User login
Clinical Case-Viewing Sessions in Dermatology: The Patient Perspective
To the Editor:
Dermatology clinical case-viewing (CCV) sessions, commonly referred to as Grand Rounds, are of core educational importance in teaching residents, fellows, and medical students. The traditional format includes the viewing of patient cases followed by resident- and faculty-led group discussions. Clinical case-viewing sessions often involve several health professionals simultaneously observing and interacting with a patient. Although these sessions are highly academically enriching, they may be ill-perceived by patients. The objective of this study was to evaluate patients’ perception of CCV sessions.
This study was approved by the Wake Forest School of Medicine (Winston-Salem, North Carolina) institutional review board and was conducted from February 2017 to August 2017. Following informed consent, 18 patients older than 18 years who were present at the Wake Forest Department of Dermatology CCV sessions were recruited. Patients were each assigned to a private clinical examination room, and CCV attendees briefly visited each room to assess the pathologic findings of interest. Patients received written quantitative surveys before and after the CCV sessions assessing their perspectives on the session (Table 1). Quantitative surveys were assessed using a 10-point Likert scale (1=least willing; 10=most willing). Patients also received qualitative surveys following the session (Table 2). Scores on a 10-item Likert scale were evaluated using a 2-tailed t test.
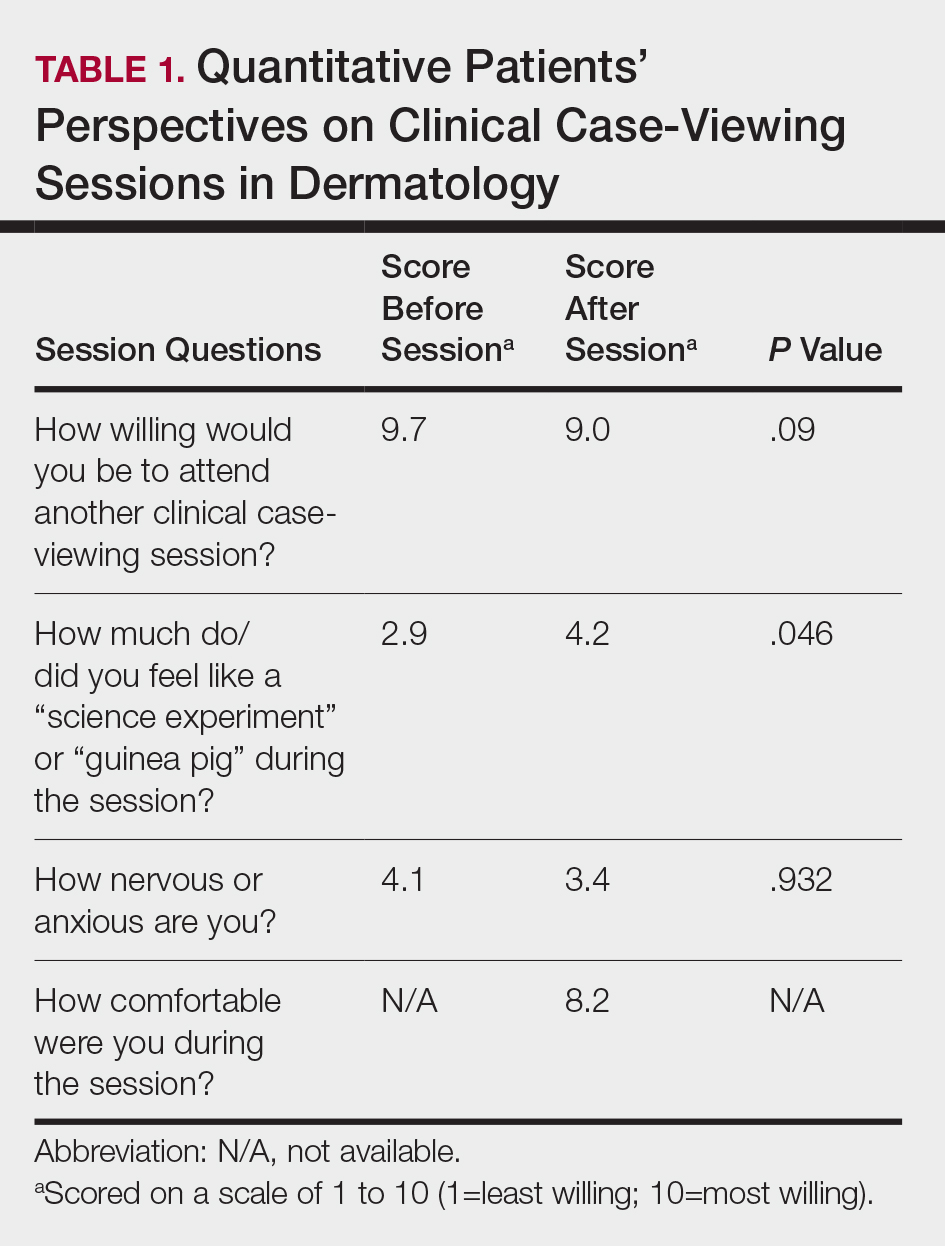
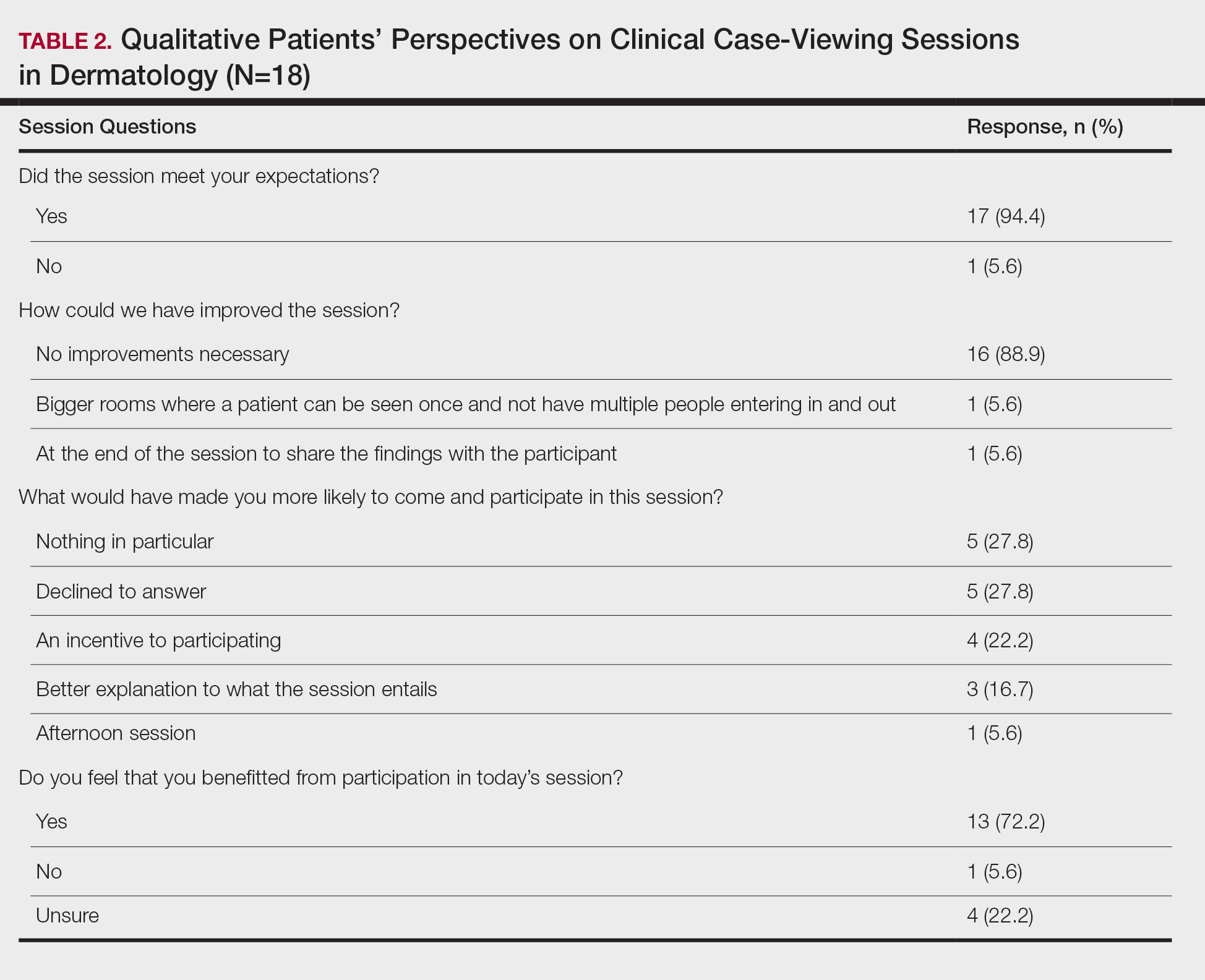
The mean age of patients was 57.6 years, and women comprised 66.7% (12/18). Patient willingness to attend CCV sessions was relatively unchanged before and after the session, with a mean willingness of 9.7 before the session and 9.0 after the session (P=.09). There was a significant difference in the extent to which patients perceived themselves as experimental subjects prior to the session compared to after the session (2.9 vs 4.2)(P=.046). Following the session, 94.4% (17/18) of patients had the impression that the session met their expectations, and 72.2% (13/18) of patients felt they directly benefitted from the session.
Clinical case-viewing sessions are the foundation of any dermatology residency program1-3; however, characterizing the sessions’ psychosocial implications on patients is important too. Although some patients did feel part of a “science experiment,” this finding may be of less importance, as patients generally considered the sessions to be a positive experience and were willing to take part again.
Limitations of the study were typical of survey-based research. All participants were patients at a single center, which may limit the generalization of the results, in addition to the small sample size. Clinical case-viewing sessions also are conducted slightly differently between dermatology programs, which may further limit the generalization of the results. Future studies may aim to assess varying CCV formats to optimize both medical education as well as patient satisfaction.
- Mehrabi D, Cruz PD Jr. Educational conferences in dermatology residency programs. J Am Acad Dermatol. 2006;55:523-524.
- Hull AL, Cullen RJ, Hekelman FP. A retrospective analysis of grand rounds in continuing medical education. J Contin Educ Health Prof. 1989;9:257-266.
- Cruz PD Jr, Chaker MB. Teaching conferences in dermatology residency programs revisited. J Am Acad of Dermatol. 1995;32:675-677.
To the Editor:
Dermatology clinical case-viewing (CCV) sessions, commonly referred to as Grand Rounds, are of core educational importance in teaching residents, fellows, and medical students. The traditional format includes the viewing of patient cases followed by resident- and faculty-led group discussions. Clinical case-viewing sessions often involve several health professionals simultaneously observing and interacting with a patient. Although these sessions are highly academically enriching, they may be ill-perceived by patients. The objective of this study was to evaluate patients’ perception of CCV sessions.
This study was approved by the Wake Forest School of Medicine (Winston-Salem, North Carolina) institutional review board and was conducted from February 2017 to August 2017. Following informed consent, 18 patients older than 18 years who were present at the Wake Forest Department of Dermatology CCV sessions were recruited. Patients were each assigned to a private clinical examination room, and CCV attendees briefly visited each room to assess the pathologic findings of interest. Patients received written quantitative surveys before and after the CCV sessions assessing their perspectives on the session (Table 1). Quantitative surveys were assessed using a 10-point Likert scale (1=least willing; 10=most willing). Patients also received qualitative surveys following the session (Table 2). Scores on a 10-item Likert scale were evaluated using a 2-tailed t test.


The mean age of patients was 57.6 years, and women comprised 66.7% (12/18). Patient willingness to attend CCV sessions was relatively unchanged before and after the session, with a mean willingness of 9.7 before the session and 9.0 after the session (P=.09). There was a significant difference in the extent to which patients perceived themselves as experimental subjects prior to the session compared to after the session (2.9 vs 4.2)(P=.046). Following the session, 94.4% (17/18) of patients had the impression that the session met their expectations, and 72.2% (13/18) of patients felt they directly benefitted from the session.
Clinical case-viewing sessions are the foundation of any dermatology residency program1-3; however, characterizing the sessions’ psychosocial implications on patients is important too. Although some patients did feel part of a “science experiment,” this finding may be of less importance, as patients generally considered the sessions to be a positive experience and were willing to take part again.
Limitations of the study were typical of survey-based research. All participants were patients at a single center, which may limit the generalization of the results, in addition to the small sample size. Clinical case-viewing sessions also are conducted slightly differently between dermatology programs, which may further limit the generalization of the results. Future studies may aim to assess varying CCV formats to optimize both medical education as well as patient satisfaction.
To the Editor:
Dermatology clinical case-viewing (CCV) sessions, commonly referred to as Grand Rounds, are of core educational importance in teaching residents, fellows, and medical students. The traditional format includes the viewing of patient cases followed by resident- and faculty-led group discussions. Clinical case-viewing sessions often involve several health professionals simultaneously observing and interacting with a patient. Although these sessions are highly academically enriching, they may be ill-perceived by patients. The objective of this study was to evaluate patients’ perception of CCV sessions.
This study was approved by the Wake Forest School of Medicine (Winston-Salem, North Carolina) institutional review board and was conducted from February 2017 to August 2017. Following informed consent, 18 patients older than 18 years who were present at the Wake Forest Department of Dermatology CCV sessions were recruited. Patients were each assigned to a private clinical examination room, and CCV attendees briefly visited each room to assess the pathologic findings of interest. Patients received written quantitative surveys before and after the CCV sessions assessing their perspectives on the session (Table 1). Quantitative surveys were assessed using a 10-point Likert scale (1=least willing; 10=most willing). Patients also received qualitative surveys following the session (Table 2). Scores on a 10-item Likert scale were evaluated using a 2-tailed t test.


The mean age of patients was 57.6 years, and women comprised 66.7% (12/18). Patient willingness to attend CCV sessions was relatively unchanged before and after the session, with a mean willingness of 9.7 before the session and 9.0 after the session (P=.09). There was a significant difference in the extent to which patients perceived themselves as experimental subjects prior to the session compared to after the session (2.9 vs 4.2)(P=.046). Following the session, 94.4% (17/18) of patients had the impression that the session met their expectations, and 72.2% (13/18) of patients felt they directly benefitted from the session.
Clinical case-viewing sessions are the foundation of any dermatology residency program1-3; however, characterizing the sessions’ psychosocial implications on patients is important too. Although some patients did feel part of a “science experiment,” this finding may be of less importance, as patients generally considered the sessions to be a positive experience and were willing to take part again.
Limitations of the study were typical of survey-based research. All participants were patients at a single center, which may limit the generalization of the results, in addition to the small sample size. Clinical case-viewing sessions also are conducted slightly differently between dermatology programs, which may further limit the generalization of the results. Future studies may aim to assess varying CCV formats to optimize both medical education as well as patient satisfaction.
- Mehrabi D, Cruz PD Jr. Educational conferences in dermatology residency programs. J Am Acad Dermatol. 2006;55:523-524.
- Hull AL, Cullen RJ, Hekelman FP. A retrospective analysis of grand rounds in continuing medical education. J Contin Educ Health Prof. 1989;9:257-266.
- Cruz PD Jr, Chaker MB. Teaching conferences in dermatology residency programs revisited. J Am Acad of Dermatol. 1995;32:675-677.
- Mehrabi D, Cruz PD Jr. Educational conferences in dermatology residency programs. J Am Acad Dermatol. 2006;55:523-524.
- Hull AL, Cullen RJ, Hekelman FP. A retrospective analysis of grand rounds in continuing medical education. J Contin Educ Health Prof. 1989;9:257-266.
- Cruz PD Jr, Chaker MB. Teaching conferences in dermatology residency programs revisited. J Am Acad of Dermatol. 1995;32:675-677.
Practice Points
- Patient willingness to attend dermatology clinical case-viewing (CCV) sessions is relatively unchanged before and after the session.
- Participants generally consider CCV sessions to be a positive experience.
A decade of telemedicine policy has advanced in just 2 weeks
The rapid spread of , which he’d never used.
But as soon as he learned that telehealth regulations had been relaxed by the Centers for Medicare & Medicaid Services and that reimbursement had been broadened, Dr. Desai, a dermatologist in private practice and his staff began to mobilize.
“Kaboom! We made the decision to start doing it,” he said in an interview. “We drafted a consent form, uploaded it to our website, called patients, changed our voice greeting, and got clarity on insurance coverage. We’ve been flying by the seat of our pants.”
“I’m doing it because I don’t have a choice at this point,” said Dr. Desai, who is a member of the American Academy of Dermatology board of directors and its coronavirus task force. “I’m very worried about continuing to be able to meet our payroll expenses for staff and overhead to keep the office open.”
“Flying by the seat of our pants” to see patients virtually
Dermatologists have long been considered pioneers in telemedicine. They have, since the 1990s, capitalized on the visual nature of the specialty to diagnose and treat skin diseases by incorporating photos, videos, and virtual-patient visits. But the pandemic has forced the hands of even holdouts like Dr. Desai, who clung to in-person consults because of confusion related to HIPAA compliance issues and the sense that teledermatology “really dehumanizes patient interaction” for him.
In fact, as of 2017, only 15% of the nation’s 11,000 or so dermatologists had implemented telehealth into their practices, according to an AAD practice survey. In the wake of COVID-19, however, that percentage has likely more than tripled, experts estimate.
Now, dermatologists are assuming the mantle of educators for other specialists who never considered telehealth before in-person visits became fraught with concerns about the spread of the virus. And some are publishing guidelines for colleagues on how to prioritize teledermatology to stem transmission and conserve personal protective equipment (PPE) and hospital beds.
User-friendly technology and the relaxed telehealth restrictions have made it fairly simple for patients and physicians to connect. Facetime and other once-prohibited platforms are all currently permissible, although physicians are encouraged to notify patients about potential privacy risks, according to an AAD teledermatology tool kit.
Teledermatology innovators
“We’ve moved 10 years in telemedicine policy in 2 weeks,” said Karen Edison, MD, of the University of Missouri, Columbia. “The federal government has really loosened the reins.”
At least half of all dermatologists in the United States have adopted telehealth since the pandemic emerged, she estimated. And most, like Dr. Desai, have done so in just the last several weeks.
“You can do about 90% of what you need to do as a dermatologist using the technology,” said Dr. Edison, who launched the first dermatology Extension for Community Healthcare Outcomes, or ECHO, program in the Midwest. That telehealth model was originally developed to connect rural general practitioners with specialists at academic medical centers or large health systems.
“People are used to taking pictures with their phones. In some ways, this crisis may change the face of our specialty,” she said in an interview.
“As we’re all practicing social distancing, I think physicians and patients are rethinking how we can access healthcare without pursuing traditional face-to-face interactions,” said Ivy Lee, MD, from the University of California, San Francisco, who is past chair of the AAD telemedicine task force and current chair of the teledermatology committee at the American Telemedicine Association. “Virtual health and telemedicine fit perfectly with that.”
Even before the pandemic, the innovative ways dermatologists were using telehealth were garnering increasing acclaim. All four clinical groups short-listed for dermatology team of the year at the BMJ Awards 2020 employed telehealth to improve patient services in the United Kingdom.
In the United States, dermatologists are joining forces to boost understanding of how telehealth can protect patients and clinicians from some of the ravages of the virus.
The Society of Dermatology Hospitalists has developed an algorithm – built on experiences its members have had caring for hospitalized patients with acute dermatologic conditions – to provide a “logical way” to triage telemedicine consults in multiple hospital settings during the coronavirus crisis, said President-Elect Daniela Kroshinsky, MD, from Massachusetts General Hospital in Boston.
Telemedicine consultation is prioritized and patients at high risk for COVID-19 exposure are identified so that exposure time and resource use are limited and patient and staff safety are maximized.
“We want to empower our colleagues in community hospitals to play a role in safely providing care for patients in need but to be mindful about preserving resources,” said Dr. Kroshinsky, who reported that the algorithm will be published imminently.
“If you don’t have to see a patient in person and can offer recommendations through telederm, you don’t need to put on a gown, gloves, mask, or goggles,” she said in an interview. “If you’re unable to assess photos, then of course you’ll use the appropriate protective wear, but it will be better if you can obtain the same result” without having to do so.
Sharing expertise
After the first week of tracking data to gauge the effectiveness of the algorithm at Massachusetts General, Dr. Kroshinsky said she is buoyed.
Of the 35 patients assessed electronically – all of whom would previously have been seen in person – only 4 ended up needing a subsequent in-person consult, she reported.
“It’s worked out great,” said Dr. Kroshinsky, who noted that the pandemic is a “nice opportunity” to test different telehealth platforms and improve quality down the line. “We never had to use any excessive PPE, beyond what was routine, and the majority of patients were able to be staffed remotely. All patients had successful outcomes.”
With telehealth more firmly established in dermatology than in most other specialties, dermatologists are now helping clinicians in other fields who are rapidly ramping up their own telemedicine offerings.
These might include obstetrics and gynecology or “any medical specialty where they need to do checkups with their patients and don’t want them coming in for nonemergent visits,” said Carrie L. Kovarik, MD, of the University of Pennsylvania, Philadelphia.
In addition to fielding many recent calls and emails from physicians seeking guidance on telehealth, Dr. Kovarik, Dr. Lee, and colleagues have published the steps required to integrate the technology into outpatient practices.
“Now that there’s a time for broad implementation, our colleagues are looking to us for help and troubleshooting advice,” said Dr. Kovarik, who is also a member of the AAD COVID-19 response task force.
Various specialties “lend themselves to telehealth, depending on how image- or data-dependent they are,” Dr. Lee said in an interview. “But all specialists thinking of limiting or shutting down their practices are thinking about how they can provide continuity of care without exposing patients or staff to the risk of contracting the coronavirus.”
After-COVID goals
In his first week of virtual patient consults, Dr. Desai said he saw about 50 patients, which is still far fewer than the 160-180 he sees in person during a normal week.
“The problem is that patients don’t really want to do telehealth. You’d think it would be a good option,” he said, “but patients hesitate because they don’t really know how to use their device.” Some have instead rescheduled in-person appointments for months down the line.
Although telehealth has enabled Dr. Desai to readily assess patients with acne, hair loss, psoriasis, rashes, warts, and eczema, he’s concerned that necessary procedures, such as biopsies and dermoscopies, could be dangerously delayed. It’s also hard to assess the texture and thickness of certain skin lesions in photos or videos, he said.
“I’m trying to stay optimistic that this will get better and we’re able to move back to taking care of patients the way we need to,” he said.
Like Dr. Desai, other dermatologists who’ve implemented telemedicine during the pandemic have largely been swayed by the relaxed CMS regulations. “It’s made all the difference,” Dr. Kovarik said. “It has brought down the anxiety level and decreased questions about platforms and concentrated them on how to code the visits.”
And although it’s difficult to envision post-COVID medical practice in the thick of the pandemic, dermatologists expect the current strides in telemedicine will stick.
“I’m hoping that telehealth use isn’t dialed back all the way to baseline” after the pandemic eases, Dr. Kovarik said. “The cat’s out of the bag, and now that it is, hopefully it won’t be put back in.”
“If there’s a silver lining to this,” Dr. Kroshinsky said, “I hope it’s that we’ll be able to innovate around health care in a fashion we wouldn’t have seen otherwise.”
A version of this article originally appeared on Medscape.com.
The rapid spread of , which he’d never used.
But as soon as he learned that telehealth regulations had been relaxed by the Centers for Medicare & Medicaid Services and that reimbursement had been broadened, Dr. Desai, a dermatologist in private practice and his staff began to mobilize.
“Kaboom! We made the decision to start doing it,” he said in an interview. “We drafted a consent form, uploaded it to our website, called patients, changed our voice greeting, and got clarity on insurance coverage. We’ve been flying by the seat of our pants.”
