User login
A Veteran Presenting With Shortness of Breath, Cough, and Leukocytosis
Case Presentation: A 62-year-old male presented with shortness of breath and a cough productive of green sputum. He had a history of hyperlipidemia, posttraumatic stress disorder, bipolar disorder, obstructive sleep apnea, and a 50 pack-year history of smoking. His medications included prazosin, melatonin, lithium, and gabapentin. He also had a significant exposure history including asbestos and chemical paints following his leave from the military. At the initial evaluation, laboratory work revealed a leukocytosis with white blood cell (WBC) count 20 k/cm3and otherwise normal transaminases, albumin, and electrolytes. A chest X-ray revealed a new left hilar mass.
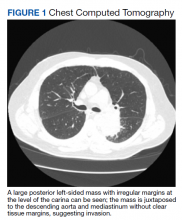
►Manisha Apte, MD, Chief Medical Resident, VA Boston Healthcare System (VABHS) and Boston Medical Center (BMC): To work up his new left hilar mass, a computed tomography (CT) of the chest was ordered (Figure 1), which revealed an apical left lower lobe mass extending into the left hilum encasing part of the ascending aorta. Enlarged mediastinal subcentimeter paratracheal and superior mediastinal lymph nodes also were identified and the pattern raised the concern for lymphangitic carcinomatosis. Dr. Fine, what do you make of the CT findings?
► Alan Fine, MD, Section of Pulmonary and Critical Care, VABHS and Professor of Medicine, Boston University School of Medicine: This mass had irregular edges with septal thickening, which may be why there was a concern for lymphangitic spread. There were no clear tissue planes to see if this process was invading the mediastinum. The mass was irregular, a single lesion, and proximal, making it consistent with a lung cancer. In fact, with his history of smoking, asbestos exposure, the numbers 1 to 10 diagnoses were lung cancer. The lack of demarcation of tissue planes supports this. There are some infections, classically actinomycosis, that do cross and invade anatomical barriers.1 But this looked like a primary lung cancer.
► Dr. Apte: The patient was referred to a pulmonologist where an additional history of night sweats and weight loss were noted. Dr. Fine, we have a patient with a newly identified lung mass, and while we have reason to suspect malignancy as you have already noted, there are many other etiologies to consider, including infections (histoplasmosis, cryptococcosis, bacterial abscess), inflammatory processes (sarcoidosis, rheumatoid nodule) and vasculitis (granulomatosis with polyangiitis). What should be the next step taken to make a diagnosis?
►Dr. Fine: For cancer specifically, we would like to both stage and make a diagnosis with one procedure. That’s part of the utility of a positron emission tomography (PET) scan: We can see lymph node involvement and stage the cancer. We must consider the patient’s comorbidities and the location of the lesion (ie, is it amenable to needle biopsy?). In this case, there are enlarged mediastinal lymph nodes, so one could perform a bronchoscopy with endobronchial ultrasound, which is a relatively noninvasive way to sample the lymph nodes to ideally stage and make a diagnosis as safely as possible. If we are considering infection, needle aspiration is not as sensitive.2
► Dr. Apte: The patient underwent a PET CT, which redemonstrated the lung mass with a loss of aortic fat plane suspicious for aortic involvement as well as lymph nodes in levels 7 and 8 that were concerning for malignancy. Subsequent bronchoscopy with biopsy and endobronchial ultrasound did not show evidence of malignancy; washing and brushing from the mass and lymph node specimens did not identify malignant cells. Benign respiratory mucosa with mild chronic inflammation was noted. Dr. Fine, given the nonspecific findings on the PET scan, negative findings on our bronchoscopy, and a negative biopsy, should we be satisfied that we have ruled out cancer?
► Dr. Fine: No, bronchoscopy has its limitations. It’s highly sensitive to the diagnosis of malignancy if you can see an endobronchial lesion, but we did not see one here. You can only go so far with the scope, and it’s not uncommon for us not to be able to make the diagnosis with bronchoscopy. Malignancy is still the most likely diagnosis, and we need to work this up further. I would perform another biopsy.
►Dr. Apte: Four weeks later, the patient presented with continued shortness of breath, fatigue, and fever. A repeat chest CT showed an opacity suggestive of pneumonia. Given the continued concern for cancer a CT-guided needle biopsy was performed and was once again negative for malignancy. The decision was made to pursue a video-assisted thorascopic surgery (VATS). Following the VATS, the patient developed rigors, fever, and tachycardia with new atrial fibrillation. While being evaluated hypercalcemia was identified, with further workup revealing a low parathyroid hormone (PTH) and low PTH-related peptide. Dr. Fine, the presence of hypercalcemia and a new arrythmia raised the possibility of sarcoidosis. Could this be sarcoidosis?
►Dr. Fine: Sarcoidosis is one of the great masqueraders in medicine. There is a type of sarcoidosis called nodular sarcoidosis where you see masslike distribution in the lung, but generally there are multiple masses and so this presentation would be atypical.3 There is also a phenomenon called sarcoidal reactions usually in the presence of cancer. Again, one tends to see multiple tiny lesions in the lung. It is certainly on the differential, but I would consider it to be less likely than cancer. It is also relatively common to develop atrial fibrillation after manipulation from a lung surgery.4 The other possibility I am concerned about is whether the mass is invading the mediastinum and involving the pericardium.
►Dr. Apte: Results from the VATS biopsy once again returned negative for malignancy and instead showed signs of focal micro-abscesses, atypical pneumocytes, and prominent neutrophils. A diagnosis of acute fibrinous organizing pneumonia (AFOP) was offered. Dr. Fine, what is AFOP?
►Dr. Fine: This is the first case of AFOP I had seen and probably the first case many in our department have seen. This is a relatively new entity with limited reported cases in the literature and is a pathological diagnosis originally recorded in autopsies from patients at the National Institutes of Health.5,6 Given the complexity of the lesion, the diagnosis is difficult to make. Most commonly, AFOP is associated with other systemic entities, most commonly hematologic malignancies like lymphomas and leukemias. It has also been associated with vasculitis and certain drugs. The mechanism is poorly understood, and although pneumonia is a part of the term, this just implies there is inflammation of the lung (ie, pneumonitis).
► Dr. Apte: Given the association of AFOP with underlying hematologic malignancies, an emphasis was placed on another finding: the patient’s increasing WBC count. The total WBC count had been 20 k/cm3 at the time of his lung mass discovery but had increased to > 40 k/cm3 with a differential of neutrophils > 80%. Flow cytometry was negative, and his peripheral smear was read as normal. Dr. Gilbert, what might explain this patient’s leukocytosis?
►Gary Gilbert, MD, Section of Hematology and Oncology, VABHS and Associate Professor of Medicine, Harvard Medical School (HMS): This patient had an elevated WBC for 4 months. Initially, the cause was likely lithium as this is known to cause a leukocytosis.7 More recently, the total WBC had increased and there were a couple of other abnormalities: A consistently elevated absolute monocyte count and a markedly elevated mature neutrophil count. These findings are consistent with a leukemoid reaction (ie, a WBC count > 50,000/µL from causes other than leukemia). The question becomes what is this a leukemoid reaction in response to? Once we have excluded a lung malignancy (a well-known common cause of a leukemoid reaction) we must consider a clonal myeloproliferative disorder. This is particularly true because many things that cause a leukemoid reaction (eg, lobar pneumonia) do not cause a persistently elevated neutrophil count. That this patient does have a persistently elevated neutrophil count suggests something abnormal about the neutrophils themselves.
► Dr. Apte: A bone marrow biopsy was performed. Dr. Gilbert, can you comment on this patient’s bone marrow biopsy and whether a myeloproliferative disorder may have played a role in the marked leukocytosis?
► Dr. Gilbert: The bone marrow biopsy was hyperplastic with myeloid predominance and normal maturation in all lineages. A deep sequencing analysis demonstrated the absence of chromosomal abnormalities or genetic mutations that are associated with myeloproliferative disorders. This excludes the possibility of a myeloproliferative disorder.
► Dr. Apte: The patient was started on 60 mg of prednisone daily, which led to marked improvement in his symptoms. He was discharged in stable condition but presented again with abdominal pain. A complete blood count once again showed increased WBC and new thrombocytosis. A CT angiogram (CTA) showed the prior lung mass with new signs of central necrosis. In the abdomen, new splenic and renal infarct were identified, along with signs of multiple arterial thrombi in the abdomen and internal and external iliac vessel wall thickening. These findings were read as concerning for a medium vessel vasculitis. Dr. Kaur, what are some of the imaging findings you would expect to see in vasculitis, and what about this patient’s CT is consistent with a medium vessel vasculitis?
► Maneet Kaur, MD, Section of Rheumatology, VABHS: Vasculitis is inflammation of the vessel wall that can lead to vascular injury and activation of the coagulation cascade. Sometimes these findings can be seen on imaging with evidence of stenosis, microaneurysms, and thrombosis distal to the stenosis. The nomenclature of vasculitis is not simple and has been revised many times. Medium-vessel vasculitis does not just affect the medium vessels (eg, visceral arteries) but can overlap with distal large vessels and smaller cutaneous vessels.
The first thing that comes to mind in this case is polyarteritis nodosa (PAN), an immune complex-mediated medium vessel disease that can involve large and small vessels in muscle, nerve, and skin. It can also present with masses.
►Dr. Apte: To address the arterial thrombi seen on CTA, arterial-brachial indices were obtained and showed bilateral occlusive disease in his distal extremities; findings that could be explained by vasculitis. His VATS biopsy pathology was reviewed for signs of vasculitis. Dr. Huang, can you review these slides for us please?
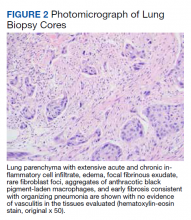
►Qin Huang, MD, Department of Pathology and Laboratory Medicine, VABHS and Assistant Professor of Pathology, HMS: This patient had a history of smoking, and there are many black pigment-laden macrophages present in the lung tissue. There were areas of hemorrhage and fibrin deposition and an overall picture of organizing pneumonia. At a lower power, you can see neutrophils everywhere, some in the form of micro-abscesses. The arterial walls did not show signs of vasculitis (Figure 2). Based on the clinical information and radiology findings, we suspected an acute infection-related pneumonia or a primary lung malignancy causing obstruction pneumonia. We suggested a rebiopsy of the lung mass to rule out a primary lung malignancy.
► Dr. Apte: Given his CT findings, a serologic rheumatologic workup including antineutrophil cytoplasmic antibody, antinuclear antibody, and rheumatoid factors were sent and returned negative. The location of arterial wall inflammation on imaging made it unamenable for biopsy. The patient began to experience bilateral temporal pain, which raised the concern for a large vessel vasculitis, specifically giant cell arteritis. Bilateral temporal artery biopsies were obtained and were not suggestive of vasculitis. Dr. Kaur, we still do not have any serologic or biopsy confirmation to support a diagnosis of vasculitis. Can we still call this a vasculitis?
►Dr. Kaur: Few things can cause the picture that was seen radiographically. A few noninflammatory causes like fibromuscular dysplasia can cause both large and small vessel stenosis, but the elevations in erythrocyte sedimentation rate and C-reactive protein along with response to steroids makes these diagnoses unlikely. Sometimes we must make a clinical diagnosis for vasculitis based on the clinical picture, and I would feel comfortable treating this patient for vasculitis.
With that said, I remain concerned that this patient also has a malignancy. His WBC increased to > 70 k/cm3 and his calcium to > 13 mg/dL. These findings are hard to explain by vasculitis alone. There are cancer-associated vasculitis, and I suspect this is the explanation here.8 His temporal pain was pointing to large vessel involvement, so he could have an undifferentiated vasculitis.
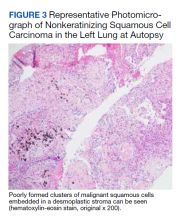
► Dr. Apte: A decision was made to empirically treat with tocilizumab, an IL-6 receptor antagonist, for an undifferentiated autoimmune disease, in addition to tapering steroids. The patient underwent a second VATS, which again revealed AFOP but no signs of malignancy. Unfortunately, he developed multiple complications over the subsequent weeks and passed away. An autopsy was requested by family members and pathology from his lung mass was reviewed. (Figure 3). Dr. Huang, can you review these slides for us?
► Dr. Huang: The left lung mass at autopsy shows nests, poorly formed clusters, and individuals of malignant neoplastic nonkeratinizing squamous cells embedded in a desmoplastic stroma in the mass center, consistent with poorly differentiated squamous cell carcinoma, and a circumscribed area of residual subacute organizing pneumonia with abscess, granulomatous changes, and early fibrosis at the periphery of this mass.
►Dr. Apte: Based on autopsy findings, the final diagnosis was poorly differentiated squamous cell carcinoma associated with subacute organizing pneumonia and medium vessel vasculitis, which presented with a severe leukocytosis ultimately thought to be a leukemoid reaction from his lung cancer.
1. Valour F, Sénéchal A, Dupieux C, et al. Actinomycosis: etiology, clinical features, diagnosis, treatment, and management. Infect Drug Resist. 2014;7:183-197. Published 2014 Jul 5. doi:10.2147/IDR.S39601
2. de Bazelaire C, Coffin A, Cohen-Zarade S, et al. CT-guided biopsies in lung infections in patients with haematological malignancies. Diagn Interv Imaging. 2013;94(2):202-215. doi:10.1016/j.diii.2012.12.008
3. Sweidan AJ, Singh NK, Stein A, Tanios M. Nodular sarcoidosis masquerading as cancer. Clin Med Insights Circ Respir Pulm Med. 2017;11:1179548417703123. Published 2017 Apr 12. doi:10.1177/1179548417703123
4. Bagheri R, Yousefi Y, Rezai R, Azemonfar V, Keshtan FG. Atrial fibrillation after lung surgery: incidence, underlying factors, and predictors. Kardiochir Torakochirurgia Pol. 2019;16(2):53-56. doi:10.5114/kitp.2019.86355
5. Lu J, Yin Q, Zha Y, et al. Acute fibrinous and organizing pneumonia: two case reports and literature review. BMC Pulm Med. 2019;19(1):141. Published 2019 Aug 5. doi:10.1186/s12890-019-0861-3
6. Beasley MB, Franks TJ, Galvin JR, Gochuico B, Travis WD. Acute fibrinous and organizing pneumonia: a histological pattern of lung injury and possible variant of diffuse alveolar damage. Arch Pathol Lab Med. 2002;126(9):1064-1070. doi:10.5858/2002-126-1064-AFAOP
7. Murphy DL, Goodwin FK, Bunney WE Jr. Leukocytosis during lithium treatment. Am J Psychiatry. 1971;127(11):1559-1561. doi:10.1176/ajp.127.11.1559
8. Fain O, Hamidou M, Cacoub P, et al. Vasculitides associated with malignancies: analysis of sixty patients. Arthritis Rheum. 2007;57(8):1473-1480. doi:10.1002/art.23085
Case Presentation: A 62-year-old male presented with shortness of breath and a cough productive of green sputum. He had a history of hyperlipidemia, posttraumatic stress disorder, bipolar disorder, obstructive sleep apnea, and a 50 pack-year history of smoking. His medications included prazosin, melatonin, lithium, and gabapentin. He also had a significant exposure history including asbestos and chemical paints following his leave from the military. At the initial evaluation, laboratory work revealed a leukocytosis with white blood cell (WBC) count 20 k/cm3and otherwise normal transaminases, albumin, and electrolytes. A chest X-ray revealed a new left hilar mass.
►Manisha Apte, MD, Chief Medical Resident, VA Boston Healthcare System (VABHS) and Boston Medical Center (BMC): To work up his new left hilar mass, a computed tomography (CT) of the chest was ordered (Figure 1), which revealed an apical left lower lobe mass extending into the left hilum encasing part of the ascending aorta. Enlarged mediastinal subcentimeter paratracheal and superior mediastinal lymph nodes also were identified and the pattern raised the concern for lymphangitic carcinomatosis. Dr. Fine, what do you make of the CT findings?
► Alan Fine, MD, Section of Pulmonary and Critical Care, VABHS and Professor of Medicine, Boston University School of Medicine: This mass had irregular edges with septal thickening, which may be why there was a concern for lymphangitic spread. There were no clear tissue planes to see if this process was invading the mediastinum. The mass was irregular, a single lesion, and proximal, making it consistent with a lung cancer. In fact, with his history of smoking, asbestos exposure, the numbers 1 to 10 diagnoses were lung cancer. The lack of demarcation of tissue planes supports this. There are some infections, classically actinomycosis, that do cross and invade anatomical barriers.1 But this looked like a primary lung cancer.
► Dr. Apte: The patient was referred to a pulmonologist where an additional history of night sweats and weight loss were noted. Dr. Fine, we have a patient with a newly identified lung mass, and while we have reason to suspect malignancy as you have already noted, there are many other etiologies to consider, including infections (histoplasmosis, cryptococcosis, bacterial abscess), inflammatory processes (sarcoidosis, rheumatoid nodule) and vasculitis (granulomatosis with polyangiitis). What should be the next step taken to make a diagnosis?
►Dr. Fine: For cancer specifically, we would like to both stage and make a diagnosis with one procedure. That’s part of the utility of a positron emission tomography (PET) scan: We can see lymph node involvement and stage the cancer. We must consider the patient’s comorbidities and the location of the lesion (ie, is it amenable to needle biopsy?). In this case, there are enlarged mediastinal lymph nodes, so one could perform a bronchoscopy with endobronchial ultrasound, which is a relatively noninvasive way to sample the lymph nodes to ideally stage and make a diagnosis as safely as possible. If we are considering infection, needle aspiration is not as sensitive.2
► Dr. Apte: The patient underwent a PET CT, which redemonstrated the lung mass with a loss of aortic fat plane suspicious for aortic involvement as well as lymph nodes in levels 7 and 8 that were concerning for malignancy. Subsequent bronchoscopy with biopsy and endobronchial ultrasound did not show evidence of malignancy; washing and brushing from the mass and lymph node specimens did not identify malignant cells. Benign respiratory mucosa with mild chronic inflammation was noted. Dr. Fine, given the nonspecific findings on the PET scan, negative findings on our bronchoscopy, and a negative biopsy, should we be satisfied that we have ruled out cancer?
► Dr. Fine: No, bronchoscopy has its limitations. It’s highly sensitive to the diagnosis of malignancy if you can see an endobronchial lesion, but we did not see one here. You can only go so far with the scope, and it’s not uncommon for us not to be able to make the diagnosis with bronchoscopy. Malignancy is still the most likely diagnosis, and we need to work this up further. I would perform another biopsy.
►Dr. Apte: Four weeks later, the patient presented with continued shortness of breath, fatigue, and fever. A repeat chest CT showed an opacity suggestive of pneumonia. Given the continued concern for cancer a CT-guided needle biopsy was performed and was once again negative for malignancy. The decision was made to pursue a video-assisted thorascopic surgery (VATS). Following the VATS, the patient developed rigors, fever, and tachycardia with new atrial fibrillation. While being evaluated hypercalcemia was identified, with further workup revealing a low parathyroid hormone (PTH) and low PTH-related peptide. Dr. Fine, the presence of hypercalcemia and a new arrythmia raised the possibility of sarcoidosis. Could this be sarcoidosis?
►Dr. Fine: Sarcoidosis is one of the great masqueraders in medicine. There is a type of sarcoidosis called nodular sarcoidosis where you see masslike distribution in the lung, but generally there are multiple masses and so this presentation would be atypical.3 There is also a phenomenon called sarcoidal reactions usually in the presence of cancer. Again, one tends to see multiple tiny lesions in the lung. It is certainly on the differential, but I would consider it to be less likely than cancer. It is also relatively common to develop atrial fibrillation after manipulation from a lung surgery.4 The other possibility I am concerned about is whether the mass is invading the mediastinum and involving the pericardium.
►Dr. Apte: Results from the VATS biopsy once again returned negative for malignancy and instead showed signs of focal micro-abscesses, atypical pneumocytes, and prominent neutrophils. A diagnosis of acute fibrinous organizing pneumonia (AFOP) was offered. Dr. Fine, what is AFOP?
►Dr. Fine: This is the first case of AFOP I had seen and probably the first case many in our department have seen. This is a relatively new entity with limited reported cases in the literature and is a pathological diagnosis originally recorded in autopsies from patients at the National Institutes of Health.5,6 Given the complexity of the lesion, the diagnosis is difficult to make. Most commonly, AFOP is associated with other systemic entities, most commonly hematologic malignancies like lymphomas and leukemias. It has also been associated with vasculitis and certain drugs. The mechanism is poorly understood, and although pneumonia is a part of the term, this just implies there is inflammation of the lung (ie, pneumonitis).
► Dr. Apte: Given the association of AFOP with underlying hematologic malignancies, an emphasis was placed on another finding: the patient’s increasing WBC count. The total WBC count had been 20 k/cm3 at the time of his lung mass discovery but had increased to > 40 k/cm3 with a differential of neutrophils > 80%. Flow cytometry was negative, and his peripheral smear was read as normal. Dr. Gilbert, what might explain this patient’s leukocytosis?
►Gary Gilbert, MD, Section of Hematology and Oncology, VABHS and Associate Professor of Medicine, Harvard Medical School (HMS): This patient had an elevated WBC for 4 months. Initially, the cause was likely lithium as this is known to cause a leukocytosis.7 More recently, the total WBC had increased and there were a couple of other abnormalities: A consistently elevated absolute monocyte count and a markedly elevated mature neutrophil count. These findings are consistent with a leukemoid reaction (ie, a WBC count > 50,000/µL from causes other than leukemia). The question becomes what is this a leukemoid reaction in response to? Once we have excluded a lung malignancy (a well-known common cause of a leukemoid reaction) we must consider a clonal myeloproliferative disorder. This is particularly true because many things that cause a leukemoid reaction (eg, lobar pneumonia) do not cause a persistently elevated neutrophil count. That this patient does have a persistently elevated neutrophil count suggests something abnormal about the neutrophils themselves.
► Dr. Apte: A bone marrow biopsy was performed. Dr. Gilbert, can you comment on this patient’s bone marrow biopsy and whether a myeloproliferative disorder may have played a role in the marked leukocytosis?
► Dr. Gilbert: The bone marrow biopsy was hyperplastic with myeloid predominance and normal maturation in all lineages. A deep sequencing analysis demonstrated the absence of chromosomal abnormalities or genetic mutations that are associated with myeloproliferative disorders. This excludes the possibility of a myeloproliferative disorder.
► Dr. Apte: The patient was started on 60 mg of prednisone daily, which led to marked improvement in his symptoms. He was discharged in stable condition but presented again with abdominal pain. A complete blood count once again showed increased WBC and new thrombocytosis. A CT angiogram (CTA) showed the prior lung mass with new signs of central necrosis. In the abdomen, new splenic and renal infarct were identified, along with signs of multiple arterial thrombi in the abdomen and internal and external iliac vessel wall thickening. These findings were read as concerning for a medium vessel vasculitis. Dr. Kaur, what are some of the imaging findings you would expect to see in vasculitis, and what about this patient’s CT is consistent with a medium vessel vasculitis?
► Maneet Kaur, MD, Section of Rheumatology, VABHS: Vasculitis is inflammation of the vessel wall that can lead to vascular injury and activation of the coagulation cascade. Sometimes these findings can be seen on imaging with evidence of stenosis, microaneurysms, and thrombosis distal to the stenosis. The nomenclature of vasculitis is not simple and has been revised many times. Medium-vessel vasculitis does not just affect the medium vessels (eg, visceral arteries) but can overlap with distal large vessels and smaller cutaneous vessels.
The first thing that comes to mind in this case is polyarteritis nodosa (PAN), an immune complex-mediated medium vessel disease that can involve large and small vessels in muscle, nerve, and skin. It can also present with masses.
►Dr. Apte: To address the arterial thrombi seen on CTA, arterial-brachial indices were obtained and showed bilateral occlusive disease in his distal extremities; findings that could be explained by vasculitis. His VATS biopsy pathology was reviewed for signs of vasculitis. Dr. Huang, can you review these slides for us please?
