User login
Want to keep cancer patients and providers safe during the pandemic? Here’s how
according to the authors of a special feature article in the Journal of the National Comprehensive Cancer Network.
Prescreening, telemedicine, and limiting procedures top the authors’ list of 10 recommendations for ensuring patient safety in U.S. oncology practices. Assuring appropriate personal proctective equipment (PPE), encouraging telecommuting, and providing wellness/stress management are a few of the ways to look out for health care worker safety during the crisis.
These recommendations were drafted to provide guidance during the rapidly evolving global pandemic that, in some cases, has deluged health care delivery systems and strained the ability of providers to assure safe and effective care, said lead author Pelin Cinar, MD, of the Hellen Diller Family Comprehensive Cancer Center at the University of California, San Francisco.
“I think we have been so overwhelmed that sometimes it’s difficult to get organized in our thought processes,” Dr. Cinar said in an interview. “So this [article] was really trying to provide some structure to each of the different steps that we should be addressing at minimum.”
Screening patients
Prescreening systems are a critical first step to ensure cancer centers are helping control community spread of the virus, according to the article. Whether done by phone or online, prescreening 1-2 days before a patient’s visit can help identify COVID-19 symptoms and exposure history, guiding whether patients need to be evaluated, monitored, or referred to an ED.
Next, screening clinics can help ensure cancer patients with COVID-19 symptoms are evaluated and tested in a unit with dedicated staff, according to the article.
“If symptomatic patients present to the cancer center for treatment after a negative prescreening assessment, they must be provided with a mask and directed to a screening clinic for evaluation and potential testing before moving forward with any cancer-directed therapy,” the article states.
Telemedicine and treatment
Telemedicine visits should be done whenever possible to avoid in-person visits, according to the article. Dr. Cinar said that her center, like other cancer centers, has seen a major uptick in these visits, which are typically done over video. In February, there were a total of 232 video visits at her center, which jumped to 1,702 in March, or an approximate 600% increase.
“Even though we had a relatively robust presence [before the pandemic], we still weren’t at a level where we are now,” Dr. Cinar said.
When it comes to cancer treatment, surgeries and procedures should be limited to essential or urgent cases, and, if possible, chemotherapy and systemic therapy regimens can be modified to allow for fewer visits to the cancer center or infusion center, according to the article.
Transitions to outpatient care can help further reduce the need for in-person visits, while intervals between scans can be increased, or biochemical markers can be used instead of scans.
Protecting providers
Health care workers providing cancer care should be assured appropriate PPE, and websites or other centralized resources should be in place to make sure workers are aware of current PPE guidelines and changes in workflow, according to the article.
The authors note that daily screening tools or temperature checks of symptomatic workers can help decrease the risk of exposure to others. The authors also recommend establishing clear rules for when health care workers with suspected or confirmed COVID-19 should be staying at home and returning to the job.
Telecommuting should be encouraged, with limited staff participating in onsite rotations to further reduce exposure risks, the article states.
Anxiety, insomnia, and distress have been reported among frontline health care workers managing patients with COVID-19, according to the article, which recommends wellness and stress management resources be available as an “invaluable resource” in cancer centers.
“We have to take care of ourselves to be able to take care of others,” Dr. Cinar said. “With PPE, you’re physically protecting yourself, while self-care, stress management, and wellness are also a big component of protecting ourselves.”
The report by Dr. Cinar and colleagues was an invited article from the NCCN Best Practices Committee. One coauthor reported relationships with Abbvie, Adaptive Biotechnologies, Aduro, and several other companies. Dr. Cinar and the remaining authors said they had no relevant conflicts of interest.
SOURCE: Cinar P et al. J Natl Compr Canc Netw. 2020 Apr 15. doi: 10.6004/jnccn.2020.7572.
according to the authors of a special feature article in the Journal of the National Comprehensive Cancer Network.
Prescreening, telemedicine, and limiting procedures top the authors’ list of 10 recommendations for ensuring patient safety in U.S. oncology practices. Assuring appropriate personal proctective equipment (PPE), encouraging telecommuting, and providing wellness/stress management are a few of the ways to look out for health care worker safety during the crisis.
These recommendations were drafted to provide guidance during the rapidly evolving global pandemic that, in some cases, has deluged health care delivery systems and strained the ability of providers to assure safe and effective care, said lead author Pelin Cinar, MD, of the Hellen Diller Family Comprehensive Cancer Center at the University of California, San Francisco.
“I think we have been so overwhelmed that sometimes it’s difficult to get organized in our thought processes,” Dr. Cinar said in an interview. “So this [article] was really trying to provide some structure to each of the different steps that we should be addressing at minimum.”
Screening patients
Prescreening systems are a critical first step to ensure cancer centers are helping control community spread of the virus, according to the article. Whether done by phone or online, prescreening 1-2 days before a patient’s visit can help identify COVID-19 symptoms and exposure history, guiding whether patients need to be evaluated, monitored, or referred to an ED.
Next, screening clinics can help ensure cancer patients with COVID-19 symptoms are evaluated and tested in a unit with dedicated staff, according to the article.
“If symptomatic patients present to the cancer center for treatment after a negative prescreening assessment, they must be provided with a mask and directed to a screening clinic for evaluation and potential testing before moving forward with any cancer-directed therapy,” the article states.
Telemedicine and treatment
Telemedicine visits should be done whenever possible to avoid in-person visits, according to the article. Dr. Cinar said that her center, like other cancer centers, has seen a major uptick in these visits, which are typically done over video. In February, there were a total of 232 video visits at her center, which jumped to 1,702 in March, or an approximate 600% increase.
“Even though we had a relatively robust presence [before the pandemic], we still weren’t at a level where we are now,” Dr. Cinar said.
When it comes to cancer treatment, surgeries and procedures should be limited to essential or urgent cases, and, if possible, chemotherapy and systemic therapy regimens can be modified to allow for fewer visits to the cancer center or infusion center, according to the article.
Transitions to outpatient care can help further reduce the need for in-person visits, while intervals between scans can be increased, or biochemical markers can be used instead of scans.
Protecting providers
Health care workers providing cancer care should be assured appropriate PPE, and websites or other centralized resources should be in place to make sure workers are aware of current PPE guidelines and changes in workflow, according to the article.
The authors note that daily screening tools or temperature checks of symptomatic workers can help decrease the risk of exposure to others. The authors also recommend establishing clear rules for when health care workers with suspected or confirmed COVID-19 should be staying at home and returning to the job.
Telecommuting should be encouraged, with limited staff participating in onsite rotations to further reduce exposure risks, the article states.
Anxiety, insomnia, and distress have been reported among frontline health care workers managing patients with COVID-19, according to the article, which recommends wellness and stress management resources be available as an “invaluable resource” in cancer centers.
“We have to take care of ourselves to be able to take care of others,” Dr. Cinar said. “With PPE, you’re physically protecting yourself, while self-care, stress management, and wellness are also a big component of protecting ourselves.”
The report by Dr. Cinar and colleagues was an invited article from the NCCN Best Practices Committee. One coauthor reported relationships with Abbvie, Adaptive Biotechnologies, Aduro, and several other companies. Dr. Cinar and the remaining authors said they had no relevant conflicts of interest.
SOURCE: Cinar P et al. J Natl Compr Canc Netw. 2020 Apr 15. doi: 10.6004/jnccn.2020.7572.
according to the authors of a special feature article in the Journal of the National Comprehensive Cancer Network.
Prescreening, telemedicine, and limiting procedures top the authors’ list of 10 recommendations for ensuring patient safety in U.S. oncology practices. Assuring appropriate personal proctective equipment (PPE), encouraging telecommuting, and providing wellness/stress management are a few of the ways to look out for health care worker safety during the crisis.
These recommendations were drafted to provide guidance during the rapidly evolving global pandemic that, in some cases, has deluged health care delivery systems and strained the ability of providers to assure safe and effective care, said lead author Pelin Cinar, MD, of the Hellen Diller Family Comprehensive Cancer Center at the University of California, San Francisco.
“I think we have been so overwhelmed that sometimes it’s difficult to get organized in our thought processes,” Dr. Cinar said in an interview. “So this [article] was really trying to provide some structure to each of the different steps that we should be addressing at minimum.”
Screening patients
Prescreening systems are a critical first step to ensure cancer centers are helping control community spread of the virus, according to the article. Whether done by phone or online, prescreening 1-2 days before a patient’s visit can help identify COVID-19 symptoms and exposure history, guiding whether patients need to be evaluated, monitored, or referred to an ED.
Next, screening clinics can help ensure cancer patients with COVID-19 symptoms are evaluated and tested in a unit with dedicated staff, according to the article.
“If symptomatic patients present to the cancer center for treatment after a negative prescreening assessment, they must be provided with a mask and directed to a screening clinic for evaluation and potential testing before moving forward with any cancer-directed therapy,” the article states.
Telemedicine and treatment
Telemedicine visits should be done whenever possible to avoid in-person visits, according to the article. Dr. Cinar said that her center, like other cancer centers, has seen a major uptick in these visits, which are typically done over video. In February, there were a total of 232 video visits at her center, which jumped to 1,702 in March, or an approximate 600% increase.
“Even though we had a relatively robust presence [before the pandemic], we still weren’t at a level where we are now,” Dr. Cinar said.
When it comes to cancer treatment, surgeries and procedures should be limited to essential or urgent cases, and, if possible, chemotherapy and systemic therapy regimens can be modified to allow for fewer visits to the cancer center or infusion center, according to the article.
Transitions to outpatient care can help further reduce the need for in-person visits, while intervals between scans can be increased, or biochemical markers can be used instead of scans.
Protecting providers
Health care workers providing cancer care should be assured appropriate PPE, and websites or other centralized resources should be in place to make sure workers are aware of current PPE guidelines and changes in workflow, according to the article.
The authors note that daily screening tools or temperature checks of symptomatic workers can help decrease the risk of exposure to others. The authors also recommend establishing clear rules for when health care workers with suspected or confirmed COVID-19 should be staying at home and returning to the job.
Telecommuting should be encouraged, with limited staff participating in onsite rotations to further reduce exposure risks, the article states.
Anxiety, insomnia, and distress have been reported among frontline health care workers managing patients with COVID-19, according to the article, which recommends wellness and stress management resources be available as an “invaluable resource” in cancer centers.
“We have to take care of ourselves to be able to take care of others,” Dr. Cinar said. “With PPE, you’re physically protecting yourself, while self-care, stress management, and wellness are also a big component of protecting ourselves.”
The report by Dr. Cinar and colleagues was an invited article from the NCCN Best Practices Committee. One coauthor reported relationships with Abbvie, Adaptive Biotechnologies, Aduro, and several other companies. Dr. Cinar and the remaining authors said they had no relevant conflicts of interest.
SOURCE: Cinar P et al. J Natl Compr Canc Netw. 2020 Apr 15. doi: 10.6004/jnccn.2020.7572.
FROM THE JOURNAL OF THE NATIONAL COMPREHENSIVE CANCER NETWORK
Cancer care ‘transformed in space of a month’ because of pandemic
, the most “revolutionary” being a deep dive into telehealth, predicts Deborah Schrag, MD, MPH, a medical oncologist specializing in gastrointestinal cancers at the Dana Farber Cancer Institute in Boston, Massachusetts.
“In the space of a month, approaches and accepted norms of cancer care delivery have been transformed of necessity,” Schrag and colleagues write in an article published in JAMA on April 13.
“Most of these changes would not have occurred without the pandemic,” they add. They predict that some changes will last after the crisis is over.
“None of us want to be thrown in the deep end.... On the other hand, sometimes it works,” Schrag told Medscape Medical News.
“The in-person visit between patient and physician has been upended,” she said.
“I don’t think there’s any going back to the way it was before because cancer patients won’t stand for it,” she said. “They’re not going to drive in to get the results of a blood test.
“I think that on balance, of course, there are situations where you need eye-to-eye contact. No one wants to have an initial oncology meeting by telehealth – doctors or patients – that’s ridiculous,” she said. “But for follow-up visits, patients are now going to be more demanding, and doctors will be more willing.”
The “essential empathy” of oncologists can still “transcend the new physical barriers presented by masks and telehealth,” Schrag and colleagues comment.
“Doctors are figuring out how to deliver empathy by Zoom,” she told Medscape Medical News. “It’s not the same, but we all convey empathy to our elderly relatives over the phone.”
Pandemic impact on oncology
While the crisis has affected all of medicine – dismantling how care is delivered and forcing clinicians to make difficult decisions regarding triage – the fact that some cancers present an immediate threat to survival means that oncology “provides a lens into the major shifts currently underway in clinical care,” Schrag and colleagues write.
They illustrate the point by highlighting systemic chemotherapy, which is provided to a large proportion of patients with advanced cancer. The pandemic has tipped the risk-benefit ratio away from treatments that have a marginal effect on quality or quantity of life, they note. It has forced an “elimination of low-value treatments that were identified by the Choosing Wisely campaign,” the authors write. Up to now, the uptake of recommendations to eliminate these treatments has been slow.
“For example, for most metastatic solid tumors, chemotherapy beyond the third regimen does not improve survival for more than a few weeks; therefore, oncologists are advising supportive care instead. For patients receiving adjuvant therapy for curable cancers, delaying initiation or abbreviating the number of cycles is appropriate. Oncologists are postponing initiation of adjuvant chemotherapy for some estrogen receptor–negative stage II breast cancers by 8 weeks and administering 6 rather than 12 cycles of adjuvant chemotherapy for stage III colorectal cancers,” Schrag and colleagues write.
On the other hand, even in the epicenters of the pandemic, thus far, oncologists are still delivering cancer treatments that have the potential to cure and cannot safely be delayed, they point out. “This includes most patients with new diagnoses of acute leukemia, high-grade lymphoma, and those with chemotherapy-responsive tumors such as testicular, ovarian, and small cell lung cancer. Despite the risks, oncologists are not modifying such treatments because these cancers are likely more lethal than COVID-19.”
It’s the cancer patients who fall in between these two extremes who pose the biggest treatment challenge during this crisis – the patients for whom a delay would have “moderate clinically important adverse influence on quality of life or survival.” In these cases, oncologists are “prescribing marginally less effective regimens that have lower risk of precipitating hospitalization,” the authors note.
These treatments include the use of “white cell growth factor, more stringent neutrophil counts for proceeding with a next cycle of therapy, and omitting use of steroids to manage nausea.” In addition, where possible, oncologists are substituting oral agents for intravenous agents and “myriad other modifications to minimize visits and hospitalizations.”
Most hospitals and outpatient infusion centers now prohibit visitors from accompanying patients, and oncologists are prioritizing conversations with patients about advance directives, healthcare proxies, and end-of-life care preferences. Yet, even here, telehealth offers a new, enhanced layer to those conversations by enabling families to gather with their loved one and the doctor, she said.
This article first appeared on Medscape.com.
, the most “revolutionary” being a deep dive into telehealth, predicts Deborah Schrag, MD, MPH, a medical oncologist specializing in gastrointestinal cancers at the Dana Farber Cancer Institute in Boston, Massachusetts.
“In the space of a month, approaches and accepted norms of cancer care delivery have been transformed of necessity,” Schrag and colleagues write in an article published in JAMA on April 13.
“Most of these changes would not have occurred without the pandemic,” they add. They predict that some changes will last after the crisis is over.
“None of us want to be thrown in the deep end.... On the other hand, sometimes it works,” Schrag told Medscape Medical News.
“The in-person visit between patient and physician has been upended,” she said.
“I don’t think there’s any going back to the way it was before because cancer patients won’t stand for it,” she said. “They’re not going to drive in to get the results of a blood test.
“I think that on balance, of course, there are situations where you need eye-to-eye contact. No one wants to have an initial oncology meeting by telehealth – doctors or patients – that’s ridiculous,” she said. “But for follow-up visits, patients are now going to be more demanding, and doctors will be more willing.”
The “essential empathy” of oncologists can still “transcend the new physical barriers presented by masks and telehealth,” Schrag and colleagues comment.
“Doctors are figuring out how to deliver empathy by Zoom,” she told Medscape Medical News. “It’s not the same, but we all convey empathy to our elderly relatives over the phone.”
Pandemic impact on oncology
While the crisis has affected all of medicine – dismantling how care is delivered and forcing clinicians to make difficult decisions regarding triage – the fact that some cancers present an immediate threat to survival means that oncology “provides a lens into the major shifts currently underway in clinical care,” Schrag and colleagues write.
They illustrate the point by highlighting systemic chemotherapy, which is provided to a large proportion of patients with advanced cancer. The pandemic has tipped the risk-benefit ratio away from treatments that have a marginal effect on quality or quantity of life, they note. It has forced an “elimination of low-value treatments that were identified by the Choosing Wisely campaign,” the authors write. Up to now, the uptake of recommendations to eliminate these treatments has been slow.
“For example, for most metastatic solid tumors, chemotherapy beyond the third regimen does not improve survival for more than a few weeks; therefore, oncologists are advising supportive care instead. For patients receiving adjuvant therapy for curable cancers, delaying initiation or abbreviating the number of cycles is appropriate. Oncologists are postponing initiation of adjuvant chemotherapy for some estrogen receptor–negative stage II breast cancers by 8 weeks and administering 6 rather than 12 cycles of adjuvant chemotherapy for stage III colorectal cancers,” Schrag and colleagues write.
On the other hand, even in the epicenters of the pandemic, thus far, oncologists are still delivering cancer treatments that have the potential to cure and cannot safely be delayed, they point out. “This includes most patients with new diagnoses of acute leukemia, high-grade lymphoma, and those with chemotherapy-responsive tumors such as testicular, ovarian, and small cell lung cancer. Despite the risks, oncologists are not modifying such treatments because these cancers are likely more lethal than COVID-19.”
It’s the cancer patients who fall in between these two extremes who pose the biggest treatment challenge during this crisis – the patients for whom a delay would have “moderate clinically important adverse influence on quality of life or survival.” In these cases, oncologists are “prescribing marginally less effective regimens that have lower risk of precipitating hospitalization,” the authors note.
These treatments include the use of “white cell growth factor, more stringent neutrophil counts for proceeding with a next cycle of therapy, and omitting use of steroids to manage nausea.” In addition, where possible, oncologists are substituting oral agents for intravenous agents and “myriad other modifications to minimize visits and hospitalizations.”
Most hospitals and outpatient infusion centers now prohibit visitors from accompanying patients, and oncologists are prioritizing conversations with patients about advance directives, healthcare proxies, and end-of-life care preferences. Yet, even here, telehealth offers a new, enhanced layer to those conversations by enabling families to gather with their loved one and the doctor, she said.
This article first appeared on Medscape.com.
, the most “revolutionary” being a deep dive into telehealth, predicts Deborah Schrag, MD, MPH, a medical oncologist specializing in gastrointestinal cancers at the Dana Farber Cancer Institute in Boston, Massachusetts.
“In the space of a month, approaches and accepted norms of cancer care delivery have been transformed of necessity,” Schrag and colleagues write in an article published in JAMA on April 13.
“Most of these changes would not have occurred without the pandemic,” they add. They predict that some changes will last after the crisis is over.
“None of us want to be thrown in the deep end.... On the other hand, sometimes it works,” Schrag told Medscape Medical News.
“The in-person visit between patient and physician has been upended,” she said.
“I don’t think there’s any going back to the way it was before because cancer patients won’t stand for it,” she said. “They’re not going to drive in to get the results of a blood test.
“I think that on balance, of course, there are situations where you need eye-to-eye contact. No one wants to have an initial oncology meeting by telehealth – doctors or patients – that’s ridiculous,” she said. “But for follow-up visits, patients are now going to be more demanding, and doctors will be more willing.”
The “essential empathy” of oncologists can still “transcend the new physical barriers presented by masks and telehealth,” Schrag and colleagues comment.
“Doctors are figuring out how to deliver empathy by Zoom,” she told Medscape Medical News. “It’s not the same, but we all convey empathy to our elderly relatives over the phone.”
Pandemic impact on oncology
While the crisis has affected all of medicine – dismantling how care is delivered and forcing clinicians to make difficult decisions regarding triage – the fact that some cancers present an immediate threat to survival means that oncology “provides a lens into the major shifts currently underway in clinical care,” Schrag and colleagues write.
They illustrate the point by highlighting systemic chemotherapy, which is provided to a large proportion of patients with advanced cancer. The pandemic has tipped the risk-benefit ratio away from treatments that have a marginal effect on quality or quantity of life, they note. It has forced an “elimination of low-value treatments that were identified by the Choosing Wisely campaign,” the authors write. Up to now, the uptake of recommendations to eliminate these treatments has been slow.
“For example, for most metastatic solid tumors, chemotherapy beyond the third regimen does not improve survival for more than a few weeks; therefore, oncologists are advising supportive care instead. For patients receiving adjuvant therapy for curable cancers, delaying initiation or abbreviating the number of cycles is appropriate. Oncologists are postponing initiation of adjuvant chemotherapy for some estrogen receptor–negative stage II breast cancers by 8 weeks and administering 6 rather than 12 cycles of adjuvant chemotherapy for stage III colorectal cancers,” Schrag and colleagues write.
On the other hand, even in the epicenters of the pandemic, thus far, oncologists are still delivering cancer treatments that have the potential to cure and cannot safely be delayed, they point out. “This includes most patients with new diagnoses of acute leukemia, high-grade lymphoma, and those with chemotherapy-responsive tumors such as testicular, ovarian, and small cell lung cancer. Despite the risks, oncologists are not modifying such treatments because these cancers are likely more lethal than COVID-19.”
It’s the cancer patients who fall in between these two extremes who pose the biggest treatment challenge during this crisis – the patients for whom a delay would have “moderate clinically important adverse influence on quality of life or survival.” In these cases, oncologists are “prescribing marginally less effective regimens that have lower risk of precipitating hospitalization,” the authors note.
These treatments include the use of “white cell growth factor, more stringent neutrophil counts for proceeding with a next cycle of therapy, and omitting use of steroids to manage nausea.” In addition, where possible, oncologists are substituting oral agents for intravenous agents and “myriad other modifications to minimize visits and hospitalizations.”
Most hospitals and outpatient infusion centers now prohibit visitors from accompanying patients, and oncologists are prioritizing conversations with patients about advance directives, healthcare proxies, and end-of-life care preferences. Yet, even here, telehealth offers a new, enhanced layer to those conversations by enabling families to gather with their loved one and the doctor, she said.
This article first appeared on Medscape.com.
Oncologists need to advocate for scarce COVID-19 resources: ASCO
both at the institutional and regional level, according to new recommendations from the American Society for Clinical Oncology (ASCO).
“There was a lot of concern from the oncology community that if a patient had cancer, they would be arbitrarily excluded from consideration for critical care resources,” said Jonathan M. Marron, MD, chair-elect of ASCO’s Ethics Committee and lead author of the recommendations.
“The hope is that we’ll never have to make any of these decisions ... but the primary reason for putting together these recommendations was that if such decisions have to be made, we hope to inform them,” he told Medscape Medical News.
Marron, who is a pediatric hematologist at Boston Children’s Hospital, says ASCO’s main recommendation is that decisions about the allocation of resources must be separated from bedside clinical care, meaning that clinicians who are caring for individual patients should not also be the ones making the allocation decisions.
“Those dueling responsibilities are a conflict of interest and make that physician unable to make an unbiased decision,” he said.
“It’s also just an unbearable burden to try and do those two things simultaneously,” he added. “It’s an incredible burden to do them individually, but it’s multifold worse to try to do them both simultaneously.”
He said the vital role of oncologists who provide treatment is to offer the kind of personalized information that triage committees need in order to make appropriate decisions.
“They should be asked – maybe even must be asked – to provide the most high-quality evidence-based data about their patients’ diagnosis and prognosis,” Marron commented. “Because oncology is evolving so rapidly, and cancer is so many different diseases, it’s impossible for someone making these decisions to know everything they would need to know about why this patient is likely to survive their cancer and this patient is not.”
He says that during the COVID-19 pandemic, concerns regarding public health transcend the well-being of individual patients and that consideration must be given to providing the maximum benefit to the greatest number of people.
“That makes perfect sense and is the appropriate and laudable goal during a public health emergency like this ... but one of the challenges is that there is this belief that a diagnosis of cancer is uniformly fatal,” Marron said.
