User login
First recommendations for cancer screening in myositis issued
AT ACR 2022
PHILADELPHIA – The first consensus screening guidelines for patients with idiopathic inflammatory myopathy (IIM) provide recommendations on risk stratification for individuals, basic and enhanced screening protocols, and screening frequency.
The recommendations, issued by the International Myositis Assessment and Clinical Studies Group (IMACS), stratify cancer risk for individual patients into low, intermediate, or high categories based on the IIM disease subtype, autoantibody status, and clinical features, reported Alexander Oldroyd, PhD, MSc, MBChB of the University of Manchester, England.
“There’s a big unmet need for cancer screening. One in four adults with myositis has cancer, either 3 years before or after a diagnosis of myositis. It’s one of the leading causes of death in these patients, and they’re overwhelmingly diagnosed at a late stage, so we need standardized approaches to get early diagnosis,” he said in an interview at the annual meeting of the American College of Rheumatology.
Sharon Kolasinski, MD, of the University of Pennsylvania in Philadelphia, said in an interview that the guideline is a welcome development for rheumatologists. Dr. Kolasinski moderated the session where Dr. Oldroyd described the guideline, but she was not involved in its formulation.
“I think that we all have wondered for a very long time: What is the optimal cancer screening for myositis patients? We all worry that the onset of their diseases is associated with a coincident cancer, or that they will develop it soon,” she said.
Dr. Oldroyd emphasized that all patients with myositis have elevated risk for cancer compared with the general population and that the guideline categories of low, intermediate, and high are relative only to patients with IIM.
International consensus
The data on which the recommendations are based come from a systematic review and meta-analysis by Dr. Oldroyd and colleagues of 69 studies on cancer risk factors and 9 on IIM-specific cancer screening.
The authors of that paper found that the dermatomyositis subtype, older age, male sex, dysphagia, cutaneous ulceration and antitranscriptional intermediary factor-1 gamma (anti-TIF1-gamma) positivity were associated with significantly increased risk of cancer.
In contrast, polymyositis and clinically amyopathic dermatomyositis subtypes, Raynaud’s phenomenon, interstitial lung disease, very high serum creatine kinase or lactate dehydrogenase levels, and positivity for anti-Jo1 or anti-EJ antibodies were associated with significantly reduced risk of cancer.
The consensus recommendations were developed with anonymous contributions from 75 expert participants in 22 countries, with additional input from 3 patient partners.
Do this
The guideline lists 18 recommendations, of which 13 are strong and 5 are conditional.
An example of a strong recommendation is number 3, based on a moderate level of evidences:
“All adult IIM patients, irrespective of cancer risk, should continue to participate in country/region-specific age and sex appropriate cancer screening programs,” the guideline recommends.
Patients with verified inclusion body myositis or juvenile-onset IIM do not, however, require routine screening for myositis-associated cancer, the guideline says (recommendations 1 and 2).
There are also recommendations that all adults with new-onset IIM be tested for myositis-specific and myositis-associated autoantibodies to assist in stratifying patients by risk category.
The guideline divides screening recommendations into basic and enhanced. The basic screening should include a comprehensive history and physical exam, complete blood count, liver functions tests, erythrocyte sedimentation rates/plasma viscosity, serum protein electrophoresis, urinalysis, and chest x-ray.
Adults with IIM who are determined to be at low risk for IIM-related cancer should have basic cancer screening at the time of IIM diagnosis. Adults with intermediate risk should undergo both basic and enhanced screening at the time of IIM diagnosis, and those with high risk should undergo enhanced screening at the time of myositis diagnosis, with basic screening annually for 3 years, the recommendations say.
Consider doing this
Conditional recommendations (“clinicians should consider ...”) include the use of PET/CT for adults at high risk for cancer when an underlying cancer has not been detected at the time of IIM diagnosis. They also include a single screening test for anti-TIF1-gamma positive dermatomyositis patients whose disease onset was after age 40 and who have at least one additional risk factor.
Also conditionally recommended are upper and lower gastrointestinal endoscopy for patients at high risk when an underlying cancer is not found at the time of IIM diagnosis, nasoendoscopy in geographical regions with elevated risk for nasopharyngeal cancers, and screening for all IIM patients with red-flag symptoms or clinical features of cancer, including unexplained weight loss, family history of cancer, smoking, unexplained fever, or night sweats.
Guided steps
“I think clinicians have a lot of questions such as, ‘well, what should I do, when should I do it?’ These are important clinical questions, and we need guidance about this. We need to balance comprehensiveness with cost-effectiveness, and we need expert opinion about what steps we should take now and which should we take later,” Dr. Kolasinski said.
The guideline development process was supported by the University of Manchester, IMACS, National Institute for Health Research (United Kingdom), National Institutes of Health, National Health Service Northern Care Alliance, The Myositis Association, Myositis UK, University of Pittsburgh, Versus Arthritis, and the Center for Musculoskeletal Research. Dr. Oldroyd and Dr. Kolasinski reported having no relevant conflicts of interest.
AT ACR 2022
PHILADELPHIA – The first consensus screening guidelines for patients with idiopathic inflammatory myopathy (IIM) provide recommendations on risk stratification for individuals, basic and enhanced screening protocols, and screening frequency.
The recommendations, issued by the International Myositis Assessment and Clinical Studies Group (IMACS), stratify cancer risk for individual patients into low, intermediate, or high categories based on the IIM disease subtype, autoantibody status, and clinical features, reported Alexander Oldroyd, PhD, MSc, MBChB of the University of Manchester, England.
“There’s a big unmet need for cancer screening. One in four adults with myositis has cancer, either 3 years before or after a diagnosis of myositis. It’s one of the leading causes of death in these patients, and they’re overwhelmingly diagnosed at a late stage, so we need standardized approaches to get early diagnosis,” he said in an interview at the annual meeting of the American College of Rheumatology.
Sharon Kolasinski, MD, of the University of Pennsylvania in Philadelphia, said in an interview that the guideline is a welcome development for rheumatologists. Dr. Kolasinski moderated the session where Dr. Oldroyd described the guideline, but she was not involved in its formulation.
“I think that we all have wondered for a very long time: What is the optimal cancer screening for myositis patients? We all worry that the onset of their diseases is associated with a coincident cancer, or that they will develop it soon,” she said.
Dr. Oldroyd emphasized that all patients with myositis have elevated risk for cancer compared with the general population and that the guideline categories of low, intermediate, and high are relative only to patients with IIM.
International consensus
The data on which the recommendations are based come from a systematic review and meta-analysis by Dr. Oldroyd and colleagues of 69 studies on cancer risk factors and 9 on IIM-specific cancer screening.
The authors of that paper found that the dermatomyositis subtype, older age, male sex, dysphagia, cutaneous ulceration and antitranscriptional intermediary factor-1 gamma (anti-TIF1-gamma) positivity were associated with significantly increased risk of cancer.
In contrast, polymyositis and clinically amyopathic dermatomyositis subtypes, Raynaud’s phenomenon, interstitial lung disease, very high serum creatine kinase or lactate dehydrogenase levels, and positivity for anti-Jo1 or anti-EJ antibodies were associated with significantly reduced risk of cancer.
The consensus recommendations were developed with anonymous contributions from 75 expert participants in 22 countries, with additional input from 3 patient partners.
Do this
The guideline lists 18 recommendations, of which 13 are strong and 5 are conditional.
An example of a strong recommendation is number 3, based on a moderate level of evidences:
“All adult IIM patients, irrespective of cancer risk, should continue to participate in country/region-specific age and sex appropriate cancer screening programs,” the guideline recommends.
Patients with verified inclusion body myositis or juvenile-onset IIM do not, however, require routine screening for myositis-associated cancer, the guideline says (recommendations 1 and 2).
There are also recommendations that all adults with new-onset IIM be tested for myositis-specific and myositis-associated autoantibodies to assist in stratifying patients by risk category.
The guideline divides screening recommendations into basic and enhanced. The basic screening should include a comprehensive history and physical exam, complete blood count, liver functions tests, erythrocyte sedimentation rates/plasma viscosity, serum protein electrophoresis, urinalysis, and chest x-ray.
Adults with IIM who are determined to be at low risk for IIM-related cancer should have basic cancer screening at the time of IIM diagnosis. Adults with intermediate risk should undergo both basic and enhanced screening at the time of IIM diagnosis, and those with high risk should undergo enhanced screening at the time of myositis diagnosis, with basic screening annually for 3 years, the recommendations say.
Consider doing this
Conditional recommendations (“clinicians should consider ...”) include the use of PET/CT for adults at high risk for cancer when an underlying cancer has not been detected at the time of IIM diagnosis. They also include a single screening test for anti-TIF1-gamma positive dermatomyositis patients whose disease onset was after age 40 and who have at least one additional risk factor.
Also conditionally recommended are upper and lower gastrointestinal endoscopy for patients at high risk when an underlying cancer is not found at the time of IIM diagnosis, nasoendoscopy in geographical regions with elevated risk for nasopharyngeal cancers, and screening for all IIM patients with red-flag symptoms or clinical features of cancer, including unexplained weight loss, family history of cancer, smoking, unexplained fever, or night sweats.
Guided steps
“I think clinicians have a lot of questions such as, ‘well, what should I do, when should I do it?’ These are important clinical questions, and we need guidance about this. We need to balance comprehensiveness with cost-effectiveness, and we need expert opinion about what steps we should take now and which should we take later,” Dr. Kolasinski said.
The guideline development process was supported by the University of Manchester, IMACS, National Institute for Health Research (United Kingdom), National Institutes of Health, National Health Service Northern Care Alliance, The Myositis Association, Myositis UK, University of Pittsburgh, Versus Arthritis, and the Center for Musculoskeletal Research. Dr. Oldroyd and Dr. Kolasinski reported having no relevant conflicts of interest.
AT ACR 2022
PHILADELPHIA – The first consensus screening guidelines for patients with idiopathic inflammatory myopathy (IIM) provide recommendations on risk stratification for individuals, basic and enhanced screening protocols, and screening frequency.
The recommendations, issued by the International Myositis Assessment and Clinical Studies Group (IMACS), stratify cancer risk for individual patients into low, intermediate, or high categories based on the IIM disease subtype, autoantibody status, and clinical features, reported Alexander Oldroyd, PhD, MSc, MBChB of the University of Manchester, England.
“There’s a big unmet need for cancer screening. One in four adults with myositis has cancer, either 3 years before or after a diagnosis of myositis. It’s one of the leading causes of death in these patients, and they’re overwhelmingly diagnosed at a late stage, so we need standardized approaches to get early diagnosis,” he said in an interview at the annual meeting of the American College of Rheumatology.
Sharon Kolasinski, MD, of the University of Pennsylvania in Philadelphia, said in an interview that the guideline is a welcome development for rheumatologists. Dr. Kolasinski moderated the session where Dr. Oldroyd described the guideline, but she was not involved in its formulation.
“I think that we all have wondered for a very long time: What is the optimal cancer screening for myositis patients? We all worry that the onset of their diseases is associated with a coincident cancer, or that they will develop it soon,” she said.
Dr. Oldroyd emphasized that all patients with myositis have elevated risk for cancer compared with the general population and that the guideline categories of low, intermediate, and high are relative only to patients with IIM.
International consensus
The data on which the recommendations are based come from a systematic review and meta-analysis by Dr. Oldroyd and colleagues of 69 studies on cancer risk factors and 9 on IIM-specific cancer screening.
The authors of that paper found that the dermatomyositis subtype, older age, male sex, dysphagia, cutaneous ulceration and antitranscriptional intermediary factor-1 gamma (anti-TIF1-gamma) positivity were associated with significantly increased risk of cancer.
In contrast, polymyositis and clinically amyopathic dermatomyositis subtypes, Raynaud’s phenomenon, interstitial lung disease, very high serum creatine kinase or lactate dehydrogenase levels, and positivity for anti-Jo1 or anti-EJ antibodies were associated with significantly reduced risk of cancer.
The consensus recommendations were developed with anonymous contributions from 75 expert participants in 22 countries, with additional input from 3 patient partners.
Do this
The guideline lists 18 recommendations, of which 13 are strong and 5 are conditional.
An example of a strong recommendation is number 3, based on a moderate level of evidences:
“All adult IIM patients, irrespective of cancer risk, should continue to participate in country/region-specific age and sex appropriate cancer screening programs,” the guideline recommends.
Patients with verified inclusion body myositis or juvenile-onset IIM do not, however, require routine screening for myositis-associated cancer, the guideline says (recommendations 1 and 2).
There are also recommendations that all adults with new-onset IIM be tested for myositis-specific and myositis-associated autoantibodies to assist in stratifying patients by risk category.
The guideline divides screening recommendations into basic and enhanced. The basic screening should include a comprehensive history and physical exam, complete blood count, liver functions tests, erythrocyte sedimentation rates/plasma viscosity, serum protein electrophoresis, urinalysis, and chest x-ray.
Adults with IIM who are determined to be at low risk for IIM-related cancer should have basic cancer screening at the time of IIM diagnosis. Adults with intermediate risk should undergo both basic and enhanced screening at the time of IIM diagnosis, and those with high risk should undergo enhanced screening at the time of myositis diagnosis, with basic screening annually for 3 years, the recommendations say.
Consider doing this
Conditional recommendations (“clinicians should consider ...”) include the use of PET/CT for adults at high risk for cancer when an underlying cancer has not been detected at the time of IIM diagnosis. They also include a single screening test for anti-TIF1-gamma positive dermatomyositis patients whose disease onset was after age 40 and who have at least one additional risk factor.
Also conditionally recommended are upper and lower gastrointestinal endoscopy for patients at high risk when an underlying cancer is not found at the time of IIM diagnosis, nasoendoscopy in geographical regions with elevated risk for nasopharyngeal cancers, and screening for all IIM patients with red-flag symptoms or clinical features of cancer, including unexplained weight loss, family history of cancer, smoking, unexplained fever, or night sweats.
Guided steps
“I think clinicians have a lot of questions such as, ‘well, what should I do, when should I do it?’ These are important clinical questions, and we need guidance about this. We need to balance comprehensiveness with cost-effectiveness, and we need expert opinion about what steps we should take now and which should we take later,” Dr. Kolasinski said.
The guideline development process was supported by the University of Manchester, IMACS, National Institute for Health Research (United Kingdom), National Institutes of Health, National Health Service Northern Care Alliance, The Myositis Association, Myositis UK, University of Pittsburgh, Versus Arthritis, and the Center for Musculoskeletal Research. Dr. Oldroyd and Dr. Kolasinski reported having no relevant conflicts of interest.
Treating deadly disease in utero called ‘revolutionary’ advance
The successful treatment of Pompe disease in utero for the first time may be the start of a new chapter for fetal therapy, researchers said.
A report published online in the New England Journal of Medicine describes in utero enzyme-replacement therapy (ERT) for infantile-onset Pompe disease.
The patient, now a toddler, is thriving, according to the researchers. Her parents previously had children with the same disorder who died.
“This treatment expands the repertoire of fetal therapies in a new direction,” Tippi MacKenzie, MD, a pediatric surgeon with University of California, San Francisco, Benioff Children’s Hospitals and a coauthor of the report, said in a news release. “As new treatments become available for children with genetic conditions, we are developing protocols to apply them before birth.”
Dr. MacKenzie codirects the University of California, San Francisco’s center for maternal-fetal precision medicine and directs the Eli and Edythe Broad Center of Regeneration Medicine and Stem Cell Research.
Pompe disease is caused by mutations in a gene that makes acid alpha-glucosidase. With limited amounts of this enzyme, dangerous amounts of glycogen accumulate in the body. Babies with infantile-onset disease typically have enlarged hearts and die by age 2 years.
The condition, which occurs in an estimated 1 in 40,000 births, is one of several early-onset lysosomal storage disorders. Patients with these diseases “are ideal candidates for prenatal therapy because organ damage starts in utero,” the researchers said.
Newborn screening can lead to early initiation of treatment with recombinant enzymes, “but this strategy does not completely prevent irreversible organ damage,” the authors said.
The patient in the new report received six prenatal ERT treatments at the Ottawa Hospital and is receiving postnatal enzyme therapy at CHEO, a pediatric hospital and research center in Ottawa.
Investigators administered alglucosidase alfa through the umbilical vein. They delivered the first infusion to the fetus at 24 weeks 5 days of gestation. They continued providing infusions at 2-week intervals through 34 weeks 5 days of gestation.
She is doing well at age 16 months, with normal cardiac and motor function, and is meeting developmental milestones, according to the news release.
The successful treatment involved collaboration among the University of California, San Francisco, where researchers are conducting a clinical trial of this treatment approach; CHEO and the Ottawa Hospital; and Duke University, Durham, N.C.
Under normal circumstances, the patient’s family would have traveled to Benioff Children’s Hospitals fetal treatment center to participate in the clinical trial, but COVID-19 restrictions led the researchers to deliver the therapy to Ottawa as part of the trial.
The University of California, San Francisco, has received U.S. Food and Drug Administration approval to treat Pompe disease and several other lysosomal storage disorders in utero as part of a phase 1 clinical trial with 10 patients. The other diseases are mucopolysaccharidosis types 1, 2, 4a, 6, and 7; Gaucher disease types 2 and 3; and Wolman disease.
Patients with Pompe disease might typically be diagnosed clinically at age 3-6 months, said study coauthor Paul Harmatz, MD, with the University of California, San Francisco. With newborn screening, the disease might be diagnosed at 1 week. But intervening before birth may be optimal, Dr. Harmatz said.
Fetal treatment appears to be “revolutionary at this point,” Dr. Harmatz said.
The research was supported by a grant from the National Institutes of Health. Sanofi Genzyme provided the enzyme for the patient.
A version of this article first appeared on Medscape.com.
The successful treatment of Pompe disease in utero for the first time may be the start of a new chapter for fetal therapy, researchers said.
A report published online in the New England Journal of Medicine describes in utero enzyme-replacement therapy (ERT) for infantile-onset Pompe disease.
The patient, now a toddler, is thriving, according to the researchers. Her parents previously had children with the same disorder who died.
“This treatment expands the repertoire of fetal therapies in a new direction,” Tippi MacKenzie, MD, a pediatric surgeon with University of California, San Francisco, Benioff Children’s Hospitals and a coauthor of the report, said in a news release. “As new treatments become available for children with genetic conditions, we are developing protocols to apply them before birth.”
Dr. MacKenzie codirects the University of California, San Francisco’s center for maternal-fetal precision medicine and directs the Eli and Edythe Broad Center of Regeneration Medicine and Stem Cell Research.
Pompe disease is caused by mutations in a gene that makes acid alpha-glucosidase. With limited amounts of this enzyme, dangerous amounts of glycogen accumulate in the body. Babies with infantile-onset disease typically have enlarged hearts and die by age 2 years.
The condition, which occurs in an estimated 1 in 40,000 births, is one of several early-onset lysosomal storage disorders. Patients with these diseases “are ideal candidates for prenatal therapy because organ damage starts in utero,” the researchers said.
Newborn screening can lead to early initiation of treatment with recombinant enzymes, “but this strategy does not completely prevent irreversible organ damage,” the authors said.
The patient in the new report received six prenatal ERT treatments at the Ottawa Hospital and is receiving postnatal enzyme therapy at CHEO, a pediatric hospital and research center in Ottawa.
Investigators administered alglucosidase alfa through the umbilical vein. They delivered the first infusion to the fetus at 24 weeks 5 days of gestation. They continued providing infusions at 2-week intervals through 34 weeks 5 days of gestation.
She is doing well at age 16 months, with normal cardiac and motor function, and is meeting developmental milestones, according to the news release.
The successful treatment involved collaboration among the University of California, San Francisco, where researchers are conducting a clinical trial of this treatment approach; CHEO and the Ottawa Hospital; and Duke University, Durham, N.C.
Under normal circumstances, the patient’s family would have traveled to Benioff Children’s Hospitals fetal treatment center to participate in the clinical trial, but COVID-19 restrictions led the researchers to deliver the therapy to Ottawa as part of the trial.
The University of California, San Francisco, has received U.S. Food and Drug Administration approval to treat Pompe disease and several other lysosomal storage disorders in utero as part of a phase 1 clinical trial with 10 patients. The other diseases are mucopolysaccharidosis types 1, 2, 4a, 6, and 7; Gaucher disease types 2 and 3; and Wolman disease.
Patients with Pompe disease might typically be diagnosed clinically at age 3-6 months, said study coauthor Paul Harmatz, MD, with the University of California, San Francisco. With newborn screening, the disease might be diagnosed at 1 week. But intervening before birth may be optimal, Dr. Harmatz said.
Fetal treatment appears to be “revolutionary at this point,” Dr. Harmatz said.
The research was supported by a grant from the National Institutes of Health. Sanofi Genzyme provided the enzyme for the patient.
A version of this article first appeared on Medscape.com.
The successful treatment of Pompe disease in utero for the first time may be the start of a new chapter for fetal therapy, researchers said.
A report published online in the New England Journal of Medicine describes in utero enzyme-replacement therapy (ERT) for infantile-onset Pompe disease.
The patient, now a toddler, is thriving, according to the researchers. Her parents previously had children with the same disorder who died.
“This treatment expands the repertoire of fetal therapies in a new direction,” Tippi MacKenzie, MD, a pediatric surgeon with University of California, San Francisco, Benioff Children’s Hospitals and a coauthor of the report, said in a news release. “As new treatments become available for children with genetic conditions, we are developing protocols to apply them before birth.”
Dr. MacKenzie codirects the University of California, San Francisco’s center for maternal-fetal precision medicine and directs the Eli and Edythe Broad Center of Regeneration Medicine and Stem Cell Research.
Pompe disease is caused by mutations in a gene that makes acid alpha-glucosidase. With limited amounts of this enzyme, dangerous amounts of glycogen accumulate in the body. Babies with infantile-onset disease typically have enlarged hearts and die by age 2 years.
The condition, which occurs in an estimated 1 in 40,000 births, is one of several early-onset lysosomal storage disorders. Patients with these diseases “are ideal candidates for prenatal therapy because organ damage starts in utero,” the researchers said.
Newborn screening can lead to early initiation of treatment with recombinant enzymes, “but this strategy does not completely prevent irreversible organ damage,” the authors said.
The patient in the new report received six prenatal ERT treatments at the Ottawa Hospital and is receiving postnatal enzyme therapy at CHEO, a pediatric hospital and research center in Ottawa.
Investigators administered alglucosidase alfa through the umbilical vein. They delivered the first infusion to the fetus at 24 weeks 5 days of gestation. They continued providing infusions at 2-week intervals through 34 weeks 5 days of gestation.
She is doing well at age 16 months, with normal cardiac and motor function, and is meeting developmental milestones, according to the news release.
The successful treatment involved collaboration among the University of California, San Francisco, where researchers are conducting a clinical trial of this treatment approach; CHEO and the Ottawa Hospital; and Duke University, Durham, N.C.
Under normal circumstances, the patient’s family would have traveled to Benioff Children’s Hospitals fetal treatment center to participate in the clinical trial, but COVID-19 restrictions led the researchers to deliver the therapy to Ottawa as part of the trial.
The University of California, San Francisco, has received U.S. Food and Drug Administration approval to treat Pompe disease and several other lysosomal storage disorders in utero as part of a phase 1 clinical trial with 10 patients. The other diseases are mucopolysaccharidosis types 1, 2, 4a, 6, and 7; Gaucher disease types 2 and 3; and Wolman disease.
Patients with Pompe disease might typically be diagnosed clinically at age 3-6 months, said study coauthor Paul Harmatz, MD, with the University of California, San Francisco. With newborn screening, the disease might be diagnosed at 1 week. But intervening before birth may be optimal, Dr. Harmatz said.
Fetal treatment appears to be “revolutionary at this point,” Dr. Harmatz said.
The research was supported by a grant from the National Institutes of Health. Sanofi Genzyme provided the enzyme for the patient.
A version of this article first appeared on Medscape.com.
Acquired Acrodermatitis Enteropathica in an Infant
Acrodermatitis enteropathica (AE) is a rare disorder of zinc metabolism that typically presents in infancy.1 Although it is clinically characterized by acral and periorificial dermatitis, alopecia, and diarrhea, only 20% of cases present with this triad.2 Zinc deficiency in AE can either be acquired or inborn (congenital). Acquired forms can occur from dietary inadequacy or malabsorption, whereas genetic causes are related to an autosomal-recessive disorder affecting zinc transporters.1 We report a case of a 3-month-old female infant with acquired AE who was successfully treated with zinc supplementation over the course of 3 weeks.
Case Report
A 3-month-old female infant presented to the emergency department with a rash of 2 weeks’ duration. She was born full term with no birth complications. The patient’s mother reported that the rash started on the cheeks, then enlarged and spread to the neck, back, and perineum. The patient also had been having diarrhea during this time. She previously had received mupirocin and cephalexin with no response to treatment. Maternal history was negative for lupus, and the mother’s diet consisted of a variety of foods but not many vegetables. The patient was exclusively breastfed, and there was no pertinent history of similar rashes occurring in other family members.
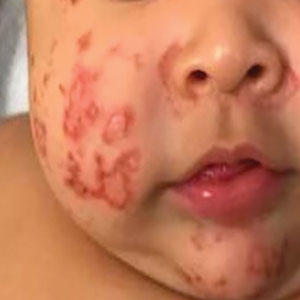
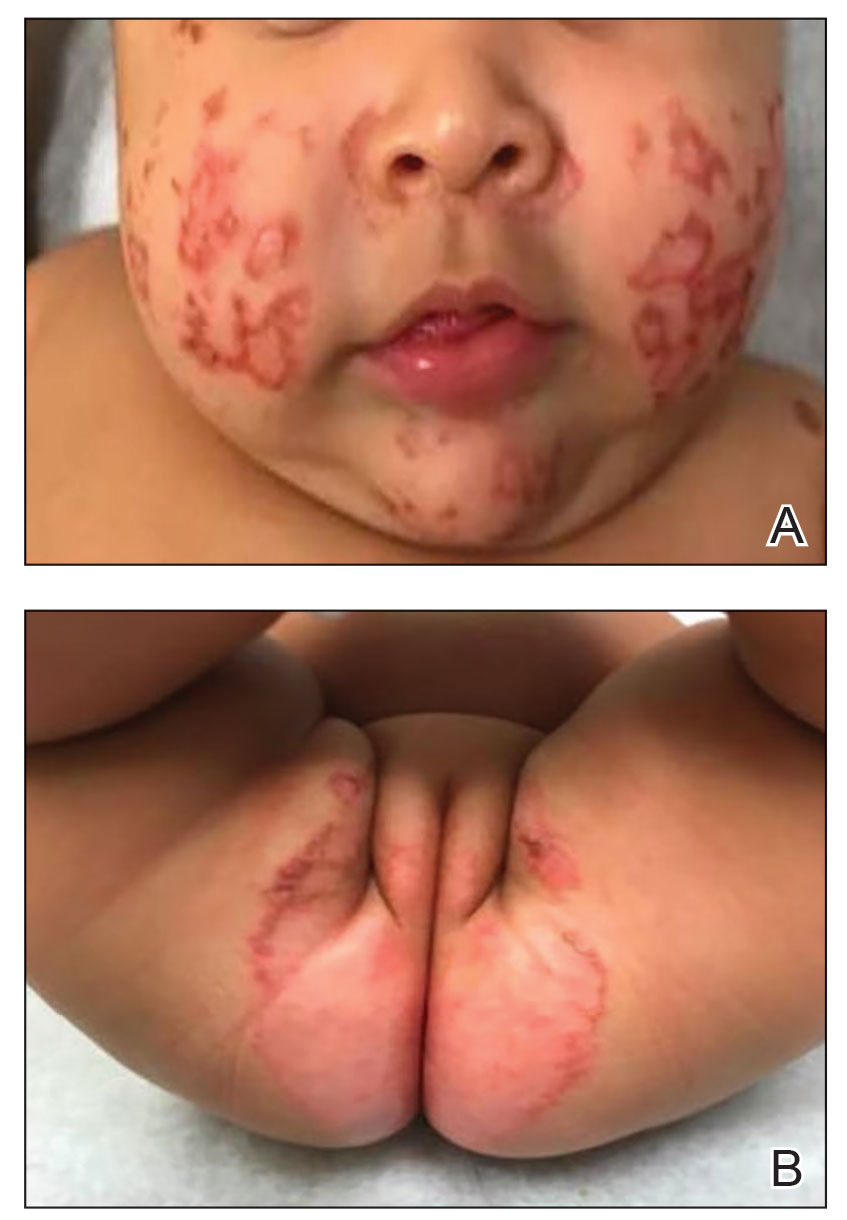
Physical examination revealed the patient had annular and polycyclic, hyperkeratotic, crusted papules and plaques on the cheeks, neck, back, and axillae, as well as the perineum/groin and perianal regions (Figure 1). The differential diagnosis at the time included neonatal lupus, zinc deficiency, and syphilis. Relevant laboratory testing and a shave biopsy of the left axilla were obtained.
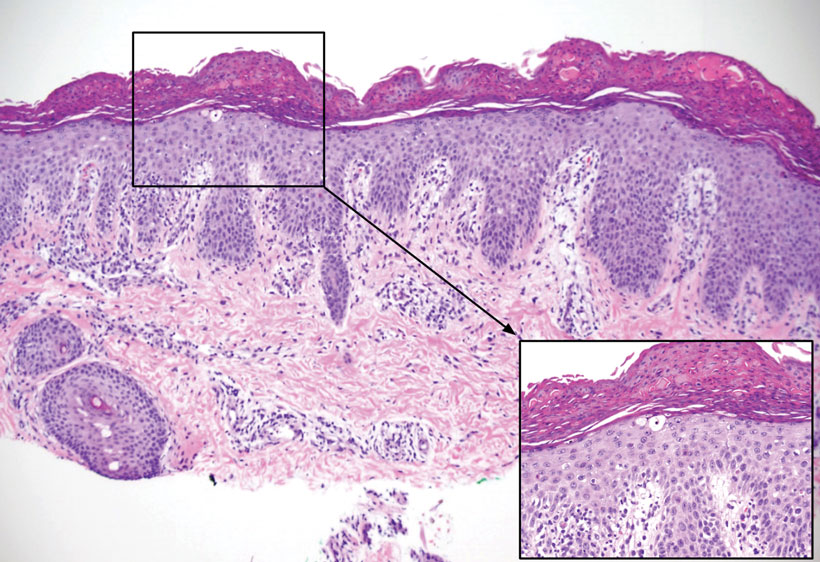
Pertinent laboratory findings included a low zinc level (23 μg/dL [reference range, 26–141 μg/dL]), low alkaline phosphatase level (74 U/L [reference range, 94–486 U/L]), and thrombocytosis (826×109/L [reference range, 150–400×109/L). Results for antinuclear antibody and anti–Sjögren syndrome–related antigen A and B antibody testing were negative. A rapid plasma reagin test was nonreactive. Histologic examination revealed psoriasiform hyperplasia with overlying confluent parakeratosis, focal spongiosis, multiple dyskeratotic keratinocytes, and mitotic figures (Figure 2). Ballooning was evident in focal cells in the subcorneal region in addition to an accompanying lymphocytic infiltrate and occasional neutrophils.
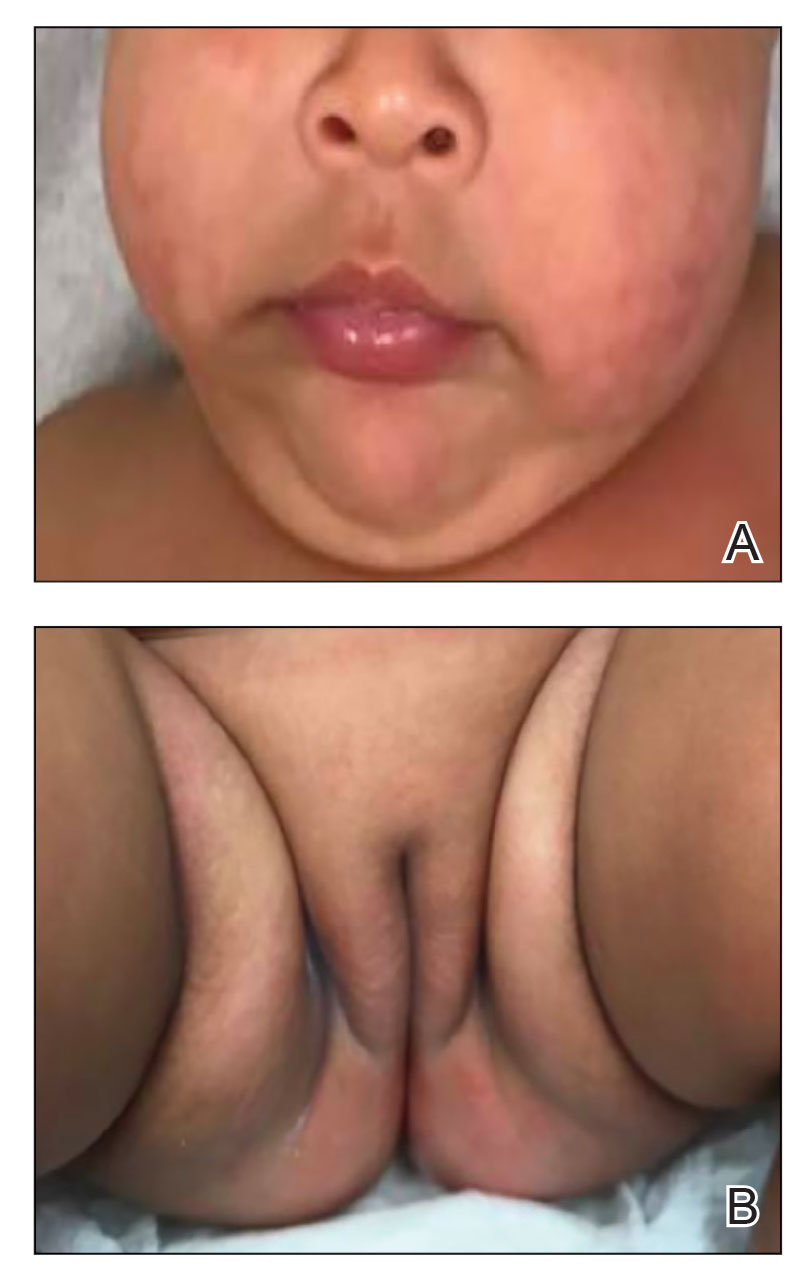
The patient was given a 10-mg/mL suspension of elemental zinc and was advised to take 1 mL (10 mg) by mouth twice daily with food. This dosage equated to 3 mg/kg/d. On follow-up 3 weeks later, the skin began to clear (Figure 3). Follow-up laboratory testing showed an increase in zinc (114 μg/dL) and alkaline phosphatase levels (313 U/L). The patient was able to discontinue the zinc supplementation, and follow-up during the next year revealed no recurrence.
Comment
Etiology of AE—Acrodermatitis enteropathica was first identified in 1942 as an acral rash associated with diarrhea3; in 1973, Barnes and Moynahan4 discovered zinc deficiency as a causal agent for these findings. The causes of AE are further subclassified as either an acquired or inborn etiology. Congenital causes commonly are seen in infants within the first few months of life, whereas acquired forms are seen at any age. Acquired forms in infants can occur from failure of the mother to secrete zinc in breast milk, low maternal serum zinc levels, or other reasons causing low nutritional intake. A single mutation in the SLC30A2 gene has been found to markedly reduce zinc concentrations in breast milk, thus causing zinc deficiency in breastfed infants.5 Other acquired forms can be caused by malabsorption, sometimes after surgery such as intestinal bypass or from intravenous nutrition without sufficient zinc.1 The congenital form of AE is an autosomal-recessive disorder occurring from mutations in the SLC39A4 gene located on band 8q24.3. Affected individuals have a decreased ability to absorb zinc in the small intestine because of defects in zinc transporters ZIP and ZnT.6 Based on our patient’s laboratory findings and history, it is believed that the zinc deficiency was acquired, as the condition normalized with repletion and has not required any supplementation in the year of follow-up. In addition, the absence of a pertinent family history supported an acquired diagnosis, which has various etiologies, whereas the congenital form primarily is a genetic disease.
Management—Treatment of AE includes supplementation with oral elemental zinc; however, there are scant evidence-based recommendations on the exact dose of zinc to be given. Generally, the recommended amount is 3 mg/kg/d.8 For individuals with the congenital form of AE, lifelong zinc supplementation is additionally recommended.9 It is important to recognize this presentation because the patient can develop worsening irritability, severe diarrhea, nail dystrophy, hair loss, immune dysfunction, and numerous ophthalmic disorders if left untreated. Acute zinc toxicity due to excess administration is rare, with symptoms of nausea and vomiting occurring with dosages of 50 to 100 mg/d. Additionally, dosages of up to 70 mg twice weekly have been provided without any toxic effect.10 In our case, 3 mg/kg/d of oral zinc supplementation proved to be effective in resolving the patient’s symptoms of acquired zinc deficiency.
Differential Diagnosis—It is important to note that deficiencies of other nutrients may present as an AE-like eruption called acrodermatitis dysmetabolica (AD). Both diseases may present with the triad of dermatitis, alopecia, and diarrhea; however, AD is associated with inborn errors of metabolism. There have been cases that describe AD in patients with a zinc deficiency in conjunction with a deficiency of branched-chain amino acids.11,12 It is important to consider AD in the differential diagnosis of an AE eruption, especially in the context of a metabolic disorder, as it may affect the treatment plan. One case described the dermatitis of AD as not responding to zinc supplementation alone, while another described improvement after increasing an isoleucine supplementation dose.11,12
Other considerations in the differential diagnoses include AE-like conditions such as biotinidase deficiency, multiple carboxylase deficiency, and essential fatty acid deficiency. An AE-like condition may present with the triad of dermatitis, alopecia, and diarrhea. However, unlike in true AE, zinc and alkaline phosphatase levels tend to be normal in these conditions. Other features seen in AE-like conditions depend on the underlying cause but often include failure to thrive, neurologic defects, ophthalmic abnormalities, and metabolic abnormalities.13
- Acrodermatitis enteropathica. National Organization for Rare Disorders. Accessed October 16, 2022. https://rarediseases.org/rare-diseases/acrodermatitis-enteropathica/
- Perafán-Riveros C, França LFS, Alves ACF, et al. Acrodermatitis enteropathica: case report and review of the literature. Pediatr Dermatol. 2002;19:426-431.
- Danbolt N. Acrodermatitis enteropathica. Br J Dermatol. 1979;100:37-40.
- Barnes PM, Moynahan EJ. Zinc deficiency in acrodermatitis enteropathica: multiple dietary intolerance treated with synthetic diet. Proc R Soc Med. 1973;66:327-329.
- Lee S, Zhou Y, Gill DL, et al. A genetic variant in SLC30A2 causes breast dysfunction during lactation by inducing ER stress, oxidative stress and epithelial barrier defects. Sci Rep. 2018;8:3542.
- Kaur S, Sangwan A, Sahu P, et al. Clinical variants of acrodermatitis enteropathica and its co-relation with genetics. Indian J Paediatr Dermatol. 2016;17:35-37.
- Dela Rosa KM, James WD. Acrodermatitis enteropathica workup. Medscape. Updated June 4, 2021. Accessed October 16, 2022. https://emedicine.medscape.com/article/1102575-workup#showall
- Ngan V, Gangakhedkar A, Oakley A. Acrodermatitis enteropathica. DermNet. Accessed October 16, 2022. https://dermnetnz.org/topics/acrodermatitis-enteropathica/
- Ranugha P, Sethi P, Veeranna S. Acrodermatitis enteropathica: the need for sustained high dose zinc supplementation. Dermatol Online J. 2018;24:13030/qt1w9002sr.
- Larson CP, Roy SK, Khan AI, et al. Zinc treatment to under-five children: applications to improve child survival and reduce burden of disease. J Health Popul Nutr. 2008;26:356-365.
- Samady JA, Schwartz RA, Shih LY, et al. Acrodermatitis enteropathica-like eruption in an infant with nonketotic hyperglycinemia. J Dermatol. 2000;27:604-608.
- Flores K, Chikowski R, Morrell DS. Acrodermatitis dysmetabolica in an infant with maple syrup urine disease. Clin Exp Dermatol. 2016;41:651-654.
- Jones L, Oakley A. Acrodermatitis enteropathica-like conditions. DermNet. Accessed August 30, 2022. https://dermnetnz.org/topics/acrodermatitis-enteropathica-like-conditions
Acrodermatitis enteropathica (AE) is a rare disorder of zinc metabolism that typically presents in infancy.1 Although it is clinically characterized by acral and periorificial dermatitis, alopecia, and diarrhea, only 20% of cases present with this triad.2 Zinc deficiency in AE can either be acquired or inborn (congenital). Acquired forms can occur from dietary inadequacy or malabsorption, whereas genetic causes are related to an autosomal-recessive disorder affecting zinc transporters.1 We report a case of a 3-month-old female infant with acquired AE who was successfully treated with zinc supplementation over the course of 3 weeks.
Case Report
A 3-month-old female infant presented to the emergency department with a rash of 2 weeks’ duration. She was born full term with no birth complications. The patient’s mother reported that the rash started on the cheeks, then enlarged and spread to the neck, back, and perineum. The patient also had been having diarrhea during this time. She previously had received mupirocin and cephalexin with no response to treatment. Maternal history was negative for lupus, and the mother’s diet consisted of a variety of foods but not many vegetables. The patient was exclusively breastfed, and there was no pertinent history of similar rashes occurring in other family members.
Physical examination revealed the patient had annular and polycyclic, hyperkeratotic, crusted papules and plaques on the cheeks, neck, back, and axillae, as well as the perineum/groin and perianal regions (Figure 1). The differential diagnosis at the time included neonatal lupus, zinc deficiency, and syphilis. Relevant laboratory testing and a shave biopsy of the left axilla were obtained.

Pertinent laboratory findings included a low zinc level (23 μg/dL [reference range, 26–141 μg/dL]), low alkaline phosphatase level (74 U/L [reference range, 94–486 U/L]), and thrombocytosis (826×109/L [reference range, 150–400×109/L). Results for antinuclear antibody and anti–Sjögren syndrome–related antigen A and B antibody testing were negative. A rapid plasma reagin test was nonreactive. Histologic examination revealed psoriasiform hyperplasia with overlying confluent parakeratosis, focal spongiosis, multiple dyskeratotic keratinocytes, and mitotic figures (Figure 2). Ballooning was evident in focal cells in the subcorneal region in addition to an accompanying lymphocytic infiltrate and occasional neutrophils.

The patient was given a 10-mg/mL suspension of elemental zinc and was advised to take 1 mL (10 mg) by mouth twice daily with food. This dosage equated to 3 mg/kg/d. On follow-up 3 weeks later, the skin began to clear (Figure 3). Follow-up laboratory testing showed an increase in zinc (114 μg/dL) and alkaline phosphatase levels (313 U/L). The patient was able to discontinue the zinc supplementation, and follow-up during the next year revealed no recurrence.

Comment
Etiology of AE—Acrodermatitis enteropathica was first identified in 1942 as an acral rash associated with diarrhea3; in 1973, Barnes and Moynahan4 discovered zinc deficiency as a causal agent for these findings. The causes of AE are further subclassified as either an acquired or inborn etiology. Congenital causes commonly are seen in infants within the first few months of life, whereas acquired forms are seen at any age. Acquired forms in infants can occur from failure of the mother to secrete zinc in breast milk, low maternal serum zinc levels, or other reasons causing low nutritional intake. A single mutation in the SLC30A2 gene has been found to markedly reduce zinc concentrations in breast milk, thus causing zinc deficiency in breastfed infants.5 Other acquired forms can be caused by malabsorption, sometimes after surgery such as intestinal bypass or from intravenous nutrition without sufficient zinc.1 The congenital form of AE is an autosomal-recessive disorder occurring from mutations in the SLC39A4 gene located on band 8q24.3. Affected individuals have a decreased ability to absorb zinc in the small intestine because of defects in zinc transporters ZIP and ZnT.6 Based on our patient’s laboratory findings and history, it is believed that the zinc deficiency was acquired, as the condition normalized with repletion and has not required any supplementation in the year of follow-up. In addition, the absence of a pertinent family history supported an acquired diagnosis, which has various etiologies, whereas the congenital form primarily is a genetic disease.
Management—Treatment of AE includes supplementation with oral elemental zinc; however, there are scant evidence-based recommendations on the exact dose of zinc to be given. Generally, the recommended amount is 3 mg/kg/d.8 For individuals with the congenital form of AE, lifelong zinc supplementation is additionally recommended.9 It is important to recognize this presentation because the patient can develop worsening irritability, severe diarrhea, nail dystrophy, hair loss, immune dysfunction, and numerous ophthalmic disorders if left untreated. Acute zinc toxicity due to excess administration is rare, with symptoms of nausea and vomiting occurring with dosages of 50 to 100 mg/d. Additionally, dosages of up to 70 mg twice weekly have been provided without any toxic effect.10 In our case, 3 mg/kg/d of oral zinc supplementation proved to be effective in resolving the patient’s symptoms of acquired zinc deficiency.
Differential Diagnosis—It is important to note that deficiencies of other nutrients may present as an AE-like eruption called acrodermatitis dysmetabolica (AD). Both diseases may present with the triad of dermatitis, alopecia, and diarrhea; however, AD is associated with inborn errors of metabolism. There have been cases that describe AD in patients with a zinc deficiency in conjunction with a deficiency of branched-chain amino acids.11,12 It is important to consider AD in the differential diagnosis of an AE eruption, especially in the context of a metabolic disorder, as it may affect the treatment plan. One case described the dermatitis of AD as not responding to zinc supplementation alone, while another described improvement after increasing an isoleucine supplementation dose.11,12
Other considerations in the differential diagnoses include AE-like conditions such as biotinidase deficiency, multiple carboxylase deficiency, and essential fatty acid deficiency. An AE-like condition may present with the triad of dermatitis, alopecia, and diarrhea. However, unlike in true AE, zinc and alkaline phosphatase levels tend to be normal in these conditions. Other features seen in AE-like conditions depend on the underlying cause but often include failure to thrive, neurologic defects, ophthalmic abnormalities, and metabolic abnormalities.13
Acrodermatitis enteropathica (AE) is a rare disorder of zinc metabolism that typically presents in infancy.1 Although it is clinically characterized by acral and periorificial dermatitis, alopecia, and diarrhea, only 20% of cases present with this triad.2 Zinc deficiency in AE can either be acquired or inborn (congenital). Acquired forms can occur from dietary inadequacy or malabsorption, whereas genetic causes are related to an autosomal-recessive disorder affecting zinc transporters.1 We report a case of a 3-month-old female infant with acquired AE who was successfully treated with zinc supplementation over the course of 3 weeks.
Case Report
A 3-month-old female infant presented to the emergency department with a rash of 2 weeks’ duration. She was born full term with no birth complications. The patient’s mother reported that the rash started on the cheeks, then enlarged and spread to the neck, back, and perineum. The patient also had been having diarrhea during this time. She previously had received mupirocin and cephalexin with no response to treatment. Maternal history was negative for lupus, and the mother’s diet consisted of a variety of foods but not many vegetables. The patient was exclusively breastfed, and there was no pertinent history of similar rashes occurring in other family members.
Physical examination revealed the patient had annular and polycyclic, hyperkeratotic, crusted papules and plaques on the cheeks, neck, back, and axillae, as well as the perineum/groin and perianal regions (Figure 1). The differential diagnosis at the time included neonatal lupus, zinc deficiency, and syphilis. Relevant laboratory testing and a shave biopsy of the left axilla were obtained.

Pertinent laboratory findings included a low zinc level (23 μg/dL [reference range, 26–141 μg/dL]), low alkaline phosphatase level (74 U/L [reference range, 94–486 U/L]), and thrombocytosis (826×109/L [reference range, 150–400×109/L). Results for antinuclear antibody and anti–Sjögren syndrome–related antigen A and B antibody testing were negative. A rapid plasma reagin test was nonreactive. Histologic examination revealed psoriasiform hyperplasia with overlying confluent parakeratosis, focal spongiosis, multiple dyskeratotic keratinocytes, and mitotic figures (Figure 2). Ballooning was evident in focal cells in the subcorneal region in addition to an accompanying lymphocytic infiltrate and occasional neutrophils.

The patient was given a 10-mg/mL suspension of elemental zinc and was advised to take 1 mL (10 mg) by mouth twice daily with food. This dosage equated to 3 mg/kg/d. On follow-up 3 weeks later, the skin began to clear (Figure 3). Follow-up laboratory testing showed an increase in zinc (114 μg/dL) and alkaline phosphatase levels (313 U/L). The patient was able to discontinue the zinc supplementation, and follow-up during the next year revealed no recurrence.

