User login
Vinorelbine survival benefit in mesothelioma overshadowed by advances in immuno-oncology
After decades of off-label use, vinorelbine finally has a randomized clinical trial supporting its efficacy as a second-line treatment of mesothelioma, an investigator reported at the annual meeting of the American Society of Clinical Oncology.
However, the development of other treatment regimens, and notably immuno-oncology approaches, are pushing this classic chemotherapy option to later lines of therapy in patients with malignant pleural mesothelioma (MPM), a speaker said at the meeting.
Adding vinorelbine to active symptom control statistically improved progression-free survival (PFS), among patients with MPM who had prior platinum-based therapy, according to results of the randomized Vinorelbine in Mesothelioma (VIM) trial.
Median PFS reached 4.2 months in the vinorelbine arm, versus 2.8 months for active symptom control alone, study results show.
That finding , coupled with safety results, supports the off-label use of vinorelbine as a treatment option for patients with relapsed MPM, according to investigator Dean Anthony Fennell, FRCP, PhD, of the University of Leicester (England).
“Vinorelbine appears to be a safe and effective treatment and could be considered as a treatment option for patients with relapsed mesothelioma,” Prof. Fennell said in his presentation at ASCO (Abstract 8507).
Changing status
While that welcome pronouncement was a long time coming, there are now other promising treatment approaches that relegate vinorelbine to a “lower priority” in the treatment algorithm, said discussant Anna K. Nowak, MBBS, FRACP, PhD, of the University of Western Australia, Nedlands.
In October 2020, the U.S. Food and Drug Administration approved the combination of ipilimumab and nivolumab for the first-line treatment of unresectable malignant pleural mesothelioma, on the basis of results from CHECKMATE-743, a randomized, open-label trial.
“Certainly, we know now that first-line ipilimumab and nivolumab is of very substantial benefit to these patients, and we still have clinical trials open in this space as well,” Prof. Nowak said in her discussion at ASCO.
There is “no doubt” that many patients with mesothelioma should at some point receive an IO agent, particularly now with recently reported randomized clinical trial evidence of an overall survival benefit, she added.
“This really pushes vinorelbine out to be a third- or fourth-line treatment,” she added, “and we know that there are usually diminishing returns from using chemotherapies further down the treatment algorithm.”
Trial details
The VIM trial described at ASCO by Prof. Fennell was a randomized, controlled phase 2 including 154 patients with MPM that had progressed after first-line chemotherapy.
“Vinorelbine has shown useful clinical activity in single-arm phase two studies, however, the specific efficacy of vinorelbine has not been evaluated in an appropriately controlled randomized trial,” Prof. Fennell said in this presentation.
Patients in the trial were randomized 2:1 to either vinorelbine plus active supportive care or active supportive care alone. Vinorelbine was given initially at 60 mg/m2 weekly every 21 days, escalated to 80 mg/m2 from cycle 2.
The median age of patients in VIM was approximately 71 years, and about 80% were male.
More partial responses were seen in the vinorelbine arm, at 3.1% of patients, compared with 1.8% for active supportive care, according to Prof. Fennell. Likewise, the rate of stable disease was higher in the vinorelbine arm, at 62.2%, versus 46.4% in the control arm.
The primary outcome of the study, PFS, was significantly improved in the vinorelbine arm, according to Dr. Fennell. The median PFS was 4.2 months in the vinorelbine arm and 2.8 months in the supportive care arm (P = .002), translating into a hazard ratio of 0.60 (95% confidence interval, 0.41-0.86), Prof. Fennell reported.
The most common grade 3-4 adverse event was neutropenia, occurring in 12.5% of the vinorelbine-treated patients and no patients managed with supportive care alone, according to the report. Other grade 3-4 adverse events occurred in fewer than 10% of patients and included dyspnea, lower respiratory infection, lymphopenia, and fatigue, among others.
Overall survival (OS) was not statistically different between vinorelbine and supportive care arms, with median OS of 9.3 months and 9.1 months, respectively.
However, a number of patients in the control arm went on to receive subsequent therapy, including 15 (or about 27%) who went into CONFIRM, a randomized phase 3 trial that, as recently reported, met its coprimary endpoints of improve OS and PFS with nivolumab vs. placebo in relapsed malignant mesothelioma.
Investigators also sought to test the hypothesis that BRCA1-negative patients might be chemoresistant, based in part on preclinical models demonstrating that BRCA1 predicted sensitivity to vinorelbine. However, there was no difference in PFS by BRCA1 expression in the VIM study, according to Prof. Fennell.
Taken together, findings of the VIM trial suggest vinorelbine is a “modestly active” agent with low cost and acceptable toxicity, according to Prof. Nowak.
“It is incumbent on us to have clear discussions with our patients on the risks and benefits of trying this as a subsequent-line therapy, in the context of this evidence that was generated as a second-line therapy,” she said in her discussant remarks on the study.
“I would say that it is a lower priority in our algorithm than cisplatin and pemetrexed, or of course, immuno-oncology agents,” she added.
Dr. Fennell reported disclosures related to AstraZeneca, Astex Therapeutics, Bayer, and multiple other pharmaceutical companies. Dr. Nowak reported disclosures with AstraZeneca, Atara Biotherapeutics, Boehringer Ingelheim, and multiple other pharmaceutical companies.
After decades of off-label use, vinorelbine finally has a randomized clinical trial supporting its efficacy as a second-line treatment of mesothelioma, an investigator reported at the annual meeting of the American Society of Clinical Oncology.
However, the development of other treatment regimens, and notably immuno-oncology approaches, are pushing this classic chemotherapy option to later lines of therapy in patients with malignant pleural mesothelioma (MPM), a speaker said at the meeting.
Adding vinorelbine to active symptom control statistically improved progression-free survival (PFS), among patients with MPM who had prior platinum-based therapy, according to results of the randomized Vinorelbine in Mesothelioma (VIM) trial.
Median PFS reached 4.2 months in the vinorelbine arm, versus 2.8 months for active symptom control alone, study results show.
That finding , coupled with safety results, supports the off-label use of vinorelbine as a treatment option for patients with relapsed MPM, according to investigator Dean Anthony Fennell, FRCP, PhD, of the University of Leicester (England).
“Vinorelbine appears to be a safe and effective treatment and could be considered as a treatment option for patients with relapsed mesothelioma,” Prof. Fennell said in his presentation at ASCO (Abstract 8507).
Changing status
While that welcome pronouncement was a long time coming, there are now other promising treatment approaches that relegate vinorelbine to a “lower priority” in the treatment algorithm, said discussant Anna K. Nowak, MBBS, FRACP, PhD, of the University of Western Australia, Nedlands.
In October 2020, the U.S. Food and Drug Administration approved the combination of ipilimumab and nivolumab for the first-line treatment of unresectable malignant pleural mesothelioma, on the basis of results from CHECKMATE-743, a randomized, open-label trial.
“Certainly, we know now that first-line ipilimumab and nivolumab is of very substantial benefit to these patients, and we still have clinical trials open in this space as well,” Prof. Nowak said in her discussion at ASCO.
There is “no doubt” that many patients with mesothelioma should at some point receive an IO agent, particularly now with recently reported randomized clinical trial evidence of an overall survival benefit, she added.
“This really pushes vinorelbine out to be a third- or fourth-line treatment,” she added, “and we know that there are usually diminishing returns from using chemotherapies further down the treatment algorithm.”
Trial details
The VIM trial described at ASCO by Prof. Fennell was a randomized, controlled phase 2 including 154 patients with MPM that had progressed after first-line chemotherapy.
“Vinorelbine has shown useful clinical activity in single-arm phase two studies, however, the specific efficacy of vinorelbine has not been evaluated in an appropriately controlled randomized trial,” Prof. Fennell said in this presentation.
Patients in the trial were randomized 2:1 to either vinorelbine plus active supportive care or active supportive care alone. Vinorelbine was given initially at 60 mg/m2 weekly every 21 days, escalated to 80 mg/m2 from cycle 2.
The median age of patients in VIM was approximately 71 years, and about 80% were male.
More partial responses were seen in the vinorelbine arm, at 3.1% of patients, compared with 1.8% for active supportive care, according to Prof. Fennell. Likewise, the rate of stable disease was higher in the vinorelbine arm, at 62.2%, versus 46.4% in the control arm.
The primary outcome of the study, PFS, was significantly improved in the vinorelbine arm, according to Dr. Fennell. The median PFS was 4.2 months in the vinorelbine arm and 2.8 months in the supportive care arm (P = .002), translating into a hazard ratio of 0.60 (95% confidence interval, 0.41-0.86), Prof. Fennell reported.
The most common grade 3-4 adverse event was neutropenia, occurring in 12.5% of the vinorelbine-treated patients and no patients managed with supportive care alone, according to the report. Other grade 3-4 adverse events occurred in fewer than 10% of patients and included dyspnea, lower respiratory infection, lymphopenia, and fatigue, among others.
Overall survival (OS) was not statistically different between vinorelbine and supportive care arms, with median OS of 9.3 months and 9.1 months, respectively.
However, a number of patients in the control arm went on to receive subsequent therapy, including 15 (or about 27%) who went into CONFIRM, a randomized phase 3 trial that, as recently reported, met its coprimary endpoints of improve OS and PFS with nivolumab vs. placebo in relapsed malignant mesothelioma.
Investigators also sought to test the hypothesis that BRCA1-negative patients might be chemoresistant, based in part on preclinical models demonstrating that BRCA1 predicted sensitivity to vinorelbine. However, there was no difference in PFS by BRCA1 expression in the VIM study, according to Prof. Fennell.
Taken together, findings of the VIM trial suggest vinorelbine is a “modestly active” agent with low cost and acceptable toxicity, according to Prof. Nowak.
“It is incumbent on us to have clear discussions with our patients on the risks and benefits of trying this as a subsequent-line therapy, in the context of this evidence that was generated as a second-line therapy,” she said in her discussant remarks on the study.
“I would say that it is a lower priority in our algorithm than cisplatin and pemetrexed, or of course, immuno-oncology agents,” she added.
Dr. Fennell reported disclosures related to AstraZeneca, Astex Therapeutics, Bayer, and multiple other pharmaceutical companies. Dr. Nowak reported disclosures with AstraZeneca, Atara Biotherapeutics, Boehringer Ingelheim, and multiple other pharmaceutical companies.
After decades of off-label use, vinorelbine finally has a randomized clinical trial supporting its efficacy as a second-line treatment of mesothelioma, an investigator reported at the annual meeting of the American Society of Clinical Oncology.
However, the development of other treatment regimens, and notably immuno-oncology approaches, are pushing this classic chemotherapy option to later lines of therapy in patients with malignant pleural mesothelioma (MPM), a speaker said at the meeting.
Adding vinorelbine to active symptom control statistically improved progression-free survival (PFS), among patients with MPM who had prior platinum-based therapy, according to results of the randomized Vinorelbine in Mesothelioma (VIM) trial.
Median PFS reached 4.2 months in the vinorelbine arm, versus 2.8 months for active symptom control alone, study results show.
That finding , coupled with safety results, supports the off-label use of vinorelbine as a treatment option for patients with relapsed MPM, according to investigator Dean Anthony Fennell, FRCP, PhD, of the University of Leicester (England).
“Vinorelbine appears to be a safe and effective treatment and could be considered as a treatment option for patients with relapsed mesothelioma,” Prof. Fennell said in his presentation at ASCO (Abstract 8507).
Changing status
While that welcome pronouncement was a long time coming, there are now other promising treatment approaches that relegate vinorelbine to a “lower priority” in the treatment algorithm, said discussant Anna K. Nowak, MBBS, FRACP, PhD, of the University of Western Australia, Nedlands.
In October 2020, the U.S. Food and Drug Administration approved the combination of ipilimumab and nivolumab for the first-line treatment of unresectable malignant pleural mesothelioma, on the basis of results from CHECKMATE-743, a randomized, open-label trial.
“Certainly, we know now that first-line ipilimumab and nivolumab is of very substantial benefit to these patients, and we still have clinical trials open in this space as well,” Prof. Nowak said in her discussion at ASCO.
There is “no doubt” that many patients with mesothelioma should at some point receive an IO agent, particularly now with recently reported randomized clinical trial evidence of an overall survival benefit, she added.
“This really pushes vinorelbine out to be a third- or fourth-line treatment,” she added, “and we know that there are usually diminishing returns from using chemotherapies further down the treatment algorithm.”
Trial details
The VIM trial described at ASCO by Prof. Fennell was a randomized, controlled phase 2 including 154 patients with MPM that had progressed after first-line chemotherapy.
“Vinorelbine has shown useful clinical activity in single-arm phase two studies, however, the specific efficacy of vinorelbine has not been evaluated in an appropriately controlled randomized trial,” Prof. Fennell said in this presentation.
Patients in the trial were randomized 2:1 to either vinorelbine plus active supportive care or active supportive care alone. Vinorelbine was given initially at 60 mg/m2 weekly every 21 days, escalated to 80 mg/m2 from cycle 2.
The median age of patients in VIM was approximately 71 years, and about 80% were male.
More partial responses were seen in the vinorelbine arm, at 3.1% of patients, compared with 1.8% for active supportive care, according to Prof. Fennell. Likewise, the rate of stable disease was higher in the vinorelbine arm, at 62.2%, versus 46.4% in the control arm.
The primary outcome of the study, PFS, was significantly improved in the vinorelbine arm, according to Dr. Fennell. The median PFS was 4.2 months in the vinorelbine arm and 2.8 months in the supportive care arm (P = .002), translating into a hazard ratio of 0.60 (95% confidence interval, 0.41-0.86), Prof. Fennell reported.
The most common grade 3-4 adverse event was neutropenia, occurring in 12.5% of the vinorelbine-treated patients and no patients managed with supportive care alone, according to the report. Other grade 3-4 adverse events occurred in fewer than 10% of patients and included dyspnea, lower respiratory infection, lymphopenia, and fatigue, among others.
Overall survival (OS) was not statistically different between vinorelbine and supportive care arms, with median OS of 9.3 months and 9.1 months, respectively.
However, a number of patients in the control arm went on to receive subsequent therapy, including 15 (or about 27%) who went into CONFIRM, a randomized phase 3 trial that, as recently reported, met its coprimary endpoints of improve OS and PFS with nivolumab vs. placebo in relapsed malignant mesothelioma.
Investigators also sought to test the hypothesis that BRCA1-negative patients might be chemoresistant, based in part on preclinical models demonstrating that BRCA1 predicted sensitivity to vinorelbine. However, there was no difference in PFS by BRCA1 expression in the VIM study, according to Prof. Fennell.
Taken together, findings of the VIM trial suggest vinorelbine is a “modestly active” agent with low cost and acceptable toxicity, according to Prof. Nowak.
“It is incumbent on us to have clear discussions with our patients on the risks and benefits of trying this as a subsequent-line therapy, in the context of this evidence that was generated as a second-line therapy,” she said in her discussant remarks on the study.
“I would say that it is a lower priority in our algorithm than cisplatin and pemetrexed, or of course, immuno-oncology agents,” she added.
Dr. Fennell reported disclosures related to AstraZeneca, Astex Therapeutics, Bayer, and multiple other pharmaceutical companies. Dr. Nowak reported disclosures with AstraZeneca, Atara Biotherapeutics, Boehringer Ingelheim, and multiple other pharmaceutical companies.
REPORTING FROM ASCO 2021
Are we there yet? Lung cancer screening – current landscape
Lung cancer is the second-most common cancer and one of the leading causes of mortality in the United States among both men and women. It accounts for almost 25% of all cancer deaths, and every year more people die of lung cancer than colon, breast, and prostate cancers combined. The American Cancer Society estimates about 235,760 new lung cancer cases and about 131,880 deaths from lung cancer in 2021.
Smoking and increasing age are the two most important risk factors for lung cancer. Lung cancer has a higher incidence among Black men than White men, and among White women compared with Black women. These differences are likely related to smoking exposure. Early diagnosis of lung cancer can improve survival, and hence screening for lung cancer in high-risk populations is desired. Among the available cancer screening tests, radiology is primarily involved in breast and lung cancer screening (LCS). In 2011, the National Lung Screening Trial (NLST) showed a benefit of annual low- dose chest CT for LCS, with about 20% reduction in lung cancer-related mortality in high-risk participants compared with chest radiographs (Aberle DR, et al. N Engl J Med. 2011 Aug 4;365[5]:395-409).
In 2013, the United States Preventative Services Task Force (USPSTF) issued a grade B recommendation in support of annual LCS by CT scan for individuals between the ages of 55-80 years with smoking history of 30 or more pack-years who are current smokers or had quit smoking in the last 15 years. Many other professional societies followed with their own recommendations with minor differences. In 2015, after the Centers for Medicare and Medicaid (CMS) decision of coverage, millions of Americans at high risk became eligible for CT LCS with no copayment or cost sharing by the patient.
The results from the European NELSON trial in 2020 augmented the NLST data showing a 24% decrease in lung cancer mortality. Nodules were measured using volume and volume doubling time rather than bidimensional axial measurements, reducing the false-positive results to 56% compared with 96% in NLST. With growing evidence of the benefits from LCS, recently USPSTF summarized with moderate certainty that annual LCS CT has moderate net benefit in people at high risk for lung cancer based on age, cumulative smoking exposure, and years since quitting smoking.
In March 2021, USPSTF has issued new recommendations with a decrease in the screening age to 50 years, and the smoking history that triggers screening to 20 pack-years (Screening for Lung Cancer: USPSTF Statement. JAMA. 2021 Mar 9;325[10]:962-70. doi: 10.1001/jama.2021.1117). These expanded eligibility criteria are projected to double the number of eligible candidates of LCS in the United States, reduce annual deaths by up to 50%, and benefit minorities and women. By widening the screening criteria to include younger individuals and who have smoked less tobacco, more lives will be saved by early detection of lung cancer. Since the NLST and NELSON trials enrolled relatively healthy people, USPSTF recommends discontinuation of screening once the person has not smoked for 15 years and in persons with any health problem that severely limits the life expectancy or the ability or willingness to undergo surgery. All screening programs must incorporate smoking cessation counseling and interventions for all the enrolled individuals who are current smokers. The USPSTF has also made recommendations on interventions to prevent the initiation of tobacco use in children and adolescents, including counseling and pharmacotherapy.
The decision to undergo LCS is inherently complex, and primary care and pulmonary physicians play a pivotal role by identifying the eligible patients, participating in shared decision-making (SDM), offering smoking cessation, ordering the CT, and managing follow-up. SDM between the patient and clinician includes a discussion of the benefits, risks, limitations, and potential harms of screening. The potential harms of screening include overdiagnosis, false-positive results, incidental findings, and the anxiety leading to further testing or follow-up. The risk of radiation exposure is markedly reduced using low-dose CT protocols compared with conventional chest CT. SDM visit also emphasizes the importance of adherence to annual screening and patient willingness and ability to undergo treatment if required. In 2015, CMS approved the addition of LCS counseling and SDM visits that are performed by physicians or qualified nonphysician practitioners (physician assistant, nurse practitioner, or clinical nurse specialist). Studies have shown that these visits improve the screening uptake rate.
To minimize the variations in the evaluation and management of screen-detected lung nodules, the American College of Radiology (ACR) developed the Lung Imaging Reporting and Data System (Lung-RADS) to be used in LCS CT reports. The latest revised version 1.1 of Lung-RADS was released in 2019. The Lung-RADS defines a positive screen and provides accepted nodule care pathways depending on their size, characteristics, and additional findings, and has been shown to decrease the rate of false-positive results in LCS. To be a designated LCS center, the department of radiology must comply with stringent requirements of technical and facility specification, with radiologist qualification, and with reporting and communication as outlined by the ACR. In addition, participation in the National LCS Registry to meet CMS quality reporting requirements is mandatory for facilities to be reimbursed by CMS.
After more than 10 years since its inception, the participation in LCS has been low. Out of 8 million eligible Americans, less than 4% have been screened (American Cancer Society, NSCLC statistics 2020) compared with breast cancer (up to 75%) (Breast Cancer: Facts and Figures 2019-2020). Adherence to annual LCS between 1-3 years in the US is only about 55%. Non-White patients, current smokers, those aged 65-73 years, and those who lack a college education are most likely to be less adherent to follow-up screening. There are hurdles at multiple levels including but not limited to patient and physician awareness, patient enrollment, adherence, follow-up, and insurance coverage. Expanding the reach of LCS in socially and economically disadvantaged, racial and ethnic minorities, and women has been even more challenging.
Significant differences exist in opinions and practices between primary care physicians (PCPs) and pulmonologists regarding referral for LCS and its benefits. Educational intervention at the PCP level aimed at awareness of USPSTF guidelines may improve utilization and adherence to screening. Increasing lung cancer awareness by community outreach programs, promoting related discussions, and providing information about available screening services to eligible population is crucial to derive the maximum benefits of LCS. Presenting decision aid tools on smartphones and online has shown to improve the participants’ knowledge of LCS, to reduce the decisional conflict, and to be acceptable among patients and providers. Implementation strategies such as involving a nonphysician provider, keeping the training on these tools brief and simple, and providing it to participants prior to the clinical encounter might be effective. Electronic medical record systems can be optimized to simplify the ordering procedure to ensure the eligibility criteria are met, to provide results to the physicians, and to direct further management of positive screen results. Most LCS programs have a nonphysician program coordinator to convey the results to the patients and physician, to send out reminders for scheduled follow up appointment, and to maintain the registry data.
In the future, newer imaging technology, and molecular biomarkers or other technologies to differentiate lung cancer more accurately from a benign nodule, and to determine its aggressiveness, will supplement the LCS to decrease false positive results. Better risk prediction models will influence screening eligibility and prognostication in a screen-detected cancer. Robust data collection from ongoing clinical programs will determine if the benefits of LCS seen in clinical trials are comparable when applied to diverse community settings.
