User login
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines define acute exacerbations of chronic obstructive pulmonary disease (AECOPD) as a sudden worsening of respiratory symptoms that require additional interventions. Exacerbations are classified as mild (treated with short-acting bronchodilators only), moderate (treated with antibiotics and/or oral corticosteroids), or severe (treatment requiring hospitalization). Exacerbations must include increased dyspnea, and other symptoms may involve increased sputum volume and purulence, cough, and a change in sputum color. These symptoms can be due to viral, bacterial, or environmental causes, with viral respiratory infections being the most common cause.1-4 However, determining the etiology of an exacerbation can be difficult based on symptoms alone and can lead to an excessive and unnecessary use of antibiotics. Only the change in sputum color is considered highly sensitive and specific for bacterial causes.1 As a result, there has been an increased interest in the use of acute biomarkers to determine whether antibiotics are necessary.
Procalcitonin (PCT) is an acute phase reactant that increases in response to inflammation, especially inflammation caused by a bacterial infection. Recent studies have suggested that PCT may be used in patients experiencing an AECOPD to reduce antibiotic use without impacting rates of treatment failure.5-9 A majority of these studies have been in the inpatient setting or a combination of inpatient and outpatient settings.
The purpose of this study was to create and to evaluate the efficacy and practicality of a PCT-based algorithm to aid emergency department (ED) clinicians in the evaluation of patients with AECOPD who do not require hospitalization. The primary outcome of this project was the rate of antibiotic prescriptions before and after the initiation of the algorithm.
Methods
This was an observational, retrospective, pre/post assessment at the Phoenix Veterans Affairs Health Care System (PVAHCS) in Arizona. Patients who were discharged from the ED with a diagnosis of an AECOPD were identified using International Classification of Diseases, Tenth Revision (ICD-10) codes. Patient charts were reviewed from November 2018 to March 2019 for the preimplementation group and from November 2019 for March 2020 in the postimplementation group. The periods were chosen to reflect similar seasons for both the pre- and postimplementation interventions. Patients were excluded from analysis if they required hospital admission, were immunocompromised, on chronic antimicrobial therapy, had no documented medical history of COPD, or if they were presenting primarily for medication refills. Information collected included the rate of antibiotic prescriptions, procalcitonin test orders, COPD GOLD classification, and 30-, 60-, and 90-day reexacerbation rates.
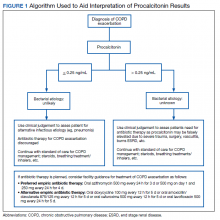
A PCT-based algorithm (Figure 1) was developed and approved by the PVAHCS Antimicrobial Stewardship Program, the Pharmacy and Therapeutics committee, and ED leadership. PCT threshold values were based on values approved by the US Food and Drug Administration and previous studies—antibiotics were discouraged for PCT levels ≤ 0.25 ng/mL but could be considered for PCT levels > 0.25 ng/mL.5,8,9 Clinicians were not required to use the algorithm, and the use of clinical judgement was encouraged. The recommended antibiotic therapies were based on previously approved PVAHCS antimicrobial stewardship guidance. To promote utilization, a PCT quick order option was added to the ED laboratory order menu.
ED clinicians were individually educated by the antimicrobial stewardship and emergency medicine pharmacists, an infectious disease physician champion, and the pharmacy resident. Clinicians were educated about PCT and its use in the setting of AECOPD to aid in the determination of bacterial infections. Each clinician received an electronic copy the algorithm and summary of the study protocol before implementation and 3 months after implementation for follow-up education. In addition, a printed copy of the algorithm was posted in multiple clinician workstations within the ED. For the first month of implementation, the project lead was available full-time in the ED to encourage algorithm use and to field questions or concerns from clinicians.
Outcome Measures
The primary outcome was the rate of antibiotic prescriptions pre- and postintervention. The safety endpoints were 30-, 60-, and 90-day reexacerbation rates. Reexacerbation rates were defined by ICD-10 codes and documentation from a primary care visit or subsequent ED visit. The secondary outcomes were the rate of PCT tests ordered and used for treatment decisions. Other areas of interest were antibiotic prescribing trends, duration of therapy, and patient COPD GOLD classification.
Statistical analysis
It was estimated that a sample size of 146 patients (73 patients/group) would provide 80% power to detect a between-group difference of 10% in the percentage of patients who were prescribed antibiotics. Categorical variables were expressed using estimates of frequency and percentages. Percentages were compared using Fisher exact tests. For all tests, the significance level was set at 0.05.
Results
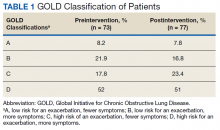
Seventy-three patients were included in the preintervention group and 77 in the postintervention group. The GOLD classification rates were similar between the groups (Table 1). In addition, > 90% of patients were White males and all patients were aged ≥ 50 years, which is characteristic of the US Department of Veterans Affairs (VA) population.
The percentage of antibiotic prescriptions decreased by 20% after implementation, falling from 83.6% before to 63.6% after the implementation (P =.01). The documented change in sputum color remained low compared with antibiotic prescriptions: 17.8% preimplementation and 16.9% postimplementation. The reduction in antibiotic prescriptions was associated with limited differences observed in 30-, 60-, and 90-day reexacerbation rates pre- and postintervention: 19.2% vs 23.4%, 12.3% vs 11.7%, and 4.1% vs 9.1%, respectively.
