% to 90% of psychiatric patients have sleep disturbances. Conversely, patients with psychiatric illness account for an estimated 40% to 50% of patients with chronic insomnia.
Furthermore, those with anxiety and depression have a fivefold increase in the likelihood of having chronic insomnia, compared with those without anxiety or depression, and numerous studies suggest that insomnia that coexists with a range of psychiatric and medical conditions benefits from the application of CBT-I.
"It is therefore worthwhile to pursue strategies for combining CBT-I and pharmacotherapy in such groups," she wrote.
However, despite the high comorbidity, insomnia is rarely independently treated with CBT-I in mood disorders, which leaves patients vulnerable to relapse of depression, as well as to morbidity associated with sleep disturbance.
"It is increasingly recognized that insomnia is often best conceptualized as a separate entity that should be managed with specific, targeted treatment rather than secondary to depression treatment," she wrote, noting that since the prevalence of comorbid insomnia increases with the severity of depression, and since insomnia increases the risk of recurrence of depression and suicide, the most important take-home lesson from the literature might well be that when patients have both major depression and insomnia, a treatment targeting both conditions is warranted.
Indeed, data increasingly suggest that CBT-I is such a treatment.
In a recent study presented at the annual meeting of the Association for Behavioral and Cognitive Therapies, 87% of 66 patients with depression whose insomnia resolved after 4 brief biweekly CBT sessions also experienced significant declines in their depression symptoms after 8 weeks of treatment – regardless of whether they were treated with an antidepressant drug or received placebo. The rate of improvement in depression symptoms in those who also experienced improvement in their insomnia was almost twice as high as in patients who did not experience improvement in their insomnia, according to the lead author, Colleen E. Carney, Ph.D., of Ryerson University, Toronto.
CBT-I in older adults
In another recent study, Nicole Lovato, Ph.D., of Flinders University, Adelaide, South Australia, and her colleagues demonstrated that 4 brief weekly CBT group-administered sessions for insomnia were effective for improving symptoms in older adults with sleep maintenance insomnia.
For that randomized controlled trial involving 118 adults with a mean age of 64 years, the investigators compared 86 CBT patients with 32 waitlist controls. At 3-month follow-up, those in the treatment group experienced significant improvements in the timing and quality of sleep, including later bedtime, earlier out-of-bed time, reduced wake after sleep onset, and improved sleep efficiency (Sleep 2014;37:117-26).
Improvements were seen on the Insomnia Severity Index, Flinders Fatigue Scale, Epworth Sleepiness Scale, Daytime Feeling and Functioning Scale, Sleep Anticipatory Anxiety Questionnaire, Dysfunctional Beliefs and Attitudes Scale, and Sleep Self-Efficacy Scale, they reported.
"These changes were supported by large effect sizes (1.14-1.54) and were significantly greater than the wait-list group both immediately following treatment and at 3-month follow-up," the investigators wrote.
The CBT intervention included bedtime restriction therapy, sleep education, and cognitive restructuring.
The group-administered treatment program used in the study, "promises to be a brief and inexpensive answer to the effective treatment of insomnia in the older population," they concluded.
Dr. Sudak’s paper also addressed CBT-I use in the elderly, who have a substantial risk of insomnia and who frequently use hypnotics for treatment of insomnia.
"Treatment with CBT-I is effective in older adults and results are more durable than medication," she said, noting that 50% of elders who receive CBT-I sustain remission for at least 2 years.
CBT-I is also effective in older adults with comorbid medical conditions; among those who are dependent on hypnotics, CBT-I helps improve subjective sleep quality and sleep onset latency. Several randomized controlled trials indicate that CBT-I "may be particularly effective in facilitating hypnotic withdrawal in older adults," she said, noting that this is important given that hypnotics are associated with falls, confusion, and constipation in this population.
She cited a study that demonstrated that the best outcomes are achieved if CBT-I is employed first, then medication added, then medication discontinued prior to the end of CBT-I (Lancet 2012;379:1129-41).
CBT-I and cost savings
Another recent study shows that in addition to improving symptoms, brief CBT-I reduces health care utilization and costs.
The medical records review of 84 outpatients with a mean age of 54 years showed that for 37 patients who completed at least three CBT session for insomnia, and 32 who completed at least three sessions and who experienced significant sleep improvement, all health care use and cost variables, with the exception of number of medications, decreased significantly or trended toward decrease after treatment.
The average decrease in CPT costs was $200 for completers and $210 for responders. No significant decreases occurred in those who did not complete therapy, Christina McCrae, Ph.D., of the University of Florida, Gainesville, and her colleagues reported in February in the Journal of Clinical Sleep Medicine.
Patients included in the study received sleep and sleep hygiene education, stimulus control therapy, sleep restriction, a 10-minute relaxation exercise, and cognitive therapy during up to 6 weekly treatment sessions led by clinical psychology graduate students and predoctoral interns.
Although the study is limited by its small sample size and non-normal data distribution, the findings underscore a need for greater dissemination of brief CBT for insomnia; as few as 3 sessions are needed for significant improvement, the therapy can be delivered by novice clinicians, and the therapy is associated with reduced costs and reduced burden of insomnia, the investigators concluded (J. Clin. Sleep Med. 2014;10:127-35).
While the cost of brief treatment, which was $460 in this study, might negate the short-term savings seen in the first 6 months after therapy, the effects of therapy are durable, so CBT for insomnia has the potential to produce substantial long-term savings, the investigator said in a press statement. They noted that this is particularly true when these results are extrapolated to the large population of insomnia patients in the U.S. health care system.
Dr. Sudak is a coauthor of the book "High-Yield Cognitive-Behavior Therapy for Brief Sessions: An Illustrated Guide" (Washington: American Psychiatric Publishing, 2010). She receives book royalties from American Psychiatric Publishing; Lippincott, Williams & Wilkins; and John Wiley & Sons. Dr. Sudak also is on an editorial board and receives honoraria from Elsevier and is a consultant for Takeda Pharmaceuticals. Dr. Lovato and Dr. McCrae reported having no disclosures.
SAN ANTONIO – Brief cognitive-behavioral therapy is particularly helpful for the treatment of insomnia, including insomnia that occurs in association with depression or other psychiatric conditions.
Even cognitive-behavioral therapy (CBT) sessions lasting only 8-10 minutes, when accompanied by informational handouts, can lead to improvements in insomnia, Dr. Donna M. Sudak, professor and director of the psychotherapy training program at Drexel University, Philadelphia, said during a premeeting workshop on high-yield brief CBT at the annual meeting of the American College of Psychiatrists.
"It’s really rapid," she said of the intervention and its effects on insomnia.
Dr. Sudak noted that in patients with depression, it often is assumed that "the insomnia component of depression really has to do with the depression itself," but in fact, treating the insomnia appears to also make a real difference in the depression, suggesting the two conditions are interrelated.
"CBT works really well, and it also may be important in terms of managing other conditions," she said.
In an article that synthesized the relevant empirical evidence related to the efficacy of CBT for insomnia (CBT-I) and the benzodiazepines and benzodiazepine-receptor antagonists often used for the treatment of insomnia, Dr. Sudak notes that chronic insomnia affects an estimated 6% to 10% of the population, and that the highly prevalent sleep disorder is accompanied by significant daytime impairment.
"Insomnia has significant consequences for daytime functioning and health-related quality of life. The disorder conveys serious occupational and economic burdens, including mood disturbance, sleepiness, fatigue, cognitive impairment, and high rates of absenteeism and ‘presenteeism,’ " she wrote.
She also noted that insomnia sufferers are at increased risk of compromised psychological and physical health.
In the article, which has been accepted for publication in the International Journal of Cognitive Psychotherapy, she notes that CBT-I, which typically involves six to eight individual or group sessions that employ strategies such as stimulus control, sleep restriction, relaxation, and cognitive restructuring, is recommended by the American Academy of Sleep Medicine and the National Institutes of Health based on the substantial support in the literature for its efficacy and effectiveness in treating primary insomnia. She cites, for example, a meta-analysis of randomized controlled trials that suggests that CBT-I has moderate to large effects with respect to improving sleep (Sleep and Biol. Rhythms 2011;9:24-34).
Other studies have found a high rate of treatment response and clinically significant remissions with CBT-I, she noted.
CBT-I for comorbid insomnia, psychiatric illness
CBT-I also is beneficial in patients with coexisting insomnia and psychiatric illness.
"An estimated 30% to 90% of psychiatric patients have sleep disturbances. Conversely, patients with psychiatric illness account for an estimated 40% to 50% of patients with chronic insomnia.
Furthermore, those with anxiety and depression have a fivefold increase in the likelihood of having chronic insomnia, compared with those without anxiety or depression, and numerous studies suggest that insomnia that coexists with a range of psychiatric and medical conditions benefits from the application of CBT-I.
"It is therefore worthwhile to pursue strategies for combining CBT-I and pharmacotherapy in such groups," she wrote.
However, despite the high comorbidity, insomnia is rarely independently treated with CBT-I in mood disorders, which leaves patients vulnerable to relapse of depression, as well as to morbidity associated with sleep disturbance.
"It is increasingly recognized that insomnia is often best conceptualized as a separate entity that should be managed with specific, targeted treatment rather than secondary to depression treatment," she wrote, noting that since the prevalence of comorbid insomnia increases with the severity of depression, and since insomnia increases the risk of recurrence of depression and suicide, the most important take-home lesson from the literature might well be that when patients have both major depression and insomnia, a treatment targeting both conditions is warranted.
Indeed, data increasingly suggest that CBT-I is such a treatment.
In a recent study presented at the annual meeting of the Association for Behavioral and Cognitive Therapies, 87% of 66 patients with depression whose insomnia resolved after 4 brief biweekly CBT sessions also experienced significant declines in their depression symptoms after 8 weeks of treatment – regardless of whether they were treated with an antidepressant drug or received placebo. The rate of improvement in depression symptoms in those who also experienced improvement in their insomnia was almost twice as high as in patients who did not experience improvement in their insomnia, according to the lead author, Colleen E. Carney, Ph.D., of Ryerson University, Toronto.
CBT-I in older adults
In another recent study, Nicole Lovato, Ph.D., of Flinders University, Adelaide, South Australia, and her colleagues demonstrated that 4 brief weekly CBT group-administered sessions for insomnia were effective for improving symptoms in older adults with sleep maintenance insomnia.
For that randomized controlled trial involving 118 adults with a mean age of 64 years, the investigators compared 86 CBT patients with 32 waitlist controls. At 3-month follow-up, those in the treatment group experienced significant improvements in the timing and quality of sleep, including later bedtime, earlier out-of-bed time, reduced wake after sleep onset, and improved sleep efficiency (Sleep 2014;37:117-26).
Improvements were seen on the Insomnia Severity Index, Flinders Fatigue Scale, Epworth Sleepiness Scale, Daytime Feeling and Functioning Scale, Sleep Anticipatory Anxiety Questionnaire, Dysfunctional Beliefs and Attitudes Scale, and Sleep Self-Efficacy Scale, they reported.
"These changes were supported by large effect sizes (1.14-1.54) and were significantly greater than the wait-list group both immediately following treatment and at 3-month follow-up," the investigators wrote.
The CBT intervention included bedtime restriction therapy, sleep education, and cognitive restructuring.
The group-administered treatment program used in the study, "promises to be a brief and inexpensive answer to the effective treatment of insomnia in the older population," they concluded.
Dr. Sudak’s paper also addressed CBT-I use in the elderly, who have a substantial risk of insomnia and who frequently use hypnotics for treatment of insomnia.
"Treatment with CBT-I is effective in older adults and results are more durable than medication," she said, noting that 50% of elders who receive CBT-I sustain remission for at least 2 years.
CBT-I is also effective in older adults with comorbid medical conditions; among those who are dependent on hypnotics, CBT-I helps improve subjective sleep quality and sleep onset latency. Several randomized controlled trials indicate that CBT-I "may be particularly effective in facilitating hypnotic withdrawal in older adults," she said, noting that this is important given that hypnotics are associated with falls, confusion, and constipation in this population.
She cited a study that demonstrated that the best outcomes are achieved if CBT-I is employed first, then medication added, then medication discontinued prior to the end of CBT-I (Lancet 2012;379:1129-41).
CBT-I and cost savings
Another recent study shows that in addition to improving symptoms, brief CBT-I reduces health care utilization and costs.
The medical records review of 84 outpatients with a mean age of 54 years showed that for 37 patients who completed at least three CBT session for insomnia, and 32 who completed at least three sessions and who experienced significant sleep improvement, all health care use and cost variables, with the exception of number of medications, decreased significantly or trended toward decrease after treatment.
The average decrease in CPT costs was $200 for completers and $210 for responders. No significant decreases occurred in those who did not complete therapy, Christina McCrae, Ph.D., of the University of Florida, Gainesville, and her colleagues reported in February in the Journal of Clinical Sleep Medicine.
Patients included in the study received sleep and sleep hygiene education, stimulus control therapy, sleep restriction, a 10-minute relaxation exercise, and cognitive therapy during up to 6 weekly treatment sessions led by clinical psychology graduate students and predoctoral interns.
Although the study is limited by its small sample size and non-normal data distribution, the findings underscore a need for greater dissemination of brief CBT for insomnia; as few as 3 sessions are needed for significant improvement, the therapy can be delivered by novice clinicians, and the therapy is associated with reduced costs and reduced burden of insomnia, the investigators concluded (J. Clin. Sleep Med. 2014;10:127-35).
While the cost of brief treatment, which was $460 in this study, might negate the short-term savings seen in the first 6 months after therapy, the effects of therapy are durable, so CBT for insomnia has the potential to produce substantial long-term savings, the investigator said in a press statement. They noted that this is particularly true when these results are extrapolated to the large population of insomnia patients in the U.S. health care system.
Dr. Sudak is a coauthor of the book "High-Yield Cognitive-Behavior Therapy for Brief Sessions: An Illustrated Guide" (Washington: American Psychiatric Publishing, 2010). She receives book royalties from American Psychiatric Publishing; Lippincott, Williams & Wilkins; and John Wiley & Sons. Dr. Sudak also is on an editorial board and receives honoraria from Elsevier and is a consultant for Takeda Pharmaceuticals. Dr. Lovato and Dr. McCrae reported having no disclosures.