Dr. Stowell and Dr. Sonavane are with the Mayo Clinic in Jacksonville, Fla.
Lung cancer is the second-most common cancer and one of the leading causes of mortality in the United States among both men and women. It accounts for almost 25% of all cancer deaths, and every year more people die of lung cancer than colon, breast, and prostate cancers combined. The American Cancer Society estimates about 235,760 new lung cancer cases and about 131,880 deaths from lung cancer in 2021.
Smoking and increasing age are the two most important risk factors for lung cancer. Lung cancer has a higher incidence among Black men than White men, and among White women compared with Black women. These differences are likely related to smoking exposure. Early diagnosis of lung cancer can improve survival, and hence screening for lung cancer in high-risk populations is desired. Among the available cancer screening tests, radiology is primarily involved in breast and lung cancer screening (LCS). In 2011, the National Lung Screening Trial (NLST) showed a benefit of annual low- dose chest CT for LCS, with about 20% reduction in lung cancer-related mortality in high-risk participants compared with chest radiographs (Aberle DR, et al. N Engl J Med. 2011 Aug 4;365[5]:395-409).
In 2013, the United States Preventative Services Task Force (USPSTF) issued a grade B recommendation in support of annual LCS by CT scan for individuals between the ages of 55-80 years with smoking history of 30 or more pack-years who are current smokers or had quit smoking in the last 15 years. Many other professional societies followed with their own recommendations with minor differences. In 2015, after the Centers for Medicare and Medicaid (CMS) decision of coverage, millions of Americans at high risk became eligible for CT LCS with no copayment or cost sharing by the patient.
The results from the European NELSON trial in 2020 augmented the NLST data showing a 24% decrease in lung cancer mortality. Nodules were measured using volume and volume doubling time rather than bidimensional axial measurements, reducing the false-positive results to 56% compared with 96% in NLST. With growing evidence of the benefits from LCS, recently USPSTF summarized with moderate certainty that annual LCS CT has moderate net benefit in people at high risk for lung cancer based on age, cumulative smoking exposure, and years since quitting smoking.
In March 2021, USPSTF has issued new recommendations with a decrease in the screening age to 50 years, and the smoking history that triggers screening to 20 pack-years (Screening for Lung Cancer: USPSTF Statement. JAMA. 2021 Mar 9;325[10]:962-70. doi: 10.1001/jama.2021.1117). These expanded eligibility criteria are projected to double the number of eligible candidates of LCS in the United States, reduce annual deaths by up to 50%, and benefit minorities and women. By widening the screening criteria to include younger individuals and who have smoked less tobacco, more lives will be saved by early detection of lung cancer. Since the NLST and NELSON trials enrolled relatively healthy people, USPSTF recommends discontinuation of screening once the person has not smoked for 15 years and in persons with any health problem that severely limits the life expectancy or the ability or willingness to undergo surgery. All screening programs must incorporate smoking cessation counseling and interventions for all the enrolled individuals who are current smokers. The USPSTF has also made recommendations on interventions to prevent the initiation of tobacco use in children and adolescents, including counseling and pharmacotherapy.
The decision to undergo LCS is inherently complex, and primary care and pulmonary physicians play a pivotal role by identifying the eligible patients, participating in shared decision-making (SDM), offering smoking cessation, ordering the CT, and managing follow-up. SDM between the patient and clinician includes a discussion of the benefits, risks, limitations, and potential harms of screening. The potential harms of screening include overdiagnosis, false-positive results, incidental findings, and the anxiety leading to further testing or follow-up. The risk of radiation exposure is markedly reduced using low-dose CT protocols compared with conventional chest CT. SDM visit also emphasizes the importance of adherence to annual screening and patient willingness and ability to undergo treatment if required. In 2015, CMS approved the addition of LCS counseling and SDM visits that are performed by physicians or qualified nonphysician practitioners (physician assistant, nurse practitioner, or clinical nurse specialist). Studies have shown that these visits improve the screening uptake rate.
To minimize the variations in the evaluation and management of screen-detected lung nodules, the American College of Radiology (ACR) developed the Lung Imaging Reporting and Data System (Lung-RADS) to be used in LCS CT reports. The latest revised version 1.1 of Lung-RADS was released in 2019. The Lung-RADS defines a positive screen and provides accepted nodule care pathways depending on their size, characteristics, and additional findings, and has been shown to decrease the rate of false-positive results in LCS. To be a designated LCS center, the department of radiology must comply with stringent requirements of technical and facility specification, with radiologist qualification, and with reporting and communication as outlined by the ACR. In addition, participation in the National LCS Registry to meet CMS quality reporting requirements is mandatory for facilities to be reimbursed by CMS.
After more than 10 years since its inception, the participation in LCS has been low. Out of 8 million eligible Americans, less than 4% have been screened (American Cancer Society, NSCLC statistics 2020) compared with breast cancer (up to 75%) (Breast Cancer: Facts and Figures 2019-2020). Adherence to annual LCS between 1-3 years in the US is only about 55%. Non-White patients, current smokers, those aged 65-73 years, and those who lack a college education are most likely to be less adherent to follow-up screening. There are hurdles at multiple levels including but not limited to patient and physician awareness, patient enrollment, adherence, follow-up, and insurance coverage. Expanding the reach of LCS in socially and economically disadvantaged, racial and ethnic minorities, and women has been even more challenging.
Significant differences exist in opinions and practices between primary care physicians (PCPs) and pulmonologists regarding referral for LCS and its benefits. Educational intervention at the PCP level aimed at awareness of USPSTF guidelines may improve utilization and adherence to screening. Increasing lung cancer awareness by community outreach programs, promoting related discussions, and providing information about available screening services to eligible population is crucial to derive the maximum benefits of LCS. Presenting decision aid tools on smartphones and online has shown to improve the participants’ knowledge of LCS, to reduce the decisional conflict, and to be acceptable among patients and providers. Implementation strategies such as involving a nonphysician provider, keeping the training on these tools brief and simple, and providing it to participants prior to the clinical encounter might be effective. Electronic medical record systems can be optimized to simplify the ordering procedure to ensure the eligibility criteria are met, to provide results to the physicians, and to direct further management of positive screen results. Most LCS programs have a nonphysician program coordinator to convey the results to the patients and physician, to send out reminders for scheduled follow up appointment, and to maintain the registry data.
In the future, newer imaging technology, and molecular biomarkers or other technologies to differentiate lung cancer more accurately from a benign nodule, and to determine its aggressiveness, will supplement the LCS to decrease false positive results. Better risk prediction models will influence screening eligibility and prognostication in a screen-detected cancer. Robust data collection from ongoing clinical programs will determine if the benefits of LCS seen in clinical trials are comparable when applied to diverse community settings.
Dr. Stowell and Dr. Sonavane are with the Mayo Clinic in Jacksonville, Fla.
Lung cancer is the second-most common cancer and one of the leading causes of mortality in the United States among both men and women. It accounts for almost 25% of all cancer deaths, and every year more people die of lung cancer than colon, breast, and prostate cancers combined. The American Cancer Society estimates about 235,760 new lung cancer cases and about 131,880 deaths from lung cancer in 2021.
Smoking and increasing age are the two most important risk factors for lung cancer. Lung cancer has a higher incidence among Black men than White men, and among White women compared with Black women. These differences are likely related to smoking exposure. Early diagnosis of lung cancer can improve survival, and hence screening for lung cancer in high-risk populations is desired. Among the available cancer screening tests, radiology is primarily involved in breast and lung cancer screening (LCS). In 2011, the National Lung Screening Trial (NLST) showed a benefit of annual low- dose chest CT for LCS, with about 20% reduction in lung cancer-related mortality in high-risk participants compared with chest radiographs (Aberle DR, et al. N Engl J Med. 2011 Aug 4;365[5]:395-409).
In 2013, the United States Preventative Services Task Force (USPSTF) issued a grade B recommendation in support of annual LCS by CT scan for individuals between the ages of 55-80 years with smoking history of 30 or more pack-years who are current smokers or had quit smoking in the last 15 years. Many other professional societies followed with their own recommendations with minor differences. In 2015, after the Centers for Medicare and Medicaid (CMS) decision of coverage, millions of Americans at high risk became eligible for CT LCS with no copayment or cost sharing by the patient.
The results from the European NELSON trial in 2020 augmented the NLST data showing a 24% decrease in lung cancer mortality. Nodules were measured using volume and volume doubling time rather than bidimensional axial measurements, reducing the false-positive results to 56% compared with 96% in NLST. With growing evidence of the benefits from LCS, recently USPSTF summarized with moderate certainty that annual LCS CT has moderate net benefit in people at high risk for lung cancer based on age, cumulative smoking exposure, and years since quitting smoking.
In March 2021, USPSTF has issued new recommendations with a decrease in the screening age to 50 years, and the smoking history that triggers screening to 20 pack-years (Screening for Lung Cancer: USPSTF Statement. JAMA. 2021 Mar 9;325[10]:962-70. doi: 10.1001/jama.2021.1117). These expanded eligibility criteria are projected to double the number of eligible candidates of LCS in the United States, reduce annual deaths by up to 50%, and benefit minorities and women. By widening the screening criteria to include younger individuals and who have smoked less tobacco, more lives will be saved by early detection of lung cancer. Since the NLST and NELSON trials enrolled relatively healthy people, USPSTF recommends discontinuation of screening once the person has not smoked for 15 years and in persons with any health problem that severely limits the life expectancy or the ability or willingness to undergo surgery. All screening programs must incorporate smoking cessation counseling and interventions for all the enrolled individuals who are current smokers. The USPSTF has also made recommendations on interventions to prevent the initiation of tobacco use in children and adolescents, including counseling and pharmacotherapy.
The decision to undergo LCS is inherently complex, and primary care and pulmonary physicians play a pivotal role by identifying the eligible patients, participating in shared decision-making (SDM), offering smoking cessation, ordering the CT, and managing follow-up. SDM between the patient and clinician includes a discussion of the benefits, risks, limitations, and potential harms of screening. The potential harms of screening include overdiagnosis, false-positive results, incidental findings, and the anxiety leading to further testing or follow-up. The risk of radiation exposure is markedly reduced using low-dose CT protocols compared with conventional chest CT. SDM visit also emphasizes the importance of adherence to annual screening and patient willingness and ability to undergo treatment if required. In 2015, CMS approved the addition of LCS counseling and SDM visits that are performed by physicians or qualified nonphysician practitioners (physician assistant, nurse practitioner, or clinical nurse specialist). Studies have shown that these visits improve the screening uptake rate.
To minimize the variations in the evaluation and management of screen-detected lung nodules, the American College of Radiology (ACR) developed the Lung Imaging Reporting and Data System (Lung-RADS) to be used in LCS CT reports. The latest revised version 1.1 of Lung-RADS was released in 2019. The Lung-RADS defines a positive screen and provides accepted nodule care pathways depending on their size, characteristics, and additional findings, and has been shown to decrease the rate of false-positive results in LCS. To be a designated LCS center, the department of radiology must comply with stringent requirements of technical and facility specification, with radiologist qualification, and with reporting and communication as outlined by the ACR. In addition, participation in the National LCS Registry to meet CMS quality reporting requirements is mandatory for facilities to be reimbursed by CMS.
After more than 10 years since its inception, the participation in LCS has been low. Out of 8 million eligible Americans, less than 4% have been screened (American Cancer Society, NSCLC statistics 2020) compared with breast cancer (up to 75%) (Breast Cancer: Facts and Figures 2019-2020). Adherence to annual LCS between 1-3 years in the US is only about 55%. Non-White patients, current smokers, those aged 65-73 years, and those who lack a college education are most likely to be less adherent to follow-up screening. There are hurdles at multiple levels including but not limited to patient and physician awareness, patient enrollment, adherence, follow-up, and insurance coverage. Expanding the reach of LCS in socially and economically disadvantaged, racial and ethnic minorities, and women has been even more challenging.
Significant differences exist in opinions and practices between primary care physicians (PCPs) and pulmonologists regarding referral for LCS and its benefits. Educational intervention at the PCP level aimed at awareness of USPSTF guidelines may improve utilization and adherence to screening. Increasing lung cancer awareness by community outreach programs, promoting related discussions, and providing information about available screening services to eligible population is crucial to derive the maximum benefits of LCS. Presenting decision aid tools on smartphones and online has shown to improve the participants’ knowledge of LCS, to reduce the decisional conflict, and to be acceptable among patients and providers. Implementation strategies such as involving a nonphysician provider, keeping the training on these tools brief and simple, and providing it to participants prior to the clinical encounter might be effective. Electronic medical record systems can be optimized to simplify the ordering procedure to ensure the eligibility criteria are met, to provide results to the physicians, and to direct further management of positive screen results. Most LCS programs have a nonphysician program coordinator to convey the results to the patients and physician, to send out reminders for scheduled follow up appointment, and to maintain the registry data.
In the future, newer imaging technology, and molecular biomarkers or other technologies to differentiate lung cancer more accurately from a benign nodule, and to determine its aggressiveness, will supplement the LCS to decrease false positive results. Better risk prediction models will influence screening eligibility and prognostication in a screen-detected cancer. Robust data collection from ongoing clinical programs will determine if the benefits of LCS seen in clinical trials are comparable when applied to diverse community settings.
Dr. Stowell and Dr. Sonavane are with the Mayo Clinic in Jacksonville, Fla.
Procalcitonin-Guided Antibiotic Prescribing for Acute Exacerbations of Chronic Obstructive Pulmonary Disease in the Emergency Department
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines define acute exacerbations of chronic obstructive pulmonary disease (AECOPD) as a sudden worsening of respiratory symptoms that require additional interventions. Exacerbations are classified as mild (treated with short-acting bronchodilators only), moderate (treated with antibiotics and/or oral corticosteroids), or severe (treatment requiring hospitalization). Exacerbations must include increased dyspnea, and other symptoms may involve increased sputum volume and purulence, cough, and a change in sputum color. These symptoms can be due to viral, bacterial, or environmental causes, with viral respiratory infections being the most common cause.1-4 However, determining the etiology of an exacerbation can be difficult based on symptoms alone and can lead to an excessive and unnecessary use of antibiotics. Only the change in sputum color is considered highly sensitive and specific for bacterial causes.1 As a result, there has been an increased interest in the use of acute biomarkers to determine whether antibiotics are necessary.
Procalcitonin (PCT) is an acute phase reactant that increases in response to inflammation, especially inflammation caused by a bacterial infection. Recent studies have suggested that PCT may be used in patients experiencing an AECOPD to reduce antibiotic use without impacting rates of treatment failure.5-9 A majority of these studies have been in the inpatient setting or a combination of inpatient and outpatient settings.
The purpose of this study was to create and to evaluate the efficacy and practicality of a PCT-based algorithm to aid emergency department (ED) clinicians in the evaluation of patients with AECOPD who do not require hospitalization. The primary outcome of this project was the rate of antibiotic prescriptions before and after the initiation of the algorithm.
Methods
This was an observational, retrospective, pre/post assessment at the Phoenix Veterans Affairs Health Care System (PVAHCS) in Arizona. Patients who were discharged from the ED with a diagnosis of an AECOPD were identified using International Classification of Diseases, Tenth Revision (ICD-10) codes. Patient charts were reviewed from November 2018 to March 2019 for the preimplementation group and from November 2019 for March 2020 in the postimplementation group. The periods were chosen to reflect similar seasons for both the pre- and postimplementation interventions. Patients were excluded from analysis if they required hospital admission, were immunocompromised, on chronic antimicrobial therapy, had no documented medical history of COPD, or if they were presenting primarily for medication refills. Information collected included the rate of antibiotic prescriptions, procalcitonin test orders, COPD GOLD classification, and 30-, 60-, and 90-day reexacerbation rates.
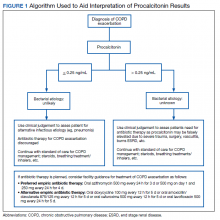
A PCT-based algorithm (Figure 1) was developed and approved by the PVAHCS Antimicrobial Stewardship Program, the Pharmacy and Therapeutics committee, and ED leadership. PCT threshold values were based on values approved by the US Food and Drug Administration and previous studies—antibiotics were discouraged for PCT levels ≤ 0.25 ng/mL but could be considered for PCT levels > 0.25 ng/mL.5,8,9 Clinicians were not required to use the algorithm, and the use of clinical judgement was encouraged. The recommended antibiotic therapies were based on previously approved PVAHCS antimicrobial stewardship guidance. To promote utilization, a PCT quick order option was added to the ED laboratory order menu.
ED clinicians were individually educated by the antimicrobial stewardship and emergency medicine pharmacists, an infectious disease physician champion, and the pharmacy resident. Clinicians were educated about PCT and its use in the setting of AECOPD to aid in the determination of bacterial infections. Each clinician received an electronic copy the algorithm and summary of the study protocol before implementation and 3 months after implementation for follow-up education. In addition, a printed copy of the algorithm was posted in multiple clinician workstations within the ED. For the first month of implementation, the project lead was available full-time in the ED to encourage algorithm use and to field questions or concerns from clinicians.
Outcome Measures
The primary outcome was the rate of antibiotic prescriptions pre- and postintervention. The safety endpoints were 30-, 60-, and 90-day reexacerbation rates. Reexacerbation rates were defined by ICD-10 codes and documentation from a primary care visit or subsequent ED visit. The secondary outcomes were the rate of PCT tests ordered and used for treatment decisions. Other areas of interest were antibiotic prescribing trends, duration of therapy, and patient COPD GOLD classification.
Statistical analysis
It was estimated that a sample size of 146 patients (73 patients/group) would provide 80% power to detect a between-group difference of 10% in the percentage of patients who were prescribed antibiotics. Categorical variables were expressed using estimates of frequency and percentages. Percentages were compared using Fisher exact tests. For all tests, the significance level was set at 0.05.
Results
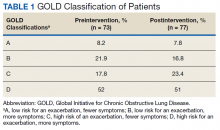
Seventy-three patients were included in the preintervention group and 77 in the postintervention group. The GOLD classification rates were similar between the groups (Table 1). In addition, > 90% of patients were White males and all patients were aged ≥ 50 years, which is characteristic of the US Department of Veterans Affairs (VA) population.
The percentage of antibiotic prescriptions decreased by 20% after implementation, falling from 83.6% before to 63.6% after the implementation (P =.01). The documented change in sputum color remained low compared with antibiotic prescriptions: 17.8% preimplementation and 16.9% postimplementation. The reduction in antibiotic prescriptions was associated with limited differences observed in 30-, 60-, and 90-day reexacerbation rates pre- and postintervention: 19.2% vs 23.4%, 12.3% vs 11.7%, and 4.1% vs 9.1%, respectively.
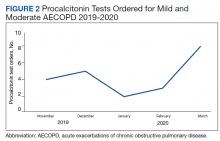
Prior to the education, introduction of the algorithm, and implementation of the PCT quick-order menu, PCT was ordered for 1.4% of AECOPD cases. Postintervention, PCT was ordered for 28.6% of mild-to-moderate AECOPD cases and used in clinical decision making per clinical documentation 81.8% of the time. PCT was used in 5 GOLD group B patients, 5 GOLD group C patients, and 7 GOLD group D patients. In all cases, PCT was < 0.25 ng/mL. In 4 cases PCT was ordered but not used: 1 GOLD group D patient refused traditional treatment with oral corticosteroids, which resulted in the clinician prescribing antibiotics, and the other 3 cases did not use PCT based on clinical decision making. The rate of PCT tests ordered for mild-to-moderate AECOPD over time is depicted in Figure 2.
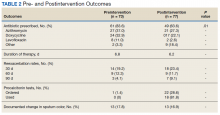
The average duration of antibiotic therapy was about 6 days pre- and postintervention. This is longer than the PVAHCS recommended duration of 5 days but is consistent with the GOLD guidelines recommended duration of 5 to 7 days.1 Azithromycin is recommended as a first-line treatment option at the PVAHCS based on the local antibiogram, and it remained the most commonly prescribed antibiotic pre- and postintervention. Outcomes of interest are detailed in Table 2.
Discussion
The implementation of PCT-guided antibiotic prescribing for patients with mild and moderate AECOPD who presented to the ED resulted in a 20% reduction in antibiotic prescriptions, falling from 83.6% before the intervention to 63.6% afterward (P = .01). The measured decrease in antibiotic prescriptions is consistent with other studies evaluating the use of acute phase reactants to guide antibiotic prescribing for AECOPD.10,11 In addition, there was no observed difference in reexacerbation rates. This adds to the increasing body of evidence that antibiotics are overprescribed in mild and moderate AECOPD.12 This is exemplified in our data by the low percentage of patients who had a documented change in sputum color; symptoms that are well known to be highly specific and sensitive for a bacterial infection in AECOPD.
Many health care providers (HCPs) in the ED were unfamiliar with PCT prior to implementation. A primary concern with this study was its impact on diagnostic stewardship. Preimplementation, ED clinicians ordered PCT 8 times for any cause. Postintervention, ED clinicians ordered PCT 180 times for any cause: 36% of these orders were for patients with AECOPD who were discharged from the ED or who required hospital admission. The other orders were for other respiratory conditions, including asthma exacerbations, pneumonia, bronchitis, sinusitis, pharyngitis, nonspecific respiratory infections, and respiratory failure.