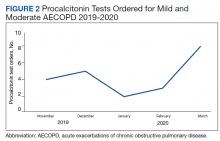
Prior to the education, introduction of the algorithm, and implementation of the PCT quick-order menu, PCT was ordered for 1.4% of AECOPD cases. Postintervention, PCT was ordered for 28.6% of mild-to-moderate AECOPD cases and used in clinical decision making per clinical documentation 81.8% of the time. PCT was used in 5 GOLD group B patients, 5 GOLD group C patients, and 7 GOLD group D patients. In all cases, PCT was < 0.25 ng/mL. In 4 cases PCT was ordered but not used: 1 GOLD group D patient refused traditional treatment with oral corticosteroids, which resulted in the clinician prescribing antibiotics, and the other 3 cases did not use PCT based on clinical decision making. The rate of PCT tests ordered for mild-to-moderate AECOPD over time is depicted in Figure 2.
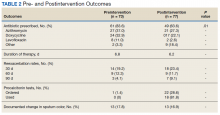
The average duration of antibiotic therapy was about 6 days pre- and postintervention. This is longer than the PVAHCS recommended duration of 5 days but is consistent with the GOLD guidelines recommended duration of 5 to 7 days.1 Azithromycin is recommended as a first-line treatment option at the PVAHCS based on the local antibiogram, and it remained the most commonly prescribed antibiotic pre- and postintervention. Outcomes of interest are detailed in Table 2.
Discussion
The implementation of PCT-guided antibiotic prescribing for patients with mild and moderate AECOPD who presented to the ED resulted in a 20% reduction in antibiotic prescriptions, falling from 83.6% before the intervention to 63.6% afterward (P = .01). The measured decrease in antibiotic prescriptions is consistent with other studies evaluating the use of acute phase reactants to guide antibiotic prescribing for AECOPD.10,11 In addition, there was no observed difference in reexacerbation rates. This adds to the increasing body of evidence that antibiotics are overprescribed in mild and moderate AECOPD.12 This is exemplified in our data by the low percentage of patients who had a documented change in sputum color; symptoms that are well known to be highly specific and sensitive for a bacterial infection in AECOPD.
Many health care providers (HCPs) in the ED were unfamiliar with PCT prior to implementation. A primary concern with this study was its impact on diagnostic stewardship. Preimplementation, ED clinicians ordered PCT 8 times for any cause. Postintervention, ED clinicians ordered PCT 180 times for any cause: 36% of these orders were for patients with AECOPD who were discharged from the ED or who required hospital admission. The other orders were for other respiratory conditions, including asthma exacerbations, pneumonia, bronchitis, sinusitis, pharyngitis, nonspecific respiratory infections, and respiratory failure.
The early phase of the COVID-19 pandemic coincided with the postintervention phase of this project. PVAHCS started preparing for the pandemic in March 2020, and the first confirmed diagnosis at the facility occurred mid-March. COVID-19 may have contributed to the sharp increase in PCT tests. There is currently no well-defined role for PCT in the diagnosis or management of COVID-19, but ED clinicians may have increased their use of PCT tests to help characterize the etiology of the large influx of patients presenting with respiratory symptoms.13
Strengths
Strengths of this project include its multimodal implementation and overall pragmatic design, which reflects real-world utilization of procalcitonin by ED HCPs. The HCPs were not mandated to follow the procalcitonin algorithm, and the use of clinical judgment was strongly encouraged. This project occurred concomitantly with the VA Infectious Disease Academic Detailing education program. The program focused on clinician education for the proper diagnosis and treatment of respiratory tract infections. In addition, viral illness packs were introduced as part of this initiative to reduce unnecessary antibiotic prescribing. The viral illness pack included standard items for symptom relief, such as saline nasal spray, cough drops, and hand sanitizer, as well as an explanation card of why the patient was not receiving antibiotics. Several studies have suggested that patients expect a prescription for an antibiotic when they present with respiratory tract symptoms, and HCPs often are compelled to maintain patient satisfaction, thus leading to unnecessary antibiotic prescriptions.14 The viral illness pack helped fulfill the patient’s expectation to receive treatment after seeking care. In addition, the project lead was available full time during the first month of PCT algorithm implementation to address questions and concerns, which may have improved HCPs overall confidence in using PCT.
Limitations
Limitations of this project include its population and its retrospective nature. The PVAHCS patient population is predominantly older, more White, and more male compared with the general civilian population, and results may not be generalizable to other populations. Data were limited to documentation in the electronic health record. The population was based on data extraction by the ICD-10 code, which may not be an accurate capture of the total population as HCPs may not select the most accurate ICD-10 code on documentation. Another potential limitation was the COVID-19 pandemic which may have resulted in HCPs ordering PCT more frequently as more patients presented to the ED with undifferentiated respiratory symptoms. Finally, there were minimal differences observed in reexacerbation rates; however, although the sample size was powered to detect a difference in antibiotic prescriptions, the sample size was not powered to detect a statistically significant difference in the primary safety outcome.
Conclusions
PCT-guided antibiotic prescribing significantly reduced the number of antibiotic prescriptions without an observable increase in reexacerbation rates for patients with mild and moderate AECOPD in the ED. This study provides a pragmatic evaluation of PCT-guided antibiotic prescribing for patients with AECOPD solely in the outpatient setting. Acute phase reactants like PCT can play a role in the management of AECOPD to reduce unnecessary antibiotic prescriptions.
1. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease: 2020 report. Accessd June 2, 2021. http://www.goldcopd.org/wp-content/uploads/2019/12/GOLD-2020-FINAL-ver1.2-03Dec19_WMV.pdf
2. George SN, Garcha DS, Mackay AJ, et al. Human rhinovirus infection during naturally occurring COPD exacerbations. Eur Respir J. 2014;44(1):87-96. doi:10.1183/09031936.00223113
3. Seemungal T, Harper-Owen R, Bhowmik A, et al. Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164(9):1618-1623. doi:10.1164/ajrccm.164.9.2105011
4. Rohde G, Wiethege A, Borg I, et al. Respiratory viruses in exacerbations of chronic obstructive pulmonary disease requiring hospitalisation: a case-control study. Thorax. 2003;58(1):37-42. doi:10.1136/thorax.58.1.37
5. Bremmer DN, Moffa MA, Ma K, et al. Acute exacerbations of chronic obstructive pulmonary disease with a low procalcitonin concentration: impact of antibiotic therapy. Clin Infect Dis. 2019;68(5):725-730. doi:10.1093/cid/ciy552
6. Mathioudakis AG, Chatzimavridou-Grigoriadou V, Corlateanu A, Vestbo J. Procalcitonin to guide antibiotic administration in COPD exacerbations: a meta-analysis. Eur Respir Rev. 2017;26(143):160073. Published 2017 Jan 31. doi:10.1183/16000617.0073-2016
7. van der Does Y, Rood PP, Haagsma JA, Patka P, van Gorp EC, Limper M. Procalcitonin-guided therapy for the initiation of antibiotics in the ED: a systematic review. Am J Emerg Med. 2016;34(7):1286-1293. doi:10.1016/j.ajem.2016.03.065
8. Huang DT, Yealy DM, Filbin MR, et al. Procalcitonin-guided use of antibiotics for lower respiratory tract infection. N Engl J Med. 2018;379(3):236-249. doi:10.1056/NEJMoa1802670
9. Picart J, Moiton MP, Gaüzère BA, Gazaille V, Combes X, DiBernardo S. Introduction of a PCT-based algorithm to guide antibiotic prescription in COPD exacerbation. Med Mal Infect. 2016;46(8):429-435. doi:10.1016/j.medmal.2016.07.008
10. Schuetz P, Chiappa V, Briel M, Greenwald JL. Procalcitonin algorithms for antibiotic therapy decisions: a systematic review of randomized controlled trials and recommendations for clinical algorithms. Arch Intern Med. 2011;171(15):1322-1331. doi:10.1001/archinternmed.2011.318
11. Butler CC, Gillespie D, White P, et al. C-reactive protein testing to guide antibiotic prescribing for COPD exacerbations. N Engl J Med. 2019;381(2):111-120. |doi:10.1056/NEJMoa1803185
12. Vollenweider DJ, Frei A, Steurer-Stey CA, Garcia-Aymerich J, Puhan MA. Antibiotics for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2018;10(10):CD010257. Published 2018 Oct 29. doi:10.1002/14651858.CD010257.pub2
13. Centers for Disease Control and Prevention. Interim clinical guidance for management of patients with confirmed coronavirus disease (COVID-19). Updated February 16, 2021. Accessed May 14, 2021. https://www.cdc.gov/coronavirus/2019ncov/hcp/clinical-guidance-management-patients.html
14. Gaarslev C, Yee M, Chan G, Fletcher-Lartey S, Khan R. A mixed methods study to understand patient expectations for antibiotics for an upper respiratory tract infection. Antimicrob Resist Infect Control. 2016;5:39. Published 2016 Oct 20. doi:10.1186/s13756-016-0134-3
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines define acute exacerbations of chronic obstructive pulmonary disease (AECOPD) as a sudden worsening of respiratory symptoms that require additional interventions. Exacerbations are classified as mild (treated with short-acting bronchodilators only), moderate (treated with antibiotics and/or oral corticosteroids), or severe (treatment requiring hospitalization). Exacerbations must include increased dyspnea, and other symptoms may involve increased sputum volume and purulence, cough, and a change in sputum color. These symptoms can be due to viral, bacterial, or environmental causes, with viral respiratory infections being the most common cause.1-4 However, determining the etiology of an exacerbation can be difficult based on symptoms alone and can lead to an excessive and unnecessary use of antibiotics. Only the change in sputum color is considered highly sensitive and specific for bacterial causes.1 As a result, there has been an increased interest in the use of acute biomarkers to determine whether antibiotics are necessary.
Procalcitonin (PCT) is an acute phase reactant that increases in response to inflammation, especially inflammation caused by a bacterial infection. Recent studies have suggested that PCT may be used in patients experiencing an AECOPD to reduce antibiotic use without impacting rates of treatment failure.5-9 A majority of these studies have been in the inpatient setting or a combination of inpatient and outpatient settings.
The purpose of this study was to create and to evaluate the efficacy and practicality of a PCT-based algorithm to aid emergency department (ED) clinicians in the evaluation of patients with AECOPD who do not require hospitalization. The primary outcome of this project was the rate of antibiotic prescriptions before and after the initiation of the algorithm.
