User login
Pandemic poses new challenges for rural doctors
These include struggling with seeing patients virtually and treating patients who have politicized the virus. Additionally, the pandemic has exposed rural practices to greater financial difficulties.
Before the pandemic some rurally based primary care physicians were already working through big challenges, such as having few local medical colleagues to consult and working in small practices with lean budgets. In fact, data gathered by the National Rural Health Association showed that there are only 40 primary care physicians per 100,000 patients in rural regions, compared with 53 in urban areas – and the number of physicians overall is 13 per 10,000 in rural areas, compared with 31 in cities.
In the prepandemic world, for some doctors, the challenges were balanced by the benefits of practicing in these sparsely populated communities with scenic, low-traffic roads. Some perks of practicing in rural areas touted by doctors included having a fast commute, being able to swim in a lake near the office before work, having a low cost of living, and feeling like they are making a difference in their communities as they treat generations of the families they see around town.
But today, new hurdles to practicing medicine in rural America created by the COVID-19 pandemic have caused the hardships to feel heavier than the joys at times for some physicians interviewed by MDedge.
Many independent rural practices in need of assistance were not able to get much from the federal Provider Relief Funds, said John M. Westfall, MD, who is director of the Robert Graham Center for Policy Studies in Family Medicine and Primary Care, in an interview.
“Rural primary care doctors function independently or in smaller critical access hospitals and community health centers,” said Dr. Westfall, who previously practiced family medicine in a small town in Colorado. “Many of these have much less financial reserves so are at risk of cutbacks and closure.”
Jacqueline W. Fincher, MD, an internist based in a tiny Georgia community along the highway between Atlanta and Augusta, said her small practice works on really thin margins and doesn’t have much cushion. At the beginning of the pandemic, all visits were down, and her practice operated at a loss. To help, Dr. Fincher and her colleagues applied for funding from the Small Business Administration’s Paycheck Protection Program (PPP) through the CARES Act.
“COVID-19 has had a tremendous impact especially on primary care practices. We live and die by volume. … Our volume in mid-March to mid-May really dropped dramatically,” explained Dr. Fincher, who is also president of the American College of Physicians. “The PPP sustained us for 2 months, enabling us to pay our staff and to remain open and get us up and running on telehealth.”
Starting up telemedicine
Experiencing spotty or no access to broadband Internet is nothing new to rural physicians, but having this problem interfere with their ability to provide care to patients is.
As much of the American health system rapidly embraced telehealth during the pandemic, obtaining access to high-speed Internet has been a major challenge for rural patients, noted Dr. Westfall.
“Some practices were able to quickly adopt some telehealth capacity with phone and video. Changes in payment for telehealth helped. But in some rural communities there was not adequate Internet bandwidth for quality video connections. And some patients did not have the means for high-speed video connections,” Dr. Westfall said.
Indeed, according to a 2019 Pew Research Center survey, 63% of rural Americans say they can access the Internet through a broadband connection at home, compared with 75% and 79% in suburban and urban areas, respectively.
In the Appalachian town of Zanesville, Ohio, for example, family physician Shelly L. Dunmyer, MD, and her colleagues discovered that many patients don’t have Internet access at home. Dr. Fincher has to go to the office to conduct telehealth visits because her own Internet access at home is unpredictable. As for patients, it may take 15 minutes for them to work out technical glitches and find good Internet reception, said Dr. Fincher. For internist Y. Ki Shin, MD, who practices in the coastal town of Montesano in Washington state, about 25% of his practice’s telehealth visits must be conducted by phone because of limitations on video, such as lack of high-speed access.
But telephone visits are often insufficient replacements for appointments via video, according to several rural physicians interviewed for this piece.
“Telehealth can be frustrating at times due to connectivity issues which can be difficult at times in the rural areas,” said Dr. Fincher. “In order for telehealth to be reasonably helpful to patients and physicians to care for people with chronic problems, the patients must have things like blood pressure monitors, glucometers, and scales to address problems like hypertension, diabetes myelitis, and congestive heart failure.”
“If you have the audio and video and the data from these devices, you’re good. If you don’t have these data, and/or don’t have the video you just can’t provide good care,” she explained.
Dr. Dunmyer and her colleagues at Medical Home Primary Care Center in Zanesville, Ohio, found a way to get around the problem of patients not being able to access Internet to participate in video visits from their homes. This involved having her patients drive into her practice’s parking lot to participate in modified telehealth visits. Staffers gave iPads to patients in their cars, and Dr. Dunmyer conducted visits from her office, about 50 yards away.
