User login
Depressed, suicidal, and brittle in her bones
CASE Broken down
Ms. E, age 20, is a college student who has had major depressive disorder for several years and a genetic bone disease (osteogenesis imperfecta, mixed type III and IV). She presents with depression, anxiety, and suicidal ideation. She reports recent worsening of her depressive symptoms, including anhedonia, excessive sleep, difficulty concentrating, and feeling overwhelmed, hopeless, and worthless. She also describes frequent thoughts of suicide with the plan of putting herself in oncoming traffic, although she has no history of suicide attempts.
Previously, her primary care physician prescribed lorazepam, 0.5 mg, as needed for anxiety, and sertraline, 100 mg/d, for depression and anxiety. She experienced only partial improvement in symptoms, however.
In addition to depressive symptoms, Ms. E describes manic symptoms lasting for as long as 3 to 5 days, including decreased need for sleep, increased energy, pressured speech, racing thoughts, distractibility, spending excessive money on cosmetics, and risking her safety—given her skeletal disorder— by participating in high-impact stage-combat classes. She denies auditory and visual hallucinations, homicidal ideation, and delusions.
The medical history is significant for osteogenesis imperfecta, which has caused 62 fractures and required 16 surgeries. Ms. E is a theater major who, despite her short stature and wheelchair use, reports enjoying her acting career and says she does not feel demoralized by her medical condition. She describes overcoming her physical disabilities with pride and confidence. However, her recent worsening mood symptoms have left her unable to concentrate and feeling overwhelmed with school.
Ms. E is voluntarily admitted to an inpatient psychiatric unit with a diagnosis of bipolar I disorder with rapid cycling, most recent episode mixed. Because of her bone fragility, the treatment team considers what would be an appropriate course of drug treatment to control bipolar symptoms while minimizing risk of bone loss.
Which medications are associated with decreased bone mineral density?
a) citalopram
b) haloperidol
c) carbamazepine
d) paliperidone
e) all of the above
The authors’ observations
Osteogenesis imperfecta is a genetic condition caused by mutations in genes implicated in collagen production. As a result, bones are brittle and prone to fracture. Different classes of psychotropics have been shown to increase risk of bone fractures through a variety of mechanisms. Clinicians often must choose appropriate pharmacotherapy for patients at high risk of fracture, including postmenopausal women, older patients, malnourished persons, and those with hormonal deficiencies leading to osteoporosis.
To assist our clinical decision-making, we reviewed the literature to establish appropriate management of a patient with increased bone fragility and new-onset bipolar disorder. We considered all classes of medications used to treat bipolar disorder, including antipsychotics, antidepressants, lithium, and anticonvulsants.
Antipsychotics
In population-based studies, prolactin-elevating antipsychotics have been associated with decreased bone mineral density and increased risk of fracture.1 Additional studies on geriatric and non-geriatric populations have supported these findings.2,3
The mechanism through which fracture risk is increased likely is related to antipsychotics’ effect on serum prolactin and cortisol levels. Antipsychotics act as antagonists on D2 receptors in the hypothalamic tubero-infundibular pathway, therefore preventing inhibition of prolactin. Long-term elevation in serum prolactin can cause loss of bone mineral density through secondary hypogonadism and direct effects on target tissues. Additional modifying factors include smoking and estrogen use.
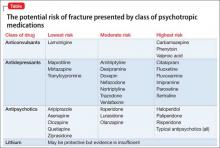
The degree to which antipsychotics increase fracture risk might be related to the degree of serum prolactin elevation.4 Antipsychotics previously have been grouped by the degree of prolactin elevation, categorizing them as high, medium, and low or no potential to elevate serum prolactin.4 Based on this classification, typical antipsychotics, risperidone, and paliperidone have the highest potential to elevate prolactin. Accordingly, antipsychotics with the lowest fracture risk are those that have the lowest risk of serum prolactin elevation: ziprasidone, asenapine, quetiapine, and clozapine. Aripiprazole may lower prolactin in some patients. This is supported by studies noting reduced bone mineral density5,6 and increased risk of fracture1 with high-potential vs low- or no-potential antipsychotics. Because of these findings, it is crucial to consider the potential risk of prolactin elevation when treating patients at increased risk of fracture. Providers should consider low/no potential antipsychotic medications before considering those with medium or high potential (Table).
Antidepressants
In a meta-analysis, antidepressants were shown to increase fracture risk by 70% to 90%.2 However, the relative risk varies by antidepressant class. Several studies have shown that selective serotonin reuptake inhibitors (SSRIs) are associated with a higher risk of fracture compared with tricyclic antidepressants (TCAs).7 In addition, antidepressants with a high affinity for the serotonin transporter, including citalopram, fluoxetine, fluvoxamine, paroxetine, sertraline, and imipramine, have been associated with greater risk of osteoporotic fracture compared with those with low affinity.8
The mechanisms by which antidepressants increase fracture risk are complex, although the strongest evidence implicates a direct effect on bone metabolism via the 5-HTT receptor. This receptor, found on osteoblasts and osteoclasts, plays an important role in bone metabolism; it is through this receptor that SSRIs might inhibit osteoblasts and promote osteoclast activity, thereby disrupting bone microarchitecture. Additional studies are needed to further describe the mechanism of the association among antidepressants, bone mineral density, and fracture risk.
Fracture risk is associated with duration of use rather than dosage. Population-based studies show a higher fracture risk for new users of TCAs compared with continuous users, and the risk of fracture with SSRIs seems to increase slightly over time.9 No association has been identified between fracture risk and antidepressant dosage. According to the literature, drugs with low affinity for the serotonin transporter, such as maprotiline and mirtazapine, likely are the safest antidepressants for patients at increased risk of fracture. Options also include other TCAs and any antidepressant with low affinity for the serotonin receptor.7,8
Lithium
Studies on lithium and bone mineral density have shown mixed results. Older studies found that lithium had a negative or no effect on bone mineral density or the parathyroid hormone level.10 More recent investigations, however, suggest that the drug has a protective effect on bone mineral density, although this has not been replicated in all studies.
In a mouse model, lithium has been shown to enhance bone formation and improve bone mass, at least in part by activation of the Wnt signaling pathway through an inhibitory effect on glycogen synthase kinase-3β.11 In humans, lithium-treated adults had lower serum alkaline phosphate, osteocalcin, and C-telopeptide levels compared with controls, suggesting a state of decreased bone remodeling and increased turnover.12 There is a paucity of clinical data on the effect of lithium on fracture risk. Additional studies are necessary to elucidate lithium’s mechanism on bone mineral density and determine the magnitude of the clinical effect.
Anticonvulsants
The association among anticonvulsants, decreased bone mineral density, and increased risk of fracture is well-established in the literature.13 However, causality is difficult to determine, because many studies were of patients with a seizure disorder, who often have additional risk factors for fracture, including seizure-related trauma, drowsiness, and slowed reflexes.
Mechanisms through which anticonvulsants increase fracture risk include increased bone resorption, secondary hypoparathyroidism, and pseudohypoparathyroidism. Markers of bone resorption were elevated in patients receiving an antiepileptic.14 This effect might be enhanced by co-administration of cytochrome P450 (CYP450) enzyme-inducing anticonvulsants and CYP450 enzyme-inhibiting medications, such as valproate. Long-term treatment with valproate may produce reduction of bone mass and increased risk of fractures; however, other studies disagree with this finding.15
In addition to CYP450-inducing effects, phenytoin, carbamezapine, and phenobarbital can increase catabolism of vitamin D, which is associated with osteomalacia.14 This results in decreased intestinal absorption of calcium, hypocalcemia, and secondary hyperparathyroidism, which also increases fracture risk. Anticonvulsants also might increase resistance to pseudohypoparathyroidism and inhibit calcitonin secretion.
Lamotrigine has not been shown to interfere with bone accrual16 and may be a safer mood stabilizer for patients at high risk of fracture. For patients at increased risk of fracture, it is important to select an anticonvulsant wisely to minimize fracture risk.
How would you treat Ms. E during her hospitalization for bipolar disorder?
a) carbamazepine
b) lithium
c) risperidone
d) mirtazapine
TREATMENT Minimizing polypharmacy
Because many pharmacotherapeutic options for managing bipolar disorder can increase the risk of fracture, clinicians must be aware of the relative risk of each class of medication and each individual drug. We initiated lithium, 300 mg, 3 times a day, to stabilize Ms. E’s mood. Although clinical data are inconclusive regarding lithium’s effect on fracture risk, we felt that the benefit of acute mood stabilization outweighed the risk of decreased bone mineral index.
We selected aripiprazole, 10 mg/d, as an adjunctive treatment because of its minimal effect on serum prolactin levels.4 We considered prescribing an antidepressant but decided against it because we were concerned about manic switching.
Polypharmacy is another important consideration for Ms. E. Several studies have identified polypharmacy, particularly with antipsychotics, as an independent risk factor for fracture.3 Therefore, we sought to minimize the number of medications Ms. E receives. Although lithium monotherapy is an option, we thought that her mood symptoms were severe enough that the risk of inadequately treating her bipolar symptoms outweighed the additional risk of fracture from dual therapy with lithium and aripiprazole. Untreated or inadequately treated depression is associated with a higher fracture risk. Therefore, we avoided prescribing >2 medications to mitigate any excessive risk of fracture from polypharmacy.
Bottom Line
Different classes of medications—antipsychotics, anticonvulsants, antidepressants, and lithium—used for treating bipolar disorder have been shown to increase risk of bone fracture through a variety of mechanisms. Anticonvulsants and prolactin-elevating antipsychotics are associated with increased fracture risk; evidence on lithium is mixed. Fracture risk with antidepressants is associated with duration of use, rather than dosage.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Howard L, Kirkwood G, Leese M. Risk of hip fracture in patients with a history of schizophrenia. Br J Psychiatry. 2007;190:129-134.
2. Takkouche B, Montes-Martínez A, Gill SS, et al. Psychotropic medications and the risk of fracture: a meta-analysis. Drug Saf. 2007;30(2):171-184.
3. Sørensen HJ, Jensen SO, Nielsen J. Schizophrenia, antipsychotics and risk of hip fracture: a population-based analysis. Eur Neuropsychopharmacol. 2013;23(8):872-878.
4. Rahman T, Clevenger CV, Kaklamani V, et al. Antipsychotic treatment in breast cancer patients. Am J Psychiatry. 2014;171(6):616-621.
5. Bilici M, Cakirbay H, Guler M, et al. Classical and atypical neuroleptics, and bone mineral density, in patients with schizophrenia. Int J Neurosci. 2002;112(7):817-828.
6. Becker D, Liver O, Mester R, et al. Risperidone, but not olanzapine, decreases bone mineral density in female premenopausal schizophrenia patients. J Clin Psychiatry. 2003;64(7):761-766.
7. Bolton JM, Metge C, Lix L, et al. Fracture risk from psychotropic medications: a population-based analysis. J Clin Psychopharmacol. 2008;28(4):384-391.
8. Verdel BM, Souverein PC, Egberts TC, et al. Use of antidepressant drugs and risk of osteoporotic and non-osteoporotic fractures. Bone. 2010;47(3):604-609.
9. Diem SJ, Ruppert K, Cauley JA. Rates of bone loss among women initiating antidepressant medication use in midlife. J Clin Endocrinol Metab. 2013;(11):4355-4363.
10. Plenge P, Rafaelsen OJ. Lithium effects on calcium, magnesium and phosphate in man: effects on balance, bone mineral content, faecal and urinary excretion. Acta Psychiatr Scand. 1982;66(5):361-373.
11. Clément-Lacroix P, Ai M, Morvan F, et al. Lrp5-independent activation of Wnt signaling by lithium chloride increases bone formation and bone mass in mice. Proc Natl Acad Sci U S A. 2005;102(48):17406-17411.
12. Zamani A, Omrani GR, Nasab MM. Lithium’s effect on bone mineral density. Bone. 2009;44(2):331-334.
13. Swanton J, Simister R, Altmann D, et al. Bone mineral density in institutionalised patients with refractory epilepsy. Seizure. 2007;16(6):538-541.
14. Pack AM, Morrell MJ. Epilepsy and bone health in adults. Epilepsy Behav. 2004;5(suppl 2):S24-S29.
15. Pack AM. Bone disease in epilepsy. Curr Neurol Neurosci Rep. 2004;4(4):329-334.
16. Sheth RD, Hermann BP. Bone mineral density with lamotrigine monotherapy for epilepsy. Pediatr Neurol. 2007;37(4):250-254.
CASE Broken down
Ms. E, age 20, is a college student who has had major depressive disorder for several years and a genetic bone disease (osteogenesis imperfecta, mixed type III and IV). She presents with depression, anxiety, and suicidal ideation. She reports recent worsening of her depressive symptoms, including anhedonia, excessive sleep, difficulty concentrating, and feeling overwhelmed, hopeless, and worthless. She also describes frequent thoughts of suicide with the plan of putting herself in oncoming traffic, although she has no history of suicide attempts.
Previously, her primary care physician prescribed lorazepam, 0.5 mg, as needed for anxiety, and sertraline, 100 mg/d, for depression and anxiety. She experienced only partial improvement in symptoms, however.
In addition to depressive symptoms, Ms. E describes manic symptoms lasting for as long as 3 to 5 days, including decreased need for sleep, increased energy, pressured speech, racing thoughts, distractibility, spending excessive money on cosmetics, and risking her safety—given her skeletal disorder— by participating in high-impact stage-combat classes. She denies auditory and visual hallucinations, homicidal ideation, and delusions.
The medical history is significant for osteogenesis imperfecta, which has caused 62 fractures and required 16 surgeries. Ms. E is a theater major who, despite her short stature and wheelchair use, reports enjoying her acting career and says she does not feel demoralized by her medical condition. She describes overcoming her physical disabilities with pride and confidence. However, her recent worsening mood symptoms have left her unable to concentrate and feeling overwhelmed with school.
Ms. E is voluntarily admitted to an inpatient psychiatric unit with a diagnosis of bipolar I disorder with rapid cycling, most recent episode mixed. Because of her bone fragility, the treatment team considers what would be an appropriate course of drug treatment to control bipolar symptoms while minimizing risk of bone loss.
Which medications are associated with decreased bone mineral density?
a) citalopram
b) haloperidol
c) carbamazepine
d) paliperidone
e) all of the above
The authors’ observations
Osteogenesis imperfecta is a genetic condition caused by mutations in genes implicated in collagen production. As a result, bones are brittle and prone to fracture. Different classes of psychotropics have been shown to increase risk of bone fractures through a variety of mechanisms. Clinicians often must choose appropriate pharmacotherapy for patients at high risk of fracture, including postmenopausal women, older patients, malnourished persons, and those with hormonal deficiencies leading to osteoporosis.
To assist our clinical decision-making, we reviewed the literature to establish appropriate management of a patient with increased bone fragility and new-onset bipolar disorder. We considered all classes of medications used to treat bipolar disorder, including antipsychotics, antidepressants, lithium, and anticonvulsants.
Antipsychotics
In population-based studies, prolactin-elevating antipsychotics have been associated with decreased bone mineral density and increased risk of fracture.1 Additional studies on geriatric and non-geriatric populations have supported these findings.2,3
The mechanism through which fracture risk is increased likely is related to antipsychotics’ effect on serum prolactin and cortisol levels. Antipsychotics act as antagonists on D2 receptors in the hypothalamic tubero-infundibular pathway, therefore preventing inhibition of prolactin. Long-term elevation in serum prolactin can cause loss of bone mineral density through secondary hypogonadism and direct effects on target tissues. Additional modifying factors include smoking and estrogen use.
The degree to which antipsychotics increase fracture risk might be related to the degree of serum prolactin elevation.4 Antipsychotics previously have been grouped by the degree of prolactin elevation, categorizing them as high, medium, and low or no potential to elevate serum prolactin.4 Based on this classification, typical antipsychotics, risperidone, and paliperidone have the highest potential to elevate prolactin. Accordingly, antipsychotics with the lowest fracture risk are those that have the lowest risk of serum prolactin elevation: ziprasidone, asenapine, quetiapine, and clozapine. Aripiprazole may lower prolactin in some patients. This is supported by studies noting reduced bone mineral density5,6 and increased risk of fracture1 with high-potential vs low- or no-potential antipsychotics. Because of these findings, it is crucial to consider the potential risk of prolactin elevation when treating patients at increased risk of fracture. Providers should consider low/no potential antipsychotic medications before considering those with medium or high potential (Table).
Antidepressants
In a meta-analysis, antidepressants were shown to increase fracture risk by 70% to 90%.2 However, the relative risk varies by antidepressant class. Several studies have shown that selective serotonin reuptake inhibitors (SSRIs) are associated with a higher risk of fracture compared with tricyclic antidepressants (TCAs).7 In addition, antidepressants with a high affinity for the serotonin transporter, including citalopram, fluoxetine, fluvoxamine, paroxetine, sertraline, and imipramine, have been associated with greater risk of osteoporotic fracture compared with those with low affinity.8
The mechanisms by which antidepressants increase fracture risk are complex, although the strongest evidence implicates a direct effect on bone metabolism via the 5-HTT receptor. This receptor, found on osteoblasts and osteoclasts, plays an important role in bone metabolism; it is through this receptor that SSRIs might inhibit osteoblasts and promote osteoclast activity, thereby disrupting bone microarchitecture. Additional studies are needed to further describe the mechanism of the association among antidepressants, bone mineral density, and fracture risk.
Fracture risk is associated with duration of use rather than dosage. Population-based studies show a higher fracture risk for new users of TCAs compared with continuous users, and the risk of fracture with SSRIs seems to increase slightly over time.9 No association has been identified between fracture risk and antidepressant dosage. According to the literature, drugs with low affinity for the serotonin transporter, such as maprotiline and mirtazapine, likely are the safest antidepressants for patients at increased risk of fracture. Options also include other TCAs and any antidepressant with low affinity for the serotonin receptor.7,8
Lithium
Studies on lithium and bone mineral density have shown mixed results. Older studies found that lithium had a negative or no effect on bone mineral density or the parathyroid hormone level.10 More recent investigations, however, suggest that the drug has a protective effect on bone mineral density, although this has not been replicated in all studies.
In a mouse model, lithium has been shown to enhance bone formation and improve bone mass, at least in part by activation of the Wnt signaling pathway through an inhibitory effect on glycogen synthase kinase-3β.11 In humans, lithium-treated adults had lower serum alkaline phosphate, osteocalcin, and C-telopeptide levels compared with controls, suggesting a state of decreased bone remodeling and increased turnover.12 There is a paucity of clinical data on the effect of lithium on fracture risk. Additional studies are necessary to elucidate lithium’s mechanism on bone mineral density and determine the magnitude of the clinical effect.
Anticonvulsants
The association among anticonvulsants, decreased bone mineral density, and increased risk of fracture is well-established in the literature.13 However, causality is difficult to determine, because many studies were of patients with a seizure disorder, who often have additional risk factors for fracture, including seizure-related trauma, drowsiness, and slowed reflexes.
Mechanisms through which anticonvulsants increase fracture risk include increased bone resorption, secondary hypoparathyroidism, and pseudohypoparathyroidism. Markers of bone resorption were elevated in patients receiving an antiepileptic.14 This effect might be enhanced by co-administration of cytochrome P450 (CYP450) enzyme-inducing anticonvulsants and CYP450 enzyme-inhibiting medications, such as valproate. Long-term treatment with valproate may produce reduction of bone mass and increased risk of fractures; however, other studies disagree with this finding.15
In addition to CYP450-inducing effects, phenytoin, carbamezapine, and phenobarbital can increase catabolism of vitamin D, which is associated with osteomalacia.14 This results in decreased intestinal absorption of calcium, hypocalcemia, and secondary hyperparathyroidism, which also increases fracture risk. Anticonvulsants also might increase resistance to pseudohypoparathyroidism and inhibit calcitonin secretion.