Methods
This was an observational, retrospective, pre/post assessment at the Phoenix Veterans Affairs Health Care System (PVAHCS) in Arizona. Patients who were discharged from the ED with a diagnosis of an AECOPD were identified using International Classification of Diseases, Tenth Revision (ICD-10) codes. Patient charts were reviewed from November 2018 to March 2019 for the preimplementation group and from November 2019 for March 2020 in the postimplementation group. The periods were chosen to reflect similar seasons for both the pre- and postimplementation interventions. Patients were excluded from analysis if they required hospital admission, were immunocompromised, on chronic antimicrobial therapy, had no documented medical history of COPD, or if they were presenting primarily for medication refills. Information collected included the rate of antibiotic prescriptions, procalcitonin test orders, COPD GOLD classification, and 30-, 60-, and 90-day reexacerbation rates.
A PCT-based algorithm (Figure 1) was developed and approved by the PVAHCS Antimicrobial Stewardship Program, the Pharmacy and Therapeutics committee, and ED leadership. PCT threshold values were based on values approved by the US Food and Drug Administration and previous studies—antibiotics were discouraged for PCT levels ≤ 0.25 ng/mL but could be considered for PCT levels > 0.25 ng/mL.5,8,9 Clinicians were not required to use the algorithm, and the use of clinical judgement was encouraged. The recommended antibiotic therapies were based on previously approved PVAHCS antimicrobial stewardship guidance. To promote utilization, a PCT quick order option was added to the ED laboratory order menu.
ED clinicians were individually educated by the antimicrobial stewardship and emergency medicine pharmacists, an infectious disease physician champion, and the pharmacy resident. Clinicians were educated about PCT and its use in the setting of AECOPD to aid in the determination of bacterial infections. Each clinician received an electronic copy the algorithm and summary of the study protocol before implementation and 3 months after implementation for follow-up education. In addition, a printed copy of the algorithm was posted in multiple clinician workstations within the ED. For the first month of implementation, the project lead was available full-time in the ED to encourage algorithm use and to field questions or concerns from clinicians.
Outcome Measures
The primary outcome was the rate of antibiotic prescriptions pre- and postintervention. The safety endpoints were 30-, 60-, and 90-day reexacerbation rates. Reexacerbation rates were defined by ICD-10 codes and documentation from a primary care visit or subsequent ED visit. The secondary outcomes were the rate of PCT tests ordered and used for treatment decisions. Other areas of interest were antibiotic prescribing trends, duration of therapy, and patient COPD GOLD classification.
Statistical analysis
It was estimated that a sample size of 146 patients (73 patients/group) would provide 80% power to detect a between-group difference of 10% in the percentage of patients who were prescribed antibiotics. Categorical variables were expressed using estimates of frequency and percentages. Percentages were compared using Fisher exact tests. For all tests, the significance level was set at 0.05.
Results
Seventy-three patients were included in the preintervention group and 77 in the postintervention group. The GOLD classification rates were similar between the groups (Table 1). In addition, > 90% of patients were White males and all patients were aged ≥ 50 years, which is characteristic of the US Department of Veterans Affairs (VA) population.
The percentage of antibiotic prescriptions decreased by 20% after implementation, falling from 83.6% before to 63.6% after the implementation (P =.01). The documented change in sputum color remained low compared with antibiotic prescriptions: 17.8% preimplementation and 16.9% postimplementation. The reduction in antibiotic prescriptions was associated with limited differences observed in 30-, 60-, and 90-day reexacerbation rates pre- and postintervention: 19.2% vs 23.4%, 12.3% vs 11.7%, and 4.1% vs 9.1%, respectively.
Prior to the education, introduction of the algorithm, and implementation of the PCT quick-order menu, PCT was ordered for 1.4% of AECOPD cases. Postintervention, PCT was ordered for 28.6% of mild-to-moderate AECOPD cases and used in clinical decision making per clinical documentation 81.8% of the time. PCT was used in 5 GOLD group B patients, 5 GOLD group C patients, and 7 GOLD group D patients. In all cases, PCT was < 0.25 ng/mL. In 4 cases PCT was ordered but not used: 1 GOLD group D patient refused traditional treatment with oral corticosteroids, which resulted in the clinician prescribing antibiotics, and the other 3 cases did not use PCT based on clinical decision making. The rate of PCT tests ordered for mild-to-moderate AECOPD over time is depicted in Figure 2.
The average duration of antibiotic therapy was about 6 days pre- and postintervention. This is longer than the PVAHCS recommended duration of 5 days but is consistent with the GOLD guidelines recommended duration of 5 to 7 days.1 Azithromycin is recommended as a first-line treatment option at the PVAHCS based on the local antibiogram, and it remained the most commonly prescribed antibiotic pre- and postintervention. Outcomes of interest are detailed in Table 2.
Discussion
The implementation of PCT-guided antibiotic prescribing for patients with mild and moderate AECOPD who presented to the ED resulted in a 20% reduction in antibiotic prescriptions, falling from 83.6% before the intervention to 63.6% afterward (P = .01). The measured decrease in antibiotic prescriptions is consistent with other studies evaluating the use of acute phase reactants to guide antibiotic prescribing for AECOPD.10,11 In addition, there was no observed difference in reexacerbation rates. This adds to the increasing body of evidence that antibiotics are overprescribed in mild and moderate AECOPD.12 This is exemplified in our data by the low percentage of patients who had a documented change in sputum color; symptoms that are well known to be highly specific and sensitive for a bacterial infection in AECOPD.