►Qin Huang, MD, Department of Pathology and Laboratory Medicine, VABHS and Assistant Professor of Pathology, HMS: This patient had a history of smoking, and there are many black pigment-laden macrophages present in the lung tissue. There were areas of hemorrhage and fibrin deposition and an overall picture of organizing pneumonia. At a lower power, you can see neutrophils everywhere, some in the form of micro-abscesses. The arterial walls did not show signs of vasculitis (Figure 2). Based on the clinical information and radiology findings, we suspected an acute infection-related pneumonia or a primary lung malignancy causing obstruction pneumonia. We suggested a rebiopsy of the lung mass to rule out a primary lung malignancy.
► Dr. Apte: Given his CT findings, a serologic rheumatologic workup including antineutrophil cytoplasmic antibody, antinuclear antibody, and rheumatoid factors were sent and returned negative. The location of arterial wall inflammation on imaging made it unamenable for biopsy. The patient began to experience bilateral temporal pain, which raised the concern for a large vessel vasculitis, specifically giant cell arteritis. Bilateral temporal artery biopsies were obtained and were not suggestive of vasculitis. Dr. Kaur, we still do not have any serologic or biopsy confirmation to support a diagnosis of vasculitis. Can we still call this a vasculitis?
►Dr. Kaur: Few things can cause the picture that was seen radiographically. A few noninflammatory causes like fibromuscular dysplasia can cause both large and small vessel stenosis, but the elevations in erythrocyte sedimentation rate and C-reactive protein along with response to steroids makes these diagnoses unlikely. Sometimes we must make a clinical diagnosis for vasculitis based on the clinical picture, and I would feel comfortable treating this patient for vasculitis.
With that said, I remain concerned that this patient also has a malignancy. His WBC increased to > 70 k/cm3 and his calcium to > 13 mg/dL. These findings are hard to explain by vasculitis alone. There are cancer-associated vasculitis, and I suspect this is the explanation here.8 His temporal pain was pointing to large vessel involvement, so he could have an undifferentiated vasculitis.
► Dr. Apte: A decision was made to empirically treat with tocilizumab, an IL-6 receptor antagonist, for an undifferentiated autoimmune disease, in addition to tapering steroids. The patient underwent a second VATS, which again revealed AFOP but no signs of malignancy. Unfortunately, he developed multiple complications over the subsequent weeks and passed away. An autopsy was requested by family members and pathology from his lung mass was reviewed. (Figure 3). Dr. Huang, can you review these slides for us?
► Dr. Huang: The left lung mass at autopsy shows nests, poorly formed clusters, and individuals of malignant neoplastic nonkeratinizing squamous cells embedded in a desmoplastic stroma in the mass center, consistent with poorly differentiated squamous cell carcinoma, and a circumscribed area of residual subacute organizing pneumonia with abscess, granulomatous changes, and early fibrosis at the periphery of this mass.
►Dr. Apte: Based on autopsy findings, the final diagnosis was poorly differentiated squamous cell carcinoma associated with subacute organizing pneumonia and medium vessel vasculitis, which presented with a severe leukocytosis ultimately thought to be a leukemoid reaction from his lung cancer.
Case Presentation: A 62-year-old male presented with shortness of breath and a cough productive of green sputum. He had a history of hyperlipidemia, posttraumatic stress disorder, bipolar disorder, obstructive sleep apnea, and a 50 pack-year history of smoking. His medications included prazosin, melatonin, lithium, and gabapentin. He also had a significant exposure history including asbestos and chemical paints following his leave from the military. At the initial evaluation, laboratory work revealed a leukocytosis with white blood cell (WBC) count 20 k/cm3and otherwise normal transaminases, albumin, and electrolytes. A chest X-ray revealed a new left hilar mass.
►Manisha Apte, MD, Chief Medical Resident, VA Boston Healthcare System (VABHS) and Boston Medical Center (BMC): To work up his new left hilar mass, a computed tomography (CT) of the chest was ordered (Figure 1), which revealed an apical left lower lobe mass extending into the left hilum encasing part of the ascending aorta. Enlarged mediastinal subcentimeter paratracheal and superior mediastinal lymph nodes also were identified and the pattern raised the concern for lymphangitic carcinomatosis. Dr. Fine, what do you make of the CT findings?
► Alan Fine, MD, Section of Pulmonary and Critical Care, VABHS and Professor of Medicine, Boston University School of Medicine: This mass had irregular edges with septal thickening, which may be why there was a concern for lymphangitic spread. There were no clear tissue planes to see if this process was invading the mediastinum. The mass was irregular, a single lesion, and proximal, making it consistent with a lung cancer. In fact, with his history of smoking, asbestos exposure, the numbers 1 to 10 diagnoses were lung cancer. The lack of demarcation of tissue planes supports this. There are some infections, classically actinomycosis, that do cross and invade anatomical barriers.1 But this looked like a primary lung cancer.
► Dr. Apte: The patient was referred to a pulmonologist where an additional history of night sweats and weight loss were noted. Dr. Fine, we have a patient with a newly identified lung mass, and while we have reason to suspect malignancy as you have already noted, there are many other etiologies to consider, including infections (histoplasmosis, cryptococcosis, bacterial abscess), inflammatory processes (sarcoidosis, rheumatoid nodule) and vasculitis (granulomatosis with polyangiitis). What should be the next step taken to make a diagnosis?
►Dr. Fine: For cancer specifically, we would like to both stage and make a diagnosis with one procedure. That’s part of the utility of a positron emission tomography (PET) scan: We can see lymph node involvement and stage the cancer. We must consider the patient’s comorbidities and the location of the lesion (ie, is it amenable to needle biopsy?). In this case, there are enlarged mediastinal lymph nodes, so one could perform a bronchoscopy with endobronchial ultrasound, which is a relatively noninvasive way to sample the lymph nodes to ideally stage and make a diagnosis as safely as possible. If we are considering infection, needle aspiration is not as sensitive.2
► Dr. Apte: The patient underwent a PET CT, which redemonstrated the lung mass with a loss of aortic fat plane suspicious for aortic involvement as well as lymph nodes in levels 7 and 8 that were concerning for malignancy. Subsequent bronchoscopy with biopsy and endobronchial ultrasound did not show evidence of malignancy; washing and brushing from the mass and lymph node specimens did not identify malignant cells. Benign respiratory mucosa with mild chronic inflammation was noted. Dr. Fine, given the nonspecific findings on the PET scan, negative findings on our bronchoscopy, and a negative biopsy, should we be satisfied that we have ruled out cancer?
► Dr. Fine: No, bronchoscopy has its limitations. It’s highly sensitive to the diagnosis of malignancy if you can see an endobronchial lesion, but we did not see one here. You can only go so far with the scope, and it’s not uncommon for us not to be able to make the diagnosis with bronchoscopy. Malignancy is still the most likely diagnosis, and we need to work this up further. I would perform another biopsy.
►Dr. Apte: Four weeks later, the patient presented with continued shortness of breath, fatigue, and fever. A repeat chest CT showed an opacity suggestive of pneumonia. Given the continued concern for cancer a CT-guided needle biopsy was performed and was once again negative for malignancy. The decision was made to pursue a video-assisted thorascopic surgery (VATS). Following the VATS, the patient developed rigors, fever, and tachycardia with new atrial fibrillation. While being evaluated hypercalcemia was identified, with further workup revealing a low parathyroid hormone (PTH) and low PTH-related peptide. Dr. Fine, the presence of hypercalcemia and a new arrythmia raised the possibility of sarcoidosis. Could this be sarcoidosis?
►Dr. Fine: Sarcoidosis is one of the great masqueraders in medicine. There is a type of sarcoidosis called nodular sarcoidosis where you see masslike distribution in the lung, but generally there are multiple masses and so this presentation would be atypical.3 There is also a phenomenon called sarcoidal reactions usually in the presence of cancer. Again, one tends to see multiple tiny lesions in the lung. It is certainly on the differential, but I would consider it to be less likely than cancer. It is also relatively common to develop atrial fibrillation after manipulation from a lung surgery.4 The other possibility I am concerned about is whether the mass is invading the mediastinum and involving the pericardium.
►Dr. Apte: Results from the VATS biopsy once again returned negative for malignancy and instead showed signs of focal micro-abscesses, atypical pneumocytes, and prominent neutrophils. A diagnosis of acute fibrinous organizing pneumonia (AFOP) was offered. Dr. Fine, what is AFOP?
►Dr. Fine: This is the first case of AFOP I had seen and probably the first case many in our department have seen. This is a relatively new entity with limited reported cases in the literature and is a pathological diagnosis originally recorded in autopsies from patients at the National Institutes of Health.5,6 Given the complexity of the lesion, the diagnosis is difficult to make. Most commonly, AFOP is associated with other systemic entities, most commonly hematologic malignancies like lymphomas and leukemias. It has also been associated with vasculitis and certain drugs. The mechanism is poorly understood, and although pneumonia is a part of the term, this just implies there is inflammation of the lung (ie, pneumonitis).
► Dr. Apte: Given the association of AFOP with underlying hematologic malignancies, an emphasis was placed on another finding: the patient’s increasing WBC count. The total WBC count had been 20 k/cm3 at the time of his lung mass discovery but had increased to > 40 k/cm3 with a differential of neutrophils > 80%. Flow cytometry was negative, and his peripheral smear was read as normal. Dr. Gilbert, what might explain this patient’s leukocytosis?
►Gary Gilbert, MD, Section of Hematology and Oncology, VABHS and Associate Professor of Medicine, Harvard Medical School (HMS): This patient had an elevated WBC for 4 months. Initially, the cause was likely lithium as this is known to cause a leukocytosis.7 More recently, the total WBC had increased and there were a couple of other abnormalities: A consistently elevated absolute monocyte count and a markedly elevated mature neutrophil count. These findings are consistent with a leukemoid reaction (ie, a WBC count > 50,000/µL from causes other than leukemia). The question becomes what is this a leukemoid reaction in response to? Once we have excluded a lung malignancy (a well-known common cause of a leukemoid reaction) we must consider a clonal myeloproliferative disorder. This is particularly true because many things that cause a leukemoid reaction (eg, lobar pneumonia) do not cause a persistently elevated neutrophil count. That this patient does have a persistently elevated neutrophil count suggests something abnormal about the neutrophils themselves.
► Dr. Apte: A bone marrow biopsy was performed. Dr. Gilbert, can you comment on this patient’s bone marrow biopsy and whether a myeloproliferative disorder may have played a role in the marked leukocytosis?
► Dr. Gilbert: The bone marrow biopsy was hyperplastic with myeloid predominance and normal maturation in all lineages. A deep sequencing analysis demonstrated the absence of chromosomal abnormalities or genetic mutations that are associated with myeloproliferative disorders. This excludes the possibility of a myeloproliferative disorder.
► Dr. Apte: The patient was started on 60 mg of prednisone daily, which led to marked improvement in his symptoms. He was discharged in stable condition but presented again with abdominal pain. A complete blood count once again showed increased WBC and new thrombocytosis. A CT angiogram (CTA) showed the prior lung mass with new signs of central necrosis. In the abdomen, new splenic and renal infarct were identified, along with signs of multiple arterial thrombi in the abdomen and internal and external iliac vessel wall thickening. These findings were read as concerning for a medium vessel vasculitis. Dr. Kaur, what are some of the imaging findings you would expect to see in vasculitis, and what about this patient’s CT is consistent with a medium vessel vasculitis?
► Maneet Kaur, MD, Section of Rheumatology, VABHS: Vasculitis is inflammation of the vessel wall that can lead to vascular injury and activation of the coagulation cascade. Sometimes these findings can be seen on imaging with evidence of stenosis, microaneurysms, and thrombosis distal to the stenosis. The nomenclature of vasculitis is not simple and has been revised many times. Medium-vessel vasculitis does not just affect the medium vessels (eg, visceral arteries) but can overlap with distal large vessels and smaller cutaneous vessels.
The first thing that comes to mind in this case is polyarteritis nodosa (PAN), an immune complex-mediated medium vessel disease that can involve large and small vessels in muscle, nerve, and skin. It can also present with masses.
►Dr. Apte: To address the arterial thrombi seen on CTA, arterial-brachial indices were obtained and showed bilateral occlusive disease in his distal extremities; findings that could be explained by vasculitis. His VATS biopsy pathology was reviewed for signs of vasculitis. Dr. Huang, can you review these slides for us please?
►Qin Huang, MD, Department of Pathology and Laboratory Medicine, VABHS and Assistant Professor of Pathology, HMS: This patient had a history of smoking, and there are many black pigment-laden macrophages present in the lung tissue. There were areas of hemorrhage and fibrin deposition and an overall picture of organizing pneumonia. At a lower power, you can see neutrophils everywhere, some in the form of micro-abscesses. The arterial walls did not show signs of vasculitis (Figure 2). Based on the clinical information and radiology findings, we suspected an acute infection-related pneumonia or a primary lung malignancy causing obstruction pneumonia. We suggested a rebiopsy of the lung mass to rule out a primary lung malignancy.
► Dr. Apte: Given his CT findings, a serologic rheumatologic workup including antineutrophil cytoplasmic antibody, antinuclear antibody, and rheumatoid factors were sent and returned negative. The location of arterial wall inflammation on imaging made it unamenable for biopsy. The patient began to experience bilateral temporal pain, which raised the concern for a large vessel vasculitis, specifically giant cell arteritis. Bilateral temporal artery biopsies were obtained and were not suggestive of vasculitis. Dr. Kaur, we still do not have any serologic or biopsy confirmation to support a diagnosis of vasculitis. Can we still call this a vasculitis?
►Dr. Kaur: Few things can cause the picture that was seen radiographically. A few noninflammatory causes like fibromuscular dysplasia can cause both large and small vessel stenosis, but the elevations in erythrocyte sedimentation rate and C-reactive protein along with response to steroids makes these diagnoses unlikely. Sometimes we must make a clinical diagnosis for vasculitis based on the clinical picture, and I would feel comfortable treating this patient for vasculitis.
With that said, I remain concerned that this patient also has a malignancy. His WBC increased to > 70 k/cm3 and his calcium to > 13 mg/dL. These findings are hard to explain by vasculitis alone. There are cancer-associated vasculitis, and I suspect this is the explanation here.8 His temporal pain was pointing to large vessel involvement, so he could have an undifferentiated vasculitis.
► Dr. Apte: A decision was made to empirically treat with tocilizumab, an IL-6 receptor antagonist, for an undifferentiated autoimmune disease, in addition to tapering steroids. The patient underwent a second VATS, which again revealed AFOP but no signs of malignancy. Unfortunately, he developed multiple complications over the subsequent weeks and passed away. An autopsy was requested by family members and pathology from his lung mass was reviewed. (Figure 3). Dr. Huang, can you review these slides for us?
► Dr. Huang: The left lung mass at autopsy shows nests, poorly formed clusters, and individuals of malignant neoplastic nonkeratinizing squamous cells embedded in a desmoplastic stroma in the mass center, consistent with poorly differentiated squamous cell carcinoma, and a circumscribed area of residual subacute organizing pneumonia with abscess, granulomatous changes, and early fibrosis at the periphery of this mass.
►Dr. Apte: Based on autopsy findings, the final diagnosis was poorly differentiated squamous cell carcinoma associated with subacute organizing pneumonia and medium vessel vasculitis, which presented with a severe leukocytosis ultimately thought to be a leukemoid reaction from his lung cancer.
1. Valour F, Sénéchal A, Dupieux C, et al. Actinomycosis: etiology, clinical features, diagnosis, treatment, and management. Infect Drug Resist. 2014;7:183-197. Published 2014 Jul 5. doi:10.2147/IDR.S39601
2. de Bazelaire C, Coffin A, Cohen-Zarade S, et al. CT-guided biopsies in lung infections in patients with haematological malignancies. Diagn Interv Imaging. 2013;94(2):202-215. doi:10.1016/j.diii.2012.12.008
3. Sweidan AJ, Singh NK, Stein A, Tanios M. Nodular sarcoidosis masquerading as cancer. Clin Med Insights Circ Respir Pulm Med. 2017;11:1179548417703123. Published 2017 Apr 12. doi:10.1177/1179548417703123
4. Bagheri R, Yousefi Y, Rezai R, Azemonfar V, Keshtan FG. Atrial fibrillation after lung surgery: incidence, underlying factors, and predictors. Kardiochir Torakochirurgia Pol. 2019;16(2):53-56. doi:10.5114/kitp.2019.86355
5. Lu J, Yin Q, Zha Y, et al. Acute fibrinous and organizing pneumonia: two case reports and literature review. BMC Pulm Med. 2019;19(1):141. Published 2019 Aug 5. doi:10.1186/s12890-019-0861-3
6. Beasley MB, Franks TJ, Galvin JR, Gochuico B, Travis WD. Acute fibrinous and organizing pneumonia: a histological pattern of lung injury and possible variant of diffuse alveolar damage. Arch Pathol Lab Med. 2002;126(9):1064-1070. doi:10.5858/2002-126-1064-AFAOP
7. Murphy DL, Goodwin FK, Bunney WE Jr. Leukocytosis during lithium treatment. Am J Psychiatry. 1971;127(11):1559-1561. doi:10.1176/ajp.127.11.1559
8. Fain O, Hamidou M, Cacoub P, et al. Vasculitides associated with malignancies: analysis of sixty patients. Arthritis Rheum. 2007;57(8):1473-1480. doi:10.1002/art.23085
1. Valour F, Sénéchal A, Dupieux C, et al. Actinomycosis: etiology, clinical features, diagnosis, treatment, and management. Infect Drug Resist. 2014;7:183-197. Published 2014 Jul 5. doi:10.2147/IDR.S39601
2. de Bazelaire C, Coffin A, Cohen-Zarade S, et al. CT-guided biopsies in lung infections in patients with haematological malignancies. Diagn Interv Imaging. 2013;94(2):202-215. doi:10.1016/j.diii.2012.12.008
3. Sweidan AJ, Singh NK, Stein A, Tanios M. Nodular sarcoidosis masquerading as cancer. Clin Med Insights Circ Respir Pulm Med. 2017;11:1179548417703123. Published 2017 Apr 12. doi:10.1177/1179548417703123
4. Bagheri R, Yousefi Y, Rezai R, Azemonfar V, Keshtan FG. Atrial fibrillation after lung surgery: incidence, underlying factors, and predictors. Kardiochir Torakochirurgia Pol. 2019;16(2):53-56. doi:10.5114/kitp.2019.86355
5. Lu J, Yin Q, Zha Y, et al. Acute fibrinous and organizing pneumonia: two case reports and literature review. BMC Pulm Med. 2019;19(1):141. Published 2019 Aug 5. doi:10.1186/s12890-019-0861-3
6. Beasley MB, Franks TJ, Galvin JR, Gochuico B, Travis WD. Acute fibrinous and organizing pneumonia: a histological pattern of lung injury and possible variant of diffuse alveolar damage. Arch Pathol Lab Med. 2002;126(9):1064-1070. doi:10.5858/2002-126-1064-AFAOP
7. Murphy DL, Goodwin FK, Bunney WE Jr. Leukocytosis during lithium treatment. Am J Psychiatry. 1971;127(11):1559-1561. doi:10.1176/ajp.127.11.1559
8. Fain O, Hamidou M, Cacoub P, et al. Vasculitides associated with malignancies: analysis of sixty patients. Arthritis Rheum. 2007;57(8):1473-1480. doi:10.1002/art.23085
Prophylactic anticoagulation tied to lower death rate in COVID
Prophylactic anticoagulation to prevent venous thromboembolism (VTE) was associated with reduced 60-day mortality in patients with COVID-19 who were ill enough to require hospitalization, a new report shows.
In a cohort study of more than 1,300 hospitalized patients with COVID-19 infection across 30 hospitals in Michigan, both prophylactic- and therapeutic-dose anticoagulation were associated with reduced in-hospital mortality; however, at 60 days, only prophylactic-dose anticoagulation remained associated with lower mortality.
And adherence was key; nonadherence, or missing 2 days or more of anticoagulation, was linked to more deaths at 60 days.
The findings, which were published online June 11 in JAMA Network Open, are final proof that a prophylactic anticoagulation strategy for the hospitalized COVID population is, indeed, the right one, Valerie M. Vaughn, MD, director of hospital medicine research at the University of Utah, Salt Lake City, said in an interview.
“We’ve probably always known that patients with COVID need prophylaxis for VTE, but we found that early on, unfortunately, that wasn’t being done,” Dr. Vaughn said.
“Now, we see that prophylactic rates have increased. We always knew to use anticoagulation prophylactically in patients who were hospitalized with infection because of their risk for VTE, so this study just drives home that proper adherence to an anticoagulation protocol improves mortality,” she said.
Dr. Vaughn was on the front lines when COVID-19 came to Michigan, where the research was conducted.
“We probably should have been anticoagulating from the get-go, but you have to remember that in the early days of COVID, the hospitals in Michigan were being overwhelmed. They didn’t have PPE. They were taking care of patients outside of their typical hospital beds or setting up field hospitals,” she said. “It was not quite as bad as New York, but at the University of Michigan, we set up four or five ICUs outside of our normal care.”
They also converted the top floor of their pediatric hospital into an ICU to take care of patients with COVID during the first surge, she added. “We didn’t know much about this disease, but faced with this influx of patients, many of whom were dying with blood clots, we had to do something.”
Some hospitals began prophylactically anticoagulating their patients, but others hesitated before adopting the strategy. “But now we feel confident that prophylactic anticoagulation, done according to the right protocol, with no interruptions in the treatment, is beneficial,” Dr. Vaughn said.
The best medication choice is enoxaparin (Lovenox), which can be given once a day, as opposed to heparin, which needs to be given via injection three times a day, she said.
“Prophylactic dose anticoagulation is typically given by an injection under the skin, but a lot of times, I’ve had patients tell me they feel like a human pin cushion and have all these bruises from being stuck with needles every day, which I can totally relate to,” she said.
“It is important for us as clinicians to explain that we’re having to poke our patients because it is good for them and will help them fight COVID,” she added. “Also having the once-a-day option is going to be a lot better for adherence, and adherence to the protocol, not missing any days, is key to the better outcome.”
Dr. Vaughn and her team reviewed the charts of 1,351 patients (48% women, 49% Black, median age 64 [range 52-75]) who were hospitalized throughout Michigan during the first several months of the COVID-19 pandemic, from March to June 2020.
Only 18 patients (1.3%) had a confirmed VTE and 219 patients (16.2%) received treatment-dose anticoagulation.
The researchers noted that use of treatment-dose anticoagulation without imaging ranged from 0% to 29% across hospitals and increased significantly over time.
Of the 1,127 patients who received anticoagulation, 392 (34.8%) missed 2 days or more of prophylaxis.
In addition, there were varying rates of missed prophylaxis among the hospitals, from 11% to 61%, but these rates decreased markedly over time.
Missed doses were associated with a higher 60-day mortality (adjusted hazard ratio, 1.31; 95% confidence interval, 1.03-1.67), but not in-hospital mortality (aHR, 0.97; 95% CI, 0.91-1.03).
Compared with no anticoagulation, receiving any dose of anticoagulation was associated with lower in-hospital mortality.
However, only prophylactic-dose anticoagulation remained associated with lower mortality at 60 days. The adjusted hazard ratio for prophylactic-dose anticoagulation was 0.71 (95% CI, 0.51-0.90), compared with 0.92 (95% CI, 0.63-1.35) for treatment-dose anticoagulation.
Study boosts confidence
Despite its limitations, the study should make clinicians more confident that the use of prophylactic anticoagulation is warranted for hospitalized patients with COVID-19, write Andrew B. Dicks, MD, and Ido Weinberg, MD, from Massachusetts General Hospital, Boston, in an invited commentary.
“Practically, we still lack the granular data we need to help guide us in patient-by-patient decision-making – such as anticoagulation agent choice, dosage, and duration of therapy – especially as dictated by acuity of patient illness,” Dr. Dicks and Dr. Weinberg note.
“While we still await the data from randomized controlled trials to guide the optimal anticoagulation dose and duration, this study adds significant merit to the previously published recommendations from several different medical organizations regarding the use of prophylactic anticoagulation in hospitalized patients with COVID-19,” Dr. Dicks told this news organization.