Comment
Etiology of AE—Acrodermatitis enteropathica was first identified in 1942 as an acral rash associated with diarrhea3; in 1973, Barnes and Moynahan4 discovered zinc deficiency as a causal agent for these findings. The causes of AE are further subclassified as either an acquired or inborn etiology. Congenital causes commonly are seen in infants within the first few months of life, whereas acquired forms are seen at any age. Acquired forms in infants can occur from failure of the mother to secrete zinc in breast milk, low maternal serum zinc levels, or other reasons causing low nutritional intake. A single mutation in the SLC30A2 gene has been found to markedly reduce zinc concentrations in breast milk, thus causing zinc deficiency in breastfed infants.5 Other acquired forms can be caused by malabsorption, sometimes after surgery such as intestinal bypass or from intravenous nutrition without sufficient zinc.1 The congenital form of AE is an autosomal-recessive disorder occurring from mutations in the SLC39A4 gene located on band 8q24.3. Affected individuals have a decreased ability to absorb zinc in the small intestine because of defects in zinc transporters ZIP and ZnT.6 Based on our patient’s laboratory findings and history, it is believed that the zinc deficiency was acquired, as the condition normalized with repletion and has not required any supplementation in the year of follow-up. In addition, the absence of a pertinent family history supported an acquired diagnosis, which has various etiologies, whereas the congenital form primarily is a genetic disease.
Management—Treatment of AE includes supplementation with oral elemental zinc; however, there are scant evidence-based recommendations on the exact dose of zinc to be given. Generally, the recommended amount is 3 mg/kg/d.8 For individuals with the congenital form of AE, lifelong zinc supplementation is additionally recommended.9 It is important to recognize this presentation because the patient can develop worsening irritability, severe diarrhea, nail dystrophy, hair loss, immune dysfunction, and numerous ophthalmic disorders if left untreated. Acute zinc toxicity due to excess administration is rare, with symptoms of nausea and vomiting occurring with dosages of 50 to 100 mg/d. Additionally, dosages of up to 70 mg twice weekly have been provided without any toxic effect.10 In our case, 3 mg/kg/d of oral zinc supplementation proved to be effective in resolving the patient’s symptoms of acquired zinc deficiency.
Differential Diagnosis—It is important to note that deficiencies of other nutrients may present as an AE-like eruption called acrodermatitis dysmetabolica (AD). Both diseases may present with the triad of dermatitis, alopecia, and diarrhea; however, AD is associated with inborn errors of metabolism. There have been cases that describe AD in patients with a zinc deficiency in conjunction with a deficiency of branched-chain amino acids.11,12 It is important to consider AD in the differential diagnosis of an AE eruption, especially in the context of a metabolic disorder, as it may affect the treatment plan. One case described the dermatitis of AD as not responding to zinc supplementation alone, while another described improvement after increasing an isoleucine supplementation dose.11,12
Other considerations in the differential diagnoses include AE-like conditions such as biotinidase deficiency, multiple carboxylase deficiency, and essential fatty acid deficiency. An AE-like condition may present with the triad of dermatitis, alopecia, and diarrhea. However, unlike in true AE, zinc and alkaline phosphatase levels tend to be normal in these conditions. Other features seen in AE-like conditions depend on the underlying cause but often include failure to thrive, neurologic defects, ophthalmic abnormalities, and metabolic abnormalities.13
- Acrodermatitis enteropathica. National Organization for Rare Disorders. Accessed October 16, 2022. https://rarediseases.org/rare-diseases/acrodermatitis-enteropathica/
- Perafán-Riveros C, França LFS, Alves ACF, et al. Acrodermatitis enteropathica: case report and review of the literature. Pediatr Dermatol. 2002;19:426-431.
- Danbolt N. Acrodermatitis enteropathica. Br J Dermatol. 1979;100:37-40.
- Barnes PM, Moynahan EJ. Zinc deficiency in acrodermatitis enteropathica: multiple dietary intolerance treated with synthetic diet. Proc R Soc Med. 1973;66:327-329.
- Lee S, Zhou Y, Gill DL, et al. A genetic variant in SLC30A2 causes breast dysfunction during lactation by inducing ER stress, oxidative stress and epithelial barrier defects. Sci Rep. 2018;8:3542.
- Kaur S, Sangwan A, Sahu P, et al. Clinical variants of acrodermatitis enteropathica and its co-relation with genetics. Indian J Paediatr Dermatol. 2016;17:35-37.
- Dela Rosa KM, James WD. Acrodermatitis enteropathica workup. Medscape. Updated June 4, 2021. Accessed October 16, 2022. https://emedicine.medscape.com/article/1102575-workup#showall
- Ngan V, Gangakhedkar A, Oakley A. Acrodermatitis enteropathica. DermNet. Accessed October 16, 2022. https://dermnetnz.org/topics/acrodermatitis-enteropathica/
- Ranugha P, Sethi P, Veeranna S. Acrodermatitis enteropathica: the need for sustained high dose zinc supplementation. Dermatol Online J. 2018;24:13030/qt1w9002sr.
- Larson CP, Roy SK, Khan AI, et al. Zinc treatment to under-five children: applications to improve child survival and reduce burden of disease. J Health Popul Nutr. 2008;26:356-365.
- Samady JA, Schwartz RA, Shih LY, et al. Acrodermatitis enteropathica-like eruption in an infant with nonketotic hyperglycinemia. J Dermatol. 2000;27:604-608.
- Flores K, Chikowski R, Morrell DS. Acrodermatitis dysmetabolica in an infant with maple syrup urine disease. Clin Exp Dermatol. 2016;41:651-654.
- Jones L, Oakley A. Acrodermatitis enteropathica-like conditions. DermNet. Accessed August 30, 2022. https://dermnetnz.org/topics/acrodermatitis-enteropathica-like-conditions
- Acrodermatitis enteropathica. National Organization for Rare Disorders. Accessed October 16, 2022. https://rarediseases.org/rare-diseases/acrodermatitis-enteropathica/
- Perafán-Riveros C, França LFS, Alves ACF, et al. Acrodermatitis enteropathica: case report and review of the literature. Pediatr Dermatol. 2002;19:426-431.
- Danbolt N. Acrodermatitis enteropathica. Br J Dermatol. 1979;100:37-40.
- Barnes PM, Moynahan EJ. Zinc deficiency in acrodermatitis enteropathica: multiple dietary intolerance treated with synthetic diet. Proc R Soc Med. 1973;66:327-329.
- Lee S, Zhou Y, Gill DL, et al. A genetic variant in SLC30A2 causes breast dysfunction during lactation by inducing ER stress, oxidative stress and epithelial barrier defects. Sci Rep. 2018;8:3542.
- Kaur S, Sangwan A, Sahu P, et al. Clinical variants of acrodermatitis enteropathica and its co-relation with genetics. Indian J Paediatr Dermatol. 2016;17:35-37.
- Dela Rosa KM, James WD. Acrodermatitis enteropathica workup. Medscape. Updated June 4, 2021. Accessed October 16, 2022. https://emedicine.medscape.com/article/1102575-workup#showall
- Ngan V, Gangakhedkar A, Oakley A. Acrodermatitis enteropathica. DermNet. Accessed October 16, 2022. https://dermnetnz.org/topics/acrodermatitis-enteropathica/
- Ranugha P, Sethi P, Veeranna S. Acrodermatitis enteropathica: the need for sustained high dose zinc supplementation. Dermatol Online J. 2018;24:13030/qt1w9002sr.
- Larson CP, Roy SK, Khan AI, et al. Zinc treatment to under-five children: applications to improve child survival and reduce burden of disease. J Health Popul Nutr. 2008;26:356-365.
- Samady JA, Schwartz RA, Shih LY, et al. Acrodermatitis enteropathica-like eruption in an infant with nonketotic hyperglycinemia. J Dermatol. 2000;27:604-608.
- Flores K, Chikowski R, Morrell DS. Acrodermatitis dysmetabolica in an infant with maple syrup urine disease. Clin Exp Dermatol. 2016;41:651-654.
- Jones L, Oakley A. Acrodermatitis enteropathica-like conditions. DermNet. Accessed August 30, 2022. https://dermnetnz.org/topics/acrodermatitis-enteropathica-like-conditions
Practice Points
- Although clinically characterized by the triad of acral and periorificial dermatitis, alopecia, and diarrhea, most cases of acrodermatitis enteropathica (AE) present with only partial features of this syndrome.
- Low levels of zinc-dependent enzymes such as alkaline phosphatase may support the diagnosis of AE.
Gene ‘cut-and-paste’ treatment could offer hope for inherited immune system diseases
An “exciting” new gene-editing strategy means those born with a rare inherited disease of the immune system could be treated by repairing a fault in their cells.
CTLA-4 is a protein produced by T cells that helps to control the activity of the immune system. Most people carry two working copies of the gene responsible for producing CTLA-4, but those who have only one functional copy produce too little of the protein to sufficiently regulate the immune system.
For patients with the condition, CTLA-4 insufficiency causes regulatory T cells to function abnormally, leading to severe autoimmunity. The authors explained that the condition also affects effector T cells and thereby “hampers their immune system’s ‘memory,’ ” meaning patients can “struggle to fight off recurring infections by the same viruses and bacteria.” In some cases, it can also lead to lymphomas.
Gene editing to ‘cut’ out faulty genes and ‘paste’ in ‘corrected’ ones
The research, published in Science Translational Medicine, and led by scientists from University College London, demonstrated in human cells and in mice that the cell fault can be repaired.
The scientists used “cut-and-paste” gene-editing techniques. First, they used the CRISPR/Cas9 system to target the faulty gene in human T cells taken from patients with CTLA-4 insufficiency, and then snip the faulty CTLA-4 gene in two. Then, to repair the errors a corrected sequence of DNA – delivered to the cell using a modified virus – was pasted over the faulty part of the gene using a cellular DNA repair mechanism known as homology-directed repair.
The authors explained that this allowed them to “preserve” important sequences within the CTLA-4 gene – known as the intron – that allow it to be switched on and off by the cell only when needed.
The outcome was “restored levels of CTLA-4 in the cells to those seen in healthy T cells,” the authors said.
Claire Booth, PhD, Mahboubian professor of gene therapy and pediatric immunology, UCL Great Ormond Street Institute of Child Health, and co–senior author, said that it was “really exciting” to think about taking this treatment forward to patients. “If we can improve their symptoms and reduce their risk of getting lymphoproliferative disease this will be a major step forward.”
In addition, the researchers were also able to improve symptoms of the disease in mice with CTLA-4 insufficiency by giving them injections of gene-edited T cells.
Technique may help tackle many conditions
The current standard treatment for CTLA-4 insufficiency is a bone marrow transplant to replace the stem cells responsible for producing T cells. However, “transplants are risky” and require high doses of chemotherapy and many weeks in hospital, the authors explained. “Older patients with CTLA-4 insufficiency are typically not well enough to tolerate the transplant procedure.”
Dr. Booth highlighted that the approach has many “positive aspects”. By correcting the patient’s T cells, “we think it can improve many of the symptoms of the disease”, she said, and added that this new approach is much less toxic than a bone marrow transplant. “Collecting the T cells is easier and correcting the T cells is easier. With this approach the amount of time in hospital the patients would need would be far less.”
Emma Morris, PhD, professor of clinical cell and gene therapy and director of UCL’s division of infection and immunity, and co–senior author, said: “Genes that play critical roles in controlling immune responses are not switched on all the time and are very tightly regulated. The technique we have used allows us to leave the natural (endogenous) mechanisms controlling gene expression intact, at the same time as correcting the mistake in the gene itself.”
The researchers explained that, although CTLA-4 insufficiency is rare, the gene editing therapy could be a proof of principle of their approach that could be adapted to tackle other conditions.
“It’s a way of correcting genetic mutations that could potentially be applicable for other diseases,” suggested Dr. Morris. “The bigger picture is it allows us to correct genes that are dysregulated or overactive, but also allows us to understand much more about gene expression and gene regulation.”
The study was funded by the Wellcome Trust, the Association for Moleculary Pathology, the Medical Research Council, Alzheimer’s Research UK, and the UCLH/UCL NIHR Biomedical Research Centre. Dr. Morris is a founder sharehold of Quell Therapeutics and has received honoraria from Orchard Therapeutics, GlaxoSmithKline, and AstraZeneca. Dr. Booth has performed ad hoc consulting in the past 3 years for SOBI and Novartis and educational material production for SOBI and Chiesi. A patent on the intronic gene editing approach has been filed in the UK. The other authors declared that they have no completing interests.
A version of this article first appeared on Medscape UK.
An “exciting” new gene-editing strategy means those born with a rare inherited disease of the immune system could be treated by repairing a fault in their cells.
CTLA-4 is a protein produced by T cells that helps to control the activity of the immune system. Most people carry two working copies of the gene responsible for producing CTLA-4, but those who have only one functional copy produce too little of the protein to sufficiently regulate the immune system.
For patients with the condition, CTLA-4 insufficiency causes regulatory T cells to function abnormally, leading to severe autoimmunity. The authors explained that the condition also affects effector T cells and thereby “hampers their immune system’s ‘memory,’ ” meaning patients can “struggle to fight off recurring infections by the same viruses and bacteria.” In some cases, it can also lead to lymphomas.
Gene editing to ‘cut’ out faulty genes and ‘paste’ in ‘corrected’ ones
The research, published in Science Translational Medicine, and led by scientists from University College London, demonstrated in human cells and in mice that the cell fault can be repaired.
The scientists used “cut-and-paste” gene-editing techniques. First, they used the CRISPR/Cas9 system to target the faulty gene in human T cells taken from patients with CTLA-4 insufficiency, and then snip the faulty CTLA-4 gene in two. Then, to repair the errors a corrected sequence of DNA – delivered to the cell using a modified virus – was pasted over the faulty part of the gene using a cellular DNA repair mechanism known as homology-directed repair.
The authors explained that this allowed them to “preserve” important sequences within the CTLA-4 gene – known as the intron – that allow it to be switched on and off by the cell only when needed.
The outcome was “restored levels of CTLA-4 in the cells to those seen in healthy T cells,” the authors said.
Claire Booth, PhD, Mahboubian professor of gene therapy and pediatric immunology, UCL Great Ormond Street Institute of Child Health, and co–senior author, said that it was “really exciting” to think about taking this treatment forward to patients. “If we can improve their symptoms and reduce their risk of getting lymphoproliferative disease this will be a major step forward.”
In addition, the researchers were also able to improve symptoms of the disease in mice with CTLA-4 insufficiency by giving them injections of gene-edited T cells.
Technique may help tackle many conditions
The current standard treatment for CTLA-4 insufficiency is a bone marrow transplant to replace the stem cells responsible for producing T cells. However, “transplants are risky” and require high doses of chemotherapy and many weeks in hospital, the authors explained. “Older patients with CTLA-4 insufficiency are typically not well enough to tolerate the transplant procedure.”
Dr. Booth highlighted that the approach has many “positive aspects”. By correcting the patient’s T cells, “we think it can improve many of the symptoms of the disease”, she said, and added that this new approach is much less toxic than a bone marrow transplant. “Collecting the T cells is easier and correcting the T cells is easier. With this approach the amount of time in hospital the patients would need would be far less.”
Emma Morris, PhD, professor of clinical cell and gene therapy and director of UCL’s division of infection and immunity, and co–senior author, said: “Genes that play critical roles in controlling immune responses are not switched on all the time and are very tightly regulated. The technique we have used allows us to leave the natural (endogenous) mechanisms controlling gene expression intact, at the same time as correcting the mistake in the gene itself.”
The researchers explained that, although CTLA-4 insufficiency is rare, the gene editing therapy could be a proof of principle of their approach that could be adapted to tackle other conditions.
“It’s a way of correcting genetic mutations that could potentially be applicable for other diseases,” suggested Dr. Morris. “The bigger picture is it allows us to correct genes that are dysregulated or overactive, but also allows us to understand much more about gene expression and gene regulation.”
The study was funded by the Wellcome Trust, the Association for Moleculary Pathology, the Medical Research Council, Alzheimer’s Research UK, and the UCLH/UCL NIHR Biomedical Research Centre. Dr. Morris is a founder sharehold of Quell Therapeutics and has received honoraria from Orchard Therapeutics, GlaxoSmithKline, and AstraZeneca. Dr. Booth has performed ad hoc consulting in the past 3 years for SOBI and Novartis and educational material production for SOBI and Chiesi. A patent on the intronic gene editing approach has been filed in the UK. The other authors declared that they have no completing interests.
A version of this article first appeared on Medscape UK.
An “exciting” new gene-editing strategy means those born with a rare inherited disease of the immune system could be treated by repairing a fault in their cells.
CTLA-4 is a protein produced by T cells that helps to control the activity of the immune system. Most people carry two working copies of the gene responsible for producing CTLA-4, but those who have only one functional copy produce too little of the protein to sufficiently regulate the immune system.
For patients with the condition, CTLA-4 insufficiency causes regulatory T cells to function abnormally, leading to severe autoimmunity. The authors explained that the condition also affects effector T cells and thereby “hampers their immune system’s ‘memory,’ ” meaning patients can “struggle to fight off recurring infections by the same viruses and bacteria.” In some cases, it can also lead to lymphomas.
Gene editing to ‘cut’ out faulty genes and ‘paste’ in ‘corrected’ ones
The research, published in Science Translational Medicine, and led by scientists from University College London, demonstrated in human cells and in mice that the cell fault can be repaired.
The scientists used “cut-and-paste” gene-editing techniques. First, they used the CRISPR/Cas9 system to target the faulty gene in human T cells taken from patients with CTLA-4 insufficiency, and then snip the faulty CTLA-4 gene in two. Then, to repair the errors a corrected sequence of DNA – delivered to the cell using a modified virus – was pasted over the faulty part of the gene using a cellular DNA repair mechanism known as homology-directed repair.
The authors explained that this allowed them to “preserve” important sequences within the CTLA-4 gene – known as the intron – that allow it to be switched on and off by the cell only when needed.
The outcome was “restored levels of CTLA-4 in the cells to those seen in healthy T cells,” the authors said.
Claire Booth, PhD, Mahboubian professor of gene therapy and pediatric immunology, UCL Great Ormond Street Institute of Child Health, and co–senior author, said that it was “really exciting” to think about taking this treatment forward to patients. “If we can improve their symptoms and reduce their risk of getting lymphoproliferative disease this will be a major step forward.”
In addition, the researchers were also able to improve symptoms of the disease in mice with CTLA-4 insufficiency by giving them injections of gene-edited T cells.
Technique may help tackle many conditions
The current standard treatment for CTLA-4 insufficiency is a bone marrow transplant to replace the stem cells responsible for producing T cells. However, “transplants are risky” and require high doses of chemotherapy and many weeks in hospital, the authors explained. “Older patients with CTLA-4 insufficiency are typically not well enough to tolerate the transplant procedure.”
Dr. Booth highlighted that the approach has many “positive aspects”. By correcting the patient’s T cells, “we think it can improve many of the symptoms of the disease”, she said, and added that this new approach is much less toxic than a bone marrow transplant. “Collecting the T cells is easier and correcting the T cells is easier. With this approach the amount of time in hospital the patients would need would be far less.”
Emma Morris, PhD, professor of clinical cell and gene therapy and director of UCL’s division of infection and immunity, and co–senior author, said: “Genes that play critical roles in controlling immune responses are not switched on all the time and are very tightly regulated. The technique we have used allows us to leave the natural (endogenous) mechanisms controlling gene expression intact, at the same time as correcting the mistake in the gene itself.”
The researchers explained that, although CTLA-4 insufficiency is rare, the gene editing therapy could be a proof of principle of their approach that could be adapted to tackle other conditions.
“It’s a way of correcting genetic mutations that could potentially be applicable for other diseases,” suggested Dr. Morris. “The bigger picture is it allows us to correct genes that are dysregulated or overactive, but also allows us to understand much more about gene expression and gene regulation.”
The study was funded by the Wellcome Trust, the Association for Moleculary Pathology, the Medical Research Council, Alzheimer’s Research UK, and the UCLH/UCL NIHR Biomedical Research Centre. Dr. Morris is a founder sharehold of Quell Therapeutics and has received honoraria from Orchard Therapeutics, GlaxoSmithKline, and AstraZeneca. Dr. Booth has performed ad hoc consulting in the past 3 years for SOBI and Novartis and educational material production for SOBI and Chiesi. A patent on the intronic gene editing approach has been filed in the UK. The other authors declared that they have no completing interests.
A version of this article first appeared on Medscape UK.
FROM SCIENCE TRANSLATIONAL MEDICINE
Goodbye ‘diabetes insipidus’, hello ‘AVP-D’ and ‘AVP-R’
An international group representing leading endocrinology associations has recommended that the name “diabetes insipidus” – which in some cases has led to harm – be changed to eliminate confusion with “diabetes mellitus” and to reflect the former condition’s pathophysiology.
The new proposed names are arginine vasopressin deficiency (AVP-D) for central (also called “cranial”) etiologies and arginine vasopressin resistance (AVP-R) for nephrogenic (kidney) etiologies.
“What we’re proposing is to rename the disease according to the pathophysiology that defines it,” statement co-author Joseph G. Verbalis, MD, professor of medicine and chief of endocrinology and metabolism at Georgetown University Medical Center, Washington, told this news organization.
The statement advises that henceforth the new names be used in manuscripts and the medical literature while keeping the old names in parentheses during a transition period, as in “AVP-deficiency (cranial diabetes insipidus)” and “AVP-resistance (nephrogenic diabetes insipidus).”
The condition formerly known as diabetes insipidus is relatively rare, occurring in about 1 person per 10-15,000 population. It is caused by either deficient production or resistance in the kidney to the hormone AVP, normally produced by the hypothalamus and stored in the pituitary gland. AVP, also called antidiuretic hormone, regulates the body’s water level and urine production by the kidney.
Both etiologies lead to extreme thirst and excessive production of urine. Common causes of the deficiency include head trauma or brain tumor, while resistance in the kidney is often congenital. It is currently treated with a synthetic form of AVP called desmopressin and fluid replacement.
What’s in a name?
The proposal to change the name by the Working Group for Renaming Diabetes Insipidus is endorsed by The Endocrine Society, European Society of Endocrinology, Pituitary Society, Society for Endocrinology, European Society for Paediatric Endocrinology, Endocrine Society of Australia, Brazilian Endocrine Society, and Japanese Endocrine Society and is under review by several other societies. It was published as a position statement in several of those society’s journals, with more to follow.
Historically, the word “diabetes,” a Greek word meaning “siphon,” was used in the 1st and 2nd century BC to describe excess flow of urine. The Latin word “mellitus” or “honey” was added in the late 17th century to describe the sweetness of the urine in the dysglycemic condition.
A century later, the Latin word “insipidus,” meaning insipid or tasteless, was coined to distinguish between the two types of polyuria, the position statement details.
In the late 19th to early 20th century, the vasopressor and antidiuretic actions of posterior pituitary extracts were discovered and used to treat people with both the central and nephrogenic etiologies, which were also recognized around that time, yet the name “diabetes insipidus” has persisted.
“From a historical perspective, the name is perfectly appropriate. At the time it was identified, and it was realized that it was different from diabetes mellitus, that was a perfectly appropriate terminology based on what was known in the late 19th century – but not now. It has persisted through the years simply because in medicine there’s a lot of inertia for change ... It’s just always been called that. If there’s not a compelling reason to change a name, generally there’s no move to change it,” Dr. Verbalis observed.
‘Dramatic cases of patient mismanagement’ due to name confusion
Unfortunately, the urgency for the change arose from tragedy. In 2009, a 22-year-old man was admitted to the orthopedics department of a London teaching hospital for a hip replacement. Despite his known panhypopituitarism and diabetes insipidus, the nurses continually checked his blood glucose but didn’t give him desmopressin or sufficient fluids. Laboratory testing showed normal glucose, but his serum sodium was 149 mmol/L. The morning after his operation, he had a fatal cardiac arrest with a serum sodium of 169 mmol/L.
“The nurses thought he had diabetes mellitus ... So that was death due to failure to recognize that diabetes insipidus is not diabetes mellitus,” Dr. Verbalis said. “If he had been admitted to endocrinology, this wouldn’t have happened. But he was admitted to orthopedics. Non-endocrinologists are not so aware of diabetes insipidus, because it is a rare disease.”
In 2016, National Health Service England issued a patient safety alert about the “risk of severe harm or death when desmopressin is omitted or delayed in patients with cranial diabetes insipidus,” citing at least four incidents within the prior 7 years where omission of desmopressin had resulted in severe dehydration and death, with another 76 cases of omission or delay that were acted on before the patients became critically ill.
Further impetus for the name change came from the results of an anonymous web-based survey of 1,034 adult and pediatric patients with central diabetes insipidus conducted between August 2021 and February 2022. Overall, 80% reported encountering situations in which their condition had been confused with diabetes mellitus by health care professionals, and 85% supported renaming the disease.
There was some divergence in opinion as to what the new name(s) should be, but clear agreement that the term “diabetes” should not be part of it.
“We’ve only become recently aware that there are dramatic cases of patient mismanagement due to the confusion caused by the word ‘diabetes.’ We think patients should have a voice. If a legitimate patient survey says over 80% think this name should be changed, then I think we as endocrinologists need to pay attention to that,” Dr. Verbalis said.
But while endocrinologists are the ones who see these patients the most often, Dr. Verbalis said a main aim of the position statement “is really to change the mindset of non-endocrinologist doctors and nurses and other health care professionals that this is not diabetes mellitus. It’s a totally different disease. And if we give it a totally different name, then I think they will better recognize that.”
As to how long Dr. Verbalis thinks it will take for the new names to catch on, he pointed out that it’s taken about a decade for the rheumatology field to fully adopt the name “granulomatosis with polyangiitis” as a replacement for “Wegener’s granulomatosis” after the eponymous physician’s Nazi ties were revealed.
“So we’re not anticipating that this is going to change terminology tomorrow. It’s a long process. We just wanted to get the process started,” he said.
Dr. Verbalis has reported consulting for Otsuka.
A version of this article first appeared on Medscape.com.
An international group representing leading endocrinology associations has recommended that the name “diabetes insipidus” – which in some cases has led to harm – be changed to eliminate confusion with “diabetes mellitus” and to reflect the former condition’s pathophysiology.
The new proposed names are arginine vasopressin deficiency (AVP-D) for central (also called “cranial”) etiologies and arginine vasopressin resistance (AVP-R) for nephrogenic (kidney) etiologies.
“What we’re proposing is to rename the disease according to the pathophysiology that defines it,” statement co-author Joseph G. Verbalis, MD, professor of medicine and chief of endocrinology and metabolism at Georgetown University Medical Center, Washington, told this news organization.
The statement advises that henceforth the new names be used in manuscripts and the medical literature while keeping the old names in parentheses during a transition period, as in “AVP-deficiency (cranial diabetes insipidus)” and “AVP-resistance (nephrogenic diabetes insipidus).”
The condition formerly known as diabetes insipidus is relatively rare, occurring in about 1 person per 10-15,000 population. It is caused by either deficient production or resistance in the kidney to the hormone AVP, normally produced by the hypothalamus and stored in the pituitary gland. AVP, also called antidiuretic hormone, regulates the body’s water level and urine production by the kidney.
Both etiologies lead to extreme thirst and excessive production of urine. Common causes of the deficiency include head trauma or brain tumor, while resistance in the kidney is often congenital. It is currently treated with a synthetic form of AVP called desmopressin and fluid replacement.
What’s in a name?
The proposal to change the name by the Working Group for Renaming Diabetes Insipidus is endorsed by The Endocrine Society, European Society of Endocrinology, Pituitary Society, Society for Endocrinology, European Society for Paediatric Endocrinology, Endocrine Society of Australia, Brazilian Endocrine Society, and Japanese Endocrine Society and is under review by several other societies. It was published as a position statement in several of those society’s journals, with more to follow.
Historically, the word “diabetes,” a Greek word meaning “siphon,” was used in the 1st and 2nd century BC to describe excess flow of urine. The Latin word “mellitus” or “honey” was added in the late 17th century to describe the sweetness of the urine in the dysglycemic condition.
A century later, the Latin word “insipidus,” meaning insipid or tasteless, was coined to distinguish between the two types of polyuria, the position statement details.
In the late 19th to early 20th century, the vasopressor and antidiuretic actions of posterior pituitary extracts were discovered and used to treat people with both the central and nephrogenic etiologies, which were also recognized around that time, yet the name “diabetes insipidus” has persisted.
“From a historical perspective, the name is perfectly appropriate. At the time it was identified, and it was realized that it was different from diabetes mellitus, that was a perfectly appropriate terminology based on what was known in the late 19th century – but not now. It has persisted through the years simply because in medicine there’s a lot of inertia for change ... It’s just always been called that. If there’s not a compelling reason to change a name, generally there’s no move to change it,” Dr. Verbalis observed.
‘Dramatic cases of patient mismanagement’ due to name confusion
Unfortunately, the urgency for the change arose from tragedy. In 2009, a 22-year-old man was admitted to the orthopedics department of a London teaching hospital for a hip replacement. Despite his known panhypopituitarism and diabetes insipidus, the nurses continually checked his blood glucose but didn’t give him desmopressin or sufficient fluids. Laboratory testing showed normal glucose, but his serum sodium was 149 mmol/L. The morning after his operation, he had a fatal cardiac arrest with a serum sodium of 169 mmol/L.
“The nurses thought he had diabetes mellitus ... So that was death due to failure to recognize that diabetes insipidus is not diabetes mellitus,” Dr. Verbalis said. “If he had been admitted to endocrinology, this wouldn’t have happened. But he was admitted to orthopedics. Non-endocrinologists are not so aware of diabetes insipidus, because it is a rare disease.”
In 2016, National Health Service England issued a patient safety alert about the “risk of severe harm or death when desmopressin is omitted or delayed in patients with cranial diabetes insipidus,” citing at least four incidents within the prior 7 years where omission of desmopressin had resulted in severe dehydration and death, with another 76 cases of omission or delay that were acted on before the patients became critically ill.
Further impetus for the name change came from the results of an anonymous web-based survey of 1,034 adult and pediatric patients with central diabetes insipidus conducted between August 2021 and February 2022. Overall, 80% reported encountering situations in which their condition had been confused with diabetes mellitus by health care professionals, and 85% supported renaming the disease.
There was some divergence in opinion as to what the new name(s) should be, but clear agreement that the term “diabetes” should not be part of it.
“We’ve only become recently aware that there are dramatic cases of patient mismanagement due to the confusion caused by the word ‘diabetes.’ We think patients should have a voice. If a legitimate patient survey says over 80% think this name should be changed, then I think we as endocrinologists need to pay attention to that,” Dr. Verbalis said.
But while endocrinologists are the ones who see these patients the most often, Dr. Verbalis said a main aim of the position statement “is really to change the mindset of non-endocrinologist doctors and nurses and other health care professionals that this is not diabetes mellitus. It’s a totally different disease. And if we give it a totally different name, then I think they will better recognize that.”
As to how long Dr. Verbalis thinks it will take for the new names to catch on, he pointed out that it’s taken about a decade for the rheumatology field to fully adopt the name “granulomatosis with polyangiitis” as a replacement for “Wegener’s granulomatosis” after the eponymous physician’s Nazi ties were revealed.
“So we’re not anticipating that this is going to change terminology tomorrow. It’s a long process. We just wanted to get the process started,” he said.
Dr. Verbalis has reported consulting for Otsuka.
A version of this article first appeared on Medscape.com.
An international group representing leading endocrinology associations has recommended that the name “diabetes insipidus” – which in some cases has led to harm – be changed to eliminate confusion with “diabetes mellitus” and to reflect the former condition’s pathophysiology.
The new proposed names are arginine vasopressin deficiency (AVP-D) for central (also called “cranial”) etiologies and arginine vasopressin resistance (AVP-R) for nephrogenic (kidney) etiologies.
“What we’re proposing is to rename the disease according to the pathophysiology that defines it,” statement co-author Joseph G. Verbalis, MD, professor of medicine and chief of endocrinology and metabolism at Georgetown University Medical Center, Washington, told this news organization.
The statement advises that henceforth the new names be used in manuscripts and the medical literature while keeping the old names in parentheses during a transition period, as in “AVP-deficiency (cranial diabetes insipidus)” and “AVP-resistance (nephrogenic diabetes insipidus).”
The condition formerly known as diabetes insipidus is relatively rare, occurring in about 1 person per 10-15,000 population. It is caused by either deficient production or resistance in the kidney to the hormone AVP, normally produced by the hypothalamus and stored in the pituitary gland. AVP, also called antidiuretic hormone, regulates the body’s water level and urine production by the kidney.
Both etiologies lead to extreme thirst and excessive production of urine. Common causes of the deficiency include head trauma or brain tumor, while resistance in the kidney is often congenital. It is currently treated with a synthetic form of AVP called desmopressin and fluid replacement.
What’s in a name?
The proposal to change the name by the Working Group for Renaming Diabetes Insipidus is endorsed by The Endocrine Society, European Society of Endocrinology, Pituitary Society, Society for Endocrinology, European Society for Paediatric Endocrinology, Endocrine Society of Australia, Brazilian Endocrine Society, and Japanese Endocrine Society and is under review by several other societies. It was published as a position statement in several of those society’s journals, with more to follow.
Historically, the word “diabetes,” a Greek word meaning “siphon,” was used in the 1st and 2nd century BC to describe excess flow of urine. The Latin word “mellitus” or “honey” was added in the late 17th century to describe the sweetness of the urine in the dysglycemic condition.
A century later, the Latin word “insipidus,” meaning insipid or tasteless, was coined to distinguish between the two types of polyuria, the position statement details.
In the late 19th to early 20th century, the vasopressor and antidiuretic actions of posterior pituitary extracts were discovered and used to treat people with both the central and nephrogenic etiologies, which were also recognized around that time, yet the name “diabetes insipidus” has persisted.
“From a historical perspective, the name is perfectly appropriate. At the time it was identified, and it was realized that it was different from diabetes mellitus, that was a perfectly appropriate terminology based on what was known in the late 19th century – but not now. It has persisted through the years simply because in medicine there’s a lot of inertia for change ... It’s just always been called that. If there’s not a compelling reason to change a name, generally there’s no move to change it,” Dr. Verbalis observed.
‘Dramatic cases of patient mismanagement’ due to name confusion
Unfortunately, the urgency for the change arose from tragedy. In 2009, a 22-year-old man was admitted to the orthopedics department of a London teaching hospital for a hip replacement. Despite his known panhypopituitarism and diabetes insipidus, the nurses continually checked his blood glucose but didn’t give him desmopressin or sufficient fluids. Laboratory testing showed normal glucose, but his serum sodium was 149 mmol/L. The morning after his operation, he had a fatal cardiac arrest with a serum sodium of 169 mmol/L.
“The nurses thought he had diabetes mellitus ... So that was death due to failure to recognize that diabetes insipidus is not diabetes mellitus,” Dr. Verbalis said. “If he had been admitted to endocrinology, this wouldn’t have happened. But he was admitted to orthopedics. Non-endocrinologists are not so aware of diabetes insipidus, because it is a rare disease.”
In 2016, National Health Service England issued a patient safety alert about the “risk of severe harm or death when desmopressin is omitted or delayed in patients with cranial diabetes insipidus,” citing at least four incidents within the prior 7 years where omission of desmopressin had resulted in severe dehydration and death, with another 76 cases of omission or delay that were acted on before the patients became critically ill.
Further impetus for the name change came from the results of an anonymous web-based survey of 1,034 adult and pediatric patients with central diabetes insipidus conducted between August 2021 and February 2022. Overall, 80% reported encountering situations in which their condition had been confused with diabetes mellitus by health care professionals, and 85% supported renaming the disease.
There was some divergence in opinion as to what the new name(s) should be, but clear agreement that the term “diabetes” should not be part of it.
“We’ve only become recently aware that there are dramatic cases of patient mismanagement due to the confusion caused by the word ‘diabetes.’ We think patients should have a voice. If a legitimate patient survey says over 80% think this name should be changed, then I think we as endocrinologists need to pay attention to that,” Dr. Verbalis said.
But while endocrinologists are the ones who see these patients the most often, Dr. Verbalis said a main aim of the position statement “is really to change the mindset of non-endocrinologist doctors and nurses and other health care professionals that this is not diabetes mellitus. It’s a totally different disease. And if we give it a totally different name, then I think they will better recognize that.”
As to how long Dr. Verbalis thinks it will take for the new names to catch on, he pointed out that it’s taken about a decade for the rheumatology field to fully adopt the name “granulomatosis with polyangiitis” as a replacement for “Wegener’s granulomatosis” after the eponymous physician’s Nazi ties were revealed.
“So we’re not anticipating that this is going to change terminology tomorrow. It’s a long process. We just wanted to get the process started,” he said.
Dr. Verbalis has reported consulting for Otsuka.
A version of this article first appeared on Medscape.com.
Diazepam nasal spray effective in Lennox-Gastaut syndrome
CINCINNATI – A new analysis of data from a phase 3 clinical trial suggests that
LGS is a severe form of epilepsy that generally begins in early childhood and has a poor prognosis and seizures that are often treatment refractory. The findings of the analysis should be encouraging to physicians who may view patients with LGS as not benefiting from treatment, said Daniel C. Tarquinio, DO, who presented the results at the 2022 annual meeting of the Child Neurology Society.
“Their response to their first appropriate weight-based rescue dose of Valtoco was essentially no different. They were subtly different, but they’re not really meaningful differences. Very few needed a second dose. In practice this is helpful because we know that kids with LGS, we think of them as having worse epilepsy, if you will. But if they need rescue, if we prescribe an appropriate rescue dose based on their weight, that the same rescue will work for them as it will for a kid that doesn’t have – quote unquote – as bad epilepsy that needs rescue,” said Dr. Tarquinio, a child neurologist and epileptologist and founder of the Center for Rare Neurological Diseases.
During the Q&A, Dr. Tarquinio was asked if there is something about the biology of LGS that would suggest it might respond differently to the drug. Dr. Tarquinio said no. “The reason we even looked at this is because many clinicians told us that their sense was [that patients with LGS] did not respond as well to rescue in general no matter what they use. This allowed us to go back and look at a controlled data set and say, at least in our controlled dataset, they respond the same,” he said.
Grace Gombolay, MD, who moderated the session, agreed that the results should be encouraging. “It seems like a lot of clinicians have the sense that Lennox-Gastaut Syndrome is a very terrible refractory epilepsy syndrome, and so doing rescue doesn’t seem to make sense if they don’t really respond. I think it’s helpful to know because there are actually studies showing that Valtoco seems to actually work in those patients, so it’s actually useful clinically to prescribe those patients and give it a shot,” said Dr. Gombolay, director of the Pediatric Neuroimmunology and Multiple Sclerosis Clinic at Emory University, Atlanta.
LGS patients may experience hundreds of seizures per day. “It’s really hard for parents to quantify, did they get better? Did the rescue help or not, because they’re still having some seizures. I think the sense is, ‘oh, this isn’t working.’ That’s probably the bias. I think this is good data that if you are able to get Valtoco for your patients, I think it’s worth a shot even in Lennox-Gastaut,” said Dr. Gombolay.
The researchers conducted a post hoc analysis of the phase 3, open-label, repeat-dose safety study of Valtoco. The study included a 12-month treatment period with visits at day 30 and every 60 days following. Patients had the option of staying on the drug following the end of the treatment period. Seizure and dosing information were obtained from a diary. The study enrolled 163 patients whose physicians believed they would need to be treated with a benzodiazepine at least once every other month to achieve seizure control. Dosing was determined by a combination of age and weight. If a second dose was required, caregivers were instructed to provide it 4-12 hours after the first dose.
In the study cohort, 47.9% of patients were aged 6-17 years. The researchers looked specifically at 73 cases of seizure clusters. In nine cases, the patient had LGS (five male, four female). Nearly all (95.9%) of LGS cluster cases were treated with a single dose and 4.1% were exposed to a second dose. Among 64 cases involving a patient with pediatric epileptic encephalopathies, 89.4% were treated with a single dose and 10.6% received a second. The safety profile was similar between patients with LGS and those with pediatric encephalopathies.
Dr. Gombolay has no relevant financial disclosures.
CINCINNATI – A new analysis of data from a phase 3 clinical trial suggests that
LGS is a severe form of epilepsy that generally begins in early childhood and has a poor prognosis and seizures that are often treatment refractory. The findings of the analysis should be encouraging to physicians who may view patients with LGS as not benefiting from treatment, said Daniel C. Tarquinio, DO, who presented the results at the 2022 annual meeting of the Child Neurology Society.
“Their response to their first appropriate weight-based rescue dose of Valtoco was essentially no different. They were subtly different, but they’re not really meaningful differences. Very few needed a second dose. In practice this is helpful because we know that kids with LGS, we think of them as having worse epilepsy, if you will. But if they need rescue, if we prescribe an appropriate rescue dose based on their weight, that the same rescue will work for them as it will for a kid that doesn’t have – quote unquote – as bad epilepsy that needs rescue,” said Dr. Tarquinio, a child neurologist and epileptologist and founder of the Center for Rare Neurological Diseases.
During the Q&A, Dr. Tarquinio was asked if there is something about the biology of LGS that would suggest it might respond differently to the drug. Dr. Tarquinio said no. “The reason we even looked at this is because many clinicians told us that their sense was [that patients with LGS] did not respond as well to rescue in general no matter what they use. This allowed us to go back and look at a controlled data set and say, at least in our controlled dataset, they respond the same,” he said.
Grace Gombolay, MD, who moderated the session, agreed that the results should be encouraging. “It seems like a lot of clinicians have the sense that Lennox-Gastaut Syndrome is a very terrible refractory epilepsy syndrome, and so doing rescue doesn’t seem to make sense if they don’t really respond. I think it’s helpful to know because there are actually studies showing that Valtoco seems to actually work in those patients, so it’s actually useful clinically to prescribe those patients and give it a shot,” said Dr. Gombolay, director of the Pediatric Neuroimmunology and Multiple Sclerosis Clinic at Emory University, Atlanta.
LGS patients may experience hundreds of seizures per day. “It’s really hard for parents to quantify, did they get better? Did the rescue help or not, because they’re still having some seizures. I think the sense is, ‘oh, this isn’t working.’ That’s probably the bias. I think this is good data that if you are able to get Valtoco for your patients, I think it’s worth a shot even in Lennox-Gastaut,” said Dr. Gombolay.
The researchers conducted a post hoc analysis of the phase 3, open-label, repeat-dose safety study of Valtoco. The study included a 12-month treatment period with visits at day 30 and every 60 days following. Patients had the option of staying on the drug following the end of the treatment period. Seizure and dosing information were obtained from a diary. The study enrolled 163 patients whose physicians believed they would need to be treated with a benzodiazepine at least once every other month to achieve seizure control. Dosing was determined by a combination of age and weight. If a second dose was required, caregivers were instructed to provide it 4-12 hours after the first dose.
In the study cohort, 47.9% of patients were aged 6-17 years. The researchers looked specifically at 73 cases of seizure clusters. In nine cases, the patient had LGS (five male, four female). Nearly all (95.9%) of LGS cluster cases were treated with a single dose and 4.1% were exposed to a second dose. Among 64 cases involving a patient with pediatric epileptic encephalopathies, 89.4% were treated with a single dose and 10.6% received a second. The safety profile was similar between patients with LGS and those with pediatric encephalopathies.
Dr. Gombolay has no relevant financial disclosures.
CINCINNATI – A new analysis of data from a phase 3 clinical trial suggests that
LGS is a severe form of epilepsy that generally begins in early childhood and has a poor prognosis and seizures that are often treatment refractory. The findings of the analysis should be encouraging to physicians who may view patients with LGS as not benefiting from treatment, said Daniel C. Tarquinio, DO, who presented the results at the 2022 annual meeting of the Child Neurology Society.
“Their response to their first appropriate weight-based rescue dose of Valtoco was essentially no different. They were subtly different, but they’re not really meaningful differences. Very few needed a second dose. In practice this is helpful because we know that kids with LGS, we think of them as having worse epilepsy, if you will. But if they need rescue, if we prescribe an appropriate rescue dose based on their weight, that the same rescue will work for them as it will for a kid that doesn’t have – quote unquote – as bad epilepsy that needs rescue,” said Dr. Tarquinio, a child neurologist and epileptologist and founder of the Center for Rare Neurological Diseases.
During the Q&A, Dr. Tarquinio was asked if there is something about the biology of LGS that would suggest it might respond differently to the drug. Dr. Tarquinio said no. “The reason we even looked at this is because many clinicians told us that their sense was [that patients with LGS] did not respond as well to rescue in general no matter what they use. This allowed us to go back and look at a controlled data set and say, at least in our controlled dataset, they respond the same,” he said.
Grace Gombolay, MD, who moderated the session, agreed that the results should be encouraging. “It seems like a lot of clinicians have the sense that Lennox-Gastaut Syndrome is a very terrible refractory epilepsy syndrome, and so doing rescue doesn’t seem to make sense if they don’t really respond. I think it’s helpful to know because there are actually studies showing that Valtoco seems to actually work in those patients, so it’s actually useful clinically to prescribe those patients and give it a shot,” said Dr. Gombolay, director of the Pediatric Neuroimmunology and Multiple Sclerosis Clinic at Emory University, Atlanta.
LGS patients may experience hundreds of seizures per day. “It’s really hard for parents to quantify, did they get better? Did the rescue help or not, because they’re still having some seizures. I think the sense is, ‘oh, this isn’t working.’ That’s probably the bias. I think this is good data that if you are able to get Valtoco for your patients, I think it’s worth a shot even in Lennox-Gastaut,” said Dr. Gombolay.
The researchers conducted a post hoc analysis of the phase 3, open-label, repeat-dose safety study of Valtoco. The study included a 12-month treatment period with visits at day 30 and every 60 days following. Patients had the option of staying on the drug following the end of the treatment period. Seizure and dosing information were obtained from a diary. The study enrolled 163 patients whose physicians believed they would need to be treated with a benzodiazepine at least once every other month to achieve seizure control. Dosing was determined by a combination of age and weight. If a second dose was required, caregivers were instructed to provide it 4-12 hours after the first dose.
In the study cohort, 47.9% of patients were aged 6-17 years. The researchers looked specifically at 73 cases of seizure clusters. In nine cases, the patient had LGS (five male, four female). Nearly all (95.9%) of LGS cluster cases were treated with a single dose and 4.1% were exposed to a second dose. Among 64 cases involving a patient with pediatric epileptic encephalopathies, 89.4% were treated with a single dose and 10.6% received a second. The safety profile was similar between patients with LGS and those with pediatric encephalopathies.
Dr. Gombolay has no relevant financial disclosures.
AT CNS 2022
The urgent need to diagnose Sanfilippo syndrome at an early age
Sanfilippo syndrome is a rare inherited neurodegenerative metabolic disorder for which there are no approved therapies. Symptoms of the more severe subtypes typically begin within the first years of life, rapidly producing serious and progressive physical and cognitive deficits. The underlying pathophysiology is targetable, but the delay in diagnosis of this as well as other lysosomal storage disorders (LSDs) is slowing progress toward effective therapies.
“Lack of awareness and the delays to diagnosis have been a real challenge for us. There is reason for cautious optimism about treatments now in or approaching clinical studies, but to evaluate efficacy on cognitive outcomes we need to enroll more children at a very young age, before loss of milestones,” according to Cara O’Neill, MD, a co-founder and chief science officer of Cure Sanfilippo Foundation.
Epidemiology and description
Sanfilippo syndrome, like the more than 50 other LSDs, is caused by a gene mutation that leads to an enzyme deficiency in the lysosome.1 In the case of Sanfilippo syndrome, also known as mucopolysaccharidosis (MPS III), there are hundreds of mutations that can lead to Sanfilippo by altering the function of one of the four genes essential to degradation of heparan sulfate.2 Lysosomal accumulation of heparan sulfate drives a broad spectrum of progressive and largely irreversible symptoms that typically begin with somatic manifestations, such as bowel dysfunction and recurrent ear and upper respiratory infections.
Impairment of the central nervous system (CNS) usually occurs early in life, halting physical and mental development. As it progresses, accumulation of heparan sulfate in a variety of cells leads to a cascade of abnormal cellular signaling and dysfunction. Disruption of these processes, which are critical for normal neurodevelopment, result in loss of the developmental skills already gained and eventually loss of brain tissue.3 Although life expectancy has improved with supportive care, survival into adulthood is typically limited to milder forms.4
Over the past several years, progress in this and other LSDs has yielded therapeutic targets, including those involving gene repair and enzyme replacement. Already approved for use in some LSDs, these therapies have also shown promise in the experimental setting for Sanfilippo syndrome, leading to several completed clinical trials.5
So far, none of these treatments has advanced beyond clinical trials in Sanfilippo syndrome, but there have been favorable changes in the markers of disease, suggesting that better methods of treatment delivery and/or more sensitive tools to measure clinical change might lead to evidence of disease attenuation. However, the promise of treatment in all cases has been to prevent, slow, or halt progression, not to reverse it. This point is important, because it indicates that degree of benefit will depend on enrolling patients early in life. Even if effective therapies are identified, few patients will benefit without strategies to accelerate diagnosis.
In fact, “one study6 reported that the average age of diagnosis for Sanfilippo syndrome has not improved over the past 30 years,” according to Dr. O’Neill. She indicated that this has been frustrating, given the availability of clinical trials on which progress is dependent. There is no widely accepted protocol for who and when to test for Sanfilippo syndrome or other LSDs, but Dr. O’Neill’s organization is among those advocating for strategies to detect these diseases earlier, including screening at birth.
Almost by definition, the clinical diagnosis of rare diseases poses a challenge. With nonspecific symptoms and a broad range of potential diagnoses, diseases with a low incidence are not the first ones that are typically considered. In the case of Sanfilippo syndrome, published studies indicate incidence rates at or below 1 per 70,000 live births.7 However, the incidence rates have been highly variable not only by geographical regions but even across neighboring countries where genetic risk would be expected to be similar.
In Europe, for example, epidemiologic studies suggest the lifetime risk of MPS IIIA is approximately two times greater in Germany and the Netherlands relative to France and Sweden.7 It is possible that the methodology for identifying cases might be a more important factor than differences in genetic risk to explain this variability. Many experts, including Dr. O’Neill, believe that prevalence figures for Sanfilippo syndrome are typically underestimates because of the frequency with which LSDs are attributed to other pathology.
“For these types of rare disorders, a clinician might only see a single case over a career, and the symptoms can vary in presentation and severity with many alternatives to consider in the differential diagnosis,” Dr. O’Neill explained. She cited case reports in which symptoms of Sanfilippo syndrome after a period of initial normal development has been initially attributed to autism, which is a comorbid feature of the disease, idiopathic developmental delay, or other nonprogressive disorders until further clinical deterioration leads to additional testing. The implication is that LSDs must be considered far earlier despite their rarity.
For the least common of the four clinical subtypes, MPS IIIC and MPS IIID, the median ages of diagnosis have ranged from 4.5 to 19 years of age.7 This is likely a reflection of a slower progression and a later onset of clinical manifestations.
For the more rapidly progressing and typically more severe subtypes, MPS IIIA and MPS IIIB, the diagnosis is typically made earlier. In one review of epidemiologic studies in different countries, the earliest reported median age at diagnosis was 2.5 years,7 a point at which significant disease progression is likely to have already occurred. If the promise of treatments in development is prevention of disease progression, disability in many patients might be substantial if the time to diagnosis is not reduced.
Screening and testing
Independent of the potential to enroll children in clinical trials, early diagnosis also advances the opportunities for supportive care to lessen the burden of the disease on patients and families. Perhaps even more important, early diagnosis is vital to family planning. Since the American pediatrician Sylvester Sanfilippo, MD, first described this syndrome in 1963,7 the genetic profile and many of the features of the disease have become well characterized.8
“One reason to emphasize the importance of early diagnosis is the heritability of this disorder. With prompt diagnosis, genetic counseling can be offered to families to provide them with critical information for future family planning and for cascade testing of other potentially affected siblings,” Dr. O’Neill reported. The inheritance pattern of Sanfilippo syndrome is autosomal recessive.3 In families with an affected child, the risk for any subsequent child to have the same disorder is 25%. The chance of a sibling to be unaffected and not a carrier is also 25%. There is a 50% chance of a sibling to be a carrier but asymptomatic. Of priorities, spreading awareness has been a critical mission of the Cure Sanfilippo Foundation since it was founded 8 years ago, according to Glenn O’Neill, the president. He and his wife, Dr. O’Neill, who is a pediatrician, founded the organization after their own child’s diagnosis of Sanfilippo syndrome. Creating awareness is fundamental to the mission of attracting funds for research, but support to patients and their families as well as early enrollment in clinical trials are among other initiatives being pursued by the foundation to improve care and prognosis.
These strategies include some novel ideas, including an algorithm based on artificial intelligence (AI) that can accelerate suspicion of Sanfilippo syndrome in advance of laboratory or genetic testing, according to Dr. O’Neill. She reported that the facial phenotype, which is observed in a high proportion of but not in all Sanfilippo patients, includes coarse facial features such as puffiness around the eyes, heavy eyebrows, full lips, and macrocephaly.9 Interpretation of photos for AI-based analysis is enhanced when combined with other clinical symptoms.

“The Foundation was involved in honing such a tool by submitting the photos that were used to teach the AI to recognize the Sanfilippo syndrome phenotype,” Dr. O’Neill said. The AI-based tool (Face2Gene.com) is available from FDNA, a company that has been involved in analyzing complex phenotypic and genomic information to guide diagnosis and therapeutic strategies for an array of diseases, not just Sanfilippo syndrome.
The preferred method for diagnosis is biochemical or genetic testing. Of these, urine testing for elevated levels of heparan sulfate glycosaminoglycans (GAG) can be useful for screening, although false-negative tests occur. Analysis of the blood can be performed to detect abnormal levels or activity of the enzymes that break down this GAG. In addition, genetic testing can be performed on blood, fibroblast, buccal swab, or saliva samples. Genetic testing of the blood is the most frequently performed.
For the four MPS III subtypes – MPS IIIA, IIIB, IIIC, and IIID – the presence of two pathogenic mutations in the SGSH (17q25.3), NAGLU (17q21.2), HGSNAT (8p11.21), and GNS (12q14.3) genes, respectively, are likely diagnostic, but enzymatic testing or GAG analysis should be performed to confirm disease status, according to Dr. O’Neill, who said that global consensus based clinical care guidelines led by the Foundation were recently accepted for publication and also include a section on the approach to diagnosis.
While laboratory testing is sensitive, urinary excretion of GAG can be variable, with the potential for ambiguous results. Typically, biochemical and genetic testing provide more reliable results for the diagnosis. They can be readily performed in utero or at the time of birth. In addition, gene panels can permit the diagnosis of multiple types of LSDs, not just Sanfilippo, making screening a cost-effective strategy to consider multiple diseases with overlapping symptoms when an LSD is suspected. Dr. O’Neill said clinical guidelines recommend confirmation of enzyme deficiency or evidence of GAG substrate accumulation as confirmatory tests when genetic testing is positive.
“Ultimately, our goal is to promote universal screening at birth for these serious genetic disorders affecting children,” Dr. O’Neill said.
“We are in a catch-22 when it comes to newborn screening. Currently our federal system requires there be an available treatment before recommending routine screening for a disease. However, it is extremely difficult to power trials with patients who are most likely to show benefit in a trial setting without that very early diagnosis. Universal newborn screening would pave the way for accelerated drug development for children,” she added.
In the meantime, Dr. O’Neill suggests that clinicians should employ a low threshold of suspicion to pursue diagnostic studies of LSDs in infants and children with developmental delays or otherwise unexplained progressive disorders.
Importantly, clinicians can now act quickly on their suspicions and order testing without concern for delays or denial by insurers through a special program, according to Dr. O’Neill. Free genetic testing, offered by the Invitae Corporation, evaluates a panel of 58 genes associated with lysosomal disorders, permitting detection of Sanfilippo syndrome and other LSDs, according to Dr. O’Neill. The Invitae testing is typically performed on 3 mL of whole blood delivered to a central testing facility.
“Results can be obtained within a few weeks or sooner. This can seem like a long wait for families, but it is much more efficient than ordering tests sequentially,” Dr. O’Neill said.
Diagnosis: Signs and symptoms
Despite the differences in progression of the MPS III subtypes, the clinical characteristics are more similar than different. In all patients, prenatal and infant development are typically normal. The initial signs of disease can be found in the newborn, such as neonatal tachypnea, through the early infancy period, such as macrocephaly. However, these are not commonly recognized until about age 1 or soon after in those with MPS IIIA and IIIB.3 Speech delay is the first developmental delay seen in most patients. In those with MPS IIIC, initial symptoms are typically detected at age 3 or later and progress more slowly.10,11 The same is likely to be true of MPS IIID, although this subtype is less well characterized than the other three.7
Although many organs can be involved, degeneration of the CNS is regarded as the most characteristic.3 In aggressive disease, this includes slower acquisition of and failure to meet developmental milestones with progressive intellectual disability, while behavioral difficulties are a more common initial compliant in children with milder disease.13,14 These behavioral changes include hyperactivity, inattention, autistic behaviors, worsening safety awareness, and in some cases aggressive behavior that can be destructive. Sleep disturbances are common.15Because of variability inherent in descriptions of relatively small numbers of patients, the characterization of each of the MPS III subgroups is based on a limited number of small studies, but most patients demonstrate behavior disorders, have coarse facial features, and develop speech delay, according to a survey conducted of published studies.7 Collectively, abnormal behavior was identified as an early symptom in 77% of those with MPS IIIA, 69% of those with IIIB, and 77% of those with IIIC.
For MPS IIIA, loss of speech was observed at a median age of 3.8 years and loss of walking ability at 10.4 years. The median survival has been reported to range between 13 and 18 years. In children with MPS IIIB, the median age of speech loss was reported to about the same age, while loss of walking ability occurred at 11 years. In one study of MPS IIIB, 24% of patients had developed dementia by age 6 years, and the reported median survival has ranged between 17 and 19 years. For MPS IIIC, the onset of clinical symptoms has been observed at a median age of 3.5 years with evidence of cognitive loss observed in 33% of children by the age of 6 years. The median survival has ranged from 19 to 34 years in three studies tracing the natural history of this MPS III subtype.
The differential diagnosis reasonably includes other types of mucopolysaccharidosis disorders with cognitive impairment, including Hurler, Hunter, or Sly syndromes, other neurodevelopmental disorders, and inborn errors of metabolism. The heterogeneity of the features makes definitive laboratory or genetic testing, rather than the effort to differentiate clinical features, appropriate for a definitive diagnosis.
Once the diagnosis is made, other examinations for the common complications of Sanfilippo syndrome are appropriate. Abdominal imaging is appropriate for detecting complications in the gastrointestinal tract, including hepatomegaly, which has been reported in more than half of patients with MPS IIIA and IIIB and in 39% of patients with IIIC.7 In patients with breathing concerns at night and/or sleep disturbance, polysomnography can be useful for identifying sleep apnea and nocturnal seizure activity. In children suspected of seizures, EEG is appropriate. In one study, 66% of patients with MPS IIIA developed seizure activity.16 This has been less commonly reported in MPS IIIB and IIIC, ranging from 8% to 13%.15
Formal hearing evaluation is indicated for any child with speech delays. Hearing loss typically develops after the newborn period in Sanfilippo and may affect peak language acquisition if not treated, according to Dr. O’Neill.
Radiographic studies for dysostosis multiplex or other skeletal abnormalities are also appropriate based on clinical presentation.
Treatment: Present and future
In the absence of treatments to improve the prognosis of Sanfilippo syndrome, current management is based on supportive care and managing organ-specific complications. However, several strategies have proven viable in experimental models and led to clinical trials. None of these therapies has reached approval yet, but several have been associated with attenuation of biomarkers of MPS III disease activity.
Of nearly 30 Sanfilippo clinical trials conducted over the past 20 years, at least 9 have now been completed.5 In addition to studying gene therapy and enzyme replacement therapy, these trials have included stem cell transplantation and substrate reduction therapy, for which the goal is to reduce synthesis of the heparan sulfate GAG to prevent accumulation.5 Of this latter approach, promising initial results with genistein, an isoflavone that breaks down heparan sulfate, reached a phase 3 evaluation.18 Although heparan sulfate levels in the CNS were non-significantly reduced over the course of the trial, the reduction was not sufficient to attenuate cognitive decline.
In other LSDs, several forms of enzyme replacement therapy are now approved. In Fabry disease, for example, recombinant alpha-galactosidase A has now been used for more than 15 years.19 Clinical benefit has not yet been demonstrated in patients with Sanfilippo syndrome because of the difficulty of delivering these therapies past the blood-brain barrier. Several strategies have been pursued. For example, intrathecal delivery of recombinant heparan-N-sulfatase reduced CNS levels of GAG heparan sulfate in one phase 2B study, but it approached but fell short of the statistical significance for the primary endpoint of predefined cognitive stabilization.20 The signal of activity and generally acceptable tolerability has encouraged further study, including an ongoing study with promising interim results of intracerebroventricular enzyme replacement in MPS IIIB, according to Dr. O’Neill.
Acceptable safety and promising activity on disease biomarkers have also been seen with gene therapy in clinical trials. In one study that showed attenuation of brain atrophy, there was moderate improvement in behavior and sleep in three of the four patients enrolled.21 Other studies using various strategies for gene delivery have also produced signals of activity against the underlying pathology, generating persistent interest in ongoing and planned clinical studies with this form of treatment.22Unmodified hematopoietic stem cell transplantation (HSCT), an approach that has demonstrated efficacy when delivered early in the course of other LSDs, such as Hurler syndrome,23 has not yet been associated with significant activity in clinical studies of MPS III, including those that initiated treatment prior to the onset of neurological symptoms.24 However, promising early results have been reported in a study of gene-modified HSCT, which overexpresses the MPS IIIA enzyme.
“The clinical trial landscape fluctuates quite a bit, so I always encourage clinicians and families to check back often for updates. Patient organizations can also be helpful for understanding the most up-to-date and emerging trial options,” Dr. O’Neill reported.
Although it is expected that the greatest benefit would be derived from treatments initiated before or very early after the onset of symptoms, based on the limited potential for reversing cognitive loss, Dr. O’Neill said that she and others are also striving to offer treatments for individuals now living with Sanfilippo syndrome.
“We have to be willing to test treatments that are symptomatic in nature. To that aim, the Cure Sanfilippo Foundation has sponsored a study of a CNS-penetrating anti-inflammatory agent in advanced-disease patients more than 4 years of age,” Dr. O’Neill said. This group of patients typically been ineligible for clinical trials in the past. Dr. O’Neill hopes to change this orientation.
“It is important to highlight that all patients deserve our efforts to improve their quality of life and alleviate suffering, regardless of how old they are or how progressed in the disease they happen to be,” she said.
However, whether the goal is enrollment before or early in disease or later in disease progression, the challenge of enrolling sufficient numbers of patients to confirm clinical activity has been and continues to be a hurdle to progress.
“Clinical studies in Sanfilippo enroll relatively small numbers of patients, often 20 or less,” said Dr. O’Neill, explaining one of the reasons why her organization has been so active in raising awareness and funding such studies. For patients and families, the Cure Sanfilippo Foundation can offer a variety of guidance and support, but information about opportunities for clinical trial participation is a key resource they provide for families and their physicians.
Conclusion
For most children with Sanfilippo syndrome, life expectancy is limited. However, the characterization of the genetic causes and the biochemistry of the subtypes has led to several viable therapeutic approaches under development. There has been progress in delivery of therapeutic enzymes to the CNS, and there is substantial optimism that more progress is coming. One issue for treatment development, is the last of a clear regulatory pathway addressing important biomarkers of pathology, such as heparan sulfate burden. Developing treatments that address this issue or impaired enzyme activity levels have promise for preventing progression, particularly if started in infancy. However, the effort to draw awareness to this disease is the first step toward accelerating the time to an early diagnosis and subsequent opportunities to enroll in clinical trials.
References
1. Sun A. Lysosomal storage disease overview. Ann Transl Med. 2018 Dec;6(24):476. doi: 10.21037/atm.2018.11.39.
2. Andrade F et al. Sanfilippo syndrome: Overall review. Pediatr Int. 2015 Jun;57(3):331-8. doi: 10.1111/ped.12636.
3. Fedele AO. Sanfilippo syndrome: Causes, consequences, and treatments. Appl Clin Genet. 2015 Nov 25;8:269-81. doi: 10.2147/TACG.S57672.
4. Lavery C et al. Mortality in patients with Sanfilippo syndrome. Orphanet J Rare Dis. 2017 Oct 23;12(1):168. doi: 10.1186/s13023-017-0717-y.
5. Pearse Y et al. A cure for Sanfilippo syndrome? A summary of current therapeutic approaches and their promise. Med Res Arch. 2020 Feb 1;8(2). doi: 10.18103/mra.v8i2.2045.
6. Kuiper GA et al. Failure to shorten the diagnostic delay in two ultrao-rphan diseases (mucopolysaccharidosis types I and III): potential causes and implication. Orphanet J Rare Dis. 2018;13:2. Doi: 10.1186/s13023-017-0733-y.
7. Zelei T et al. Epidemiology of Sanfilippo syndrome: Results of a systematic literature review. Orphanet J Rare Dis. 2018 Apr 10;13(1):53. doi: 10.1186/s13023-018-0796-4.
8. Wagner VF, Northrup H. Mucopolysaccaharidosis type III. Gene Reviews. 2019 Sep 19. University of Washington, Seattle. https://www.ncbi.nlm.nih.gov/books/NBK546574/8.
9. O’Neill C et al. Natural history of facial features observed in Sanfilippo syndrome (MPS IIIB) using a next generation phenotyping tool. Mol Genet Metab. 2019 Feb;126:S112.
10. Ruijter GJ et al. Clinical and genetic spectrum of Sanfilippo type C (MPS IIIC) disease in the Netherlands. Mol Genet Metab. 2008 Feb;93(2):104-11. doi: 10.1016/j.ymgme.2007.09.011.
11. Valstar MJ et al. Mucopolysaccharidosis type IIID: 12 new patients and 15 novel mutations. Hum Mutat. 2010 May;31(5):E1348-60. doi: 10.1002/humu.21234.
12. Nijmeijer SCM. The attenuated end of phenotypic spectrum in MPS III: from late-onset stable cognitive impairment to non-neuronopathic phenotype. Orphanet J Rare Dis. 2019;14:249. Doi10.1186/s13023-019-1232-0.
13. Nidiffer FD, Kelly TE. Developmental and degenerative patterns associated with cognitive, behavioural and motor difficulties in the Sanfilippo syndrome: An epidemiological study. J Ment Defic Res. 1983 Sep;27 (Pt 3):185-203. doi: 10.1111/j.1365-2788.1983.tb00291.x.
14. Bax MC, Colville GA. Behaviour in mucopolysaccharide disorders. Arch Dis Child. 1995 Jul;73(1):77-81. doi: 10.1136/adc.73.1.77.
15. Fraser J et al. Sleep disturbance in mucopolysaccharidosis type III (Sanfilippo syndrome): A survey of managing clinicians. Clin Genet. 2002 Nov;62(5):418-21. doi: 10.1034/j.1399-0004.2002.620512.x.
16. Valstar MJ et al. Mucopolysaccharidosis type IIIA: Clinical spectrum and genotype-phenotype correlations. Ann Neurol. 2010 Dec;68(6):876-87. doi: 10.1002/ana.22092.
17. Heron B et al. Incidence and natural history of mucopolysaccharidosis type III in France and comparison with United Kingdom and Greece. Am J Med Genet A. 2011 Jan;155A(1):58-68. doi: 10.1002/ajmg.a.33779.
18. Delgadillo V et al. Genistein supplementation in patients affected by Sanfilippo disease. J Inherit Metab Dis. 2011 Oct;34(5):1039-44. doi: 10.1007/s10545-011-9342-4.
19. van der Veen SJ et al. Developments in the treatment of Fabry disease. J Inherit Metab Dis. 2020 Sep;43(5):908-21. doi: 10.1002/jimd.12228.
20. Wijburg FA et al. Intrathecal heparan-N-sulfatase in patients with Sanfilippo syndrome type A: A phase IIb randomized trial. Mol Genet Metab. 2019 Feb;126(2):121-30. doi: 10.1016/j.ymgme.2018.10.006.
21. Tardieu M et al. Intracerebral administration of adeno-associated viral vector serotype rh.10 carrying human SGSH and SUMF1 cDNAs in children with mucopolysaccharidosis type IIIA disease: Results of a phase I/II trial. Hum Gene Ther. 2014 Jun;25(6):506-16. doi: 10.1089/hum.2013.238.
22. Marco S et al. In vivo gene therapy for mucopolysaccharidosis type III (Sanfilippo syndrome): A new treatment horizon. Hum Gene Ther. 2019 Oct;30(10):1211-1121. doi: 10.1089/hum.2019.217.
23. Taylor M et al. Hematopoietic stem cell transplantation for mucopolysaccharidoses: Past, present, and future. Biol Blood Marrow Transplant. 2019 Jul;25(7):e226-e246. doi: 10.1016/j.bbmt.2019.02.012.
24. Sivakumur P, Wraith JE. Bone marrow transplantation in mucopolysaccharidosis type IIIA: A comparison of an early treated patient with his untreated sibling. J Inherit Metab Dis. 1999 Oct;22(7):849-50. doi: 10.1023/a:1005526628598.
Sanfilippo syndrome is a rare inherited neurodegenerative metabolic disorder for which there are no approved therapies. Symptoms of the more severe subtypes typically begin within the first years of life, rapidly producing serious and progressive physical and cognitive deficits. The underlying pathophysiology is targetable, but the delay in diagnosis of this as well as other lysosomal storage disorders (LSDs) is slowing progress toward effective therapies.
“Lack of awareness and the delays to diagnosis have been a real challenge for us. There is reason for cautious optimism about treatments now in or approaching clinical studies, but to evaluate efficacy on cognitive outcomes we need to enroll more children at a very young age, before loss of milestones,” according to Cara O’Neill, MD, a co-founder and chief science officer of Cure Sanfilippo Foundation.
Epidemiology and description
Sanfilippo syndrome, like the more than 50 other LSDs, is caused by a gene mutation that leads to an enzyme deficiency in the lysosome.1 In the case of Sanfilippo syndrome, also known as mucopolysaccharidosis (MPS III), there are hundreds of mutations that can lead to Sanfilippo by altering the function of one of the four genes essential to degradation of heparan sulfate.2 Lysosomal accumulation of heparan sulfate drives a broad spectrum of progressive and largely irreversible symptoms that typically begin with somatic manifestations, such as bowel dysfunction and recurrent ear and upper respiratory infections.
Impairment of the central nervous system (CNS) usually occurs early in life, halting physical and mental development. As it progresses, accumulation of heparan sulfate in a variety of cells leads to a cascade of abnormal cellular signaling and dysfunction. Disruption of these processes, which are critical for normal neurodevelopment, result in loss of the developmental skills already gained and eventually loss of brain tissue.3 Although life expectancy has improved with supportive care, survival into adulthood is typically limited to milder forms.4
Over the past several years, progress in this and other LSDs has yielded therapeutic targets, including those involving gene repair and enzyme replacement. Already approved for use in some LSDs, these therapies have also shown promise in the experimental setting for Sanfilippo syndrome, leading to several completed clinical trials.5
So far, none of these treatments has advanced beyond clinical trials in Sanfilippo syndrome, but there have been favorable changes in the markers of disease, suggesting that better methods of treatment delivery and/or more sensitive tools to measure clinical change might lead to evidence of disease attenuation. However, the promise of treatment in all cases has been to prevent, slow, or halt progression, not to reverse it. This point is important, because it indicates that degree of benefit will depend on enrolling patients early in life. Even if effective therapies are identified, few patients will benefit without strategies to accelerate diagnosis.
In fact, “one study6 reported that the average age of diagnosis for Sanfilippo syndrome has not improved over the past 30 years,” according to Dr. O’Neill. She indicated that this has been frustrating, given the availability of clinical trials on which progress is dependent. There is no widely accepted protocol for who and when to test for Sanfilippo syndrome or other LSDs, but Dr. O’Neill’s organization is among those advocating for strategies to detect these diseases earlier, including screening at birth.
Almost by definition, the clinical diagnosis of rare diseases poses a challenge. With nonspecific symptoms and a broad range of potential diagnoses, diseases with a low incidence are not the first ones that are typically considered. In the case of Sanfilippo syndrome, published studies indicate incidence rates at or below 1 per 70,000 live births.7 However, the incidence rates have been highly variable not only by geographical regions but even across neighboring countries where genetic risk would be expected to be similar.
In Europe, for example, epidemiologic studies suggest the lifetime risk of MPS IIIA is approximately two times greater in Germany and the Netherlands relative to France and Sweden.7 It is possible that the methodology for identifying cases might be a more important factor than differences in genetic risk to explain this variability. Many experts, including Dr. O’Neill, believe that prevalence figures for Sanfilippo syndrome are typically underestimates because of the frequency with which LSDs are attributed to other pathology.
“For these types of rare disorders, a clinician might only see a single case over a career, and the symptoms can vary in presentation and severity with many alternatives to consider in the differential diagnosis,” Dr. O’Neill explained. She cited case reports in which symptoms of Sanfilippo syndrome after a period of initial normal development has been initially attributed to autism, which is a comorbid feature of the disease, idiopathic developmental delay, or other nonprogressive disorders until further clinical deterioration leads to additional testing. The implication is that LSDs must be considered far earlier despite their rarity.
For the least common of the four clinical subtypes, MPS IIIC and MPS IIID, the median ages of diagnosis have ranged from 4.5 to 19 years of age.7 This is likely a reflection of a slower progression and a later onset of clinical manifestations.
For the more rapidly progressing and typically more severe subtypes, MPS IIIA and MPS IIIB, the diagnosis is typically made earlier. In one review of epidemiologic studies in different countries, the earliest reported median age at diagnosis was 2.5 years,7 a point at which significant disease progression is likely to have already occurred. If the promise of treatments in development is prevention of disease progression, disability in many patients might be substantial if the time to diagnosis is not reduced.
Screening and testing
Independent of the potential to enroll children in clinical trials, early diagnosis also advances the opportunities for supportive care to lessen the burden of the disease on patients and families. Perhaps even more important, early diagnosis is vital to family planning. Since the American pediatrician Sylvester Sanfilippo, MD, first described this syndrome in 1963,7 the genetic profile and many of the features of the disease have become well characterized.8
“One reason to emphasize the importance of early diagnosis is the heritability of this disorder. With prompt diagnosis, genetic counseling can be offered to families to provide them with critical information for future family planning and for cascade testing of other potentially affected siblings,” Dr. O’Neill reported. The inheritance pattern of Sanfilippo syndrome is autosomal recessive.3 In families with an affected child, the risk for any subsequent child to have the same disorder is 25%. The chance of a sibling to be unaffected and not a carrier is also 25%. There is a 50% chance of a sibling to be a carrier but asymptomatic. Of priorities, spreading awareness has been a critical mission of the Cure Sanfilippo Foundation since it was founded 8 years ago, according to Glenn O’Neill, the president. He and his wife, Dr. O’Neill, who is a pediatrician, founded the organization after their own child’s diagnosis of Sanfilippo syndrome. Creating awareness is fundamental to the mission of attracting funds for research, but support to patients and their families as well as early enrollment in clinical trials are among other initiatives being pursued by the foundation to improve care and prognosis.
These strategies include some novel ideas, including an algorithm based on artificial intelligence (AI) that can accelerate suspicion of Sanfilippo syndrome in advance of laboratory or genetic testing, according to Dr. O’Neill. She reported that the facial phenotype, which is observed in a high proportion of but not in all Sanfilippo patients, includes coarse facial features such as puffiness around the eyes, heavy eyebrows, full lips, and macrocephaly.9 Interpretation of photos for AI-based analysis is enhanced when combined with other clinical symptoms.