The early phase of the COVID-19 pandemic coincided with the postintervention phase of this project. PVAHCS started preparing for the pandemic in March 2020, and the first confirmed diagnosis at the facility occurred mid-March. COVID-19 may have contributed to the sharp increase in PCT tests. There is currently no well-defined role for PCT in the diagnosis or management of COVID-19, but ED clinicians may have increased their use of PCT tests to help characterize the etiology of the large influx of patients presenting with respiratory symptoms.13
Strengths
Strengths of this project include its multimodal implementation and overall pragmatic design, which reflects real-world utilization of procalcitonin by ED HCPs. The HCPs were not mandated to follow the procalcitonin algorithm, and the use of clinical judgment was strongly encouraged. This project occurred concomitantly with the VA Infectious Disease Academic Detailing education program. The program focused on clinician education for the proper diagnosis and treatment of respiratory tract infections. In addition, viral illness packs were introduced as part of this initiative to reduce unnecessary antibiotic prescribing. The viral illness pack included standard items for symptom relief, such as saline nasal spray, cough drops, and hand sanitizer, as well as an explanation card of why the patient was not receiving antibiotics. Several studies have suggested that patients expect a prescription for an antibiotic when they present with respiratory tract symptoms, and HCPs often are compelled to maintain patient satisfaction, thus leading to unnecessary antibiotic prescriptions.14 The viral illness pack helped fulfill the patient’s expectation to receive treatment after seeking care. In addition, the project lead was available full time during the first month of PCT algorithm implementation to address questions and concerns, which may have improved HCPs overall confidence in using PCT.
Limitations
Limitations of this project include its population and its retrospective nature. The PVAHCS patient population is predominantly older, more White, and more male compared with the general civilian population, and results may not be generalizable to other populations. Data were limited to documentation in the electronic health record. The population was based on data extraction by the ICD-10 code, which may not be an accurate capture of the total population as HCPs may not select the most accurate ICD-10 code on documentation. Another potential limitation was the COVID-19 pandemic which may have resulted in HCPs ordering PCT more frequently as more patients presented to the ED with undifferentiated respiratory symptoms. Finally, there were minimal differences observed in reexacerbation rates; however, although the sample size was powered to detect a difference in antibiotic prescriptions, the sample size was not powered to detect a statistically significant difference in the primary safety outcome.
Conclusions
PCT-guided antibiotic prescribing significantly reduced the number of antibiotic prescriptions without an observable increase in reexacerbation rates for patients with mild and moderate AECOPD in the ED. This study provides a pragmatic evaluation of PCT-guided antibiotic prescribing for patients with AECOPD solely in the outpatient setting. Acute phase reactants like PCT can play a role in the management of AECOPD to reduce unnecessary antibiotic prescriptions.
1. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease: 2020 report. Accessd June 2, 2021. http://www.goldcopd.org/wp-content/uploads/2019/12/GOLD-2020-FINAL-ver1.2-03Dec19_WMV.pdf
2. George SN, Garcha DS, Mackay AJ, et al. Human rhinovirus infection during naturally occurring COPD exacerbations. Eur Respir J. 2014;44(1):87-96. doi:10.1183/09031936.00223113
3. Seemungal T, Harper-Owen R, Bhowmik A, et al. Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164(9):1618-1623. doi:10.1164/ajrccm.164.9.2105011
4. Rohde G, Wiethege A, Borg I, et al. Respiratory viruses in exacerbations of chronic obstructive pulmonary disease requiring hospitalisation: a case-control study. Thorax. 2003;58(1):37-42. doi:10.1136/thorax.58.1.37
5. Bremmer DN, Moffa MA, Ma K, et al. Acute exacerbations of chronic obstructive pulmonary disease with a low procalcitonin concentration: impact of antibiotic therapy. Clin Infect Dis. 2019;68(5):725-730. doi:10.1093/cid/ciy552
6. Mathioudakis AG, Chatzimavridou-Grigoriadou V, Corlateanu A, Vestbo J. Procalcitonin to guide antibiotic administration in COPD exacerbations: a meta-analysis. Eur Respir Rev. 2017;26(143):160073. Published 2017 Jan 31. doi:10.1183/16000617.0073-2016
7. van der Does Y, Rood PP, Haagsma JA, Patka P, van Gorp EC, Limper M. Procalcitonin-guided therapy for the initiation of antibiotics in the ED: a systematic review. Am J Emerg Med. 2016;34(7):1286-1293. doi:10.1016/j.ajem.2016.03.065
8. Huang DT, Yealy DM, Filbin MR, et al. Procalcitonin-guided use of antibiotics for lower respiratory tract infection. N Engl J Med. 2018;379(3):236-249. doi:10.1056/NEJMoa1802670
9. Picart J, Moiton MP, Gaüzère BA, Gazaille V, Combes X, DiBernardo S. Introduction of a PCT-based algorithm to guide antibiotic prescription in COPD exacerbation. Med Mal Infect. 2016;46(8):429-435. doi:10.1016/j.medmal.2016.07.008
10. Schuetz P, Chiappa V, Briel M, Greenwald JL. Procalcitonin algorithms for antibiotic therapy decisions: a systematic review of randomized controlled trials and recommendations for clinical algorithms. Arch Intern Med. 2011;171(15):1322-1331. doi:10.1001/archinternmed.2011.318
11. Butler CC, Gillespie D, White P, et al. C-reactive protein testing to guide antibiotic prescribing for COPD exacerbations. N Engl J Med. 2019;381(2):111-120. |doi:10.1056/NEJMoa1803185
12. Vollenweider DJ, Frei A, Steurer-Stey CA, Garcia-Aymerich J, Puhan MA. Antibiotics for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2018;10(10):CD010257. Published 2018 Oct 29. doi:10.1002/14651858.CD010257.pub2
13. Centers for Disease Control and Prevention. Interim clinical guidance for management of patients with confirmed coronavirus disease (COVID-19). Updated February 16, 2021. Accessed May 14, 2021. https://www.cdc.gov/coronavirus/2019ncov/hcp/clinical-guidance-management-patients.html
14. Gaarslev C, Yee M, Chan G, Fletcher-Lartey S, Khan R. A mixed methods study to understand patient expectations for antibiotics for an upper respiratory tract infection. Antimicrob Resist Infect Control. 2016;5:39. Published 2016 Oct 20. doi:10.1186/s13756-016-0134-3
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines define acute exacerbations of chronic obstructive pulmonary disease (AECOPD) as a sudden worsening of respiratory symptoms that require additional interventions. Exacerbations are classified as mild (treated with short-acting bronchodilators only), moderate (treated with antibiotics and/or oral corticosteroids), or severe (treatment requiring hospitalization). Exacerbations must include increased dyspnea, and other symptoms may involve increased sputum volume and purulence, cough, and a change in sputum color. These symptoms can be due to viral, bacterial, or environmental causes, with viral respiratory infections being the most common cause.1-4 However, determining the etiology of an exacerbation can be difficult based on symptoms alone and can lead to an excessive and unnecessary use of antibiotics. Only the change in sputum color is considered highly sensitive and specific for bacterial causes.1 As a result, there has been an increased interest in the use of acute biomarkers to determine whether antibiotics are necessary.
Procalcitonin (PCT) is an acute phase reactant that increases in response to inflammation, especially inflammation caused by a bacterial infection. Recent studies have suggested that PCT may be used in patients experiencing an AECOPD to reduce antibiotic use without impacting rates of treatment failure.5-9 A majority of these studies have been in the inpatient setting or a combination of inpatient and outpatient settings.
The purpose of this study was to create and to evaluate the efficacy and practicality of a PCT-based algorithm to aid emergency department (ED) clinicians in the evaluation of patients with AECOPD who do not require hospitalization. The primary outcome of this project was the rate of antibiotic prescriptions before and after the initiation of the algorithm.
Methods
This was an observational, retrospective, pre/post assessment at the Phoenix Veterans Affairs Health Care System (PVAHCS) in Arizona. Patients who were discharged from the ED with a diagnosis of an AECOPD were identified using International Classification of Diseases, Tenth Revision (ICD-10) codes. Patient charts were reviewed from November 2018 to March 2019 for the preimplementation group and from November 2019 for March 2020 in the postimplementation group. The periods were chosen to reflect similar seasons for both the pre- and postimplementation interventions. Patients were excluded from analysis if they required hospital admission, were immunocompromised, on chronic antimicrobial therapy, had no documented medical history of COPD, or if they were presenting primarily for medication refills. Information collected included the rate of antibiotic prescriptions, procalcitonin test orders, COPD GOLD classification, and 30-, 60-, and 90-day reexacerbation rates.
A PCT-based algorithm (Figure 1) was developed and approved by the PVAHCS Antimicrobial Stewardship Program, the Pharmacy and Therapeutics committee, and ED leadership. PCT threshold values were based on values approved by the US Food and Drug Administration and previous studies—antibiotics were discouraged for PCT levels ≤ 0.25 ng/mL but could be considered for PCT levels > 0.25 ng/mL.5,8,9 Clinicians were not required to use the algorithm, and the use of clinical judgement was encouraged. The recommended antibiotic therapies were based on previously approved PVAHCS antimicrobial stewardship guidance. To promote utilization, a PCT quick order option was added to the ED laboratory order menu.
ED clinicians were individually educated by the antimicrobial stewardship and emergency medicine pharmacists, an infectious disease physician champion, and the pharmacy resident. Clinicians were educated about PCT and its use in the setting of AECOPD to aid in the determination of bacterial infections. Each clinician received an electronic copy the algorithm and summary of the study protocol before implementation and 3 months after implementation for follow-up education. In addition, a printed copy of the algorithm was posted in multiple clinician workstations within the ED. For the first month of implementation, the project lead was available full-time in the ED to encourage algorithm use and to field questions or concerns from clinicians.
Outcome Measures
The primary outcome was the rate of antibiotic prescriptions pre- and postintervention. The safety endpoints were 30-, 60-, and 90-day reexacerbation rates. Reexacerbation rates were defined by ICD-10 codes and documentation from a primary care visit or subsequent ED visit. The secondary outcomes were the rate of PCT tests ordered and used for treatment decisions. Other areas of interest were antibiotic prescribing trends, duration of therapy, and patient COPD GOLD classification.
Statistical analysis
It was estimated that a sample size of 146 patients (73 patients/group) would provide 80% power to detect a between-group difference of 10% in the percentage of patients who were prescribed antibiotics. Categorical variables were expressed using estimates of frequency and percentages. Percentages were compared using Fisher exact tests. For all tests, the significance level was set at 0.05.
Results
Seventy-three patients were included in the preintervention group and 77 in the postintervention group. The GOLD classification rates were similar between the groups (Table 1). In addition, > 90% of patients were White males and all patients were aged ≥ 50 years, which is characteristic of the US Department of Veterans Affairs (VA) population.
The percentage of antibiotic prescriptions decreased by 20% after implementation, falling from 83.6% before to 63.6% after the implementation (P =.01). The documented change in sputum color remained low compared with antibiotic prescriptions: 17.8% preimplementation and 16.9% postimplementation. The reduction in antibiotic prescriptions was associated with limited differences observed in 30-, 60-, and 90-day reexacerbation rates pre- and postintervention: 19.2% vs 23.4%, 12.3% vs 11.7%, and 4.1% vs 9.1%, respectively.
Prior to the education, introduction of the algorithm, and implementation of the PCT quick-order menu, PCT was ordered for 1.4% of AECOPD cases. Postintervention, PCT was ordered for 28.6% of mild-to-moderate AECOPD cases and used in clinical decision making per clinical documentation 81.8% of the time. PCT was used in 5 GOLD group B patients, 5 GOLD group C patients, and 7 GOLD group D patients. In all cases, PCT was < 0.25 ng/mL. In 4 cases PCT was ordered but not used: 1 GOLD group D patient refused traditional treatment with oral corticosteroids, which resulted in the clinician prescribing antibiotics, and the other 3 cases did not use PCT based on clinical decision making. The rate of PCT tests ordered for mild-to-moderate AECOPD over time is depicted in Figure 2.
The average duration of antibiotic therapy was about 6 days pre- and postintervention. This is longer than the PVAHCS recommended duration of 5 days but is consistent with the GOLD guidelines recommended duration of 5 to 7 days.1 Azithromycin is recommended as a first-line treatment option at the PVAHCS based on the local antibiogram, and it remained the most commonly prescribed antibiotic pre- and postintervention. Outcomes of interest are detailed in Table 2.
Discussion
The implementation of PCT-guided antibiotic prescribing for patients with mild and moderate AECOPD who presented to the ED resulted in a 20% reduction in antibiotic prescriptions, falling from 83.6% before the intervention to 63.6% afterward (P = .01). The measured decrease in antibiotic prescriptions is consistent with other studies evaluating the use of acute phase reactants to guide antibiotic prescribing for AECOPD.10,11 In addition, there was no observed difference in reexacerbation rates. This adds to the increasing body of evidence that antibiotics are overprescribed in mild and moderate AECOPD.12 This is exemplified in our data by the low percentage of patients who had a documented change in sputum color; symptoms that are well known to be highly specific and sensitive for a bacterial infection in AECOPD.
Many health care providers (HCPs) in the ED were unfamiliar with PCT prior to implementation. A primary concern with this study was its impact on diagnostic stewardship. Preimplementation, ED clinicians ordered PCT 8 times for any cause. Postintervention, ED clinicians ordered PCT 180 times for any cause: 36% of these orders were for patients with AECOPD who were discharged from the ED or who required hospital admission. The other orders were for other respiratory conditions, including asthma exacerbations, pneumonia, bronchitis, sinusitis, pharyngitis, nonspecific respiratory infections, and respiratory failure.
The early phase of the COVID-19 pandemic coincided with the postintervention phase of this project. PVAHCS started preparing for the pandemic in March 2020, and the first confirmed diagnosis at the facility occurred mid-March. COVID-19 may have contributed to the sharp increase in PCT tests. There is currently no well-defined role for PCT in the diagnosis or management of COVID-19, but ED clinicians may have increased their use of PCT tests to help characterize the etiology of the large influx of patients presenting with respiratory symptoms.13
Strengths
Strengths of this project include its multimodal implementation and overall pragmatic design, which reflects real-world utilization of procalcitonin by ED HCPs. The HCPs were not mandated to follow the procalcitonin algorithm, and the use of clinical judgment was strongly encouraged. This project occurred concomitantly with the VA Infectious Disease Academic Detailing education program. The program focused on clinician education for the proper diagnosis and treatment of respiratory tract infections. In addition, viral illness packs were introduced as part of this initiative to reduce unnecessary antibiotic prescribing. The viral illness pack included standard items for symptom relief, such as saline nasal spray, cough drops, and hand sanitizer, as well as an explanation card of why the patient was not receiving antibiotics. Several studies have suggested that patients expect a prescription for an antibiotic when they present with respiratory tract symptoms, and HCPs often are compelled to maintain patient satisfaction, thus leading to unnecessary antibiotic prescriptions.14 The viral illness pack helped fulfill the patient’s expectation to receive treatment after seeking care. In addition, the project lead was available full time during the first month of PCT algorithm implementation to address questions and concerns, which may have improved HCPs overall confidence in using PCT.
Limitations
Limitations of this project include its population and its retrospective nature. The PVAHCS patient population is predominantly older, more White, and more male compared with the general civilian population, and results may not be generalizable to other populations. Data were limited to documentation in the electronic health record. The population was based on data extraction by the ICD-10 code, which may not be an accurate capture of the total population as HCPs may not select the most accurate ICD-10 code on documentation. Another potential limitation was the COVID-19 pandemic which may have resulted in HCPs ordering PCT more frequently as more patients presented to the ED with undifferentiated respiratory symptoms. Finally, there were minimal differences observed in reexacerbation rates; however, although the sample size was powered to detect a difference in antibiotic prescriptions, the sample size was not powered to detect a statistically significant difference in the primary safety outcome.
Conclusions
PCT-guided antibiotic prescribing significantly reduced the number of antibiotic prescriptions without an observable increase in reexacerbation rates for patients with mild and moderate AECOPD in the ED. This study provides a pragmatic evaluation of PCT-guided antibiotic prescribing for patients with AECOPD solely in the outpatient setting. Acute phase reactants like PCT can play a role in the management of AECOPD to reduce unnecessary antibiotic prescriptions.
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines define acute exacerbations of chronic obstructive pulmonary disease (AECOPD) as a sudden worsening of respiratory symptoms that require additional interventions. Exacerbations are classified as mild (treated with short-acting bronchodilators only), moderate (treated with antibiotics and/or oral corticosteroids), or severe (treatment requiring hospitalization). Exacerbations must include increased dyspnea, and other symptoms may involve increased sputum volume and purulence, cough, and a change in sputum color. These symptoms can be due to viral, bacterial, or environmental causes, with viral respiratory infections being the most common cause.1-4 However, determining the etiology of an exacerbation can be difficult based on symptoms alone and can lead to an excessive and unnecessary use of antibiotics. Only the change in sputum color is considered highly sensitive and specific for bacterial causes.1 As a result, there has been an increased interest in the use of acute biomarkers to determine whether antibiotics are necessary.
Procalcitonin (PCT) is an acute phase reactant that increases in response to inflammation, especially inflammation caused by a bacterial infection. Recent studies have suggested that PCT may be used in patients experiencing an AECOPD to reduce antibiotic use without impacting rates of treatment failure.5-9 A majority of these studies have been in the inpatient setting or a combination of inpatient and outpatient settings.
The purpose of this study was to create and to evaluate the efficacy and practicality of a PCT-based algorithm to aid emergency department (ED) clinicians in the evaluation of patients with AECOPD who do not require hospitalization. The primary outcome of this project was the rate of antibiotic prescriptions before and after the initiation of the algorithm.
Methods
This was an observational, retrospective, pre/post assessment at the Phoenix Veterans Affairs Health Care System (PVAHCS) in Arizona. Patients who were discharged from the ED with a diagnosis of an AECOPD were identified using International Classification of Diseases, Tenth Revision (ICD-10) codes. Patient charts were reviewed from November 2018 to March 2019 for the preimplementation group and from November 2019 for March 2020 in the postimplementation group. The periods were chosen to reflect similar seasons for both the pre- and postimplementation interventions. Patients were excluded from analysis if they required hospital admission, were immunocompromised, on chronic antimicrobial therapy, had no documented medical history of COPD, or if they were presenting primarily for medication refills. Information collected included the rate of antibiotic prescriptions, procalcitonin test orders, COPD GOLD classification, and 30-, 60-, and 90-day reexacerbation rates.
A PCT-based algorithm (Figure 1) was developed and approved by the PVAHCS Antimicrobial Stewardship Program, the Pharmacy and Therapeutics committee, and ED leadership. PCT threshold values were based on values approved by the US Food and Drug Administration and previous studies—antibiotics were discouraged for PCT levels ≤ 0.25 ng/mL but could be considered for PCT levels > 0.25 ng/mL.5,8,9 Clinicians were not required to use the algorithm, and the use of clinical judgement was encouraged. The recommended antibiotic therapies were based on previously approved PVAHCS antimicrobial stewardship guidance. To promote utilization, a PCT quick order option was added to the ED laboratory order menu.
ED clinicians were individually educated by the antimicrobial stewardship and emergency medicine pharmacists, an infectious disease physician champion, and the pharmacy resident. Clinicians were educated about PCT and its use in the setting of AECOPD to aid in the determination of bacterial infections. Each clinician received an electronic copy the algorithm and summary of the study protocol before implementation and 3 months after implementation for follow-up education. In addition, a printed copy of the algorithm was posted in multiple clinician workstations within the ED. For the first month of implementation, the project lead was available full-time in the ED to encourage algorithm use and to field questions or concerns from clinicians.
Outcome Measures
The primary outcome was the rate of antibiotic prescriptions pre- and postintervention. The safety endpoints were 30-, 60-, and 90-day reexacerbation rates. Reexacerbation rates were defined by ICD-10 codes and documentation from a primary care visit or subsequent ED visit. The secondary outcomes were the rate of PCT tests ordered and used for treatment decisions. Other areas of interest were antibiotic prescribing trends, duration of therapy, and patient COPD GOLD classification.
Statistical analysis
It was estimated that a sample size of 146 patients (73 patients/group) would provide 80% power to detect a between-group difference of 10% in the percentage of patients who were prescribed antibiotics. Categorical variables were expressed using estimates of frequency and percentages. Percentages were compared using Fisher exact tests. For all tests, the significance level was set at 0.05.
Results
Seventy-three patients were included in the preintervention group and 77 in the postintervention group. The GOLD classification rates were similar between the groups (Table 1). In addition, > 90% of patients were White males and all patients were aged ≥ 50 years, which is characteristic of the US Department of Veterans Affairs (VA) population.
The percentage of antibiotic prescriptions decreased by 20% after implementation, falling from 83.6% before to 63.6% after the implementation (P =.01). The documented change in sputum color remained low compared with antibiotic prescriptions: 17.8% preimplementation and 16.9% postimplementation. The reduction in antibiotic prescriptions was associated with limited differences observed in 30-, 60-, and 90-day reexacerbation rates pre- and postintervention: 19.2% vs 23.4%, 12.3% vs 11.7%, and 4.1% vs 9.1%, respectively.
Prior to the education, introduction of the algorithm, and implementation of the PCT quick-order menu, PCT was ordered for 1.4% of AECOPD cases. Postintervention, PCT was ordered for 28.6% of mild-to-moderate AECOPD cases and used in clinical decision making per clinical documentation 81.8% of the time. PCT was used in 5 GOLD group B patients, 5 GOLD group C patients, and 7 GOLD group D patients. In all cases, PCT was < 0.25 ng/mL. In 4 cases PCT was ordered but not used: 1 GOLD group D patient refused traditional treatment with oral corticosteroids, which resulted in the clinician prescribing antibiotics, and the other 3 cases did not use PCT based on clinical decision making. The rate of PCT tests ordered for mild-to-moderate AECOPD over time is depicted in Figure 2.