The study was supported by Blue Cross and Blue Shield of Michigan and Blue Care Network as part of their Value Partnerships program. Dr. Vaughn has reported receiving speaking fees from Thermo Fisher Scientific. Dr. Dicks and Dr. Weinberg have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Prophylactic anticoagulation to prevent venous thromboembolism (VTE) was associated with reduced 60-day mortality in patients with COVID-19 who were ill enough to require hospitalization, a new report shows.
In a cohort study of more than 1,300 hospitalized patients with COVID-19 infection across 30 hospitals in Michigan, both prophylactic- and therapeutic-dose anticoagulation were associated with reduced in-hospital mortality; however, at 60 days, only prophylactic-dose anticoagulation remained associated with lower mortality.
And adherence was key; nonadherence, or missing 2 days or more of anticoagulation, was linked to more deaths at 60 days.
The findings, which were published online June 11 in JAMA Network Open, are final proof that a prophylactic anticoagulation strategy for the hospitalized COVID population is, indeed, the right one, Valerie M. Vaughn, MD, director of hospital medicine research at the University of Utah, Salt Lake City, said in an interview.
“We’ve probably always known that patients with COVID need prophylaxis for VTE, but we found that early on, unfortunately, that wasn’t being done,” Dr. Vaughn said.
“Now, we see that prophylactic rates have increased. We always knew to use anticoagulation prophylactically in patients who were hospitalized with infection because of their risk for VTE, so this study just drives home that proper adherence to an anticoagulation protocol improves mortality,” she said.
Dr. Vaughn was on the front lines when COVID-19 came to Michigan, where the research was conducted.
“We probably should have been anticoagulating from the get-go, but you have to remember that in the early days of COVID, the hospitals in Michigan were being overwhelmed. They didn’t have PPE. They were taking care of patients outside of their typical hospital beds or setting up field hospitals,” she said. “It was not quite as bad as New York, but at the University of Michigan, we set up four or five ICUs outside of our normal care.”
They also converted the top floor of their pediatric hospital into an ICU to take care of patients with COVID during the first surge, she added. “We didn’t know much about this disease, but faced with this influx of patients, many of whom were dying with blood clots, we had to do something.”
Some hospitals began prophylactically anticoagulating their patients, but others hesitated before adopting the strategy. “But now we feel confident that prophylactic anticoagulation, done according to the right protocol, with no interruptions in the treatment, is beneficial,” Dr. Vaughn said.
The best medication choice is enoxaparin (Lovenox), which can be given once a day, as opposed to heparin, which needs to be given via injection three times a day, she said.
“Prophylactic dose anticoagulation is typically given by an injection under the skin, but a lot of times, I’ve had patients tell me they feel like a human pin cushion and have all these bruises from being stuck with needles every day, which I can totally relate to,” she said.
“It is important for us as clinicians to explain that we’re having to poke our patients because it is good for them and will help them fight COVID,” she added. “Also having the once-a-day option is going to be a lot better for adherence, and adherence to the protocol, not missing any days, is key to the better outcome.”
Dr. Vaughn and her team reviewed the charts of 1,351 patients (48% women, 49% Black, median age 64 [range 52-75]) who were hospitalized throughout Michigan during the first several months of the COVID-19 pandemic, from March to June 2020.
Only 18 patients (1.3%) had a confirmed VTE and 219 patients (16.2%) received treatment-dose anticoagulation.
The researchers noted that use of treatment-dose anticoagulation without imaging ranged from 0% to 29% across hospitals and increased significantly over time.
Of the 1,127 patients who received anticoagulation, 392 (34.8%) missed 2 days or more of prophylaxis.
In addition, there were varying rates of missed prophylaxis among the hospitals, from 11% to 61%, but these rates decreased markedly over time.
Missed doses were associated with a higher 60-day mortality (adjusted hazard ratio, 1.31; 95% confidence interval, 1.03-1.67), but not in-hospital mortality (aHR, 0.97; 95% CI, 0.91-1.03).
Compared with no anticoagulation, receiving any dose of anticoagulation was associated with lower in-hospital mortality.
However, only prophylactic-dose anticoagulation remained associated with lower mortality at 60 days. The adjusted hazard ratio for prophylactic-dose anticoagulation was 0.71 (95% CI, 0.51-0.90), compared with 0.92 (95% CI, 0.63-1.35) for treatment-dose anticoagulation.
Study boosts confidence
Despite its limitations, the study should make clinicians more confident that the use of prophylactic anticoagulation is warranted for hospitalized patients with COVID-19, write Andrew B. Dicks, MD, and Ido Weinberg, MD, from Massachusetts General Hospital, Boston, in an invited commentary.
“Practically, we still lack the granular data we need to help guide us in patient-by-patient decision-making – such as anticoagulation agent choice, dosage, and duration of therapy – especially as dictated by acuity of patient illness,” Dr. Dicks and Dr. Weinberg note.
“While we still await the data from randomized controlled trials to guide the optimal anticoagulation dose and duration, this study adds significant merit to the previously published recommendations from several different medical organizations regarding the use of prophylactic anticoagulation in hospitalized patients with COVID-19,” Dr. Dicks told this news organization.
The study was supported by Blue Cross and Blue Shield of Michigan and Blue Care Network as part of their Value Partnerships program. Dr. Vaughn has reported receiving speaking fees from Thermo Fisher Scientific. Dr. Dicks and Dr. Weinberg have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Prophylactic anticoagulation to prevent venous thromboembolism (VTE) was associated with reduced 60-day mortality in patients with COVID-19 who were ill enough to require hospitalization, a new report shows.
In a cohort study of more than 1,300 hospitalized patients with COVID-19 infection across 30 hospitals in Michigan, both prophylactic- and therapeutic-dose anticoagulation were associated with reduced in-hospital mortality; however, at 60 days, only prophylactic-dose anticoagulation remained associated with lower mortality.
And adherence was key; nonadherence, or missing 2 days or more of anticoagulation, was linked to more deaths at 60 days.
The findings, which were published online June 11 in JAMA Network Open, are final proof that a prophylactic anticoagulation strategy for the hospitalized COVID population is, indeed, the right one, Valerie M. Vaughn, MD, director of hospital medicine research at the University of Utah, Salt Lake City, said in an interview.
“We’ve probably always known that patients with COVID need prophylaxis for VTE, but we found that early on, unfortunately, that wasn’t being done,” Dr. Vaughn said.
“Now, we see that prophylactic rates have increased. We always knew to use anticoagulation prophylactically in patients who were hospitalized with infection because of their risk for VTE, so this study just drives home that proper adherence to an anticoagulation protocol improves mortality,” she said.
Dr. Vaughn was on the front lines when COVID-19 came to Michigan, where the research was conducted.
“We probably should have been anticoagulating from the get-go, but you have to remember that in the early days of COVID, the hospitals in Michigan were being overwhelmed. They didn’t have PPE. They were taking care of patients outside of their typical hospital beds or setting up field hospitals,” she said. “It was not quite as bad as New York, but at the University of Michigan, we set up four or five ICUs outside of our normal care.”
They also converted the top floor of their pediatric hospital into an ICU to take care of patients with COVID during the first surge, she added. “We didn’t know much about this disease, but faced with this influx of patients, many of whom were dying with blood clots, we had to do something.”
Some hospitals began prophylactically anticoagulating their patients, but others hesitated before adopting the strategy. “But now we feel confident that prophylactic anticoagulation, done according to the right protocol, with no interruptions in the treatment, is beneficial,” Dr. Vaughn said.
The best medication choice is enoxaparin (Lovenox), which can be given once a day, as opposed to heparin, which needs to be given via injection three times a day, she said.
“Prophylactic dose anticoagulation is typically given by an injection under the skin, but a lot of times, I’ve had patients tell me they feel like a human pin cushion and have all these bruises from being stuck with needles every day, which I can totally relate to,” she said.
“It is important for us as clinicians to explain that we’re having to poke our patients because it is good for them and will help them fight COVID,” she added. “Also having the once-a-day option is going to be a lot better for adherence, and adherence to the protocol, not missing any days, is key to the better outcome.”
Dr. Vaughn and her team reviewed the charts of 1,351 patients (48% women, 49% Black, median age 64 [range 52-75]) who were hospitalized throughout Michigan during the first several months of the COVID-19 pandemic, from March to June 2020.
Only 18 patients (1.3%) had a confirmed VTE and 219 patients (16.2%) received treatment-dose anticoagulation.
The researchers noted that use of treatment-dose anticoagulation without imaging ranged from 0% to 29% across hospitals and increased significantly over time.
Of the 1,127 patients who received anticoagulation, 392 (34.8%) missed 2 days or more of prophylaxis.
In addition, there were varying rates of missed prophylaxis among the hospitals, from 11% to 61%, but these rates decreased markedly over time.
Missed doses were associated with a higher 60-day mortality (adjusted hazard ratio, 1.31; 95% confidence interval, 1.03-1.67), but not in-hospital mortality (aHR, 0.97; 95% CI, 0.91-1.03).
Compared with no anticoagulation, receiving any dose of anticoagulation was associated with lower in-hospital mortality.
However, only prophylactic-dose anticoagulation remained associated with lower mortality at 60 days. The adjusted hazard ratio for prophylactic-dose anticoagulation was 0.71 (95% CI, 0.51-0.90), compared with 0.92 (95% CI, 0.63-1.35) for treatment-dose anticoagulation.
Study boosts confidence
Despite its limitations, the study should make clinicians more confident that the use of prophylactic anticoagulation is warranted for hospitalized patients with COVID-19, write Andrew B. Dicks, MD, and Ido Weinberg, MD, from Massachusetts General Hospital, Boston, in an invited commentary.
“Practically, we still lack the granular data we need to help guide us in patient-by-patient decision-making – such as anticoagulation agent choice, dosage, and duration of therapy – especially as dictated by acuity of patient illness,” Dr. Dicks and Dr. Weinberg note.
“While we still await the data from randomized controlled trials to guide the optimal anticoagulation dose and duration, this study adds significant merit to the previously published recommendations from several different medical organizations regarding the use of prophylactic anticoagulation in hospitalized patients with COVID-19,” Dr. Dicks told this news organization.
The study was supported by Blue Cross and Blue Shield of Michigan and Blue Care Network as part of their Value Partnerships program. Dr. Vaughn has reported receiving speaking fees from Thermo Fisher Scientific. Dr. Dicks and Dr. Weinberg have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
U.S., international MIS-C studies yield disparate results
That requires rapid pragmatic evaluation of therapies. Two real-world observational studies published online June 16 in The New England Journal of Medicine do that, with differing results.
In the Overcoming COVID-19 study, investigators assessed initial therapy and outcomes for patients with MIS-C using surveillance data from 58 pediatric hospitals nationwide.
The results suggest that patients with MIS-C who were younger than 21 years of age and who were initially treated with intravenous immunoglobulin (IVIG) plus glucocorticoids fared better in terms of cardiovascular function.
The study included 518 children (median age, 8.7 years) who were admitted to the hospital between March and October 2020 and who received at least one immunomodulatory therapy. In a propensity score–matched analysis, those given IVIG plus glucocorticoids (n = 103) had a lower risk for the primary outcome of cardiovascular dysfunction on or after day 2 than those given IVIG alone (n = 103), at 17% versus 31% (risk ratio, 0.56; 95% confidence interval, 0.34-0.94).
Risks for individual aspects of the study’s composite outcome were also lower with IVIG plus glucocorticoids. Left ventricular dysfunction occurred in 8% and 17%, respectively (RR, 0.46; 95% CI, 0.19-1.15). Shock requiring vasopressor use emerged in 13% and 24%, respectively (RR, 0.54; 95% CI, 0.29-1.00).
In addition, there were fewer cases in which adjunctive therapy was given on day one among those who received combination therapy than among those who received IVIG alone, at 34% versus 70% (RR, 0.49; 95% CI, 0.36-0.65), but the risk for fever was not lower on or after day two (31% and 40%, respectively; RR, 0.78; 95% CI, 0.53-1.13).
Lead author Mary Beth F. Son, MD, director of the rheumatology program at Boston Children’s Hospital, who is also associate professor of pediatrics at Harvard Medical School, stressed that the study did not assess which MIS-C patients should receive treatment. “Rather, we studied children who had been treated with one of two initial regimens and then assessed short-term outcomes,” she told this news organization.
Going forward, it will be important to study which children should receive immunomodulatory treatment, Dr. Son said. “Specifically, can the less ill children receive IVIG alone or no treatment? This is an unanswered question at the moment, which could be addressed with a randomized controlled trial.”
Future directions, she added, will include assessing long-term cardiac outcomes for patients with MIS-C as well as studying outpatient regimens, especially those that involve steroids.
Earlier this year, French investigators found better outcomes with combined corticosteroids and IVIG than with IVIG alone. They suggested that combination therapy should be the standard of care, given the present state of therapeutic knowledge.
Maybe not so standard
Different results emerged, however, from an international study of MIS-C that compared three, rather than two, treatment approaches. Collaborators from the Best Available Treatment Study for MIS-C (BATS) evaluated data for 614 children with suspected MIS-C between June 2020 and February 2021 in 32 countries and found no substantial differences in recovery among children whose primary treatment was IVIG alone, IVIG plus glucocorticoids, or glucocorticoids alone.
The study by Andrew J. McArdle, MB BChir, MSC, a clinical research fellow at Imperial College London, and colleagues was published June 16 in The New England Journal of Medicine.
In the BATS cohort, 246 received IVIG alone, 208 received IVIG plus glucocorticoids, and 99 received glucocorticoids alone. Twenty-two patients received other combinations, including biologics, and 39 received no immunomodulatory therapy.
Among patients who were included in the primary analysis, death occurred or inotropic or ventilatory support was employed in 56 of 180 of the patients who received IVIG plus glucocorticoids, compared with 44 of 211 patients treated with IVIG alone, for an adjusted odds ratio (aOR) of 0.77 (95% CI, 0.33-1.82). Among those who received glucocorticoids alone, 17 of 83 met the primary endpoint of death or inotropic or ventilatory support, for an aOR relative to IVIG alone of 0.54 (95% CI, 0.22-1.33).
After adjustments, the likelihood for reduced disease severity was similar in the two groups relative to IVIG alone, at 0.90 for IVIG plus glucocorticoids and 0.93 for glucocorticoids alone. Time to reduction in disease severity was also comparable across all groups.
Some of the differences between the U.S. study and the global studies could be the result of the larger size of the international cohort and possibly a difference in the strains of virus in the United States and abroad, according to S. Sexson Tejtel, MD, PhD, MPH, a pediatric cardiologist at Texas Children’s Hospital and an assistant professor at Baylor College of Medicine, Houston, Texas. “Some strains make children sicker than others, and they’re going to need more treatment,” said Dr. Sexson Tejtel, who was not involved in either study.
Dr. Sexson Tejtel also noted that the U.S. researchers did not assess outcomes among children treated with steroids alone. “It would be interesting to know what steroids alone look like in the U.S. MIS-C population,” she said in an interview.
BATS corresponding author Michael Levin, MBE, PhD, FRCPCH, an Imperial College professor of pediatrics and international child health, told this news organization that the differing results may have arisen because of the international study’s three-treatment focus, its wider spectrum of patients, and its different endpoints: Death and inotropic support on or after day 2, versus echocardiographic left ventricular dysfunction or inotropic usage.
Regardless of the differences between the two studies, neither establishes the most effective single or combination treatment, writes Roberta L. DeBiasi, MD, of the Division of Pediatric Infectious Diseases at Children’s National Hospital and Research Institute and George Washington University, Washington, in an accompanying editorial. “Specifically, neither study was powered to include an evaluation of approaches that steer away from broad immunosuppression with glucocorticoids and that focus on more targeted and titratable treatments with biologic agents, such as anakinra and infliximab,” she writes.
Dr. DeBiasi adds that long-term follow-up studies of cardiac and noncardiac outcomes in these patients will launch soon. “Meanwhile, continued collaboration across centers is essential to decreasing the short-term incidence of death and complications,” she writes.
“It will be interesting as we apply results from these studies as they come out to see how they change our practice,” Dr. Sexson Tejtel said. “And it would be good to have some randomized clinical trials.”
For Dr. Levin, the bottom line is that all three treatments are associated with recovery for a majority of children. “This is good news for clinicians who have been guessing which treatment to use,” he said. “Both studies are attempts to provide doctors with some evidence on which to base treatment decisions and are not the final answer. Our study is ongoing, and with larger numbers of patients it may give clearer answers.”
The Overcoming COVID-19 study was funded by the U.S. Centers for Disease Control and Prevention. Several coauthors have reported support from industry outside of the submitted work. BATS was funded by the European Union’s Horizons 2020 Program. The study authors have disclosed no relevant financial relationships. One coauthor’s spouse is employed by GlaxoSmithKline. Dr. DeBiasi and Dr. Sexson Tejtel have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
That requires rapid pragmatic evaluation of therapies. Two real-world observational studies published online June 16 in The New England Journal of Medicine do that, with differing results.
In the Overcoming COVID-19 study, investigators assessed initial therapy and outcomes for patients with MIS-C using surveillance data from 58 pediatric hospitals nationwide.
The results suggest that patients with MIS-C who were younger than 21 years of age and who were initially treated with intravenous immunoglobulin (IVIG) plus glucocorticoids fared better in terms of cardiovascular function.
The study included 518 children (median age, 8.7 years) who were admitted to the hospital between March and October 2020 and who received at least one immunomodulatory therapy. In a propensity score–matched analysis, those given IVIG plus glucocorticoids (n = 103) had a lower risk for the primary outcome of cardiovascular dysfunction on or after day 2 than those given IVIG alone (n = 103), at 17% versus 31% (risk ratio, 0.56; 95% confidence interval, 0.34-0.94).
Risks for individual aspects of the study’s composite outcome were also lower with IVIG plus glucocorticoids. Left ventricular dysfunction occurred in 8% and 17%, respectively (RR, 0.46; 95% CI, 0.19-1.15). Shock requiring vasopressor use emerged in 13% and 24%, respectively (RR, 0.54; 95% CI, 0.29-1.00).
In addition, there were fewer cases in which adjunctive therapy was given on day one among those who received combination therapy than among those who received IVIG alone, at 34% versus 70% (RR, 0.49; 95% CI, 0.36-0.65), but the risk for fever was not lower on or after day two (31% and 40%, respectively; RR, 0.78; 95% CI, 0.53-1.13).
Lead author Mary Beth F. Son, MD, director of the rheumatology program at Boston Children’s Hospital, who is also associate professor of pediatrics at Harvard Medical School, stressed that the study did not assess which MIS-C patients should receive treatment. “Rather, we studied children who had been treated with one of two initial regimens and then assessed short-term outcomes,” she told this news organization.
Going forward, it will be important to study which children should receive immunomodulatory treatment, Dr. Son said. “Specifically, can the less ill children receive IVIG alone or no treatment? This is an unanswered question at the moment, which could be addressed with a randomized controlled trial.”
Future directions, she added, will include assessing long-term cardiac outcomes for patients with MIS-C as well as studying outpatient regimens, especially those that involve steroids.
Earlier this year, French investigators found better outcomes with combined corticosteroids and IVIG than with IVIG alone. They suggested that combination therapy should be the standard of care, given the present state of therapeutic knowledge.
Maybe not so standard
Different results emerged, however, from an international study of MIS-C that compared three, rather than two, treatment approaches. Collaborators from the Best Available Treatment Study for MIS-C (BATS) evaluated data for 614 children with suspected MIS-C between June 2020 and February 2021 in 32 countries and found no substantial differences in recovery among children whose primary treatment was IVIG alone, IVIG plus glucocorticoids, or glucocorticoids alone.
The study by Andrew J. McArdle, MB BChir, MSC, a clinical research fellow at Imperial College London, and colleagues was published June 16 in The New England Journal of Medicine.
In the BATS cohort, 246 received IVIG alone, 208 received IVIG plus glucocorticoids, and 99 received glucocorticoids alone. Twenty-two patients received other combinations, including biologics, and 39 received no immunomodulatory therapy.
Among patients who were included in the primary analysis, death occurred or inotropic or ventilatory support was employed in 56 of 180 of the patients who received IVIG plus glucocorticoids, compared with 44 of 211 patients treated with IVIG alone, for an adjusted odds ratio (aOR) of 0.77 (95% CI, 0.33-1.82). Among those who received glucocorticoids alone, 17 of 83 met the primary endpoint of death or inotropic or ventilatory support, for an aOR relative to IVIG alone of 0.54 (95% CI, 0.22-1.33).
After adjustments, the likelihood for reduced disease severity was similar in the two groups relative to IVIG alone, at 0.90 for IVIG plus glucocorticoids and 0.93 for glucocorticoids alone. Time to reduction in disease severity was also comparable across all groups.
Some of the differences between the U.S. study and the global studies could be the result of the larger size of the international cohort and possibly a difference in the strains of virus in the United States and abroad, according to S. Sexson Tejtel, MD, PhD, MPH, a pediatric cardiologist at Texas Children’s Hospital and an assistant professor at Baylor College of Medicine, Houston, Texas. “Some strains make children sicker than others, and they’re going to need more treatment,” said Dr. Sexson Tejtel, who was not involved in either study.
Dr. Sexson Tejtel also noted that the U.S. researchers did not assess outcomes among children treated with steroids alone. “It would be interesting to know what steroids alone look like in the U.S. MIS-C population,” she said in an interview.
BATS corresponding author Michael Levin, MBE, PhD, FRCPCH, an Imperial College professor of pediatrics and international child health, told this news organization that the differing results may have arisen because of the international study’s three-treatment focus, its wider spectrum of patients, and its different endpoints: Death and inotropic support on or after day 2, versus echocardiographic left ventricular dysfunction or inotropic usage.
Regardless of the differences between the two studies, neither establishes the most effective single or combination treatment, writes Roberta L. DeBiasi, MD, of the Division of Pediatric Infectious Diseases at Children’s National Hospital and Research Institute and George Washington University, Washington, in an accompanying editorial. “Specifically, neither study was powered to include an evaluation of approaches that steer away from broad immunosuppression with glucocorticoids and that focus on more targeted and titratable treatments with biologic agents, such as anakinra and infliximab,” she writes.
Dr. DeBiasi adds that long-term follow-up studies of cardiac and noncardiac outcomes in these patients will launch soon. “Meanwhile, continued collaboration across centers is essential to decreasing the short-term incidence of death and complications,” she writes.
“It will be interesting as we apply results from these studies as they come out to see how they change our practice,” Dr. Sexson Tejtel said. “And it would be good to have some randomized clinical trials.”
For Dr. Levin, the bottom line is that all three treatments are associated with recovery for a majority of children. “This is good news for clinicians who have been guessing which treatment to use,” he said. “Both studies are attempts to provide doctors with some evidence on which to base treatment decisions and are not the final answer. Our study is ongoing, and with larger numbers of patients it may give clearer answers.”
The Overcoming COVID-19 study was funded by the U.S. Centers for Disease Control and Prevention. Several coauthors have reported support from industry outside of the submitted work. BATS was funded by the European Union’s Horizons 2020 Program. The study authors have disclosed no relevant financial relationships. One coauthor’s spouse is employed by GlaxoSmithKline. Dr. DeBiasi and Dr. Sexson Tejtel have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
That requires rapid pragmatic evaluation of therapies. Two real-world observational studies published online June 16 in The New England Journal of Medicine do that, with differing results.
In the Overcoming COVID-19 study, investigators assessed initial therapy and outcomes for patients with MIS-C using surveillance data from 58 pediatric hospitals nationwide.
The results suggest that patients with MIS-C who were younger than 21 years of age and who were initially treated with intravenous immunoglobulin (IVIG) plus glucocorticoids fared better in terms of cardiovascular function.
The study included 518 children (median age, 8.7 years) who were admitted to the hospital between March and October 2020 and who received at least one immunomodulatory therapy. In a propensity score–matched analysis, those given IVIG plus glucocorticoids (n = 103) had a lower risk for the primary outcome of cardiovascular dysfunction on or after day 2 than those given IVIG alone (n = 103), at 17% versus 31% (risk ratio, 0.56; 95% confidence interval, 0.34-0.94).