Lamotrigine has not been shown to interfere with bone accrual16 and may be a safer mood stabilizer for patients at high risk of fracture. For patients at increased risk of fracture, it is important to select an anticonvulsant wisely to minimize fracture risk.
How would you treat Ms. E during her hospitalization for bipolar disorder?
a) carbamazepine
b) lithium
c) risperidone
d) mirtazapine
TREATMENT Minimizing polypharmacy
Because many pharmacotherapeutic options for managing bipolar disorder can increase the risk of fracture, clinicians must be aware of the relative risk of each class of medication and each individual drug. We initiated lithium, 300 mg, 3 times a day, to stabilize Ms. E’s mood. Although clinical data are inconclusive regarding lithium’s effect on fracture risk, we felt that the benefit of acute mood stabilization outweighed the risk of decreased bone mineral index.
We selected aripiprazole, 10 mg/d, as an adjunctive treatment because of its minimal effect on serum prolactin levels.4 We considered prescribing an antidepressant but decided against it because we were concerned about manic switching.
Polypharmacy is another important consideration for Ms. E. Several studies have identified polypharmacy, particularly with antipsychotics, as an independent risk factor for fracture.3 Therefore, we sought to minimize the number of medications Ms. E receives. Although lithium monotherapy is an option, we thought that her mood symptoms were severe enough that the risk of inadequately treating her bipolar symptoms outweighed the additional risk of fracture from dual therapy with lithium and aripiprazole. Untreated or inadequately treated depression is associated with a higher fracture risk. Therefore, we avoided prescribing >2 medications to mitigate any excessive risk of fracture from polypharmacy.
Bottom Line
Different classes of medications—antipsychotics, anticonvulsants, antidepressants, and lithium—used for treating bipolar disorder have been shown to increase risk of bone fracture through a variety of mechanisms. Anticonvulsants and prolactin-elevating antipsychotics are associated with increased fracture risk; evidence on lithium is mixed. Fracture risk with antidepressants is associated with duration of use, rather than dosage.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE Broken down
Ms. E, age 20, is a college student who has had major depressive disorder for several years and a genetic bone disease (osteogenesis imperfecta, mixed type III and IV). She presents with depression, anxiety, and suicidal ideation. She reports recent worsening of her depressive symptoms, including anhedonia, excessive sleep, difficulty concentrating, and feeling overwhelmed, hopeless, and worthless. She also describes frequent thoughts of suicide with the plan of putting herself in oncoming traffic, although she has no history of suicide attempts.
Previously, her primary care physician prescribed lorazepam, 0.5 mg, as needed for anxiety, and sertraline, 100 mg/d, for depression and anxiety. She experienced only partial improvement in symptoms, however.
In addition to depressive symptoms, Ms. E describes manic symptoms lasting for as long as 3 to 5 days, including decreased need for sleep, increased energy, pressured speech, racing thoughts, distractibility, spending excessive money on cosmetics, and risking her safety—given her skeletal disorder— by participating in high-impact stage-combat classes. She denies auditory and visual hallucinations, homicidal ideation, and delusions.
The medical history is significant for osteogenesis imperfecta, which has caused 62 fractures and required 16 surgeries. Ms. E is a theater major who, despite her short stature and wheelchair use, reports enjoying her acting career and says she does not feel demoralized by her medical condition. She describes overcoming her physical disabilities with pride and confidence. However, her recent worsening mood symptoms have left her unable to concentrate and feeling overwhelmed with school.
Ms. E is voluntarily admitted to an inpatient psychiatric unit with a diagnosis of bipolar I disorder with rapid cycling, most recent episode mixed. Because of her bone fragility, the treatment team considers what would be an appropriate course of drug treatment to control bipolar symptoms while minimizing risk of bone loss.
Which medications are associated with decreased bone mineral density?
a) citalopram
b) haloperidol
c) carbamazepine
d) paliperidone
e) all of the above
The authors’ observations
Osteogenesis imperfecta is a genetic condition caused by mutations in genes implicated in collagen production. As a result, bones are brittle and prone to fracture. Different classes of psychotropics have been shown to increase risk of bone fractures through a variety of mechanisms. Clinicians often must choose appropriate pharmacotherapy for patients at high risk of fracture, including postmenopausal women, older patients, malnourished persons, and those with hormonal deficiencies leading to osteoporosis.
To assist our clinical decision-making, we reviewed the literature to establish appropriate management of a patient with increased bone fragility and new-onset bipolar disorder. We considered all classes of medications used to treat bipolar disorder, including antipsychotics, antidepressants, lithium, and anticonvulsants.
Antipsychotics
In population-based studies, prolactin-elevating antipsychotics have been associated with decreased bone mineral density and increased risk of fracture.1 Additional studies on geriatric and non-geriatric populations have supported these findings.2,3
The mechanism through which fracture risk is increased likely is related to antipsychotics’ effect on serum prolactin and cortisol levels. Antipsychotics act as antagonists on D2 receptors in the hypothalamic tubero-infundibular pathway, therefore preventing inhibition of prolactin. Long-term elevation in serum prolactin can cause loss of bone mineral density through secondary hypogonadism and direct effects on target tissues. Additional modifying factors include smoking and estrogen use.
The degree to which antipsychotics increase fracture risk might be related to the degree of serum prolactin elevation.4 Antipsychotics previously have been grouped by the degree of prolactin elevation, categorizing them as high, medium, and low or no potential to elevate serum prolactin.4 Based on this classification, typical antipsychotics, risperidone, and paliperidone have the highest potential to elevate prolactin. Accordingly, antipsychotics with the lowest fracture risk are those that have the lowest risk of serum prolactin elevation: ziprasidone, asenapine, quetiapine, and clozapine. Aripiprazole may lower prolactin in some patients. This is supported by studies noting reduced bone mineral density5,6 and increased risk of fracture1 with high-potential vs low- or no-potential antipsychotics. Because of these findings, it is crucial to consider the potential risk of prolactin elevation when treating patients at increased risk of fracture. Providers should consider low/no potential antipsychotic medications before considering those with medium or high potential (Table).
Antidepressants
In a meta-analysis, antidepressants were shown to increase fracture risk by 70% to 90%.2 However, the relative risk varies by antidepressant class. Several studies have shown that selective serotonin reuptake inhibitors (SSRIs) are associated with a higher risk of fracture compared with tricyclic antidepressants (TCAs).7 In addition, antidepressants with a high affinity for the serotonin transporter, including citalopram, fluoxetine, fluvoxamine, paroxetine, sertraline, and imipramine, have been associated with greater risk of osteoporotic fracture compared with those with low affinity.8
The mechanisms by which antidepressants increase fracture risk are complex, although the strongest evidence implicates a direct effect on bone metabolism via the 5-HTT receptor. This receptor, found on osteoblasts and osteoclasts, plays an important role in bone metabolism; it is through this receptor that SSRIs might inhibit osteoblasts and promote osteoclast activity, thereby disrupting bone microarchitecture. Additional studies are needed to further describe the mechanism of the association among antidepressants, bone mineral density, and fracture risk.
Fracture risk is associated with duration of use rather than dosage. Population-based studies show a higher fracture risk for new users of TCAs compared with continuous users, and the risk of fracture with SSRIs seems to increase slightly over time.9 No association has been identified between fracture risk and antidepressant dosage. According to the literature, drugs with low affinity for the serotonin transporter, such as maprotiline and mirtazapine, likely are the safest antidepressants for patients at increased risk of fracture. Options also include other TCAs and any antidepressant with low affinity for the serotonin receptor.7,8
Lithium
Studies on lithium and bone mineral density have shown mixed results. Older studies found that lithium had a negative or no effect on bone mineral density or the parathyroid hormone level.10 More recent investigations, however, suggest that the drug has a protective effect on bone mineral density, although this has not been replicated in all studies.
In a mouse model, lithium has been shown to enhance bone formation and improve bone mass, at least in part by activation of the Wnt signaling pathway through an inhibitory effect on glycogen synthase kinase-3β.11 In humans, lithium-treated adults had lower serum alkaline phosphate, osteocalcin, and C-telopeptide levels compared with controls, suggesting a state of decreased bone remodeling and increased turnover.12 There is a paucity of clinical data on the effect of lithium on fracture risk. Additional studies are necessary to elucidate lithium’s mechanism on bone mineral density and determine the magnitude of the clinical effect.
Anticonvulsants
The association among anticonvulsants, decreased bone mineral density, and increased risk of fracture is well-established in the literature.13 However, causality is difficult to determine, because many studies were of patients with a seizure disorder, who often have additional risk factors for fracture, including seizure-related trauma, drowsiness, and slowed reflexes.
Mechanisms through which anticonvulsants increase fracture risk include increased bone resorption, secondary hypoparathyroidism, and pseudohypoparathyroidism. Markers of bone resorption were elevated in patients receiving an antiepileptic.14 This effect might be enhanced by co-administration of cytochrome P450 (CYP450) enzyme-inducing anticonvulsants and CYP450 enzyme-inhibiting medications, such as valproate. Long-term treatment with valproate may produce reduction of bone mass and increased risk of fractures; however, other studies disagree with this finding.15
In addition to CYP450-inducing effects, phenytoin, carbamezapine, and phenobarbital can increase catabolism of vitamin D, which is associated with osteomalacia.14 This results in decreased intestinal absorption of calcium, hypocalcemia, and secondary hyperparathyroidism, which also increases fracture risk. Anticonvulsants also might increase resistance to pseudohypoparathyroidism and inhibit calcitonin secretion.
Lamotrigine has not been shown to interfere with bone accrual16 and may be a safer mood stabilizer for patients at high risk of fracture. For patients at increased risk of fracture, it is important to select an anticonvulsant wisely to minimize fracture risk.
How would you treat Ms. E during her hospitalization for bipolar disorder?
a) carbamazepine
b) lithium
c) risperidone
d) mirtazapine
TREATMENT Minimizing polypharmacy
Because many pharmacotherapeutic options for managing bipolar disorder can increase the risk of fracture, clinicians must be aware of the relative risk of each class of medication and each individual drug. We initiated lithium, 300 mg, 3 times a day, to stabilize Ms. E’s mood. Although clinical data are inconclusive regarding lithium’s effect on fracture risk, we felt that the benefit of acute mood stabilization outweighed the risk of decreased bone mineral index.
We selected aripiprazole, 10 mg/d, as an adjunctive treatment because of its minimal effect on serum prolactin levels.4 We considered prescribing an antidepressant but decided against it because we were concerned about manic switching.
Polypharmacy is another important consideration for Ms. E. Several studies have identified polypharmacy, particularly with antipsychotics, as an independent risk factor for fracture.3 Therefore, we sought to minimize the number of medications Ms. E receives. Although lithium monotherapy is an option, we thought that her mood symptoms were severe enough that the risk of inadequately treating her bipolar symptoms outweighed the additional risk of fracture from dual therapy with lithium and aripiprazole. Untreated or inadequately treated depression is associated with a higher fracture risk. Therefore, we avoided prescribing >2 medications to mitigate any excessive risk of fracture from polypharmacy.
Bottom Line
Different classes of medications—antipsychotics, anticonvulsants, antidepressants, and lithium—used for treating bipolar disorder have been shown to increase risk of bone fracture through a variety of mechanisms. Anticonvulsants and prolactin-elevating antipsychotics are associated with increased fracture risk; evidence on lithium is mixed. Fracture risk with antidepressants is associated with duration of use, rather than dosage.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Howard L, Kirkwood G, Leese M. Risk of hip fracture in patients with a history of schizophrenia. Br J Psychiatry. 2007;190:129-134.
2. Takkouche B, Montes-Martínez A, Gill SS, et al. Psychotropic medications and the risk of fracture: a meta-analysis. Drug Saf. 2007;30(2):171-184.
3. Sørensen HJ, Jensen SO, Nielsen J. Schizophrenia, antipsychotics and risk of hip fracture: a population-based analysis. Eur Neuropsychopharmacol. 2013;23(8):872-878.
4. Rahman T, Clevenger CV, Kaklamani V, et al. Antipsychotic treatment in breast cancer patients. Am J Psychiatry. 2014;171(6):616-621.
5. Bilici M, Cakirbay H, Guler M, et al. Classical and atypical neuroleptics, and bone mineral density, in patients with schizophrenia. Int J Neurosci. 2002;112(7):817-828.
6. Becker D, Liver O, Mester R, et al. Risperidone, but not olanzapine, decreases bone mineral density in female premenopausal schizophrenia patients. J Clin Psychiatry. 2003;64(7):761-766.
7. Bolton JM, Metge C, Lix L, et al. Fracture risk from psychotropic medications: a population-based analysis. J Clin Psychopharmacol. 2008;28(4):384-391.
8. Verdel BM, Souverein PC, Egberts TC, et al. Use of antidepressant drugs and risk of osteoporotic and non-osteoporotic fractures. Bone. 2010;47(3):604-609.
9. Diem SJ, Ruppert K, Cauley JA. Rates of bone loss among women initiating antidepressant medication use in midlife. J Clin Endocrinol Metab. 2013;(11):4355-4363.
10. Plenge P, Rafaelsen OJ. Lithium effects on calcium, magnesium and phosphate in man: effects on balance, bone mineral content, faecal and urinary excretion. Acta Psychiatr Scand. 1982;66(5):361-373.
11. Clément-Lacroix P, Ai M, Morvan F, et al. Lrp5-independent activation of Wnt signaling by lithium chloride increases bone formation and bone mass in mice. Proc Natl Acad Sci U S A. 2005;102(48):17406-17411.
12. Zamani A, Omrani GR, Nasab MM. Lithium’s effect on bone mineral density. Bone. 2009;44(2):331-334.
13. Swanton J, Simister R, Altmann D, et al. Bone mineral density in institutionalised patients with refractory epilepsy. Seizure. 2007;16(6):538-541.
14. Pack AM, Morrell MJ. Epilepsy and bone health in adults. Epilepsy Behav. 2004;5(suppl 2):S24-S29.
15. Pack AM. Bone disease in epilepsy. Curr Neurol Neurosci Rep. 2004;4(4):329-334.
16. Sheth RD, Hermann BP. Bone mineral density with lamotrigine monotherapy for epilepsy. Pediatr Neurol. 2007;37(4):250-254.
1. Howard L, Kirkwood G, Leese M. Risk of hip fracture in patients with a history of schizophrenia. Br J Psychiatry. 2007;190:129-134.
2. Takkouche B, Montes-Martínez A, Gill SS, et al. Psychotropic medications and the risk of fracture: a meta-analysis. Drug Saf. 2007;30(2):171-184.
3. Sørensen HJ, Jensen SO, Nielsen J. Schizophrenia, antipsychotics and risk of hip fracture: a population-based analysis. Eur Neuropsychopharmacol. 2013;23(8):872-878.
4. Rahman T, Clevenger CV, Kaklamani V, et al. Antipsychotic treatment in breast cancer patients. Am J Psychiatry. 2014;171(6):616-621.
5. Bilici M, Cakirbay H, Guler M, et al. Classical and atypical neuroleptics, and bone mineral density, in patients with schizophrenia. Int J Neurosci. 2002;112(7):817-828.
6. Becker D, Liver O, Mester R, et al. Risperidone, but not olanzapine, decreases bone mineral density in female premenopausal schizophrenia patients. J Clin Psychiatry. 2003;64(7):761-766.
7. Bolton JM, Metge C, Lix L, et al. Fracture risk from psychotropic medications: a population-based analysis. J Clin Psychopharmacol. 2008;28(4):384-391.
8. Verdel BM, Souverein PC, Egberts TC, et al. Use of antidepressant drugs and risk of osteoporotic and non-osteoporotic fractures. Bone. 2010;47(3):604-609.
9. Diem SJ, Ruppert K, Cauley JA. Rates of bone loss among women initiating antidepressant medication use in midlife. J Clin Endocrinol Metab. 2013;(11):4355-4363.
10. Plenge P, Rafaelsen OJ. Lithium effects on calcium, magnesium and phosphate in man: effects on balance, bone mineral content, faecal and urinary excretion. Acta Psychiatr Scand. 1982;66(5):361-373.
11. Clément-Lacroix P, Ai M, Morvan F, et al. Lrp5-independent activation of Wnt signaling by lithium chloride increases bone formation and bone mass in mice. Proc Natl Acad Sci U S A. 2005;102(48):17406-17411.
12. Zamani A, Omrani GR, Nasab MM. Lithium’s effect on bone mineral density. Bone. 2009;44(2):331-334.
13. Swanton J, Simister R, Altmann D, et al. Bone mineral density in institutionalised patients with refractory epilepsy. Seizure. 2007;16(6):538-541.
14. Pack AM, Morrell MJ. Epilepsy and bone health in adults. Epilepsy Behav. 2004;5(suppl 2):S24-S29.
15. Pack AM. Bone disease in epilepsy. Curr Neurol Neurosci Rep. 2004;4(4):329-334.
16. Sheth RD, Hermann BP. Bone mineral density with lamotrigine monotherapy for epilepsy. Pediatr Neurol. 2007;37(4):250-254.
8 tests rolled into a mnemonic to detect weakness in suspected conversion disorder
DSM-5 criteria for conversion disorder (or functional neurological symptom disorder) requires findings that are incompatible with recognized neurologic or medical conditions.1 Knowledge of signs specific to conversion disorder may help you diagnose the illness with confidence.
We review signs suggestive of conversion disorder. These can be remembered using the mnemonic How About Finding Some Conversion Weakness [in an otherwise] Strong Guy/Gal? (Table2).
Inconsistencies in motor function can be observed on examination. Signs may be consciously or unconsciously produced. Although most of the tests mentioned have high positive and negative predictive values (noted in the Table2) they have limited sensitivity and specificity,3 and the presence of a positive sign does not exclude the possibility of comorbid disease.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Stone J, LaFrance WC Jr, Levenson JL, et al. Issues for DSM- 5: conversion disorder. Am J Psychiatry. 2010;167(6):626-627.
2. Daum C, Hubschmid M, Aybek S. The value of ‘positive’ clinical signs for weakness, sensory and gait disorders in conversion disorder: a systematic and narrative review. J Neurol Neurosurg Psychiatry. 2014;85(2):180-190.
3. Stone J, Carson A, Sharpe M. Functional symptoms and signs in neurology: assessment and diagnosis. J Neurol Neurosurg Psychiatry. 2005;76(suppl 1):i2-i12.
DSM-5 criteria for conversion disorder (or functional neurological symptom disorder) requires findings that are incompatible with recognized neurologic or medical conditions.1 Knowledge of signs specific to conversion disorder may help you diagnose the illness with confidence.
We review signs suggestive of conversion disorder. These can be remembered using the mnemonic How About Finding Some Conversion Weakness [in an otherwise] Strong Guy/Gal? (Table2).
Inconsistencies in motor function can be observed on examination. Signs may be consciously or unconsciously produced. Although most of the tests mentioned have high positive and negative predictive values (noted in the Table2) they have limited sensitivity and specificity,3 and the presence of a positive sign does not exclude the possibility of comorbid disease.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
DSM-5 criteria for conversion disorder (or functional neurological symptom disorder) requires findings that are incompatible with recognized neurologic or medical conditions.1 Knowledge of signs specific to conversion disorder may help you diagnose the illness with confidence.
We review signs suggestive of conversion disorder. These can be remembered using the mnemonic How About Finding Some Conversion Weakness [in an otherwise] Strong Guy/Gal? (Table2).
Inconsistencies in motor function can be observed on examination. Signs may be consciously or unconsciously produced. Although most of the tests mentioned have high positive and negative predictive values (noted in the Table2) they have limited sensitivity and specificity,3 and the presence of a positive sign does not exclude the possibility of comorbid disease.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Stone J, LaFrance WC Jr, Levenson JL, et al. Issues for DSM- 5: conversion disorder. Am J Psychiatry. 2010;167(6):626-627.