“It’s certainly conceivable that it would be a better use of resources to give the last ventilator to a young, otherwise healthy patient rather than a patient with multiply recurrent progressive metastatic cancer,” he continued. “However, we want to ensure that there is at least a discussion where that information is made available, rather than just saying, ‘She’s got cancer. She’s a lost cause.’ ”
Cancer patients are doing very well
Concerns about cancer misconceptions have been circulating in the oncology community since the start of the pandemic. “It’s really important that people understand that cancer patients are doing very well nowadays, and even with a diagnosis of cancer, they can potentially live for many years,” Anne Chiang, MD, PhD, from the Smilow Cancer Network, New Haven, Connecticut, told Medscape Medical News in a recent interview.
Thus far, even in hard-hit New York City, fears that cancer patients may not be receiving appropriate care have not materialized, according to Mark Robson, MD, a medical oncologist at Memorial Sloan Kettering Cancer Center (MSKCC). “I would emphasize that cancer patients are ABSOLUTELY getting the care they need, including patients with metastatic disease,” he recently tweeted. “NOONE at @sloan_kettering (or anywhere else) is being ‘triaged’ because of advanced cancer. Period.”
Robson told Medscape Medical News that although MSKCC continues to provide oncology care to patients with cancer, “we are [also] treating them if they develop COVID. ... I am trying to help pivot the institution towards care in this setting.”
He said he agrees with Craig Spencer, MD, MPH, director of global health in emergency medicine at the New York–Presbyterian/Columbia University Medical Center, who recently tweeted, “If you need a ventilator, you get a ventilator. Let’s be clear – this isn’t being ‘rationed.’ ”
Marron emphasized that an important safeguard against uninformed decision making is appropriate planning. For hospitalized patients, this means oncologists who provide treatment should offer information even before it is requested. But he said the “duty to plan” begins long before that.
“Clinicians haven’t always been great at talking about death and long-term outcomes with their patients, but this really cranks up the importance of having those conversations, and having them early, even though it’s incredibly hard. If someone has expressed that they would never want to be put on a ventilator, it’s important now even more so that is made clear,” he said.
He said early responses to the ASCO statement suggest that it has calmed some concerns in the oncology community, “but it still remains to be seen whether individual institutions will take this to heart, because this unto itself cannot enforce anything – it is up to individual institutions. I am hopeful this will get to the people it needs to get to.”
This article first appeared on Medscape.com.
both at the institutional and regional level, according to new recommendations from the American Society for Clinical Oncology (ASCO).
“There was a lot of concern from the oncology community that if a patient had cancer, they would be arbitrarily excluded from consideration for critical care resources,” said Jonathan M. Marron, MD, chair-elect of ASCO’s Ethics Committee and lead author of the recommendations.
“The hope is that we’ll never have to make any of these decisions ... but the primary reason for putting together these recommendations was that if such decisions have to be made, we hope to inform them,” he told Medscape Medical News.
Marron, who is a pediatric hematologist at Boston Children’s Hospital, says ASCO’s main recommendation is that decisions about the allocation of resources must be separated from bedside clinical care, meaning that clinicians who are caring for individual patients should not also be the ones making the allocation decisions.
“Those dueling responsibilities are a conflict of interest and make that physician unable to make an unbiased decision,” he said.
“It’s also just an unbearable burden to try and do those two things simultaneously,” he added. “It’s an incredible burden to do them individually, but it’s multifold worse to try to do them both simultaneously.”
He said the vital role of oncologists who provide treatment is to offer the kind of personalized information that triage committees need in order to make appropriate decisions.
“They should be asked – maybe even must be asked – to provide the most high-quality evidence-based data about their patients’ diagnosis and prognosis,” Marron commented. “Because oncology is evolving so rapidly, and cancer is so many different diseases, it’s impossible for someone making these decisions to know everything they would need to know about why this patient is likely to survive their cancer and this patient is not.”
He says that during the COVID-19 pandemic, concerns regarding public health transcend the well-being of individual patients and that consideration must be given to providing the maximum benefit to the greatest number of people.
“That makes perfect sense and is the appropriate and laudable goal during a public health emergency like this ... but one of the challenges is that there is this belief that a diagnosis of cancer is uniformly fatal,” Marron said.
“It’s certainly conceivable that it would be a better use of resources to give the last ventilator to a young, otherwise healthy patient rather than a patient with multiply recurrent progressive metastatic cancer,” he continued. “However, we want to ensure that there is at least a discussion where that information is made available, rather than just saying, ‘She’s got cancer. She’s a lost cause.’ ”
Cancer patients are doing very well
Concerns about cancer misconceptions have been circulating in the oncology community since the start of the pandemic. “It’s really important that people understand that cancer patients are doing very well nowadays, and even with a diagnosis of cancer, they can potentially live for many years,” Anne Chiang, MD, PhD, from the Smilow Cancer Network, New Haven, Connecticut, told Medscape Medical News in a recent interview.
Thus far, even in hard-hit New York City, fears that cancer patients may not be receiving appropriate care have not materialized, according to Mark Robson, MD, a medical oncologist at Memorial Sloan Kettering Cancer Center (MSKCC). “I would emphasize that cancer patients are ABSOLUTELY getting the care they need, including patients with metastatic disease,” he recently tweeted. “NOONE at @sloan_kettering (or anywhere else) is being ‘triaged’ because of advanced cancer. Period.”
Robson told Medscape Medical News that although MSKCC continues to provide oncology care to patients with cancer, “we are [also] treating them if they develop COVID. ... I am trying to help pivot the institution towards care in this setting.”
He said he agrees with Craig Spencer, MD, MPH, director of global health in emergency medicine at the New York–Presbyterian/Columbia University Medical Center, who recently tweeted, “If you need a ventilator, you get a ventilator. Let’s be clear – this isn’t being ‘rationed.’ ”
Marron emphasized that an important safeguard against uninformed decision making is appropriate planning. For hospitalized patients, this means oncologists who provide treatment should offer information even before it is requested. But he said the “duty to plan” begins long before that.
“Clinicians haven’t always been great at talking about death and long-term outcomes with their patients, but this really cranks up the importance of having those conversations, and having them early, even though it’s incredibly hard. If someone has expressed that they would never want to be put on a ventilator, it’s important now even more so that is made clear,” he said.
He said early responses to the ASCO statement suggest that it has calmed some concerns in the oncology community, “but it still remains to be seen whether individual institutions will take this to heart, because this unto itself cannot enforce anything – it is up to individual institutions. I am hopeful this will get to the people it needs to get to.”
This article first appeared on Medscape.com.
both at the institutional and regional level, according to new recommendations from the American Society for Clinical Oncology (ASCO).
“There was a lot of concern from the oncology community that if a patient had cancer, they would be arbitrarily excluded from consideration for critical care resources,” said Jonathan M. Marron, MD, chair-elect of ASCO’s Ethics Committee and lead author of the recommendations.
“The hope is that we’ll never have to make any of these decisions ... but the primary reason for putting together these recommendations was that if such decisions have to be made, we hope to inform them,” he told Medscape Medical News.
Marron, who is a pediatric hematologist at Boston Children’s Hospital, says ASCO’s main recommendation is that decisions about the allocation of resources must be separated from bedside clinical care, meaning that clinicians who are caring for individual patients should not also be the ones making the allocation decisions.
“Those dueling responsibilities are a conflict of interest and make that physician unable to make an unbiased decision,” he said.
“It’s also just an unbearable burden to try and do those two things simultaneously,” he added. “It’s an incredible burden to do them individually, but it’s multifold worse to try to do them both simultaneously.”
He said the vital role of oncologists who provide treatment is to offer the kind of personalized information that triage committees need in order to make appropriate decisions.
“They should be asked – maybe even must be asked – to provide the most high-quality evidence-based data about their patients’ diagnosis and prognosis,” Marron commented. “Because oncology is evolving so rapidly, and cancer is so many different diseases, it’s impossible for someone making these decisions to know everything they would need to know about why this patient is likely to survive their cancer and this patient is not.”
He says that during the COVID-19 pandemic, concerns regarding public health transcend the well-being of individual patients and that consideration must be given to providing the maximum benefit to the greatest number of people.
“That makes perfect sense and is the appropriate and laudable goal during a public health emergency like this ... but one of the challenges is that there is this belief that a diagnosis of cancer is uniformly fatal,” Marron said.
“It’s certainly conceivable that it would be a better use of resources to give the last ventilator to a young, otherwise healthy patient rather than a patient with multiply recurrent progressive metastatic cancer,” he continued. “However, we want to ensure that there is at least a discussion where that information is made available, rather than just saying, ‘She’s got cancer. She’s a lost cause.’ ”
Cancer patients are doing very well
Concerns about cancer misconceptions have been circulating in the oncology community since the start of the pandemic. “It’s really important that people understand that cancer patients are doing very well nowadays, and even with a diagnosis of cancer, they can potentially live for many years,” Anne Chiang, MD, PhD, from the Smilow Cancer Network, New Haven, Connecticut, told Medscape Medical News in a recent interview.
Thus far, even in hard-hit New York City, fears that cancer patients may not be receiving appropriate care have not materialized, according to Mark Robson, MD, a medical oncologist at Memorial Sloan Kettering Cancer Center (MSKCC). “I would emphasize that cancer patients are ABSOLUTELY getting the care they need, including patients with metastatic disease,” he recently tweeted. “NOONE at @sloan_kettering (or anywhere else) is being ‘triaged’ because of advanced cancer. Period.”
Robson told Medscape Medical News that although MSKCC continues to provide oncology care to patients with cancer, “we are [also] treating them if they develop COVID. ... I am trying to help pivot the institution towards care in this setting.”
He said he agrees with Craig Spencer, MD, MPH, director of global health in emergency medicine at the New York–Presbyterian/Columbia University Medical Center, who recently tweeted, “If you need a ventilator, you get a ventilator. Let’s be clear – this isn’t being ‘rationed.’ ”
Marron emphasized that an important safeguard against uninformed decision making is appropriate planning. For hospitalized patients, this means oncologists who provide treatment should offer information even before it is requested. But he said the “duty to plan” begins long before that.
“Clinicians haven’t always been great at talking about death and long-term outcomes with their patients, but this really cranks up the importance of having those conversations, and having them early, even though it’s incredibly hard. If someone has expressed that they would never want to be put on a ventilator, it’s important now even more so that is made clear,” he said.
He said early responses to the ASCO statement suggest that it has calmed some concerns in the oncology community, “but it still remains to be seen whether individual institutions will take this to heart, because this unto itself cannot enforce anything – it is up to individual institutions. I am hopeful this will get to the people it needs to get to.”
This article first appeared on Medscape.com.
COVID-19 causes financial woes for GI practices
On a typical clinic day, Will Bulsiewicz, MD, a Charleston, S.C.–based gastroenterologist, used to see 22 patients, while other days were filled with up to 16 procedures.
Since COVID-19 however, things have vastly changed. Dr. Bulsiewicz now visits with all clinic patients through telehealth, and the volume has dipped to between zero and six patients per day. His three-doctor practice has also experienced a more than 90% reduction in endoscopy volume.
“Naturally, this has been devastating,” Dr. Bulsiewicz said in an interview. “Our practice was started in 1984, and we had a business model that we used for the history of our practice. That practice model was upended in a matter of 2 weeks.”
Dr. Bulsiewicz is far from alone. Community GI practices across the country are experiencing similar financial distress in the face of COVID-19. In addition to a decrease in patient referrals, the Centers for Medicare & Medicaid Services has requested that all elective esophagogastroduodenoscopies, colonoscopies, endoscopies, surgeries, and procedures be delayed during the coronavirus outbreak to conserve critical equipment and limit virus exposure. The guidance aligns with recent recommendations issued by American Gastroenterological Association, American Association for the Study of Liver Diseases, American College of Gastroenterology, and American Society for Gastrointestinal Endoscopy. The lack of patients has led to plummeting revenue for many GI practices and resulted in layoffs, reduced hours, and limited salaries in order to keep practices afloat.
“We’ve had to make drastic changes in the way we work,” said Rajeev Jain, MD, AGAF, a Dallas-based gastroenterologist. “The way private practices are economically set up, they don’t have large reserves of capital or liquidity. We’re not like Apple or these big companies that have these massive cushions. It’s one thing when you have a downturn in the economy and less people come to get care, but when you have a complete shutdown, your revenue stream to pay your bills is literally dried up.”
Dr. Jain’s practice is part of Texas Digestive Disease Consultants (TDDC), which provides GI care for patients in Texas and Louisiana. TDDC is part of GI Alliance, a private equity–based consolidation of practices that includes several states and more than 350 GIs. The management services organization is a collaboration between the PE firm and the partner physicians. Since the COVID-19 outbreak, Dr. Jain said his practice has seen a dramatic drop in patients. Normally, Dr. Jain would perform between 25 and 30 outpatient scopes over the course of 2 days, he said. On a recent Monday, he performed two procedures. To preserve cash flow, Dr. Jain said he and his senior partners are not taking an income right now. Some employees were recently furloughed and laid off.
“I never in my life thought that I would have to lay off people because of an economic issue,” Dr. Jain said. “That’s psychological strain that as a physician owner you feel because these are people that you work with on a day-to-day basis and you don’t want them suffering either. That’s been a tough thing.
James S. Leavitt, MD, said his 17-physician center in Miami, Fla., has furloughed about half its staff. The center is part of Gastro Health, a private equity firm–based medical group with more than 250 providers in four states. Dr. Leavitt, president and chief clinical officer for Gastro Health, said his center has gone from about 150 patients per day to 5 or fewer, while procedures have dropped from more than 100 a day to maybe 5.
Having partnered with a private equity firm, however, Dr. Leavitt believes his practice is bettered situated to manage the health crisis and address financial challenges.
“It’s made us better prepared to weather the storm. We have a very high-powered, sophisticated administration and much broader base and access to capital. [For example,] we had a lot of depth in management so that we could roll out a robust televisit program in a week in four states with over 250 doctors.”
From a business standpoint, however, certain goals for the company are on hold, he said, such as closing on potential acquisitions.
Telemedicine works well for many patients, particularly for follow-up patients and for patients who have an established relationship with Dr. Leavitt, he said. There are limitations of course, he noted.
“If I were a dermatologist, maybe I could see the skin rash, but you can’t examine the patient,” he said. “There are certain things you can’t do. If a patient has significant abdominal pain, a televisit isn’t the greatest.”
That’s why Dr. Leavitt’s care center remains open for the handful of patients who must be seen in-person, he said. Those patients are screened beforehand and their temperatures taken before treatment.
Dr. Bulsiewicz’s practice made the transition to telehealth after never having used the modality before COVID-19.
“This was a scramble,” said Dr. Bulsiewicz, who posts about COVID-19 on social media. “We started from zero knowledge to implementation in less than a week.”
Overall, the switch went smoothly, but Dr. Bulsiewicz said reimbursement challenges come with telehealth.
“The billing is not the same,” he said. “You’re doing the same work or more, and you’re taking a reduced fee because of the antiquated fee structure that is forcing you to apply the typical rules of an office encounter.”
He hopes CMS will alter the reimbursement schedule to temporarily pay on par with traditional evaluation and management codes based on medical complexity as opposed to documentation of physical exam. CMS has already expanded Medicare telehealth coverage to cover a wider range of health care services in light of the COVID-19 crisis and also broadened the range of communication tools that can be used, according to a March announcement.
In the meantime, many practices have applied for financial assistance programs. The AGA recently pushed the government for additional assistance to help struggling practices.
Dr. Jain hopes these assistance programs roll out quickly.
“If these don’t get out there quick enough and big enough, we are going to see a massive wave of loss of independent practices and/or consolidation,” he said. “I fear a death to small, independent practices because they’re not going to have the financial wherewithal to tolerate this for too long.”
On a typical clinic day, Will Bulsiewicz, MD, a Charleston, S.C.–based gastroenterologist, used to see 22 patients, while other days were filled with up to 16 procedures.
Since COVID-19 however, things have vastly changed. Dr. Bulsiewicz now visits with all clinic patients through telehealth, and the volume has dipped to between zero and six patients per day. His three-doctor practice has also experienced a more than 90% reduction in endoscopy volume.
“Naturally, this has been devastating,” Dr. Bulsiewicz said in an interview. “Our practice was started in 1984, and we had a business model that we used for the history of our practice. That practice model was upended in a matter of 2 weeks.”
Dr. Bulsiewicz is far from alone. Community GI practices across the country are experiencing similar financial distress in the face of COVID-19. In addition to a decrease in patient referrals, the Centers for Medicare & Medicaid Services has requested that all elective esophagogastroduodenoscopies, colonoscopies, endoscopies, surgeries, and procedures be delayed during the coronavirus outbreak to conserve critical equipment and limit virus exposure. The guidance aligns with recent recommendations issued by American Gastroenterological Association, American Association for the Study of Liver Diseases, American College of Gastroenterology, and American Society for Gastrointestinal Endoscopy. The lack of patients has led to plummeting revenue for many GI practices and resulted in layoffs, reduced hours, and limited salaries in order to keep practices afloat.
“We’ve had to make drastic changes in the way we work,” said Rajeev Jain, MD, AGAF, a Dallas-based gastroenterologist. “The way private practices are economically set up, they don’t have large reserves of capital or liquidity. We’re not like Apple or these big companies that have these massive cushions. It’s one thing when you have a downturn in the economy and less people come to get care, but when you have a complete shutdown, your revenue stream to pay your bills is literally dried up.”
Dr. Jain’s practice is part of Texas Digestive Disease Consultants (TDDC), which provides GI care for patients in Texas and Louisiana. TDDC is part of GI Alliance, a private equity–based consolidation of practices that includes several states and more than 350 GIs. The management services organization is a collaboration between the PE firm and the partner physicians. Since the COVID-19 outbreak, Dr. Jain said his practice has seen a dramatic drop in patients. Normally, Dr. Jain would perform between 25 and 30 outpatient scopes over the course of 2 days, he said. On a recent Monday, he performed two procedures. To preserve cash flow, Dr. Jain said he and his senior partners are not taking an income right now. Some employees were recently furloughed and laid off.
“I never in my life thought that I would have to lay off people because of an economic issue,” Dr. Jain said. “That’s psychological strain that as a physician owner you feel because these are people that you work with on a day-to-day basis and you don’t want them suffering either. That’s been a tough thing.
James S. Leavitt, MD, said his 17-physician center in Miami, Fla., has furloughed about half its staff. The center is part of Gastro Health, a private equity firm–based medical group with more than 250 providers in four states. Dr. Leavitt, president and chief clinical officer for Gastro Health, said his center has gone from about 150 patients per day to 5 or fewer, while procedures have dropped from more than 100 a day to maybe 5.
Having partnered with a private equity firm, however, Dr. Leavitt believes his practice is bettered situated to manage the health crisis and address financial challenges.
“It’s made us better prepared to weather the storm. We have a very high-powered, sophisticated administration and much broader base and access to capital. [For example,] we had a lot of depth in management so that we could roll out a robust televisit program in a week in four states with over 250 doctors.”
From a business standpoint, however, certain goals for the company are on hold, he said, such as closing on potential acquisitions.
Telemedicine works well for many patients, particularly for follow-up patients and for patients who have an established relationship with Dr. Leavitt, he said. There are limitations of course, he noted.
“If I were a dermatologist, maybe I could see the skin rash, but you can’t examine the patient,” he said. “There are certain things you can’t do. If a patient has significant abdominal pain, a televisit isn’t the greatest.”
That’s why Dr. Leavitt’s care center remains open for the handful of patients who must be seen in-person, he said. Those patients are screened beforehand and their temperatures taken before treatment.
Dr. Bulsiewicz’s practice made the transition to telehealth after never having used the modality before COVID-19.
“This was a scramble,” said Dr. Bulsiewicz, who posts about COVID-19 on social media. “We started from zero knowledge to implementation in less than a week.”
Overall, the switch went smoothly, but Dr. Bulsiewicz said reimbursement challenges come with telehealth.
“The billing is not the same,” he said. “You’re doing the same work or more, and you’re taking a reduced fee because of the antiquated fee structure that is forcing you to apply the typical rules of an office encounter.”
He hopes CMS will alter the reimbursement schedule to temporarily pay on par with traditional evaluation and management codes based on medical complexity as opposed to documentation of physical exam. CMS has already expanded Medicare telehealth coverage to cover a wider range of health care services in light of the COVID-19 crisis and also broadened the range of communication tools that can be used, according to a March announcement.
In the meantime, many practices have applied for financial assistance programs. The AGA recently pushed the government for additional assistance to help struggling practices.
Dr. Jain hopes these assistance programs roll out quickly.
“If these don’t get out there quick enough and big enough, we are going to see a massive wave of loss of independent practices and/or consolidation,” he said. “I fear a death to small, independent practices because they’re not going to have the financial wherewithal to tolerate this for too long.”
On a typical clinic day, Will Bulsiewicz, MD, a Charleston, S.C.–based gastroenterologist, used to see 22 patients, while other days were filled with up to 16 procedures.
Since COVID-19 however, things have vastly changed. Dr. Bulsiewicz now visits with all clinic patients through telehealth, and the volume has dipped to between zero and six patients per day. His three-doctor practice has also experienced a more than 90% reduction in endoscopy volume.
“Naturally, this has been devastating,” Dr. Bulsiewicz said in an interview. “Our practice was started in 1984, and we had a business model that we used for the history of our practice. That practice model was upended in a matter of 2 weeks.”
Dr. Bulsiewicz is far from alone. Community GI practices across the country are experiencing similar financial distress in the face of COVID-19. In addition to a decrease in patient referrals, the Centers for Medicare & Medicaid Services has requested that all elective esophagogastroduodenoscopies, colonoscopies, endoscopies, surgeries, and procedures be delayed during the coronavirus outbreak to conserve critical equipment and limit virus exposure. The guidance aligns with recent recommendations issued by American Gastroenterological Association, American Association for the Study of Liver Diseases, American College of Gastroenterology, and American Society for Gastrointestinal Endoscopy. The lack of patients has led to plummeting revenue for many GI practices and resulted in layoffs, reduced hours, and limited salaries in order to keep practices afloat.
“We’ve had to make drastic changes in the way we work,” said Rajeev Jain, MD, AGAF, a Dallas-based gastroenterologist. “The way private practices are economically set up, they don’t have large reserves of capital or liquidity. We’re not like Apple or these big companies that have these massive cushions. It’s one thing when you have a downturn in the economy and less people come to get care, but when you have a complete shutdown, your revenue stream to pay your bills is literally dried up.”
Dr. Jain’s practice is part of Texas Digestive Disease Consultants (TDDC), which provides GI care for patients in Texas and Louisiana. TDDC is part of GI Alliance, a private equity–based consolidation of practices that includes several states and more than 350 GIs. The management services organization is a collaboration between the PE firm and the partner physicians. Since the COVID-19 outbreak, Dr. Jain said his practice has seen a dramatic drop in patients. Normally, Dr. Jain would perform between 25 and 30 outpatient scopes over the course of 2 days, he said. On a recent Monday, he performed two procedures. To preserve cash flow, Dr. Jain said he and his senior partners are not taking an income right now. Some employees were recently furloughed and laid off.
“I never in my life thought that I would have to lay off people because of an economic issue,” Dr. Jain said. “That’s psychological strain that as a physician owner you feel because these are people that you work with on a day-to-day basis and you don’t want them suffering either. That’s been a tough thing.
James S. Leavitt, MD, said his 17-physician center in Miami, Fla., has furloughed about half its staff. The center is part of Gastro Health, a private equity firm–based medical group with more than 250 providers in four states. Dr. Leavitt, president and chief clinical officer for Gastro Health, said his center has gone from about 150 patients per day to 5 or fewer, while procedures have dropped from more than 100 a day to maybe 5.
Having partnered with a private equity firm, however, Dr. Leavitt believes his practice is bettered situated to manage the health crisis and address financial challenges.
“It’s made us better prepared to weather the storm. We have a very high-powered, sophisticated administration and much broader base and access to capital. [For example,] we had a lot of depth in management so that we could roll out a robust televisit program in a week in four states with over 250 doctors.”
From a business standpoint, however, certain goals for the company are on hold, he said, such as closing on potential acquisitions.
Telemedicine works well for many patients, particularly for follow-up patients and for patients who have an established relationship with Dr. Leavitt, he said. There are limitations of course, he noted.