“The Foundation was involved in honing such a tool by submitting the photos that were used to teach the AI to recognize the Sanfilippo syndrome phenotype,” Dr. O’Neill said. The AI-based tool (Face2Gene.com) is available from FDNA, a company that has been involved in analyzing complex phenotypic and genomic information to guide diagnosis and therapeutic strategies for an array of diseases, not just Sanfilippo syndrome.
The preferred method for diagnosis is biochemical or genetic testing. Of these, urine testing for elevated levels of heparan sulfate glycosaminoglycans (GAG) can be useful for screening, although false-negative tests occur. Analysis of the blood can be performed to detect abnormal levels or activity of the enzymes that break down this GAG. In addition, genetic testing can be performed on blood, fibroblast, buccal swab, or saliva samples. Genetic testing of the blood is the most frequently performed.
For the four MPS III subtypes – MPS IIIA, IIIB, IIIC, and IIID – the presence of two pathogenic mutations in the SGSH (17q25.3), NAGLU (17q21.2), HGSNAT (8p11.21), and GNS (12q14.3) genes, respectively, are likely diagnostic, but enzymatic testing or GAG analysis should be performed to confirm disease status, according to Dr. O’Neill, who said that global consensus based clinical care guidelines led by the Foundation were recently accepted for publication and also include a section on the approach to diagnosis.
While laboratory testing is sensitive, urinary excretion of GAG can be variable, with the potential for ambiguous results. Typically, biochemical and genetic testing provide more reliable results for the diagnosis. They can be readily performed in utero or at the time of birth. In addition, gene panels can permit the diagnosis of multiple types of LSDs, not just Sanfilippo, making screening a cost-effective strategy to consider multiple diseases with overlapping symptoms when an LSD is suspected. Dr. O’Neill said clinical guidelines recommend confirmation of enzyme deficiency or evidence of GAG substrate accumulation as confirmatory tests when genetic testing is positive.
“Ultimately, our goal is to promote universal screening at birth for these serious genetic disorders affecting children,” Dr. O’Neill said.
“We are in a catch-22 when it comes to newborn screening. Currently our federal system requires there be an available treatment before recommending routine screening for a disease. However, it is extremely difficult to power trials with patients who are most likely to show benefit in a trial setting without that very early diagnosis. Universal newborn screening would pave the way for accelerated drug development for children,” she added.
In the meantime, Dr. O’Neill suggests that clinicians should employ a low threshold of suspicion to pursue diagnostic studies of LSDs in infants and children with developmental delays or otherwise unexplained progressive disorders.
Importantly, clinicians can now act quickly on their suspicions and order testing without concern for delays or denial by insurers through a special program, according to Dr. O’Neill. Free genetic testing, offered by the Invitae Corporation, evaluates a panel of 58 genes associated with lysosomal disorders, permitting detection of Sanfilippo syndrome and other LSDs, according to Dr. O’Neill. The Invitae testing is typically performed on 3 mL of whole blood delivered to a central testing facility.
“Results can be obtained within a few weeks or sooner. This can seem like a long wait for families, but it is much more efficient than ordering tests sequentially,” Dr. O’Neill said.
Diagnosis: Signs and symptoms
Despite the differences in progression of the MPS III subtypes, the clinical characteristics are more similar than different. In all patients, prenatal and infant development are typically normal. The initial signs of disease can be found in the newborn, such as neonatal tachypnea, through the early infancy period, such as macrocephaly. However, these are not commonly recognized until about age 1 or soon after in those with MPS IIIA and IIIB.3 Speech delay is the first developmental delay seen in most patients. In those with MPS IIIC, initial symptoms are typically detected at age 3 or later and progress more slowly.10,11 The same is likely to be true of MPS IIID, although this subtype is less well characterized than the other three.7
Although many organs can be involved, degeneration of the CNS is regarded as the most characteristic.3 In aggressive disease, this includes slower acquisition of and failure to meet developmental milestones with progressive intellectual disability, while behavioral difficulties are a more common initial compliant in children with milder disease.13,14 These behavioral changes include hyperactivity, inattention, autistic behaviors, worsening safety awareness, and in some cases aggressive behavior that can be destructive. Sleep disturbances are common.15Because of variability inherent in descriptions of relatively small numbers of patients, the characterization of each of the MPS III subgroups is based on a limited number of small studies, but most patients demonstrate behavior disorders, have coarse facial features, and develop speech delay, according to a survey conducted of published studies.7 Collectively, abnormal behavior was identified as an early symptom in 77% of those with MPS IIIA, 69% of those with IIIB, and 77% of those with IIIC.
For MPS IIIA, loss of speech was observed at a median age of 3.8 years and loss of walking ability at 10.4 years. The median survival has been reported to range between 13 and 18 years. In children with MPS IIIB, the median age of speech loss was reported to about the same age, while loss of walking ability occurred at 11 years. In one study of MPS IIIB, 24% of patients had developed dementia by age 6 years, and the reported median survival has ranged between 17 and 19 years. For MPS IIIC, the onset of clinical symptoms has been observed at a median age of 3.5 years with evidence of cognitive loss observed in 33% of children by the age of 6 years. The median survival has ranged from 19 to 34 years in three studies tracing the natural history of this MPS III subtype.
The differential diagnosis reasonably includes other types of mucopolysaccharidosis disorders with cognitive impairment, including Hurler, Hunter, or Sly syndromes, other neurodevelopmental disorders, and inborn errors of metabolism. The heterogeneity of the features makes definitive laboratory or genetic testing, rather than the effort to differentiate clinical features, appropriate for a definitive diagnosis.
Once the diagnosis is made, other examinations for the common complications of Sanfilippo syndrome are appropriate. Abdominal imaging is appropriate for detecting complications in the gastrointestinal tract, including hepatomegaly, which has been reported in more than half of patients with MPS IIIA and IIIB and in 39% of patients with IIIC.7 In patients with breathing concerns at night and/or sleep disturbance, polysomnography can be useful for identifying sleep apnea and nocturnal seizure activity. In children suspected of seizures, EEG is appropriate. In one study, 66% of patients with MPS IIIA developed seizure activity.16 This has been less commonly reported in MPS IIIB and IIIC, ranging from 8% to 13%.15
Formal hearing evaluation is indicated for any child with speech delays. Hearing loss typically develops after the newborn period in Sanfilippo and may affect peak language acquisition if not treated, according to Dr. O’Neill.
Radiographic studies for dysostosis multiplex or other skeletal abnormalities are also appropriate based on clinical presentation.
Treatment: Present and future
In the absence of treatments to improve the prognosis of Sanfilippo syndrome, current management is based on supportive care and managing organ-specific complications. However, several strategies have proven viable in experimental models and led to clinical trials. None of these therapies has reached approval yet, but several have been associated with attenuation of biomarkers of MPS III disease activity.
Of nearly 30 Sanfilippo clinical trials conducted over the past 20 years, at least 9 have now been completed.5 In addition to studying gene therapy and enzyme replacement therapy, these trials have included stem cell transplantation and substrate reduction therapy, for which the goal is to reduce synthesis of the heparan sulfate GAG to prevent accumulation.5 Of this latter approach, promising initial results with genistein, an isoflavone that breaks down heparan sulfate, reached a phase 3 evaluation.18 Although heparan sulfate levels in the CNS were non-significantly reduced over the course of the trial, the reduction was not sufficient to attenuate cognitive decline.
In other LSDs, several forms of enzyme replacement therapy are now approved. In Fabry disease, for example, recombinant alpha-galactosidase A has now been used for more than 15 years.19 Clinical benefit has not yet been demonstrated in patients with Sanfilippo syndrome because of the difficulty of delivering these therapies past the blood-brain barrier. Several strategies have been pursued. For example, intrathecal delivery of recombinant heparan-N-sulfatase reduced CNS levels of GAG heparan sulfate in one phase 2B study, but it approached but fell short of the statistical significance for the primary endpoint of predefined cognitive stabilization.20 The signal of activity and generally acceptable tolerability has encouraged further study, including an ongoing study with promising interim results of intracerebroventricular enzyme replacement in MPS IIIB, according to Dr. O’Neill.
Acceptable safety and promising activity on disease biomarkers have also been seen with gene therapy in clinical trials. In one study that showed attenuation of brain atrophy, there was moderate improvement in behavior and sleep in three of the four patients enrolled.21 Other studies using various strategies for gene delivery have also produced signals of activity against the underlying pathology, generating persistent interest in ongoing and planned clinical studies with this form of treatment.22Unmodified hematopoietic stem cell transplantation (HSCT), an approach that has demonstrated efficacy when delivered early in the course of other LSDs, such as Hurler syndrome,23 has not yet been associated with significant activity in clinical studies of MPS III, including those that initiated treatment prior to the onset of neurological symptoms.24 However, promising early results have been reported in a study of gene-modified HSCT, which overexpresses the MPS IIIA enzyme.
“The clinical trial landscape fluctuates quite a bit, so I always encourage clinicians and families to check back often for updates. Patient organizations can also be helpful for understanding the most up-to-date and emerging trial options,” Dr. O’Neill reported.
Although it is expected that the greatest benefit would be derived from treatments initiated before or very early after the onset of symptoms, based on the limited potential for reversing cognitive loss, Dr. O’Neill said that she and others are also striving to offer treatments for individuals now living with Sanfilippo syndrome.
“We have to be willing to test treatments that are symptomatic in nature. To that aim, the Cure Sanfilippo Foundation has sponsored a study of a CNS-penetrating anti-inflammatory agent in advanced-disease patients more than 4 years of age,” Dr. O’Neill said. This group of patients typically been ineligible for clinical trials in the past. Dr. O’Neill hopes to change this orientation.
“It is important to highlight that all patients deserve our efforts to improve their quality of life and alleviate suffering, regardless of how old they are or how progressed in the disease they happen to be,” she said.
However, whether the goal is enrollment before or early in disease or later in disease progression, the challenge of enrolling sufficient numbers of patients to confirm clinical activity has been and continues to be a hurdle to progress.
“Clinical studies in Sanfilippo enroll relatively small numbers of patients, often 20 or less,” said Dr. O’Neill, explaining one of the reasons why her organization has been so active in raising awareness and funding such studies. For patients and families, the Cure Sanfilippo Foundation can offer a variety of guidance and support, but information about opportunities for clinical trial participation is a key resource they provide for families and their physicians.
Conclusion
For most children with Sanfilippo syndrome, life expectancy is limited. However, the characterization of the genetic causes and the biochemistry of the subtypes has led to several viable therapeutic approaches under development. There has been progress in delivery of therapeutic enzymes to the CNS, and there is substantial optimism that more progress is coming. One issue for treatment development, is the last of a clear regulatory pathway addressing important biomarkers of pathology, such as heparan sulfate burden. Developing treatments that address this issue or impaired enzyme activity levels have promise for preventing progression, particularly if started in infancy. However, the effort to draw awareness to this disease is the first step toward accelerating the time to an early diagnosis and subsequent opportunities to enroll in clinical trials.
References
1. Sun A. Lysosomal storage disease overview. Ann Transl Med. 2018 Dec;6(24):476. doi: 10.21037/atm.2018.11.39.
2. Andrade F et al. Sanfilippo syndrome: Overall review. Pediatr Int. 2015 Jun;57(3):331-8. doi: 10.1111/ped.12636.
3. Fedele AO. Sanfilippo syndrome: Causes, consequences, and treatments. Appl Clin Genet. 2015 Nov 25;8:269-81. doi: 10.2147/TACG.S57672.
4. Lavery C et al. Mortality in patients with Sanfilippo syndrome. Orphanet J Rare Dis. 2017 Oct 23;12(1):168. doi: 10.1186/s13023-017-0717-y.
5. Pearse Y et al. A cure for Sanfilippo syndrome? A summary of current therapeutic approaches and their promise. Med Res Arch. 2020 Feb 1;8(2). doi: 10.18103/mra.v8i2.2045.
6. Kuiper GA et al. Failure to shorten the diagnostic delay in two ultrao-rphan diseases (mucopolysaccharidosis types I and III): potential causes and implication. Orphanet J Rare Dis. 2018;13:2. Doi: 10.1186/s13023-017-0733-y.
7. Zelei T et al. Epidemiology of Sanfilippo syndrome: Results of a systematic literature review. Orphanet J Rare Dis. 2018 Apr 10;13(1):53. doi: 10.1186/s13023-018-0796-4.
8. Wagner VF, Northrup H. Mucopolysaccaharidosis type III. Gene Reviews. 2019 Sep 19. University of Washington, Seattle. https://www.ncbi.nlm.nih.gov/books/NBK546574/8.
9. O’Neill C et al. Natural history of facial features observed in Sanfilippo syndrome (MPS IIIB) using a next generation phenotyping tool. Mol Genet Metab. 2019 Feb;126:S112.
10. Ruijter GJ et al. Clinical and genetic spectrum of Sanfilippo type C (MPS IIIC) disease in the Netherlands. Mol Genet Metab. 2008 Feb;93(2):104-11. doi: 10.1016/j.ymgme.2007.09.011.
11. Valstar MJ et al. Mucopolysaccharidosis type IIID: 12 new patients and 15 novel mutations. Hum Mutat. 2010 May;31(5):E1348-60. doi: 10.1002/humu.21234.
12. Nijmeijer SCM. The attenuated end of phenotypic spectrum in MPS III: from late-onset stable cognitive impairment to non-neuronopathic phenotype. Orphanet J Rare Dis. 2019;14:249. Doi10.1186/s13023-019-1232-0.
13. Nidiffer FD, Kelly TE. Developmental and degenerative patterns associated with cognitive, behavioural and motor difficulties in the Sanfilippo syndrome: An epidemiological study. J Ment Defic Res. 1983 Sep;27 (Pt 3):185-203. doi: 10.1111/j.1365-2788.1983.tb00291.x.
14. Bax MC, Colville GA. Behaviour in mucopolysaccharide disorders. Arch Dis Child. 1995 Jul;73(1):77-81. doi: 10.1136/adc.73.1.77.
15. Fraser J et al. Sleep disturbance in mucopolysaccharidosis type III (Sanfilippo syndrome): A survey of managing clinicians. Clin Genet. 2002 Nov;62(5):418-21. doi: 10.1034/j.1399-0004.2002.620512.x.
16. Valstar MJ et al. Mucopolysaccharidosis type IIIA: Clinical spectrum and genotype-phenotype correlations. Ann Neurol. 2010 Dec;68(6):876-87. doi: 10.1002/ana.22092.
17. Heron B et al. Incidence and natural history of mucopolysaccharidosis type III in France and comparison with United Kingdom and Greece. Am J Med Genet A. 2011 Jan;155A(1):58-68. doi: 10.1002/ajmg.a.33779.
18. Delgadillo V et al. Genistein supplementation in patients affected by Sanfilippo disease. J Inherit Metab Dis. 2011 Oct;34(5):1039-44. doi: 10.1007/s10545-011-9342-4.
19. van der Veen SJ et al. Developments in the treatment of Fabry disease. J Inherit Metab Dis. 2020 Sep;43(5):908-21. doi: 10.1002/jimd.12228.
20. Wijburg FA et al. Intrathecal heparan-N-sulfatase in patients with Sanfilippo syndrome type A: A phase IIb randomized trial. Mol Genet Metab. 2019 Feb;126(2):121-30. doi: 10.1016/j.ymgme.2018.10.006.
21. Tardieu M et al. Intracerebral administration of adeno-associated viral vector serotype rh.10 carrying human SGSH and SUMF1 cDNAs in children with mucopolysaccharidosis type IIIA disease: Results of a phase I/II trial. Hum Gene Ther. 2014 Jun;25(6):506-16. doi: 10.1089/hum.2013.238.
22. Marco S et al. In vivo gene therapy for mucopolysaccharidosis type III (Sanfilippo syndrome): A new treatment horizon. Hum Gene Ther. 2019 Oct;30(10):1211-1121. doi: 10.1089/hum.2019.217.
23. Taylor M et al. Hematopoietic stem cell transplantation for mucopolysaccharidoses: Past, present, and future. Biol Blood Marrow Transplant. 2019 Jul;25(7):e226-e246. doi: 10.1016/j.bbmt.2019.02.012.
24. Sivakumur P, Wraith JE. Bone marrow transplantation in mucopolysaccharidosis type IIIA: A comparison of an early treated patient with his untreated sibling. J Inherit Metab Dis. 1999 Oct;22(7):849-50. doi: 10.1023/a:1005526628598.
Sanfilippo syndrome is a rare inherited neurodegenerative metabolic disorder for which there are no approved therapies. Symptoms of the more severe subtypes typically begin within the first years of life, rapidly producing serious and progressive physical and cognitive deficits. The underlying pathophysiology is targetable, but the delay in diagnosis of this as well as other lysosomal storage disorders (LSDs) is slowing progress toward effective therapies.
“Lack of awareness and the delays to diagnosis have been a real challenge for us. There is reason for cautious optimism about treatments now in or approaching clinical studies, but to evaluate efficacy on cognitive outcomes we need to enroll more children at a very young age, before loss of milestones,” according to Cara O’Neill, MD, a co-founder and chief science officer of Cure Sanfilippo Foundation.
Epidemiology and description
Sanfilippo syndrome, like the more than 50 other LSDs, is caused by a gene mutation that leads to an enzyme deficiency in the lysosome.1 In the case of Sanfilippo syndrome, also known as mucopolysaccharidosis (MPS III), there are hundreds of mutations that can lead to Sanfilippo by altering the function of one of the four genes essential to degradation of heparan sulfate.2 Lysosomal accumulation of heparan sulfate drives a broad spectrum of progressive and largely irreversible symptoms that typically begin with somatic manifestations, such as bowel dysfunction and recurrent ear and upper respiratory infections.
Impairment of the central nervous system (CNS) usually occurs early in life, halting physical and mental development. As it progresses, accumulation of heparan sulfate in a variety of cells leads to a cascade of abnormal cellular signaling and dysfunction. Disruption of these processes, which are critical for normal neurodevelopment, result in loss of the developmental skills already gained and eventually loss of brain tissue.3 Although life expectancy has improved with supportive care, survival into adulthood is typically limited to milder forms.4
Over the past several years, progress in this and other LSDs has yielded therapeutic targets, including those involving gene repair and enzyme replacement. Already approved for use in some LSDs, these therapies have also shown promise in the experimental setting for Sanfilippo syndrome, leading to several completed clinical trials.5
So far, none of these treatments has advanced beyond clinical trials in Sanfilippo syndrome, but there have been favorable changes in the markers of disease, suggesting that better methods of treatment delivery and/or more sensitive tools to measure clinical change might lead to evidence of disease attenuation. However, the promise of treatment in all cases has been to prevent, slow, or halt progression, not to reverse it. This point is important, because it indicates that degree of benefit will depend on enrolling patients early in life. Even if effective therapies are identified, few patients will benefit without strategies to accelerate diagnosis.
In fact, “one study6 reported that the average age of diagnosis for Sanfilippo syndrome has not improved over the past 30 years,” according to Dr. O’Neill. She indicated that this has been frustrating, given the availability of clinical trials on which progress is dependent. There is no widely accepted protocol for who and when to test for Sanfilippo syndrome or other LSDs, but Dr. O’Neill’s organization is among those advocating for strategies to detect these diseases earlier, including screening at birth.
Almost by definition, the clinical diagnosis of rare diseases poses a challenge. With nonspecific symptoms and a broad range of potential diagnoses, diseases with a low incidence are not the first ones that are typically considered. In the case of Sanfilippo syndrome, published studies indicate incidence rates at or below 1 per 70,000 live births.7 However, the incidence rates have been highly variable not only by geographical regions but even across neighboring countries where genetic risk would be expected to be similar.
In Europe, for example, epidemiologic studies suggest the lifetime risk of MPS IIIA is approximately two times greater in Germany and the Netherlands relative to France and Sweden.7 It is possible that the methodology for identifying cases might be a more important factor than differences in genetic risk to explain this variability. Many experts, including Dr. O’Neill, believe that prevalence figures for Sanfilippo syndrome are typically underestimates because of the frequency with which LSDs are attributed to other pathology.
“For these types of rare disorders, a clinician might only see a single case over a career, and the symptoms can vary in presentation and severity with many alternatives to consider in the differential diagnosis,” Dr. O’Neill explained. She cited case reports in which symptoms of Sanfilippo syndrome after a period of initial normal development has been initially attributed to autism, which is a comorbid feature of the disease, idiopathic developmental delay, or other nonprogressive disorders until further clinical deterioration leads to additional testing. The implication is that LSDs must be considered far earlier despite their rarity.
For the least common of the four clinical subtypes, MPS IIIC and MPS IIID, the median ages of diagnosis have ranged from 4.5 to 19 years of age.7 This is likely a reflection of a slower progression and a later onset of clinical manifestations.
For the more rapidly progressing and typically more severe subtypes, MPS IIIA and MPS IIIB, the diagnosis is typically made earlier. In one review of epidemiologic studies in different countries, the earliest reported median age at diagnosis was 2.5 years,7 a point at which significant disease progression is likely to have already occurred. If the promise of treatments in development is prevention of disease progression, disability in many patients might be substantial if the time to diagnosis is not reduced.
Screening and testing
Independent of the potential to enroll children in clinical trials, early diagnosis also advances the opportunities for supportive care to lessen the burden of the disease on patients and families. Perhaps even more important, early diagnosis is vital to family planning. Since the American pediatrician Sylvester Sanfilippo, MD, first described this syndrome in 1963,7 the genetic profile and many of the features of the disease have become well characterized.8
“One reason to emphasize the importance of early diagnosis is the heritability of this disorder. With prompt diagnosis, genetic counseling can be offered to families to provide them with critical information for future family planning and for cascade testing of other potentially affected siblings,” Dr. O’Neill reported. The inheritance pattern of Sanfilippo syndrome is autosomal recessive.3 In families with an affected child, the risk for any subsequent child to have the same disorder is 25%. The chance of a sibling to be unaffected and not a carrier is also 25%. There is a 50% chance of a sibling to be a carrier but asymptomatic. Of priorities, spreading awareness has been a critical mission of the Cure Sanfilippo Foundation since it was founded 8 years ago, according to Glenn O’Neill, the president. He and his wife, Dr. O’Neill, who is a pediatrician, founded the organization after their own child’s diagnosis of Sanfilippo syndrome. Creating awareness is fundamental to the mission of attracting funds for research, but support to patients and their families as well as early enrollment in clinical trials are among other initiatives being pursued by the foundation to improve care and prognosis.
These strategies include some novel ideas, including an algorithm based on artificial intelligence (AI) that can accelerate suspicion of Sanfilippo syndrome in advance of laboratory or genetic testing, according to Dr. O’Neill. She reported that the facial phenotype, which is observed in a high proportion of but not in all Sanfilippo patients, includes coarse facial features such as puffiness around the eyes, heavy eyebrows, full lips, and macrocephaly.9 Interpretation of photos for AI-based analysis is enhanced when combined with other clinical symptoms.