2. Daum C, Hubschmid M, Aybek S. The value of ‘positive’ clinical signs for weakness, sensory and gait disorders in conversion disorder: a systematic and narrative review. J Neurol Neurosurg Psychiatry. 2014;85(2):180-190.
3. Stone J, Carson A, Sharpe M. Functional symptoms and signs in neurology: assessment and diagnosis. J Neurol Neurosurg Psychiatry. 2005;76(suppl 1):i2-i12.
1. Stone J, LaFrance WC Jr, Levenson JL, et al. Issues for DSM- 5: conversion disorder. Am J Psychiatry. 2010;167(6):626-627.
2. Daum C, Hubschmid M, Aybek S. The value of ‘positive’ clinical signs for weakness, sensory and gait disorders in conversion disorder: a systematic and narrative review. J Neurol Neurosurg Psychiatry. 2014;85(2):180-190.
3. Stone J, Carson A, Sharpe M. Functional symptoms and signs in neurology: assessment and diagnosis. J Neurol Neurosurg Psychiatry. 2005;76(suppl 1):i2-i12.
A young man with psychosis whose heart is racing
Case Agitated and violent
Mr. C, age 19, presents with anxiety, agitation, isolation, social withdrawal, and paranoia. He is admitted to the inpatient unit after attempting to punch his father and place him in a headlock. Mr. C has no history of mental illness, no significant medical history, and no significant family history of mental illness.
The treatment team determines that this is Mr. C’s first psychotic break. He is given a diagnosis of psychosis, not otherwise specified and started on risperidone, titrated to 2 mg/d, later discontinued secondary to tachycardia. He is then started on haloperidol, 5 mg/d titrated to 10 mg/d, and psychotic symptoms abate. Mr. C is discharged with a plan to receive follow-up care at an outpatient mental health center.
One year later, Mr. C is readmitted with a similar presentation: paranoia, agitation, anxiety, and isolation. After discharge, he starts an intensive outpatient program (IOP) for long-term treatment of adults who have a diagnosis of a schizophrenia spectrum disorder.
Several medication trials ensue, including risperidone, escitalopram, citalopram, fluphenazine, lorazepam, quetiapine, and haloperidol. Despite these trials over the course of 2 years, Mr. C continues to display paranoia and agitation, and is unable to resume academic and community activities. Within the IOP, Mr. C is placed in a vocational training program and struggles to remain stable enough to continue his job at a small greenhouse.
Concurrently, Mr. C is noted to be abusing alcohol. After the IOP treatment team expresses concern about his abuse, he reduces alcohol intake and he and his parents are educated on the impact of alcohol use on schizophrenia.
Which treatment option would you choose next?
a) initiate a trial of clozapine
b) try a long-acting injectable antipsychotic
c) recommend inpatient treatment
The authors’ observations
Clozapine is an atypical antipsychotic that is FDA-approved for treatment-resistant schizophrenia; it also helps reduce recurrent suicidal behavior in patients with schizophrenia or schizoaffective disorder.
Clozapine works by blocking D2 receptors, thereby reducing positive symptoms. It also blocks serotonin 2A receptors, which enhances dopamine release in certain brain regions, thereby reducing motor side effects. Interactions at 5-HT2C and 5-HT1A receptors may address cognitive and affective symptoms. Clozapine can help relieve negative symptoms and can decrease aggression. Because it has a low risk of tardive dyskinesia, clozapine is useful when treating patients with treatment-resistant schizophrenia.1-3
Treatment Quick heart rate
Mr. C’s IOP treatment team considers a clozapine trial because previous medication trials failed. All paperwork for the registry and screening labs are completed and Mr. C is started on clozapine.
Mr. C’s clozapine dosages are:
• Days 1 to 9: 25 mg/d
• Days 10 to 16: 50 mg/d
• Days 17 to 23: 75 mg/d
• Days 24 to 32: 100 mg/d
• Days 33 to 37: 125 mg/d
• Day 38: 150 mg/d.
On Day 45 of the clozapine trial, Mr. C is increasingly paranoid toward his father and thinks that his father is controlling his thoughts. Mr. C tells the attending psychiatrist that he ingested a handful of clonazepam and considered putting a bag over his head with the intent to commit suicide. Mr. C is admitted to the inpatient unit.
Admission vitals recorded a heart rate of 72 beats per minute but, later that day, the rate was recorded in the vital sign book as 137 beats per minute. The treatment team considers dehydration, anxiety, and staff error; Mr. C is observed carefully. Over the next 2 days, heart rate remains between 102 and 119 beats per minute.
Because of persistent tachycardia, the team orders lab studies, a medical consult, and an electrocardiogram (ECG). Thyroid panel, electrolytes, and clozapine level are within normal limits; ECG is unremarkable.
Although tachycardia is a known side effect of clozapine,3,4 we order an echocardiogram because of Mr. C’s young age and non-diagnostic laboratory workup. The echo study demonstrates reduced left-ventricular ejection fraction (LVEF) of 45%. Tests for HIV infection and Lyme disease are negative. The cardiology team diagnoses cardiomyopathy of unknown origin.
Although Mr. C has a history of alcohol abuse, the cardiology team believes that alcohol consumption does not adequately explain the cardiomyopathy, given his young age and the limited number of lifetime drinking-years (approximately 4 or 5); the team determines that clozapine is causing secondary cardiomyopathy and tachycardia, leading to reduced LVEF. Clozapine is stopped because the recommended treatment for toxic secondary cardiomyopathy is to remove the offending agent. At this point, the clozapine dosage is 250 mg/d.
At the medical team’s recommendation, Mr. C is started on metoprolol, a beta blocker, at 25 mg/d.
The etiology of secondary cardiomyopathy includes all of the following except:
a) tachycardia-induced
b) autoimmune
c) radiation-induced
d) infiltrative
e) endomyocardial
The authors’ observations
Cardiomyopathies are diseases of the heart muscle causing mechanical and electrical dysfunction. This group of diseases has a range of symptoms, causes, and treatments. Disease manifests typically as arrhythmia, systolic dysfunction, or diastolic dysfunction. Classification systems are based on origin, anatomy, physiology, primary treatments, method of diagnosis, biopsy, histopathology, and symptomatic state.
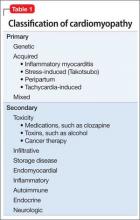
The American Heart Association Scientific Statement5 distinguishes cardiomyopathies by degree of organ involvement. Diseases confined to the heart are defined as primary cardiomyopathy, which may have a genetic, acquired, or mixed cause. Acquired causes include inflammatory (myocarditis), stress (Takotsubo), peripartum, and tachycardia. Cardiomyopathies that are part of generalized systemic disorders are defined as secondary cardiomyopathy (Table 1).
Secondary cardiomyopathies have many causes. These include toxicity (medications or alcohol), cancer therapy, infiltrative, storage disease, and endomyocardial, inflammatory, autoimmune, endocrine, and neurologic diseases.5
Evaluation of suspected cardiomyopathy begins with a history and physical focused on identifying causative factors. Selective testing, based on pretest probabilities, might include lab testing, ECG, and echocardiography, and can narrow the differential diagnosis. When toxin-induced cardiomyopathy is suspected, withdrawing the toxin and monitoring for improvement is recommended. The treatment and prognosis for cardiomyopathies vary, based on the cause.6
Review of the literature
After 23 cases of fatal and non-fatal myocarditis were found in a study of 8,000 patients starting clozapine,7 manufacturers in Australia introduced clinical guidelines. Before initiating clozapine, they recommended, clinicians should:
• screen for cardiac symptoms
• screen for a family history of heart disease
• obtain baseline ECG
• obtain baseline markers of myocardial damage (troponin assay and serum creatinine)
• obtain baseline echocardiogram
• repeat cardiac monitoring after the first and second week and then repeat in 6 months
• maintain a high degree of vigilance for signs and symptoms of cardiac toxicity throughout clozapine treatment.8,9
After studying 38 cases of clozapine-induced myocarditis—3 fatal— Ronaldson et al10 listed primary diagnostic features as:
• tachycardia (heart rate >100 beats per minute)
• heart rate >120 beats per minute
• temperature >37°C
• chest pain
• troponin I/T level >2 ng/mL
• C-reactive protein (CRP) > 100 mg/L
• erythrocyte sedimentation rate >50 mm/h.
Among non-fatal cases, symptoms abated after clozapine was discontinued. In 36 of the 38 cases, symptoms emerged 14 to 22 days after clozapine was started. For tachycardia to be considered a diagnostic feature, it must persist for at least 24 hours; if the heart rate is ≥120 beats per minute, however, persistence is not a criterion. It was thought that elevated CRP might herald disease onset; the authors suggest that CRP >50 mg/L should warrant increased monitoring with daily ECG and troponin levels.
Authors’ recommendations include:
• measuring troponin and CRP and order an ECG at baseline and at 7, 14, 21, and 28 days
• examining patient for signs and symptoms of illness at these same intervals
• considering chest pain or fever as an indicator of cardiomyopathy
• asking patients to report any illness during this 4-week period
• if ECG is abnormal or troponin elevated, decreasing clozapine pending further investigation.10
When medications fail
We had to discontinue Mr. C’s clozapine, which meant that the therapeutic relationship established between him and the psychology fellow became an important and, at times, the only bond between him and the medical team while olanzapine was initiated. The alliance between patient and clinician is an important factor for positive prognosis in mental health treatment.11-13 Priebe and McCabe14 asked if the therapeutic relationship in psychiatry is “the basis of therapy or therapy itself?” In a review of studies that used an operationalized measurement of the therapeutic relationship in treating severe mental illness, the authors concluded that the therapeutic relationship is a reliable predictor of outcome.15
In Mr. C’s case, the psychology fellow, who also works with the Partial Hospitalization Program/Intensive Outpatient Program (PHP/IOP), joined the treatment team on the inpatient unit a few days into hospitalization. Eleven meetings, including a discharge session, were held between the psychology fellow and the patient during the inpatient hospitalization. Mr. C also participated in a daily group session, facilitated by the psychology fellow.
Maintaining recognition of the boundary disturbance that characterizes schizophrenic psychoses was important for Mr. C. As Auerhahn and Moskowitz16 wrote, the inpatient therapist can be transformed by the schizophrenia patient into the all-knowing, all-powerful early mother, which could contribute to substantial improvement in the patient’s functioning and report of symptoms, only to have the patient’s symptoms return after discharge.
In an effort to evaluate the duration, frequency, and intensity of Mr. C’s symptom experience, a goal of Mr. C’s hospitalization was to attach words to his internal states, including mood and intensity of paranoid ideation. We showed Mr. C directly and indirectly that reporting intensification of symptoms and decreased functioning would not result in abandonment or punishment, and worked to demonstrate through our actions that the treatment team differs from Mr. C’s view of the world as dangerous and others as hostile and omnipotent.
Treatment Developing language
Initially, Mr. C gives a number (from 1 to 10) to describe his mood, 10 being the happiest he has ever felt and 1 being the most depressed. The treatment team discusses how important it is that Mr. C know his feelings and be able to convey to others how he feels.
Over time, Mr. C is encouraged to attach a feeling word to the number, and by discharge, he stops using numbers and responds to inquiries about his feelings with a mood word. This practice has been reinforced with the patient in the IOP program, allowing him to continue practicing linking his internal state with feeling words.
During hospitalization, Mr. C becomes more vocal about his level of paranoia and is now more likely to seek support when he first experiences a paranoid thought, rather than waiting until after he is paranoid and agitated. Mr. C is encouraged to monitor his thoughts and feelings, and to practice coping strategies he has identified as helpful, including deep breathing, meditation, listening to music, and reminding himself that he is safe.
The treatment team responds to Mr. C’s reports of paranoid ideation (eg, “Some of the other patients were talking about me today”) by processing the affect, and hypothesizing other explanations for these events to slow down “jumping to conclusions,” which is a common part of the paranoid experience.17 Additionally, all meetings with the cardiology team are processed and Mr. C receives psychoeducation about his heart function. Joint sessions with the psychiatry resident and psychology fellow allow Mr. C to ask medical questions and immediately process his reactions, which likely ameliorated his anxiety and allowed him to continue connecting with, identifying, and verbalizing his internal experiences. Given his history of paranoia, sessions also showed that Mr. C is an active participant in his treatment, with the hope of lessening his belief that bad things happen to him and that they are out of his control.
We maintain frequent contact with Mr. C’s parents to update them on their son’s functioning and to discuss treatment interventions that were helpful and the family could implement when Mr. C returns home. Discharge medications are discussed.
After 24 days in the inpatient unit, Mr. C is discharged to the IOP program. The psychology fellow walks Mr. C to the IOP program, where he transitioned immediately from inpatient to the IOP daily schedule of groups and an appointment with the program psychiatrist. The psychology fellow also arranged for and participated in the family meeting with Mr. C’s parents, sister, and treatment providers in the IOP program after his first day back at the IOP.
Throughout his hospitalization, Mr. C had no symptoms of cardiomyopathy, without exercise intolerance, shortness of breath, fatigue, or fever. He is discharged with follow-up care at his outpatient program at the PHP level of care and a follow-up echocardiogram and cardiology appointment are scheduled for 6 weeks later.
The authors' observations
Throughout Mr. C’s hospitalization, the intersections among psychiatry, psychology, cardiology, and internal medicine were apparent and necessary for treatment. No one specialty was able to completely direct this patient’s care without the expertise of, and input from, others. When it looked like all medications had failed, the relationship between the patient and the psychology fellow and the application of previously learned coping strategies prevented acute decompensation.
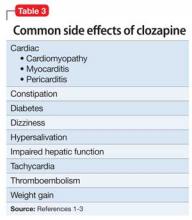
Clozapine is FDA-approved for treatment-resistant schizophrenia and often is a last resort to help patients remain stable. When clozapine is chosen, it is important to be aware of its side-effect profile (Table 2,1 and Table 3,1-3) and the need for monitoring. The importance of relying on colleagues from other specialties to assist in the effective monitoring process cannot be overstated. This multidisciplinary team ensured that Mr. C did not experience acute decompensation during this process. Cardiac function improved, with an LVEF of 50% after clozapine was discontinued. Mr. C has not needed hospitalization again.
Outcome Stability achieved
Mr. C is successfully discharged from the inpatient service after 24 days in the hospital on the following regimen: olanzapine, 20 mg/d; duloxetine 60 mg/d; benztropine, 0.5 mg/d; haloperidol, 20 mg/d; metoprolol, 25 mg/d; clonazepam, 0.25 mg/d; quetiapine, 50 mg/d; and chlorpromazine, 50 mg as needed for agitation and paranoia. He is given a diagnosis of toxic secondary cardiomyopathy due to clozapine, and remains asymptomatic from a cardiac perspective after discontinuing clozapine.
Follow-up appointment with cardiology and repeat echocardiography were scheduled for 6 weeks after discharge. The follow-up echocardiogram showed improvement (LVEF, 50%). Mr. C continues to do well and remains a client at the IOP program.
Bottom Line
Clozapine often is used as a last resort for patients with treatment-resistant schizophrenia, but its side-effect profile requires careful management and monitoring. If a patient taking clozapine shows tachycardia, consider cardiomyopathy. Evaluation might include lab testing, electrocardiography, and echocardiography. Symptoms often resolve when clozapine is discontinued.
Related Resources
• Citrome L. Clozapine for schizophrenia: life-threatening or life-saving treatment? Current Psychiatry. 2009;8(12):56-63.
• Layland JJ, Liew D, Prior DL. Clozapine-induced cardiotoxicity: a clinical update. Med J Aust. 2009;190(4):190-192.
Drug Brand Names
Benztropine • Cogentin Fluphenazine • Prolixin
Chlorpromazine • Thorazine Haloperidol • Haldol
Citalopram • Celexa Lorazepam • Ativan
Clonazepam • Klonopin Metoprolol • Lopressor
Clozapine • Clozaril Olanzapine • Zyprexa
Duloxetine • Cymbalta Quetiapine • Seroquel
Escitalopram • Lexapro Risperidone • Risperdal
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Clozaril [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2013.
2. Stahl SM. Clozapine. In: Stahl SM. The prescriber’s guide: Stahl’s essential psychopharmacology. 3rd ed. New York, NY: Cambridge University Press; 2009:113-118.
3. Young CR, Bowers MB Jr, Mazure CM. Management of the adverse effects of clozapine. Schizophr Bull. 1998;24(3):381-388.
4. Lang UE, Willbring M, von Golitschek R, et al. Clozapine-induced myocarditis after long-term treatment: case presentation and clinical perspectives. J Psychopharmacol. 2008;22(5):576-580.
5. Maron BJ, Towbin JA, Thiene G, et al; American Heart Association; Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; Council on Epidemiology and Prevention. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association scientific statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation. 2006;113(14):1807-1816.
6. Hare JM. The dilated, restrictive, and infiltrative cardiomyopathies. In: Braunwald’s heart disease: a textbook of cardiovascular medicine. 9th ed. Bonow RO, Mann DL, Zipes DP, eds. New York, NY: Elsevier; 2012:1561-1581.
7. Kilian JG, Kerr K, Lawrence C, et al. Myocarditis and cardiomyopathy associated with clozapine. Lancet. 1999;354(9193):1841-1845.
8. Clopine [package insert]. Aukland, New Zealand: Douglas Pharmaceuticals; 2014.
9. Killian JG, Kerr K, Lawrence C, et al. Myocarditis and cardiomyopathy associated with clozapine. Lancet. 1999; 354(9193):1841-1845.
10. Ronaldson KJ, Taylor AJ, Fitzgerald PB, et al. Diagnostic characteristics of clozapine-induced myocarditis identified by an analysis of 38 cases and 47 controls. J Clin Psychiatry. 2010;71(8):976-981.
11. Rogers CR. On becoming a person: a therapist’s view of psychotherapy. New York, NY: Houghton Mifflin; 1961.
12. Horvath AO, Symonds BD. Relation between a working alliance and outcome in psychotherapy: a meta-analysis. Journal of Counseling Psychology. 1991;38(2):139-149.
13. Krupnick JL, Sotsky SM, Simmens S, et al. The role of the therapeutic alliance in psychotherapy and pharmacotherapy outcome: Findings in the National Institute of Mental Health Treatment of Depression Collaborative Research Program. Journal of Consulting and Clinical Psychology. 1996;64(3):532-539.
14. Priebe S, McCabe R. Therapeutic relationships in psychiatry: the basis of therapy or therapy in itself? Int Rev Psychiatry. 2008;20(6):521-526.
15. McCabe R, Priebe S. The therapeutic relationship in the treatment of severe mental illness: a review of methods and findings. Int J Soc Psychiatry. 2004;50(2):115-128.
16. Auerhahn NC, Moskowitz MB. Merger fantasies in individual inpatient therapy with schizophrenic patient. Psychoanalytic Psychology. 1984;1(2):131-148.
17. Penn DL, Roberts DL, Combs D, et al. Best practices: The development of the Social Cognition and Interaction Training program for schizophrenia spectrum disorders. Psychiatr Serv. 2007;58(4):449-451.
Case Agitated and violent
Mr. C, age 19, presents with anxiety, agitation, isolation, social withdrawal, and paranoia. He is admitted to the inpatient unit after attempting to punch his father and place him in a headlock. Mr. C has no history of mental illness, no significant medical history, and no significant family history of mental illness.
The treatment team determines that this is Mr. C’s first psychotic break. He is given a diagnosis of psychosis, not otherwise specified and started on risperidone, titrated to 2 mg/d, later discontinued secondary to tachycardia. He is then started on haloperidol, 5 mg/d titrated to 10 mg/d, and psychotic symptoms abate. Mr. C is discharged with a plan to receive follow-up care at an outpatient mental health center.
One year later, Mr. C is readmitted with a similar presentation: paranoia, agitation, anxiety, and isolation. After discharge, he starts an intensive outpatient program (IOP) for long-term treatment of adults who have a diagnosis of a schizophrenia spectrum disorder.