SAN ANTONIO – Brief cognitive-behavioral therapy is particularly helpful for the treatment of insomnia, including insomnia that occurs in association with depression or other psychiatric conditions.
Even cognitive-behavioral therapy (CBT) sessions lasting only 8-10 minutes, when accompanied by informational handouts, can lead to improvements in insomnia, Dr. Donna M. Sudak, professor and director of the psychotherapy training program at Drexel University, Philadelphia, said during a premeeting workshop on high-yield brief CBT at the annual meeting of the American College of Psychiatrists.
"It’s really rapid," she said of the intervention and its effects on insomnia.
Dr. Sudak noted that in patients with depression, it often is assumed that "the insomnia component of depression really has to do with the depression itself," but in fact, treating the insomnia appears to also make a real difference in the depression, suggesting the two conditions are interrelated.
"CBT works really well, and it also may be important in terms of managing other conditions," she said.
In an article that synthesized the relevant empirical evidence related to the efficacy of CBT for insomnia (CBT-I) and the benzodiazepines and benzodiazepine-receptor antagonists often used for the treatment of insomnia, Dr. Sudak notes that chronic insomnia affects an estimated 6% to 10% of the population, and that the highly prevalent sleep disorder is accompanied by significant daytime impairment.
"Insomnia has significant consequences for daytime functioning and health-related quality of life. The disorder conveys serious occupational and economic burdens, including mood disturbance, sleepiness, fatigue, cognitive impairment, and high rates of absenteeism and ‘presenteeism,’ " she wrote.
She also noted that insomnia sufferers are at increased risk of compromised psychological and physical health.
In the article, which has been accepted for publication in the International Journal of Cognitive Psychotherapy, she notes that CBT-I, which typically involves six to eight individual or group sessions that employ strategies such as stimulus control, sleep restriction, relaxation, and cognitive restructuring, is recommended by the American Academy of Sleep Medicine and the National Institutes of Health based on the substantial support in the literature for its efficacy and effectiveness in treating primary insomnia. She cites, for example, a meta-analysis of randomized controlled trials that suggests that CBT-I has moderate to large effects with respect to improving sleep (Sleep and Biol. Rhythms 2011;9:24-34).
Other studies have found a high rate of treatment response and clinically significant remissions with CBT-I, she noted.
CBT-I for comorbid insomnia, psychiatric illness
CBT-I also is beneficial in patients with coexisting insomnia and psychiatric illness.
"An estimated 30% to 90% of psychiatric patients have sleep disturbances. Conversely, patients with psychiatric illness account for an estimated 40% to 50% of patients with chronic insomnia.
Furthermore, those with anxiety and depression have a fivefold increase in the likelihood of having chronic insomnia, compared with those without anxiety or depression, and numerous studies suggest that insomnia that coexists with a range of psychiatric and medical conditions benefits from the application of CBT-I.
"It is therefore worthwhile to pursue strategies for combining CBT-I and pharmacotherapy in such groups," she wrote.
However, despite the high comorbidity, insomnia is rarely independently treated with CBT-I in mood disorders, which leaves patients vulnerable to relapse of depression, as well as to morbidity associated with sleep disturbance.
"It is increasingly recognized that insomnia is often best conceptualized as a separate entity that should be managed with specific, targeted treatment rather than secondary to depression treatment," she wrote, noting that since the prevalence of comorbid insomnia increases with the severity of depression, and since insomnia increases the risk of recurrence of depression and suicide, the most important take-home lesson from the literature might well be that when patients have both major depression and insomnia, a treatment targeting both conditions is warranted.
Indeed, data increasingly suggest that CBT-I is such a treatment.
In a recent study presented at the annual meeting of the Association for Behavioral and Cognitive Therapies, 87% of 66 patients with depression whose insomnia resolved after 4 brief biweekly CBT sessions also experienced significant declines in their depression symptoms after 8 weeks of treatment – regardless of whether they were treated with an antidepressant drug or received placebo. The rate of improvement in depression symptoms in those who also experienced improvement in their insomnia was almost twice as high as in patients who did not experience improvement in their insomnia, according to the lead author, Colleen E. Carney, Ph.D., of Ryerson University, Toronto.
CBT-I in older adults
In another recent study, Nicole Lovato, Ph.D., of Flinders University, Adelaide, South Australia, and her colleagues demonstrated that 4 brief weekly CBT group-administered sessions for insomnia were effective for improving symptoms in older adults with sleep maintenance insomnia.
For that randomized controlled trial involving 118 adults with a mean age of 64 years, the investigators compared 86 CBT patients with 32 waitlist controls. At 3-month follow-up, those in the treatment group experienced significant improvements in the timing and quality of sleep, including later bedtime, earlier out-of-bed time, reduced wake after sleep onset, and improved sleep efficiency (Sleep 2014;37:117-26).
Improvements were seen on the Insomnia Severity Index, Flinders Fatigue Scale, Epworth Sleepiness Scale, Daytime Feeling and Functioning Scale, Sleep Anticipatory Anxiety Questionnaire, Dysfunctional Beliefs and Attitudes Scale, and Sleep Self-Efficacy Scale, they reported.
"These changes were supported by large effect sizes (1.14-1.54) and were significantly greater than the wait-list group both immediately following treatment and at 3-month follow-up," the investigators wrote.
The CBT intervention included bedtime restriction therapy, sleep education, and cognitive restructuring.
The group-administered treatment program used in the study, "promises to be a brief and inexpensive answer to the effective treatment of insomnia in the older population," they concluded.
Dr. Sudak’s paper also addressed CBT-I use in the elderly, who have a substantial risk of insomnia and who frequently use hypnotics for treatment of insomnia.
"Treatment with CBT-I is effective in older adults and results are more durable than medication," she said, noting that 50% of elders who receive CBT-I sustain remission for at least 2 years.
CBT-I is also effective in older adults with comorbid medical conditions; among those who are dependent on hypnotics, CBT-I helps improve subjective sleep quality and sleep onset latency. Several randomized controlled trials indicate that CBT-I "may be particularly effective in facilitating hypnotic withdrawal in older adults," she said, noting that this is important given that hypnotics are associated with falls, confusion, and constipation in this population.
She cited a study that demonstrated that the best outcomes are achieved if CBT-I is employed first, then medication added, then medication discontinued prior to the end of CBT-I (Lancet 2012;379:1129-41).
CBT-I and cost savings
Another recent study shows that in addition to improving symptoms, brief CBT-I reduces health care utilization and costs.
The medical records review of 84 outpatients with a mean age of 54 years showed that for 37 patients who completed at least three CBT session for insomnia, and 32 who completed at least three sessions and who experienced significant sleep improvement, all health care use and cost variables, with the exception of number of medications, decreased significantly or trended toward decrease after treatment.
The average decrease in CPT costs was $200 for completers and $210 for responders. No significant decreases occurred in those who did not complete therapy, Christina McCrae, Ph.D., of the University of Florida, Gainesville, and her colleagues reported in February in the Journal of Clinical Sleep Medicine.
Patients included in the study received sleep and sleep hygiene education, stimulus control therapy, sleep restriction, a 10-minute relaxation exercise, and cognitive therapy during up to 6 weekly treatment sessions led by clinical psychology graduate students and predoctoral interns.
Although the study is limited by its small sample size and non-normal data distribution, the findings underscore a need for greater dissemination of brief CBT for insomnia; as few as 3 sessions are needed for significant improvement, the therapy can be delivered by novice clinicians, and the therapy is associated with reduced costs and reduced burden of insomnia, the investigators concluded (J. Clin. Sleep Med. 2014;10:127-35).
While the cost of brief treatment, which was $460 in this study, might negate the short-term savings seen in the first 6 months after therapy, the effects of therapy are durable, so CBT for insomnia has the potential to produce substantial long-term savings, the investigator said in a press statement. They noted that this is particularly true when these results are extrapolated to the large population of insomnia patients in the U.S. health care system.
Dr. Sudak is a coauthor of the book "High-Yield Cognitive-Behavior Therapy for Brief Sessions: An Illustrated Guide" (Washington: American Psychiatric Publishing, 2010). She receives book royalties from American Psychiatric Publishing; Lippincott, Williams & Wilkins; and John Wiley & Sons. Dr. Sudak also is on an editorial board and receives honoraria from Elsevier and is a consultant for Takeda Pharmaceuticals. Dr. Lovato and Dr. McCrae reported having no disclosures.
AT THE AMERICAN COLLEGE OF PSYCHIATRISTS MEETING
Intervention manages cardiac patients with depression, anxiety
A low-intensity intervention aimed at managing cardiac patients who have concomitant depression or anxiety improved mental-health–related quality of life when compared with usual care, according to a report published online April 14 in JAMA Internal Medicine.
The MOSAIC (Management of Sadness and Anxiety in Cardiology) clinical trial involved 183 patients hospitalized at a single urban academic medical center for acute coronary syndrome, heart failure, or arrhythmia during a 2-year period and who were found to have coexisting depression, generalized anxiety disorder, and/or panic disorder. The mean age of the participants was 60.5 years, 90% were white, 61% were employed, 53% were women (JAMA Intern. Med. 2014 April 14 [doi:10.1001/jamainternmend.2014.739]).
They were randomly assigned to receive either usual care or a telephone-based intervention in which a part-time social work care manager coordinated care among psychiatrists, inpatient medical providers, and outpatient medical providers; provided cognitive-behavioral therapy specific to the patient’s condition; and monitored patient symptoms for the 6-month duration of the intervention, said Dr. Jeff C. Huffman, director of cardiac psychiatry research program at Massachusetts General Hospital, and who is in the department of psychiatry at Harvard Medical School, Boston, and his associates.
Patients in the intervention group showed a significantly greater improvement than did those who received usual care in the primary outcome measure: mental-health-related quality of life (QOL), as measured by the Medical Outcomes Study Short Form-12 Mental Component Score. They also were remarkably more likely to receive treatment deemed "adequate" for their psychiatric disorders (75% vs 7%), and to show significantly greater improvement in Patient Health Questionnaire-9 scores; in overall function, as measured on the Duke Activity Status Index; and in health care–related QOL, as measured by EuroQol 5-Domain Instrument scores.
No significant differences were found between the two study groups in cardiac readmission rates at 6 months, but the mean time to readmission was significantly longer for the intervention group (92.4 days) than for the usual care group (62.5 days), Dr. Huffman and his associates said.
The investigators noted that their study involved typical patients seen for cardiac care – including many with serious medical issues and some who declined psychiatric treatment – and so should reflect results that would be found in real-world settings. In addition, using a social worker instead of a nurse as care manager and using telephone rather than in-person contacts substantially saved on costs.
However, Dr. Huffman and his colleagues cited several limitations. For example, the study was conducted in an academic medical center with mostly white patients. Also, those who delivered the intervention had experience with that population and with collaborative care programs.
Still, they expressed optimism about the intervention’s potential. "This intervention seems to have substantial promise as an adjunct or alternative to standard [collaborative care] paradigms," they wrote. "We found that a single care manager was able to coordinate care of three psychiatric conditions in patients with a wide range of cardiac diagnoses living within and outside the metropolitan area of the hospital."
This work was supported in part by the American Heart Association. No relevant financial conflicts of interest were reported.
A low-intensity intervention aimed at managing cardiac patients who have concomitant depression or anxiety improved mental-health–related quality of life when compared with usual care, according to a report published online April 14 in JAMA Internal Medicine.
The MOSAIC (Management of Sadness and Anxiety in Cardiology) clinical trial involved 183 patients hospitalized at a single urban academic medical center for acute coronary syndrome, heart failure, or arrhythmia during a 2-year period and who were found to have coexisting depression, generalized anxiety disorder, and/or panic disorder. The mean age of the participants was 60.5 years, 90% were white, 61% were employed, 53% were women (JAMA Intern. Med. 2014 April 14 [doi:10.1001/jamainternmend.2014.739]).
They were randomly assigned to receive either usual care or a telephone-based intervention in which a part-time social work care manager coordinated care among psychiatrists, inpatient medical providers, and outpatient medical providers; provided cognitive-behavioral therapy specific to the patient’s condition; and monitored patient symptoms for the 6-month duration of the intervention, said Dr. Jeff C. Huffman, director of cardiac psychiatry research program at Massachusetts General Hospital, and who is in the department of psychiatry at Harvard Medical School, Boston, and his associates.
Patients in the intervention group showed a significantly greater improvement than did those who received usual care in the primary outcome measure: mental-health-related quality of life (QOL), as measured by the Medical Outcomes Study Short Form-12 Mental Component Score. They also were remarkably more likely to receive treatment deemed "adequate" for their psychiatric disorders (75% vs 7%), and to show significantly greater improvement in Patient Health Questionnaire-9 scores; in overall function, as measured on the Duke Activity Status Index; and in health care–related QOL, as measured by EuroQol 5-Domain Instrument scores.
No significant differences were found between the two study groups in cardiac readmission rates at 6 months, but the mean time to readmission was significantly longer for the intervention group (92.4 days) than for the usual care group (62.5 days), Dr. Huffman and his associates said.
The investigators noted that their study involved typical patients seen for cardiac care – including many with serious medical issues and some who declined psychiatric treatment – and so should reflect results that would be found in real-world settings. In addition, using a social worker instead of a nurse as care manager and using telephone rather than in-person contacts substantially saved on costs.
However, Dr. Huffman and his colleagues cited several limitations. For example, the study was conducted in an academic medical center with mostly white patients. Also, those who delivered the intervention had experience with that population and with collaborative care programs.
Still, they expressed optimism about the intervention’s potential. "This intervention seems to have substantial promise as an adjunct or alternative to standard [collaborative care] paradigms," they wrote. "We found that a single care manager was able to coordinate care of three psychiatric conditions in patients with a wide range of cardiac diagnoses living within and outside the metropolitan area of the hospital."
This work was supported in part by the American Heart Association. No relevant financial conflicts of interest were reported.
A low-intensity intervention aimed at managing cardiac patients who have concomitant depression or anxiety improved mental-health–related quality of life when compared with usual care, according to a report published online April 14 in JAMA Internal Medicine.