“The Foundation was involved in honing such a tool by submitting the photos that were used to teach the AI to recognize the Sanfilippo syndrome phenotype,” Dr. O’Neill said. The AI-based tool (Face2Gene.com) is available from FDNA, a company that has been involved in analyzing complex phenotypic and genomic information to guide diagnosis and therapeutic strategies for an array of diseases, not just Sanfilippo syndrome.
The preferred method for diagnosis is biochemical or genetic testing. Of these, urine testing for elevated levels of heparan sulfate glycosaminoglycans (GAG) can be useful for screening, although false-negative tests occur. Analysis of the blood can be performed to detect abnormal levels or activity of the enzymes that break down this GAG. In addition, genetic testing can be performed on blood, fibroblast, buccal swab, or saliva samples. Genetic testing of the blood is the most frequently performed.
For the four MPS III subtypes – MPS IIIA, IIIB, IIIC, and IIID – the presence of two pathogenic mutations in the SGSH (17q25.3), NAGLU (17q21.2), HGSNAT (8p11.21), and GNS (12q14.3) genes, respectively, are likely diagnostic, but enzymatic testing or GAG analysis should be performed to confirm disease status, according to Dr. O’Neill, who said that global consensus based clinical care guidelines led by the Foundation were recently accepted for publication and also include a section on the approach to diagnosis.
While laboratory testing is sensitive, urinary excretion of GAG can be variable, with the potential for ambiguous results. Typically, biochemical and genetic testing provide more reliable results for the diagnosis. They can be readily performed in utero or at the time of birth. In addition, gene panels can permit the diagnosis of multiple types of LSDs, not just Sanfilippo, making screening a cost-effective strategy to consider multiple diseases with overlapping symptoms when an LSD is suspected. Dr. O’Neill said clinical guidelines recommend confirmation of enzyme deficiency or evidence of GAG substrate accumulation as confirmatory tests when genetic testing is positive.
“Ultimately, our goal is to promote universal screening at birth for these serious genetic disorders affecting children,” Dr. O’Neill said.
“We are in a catch-22 when it comes to newborn screening. Currently our federal system requires there be an available treatment before recommending routine screening for a disease. However, it is extremely difficult to power trials with patients who are most likely to show benefit in a trial setting without that very early diagnosis. Universal newborn screening would pave the way for accelerated drug development for children,” she added.
In the meantime, Dr. O’Neill suggests that clinicians should employ a low threshold of suspicion to pursue diagnostic studies of LSDs in infants and children with developmental delays or otherwise unexplained progressive disorders.
Importantly, clinicians can now act quickly on their suspicions and order testing without concern for delays or denial by insurers through a special program, according to Dr. O’Neill. Free genetic testing, offered by the Invitae Corporation, evaluates a panel of 58 genes associated with lysosomal disorders, permitting detection of Sanfilippo syndrome and other LSDs, according to Dr. O’Neill. The Invitae testing is typically performed on 3 mL of whole blood delivered to a central testing facility.
“Results can be obtained within a few weeks or sooner. This can seem like a long wait for families, but it is much more efficient than ordering tests sequentially,” Dr. O’Neill said.
Diagnosis: Signs and symptoms
Despite the differences in progression of the MPS III subtypes, the clinical characteristics are more similar than different. In all patients, prenatal and infant development are typically normal. The initial signs of disease can be found in the newborn, such as neonatal tachypnea, through the early infancy period, such as macrocephaly. However, these are not commonly recognized until about age 1 or soon after in those with MPS IIIA and IIIB.3 Speech delay is the first developmental delay seen in most patients. In those with MPS IIIC, initial symptoms are typically detected at age 3 or later and progress more slowly.10,11 The same is likely to be true of MPS IIID, although this subtype is less well characterized than the other three.7
Although many organs can be involved, degeneration of the CNS is regarded as the most characteristic.3 In aggressive disease, this includes slower acquisition of and failure to meet developmental milestones with progressive intellectual disability, while behavioral difficulties are a more common initial compliant in children with milder disease.13,14 These behavioral changes include hyperactivity, inattention, autistic behaviors, worsening safety awareness, and in some cases aggressive behavior that can be destructive. Sleep disturbances are common.15Because of variability inherent in descriptions of relatively small numbers of patients, the characterization of each of the MPS III subgroups is based on a limited number of small studies, but most patients demonstrate behavior disorders, have coarse facial features, and develop speech delay, according to a survey conducted of published studies.7 Collectively, abnormal behavior was identified as an early symptom in 77% of those with MPS IIIA, 69% of those with IIIB, and 77% of those with IIIC.
For MPS IIIA, loss of speech was observed at a median age of 3.8 years and loss of walking ability at 10.4 years. The median survival has been reported to range between 13 and 18 years. In children with MPS IIIB, the median age of speech loss was reported to about the same age, while loss of walking ability occurred at 11 years. In one study of MPS IIIB, 24% of patients had developed dementia by age 6 years, and the reported median survival has ranged between 17 and 19 years. For MPS IIIC, the onset of clinical symptoms has been observed at a median age of 3.5 years with evidence of cognitive loss observed in 33% of children by the age of 6 years. The median survival has ranged from 19 to 34 years in three studies tracing the natural history of this MPS III subtype.
The differential diagnosis reasonably includes other types of mucopolysaccharidosis disorders with cognitive impairment, including Hurler, Hunter, or Sly syndromes, other neurodevelopmental disorders, and inborn errors of metabolism. The heterogeneity of the features makes definitive laboratory or genetic testing, rather than the effort to differentiate clinical features, appropriate for a definitive diagnosis.
Once the diagnosis is made, other examinations for the common complications of Sanfilippo syndrome are appropriate. Abdominal imaging is appropriate for detecting complications in the gastrointestinal tract, including hepatomegaly, which has been reported in more than half of patients with MPS IIIA and IIIB and in 39% of patients with IIIC.7 In patients with breathing concerns at night and/or sleep disturbance, polysomnography can be useful for identifying sleep apnea and nocturnal seizure activity. In children suspected of seizures, EEG is appropriate. In one study, 66% of patients with MPS IIIA developed seizure activity.16 This has been less commonly reported in MPS IIIB and IIIC, ranging from 8% to 13%.15
Formal hearing evaluation is indicated for any child with speech delays. Hearing loss typically develops after the newborn period in Sanfilippo and may affect peak language acquisition if not treated, according to Dr. O’Neill.
Radiographic studies for dysostosis multiplex or other skeletal abnormalities are also appropriate based on clinical presentation.
Treatment: Present and future
In the absence of treatments to improve the prognosis of Sanfilippo syndrome, current management is based on supportive care and managing organ-specific complications. However, several strategies have proven viable in experimental models and led to clinical trials. None of these therapies has reached approval yet, but several have been associated with attenuation of biomarkers of MPS III disease activity.
Of nearly 30 Sanfilippo clinical trials conducted over the past 20 years, at least 9 have now been completed.5 In addition to studying gene therapy and enzyme replacement therapy, these trials have included stem cell transplantation and substrate reduction therapy, for which the goal is to reduce synthesis of the heparan sulfate GAG to prevent accumulation.5 Of this latter approach, promising initial results with genistein, an isoflavone that breaks down heparan sulfate, reached a phase 3 evaluation.18 Although heparan sulfate levels in the CNS were non-significantly reduced over the course of the trial, the reduction was not sufficient to attenuate cognitive decline.
In other LSDs, several forms of enzyme replacement therapy are now approved. In Fabry disease, for example, recombinant alpha-galactosidase A has now been used for more than 15 years.19 Clinical benefit has not yet been demonstrated in patients with Sanfilippo syndrome because of the difficulty of delivering these therapies past the blood-brain barrier. Several strategies have been pursued. For example, intrathecal delivery of recombinant heparan-N-sulfatase reduced CNS levels of GAG heparan sulfate in one phase 2B study, but it approached but fell short of the statistical significance for the primary endpoint of predefined cognitive stabilization.20 The signal of activity and generally acceptable tolerability has encouraged further study, including an ongoing study with promising interim results of intracerebroventricular enzyme replacement in MPS IIIB, according to Dr. O’Neill.
Acceptable safety and promising activity on disease biomarkers have also been seen with gene therapy in clinical trials. In one study that showed attenuation of brain atrophy, there was moderate improvement in behavior and sleep in three of the four patients enrolled.21 Other studies using various strategies for gene delivery have also produced signals of activity against the underlying pathology, generating persistent interest in ongoing and planned clinical studies with this form of treatment.22Unmodified hematopoietic stem cell transplantation (HSCT), an approach that has demonstrated efficacy when delivered early in the course of other LSDs, such as Hurler syndrome,23 has not yet been associated with significant activity in clinical studies of MPS III, including those that initiated treatment prior to the onset of neurological symptoms.24 However, promising early results have been reported in a study of gene-modified HSCT, which overexpresses the MPS IIIA enzyme.
“The clinical trial landscape fluctuates quite a bit, so I always encourage clinicians and families to check back often for updates. Patient organizations can also be helpful for understanding the most up-to-date and emerging trial options,” Dr. O’Neill reported.
Although it is expected that the greatest benefit would be derived from treatments initiated before or very early after the onset of symptoms, based on the limited potential for reversing cognitive loss, Dr. O’Neill said that she and others are also striving to offer treatments for individuals now living with Sanfilippo syndrome.
“We have to be willing to test treatments that are symptomatic in nature. To that aim, the Cure Sanfilippo Foundation has sponsored a study of a CNS-penetrating anti-inflammatory agent in advanced-disease patients more than 4 years of age,” Dr. O’Neill said. This group of patients typically been ineligible for clinical trials in the past. Dr. O’Neill hopes to change this orientation.
“It is important to highlight that all patients deserve our efforts to improve their quality of life and alleviate suffering, regardless of how old they are or how progressed in the disease they happen to be,” she said.
However, whether the goal is enrollment before or early in disease or later in disease progression, the challenge of enrolling sufficient numbers of patients to confirm clinical activity has been and continues to be a hurdle to progress.
“Clinical studies in Sanfilippo enroll relatively small numbers of patients, often 20 or less,” said Dr. O’Neill, explaining one of the reasons why her organization has been so active in raising awareness and funding such studies. For patients and families, the Cure Sanfilippo Foundation can offer a variety of guidance and support, but information about opportunities for clinical trial participation is a key resource they provide for families and their physicians.
Conclusion
For most children with Sanfilippo syndrome, life expectancy is limited. However, the characterization of the genetic causes and the biochemistry of the subtypes has led to several viable therapeutic approaches under development. There has been progress in delivery of therapeutic enzymes to the CNS, and there is substantial optimism that more progress is coming. One issue for treatment development, is the last of a clear regulatory pathway addressing important biomarkers of pathology, such as heparan sulfate burden. Developing treatments that address this issue or impaired enzyme activity levels have promise for preventing progression, particularly if started in infancy. However, the effort to draw awareness to this disease is the first step toward accelerating the time to an early diagnosis and subsequent opportunities to enroll in clinical trials.
References
1. Sun A. Lysosomal storage disease overview. Ann Transl Med. 2018 Dec;6(24):476. doi: 10.21037/atm.2018.11.39.
2. Andrade F et al. Sanfilippo syndrome: Overall review. Pediatr Int. 2015 Jun;57(3):331-8. doi: 10.1111/ped.12636.
3. Fedele AO. Sanfilippo syndrome: Causes, consequences, and treatments. Appl Clin Genet. 2015 Nov 25;8:269-81. doi: 10.2147/TACG.S57672.
4. Lavery C et al. Mortality in patients with Sanfilippo syndrome. Orphanet J Rare Dis. 2017 Oct 23;12(1):168. doi: 10.1186/s13023-017-0717-y.
5. Pearse Y et al. A cure for Sanfilippo syndrome? A summary of current therapeutic approaches and their promise. Med Res Arch. 2020 Feb 1;8(2). doi: 10.18103/mra.v8i2.2045.
6. Kuiper GA et al. Failure to shorten the diagnostic delay in two ultrao-rphan diseases (mucopolysaccharidosis types I and III): potential causes and implication. Orphanet J Rare Dis. 2018;13:2. Doi: 10.1186/s13023-017-0733-y.
7. Zelei T et al. Epidemiology of Sanfilippo syndrome: Results of a systematic literature review. Orphanet J Rare Dis. 2018 Apr 10;13(1):53. doi: 10.1186/s13023-018-0796-4.
8. Wagner VF, Northrup H. Mucopolysaccaharidosis type III. Gene Reviews. 2019 Sep 19. University of Washington, Seattle. https://www.ncbi.nlm.nih.gov/books/NBK546574/8.
9. O’Neill C et al. Natural history of facial features observed in Sanfilippo syndrome (MPS IIIB) using a next generation phenotyping tool. Mol Genet Metab. 2019 Feb;126:S112.
10. Ruijter GJ et al. Clinical and genetic spectrum of Sanfilippo type C (MPS IIIC) disease in the Netherlands. Mol Genet Metab. 2008 Feb;93(2):104-11. doi: 10.1016/j.ymgme.2007.09.011.
11. Valstar MJ et al. Mucopolysaccharidosis type IIID: 12 new patients and 15 novel mutations. Hum Mutat. 2010 May;31(5):E1348-60. doi: 10.1002/humu.21234.
12. Nijmeijer SCM. The attenuated end of phenotypic spectrum in MPS III: from late-onset stable cognitive impairment to non-neuronopathic phenotype. Orphanet J Rare Dis. 2019;14:249. Doi10.1186/s13023-019-1232-0.
13. Nidiffer FD, Kelly TE. Developmental and degenerative patterns associated with cognitive, behavioural and motor difficulties in the Sanfilippo syndrome: An epidemiological study. J Ment Defic Res. 1983 Sep;27 (Pt 3):185-203. doi: 10.1111/j.1365-2788.1983.tb00291.x.
14. Bax MC, Colville GA. Behaviour in mucopolysaccharide disorders. Arch Dis Child. 1995 Jul;73(1):77-81. doi: 10.1136/adc.73.1.77.
15. Fraser J et al. Sleep disturbance in mucopolysaccharidosis type III (Sanfilippo syndrome): A survey of managing clinicians. Clin Genet. 2002 Nov;62(5):418-21. doi: 10.1034/j.1399-0004.2002.620512.x.
16. Valstar MJ et al. Mucopolysaccharidosis type IIIA: Clinical spectrum and genotype-phenotype correlations. Ann Neurol. 2010 Dec;68(6):876-87. doi: 10.1002/ana.22092.
17. Heron B et al. Incidence and natural history of mucopolysaccharidosis type III in France and comparison with United Kingdom and Greece. Am J Med Genet A. 2011 Jan;155A(1):58-68. doi: 10.1002/ajmg.a.33779.
18. Delgadillo V et al. Genistein supplementation in patients affected by Sanfilippo disease. J Inherit Metab Dis. 2011 Oct;34(5):1039-44. doi: 10.1007/s10545-011-9342-4.
19. van der Veen SJ et al. Developments in the treatment of Fabry disease. J Inherit Metab Dis. 2020 Sep;43(5):908-21. doi: 10.1002/jimd.12228.
20. Wijburg FA et al. Intrathecal heparan-N-sulfatase in patients with Sanfilippo syndrome type A: A phase IIb randomized trial. Mol Genet Metab. 2019 Feb;126(2):121-30. doi: 10.1016/j.ymgme.2018.10.006.
21. Tardieu M et al. Intracerebral administration of adeno-associated viral vector serotype rh.10 carrying human SGSH and SUMF1 cDNAs in children with mucopolysaccharidosis type IIIA disease: Results of a phase I/II trial. Hum Gene Ther. 2014 Jun;25(6):506-16. doi: 10.1089/hum.2013.238.
22. Marco S et al. In vivo gene therapy for mucopolysaccharidosis type III (Sanfilippo syndrome): A new treatment horizon. Hum Gene Ther. 2019 Oct;30(10):1211-1121. doi: 10.1089/hum.2019.217.
23. Taylor M et al. Hematopoietic stem cell transplantation for mucopolysaccharidoses: Past, present, and future. Biol Blood Marrow Transplant. 2019 Jul;25(7):e226-e246. doi: 10.1016/j.bbmt.2019.02.012.
24. Sivakumur P, Wraith JE. Bone marrow transplantation in mucopolysaccharidosis type IIIA: A comparison of an early treated patient with his untreated sibling. J Inherit Metab Dis. 1999 Oct;22(7):849-50. doi: 10.1023/a:1005526628598.
Novel gene-based therapies for neuromuscular diseases
Neuromuscular diseases (NMDs) are a broad classification of heterogeneous groups of disorders characterized by progressive muscle weakness resulting from muscle or nerve dysfunction.1 Diagnosis is based on symptoms and a full medical history, as well as on muscle and imaging tests (including electromyography, nerve-conduction studies, magnetic resonance imaging, muscle biopsy, and blood tests) to confirm or rule out specific NMDs.2 Early diagnosis of NMDs can be difficult because symptoms overlap with those of many other diseases.
Although individually, NMDs are rare, collectively, they affect approximately 250,000 people in the United States. Disease types vary in regard to cause, symptoms, prevalence, age of onset, progression, and severity. Functional impairment from any NMD can lead to lifelong morbidities and shortened life expectancy.1,3
Treatment options for NMDs are limited; most target symptoms, not disease progression. Although there is a need for safe and effective gene-based therapies for NMDs, there are challenges to developing and delivering such treatments that have impeded clinical success. These include a lack of understanding about disease pathology and drug targets, limited animal model systems, and few reliable biomarkers that are predictive of therapeutic success.4,5
Notwithstanding that challenges remain, our understanding of gene expression in NMDs has greatly advanced in the past few decades. This progress has translated into promising results in the gene-therapy field – thereby setting the stage for therapeutic approaches that use novel gene-delivery and gene-manipulation tools.6 These novel approaches include nonviral strategies, such as antisense oligonucleotides (ASOs), and viral-based strategies, such as adeno-associated virus (AAV)-mediated gene silencing and AAV-mediated gene delivery.
In this article, we highlight advancements in the clinical development of gene-based therapies for NMDs. We focus on amyotrophic lateral sclerosis (ALS), spinal muscular atrophy (SMA), and Duchenne muscular dystrophy (DMD) because of recent clinical successes in developing such therapies.1,6,7 We also catalog completed and ongoing clinical trials for ALS, SMA, and DMD (Tables 1-3).
Amyotrophic lateral sclerosis
ALS is caused by progressive degeneration of upper- and lower-motor neurons, which eventually leads to respiratory failure and death 3 to 5 years after disease onset.7-9 There are two subtypes: Familial ALS (10% of cases) and sporadic ALS (90% of cases). Commonly mutated ALS-associated genes6,8 are:
- Superoxide dismutase type 1 (SOD1).
- Chromosome 9 open reading frame 72 (C9orf72).
- Transactive response DNA-binding protein 43 (TARDBP).
- Fused in sarcoma (FUS).
SOD1-targeted therapy is being studied, with early evidence of clinical success. Mutations in SOD1 account for 10% to 20% of familial ALS cases and 1% to 2% of sporadic ALS cases.6,10 10 Mutations in C9orf72 account for 25 to 40% of familial ALS cases and 7% of sporadic ALS cases.8,9,11 Mutations in TARDBP account for 3% of familial ALS cases and 2% of sporadic cases.12 Mutations in FUS account for 4% of familial ALS cases and 1% of sporadic cases. Overall, these mutant proteins can trigger neurotoxicity, thus inducing motor-neuron death.6,10
Treatment of ALS
Two treatments for ALS are Food and Drug Administration approved: riluzole (Rilutek), approved in 1995, and edaravone (Radicava), approved in 2017.
Riluzole is an oral anti-excitotoxic glutamate antagonist.11 Approval of riluzole was based on the results of two studies that demonstrated a 2- to 3-month survival benefit.10,14 For patients who have difficulty swallowing, an oral suspension (Tiglutik, approved in 2018) and an oral film (Exservan, approved in 2019) are available.
Edaravone is a free-radical scavenger that decreases oxidative stress and is administered intravenously (IV).9,13,14 Findings from clinical trials suggest functional improvement or slower decline in function for some patients.
Although these two agents demonstrate modest therapeutic benefit, neither reverses progression of disease.10,14
Gene-based therapy for ALS
Many non-viral strategies, including antisense oligonucleotide (ASO), monoclonal antibodies, reverse transcriptase inhibitors, and HGF gene replacement therapy are used as therapeutic approaches to SOD1, C9orf72, and FUS gene mutations in ALS patients, and are being evaluated in clinical studies14,15 (Table 113-17).
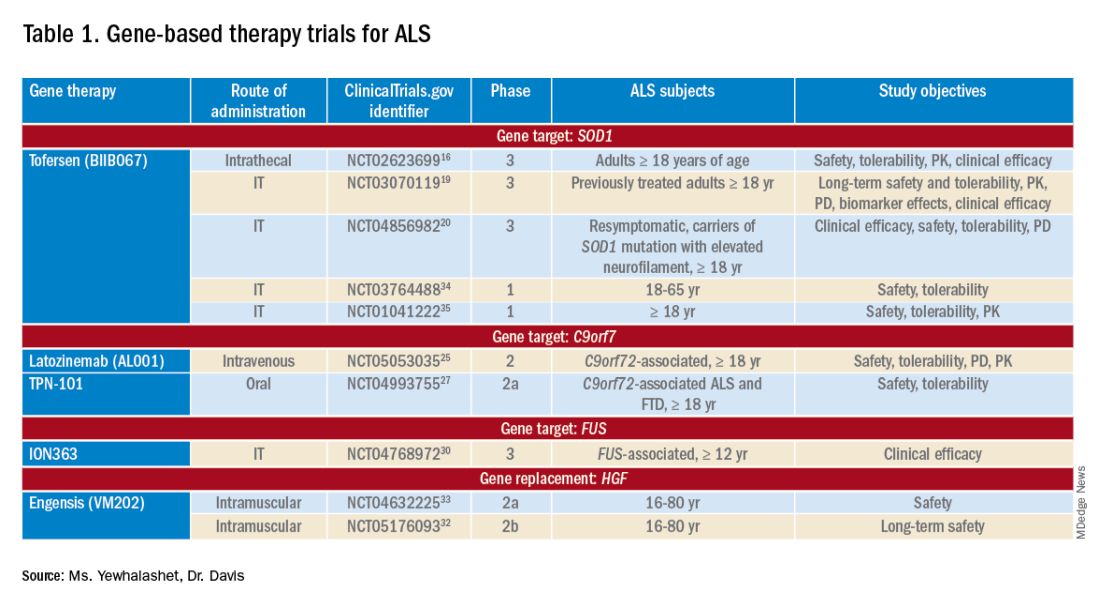
Tofersen, also known as BIIB067, is an investigational ASO, administered by intrathecal (IT) injection, that binds to SOD1 mRNA, thus reducing its protein levels.16 Tofersen was evaluated in the VALOR phase 3 study (ClinicalTrials.gov Identifier: NCT02623699), a three-part randomized, double-blind, placebo-controlled trial: single ascending dose (Part A), multiple ascending dose (B), and fixed dose (C).10 In Parts A and B, 48 participants received five IT injections of tofersen or placebo over 12 weeks and were followed for an additional 12 weeks. Reduction in SOD1 protein production and neurofilament level in cerebrospinal fluid (CSF) (a potential biomarker of motor-neuron degeneration) was observed, which determined the fixed-dose for Part C.16,17
Part C examined the efficacy, safety and tolerability, pharmacokinetics (PK), and pharmacodynamics (PD) of tofersen, compared with placebo, in adults with ALS who had a confirmed SOD1 mutation.17 A total of 108 participants were enrolled; 60 were identified as “faster-progressing”; 48, as “slower-progressing.”18 The primary endpoint of Part C was change from baseline to Week 28 on the Revised ALS Functional Rating Scale (ALSFRS-R) total score. (ALSFRS-R measures overall clinical effect; the score ranges from 0 [no function] to 4 [full function].17)
Tofersen failed to meet the primary efficacy outcome because statistically significant findings were lacking in the faster-progressing population, as measured by joint-rank analysis (difference of 1.2 on the ALSFRS-R score; P = .97). However, trends favoring tofersen were observed across key secondary clinical outcome measures18:
- Change from baseline in CSF SOD1 protein concentration.17 Percent reduction in the total SOD1 protein level was much higher in the tofersen-treated group than in the control group (38% more than controls in the faster-progressing population; 26% more than controls in the slower-progressing population).18
- Change from baseline in neurofilament light-chain concentration in plasma.17,18 Percent reduction in the level of neurofilament light chain was also observed to be higher in the tofersen-treated group than in the control group (67% more than controls in the faster-progressing population and 48% more than controls in the slower-progressing population).18
Because of these encouraging results, VALOR participants were moved to the ongoing open-label extension trial of tofersen (ClinicalTri-als.gov Identifier: NCT03070119), in which both groups were treated with the active agent.
These data suggest that early tofersen treatment might slow decline in faster-progressing patients and stabilize clinical function in slower-progressing patients.18,19 Overall, most adverse events (AEs) in the trial among patients receiving active treatment were of mild or moderate severity, and were largely consistent with either disease progression or lumbar puncture–related complications.18
Because data from VALOR suggested potential benefit from tofersen, the ATLAS trial (ClinicalTrials.gov Identifier: NCT04856982) is investigating the clinical value of presymptomatic treatment and the optimal timing of initiation of therapy.20,21 ATLAS is a phase 3, randomized, placebo-controlled trial that examines the clinical efficacy, safety, and tolerability of tofersen in presymptomatic adult carriers of SOD1 mutation who have an elevated neurofilament light-chain concentration.21 ATLAS will also evaluate the efficacy of tofersen when initiated before, rather than after, ALS manifests clinically. Enrollment is still open for this trial.20,21
Latozinemab, also known as AL001, is a first-in-class monoclonal antibody, administered by IV infusion, that elevates levels of progranulin, a key regulator of the immune activity and lysosomal function in the brain.22,23 Latozinemab limits progranulin endocytosis and degradation by sortilin inhibition.22 Progranulin gene mutations can reduce progranulin expression (by 50 to 70 percent reduction), which may cause neuro-degeneration due to abnormal accumulation of TAR-DNA-binding protein 43 (TDP-43) in the brain cells.22,24 TDP-43 pathology has also been shown to be associated with C9orf72 mutations.23 Although the mechanism is not fully understood, the role of progranulin deficiency in TDP-43 pathology is believed to be associated with neurodegenerative diseases like ALS.11,23,24,43 Previous animal models of chronic neurodegenera-tion have demonstrated how increased progranulin levels can be protective against TDP-43 pathology, increasing neuronal development and survival, thus potentially slowing disease progression.23,24,43 Currently, latozinemab is being investigated in a randomized, double-blind, placebo-controlled, multicenter phase 2 trial (ClinicalTrials.gov Identifier: NCT05053035). Approximately, 45 C90rf72-associated ALS participants (≥ 18 years of age) will receive latozinemab or placebo infusions every 4 weeks (for 24 weeks). Study endpoints include safety, tolerability, PK, PD, as well as plasma, and CSF progranulin levels.25 In previous studies, latozinemab demonstrated encouraging results in frontotemporal dementia (FTD) patients who carry a progranulin mutation. Because FTD was revealed to have significant genetic overlap with ALS, there is disease-modifying potential for latozinemab in ALS patients.23,24
TPN-101 is a nucleoside analog reverse transcriptase inhibitor, administered orally, that was originally developed for human immunodeficiency virus (HIV) treatment. However, due to recent findings suggesting retrotransposon activity contributing to neurodegeneration in TDP-43 mediated diseases, including ALS and FTD, TNP-101 is being repurposed.26 The safety and tolerability of TNP-101 are currently being evaluated in C9orf72-associated ALS and FTD patients (≥ 18 years of age). The study is a randomized, double-blind, placebo-controlled paral-lel-group phase 2a trial (ClinicalTrials.gov Identifier: NCT04993755) The study includes a screening period of 6 weeks, double-blind treatment period of 24 weeks, an open-label treatment period of 24 weeks, and 4 weeks of the post-treatment follow-up visit. Study endpoints include the incidence and severity of spontaneously reported treatment-emergent adverse events (TEAEs) associated with TNP-101 and placebo for a to-tal of 48 weeks.27
ION363 is an investigational ASO, administered by IT injection, that selectively targets one of the FUS mutations (p.P525L), which is responsible for earlier disease onset and rapid ALS progression.28,29 The clinical efficacy of ION363, specifically in clinical function and survival is being assessed in FUS-associated ALS patients (≥ 12 years of age). This randomized phase 3 study (ClinicalTrials.gov Identifier: NCT04768972) includes two parts; part 1 will consist of participants receiving a multi-dose regimen (1 dose every 4-12 weeks) of ION363 or placebo for 61 weeks followed by an open-label extension treatment period in part 2, which will consist of participants receiving ION363 (every 12 weeks) for 85 weeks. The primary endpoint of the study is the change from baseline to day 505 in functional impairment, using ALS Functional Rating Scale-Revised (ALSFRS-R). This measures functional disease severity, specifically in bulbar function, gross motor skills, fine motor skills, and respiratory. The score for all 12 questions can range from 0 (no function) to 4 (full function) with a total possible score of 48.30
Engensis, also known as VM202, is a non-viral gene therapy, administered by intramuscular (IM) injection, that uses a plasmid to deliver the hepatocyte growth factor (HGF) gene to promote HGF protein production. The HGF protein plays a role in angiogenesis, the previous of muscle atrophy, and the promotion of neuronal survival and growth. Based on preclinical studies, increasing HGF protein production has been shown to reduce neurodegeneration, thus potentially halting or slowing ALS progression.31 Currently, the safety of engensis is being evaluated in ALS patients (18-80 years of age) in the REViVALS phase 2a (ClinicalTrials.gov Identifier: NCT04632225)/2b (ClinicalTrial.gov Identifier: NCT05176093).32,33 The ReViVALS trial is a double-blind, randomized, placebo-controlled, multi-center study. The phase 2a study endpoints include the incidence of TEAEs, treatment-emergent serious adverse events (TESAEs), injection site reactions, and clinically significant labor-atory values post-treatment (engensis vs placebo group) for 180 days.33 A phase 2b study will evaluate the long-term safety of engensis for an additional 6 months. Study endpoints include the incidence of AEs, changes from baseline in ALSFRS-R scores to evaluate improvement in muscle function, changes from baseline in quality of life using the ALS patient assessment questionnaire, time to all-cause mortality compared to placebo, etc.32
Spinal muscular atrophy
SMA is a hereditary lower motor-neuron disease caused (in 95% of cases) by deletions or, less commonly, by mutations of the survival motor neuron 1 (SMN1) gene on chromosome 5q13 that encodes the SMN protein.6 Reduction in expression of the SMN protein causes motor neurons to degenerate.36-38 Because of a large inverted duplication in chromosome 5q, two variants of SMN (SMN1 and SMN2) exist on each allele. The paralog gene, SMN2, also produces the SMN protein – although at a lower level (10% to 20% of total SMN protein production) than SMN1 does.
A single nucleotide substitution in SMN2 alters splicing and suppresses transcription of exon 7, resulting in a shortened mRNA strand that yields a truncated SMN protein product.6,37,39 SMA is classified based on age of onset and maximum motor abilities achieved, ranging from the most severe (Type 0) to mildest (Type 4) disease.36,40 Because SMA patients lack functional SMN1 (due to polymorphisms), disease severity is determined by copy numbers of SMN2.6,39
Gene-based therapy for SMA
Three FDA-approved SMN treatments demonstrate clinically meaningful benefit in SMA: SMN2-targeting nusinersen [Spinraza] and risdiplam [Evrysdi], and SMN1-targeting onasemnogene abeparvovec-xioi [Zolgensma]38 Additional approaches to SMA treatment are through SMN-independent therapies, which target muscle and nerve function. Research has strongly suggested that combined SMA therapies, specifically approved SMN-targeted and investigational SMN-independent treatments, such as GYM329 (also known as RO7204239) may be the best strategy to treat all ages, stages, and types of SMA.41 (Table 226-41).
Agents that modulate SMN2. Nusinersen, approved by the FDA in 2016, was the first treatment indicated for all SMA types in pediatric and adult patients.42 The agent is an ASO that targets exon 7 of SMN2, thus stabilizing transcription. Inclusion of exon 7 increases SMN protein production, improving motor function.6,38 Nusinersen is a lifelong treatment that requires IT administration every 4 months because it cannot cross the blood-brain barrier.38,43
Pivotal clinical studies that led to approval of nusinersen include CHERISH (ClinicalTrial.gov Identifier: NCT02292537) and ENDEAR (ClinicalTrial.gov Identifier: NCT02193074) studies.
CHERISH was a phase 3, randomized, double-blind, sham procedure–controlled trial that examined the clinical efficacy and safety of nusinersen in 126 participants with later-onset SMA (2-12 years of age). The primary endpoint was the change from baseline using the Hammersmith Functional Motor Scale Expanded (HFMSE) at 15 months. HFMSE looks at 33 activities to assess improvement in motor function. The study met the primary efficacy outcome, demonstrating statistically significant (P = .0000001) improvement in overall motor function. The nusinersen group showed a 3.9-point increase in the HFMSE score from baseline, which indicates improvement, compared with a 1.0-point decline from baseline in the control group.46,47
ENDEAR was also a randomized, double-blind, sham procedure–controlled phase 3 trial, which investigated the efficacy and safety of nusinersen in 121 participants with early-onset SMA Type 1 (≤ 210 days of age). Coprimary endpoints were:
- Percentage of motor milestones responders, as determined using Section 2 of the Hammersmith Infant Neurological Examination–Part 2.
- Event-free survival (that is, avoidance of combined endpoint of death or permanent ventilation).
ENDEAR met the first primary efficacy outcome, demonstrating statistically significant (P < .0001) improvement in motor milestones (head control, rolling, independent sitting, and standing). By 13 months of age, approximately 51% of nusinersen-treated participants showed improvement, compared with none in the control group.46,47
The second primary endpoint was also met, with a statistically significant (P = .005) 47% decrease in mortality or permanent ventilation use.46-48
The NURTURE (ClinicalTrial.gov Identifier: NCT02386553) study is also investigating the efficacy and safety of nusinersen. An ongoing, open-label, supportive phase 2 trial, NURTURE is evaluating the efficacy and safety of multiple doses of nusinersen in 25 presymptomatic SMA patients (≤ 6 weeks of age). The primary endpoint of this study is time to death or respiratory intervention.49 Interim results demonstrate that 100% of presymptomatic infants are functioning without respiratory intervention after median follow-up of 2.9 years.46-48
Although nusinersen has been shown to be generally safe in clinical studies, development of lumbar puncture–related complications, as well as the need for sedation during IT administration, might affect treatment tolerability in some patients.39
Risdiplam was approved by the FDA in 2020 as the first orally administered small-molecule treatment of SMA (for patients ≤ 2 months of age).52 Risdiplam is a SMN2 splicing modifier, binding to the 5’ splice site of intron 7 and exonic splicing enhancer 2 in exon 7 of SMN2 pre-mRNA. This alternative splicing increases efficiency in SMN2 gene transcription, thus increasing SMN protein production in motor-neuron cells.36 An important advantage of risdiplam is the convenience of oral administration: A large percentage of SMA patients (that is, those with Type 2 disease) have severe scoliosis, which can further complicate therapy or deter patients from using a treatment that is administered through the IT route.40
FDA approval of risdiplam was based on clinical data from two pivotal studies, FIREFISH (ClinicalTrial.gov Identifier: NCT02913482) and SUNFISH (ClinicalTrial.gov Identifier: NCT02908685).53-54
FIREFISH is an open-label, phase 2/3 ongoing trial in infants (1-7 months of age) with SMA Type 1. The study comprises two parts; Part 1 determined the dose of risdiplam used in Part 2, which assessed the efficacy and safety of risdiplam for 24 months. The primary endpoint was the percentage of infants sitting without support for 5 seconds after 12 months of treatment using the gross motor scale of the Bayley Scales of Infant and Toddler Development–Third Edition. A statistically significant (P < .0001) therapeutic benefit was observed in motor milestones. Approximately 29% of infants achieved the motor milestone of independent sitting for 5 seconds, which had not been observed in the natural history of SMA.53-55
SUNFISH is an ongoing randomized, double-blind, placebo-controlled trial of risdiplam in adult and pediatric patients with SMA Types 2 and 3 (2-25 years old). This phase 2/3 study comprises two parts: Part 1 determined the dose (for 12 weeks) to be used for confirmatory Part 2 (for 12 to 24 months). The primary endpoint was the change from baseline on the 32-item Motor Function Measure at 12 months. The study met its primary endpoint, demonstrating statistically significant (P = .0156) improvement in motor function scores, with a 1.36-point increase in the risdiplam group, compared with a 0.19-point decrease in the control group.54,55
Ongoing risdiplam clinical trials also include JEWELFISH (ClinicalTrial.gov Identifier: NCT03032172) and RAINBOW (ClinicalTrial.gov Identifier: NCT03779334).56-57 JEWELFISH is an open-label, phase 2 trial assessing the safety of risdiplam in patients (6 months to 60 years old) who received prior treatment. The study has completed recruitment; results are pending.56 RAINBOW is an ongoing, open-label, single-arm, phase 2 trial, evaluating the clinical efficacy and safety of risdiplam in SMA-presymptomatic newborns (≤ 6 weeks old). The study is open for enrollment.57 Overall, interim results for JEWELFISH and RAINBOW appear promising.
In addition, combined SMA therapies, specifically risdiplam and GYM329 are currently being investigated to address the underlying cause and symptoms of SMA concurrently.58 GYM329, is an investigational anti-myostatin antibody, selectively binding preforms of myostatin - pro-myostatin and latent myostatin, thus improving muscle mass and strength for SMA patients.59 The safety and efficacy of GYM329 in combination with risdiplam is currently being investigated in 180 ambulant participants with SMA (2-10 years of age) in the MANATEE (ClinicalTrial.gov Identifier: NCT05115110) phase 2/3 trial. The MANATEE study is a two-part, seamless, randomized, placebo-controlled, double-blind trial. Part 1 will assess the safety of the combination treatment in approximately 36 participants; participants will receive both GYM329 (every 4 weeks) by subcutaneous (SC) injection into the abdomen and risdiplam (once per day) for 24 weeks followed by a 72-week open-label treatment period. 54,58 The outcome measures include the incidence of AEs, percentage change from baseline in the contractile area of skeletal muscle (in dominant thigh and calf), change from baseline in RHS total score, and incidence of change from baseline in serum concentration (total myostatin, free latent myostatin, and mature myostatin) etc.54 Part 2 will be conducted on 144 participants, specifically assessing the efficacy and safety of the optimal dose of GYM329 selected from Part 1 (combined with risdiplam) for 72 weeks. Once the treatment period is completed in either part, participants can partake in a 2-year open-label extension period.54,58 Other outcome measures include change from baseline in lean muscle mass (assessed by full body dual-energy X- ray absorptiometry (DXA) scan), in time taken to walk/run 10 meters (measured by RHS), in time taken to rise from the floor (measured by RHS), etc.54 Overall, this combination treatment has the potential to further improve SMA patient outcomes and will be further investigated in other patient populations (including non-ambulant patients and a broader age range) in the future.58
An agent that alters SMN1 expression. Onasemnogene abeparvovec-xioi, FDA approved in 2019, was the first gene-replacement therapy indicated for treating SMA in children ≤ 2 years old.60 Treatment utilizes an AAV vector type 9 (AAV9) to deliver a functional copy of SMN1 into target motor-neuron cells, thus increasing SMN protein production and improving motor function. This AAV serotype is ideal because it crosses the blood-brain barrier. Treatment is administered as a one-time IV fusion.38,39,43
FDA approval was based on the STR1VE (ClinicalTrial.gov Identifier: NCT03306277) phase 3 study and START (ClinicalTrial.gov Identifier: NCT02122952) phase 1 study.61,62 START was the first trial to investigate the safety and efficacy of onasemnogene abeparvovec-xioi in SMA Type 1 infants (< 6 months old). Results demonstrated remarkable clinical benefit, including 100% permanent ventilation-free survival and a 92% (11 of 12 patients) rate of improvement in motor function. Improvement in development milestones was also observed: 92% (11 of 12 patients) could sit without support for 5 seconds and 75% (9 of 12) could sit without support for 30 seconds.14,61,63
The efficacy of onasemnogene abeparvovec-xioi seen in STR1VE was consistent with what was observed in START. STRIVE, a phase 3 open-label, single-dose trial, examined treatment efficacy and safety in 22 symptomatic infants (< 6 months old) with SMA Type 1 (one or two SMN2 copies). The primary endpoint was 30 seconds of independent sitting and event-free survival. Patients were followed for as long as 18 months. Treatment showed statistically significant (P < .0001) improvement in motor milestone development and event-free survival, which had not been observed in SMA Type 1 historically. Approximately 59% (13 of 22 patients) could sit independently for 30 seconds at 18 months of age. At 14 months of age, 91% (20 of 22 patients) were alive and achieved independence from ventilatory support.34,35,53
Although many clinical studies suggest that onasemnogene abeparvovec-xioi can slow disease progression, the benefits and risks of long-term effects are still unknown. A 15-year observational study is investigating the long-term therapeutic effects and potential complications of onasemnogene abeparvovec-xioi. Participants in START were invited to enroll in this long-term follow-up study (ClinicalTrial.gov Identifier: NCT04042025).66-67
Duchenne muscular dystrophy
DMD is the most common muscular dystrophy of childhood. With an X-linked pattern of inheritance, DMD is seen mostly in young males (1 in every 3,500 male births).38,39,73 DMD is caused by mutation of the dystrophin encoding gene, or DMD, on the X chromosome. Deletion of one or more exons of DMD prevents production of the dystrophin protein, which leads to muscle degeneration.38,39,43 Common DMD deletion hotspots are exon 51 (20% of cases), exon 53 (13% of cases), exon 44 (11% of cases), and exon 45 (12% of cases).74 Nonsense mutations, which account for another 10% of DMD cases, occur when premature termination codons are found in the DMD gene. Those mutations yield truncated dystrophin protein products.39,66
Therapy for DMD
There are many therapeutic options for DMD, including deflazacort (Emflaza), FDA approved in 2017, which has been shown to reduce inflammation and immune system activity in DMD patients (≥ 5 years old). Deflazacort is a corticosteroid prodrug; its active metabolite acts on the glucocorticoid receptor to exert anti-inflammatory and immunosuppressive effects. Studies have shown that muscle strength scores over 6-12 months and average time to loss of ambulation numerically favored deflazacort over placebo.74,75
Gene-based therapy for DMD
Mutation-specific therapeutic approaches, such as exon skipping and nonsense suppression, have shown promise for the treatment of DMD (Table 358-79):
- ASO-mediated exon skipping allows one or more exons to be omitted from the mutated DMD mRNA.74,75 Effective FDA-approved ASOs include golodirsen [Vyondys 53], viltolarsen [Viltepso], and casimersen [Amondys 45].74
- An example of therapeutic suppression of nonsense mutations is ataluren [Translarna], an investigational agent that can promote premature termination codon read-through in DMD patients.66
Another potential treatment approach is through the use of AAV gene transfer to treat DMD. However, because DMD is too large for the AAV vector (packaging size, 5.0 kb), microdystrophin genes (3.5-4 kb, are used as an alternative to fit into a single AAV vector.39,76
Exon skipping targeting exon 51. Eteplirsen, approved in 2016, is indicated for the treatment of DMD patients with the confirmed DMD gene mutation that is amenable to exon 51 skipping. Eteplirsen binds to exon 51 of dystrophin pre-mRNA, causing it to be skipped, thus, restoring the reading frame in patients with DMD gene mutation amenable to exon 51 skipping. This exclusion promotes dystrophin production. Though the dystrophin protein is still functional, it is shortened.38,77 Treatment is administered IV, once a week (over 35-60 minutes). Eteplirsen’s accelerated approval was based on 3 clinical studies (ClinicalTrial.gov Identifier: NCT01396239, NCT01540409, and NCT00844597.) 78-81 The data demonstrated an increased expression of dystrophin in skeletal muscles in some DMD patients treated with eteplirsen. Though the clinical benefit of eteplirsen (including improved motor function) was not established, it was concluded by the FDA that the data were reasonably likely to predict clinical benefit. Continued approval for this indication may depend on the verification of a clinical benefit in confirmatory trials. Ongoing clinical trials include (ClinicalTrial.gov Identifier: NCT03992430 (MIS51ON), NCT03218995, and NCT03218995).77,81,82
Vesleteplirsen, is an investigational agent that is designed for DMD patients who are amendable to exon 51 skip-ping. The mechanism of action of vesleteplirsen appears to be similar to that of eteplirsen.83 The ongoing MOMENTUM (ClinicalTrial.gov Identifier: NCT04004065) phase 2 trial is assessing the safety and tolerability of vesleteplirsen at multiple-ascending dose levels (administered via IV infusion) in 60 participants (7-21 years of age). The study consists of two parts; participants receive escalating dose levels of vesleteplirsen (every 4 weeks) for 72 weeks during part A and participants receive the selected doses from part A (every 4 weeks) for 2 years during part B. Study endpoints include the number of AEs (up to 75 weeks) and the change from baseline to week 28 in dystrophin protein level. 84 Serious AEs of reversible hypomagnesemia were observed in part B, and as a result, the study protocol was amended to include magnesium supplementation and monitoring of magnesium levels.83
Exon skipping targeting exon 53. Golodirsen, FDA approved in 2019, is indicated for the treatment of DMD in patients who have a confirmed DMD mutation that is amenable to exon 53 skipping. The mechanism of action is similar to eteplirsen, however, golodirsen is designed to bind to exon 53.38,39 Treatment is administered by IV infusion over 35-60 minutes.
Approval of golodirsen was based primarily on a two-part, phase 1/2 clinical trial (ClinicalTrial.gov Identifier: NCT02310906). Part 1 was a randomized, placebo-controlled, dose-titration study that assessed multiple-dose efficacy in 12 DMD male patients, 6 to 15 years old, with deletions that were amenable to exon 53 skipping.
Part 2 was an open-label trial in 12 DMD patients from Part 1 of the trial plus 13 newly enrolled male DMD patients who were also amenable to exon 53 skipping and who had not already received treatment. Primary endpoints were change from baseline in total distance walked during the 6-minute walk test at Week 144 and dystrophin protein levels (measured by western blot testing) at Week 48. A statistically significant increase in the mean dystrophin level was observed, from a baseline 0.10% mean dystrophin level to a 1.02% mean dystrophin level after 48 weeks of treatment (P < .001). Common reported adverse events associated with golodirsen were headache, fever, abdominal pain, rash, and dermatitis. Renal toxicity was observed in preclinical studies of golodirsen but not in clinical studies.80,85
Viltolarsen, approved in 2020, is also indicated for the treatment of DMD in patients with deletions amenable to exon 53 skipping. The mechanism of action and administration (IV infusion over 60 minutes) are similar to that of golodirsen.
Approval of viltolarsen was based on two phase 2 clinical trials (ClinicalTrial.gov Identifier: NCT02740972 and NCT03167255) in a total of 32 patients. NCT02740972 was a randomized, double-blind, placebo-controlled, dose-finding study that evaluated the clinical efficacy of viltolarsen in 16 male DMD patients (4-9 years old) for 24 weeks.
NCT03167255 was an open-label study that evaluated the safety and tolerability of viltolarsen in DMD male patients (5-18 years old) for 192 weeks. The efficacy endpoint was the change in dystrophin production from baseline after 24 weeks of treatment. A statistically significant increase in the mean dystrophin level was observed, from a 0.6% mean dystrophin level at baseline to a 5.9% mean dystrophin level at Week 25 (P = .01). The most common adverse events observed were upper respiratory tract infection, cough, fever, and injection-site reaction.86-87
Exon skipping targeting exon 45. Casimersen was approved in 2021 for the treatment of DMD in patients with deletions amenable to exon 45 skipping.88 Treatment is administered by IV infusion over 30-60 minutes. Approval was based on an increase in dystrophin production in skeletal muscle in treated patients. Clinical benefit was reported in interim results from the ESSENCE (ClinicalTrial.gov Identifier: NCT02500381) study, an ongoing double-blind, placebo-controlled phase 3 trial that is evaluating the efficacy of casimersen, compared with placebo, in male participants (6-13 years old) for 48 weeks. Efficacy is based on the change from baseline dystrophin intensity level, determined by immunohistochemistry, at Week 48.
Interim results from ESSENCE show a statistically significant increase in dystrophin production in the casimersen group, from a 0.9% mean dystrophin level at baseline to a 1.7% mean dystrophin level at Week 48 (P = .004); in the control group, a 0.54% mean dystrophin level at baseline increased to a 0.76% mean dystrophin level at Week 48 (P = .09). Common adverse events have included respiratory tract infection, headache, arthralgia, fever, and oropharyngeal pain. Renal toxicity was observed in preclinical data but not in clinical studies.60,84
Targeting nonsense mutations. Ataluren is an investigational, orally administered nonsense mutation suppression therapy (through the read-through of stop codons).37 Early clinical evidence supporting the use of ataluren in DMD was seen in an open-label, dose-ranging, phase 2a study (ClinicalTrial.gov Identifier: NCT00264888) in male DMD patients (≥ 5 years old) caused by nonsense mutation. The study demonstrated a modest (61% ) increase in dystrophin expression in 23 of 38 patients after 28 days of treatment.37,91,92
However, a phase 2b randomized, double-blind, placebo-controlled trial (ClinicalTrial.gov Identifier: NCT00592553) and a subsequent confirmatory ACT DMD phase 3 study (ClinicalTrial.gov Identifier: NCT01826487) did not meet their primary endpoint of improvement in ambulation after 48 weeks as measured by the 6-minute walk test.37,93,94 In ACT DMD, approximately 74% of the ataluren group did not experience disease progression, compared with 56% of the control group (P = 0386), measured by a change in the 6-minute walk test, which assessed ambulatory decline.37,95
Based on limited data showing that ataluren is effective and well tolerated, the European Medicines Agency has given conditional approval for clinical use of the drug in Europe. However, ataluren was rejected by the FDA as a candidate therapy for DMD in the United States.22 Late-stage clinical studies of ataluren are ongoing in the United States.
AAV gene transfer with microdystrophin. Limitations on traditional gene-replacement therapy prompted exploration of gene-editing strategies for treating DMD, including using AAV-based vectors to transfer microdystrophin, an engineered version of DMD, into target muscles.43 The microdystrophin gene is designed to produce a functional, truncated form of dystrophin, thus improving muscular function.
There are 3 ongoing investigational microdystrophin gene therapies that are in clinical development (ClinicalTrial.gov Identifier: NCT03368742 (IGNITE DMD), NCT04281485 (CIFFREO), and NCT05096221 (EMBARK)).38,82
IGNITE DMD is a phase 1/2 randomized, controlled, single-ascending dose trial evaluating the safety and efficacy of a SGT-001, single IV infusion of AAV9 vector containing a microdystrophin construct in DMD patients (4-17 years old) for 12 months. At the conclusion of the trial, treatment and control groups will be followed for 5 years. The primary efficacy endpoint is the change from baseline in microdystrophin protein production in muscle-biopsy material, using western blot testing.96 Long-term interim data on biopsy findings from three patients demonstrated clinical evidence of durable microdystrophin protein expression after 2 years of treatment.96,97
The CIFFREO trial will assess the safety and efficacy of the PF-06939926 microdystrophin gene therapy, an investigational AAV9 containing microdystrophin, in approximately 99 ambulatory DMD patients (4-7 years of age). The study is a randomized, double-blind, placebo-controlled, multicenter phase 3 trial. The primary efficacy end-point is the change from baseline in the North Star Ambulatory Assessment (NSAA), which measures gross motor function. This will be assessed at 52 weeks; all study participants will be followed for a total of 5 years post-treatment.98,99,100 Due to unexpected patient death (in a non-ambulatory cohort) in the phase 1b (in a non-ambulatory cohort) in the phase 1b (ClinicalTrial.gov Identifier: (NCT03362502) trial, microdystrophin gene therapy was immediately placed on clinical hold.101,102 The amended study protocol required that all participants undergo one week of in-hospital observation after receiving treatment.102
The EMBARK study is a global, randomized, double-blind, placebo-controlled, phase 3 trial that is evaluating the safety and efficacy of SRP-9001, which is a rAAVrh74.MHCK7.microdystrophin gene therapy. The AAV vector (rAAVrh74) contains the microdystrophin construct, driven by the skeletal and cardiac muscle–specific promoter, MHCK7.98,99 In the EMBARK study, approximately 120 participants with DMD (4-7 years of age) will be enrolled. The primary efficacy endpoint includes the change from baseline to week 52 in the NSAA total score.99 Based on SRP-9001, data demonstrating consistent statistically significant functional improvements in NSAA total scores and timed function tests (after one-year post- treatment) in DMD patients from previous studies and an integrated analysis from multiple studies (ClinicalTrial.gov Identifier: NCT03375164, NCT03769116, and NCT04626674), the ongoing EMBARK has great promise.103,104
Challenges ahead, but advancements realized
Novel gene-based therapies show significant potential for transforming the treatment of NMDs. The complex pathologies of NMDs have been a huge challenge to disease management in an area once considered unremediable by gene-based therapy. However, advancements in precision medicine – specifically, gene-delivery systems (for example, AAV9 and AAVrh74 vectors) combined with gene modification strategies (ASOs and AAV-mediated silencing) – have the potential to, first, revolutionize standards of care for sporadic and inherited NMDs and, second, significantly reduce disease burden.6
What will be determined to be the “best” therapeutic approach will, likely, vary from NMD to NMD; further investigation is required to determine which agents offer optimal clinical efficacy and safety profiles.43 Furthermore, the key to therapeutic success will continue to be early detection and diagnosis – first, by better understanding disease pathology and drug targets and, second, by validation of reliable biomarkers that are predictive of therapeutic benefit.4,5
To sum up, development challenges remain, but therapeutic approaches to ALS, SMA, and DMD that utilize novel gene-delivery and gene-manipulation tools show great promise.
Ms. Yewhalashet is a student in the masters of business and science program, with a concentration in healthcare economics, at Keck Graduate Institute Henry E. Riggs School of Applied Life Sciences, Claremont, Calif. Dr. Davis is professor of practice in clinical and regulatory affairs, Keck Graduate Institute Henry E. Riggs School of Applied Life Sciences.
References
1. Aitken M et al. Understanding neuromuscular disease care. IQVIA [Internet]. Oct 30, 2018. Accessed Mar 1, 2022. https://www.iqvia.com/insights/the-iqvia-institute/reports/understanding-neuromuscular-disease-care.
2. National Institute of Neurological Disorders and Stroke. Neurological diagnostic tests and procedures fact sheet. Updated Nov 15, 2021. Ac-cessed Mar 1, 2022. http://www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Fact-Sheets/Neurological-Diagnostic-Tests-and-Procedures-Fact.
3. Deenen JCW et al. The epidemiology of neuromuscular disorders: A comprehensive overview of the literature. J Neuromuscul Dis. 2015;2(1):73-85.
4. Cavazzoni P. The path forward: Advancing treatments and cures for neurodegenerative diseases. U.S. Food and Drug Administration. Jul 29, 2021. Accessed Mar 1, 2022. http://www.fda.gov/news-events/congressional-testimony/path-forward-advancing-treatments-and-cures-neurodegenerative-diseases-07292021.
5. Martier R, Konstantinova P. Gene therapy for neurodegenerative diseases: Slowing down the ticking clock. Front Neurosci. 2020 Sep 18;14:580179. doi: 10.3389/fnins.2020.580179.
6. Sun J, Roy S. Gene-based therapies for neurodegenerative diseases. Nat Neurosci. 2021 Mar;24(3):297-311. doi:10.1038/s41593-020-00778-1.
7. Amado DA, Davidson BL. Gene therapy for ALS: A review. Mol Ther. 2021 Dec 1;29(12):3345-58. doi:10.1016/j.ymthe.2021.04.008.
8. Yun Y, Ha Y. CRISPR/Cas9-mediated gene correction to understand ALS. Int J Mol Sci. 2020;21(11):3801. doi:10.3390/ijms21113801.
9. National Institute of Neurological Disorders and Stroke. Amyotrophic lateral sclerosis (ALS) fact sheet. Updated Nov 15, 2021. Accessed Mar 1, 2022. http://www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Fact-Sheets/Amyotrophic-Lateral-Sclerosis-ALS-Fact-Sheet.
10. Cappella M et al. Gene therapy for ALS – A perspective. Int J Mol Sci. 2019;20(18):4388. doi:10.3390/ijms20184388.
11. Abramzon YA, Fratta P, Traynor BJ, Chia R. The Overlapping Genetics of Amyotrophic Lateral Sclerosis and Frontotemporal Dementia. Front Neurosci. 2020;14. Accessed August 18, 2022. https://www.frontiersin.org/articles/10.3389/fnins.2020.00042
12. Giannini M, Bayona-Feliu A, Sproviero D, Barroso SI, Cereda C, Aguilera A. TDP-43 mutations link Amyotrophic Lateral Sclerosis with R-loop homeostasis and R loop-mediated DNA damage. PLOS Genet. 2020;16(12):e1009260. doi:10.1371/journal.pgen.1009260
13. FDA-approved drugs for treating ALS. The ALS Association [Internet]. Accessed Mar 1, 2022. http://www.als.org/navigating-als/living-with-als/fda-approved-drugs.
14. Jensen TL et al. Current and future prospects for gene therapy for rare genetic diseases affecting the brain and spinal cord. Front Mol Neurosci. 2021 Oct 6;14:695937. doi:10.3389/fnmol.2021.695937.
15. ALS Gene Targeted Therapies. The ALS Association. Accessed August 22, 2022. https://www.als.org/understanding-als/who-gets-als/genetic-testing/als-gene-targeted-therapies
16. Tofersen for ALS clears phase 1/2 trial, now in phase 3. Advances in Motion. Massachusetts General Hospital [Internet]. Sep 30, 2020. Accessed Mar 1, 2022. https://advances.massgeneral.org/neuro/journal.aspx?id=1699.17. Biogen. A study to evaluate the efficacy, safety, tol-erability, pharmacokinetics, and pharmacodynamics of BIIB067 administered to adult subjects with amyotrophic lateral sclerosis and confirmed superoxide dismutase 1 mutation. ClinicalTrials.gov Identifier: NCT02623699. Updated Jul 25, 2021. Accessed Feb 17, 2022. https://clinicaltrials.gov/ct2/show/NCT02623699.
18. Biogen. Biogen announces topline results from the tofersen phase 3 study and its open-label Extension in SOD1-ALS. Press release. Oct 17, 2021. Accessed Mar 1, 2022. https://investors.biogen.com/news-releases/news-release-details/biogen-announces-topline-results-tofersen-phase-3-study-and-its.
19. Biogen. An extension study to assess the long-term safety, tolerability, pharmacokinetics, and effect on disease progression of BIIB067 ad-ministered to previously treated adults with amyotrophic lateral sclerosis caused by superoxide dismutase 1 mutation. ClinicalTrials.gov Identi-fier: NCT03070119. Updated Sep 10, 2021. Accessed Feb 17, 2022. https://clinicaltrials.gov/ct2/show/NCT03070119.
20. MS MW. #AANAM – ATLAS Trial to Assess Tofersen in Presymptomatic SOD1 ALS. Accessed February 19, 2022. https://alsnewstoday.com/news-posts/2021/04/23/aanam-atlas-clinical-trial- tofersen-presymptomatic-sod1-als-patients/
21.Biogen. A phase 3 randomized, placebo-controlled trial with a longitudinal natural history run-in and open-label extension to evaluate BIIB067 initiated in clinically presymptomatic adults with a confirmed superoxide dismutase 1 mutation. ClinicalTrials.gov Identifier: NCT04856982. Updated Feb 18, 2022. Accessed Mar 1, 2022. https://clinicaltrials.gov/ct2/show/NCT04856982.
22. Latozinemab | ALZFORUM. Accessed August 19, 2022. https://www.alzforum.org/therapeutics/latozinemab
23. Alector Presents AL001 (latozinemab) Data from the FTD-C9orf72 Cohort of the INFRONT-2 Phase 2 Clinical Trial | Alector. Accessed August 18, 2022. https://investors.alector.com/news- releas-es/news-release-details/alector-presents-al001-latozinemab-data-ftd-c9orf72-cohort/
24. Alector Announces First Participant Dosed in Phase 2 Study Evaluating AL001 in Amyotrophic Lateral Sclerosis (ALS) | Alector. Accessed August 18, 2022. https://investors.alector.com/news- releases/news-release-details/lector-announces-first-participant-dosed-phase-2-study-0/ 25. A Phase 2 Study to Evaluate AL001 in C9orf72-Associated ALS - Full Text View - ClinicalTrials.gov. Accessed August 19, 2022. https://clinicaltrials.gov/ct2/show/NCT05053035
26.TPN-101 | ALZFORUM. Accessed August 19, 2022. https://www.alzforum.org/therapeutics/tpn- 101
27. Transposon Therapeutics, Inc. A Phase 2a Study of TPN-101 in Patients With Amyotrophic Lateral Sclerosis (ALS) and/or Frontotemporal Dementia (FTD) Associated With Hexanucleotide Repeat Expansion in the C9orf72 Gene (C9ORF72 ALS/FTD). clinicaltrials.gov; 2022. Ac-cessed August 17, 2022. https://clinicaltrials.gov/ct2/show/NCT04993755
28. Kerk SY, Bai Y, Smith J, et al. Homozygous ALS-linked FUS P525L mutations cell- autonomously perturb transcriptome profile and chem-oreceptor signaling in human iPSC microglia. Stem Cell Rep. 2022;17(3):678-692. doi:10.1016/j.stemcr.2022.01.004
29. ION363 | ALZFORUM. Accessed August 19, 2022. https://www.alzforum.org/therapeutics/ion363 30. Ionis Pharmaceuticals, Inc. A Phase 1-3 Study to Evaluate the Efficacy, Safety, Pharmacokinetics and Pharmacodynamics of Intrathecally Administered ION363 in Amyo-trophic Lateral Sclerosis Patients With Fused in Sarcoma Mutations (FUS-ALS). clinicaltrials.gov; 2022. Accessed August 18, 2022. https://clinicaltrials.gov/ct2/show/NCT04768972
31. PhD LF. Engensis (VM202) - ALS News Today. Accessed August 19, 2022. https://alsnewstoday.com/vm202/
32. Helixmith Co., Ltd. A 6-Month Extension Study Following Protocol VMALS-002-2 (A Phase 2a, Double-Blind, Randomized, Place-bo-Controlled, Multicenter Study to Assess the Safety of Engensis in Participants With Amyotrophic Lateral Sclerosis). clinicaltrials.gov; 2022. Accessed August 18, 2022. https://clinicaltrials.gov/ct2/show/NCT05176093 33. Safety of Engensis in Participants With Amyotrophic Lateral Sclerosis - Full Text View - ClinicalTrials.gov. Accessed August 19, 2022. https://clinicaltrials.gov/ct2/show/NCT04632225
34. Biogen. A phase 1, safety, tolerability, and distribution study of a microdose of radiolabeled BIIB067 co-administered with BIIB067 to healthy adults. ClinicalTrials.gov Identifier: NCT03764488. Updated Jul 19, 2021. Accessed Mar 1, 2022. https://clinicaltrials.gov/ct2/show/NCT03764488.
35. Ionis Pharmaceuticals Inc. A phase 1, double-blind, placebo-controlled, dose-escalation study of the safety, tolerability, and pharmacokinet-ics of ISIS 333611 administered intrathecally to patients with familial amyotrophic lateral sclerosis due to superoxide dismutase 1 gene muta-tions. ClinicalTrials.gov Identifier: NCT01041222. Updated Apr 13, 2012. Accessed Mar 1, 2022. https://clinicaltrials.gov/ct2/show/NCT01041222.
36. Messina S, Sframeli M. New treatments in spinal muscular atrophy: Positive results and new challenges. J Clin Med. 2020;9(7):2222. doi:10.3390/jcm9072222.
37. Scoto M et al. Genetic therapies for inherited neuromuscular disorders. Lancet Child Adolesc Health. 2018 Aug;2(8):600-9. doi:10.1016/S2352-4642(18)30140-8.
38. Abreu NJ, Waldrop MA. Overview of gene therapy in spinal muscular atrophy and Duchenne muscular dystrophy. Pediatr Pulmonol. 2021 Apr;56(4):710-20. doi:10.1002/ppul.25055.
39. Brandsema J, Cappa R. Genetically targeted therapies for inherited neuromuscular disorders. Practical Neurology [Internet]. Jul/Aug 2021:69-73. Accessed Mar 1, 2022. https://practicalneurology.com/articles/2021-july-aug/genetically-targeted-therapies-for-inherited-neuromuscular-disorders/pdf.
40. Ojala KS et al. In search of a cure: The development of therapeutics to alter the progression of spinal muscular atrophy. Brain Sci. 2021;11(2):194. doi:10.3390/brainsci11020194.
41. McCall S. Cure SMA Releases Updated Drug Pipeline. Cure SMA. Published December 13, 2021. Accessed August 21, 2022. https://www.curesma.org/cure-sma-releases-updated-drug-pipeline- 2021/ 42. FDA approves first drug for spinal muscular atrophy. U.S. Food and Drug Administration. News release. Dec 23, 2016. Accessed Mar 1, 2022. http://www.fda.gov/news-events/press-announcements/fda-approves-first-drug-spinal-muscular-atrophy.43. Kirschner J. Postnatal gene therapy for neuromuscular diseases – Opportunities and limitations. J Perinat Med. 2021 Sep;49(8):1011-5. doi:10.1515/jpm-2021-0435.
43. Terryn J, Verfaillie CM, Van Damme P. Tweaking Progranulin Expression: Therapeutic Avenues and Opportunities. Front Mol Neurosci. 2021;14. Accessed September 4, 2022. https://www.frontiersin.org/articles/10.3389/fnmol.2021.71303144.
44. Biogen. A phase 3, randomized, double-blind, sham-procedure controlled study to assess the clinical efficacy and safety of ISIS 396443 administered intrathecally in patients with later-onset spinal muscular atrophy. ClinicalTrials.gov Identifier: NCT02292537. Updated Feb 17, 2021. Accessed Mar 1, 2022. https://clinicaltrials.gov/ct2/show/study/NCT02292537.
45. Why Spinraza/later-onset studies. SPINRAZA® (nusinersen) [Internet]. Accessed Mar 1, 2022. www.spinraza.com/en_us/home/why-spinraza/later-onset-studies.html#scroll-tabs.
46. Biogen. A Phase 3, Randomized, Double-Blind, Sham-Procedure Controlled Study to Assess the Clinical Efficacy and Safety of ISIS 396443 Administered Intrathecally in Patients With Infantile- Onset Spinal Muscular Atrophy. clinicaltrials.gov; 2021. Accessed February 10, 2022. https://clinicaltrials.gov/ct2/show/results/NCT02193074
47. Early-onset SMA (Type 1) | SPINRAZA® (nusinersen). Accessed Mar 1, 2022. https://www.spinraza-hcp.com/en_us/home/why-spinraza/about-spinraza.html.
48. Finkel RS et al; ENDEAR Study Group. Nusinersen versus sham control in infantile-onset spinal muscular atrophy. N Engl J Med. 2017;377(18):1723-32. doi: 10.1056/NEJMoa1702752.
49. Biogen. An open-label study to assess the efficacy, safety, tolerability, and pharmacokinetics of multiple doses of ISIS 396443 delivered intrathecally to subjects with genetically diagnosed and presymptomatic spinal muscular atrophy. ClinicalTrials.gov Identifier: NCT02386553. Updated Nov 18, 2021. Accessed Mar 1, 2022. https://clinicaltrials.gov/ct2/show/NCT02386553.
50. De Vivo DC et al; NURTURE Study Group. Nusinersen initiated in infants during the presymptomatic stage of spinal muscular atrophy: In-terim efficacy and safety results from the phase 2 NURTURE study. Neuromuscul Disord. 2019 Nov;29(11):842-56. doi:10.1016/j.nmd.2019.09.007.
51. Why Spinraza/presymptomatic study. SPINRAZA® (nusinersen) [Internet]. Accessed Feb 22, 2022. www.spinraza.com/en_us/home/why-spinraza/presymptomatic-study.html#scroll-tabs.
52. FDA approves oral treatment for spinal muscular atrophy. U.S. Food and Drug Administration. News release. Aug 7, 2020. Accessed Mar 1, 2022. http://www.fda.gov/news-events/press-announcements/fda-approves-oral-treatment-spinal-muscular-atrophy.
53. Hoffmann-La Roche. A two-part seamless, open-label, multicenter study to investigate the safety, tolerability, pharmacokinetics, pharmaco-dynamics and efficacy of risdiplam (RO7034067) in infants with type 1 spinal muscular atrophy. ClinicalTrials.gov Identifier: NCT02913482. Updated Jan 21, 2022. Accessed Mar 1, 2022. https://clinicaltrials.gov/ct2/show/NCT02913482.
54. Hoffmann-La Roche. A two-part seamless, multi-center randomized, placebo-controlled, double-blind study to investigate the safety, tolera-bility, pharmacokinetics, pharmacodynamics and efficacy of risdiplam (RO7034067) in type 2 and 3 spinal muscular atrophy patients. Clinical-Trials.gov Identifier: NCT02908685. Updated Dec 28, 2021. Accessed Mar 1, 2022. https://clinicaltrials.gov/ct2/show/NCT02908685.
55. Genentech. Genentech’s risdiplam shows significant improvement in survival and motor milestones in infants with type 1 spinal muscular atrophy (SMA). Press release. Apr 27, 2020. Accessed Mar 1, 2022. http://www.gene.com/media/press-releases/14847/2020-04-27/genentechs-risdiplam-shows-significant-i
56. Hoffmann-La Roche. An open-label study to investigate the safety, tolerability, and pharmacokinetics/pharmacodynamics of risdiplam (RO7034067) in adult and pediatric patients with spinal muscular atrophy. ClinicalTrials.gov Identifier: NCT03032172. Updated Jan 27, 2022. Accessed Mar 1, 2022. https://clinicaltrials.gov/ct2/show/NCT03032172.
57. Hoffmann-La Roche. An open-label study of risdiplam in infants with genetically diagnosed and presymptomatic spinal muscular atrophy. ClinicalTrials.gov Identifier: NCT03779334. Updated Jan 27, 2022. Accessed Mar 1, 2022. https://clinicaltrials.gov/ct2/show/NCT03779334.
58. McCall S. Update on Genentech/Roche Initiation of MANATEE Clinical Study. Cure SMA. Published October 20, 2021. Accessed August 20, 2022. https://www.curesma.org/update-on- genentech-roche-initiation-of-manatee-clinical-study/
59. Abati E, Manini A, Comi GP, Corti S. Inhibition of myostatin and related signaling pathways for the treatment of muscle atrophy in motor neuron diseases. Cell Mol Life Sci. 2022;79(7):374. doi:10.1007/s00018-022-04408-w
60. FDA approves innovative gene therapy to treat pediatric patients with spinal muscular atrophy, a rare disease and leading genetic cause of infant mortality. U.S. Food and Drug Administration. News release. May 24, 2019. Accessed Mar 1, 2022. http://www.fda.gov/news-events/press-announcements/fda-approves-innovative-gene-therapy-treat-pediatric-patients-spinal-muscular-atrophy-rare-disease.
61. Novartis Gene Therapies. Phase I gene transfer clinical trial for spinal muscular atrophy type 1 delivering AVXS-101. ClinicalTrials.gov Identifier: NCT02122952. Updated Jun 14, 2021. Accessed Mar 1, 2022. https://clinicaltrials.gov/ct2/show/NCT02122952.
62. Novartis Gene Therapies. Phase 3, open-label, single-arm, single-dose gene replacement therapy clinical trial for patients with spinal mus-cular atrophy type 1 with one or two SMN2 copies delivering AVXS-101 by intravenous infusion. ClinicalTrials.gov Identifier: NCT03306277. Updated Jun 14, 2021. Accessed Feb 21, 2022. https://clinicaltrials.gov/ct2/show/NCT03306277.
63. Mendell JR et al. Single-dose gene-replacement therapy for spinal muscular atrophy. N Engl J Med. 2017;377(18):1713-22. doi:10.1056/NEJMoa1706198.
64. Symptomatic study results. ZOLGENSMA [Internet]. Updated Nov 2021. Accessed Mar 1, 2022. Error! Hyperlink reference not valid..
65. Novartis Gene Therapies. A global study of a single, one-time dose of AVXS-101 delivered to infants with genetically diagnosed and pre-symptomatic spinal muscular atrophy with multiple copies of SMN2. ClinicalTrials.gov Identifier: NCT03505099. Updated Jan 1, 2022. Ac-cessed Mar 1, 2022. https://clinicaltrials.gov/ct2/show/NCT03505099.
66. Chiu W et al. Current genetics and potential gene-targeting therapeutics for neuromuscular diseases. Int J Mol Sci. 2020 Dec;21(24):9589. doi:10.3390/ijms21249589.
67. Novartis Gene Therapies. A long-term follow-up study of patients in the clinical trials for spinal muscular atrophy receiving AVXS-101. Clini-calTrials.gov Identifier: NCT04042025. Updated Jun 9, 2021. Accessed Mar 1, 2022. https://clinicaltrials.gov/ct2/show/NCT04042025.
68. Novartis Gene Therapies. Phase 3, open-label, single-arm, single-dose gene replacement therapy clinical trial for patients with spinal mus-cular atrophy type 1 with one or two SMN2 copies delivering AVXS-101 by intravenous infusion. ClinicalTrials.gov Identifier: NCT0383718. Up-dated Jan 11, 2022. Accessed Mar 1, 2022. https://clinicaltrials.gov/ct2/show/NCT03837184.
69. Biogen. An open-label, dose escalation study to assess the safety, tolerability and dose-range finding of multiple doses of ISIS 396443 de-livered intrathecally to patients with spinal muscular atrophy. ClinicalTrials.gov Identifier: NCT01703988. Updated Apr 13, 2021. Accessed Mar 1, 2022. https://clinicaltrials.gov/ct2/show/NCT01703988.
70. Biogen. A study to assess the efficacy, safety, tolerability, and pharmacokinetics of multiple doses of ISIS 396443 delivered intrathecally to patients with infantile-onset spinal muscular atrophy. ClinicalTrials.gov Identifier: NCT01839656. Updated Feb 17, 2021. Accessed Mar 1, 2022. https://clinicaltrials.gov/ct2/show/NCT01839656.
71. Biogen. An open-label extension study for patients with spinal muscular atrophy who previously participated in investigational studies of ISIS 396443. ClinicalTrials.gov Identifier: NCT02594124. Updated Nov 15, 2021. Accessed Mar 1, 2022. https://clinicaltrials.gov/ct2/show/NCT02594124.
72. Biogen. Escalating dose and randomized, controlled study of nusinersen (BIIB058) in participants with spinal muscular atrophy. ClinicalTri-als.gov Identifier: NCT04089566. Updated Feb 24, 2022. Accessed Mar 1, 2022. https://clinicaltrials.gov/ct2/show/NCT04089566.
73. National Center for Advancing Translational Sciences. Duchenne muscular dystrophy. Genetic and Rare Diseases Information Center. Up-dated Nov 2, 2020. Accessed Mar 1, 2022. https://rarediseases.info.nih.gov/diseases/6291/duchenne-muscular-dystrophy.
74. Matsuo M. Antisense oligonucleotide-mediated exon-skipping therapies: Precision medicine spreading from Duchenne muscular dystrophy. JMA J. 2021 Jul 15;4(3):232-40. doi:10.31662/jmaj.2021-0019.
75. FDA approves drug to treat Duchenne muscular dystrophy. U.S. Food and Drug Administration. News release. Feb 9, 2017. Accessed Mar 1, 2022. http://www.fda.gov/news-events/press-announcements/fda-approves-drug-treat-duchenne-muscular-dystrophy.74.
76. Duan D. Dystrophin gene replacement and gene repair therapy for Duchenne muscular dystrophy in 2016: An interview. Hum Gene Ther Clin Dev. 2016 Mar;27(1):9-18. doi:10.1089/humc.2016.001.
77. EXONDYS 51®. Parent Project Muscular Dystrophy. Accessed August 21, 2022. https://www.parentprojectmd.org/drug-development-pipeline/exondys-51/
78. Sarepta Therapeutics, Inc. A Randomized, Double-Blind, Placebo-Controlled, Multiple Dose Efficacy, Safety, Tolerability and Pharmacoki-netics Study of AVI-4658(Eteplirsen),in the Treatment of Ambulant Subjects With Duchenne Muscular Dystrophy. clinicaltrials.gov; 2020. Ac-cessed August 18, 2022. https://clinicaltrials.gov/ct2/show/NCT01396239
79. Sarepta Therapeutics, Inc. Clinical Study to Assess the Safety Fo AVI-4658 in Subjects With Duchenne Muscular Dystrophy Due to a Frame-Shift Mutation Amenable to Correction by Skipping Exon 51. clinicaltrials.gov; 2015. Accessed August 18, 2022. https://clinicaltrials.gov/ct2/show/study/NCT00844597
80. Sarepta Therapeutics, Inc. A 2-part, randomized, double-blind, placebo-controlled, dose-titration, safety, tolerability, and pharmacokinetics study (Part 1) followed by an open-label efficacy and safety evaluation (Part 2) of SRP-4053 in patients with Duchenne muscular dystrophy amenable to exon 53 skipping. ClinicalTrials.gov Identifier: NCT02310906. Updated Oct 19, 2020. Accessed Mar 1, 2022. https://clinicaltrials.gov/ct2/show/results/NCT02310906.
81. Commissioner O of the. FDA grants accelerated approval to first drug for Duchenne muscular dystrophy. FDA. Published March 24, 2020. Accessed August 21, 2022. hDuchenne Muscular Dystrophy Amenable to Exon 51-Skipping Treatment. clinicaltrials.gov; 2022. Accessed Au-gust 18, 2022. https://clinicaltrials.gov/ct2/show/NCT04004065
109. National Center of Neurology and Psychiatry, Japan. Exploratory study of NS-065/NCNP-01 in Duchenne muscular dystrophy. ClinicalTri-als.gov Identifier: NCT02081625; Updated Feb 26, 2020. Accessed Mar 2, 2022. https://clinicaltrialsttps://www.fda.gov/news-events/press-announcements/fda-grants-accelerated-approval-first-drug-duchenne-muscular- dys-trophy
82. Duchenne Drug Development Pipeline. Parent Project Muscular Dystrophy. Accessed August 21, 2022. https://www.parentprojectmd.org/duchenne-drug-development-pipeline/
83. Sarepta Therapeutics Provides Update on SRP-5051 for the Treatment of Duchenne Muscular Dystrophy | Sarepta Therapeutics, Inc. Ac-cessed August 22, 2022. https://investorrelations.sarepta.com/news-releases/news-release-details/sarepta-therapeutics- pro-vides-update-srp-5051-treatment-duchenne
84. Sarepta Therapeutics, Inc. An Open-Label Extension Study for Patients With Duchenne Muscular Dystrophy Who Participated in Studies of SRP-5051. clinicaltrials.gov; 2021. Accessed August 18, 2022. https://clinicaltrials.gov/ct2/show/NCT03675126
85. VYONDYS 53. Prescribing information. Sarepta Therapeutics Inc.; 2019. Accessed Mar 2, 2022. http://www.accessdata.fda.gov/drugsatfda_docs/label/2019/211970s000lbl.pdf.
86. NS Pharma Inc. Long-term use of viltolarsen in boys with Duchenne muscular dystrophy in clinical practice (VILT-502). ClinicalTrials.gov Identifier: NCT04687020. Updated Nov 22, 2021. Accessed Mar 1, 2022. https://clinicaltrials.gov/ct2/show/NCT04687020.
87. VILTEPSO. Prescribing information. NS Pharma; 2020. Accessed Mar 2, 2022. http://www.accessdata.fda.gov/drugsatfda_docs/label/2020/212154s000lbl.pdf.
88. FDA approves targeted treatment for rare Duchenne muscular dystrophy mutation. U.S. Food and Drug Administration. News release. Feb 25, 2021. Accessed Mar 1, 2022. http://www.fda.gov/news-events/press-announcements/fda-approves-targeted-treatment-rare-duchenne-muscular-dystrophy-mutation-0.
89. Sarepta Therapeutics Inc. A double-blind, placebo-controlled, multi-center study with an open-label extension to evaluate the efficacy and safety of SRP-4045 and SRP-4053 in patients with Duchenne muscular dystrophy. Clinicaltrials.gov Identifier: NCT02500381. Updated Aug 19, 2021. Accessed Mar 1, 2022. https://clinicaltrials.gov/ct2/show/NCT02500381.
90. AMONDYS 45. Prescribing information. Sarepta Therapeutics Inc.; 2021. Accessed Feb 22, 2022. http://www.accessdata.fda.gov/drugsatfda_docs/label/2021/213026lbl.pdf.
91. Finkel RS et al. Phase 2a study of ataluren-mediated dystrophin production in patients with nonsense mutation Duchenne muscular dys-trophy. PLoS ONE. 2013;8(12):e81302. doi:10.1371/journal.pone.0081302.
92. PTC Therapeutics. A phase 2 study of PTC124 as an oral treatment for nonsense-mutation-mediated Duchenne muscular dystrophy. Clini-calTrials.gov Identifier: NCT00264888. Updated Jan 14, 2009. Accessed Mar 1, 2022. https://clinicaltrials.gov/ct2/show/NCT00264888.
93. PTC Therapeutics. A phase 2B efficacy and safety study of PTC124 in subjects with nonsense-mutation-mediated Duchenne and Becker muscular dystrophy. ClinicalTrials.gov Identifier: NCT00592553. Updated Apr 7, 2020. Accessed Mar 1, 2022. https://clinicaltrials.gov/ct2/show/NCT00592553.
94. PTC Therapeutics. A phase 3 efficacy and safety study of ataluren in patients with nonsense mutation dystrophinopathy. ClinicalTrials.gov Identifier: NCT01826487. Updated Aug 4, 2020. Accessed Mar 1, 2022. https://clinicaltrials.gov/ct2/show/NCT01826487.
95. Bushby K et al; PTC124-GD-007-DMD Study Group. Ataluren treatment of patients with nonsense mutation dystrophinopathy. Muscle Nerve. 2014 Oct;50(4):477-87. doi:10.1002/mus.24332.
96. Solid Biosciences LLC. A randomized, controlled, open-label, single-ascending dose, phase I/II study to investigate the safety and tolerabil-ity, and efficacy of intravenous SGT-001 in male adolescents and children with Duchenne muscular dystrophy. ClinicalTrials.gov Identifier: NCT03368742. Updated Aug 24, 2021. Accessed Mar 1, 2022. https://clinicaltrials.gov/ct2/show/NCT03368742.
97. Solid Biosciences reports 1.5-year data from patients in the ongoing IGNITE DMD phase I/II clinical trial of SGT-001. Press release. Solid Biosciences. Sep 27, 2021. Accessed Mar 2, 2022. http://www.solidbio.com/about/media/press-releases/solid-biosciences-reports-1-5-year-data-from-patients-in-the-ongoing-ignite-dmd-phase-i-ii-clinical-trial-of-sgt-001.
98. Potter RA et al. Dose-escalation study of systemically delivered rAAVrh74.MHCK7.microdystrophin in the mdx mouse model of Duchenne muscular dystrophy. Hum Gene Ther. 2021 Apr;32(7-8):375-89. doi:10.1089/hum.2019.255.
99. Sarepta Therapeutics, Inc. A Phase 3 Multinational, Randomized, Double-Blind, Placebo- Controlled Systemic Gene Delivery Study to Evaluate the Safety and Efficacy of SRP-9001 in Patients With Duchenne Muscular Dystrophy (EMBARK). clinicaltrials.gov; 2022. Accessed August 18, 2022. https://clinicaltrials.gov/ct2/show/NCT05096221
100. Pfizer. A PHASE 3, MULTICENTER, RANDOMIZED, DOUBLE-BLIND, PLACEBO CONTROLLED STUDY TO EVALUATE THE SAFETY AND EFFICACY OF PF 06939926 FOR THE TREATMENT OF DUCHENNE MUSCULAR DYSTROPHY. clinicaltrials.gov; 2022. Accessed August 18, 2022. https://clinicaltrials.gov/ct2/show/NCT04281485
101. Pfizer. A phase 1B multicenter open-label, single ascending dose study to evaluate the safety and tolerability of PF-06939926 in ambula-tory and non-ambulatory subjects with Duchenne muscular dystrophy. ClinicalTrials.gov Identifier: NCT03362502. Updated Mar 2, 2022. Ac-cessed Mar 2, 2022. https://clinicaltrials.gov/ct2/show/NCT03362502.
102. MS MW. Phase 3 CIFFREO DMD Gene Therapy Trial Slated to Begin in June in US. Accessed August 21, 2022. https://musculardystrophynews.com/news/phase-3-trial-of-pfizers-gene-therapy- expected-to-open-in-us-in-june/
103. SRP-9001. Parent Project Muscular Dystrophy. Accessed August 22, 2022. https://www.parentprojectmd.org/drug-development-pipeline/srp-9001-micro-dystrophin-gene- transfer/
104. Sarepta Therapeutics’ Investigational Gene Therapy SRP-9001 for Duchenne Muscular Dystrophy Demonstrates Significant Functional Improvements Across Multiple Studies | Sarepta Therapeutics, Inc. Accessed August 22, 2022. https://investorrelations.sarepta.com/news-releases/news-release- details/sarepta-therapeutics-investigational-gene-therapy-srp-9001
105. Sarepta Therapeutics, Inc. An Open-Label Safety, Tolerability, and Efficacy Study of Eteplirsen in Patients With Duchenne Muscular Dys-trophy Who Have Completed Study 4658-102.clinicaltrials.gov; 2022. Accessed August 18, 2022. https://clinicaltrials.gov/ct2/show/NCT03985878
106. Sarepta Therapeutics, Inc. An Open-Label Safety, Tolerability, and Pharmacokinetics Study of Eteplirsen in Young Patients With Duchenne Mus-cular Dystrophy Amenable to Exon 51 Skipping. clinicaltrials.gov; 2021. Accessed August 18, 2022. https://clinicaltrials.gov/ct2/show/NCT03218995
107.Sarepta Therapeutics, Inc. A Randomized, Double-Blind, Dose Finding and Comparison Study of the Safety and Efficacy of a High Dose of Eteplirsen, Preceded by an Open-Label Dose Escalation, in Patients With Duchenne Muscular Dystrophy With Deletion Mutations Amenable to Exon 51 Skipping. clinicaltrials.gov; 2022. Accessed August 18, 2022. https://clinicaltrials.gov/ct2/show/NCT03992430
108. Sarepta Therapeutics, Inc. A Phase 2, Two-Part, Multiple-Ascending-Dose Study of SRP-5051 for Dose Determination, Then Dose Ex-pansion, in Patients With .gov/ct2/show/NCT02081625.
110. NS Pharma Inc. A phase II, dose finding study to assess the safety, tolerability, pharmacokinetics, and pharmacodynamics of NS-065/NCNP-01 in boys with Duchenne muscular dystrophy (DMD). ClinicalTrials.gov Identifier: NCT02740972. Updated Dec 7, 2021. Ac-cessed Mar 2, 2022. https://clinicaltrials.gov/ct2/show/NCT02740972.
111. NS Pharma Inc. A phase II, open-label, extension study to assess the safety and efficacy of NS-065/NCNP-01 in boys with Duchenne muscular dystrophy (DMD). ClinicalTrials.gov Identifier: NCT03167255. Updated Nov 24, 2021. Accessed Mar 2, 2022. https://clinicaltrials.gov/ct2/show/NCT03167255.
112. NS Pharma Inc. A phase 2 open label study to assess the safety, tolerability, and efficacy of viltolarsen in ambulant and non-ambulant boys with Duchenne muscular dystrophy (DMD) compared with natural history controls. ClinicalTrials.gov Identifier: NCT04956289. Updated Feb 1, 2022. Accessed Mar 2, 2022. https://clinicaltrials.gov/ct2/show/NCT04956289.
113. NS Pharma Inc. A phase 3 randomized, double-blind, placebo-controlled, multi-center study to assess the efficacy and safety of viltolarsen in ambulant boys with Duchenne muscular dystrophy (DMD). ClinicalTrials.gov Identifier: NCT04060199. Updated Nov 16, 2021. Accessed Mar 2, 2022. https://clinicaltrials.gov/ct2/show/NCT04060199.
114. NS Pharma Inc. A phase 3, multi-center, open-label extension study to assess the safety and efficacy of viltolarsen in ambulant boys with Duchenne muscular dystrophy (DMD). ClinicalTrials.gov Identifier: NCT04768062. Updated Nov 16, 2021. Accessed Mar 2, 2022. https://clinicaltrials.gov/ct2/show/NCT04768062.
115. Sarepta Therapeutics Inc. A randomized, double-blind, placebo-controlled, dose-titration, safety, tolerability, and pharmacokinetics study followed by an open-label safety and efficacy evaluation of SRP-4045 in advanced-stage patients with Duchenne muscular dystrophy amena-ble to exon 45 skipping. ClinicalTrials.gov Identifier: NCT02530905. Updated May 17, 2021. Accessed Mar 2, 2022. https://clinicaltrials.gov/ct2/show/NCT02530905.
116. Sarepta Therapeutics Inc. Long-term, open-label extension study for patients with Duchenne muscular dystrophy enrolled in clinical trials evaluating casimersen or golodirsen. ClinicalTrials.gov Identifier: NCT03532542. Updated Dec 20, 2021. Accessed Mar 2, 2022. https://clinicaltrials.gov/ct2/show/NCT03532542.
117. PTC Therapeutics. A phase 2 study of the safety, pharmacokinetics, and pharmacodynamics of ataluren (PTC124®) in patients aged ≥2 to <5 years old with nonsense mutation dystrophinopathy. ClinicalTrials.gov Identifier: NCT02819557. Updated Aug 28, 2020. Accessed Mar 2, 2022. https://clinicaltrials.gov/ct2/show/NCT02819557.
118. PTC Therapeutics. Phase 2, non-interventional, clinical study to assess dystrophin levels in subjects with nonsense mutation Duchenne muscular dystrophy who have been treated with ataluren for ≥ 9 months. ClinicalTrials.gov Identifier: NCT03796637. Updated Apr 10, 2020. Accessed Mar 2, 2022. https://clinicaltrials.gov/ct2/show/NCT03796637.
119. PTC Therapeutics. An Open-Label Study Evaluating the Safety and Pharmacokinetics of Ataluren in Children From ≥6 Months to <2 Years of Age With Nonsense Mutation Duchenne Muscular Dystrophy. clinicaltrials.gov; 2022. Accessed August 18, 2022. https://clinicaltrials.gov/ct2/show/NCT04336826 120. PTC Therapeutics. An open-label study for previously treated ataluren (PTC124®) pa-tients with nonsense mutation dystrophinopathy. ClinicalTrials.gov Identifier: NCT01557400. Updated Nov 25, 2020. Accessed Feb 21, 2022. https://clinicaltrials.gov/ct2/show/NCT01557400.
121. PTC Therapeutics. An open-label, safety study for ataluren (PTC124) patients with nonsense mutation dystrophinopathy. ClinicalTrials.gov Identifier: NCT01247207. Updated Feb 16, 2022. Accessed Mar 2, 2022. https://clinicaltrials.gov/ct2/show/NCT01247207.
122. PTC Therapeutics. A phase 3, randomized, double-blind, placebo-controlled efficacy and safety study of ataluren in patients with non-sense mutation Duchenne muscular dystrophy and open-label extension. ClinicalTrials.gov Identifier: NCT03179631. Updated Feb 8, 2022. Accessed Mar 2, 2022. https://clinicaltrials.gov/ct2/show/NCT03179631.
123. Sarepta Therapeutics, Inc. An Open-Label, Systemic Gene Delivery Study Using Commercial Process Material to Evaluate the Safety of and Expression From SRP-9001 in Subjects With Duchenne Muscular Dystrophy (ENDEAVOR). clinicaltrials.gov; 2022. Accessed August 18, 2022. https://clinicaltrials.gov/ct2/show/NCT04626674
124. Sarepta Therapeutics, Inc. Systemic Gene Delivery Phase I/IIa Clinical Trial for Duchenne Muscular Dystrophy Using RAA-Vrh74.MHCK7.Micro-Dystrophin (MicroDys-IV-001). clinicaltrials.gov; 2022. Accessed August 18, 2022. https://clinicaltrials.gov/ct2/show/NCT03375164
125. Sarepta Therapeutics Inc. A multicenter, randomized, double-blind, placebo-controlled trial for Duchenne muscular dystrophy using SRP-9001. ClinicalTrials.gov Identifier: NCT03769116. Updated Dec 2021. Accessed Mar 2, 2022. https://clinicaltrials.gov/ct2/show/NCT03769116.
126. Hoffmann-La Roche. A Two-Part, Seamless, Multi-Center, Randomized, Placebo-Controlled, Double-Blind Study to Investigate the Safety, Tolerability, Pharmacokinetics, Pharmacodynamics and Efficacy of RO7204239 in Combination With Risdiplam (RO7034067) in Ambulant Pa-tients With Spinal Muscular Atrophy. clinicaltrials.gov; 2022. Accessed September 1, 2022. https://clinicaltrials.gov/ct2/show/NCT05115110
Neuromuscular diseases (NMDs) are a broad classification of heterogeneous groups of disorders characterized by progressive muscle weakness resulting from muscle or nerve dysfunction.1 Diagnosis is based on symptoms and a full medical history, as well as on muscle and imaging tests (including electromyography, nerve-conduction studies, magnetic resonance imaging, muscle biopsy, and blood tests) to confirm or rule out specific NMDs.2 Early diagnosis of NMDs can be difficult because symptoms overlap with those of many other diseases.
Although individually, NMDs are rare, collectively, they affect approximately 250,000 people in the United States. Disease types vary in regard to cause, symptoms, prevalence, age of onset, progression, and severity. Functional impairment from any NMD can lead to lifelong morbidities and shortened life expectancy.1,3
Treatment options for NMDs are limited; most target symptoms, not disease progression. Although there is a need for safe and effective gene-based therapies for NMDs, there are challenges to developing and delivering such treatments that have impeded clinical success. These include a lack of understanding about disease pathology and drug targets, limited animal model systems, and few reliable biomarkers that are predictive of therapeutic success.4,5
Notwithstanding that challenges remain, our understanding of gene expression in NMDs has greatly advanced in the past few decades. This progress has translated into promising results in the gene-therapy field – thereby setting the stage for therapeutic approaches that use novel gene-delivery and gene-manipulation tools.6 These novel approaches include nonviral strategies, such as antisense oligonucleotides (ASOs), and viral-based strategies, such as adeno-associated virus (AAV)-mediated gene silencing and AAV-mediated gene delivery.
In this article, we highlight advancements in the clinical development of gene-based therapies for NMDs. We focus on amyotrophic lateral sclerosis (ALS), spinal muscular atrophy (SMA), and Duchenne muscular dystrophy (DMD) because of recent clinical successes in developing such therapies.1,6,7 We also catalog completed and ongoing clinical trials for ALS, SMA, and DMD (Tables 1-3).
Amyotrophic lateral sclerosis
ALS is caused by progressive degeneration of upper- and lower-motor neurons, which eventually leads to respiratory failure and death 3 to 5 years after disease onset.7-9 There are two subtypes: Familial ALS (10% of cases) and sporadic ALS (90% of cases). Commonly mutated ALS-associated genes6,8 are:
- Superoxide dismutase type 1 (SOD1).
- Chromosome 9 open reading frame 72 (C9orf72).
- Transactive response DNA-binding protein 43 (TARDBP).
- Fused in sarcoma (FUS).
SOD1-targeted therapy is being studied, with early evidence of clinical success. Mutations in SOD1 account for 10% to 20% of familial ALS cases and 1% to 2% of sporadic ALS cases.6,10 10 Mutations in C9orf72 account for 25 to 40% of familial ALS cases and 7% of sporadic ALS cases.8,9,11 Mutations in TARDBP account for 3% of familial ALS cases and 2% of sporadic cases.12 Mutations in FUS account for 4% of familial ALS cases and 1% of sporadic cases. Overall, these mutant proteins can trigger neurotoxicity, thus inducing motor-neuron death.6,10
Treatment of ALS
Two treatments for ALS are Food and Drug Administration approved: riluzole (Rilutek), approved in 1995, and edaravone (Radicava), approved in 2017.
Riluzole is an oral anti-excitotoxic glutamate antagonist.11 Approval of riluzole was based on the results of two studies that demonstrated a 2- to 3-month survival benefit.10,14 For patients who have difficulty swallowing, an oral suspension (Tiglutik, approved in 2018) and an oral film (Exservan, approved in 2019) are available.
Edaravone is a free-radical scavenger that decreases oxidative stress and is administered intravenously (IV).9,13,14 Findings from clinical trials suggest functional improvement or slower decline in function for some patients.
Although these two agents demonstrate modest therapeutic benefit, neither reverses progression of disease.10,14
Gene-based therapy for ALS
Many non-viral strategies, including antisense oligonucleotide (ASO), monoclonal antibodies, reverse transcriptase inhibitors, and HGF gene replacement therapy are used as therapeutic approaches to SOD1, C9orf72, and FUS gene mutations in ALS patients, and are being evaluated in clinical studies14,15 (Table 113-17).