Several medication trials ensue, including risperidone, escitalopram, citalopram, fluphenazine, lorazepam, quetiapine, and haloperidol. Despite these trials over the course of 2 years, Mr. C continues to display paranoia and agitation, and is unable to resume academic and community activities. Within the IOP, Mr. C is placed in a vocational training program and struggles to remain stable enough to continue his job at a small greenhouse.
Concurrently, Mr. C is noted to be abusing alcohol. After the IOP treatment team expresses concern about his abuse, he reduces alcohol intake and he and his parents are educated on the impact of alcohol use on schizophrenia.
Which treatment option would you choose next?
a) initiate a trial of clozapine
b) try a long-acting injectable antipsychotic
c) recommend inpatient treatment
The authors’ observations
Clozapine is an atypical antipsychotic that is FDA-approved for treatment-resistant schizophrenia; it also helps reduce recurrent suicidal behavior in patients with schizophrenia or schizoaffective disorder.
Clozapine works by blocking D2 receptors, thereby reducing positive symptoms. It also blocks serotonin 2A receptors, which enhances dopamine release in certain brain regions, thereby reducing motor side effects. Interactions at 5-HT2C and 5-HT1A receptors may address cognitive and affective symptoms. Clozapine can help relieve negative symptoms and can decrease aggression. Because it has a low risk of tardive dyskinesia, clozapine is useful when treating patients with treatment-resistant schizophrenia.1-3
Treatment Quick heart rate
Mr. C’s IOP treatment team considers a clozapine trial because previous medication trials failed. All paperwork for the registry and screening labs are completed and Mr. C is started on clozapine.
Mr. C’s clozapine dosages are:
• Days 1 to 9: 25 mg/d
• Days 10 to 16: 50 mg/d
• Days 17 to 23: 75 mg/d
• Days 24 to 32: 100 mg/d
• Days 33 to 37: 125 mg/d
• Day 38: 150 mg/d.
On Day 45 of the clozapine trial, Mr. C is increasingly paranoid toward his father and thinks that his father is controlling his thoughts. Mr. C tells the attending psychiatrist that he ingested a handful of clonazepam and considered putting a bag over his head with the intent to commit suicide. Mr. C is admitted to the inpatient unit.
Admission vitals recorded a heart rate of 72 beats per minute but, later that day, the rate was recorded in the vital sign book as 137 beats per minute. The treatment team considers dehydration, anxiety, and staff error; Mr. C is observed carefully. Over the next 2 days, heart rate remains between 102 and 119 beats per minute.
Because of persistent tachycardia, the team orders lab studies, a medical consult, and an electrocardiogram (ECG). Thyroid panel, electrolytes, and clozapine level are within normal limits; ECG is unremarkable.
Although tachycardia is a known side effect of clozapine,3,4 we order an echocardiogram because of Mr. C’s young age and non-diagnostic laboratory workup. The echo study demonstrates reduced left-ventricular ejection fraction (LVEF) of 45%. Tests for HIV infection and Lyme disease are negative. The cardiology team diagnoses cardiomyopathy of unknown origin.
Although Mr. C has a history of alcohol abuse, the cardiology team believes that alcohol consumption does not adequately explain the cardiomyopathy, given his young age and the limited number of lifetime drinking-years (approximately 4 or 5); the team determines that clozapine is causing secondary cardiomyopathy and tachycardia, leading to reduced LVEF. Clozapine is stopped because the recommended treatment for toxic secondary cardiomyopathy is to remove the offending agent. At this point, the clozapine dosage is 250 mg/d.
At the medical team’s recommendation, Mr. C is started on metoprolol, a beta blocker, at 25 mg/d.
The etiology of secondary cardiomyopathy includes all of the following except:
a) tachycardia-induced
b) autoimmune
c) radiation-induced
d) infiltrative
e) endomyocardial
The authors’ observations
Cardiomyopathies are diseases of the heart muscle causing mechanical and electrical dysfunction. This group of diseases has a range of symptoms, causes, and treatments. Disease manifests typically as arrhythmia, systolic dysfunction, or diastolic dysfunction. Classification systems are based on origin, anatomy, physiology, primary treatments, method of diagnosis, biopsy, histopathology, and symptomatic state.
The American Heart Association Scientific Statement5 distinguishes cardiomyopathies by degree of organ involvement. Diseases confined to the heart are defined as primary cardiomyopathy, which may have a genetic, acquired, or mixed cause. Acquired causes include inflammatory (myocarditis), stress (Takotsubo), peripartum, and tachycardia. Cardiomyopathies that are part of generalized systemic disorders are defined as secondary cardiomyopathy (Table 1).
Secondary cardiomyopathies have many causes. These include toxicity (medications or alcohol), cancer therapy, infiltrative, storage disease, and endomyocardial, inflammatory, autoimmune, endocrine, and neurologic diseases.5
Evaluation of suspected cardiomyopathy begins with a history and physical focused on identifying causative factors. Selective testing, based on pretest probabilities, might include lab testing, ECG, and echocardiography, and can narrow the differential diagnosis. When toxin-induced cardiomyopathy is suspected, withdrawing the toxin and monitoring for improvement is recommended. The treatment and prognosis for cardiomyopathies vary, based on the cause.6
Review of the literature
After 23 cases of fatal and non-fatal myocarditis were found in a study of 8,000 patients starting clozapine,7 manufacturers in Australia introduced clinical guidelines. Before initiating clozapine, they recommended, clinicians should:
• screen for cardiac symptoms
• screen for a family history of heart disease
• obtain baseline ECG
• obtain baseline markers of myocardial damage (troponin assay and serum creatinine)
• obtain baseline echocardiogram
• repeat cardiac monitoring after the first and second week and then repeat in 6 months
• maintain a high degree of vigilance for signs and symptoms of cardiac toxicity throughout clozapine treatment.8,9
After studying 38 cases of clozapine-induced myocarditis—3 fatal— Ronaldson et al10 listed primary diagnostic features as:
• tachycardia (heart rate >100 beats per minute)
• heart rate >120 beats per minute
• temperature >37°C
• chest pain
• troponin I/T level >2 ng/mL
• C-reactive protein (CRP) > 100 mg/L
• erythrocyte sedimentation rate >50 mm/h.
Among non-fatal cases, symptoms abated after clozapine was discontinued. In 36 of the 38 cases, symptoms emerged 14 to 22 days after clozapine was started. For tachycardia to be considered a diagnostic feature, it must persist for at least 24 hours; if the heart rate is ≥120 beats per minute, however, persistence is not a criterion. It was thought that elevated CRP might herald disease onset; the authors suggest that CRP >50 mg/L should warrant increased monitoring with daily ECG and troponin levels.
Authors’ recommendations include:
• measuring troponin and CRP and order an ECG at baseline and at 7, 14, 21, and 28 days
• examining patient for signs and symptoms of illness at these same intervals
• considering chest pain or fever as an indicator of cardiomyopathy
• asking patients to report any illness during this 4-week period
• if ECG is abnormal or troponin elevated, decreasing clozapine pending further investigation.10
When medications fail
We had to discontinue Mr. C’s clozapine, which meant that the therapeutic relationship established between him and the psychology fellow became an important and, at times, the only bond between him and the medical team while olanzapine was initiated. The alliance between patient and clinician is an important factor for positive prognosis in mental health treatment.11-13 Priebe and McCabe14 asked if the therapeutic relationship in psychiatry is “the basis of therapy or therapy itself?” In a review of studies that used an operationalized measurement of the therapeutic relationship in treating severe mental illness, the authors concluded that the therapeutic relationship is a reliable predictor of outcome.15
In Mr. C’s case, the psychology fellow, who also works with the Partial Hospitalization Program/Intensive Outpatient Program (PHP/IOP), joined the treatment team on the inpatient unit a few days into hospitalization. Eleven meetings, including a discharge session, were held between the psychology fellow and the patient during the inpatient hospitalization. Mr. C also participated in a daily group session, facilitated by the psychology fellow.
Maintaining recognition of the boundary disturbance that characterizes schizophrenic psychoses was important for Mr. C. As Auerhahn and Moskowitz16 wrote, the inpatient therapist can be transformed by the schizophrenia patient into the all-knowing, all-powerful early mother, which could contribute to substantial improvement in the patient’s functioning and report of symptoms, only to have the patient’s symptoms return after discharge.
In an effort to evaluate the duration, frequency, and intensity of Mr. C’s symptom experience, a goal of Mr. C’s hospitalization was to attach words to his internal states, including mood and intensity of paranoid ideation. We showed Mr. C directly and indirectly that reporting intensification of symptoms and decreased functioning would not result in abandonment or punishment, and worked to demonstrate through our actions that the treatment team differs from Mr. C’s view of the world as dangerous and others as hostile and omnipotent.
Treatment Developing language
Initially, Mr. C gives a number (from 1 to 10) to describe his mood, 10 being the happiest he has ever felt and 1 being the most depressed. The treatment team discusses how important it is that Mr. C know his feelings and be able to convey to others how he feels.
Over time, Mr. C is encouraged to attach a feeling word to the number, and by discharge, he stops using numbers and responds to inquiries about his feelings with a mood word. This practice has been reinforced with the patient in the IOP program, allowing him to continue practicing linking his internal state with feeling words.
During hospitalization, Mr. C becomes more vocal about his level of paranoia and is now more likely to seek support when he first experiences a paranoid thought, rather than waiting until after he is paranoid and agitated. Mr. C is encouraged to monitor his thoughts and feelings, and to practice coping strategies he has identified as helpful, including deep breathing, meditation, listening to music, and reminding himself that he is safe.
The treatment team responds to Mr. C’s reports of paranoid ideation (eg, “Some of the other patients were talking about me today”) by processing the affect, and hypothesizing other explanations for these events to slow down “jumping to conclusions,” which is a common part of the paranoid experience.17 Additionally, all meetings with the cardiology team are processed and Mr. C receives psychoeducation about his heart function. Joint sessions with the psychiatry resident and psychology fellow allow Mr. C to ask medical questions and immediately process his reactions, which likely ameliorated his anxiety and allowed him to continue connecting with, identifying, and verbalizing his internal experiences. Given his history of paranoia, sessions also showed that Mr. C is an active participant in his treatment, with the hope of lessening his belief that bad things happen to him and that they are out of his control.
We maintain frequent contact with Mr. C’s parents to update them on their son’s functioning and to discuss treatment interventions that were helpful and the family could implement when Mr. C returns home. Discharge medications are discussed.
After 24 days in the inpatient unit, Mr. C is discharged to the IOP program. The psychology fellow walks Mr. C to the IOP program, where he transitioned immediately from inpatient to the IOP daily schedule of groups and an appointment with the program psychiatrist. The psychology fellow also arranged for and participated in the family meeting with Mr. C’s parents, sister, and treatment providers in the IOP program after his first day back at the IOP.
Throughout his hospitalization, Mr. C had no symptoms of cardiomyopathy, without exercise intolerance, shortness of breath, fatigue, or fever. He is discharged with follow-up care at his outpatient program at the PHP level of care and a follow-up echocardiogram and cardiology appointment are scheduled for 6 weeks later.
The authors' observations
Throughout Mr. C’s hospitalization, the intersections among psychiatry, psychology, cardiology, and internal medicine were apparent and necessary for treatment. No one specialty was able to completely direct this patient’s care without the expertise of, and input from, others. When it looked like all medications had failed, the relationship between the patient and the psychology fellow and the application of previously learned coping strategies prevented acute decompensation.
Clozapine is FDA-approved for treatment-resistant schizophrenia and often is a last resort to help patients remain stable. When clozapine is chosen, it is important to be aware of its side-effect profile (Table 2,1 and Table 3,1-3) and the need for monitoring. The importance of relying on colleagues from other specialties to assist in the effective monitoring process cannot be overstated. This multidisciplinary team ensured that Mr. C did not experience acute decompensation during this process. Cardiac function improved, with an LVEF of 50% after clozapine was discontinued. Mr. C has not needed hospitalization again.
Outcome Stability achieved
Mr. C is successfully discharged from the inpatient service after 24 days in the hospital on the following regimen: olanzapine, 20 mg/d; duloxetine 60 mg/d; benztropine, 0.5 mg/d; haloperidol, 20 mg/d; metoprolol, 25 mg/d; clonazepam, 0.25 mg/d; quetiapine, 50 mg/d; and chlorpromazine, 50 mg as needed for agitation and paranoia. He is given a diagnosis of toxic secondary cardiomyopathy due to clozapine, and remains asymptomatic from a cardiac perspective after discontinuing clozapine.
Follow-up appointment with cardiology and repeat echocardiography were scheduled for 6 weeks after discharge. The follow-up echocardiogram showed improvement (LVEF, 50%). Mr. C continues to do well and remains a client at the IOP program.
Bottom Line
Clozapine often is used as a last resort for patients with treatment-resistant schizophrenia, but its side-effect profile requires careful management and monitoring. If a patient taking clozapine shows tachycardia, consider cardiomyopathy. Evaluation might include lab testing, electrocardiography, and echocardiography. Symptoms often resolve when clozapine is discontinued.
Related Resources
• Citrome L. Clozapine for schizophrenia: life-threatening or life-saving treatment? Current Psychiatry. 2009;8(12):56-63.
• Layland JJ, Liew D, Prior DL. Clozapine-induced cardiotoxicity: a clinical update. Med J Aust. 2009;190(4):190-192.
Drug Brand Names
Benztropine • Cogentin Fluphenazine • Prolixin
Chlorpromazine • Thorazine Haloperidol • Haldol
Citalopram • Celexa Lorazepam • Ativan
Clonazepam • Klonopin Metoprolol • Lopressor
Clozapine • Clozaril Olanzapine • Zyprexa
Duloxetine • Cymbalta Quetiapine • Seroquel
Escitalopram • Lexapro Risperidone • Risperdal
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Case Agitated and violent
Mr. C, age 19, presents with anxiety, agitation, isolation, social withdrawal, and paranoia. He is admitted to the inpatient unit after attempting to punch his father and place him in a headlock. Mr. C has no history of mental illness, no significant medical history, and no significant family history of mental illness.
The treatment team determines that this is Mr. C’s first psychotic break. He is given a diagnosis of psychosis, not otherwise specified and started on risperidone, titrated to 2 mg/d, later discontinued secondary to tachycardia. He is then started on haloperidol, 5 mg/d titrated to 10 mg/d, and psychotic symptoms abate. Mr. C is discharged with a plan to receive follow-up care at an outpatient mental health center.
One year later, Mr. C is readmitted with a similar presentation: paranoia, agitation, anxiety, and isolation. After discharge, he starts an intensive outpatient program (IOP) for long-term treatment of adults who have a diagnosis of a schizophrenia spectrum disorder.
Several medication trials ensue, including risperidone, escitalopram, citalopram, fluphenazine, lorazepam, quetiapine, and haloperidol. Despite these trials over the course of 2 years, Mr. C continues to display paranoia and agitation, and is unable to resume academic and community activities. Within the IOP, Mr. C is placed in a vocational training program and struggles to remain stable enough to continue his job at a small greenhouse.
Concurrently, Mr. C is noted to be abusing alcohol. After the IOP treatment team expresses concern about his abuse, he reduces alcohol intake and he and his parents are educated on the impact of alcohol use on schizophrenia.
Which treatment option would you choose next?
a) initiate a trial of clozapine
b) try a long-acting injectable antipsychotic
c) recommend inpatient treatment
The authors’ observations
Clozapine is an atypical antipsychotic that is FDA-approved for treatment-resistant schizophrenia; it also helps reduce recurrent suicidal behavior in patients with schizophrenia or schizoaffective disorder.
Clozapine works by blocking D2 receptors, thereby reducing positive symptoms. It also blocks serotonin 2A receptors, which enhances dopamine release in certain brain regions, thereby reducing motor side effects. Interactions at 5-HT2C and 5-HT1A receptors may address cognitive and affective symptoms. Clozapine can help relieve negative symptoms and can decrease aggression. Because it has a low risk of tardive dyskinesia, clozapine is useful when treating patients with treatment-resistant schizophrenia.1-3
Treatment Quick heart rate
Mr. C’s IOP treatment team considers a clozapine trial because previous medication trials failed. All paperwork for the registry and screening labs are completed and Mr. C is started on clozapine.
Mr. C’s clozapine dosages are:
• Days 1 to 9: 25 mg/d
• Days 10 to 16: 50 mg/d
• Days 17 to 23: 75 mg/d
• Days 24 to 32: 100 mg/d
• Days 33 to 37: 125 mg/d
• Day 38: 150 mg/d.
On Day 45 of the clozapine trial, Mr. C is increasingly paranoid toward his father and thinks that his father is controlling his thoughts. Mr. C tells the attending psychiatrist that he ingested a handful of clonazepam and considered putting a bag over his head with the intent to commit suicide. Mr. C is admitted to the inpatient unit.
Admission vitals recorded a heart rate of 72 beats per minute but, later that day, the rate was recorded in the vital sign book as 137 beats per minute. The treatment team considers dehydration, anxiety, and staff error; Mr. C is observed carefully. Over the next 2 days, heart rate remains between 102 and 119 beats per minute.
Because of persistent tachycardia, the team orders lab studies, a medical consult, and an electrocardiogram (ECG). Thyroid panel, electrolytes, and clozapine level are within normal limits; ECG is unremarkable.
Although tachycardia is a known side effect of clozapine,3,4 we order an echocardiogram because of Mr. C’s young age and non-diagnostic laboratory workup. The echo study demonstrates reduced left-ventricular ejection fraction (LVEF) of 45%. Tests for HIV infection and Lyme disease are negative. The cardiology team diagnoses cardiomyopathy of unknown origin.
Although Mr. C has a history of alcohol abuse, the cardiology team believes that alcohol consumption does not adequately explain the cardiomyopathy, given his young age and the limited number of lifetime drinking-years (approximately 4 or 5); the team determines that clozapine is causing secondary cardiomyopathy and tachycardia, leading to reduced LVEF. Clozapine is stopped because the recommended treatment for toxic secondary cardiomyopathy is to remove the offending agent. At this point, the clozapine dosage is 250 mg/d.
At the medical team’s recommendation, Mr. C is started on metoprolol, a beta blocker, at 25 mg/d.
The etiology of secondary cardiomyopathy includes all of the following except:
a) tachycardia-induced
b) autoimmune
c) radiation-induced
d) infiltrative
e) endomyocardial
The authors’ observations
Cardiomyopathies are diseases of the heart muscle causing mechanical and electrical dysfunction. This group of diseases has a range of symptoms, causes, and treatments. Disease manifests typically as arrhythmia, systolic dysfunction, or diastolic dysfunction. Classification systems are based on origin, anatomy, physiology, primary treatments, method of diagnosis, biopsy, histopathology, and symptomatic state.
The American Heart Association Scientific Statement5 distinguishes cardiomyopathies by degree of organ involvement. Diseases confined to the heart are defined as primary cardiomyopathy, which may have a genetic, acquired, or mixed cause. Acquired causes include inflammatory (myocarditis), stress (Takotsubo), peripartum, and tachycardia. Cardiomyopathies that are part of generalized systemic disorders are defined as secondary cardiomyopathy (Table 1).