The MOSAIC (Management of Sadness and Anxiety in Cardiology) clinical trial involved 183 patients hospitalized at a single urban academic medical center for acute coronary syndrome, heart failure, or arrhythmia during a 2-year period and who were found to have coexisting depression, generalized anxiety disorder, and/or panic disorder. The mean age of the participants was 60.5 years, 90% were white, 61% were employed, 53% were women (JAMA Intern. Med. 2014 April 14 [doi:10.1001/jamainternmend.2014.739]).
They were randomly assigned to receive either usual care or a telephone-based intervention in which a part-time social work care manager coordinated care among psychiatrists, inpatient medical providers, and outpatient medical providers; provided cognitive-behavioral therapy specific to the patient’s condition; and monitored patient symptoms for the 6-month duration of the intervention, said Dr. Jeff C. Huffman, director of cardiac psychiatry research program at Massachusetts General Hospital, and who is in the department of psychiatry at Harvard Medical School, Boston, and his associates.
Patients in the intervention group showed a significantly greater improvement than did those who received usual care in the primary outcome measure: mental-health-related quality of life (QOL), as measured by the Medical Outcomes Study Short Form-12 Mental Component Score. They also were remarkably more likely to receive treatment deemed "adequate" for their psychiatric disorders (75% vs 7%), and to show significantly greater improvement in Patient Health Questionnaire-9 scores; in overall function, as measured on the Duke Activity Status Index; and in health care–related QOL, as measured by EuroQol 5-Domain Instrument scores.
No significant differences were found between the two study groups in cardiac readmission rates at 6 months, but the mean time to readmission was significantly longer for the intervention group (92.4 days) than for the usual care group (62.5 days), Dr. Huffman and his associates said.
The investigators noted that their study involved typical patients seen for cardiac care – including many with serious medical issues and some who declined psychiatric treatment – and so should reflect results that would be found in real-world settings. In addition, using a social worker instead of a nurse as care manager and using telephone rather than in-person contacts substantially saved on costs.
However, Dr. Huffman and his colleagues cited several limitations. For example, the study was conducted in an academic medical center with mostly white patients. Also, those who delivered the intervention had experience with that population and with collaborative care programs.
Still, they expressed optimism about the intervention’s potential. "This intervention seems to have substantial promise as an adjunct or alternative to standard [collaborative care] paradigms," they wrote. "We found that a single care manager was able to coordinate care of three psychiatric conditions in patients with a wide range of cardiac diagnoses living within and outside the metropolitan area of the hospital."
This work was supported in part by the American Heart Association. No relevant financial conflicts of interest were reported.
FROM JAMA INTERNAL MEDICINE
Major finding: Patients in the intervention group showed a significantly greater improvement than did those who received usual care in the primary outcome measure, mental-health–related QOL, as well as being remarkably more likely to receive treatment deemed "adequate" for their psychiatric disorder (75% vs. 7%) and showing significantly greater improvement in overall function and health-related QOL.
Data source: A randomized clinical trial comparing psychiatric outcomes after 6 months of usual care vs. a collaborative care intervention, involving 183 cardiac patients who had concomitant depression or anxiety.
Disclosures: This work was supported in part by the American Heart Association. No relevant financial conflicts of interest were reported.
Ideal agent for insomnia not always clear cut
LAS VEGAS – Many patients with insomnia reach for certain dietary supplements and herbal preparations for relief, but their efficacies have not been established in well-controlled studies.
"Dietary supplements and herbal preparations not regulated by the FDA [Food and Drug Administration]," Dr. Karl Doghramji said at the annual psychopharmacology update held by the Nevada Psychiatric Association. "There is some question about the purity of these agents, and also about the active ingredient. There are many ingredients in these so-called nutraceutical compounds. Which is the active ingredient? We’re not quite sure."
If clinicians recommend agents whose effectiveness is not well-established, "are we delaying treatment for insomnia and other conditions, which may have a negative impact in daytime performance and may impair mood?" asked Dr. Doghramji, professor of psychiatry, neurology, and medicine at Thomas Jefferson University, Philadelphia. "That’s a concern."
One of the more commonly used natural supplements for insomnia is valerian in a dose of 400-450 mg/day. This herb is believed to have some anxiolytic, muscle relaxant, and sleep-promoting properties, "yet data regarding efficacy are mixed," said Dr. Doghramji, who also directs the university’s Sleep Disorders Center. "Safety data are scant, yet side effects appear to be rare and mild, primarily GI irritation and headache. There are case reports of hepatotoxicity in persons taking herbal products containing valerian."
He described melatonin as the most commonly used natural supplement for insomnia. A review of 139 published studies commissioned by the Agency for Healthcare Research and Quality suggests that melatonin has no effectiveness in the treatment of everyday regular insomnia (AHRQ Publication No. 05-E002-2; 2004). "But, some evidence suggests that it is effective in treating delayed sleep-phase syndrome with short-term use," Dr. Doghramji noted. "On the other hand, evidence suggests that melatonin is not effective in treating most secondary sleep disorders with short-term use, and no evidence suggests that melatonin is effective in alleviating the sleep disturbance aspect of jet lag and shift-work disorder."
Certain prescription agents might benefit patients with insomnia, he continued. FDA nonapproved agents for insomnia include sedating antidepressants, antipsychotics, and anticonvulsants. FDA-approved hypnotics include benzodiazepine-receptor agonists, melatonin-receptor agonists, and H1-receptor antagonists.
"At appropriate doses, sedating antidepressants are effective for mood and anxiety disorders; there is a low abuse risk, low cost, and there is a large dose range," Dr. Doghramji said. "One of the disadvantages is that they tend to be long acting and have anticholinergic and antihistaminic side effects." A 42-day controlled study of doxepin 25-50 mg found that the agent did not produce any change in terms of sleep latency (J. Clin. Psychiatry 2001;62:453-63). However, "it did increase their total sleep time, suggesting that they didn’t necessarily fall asleep more quickly, but they had fewer awakenings after they did fall asleep," he said. "So, if doxepin is to be used for insomnia, it seems to be best suited for the insomnia characterized by middle of the night awakening and late morning insomnia."
A 2-week study that compared trazodone with zolpidem in primary insomnia demonstrated that trazodone did seem to help people fall asleep more quickly in the first week or so (Hum. Psychopharmacol. 1998;13:191-8). "It also helped them feel as though they had slept longer," said Dr. Doghramji, who was not involved with the study. "The problem was, tolerance occurred within 2 weeks. The issue there is, should you increase the dosage or keep the same dosage for a while? We don’t have a lot of data on this."
From a pharmacokinetic standpoint, trazodone has a long half-life (5-12 hours) and features a complex set of pharmacodynamics. "It not only has some serotonergic potential, it has some histaminic potential, making it an agent that can have multiple side effects, so be careful with it," he said.
Clinicians likely use benzodiazepine receptor agonists more than any other agent for insomnia. These include the benzodiazepines, such as estazolam, flurazepam, quazepam, temazepam, and triazolam; and the non-benzodiazepines (also known as selective benzodiazepine receptor agonists) such as zaleplon, zolpidem and its various preparations (oral, sublingual, and oral spray); and eszopiclone. Adverse effects may include daytime sedation, psychomotor and cognitive impairment (depending on dose and half-life), rebound insomnia, and respiratory depression in vulnerable populations. All of these are classified as schedule IV controlled substance by the Drug Enforcement Administration. New drugs, which do not have a DEA schedule classification, include ramelteon, a melatonin receptor-agonist, and low-dose doxepin.
Choosing which antidepressant agent to use for a patient with depression and comorbid insomnia poses a certain clinical dilemma, Dr. Doghramji concluded. "Do you start with a sedating agent when your patient is both depressed and cannot sleep? Or do you put them on any old agent, regardless of whether it’s sedating or not? Unfortunately, at this point, there are not a lot of data guiding us on this."
Dr. Doghramji disclosed that he is a consultant for UCB, Teva Pharmaceuticals, Vanda Pharmaceuticals, and Jazz Pharmaceuticals, and that he holds stock in Merck.
LAS VEGAS – Many patients with insomnia reach for certain dietary supplements and herbal preparations for relief, but their efficacies have not been established in well-controlled studies.
"Dietary supplements and herbal preparations not regulated by the FDA [Food and Drug Administration]," Dr. Karl Doghramji said at the annual psychopharmacology update held by the Nevada Psychiatric Association. "There is some question about the purity of these agents, and also about the active ingredient. There are many ingredients in these so-called nutraceutical compounds. Which is the active ingredient? We’re not quite sure."
If clinicians recommend agents whose effectiveness is not well-established, "are we delaying treatment for insomnia and other conditions, which may have a negative impact in daytime performance and may impair mood?" asked Dr. Doghramji, professor of psychiatry, neurology, and medicine at Thomas Jefferson University, Philadelphia. "That’s a concern."
One of the more commonly used natural supplements for insomnia is valerian in a dose of 400-450 mg/day. This herb is believed to have some anxiolytic, muscle relaxant, and sleep-promoting properties, "yet data regarding efficacy are mixed," said Dr. Doghramji, who also directs the university’s Sleep Disorders Center. "Safety data are scant, yet side effects appear to be rare and mild, primarily GI irritation and headache. There are case reports of hepatotoxicity in persons taking herbal products containing valerian."
He described melatonin as the most commonly used natural supplement for insomnia. A review of 139 published studies commissioned by the Agency for Healthcare Research and Quality suggests that melatonin has no effectiveness in the treatment of everyday regular insomnia (AHRQ Publication No. 05-E002-2; 2004). "But, some evidence suggests that it is effective in treating delayed sleep-phase syndrome with short-term use," Dr. Doghramji noted. "On the other hand, evidence suggests that melatonin is not effective in treating most secondary sleep disorders with short-term use, and no evidence suggests that melatonin is effective in alleviating the sleep disturbance aspect of jet lag and shift-work disorder."
Certain prescription agents might benefit patients with insomnia, he continued. FDA nonapproved agents for insomnia include sedating antidepressants, antipsychotics, and anticonvulsants. FDA-approved hypnotics include benzodiazepine-receptor agonists, melatonin-receptor agonists, and H1-receptor antagonists.
"At appropriate doses, sedating antidepressants are effective for mood and anxiety disorders; there is a low abuse risk, low cost, and there is a large dose range," Dr. Doghramji said. "One of the disadvantages is that they tend to be long acting and have anticholinergic and antihistaminic side effects." A 42-day controlled study of doxepin 25-50 mg found that the agent did not produce any change in terms of sleep latency (J. Clin. Psychiatry 2001;62:453-63). However, "it did increase their total sleep time, suggesting that they didn’t necessarily fall asleep more quickly, but they had fewer awakenings after they did fall asleep," he said. "So, if doxepin is to be used for insomnia, it seems to be best suited for the insomnia characterized by middle of the night awakening and late morning insomnia."
A 2-week study that compared trazodone with zolpidem in primary insomnia demonstrated that trazodone did seem to help people fall asleep more quickly in the first week or so (Hum. Psychopharmacol. 1998;13:191-8). "It also helped them feel as though they had slept longer," said Dr. Doghramji, who was not involved with the study. "The problem was, tolerance occurred within 2 weeks. The issue there is, should you increase the dosage or keep the same dosage for a while? We don’t have a lot of data on this."
From a pharmacokinetic standpoint, trazodone has a long half-life (5-12 hours) and features a complex set of pharmacodynamics. "It not only has some serotonergic potential, it has some histaminic potential, making it an agent that can have multiple side effects, so be careful with it," he said.
Clinicians likely use benzodiazepine receptor agonists more than any other agent for insomnia. These include the benzodiazepines, such as estazolam, flurazepam, quazepam, temazepam, and triazolam; and the non-benzodiazepines (also known as selective benzodiazepine receptor agonists) such as zaleplon, zolpidem and its various preparations (oral, sublingual, and oral spray); and eszopiclone. Adverse effects may include daytime sedation, psychomotor and cognitive impairment (depending on dose and half-life), rebound insomnia, and respiratory depression in vulnerable populations. All of these are classified as schedule IV controlled substance by the Drug Enforcement Administration. New drugs, which do not have a DEA schedule classification, include ramelteon, a melatonin receptor-agonist, and low-dose doxepin.
Choosing which antidepressant agent to use for a patient with depression and comorbid insomnia poses a certain clinical dilemma, Dr. Doghramji concluded. "Do you start with a sedating agent when your patient is both depressed and cannot sleep? Or do you put them on any old agent, regardless of whether it’s sedating or not? Unfortunately, at this point, there are not a lot of data guiding us on this."
Dr. Doghramji disclosed that he is a consultant for UCB, Teva Pharmaceuticals, Vanda Pharmaceuticals, and Jazz Pharmaceuticals, and that he holds stock in Merck.
LAS VEGAS – Many patients with insomnia reach for certain dietary supplements and herbal preparations for relief, but their efficacies have not been established in well-controlled studies.
"Dietary supplements and herbal preparations not regulated by the FDA [Food and Drug Administration]," Dr. Karl Doghramji said at the annual psychopharmacology update held by the Nevada Psychiatric Association. "There is some question about the purity of these agents, and also about the active ingredient. There are many ingredients in these so-called nutraceutical compounds. Which is the active ingredient? We’re not quite sure."
If clinicians recommend agents whose effectiveness is not well-established, "are we delaying treatment for insomnia and other conditions, which may have a negative impact in daytime performance and may impair mood?" asked Dr. Doghramji, professor of psychiatry, neurology, and medicine at Thomas Jefferson University, Philadelphia. "That’s a concern."
One of the more commonly used natural supplements for insomnia is valerian in a dose of 400-450 mg/day. This herb is believed to have some anxiolytic, muscle relaxant, and sleep-promoting properties, "yet data regarding efficacy are mixed," said Dr. Doghramji, who also directs the university’s Sleep Disorders Center. "Safety data are scant, yet side effects appear to be rare and mild, primarily GI irritation and headache. There are case reports of hepatotoxicity in persons taking herbal products containing valerian."
He described melatonin as the most commonly used natural supplement for insomnia. A review of 139 published studies commissioned by the Agency for Healthcare Research and Quality suggests that melatonin has no effectiveness in the treatment of everyday regular insomnia (AHRQ Publication No. 05-E002-2; 2004). "But, some evidence suggests that it is effective in treating delayed sleep-phase syndrome with short-term use," Dr. Doghramji noted. "On the other hand, evidence suggests that melatonin is not effective in treating most secondary sleep disorders with short-term use, and no evidence suggests that melatonin is effective in alleviating the sleep disturbance aspect of jet lag and shift-work disorder."