“We were even doing Medicare wellness visits: Instead of asking them to get up and move around the room, we would sit at the window and wave at them, ask them to get out, walk around the car. We were able to check mobility and all kinds of things that we’d normally do in the office,” Dr. Dunmyer explained in an interview.
The family physician noted that her practice is now conducting fewer parking lot visits since her office is allowing in-person appointments, but that they’re still an option for her patients.
Treating political adversaries
Some rural physicians have experienced strained relationships with patients for reasons other than technology – stark differences in opinion over the pandemic itself. Certain patients are following President Trump’s lead and questioning everything from the pandemic death toll to preventive measures recommended by scientists and medical experts, physicians interviewed by MDedge said.
Patients everywhere share these viewpoints, of course, but research and election results confirm that rural areas are more receptive to conservative viewpoints. In 2018, a Pew Research Center survey reported that rural and urban areas are “becoming more polarized politically,” and “rural areas tend to have a higher concentration of Republicans and Republican-leaning independents.” For example, 40% of rural respondents reported “very warm” or “somewhat warm” feelings toward Donald Trump, compared with just 19% in urban areas.
Dr. Shin has struggled to cope with patients who want to argue about pandemic safety precautions like wearing masks and seem to question whether systemic racism exists.
“We are seeing a lot more people who feel that this pandemic is not real, that it’s a political and not-true infection,” he said in an interview. “We’ve had patients who were angry at us because we made them wear masks, and some were demanding hydroxychloroquine and wanted to have an argument because we’re not going to prescribe it for them.”
In one situation, which he found especially disturbing, Dr. Shin had to leave the exam room because a patient wouldn’t stop challenging him regarding the pandemic. Things have gotten so bad that Dr. Shin has even questioned whether he wants to continue his long career in his small town because of local political attitudes such as opposition to mask-wearing and social distancing.
“Mr. Trump’s misinformation on this pandemic made my job much more difficult. As a minority, I feel less safe in my community than ever,” said Dr. Shin, who described himself as Asian American.
Despite these new stressors, Dr. Shin has experienced some joyful moments while practicing medicine in the pandemic.
He said a recent home visit to a patient who had been hospitalized for over 3 months and nearly died helped him put political disputes with his patients into perspective.
“He was discharged home but is bedbound. He had gangrene on his toes, and I could not fully examine him using video,” Dr. Shin recalled. “It was tricky to find the house, but a very large Trump sign was very helpful in locating it. It was a good visit: He was happy to see me, and I was happy to see that he was doing okay at home.”
“I need to remind myself that supporting Mr. Trump does not always mean that my patient supports Mr. Trump’s view on the pandemic and the race issues in our country,” Dr. Shin added.
The Washington-based internist said he also tells himself that, even if his patients refuse to follow his strong advice regarding pandemic precautions, it does not mean he has failed as a doctor.
“I need to continue to educate patients about the dangers of COVID infection but cannot be angry if they don’t choose to follow my recommendations,” he noted.
Dr. Fincher says her close connection with patients has allowed her to smooth over politically charged claims about the pandemic in the town of Thomson, Georgia, with a population 6,800.
“I have a sense that, even though we may differ in our understanding of some basic facts, they appreciate what I say since we have a long-term relationship built on trust,” she said. This kind of trust, Dr. Fincher suggested, may be more common than in urban areas where there’s a larger supply of physicians, and patients don’t see the same doctors for long periods of time.
“It’s more meaningful when it comes from me, rather than doctors who are [new to patients] every year when their employer changes their insurance,” she noted.
These include struggling with seeing patients virtually and treating patients who have politicized the virus. Additionally, the pandemic has exposed rural practices to greater financial difficulties.
Before the pandemic some rurally based primary care physicians were already working through big challenges, such as having few local medical colleagues to consult and working in small practices with lean budgets. In fact, data gathered by the National Rural Health Association showed that there are only 40 primary care physicians per 100,000 patients in rural regions, compared with 53 in urban areas – and the number of physicians overall is 13 per 10,000 in rural areas, compared with 31 in cities.
In the prepandemic world, for some doctors, the challenges were balanced by the benefits of practicing in these sparsely populated communities with scenic, low-traffic roads. Some perks of practicing in rural areas touted by doctors included having a fast commute, being able to swim in a lake near the office before work, having a low cost of living, and feeling like they are making a difference in their communities as they treat generations of the families they see around town.