“I’m doing it because I don’t have a choice at this point,” said Dr. Desai, who is a member of the American Academy of Dermatology board of directors and its coronavirus task force. “I’m very worried about continuing to be able to meet our payroll expenses for staff and overhead to keep the office open.”
“Flying by the seat of our pants” to see patients virtually
Dermatologists have long been considered pioneers in telemedicine. They have, since the 1990s, capitalized on the visual nature of the specialty to diagnose and treat skin diseases by incorporating photos, videos, and virtual-patient visits. But the pandemic has forced the hands of even holdouts like Dr. Desai, who clung to in-person consults because of confusion related to HIPAA compliance issues and the sense that teledermatology “really dehumanizes patient interaction” for him.
In fact, as of 2017, only 15% of the nation’s 11,000 or so dermatologists had implemented telehealth into their practices, according to an AAD practice survey. In the wake of COVID-19, however, that percentage has likely more than tripled, experts estimate.
Now, dermatologists are assuming the mantle of educators for other specialists who never considered telehealth before in-person visits became fraught with concerns about the spread of the virus. And some are publishing guidelines for colleagues on how to prioritize teledermatology to stem transmission and conserve personal protective equipment (PPE) and hospital beds.
User-friendly technology and the relaxed telehealth restrictions have made it fairly simple for patients and physicians to connect. Facetime and other once-prohibited platforms are all currently permissible, although physicians are encouraged to notify patients about potential privacy risks, according to an AAD teledermatology tool kit.
Teledermatology innovators
“We’ve moved 10 years in telemedicine policy in 2 weeks,” said Karen Edison, MD, of the University of Missouri, Columbia. “The federal government has really loosened the reins.”
At least half of all dermatologists in the United States have adopted telehealth since the pandemic emerged, she estimated. And most, like Dr. Desai, have done so in just the last several weeks.
“You can do about 90% of what you need to do as a dermatologist using the technology,” said Dr. Edison, who launched the first dermatology Extension for Community Healthcare Outcomes, or ECHO, program in the Midwest. That telehealth model was originally developed to connect rural general practitioners with specialists at academic medical centers or large health systems.
“People are used to taking pictures with their phones. In some ways, this crisis may change the face of our specialty,” she said in an interview.
“As we’re all practicing social distancing, I think physicians and patients are rethinking how we can access healthcare without pursuing traditional face-to-face interactions,” said Ivy Lee, MD, from the University of California, San Francisco, who is past chair of the AAD telemedicine task force and current chair of the teledermatology committee at the American Telemedicine Association. “Virtual health and telemedicine fit perfectly with that.”
Even before the pandemic, the innovative ways dermatologists were using telehealth were garnering increasing acclaim. All four clinical groups short-listed for dermatology team of the year at the BMJ Awards 2020 employed telehealth to improve patient services in the United Kingdom.
In the United States, dermatologists are joining forces to boost understanding of how telehealth can protect patients and clinicians from some of the ravages of the virus.
The Society of Dermatology Hospitalists has developed an algorithm – built on experiences its members have had caring for hospitalized patients with acute dermatologic conditions – to provide a “logical way” to triage telemedicine consults in multiple hospital settings during the coronavirus crisis, said President-Elect Daniela Kroshinsky, MD, from Massachusetts General Hospital in Boston.
Telemedicine consultation is prioritized and patients at high risk for COVID-19 exposure are identified so that exposure time and resource use are limited and patient and staff safety are maximized.
“We want to empower our colleagues in community hospitals to play a role in safely providing care for patients in need but to be mindful about preserving resources,” said Dr. Kroshinsky, who reported that the algorithm will be published imminently.
“If you don’t have to see a patient in person and can offer recommendations through telederm, you don’t need to put on a gown, gloves, mask, or goggles,” she said in an interview. “If you’re unable to assess photos, then of course you’ll use the appropriate protective wear, but it will be better if you can obtain the same result” without having to do so.
Sharing expertise
After the first week of tracking data to gauge the effectiveness of the algorithm at Massachusetts General, Dr. Kroshinsky said she is buoyed.
Of the 35 patients assessed electronically – all of whom would previously have been seen in person – only 4 ended up needing a subsequent in-person consult, she reported.
“It’s worked out great,” said Dr. Kroshinsky, who noted that the pandemic is a “nice opportunity” to test different telehealth platforms and improve quality down the line. “We never had to use any excessive PPE, beyond what was routine, and the majority of patients were able to be staffed remotely. All patients had successful outcomes.”
With telehealth more firmly established in dermatology than in most other specialties, dermatologists are now helping clinicians in other fields who are rapidly ramping up their own telemedicine offerings.
These might include obstetrics and gynecology or “any medical specialty where they need to do checkups with their patients and don’t want them coming in for nonemergent visits,” said Carrie L. Kovarik, MD, of the University of Pennsylvania, Philadelphia.
In addition to fielding many recent calls and emails from physicians seeking guidance on telehealth, Dr. Kovarik, Dr. Lee, and colleagues have published the steps required to integrate the technology into outpatient practices.
“Now that there’s a time for broad implementation, our colleagues are looking to us for help and troubleshooting advice,” said Dr. Kovarik, who is also a member of the AAD COVID-19 response task force.
Various specialties “lend themselves to telehealth, depending on how image- or data-dependent they are,” Dr. Lee said in an interview. “But all specialists thinking of limiting or shutting down their practices are thinking about how they can provide continuity of care without exposing patients or staff to the risk of contracting the coronavirus.”
After-COVID goals
In his first week of virtual patient consults, Dr. Desai said he saw about 50 patients, which is still far fewer than the 160-180 he sees in person during a normal week.
“The problem is that patients don’t really want to do telehealth. You’d think it would be a good option,” he said, “but patients hesitate because they don’t really know how to use their device.” Some have instead rescheduled in-person appointments for months down the line.
Although telehealth has enabled Dr. Desai to readily assess patients with acne, hair loss, psoriasis, rashes, warts, and eczema, he’s concerned that necessary procedures, such as biopsies and dermoscopies, could be dangerously delayed. It’s also hard to assess the texture and thickness of certain skin lesions in photos or videos, he said.
“I’m trying to stay optimistic that this will get better and we’re able to move back to taking care of patients the way we need to,” he said.
Like Dr. Desai, other dermatologists who’ve implemented telemedicine during the pandemic have largely been swayed by the relaxed CMS regulations. “It’s made all the difference,” Dr. Kovarik said. “It has brought down the anxiety level and decreased questions about platforms and concentrated them on how to code the visits.”
And although it’s difficult to envision post-COVID medical practice in the thick of the pandemic, dermatologists expect the current strides in telemedicine will stick.
“I’m hoping that telehealth use isn’t dialed back all the way to baseline” after the pandemic eases, Dr. Kovarik said. “The cat’s out of the bag, and now that it is, hopefully it won’t be put back in.”
“If there’s a silver lining to this,” Dr. Kroshinsky said, “I hope it’s that we’ll be able to innovate around health care in a fashion we wouldn’t have seen otherwise.”
A version of this article originally appeared on Medscape.com.
The rapid spread of , which he’d never used.
But as soon as he learned that telehealth regulations had been relaxed by the Centers for Medicare & Medicaid Services and that reimbursement had been broadened, Dr. Desai, a dermatologist in private practice and his staff began to mobilize.
“Kaboom! We made the decision to start doing it,” he said in an interview. “We drafted a consent form, uploaded it to our website, called patients, changed our voice greeting, and got clarity on insurance coverage. We’ve been flying by the seat of our pants.”
“I’m doing it because I don’t have a choice at this point,” said Dr. Desai, who is a member of the American Academy of Dermatology board of directors and its coronavirus task force. “I’m very worried about continuing to be able to meet our payroll expenses for staff and overhead to keep the office open.”
“Flying by the seat of our pants” to see patients virtually
Dermatologists have long been considered pioneers in telemedicine. They have, since the 1990s, capitalized on the visual nature of the specialty to diagnose and treat skin diseases by incorporating photos, videos, and virtual-patient visits. But the pandemic has forced the hands of even holdouts like Dr. Desai, who clung to in-person consults because of confusion related to HIPAA compliance issues and the sense that teledermatology “really dehumanizes patient interaction” for him.
In fact, as of 2017, only 15% of the nation’s 11,000 or so dermatologists had implemented telehealth into their practices, according to an AAD practice survey. In the wake of COVID-19, however, that percentage has likely more than tripled, experts estimate.
Now, dermatologists are assuming the mantle of educators for other specialists who never considered telehealth before in-person visits became fraught with concerns about the spread of the virus. And some are publishing guidelines for colleagues on how to prioritize teledermatology to stem transmission and conserve personal protective equipment (PPE) and hospital beds.
User-friendly technology and the relaxed telehealth restrictions have made it fairly simple for patients and physicians to connect. Facetime and other once-prohibited platforms are all currently permissible, although physicians are encouraged to notify patients about potential privacy risks, according to an AAD teledermatology tool kit.
Teledermatology innovators
“We’ve moved 10 years in telemedicine policy in 2 weeks,” said Karen Edison, MD, of the University of Missouri, Columbia. “The federal government has really loosened the reins.”
At least half of all dermatologists in the United States have adopted telehealth since the pandemic emerged, she estimated. And most, like Dr. Desai, have done so in just the last several weeks.
“You can do about 90% of what you need to do as a dermatologist using the technology,” said Dr. Edison, who launched the first dermatology Extension for Community Healthcare Outcomes, or ECHO, program in the Midwest. That telehealth model was originally developed to connect rural general practitioners with specialists at academic medical centers or large health systems.
“People are used to taking pictures with their phones. In some ways, this crisis may change the face of our specialty,” she said in an interview.
“As we’re all practicing social distancing, I think physicians and patients are rethinking how we can access healthcare without pursuing traditional face-to-face interactions,” said Ivy Lee, MD, from the University of California, San Francisco, who is past chair of the AAD telemedicine task force and current chair of the teledermatology committee at the American Telemedicine Association. “Virtual health and telemedicine fit perfectly with that.”
Even before the pandemic, the innovative ways dermatologists were using telehealth were garnering increasing acclaim. All four clinical groups short-listed for dermatology team of the year at the BMJ Awards 2020 employed telehealth to improve patient services in the United Kingdom.
In the United States, dermatologists are joining forces to boost understanding of how telehealth can protect patients and clinicians from some of the ravages of the virus.
The Society of Dermatology Hospitalists has developed an algorithm – built on experiences its members have had caring for hospitalized patients with acute dermatologic conditions – to provide a “logical way” to triage telemedicine consults in multiple hospital settings during the coronavirus crisis, said President-Elect Daniela Kroshinsky, MD, from Massachusetts General Hospital in Boston.
Telemedicine consultation is prioritized and patients at high risk for COVID-19 exposure are identified so that exposure time and resource use are limited and patient and staff safety are maximized.
“We want to empower our colleagues in community hospitals to play a role in safely providing care for patients in need but to be mindful about preserving resources,” said Dr. Kroshinsky, who reported that the algorithm will be published imminently.
“If you don’t have to see a patient in person and can offer recommendations through telederm, you don’t need to put on a gown, gloves, mask, or goggles,” she said in an interview. “If you’re unable to assess photos, then of course you’ll use the appropriate protective wear, but it will be better if you can obtain the same result” without having to do so.
Sharing expertise
After the first week of tracking data to gauge the effectiveness of the algorithm at Massachusetts General, Dr. Kroshinsky said she is buoyed.
Of the 35 patients assessed electronically – all of whom would previously have been seen in person – only 4 ended up needing a subsequent in-person consult, she reported.
“It’s worked out great,” said Dr. Kroshinsky, who noted that the pandemic is a “nice opportunity” to test different telehealth platforms and improve quality down the line. “We never had to use any excessive PPE, beyond what was routine, and the majority of patients were able to be staffed remotely. All patients had successful outcomes.”
With telehealth more firmly established in dermatology than in most other specialties, dermatologists are now helping clinicians in other fields who are rapidly ramping up their own telemedicine offerings.
These might include obstetrics and gynecology or “any medical specialty where they need to do checkups with their patients and don’t want them coming in for nonemergent visits,” said Carrie L. Kovarik, MD, of the University of Pennsylvania, Philadelphia.
In addition to fielding many recent calls and emails from physicians seeking guidance on telehealth, Dr. Kovarik, Dr. Lee, and colleagues have published the steps required to integrate the technology into outpatient practices.
“Now that there’s a time for broad implementation, our colleagues are looking to us for help and troubleshooting advice,” said Dr. Kovarik, who is also a member of the AAD COVID-19 response task force.
Various specialties “lend themselves to telehealth, depending on how image- or data-dependent they are,” Dr. Lee said in an interview. “But all specialists thinking of limiting or shutting down their practices are thinking about how they can provide continuity of care without exposing patients or staff to the risk of contracting the coronavirus.”
After-COVID goals
In his first week of virtual patient consults, Dr. Desai said he saw about 50 patients, which is still far fewer than the 160-180 he sees in person during a normal week.
“The problem is that patients don’t really want to do telehealth. You’d think it would be a good option,” he said, “but patients hesitate because they don’t really know how to use their device.” Some have instead rescheduled in-person appointments for months down the line.
Although telehealth has enabled Dr. Desai to readily assess patients with acne, hair loss, psoriasis, rashes, warts, and eczema, he’s concerned that necessary procedures, such as biopsies and dermoscopies, could be dangerously delayed. It’s also hard to assess the texture and thickness of certain skin lesions in photos or videos, he said.
“I’m trying to stay optimistic that this will get better and we’re able to move back to taking care of patients the way we need to,” he said.
Like Dr. Desai, other dermatologists who’ve implemented telemedicine during the pandemic have largely been swayed by the relaxed CMS regulations. “It’s made all the difference,” Dr. Kovarik said. “It has brought down the anxiety level and decreased questions about platforms and concentrated them on how to code the visits.”
And although it’s difficult to envision post-COVID medical practice in the thick of the pandemic, dermatologists expect the current strides in telemedicine will stick.
“I’m hoping that telehealth use isn’t dialed back all the way to baseline” after the pandemic eases, Dr. Kovarik said. “The cat’s out of the bag, and now that it is, hopefully it won’t be put back in.”
“If there’s a silver lining to this,” Dr. Kroshinsky said, “I hope it’s that we’ll be able to innovate around health care in a fashion we wouldn’t have seen otherwise.”
A version of this article originally appeared on Medscape.com.
‘Brutal’ plan to restrict palliative radiation during pandemic
A major comprehensive cancer center at the epicenter of the New York City COVID-19 storm is preparing to scale back palliative radiation therapy (RT), anticipating a focus on only oncologic emergencies.
“We’re not there yet, but we’re anticipating when the time comes in the next few weeks that we will have a system in place so we are able to handle it,” Jonathan Yang, MD, PhD, of Memorial Sloan Kettering Cancer Center (MSKCC) in New York City, told Medscape Medical News.
Yang and an expert panel of colleagues reviewed high-impact evidence, prior systematic reviews, and national guidelines to compile a set of recommendations for triage and shortened palliative rRT at their center, should the need arise.
The recommendations on palliative radiotherapy for oncologic emergencies in the setting of COVID-19 appear in a preprint version in Advances in Radiation Oncology, released by the American Society of Radiation Oncology.
Yang says the recommendations are a careful balance between the risk of COVID-19 exposure of staff and patients with the potential morbidity of delaying treatment.
“Everyone is conscious of decisions about whether patients need treatment now or can wait,” he told Medscape Medical News. “It’s a juggling act every single day, but by having this guideline in place, when we face the situation where we do have to make decisions, is helpful.”
The document aims to enable swift decisions based on best practice, including a three-tiered system prioritizing only “clinically urgent cases, in which delaying treatment would result in compromised outcomes or serious morbidity.”
“It’s brutal, that’s the only word for it. Not that I disagree with it,” commented Padraig Warde, MB BCh, professor, Department of Radiation Oncology, University of Toronto, and radiation oncologist, Princess Margaret Cancer Centre, Toronto, Ontario, Canada.
Like many places, Toronto is not yet experiencing the COVID-19 burden of New York City, but Warde says the MSKCC guideline is useful for everyone. “Other centers should review it and see how they could deal with resource limitations,” he said. “It’s sobering and sad, but if you don’t have the staff to treat all patients, which particular patients do you choose to treat?”
In a nutshell, the MSKCC recommendations defines Tier 1 patients as having oncologic emergencies that require palliative RT, including “cord compression, symptomatic brain metastases requiring whole-brain radiotherapy, life-threatening tumor bleeding, and malignant airway obstruction.”
According to the decision-making guideline, patients in Tiers 2 and 3 would have their palliative RT delayed. This would include Tier 2 patients whose needs are not classified as emergencies, but who have either symptomatic disease for which RT is usually the standard of care or asymptomatic disease for which RT is recommended “to prevent imminent functional deficits.” Tier 3 would be symptomatic or asymptomatic patients for whom RT is “one of the effective treatment options.”
“Rationing is always very difficult because as physicians you always want to do everything you can for your patients but we really have to strike the balance on when to do what, said Yang. The plan that he authored anticipates both reduced availability of radiation therapists as well as aggressive attempts to limit patients’ infection exposure.
“If a patient’s radiation is being considered for delay due to COVID-19, other means are utilized to achieve the goal of palliation in the interim, and in addition to the tier system, this decision is also made on a case-by-case basis with departmental discussion on the risks and benefits,” he explained.
“There are layers of checks and balances for these decisions...Obviously for oncologic emergencies, radiation will be implemented. However for less urgent situations, bringing them into the hospital when there are other ways to achieve the same goal, potential risk of exposure to COVID-19 is higher than the benefit we would be able to provide.”
The document also recommends shorter courses of RT when radiation is deemed appropriate.
“We have good evidence showing shorter courses of radiation can effectively treat the goal of palliation compared to longer courses of radiation,” he explained. “Going through this pandemic actually forces radiation oncologists in the United States to put that evidence into practice. It’s not suboptimal care in the sense that we are achieving the same goal — palliation. This paper is to remind people there are equally effective courses of palliation we can be using.”
“[There’s] nothing like a crisis to get people to do the right thing,” commented Louis Potters, MD, professor and chair of radiation medicine at the Feinstein Institutes, the research arm of Northwell Health, New York’s largest healthcare provider.
Northwell Health has been at the epicenter of the New York outbreak of COVID-19. Potters writes on an ASTRO blog that, as of March 26, Northwell Health “has diagnosed 4399 positive COVID-19 patients, which is about 20% of New York state and 1.2% of all cases in the world. All cancer surgery was discontinued as of March 20 and all of our 23 hospitals are seeing COVID-19 admissions, and ICU care became the primary focus of the entire system. As of today, we have reserved one floor in two hospitals for non-COVID care such as trauma. That’s it.”
Before the crisis, radiation medicine at Northwell consisted of eight separate locations treating on average 280 EBRT cases a day, not including SBRT/SRS and brachytherapy cases. “That of course was 3 weeks ago,” he notes.
Commenting on the recommendations from the MSKCC group, Potters told Medscape Medical News that the primary goal “was to document what are acceptable alternatives for accelerated care.”
“Ironically, these guidelines represent best practices with evidence that — in a non–COVID-19 world — make sense for the majority of patients requiring palliative radiotherapy,” he said.
Potters said there has been hesitance to transition to shorter radiation treatments for several reasons.
“Historically, palliative radiotherapy has been delivered over 2 to 4 weeks with good results. And, as is typical in medicine, the transition to shorter course care is slowed by financial incentives to protract care,” he explained.
“In a value-based future where payment is based on outcomes, this transition to shorter care will evolve very quickly. But given the current COVID-19 crisis, and the risk to patients and staff, the incentive for shorter treatment courses has been thrust upon us and the MSKCC outline helps to define how to do this safely and with evidence-based expected efficacy.”
This article first appeared on Medscape.com.
A major comprehensive cancer center at the epicenter of the New York City COVID-19 storm is preparing to scale back palliative radiation therapy (RT), anticipating a focus on only oncologic emergencies.
“We’re not there yet, but we’re anticipating when the time comes in the next few weeks that we will have a system in place so we are able to handle it,” Jonathan Yang, MD, PhD, of Memorial Sloan Kettering Cancer Center (MSKCC) in New York City, told Medscape Medical News.
Yang and an expert panel of colleagues reviewed high-impact evidence, prior systematic reviews, and national guidelines to compile a set of recommendations for triage and shortened palliative rRT at their center, should the need arise.
The recommendations on palliative radiotherapy for oncologic emergencies in the setting of COVID-19 appear in a preprint version in Advances in Radiation Oncology, released by the American Society of Radiation Oncology.
Yang says the recommendations are a careful balance between the risk of COVID-19 exposure of staff and patients with the potential morbidity of delaying treatment.
“Everyone is conscious of decisions about whether patients need treatment now or can wait,” he told Medscape Medical News. “It’s a juggling act every single day, but by having this guideline in place, when we face the situation where we do have to make decisions, is helpful.”
The document aims to enable swift decisions based on best practice, including a three-tiered system prioritizing only “clinically urgent cases, in which delaying treatment would result in compromised outcomes or serious morbidity.”
“It’s brutal, that’s the only word for it. Not that I disagree with it,” commented Padraig Warde, MB BCh, professor, Department of Radiation Oncology, University of Toronto, and radiation oncologist, Princess Margaret Cancer Centre, Toronto, Ontario, Canada.
Like many places, Toronto is not yet experiencing the COVID-19 burden of New York City, but Warde says the MSKCC guideline is useful for everyone. “Other centers should review it and see how they could deal with resource limitations,” he said. “It’s sobering and sad, but if you don’t have the staff to treat all patients, which particular patients do you choose to treat?”
In a nutshell, the MSKCC recommendations defines Tier 1 patients as having oncologic emergencies that require palliative RT, including “cord compression, symptomatic brain metastases requiring whole-brain radiotherapy, life-threatening tumor bleeding, and malignant airway obstruction.”
According to the decision-making guideline, patients in Tiers 2 and 3 would have their palliative RT delayed. This would include Tier 2 patients whose needs are not classified as emergencies, but who have either symptomatic disease for which RT is usually the standard of care or asymptomatic disease for which RT is recommended “to prevent imminent functional deficits.” Tier 3 would be symptomatic or asymptomatic patients for whom RT is “one of the effective treatment options.”
“Rationing is always very difficult because as physicians you always want to do everything you can for your patients but we really have to strike the balance on when to do what, said Yang. The plan that he authored anticipates both reduced availability of radiation therapists as well as aggressive attempts to limit patients’ infection exposure.
“If a patient’s radiation is being considered for delay due to COVID-19, other means are utilized to achieve the goal of palliation in the interim, and in addition to the tier system, this decision is also made on a case-by-case basis with departmental discussion on the risks and benefits,” he explained.
“There are layers of checks and balances for these decisions...Obviously for oncologic emergencies, radiation will be implemented. However for less urgent situations, bringing them into the hospital when there are other ways to achieve the same goal, potential risk of exposure to COVID-19 is higher than the benefit we would be able to provide.”
The document also recommends shorter courses of RT when radiation is deemed appropriate.
“We have good evidence showing shorter courses of radiation can effectively treat the goal of palliation compared to longer courses of radiation,” he explained. “Going through this pandemic actually forces radiation oncologists in the United States to put that evidence into practice. It’s not suboptimal care in the sense that we are achieving the same goal — palliation. This paper is to remind people there are equally effective courses of palliation we can be using.”
“[There’s] nothing like a crisis to get people to do the right thing,” commented Louis Potters, MD, professor and chair of radiation medicine at the Feinstein Institutes, the research arm of Northwell Health, New York’s largest healthcare provider.