Many health care providers (HCPs) in the ED were unfamiliar with PCT prior to implementation. A primary concern with this study was its impact on diagnostic stewardship. Preimplementation, ED clinicians ordered PCT 8 times for any cause. Postintervention, ED clinicians ordered PCT 180 times for any cause: 36% of these orders were for patients with AECOPD who were discharged from the ED or who required hospital admission. The other orders were for other respiratory conditions, including asthma exacerbations, pneumonia, bronchitis, sinusitis, pharyngitis, nonspecific respiratory infections, and respiratory failure.
The early phase of the COVID-19 pandemic coincided with the postintervention phase of this project. PVAHCS started preparing for the pandemic in March 2020, and the first confirmed diagnosis at the facility occurred mid-March. COVID-19 may have contributed to the sharp increase in PCT tests. There is currently no well-defined role for PCT in the diagnosis or management of COVID-19, but ED clinicians may have increased their use of PCT tests to help characterize the etiology of the large influx of patients presenting with respiratory symptoms.13
Strengths
Strengths of this project include its multimodal implementation and overall pragmatic design, which reflects real-world utilization of procalcitonin by ED HCPs. The HCPs were not mandated to follow the procalcitonin algorithm, and the use of clinical judgment was strongly encouraged. This project occurred concomitantly with the VA Infectious Disease Academic Detailing education program. The program focused on clinician education for the proper diagnosis and treatment of respiratory tract infections. In addition, viral illness packs were introduced as part of this initiative to reduce unnecessary antibiotic prescribing. The viral illness pack included standard items for symptom relief, such as saline nasal spray, cough drops, and hand sanitizer, as well as an explanation card of why the patient was not receiving antibiotics. Several studies have suggested that patients expect a prescription for an antibiotic when they present with respiratory tract symptoms, and HCPs often are compelled to maintain patient satisfaction, thus leading to unnecessary antibiotic prescriptions.14 The viral illness pack helped fulfill the patient’s expectation to receive treatment after seeking care. In addition, the project lead was available full time during the first month of PCT algorithm implementation to address questions and concerns, which may have improved HCPs overall confidence in using PCT.
Limitations
Limitations of this project include its population and its retrospective nature. The PVAHCS patient population is predominantly older, more White, and more male compared with the general civilian population, and results may not be generalizable to other populations. Data were limited to documentation in the electronic health record. The population was based on data extraction by the ICD-10 code, which may not be an accurate capture of the total population as HCPs may not select the most accurate ICD-10 code on documentation. Another potential limitation was the COVID-19 pandemic which may have resulted in HCPs ordering PCT more frequently as more patients presented to the ED with undifferentiated respiratory symptoms. Finally, there were minimal differences observed in reexacerbation rates; however, although the sample size was powered to detect a difference in antibiotic prescriptions, the sample size was not powered to detect a statistically significant difference in the primary safety outcome.
Conclusions
PCT-guided antibiotic prescribing significantly reduced the number of antibiotic prescriptions without an observable increase in reexacerbation rates for patients with mild and moderate AECOPD in the ED. This study provides a pragmatic evaluation of PCT-guided antibiotic prescribing for patients with AECOPD solely in the outpatient setting. Acute phase reactants like PCT can play a role in the management of AECOPD to reduce unnecessary antibiotic prescriptions.
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines define acute exacerbations of chronic obstructive pulmonary disease (AECOPD) as a sudden worsening of respiratory symptoms that require additional interventions. Exacerbations are classified as mild (treated with short-acting bronchodilators only), moderate (treated with antibiotics and/or oral corticosteroids), or severe (treatment requiring hospitalization). Exacerbations must include increased dyspnea, and other symptoms may involve increased sputum volume and purulence, cough, and a change in sputum color. These symptoms can be due to viral, bacterial, or environmental causes, with viral respiratory infections being the most common cause.1-4 However, determining the etiology of an exacerbation can be difficult based on symptoms alone and can lead to an excessive and unnecessary use of antibiotics. Only the change in sputum color is considered highly sensitive and specific for bacterial causes.1 As a result, there has been an increased interest in the use of acute biomarkers to determine whether antibiotics are necessary.
Procalcitonin (PCT) is an acute phase reactant that increases in response to inflammation, especially inflammation caused by a bacterial infection. Recent studies have suggested that PCT may be used in patients experiencing an AECOPD to reduce antibiotic use without impacting rates of treatment failure.5-9 A majority of these studies have been in the inpatient setting or a combination of inpatient and outpatient settings.
The purpose of this study was to create and to evaluate the efficacy and practicality of a PCT-based algorithm to aid emergency department (ED) clinicians in the evaluation of patients with AECOPD who do not require hospitalization. The primary outcome of this project was the rate of antibiotic prescriptions before and after the initiation of the algorithm.