But today, new hurdles to practicing medicine in rural America created by the COVID-19 pandemic have caused the hardships to feel heavier than the joys at times for some physicians interviewed by MDedge.
Many independent rural practices in need of assistance were not able to get much from the federal Provider Relief Funds, said John M. Westfall, MD, who is director of the Robert Graham Center for Policy Studies in Family Medicine and Primary Care, in an interview.
“Rural primary care doctors function independently or in smaller critical access hospitals and community health centers,” said Dr. Westfall, who previously practiced family medicine in a small town in Colorado. “Many of these have much less financial reserves so are at risk of cutbacks and closure.”
Jacqueline W. Fincher, MD, an internist based in a tiny Georgia community along the highway between Atlanta and Augusta, said her small practice works on really thin margins and doesn’t have much cushion. At the beginning of the pandemic, all visits were down, and her practice operated at a loss. To help, Dr. Fincher and her colleagues applied for funding from the Small Business Administration’s Paycheck Protection Program (PPP) through the CARES Act.
“COVID-19 has had a tremendous impact especially on primary care practices. We live and die by volume. … Our volume in mid-March to mid-May really dropped dramatically,” explained Dr. Fincher, who is also president of the American College of Physicians. “The PPP sustained us for 2 months, enabling us to pay our staff and to remain open and get us up and running on telehealth.”
Starting up telemedicine
Experiencing spotty or no access to broadband Internet is nothing new to rural physicians, but having this problem interfere with their ability to provide care to patients is.
As much of the American health system rapidly embraced telehealth during the pandemic, obtaining access to high-speed Internet has been a major challenge for rural patients, noted Dr. Westfall.
“Some practices were able to quickly adopt some telehealth capacity with phone and video. Changes in payment for telehealth helped. But in some rural communities there was not adequate Internet bandwidth for quality video connections. And some patients did not have the means for high-speed video connections,” Dr. Westfall said.
Indeed, according to a 2019 Pew Research Center survey, 63% of rural Americans say they can access the Internet through a broadband connection at home, compared with 75% and 79% in suburban and urban areas, respectively.
In the Appalachian town of Zanesville, Ohio, for example, family physician Shelly L. Dunmyer, MD, and her colleagues discovered that many patients don’t have Internet access at home. Dr. Fincher has to go to the office to conduct telehealth visits because her own Internet access at home is unpredictable. As for patients, it may take 15 minutes for them to work out technical glitches and find good Internet reception, said Dr. Fincher. For internist Y. Ki Shin, MD, who practices in the coastal town of Montesano in Washington state, about 25% of his practice’s telehealth visits must be conducted by phone because of limitations on video, such as lack of high-speed access.
But telephone visits are often insufficient replacements for appointments via video, according to several rural physicians interviewed for this piece.
“Telehealth can be frustrating at times due to connectivity issues which can be difficult at times in the rural areas,” said Dr. Fincher. “In order for telehealth to be reasonably helpful to patients and physicians to care for people with chronic problems, the patients must have things like blood pressure monitors, glucometers, and scales to address problems like hypertension, diabetes myelitis, and congestive heart failure.”
“If you have the audio and video and the data from these devices, you’re good. If you don’t have these data, and/or don’t have the video you just can’t provide good care,” she explained.
Dr. Dunmyer and her colleagues at Medical Home Primary Care Center in Zanesville, Ohio, found a way to get around the problem of patients not being able to access Internet to participate in video visits from their homes. This involved having her patients drive into her practice’s parking lot to participate in modified telehealth visits. Staffers gave iPads to patients in their cars, and Dr. Dunmyer conducted visits from her office, about 50 yards away.
“We were even doing Medicare wellness visits: Instead of asking them to get up and move around the room, we would sit at the window and wave at them, ask them to get out, walk around the car. We were able to check mobility and all kinds of things that we’d normally do in the office,” Dr. Dunmyer explained in an interview.
The family physician noted that her practice is now conducting fewer parking lot visits since her office is allowing in-person appointments, but that they’re still an option for her patients.
Treating political adversaries
Some rural physicians have experienced strained relationships with patients for reasons other than technology – stark differences in opinion over the pandemic itself. Certain patients are following President Trump’s lead and questioning everything from the pandemic death toll to preventive measures recommended by scientists and medical experts, physicians interviewed by MDedge said.