Secondary cardiomyopathies have many causes. These include toxicity (medications or alcohol), cancer therapy, infiltrative, storage disease, and endomyocardial, inflammatory, autoimmune, endocrine, and neurologic diseases.5
Evaluation of suspected cardiomyopathy begins with a history and physical focused on identifying causative factors. Selective testing, based on pretest probabilities, might include lab testing, ECG, and echocardiography, and can narrow the differential diagnosis. When toxin-induced cardiomyopathy is suspected, withdrawing the toxin and monitoring for improvement is recommended. The treatment and prognosis for cardiomyopathies vary, based on the cause.6
Review of the literature
After 23 cases of fatal and non-fatal myocarditis were found in a study of 8,000 patients starting clozapine,7 manufacturers in Australia introduced clinical guidelines. Before initiating clozapine, they recommended, clinicians should:
• screen for cardiac symptoms
• screen for a family history of heart disease
• obtain baseline ECG
• obtain baseline markers of myocardial damage (troponin assay and serum creatinine)
• obtain baseline echocardiogram
• repeat cardiac monitoring after the first and second week and then repeat in 6 months
• maintain a high degree of vigilance for signs and symptoms of cardiac toxicity throughout clozapine treatment.8,9
After studying 38 cases of clozapine-induced myocarditis—3 fatal— Ronaldson et al10 listed primary diagnostic features as:
• tachycardia (heart rate >100 beats per minute)
• heart rate >120 beats per minute
• temperature >37°C
• chest pain
• troponin I/T level >2 ng/mL
• C-reactive protein (CRP) > 100 mg/L
• erythrocyte sedimentation rate >50 mm/h.
Among non-fatal cases, symptoms abated after clozapine was discontinued. In 36 of the 38 cases, symptoms emerged 14 to 22 days after clozapine was started. For tachycardia to be considered a diagnostic feature, it must persist for at least 24 hours; if the heart rate is ≥120 beats per minute, however, persistence is not a criterion. It was thought that elevated CRP might herald disease onset; the authors suggest that CRP >50 mg/L should warrant increased monitoring with daily ECG and troponin levels.
Authors’ recommendations include:
• measuring troponin and CRP and order an ECG at baseline and at 7, 14, 21, and 28 days
• examining patient for signs and symptoms of illness at these same intervals
• considering chest pain or fever as an indicator of cardiomyopathy
• asking patients to report any illness during this 4-week period
• if ECG is abnormal or troponin elevated, decreasing clozapine pending further investigation.10
When medications fail
We had to discontinue Mr. C’s clozapine, which meant that the therapeutic relationship established between him and the psychology fellow became an important and, at times, the only bond between him and the medical team while olanzapine was initiated. The alliance between patient and clinician is an important factor for positive prognosis in mental health treatment.11-13 Priebe and McCabe14 asked if the therapeutic relationship in psychiatry is “the basis of therapy or therapy itself?” In a review of studies that used an operationalized measurement of the therapeutic relationship in treating severe mental illness, the authors concluded that the therapeutic relationship is a reliable predictor of outcome.15
In Mr. C’s case, the psychology fellow, who also works with the Partial Hospitalization Program/Intensive Outpatient Program (PHP/IOP), joined the treatment team on the inpatient unit a few days into hospitalization. Eleven meetings, including a discharge session, were held between the psychology fellow and the patient during the inpatient hospitalization. Mr. C also participated in a daily group session, facilitated by the psychology fellow.
Maintaining recognition of the boundary disturbance that characterizes schizophrenic psychoses was important for Mr. C. As Auerhahn and Moskowitz16 wrote, the inpatient therapist can be transformed by the schizophrenia patient into the all-knowing, all-powerful early mother, which could contribute to substantial improvement in the patient’s functioning and report of symptoms, only to have the patient’s symptoms return after discharge.
In an effort to evaluate the duration, frequency, and intensity of Mr. C’s symptom experience, a goal of Mr. C’s hospitalization was to attach words to his internal states, including mood and intensity of paranoid ideation. We showed Mr. C directly and indirectly that reporting intensification of symptoms and decreased functioning would not result in abandonment or punishment, and worked to demonstrate through our actions that the treatment team differs from Mr. C’s view of the world as dangerous and others as hostile and omnipotent.
Treatment Developing language
Initially, Mr. C gives a number (from 1 to 10) to describe his mood, 10 being the happiest he has ever felt and 1 being the most depressed. The treatment team discusses how important it is that Mr. C know his feelings and be able to convey to others how he feels.
Over time, Mr. C is encouraged to attach a feeling word to the number, and by discharge, he stops using numbers and responds to inquiries about his feelings with a mood word. This practice has been reinforced with the patient in the IOP program, allowing him to continue practicing linking his internal state with feeling words.
During hospitalization, Mr. C becomes more vocal about his level of paranoia and is now more likely to seek support when he first experiences a paranoid thought, rather than waiting until after he is paranoid and agitated. Mr. C is encouraged to monitor his thoughts and feelings, and to practice coping strategies he has identified as helpful, including deep breathing, meditation, listening to music, and reminding himself that he is safe.
The treatment team responds to Mr. C’s reports of paranoid ideation (eg, “Some of the other patients were talking about me today”) by processing the affect, and hypothesizing other explanations for these events to slow down “jumping to conclusions,” which is a common part of the paranoid experience.17 Additionally, all meetings with the cardiology team are processed and Mr. C receives psychoeducation about his heart function. Joint sessions with the psychiatry resident and psychology fellow allow Mr. C to ask medical questions and immediately process his reactions, which likely ameliorated his anxiety and allowed him to continue connecting with, identifying, and verbalizing his internal experiences. Given his history of paranoia, sessions also showed that Mr. C is an active participant in his treatment, with the hope of lessening his belief that bad things happen to him and that they are out of his control.
We maintain frequent contact with Mr. C’s parents to update them on their son’s functioning and to discuss treatment interventions that were helpful and the family could implement when Mr. C returns home. Discharge medications are discussed.
After 24 days in the inpatient unit, Mr. C is discharged to the IOP program. The psychology fellow walks Mr. C to the IOP program, where he transitioned immediately from inpatient to the IOP daily schedule of groups and an appointment with the program psychiatrist. The psychology fellow also arranged for and participated in the family meeting with Mr. C’s parents, sister, and treatment providers in the IOP program after his first day back at the IOP.
Throughout his hospitalization, Mr. C had no symptoms of cardiomyopathy, without exercise intolerance, shortness of breath, fatigue, or fever. He is discharged with follow-up care at his outpatient program at the PHP level of care and a follow-up echocardiogram and cardiology appointment are scheduled for 6 weeks later.
The authors' observations
Throughout Mr. C’s hospitalization, the intersections among psychiatry, psychology, cardiology, and internal medicine were apparent and necessary for treatment. No one specialty was able to completely direct this patient’s care without the expertise of, and input from, others. When it looked like all medications had failed, the relationship between the patient and the psychology fellow and the application of previously learned coping strategies prevented acute decompensation.
Clozapine is FDA-approved for treatment-resistant schizophrenia and often is a last resort to help patients remain stable. When clozapine is chosen, it is important to be aware of its side-effect profile (Table 2,1 and Table 3,1-3) and the need for monitoring. The importance of relying on colleagues from other specialties to assist in the effective monitoring process cannot be overstated. This multidisciplinary team ensured that Mr. C did not experience acute decompensation during this process. Cardiac function improved, with an LVEF of 50% after clozapine was discontinued. Mr. C has not needed hospitalization again.
Outcome Stability achieved
Mr. C is successfully discharged from the inpatient service after 24 days in the hospital on the following regimen: olanzapine, 20 mg/d; duloxetine 60 mg/d; benztropine, 0.5 mg/d; haloperidol, 20 mg/d; metoprolol, 25 mg/d; clonazepam, 0.25 mg/d; quetiapine, 50 mg/d; and chlorpromazine, 50 mg as needed for agitation and paranoia. He is given a diagnosis of toxic secondary cardiomyopathy due to clozapine, and remains asymptomatic from a cardiac perspective after discontinuing clozapine.
Follow-up appointment with cardiology and repeat echocardiography were scheduled for 6 weeks after discharge. The follow-up echocardiogram showed improvement (LVEF, 50%). Mr. C continues to do well and remains a client at the IOP program.
Bottom Line
Clozapine often is used as a last resort for patients with treatment-resistant schizophrenia, but its side-effect profile requires careful management and monitoring. If a patient taking clozapine shows tachycardia, consider cardiomyopathy. Evaluation might include lab testing, electrocardiography, and echocardiography. Symptoms often resolve when clozapine is discontinued.
Related Resources
• Citrome L. Clozapine for schizophrenia: life-threatening or life-saving treatment? Current Psychiatry. 2009;8(12):56-63.
• Layland JJ, Liew D, Prior DL. Clozapine-induced cardiotoxicity: a clinical update. Med J Aust. 2009;190(4):190-192.
Drug Brand Names
Benztropine • Cogentin Fluphenazine • Prolixin
Chlorpromazine • Thorazine Haloperidol • Haldol
Citalopram • Celexa Lorazepam • Ativan
Clonazepam • Klonopin Metoprolol • Lopressor
Clozapine • Clozaril Olanzapine • Zyprexa
Duloxetine • Cymbalta Quetiapine • Seroquel
Escitalopram • Lexapro Risperidone • Risperdal
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Clozaril [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2013.
2. Stahl SM. Clozapine. In: Stahl SM. The prescriber’s guide: Stahl’s essential psychopharmacology. 3rd ed. New York, NY: Cambridge University Press; 2009:113-118.
3. Young CR, Bowers MB Jr, Mazure CM. Management of the adverse effects of clozapine. Schizophr Bull. 1998;24(3):381-388.
4. Lang UE, Willbring M, von Golitschek R, et al. Clozapine-induced myocarditis after long-term treatment: case presentation and clinical perspectives. J Psychopharmacol. 2008;22(5):576-580.
5. Maron BJ, Towbin JA, Thiene G, et al; American Heart Association; Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; Council on Epidemiology and Prevention. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association scientific statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation. 2006;113(14):1807-1816.
6. Hare JM. The dilated, restrictive, and infiltrative cardiomyopathies. In: Braunwald’s heart disease: a textbook of cardiovascular medicine. 9th ed. Bonow RO, Mann DL, Zipes DP, eds. New York, NY: Elsevier; 2012:1561-1581.
7. Kilian JG, Kerr K, Lawrence C, et al. Myocarditis and cardiomyopathy associated with clozapine. Lancet. 1999;354(9193):1841-1845.
8. Clopine [package insert]. Aukland, New Zealand: Douglas Pharmaceuticals; 2014.
9. Killian JG, Kerr K, Lawrence C, et al. Myocarditis and cardiomyopathy associated with clozapine. Lancet. 1999; 354(9193):1841-1845.
10. Ronaldson KJ, Taylor AJ, Fitzgerald PB, et al. Diagnostic characteristics of clozapine-induced myocarditis identified by an analysis of 38 cases and 47 controls. J Clin Psychiatry. 2010;71(8):976-981.
11. Rogers CR. On becoming a person: a therapist’s view of psychotherapy. New York, NY: Houghton Mifflin; 1961.
12. Horvath AO, Symonds BD. Relation between a working alliance and outcome in psychotherapy: a meta-analysis. Journal of Counseling Psychology. 1991;38(2):139-149.
13. Krupnick JL, Sotsky SM, Simmens S, et al. The role of the therapeutic alliance in psychotherapy and pharmacotherapy outcome: Findings in the National Institute of Mental Health Treatment of Depression Collaborative Research Program. Journal of Consulting and Clinical Psychology. 1996;64(3):532-539.
14. Priebe S, McCabe R. Therapeutic relationships in psychiatry: the basis of therapy or therapy in itself? Int Rev Psychiatry. 2008;20(6):521-526.
15. McCabe R, Priebe S. The therapeutic relationship in the treatment of severe mental illness: a review of methods and findings. Int J Soc Psychiatry. 2004;50(2):115-128.
16. Auerhahn NC, Moskowitz MB. Merger fantasies in individual inpatient therapy with schizophrenic patient. Psychoanalytic Psychology. 1984;1(2):131-148.
17. Penn DL, Roberts DL, Combs D, et al. Best practices: The development of the Social Cognition and Interaction Training program for schizophrenia spectrum disorders. Psychiatr Serv. 2007;58(4):449-451.
1. Clozaril [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2013.
2. Stahl SM. Clozapine. In: Stahl SM. The prescriber’s guide: Stahl’s essential psychopharmacology. 3rd ed. New York, NY: Cambridge University Press; 2009:113-118.
3. Young CR, Bowers MB Jr, Mazure CM. Management of the adverse effects of clozapine. Schizophr Bull. 1998;24(3):381-388.
4. Lang UE, Willbring M, von Golitschek R, et al. Clozapine-induced myocarditis after long-term treatment: case presentation and clinical perspectives. J Psychopharmacol. 2008;22(5):576-580.
5. Maron BJ, Towbin JA, Thiene G, et al; American Heart Association; Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; Council on Epidemiology and Prevention. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association scientific statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation. 2006;113(14):1807-1816.
6. Hare JM. The dilated, restrictive, and infiltrative cardiomyopathies. In: Braunwald’s heart disease: a textbook of cardiovascular medicine. 9th ed. Bonow RO, Mann DL, Zipes DP, eds. New York, NY: Elsevier; 2012:1561-1581.
7. Kilian JG, Kerr K, Lawrence C, et al. Myocarditis and cardiomyopathy associated with clozapine. Lancet. 1999;354(9193):1841-1845.
8. Clopine [package insert]. Aukland, New Zealand: Douglas Pharmaceuticals; 2014.
9. Killian JG, Kerr K, Lawrence C, et al. Myocarditis and cardiomyopathy associated with clozapine. Lancet. 1999; 354(9193):1841-1845.
10. Ronaldson KJ, Taylor AJ, Fitzgerald PB, et al. Diagnostic characteristics of clozapine-induced myocarditis identified by an analysis of 38 cases and 47 controls. J Clin Psychiatry. 2010;71(8):976-981.
11. Rogers CR. On becoming a person: a therapist’s view of psychotherapy. New York, NY: Houghton Mifflin; 1961.
12. Horvath AO, Symonds BD. Relation between a working alliance and outcome in psychotherapy: a meta-analysis. Journal of Counseling Psychology. 1991;38(2):139-149.
13. Krupnick JL, Sotsky SM, Simmens S, et al. The role of the therapeutic alliance in psychotherapy and pharmacotherapy outcome: Findings in the National Institute of Mental Health Treatment of Depression Collaborative Research Program. Journal of Consulting and Clinical Psychology. 1996;64(3):532-539.
14. Priebe S, McCabe R. Therapeutic relationships in psychiatry: the basis of therapy or therapy in itself? Int Rev Psychiatry. 2008;20(6):521-526.
15. McCabe R, Priebe S. The therapeutic relationship in the treatment of severe mental illness: a review of methods and findings. Int J Soc Psychiatry. 2004;50(2):115-128.
16. Auerhahn NC, Moskowitz MB. Merger fantasies in individual inpatient therapy with schizophrenic patient. Psychoanalytic Psychology. 1984;1(2):131-148.
17. Penn DL, Roberts DL, Combs D, et al. Best practices: The development of the Social Cognition and Interaction Training program for schizophrenia spectrum disorders. Psychiatr Serv. 2007;58(4):449-451.
Depression may run with opiate use in patients with ankylosing spondylitis
NEW YORK – Patients with ankylosing spondylitis who used opiate analgesics were five times more likely to report depression than were those who did not, in a retrospective case-control study of 611 patients.
The relationship between opiate usage and depression was significant even though those who took antidepressants or anxiolytics were significantly less likely to use opiates, according to Dr. Jonathan D. Dau of the department of rheumatology at the University of Texas, Houston.
In the study, there were several highly significant distinctions between those who used opiate analgesics and those who did not, but none could be connected to inflammatory activity, Dr. Dau said.
"None of the objective measures of AS [ankylosing spondylitis] disease activity or progression were found to be associated with opiate usage. This adds confirmation to the hypothesis that pain associated with AS may develop from sources other than spinal inflammation alone," he said.
In data presented at the joint meetings of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis and the Spondyloarthritis Research & Treatment Network, a large variety of parameters were compared between the 87 patients who took opiate analgesics intermittently (91%) or continuously (9%) and the remaining 524 who never took opiate analgesics over a follow-up period of up to 4 years. Three centers in addition to the University of Texas contributed AS patients to the analysis. The mean disease duration was 17.6 years.
Although there were no significant differences between users and nonusers of opiates for radiographic severity, as measured with the modified Stoke Ankylosing Spondylitis Spinal Score, or inflammation, as measured with C-reactive protein levels or erythrocyte sedimentation rate, opiate users scored high on subjective measures. Specifically, the odds ratio for a Bath AS Disease Activity Index score of 4 or greater was 5.460 (P less than .0001). The OR for a high patient global pain assessment was 4.240 (P less than .0001).
As for depression, the OR for opiate users relative to nonopiate users was 5.907 (P less than .0001) by self-report and 3.071 (P less than .0001) by the Center for Epidemiologic Study Depression scale. The authors also reported an OR for smoking among opiate users of 2.125 (P = .0018).
Conversely, an analysis of concomitant medication use found that antidepressants correlated with a 63% reduction (OR, 0.371; P = .0004) in the likelihood of opiate use. Anxiolytics correlated with a nearly 90% reduction (OR, 0.124; P less than .0001). There was no significant association with opiate use and use of either NSAIDs or tumor necrosis factor inhibitors.
However, the study found that opiate users were almost three times more likely to be taking prednisone (OR, 2.996; P = .0073) and more than eight times more likely to be taking muscle relaxants (OR, 8.458; P less than .0001). Dr. Dau observed that muscle relaxants on top of opiates "may provide a greater magnitude of pain relief taken together than when taken alone."
Because of the concerns about use of opiates, particularly their propensity to induce dependence, Dr. Dau suggested that it is important to further explore why some patients take these agents in addition to treatments targeted at disease activity. While standard medications such as NSAIDs and TNF inhibitors have been shown to relieve pain, they do not control somatic pain for all patients.
"This is especially true when the pain stems from processes other than inflammation," reported Dr. Dau. More data are needed to determine whether control of depression through antidepressants is a factor for reducing opiate use, he said.
Dr. Dau reported no relevant financial disclosures.
NEW YORK – Patients with ankylosing spondylitis who used opiate analgesics were five times more likely to report depression than were those who did not, in a retrospective case-control study of 611 patients.
The relationship between opiate usage and depression was significant even though those who took antidepressants or anxiolytics were significantly less likely to use opiates, according to Dr. Jonathan D. Dau of the department of rheumatology at the University of Texas, Houston.
In the study, there were several highly significant distinctions between those who used opiate analgesics and those who did not, but none could be connected to inflammatory activity, Dr. Dau said.
"None of the objective measures of AS [ankylosing spondylitis] disease activity or progression were found to be associated with opiate usage. This adds confirmation to the hypothesis that pain associated with AS may develop from sources other than spinal inflammation alone," he said.
In data presented at the joint meetings of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis and the Spondyloarthritis Research & Treatment Network, a large variety of parameters were compared between the 87 patients who took opiate analgesics intermittently (91%) or continuously (9%) and the remaining 524 who never took opiate analgesics over a follow-up period of up to 4 years. Three centers in addition to the University of Texas contributed AS patients to the analysis. The mean disease duration was 17.6 years.
Although there were no significant differences between users and nonusers of opiates for radiographic severity, as measured with the modified Stoke Ankylosing Spondylitis Spinal Score, or inflammation, as measured with C-reactive protein levels or erythrocyte sedimentation rate, opiate users scored high on subjective measures. Specifically, the odds ratio for a Bath AS Disease Activity Index score of 4 or greater was 5.460 (P less than .0001). The OR for a high patient global pain assessment was 4.240 (P less than .0001).
As for depression, the OR for opiate users relative to nonopiate users was 5.907 (P less than .0001) by self-report and 3.071 (P less than .0001) by the Center for Epidemiologic Study Depression scale. The authors also reported an OR for smoking among opiate users of 2.125 (P = .0018).
Conversely, an analysis of concomitant medication use found that antidepressants correlated with a 63% reduction (OR, 0.371; P = .0004) in the likelihood of opiate use. Anxiolytics correlated with a nearly 90% reduction (OR, 0.124; P less than .0001). There was no significant association with opiate use and use of either NSAIDs or tumor necrosis factor inhibitors.