Risks for individual aspects of the study’s composite outcome were also lower with IVIG plus glucocorticoids. Left ventricular dysfunction occurred in 8% and 17%, respectively (RR, 0.46; 95% CI, 0.19-1.15). Shock requiring vasopressor use emerged in 13% and 24%, respectively (RR, 0.54; 95% CI, 0.29-1.00).
In addition, there were fewer cases in which adjunctive therapy was given on day one among those who received combination therapy than among those who received IVIG alone, at 34% versus 70% (RR, 0.49; 95% CI, 0.36-0.65), but the risk for fever was not lower on or after day two (31% and 40%, respectively; RR, 0.78; 95% CI, 0.53-1.13).
Lead author Mary Beth F. Son, MD, director of the rheumatology program at Boston Children’s Hospital, who is also associate professor of pediatrics at Harvard Medical School, stressed that the study did not assess which MIS-C patients should receive treatment. “Rather, we studied children who had been treated with one of two initial regimens and then assessed short-term outcomes,” she told this news organization.
Going forward, it will be important to study which children should receive immunomodulatory treatment, Dr. Son said. “Specifically, can the less ill children receive IVIG alone or no treatment? This is an unanswered question at the moment, which could be addressed with a randomized controlled trial.”
Future directions, she added, will include assessing long-term cardiac outcomes for patients with MIS-C as well as studying outpatient regimens, especially those that involve steroids.
Earlier this year, French investigators found better outcomes with combined corticosteroids and IVIG than with IVIG alone. They suggested that combination therapy should be the standard of care, given the present state of therapeutic knowledge.
Maybe not so standard
Different results emerged, however, from an international study of MIS-C that compared three, rather than two, treatment approaches. Collaborators from the Best Available Treatment Study for MIS-C (BATS) evaluated data for 614 children with suspected MIS-C between June 2020 and February 2021 in 32 countries and found no substantial differences in recovery among children whose primary treatment was IVIG alone, IVIG plus glucocorticoids, or glucocorticoids alone.
The study by Andrew J. McArdle, MB BChir, MSC, a clinical research fellow at Imperial College London, and colleagues was published June 16 in The New England Journal of Medicine.
In the BATS cohort, 246 received IVIG alone, 208 received IVIG plus glucocorticoids, and 99 received glucocorticoids alone. Twenty-two patients received other combinations, including biologics, and 39 received no immunomodulatory therapy.
Among patients who were included in the primary analysis, death occurred or inotropic or ventilatory support was employed in 56 of 180 of the patients who received IVIG plus glucocorticoids, compared with 44 of 211 patients treated with IVIG alone, for an adjusted odds ratio (aOR) of 0.77 (95% CI, 0.33-1.82). Among those who received glucocorticoids alone, 17 of 83 met the primary endpoint of death or inotropic or ventilatory support, for an aOR relative to IVIG alone of 0.54 (95% CI, 0.22-1.33).
After adjustments, the likelihood for reduced disease severity was similar in the two groups relative to IVIG alone, at 0.90 for IVIG plus glucocorticoids and 0.93 for glucocorticoids alone. Time to reduction in disease severity was also comparable across all groups.
Some of the differences between the U.S. study and the global studies could be the result of the larger size of the international cohort and possibly a difference in the strains of virus in the United States and abroad, according to S. Sexson Tejtel, MD, PhD, MPH, a pediatric cardiologist at Texas Children’s Hospital and an assistant professor at Baylor College of Medicine, Houston, Texas. “Some strains make children sicker than others, and they’re going to need more treatment,” said Dr. Sexson Tejtel, who was not involved in either study.
Dr. Sexson Tejtel also noted that the U.S. researchers did not assess outcomes among children treated with steroids alone. “It would be interesting to know what steroids alone look like in the U.S. MIS-C population,” she said in an interview.
BATS corresponding author Michael Levin, MBE, PhD, FRCPCH, an Imperial College professor of pediatrics and international child health, told this news organization that the differing results may have arisen because of the international study’s three-treatment focus, its wider spectrum of patients, and its different endpoints: Death and inotropic support on or after day 2, versus echocardiographic left ventricular dysfunction or inotropic usage.
Regardless of the differences between the two studies, neither establishes the most effective single or combination treatment, writes Roberta L. DeBiasi, MD, of the Division of Pediatric Infectious Diseases at Children’s National Hospital and Research Institute and George Washington University, Washington, in an accompanying editorial. “Specifically, neither study was powered to include an evaluation of approaches that steer away from broad immunosuppression with glucocorticoids and that focus on more targeted and titratable treatments with biologic agents, such as anakinra and infliximab,” she writes.
Dr. DeBiasi adds that long-term follow-up studies of cardiac and noncardiac outcomes in these patients will launch soon. “Meanwhile, continued collaboration across centers is essential to decreasing the short-term incidence of death and complications,” she writes.
“It will be interesting as we apply results from these studies as they come out to see how they change our practice,” Dr. Sexson Tejtel said. “And it would be good to have some randomized clinical trials.”
For Dr. Levin, the bottom line is that all three treatments are associated with recovery for a majority of children. “This is good news for clinicians who have been guessing which treatment to use,” he said. “Both studies are attempts to provide doctors with some evidence on which to base treatment decisions and are not the final answer. Our study is ongoing, and with larger numbers of patients it may give clearer answers.”
The Overcoming COVID-19 study was funded by the U.S. Centers for Disease Control and Prevention. Several coauthors have reported support from industry outside of the submitted work. BATS was funded by the European Union’s Horizons 2020 Program. The study authors have disclosed no relevant financial relationships. One coauthor’s spouse is employed by GlaxoSmithKline. Dr. DeBiasi and Dr. Sexson Tejtel have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Fewer dangerous COPD flare-ups during COVID-19
Public health precautions meant to reduce the spread of COVID-19 may have had an unintended but happy side effect.
They may also have benefited individuals who have chronic obstructive pulmonary disease (COPD), according to a new study.
During the pandemic, admissions for COPD flare-ups dropped dramatically – by 53% – at University of Maryland Medical System hospitals.
Researchers at the university suspect this was the result of a drop in circulating seasonal respiratory viruses, such as influenza. They theorized that stay-at-home orders, social distancing, mask mandates, and strict limits on large gatherings reduced exposure not only to COVID but also to other respiratory infections.
“Our study shows there’s a silver lining to the behavior changes beyond protecting against COVID-19,” said senior author Robert Reed, MD, a pulmonologist and professor of medicine.
COPD is a group of lung diseases that worsen over time and make it hard to breathe. Before the pandemic, they were the fourth-leading cause of death worldwide, commonly triggered by tobacco smoke and dirty air. Nearly half of flare-ups are caused by seasonal respiratory viruses.
For the study, the researchers analyzed data from 13 UMMS hospitals, comparing weekly admissions for COPD in 2018 and 2019, with admissions after April 1, 2020, when COVID-19 public health measures were introduced. Investigators chose the same six-month period in each year for comparison – April 1 to Sept. 30.
The findings were matched against U.S. federal data on respiratory viral trends between Jan. 1, 2018, and Oct. 1, 2020.
As significant as was the system’s 53% drop in COPD admissions during the pandemic, there was also a 36% decline in weekly admissions for such serious conditions as congestive heart failure, diabetes and heart attack, said co–lead author Jennifer So, MD. She’s an assistant professor of medicine and COPD specialist.
The researchers warned that a full return to normal may again expose COPD patients to the familiar seasonal triggers.
“If we completely eliminate masks and distancing during cold and flu season, we’ll allow all those viruses that have been effectively suppressed to come raging back,” Dr. Reed said in a university news release. “There could be a lot of illness.”
He noted that the study did not assess which measures tamed seasonal viruses. But, Dr. Reed added, “a simple thing like wearing a mask while riding on public transit or working from home when you’re sick with a cold could go a long way to reduce virus exposure.”
Dr. So said it is a cultural norm in her native South Korea to wear masks during the winter.
“The COVID-19 pandemic has helped a lot of people around the world become more aware of the role of masking and social distancing to reduce the spread of disease,” she said in the release.
The findings were recently published in the preprint server medRxiv and have not yet been peer reviewed.
The U.S. Centers for Disease Control and Prevention has more information on COVID-19 and chronic lung diseases.
A version of this article first appeared on WebMD.com.
Public health precautions meant to reduce the spread of COVID-19 may have had an unintended but happy side effect.
They may also have benefited individuals who have chronic obstructive pulmonary disease (COPD), according to a new study.
During the pandemic, admissions for COPD flare-ups dropped dramatically – by 53% – at University of Maryland Medical System hospitals.
Researchers at the university suspect this was the result of a drop in circulating seasonal respiratory viruses, such as influenza. They theorized that stay-at-home orders, social distancing, mask mandates, and strict limits on large gatherings reduced exposure not only to COVID but also to other respiratory infections.
“Our study shows there’s a silver lining to the behavior changes beyond protecting against COVID-19,” said senior author Robert Reed, MD, a pulmonologist and professor of medicine.
COPD is a group of lung diseases that worsen over time and make it hard to breathe. Before the pandemic, they were the fourth-leading cause of death worldwide, commonly triggered by tobacco smoke and dirty air. Nearly half of flare-ups are caused by seasonal respiratory viruses.
For the study, the researchers analyzed data from 13 UMMS hospitals, comparing weekly admissions for COPD in 2018 and 2019, with admissions after April 1, 2020, when COVID-19 public health measures were introduced. Investigators chose the same six-month period in each year for comparison – April 1 to Sept. 30.
The findings were matched against U.S. federal data on respiratory viral trends between Jan. 1, 2018, and Oct. 1, 2020.
As significant as was the system’s 53% drop in COPD admissions during the pandemic, there was also a 36% decline in weekly admissions for such serious conditions as congestive heart failure, diabetes and heart attack, said co–lead author Jennifer So, MD. She’s an assistant professor of medicine and COPD specialist.
The researchers warned that a full return to normal may again expose COPD patients to the familiar seasonal triggers.
“If we completely eliminate masks and distancing during cold and flu season, we’ll allow all those viruses that have been effectively suppressed to come raging back,” Dr. Reed said in a university news release. “There could be a lot of illness.”
He noted that the study did not assess which measures tamed seasonal viruses. But, Dr. Reed added, “a simple thing like wearing a mask while riding on public transit or working from home when you’re sick with a cold could go a long way to reduce virus exposure.”
Dr. So said it is a cultural norm in her native South Korea to wear masks during the winter.
“The COVID-19 pandemic has helped a lot of people around the world become more aware of the role of masking and social distancing to reduce the spread of disease,” she said in the release.
The findings were recently published in the preprint server medRxiv and have not yet been peer reviewed.
The U.S. Centers for Disease Control and Prevention has more information on COVID-19 and chronic lung diseases.
A version of this article first appeared on WebMD.com.
Public health precautions meant to reduce the spread of COVID-19 may have had an unintended but happy side effect.
They may also have benefited individuals who have chronic obstructive pulmonary disease (COPD), according to a new study.
During the pandemic, admissions for COPD flare-ups dropped dramatically – by 53% – at University of Maryland Medical System hospitals.
Researchers at the university suspect this was the result of a drop in circulating seasonal respiratory viruses, such as influenza. They theorized that stay-at-home orders, social distancing, mask mandates, and strict limits on large gatherings reduced exposure not only to COVID but also to other respiratory infections.
“Our study shows there’s a silver lining to the behavior changes beyond protecting against COVID-19,” said senior author Robert Reed, MD, a pulmonologist and professor of medicine.
COPD is a group of lung diseases that worsen over time and make it hard to breathe. Before the pandemic, they were the fourth-leading cause of death worldwide, commonly triggered by tobacco smoke and dirty air. Nearly half of flare-ups are caused by seasonal respiratory viruses.
For the study, the researchers analyzed data from 13 UMMS hospitals, comparing weekly admissions for COPD in 2018 and 2019, with admissions after April 1, 2020, when COVID-19 public health measures were introduced. Investigators chose the same six-month period in each year for comparison – April 1 to Sept. 30.
The findings were matched against U.S. federal data on respiratory viral trends between Jan. 1, 2018, and Oct. 1, 2020.
As significant as was the system’s 53% drop in COPD admissions during the pandemic, there was also a 36% decline in weekly admissions for such serious conditions as congestive heart failure, diabetes and heart attack, said co–lead author Jennifer So, MD. She’s an assistant professor of medicine and COPD specialist.
The researchers warned that a full return to normal may again expose COPD patients to the familiar seasonal triggers.
“If we completely eliminate masks and distancing during cold and flu season, we’ll allow all those viruses that have been effectively suppressed to come raging back,” Dr. Reed said in a university news release. “There could be a lot of illness.”
He noted that the study did not assess which measures tamed seasonal viruses. But, Dr. Reed added, “a simple thing like wearing a mask while riding on public transit or working from home when you’re sick with a cold could go a long way to reduce virus exposure.”
Dr. So said it is a cultural norm in her native South Korea to wear masks during the winter.
“The COVID-19 pandemic has helped a lot of people around the world become more aware of the role of masking and social distancing to reduce the spread of disease,” she said in the release.
The findings were recently published in the preprint server medRxiv and have not yet been peer reviewed.
The U.S. Centers for Disease Control and Prevention has more information on COVID-19 and chronic lung diseases.
A version of this article first appeared on WebMD.com.
New biomarkers may predict interstitial lung disease progression in patients with systemic sclerosis
Quantitative assessment of the extent of interstitial lung disease in patients with systemic sclerosis and levels of certain proteins in bronchoalveolar lavage samples have potential for predicting mortality and disease progression, according to two analyses of data from the Scleroderma Lung Study I and II.
The analyses, presented at the annual European Congress of Rheumatology, aim to improve current prognostic abilities in patients with systemic sclerosis–interstitial lung disease (SSc-ILD). Although forced vital capacity is commonly used as a biomarker for survival in many SSc-ILD trials, other factors can affect FVC, such as respiratory muscle weakness and skin fibrosis. Further, FVC correlates poorly with patient-reported outcomes, explained first author Elizabeth Volkmann, MD, director of the scleroderma program at the University of California, Los Angeles, and the founder and codirector of the UCLA connective tissue disease–related interstitial lung disease program.
Dr. Volkmann presented two studies that investigated the potential of radiographic and protein biomarkers for predicting mortality and identifying patients at risk for ILD progression. The biomarkers may also help to identify patients who would benefit most from immunosuppressive therapy.
The first study found that tracking the quantitative extent of ILD (QILD) over time with high-resolution CT (HRCT) predicted poorer outcomes and could therefore act as a surrogate endpoint for mortality among patients with SSc-ILD. The other study identified associations between specific proteins from bronchoalveolar lavage (BAL) and the likelihood of ILD progression, although some associations were treatment dependent.
Jacob M. van Laar, MD, PhD, professor of rheumatology at the University Medical Center Utrecht (the Netherlands), who was not involved in the study, found the results intriguing and noted the importance of further validation in research before these biomarkers are considered for clinical use.
“It would be wonderful if we can tailor therapy based on BAL biomarkers in the future, as clinicians often struggle to decide on selection, timing, and duration of immunosuppressive treatment,” Dr. van Laar told this news organization. “This has become even more relevant with the introduction of new drugs such as nintedanib.”
Extent of ILD progression as a surrogate for mortality
Scleroderma Lung Study I involved 158 patients with SSc-ILD who were randomly assigned to receive either cyclophosphamide or placebo for 12 months. Scleroderma Lung Study II included 142 patients with SSc-ILD who were randomly assigned to receive either mycophenolate for 24 months or cyclophosphamide for 12 months followed by placebo for 12 months.
The researchers calculated QILD in the whole lung at baseline, at 12 months in the first trial, and at 24 months in the second trial. However, only 82 participants from the first trial and 90 participants from the second trial underwent HRCT. Demographic and disease characteristics were similar between the two groups on follow-up scans.
Follow-up continued for 12 years for patients in the first trial and 8 years in the second. The researchers compared survival rates between the 41% of participants from the first study and 31% of participants from the second study who had poorer QILD scores (at least a 2% increase) with the participants who had stable or improved scores (less than 2% increase).
Participants from both trials had significantly poorer long-term survival if their QILD scores had increased by at least 2% at follow-up (P = .01 for I; P = .019 for II). The association was no longer significant after adjustment for baseline FVC, age, and modified Rodnan skin score in the first trial (hazard ratio, 1.98; P = .089), but it remained significant for participants of the second trial (HR, 3.86; P = .014).
“Data from two independent trial cohorts demonstrated that radiographic progression of SSc-ILD at 1 and 2 years is associated with worse long-term survival,” Dr. Volkmann told attendees.
However, FVC did not significantly predict risk of mortality in either trial.
“To me, the most striking finding from the first study was that change in QILD performed better as a predictor of survival than change in FVC,” Dr. van Laar said in an interview. “This indicates QILD is fit for purpose and worth including in future clinical trials.”
Limitations of the study included lack of HRCT for all participants in the trials and the difference in timing (1 year and 2 years) of HRCT assessment between the two trials. The greater hazard ratio for worsened QILD in the second trial may suggest that assessment at 2 years provides more reliable data as a biomarker, Dr. Volkmann said.
“QILD may represent a better proxy for how a patient feels, functions, and survives than FVC,” she said.
Treatment-dependent biomarkers for worsening lung fibrosis
In the second study, the researchers looked for any associations between changes in the radiographic extent of SSc-ILD and 68 proteins from BAL.
“Being able to risk-stratify patients with interstitial lung disease at the time of diagnosis and predict which patients are likely to have a stable versus progressive disease course is critical for making important treatment decisions for these patients,” Dr. Volkmann told attendees.
The second study she presented involved Scleroderma Lung Study I. Of the 158 participants, 144 underwent a bronchoscopy, yielding BAL protein samples from 103 participants. The researchers determined the extent of radiographic fibrosis in the whole lung with quantitative imaging analysis of HRCT of the chest at baseline and 12 months.
Although the researchers identified several statistically significant associations between certain proteins and changes in radiographic fibrosis, “baseline protein levels were differentially associated with the course of ILD based on treatment status,” she told attendees.
For example, increased levels of the following proteins were linked to poor radiographic fibrosis scores for patients who received placebo:
- Granulocyte-macrophage colony-stimulating factor
- Interleukin-1
- Monocyte chemoattractant protein–3
- Chemokine ligand–5
- Transforming growth factor–beta
- Hepatocyte growth factor
- Stem cell factor
- IL-4
- TGF-alpha
Yet increases in these proteins predicted improvement in radiographic fibrosis in patients who had taken cyclophosphamide.
Independently of treatment, the researchers also identified an association between higher levels of fractalkine and poorer radiographic fibrosis scores and between higher IL-7 levels and improved radiographic fibrosis scores.
After adjusting for treatment arm and baseline severity of ILD, significant associations remained between change in radiographic fibrosis score and IL-1, MCP-3, surfactant protein C, IL-7 and CCL-5 levels.
“Biomarker discovery is really central to our ability to risk stratify patients with SSc-ILD,” Dr. Volkmann told attendees. “Understanding how biomarkers predict outcomes in treated and untreated patients may improve personalized medicine to patients with SSc-ILD and could also reveal novel treatment targets.”
Dr. van Laar said in an interview that this study’s biggest strength lay in its large sample size and in the comprehensiveness of the biomarkers studied.
“The findings are interesting from a research perspective and potentially relevant for clinical practice, but the utility of measuring biomarkers in BAL should be further studied for predictive value on clinical endpoints,” Dr. van Laar said. “BAL is an invasive procedure [that] is not routinely done.”
The research was funded by the National Institutes of Health. Dr. Volkmann has consulted for Boehringer Ingelheim and received grant funding from Corbus, Forbius, and Kadmon. Dr. van Laar has received grant funding or personal fees from Arthrogen, Arxx Therapeutics, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Gesynta, Leadiant, Merck Sharp & Dohme, Roche, Sanofi, and Thermofisher.
A version of this article first appeared on Medscape.com.
Quantitative assessment of the extent of interstitial lung disease in patients with systemic sclerosis and levels of certain proteins in bronchoalveolar lavage samples have potential for predicting mortality and disease progression, according to two analyses of data from the Scleroderma Lung Study I and II.
The analyses, presented at the annual European Congress of Rheumatology, aim to improve current prognostic abilities in patients with systemic sclerosis–interstitial lung disease (SSc-ILD). Although forced vital capacity is commonly used as a biomarker for survival in many SSc-ILD trials, other factors can affect FVC, such as respiratory muscle weakness and skin fibrosis. Further, FVC correlates poorly with patient-reported outcomes, explained first author Elizabeth Volkmann, MD, director of the scleroderma program at the University of California, Los Angeles, and the founder and codirector of the UCLA connective tissue disease–related interstitial lung disease program.
Dr. Volkmann presented two studies that investigated the potential of radiographic and protein biomarkers for predicting mortality and identifying patients at risk for ILD progression. The biomarkers may also help to identify patients who would benefit most from immunosuppressive therapy.
The first study found that tracking the quantitative extent of ILD (QILD) over time with high-resolution CT (HRCT) predicted poorer outcomes and could therefore act as a surrogate endpoint for mortality among patients with SSc-ILD. The other study identified associations between specific proteins from bronchoalveolar lavage (BAL) and the likelihood of ILD progression, although some associations were treatment dependent.
Jacob M. van Laar, MD, PhD, professor of rheumatology at the University Medical Center Utrecht (the Netherlands), who was not involved in the study, found the results intriguing and noted the importance of further validation in research before these biomarkers are considered for clinical use.
“It would be wonderful if we can tailor therapy based on BAL biomarkers in the future, as clinicians often struggle to decide on selection, timing, and duration of immunosuppressive treatment,” Dr. van Laar told this news organization. “This has become even more relevant with the introduction of new drugs such as nintedanib.”
Extent of ILD progression as a surrogate for mortality
Scleroderma Lung Study I involved 158 patients with SSc-ILD who were randomly assigned to receive either cyclophosphamide or placebo for 12 months. Scleroderma Lung Study II included 142 patients with SSc-ILD who were randomly assigned to receive either mycophenolate for 24 months or cyclophosphamide for 12 months followed by placebo for 12 months.
The researchers calculated QILD in the whole lung at baseline, at 12 months in the first trial, and at 24 months in the second trial. However, only 82 participants from the first trial and 90 participants from the second trial underwent HRCT. Demographic and disease characteristics were similar between the two groups on follow-up scans.
Follow-up continued for 12 years for patients in the first trial and 8 years in the second. The researchers compared survival rates between the 41% of participants from the first study and 31% of participants from the second study who had poorer QILD scores (at least a 2% increase) with the participants who had stable or improved scores (less than 2% increase).
Participants from both trials had significantly poorer long-term survival if their QILD scores had increased by at least 2% at follow-up (P = .01 for I; P = .019 for II). The association was no longer significant after adjustment for baseline FVC, age, and modified Rodnan skin score in the first trial (hazard ratio, 1.98; P = .089), but it remained significant for participants of the second trial (HR, 3.86; P = .014).
“Data from two independent trial cohorts demonstrated that radiographic progression of SSc-ILD at 1 and 2 years is associated with worse long-term survival,” Dr. Volkmann told attendees.
However, FVC did not significantly predict risk of mortality in either trial.
“To me, the most striking finding from the first study was that change in QILD performed better as a predictor of survival than change in FVC,” Dr. van Laar said in an interview. “This indicates QILD is fit for purpose and worth including in future clinical trials.”
Limitations of the study included lack of HRCT for all participants in the trials and the difference in timing (1 year and 2 years) of HRCT assessment between the two trials. The greater hazard ratio for worsened QILD in the second trial may suggest that assessment at 2 years provides more reliable data as a biomarker, Dr. Volkmann said.
“QILD may represent a better proxy for how a patient feels, functions, and survives than FVC,” she said.
Treatment-dependent biomarkers for worsening lung fibrosis
In the second study, the researchers looked for any associations between changes in the radiographic extent of SSc-ILD and 68 proteins from BAL.
“Being able to risk-stratify patients with interstitial lung disease at the time of diagnosis and predict which patients are likely to have a stable versus progressive disease course is critical for making important treatment decisions for these patients,” Dr. Volkmann told attendees.
The second study she presented involved Scleroderma Lung Study I. Of the 158 participants, 144 underwent a bronchoscopy, yielding BAL protein samples from 103 participants. The researchers determined the extent of radiographic fibrosis in the whole lung with quantitative imaging analysis of HRCT of the chest at baseline and 12 months.