Northwell Health has been at the epicenter of the New York outbreak of COVID-19. Potters writes on an ASTRO blog that, as of March 26, Northwell Health “has diagnosed 4399 positive COVID-19 patients, which is about 20% of New York state and 1.2% of all cases in the world. All cancer surgery was discontinued as of March 20 and all of our 23 hospitals are seeing COVID-19 admissions, and ICU care became the primary focus of the entire system. As of today, we have reserved one floor in two hospitals for non-COVID care such as trauma. That’s it.”
Before the crisis, radiation medicine at Northwell consisted of eight separate locations treating on average 280 EBRT cases a day, not including SBRT/SRS and brachytherapy cases. “That of course was 3 weeks ago,” he notes.
Commenting on the recommendations from the MSKCC group, Potters told Medscape Medical News that the primary goal “was to document what are acceptable alternatives for accelerated care.”
“Ironically, these guidelines represent best practices with evidence that — in a non–COVID-19 world — make sense for the majority of patients requiring palliative radiotherapy,” he said.
Potters said there has been hesitance to transition to shorter radiation treatments for several reasons.
“Historically, palliative radiotherapy has been delivered over 2 to 4 weeks with good results. And, as is typical in medicine, the transition to shorter course care is slowed by financial incentives to protract care,” he explained.
“In a value-based future where payment is based on outcomes, this transition to shorter care will evolve very quickly. But given the current COVID-19 crisis, and the risk to patients and staff, the incentive for shorter treatment courses has been thrust upon us and the MSKCC outline helps to define how to do this safely and with evidence-based expected efficacy.”
This article first appeared on Medscape.com.
A major comprehensive cancer center at the epicenter of the New York City COVID-19 storm is preparing to scale back palliative radiation therapy (RT), anticipating a focus on only oncologic emergencies.
“We’re not there yet, but we’re anticipating when the time comes in the next few weeks that we will have a system in place so we are able to handle it,” Jonathan Yang, MD, PhD, of Memorial Sloan Kettering Cancer Center (MSKCC) in New York City, told Medscape Medical News.
Yang and an expert panel of colleagues reviewed high-impact evidence, prior systematic reviews, and national guidelines to compile a set of recommendations for triage and shortened palliative rRT at their center, should the need arise.
The recommendations on palliative radiotherapy for oncologic emergencies in the setting of COVID-19 appear in a preprint version in Advances in Radiation Oncology, released by the American Society of Radiation Oncology.
Yang says the recommendations are a careful balance between the risk of COVID-19 exposure of staff and patients with the potential morbidity of delaying treatment.
“Everyone is conscious of decisions about whether patients need treatment now or can wait,” he told Medscape Medical News. “It’s a juggling act every single day, but by having this guideline in place, when we face the situation where we do have to make decisions, is helpful.”
The document aims to enable swift decisions based on best practice, including a three-tiered system prioritizing only “clinically urgent cases, in which delaying treatment would result in compromised outcomes or serious morbidity.”
“It’s brutal, that’s the only word for it. Not that I disagree with it,” commented Padraig Warde, MB BCh, professor, Department of Radiation Oncology, University of Toronto, and radiation oncologist, Princess Margaret Cancer Centre, Toronto, Ontario, Canada.
Like many places, Toronto is not yet experiencing the COVID-19 burden of New York City, but Warde says the MSKCC guideline is useful for everyone. “Other centers should review it and see how they could deal with resource limitations,” he said. “It’s sobering and sad, but if you don’t have the staff to treat all patients, which particular patients do you choose to treat?”
In a nutshell, the MSKCC recommendations defines Tier 1 patients as having oncologic emergencies that require palliative RT, including “cord compression, symptomatic brain metastases requiring whole-brain radiotherapy, life-threatening tumor bleeding, and malignant airway obstruction.”
According to the decision-making guideline, patients in Tiers 2 and 3 would have their palliative RT delayed. This would include Tier 2 patients whose needs are not classified as emergencies, but who have either symptomatic disease for which RT is usually the standard of care or asymptomatic disease for which RT is recommended “to prevent imminent functional deficits.” Tier 3 would be symptomatic or asymptomatic patients for whom RT is “one of the effective treatment options.”
“Rationing is always very difficult because as physicians you always want to do everything you can for your patients but we really have to strike the balance on when to do what, said Yang. The plan that he authored anticipates both reduced availability of radiation therapists as well as aggressive attempts to limit patients’ infection exposure.
“If a patient’s radiation is being considered for delay due to COVID-19, other means are utilized to achieve the goal of palliation in the interim, and in addition to the tier system, this decision is also made on a case-by-case basis with departmental discussion on the risks and benefits,” he explained.
“There are layers of checks and balances for these decisions...Obviously for oncologic emergencies, radiation will be implemented. However for less urgent situations, bringing them into the hospital when there are other ways to achieve the same goal, potential risk of exposure to COVID-19 is higher than the benefit we would be able to provide.”
The document also recommends shorter courses of RT when radiation is deemed appropriate.
“We have good evidence showing shorter courses of radiation can effectively treat the goal of palliation compared to longer courses of radiation,” he explained. “Going through this pandemic actually forces radiation oncologists in the United States to put that evidence into practice. It’s not suboptimal care in the sense that we are achieving the same goal — palliation. This paper is to remind people there are equally effective courses of palliation we can be using.”
“[There’s] nothing like a crisis to get people to do the right thing,” commented Louis Potters, MD, professor and chair of radiation medicine at the Feinstein Institutes, the research arm of Northwell Health, New York’s largest healthcare provider.
Northwell Health has been at the epicenter of the New York outbreak of COVID-19. Potters writes on an ASTRO blog that, as of March 26, Northwell Health “has diagnosed 4399 positive COVID-19 patients, which is about 20% of New York state and 1.2% of all cases in the world. All cancer surgery was discontinued as of March 20 and all of our 23 hospitals are seeing COVID-19 admissions, and ICU care became the primary focus of the entire system. As of today, we have reserved one floor in two hospitals for non-COVID care such as trauma. That’s it.”
Before the crisis, radiation medicine at Northwell consisted of eight separate locations treating on average 280 EBRT cases a day, not including SBRT/SRS and brachytherapy cases. “That of course was 3 weeks ago,” he notes.
Commenting on the recommendations from the MSKCC group, Potters told Medscape Medical News that the primary goal “was to document what are acceptable alternatives for accelerated care.”
“Ironically, these guidelines represent best practices with evidence that — in a non–COVID-19 world — make sense for the majority of patients requiring palliative radiotherapy,” he said.
Potters said there has been hesitance to transition to shorter radiation treatments for several reasons.
“Historically, palliative radiotherapy has been delivered over 2 to 4 weeks with good results. And, as is typical in medicine, the transition to shorter course care is slowed by financial incentives to protract care,” he explained.
“In a value-based future where payment is based on outcomes, this transition to shorter care will evolve very quickly. But given the current COVID-19 crisis, and the risk to patients and staff, the incentive for shorter treatment courses has been thrust upon us and the MSKCC outline helps to define how to do this safely and with evidence-based expected efficacy.”
This article first appeared on Medscape.com.
Advice from the front lines: How cancer centers can cope with COVID-19
according to the medical director of a cancer care alliance in the first U.S. epicenter of the coronavirus outbreak.
Jennie R. Crews, MD, the medical director of the Seattle Cancer Care Alliance (SCCA), discussed the SCCA experience and offered advice for other cancer centers in a webinar hosted by the Association of Community Cancer Centers.
Dr. Crews highlighted the SCCA’s use of algorithms to predict which patients can be managed via telehealth and which require face-to-face visits, human resource issues that arose at SCCA, screening and testing procedures, and the importance of communication with patients, caregivers, and staff.
Communication
Dr. Crews stressed the value of clear, regular, and internally consistent staff communication in a variety of formats. SCCA sends daily email blasts to their personnel regarding policies and procedures, which are archived on the SCCA intranet site.
SCCA also holds weekly town hall meetings at which leaders respond to staff questions regarding practical matters they have encountered and future plans. Providers’ up-to-the-minute familiarity with policies and procedures enables all team members to uniformly and clearly communicate to patients and caregivers.
Dr. Crews emphasized the value of consistency and “over-communication” in projecting confidence and preparedness to patients and caregivers during an unsettling time. SCCA has developed fact sheets, posted current information on the SCCA website, and provided education during doorway screenings.
Screening and testing
All SCCA staff members are screened daily at the practice entrance so they have personal experience with the process utilized for patients. Because symptoms associated with coronavirus infection may overlap with cancer treatment–related complaints, SCCA clinicians have expanded the typical coronavirus screening questionnaire for patients on cancer treatment.
Patients with ambiguous symptoms are masked, taken to a physically separate area of the SCCA clinics, and screened further by an advanced practice provider. The patients are then triaged to either the clinic for treatment or to the emergency department for further triage and care.
Although testing processes and procedures have been modified, Dr. Crews advised codifying those policies and procedures, including notification of results and follow-up for both patients and staff. Dr. Crews also stressed the importance of clearly articulated return-to-work policies for staff who have potential exposure and/or positive test results.
At the University of Washington’s virology laboratory, they have a test turnaround time of less than 12 hours.
Planning ahead
Dr. Crews highlighted the importance of community-based surge planning, utilizing predictive models to assess inpatient capacity requirements and potential repurposing of providers.
The SCCA is prepared to close selected community sites and shift personnel to other locations if personnel needs cannot be met because of illness or quarantine. Contingency plans include specialized pharmacy services for patients requiring chemotherapy.
The SCCA has not yet experienced shortages of personal protective equipment (PPE). However, Dr. Crews said staff require detailed education regarding the use of PPE in order to safeguard the supply while providing maximal staff protection.
Helping the helpers
During the pandemic, SCCA has dealt with a variety of challenging human resource issues, including:
- Extending sick time beyond what was previously “stored” in staff members’ earned time off.
- Childcare during an extended hiatus in school and daycare schedules.
- Programs to maintain and/or restore employee wellness (including staff-centered support services, spiritual care, mindfulness exercises, and town halls).
Dr. Crews also discussed recruitment of community resources to provide meals for staff from local restaurants with restricted hours and transportation resources for staff and patients, as visitors are restricted (currently one per patient).
Managing care
Dr. Crews noted that the University of Washington had a foundational structure for a telehealth program prior to the pandemic. Their telehealth committee enabled SCCA to scale up the service quickly with their academic partners, including training modules for and certification of providers, outfitting off-site personnel with dedicated lines and hardware, and provision of personal Zoom accounts.
SCCA also devised algorithms for determining when face-to-face visits, remote management, or deferred visits are appropriate in various scenarios. The algorithms were developed by disease-specialized teams.
As a general rule, routine chemotherapy and radiation are administered on schedule. On-treatment and follow-up office visits are conducted via telehealth if possible. In some cases, initiation of chemotherapy and radiation has been delayed, and screening services have been suspended.
In response to questions about palliative care during the pandemic, Dr. Crews said SCCA has encouraged their patients to complete, review, or update their advance directives. The SCCA has not had the need to resuscitate a coronavirus-infected outpatient but has instituted policies for utilizing full PPE on any patient requiring resuscitation.
In her closing remarks, Dr. Crews stressed that the response to COVID-19 in Washington state has required an intense collaboration among colleagues, the community, and government leaders, as the actions required extended far beyond medical decision makers alone.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
according to the medical director of a cancer care alliance in the first U.S. epicenter of the coronavirus outbreak.
Jennie R. Crews, MD, the medical director of the Seattle Cancer Care Alliance (SCCA), discussed the SCCA experience and offered advice for other cancer centers in a webinar hosted by the Association of Community Cancer Centers.
Dr. Crews highlighted the SCCA’s use of algorithms to predict which patients can be managed via telehealth and which require face-to-face visits, human resource issues that arose at SCCA, screening and testing procedures, and the importance of communication with patients, caregivers, and staff.
Communication
Dr. Crews stressed the value of clear, regular, and internally consistent staff communication in a variety of formats. SCCA sends daily email blasts to their personnel regarding policies and procedures, which are archived on the SCCA intranet site.
SCCA also holds weekly town hall meetings at which leaders respond to staff questions regarding practical matters they have encountered and future plans. Providers’ up-to-the-minute familiarity with policies and procedures enables all team members to uniformly and clearly communicate to patients and caregivers.
Dr. Crews emphasized the value of consistency and “over-communication” in projecting confidence and preparedness to patients and caregivers during an unsettling time. SCCA has developed fact sheets, posted current information on the SCCA website, and provided education during doorway screenings.
Screening and testing
All SCCA staff members are screened daily at the practice entrance so they have personal experience with the process utilized for patients. Because symptoms associated with coronavirus infection may overlap with cancer treatment–related complaints, SCCA clinicians have expanded the typical coronavirus screening questionnaire for patients on cancer treatment.
Patients with ambiguous symptoms are masked, taken to a physically separate area of the SCCA clinics, and screened further by an advanced practice provider. The patients are then triaged to either the clinic for treatment or to the emergency department for further triage and care.
Although testing processes and procedures have been modified, Dr. Crews advised codifying those policies and procedures, including notification of results and follow-up for both patients and staff. Dr. Crews also stressed the importance of clearly articulated return-to-work policies for staff who have potential exposure and/or positive test results.
At the University of Washington’s virology laboratory, they have a test turnaround time of less than 12 hours.
Planning ahead
Dr. Crews highlighted the importance of community-based surge planning, utilizing predictive models to assess inpatient capacity requirements and potential repurposing of providers.
The SCCA is prepared to close selected community sites and shift personnel to other locations if personnel needs cannot be met because of illness or quarantine. Contingency plans include specialized pharmacy services for patients requiring chemotherapy.
The SCCA has not yet experienced shortages of personal protective equipment (PPE). However, Dr. Crews said staff require detailed education regarding the use of PPE in order to safeguard the supply while providing maximal staff protection.
Helping the helpers
During the pandemic, SCCA has dealt with a variety of challenging human resource issues, including:
- Extending sick time beyond what was previously “stored” in staff members’ earned time off.
- Childcare during an extended hiatus in school and daycare schedules.
- Programs to maintain and/or restore employee wellness (including staff-centered support services, spiritual care, mindfulness exercises, and town halls).
Dr. Crews also discussed recruitment of community resources to provide meals for staff from local restaurants with restricted hours and transportation resources for staff and patients, as visitors are restricted (currently one per patient).
Managing care
Dr. Crews noted that the University of Washington had a foundational structure for a telehealth program prior to the pandemic. Their telehealth committee enabled SCCA to scale up the service quickly with their academic partners, including training modules for and certification of providers, outfitting off-site personnel with dedicated lines and hardware, and provision of personal Zoom accounts.
SCCA also devised algorithms for determining when face-to-face visits, remote management, or deferred visits are appropriate in various scenarios. The algorithms were developed by disease-specialized teams.
As a general rule, routine chemotherapy and radiation are administered on schedule. On-treatment and follow-up office visits are conducted via telehealth if possible. In some cases, initiation of chemotherapy and radiation has been delayed, and screening services have been suspended.
In response to questions about palliative care during the pandemic, Dr. Crews said SCCA has encouraged their patients to complete, review, or update their advance directives. The SCCA has not had the need to resuscitate a coronavirus-infected outpatient but has instituted policies for utilizing full PPE on any patient requiring resuscitation.
In her closing remarks, Dr. Crews stressed that the response to COVID-19 in Washington state has required an intense collaboration among colleagues, the community, and government leaders, as the actions required extended far beyond medical decision makers alone.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
according to the medical director of a cancer care alliance in the first U.S. epicenter of the coronavirus outbreak.
Jennie R. Crews, MD, the medical director of the Seattle Cancer Care Alliance (SCCA), discussed the SCCA experience and offered advice for other cancer centers in a webinar hosted by the Association of Community Cancer Centers.
Dr. Crews highlighted the SCCA’s use of algorithms to predict which patients can be managed via telehealth and which require face-to-face visits, human resource issues that arose at SCCA, screening and testing procedures, and the importance of communication with patients, caregivers, and staff.
Communication
Dr. Crews stressed the value of clear, regular, and internally consistent staff communication in a variety of formats. SCCA sends daily email blasts to their personnel regarding policies and procedures, which are archived on the SCCA intranet site.
SCCA also holds weekly town hall meetings at which leaders respond to staff questions regarding practical matters they have encountered and future plans. Providers’ up-to-the-minute familiarity with policies and procedures enables all team members to uniformly and clearly communicate to patients and caregivers.
Dr. Crews emphasized the value of consistency and “over-communication” in projecting confidence and preparedness to patients and caregivers during an unsettling time. SCCA has developed fact sheets, posted current information on the SCCA website, and provided education during doorway screenings.
Screening and testing
All SCCA staff members are screened daily at the practice entrance so they have personal experience with the process utilized for patients. Because symptoms associated with coronavirus infection may overlap with cancer treatment–related complaints, SCCA clinicians have expanded the typical coronavirus screening questionnaire for patients on cancer treatment.
Patients with ambiguous symptoms are masked, taken to a physically separate area of the SCCA clinics, and screened further by an advanced practice provider. The patients are then triaged to either the clinic for treatment or to the emergency department for further triage and care.
Although testing processes and procedures have been modified, Dr. Crews advised codifying those policies and procedures, including notification of results and follow-up for both patients and staff. Dr. Crews also stressed the importance of clearly articulated return-to-work policies for staff who have potential exposure and/or positive test results.
At the University of Washington’s virology laboratory, they have a test turnaround time of less than 12 hours.
Planning ahead
Dr. Crews highlighted the importance of community-based surge planning, utilizing predictive models to assess inpatient capacity requirements and potential repurposing of providers.
The SCCA is prepared to close selected community sites and shift personnel to other locations if personnel needs cannot be met because of illness or quarantine. Contingency plans include specialized pharmacy services for patients requiring chemotherapy.
The SCCA has not yet experienced shortages of personal protective equipment (PPE). However, Dr. Crews said staff require detailed education regarding the use of PPE in order to safeguard the supply while providing maximal staff protection.
Helping the helpers
During the pandemic, SCCA has dealt with a variety of challenging human resource issues, including:
- Extending sick time beyond what was previously “stored” in staff members’ earned time off.
- Childcare during an extended hiatus in school and daycare schedules.
- Programs to maintain and/or restore employee wellness (including staff-centered support services, spiritual care, mindfulness exercises, and town halls).
Dr. Crews also discussed recruitment of community resources to provide meals for staff from local restaurants with restricted hours and transportation resources for staff and patients, as visitors are restricted (currently one per patient).
Managing care
Dr. Crews noted that the University of Washington had a foundational structure for a telehealth program prior to the pandemic. Their telehealth committee enabled SCCA to scale up the service quickly with their academic partners, including training modules for and certification of providers, outfitting off-site personnel with dedicated lines and hardware, and provision of personal Zoom accounts.
SCCA also devised algorithms for determining when face-to-face visits, remote management, or deferred visits are appropriate in various scenarios. The algorithms were developed by disease-specialized teams.
As a general rule, routine chemotherapy and radiation are administered on schedule. On-treatment and follow-up office visits are conducted via telehealth if possible. In some cases, initiation of chemotherapy and radiation has been delayed, and screening services have been suspended.
In response to questions about palliative care during the pandemic, Dr. Crews said SCCA has encouraged their patients to complete, review, or update their advance directives. The SCCA has not had the need to resuscitate a coronavirus-infected outpatient but has instituted policies for utilizing full PPE on any patient requiring resuscitation.
In her closing remarks, Dr. Crews stressed that the response to COVID-19 in Washington state has required an intense collaboration among colleagues, the community, and government leaders, as the actions required extended far beyond medical decision makers alone.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
No staff COVID-19 diagnoses after plan at Chinese cancer center
Short-term results
No staff members or patients were diagnosed with COVID-19 after “strict protective measures” for screening and managing patients were implemented at the National Cancer Center/Cancer Hospital, Chinese Academy of Sciences, in Beijing, according to a report published online April 1 in JAMA Oncology.
However, the time period for the analysis, which included nearly 3000 patients, was short — only about 3 weeks (February 12 to March 3). Also, Beijing is more than 1100 kilometers from Wuhan, the center of the Chinese outbreak of COVID-19.
The Beijing cancer hospital implemented a multipronged safety plan in February in order to “avoid COVID-19 related nosocomial cross-infection between patients and medical staff,” explain the authors, led by medical oncologist Zhijie Wang, MD.
Notably, “all of the measures taken in China are actively being implemented and used in major oncology centers in the United States,” Robert Carlson, MD, chief executive officer, National Comprehensive Cancer Network (NCCN), told Medscape Medical News.
John Greene, MD, section chief, Infectious Disease and Tropical Medicine, Moffitt Cancer Center, Tampa, Florida, pointed out that the Chinese safety plan, which is full of “good measures,” is being largely used at his center. However, he observed that one tool — doing a temperature check at the hospital front door — is not well supported by most of the literature. “It gives good optics and looks like you are doing the most you possibly can, but scientifically it may not be as effective [as other screening measures],” he said.
The Chinese plan consists of four broad elements
First, the above-mentioned on-site temperature tests are performed at the entrances of the hospital, outpatient clinic, and wards. Contact and travel histories related to the Wuhan epidemic area are also established and recorded.
Second, an outpatient appointment scheduling system allows both online scheduling and on-site registration. Online consultation channels are open daily, featuring instruction on medication taking and cancer-related symptom management. These “substantially reduced the flow of people in the hospital,” write the authors. On-site patients must wear a mask and have their own disinfectant.
Third, for patients with cancer preparing to be admitted to hospital, symptoms associated with COVID-19, such as fever and cough, are recorded. Mandatory blood tests and CT scans of the lungs are performed. COVID-19 virus nucleic acid tests are performed for patients with suspected pneumonia on imaging.
Fourth, some anticancer drugs conventionally administered by infusion have been changed to oral administration, such as etoposide and vinorelbine. For adjuvant or maintenance chemotherapy, the infusion intervals were appropriately prolonged depending on patients’ conditions.
Eight out of 2,900 patients had imaging suspicious for infection
The Chinese authors report that a total of 2,944 patients with cancer were seen for clinic consultation and treatment in the wards (2795 outpatients and 149 inpatients).
Patients with cancer are believed to have a higher probability of severe illness and increased mortality compared with the healthy population once infected with COVID-19, point out the authors.
Under the new “strict screening strategy,” 27 patients showed radiologic manifestations of inflammatory changes or multiple-site exudative pneumonia in the lungs, including eight suspected of having COVID-19 infection. “Fortunately, negative results from nucleic acid testing ultimately excluded COVID-19 infection in all these patients,” the authors report.
However, two of these patients “presented with recovered pneumonia after symptomatic treatment.” Commenting on this finding, Moffitt’s Greene said that may mean these two patients were tested and found to be positive but were early in the infection and not yet shedding the virus, or they were infected after the initial negative result.
Greene said his center has implemented some measures not mentioned in the Chinese plan. For example, the Florida center no longer allows inpatient visitation. Also, one third of staff now work from home, resulting in less social interaction. Social distancing in meetings, the cafeteria, and hallways is being observed “aggressively,” and most meetings are now on Zoom, he said.
Moffitt has not been hard hit with COVID-19 and is at level one preparedness, the lowest rung. The center has performed 60 tests to date, with only one positive for the virus (< 2%), Greene told Medscape Medical News.
Currently, in the larger Tampa Bay community setting, about 12% of tests are positive.
The low percentage found among the Moffitt patients “tells you that a lot of cancer patients have fever and respiratory symptoms due to other viruses and, more importantly, other reasons, whether it’s their immunotherapy or chemotherapy or their cancer,” said Greene.
NCCN’s Carlson said the publication of the Chinese data was a good sign in terms of international science.
“This is a strong example of how the global oncology community rapidly shares information and experience whenever it makes a difference in patient care,” he commented.