Certain prescription agents might benefit patients with insomnia, he continued. FDA nonapproved agents for insomnia include sedating antidepressants, antipsychotics, and anticonvulsants. FDA-approved hypnotics include benzodiazepine-receptor agonists, melatonin-receptor agonists, and H1-receptor antagonists.
"At appropriate doses, sedating antidepressants are effective for mood and anxiety disorders; there is a low abuse risk, low cost, and there is a large dose range," Dr. Doghramji said. "One of the disadvantages is that they tend to be long acting and have anticholinergic and antihistaminic side effects." A 42-day controlled study of doxepin 25-50 mg found that the agent did not produce any change in terms of sleep latency (J. Clin. Psychiatry 2001;62:453-63). However, "it did increase their total sleep time, suggesting that they didn’t necessarily fall asleep more quickly, but they had fewer awakenings after they did fall asleep," he said. "So, if doxepin is to be used for insomnia, it seems to be best suited for the insomnia characterized by middle of the night awakening and late morning insomnia."
A 2-week study that compared trazodone with zolpidem in primary insomnia demonstrated that trazodone did seem to help people fall asleep more quickly in the first week or so (Hum. Psychopharmacol. 1998;13:191-8). "It also helped them feel as though they had slept longer," said Dr. Doghramji, who was not involved with the study. "The problem was, tolerance occurred within 2 weeks. The issue there is, should you increase the dosage or keep the same dosage for a while? We don’t have a lot of data on this."
From a pharmacokinetic standpoint, trazodone has a long half-life (5-12 hours) and features a complex set of pharmacodynamics. "It not only has some serotonergic potential, it has some histaminic potential, making it an agent that can have multiple side effects, so be careful with it," he said.
Clinicians likely use benzodiazepine receptor agonists more than any other agent for insomnia. These include the benzodiazepines, such as estazolam, flurazepam, quazepam, temazepam, and triazolam; and the non-benzodiazepines (also known as selective benzodiazepine receptor agonists) such as zaleplon, zolpidem and its various preparations (oral, sublingual, and oral spray); and eszopiclone. Adverse effects may include daytime sedation, psychomotor and cognitive impairment (depending on dose and half-life), rebound insomnia, and respiratory depression in vulnerable populations. All of these are classified as schedule IV controlled substance by the Drug Enforcement Administration. New drugs, which do not have a DEA schedule classification, include ramelteon, a melatonin receptor-agonist, and low-dose doxepin.
Choosing which antidepressant agent to use for a patient with depression and comorbid insomnia poses a certain clinical dilemma, Dr. Doghramji concluded. "Do you start with a sedating agent when your patient is both depressed and cannot sleep? Or do you put them on any old agent, regardless of whether it’s sedating or not? Unfortunately, at this point, there are not a lot of data guiding us on this."
Dr. Doghramji disclosed that he is a consultant for UCB, Teva Pharmaceuticals, Vanda Pharmaceuticals, and Jazz Pharmaceuticals, and that he holds stock in Merck.
EXPERT ANALYSIS AT THE NPA PSYCHOPHARMACOLOGY UPDATE
A different take on sensory deprivation
Recently, my local newspaper featured a story by Julie Scharper entitled "What I found in the sensory-deprivation chamber" (Baltimore Sun, Jan. 11, 2014) about a new local business known as a flotation spa, a trend currently spreading across the East Coast. For $50 an hour (or $70 for 90 minutes), the customer is escorted to a dark and quiet room, where she floats nude in a body-temperature pool of Epsom salts.
In this environment, the client reportedly enters a "drug-free altered state" intended to soothe aches and tension, as well as ease sleep problems. The author described her experience in the pool, during which she had a vivid daydream of a woman playing a red piano. Soon, a new idea for a children’s book sprang into her mind, and she left the session relaxed and filled with creative energy.
A while ago I reviewed some of the old research about sensory deprivation. Psychologists and psychiatrists began studying this topic following the Korean War, when the Central Intelligence Agency and the Department of Defense wanted to learn more about conditions that would make people more susceptible to brainwashing. They placed people in baths of water while covering their eyes and ears, and encased their limbs in protective coverings to minimize tactile input. The subjects were then interviewed about their experiences and were monitored through EEGs. They reported many disturbances, such as alterations in concentration and attention, illusions, anxiety and panic, and perceptual disturbances inaccurately described as hallucinations.
All of these symptoms resolved spontaneously after the subjects were removed from the deprivation chamber. This amorphous constellation of sensations was later given the label "special housing unit" (SHU) syndrome when it occurred in prisoners held in long-term segregation. One longstanding opponent of long-term segregation, who also frequently appeared as an expert in suits against control unit prisons, referred to sensory deprivation as "toxic" to brain functioning and a cause of stupor and delirium in segregated prisoners.
Critics of this theory, and I count myself among them, point out that current control unit conditions are hardly anything like a sensory deprivation chamber. Although segregation is less noisy and stimulating than a general population tier, it is hardly without distractions. Segregated inmates still have access to mail and recreation if they are not segregated for disciplinary reasons. They have contact with other people, although not always other inmates. Most facilities do regular rounds to check on prisoners in segregated tiers, and confined inmates can still have access to psychiatric services. In contrast to the SHU syndrome proponents, I rarely see psychological deterioration in segregated prisoners. There are even inmates who request segregated confinement specifically because it is less stimulating than general population.
So what makes sensory deprivation "cruel and unusual" to some but a source of energy and relaxation to others?
Expectation counts for a lot. Inmates placed in disciplinary segregation are not happy to be there – they are cut off from visits and ready access to the telephone, as well as certain personal property like a radio or television if they had one. A disciplinary segregation inmate enters the cell with the expectation that the experience will be punishment. In contrast, a flotation spa client is prepared for the experience by being told what positive experiences to expect and that these positive effects will carry over after the spa session ends.
Similarly, when I interview prisoners, I find that the most successful ones are those who are able to shift their own individual mindsets from an expectation of punishment to one of anticipated opportunity. The punishment mindset ("I’m here for no reason, so I’m going to spend my time complaining") is a hefty barrier to rehabilitation. An inmate who is able to accept the reality of his confinement and his responsibility for it ("I put myself here, so I better make my time work for me") will find ways to adapt psychologically, regardless of sentence length.
Reading about flotation spas brought on a weird sense of deja vu for me, but it also was a useful reminder of the effects of expectation and outlook for managing many life experiences.
Dr. Hanson is a forensic psychiatrist and coauthor of "Shrink Rap: Three Psychiatrists Explain Their Work" (Baltimore: The Johns Hopkins University Press, 2011). The opinions expressed are those of the author only, and do not represent those of any of Dr. Hanson’s employers or consultees, including the Maryland Department of Health and Mental Hygiene or the Maryland Division of Correction.
Recently, my local newspaper featured a story by Julie Scharper entitled "What I found in the sensory-deprivation chamber" (Baltimore Sun, Jan. 11, 2014) about a new local business known as a flotation spa, a trend currently spreading across the East Coast. For $50 an hour (or $70 for 90 minutes), the customer is escorted to a dark and quiet room, where she floats nude in a body-temperature pool of Epsom salts.
In this environment, the client reportedly enters a "drug-free altered state" intended to soothe aches and tension, as well as ease sleep problems. The author described her experience in the pool, during which she had a vivid daydream of a woman playing a red piano. Soon, a new idea for a children’s book sprang into her mind, and she left the session relaxed and filled with creative energy.
A while ago I reviewed some of the old research about sensory deprivation. Psychologists and psychiatrists began studying this topic following the Korean War, when the Central Intelligence Agency and the Department of Defense wanted to learn more about conditions that would make people more susceptible to brainwashing. They placed people in baths of water while covering their eyes and ears, and encased their limbs in protective coverings to minimize tactile input. The subjects were then interviewed about their experiences and were monitored through EEGs. They reported many disturbances, such as alterations in concentration and attention, illusions, anxiety and panic, and perceptual disturbances inaccurately described as hallucinations.
All of these symptoms resolved spontaneously after the subjects were removed from the deprivation chamber. This amorphous constellation of sensations was later given the label "special housing unit" (SHU) syndrome when it occurred in prisoners held in long-term segregation. One longstanding opponent of long-term segregation, who also frequently appeared as an expert in suits against control unit prisons, referred to sensory deprivation as "toxic" to brain functioning and a cause of stupor and delirium in segregated prisoners.
Critics of this theory, and I count myself among them, point out that current control unit conditions are hardly anything like a sensory deprivation chamber. Although segregation is less noisy and stimulating than a general population tier, it is hardly without distractions. Segregated inmates still have access to mail and recreation if they are not segregated for disciplinary reasons. They have contact with other people, although not always other inmates. Most facilities do regular rounds to check on prisoners in segregated tiers, and confined inmates can still have access to psychiatric services. In contrast to the SHU syndrome proponents, I rarely see psychological deterioration in segregated prisoners. There are even inmates who request segregated confinement specifically because it is less stimulating than general population.
So what makes sensory deprivation "cruel and unusual" to some but a source of energy and relaxation to others?
Expectation counts for a lot. Inmates placed in disciplinary segregation are not happy to be there – they are cut off from visits and ready access to the telephone, as well as certain personal property like a radio or television if they had one. A disciplinary segregation inmate enters the cell with the expectation that the experience will be punishment. In contrast, a flotation spa client is prepared for the experience by being told what positive experiences to expect and that these positive effects will carry over after the spa session ends.
Similarly, when I interview prisoners, I find that the most successful ones are those who are able to shift their own individual mindsets from an expectation of punishment to one of anticipated opportunity. The punishment mindset ("I’m here for no reason, so I’m going to spend my time complaining") is a hefty barrier to rehabilitation. An inmate who is able to accept the reality of his confinement and his responsibility for it ("I put myself here, so I better make my time work for me") will find ways to adapt psychologically, regardless of sentence length.
Reading about flotation spas brought on a weird sense of deja vu for me, but it also was a useful reminder of the effects of expectation and outlook for managing many life experiences.
Dr. Hanson is a forensic psychiatrist and coauthor of "Shrink Rap: Three Psychiatrists Explain Their Work" (Baltimore: The Johns Hopkins University Press, 2011). The opinions expressed are those of the author only, and do not represent those of any of Dr. Hanson’s employers or consultees, including the Maryland Department of Health and Mental Hygiene or the Maryland Division of Correction.
Recently, my local newspaper featured a story by Julie Scharper entitled "What I found in the sensory-deprivation chamber" (Baltimore Sun, Jan. 11, 2014) about a new local business known as a flotation spa, a trend currently spreading across the East Coast. For $50 an hour (or $70 for 90 minutes), the customer is escorted to a dark and quiet room, where she floats nude in a body-temperature pool of Epsom salts.
In this environment, the client reportedly enters a "drug-free altered state" intended to soothe aches and tension, as well as ease sleep problems. The author described her experience in the pool, during which she had a vivid daydream of a woman playing a red piano. Soon, a new idea for a children’s book sprang into her mind, and she left the session relaxed and filled with creative energy.
A while ago I reviewed some of the old research about sensory deprivation. Psychologists and psychiatrists began studying this topic following the Korean War, when the Central Intelligence Agency and the Department of Defense wanted to learn more about conditions that would make people more susceptible to brainwashing. They placed people in baths of water while covering their eyes and ears, and encased their limbs in protective coverings to minimize tactile input. The subjects were then interviewed about their experiences and were monitored through EEGs. They reported many disturbances, such as alterations in concentration and attention, illusions, anxiety and panic, and perceptual disturbances inaccurately described as hallucinations.
All of these symptoms resolved spontaneously after the subjects were removed from the deprivation chamber. This amorphous constellation of sensations was later given the label "special housing unit" (SHU) syndrome when it occurred in prisoners held in long-term segregation. One longstanding opponent of long-term segregation, who also frequently appeared as an expert in suits against control unit prisons, referred to sensory deprivation as "toxic" to brain functioning and a cause of stupor and delirium in segregated prisoners.
Critics of this theory, and I count myself among them, point out that current control unit conditions are hardly anything like a sensory deprivation chamber. Although segregation is less noisy and stimulating than a general population tier, it is hardly without distractions. Segregated inmates still have access to mail and recreation if they are not segregated for disciplinary reasons. They have contact with other people, although not always other inmates. Most facilities do regular rounds to check on prisoners in segregated tiers, and confined inmates can still have access to psychiatric services. In contrast to the SHU syndrome proponents, I rarely see psychological deterioration in segregated prisoners. There are even inmates who request segregated confinement specifically because it is less stimulating than general population.
So what makes sensory deprivation "cruel and unusual" to some but a source of energy and relaxation to others?
Expectation counts for a lot. Inmates placed in disciplinary segregation are not happy to be there – they are cut off from visits and ready access to the telephone, as well as certain personal property like a radio or television if they had one. A disciplinary segregation inmate enters the cell with the expectation that the experience will be punishment. In contrast, a flotation spa client is prepared for the experience by being told what positive experiences to expect and that these positive effects will carry over after the spa session ends.
Similarly, when I interview prisoners, I find that the most successful ones are those who are able to shift their own individual mindsets from an expectation of punishment to one of anticipated opportunity. The punishment mindset ("I’m here for no reason, so I’m going to spend my time complaining") is a hefty barrier to rehabilitation. An inmate who is able to accept the reality of his confinement and his responsibility for it ("I put myself here, so I better make my time work for me") will find ways to adapt psychologically, regardless of sentence length.
Reading about flotation spas brought on a weird sense of deja vu for me, but it also was a useful reminder of the effects of expectation and outlook for managing many life experiences.
Dr. Hanson is a forensic psychiatrist and coauthor of "Shrink Rap: Three Psychiatrists Explain Their Work" (Baltimore: The Johns Hopkins University Press, 2011). The opinions expressed are those of the author only, and do not represent those of any of Dr. Hanson’s employers or consultees, including the Maryland Department of Health and Mental Hygiene or the Maryland Division of Correction.
Who are the mentally ill? Take a survey
I’ve been a psychiatrist for more than 2 decades, and as such, I treat patients who suffer from mental illnesses. I will be the first to admit that when I heard President Obama say we need to keep guns out of the hands of the mentally ill, I had no idea who he was talking about. I believe we should take guns out of the hands of almost everyone.