The average duration of antibiotic therapy was about 6 days pre- and postintervention. This is longer than the PVAHCS recommended duration of 5 days but is consistent with the GOLD guidelines recommended duration of 5 to 7 days.1 Azithromycin is recommended as a first-line treatment option at the PVAHCS based on the local antibiogram, and it remained the most commonly prescribed antibiotic pre- and postintervention. Outcomes of interest are detailed in Table 2.
Discussion
The implementation of PCT-guided antibiotic prescribing for patients with mild and moderate AECOPD who presented to the ED resulted in a 20% reduction in antibiotic prescriptions, falling from 83.6% before the intervention to 63.6% afterward (P = .01). The measured decrease in antibiotic prescriptions is consistent with other studies evaluating the use of acute phase reactants to guide antibiotic prescribing for AECOPD.10,11 In addition, there was no observed difference in reexacerbation rates. This adds to the increasing body of evidence that antibiotics are overprescribed in mild and moderate AECOPD.12 This is exemplified in our data by the low percentage of patients who had a documented change in sputum color; symptoms that are well known to be highly specific and sensitive for a bacterial infection in AECOPD.
Many health care providers (HCPs) in the ED were unfamiliar with PCT prior to implementation. A primary concern with this study was its impact on diagnostic stewardship. Preimplementation, ED clinicians ordered PCT 8 times for any cause. Postintervention, ED clinicians ordered PCT 180 times for any cause: 36% of these orders were for patients with AECOPD who were discharged from the ED or who required hospital admission. The other orders were for other respiratory conditions, including asthma exacerbations, pneumonia, bronchitis, sinusitis, pharyngitis, nonspecific respiratory infections, and respiratory failure.
The early phase of the COVID-19 pandemic coincided with the postintervention phase of this project. PVAHCS started preparing for the pandemic in March 2020, and the first confirmed diagnosis at the facility occurred mid-March. COVID-19 may have contributed to the sharp increase in PCT tests. There is currently no well-defined role for PCT in the diagnosis or management of COVID-19, but ED clinicians may have increased their use of PCT tests to help characterize the etiology of the large influx of patients presenting with respiratory symptoms.13
Strengths
Strengths of this project include its multimodal implementation and overall pragmatic design, which reflects real-world utilization of procalcitonin by ED HCPs. The HCPs were not mandated to follow the procalcitonin algorithm, and the use of clinical judgment was strongly encouraged. This project occurred concomitantly with the VA Infectious Disease Academic Detailing education program. The program focused on clinician education for the proper diagnosis and treatment of respiratory tract infections. In addition, viral illness packs were introduced as part of this initiative to reduce unnecessary antibiotic prescribing. The viral illness pack included standard items for symptom relief, such as saline nasal spray, cough drops, and hand sanitizer, as well as an explanation card of why the patient was not receiving antibiotics. Several studies have suggested that patients expect a prescription for an antibiotic when they present with respiratory tract symptoms, and HCPs often are compelled to maintain patient satisfaction, thus leading to unnecessary antibiotic prescriptions.14 The viral illness pack helped fulfill the patient’s expectation to receive treatment after seeking care. In addition, the project lead was available full time during the first month of PCT algorithm implementation to address questions and concerns, which may have improved HCPs overall confidence in using PCT.
Limitations
Limitations of this project include its population and its retrospective nature. The PVAHCS patient population is predominantly older, more White, and more male compared with the general civilian population, and results may not be generalizable to other populations. Data were limited to documentation in the electronic health record. The population was based on data extraction by the ICD-10 code, which may not be an accurate capture of the total population as HCPs may not select the most accurate ICD-10 code on documentation. Another potential limitation was the COVID-19 pandemic which may have resulted in HCPs ordering PCT more frequently as more patients presented to the ED with undifferentiated respiratory symptoms. Finally, there were minimal differences observed in reexacerbation rates; however, although the sample size was powered to detect a difference in antibiotic prescriptions, the sample size was not powered to detect a statistically significant difference in the primary safety outcome.
Conclusions
PCT-guided antibiotic prescribing significantly reduced the number of antibiotic prescriptions without an observable increase in reexacerbation rates for patients with mild and moderate AECOPD in the ED. This study provides a pragmatic evaluation of PCT-guided antibiotic prescribing for patients with AECOPD solely in the outpatient setting. Acute phase reactants like PCT can play a role in the management of AECOPD to reduce unnecessary antibiotic prescriptions.
1. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease: 2020 report. Accessd June 2, 2021. http://www.goldcopd.org/wp-content/uploads/2019/12/GOLD-2020-FINAL-ver1.2-03Dec19_WMV.pdf
2. George SN, Garcha DS, Mackay AJ, et al. Human rhinovirus infection during naturally occurring COPD exacerbations. Eur Respir J. 2014;44(1):87-96. doi:10.1183/09031936.00223113
3. Seemungal T, Harper-Owen R, Bhowmik A, et al. Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164(9):1618-1623. doi:10.1164/ajrccm.164.9.2105011
4. Rohde G, Wiethege A, Borg I, et al. Respiratory viruses in exacerbations of chronic obstructive pulmonary disease requiring hospitalisation: a case-control study. Thorax. 2003;58(1):37-42. doi:10.1136/thorax.58.1.37
5. Bremmer DN, Moffa MA, Ma K, et al. Acute exacerbations of chronic obstructive pulmonary disease with a low procalcitonin concentration: impact of antibiotic therapy. Clin Infect Dis. 2019;68(5):725-730. doi:10.1093/cid/ciy552
6. Mathioudakis AG, Chatzimavridou-Grigoriadou V, Corlateanu A, Vestbo J. Procalcitonin to guide antibiotic administration in COPD exacerbations: a meta-analysis. Eur Respir Rev. 2017;26(143):160073. Published 2017 Jan 31. doi:10.1183/16000617.0073-2016
7. van der Does Y, Rood PP, Haagsma JA, Patka P, van Gorp EC, Limper M. Procalcitonin-guided therapy for the initiation of antibiotics in the ED: a systematic review. Am J Emerg Med. 2016;34(7):1286-1293. doi:10.1016/j.ajem.2016.03.065
8. Huang DT, Yealy DM, Filbin MR, et al. Procalcitonin-guided use of antibiotics for lower respiratory tract infection. N Engl J Med. 2018;379(3):236-249. doi:10.1056/NEJMoa1802670
9. Picart J, Moiton MP, Gaüzère BA, Gazaille V, Combes X, DiBernardo S. Introduction of a PCT-based algorithm to guide antibiotic prescription in COPD exacerbation. Med Mal Infect. 2016;46(8):429-435. doi:10.1016/j.medmal.2016.07.008
10. Schuetz P, Chiappa V, Briel M, Greenwald JL. Procalcitonin algorithms for antibiotic therapy decisions: a systematic review of randomized controlled trials and recommendations for clinical algorithms. Arch Intern Med. 2011;171(15):1322-1331. doi:10.1001/archinternmed.2011.318
11. Butler CC, Gillespie D, White P, et al. C-reactive protein testing to guide antibiotic prescribing for COPD exacerbations. N Engl J Med. 2019;381(2):111-120. |doi:10.1056/NEJMoa1803185
12. Vollenweider DJ, Frei A, Steurer-Stey CA, Garcia-Aymerich J, Puhan MA. Antibiotics for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2018;10(10):CD010257. Published 2018 Oct 29. doi:10.1002/14651858.CD010257.pub2
13. Centers for Disease Control and Prevention. Interim clinical guidance for management of patients with confirmed coronavirus disease (COVID-19). Updated February 16, 2021. Accessed May 14, 2021. https://www.cdc.gov/coronavirus/2019ncov/hcp/clinical-guidance-management-patients.html
14. Gaarslev C, Yee M, Chan G, Fletcher-Lartey S, Khan R. A mixed methods study to understand patient expectations for antibiotics for an upper respiratory tract infection. Antimicrob Resist Infect Control. 2016;5:39. Published 2016 Oct 20. doi:10.1186/s13756-016-0134-3
1. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease: 2020 report. Accessd June 2, 2021. http://www.goldcopd.org/wp-content/uploads/2019/12/GOLD-2020-FINAL-ver1.2-03Dec19_WMV.pdf
2. George SN, Garcha DS, Mackay AJ, et al. Human rhinovirus infection during naturally occurring COPD exacerbations. Eur Respir J. 2014;44(1):87-96. doi:10.1183/09031936.00223113
3. Seemungal T, Harper-Owen R, Bhowmik A, et al. Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164(9):1618-1623. doi:10.1164/ajrccm.164.9.2105011
4. Rohde G, Wiethege A, Borg I, et al. Respiratory viruses in exacerbations of chronic obstructive pulmonary disease requiring hospitalisation: a case-control study. Thorax. 2003;58(1):37-42. doi:10.1136/thorax.58.1.37
5. Bremmer DN, Moffa MA, Ma K, et al. Acute exacerbations of chronic obstructive pulmonary disease with a low procalcitonin concentration: impact of antibiotic therapy. Clin Infect Dis. 2019;68(5):725-730. doi:10.1093/cid/ciy552
6. Mathioudakis AG, Chatzimavridou-Grigoriadou V, Corlateanu A, Vestbo J. Procalcitonin to guide antibiotic administration in COPD exacerbations: a meta-analysis. Eur Respir Rev. 2017;26(143):160073. Published 2017 Jan 31. doi:10.1183/16000617.0073-2016
7. van der Does Y, Rood PP, Haagsma JA, Patka P, van Gorp EC, Limper M. Procalcitonin-guided therapy for the initiation of antibiotics in the ED: a systematic review. Am J Emerg Med. 2016;34(7):1286-1293. doi:10.1016/j.ajem.2016.03.065
8. Huang DT, Yealy DM, Filbin MR, et al. Procalcitonin-guided use of antibiotics for lower respiratory tract infection. N Engl J Med. 2018;379(3):236-249. doi:10.1056/NEJMoa1802670
9. Picart J, Moiton MP, Gaüzère BA, Gazaille V, Combes X, DiBernardo S. Introduction of a PCT-based algorithm to guide antibiotic prescription in COPD exacerbation. Med Mal Infect. 2016;46(8):429-435. doi:10.1016/j.medmal.2016.07.008
10. Schuetz P, Chiappa V, Briel M, Greenwald JL. Procalcitonin algorithms for antibiotic therapy decisions: a systematic review of randomized controlled trials and recommendations for clinical algorithms. Arch Intern Med. 2011;171(15):1322-1331. doi:10.1001/archinternmed.2011.318
11. Butler CC, Gillespie D, White P, et al. C-reactive protein testing to guide antibiotic prescribing for COPD exacerbations. N Engl J Med. 2019;381(2):111-120. |doi:10.1056/NEJMoa1803185
12. Vollenweider DJ, Frei A, Steurer-Stey CA, Garcia-Aymerich J, Puhan MA. Antibiotics for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2018;10(10):CD010257. Published 2018 Oct 29. doi:10.1002/14651858.CD010257.pub2
13. Centers for Disease Control and Prevention. Interim clinical guidance for management of patients with confirmed coronavirus disease (COVID-19). Updated February 16, 2021. Accessed May 14, 2021. https://www.cdc.gov/coronavirus/2019ncov/hcp/clinical-guidance-management-patients.html
14. Gaarslev C, Yee M, Chan G, Fletcher-Lartey S, Khan R. A mixed methods study to understand patient expectations for antibiotics for an upper respiratory tract infection. Antimicrob Resist Infect Control. 2016;5:39. Published 2016 Oct 20. doi:10.1186/s13756-016-0134-3
NIAID advances universal flu vaccine candidate into phase 1 trial
Last month, U.S. government researchers began a test of an experimental influenza vaccine that they hope will provide long-lasting immunity against multiple strains of the virus. Their project adds to the many approaches that have been tried in the decades-long quest for a universal flu shot.
For the first time, the National Institute of Allergy and Infectious Diseases (NIAID) is testing an investigational flu vaccine, known as FluMos-v1, on people. Researchers in recent years have targeted the stalk or stem of an influenza surface protein called hemagglutinin (HA) in trying to develop better flu vaccines. NIAID said FluMos-v1 is designed to spark production of antibodies against the HA protein from different virus strains, which could make it superior to vaccines now available, NIAID said.
“It could be longer lasting than the traditional flu vaccine and give us what we call super seasonal protection that might go beyond just one flu season to next year’s or the year after, or offer additional protection in a pandemic setting,” Alicia T. Widge, MD, of NIAID’s Vaccine Research Center, who is the principal investigator of the trial, said in an interview.
The phase 1 study (NCT04896086) aims to enroll 35 participants, 15 of whom will receive a single intramuscular injection of a comparator treatment, Flucelvax, which has already been approved by the U.S. Food and Drug Administration. The FluMos-v1 group will start with five participants who will receive one 20-μg dose. If no safety problems emerge at that dosage, another 15 volunteers will receive one 60-μg dose of the investigational vaccine.
The incorporation of a comparator group in the phase 1 study may help investigators get an early idea of how well FluMos-v1 compares to a marketed product, Dr. Widge said. The test will be carried out through the National Institutes of Health Clinical Center.
‘Renaissance’ of flu-vaccine research?
Currently, flu vaccines are reformulated each year in an attempt to match the dominant strain for the upcoming season, an effort that often falls notably short. The estimated vaccine effectiveness rate in the United States has ranged from a low of 19% to a high of 60% in recent years, according to the Centers for Disease Control and Prevention.
Scientists have been working for decades on a universal flu vaccine that would offer better results but haven’t yet identified the right strategy to outwit mutations in the virus. Recent setbacks include BiondVax Pharmaceuticals’ October 2020 announcement of a failed phase 3 trial of its experimental M-001 universal flu vaccine candidate.
But advances in understanding the immune system may set the stage for a “renaissance” in efforts to develop a universal flu vaccine, Michael Osterholm, PhD, MPH, director of the Center for Infectious Disease Research and Policy at the University of Minnesota, said in an interview.
The COVID-19 pandemic has spurred greater interest in the need to develop a universal flu vaccine, he said. Dr. Osterholm said he is “more optimistic now than ever” about the chances for developing vaccines that can fend off multiple strains over longer periods, although the goal of a shot that can ward off influenza in all cases may remain elusive.
“How good can we make them? Will they ever be really universal? Will they have long periods of protection? I don’t think any of us know that yet,” Dr. Osterholm said. “But this is not the influenza vaccine world of 5 or 7 years ago.”
The mRNA technology used to develop the world’s first approved COVID-19 vaccines, for example, may be applied against influenza, Dr. Osterholm said.
In January 2021, Moderna announced plans to test three development candidates for a seasonal influenza vaccine and aims to start a phase 1 study this year. In an April interview on CNBC’s Squawk Box program, Moderna’s chief executive, Stephané Bancel, spoke about the company’s plans to eventually create a combination vaccine for SARS-Cov-2 and flu viruses.
SARS-CoV-2 “is not going away.” Like flu, this virus will persist and change forms, Ms. Bancel said. Creating a flu shot that outperforms the existing ones would boost confidence in influenza vaccines, which many people now skip, Ms. Bancel said. People might someday be able to get a combination of this more effective flu shot with a COVID-19 vaccine booster in their local pharmacies.
“You can take one dose and then have a nice winter,” Ms. Bancel said of Moderna’s goal for a combination vaccine.
A version of this article first appeared on Medscape.com.
Last month, U.S. government researchers began a test of an experimental influenza vaccine that they hope will provide long-lasting immunity against multiple strains of the virus. Their project adds to the many approaches that have been tried in the decades-long quest for a universal flu shot.
For the first time, the National Institute of Allergy and Infectious Diseases (NIAID) is testing an investigational flu vaccine, known as FluMos-v1, on people. Researchers in recent years have targeted the stalk or stem of an influenza surface protein called hemagglutinin (HA) in trying to develop better flu vaccines. NIAID said FluMos-v1 is designed to spark production of antibodies against the HA protein from different virus strains, which could make it superior to vaccines now available, NIAID said.
“It could be longer lasting than the traditional flu vaccine and give us what we call super seasonal protection that might go beyond just one flu season to next year’s or the year after, or offer additional protection in a pandemic setting,” Alicia T. Widge, MD, of NIAID’s Vaccine Research Center, who is the principal investigator of the trial, said in an interview.
The phase 1 study (NCT04896086) aims to enroll 35 participants, 15 of whom will receive a single intramuscular injection of a comparator treatment, Flucelvax, which has already been approved by the U.S. Food and Drug Administration. The FluMos-v1 group will start with five participants who will receive one 20-μg dose. If no safety problems emerge at that dosage, another 15 volunteers will receive one 60-μg dose of the investigational vaccine.
The incorporation of a comparator group in the phase 1 study may help investigators get an early idea of how well FluMos-v1 compares to a marketed product, Dr. Widge said. The test will be carried out through the National Institutes of Health Clinical Center.
‘Renaissance’ of flu-vaccine research?
Currently, flu vaccines are reformulated each year in an attempt to match the dominant strain for the upcoming season, an effort that often falls notably short. The estimated vaccine effectiveness rate in the United States has ranged from a low of 19% to a high of 60% in recent years, according to the Centers for Disease Control and Prevention.
Scientists have been working for decades on a universal flu vaccine that would offer better results but haven’t yet identified the right strategy to outwit mutations in the virus. Recent setbacks include BiondVax Pharmaceuticals’ October 2020 announcement of a failed phase 3 trial of its experimental M-001 universal flu vaccine candidate.
But advances in understanding the immune system may set the stage for a “renaissance” in efforts to develop a universal flu vaccine, Michael Osterholm, PhD, MPH, director of the Center for Infectious Disease Research and Policy at the University of Minnesota, said in an interview.
The COVID-19 pandemic has spurred greater interest in the need to develop a universal flu vaccine, he said. Dr. Osterholm said he is “more optimistic now than ever” about the chances for developing vaccines that can fend off multiple strains over longer periods, although the goal of a shot that can ward off influenza in all cases may remain elusive.
“How good can we make them? Will they ever be really universal? Will they have long periods of protection? I don’t think any of us know that yet,” Dr. Osterholm said. “But this is not the influenza vaccine world of 5 or 7 years ago.”
The mRNA technology used to develop the world’s first approved COVID-19 vaccines, for example, may be applied against influenza, Dr. Osterholm said.
In January 2021, Moderna announced plans to test three development candidates for a seasonal influenza vaccine and aims to start a phase 1 study this year. In an April interview on CNBC’s Squawk Box program, Moderna’s chief executive, Stephané Bancel, spoke about the company’s plans to eventually create a combination vaccine for SARS-Cov-2 and flu viruses.
SARS-CoV-2 “is not going away.” Like flu, this virus will persist and change forms, Ms. Bancel said. Creating a flu shot that outperforms the existing ones would boost confidence in influenza vaccines, which many people now skip, Ms. Bancel said. People might someday be able to get a combination of this more effective flu shot with a COVID-19 vaccine booster in their local pharmacies.
“You can take one dose and then have a nice winter,” Ms. Bancel said of Moderna’s goal for a combination vaccine.
A version of this article first appeared on Medscape.com.
Last month, U.S. government researchers began a test of an experimental influenza vaccine that they hope will provide long-lasting immunity against multiple strains of the virus. Their project adds to the many approaches that have been tried in the decades-long quest for a universal flu shot.
For the first time, the National Institute of Allergy and Infectious Diseases (NIAID) is testing an investigational flu vaccine, known as FluMos-v1, on people. Researchers in recent years have targeted the stalk or stem of an influenza surface protein called hemagglutinin (HA) in trying to develop better flu vaccines. NIAID said FluMos-v1 is designed to spark production of antibodies against the HA protein from different virus strains, which could make it superior to vaccines now available, NIAID said.
“It could be longer lasting than the traditional flu vaccine and give us what we call super seasonal protection that might go beyond just one flu season to next year’s or the year after, or offer additional protection in a pandemic setting,” Alicia T. Widge, MD, of NIAID’s Vaccine Research Center, who is the principal investigator of the trial, said in an interview.
The phase 1 study (NCT04896086) aims to enroll 35 participants, 15 of whom will receive a single intramuscular injection of a comparator treatment, Flucelvax, which has already been approved by the U.S. Food and Drug Administration. The FluMos-v1 group will start with five participants who will receive one 20-μg dose. If no safety problems emerge at that dosage, another 15 volunteers will receive one 60-μg dose of the investigational vaccine.
The incorporation of a comparator group in the phase 1 study may help investigators get an early idea of how well FluMos-v1 compares to a marketed product, Dr. Widge said. The test will be carried out through the National Institutes of Health Clinical Center.
‘Renaissance’ of flu-vaccine research?
Currently, flu vaccines are reformulated each year in an attempt to match the dominant strain for the upcoming season, an effort that often falls notably short. The estimated vaccine effectiveness rate in the United States has ranged from a low of 19% to a high of 60% in recent years, according to the Centers for Disease Control and Prevention.
Scientists have been working for decades on a universal flu vaccine that would offer better results but haven’t yet identified the right strategy to outwit mutations in the virus. Recent setbacks include BiondVax Pharmaceuticals’ October 2020 announcement of a failed phase 3 trial of its experimental M-001 universal flu vaccine candidate.
But advances in understanding the immune system may set the stage for a “renaissance” in efforts to develop a universal flu vaccine, Michael Osterholm, PhD, MPH, director of the Center for Infectious Disease Research and Policy at the University of Minnesota, said in an interview.
The COVID-19 pandemic has spurred greater interest in the need to develop a universal flu vaccine, he said. Dr. Osterholm said he is “more optimistic now than ever” about the chances for developing vaccines that can fend off multiple strains over longer periods, although the goal of a shot that can ward off influenza in all cases may remain elusive.