The authors, as well as Carlson and Greene, have reported no relevant financial relationships.
This article first appeared on Medscape.com.
Short-term results
Short-term results
No staff members or patients were diagnosed with COVID-19 after “strict protective measures” for screening and managing patients were implemented at the National Cancer Center/Cancer Hospital, Chinese Academy of Sciences, in Beijing, according to a report published online April 1 in JAMA Oncology.
However, the time period for the analysis, which included nearly 3000 patients, was short — only about 3 weeks (February 12 to March 3). Also, Beijing is more than 1100 kilometers from Wuhan, the center of the Chinese outbreak of COVID-19.
The Beijing cancer hospital implemented a multipronged safety plan in February in order to “avoid COVID-19 related nosocomial cross-infection between patients and medical staff,” explain the authors, led by medical oncologist Zhijie Wang, MD.
Notably, “all of the measures taken in China are actively being implemented and used in major oncology centers in the United States,” Robert Carlson, MD, chief executive officer, National Comprehensive Cancer Network (NCCN), told Medscape Medical News.
John Greene, MD, section chief, Infectious Disease and Tropical Medicine, Moffitt Cancer Center, Tampa, Florida, pointed out that the Chinese safety plan, which is full of “good measures,” is being largely used at his center. However, he observed that one tool — doing a temperature check at the hospital front door — is not well supported by most of the literature. “It gives good optics and looks like you are doing the most you possibly can, but scientifically it may not be as effective [as other screening measures],” he said.
The Chinese plan consists of four broad elements
First, the above-mentioned on-site temperature tests are performed at the entrances of the hospital, outpatient clinic, and wards. Contact and travel histories related to the Wuhan epidemic area are also established and recorded.
Second, an outpatient appointment scheduling system allows both online scheduling and on-site registration. Online consultation channels are open daily, featuring instruction on medication taking and cancer-related symptom management. These “substantially reduced the flow of people in the hospital,” write the authors. On-site patients must wear a mask and have their own disinfectant.
Third, for patients with cancer preparing to be admitted to hospital, symptoms associated with COVID-19, such as fever and cough, are recorded. Mandatory blood tests and CT scans of the lungs are performed. COVID-19 virus nucleic acid tests are performed for patients with suspected pneumonia on imaging.
Fourth, some anticancer drugs conventionally administered by infusion have been changed to oral administration, such as etoposide and vinorelbine. For adjuvant or maintenance chemotherapy, the infusion intervals were appropriately prolonged depending on patients’ conditions.
Eight out of 2,900 patients had imaging suspicious for infection
The Chinese authors report that a total of 2,944 patients with cancer were seen for clinic consultation and treatment in the wards (2795 outpatients and 149 inpatients).
Patients with cancer are believed to have a higher probability of severe illness and increased mortality compared with the healthy population once infected with COVID-19, point out the authors.
Under the new “strict screening strategy,” 27 patients showed radiologic manifestations of inflammatory changes or multiple-site exudative pneumonia in the lungs, including eight suspected of having COVID-19 infection. “Fortunately, negative results from nucleic acid testing ultimately excluded COVID-19 infection in all these patients,” the authors report.
However, two of these patients “presented with recovered pneumonia after symptomatic treatment.” Commenting on this finding, Moffitt’s Greene said that may mean these two patients were tested and found to be positive but were early in the infection and not yet shedding the virus, or they were infected after the initial negative result.
Greene said his center has implemented some measures not mentioned in the Chinese plan. For example, the Florida center no longer allows inpatient visitation. Also, one third of staff now work from home, resulting in less social interaction. Social distancing in meetings, the cafeteria, and hallways is being observed “aggressively,” and most meetings are now on Zoom, he said.
Moffitt has not been hard hit with COVID-19 and is at level one preparedness, the lowest rung. The center has performed 60 tests to date, with only one positive for the virus (< 2%), Greene told Medscape Medical News.
Currently, in the larger Tampa Bay community setting, about 12% of tests are positive.
The low percentage found among the Moffitt patients “tells you that a lot of cancer patients have fever and respiratory symptoms due to other viruses and, more importantly, other reasons, whether it’s their immunotherapy or chemotherapy or their cancer,” said Greene.
NCCN’s Carlson said the publication of the Chinese data was a good sign in terms of international science.
“This is a strong example of how the global oncology community rapidly shares information and experience whenever it makes a difference in patient care,” he commented.
The authors, as well as Carlson and Greene, have reported no relevant financial relationships.
This article first appeared on Medscape.com.
No staff members or patients were diagnosed with COVID-19 after “strict protective measures” for screening and managing patients were implemented at the National Cancer Center/Cancer Hospital, Chinese Academy of Sciences, in Beijing, according to a report published online April 1 in JAMA Oncology.
However, the time period for the analysis, which included nearly 3000 patients, was short — only about 3 weeks (February 12 to March 3). Also, Beijing is more than 1100 kilometers from Wuhan, the center of the Chinese outbreak of COVID-19.
The Beijing cancer hospital implemented a multipronged safety plan in February in order to “avoid COVID-19 related nosocomial cross-infection between patients and medical staff,” explain the authors, led by medical oncologist Zhijie Wang, MD.
Notably, “all of the measures taken in China are actively being implemented and used in major oncology centers in the United States,” Robert Carlson, MD, chief executive officer, National Comprehensive Cancer Network (NCCN), told Medscape Medical News.
John Greene, MD, section chief, Infectious Disease and Tropical Medicine, Moffitt Cancer Center, Tampa, Florida, pointed out that the Chinese safety plan, which is full of “good measures,” is being largely used at his center. However, he observed that one tool — doing a temperature check at the hospital front door — is not well supported by most of the literature. “It gives good optics and looks like you are doing the most you possibly can, but scientifically it may not be as effective [as other screening measures],” he said.
The Chinese plan consists of four broad elements
First, the above-mentioned on-site temperature tests are performed at the entrances of the hospital, outpatient clinic, and wards. Contact and travel histories related to the Wuhan epidemic area are also established and recorded.
Second, an outpatient appointment scheduling system allows both online scheduling and on-site registration. Online consultation channels are open daily, featuring instruction on medication taking and cancer-related symptom management. These “substantially reduced the flow of people in the hospital,” write the authors. On-site patients must wear a mask and have their own disinfectant.
Third, for patients with cancer preparing to be admitted to hospital, symptoms associated with COVID-19, such as fever and cough, are recorded. Mandatory blood tests and CT scans of the lungs are performed. COVID-19 virus nucleic acid tests are performed for patients with suspected pneumonia on imaging.
Fourth, some anticancer drugs conventionally administered by infusion have been changed to oral administration, such as etoposide and vinorelbine. For adjuvant or maintenance chemotherapy, the infusion intervals were appropriately prolonged depending on patients’ conditions.
Eight out of 2,900 patients had imaging suspicious for infection
The Chinese authors report that a total of 2,944 patients with cancer were seen for clinic consultation and treatment in the wards (2795 outpatients and 149 inpatients).
Patients with cancer are believed to have a higher probability of severe illness and increased mortality compared with the healthy population once infected with COVID-19, point out the authors.
Under the new “strict screening strategy,” 27 patients showed radiologic manifestations of inflammatory changes or multiple-site exudative pneumonia in the lungs, including eight suspected of having COVID-19 infection. “Fortunately, negative results from nucleic acid testing ultimately excluded COVID-19 infection in all these patients,” the authors report.
However, two of these patients “presented with recovered pneumonia after symptomatic treatment.” Commenting on this finding, Moffitt’s Greene said that may mean these two patients were tested and found to be positive but were early in the infection and not yet shedding the virus, or they were infected after the initial negative result.
Greene said his center has implemented some measures not mentioned in the Chinese plan. For example, the Florida center no longer allows inpatient visitation. Also, one third of staff now work from home, resulting in less social interaction. Social distancing in meetings, the cafeteria, and hallways is being observed “aggressively,” and most meetings are now on Zoom, he said.
Moffitt has not been hard hit with COVID-19 and is at level one preparedness, the lowest rung. The center has performed 60 tests to date, with only one positive for the virus (< 2%), Greene told Medscape Medical News.
Currently, in the larger Tampa Bay community setting, about 12% of tests are positive.
The low percentage found among the Moffitt patients “tells you that a lot of cancer patients have fever and respiratory symptoms due to other viruses and, more importantly, other reasons, whether it’s their immunotherapy or chemotherapy or their cancer,” said Greene.
NCCN’s Carlson said the publication of the Chinese data was a good sign in terms of international science.
“This is a strong example of how the global oncology community rapidly shares information and experience whenever it makes a difference in patient care,” he commented.
The authors, as well as Carlson and Greene, have reported no relevant financial relationships.
This article first appeared on Medscape.com.
Maintaining cancer care in the face of COVID-19
Medical oncologist Anne Chiang, MD, PhD, is scrambling to maintain cancer care in New Haven, Connecticut, while COVID-19 advances unrelentingly. As deputy chief medical officer of the Smilow Cancer Network, the largest cancer care delivery system in Connecticut and Rhode Island, she has no illusions about dodging what’s unfolding just 2 hours down the road in New York City.
“They’re trying their best to continue active cancer treatment but it’s getting harder,” she says of her colleagues in the thick of the pandemic. “We have to be prepared for it here.”
In anticipation of what’s coming, her team has just emptied the top three floors of the Smilow Cancer Hospital, moving 60 patients by ambulance and other medical transport to a different hospital nearby.
The move frees the Smilow Cancer hospital’s negative-pressure wards for the anticipated wave of COVID-19 patients. It will keep the virus sealed off from the rest of the hospital. But in other locations it’s harder to shield patients with cancer from the infection.
Around the state, Smilow Cancer Network’s affiliated hospitals are already treating a growing number of COVID-19 patients, especially at Greenwich Hospital, right on the border with New York state.
To protect patients with cancer, who are among the most vulnerable to the virus, oncologists are embracing telemedicine to allow most patients to stay home.
“We’re really concentrating on decreasing the risk to these patients, with a widespread massive-scale conversion to telehealth,” said Chiang. “This is something that, in the space of about a week, has transformed the care of our patients — it’s a really amazing transformation.”
If anything good comes out of the COVID-19 pandemic, it will be this global adoption of virtual healthcare.
Across the US border in Canada, the medical director of Toronto’s Princess Margaret Cancer Centre is directing a similar transformation.
“We have converted probably about 70% to 80% of our clinic visits to virtual visits,” says radiation oncologist Mary Gospodarowicz, MD.
“We have three priorities: number one, to keep our patients safe; number two, to keep our staff safe, because if staff are sick we won’t be treating anybody; and number three, to treat as many patients with cancer as possible.”
Gospodarowicz woke up last week to a local headline about a woman whose mastectomy had been canceled “because of the coronavirus.” The story exposed the many layers of the COVID-19 crisis. “A lot of hospitals have canceled elective surgeries,” she acknowledged. “For patients who have treatment or surgery deferred, we have a database and we’ll make sure we look after those patients eventually. We have a priority system, so low-risk prostate cancer, very low-risk breast cancer patients are waiting. All the urgent head and neck, breast, and other higher priority surgeries are still being done, but it just depends how it goes. The situation changes every day.”
It’s similar in Los Angeles, at the University of Southern California, says Elizabeth David, MD, a cardiothoracic surgeon with Keck Medicine.
“For thoracic, we just had a conference call with about 30 surgeons around the country going through really nitty-gritty specifics to help with our decision making about what could wait without detriment to the patient – hopefully – and what should be done now,” she told Medscape Medical News.
“There are some hospitals where they are not doing anything but life and death emergency operations, whereas we are still doing our emergent cancer operations in our institution, but we all know – and patients know – that could change from one day to the next. They may think they’re having surgery tomorrow but may get a call saying we can’t do it,” David said.
Many of David’s patients have non–small cell lung cancer, putting them at particular risk with a pulmonary infection like COVID-19. For now, she says delivery of postsurgical chemotherapy and radiotherapy has not been impacted in her area, but her videoconference discussions with patients are much longer – and harder – these days.
“I’ve been in practice a while now and I’ve had numerous conversations with patients this week that I never trained for, and I’ve never known anyone else who has. It’s really hard as a provider to know what to say,” she said.
In cardiothoracic surgery, David said guidance on clinical decision making is coming from the American College of Surgeons, Society of Thoracic Surgery, and American Association of Thoracic Surgeons. Yet, she says each patient is being assessed – and reassessed – individually.
“You have to balance the risk of delaying the intervention with supply issues, hospital exposure issues, the danger to the patient of being in the hospital environment – there’s just so many factors. We’re spending so much time talking through cases, and a lot of times we’re talking about cases we already talked about, but we’re just making sure that based on today’s numbers we should still be moving forward,” she commented.
In Connecticut, Chiang said treatment decisions are also mostly on a case-by-case basis at the moment, although more standardized guidelines are being worked out.
“Our disease teams have been really proactive in terms of offering alternative solutions to patients, creative ways to basically keep them out of the hospital and also reduce the immunosuppressive regimens that we give them,” she said.
Examples include offering endocrine therapy to patients who can’t get breast cancer surgery, or offering alternative drug regimens and dosing schedules. “At this point we haven’t needed to ration actual treatment – patients are continuing to get active therapy if that’s appropriate – it’s more about how can we protect them,” she said. “It’s a complex puzzle of moving pieces.”
In Toronto, Gospodarowicz says newly published medical and radiation oncology guidelines from France are the backbone of her hospital’s policy discussions about treating cancer and protecting patients from COVID-19.
While patients’ concerns are understandable, she says even in the current hot spots of infection, it’s encouraging to know that cancer patients are not being forgotten.
“I recently had email communication with a radiation oncologist in Brescia, one of the worst-affected areas in Italy, and he told me the radiotherapy department has been 60% to 70% capacity, so they still treat 70% these patients, just taking precautions and separating the COVID-positive and negative ones. When we read the stats it looks horrible, but life still goes on and people are still being treated,” she said.
Although telemedicine offers meaningful solutions to the COVID-19 crisis in North America, it may not be possible in other parts of the world.
Web consultations were only just approved in Brazil this week. “We are still discussing how to make it official and reimbursed,” says Rachel Riechelmann, MD, head of clinical oncology at AC Camargo Cancer Center in São Paulo.
To minimize infection risk for patients, Riechelmann says her hospital is doing the following: postponing surgeries in cases where there is good evidence of neoadjuvant treatment, such as total neoadjuvant therapy for rectal cancer; avoiding adjuvant chemo for stage 2 colon cancer; moving to hypofractionated radiotherapy if possible; adopting watchful waiting in grade 1 nonfunctional neuroendocrine tumors; and postponing follow-up visits.
“We do our best,” she wrote in an email. “We keep treating cancer if treatment cannot wait.”
Riechelmann’s center has just launched a trial of hydroxychloroquine and tocilizumab therapy in patients with cancer who have severe COVID-19 and acute respiratory distress syndrome (ARDS).
Meanwhile in New Haven, Chiang says for patients with cancer who are infected with COVID-19, her team is also prognosticating about the fair allocation of limited resources such as ventilators.
“If it ever gets to the point where somebody has to choose between a cancer patient and a noncancer patient in providing life support, it’s really important that people understand that cancer patients are doing very well nowadays and even with a diagnosis of cancer they can potentially live for many years, so that shouldn’t necessarily be a decision-point,” she emphasized.
This article first appeared on Medscape.com.
Medical oncologist Anne Chiang, MD, PhD, is scrambling to maintain cancer care in New Haven, Connecticut, while COVID-19 advances unrelentingly. As deputy chief medical officer of the Smilow Cancer Network, the largest cancer care delivery system in Connecticut and Rhode Island, she has no illusions about dodging what’s unfolding just 2 hours down the road in New York City.
“They’re trying their best to continue active cancer treatment but it’s getting harder,” she says of her colleagues in the thick of the pandemic. “We have to be prepared for it here.”
In anticipation of what’s coming, her team has just emptied the top three floors of the Smilow Cancer Hospital, moving 60 patients by ambulance and other medical transport to a different hospital nearby.
The move frees the Smilow Cancer hospital’s negative-pressure wards for the anticipated wave of COVID-19 patients. It will keep the virus sealed off from the rest of the hospital. But in other locations it’s harder to shield patients with cancer from the infection.
Around the state, Smilow Cancer Network’s affiliated hospitals are already treating a growing number of COVID-19 patients, especially at Greenwich Hospital, right on the border with New York state.
To protect patients with cancer, who are among the most vulnerable to the virus, oncologists are embracing telemedicine to allow most patients to stay home.
“We’re really concentrating on decreasing the risk to these patients, with a widespread massive-scale conversion to telehealth,” said Chiang. “This is something that, in the space of about a week, has transformed the care of our patients — it’s a really amazing transformation.”
If anything good comes out of the COVID-19 pandemic, it will be this global adoption of virtual healthcare.
Across the US border in Canada, the medical director of Toronto’s Princess Margaret Cancer Centre is directing a similar transformation.
“We have converted probably about 70% to 80% of our clinic visits to virtual visits,” says radiation oncologist Mary Gospodarowicz, MD.
“We have three priorities: number one, to keep our patients safe; number two, to keep our staff safe, because if staff are sick we won’t be treating anybody; and number three, to treat as many patients with cancer as possible.”
Gospodarowicz woke up last week to a local headline about a woman whose mastectomy had been canceled “because of the coronavirus.” The story exposed the many layers of the COVID-19 crisis. “A lot of hospitals have canceled elective surgeries,” she acknowledged. “For patients who have treatment or surgery deferred, we have a database and we’ll make sure we look after those patients eventually. We have a priority system, so low-risk prostate cancer, very low-risk breast cancer patients are waiting. All the urgent head and neck, breast, and other higher priority surgeries are still being done, but it just depends how it goes. The situation changes every day.”
It’s similar in Los Angeles, at the University of Southern California, says Elizabeth David, MD, a cardiothoracic surgeon with Keck Medicine.
“For thoracic, we just had a conference call with about 30 surgeons around the country going through really nitty-gritty specifics to help with our decision making about what could wait without detriment to the patient – hopefully – and what should be done now,” she told Medscape Medical News.
“There are some hospitals where they are not doing anything but life and death emergency operations, whereas we are still doing our emergent cancer operations in our institution, but we all know – and patients know – that could change from one day to the next. They may think they’re having surgery tomorrow but may get a call saying we can’t do it,” David said.
Many of David’s patients have non–small cell lung cancer, putting them at particular risk with a pulmonary infection like COVID-19. For now, she says delivery of postsurgical chemotherapy and radiotherapy has not been impacted in her area, but her videoconference discussions with patients are much longer – and harder – these days.
“I’ve been in practice a while now and I’ve had numerous conversations with patients this week that I never trained for, and I’ve never known anyone else who has. It’s really hard as a provider to know what to say,” she said.
In cardiothoracic surgery, David said guidance on clinical decision making is coming from the American College of Surgeons, Society of Thoracic Surgery, and American Association of Thoracic Surgeons. Yet, she says each patient is being assessed – and reassessed – individually.
“You have to balance the risk of delaying the intervention with supply issues, hospital exposure issues, the danger to the patient of being in the hospital environment – there’s just so many factors. We’re spending so much time talking through cases, and a lot of times we’re talking about cases we already talked about, but we’re just making sure that based on today’s numbers we should still be moving forward,” she commented.
In Connecticut, Chiang said treatment decisions are also mostly on a case-by-case basis at the moment, although more standardized guidelines are being worked out.
“Our disease teams have been really proactive in terms of offering alternative solutions to patients, creative ways to basically keep them out of the hospital and also reduce the immunosuppressive regimens that we give them,” she said.
Examples include offering endocrine therapy to patients who can’t get breast cancer surgery, or offering alternative drug regimens and dosing schedules. “At this point we haven’t needed to ration actual treatment – patients are continuing to get active therapy if that’s appropriate – it’s more about how can we protect them,” she said. “It’s a complex puzzle of moving pieces.”
In Toronto, Gospodarowicz says newly published medical and radiation oncology guidelines from France are the backbone of her hospital’s policy discussions about treating cancer and protecting patients from COVID-19.
While patients’ concerns are understandable, she says even in the current hot spots of infection, it’s encouraging to know that cancer patients are not being forgotten.
“I recently had email communication with a radiation oncologist in Brescia, one of the worst-affected areas in Italy, and he told me the radiotherapy department has been 60% to 70% capacity, so they still treat 70% these patients, just taking precautions and separating the COVID-positive and negative ones. When we read the stats it looks horrible, but life still goes on and people are still being treated,” she said.
Although telemedicine offers meaningful solutions to the COVID-19 crisis in North America, it may not be possible in other parts of the world.
Web consultations were only just approved in Brazil this week. “We are still discussing how to make it official and reimbursed,” says Rachel Riechelmann, MD, head of clinical oncology at AC Camargo Cancer Center in São Paulo.
To minimize infection risk for patients, Riechelmann says her hospital is doing the following: postponing surgeries in cases where there is good evidence of neoadjuvant treatment, such as total neoadjuvant therapy for rectal cancer; avoiding adjuvant chemo for stage 2 colon cancer; moving to hypofractionated radiotherapy if possible; adopting watchful waiting in grade 1 nonfunctional neuroendocrine tumors; and postponing follow-up visits.
“We do our best,” she wrote in an email. “We keep treating cancer if treatment cannot wait.”
Riechelmann’s center has just launched a trial of hydroxychloroquine and tocilizumab therapy in patients with cancer who have severe COVID-19 and acute respiratory distress syndrome (ARDS).
Meanwhile in New Haven, Chiang says for patients with cancer who are infected with COVID-19, her team is also prognosticating about the fair allocation of limited resources such as ventilators.
“If it ever gets to the point where somebody has to choose between a cancer patient and a noncancer patient in providing life support, it’s really important that people understand that cancer patients are doing very well nowadays and even with a diagnosis of cancer they can potentially live for many years, so that shouldn’t necessarily be a decision-point,” she emphasized.
This article first appeared on Medscape.com.
Medical oncologist Anne Chiang, MD, PhD, is scrambling to maintain cancer care in New Haven, Connecticut, while COVID-19 advances unrelentingly. As deputy chief medical officer of the Smilow Cancer Network, the largest cancer care delivery system in Connecticut and Rhode Island, she has no illusions about dodging what’s unfolding just 2 hours down the road in New York City.
“They’re trying their best to continue active cancer treatment but it’s getting harder,” she says of her colleagues in the thick of the pandemic. “We have to be prepared for it here.”
In anticipation of what’s coming, her team has just emptied the top three floors of the Smilow Cancer Hospital, moving 60 patients by ambulance and other medical transport to a different hospital nearby.
The move frees the Smilow Cancer hospital’s negative-pressure wards for the anticipated wave of COVID-19 patients. It will keep the virus sealed off from the rest of the hospital. But in other locations it’s harder to shield patients with cancer from the infection.
Around the state, Smilow Cancer Network’s affiliated hospitals are already treating a growing number of COVID-19 patients, especially at Greenwich Hospital, right on the border with New York state.
To protect patients with cancer, who are among the most vulnerable to the virus, oncologists are embracing telemedicine to allow most patients to stay home.
“We’re really concentrating on decreasing the risk to these patients, with a widespread massive-scale conversion to telehealth,” said Chiang. “This is something that, in the space of about a week, has transformed the care of our patients — it’s a really amazing transformation.”
If anything good comes out of the COVID-19 pandemic, it will be this global adoption of virtual healthcare.
Across the US border in Canada, the medical director of Toronto’s Princess Margaret Cancer Centre is directing a similar transformation.
“We have converted probably about 70% to 80% of our clinic visits to virtual visits,” says radiation oncologist Mary Gospodarowicz, MD.