The term "mental illness" gets bandied about quite freely, and the designation of mental illness buys both stigma and entitlements. The mentally ill can take their service dogs on planes for free. This is not true for those without a diagnosis; the same dog is just a pet on a plane, and he goes in cargo for a fee. Psychiatric diagnoses sometimes allow access to good things, including extra time to take exams, smaller classes with one-on-one aides for emotionally disturbed children, monthly disability payments and Medicare benefits from the government, as well as admission to day programs and vocational rehabilitation, and sometimes even housing. Those who meet criteria for specific disorders may be able to use their health insurance benefits to pay for psychotherapy. But are all those people the mentally ill?
Mental illness is more frequently a term that is associated with stigma and the assumption of limitations. The designation might restrict a person from owning a gun, driving a car, holding certain jobs, or even – as Andrew Solomon pointed out in a New York Times editorial – passing through the country on the way to a vacation cruise ship. The National Alliance on Mental Illness posts on its website that one in four Americans are affected by these issues and lists the specific disorders that qualify. Do we all agree that everyone with these specific diagnoses are the mentally ill?
I thought I’d ask the question, but I’m not an academic psychiatrist. I hear it takes time and money to conduct surveys through the usual channels; there are grants to be written and submitted, institutional review boards to approach, instruments to validate, a population to identify, and statistics to be analyzed. That process could take months, if not years, and I actually wanted to know this right away, so I decided to ask my questions as a social media experiment. It’s not science, but it’s fast and it’s free.
I designed some questions to look at different aspects of what might constitute the public opinion of mental illness. Is it defined by who delivers the treatment? By the medications a patient takes? By the patient’s diagnosis? By behaviors? By time spent in a hospital? I put the questions together on a free Google form, and posted the survey to my free Shrink Rap blogger site, and began to circulate the survey on Twitter, Facebook, and listservs, and then I asked others to retweet it, which many kindly did. With my budget of $0, the only cost was my uncompensated time, and the weather was kind enough to oblige me; it snowed here in Maryland recently. Many of my patients cancelled and my time was uncompensated, anyway. The question remained, Would someone see a tweet, click through to read a blog post, and then take a survey?
The form went up, and Dr. Steve Daviss, my colleague in our Accessible Psychiatry Project, immediately texted me. The survey is offensive and should come down. It gives credence to those who want to paint the mentally ill with a single brush stroke, and at a minimum, I should ask about "people with mental illness."
I hoped respondents would understand that I was not in favor of such terminology and decided to leave it up for a little while, with the idea that I would take the survey down if commenters complained. Hundreds of people have now taken the survey. No one has yet complained about my use of the term, but several people – among them some psychiatrists – have told me the questions made them uncomfortable. Let’s face it, labeling people as mentally ill is uncomfortable.
With that as a prelude, I invite you to be part of this social media experiment and to add your voice to the question, "Who are the mentally ill?" The survey takes roughly 3 minutes. Click here to take the survey. I’ll also invite you to share the link with your family, followers, friends, circles, and listservs, but I will ask that each person take the survey only once. Thank you for participating, and I welcome your feedback.
Dr. Miller is a coauthor of "Shrink Rap: Three Psychiatrists Explain Their Work" (Baltimore: the Johns Hopkins University Press, 2011).
I’ve been a psychiatrist for more than 2 decades, and as such, I treat patients who suffer from mental illnesses. I will be the first to admit that when I heard President Obama say we need to keep guns out of the hands of the mentally ill, I had no idea who he was talking about. I believe we should take guns out of the hands of almost everyone.
The term "mental illness" gets bandied about quite freely, and the designation of mental illness buys both stigma and entitlements. The mentally ill can take their service dogs on planes for free. This is not true for those without a diagnosis; the same dog is just a pet on a plane, and he goes in cargo for a fee. Psychiatric diagnoses sometimes allow access to good things, including extra time to take exams, smaller classes with one-on-one aides for emotionally disturbed children, monthly disability payments and Medicare benefits from the government, as well as admission to day programs and vocational rehabilitation, and sometimes even housing. Those who meet criteria for specific disorders may be able to use their health insurance benefits to pay for psychotherapy. But are all those people the mentally ill?
Mental illness is more frequently a term that is associated with stigma and the assumption of limitations. The designation might restrict a person from owning a gun, driving a car, holding certain jobs, or even – as Andrew Solomon pointed out in a New York Times editorial – passing through the country on the way to a vacation cruise ship. The National Alliance on Mental Illness posts on its website that one in four Americans are affected by these issues and lists the specific disorders that qualify. Do we all agree that everyone with these specific diagnoses are the mentally ill?
I thought I’d ask the question, but I’m not an academic psychiatrist. I hear it takes time and money to conduct surveys through the usual channels; there are grants to be written and submitted, institutional review boards to approach, instruments to validate, a population to identify, and statistics to be analyzed. That process could take months, if not years, and I actually wanted to know this right away, so I decided to ask my questions as a social media experiment. It’s not science, but it’s fast and it’s free.
I designed some questions to look at different aspects of what might constitute the public opinion of mental illness. Is it defined by who delivers the treatment? By the medications a patient takes? By the patient’s diagnosis? By behaviors? By time spent in a hospital? I put the questions together on a free Google form, and posted the survey to my free Shrink Rap blogger site, and began to circulate the survey on Twitter, Facebook, and listservs, and then I asked others to retweet it, which many kindly did. With my budget of $0, the only cost was my uncompensated time, and the weather was kind enough to oblige me; it snowed here in Maryland recently. Many of my patients cancelled and my time was uncompensated, anyway. The question remained, Would someone see a tweet, click through to read a blog post, and then take a survey?
The form went up, and Dr. Steve Daviss, my colleague in our Accessible Psychiatry Project, immediately texted me. The survey is offensive and should come down. It gives credence to those who want to paint the mentally ill with a single brush stroke, and at a minimum, I should ask about "people with mental illness."
I hoped respondents would understand that I was not in favor of such terminology and decided to leave it up for a little while, with the idea that I would take the survey down if commenters complained. Hundreds of people have now taken the survey. No one has yet complained about my use of the term, but several people – among them some psychiatrists – have told me the questions made them uncomfortable. Let’s face it, labeling people as mentally ill is uncomfortable.
With that as a prelude, I invite you to be part of this social media experiment and to add your voice to the question, "Who are the mentally ill?" The survey takes roughly 3 minutes. Click here to take the survey. I’ll also invite you to share the link with your family, followers, friends, circles, and listservs, but I will ask that each person take the survey only once. Thank you for participating, and I welcome your feedback.
Dr. Miller is a coauthor of "Shrink Rap: Three Psychiatrists Explain Their Work" (Baltimore: the Johns Hopkins University Press, 2011).
I’ve been a psychiatrist for more than 2 decades, and as such, I treat patients who suffer from mental illnesses. I will be the first to admit that when I heard President Obama say we need to keep guns out of the hands of the mentally ill, I had no idea who he was talking about. I believe we should take guns out of the hands of almost everyone.
The term "mental illness" gets bandied about quite freely, and the designation of mental illness buys both stigma and entitlements. The mentally ill can take their service dogs on planes for free. This is not true for those without a diagnosis; the same dog is just a pet on a plane, and he goes in cargo for a fee. Psychiatric diagnoses sometimes allow access to good things, including extra time to take exams, smaller classes with one-on-one aides for emotionally disturbed children, monthly disability payments and Medicare benefits from the government, as well as admission to day programs and vocational rehabilitation, and sometimes even housing. Those who meet criteria for specific disorders may be able to use their health insurance benefits to pay for psychotherapy. But are all those people the mentally ill?
Mental illness is more frequently a term that is associated with stigma and the assumption of limitations. The designation might restrict a person from owning a gun, driving a car, holding certain jobs, or even – as Andrew Solomon pointed out in a New York Times editorial – passing through the country on the way to a vacation cruise ship. The National Alliance on Mental Illness posts on its website that one in four Americans are affected by these issues and lists the specific disorders that qualify. Do we all agree that everyone with these specific diagnoses are the mentally ill?
I thought I’d ask the question, but I’m not an academic psychiatrist. I hear it takes time and money to conduct surveys through the usual channels; there are grants to be written and submitted, institutional review boards to approach, instruments to validate, a population to identify, and statistics to be analyzed. That process could take months, if not years, and I actually wanted to know this right away, so I decided to ask my questions as a social media experiment. It’s not science, but it’s fast and it’s free.
I designed some questions to look at different aspects of what might constitute the public opinion of mental illness. Is it defined by who delivers the treatment? By the medications a patient takes? By the patient’s diagnosis? By behaviors? By time spent in a hospital? I put the questions together on a free Google form, and posted the survey to my free Shrink Rap blogger site, and began to circulate the survey on Twitter, Facebook, and listservs, and then I asked others to retweet it, which many kindly did. With my budget of $0, the only cost was my uncompensated time, and the weather was kind enough to oblige me; it snowed here in Maryland recently. Many of my patients cancelled and my time was uncompensated, anyway. The question remained, Would someone see a tweet, click through to read a blog post, and then take a survey?
The form went up, and Dr. Steve Daviss, my colleague in our Accessible Psychiatry Project, immediately texted me. The survey is offensive and should come down. It gives credence to those who want to paint the mentally ill with a single brush stroke, and at a minimum, I should ask about "people with mental illness."
I hoped respondents would understand that I was not in favor of such terminology and decided to leave it up for a little while, with the idea that I would take the survey down if commenters complained. Hundreds of people have now taken the survey. No one has yet complained about my use of the term, but several people – among them some psychiatrists – have told me the questions made them uncomfortable. Let’s face it, labeling people as mentally ill is uncomfortable.
With that as a prelude, I invite you to be part of this social media experiment and to add your voice to the question, "Who are the mentally ill?" The survey takes roughly 3 minutes. Click here to take the survey. I’ll also invite you to share the link with your family, followers, friends, circles, and listservs, but I will ask that each person take the survey only once. Thank you for participating, and I welcome your feedback.
Dr. Miller is a coauthor of "Shrink Rap: Three Psychiatrists Explain Their Work" (Baltimore: the Johns Hopkins University Press, 2011).
Depression accounts for psoriatics’ increased MI risk
SAN DIEGO – Depression is an independent risk factor for acute myocardial infarction in patients with psoriasis or psoriatic arthritis, a large population-based cohort study indicates.
In this study of more than 10,000 British Columbians with psoriasis and/or psoriatic arthritis, the increased risk of MI was confined to the patient subset having comorbid depression, Lindsay C. Burns, Ph.D., reported at the annual meeting of the American College of Rheumatology.
"These data underscore the need to actively screen for depression among psoriasis and psoriatic arthritis patients and closely monitor cardiovascular health in this high-risk group to improve long-term survival," declared Dr. Burns of the University of British Columbia, Vancouver.
She and her coinvestigators mined the comprehensive health records available for 4.1 million adults through British Columbia’s universal medical insurance coverage system in order to identify all 10,041 patients who were diagnosed with psoriasis by a dermatologist or psoriatic arthritis by a rheumatologist during 1996-2006, and who at that time had no history of MI. The patients were matched by age, gender, and years of follow-up with 47,415 controls.
Acute MI occurred in 268 patients with psoriasis or psoriatic arthritis, for an incidence rate of 5.8 cases per 1,000 person-years. The incidence rate of physician-diagnosed depression was 3.4 per 1,000 person-years in the psoriatic group, with a 10-year prevalence of 21.6%.
In a multivariate regression analysis adjusted for comorbid conditions, socioeconomic status, health resource utilization, age, and gender, individuals with psoriasis or psoriatic arthritis were 26% more likely to be depressed than controls. Diagnosis of depression in psoriatic patients during the follow-up period increased their risk of having an MI by an adjusted 80% compared with psoriatic patients without the psychiatric diagnosis.
Psoriatic subjects without depression had a statistically nonsignificant 10% increased risk of MI compared with nonpsoriatic controls. In contrast, psoriatic patients with diagnosed depression had a 60% greater MI risk than controls, according to Dr. Burns.
The study was funded by the Arthritis Research Center of Canada. Dr. Burns reported having no financial conflicts of interest.
SAN DIEGO – Depression is an independent risk factor for acute myocardial infarction in patients with psoriasis or psoriatic arthritis, a large population-based cohort study indicates.
In this study of more than 10,000 British Columbians with psoriasis and/or psoriatic arthritis, the increased risk of MI was confined to the patient subset having comorbid depression, Lindsay C. Burns, Ph.D., reported at the annual meeting of the American College of Rheumatology.
"These data underscore the need to actively screen for depression among psoriasis and psoriatic arthritis patients and closely monitor cardiovascular health in this high-risk group to improve long-term survival," declared Dr. Burns of the University of British Columbia, Vancouver.
She and her coinvestigators mined the comprehensive health records available for 4.1 million adults through British Columbia’s universal medical insurance coverage system in order to identify all 10,041 patients who were diagnosed with psoriasis by a dermatologist or psoriatic arthritis by a rheumatologist during 1996-2006, and who at that time had no history of MI. The patients were matched by age, gender, and years of follow-up with 47,415 controls.
Acute MI occurred in 268 patients with psoriasis or psoriatic arthritis, for an incidence rate of 5.8 cases per 1,000 person-years. The incidence rate of physician-diagnosed depression was 3.4 per 1,000 person-years in the psoriatic group, with a 10-year prevalence of 21.6%.
In a multivariate regression analysis adjusted for comorbid conditions, socioeconomic status, health resource utilization, age, and gender, individuals with psoriasis or psoriatic arthritis were 26% more likely to be depressed than controls. Diagnosis of depression in psoriatic patients during the follow-up period increased their risk of having an MI by an adjusted 80% compared with psoriatic patients without the psychiatric diagnosis.
Psoriatic subjects without depression had a statistically nonsignificant 10% increased risk of MI compared with nonpsoriatic controls. In contrast, psoriatic patients with diagnosed depression had a 60% greater MI risk than controls, according to Dr. Burns.
The study was funded by the Arthritis Research Center of Canada. Dr. Burns reported having no financial conflicts of interest.
SAN DIEGO – Depression is an independent risk factor for acute myocardial infarction in patients with psoriasis or psoriatic arthritis, a large population-based cohort study indicates.