“How good can we make them? Will they ever be really universal? Will they have long periods of protection? I don’t think any of us know that yet,” Dr. Osterholm said. “But this is not the influenza vaccine world of 5 or 7 years ago.”
The mRNA technology used to develop the world’s first approved COVID-19 vaccines, for example, may be applied against influenza, Dr. Osterholm said.
In January 2021, Moderna announced plans to test three development candidates for a seasonal influenza vaccine and aims to start a phase 1 study this year. In an April interview on CNBC’s Squawk Box program, Moderna’s chief executive, Stephané Bancel, spoke about the company’s plans to eventually create a combination vaccine for SARS-Cov-2 and flu viruses.
SARS-CoV-2 “is not going away.” Like flu, this virus will persist and change forms, Ms. Bancel said. Creating a flu shot that outperforms the existing ones would boost confidence in influenza vaccines, which many people now skip, Ms. Bancel said. People might someday be able to get a combination of this more effective flu shot with a COVID-19 vaccine booster in their local pharmacies.
“You can take one dose and then have a nice winter,” Ms. Bancel said of Moderna’s goal for a combination vaccine.
A version of this article first appeared on Medscape.com.
Novel study links air pollution to increased risk of rheumatoid arthritis flares
Pollution appears to trigger inflammation
In patients with rheumatoid arthritis, exposure to air pollution is associated with both elevated levels of C-reactive protein (CRP) and increased risk of arthritis flares, according to a novel longitudinal study presented at the annual European Congress of Rheumatology.
The data revealed “a striking association between air pollution and increased CRP levels and risk of an arthritis flare,” reported first author Giovanni Adami, MD, DSc, of the rheumatology unit at the University of Verona (Italy).
The excess risk of elevated CRP and flares began “at very low levels of exposure, even those below commonly used thresholds for risk to human health,” he added.
Study details
Researchers collected data on 888 patients with RA from numerous patient visits in the context of more than 13,000 air pollution records. The CRP levels and RA flares were evaluated in the context of air pollution monitoring that is performed on a daily basis at several sites in the city of Verona where the study was conducted. Verona is an industrial city in northern Italy that has high but variable levels of air pollution based on factory activity and weather conditions.
Patients with RA who provided clinical data for this study were matched by their proximity to specific air pollution monitoring sites. By linking CRP levels and disease activity to air pollution levels over multiple follow-up visits, the design allowed the RA study participants “to serve as their own controls,” Dr. Adami explained.
At each patient visit during the study, CRP levels were measured and disease activity assessed. Patients were considered to have elevated CRP when levels were 5 mg/L or higher. The presence of an RA flare was defined by a 1.2-point increase or more in 28-joint Disease Activity Score using CRP (DAS28-CRP).
Both the CRP level and the presence or absence of a flare were evaluated in relationship to the patient’s specific local air pollution levels in the prior 60 days.
Increased levels of CRP, a surrogate for inflammatory activity, and increased disease activity, were both associated with elevated exposure to air pollutants prior to an office visit. These associations remained statistically significant when evaluated by specific air pollutants such as carbon monoxide (CO), nitrogen oxides (NO2, NO), small particulate matter (PM10; particles ≤ 10 mcm), and ozone (O3).
The relationship between increased exposure to air pollution contaminants and elevated CRP was supported by a dose effect. In the case of PM10, for example, the odds ratio of having elevated CRP was increased by only about 25% (OR, 1.25) when mean levels were 30 mcg/m3 or lower in the period prior to the office visit. This rose incrementally for higher mean levels of PM10, reaching 70% (OR, 1.70) for levels > 50 mcg/m3.
The researchers detected statistically significant differences in mean and area-under-the curve (AUC) values of most air pollutants in the 60 days prior to office visits when patients had a flare versus when disease activity was low. For example, the difference in mean and AUC levels in the period prior to a flare relative to a period with low disease activity was significant for CO (P = .001 for both) and NO and NO2 (P = .003 for both), and O3 (P = .002 and P = .001, respectively). For PM10, P values were .011 and .005, respectively.
“Remarkably, we found that the cumulative exposure to NO2 in the 60 days preceding a flare was approximately 500 mcg/m3 higher than the low disease activity visit, an exposure that equates to approximately 200 passively smoked cigarettes,” Dr. Adami reported.
Trying to confirm causality of association
Dr. Adami’s study is not the first study to link air pollution to risk of RA. Several have suggested that air pollution is a risk factor for developing joint disease, but a recently published study conducted in Kuwait associated greater disease activity with NO2 and another air pollutant, sulfur dioxide (SO2), although not CO, PM10, or O3.
A coauthor of that study, which evaluated pollution in regard to disease activity on DAS score, Adeeba Al-Herz, MD, a rheumatology consultant at Al-Amiri Hospital, Kuwait City, said in an interview, “We proved the correlation between them but not the causality.”
However, she believes that this is an important area of inquiry.
“We are working now on another paper in which we studied a causal relationship between the two, meaning that we are evaluating whether SO2 and NO2 trigger RA activity,” Dr. Al-Herz said. That study is now complete, and the manuscript is being written.
The magnitude of the association in these two studies suggest that there might be a clinical message if causality can be confirmed, according to Dr. Adami. Although there are many reasons to seek to reduce and avoid air pollution, these data suggest risk of a proinflammatory state might be one of them.
Dr. Adami believes that the evidence of an adverse effect on patients with RA is strong.
“In order to reduce the burden of RA, public and environmental health policy makers should aim to diminish gaseous and particulate matter emissions to a larger extent than currently recommended,” he said.
In an interview after his presentation, Dr. Adami suggested that the risk of an inflammatory response and increases in arthritis flares from air pollution is not surprising. Previous studies have linked cigarette smoking to both.
“The mechanisms underlying the development of inflammation are very similar. Indeed, the toxic components contained in cigarette smoking are largely shared with diesel exhaust and fossil fuel combustion,” he said.
Although causality between air pollution and arthritis flares cannot be confirmed in these data, a basis for suspecting a causal relationship is supported by “plenty of in vitro and animal studies,” according to Dr. Adami.
On the basis of these studies, several mechanisms have been postulated.
“As an example, exposure to air pollution can promote the activation of the bronchus-associated lymphoid tissue (BALT), which can trigger the activation of the transcription factor nuclear factor-kappaB,” he said. This, in turn, can “lead to the secretion of proinflammatory cytokines, such as tumor necrosis factor–alpha and interleukin-1.”
Another theory is that posttranslational modification of proteins in the lung, a process called citrullination, “can lead to production of autoantibodies known to have a pathogenic role in RA,” he added.
Proving a causal relationship, however, is difficult.
“We certainly cannot conduct a randomized clinical trial on that and voluntarily expose some patients to pollution. Thus, we need to rely on observational data,” Dr. Adami said.
Of strategies being considered to generate evidence of a causal relationship between pollution and the exacerbation of RA, “we certainly will try to study those patients that move from a highly polluted area to a greener zone and vice versa,” he said. This will allow us “to explore what happens when the exposure to pollution changes dramatically in a short period of time.”
In the meantime, “given what is known to date, I would certainly advise my RA patients to avoid exposure to air pollution,” Dr. Adami said. He acknowledged there is no proof that this will help patients to reduce the risk of flares, but there are already many good reasons to minimize exposure to air pollution.
Dr. Adami and Dr. Al-Herz report no potential conflicts of interest.
Pollution appears to trigger inflammation
Pollution appears to trigger inflammation
In patients with rheumatoid arthritis, exposure to air pollution is associated with both elevated levels of C-reactive protein (CRP) and increased risk of arthritis flares, according to a novel longitudinal study presented at the annual European Congress of Rheumatology.
The data revealed “a striking association between air pollution and increased CRP levels and risk of an arthritis flare,” reported first author Giovanni Adami, MD, DSc, of the rheumatology unit at the University of Verona (Italy).
The excess risk of elevated CRP and flares began “at very low levels of exposure, even those below commonly used thresholds for risk to human health,” he added.
Study details
Researchers collected data on 888 patients with RA from numerous patient visits in the context of more than 13,000 air pollution records. The CRP levels and RA flares were evaluated in the context of air pollution monitoring that is performed on a daily basis at several sites in the city of Verona where the study was conducted. Verona is an industrial city in northern Italy that has high but variable levels of air pollution based on factory activity and weather conditions.
Patients with RA who provided clinical data for this study were matched by their proximity to specific air pollution monitoring sites. By linking CRP levels and disease activity to air pollution levels over multiple follow-up visits, the design allowed the RA study participants “to serve as their own controls,” Dr. Adami explained.
At each patient visit during the study, CRP levels were measured and disease activity assessed. Patients were considered to have elevated CRP when levels were 5 mg/L or higher. The presence of an RA flare was defined by a 1.2-point increase or more in 28-joint Disease Activity Score using CRP (DAS28-CRP).
Both the CRP level and the presence or absence of a flare were evaluated in relationship to the patient’s specific local air pollution levels in the prior 60 days.
Increased levels of CRP, a surrogate for inflammatory activity, and increased disease activity, were both associated with elevated exposure to air pollutants prior to an office visit. These associations remained statistically significant when evaluated by specific air pollutants such as carbon monoxide (CO), nitrogen oxides (NO2, NO), small particulate matter (PM10; particles ≤ 10 mcm), and ozone (O3).
The relationship between increased exposure to air pollution contaminants and elevated CRP was supported by a dose effect. In the case of PM10, for example, the odds ratio of having elevated CRP was increased by only about 25% (OR, 1.25) when mean levels were 30 mcg/m3 or lower in the period prior to the office visit. This rose incrementally for higher mean levels of PM10, reaching 70% (OR, 1.70) for levels > 50 mcg/m3.
The researchers detected statistically significant differences in mean and area-under-the curve (AUC) values of most air pollutants in the 60 days prior to office visits when patients had a flare versus when disease activity was low. For example, the difference in mean and AUC levels in the period prior to a flare relative to a period with low disease activity was significant for CO (P = .001 for both) and NO and NO2 (P = .003 for both), and O3 (P = .002 and P = .001, respectively). For PM10, P values were .011 and .005, respectively.
“Remarkably, we found that the cumulative exposure to NO2 in the 60 days preceding a flare was approximately 500 mcg/m3 higher than the low disease activity visit, an exposure that equates to approximately 200 passively smoked cigarettes,” Dr. Adami reported.
Trying to confirm causality of association
Dr. Adami’s study is not the first study to link air pollution to risk of RA. Several have suggested that air pollution is a risk factor for developing joint disease, but a recently published study conducted in Kuwait associated greater disease activity with NO2 and another air pollutant, sulfur dioxide (SO2), although not CO, PM10, or O3.
A coauthor of that study, which evaluated pollution in regard to disease activity on DAS score, Adeeba Al-Herz, MD, a rheumatology consultant at Al-Amiri Hospital, Kuwait City, said in an interview, “We proved the correlation between them but not the causality.”
However, she believes that this is an important area of inquiry.
“We are working now on another paper in which we studied a causal relationship between the two, meaning that we are evaluating whether SO2 and NO2 trigger RA activity,” Dr. Al-Herz said. That study is now complete, and the manuscript is being written.
The magnitude of the association in these two studies suggest that there might be a clinical message if causality can be confirmed, according to Dr. Adami. Although there are many reasons to seek to reduce and avoid air pollution, these data suggest risk of a proinflammatory state might be one of them.
Dr. Adami believes that the evidence of an adverse effect on patients with RA is strong.
“In order to reduce the burden of RA, public and environmental health policy makers should aim to diminish gaseous and particulate matter emissions to a larger extent than currently recommended,” he said.
In an interview after his presentation, Dr. Adami suggested that the risk of an inflammatory response and increases in arthritis flares from air pollution is not surprising. Previous studies have linked cigarette smoking to both.
“The mechanisms underlying the development of inflammation are very similar. Indeed, the toxic components contained in cigarette smoking are largely shared with diesel exhaust and fossil fuel combustion,” he said.
Although causality between air pollution and arthritis flares cannot be confirmed in these data, a basis for suspecting a causal relationship is supported by “plenty of in vitro and animal studies,” according to Dr. Adami.
On the basis of these studies, several mechanisms have been postulated.
“As an example, exposure to air pollution can promote the activation of the bronchus-associated lymphoid tissue (BALT), which can trigger the activation of the transcription factor nuclear factor-kappaB,” he said. This, in turn, can “lead to the secretion of proinflammatory cytokines, such as tumor necrosis factor–alpha and interleukin-1.”
Another theory is that posttranslational modification of proteins in the lung, a process called citrullination, “can lead to production of autoantibodies known to have a pathogenic role in RA,” he added.
Proving a causal relationship, however, is difficult.
“We certainly cannot conduct a randomized clinical trial on that and voluntarily expose some patients to pollution. Thus, we need to rely on observational data,” Dr. Adami said.
Of strategies being considered to generate evidence of a causal relationship between pollution and the exacerbation of RA, “we certainly will try to study those patients that move from a highly polluted area to a greener zone and vice versa,” he said. This will allow us “to explore what happens when the exposure to pollution changes dramatically in a short period of time.”
In the meantime, “given what is known to date, I would certainly advise my RA patients to avoid exposure to air pollution,” Dr. Adami said. He acknowledged there is no proof that this will help patients to reduce the risk of flares, but there are already many good reasons to minimize exposure to air pollution.
Dr. Adami and Dr. Al-Herz report no potential conflicts of interest.
In patients with rheumatoid arthritis, exposure to air pollution is associated with both elevated levels of C-reactive protein (CRP) and increased risk of arthritis flares, according to a novel longitudinal study presented at the annual European Congress of Rheumatology.
The data revealed “a striking association between air pollution and increased CRP levels and risk of an arthritis flare,” reported first author Giovanni Adami, MD, DSc, of the rheumatology unit at the University of Verona (Italy).
The excess risk of elevated CRP and flares began “at very low levels of exposure, even those below commonly used thresholds for risk to human health,” he added.
Study details
Researchers collected data on 888 patients with RA from numerous patient visits in the context of more than 13,000 air pollution records. The CRP levels and RA flares were evaluated in the context of air pollution monitoring that is performed on a daily basis at several sites in the city of Verona where the study was conducted. Verona is an industrial city in northern Italy that has high but variable levels of air pollution based on factory activity and weather conditions.
Patients with RA who provided clinical data for this study were matched by their proximity to specific air pollution monitoring sites. By linking CRP levels and disease activity to air pollution levels over multiple follow-up visits, the design allowed the RA study participants “to serve as their own controls,” Dr. Adami explained.
At each patient visit during the study, CRP levels were measured and disease activity assessed. Patients were considered to have elevated CRP when levels were 5 mg/L or higher. The presence of an RA flare was defined by a 1.2-point increase or more in 28-joint Disease Activity Score using CRP (DAS28-CRP).
Both the CRP level and the presence or absence of a flare were evaluated in relationship to the patient’s specific local air pollution levels in the prior 60 days.
Increased levels of CRP, a surrogate for inflammatory activity, and increased disease activity, were both associated with elevated exposure to air pollutants prior to an office visit. These associations remained statistically significant when evaluated by specific air pollutants such as carbon monoxide (CO), nitrogen oxides (NO2, NO), small particulate matter (PM10; particles ≤ 10 mcm), and ozone (O3).
The relationship between increased exposure to air pollution contaminants and elevated CRP was supported by a dose effect. In the case of PM10, for example, the odds ratio of having elevated CRP was increased by only about 25% (OR, 1.25) when mean levels were 30 mcg/m3 or lower in the period prior to the office visit. This rose incrementally for higher mean levels of PM10, reaching 70% (OR, 1.70) for levels > 50 mcg/m3.
The researchers detected statistically significant differences in mean and area-under-the curve (AUC) values of most air pollutants in the 60 days prior to office visits when patients had a flare versus when disease activity was low. For example, the difference in mean and AUC levels in the period prior to a flare relative to a period with low disease activity was significant for CO (P = .001 for both) and NO and NO2 (P = .003 for both), and O3 (P = .002 and P = .001, respectively). For PM10, P values were .011 and .005, respectively.
“Remarkably, we found that the cumulative exposure to NO2 in the 60 days preceding a flare was approximately 500 mcg/m3 higher than the low disease activity visit, an exposure that equates to approximately 200 passively smoked cigarettes,” Dr. Adami reported.
Trying to confirm causality of association
Dr. Adami’s study is not the first study to link air pollution to risk of RA. Several have suggested that air pollution is a risk factor for developing joint disease, but a recently published study conducted in Kuwait associated greater disease activity with NO2 and another air pollutant, sulfur dioxide (SO2), although not CO, PM10, or O3.
A coauthor of that study, which evaluated pollution in regard to disease activity on DAS score, Adeeba Al-Herz, MD, a rheumatology consultant at Al-Amiri Hospital, Kuwait City, said in an interview, “We proved the correlation between them but not the causality.”
However, she believes that this is an important area of inquiry.
“We are working now on another paper in which we studied a causal relationship between the two, meaning that we are evaluating whether SO2 and NO2 trigger RA activity,” Dr. Al-Herz said. That study is now complete, and the manuscript is being written.
The magnitude of the association in these two studies suggest that there might be a clinical message if causality can be confirmed, according to Dr. Adami. Although there are many reasons to seek to reduce and avoid air pollution, these data suggest risk of a proinflammatory state might be one of them.
Dr. Adami believes that the evidence of an adverse effect on patients with RA is strong.
“In order to reduce the burden of RA, public and environmental health policy makers should aim to diminish gaseous and particulate matter emissions to a larger extent than currently recommended,” he said.
In an interview after his presentation, Dr. Adami suggested that the risk of an inflammatory response and increases in arthritis flares from air pollution is not surprising. Previous studies have linked cigarette smoking to both.
“The mechanisms underlying the development of inflammation are very similar. Indeed, the toxic components contained in cigarette smoking are largely shared with diesel exhaust and fossil fuel combustion,” he said.
Although causality between air pollution and arthritis flares cannot be confirmed in these data, a basis for suspecting a causal relationship is supported by “plenty of in vitro and animal studies,” according to Dr. Adami.
On the basis of these studies, several mechanisms have been postulated.
“As an example, exposure to air pollution can promote the activation of the bronchus-associated lymphoid tissue (BALT), which can trigger the activation of the transcription factor nuclear factor-kappaB,” he said. This, in turn, can “lead to the secretion of proinflammatory cytokines, such as tumor necrosis factor–alpha and interleukin-1.”
Another theory is that posttranslational modification of proteins in the lung, a process called citrullination, “can lead to production of autoantibodies known to have a pathogenic role in RA,” he added.
Proving a causal relationship, however, is difficult.
“We certainly cannot conduct a randomized clinical trial on that and voluntarily expose some patients to pollution. Thus, we need to rely on observational data,” Dr. Adami said.
Of strategies being considered to generate evidence of a causal relationship between pollution and the exacerbation of RA, “we certainly will try to study those patients that move from a highly polluted area to a greener zone and vice versa,” he said. This will allow us “to explore what happens when the exposure to pollution changes dramatically in a short period of time.”
In the meantime, “given what is known to date, I would certainly advise my RA patients to avoid exposure to air pollution,” Dr. Adami said. He acknowledged there is no proof that this will help patients to reduce the risk of flares, but there are already many good reasons to minimize exposure to air pollution.
Dr. Adami and Dr. Al-Herz report no potential conflicts of interest.
FROM THE EULAR 2021 CONGRESS
Collaborative effort reduces COPD readmissions, costs
Medicare exacts a penalty whenever it deems that hospitals have too many patients with chronic obstructive pulmonary disease who have been re-admitted within 30 days of discharge for care related to the disease. For acute-care hospitals the solution to reducing chronic obstructive pulmonary disease (COPD) re-admissions has been elusive, but members of a COPD chronic care management collaborative think they have found at least a partial solution.
Among 33 centers participating in the performance improvement program, the aggregated cost avoidance for emergency department (ED) visits was estimated at $351,000, and the savings for hospital re-visits avoided was an estimated $2.6 million, reported Valerie Press, MD, MPH, from the University of Chicago, and co-authors from the health care performance-improvement company Vizient.
The investigators described their chronic care management collaborative in a thematic poster presented during the American Thoracic Society’s virtual international conference (Abstract A1688).
“I’ve been working in the space of COPD re-admissions pretty much since Medicare started its penalty program,” Dr. Press said in an interview.
“At both my own institution and nationally, we’ve been trying to understand the policy that went into place to reduce what was considered to be excessive readmissions after a COPD admission, but there really wasn’t a lot of evidence to suggest how to do this at the time the policy went into place,” she said.
The Centers for Medicare & Medicaid Services (CMS) initiated its Hospital Readmission Reduction Program for COPD in 2014.
“The challenge with COPD is that we have not found really successful interventions to decrease readmissions,” commented Laura C. Myers, MD, MPH, in an interview. Dr. Myers, who studies optimal care delivery models for patients with COPD at Kaiser Permanente Northern California in Oakland, was not involved in the study.
She said that although the aggregate cost savings in the study by Dr. Press and colleagues are relatively modest, “if you extrapolate across the country, then those numbers could potentially be impressive.”
Collaboration details
Dr. Press was a subject matter expert for the collaborative, which included 47 Vizient member sites in the Southeast, Southwest, Midwest, and Northeast and Northwest coasts. Of these centers, 33 completed both parts of the collaboration.
The program included bi-monthly didactic sessions and site report and discussion sessions with peer-to-peer networking for a total of 6 months. During the sessions, meeting participants discussed best practices, received expert coaching, and provided progress updates on performance improvement projects.