Tofersen, also known as BIIB067, is an investigational ASO, administered by intrathecal (IT) injection, that binds to SOD1 mRNA, thus reducing its protein levels.16 Tofersen was evaluated in the VALOR phase 3 study (ClinicalTrials.gov Identifier: NCT02623699), a three-part randomized, double-blind, placebo-controlled trial: single ascending dose (Part A), multiple ascending dose (B), and fixed dose (C).10 In Parts A and B, 48 participants received five IT injections of tofersen or placebo over 12 weeks and were followed for an additional 12 weeks. Reduction in SOD1 protein production and neurofilament level in cerebrospinal fluid (CSF) (a potential biomarker of motor-neuron degeneration) was observed, which determined the fixed-dose for Part C.16,17
Part C examined the efficacy, safety and tolerability, pharmacokinetics (PK), and pharmacodynamics (PD) of tofersen, compared with placebo, in adults with ALS who had a confirmed SOD1 mutation.17 A total of 108 participants were enrolled; 60 were identified as “faster-progressing”; 48, as “slower-progressing.”18 The primary endpoint of Part C was change from baseline to Week 28 on the Revised ALS Functional Rating Scale (ALSFRS-R) total score. (ALSFRS-R measures overall clinical effect; the score ranges from 0 [no function] to 4 [full function].17)
Tofersen failed to meet the primary efficacy outcome because statistically significant findings were lacking in the faster-progressing population, as measured by joint-rank analysis (difference of 1.2 on the ALSFRS-R score; P = .97). However, trends favoring tofersen were observed across key secondary clinical outcome measures18:
- Change from baseline in CSF SOD1 protein concentration.17 Percent reduction in the total SOD1 protein level was much higher in the tofersen-treated group than in the control group (38% more than controls in the faster-progressing population; 26% more than controls in the slower-progressing population).18
- Change from baseline in neurofilament light-chain concentration in plasma.17,18 Percent reduction in the level of neurofilament light chain was also observed to be higher in the tofersen-treated group than in the control group (67% more than controls in the faster-progressing population and 48% more than controls in the slower-progressing population).18
Because of these encouraging results, VALOR participants were moved to the ongoing open-label extension trial of tofersen (ClinicalTri-als.gov Identifier: NCT03070119), in which both groups were treated with the active agent.
These data suggest that early tofersen treatment might slow decline in faster-progressing patients and stabilize clinical function in slower-progressing patients.18,19 Overall, most adverse events (AEs) in the trial among patients receiving active treatment were of mild or moderate severity, and were largely consistent with either disease progression or lumbar puncture–related complications.18
Because data from VALOR suggested potential benefit from tofersen, the ATLAS trial (ClinicalTrials.gov Identifier: NCT04856982) is investigating the clinical value of presymptomatic treatment and the optimal timing of initiation of therapy.20,21 ATLAS is a phase 3, randomized, placebo-controlled trial that examines the clinical efficacy, safety, and tolerability of tofersen in presymptomatic adult carriers of SOD1 mutation who have an elevated neurofilament light-chain concentration.21 ATLAS will also evaluate the efficacy of tofersen when initiated before, rather than after, ALS manifests clinically. Enrollment is still open for this trial.20,21
Latozinemab, also known as AL001, is a first-in-class monoclonal antibody, administered by IV infusion, that elevates levels of progranulin, a key regulator of the immune activity and lysosomal function in the brain.22,23 Latozinemab limits progranulin endocytosis and degradation by sortilin inhibition.22 Progranulin gene mutations can reduce progranulin expression (by 50 to 70 percent reduction), which may cause neuro-degeneration due to abnormal accumulation of TAR-DNA-binding protein 43 (TDP-43) in the brain cells.22,24 TDP-43 pathology has also been shown to be associated with C9orf72 mutations.23 Although the mechanism is not fully understood, the role of progranulin deficiency in TDP-43 pathology is believed to be associated with neurodegenerative diseases like ALS.11,23,24,43 Previous animal models of chronic neurodegenera-tion have demonstrated how increased progranulin levels can be protective against TDP-43 pathology, increasing neuronal development and survival, thus potentially slowing disease progression.23,24,43 Currently, latozinemab is being investigated in a randomized, double-blind, placebo-controlled, multicenter phase 2 trial (ClinicalTrials.gov Identifier: NCT05053035). Approximately, 45 C90rf72-associated ALS participants (≥ 18 years of age) will receive latozinemab or placebo infusions every 4 weeks (for 24 weeks). Study endpoints include safety, tolerability, PK, PD, as well as plasma, and CSF progranulin levels.25 In previous studies, latozinemab demonstrated encouraging results in frontotemporal dementia (FTD) patients who carry a progranulin mutation. Because FTD was revealed to have significant genetic overlap with ALS, there is disease-modifying potential for latozinemab in ALS patients.23,24
TPN-101 is a nucleoside analog reverse transcriptase inhibitor, administered orally, that was originally developed for human immunodeficiency virus (HIV) treatment. However, due to recent findings suggesting retrotransposon activity contributing to neurodegeneration in TDP-43 mediated diseases, including ALS and FTD, TNP-101 is being repurposed.26 The safety and tolerability of TNP-101 are currently being evaluated in C9orf72-associated ALS and FTD patients (≥ 18 years of age). The study is a randomized, double-blind, placebo-controlled paral-lel-group phase 2a trial (ClinicalTrials.gov Identifier: NCT04993755) The study includes a screening period of 6 weeks, double-blind treatment period of 24 weeks, an open-label treatment period of 24 weeks, and 4 weeks of the post-treatment follow-up visit. Study endpoints include the incidence and severity of spontaneously reported treatment-emergent adverse events (TEAEs) associated with TNP-101 and placebo for a to-tal of 48 weeks.27
ION363 is an investigational ASO, administered by IT injection, that selectively targets one of the FUS mutations (p.P525L), which is responsible for earlier disease onset and rapid ALS progression.28,29 The clinical efficacy of ION363, specifically in clinical function and survival is being assessed in FUS-associated ALS patients (≥ 12 years of age). This randomized phase 3 study (ClinicalTrials.gov Identifier: NCT04768972) includes two parts; part 1 will consist of participants receiving a multi-dose regimen (1 dose every 4-12 weeks) of ION363 or placebo for 61 weeks followed by an open-label extension treatment period in part 2, which will consist of participants receiving ION363 (every 12 weeks) for 85 weeks. The primary endpoint of the study is the change from baseline to day 505 in functional impairment, using ALS Functional Rating Scale-Revised (ALSFRS-R). This measures functional disease severity, specifically in bulbar function, gross motor skills, fine motor skills, and respiratory. The score for all 12 questions can range from 0 (no function) to 4 (full function) with a total possible score of 48.30
Engensis, also known as VM202, is a non-viral gene therapy, administered by intramuscular (IM) injection, that uses a plasmid to deliver the hepatocyte growth factor (HGF) gene to promote HGF protein production. The HGF protein plays a role in angiogenesis, the previous of muscle atrophy, and the promotion of neuronal survival and growth. Based on preclinical studies, increasing HGF protein production has been shown to reduce neurodegeneration, thus potentially halting or slowing ALS progression.31 Currently, the safety of engensis is being evaluated in ALS patients (18-80 years of age) in the REViVALS phase 2a (ClinicalTrials.gov Identifier: NCT04632225)/2b (ClinicalTrial.gov Identifier: NCT05176093).32,33 The ReViVALS trial is a double-blind, randomized, placebo-controlled, multi-center study. The phase 2a study endpoints include the incidence of TEAEs, treatment-emergent serious adverse events (TESAEs), injection site reactions, and clinically significant labor-atory values post-treatment (engensis vs placebo group) for 180 days.33 A phase 2b study will evaluate the long-term safety of engensis for an additional 6 months. Study endpoints include the incidence of AEs, changes from baseline in ALSFRS-R scores to evaluate improvement in muscle function, changes from baseline in quality of life using the ALS patient assessment questionnaire, time to all-cause mortality compared to placebo, etc.32
Spinal muscular atrophy
SMA is a hereditary lower motor-neuron disease caused (in 95% of cases) by deletions or, less commonly, by mutations of the survival motor neuron 1 (SMN1) gene on chromosome 5q13 that encodes the SMN protein.6 Reduction in expression of the SMN protein causes motor neurons to degenerate.36-38 Because of a large inverted duplication in chromosome 5q, two variants of SMN (SMN1 and SMN2) exist on each allele. The paralog gene, SMN2, also produces the SMN protein – although at a lower level (10% to 20% of total SMN protein production) than SMN1 does.
A single nucleotide substitution in SMN2 alters splicing and suppresses transcription of exon 7, resulting in a shortened mRNA strand that yields a truncated SMN protein product.6,37,39 SMA is classified based on age of onset and maximum motor abilities achieved, ranging from the most severe (Type 0) to mildest (Type 4) disease.36,40 Because SMA patients lack functional SMN1 (due to polymorphisms), disease severity is determined by copy numbers of SMN2.6,39
Gene-based therapy for SMA
Three FDA-approved SMN treatments demonstrate clinically meaningful benefit in SMA: SMN2-targeting nusinersen [Spinraza] and risdiplam [Evrysdi], and SMN1-targeting onasemnogene abeparvovec-xioi [Zolgensma]38 Additional approaches to SMA treatment are through SMN-independent therapies, which target muscle and nerve function. Research has strongly suggested that combined SMA therapies, specifically approved SMN-targeted and investigational SMN-independent treatments, such as GYM329 (also known as RO7204239) may be the best strategy to treat all ages, stages, and types of SMA.41 (Table 226-41).

Agents that modulate SMN2. Nusinersen, approved by the FDA in 2016, was the first treatment indicated for all SMA types in pediatric and adult patients.42 The agent is an ASO that targets exon 7 of SMN2, thus stabilizing transcription. Inclusion of exon 7 increases SMN protein production, improving motor function.6,38 Nusinersen is a lifelong treatment that requires IT administration every 4 months because it cannot cross the blood-brain barrier.38,43
Pivotal clinical studies that led to approval of nusinersen include CHERISH (ClinicalTrial.gov Identifier: NCT02292537) and ENDEAR (ClinicalTrial.gov Identifier: NCT02193074) studies.
CHERISH was a phase 3, randomized, double-blind, sham procedure–controlled trial that examined the clinical efficacy and safety of nusinersen in 126 participants with later-onset SMA (2-12 years of age). The primary endpoint was the change from baseline using the Hammersmith Functional Motor Scale Expanded (HFMSE) at 15 months. HFMSE looks at 33 activities to assess improvement in motor function. The study met the primary efficacy outcome, demonstrating statistically significant (P = .0000001) improvement in overall motor function. The nusinersen group showed a 3.9-point increase in the HFMSE score from baseline, which indicates improvement, compared with a 1.0-point decline from baseline in the control group.46,47
ENDEAR was also a randomized, double-blind, sham procedure–controlled phase 3 trial, which investigated the efficacy and safety of nusinersen in 121 participants with early-onset SMA Type 1 (≤ 210 days of age). Coprimary endpoints were:
- Percentage of motor milestones responders, as determined using Section 2 of the Hammersmith Infant Neurological Examination–Part 2.
- Event-free survival (that is, avoidance of combined endpoint of death or permanent ventilation).
ENDEAR met the first primary efficacy outcome, demonstrating statistically significant (P < .0001) improvement in motor milestones (head control, rolling, independent sitting, and standing). By 13 months of age, approximately 51% of nusinersen-treated participants showed improvement, compared with none in the control group.46,47
The second primary endpoint was also met, with a statistically significant (P = .005) 47% decrease in mortality or permanent ventilation use.46-48
The NURTURE (ClinicalTrial.gov Identifier: NCT02386553) study is also investigating the efficacy and safety of nusinersen. An ongoing, open-label, supportive phase 2 trial, NURTURE is evaluating the efficacy and safety of multiple doses of nusinersen in 25 presymptomatic SMA patients (≤ 6 weeks of age). The primary endpoint of this study is time to death or respiratory intervention.49 Interim results demonstrate that 100% of presymptomatic infants are functioning without respiratory intervention after median follow-up of 2.9 years.46-48
Although nusinersen has been shown to be generally safe in clinical studies, development of lumbar puncture–related complications, as well as the need for sedation during IT administration, might affect treatment tolerability in some patients.39
Risdiplam was approved by the FDA in 2020 as the first orally administered small-molecule treatment of SMA (for patients ≤ 2 months of age).52 Risdiplam is a SMN2 splicing modifier, binding to the 5’ splice site of intron 7 and exonic splicing enhancer 2 in exon 7 of SMN2 pre-mRNA. This alternative splicing increases efficiency in SMN2 gene transcription, thus increasing SMN protein production in motor-neuron cells.36 An important advantage of risdiplam is the convenience of oral administration: A large percentage of SMA patients (that is, those with Type 2 disease) have severe scoliosis, which can further complicate therapy or deter patients from using a treatment that is administered through the IT route.40
FDA approval of risdiplam was based on clinical data from two pivotal studies, FIREFISH (ClinicalTrial.gov Identifier: NCT02913482) and SUNFISH (ClinicalTrial.gov Identifier: NCT02908685).53-54
FIREFISH is an open-label, phase 2/3 ongoing trial in infants (1-7 months of age) with SMA Type 1. The study comprises two parts; Part 1 determined the dose of risdiplam used in Part 2, which assessed the efficacy and safety of risdiplam for 24 months. The primary endpoint was the percentage of infants sitting without support for 5 seconds after 12 months of treatment using the gross motor scale of the Bayley Scales of Infant and Toddler Development–Third Edition. A statistically significant (P < .0001) therapeutic benefit was observed in motor milestones. Approximately 29% of infants achieved the motor milestone of independent sitting for 5 seconds, which had not been observed in the natural history of SMA.53-55
SUNFISH is an ongoing randomized, double-blind, placebo-controlled trial of risdiplam in adult and pediatric patients with SMA Types 2 and 3 (2-25 years old). This phase 2/3 study comprises two parts: Part 1 determined the dose (for 12 weeks) to be used for confirmatory Part 2 (for 12 to 24 months). The primary endpoint was the change from baseline on the 32-item Motor Function Measure at 12 months. The study met its primary endpoint, demonstrating statistically significant (P = .0156) improvement in motor function scores, with a 1.36-point increase in the risdiplam group, compared with a 0.19-point decrease in the control group.54,55
Ongoing risdiplam clinical trials also include JEWELFISH (ClinicalTrial.gov Identifier: NCT03032172) and RAINBOW (ClinicalTrial.gov Identifier: NCT03779334).56-57 JEWELFISH is an open-label, phase 2 trial assessing the safety of risdiplam in patients (6 months to 60 years old) who received prior treatment. The study has completed recruitment; results are pending.56 RAINBOW is an ongoing, open-label, single-arm, phase 2 trial, evaluating the clinical efficacy and safety of risdiplam in SMA-presymptomatic newborns (≤ 6 weeks old). The study is open for enrollment.57 Overall, interim results for JEWELFISH and RAINBOW appear promising.
In addition, combined SMA therapies, specifically risdiplam and GYM329 are currently being investigated to address the underlying cause and symptoms of SMA concurrently.58 GYM329, is an investigational anti-myostatin antibody, selectively binding preforms of myostatin - pro-myostatin and latent myostatin, thus improving muscle mass and strength for SMA patients.59 The safety and efficacy of GYM329 in combination with risdiplam is currently being investigated in 180 ambulant participants with SMA (2-10 years of age) in the MANATEE (ClinicalTrial.gov Identifier: NCT05115110) phase 2/3 trial. The MANATEE study is a two-part, seamless, randomized, placebo-controlled, double-blind trial. Part 1 will assess the safety of the combination treatment in approximately 36 participants; participants will receive both GYM329 (every 4 weeks) by subcutaneous (SC) injection into the abdomen and risdiplam (once per day) for 24 weeks followed by a 72-week open-label treatment period. 54,58 The outcome measures include the incidence of AEs, percentage change from baseline in the contractile area of skeletal muscle (in dominant thigh and calf), change from baseline in RHS total score, and incidence of change from baseline in serum concentration (total myostatin, free latent myostatin, and mature myostatin) etc.54 Part 2 will be conducted on 144 participants, specifically assessing the efficacy and safety of the optimal dose of GYM329 selected from Part 1 (combined with risdiplam) for 72 weeks. Once the treatment period is completed in either part, participants can partake in a 2-year open-label extension period.54,58 Other outcome measures include change from baseline in lean muscle mass (assessed by full body dual-energy X- ray absorptiometry (DXA) scan), in time taken to walk/run 10 meters (measured by RHS), in time taken to rise from the floor (measured by RHS), etc.54 Overall, this combination treatment has the potential to further improve SMA patient outcomes and will be further investigated in other patient populations (including non-ambulant patients and a broader age range) in the future.58
An agent that alters SMN1 expression. Onasemnogene abeparvovec-xioi, FDA approved in 2019, was the first gene-replacement therapy indicated for treating SMA in children ≤ 2 years old.60 Treatment utilizes an AAV vector type 9 (AAV9) to deliver a functional copy of SMN1 into target motor-neuron cells, thus increasing SMN protein production and improving motor function. This AAV serotype is ideal because it crosses the blood-brain barrier. Treatment is administered as a one-time IV fusion.38,39,43
FDA approval was based on the STR1VE (ClinicalTrial.gov Identifier: NCT03306277) phase 3 study and START (ClinicalTrial.gov Identifier: NCT02122952) phase 1 study.61,62 START was the first trial to investigate the safety and efficacy of onasemnogene abeparvovec-xioi in SMA Type 1 infants (< 6 months old). Results demonstrated remarkable clinical benefit, including 100% permanent ventilation-free survival and a 92% (11 of 12 patients) rate of improvement in motor function. Improvement in development milestones was also observed: 92% (11 of 12 patients) could sit without support for 5 seconds and 75% (9 of 12) could sit without support for 30 seconds.14,61,63
The efficacy of onasemnogene abeparvovec-xioi seen in STR1VE was consistent with what was observed in START. STRIVE, a phase 3 open-label, single-dose trial, examined treatment efficacy and safety in 22 symptomatic infants (< 6 months old) with SMA Type 1 (one or two SMN2 copies). The primary endpoint was 30 seconds of independent sitting and event-free survival. Patients were followed for as long as 18 months. Treatment showed statistically significant (P < .0001) improvement in motor milestone development and event-free survival, which had not been observed in SMA Type 1 historically. Approximately 59% (13 of 22 patients) could sit independently for 30 seconds at 18 months of age. At 14 months of age, 91% (20 of 22 patients) were alive and achieved independence from ventilatory support.34,35,53
Although many clinical studies suggest that onasemnogene abeparvovec-xioi can slow disease progression, the benefits and risks of long-term effects are still unknown. A 15-year observational study is investigating the long-term therapeutic effects and potential complications of onasemnogene abeparvovec-xioi. Participants in START were invited to enroll in this long-term follow-up study (ClinicalTrial.gov Identifier: NCT04042025).66-67
Duchenne muscular dystrophy
DMD is the most common muscular dystrophy of childhood. With an X-linked pattern of inheritance, DMD is seen mostly in young males (1 in every 3,500 male births).38,39,73 DMD is caused by mutation of the dystrophin encoding gene, or DMD, on the X chromosome. Deletion of one or more exons of DMD prevents production of the dystrophin protein, which leads to muscle degeneration.38,39,43 Common DMD deletion hotspots are exon 51 (20% of cases), exon 53 (13% of cases), exon 44 (11% of cases), and exon 45 (12% of cases).74 Nonsense mutations, which account for another 10% of DMD cases, occur when premature termination codons are found in the DMD gene. Those mutations yield truncated dystrophin protein products.39,66
Therapy for DMD
There are many therapeutic options for DMD, including deflazacort (Emflaza), FDA approved in 2017, which has been shown to reduce inflammation and immune system activity in DMD patients (≥ 5 years old). Deflazacort is a corticosteroid prodrug; its active metabolite acts on the glucocorticoid receptor to exert anti-inflammatory and immunosuppressive effects. Studies have shown that muscle strength scores over 6-12 months and average time to loss of ambulation numerically favored deflazacort over placebo.74,75
Gene-based therapy for DMD
Mutation-specific therapeutic approaches, such as exon skipping and nonsense suppression, have shown promise for the treatment of DMD (Table 358-79):