“If I were a dermatologist, maybe I could see the skin rash, but you can’t examine the patient,” he said. “There are certain things you can’t do. If a patient has significant abdominal pain, a televisit isn’t the greatest.”
That’s why Dr. Leavitt’s care center remains open for the handful of patients who must be seen in-person, he said. Those patients are screened beforehand and their temperatures taken before treatment.
Dr. Bulsiewicz’s practice made the transition to telehealth after never having used the modality before COVID-19.
“This was a scramble,” said Dr. Bulsiewicz, who posts about COVID-19 on social media. “We started from zero knowledge to implementation in less than a week.”
Overall, the switch went smoothly, but Dr. Bulsiewicz said reimbursement challenges come with telehealth.
“The billing is not the same,” he said. “You’re doing the same work or more, and you’re taking a reduced fee because of the antiquated fee structure that is forcing you to apply the typical rules of an office encounter.”
He hopes CMS will alter the reimbursement schedule to temporarily pay on par with traditional evaluation and management codes based on medical complexity as opposed to documentation of physical exam. CMS has already expanded Medicare telehealth coverage to cover a wider range of health care services in light of the COVID-19 crisis and also broadened the range of communication tools that can be used, according to a March announcement.
In the meantime, many practices have applied for financial assistance programs. The AGA recently pushed the government for additional assistance to help struggling practices.
Dr. Jain hopes these assistance programs roll out quickly.
“If these don’t get out there quick enough and big enough, we are going to see a massive wave of loss of independent practices and/or consolidation,” he said. “I fear a death to small, independent practices because they’re not going to have the financial wherewithal to tolerate this for too long.”
CMS implements temporary regulatory changes to aid COVID-19 response
The Centers for Medicare & Medicaid Services has announced a wide range of temporary regulatory moves aimed at helping hospitals and health systems handle the surge of COVID-19 patients.
“We are waiving a wide and unprecedented range of regulatory requirements to equip the American health care system with maximum flexibility to deal with an influx of cases,” CMS Administrator Seema Verma said during a March 30 conference call with reporters. “Many health care systems may not need these waivers and they shouldn’t use them if the situation doesn’t warrant it. But the flexibilities are there if it does. At a time of crisis, no regulatory barriers should stand in the way of patient care.”
Among the changes is an expansion of the venues in which health care systems and hospitals can provide services.
Federal regulations call for hospitals to provide services within their own buildings, raising concerns as to whether there will be enough capacity to handle the anticipated COVID-19 caseload.
“Under CMS’s temporary new rules, hospitals will be able to transfer patients to outside facilities, such as ambulatory surgery centers, inpatient rehabilitation hospitals, hotels, and dormitories, while still receiving hospital payments under Medicare,” the CMS stated in a fact sheet highlighting the regulatory changes.
Ambulatory surgery centers will have the option to contract with local health care systems to provide hospital services or they can enroll and bill as hospitals during the emergency, the fact sheet noted. They will be able to perform hospital services such as cancer procedures, trauma surgeries, and other essential surgeries.
CMS also is waiving the limit on the number of beds a doctor-owned hospital can have.
For Medicare patients who may be homebound, CMS will now pay for a laboratory technician to make a home visit to collect a specimen for COVID-19 testing, and hospitals will be able to conduct testing in homes or other community-based settings under certain circumstances.
In addition, CMS is taking actions aimed at expanding the health care workforce.
For instance, the agency is issuing a “blanket waiver” that allows hospitals to provide benefits to medical staff, including multiple daily meals, laundry service for personal clothing, or child care services while the staff is at the hospital providing patient care, according to the fact sheet. Teaching hospitals will also receive more flexibility in using residents to provide health care services under the virtual direction of a teaching physician, who may be available through audio/video technology.
CMS also is temporarily eliminating paperwork requirements and allowing greater use of verbal orders so that clinicians can spend more time on direct patient care.
Another change announced deals with the Merit-based Incentive Payment System (MIPS) track of the Quality Payment Program. Clinicians affected by the pandemic can request reweighting of the MIPS performance categories for the 2019 performance year, which will allow clinicians who are unable to submit MIPS data in the current submission period to request reweighting and receive a neutral payment adjustment in the 2021 payment year.
CMS also added an option for calendar year 2020 in the improvement activity category. Clinicians will receive credit if they are participating in a clinical trial that uses a drug or biological product to treat a COVID-19 patient and they report the findings to a clinical trial repository or registry.
On the device/equipment side, Medicare will cover respiratory-related devices and equipment “for any medical reason determined by clinicians,” according to the fact sheet, rather than only under certain circumstances.
And on the telehealth side, CMS is expanding the number of services that it will pay for via telehealth by more than 80, including ED visits, initial nursing facility and discharge visits, and home visits, which must be provided by a clinician that is allowed to provide telehealth. CMS will allow the use of commonly available interactive apps with audio and video, as well as audio-only phones.
CMS noted that providers can report telehealth for new and established patients, even if the billing code is specific for established patients only. CMS also has removed requirements regarding documentation of medical history and/or physical examination in the medical records during the COVID-19 crisis to help facilitate the use of telehealth for evaluation and management visits.
To help practices financially, providers who participate in Medicare fee-for-service will be able to request up to a 100% advance on their Medicare payments for a 3-month period. Repayment begins 120 days after the advance payment is received and must be paid within 210 days of the payment. Repayment will be automatically deducted from claims processed.
The agency also included new exceptions to the Stark Law. Some examples include the ability for hospitals to pay above or below fair market value to rent equipment or receive services from physicians and the allowance of health care providers to provide financial assistance to each other to ensure continuity of operations.
*This story was updated on 4/17/2020.
The Centers for Medicare & Medicaid Services has announced a wide range of temporary regulatory moves aimed at helping hospitals and health systems handle the surge of COVID-19 patients.
“We are waiving a wide and unprecedented range of regulatory requirements to equip the American health care system with maximum flexibility to deal with an influx of cases,” CMS Administrator Seema Verma said during a March 30 conference call with reporters. “Many health care systems may not need these waivers and they shouldn’t use them if the situation doesn’t warrant it. But the flexibilities are there if it does. At a time of crisis, no regulatory barriers should stand in the way of patient care.”
Among the changes is an expansion of the venues in which health care systems and hospitals can provide services.
Federal regulations call for hospitals to provide services within their own buildings, raising concerns as to whether there will be enough capacity to handle the anticipated COVID-19 caseload.
“Under CMS’s temporary new rules, hospitals will be able to transfer patients to outside facilities, such as ambulatory surgery centers, inpatient rehabilitation hospitals, hotels, and dormitories, while still receiving hospital payments under Medicare,” the CMS stated in a fact sheet highlighting the regulatory changes.
Ambulatory surgery centers will have the option to contract with local health care systems to provide hospital services or they can enroll and bill as hospitals during the emergency, the fact sheet noted. They will be able to perform hospital services such as cancer procedures, trauma surgeries, and other essential surgeries.
CMS also is waiving the limit on the number of beds a doctor-owned hospital can have.
For Medicare patients who may be homebound, CMS will now pay for a laboratory technician to make a home visit to collect a specimen for COVID-19 testing, and hospitals will be able to conduct testing in homes or other community-based settings under certain circumstances.
In addition, CMS is taking actions aimed at expanding the health care workforce.
For instance, the agency is issuing a “blanket waiver” that allows hospitals to provide benefits to medical staff, including multiple daily meals, laundry service for personal clothing, or child care services while the staff is at the hospital providing patient care, according to the fact sheet. Teaching hospitals will also receive more flexibility in using residents to provide health care services under the virtual direction of a teaching physician, who may be available through audio/video technology.
CMS also is temporarily eliminating paperwork requirements and allowing greater use of verbal orders so that clinicians can spend more time on direct patient care.
Another change announced deals with the Merit-based Incentive Payment System (MIPS) track of the Quality Payment Program. Clinicians affected by the pandemic can request reweighting of the MIPS performance categories for the 2019 performance year, which will allow clinicians who are unable to submit MIPS data in the current submission period to request reweighting and receive a neutral payment adjustment in the 2021 payment year.
CMS also added an option for calendar year 2020 in the improvement activity category. Clinicians will receive credit if they are participating in a clinical trial that uses a drug or biological product to treat a COVID-19 patient and they report the findings to a clinical trial repository or registry.
On the device/equipment side, Medicare will cover respiratory-related devices and equipment “for any medical reason determined by clinicians,” according to the fact sheet, rather than only under certain circumstances.
And on the telehealth side, CMS is expanding the number of services that it will pay for via telehealth by more than 80, including ED visits, initial nursing facility and discharge visits, and home visits, which must be provided by a clinician that is allowed to provide telehealth. CMS will allow the use of commonly available interactive apps with audio and video, as well as audio-only phones.
CMS noted that providers can report telehealth for new and established patients, even if the billing code is specific for established patients only. CMS also has removed requirements regarding documentation of medical history and/or physical examination in the medical records during the COVID-19 crisis to help facilitate the use of telehealth for evaluation and management visits.
To help practices financially, providers who participate in Medicare fee-for-service will be able to request up to a 100% advance on their Medicare payments for a 3-month period. Repayment begins 120 days after the advance payment is received and must be paid within 210 days of the payment. Repayment will be automatically deducted from claims processed.
The agency also included new exceptions to the Stark Law. Some examples include the ability for hospitals to pay above or below fair market value to rent equipment or receive services from physicians and the allowance of health care providers to provide financial assistance to each other to ensure continuity of operations.
*This story was updated on 4/17/2020.
The Centers for Medicare & Medicaid Services has announced a wide range of temporary regulatory moves aimed at helping hospitals and health systems handle the surge of COVID-19 patients.
“We are waiving a wide and unprecedented range of regulatory requirements to equip the American health care system with maximum flexibility to deal with an influx of cases,” CMS Administrator Seema Verma said during a March 30 conference call with reporters. “Many health care systems may not need these waivers and they shouldn’t use them if the situation doesn’t warrant it. But the flexibilities are there if it does. At a time of crisis, no regulatory barriers should stand in the way of patient care.”
Among the changes is an expansion of the venues in which health care systems and hospitals can provide services.
Federal regulations call for hospitals to provide services within their own buildings, raising concerns as to whether there will be enough capacity to handle the anticipated COVID-19 caseload.
“Under CMS’s temporary new rules, hospitals will be able to transfer patients to outside facilities, such as ambulatory surgery centers, inpatient rehabilitation hospitals, hotels, and dormitories, while still receiving hospital payments under Medicare,” the CMS stated in a fact sheet highlighting the regulatory changes.
Ambulatory surgery centers will have the option to contract with local health care systems to provide hospital services or they can enroll and bill as hospitals during the emergency, the fact sheet noted. They will be able to perform hospital services such as cancer procedures, trauma surgeries, and other essential surgeries.
CMS also is waiving the limit on the number of beds a doctor-owned hospital can have.
For Medicare patients who may be homebound, CMS will now pay for a laboratory technician to make a home visit to collect a specimen for COVID-19 testing, and hospitals will be able to conduct testing in homes or other community-based settings under certain circumstances.
In addition, CMS is taking actions aimed at expanding the health care workforce.
For instance, the agency is issuing a “blanket waiver” that allows hospitals to provide benefits to medical staff, including multiple daily meals, laundry service for personal clothing, or child care services while the staff is at the hospital providing patient care, according to the fact sheet. Teaching hospitals will also receive more flexibility in using residents to provide health care services under the virtual direction of a teaching physician, who may be available through audio/video technology.
CMS also is temporarily eliminating paperwork requirements and allowing greater use of verbal orders so that clinicians can spend more time on direct patient care.
Another change announced deals with the Merit-based Incentive Payment System (MIPS) track of the Quality Payment Program. Clinicians affected by the pandemic can request reweighting of the MIPS performance categories for the 2019 performance year, which will allow clinicians who are unable to submit MIPS data in the current submission period to request reweighting and receive a neutral payment adjustment in the 2021 payment year.
CMS also added an option for calendar year 2020 in the improvement activity category. Clinicians will receive credit if they are participating in a clinical trial that uses a drug or biological product to treat a COVID-19 patient and they report the findings to a clinical trial repository or registry.
On the device/equipment side, Medicare will cover respiratory-related devices and equipment “for any medical reason determined by clinicians,” according to the fact sheet, rather than only under certain circumstances.
And on the telehealth side, CMS is expanding the number of services that it will pay for via telehealth by more than 80, including ED visits, initial nursing facility and discharge visits, and home visits, which must be provided by a clinician that is allowed to provide telehealth. CMS will allow the use of commonly available interactive apps with audio and video, as well as audio-only phones.
CMS noted that providers can report telehealth for new and established patients, even if the billing code is specific for established patients only. CMS also has removed requirements regarding documentation of medical history and/or physical examination in the medical records during the COVID-19 crisis to help facilitate the use of telehealth for evaluation and management visits.
To help practices financially, providers who participate in Medicare fee-for-service will be able to request up to a 100% advance on their Medicare payments for a 3-month period. Repayment begins 120 days after the advance payment is received and must be paid within 210 days of the payment. Repayment will be automatically deducted from claims processed.
The agency also included new exceptions to the Stark Law. Some examples include the ability for hospitals to pay above or below fair market value to rent equipment or receive services from physicians and the allowance of health care providers to provide financial assistance to each other to ensure continuity of operations.
*This story was updated on 4/17/2020.
ABIM grants MOC extension
Physicians will not lose their certification if they are unable to complete maintenance of certification requirements in 2020, the American Board of Internal Medicine announced.
ABIM President Richard Baron, MD, said in a letter sent to all diplomates.
Additionally, physicians “currently in their grace year will also be afforded an additional grace year in 2021,” the letter continued.
ABIM noted that many assessments were planned for the fall of 2020 and the organization will continue to offer them as planned for physicians who are able to take them. It added that more assessment dates for 2020 and 2021 will be sent out later this year.
“The next few weeks and months will challenge our health care system and country like never before,” Dr. Baron stated. “Our many internal medicine colleagues – and the clinical teams that support them – have been heroic in their response, often selflessly putting their own personal safety at risk while using their superb skills to provide care for others. They have inspired all of us.”
Physicians will not lose their certification if they are unable to complete maintenance of certification requirements in 2020, the American Board of Internal Medicine announced.
ABIM President Richard Baron, MD, said in a letter sent to all diplomates.
Additionally, physicians “currently in their grace year will also be afforded an additional grace year in 2021,” the letter continued.
ABIM noted that many assessments were planned for the fall of 2020 and the organization will continue to offer them as planned for physicians who are able to take them. It added that more assessment dates for 2020 and 2021 will be sent out later this year.
“The next few weeks and months will challenge our health care system and country like never before,” Dr. Baron stated. “Our many internal medicine colleagues – and the clinical teams that support them – have been heroic in their response, often selflessly putting their own personal safety at risk while using their superb skills to provide care for others. They have inspired all of us.”
Physicians will not lose their certification if they are unable to complete maintenance of certification requirements in 2020, the American Board of Internal Medicine announced.
ABIM President Richard Baron, MD, said in a letter sent to all diplomates.
Additionally, physicians “currently in their grace year will also be afforded an additional grace year in 2021,” the letter continued.
ABIM noted that many assessments were planned for the fall of 2020 and the organization will continue to offer them as planned for physicians who are able to take them. It added that more assessment dates for 2020 and 2021 will be sent out later this year.
“The next few weeks and months will challenge our health care system and country like never before,” Dr. Baron stated. “Our many internal medicine colleagues – and the clinical teams that support them – have been heroic in their response, often selflessly putting their own personal safety at risk while using their superb skills to provide care for others. They have inspired all of us.”
Learning to live with COVID-19: Postpandemic life will be reflected in how effectively we leverage this crisis
While often compared with the Spanish influenza contagion of 1918, the current COVID-19 pandemic is arguably unprecedented in scale and scope, global reach, and the rate at which it has spread across the world.
Unprecedented times
The United States now has the greatest burden of COVID-19 disease worldwide.1 Although Boston has thus far been spared the full force of the disease’s impact, it is likely only a matter of time before it reaches here. To prepare for the imminent surge, we at Tufts Medical Center defined 4 short-term strategic imperatives to help guide our COVID-19 preparedness. Having a single unified strategy across our organization has helped to maintain focus and consistency in the messaging amidst all of the uncertainty. Our focus areas are outlined below.
1 Flatten the curve
This term refers to the use of “social distancing” and community isolation measures to keep the number of disease cases at a manageable level. COVID-19 is spread almost exclusively through contact with contaminated respiratory droplets. While several categories of risk have been described, the US Centers for Disease Control and Prevention (CDC) defines disease “exposure” as face-to-face contact within 6 feet of an infected individual for more than 15 minutes without wearing a mask.2 Intervening at all 3 of these touchpoints effectively reduces transmission. Interventions include limiting in-person meetings, increasing the space between individuals (both providers and patients), and routinely using personal protective equipment (PPE).
Another effective strategy is to divide frontline providers into smaller units or teams to limit cross-contamination: the inpatient team versus the outpatient team, the day team versus the night team, the “on” team versus the “off” team. If the infection lays one team low, other providers can step in until they recover and return to work.
Visitor policies should be developed and strictly implemented. Many institutions do allow one support person in labor and delivery (L&D) regardless of the patient’s COVID-19 status, although that person should not be symptomatic or COVID-19 positive. Whether to test all patients and support persons for COVID-19 on arrival at L&D remains controversial.3 At a minimum, these individuals should be screened for symptoms. Although it was a major focus of initial preventative efforts, taking a travel and exposure history is no longer informative as the virus is now endemic and community spread is common.
Initial preventative efforts focused also on high-risk patients, but routine use of PPE for all encounters clearly is more effective because of the high rate of asymptomatic shedding. The virus can survive suspended in the air for up to 2 hours following an aerosol-generating procedure (AGP) and on surfaces for several hours or even days. Practices such as regular handwashing, cleaning of exposed work surfaces, and avoiding face touching should by now be part of our everyday routine.
Institutions throughout the United States have established inpatient COVID-19 units—so-called “dirty” units—with mixed success. As the pandemic spreads and the number of patients with asymptomatic shedding increases, it is harder to determine who is and who is not infected. Cross-contamination has rendered this approach largely ineffective. Whether this will change with the introduction of rapid point-of-care testing remains to be seen.
Continue to: 2 Preserve PPE...
2 Preserve PPE
PPE use is effective in reducing transmission. This includes tier 1 PPE with or without enhanced droplet precaution (surgical mask, eye protection, gloves, yellow gown) and tier 2 PPE (tier 1 plus N95 respirators or powered air-purifying respirators [PAPR]). Given the acute PPE shortage in many parts of the country, appropriate use of PPE is critical to maintain an adequate supply. For example, tier 2 PPE is required only in the setting of an AGP. This includes intubation and, in our determination, the second stage of labor for COVID-19–positive patients and patients under investigation (PUIs); we do not employ tier 2 PPE for all patients in the second stage of labor, although some hospitals endorse this practice.
Creative solutions to the impending PPE shortage abound, such as the use of 3D printers to make face shields and novel techniques to sterilize and reuse N95 respirators.
3 Create capacity
In the absence of effective treatment for COVID-19 and with a vaccine still many months away, supportive care is critical. The pulmonary sequelae with cytokine storm and acute hypoxemia can come on quickly, require urgent mechanical ventilatory support, and take several weeks to resolve.
Our ability to create inpatient capacity to accommodate ill patients, monitor them closely, and intubate early will likely be the most critical driver of the case fatality rate. This requires deferring outpatient visits (or doing them via telemedicine), expanding intensive care unit capabilities (especially ventilator beds), and canceling elective surgeries. What constitutes “elective surgery” is not always clear. Our institution, for example, regards abortion services as essential and not elective, but this is not the case throughout the United States.
Creating capacity also refers to staffing. Where necessary, providers should be retrained and redeployed. This may require emergency credentialing of providers in areas outside their usual clinical practice and permission may be needed from the Accreditation Council for Graduate Medical Education to engage trainees outside their usual duty hours.
4 Support and protect your workforce
Everyone is anxious, and people convey their anxiety in different ways. I have found it helpful to acknowledge those feelings and provide a forum for staff to express and share their anxieties. That said, hospitals are not a democracy. While staff members should be encouraged to ask questions and voice their opinions, everyone is expected to follow protocol regarding patient care.
Celebrating small successes and finding creative ways to alleviate the stress and inject humor can help. Most institutions are using electronic conferencing platforms (such as Zoom or Microsoft Teams) to stay in touch and to continue education initiatives through interactive didactic sessions, grand rounds, morbidity and mortality conferences, and e-journal clubs. These are also a great platform for social events, such as w(h)ine and book clubs and virtual karaoke.
Since many ObGyn providers are women, the closure of day-care centers and schools is particularly challenging. Share best practices among your staff on how to address this problem, such as alternating on-call shifts or matching providers needing day care with ‘furloughed’ college students who are looking to keep busy and make a little money.
Continue to: Avoid overcommunicating...
Avoid overcommunicating
Clear, concise, and timely communication is key. This can be challenging given the rapidly evolving science of COVID-19 and the daily barrage of information from both reliable and unreliable sources. Setting up regular online meetings with your faculty 2 or 3 times per week can keep people informed, promote engagement, and boost morale.
If an urgent e-mail announcement is needed, keep the message focused. Highlight only updated information and changes to existing policies and guidelines. And consider adding a brief anecdote to illustrate the staff’s creativity and resilience: a “best catch” story, for example, or a staff member who started a “commit to sit” program (spending time in the room with patients who want company but are not able to have their family in attendance).
Look to the future
COVID-19 will pass. Herd immunity will inevitably develop. The question is how quickly and at what cost. Children delivered today are being born into a society already profoundly altered by COVID-19. Some have started to call them Generation C.
Exactly what life will look like at the back end of this pandemic depends on how effectively we leverage this crisis. There are numerous opportunities to change the way we think about health care and educate the next generation of providers. These include increasing the use of telehealth and remote education, redesigning our traditional prenatal care paradigms, and reinforcing the importance of preventive medicine. This is an opportunity to put the “health” back into “health care.”
Look after yourself
Amid all the chaos and uncertainty, do not forget to take care of yourself and your family. Be calm, be kind, and be flexible. Stay safe.
- Kommenda N, Gutierrez P, Adolphe J. Coronavirus world map: which countries have the most cases and deaths? The Guardian. April 1, 2020. https://www.theguardian.com/world/2020/mar/31/coronavirus-mapped-which-countries-have-the-most-cases-and-deaths. Accessed April 1, 2020.
- Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19).Interim US guidance for risk assessment and public health management of healthcare personnel with potential exposure in a healthcare setting to patients with coronavirus disease (COVID-19). https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-risk-assesment-hcp.html. Accessed April 1, 2020.
- Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19). Evaluating and testing persons for coronavirus disease 2020 (COVID-19). https://www.cdc.gov/coronavirus/2019-nCoV/hcp/clinical-criteria.html. Accessed April 1, 2020.
While often compared with the Spanish influenza contagion of 1918, the current COVID-19 pandemic is arguably unprecedented in scale and scope, global reach, and the rate at which it has spread across the world.
Unprecedented times
The United States now has the greatest burden of COVID-19 disease worldwide.1 Although Boston has thus far been spared the full force of the disease’s impact, it is likely only a matter of time before it reaches here. To prepare for the imminent surge, we at Tufts Medical Center defined 4 short-term strategic imperatives to help guide our COVID-19 preparedness. Having a single unified strategy across our organization has helped to maintain focus and consistency in the messaging amidst all of the uncertainty. Our focus areas are outlined below.
1 Flatten the curve
This term refers to the use of “social distancing” and community isolation measures to keep the number of disease cases at a manageable level. COVID-19 is spread almost exclusively through contact with contaminated respiratory droplets. While several categories of risk have been described, the US Centers for Disease Control and Prevention (CDC) defines disease “exposure” as face-to-face contact within 6 feet of an infected individual for more than 15 minutes without wearing a mask.2 Intervening at all 3 of these touchpoints effectively reduces transmission. Interventions include limiting in-person meetings, increasing the space between individuals (both providers and patients), and routinely using personal protective equipment (PPE).
Another effective strategy is to divide frontline providers into smaller units or teams to limit cross-contamination: the inpatient team versus the outpatient team, the day team versus the night team, the “on” team versus the “off” team. If the infection lays one team low, other providers can step in until they recover and return to work.