“The goal was for them to identify the gaps or needs they had at their hospitals or practices, and then to try to put in place one or more interventions,” Dr. Press said. “This wasn’t a research program. It wasn’t standardized, and not all hospitals had to do the same program.”
The participants submitted reports for baseline and post-collaboration periods on both an intervention’s “reach,” defined as the percentage of patients who received a specified intervention, and on two outcome measures.
The interventions measured included spirometry, follow-up visits scheduled within 7 to 14 days of discharge, patients receiving COPD education, pulmonary referrals, and adherence to the COPD clinical pathway.
The outcome measures were the rate of COPD-related ED visits and hospital readmissions.
Revisits reduced
At the end of the program, 83% of participating sites had reductions in either ED visits or readmissions, and of this group, five sites had decreases in both measures.
Among all sites with improved metrics, the average rate of COPD-related ED revisits declined from 12.7% to 9%, and average inpatient readmissions declined from 20.1% to 15.6%.
As noted, the estimated cost savings in ED revisits avoided was $351,00, and the estimated savings in hospital readmission costs was $2.6 million.
“Although the centers didn’t have to participate in both parts, we did see in our results that the programs that participated fully had better results,” Dr. Press said.
“Historically, we’ve had such difficulty in decreasing COPD readmissions, and it’s nice to see something that actually works, both for patients and for conserving health resources,” Dr. Myers commented.
The study was supported by Vizient. Dr. Press disclosed honoraria from the company in her role as subject matter expert. Dr. Myers reported no conflicts of interest.
Medicare exacts a penalty whenever it deems that hospitals have too many patients with chronic obstructive pulmonary disease who have been re-admitted within 30 days of discharge for care related to the disease. For acute-care hospitals the solution to reducing chronic obstructive pulmonary disease (COPD) re-admissions has been elusive, but members of a COPD chronic care management collaborative think they have found at least a partial solution.
Among 33 centers participating in the performance improvement program, the aggregated cost avoidance for emergency department (ED) visits was estimated at $351,000, and the savings for hospital re-visits avoided was an estimated $2.6 million, reported Valerie Press, MD, MPH, from the University of Chicago, and co-authors from the health care performance-improvement company Vizient.
The investigators described their chronic care management collaborative in a thematic poster presented during the American Thoracic Society’s virtual international conference (Abstract A1688).
“I’ve been working in the space of COPD re-admissions pretty much since Medicare started its penalty program,” Dr. Press said in an interview.
“At both my own institution and nationally, we’ve been trying to understand the policy that went into place to reduce what was considered to be excessive readmissions after a COPD admission, but there really wasn’t a lot of evidence to suggest how to do this at the time the policy went into place,” she said.
The Centers for Medicare & Medicaid Services (CMS) initiated its Hospital Readmission Reduction Program for COPD in 2014.
“The challenge with COPD is that we have not found really successful interventions to decrease readmissions,” commented Laura C. Myers, MD, MPH, in an interview. Dr. Myers, who studies optimal care delivery models for patients with COPD at Kaiser Permanente Northern California in Oakland, was not involved in the study.
She said that although the aggregate cost savings in the study by Dr. Press and colleagues are relatively modest, “if you extrapolate across the country, then those numbers could potentially be impressive.”
Collaboration details
Dr. Press was a subject matter expert for the collaborative, which included 47 Vizient member sites in the Southeast, Southwest, Midwest, and Northeast and Northwest coasts. Of these centers, 33 completed both parts of the collaboration.
The program included bi-monthly didactic sessions and site report and discussion sessions with peer-to-peer networking for a total of 6 months. During the sessions, meeting participants discussed best practices, received expert coaching, and provided progress updates on performance improvement projects.
“The goal was for them to identify the gaps or needs they had at their hospitals or practices, and then to try to put in place one or more interventions,” Dr. Press said. “This wasn’t a research program. It wasn’t standardized, and not all hospitals had to do the same program.”
The participants submitted reports for baseline and post-collaboration periods on both an intervention’s “reach,” defined as the percentage of patients who received a specified intervention, and on two outcome measures.
The interventions measured included spirometry, follow-up visits scheduled within 7 to 14 days of discharge, patients receiving COPD education, pulmonary referrals, and adherence to the COPD clinical pathway.
The outcome measures were the rate of COPD-related ED visits and hospital readmissions.
Revisits reduced
At the end of the program, 83% of participating sites had reductions in either ED visits or readmissions, and of this group, five sites had decreases in both measures.
Among all sites with improved metrics, the average rate of COPD-related ED revisits declined from 12.7% to 9%, and average inpatient readmissions declined from 20.1% to 15.6%.
As noted, the estimated cost savings in ED revisits avoided was $351,00, and the estimated savings in hospital readmission costs was $2.6 million.
“Although the centers didn’t have to participate in both parts, we did see in our results that the programs that participated fully had better results,” Dr. Press said.
“Historically, we’ve had such difficulty in decreasing COPD readmissions, and it’s nice to see something that actually works, both for patients and for conserving health resources,” Dr. Myers commented.
The study was supported by Vizient. Dr. Press disclosed honoraria from the company in her role as subject matter expert. Dr. Myers reported no conflicts of interest.
Medicare exacts a penalty whenever it deems that hospitals have too many patients with chronic obstructive pulmonary disease who have been re-admitted within 30 days of discharge for care related to the disease. For acute-care hospitals the solution to reducing chronic obstructive pulmonary disease (COPD) re-admissions has been elusive, but members of a COPD chronic care management collaborative think they have found at least a partial solution.
Among 33 centers participating in the performance improvement program, the aggregated cost avoidance for emergency department (ED) visits was estimated at $351,000, and the savings for hospital re-visits avoided was an estimated $2.6 million, reported Valerie Press, MD, MPH, from the University of Chicago, and co-authors from the health care performance-improvement company Vizient.
The investigators described their chronic care management collaborative in a thematic poster presented during the American Thoracic Society’s virtual international conference (Abstract A1688).
“I’ve been working in the space of COPD re-admissions pretty much since Medicare started its penalty program,” Dr. Press said in an interview.
“At both my own institution and nationally, we’ve been trying to understand the policy that went into place to reduce what was considered to be excessive readmissions after a COPD admission, but there really wasn’t a lot of evidence to suggest how to do this at the time the policy went into place,” she said.
The Centers for Medicare & Medicaid Services (CMS) initiated its Hospital Readmission Reduction Program for COPD in 2014.
“The challenge with COPD is that we have not found really successful interventions to decrease readmissions,” commented Laura C. Myers, MD, MPH, in an interview. Dr. Myers, who studies optimal care delivery models for patients with COPD at Kaiser Permanente Northern California in Oakland, was not involved in the study.
She said that although the aggregate cost savings in the study by Dr. Press and colleagues are relatively modest, “if you extrapolate across the country, then those numbers could potentially be impressive.”
Collaboration details
Dr. Press was a subject matter expert for the collaborative, which included 47 Vizient member sites in the Southeast, Southwest, Midwest, and Northeast and Northwest coasts. Of these centers, 33 completed both parts of the collaboration.
The program included bi-monthly didactic sessions and site report and discussion sessions with peer-to-peer networking for a total of 6 months. During the sessions, meeting participants discussed best practices, received expert coaching, and provided progress updates on performance improvement projects.
“The goal was for them to identify the gaps or needs they had at their hospitals or practices, and then to try to put in place one or more interventions,” Dr. Press said. “This wasn’t a research program. It wasn’t standardized, and not all hospitals had to do the same program.”
The participants submitted reports for baseline and post-collaboration periods on both an intervention’s “reach,” defined as the percentage of patients who received a specified intervention, and on two outcome measures.
The interventions measured included spirometry, follow-up visits scheduled within 7 to 14 days of discharge, patients receiving COPD education, pulmonary referrals, and adherence to the COPD clinical pathway.
The outcome measures were the rate of COPD-related ED visits and hospital readmissions.
Revisits reduced
At the end of the program, 83% of participating sites had reductions in either ED visits or readmissions, and of this group, five sites had decreases in both measures.
Among all sites with improved metrics, the average rate of COPD-related ED revisits declined from 12.7% to 9%, and average inpatient readmissions declined from 20.1% to 15.6%.
As noted, the estimated cost savings in ED revisits avoided was $351,00, and the estimated savings in hospital readmission costs was $2.6 million.
“Although the centers didn’t have to participate in both parts, we did see in our results that the programs that participated fully had better results,” Dr. Press said.
“Historically, we’ve had such difficulty in decreasing COPD readmissions, and it’s nice to see something that actually works, both for patients and for conserving health resources,” Dr. Myers commented.
The study was supported by Vizient. Dr. Press disclosed honoraria from the company in her role as subject matter expert. Dr. Myers reported no conflicts of interest.
FROM ATS 2021
Secondhand smoke in childhood and adulthood linked to increased risk of rheumatoid arthritis
Secondhand smoke exposure in both childhood and adulthood is associated with an increased risk of rheumatoid arthritis in women, according to a study presented at the annual European Congress of Rheumatology.
“These results suggest that smoking by-products, whether actively or passively inhaled or absorbed, could generate autoimmunity, at least towards antigens involved in rheumatoid arthritis pathogenesis,” said Yann Nguyen, MD, MPH, of the center for research in epidemiology and population health at the University of Paris-Saclay in Villejuif and of Beaujon Hospital at the University of Paris in Clichy, France.
Previous research has already repeatedly implicated smoking as a risk factor for rheumatoid arthritis positive for anticitrullinated protein antibodies (ACPA), especially in those who have the HLA-DRB1-shared epitope (SE) alleles, Dr. Nguyen explained to attendees. This study looked at whether exposure to others’ smoke had any similar associations.
The researchers relied on the French prospective cohort study known as E3N-EPIC (Etude Epidémiologique auprès de femmes de la Mutuelle Générale de l’Education Nationale), which is designed to examine potential associations between environmental factors and chronic disease. Of the 98,995 healthy French women the longitudinal study has tracked since 1990, this study included 79,806 participants with an average age of 49 years. A total of 698 women developed rheumatoid arthritis during the study an average of 11.7 years after baseline.
Exposure to secondhand smoke, or passive smoking, in childhood was defined as spending several hours a day in a smoky room as a child, based on participants’ self-report. Adult exposure to passive smoking referred to women’s self-report of spending at least 1 hour a day around actively smoking adults. Researchers further stratified participants according to whether they currently smoke, have never smoked, or used to smoke. Additional covariates in the fully adjusted models included body mass index and educational level.
About one in seven of the women (13.5%) reported exposure to childhood passive smoking, and just over half (53.6%) reported passive smoking exposure as adults. Overall, 58.9% of participants had secondhand exposure in adulthood or childhood, and 8.25% had both.
A positive association existed between childhood exposure and rheumatoid arthritis in the unadjusted and adjusted models. In the fully adjusted model, the risk of rheumatoid arthritis was 1.24 times greater overall for those exposed to secondhand smoke in childhood compared with those who had no exposure. The risk was even greater, however, among women who had never smoked (hazard ratio, 1.42), and the association was not statistically significant in women who had ever smoked.
Similarly, risk of rheumatoid arthritis was greater among those women reporting exposure to passive smoking in adulthood in the unadjusted and adjusted models (HR, 1.19 after adjustment). Once again, women who had never smoked had a modestly higher increased risk (HR, 1.27) if they had secondhand smoke exposure in adulthood, but no statistically significant association existed for women who were current or former smokers.
Although research had previously shown the association between active smoking and rheumatoid arthritis, these new findings suggest clinicians need to emphasize to their patients this additional negative effect from smoking.
Dr. Nguyen, Dr. Carmona, and Dr. Schulze-Koops have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Secondhand smoke exposure in both childhood and adulthood is associated with an increased risk of rheumatoid arthritis in women, according to a study presented at the annual European Congress of Rheumatology.
“These results suggest that smoking by-products, whether actively or passively inhaled or absorbed, could generate autoimmunity, at least towards antigens involved in rheumatoid arthritis pathogenesis,” said Yann Nguyen, MD, MPH, of the center for research in epidemiology and population health at the University of Paris-Saclay in Villejuif and of Beaujon Hospital at the University of Paris in Clichy, France.
Previous research has already repeatedly implicated smoking as a risk factor for rheumatoid arthritis positive for anticitrullinated protein antibodies (ACPA), especially in those who have the HLA-DRB1-shared epitope (SE) alleles, Dr. Nguyen explained to attendees. This study looked at whether exposure to others’ smoke had any similar associations.
The researchers relied on the French prospective cohort study known as E3N-EPIC (Etude Epidémiologique auprès de femmes de la Mutuelle Générale de l’Education Nationale), which is designed to examine potential associations between environmental factors and chronic disease. Of the 98,995 healthy French women the longitudinal study has tracked since 1990, this study included 79,806 participants with an average age of 49 years. A total of 698 women developed rheumatoid arthritis during the study an average of 11.7 years after baseline.
Exposure to secondhand smoke, or passive smoking, in childhood was defined as spending several hours a day in a smoky room as a child, based on participants’ self-report. Adult exposure to passive smoking referred to women’s self-report of spending at least 1 hour a day around actively smoking adults. Researchers further stratified participants according to whether they currently smoke, have never smoked, or used to smoke. Additional covariates in the fully adjusted models included body mass index and educational level.
About one in seven of the women (13.5%) reported exposure to childhood passive smoking, and just over half (53.6%) reported passive smoking exposure as adults. Overall, 58.9% of participants had secondhand exposure in adulthood or childhood, and 8.25% had both.
A positive association existed between childhood exposure and rheumatoid arthritis in the unadjusted and adjusted models. In the fully adjusted model, the risk of rheumatoid arthritis was 1.24 times greater overall for those exposed to secondhand smoke in childhood compared with those who had no exposure. The risk was even greater, however, among women who had never smoked (hazard ratio, 1.42), and the association was not statistically significant in women who had ever smoked.
Similarly, risk of rheumatoid arthritis was greater among those women reporting exposure to passive smoking in adulthood in the unadjusted and adjusted models (HR, 1.19 after adjustment). Once again, women who had never smoked had a modestly higher increased risk (HR, 1.27) if they had secondhand smoke exposure in adulthood, but no statistically significant association existed for women who were current or former smokers.
Although research had previously shown the association between active smoking and rheumatoid arthritis, these new findings suggest clinicians need to emphasize to their patients this additional negative effect from smoking.
Dr. Nguyen, Dr. Carmona, and Dr. Schulze-Koops have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Secondhand smoke exposure in both childhood and adulthood is associated with an increased risk of rheumatoid arthritis in women, according to a study presented at the annual European Congress of Rheumatology.
“These results suggest that smoking by-products, whether actively or passively inhaled or absorbed, could generate autoimmunity, at least towards antigens involved in rheumatoid arthritis pathogenesis,” said Yann Nguyen, MD, MPH, of the center for research in epidemiology and population health at the University of Paris-Saclay in Villejuif and of Beaujon Hospital at the University of Paris in Clichy, France.
Previous research has already repeatedly implicated smoking as a risk factor for rheumatoid arthritis positive for anticitrullinated protein antibodies (ACPA), especially in those who have the HLA-DRB1-shared epitope (SE) alleles, Dr. Nguyen explained to attendees. This study looked at whether exposure to others’ smoke had any similar associations.
The researchers relied on the French prospective cohort study known as E3N-EPIC (Etude Epidémiologique auprès de femmes de la Mutuelle Générale de l’Education Nationale), which is designed to examine potential associations between environmental factors and chronic disease. Of the 98,995 healthy French women the longitudinal study has tracked since 1990, this study included 79,806 participants with an average age of 49 years. A total of 698 women developed rheumatoid arthritis during the study an average of 11.7 years after baseline.
Exposure to secondhand smoke, or passive smoking, in childhood was defined as spending several hours a day in a smoky room as a child, based on participants’ self-report. Adult exposure to passive smoking referred to women’s self-report of spending at least 1 hour a day around actively smoking adults. Researchers further stratified participants according to whether they currently smoke, have never smoked, or used to smoke. Additional covariates in the fully adjusted models included body mass index and educational level.
About one in seven of the women (13.5%) reported exposure to childhood passive smoking, and just over half (53.6%) reported passive smoking exposure as adults. Overall, 58.9% of participants had secondhand exposure in adulthood or childhood, and 8.25% had both.
A positive association existed between childhood exposure and rheumatoid arthritis in the unadjusted and adjusted models. In the fully adjusted model, the risk of rheumatoid arthritis was 1.24 times greater overall for those exposed to secondhand smoke in childhood compared with those who had no exposure. The risk was even greater, however, among women who had never smoked (hazard ratio, 1.42), and the association was not statistically significant in women who had ever smoked.
Similarly, risk of rheumatoid arthritis was greater among those women reporting exposure to passive smoking in adulthood in the unadjusted and adjusted models (HR, 1.19 after adjustment). Once again, women who had never smoked had a modestly higher increased risk (HR, 1.27) if they had secondhand smoke exposure in adulthood, but no statistically significant association existed for women who were current or former smokers.
Although research had previously shown the association between active smoking and rheumatoid arthritis, these new findings suggest clinicians need to emphasize to their patients this additional negative effect from smoking.
Dr. Nguyen, Dr. Carmona, and Dr. Schulze-Koops have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE EULAR 2021 CONGRESS
Gene variant confirmed as strong predictor of lung disease in RA
Carriers have more than twofold greater risk
Patients with rheumatoid arthritis who carry a specific allele of the gene MUC5B have about double the risk of developing interstitial lung disease when compared with noncarriers, according to a large Finnish biobank study presented at the annual European Congress of Rheumatology.
“The risk difference [or carriers relative to noncarriers] started at about age 65, with a bigger difference [for] men than women,” reported Antti Palomäki, MD, PhD, of the center for rheumatology and clinical immunology at Turku (Finland) University.
The gain-of-function MUC5B variant, which encodes mucin 5B, was first linked to RA-associated interstitial lung disease (ILD) more than 3 years ago. At that time, it was already a known genetic risk factor for idiopathic pulmonary fibrosis in the general population. The new data confirm the association in a longitudinal analysis of a large biobank and suggest the association might have clinical utility.
“This is not ready for clinical practice at the moment. We do not yet know whether we can change therapy to reduce risk,” Dr. Palomäki said, adding “in the future we can look.”
One question that might be asked in clinical studies using MUC5B as a tool to assess and modify risk of ILD in patients with RA is whether one therapy is better than another in avoiding or delaying development of lung fibrosis. Dr. Palomäki noted that biologics, for example, might be a more favorable choice in patients with RA who are at high risk of developing ILD.
The association of the MUC5B variant with increased ILD incidence in patients with RA was drawn from a data set known as FinnGen, a biobank collection of epidemiologic cohorts and hospital samples with genotypes of about 10% of the Finnish population. Follow-up extends to 46 years in some of these individuals.
When 248,4000 individuals in this data set were evaluated, 5,534 had a diagnosis of RA. Of these, 178 (3.2%) developed ILD. About 20% of both those with and without RA were MUC5B variant carriers, meaning the remainder were not.
Sex and age factor into lifetime risk
In patients with RA, the lifetime rate of ILD among MUC5B variant carriers was 16.8% versus only 6.1% among noncarriers. This finding translated into a hazard ratio for ILD of 2.27 (95% confidence interval, 1.75–2.96) for variant carriers versus noncarriers.
The lifetime rate of ILD in patients with RA was greater in men versus women regardless of carrier status (18.5% vs. 8.5%). For women, the lifetime rate was lower for carriers, although the difference relative to female noncarriers was greater (14.5% vs. 4.7%).
ILD, whether in the general population or in patients with RA, is a disease of advancing age. When Dr. Palomäki showed a graph, the rise in ILD incidence did not start in any population, whether those with or without RA and regardless of carrier status, until about age 55. In those without RA and in noncarriers of the variant, ILD incidence remained low and began a discernible climb at around age 70.
In those who did not have RA but were positive for the variant, the rates rose more than twice as fast, particularly after age 70. In people who had RA but not the variant, the rate of ILD was greater than in patients who carried the variant without RA, starting the climb earlier and rising more steeply with age. In those with RA and the variant, the climb in ILD incidence rose rapidly after age 65 years even though the incidence remained fairly similar between all of these groups at age 60.
Putting the findings into context
The need to develop ways to prevent ILD in RA is urgent. ILD is one of the most common extraarticular manifestations of RA, developing in up to 60% of patients with RA in older age groups when evaluated with imaging, according to Dr. Palomäki. Although it develops into a clinically significant complication in only about 10% of these patients, ILD still is a significant cause of illness and death in elderly patients with RA.
In the 2018 study that first linked the MUC5B variant to RA-ILD, the investigators also found that the variant was associated with an increased likelihood of developing the usual interstitial pneumonia type of ILD on imaging. David Schwartz, MD, professor of medicine, pulmonary sciences, and critical care and chair of the department of medicine at the University of Colorado at Denver, Aurora, was a senior author of that study. He said these findings build on the 2018 study.
“While the gain-of-function MUC5B promoter variant is important in predicting who will develop RA-ILD, these findings also suggest that MUC5B may be involved in the etiology of RA-ILD, at least for those with the MUC5B variant,” he said.
“The study also raises the possibility that there are several subtypes of RA-ILD, and the subtype that is driven by MUC5B may respond differently to RA biologics or therapeutic agents to treat ILD,” he added.
In the discussion following the presentation by Dr. Palomäki, others agreed, with that statement including Dr. Palomäki. He expressed interest in clinical studies comparing different classes of RA therapies for their relative impact on the risk of developing ILD.Dr. Palomäki reported financial relationships with AbbVie, Merck, Pfizer, and Sanofi. Dr. Schwartz is the founder of Eleven P15, which is developing methods for early diagnosis and treatment of pulmonary fibrosis.