“We have three priorities: number one, to keep our patients safe; number two, to keep our staff safe, because if staff are sick we won’t be treating anybody; and number three, to treat as many patients with cancer as possible.”
Gospodarowicz woke up last week to a local headline about a woman whose mastectomy had been canceled “because of the coronavirus.” The story exposed the many layers of the COVID-19 crisis. “A lot of hospitals have canceled elective surgeries,” she acknowledged. “For patients who have treatment or surgery deferred, we have a database and we’ll make sure we look after those patients eventually. We have a priority system, so low-risk prostate cancer, very low-risk breast cancer patients are waiting. All the urgent head and neck, breast, and other higher priority surgeries are still being done, but it just depends how it goes. The situation changes every day.”
It’s similar in Los Angeles, at the University of Southern California, says Elizabeth David, MD, a cardiothoracic surgeon with Keck Medicine.
“For thoracic, we just had a conference call with about 30 surgeons around the country going through really nitty-gritty specifics to help with our decision making about what could wait without detriment to the patient – hopefully – and what should be done now,” she told Medscape Medical News.
“There are some hospitals where they are not doing anything but life and death emergency operations, whereas we are still doing our emergent cancer operations in our institution, but we all know – and patients know – that could change from one day to the next. They may think they’re having surgery tomorrow but may get a call saying we can’t do it,” David said.
Many of David’s patients have non–small cell lung cancer, putting them at particular risk with a pulmonary infection like COVID-19. For now, she says delivery of postsurgical chemotherapy and radiotherapy has not been impacted in her area, but her videoconference discussions with patients are much longer – and harder – these days.
“I’ve been in practice a while now and I’ve had numerous conversations with patients this week that I never trained for, and I’ve never known anyone else who has. It’s really hard as a provider to know what to say,” she said.
In cardiothoracic surgery, David said guidance on clinical decision making is coming from the American College of Surgeons, Society of Thoracic Surgery, and American Association of Thoracic Surgeons. Yet, she says each patient is being assessed – and reassessed – individually.
“You have to balance the risk of delaying the intervention with supply issues, hospital exposure issues, the danger to the patient of being in the hospital environment – there’s just so many factors. We’re spending so much time talking through cases, and a lot of times we’re talking about cases we already talked about, but we’re just making sure that based on today’s numbers we should still be moving forward,” she commented.
In Connecticut, Chiang said treatment decisions are also mostly on a case-by-case basis at the moment, although more standardized guidelines are being worked out.
“Our disease teams have been really proactive in terms of offering alternative solutions to patients, creative ways to basically keep them out of the hospital and also reduce the immunosuppressive regimens that we give them,” she said.
Examples include offering endocrine therapy to patients who can’t get breast cancer surgery, or offering alternative drug regimens and dosing schedules. “At this point we haven’t needed to ration actual treatment – patients are continuing to get active therapy if that’s appropriate – it’s more about how can we protect them,” she said. “It’s a complex puzzle of moving pieces.”
In Toronto, Gospodarowicz says newly published medical and radiation oncology guidelines from France are the backbone of her hospital’s policy discussions about treating cancer and protecting patients from COVID-19.
While patients’ concerns are understandable, she says even in the current hot spots of infection, it’s encouraging to know that cancer patients are not being forgotten.
“I recently had email communication with a radiation oncologist in Brescia, one of the worst-affected areas in Italy, and he told me the radiotherapy department has been 60% to 70% capacity, so they still treat 70% these patients, just taking precautions and separating the COVID-positive and negative ones. When we read the stats it looks horrible, but life still goes on and people are still being treated,” she said.
Although telemedicine offers meaningful solutions to the COVID-19 crisis in North America, it may not be possible in other parts of the world.
Web consultations were only just approved in Brazil this week. “We are still discussing how to make it official and reimbursed,” says Rachel Riechelmann, MD, head of clinical oncology at AC Camargo Cancer Center in São Paulo.
To minimize infection risk for patients, Riechelmann says her hospital is doing the following: postponing surgeries in cases where there is good evidence of neoadjuvant treatment, such as total neoadjuvant therapy for rectal cancer; avoiding adjuvant chemo for stage 2 colon cancer; moving to hypofractionated radiotherapy if possible; adopting watchful waiting in grade 1 nonfunctional neuroendocrine tumors; and postponing follow-up visits.
“We do our best,” she wrote in an email. “We keep treating cancer if treatment cannot wait.”
Riechelmann’s center has just launched a trial of hydroxychloroquine and tocilizumab therapy in patients with cancer who have severe COVID-19 and acute respiratory distress syndrome (ARDS).
Meanwhile in New Haven, Chiang says for patients with cancer who are infected with COVID-19, her team is also prognosticating about the fair allocation of limited resources such as ventilators.
“If it ever gets to the point where somebody has to choose between a cancer patient and a noncancer patient in providing life support, it’s really important that people understand that cancer patients are doing very well nowadays and even with a diagnosis of cancer they can potentially live for many years, so that shouldn’t necessarily be a decision-point,” she emphasized.
This article first appeared on Medscape.com.
Firings, furloughs, and pay cuts in advance of COVID-19 surge
Doctors at a Boston-area hospital learned via video conferencing that they would be receiving a 20% pay cut – a slap in the face at the precise moment that those on the front lines of the COVID-19 pandemic need a pat on the back (and more N95 respirators).
But Steward Health Care System*, which runs the hospital and dozens of others around the country, did the math and decided that the pay cuts were necessary to survive what they called “a seismic shock to our system.” They also announced furloughs for a large number of their nonclinical staff.
Spirits sank after the announcement. “It was devastating,” said one Boston doctor, who works for Steward and asked not to be identified for fear of retribution. “I didn’t say much during the call because I was so panicked, and I didn’t want to be crying on the call.”
Someone else did speak up, a senior colleague who warned that such a cut would kill morale at a time when physicians were already feeling vulnerable because of other shortages, including personal protective equipment. (Requests for interviews with Steward Health Care System executives were declined.)
Furloughs, layoffs, and even firings are happening elsewhere too. Hospitals in virus hotspots have already come up short on beds and face masks. Now a shortage of cash is prompting many to fire some of their health care workers, furlough them temporarily, or – like Steward Health Care System – slash their pay checks.
Despite almost $200 billion earmarked for hospital systems in the recently passed federal stimulus package, many hospitals are still in dire financial straits. Most make the majority of their money through so-called elective procedures, such as knee replacements and cataract surgeries, almost all of which have been postponed in order to conserve personal protective equipment and minimize spread of the virus. Those cancellations translate to a significant financial hit.
On top of that, hospitals will lose an average of $1,800 on every COVID-19 case, according to projections by Strata Decision Technology, a health care financial planning and analytic company. Some, they estimate, may lose much more, between $6,000 and $8,000 per patient. And hospitals were already hurting. According to a report from Bloomberg, at least 30 hospitals entered bankruptcy in 2019.
“This pressure on institutions to control costs has been around for several years,” said Steve Lefar, executive director of the data science division of Strata Decision Technology and lead author of the study. “This is just making it incredibly acute for them.”
Many hospital executives are bracing for months of hardship, leading to wrenching decisions to furlough or lay off staff, suspend bonuses, or cut pay – even as some short-staffed hospitals in COVID-19 hotspots are issuing pleas for doctors to come out of retirement.
Forward thinking?
While most furloughs and layoffs so far have affected people who don’t work directly with patients, many on the front lines have been hit with pay cuts or withheld bonuses or retirement contributions. In Massachusetts, the state’s medical society has asked Governor Charlie Baker for financial relief for health care workers in the form of grants, no-interest or forgivable small-business loans for physician practices, and deferment of medical student loan payments.
At St. Alexius Hospital in St. Louis, Sonny Saggar, MD, was fired as CEO after he clashed with a bankruptcy trustee. Dr. Saggar had proposed offering open beds to other hospital systems during the pandemic – an idea that, he said, was turned down out of concern for the bottom line.
“This is one of those times where we need to put down our search for profit and money and just look after people’s lives. We’re supposed to have that calling in health care,” said Dr. Saggar, who has since been reinstated as chief strategy officer and director of the COVID task force and ED. He noted that he and the trustee have resolved differences over funding.
At St. Claire HealthCare in Morehead, Ky., 300 employees who were not involved in direct patient care – a quarter of the hospital’s staff – have been furloughed, something Donald Lloyd II, St. Claire HealthCare’s CEO as of May 1, described as forward thinking.
To prepare for the influx of COVID-19 patients, the hospital shut down elective procedures early. “Prudence dictates the need to be extremely proactive,” Mr. Lloyd said. “We need to devote our limited resources to frontline clinical teams.”
Other hospitals are making similar moves, although many are not doing so publicly. Mr. Lloyd decided to put out a press release because he found it offensive that the federal government was “bailing out airlines and cruise lines before our frontline men and women caring for patients.”
Massachusetts-based Atrius Health, for instance, placed many staffers on a 1-month furlough, while simultaneously withholding a percentage of working physicians’ paychecks, saying that they plan to pay them back at a later date. TriHealth, in Cincinnati, looked elsewhere for ways to save money. Instead of cutting physician salaries, 11 executives took a 20% pay cut.
There are both better and worse ways to go about such staff reductions, according to Mr. Lefar. If reductions have to be made, it would be best if CEOs keep cuts as far away as possible from the front lines of patient care.
“My bias is to start with pay reductions for high-paid executives, then furloughs, and beyond that layoffs,” he said. (Furloughs allow employees to be brought back and receive unemployment benefits while not working.) “Anyone related to patient care – these are the people who are getting the country through this, these are the heroes.”
After the pandemic
Large hospital systems that can designate separate buildings for COVID-19 care may fare best financially, Mr. Lefar said. By retaining a clean, noninfectious facility, such setups could allow for an earlier return to regular procedures – as long as rapid COVID-19 testing becomes available.
Smaller hospitals, nearly half of which run at a financial loss, according to the Chartis Center for Rural Health, face the additional burdens of both limited capacity and a limited ability to separate COVID-19 care.
Mostly, Mr. Lefar said, it’s a matter of doing whatever is necessary to get through the worst of it. “A lot of what is deemed elective or scheduled will come back,” he said. “Right now it’s crisis mode. ... I think it’s going to be a rough 6-9 months, but we will get back to it.”
*Correction, 4/7/20: An earlier version of this article misstated the name of a hospital in the Boston area run by Steward Health Care System.
A version of this article originally appeared on Medscape.com.
Doctors at a Boston-area hospital learned via video conferencing that they would be receiving a 20% pay cut – a slap in the face at the precise moment that those on the front lines of the COVID-19 pandemic need a pat on the back (and more N95 respirators).
But Steward Health Care System*, which runs the hospital and dozens of others around the country, did the math and decided that the pay cuts were necessary to survive what they called “a seismic shock to our system.” They also announced furloughs for a large number of their nonclinical staff.
Spirits sank after the announcement. “It was devastating,” said one Boston doctor, who works for Steward and asked not to be identified for fear of retribution. “I didn’t say much during the call because I was so panicked, and I didn’t want to be crying on the call.”
Someone else did speak up, a senior colleague who warned that such a cut would kill morale at a time when physicians were already feeling vulnerable because of other shortages, including personal protective equipment. (Requests for interviews with Steward Health Care System executives were declined.)
Furloughs, layoffs, and even firings are happening elsewhere too. Hospitals in virus hotspots have already come up short on beds and face masks. Now a shortage of cash is prompting many to fire some of their health care workers, furlough them temporarily, or – like Steward Health Care System – slash their pay checks.
Despite almost $200 billion earmarked for hospital systems in the recently passed federal stimulus package, many hospitals are still in dire financial straits. Most make the majority of their money through so-called elective procedures, such as knee replacements and cataract surgeries, almost all of which have been postponed in order to conserve personal protective equipment and minimize spread of the virus. Those cancellations translate to a significant financial hit.
On top of that, hospitals will lose an average of $1,800 on every COVID-19 case, according to projections by Strata Decision Technology, a health care financial planning and analytic company. Some, they estimate, may lose much more, between $6,000 and $8,000 per patient. And hospitals were already hurting. According to a report from Bloomberg, at least 30 hospitals entered bankruptcy in 2019.
“This pressure on institutions to control costs has been around for several years,” said Steve Lefar, executive director of the data science division of Strata Decision Technology and lead author of the study. “This is just making it incredibly acute for them.”
Many hospital executives are bracing for months of hardship, leading to wrenching decisions to furlough or lay off staff, suspend bonuses, or cut pay – even as some short-staffed hospitals in COVID-19 hotspots are issuing pleas for doctors to come out of retirement.
Forward thinking?
While most furloughs and layoffs so far have affected people who don’t work directly with patients, many on the front lines have been hit with pay cuts or withheld bonuses or retirement contributions. In Massachusetts, the state’s medical society has asked Governor Charlie Baker for financial relief for health care workers in the form of grants, no-interest or forgivable small-business loans for physician practices, and deferment of medical student loan payments.
At St. Alexius Hospital in St. Louis, Sonny Saggar, MD, was fired as CEO after he clashed with a bankruptcy trustee. Dr. Saggar had proposed offering open beds to other hospital systems during the pandemic – an idea that, he said, was turned down out of concern for the bottom line.
“This is one of those times where we need to put down our search for profit and money and just look after people’s lives. We’re supposed to have that calling in health care,” said Dr. Saggar, who has since been reinstated as chief strategy officer and director of the COVID task force and ED. He noted that he and the trustee have resolved differences over funding.
At St. Claire HealthCare in Morehead, Ky., 300 employees who were not involved in direct patient care – a quarter of the hospital’s staff – have been furloughed, something Donald Lloyd II, St. Claire HealthCare’s CEO as of May 1, described as forward thinking.
To prepare for the influx of COVID-19 patients, the hospital shut down elective procedures early. “Prudence dictates the need to be extremely proactive,” Mr. Lloyd said. “We need to devote our limited resources to frontline clinical teams.”
Other hospitals are making similar moves, although many are not doing so publicly. Mr. Lloyd decided to put out a press release because he found it offensive that the federal government was “bailing out airlines and cruise lines before our frontline men and women caring for patients.”
Massachusetts-based Atrius Health, for instance, placed many staffers on a 1-month furlough, while simultaneously withholding a percentage of working physicians’ paychecks, saying that they plan to pay them back at a later date. TriHealth, in Cincinnati, looked elsewhere for ways to save money. Instead of cutting physician salaries, 11 executives took a 20% pay cut.
There are both better and worse ways to go about such staff reductions, according to Mr. Lefar. If reductions have to be made, it would be best if CEOs keep cuts as far away as possible from the front lines of patient care.
“My bias is to start with pay reductions for high-paid executives, then furloughs, and beyond that layoffs,” he said. (Furloughs allow employees to be brought back and receive unemployment benefits while not working.) “Anyone related to patient care – these are the people who are getting the country through this, these are the heroes.”
After the pandemic
Large hospital systems that can designate separate buildings for COVID-19 care may fare best financially, Mr. Lefar said. By retaining a clean, noninfectious facility, such setups could allow for an earlier return to regular procedures – as long as rapid COVID-19 testing becomes available.
Smaller hospitals, nearly half of which run at a financial loss, according to the Chartis Center for Rural Health, face the additional burdens of both limited capacity and a limited ability to separate COVID-19 care.
Mostly, Mr. Lefar said, it’s a matter of doing whatever is necessary to get through the worst of it. “A lot of what is deemed elective or scheduled will come back,” he said. “Right now it’s crisis mode. ... I think it’s going to be a rough 6-9 months, but we will get back to it.”
*Correction, 4/7/20: An earlier version of this article misstated the name of a hospital in the Boston area run by Steward Health Care System.
A version of this article originally appeared on Medscape.com.
Doctors at a Boston-area hospital learned via video conferencing that they would be receiving a 20% pay cut – a slap in the face at the precise moment that those on the front lines of the COVID-19 pandemic need a pat on the back (and more N95 respirators).
But Steward Health Care System*, which runs the hospital and dozens of others around the country, did the math and decided that the pay cuts were necessary to survive what they called “a seismic shock to our system.” They also announced furloughs for a large number of their nonclinical staff.
Spirits sank after the announcement. “It was devastating,” said one Boston doctor, who works for Steward and asked not to be identified for fear of retribution. “I didn’t say much during the call because I was so panicked, and I didn’t want to be crying on the call.”
Someone else did speak up, a senior colleague who warned that such a cut would kill morale at a time when physicians were already feeling vulnerable because of other shortages, including personal protective equipment. (Requests for interviews with Steward Health Care System executives were declined.)
Furloughs, layoffs, and even firings are happening elsewhere too. Hospitals in virus hotspots have already come up short on beds and face masks. Now a shortage of cash is prompting many to fire some of their health care workers, furlough them temporarily, or – like Steward Health Care System – slash their pay checks.
Despite almost $200 billion earmarked for hospital systems in the recently passed federal stimulus package, many hospitals are still in dire financial straits. Most make the majority of their money through so-called elective procedures, such as knee replacements and cataract surgeries, almost all of which have been postponed in order to conserve personal protective equipment and minimize spread of the virus. Those cancellations translate to a significant financial hit.
On top of that, hospitals will lose an average of $1,800 on every COVID-19 case, according to projections by Strata Decision Technology, a health care financial planning and analytic company. Some, they estimate, may lose much more, between $6,000 and $8,000 per patient. And hospitals were already hurting. According to a report from Bloomberg, at least 30 hospitals entered bankruptcy in 2019.
“This pressure on institutions to control costs has been around for several years,” said Steve Lefar, executive director of the data science division of Strata Decision Technology and lead author of the study. “This is just making it incredibly acute for them.”
Many hospital executives are bracing for months of hardship, leading to wrenching decisions to furlough or lay off staff, suspend bonuses, or cut pay – even as some short-staffed hospitals in COVID-19 hotspots are issuing pleas for doctors to come out of retirement.
Forward thinking?
While most furloughs and layoffs so far have affected people who don’t work directly with patients, many on the front lines have been hit with pay cuts or withheld bonuses or retirement contributions. In Massachusetts, the state’s medical society has asked Governor Charlie Baker for financial relief for health care workers in the form of grants, no-interest or forgivable small-business loans for physician practices, and deferment of medical student loan payments.
At St. Alexius Hospital in St. Louis, Sonny Saggar, MD, was fired as CEO after he clashed with a bankruptcy trustee. Dr. Saggar had proposed offering open beds to other hospital systems during the pandemic – an idea that, he said, was turned down out of concern for the bottom line.
“This is one of those times where we need to put down our search for profit and money and just look after people’s lives. We’re supposed to have that calling in health care,” said Dr. Saggar, who has since been reinstated as chief strategy officer and director of the COVID task force and ED. He noted that he and the trustee have resolved differences over funding.
At St. Claire HealthCare in Morehead, Ky., 300 employees who were not involved in direct patient care – a quarter of the hospital’s staff – have been furloughed, something Donald Lloyd II, St. Claire HealthCare’s CEO as of May 1, described as forward thinking.
To prepare for the influx of COVID-19 patients, the hospital shut down elective procedures early. “Prudence dictates the need to be extremely proactive,” Mr. Lloyd said. “We need to devote our limited resources to frontline clinical teams.”
Other hospitals are making similar moves, although many are not doing so publicly. Mr. Lloyd decided to put out a press release because he found it offensive that the federal government was “bailing out airlines and cruise lines before our frontline men and women caring for patients.”
Massachusetts-based Atrius Health, for instance, placed many staffers on a 1-month furlough, while simultaneously withholding a percentage of working physicians’ paychecks, saying that they plan to pay them back at a later date. TriHealth, in Cincinnati, looked elsewhere for ways to save money. Instead of cutting physician salaries, 11 executives took a 20% pay cut.
There are both better and worse ways to go about such staff reductions, according to Mr. Lefar. If reductions have to be made, it would be best if CEOs keep cuts as far away as possible from the front lines of patient care.
“My bias is to start with pay reductions for high-paid executives, then furloughs, and beyond that layoffs,” he said. (Furloughs allow employees to be brought back and receive unemployment benefits while not working.) “Anyone related to patient care – these are the people who are getting the country through this, these are the heroes.”
After the pandemic
Large hospital systems that can designate separate buildings for COVID-19 care may fare best financially, Mr. Lefar said. By retaining a clean, noninfectious facility, such setups could allow for an earlier return to regular procedures – as long as rapid COVID-19 testing becomes available.
Smaller hospitals, nearly half of which run at a financial loss, according to the Chartis Center for Rural Health, face the additional burdens of both limited capacity and a limited ability to separate COVID-19 care.
Mostly, Mr. Lefar said, it’s a matter of doing whatever is necessary to get through the worst of it. “A lot of what is deemed elective or scheduled will come back,” he said. “Right now it’s crisis mode. ... I think it’s going to be a rough 6-9 months, but we will get back to it.”
*Correction, 4/7/20: An earlier version of this article misstated the name of a hospital in the Boston area run by Steward Health Care System.
A version of this article originally appeared on Medscape.com.
U.S. lifts visa halt to boost COVID-19 physician workforce
New information from the US State Department indicates that it is lifting the suspension on visas for foreign-trained medical professionals, a move that has promise for boosting the US physician workforce battling COVID-19.
The move may also help physicians extend their visas.
The communication late last week follows a March 18 announcement that, because of COVID-19, the United States was suspending routine processing of immigrant and nonimmigrant visas, including the J and H visas, at embassies and consulates worldwide.
As reported by Medscape Medical News, the Educational Commission for Foreign Medical Graduates (ECFMG) appealed to the State Department to lift the suspension, noting that 4222 graduates of medical schools outside the United States who had matched into residencies in the United States and were ready to start on July 1 would not get the visas most of them need to begin training.
The State Department lifted the suspensions and issued this update:
“We encourage medical professionals with an approved US non-immigrant or immigrant visa petition (I-129, I-140, or similar) or a certificate of eligibility in an approved exchange visitor program (DS-2019), particularly those working to treat or mitigate the effects of COVID-19, to review the website of their nearest embassy or consulate for procedures to request a visa appointment.”
The State Department also issued guidance for foreign medical professionals already in the United States:
“J-1 Alien Physicians (medical residents) may consult with their program sponsor, ECFMG, to extend their programs in the United States. Generally, a J-1 program for a foreign medical resident can be extended one year at a time for up to seven years.
“Note that the expiration date on a US visa does not determine how long one can be in the United States. The way to confirm one’s required departure date is here : https://i94.cbp.dhs.gov/I94/#/home.
“Those who need to extend their stay or adjust their visa status must apply with USCIS (US Citizenship and Immigration Services).”
Complications Still Exist
ECFMG’s CEO, William W. Pinsky, MD, told Medscape Medical News that, although they welcomed the news from the State Department, there are still unanswered questions.
ECFMG explained that J-1 visas are currently granted only 30 days before the residency program begins.
However, travel to the United States may still be difficult in June, Pinsky said, and physicians may need to be quarantined for 2 weeks upon arrival.
“We’re still having some discussion with the Department of State on whether that regulation could be relaxed and they could come in earlier,” he said.
He cautioned that even after a J-1 visa application is made, the physician’s home country has to endorse the application.
Pinsky said he did not yet know whether that would be a problem.
He also said that, in response to New York’s plea for more healthcare workers, ECFMG is offering to verify education and licensing credentials for physicians educated outside the United States at no cost.
Individual hospitals and regulatory authorities can decide whether there may be roles in some capacity for physicians who have graduated from medical school, even if they have not completed residency or have not been licensed, he said.
This article first appeared on Medscape.com.
New information from the US State Department indicates that it is lifting the suspension on visas for foreign-trained medical professionals, a move that has promise for boosting the US physician workforce battling COVID-19.
The move may also help physicians extend their visas.
The communication late last week follows a March 18 announcement that, because of COVID-19, the United States was suspending routine processing of immigrant and nonimmigrant visas, including the J and H visas, at embassies and consulates worldwide.