Although the researchers identified several statistically significant associations between certain proteins and changes in radiographic fibrosis, “baseline protein levels were differentially associated with the course of ILD based on treatment status,” she told attendees.
For example, increased levels of the following proteins were linked to poor radiographic fibrosis scores for patients who received placebo:
- Granulocyte-macrophage colony-stimulating factor
- Interleukin-1
- Monocyte chemoattractant protein–3
- Chemokine ligand–5
- Transforming growth factor–beta
- Hepatocyte growth factor
- Stem cell factor
- IL-4
- TGF-alpha
Yet increases in these proteins predicted improvement in radiographic fibrosis in patients who had taken cyclophosphamide.
Independently of treatment, the researchers also identified an association between higher levels of fractalkine and poorer radiographic fibrosis scores and between higher IL-7 levels and improved radiographic fibrosis scores.
After adjusting for treatment arm and baseline severity of ILD, significant associations remained between change in radiographic fibrosis score and IL-1, MCP-3, surfactant protein C, IL-7 and CCL-5 levels.
“Biomarker discovery is really central to our ability to risk stratify patients with SSc-ILD,” Dr. Volkmann told attendees. “Understanding how biomarkers predict outcomes in treated and untreated patients may improve personalized medicine to patients with SSc-ILD and could also reveal novel treatment targets.”
Dr. van Laar said in an interview that this study’s biggest strength lay in its large sample size and in the comprehensiveness of the biomarkers studied.
“The findings are interesting from a research perspective and potentially relevant for clinical practice, but the utility of measuring biomarkers in BAL should be further studied for predictive value on clinical endpoints,” Dr. van Laar said. “BAL is an invasive procedure [that] is not routinely done.”
The research was funded by the National Institutes of Health. Dr. Volkmann has consulted for Boehringer Ingelheim and received grant funding from Corbus, Forbius, and Kadmon. Dr. van Laar has received grant funding or personal fees from Arthrogen, Arxx Therapeutics, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Gesynta, Leadiant, Merck Sharp & Dohme, Roche, Sanofi, and Thermofisher.
A version of this article first appeared on Medscape.com.
Quantitative assessment of the extent of interstitial lung disease in patients with systemic sclerosis and levels of certain proteins in bronchoalveolar lavage samples have potential for predicting mortality and disease progression, according to two analyses of data from the Scleroderma Lung Study I and II.
The analyses, presented at the annual European Congress of Rheumatology, aim to improve current prognostic abilities in patients with systemic sclerosis–interstitial lung disease (SSc-ILD). Although forced vital capacity is commonly used as a biomarker for survival in many SSc-ILD trials, other factors can affect FVC, such as respiratory muscle weakness and skin fibrosis. Further, FVC correlates poorly with patient-reported outcomes, explained first author Elizabeth Volkmann, MD, director of the scleroderma program at the University of California, Los Angeles, and the founder and codirector of the UCLA connective tissue disease–related interstitial lung disease program.
Dr. Volkmann presented two studies that investigated the potential of radiographic and protein biomarkers for predicting mortality and identifying patients at risk for ILD progression. The biomarkers may also help to identify patients who would benefit most from immunosuppressive therapy.
The first study found that tracking the quantitative extent of ILD (QILD) over time with high-resolution CT (HRCT) predicted poorer outcomes and could therefore act as a surrogate endpoint for mortality among patients with SSc-ILD. The other study identified associations between specific proteins from bronchoalveolar lavage (BAL) and the likelihood of ILD progression, although some associations were treatment dependent.
Jacob M. van Laar, MD, PhD, professor of rheumatology at the University Medical Center Utrecht (the Netherlands), who was not involved in the study, found the results intriguing and noted the importance of further validation in research before these biomarkers are considered for clinical use.
“It would be wonderful if we can tailor therapy based on BAL biomarkers in the future, as clinicians often struggle to decide on selection, timing, and duration of immunosuppressive treatment,” Dr. van Laar told this news organization. “This has become even more relevant with the introduction of new drugs such as nintedanib.”
Extent of ILD progression as a surrogate for mortality
Scleroderma Lung Study I involved 158 patients with SSc-ILD who were randomly assigned to receive either cyclophosphamide or placebo for 12 months. Scleroderma Lung Study II included 142 patients with SSc-ILD who were randomly assigned to receive either mycophenolate for 24 months or cyclophosphamide for 12 months followed by placebo for 12 months.
The researchers calculated QILD in the whole lung at baseline, at 12 months in the first trial, and at 24 months in the second trial. However, only 82 participants from the first trial and 90 participants from the second trial underwent HRCT. Demographic and disease characteristics were similar between the two groups on follow-up scans.
Follow-up continued for 12 years for patients in the first trial and 8 years in the second. The researchers compared survival rates between the 41% of participants from the first study and 31% of participants from the second study who had poorer QILD scores (at least a 2% increase) with the participants who had stable or improved scores (less than 2% increase).
Participants from both trials had significantly poorer long-term survival if their QILD scores had increased by at least 2% at follow-up (P = .01 for I; P = .019 for II). The association was no longer significant after adjustment for baseline FVC, age, and modified Rodnan skin score in the first trial (hazard ratio, 1.98; P = .089), but it remained significant for participants of the second trial (HR, 3.86; P = .014).
“Data from two independent trial cohorts demonstrated that radiographic progression of SSc-ILD at 1 and 2 years is associated with worse long-term survival,” Dr. Volkmann told attendees.
However, FVC did not significantly predict risk of mortality in either trial.
“To me, the most striking finding from the first study was that change in QILD performed better as a predictor of survival than change in FVC,” Dr. van Laar said in an interview. “This indicates QILD is fit for purpose and worth including in future clinical trials.”
Limitations of the study included lack of HRCT for all participants in the trials and the difference in timing (1 year and 2 years) of HRCT assessment between the two trials. The greater hazard ratio for worsened QILD in the second trial may suggest that assessment at 2 years provides more reliable data as a biomarker, Dr. Volkmann said.
“QILD may represent a better proxy for how a patient feels, functions, and survives than FVC,” she said.
Treatment-dependent biomarkers for worsening lung fibrosis
In the second study, the researchers looked for any associations between changes in the radiographic extent of SSc-ILD and 68 proteins from BAL.
“Being able to risk-stratify patients with interstitial lung disease at the time of diagnosis and predict which patients are likely to have a stable versus progressive disease course is critical for making important treatment decisions for these patients,” Dr. Volkmann told attendees.
The second study she presented involved Scleroderma Lung Study I. Of the 158 participants, 144 underwent a bronchoscopy, yielding BAL protein samples from 103 participants. The researchers determined the extent of radiographic fibrosis in the whole lung with quantitative imaging analysis of HRCT of the chest at baseline and 12 months.
Although the researchers identified several statistically significant associations between certain proteins and changes in radiographic fibrosis, “baseline protein levels were differentially associated with the course of ILD based on treatment status,” she told attendees.
For example, increased levels of the following proteins were linked to poor radiographic fibrosis scores for patients who received placebo:
- Granulocyte-macrophage colony-stimulating factor
- Interleukin-1
- Monocyte chemoattractant protein–3
- Chemokine ligand–5
- Transforming growth factor–beta
- Hepatocyte growth factor
- Stem cell factor
- IL-4
- TGF-alpha
Yet increases in these proteins predicted improvement in radiographic fibrosis in patients who had taken cyclophosphamide.
Independently of treatment, the researchers also identified an association between higher levels of fractalkine and poorer radiographic fibrosis scores and between higher IL-7 levels and improved radiographic fibrosis scores.
After adjusting for treatment arm and baseline severity of ILD, significant associations remained between change in radiographic fibrosis score and IL-1, MCP-3, surfactant protein C, IL-7 and CCL-5 levels.
“Biomarker discovery is really central to our ability to risk stratify patients with SSc-ILD,” Dr. Volkmann told attendees. “Understanding how biomarkers predict outcomes in treated and untreated patients may improve personalized medicine to patients with SSc-ILD and could also reveal novel treatment targets.”
Dr. van Laar said in an interview that this study’s biggest strength lay in its large sample size and in the comprehensiveness of the biomarkers studied.
“The findings are interesting from a research perspective and potentially relevant for clinical practice, but the utility of measuring biomarkers in BAL should be further studied for predictive value on clinical endpoints,” Dr. van Laar said. “BAL is an invasive procedure [that] is not routinely done.”
The research was funded by the National Institutes of Health. Dr. Volkmann has consulted for Boehringer Ingelheim and received grant funding from Corbus, Forbius, and Kadmon. Dr. van Laar has received grant funding or personal fees from Arthrogen, Arxx Therapeutics, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Gesynta, Leadiant, Merck Sharp & Dohme, Roche, Sanofi, and Thermofisher.
A version of this article first appeared on Medscape.com.
Third COVID-19 vaccine dose helped some transplant recipients
All of those with low titers before the third dose had high titers after receiving the additional shot, but only about 33% of those with negative initial responses had detectable antibodies after the third dose, according to the paper, published in Annals of Internal Medicine.
Researchers at Johns Hopkins, Baltimore, who keep a COVID-19 vaccine registry, perform antibody tests on all registry subjects and inform them of their results. Registry participants were asked to inform the research team if they received a third dose, and, the research team tracked the immune responses of those who did.
The participants in this case series had low antibody levels and received a third dose of the vaccine on their own between March 20 and May 10 of 2021.
Third dose results
In this cases series – thought to be the first to look at third vaccine shots in this type of patient group – all six of those who had low antibody titers before the third dose had high-positive titers after the third dose.
Of the 24 individuals who had negative antibody titers before the third dose, just 6 had high titers after the third dose.
Two of the participants had low-positive titers, and 16 were negative.
“Several of those boosted very nicely into ranges seen, using these assays, in healthy persons,” said William Werbel, MD, a fellow in infectious disease at Johns Hopkins Medicine, Baltimore, who helped lead the study. Those with negative levels, even if they responded, tended to have lower titers, he said.
“The benefits at least from an antibody perspective were not the same for everybody and so this is obviously something that needs to be considered when thinking about selecting patients” for a COVID-19 prevention strategy, he said.
Reactions to the vaccine were low to moderate, such as some arm pain and fatigue.
“Showing that something is safe in that special, vulnerable population is important,” Dr. Werbel said. “We’re all wanting to make sure that we’re doing no harm.”
Dr. Werbel noted that there was no pattern in the small series based on the organ transplanted or in the vaccines used. As their third shot, 15 of the patients received the Johnson & Johnson vaccine; 9 received Moderna; and 6 received Pfizer-BioNTech.
Welcome news, but larger studies needed
“To think that a third dose could confer protection for a significant number of people is of course extremely welcome news,” said Christian Larsen, MD, DPhil, professor of surgery in the transplantation division at Emory University, Atlanta, who was not involved in the study. “It’s the easiest conceivable next intervention.”
He added, “We just want studies to confirm that – larger studies.”
Dr. Werbel stressed the importance of looking at third doses in these patients in a more controlled fashion in a randomized trial, to more carefully monitor safety and how patients fare when starting with one type of vaccine and switching to another, for example.
Richard Wender, MD, chair of family medicine and community health at the University of Pennsylvania, Philadelphia, said the findings are a reminder that there is still a lot that is unknown about COVID-19 and vaccination.
“We still don’t know who will or will not benefit from a third dose,” he said. “And our knowledge is evolving. For example, a recent study suggested that people with previous infection and who are vaccinated may have better and longer protection than people with vaccination alone. We’re still learning.”
He added that specialists, not primary care clinicians, should be relied upon to respond to this emerging vaccination data. Primary care doctors are very busy in other ways – such as in getting children caught up on vaccinations and helping adults return to managing their chronic diseases, Dr. Wender noted.
“Their focus needs to be on helping to overcome hesitancy, mistrust, lack of information, or antivaccination sentiment to help more people feel comfortable being vaccinated – this is a lot of work and needs constant focus. In short, primary care clinicians need to focus chiefly on the unvaccinated,” he said.
“Monitoring immunization recommendations for unique at-risk populations should be the chief responsibility of teams providing subspecialty care, [such as for] transplant patients, people with chronic kidney disease, cancer patients, and people with other chronic illnesses. This will allow primary care clinicians to tackle their many complex jobs.”
Possible solutions for those with low antibody responses
Dr. Larsen said that those with ongoing low antibody responses might still have other immune responses, such as a T-cell response. Such patients also could consider changing their vaccine type, he said.
“At the more significant intervention level, there may be circumstances where one could change the immunosuppressive drugs in a controlled way that might allow a better response,” suggested Dr. Larsen. “That’s obviously going to be something that requires a lot more thought and careful study.”
Dr. Werbel said that other options might need to be considered for those having no response following a third dose. One possibility is trying a vaccine with an adjuvant, such as the Novavax version, which might be more widely available soon.
“If you’re given a third dose of a very immunogenic vaccine – something that should work – and you just have no antibody development, it seems relatively unlikely that doing the same thing again is going to help you from that perspective, and for all we know might expose you to more risk,” Dr. Werbel noted.
Participant details
None of the 30 patients were thought to have ever had COVID-19. On average, patients had received their transplant 4.5 years before their original vaccination. In 25 patients, maintenance immunosuppression included tacrolimus or cyclosporine along with mycophenolate. Corticosteroids were also used for 24 patients, sirolimus was used for one patient, and belatacept was used for another patient.
Fifty-seven percent of patients had received the Pfizer/BioNTech vaccine originally, and 43% the Moderna vaccine. Most of the patients were kidney recipients, with two heart, three liver, one lung, one pancreas and one kidney-pancreas.
Dr. Werbel, Dr. Wender, and Dr. Larsen reported no relevant disclosures.
All of those with low titers before the third dose had high titers after receiving the additional shot, but only about 33% of those with negative initial responses had detectable antibodies after the third dose, according to the paper, published in Annals of Internal Medicine.
Researchers at Johns Hopkins, Baltimore, who keep a COVID-19 vaccine registry, perform antibody tests on all registry subjects and inform them of their results. Registry participants were asked to inform the research team if they received a third dose, and, the research team tracked the immune responses of those who did.
The participants in this case series had low antibody levels and received a third dose of the vaccine on their own between March 20 and May 10 of 2021.
Third dose results
In this cases series – thought to be the first to look at third vaccine shots in this type of patient group – all six of those who had low antibody titers before the third dose had high-positive titers after the third dose.
Of the 24 individuals who had negative antibody titers before the third dose, just 6 had high titers after the third dose.
Two of the participants had low-positive titers, and 16 were negative.
“Several of those boosted very nicely into ranges seen, using these assays, in healthy persons,” said William Werbel, MD, a fellow in infectious disease at Johns Hopkins Medicine, Baltimore, who helped lead the study. Those with negative levels, even if they responded, tended to have lower titers, he said.
“The benefits at least from an antibody perspective were not the same for everybody and so this is obviously something that needs to be considered when thinking about selecting patients” for a COVID-19 prevention strategy, he said.
Reactions to the vaccine were low to moderate, such as some arm pain and fatigue.
“Showing that something is safe in that special, vulnerable population is important,” Dr. Werbel said. “We’re all wanting to make sure that we’re doing no harm.”
Dr. Werbel noted that there was no pattern in the small series based on the organ transplanted or in the vaccines used. As their third shot, 15 of the patients received the Johnson & Johnson vaccine; 9 received Moderna; and 6 received Pfizer-BioNTech.
Welcome news, but larger studies needed
“To think that a third dose could confer protection for a significant number of people is of course extremely welcome news,” said Christian Larsen, MD, DPhil, professor of surgery in the transplantation division at Emory University, Atlanta, who was not involved in the study. “It’s the easiest conceivable next intervention.”
He added, “We just want studies to confirm that – larger studies.”
Dr. Werbel stressed the importance of looking at third doses in these patients in a more controlled fashion in a randomized trial, to more carefully monitor safety and how patients fare when starting with one type of vaccine and switching to another, for example.
Richard Wender, MD, chair of family medicine and community health at the University of Pennsylvania, Philadelphia, said the findings are a reminder that there is still a lot that is unknown about COVID-19 and vaccination.
“We still don’t know who will or will not benefit from a third dose,” he said. “And our knowledge is evolving. For example, a recent study suggested that people with previous infection and who are vaccinated may have better and longer protection than people with vaccination alone. We’re still learning.”
He added that specialists, not primary care clinicians, should be relied upon to respond to this emerging vaccination data. Primary care doctors are very busy in other ways – such as in getting children caught up on vaccinations and helping adults return to managing their chronic diseases, Dr. Wender noted.
“Their focus needs to be on helping to overcome hesitancy, mistrust, lack of information, or antivaccination sentiment to help more people feel comfortable being vaccinated – this is a lot of work and needs constant focus. In short, primary care clinicians need to focus chiefly on the unvaccinated,” he said.
“Monitoring immunization recommendations for unique at-risk populations should be the chief responsibility of teams providing subspecialty care, [such as for] transplant patients, people with chronic kidney disease, cancer patients, and people with other chronic illnesses. This will allow primary care clinicians to tackle their many complex jobs.”
Possible solutions for those with low antibody responses
Dr. Larsen said that those with ongoing low antibody responses might still have other immune responses, such as a T-cell response. Such patients also could consider changing their vaccine type, he said.
“At the more significant intervention level, there may be circumstances where one could change the immunosuppressive drugs in a controlled way that might allow a better response,” suggested Dr. Larsen. “That’s obviously going to be something that requires a lot more thought and careful study.”
Dr. Werbel said that other options might need to be considered for those having no response following a third dose. One possibility is trying a vaccine with an adjuvant, such as the Novavax version, which might be more widely available soon.
“If you’re given a third dose of a very immunogenic vaccine – something that should work – and you just have no antibody development, it seems relatively unlikely that doing the same thing again is going to help you from that perspective, and for all we know might expose you to more risk,” Dr. Werbel noted.
Participant details
None of the 30 patients were thought to have ever had COVID-19. On average, patients had received their transplant 4.5 years before their original vaccination. In 25 patients, maintenance immunosuppression included tacrolimus or cyclosporine along with mycophenolate. Corticosteroids were also used for 24 patients, sirolimus was used for one patient, and belatacept was used for another patient.
Fifty-seven percent of patients had received the Pfizer/BioNTech vaccine originally, and 43% the Moderna vaccine. Most of the patients were kidney recipients, with two heart, three liver, one lung, one pancreas and one kidney-pancreas.
Dr. Werbel, Dr. Wender, and Dr. Larsen reported no relevant disclosures.
All of those with low titers before the third dose had high titers after receiving the additional shot, but only about 33% of those with negative initial responses had detectable antibodies after the third dose, according to the paper, published in Annals of Internal Medicine.
Researchers at Johns Hopkins, Baltimore, who keep a COVID-19 vaccine registry, perform antibody tests on all registry subjects and inform them of their results. Registry participants were asked to inform the research team if they received a third dose, and, the research team tracked the immune responses of those who did.
The participants in this case series had low antibody levels and received a third dose of the vaccine on their own between March 20 and May 10 of 2021.
Third dose results
In this cases series – thought to be the first to look at third vaccine shots in this type of patient group – all six of those who had low antibody titers before the third dose had high-positive titers after the third dose.
Of the 24 individuals who had negative antibody titers before the third dose, just 6 had high titers after the third dose.
Two of the participants had low-positive titers, and 16 were negative.
“Several of those boosted very nicely into ranges seen, using these assays, in healthy persons,” said William Werbel, MD, a fellow in infectious disease at Johns Hopkins Medicine, Baltimore, who helped lead the study. Those with negative levels, even if they responded, tended to have lower titers, he said.
“The benefits at least from an antibody perspective were not the same for everybody and so this is obviously something that needs to be considered when thinking about selecting patients” for a COVID-19 prevention strategy, he said.
Reactions to the vaccine were low to moderate, such as some arm pain and fatigue.
“Showing that something is safe in that special, vulnerable population is important,” Dr. Werbel said. “We’re all wanting to make sure that we’re doing no harm.”
Dr. Werbel noted that there was no pattern in the small series based on the organ transplanted or in the vaccines used. As their third shot, 15 of the patients received the Johnson & Johnson vaccine; 9 received Moderna; and 6 received Pfizer-BioNTech.
Welcome news, but larger studies needed
“To think that a third dose could confer protection for a significant number of people is of course extremely welcome news,” said Christian Larsen, MD, DPhil, professor of surgery in the transplantation division at Emory University, Atlanta, who was not involved in the study. “It’s the easiest conceivable next intervention.”
He added, “We just want studies to confirm that – larger studies.”
Dr. Werbel stressed the importance of looking at third doses in these patients in a more controlled fashion in a randomized trial, to more carefully monitor safety and how patients fare when starting with one type of vaccine and switching to another, for example.
Richard Wender, MD, chair of family medicine and community health at the University of Pennsylvania, Philadelphia, said the findings are a reminder that there is still a lot that is unknown about COVID-19 and vaccination.
“We still don’t know who will or will not benefit from a third dose,” he said. “And our knowledge is evolving. For example, a recent study suggested that people with previous infection and who are vaccinated may have better and longer protection than people with vaccination alone. We’re still learning.”
He added that specialists, not primary care clinicians, should be relied upon to respond to this emerging vaccination data. Primary care doctors are very busy in other ways – such as in getting children caught up on vaccinations and helping adults return to managing their chronic diseases, Dr. Wender noted.
“Their focus needs to be on helping to overcome hesitancy, mistrust, lack of information, or antivaccination sentiment to help more people feel comfortable being vaccinated – this is a lot of work and needs constant focus. In short, primary care clinicians need to focus chiefly on the unvaccinated,” he said.
“Monitoring immunization recommendations for unique at-risk populations should be the chief responsibility of teams providing subspecialty care, [such as for] transplant patients, people with chronic kidney disease, cancer patients, and people with other chronic illnesses. This will allow primary care clinicians to tackle their many complex jobs.”
Possible solutions for those with low antibody responses
Dr. Larsen said that those with ongoing low antibody responses might still have other immune responses, such as a T-cell response. Such patients also could consider changing their vaccine type, he said.
“At the more significant intervention level, there may be circumstances where one could change the immunosuppressive drugs in a controlled way that might allow a better response,” suggested Dr. Larsen. “That’s obviously going to be something that requires a lot more thought and careful study.”
Dr. Werbel said that other options might need to be considered for those having no response following a third dose. One possibility is trying a vaccine with an adjuvant, such as the Novavax version, which might be more widely available soon.
“If you’re given a third dose of a very immunogenic vaccine – something that should work – and you just have no antibody development, it seems relatively unlikely that doing the same thing again is going to help you from that perspective, and for all we know might expose you to more risk,” Dr. Werbel noted.
Participant details
None of the 30 patients were thought to have ever had COVID-19. On average, patients had received their transplant 4.5 years before their original vaccination. In 25 patients, maintenance immunosuppression included tacrolimus or cyclosporine along with mycophenolate. Corticosteroids were also used for 24 patients, sirolimus was used for one patient, and belatacept was used for another patient.
Fifty-seven percent of patients had received the Pfizer/BioNTech vaccine originally, and 43% the Moderna vaccine. Most of the patients were kidney recipients, with two heart, three liver, one lung, one pancreas and one kidney-pancreas.
Dr. Werbel, Dr. Wender, and Dr. Larsen reported no relevant disclosures.
Conflicting medical opinions: Black lungs, Big Coal, and bias
In 2008, the U.S. Department of Labor (DOL) paid for Tony Adams, a 48-year-old coal miner, to have a chest x-ray. His doctor found stage I black lung disease. Yet Mr. Adams’ claim for medical benefits was denied. This was because the insurance group that represented his employer hired a different – more credentialed – doctor as its medical expert. That doctor said he saw no such evidence. The judge ruled in favor of the mining company on the basis of the latter’s “expertise.”
Before he died 5 years later, at age 53, Mr. Adams went through this process again. In fact, he did it four more times. Each time, his doctor found evidence of black lung, but the company’s medical expert did not. He died without receiving benefits. Among the causes of death listed on his autopsy were cardiopulmonary arrest and coal worker’s pneumoconiosis (CWP): black lung.