- ASO-mediated exon skipping allows one or more exons to be omitted from the mutated DMD mRNA.74,75 Effective FDA-approved ASOs include golodirsen [Vyondys 53], viltolarsen [Viltepso], and casimersen [Amondys 45].74
- An example of therapeutic suppression of nonsense mutations is ataluren [Translarna], an investigational agent that can promote premature termination codon read-through in DMD patients.66
Another potential treatment approach is through the use of AAV gene transfer to treat DMD. However, because DMD is too large for the AAV vector (packaging size, 5.0 kb), microdystrophin genes (3.5-4 kb, are used as an alternative to fit into a single AAV vector.39,76
Exon skipping targeting exon 51. Eteplirsen, approved in 2016, is indicated for the treatment of DMD patients with the confirmed DMD gene mutation that is amenable to exon 51 skipping. Eteplirsen binds to exon 51 of dystrophin pre-mRNA, causing it to be skipped, thus, restoring the reading frame in patients with DMD gene mutation amenable to exon 51 skipping. This exclusion promotes dystrophin production. Though the dystrophin protein is still functional, it is shortened.38,77 Treatment is administered IV, once a week (over 35-60 minutes). Eteplirsen’s accelerated approval was based on 3 clinical studies (ClinicalTrial.gov Identifier: NCT01396239, NCT01540409, and NCT00844597.) 78-81 The data demonstrated an increased expression of dystrophin in skeletal muscles in some DMD patients treated with eteplirsen. Though the clinical benefit of eteplirsen (including improved motor function) was not established, it was concluded by the FDA that the data were reasonably likely to predict clinical benefit. Continued approval for this indication may depend on the verification of a clinical benefit in confirmatory trials. Ongoing clinical trials include (ClinicalTrial.gov Identifier: NCT03992430 (MIS51ON), NCT03218995, and NCT03218995).77,81,82
Vesleteplirsen, is an investigational agent that is designed for DMD patients who are amendable to exon 51 skip-ping. The mechanism of action of vesleteplirsen appears to be similar to that of eteplirsen.83 The ongoing MOMENTUM (ClinicalTrial.gov Identifier: NCT04004065) phase 2 trial is assessing the safety and tolerability of vesleteplirsen at multiple-ascending dose levels (administered via IV infusion) in 60 participants (7-21 years of age). The study consists of two parts; participants receive escalating dose levels of vesleteplirsen (every 4 weeks) for 72 weeks during part A and participants receive the selected doses from part A (every 4 weeks) for 2 years during part B. Study endpoints include the number of AEs (up to 75 weeks) and the change from baseline to week 28 in dystrophin protein level. 84 Serious AEs of reversible hypomagnesemia were observed in part B, and as a result, the study protocol was amended to include magnesium supplementation and monitoring of magnesium levels.83
Exon skipping targeting exon 53. Golodirsen, FDA approved in 2019, is indicated for the treatment of DMD in patients who have a confirmed DMD mutation that is amenable to exon 53 skipping. The mechanism of action is similar to eteplirsen, however, golodirsen is designed to bind to exon 53.38,39 Treatment is administered by IV infusion over 35-60 minutes.
Approval of golodirsen was based primarily on a two-part, phase 1/2 clinical trial (ClinicalTrial.gov Identifier: NCT02310906). Part 1 was a randomized, placebo-controlled, dose-titration study that assessed multiple-dose efficacy in 12 DMD male patients, 6 to 15 years old, with deletions that were amenable to exon 53 skipping.
Part 2 was an open-label trial in 12 DMD patients from Part 1 of the trial plus 13 newly enrolled male DMD patients who were also amenable to exon 53 skipping and who had not already received treatment. Primary endpoints were change from baseline in total distance walked during the 6-minute walk test at Week 144 and dystrophin protein levels (measured by western blot testing) at Week 48. A statistically significant increase in the mean dystrophin level was observed, from a baseline 0.10% mean dystrophin level to a 1.02% mean dystrophin level after 48 weeks of treatment (P < .001). Common reported adverse events associated with golodirsen were headache, fever, abdominal pain, rash, and dermatitis. Renal toxicity was observed in preclinical studies of golodirsen but not in clinical studies.80,85
Viltolarsen, approved in 2020, is also indicated for the treatment of DMD in patients with deletions amenable to exon 53 skipping. The mechanism of action and administration (IV infusion over 60 minutes) are similar to that of golodirsen.
Approval of viltolarsen was based on two phase 2 clinical trials (ClinicalTrial.gov Identifier: NCT02740972 and NCT03167255) in a total of 32 patients. NCT02740972 was a randomized, double-blind, placebo-controlled, dose-finding study that evaluated the clinical efficacy of viltolarsen in 16 male DMD patients (4-9 years old) for 24 weeks.
NCT03167255 was an open-label study that evaluated the safety and tolerability of viltolarsen in DMD male patients (5-18 years old) for 192 weeks. The efficacy endpoint was the change in dystrophin production from baseline after 24 weeks of treatment. A statistically significant increase in the mean dystrophin level was observed, from a 0.6% mean dystrophin level at baseline to a 5.9% mean dystrophin level at Week 25 (P = .01). The most common adverse events observed were upper respiratory tract infection, cough, fever, and injection-site reaction.86-87
Exon skipping targeting exon 45. Casimersen was approved in 2021 for the treatment of DMD in patients with deletions amenable to exon 45 skipping.88 Treatment is administered by IV infusion over 30-60 minutes. Approval was based on an increase in dystrophin production in skeletal muscle in treated patients. Clinical benefit was reported in interim results from the ESSENCE (ClinicalTrial.gov Identifier: NCT02500381) study, an ongoing double-blind, placebo-controlled phase 3 trial that is evaluating the efficacy of casimersen, compared with placebo, in male participants (6-13 years old) for 48 weeks. Efficacy is based on the change from baseline dystrophin intensity level, determined by immunohistochemistry, at Week 48.
Interim results from ESSENCE show a statistically significant increase in dystrophin production in the casimersen group, from a 0.9% mean dystrophin level at baseline to a 1.7% mean dystrophin level at Week 48 (P = .004); in the control group, a 0.54% mean dystrophin level at baseline increased to a 0.76% mean dystrophin level at Week 48 (P = .09). Common adverse events have included respiratory tract infection, headache, arthralgia, fever, and oropharyngeal pain. Renal toxicity was observed in preclinical data but not in clinical studies.60,84
Targeting nonsense mutations. Ataluren is an investigational, orally administered nonsense mutation suppression therapy (through the read-through of stop codons).37 Early clinical evidence supporting the use of ataluren in DMD was seen in an open-label, dose-ranging, phase 2a study (ClinicalTrial.gov Identifier: NCT00264888) in male DMD patients (≥ 5 years old) caused by nonsense mutation. The study demonstrated a modest (61% ) increase in dystrophin expression in 23 of 38 patients after 28 days of treatment.37,91,92
However, a phase 2b randomized, double-blind, placebo-controlled trial (ClinicalTrial.gov Identifier: NCT00592553) and a subsequent confirmatory ACT DMD phase 3 study (ClinicalTrial.gov Identifier: NCT01826487) did not meet their primary endpoint of improvement in ambulation after 48 weeks as measured by the 6-minute walk test.37,93,94 In ACT DMD, approximately 74% of the ataluren group did not experience disease progression, compared with 56% of the control group (P = 0386), measured by a change in the 6-minute walk test, which assessed ambulatory decline.37,95
Based on limited data showing that ataluren is effective and well tolerated, the European Medicines Agency has given conditional approval for clinical use of the drug in Europe. However, ataluren was rejected by the FDA as a candidate therapy for DMD in the United States.22 Late-stage clinical studies of ataluren are ongoing in the United States.
AAV gene transfer with microdystrophin. Limitations on traditional gene-replacement therapy prompted exploration of gene-editing strategies for treating DMD, including using AAV-based vectors to transfer microdystrophin, an engineered version of DMD, into target muscles.43 The microdystrophin gene is designed to produce a functional, truncated form of dystrophin, thus improving muscular function.
There are 3 ongoing investigational microdystrophin gene therapies that are in clinical development (ClinicalTrial.gov Identifier: NCT03368742 (IGNITE DMD), NCT04281485 (CIFFREO), and NCT05096221 (EMBARK)).38,82
IGNITE DMD is a phase 1/2 randomized, controlled, single-ascending dose trial evaluating the safety and efficacy of a SGT-001, single IV infusion of AAV9 vector containing a microdystrophin construct in DMD patients (4-17 years old) for 12 months. At the conclusion of the trial, treatment and control groups will be followed for 5 years. The primary efficacy endpoint is the change from baseline in microdystrophin protein production in muscle-biopsy material, using western blot testing.96 Long-term interim data on biopsy findings from three patients demonstrated clinical evidence of durable microdystrophin protein expression after 2 years of treatment.96,97
The CIFFREO trial will assess the safety and efficacy of the PF-06939926 microdystrophin gene therapy, an investigational AAV9 containing microdystrophin, in approximately 99 ambulatory DMD patients (4-7 years of age). The study is a randomized, double-blind, placebo-controlled, multicenter phase 3 trial. The primary efficacy end-point is the change from baseline in the North Star Ambulatory Assessment (NSAA), which measures gross motor function. This will be assessed at 52 weeks; all study participants will be followed for a total of 5 years post-treatment.98,99,100 Due to unexpected patient death (in a non-ambulatory cohort) in the phase 1b (in a non-ambulatory cohort) in the phase 1b (ClinicalTrial.gov Identifier: (NCT03362502) trial, microdystrophin gene therapy was immediately placed on clinical hold.101,102 The amended study protocol required that all participants undergo one week of in-hospital observation after receiving treatment.102
The EMBARK study is a global, randomized, double-blind, placebo-controlled, phase 3 trial that is evaluating the safety and efficacy of SRP-9001, which is a rAAVrh74.MHCK7.microdystrophin gene therapy. The AAV vector (rAAVrh74) contains the microdystrophin construct, driven by the skeletal and cardiac muscle–specific promoter, MHCK7.98,99 In the EMBARK study, approximately 120 participants with DMD (4-7 years of age) will be enrolled. The primary efficacy endpoint includes the change from baseline to week 52 in the NSAA total score.99 Based on SRP-9001, data demonstrating consistent statistically significant functional improvements in NSAA total scores and timed function tests (after one-year post- treatment) in DMD patients from previous studies and an integrated analysis from multiple studies (ClinicalTrial.gov Identifier: NCT03375164, NCT03769116, and NCT04626674), the ongoing EMBARK has great promise.103,104
Challenges ahead, but advancements realized
Novel gene-based therapies show significant potential for transforming the treatment of NMDs. The complex pathologies of NMDs have been a huge challenge to disease management in an area once considered unremediable by gene-based therapy. However, advancements in precision medicine – specifically, gene-delivery systems (for example, AAV9 and AAVrh74 vectors) combined with gene modification strategies (ASOs and AAV-mediated silencing) – have the potential to, first, revolutionize standards of care for sporadic and inherited NMDs and, second, significantly reduce disease burden.6
What will be determined to be the “best” therapeutic approach will, likely, vary from NMD to NMD; further investigation is required to determine which agents offer optimal clinical efficacy and safety profiles.43 Furthermore, the key to therapeutic success will continue to be early detection and diagnosis – first, by better understanding disease pathology and drug targets and, second, by validation of reliable biomarkers that are predictive of therapeutic benefit.4,5
To sum up, development challenges remain, but therapeutic approaches to ALS, SMA, and DMD that utilize novel gene-delivery and gene-manipulation tools show great promise.
Ms. Yewhalashet is a student in the masters of business and science program, with a concentration in healthcare economics, at Keck Graduate Institute Henry E. Riggs School of Applied Life Sciences, Claremont, Calif. Dr. Davis is professor of practice in clinical and regulatory affairs, Keck Graduate Institute Henry E. Riggs School of Applied Life Sciences.
References
1. Aitken M et al. Understanding neuromuscular disease care. IQVIA [Internet]. Oct 30, 2018. Accessed Mar 1, 2022. https://www.iqvia.com/insights/the-iqvia-institute/reports/understanding-neuromuscular-disease-care.
2. National Institute of Neurological Disorders and Stroke. Neurological diagnostic tests and procedures fact sheet. Updated Nov 15, 2021. Ac-cessed Mar 1, 2022. http://www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Fact-Sheets/Neurological-Diagnostic-Tests-and-Procedures-Fact.
3. Deenen JCW et al. The epidemiology of neuromuscular disorders: A comprehensive overview of the literature. J Neuromuscul Dis. 2015;2(1):73-85.
4. Cavazzoni P. The path forward: Advancing treatments and cures for neurodegenerative diseases. U.S. Food and Drug Administration. Jul 29, 2021. Accessed Mar 1, 2022. http://www.fda.gov/news-events/congressional-testimony/path-forward-advancing-treatments-and-cures-neurodegenerative-diseases-07292021.
5. Martier R, Konstantinova P. Gene therapy for neurodegenerative diseases: Slowing down the ticking clock. Front Neurosci. 2020 Sep 18;14:580179. doi: 10.3389/fnins.2020.580179.
6. Sun J, Roy S. Gene-based therapies for neurodegenerative diseases. Nat Neurosci. 2021 Mar;24(3):297-311. doi:10.1038/s41593-020-00778-1.
7. Amado DA, Davidson BL. Gene therapy for ALS: A review. Mol Ther. 2021 Dec 1;29(12):3345-58. doi:10.1016/j.ymthe.2021.04.008.
8. Yun Y, Ha Y. CRISPR/Cas9-mediated gene correction to understand ALS. Int J Mol Sci. 2020;21(11):3801. doi:10.3390/ijms21113801.
9. National Institute of Neurological Disorders and Stroke. Amyotrophic lateral sclerosis (ALS) fact sheet. Updated Nov 15, 2021. Accessed Mar 1, 2022. http://www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Fact-Sheets/Amyotrophic-Lateral-Sclerosis-ALS-Fact-Sheet.
10. Cappella M et al. Gene therapy for ALS – A perspective. Int J Mol Sci. 2019;20(18):4388. doi:10.3390/ijms20184388.
11. Abramzon YA, Fratta P, Traynor BJ, Chia R. The Overlapping Genetics of Amyotrophic Lateral Sclerosis and Frontotemporal Dementia. Front Neurosci. 2020;14. Accessed August 18, 2022. https://www.frontiersin.org/articles/10.3389/fnins.2020.00042
12. Giannini M, Bayona-Feliu A, Sproviero D, Barroso SI, Cereda C, Aguilera A. TDP-43 mutations link Amyotrophic Lateral Sclerosis with R-loop homeostasis and R loop-mediated DNA damage. PLOS Genet. 2020;16(12):e1009260. doi:10.1371/journal.pgen.1009260
13. FDA-approved drugs for treating ALS. The ALS Association [Internet]. Accessed Mar 1, 2022. http://www.als.org/navigating-als/living-with-als/fda-approved-drugs.
14. Jensen TL et al. Current and future prospects for gene therapy for rare genetic diseases affecting the brain and spinal cord. Front Mol Neurosci. 2021 Oct 6;14:695937. doi:10.3389/fnmol.2021.695937.
15. ALS Gene Targeted Therapies. The ALS Association. Accessed August 22, 2022. https://www.als.org/understanding-als/who-gets-als/genetic-testing/als-gene-targeted-therapies
16. Tofersen for ALS clears phase 1/2 trial, now in phase 3. Advances in Motion. Massachusetts General Hospital [Internet]. Sep 30, 2020. Accessed Mar 1, 2022. https://advances.massgeneral.org/neuro/journal.aspx?id=1699.17. Biogen. A study to evaluate the efficacy, safety, tol-erability, pharmacokinetics, and pharmacodynamics of BIIB067 administered to adult subjects with amyotrophic lateral sclerosis and confirmed superoxide dismutase 1 mutation. ClinicalTrials.gov Identifier: NCT02623699. Updated Jul 25, 2021. Accessed Feb 17, 2022. https://clinicaltrials.gov/ct2/show/NCT02623699.
18. Biogen. Biogen announces topline results from the tofersen phase 3 study and its open-label Extension in SOD1-ALS. Press release. Oct 17, 2021. Accessed Mar 1, 2022. https://investors.biogen.com/news-releases/news-release-details/biogen-announces-topline-results-tofersen-phase-3-study-and-its.
19. Biogen. An extension study to assess the long-term safety, tolerability, pharmacokinetics, and effect on disease progression of BIIB067 ad-ministered to previously treated adults with amyotrophic lateral sclerosis caused by superoxide dismutase 1 mutation. ClinicalTrials.gov Identi-fier: NCT03070119. Updated Sep 10, 2021. Accessed Feb 17, 2022. https://clinicaltrials.gov/ct2/show/NCT03070119.
20. MS MW. #AANAM – ATLAS Trial to Assess Tofersen in Presymptomatic SOD1 ALS. Accessed February 19, 2022. https://alsnewstoday.com/news-posts/2021/04/23/aanam-atlas-clinical-trial- tofersen-presymptomatic-sod1-als-patients/
21.Biogen. A phase 3 randomized, placebo-controlled trial with a longitudinal natural history run-in and open-label extension to evaluate BIIB067 initiated in clinically presymptomatic adults with a confirmed superoxide dismutase 1 mutation. ClinicalTrials.gov Identifier: NCT04856982. Updated Feb 18, 2022. Accessed Mar 1, 2022. https://clinicaltrials.gov/ct2/show/NCT04856982.
22. Latozinemab | ALZFORUM. Accessed August 19, 2022. https://www.alzforum.org/therapeutics/latozinemab
23. Alector Presents AL001 (latozinemab) Data from the FTD-C9orf72 Cohort of the INFRONT-2 Phase 2 Clinical Trial | Alector. Accessed August 18, 2022. https://investors.alector.com/news- releas-es/news-release-details/alector-presents-al001-latozinemab-data-ftd-c9orf72-cohort/
24. Alector Announces First Participant Dosed in Phase 2 Study Evaluating AL001 in Amyotrophic Lateral Sclerosis (ALS) | Alector. Accessed August 18, 2022. https://investors.alector.com/news- releases/news-release-details/lector-announces-first-participant-dosed-phase-2-study-0/ 25. A Phase 2 Study to Evaluate AL001 in C9orf72-Associated ALS - Full Text View - ClinicalTrials.gov. Accessed August 19, 2022. https://clinicaltrials.gov/ct2/show/NCT05053035
26.TPN-101 | ALZFORUM. Accessed August 19, 2022. https://www.alzforum.org/therapeutics/tpn- 101
27. Transposon Therapeutics, Inc. A Phase 2a Study of TPN-101 in Patients With Amyotrophic Lateral Sclerosis (ALS) and/or Frontotemporal Dementia (FTD) Associated With Hexanucleotide Repeat Expansion in the C9orf72 Gene (C9ORF72 ALS/FTD). clinicaltrials.gov; 2022. Ac-cessed August 17, 2022. https://clinicaltrials.gov/ct2/show/NCT04993755
28. Kerk SY, Bai Y, Smith J, et al. Homozygous ALS-linked FUS P525L mutations cell- autonomously perturb transcriptome profile and chem-oreceptor signaling in human iPSC microglia. Stem Cell Rep. 2022;17(3):678-692. doi:10.1016/j.stemcr.2022.01.004
29. ION363 | ALZFORUM. Accessed August 19, 2022. https://www.alzforum.org/therapeutics/ion363 30. Ionis Pharmaceuticals, Inc. A Phase 1-3 Study to Evaluate the Efficacy, Safety, Pharmacokinetics and Pharmacodynamics of Intrathecally Administered ION363 in Amyo-trophic Lateral Sclerosis Patients With Fused in Sarcoma Mutations (FUS-ALS). clinicaltrials.gov; 2022. Accessed August 18, 2022. https://clinicaltrials.gov/ct2/show/NCT04768972
31. PhD LF. Engensis (VM202) - ALS News Today. Accessed August 19, 2022. https://alsnewstoday.com/vm202/
32. Helixmith Co., Ltd. A 6-Month Extension Study Following Protocol VMALS-002-2 (A Phase 2a, Double-Blind, Randomized, Place-bo-Controlled, Multicenter Study to Assess the Safety of Engensis in Participants With Amyotrophic Lateral Sclerosis). clinicaltrials.gov; 2022. Accessed August 18, 2022. https://clinicaltrials.gov/ct2/show/NCT05176093 33. Safety of Engensis in Participants With Amyotrophic Lateral Sclerosis - Full Text View - ClinicalTrials.gov. Accessed August 19, 2022. https://clinicaltrials.gov/ct2/show/NCT04632225
34. Biogen. A phase 1, safety, tolerability, and distribution study of a microdose of radiolabeled BIIB067 co-administered with BIIB067 to healthy adults. ClinicalTrials.gov Identifier: NCT03764488. Updated Jul 19, 2021. Accessed Mar 1, 2022. https://clinicaltrials.gov/ct2/show/NCT03764488.
35. Ionis Pharmaceuticals Inc. A phase 1, double-blind, placebo-controlled, dose-escalation study of the safety, tolerability, and pharmacokinet-ics of ISIS 333611 administered intrathecally to patients with familial amyotrophic lateral sclerosis due to superoxide dismutase 1 gene muta-tions. ClinicalTrials.gov Identifier: NCT01041222. Updated Apr 13, 2012. Accessed Mar 1, 2022. https://clinicaltrials.gov/ct2/show/NCT01041222.
36. Messina S, Sframeli M. New treatments in spinal muscular atrophy: Positive results and new challenges. J Clin Med. 2020;9(7):2222. doi:10.3390/jcm9072222.
37. Scoto M et al. Genetic therapies for inherited neuromuscular disorders. Lancet Child Adolesc Health. 2018 Aug;2(8):600-9. doi:10.1016/S2352-4642(18)30140-8.
38. Abreu NJ, Waldrop MA. Overview of gene therapy in spinal muscular atrophy and Duchenne muscular dystrophy. Pediatr Pulmonol. 2021 Apr;56(4):710-20. doi:10.1002/ppul.25055.
39. Brandsema J, Cappa R. Genetically targeted therapies for inherited neuromuscular disorders. Practical Neurology [Internet]. Jul/Aug 2021:69-73. Accessed Mar 1, 2022. https://practicalneurology.com/articles/2021-july-aug/genetically-targeted-therapies-for-inherited-neuromuscular-disorders/pdf.
40. Ojala KS et al. In search of a cure: The development of therapeutics to alter the progression of spinal muscular atrophy. Brain Sci. 2021;11(2):194. doi:10.3390/brainsci11020194.
41. McCall S. Cure SMA Releases Updated Drug Pipeline. Cure SMA. Published December 13, 2021. Accessed August 21, 2022. https://www.curesma.org/cure-sma-releases-updated-drug-pipeline- 2021/ 42. FDA approves first drug for spinal muscular atrophy. U.S. Food and Drug Administration. News release. Dec 23, 2016. Accessed Mar 1, 2022. http://www.fda.gov/news-events/press-announcements/fda-approves-first-drug-spinal-muscular-atrophy.43. Kirschner J. Postnatal gene therapy for neuromuscular diseases – Opportunities and limitations. J Perinat Med. 2021 Sep;49(8):1011-5. doi:10.1515/jpm-2021-0435.
43. Terryn J, Verfaillie CM, Van Damme P. Tweaking Progranulin Expression: Therapeutic Avenues and Opportunities. Front Mol Neurosci. 2021;14. Accessed September 4, 2022. https://www.frontiersin.org/articles/10.3389/fnmol.2021.71303144.
44. Biogen. A phase 3, randomized, double-blind, sham-procedure controlled study to assess the clinical efficacy and safety of ISIS 396443 administered intrathecally in patients with later-onset spinal muscular atrophy. ClinicalTrials.gov Identifier: NCT02292537. Updated Feb 17, 2021. Accessed Mar 1, 2022. https://clinicaltrials.gov/ct2/show/study/NCT02292537.
45. Why Spinraza/later-onset studies. SPINRAZA® (nusinersen) [Internet]. Accessed Mar 1, 2022. www.spinraza.com/en_us/home/why-spinraza/later-onset-studies.html#scroll-tabs.
46. Biogen. A Phase 3, Randomized, Double-Blind, Sham-Procedure Controlled Study to Assess the Clinical Efficacy and Safety of ISIS 396443 Administered Intrathecally in Patients With Infantile- Onset Spinal Muscular Atrophy. clinicaltrials.gov; 2021. Accessed February 10, 2022. https://clinicaltrials.gov/ct2/show/results/NCT02193074
47. Early-onset SMA (Type 1) | SPINRAZA® (nusinersen). Accessed Mar 1, 2022. https://www.spinraza-hcp.com/en_us/home/why-spinraza/about-spinraza.html.
48. Finkel RS et al; ENDEAR Study Group. Nusinersen versus sham control in infantile-onset spinal muscular atrophy. N Engl J Med. 2017;377(18):1723-32. doi: 10.1056/NEJMoa1702752.
49. Biogen. An open-label study to assess the efficacy, safety, tolerability, and pharmacokinetics of multiple doses of ISIS 396443 delivered intrathecally to subjects with genetically diagnosed and presymptomatic spinal muscular atrophy. ClinicalTrials.gov Identifier: NCT02386553. Updated Nov 18, 2021. Accessed Mar 1, 2022. https://clinicaltrials.gov/ct2/show/NCT02386553.
50. De Vivo DC et al; NURTURE Study Group. Nusinersen initiated in infants during the presymptomatic stage of spinal muscular atrophy: In-terim efficacy and safety results from the phase 2 NURTURE study. Neuromuscul Disord. 2019 Nov;29(11):842-56. doi:10.1016/j.nmd.2019.09.007.
51. Why Spinraza/presymptomatic study. SPINRAZA® (nusinersen) [Internet]. Accessed Feb 22, 2022. www.spinraza.com/en_us/home/why-spinraza/presymptomatic-study.html#scroll-tabs.
52. FDA approves oral treatment for spinal muscular atrophy. U.S. Food and Drug Administration. News release. Aug 7, 2020. Accessed Mar 1, 2022. http://www.fda.gov/news-events/press-announcements/fda-approves-oral-treatment-spinal-muscular-atrophy.
53. Hoffmann-La Roche. A two-part seamless, open-label, multicenter study to investigate the safety, tolerability, pharmacokinetics, pharmaco-dynamics and efficacy of risdiplam (RO7034067) in infants with type 1 spinal muscular atrophy. ClinicalTrials.gov Identifier: NCT02913482. Updated Jan 21, 2022. Accessed Mar 1, 2022. https://clinicaltrials.gov/ct2/show/NCT02913482.
54. Hoffmann-La Roche. A two-part seamless, multi-center randomized, placebo-controlled, double-blind study to investigate the safety, tolera-bility, pharmacokinetics, pharmacodynamics and efficacy of risdiplam (RO7034067) in type 2 and 3 spinal muscular atrophy patients. Clinical-Trials.gov Identifier: NCT02908685. Updated Dec 28, 2021. Accessed Mar 1, 2022. https://clinicaltrials.gov/ct2/show/NCT02908685.
55. Genentech. Genentech’s risdiplam shows significant improvement in survival and motor milestones in infants with type 1 spinal muscular atrophy (SMA). Press release. Apr 27, 2020. Accessed Mar 1, 2022. http://www.gene.com/media/press-releases/14847/2020-04-27/genentechs-risdiplam-shows-significant-i
56. Hoffmann-La Roche. An open-label study to investigate the safety, tolerability, and pharmacokinetics/pharmacodynamics of risdiplam (RO7034067) in adult and pediatric patients with spinal muscular atrophy. ClinicalTrials.gov Identifier: NCT03032172. Updated Jan 27, 2022. Accessed Mar 1, 2022. https://clinicaltrials.gov/ct2/show/NCT03032172.
57. Hoffmann-La Roche. An open-label study of risdiplam in infants with genetically diagnosed and presymptomatic spinal muscular atrophy. ClinicalTrials.gov Identifier: NCT03779334. Updated Jan 27, 2022. Accessed Mar 1, 2022. https://clinicaltrials.gov/ct2/show/NCT03779334.
58. McCall S. Update on Genentech/Roche Initiation of MANATEE Clinical Study. Cure SMA. Published October 20, 2021. Accessed August 20, 2022. https://www.curesma.org/update-on- genentech-roche-initiation-of-manatee-clinical-study/
59. Abati E, Manini A, Comi GP, Corti S. Inhibition of myostatin and related signaling pathways for the treatment of muscle atrophy in motor neuron diseases. Cell Mol Life Sci. 2022;79(7):374. doi:10.1007/s00018-022-04408-w
60. FDA approves innovative gene therapy to treat pediatric patients with spinal muscular atrophy, a rare disease and leading genetic cause of infant mortality. U.S. Food and Drug Administration. News release. May 24, 2019. Accessed Mar 1, 2022. http://www.fda.gov/news-events/press-announcements/fda-approves-innovative-gene-therapy-treat-pediatric-patients-spinal-muscular-atrophy-rare-disease.
61. Novartis Gene Therapies. Phase I gene transfer clinical trial for spinal muscular atrophy type 1 delivering AVXS-101. ClinicalTrials.gov Identifier: NCT02122952. Updated Jun 14, 2021. Accessed Mar 1, 2022. https://clinicaltrials.gov/ct2/show/NCT02122952.
62. Novartis Gene Therapies. Phase 3, open-label, single-arm, single-dose gene replacement therapy clinical trial for patients with spinal mus-cular atrophy type 1 with one or two SMN2 copies delivering AVXS-101 by intravenous infusion. ClinicalTrials.gov Identifier: NCT03306277. Updated Jun 14, 2021. Accessed Feb 21, 2022. https://clinicaltrials.gov/ct2/show/NCT03306277.
63. Mendell JR et al. Single-dose gene-replacement therapy for spinal muscular atrophy. N Engl J Med. 2017;377(18):1713-22. doi:10.1056/NEJMoa1706198.
64. Symptomatic study results. ZOLGENSMA [Internet]. Updated Nov 2021. Accessed Mar 1, 2022. Error! Hyperlink reference not valid..
65. Novartis Gene Therapies. A global study of a single, one-time dose of AVXS-101 delivered to infants with genetically diagnosed and pre-symptomatic spinal muscular atrophy with multiple copies of SMN2. ClinicalTrials.gov Identifier: NCT03505099. Updated Jan 1, 2022. Ac-cessed Mar 1, 2022. https://clinicaltrials.gov/ct2/show/NCT03505099.
66. Chiu W et al. Current genetics and potential gene-targeting therapeutics for neuromuscular diseases. Int J Mol Sci. 2020 Dec;21(24):9589. doi:10.3390/ijms21249589.
67. Novartis Gene Therapies. A long-term follow-up study of patients in the clinical trials for spinal muscular atrophy receiving AVXS-101. Clini-calTrials.gov Identifier: NCT04042025. Updated Jun 9, 2021. Accessed Mar 1, 2022. https://clinicaltrials.gov/ct2/show/NCT04042025.
68. Novartis Gene Therapies. Phase 3, open-label, single-arm, single-dose gene replacement therapy clinical trial for patients with spinal mus-cular atrophy type 1 with one or two SMN2 copies delivering AVXS-101 by intravenous infusion. ClinicalTrials.gov Identifier: NCT0383718. Up-dated Jan 11, 2022. Accessed Mar 1, 2022. https://clinicaltrials.gov/ct2/show/NCT03837184.
69. Biogen. An open-label, dose escalation study to assess the safety, tolerability and dose-range finding of multiple doses of ISIS 396443 de-livered intrathecally to patients with spinal muscular atrophy. ClinicalTrials.gov Identifier: NCT01703988. Updated Apr 13, 2021. Accessed Mar 1, 2022. https://clinicaltrials.gov/ct2/show/NCT01703988.
70. Biogen. A study to assess the efficacy, safety, tolerability, and pharmacokinetics of multiple doses of ISIS 396443 delivered intrathecally to patients with infantile-onset spinal muscular atrophy. ClinicalTrials.gov Identifier: NCT01839656. Updated Feb 17, 2021. Accessed Mar 1, 2022. https://clinicaltrials.gov/ct2/show/NCT01839656.
71. Biogen. An open-label extension study for patients with spinal muscular atrophy who previously participated in investigational studies of ISIS 396443. ClinicalTrials.gov Identifier: NCT02594124. Updated Nov 15, 2021. Accessed Mar 1, 2022. https://clinicaltrials.gov/ct2/show/NCT02594124.
72. Biogen. Escalating dose and randomized, controlled study of nusinersen (BIIB058) in participants with spinal muscular atrophy. ClinicalTri-als.gov Identifier: NCT04089566. Updated Feb 24, 2022. Accessed Mar 1, 2022. https://clinicaltrials.gov/ct2/show/NCT04089566.
73. National Center for Advancing Translational Sciences. Duchenne muscular dystrophy. Genetic and Rare Diseases Information Center. Up-dated Nov 2, 2020. Accessed Mar 1, 2022. https://rarediseases.info.nih.gov/diseases/6291/duchenne-muscular-dystrophy.
74. Matsuo M. Antisense oligonucleotide-mediated exon-skipping therapies: Precision medicine spreading from Duchenne muscular dystrophy. JMA J. 2021 Jul 15;4(3):232-40. doi:10.31662/jmaj.2021-0019.
75. FDA approves drug to treat Duchenne muscular dystrophy. U.S. Food and Drug Administration. News release. Feb 9, 2017. Accessed Mar 1, 2022. http://www.fda.gov/news-events/press-announcements/fda-approves-drug-treat-duchenne-muscular-dystrophy.74.
76. Duan D. Dystrophin gene replacement and gene repair therapy for Duchenne muscular dystrophy in 2016: An interview. Hum Gene Ther Clin Dev. 2016 Mar;27(1):9-18. doi:10.1089/humc.2016.001.
77. EXONDYS 51®. Parent Project Muscular Dystrophy. Accessed August 21, 2022. https://www.parentprojectmd.org/drug-development-pipeline/exondys-51/
78. Sarepta Therapeutics, Inc. A Randomized, Double-Blind, Placebo-Controlled, Multiple Dose Efficacy, Safety, Tolerability and Pharmacoki-netics Study of AVI-4658(Eteplirsen),in the Treatment of Ambulant Subjects With Duchenne Muscular Dystrophy. clinicaltrials.gov; 2020. Ac-cessed August 18, 2022. https://clinicaltrials.gov/ct2/show/NCT01396239
79. Sarepta Therapeutics, Inc. Clinical Study to Assess the Safety Fo AVI-4658 in Subjects With Duchenne Muscular Dystrophy Due to a Frame-Shift Mutation Amenable to Correction by Skipping Exon 51. clinicaltrials.gov; 2015. Accessed August 18, 2022. https://clinicaltrials.gov/ct2/show/study/NCT00844597
80. Sarepta Therapeutics, Inc. A 2-part, randomized, double-blind, placebo-controlled, dose-titration, safety, tolerability, and pharmacokinetics study (Part 1) followed by an open-label efficacy and safety evaluation (Part 2) of SRP-4053 in patients with Duchenne muscular dystrophy amenable to exon 53 skipping. ClinicalTrials.gov Identifier: NCT02310906. Updated Oct 19, 2020. Accessed Mar 1, 2022. https://clinicaltrials.gov/ct2/show/results/NCT02310906.
81. Commissioner O of the. FDA grants accelerated approval to first drug for Duchenne muscular dystrophy. FDA. Published March 24, 2020. Accessed August 21, 2022. hDuchenne Muscular Dystrophy Amenable to Exon 51-Skipping Treatment. clinicaltrials.gov; 2022. Accessed Au-gust 18, 2022. https://clinicaltrials.gov/ct2/show/NCT04004065
109. National Center of Neurology and Psychiatry, Japan. Exploratory study of NS-065/NCNP-01 in Duchenne muscular dystrophy. ClinicalTri-als.gov Identifier: NCT02081625; Updated Feb 26, 2020. Accessed Mar 2, 2022. https://clinicaltrialsttps://www.fda.gov/news-events/press-announcements/fda-grants-accelerated-approval-first-drug-duchenne-muscular- dys-trophy
82. Duchenne Drug Development Pipeline. Parent Project Muscular Dystrophy. Accessed August 21, 2022. https://www.parentprojectmd.org/duchenne-drug-development-pipeline/
83. Sarepta Therapeutics Provides Update on SRP-5051 for the Treatment of Duchenne Muscular Dystrophy | Sarepta Therapeutics, Inc. Ac-cessed August 22, 2022. https://investorrelations.sarepta.com/news-releases/news-release-details/sarepta-therapeutics- pro-vides-update-srp-5051-treatment-duchenne
84. Sarepta Therapeutics, Inc. An Open-Label Extension Study for Patients With Duchenne Muscular Dystrophy Who Participated in Studies of SRP-5051. clinicaltrials.gov; 2021. Accessed August 18, 2022. https://clinicaltrials.gov/ct2/show/NCT03675126
85. VYONDYS 53. Prescribing information. Sarepta Therapeutics Inc.; 2019. Accessed Mar 2, 2022. http://www.accessdata.fda.gov/drugsatfda_docs/label/2019/211970s000lbl.pdf.
86. NS Pharma Inc. Long-term use of viltolarsen in boys with Duchenne muscular dystrophy in clinical practice (VILT-502). ClinicalTrials.gov Identifier: NCT04687020. Updated Nov 22, 2021. Accessed Mar 1, 2022. https://clinicaltrials.gov/ct2/show/NCT04687020.
87. VILTEPSO. Prescribing information. NS Pharma; 2020. Accessed Mar 2, 2022. http://www.accessdata.fda.gov/drugsatfda_docs/label/2020/212154s000lbl.pdf.
88. FDA approves targeted treatment for rare Duchenne muscular dystrophy mutation. U.S. Food and Drug Administration. News release. Feb 25, 2021. Accessed Mar 1, 2022. http://www.fda.gov/news-events/press-announcements/fda-approves-targeted-treatment-rare-duchenne-muscular-dystrophy-mutation-0.
89. Sarepta Therapeutics Inc. A double-blind, placebo-controlled, multi-center study with an open-label extension to evaluate the efficacy and safety of SRP-4045 and SRP-4053 in patients with Duchenne muscular dystrophy. Clinicaltrials.gov Identifier: NCT02500381. Updated Aug 19, 2021. Accessed Mar 1, 2022. https://clinicaltrials.gov/ct2/show/NCT02500381.
90. AMONDYS 45. Prescribing information. Sarepta Therapeutics Inc.; 2021. Accessed Feb 22, 2022. http://www.accessdata.fda.gov/drugsatfda_docs/label/2021/213026lbl.pdf.
91. Finkel RS et al. Phase 2a study of ataluren-mediated dystrophin production in patients with nonsense mutation Duchenne muscular dys-trophy. PLoS ONE. 2013;8(12):e81302. doi:10.1371/journal.pone.0081302.
92. PTC Therapeutics. A phase 2 study of PTC124 as an oral treatment for nonsense-mutation-mediated Duchenne muscular dystrophy. Clini-calTrials.gov Identifier: NCT00264888. Updated Jan 14, 2009. Accessed Mar 1, 2022. https://clinicaltrials.gov/ct2/show/NCT00264888.
93. PTC Therapeutics. A phase 2B efficacy and safety study of PTC124 in subjects with nonsense-mutation-mediated Duchenne and Becker muscular dystrophy. ClinicalTrials.gov Identifier: NCT00592553. Updated Apr 7, 2020. Accessed Mar 1, 2022. https://clinicaltrials.gov/ct2/show/NCT00592553.
94. PTC Therapeutics. A phase 3 efficacy and safety study of ataluren in patients with nonsense mutation dystrophinopathy. ClinicalTrials.gov Identifier: NCT01826487. Updated Aug 4, 2020. Accessed Mar 1, 2022. https://clinicaltrials.gov/ct2/show/NCT01826487.
95. Bushby K et al; PTC124-GD-007-DMD Study Group. Ataluren treatment of patients with nonsense mutation dystrophinopathy. Muscle Nerve. 2014 Oct;50(4):477-87. doi:10.1002/mus.24332.
96. Solid Biosciences LLC. A randomized, controlled, open-label, single-ascending dose, phase I/II study to investigate the safety and tolerabil-ity, and efficacy of intravenous SGT-001 in male adolescents and children with Duchenne muscular dystrophy. ClinicalTrials.gov Identifier: NCT03368742. Updated Aug 24, 2021. Accessed Mar 1, 2022. https://clinicaltrials.gov/ct2/show/NCT03368742.
97. Solid Biosciences reports 1.5-year data from patients in the ongoing IGNITE DMD phase I/II clinical trial of SGT-001. Press release. Solid Biosciences. Sep 27, 2021. Accessed Mar 2, 2022. http://www.solidbio.com/about/media/press-releases/solid-biosciences-reports-1-5-year-data-from-patients-in-the-ongoing-ignite-dmd-phase-i-ii-clinical-trial-of-sgt-001.
98. Potter RA et al. Dose-escalation study of systemically delivered rAAVrh74.MHCK7.microdystrophin in the mdx mouse model of Duchenne muscular dystrophy. Hum Gene Ther. 2021 Apr;32(7-8):375-89. doi:10.1089/hum.2019.255.
99. Sarepta Therapeutics, Inc. A Phase 3 Multinational, Randomized, Double-Blind, Placebo- Controlled Systemic Gene Delivery Study to Evaluate the Safety and Efficacy of SRP-9001 in Patients With Duchenne Muscular Dystrophy (EMBARK). clinicaltrials.gov; 2022. Accessed August 18, 2022. https://clinicaltrials.gov/ct2/show/NCT05096221
100. Pfizer. A PHASE 3, MULTICENTER, RANDOMIZED, DOUBLE-BLIND, PLACEBO CONTROLLED STUDY TO EVALUATE THE SAFETY AND EFFICACY OF PF 06939926 FOR THE TREATMENT OF DUCHENNE MUSCULAR DYSTROPHY. clinicaltrials.gov; 2022. Accessed August 18, 2022. https://clinicaltrials.gov/ct2/show/NCT04281485
101. Pfizer. A phase 1B multicenter open-label, single ascending dose study to evaluate the safety and tolerability of PF-06939926 in ambula-tory and non-ambulatory subjects with Duchenne muscular dystrophy. ClinicalTrials.gov Identifier: NCT03362502. Updated Mar 2, 2022. Ac-cessed Mar 2, 2022. https://clinicaltrials.gov/ct2/show/NCT03362502.
102. MS MW. Phase 3 CIFFREO DMD Gene Therapy Trial Slated to Begin in June in US. Accessed August 21, 2022. https://musculardystrophynews.com/news/phase-3-trial-of-pfizers-gene-therapy- expected-to-open-in-us-in-june/
103. SRP-9001. Parent Project Muscular Dystrophy. Accessed August 22, 2022. https://www.parentprojectmd.org/drug-development-pipeline/srp-9001-micro-dystrophin-gene- transfer/
104. Sarepta Therapeutics’ Investigational Gene Therapy SRP-9001 for Duchenne Muscular Dystrophy Demonstrates Significant Functional Improvements Across Multiple Studies | Sarepta Therapeutics, Inc. Accessed August 22, 2022. https://investorrelations.sarepta.com/news-releases/news-release- details/sarepta-therapeutics-investigational-gene-therapy-srp-9001
105. Sarepta Therapeutics, Inc. An Open-Label Safety, Tolerability, and Efficacy Study of Eteplirsen in Patients With Duchenne Muscular Dys-trophy Who Have Completed Study 4658-102.clinicaltrials.gov; 2022. Accessed August 18, 2022. https://clinicaltrials.gov/ct2/show/NCT03985878
106. Sarepta Therapeutics, Inc. An Open-Label Safety, Tolerability, and Pharmacokinetics Study of Eteplirsen in Young Patients With Duchenne Mus-cular Dystrophy Amenable to Exon 51 Skipping. clinicaltrials.gov; 2021. Accessed August 18, 2022. https://clinicaltrials.gov/ct2/show/NCT03218995
107.Sarepta Therapeutics, Inc. A Randomized, Double-Blind, Dose Finding and Comparison Study of the Safety and Efficacy of a High Dose of Eteplirsen, Preceded by an Open-Label Dose Escalation, in Patients With Duchenne Muscular Dystrophy With Deletion Mutations Amenable to Exon 51 Skipping. clinicaltrials.gov; 2022. Accessed August 18, 2022. https://clinicaltrials.gov/ct2/show/NCT03992430
108. Sarepta Therapeutics, Inc. A Phase 2, Two-Part, Multiple-Ascending-Dose Study of SRP-5051 for Dose Determination, Then Dose Ex-pansion, in Patients With .gov/ct2/show/NCT02081625.
110. NS Pharma Inc. A phase II, dose finding study to assess the safety, tolerability, pharmacokinetics, and pharmacodynamics of NS-065/NCNP-01 in boys with Duchenne muscular dystrophy (DMD). ClinicalTrials.gov Identifier: NCT02740972. Updated Dec 7, 2021. Ac-cessed Mar 2, 2022. https://clinicaltrials.gov/ct2/show/NCT02740972.
111. NS Pharma Inc. A phase II, open-label, extension study to assess the safety and efficacy of NS-065/NCNP-01 in boys with Duchenne muscular dystrophy (DMD). ClinicalTrials.gov Identifier: NCT03167255. Updated Nov 24, 2021. Accessed Mar 2, 2022. https://clinicaltrials.gov/ct2/show/NCT03167255.
112. NS Pharma Inc. A phase 2 open label study to assess the safety, tolerability, and efficacy of viltolarsen in ambulant and non-ambulant boys with Duchenne muscular dystrophy (DMD) compared with natural history controls. ClinicalTrials.gov Identifier: NCT04956289. Updated Feb 1, 2022. Accessed Mar 2, 2022. https://clinicaltrials.gov/ct2/show/NCT04956289.
113. NS Pharma Inc. A phase 3 randomized, double-blind, placebo-controlled, multi-center study to assess the efficacy and safety of viltolarsen in ambulant boys with Duchenne muscular dystrophy (DMD). ClinicalTrials.gov Identifier: NCT04060199. Updated Nov 16, 2021. Accessed Mar 2, 2022. https://clinicaltrials.gov/ct2/show/NCT04060199.
114. NS Pharma Inc. A phase 3, multi-center, open-label extension study to assess the safety and efficacy of viltolarsen in ambulant boys with Duchenne muscular dystrophy (DMD). ClinicalTrials.gov Identifier: NCT04768062. Updated Nov 16, 2021. Accessed Mar 2, 2022. https://clinicaltrials.gov/ct2/show/NCT04768062.
115. Sarepta Therapeutics Inc. A randomized, double-blind, placebo-controlled, dose-titration, safety, tolerability, and pharmacokinetics study followed by an open-label safety and efficacy evaluation of SRP-4045 in advanced-stage patients with Duchenne muscular dystrophy amena-ble to exon 45 skipping. ClinicalTrials.gov Identifier: NCT02530905. Updated May 17, 2021. Accessed Mar 2, 2022. https://clinicaltrials.gov/ct2/show/NCT02530905.
116. Sarepta Therapeutics Inc. Long-term, open-label extension study for patients with Duchenne muscular dystrophy enrolled in clinical trials evaluating casimersen or golodirsen. ClinicalTrials.gov Identifier: NCT03532542. Updated Dec 20, 2021. Accessed Mar 2, 2022. https://clinicaltrials.gov/ct2/show/NCT03532542.
117. PTC Therapeutics. A phase 2 study of the safety, pharmacokinetics, and pharmacodynamics of ataluren (PTC124®) in patients aged ≥2 to <5 years old with nonsense mutation dystrophinopathy. ClinicalTrials.gov Identifier: NCT02819557. Updated Aug 28, 2020. Accessed Mar 2, 2022. https://clinicaltrials.gov/ct2/show/NCT02819557.
118. PTC Therapeutics. Phase 2, non-interventional, clinical study to assess dystrophin levels in subjects with nonsense mutation Duchenne muscular dystrophy who have been treated with ataluren for ≥ 9 months. ClinicalTrials.gov Identifier: NCT03796637. Updated Apr 10, 2020. Accessed Mar 2, 2022. https://clinicaltrials.gov/ct2/show/NCT03796637.
119. PTC Therapeutics. An Open-Label Study Evaluating the Safety and Pharmacokinetics of Ataluren in Children From ≥6 Months to <2 Years of Age With Nonsense Mutation Duchenne Muscular Dystrophy. clinicaltrials.gov; 2022. Accessed August 18, 2022. https://clinicaltrials.gov/ct2/show/NCT04336826 120. PTC Therapeutics. An open-label study for previously treated ataluren (PTC124®) pa-tients with nonsense mutation dystrophinopathy. ClinicalTrials.gov Identifier: NCT01557400. Updated Nov 25, 2020. Accessed Feb 21, 2022. https://clinicaltrials.gov/ct2/show/NCT01557400.
121. PTC Therapeutics. An open-label, safety study for ataluren (PTC124) patients with nonsense mutation dystrophinopathy. ClinicalTrials.gov Identifier: NCT01247207. Updated Feb 16, 2022. Accessed Mar 2, 2022. https://clinicaltrials.gov/ct2/show/NCT01247207.
122. PTC Therapeutics. A phase 3, randomized, double-blind, placebo-controlled efficacy and safety study of ataluren in patients with non-sense mutation Duchenne muscular dystrophy and open-label extension. ClinicalTrials.gov Identifier: NCT03179631. Updated Feb 8, 2022. Accessed Mar 2, 2022. https://clinicaltrials.gov/ct2/show/NCT03179631.
123. Sarepta Therapeutics, Inc. An Open-Label, Systemic Gene Delivery Study Using Commercial Process Material to Evaluate the Safety of and Expression From SRP-9001 in Subjects With Duchenne Muscular Dystrophy (ENDEAVOR). clinicaltrials.gov; 2022. Accessed August 18, 2022. https://clinicaltrials.gov/ct2/show/NCT04626674
124. Sarepta Therapeutics, Inc. Systemic Gene Delivery Phase I/IIa Clinical Trial for Duchenne Muscular Dystrophy Using RAA-Vrh74.MHCK7.Micro-Dystrophin (MicroDys-IV-001). clinicaltrials.gov; 2022. Accessed August 18, 2022. https://clinicaltrials.gov/ct2/show/NCT03375164
125. Sarepta Therapeutics Inc. A multicenter, randomized, double-blind, placebo-controlled trial for Duchenne muscular dystrophy using SRP-9001. ClinicalTrials.gov Identifier: NCT03769116. Updated Dec 2021. Accessed Mar 2, 2022. https://clinicaltrials.gov/ct2/show/NCT03769116.
126. Hoffmann-La Roche. A Two-Part, Seamless, Multi-Center, Randomized, Placebo-Controlled, Double-Blind Study to Investigate the Safety, Tolerability, Pharmacokinetics, Pharmacodynamics and Efficacy of RO7204239 in Combination With Risdiplam (RO7034067) in Ambulant Pa-tients With Spinal Muscular Atrophy. clinicaltrials.gov; 2022. Accessed September 1, 2022. https://clinicaltrials.gov/ct2/show/NCT05115110
Neuromuscular diseases (NMDs) are a broad classification of heterogeneous groups of disorders characterized by progressive muscle weakness resulting from muscle or nerve dysfunction.1 Diagnosis is based on symptoms and a full medical history, as well as on muscle and imaging tests (including electromyography, nerve-conduction studies, magnetic resonance imaging, muscle biopsy, and blood tests) to confirm or rule out specific NMDs.2 Early diagnosis of NMDs can be difficult because symptoms overlap with those of many other diseases.
Although individually, NMDs are rare, collectively, they affect approximately 250,000 people in the United States. Disease types vary in regard to cause, symptoms, prevalence, age of onset, progression, and severity. Functional impairment from any NMD can lead to lifelong morbidities and shortened life expectancy.1,3
Treatment options for NMDs are limited; most target symptoms, not disease progression. Although there is a need for safe and effective gene-based therapies for NMDs, there are challenges to developing and delivering such treatments that have impeded clinical success. These include a lack of understanding about disease pathology and drug targets, limited animal model systems, and few reliable biomarkers that are predictive of therapeutic success.4,5
Notwithstanding that challenges remain, our understanding of gene expression in NMDs has greatly advanced in the past few decades. This progress has translated into promising results in the gene-therapy field – thereby setting the stage for therapeutic approaches that use novel gene-delivery and gene-manipulation tools.6 These novel approaches include nonviral strategies, such as antisense oligonucleotides (ASOs), and viral-based strategies, such as adeno-associated virus (AAV)-mediated gene silencing and AAV-mediated gene delivery.
In this article, we highlight advancements in the clinical development of gene-based therapies for NMDs. We focus on amyotrophic lateral sclerosis (ALS), spinal muscular atrophy (SMA), and Duchenne muscular dystrophy (DMD) because of recent clinical successes in developing such therapies.1,6,7 We also catalog completed and ongoing clinical trials for ALS, SMA, and DMD (Tables 1-3).
Amyotrophic lateral sclerosis
ALS is caused by progressive degeneration of upper- and lower-motor neurons, which eventually leads to respiratory failure and death 3 to 5 years after disease onset.7-9 There are two subtypes: Familial ALS (10% of cases) and sporadic ALS (90% of cases). Commonly mutated ALS-associated genes6,8 are:
- Superoxide dismutase type 1 (SOD1).
- Chromosome 9 open reading frame 72 (C9orf72).
- Transactive response DNA-binding protein 43 (TARDBP).
- Fused in sarcoma (FUS).
SOD1-targeted therapy is being studied, with early evidence of clinical success. Mutations in SOD1 account for 10% to 20% of familial ALS cases and 1% to 2% of sporadic ALS cases.6,10 10 Mutations in C9orf72 account for 25 to 40% of familial ALS cases and 7% of sporadic ALS cases.8,9,11 Mutations in TARDBP account for 3% of familial ALS cases and 2% of sporadic cases.12 Mutations in FUS account for 4% of familial ALS cases and 1% of sporadic cases. Overall, these mutant proteins can trigger neurotoxicity, thus inducing motor-neuron death.6,10
Treatment of ALS
Two treatments for ALS are Food and Drug Administration approved: riluzole (Rilutek), approved in 1995, and edaravone (Radicava), approved in 2017.
Riluzole is an oral anti-excitotoxic glutamate antagonist.11 Approval of riluzole was based on the results of two studies that demonstrated a 2- to 3-month survival benefit.10,14 For patients who have difficulty swallowing, an oral suspension (Tiglutik, approved in 2018) and an oral film (Exservan, approved in 2019) are available.
Edaravone is a free-radical scavenger that decreases oxidative stress and is administered intravenously (IV).9,13,14 Findings from clinical trials suggest functional improvement or slower decline in function for some patients.
Although these two agents demonstrate modest therapeutic benefit, neither reverses progression of disease.10,14
Gene-based therapy for ALS
Many non-viral strategies, including antisense oligonucleotide (ASO), monoclonal antibodies, reverse transcriptase inhibitors, and HGF gene replacement therapy are used as therapeutic approaches to SOD1, C9orf72, and FUS gene mutations in ALS patients, and are being evaluated in clinical studies14,15 (Table 113-17).

Tofersen, also known as BIIB067, is an investigational ASO, administered by intrathecal (IT) injection, that binds to SOD1 mRNA, thus reducing its protein levels.16 Tofersen was evaluated in the VALOR phase 3 study (ClinicalTrials.gov Identifier: NCT02623699), a three-part randomized, double-blind, placebo-controlled trial: single ascending dose (Part A), multiple ascending dose (B), and fixed dose (C).10 In Parts A and B, 48 participants received five IT injections of tofersen or placebo over 12 weeks and were followed for an additional 12 weeks. Reduction in SOD1 protein production and neurofilament level in cerebrospinal fluid (CSF) (a potential biomarker of motor-neuron degeneration) was observed, which determined the fixed-dose for Part C.16,17
Part C examined the efficacy, safety and tolerability, pharmacokinetics (PK), and pharmacodynamics (PD) of tofersen, compared with placebo, in adults with ALS who had a confirmed SOD1 mutation.17 A total of 108 participants were enrolled; 60 were identified as “faster-progressing”; 48, as “slower-progressing.”18 The primary endpoint of Part C was change from baseline to Week 28 on the Revised ALS Functional Rating Scale (ALSFRS-R) total score. (ALSFRS-R measures overall clinical effect; the score ranges from 0 [no function] to 4 [full function].17)
Tofersen failed to meet the primary efficacy outcome because statistically significant findings were lacking in the faster-progressing population, as measured by joint-rank analysis (difference of 1.2 on the ALSFRS-R score; P = .97). However, trends favoring tofersen were observed across key secondary clinical outcome measures18:
- Change from baseline in CSF SOD1 protein concentration.17 Percent reduction in the total SOD1 protein level was much higher in the tofersen-treated group than in the control group (38% more than controls in the faster-progressing population; 26% more than controls in the slower-progressing population).18
- Change from baseline in neurofilament light-chain concentration in plasma.17,18 Percent reduction in the level of neurofilament light chain was also observed to be higher in the tofersen-treated group than in the control group (67% more than controls in the faster-progressing population and 48% more than controls in the slower-progressing population).18
Because of these encouraging results, VALOR participants were moved to the ongoing open-label extension trial of tofersen (ClinicalTri-als.gov Identifier: NCT03070119), in which both groups were treated with the active agent.
These data suggest that early tofersen treatment might slow decline in faster-progressing patients and stabilize clinical function in slower-progressing patients.18,19 Overall, most adverse events (AEs) in the trial among patients receiving active treatment were of mild or moderate severity, and were largely consistent with either disease progression or lumbar puncture–related complications.18
Because data from VALOR suggested potential benefit from tofersen, the ATLAS trial (ClinicalTrials.gov Identifier: NCT04856982) is investigating the clinical value of presymptomatic treatment and the optimal timing of initiation of therapy.20,21 ATLAS is a phase 3, randomized, placebo-controlled trial that examines the clinical efficacy, safety, and tolerability of tofersen in presymptomatic adult carriers of SOD1 mutation who have an elevated neurofilament light-chain concentration.21 ATLAS will also evaluate the efficacy of tofersen when initiated before, rather than after, ALS manifests clinically. Enrollment is still open for this trial.20,21
Latozinemab, also known as AL001, is a first-in-class monoclonal antibody, administered by IV infusion, that elevates levels of progranulin, a key regulator of the immune activity and lysosomal function in the brain.22,23 Latozinemab limits progranulin endocytosis and degradation by sortilin inhibition.22 Progranulin gene mutations can reduce progranulin expression (by 50 to 70 percent reduction), which may cause neuro-degeneration due to abnormal accumulation of TAR-DNA-binding protein 43 (TDP-43) in the brain cells.22,24 TDP-43 pathology has also been shown to be associated with C9orf72 mutations.23 Although the mechanism is not fully understood, the role of progranulin deficiency in TDP-43 pathology is believed to be associated with neurodegenerative diseases like ALS.11,23,24,43 Previous animal models of chronic neurodegenera-tion have demonstrated how increased progranulin levels can be protective against TDP-43 pathology, increasing neuronal development and survival, thus potentially slowing disease progression.23,24,43 Currently, latozinemab is being investigated in a randomized, double-blind, placebo-controlled, multicenter phase 2 trial (ClinicalTrials.gov Identifier: NCT05053035). Approximately, 45 C90rf72-associated ALS participants (≥ 18 years of age) will receive latozinemab or placebo infusions every 4 weeks (for 24 weeks). Study endpoints include safety, tolerability, PK, PD, as well as plasma, and CSF progranulin levels.25 In previous studies, latozinemab demonstrated encouraging results in frontotemporal dementia (FTD) patients who carry a progranulin mutation. Because FTD was revealed to have significant genetic overlap with ALS, there is disease-modifying potential for latozinemab in ALS patients.23,24
TPN-101 is a nucleoside analog reverse transcriptase inhibitor, administered orally, that was originally developed for human immunodeficiency virus (HIV) treatment. However, due to recent findings suggesting retrotransposon activity contributing to neurodegeneration in TDP-43 mediated diseases, including ALS and FTD, TNP-101 is being repurposed.26 The safety and tolerability of TNP-101 are currently being evaluated in C9orf72-associated ALS and FTD patients (≥ 18 years of age). The study is a randomized, double-blind, placebo-controlled paral-lel-group phase 2a trial (ClinicalTrials.gov Identifier: NCT04993755) The study includes a screening period of 6 weeks, double-blind treatment period of 24 weeks, an open-label treatment period of 24 weeks, and 4 weeks of the post-treatment follow-up visit. Study endpoints include the incidence and severity of spontaneously reported treatment-emergent adverse events (TEAEs) associated with TNP-101 and placebo for a to-tal of 48 weeks.27
ION363 is an investigational ASO, administered by IT injection, that selectively targets one of the FUS mutations (p.P525L), which is responsible for earlier disease onset and rapid ALS progression.28,29 The clinical efficacy of ION363, specifically in clinical function and survival is being assessed in FUS-associated ALS patients (≥ 12 years of age). This randomized phase 3 study (ClinicalTrials.gov Identifier: NCT04768972) includes two parts; part 1 will consist of participants receiving a multi-dose regimen (1 dose every 4-12 weeks) of ION363 or placebo for 61 weeks followed by an open-label extension treatment period in part 2, which will consist of participants receiving ION363 (every 12 weeks) for 85 weeks. The primary endpoint of the study is the change from baseline to day 505 in functional impairment, using ALS Functional Rating Scale-Revised (ALSFRS-R). This measures functional disease severity, specifically in bulbar function, gross motor skills, fine motor skills, and respiratory. The score for all 12 questions can range from 0 (no function) to 4 (full function) with a total possible score of 48.30
Engensis, also known as VM202, is a non-viral gene therapy, administered by intramuscular (IM) injection, that uses a plasmid to deliver the hepatocyte growth factor (HGF) gene to promote HGF protein production. The HGF protein plays a role in angiogenesis, the previous of muscle atrophy, and the promotion of neuronal survival and growth. Based on preclinical studies, increasing HGF protein production has been shown to reduce neurodegeneration, thus potentially halting or slowing ALS progression.31 Currently, the safety of engensis is being evaluated in ALS patients (18-80 years of age) in the REViVALS phase 2a (ClinicalTrials.gov Identifier: NCT04632225)/2b (ClinicalTrial.gov Identifier: NCT05176093).32,33 The ReViVALS trial is a double-blind, randomized, placebo-controlled, multi-center study. The phase 2a study endpoints include the incidence of TEAEs, treatment-emergent serious adverse events (TESAEs), injection site reactions, and clinically significant labor-atory values post-treatment (engensis vs placebo group) for 180 days.33 A phase 2b study will evaluate the long-term safety of engensis for an additional 6 months. Study endpoints include the incidence of AEs, changes from baseline in ALSFRS-R scores to evaluate improvement in muscle function, changes from baseline in quality of life using the ALS patient assessment questionnaire, time to all-cause mortality compared to placebo, etc.32
Spinal muscular atrophy
SMA is a hereditary lower motor-neuron disease caused (in 95% of cases) by deletions or, less commonly, by mutations of the survival motor neuron 1 (SMN1) gene on chromosome 5q13 that encodes the SMN protein.6 Reduction in expression of the SMN protein causes motor neurons to degenerate.36-38 Because of a large inverted duplication in chromosome 5q, two variants of SMN (SMN1 and SMN2) exist on each allele. The paralog gene, SMN2, also produces the SMN protein – although at a lower level (10% to 20% of total SMN protein production) than SMN1 does.
A single nucleotide substitution in SMN2 alters splicing and suppresses transcription of exon 7, resulting in a shortened mRNA strand that yields a truncated SMN protein product.6,37,39 SMA is classified based on age of onset and maximum motor abilities achieved, ranging from the most severe (Type 0) to mildest (Type 4) disease.36,40 Because SMA patients lack functional SMN1 (due to polymorphisms), disease severity is determined by copy numbers of SMN2.6,39
Gene-based therapy for SMA
Three FDA-approved SMN treatments demonstrate clinically meaningful benefit in SMA: SMN2-targeting nusinersen [Spinraza] and risdiplam [Evrysdi], and SMN1-targeting onasemnogene abeparvovec-xioi [Zolgensma]38 Additional approaches to SMA treatment are through SMN-independent therapies, which target muscle and nerve function. Research has strongly suggested that combined SMA therapies, specifically approved SMN-targeted and investigational SMN-independent treatments, such as GYM329 (also known as RO7204239) may be the best strategy to treat all ages, stages, and types of SMA.41 (Table 226-41).

Agents that modulate SMN2. Nusinersen, approved by the FDA in 2016, was the first treatment indicated for all SMA types in pediatric and adult patients.42 The agent is an ASO that targets exon 7 of SMN2, thus stabilizing transcription. Inclusion of exon 7 increases SMN protein production, improving motor function.6,38 Nusinersen is a lifelong treatment that requires IT administration every 4 months because it cannot cross the blood-brain barrier.38,43
Pivotal clinical studies that led to approval of nusinersen include CHERISH (ClinicalTrial.gov Identifier: NCT02292537) and ENDEAR (ClinicalTrial.gov Identifier: NCT02193074) studies.
CHERISH was a phase 3, randomized, double-blind, sham procedure–controlled trial that examined the clinical efficacy and safety of nusinersen in 126 participants with later-onset SMA (2-12 years of age). The primary endpoint was the change from baseline using the Hammersmith Functional Motor Scale Expanded (HFMSE) at 15 months. HFMSE looks at 33 activities to assess improvement in motor function. The study met the primary efficacy outcome, demonstrating statistically significant (P = .0000001) improvement in overall motor function. The nusinersen group showed a 3.9-point increase in the HFMSE score from baseline, which indicates improvement, compared with a 1.0-point decline from baseline in the control group.46,47
ENDEAR was also a randomized, double-blind, sham procedure–controlled phase 3 trial, which investigated the efficacy and safety of nusinersen in 121 participants with early-onset SMA Type 1 (≤ 210 days of age). Coprimary endpoints were:
- Percentage of motor milestones responders, as determined using Section 2 of the Hammersmith Infant Neurological Examination–Part 2.
- Event-free survival (that is, avoidance of combined endpoint of death or permanent ventilation).
ENDEAR met the first primary efficacy outcome, demonstrating statistically significant (P < .0001) improvement in motor milestones (head control, rolling, independent sitting, and standing). By 13 months of age, approximately 51% of nusinersen-treated participants showed improvement, compared with none in the control group.46,47
The second primary endpoint was also met, with a statistically significant (P = .005) 47% decrease in mortality or permanent ventilation use.46-48
The NURTURE (ClinicalTrial.gov Identifier: NCT02386553) study is also investigating the efficacy and safety of nusinersen. An ongoing, open-label, supportive phase 2 trial, NURTURE is evaluating the efficacy and safety of multiple doses of nusinersen in 25 presymptomatic SMA patients (≤ 6 weeks of age). The primary endpoint of this study is time to death or respiratory intervention.49 Interim results demonstrate that 100% of presymptomatic infants are functioning without respiratory intervention after median follow-up of 2.9 years.46-48
Although nusinersen has been shown to be generally safe in clinical studies, development of lumbar puncture–related complications, as well as the need for sedation during IT administration, might affect treatment tolerability in some patients.39
Risdiplam was approved by the FDA in 2020 as the first orally administered small-molecule treatment of SMA (for patients ≤ 2 months of age).52 Risdiplam is a SMN2 splicing modifier, binding to the 5’ splice site of intron 7 and exonic splicing enhancer 2 in exon 7 of SMN2 pre-mRNA. This alternative splicing increases efficiency in SMN2 gene transcription, thus increasing SMN protein production in motor-neuron cells.36 An important advantage of risdiplam is the convenience of oral administration: A large percentage of SMA patients (that is, those with Type 2 disease) have severe scoliosis, which can further complicate therapy or deter patients from using a treatment that is administered through the IT route.40
FDA approval of risdiplam was based on clinical data from two pivotal studies, FIREFISH (ClinicalTrial.gov Identifier: NCT02913482) and SUNFISH (ClinicalTrial.gov Identifier: NCT02908685).53-54
FIREFISH is an open-label, phase 2/3 ongoing trial in infants (1-7 months of age) with SMA Type 1. The study comprises two parts; Part 1 determined the dose of risdiplam used in Part 2, which assessed the efficacy and safety of risdiplam for 24 months. The primary endpoint was the percentage of infants sitting without support for 5 seconds after 12 months of treatment using the gross motor scale of the Bayley Scales of Infant and Toddler Development–Third Edition. A statistically significant (P < .0001) therapeutic benefit was observed in motor milestones. Approximately 29% of infants achieved the motor milestone of independent sitting for 5 seconds, which had not been observed in the natural history of SMA.53-55
SUNFISH is an ongoing randomized, double-blind, placebo-controlled trial of risdiplam in adult and pediatric patients with SMA Types 2 and 3 (2-25 years old). This phase 2/3 study comprises two parts: Part 1 determined the dose (for 12 weeks) to be used for confirmatory Part 2 (for 12 to 24 months). The primary endpoint was the change from baseline on the 32-item Motor Function Measure at 12 months. The study met its primary endpoint, demonstrating statistically significant (P = .0156) improvement in motor function scores, with a 1.36-point increase in the risdiplam group, compared with a 0.19-point decrease in the control group.54,55
Ongoing risdiplam clinical trials also include JEWELFISH (ClinicalTrial.gov Identifier: NCT03032172) and RAINBOW (ClinicalTrial.gov Identifier: NCT03779334).56-57 JEWELFISH is an open-label, phase 2 trial assessing the safety of risdiplam in patients (6 months to 60 years old) who received prior treatment. The study has completed recruitment; results are pending.56 RAINBOW is an ongoing, open-label, single-arm, phase 2 trial, evaluating the clinical efficacy and safety of risdiplam in SMA-presymptomatic newborns (≤ 6 weeks old). The study is open for enrollment.57 Overall, interim results for JEWELFISH and RAINBOW appear promising.
In addition, combined SMA therapies, specifically risdiplam and GYM329 are currently being investigated to address the underlying cause and symptoms of SMA concurrently.58 GYM329, is an investigational anti-myostatin antibody, selectively binding preforms of myostatin - pro-myostatin and latent myostatin, thus improving muscle mass and strength for SMA patients.59 The safety and efficacy of GYM329 in combination with risdiplam is currently being investigated in 180 ambulant participants with SMA (2-10 years of age) in the MANATEE (ClinicalTrial.gov Identifier: NCT05115110) phase 2/3 trial. The MANATEE study is a two-part, seamless, randomized, placebo-controlled, double-blind trial. Part 1 will assess the safety of the combination treatment in approximately 36 participants; participants will receive both GYM329 (every 4 weeks) by subcutaneous (SC) injection into the abdomen and risdiplam (once per day) for 24 weeks followed by a 72-week open-label treatment period. 54,58 The outcome measures include the incidence of AEs, percentage change from baseline in the contractile area of skeletal muscle (in dominant thigh and calf), change from baseline in RHS total score, and incidence of change from baseline in serum concentration (total myostatin, free latent myostatin, and mature myostatin) etc.54 Part 2 will be conducted on 144 participants, specifically assessing the efficacy and safety of the optimal dose of GYM329 selected from Part 1 (combined with risdiplam) for 72 weeks. Once the treatment period is completed in either part, participants can partake in a 2-year open-label extension period.54,58 Other outcome measures include change from baseline in lean muscle mass (assessed by full body dual-energy X- ray absorptiometry (DXA) scan), in time taken to walk/run 10 meters (measured by RHS), in time taken to rise from the floor (measured by RHS), etc.54 Overall, this combination treatment has the potential to further improve SMA patient outcomes and will be further investigated in other patient populations (including non-ambulant patients and a broader age range) in the future.58
An agent that alters SMN1 expression. Onasemnogene abeparvovec-xioi, FDA approved in 2019, was the first gene-replacement therapy indicated for treating SMA in children ≤ 2 years old.60 Treatment utilizes an AAV vector type 9 (AAV9) to deliver a functional copy of SMN1 into target motor-neuron cells, thus increasing SMN protein production and improving motor function. This AAV serotype is ideal because it crosses the blood-brain barrier. Treatment is administered as a one-time IV fusion.38,39,43
FDA approval was based on the STR1VE (ClinicalTrial.gov Identifier: NCT03306277) phase 3 study and START (ClinicalTrial.gov Identifier: NCT02122952) phase 1 study.61,62 START was the first trial to investigate the safety and efficacy of onasemnogene abeparvovec-xioi in SMA Type 1 infants (< 6 months old). Results demonstrated remarkable clinical benefit, including 100% permanent ventilation-free survival and a 92% (11 of 12 patients) rate of improvement in motor function. Improvement in development milestones was also observed: 92% (11 of 12 patients) could sit without support for 5 seconds and 75% (9 of 12) could sit without support for 30 seconds.14,61,63
The efficacy of onasemnogene abeparvovec-xioi seen in STR1VE was consistent with what was observed in START. STRIVE, a phase 3 open-label, single-dose trial, examined treatment efficacy and safety in 22 symptomatic infants (< 6 months old) with SMA Type 1 (one or two SMN2 copies). The primary endpoint was 30 seconds of independent sitting and event-free survival. Patients were followed for as long as 18 months. Treatment showed statistically significant (P < .0001) improvement in motor milestone development and event-free survival, which had not been observed in SMA Type 1 historically. Approximately 59% (13 of 22 patients) could sit independently for 30 seconds at 18 months of age. At 14 months of age, 91% (20 of 22 patients) were alive and achieved independence from ventilatory support.34,35,53
Although many clinical studies suggest that onasemnogene abeparvovec-xioi can slow disease progression, the benefits and risks of long-term effects are still unknown. A 15-year observational study is investigating the long-term therapeutic effects and potential complications of onasemnogene abeparvovec-xioi. Participants in START were invited to enroll in this long-term follow-up study (ClinicalTrial.gov Identifier: NCT04042025).66-67
Duchenne muscular dystrophy
DMD is the most common muscular dystrophy of childhood. With an X-linked pattern of inheritance, DMD is seen mostly in young males (1 in every 3,500 male births).38,39,73 DMD is caused by mutation of the dystrophin encoding gene, or DMD, on the X chromosome. Deletion of one or more exons of DMD prevents production of the dystrophin protein, which leads to muscle degeneration.38,39,43 Common DMD deletion hotspots are exon 51 (20% of cases), exon 53 (13% of cases), exon 44 (11% of cases), and exon 45 (12% of cases).74 Nonsense mutations, which account for another 10% of DMD cases, occur when premature termination codons are found in the DMD gene. Those mutations yield truncated dystrophin protein products.39,66
Therapy for DMD
There are many therapeutic options for DMD, including deflazacort (Emflaza), FDA approved in 2017, which has been shown to reduce inflammation and immune system activity in DMD patients (≥ 5 years old). Deflazacort is a corticosteroid prodrug; its active metabolite acts on the glucocorticoid receptor to exert anti-inflammatory and immunosuppressive effects. Studies have shown that muscle strength scores over 6-12 months and average time to loss of ambulation numerically favored deflazacort over placebo.74,75
Gene-based therapy for DMD
Mutation-specific therapeutic approaches, such as exon skipping and nonsense suppression, have shown promise for the treatment of DMD (Table 358-79):