Patients everywhere share these viewpoints, of course, but research and election results confirm that rural areas are more receptive to conservative viewpoints. In 2018, a Pew Research Center survey reported that rural and urban areas are “becoming more polarized politically,” and “rural areas tend to have a higher concentration of Republicans and Republican-leaning independents.” For example, 40% of rural respondents reported “very warm” or “somewhat warm” feelings toward Donald Trump, compared with just 19% in urban areas.
Dr. Shin has struggled to cope with patients who want to argue about pandemic safety precautions like wearing masks and seem to question whether systemic racism exists.
“We are seeing a lot more people who feel that this pandemic is not real, that it’s a political and not-true infection,” he said in an interview. “We’ve had patients who were angry at us because we made them wear masks, and some were demanding hydroxychloroquine and wanted to have an argument because we’re not going to prescribe it for them.”
In one situation, which he found especially disturbing, Dr. Shin had to leave the exam room because a patient wouldn’t stop challenging him regarding the pandemic. Things have gotten so bad that Dr. Shin has even questioned whether he wants to continue his long career in his small town because of local political attitudes such as opposition to mask-wearing and social distancing.
“Mr. Trump’s misinformation on this pandemic made my job much more difficult. As a minority, I feel less safe in my community than ever,” said Dr. Shin, who described himself as Asian American.
Despite these new stressors, Dr. Shin has experienced some joyful moments while practicing medicine in the pandemic.
He said a recent home visit to a patient who had been hospitalized for over 3 months and nearly died helped him put political disputes with his patients into perspective.
“He was discharged home but is bedbound. He had gangrene on his toes, and I could not fully examine him using video,” Dr. Shin recalled. “It was tricky to find the house, but a very large Trump sign was very helpful in locating it. It was a good visit: He was happy to see me, and I was happy to see that he was doing okay at home.”
“I need to remind myself that supporting Mr. Trump does not always mean that my patient supports Mr. Trump’s view on the pandemic and the race issues in our country,” Dr. Shin added.
The Washington-based internist said he also tells himself that, even if his patients refuse to follow his strong advice regarding pandemic precautions, it does not mean he has failed as a doctor.
“I need to continue to educate patients about the dangers of COVID infection but cannot be angry if they don’t choose to follow my recommendations,” he noted.
Dr. Fincher says her close connection with patients has allowed her to smooth over politically charged claims about the pandemic in the town of Thomson, Georgia, with a population 6,800.
“I have a sense that, even though we may differ in our understanding of some basic facts, they appreciate what I say since we have a long-term relationship built on trust,” she said. This kind of trust, Dr. Fincher suggested, may be more common than in urban areas where there’s a larger supply of physicians, and patients don’t see the same doctors for long periods of time.
“It’s more meaningful when it comes from me, rather than doctors who are [new to patients] every year when their employer changes their insurance,” she noted.
These include struggling with seeing patients virtually and treating patients who have politicized the virus. Additionally, the pandemic has exposed rural practices to greater financial difficulties.
Before the pandemic some rurally based primary care physicians were already working through big challenges, such as having few local medical colleagues to consult and working in small practices with lean budgets. In fact, data gathered by the National Rural Health Association showed that there are only 40 primary care physicians per 100,000 patients in rural regions, compared with 53 in urban areas – and the number of physicians overall is 13 per 10,000 in rural areas, compared with 31 in cities.
In the prepandemic world, for some doctors, the challenges were balanced by the benefits of practicing in these sparsely populated communities with scenic, low-traffic roads. Some perks of practicing in rural areas touted by doctors included having a fast commute, being able to swim in a lake near the office before work, having a low cost of living, and feeling like they are making a difference in their communities as they treat generations of the families they see around town.
But today, new hurdles to practicing medicine in rural America created by the COVID-19 pandemic have caused the hardships to feel heavier than the joys at times for some physicians interviewed by MDedge.
Many independent rural practices in need of assistance were not able to get much from the federal Provider Relief Funds, said John M. Westfall, MD, who is director of the Robert Graham Center for Policy Studies in Family Medicine and Primary Care, in an interview.
“Rural primary care doctors function independently or in smaller critical access hospitals and community health centers,” said Dr. Westfall, who previously practiced family medicine in a small town in Colorado. “Many of these have much less financial reserves so are at risk of cutbacks and closure.”