Since his death in 2013, two judges have awarded Mr. Adams’ benefits to his widow, Linda. Both times, the mining company appealed the decision, most recently in December 2020. She’s not giving up. “Two weeks before he died, he told me, ‘I’m going to die of black lung,’ ” Linda recalled. “‘But I don’t want you to give up on black lung. There are too many people screwing these miners out of what they deserve.’”
There has long been suspicion among miners and their advocates that doctors used by coal companies to fight claims like Mr. Adams’ are in the pocket of “Big Coal.” At the very least, some say these physicians are swayed by their client’s preference when reading a coal miner’s chest x-ray. A recent study published in Annals of the American Thoracic Society provides empirical evidence that these doctors’ conflict of interest – namely, that parties representing coal companies hired them – appears to influence their medical opinion.
Proof of a ‘broken system’
The Annals study examined 63,780 radiograph classifications made by 264 physicians – all certified as B-readers, a certification by the National Institute for Occupational Safety and Health (NIOSH) for physicians who demonstrate proficiency in classifying radiographs of pneumoconiosis. The results showed that doctors hired by miners identified black lung 49% of the time; those hired by coal companies identified black lung only 15% of the time.
The study also found that B-readers contracted by employers read results differently for different clients. The same doctors were significantly less likely to say a miner’s lungs were negative for CWP when they were hired by the DOL (77.2%) than when they were hired by a coal company or its insurers (90.2%).
The bias does appear to work both ways: B-readers hired by miners and miners’ attorneys were more likely to find evidence of black lung when they worked with plaintiffs. However, a much higher number of doctors appeared to be biased in favor of the companies. “There were 3X more B-readers providing 8X more classifications among those affiliated with employers compared to those affiliated with miners,” the study concluded.
The authors suggest that one reason for this was the difference in pay. Some company-hired doctors made as much as $750 per reading, about 10 times what miner-hired doctors were paid.
“We knew [about the potential bias] from our work over the decades taking care of these guys,” said Robert A. Cohen, MD, a pulmonologist and the study’s senior author. “But then you see it with P values that are incredibly statistically significant ...”
The study finally put numbers to a problem that many working with black lung claims had always assumed. Those within the system are accustomed to seeing names of the same doctors on documents and reports, with little to no overlap between those hired by the defense and the plaintiffs.
“The vast majority of the time, we know what a report will say based on the doctor’s name,” said Evan Smith, JD, advocacy director at AppalReD Legal Aid, in Prestonsburg, Ky.. It is far more surprising, he said, when a defense-hired doctor agrees with a miner-hired doctor.
Over the years, Katherine DePonte, MD, a radiologist and B-reader in West Virginia, has often seen an “almost textbook appearance” of CWP, only to later learn that “another radiologist read it as negative.” She explained, “They would use some other term, like ‘old granulomatous disease.’”
Employer-hired doctors often do acknowledge the same lung damage on the radiograph as miner-hired docs; they simply don’t attribute it to coal dust. Common “alternative diagnoses” include chronic obstructive pulmonary disease or histoplasmosis. “I know a number don’t believe this disease of coal worker pneumoconiosis exists [at all],” Dr. DePonte said.
What’s inarguable is that, even as coal mining in Appalachia is on the decline, black lung disease is on the rise. NIOSH now estimates that it affects over 20% of long-term (25+ years) coal workers in central Appalachia. That’s the highest prevalence in a quarter of a century.
Mr. Smith said that at its most basic level, these doctors’ conflicts of interest “lead to people who have the disease that these benefits are for, having them denied.” People like Tony Adams. Whether the doctors involved are complicit or just conservative, critics say they have become a fixture of a broken system.
Financial bias or difference of opinion?
Broken system or not, evidence suggests that the problem can’t be blamed solely on medical experts. Dr. DePonte primarily reads for the DOL and miners. “Not that I necessarily chose that,” she said. “You get pigeonholed.”
Some say that the bias demonstrated by the Annals study is at least partially driven by the litigation process itself. It is an adversarial system. As such, attorneys on both sides are naturally inclined to seek out doctors who will best support their clients’ cases. Doctors with a legitimately conservative perspective on what constitutes black lung are more sought after by the coal companies’ attorneys.
“It can often be impossible to tell whether the money is driving a change in the behavior or if the behavior is causing them to be sought out,” said Matt McCoy, PhD, a medical ethicist who specializes in conflicts of interest at the University of Pennsylvania, Philadelphia.
Although some believe that certain doctors are driven purely by financial incentive and offer a specific reading to secure repeat business, B-readers can end up working exclusively for companies because of other reasons. Wes Addington, JD, an attorney at the Appalachian Citizens’ Law Center, Whitesburg, Ky., said some doctors appear to have an authentically different – often antiquated – view of the disease.
Perhaps the most extreme example is Paul Wheeler, MD, a highly credentialed Johns Hopkins radiologist who was exposed for false medical testimony in Chris Hamby’s 2013 Pulitzer Prize reporting. In 1,500 readings, Dr. Wheeler never diagnosed a single case of severe black lung. And yet, Dr. Cohen, Mr. Addington, Mr. Smith, and other experts all agree that Dr. Wheeler appeared to wholeheartedly believe that his view of black lung was accurate. That made him a valuable asset to mining companies.
Since Dr. Wheeler’s exposure, there has been a greater sense of accountability among B-readers, said John Cline, JD, a West Virginia–based attorney who represents miners with federal black lung claims. “Radiologists were thinking, ‘Somebody could be watching me.’ Even if they thought they were doing this in the shadows, it made people more cautious,” he said.
The data used in the Annals study predate Mr. Hamby’s investigation, going back to 2000. Thus, it is possible that, as Mr. Cline argues, things may be different now. However, Lee S. Friedman, PhD, associate professor at the University of Illinois at Chicago, who is the lead author of the study, remains skeptical.
“While the Wheeler case might have dampened some physicians [who were] completely skewing their readings always negative, I think it’s premature or incorrect” to say it resolved the issue, he said. “Did they all change their behavior the morning after? It doesn’t seem likely, given the evidence of financial conflicts of interest and behavior that’s been demonstrated.”
Skewing the evidence?
Mr. Hamby’s 2013 reporting also revealed that even when company-hired doctors did diagnose CWP, law firms were burying those readings. In 2016, the DOL attempted to stop this practice. The agency made suppression of written evidence illegal – emphasis on written.
Law firms can’t hide positive reports, but they can prevent them. Dr. Cohen explained that now, “a doctor on the phone says, ‘I will read this as positive.’ Then the company says, ‘No, thank you,’ we will send you a check.”
This practice was confirmed by Kim Adcock, MD, a retired radiologist and B-reader in Littleton, Colo., who primarily reads for 26 law firms. Some of his clients want a report no matter how he reads the radiograph. However, some want him to call them first if he’s going to read the radiograph as positive. Dr. Adcock said this practice skews the dataset to make company-hired docs appear to read more negatively than they actually do.
Because the dataset used in the study is from the Federal Black Lung Program (FBLP), it includes only readings that made it to court. Dr. Adcock said he reads approximately 2,000 radiographs a year, although only a few of his readings appeared in the study’s dataset, according to a search by Dr. Friedman. This difference is likely because the study evaluated only readings between 2000 and 2013, the year Dr. Adcock started B-reading.
“I think it’s important to get a message that, to a certain extent, contravenes this paper. Yes, we should have some reservations about the conclusions,” Dr. Adcock explained. “There are people out there attempting to do the best job they could do.”
Law firms shopping for the reading they want and censoring the ones they don’t might alter the FBLP data, but experts say that doesn’t change the underlying problem. “In any case like this, where you’re looking at individuals going up against corporations,” Dr. McCoy said, “[corporations] are able to marshal their resources and hire more officials in a way claimants can’t, and that’s a baseline concern here.”
Battling bias
Admitting bias is notoriously difficult; thus, it isn’t surprising that many doctors involved refuse to believe they are influenced by money, incentives, or other biases. Dr. DePonte said she’s not swayed by money, nor does she actively take a pro-miner stance. She views herself as more of an advocate for accuracy. However, she did say that it has traditionally been far more difficult for miners to prove their cases, a problem that has improved with new regulations in recent years.
In Colorado, Dr. Adcock’s approach is to stay as far removed from the litigation process as possible. He said he has limited understanding of how his reports are used or how claims are filed and awarded. He leans heavily on his initial – almost instantaneous – impression of a chest x-ray.
Dr. DePonte and Dr. Adcock were both hired as experts on Tony Adams’ case. In 2008, Dr. DePonte read his chest x-ray as positive for early-stage black lung (1/0). Dr. Adcock also read two of Adams’ four chest x-rays, one in 2009 and the other in 2013. He read them as negative. When asked about the case, which autopsy confirmed as black lung, Dr. Adcock explained that positive histopathology doesn’t mean the radiograph reading was wrong, only that the disease didn’t show on that radiograph. He said his “highest ambition” is to be “an objective finder of fact” and that he trusts the process to work out the truth.
That process didn’t work in time for Tony Adams. Dr. Friedman argues that people who provide expert testimony have an ethical responsibility to know how their testimony is being used; to do otherwise, he says, is “willful ignorance.” Still, the Annals study authors, along with Dr. DePonte, Mr. Cline, and West Virginia attorney Sam Petsonk, say that the process is getting fairer, thanks to new policies developed over the past 5 years by the DOL.
“The DOL has worked very hard to reconcile the final award rate (around 30%) with the incidence of disease in the population (between 20% and 25%),” Mr. Petsonk said. Although the study calls into question the integrity of the system and the doctors within it, it’s critical for miners to know that the system is working and that they can get benefits, he explained. Many fear that cynicism about the system drives miners away and causes them to resort to Social Security or long-term disability.
Fixing what’s broken
The Annals study’s authors propose some solutions to the problems they quantified. The first is a sort of “super panel” that collectively evaluates readings. Although a completely unbiased panel would be nice, such impartiality is likely unsustainable, Mr. Smith said. He believes that over time, the panel would become vulnerable to politics and would work in favor of the companies.
Even without a panel, a method to provide greater transparency could be a great start, some suggest. The DOL could make the entire FBLP database public and analyze it annually. The authors also propose a flat fee for readings. Even now, Dr. Adcock said he doesn’t make anywhere close to the upper limit of $750 per readings. “My understanding is around $125 is a pretty characteristic fee [for reading a chest x-ray],” he elaborated. “Everyone I’ve had a conversation with is within 25 bucks [of that].”
That said, Dr. Adcock is not currently listed among the heavy readers who appear in the data used for the study; it’s possible that his experience is not representative. Some readers who were included in that dataset read more than 10 times the average number of classifications per reader – the average was 242 classifications – and read 95% of chest x-rays as negative, according to Dr. Friedman. This news organization obtained the names of two doctors whose readings were 95% negative on a high volume of cases. Neither agreed to an interview.
It’s possible that if the dataset had included readings from more recent years, Dr. Adcock would have appeared more frequently, given his personal estimates. That’s why the study authors recommend that the DOL conduct this kind of analysis annually in order to get an accurate picture of who is contributing to these cases, in what way, and how often. By doing so, readers who appear biased could be identified and addressed with more regularity, Dr. Friedman said.
Even if the rate were more consistent and the data were more frequently analyzed, the very nature of the adversarial system will put any potential solution at risk. “I’m not sure there’s a foolproof system that can be devised that can’t be corrupted in time,” Mr. Cline said.
A version of this article first appeared on Medscape.com.
In 2008, the U.S. Department of Labor (DOL) paid for Tony Adams, a 48-year-old coal miner, to have a chest x-ray. His doctor found stage I black lung disease. Yet Mr. Adams’ claim for medical benefits was denied. This was because the insurance group that represented his employer hired a different – more credentialed – doctor as its medical expert. That doctor said he saw no such evidence. The judge ruled in favor of the mining company on the basis of the latter’s “expertise.”
Before he died 5 years later, at age 53, Mr. Adams went through this process again. In fact, he did it four more times. Each time, his doctor found evidence of black lung, but the company’s medical expert did not. He died without receiving benefits. Among the causes of death listed on his autopsy were cardiopulmonary arrest and coal worker’s pneumoconiosis (CWP): black lung.
Since his death in 2013, two judges have awarded Mr. Adams’ benefits to his widow, Linda. Both times, the mining company appealed the decision, most recently in December 2020. She’s not giving up. “Two weeks before he died, he told me, ‘I’m going to die of black lung,’ ” Linda recalled. “‘But I don’t want you to give up on black lung. There are too many people screwing these miners out of what they deserve.’”
There has long been suspicion among miners and their advocates that doctors used by coal companies to fight claims like Mr. Adams’ are in the pocket of “Big Coal.” At the very least, some say these physicians are swayed by their client’s preference when reading a coal miner’s chest x-ray. A recent study published in Annals of the American Thoracic Society provides empirical evidence that these doctors’ conflict of interest – namely, that parties representing coal companies hired them – appears to influence their medical opinion.
Proof of a ‘broken system’
The Annals study examined 63,780 radiograph classifications made by 264 physicians – all certified as B-readers, a certification by the National Institute for Occupational Safety and Health (NIOSH) for physicians who demonstrate proficiency in classifying radiographs of pneumoconiosis. The results showed that doctors hired by miners identified black lung 49% of the time; those hired by coal companies identified black lung only 15% of the time.
The study also found that B-readers contracted by employers read results differently for different clients. The same doctors were significantly less likely to say a miner’s lungs were negative for CWP when they were hired by the DOL (77.2%) than when they were hired by a coal company or its insurers (90.2%).
The bias does appear to work both ways: B-readers hired by miners and miners’ attorneys were more likely to find evidence of black lung when they worked with plaintiffs. However, a much higher number of doctors appeared to be biased in favor of the companies. “There were 3X more B-readers providing 8X more classifications among those affiliated with employers compared to those affiliated with miners,” the study concluded.
The authors suggest that one reason for this was the difference in pay. Some company-hired doctors made as much as $750 per reading, about 10 times what miner-hired doctors were paid.
“We knew [about the potential bias] from our work over the decades taking care of these guys,” said Robert A. Cohen, MD, a pulmonologist and the study’s senior author. “But then you see it with P values that are incredibly statistically significant ...”
The study finally put numbers to a problem that many working with black lung claims had always assumed. Those within the system are accustomed to seeing names of the same doctors on documents and reports, with little to no overlap between those hired by the defense and the plaintiffs.
“The vast majority of the time, we know what a report will say based on the doctor’s name,” said Evan Smith, JD, advocacy director at AppalReD Legal Aid, in Prestonsburg, Ky.. It is far more surprising, he said, when a defense-hired doctor agrees with a miner-hired doctor.
Over the years, Katherine DePonte, MD, a radiologist and B-reader in West Virginia, has often seen an “almost textbook appearance” of CWP, only to later learn that “another radiologist read it as negative.” She explained, “They would use some other term, like ‘old granulomatous disease.’”
Employer-hired doctors often do acknowledge the same lung damage on the radiograph as miner-hired docs; they simply don’t attribute it to coal dust. Common “alternative diagnoses” include chronic obstructive pulmonary disease or histoplasmosis. “I know a number don’t believe this disease of coal worker pneumoconiosis exists [at all],” Dr. DePonte said.
What’s inarguable is that, even as coal mining in Appalachia is on the decline, black lung disease is on the rise. NIOSH now estimates that it affects over 20% of long-term (25+ years) coal workers in central Appalachia. That’s the highest prevalence in a quarter of a century.
Mr. Smith said that at its most basic level, these doctors’ conflicts of interest “lead to people who have the disease that these benefits are for, having them denied.” People like Tony Adams. Whether the doctors involved are complicit or just conservative, critics say they have become a fixture of a broken system.
Financial bias or difference of opinion?
Broken system or not, evidence suggests that the problem can’t be blamed solely on medical experts. Dr. DePonte primarily reads for the DOL and miners. “Not that I necessarily chose that,” she said. “You get pigeonholed.”
Some say that the bias demonstrated by the Annals study is at least partially driven by the litigation process itself. It is an adversarial system. As such, attorneys on both sides are naturally inclined to seek out doctors who will best support their clients’ cases. Doctors with a legitimately conservative perspective on what constitutes black lung are more sought after by the coal companies’ attorneys.
“It can often be impossible to tell whether the money is driving a change in the behavior or if the behavior is causing them to be sought out,” said Matt McCoy, PhD, a medical ethicist who specializes in conflicts of interest at the University of Pennsylvania, Philadelphia.
Although some believe that certain doctors are driven purely by financial incentive and offer a specific reading to secure repeat business, B-readers can end up working exclusively for companies because of other reasons. Wes Addington, JD, an attorney at the Appalachian Citizens’ Law Center, Whitesburg, Ky., said some doctors appear to have an authentically different – often antiquated – view of the disease.
Perhaps the most extreme example is Paul Wheeler, MD, a highly credentialed Johns Hopkins radiologist who was exposed for false medical testimony in Chris Hamby’s 2013 Pulitzer Prize reporting. In 1,500 readings, Dr. Wheeler never diagnosed a single case of severe black lung. And yet, Dr. Cohen, Mr. Addington, Mr. Smith, and other experts all agree that Dr. Wheeler appeared to wholeheartedly believe that his view of black lung was accurate. That made him a valuable asset to mining companies.
Since Dr. Wheeler’s exposure, there has been a greater sense of accountability among B-readers, said John Cline, JD, a West Virginia–based attorney who represents miners with federal black lung claims. “Radiologists were thinking, ‘Somebody could be watching me.’ Even if they thought they were doing this in the shadows, it made people more cautious,” he said.
The data used in the Annals study predate Mr. Hamby’s investigation, going back to 2000. Thus, it is possible that, as Mr. Cline argues, things may be different now. However, Lee S. Friedman, PhD, associate professor at the University of Illinois at Chicago, who is the lead author of the study, remains skeptical.
“While the Wheeler case might have dampened some physicians [who were] completely skewing their readings always negative, I think it’s premature or incorrect” to say it resolved the issue, he said. “Did they all change their behavior the morning after? It doesn’t seem likely, given the evidence of financial conflicts of interest and behavior that’s been demonstrated.”
Skewing the evidence?
Mr. Hamby’s 2013 reporting also revealed that even when company-hired doctors did diagnose CWP, law firms were burying those readings. In 2016, the DOL attempted to stop this practice. The agency made suppression of written evidence illegal – emphasis on written.
Law firms can’t hide positive reports, but they can prevent them. Dr. Cohen explained that now, “a doctor on the phone says, ‘I will read this as positive.’ Then the company says, ‘No, thank you,’ we will send you a check.”
This practice was confirmed by Kim Adcock, MD, a retired radiologist and B-reader in Littleton, Colo., who primarily reads for 26 law firms. Some of his clients want a report no matter how he reads the radiograph. However, some want him to call them first if he’s going to read the radiograph as positive. Dr. Adcock said this practice skews the dataset to make company-hired docs appear to read more negatively than they actually do.
Because the dataset used in the study is from the Federal Black Lung Program (FBLP), it includes only readings that made it to court. Dr. Adcock said he reads approximately 2,000 radiographs a year, although only a few of his readings appeared in the study’s dataset, according to a search by Dr. Friedman. This difference is likely because the study evaluated only readings between 2000 and 2013, the year Dr. Adcock started B-reading.
“I think it’s important to get a message that, to a certain extent, contravenes this paper. Yes, we should have some reservations about the conclusions,” Dr. Adcock explained. “There are people out there attempting to do the best job they could do.”
Law firms shopping for the reading they want and censoring the ones they don’t might alter the FBLP data, but experts say that doesn’t change the underlying problem. “In any case like this, where you’re looking at individuals going up against corporations,” Dr. McCoy said, “[corporations] are able to marshal their resources and hire more officials in a way claimants can’t, and that’s a baseline concern here.”
Battling bias
Admitting bias is notoriously difficult; thus, it isn’t surprising that many doctors involved refuse to believe they are influenced by money, incentives, or other biases. Dr. DePonte said she’s not swayed by money, nor does she actively take a pro-miner stance. She views herself as more of an advocate for accuracy. However, she did say that it has traditionally been far more difficult for miners to prove their cases, a problem that has improved with new regulations in recent years.
In Colorado, Dr. Adcock’s approach is to stay as far removed from the litigation process as possible. He said he has limited understanding of how his reports are used or how claims are filed and awarded. He leans heavily on his initial – almost instantaneous – impression of a chest x-ray.
Dr. DePonte and Dr. Adcock were both hired as experts on Tony Adams’ case. In 2008, Dr. DePonte read his chest x-ray as positive for early-stage black lung (1/0). Dr. Adcock also read two of Adams’ four chest x-rays, one in 2009 and the other in 2013. He read them as negative. When asked about the case, which autopsy confirmed as black lung, Dr. Adcock explained that positive histopathology doesn’t mean the radiograph reading was wrong, only that the disease didn’t show on that radiograph. He said his “highest ambition” is to be “an objective finder of fact” and that he trusts the process to work out the truth.
That process didn’t work in time for Tony Adams. Dr. Friedman argues that people who provide expert testimony have an ethical responsibility to know how their testimony is being used; to do otherwise, he says, is “willful ignorance.” Still, the Annals study authors, along with Dr. DePonte, Mr. Cline, and West Virginia attorney Sam Petsonk, say that the process is getting fairer, thanks to new policies developed over the past 5 years by the DOL.
“The DOL has worked very hard to reconcile the final award rate (around 30%) with the incidence of disease in the population (between 20% and 25%),” Mr. Petsonk said. Although the study calls into question the integrity of the system and the doctors within it, it’s critical for miners to know that the system is working and that they can get benefits, he explained. Many fear that cynicism about the system drives miners away and causes them to resort to Social Security or long-term disability.
Fixing what’s broken
The Annals study’s authors propose some solutions to the problems they quantified. The first is a sort of “super panel” that collectively evaluates readings. Although a completely unbiased panel would be nice, such impartiality is likely unsustainable, Mr. Smith said. He believes that over time, the panel would become vulnerable to politics and would work in favor of the companies.
Even without a panel, a method to provide greater transparency could be a great start, some suggest. The DOL could make the entire FBLP database public and analyze it annually. The authors also propose a flat fee for readings. Even now, Dr. Adcock said he doesn’t make anywhere close to the upper limit of $750 per readings. “My understanding is around $125 is a pretty characteristic fee [for reading a chest x-ray],” he elaborated. “Everyone I’ve had a conversation with is within 25 bucks [of that].”
That said, Dr. Adcock is not currently listed among the heavy readers who appear in the data used for the study; it’s possible that his experience is not representative. Some readers who were included in that dataset read more than 10 times the average number of classifications per reader – the average was 242 classifications – and read 95% of chest x-rays as negative, according to Dr. Friedman. This news organization obtained the names of two doctors whose readings were 95% negative on a high volume of cases. Neither agreed to an interview.
It’s possible that if the dataset had included readings from more recent years, Dr. Adcock would have appeared more frequently, given his personal estimates. That’s why the study authors recommend that the DOL conduct this kind of analysis annually in order to get an accurate picture of who is contributing to these cases, in what way, and how often. By doing so, readers who appear biased could be identified and addressed with more regularity, Dr. Friedman said.
Even if the rate were more consistent and the data were more frequently analyzed, the very nature of the adversarial system will put any potential solution at risk. “I’m not sure there’s a foolproof system that can be devised that can’t be corrupted in time,” Mr. Cline said.
A version of this article first appeared on Medscape.com.
In 2008, the U.S. Department of Labor (DOL) paid for Tony Adams, a 48-year-old coal miner, to have a chest x-ray. His doctor found stage I black lung disease. Yet Mr. Adams’ claim for medical benefits was denied. This was because the insurance group that represented his employer hired a different – more credentialed – doctor as its medical expert. That doctor said he saw no such evidence. The judge ruled in favor of the mining company on the basis of the latter’s “expertise.”
Before he died 5 years later, at age 53, Mr. Adams went through this process again. In fact, he did it four more times. Each time, his doctor found evidence of black lung, but the company’s medical expert did not. He died without receiving benefits. Among the causes of death listed on his autopsy were cardiopulmonary arrest and coal worker’s pneumoconiosis (CWP): black lung.