- ASO-mediated exon skipping allows one or more exons to be omitted from the mutated DMD mRNA.74,75 Effective FDA-approved ASOs include golodirsen [Vyondys 53], viltolarsen [Viltepso], and casimersen [Amondys 45].74
- An example of therapeutic suppression of nonsense mutations is ataluren [Translarna], an investigational agent that can promote premature termination codon read-through in DMD patients.66
Another potential treatment approach is through the use of AAV gene transfer to treat DMD. However, because DMD is too large for the AAV vector (packaging size, 5.0 kb), microdystrophin genes (3.5-4 kb, are used as an alternative to fit into a single AAV vector.39,76
Exon skipping targeting exon 51. Eteplirsen, approved in 2016, is indicated for the treatment of DMD patients with the confirmed DMD gene mutation that is amenable to exon 51 skipping. Eteplirsen binds to exon 51 of dystrophin pre-mRNA, causing it to be skipped, thus, restoring the reading frame in patients with DMD gene mutation amenable to exon 51 skipping. This exclusion promotes dystrophin production. Though the dystrophin protein is still functional, it is shortened.38,77 Treatment is administered IV, once a week (over 35-60 minutes). Eteplirsen’s accelerated approval was based on 3 clinical studies (ClinicalTrial.gov Identifier: NCT01396239, NCT01540409, and NCT00844597.) 78-81 The data demonstrated an increased expression of dystrophin in skeletal muscles in some DMD patients treated with eteplirsen. Though the clinical benefit of eteplirsen (including improved motor function) was not established, it was concluded by the FDA that the data were reasonably likely to predict clinical benefit. Continued approval for this indication may depend on the verification of a clinical benefit in confirmatory trials. Ongoing clinical trials include (ClinicalTrial.gov Identifier: NCT03992430 (MIS51ON), NCT03218995, and NCT03218995).77,81,82
Vesleteplirsen, is an investigational agent that is designed for DMD patients who are amendable to exon 51 skip-ping. The mechanism of action of vesleteplirsen appears to be similar to that of eteplirsen.83 The ongoing MOMENTUM (ClinicalTrial.gov Identifier: NCT04004065) phase 2 trial is assessing the safety and tolerability of vesleteplirsen at multiple-ascending dose levels (administered via IV infusion) in 60 participants (7-21 years of age). The study consists of two parts; participants receive escalating dose levels of vesleteplirsen (every 4 weeks) for 72 weeks during part A and participants receive the selected doses from part A (every 4 weeks) for 2 years during part B. Study endpoints include the number of AEs (up to 75 weeks) and the change from baseline to week 28 in dystrophin protein level. 84 Serious AEs of reversible hypomagnesemia were observed in part B, and as a result, the study protocol was amended to include magnesium supplementation and monitoring of magnesium levels.83
Exon skipping targeting exon 53. Golodirsen, FDA approved in 2019, is indicated for the treatment of DMD in patients who have a confirmed DMD mutation that is amenable to exon 53 skipping. The mechanism of action is similar to eteplirsen, however, golodirsen is designed to bind to exon 53.38,39 Treatment is administered by IV infusion over 35-60 minutes.
Approval of golodirsen was based primarily on a two-part, phase 1/2 clinical trial (ClinicalTrial.gov Identifier: NCT02310906). Part 1 was a randomized, placebo-controlled, dose-titration study that assessed multiple-dose efficacy in 12 DMD male patients, 6 to 15 years old, with deletions that were amenable to exon 53 skipping.
Part 2 was an open-label trial in 12 DMD patients from Part 1 of the trial plus 13 newly enrolled male DMD patients who were also amenable to exon 53 skipping and who had not already received treatment. Primary endpoints were change from baseline in total distance walked during the 6-minute walk test at Week 144 and dystrophin protein levels (measured by western blot testing) at Week 48. A statistically significant increase in the mean dystrophin level was observed, from a baseline 0.10% mean dystrophin level to a 1.02% mean dystrophin level after 48 weeks of treatment (P < .001). Common reported adverse events associated with golodirsen were headache, fever, abdominal pain, rash, and dermatitis. Renal toxicity was observed in preclinical studies of golodirsen but not in clinical studies.80,85
Viltolarsen, approved in 2020, is also indicated for the treatment of DMD in patients with deletions amenable to exon 53 skipping. The mechanism of action and administration (IV infusion over 60 minutes) are similar to that of golodirsen.
Approval of viltolarsen was based on two phase 2 clinical trials (ClinicalTrial.gov Identifier: NCT02740972 and NCT03167255) in a total of 32 patients. NCT02740972 was a randomized, double-blind, placebo-controlled, dose-finding study that evaluated the clinical efficacy of viltolarsen in 16 male DMD patients (4-9 years old) for 24 weeks.
NCT03167255 was an open-label study that evaluated the safety and tolerability of viltolarsen in DMD male patients (5-18 years old) for 192 weeks. The efficacy endpoint was the change in dystrophin production from baseline after 24 weeks of treatment. A statistically significant increase in the mean dystrophin level was observed, from a 0.6% mean dystrophin level at baseline to a 5.9% mean dystrophin level at Week 25 (P = .01). The most common adverse events observed were upper respiratory tract infection, cough, fever, and injection-site reaction.86-87
Exon skipping targeting exon 45. Casimersen was approved in 2021 for the treatment of DMD in patients with deletions amenable to exon 45 skipping.88 Treatment is administered by IV infusion over 30-60 minutes. Approval was based on an increase in dystrophin production in skeletal muscle in treated patients. Clinical benefit was reported in interim results from the ESSENCE (ClinicalTrial.gov Identifier: NCT02500381) study, an ongoing double-blind, placebo-controlled phase 3 trial that is evaluating the efficacy of casimersen, compared with placebo, in male participants (6-13 years old) for 48 weeks. Efficacy is based on the change from baseline dystrophin intensity level, determined by immunohistochemistry, at Week 48.
Interim results from ESSENCE show a statistically significant increase in dystrophin production in the casimersen group, from a 0.9% mean dystrophin level at baseline to a 1.7% mean dystrophin level at Week 48 (P = .004); in the control group, a 0.54% mean dystrophin level at baseline increased to a 0.76% mean dystrophin level at Week 48 (P = .09). Common adverse events have included respiratory tract infection, headache, arthralgia, fever, and oropharyngeal pain. Renal toxicity was observed in preclinical data but not in clinical studies.60,84
Targeting nonsense mutations. Ataluren is an investigational, orally administered nonsense mutation suppression therapy (through the read-through of stop codons).37 Early clinical evidence supporting the use of ataluren in DMD was seen in an open-label, dose-ranging, phase 2a study (ClinicalTrial.gov Identifier: NCT00264888) in male DMD patients (≥ 5 years old) caused by nonsense mutation. The study demonstrated a modest (61% ) increase in dystrophin expression in 23 of 38 patients after 28 days of treatment.37,91,92
However, a phase 2b randomized, double-blind, placebo-controlled trial (ClinicalTrial.gov Identifier: NCT00592553) and a subsequent confirmatory ACT DMD phase 3 study (ClinicalTrial.gov Identifier: NCT01826487) did not meet their primary endpoint of improvement in ambulation after 48 weeks as measured by the 6-minute walk test.37,93,94 In ACT DMD, approximately 74% of the ataluren group did not experience disease progression, compared with 56% of the control group (P = 0386), measured by a change in the 6-minute walk test, which assessed ambulatory decline.37,95
Based on limited data showing that ataluren is effective and well tolerated, the European Medicines Agency has given conditional approval for clinical use of the drug in Europe. However, ataluren was rejected by the FDA as a candidate therapy for DMD in the United States.22 Late-stage clinical studies of ataluren are ongoing in the United States.
AAV gene transfer with microdystrophin. Limitations on traditional gene-replacement therapy prompted exploration of gene-editing strategies for treating DMD, including using AAV-based vectors to transfer microdystrophin, an engineered version of DMD, into target muscles.43 The microdystrophin gene is designed to produce a functional, truncated form of dystrophin, thus improving muscular function.
There are 3 ongoing investigational microdystrophin gene therapies that are in clinical development (ClinicalTrial.gov Identifier: NCT03368742 (IGNITE DMD), NCT04281485 (CIFFREO), and NCT05096221 (EMBARK)).38,82
IGNITE DMD is a phase 1/2 randomized, controlled, single-ascending dose trial evaluating the safety and efficacy of a SGT-001, single IV infusion of AAV9 vector containing a microdystrophin construct in DMD patients (4-17 years old) for 12 months. At the conclusion of the trial, treatment and control groups will be followed for 5 years. The primary efficacy endpoint is the change from baseline in microdystrophin protein production in muscle-biopsy material, using western blot testing.96 Long-term interim data on biopsy findings from three patients demonstrated clinical evidence of durable microdystrophin protein expression after 2 years of treatment.96,97
The CIFFREO trial will assess the safety and efficacy of the PF-06939926 microdystrophin gene therapy, an investigational AAV9 containing microdystrophin, in approximately 99 ambulatory DMD patients (4-7 years of age). The study is a randomized, double-blind, placebo-controlled, multicenter phase 3 trial. The primary efficacy end-point is the change from baseline in the North Star Ambulatory Assessment (NSAA), which measures gross motor function. This will be assessed at 52 weeks; all study participants will be followed for a total of 5 years post-treatment.98,99,100 Due to unexpected patient death (in a non-ambulatory cohort) in the phase 1b (in a non-ambulatory cohort) in the phase 1b (ClinicalTrial.gov Identifier: (NCT03362502) trial, microdystrophin gene therapy was immediately placed on clinical hold.101,102 The amended study protocol required that all participants undergo one week of in-hospital observation after receiving treatment.102
The EMBARK study is a global, randomized, double-blind, placebo-controlled, phase 3 trial that is evaluating the safety and efficacy of SRP-9001, which is a rAAVrh74.MHCK7.microdystrophin gene therapy. The AAV vector (rAAVrh74) contains the microdystrophin construct, driven by the skeletal and cardiac muscle–specific promoter, MHCK7.98,99 In the EMBARK study, approximately 120 participants with DMD (4-7 years of age) will be enrolled. The primary efficacy endpoint includes the change from baseline to week 52 in the NSAA total score.99 Based on SRP-9001, data demonstrating consistent statistically significant functional improvements in NSAA total scores and timed function tests (after one-year post- treatment) in DMD patients from previous studies and an integrated analysis from multiple studies (ClinicalTrial.gov Identifier: NCT03375164, NCT03769116, and NCT04626674), the ongoing EMBARK has great promise.103,104
Challenges ahead, but advancements realized
Novel gene-based therapies show significant potential for transforming the treatment of NMDs. The complex pathologies of NMDs have been a huge challenge to disease management in an area once considered unremediable by gene-based therapy. However, advancements in precision medicine – specifically, gene-delivery systems (for example, AAV9 and AAVrh74 vectors) combined with gene modification strategies (ASOs and AAV-mediated silencing) – have the potential to, first, revolutionize standards of care for sporadic and inherited NMDs and, second, significantly reduce disease burden.6
What will be determined to be the “best” therapeutic approach will, likely, vary from NMD to NMD; further investigation is required to determine which agents offer optimal clinical efficacy and safety profiles.43 Furthermore, the key to therapeutic success will continue to be early detection and diagnosis – first, by better understanding disease pathology and drug targets and, second, by validation of reliable biomarkers that are predictive of therapeutic benefit.4,5
To sum up, development challenges remain, but therapeutic approaches to ALS, SMA, and DMD that utilize novel gene-delivery and gene-manipulation tools show great promise.
Ms. Yewhalashet is a student in the masters of business and science program, with a concentration in healthcare economics, at Keck Graduate Institute Henry E. Riggs School of Applied Life Sciences, Claremont, Calif. Dr. Davis is professor of practice in clinical and regulatory affairs, Keck Graduate Institute Henry E. Riggs School of Applied Life Sciences.
References
1. Aitken M et al. Understanding neuromuscular disease care. IQVIA [Internet]. Oct 30, 2018. Accessed Mar 1, 2022. https://www.iqvia.com/insights/the-iqvia-institute/reports/understanding-neuromuscular-disease-care.
2. National Institute of Neurological Disorders and Stroke. Neurological diagnostic tests and procedures fact sheet. Updated Nov 15, 2021. Ac-cessed Mar 1, 2022. http://www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Fact-Sheets/Neurological-Diagnostic-Tests-and-Procedures-Fact.
3. Deenen JCW et al. The epidemiology of neuromuscular disorders: A comprehensive overview of the literature. J Neuromuscul Dis. 2015;2(1):73-85.
4. Cavazzoni P. The path forward: Advancing treatments and cures for neurodegenerative diseases. U.S. Food and Drug Administration. Jul 29, 2021. Accessed Mar 1, 2022. http://www.fda.gov/news-events/congressional-testimony/path-forward-advancing-treatments-and-cures-neurodegenerative-diseases-07292021.
5. Martier R, Konstantinova P. Gene therapy for neurodegenerative diseases: Slowing down the ticking clock. Front Neurosci. 2020 Sep 18;14:580179. doi: 10.3389/fnins.2020.580179.
6. Sun J, Roy S. Gene-based therapies for neurodegenerative diseases. Nat Neurosci. 2021 Mar;24(3):297-311. doi:10.1038/s41593-020-00778-1.
7. Amado DA, Davidson BL. Gene therapy for ALS: A review. Mol Ther. 2021 Dec 1;29(12):3345-58. doi:10.1016/j.ymthe.2021.04.008.
8. Yun Y, Ha Y. CRISPR/Cas9-mediated gene correction to understand ALS. Int J Mol Sci. 2020;21(11):3801. doi:10.3390/ijms21113801.
9. National Institute of Neurological Disorders and Stroke. Amyotrophic lateral sclerosis (ALS) fact sheet. Updated Nov 15, 2021. Accessed Mar 1, 2022. http://www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Fact-Sheets/Amyotrophic-Lateral-Sclerosis-ALS-Fact-Sheet.
10. Cappella M et al. Gene therapy for ALS – A perspective. Int J Mol Sci. 2019;20(18):4388. doi:10.3390/ijms20184388.
11. Abramzon YA, Fratta P, Traynor BJ, Chia R. The Overlapping Genetics of Amyotrophic Lateral Sclerosis and Frontotemporal Dementia. Front Neurosci. 2020;14. Accessed August 18, 2022. https://www.frontiersin.org/articles/10.3389/fnins.2020.00042
12. Giannini M, Bayona-Feliu A, Sproviero D, Barroso SI, Cereda C, Aguilera A. TDP-43 mutations link Amyotrophic Lateral Sclerosis with R-loop homeostasis and R loop-mediated DNA damage. PLOS Genet. 2020;16(12):e1009260. doi:10.1371/journal.pgen.1009260
13. FDA-approved drugs for treating ALS. The ALS Association [Internet]. Accessed Mar 1, 2022. http://www.als.org/navigating-als/living-with-als/fda-approved-drugs.
14. Jensen TL et al. Current and future prospects for gene therapy for rare genetic diseases affecting the brain and spinal cord. Front Mol Neurosci. 2021 Oct 6;14:695937. doi:10.3389/fnmol.2021.695937.
15. ALS Gene Targeted Therapies. The ALS Association. Accessed August 22, 2022. https://www.als.org/understanding-als/who-gets-als/genetic-testing/als-gene-targeted-therapies
16. Tofersen for ALS clears phase 1/2 trial, now in phase 3. Advances in Motion. Massachusetts General Hospital [Internet]. Sep 30, 2020. Accessed Mar 1, 2022. https://advances.massgeneral.org/neuro/journal.aspx?id=1699.17. Biogen. A study to evaluate the efficacy, safety, tol-erability, pharmacokinetics, and pharmacodynamics of BIIB067 administered to adult subjects with amyotrophic lateral sclerosis and confirmed superoxide dismutase 1 mutation. ClinicalTrials.gov Identifier: NCT02623699. Updated Jul 25, 2021. Accessed Feb 17, 2022. https://clinicaltrials.gov/ct2/show/NCT02623699.
18. Biogen. Biogen announces topline results from the tofersen phase 3 study and its open-label Extension in SOD1-ALS. Press release. Oct 17, 2021. Accessed Mar 1, 2022. https://investors.biogen.com/news-releases/news-release-details/biogen-announces-topline-results-tofersen-phase-3-study-and-its.
19. Biogen. An extension study to assess the long-term safety, tolerability, pharmacokinetics, and effect on disease progression of BIIB067 ad-ministered to previously treated adults with amyotrophic lateral sclerosis caused by superoxide dismutase 1 mutation. ClinicalTrials.gov Identi-fier: NCT03070119. Updated Sep 10, 2021. Accessed Feb 17, 2022. https://clinicaltrials.gov/ct2/show/NCT03070119.
20. MS MW. #AANAM – ATLAS Trial to Assess Tofersen in Presymptomatic SOD1 ALS. Accessed February 19, 2022. https://alsnewstoday.com/news-posts/2021/04/23/aanam-atlas-clinical-trial- tofersen-presymptomatic-sod1-als-patients/
21.Biogen. A phase 3 randomized, placebo-controlled trial with a longitudinal natural history run-in and open-label extension to evaluate BIIB067 initiated in clinically presymptomatic adults with a confirmed superoxide dismutase 1 mutation. ClinicalTrials.gov Identifier: NCT04856982. Updated Feb 18, 2022. Accessed Mar 1, 2022. https://clinicaltrials.gov/ct2/show/NCT04856982.
22. Latozinemab | ALZFORUM. Accessed August 19, 2022. https://www.alzforum.org/therapeutics/latozinemab
23. Alector Presents AL001 (latozinemab) Data from the FTD-C9orf72 Cohort of the INFRONT-2 Phase 2 Clinical Trial | Alector. Accessed August 18, 2022. https://investors.alector.com/news- releas-es/news-release-details/alector-presents-al001-latozinemab-data-ftd-c9orf72-cohort/
24. Alector Announces First Participant Dosed in Phase 2 Study Evaluating AL001 in Amyotrophic Lateral Sclerosis (ALS) | Alector. Accessed August 18, 2022. https://investors.alector.com/news- releases/news-release-details/lector-announces-first-participant-dosed-phase-2-study-0/ 25. A Phase 2 Study to Evaluate AL001 in C9orf72-Associated ALS - Full Text View - ClinicalTrials.gov. Accessed August 19, 2022. https://clinicaltrials.gov/ct2/show/NCT05053035
26.TPN-101 | ALZFORUM. Accessed August 19, 2022. https://www.alzforum.org/therapeutics/tpn- 101
27. Transposon Therapeutics, Inc. A Phase 2a Study of TPN-101 in Patients With Amyotrophic Lateral Sclerosis (ALS) and/or Frontotemporal Dementia (FTD) Associated With Hexanucleotide Repeat Expansion in the C9orf72 Gene (C9ORF72 ALS/FTD). clinicaltrials.gov; 2022. Ac-cessed August 17, 2022. https://clinicaltrials.gov/ct2/show/NCT04993755
28. Kerk SY, Bai Y, Smith J, et al. Homozygous ALS-linked FUS P525L mutations cell- autonomously perturb transcriptome profile and chem-oreceptor signaling in human iPSC microglia. Stem Cell Rep. 2022;17(3):678-692. doi:10.1016/j.stemcr.2022.01.004
29. ION363 | ALZFORUM. Accessed August 19, 2022. https://www.alzforum.org/therapeutics/ion363 30. Ionis Pharmaceuticals, Inc. A Phase 1-3 Study to Evaluate the Efficacy, Safety, Pharmacokinetics and Pharmacodynamics of Intrathecally Administered ION363 in Amyo-trophic Lateral Sclerosis Patients With Fused in Sarcoma Mutations (FUS-ALS). clinicaltrials.gov; 2022. Accessed August 18, 2022. https://clinicaltrials.gov/ct2/show/NCT04768972
31. PhD LF. Engensis (VM202) - ALS News Today. Accessed August 19, 2022. https://alsnewstoday.com/vm202/
32. Helixmith Co., Ltd. A 6-Month Extension Study Following Protocol VMALS-002-2 (A Phase 2a, Double-Blind, Randomized, Place-bo-Controlled, Multicenter Study to Assess the Safety of Engensis in Participants With Amyotrophic Lateral Sclerosis). clinicaltrials.gov; 2022. Accessed August 18, 2022. https://clinicaltrials.gov/ct2/show/NCT05176093 33. Safety of Engensis in Participants With Amyotrophic Lateral Sclerosis - Full Text View - ClinicalTrials.gov. Accessed August 19, 2022. https://clinicaltrials.gov/ct2/show/NCT04632225
34. Biogen. A phase 1, safety, tolerability, and distribution study of a microdose of radiolabeled BIIB067 co-administered with BIIB067 to healthy adults. ClinicalTrials.gov Identifier: NCT03764488. Updated Jul 19, 2021. Accessed Mar 1, 2022. https://clinicaltrials.gov/ct2/show/NCT03764488.
35. Ionis Pharmaceuticals Inc. A phase 1, double-blind, placebo-controlled, dose-escalation study of the safety, tolerability, and pharmacokinet-ics of ISIS 333611 administered intrathecally to patients with familial amyotrophic lateral sclerosis due to superoxide dismutase 1 gene muta-tions. ClinicalTrials.gov Identifier: NCT01041222. Updated Apr 13, 2012. Accessed Mar 1, 2022. https://clinicaltrials.gov/ct2/show/NCT01041222.
36. Messina S, Sframeli M. New treatments in spinal muscular atrophy: Positive results and new challenges. J Clin Med. 2020;9(7):2222. doi:10.3390/jcm9072222.
37. Scoto M et al. Genetic therapies for inherited neuromuscular disorders. Lancet Child Adolesc Health. 2018 Aug;2(8):600-9. doi:10.1016/S2352-4642(18)30140-8.
38. Abreu NJ, Waldrop MA. Overview of gene therapy in spinal muscular atrophy and Duchenne muscular dystrophy. Pediatr Pulmonol. 2021 Apr;56(4):710-20. doi:10.1002/ppul.25055.
39. Brandsema J, Cappa R. Genetically targeted therapies for inherited neuromuscular disorders. Practical Neurology [Internet]. Jul/Aug 2021:69-73. Accessed Mar 1, 2022. https://practicalneurology.com/articles/2021-july-aug/genetically-targeted-therapies-for-inherited-neuromuscular-disorders/pdf.
40. Ojala KS et al. In search of a cure: The development of therapeutics to alter the progression of spinal muscular atrophy. Brain Sci. 2021;11(2):194. doi:10.3390/brainsci11020194.
41. McCall S. Cure SMA Releases Updated Drug Pipeline. Cure SMA. Published December 13, 2021. Accessed August 21, 2022. https://www.curesma.org/cure-sma-releases-updated-drug-pipeline- 2021/ 42. FDA approves first drug for spinal muscular atrophy. U.S. Food and Drug Administration. News release. Dec 23, 2016. Accessed Mar 1, 2022. http://www.fda.gov/news-events/press-announcements/fda-approves-first-drug-spinal-muscular-atrophy.43. Kirschner J. Postnatal gene therapy for neuromuscular diseases – Opportunities and limitations. J Perinat Med. 2021 Sep;49(8):1011-5. doi:10.1515/jpm-2021-0435.
43. Terryn J, Verfaillie CM, Van Damme P. Tweaking Progranulin Expression: Therapeutic Avenues and Opportunities. Front Mol Neurosci. 2021;14. Accessed September 4, 2022. https://www.frontiersin.org/articles/10.3389/fnmol.2021.71303144.
44. Biogen. A phase 3, randomized, double-blind, sham-procedure controlled study to assess the clinical efficacy and safety of ISIS 396443 administered intrathecally in patients with later-onset spinal muscular atrophy. ClinicalTrials.gov Identifier: NCT02292537. Updated Feb 17, 2021. Accessed Mar 1, 2022. https://clinicaltrials.gov/ct2/show/study/NCT02292537.
45. Why Spinraza/later-onset studies. SPINRAZA® (nusinersen) [Internet]. Accessed Mar 1, 2022. www.spinraza.com/en_us/home/why-spinraza/later-onset-studies.html#scroll-tabs.
46. Biogen. A Phase 3, Randomized, Double-Blind, Sham-Procedure Controlled Study to Assess the Clinical Efficacy and Safety of ISIS 396443 Administered Intrathecally in Patients With Infantile- Onset Spinal Muscular Atrophy. clinicaltrials.gov; 2021. Accessed February 10, 2022. https://clinicaltrials.gov/ct2/show/results/NCT02193074
47. Early-onset SMA (Type 1) | SPINRAZA® (nusinersen). Accessed Mar 1, 2022. https://www.spinraza-hcp.com/en_us/home/why-spinraza/about-spinraza.html.
48. Finkel RS et al; ENDEAR Study Group. Nusinersen versus sham control in infantile-onset spinal muscular atrophy. N Engl J Med. 2017;377(18):1723-32. doi: 10.1056/NEJMoa1702752.
49. Biogen. An open-label study to assess the efficacy, safety, tolerability, and pharmacokinetics of multiple doses of ISIS 396443 delivered intrathecally to subjects with genetically diagnosed and presymptomatic spinal muscular atrophy. ClinicalTrials.gov Identifier: NCT02386553. Updated Nov 18, 2021. Accessed Mar 1, 2022. https://clinicaltrials.gov/ct2/show/NCT02386553.
50. De Vivo DC et al; NURTURE Study Group. Nusinersen initiated in infants during the presymptomatic stage of spinal muscular atrophy: In-terim efficacy and safety results from the phase 2 NURTURE study. Neuromuscul Disord. 2019 Nov;29(11):842-56. doi:10.1016/j.nmd.2019.09.007.
51. Why Spinraza/presymptomatic study. SPINRAZA® (nusinersen) [Internet]. Accessed Feb 22, 2022. www.spinraza.com/en_us/home/why-spinraza/presymptomatic-study.html#scroll-tabs.
52. FDA approves oral treatment for spinal muscular atrophy. U.S. Food and Drug Administration. News release. Aug 7, 2020. Accessed Mar 1, 2022. http://www.fda.gov/news-events/press-announcements/fda-approves-oral-treatment-spinal-muscular-atrophy.
53. Hoffmann-La Roche. A two-part seamless, open-label, multicenter study to investigate the safety, tolerability, pharmacokinetics, pharmaco-dynamics and efficacy of risdiplam (RO7034067) in infants with type 1 spinal muscular atrophy. ClinicalTrials.gov Identifier: NCT02913482. Updated Jan 21, 2022. Accessed Mar 1, 2022. https://clinicaltrials.gov/ct2/show/NCT02913482.
54. Hoffmann-La Roche. A two-part seamless, multi-center randomized, placebo-controlled, double-blind study to investigate the safety, tolera-bility, pharmacokinetics, pharmacodynamics and efficacy of risdiplam (RO7034067) in type 2 and 3 spinal muscular atrophy patients. Clinical-Trials.gov Identifier: NCT02908685. Updated Dec 28, 2021. Accessed Mar 1, 2022. https://clinicaltrials.gov/ct2/show/NCT02908685.
55. Genentech. Genentech’s risdiplam shows significant improvement in survival and motor milestones in infants with type 1 spinal muscular atrophy (SMA). Press release. Apr 27, 2020. Accessed Mar 1, 2022. http://www.gene.com/media/press-releases/14847/2020-04-27/genentechs-risdiplam-shows-significant-i
56. Hoffmann-La Roche. An open-label study to investigate the safety, tolerability, and pharmacokinetics/pharmacodynamics of risdiplam (RO7034067) in adult and pediatric patients with spinal muscular atrophy. ClinicalTrials.gov Identifier: NCT03032172. Updated Jan 27, 2022. Accessed Mar 1, 2022. https://clinicaltrials.gov/ct2/show/NCT03032172.
57. Hoffmann-La Roche. An open-label study of risdiplam in infants with genetically diagnosed and presymptomatic spinal muscular atrophy. ClinicalTrials.gov Identifier: NCT03779334. Updated Jan 27, 2022. Accessed Mar 1, 2022. https://clinicaltrials.gov/ct2/show/NCT03779334.
58. McCall S. Update on Genentech/Roche Initiation of MANATEE Clinical Study. Cure SMA. Published October 20, 2021. Accessed August 20, 2022. https://www.curesma.org/update-on- genentech-roche-initiation-of-manatee-clinical-study/
59. Abati E, Manini A, Comi GP, Corti S. Inhibition of myostatin and related signaling pathways for the treatment of muscle atrophy in motor neuron diseases. Cell Mol Life Sci. 2022;79(7):374. doi:10.1007/s00018-022-04408-w
60. FDA approves innovative gene therapy to treat pediatric patients with spinal muscular atrophy, a rare disease and leading genetic cause of infant mortality. U.S. Food and Drug Administration. News release. May 24, 2019. Accessed Mar 1, 2022. http://www.fda.gov/news-events/press-announcements/fda-approves-innovative-gene-therapy-treat-pediatric-patients-spinal-muscular-atrophy-rare-disease.
61. Novartis Gene Therapies. Phase I gene transfer clinical trial for spinal muscular atrophy type 1 delivering AVXS-101. ClinicalTrials.gov Identifier: NCT02122952. Updated Jun 14, 2021. Accessed Mar 1, 2022. https://clinicaltrials.gov/ct2/show/NCT02122952.
62. Novartis Gene Therapies. Phase 3, open-label, single-arm, single-dose gene replacement therapy clinical trial for patients with spinal mus-cular atrophy type 1 with one or two SMN2 copies delivering AVXS-101 by intravenous infusion. ClinicalTrials.gov Identifier: NCT03306277. Updated Jun 14, 2021. Accessed Feb 21, 2022. https://clinicaltrials.gov/ct2/show/NCT03306277.
63. Mendell JR et al. Single-dose gene-replacement therapy for spinal muscular atrophy. N Engl J Med. 2017;377(18):1713-22. doi:10.1056/NEJMoa1706198.
64. Symptomatic study results. ZOLGENSMA [Internet]. Updated Nov 2021. Accessed Mar 1, 2022. Error! Hyperlink reference not valid..
65. Novartis Gene Therapies. A global study of a single, one-time dose of AVXS-101 delivered to infants with genetically diagnosed and pre-symptomatic spinal muscular atrophy with multiple copies of SMN2. ClinicalTrials.gov Identifier: NCT03505099. Updated Jan 1, 2022. Ac-cessed Mar 1, 2022. https://clinicaltrials.gov/ct2/show/NCT03505099.
66. Chiu W et al. Current genetics and potential gene-targeting therapeutics for neuromuscular diseases. Int J Mol Sci. 2020 Dec;21(24):9589. doi:10.3390/ijms21249589.
67. Novartis Gene Therapies. A long-term follow-up study of patients in the clinical trials for spinal muscular atrophy receiving AVXS-101. Clini-calTrials.gov Identifier: NCT04042025. Updated Jun 9, 2021. Accessed Mar 1, 2022. https://clinicaltrials.gov/ct2/show/NCT04042025.
68. Novartis Gene Therapies. Phase 3, open-label, single-arm, single-dose gene replacement therapy clinical trial for patients with spinal mus-cular atrophy type 1 with one or two SMN2 copies delivering AVXS-101 by intravenous infusion. ClinicalTrials.gov Identifier: NCT0383718. Up-dated Jan 11, 2022. Accessed Mar 1, 2022. https://clinicaltrials.gov/ct2/show/NCT03837184.
69. Biogen. An open-label, dose escalation study to assess the safety, tolerability and dose-range finding of multiple doses of ISIS 396443 de-livered intrathecally to patients with spinal muscular atrophy. ClinicalTrials.gov Identifier: NCT01703988. Updated Apr 13, 2021. Accessed Mar 1, 2022. https://clinicaltrials.gov/ct2/show/NCT01703988.
70. Biogen. A study to assess the efficacy, safety, tolerability, and pharmacokinetics of multiple doses of ISIS 396443 delivered intrathecally to patients with infantile-onset spinal muscular atrophy. ClinicalTrials.gov Identifier: NCT01839656. Updated Feb 17, 2021. Accessed Mar 1, 2022. https://clinicaltrials.gov/ct2/show/NCT01839656.
71. Biogen. An open-label extension study for patients with spinal muscular atrophy who previously participated in investigational studies of ISIS 396443. ClinicalTrials.gov Identifier: NCT02594124. Updated Nov 15, 2021. Accessed Mar 1, 2022. https://clinicaltrials.gov/ct2/show/NCT02594124.
72. Biogen. Escalating dose and randomized, controlled study of nusinersen (BIIB058) in participants with spinal muscular atrophy. ClinicalTri-als.gov Identifier: NCT04089566. Updated Feb 24, 2022. Accessed Mar 1, 2022. https://clinicaltrials.gov/ct2/show/NCT04089566.
73. National Center for Advancing Translational Sciences. Duchenne muscular dystrophy. Genetic and Rare Diseases Information Center. Up-dated Nov 2, 2020. Accessed Mar 1, 2022. https://rarediseases.info.nih.gov/diseases/6291/duchenne-muscular-dystrophy.
74. Matsuo M. Antisense oligonucleotide-mediated exon-skipping therapies: Precision medicine spreading from Duchenne muscular dystrophy. JMA J. 2021 Jul 15;4(3):232-40. doi:10.31662/jmaj.2021-0019.
75. FDA approves drug to treat Duchenne muscular dystrophy. U.S. Food and Drug Administration. News release. Feb 9, 2017. Accessed Mar 1, 2022. http://www.fda.gov/news-events/press-announcements/fda-approves-drug-treat-duchenne-muscular-dystrophy.74.
76. Duan D. Dystrophin gene replacement and gene repair therapy for Duchenne muscular dystrophy in 2016: An interview. Hum Gene Ther Clin Dev. 2016 Mar;27(1):9-18. doi:10.1089/humc.2016.001.
77. EXONDYS 51®. Parent Project Muscular Dystrophy. Accessed August 21, 2022. https://www.parentprojectmd.org/drug-development-pipeline/exondys-51/
78. Sarepta Therapeutics, Inc. A Randomized, Double-Blind, Placebo-Controlled, Multiple Dose Efficacy, Safety, Tolerability and Pharmacoki-netics Study of AVI-4658(Eteplirsen),in the Treatment of Ambulant Subjects With Duchenne Muscular Dystrophy. clinicaltrials.gov; 2020. Ac-cessed August 18, 2022. https://clinicaltrials.gov/ct2/show/NCT01396239
79. Sarepta Therapeutics, Inc. Clinical Study to Assess the Safety Fo AVI-4658 in Subjects With Duchenne Muscular Dystrophy Due to a Frame-Shift Mutation Amenable to Correction by Skipping Exon 51. clinicaltrials.gov; 2015. Accessed August 18, 2022. https://clinicaltrials.gov/ct2/show/study/NCT00844597
80. Sarepta Therapeutics, Inc. A 2-part, randomized, double-blind, placebo-controlled, dose-titration, safety, tolerability, and pharmacokinetics study (Part 1) followed by an open-label efficacy and safety evaluation (Part 2) of SRP-4053 in patients with Duchenne muscular dystrophy amenable to exon 53 skipping. ClinicalTrials.gov Identifier: NCT02310906. Updated Oct 19, 2020. Accessed Mar 1, 2022. https://clinicaltrials.gov/ct2/show/results/NCT02310906.
81. Commissioner O of the. FDA grants accelerated approval to first drug for Duchenne muscular dystrophy. FDA. Published March 24, 2020. Accessed August 21, 2022. hDuchenne Muscular Dystrophy Amenable to Exon 51-Skipping Treatment. clinicaltrials.gov; 2022. Accessed Au-gust 18, 2022. https://clinicaltrials.gov/ct2/show/NCT04004065
109. National Center of Neurology and Psychiatry, Japan. Exploratory study of NS-065/NCNP-01 in Duchenne muscular dystrophy. ClinicalTri-als.gov Identifier: NCT02081625; Updated Feb 26, 2020. Accessed Mar 2, 2022. https://clinicaltrialsttps://www.fda.gov/news-events/press-announcements/fda-grants-accelerated-approval-first-drug-duchenne-muscular- dys-trophy
82. Duchenne Drug Development Pipeline. Parent Project Muscular Dystrophy. Accessed August 21, 2022. https://www.parentprojectmd.org/duchenne-drug-development-pipeline/
83. Sarepta Therapeutics Provides Update on SRP-5051 for the Treatment of Duchenne Muscular Dystrophy | Sarepta Therapeutics, Inc. Ac-cessed August 22, 2022. https://investorrelations.sarepta.com/news-releases/news-release-details/sarepta-therapeutics- pro-vides-update-srp-5051-treatment-duchenne
84. Sarepta Therapeutics, Inc. An Open-Label Extension Study for Patients With Duchenne Muscular Dystrophy Who Participated in Studies of SRP-5051. clinicaltrials.gov; 2021. Accessed August 18, 2022. https://clinicaltrials.gov/ct2/show/NCT03675126
85. VYONDYS 53. Prescribing information. Sarepta Therapeutics Inc.; 2019. Accessed Mar 2, 2022. http://www.accessdata.fda.gov/drugsatfda_docs/label/2019/211970s000lbl.pdf.
86. NS Pharma Inc. Long-term use of viltolarsen in boys with Duchenne muscular dystrophy in clinical practice (VILT-502). ClinicalTrials.gov Identifier: NCT04687020. Updated Nov 22, 2021. Accessed Mar 1, 2022. https://clinicaltrials.gov/ct2/show/NCT04687020.
87. VILTEPSO. Prescribing information. NS Pharma; 2020. Accessed Mar 2, 2022. http://www.accessdata.fda.gov/drugsatfda_docs/label/2020/212154s000lbl.pdf.
88. FDA approves targeted treatment for rare Duchenne muscular dystrophy mutation. U.S. Food and Drug Administration. News release. Feb 25, 2021. Accessed Mar 1, 2022. http://www.fda.gov/news-events/press-announcements/fda-approves-targeted-treatment-rare-duchenne-muscular-dystrophy-mutation-0.
89. Sarepta Therapeutics Inc. A double-blind, placebo-controlled, multi-center study with an open-label extension to evaluate the efficacy and safety of SRP-4045 and SRP-4053 in patients with Duchenne muscular dystrophy. Clinicaltrials.gov Identifier: NCT02500381. Updated Aug 19, 2021. Accessed Mar 1, 2022. https://clinicaltrials.gov/ct2/show/NCT02500381.
90. AMONDYS 45. Prescribing information. Sarepta Therapeutics Inc.; 2021. Accessed Feb 22, 2022. http://www.accessdata.fda.gov/drugsatfda_docs/label/2021/213026lbl.pdf.
91. Finkel RS et al. Phase 2a study of ataluren-mediated dystrophin production in patients with nonsense mutation Duchenne muscular dys-trophy. PLoS ONE. 2013;8(12):e81302. doi:10.1371/journal.pone.0081302.
92. PTC Therapeutics. A phase 2 study of PTC124 as an oral treatment for nonsense-mutation-mediated Duchenne muscular dystrophy. Clini-calTrials.gov Identifier: NCT00264888. Updated Jan 14, 2009. Accessed Mar 1, 2022. https://clinicaltrials.gov/ct2/show/NCT00264888.
93. PTC Therapeutics. A phase 2B efficacy and safety study of PTC124 in subjects with nonsense-mutation-mediated Duchenne and Becker muscular dystrophy. ClinicalTrials.gov Identifier: NCT00592553. Updated Apr 7, 2020. Accessed Mar 1, 2022. https://clinicaltrials.gov/ct2/show/NCT00592553.
94. PTC Therapeutics. A phase 3 efficacy and safety study of ataluren in patients with nonsense mutation dystrophinopathy. ClinicalTrials.gov Identifier: NCT01826487. Updated Aug 4, 2020. Accessed Mar 1, 2022. https://clinicaltrials.gov/ct2/show/NCT01826487.
95. Bushby K et al; PTC124-GD-007-DMD Study Group. Ataluren treatment of patients with nonsense mutation dystrophinopathy. Muscle Nerve. 2014 Oct;50(4):477-87. doi:10.1002/mus.24332.
96. Solid Biosciences LLC. A randomized, controlled, open-label, single-ascending dose, phase I/II study to investigate the safety and tolerabil-ity, and efficacy of intravenous SGT-001 in male adolescents and children with Duchenne muscular dystrophy. ClinicalTrials.gov Identifier: NCT03368742. Updated Aug 24, 2021. Accessed Mar 1, 2022. https://clinicaltrials.gov/ct2/show/NCT03368742.
97. Solid Biosciences reports 1.5-year data from patients in the ongoing IGNITE DMD phase I/II clinical trial of SGT-001. Press release. Solid Biosciences. Sep 27, 2021. Accessed Mar 2, 2022. http://www.solidbio.com/about/media/press-releases/solid-biosciences-reports-1-5-year-data-from-patients-in-the-ongoing-ignite-dmd-phase-i-ii-clinical-trial-of-sgt-001.
98. Potter RA et al. Dose-escalation study of systemically delivered rAAVrh74.MHCK7.microdystrophin in the mdx mouse model of Duchenne muscular dystrophy. Hum Gene Ther. 2021 Apr;32(7-8):375-89. doi:10.1089/hum.2019.255.
99. Sarepta Therapeutics, Inc. A Phase 3 Multinational, Randomized, Double-Blind, Placebo- Controlled Systemic Gene Delivery Study to Evaluate the Safety and Efficacy of SRP-9001 in Patients With Duchenne Muscular Dystrophy (EMBARK). clinicaltrials.gov; 2022. Accessed August 18, 2022. https://clinicaltrials.gov/ct2/show/NCT05096221
100. Pfizer. A PHASE 3, MULTICENTER, RANDOMIZED, DOUBLE-BLIND, PLACEBO CONTROLLED STUDY TO EVALUATE THE SAFETY AND EFFICACY OF PF 06939926 FOR THE TREATMENT OF DUCHENNE MUSCULAR DYSTROPHY. clinicaltrials.gov; 2022. Accessed August 18, 2022. https://clinicaltrials.gov/ct2/show/NCT04281485
101. Pfizer. A phase 1B multicenter open-label, single ascending dose study to evaluate the safety and tolerability of PF-06939926 in ambula-tory and non-ambulatory subjects with Duchenne muscular dystrophy. ClinicalTrials.gov Identifier: NCT03362502. Updated Mar 2, 2022. Ac-cessed Mar 2, 2022. https://clinicaltrials.gov/ct2/show/NCT03362502.
102. MS MW. Phase 3 CIFFREO DMD Gene Therapy Trial Slated to Begin in June in US. Accessed August 21, 2022. https://musculardystrophynews.com/news/phase-3-trial-of-pfizers-gene-therapy- expected-to-open-in-us-in-june/
103. SRP-9001. Parent Project Muscular Dystrophy. Accessed August 22, 2022. https://www.parentprojectmd.org/drug-development-pipeline/srp-9001-micro-dystrophin-gene- transfer/
104. Sarepta Therapeutics’ Investigational Gene Therapy SRP-9001 for Duchenne Muscular Dystrophy Demonstrates Significant Functional Improvements Across Multiple Studies | Sarepta Therapeutics, Inc. Accessed August 22, 2022. https://investorrelations.sarepta.com/news-releases/news-release- details/sarepta-therapeutics-investigational-gene-therapy-srp-9001
105. Sarepta Therapeutics, Inc. An Open-Label Safety, Tolerability, and Efficacy Study of Eteplirsen in Patients With Duchenne Muscular Dys-trophy Who Have Completed Study 4658-102.clinicaltrials.gov; 2022. Accessed August 18, 2022. https://clinicaltrials.gov/ct2/show/NCT03985878
106. Sarepta Therapeutics, Inc. An Open-Label Safety, Tolerability, and Pharmacokinetics Study of Eteplirsen in Young Patients With Duchenne Mus-cular Dystrophy Amenable to Exon 51 Skipping. clinicaltrials.gov; 2021. Accessed August 18, 2022. https://clinicaltrials.gov/ct2/show/NCT03218995
107.Sarepta Therapeutics, Inc. A Randomized, Double-Blind, Dose Finding and Comparison Study of the Safety and Efficacy of a High Dose of Eteplirsen, Preceded by an Open-Label Dose Escalation, in Patients With Duchenne Muscular Dystrophy With Deletion Mutations Amenable to Exon 51 Skipping. clinicaltrials.gov; 2022. Accessed August 18, 2022. https://clinicaltrials.gov/ct2/show/NCT03992430
108. Sarepta Therapeutics, Inc. A Phase 2, Two-Part, Multiple-Ascending-Dose Study of SRP-5051 for Dose Determination, Then Dose Ex-pansion, in Patients With .gov/ct2/show/NCT02081625.
110. NS Pharma Inc. A phase II, dose finding study to assess the safety, tolerability, pharmacokinetics, and pharmacodynamics of NS-065/NCNP-01 in boys with Duchenne muscular dystrophy (DMD). ClinicalTrials.gov Identifier: NCT02740972. Updated Dec 7, 2021. Ac-cessed Mar 2, 2022. https://clinicaltrials.gov/ct2/show/NCT02740972.
111. NS Pharma Inc. A phase II, open-label, extension study to assess the safety and efficacy of NS-065/NCNP-01 in boys with Duchenne muscular dystrophy (DMD). ClinicalTrials.gov Identifier: NCT03167255. Updated Nov 24, 2021. Accessed Mar 2, 2022. https://clinicaltrials.gov/ct2/show/NCT03167255.
112. NS Pharma Inc. A phase 2 open label study to assess the safety, tolerability, and efficacy of viltolarsen in ambulant and non-ambulant boys with Duchenne muscular dystrophy (DMD) compared with natural history controls. ClinicalTrials.gov Identifier: NCT04956289. Updated Feb 1, 2022. Accessed Mar 2, 2022. https://clinicaltrials.gov/ct2/show/NCT04956289.
113. NS Pharma Inc. A phase 3 randomized, double-blind, placebo-controlled, multi-center study to assess the efficacy and safety of viltolarsen in ambulant boys with Duchenne muscular dystrophy (DMD). ClinicalTrials.gov Identifier: NCT04060199. Updated Nov 16, 2021. Accessed Mar 2, 2022. https://clinicaltrials.gov/ct2/show/NCT04060199.
114. NS Pharma Inc. A phase 3, multi-center, open-label extension study to assess the safety and efficacy of viltolarsen in ambulant boys with Duchenne muscular dystrophy (DMD). ClinicalTrials.gov Identifier: NCT04768062. Updated Nov 16, 2021. Accessed Mar 2, 2022. https://clinicaltrials.gov/ct2/show/NCT04768062.
115. Sarepta Therapeutics Inc. A randomized, double-blind, placebo-controlled, dose-titration, safety, tolerability, and pharmacokinetics study followed by an open-label safety and efficacy evaluation of SRP-4045 in advanced-stage patients with Duchenne muscular dystrophy amena-ble to exon 45 skipping. ClinicalTrials.gov Identifier: NCT02530905. Updated May 17, 2021. Accessed Mar 2, 2022. https://clinicaltrials.gov/ct2/show/NCT02530905.
116. Sarepta Therapeutics Inc. Long-term, open-label extension study for patients with Duchenne muscular dystrophy enrolled in clinical trials evaluating casimersen or golodirsen. ClinicalTrials.gov Identifier: NCT03532542. Updated Dec 20, 2021. Accessed Mar 2, 2022. https://clinicaltrials.gov/ct2/show/NCT03532542.
117. PTC Therapeutics. A phase 2 study of the safety, pharmacokinetics, and pharmacodynamics of ataluren (PTC124®) in patients aged ≥2 to <5 years old with nonsense mutation dystrophinopathy. ClinicalTrials.gov Identifier: NCT02819557. Updated Aug 28, 2020. Accessed Mar 2, 2022. https://clinicaltrials.gov/ct2/show/NCT02819557.
118. PTC Therapeutics. Phase 2, non-interventional, clinical study to assess dystrophin levels in subjects with nonsense mutation Duchenne muscular dystrophy who have been treated with ataluren for ≥ 9 months. ClinicalTrials.gov Identifier: NCT03796637. Updated Apr 10, 2020. Accessed Mar 2, 2022. https://clinicaltrials.gov/ct2/show/NCT03796637.
119. PTC Therapeutics. An Open-Label Study Evaluating the Safety and Pharmacokinetics of Ataluren in Children From ≥6 Months to <2 Years of Age With Nonsense Mutation Duchenne Muscular Dystrophy. clinicaltrials.gov; 2022. Accessed August 18, 2022. https://clinicaltrials.gov/ct2/show/NCT04336826 120. PTC Therapeutics. An open-label study for previously treated ataluren (PTC124®) pa-tients with nonsense mutation dystrophinopathy. ClinicalTrials.gov Identifier: NCT01557400. Updated Nov 25, 2020. Accessed Feb 21, 2022. https://clinicaltrials.gov/ct2/show/NCT01557400.
121. PTC Therapeutics. An open-label, safety study for ataluren (PTC124) patients with nonsense mutation dystrophinopathy. ClinicalTrials.gov Identifier: NCT01247207. Updated Feb 16, 2022. Accessed Mar 2, 2022. https://clinicaltrials.gov/ct2/show/NCT01247207.
122. PTC Therapeutics. A phase 3, randomized, double-blind, placebo-controlled efficacy and safety study of ataluren in patients with non-sense mutation Duchenne muscular dystrophy and open-label extension. ClinicalTrials.gov Identifier: NCT03179631. Updated Feb 8, 2022. Accessed Mar 2, 2022. https://clinicaltrials.gov/ct2/show/NCT03179631.
123. Sarepta Therapeutics, Inc. An Open-Label, Systemic Gene Delivery Study Using Commercial Process Material to Evaluate the Safety of and Expression From SRP-9001 in Subjects With Duchenne Muscular Dystrophy (ENDEAVOR). clinicaltrials.gov; 2022. Accessed August 18, 2022. https://clinicaltrials.gov/ct2/show/NCT04626674
124. Sarepta Therapeutics, Inc. Systemic Gene Delivery Phase I/IIa Clinical Trial for Duchenne Muscular Dystrophy Using RAA-Vrh74.MHCK7.Micro-Dystrophin (MicroDys-IV-001). clinicaltrials.gov; 2022. Accessed August 18, 2022. https://clinicaltrials.gov/ct2/show/NCT03375164
125. Sarepta Therapeutics Inc. A multicenter, randomized, double-blind, placebo-controlled trial for Duchenne muscular dystrophy using SRP-9001. ClinicalTrials.gov Identifier: NCT03769116. Updated Dec 2021. Accessed Mar 2, 2022. https://clinicaltrials.gov/ct2/show/NCT03769116.
126. Hoffmann-La Roche. A Two-Part, Seamless, Multi-Center, Randomized, Placebo-Controlled, Double-Blind Study to Investigate the Safety, Tolerability, Pharmacokinetics, Pharmacodynamics and Efficacy of RO7204239 in Combination With Risdiplam (RO7034067) in Ambulant Pa-tients With Spinal Muscular Atrophy. clinicaltrials.gov; 2022. Accessed September 1, 2022. https://clinicaltrials.gov/ct2/show/NCT05115110
Staying alert for patients with narcolepsy
Almost half of Americans report feeling daytime sleepiness on at least 3 days per week. For most patients, this sleepiness results from insufficient nighttime sleep. But a minority of these patients have narcolepsy, a chronic neurologic disorder that impairs the brain’s control of sleep-wake cycles. This disorder often goes undiagnosed, but neurologists can make a significant difference by learning how to recognize and treat it.
What is narcolepsy?
Narcolepsy is characterized by excessive daytime sleepiness (EDS) and sudden attacks of sleep. Patients have difficulty staying awake for long periods of time, and the disorder can make performing daily tasks difficult. Problems with concentration and alertness are common.
Narcolepsy is considered to have two subtypes. Patients with narcolepsy type 1 also have cataplexy, a sudden loss of muscle tone. Attacks of cataplexy are triggered by strong, usually positive, emotions. These attacks have manifestations ranging from slurred speech to complete weakness of most muscles. Patients with narcolepsy type 2, however, do not have cataplexy.
Dysregulation of rapid eye movement (REM) sleep, which is when most dreaming occurs, is another symptom of narcolepsy. The transition to REM sleep is quicker in patients with narcolepsy and usually occurs within 15 minutes of sleep onset. A related symptom is sleep paralysis, an inability to move while falling asleep or waking up. This symptom resembles a state that normally occurs during REM sleep.
Hallucinations also are common in patients with narcolepsy and can be especially vivid. Hypnagogic hallucinations occur during the transition to sleep, and hypnopompic hallucinations arise while the patient is waking up. Patients may think they see a stranger in their bedroom, and children sometimes report seeing animals.
Although it is easy for patients with narcolepsy to fall asleep at night, they often have disrupted sleep. Patients have frequent, brief arousals throughout the night that may become disturbing. Dream content often is affected in narcolepsy, too. Patients have described lucid dreams of flying or out-of-body experiences. After such intense dreams, patients often feel that their sleep has not been restful.
Criteria and diagnosis
To receive a diagnosis of narcolepsy type 1, a patient must have EDS that persists for at least 3 months and at least one of the following two features: cataplexy and objective evidence of quick sleep onset and early start of REM sleep or low cerebrospinal fluid (CSF) levels (that is, less than 110 pg/mL) of hypocretin. Hypocretin, also known as orexin, is a neuropeptide that regulates wakefulness and arousal.
Patients must meet five criteria to receive a diagnosis of narcolepsy type 2. They must have EDS that persists for at least 3 months. They must have test results that show quick sleep onset and early start of REM sleep. They must have no cataplexy. Their CSF levels of hypocretin must be normal or unknown. Finally, they must have no other conditions that provide a better explanation for their symptoms and test results.
“The diagnosis of narcolepsy is made primarily by history on the clinical features of the disorder,” said Michael J. Thorpy, MB, ChB, professor of neurology at Albert Einstein College of Medicine and director of the Sleep–Wake Disorders Center at Montefiore Medical Center in New York. When narcolepsy is suspected, testing is required to confirm the diagnosis. The patient should undergo all-night polysomnographic (PSG) testing, followed by a daytime multiple sleep latency test (MSLT). Measurement of CSF hypocretin can be diagnostic but is performed mainly in the research setting and is not common in the clinical setting, said Dr. Thorpy.
Patients with narcolepsy typically fall asleep in an average of less than 8 minutes during the nap opportunities of the MSLT. They also have at least two sleep-onset REM periods. “A new change in the diagnostic classification is that a sleep-onset REM period on the preceding night’s PSG can count as one of the two sleep-onset REM periods required for diagnosis,” said Dr. Thorpy.
“In the case of type 1 narcolepsy, the history is usually pretty clear, and the MSLT is usually positive, in the sense that it is consistent with a narcolepsy pattern,” said Thomas E. Scammell, MD, professor of neurology at Harvard Medical School and Beth Israel Deaconess Medical Center in Boston. “The PSG is also important, because other factors that disrupt the patient’s nighttime sleep (such as obstructive sleep apnea and periodic limb movements) must be ruled out, especially in type 2 narcolepsy,” said Dr. Scammell.
Early sleep onset, late diagnosis
Diagnostic delay is a common problem for patients with narcolepsy. Although the median age of onset is 16 years, a patient typically does not receive the appropriate diagnosis until adulthood. “It takes, on average, somewhere between 8 and 12 years for a patient to get a diagnosis of narcolepsy,” said Dr. Thorpy. Growing awareness and an increase in the number of sleep disorder centers have reduced but not eliminated the diagnostic delay.
Children with narcolepsy are often misdiagnosed. “One of the most common misdiagnoses in childhood is ADHD, because sleepiness in children differs from that in adults,” said Dr. Thorpy. Sleepy children often become hyperactive and display increased impulsivity, he explained. Stimulants prescribed for ADHD tend to mask the symptoms of narcolepsy and delay the correct diagnosis. Mood disorders, behavioral disorders, and psychogenic disorders are other common misdiagnoses for children with narcolepsy.
But when it comes to adults, sometimes patients themselves contribute to the diagnostic delay. EDS is “such a pervasive feeling that I think a lot of people just don’t make much of it,” said Dr. Scammell. The symptom is easily ascribed to insufficient sleep or a difficult work schedule. “It may take them months to get to see a doctor,” said Dr. Scammell.
Behavioral treatments
Nonpharmacologic treatments are one component of care for patients with narcolepsy. Patients must maintain a regular sleep-wake schedule and ensure that they are in bed for no less than 8 hours per night, said Dr. Thorpy. Taking no more than two daytime naps of less than 20 minutes each can help relieve some of the sleepiness, he added.
In addition to ensuring an adequate amount of sleep, it is important to promote good quality sleep, said Dr. Scammell. To do this, clinicians should address any conditions such as sleep apnea that disrupt patients’ sleep, he added.
Patients also tend to avoid situations that are likely to entail the emotional stimuli that could precipitate cataplexy. Some avoid laughter or try to suppress their emotions. “That’s not good,” said Kiran Maski, MD, MPH, assistant professor of neurology at Harvard Medical School and neurologist and sleep physician at Boston Children’s Hospital. “We worry that that might be a risk factor for depression or social isolation.” Cognitive-behavioral therapy can help patients with narcolepsy gradually increase their comfort with and exposure to social situations.
Although behavioral treatments are helpful, they are not sufficient to control all the symptoms of narcolepsy. Most patients require pharmacologic treatments, which are the most effective treatments for narcolepsy, said Dr. Thorpy.
Pharmacologic treatments
Previously, neurologists relied on the stimulants methylphenidate and amphetamine, which primarily treated patients’ EDS. But the field is moving away from these drugs because of their tendency to induce side effects and their potential for abuse, said Dr. Thorpy. In this context, modafinil and armodafinil became the mainstay for promoting alertness in patients with narcolepsy.
In recent years, newer medications have emerged that have slightly greater efficacy and better safety profiles than modafinil and armodafinil. Solriamfetol (Sunosi, Jazz Pharmaceuticals), for example, is effective for EDS but does not affect cataplexy. Pitolisant (Wakix, Harmony Biosciences), on the other hand, effectively treats EDS and cataplexy.
Sodium oxybate (Xyrem, Jazz Pharmaceuticals) is the only medication that treats all the symptoms of narcolepsy, said Dr. Thorpy. “That treats the sleepiness, the cataplexy, and the disturbed nocturnal sleep,” he added. Sodium oxybate also appears to reduce sleep paralysis, hallucinations, and disturbed dreams.
A potential concern about sodium oxybate, which has been used since approximately 2000, is its high sodium load. A new formulation called low-sodium oxybate (Xywav, Jazz Pharmaceuticals) “has a slightly better safety profile, particularly in people who have cardiovascular or renal disease,” said Dr. Thorpy. “This is tending to take over the role of regular sodium oxybate.”
Many clinicians who treat patients with narcolepsy develop their own approaches, but the choice of treatment generally depends on the patient’s symptoms, said Dr. Scammell. Modafinil is a good first choice for patients with mild to moderate sleepiness, he added. Pitolisant is another good choice for these patients but is more expensive. Both drugs are well tolerated.
Clinicians can consider solriamfetol and amphetamine for patients with moderate to severe sleepiness. “I generally consider the oxybates to be a second line,” said Dr. Scammell. Although these drugs may be the most effective, and they do help patients a great deal, they have a higher prevalence of side effects and are more expensive, he added. “If we can get good results with something gentle and simple like modafinil, that would be great.”
“There are differences of opinion as to what the first-line treatments are,” said Dr. Thorpy. Some patients prefer to use the traditional stimulants as first-line treatments, but others prefer to avoid them because of their adverse effects. They favor the newer, and unfortunately more expensive, medications instead. But there is no consensus among clinicians about which of the newer medications to use. “There’s no standard treatment, and it’s very hard to develop an algorithm that is acceptable to most physicians treating patients with narcolepsy,” said Dr. Thorpy. Treatment response varies, as well. Some patients respond extremely well to treatment, but clinical trials indicate that even optimal therapy helps patients achieve about 70% of the normal level of alertness. “If they’re sedentary, sitting in a boring meeting or at the computer, they can still fall asleep, even with our current medications,” said Dr. Scammell.
“The hardest symptom of all to treat is the EDS,” agreed Dr. Thorpy. Most patients cannot be treated with one medication alone, and polypharmacy tends to be necessary, he added. Typically, this means the addition of another medication to the regimen to maximize alertness. For other patients, cataplexy is difficult to control, and adding an anticataplectic medication is appropriate. Still, most patients can control their cataplexy with one drug, either oxybate or pitolisant, said Dr. Thorpy.
Investigational treatments
Researchers are trying to develop new medicines with greater potency, and several medications are under investigation. Early studies have shown that reboxetine, an antidepressant medication that affects dopamine and norepinephrine activity, is an effective treatment for EDS and cataplexy. Ongoing phase 3 studies are examining reboxetine for EDS. Another drug known as FT-218 is a once-nightly formulation of sodium oxybate, unlike the twice-nightly formulations of the drug that currently are available. In a phase 3 trial, the drug was associated with significant improvements in wakefulness and reductions in attacks of cataplexy. Avadel, which is developing the drug, submitted it to the U.S. Food and Drug Administration for approval in 2021, but the agency has not yet made a decision about it.
Researchers and patients alike have high hopes for medications that activate the orexin receptors. Orexin stimulates the wake-promoting neurons in the brain. Narcolepsy, and particularly narcolepsy type 1, is characterized by a loss of hypocretin cells in the central nervous system. The loss of these cells promotes sleepiness and disturbed REM sleep. To counteract this loss of cells, several companies are investigating new orexin agonists.
One such medication is TAK-994, which was developed by Takeda. The drug showed great promise for treating EDS and cataplexy, said Dr. Thorpy. But when phase 3 studies suggested that TAK-994 was associated with hepatotoxicity, the company terminated the studies. Nevertheless, other orexin agonists, including Takeda’s TAK-861, are under investigation.
“If we can restore orexin signaling, it could be like giving insulin to type 1 diabetics,” said Dr. Scammell. This class of medications could provide substantial improvements in sleepiness and other symptoms, he added. “I think when orexin agonists become available, it’s going to be quite transformative.” But these drugs are still in early development and will not be available in clinical practice for several years.
Common psychological comorbidities
Certain comorbidities are prevalent among patients with narcolepsy, and psychiatric disorders tend to be the most common. These comorbidities may complicate the management of narcolepsy. Nevertheless, they often are significant enough to require management in their own right, said Dr. Thorpy.
Depression is likely twice as common among patients with narcolepsy than among the general population, said Dr. Scammell. “Whether this is an actual neurobiologic feature of the disease, or whether it is just a reaction to having a challenging disorder isn’t entirely clear,” he added. “But it doesn’t get the attention or treatment that it deserves.”
Partnering with a psychologist or psychiatrist is important because many treatments can exacerbate mood disorders, said Dr. Maski. In general, stimulants, for example, can worsen depression and anxiety and are associated with increased suicide risk. “We oftentimes are using high-dose stimulants in patients, so mood has to be really carefully monitored and managed,” Dr. Maski added.
Cases of depression and suicidal ideation were reported in clinical trials of sodium oxybate. Although these serious adverse events were rare, patients must be monitored very closely even on treatments specifically approved for narcolepsy, said Dr. Maski. Mood disturbances are reported less frequently with modafinil and pitolisant than with stimulants, she noted.
Many times, patients need to take an antidepressant medication, but these drugs could affect the medicines administered for narcolepsy, said Dr. Thorpy. Pitolisant, in particular, may be adversely affected by current antidepressant medications. The only remedies are to change from pitolisant to another narcolepsy medication or to use an antidepressant that does not have histamine 1 receptor antagonism or affect the QTc interval.
Anxiety also is prevalent among patients with narcolepsy, and it can be worsened by traditional stimulants. These drugs also can increase the likelihood of irritability or obsessive-compulsive tendencies. “Traditional stimulants would be best avoided in these patients who have significant anxiety,” said Dr. Thorpy.
The social burden of narcolepsy
The burden of narcolepsy extends beyond psychiatric comorbidities into the social sphere. “Patients with narcolepsy do have greater difficulties in terms of social and interpersonal relationships,” said Dr. Thorpy. The disorder reduces patients’ quality of life, and educational difficulties and job loss are common in this population. “It’s a lifelong, incurable disorder, and these patients suffer an immense burden throughout their life because of the sleepiness that … affects their cognitive abilities,” said Dr. Thorpy.
“There’s an increased reporting of what probably amounts to social isolation,” said Dr. Maski. Patients often report that they must prioritize activities or events because they do not have the energy or alertness to participate in all of them. For instance, adolescents with narcolepsy frequently say that they must forgo after-school extracurricular activities because they need to prioritize studying and getting enough sleep. “Those priorities take away from their normal social life and events that they would like to participate in,” said Dr. Maski.
Another problem is that patients have the impression that others do not understand their condition. They are afraid that they will be perceived as lazy, uninterested, or unmotivated if they fall asleep. “Sometimes they withdraw from social events because they don’t want to be perceived in such a way,” said Dr. Maski. She and her colleagues encourage patients to participate in selected after-school events and to engage in social activities they find meaningful to maintain social networks.
An unpublished study of more than 300 patients with narcolepsy examined the effect of the disorder on patients’ social lives. At the end of the day, many patients “crash and burn,” said Dr. Scammell. Consequently, they do not have as much energy for social activities.
This lack of energy affects patients’ social relationships. The study suggests that patients with narcolepsy do not have as many friends as the general population does. Nevertheless, the frequency of close relationships and marriage was similar between patients with narcolepsy and the general population. “What people are doing is putting their energy into these close relationships, rather than having lots of friends and socializing a lot,” said Dr. Scammell. “I found that heartening, that people were doing their best and developed those close relationships,” which are vitally important for many reasons, he added.
The study, which has been submitted for publication, also asked patients about their sex lives. Many patients reported having had cataplexy during sex, and others reported that their medications caused problems with their sex lives. “Their doctors never ask about these things, and many patients actually would like their doctor to ask about them more,” said Dr. Scammell.
In addition, narcolepsy significantly affects a patient’s ability to drive. Patients with narcolepsy have a three- to fourfold increased risk of car accidents, said Dr. Scammell. This increased risk likely results from patients’ EDS.
But as important as this issue is for patients’ lives, there is no consensus on how to counsel patients about driving, said Dr. Maski. “For instance, it is not really clear if there is value in doing a maintenance of wakefulness test before allowing patients with narcolepsy to drive,” she said. The test is not validated in children or adolescents, which raises questions about how to advise beginning drivers with narcolepsy. “It’s not really clear that passing your maintenance of wakefulness test increases your safety behind the wheel,” said Dr. Maski.
“It’s the rare person with narcolepsy who can easily and safely do a 2-hour drive by themselves,” said Dr. Scammell. Patients must determine what their own limits are, and it is important for clinicians to discuss reasonable limits honestly with their patients. “I almost never would push to have somebody’s license taken away,” said Dr. Scammell. “But there are patients who only can drive around town for short errands, and if it’s anything more than half an hour, they start getting drowsy.”
There is a need for a public awareness campaign about narcolepsy, Dr. Scammell added. Such a campaign was carried out in Italy several years ago, and it included cartoons and TV segments. “It got a lot of people’s attention, and there was a real spike in new and correct diagnoses of narcolepsy,” said Dr. Scammell. But such a broad campaign is expensive, while narcolepsy is rare, and it might not be feasible to reach out to the general population. “But I certainly think it’s worth targeting doctors who are likely to see patients with sleepiness: neurologists, psychiatrists and psychologists, and primary care doctors,” said Dr. Scammell.
Almost half of Americans report feeling daytime sleepiness on at least 3 days per week. For most patients, this sleepiness results from insufficient nighttime sleep. But a minority of these patients have narcolepsy, a chronic neurologic disorder that impairs the brain’s control of sleep-wake cycles. This disorder often goes undiagnosed, but neurologists can make a significant difference by learning how to recognize and treat it.
What is narcolepsy?
Narcolepsy is characterized by excessive daytime sleepiness (EDS) and sudden attacks of sleep. Patients have difficulty staying awake for long periods of time, and the disorder can make performing daily tasks difficult. Problems with concentration and alertness are common.
Narcolepsy is considered to have two subtypes. Patients with narcolepsy type 1 also have cataplexy, a sudden loss of muscle tone. Attacks of cataplexy are triggered by strong, usually positive, emotions. These attacks have manifestations ranging from slurred speech to complete weakness of most muscles. Patients with narcolepsy type 2, however, do not have cataplexy.
Dysregulation of rapid eye movement (REM) sleep, which is when most dreaming occurs, is another symptom of narcolepsy. The transition to REM sleep is quicker in patients with narcolepsy and usually occurs within 15 minutes of sleep onset. A related symptom is sleep paralysis, an inability to move while falling asleep or waking up. This symptom resembles a state that normally occurs during REM sleep.
Hallucinations also are common in patients with narcolepsy and can be especially vivid. Hypnagogic hallucinations occur during the transition to sleep, and hypnopompic hallucinations arise while the patient is waking up. Patients may think they see a stranger in their bedroom, and children sometimes report seeing animals.
Although it is easy for patients with narcolepsy to fall asleep at night, they often have disrupted sleep. Patients have frequent, brief arousals throughout the night that may become disturbing. Dream content often is affected in narcolepsy, too. Patients have described lucid dreams of flying or out-of-body experiences. After such intense dreams, patients often feel that their sleep has not been restful.
Criteria and diagnosis
To receive a diagnosis of narcolepsy type 1, a patient must have EDS that persists for at least 3 months and at least one of the following two features: cataplexy and objective evidence of quick sleep onset and early start of REM sleep or low cerebrospinal fluid (CSF) levels (that is, less than 110 pg/mL) of hypocretin. Hypocretin, also known as orexin, is a neuropeptide that regulates wakefulness and arousal.
Patients must meet five criteria to receive a diagnosis of narcolepsy type 2. They must have EDS that persists for at least 3 months. They must have test results that show quick sleep onset and early start of REM sleep. They must have no cataplexy. Their CSF levels of hypocretin must be normal or unknown. Finally, they must have no other conditions that provide a better explanation for their symptoms and test results.
“The diagnosis of narcolepsy is made primarily by history on the clinical features of the disorder,” said Michael J. Thorpy, MB, ChB, professor of neurology at Albert Einstein College of Medicine and director of the Sleep–Wake Disorders Center at Montefiore Medical Center in New York. When narcolepsy is suspected, testing is required to confirm the diagnosis. The patient should undergo all-night polysomnographic (PSG) testing, followed by a daytime multiple sleep latency test (MSLT). Measurement of CSF hypocretin can be diagnostic but is performed mainly in the research setting and is not common in the clinical setting, said Dr. Thorpy.
Patients with narcolepsy typically fall asleep in an average of less than 8 minutes during the nap opportunities of the MSLT. They also have at least two sleep-onset REM periods. “A new change in the diagnostic classification is that a sleep-onset REM period on the preceding night’s PSG can count as one of the two sleep-onset REM periods required for diagnosis,” said Dr. Thorpy.
“In the case of type 1 narcolepsy, the history is usually pretty clear, and the MSLT is usually positive, in the sense that it is consistent with a narcolepsy pattern,” said Thomas E. Scammell, MD, professor of neurology at Harvard Medical School and Beth Israel Deaconess Medical Center in Boston. “The PSG is also important, because other factors that disrupt the patient’s nighttime sleep (such as obstructive sleep apnea and periodic limb movements) must be ruled out, especially in type 2 narcolepsy,” said Dr. Scammell.
Early sleep onset, late diagnosis
Diagnostic delay is a common problem for patients with narcolepsy. Although the median age of onset is 16 years, a patient typically does not receive the appropriate diagnosis until adulthood. “It takes, on average, somewhere between 8 and 12 years for a patient to get a diagnosis of narcolepsy,” said Dr. Thorpy. Growing awareness and an increase in the number of sleep disorder centers have reduced but not eliminated the diagnostic delay.