As reported by Medscape Medical News, the Educational Commission for Foreign Medical Graduates (ECFMG) appealed to the State Department to lift the suspension, noting that 4222 graduates of medical schools outside the United States who had matched into residencies in the United States and were ready to start on July 1 would not get the visas most of them need to begin training.
The State Department lifted the suspensions and issued this update:
“We encourage medical professionals with an approved US non-immigrant or immigrant visa petition (I-129, I-140, or similar) or a certificate of eligibility in an approved exchange visitor program (DS-2019), particularly those working to treat or mitigate the effects of COVID-19, to review the website of their nearest embassy or consulate for procedures to request a visa appointment.”
The State Department also issued guidance for foreign medical professionals already in the United States:
“J-1 Alien Physicians (medical residents) may consult with their program sponsor, ECFMG, to extend their programs in the United States. Generally, a J-1 program for a foreign medical resident can be extended one year at a time for up to seven years.
“Note that the expiration date on a US visa does not determine how long one can be in the United States. The way to confirm one’s required departure date is here : https://i94.cbp.dhs.gov/I94/#/home.
“Those who need to extend their stay or adjust their visa status must apply with USCIS (US Citizenship and Immigration Services).”
Complications Still Exist
ECFMG’s CEO, William W. Pinsky, MD, told Medscape Medical News that, although they welcomed the news from the State Department, there are still unanswered questions.
ECFMG explained that J-1 visas are currently granted only 30 days before the residency program begins.
However, travel to the United States may still be difficult in June, Pinsky said, and physicians may need to be quarantined for 2 weeks upon arrival.
“We’re still having some discussion with the Department of State on whether that regulation could be relaxed and they could come in earlier,” he said.
He cautioned that even after a J-1 visa application is made, the physician’s home country has to endorse the application.
Pinsky said he did not yet know whether that would be a problem.
He also said that, in response to New York’s plea for more healthcare workers, ECFMG is offering to verify education and licensing credentials for physicians educated outside the United States at no cost.
Individual hospitals and regulatory authorities can decide whether there may be roles in some capacity for physicians who have graduated from medical school, even if they have not completed residency or have not been licensed, he said.
This article first appeared on Medscape.com.
New information from the US State Department indicates that it is lifting the suspension on visas for foreign-trained medical professionals, a move that has promise for boosting the US physician workforce battling COVID-19.
The move may also help physicians extend their visas.
The communication late last week follows a March 18 announcement that, because of COVID-19, the United States was suspending routine processing of immigrant and nonimmigrant visas, including the J and H visas, at embassies and consulates worldwide.
As reported by Medscape Medical News, the Educational Commission for Foreign Medical Graduates (ECFMG) appealed to the State Department to lift the suspension, noting that 4222 graduates of medical schools outside the United States who had matched into residencies in the United States and were ready to start on July 1 would not get the visas most of them need to begin training.
The State Department lifted the suspensions and issued this update:
“We encourage medical professionals with an approved US non-immigrant or immigrant visa petition (I-129, I-140, or similar) or a certificate of eligibility in an approved exchange visitor program (DS-2019), particularly those working to treat or mitigate the effects of COVID-19, to review the website of their nearest embassy or consulate for procedures to request a visa appointment.”
The State Department also issued guidance for foreign medical professionals already in the United States:
“J-1 Alien Physicians (medical residents) may consult with their program sponsor, ECFMG, to extend their programs in the United States. Generally, a J-1 program for a foreign medical resident can be extended one year at a time for up to seven years.
“Note that the expiration date on a US visa does not determine how long one can be in the United States. The way to confirm one’s required departure date is here : https://i94.cbp.dhs.gov/I94/#/home.
“Those who need to extend their stay or adjust their visa status must apply with USCIS (US Citizenship and Immigration Services).”
Complications Still Exist
ECFMG’s CEO, William W. Pinsky, MD, told Medscape Medical News that, although they welcomed the news from the State Department, there are still unanswered questions.
ECFMG explained that J-1 visas are currently granted only 30 days before the residency program begins.
However, travel to the United States may still be difficult in June, Pinsky said, and physicians may need to be quarantined for 2 weeks upon arrival.
“We’re still having some discussion with the Department of State on whether that regulation could be relaxed and they could come in earlier,” he said.
He cautioned that even after a J-1 visa application is made, the physician’s home country has to endorse the application.
Pinsky said he did not yet know whether that would be a problem.
He also said that, in response to New York’s plea for more healthcare workers, ECFMG is offering to verify education and licensing credentials for physicians educated outside the United States at no cost.
Individual hospitals and regulatory authorities can decide whether there may be roles in some capacity for physicians who have graduated from medical school, even if they have not completed residency or have not been licensed, he said.
This article first appeared on Medscape.com.
Physician couples draft wills, face tough questions amid COVID-19
Not long ago, weekends for Cornelia Griggs, MD, meant making trips to the grocery store, chasing after two active toddlers, and eating brunch with her husband after a busy work week. But life has changed dramatically for the family since the spread of COVID-19. On a recent weekend, Dr. Griggs and her husband, Robert Goldstone, MD, spent their days off drafting a will.
“We’re both doctors, and we know that health care workers have an increased risk of contracting COVID,” said Dr. Griggs, a pediatric surgery fellow at Columbia University Irving Medical Center in New York. “It felt like the responsible thing to do: Have a will in place to make sure our wishes are clear about who would manage our property and assets, and who would take care of our kids – God forbid.”
Outlining their final wishes is among many difficult decisions the doctors, both 36, have been forced to make in recent weeks. Dr. Goldstone, a general surgeon at Massachusetts General Hospital in Boston, is no longer returning to New York during his time off, said Dr. Griggs, who has had known COVID-19 exposures. The couple’s children, aged 4 and almost 2, are temporarily living with their grandparents in Connecticut to decrease their exposure risk.
“I felt like it was safer for all of them to be there while I was going back and forth from the hospital,” Dr. Griggs said. “My husband is in Boston. The kids are in Connecticut and I’m in New York. That inherently is hard because our whole family is split up. I don’t know when it will be safe for me to see them again.”
Health professional couples across the country are facing similar challenges as they navigate the risk of contracting COVID-19 at work, while trying to protect their families at home. From childcare dilemmas to quarantine quandaries to end-of-life considerations, partners who work in health care are confronting tough questions as the pandemic continues.
The biggest challenge is the uncertainty, says Angela Weyand, MD, an Ann Arbor, Mich.–based pediatric hematologist/oncologist who shares two young daughters with husband Ted Claflin, MD, a physical medicine and rehabilitation physician. Dr. Weyand said she and her husband are primarily working remotely now, but she knows that one or both could be deployed to the hospital to help care for patients, if the need arises. Nearby Detroit has been labeled a coronavirus “hot spot” by the U.S. Surgeon General.
“Right now, I think our biggest fear is spreading coronavirus to those we love, especially those in higher risk groups,” she said. “At the same time, we are also concerned about our own health and our future ability to be there for our children, a fear that, thankfully, neither one of us has ever had to face before. We are trying to take things one day at a time, acknowledging all that we have to be grateful for, and also learning to accept that many things right now are outside of our control.”
Dr. Weyand, 38, and her husband, 40, finalized their wills in March.
“We have been working on them for quite some time, but before now, there has never been any urgency,” Dr. Weyand said. “Hearing about the high rate of infection in health care workers and the increasing number of deaths in young healthy people made us realize that this should be a priority.”
Dallas internist Bethany Agusala, MD, 36, and her husband, Kartik Agusala, MD, 41, a cardiologist, recently spent time engaged in the same activity. The couple, who work for the University of Texas Southwestern Medical Center, have two children, aged 2 and 4.
“The chances are hopefully small that something bad would happen to either one of us, but it just seemed like a good time to get [a will] in place,” Dr. Bethany Agusala said in an interview. “It’s never an easy thing to think about.
Pediatric surgeon Chethan Sathya, MD, 34, and his wife, 31, a physician assistant, have vastly altered their home routine to prevent the risk of exposure to their 16-month-old daughter. Dr. Sathya works for the Northwell Health System in New York, which has hundreds of hospitalized patients with COVID-19, Dr. Sathya said in an interview. He did not want to disclose his wife's name or institution, but said she works in a COVID-19 unit at a New York hospital.
When his wife returns home, she removes all of her clothes and places them in a bag, showers, and then isolates herself in the bedroom. Dr. Sathya brings his wife meals and then remains in a different room with their baby.
“It’s only been a few days,” he said. “We’re going to decide: Does she just stay in one room at all times or when she doesn’t work for a few days then after 1 day, can she come out? Should she get a hotel room elsewhere? These are the considerations.”
They employ an older nanny whom they also worry about, and with whom they try to limit contact, said Dr. Sathya, who practices at Cohen Children’s Medical Center. In a matter of weeks, Dr. Sathya anticipates he will be called upon to assist in some form with the COVID crisis.
“We haven’t figured that out. I’m not sure what we’ll do,” he said. “There is no perfect solution. You have to adapt. It’s very difficult to do so when you’re living in a condo in New York.”
For Dr. Griggs, life is much quieter at home without her husband and two “laughing, wiggly,” toddlers. Weekends are now defined by resting, video calls with her family, and exercising, when it’s safe, said Dr. Griggs, who recently penned a New York Times opinion piece about the pandemic and is also active on social media regarding personal protective equipment. She calls her husband her “rock” who never fails to put a smile on her face when they chat from across the miles. Her advice for other health care couples is to take it “one day at a time.”
“Don’t try to make plans weeks in advance or let your mind go to a dark place,” she said. “It’s so easy to feel overwhelmed. The only way to get through this is to focus on surviving each day.”
Editor's Note, 3/31/20: Due to incorrect information provided, the hospital where Dr. Sathya's wife works was misidentified. We have removed the name of that hospital. The story does not include his wife's employer, because Dr. Sathya did not have permission to disclose her workplace and she wishes to remain anonymous.
Not long ago, weekends for Cornelia Griggs, MD, meant making trips to the grocery store, chasing after two active toddlers, and eating brunch with her husband after a busy work week. But life has changed dramatically for the family since the spread of COVID-19. On a recent weekend, Dr. Griggs and her husband, Robert Goldstone, MD, spent their days off drafting a will.
“We’re both doctors, and we know that health care workers have an increased risk of contracting COVID,” said Dr. Griggs, a pediatric surgery fellow at Columbia University Irving Medical Center in New York. “It felt like the responsible thing to do: Have a will in place to make sure our wishes are clear about who would manage our property and assets, and who would take care of our kids – God forbid.”
Outlining their final wishes is among many difficult decisions the doctors, both 36, have been forced to make in recent weeks. Dr. Goldstone, a general surgeon at Massachusetts General Hospital in Boston, is no longer returning to New York during his time off, said Dr. Griggs, who has had known COVID-19 exposures. The couple’s children, aged 4 and almost 2, are temporarily living with their grandparents in Connecticut to decrease their exposure risk.
“I felt like it was safer for all of them to be there while I was going back and forth from the hospital,” Dr. Griggs said. “My husband is in Boston. The kids are in Connecticut and I’m in New York. That inherently is hard because our whole family is split up. I don’t know when it will be safe for me to see them again.”
Health professional couples across the country are facing similar challenges as they navigate the risk of contracting COVID-19 at work, while trying to protect their families at home. From childcare dilemmas to quarantine quandaries to end-of-life considerations, partners who work in health care are confronting tough questions as the pandemic continues.
The biggest challenge is the uncertainty, says Angela Weyand, MD, an Ann Arbor, Mich.–based pediatric hematologist/oncologist who shares two young daughters with husband Ted Claflin, MD, a physical medicine and rehabilitation physician. Dr. Weyand said she and her husband are primarily working remotely now, but she knows that one or both could be deployed to the hospital to help care for patients, if the need arises. Nearby Detroit has been labeled a coronavirus “hot spot” by the U.S. Surgeon General.
“Right now, I think our biggest fear is spreading coronavirus to those we love, especially those in higher risk groups,” she said. “At the same time, we are also concerned about our own health and our future ability to be there for our children, a fear that, thankfully, neither one of us has ever had to face before. We are trying to take things one day at a time, acknowledging all that we have to be grateful for, and also learning to accept that many things right now are outside of our control.”
Dr. Weyand, 38, and her husband, 40, finalized their wills in March.
“We have been working on them for quite some time, but before now, there has never been any urgency,” Dr. Weyand said. “Hearing about the high rate of infection in health care workers and the increasing number of deaths in young healthy people made us realize that this should be a priority.”
Dallas internist Bethany Agusala, MD, 36, and her husband, Kartik Agusala, MD, 41, a cardiologist, recently spent time engaged in the same activity. The couple, who work for the University of Texas Southwestern Medical Center, have two children, aged 2 and 4.
“The chances are hopefully small that something bad would happen to either one of us, but it just seemed like a good time to get [a will] in place,” Dr. Bethany Agusala said in an interview. “It’s never an easy thing to think about.
Pediatric surgeon Chethan Sathya, MD, 34, and his wife, 31, a physician assistant, have vastly altered their home routine to prevent the risk of exposure to their 16-month-old daughter. Dr. Sathya works for the Northwell Health System in New York, which has hundreds of hospitalized patients with COVID-19, Dr. Sathya said in an interview. He did not want to disclose his wife's name or institution, but said she works in a COVID-19 unit at a New York hospital.
When his wife returns home, she removes all of her clothes and places them in a bag, showers, and then isolates herself in the bedroom. Dr. Sathya brings his wife meals and then remains in a different room with their baby.
“It’s only been a few days,” he said. “We’re going to decide: Does she just stay in one room at all times or when she doesn’t work for a few days then after 1 day, can she come out? Should she get a hotel room elsewhere? These are the considerations.”
They employ an older nanny whom they also worry about, and with whom they try to limit contact, said Dr. Sathya, who practices at Cohen Children’s Medical Center. In a matter of weeks, Dr. Sathya anticipates he will be called upon to assist in some form with the COVID crisis.
“We haven’t figured that out. I’m not sure what we’ll do,” he said. “There is no perfect solution. You have to adapt. It’s very difficult to do so when you’re living in a condo in New York.”
For Dr. Griggs, life is much quieter at home without her husband and two “laughing, wiggly,” toddlers. Weekends are now defined by resting, video calls with her family, and exercising, when it’s safe, said Dr. Griggs, who recently penned a New York Times opinion piece about the pandemic and is also active on social media regarding personal protective equipment. She calls her husband her “rock” who never fails to put a smile on her face when they chat from across the miles. Her advice for other health care couples is to take it “one day at a time.”

“Don’t try to make plans weeks in advance or let your mind go to a dark place,” she said. “It’s so easy to feel overwhelmed. The only way to get through this is to focus on surviving each day.”
Editor's Note, 3/31/20: Due to incorrect information provided, the hospital where Dr. Sathya's wife works was misidentified. We have removed the name of that hospital. The story does not include his wife's employer, because Dr. Sathya did not have permission to disclose her workplace and she wishes to remain anonymous.
Not long ago, weekends for Cornelia Griggs, MD, meant making trips to the grocery store, chasing after two active toddlers, and eating brunch with her husband after a busy work week. But life has changed dramatically for the family since the spread of COVID-19. On a recent weekend, Dr. Griggs and her husband, Robert Goldstone, MD, spent their days off drafting a will.
“We’re both doctors, and we know that health care workers have an increased risk of contracting COVID,” said Dr. Griggs, a pediatric surgery fellow at Columbia University Irving Medical Center in New York. “It felt like the responsible thing to do: Have a will in place to make sure our wishes are clear about who would manage our property and assets, and who would take care of our kids – God forbid.”
Outlining their final wishes is among many difficult decisions the doctors, both 36, have been forced to make in recent weeks. Dr. Goldstone, a general surgeon at Massachusetts General Hospital in Boston, is no longer returning to New York during his time off, said Dr. Griggs, who has had known COVID-19 exposures. The couple’s children, aged 4 and almost 2, are temporarily living with their grandparents in Connecticut to decrease their exposure risk.
“I felt like it was safer for all of them to be there while I was going back and forth from the hospital,” Dr. Griggs said. “My husband is in Boston. The kids are in Connecticut and I’m in New York. That inherently is hard because our whole family is split up. I don’t know when it will be safe for me to see them again.”
Health professional couples across the country are facing similar challenges as they navigate the risk of contracting COVID-19 at work, while trying to protect their families at home. From childcare dilemmas to quarantine quandaries to end-of-life considerations, partners who work in health care are confronting tough questions as the pandemic continues.
The biggest challenge is the uncertainty, says Angela Weyand, MD, an Ann Arbor, Mich.–based pediatric hematologist/oncologist who shares two young daughters with husband Ted Claflin, MD, a physical medicine and rehabilitation physician. Dr. Weyand said she and her husband are primarily working remotely now, but she knows that one or both could be deployed to the hospital to help care for patients, if the need arises. Nearby Detroit has been labeled a coronavirus “hot spot” by the U.S. Surgeon General.
“Right now, I think our biggest fear is spreading coronavirus to those we love, especially those in higher risk groups,” she said. “At the same time, we are also concerned about our own health and our future ability to be there for our children, a fear that, thankfully, neither one of us has ever had to face before. We are trying to take things one day at a time, acknowledging all that we have to be grateful for, and also learning to accept that many things right now are outside of our control.”
Dr. Weyand, 38, and her husband, 40, finalized their wills in March.
“We have been working on them for quite some time, but before now, there has never been any urgency,” Dr. Weyand said. “Hearing about the high rate of infection in health care workers and the increasing number of deaths in young healthy people made us realize that this should be a priority.”
Dallas internist Bethany Agusala, MD, 36, and her husband, Kartik Agusala, MD, 41, a cardiologist, recently spent time engaged in the same activity. The couple, who work for the University of Texas Southwestern Medical Center, have two children, aged 2 and 4.
“The chances are hopefully small that something bad would happen to either one of us, but it just seemed like a good time to get [a will] in place,” Dr. Bethany Agusala said in an interview. “It’s never an easy thing to think about.
Pediatric surgeon Chethan Sathya, MD, 34, and his wife, 31, a physician assistant, have vastly altered their home routine to prevent the risk of exposure to their 16-month-old daughter. Dr. Sathya works for the Northwell Health System in New York, which has hundreds of hospitalized patients with COVID-19, Dr. Sathya said in an interview. He did not want to disclose his wife's name or institution, but said she works in a COVID-19 unit at a New York hospital.
When his wife returns home, she removes all of her clothes and places them in a bag, showers, and then isolates herself in the bedroom. Dr. Sathya brings his wife meals and then remains in a different room with their baby.
“It’s only been a few days,” he said. “We’re going to decide: Does she just stay in one room at all times or when she doesn’t work for a few days then after 1 day, can she come out? Should she get a hotel room elsewhere? These are the considerations.”
They employ an older nanny whom they also worry about, and with whom they try to limit contact, said Dr. Sathya, who practices at Cohen Children’s Medical Center. In a matter of weeks, Dr. Sathya anticipates he will be called upon to assist in some form with the COVID crisis.
“We haven’t figured that out. I’m not sure what we’ll do,” he said. “There is no perfect solution. You have to adapt. It’s very difficult to do so when you’re living in a condo in New York.”
For Dr. Griggs, life is much quieter at home without her husband and two “laughing, wiggly,” toddlers. Weekends are now defined by resting, video calls with her family, and exercising, when it’s safe, said Dr. Griggs, who recently penned a New York Times opinion piece about the pandemic and is also active on social media regarding personal protective equipment. She calls her husband her “rock” who never fails to put a smile on her face when they chat from across the miles. Her advice for other health care couples is to take it “one day at a time.”

“Don’t try to make plans weeks in advance or let your mind go to a dark place,” she said. “It’s so easy to feel overwhelmed. The only way to get through this is to focus on surviving each day.”
Editor's Note, 3/31/20: Due to incorrect information provided, the hospital where Dr. Sathya's wife works was misidentified. We have removed the name of that hospital. The story does not include his wife's employer, because Dr. Sathya did not have permission to disclose her workplace and she wishes to remain anonymous.
Use of an Electronic Alert Tool to Prevent Readmissions Following Coronary Artery Bypass Graft Surgery
From the University of North Carolina at Wilmington School of Nursing (Dr. Smith and Dr. Turrise), the New Hanover Regional Medical Center Heart Center (Mr. Jordan), the Coastal Carolinas Health Alliance and Coastal Connect Health Information Exchange (Ms. Robertson), and Coastal Thoracic Surgical Associates (Dr. Kane), Wilmington, NC.
Abstract
Objective: Cardiothoracic (CT) surgeons at our medical center were not receiving timely notification when their coronary artery bypass graft (CABG) surgery patients were admitted to the medical center or to other hospitals. The CT surgical team worked with a health alliance in southeastern North Carolina to implement health information exchange (HIE) real-time electronic notifications for their CABG patients who presented to the hospital’s emergency department (ED) or any ED affiliated with the medical center. The alert tool notifies team members about patient encounters, driving timely clinical engagement.
Methods: The CT team provided the HIE team with the names of CABG surgery patients, which were loaded into the alert tool. When a patient on the list presented to the hospital ED or its affiliates, the alert tool sent a real-time electronic notification to the Cardiac Surgical Services nurse coordinator. This intervention prompted the assessment and disposition of CABG patients, while in the ED, by the CT surgical team.
Results: Over a 16-month period (September 2017-December 2018), the names of 614 post-CABG patients were input into the HIE for tracking. Of these patients, 47 were treated and discharged from the ED; 31 were admitted for observation; 44 were readmitted for inpatient care; and 492 did not have a qualifying event requiring a notification alert. Following implementation of this practice change, the 30-day readmission rate for patients who underwent CABG at our institution decreased from 10% to 7.2%.
Conclusion: Utilizing a real-time alert tool resulted in immediate notification of the CT team when 1 of their patients presented to the ED. This afforded the CT surgical team an opportunity to intervene in the care of their patients, which in turn led to improved quality of care, physician communication and collaboration, and patient outcomes, such as preventable 30-day readmissions.
Keywords: electronic health record; real-time electronic notification; CABG; process improvement.
Unplanned 30-day hospital readmissions of patients who have undergone coronary artery bypass graft (CABG) surgery contribute to higher overall health care costs. CABG is 1 of the conditions/procedures that the Centers for Medicare and Medicaid Services (CMS) monitors for excess readmissions.1 Readmission rates for CABG-related conditions at 30 days post-surgery are reported to be between 16% and 20% for US hospitals.2 Readmissions are not only financially costly, but also have been associated with worse patient outcomes and decreased patient satisfaction.3 Common diagnoses for post-CABG admission include atrial fibrillation, pleural effusion, and wound infection.
The facility where this project was implemented had a 10% post-CABG admission rate for patients across all payers. While this rate is below the national average of 13.2%, the cardiothoracic (CT) surgical team was not being notified in a timely manner when their post-CABG patients were readmitted. The Lean team used the A3 problem-solving process to develop strategies that would reduce these readmissions and improve the care of their patients.
We explored the use of electronic alerts in managing post-CABG patients by conducting a literature search using the terms electronic alerts in patient care, patient engagement in the emergency department, electronic alerts in CABG, real-time notifications to prevent readmission, and CABG readmission. Databases searched were PubMed, Google Scholar, Cumulative Index of Nursing and Allied Health Literature, ProQuest, and ScienceDirect. This search resulted in studies focused on the use of electronic health record (EHR) alerts as a clinical decision-support tool; for example, patient demographic and assessment data are entered into the EHR, and the clinician is prompted with “performance” recommendations (eg, consider electrocardiogram and aspirin).4 In a paper by Engelman and Benjamin,5 the authors discuss the importance of the engaged physician and note that, in their emergency department (ED), an electronic notification is sent when a postoperative patient presents; however, the notification goes to the inpatient service for timely review and disposition. There was no literature that discussed the use of an electronic alert tool as a real-time patient engagement strategy that resulted in a practice change specific to the CT surgical team.