Since his death in 2013, two judges have awarded Mr. Adams’ benefits to his widow, Linda. Both times, the mining company appealed the decision, most recently in December 2020. She’s not giving up. “Two weeks before he died, he told me, ‘I’m going to die of black lung,’ ” Linda recalled. “‘But I don’t want you to give up on black lung. There are too many people screwing these miners out of what they deserve.’”
There has long been suspicion among miners and their advocates that doctors used by coal companies to fight claims like Mr. Adams’ are in the pocket of “Big Coal.” At the very least, some say these physicians are swayed by their client’s preference when reading a coal miner’s chest x-ray. A recent study published in Annals of the American Thoracic Society provides empirical evidence that these doctors’ conflict of interest – namely, that parties representing coal companies hired them – appears to influence their medical opinion.
Proof of a ‘broken system’
The Annals study examined 63,780 radiograph classifications made by 264 physicians – all certified as B-readers, a certification by the National Institute for Occupational Safety and Health (NIOSH) for physicians who demonstrate proficiency in classifying radiographs of pneumoconiosis. The results showed that doctors hired by miners identified black lung 49% of the time; those hired by coal companies identified black lung only 15% of the time.
The study also found that B-readers contracted by employers read results differently for different clients. The same doctors were significantly less likely to say a miner’s lungs were negative for CWP when they were hired by the DOL (77.2%) than when they were hired by a coal company or its insurers (90.2%).
The bias does appear to work both ways: B-readers hired by miners and miners’ attorneys were more likely to find evidence of black lung when they worked with plaintiffs. However, a much higher number of doctors appeared to be biased in favor of the companies. “There were 3X more B-readers providing 8X more classifications among those affiliated with employers compared to those affiliated with miners,” the study concluded.
The authors suggest that one reason for this was the difference in pay. Some company-hired doctors made as much as $750 per reading, about 10 times what miner-hired doctors were paid.
“We knew [about the potential bias] from our work over the decades taking care of these guys,” said Robert A. Cohen, MD, a pulmonologist and the study’s senior author. “But then you see it with P values that are incredibly statistically significant ...”
The study finally put numbers to a problem that many working with black lung claims had always assumed. Those within the system are accustomed to seeing names of the same doctors on documents and reports, with little to no overlap between those hired by the defense and the plaintiffs.
“The vast majority of the time, we know what a report will say based on the doctor’s name,” said Evan Smith, JD, advocacy director at AppalReD Legal Aid, in Prestonsburg, Ky.. It is far more surprising, he said, when a defense-hired doctor agrees with a miner-hired doctor.
Over the years, Katherine DePonte, MD, a radiologist and B-reader in West Virginia, has often seen an “almost textbook appearance” of CWP, only to later learn that “another radiologist read it as negative.” She explained, “They would use some other term, like ‘old granulomatous disease.’”
Employer-hired doctors often do acknowledge the same lung damage on the radiograph as miner-hired docs; they simply don’t attribute it to coal dust. Common “alternative diagnoses” include chronic obstructive pulmonary disease or histoplasmosis. “I know a number don’t believe this disease of coal worker pneumoconiosis exists [at all],” Dr. DePonte said.
What’s inarguable is that, even as coal mining in Appalachia is on the decline, black lung disease is on the rise. NIOSH now estimates that it affects over 20% of long-term (25+ years) coal workers in central Appalachia. That’s the highest prevalence in a quarter of a century.
Mr. Smith said that at its most basic level, these doctors’ conflicts of interest “lead to people who have the disease that these benefits are for, having them denied.” People like Tony Adams. Whether the doctors involved are complicit or just conservative, critics say they have become a fixture of a broken system.
Financial bias or difference of opinion?
Broken system or not, evidence suggests that the problem can’t be blamed solely on medical experts. Dr. DePonte primarily reads for the DOL and miners. “Not that I necessarily chose that,” she said. “You get pigeonholed.”
Some say that the bias demonstrated by the Annals study is at least partially driven by the litigation process itself. It is an adversarial system. As such, attorneys on both sides are naturally inclined to seek out doctors who will best support their clients’ cases. Doctors with a legitimately conservative perspective on what constitutes black lung are more sought after by the coal companies’ attorneys.
“It can often be impossible to tell whether the money is driving a change in the behavior or if the behavior is causing them to be sought out,” said Matt McCoy, PhD, a medical ethicist who specializes in conflicts of interest at the University of Pennsylvania, Philadelphia.
Although some believe that certain doctors are driven purely by financial incentive and offer a specific reading to secure repeat business, B-readers can end up working exclusively for companies because of other reasons. Wes Addington, JD, an attorney at the Appalachian Citizens’ Law Center, Whitesburg, Ky., said some doctors appear to have an authentically different – often antiquated – view of the disease.
Perhaps the most extreme example is Paul Wheeler, MD, a highly credentialed Johns Hopkins radiologist who was exposed for false medical testimony in Chris Hamby’s 2013 Pulitzer Prize reporting. In 1,500 readings, Dr. Wheeler never diagnosed a single case of severe black lung. And yet, Dr. Cohen, Mr. Addington, Mr. Smith, and other experts all agree that Dr. Wheeler appeared to wholeheartedly believe that his view of black lung was accurate. That made him a valuable asset to mining companies.
Since Dr. Wheeler’s exposure, there has been a greater sense of accountability among B-readers, said John Cline, JD, a West Virginia–based attorney who represents miners with federal black lung claims. “Radiologists were thinking, ‘Somebody could be watching me.’ Even if they thought they were doing this in the shadows, it made people more cautious,” he said.
The data used in the Annals study predate Mr. Hamby’s investigation, going back to 2000. Thus, it is possible that, as Mr. Cline argues, things may be different now. However, Lee S. Friedman, PhD, associate professor at the University of Illinois at Chicago, who is the lead author of the study, remains skeptical.
“While the Wheeler case might have dampened some physicians [who were] completely skewing their readings always negative, I think it’s premature or incorrect” to say it resolved the issue, he said. “Did they all change their behavior the morning after? It doesn’t seem likely, given the evidence of financial conflicts of interest and behavior that’s been demonstrated.”
Skewing the evidence?
Mr. Hamby’s 2013 reporting also revealed that even when company-hired doctors did diagnose CWP, law firms were burying those readings. In 2016, the DOL attempted to stop this practice. The agency made suppression of written evidence illegal – emphasis on written.
Law firms can’t hide positive reports, but they can prevent them. Dr. Cohen explained that now, “a doctor on the phone says, ‘I will read this as positive.’ Then the company says, ‘No, thank you,’ we will send you a check.”
This practice was confirmed by Kim Adcock, MD, a retired radiologist and B-reader in Littleton, Colo., who primarily reads for 26 law firms. Some of his clients want a report no matter how he reads the radiograph. However, some want him to call them first if he’s going to read the radiograph as positive. Dr. Adcock said this practice skews the dataset to make company-hired docs appear to read more negatively than they actually do.
Because the dataset used in the study is from the Federal Black Lung Program (FBLP), it includes only readings that made it to court. Dr. Adcock said he reads approximately 2,000 radiographs a year, although only a few of his readings appeared in the study’s dataset, according to a search by Dr. Friedman. This difference is likely because the study evaluated only readings between 2000 and 2013, the year Dr. Adcock started B-reading.
“I think it’s important to get a message that, to a certain extent, contravenes this paper. Yes, we should have some reservations about the conclusions,” Dr. Adcock explained. “There are people out there attempting to do the best job they could do.”
Law firms shopping for the reading they want and censoring the ones they don’t might alter the FBLP data, but experts say that doesn’t change the underlying problem. “In any case like this, where you’re looking at individuals going up against corporations,” Dr. McCoy said, “[corporations] are able to marshal their resources and hire more officials in a way claimants can’t, and that’s a baseline concern here.”
Battling bias
Admitting bias is notoriously difficult; thus, it isn’t surprising that many doctors involved refuse to believe they are influenced by money, incentives, or other biases. Dr. DePonte said she’s not swayed by money, nor does she actively take a pro-miner stance. She views herself as more of an advocate for accuracy. However, she did say that it has traditionally been far more difficult for miners to prove their cases, a problem that has improved with new regulations in recent years.
In Colorado, Dr. Adcock’s approach is to stay as far removed from the litigation process as possible. He said he has limited understanding of how his reports are used or how claims are filed and awarded. He leans heavily on his initial – almost instantaneous – impression of a chest x-ray.
Dr. DePonte and Dr. Adcock were both hired as experts on Tony Adams’ case. In 2008, Dr. DePonte read his chest x-ray as positive for early-stage black lung (1/0). Dr. Adcock also read two of Adams’ four chest x-rays, one in 2009 and the other in 2013. He read them as negative. When asked about the case, which autopsy confirmed as black lung, Dr. Adcock explained that positive histopathology doesn’t mean the radiograph reading was wrong, only that the disease didn’t show on that radiograph. He said his “highest ambition” is to be “an objective finder of fact” and that he trusts the process to work out the truth.
That process didn’t work in time for Tony Adams. Dr. Friedman argues that people who provide expert testimony have an ethical responsibility to know how their testimony is being used; to do otherwise, he says, is “willful ignorance.” Still, the Annals study authors, along with Dr. DePonte, Mr. Cline, and West Virginia attorney Sam Petsonk, say that the process is getting fairer, thanks to new policies developed over the past 5 years by the DOL.
“The DOL has worked very hard to reconcile the final award rate (around 30%) with the incidence of disease in the population (between 20% and 25%),” Mr. Petsonk said. Although the study calls into question the integrity of the system and the doctors within it, it’s critical for miners to know that the system is working and that they can get benefits, he explained. Many fear that cynicism about the system drives miners away and causes them to resort to Social Security or long-term disability.
Fixing what’s broken
The Annals study’s authors propose some solutions to the problems they quantified. The first is a sort of “super panel” that collectively evaluates readings. Although a completely unbiased panel would be nice, such impartiality is likely unsustainable, Mr. Smith said. He believes that over time, the panel would become vulnerable to politics and would work in favor of the companies.
Even without a panel, a method to provide greater transparency could be a great start, some suggest. The DOL could make the entire FBLP database public and analyze it annually. The authors also propose a flat fee for readings. Even now, Dr. Adcock said he doesn’t make anywhere close to the upper limit of $750 per readings. “My understanding is around $125 is a pretty characteristic fee [for reading a chest x-ray],” he elaborated. “Everyone I’ve had a conversation with is within 25 bucks [of that].”
That said, Dr. Adcock is not currently listed among the heavy readers who appear in the data used for the study; it’s possible that his experience is not representative. Some readers who were included in that dataset read more than 10 times the average number of classifications per reader – the average was 242 classifications – and read 95% of chest x-rays as negative, according to Dr. Friedman. This news organization obtained the names of two doctors whose readings were 95% negative on a high volume of cases. Neither agreed to an interview.
It’s possible that if the dataset had included readings from more recent years, Dr. Adcock would have appeared more frequently, given his personal estimates. That’s why the study authors recommend that the DOL conduct this kind of analysis annually in order to get an accurate picture of who is contributing to these cases, in what way, and how often. By doing so, readers who appear biased could be identified and addressed with more regularity, Dr. Friedman said.
Even if the rate were more consistent and the data were more frequently analyzed, the very nature of the adversarial system will put any potential solution at risk. “I’m not sure there’s a foolproof system that can be devised that can’t be corrupted in time,” Mr. Cline said.
A version of this article first appeared on Medscape.com.
COVID-19 death toll higher for international medical graduates
researchers report.
“I’ve always felt that international medical graduates [IMGs] in America are largely invisible,” said senior author Abraham Verghese, MD, MFA, an infectious disease specialist at Stanford (Calif.) University. “Everyone is aware that there are foreign doctors, but very few are aware of how many there are and also how vital they are to providing health care in America.”
IMGs made up 25% of all U.S. physicians in 2020 but accounted for 45% of those whose deaths had been attributed to COVID-19 through Nov. 23, 2020, Deendayal Dinakarpandian, MD, PhD, clinical associate professor of medicine at Stanford (Calif.) University, and colleagues report in JAMA Network Open.
IMGs are more likely to work in places where the incidence of COVID-19 is high and in facilities with fewer resources, Dr. Verghese said in an interview. “So, it’s not surprising that they were on the front lines when this thing came along,” he said.
To see whether their vulnerability affected their risk for death, Dr. Dinakarpandian and colleagues collected data from Nov. 23, 2020, from three sources of information regarding deaths among physicians: MedPage Today, which used investigative and voluntary reporting; Medscape, which used voluntary reporting of verifiable information; and a collaboration of The Guardian and Kaiser Health News, which used investigative reporting.
The Medscape project was launched on April 1, 2020. The MedPage Today and The Guardian/Kaiser Health News projects were launched on April 8, 2020.
Dr. Verghese and colleagues researched obituaries and news articles referenced by the three projects to verify their data. They used DocInfo to ascertain the deceased physicians’ medical schools.
After eliminating duplications from the lists, the researchers counted 132 physician deaths in 28 states. Of these, 59 physicians had graduated from medical schools outside the United States, a death toll 1.8 times higher than the proportion of IMGs among U.S. physicians (95% confidence interval, 1.52-2.21; P < .001).
New York, New Jersey, and Florida accounted for 66% of the deaths among IMGs but for only 45% of the deaths among U.S. medical school graduates.
Within each state, the proportion of IMGs among deceased physicians was not statistically different from their proportion among physicians in those states, with the exception of New York.
Two-thirds of the physicians’ deaths occurred in states where IMGs make up a larger proportion of physicians than in the nation as a whole. In these states, the incidence of COVID-19 was high at the start of the pandemic.
In New York, IMGs accounted for 60% of physician deaths, which was 1.62 times higher (95% CI, 1.26-2.09; P = .005) than the 37% among New York physicians overall.
Physicians who were trained abroad frequently can’t get into the most prestigious residency programs or into the highest paid specialties and are more likely to serve in primary care, Dr. Verghese said. Overall, 60% of the physicians who died of COVID-19 worked in primary care.
IMGs often staff hospitals serving low-income communities and communities of color, which were hardest hit by the pandemic and where personal protective equipment was hard to obtain, said Dr. Verghese.
In addition to these risks, IMGs sometimes endure racism, said Dr. Verghese, who obtained his medical degree at Madras Medical College, Chennai, India. “We’ve actually seen in the COVID era, in keeping with the sort of political tone that was set in Washington, that there’s been a lot more abuses of both foreign physicians and foreign looking physicians – even if they’re not foreign trained – and nurses by patients who have been given license. And I want to acknowledge the heroism of all these physicians.”
The study was partially funded by the Presence Center at Stanford. Dr. Verghese is a regular contributor to Medscape. He served on the advisory board for Gilead Sciences, serves as a speaker or a member of a speakers bureau for Leigh Bureau, and receives royalties from Penguin Random House and Simon & Schuster.
A version of this article first appeared on Medscape.com.
researchers report.
“I’ve always felt that international medical graduates [IMGs] in America are largely invisible,” said senior author Abraham Verghese, MD, MFA, an infectious disease specialist at Stanford (Calif.) University. “Everyone is aware that there are foreign doctors, but very few are aware of how many there are and also how vital they are to providing health care in America.”
IMGs made up 25% of all U.S. physicians in 2020 but accounted for 45% of those whose deaths had been attributed to COVID-19 through Nov. 23, 2020, Deendayal Dinakarpandian, MD, PhD, clinical associate professor of medicine at Stanford (Calif.) University, and colleagues report in JAMA Network Open.
IMGs are more likely to work in places where the incidence of COVID-19 is high and in facilities with fewer resources, Dr. Verghese said in an interview. “So, it’s not surprising that they were on the front lines when this thing came along,” he said.
To see whether their vulnerability affected their risk for death, Dr. Dinakarpandian and colleagues collected data from Nov. 23, 2020, from three sources of information regarding deaths among physicians: MedPage Today, which used investigative and voluntary reporting; Medscape, which used voluntary reporting of verifiable information; and a collaboration of The Guardian and Kaiser Health News, which used investigative reporting.
The Medscape project was launched on April 1, 2020. The MedPage Today and The Guardian/Kaiser Health News projects were launched on April 8, 2020.
Dr. Verghese and colleagues researched obituaries and news articles referenced by the three projects to verify their data. They used DocInfo to ascertain the deceased physicians’ medical schools.
After eliminating duplications from the lists, the researchers counted 132 physician deaths in 28 states. Of these, 59 physicians had graduated from medical schools outside the United States, a death toll 1.8 times higher than the proportion of IMGs among U.S. physicians (95% confidence interval, 1.52-2.21; P < .001).
New York, New Jersey, and Florida accounted for 66% of the deaths among IMGs but for only 45% of the deaths among U.S. medical school graduates.
Within each state, the proportion of IMGs among deceased physicians was not statistically different from their proportion among physicians in those states, with the exception of New York.
Two-thirds of the physicians’ deaths occurred in states where IMGs make up a larger proportion of physicians than in the nation as a whole. In these states, the incidence of COVID-19 was high at the start of the pandemic.
In New York, IMGs accounted for 60% of physician deaths, which was 1.62 times higher (95% CI, 1.26-2.09; P = .005) than the 37% among New York physicians overall.
Physicians who were trained abroad frequently can’t get into the most prestigious residency programs or into the highest paid specialties and are more likely to serve in primary care, Dr. Verghese said. Overall, 60% of the physicians who died of COVID-19 worked in primary care.
IMGs often staff hospitals serving low-income communities and communities of color, which were hardest hit by the pandemic and where personal protective equipment was hard to obtain, said Dr. Verghese.
In addition to these risks, IMGs sometimes endure racism, said Dr. Verghese, who obtained his medical degree at Madras Medical College, Chennai, India. “We’ve actually seen in the COVID era, in keeping with the sort of political tone that was set in Washington, that there’s been a lot more abuses of both foreign physicians and foreign looking physicians – even if they’re not foreign trained – and nurses by patients who have been given license. And I want to acknowledge the heroism of all these physicians.”
The study was partially funded by the Presence Center at Stanford. Dr. Verghese is a regular contributor to Medscape. He served on the advisory board for Gilead Sciences, serves as a speaker or a member of a speakers bureau for Leigh Bureau, and receives royalties from Penguin Random House and Simon & Schuster.
A version of this article first appeared on Medscape.com.
researchers report.
“I’ve always felt that international medical graduates [IMGs] in America are largely invisible,” said senior author Abraham Verghese, MD, MFA, an infectious disease specialist at Stanford (Calif.) University. “Everyone is aware that there are foreign doctors, but very few are aware of how many there are and also how vital they are to providing health care in America.”
IMGs made up 25% of all U.S. physicians in 2020 but accounted for 45% of those whose deaths had been attributed to COVID-19 through Nov. 23, 2020, Deendayal Dinakarpandian, MD, PhD, clinical associate professor of medicine at Stanford (Calif.) University, and colleagues report in JAMA Network Open.
IMGs are more likely to work in places where the incidence of COVID-19 is high and in facilities with fewer resources, Dr. Verghese said in an interview. “So, it’s not surprising that they were on the front lines when this thing came along,” he said.
To see whether their vulnerability affected their risk for death, Dr. Dinakarpandian and colleagues collected data from Nov. 23, 2020, from three sources of information regarding deaths among physicians: MedPage Today, which used investigative and voluntary reporting; Medscape, which used voluntary reporting of verifiable information; and a collaboration of The Guardian and Kaiser Health News, which used investigative reporting.
The Medscape project was launched on April 1, 2020. The MedPage Today and The Guardian/Kaiser Health News projects were launched on April 8, 2020.
Dr. Verghese and colleagues researched obituaries and news articles referenced by the three projects to verify their data. They used DocInfo to ascertain the deceased physicians’ medical schools.
After eliminating duplications from the lists, the researchers counted 132 physician deaths in 28 states. Of these, 59 physicians had graduated from medical schools outside the United States, a death toll 1.8 times higher than the proportion of IMGs among U.S. physicians (95% confidence interval, 1.52-2.21; P < .001).
New York, New Jersey, and Florida accounted for 66% of the deaths among IMGs but for only 45% of the deaths among U.S. medical school graduates.
Within each state, the proportion of IMGs among deceased physicians was not statistically different from their proportion among physicians in those states, with the exception of New York.
Two-thirds of the physicians’ deaths occurred in states where IMGs make up a larger proportion of physicians than in the nation as a whole. In these states, the incidence of COVID-19 was high at the start of the pandemic.
In New York, IMGs accounted for 60% of physician deaths, which was 1.62 times higher (95% CI, 1.26-2.09; P = .005) than the 37% among New York physicians overall.
Physicians who were trained abroad frequently can’t get into the most prestigious residency programs or into the highest paid specialties and are more likely to serve in primary care, Dr. Verghese said. Overall, 60% of the physicians who died of COVID-19 worked in primary care.
IMGs often staff hospitals serving low-income communities and communities of color, which were hardest hit by the pandemic and where personal protective equipment was hard to obtain, said Dr. Verghese.
In addition to these risks, IMGs sometimes endure racism, said Dr. Verghese, who obtained his medical degree at Madras Medical College, Chennai, India. “We’ve actually seen in the COVID era, in keeping with the sort of political tone that was set in Washington, that there’s been a lot more abuses of both foreign physicians and foreign looking physicians – even if they’re not foreign trained – and nurses by patients who have been given license. And I want to acknowledge the heroism of all these physicians.”
The study was partially funded by the Presence Center at Stanford. Dr. Verghese is a regular contributor to Medscape. He served on the advisory board for Gilead Sciences, serves as a speaker or a member of a speakers bureau for Leigh Bureau, and receives royalties from Penguin Random House and Simon & Schuster.
A version of this article first appeared on Medscape.com.
More evidence links COVID vaccines to rare cases of myocarditis in youth
a Centers for Disease Control and Prevention expert reported on June 10, detailing data on cases of myocarditis and pericarditis detected through a government safety system.
The side effect seems to be more common in teen boys and young men than in older adults and women and may occur in 16 cases for every 1 million people who got a second dose, said Tom Shimabukuro, MD, MPH, deputy director of the CDC’s Immunization Safety Office, who presented information on the cases at a meeting of an expert panel that advises the U.S. Food and Drug Administration on vaccines.
Telltale symptoms include chest pain, shortness of breath, and fever.
William Schaffner, MD, an infectious diseases specialist from Vanderbilt University, Nashville, Tenn., thinks certain characteristics are pointing toward a “rare, but real” signal. First, the events are clustering, occurring within days of vaccination. Second, they tend to be more common in males and younger people. Third, he says, the number of events is above the so-called “background rate” – the cases that could be expected in this age group even without vaccination.
“I don’t think we’re quite there yet. We haven’t tied a ribbon around it, but I think the data are trending in that direction,” he said.
The issue of myocarditis weighed heavily on the Vaccines and Related Biological Products Advisory Committee’s considerations of what kind and how much data might be needed to green light use of a vaccine for COVID in children.
Because the rates of hospitalization for COVID are low in kids, some felt that the FDA should require at least a year of study of the vaccines in clinical trials, the amount of data typically required for full approval, instead of the 2 months currently required for emergency use authorization. Others wondered whether the risks of vaccination – as low as they are – might outweigh the benefits in this age group.
“I don’t really see this as an emergency in children,” said committee member Michael Kurilla, MD, PhD, the director of clinical innovation at the National Institutes of Health. Dr. Kurilla, however, did say he thought having an expanded access program for children at high risk might make sense.
Most of the young adults who experienced myocarditis recovered quickly, though three needed intensive care and rehabilitation after their episodes. Among cases with known outcomes, 81% got better and 19% still have ongoing symptoms.
Adverse events reports
The data on myocarditis come from the Vaccine Adverse Events Reporting System, or VAERS, a database of health problems reported after vaccination. This reporting system, open to anyone, has benefits and limits. It gives the CDC and FDA the ability to rapidly detect potential safety issues, and it is large enough that it can detect rare events, something that’s beyond the power of even large clinical trials.
But it is observational, so that there’s no way to know if problems reported were caused by the vaccines or a coincidence.
But because VAERS works on an honor system, it can also be spammed, and it carries the bias of the person who’s doing the reporting, from clinicians to average patients. For that reason, Dr. Shimabukuro said they are actively investigating and confirming each report they get.