Visitor policies should be developed and strictly implemented. Many institutions do allow one support person in labor and delivery (L&D) regardless of the patient’s COVID-19 status, although that person should not be symptomatic or COVID-19 positive. Whether to test all patients and support persons for COVID-19 on arrival at L&D remains controversial.3 At a minimum, these individuals should be screened for symptoms. Although it was a major focus of initial preventative efforts, taking a travel and exposure history is no longer informative as the virus is now endemic and community spread is common.
Initial preventative efforts focused also on high-risk patients, but routine use of PPE for all encounters clearly is more effective because of the high rate of asymptomatic shedding. The virus can survive suspended in the air for up to 2 hours following an aerosol-generating procedure (AGP) and on surfaces for several hours or even days. Practices such as regular handwashing, cleaning of exposed work surfaces, and avoiding face touching should by now be part of our everyday routine.
Institutions throughout the United States have established inpatient COVID-19 units—so-called “dirty” units—with mixed success. As the pandemic spreads and the number of patients with asymptomatic shedding increases, it is harder to determine who is and who is not infected. Cross-contamination has rendered this approach largely ineffective. Whether this will change with the introduction of rapid point-of-care testing remains to be seen.
Continue to: 2 Preserve PPE...
2 Preserve PPE
PPE use is effective in reducing transmission. This includes tier 1 PPE with or without enhanced droplet precaution (surgical mask, eye protection, gloves, yellow gown) and tier 2 PPE (tier 1 plus N95 respirators or powered air-purifying respirators [PAPR]). Given the acute PPE shortage in many parts of the country, appropriate use of PPE is critical to maintain an adequate supply. For example, tier 2 PPE is required only in the setting of an AGP. This includes intubation and, in our determination, the second stage of labor for COVID-19–positive patients and patients under investigation (PUIs); we do not employ tier 2 PPE for all patients in the second stage of labor, although some hospitals endorse this practice.
Creative solutions to the impending PPE shortage abound, such as the use of 3D printers to make face shields and novel techniques to sterilize and reuse N95 respirators.
3 Create capacity
In the absence of effective treatment for COVID-19 and with a vaccine still many months away, supportive care is critical. The pulmonary sequelae with cytokine storm and acute hypoxemia can come on quickly, require urgent mechanical ventilatory support, and take several weeks to resolve.
Our ability to create inpatient capacity to accommodate ill patients, monitor them closely, and intubate early will likely be the most critical driver of the case fatality rate. This requires deferring outpatient visits (or doing them via telemedicine), expanding intensive care unit capabilities (especially ventilator beds), and canceling elective surgeries. What constitutes “elective surgery” is not always clear. Our institution, for example, regards abortion services as essential and not elective, but this is not the case throughout the United States.
Creating capacity also refers to staffing. Where necessary, providers should be retrained and redeployed. This may require emergency credentialing of providers in areas outside their usual clinical practice and permission may be needed from the Accreditation Council for Graduate Medical Education to engage trainees outside their usual duty hours.
4 Support and protect your workforce
Everyone is anxious, and people convey their anxiety in different ways. I have found it helpful to acknowledge those feelings and provide a forum for staff to express and share their anxieties. That said, hospitals are not a democracy. While staff members should be encouraged to ask questions and voice their opinions, everyone is expected to follow protocol regarding patient care.
Celebrating small successes and finding creative ways to alleviate the stress and inject humor can help. Most institutions are using electronic conferencing platforms (such as Zoom or Microsoft Teams) to stay in touch and to continue education initiatives through interactive didactic sessions, grand rounds, morbidity and mortality conferences, and e-journal clubs. These are also a great platform for social events, such as w(h)ine and book clubs and virtual karaoke.
Since many ObGyn providers are women, the closure of day-care centers and schools is particularly challenging. Share best practices among your staff on how to address this problem, such as alternating on-call shifts or matching providers needing day care with ‘furloughed’ college students who are looking to keep busy and make a little money.
Continue to: Avoid overcommunicating...
Avoid overcommunicating
Clear, concise, and timely communication is key. This can be challenging given the rapidly evolving science of COVID-19 and the daily barrage of information from both reliable and unreliable sources. Setting up regular online meetings with your faculty 2 or 3 times per week can keep people informed, promote engagement, and boost morale.
If an urgent e-mail announcement is needed, keep the message focused. Highlight only updated information and changes to existing policies and guidelines. And consider adding a brief anecdote to illustrate the staff’s creativity and resilience: a “best catch” story, for example, or a staff member who started a “commit to sit” program (spending time in the room with patients who want company but are not able to have their family in attendance).
Look to the future
COVID-19 will pass. Herd immunity will inevitably develop. The question is how quickly and at what cost. Children delivered today are being born into a society already profoundly altered by COVID-19. Some have started to call them Generation C.
Exactly what life will look like at the back end of this pandemic depends on how effectively we leverage this crisis. There are numerous opportunities to change the way we think about health care and educate the next generation of providers. These include increasing the use of telehealth and remote education, redesigning our traditional prenatal care paradigms, and reinforcing the importance of preventive medicine. This is an opportunity to put the “health” back into “health care.”
Look after yourself
Amid all the chaos and uncertainty, do not forget to take care of yourself and your family. Be calm, be kind, and be flexible. Stay safe.
While often compared with the Spanish influenza contagion of 1918, the current COVID-19 pandemic is arguably unprecedented in scale and scope, global reach, and the rate at which it has spread across the world.
Unprecedented times
The United States now has the greatest burden of COVID-19 disease worldwide.1 Although Boston has thus far been spared the full force of the disease’s impact, it is likely only a matter of time before it reaches here. To prepare for the imminent surge, we at Tufts Medical Center defined 4 short-term strategic imperatives to help guide our COVID-19 preparedness. Having a single unified strategy across our organization has helped to maintain focus and consistency in the messaging amidst all of the uncertainty. Our focus areas are outlined below.
1 Flatten the curve
This term refers to the use of “social distancing” and community isolation measures to keep the number of disease cases at a manageable level. COVID-19 is spread almost exclusively through contact with contaminated respiratory droplets. While several categories of risk have been described, the US Centers for Disease Control and Prevention (CDC) defines disease “exposure” as face-to-face contact within 6 feet of an infected individual for more than 15 minutes without wearing a mask.2 Intervening at all 3 of these touchpoints effectively reduces transmission. Interventions include limiting in-person meetings, increasing the space between individuals (both providers and patients), and routinely using personal protective equipment (PPE).
Another effective strategy is to divide frontline providers into smaller units or teams to limit cross-contamination: the inpatient team versus the outpatient team, the day team versus the night team, the “on” team versus the “off” team. If the infection lays one team low, other providers can step in until they recover and return to work.
Visitor policies should be developed and strictly implemented. Many institutions do allow one support person in labor and delivery (L&D) regardless of the patient’s COVID-19 status, although that person should not be symptomatic or COVID-19 positive. Whether to test all patients and support persons for COVID-19 on arrival at L&D remains controversial.3 At a minimum, these individuals should be screened for symptoms. Although it was a major focus of initial preventative efforts, taking a travel and exposure history is no longer informative as the virus is now endemic and community spread is common.
Initial preventative efforts focused also on high-risk patients, but routine use of PPE for all encounters clearly is more effective because of the high rate of asymptomatic shedding. The virus can survive suspended in the air for up to 2 hours following an aerosol-generating procedure (AGP) and on surfaces for several hours or even days. Practices such as regular handwashing, cleaning of exposed work surfaces, and avoiding face touching should by now be part of our everyday routine.
Institutions throughout the United States have established inpatient COVID-19 units—so-called “dirty” units—with mixed success. As the pandemic spreads and the number of patients with asymptomatic shedding increases, it is harder to determine who is and who is not infected. Cross-contamination has rendered this approach largely ineffective. Whether this will change with the introduction of rapid point-of-care testing remains to be seen.
Continue to: 2 Preserve PPE...
2 Preserve PPE
PPE use is effective in reducing transmission. This includes tier 1 PPE with or without enhanced droplet precaution (surgical mask, eye protection, gloves, yellow gown) and tier 2 PPE (tier 1 plus N95 respirators or powered air-purifying respirators [PAPR]). Given the acute PPE shortage in many parts of the country, appropriate use of PPE is critical to maintain an adequate supply. For example, tier 2 PPE is required only in the setting of an AGP. This includes intubation and, in our determination, the second stage of labor for COVID-19–positive patients and patients under investigation (PUIs); we do not employ tier 2 PPE for all patients in the second stage of labor, although some hospitals endorse this practice.
Creative solutions to the impending PPE shortage abound, such as the use of 3D printers to make face shields and novel techniques to sterilize and reuse N95 respirators.
3 Create capacity
In the absence of effective treatment for COVID-19 and with a vaccine still many months away, supportive care is critical. The pulmonary sequelae with cytokine storm and acute hypoxemia can come on quickly, require urgent mechanical ventilatory support, and take several weeks to resolve.
Our ability to create inpatient capacity to accommodate ill patients, monitor them closely, and intubate early will likely be the most critical driver of the case fatality rate. This requires deferring outpatient visits (or doing them via telemedicine), expanding intensive care unit capabilities (especially ventilator beds), and canceling elective surgeries. What constitutes “elective surgery” is not always clear. Our institution, for example, regards abortion services as essential and not elective, but this is not the case throughout the United States.
Creating capacity also refers to staffing. Where necessary, providers should be retrained and redeployed. This may require emergency credentialing of providers in areas outside their usual clinical practice and permission may be needed from the Accreditation Council for Graduate Medical Education to engage trainees outside their usual duty hours.
4 Support and protect your workforce
Everyone is anxious, and people convey their anxiety in different ways. I have found it helpful to acknowledge those feelings and provide a forum for staff to express and share their anxieties. That said, hospitals are not a democracy. While staff members should be encouraged to ask questions and voice their opinions, everyone is expected to follow protocol regarding patient care.
Celebrating small successes and finding creative ways to alleviate the stress and inject humor can help. Most institutions are using electronic conferencing platforms (such as Zoom or Microsoft Teams) to stay in touch and to continue education initiatives through interactive didactic sessions, grand rounds, morbidity and mortality conferences, and e-journal clubs. These are also a great platform for social events, such as w(h)ine and book clubs and virtual karaoke.
Since many ObGyn providers are women, the closure of day-care centers and schools is particularly challenging. Share best practices among your staff on how to address this problem, such as alternating on-call shifts or matching providers needing day care with ‘furloughed’ college students who are looking to keep busy and make a little money.
Continue to: Avoid overcommunicating...
Avoid overcommunicating
Clear, concise, and timely communication is key. This can be challenging given the rapidly evolving science of COVID-19 and the daily barrage of information from both reliable and unreliable sources. Setting up regular online meetings with your faculty 2 or 3 times per week can keep people informed, promote engagement, and boost morale.
If an urgent e-mail announcement is needed, keep the message focused. Highlight only updated information and changes to existing policies and guidelines. And consider adding a brief anecdote to illustrate the staff’s creativity and resilience: a “best catch” story, for example, or a staff member who started a “commit to sit” program (spending time in the room with patients who want company but are not able to have their family in attendance).
Look to the future
COVID-19 will pass. Herd immunity will inevitably develop. The question is how quickly and at what cost. Children delivered today are being born into a society already profoundly altered by COVID-19. Some have started to call them Generation C.
Exactly what life will look like at the back end of this pandemic depends on how effectively we leverage this crisis. There are numerous opportunities to change the way we think about health care and educate the next generation of providers. These include increasing the use of telehealth and remote education, redesigning our traditional prenatal care paradigms, and reinforcing the importance of preventive medicine. This is an opportunity to put the “health” back into “health care.”
Look after yourself
Amid all the chaos and uncertainty, do not forget to take care of yourself and your family. Be calm, be kind, and be flexible. Stay safe.
- Kommenda N, Gutierrez P, Adolphe J. Coronavirus world map: which countries have the most cases and deaths? The Guardian. April 1, 2020. https://www.theguardian.com/world/2020/mar/31/coronavirus-mapped-which-countries-have-the-most-cases-and-deaths. Accessed April 1, 2020.
- Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19).Interim US guidance for risk assessment and public health management of healthcare personnel with potential exposure in a healthcare setting to patients with coronavirus disease (COVID-19). https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-risk-assesment-hcp.html. Accessed April 1, 2020.
- Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19). Evaluating and testing persons for coronavirus disease 2020 (COVID-19). https://www.cdc.gov/coronavirus/2019-nCoV/hcp/clinical-criteria.html. Accessed April 1, 2020.
- Kommenda N, Gutierrez P, Adolphe J. Coronavirus world map: which countries have the most cases and deaths? The Guardian. April 1, 2020. https://www.theguardian.com/world/2020/mar/31/coronavirus-mapped-which-countries-have-the-most-cases-and-deaths. Accessed April 1, 2020.
- Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19).Interim US guidance for risk assessment and public health management of healthcare personnel with potential exposure in a healthcare setting to patients with coronavirus disease (COVID-19). https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-risk-assesment-hcp.html. Accessed April 1, 2020.
- Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19). Evaluating and testing persons for coronavirus disease 2020 (COVID-19). https://www.cdc.gov/coronavirus/2019-nCoV/hcp/clinical-criteria.html. Accessed April 1, 2020.
Concerns for clinicians over 65 grow in the face of COVID-19
When Judith Salerno, MD, heard that New York was calling for volunteer clinicians to assist with the COVID-19 response, she didn’t hesitate to sign up.
Although Dr. Salerno, 68, has held administrative, research, and policy roles for 25 years, she has kept her medical license active and always found ways to squeeze some clinical work into her busy schedule.
“I have what I could consider ‘rusty’ clinical skills, but pretty good clinical judgment,” said Dr. Salerno, president of the New York Academy of Medicine. “I thought in this situation that I could resurrect and hone those skills, even if it was just taking care of routine patients and working on a team, there was a lot of good I can do.”
Dr. Salerno is among 80,000 health care professionals who have volunteered to work temporarily in New York during the COVID-19 pandemic as of March 31, 2020, according to New York state officials. In mid-March, New York Governor Andrew Cuomo (D) issued a plea for retired physicians and nurses to help the state by signing up for on-call work. Other states have made similar appeals for retired health care professionals to return to medicine in an effort to relieve overwhelmed hospital staffs and aid capacity if health care workers become ill. Such redeployments, however, are raising concerns about exposing senior physicians to a virus that causes more severe illness in individuals aged over 65 years and kills them at a higher rate.
At the same time, a significant portion of the current health care workforce is aged 55 years and older, placing them at higher risk for serious illness, hospitalization, and death from COVID-19, said Douglas O. Staiger, PhD, a researcher and economics professor at Dartmouth College, Hanover, N.H. Dr. Staiger recently coauthored a viewpoint in JAMA called “Older clinicians and the surge in novel coronavirus disease 2019,” which outlines the risks and mortality rates from the novel coronavirus among patients aged 55 years and older.
Among the 1.2 million practicing physicians in the United States, about 20% are aged 55-64 years and an estimated 9% are 65 years or older, according to the paper. Of the nation’s nearly 2 million registered nurses employed in hospitals, about 19% are aged 55-64 years, and an estimated 3% are aged 65 years or older.
“In some metro areas, this proportion is even higher,” Dr. Staiger said in an interview. “Hospitals and other health care providers should consider ways of utilizing older clinicians’ skills and experience in a way that minimizes their risk of exposure to COVID-19, such as transferring them from jobs interacting with patients to more supervisory, administrative, or telehealth roles. This is increasingly important as retired physicians and nurses are being asked to return to the workforce.”
Protecting staff, screening volunteers
Hematologist-oncologist David H. Henry, MD, said his eight-physician group practice at Pennsylvania Hospital, Philadelphia, has already taken steps to protect him from COVID exposure.
At the request of his younger colleagues, Dr. Henry, 69, said he is no longer seeing patients in the hospital where there is increased exposure risk to the virus. He and the staff also limit their time in the office to 2-3 days a week and practice telemedicine the rest of the week, Dr. Henry said in an interview.
“Whether you’re a person trying to stay at home because you’re quote ‘nonessential,’ or you’re a health care worker and you have to keep seeing patients to some extent, the less we’re face to face with others the better,” said Dr. Henry, who hosts the Blood & Cancer podcast for MDedge News. “There’s an extreme and a middle ground. If they told me just to stay home that wouldn’t help anybody. If they said, ‘business as usual,’ that would be wrong. This is a middle strategy, which is reasonable, rational, and will help dial this dangerous time down as fast as possible.”
On a recent weekend when Dr. Henry would normally have been on call in the hospital, he took phone calls for his colleagues at home while they saw patients in the hospital. This included calls with patients who had questions and consultation calls with other physicians.
“They are helping me and I am helping them,” Dr. Henry said. “Taking those calls makes it easier for my partners to see all those patients. We all want to help and be there, within reason. You want to step up an do your job, but you want to be safe.”
Peter D. Quinn, DMD, MD, chief executive physician of the Penn Medicine Medical Group, said safeguarding the health of its workforce is a top priority as Penn Medicine works to fight the COVID-19 pandemic.
“This includes ensuring that all employees adhere to Centers for Disease Control and Penn Medicine infection prevention guidance as they continue their normal clinical work,” Dr. Quinn said in an interview. “Though age alone is not a criterion to remove frontline staff from direct clinical care during the COVID-19 outbreak, certain conditions such as cardiac or lung disease may be, and clinicians who have concerns are urged to speak with their leadership about options to fill clinical or support roles remotely.”
Meanwhile, for states calling on retired health professionals to assist during the pandemic, thorough screenings that identify high-risk volunteers are essential to protect vulnerable clinicians, said Nathaniel Hibbs, DO, president of the Colorado chapter of the American College of Emergency Physicians.
After Colorado issued a statewide request for retired clinicians to help, Dr. Hibbs became concerned that the state’s website initially included only a basic set of questions for interested volunteers.
“It didn’t have screening questions for prior health problems, comorbidities, or things like high blood pressure, heart disease, lung disease – the high-risk factors that we associate with bad outcomes if people get infected with COVID,” Dr. Hibbs said in an interview.
To address this, Dr. Hibbs and associates recently provided recommendations to the state about its screening process that advised collecting more health information from volunteers and considering lower-risk assignments for high-risk individuals. State officials indicated they would strongly consider the recommendations, Dr. Hibbs said.
The Colorado Department of Public Health & Environment did not respond to messages seeking comment. Officials at the New York State Department of Health declined to be interviewed for this article but confirmed that they are reviewing the age and background of all volunteers, and individual hospitals will also review each volunteer to find suitable jobs.
The American Medical Association on March 30 issued guidance for retired physicians about rejoining the workforce to help with the COVID response. The guidance outlines license considerations, contribution options, professional liability considerations, and questions to ask volunteer coordinators.
“Throughout the COVID-19 pandemic, many physicians over the age of 65 will provide care to patients,” AMA President Patrice A. Harris, MD, said in a statement. “Whether ‘senior’ physicians should be on the front line of patient care at this time is a complex issue that must balance several factors against the benefit these physicians can provide. As with all people in high-risk age groups, careful consideration must be given to the health and safety of retired physicians and their immediate family members, especially those with chronic medical conditions.”
Tapping talent, sharing knowledge
When Barbara L. Schuster, MD, 69, filled out paperwork to join the Georgia Medical Reserve Corps, she answered a range of questions, including inquiries about her age, specialty, licensing, and whether she had any major medical conditions.
“They sent out instructions that said, if you are over the age of 60, we really don’t want you to be doing inpatient or ambulatory with active patients,” said Dr. Schuster, a retired medical school dean in the Athens, Ga., area. “Unless they get to a point where it’s going to be you or nobody, I think that they try to protect us for both our sake and also theirs.”
Dr. Schuster opted for telehealth or administrative duties, but has not yet been called upon to help. The Athens area has not seen high numbers of COVID-19 patients, compared with other parts of the country, and there have not been many volunteer opportunities for physicians thus far, she said. In the meantime, Dr. Schuster has found other ways to give her time, such as answering questions from community members on both COVID-19 and non–COVID-19 topics, and offering guidance to medical students.
“I’ve spent an increasing number of hours on Zoom, Skype, or FaceTime meeting with them to talk about various issues,” Dr. Schuster said.
As hospitals and organizations ramp up pandemic preparation, now is the time to consider roles for older clinicians and how they can best contribute, said Peter I. Buerhaus, PhD, RN, a nurse and director of the Center for Interdisciplinary Health Workforce Studies at Montana State University, Bozeman, Mont. Dr. Buerhaus was the first author of the recent JAMA viewpoint “Older clinicians and the surge in novel coronavirus 2019.”
“It’s important for hospitals that are anticipating a surge of critically ill patients to assess their workforce’s capability, including the proportion of older clinicians,” he said. “Is there something organizations can do differently to lessen older physicians’ and nurses’ direct patient contact and reduce their risk of infection?”
Dr. Buerhaus’ JAMA piece offers a range of ideas and assignments for older clinicians during the pandemic, including consulting with younger staff, advising on resources, assisting with clinical and organizational problem solving, aiding clinicians and managers with challenging decisions, consulting with patient families, advising managers and executives, being public spokespersons, and working with public and community health organizations.
“Older clinicians are at increased risk of becoming seriously ill if infected, but yet they’re also the ones who perhaps some of the best minds and experiences to help organizations combat the pandemic,” Dr. Buerhaus said. “These clinicians have great backgrounds and skills and 20, 30, 40 years of experience to draw on, including dealing with prior medical emergencies. I would hope that organizations, if they can, use the time before becoming a hotspot as an opportunity where the younger workforce could be teamed up with some of the older clinicians and learn as much as possible. It’s a great opportunity to share this wealth of knowledge with the workforce that will carry on after the pandemic.”
Since responding to New York’s call for volunteers, Dr. Salerno has been assigned to a palliative care inpatient team at a Manhattan hospital where she is working with large numbers of ICU patients and their families.
“My experience as a geriatrician helps me in talking with anxious and concerned families, especially when they are unable to see or communicate with their critically ill loved ones,” she said.
Before she was assigned the post, Dr. Salerno said she heard concerns from her adult children, who would prefer their mom take on a volunteer telehealth role. At the time, Dr. Salerno said she was not opposed to a telehealth assignment, but stressed to her family that she would go where she was needed.
“I’m healthy enough to run an organization, work long hours, long weeks; I have the stamina. The only thing working against me is age,” she said. “To say I’m not concerned is not honest. Of course I’m concerned. Am I afraid? No. I’m hoping that we can all be kept safe.”
When Judith Salerno, MD, heard that New York was calling for volunteer clinicians to assist with the COVID-19 response, she didn’t hesitate to sign up.
Although Dr. Salerno, 68, has held administrative, research, and policy roles for 25 years, she has kept her medical license active and always found ways to squeeze some clinical work into her busy schedule.
“I have what I could consider ‘rusty’ clinical skills, but pretty good clinical judgment,” said Dr. Salerno, president of the New York Academy of Medicine. “I thought in this situation that I could resurrect and hone those skills, even if it was just taking care of routine patients and working on a team, there was a lot of good I can do.”
Dr. Salerno is among 80,000 health care professionals who have volunteered to work temporarily in New York during the COVID-19 pandemic as of March 31, 2020, according to New York state officials. In mid-March, New York Governor Andrew Cuomo (D) issued a plea for retired physicians and nurses to help the state by signing up for on-call work. Other states have made similar appeals for retired health care professionals to return to medicine in an effort to relieve overwhelmed hospital staffs and aid capacity if health care workers become ill. Such redeployments, however, are raising concerns about exposing senior physicians to a virus that causes more severe illness in individuals aged over 65 years and kills them at a higher rate.
At the same time, a significant portion of the current health care workforce is aged 55 years and older, placing them at higher risk for serious illness, hospitalization, and death from COVID-19, said Douglas O. Staiger, PhD, a researcher and economics professor at Dartmouth College, Hanover, N.H. Dr. Staiger recently coauthored a viewpoint in JAMA called “Older clinicians and the surge in novel coronavirus disease 2019,” which outlines the risks and mortality rates from the novel coronavirus among patients aged 55 years and older.
Among the 1.2 million practicing physicians in the United States, about 20% are aged 55-64 years and an estimated 9% are 65 years or older, according to the paper. Of the nation’s nearly 2 million registered nurses employed in hospitals, about 19% are aged 55-64 years, and an estimated 3% are aged 65 years or older.