Children with narcolepsy are often misdiagnosed. “One of the most common misdiagnoses in childhood is ADHD, because sleepiness in children differs from that in adults,” said Dr. Thorpy. Sleepy children often become hyperactive and display increased impulsivity, he explained. Stimulants prescribed for ADHD tend to mask the symptoms of narcolepsy and delay the correct diagnosis. Mood disorders, behavioral disorders, and psychogenic disorders are other common misdiagnoses for children with narcolepsy.
But when it comes to adults, sometimes patients themselves contribute to the diagnostic delay. EDS is “such a pervasive feeling that I think a lot of people just don’t make much of it,” said Dr. Scammell. The symptom is easily ascribed to insufficient sleep or a difficult work schedule. “It may take them months to get to see a doctor,” said Dr. Scammell.
Behavioral treatments
Nonpharmacologic treatments are one component of care for patients with narcolepsy. Patients must maintain a regular sleep-wake schedule and ensure that they are in bed for no less than 8 hours per night, said Dr. Thorpy. Taking no more than two daytime naps of less than 20 minutes each can help relieve some of the sleepiness, he added.
In addition to ensuring an adequate amount of sleep, it is important to promote good quality sleep, said Dr. Scammell. To do this, clinicians should address any conditions such as sleep apnea that disrupt patients’ sleep, he added.
Patients also tend to avoid situations that are likely to entail the emotional stimuli that could precipitate cataplexy. Some avoid laughter or try to suppress their emotions. “That’s not good,” said Kiran Maski, MD, MPH, assistant professor of neurology at Harvard Medical School and neurologist and sleep physician at Boston Children’s Hospital. “We worry that that might be a risk factor for depression or social isolation.” Cognitive-behavioral therapy can help patients with narcolepsy gradually increase their comfort with and exposure to social situations.
Although behavioral treatments are helpful, they are not sufficient to control all the symptoms of narcolepsy. Most patients require pharmacologic treatments, which are the most effective treatments for narcolepsy, said Dr. Thorpy.
Pharmacologic treatments
Previously, neurologists relied on the stimulants methylphenidate and amphetamine, which primarily treated patients’ EDS. But the field is moving away from these drugs because of their tendency to induce side effects and their potential for abuse, said Dr. Thorpy. In this context, modafinil and armodafinil became the mainstay for promoting alertness in patients with narcolepsy.
In recent years, newer medications have emerged that have slightly greater efficacy and better safety profiles than modafinil and armodafinil. Solriamfetol (Sunosi, Jazz Pharmaceuticals), for example, is effective for EDS but does not affect cataplexy. Pitolisant (Wakix, Harmony Biosciences), on the other hand, effectively treats EDS and cataplexy.
Sodium oxybate (Xyrem, Jazz Pharmaceuticals) is the only medication that treats all the symptoms of narcolepsy, said Dr. Thorpy. “That treats the sleepiness, the cataplexy, and the disturbed nocturnal sleep,” he added. Sodium oxybate also appears to reduce sleep paralysis, hallucinations, and disturbed dreams.
A potential concern about sodium oxybate, which has been used since approximately 2000, is its high sodium load. A new formulation called low-sodium oxybate (Xywav, Jazz Pharmaceuticals) “has a slightly better safety profile, particularly in people who have cardiovascular or renal disease,” said Dr. Thorpy. “This is tending to take over the role of regular sodium oxybate.”
Many clinicians who treat patients with narcolepsy develop their own approaches, but the choice of treatment generally depends on the patient’s symptoms, said Dr. Scammell. Modafinil is a good first choice for patients with mild to moderate sleepiness, he added. Pitolisant is another good choice for these patients but is more expensive. Both drugs are well tolerated.
Clinicians can consider solriamfetol and amphetamine for patients with moderate to severe sleepiness. “I generally consider the oxybates to be a second line,” said Dr. Scammell. Although these drugs may be the most effective, and they do help patients a great deal, they have a higher prevalence of side effects and are more expensive, he added. “If we can get good results with something gentle and simple like modafinil, that would be great.”
“There are differences of opinion as to what the first-line treatments are,” said Dr. Thorpy. Some patients prefer to use the traditional stimulants as first-line treatments, but others prefer to avoid them because of their adverse effects. They favor the newer, and unfortunately more expensive, medications instead. But there is no consensus among clinicians about which of the newer medications to use. “There’s no standard treatment, and it’s very hard to develop an algorithm that is acceptable to most physicians treating patients with narcolepsy,” said Dr. Thorpy. Treatment response varies, as well. Some patients respond extremely well to treatment, but clinical trials indicate that even optimal therapy helps patients achieve about 70% of the normal level of alertness. “If they’re sedentary, sitting in a boring meeting or at the computer, they can still fall asleep, even with our current medications,” said Dr. Scammell.
“The hardest symptom of all to treat is the EDS,” agreed Dr. Thorpy. Most patients cannot be treated with one medication alone, and polypharmacy tends to be necessary, he added. Typically, this means the addition of another medication to the regimen to maximize alertness. For other patients, cataplexy is difficult to control, and adding an anticataplectic medication is appropriate. Still, most patients can control their cataplexy with one drug, either oxybate or pitolisant, said Dr. Thorpy.
Investigational treatments
Researchers are trying to develop new medicines with greater potency, and several medications are under investigation. Early studies have shown that reboxetine, an antidepressant medication that affects dopamine and norepinephrine activity, is an effective treatment for EDS and cataplexy. Ongoing phase 3 studies are examining reboxetine for EDS. Another drug known as FT-218 is a once-nightly formulation of sodium oxybate, unlike the twice-nightly formulations of the drug that currently are available. In a phase 3 trial, the drug was associated with significant improvements in wakefulness and reductions in attacks of cataplexy. Avadel, which is developing the drug, submitted it to the U.S. Food and Drug Administration for approval in 2021, but the agency has not yet made a decision about it.
Researchers and patients alike have high hopes for medications that activate the orexin receptors. Orexin stimulates the wake-promoting neurons in the brain. Narcolepsy, and particularly narcolepsy type 1, is characterized by a loss of hypocretin cells in the central nervous system. The loss of these cells promotes sleepiness and disturbed REM sleep. To counteract this loss of cells, several companies are investigating new orexin agonists.
One such medication is TAK-994, which was developed by Takeda. The drug showed great promise for treating EDS and cataplexy, said Dr. Thorpy. But when phase 3 studies suggested that TAK-994 was associated with hepatotoxicity, the company terminated the studies. Nevertheless, other orexin agonists, including Takeda’s TAK-861, are under investigation.
“If we can restore orexin signaling, it could be like giving insulin to type 1 diabetics,” said Dr. Scammell. This class of medications could provide substantial improvements in sleepiness and other symptoms, he added. “I think when orexin agonists become available, it’s going to be quite transformative.” But these drugs are still in early development and will not be available in clinical practice for several years.
Common psychological comorbidities
Certain comorbidities are prevalent among patients with narcolepsy, and psychiatric disorders tend to be the most common. These comorbidities may complicate the management of narcolepsy. Nevertheless, they often are significant enough to require management in their own right, said Dr. Thorpy.
Depression is likely twice as common among patients with narcolepsy than among the general population, said Dr. Scammell. “Whether this is an actual neurobiologic feature of the disease, or whether it is just a reaction to having a challenging disorder isn’t entirely clear,” he added. “But it doesn’t get the attention or treatment that it deserves.”
Partnering with a psychologist or psychiatrist is important because many treatments can exacerbate mood disorders, said Dr. Maski. In general, stimulants, for example, can worsen depression and anxiety and are associated with increased suicide risk. “We oftentimes are using high-dose stimulants in patients, so mood has to be really carefully monitored and managed,” Dr. Maski added.
Cases of depression and suicidal ideation were reported in clinical trials of sodium oxybate. Although these serious adverse events were rare, patients must be monitored very closely even on treatments specifically approved for narcolepsy, said Dr. Maski. Mood disturbances are reported less frequently with modafinil and pitolisant than with stimulants, she noted.
Many times, patients need to take an antidepressant medication, but these drugs could affect the medicines administered for narcolepsy, said Dr. Thorpy. Pitolisant, in particular, may be adversely affected by current antidepressant medications. The only remedies are to change from pitolisant to another narcolepsy medication or to use an antidepressant that does not have histamine 1 receptor antagonism or affect the QTc interval.
Anxiety also is prevalent among patients with narcolepsy, and it can be worsened by traditional stimulants. These drugs also can increase the likelihood of irritability or obsessive-compulsive tendencies. “Traditional stimulants would be best avoided in these patients who have significant anxiety,” said Dr. Thorpy.
The social burden of narcolepsy
The burden of narcolepsy extends beyond psychiatric comorbidities into the social sphere. “Patients with narcolepsy do have greater difficulties in terms of social and interpersonal relationships,” said Dr. Thorpy. The disorder reduces patients’ quality of life, and educational difficulties and job loss are common in this population. “It’s a lifelong, incurable disorder, and these patients suffer an immense burden throughout their life because of the sleepiness that … affects their cognitive abilities,” said Dr. Thorpy.
“There’s an increased reporting of what probably amounts to social isolation,” said Dr. Maski. Patients often report that they must prioritize activities or events because they do not have the energy or alertness to participate in all of them. For instance, adolescents with narcolepsy frequently say that they must forgo after-school extracurricular activities because they need to prioritize studying and getting enough sleep. “Those priorities take away from their normal social life and events that they would like to participate in,” said Dr. Maski.
Another problem is that patients have the impression that others do not understand their condition. They are afraid that they will be perceived as lazy, uninterested, or unmotivated if they fall asleep. “Sometimes they withdraw from social events because they don’t want to be perceived in such a way,” said Dr. Maski. She and her colleagues encourage patients to participate in selected after-school events and to engage in social activities they find meaningful to maintain social networks.
An unpublished study of more than 300 patients with narcolepsy examined the effect of the disorder on patients’ social lives. At the end of the day, many patients “crash and burn,” said Dr. Scammell. Consequently, they do not have as much energy for social activities.
This lack of energy affects patients’ social relationships. The study suggests that patients with narcolepsy do not have as many friends as the general population does. Nevertheless, the frequency of close relationships and marriage was similar between patients with narcolepsy and the general population. “What people are doing is putting their energy into these close relationships, rather than having lots of friends and socializing a lot,” said Dr. Scammell. “I found that heartening, that people were doing their best and developed those close relationships,” which are vitally important for many reasons, he added.
The study, which has been submitted for publication, also asked patients about their sex lives. Many patients reported having had cataplexy during sex, and others reported that their medications caused problems with their sex lives. “Their doctors never ask about these things, and many patients actually would like their doctor to ask about them more,” said Dr. Scammell.
In addition, narcolepsy significantly affects a patient’s ability to drive. Patients with narcolepsy have a three- to fourfold increased risk of car accidents, said Dr. Scammell. This increased risk likely results from patients’ EDS.
But as important as this issue is for patients’ lives, there is no consensus on how to counsel patients about driving, said Dr. Maski. “For instance, it is not really clear if there is value in doing a maintenance of wakefulness test before allowing patients with narcolepsy to drive,” she said. The test is not validated in children or adolescents, which raises questions about how to advise beginning drivers with narcolepsy. “It’s not really clear that passing your maintenance of wakefulness test increases your safety behind the wheel,” said Dr. Maski.
“It’s the rare person with narcolepsy who can easily and safely do a 2-hour drive by themselves,” said Dr. Scammell. Patients must determine what their own limits are, and it is important for clinicians to discuss reasonable limits honestly with their patients. “I almost never would push to have somebody’s license taken away,” said Dr. Scammell. “But there are patients who only can drive around town for short errands, and if it’s anything more than half an hour, they start getting drowsy.”
There is a need for a public awareness campaign about narcolepsy, Dr. Scammell added. Such a campaign was carried out in Italy several years ago, and it included cartoons and TV segments. “It got a lot of people’s attention, and there was a real spike in new and correct diagnoses of narcolepsy,” said Dr. Scammell. But such a broad campaign is expensive, while narcolepsy is rare, and it might not be feasible to reach out to the general population. “But I certainly think it’s worth targeting doctors who are likely to see patients with sleepiness: neurologists, psychiatrists and psychologists, and primary care doctors,” said Dr. Scammell.
Almost half of Americans report feeling daytime sleepiness on at least 3 days per week. For most patients, this sleepiness results from insufficient nighttime sleep. But a minority of these patients have narcolepsy, a chronic neurologic disorder that impairs the brain’s control of sleep-wake cycles. This disorder often goes undiagnosed, but neurologists can make a significant difference by learning how to recognize and treat it.
What is narcolepsy?
Narcolepsy is characterized by excessive daytime sleepiness (EDS) and sudden attacks of sleep. Patients have difficulty staying awake for long periods of time, and the disorder can make performing daily tasks difficult. Problems with concentration and alertness are common.
Narcolepsy is considered to have two subtypes. Patients with narcolepsy type 1 also have cataplexy, a sudden loss of muscle tone. Attacks of cataplexy are triggered by strong, usually positive, emotions. These attacks have manifestations ranging from slurred speech to complete weakness of most muscles. Patients with narcolepsy type 2, however, do not have cataplexy.
Dysregulation of rapid eye movement (REM) sleep, which is when most dreaming occurs, is another symptom of narcolepsy. The transition to REM sleep is quicker in patients with narcolepsy and usually occurs within 15 minutes of sleep onset. A related symptom is sleep paralysis, an inability to move while falling asleep or waking up. This symptom resembles a state that normally occurs during REM sleep.
Hallucinations also are common in patients with narcolepsy and can be especially vivid. Hypnagogic hallucinations occur during the transition to sleep, and hypnopompic hallucinations arise while the patient is waking up. Patients may think they see a stranger in their bedroom, and children sometimes report seeing animals.
Although it is easy for patients with narcolepsy to fall asleep at night, they often have disrupted sleep. Patients have frequent, brief arousals throughout the night that may become disturbing. Dream content often is affected in narcolepsy, too. Patients have described lucid dreams of flying or out-of-body experiences. After such intense dreams, patients often feel that their sleep has not been restful.
Criteria and diagnosis
To receive a diagnosis of narcolepsy type 1, a patient must have EDS that persists for at least 3 months and at least one of the following two features: cataplexy and objective evidence of quick sleep onset and early start of REM sleep or low cerebrospinal fluid (CSF) levels (that is, less than 110 pg/mL) of hypocretin. Hypocretin, also known as orexin, is a neuropeptide that regulates wakefulness and arousal.
Patients must meet five criteria to receive a diagnosis of narcolepsy type 2. They must have EDS that persists for at least 3 months. They must have test results that show quick sleep onset and early start of REM sleep. They must have no cataplexy. Their CSF levels of hypocretin must be normal or unknown. Finally, they must have no other conditions that provide a better explanation for their symptoms and test results.
“The diagnosis of narcolepsy is made primarily by history on the clinical features of the disorder,” said Michael J. Thorpy, MB, ChB, professor of neurology at Albert Einstein College of Medicine and director of the Sleep–Wake Disorders Center at Montefiore Medical Center in New York. When narcolepsy is suspected, testing is required to confirm the diagnosis. The patient should undergo all-night polysomnographic (PSG) testing, followed by a daytime multiple sleep latency test (MSLT). Measurement of CSF hypocretin can be diagnostic but is performed mainly in the research setting and is not common in the clinical setting, said Dr. Thorpy.
Patients with narcolepsy typically fall asleep in an average of less than 8 minutes during the nap opportunities of the MSLT. They also have at least two sleep-onset REM periods. “A new change in the diagnostic classification is that a sleep-onset REM period on the preceding night’s PSG can count as one of the two sleep-onset REM periods required for diagnosis,” said Dr. Thorpy.
“In the case of type 1 narcolepsy, the history is usually pretty clear, and the MSLT is usually positive, in the sense that it is consistent with a narcolepsy pattern,” said Thomas E. Scammell, MD, professor of neurology at Harvard Medical School and Beth Israel Deaconess Medical Center in Boston. “The PSG is also important, because other factors that disrupt the patient’s nighttime sleep (such as obstructive sleep apnea and periodic limb movements) must be ruled out, especially in type 2 narcolepsy,” said Dr. Scammell.
Early sleep onset, late diagnosis
Diagnostic delay is a common problem for patients with narcolepsy. Although the median age of onset is 16 years, a patient typically does not receive the appropriate diagnosis until adulthood. “It takes, on average, somewhere between 8 and 12 years for a patient to get a diagnosis of narcolepsy,” said Dr. Thorpy. Growing awareness and an increase in the number of sleep disorder centers have reduced but not eliminated the diagnostic delay.
Children with narcolepsy are often misdiagnosed. “One of the most common misdiagnoses in childhood is ADHD, because sleepiness in children differs from that in adults,” said Dr. Thorpy. Sleepy children often become hyperactive and display increased impulsivity, he explained. Stimulants prescribed for ADHD tend to mask the symptoms of narcolepsy and delay the correct diagnosis. Mood disorders, behavioral disorders, and psychogenic disorders are other common misdiagnoses for children with narcolepsy.
But when it comes to adults, sometimes patients themselves contribute to the diagnostic delay. EDS is “such a pervasive feeling that I think a lot of people just don’t make much of it,” said Dr. Scammell. The symptom is easily ascribed to insufficient sleep or a difficult work schedule. “It may take them months to get to see a doctor,” said Dr. Scammell.
Behavioral treatments
Nonpharmacologic treatments are one component of care for patients with narcolepsy. Patients must maintain a regular sleep-wake schedule and ensure that they are in bed for no less than 8 hours per night, said Dr. Thorpy. Taking no more than two daytime naps of less than 20 minutes each can help relieve some of the sleepiness, he added.
In addition to ensuring an adequate amount of sleep, it is important to promote good quality sleep, said Dr. Scammell. To do this, clinicians should address any conditions such as sleep apnea that disrupt patients’ sleep, he added.
Patients also tend to avoid situations that are likely to entail the emotional stimuli that could precipitate cataplexy. Some avoid laughter or try to suppress their emotions. “That’s not good,” said Kiran Maski, MD, MPH, assistant professor of neurology at Harvard Medical School and neurologist and sleep physician at Boston Children’s Hospital. “We worry that that might be a risk factor for depression or social isolation.” Cognitive-behavioral therapy can help patients with narcolepsy gradually increase their comfort with and exposure to social situations.
Although behavioral treatments are helpful, they are not sufficient to control all the symptoms of narcolepsy. Most patients require pharmacologic treatments, which are the most effective treatments for narcolepsy, said Dr. Thorpy.
Pharmacologic treatments
Previously, neurologists relied on the stimulants methylphenidate and amphetamine, which primarily treated patients’ EDS. But the field is moving away from these drugs because of their tendency to induce side effects and their potential for abuse, said Dr. Thorpy. In this context, modafinil and armodafinil became the mainstay for promoting alertness in patients with narcolepsy.
In recent years, newer medications have emerged that have slightly greater efficacy and better safety profiles than modafinil and armodafinil. Solriamfetol (Sunosi, Jazz Pharmaceuticals), for example, is effective for EDS but does not affect cataplexy. Pitolisant (Wakix, Harmony Biosciences), on the other hand, effectively treats EDS and cataplexy.
Sodium oxybate (Xyrem, Jazz Pharmaceuticals) is the only medication that treats all the symptoms of narcolepsy, said Dr. Thorpy. “That treats the sleepiness, the cataplexy, and the disturbed nocturnal sleep,” he added. Sodium oxybate also appears to reduce sleep paralysis, hallucinations, and disturbed dreams.
A potential concern about sodium oxybate, which has been used since approximately 2000, is its high sodium load. A new formulation called low-sodium oxybate (Xywav, Jazz Pharmaceuticals) “has a slightly better safety profile, particularly in people who have cardiovascular or renal disease,” said Dr. Thorpy. “This is tending to take over the role of regular sodium oxybate.”
Many clinicians who treat patients with narcolepsy develop their own approaches, but the choice of treatment generally depends on the patient’s symptoms, said Dr. Scammell. Modafinil is a good first choice for patients with mild to moderate sleepiness, he added. Pitolisant is another good choice for these patients but is more expensive. Both drugs are well tolerated.
Clinicians can consider solriamfetol and amphetamine for patients with moderate to severe sleepiness. “I generally consider the oxybates to be a second line,” said Dr. Scammell. Although these drugs may be the most effective, and they do help patients a great deal, they have a higher prevalence of side effects and are more expensive, he added. “If we can get good results with something gentle and simple like modafinil, that would be great.”
“There are differences of opinion as to what the first-line treatments are,” said Dr. Thorpy. Some patients prefer to use the traditional stimulants as first-line treatments, but others prefer to avoid them because of their adverse effects. They favor the newer, and unfortunately more expensive, medications instead. But there is no consensus among clinicians about which of the newer medications to use. “There’s no standard treatment, and it’s very hard to develop an algorithm that is acceptable to most physicians treating patients with narcolepsy,” said Dr. Thorpy. Treatment response varies, as well. Some patients respond extremely well to treatment, but clinical trials indicate that even optimal therapy helps patients achieve about 70% of the normal level of alertness. “If they’re sedentary, sitting in a boring meeting or at the computer, they can still fall asleep, even with our current medications,” said Dr. Scammell.
“The hardest symptom of all to treat is the EDS,” agreed Dr. Thorpy. Most patients cannot be treated with one medication alone, and polypharmacy tends to be necessary, he added. Typically, this means the addition of another medication to the regimen to maximize alertness. For other patients, cataplexy is difficult to control, and adding an anticataplectic medication is appropriate. Still, most patients can control their cataplexy with one drug, either oxybate or pitolisant, said Dr. Thorpy.
Investigational treatments
Researchers are trying to develop new medicines with greater potency, and several medications are under investigation. Early studies have shown that reboxetine, an antidepressant medication that affects dopamine and norepinephrine activity, is an effective treatment for EDS and cataplexy. Ongoing phase 3 studies are examining reboxetine for EDS. Another drug known as FT-218 is a once-nightly formulation of sodium oxybate, unlike the twice-nightly formulations of the drug that currently are available. In a phase 3 trial, the drug was associated with significant improvements in wakefulness and reductions in attacks of cataplexy. Avadel, which is developing the drug, submitted it to the U.S. Food and Drug Administration for approval in 2021, but the agency has not yet made a decision about it.
Researchers and patients alike have high hopes for medications that activate the orexin receptors. Orexin stimulates the wake-promoting neurons in the brain. Narcolepsy, and particularly narcolepsy type 1, is characterized by a loss of hypocretin cells in the central nervous system. The loss of these cells promotes sleepiness and disturbed REM sleep. To counteract this loss of cells, several companies are investigating new orexin agonists.
One such medication is TAK-994, which was developed by Takeda. The drug showed great promise for treating EDS and cataplexy, said Dr. Thorpy. But when phase 3 studies suggested that TAK-994 was associated with hepatotoxicity, the company terminated the studies. Nevertheless, other orexin agonists, including Takeda’s TAK-861, are under investigation.
“If we can restore orexin signaling, it could be like giving insulin to type 1 diabetics,” said Dr. Scammell. This class of medications could provide substantial improvements in sleepiness and other symptoms, he added. “I think when orexin agonists become available, it’s going to be quite transformative.” But these drugs are still in early development and will not be available in clinical practice for several years.
Common psychological comorbidities
Certain comorbidities are prevalent among patients with narcolepsy, and psychiatric disorders tend to be the most common. These comorbidities may complicate the management of narcolepsy. Nevertheless, they often are significant enough to require management in their own right, said Dr. Thorpy.
Depression is likely twice as common among patients with narcolepsy than among the general population, said Dr. Scammell. “Whether this is an actual neurobiologic feature of the disease, or whether it is just a reaction to having a challenging disorder isn’t entirely clear,” he added. “But it doesn’t get the attention or treatment that it deserves.”
Partnering with a psychologist or psychiatrist is important because many treatments can exacerbate mood disorders, said Dr. Maski. In general, stimulants, for example, can worsen depression and anxiety and are associated with increased suicide risk. “We oftentimes are using high-dose stimulants in patients, so mood has to be really carefully monitored and managed,” Dr. Maski added.
Cases of depression and suicidal ideation were reported in clinical trials of sodium oxybate. Although these serious adverse events were rare, patients must be monitored very closely even on treatments specifically approved for narcolepsy, said Dr. Maski. Mood disturbances are reported less frequently with modafinil and pitolisant than with stimulants, she noted.
Many times, patients need to take an antidepressant medication, but these drugs could affect the medicines administered for narcolepsy, said Dr. Thorpy. Pitolisant, in particular, may be adversely affected by current antidepressant medications. The only remedies are to change from pitolisant to another narcolepsy medication or to use an antidepressant that does not have histamine 1 receptor antagonism or affect the QTc interval.
Anxiety also is prevalent among patients with narcolepsy, and it can be worsened by traditional stimulants. These drugs also can increase the likelihood of irritability or obsessive-compulsive tendencies. “Traditional stimulants would be best avoided in these patients who have significant anxiety,” said Dr. Thorpy.
The social burden of narcolepsy
The burden of narcolepsy extends beyond psychiatric comorbidities into the social sphere. “Patients with narcolepsy do have greater difficulties in terms of social and interpersonal relationships,” said Dr. Thorpy. The disorder reduces patients’ quality of life, and educational difficulties and job loss are common in this population. “It’s a lifelong, incurable disorder, and these patients suffer an immense burden throughout their life because of the sleepiness that … affects their cognitive abilities,” said Dr. Thorpy.
“There’s an increased reporting of what probably amounts to social isolation,” said Dr. Maski. Patients often report that they must prioritize activities or events because they do not have the energy or alertness to participate in all of them. For instance, adolescents with narcolepsy frequently say that they must forgo after-school extracurricular activities because they need to prioritize studying and getting enough sleep. “Those priorities take away from their normal social life and events that they would like to participate in,” said Dr. Maski.
Another problem is that patients have the impression that others do not understand their condition. They are afraid that they will be perceived as lazy, uninterested, or unmotivated if they fall asleep. “Sometimes they withdraw from social events because they don’t want to be perceived in such a way,” said Dr. Maski. She and her colleagues encourage patients to participate in selected after-school events and to engage in social activities they find meaningful to maintain social networks.
An unpublished study of more than 300 patients with narcolepsy examined the effect of the disorder on patients’ social lives. At the end of the day, many patients “crash and burn,” said Dr. Scammell. Consequently, they do not have as much energy for social activities.
This lack of energy affects patients’ social relationships. The study suggests that patients with narcolepsy do not have as many friends as the general population does. Nevertheless, the frequency of close relationships and marriage was similar between patients with narcolepsy and the general population. “What people are doing is putting their energy into these close relationships, rather than having lots of friends and socializing a lot,” said Dr. Scammell. “I found that heartening, that people were doing their best and developed those close relationships,” which are vitally important for many reasons, he added.
The study, which has been submitted for publication, also asked patients about their sex lives. Many patients reported having had cataplexy during sex, and others reported that their medications caused problems with their sex lives. “Their doctors never ask about these things, and many patients actually would like their doctor to ask about them more,” said Dr. Scammell.
In addition, narcolepsy significantly affects a patient’s ability to drive. Patients with narcolepsy have a three- to fourfold increased risk of car accidents, said Dr. Scammell. This increased risk likely results from patients’ EDS.
But as important as this issue is for patients’ lives, there is no consensus on how to counsel patients about driving, said Dr. Maski. “For instance, it is not really clear if there is value in doing a maintenance of wakefulness test before allowing patients with narcolepsy to drive,” she said. The test is not validated in children or adolescents, which raises questions about how to advise beginning drivers with narcolepsy. “It’s not really clear that passing your maintenance of wakefulness test increases your safety behind the wheel,” said Dr. Maski.
“It’s the rare person with narcolepsy who can easily and safely do a 2-hour drive by themselves,” said Dr. Scammell. Patients must determine what their own limits are, and it is important for clinicians to discuss reasonable limits honestly with their patients. “I almost never would push to have somebody’s license taken away,” said Dr. Scammell. “But there are patients who only can drive around town for short errands, and if it’s anything more than half an hour, they start getting drowsy.”
There is a need for a public awareness campaign about narcolepsy, Dr. Scammell added. Such a campaign was carried out in Italy several years ago, and it included cartoons and TV segments. “It got a lot of people’s attention, and there was a real spike in new and correct diagnoses of narcolepsy,” said Dr. Scammell. But such a broad campaign is expensive, while narcolepsy is rare, and it might not be feasible to reach out to the general population. “But I certainly think it’s worth targeting doctors who are likely to see patients with sleepiness: neurologists, psychiatrists and psychologists, and primary care doctors,” said Dr. Scammell.
NORD Rare Disease Centers of Excellence: A new network seeks to break down barriers in rare disease care
In November 2021, the National Organization for Rare Disorders (NORD) announced that it had designated 31 institutions across the United States as “NORD Rare Disease Centers of Excellence.” More than just a stamp of approval, the new NORD network aims to change the way rare diseases are diagnosed and treated, creating more efficient pathways for collaboration among physicians, while helping patients get better care closer to home.
To understand better how the nascent network can benefit patients and clinicians, Neurology Reviews/MDedge Neurology spoke with Ed Neilan, MD, PhD, NORD’s chief scientific and medical officer. Dr. Neilan, a pediatrician and geneticist, is a former president of the medical staff at Boston Children’s Hospital and also served as head of global medical affairs for rare neurology at Sanofi Genzyme.
How did NORD choose its 31 centers?
We were looking for places that had both broad capabilities and deep expertise, where it was reasonable to expect that a patient with almost any condition could go and, without too many missteps or delays, get the right diagnosis or the right treatment. We also sought sites that were educating the next generation of rare disease specialists across departments. The sites had to be involved in research, because that moves the field forward, and sometimes it’s the only way to get a really impactful treatment for the 95% of rare diseases that don’t have an FDA-approved treatment. NORD sent a letter inviting different centers to apply, along with an application that had 120 questions. Most of the questions sought information about what kinds of expertise or services were available on-site, so that patients don’t have to go elsewhere to get, let’s say, a brain MRI scan or to see an immunologist. We wanted each site to be a place where you could go for almost any problem, at any age, and expect that while you’re being seen, and receiving treatment, it can also contribute to the education of the next generation of rare disease specialists and to research.
Several of the members of the network comprise more than one institution: They’re a children’s hospital combined with another facility.
Children’s hospitals, which are highly specialized and able to care for rare things in children, couldn’t apply by themselves. They had to apply in partnership with a center that could provide adult care as patients got older; otherwise, their care model would be incomplete. We’ve had some small victories already just by asking these questions and outlining this sort of approach. At one institution in the Great Plains, the director told us that he had been trying for years to get permission to hire someone who could make appointments across three different hospitals – a children’s hospital and two adult hospitals. He’d wanted to ensure that patients with rare and genetic diseases were seen in the appropriate places, and thanks to the NORD designation, he finally can. Now, regardless of age, the same office staff can handle the arrangements, and the patient will be scheduled in the right place.
You make clear that these are different from disease-specific centers of excellence – you specifically chose the 31 centers for their breadth of expertise. There’s no way to represent all 7,000 rare diseases equally, and disease-specific centers of excellence, which already exist for hemophilia, muscular dystrophy, cystic fibrosis, and some other conditions, have a very important role. We’re not aiming to compete with any other existing resources. What we are seeking to do is to fill the unmet need of, “What if there are no such designations for the disease that you’re concerned about?” Our goal was to find places that could help with unanswered questions, whether diagnostic questions or treatment questions. To identify places where a patient could reasonably expect to go and have a deeper dive – maybe an interdisciplinary deep dive.
The delay to diagnosis can be years in rare diseases. How can the network help speed up diagnoses?
With all these experts on different diseases, we hope to develop some better diagnostic algorithms within the network. Another thing we can do is to share resources. With 31 sites, everybody’s seeing patients with unknown diagnoses. Everyone is seeing patients for whom they would maybe like to get a whole genome done, or a whole exome done, but they are often encountering stiff resistance from insurance companies.
Meanwhile some sites, but not all 31, have multimillion-dollar grants to do sequencing and other kinds of advanced diagnostic tests to solve unknown cases. And there are people at those sites who say, “We need more samples. Can you get us samples from the other sites?”
One of the main things we aim to do is share information, including information about available diagnostic resources. We want all 31 sites to know which sites have funding and programs that enable them to study samples for other sites. We also want to know what criteria they’re putting on it. Someone might say: “I’ve got a grant to sequence genomes for people with unexplained seizures. Send me all your unexplained seizures.” Somebody else might have a grant for unexplained GI diseases. So, we want to put on our intranet a resource for the 31 sites, kind of a cookbook for – when if you can’t get it paid for by insurance, but you really think you need a particular special test – who might be able to do it for you within the network.
This would seem to benefit research across sites as well.
Yes, but we also want to share clinical advice and expertise for direct patient benefit. So, it doesn’t always have to fulfill the goals of a specific research project. For example, we might be able to create an undiagnosed patient quality improvement database across all 31 sites that could compliantly let Drs. X and Y know that they’re each seeing a patient with the same rare thing.
But let’s say you want to move the field forward by discovering a new disease. Rare genetic diseases are now being discovered at the rate of about 250 a year, so about 5 per week across the world. With two or three unrelated patients who have the same disease and a whole exome sequence, you can potentially discover a disease. Maybe you’ve found one unique patient with a genetic variant of possible significance, but you can’t be 100% sure, and you may not be able to convince your colleagues, or journal editors, until you find other cases. You need those two or three ultrarare patients. Within this network, a lot of sites want to share information about their ultrarare patients and be able to put together additional instances of the same thing, to prove that it is a real disease, to learn more about it and how to diagnose, manage, and treat it.
Part of the idea with a nationwide network is that patients aren’t going to have to move around among these centers of excellence, is that correct? They’re going to be seen at the closest ones, and it’s the expertise that is mobile.
Yes, that’s right. While we can’t eliminate the need for travel, what we are trying to do is increase the sharing of expertise, to improve results for patients while limiting the need for traveling very long distances. As a geneticist I’ve been on both the requesting and the receiving end of consultations with doctors at other sites, sometimes very far away, especially for ultrarare conditions for which any one physician’s experience is limited. We all try to honor these sorts of requests, but insurance doesn’t reimburse it and so hospitals don’t give doctors much credit for it.
We want to ultimately find ways to incentivize this type of collaboration. Hopefully we can get agreements with insurance companies to allow intersite consultations within our network, recognizing that they don’t want to pay for the patient to be seen out of state, but you also want the patient to get the best possible medical advice. This might require legislative changes in the long run. But what we can do more readily is create a culture within this network of mutual consultation and sharing of clinical experience. Outside of such a network, the idea of “cold calling” somebody, whom you may never have met, and asking them for help and free advice is a little bit of a bar, right? We want to lower that bar.
Can patients get telemedicine consults with physicians across the network?
NORD supports having telemedicine options for everybody regardless of diagnosis, rare or not, and we support legislation that would continue access and reimbursement for telemedicine post pandemic. I hope we can get that, or at least preserve telemedicine for rare diseases, for which there are often not enough, or sometimes not any, expert providers in the same state. Ultimately, we want patients to be able to get the expert assessments and advice they need. For rare diseases, that sometimes means battling back and forth with an insurance provider, seeking permission to see an expert clinician a thousand miles away. By sharing medical expertise, and through telemedicine when that’s allowed, we hope to reduce the need for that. But the telemedicine environment is still evolving and somewhat uncertain.
How will the network’s physician collaborations take place?
One of the important things NORD is providing to the network is an information technology setup and intranet across the 31 sites. That intranet is where center staff will go to access the network’s internal resources, including live and recorded case conferences. In those case conferences you can present a case you haven’t been able to solve. Experts you may have only heard of by reputation will now be streamed to your computer as part of the nationwide network. It benefits the patient because you get additional expert opinions, but it also benefits the physicians because we have this collegial space for discussion and learning. We’ll be linked by frequent meetings – some in person, most virtual – a common culture, and a common intranet.
On the intranet, we will also have a growing set of useful databases, links, and documents that are available to all members. These will be progressively updated with help from experts at the centers, so that clinicians can more directly learn from each other, instead of separately reinventing the wheel. The way things usually work, when you see a patient with an ultrarare condition that you’re not that familiar with, is that you tell them what little you can, then schedule them to come back in a few weeks. In the meantime, usually in your off time, you spend hours searching PubMed and other sources and you try to piece things together, to figure out what’s known that might help your patient. But imagine that this has already been figured out by someone else in the network. You can see on the network a list of articles the other expert read and found helpful in addressing this problem. And you then reach out directly to that other expert.
In recent months you’ve had one-on-one meetings with all 31 directors at the sites, and after that you convened 11 working groups. What are you trying to achieve?
Once the sites were chosen, we aimed to talk quickly and honestly about what everyone needed, what everyone saw as the biggest problems to tackle in rare diseases. Two things were very rewarding about those phone calls: one, all the centers were very enthusiastic, and two, they pretty much all agreed on what the key unmet needs are for rare disease patients and the practitioners trying to help them. So, we empaneled working groups of expert volunteers enthusiastic to work on each of those problems. These groups collectively comprise more than 200 volunteers – faculty, staff, and trainees – from the different sites nationwide. Each group is working on a key unmet need in rare diseases, and each group will be given its own space on our file-sharing platform, where they can share information and co-develop new ideas and documents. When something they produce is good enough to start to be a practice resource, such as a draft treatment guideline that the working group now wants to try in the real world, but it’s not yet ready to be published, they can share it and have it tested by all 31 sites through the dedicated intranet we are building for the network.
Jennie Smith is a freelance journalist specializing in medicine and health.
In November 2021, the National Organization for Rare Disorders (NORD) announced that it had designated 31 institutions across the United States as “NORD Rare Disease Centers of Excellence.” More than just a stamp of approval, the new NORD network aims to change the way rare diseases are diagnosed and treated, creating more efficient pathways for collaboration among physicians, while helping patients get better care closer to home.
To understand better how the nascent network can benefit patients and clinicians, Neurology Reviews/MDedge Neurology spoke with Ed Neilan, MD, PhD, NORD’s chief scientific and medical officer. Dr. Neilan, a pediatrician and geneticist, is a former president of the medical staff at Boston Children’s Hospital and also served as head of global medical affairs for rare neurology at Sanofi Genzyme.
How did NORD choose its 31 centers?
We were looking for places that had both broad capabilities and deep expertise, where it was reasonable to expect that a patient with almost any condition could go and, without too many missteps or delays, get the right diagnosis or the right treatment. We also sought sites that were educating the next generation of rare disease specialists across departments. The sites had to be involved in research, because that moves the field forward, and sometimes it’s the only way to get a really impactful treatment for the 95% of rare diseases that don’t have an FDA-approved treatment. NORD sent a letter inviting different centers to apply, along with an application that had 120 questions. Most of the questions sought information about what kinds of expertise or services were available on-site, so that patients don’t have to go elsewhere to get, let’s say, a brain MRI scan or to see an immunologist. We wanted each site to be a place where you could go for almost any problem, at any age, and expect that while you’re being seen, and receiving treatment, it can also contribute to the education of the next generation of rare disease specialists and to research.
Several of the members of the network comprise more than one institution: They’re a children’s hospital combined with another facility.
Children’s hospitals, which are highly specialized and able to care for rare things in children, couldn’t apply by themselves. They had to apply in partnership with a center that could provide adult care as patients got older; otherwise, their care model would be incomplete. We’ve had some small victories already just by asking these questions and outlining this sort of approach. At one institution in the Great Plains, the director told us that he had been trying for years to get permission to hire someone who could make appointments across three different hospitals – a children’s hospital and two adult hospitals. He’d wanted to ensure that patients with rare and genetic diseases were seen in the appropriate places, and thanks to the NORD designation, he finally can. Now, regardless of age, the same office staff can handle the arrangements, and the patient will be scheduled in the right place.
You make clear that these are different from disease-specific centers of excellence – you specifically chose the 31 centers for their breadth of expertise. There’s no way to represent all 7,000 rare diseases equally, and disease-specific centers of excellence, which already exist for hemophilia, muscular dystrophy, cystic fibrosis, and some other conditions, have a very important role. We’re not aiming to compete with any other existing resources. What we are seeking to do is to fill the unmet need of, “What if there are no such designations for the disease that you’re concerned about?” Our goal was to find places that could help with unanswered questions, whether diagnostic questions or treatment questions. To identify places where a patient could reasonably expect to go and have a deeper dive – maybe an interdisciplinary deep dive.
The delay to diagnosis can be years in rare diseases. How can the network help speed up diagnoses?
With all these experts on different diseases, we hope to develop some better diagnostic algorithms within the network. Another thing we can do is to share resources. With 31 sites, everybody’s seeing patients with unknown diagnoses. Everyone is seeing patients for whom they would maybe like to get a whole genome done, or a whole exome done, but they are often encountering stiff resistance from insurance companies.
Meanwhile some sites, but not all 31, have multimillion-dollar grants to do sequencing and other kinds of advanced diagnostic tests to solve unknown cases. And there are people at those sites who say, “We need more samples. Can you get us samples from the other sites?”
One of the main things we aim to do is share information, including information about available diagnostic resources. We want all 31 sites to know which sites have funding and programs that enable them to study samples for other sites. We also want to know what criteria they’re putting on it. Someone might say: “I’ve got a grant to sequence genomes for people with unexplained seizures. Send me all your unexplained seizures.” Somebody else might have a grant for unexplained GI diseases. So, we want to put on our intranet a resource for the 31 sites, kind of a cookbook for – when if you can’t get it paid for by insurance, but you really think you need a particular special test – who might be able to do it for you within the network.
This would seem to benefit research across sites as well.
Yes, but we also want to share clinical advice and expertise for direct patient benefit. So, it doesn’t always have to fulfill the goals of a specific research project. For example, we might be able to create an undiagnosed patient quality improvement database across all 31 sites that could compliantly let Drs. X and Y know that they’re each seeing a patient with the same rare thing.
But let’s say you want to move the field forward by discovering a new disease. Rare genetic diseases are now being discovered at the rate of about 250 a year, so about 5 per week across the world. With two or three unrelated patients who have the same disease and a whole exome sequence, you can potentially discover a disease. Maybe you’ve found one unique patient with a genetic variant of possible significance, but you can’t be 100% sure, and you may not be able to convince your colleagues, or journal editors, until you find other cases. You need those two or three ultrarare patients. Within this network, a lot of sites want to share information about their ultrarare patients and be able to put together additional instances of the same thing, to prove that it is a real disease, to learn more about it and how to diagnose, manage, and treat it.
Part of the idea with a nationwide network is that patients aren’t going to have to move around among these centers of excellence, is that correct? They’re going to be seen at the closest ones, and it’s the expertise that is mobile.
Yes, that’s right. While we can’t eliminate the need for travel, what we are trying to do is increase the sharing of expertise, to improve results for patients while limiting the need for traveling very long distances. As a geneticist I’ve been on both the requesting and the receiving end of consultations with doctors at other sites, sometimes very far away, especially for ultrarare conditions for which any one physician’s experience is limited. We all try to honor these sorts of requests, but insurance doesn’t reimburse it and so hospitals don’t give doctors much credit for it.
We want to ultimately find ways to incentivize this type of collaboration. Hopefully we can get agreements with insurance companies to allow intersite consultations within our network, recognizing that they don’t want to pay for the patient to be seen out of state, but you also want the patient to get the best possible medical advice. This might require legislative changes in the long run. But what we can do more readily is create a culture within this network of mutual consultation and sharing of clinical experience. Outside of such a network, the idea of “cold calling” somebody, whom you may never have met, and asking them for help and free advice is a little bit of a bar, right? We want to lower that bar.
Can patients get telemedicine consults with physicians across the network?
NORD supports having telemedicine options for everybody regardless of diagnosis, rare or not, and we support legislation that would continue access and reimbursement for telemedicine post pandemic. I hope we can get that, or at least preserve telemedicine for rare diseases, for which there are often not enough, or sometimes not any, expert providers in the same state. Ultimately, we want patients to be able to get the expert assessments and advice they need. For rare diseases, that sometimes means battling back and forth with an insurance provider, seeking permission to see an expert clinician a thousand miles away. By sharing medical expertise, and through telemedicine when that’s allowed, we hope to reduce the need for that. But the telemedicine environment is still evolving and somewhat uncertain.
How will the network’s physician collaborations take place?
One of the important things NORD is providing to the network is an information technology setup and intranet across the 31 sites. That intranet is where center staff will go to access the network’s internal resources, including live and recorded case conferences. In those case conferences you can present a case you haven’t been able to solve. Experts you may have only heard of by reputation will now be streamed to your computer as part of the nationwide network. It benefits the patient because you get additional expert opinions, but it also benefits the physicians because we have this collegial space for discussion and learning. We’ll be linked by frequent meetings – some in person, most virtual – a common culture, and a common intranet.
On the intranet, we will also have a growing set of useful databases, links, and documents that are available to all members. These will be progressively updated with help from experts at the centers, so that clinicians can more directly learn from each other, instead of separately reinventing the wheel. The way things usually work, when you see a patient with an ultrarare condition that you’re not that familiar with, is that you tell them what little you can, then schedule them to come back in a few weeks. In the meantime, usually in your off time, you spend hours searching PubMed and other sources and you try to piece things together, to figure out what’s known that might help your patient. But imagine that this has already been figured out by someone else in the network. You can see on the network a list of articles the other expert read and found helpful in addressing this problem. And you then reach out directly to that other expert.
In recent months you’ve had one-on-one meetings with all 31 directors at the sites, and after that you convened 11 working groups. What are you trying to achieve?
Once the sites were chosen, we aimed to talk quickly and honestly about what everyone needed, what everyone saw as the biggest problems to tackle in rare diseases. Two things were very rewarding about those phone calls: one, all the centers were very enthusiastic, and two, they pretty much all agreed on what the key unmet needs are for rare disease patients and the practitioners trying to help them. So, we empaneled working groups of expert volunteers enthusiastic to work on each of those problems. These groups collectively comprise more than 200 volunteers – faculty, staff, and trainees – from the different sites nationwide. Each group is working on a key unmet need in rare diseases, and each group will be given its own space on our file-sharing platform, where they can share information and co-develop new ideas and documents. When something they produce is good enough to start to be a practice resource, such as a draft treatment guideline that the working group now wants to try in the real world, but it’s not yet ready to be published, they can share it and have it tested by all 31 sites through the dedicated intranet we are building for the network.
Jennie Smith is a freelance journalist specializing in medicine and health.
In November 2021, the National Organization for Rare Disorders (NORD) announced that it had designated 31 institutions across the United States as “NORD Rare Disease Centers of Excellence.” More than just a stamp of approval, the new NORD network aims to change the way rare diseases are diagnosed and treated, creating more efficient pathways for collaboration among physicians, while helping patients get better care closer to home.
To understand better how the nascent network can benefit patients and clinicians, Neurology Reviews/MDedge Neurology spoke with Ed Neilan, MD, PhD, NORD’s chief scientific and medical officer. Dr. Neilan, a pediatrician and geneticist, is a former president of the medical staff at Boston Children’s Hospital and also served as head of global medical affairs for rare neurology at Sanofi Genzyme.
How did NORD choose its 31 centers?
We were looking for places that had both broad capabilities and deep expertise, where it was reasonable to expect that a patient with almost any condition could go and, without too many missteps or delays, get the right diagnosis or the right treatment. We also sought sites that were educating the next generation of rare disease specialists across departments. The sites had to be involved in research, because that moves the field forward, and sometimes it’s the only way to get a really impactful treatment for the 95% of rare diseases that don’t have an FDA-approved treatment. NORD sent a letter inviting different centers to apply, along with an application that had 120 questions. Most of the questions sought information about what kinds of expertise or services were available on-site, so that patients don’t have to go elsewhere to get, let’s say, a brain MRI scan or to see an immunologist. We wanted each site to be a place where you could go for almost any problem, at any age, and expect that while you’re being seen, and receiving treatment, it can also contribute to the education of the next generation of rare disease specialists and to research.
Several of the members of the network comprise more than one institution: They’re a children’s hospital combined with another facility.
Children’s hospitals, which are highly specialized and able to care for rare things in children, couldn’t apply by themselves. They had to apply in partnership with a center that could provide adult care as patients got older; otherwise, their care model would be incomplete. We’ve had some small victories already just by asking these questions and outlining this sort of approach. At one institution in the Great Plains, the director told us that he had been trying for years to get permission to hire someone who could make appointments across three different hospitals – a children’s hospital and two adult hospitals. He’d wanted to ensure that patients with rare and genetic diseases were seen in the appropriate places, and thanks to the NORD designation, he finally can. Now, regardless of age, the same office staff can handle the arrangements, and the patient will be scheduled in the right place.
You make clear that these are different from disease-specific centers of excellence – you specifically chose the 31 centers for their breadth of expertise. There’s no way to represent all 7,000 rare diseases equally, and disease-specific centers of excellence, which already exist for hemophilia, muscular dystrophy, cystic fibrosis, and some other conditions, have a very important role. We’re not aiming to compete with any other existing resources. What we are seeking to do is to fill the unmet need of, “What if there are no such designations for the disease that you’re concerned about?” Our goal was to find places that could help with unanswered questions, whether diagnostic questions or treatment questions. To identify places where a patient could reasonably expect to go and have a deeper dive – maybe an interdisciplinary deep dive.
The delay to diagnosis can be years in rare diseases. How can the network help speed up diagnoses?
With all these experts on different diseases, we hope to develop some better diagnostic algorithms within the network. Another thing we can do is to share resources. With 31 sites, everybody’s seeing patients with unknown diagnoses. Everyone is seeing patients for whom they would maybe like to get a whole genome done, or a whole exome done, but they are often encountering stiff resistance from insurance companies.
Meanwhile some sites, but not all 31, have multimillion-dollar grants to do sequencing and other kinds of advanced diagnostic tests to solve unknown cases. And there are people at those sites who say, “We need more samples. Can you get us samples from the other sites?”
One of the main things we aim to do is share information, including information about available diagnostic resources. We want all 31 sites to know which sites have funding and programs that enable them to study samples for other sites. We also want to know what criteria they’re putting on it. Someone might say: “I’ve got a grant to sequence genomes for people with unexplained seizures. Send me all your unexplained seizures.” Somebody else might have a grant for unexplained GI diseases. So, we want to put on our intranet a resource for the 31 sites, kind of a cookbook for – when if you can’t get it paid for by insurance, but you really think you need a particular special test – who might be able to do it for you within the network.
This would seem to benefit research across sites as well.
Yes, but we also want to share clinical advice and expertise for direct patient benefit. So, it doesn’t always have to fulfill the goals of a specific research project. For example, we might be able to create an undiagnosed patient quality improvement database across all 31 sites that could compliantly let Drs. X and Y know that they’re each seeing a patient with the same rare thing.
But let’s say you want to move the field forward by discovering a new disease. Rare genetic diseases are now being discovered at the rate of about 250 a year, so about 5 per week across the world. With two or three unrelated patients who have the same disease and a whole exome sequence, you can potentially discover a disease. Maybe you’ve found one unique patient with a genetic variant of possible significance, but you can’t be 100% sure, and you may not be able to convince your colleagues, or journal editors, until you find other cases. You need those two or three ultrarare patients. Within this network, a lot of sites want to share information about their ultrarare patients and be able to put together additional instances of the same thing, to prove that it is a real disease, to learn more about it and how to diagnose, manage, and treat it.
Part of the idea with a nationwide network is that patients aren’t going to have to move around among these centers of excellence, is that correct? They’re going to be seen at the closest ones, and it’s the expertise that is mobile.
Yes, that’s right. While we can’t eliminate the need for travel, what we are trying to do is increase the sharing of expertise, to improve results for patients while limiting the need for traveling very long distances. As a geneticist I’ve been on both the requesting and the receiving end of consultations with doctors at other sites, sometimes very far away, especially for ultrarare conditions for which any one physician’s experience is limited. We all try to honor these sorts of requests, but insurance doesn’t reimburse it and so hospitals don’t give doctors much credit for it.
We want to ultimately find ways to incentivize this type of collaboration. Hopefully we can get agreements with insurance companies to allow intersite consultations within our network, recognizing that they don’t want to pay for the patient to be seen out of state, but you also want the patient to get the best possible medical advice. This might require legislative changes in the long run. But what we can do more readily is create a culture within this network of mutual consultation and sharing of clinical experience. Outside of such a network, the idea of “cold calling” somebody, whom you may never have met, and asking them for help and free advice is a little bit of a bar, right? We want to lower that bar.
Can patients get telemedicine consults with physicians across the network?
NORD supports having telemedicine options for everybody regardless of diagnosis, rare or not, and we support legislation that would continue access and reimbursement for telemedicine post pandemic. I hope we can get that, or at least preserve telemedicine for rare diseases, for which there are often not enough, or sometimes not any, expert providers in the same state. Ultimately, we want patients to be able to get the expert assessments and advice they need. For rare diseases, that sometimes means battling back and forth with an insurance provider, seeking permission to see an expert clinician a thousand miles away. By sharing medical expertise, and through telemedicine when that’s allowed, we hope to reduce the need for that. But the telemedicine environment is still evolving and somewhat uncertain.
How will the network’s physician collaborations take place?
One of the important things NORD is providing to the network is an information technology setup and intranet across the 31 sites. That intranet is where center staff will go to access the network’s internal resources, including live and recorded case conferences. In those case conferences you can present a case you haven’t been able to solve. Experts you may have only heard of by reputation will now be streamed to your computer as part of the nationwide network. It benefits the patient because you get additional expert opinions, but it also benefits the physicians because we have this collegial space for discussion and learning. We’ll be linked by frequent meetings – some in person, most virtual – a common culture, and a common intranet.
On the intranet, we will also have a growing set of useful databases, links, and documents that are available to all members. These will be progressively updated with help from experts at the centers, so that clinicians can more directly learn from each other, instead of separately reinventing the wheel. The way things usually work, when you see a patient with an ultrarare condition that you’re not that familiar with, is that you tell them what little you can, then schedule them to come back in a few weeks. In the meantime, usually in your off time, you spend hours searching PubMed and other sources and you try to piece things together, to figure out what’s known that might help your patient. But imagine that this has already been figured out by someone else in the network. You can see on the network a list of articles the other expert read and found helpful in addressing this problem. And you then reach out directly to that other expert.
In recent months you’ve had one-on-one meetings with all 31 directors at the sites, and after that you convened 11 working groups. What are you trying to achieve?
Once the sites were chosen, we aimed to talk quickly and honestly about what everyone needed, what everyone saw as the biggest problems to tackle in rare diseases. Two things were very rewarding about those phone calls: one, all the centers were very enthusiastic, and two, they pretty much all agreed on what the key unmet needs are for rare disease patients and the practitioners trying to help them. So, we empaneled working groups of expert volunteers enthusiastic to work on each of those problems. These groups collectively comprise more than 200 volunteers – faculty, staff, and trainees – from the different sites nationwide. Each group is working on a key unmet need in rare diseases, and each group will be given its own space on our file-sharing platform, where they can share information and co-develop new ideas and documents. When something they produce is good enough to start to be a practice resource, such as a draft treatment guideline that the working group now wants to try in the real world, but it’s not yet ready to be published, they can share it and have it tested by all 31 sites through the dedicated intranet we are building for the network.
Jennie Smith is a freelance journalist specializing in medicine and health.