In this study of more than 10,000 British Columbians with psoriasis and/or psoriatic arthritis, the increased risk of MI was confined to the patient subset having comorbid depression, Lindsay C. Burns, Ph.D., reported at the annual meeting of the American College of Rheumatology.
"These data underscore the need to actively screen for depression among psoriasis and psoriatic arthritis patients and closely monitor cardiovascular health in this high-risk group to improve long-term survival," declared Dr. Burns of the University of British Columbia, Vancouver.
She and her coinvestigators mined the comprehensive health records available for 4.1 million adults through British Columbia’s universal medical insurance coverage system in order to identify all 10,041 patients who were diagnosed with psoriasis by a dermatologist or psoriatic arthritis by a rheumatologist during 1996-2006, and who at that time had no history of MI. The patients were matched by age, gender, and years of follow-up with 47,415 controls.
Acute MI occurred in 268 patients with psoriasis or psoriatic arthritis, for an incidence rate of 5.8 cases per 1,000 person-years. The incidence rate of physician-diagnosed depression was 3.4 per 1,000 person-years in the psoriatic group, with a 10-year prevalence of 21.6%.
In a multivariate regression analysis adjusted for comorbid conditions, socioeconomic status, health resource utilization, age, and gender, individuals with psoriasis or psoriatic arthritis were 26% more likely to be depressed than controls. Diagnosis of depression in psoriatic patients during the follow-up period increased their risk of having an MI by an adjusted 80% compared with psoriatic patients without the psychiatric diagnosis.
Psoriatic subjects without depression had a statistically nonsignificant 10% increased risk of MI compared with nonpsoriatic controls. In contrast, psoriatic patients with diagnosed depression had a 60% greater MI risk than controls, according to Dr. Burns.
The study was funded by the Arthritis Research Center of Canada. Dr. Burns reported having no financial conflicts of interest.
AT THE ACR ANNUAL MEETING
Major finding: Patients with psoriasis or psoriatic arthritis who developed comorbid depression had a highly significant 80% increased risk of acute MI during follow-up, compared with those without depression.
Data source: This was a population-based cohort study involving 10,041 adults diagnosed with psoriasis or psoriatic arthritis in British Columbia during 1996-2006 and more than 47,000 controls.
Disclosures: The study was funded by the Arthritis Research Center of Canada. Dr. Burns reported having no financial conflicts of interest.
Depression, anxiety common in SLE patients
Nearly half of patients with systemic lupus erythematosus had at least one mood or anxiety disorder, and slightly more than one-third had at least one personality disorder, the results from a small cross-sectional study demonstrated.
"When our results, including the onset time of mood or anxiety disorders, are considered, these disorders seem to be secondary to the severity of lupus rather than play a primary role in the exacerbation of SLE," Turkish researchers led by Dr. Faruk Uguz wrote. "However, it is unclear whether there is a biological basis of this condition or psychological reaction to the severity of SLE."
Dr. Uguz, a psychiatrist with the University of Necmettin Erbakan, Konya, Turkey, and his associates evaluated 45 consecutive patients with SLE who were admitted to the university’s rheumatology outpatient clinic. They also included a control group of 60 hospital personnel and their relatives who were matched for the sociodemographic characteristics of the SLE patients.
Exclusion criteria included illiteracy, cognitive incompetence, a history of schizophrenia or related psychotic disorders, a history of neurologic diseases or severe neurologic manifestations of SLE, concomitant serious medical illnesses such as uncontrolled endocrine abnormalities, cardiovascular disease, movement disorder, and the use of psychotropic medication within the last 4 weeks.
The researchers used the American College of Rheumatology revised criteria for SLE to diagnose SLE, the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition/Clinical Version to diagnose mood and anxiety disorders, and the Structured Clinical Interview for DSM, Revised Third Edition to diagnose personality disorders (Compr. Psychiatry 2013;54:341-5). Psychiatric interviews were conducted by psychiatrists with at least 4 years of experience with psychiatric disorders and diagnostic instruments.
The mean age of the study participants was 40 years. Most (91%) were female, married (85%), and unemployed (88%). The disease duration was a mean of 67 months in the patient group. Dr. Uguz and his colleagues reported that 21 people in the patient group (47%) had at least one mood or anxiety disorder and 16 (36%) had any personality disorder. By contrast, 10 people in the control group (17%) had at least one mood or anxiety disorder and 7 (12%) had any personality disorder.
Among SLE patients, the most common psychiatric disorders were major depression (22%) and generalized anxiety disorder (16%). The conditions affected 5% and 3% of control subjects, respectively.
"Being of a cross-sectional nature, the present study is limited by an insufficiency to indicate whether the evaluated Axis I or Axis II psychiatric disorders have causal relevance to SLE," the researchers wrote. "It is unclear whether Axis I disorders are a contributing factor that may exacerbate SLE, or are secondary to the development of a more active SLE."
Another limitation of the study is its small sample size, they said, as well as its failure to determine whether a patient had a family history of mental disorders. "Further controlled studies with larger sample sizes should be conducted to investigate long-term effects of psychiatric disorders and their treatments in the course of SLE," the investigators concluded.
Nearly half of patients with systemic lupus erythematosus had at least one mood or anxiety disorder, and slightly more than one-third had at least one personality disorder, the results from a small cross-sectional study demonstrated.
"When our results, including the onset time of mood or anxiety disorders, are considered, these disorders seem to be secondary to the severity of lupus rather than play a primary role in the exacerbation of SLE," Turkish researchers led by Dr. Faruk Uguz wrote. "However, it is unclear whether there is a biological basis of this condition or psychological reaction to the severity of SLE."
Dr. Uguz, a psychiatrist with the University of Necmettin Erbakan, Konya, Turkey, and his associates evaluated 45 consecutive patients with SLE who were admitted to the university’s rheumatology outpatient clinic. They also included a control group of 60 hospital personnel and their relatives who were matched for the sociodemographic characteristics of the SLE patients.
Exclusion criteria included illiteracy, cognitive incompetence, a history of schizophrenia or related psychotic disorders, a history of neurologic diseases or severe neurologic manifestations of SLE, concomitant serious medical illnesses such as uncontrolled endocrine abnormalities, cardiovascular disease, movement disorder, and the use of psychotropic medication within the last 4 weeks.
The researchers used the American College of Rheumatology revised criteria for SLE to diagnose SLE, the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition/Clinical Version to diagnose mood and anxiety disorders, and the Structured Clinical Interview for DSM, Revised Third Edition to diagnose personality disorders (Compr. Psychiatry 2013;54:341-5). Psychiatric interviews were conducted by psychiatrists with at least 4 years of experience with psychiatric disorders and diagnostic instruments.
The mean age of the study participants was 40 years. Most (91%) were female, married (85%), and unemployed (88%). The disease duration was a mean of 67 months in the patient group. Dr. Uguz and his colleagues reported that 21 people in the patient group (47%) had at least one mood or anxiety disorder and 16 (36%) had any personality disorder. By contrast, 10 people in the control group (17%) had at least one mood or anxiety disorder and 7 (12%) had any personality disorder.
Among SLE patients, the most common psychiatric disorders were major depression (22%) and generalized anxiety disorder (16%). The conditions affected 5% and 3% of control subjects, respectively.
"Being of a cross-sectional nature, the present study is limited by an insufficiency to indicate whether the evaluated Axis I or Axis II psychiatric disorders have causal relevance to SLE," the researchers wrote. "It is unclear whether Axis I disorders are a contributing factor that may exacerbate SLE, or are secondary to the development of a more active SLE."
Another limitation of the study is its small sample size, they said, as well as its failure to determine whether a patient had a family history of mental disorders. "Further controlled studies with larger sample sizes should be conducted to investigate long-term effects of psychiatric disorders and their treatments in the course of SLE," the investigators concluded.
Nearly half of patients with systemic lupus erythematosus had at least one mood or anxiety disorder, and slightly more than one-third had at least one personality disorder, the results from a small cross-sectional study demonstrated.
"When our results, including the onset time of mood or anxiety disorders, are considered, these disorders seem to be secondary to the severity of lupus rather than play a primary role in the exacerbation of SLE," Turkish researchers led by Dr. Faruk Uguz wrote. "However, it is unclear whether there is a biological basis of this condition or psychological reaction to the severity of SLE."
Dr. Uguz, a psychiatrist with the University of Necmettin Erbakan, Konya, Turkey, and his associates evaluated 45 consecutive patients with SLE who were admitted to the university’s rheumatology outpatient clinic. They also included a control group of 60 hospital personnel and their relatives who were matched for the sociodemographic characteristics of the SLE patients.
Exclusion criteria included illiteracy, cognitive incompetence, a history of schizophrenia or related psychotic disorders, a history of neurologic diseases or severe neurologic manifestations of SLE, concomitant serious medical illnesses such as uncontrolled endocrine abnormalities, cardiovascular disease, movement disorder, and the use of psychotropic medication within the last 4 weeks.
The researchers used the American College of Rheumatology revised criteria for SLE to diagnose SLE, the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition/Clinical Version to diagnose mood and anxiety disorders, and the Structured Clinical Interview for DSM, Revised Third Edition to diagnose personality disorders (Compr. Psychiatry 2013;54:341-5). Psychiatric interviews were conducted by psychiatrists with at least 4 years of experience with psychiatric disorders and diagnostic instruments.
The mean age of the study participants was 40 years. Most (91%) were female, married (85%), and unemployed (88%). The disease duration was a mean of 67 months in the patient group. Dr. Uguz and his colleagues reported that 21 people in the patient group (47%) had at least one mood or anxiety disorder and 16 (36%) had any personality disorder. By contrast, 10 people in the control group (17%) had at least one mood or anxiety disorder and 7 (12%) had any personality disorder.
Among SLE patients, the most common psychiatric disorders were major depression (22%) and generalized anxiety disorder (16%). The conditions affected 5% and 3% of control subjects, respectively.
"Being of a cross-sectional nature, the present study is limited by an insufficiency to indicate whether the evaluated Axis I or Axis II psychiatric disorders have causal relevance to SLE," the researchers wrote. "It is unclear whether Axis I disorders are a contributing factor that may exacerbate SLE, or are secondary to the development of a more active SLE."
Another limitation of the study is its small sample size, they said, as well as its failure to determine whether a patient had a family history of mental disorders. "Further controlled studies with larger sample sizes should be conducted to investigate long-term effects of psychiatric disorders and their treatments in the course of SLE," the investigators concluded.
FROM COMPREHENSIVE PSYCHIATRY
Major finding: Among patients with systemic lupus erythematosus, 47% had at least one mood or anxiety disorder, and 36% had at least one personality disorder.
Data source: A study of 45 patients with SLE and 60 matched controls who underwent formal testing for mood and anxiety disorders and personality disorders.
Disclosures: The researchers said they had no relevant financial conflicts.
Family narratives and the intergenerational transmission of resilience
Carmen Bugan read her poems to her family. Her father had been imprisoned by Securitate, the Romanian secret police, for anticommunist rhetoric that he distributed on leaflets to people’s mailboxes. Securitate tracked him down by examining the leaflets for identifying typescript that they linked to one of his typewriters. He buried his other typewriter in the garden to escape detection. He would dig it up when he wanted to write serious anticommunist literature, then rebury it again in the garden.
"It is not important that the poem stays or goes," Carmen writes. "I discover a way to relieve our family’s suffering even though when I read the poems to Mom and my sister it seems that I create more pain at first.
"Mom loves the words, loves explanations of feelings to negotiate pain, and I can provide this for her. My sister says her feelings are exteriorized, articulated by the emotions in the poem and I can help bring things out" ("Burying the Typewriter," Minneapolis: Graywolf Press, 2012, p. 124).
Carmen created a poetic narrative to help her family manage their suffering. In this way, she helped her family become close and share a sense of belonging together. Carmen was able to transmute the family’s experience of trauma into a story that articulated their survival. Her poems became a written narrative of her family’s history. Resilience was created and passed along through the generations. This is the intergenerational transmission of resilience.
Intergenerational transmission has been shown in trauma; antisocial behavior; violence; religion; politics; substance abuse (J. Res. Adolesc. 1995;5:225-52); depression (J. Fam. Psychol. 2003;17:545-56); attachment (Psychol. Bull. 1995;117:387-403); perfectionism (J. Fam. Psychol. 2005;19:358-66); poverty; being on welfare; teenage pregnancy; education; and family life trajectories ("Intergenerational Transmission of Behavioral Patterns: Similarity of Parents’ and Children’s Family-Life Trajectories," Netherlands Interdisciplinary Demographical Institute, The Hague, 2006).
In short, there is evidence for the intergenerational transmission of everything bad. It is time to create evidence of the intergenerational transmission of resilience.
Researchers who study intergenerational legacies have discovered that children who know the most about their families have a strong sense of control over their lives, higher self-esteem, and the strongest "intergenerational self," compared with children who know less about their families. Marshall P. Duke, Ph.D., and his colleagues developed a measure called "Do You Know?" that asks children questions about their family. Examples of questions are "Do you know where your grandparents grew up? Do you know where your mom and dad went to high school?" (Psychotherapy 2008;45,268-72).
Dr. Duke identifies three common family narratives:
• The ascending family narrative: "Son, when we came to this country, we had nothing. Our family worked. We opened a store. Your grandfather went to high school. Your father went to college. And now you ... "
• The descending narrative: "Sweetheart, we used to have it all. Then we lost everything."
• The oscillating family narrative: "Dear, let me tell you, we’ve had ups and downs in our family. We built a family business. Your grandfather was a pillar of the community. Your mother was on the board of the hospital. But we also had setbacks. You had an uncle who was once arrested. We had a house burn down. Your father lost a job. But no matter what happened, we always stuck together as a family."
Healing narratives are prominent in American Indian and folk medicine traditions but also exist in modern medicine. In psychiatry, one of the tenets of the Recovery Movement is to focus on strengths and a positive sense of identity that is not linked to a psychiatric diagnosis. Communities such as Alcoholics Anonymous, Narcotics Anonymous, and Al-Anon foster resilience through communion and sharing. Narrative therapy, developed by Australian therapist Michael White and his collaborator David Epston of New Zealand in 1989 (Context 2009;105:57-58), is a type of psychotherapy that seeks and promotes a healthy, successful personal narrative to replace a dominant repressive illness narrative.