“In some metro areas, this proportion is even higher,” Dr. Staiger said in an interview. “Hospitals and other health care providers should consider ways of utilizing older clinicians’ skills and experience in a way that minimizes their risk of exposure to COVID-19, such as transferring them from jobs interacting with patients to more supervisory, administrative, or telehealth roles. This is increasingly important as retired physicians and nurses are being asked to return to the workforce.”
Protecting staff, screening volunteers
Hematologist-oncologist David H. Henry, MD, said his eight-physician group practice at Pennsylvania Hospital, Philadelphia, has already taken steps to protect him from COVID exposure.
At the request of his younger colleagues, Dr. Henry, 69, said he is no longer seeing patients in the hospital where there is increased exposure risk to the virus. He and the staff also limit their time in the office to 2-3 days a week and practice telemedicine the rest of the week, Dr. Henry said in an interview.
“Whether you’re a person trying to stay at home because you’re quote ‘nonessential,’ or you’re a health care worker and you have to keep seeing patients to some extent, the less we’re face to face with others the better,” said Dr. Henry, who hosts the Blood & Cancer podcast for MDedge News. “There’s an extreme and a middle ground. If they told me just to stay home that wouldn’t help anybody. If they said, ‘business as usual,’ that would be wrong. This is a middle strategy, which is reasonable, rational, and will help dial this dangerous time down as fast as possible.”
On a recent weekend when Dr. Henry would normally have been on call in the hospital, he took phone calls for his colleagues at home while they saw patients in the hospital. This included calls with patients who had questions and consultation calls with other physicians.
“They are helping me and I am helping them,” Dr. Henry said. “Taking those calls makes it easier for my partners to see all those patients. We all want to help and be there, within reason. You want to step up an do your job, but you want to be safe.”
Peter D. Quinn, DMD, MD, chief executive physician of the Penn Medicine Medical Group, said safeguarding the health of its workforce is a top priority as Penn Medicine works to fight the COVID-19 pandemic.
“This includes ensuring that all employees adhere to Centers for Disease Control and Penn Medicine infection prevention guidance as they continue their normal clinical work,” Dr. Quinn said in an interview. “Though age alone is not a criterion to remove frontline staff from direct clinical care during the COVID-19 outbreak, certain conditions such as cardiac or lung disease may be, and clinicians who have concerns are urged to speak with their leadership about options to fill clinical or support roles remotely.”
Meanwhile, for states calling on retired health professionals to assist during the pandemic, thorough screenings that identify high-risk volunteers are essential to protect vulnerable clinicians, said Nathaniel Hibbs, DO, president of the Colorado chapter of the American College of Emergency Physicians.
After Colorado issued a statewide request for retired clinicians to help, Dr. Hibbs became concerned that the state’s website initially included only a basic set of questions for interested volunteers.
“It didn’t have screening questions for prior health problems, comorbidities, or things like high blood pressure, heart disease, lung disease – the high-risk factors that we associate with bad outcomes if people get infected with COVID,” Dr. Hibbs said in an interview.
To address this, Dr. Hibbs and associates recently provided recommendations to the state about its screening process that advised collecting more health information from volunteers and considering lower-risk assignments for high-risk individuals. State officials indicated they would strongly consider the recommendations, Dr. Hibbs said.
The Colorado Department of Public Health & Environment did not respond to messages seeking comment. Officials at the New York State Department of Health declined to be interviewed for this article but confirmed that they are reviewing the age and background of all volunteers, and individual hospitals will also review each volunteer to find suitable jobs.
The American Medical Association on March 30 issued guidance for retired physicians about rejoining the workforce to help with the COVID response. The guidance outlines license considerations, contribution options, professional liability considerations, and questions to ask volunteer coordinators.
“Throughout the COVID-19 pandemic, many physicians over the age of 65 will provide care to patients,” AMA President Patrice A. Harris, MD, said in a statement. “Whether ‘senior’ physicians should be on the front line of patient care at this time is a complex issue that must balance several factors against the benefit these physicians can provide. As with all people in high-risk age groups, careful consideration must be given to the health and safety of retired physicians and their immediate family members, especially those with chronic medical conditions.”
Tapping talent, sharing knowledge
When Barbara L. Schuster, MD, 69, filled out paperwork to join the Georgia Medical Reserve Corps, she answered a range of questions, including inquiries about her age, specialty, licensing, and whether she had any major medical conditions.
“They sent out instructions that said, if you are over the age of 60, we really don’t want you to be doing inpatient or ambulatory with active patients,” said Dr. Schuster, a retired medical school dean in the Athens, Ga., area. “Unless they get to a point where it’s going to be you or nobody, I think that they try to protect us for both our sake and also theirs.”
Dr. Schuster opted for telehealth or administrative duties, but has not yet been called upon to help. The Athens area has not seen high numbers of COVID-19 patients, compared with other parts of the country, and there have not been many volunteer opportunities for physicians thus far, she said. In the meantime, Dr. Schuster has found other ways to give her time, such as answering questions from community members on both COVID-19 and non–COVID-19 topics, and offering guidance to medical students.
“I’ve spent an increasing number of hours on Zoom, Skype, or FaceTime meeting with them to talk about various issues,” Dr. Schuster said.
As hospitals and organizations ramp up pandemic preparation, now is the time to consider roles for older clinicians and how they can best contribute, said Peter I. Buerhaus, PhD, RN, a nurse and director of the Center for Interdisciplinary Health Workforce Studies at Montana State University, Bozeman, Mont. Dr. Buerhaus was the first author of the recent JAMA viewpoint “Older clinicians and the surge in novel coronavirus 2019.”
“It’s important for hospitals that are anticipating a surge of critically ill patients to assess their workforce’s capability, including the proportion of older clinicians,” he said. “Is there something organizations can do differently to lessen older physicians’ and nurses’ direct patient contact and reduce their risk of infection?”
Dr. Buerhaus’ JAMA piece offers a range of ideas and assignments for older clinicians during the pandemic, including consulting with younger staff, advising on resources, assisting with clinical and organizational problem solving, aiding clinicians and managers with challenging decisions, consulting with patient families, advising managers and executives, being public spokespersons, and working with public and community health organizations.
“Older clinicians are at increased risk of becoming seriously ill if infected, but yet they’re also the ones who perhaps some of the best minds and experiences to help organizations combat the pandemic,” Dr. Buerhaus said. “These clinicians have great backgrounds and skills and 20, 30, 40 years of experience to draw on, including dealing with prior medical emergencies. I would hope that organizations, if they can, use the time before becoming a hotspot as an opportunity where the younger workforce could be teamed up with some of the older clinicians and learn as much as possible. It’s a great opportunity to share this wealth of knowledge with the workforce that will carry on after the pandemic.”
Since responding to New York’s call for volunteers, Dr. Salerno has been assigned to a palliative care inpatient team at a Manhattan hospital where she is working with large numbers of ICU patients and their families.
“My experience as a geriatrician helps me in talking with anxious and concerned families, especially when they are unable to see or communicate with their critically ill loved ones,” she said.
Before she was assigned the post, Dr. Salerno said she heard concerns from her adult children, who would prefer their mom take on a volunteer telehealth role. At the time, Dr. Salerno said she was not opposed to a telehealth assignment, but stressed to her family that she would go where she was needed.
“I’m healthy enough to run an organization, work long hours, long weeks; I have the stamina. The only thing working against me is age,” she said. “To say I’m not concerned is not honest. Of course I’m concerned. Am I afraid? No. I’m hoping that we can all be kept safe.”
When Judith Salerno, MD, heard that New York was calling for volunteer clinicians to assist with the COVID-19 response, she didn’t hesitate to sign up.
Although Dr. Salerno, 68, has held administrative, research, and policy roles for 25 years, she has kept her medical license active and always found ways to squeeze some clinical work into her busy schedule.
“I have what I could consider ‘rusty’ clinical skills, but pretty good clinical judgment,” said Dr. Salerno, president of the New York Academy of Medicine. “I thought in this situation that I could resurrect and hone those skills, even if it was just taking care of routine patients and working on a team, there was a lot of good I can do.”
Dr. Salerno is among 80,000 health care professionals who have volunteered to work temporarily in New York during the COVID-19 pandemic as of March 31, 2020, according to New York state officials. In mid-March, New York Governor Andrew Cuomo (D) issued a plea for retired physicians and nurses to help the state by signing up for on-call work. Other states have made similar appeals for retired health care professionals to return to medicine in an effort to relieve overwhelmed hospital staffs and aid capacity if health care workers become ill. Such redeployments, however, are raising concerns about exposing senior physicians to a virus that causes more severe illness in individuals aged over 65 years and kills them at a higher rate.
At the same time, a significant portion of the current health care workforce is aged 55 years and older, placing them at higher risk for serious illness, hospitalization, and death from COVID-19, said Douglas O. Staiger, PhD, a researcher and economics professor at Dartmouth College, Hanover, N.H. Dr. Staiger recently coauthored a viewpoint in JAMA called “Older clinicians and the surge in novel coronavirus disease 2019,” which outlines the risks and mortality rates from the novel coronavirus among patients aged 55 years and older.
Among the 1.2 million practicing physicians in the United States, about 20% are aged 55-64 years and an estimated 9% are 65 years or older, according to the paper. Of the nation’s nearly 2 million registered nurses employed in hospitals, about 19% are aged 55-64 years, and an estimated 3% are aged 65 years or older.
“In some metro areas, this proportion is even higher,” Dr. Staiger said in an interview. “Hospitals and other health care providers should consider ways of utilizing older clinicians’ skills and experience in a way that minimizes their risk of exposure to COVID-19, such as transferring them from jobs interacting with patients to more supervisory, administrative, or telehealth roles. This is increasingly important as retired physicians and nurses are being asked to return to the workforce.”
Protecting staff, screening volunteers
Hematologist-oncologist David H. Henry, MD, said his eight-physician group practice at Pennsylvania Hospital, Philadelphia, has already taken steps to protect him from COVID exposure.
At the request of his younger colleagues, Dr. Henry, 69, said he is no longer seeing patients in the hospital where there is increased exposure risk to the virus. He and the staff also limit their time in the office to 2-3 days a week and practice telemedicine the rest of the week, Dr. Henry said in an interview.
“Whether you’re a person trying to stay at home because you’re quote ‘nonessential,’ or you’re a health care worker and you have to keep seeing patients to some extent, the less we’re face to face with others the better,” said Dr. Henry, who hosts the Blood & Cancer podcast for MDedge News. “There’s an extreme and a middle ground. If they told me just to stay home that wouldn’t help anybody. If they said, ‘business as usual,’ that would be wrong. This is a middle strategy, which is reasonable, rational, and will help dial this dangerous time down as fast as possible.”
On a recent weekend when Dr. Henry would normally have been on call in the hospital, he took phone calls for his colleagues at home while they saw patients in the hospital. This included calls with patients who had questions and consultation calls with other physicians.
“They are helping me and I am helping them,” Dr. Henry said. “Taking those calls makes it easier for my partners to see all those patients. We all want to help and be there, within reason. You want to step up an do your job, but you want to be safe.”
Peter D. Quinn, DMD, MD, chief executive physician of the Penn Medicine Medical Group, said safeguarding the health of its workforce is a top priority as Penn Medicine works to fight the COVID-19 pandemic.
“This includes ensuring that all employees adhere to Centers for Disease Control and Penn Medicine infection prevention guidance as they continue their normal clinical work,” Dr. Quinn said in an interview. “Though age alone is not a criterion to remove frontline staff from direct clinical care during the COVID-19 outbreak, certain conditions such as cardiac or lung disease may be, and clinicians who have concerns are urged to speak with their leadership about options to fill clinical or support roles remotely.”
Meanwhile, for states calling on retired health professionals to assist during the pandemic, thorough screenings that identify high-risk volunteers are essential to protect vulnerable clinicians, said Nathaniel Hibbs, DO, president of the Colorado chapter of the American College of Emergency Physicians.
After Colorado issued a statewide request for retired clinicians to help, Dr. Hibbs became concerned that the state’s website initially included only a basic set of questions for interested volunteers.
“It didn’t have screening questions for prior health problems, comorbidities, or things like high blood pressure, heart disease, lung disease – the high-risk factors that we associate with bad outcomes if people get infected with COVID,” Dr. Hibbs said in an interview.
To address this, Dr. Hibbs and associates recently provided recommendations to the state about its screening process that advised collecting more health information from volunteers and considering lower-risk assignments for high-risk individuals. State officials indicated they would strongly consider the recommendations, Dr. Hibbs said.
The Colorado Department of Public Health & Environment did not respond to messages seeking comment. Officials at the New York State Department of Health declined to be interviewed for this article but confirmed that they are reviewing the age and background of all volunteers, and individual hospitals will also review each volunteer to find suitable jobs.
The American Medical Association on March 30 issued guidance for retired physicians about rejoining the workforce to help with the COVID response. The guidance outlines license considerations, contribution options, professional liability considerations, and questions to ask volunteer coordinators.
“Throughout the COVID-19 pandemic, many physicians over the age of 65 will provide care to patients,” AMA President Patrice A. Harris, MD, said in a statement. “Whether ‘senior’ physicians should be on the front line of patient care at this time is a complex issue that must balance several factors against the benefit these physicians can provide. As with all people in high-risk age groups, careful consideration must be given to the health and safety of retired physicians and their immediate family members, especially those with chronic medical conditions.”
Tapping talent, sharing knowledge
When Barbara L. Schuster, MD, 69, filled out paperwork to join the Georgia Medical Reserve Corps, she answered a range of questions, including inquiries about her age, specialty, licensing, and whether she had any major medical conditions.
“They sent out instructions that said, if you are over the age of 60, we really don’t want you to be doing inpatient or ambulatory with active patients,” said Dr. Schuster, a retired medical school dean in the Athens, Ga., area. “Unless they get to a point where it’s going to be you or nobody, I think that they try to protect us for both our sake and also theirs.”
Dr. Schuster opted for telehealth or administrative duties, but has not yet been called upon to help. The Athens area has not seen high numbers of COVID-19 patients, compared with other parts of the country, and there have not been many volunteer opportunities for physicians thus far, she said. In the meantime, Dr. Schuster has found other ways to give her time, such as answering questions from community members on both COVID-19 and non–COVID-19 topics, and offering guidance to medical students.
“I’ve spent an increasing number of hours on Zoom, Skype, or FaceTime meeting with them to talk about various issues,” Dr. Schuster said.
As hospitals and organizations ramp up pandemic preparation, now is the time to consider roles for older clinicians and how they can best contribute, said Peter I. Buerhaus, PhD, RN, a nurse and director of the Center for Interdisciplinary Health Workforce Studies at Montana State University, Bozeman, Mont. Dr. Buerhaus was the first author of the recent JAMA viewpoint “Older clinicians and the surge in novel coronavirus 2019.”
“It’s important for hospitals that are anticipating a surge of critically ill patients to assess their workforce’s capability, including the proportion of older clinicians,” he said. “Is there something organizations can do differently to lessen older physicians’ and nurses’ direct patient contact and reduce their risk of infection?”
Dr. Buerhaus’ JAMA piece offers a range of ideas and assignments for older clinicians during the pandemic, including consulting with younger staff, advising on resources, assisting with clinical and organizational problem solving, aiding clinicians and managers with challenging decisions, consulting with patient families, advising managers and executives, being public spokespersons, and working with public and community health organizations.
“Older clinicians are at increased risk of becoming seriously ill if infected, but yet they’re also the ones who perhaps some of the best minds and experiences to help organizations combat the pandemic,” Dr. Buerhaus said. “These clinicians have great backgrounds and skills and 20, 30, 40 years of experience to draw on, including dealing with prior medical emergencies. I would hope that organizations, if they can, use the time before becoming a hotspot as an opportunity where the younger workforce could be teamed up with some of the older clinicians and learn as much as possible. It’s a great opportunity to share this wealth of knowledge with the workforce that will carry on after the pandemic.”
Since responding to New York’s call for volunteers, Dr. Salerno has been assigned to a palliative care inpatient team at a Manhattan hospital where she is working with large numbers of ICU patients and their families.
“My experience as a geriatrician helps me in talking with anxious and concerned families, especially when they are unable to see or communicate with their critically ill loved ones,” she said.
Before she was assigned the post, Dr. Salerno said she heard concerns from her adult children, who would prefer their mom take on a volunteer telehealth role. At the time, Dr. Salerno said she was not opposed to a telehealth assignment, but stressed to her family that she would go where she was needed.
“I’m healthy enough to run an organization, work long hours, long weeks; I have the stamina. The only thing working against me is age,” she said. “To say I’m not concerned is not honest. Of course I’m concerned. Am I afraid? No. I’m hoping that we can all be kept safe.”
Conducting cancer trials amid the COVID-19 pandemic
More than three-quarters of cancer clinical research programs have experienced operational changes during the COVID-19 pandemic, according to a survey conducted by the Association of Community Cancer Centers (ACCC) during a recent webinar.
The webinar included insights into how some cancer research programs have adapted to the pandemic, a review of guidance for conducting cancer trials during this time, and a discussion of how the cancer research landscape may be affected by COVID-19 going forward.
The webinar was led by Randall A. Oyer, MD, president of the ACCC and medical director of the oncology program at Penn Medicine Lancaster General Health in Pennsylvania.
The impact of COVID-19 on cancer research
Dr. Oyer observed that planning and implementation for COVID-19–related illness at U.S. health care institutions has had a predictable effect of limiting patient access and staff availability for nonessential services.
Coronavirus-related exposure and/or illness has relegated cancer research to a lower-level priority. As a result, ACCC institutions have made adjustments in their cancer research programs, including moving clinical research coordinators off-campus and deploying them in clinical areas.
New clinical trials have not been opened. In some cases, new accruals have been halted, particularly for registry, prevention, and symptom control trials.
Standards that have changed and those that have not
Guidance documents for conducting clinical trials during the pandemic have been developed by the Food and Drug Administration, the National Cancer Institute’s Cancer Therapy Evaluation Program and Central Institutional Review Board, and the National Institutes of Health’s Office of Extramural Research. Industry sponsors and parent institutions of research programs have also disseminated guidance.
Among other topics, guidance documents have addressed:
- How COVID-19-related protocol deviations will be judged at monitoring visits and audits
- Missed office visits and endpoint evaluations
- Providing investigational oral medications to patients via mail and potential issues of medication unavailability
- Processes for patients to have interim visits with providers at external institutions, including providers who may not be personally engaged in or credentialed for the research trial
- Potential delays in submitting protocol amendments for institutional review board (IRB) review
- Recommendations for patients confirmed or suspected of having a coronavirus infection.
Dr. Oyer emphasized that patient safety must remain the highest priority for patient management, on or off study. He advised continuing investigational therapy when potential benefit from treatment is anticipated and identifying alternative methods to face-to-face visits for monitoring and access to treatment.
Dr. Oyer urged programs to:
- Maintain good clinical practice standards
- Consult with sponsors and IRBs when questions arise but implement changes that affect patient safety prior to IRB review if necessary
- Document all deviations and COVID-19 related adaptations in a log or spreadsheet in anticipation of future questions from sponsors, monitors, and other entities.
New questions and considerations
In the short-term, Dr. Oyer predicts fewer available trials and a decreased rate of accrual to existing studies. This may result in delays in trial completion and the possibility of redesign for some trials.
He predicts the emergence of COVID-19-focused research questions, including those assessing the course of coronavirus infection in various malignant settings and the impact of cancer-directed treatments and supportive care interventions (e.g., treatment for graft-versus-host disease) on response to COVID-19.
To facilitate developing a clinically and research-relevant database, Dr. Oyer stressed the importance of documentation in the research record, reporting infections as serious adverse events. Documentation should specify whether the infection was confirmed or suspected coronavirus or related to another organism.
In general, when coronavirus infection is strongly suspected, Dr. Oyer said investigational treatments should be interrupted, but study-specific criteria will be forthcoming on that issue.
Looking to the future
For patients with advanced cancers, clinical trials provide an important option for hope and clinical benefit. Disrupting the conduct of clinical trials could endanger the lives of participants and delay the emergence of promising treatments and diagnostic tests.
When the coronavirus pandemic recedes, advancing knowledge and treatments for cancer will demand renewed commitment across the oncology care community.
Going forward, Dr. Oyer advised that clinical research staff protect their own health and the safety of trial participants. He encouraged programs to work with sponsors and IRBs to solve logistical problems and clarify individual issues.
He was optimistic that resumption of more normal conduct of studies will enable the successful completion of ongoing trials, enhanced by the creative solutions that were devised during the crisis and by additional prospective, clinically annotated, carefully recorded data from academic and community research sites.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
More than three-quarters of cancer clinical research programs have experienced operational changes during the COVID-19 pandemic, according to a survey conducted by the Association of Community Cancer Centers (ACCC) during a recent webinar.
The webinar included insights into how some cancer research programs have adapted to the pandemic, a review of guidance for conducting cancer trials during this time, and a discussion of how the cancer research landscape may be affected by COVID-19 going forward.
The webinar was led by Randall A. Oyer, MD, president of the ACCC and medical director of the oncology program at Penn Medicine Lancaster General Health in Pennsylvania.
The impact of COVID-19 on cancer research
Dr. Oyer observed that planning and implementation for COVID-19–related illness at U.S. health care institutions has had a predictable effect of limiting patient access and staff availability for nonessential services.
Coronavirus-related exposure and/or illness has relegated cancer research to a lower-level priority. As a result, ACCC institutions have made adjustments in their cancer research programs, including moving clinical research coordinators off-campus and deploying them in clinical areas.
New clinical trials have not been opened. In some cases, new accruals have been halted, particularly for registry, prevention, and symptom control trials.
Standards that have changed and those that have not
Guidance documents for conducting clinical trials during the pandemic have been developed by the Food and Drug Administration, the National Cancer Institute’s Cancer Therapy Evaluation Program and Central Institutional Review Board, and the National Institutes of Health’s Office of Extramural Research. Industry sponsors and parent institutions of research programs have also disseminated guidance.
Among other topics, guidance documents have addressed:
- How COVID-19-related protocol deviations will be judged at monitoring visits and audits
- Missed office visits and endpoint evaluations
- Providing investigational oral medications to patients via mail and potential issues of medication unavailability
- Processes for patients to have interim visits with providers at external institutions, including providers who may not be personally engaged in or credentialed for the research trial
- Potential delays in submitting protocol amendments for institutional review board (IRB) review
- Recommendations for patients confirmed or suspected of having a coronavirus infection.
Dr. Oyer emphasized that patient safety must remain the highest priority for patient management, on or off study. He advised continuing investigational therapy when potential benefit from treatment is anticipated and identifying alternative methods to face-to-face visits for monitoring and access to treatment.
Dr. Oyer urged programs to:
- Maintain good clinical practice standards
- Consult with sponsors and IRBs when questions arise but implement changes that affect patient safety prior to IRB review if necessary
- Document all deviations and COVID-19 related adaptations in a log or spreadsheet in anticipation of future questions from sponsors, monitors, and other entities.
New questions and considerations
In the short-term, Dr. Oyer predicts fewer available trials and a decreased rate of accrual to existing studies. This may result in delays in trial completion and the possibility of redesign for some trials.
He predicts the emergence of COVID-19-focused research questions, including those assessing the course of coronavirus infection in various malignant settings and the impact of cancer-directed treatments and supportive care interventions (e.g., treatment for graft-versus-host disease) on response to COVID-19.
To facilitate developing a clinically and research-relevant database, Dr. Oyer stressed the importance of documentation in the research record, reporting infections as serious adverse events. Documentation should specify whether the infection was confirmed or suspected coronavirus or related to another organism.
In general, when coronavirus infection is strongly suspected, Dr. Oyer said investigational treatments should be interrupted, but study-specific criteria will be forthcoming on that issue.
Looking to the future
For patients with advanced cancers, clinical trials provide an important option for hope and clinical benefit. Disrupting the conduct of clinical trials could endanger the lives of participants and delay the emergence of promising treatments and diagnostic tests.
When the coronavirus pandemic recedes, advancing knowledge and treatments for cancer will demand renewed commitment across the oncology care community.
Going forward, Dr. Oyer advised that clinical research staff protect their own health and the safety of trial participants. He encouraged programs to work with sponsors and IRBs to solve logistical problems and clarify individual issues.