Methods
This was an observational, retrospective, pre/post assessment at the Phoenix Veterans Affairs Health Care System (PVAHCS) in Arizona. Patients who were discharged from the ED with a diagnosis of an AECOPD were identified using International Classification of Diseases, Tenth Revision (ICD-10) codes. Patient charts were reviewed from November 2018 to March 2019 for the preimplementation group and from November 2019 for March 2020 in the postimplementation group. The periods were chosen to reflect similar seasons for both the pre- and postimplementation interventions. Patients were excluded from analysis if they required hospital admission, were immunocompromised, on chronic antimicrobial therapy, had no documented medical history of COPD, or if they were presenting primarily for medication refills. Information collected included the rate of antibiotic prescriptions, procalcitonin test orders, COPD GOLD classification, and 30-, 60-, and 90-day reexacerbation rates.
A PCT-based algorithm (Figure 1) was developed and approved by the PVAHCS Antimicrobial Stewardship Program, the Pharmacy and Therapeutics committee, and ED leadership. PCT threshold values were based on values approved by the US Food and Drug Administration and previous studies—antibiotics were discouraged for PCT levels ≤ 0.25 ng/mL but could be considered for PCT levels > 0.25 ng/mL.5,8,9 Clinicians were not required to use the algorithm, and the use of clinical judgement was encouraged. The recommended antibiotic therapies were based on previously approved PVAHCS antimicrobial stewardship guidance. To promote utilization, a PCT quick order option was added to the ED laboratory order menu.
ED clinicians were individually educated by the antimicrobial stewardship and emergency medicine pharmacists, an infectious disease physician champion, and the pharmacy resident. Clinicians were educated about PCT and its use in the setting of AECOPD to aid in the determination of bacterial infections. Each clinician received an electronic copy the algorithm and summary of the study protocol before implementation and 3 months after implementation for follow-up education. In addition, a printed copy of the algorithm was posted in multiple clinician workstations within the ED. For the first month of implementation, the project lead was available full-time in the ED to encourage algorithm use and to field questions or concerns from clinicians.
Outcome Measures
The primary outcome was the rate of antibiotic prescriptions pre- and postintervention. The safety endpoints were 30-, 60-, and 90-day reexacerbation rates. Reexacerbation rates were defined by ICD-10 codes and documentation from a primary care visit or subsequent ED visit. The secondary outcomes were the rate of PCT tests ordered and used for treatment decisions. Other areas of interest were antibiotic prescribing trends, duration of therapy, and patient COPD GOLD classification.
Statistical analysis
It was estimated that a sample size of 146 patients (73 patients/group) would provide 80% power to detect a between-group difference of 10% in the percentage of patients who were prescribed antibiotics. Categorical variables were expressed using estimates of frequency and percentages. Percentages were compared using Fisher exact tests. For all tests, the significance level was set at 0.05.
Results
Seventy-three patients were included in the preintervention group and 77 in the postintervention group. The GOLD classification rates were similar between the groups (Table 1). In addition, > 90% of patients were White males and all patients were aged ≥ 50 years, which is characteristic of the US Department of Veterans Affairs (VA) population.
The percentage of antibiotic prescriptions decreased by 20% after implementation, falling from 83.6% before to 63.6% after the implementation (P =.01). The documented change in sputum color remained low compared with antibiotic prescriptions: 17.8% preimplementation and 16.9% postimplementation. The reduction in antibiotic prescriptions was associated with limited differences observed in 30-, 60-, and 90-day reexacerbation rates pre- and postintervention: 19.2% vs 23.4%, 12.3% vs 11.7%, and 4.1% vs 9.1%, respectively.
Prior to the education, introduction of the algorithm, and implementation of the PCT quick-order menu, PCT was ordered for 1.4% of AECOPD cases. Postintervention, PCT was ordered for 28.6% of mild-to-moderate AECOPD cases and used in clinical decision making per clinical documentation 81.8% of the time. PCT was used in 5 GOLD group B patients, 5 GOLD group C patients, and 7 GOLD group D patients. In all cases, PCT was < 0.25 ng/mL. In 4 cases PCT was ordered but not used: 1 GOLD group D patient refused traditional treatment with oral corticosteroids, which resulted in the clinician prescribing antibiotics, and the other 3 cases did not use PCT based on clinical decision making. The rate of PCT tests ordered for mild-to-moderate AECOPD over time is depicted in Figure 2.
The average duration of antibiotic therapy was about 6 days pre- and postintervention. This is longer than the PVAHCS recommended duration of 5 days but is consistent with the GOLD guidelines recommended duration of 5 to 7 days.1 Azithromycin is recommended as a first-line treatment option at the PVAHCS based on the local antibiogram, and it remained the most commonly prescribed antibiotic pre- and postintervention. Outcomes of interest are detailed in Table 2.
Discussion
The implementation of PCT-guided antibiotic prescribing for patients with mild and moderate AECOPD who presented to the ED resulted in a 20% reduction in antibiotic prescriptions, falling from 83.6% before the intervention to 63.6% afterward (P = .01). The measured decrease in antibiotic prescriptions is consistent with other studies evaluating the use of acute phase reactants to guide antibiotic prescribing for AECOPD.10,11 In addition, there was no observed difference in reexacerbation rates. This adds to the increasing body of evidence that antibiotics are overprescribed in mild and moderate AECOPD.12 This is exemplified in our data by the low percentage of patients who had a documented change in sputum color; symptoms that are well known to be highly specific and sensitive for a bacterial infection in AECOPD.