Out of more than 12 million doses administered to youth ages 16-24, the CDC says it has 275 reports of heart inflammation following vaccination in this age group. The CDC has analyzed a total 475 cases of myocarditis after vaccination in people under age 30 that were reported to VAERS.
The vaccines linked to the events are the mRNA vaccines made by Pfizer and Moderna. The only vaccines currently authorized for use in adolescents are made by Pfizer. Because the Pfizer vaccine was authorized for use in kids as young as 12 last month, there’s not yet enough data to draw conclusions about the risk of myocarditis in kids ages 12-15.
Younger age groups have only received about 9% of the total doses of the vaccine so far, but they represent about 50% of the myocarditis cases reported after vaccination. “We clearly have an imbalance there,” Dr. Shimabukuro said.
The number of events in this age group appears to be above the rate that would be expected for these age groups without vaccines in the picture, he said, explaining that the number of events are in line with similar adverse events seen in young people in Israel and reported by the Department of Defense. Israel found the incidence of myocarditis after vaccination was 50 cases per million for men ages 18-30.
More study needed
Another system tracking adverse events through hospitals, the Vaccine Safety Datalink, didn’t show reports of heart inflammation above numbers that are normally seen in the population, but it did show that inflammation was more likely after a second dose of the vaccine.
“Should this be included in informed consent?” asked Cody Meissner, MD, a pediatric infectious disease specialist at Tufts University, Boston, and a member of the FDA committee.
“I think it’s hard to deny there seem to be some [events that seem] to be occurring in terms of myocarditis,” he said.
Dr. Meissner said later in the committee’s discussion that his own hospital had recently admitted a 12-year-old boy who developed heart swelling 2 days after the second dose of vaccine with a high level of troponin, an enzyme that indicates damage to the heart. His level was over 9. “A very high level,” Dr. Meissner said.
“Will there be scarring to the myocardium? Will there be a predisposition to arrhythmias later on? Will there be an early onset of heart failure? We think that’s unlikely, but [we] don’t know that,” he said.
The CDC has scheduled an emergency meeting next week to convene an expert panel on immunization practices to further review the events.
In addition to the information presented at the FDA’s meeting, doctors at Oregon Health & Science University, Portland, recently described seven cases in teens – all boys – who developed heart inflammation within 4 days of getting the second dose of the Pfizer vaccine.
The study was published June 10 in Pediatrics. All the boys were hospitalized and treated with anti-inflammatory medications including NSAIDs and steroids. Most were discharged within a few days and all recovered from their symptoms.
A version of this article first appeared on Medscape.com.
a Centers for Disease Control and Prevention expert reported on June 10, detailing data on cases of myocarditis and pericarditis detected through a government safety system.
The side effect seems to be more common in teen boys and young men than in older adults and women and may occur in 16 cases for every 1 million people who got a second dose, said Tom Shimabukuro, MD, MPH, deputy director of the CDC’s Immunization Safety Office, who presented information on the cases at a meeting of an expert panel that advises the U.S. Food and Drug Administration on vaccines.
Telltale symptoms include chest pain, shortness of breath, and fever.
William Schaffner, MD, an infectious diseases specialist from Vanderbilt University, Nashville, Tenn., thinks certain characteristics are pointing toward a “rare, but real” signal. First, the events are clustering, occurring within days of vaccination. Second, they tend to be more common in males and younger people. Third, he says, the number of events is above the so-called “background rate” – the cases that could be expected in this age group even without vaccination.
“I don’t think we’re quite there yet. We haven’t tied a ribbon around it, but I think the data are trending in that direction,” he said.
The issue of myocarditis weighed heavily on the Vaccines and Related Biological Products Advisory Committee’s considerations of what kind and how much data might be needed to green light use of a vaccine for COVID in children.
Because the rates of hospitalization for COVID are low in kids, some felt that the FDA should require at least a year of study of the vaccines in clinical trials, the amount of data typically required for full approval, instead of the 2 months currently required for emergency use authorization. Others wondered whether the risks of vaccination – as low as they are – might outweigh the benefits in this age group.
“I don’t really see this as an emergency in children,” said committee member Michael Kurilla, MD, PhD, the director of clinical innovation at the National Institutes of Health. Dr. Kurilla, however, did say he thought having an expanded access program for children at high risk might make sense.
Most of the young adults who experienced myocarditis recovered quickly, though three needed intensive care and rehabilitation after their episodes. Among cases with known outcomes, 81% got better and 19% still have ongoing symptoms.
Adverse events reports
The data on myocarditis come from the Vaccine Adverse Events Reporting System, or VAERS, a database of health problems reported after vaccination. This reporting system, open to anyone, has benefits and limits. It gives the CDC and FDA the ability to rapidly detect potential safety issues, and it is large enough that it can detect rare events, something that’s beyond the power of even large clinical trials.
But it is observational, so that there’s no way to know if problems reported were caused by the vaccines or a coincidence.
But because VAERS works on an honor system, it can also be spammed, and it carries the bias of the person who’s doing the reporting, from clinicians to average patients. For that reason, Dr. Shimabukuro said they are actively investigating and confirming each report they get.
Out of more than 12 million doses administered to youth ages 16-24, the CDC says it has 275 reports of heart inflammation following vaccination in this age group. The CDC has analyzed a total 475 cases of myocarditis after vaccination in people under age 30 that were reported to VAERS.
The vaccines linked to the events are the mRNA vaccines made by Pfizer and Moderna. The only vaccines currently authorized for use in adolescents are made by Pfizer. Because the Pfizer vaccine was authorized for use in kids as young as 12 last month, there’s not yet enough data to draw conclusions about the risk of myocarditis in kids ages 12-15.
Younger age groups have only received about 9% of the total doses of the vaccine so far, but they represent about 50% of the myocarditis cases reported after vaccination. “We clearly have an imbalance there,” Dr. Shimabukuro said.
The number of events in this age group appears to be above the rate that would be expected for these age groups without vaccines in the picture, he said, explaining that the number of events are in line with similar adverse events seen in young people in Israel and reported by the Department of Defense. Israel found the incidence of myocarditis after vaccination was 50 cases per million for men ages 18-30.
More study needed
Another system tracking adverse events through hospitals, the Vaccine Safety Datalink, didn’t show reports of heart inflammation above numbers that are normally seen in the population, but it did show that inflammation was more likely after a second dose of the vaccine.
“Should this be included in informed consent?” asked Cody Meissner, MD, a pediatric infectious disease specialist at Tufts University, Boston, and a member of the FDA committee.
“I think it’s hard to deny there seem to be some [events that seem] to be occurring in terms of myocarditis,” he said.
Dr. Meissner said later in the committee’s discussion that his own hospital had recently admitted a 12-year-old boy who developed heart swelling 2 days after the second dose of vaccine with a high level of troponin, an enzyme that indicates damage to the heart. His level was over 9. “A very high level,” Dr. Meissner said.
“Will there be scarring to the myocardium? Will there be a predisposition to arrhythmias later on? Will there be an early onset of heart failure? We think that’s unlikely, but [we] don’t know that,” he said.
The CDC has scheduled an emergency meeting next week to convene an expert panel on immunization practices to further review the events.
In addition to the information presented at the FDA’s meeting, doctors at Oregon Health & Science University, Portland, recently described seven cases in teens – all boys – who developed heart inflammation within 4 days of getting the second dose of the Pfizer vaccine.
The study was published June 10 in Pediatrics. All the boys were hospitalized and treated with anti-inflammatory medications including NSAIDs and steroids. Most were discharged within a few days and all recovered from their symptoms.
A version of this article first appeared on Medscape.com.
a Centers for Disease Control and Prevention expert reported on June 10, detailing data on cases of myocarditis and pericarditis detected through a government safety system.
The side effect seems to be more common in teen boys and young men than in older adults and women and may occur in 16 cases for every 1 million people who got a second dose, said Tom Shimabukuro, MD, MPH, deputy director of the CDC’s Immunization Safety Office, who presented information on the cases at a meeting of an expert panel that advises the U.S. Food and Drug Administration on vaccines.
Telltale symptoms include chest pain, shortness of breath, and fever.
William Schaffner, MD, an infectious diseases specialist from Vanderbilt University, Nashville, Tenn., thinks certain characteristics are pointing toward a “rare, but real” signal. First, the events are clustering, occurring within days of vaccination. Second, they tend to be more common in males and younger people. Third, he says, the number of events is above the so-called “background rate” – the cases that could be expected in this age group even without vaccination.
“I don’t think we’re quite there yet. We haven’t tied a ribbon around it, but I think the data are trending in that direction,” he said.
The issue of myocarditis weighed heavily on the Vaccines and Related Biological Products Advisory Committee’s considerations of what kind and how much data might be needed to green light use of a vaccine for COVID in children.
Because the rates of hospitalization for COVID are low in kids, some felt that the FDA should require at least a year of study of the vaccines in clinical trials, the amount of data typically required for full approval, instead of the 2 months currently required for emergency use authorization. Others wondered whether the risks of vaccination – as low as they are – might outweigh the benefits in this age group.
“I don’t really see this as an emergency in children,” said committee member Michael Kurilla, MD, PhD, the director of clinical innovation at the National Institutes of Health. Dr. Kurilla, however, did say he thought having an expanded access program for children at high risk might make sense.
Most of the young adults who experienced myocarditis recovered quickly, though three needed intensive care and rehabilitation after their episodes. Among cases with known outcomes, 81% got better and 19% still have ongoing symptoms.
Adverse events reports
The data on myocarditis come from the Vaccine Adverse Events Reporting System, or VAERS, a database of health problems reported after vaccination. This reporting system, open to anyone, has benefits and limits. It gives the CDC and FDA the ability to rapidly detect potential safety issues, and it is large enough that it can detect rare events, something that’s beyond the power of even large clinical trials.
But it is observational, so that there’s no way to know if problems reported were caused by the vaccines or a coincidence.
But because VAERS works on an honor system, it can also be spammed, and it carries the bias of the person who’s doing the reporting, from clinicians to average patients. For that reason, Dr. Shimabukuro said they are actively investigating and confirming each report they get.
Out of more than 12 million doses administered to youth ages 16-24, the CDC says it has 275 reports of heart inflammation following vaccination in this age group. The CDC has analyzed a total 475 cases of myocarditis after vaccination in people under age 30 that were reported to VAERS.
The vaccines linked to the events are the mRNA vaccines made by Pfizer and Moderna. The only vaccines currently authorized for use in adolescents are made by Pfizer. Because the Pfizer vaccine was authorized for use in kids as young as 12 last month, there’s not yet enough data to draw conclusions about the risk of myocarditis in kids ages 12-15.
Younger age groups have only received about 9% of the total doses of the vaccine so far, but they represent about 50% of the myocarditis cases reported after vaccination. “We clearly have an imbalance there,” Dr. Shimabukuro said.
The number of events in this age group appears to be above the rate that would be expected for these age groups without vaccines in the picture, he said, explaining that the number of events are in line with similar adverse events seen in young people in Israel and reported by the Department of Defense. Israel found the incidence of myocarditis after vaccination was 50 cases per million for men ages 18-30.
More study needed
Another system tracking adverse events through hospitals, the Vaccine Safety Datalink, didn’t show reports of heart inflammation above numbers that are normally seen in the population, but it did show that inflammation was more likely after a second dose of the vaccine.
“Should this be included in informed consent?” asked Cody Meissner, MD, a pediatric infectious disease specialist at Tufts University, Boston, and a member of the FDA committee.
“I think it’s hard to deny there seem to be some [events that seem] to be occurring in terms of myocarditis,” he said.
Dr. Meissner said later in the committee’s discussion that his own hospital had recently admitted a 12-year-old boy who developed heart swelling 2 days after the second dose of vaccine with a high level of troponin, an enzyme that indicates damage to the heart. His level was over 9. “A very high level,” Dr. Meissner said.
“Will there be scarring to the myocardium? Will there be a predisposition to arrhythmias later on? Will there be an early onset of heart failure? We think that’s unlikely, but [we] don’t know that,” he said.
The CDC has scheduled an emergency meeting next week to convene an expert panel on immunization practices to further review the events.
In addition to the information presented at the FDA’s meeting, doctors at Oregon Health & Science University, Portland, recently described seven cases in teens – all boys – who developed heart inflammation within 4 days of getting the second dose of the Pfizer vaccine.
The study was published June 10 in Pediatrics. All the boys were hospitalized and treated with anti-inflammatory medications including NSAIDs and steroids. Most were discharged within a few days and all recovered from their symptoms.
A version of this article first appeared on Medscape.com.
LDCT lung cancer screening may ID aortic stenosis risk
says new research published in Annals of Internal Medicine.
Aortic stenosis is one of the most common valve disease problems and is characterized by the narrowing of the aortic valve opening, according to the American Heart Association. The condition impedes the delivery of blood from the heart to the body.
Researchers found that LDCT, which according to the Centers for Disease Control and Prevention is the only recommended screening test for lung cancer, also can be used to identify aortic valve calcification – a condition in which calcium deposits form on the aortic valve, narrowing it.
Since cardiovascular events and lung cancer are known to have the same modifiable risk factors, people screened for lung cancer could also be diagnosed with cardiovascular diseases, the authors noted in their paper.
Furthermore, a 2019 study published in the Journal of Thoracic Imaging found that LDCT can be useful for identifying not just lung cancer, but the early stages of chronic obstructive pulmonary disease and coronary artery disease.
“LDCT has been described as useful for identifying the early stages of chronic obstructive pulmonary disease and coronary artery disease, but it can also [screen for] calcified aortic valve [which corresponds] with the risk of severe aortic stenosis,” study author Marcin Fijalkowski, MD, PhD, of the Medical University of Gdansk, said in an interview. “This additional evaluation is not time-consuming and is easy to perform.”
Methods and results
For the study, Dr. Fijalkowski and his colleagues examined data from 6,631 people between the ages of 50 and 80 years of age with a smoking history of 30 or more pack-years. The group was enrolled in the MOLTEST BIS lung cancer screening program between 2016 and 2018, which assessed the usefulness of LDCT performed during lung cancer screening in determining the degree of aortic valve calcification as an additional finding. The researchers arbitrarily determined a calcium score of 900 as a cutoff point indicating a positive test result. Positive patients were sent for an echocardiogram for confirmation of diagnosis.
Aortic valve calcification was identified in 869 patients, 13.1% of the group. Sixty-eight participants, which is about 8% of this group, were identified as having a calcium score of 900 at least and were referred for echocardiography to confirm these results. Of this group, 0.5% were diagnosed with at least moderate aortic stenosis after receiving an echocardiogram. About 55% of the participants with this condition were unaware of their valvular heart disease, including 23% with a severe form of the disease.
Study identified patients who had not been aware of disease
Dr. Fijalkowski said while he was not surprised by the findings, he was surprised that the study may have saved some of the participants’ lives.
“We were expecting the same degree of calcification of aortic valve and correlation with aortic stenosis severity, but what surprised us was that half of diagnosed patients were not aware of disease,” he said. “This additional finding was lifesaving.”
In the paper, the authors noted that cardiology societies do not yet recognize LDCT as a diagnostic tool for aortic stenosis. Based on their findings, they propose that aortic valve calcification become a routine assessment procedure in the LDCT protocol for lung cancer screening.
Findings are ‘important’ but not practice changing
Salim S. Virani, MD, FACC, who was not involved in the study, said this new research is important.
The analyses were done well and push the needle further in a direction that suggests “when we are doing imaging for one reason, we should use the totality of information that we have available,” he noted.
“I mean, if you are looking at a lung nodule, if you see an aortic valve that’s very calcified, then it should prompt you to at least ask the patient about some symptoms related to that,” Dr. Virani explained.
However, he said more research is needed on a larger population before LDCT can be considered a diagnostic tool for aortic stenosis.
“I think we have to understand that this study was done in a very specific group of patients,” said Dr. Virani, professor in the sections of cardiology and cardiovascular research at Baylor College of Medicine, Houston. “If you were to do it in a population that was much younger, with much lower risk of even lung cancer, then the yield of a CT to pick up aortic stenosis would be lower.”
Before any practice changes are made regarding LDCT and the diagnosis of aortic stenosis, there needs to be more research on how many people in the general population are getting non–cardiology-related chest imaging and then come up with a population-based metric as to what calcium score cutoff could be used, he said.
Dr. Fijalkowski said he believes the results of his study will encourage physicians to focus not only on pulmonary nodules but also to look for additional things such as aortic valve calcification.
The experts did not disclose any relevant financial relationships.
says new research published in Annals of Internal Medicine.
Aortic stenosis is one of the most common valve disease problems and is characterized by the narrowing of the aortic valve opening, according to the American Heart Association. The condition impedes the delivery of blood from the heart to the body.
Researchers found that LDCT, which according to the Centers for Disease Control and Prevention is the only recommended screening test for lung cancer, also can be used to identify aortic valve calcification – a condition in which calcium deposits form on the aortic valve, narrowing it.
Since cardiovascular events and lung cancer are known to have the same modifiable risk factors, people screened for lung cancer could also be diagnosed with cardiovascular diseases, the authors noted in their paper.
Furthermore, a 2019 study published in the Journal of Thoracic Imaging found that LDCT can be useful for identifying not just lung cancer, but the early stages of chronic obstructive pulmonary disease and coronary artery disease.
“LDCT has been described as useful for identifying the early stages of chronic obstructive pulmonary disease and coronary artery disease, but it can also [screen for] calcified aortic valve [which corresponds] with the risk of severe aortic stenosis,” study author Marcin Fijalkowski, MD, PhD, of the Medical University of Gdansk, said in an interview. “This additional evaluation is not time-consuming and is easy to perform.”
Methods and results
For the study, Dr. Fijalkowski and his colleagues examined data from 6,631 people between the ages of 50 and 80 years of age with a smoking history of 30 or more pack-years. The group was enrolled in the MOLTEST BIS lung cancer screening program between 2016 and 2018, which assessed the usefulness of LDCT performed during lung cancer screening in determining the degree of aortic valve calcification as an additional finding. The researchers arbitrarily determined a calcium score of 900 as a cutoff point indicating a positive test result. Positive patients were sent for an echocardiogram for confirmation of diagnosis.
Aortic valve calcification was identified in 869 patients, 13.1% of the group. Sixty-eight participants, which is about 8% of this group, were identified as having a calcium score of 900 at least and were referred for echocardiography to confirm these results. Of this group, 0.5% were diagnosed with at least moderate aortic stenosis after receiving an echocardiogram. About 55% of the participants with this condition were unaware of their valvular heart disease, including 23% with a severe form of the disease.
Study identified patients who had not been aware of disease
Dr. Fijalkowski said while he was not surprised by the findings, he was surprised that the study may have saved some of the participants’ lives.
“We were expecting the same degree of calcification of aortic valve and correlation with aortic stenosis severity, but what surprised us was that half of diagnosed patients were not aware of disease,” he said. “This additional finding was lifesaving.”
In the paper, the authors noted that cardiology societies do not yet recognize LDCT as a diagnostic tool for aortic stenosis. Based on their findings, they propose that aortic valve calcification become a routine assessment procedure in the LDCT protocol for lung cancer screening.
Findings are ‘important’ but not practice changing
Salim S. Virani, MD, FACC, who was not involved in the study, said this new research is important.
The analyses were done well and push the needle further in a direction that suggests “when we are doing imaging for one reason, we should use the totality of information that we have available,” he noted.
“I mean, if you are looking at a lung nodule, if you see an aortic valve that’s very calcified, then it should prompt you to at least ask the patient about some symptoms related to that,” Dr. Virani explained.
However, he said more research is needed on a larger population before LDCT can be considered a diagnostic tool for aortic stenosis.
“I think we have to understand that this study was done in a very specific group of patients,” said Dr. Virani, professor in the sections of cardiology and cardiovascular research at Baylor College of Medicine, Houston. “If you were to do it in a population that was much younger, with much lower risk of even lung cancer, then the yield of a CT to pick up aortic stenosis would be lower.”
Before any practice changes are made regarding LDCT and the diagnosis of aortic stenosis, there needs to be more research on how many people in the general population are getting non–cardiology-related chest imaging and then come up with a population-based metric as to what calcium score cutoff could be used, he said.
Dr. Fijalkowski said he believes the results of his study will encourage physicians to focus not only on pulmonary nodules but also to look for additional things such as aortic valve calcification.
The experts did not disclose any relevant financial relationships.
says new research published in Annals of Internal Medicine.
Aortic stenosis is one of the most common valve disease problems and is characterized by the narrowing of the aortic valve opening, according to the American Heart Association. The condition impedes the delivery of blood from the heart to the body.
Researchers found that LDCT, which according to the Centers for Disease Control and Prevention is the only recommended screening test for lung cancer, also can be used to identify aortic valve calcification – a condition in which calcium deposits form on the aortic valve, narrowing it.
Since cardiovascular events and lung cancer are known to have the same modifiable risk factors, people screened for lung cancer could also be diagnosed with cardiovascular diseases, the authors noted in their paper.
Furthermore, a 2019 study published in the Journal of Thoracic Imaging found that LDCT can be useful for identifying not just lung cancer, but the early stages of chronic obstructive pulmonary disease and coronary artery disease.
“LDCT has been described as useful for identifying the early stages of chronic obstructive pulmonary disease and coronary artery disease, but it can also [screen for] calcified aortic valve [which corresponds] with the risk of severe aortic stenosis,” study author Marcin Fijalkowski, MD, PhD, of the Medical University of Gdansk, said in an interview. “This additional evaluation is not time-consuming and is easy to perform.”
Methods and results
For the study, Dr. Fijalkowski and his colleagues examined data from 6,631 people between the ages of 50 and 80 years of age with a smoking history of 30 or more pack-years. The group was enrolled in the MOLTEST BIS lung cancer screening program between 2016 and 2018, which assessed the usefulness of LDCT performed during lung cancer screening in determining the degree of aortic valve calcification as an additional finding. The researchers arbitrarily determined a calcium score of 900 as a cutoff point indicating a positive test result. Positive patients were sent for an echocardiogram for confirmation of diagnosis.
Aortic valve calcification was identified in 869 patients, 13.1% of the group. Sixty-eight participants, which is about 8% of this group, were identified as having a calcium score of 900 at least and were referred for echocardiography to confirm these results. Of this group, 0.5% were diagnosed with at least moderate aortic stenosis after receiving an echocardiogram. About 55% of the participants with this condition were unaware of their valvular heart disease, including 23% with a severe form of the disease.
Study identified patients who had not been aware of disease
Dr. Fijalkowski said while he was not surprised by the findings, he was surprised that the study may have saved some of the participants’ lives.
“We were expecting the same degree of calcification of aortic valve and correlation with aortic stenosis severity, but what surprised us was that half of diagnosed patients were not aware of disease,” he said. “This additional finding was lifesaving.”
In the paper, the authors noted that cardiology societies do not yet recognize LDCT as a diagnostic tool for aortic stenosis. Based on their findings, they propose that aortic valve calcification become a routine assessment procedure in the LDCT protocol for lung cancer screening.
Findings are ‘important’ but not practice changing
Salim S. Virani, MD, FACC, who was not involved in the study, said this new research is important.
The analyses were done well and push the needle further in a direction that suggests “when we are doing imaging for one reason, we should use the totality of information that we have available,” he noted.
“I mean, if you are looking at a lung nodule, if you see an aortic valve that’s very calcified, then it should prompt you to at least ask the patient about some symptoms related to that,” Dr. Virani explained.
However, he said more research is needed on a larger population before LDCT can be considered a diagnostic tool for aortic stenosis.
“I think we have to understand that this study was done in a very specific group of patients,” said Dr. Virani, professor in the sections of cardiology and cardiovascular research at Baylor College of Medicine, Houston. “If you were to do it in a population that was much younger, with much lower risk of even lung cancer, then the yield of a CT to pick up aortic stenosis would be lower.”
Before any practice changes are made regarding LDCT and the diagnosis of aortic stenosis, there needs to be more research on how many people in the general population are getting non–cardiology-related chest imaging and then come up with a population-based metric as to what calcium score cutoff could be used, he said.
Dr. Fijalkowski said he believes the results of his study will encourage physicians to focus not only on pulmonary nodules but also to look for additional things such as aortic valve calcification.
The experts did not disclose any relevant financial relationships.
FROM ANNALS OF INTERNAL MEDICINE