He was optimistic that resumption of more normal conduct of studies will enable the successful completion of ongoing trials, enhanced by the creative solutions that were devised during the crisis and by additional prospective, clinically annotated, carefully recorded data from academic and community research sites.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
More than three-quarters of cancer clinical research programs have experienced operational changes during the COVID-19 pandemic, according to a survey conducted by the Association of Community Cancer Centers (ACCC) during a recent webinar.
The webinar included insights into how some cancer research programs have adapted to the pandemic, a review of guidance for conducting cancer trials during this time, and a discussion of how the cancer research landscape may be affected by COVID-19 going forward.
The webinar was led by Randall A. Oyer, MD, president of the ACCC and medical director of the oncology program at Penn Medicine Lancaster General Health in Pennsylvania.
The impact of COVID-19 on cancer research
Dr. Oyer observed that planning and implementation for COVID-19–related illness at U.S. health care institutions has had a predictable effect of limiting patient access and staff availability for nonessential services.
Coronavirus-related exposure and/or illness has relegated cancer research to a lower-level priority. As a result, ACCC institutions have made adjustments in their cancer research programs, including moving clinical research coordinators off-campus and deploying them in clinical areas.
New clinical trials have not been opened. In some cases, new accruals have been halted, particularly for registry, prevention, and symptom control trials.
Standards that have changed and those that have not
Guidance documents for conducting clinical trials during the pandemic have been developed by the Food and Drug Administration, the National Cancer Institute’s Cancer Therapy Evaluation Program and Central Institutional Review Board, and the National Institutes of Health’s Office of Extramural Research. Industry sponsors and parent institutions of research programs have also disseminated guidance.
Among other topics, guidance documents have addressed:
- How COVID-19-related protocol deviations will be judged at monitoring visits and audits
- Missed office visits and endpoint evaluations
- Providing investigational oral medications to patients via mail and potential issues of medication unavailability
- Processes for patients to have interim visits with providers at external institutions, including providers who may not be personally engaged in or credentialed for the research trial
- Potential delays in submitting protocol amendments for institutional review board (IRB) review
- Recommendations for patients confirmed or suspected of having a coronavirus infection.
Dr. Oyer emphasized that patient safety must remain the highest priority for patient management, on or off study. He advised continuing investigational therapy when potential benefit from treatment is anticipated and identifying alternative methods to face-to-face visits for monitoring and access to treatment.
Dr. Oyer urged programs to:
- Maintain good clinical practice standards
- Consult with sponsors and IRBs when questions arise but implement changes that affect patient safety prior to IRB review if necessary
- Document all deviations and COVID-19 related adaptations in a log or spreadsheet in anticipation of future questions from sponsors, monitors, and other entities.
New questions and considerations
In the short-term, Dr. Oyer predicts fewer available trials and a decreased rate of accrual to existing studies. This may result in delays in trial completion and the possibility of redesign for some trials.
He predicts the emergence of COVID-19-focused research questions, including those assessing the course of coronavirus infection in various malignant settings and the impact of cancer-directed treatments and supportive care interventions (e.g., treatment for graft-versus-host disease) on response to COVID-19.
To facilitate developing a clinically and research-relevant database, Dr. Oyer stressed the importance of documentation in the research record, reporting infections as serious adverse events. Documentation should specify whether the infection was confirmed or suspected coronavirus or related to another organism.
In general, when coronavirus infection is strongly suspected, Dr. Oyer said investigational treatments should be interrupted, but study-specific criteria will be forthcoming on that issue.
Looking to the future
For patients with advanced cancers, clinical trials provide an important option for hope and clinical benefit. Disrupting the conduct of clinical trials could endanger the lives of participants and delay the emergence of promising treatments and diagnostic tests.
When the coronavirus pandemic recedes, advancing knowledge and treatments for cancer will demand renewed commitment across the oncology care community.
Going forward, Dr. Oyer advised that clinical research staff protect their own health and the safety of trial participants. He encouraged programs to work with sponsors and IRBs to solve logistical problems and clarify individual issues.
He was optimistic that resumption of more normal conduct of studies will enable the successful completion of ongoing trials, enhanced by the creative solutions that were devised during the crisis and by additional prospective, clinically annotated, carefully recorded data from academic and community research sites.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
Concordance Between Dermatologist Self-reported and Industry-Reported Interactions at a National Dermatology Conference
Interactions between industry and physicians, including dermatologists, are widely prevalent.1-3 Proper reporting of industry relationships is essential for transparency, objectivity, and management of potential biases and conflicts of interest. There has been increasing public scrutiny regarding these interactions.
The Physician Payments Sunshine Act established Open Payments (OP), a publicly available database that collects and displays industry-reported physician-industry interactions.4,5 For the medical community and public, the OP database may be used to assess transparency by comparing the data with physician self-disclosures. There is a paucity of studies in the literature examining the concordance of industry-reported disclosures and physician self-reported data, with even fewer studies utilizing OP as a source of industry disclosures, and none exists for dermatology.6-12 It also is not clear to what extent the OP database captures all possible dermatologist-industry interactions, as the Sunshine Act only mandates reporting by applicable US-based manufacturers and group purchasing organizations that produce or purchase drugs or devices that require a prescription and are reimbursable by a government-run health care program.5 As a result, certain companies, such as cosmeceuticals, may not be represented.
In this study we aimed to evaluate the concordance of dermatologist self-disclosure of industry relationships and those reported on OP. Specifically, we focused on interactions disclosed by presenters at the American Academy of Dermatology (AAD) 73rd Annual Meeting in San Francisco, California (March 20–24, 2015), and those by industry in the 2014 OP database.
Methods
In this retrospective cohort study, we compared publicly available data from the OP database to presenter disclosures found in the publicly available AAD 73rd Annual Meeting program (AADMP). The AAD required speakers to disclose financial relationships with industry within the 12 months preceding the presentation, as outlined in the Accreditation Council for Continuing Medical Education guidelines.13 All AAD presenters who were dermatologists practicing in the United States were included in the analysis, whereas residents, fellows, nonphysicians, nondermatologist physicians, and international dermatologists were excluded.
We examined general, research, and associated research payments to specific dermatologists using the 2014 OP data, which contained industry payments made between January 1 and December 31, 2014. Open Payments defined research payments as direct payment to the physician for different types of research activities and associated research payments as indirect payments made to a research institution or entity where the physician was named the principal investigator.5 We chose the 2014 database because it most closely matched the period of required disclosures defined by the AAD for the 2015 meeting. Our review of the OP data occurred after the June 2016 update and thus included the most accurate and up-to-date financial interactions.
We conducted our analysis in 2 major steps. First, we determined whether each industry interaction reported in the OP database was present in the AADMP, which provided an assessment of interaction-level concordance. Second, we determined whether all the industry interactions for any given dermatologist listed in the OP also were present in AADMP, which provided an assessment of dermatologist-level concordance.
First, to establish interaction-level concordance for each industry interaction, the company name and the type of interaction (eg, consultant, speaker, investigator) listed in the AADMP were compared with the data in OP to verify a match. Each interaction was assigned into one of the categories of concordant disclosure (a match of both the company name and type of interaction details in OP and the AADMP), overdisclosure (the presence of an AADMP interaction not found in OP, such as an additional type of interaction or company), or underdisclosure (a company name or type of interaction found in OP but not reported in the AADMP). For underdisclosure, we further classified into company present or company absent based on whether the dermatologist disclosed any relationship with a particular company in the AADMP. We considered the type of interaction to be matching if they were identical or similar in nature (eg, consulting in OP and advisory board in the AADMP), as the types of interactions are reported differently in OP and the AADMP. Otherwise, if they were not similar enough (eg, education in OP and stockholder in the AADMP), it was classified as underdisclosure. Some types of interactions reported in OP were not available on the AAD disclosure form. For example, food and beverage as well as travel and lodging were types of interactions in OP that did not exist in the AADMP. These 2 types of interactions comprised a large majority of OP payment entries but only accounted for a small percentage of the payment amount. Analysis was performed both including and excluding interactions for food, beverage, travel, and lodging (f/b/t/l) to best account for differences in interaction categories between OP and the AADMP.
Second, each dermatologist was assigned to an overall disclosure category of dermatologist-level concordance based on the status for all his/her interactions. Categories included no disclosure (no industry interactions in OP and the AADMP), concordant (all industry interactions reported in OP and the AADMP match), overdisclosure only (no industry interactions on OP but self-reported interactions present in the AADMP), and discordant (not all OP interactions were disclosed in the AADMP). The discordant category was further divided into with overdisclosure and without overdisclosure, depending on the presence or absence of industry relationships listed in the AADMP but not in OP, respectively.
To ensure uniformity, one individual (A.F.S.) reviewed and collected the data from OP and the AADMP. Information on gender and academic affiliation of study participants was obtained from information listed in the AADMP and Google searches. Data management was performed with Microsoft Excel software (Microsoft Excel 2010, Version 14.0, Microsoft Corporation). The New York University School of Medicine’s (New York, New York) institutional review board exempted this study.
Results
Of the 938 presenters listed in the AADMP, 768 individuals met the inclusion criteria. The most commonly cited type of relationship with industry listed in the AADMP was serving as an investigator, consultant, or advisory board member, comprising 34%, 26%, and 18%, respectively (Table 1). The forms of payment most frequently reported in the AADMP were honoraria and grants/research funding, comprising 49% and 25%, respectively (Table 2).
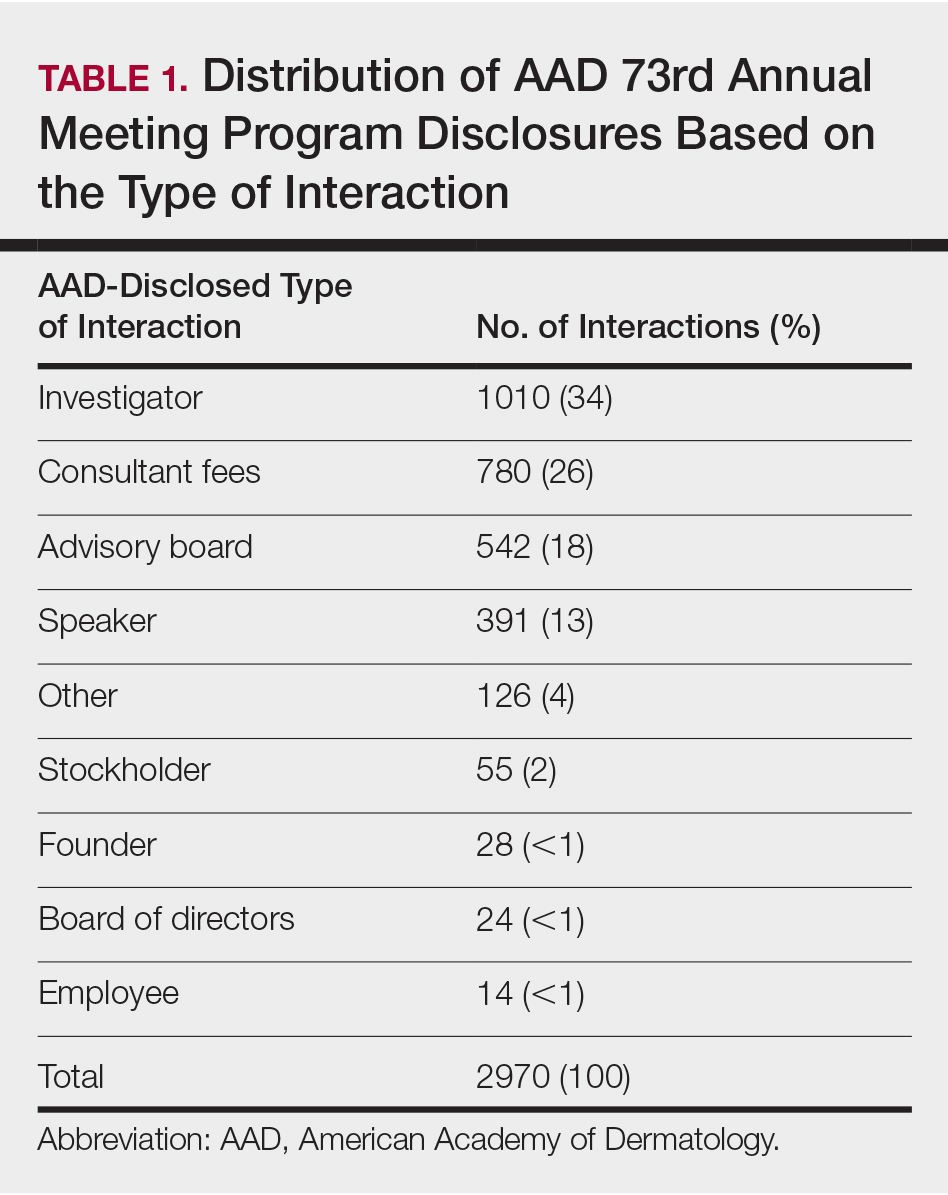
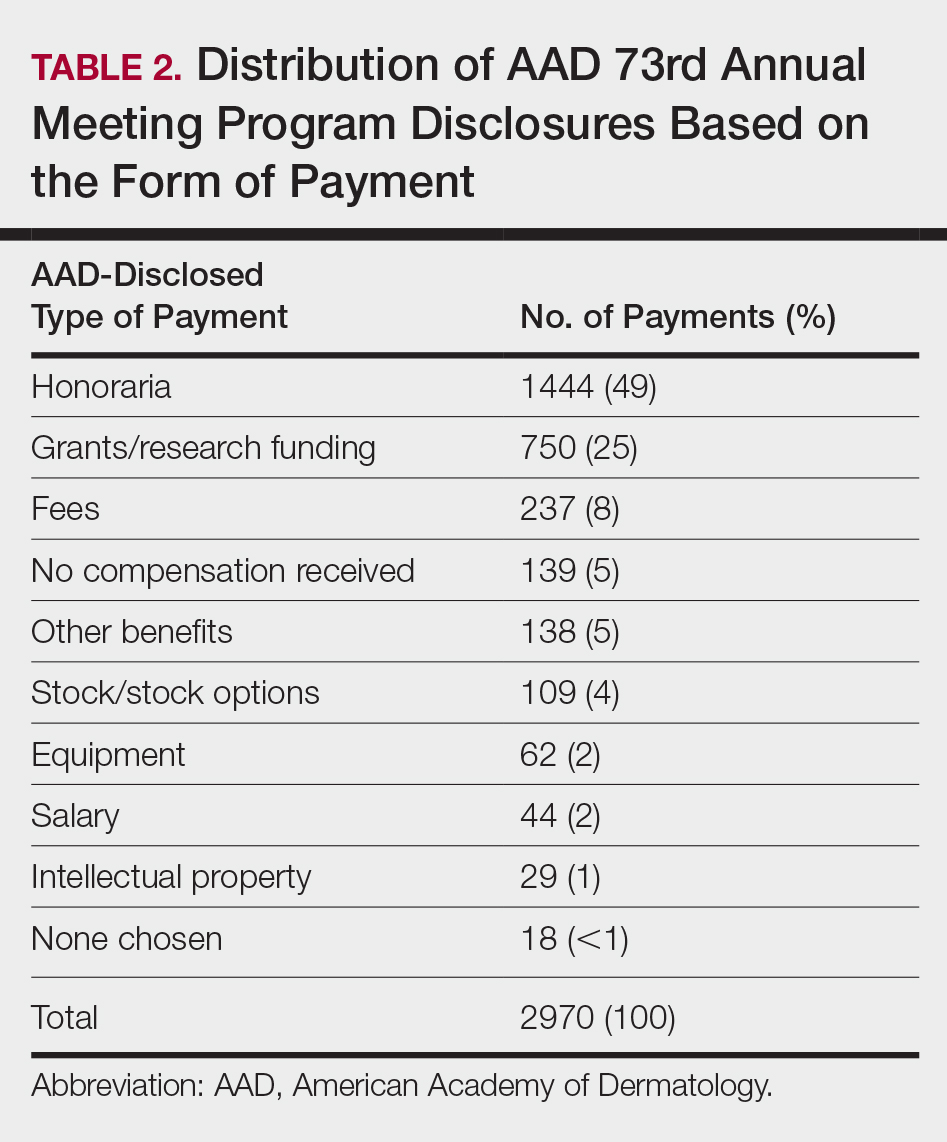
In 2014, there were a total of 20,761 industry payments totaling $35,627,365 for general, research, and associated research payments in the OP database related to the dermatologists who met inclusion criteria. There were 8678 payments totaling $466,622 for food and beverage and 3238 payments totaling $1,357,770 for travel and lodging. After excluding payments for f/b/t/l, there were 8845 payments totaling $33,802,973, with highest percentages of payment amounts for associated research (67.1%), consulting fees (11.5%), research (7.9%), and speaker fees (7.2%)(Table 3). For presenters with industry payments, the range of disbursements excluding f/b/t/l was $6.52 to $1,933,705, with a mean (standard deviation) of $107,997 ($249,941), a median of $18,247, and an interquartile range of $3422 to $97,375 (data not shown).
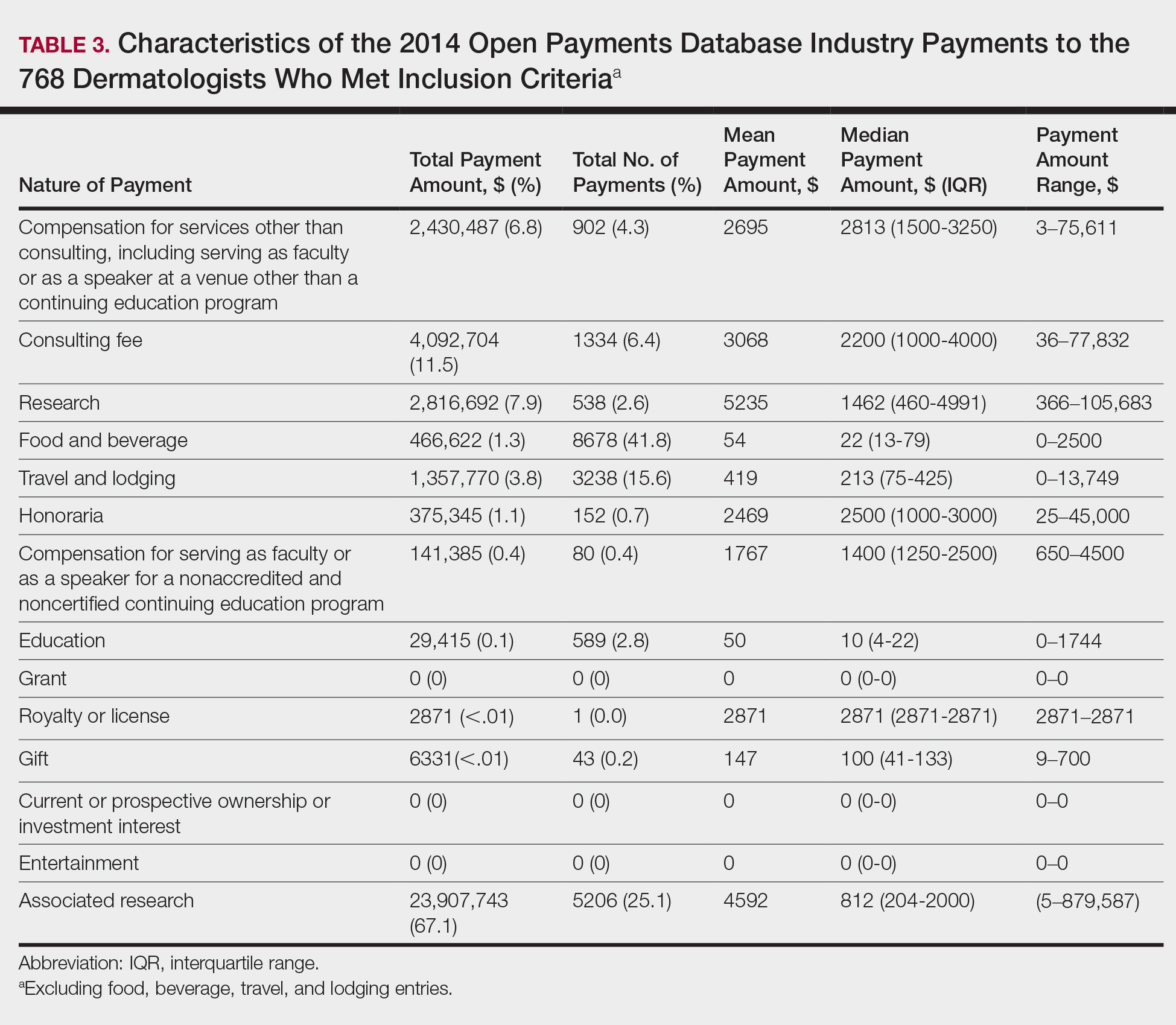
In assessing interaction-level concordance, 63% of all payment amounts in OP were classified as concordant disclosures. Regarding the number of OP payments, 27% were concordant disclosures, 34% were underdisclosures due to f/b/t/l payments, and 39% were underdisclosures due to non–f/b/t/l payments. When f/b/t/l payment entries in OP were excluded, the status of concordant disclosure for the amount and number of OP payments increased to 66% ($22,242,638) and 63% (5549), respectively. The percentage of payment entries with concordant disclosure status ranged from 43% to 71% depending on the payment amount. Payment entries at both ends of the spectrum had the lowest concordant disclosure rates, with 43% for payment entries between $0.01 and $100 and 58% for entries greater than $100,000 (Table 4). The concordance status also differed by the type of interactions. None of the OP payments for gift and royalty or license were disclosed in the AADMP, as there were no suitable corresponding categories. The proportion of payments with concordant disclosure for honoraria (45%), education (48%), and associated research (61%) was lower than the proportion of payments with concordant disclosure for research (90%), speaker fees (75%–79%), and consulting fees (74%)(Table 5).