However, the study found that opiate users were almost three times more likely to be taking prednisone (OR, 2.996; P = .0073) and more than eight times more likely to be taking muscle relaxants (OR, 8.458; P less than .0001). Dr. Dau observed that muscle relaxants on top of opiates "may provide a greater magnitude of pain relief taken together than when taken alone."
Because of the concerns about use of opiates, particularly their propensity to induce dependence, Dr. Dau suggested that it is important to further explore why some patients take these agents in addition to treatments targeted at disease activity. While standard medications such as NSAIDs and TNF inhibitors have been shown to relieve pain, they do not control somatic pain for all patients.
"This is especially true when the pain stems from processes other than inflammation," reported Dr. Dau. More data are needed to determine whether control of depression through antidepressants is a factor for reducing opiate use, he said.
Dr. Dau reported no relevant financial disclosures.
NEW YORK – Patients with ankylosing spondylitis who used opiate analgesics were five times more likely to report depression than were those who did not, in a retrospective case-control study of 611 patients.
The relationship between opiate usage and depression was significant even though those who took antidepressants or anxiolytics were significantly less likely to use opiates, according to Dr. Jonathan D. Dau of the department of rheumatology at the University of Texas, Houston.
In the study, there were several highly significant distinctions between those who used opiate analgesics and those who did not, but none could be connected to inflammatory activity, Dr. Dau said.
"None of the objective measures of AS [ankylosing spondylitis] disease activity or progression were found to be associated with opiate usage. This adds confirmation to the hypothesis that pain associated with AS may develop from sources other than spinal inflammation alone," he said.
In data presented at the joint meetings of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis and the Spondyloarthritis Research & Treatment Network, a large variety of parameters were compared between the 87 patients who took opiate analgesics intermittently (91%) or continuously (9%) and the remaining 524 who never took opiate analgesics over a follow-up period of up to 4 years. Three centers in addition to the University of Texas contributed AS patients to the analysis. The mean disease duration was 17.6 years.
Although there were no significant differences between users and nonusers of opiates for radiographic severity, as measured with the modified Stoke Ankylosing Spondylitis Spinal Score, or inflammation, as measured with C-reactive protein levels or erythrocyte sedimentation rate, opiate users scored high on subjective measures. Specifically, the odds ratio for a Bath AS Disease Activity Index score of 4 or greater was 5.460 (P less than .0001). The OR for a high patient global pain assessment was 4.240 (P less than .0001).
As for depression, the OR for opiate users relative to nonopiate users was 5.907 (P less than .0001) by self-report and 3.071 (P less than .0001) by the Center for Epidemiologic Study Depression scale. The authors also reported an OR for smoking among opiate users of 2.125 (P = .0018).
Conversely, an analysis of concomitant medication use found that antidepressants correlated with a 63% reduction (OR, 0.371; P = .0004) in the likelihood of opiate use. Anxiolytics correlated with a nearly 90% reduction (OR, 0.124; P less than .0001). There was no significant association with opiate use and use of either NSAIDs or tumor necrosis factor inhibitors.
However, the study found that opiate users were almost three times more likely to be taking prednisone (OR, 2.996; P = .0073) and more than eight times more likely to be taking muscle relaxants (OR, 8.458; P less than .0001). Dr. Dau observed that muscle relaxants on top of opiates "may provide a greater magnitude of pain relief taken together than when taken alone."
Because of the concerns about use of opiates, particularly their propensity to induce dependence, Dr. Dau suggested that it is important to further explore why some patients take these agents in addition to treatments targeted at disease activity. While standard medications such as NSAIDs and TNF inhibitors have been shown to relieve pain, they do not control somatic pain for all patients.
"This is especially true when the pain stems from processes other than inflammation," reported Dr. Dau. More data are needed to determine whether control of depression through antidepressants is a factor for reducing opiate use, he said.
Dr. Dau reported no relevant financial disclosures.
AT THE 2014 GRAPPA AND SPARTAN ANNUAL MEETINGS
Key clinical point: Patients with ankylosing spondylitis who use opiates are far more likely to self-report depression than are those who do not use opiates, with declining use of opiates in those on antidepressants or anxiolytics.
Major finding: Depression was significantly more likely to occur among opiate users relative to nonopiate users by self-report (OR, 5.907; P less than .0001) and by the Center for Epidemiologic Study Depression scale (OR, 3.071; P less than .0001).
Data source: A retrospective, case-control study of 611 patients with AS.
Disclosures: Dr. Dau reported no relevant financial disclosures.
Eczema linked to increased suicidal thoughts in teens
Adolescents with eczema were significantly more likely to report suicidal ideation than those who did not have eczema, based on data from a population-based study of more than 3,000 adolescents. The findings were published in the Journal of Investigative Dermatology.
Data from previous studies have shown an increased suicide risk in patients with skin disorders, but the impact of eczema on adolescents in particular has not been well studied, wrote Dr. Jon A. Halvorsen of the department of dermatology at the University of Oslo and his colleagues (J. Invest. Dermatol. 2014;134:1847-54).
The researchers reviewed survey data from 3,556 adolescents aged 18-19 years collected as part of the Oslo section of the Youths 2004 study.
The overall prevalence of eczema was 10% (12% in girls and 7% in boys). The overall incidence of suicidal ideation was 11%, but 16% of adolescents with eczema reported suicidal ideation vs. 9% of those with no eczema (odds ratio, 1.87). The association was even stronger in adolescents who reported eczema with itching (OR, 3.57), compared with those who did not report itching (OR, 1.06).
"The risk of suicidal ideation increased nearly fourfold in this group," the researchers noted. "Our data suggest that itch is a major predictor of psychological problems and may be a greater risk factor for these problems than chronic eczema without itch."
In addition, adolescents with eczema were significantly more likely than those without eczema to report mental health problems (assessed by the Strengths and Difficulties Questionnaire) and mental distress (assessed by the Hopkins Symptom Checklist 10) and these associations were even more significant in adolescents who reported eczema with itch, compared with those who had eczema without itch.
The study was limited by the use of self-reports, but its strengths include a large sample size with high participation, the researchers said. The results suggest that "both treatment of eczema and psychological support in this high-risk group may help to reduce suicide and mental health problems in this vulnerable population," they added.
The researchers reported no financial conflicts.
Adolescents with eczema were significantly more likely to report suicidal ideation than those who did not have eczema, based on data from a population-based study of more than 3,000 adolescents. The findings were published in the Journal of Investigative Dermatology.
Data from previous studies have shown an increased suicide risk in patients with skin disorders, but the impact of eczema on adolescents in particular has not been well studied, wrote Dr. Jon A. Halvorsen of the department of dermatology at the University of Oslo and his colleagues (J. Invest. Dermatol. 2014;134:1847-54).
The researchers reviewed survey data from 3,556 adolescents aged 18-19 years collected as part of the Oslo section of the Youths 2004 study.
The overall prevalence of eczema was 10% (12% in girls and 7% in boys). The overall incidence of suicidal ideation was 11%, but 16% of adolescents with eczema reported suicidal ideation vs. 9% of those with no eczema (odds ratio, 1.87). The association was even stronger in adolescents who reported eczema with itching (OR, 3.57), compared with those who did not report itching (OR, 1.06).
"The risk of suicidal ideation increased nearly fourfold in this group," the researchers noted. "Our data suggest that itch is a major predictor of psychological problems and may be a greater risk factor for these problems than chronic eczema without itch."
In addition, adolescents with eczema were significantly more likely than those without eczema to report mental health problems (assessed by the Strengths and Difficulties Questionnaire) and mental distress (assessed by the Hopkins Symptom Checklist 10) and these associations were even more significant in adolescents who reported eczema with itch, compared with those who had eczema without itch.
The study was limited by the use of self-reports, but its strengths include a large sample size with high participation, the researchers said. The results suggest that "both treatment of eczema and psychological support in this high-risk group may help to reduce suicide and mental health problems in this vulnerable population," they added.
The researchers reported no financial conflicts.
Adolescents with eczema were significantly more likely to report suicidal ideation than those who did not have eczema, based on data from a population-based study of more than 3,000 adolescents. The findings were published in the Journal of Investigative Dermatology.
Data from previous studies have shown an increased suicide risk in patients with skin disorders, but the impact of eczema on adolescents in particular has not been well studied, wrote Dr. Jon A. Halvorsen of the department of dermatology at the University of Oslo and his colleagues (J. Invest. Dermatol. 2014;134:1847-54).
The researchers reviewed survey data from 3,556 adolescents aged 18-19 years collected as part of the Oslo section of the Youths 2004 study.
The overall prevalence of eczema was 10% (12% in girls and 7% in boys). The overall incidence of suicidal ideation was 11%, but 16% of adolescents with eczema reported suicidal ideation vs. 9% of those with no eczema (odds ratio, 1.87). The association was even stronger in adolescents who reported eczema with itching (OR, 3.57), compared with those who did not report itching (OR, 1.06).
"The risk of suicidal ideation increased nearly fourfold in this group," the researchers noted. "Our data suggest that itch is a major predictor of psychological problems and may be a greater risk factor for these problems than chronic eczema without itch."
In addition, adolescents with eczema were significantly more likely than those without eczema to report mental health problems (assessed by the Strengths and Difficulties Questionnaire) and mental distress (assessed by the Hopkins Symptom Checklist 10) and these associations were even more significant in adolescents who reported eczema with itch, compared with those who had eczema without itch.
The study was limited by the use of self-reports, but its strengths include a large sample size with high participation, the researchers said. The results suggest that "both treatment of eczema and psychological support in this high-risk group may help to reduce suicide and mental health problems in this vulnerable population," they added.
The researchers reported no financial conflicts.
FROM THE JOURNAL OF INVESTIGATIVE DERMATOLOGY
Key clinical point: Treating eczema and providing psychological support might help reduce suicidal ideation in vulnerable adolescent populations.
Major finding: Sixteen percent of adolescents with eczema reported suicidal ideation, compared with 9% of those without eczema (odds ratio, 1.87), and the association was even stronger in those reporting itching (OR, 3.57) compared with those without itching (OR, 1.06).
Data source: A population-based study of 3,556 adolescents aged 18-19 years.
Disclosures: The researchers reported no financial conflicts.
CBT-informed psychotherapy benefits patients with psychogenic nonepileptic seizures
Cognitive behavioral therapy–informed psychotherapy was associated with significant seizure reduction and improved comorbid symptoms and global functioning in patients with psychogenic nonepileptic seizures in a multicenter, pilot, randomized clinical trial.
In 38 patients with psychogenic nonepileptic seizures (PNES) who were randomized to receive treatment with flexible-dose sertraline hydrochloride only, cognitive behavioral therapy–informed psychotherapy (CBT-IP) only, CBT-IP along with sertraline, or treatment as usual, CBT-IP-only was associated with a 51.4% reduction in total monthly seizures and significant improvement in depression, anxiety, quality of life, and global functioning measures. Combined CBT-IP and sertraline was associated with a 59.3% reduction in total monthly seizures and with significant improvement in global functioning, Dr. W. Curt LaFrance Jr. of Brown University, Providence, R.I., and his colleagues reported July 2 in JAMA Psychiatry on behalf of the NES Treatment Trial Consortium.
The patients were treated at three academic medical centers with mental health clinicians specially trained to treat outpatients with PNES, which is the most common type of conversion disorder, and which is as disabling as epilepsy. They were followed for 16 weeks. No significant seizure reduction was seen in those treated with sertraline only or with usual care, the investigators reported (JAMA Psychiatry 2014 July 2 [doi: 10.1001/jamapsychiatry.2014.817]).
The findings of this study, which address only the effect of treatment in the phase of gaining control of seizures, support the use of this type of manualized psychotherapy for patients with PNES, they concluded, noting that the durability of treatment will be assessed in future studies.
This study was supported by the American Epilepsy Society and the Research Infrastructure Award from the Epilepsy Foundation. Dr. LaFrance reported receiving research support from government institutes and epilepsy organizations and foundations, serving on the editorial boards of Epilepsia and Epilepsy & Behavior, and providing medicolegal expert testimony. Several coauthors reported receiving research support from, serving on an advisory board of, or receiving honoraria from several pharmaceutical companies.
Cognitive behavioral therapy–informed psychotherapy was associated with significant seizure reduction and improved comorbid symptoms and global functioning in patients with psychogenic nonepileptic seizures in a multicenter, pilot, randomized clinical trial.
In 38 patients with psychogenic nonepileptic seizures (PNES) who were randomized to receive treatment with flexible-dose sertraline hydrochloride only, cognitive behavioral therapy–informed psychotherapy (CBT-IP) only, CBT-IP along with sertraline, or treatment as usual, CBT-IP-only was associated with a 51.4% reduction in total monthly seizures and significant improvement in depression, anxiety, quality of life, and global functioning measures. Combined CBT-IP and sertraline was associated with a 59.3% reduction in total monthly seizures and with significant improvement in global functioning, Dr. W. Curt LaFrance Jr. of Brown University, Providence, R.I., and his colleagues reported July 2 in JAMA Psychiatry on behalf of the NES Treatment Trial Consortium.
The patients were treated at three academic medical centers with mental health clinicians specially trained to treat outpatients with PNES, which is the most common type of conversion disorder, and which is as disabling as epilepsy. They were followed for 16 weeks. No significant seizure reduction was seen in those treated with sertraline only or with usual care, the investigators reported (JAMA Psychiatry 2014 July 2 [doi: 10.1001/jamapsychiatry.2014.817]).
The findings of this study, which address only the effect of treatment in the phase of gaining control of seizures, support the use of this type of manualized psychotherapy for patients with PNES, they concluded, noting that the durability of treatment will be assessed in future studies.
This study was supported by the American Epilepsy Society and the Research Infrastructure Award from the Epilepsy Foundation. Dr. LaFrance reported receiving research support from government institutes and epilepsy organizations and foundations, serving on the editorial boards of Epilepsia and Epilepsy & Behavior, and providing medicolegal expert testimony. Several coauthors reported receiving research support from, serving on an advisory board of, or receiving honoraria from several pharmaceutical companies.
Cognitive behavioral therapy–informed psychotherapy was associated with significant seizure reduction and improved comorbid symptoms and global functioning in patients with psychogenic nonepileptic seizures in a multicenter, pilot, randomized clinical trial.
In 38 patients with psychogenic nonepileptic seizures (PNES) who were randomized to receive treatment with flexible-dose sertraline hydrochloride only, cognitive behavioral therapy–informed psychotherapy (CBT-IP) only, CBT-IP along with sertraline, or treatment as usual, CBT-IP-only was associated with a 51.4% reduction in total monthly seizures and significant improvement in depression, anxiety, quality of life, and global functioning measures. Combined CBT-IP and sertraline was associated with a 59.3% reduction in total monthly seizures and with significant improvement in global functioning, Dr. W. Curt LaFrance Jr. of Brown University, Providence, R.I., and his colleagues reported July 2 in JAMA Psychiatry on behalf of the NES Treatment Trial Consortium.
The patients were treated at three academic medical centers with mental health clinicians specially trained to treat outpatients with PNES, which is the most common type of conversion disorder, and which is as disabling as epilepsy. They were followed for 16 weeks. No significant seizure reduction was seen in those treated with sertraline only or with usual care, the investigators reported (JAMA Psychiatry 2014 July 2 [doi: 10.1001/jamapsychiatry.2014.817]).
The findings of this study, which address only the effect of treatment in the phase of gaining control of seizures, support the use of this type of manualized psychotherapy for patients with PNES, they concluded, noting that the durability of treatment will be assessed in future studies.
This study was supported by the American Epilepsy Society and the Research Infrastructure Award from the Epilepsy Foundation. Dr. LaFrance reported receiving research support from government institutes and epilepsy organizations and foundations, serving on the editorial boards of Epilepsia and Epilepsy & Behavior, and providing medicolegal expert testimony. Several coauthors reported receiving research support from, serving on an advisory board of, or receiving honoraria from several pharmaceutical companies.
FROM JAMA PSYCHIATRY
Key clinical point: CBT-informed psychotherapy appears to help patients to gain control of psychogenic nonepileptic seizures, the second stage of their management.
Major finding: CBT-IP only and CBT-IP with sertraline were associated with 51.4% and 59.3% reductions, respectively, in total monthly seizures.
Data source: A multicenter, pilot, randomized clinical trial involving 38 patients.
Disclosures: This study was supported by the American Epilepsy Society and the Research Infrastructure Award from the Epilepsy Foundation. Dr. LaFrance reported receiving research support from government institutes and epilepsy organizations and foundations, serving on the editorial boards of Epilepsia and Epilepsy & Behavior, and providing medicolegal expert testimony. Several coauthors reported receiving research support, serving on an advisory board, or receiving honoraria from several pharmaceutical companies.
Poor oral hygiene in the mentally ill: Be aware of the problem, and intervene
Poor oral health is common among mentally ill people and is related to inadequate nutrition, poor self-care, substance abuse, and medication side effects.1 Poor oral hygiene is a significant problem because it results in dental pathology that has an adverse influence on the whole body.
Compared with the general population, mentally ill patients are 3 times more likely to have their teeth removed.2 In a survey of mentally ill adults, 92% were found to have tooth decay—of which 23% were untreated and 40% smoked tobacco.3 Approximately 9% have periodontal disease, which most often occurs in those who smoke cigarettes.4
Lifestyle contributors
Drug abuse facilitates dental diseases, as evidenced by the high rate of caries among methamphetamine users.5 The drug induces xerostomia, encouraging users to drink sweetened beverages; this, combined with limited oral care, results in profound dental decay (“meth mouth”). Oral cocaine users often exhibit dental erosions or abrasions, gingival lacerations or necrosis, and mucosal lesions. Smoking Cannabis is associated with an increased rate of gingivitis, alveolar bone loss, leukoplakia, and oral papilloma or other cancers.5 Heroin users are at increased risk of tooth decay, periodontal disease, and oral infection.5
Alcohol consumption increases the risk of oral cancer. Long-term alcohol use suppresses bone marrow function, causing leukopenia and resulting in immunosuppression and an increased incidence of dental infections.6 Excessive alcohol consumption also can cause thrombocytopenia and bleeding, which can complicate dental procedures.
Smoking cigarettes increases the incidence of periodontal disease, especially necrotizing gingivitis and candidiasis.7 Ninety percent of patients with schizophrenia smoke—compared with up to 70% of patients with other psychiatric disorders, and 19% of the general population.7,8 Physiologic aspects of schizophrenia reinforce the smoking habit.7
Somatic ailments. Psychiatric disorders are strongly associated with diabetes, obesity, hypertension, stroke, heart disease, and arthritis, all of which contribute to oral pathology. Older age, greater dysfunction, longer duration of illness, and smoking are predictors of adverse dental outcomes.
Anxiety, depression, stress—all of these these disorders increase the circulating level of cortisol, thus raising the risk that periodontal disease will progress.9 Periodontitis increases the risk of stroke and heart attack by accelerating atherosclerotic plaque formation.10 Depression, anxiety, and substance abuse can lead to temporomandibular disorders that cause pain and restrict jaw movement.11 Stressed patients may experience muscle tension and bruxism, which can lead to temporomandibular joint discomfort.
Eating disorders. Patients who induce vomiting may exhibit enamel erosions (especially on the anterior maxillary teeth), increased tooth hypersensitivity, decay, and wear on dental restorative work.
Atypical odontalgia, characterized by chronic, burning pain in teeth and gums, is associated with depression and anxiety.11 Misdiagnosis can result in extractions or procedures without an appropriate indication and failure to alleviate the pain.
Medication side effects. Xerostomia can increase the risk for caries, periodontal disease, and oral infections such as candidiasis, glossitis, stomatitis, and parotitis.9 Extrapyramidal side effects (tardive dyskinesia, dystonia) may cause tooth damage and make managing dentures difficult.6
What to tell patients, and what you can do for them
Encourage your patients to reduce their sugar intake, brush and floss regularly, and work to stop smoking or ingesting substances of abuse. Teach appropriate hygiene and nutrition, which reduces the risk of dental caries, infection, and related problems. Recommend periodic oral health screening and how to secure such dental care.