Jacqueline W. Fincher, MD, an internist based in a tiny Georgia community along the highway between Atlanta and Augusta, said her small practice works on really thin margins and doesn’t have much cushion. At the beginning of the pandemic, all visits were down, and her practice operated at a loss. To help, Dr. Fincher and her colleagues applied for funding from the Small Business Administration’s Paycheck Protection Program (PPP) through the CARES Act.
“COVID-19 has had a tremendous impact especially on primary care practices. We live and die by volume. … Our volume in mid-March to mid-May really dropped dramatically,” explained Dr. Fincher, who is also president of the American College of Physicians. “The PPP sustained us for 2 months, enabling us to pay our staff and to remain open and get us up and running on telehealth.”
Starting up telemedicine
Experiencing spotty or no access to broadband Internet is nothing new to rural physicians, but having this problem interfere with their ability to provide care to patients is.
As much of the American health system rapidly embraced telehealth during the pandemic, obtaining access to high-speed Internet has been a major challenge for rural patients, noted Dr. Westfall.
“Some practices were able to quickly adopt some telehealth capacity with phone and video. Changes in payment for telehealth helped. But in some rural communities there was not adequate Internet bandwidth for quality video connections. And some patients did not have the means for high-speed video connections,” Dr. Westfall said.
Indeed, according to a 2019 Pew Research Center survey, 63% of rural Americans say they can access the Internet through a broadband connection at home, compared with 75% and 79% in suburban and urban areas, respectively.
In the Appalachian town of Zanesville, Ohio, for example, family physician Shelly L. Dunmyer, MD, and her colleagues discovered that many patients don’t have Internet access at home. Dr. Fincher has to go to the office to conduct telehealth visits because her own Internet access at home is unpredictable. As for patients, it may take 15 minutes for them to work out technical glitches and find good Internet reception, said Dr. Fincher. For internist Y. Ki Shin, MD, who practices in the coastal town of Montesano in Washington state, about 25% of his practice’s telehealth visits must be conducted by phone because of limitations on video, such as lack of high-speed access.
But telephone visits are often insufficient replacements for appointments via video, according to several rural physicians interviewed for this piece.
“Telehealth can be frustrating at times due to connectivity issues which can be difficult at times in the rural areas,” said Dr. Fincher. “In order for telehealth to be reasonably helpful to patients and physicians to care for people with chronic problems, the patients must have things like blood pressure monitors, glucometers, and scales to address problems like hypertension, diabetes myelitis, and congestive heart failure.”
“If you have the audio and video and the data from these devices, you’re good. If you don’t have these data, and/or don’t have the video you just can’t provide good care,” she explained.
Dr. Dunmyer and her colleagues at Medical Home Primary Care Center in Zanesville, Ohio, found a way to get around the problem of patients not being able to access Internet to participate in video visits from their homes. This involved having her patients drive into her practice’s parking lot to participate in modified telehealth visits. Staffers gave iPads to patients in their cars, and Dr. Dunmyer conducted visits from her office, about 50 yards away.
“We were even doing Medicare wellness visits: Instead of asking them to get up and move around the room, we would sit at the window and wave at them, ask them to get out, walk around the car. We were able to check mobility and all kinds of things that we’d normally do in the office,” Dr. Dunmyer explained in an interview.
The family physician noted that her practice is now conducting fewer parking lot visits since her office is allowing in-person appointments, but that they’re still an option for her patients.
Treating political adversaries
Some rural physicians have experienced strained relationships with patients for reasons other than technology – stark differences in opinion over the pandemic itself. Certain patients are following President Trump’s lead and questioning everything from the pandemic death toll to preventive measures recommended by scientists and medical experts, physicians interviewed by MDedge said.
Patients everywhere share these viewpoints, of course, but research and election results confirm that rural areas are more receptive to conservative viewpoints. In 2018, a Pew Research Center survey reported that rural and urban areas are “becoming more polarized politically,” and “rural areas tend to have a higher concentration of Republicans and Republican-leaning independents.” For example, 40% of rural respondents reported “very warm” or “somewhat warm” feelings toward Donald Trump, compared with just 19% in urban areas.
Dr. Shin has struggled to cope with patients who want to argue about pandemic safety precautions like wearing masks and seem to question whether systemic racism exists.
“We are seeing a lot more people who feel that this pandemic is not real, that it’s a political and not-true infection,” he said in an interview. “We’ve had patients who were angry at us because we made them wear masks, and some were demanding hydroxychloroquine and wanted to have an argument because we’re not going to prescribe it for them.”