Our process improvement project focused on alerting the CT surgical team when a post-CABG patient presented to the ED, allowing them to evaluate the patient in real time and determine whether the chief complaint was related to the CABG and whether further evaluation by the CT surgeon was required. Specifically, we wanted to determine whether a real-time electronic alert that notified the CT surgical team about post-op CABG patients presenting to the ED would result in timely patient engagement, avoidance of readmissions, and an enhanced patient experience. During this project, alerts were sent to the CT surgical team notifying them of a post-CABG patient presenting to the ED or being directly admitted from home on physician orders, a provider’s office, or inpatient rehabilitation; however, the focus of this article is specifically on the notification regarding post-CABG patients presenting to the ED.
Prior to implementing the electronic notification project, the team developed and implemented several internal and external readmission reduction and prevention strategies for CABG patients. An in-house strategy involved a process whereby patients would receive their discharge medications prior to being discharged from the hospital post-CABG, thereby avoiding potential delays in the patient obtaining medications. When examining post-CABG patient readmissions, the primary conditions that led to readmission were fluid overload, pleural effusion, and atrial fibrillation. As such, a second in-house strategy was developed for post-CABG patients presenting to the ED with atrial fibrillation. The newly established protocol allowed patients to be monitored and treated in the cardiac observation unit. In addition, external strategies, including an outpatient furosemide protocol for home health nurses and an outpatient thoracentesis program and order set, were established (eg, for patients with congestive heart failure, shortness of breath).
Methods
Setting
The regional medical center where this project was implemented is the ninth largest hospital in North Carolina and the largest county-owned public hospital in the state. It is a tertiary care center and teaching hospital with 3 hospital campuses and 855 licensed beds. The medical center was included in the 100 Safecare Hospitals list by the Safecare Group; received a grade “A” Hospital Safety Score from the Leapfrog Group; and is 1 of America’s Top 100 Hospitals for Patient Experience.
Real-Time Notification Project
A regional hospital alliance in southeastern North Carolina established a health information exchange (HIE) with its member hospitals and office-based physicians to enable electronic exchange of patient information to improve quality, safety, and efficiency in health care delivery. Our medical center is part of this alliance. The HIE is a digital platform that facilitates the sharing of information between disparate connected EHR systems, and offers a portal for practices and hospitals to access patient information across North Carolina, South Carolina (via SC HIE), and nationwide (select dialysis centers). More specifically, approved providers and team members are able to access, in real time, patient-care encounter documents from other care settings (eg, acute, post-acute, ambulatory) via the HIE. Additionally, approved care entities can query-retrieve web portal information to support patient outcome improvement strategies. A partnership discussion highlighted the opportunity to utilize the HIE’s capabilities, such as real-time notification, to facilitate workflow (eg, when a patient presents to the ED, the HIE can provide access to health information at the point of care). In this capacity, the alert tool notifies care team members about patient encounters to drive timely clinical engagement for care transitions.
In January 2017, we began discussions on using the HIE to facilitate real-time electronic tracking in the Cardiac Surgical Services department at our medical center. Persons involved in these discussions included the cardiovascular (CV) team (comprised of case managers, department managers and coordinators, program coordinators, administrators, and support services [eg, pre-admission testing and home health staff]) and CT surgeons. At that time, CABG readmissions were manually tracked, and the real-time notification tool was being used in other departments (eg, in case management for tracking readmissions). The entire team was part of the initial decision meeting to pursue this possibility. The CV team reached consensus in June 2017 and proposed extending the use of the alert tool to the post-CABG population presenting to the ED (or any ED affiliated with the medical center) or admitted directly to the medical center.
The HIE staff met with the Cardiac Surgical Services team to tailor and develop the logistics of the project, such as who would be notified and how. The goals of the project were to support appropriate care intervention, reduce preventable hospital readmissions, and improve quality of care through enhanced provider communication and engagement. To achieve these goals, on the day of discharge the Cardiac Surgical Services coordinator provided the HIE team with the names of patients who had undergone CABG surgery. This patient list was loaded into the alert tool and continually updated. At 31 days, patient names were removed from the list. When a patient on the list presented to the hospital ED, the alert tool sent 2 real-time electronic notifications, an email and a text message, to the Cardiac Surgical Services coordinator, noting that a patient event occurred. Personal information was not included in the alert in order to protect patient information and comply with Health Insurance Portability and Accountability Act regulations.
The alert prompted the Cardiac Surgical Services coordinator to securely access patient information to identify and, if necessary, visit the patient. Then, based on the information gathered by the Cardiac Surgical Services coordinator, a Situation-Background-Assessment-Recommendation report was relayed to the CT surgeon, who then determined whether intervention by the CT surgical team was warranted. This process, on average, took approximately 30 minutes to complete. This was a key change in processes, one that allowed post-CABG patients to be seen by the CT surgical team while in the ED. If the issue was related to the CABG surgery, the CT surgeons could then determine an appropriate course of action, including admission or implementation of another protocol, such as the home furosemide protocol. For patients directly admitted, the surgeon contacted the admitting provider to discuss the level of care required (ie, observation or inpatient admission and treatment).
Biweekly CV team meetings were conducted during the implementation of the real-time notification alert tool. At each meeting, updates were provided on notifications received, patients who were missed by the notification process, and how well the real-time alerts were working to enhance care and appropriate disposition.
Measurements
Clinical performance data included total notifications, total number of ED visits, ED disposition (inpatient admission, observation, discharge), total number of direct admissions, direct admissions to observation, direct inpatient admissions, and patients missed by the notification process (eg, due to data entry errors, omissions of information [suffix of junior or senior], as well as programming bugs). Finally, the number of observation admissions converted to inpatient admissions was collected and further analyzed to inform needed process changes.
The Cardiac Surgical Services coordinator collected, entered, and maintained data using Excel. Data were obtained from the EHR, recorded in Excel, and analyzed using basic descriptive statistics in an ongoing fashion. Particular attention was focused on problems with the notification process (eg, patients being missed due to errors in data entry) and summarizing information to keep the Cardiac Surgical Services team updated on the progress of the process improvement. This project did not require staff protections or considerations, and because this was not a research study Institutional Review Board approval was not required.
Results
This practice change was implemented in September 2017 and led to improvements in care quality, as evidenced by improved physician communication and collaboration. In the 16-month period from implementation through December 2018, the names of 614 post-CABG patients were input into the HIE for tracking. Of these patients, 47 were treated and discharged from the ED; 31 were admitted for observation; and 44 were readmitted for inpatient care. The remaining 492 patients did not have a qualifying event requiring a notification alert. Clinical performance data from this period included 70 ED visits, 21 direct admissions, 19 direct admissions to observation, 5 patients missed by the notification process, and 4 observation admissions converted to inpatient admissions. A reduction in the CABG readmission rate from 10% in September 2017 to 7.2% in December 2018 was also noted.
Discussion
The aim of this process improvement project was to determine whether a real-time electronic alert that notified the CT surgical team about post-op CABG patients presenting to the ED would result in timely patient engagement, avoidance of readmissions, and an enhanced patient experience. This practice change has been successful, following 16 months of implementation and process refinement. Integrating a real-time electronic alert with a supporting action plan and care protocols resulted in timely patient engagement and avoidance of readmission of post-CABG patients.
Early notification of possible post-CABG readmissions became a standard-of-care process within the Cardiac Surgical Services department, with expansion to all CT post-op patients. Leveraging HIE technology to support quality improvement processes was also viewed by other departments as relevant and beneficial. For example, the hospital stroke and orthopedic-spine teams established their own processes for receiving real-time alerts.
There were several lessons learned during this project. First, gaining 100% physician buy-in to collaborative communication proved to be critical to the project’s success. The CV team was surprised by the length of time (approximately 8-10 months) it took for the practice change to be adopted by the physicians. In part, some of this delay in adoption resulted from medical staff turnover, primarily in the medical resident training rotations. Collaborative communication was key. The CT surgeons spoke with ED leadership and hospitalist services to explain the readmission reduction project and the use of an electronic alert tool. The CT surgeons also communicated to the ED physicians, hospitalists, and cardiologists that the Cardiac Surgical Services coordinator would be involved in the process and discussions regarding patientss care. Additionally, the CT surgeons authored the furosemide protocol and then committed to its use in the home health setting, further highlighting the role of collaborative communication in avoiding readmissions.
Another key step in this quality improvement project was determining who should receive the alert notifications. At the onset of the project, all notifications were sent to 1 person, the Cardiac Surgical Services coordinator. While this seemed logical in the initial stage of the project, it was unsustainable, as the receipt of the alert and the subsequent notification of the CT surgeon depended on 1 person and their availability. Approximately 10 months into the project, the notification process was further refined, with the cardiovascular intensive care unit charge nurse becoming the point of contact for the alerts. The Cardiac Surgical Services coordinator, in collaboration with nursing leaders and CT surgeons, completed a Lean Standard Work template outlining the major steps and the associated responsibilities (for the cardiovascular intensive care unit charge nurse, CT surgeon and on-call surgeon, Cardiac Surgical Services coordinator) in the process of receiving notifications, collecting patient assessment data, and reporting notifications to the CT surgeons.
Establishing adequate support mechanisms during a practice change is also important. For instance, we had to dedicate personnel time for data collection and analysis and involve additional nursing or other qualified personnel in the new process to avoid depending on a single person for the project’s success. Additional considerations were establishing criteria for surgeon notification and defining an appropriate time frame for notification (eg, urgent versus next-day notifications). We accomplished these activities approximately 10 months into the project, after it became apparent at CV team meeting discussions that further clarification of criteria and timelines was needed.
Some aspects of the project unfolded as planned, while others presented opportunities for improvement. For example, the alert notification process worked as envisioned; however, as previously mentioned, the process needed to be more inclusive to ensure there is always a charge nurse on duty to receive the alert notification, rather than just the Cardiac Surgical Services coordinator, who may not always be at the hospital. The outpatient thoracentesis program was well planned and effectively implemented. This program provided an avenue for patients who had symptoms of pleural effusion to be treated in an outpatient setting, rather than requiring an inpatient stay. Opportunities for improvement included addressing the inconsistent use of the home health furosemide protocol (developed in 2016), and the need for continued interprofessional and interdepartmental communication and coordination. For example, we had to inform the ED physicians and staff who rotate or are new to the ED about established processes and protocols in place for managing post-CABG patients who present to the ED.
The primary limitation of this project was the inability to measure the enhanced patient experience, which was 1 of the stated project goals. This goal became secondary because of more pressing issues, specifically, interorganizational collaboration (eg, hospital EHR, HIE, and CT surgical team) and tailoring the functionality of the electronic alert tool to the project. Developing and implementing measures of enhanced patient experience were not feasible during this implementation. Additionally, because this was not a research study, it was not possible to determine cause and effect or to control for confounders, such as a sicker, older cohort with more comorbid conditions, during the comparison period. Finally, although this process improvement project was conducted at a regional medical center that is the only facility performing CABG within the region, patients may have presented to another facility for an event that led to a readmission. Because readmissions to other facilities could not be captured, it is possible that the actual readmission rate was higher than the rate reported here.
Conclusions and Implications
Utilizing a real-time alert from the HIE to the CT surgical team resulted in CT surgeons being immediately made aware when their patients presented to the ED, allowing the CT surgical team the opportunity to intervene, as appropriate, in the care of their patients. Furthermore, this real-time notification and intervention resulted in timely patient engagement and, in some cases, avoidance of readmissions. Currently, patients are monitored for readmission within 30 days of discharge. In the future, the time will expand to 91 days, in preparation for participation in the CMS bundle payment program for CABG surgery.
This practice change can be used in organizations that do not have or participate in a HIE. In fact, these real-time alert applications may be available through an EHR already in use within the organization. The use of the alert requires collaborative communication and having supporting protocols in place to guide decision-making and care of post-CABG patients presenting to the ED.
There appears to be a gap in the literature discussing the use of an electronic alert tool as a real-time patient engagement strategy for post-CABG patients presenting to the ED. As such, this project contributes important results and lessons learned for other hospital service lines/departments that might consider implementing a similar process. Next steps include designing and conducting methodologically rigorous research studies based on this process improvement project to examine mortality rates as an outcome, and designing a more specific measure of patient experience, as the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey only provides hospital-level data.
Corresponding author: Stephanie D. Smith, PhD, RN, UNCW School of Nursing, 601 South College Road, Wilmington, NC 28403; [email protected].
Funding disclosures: None.
1. Hannan EL, Zhong Y, Lahey SJ, et al. 30-day readmissions after coronary artery bypass graft surgery in New York State. JACC Cardiovasc Interv. 2011;4:569-576.
2. Feng TR, White R, Gaber-Baylis L, et al. Coronary artery bypass graft readmission rates and risk factors- A retrospective cohort study. Int J Surg. 2018;54 (Part A):7-17.
3. Donndorf P, Kaminski A. “Return to sender” or “consider it done”?! The importance of reducing hospital readmission after coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2017;154:1298-1299.
4. Sequist TD, Morong SM, Marston A, et al. Electronic risk alerts to improve primary care management of chest pain: A randomized, controlled trial. J Gen Intern Med. 2012;27:438-444.
5. Engelman D, Benjamin EM. Physician engagement: The “secret sauce” to success in bundled health care. Am J Med Qual. 2018;33:100-102.
From the University of North Carolina at Wilmington School of Nursing (Dr. Smith and Dr. Turrise), the New Hanover Regional Medical Center Heart Center (Mr. Jordan), the Coastal Carolinas Health Alliance and Coastal Connect Health Information Exchange (Ms. Robertson), and Coastal Thoracic Surgical Associates (Dr. Kane), Wilmington, NC.
Abstract
Objective: Cardiothoracic (CT) surgeons at our medical center were not receiving timely notification when their coronary artery bypass graft (CABG) surgery patients were admitted to the medical center or to other hospitals. The CT surgical team worked with a health alliance in southeastern North Carolina to implement health information exchange (HIE) real-time electronic notifications for their CABG patients who presented to the hospital’s emergency department (ED) or any ED affiliated with the medical center. The alert tool notifies team members about patient encounters, driving timely clinical engagement.
Methods: The CT team provided the HIE team with the names of CABG surgery patients, which were loaded into the alert tool. When a patient on the list presented to the hospital ED or its affiliates, the alert tool sent a real-time electronic notification to the Cardiac Surgical Services nurse coordinator. This intervention prompted the assessment and disposition of CABG patients, while in the ED, by the CT surgical team.
Results: Over a 16-month period (September 2017-December 2018), the names of 614 post-CABG patients were input into the HIE for tracking. Of these patients, 47 were treated and discharged from the ED; 31 were admitted for observation; 44 were readmitted for inpatient care; and 492 did not have a qualifying event requiring a notification alert. Following implementation of this practice change, the 30-day readmission rate for patients who underwent CABG at our institution decreased from 10% to 7.2%.
Conclusion: Utilizing a real-time alert tool resulted in immediate notification of the CT team when 1 of their patients presented to the ED. This afforded the CT surgical team an opportunity to intervene in the care of their patients, which in turn led to improved quality of care, physician communication and collaboration, and patient outcomes, such as preventable 30-day readmissions.
Keywords: electronic health record; real-time electronic notification; CABG; process improvement.
Unplanned 30-day hospital readmissions of patients who have undergone coronary artery bypass graft (CABG) surgery contribute to higher overall health care costs. CABG is 1 of the conditions/procedures that the Centers for Medicare and Medicaid Services (CMS) monitors for excess readmissions.1 Readmission rates for CABG-related conditions at 30 days post-surgery are reported to be between 16% and 20% for US hospitals.2 Readmissions are not only financially costly, but also have been associated with worse patient outcomes and decreased patient satisfaction.3 Common diagnoses for post-CABG admission include atrial fibrillation, pleural effusion, and wound infection.
The facility where this project was implemented had a 10% post-CABG admission rate for patients across all payers. While this rate is below the national average of 13.2%, the cardiothoracic (CT) surgical team was not being notified in a timely manner when their post-CABG patients were readmitted. The Lean team used the A3 problem-solving process to develop strategies that would reduce these readmissions and improve the care of their patients.
We explored the use of electronic alerts in managing post-CABG patients by conducting a literature search using the terms electronic alerts in patient care, patient engagement in the emergency department, electronic alerts in CABG, real-time notifications to prevent readmission, and CABG readmission. Databases searched were PubMed, Google Scholar, Cumulative Index of Nursing and Allied Health Literature, ProQuest, and ScienceDirect. This search resulted in studies focused on the use of electronic health record (EHR) alerts as a clinical decision-support tool; for example, patient demographic and assessment data are entered into the EHR, and the clinician is prompted with “performance” recommendations (eg, consider electrocardiogram and aspirin).4 In a paper by Engelman and Benjamin,5 the authors discuss the importance of the engaged physician and note that, in their emergency department (ED), an electronic notification is sent when a postoperative patient presents; however, the notification goes to the inpatient service for timely review and disposition. There was no literature that discussed the use of an electronic alert tool as a real-time patient engagement strategy that resulted in a practice change specific to the CT surgical team.
Our process improvement project focused on alerting the CT surgical team when a post-CABG patient presented to the ED, allowing them to evaluate the patient in real time and determine whether the chief complaint was related to the CABG and whether further evaluation by the CT surgeon was required. Specifically, we wanted to determine whether a real-time electronic alert that notified the CT surgical team about post-op CABG patients presenting to the ED would result in timely patient engagement, avoidance of readmissions, and an enhanced patient experience. During this project, alerts were sent to the CT surgical team notifying them of a post-CABG patient presenting to the ED or being directly admitted from home on physician orders, a provider’s office, or inpatient rehabilitation; however, the focus of this article is specifically on the notification regarding post-CABG patients presenting to the ED.
Prior to implementing the electronic notification project, the team developed and implemented several internal and external readmission reduction and prevention strategies for CABG patients. An in-house strategy involved a process whereby patients would receive their discharge medications prior to being discharged from the hospital post-CABG, thereby avoiding potential delays in the patient obtaining medications. When examining post-CABG patient readmissions, the primary conditions that led to readmission were fluid overload, pleural effusion, and atrial fibrillation. As such, a second in-house strategy was developed for post-CABG patients presenting to the ED with atrial fibrillation. The newly established protocol allowed patients to be monitored and treated in the cardiac observation unit. In addition, external strategies, including an outpatient furosemide protocol for home health nurses and an outpatient thoracentesis program and order set, were established (eg, for patients with congestive heart failure, shortness of breath).
Methods
Setting
The regional medical center where this project was implemented is the ninth largest hospital in North Carolina and the largest county-owned public hospital in the state. It is a tertiary care center and teaching hospital with 3 hospital campuses and 855 licensed beds. The medical center was included in the 100 Safecare Hospitals list by the Safecare Group; received a grade “A” Hospital Safety Score from the Leapfrog Group; and is 1 of America’s Top 100 Hospitals for Patient Experience.
Real-Time Notification Project
A regional hospital alliance in southeastern North Carolina established a health information exchange (HIE) with its member hospitals and office-based physicians to enable electronic exchange of patient information to improve quality, safety, and efficiency in health care delivery. Our medical center is part of this alliance. The HIE is a digital platform that facilitates the sharing of information between disparate connected EHR systems, and offers a portal for practices and hospitals to access patient information across North Carolina, South Carolina (via SC HIE), and nationwide (select dialysis centers). More specifically, approved providers and team members are able to access, in real time, patient-care encounter documents from other care settings (eg, acute, post-acute, ambulatory) via the HIE. Additionally, approved care entities can query-retrieve web portal information to support patient outcome improvement strategies. A partnership discussion highlighted the opportunity to utilize the HIE’s capabilities, such as real-time notification, to facilitate workflow (eg, when a patient presents to the ED, the HIE can provide access to health information at the point of care). In this capacity, the alert tool notifies care team members about patient encounters to drive timely clinical engagement for care transitions.
In January 2017, we began discussions on using the HIE to facilitate real-time electronic tracking in the Cardiac Surgical Services department at our medical center. Persons involved in these discussions included the cardiovascular (CV) team (comprised of case managers, department managers and coordinators, program coordinators, administrators, and support services [eg, pre-admission testing and home health staff]) and CT surgeons. At that time, CABG readmissions were manually tracked, and the real-time notification tool was being used in other departments (eg, in case management for tracking readmissions). The entire team was part of the initial decision meeting to pursue this possibility. The CV team reached consensus in June 2017 and proposed extending the use of the alert tool to the post-CABG population presenting to the ED (or any ED affiliated with the medical center) or admitted directly to the medical center.
The HIE staff met with the Cardiac Surgical Services team to tailor and develop the logistics of the project, such as who would be notified and how. The goals of the project were to support appropriate care intervention, reduce preventable hospital readmissions, and improve quality of care through enhanced provider communication and engagement. To achieve these goals, on the day of discharge the Cardiac Surgical Services coordinator provided the HIE team with the names of patients who had undergone CABG surgery. This patient list was loaded into the alert tool and continually updated. At 31 days, patient names were removed from the list. When a patient on the list presented to the hospital ED, the alert tool sent 2 real-time electronic notifications, an email and a text message, to the Cardiac Surgical Services coordinator, noting that a patient event occurred. Personal information was not included in the alert in order to protect patient information and comply with Health Insurance Portability and Accountability Act regulations.
The alert prompted the Cardiac Surgical Services coordinator to securely access patient information to identify and, if necessary, visit the patient. Then, based on the information gathered by the Cardiac Surgical Services coordinator, a Situation-Background-Assessment-Recommendation report was relayed to the CT surgeon, who then determined whether intervention by the CT surgical team was warranted. This process, on average, took approximately 30 minutes to complete. This was a key change in processes, one that allowed post-CABG patients to be seen by the CT surgical team while in the ED. If the issue was related to the CABG surgery, the CT surgeons could then determine an appropriate course of action, including admission or implementation of another protocol, such as the home furosemide protocol. For patients directly admitted, the surgeon contacted the admitting provider to discuss the level of care required (ie, observation or inpatient admission and treatment).
Biweekly CV team meetings were conducted during the implementation of the real-time notification alert tool. At each meeting, updates were provided on notifications received, patients who were missed by the notification process, and how well the real-time alerts were working to enhance care and appropriate disposition.
Measurements
Clinical performance data included total notifications, total number of ED visits, ED disposition (inpatient admission, observation, discharge), total number of direct admissions, direct admissions to observation, direct inpatient admissions, and patients missed by the notification process (eg, due to data entry errors, omissions of information [suffix of junior or senior], as well as programming bugs). Finally, the number of observation admissions converted to inpatient admissions was collected and further analyzed to inform needed process changes.
The Cardiac Surgical Services coordinator collected, entered, and maintained data using Excel. Data were obtained from the EHR, recorded in Excel, and analyzed using basic descriptive statistics in an ongoing fashion. Particular attention was focused on problems with the notification process (eg, patients being missed due to errors in data entry) and summarizing information to keep the Cardiac Surgical Services team updated on the progress of the process improvement. This project did not require staff protections or considerations, and because this was not a research study Institutional Review Board approval was not required.
Results
This practice change was implemented in September 2017 and led to improvements in care quality, as evidenced by improved physician communication and collaboration. In the 16-month period from implementation through December 2018, the names of 614 post-CABG patients were input into the HIE for tracking. Of these patients, 47 were treated and discharged from the ED; 31 were admitted for observation; and 44 were readmitted for inpatient care. The remaining 492 patients did not have a qualifying event requiring a notification alert. Clinical performance data from this period included 70 ED visits, 21 direct admissions, 19 direct admissions to observation, 5 patients missed by the notification process, and 4 observation admissions converted to inpatient admissions. A reduction in the CABG readmission rate from 10% in September 2017 to 7.2% in December 2018 was also noted.