From your position of familiarity with patients’ psychopharmacotherapy, make an effort to personalize and adjust their regimens when dental disease is present to address concerns about oral health that can be caused by medication side effects.
A multidisciplinary approach with patient advocacy, involving you and the patient’s dentist and primary care physician, facilitates health care and works to offer the patient access to global medical services.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Mental Illness Fellowship of Australia Inc. Overview of the oral health of people affected by mental illness. http:// www.wfmh.com/links/external-contacts/mental-illness-fellowship-of-australia. Accessed June 18, 2014.
2. Kisely S, Quek LH, Pais J, et al. Advanced dental disease in people with severe mental illness: systematic review and meta-analysis. Br J Psychiatry. 2011;199(3):187-193.
3. Dental caries (tooth decay) in adults (age 20 to 64). National Institute of Dental and Craniofacial Research. http:// www.nidcr.nih.gov/DataStatistics/FindDataByTopic/ DentalCaries/DentalCariesAdults20to64.htm. Updated January 6, 2014. Accessed June 18, 2014.
4. Peridontal disease in adults (age 20 to 64). National Institute of Dental and Craniofacial Research. http://www.nidcr. nih.gov/DataStatistics/FindDataByTopic/GumDisease/ PeriodontaldiseaseAdults20to64.htm. Updated January 6, 2014. Accessed June 18, 2014.
5. Maloney WJ. The significance of illicit drug use to dental practice. http://www.webmedcentral.com/wmcpdf/ Article_WMC00455.pdf. Published July 28, 2010. Accessed June 18, 2014.
6. Oral health care for people with mental problems: guidelines and recommendations. British Society for Disability and Oral Health. http://www.bsdh.org.uk/guidelines/ mental.pdf. Updated January 2000. Accessed June 18, 2014.
7. Lohr JB, Flynn K. Smoking and schizophrenia. Schizophr Res. 1992;8(2):93-102.
8. Centers for Disease Control and Prevention (CDC). Vital signs: current cigarette smoking among adults aged ≥18 years–United States, 2005-2010. MMWR Morb Mortal Wkly Rep. 2011;60(35):1207-1212.
9. Yoffee L. The link between oral health and medical illness. http://www.everydayhealth.com/dental-health/oral-conditions/oral-health-and-other-diseases.aspx. Updated November 9, 2012. Accessed June 18, 2014.
10. Demmer RT, Desvarieux M. Periodontal infections and cardiovascular disease: the heart of the matter. J Am Dent Assoc. 2006;137(suppl 2):14S-20S; quiz 38S.
11. Mental illness and the dental patient. American Dental Hygienists’ Association. http://www.adha.org/ce-course-10. Accessed June 18, 2014.
Poor oral health is common among mentally ill people and is related to inadequate nutrition, poor self-care, substance abuse, and medication side effects.1 Poor oral hygiene is a significant problem because it results in dental pathology that has an adverse influence on the whole body.
Compared with the general population, mentally ill patients are 3 times more likely to have their teeth removed.2 In a survey of mentally ill adults, 92% were found to have tooth decay—of which 23% were untreated and 40% smoked tobacco.3 Approximately 9% have periodontal disease, which most often occurs in those who smoke cigarettes.4
Lifestyle contributors
Drug abuse facilitates dental diseases, as evidenced by the high rate of caries among methamphetamine users.5 The drug induces xerostomia, encouraging users to drink sweetened beverages; this, combined with limited oral care, results in profound dental decay (“meth mouth”). Oral cocaine users often exhibit dental erosions or abrasions, gingival lacerations or necrosis, and mucosal lesions. Smoking Cannabis is associated with an increased rate of gingivitis, alveolar bone loss, leukoplakia, and oral papilloma or other cancers.5 Heroin users are at increased risk of tooth decay, periodontal disease, and oral infection.5
Alcohol consumption increases the risk of oral cancer. Long-term alcohol use suppresses bone marrow function, causing leukopenia and resulting in immunosuppression and an increased incidence of dental infections.6 Excessive alcohol consumption also can cause thrombocytopenia and bleeding, which can complicate dental procedures.
Smoking cigarettes increases the incidence of periodontal disease, especially necrotizing gingivitis and candidiasis.7 Ninety percent of patients with schizophrenia smoke—compared with up to 70% of patients with other psychiatric disorders, and 19% of the general population.7,8 Physiologic aspects of schizophrenia reinforce the smoking habit.7
Somatic ailments. Psychiatric disorders are strongly associated with diabetes, obesity, hypertension, stroke, heart disease, and arthritis, all of which contribute to oral pathology. Older age, greater dysfunction, longer duration of illness, and smoking are predictors of adverse dental outcomes.
Anxiety, depression, stress—all of these these disorders increase the circulating level of cortisol, thus raising the risk that periodontal disease will progress.9 Periodontitis increases the risk of stroke and heart attack by accelerating atherosclerotic plaque formation.10 Depression, anxiety, and substance abuse can lead to temporomandibular disorders that cause pain and restrict jaw movement.11 Stressed patients may experience muscle tension and bruxism, which can lead to temporomandibular joint discomfort.
Eating disorders. Patients who induce vomiting may exhibit enamel erosions (especially on the anterior maxillary teeth), increased tooth hypersensitivity, decay, and wear on dental restorative work.
Atypical odontalgia, characterized by chronic, burning pain in teeth and gums, is associated with depression and anxiety.11 Misdiagnosis can result in extractions or procedures without an appropriate indication and failure to alleviate the pain.
Medication side effects. Xerostomia can increase the risk for caries, periodontal disease, and oral infections such as candidiasis, glossitis, stomatitis, and parotitis.9 Extrapyramidal side effects (tardive dyskinesia, dystonia) may cause tooth damage and make managing dentures difficult.6
What to tell patients, and what you can do for them
Encourage your patients to reduce their sugar intake, brush and floss regularly, and work to stop smoking or ingesting substances of abuse. Teach appropriate hygiene and nutrition, which reduces the risk of dental caries, infection, and related problems. Recommend periodic oral health screening and how to secure such dental care.
From your position of familiarity with patients’ psychopharmacotherapy, make an effort to personalize and adjust their regimens when dental disease is present to address concerns about oral health that can be caused by medication side effects.
A multidisciplinary approach with patient advocacy, involving you and the patient’s dentist and primary care physician, facilitates health care and works to offer the patient access to global medical services.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Poor oral health is common among mentally ill people and is related to inadequate nutrition, poor self-care, substance abuse, and medication side effects.1 Poor oral hygiene is a significant problem because it results in dental pathology that has an adverse influence on the whole body.
Compared with the general population, mentally ill patients are 3 times more likely to have their teeth removed.2 In a survey of mentally ill adults, 92% were found to have tooth decay—of which 23% were untreated and 40% smoked tobacco.3 Approximately 9% have periodontal disease, which most often occurs in those who smoke cigarettes.4
Lifestyle contributors
Drug abuse facilitates dental diseases, as evidenced by the high rate of caries among methamphetamine users.5 The drug induces xerostomia, encouraging users to drink sweetened beverages; this, combined with limited oral care, results in profound dental decay (“meth mouth”). Oral cocaine users often exhibit dental erosions or abrasions, gingival lacerations or necrosis, and mucosal lesions. Smoking Cannabis is associated with an increased rate of gingivitis, alveolar bone loss, leukoplakia, and oral papilloma or other cancers.5 Heroin users are at increased risk of tooth decay, periodontal disease, and oral infection.5
Alcohol consumption increases the risk of oral cancer. Long-term alcohol use suppresses bone marrow function, causing leukopenia and resulting in immunosuppression and an increased incidence of dental infections.6 Excessive alcohol consumption also can cause thrombocytopenia and bleeding, which can complicate dental procedures.
Smoking cigarettes increases the incidence of periodontal disease, especially necrotizing gingivitis and candidiasis.7 Ninety percent of patients with schizophrenia smoke—compared with up to 70% of patients with other psychiatric disorders, and 19% of the general population.7,8 Physiologic aspects of schizophrenia reinforce the smoking habit.7
Somatic ailments. Psychiatric disorders are strongly associated with diabetes, obesity, hypertension, stroke, heart disease, and arthritis, all of which contribute to oral pathology. Older age, greater dysfunction, longer duration of illness, and smoking are predictors of adverse dental outcomes.
Anxiety, depression, stress—all of these these disorders increase the circulating level of cortisol, thus raising the risk that periodontal disease will progress.9 Periodontitis increases the risk of stroke and heart attack by accelerating atherosclerotic plaque formation.10 Depression, anxiety, and substance abuse can lead to temporomandibular disorders that cause pain and restrict jaw movement.11 Stressed patients may experience muscle tension and bruxism, which can lead to temporomandibular joint discomfort.
Eating disorders. Patients who induce vomiting may exhibit enamel erosions (especially on the anterior maxillary teeth), increased tooth hypersensitivity, decay, and wear on dental restorative work.
Atypical odontalgia, characterized by chronic, burning pain in teeth and gums, is associated with depression and anxiety.11 Misdiagnosis can result in extractions or procedures without an appropriate indication and failure to alleviate the pain.
Medication side effects. Xerostomia can increase the risk for caries, periodontal disease, and oral infections such as candidiasis, glossitis, stomatitis, and parotitis.9 Extrapyramidal side effects (tardive dyskinesia, dystonia) may cause tooth damage and make managing dentures difficult.6
What to tell patients, and what you can do for them
Encourage your patients to reduce their sugar intake, brush and floss regularly, and work to stop smoking or ingesting substances of abuse. Teach appropriate hygiene and nutrition, which reduces the risk of dental caries, infection, and related problems. Recommend periodic oral health screening and how to secure such dental care.
From your position of familiarity with patients’ psychopharmacotherapy, make an effort to personalize and adjust their regimens when dental disease is present to address concerns about oral health that can be caused by medication side effects.
A multidisciplinary approach with patient advocacy, involving you and the patient’s dentist and primary care physician, facilitates health care and works to offer the patient access to global medical services.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Mental Illness Fellowship of Australia Inc. Overview of the oral health of people affected by mental illness. http:// www.wfmh.com/links/external-contacts/mental-illness-fellowship-of-australia. Accessed June 18, 2014.
2. Kisely S, Quek LH, Pais J, et al. Advanced dental disease in people with severe mental illness: systematic review and meta-analysis. Br J Psychiatry. 2011;199(3):187-193.
3. Dental caries (tooth decay) in adults (age 20 to 64). National Institute of Dental and Craniofacial Research. http:// www.nidcr.nih.gov/DataStatistics/FindDataByTopic/ DentalCaries/DentalCariesAdults20to64.htm. Updated January 6, 2014. Accessed June 18, 2014.
4. Peridontal disease in adults (age 20 to 64). National Institute of Dental and Craniofacial Research. http://www.nidcr. nih.gov/DataStatistics/FindDataByTopic/GumDisease/ PeriodontaldiseaseAdults20to64.htm. Updated January 6, 2014. Accessed June 18, 2014.
5. Maloney WJ. The significance of illicit drug use to dental practice. http://www.webmedcentral.com/wmcpdf/ Article_WMC00455.pdf. Published July 28, 2010. Accessed June 18, 2014.
6. Oral health care for people with mental problems: guidelines and recommendations. British Society for Disability and Oral Health. http://www.bsdh.org.uk/guidelines/ mental.pdf. Updated January 2000. Accessed June 18, 2014.
7. Lohr JB, Flynn K. Smoking and schizophrenia. Schizophr Res. 1992;8(2):93-102.
8. Centers for Disease Control and Prevention (CDC). Vital signs: current cigarette smoking among adults aged ≥18 years–United States, 2005-2010. MMWR Morb Mortal Wkly Rep. 2011;60(35):1207-1212.
9. Yoffee L. The link between oral health and medical illness. http://www.everydayhealth.com/dental-health/oral-conditions/oral-health-and-other-diseases.aspx. Updated November 9, 2012. Accessed June 18, 2014.
10. Demmer RT, Desvarieux M. Periodontal infections and cardiovascular disease: the heart of the matter. J Am Dent Assoc. 2006;137(suppl 2):14S-20S; quiz 38S.
11. Mental illness and the dental patient. American Dental Hygienists’ Association. http://www.adha.org/ce-course-10. Accessed June 18, 2014.
1. Mental Illness Fellowship of Australia Inc. Overview of the oral health of people affected by mental illness. http:// www.wfmh.com/links/external-contacts/mental-illness-fellowship-of-australia. Accessed June 18, 2014.
2. Kisely S, Quek LH, Pais J, et al. Advanced dental disease in people with severe mental illness: systematic review and meta-analysis. Br J Psychiatry. 2011;199(3):187-193.
3. Dental caries (tooth decay) in adults (age 20 to 64). National Institute of Dental and Craniofacial Research. http:// www.nidcr.nih.gov/DataStatistics/FindDataByTopic/ DentalCaries/DentalCariesAdults20to64.htm. Updated January 6, 2014. Accessed June 18, 2014.
4. Peridontal disease in adults (age 20 to 64). National Institute of Dental and Craniofacial Research. http://www.nidcr. nih.gov/DataStatistics/FindDataByTopic/GumDisease/ PeriodontaldiseaseAdults20to64.htm. Updated January 6, 2014. Accessed June 18, 2014.
5. Maloney WJ. The significance of illicit drug use to dental practice. http://www.webmedcentral.com/wmcpdf/ Article_WMC00455.pdf. Published July 28, 2010. Accessed June 18, 2014.
6. Oral health care for people with mental problems: guidelines and recommendations. British Society for Disability and Oral Health. http://www.bsdh.org.uk/guidelines/ mental.pdf. Updated January 2000. Accessed June 18, 2014.
7. Lohr JB, Flynn K. Smoking and schizophrenia. Schizophr Res. 1992;8(2):93-102.
8. Centers for Disease Control and Prevention (CDC). Vital signs: current cigarette smoking among adults aged ≥18 years–United States, 2005-2010. MMWR Morb Mortal Wkly Rep. 2011;60(35):1207-1212.
9. Yoffee L. The link between oral health and medical illness. http://www.everydayhealth.com/dental-health/oral-conditions/oral-health-and-other-diseases.aspx. Updated November 9, 2012. Accessed June 18, 2014.
10. Demmer RT, Desvarieux M. Periodontal infections and cardiovascular disease: the heart of the matter. J Am Dent Assoc. 2006;137(suppl 2):14S-20S; quiz 38S.
11. Mental illness and the dental patient. American Dental Hygienists’ Association. http://www.adha.org/ce-course-10. Accessed June 18, 2014.
Sleep apnea linked to worsened neurocognitive function in Hispanic women
MINNEAPOLIS – Sleep apnea is associated with neurocognitive dysfunction in adult Hispanics living in the United States, research presented at the annual meeting of the Associated Professional Sleep Societies shows.
In unadjusted models, sleep apnea, as assessed by the apnea-hypopnea index (AHI), was inversely associated with neurocognitive dysfunction in both men and women.
"However, after adjusting for covariates, these interactions were attenuated," reported Dr. Alberto R. Ramos. "In a fully adjusted model accounting for age, BMI [body mass index], tobacco use, depression and anxiety scores, stroke, diabetes, hypertension, and field center tested, this association was seen only in women, but not in men."
Dr. Ramos of the department of clinical neurology at the University of Miami and his colleagues conducted a cross-sectional analysis of 9,714 Hispanic men and women between the ages of 45 and 74 who were participants in the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). The researchers sought to determine the extent to which sleep apnea contributes to neurocognitive dysfunction in a representative sample of Hispanic men and women residing in the United States.
Subjects were administered multiple neurocognitive tests, assessing learning, recall, word fluency, and digit symbol substitution. Obstructive sleep apnea was determined objectively using the apnea risk evaluation system and defined by the apnea-hypopnea index (AHI), and subjectively using standardized sleep symptom scales.
The mean AHI was 8.9, 11.5 for men and 6.8 for women (P less than 0.001 for difference between genders). With increasing age, the AHI significantly increased, too, from 7.4 in subjects aged 45 to 54, to 11.5 in those aged 65 to 74 (P less than 0.001).
Dementia prevalence is on the rise, particularly among Hispanics/Latinos who are up to 3.3 times more likely to meet diagnostic criteria for advanced dementia, compared with non-Hispanic whites, Dr. Ramos reported.
Dr. Ramos reported having no disclosures. The study received support from multiple National Heart, Lung, and Blood Institute grants.
MINNEAPOLIS – Sleep apnea is associated with neurocognitive dysfunction in adult Hispanics living in the United States, research presented at the annual meeting of the Associated Professional Sleep Societies shows.
In unadjusted models, sleep apnea, as assessed by the apnea-hypopnea index (AHI), was inversely associated with neurocognitive dysfunction in both men and women.
"However, after adjusting for covariates, these interactions were attenuated," reported Dr. Alberto R. Ramos. "In a fully adjusted model accounting for age, BMI [body mass index], tobacco use, depression and anxiety scores, stroke, diabetes, hypertension, and field center tested, this association was seen only in women, but not in men."
Dr. Ramos of the department of clinical neurology at the University of Miami and his colleagues conducted a cross-sectional analysis of 9,714 Hispanic men and women between the ages of 45 and 74 who were participants in the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). The researchers sought to determine the extent to which sleep apnea contributes to neurocognitive dysfunction in a representative sample of Hispanic men and women residing in the United States.
Subjects were administered multiple neurocognitive tests, assessing learning, recall, word fluency, and digit symbol substitution. Obstructive sleep apnea was determined objectively using the apnea risk evaluation system and defined by the apnea-hypopnea index (AHI), and subjectively using standardized sleep symptom scales.
The mean AHI was 8.9, 11.5 for men and 6.8 for women (P less than 0.001 for difference between genders). With increasing age, the AHI significantly increased, too, from 7.4 in subjects aged 45 to 54, to 11.5 in those aged 65 to 74 (P less than 0.001).
Dementia prevalence is on the rise, particularly among Hispanics/Latinos who are up to 3.3 times more likely to meet diagnostic criteria for advanced dementia, compared with non-Hispanic whites, Dr. Ramos reported.
Dr. Ramos reported having no disclosures. The study received support from multiple National Heart, Lung, and Blood Institute grants.
MINNEAPOLIS – Sleep apnea is associated with neurocognitive dysfunction in adult Hispanics living in the United States, research presented at the annual meeting of the Associated Professional Sleep Societies shows.
In unadjusted models, sleep apnea, as assessed by the apnea-hypopnea index (AHI), was inversely associated with neurocognitive dysfunction in both men and women.
"However, after adjusting for covariates, these interactions were attenuated," reported Dr. Alberto R. Ramos. "In a fully adjusted model accounting for age, BMI [body mass index], tobacco use, depression and anxiety scores, stroke, diabetes, hypertension, and field center tested, this association was seen only in women, but not in men."
Dr. Ramos of the department of clinical neurology at the University of Miami and his colleagues conducted a cross-sectional analysis of 9,714 Hispanic men and women between the ages of 45 and 74 who were participants in the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). The researchers sought to determine the extent to which sleep apnea contributes to neurocognitive dysfunction in a representative sample of Hispanic men and women residing in the United States.
Subjects were administered multiple neurocognitive tests, assessing learning, recall, word fluency, and digit symbol substitution. Obstructive sleep apnea was determined objectively using the apnea risk evaluation system and defined by the apnea-hypopnea index (AHI), and subjectively using standardized sleep symptom scales.
The mean AHI was 8.9, 11.5 for men and 6.8 for women (P less than 0.001 for difference between genders). With increasing age, the AHI significantly increased, too, from 7.4 in subjects aged 45 to 54, to 11.5 in those aged 65 to 74 (P less than 0.001).