How can the psychiatrist, during a routine office visit, help patients develop a positive, resilient family narrative? Patients can benefit from an exploration of patterns of behavior or ways of relating that might have been passed down through the generations. Understanding the motivations, difficulties, and aspirations of their parents and grandparents provides patients with a historical perspective on their current difficulties. If patients can understand their difficulties in the context of the larger family system, they develop a more nuanced and less harsh understanding of the challenges they face.
When Sarah presented with depression, it became clear that her family dynamics were troubling. She felt happy and competent at work. In passing, she remarked that she felt intimidated by her teenage daughter, so I inquired about her family system to see what generational narratives might be at play. Over several sessions, we uncovered the covert negative messages she had received as a child. She had fought not to pass these on to her children, by being "more permissive and hands off." In response, her children chided her for being overly anxious, sensing that she was conflicted and troubled, although the source remained mysterious to everyone. Using a family systems approach to understand the intergenerational inheritance, the family came to understand the strong generational forces at work. This lessened her guilt and anguish, and increased the children’s understanding and empathy for their mother.
A family systems approach allows a family legacy to be revealed, reworked, and rewritten. A new family narrative that carries the family forward and allows the telling of a positive family narrative can be created. We can guide patients to find the positive aspects of their family stories and thus promote family resilience.
Here are a few questions we should ask our patients: "What did your parents teach you that you want to pass along? What values did your parents have? How have you lived or not lived those values? How has the relationship with your parents affected your relationship with your children? How did your parents resolve problems, and how do you resolve problems? How do your children resolve problems? What were the motivations that drove your parents? What countries do your relatives come from? What was it like for them growing up? Did they experience deprivation? War? How has that affected you and your siblings? Are there family secrets? What do you want to take away from this legacy? What do you want to pass along to the next generation?" Asking these questions allows the patient to see their current struggles and conflicts with a longer lens.
The novelist Laila Lalami, who did not know her mother, was surprised when her husband gave her a DNA test kit so that she could find out her genetic inheritance. When the results came in, Laila remarked: "So it was that, in just a few moments, I found myself returning to those childhood days when I used to dream up different families, and different fates, for my mother. What science gave me, in the end, was no different from what my own imagination had fed me for many years – stories. The search was not over. The search would never be over. And not even science could help fill out the abyss I grew up with. Only stories could." ("My Fictional Grandparents," The New York Times, July 26, 2013)
We are all part of our own family narrative that stretches back in time and forward into the future. We are creating a family story for ourselves in the present that our children will carry forward with them into their future. These narratives have many strands. Let’s help our patients pick out the strands that help them build family resilience.
Dr. Heru is with the department of psychiatry at the University of Colorado at Denver, Aurora. She is editor of the recently published book, "Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals" (New York: Routledge, 2013).
Carmen Bugan read her poems to her family. Her father had been imprisoned by Securitate, the Romanian secret police, for anticommunist rhetoric that he distributed on leaflets to people’s mailboxes. Securitate tracked him down by examining the leaflets for identifying typescript that they linked to one of his typewriters. He buried his other typewriter in the garden to escape detection. He would dig it up when he wanted to write serious anticommunist literature, then rebury it again in the garden.
"It is not important that the poem stays or goes," Carmen writes. "I discover a way to relieve our family’s suffering even though when I read the poems to Mom and my sister it seems that I create more pain at first.
"Mom loves the words, loves explanations of feelings to negotiate pain, and I can provide this for her. My sister says her feelings are exteriorized, articulated by the emotions in the poem and I can help bring things out" ("Burying the Typewriter," Minneapolis: Graywolf Press, 2012, p. 124).
Carmen created a poetic narrative to help her family manage their suffering. In this way, she helped her family become close and share a sense of belonging together. Carmen was able to transmute the family’s experience of trauma into a story that articulated their survival. Her poems became a written narrative of her family’s history. Resilience was created and passed along through the generations. This is the intergenerational transmission of resilience.
Intergenerational transmission has been shown in trauma; antisocial behavior; violence; religion; politics; substance abuse (J. Res. Adolesc. 1995;5:225-52); depression (J. Fam. Psychol. 2003;17:545-56); attachment (Psychol. Bull. 1995;117:387-403); perfectionism (J. Fam. Psychol. 2005;19:358-66); poverty; being on welfare; teenage pregnancy; education; and family life trajectories ("Intergenerational Transmission of Behavioral Patterns: Similarity of Parents’ and Children’s Family-Life Trajectories," Netherlands Interdisciplinary Demographical Institute, The Hague, 2006).
In short, there is evidence for the intergenerational transmission of everything bad. It is time to create evidence of the intergenerational transmission of resilience.
Researchers who study intergenerational legacies have discovered that children who know the most about their families have a strong sense of control over their lives, higher self-esteem, and the strongest "intergenerational self," compared with children who know less about their families. Marshall P. Duke, Ph.D., and his colleagues developed a measure called "Do You Know?" that asks children questions about their family. Examples of questions are "Do you know where your grandparents grew up? Do you know where your mom and dad went to high school?" (Psychotherapy 2008;45,268-72).
Dr. Duke identifies three common family narratives:
• The ascending family narrative: "Son, when we came to this country, we had nothing. Our family worked. We opened a store. Your grandfather went to high school. Your father went to college. And now you ... "
• The descending narrative: "Sweetheart, we used to have it all. Then we lost everything."
• The oscillating family narrative: "Dear, let me tell you, we’ve had ups and downs in our family. We built a family business. Your grandfather was a pillar of the community. Your mother was on the board of the hospital. But we also had setbacks. You had an uncle who was once arrested. We had a house burn down. Your father lost a job. But no matter what happened, we always stuck together as a family."
Healing narratives are prominent in American Indian and folk medicine traditions but also exist in modern medicine. In psychiatry, one of the tenets of the Recovery Movement is to focus on strengths and a positive sense of identity that is not linked to a psychiatric diagnosis. Communities such as Alcoholics Anonymous, Narcotics Anonymous, and Al-Anon foster resilience through communion and sharing. Narrative therapy, developed by Australian therapist Michael White and his collaborator David Epston of New Zealand in 1989 (Context 2009;105:57-58), is a type of psychotherapy that seeks and promotes a healthy, successful personal narrative to replace a dominant repressive illness narrative.
How can the psychiatrist, during a routine office visit, help patients develop a positive, resilient family narrative? Patients can benefit from an exploration of patterns of behavior or ways of relating that might have been passed down through the generations. Understanding the motivations, difficulties, and aspirations of their parents and grandparents provides patients with a historical perspective on their current difficulties. If patients can understand their difficulties in the context of the larger family system, they develop a more nuanced and less harsh understanding of the challenges they face.
When Sarah presented with depression, it became clear that her family dynamics were troubling. She felt happy and competent at work. In passing, she remarked that she felt intimidated by her teenage daughter, so I inquired about her family system to see what generational narratives might be at play. Over several sessions, we uncovered the covert negative messages she had received as a child. She had fought not to pass these on to her children, by being "more permissive and hands off." In response, her children chided her for being overly anxious, sensing that she was conflicted and troubled, although the source remained mysterious to everyone. Using a family systems approach to understand the intergenerational inheritance, the family came to understand the strong generational forces at work. This lessened her guilt and anguish, and increased the children’s understanding and empathy for their mother.
A family systems approach allows a family legacy to be revealed, reworked, and rewritten. A new family narrative that carries the family forward and allows the telling of a positive family narrative can be created. We can guide patients to find the positive aspects of their family stories and thus promote family resilience.
Here are a few questions we should ask our patients: "What did your parents teach you that you want to pass along? What values did your parents have? How have you lived or not lived those values? How has the relationship with your parents affected your relationship with your children? How did your parents resolve problems, and how do you resolve problems? How do your children resolve problems? What were the motivations that drove your parents? What countries do your relatives come from? What was it like for them growing up? Did they experience deprivation? War? How has that affected you and your siblings? Are there family secrets? What do you want to take away from this legacy? What do you want to pass along to the next generation?" Asking these questions allows the patient to see their current struggles and conflicts with a longer lens.
The novelist Laila Lalami, who did not know her mother, was surprised when her husband gave her a DNA test kit so that she could find out her genetic inheritance. When the results came in, Laila remarked: "So it was that, in just a few moments, I found myself returning to those childhood days when I used to dream up different families, and different fates, for my mother. What science gave me, in the end, was no different from what my own imagination had fed me for many years – stories. The search was not over. The search would never be over. And not even science could help fill out the abyss I grew up with. Only stories could." ("My Fictional Grandparents," The New York Times, July 26, 2013)
We are all part of our own family narrative that stretches back in time and forward into the future. We are creating a family story for ourselves in the present that our children will carry forward with them into their future. These narratives have many strands. Let’s help our patients pick out the strands that help them build family resilience.
Dr. Heru is with the department of psychiatry at the University of Colorado at Denver, Aurora. She is editor of the recently published book, "Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals" (New York: Routledge, 2013).
Carmen Bugan read her poems to her family. Her father had been imprisoned by Securitate, the Romanian secret police, for anticommunist rhetoric that he distributed on leaflets to people’s mailboxes. Securitate tracked him down by examining the leaflets for identifying typescript that they linked to one of his typewriters. He buried his other typewriter in the garden to escape detection. He would dig it up when he wanted to write serious anticommunist literature, then rebury it again in the garden.
"It is not important that the poem stays or goes," Carmen writes. "I discover a way to relieve our family’s suffering even though when I read the poems to Mom and my sister it seems that I create more pain at first.
"Mom loves the words, loves explanations of feelings to negotiate pain, and I can provide this for her. My sister says her feelings are exteriorized, articulated by the emotions in the poem and I can help bring things out" ("Burying the Typewriter," Minneapolis: Graywolf Press, 2012, p. 124).
Carmen created a poetic narrative to help her family manage their suffering. In this way, she helped her family become close and share a sense of belonging together. Carmen was able to transmute the family’s experience of trauma into a story that articulated their survival. Her poems became a written narrative of her family’s history. Resilience was created and passed along through the generations. This is the intergenerational transmission of resilience.
Intergenerational transmission has been shown in trauma; antisocial behavior; violence; religion; politics; substance abuse (J. Res. Adolesc. 1995;5:225-52); depression (J. Fam. Psychol. 2003;17:545-56); attachment (Psychol. Bull. 1995;117:387-403); perfectionism (J. Fam. Psychol. 2005;19:358-66); poverty; being on welfare; teenage pregnancy; education; and family life trajectories ("Intergenerational Transmission of Behavioral Patterns: Similarity of Parents’ and Children’s Family-Life Trajectories," Netherlands Interdisciplinary Demographical Institute, The Hague, 2006).
In short, there is evidence for the intergenerational transmission of everything bad. It is time to create evidence of the intergenerational transmission of resilience.
Researchers who study intergenerational legacies have discovered that children who know the most about their families have a strong sense of control over their lives, higher self-esteem, and the strongest "intergenerational self," compared with children who know less about their families. Marshall P. Duke, Ph.D., and his colleagues developed a measure called "Do You Know?" that asks children questions about their family. Examples of questions are "Do you know where your grandparents grew up? Do you know where your mom and dad went to high school?" (Psychotherapy 2008;45,268-72).
Dr. Duke identifies three common family narratives:
• The ascending family narrative: "Son, when we came to this country, we had nothing. Our family worked. We opened a store. Your grandfather went to high school. Your father went to college. And now you ... "
• The descending narrative: "Sweetheart, we used to have it all. Then we lost everything."
• The oscillating family narrative: "Dear, let me tell you, we’ve had ups and downs in our family. We built a family business. Your grandfather was a pillar of the community. Your mother was on the board of the hospital. But we also had setbacks. You had an uncle who was once arrested. We had a house burn down. Your father lost a job. But no matter what happened, we always stuck together as a family."
Healing narratives are prominent in American Indian and folk medicine traditions but also exist in modern medicine. In psychiatry, one of the tenets of the Recovery Movement is to focus on strengths and a positive sense of identity that is not linked to a psychiatric diagnosis. Communities such as Alcoholics Anonymous, Narcotics Anonymous, and Al-Anon foster resilience through communion and sharing. Narrative therapy, developed by Australian therapist Michael White and his collaborator David Epston of New Zealand in 1989 (Context 2009;105:57-58), is a type of psychotherapy that seeks and promotes a healthy, successful personal narrative to replace a dominant repressive illness narrative.
How can the psychiatrist, during a routine office visit, help patients develop a positive, resilient family narrative? Patients can benefit from an exploration of patterns of behavior or ways of relating that might have been passed down through the generations. Understanding the motivations, difficulties, and aspirations of their parents and grandparents provides patients with a historical perspective on their current difficulties. If patients can understand their difficulties in the context of the larger family system, they develop a more nuanced and less harsh understanding of the challenges they face.
When Sarah presented with depression, it became clear that her family dynamics were troubling. She felt happy and competent at work. In passing, she remarked that she felt intimidated by her teenage daughter, so I inquired about her family system to see what generational narratives might be at play. Over several sessions, we uncovered the covert negative messages she had received as a child. She had fought not to pass these on to her children, by being "more permissive and hands off." In response, her children chided her for being overly anxious, sensing that she was conflicted and troubled, although the source remained mysterious to everyone. Using a family systems approach to understand the intergenerational inheritance, the family came to understand the strong generational forces at work. This lessened her guilt and anguish, and increased the children’s understanding and empathy for their mother.
A family systems approach allows a family legacy to be revealed, reworked, and rewritten. A new family narrative that carries the family forward and allows the telling of a positive family narrative can be created. We can guide patients to find the positive aspects of their family stories and thus promote family resilience.
Here are a few questions we should ask our patients: "What did your parents teach you that you want to pass along? What values did your parents have? How have you lived or not lived those values? How has the relationship with your parents affected your relationship with your children? How did your parents resolve problems, and how do you resolve problems? How do your children resolve problems? What were the motivations that drove your parents? What countries do your relatives come from? What was it like for them growing up? Did they experience deprivation? War? How has that affected you and your siblings? Are there family secrets? What do you want to take away from this legacy? What do you want to pass along to the next generation?" Asking these questions allows the patient to see their current struggles and conflicts with a longer lens.