Carriers have more than twofold greater risk
Carriers have more than twofold greater risk
Patients with rheumatoid arthritis who carry a specific allele of the gene MUC5B have about double the risk of developing interstitial lung disease when compared with noncarriers, according to a large Finnish biobank study presented at the annual European Congress of Rheumatology.
“The risk difference [or carriers relative to noncarriers] started at about age 65, with a bigger difference [for] men than women,” reported Antti Palomäki, MD, PhD, of the center for rheumatology and clinical immunology at Turku (Finland) University.
The gain-of-function MUC5B variant, which encodes mucin 5B, was first linked to RA-associated interstitial lung disease (ILD) more than 3 years ago. At that time, it was already a known genetic risk factor for idiopathic pulmonary fibrosis in the general population. The new data confirm the association in a longitudinal analysis of a large biobank and suggest the association might have clinical utility.
“This is not ready for clinical practice at the moment. We do not yet know whether we can change therapy to reduce risk,” Dr. Palomäki said, adding “in the future we can look.”
One question that might be asked in clinical studies using MUC5B as a tool to assess and modify risk of ILD in patients with RA is whether one therapy is better than another in avoiding or delaying development of lung fibrosis. Dr. Palomäki noted that biologics, for example, might be a more favorable choice in patients with RA who are at high risk of developing ILD.
The association of the MUC5B variant with increased ILD incidence in patients with RA was drawn from a data set known as FinnGen, a biobank collection of epidemiologic cohorts and hospital samples with genotypes of about 10% of the Finnish population. Follow-up extends to 46 years in some of these individuals.
When 248,4000 individuals in this data set were evaluated, 5,534 had a diagnosis of RA. Of these, 178 (3.2%) developed ILD. About 20% of both those with and without RA were MUC5B variant carriers, meaning the remainder were not.
Sex and age factor into lifetime risk
In patients with RA, the lifetime rate of ILD among MUC5B variant carriers was 16.8% versus only 6.1% among noncarriers. This finding translated into a hazard ratio for ILD of 2.27 (95% confidence interval, 1.75–2.96) for variant carriers versus noncarriers.
The lifetime rate of ILD in patients with RA was greater in men versus women regardless of carrier status (18.5% vs. 8.5%). For women, the lifetime rate was lower for carriers, although the difference relative to female noncarriers was greater (14.5% vs. 4.7%).
ILD, whether in the general population or in patients with RA, is a disease of advancing age. When Dr. Palomäki showed a graph, the rise in ILD incidence did not start in any population, whether those with or without RA and regardless of carrier status, until about age 55. In those without RA and in noncarriers of the variant, ILD incidence remained low and began a discernible climb at around age 70.
In those who did not have RA but were positive for the variant, the rates rose more than twice as fast, particularly after age 70. In people who had RA but not the variant, the rate of ILD was greater than in patients who carried the variant without RA, starting the climb earlier and rising more steeply with age. In those with RA and the variant, the climb in ILD incidence rose rapidly after age 65 years even though the incidence remained fairly similar between all of these groups at age 60.
Putting the findings into context
The need to develop ways to prevent ILD in RA is urgent. ILD is one of the most common extraarticular manifestations of RA, developing in up to 60% of patients with RA in older age groups when evaluated with imaging, according to Dr. Palomäki. Although it develops into a clinically significant complication in only about 10% of these patients, ILD still is a significant cause of illness and death in elderly patients with RA.
In the 2018 study that first linked the MUC5B variant to RA-ILD, the investigators also found that the variant was associated with an increased likelihood of developing the usual interstitial pneumonia type of ILD on imaging. David Schwartz, MD, professor of medicine, pulmonary sciences, and critical care and chair of the department of medicine at the University of Colorado at Denver, Aurora, was a senior author of that study. He said these findings build on the 2018 study.
“While the gain-of-function MUC5B promoter variant is important in predicting who will develop RA-ILD, these findings also suggest that MUC5B may be involved in the etiology of RA-ILD, at least for those with the MUC5B variant,” he said.
“The study also raises the possibility that there are several subtypes of RA-ILD, and the subtype that is driven by MUC5B may respond differently to RA biologics or therapeutic agents to treat ILD,” he added.
In the discussion following the presentation by Dr. Palomäki, others agreed, with that statement including Dr. Palomäki. He expressed interest in clinical studies comparing different classes of RA therapies for their relative impact on the risk of developing ILD.Dr. Palomäki reported financial relationships with AbbVie, Merck, Pfizer, and Sanofi. Dr. Schwartz is the founder of Eleven P15, which is developing methods for early diagnosis and treatment of pulmonary fibrosis.
Patients with rheumatoid arthritis who carry a specific allele of the gene MUC5B have about double the risk of developing interstitial lung disease when compared with noncarriers, according to a large Finnish biobank study presented at the annual European Congress of Rheumatology.
“The risk difference [or carriers relative to noncarriers] started at about age 65, with a bigger difference [for] men than women,” reported Antti Palomäki, MD, PhD, of the center for rheumatology and clinical immunology at Turku (Finland) University.
The gain-of-function MUC5B variant, which encodes mucin 5B, was first linked to RA-associated interstitial lung disease (ILD) more than 3 years ago. At that time, it was already a known genetic risk factor for idiopathic pulmonary fibrosis in the general population. The new data confirm the association in a longitudinal analysis of a large biobank and suggest the association might have clinical utility.
“This is not ready for clinical practice at the moment. We do not yet know whether we can change therapy to reduce risk,” Dr. Palomäki said, adding “in the future we can look.”
One question that might be asked in clinical studies using MUC5B as a tool to assess and modify risk of ILD in patients with RA is whether one therapy is better than another in avoiding or delaying development of lung fibrosis. Dr. Palomäki noted that biologics, for example, might be a more favorable choice in patients with RA who are at high risk of developing ILD.
The association of the MUC5B variant with increased ILD incidence in patients with RA was drawn from a data set known as FinnGen, a biobank collection of epidemiologic cohorts and hospital samples with genotypes of about 10% of the Finnish population. Follow-up extends to 46 years in some of these individuals.
When 248,4000 individuals in this data set were evaluated, 5,534 had a diagnosis of RA. Of these, 178 (3.2%) developed ILD. About 20% of both those with and without RA were MUC5B variant carriers, meaning the remainder were not.
Sex and age factor into lifetime risk
In patients with RA, the lifetime rate of ILD among MUC5B variant carriers was 16.8% versus only 6.1% among noncarriers. This finding translated into a hazard ratio for ILD of 2.27 (95% confidence interval, 1.75–2.96) for variant carriers versus noncarriers.
The lifetime rate of ILD in patients with RA was greater in men versus women regardless of carrier status (18.5% vs. 8.5%). For women, the lifetime rate was lower for carriers, although the difference relative to female noncarriers was greater (14.5% vs. 4.7%).
ILD, whether in the general population or in patients with RA, is a disease of advancing age. When Dr. Palomäki showed a graph, the rise in ILD incidence did not start in any population, whether those with or without RA and regardless of carrier status, until about age 55. In those without RA and in noncarriers of the variant, ILD incidence remained low and began a discernible climb at around age 70.
In those who did not have RA but were positive for the variant, the rates rose more than twice as fast, particularly after age 70. In people who had RA but not the variant, the rate of ILD was greater than in patients who carried the variant without RA, starting the climb earlier and rising more steeply with age. In those with RA and the variant, the climb in ILD incidence rose rapidly after age 65 years even though the incidence remained fairly similar between all of these groups at age 60.
Putting the findings into context
The need to develop ways to prevent ILD in RA is urgent. ILD is one of the most common extraarticular manifestations of RA, developing in up to 60% of patients with RA in older age groups when evaluated with imaging, according to Dr. Palomäki. Although it develops into a clinically significant complication in only about 10% of these patients, ILD still is a significant cause of illness and death in elderly patients with RA.
In the 2018 study that first linked the MUC5B variant to RA-ILD, the investigators also found that the variant was associated with an increased likelihood of developing the usual interstitial pneumonia type of ILD on imaging. David Schwartz, MD, professor of medicine, pulmonary sciences, and critical care and chair of the department of medicine at the University of Colorado at Denver, Aurora, was a senior author of that study. He said these findings build on the 2018 study.
“While the gain-of-function MUC5B promoter variant is important in predicting who will develop RA-ILD, these findings also suggest that MUC5B may be involved in the etiology of RA-ILD, at least for those with the MUC5B variant,” he said.
“The study also raises the possibility that there are several subtypes of RA-ILD, and the subtype that is driven by MUC5B may respond differently to RA biologics or therapeutic agents to treat ILD,” he added.
In the discussion following the presentation by Dr. Palomäki, others agreed, with that statement including Dr. Palomäki. He expressed interest in clinical studies comparing different classes of RA therapies for their relative impact on the risk of developing ILD.Dr. Palomäki reported financial relationships with AbbVie, Merck, Pfizer, and Sanofi. Dr. Schwartz is the founder of Eleven P15, which is developing methods for early diagnosis and treatment of pulmonary fibrosis.
FROM THE EULAR 2021 CONGRESS
Nintedanib slows interstitial lung disease in RA patients
Subgroup analysis from INBUILD trial finds results similar to overall study cohort
In a new subgroup analysis of a previously published multinational trial, the preservation of lung function with nintedanib (Ofev) was about the same in patients with interstitial lung disease related to rheumatoid arthritis (RA-ILD) as it was in patients with other etiologies, according to data presented at the annual European Congress of Rheumatology.
“There was no significant heterogeneity across any of several characteristics we evaluated,” reported Clive Kelly, MBBS, of the Institute of Cellular Medicine at Newcastle University (England).
The INBUILD trial, which enrolled more than 600 patients in 15 countries with a range of fibrosing lung diseases, was published almost 2 years ago. On the primary endpoint of rate of decline in forced vital capacity (FVC), the medians were –80.8 mL per year among those randomized to nintedanib and –187.8 mL per year (P < .001) on placebo.
The INBUILD study provided evidence that fibrosing lung diseases have a common pathobiologic mechanism that can be slowed by targeting intracellular kinases. Nintedanib inhibits several growth factor receptors as well as nonreceptor tyrosine kinases, but its exact mechanism for slowing fibrosing lung diseases remains unclear. Initially approved for, nintedanib received approvals from the FDA for systemic sclerosis–associated ILD in 2019 and for chronic fibrosing ILD with progressive phenotypes in 2020 after being initially approved for the treatment of idiopathic pulmonary fibrosis in 2014.
When asked for comment, Paul F. Dellaripa, MD, an associate professor of medicine in the division of rheumatology, immunology, and allergy at Harvard Medical School, Boston, indicated these data are helpful in considering strategies for RA patients with ILD, but he encouraged collaboration between joint and lung specialists.
“Antifibrotic agents for patients with progressive ILD in autoimmune diseases like RA is a welcome addition to our care of this challenging complication,” said Dr. Dellaripa, who has published frequently on the diagnosis and treatment of lung diseases associated with RA. Yet, treatment must be individualized, he added.
“It will be incumbent for rheumatologists to incorporate lung health as a critical part of patient care and work closely with pulmonologists to consider when to institute antifibrotic therapy in patients with ILD,” he said.
Details of subanalysis
In the RA-ILD subpopulation of 89 patients, there was no further decline in FVC from 24 weeks after randomization to the end of 52 weeks for those on nintedanib, but the decline remained steady over the full course of follow-up among those in the placebo group. At 52 weeks, the decline in the placebo group reached –200 mL at the end of 52 weeks. As a result, the between-group relative reduction in FVC at 52 weeks of 116.7 mL favoring nintedanib over placebo (P < .037) slightly exceeded the 107-mL reduction (P < .001) observed in the overall INBUILD study population.
Among other subgroups the investigators evaluated, outcomes with nintedanib did not differ when patients were split into groups with higher or lower baseline levels of high-sensitivity C-reactive protein, regardless of whether the groups were defined by levels above and below 1 mg/L or 3 mg/L. The same was true for those who were taking nonbiologic disease-modifying antirheumatic drugs or glucocorticoids.
However, for these latter analyses, Dr. Kelly conceded that the differences were based on small numbers of patients and so cannot be considered conclusive.
The adverse event most closely associated with nintedanib in the RA-ILD population was diarrhea, just as in the overall study, and it was more than twice as frequent in the RA-ILD patients receiving the active therapy, compared with placebo (54.8% vs. 25.5%). Nausea was also more common (21.4% vs. 10.6%), and so was decreased appetite (11.9% vs. 2.1%) and weight reduction (9.5% vs. 2.1%).
Lung-related adverse events, such as bronchiolitis (21.4% vs. 17.0%) and dyspnea (11.9% vs. 10.6%), were only slightly more frequent in the nintedanib group. Nasopharyngitis (7.1% vs. 12.8%) was less common. Side effects leading to treatment discontinuation were higher on nintedanib (19.0% vs. 12.8%)
The RA-ILD subgroup represented 13.4% of those randomized in INBUILD. The mean time since diagnosis of RA was about 10 years. More than 60% were smokers or former smokers. At baseline, the mean FVC of predicted was 71%. More than 85% had a usual interstitial pneumonia (UIP) radiologic pattern.
Acute exacerbations and death were not evaluated in the RA-ILD subpopulation, but these were secondary endpoints in the published INBUILD study according to the presence or absence of a UIP-like fibrotic pattern. For the combined endpoint of acute exacerbation of ILD or death, the protection associated with nintedanib approached statistical significance for the population overall (odds ratio, 0.68; 95% confidence interval, 0.46-1.01) and reached significance for those with a UIP pattern (OR, 0.61; 95% CI, 0.38-0.98).
Nintedanib led to lower death rates at 52 weeks in the overall population (8.1% vs. 11.5% with placebo) and in the group with a UIP pattern (9.7% vs. 15.0% with placebo).
Dr. Kelly has financial relationships with multiple pharmaceutical companies, including Boehringer Ingelheim, which provided funding for INBUILD and this subpopulation analysis. Dr. Dellaripa reported financial relationships with Bristol-Myers Squibb and Genentech.
Subgroup analysis from INBUILD trial finds results similar to overall study cohort
Subgroup analysis from INBUILD trial finds results similar to overall study cohort
In a new subgroup analysis of a previously published multinational trial, the preservation of lung function with nintedanib (Ofev) was about the same in patients with interstitial lung disease related to rheumatoid arthritis (RA-ILD) as it was in patients with other etiologies, according to data presented at the annual European Congress of Rheumatology.
“There was no significant heterogeneity across any of several characteristics we evaluated,” reported Clive Kelly, MBBS, of the Institute of Cellular Medicine at Newcastle University (England).
The INBUILD trial, which enrolled more than 600 patients in 15 countries with a range of fibrosing lung diseases, was published almost 2 years ago. On the primary endpoint of rate of decline in forced vital capacity (FVC), the medians were –80.8 mL per year among those randomized to nintedanib and –187.8 mL per year (P < .001) on placebo.
The INBUILD study provided evidence that fibrosing lung diseases have a common pathobiologic mechanism that can be slowed by targeting intracellular kinases. Nintedanib inhibits several growth factor receptors as well as nonreceptor tyrosine kinases, but its exact mechanism for slowing fibrosing lung diseases remains unclear. Initially approved for, nintedanib received approvals from the FDA for systemic sclerosis–associated ILD in 2019 and for chronic fibrosing ILD with progressive phenotypes in 2020 after being initially approved for the treatment of idiopathic pulmonary fibrosis in 2014.
When asked for comment, Paul F. Dellaripa, MD, an associate professor of medicine in the division of rheumatology, immunology, and allergy at Harvard Medical School, Boston, indicated these data are helpful in considering strategies for RA patients with ILD, but he encouraged collaboration between joint and lung specialists.
“Antifibrotic agents for patients with progressive ILD in autoimmune diseases like RA is a welcome addition to our care of this challenging complication,” said Dr. Dellaripa, who has published frequently on the diagnosis and treatment of lung diseases associated with RA. Yet, treatment must be individualized, he added.
“It will be incumbent for rheumatologists to incorporate lung health as a critical part of patient care and work closely with pulmonologists to consider when to institute antifibrotic therapy in patients with ILD,” he said.
Details of subanalysis
In the RA-ILD subpopulation of 89 patients, there was no further decline in FVC from 24 weeks after randomization to the end of 52 weeks for those on nintedanib, but the decline remained steady over the full course of follow-up among those in the placebo group. At 52 weeks, the decline in the placebo group reached –200 mL at the end of 52 weeks. As a result, the between-group relative reduction in FVC at 52 weeks of 116.7 mL favoring nintedanib over placebo (P < .037) slightly exceeded the 107-mL reduction (P < .001) observed in the overall INBUILD study population.
Among other subgroups the investigators evaluated, outcomes with nintedanib did not differ when patients were split into groups with higher or lower baseline levels of high-sensitivity C-reactive protein, regardless of whether the groups were defined by levels above and below 1 mg/L or 3 mg/L. The same was true for those who were taking nonbiologic disease-modifying antirheumatic drugs or glucocorticoids.
However, for these latter analyses, Dr. Kelly conceded that the differences were based on small numbers of patients and so cannot be considered conclusive.
The adverse event most closely associated with nintedanib in the RA-ILD population was diarrhea, just as in the overall study, and it was more than twice as frequent in the RA-ILD patients receiving the active therapy, compared with placebo (54.8% vs. 25.5%). Nausea was also more common (21.4% vs. 10.6%), and so was decreased appetite (11.9% vs. 2.1%) and weight reduction (9.5% vs. 2.1%).
Lung-related adverse events, such as bronchiolitis (21.4% vs. 17.0%) and dyspnea (11.9% vs. 10.6%), were only slightly more frequent in the nintedanib group. Nasopharyngitis (7.1% vs. 12.8%) was less common. Side effects leading to treatment discontinuation were higher on nintedanib (19.0% vs. 12.8%)
The RA-ILD subgroup represented 13.4% of those randomized in INBUILD. The mean time since diagnosis of RA was about 10 years. More than 60% were smokers or former smokers. At baseline, the mean FVC of predicted was 71%. More than 85% had a usual interstitial pneumonia (UIP) radiologic pattern.
Acute exacerbations and death were not evaluated in the RA-ILD subpopulation, but these were secondary endpoints in the published INBUILD study according to the presence or absence of a UIP-like fibrotic pattern. For the combined endpoint of acute exacerbation of ILD or death, the protection associated with nintedanib approached statistical significance for the population overall (odds ratio, 0.68; 95% confidence interval, 0.46-1.01) and reached significance for those with a UIP pattern (OR, 0.61; 95% CI, 0.38-0.98).
Nintedanib led to lower death rates at 52 weeks in the overall population (8.1% vs. 11.5% with placebo) and in the group with a UIP pattern (9.7% vs. 15.0% with placebo).
Dr. Kelly has financial relationships with multiple pharmaceutical companies, including Boehringer Ingelheim, which provided funding for INBUILD and this subpopulation analysis. Dr. Dellaripa reported financial relationships with Bristol-Myers Squibb and Genentech.
In a new subgroup analysis of a previously published multinational trial, the preservation of lung function with nintedanib (Ofev) was about the same in patients with interstitial lung disease related to rheumatoid arthritis (RA-ILD) as it was in patients with other etiologies, according to data presented at the annual European Congress of Rheumatology.
“There was no significant heterogeneity across any of several characteristics we evaluated,” reported Clive Kelly, MBBS, of the Institute of Cellular Medicine at Newcastle University (England).
The INBUILD trial, which enrolled more than 600 patients in 15 countries with a range of fibrosing lung diseases, was published almost 2 years ago. On the primary endpoint of rate of decline in forced vital capacity (FVC), the medians were –80.8 mL per year among those randomized to nintedanib and –187.8 mL per year (P < .001) on placebo.
The INBUILD study provided evidence that fibrosing lung diseases have a common pathobiologic mechanism that can be slowed by targeting intracellular kinases. Nintedanib inhibits several growth factor receptors as well as nonreceptor tyrosine kinases, but its exact mechanism for slowing fibrosing lung diseases remains unclear. Initially approved for, nintedanib received approvals from the FDA for systemic sclerosis–associated ILD in 2019 and for chronic fibrosing ILD with progressive phenotypes in 2020 after being initially approved for the treatment of idiopathic pulmonary fibrosis in 2014.
When asked for comment, Paul F. Dellaripa, MD, an associate professor of medicine in the division of rheumatology, immunology, and allergy at Harvard Medical School, Boston, indicated these data are helpful in considering strategies for RA patients with ILD, but he encouraged collaboration between joint and lung specialists.
“Antifibrotic agents for patients with progressive ILD in autoimmune diseases like RA is a welcome addition to our care of this challenging complication,” said Dr. Dellaripa, who has published frequently on the diagnosis and treatment of lung diseases associated with RA. Yet, treatment must be individualized, he added.
“It will be incumbent for rheumatologists to incorporate lung health as a critical part of patient care and work closely with pulmonologists to consider when to institute antifibrotic therapy in patients with ILD,” he said.
Details of subanalysis
In the RA-ILD subpopulation of 89 patients, there was no further decline in FVC from 24 weeks after randomization to the end of 52 weeks for those on nintedanib, but the decline remained steady over the full course of follow-up among those in the placebo group. At 52 weeks, the decline in the placebo group reached –200 mL at the end of 52 weeks. As a result, the between-group relative reduction in FVC at 52 weeks of 116.7 mL favoring nintedanib over placebo (P < .037) slightly exceeded the 107-mL reduction (P < .001) observed in the overall INBUILD study population.