In one situation, which he found especially disturbing, Dr. Shin had to leave the exam room because a patient wouldn’t stop challenging him regarding the pandemic. Things have gotten so bad that Dr. Shin has even questioned whether he wants to continue his long career in his small town because of local political attitudes such as opposition to mask-wearing and social distancing.
“Mr. Trump’s misinformation on this pandemic made my job much more difficult. As a minority, I feel less safe in my community than ever,” said Dr. Shin, who described himself as Asian American.
Despite these new stressors, Dr. Shin has experienced some joyful moments while practicing medicine in the pandemic.
He said a recent home visit to a patient who had been hospitalized for over 3 months and nearly died helped him put political disputes with his patients into perspective.
“He was discharged home but is bedbound. He had gangrene on his toes, and I could not fully examine him using video,” Dr. Shin recalled. “It was tricky to find the house, but a very large Trump sign was very helpful in locating it. It was a good visit: He was happy to see me, and I was happy to see that he was doing okay at home.”
“I need to remind myself that supporting Mr. Trump does not always mean that my patient supports Mr. Trump’s view on the pandemic and the race issues in our country,” Dr. Shin added.
The Washington-based internist said he also tells himself that, even if his patients refuse to follow his strong advice regarding pandemic precautions, it does not mean he has failed as a doctor.
“I need to continue to educate patients about the dangers of COVID infection but cannot be angry if they don’t choose to follow my recommendations,” he noted.
Dr. Fincher says her close connection with patients has allowed her to smooth over politically charged claims about the pandemic in the town of Thomson, Georgia, with a population 6,800.
“I have a sense that, even though we may differ in our understanding of some basic facts, they appreciate what I say since we have a long-term relationship built on trust,” she said. This kind of trust, Dr. Fincher suggested, may be more common than in urban areas where there’s a larger supply of physicians, and patients don’t see the same doctors for long periods of time.
“It’s more meaningful when it comes from me, rather than doctors who are [new to patients] every year when their employer changes their insurance,” she noted.
Revamping mentorship in medicine
Why the current system fails underrepresented physicians — and tips to improve it
Mentoring is often promoted as an organizational practice to promote diversity and inclusion. New or established group members who want to further their careers look for a mentor to guide them toward success within a system by amplifying their strengths and accomplishments and defending and promoting them when necessary. But how can mentoring work if there isn’t a mentor?
For underrepresented groups or marginalized physicians, it too often looks as if there are no mentors who understand the struggles of being a racial or ethnic minority group member or mentors who are even cognizant of those struggles. Mentoring is a practice that occurs within the overarching systems of practice groups, academic departments, hospitals, medicine, and society at large. These systems frequently carry the legacies of bias, discrimination, and exclusion. The mentoring itself that takes place within a biased system risks perpetuating institutional bias, exclusion, or a sense of unworthiness in the mentee. It is stressful for any person with a minority background or even a minority interest to feel that there’s no one to emulate in their immediate working environment. When that is the case, a natural question follows: “Do I even belong here?”
Before departments and psychiatric practices turn to old, surface-level solutions like using mentorship to appear more welcoming to underrepresented groups, leaders must explicitly evaluate their track record of mentorship within their system and determine whether mentorship has been used to protect the status quo or move the culture forward. As mentorship is inherently an imbalanced relationship, there must be department- or group-level reflection about the diversity of mentors and also their examinations of mentors’ own preconceived notions of who will make a “good” mentee.
At the most basic level, leaders can examine whether there are gaps in who is mentored and who is not. Other parts of mentoring relationships should also be examined: a) How can mentoring happen if there is a dearth of underrepresented groups in the department? b) What type of mentoring style is favored? Do departments/groups look for a natural fit between mentor and mentee or are they matched based on interests, ideals, and goals? and c) How is the worthiness for mentorship determined?
One example is the fraught process of evaluating “worthiness” among residents. Prospective mentors frequently divide trainees unofficially into a top-tier candidates, middle-tier performers who may be overlooked, and a bottom tier who are avoided when it comes to mentorship. Because this division is informal and usually based on extremely early perceptions of trainees’ aptitude and openness, the process can be subject to an individual mentor’s conscious and unconscious bias, which then plays a large role in perpetuating systemic racism. When it comes to these informal but often rigid divisions, it can be hard to fall from the top when mentees are buoyed by good will, frequent opportunities to shine, and the mentor’s reputation. Likewise,
Below are three recommendations to consider for improving mentorship within departments:
1) Consider opportunities for senior mentors and potential mentees to interact with one another outside of assigned duties so that some mentorship relationships can be formed organically.