The novelist Laila Lalami, who did not know her mother, was surprised when her husband gave her a DNA test kit so that she could find out her genetic inheritance. When the results came in, Laila remarked: "So it was that, in just a few moments, I found myself returning to those childhood days when I used to dream up different families, and different fates, for my mother. What science gave me, in the end, was no different from what my own imagination had fed me for many years – stories. The search was not over. The search would never be over. And not even science could help fill out the abyss I grew up with. Only stories could." ("My Fictional Grandparents," The New York Times, July 26, 2013)
We are all part of our own family narrative that stretches back in time and forward into the future. We are creating a family story for ourselves in the present that our children will carry forward with them into their future. These narratives have many strands. Let’s help our patients pick out the strands that help them build family resilience.
Dr. Heru is with the department of psychiatry at the University of Colorado at Denver, Aurora. She is editor of the recently published book, "Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals" (New York: Routledge, 2013).
Novel intervention improves outcomes in homeless schizophrenia patients
HOLLYWOOD, FLA. – A manualized psychosocial intervention known as customized adherence enhancement coupled with long-acting injectable antipsychotic medication produced marked improvement in adherence to concomitant oral medication as well as in psychiatric symptoms and functional status in urban homeless people with schizophrenia in a small prospective observational study.
Moreover, the intervention also achieved an impressive improvement in a domain not often considered in psychiatric studies, but one of great practical value to patients and their families: housing conditions, Dr. Martha Sajatovic said at a meeting of the New Clinical Drug Evaluation Unit sponsored by the National Institute of Mental Health
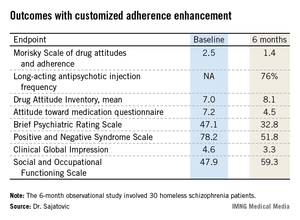
The mean time spent in suboptimal housing – living outdoors or in jail – changed from 56% during the 6 months prior to study enrollment to 41% in the first 3 months of the intervention and a mere 14% in the second 3 months of the study, reported Dr. Sajatovic, professor of psychiatry and neurology at Case Western Reserve University, Cleveland.
"What I think is most interesting about our study are the housing data. Our intervention did not include anything about housing – no housing placement or outreach or anything like that. People used whatever was available in the community. And Cleveland has the dubious distinction of being one of the poorest cities in America, so we have services, but not a lot of them," she noted.
Customized adherence enhancement (CAE) is a needs-based, flexibly dosed psychosocial intervention originally developed by Dr. Sajatovic and her colleagues to improve medication adherence in individuals with bipolar disorder receiving antipsychotic therapy. Following a 6-month study demonstrating its benefits in this population (Bipolar Disord. 2012;14:291-300), the investigators adapted the manualized CAE program for application in a particularly challenging patient population: homeless people with schizophrenia/schizoaffective disorder and poor adherence to their prescribed oral antipsychotic medication.
This 6-month, observational, uncontrolled study involved 30 homeless schizophrenic patients. The CAE consisted of once-monthly brief interventions matched to core adherence vulnerabilities identified at baseline. The CAE entailed psychoeducation, modified motivational enhancement therapy, and coaching on communication with providers and medication routines.
At the time of the monthly CAE session, patients also received a dose of long-acting injectable antipsychotic medication. Because the study was funded by a small nonprofit charitable organization, investigators employed the least expensive long-acting antipsychotic available: intramuscular haloperidol decanoate. This resulted in a high rate of side effects, most prominently restlessness because of akathisia in 40% of subjects, dry mouth in 33%, and muscle twitching in 33%. The mean monthly dose at 24 weeks was 68 mg, with a range of 50-100 mg.
The study population was typical of homeless individuals with major mental illness. Their mean age was 42 years, roughly half were women, 97% had a history of substance abuse, 97% had a history of incarceration, most subjects had not finished high school, and 70% were single or never married.
All patients remained on oral medication in addition to their new long-acting injectable antipsychotic regimen. Adherence to oral medication improved dramatically during the 6-month intervention: At baseline, patients had missed 46% of their oral medication during the past month, compared with just 10% at week 25. Adherence to long-acting injectable therapy was good: 76% at 6 months.
Twenty of 30 patients completed the 6-month study. Of the 10 nonfinishers, 3 were incarcerated, 2 relocated elsewhere, 1 discontinued because of drug-induced akathisia, and 4 were lost to follow-up.
Participants showed significant improvement in numerous measures of adherence behavior, treatment attitudes, psychiatric symptoms, and functioning (see chart).
For Dr. Sajatovic, the take-home message from this study was clear: "I would say that when we think about adherence, we can talk about relapse, but it’s very hard to have relapse as an outcome measure in these kinds of patients. They start out in relapse. So we should also consider those outcome components that patients and families really value, like functioning and housing."
Discussant Dr. Stephen R. Marder concurred: "Adherence is not necessarily the goal; it’s the path to better outcomes. And looking at things that are distal to relapse and symptoms – things like housing – shows an advantage. We need to tell patients that preventing relapse is just one of the reasons to be adherent. Long-term outcomes, whether it’s housing, work, school, or independent living, seem to be better for people who are adherent to medication. With adherence, rehabilitation can start.
"Without adherence, it’s really impossible to do," commented Dr. Marder, professor of psychiatry and behavioral sciences and director of the section on psychosis at the University of California, Los Angeles, Neuropsychiatric Institute.
He serves as a consultant to or recipient of research funding from roughly a dozen pharmaceutical companies. Dr. Sajatovic reported having no conflicts of interest.
HOLLYWOOD, FLA. – A manualized psychosocial intervention known as customized adherence enhancement coupled with long-acting injectable antipsychotic medication produced marked improvement in adherence to concomitant oral medication as well as in psychiatric symptoms and functional status in urban homeless people with schizophrenia in a small prospective observational study.
Moreover, the intervention also achieved an impressive improvement in a domain not often considered in psychiatric studies, but one of great practical value to patients and their families: housing conditions, Dr. Martha Sajatovic said at a meeting of the New Clinical Drug Evaluation Unit sponsored by the National Institute of Mental Health

The mean time spent in suboptimal housing – living outdoors or in jail – changed from 56% during the 6 months prior to study enrollment to 41% in the first 3 months of the intervention and a mere 14% in the second 3 months of the study, reported Dr. Sajatovic, professor of psychiatry and neurology at Case Western Reserve University, Cleveland.
"What I think is most interesting about our study are the housing data. Our intervention did not include anything about housing – no housing placement or outreach or anything like that. People used whatever was available in the community. And Cleveland has the dubious distinction of being one of the poorest cities in America, so we have services, but not a lot of them," she noted.
Customized adherence enhancement (CAE) is a needs-based, flexibly dosed psychosocial intervention originally developed by Dr. Sajatovic and her colleagues to improve medication adherence in individuals with bipolar disorder receiving antipsychotic therapy. Following a 6-month study demonstrating its benefits in this population (Bipolar Disord. 2012;14:291-300), the investigators adapted the manualized CAE program for application in a particularly challenging patient population: homeless people with schizophrenia/schizoaffective disorder and poor adherence to their prescribed oral antipsychotic medication.
This 6-month, observational, uncontrolled study involved 30 homeless schizophrenic patients. The CAE consisted of once-monthly brief interventions matched to core adherence vulnerabilities identified at baseline. The CAE entailed psychoeducation, modified motivational enhancement therapy, and coaching on communication with providers and medication routines.
At the time of the monthly CAE session, patients also received a dose of long-acting injectable antipsychotic medication. Because the study was funded by a small nonprofit charitable organization, investigators employed the least expensive long-acting antipsychotic available: intramuscular haloperidol decanoate. This resulted in a high rate of side effects, most prominently restlessness because of akathisia in 40% of subjects, dry mouth in 33%, and muscle twitching in 33%. The mean monthly dose at 24 weeks was 68 mg, with a range of 50-100 mg.
The study population was typical of homeless individuals with major mental illness. Their mean age was 42 years, roughly half were women, 97% had a history of substance abuse, 97% had a history of incarceration, most subjects had not finished high school, and 70% were single or never married.
All patients remained on oral medication in addition to their new long-acting injectable antipsychotic regimen. Adherence to oral medication improved dramatically during the 6-month intervention: At baseline, patients had missed 46% of their oral medication during the past month, compared with just 10% at week 25. Adherence to long-acting injectable therapy was good: 76% at 6 months.
Twenty of 30 patients completed the 6-month study. Of the 10 nonfinishers, 3 were incarcerated, 2 relocated elsewhere, 1 discontinued because of drug-induced akathisia, and 4 were lost to follow-up.
Participants showed significant improvement in numerous measures of adherence behavior, treatment attitudes, psychiatric symptoms, and functioning (see chart).
For Dr. Sajatovic, the take-home message from this study was clear: "I would say that when we think about adherence, we can talk about relapse, but it’s very hard to have relapse as an outcome measure in these kinds of patients. They start out in relapse. So we should also consider those outcome components that patients and families really value, like functioning and housing."
Discussant Dr. Stephen R. Marder concurred: "Adherence is not necessarily the goal; it’s the path to better outcomes. And looking at things that are distal to relapse and symptoms – things like housing – shows an advantage. We need to tell patients that preventing relapse is just one of the reasons to be adherent. Long-term outcomes, whether it’s housing, work, school, or independent living, seem to be better for people who are adherent to medication. With adherence, rehabilitation can start.
"Without adherence, it’s really impossible to do," commented Dr. Marder, professor of psychiatry and behavioral sciences and director of the section on psychosis at the University of California, Los Angeles, Neuropsychiatric Institute.
He serves as a consultant to or recipient of research funding from roughly a dozen pharmaceutical companies. Dr. Sajatovic reported having no conflicts of interest.
HOLLYWOOD, FLA. – A manualized psychosocial intervention known as customized adherence enhancement coupled with long-acting injectable antipsychotic medication produced marked improvement in adherence to concomitant oral medication as well as in psychiatric symptoms and functional status in urban homeless people with schizophrenia in a small prospective observational study.
Moreover, the intervention also achieved an impressive improvement in a domain not often considered in psychiatric studies, but one of great practical value to patients and their families: housing conditions, Dr. Martha Sajatovic said at a meeting of the New Clinical Drug Evaluation Unit sponsored by the National Institute of Mental Health

The mean time spent in suboptimal housing – living outdoors or in jail – changed from 56% during the 6 months prior to study enrollment to 41% in the first 3 months of the intervention and a mere 14% in the second 3 months of the study, reported Dr. Sajatovic, professor of psychiatry and neurology at Case Western Reserve University, Cleveland.
"What I think is most interesting about our study are the housing data. Our intervention did not include anything about housing – no housing placement or outreach or anything like that. People used whatever was available in the community. And Cleveland has the dubious distinction of being one of the poorest cities in America, so we have services, but not a lot of them," she noted.
Customized adherence enhancement (CAE) is a needs-based, flexibly dosed psychosocial intervention originally developed by Dr. Sajatovic and her colleagues to improve medication adherence in individuals with bipolar disorder receiving antipsychotic therapy. Following a 6-month study demonstrating its benefits in this population (Bipolar Disord. 2012;14:291-300), the investigators adapted the manualized CAE program for application in a particularly challenging patient population: homeless people with schizophrenia/schizoaffective disorder and poor adherence to their prescribed oral antipsychotic medication.
This 6-month, observational, uncontrolled study involved 30 homeless schizophrenic patients. The CAE consisted of once-monthly brief interventions matched to core adherence vulnerabilities identified at baseline. The CAE entailed psychoeducation, modified motivational enhancement therapy, and coaching on communication with providers and medication routines.
At the time of the monthly CAE session, patients also received a dose of long-acting injectable antipsychotic medication. Because the study was funded by a small nonprofit charitable organization, investigators employed the least expensive long-acting antipsychotic available: intramuscular haloperidol decanoate. This resulted in a high rate of side effects, most prominently restlessness because of akathisia in 40% of subjects, dry mouth in 33%, and muscle twitching in 33%. The mean monthly dose at 24 weeks was 68 mg, with a range of 50-100 mg.
The study population was typical of homeless individuals with major mental illness. Their mean age was 42 years, roughly half were women, 97% had a history of substance abuse, 97% had a history of incarceration, most subjects had not finished high school, and 70% were single or never married.
All patients remained on oral medication in addition to their new long-acting injectable antipsychotic regimen. Adherence to oral medication improved dramatically during the 6-month intervention: At baseline, patients had missed 46% of their oral medication during the past month, compared with just 10% at week 25. Adherence to long-acting injectable therapy was good: 76% at 6 months.
Twenty of 30 patients completed the 6-month study. Of the 10 nonfinishers, 3 were incarcerated, 2 relocated elsewhere, 1 discontinued because of drug-induced akathisia, and 4 were lost to follow-up.
Participants showed significant improvement in numerous measures of adherence behavior, treatment attitudes, psychiatric symptoms, and functioning (see chart).
For Dr. Sajatovic, the take-home message from this study was clear: "I would say that when we think about adherence, we can talk about relapse, but it’s very hard to have relapse as an outcome measure in these kinds of patients. They start out in relapse. So we should also consider those outcome components that patients and families really value, like functioning and housing."
Discussant Dr. Stephen R. Marder concurred: "Adherence is not necessarily the goal; it’s the path to better outcomes. And looking at things that are distal to relapse and symptoms – things like housing – shows an advantage. We need to tell patients that preventing relapse is just one of the reasons to be adherent. Long-term outcomes, whether it’s housing, work, school, or independent living, seem to be better for people who are adherent to medication. With adherence, rehabilitation can start.
"Without adherence, it’s really impossible to do," commented Dr. Marder, professor of psychiatry and behavioral sciences and director of the section on psychosis at the University of California, Los Angeles, Neuropsychiatric Institute.
He serves as a consultant to or recipient of research funding from roughly a dozen pharmaceutical companies. Dr. Sajatovic reported having no conflicts of interest.
AT THE NCDEU MEETING
Major finding: Homeless patients with schizophrenia missed 46% of their oral medication in the month prior to a customized psychosocial intervention. After the intervention, the percentage of missed medication dropped sharply to just 10% in the final month of a 6-month study.
Data source: This was a 6-month, prospective, uncontrolled study involving 30 homeless individuals with schizophrenia or schizoaffective disorder.
Disclosures: The study was supported by a grant from the Reuter Foundation. The presenter reported having no financial conflicts.