Many health care providers (HCPs) in the ED were unfamiliar with PCT prior to implementation. A primary concern with this study was its impact on diagnostic stewardship. Preimplementation, ED clinicians ordered PCT 8 times for any cause. Postintervention, ED clinicians ordered PCT 180 times for any cause: 36% of these orders were for patients with AECOPD who were discharged from the ED or who required hospital admission. The other orders were for other respiratory conditions, including asthma exacerbations, pneumonia, bronchitis, sinusitis, pharyngitis, nonspecific respiratory infections, and respiratory failure.
The early phase of the COVID-19 pandemic coincided with the postintervention phase of this project. PVAHCS started preparing for the pandemic in March 2020, and the first confirmed diagnosis at the facility occurred mid-March. COVID-19 may have contributed to the sharp increase in PCT tests. There is currently no well-defined role for PCT in the diagnosis or management of COVID-19, but ED clinicians may have increased their use of PCT tests to help characterize the etiology of the large influx of patients presenting with respiratory symptoms.13
Strengths
Strengths of this project include its multimodal implementation and overall pragmatic design, which reflects real-world utilization of procalcitonin by ED HCPs. The HCPs were not mandated to follow the procalcitonin algorithm, and the use of clinical judgment was strongly encouraged. This project occurred concomitantly with the VA Infectious Disease Academic Detailing education program. The program focused on clinician education for the proper diagnosis and treatment of respiratory tract infections. In addition, viral illness packs were introduced as part of this initiative to reduce unnecessary antibiotic prescribing. The viral illness pack included standard items for symptom relief, such as saline nasal spray, cough drops, and hand sanitizer, as well as an explanation card of why the patient was not receiving antibiotics. Several studies have suggested that patients expect a prescription for an antibiotic when they present with respiratory tract symptoms, and HCPs often are compelled to maintain patient satisfaction, thus leading to unnecessary antibiotic prescriptions.14 The viral illness pack helped fulfill the patient’s expectation to receive treatment after seeking care. In addition, the project lead was available full time during the first month of PCT algorithm implementation to address questions and concerns, which may have improved HCPs overall confidence in using PCT.
Limitations
Limitations of this project include its population and its retrospective nature. The PVAHCS patient population is predominantly older, more White, and more male compared with the general civilian population, and results may not be generalizable to other populations. Data were limited to documentation in the electronic health record. The population was based on data extraction by the ICD-10 code, which may not be an accurate capture of the total population as HCPs may not select the most accurate ICD-10 code on documentation. Another potential limitation was the COVID-19 pandemic which may have resulted in HCPs ordering PCT more frequently as more patients presented to the ED with undifferentiated respiratory symptoms. Finally, there were minimal differences observed in reexacerbation rates; however, although the sample size was powered to detect a difference in antibiotic prescriptions, the sample size was not powered to detect a statistically significant difference in the primary safety outcome.
Conclusions
PCT-guided antibiotic prescribing significantly reduced the number of antibiotic prescriptions without an observable increase in reexacerbation rates for patients with mild and moderate AECOPD in the ED. This study provides a pragmatic evaluation of PCT-guided antibiotic prescribing for patients with AECOPD solely in the outpatient setting. Acute phase reactants like PCT can play a role in the management of AECOPD to reduce unnecessary antibiotic prescriptions.
1. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease: 2020 report. Accessd June 2, 2021. http://www.goldcopd.org/wp-content/uploads/2019/12/GOLD-2020-FINAL-ver1.2-03Dec19_WMV.pdf
2. George SN, Garcha DS, Mackay AJ, et al. Human rhinovirus infection during naturally occurring COPD exacerbations. Eur Respir J. 2014;44(1):87-96. doi:10.1183/09031936.00223113
3. Seemungal T, Harper-Owen R, Bhowmik A, et al. Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164(9):1618-1623. doi:10.1164/ajrccm.164.9.2105011
4. Rohde G, Wiethege A, Borg I, et al. Respiratory viruses in exacerbations of chronic obstructive pulmonary disease requiring hospitalisation: a case-control study. Thorax. 2003;58(1):37-42. doi:10.1136/thorax.58.1.37
5. Bremmer DN, Moffa MA, Ma K, et al. Acute exacerbations of chronic obstructive pulmonary disease with a low procalcitonin concentration: impact of antibiotic therapy. Clin Infect Dis. 2019;68(5):725-730. doi:10.1093/cid/ciy552