Discussion
The aim of this process improvement project was to determine whether a real-time electronic alert that notified the CT surgical team about post-op CABG patients presenting to the ED would result in timely patient engagement, avoidance of readmissions, and an enhanced patient experience. This practice change has been successful, following 16 months of implementation and process refinement. Integrating a real-time electronic alert with a supporting action plan and care protocols resulted in timely patient engagement and avoidance of readmission of post-CABG patients.
Early notification of possible post-CABG readmissions became a standard-of-care process within the Cardiac Surgical Services department, with expansion to all CT post-op patients. Leveraging HIE technology to support quality improvement processes was also viewed by other departments as relevant and beneficial. For example, the hospital stroke and orthopedic-spine teams established their own processes for receiving real-time alerts.
There were several lessons learned during this project. First, gaining 100% physician buy-in to collaborative communication proved to be critical to the project’s success. The CV team was surprised by the length of time (approximately 8-10 months) it took for the practice change to be adopted by the physicians. In part, some of this delay in adoption resulted from medical staff turnover, primarily in the medical resident training rotations. Collaborative communication was key. The CT surgeons spoke with ED leadership and hospitalist services to explain the readmission reduction project and the use of an electronic alert tool. The CT surgeons also communicated to the ED physicians, hospitalists, and cardiologists that the Cardiac Surgical Services coordinator would be involved in the process and discussions regarding patientss care. Additionally, the CT surgeons authored the furosemide protocol and then committed to its use in the home health setting, further highlighting the role of collaborative communication in avoiding readmissions.
Another key step in this quality improvement project was determining who should receive the alert notifications. At the onset of the project, all notifications were sent to 1 person, the Cardiac Surgical Services coordinator. While this seemed logical in the initial stage of the project, it was unsustainable, as the receipt of the alert and the subsequent notification of the CT surgeon depended on 1 person and their availability. Approximately 10 months into the project, the notification process was further refined, with the cardiovascular intensive care unit charge nurse becoming the point of contact for the alerts. The Cardiac Surgical Services coordinator, in collaboration with nursing leaders and CT surgeons, completed a Lean Standard Work template outlining the major steps and the associated responsibilities (for the cardiovascular intensive care unit charge nurse, CT surgeon and on-call surgeon, Cardiac Surgical Services coordinator) in the process of receiving notifications, collecting patient assessment data, and reporting notifications to the CT surgeons.
Establishing adequate support mechanisms during a practice change is also important. For instance, we had to dedicate personnel time for data collection and analysis and involve additional nursing or other qualified personnel in the new process to avoid depending on a single person for the project’s success. Additional considerations were establishing criteria for surgeon notification and defining an appropriate time frame for notification (eg, urgent versus next-day notifications). We accomplished these activities approximately 10 months into the project, after it became apparent at CV team meeting discussions that further clarification of criteria and timelines was needed.
Some aspects of the project unfolded as planned, while others presented opportunities for improvement. For example, the alert notification process worked as envisioned; however, as previously mentioned, the process needed to be more inclusive to ensure there is always a charge nurse on duty to receive the alert notification, rather than just the Cardiac Surgical Services coordinator, who may not always be at the hospital. The outpatient thoracentesis program was well planned and effectively implemented. This program provided an avenue for patients who had symptoms of pleural effusion to be treated in an outpatient setting, rather than requiring an inpatient stay. Opportunities for improvement included addressing the inconsistent use of the home health furosemide protocol (developed in 2016), and the need for continued interprofessional and interdepartmental communication and coordination. For example, we had to inform the ED physicians and staff who rotate or are new to the ED about established processes and protocols in place for managing post-CABG patients who present to the ED.
The primary limitation of this project was the inability to measure the enhanced patient experience, which was 1 of the stated project goals. This goal became secondary because of more pressing issues, specifically, interorganizational collaboration (eg, hospital EHR, HIE, and CT surgical team) and tailoring the functionality of the electronic alert tool to the project. Developing and implementing measures of enhanced patient experience were not feasible during this implementation. Additionally, because this was not a research study, it was not possible to determine cause and effect or to control for confounders, such as a sicker, older cohort with more comorbid conditions, during the comparison period. Finally, although this process improvement project was conducted at a regional medical center that is the only facility performing CABG within the region, patients may have presented to another facility for an event that led to a readmission. Because readmissions to other facilities could not be captured, it is possible that the actual readmission rate was higher than the rate reported here.
Conclusions and Implications
Utilizing a real-time alert from the HIE to the CT surgical team resulted in CT surgeons being immediately made aware when their patients presented to the ED, allowing the CT surgical team the opportunity to intervene, as appropriate, in the care of their patients. Furthermore, this real-time notification and intervention resulted in timely patient engagement and, in some cases, avoidance of readmissions. Currently, patients are monitored for readmission within 30 days of discharge. In the future, the time will expand to 91 days, in preparation for participation in the CMS bundle payment program for CABG surgery.
This practice change can be used in organizations that do not have or participate in a HIE. In fact, these real-time alert applications may be available through an EHR already in use within the organization. The use of the alert requires collaborative communication and having supporting protocols in place to guide decision-making and care of post-CABG patients presenting to the ED.
There appears to be a gap in the literature discussing the use of an electronic alert tool as a real-time patient engagement strategy for post-CABG patients presenting to the ED. As such, this project contributes important results and lessons learned for other hospital service lines/departments that might consider implementing a similar process. Next steps include designing and conducting methodologically rigorous research studies based on this process improvement project to examine mortality rates as an outcome, and designing a more specific measure of patient experience, as the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey only provides hospital-level data.
Corresponding author: Stephanie D. Smith, PhD, RN, UNCW School of Nursing, 601 South College Road, Wilmington, NC 28403; [email protected].
Funding disclosures: None.
From the University of North Carolina at Wilmington School of Nursing (Dr. Smith and Dr. Turrise), the New Hanover Regional Medical Center Heart Center (Mr. Jordan), the Coastal Carolinas Health Alliance and Coastal Connect Health Information Exchange (Ms. Robertson), and Coastal Thoracic Surgical Associates (Dr. Kane), Wilmington, NC.
Abstract
Objective: Cardiothoracic (CT) surgeons at our medical center were not receiving timely notification when their coronary artery bypass graft (CABG) surgery patients were admitted to the medical center or to other hospitals. The CT surgical team worked with a health alliance in southeastern North Carolina to implement health information exchange (HIE) real-time electronic notifications for their CABG patients who presented to the hospital’s emergency department (ED) or any ED affiliated with the medical center. The alert tool notifies team members about patient encounters, driving timely clinical engagement.
Methods: The CT team provided the HIE team with the names of CABG surgery patients, which were loaded into the alert tool. When a patient on the list presented to the hospital ED or its affiliates, the alert tool sent a real-time electronic notification to the Cardiac Surgical Services nurse coordinator. This intervention prompted the assessment and disposition of CABG patients, while in the ED, by the CT surgical team.
Results: Over a 16-month period (September 2017-December 2018), the names of 614 post-CABG patients were input into the HIE for tracking. Of these patients, 47 were treated and discharged from the ED; 31 were admitted for observation; 44 were readmitted for inpatient care; and 492 did not have a qualifying event requiring a notification alert. Following implementation of this practice change, the 30-day readmission rate for patients who underwent CABG at our institution decreased from 10% to 7.2%.
Conclusion: Utilizing a real-time alert tool resulted in immediate notification of the CT team when 1 of their patients presented to the ED. This afforded the CT surgical team an opportunity to intervene in the care of their patients, which in turn led to improved quality of care, physician communication and collaboration, and patient outcomes, such as preventable 30-day readmissions.
Keywords: electronic health record; real-time electronic notification; CABG; process improvement.
Unplanned 30-day hospital readmissions of patients who have undergone coronary artery bypass graft (CABG) surgery contribute to higher overall health care costs. CABG is 1 of the conditions/procedures that the Centers for Medicare and Medicaid Services (CMS) monitors for excess readmissions.1 Readmission rates for CABG-related conditions at 30 days post-surgery are reported to be between 16% and 20% for US hospitals.2 Readmissions are not only financially costly, but also have been associated with worse patient outcomes and decreased patient satisfaction.3 Common diagnoses for post-CABG admission include atrial fibrillation, pleural effusion, and wound infection.
The facility where this project was implemented had a 10% post-CABG admission rate for patients across all payers. While this rate is below the national average of 13.2%, the cardiothoracic (CT) surgical team was not being notified in a timely manner when their post-CABG patients were readmitted. The Lean team used the A3 problem-solving process to develop strategies that would reduce these readmissions and improve the care of their patients.
We explored the use of electronic alerts in managing post-CABG patients by conducting a literature search using the terms electronic alerts in patient care, patient engagement in the emergency department, electronic alerts in CABG, real-time notifications to prevent readmission, and CABG readmission. Databases searched were PubMed, Google Scholar, Cumulative Index of Nursing and Allied Health Literature, ProQuest, and ScienceDirect. This search resulted in studies focused on the use of electronic health record (EHR) alerts as a clinical decision-support tool; for example, patient demographic and assessment data are entered into the EHR, and the clinician is prompted with “performance” recommendations (eg, consider electrocardiogram and aspirin).4 In a paper by Engelman and Benjamin,5 the authors discuss the importance of the engaged physician and note that, in their emergency department (ED), an electronic notification is sent when a postoperative patient presents; however, the notification goes to the inpatient service for timely review and disposition. There was no literature that discussed the use of an electronic alert tool as a real-time patient engagement strategy that resulted in a practice change specific to the CT surgical team.
Our process improvement project focused on alerting the CT surgical team when a post-CABG patient presented to the ED, allowing them to evaluate the patient in real time and determine whether the chief complaint was related to the CABG and whether further evaluation by the CT surgeon was required. Specifically, we wanted to determine whether a real-time electronic alert that notified the CT surgical team about post-op CABG patients presenting to the ED would result in timely patient engagement, avoidance of readmissions, and an enhanced patient experience. During this project, alerts were sent to the CT surgical team notifying them of a post-CABG patient presenting to the ED or being directly admitted from home on physician orders, a provider’s office, or inpatient rehabilitation; however, the focus of this article is specifically on the notification regarding post-CABG patients presenting to the ED.
Prior to implementing the electronic notification project, the team developed and implemented several internal and external readmission reduction and prevention strategies for CABG patients. An in-house strategy involved a process whereby patients would receive their discharge medications prior to being discharged from the hospital post-CABG, thereby avoiding potential delays in the patient obtaining medications. When examining post-CABG patient readmissions, the primary conditions that led to readmission were fluid overload, pleural effusion, and atrial fibrillation. As such, a second in-house strategy was developed for post-CABG patients presenting to the ED with atrial fibrillation. The newly established protocol allowed patients to be monitored and treated in the cardiac observation unit. In addition, external strategies, including an outpatient furosemide protocol for home health nurses and an outpatient thoracentesis program and order set, were established (eg, for patients with congestive heart failure, shortness of breath).
Methods
Setting
The regional medical center where this project was implemented is the ninth largest hospital in North Carolina and the largest county-owned public hospital in the state. It is a tertiary care center and teaching hospital with 3 hospital campuses and 855 licensed beds. The medical center was included in the 100 Safecare Hospitals list by the Safecare Group; received a grade “A” Hospital Safety Score from the Leapfrog Group; and is 1 of America’s Top 100 Hospitals for Patient Experience.
Real-Time Notification Project
A regional hospital alliance in southeastern North Carolina established a health information exchange (HIE) with its member hospitals and office-based physicians to enable electronic exchange of patient information to improve quality, safety, and efficiency in health care delivery. Our medical center is part of this alliance. The HIE is a digital platform that facilitates the sharing of information between disparate connected EHR systems, and offers a portal for practices and hospitals to access patient information across North Carolina, South Carolina (via SC HIE), and nationwide (select dialysis centers). More specifically, approved providers and team members are able to access, in real time, patient-care encounter documents from other care settings (eg, acute, post-acute, ambulatory) via the HIE. Additionally, approved care entities can query-retrieve web portal information to support patient outcome improvement strategies. A partnership discussion highlighted the opportunity to utilize the HIE’s capabilities, such as real-time notification, to facilitate workflow (eg, when a patient presents to the ED, the HIE can provide access to health information at the point of care). In this capacity, the alert tool notifies care team members about patient encounters to drive timely clinical engagement for care transitions.
In January 2017, we began discussions on using the HIE to facilitate real-time electronic tracking in the Cardiac Surgical Services department at our medical center. Persons involved in these discussions included the cardiovascular (CV) team (comprised of case managers, department managers and coordinators, program coordinators, administrators, and support services [eg, pre-admission testing and home health staff]) and CT surgeons. At that time, CABG readmissions were manually tracked, and the real-time notification tool was being used in other departments (eg, in case management for tracking readmissions). The entire team was part of the initial decision meeting to pursue this possibility. The CV team reached consensus in June 2017 and proposed extending the use of the alert tool to the post-CABG population presenting to the ED (or any ED affiliated with the medical center) or admitted directly to the medical center.
The HIE staff met with the Cardiac Surgical Services team to tailor and develop the logistics of the project, such as who would be notified and how. The goals of the project were to support appropriate care intervention, reduce preventable hospital readmissions, and improve quality of care through enhanced provider communication and engagement. To achieve these goals, on the day of discharge the Cardiac Surgical Services coordinator provided the HIE team with the names of patients who had undergone CABG surgery. This patient list was loaded into the alert tool and continually updated. At 31 days, patient names were removed from the list. When a patient on the list presented to the hospital ED, the alert tool sent 2 real-time electronic notifications, an email and a text message, to the Cardiac Surgical Services coordinator, noting that a patient event occurred. Personal information was not included in the alert in order to protect patient information and comply with Health Insurance Portability and Accountability Act regulations.
The alert prompted the Cardiac Surgical Services coordinator to securely access patient information to identify and, if necessary, visit the patient. Then, based on the information gathered by the Cardiac Surgical Services coordinator, a Situation-Background-Assessment-Recommendation report was relayed to the CT surgeon, who then determined whether intervention by the CT surgical team was warranted. This process, on average, took approximately 30 minutes to complete. This was a key change in processes, one that allowed post-CABG patients to be seen by the CT surgical team while in the ED. If the issue was related to the CABG surgery, the CT surgeons could then determine an appropriate course of action, including admission or implementation of another protocol, such as the home furosemide protocol. For patients directly admitted, the surgeon contacted the admitting provider to discuss the level of care required (ie, observation or inpatient admission and treatment).
Biweekly CV team meetings were conducted during the implementation of the real-time notification alert tool. At each meeting, updates were provided on notifications received, patients who were missed by the notification process, and how well the real-time alerts were working to enhance care and appropriate disposition.
Measurements
Clinical performance data included total notifications, total number of ED visits, ED disposition (inpatient admission, observation, discharge), total number of direct admissions, direct admissions to observation, direct inpatient admissions, and patients missed by the notification process (eg, due to data entry errors, omissions of information [suffix of junior or senior], as well as programming bugs). Finally, the number of observation admissions converted to inpatient admissions was collected and further analyzed to inform needed process changes.
The Cardiac Surgical Services coordinator collected, entered, and maintained data using Excel. Data were obtained from the EHR, recorded in Excel, and analyzed using basic descriptive statistics in an ongoing fashion. Particular attention was focused on problems with the notification process (eg, patients being missed due to errors in data entry) and summarizing information to keep the Cardiac Surgical Services team updated on the progress of the process improvement. This project did not require staff protections or considerations, and because this was not a research study Institutional Review Board approval was not required.
Results
This practice change was implemented in September 2017 and led to improvements in care quality, as evidenced by improved physician communication and collaboration. In the 16-month period from implementation through December 2018, the names of 614 post-CABG patients were input into the HIE for tracking. Of these patients, 47 were treated and discharged from the ED; 31 were admitted for observation; and 44 were readmitted for inpatient care. The remaining 492 patients did not have a qualifying event requiring a notification alert. Clinical performance data from this period included 70 ED visits, 21 direct admissions, 19 direct admissions to observation, 5 patients missed by the notification process, and 4 observation admissions converted to inpatient admissions. A reduction in the CABG readmission rate from 10% in September 2017 to 7.2% in December 2018 was also noted.
Discussion
The aim of this process improvement project was to determine whether a real-time electronic alert that notified the CT surgical team about post-op CABG patients presenting to the ED would result in timely patient engagement, avoidance of readmissions, and an enhanced patient experience. This practice change has been successful, following 16 months of implementation and process refinement. Integrating a real-time electronic alert with a supporting action plan and care protocols resulted in timely patient engagement and avoidance of readmission of post-CABG patients.
Early notification of possible post-CABG readmissions became a standard-of-care process within the Cardiac Surgical Services department, with expansion to all CT post-op patients. Leveraging HIE technology to support quality improvement processes was also viewed by other departments as relevant and beneficial. For example, the hospital stroke and orthopedic-spine teams established their own processes for receiving real-time alerts.
There were several lessons learned during this project. First, gaining 100% physician buy-in to collaborative communication proved to be critical to the project’s success. The CV team was surprised by the length of time (approximately 8-10 months) it took for the practice change to be adopted by the physicians. In part, some of this delay in adoption resulted from medical staff turnover, primarily in the medical resident training rotations. Collaborative communication was key. The CT surgeons spoke with ED leadership and hospitalist services to explain the readmission reduction project and the use of an electronic alert tool. The CT surgeons also communicated to the ED physicians, hospitalists, and cardiologists that the Cardiac Surgical Services coordinator would be involved in the process and discussions regarding patientss care. Additionally, the CT surgeons authored the furosemide protocol and then committed to its use in the home health setting, further highlighting the role of collaborative communication in avoiding readmissions.
Another key step in this quality improvement project was determining who should receive the alert notifications. At the onset of the project, all notifications were sent to 1 person, the Cardiac Surgical Services coordinator. While this seemed logical in the initial stage of the project, it was unsustainable, as the receipt of the alert and the subsequent notification of the CT surgeon depended on 1 person and their availability. Approximately 10 months into the project, the notification process was further refined, with the cardiovascular intensive care unit charge nurse becoming the point of contact for the alerts. The Cardiac Surgical Services coordinator, in collaboration with nursing leaders and CT surgeons, completed a Lean Standard Work template outlining the major steps and the associated responsibilities (for the cardiovascular intensive care unit charge nurse, CT surgeon and on-call surgeon, Cardiac Surgical Services coordinator) in the process of receiving notifications, collecting patient assessment data, and reporting notifications to the CT surgeons.
Establishing adequate support mechanisms during a practice change is also important. For instance, we had to dedicate personnel time for data collection and analysis and involve additional nursing or other qualified personnel in the new process to avoid depending on a single person for the project’s success. Additional considerations were establishing criteria for surgeon notification and defining an appropriate time frame for notification (eg, urgent versus next-day notifications). We accomplished these activities approximately 10 months into the project, after it became apparent at CV team meeting discussions that further clarification of criteria and timelines was needed.
Some aspects of the project unfolded as planned, while others presented opportunities for improvement. For example, the alert notification process worked as envisioned; however, as previously mentioned, the process needed to be more inclusive to ensure there is always a charge nurse on duty to receive the alert notification, rather than just the Cardiac Surgical Services coordinator, who may not always be at the hospital. The outpatient thoracentesis program was well planned and effectively implemented. This program provided an avenue for patients who had symptoms of pleural effusion to be treated in an outpatient setting, rather than requiring an inpatient stay. Opportunities for improvement included addressing the inconsistent use of the home health furosemide protocol (developed in 2016), and the need for continued interprofessional and interdepartmental communication and coordination. For example, we had to inform the ED physicians and staff who rotate or are new to the ED about established processes and protocols in place for managing post-CABG patients who present to the ED.
The primary limitation of this project was the inability to measure the enhanced patient experience, which was 1 of the stated project goals. This goal became secondary because of more pressing issues, specifically, interorganizational collaboration (eg, hospital EHR, HIE, and CT surgical team) and tailoring the functionality of the electronic alert tool to the project. Developing and implementing measures of enhanced patient experience were not feasible during this implementation. Additionally, because this was not a research study, it was not possible to determine cause and effect or to control for confounders, such as a sicker, older cohort with more comorbid conditions, during the comparison period. Finally, although this process improvement project was conducted at a regional medical center that is the only facility performing CABG within the region, patients may have presented to another facility for an event that led to a readmission. Because readmissions to other facilities could not be captured, it is possible that the actual readmission rate was higher than the rate reported here.
Conclusions and Implications
Utilizing a real-time alert from the HIE to the CT surgical team resulted in CT surgeons being immediately made aware when their patients presented to the ED, allowing the CT surgical team the opportunity to intervene, as appropriate, in the care of their patients. Furthermore, this real-time notification and intervention resulted in timely patient engagement and, in some cases, avoidance of readmissions. Currently, patients are monitored for readmission within 30 days of discharge. In the future, the time will expand to 91 days, in preparation for participation in the CMS bundle payment program for CABG surgery.
This practice change can be used in organizations that do not have or participate in a HIE. In fact, these real-time alert applications may be available through an EHR already in use within the organization. The use of the alert requires collaborative communication and having supporting protocols in place to guide decision-making and care of post-CABG patients presenting to the ED.
There appears to be a gap in the literature discussing the use of an electronic alert tool as a real-time patient engagement strategy for post-CABG patients presenting to the ED. As such, this project contributes important results and lessons learned for other hospital service lines/departments that might consider implementing a similar process. Next steps include designing and conducting methodologically rigorous research studies based on this process improvement project to examine mortality rates as an outcome, and designing a more specific measure of patient experience, as the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey only provides hospital-level data.
Corresponding author: Stephanie D. Smith, PhD, RN, UNCW School of Nursing, 601 South College Road, Wilmington, NC 28403; [email protected].
Funding disclosures: None.
1. Hannan EL, Zhong Y, Lahey SJ, et al. 30-day readmissions after coronary artery bypass graft surgery in New York State. JACC Cardiovasc Interv. 2011;4:569-576.
2. Feng TR, White R, Gaber-Baylis L, et al. Coronary artery bypass graft readmission rates and risk factors- A retrospective cohort study. Int J Surg. 2018;54 (Part A):7-17.
3. Donndorf P, Kaminski A. “Return to sender” or “consider it done”?! The importance of reducing hospital readmission after coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2017;154:1298-1299.
4. Sequist TD, Morong SM, Marston A, et al. Electronic risk alerts to improve primary care management of chest pain: A randomized, controlled trial. J Gen Intern Med. 2012;27:438-444.
5. Engelman D, Benjamin EM. Physician engagement: The “secret sauce” to success in bundled health care. Am J Med Qual. 2018;33:100-102.
1. Hannan EL, Zhong Y, Lahey SJ, et al. 30-day readmissions after coronary artery bypass graft surgery in New York State. JACC Cardiovasc Interv. 2011;4:569-576.
2. Feng TR, White R, Gaber-Baylis L, et al. Coronary artery bypass graft readmission rates and risk factors- A retrospective cohort study. Int J Surg. 2018;54 (Part A):7-17.
3. Donndorf P, Kaminski A. “Return to sender” or “consider it done”?! The importance of reducing hospital readmission after coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2017;154:1298-1299.
4. Sequist TD, Morong SM, Marston A, et al. Electronic risk alerts to improve primary care management of chest pain: A randomized, controlled trial. J Gen Intern Med. 2012;27:438-444.
5. Engelman D, Benjamin EM. Physician engagement: The “secret sauce” to success in bundled health care. Am J Med Qual. 2018;33:100-102.