Among other subgroups the investigators evaluated, outcomes with nintedanib did not differ when patients were split into groups with higher or lower baseline levels of high-sensitivity C-reactive protein, regardless of whether the groups were defined by levels above and below 1 mg/L or 3 mg/L. The same was true for those who were taking nonbiologic disease-modifying antirheumatic drugs or glucocorticoids.
However, for these latter analyses, Dr. Kelly conceded that the differences were based on small numbers of patients and so cannot be considered conclusive.
The adverse event most closely associated with nintedanib in the RA-ILD population was diarrhea, just as in the overall study, and it was more than twice as frequent in the RA-ILD patients receiving the active therapy, compared with placebo (54.8% vs. 25.5%). Nausea was also more common (21.4% vs. 10.6%), and so was decreased appetite (11.9% vs. 2.1%) and weight reduction (9.5% vs. 2.1%).
Lung-related adverse events, such as bronchiolitis (21.4% vs. 17.0%) and dyspnea (11.9% vs. 10.6%), were only slightly more frequent in the nintedanib group. Nasopharyngitis (7.1% vs. 12.8%) was less common. Side effects leading to treatment discontinuation were higher on nintedanib (19.0% vs. 12.8%)
The RA-ILD subgroup represented 13.4% of those randomized in INBUILD. The mean time since diagnosis of RA was about 10 years. More than 60% were smokers or former smokers. At baseline, the mean FVC of predicted was 71%. More than 85% had a usual interstitial pneumonia (UIP) radiologic pattern.
Acute exacerbations and death were not evaluated in the RA-ILD subpopulation, but these were secondary endpoints in the published INBUILD study according to the presence or absence of a UIP-like fibrotic pattern. For the combined endpoint of acute exacerbation of ILD or death, the protection associated with nintedanib approached statistical significance for the population overall (odds ratio, 0.68; 95% confidence interval, 0.46-1.01) and reached significance for those with a UIP pattern (OR, 0.61; 95% CI, 0.38-0.98).
Nintedanib led to lower death rates at 52 weeks in the overall population (8.1% vs. 11.5% with placebo) and in the group with a UIP pattern (9.7% vs. 15.0% with placebo).
Dr. Kelly has financial relationships with multiple pharmaceutical companies, including Boehringer Ingelheim, which provided funding for INBUILD and this subpopulation analysis. Dr. Dellaripa reported financial relationships with Bristol-Myers Squibb and Genentech.
FROM THE EULAR 2021 CONGRESS
Trastuzumab deruxtecan-related lung disease in MBC patients can occur anytime in first year
Although rates are generally low, interstitial lung disease (ILD) can occur at any point in the first year of treatment with trastuzumab deruxtecan (T-DXd) for HER2-positive metastatic breast cancer (MBC).
That’s according to a pooled analysis of three early clinical trials with the drug that was reported at the European Society for Medical Oncology (ESMO): Breast Cancer virtual meeting.
Over a 5-year analysis period, the rate of any grade of ILD was 15.5%. The majority (79%) of those events were grade 1 or 2, observed pulmonologist Charles A. Powell, MD, of Icahn School of Medicine at Mount Sinai in New York, who presented the findings.
Of the 245 patients who were included in the analysis, 38 had an ILD event deemed related to treatment. A respective 9 (3.7%) and 21 (8.6%) had events graded as 1 or 2, 1 patient each (0.4%) had a grade 3 or 4 event, and 6 (2.4%) patients had a grade 5 event.
The timing of the first identified ILD event varied from 1.1 months to 20.8 months, given a median of 5.6 months overall. “This highlights an opportunity for more timely detection of ILD,” Dr. Powell suggested. He added that in almost all (97%) cases, ILD occurred before 12 months and the risk may even decrease over time “suggesting that the risk is not cumulative.”
He cautioned, however: “It is important to note that this analysis is exploratory and hypothesis generating in nature.”
ILD occurs with other cancer drugs
ILD is not just associated with T-DXd treatment, said the invited discussant for the trial, Harold J. Burstein, MD, PhD, of the Dana-Farber Cancer Institute in Boston.
“It’s important for clinicians to remember that ILD/pneumonitis is an uncommon, but potentially very serious side effect that affects many breast cancer treatments,” he said.
That not only includes T-DXd, but other newer drugs such the cyclin dependent kinase (CDK) 4/6 inhibitors and immune checkpoint inhibitors, as well as other older more established drugs including taxanes, cyclophosphamide and even the mTOR inhibitor everolimus.
“Both clinicians and patients need to be aware of this risk. It’s part of the differential diagnosis for any patient who develops either ground glass changes or other infiltrates on a CT scan, or who has symptoms,” Dr. Burstein added.
Investigating ILD in T-DXd trials
T-DXd (Enhertu) is an anti-HER2-antibody drug conjugate that contains a humanized anti-HER2 IgG1 monoclonal antibody akin to trastuzumab that is linked to DXd, a topoisomerase I inhibitor that is a derivative of exatecan.
It has been approved for use in patients with HER2-positive metastatic breast cancer after two other HER2 treatments fail in the United States and Europe, and after chemotherapy in Japan, noted Dr. Powell. This is largely due to the results from the phase 2, open-label DESTINY-Breast01 trial.
“In breast cancer, T-DXd continues to demonstrate clinically meaningful efficacy with a median duration of response of more than 20 months in a heavily pretreated population,” he said. Objective response rates seen in the DESTINY-Breast01 trial were around 60%, and the median progression-free survival was a little over 19 months.
To look at the issue of drug-related ILD events in patients treated with T-DXd for HER2-positive MBC, an independent adjudication committee was formed to look at all the imaging and clinical data from the DESTINY-Breast01 trial and two single-arm phase 1 trials (NCT02564900 and NCT03383692).
In all, data on 245 patients who had been treated with T-DXd at the approved dose of 5.4 mg/kg in those trials between August 2015 and June 2020 were analyzed.
Dealing with lung toxicity
“We are getting new drugs to improve the treatment of cancer, but they always come with a price in terms of toxicity,” observed David Cameron, MD, professor of medical oncology at Edinburgh University in Scotland. Dr. Cameron chaired the session.
“Several measures were taken to identify and mitigate ILD,” across all the T-DXd studies, Dr. Powell explained. As well as the independent adjudication committee, available guidelines were followed and updated on how to diagnose and treat drug-induced lung injuries, and a “safe use” campaign was run in 2019.
Many patients in the early MBC studies were recruited before these measures were in place, such as the use of systemic steroids to manage low-grade events.
The bottom line, however, is that if a patient develops ILD then treatment should be stopped, Dr. Powell said. “Patients with grade 1 events may restart once the ILD has resolved, but those with grade 2 to 4 events must discontinue treatment.”
Dr. Powell concluded: “The overall clinical data support the positive risk-benefit profile of T-DXd. Phase 3 randomized controlled trials in breast cancer are ongoing.”
ILD also seen in monarchE trial with abemaciclib
Data on ILD events seen in the phase 3 monarchE trial were also reported separately at the ESMO Breast Cancer virtual meeting. The analysis population included 2,971 patients who had been treated with the CDK 4/6 inhibitor abemaciclib (Verzenio) together with endocrine therapy and 2,800 who had received endocrine therapy alone in the early-stage, adjuvant advanced breast cancer setting.
Most ILD (97%) events that occurred were single occurrences, with any grade of ILD occurring in a higher percentage of patients treated with abemaciclib with endocrine therapy than endocrine therapy alone (2.9% vs. 1.2%). Grade 3 events occurred in a respective 0.4% and 0.0% of patients.
So who’s at risk?
The risk factors for ILD and pneumonitis are not well characterized with either of the two drugs discussed, Dr. Burstein observed.
“In the abemaciclib experience, it looked like obesity might be a predisposing factor, with trastuzumab deruxtecan, it looked like patients of Asian ancestry were greater risk, but we need more data to really understand who’s at jeopardy.”
Dr. Burstein observed: “This is something patients need to be aware of as they’re contemplating this treatment.”
While data to prove the benefit of the drug need to mature, Dr. Burstein “would likely discontinue therapy” if a patient were to develop ILD or pneumonitis and treat accordingly.
As for T-DXd, he said: “It’s important that patients know that lung disease is a potentially severe side effect of treatment and that any respiratory symptoms need to be jumped on quickly.”
While prospective studies are now needed, and the phase 3 data should help to better understand the risk of ILD with T-DXd, Dr. Burstein believes it will be important to develop algorithms to ensure the safe administration of the drug.
These algorithms should include “appropriate surveillance and monitoring, especially as we think about trying to move this drug forward into the early stage setting where we’re using it in women who have favorable prognosis, and potentially curative situations for breast cancer.”
The trastuzumab deruxtecan trials were cosponsored by Daiichi Sankyo and AstraZeneca. The monarchE trial was supported by Eli Lilly.
Dr. Powell acknowledged receiving personal fees for acting as an advisory or consultant to both companies as well as to Voluntis. Dr. Burstein had nothing to disclose, and Dr. Cameron had no relevant financial interests in the data being presented.
Although rates are generally low, interstitial lung disease (ILD) can occur at any point in the first year of treatment with trastuzumab deruxtecan (T-DXd) for HER2-positive metastatic breast cancer (MBC).
That’s according to a pooled analysis of three early clinical trials with the drug that was reported at the European Society for Medical Oncology (ESMO): Breast Cancer virtual meeting.
Over a 5-year analysis period, the rate of any grade of ILD was 15.5%. The majority (79%) of those events were grade 1 or 2, observed pulmonologist Charles A. Powell, MD, of Icahn School of Medicine at Mount Sinai in New York, who presented the findings.
Of the 245 patients who were included in the analysis, 38 had an ILD event deemed related to treatment. A respective 9 (3.7%) and 21 (8.6%) had events graded as 1 or 2, 1 patient each (0.4%) had a grade 3 or 4 event, and 6 (2.4%) patients had a grade 5 event.
The timing of the first identified ILD event varied from 1.1 months to 20.8 months, given a median of 5.6 months overall. “This highlights an opportunity for more timely detection of ILD,” Dr. Powell suggested. He added that in almost all (97%) cases, ILD occurred before 12 months and the risk may even decrease over time “suggesting that the risk is not cumulative.”
He cautioned, however: “It is important to note that this analysis is exploratory and hypothesis generating in nature.”
ILD occurs with other cancer drugs
ILD is not just associated with T-DXd treatment, said the invited discussant for the trial, Harold J. Burstein, MD, PhD, of the Dana-Farber Cancer Institute in Boston.
“It’s important for clinicians to remember that ILD/pneumonitis is an uncommon, but potentially very serious side effect that affects many breast cancer treatments,” he said.
That not only includes T-DXd, but other newer drugs such the cyclin dependent kinase (CDK) 4/6 inhibitors and immune checkpoint inhibitors, as well as other older more established drugs including taxanes, cyclophosphamide and even the mTOR inhibitor everolimus.
“Both clinicians and patients need to be aware of this risk. It’s part of the differential diagnosis for any patient who develops either ground glass changes or other infiltrates on a CT scan, or who has symptoms,” Dr. Burstein added.
Investigating ILD in T-DXd trials
T-DXd (Enhertu) is an anti-HER2-antibody drug conjugate that contains a humanized anti-HER2 IgG1 monoclonal antibody akin to trastuzumab that is linked to DXd, a topoisomerase I inhibitor that is a derivative of exatecan.
It has been approved for use in patients with HER2-positive metastatic breast cancer after two other HER2 treatments fail in the United States and Europe, and after chemotherapy in Japan, noted Dr. Powell. This is largely due to the results from the phase 2, open-label DESTINY-Breast01 trial.
“In breast cancer, T-DXd continues to demonstrate clinically meaningful efficacy with a median duration of response of more than 20 months in a heavily pretreated population,” he said. Objective response rates seen in the DESTINY-Breast01 trial were around 60%, and the median progression-free survival was a little over 19 months.
To look at the issue of drug-related ILD events in patients treated with T-DXd for HER2-positive MBC, an independent adjudication committee was formed to look at all the imaging and clinical data from the DESTINY-Breast01 trial and two single-arm phase 1 trials (NCT02564900 and NCT03383692).
In all, data on 245 patients who had been treated with T-DXd at the approved dose of 5.4 mg/kg in those trials between August 2015 and June 2020 were analyzed.
Dealing with lung toxicity
“We are getting new drugs to improve the treatment of cancer, but they always come with a price in terms of toxicity,” observed David Cameron, MD, professor of medical oncology at Edinburgh University in Scotland. Dr. Cameron chaired the session.
“Several measures were taken to identify and mitigate ILD,” across all the T-DXd studies, Dr. Powell explained. As well as the independent adjudication committee, available guidelines were followed and updated on how to diagnose and treat drug-induced lung injuries, and a “safe use” campaign was run in 2019.
Many patients in the early MBC studies were recruited before these measures were in place, such as the use of systemic steroids to manage low-grade events.
The bottom line, however, is that if a patient develops ILD then treatment should be stopped, Dr. Powell said. “Patients with grade 1 events may restart once the ILD has resolved, but those with grade 2 to 4 events must discontinue treatment.”
Dr. Powell concluded: “The overall clinical data support the positive risk-benefit profile of T-DXd. Phase 3 randomized controlled trials in breast cancer are ongoing.”
ILD also seen in monarchE trial with abemaciclib
Data on ILD events seen in the phase 3 monarchE trial were also reported separately at the ESMO Breast Cancer virtual meeting. The analysis population included 2,971 patients who had been treated with the CDK 4/6 inhibitor abemaciclib (Verzenio) together with endocrine therapy and 2,800 who had received endocrine therapy alone in the early-stage, adjuvant advanced breast cancer setting.
Most ILD (97%) events that occurred were single occurrences, with any grade of ILD occurring in a higher percentage of patients treated with abemaciclib with endocrine therapy than endocrine therapy alone (2.9% vs. 1.2%). Grade 3 events occurred in a respective 0.4% and 0.0% of patients.
So who’s at risk?
The risk factors for ILD and pneumonitis are not well characterized with either of the two drugs discussed, Dr. Burstein observed.
“In the abemaciclib experience, it looked like obesity might be a predisposing factor, with trastuzumab deruxtecan, it looked like patients of Asian ancestry were greater risk, but we need more data to really understand who’s at jeopardy.”
Dr. Burstein observed: “This is something patients need to be aware of as they’re contemplating this treatment.”
While data to prove the benefit of the drug need to mature, Dr. Burstein “would likely discontinue therapy” if a patient were to develop ILD or pneumonitis and treat accordingly.
As for T-DXd, he said: “It’s important that patients know that lung disease is a potentially severe side effect of treatment and that any respiratory symptoms need to be jumped on quickly.”
While prospective studies are now needed, and the phase 3 data should help to better understand the risk of ILD with T-DXd, Dr. Burstein believes it will be important to develop algorithms to ensure the safe administration of the drug.
These algorithms should include “appropriate surveillance and monitoring, especially as we think about trying to move this drug forward into the early stage setting where we’re using it in women who have favorable prognosis, and potentially curative situations for breast cancer.”
The trastuzumab deruxtecan trials were cosponsored by Daiichi Sankyo and AstraZeneca. The monarchE trial was supported by Eli Lilly.
Dr. Powell acknowledged receiving personal fees for acting as an advisory or consultant to both companies as well as to Voluntis. Dr. Burstein had nothing to disclose, and Dr. Cameron had no relevant financial interests in the data being presented.
Although rates are generally low, interstitial lung disease (ILD) can occur at any point in the first year of treatment with trastuzumab deruxtecan (T-DXd) for HER2-positive metastatic breast cancer (MBC).
That’s according to a pooled analysis of three early clinical trials with the drug that was reported at the European Society for Medical Oncology (ESMO): Breast Cancer virtual meeting.
Over a 5-year analysis period, the rate of any grade of ILD was 15.5%. The majority (79%) of those events were grade 1 or 2, observed pulmonologist Charles A. Powell, MD, of Icahn School of Medicine at Mount Sinai in New York, who presented the findings.
Of the 245 patients who were included in the analysis, 38 had an ILD event deemed related to treatment. A respective 9 (3.7%) and 21 (8.6%) had events graded as 1 or 2, 1 patient each (0.4%) had a grade 3 or 4 event, and 6 (2.4%) patients had a grade 5 event.
The timing of the first identified ILD event varied from 1.1 months to 20.8 months, given a median of 5.6 months overall. “This highlights an opportunity for more timely detection of ILD,” Dr. Powell suggested. He added that in almost all (97%) cases, ILD occurred before 12 months and the risk may even decrease over time “suggesting that the risk is not cumulative.”
He cautioned, however: “It is important to note that this analysis is exploratory and hypothesis generating in nature.”
ILD occurs with other cancer drugs
ILD is not just associated with T-DXd treatment, said the invited discussant for the trial, Harold J. Burstein, MD, PhD, of the Dana-Farber Cancer Institute in Boston.
“It’s important for clinicians to remember that ILD/pneumonitis is an uncommon, but potentially very serious side effect that affects many breast cancer treatments,” he said.
That not only includes T-DXd, but other newer drugs such the cyclin dependent kinase (CDK) 4/6 inhibitors and immune checkpoint inhibitors, as well as other older more established drugs including taxanes, cyclophosphamide and even the mTOR inhibitor everolimus.
“Both clinicians and patients need to be aware of this risk. It’s part of the differential diagnosis for any patient who develops either ground glass changes or other infiltrates on a CT scan, or who has symptoms,” Dr. Burstein added.
Investigating ILD in T-DXd trials
T-DXd (Enhertu) is an anti-HER2-antibody drug conjugate that contains a humanized anti-HER2 IgG1 monoclonal antibody akin to trastuzumab that is linked to DXd, a topoisomerase I inhibitor that is a derivative of exatecan.
It has been approved for use in patients with HER2-positive metastatic breast cancer after two other HER2 treatments fail in the United States and Europe, and after chemotherapy in Japan, noted Dr. Powell. This is largely due to the results from the phase 2, open-label DESTINY-Breast01 trial.
“In breast cancer, T-DXd continues to demonstrate clinically meaningful efficacy with a median duration of response of more than 20 months in a heavily pretreated population,” he said. Objective response rates seen in the DESTINY-Breast01 trial were around 60%, and the median progression-free survival was a little over 19 months.
To look at the issue of drug-related ILD events in patients treated with T-DXd for HER2-positive MBC, an independent adjudication committee was formed to look at all the imaging and clinical data from the DESTINY-Breast01 trial and two single-arm phase 1 trials (NCT02564900 and NCT03383692).
In all, data on 245 patients who had been treated with T-DXd at the approved dose of 5.4 mg/kg in those trials between August 2015 and June 2020 were analyzed.
Dealing with lung toxicity
“We are getting new drugs to improve the treatment of cancer, but they always come with a price in terms of toxicity,” observed David Cameron, MD, professor of medical oncology at Edinburgh University in Scotland. Dr. Cameron chaired the session.
“Several measures were taken to identify and mitigate ILD,” across all the T-DXd studies, Dr. Powell explained. As well as the independent adjudication committee, available guidelines were followed and updated on how to diagnose and treat drug-induced lung injuries, and a “safe use” campaign was run in 2019.
Many patients in the early MBC studies were recruited before these measures were in place, such as the use of systemic steroids to manage low-grade events.
The bottom line, however, is that if a patient develops ILD then treatment should be stopped, Dr. Powell said. “Patients with grade 1 events may restart once the ILD has resolved, but those with grade 2 to 4 events must discontinue treatment.”
Dr. Powell concluded: “The overall clinical data support the positive risk-benefit profile of T-DXd. Phase 3 randomized controlled trials in breast cancer are ongoing.”
ILD also seen in monarchE trial with abemaciclib
Data on ILD events seen in the phase 3 monarchE trial were also reported separately at the ESMO Breast Cancer virtual meeting. The analysis population included 2,971 patients who had been treated with the CDK 4/6 inhibitor abemaciclib (Verzenio) together with endocrine therapy and 2,800 who had received endocrine therapy alone in the early-stage, adjuvant advanced breast cancer setting.
Most ILD (97%) events that occurred were single occurrences, with any grade of ILD occurring in a higher percentage of patients treated with abemaciclib with endocrine therapy than endocrine therapy alone (2.9% vs. 1.2%). Grade 3 events occurred in a respective 0.4% and 0.0% of patients.
So who’s at risk?
The risk factors for ILD and pneumonitis are not well characterized with either of the two drugs discussed, Dr. Burstein observed.
“In the abemaciclib experience, it looked like obesity might be a predisposing factor, with trastuzumab deruxtecan, it looked like patients of Asian ancestry were greater risk, but we need more data to really understand who’s at jeopardy.”
Dr. Burstein observed: “This is something patients need to be aware of as they’re contemplating this treatment.”
While data to prove the benefit of the drug need to mature, Dr. Burstein “would likely discontinue therapy” if a patient were to develop ILD or pneumonitis and treat accordingly.
As for T-DXd, he said: “It’s important that patients know that lung disease is a potentially severe side effect of treatment and that any respiratory symptoms need to be jumped on quickly.”
While prospective studies are now needed, and the phase 3 data should help to better understand the risk of ILD with T-DXd, Dr. Burstein believes it will be important to develop algorithms to ensure the safe administration of the drug.
These algorithms should include “appropriate surveillance and monitoring, especially as we think about trying to move this drug forward into the early stage setting where we’re using it in women who have favorable prognosis, and potentially curative situations for breast cancer.”
The trastuzumab deruxtecan trials were cosponsored by Daiichi Sankyo and AstraZeneca. The monarchE trial was supported by Eli Lilly.
Dr. Powell acknowledged receiving personal fees for acting as an advisory or consultant to both companies as well as to Voluntis. Dr. Burstein had nothing to disclose, and Dr. Cameron had no relevant financial interests in the data being presented.
FROM ESMO BREAST CANCER 2021