Dementia prevalence is on the rise, particularly among Hispanics/Latinos who are up to 3.3 times more likely to meet diagnostic criteria for advanced dementia, compared with non-Hispanic whites, Dr. Ramos reported.
Dr. Ramos reported having no disclosures. The study received support from multiple National Heart, Lung, and Blood Institute grants.
AT SLEEP 2014
Key clinical point: Hispanic women patients diagnosed with sleep apnea should be watched closely for neurocognitive dysfunction.
Major finding: The apnea-hypopnea index was inversely associated with neurocognitive function in a large sample of Hispanics in the United States. The association was attenuated by age and education and after full adjustment was only seen in women, not men.
Data source: Cross-sectional analysis of 9,714 U.S. Hispanics aged 45-74.
Disclosures: Dr. Ramos reported having no disclosures. The study received support from multiple National Heart, Lung, and Blood Institute grants.
Impact of poor sleep on GPA equal to binge drinking for college students
MINNEAPOLIS – College students who do not get enough sleep experience an impact on their academic performance that is on par with binge drinking or regular marijuana use, two researchers say.
"The cultural assumption is that college is a time of bad sleep, and that all-nighters fueled by energy drinks and cheap pizza are just an inherent part of what it means to be a student," investigators J. Roxanne Prichard, Ph.D., and Monica Hartmann, Ph.D., reported at the annual meeting of the Associated Professional Sleep Societies.
But they say that assumption is shortsighted and wrong. "Well-rested students perform better academically and are healthier physically and psychologically," the researchers said.
Dr. Prichard of the department of neuroscience at the University of St. Thomas, St. Paul, Minn., and Dr. Hartmann,professor of economics at the university analyzed data from the Spring 2009 NCHA (the American College Health Association National College Health Assessment), which included survey information from 72, 966 students, 63% of whom were female and 75% of whom were white.
Students who participated in the survey were asked about their physical and mental health, sexual activity, and substance use, among other issues. They also were asked whether they’ve had sleep problems or had been diagnosed with a sleep disorder or insomnia.
Using data from more than 43,000 respondents, the researchers attempted to evaluate factors that predicted academic problems, including dropping a course, earning a lower course grade, and having a lower cumulative grade point average. The researchers examined those impacts for all students but focused on freshmen, because first-year performance "has such a large effect on retention rates and thus the economic stability for the institution of higher education," the researchers said. They found that sleep timing and sleep-related problems in college students were a strong predictor of academic problems, even after they controlled for other factors that might have had an impact, including clinical depression, feeling isolated, and a diagnosis of a learning disability or chronic health issue.
Students who earned "A" grades reported experiencing fewer of the following sleep issues: early awakenings, feeling sleepy during the day, going to bed early because they could not stay awake, or having trouble falling asleep. Students with worse grades tended to report more sleep issues.
Sleep problems had about the same impact on GPA as did binge drinking and marijuana use, the authors reported. In freshmen, poor sleep was an independent predictor of whether a student would drop or withdraw from a course. The authors adjusted their analysis to account for race, gender, work hours, chronic illness, and psychiatric problems such as anxiety.
Reducing sleep problems might have had a greater impact than reducing binge drinking or marijuana use, they said. For instance, improving sleep on just 1 night a week reduced the probability that a freshman drops a course by about 15%, the authors found.
Dr. Prichard and Dr. Hartmann also tried to gauge the effect that sleep disturbances in college eventually might have on the university’s ability to keep the student and the student’s future earnings potential. They determined that a sleep screening program that identified students at risk and led to treatment would be cost effective, even for the smallest universities. "Identifying and treating students with undiagnosed sleep problems early on in a student’s career economically benefits the university through increased retention and increases the students’ lifetime earning potential," they said.
But they noted that most institutions of higher learning do not pay much attention to students’ sleep habits. Rarely is there any time or money devoted to improving sleep, and if there is, it’s much less than the amount spent to address learning disabilities, substance abuse, and contagious illness, the researchers said. They encouraged a reexamination of the resources directed toward sleep quality in this population.
Dr. Prichard and Dr. Hartmann reported no conflicts. They received no outside funding for the study.
On Twitter @aliciaault
MINNEAPOLIS – College students who do not get enough sleep experience an impact on their academic performance that is on par with binge drinking or regular marijuana use, two researchers say.
"The cultural assumption is that college is a time of bad sleep, and that all-nighters fueled by energy drinks and cheap pizza are just an inherent part of what it means to be a student," investigators J. Roxanne Prichard, Ph.D., and Monica Hartmann, Ph.D., reported at the annual meeting of the Associated Professional Sleep Societies.
But they say that assumption is shortsighted and wrong. "Well-rested students perform better academically and are healthier physically and psychologically," the researchers said.
Dr. Prichard of the department of neuroscience at the University of St. Thomas, St. Paul, Minn., and Dr. Hartmann,professor of economics at the university analyzed data from the Spring 2009 NCHA (the American College Health Association National College Health Assessment), which included survey information from 72, 966 students, 63% of whom were female and 75% of whom were white.
Students who participated in the survey were asked about their physical and mental health, sexual activity, and substance use, among other issues. They also were asked whether they’ve had sleep problems or had been diagnosed with a sleep disorder or insomnia.
Using data from more than 43,000 respondents, the researchers attempted to evaluate factors that predicted academic problems, including dropping a course, earning a lower course grade, and having a lower cumulative grade point average. The researchers examined those impacts for all students but focused on freshmen, because first-year performance "has such a large effect on retention rates and thus the economic stability for the institution of higher education," the researchers said. They found that sleep timing and sleep-related problems in college students were a strong predictor of academic problems, even after they controlled for other factors that might have had an impact, including clinical depression, feeling isolated, and a diagnosis of a learning disability or chronic health issue.
Students who earned "A" grades reported experiencing fewer of the following sleep issues: early awakenings, feeling sleepy during the day, going to bed early because they could not stay awake, or having trouble falling asleep. Students with worse grades tended to report more sleep issues.
Sleep problems had about the same impact on GPA as did binge drinking and marijuana use, the authors reported. In freshmen, poor sleep was an independent predictor of whether a student would drop or withdraw from a course. The authors adjusted their analysis to account for race, gender, work hours, chronic illness, and psychiatric problems such as anxiety.
Reducing sleep problems might have had a greater impact than reducing binge drinking or marijuana use, they said. For instance, improving sleep on just 1 night a week reduced the probability that a freshman drops a course by about 15%, the authors found.
Dr. Prichard and Dr. Hartmann also tried to gauge the effect that sleep disturbances in college eventually might have on the university’s ability to keep the student and the student’s future earnings potential. They determined that a sleep screening program that identified students at risk and led to treatment would be cost effective, even for the smallest universities. "Identifying and treating students with undiagnosed sleep problems early on in a student’s career economically benefits the university through increased retention and increases the students’ lifetime earning potential," they said.
But they noted that most institutions of higher learning do not pay much attention to students’ sleep habits. Rarely is there any time or money devoted to improving sleep, and if there is, it’s much less than the amount spent to address learning disabilities, substance abuse, and contagious illness, the researchers said. They encouraged a reexamination of the resources directed toward sleep quality in this population.
Dr. Prichard and Dr. Hartmann reported no conflicts. They received no outside funding for the study.
On Twitter @aliciaault
MINNEAPOLIS – College students who do not get enough sleep experience an impact on their academic performance that is on par with binge drinking or regular marijuana use, two researchers say.
"The cultural assumption is that college is a time of bad sleep, and that all-nighters fueled by energy drinks and cheap pizza are just an inherent part of what it means to be a student," investigators J. Roxanne Prichard, Ph.D., and Monica Hartmann, Ph.D., reported at the annual meeting of the Associated Professional Sleep Societies.
But they say that assumption is shortsighted and wrong. "Well-rested students perform better academically and are healthier physically and psychologically," the researchers said.
Dr. Prichard of the department of neuroscience at the University of St. Thomas, St. Paul, Minn., and Dr. Hartmann,professor of economics at the university analyzed data from the Spring 2009 NCHA (the American College Health Association National College Health Assessment), which included survey information from 72, 966 students, 63% of whom were female and 75% of whom were white.
Students who participated in the survey were asked about their physical and mental health, sexual activity, and substance use, among other issues. They also were asked whether they’ve had sleep problems or had been diagnosed with a sleep disorder or insomnia.
Using data from more than 43,000 respondents, the researchers attempted to evaluate factors that predicted academic problems, including dropping a course, earning a lower course grade, and having a lower cumulative grade point average. The researchers examined those impacts for all students but focused on freshmen, because first-year performance "has such a large effect on retention rates and thus the economic stability for the institution of higher education," the researchers said. They found that sleep timing and sleep-related problems in college students were a strong predictor of academic problems, even after they controlled for other factors that might have had an impact, including clinical depression, feeling isolated, and a diagnosis of a learning disability or chronic health issue.
Students who earned "A" grades reported experiencing fewer of the following sleep issues: early awakenings, feeling sleepy during the day, going to bed early because they could not stay awake, or having trouble falling asleep. Students with worse grades tended to report more sleep issues.
Sleep problems had about the same impact on GPA as did binge drinking and marijuana use, the authors reported. In freshmen, poor sleep was an independent predictor of whether a student would drop or withdraw from a course. The authors adjusted their analysis to account for race, gender, work hours, chronic illness, and psychiatric problems such as anxiety.
Reducing sleep problems might have had a greater impact than reducing binge drinking or marijuana use, they said. For instance, improving sleep on just 1 night a week reduced the probability that a freshman drops a course by about 15%, the authors found.
Dr. Prichard and Dr. Hartmann also tried to gauge the effect that sleep disturbances in college eventually might have on the university’s ability to keep the student and the student’s future earnings potential. They determined that a sleep screening program that identified students at risk and led to treatment would be cost effective, even for the smallest universities. "Identifying and treating students with undiagnosed sleep problems early on in a student’s career economically benefits the university through increased retention and increases the students’ lifetime earning potential," they said.
But they noted that most institutions of higher learning do not pay much attention to students’ sleep habits. Rarely is there any time or money devoted to improving sleep, and if there is, it’s much less than the amount spent to address learning disabilities, substance abuse, and contagious illness, the researchers said. They encouraged a reexamination of the resources directed toward sleep quality in this population.
Dr. Prichard and Dr. Hartmann reported no conflicts. They received no outside funding for the study.
On Twitter @aliciaault
FROM SLEEP 2014
Key clinical point: Interventions aimed at improving sleep hygiene for college students are needed.
Major finding: Insomnia and other sleep disturbances are independent risk factors for poor academic performance, on par with binge drinking or marijuana use.
Data source: An analysis of 43,000 responses to the American College Health Association National College Health survey.
Disclosures: Dr. Prichard and Dr. Hartmann reported no conflicts. They received no outside funding for the study.
Insomnia increases risk of retirement because of poor health or disability
MINNEAPOLIS – Midlife insomnia increases the likelihood of retiring because of poor health or disability, a longitudinal cohort study among 1,590 Wisconsin state employees showed.
Previous research has established that insomniacs retire earlier than peers without this sleep disorder, according to lead researcher Lauren Hale, Ph.D., of the public health program, Stony Brook (N.Y.) University. But the reasons for retiring are unclear.
She and her colleagues analyzed data from the REST (Retirement and Sleep Trajectories Study) cohort, a mixed group of blue- and white-collar Wisconsin state employees who were followed from midlife onward and completed questionnaires probing their reasons for retiring.
Overall, 41% of the participants had insomnia at approximately 50 years of age based on their report of often or almost always experiencing at least one of four symptoms of the disorder, Dr. Hale reported at the annual meeting of the Associated Professional Sleep Societies.
As of 2013, two-thirds of the entire cohort had retired, most commonly citing reasons of wanting to do other things, being financially secure, and wanting more time to spend with family and friends.
But after the data were adjusted for covariates, insomnia was most strongly and significantly associated with retiring because of poor health or disability (P less than .001). And the more insomnia symptoms a participant reported, the higher his or her risk of retiring for this reason; those reporting three or four symptoms had approximately twice the risk of peers without insomnia.
Surprisingly, insomnia did not increase the risk of retirement because of being laid off (as might be expected if employees were frequently late to work because of sleep loss) or retirement because of needing to care for a family member (a stressor that might be expected to lead to insomnia), according to Dr. Hale.
"We confirmed our hypothesis that the leading reason that people who have insomnia symptoms in early life are retiring earlier is due to poor health or disability as they are getting older," she commented.
"Now, there is still work to be done; the temporal sequencing is not 100% clear," said Dr. Hale.
Although it appears that the insomnia is preceding poor health, which then triggers early retirement, it is also possible that the poor health comes first and gives rise to insomnia, ultimately leading to the decision to retire, she explained. And there is a third possibility. "There are of course many unmeasured health variables that we didn’t include in the models that might be preceding the insomnia at age 50 and then leading to early retirement. And of course there are a range of unmeasured factors – social, psychosocial, cultural – that could be leading to both concurrent insomnia and poor health, that lead to early retirement," she explained. "So we hope to probe into that in the future."
In an interview, session chair Dr. Nalaka S. Gooneratne of the department of medicine atthe Hospital of the University of Pennsylvania and the Presbyterian Medical Center of Philadelphia, said: "I think these findings have very important public health ramifications. It’s very important for the field of sleep to not just look at the effects of sleep on immediate disease outcomes, but also on public health factors such as retirement and quality of life for retirees, and maximizing functional status and independence for older adults.
"Some of this data is really quite intriguing, suggesting there are hitherto unappreciated links between sleep and the decisions people make later on in their life about how they want to manage their retirement and finances," he added. "I think it’s very important to fund and explore research on the links between aging and sleep, especially with the growing numbers of older adults in the population."
Dr. Hale disclosed no relevant conflicts of interest.
MINNEAPOLIS – Midlife insomnia increases the likelihood of retiring because of poor health or disability, a longitudinal cohort study among 1,590 Wisconsin state employees showed.
Previous research has established that insomniacs retire earlier than peers without this sleep disorder, according to lead researcher Lauren Hale, Ph.D., of the public health program, Stony Brook (N.Y.) University. But the reasons for retiring are unclear.
She and her colleagues analyzed data from the REST (Retirement and Sleep Trajectories Study) cohort, a mixed group of blue- and white-collar Wisconsin state employees who were followed from midlife onward and completed questionnaires probing their reasons for retiring.
Overall, 41% of the participants had insomnia at approximately 50 years of age based on their report of often or almost always experiencing at least one of four symptoms of the disorder, Dr. Hale reported at the annual meeting of the Associated Professional Sleep Societies.
As of 2013, two-thirds of the entire cohort had retired, most commonly citing reasons of wanting to do other things, being financially secure, and wanting more time to spend with family and friends.
But after the data were adjusted for covariates, insomnia was most strongly and significantly associated with retiring because of poor health or disability (P less than .001). And the more insomnia symptoms a participant reported, the higher his or her risk of retiring for this reason; those reporting three or four symptoms had approximately twice the risk of peers without insomnia.
Surprisingly, insomnia did not increase the risk of retirement because of being laid off (as might be expected if employees were frequently late to work because of sleep loss) or retirement because of needing to care for a family member (a stressor that might be expected to lead to insomnia), according to Dr. Hale.
"We confirmed our hypothesis that the leading reason that people who have insomnia symptoms in early life are retiring earlier is due to poor health or disability as they are getting older," she commented.
"Now, there is still work to be done; the temporal sequencing is not 100% clear," said Dr. Hale.
Although it appears that the insomnia is preceding poor health, which then triggers early retirement, it is also possible that the poor health comes first and gives rise to insomnia, ultimately leading to the decision to retire, she explained. And there is a third possibility. "There are of course many unmeasured health variables that we didn’t include in the models that might be preceding the insomnia at age 50 and then leading to early retirement. And of course there are a range of unmeasured factors – social, psychosocial, cultural – that could be leading to both concurrent insomnia and poor health, that lead to early retirement," she explained. "So we hope to probe into that in the future."
In an interview, session chair Dr. Nalaka S. Gooneratne of the department of medicine atthe Hospital of the University of Pennsylvania and the Presbyterian Medical Center of Philadelphia, said: "I think these findings have very important public health ramifications. It’s very important for the field of sleep to not just look at the effects of sleep on immediate disease outcomes, but also on public health factors such as retirement and quality of life for retirees, and maximizing functional status and independence for older adults.
"Some of this data is really quite intriguing, suggesting there are hitherto unappreciated links between sleep and the decisions people make later on in their life about how they want to manage their retirement and finances," he added. "I think it’s very important to fund and explore research on the links between aging and sleep, especially with the growing numbers of older adults in the population."
Dr. Hale disclosed no relevant conflicts of interest.
MINNEAPOLIS – Midlife insomnia increases the likelihood of retiring because of poor health or disability, a longitudinal cohort study among 1,590 Wisconsin state employees showed.
Previous research has established that insomniacs retire earlier than peers without this sleep disorder, according to lead researcher Lauren Hale, Ph.D., of the public health program, Stony Brook (N.Y.) University. But the reasons for retiring are unclear.
She and her colleagues analyzed data from the REST (Retirement and Sleep Trajectories Study) cohort, a mixed group of blue- and white-collar Wisconsin state employees who were followed from midlife onward and completed questionnaires probing their reasons for retiring.
Overall, 41% of the participants had insomnia at approximately 50 years of age based on their report of often or almost always experiencing at least one of four symptoms of the disorder, Dr. Hale reported at the annual meeting of the Associated Professional Sleep Societies.
As of 2013, two-thirds of the entire cohort had retired, most commonly citing reasons of wanting to do other things, being financially secure, and wanting more time to spend with family and friends.
But after the data were adjusted for covariates, insomnia was most strongly and significantly associated with retiring because of poor health or disability (P less than .001). And the more insomnia symptoms a participant reported, the higher his or her risk of retiring for this reason; those reporting three or four symptoms had approximately twice the risk of peers without insomnia.
Surprisingly, insomnia did not increase the risk of retirement because of being laid off (as might be expected if employees were frequently late to work because of sleep loss) or retirement because of needing to care for a family member (a stressor that might be expected to lead to insomnia), according to Dr. Hale.
"We confirmed our hypothesis that the leading reason that people who have insomnia symptoms in early life are retiring earlier is due to poor health or disability as they are getting older," she commented.
"Now, there is still work to be done; the temporal sequencing is not 100% clear," said Dr. Hale.
Although it appears that the insomnia is preceding poor health, which then triggers early retirement, it is also possible that the poor health comes first and gives rise to insomnia, ultimately leading to the decision to retire, she explained. And there is a third possibility. "There are of course many unmeasured health variables that we didn’t include in the models that might be preceding the insomnia at age 50 and then leading to early retirement. And of course there are a range of unmeasured factors – social, psychosocial, cultural – that could be leading to both concurrent insomnia and poor health, that lead to early retirement," she explained. "So we hope to probe into that in the future."
In an interview, session chair Dr. Nalaka S. Gooneratne of the department of medicine atthe Hospital of the University of Pennsylvania and the Presbyterian Medical Center of Philadelphia, said: "I think these findings have very important public health ramifications. It’s very important for the field of sleep to not just look at the effects of sleep on immediate disease outcomes, but also on public health factors such as retirement and quality of life for retirees, and maximizing functional status and independence for older adults.
"Some of this data is really quite intriguing, suggesting there are hitherto unappreciated links between sleep and the decisions people make later on in their life about how they want to manage their retirement and finances," he added. "I think it’s very important to fund and explore research on the links between aging and sleep, especially with the growing numbers of older adults in the population."
Dr. Hale disclosed no relevant conflicts of interest.
AT SLEEP 2014
Key clinical point: Addressing the insomnia problems of patients aged 50 and older could have important public health implications.
Major finding: People who had insomnia in midlife were more likely than unaffected peers to report that they had retired because of poor health or disability (P less than .001).
Data source: A longitudinal cohort study among 1,590 Wisconsin state employees.
Disclosures: Dr. Hale disclosed no relevant conflicts of interest.