6. Mathioudakis AG, Chatzimavridou-Grigoriadou V, Corlateanu A, Vestbo J. Procalcitonin to guide antibiotic administration in COPD exacerbations: a meta-analysis. Eur Respir Rev. 2017;26(143):160073. Published 2017 Jan 31. doi:10.1183/16000617.0073-2016
7. van der Does Y, Rood PP, Haagsma JA, Patka P, van Gorp EC, Limper M. Procalcitonin-guided therapy for the initiation of antibiotics in the ED: a systematic review. Am J Emerg Med. 2016;34(7):1286-1293. doi:10.1016/j.ajem.2016.03.065
8. Huang DT, Yealy DM, Filbin MR, et al. Procalcitonin-guided use of antibiotics for lower respiratory tract infection. N Engl J Med. 2018;379(3):236-249. doi:10.1056/NEJMoa1802670
9. Picart J, Moiton MP, Gaüzère BA, Gazaille V, Combes X, DiBernardo S. Introduction of a PCT-based algorithm to guide antibiotic prescription in COPD exacerbation. Med Mal Infect. 2016;46(8):429-435. doi:10.1016/j.medmal.2016.07.008
10. Schuetz P, Chiappa V, Briel M, Greenwald JL. Procalcitonin algorithms for antibiotic therapy decisions: a systematic review of randomized controlled trials and recommendations for clinical algorithms. Arch Intern Med. 2011;171(15):1322-1331. doi:10.1001/archinternmed.2011.318
11. Butler CC, Gillespie D, White P, et al. C-reactive protein testing to guide antibiotic prescribing for COPD exacerbations. N Engl J Med. 2019;381(2):111-120. |doi:10.1056/NEJMoa1803185
12. Vollenweider DJ, Frei A, Steurer-Stey CA, Garcia-Aymerich J, Puhan MA. Antibiotics for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2018;10(10):CD010257. Published 2018 Oct 29. doi:10.1002/14651858.CD010257.pub2
13. Centers for Disease Control and Prevention. Interim clinical guidance for management of patients with confirmed coronavirus disease (COVID-19). Updated February 16, 2021. Accessed May 14, 2021. https://www.cdc.gov/coronavirus/2019ncov/hcp/clinical-guidance-management-patients.html
14. Gaarslev C, Yee M, Chan G, Fletcher-Lartey S, Khan R. A mixed methods study to understand patient expectations for antibiotics for an upper respiratory tract infection. Antimicrob Resist Infect Control. 2016;5:39. Published 2016 Oct 20. doi:10.1186/s13756-016-0134-3
1. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease: 2020 report. Accessd June 2, 2021. http://www.goldcopd.org/wp-content/uploads/2019/12/GOLD-2020-FINAL-ver1.2-03Dec19_WMV.pdf
2. George SN, Garcha DS, Mackay AJ, et al. Human rhinovirus infection during naturally occurring COPD exacerbations. Eur Respir J. 2014;44(1):87-96. doi:10.1183/09031936.00223113
3. Seemungal T, Harper-Owen R, Bhowmik A, et al. Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164(9):1618-1623. doi:10.1164/ajrccm.164.9.2105011
4. Rohde G, Wiethege A, Borg I, et al. Respiratory viruses in exacerbations of chronic obstructive pulmonary disease requiring hospitalisation: a case-control study. Thorax. 2003;58(1):37-42. doi:10.1136/thorax.58.1.37
5. Bremmer DN, Moffa MA, Ma K, et al. Acute exacerbations of chronic obstructive pulmonary disease with a low procalcitonin concentration: impact of antibiotic therapy. Clin Infect Dis. 2019;68(5):725-730. doi:10.1093/cid/ciy552
6. Mathioudakis AG, Chatzimavridou-Grigoriadou V, Corlateanu A, Vestbo J. Procalcitonin to guide antibiotic administration in COPD exacerbations: a meta-analysis. Eur Respir Rev. 2017;26(143):160073. Published 2017 Jan 31. doi:10.1183/16000617.0073-2016
7. van der Does Y, Rood PP, Haagsma JA, Patka P, van Gorp EC, Limper M. Procalcitonin-guided therapy for the initiation of antibiotics in the ED: a systematic review. Am J Emerg Med. 2016;34(7):1286-1293. doi:10.1016/j.ajem.2016.03.065
8. Huang DT, Yealy DM, Filbin MR, et al. Procalcitonin-guided use of antibiotics for lower respiratory tract infection. N Engl J Med. 2018;379(3):236-249. doi:10.1056/NEJMoa1802670
9. Picart J, Moiton MP, Gaüzère BA, Gazaille V, Combes X, DiBernardo S. Introduction of a PCT-based algorithm to guide antibiotic prescription in COPD exacerbation. Med Mal Infect. 2016;46(8):429-435. doi:10.1016/j.medmal.2016.07.008
10. Schuetz P, Chiappa V, Briel M, Greenwald JL. Procalcitonin algorithms for antibiotic therapy decisions: a systematic review of randomized controlled trials and recommendations for clinical algorithms. Arch Intern Med. 2011;171(15):1322-1331. doi:10.1001/archinternmed.2011.318
11. Butler CC, Gillespie D, White P, et al. C-reactive protein testing to guide antibiotic prescribing for COPD exacerbations. N Engl J Med. 2019;381(2):111-120. |doi:10.1056/NEJMoa1803185
12. Vollenweider DJ, Frei A, Steurer-Stey CA, Garcia-Aymerich J, Puhan MA. Antibiotics for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2018;10(10):CD010257. Published 2018 Oct 29. doi:10.1002/14651858.CD010257.pub2
13. Centers for Disease Control and Prevention. Interim clinical guidance for management of patients with confirmed coronavirus disease (COVID-19). Updated February 16, 2021. Accessed May 14, 2021. https://www.cdc.gov/coronavirus/2019ncov/hcp/clinical-guidance-management-patients.html
14. Gaarslev C, Yee M, Chan G, Fletcher-Lartey S, Khan R. A mixed methods study to understand patient expectations for antibiotics for an upper respiratory tract infection. Antimicrob Resist Infect Control. 2016;5:39. Published 2016 Oct 20. doi:10.1186/s13756-016-0134-3