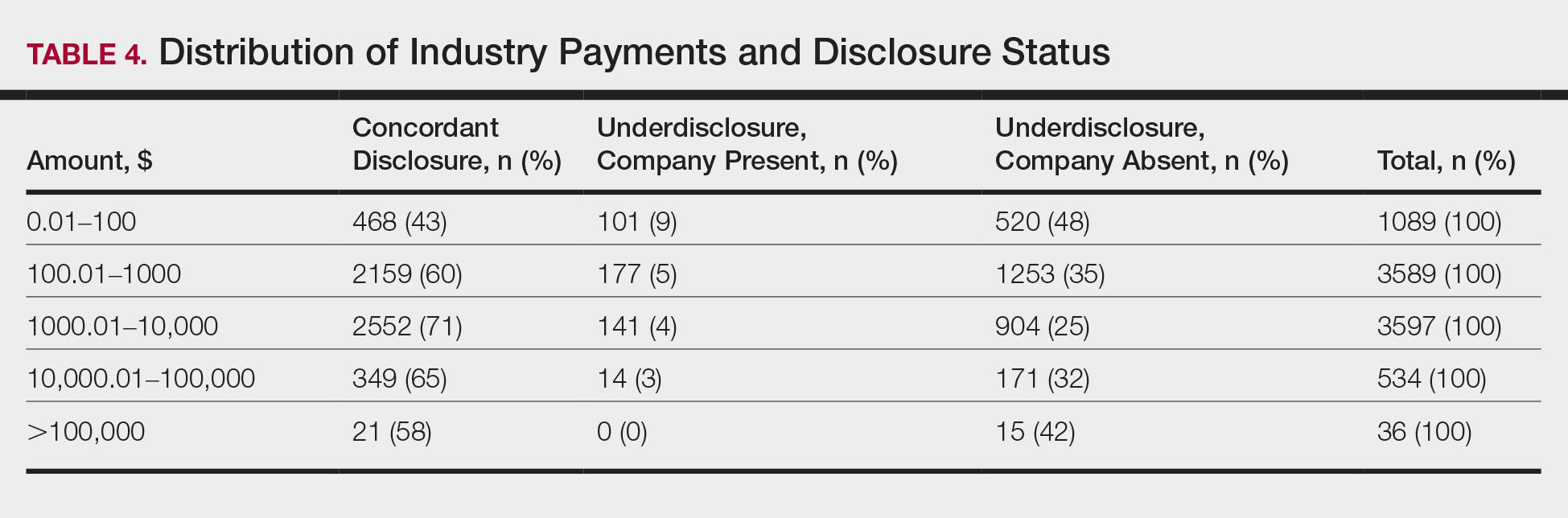
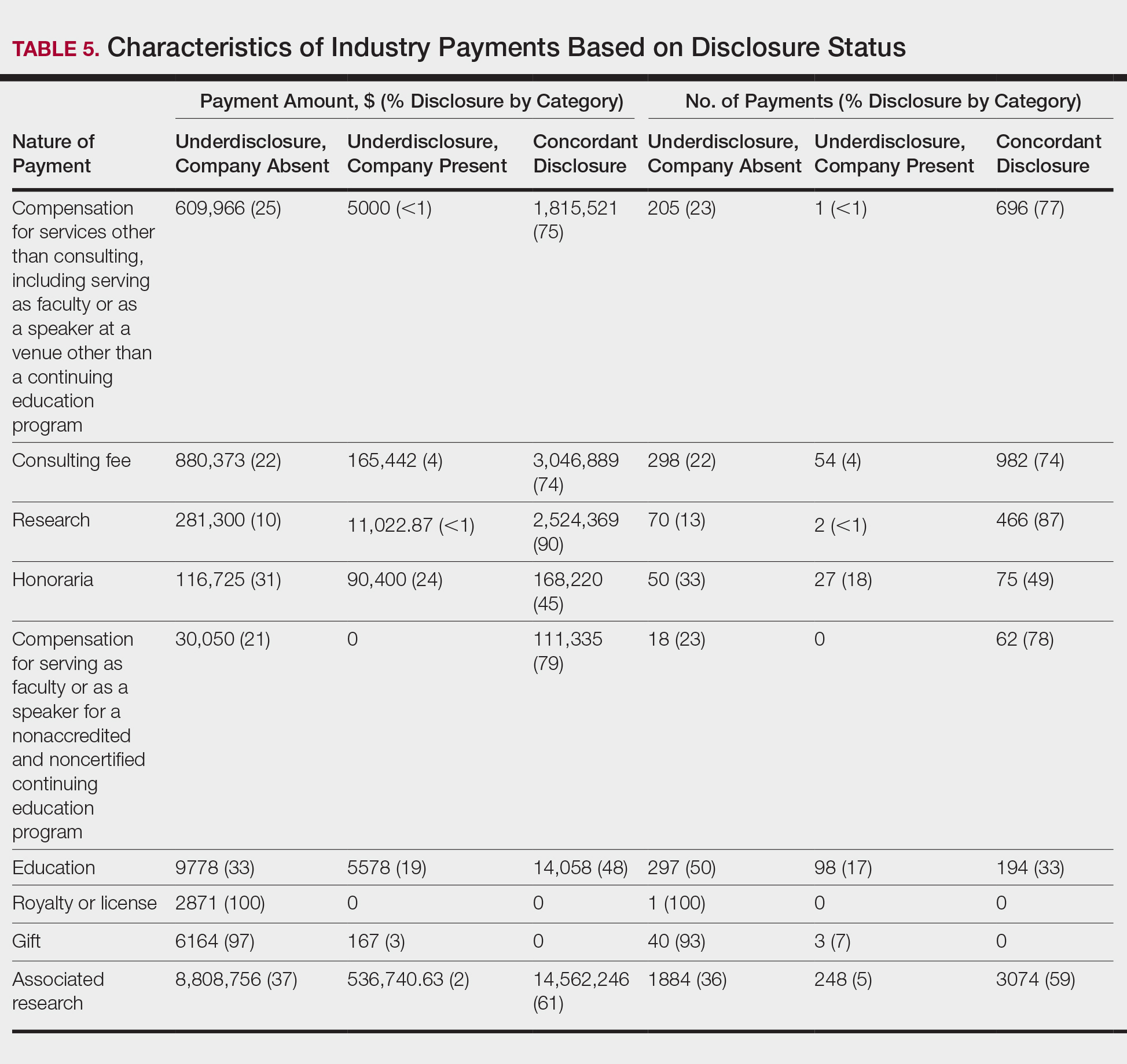
In assessing dermatologist-level concordance including all OP entries, the number of dermatologists with no disclosure, overdisclosure only, concordant disclosure, discordant with overdisclosure, and discordant without overdisclosure statuses were 234 (30%), 70 (9%), 9 (1%), 251 (33%), and 204 (27%), respectively. When f/b/t/l entries were excluded, those figures changed to 347 (45%), 108 (14%), 79 (10%), 157 (20%), and 77 (10%), respectively. The characteristics of these dermatologists and their associated industry interactions by disclosure status are shown in the eTable. Dermatologists in the discordant with overdisclosure group had the highest median number and amount of OP payments, followed by those in the concordant disclosure and discordant without overdisclosure groups. Additionally, discordant with overdisclosure dermatologists also had the highest median and mean number of unique industry interactions not on OP, followed by those in the overdisclosure only and no disclosure groups. Academic and private practice settings did not impact dermatologists’ disclosure status. The percentage of female and male dermatologists in the discordant group was 25% and 36%, respectively.
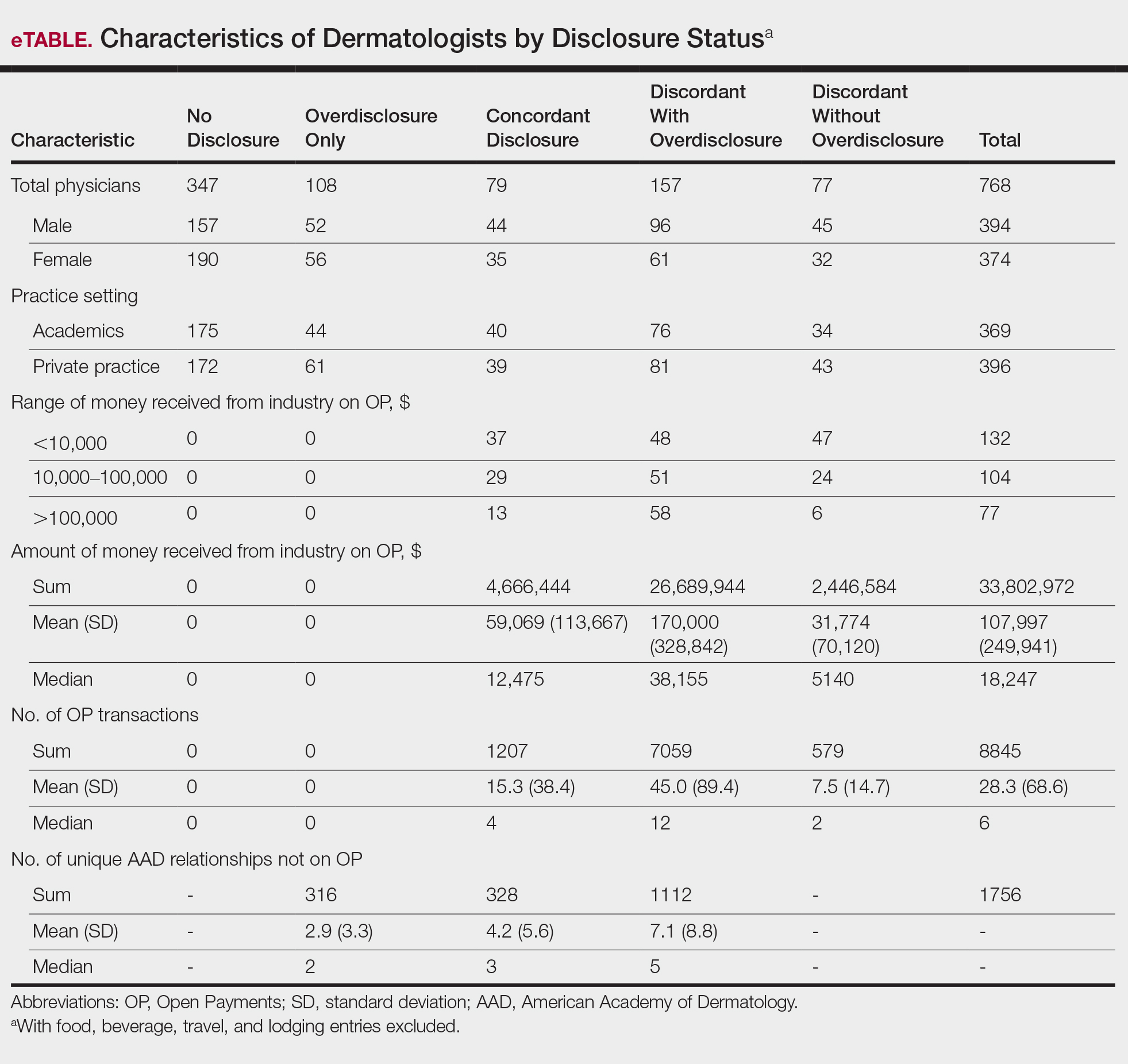
Dermatologists reported a total of 1756 unique industry relationships in the AADMP that were not found on OP. Of these, 1440 (82%) relationships were from 236 dermatologists who had industry payments on OP. The remaining 316 relationships were from 108 dermatologists who had no payments on OP. Although 114 companies reported payments to dermatologists on OP, dermatologists in the AADMP reported interactions with an additional 430 companies.
Comment
In this study, we demonstrated discordance between dermatologist self-reported financial interactions in the AADMP compared with those reported by industry via OP. After excluding f/b/t/l entries, approximately two-thirds of the total amount and number of payments in OP were disclosed, while 31% of dermatologists had discordant disclosure status.
Prior investigations in other medical fields showed high discrepancy rates between industry-reported and physician-reported relationships ranging from 23% to 62%, with studies utilizing various methodologies.6-9,11,12,14,15 Only a few studies have utilized the OP database.8,12,15 Thompson et al12 compared OP payment data with physician financial disclosure at an annual gynecology scientific meeting and found although 209 of 335 (62%) physicians had interactions listed in the OP database, only 24 (7%) listed at least 1 company in the meeting financial disclosure section. Of these 24 physicians, only 5 (21%) accurately disclosed financial relationships with all of the companies listed in OP. The investigators found 129 (38.5%) physicians and 33.7% of the $1.99 million OP payments had concordant disclosure status. When they excluded physicians who received less than $100, 53% of individuals had concordant disclosure.12 Hannon et al8 reported on inconsistencies between disclosures in the OP database and the American Academy of Orthopedic Surgeons Annual Meeting and found 259 (23%) of 1113 physicians meeting inclusion criteria had financial interactions listed in the OP database that were not reported in the meeting disclosures. Yee et al15 also utilized the OP database and compared it with author disclosures in 3 major ophthalmology journals.Of 670 authors, 367 (54.8%) had complete concordance, with 68 (10.1%) more reporting additional overdisclosures, leading to a discordant with underdisclosure rate of 35.1%. Additionally, $1.46 million (44.6%) of the $3.27 million OP payments had concordant disclosure status.15 Other studies compared individual companies’ online reports of physician payments with physician self-disclosures in annual meeting programs, clinical guidelines, and peer-reviewed publications.6,7,9,11,14
Our study demonstrated variation in disclosure status. Compared with other groups, dermatologists in the discordant with overdisclosure group on average had more interactions with and received higher payments from industry, which is consistent with studies in the orthopedic surgery literature.8,9 Male dermatologists had 11% more discordant disclosures than their female counterparts, which may be influenced by men having more industry interactions than women.3 Although small industry payments possessed the lowest concordant rate in our study, which has been observed,12 payments greater than $100,000 had the second-lowest concordance rate at 58%, which may be skewed by the small sample size. Rates of concordant disclosure differed among types of interactions, such as between research and associated research payments. This particular difference may be attributed to the incorrect listing of dermatologists as principal investigators or reduced awareness of payments, as associated research payments were made to the institution and not the individual.
Reasons for discrepancies between industry-reported and dermatologist-reported disclosures may include reporting time differences, lack of physician awareness of OP, industry reporting inaccuracies, dearth of contextual information associated with individual payment entries, and misunderstandings. Prior research demonstrated that the most common reasons for physician nondisclosure included misunderstanding disclosure requirements, unintentional omission of payment, and a lack of relationship between the industry payment and presentation topic.9,12 These factors likely contributed to the disclosure inconsistencies in our study. Similarly high rates of inconsistencies across different specialties suggest systemic concerns.
We found a substantial number of dermatologist-industry interactions listed in the AADMP that were not captured by OP, with 108 dermatologists (35%) having overdisclosures even when excluding f/b/t/l entries. The number of companies in these overdisclosures approximated 4 times the number of companies on OP. Other studies have also observed physician-industry interactions not displayed on OP.8,12,15 Because the Sunshine Act requires reporting only by certain companies, interactions surrounding products such as over-the-counter merchandise, cosmetics, lasers, novel devices, and new medications are generally not included. Further, OP may not capture nonmonetary industry relationships.
There were several limitations to this study. The most notable limitation was the differences in the categorizations of industry relationships by OP and the AADMP. These differences can overemphasize some types of interactions at the expense of other types, such as f/b/t/l. As such, analyses were repeated after excluding f/b/t/l. Another limitation was the inexact overlap of time frames for OP and the AADMP, which may have led to discrepancies. However, we used the best available data and expect the vast majority of interactions to have occurred by the AAD disclosure deadline. It is possible that the presenters may have had a more updated conflict-of-interest disclosure slide at the time of the meeting presentation. The most important limitation was that we were unable to determine whether discrepancies resulted from underreporting by dermatologists or inaccurate reporting by industry. It was unlikely that OP or the AADMP alone completely represented all dermatologist-industry financial relationships.
Conclusion
With a growing emphasis on physician-industry transparency, we identified rates of differences in dermatology consistent with those in other medical fields when comparing the publicly available OP database with disclosures at national conferences. Although the differences in the categorization and requirements for disclosure between the OP database and the AADMP may account for some of the discordance, dermatologists should be aware of potentially negative public perceptions regarding the transparency and prevalence of physician-industry interactions.
Acknowledgment
The first two authors contributed equally to this research/article
- Campbell EG, Gruen RL, Mountford J, et al. A national survey of physician-industry relationships. N Engl J Med. 2007;356:1742-1750.
- Marshall DC, Jackson ME, Hattangadi-Gluth JA. Disclosure of industry payments to physicians: an epidemiologic analysis of early data from the open payments program. Mayo Clin Proc. 2016;91:84-96.
- Feng H, Wu P, Leger M. Exploring the industry-dermatologist financial relationship: insight from the open payment data. JAMA Dermatol. 2016;152:1307-1313.
- Kirschner NM, Sulmasy LS, Kesselheim AS. Health policy basics: the physician payment Sunshine Act and the open payments program. Ann Intern Med. 2014;161:519-521.
- Search Open Payment. Centers for Medicare & Medicaid Services. https://openpaymentsdata.cms.gov. Accessed October 21, 2019.
- Buerba RA, Fu MC, Grauer JN. Discrepancies in spine surgeon conflict of interest disclosures between a national meeting and physician payment listings on device manufacturer web sites. Spine J. 2013;13:1780-1788.
- Chimonas S, Frosch Z, Rothman DJ. From disclosure to transparency: the use of company payment data. Arch Intern Med. 2011;171:81-86.
- Hannon CP, Chalmers PN, Carpiniello MF, et al. Inconsistencies between physician-reported disclosures at the AAOS annual meeting and industry-reported financial disclosures in the open payments database. J Bone Joint Surg. 2016;98:E90.
- Okike K, Kocher MS, Wei EX, et al. Accuracy of conflict-of-interest disclosures reported by physicians. N Engl J Med. 2009;361:1466-1474.
- Ramm O, Brubaker L. Conflicts-of-interest disclosures at the 2010 AUGS Scientific Meeting. Female Pelvic Med Reconstr Surg. 2012;18:79-81.
- Tanzer D, Smith K, Tanzer M. American Academy of Orthopaedic Surgeons disclosure policy fails to accurately inform its members of potential conflicts of interest. Am J Orthop (Belle Mead NJ). 2015;44:E207-E210.
- Thompson JC, Volpe KA, Bridgewater LK, et al. Sunshine Act: shedding light on inaccurate disclosures at a gynecologic annual meeting. Am J Obstet Gynecol. 2016;215:661.
- Disclosure of Potential Conflicts of Interest. American Academy of Dermatology and AAD Association Web site. https://aad.org/Forms/Policies/Uploads/AR/
AR%20Disclosure%20of%20Potential%20Conflicts%
20of%20Interest-2.pdf. Accessed October 21, 2019. - Hockenberry JM, Weigel P, Auerbach A, et al. Financial payments by orthopedic device makers to orthopedic surgeons. Arch Intern Med. 2011;171:1759-1765.
- Yee C, Greenberg PB, Margo CE, et al. Financial disclosures in academic publications and the Sunshine Act: a concordance dtudy. Br J Med Med Res. 2015;10:1-6.
Interactions between industry and physicians, including dermatologists, are widely prevalent.1-3 Proper reporting of industry relationships is essential for transparency, objectivity, and management of potential biases and conflicts of interest. There has been increasing public scrutiny regarding these interactions.
The Physician Payments Sunshine Act established Open Payments (OP), a publicly available database that collects and displays industry-reported physician-industry interactions.4,5 For the medical community and public, the OP database may be used to assess transparency by comparing the data with physician self-disclosures. There is a paucity of studies in the literature examining the concordance of industry-reported disclosures and physician self-reported data, with even fewer studies utilizing OP as a source of industry disclosures, and none exists for dermatology.6-12 It also is not clear to what extent the OP database captures all possible dermatologist-industry interactions, as the Sunshine Act only mandates reporting by applicable US-based manufacturers and group purchasing organizations that produce or purchase drugs or devices that require a prescription and are reimbursable by a government-run health care program.5 As a result, certain companies, such as cosmeceuticals, may not be represented.
In this study we aimed to evaluate the concordance of dermatologist self-disclosure of industry relationships and those reported on OP. Specifically, we focused on interactions disclosed by presenters at the American Academy of Dermatology (AAD) 73rd Annual Meeting in San Francisco, California (March 20–24, 2015), and those by industry in the 2014 OP database.
Methods
In this retrospective cohort study, we compared publicly available data from the OP database to presenter disclosures found in the publicly available AAD 73rd Annual Meeting program (AADMP). The AAD required speakers to disclose financial relationships with industry within the 12 months preceding the presentation, as outlined in the Accreditation Council for Continuing Medical Education guidelines.13 All AAD presenters who were dermatologists practicing in the United States were included in the analysis, whereas residents, fellows, nonphysicians, nondermatologist physicians, and international dermatologists were excluded.
We examined general, research, and associated research payments to specific dermatologists using the 2014 OP data, which contained industry payments made between January 1 and December 31, 2014. Open Payments defined research payments as direct payment to the physician for different types of research activities and associated research payments as indirect payments made to a research institution or entity where the physician was named the principal investigator.5 We chose the 2014 database because it most closely matched the period of required disclosures defined by the AAD for the 2015 meeting. Our review of the OP data occurred after the June 2016 update and thus included the most accurate and up-to-date financial interactions.
We conducted our analysis in 2 major steps. First, we determined whether each industry interaction reported in the OP database was present in the AADMP, which provided an assessment of interaction-level concordance. Second, we determined whether all the industry interactions for any given dermatologist listed in the OP also were present in AADMP, which provided an assessment of dermatologist-level concordance.
First, to establish interaction-level concordance for each industry interaction, the company name and the type of interaction (eg, consultant, speaker, investigator) listed in the AADMP were compared with the data in OP to verify a match. Each interaction was assigned into one of the categories of concordant disclosure (a match of both the company name and type of interaction details in OP and the AADMP), overdisclosure (the presence of an AADMP interaction not found in OP, such as an additional type of interaction or company), or underdisclosure (a company name or type of interaction found in OP but not reported in the AADMP). For underdisclosure, we further classified into company present or company absent based on whether the dermatologist disclosed any relationship with a particular company in the AADMP. We considered the type of interaction to be matching if they were identical or similar in nature (eg, consulting in OP and advisory board in the AADMP), as the types of interactions are reported differently in OP and the AADMP. Otherwise, if they were not similar enough (eg, education in OP and stockholder in the AADMP), it was classified as underdisclosure. Some types of interactions reported in OP were not available on the AAD disclosure form. For example, food and beverage as well as travel and lodging were types of interactions in OP that did not exist in the AADMP. These 2 types of interactions comprised a large majority of OP payment entries but only accounted for a small percentage of the payment amount. Analysis was performed both including and excluding interactions for food, beverage, travel, and lodging (f/b/t/l) to best account for differences in interaction categories between OP and the AADMP.
Second, each dermatologist was assigned to an overall disclosure category of dermatologist-level concordance based on the status for all his/her interactions. Categories included no disclosure (no industry interactions in OP and the AADMP), concordant (all industry interactions reported in OP and the AADMP match), overdisclosure only (no industry interactions on OP but self-reported interactions present in the AADMP), and discordant (not all OP interactions were disclosed in the AADMP). The discordant category was further divided into with overdisclosure and without overdisclosure, depending on the presence or absence of industry relationships listed in the AADMP but not in OP, respectively.
To ensure uniformity, one individual (A.F.S.) reviewed and collected the data from OP and the AADMP. Information on gender and academic affiliation of study participants was obtained from information listed in the AADMP and Google searches. Data management was performed with Microsoft Excel software (Microsoft Excel 2010, Version 14.0, Microsoft Corporation). The New York University School of Medicine’s (New York, New York) institutional review board exempted this study.
Results
Of the 938 presenters listed in the AADMP, 768 individuals met the inclusion criteria. The most commonly cited type of relationship with industry listed in the AADMP was serving as an investigator, consultant, or advisory board member, comprising 34%, 26%, and 18%, respectively (Table 1). The forms of payment most frequently reported in the AADMP were honoraria and grants/research funding, comprising 49% and 25%, respectively (Table 2).


In 2014, there were a total of 20,761 industry payments totaling $35,627,365 for general, research, and associated research payments in the OP database related to the dermatologists who met inclusion criteria. There were 8678 payments totaling $466,622 for food and beverage and 3238 payments totaling $1,357,770 for travel and lodging. After excluding payments for f/b/t/l, there were 8845 payments totaling $33,802,973, with highest percentages of payment amounts for associated research (67.1%), consulting fees (11.5%), research (7.9%), and speaker fees (7.2%)(Table 3). For presenters with industry payments, the range of disbursements excluding f/b/t/l was $6.52 to $1,933,705, with a mean (standard deviation) of $107,997 ($249,941), a median of $18,247, and an interquartile range of $3422 to $97,375 (data not shown).

In assessing interaction-level concordance, 63% of all payment amounts in OP were classified as concordant disclosures. Regarding the number of OP payments, 27% were concordant disclosures, 34% were underdisclosures due to f/b/t/l payments, and 39% were underdisclosures due to non–f/b/t/l payments. When f/b/t/l payment entries in OP were excluded, the status of concordant disclosure for the amount and number of OP payments increased to 66% ($22,242,638) and 63% (5549), respectively. The percentage of payment entries with concordant disclosure status ranged from 43% to 71% depending on the payment amount. Payment entries at both ends of the spectrum had the lowest concordant disclosure rates, with 43% for payment entries between $0.01 and $100 and 58% for entries greater than $100,000 (Table 4). The concordance status also differed by the type of interactions. None of the OP payments for gift and royalty or license were disclosed in the AADMP, as there were no suitable corresponding categories. The proportion of payments with concordant disclosure for honoraria (45%), education (48%), and associated research (61%) was lower than the proportion of payments with concordant disclosure for research (90%), speaker fees (75%–79%), and consulting fees (74%)(Table 5).


In assessing dermatologist-level concordance including all OP entries, the number of dermatologists with no disclosure, overdisclosure only, concordant disclosure, discordant with overdisclosure, and discordant without overdisclosure statuses were 234 (30%), 70 (9%), 9 (1%), 251 (33%), and 204 (27%), respectively. When f/b/t/l entries were excluded, those figures changed to 347 (45%), 108 (14%), 79 (10%), 157 (20%), and 77 (10%), respectively. The characteristics of these dermatologists and their associated industry interactions by disclosure status are shown in the eTable. Dermatologists in the discordant with overdisclosure group had the highest median number and amount of OP payments, followed by those in the concordant disclosure and discordant without overdisclosure groups. Additionally, discordant with overdisclosure dermatologists also had the highest median and mean number of unique industry interactions not on OP, followed by those in the overdisclosure only and no disclosure groups. Academic and private practice settings did not impact dermatologists’ disclosure status. The percentage of female and male dermatologists in the discordant group was 25% and 36%, respectively.

Dermatologists reported a total of 1756 unique industry relationships in the AADMP that were not found on OP. Of these, 1440 (82%) relationships were from 236 dermatologists who had industry payments on OP. The remaining 316 relationships were from 108 dermatologists who had no payments on OP. Although 114 companies reported payments to dermatologists on OP, dermatologists in the AADMP reported interactions with an additional 430 companies.
Comment
In this study, we demonstrated discordance between dermatologist self-reported financial interactions in the AADMP compared with those reported by industry via OP. After excluding f/b/t/l entries, approximately two-thirds of the total amount and number of payments in OP were disclosed, while 31% of dermatologists had discordant disclosure status.
Prior investigations in other medical fields showed high discrepancy rates between industry-reported and physician-reported relationships ranging from 23% to 62%, with studies utilizing various methodologies.6-9,11,12,14,15 Only a few studies have utilized the OP database.8,12,15 Thompson et al12 compared OP payment data with physician financial disclosure at an annual gynecology scientific meeting and found although 209 of 335 (62%) physicians had interactions listed in the OP database, only 24 (7%) listed at least 1 company in the meeting financial disclosure section. Of these 24 physicians, only 5 (21%) accurately disclosed financial relationships with all of the companies listed in OP. The investigators found 129 (38.5%) physicians and 33.7% of the $1.99 million OP payments had concordant disclosure status. When they excluded physicians who received less than $100, 53% of individuals had concordant disclosure.12 Hannon et al8 reported on inconsistencies between disclosures in the OP database and the American Academy of Orthopedic Surgeons Annual Meeting and found 259 (23%) of 1113 physicians meeting inclusion criteria had financial interactions listed in the OP database that were not reported in the meeting disclosures. Yee et al15 also utilized the OP database and compared it with author disclosures in 3 major ophthalmology journals.Of 670 authors, 367 (54.8%) had complete concordance, with 68 (10.1%) more reporting additional overdisclosures, leading to a discordant with underdisclosure rate of 35.1%. Additionally, $1.46 million (44.6%) of the $3.27 million OP payments had concordant disclosure status.15 Other studies compared individual companies’ online reports of physician payments with physician self-disclosures in annual meeting programs, clinical guidelines, and peer-reviewed publications.6,7,9,11,14
Our study demonstrated variation in disclosure status. Compared with other groups, dermatologists in the discordant with overdisclosure group on average had more interactions with and received higher payments from industry, which is consistent with studies in the orthopedic surgery literature.8,9 Male dermatologists had 11% more discordant disclosures than their female counterparts, which may be influenced by men having more industry interactions than women.3 Although small industry payments possessed the lowest concordant rate in our study, which has been observed,12 payments greater than $100,000 had the second-lowest concordance rate at 58%, which may be skewed by the small sample size. Rates of concordant disclosure differed among types of interactions, such as between research and associated research payments. This particular difference may be attributed to the incorrect listing of dermatologists as principal investigators or reduced awareness of payments, as associated research payments were made to the institution and not the individual.
Reasons for discrepancies between industry-reported and dermatologist-reported disclosures may include reporting time differences, lack of physician awareness of OP, industry reporting inaccuracies, dearth of contextual information associated with individual payment entries, and misunderstandings. Prior research demonstrated that the most common reasons for physician nondisclosure included misunderstanding disclosure requirements, unintentional omission of payment, and a lack of relationship between the industry payment and presentation topic.9,12 These factors likely contributed to the disclosure inconsistencies in our study. Similarly high rates of inconsistencies across different specialties suggest systemic concerns.
We found a substantial number of dermatologist-industry interactions listed in the AADMP that were not captured by OP, with 108 dermatologists (35%) having overdisclosures even when excluding f/b/t/l entries. The number of companies in these overdisclosures approximated 4 times the number of companies on OP. Other studies have also observed physician-industry interactions not displayed on OP.8,12,15 Because the Sunshine Act requires reporting only by certain companies, interactions surrounding products such as over-the-counter merchandise, cosmetics, lasers, novel devices, and new medications are generally not included. Further, OP may not capture nonmonetary industry relationships.
There were several limitations to this study. The most notable limitation was the differences in the categorizations of industry relationships by OP and the AADMP. These differences can overemphasize some types of interactions at the expense of other types, such as f/b/t/l. As such, analyses were repeated after excluding f/b/t/l. Another limitation was the inexact overlap of time frames for OP and the AADMP, which may have led to discrepancies. However, we used the best available data and expect the vast majority of interactions to have occurred by the AAD disclosure deadline. It is possible that the presenters may have had a more updated conflict-of-interest disclosure slide at the time of the meeting presentation. The most important limitation was that we were unable to determine whether discrepancies resulted from underreporting by dermatologists or inaccurate reporting by industry. It was unlikely that OP or the AADMP alone completely represented all dermatologist-industry financial relationships.
Conclusion
With a growing emphasis on physician-industry transparency, we identified rates of differences in dermatology consistent with those in other medical fields when comparing the publicly available OP database with disclosures at national conferences. Although the differences in the categorization and requirements for disclosure between the OP database and the AADMP may account for some of the discordance, dermatologists should be aware of potentially negative public perceptions regarding the transparency and prevalence of physician-industry interactions.
Acknowledgment
The first two authors contributed equally to this research/article
Interactions between industry and physicians, including dermatologists, are widely prevalent.1-3 Proper reporting of industry relationships is essential for transparency, objectivity, and management of potential biases and conflicts of interest. There has been increasing public scrutiny regarding these interactions.
The Physician Payments Sunshine Act established Open Payments (OP), a publicly available database that collects and displays industry-reported physician-industry interactions.4,5 For the medical community and public, the OP database may be used to assess transparency by comparing the data with physician self-disclosures. There is a paucity of studies in the literature examining the concordance of industry-reported disclosures and physician self-reported data, with even fewer studies utilizing OP as a source of industry disclosures, and none exists for dermatology.6-12 It also is not clear to what extent the OP database captures all possible dermatologist-industry interactions, as the Sunshine Act only mandates reporting by applicable US-based manufacturers and group purchasing organizations that produce or purchase drugs or devices that require a prescription and are reimbursable by a government-run health care program.5 As a result, certain companies, such as cosmeceuticals, may not be represented.
In this study we aimed to evaluate the concordance of dermatologist self-disclosure of industry relationships and those reported on OP. Specifically, we focused on interactions disclosed by presenters at the American Academy of Dermatology (AAD) 73rd Annual Meeting in San Francisco, California (March 20–24, 2015), and those by industry in the 2014 OP database.
Methods
In this retrospective cohort study, we compared publicly available data from the OP database to presenter disclosures found in the publicly available AAD 73rd Annual Meeting program (AADMP). The AAD required speakers to disclose financial relationships with industry within the 12 months preceding the presentation, as outlined in the Accreditation Council for Continuing Medical Education guidelines.13 All AAD presenters who were dermatologists practicing in the United States were included in the analysis, whereas residents, fellows, nonphysicians, nondermatologist physicians, and international dermatologists were excluded.
We examined general, research, and associated research payments to specific dermatologists using the 2014 OP data, which contained industry payments made between January 1 and December 31, 2014. Open Payments defined research payments as direct payment to the physician for different types of research activities and associated research payments as indirect payments made to a research institution or entity where the physician was named the principal investigator.5 We chose the 2014 database because it most closely matched the period of required disclosures defined by the AAD for the 2015 meeting. Our review of the OP data occurred after the June 2016 update and thus included the most accurate and up-to-date financial interactions.
We conducted our analysis in 2 major steps. First, we determined whether each industry interaction reported in the OP database was present in the AADMP, which provided an assessment of interaction-level concordance. Second, we determined whether all the industry interactions for any given dermatologist listed in the OP also were present in AADMP, which provided an assessment of dermatologist-level concordance.
First, to establish interaction-level concordance for each industry interaction, the company name and the type of interaction (eg, consultant, speaker, investigator) listed in the AADMP were compared with the data in OP to verify a match. Each interaction was assigned into one of the categories of concordant disclosure (a match of both the company name and type of interaction details in OP and the AADMP), overdisclosure (the presence of an AADMP interaction not found in OP, such as an additional type of interaction or company), or underdisclosure (a company name or type of interaction found in OP but not reported in the AADMP). For underdisclosure, we further classified into company present or company absent based on whether the dermatologist disclosed any relationship with a particular company in the AADMP. We considered the type of interaction to be matching if they were identical or similar in nature (eg, consulting in OP and advisory board in the AADMP), as the types of interactions are reported differently in OP and the AADMP. Otherwise, if they were not similar enough (eg, education in OP and stockholder in the AADMP), it was classified as underdisclosure. Some types of interactions reported in OP were not available on the AAD disclosure form. For example, food and beverage as well as travel and lodging were types of interactions in OP that did not exist in the AADMP. These 2 types of interactions comprised a large majority of OP payment entries but only accounted for a small percentage of the payment amount. Analysis was performed both including and excluding interactions for food, beverage, travel, and lodging (f/b/t/l) to best account for differences in interaction categories between OP and the AADMP.
Second, each dermatologist was assigned to an overall disclosure category of dermatologist-level concordance based on the status for all his/her interactions. Categories included no disclosure (no industry interactions in OP and the AADMP), concordant (all industry interactions reported in OP and the AADMP match), overdisclosure only (no industry interactions on OP but self-reported interactions present in the AADMP), and discordant (not all OP interactions were disclosed in the AADMP). The discordant category was further divided into with overdisclosure and without overdisclosure, depending on the presence or absence of industry relationships listed in the AADMP but not in OP, respectively.
To ensure uniformity, one individual (A.F.S.) reviewed and collected the data from OP and the AADMP. Information on gender and academic affiliation of study participants was obtained from information listed in the AADMP and Google searches. Data management was performed with Microsoft Excel software (Microsoft Excel 2010, Version 14.0, Microsoft Corporation). The New York University School of Medicine’s (New York, New York) institutional review board exempted this study.
Results
Of the 938 presenters listed in the AADMP, 768 individuals met the inclusion criteria. The most commonly cited type of relationship with industry listed in the AADMP was serving as an investigator, consultant, or advisory board member, comprising 34%, 26%, and 18%, respectively (Table 1). The forms of payment most frequently reported in the AADMP were honoraria and grants/research funding, comprising 49% and 25%, respectively (Table 2).


In 2014, there were a total of 20,761 industry payments totaling $35,627,365 for general, research, and associated research payments in the OP database related to the dermatologists who met inclusion criteria. There were 8678 payments totaling $466,622 for food and beverage and 3238 payments totaling $1,357,770 for travel and lodging. After excluding payments for f/b/t/l, there were 8845 payments totaling $33,802,973, with highest percentages of payment amounts for associated research (67.1%), consulting fees (11.5%), research (7.9%), and speaker fees (7.2%)(Table 3). For presenters with industry payments, the range of disbursements excluding f/b/t/l was $6.52 to $1,933,705, with a mean (standard deviation) of $107,997 ($249,941), a median of $18,247, and an interquartile range of $3422 to $97,375 (data not shown).

In assessing interaction-level concordance, 63% of all payment amounts in OP were classified as concordant disclosures. Regarding the number of OP payments, 27% were concordant disclosures, 34% were underdisclosures due to f/b/t/l payments, and 39% were underdisclosures due to non–f/b/t/l payments. When f/b/t/l payment entries in OP were excluded, the status of concordant disclosure for the amount and number of OP payments increased to 66% ($22,242,638) and 63% (5549), respectively. The percentage of payment entries with concordant disclosure status ranged from 43% to 71% depending on the payment amount. Payment entries at both ends of the spectrum had the lowest concordant disclosure rates, with 43% for payment entries between $0.01 and $100 and 58% for entries greater than $100,000 (Table 4). The concordance status also differed by the type of interactions. None of the OP payments for gift and royalty or license were disclosed in the AADMP, as there were no suitable corresponding categories. The proportion of payments with concordant disclosure for honoraria (45%), education (48%), and associated research (61%) was lower than the proportion of payments with concordant disclosure for research (90%), speaker fees (75%–79%), and consulting fees (74%)(Table 5).


In assessing dermatologist-level concordance including all OP entries, the number of dermatologists with no disclosure, overdisclosure only, concordant disclosure, discordant with overdisclosure, and discordant without overdisclosure statuses were 234 (30%), 70 (9%), 9 (1%), 251 (33%), and 204 (27%), respectively. When f/b/t/l entries were excluded, those figures changed to 347 (45%), 108 (14%), 79 (10%), 157 (20%), and 77 (10%), respectively. The characteristics of these dermatologists and their associated industry interactions by disclosure status are shown in the eTable. Dermatologists in the discordant with overdisclosure group had the highest median number and amount of OP payments, followed by those in the concordant disclosure and discordant without overdisclosure groups. Additionally, discordant with overdisclosure dermatologists also had the highest median and mean number of unique industry interactions not on OP, followed by those in the overdisclosure only and no disclosure groups. Academic and private practice settings did not impact dermatologists’ disclosure status. The percentage of female and male dermatologists in the discordant group was 25% and 36%, respectively.

Dermatologists reported a total of 1756 unique industry relationships in the AADMP that were not found on OP. Of these, 1440 (82%) relationships were from 236 dermatologists who had industry payments on OP. The remaining 316 relationships were from 108 dermatologists who had no payments on OP. Although 114 companies reported payments to dermatologists on OP, dermatologists in the AADMP reported interactions with an additional 430 companies.
Comment
In this study, we demonstrated discordance between dermatologist self-reported financial interactions in the AADMP compared with those reported by industry via OP. After excluding f/b/t/l entries, approximately two-thirds of the total amount and number of payments in OP were disclosed, while 31% of dermatologists had discordant disclosure status.
Prior investigations in other medical fields showed high discrepancy rates between industry-reported and physician-reported relationships ranging from 23% to 62%, with studies utilizing various methodologies.6-9,11,12,14,15 Only a few studies have utilized the OP database.8,12,15 Thompson et al12 compared OP payment data with physician financial disclosure at an annual gynecology scientific meeting and found although 209 of 335 (62%) physicians had interactions listed in the OP database, only 24 (7%) listed at least 1 company in the meeting financial disclosure section. Of these 24 physicians, only 5 (21%) accurately disclosed financial relationships with all of the companies listed in OP. The investigators found 129 (38.5%) physicians and 33.7% of the $1.99 million OP payments had concordant disclosure status. When they excluded physicians who received less than $100, 53% of individuals had concordant disclosure.12 Hannon et al8 reported on inconsistencies between disclosures in the OP database and the American Academy of Orthopedic Surgeons Annual Meeting and found 259 (23%) of 1113 physicians meeting inclusion criteria had financial interactions listed in the OP database that were not reported in the meeting disclosures. Yee et al15 also utilized the OP database and compared it with author disclosures in 3 major ophthalmology journals.Of 670 authors, 367 (54.8%) had complete concordance, with 68 (10.1%) more reporting additional overdisclosures, leading to a discordant with underdisclosure rate of 35.1%. Additionally, $1.46 million (44.6%) of the $3.27 million OP payments had concordant disclosure status.15 Other studies compared individual companies’ online reports of physician payments with physician self-disclosures in annual meeting programs, clinical guidelines, and peer-reviewed publications.6,7,9,11,14
Our study demonstrated variation in disclosure status. Compared with other groups, dermatologists in the discordant with overdisclosure group on average had more interactions with and received higher payments from industry, which is consistent with studies in the orthopedic surgery literature.8,9 Male dermatologists had 11% more discordant disclosures than their female counterparts, which may be influenced by men having more industry interactions than women.3 Although small industry payments possessed the lowest concordant rate in our study, which has been observed,12 payments greater than $100,000 had the second-lowest concordance rate at 58%, which may be skewed by the small sample size. Rates of concordant disclosure differed among types of interactions, such as between research and associated research payments. This particular difference may be attributed to the incorrect listing of dermatologists as principal investigators or reduced awareness of payments, as associated research payments were made to the institution and not the individual.
Reasons for discrepancies between industry-reported and dermatologist-reported disclosures may include reporting time differences, lack of physician awareness of OP, industry reporting inaccuracies, dearth of contextual information associated with individual payment entries, and misunderstandings. Prior research demonstrated that the most common reasons for physician nondisclosure included misunderstanding disclosure requirements, unintentional omission of payment, and a lack of relationship between the industry payment and presentation topic.9,12 These factors likely contributed to the disclosure inconsistencies in our study. Similarly high rates of inconsistencies across different specialties suggest systemic concerns.
We found a substantial number of dermatologist-industry interactions listed in the AADMP that were not captured by OP, with 108 dermatologists (35%) having overdisclosures even when excluding f/b/t/l entries. The number of companies in these overdisclosures approximated 4 times the number of companies on OP. Other studies have also observed physician-industry interactions not displayed on OP.8,12,15 Because the Sunshine Act requires reporting only by certain companies, interactions surrounding products such as over-the-counter merchandise, cosmetics, lasers, novel devices, and new medications are generally not included. Further, OP may not capture nonmonetary industry relationships.
There were several limitations to this study. The most notable limitation was the differences in the categorizations of industry relationships by OP and the AADMP. These differences can overemphasize some types of interactions at the expense of other types, such as f/b/t/l. As such, analyses were repeated after excluding f/b/t/l. Another limitation was the inexact overlap of time frames for OP and the AADMP, which may have led to discrepancies. However, we used the best available data and expect the vast majority of interactions to have occurred by the AAD disclosure deadline. It is possible that the presenters may have had a more updated conflict-of-interest disclosure slide at the time of the meeting presentation. The most important limitation was that we were unable to determine whether discrepancies resulted from underreporting by dermatologists or inaccurate reporting by industry. It was unlikely that OP or the AADMP alone completely represented all dermatologist-industry financial relationships.
Conclusion
With a growing emphasis on physician-industry transparency, we identified rates of differences in dermatology consistent with those in other medical fields when comparing the publicly available OP database with disclosures at national conferences. Although the differences in the categorization and requirements for disclosure between the OP database and the AADMP may account for some of the discordance, dermatologists should be aware of potentially negative public perceptions regarding the transparency and prevalence of physician-industry interactions.
Acknowledgment
The first two authors contributed equally to this research/article
- Campbell EG, Gruen RL, Mountford J, et al. A national survey of physician-industry relationships. N Engl J Med. 2007;356:1742-1750.
- Marshall DC, Jackson ME, Hattangadi-Gluth JA. Disclosure of industry payments to physicians: an epidemiologic analysis of early data from the open payments program. Mayo Clin Proc. 2016;91:84-96.
- Feng H, Wu P, Leger M. Exploring the industry-dermatologist financial relationship: insight from the open payment data. JAMA Dermatol. 2016;152:1307-1313.
- Kirschner NM, Sulmasy LS, Kesselheim AS. Health policy basics: the physician payment Sunshine Act and the open payments program. Ann Intern Med. 2014;161:519-521.
- Search Open Payment. Centers for Medicare & Medicaid Services. https://openpaymentsdata.cms.gov. Accessed October 21, 2019.
- Buerba RA, Fu MC, Grauer JN. Discrepancies in spine surgeon conflict of interest disclosures between a national meeting and physician payment listings on device manufacturer web sites. Spine J. 2013;13:1780-1788.
- Chimonas S, Frosch Z, Rothman DJ. From disclosure to transparency: the use of company payment data. Arch Intern Med. 2011;171:81-86.
- Hannon CP, Chalmers PN, Carpiniello MF, et al. Inconsistencies between physician-reported disclosures at the AAOS annual meeting and industry-reported financial disclosures in the open payments database. J Bone Joint Surg. 2016;98:E90.
- Okike K, Kocher MS, Wei EX, et al. Accuracy of conflict-of-interest disclosures reported by physicians. N Engl J Med. 2009;361:1466-1474.
- Ramm O, Brubaker L. Conflicts-of-interest disclosures at the 2010 AUGS Scientific Meeting. Female Pelvic Med Reconstr Surg. 2012;18:79-81.
- Tanzer D, Smith K, Tanzer M. American Academy of Orthopaedic Surgeons disclosure policy fails to accurately inform its members of potential conflicts of interest. Am J Orthop (Belle Mead NJ). 2015;44:E207-E210.
- Thompson JC, Volpe KA, Bridgewater LK, et al. Sunshine Act: shedding light on inaccurate disclosures at a gynecologic annual meeting. Am J Obstet Gynecol. 2016;215:661.
- Disclosure of Potential Conflicts of Interest. American Academy of Dermatology and AAD Association Web site. https://aad.org/Forms/Policies/Uploads/AR/
AR%20Disclosure%20of%20Potential%20Conflicts%
20of%20Interest-2.pdf. Accessed October 21, 2019. - Hockenberry JM, Weigel P, Auerbach A, et al. Financial payments by orthopedic device makers to orthopedic surgeons. Arch Intern Med. 2011;171:1759-1765.
- Yee C, Greenberg PB, Margo CE, et al. Financial disclosures in academic publications and the Sunshine Act: a concordance dtudy. Br J Med Med Res. 2015;10:1-6.
- Campbell EG, Gruen RL, Mountford J, et al. A national survey of physician-industry relationships. N Engl J Med. 2007;356:1742-1750.
- Marshall DC, Jackson ME, Hattangadi-Gluth JA. Disclosure of industry payments to physicians: an epidemiologic analysis of early data from the open payments program. Mayo Clin Proc. 2016;91:84-96.
- Feng H, Wu P, Leger M. Exploring the industry-dermatologist financial relationship: insight from the open payment data. JAMA Dermatol. 2016;152:1307-1313.
- Kirschner NM, Sulmasy LS, Kesselheim AS. Health policy basics: the physician payment Sunshine Act and the open payments program. Ann Intern Med. 2014;161:519-521.
- Search Open Payment. Centers for Medicare & Medicaid Services. https://openpaymentsdata.cms.gov. Accessed October 21, 2019.
- Buerba RA, Fu MC, Grauer JN. Discrepancies in spine surgeon conflict of interest disclosures between a national meeting and physician payment listings on device manufacturer web sites. Spine J. 2013;13:1780-1788.
- Chimonas S, Frosch Z, Rothman DJ. From disclosure to transparency: the use of company payment data. Arch Intern Med. 2011;171:81-86.
- Hannon CP, Chalmers PN, Carpiniello MF, et al. Inconsistencies between physician-reported disclosures at the AAOS annual meeting and industry-reported financial disclosures in the open payments database. J Bone Joint Surg. 2016;98:E90.
- Okike K, Kocher MS, Wei EX, et al. Accuracy of conflict-of-interest disclosures reported by physicians. N Engl J Med. 2009;361:1466-1474.
- Ramm O, Brubaker L. Conflicts-of-interest disclosures at the 2010 AUGS Scientific Meeting. Female Pelvic Med Reconstr Surg. 2012;18:79-81.
- Tanzer D, Smith K, Tanzer M. American Academy of Orthopaedic Surgeons disclosure policy fails to accurately inform its members of potential conflicts of interest. Am J Orthop (Belle Mead NJ). 2015;44:E207-E210.
- Thompson JC, Volpe KA, Bridgewater LK, et al. Sunshine Act: shedding light on inaccurate disclosures at a gynecologic annual meeting. Am J Obstet Gynecol. 2016;215:661.
- Disclosure of Potential Conflicts of Interest. American Academy of Dermatology and AAD Association Web site. https://aad.org/Forms/Policies/Uploads/AR/
AR%20Disclosure%20of%20Potential%20Conflicts%
20of%20Interest-2.pdf. Accessed October 21, 2019. - Hockenberry JM, Weigel P, Auerbach A, et al. Financial payments by orthopedic device makers to orthopedic surgeons. Arch Intern Med. 2011;171:1759-1765.
- Yee C, Greenberg PB, Margo CE, et al. Financial disclosures in academic publications and the Sunshine Act: a concordance dtudy. Br J Med Med Res. 2015;10:1-6.
Practice Points
- There is heightening public attention to conflicts of interest since the start of the government-mandated reporting of physician-industry interactions.
- When compared with an industry-reported physician-interaction database, approximately two-thirds of dermatologists who presented at a national dermatology conference self-disclosed all interactions.
- This rate of discordance is consistent with other specialties, but it may reflect differences in the database reporting methods.