2) Review when mentorship relationships have been ineffective or unsuccessful versus productive and useful for both participants.
3) Increase opportunities for adjunct or former faculty who remain connected to the institution to also be mentors. This approach would open up more possibilities and could increase the diversity of available mentors.
If mentorship is to be part of the armamentarium for promoting equity within academia and workplaces alike, it must be examined and changed to meet the new reality.
Dr. Posada is assistant clinical professor, department of psychiatry and behavioral sciences at George Washington University in Washington. She also serves as staff physician at George Washington Medical Faculty Associates, also in Washington. She disclosed no relevant financial relationships. Dr. Forrester is consultation-liaison psychiatry fellowship training director at the University of Maryland, Baltimore. She disclosed no relevant financial relationships.
Why the current system fails underrepresented physicians — and tips to improve it
Why the current system fails underrepresented physicians — and tips to improve it
Mentoring is often promoted as an organizational practice to promote diversity and inclusion. New or established group members who want to further their careers look for a mentor to guide them toward success within a system by amplifying their strengths and accomplishments and defending and promoting them when necessary. But how can mentoring work if there isn’t a mentor?
For underrepresented groups or marginalized physicians, it too often looks as if there are no mentors who understand the struggles of being a racial or ethnic minority group member or mentors who are even cognizant of those struggles. Mentoring is a practice that occurs within the overarching systems of practice groups, academic departments, hospitals, medicine, and society at large. These systems frequently carry the legacies of bias, discrimination, and exclusion. The mentoring itself that takes place within a biased system risks perpetuating institutional bias, exclusion, or a sense of unworthiness in the mentee. It is stressful for any person with a minority background or even a minority interest to feel that there’s no one to emulate in their immediate working environment. When that is the case, a natural question follows: “Do I even belong here?”
Before departments and psychiatric practices turn to old, surface-level solutions like using mentorship to appear more welcoming to underrepresented groups, leaders must explicitly evaluate their track record of mentorship within their system and determine whether mentorship has been used to protect the status quo or move the culture forward. As mentorship is inherently an imbalanced relationship, there must be department- or group-level reflection about the diversity of mentors and also their examinations of mentors’ own preconceived notions of who will make a “good” mentee.
At the most basic level, leaders can examine whether there are gaps in who is mentored and who is not. Other parts of mentoring relationships should also be examined: a) How can mentoring happen if there is a dearth of underrepresented groups in the department? b) What type of mentoring style is favored? Do departments/groups look for a natural fit between mentor and mentee or are they matched based on interests, ideals, and goals? and c) How is the worthiness for mentorship determined?
One example is the fraught process of evaluating “worthiness” among residents. Prospective mentors frequently divide trainees unofficially into a top-tier candidates, middle-tier performers who may be overlooked, and a bottom tier who are avoided when it comes to mentorship. Because this division is informal and usually based on extremely early perceptions of trainees’ aptitude and openness, the process can be subject to an individual mentor’s conscious and unconscious bias, which then plays a large role in perpetuating systemic racism. When it comes to these informal but often rigid divisions, it can be hard to fall from the top when mentees are buoyed by good will, frequent opportunities to shine, and the mentor’s reputation. Likewise,
Below are three recommendations to consider for improving mentorship within departments:
1) Consider opportunities for senior mentors and potential mentees to interact with one another outside of assigned duties so that some mentorship relationships can be formed organically.
2) Review when mentorship relationships have been ineffective or unsuccessful versus productive and useful for both participants.
3) Increase opportunities for adjunct or former faculty who remain connected to the institution to also be mentors. This approach would open up more possibilities and could increase the diversity of available mentors.
If mentorship is to be part of the armamentarium for promoting equity within academia and workplaces alike, it must be examined and changed to meet the new reality.
Dr. Posada is assistant clinical professor, department of psychiatry and behavioral sciences at George Washington University in Washington. She also serves as staff physician at George Washington Medical Faculty Associates, also in Washington. She disclosed no relevant financial relationships. Dr. Forrester is consultation-liaison psychiatry fellowship training director at the University of Maryland, Baltimore. She disclosed no relevant financial relationships.
Mentoring is often promoted as an organizational practice to promote diversity and inclusion. New or established group members who want to further their careers look for a mentor to guide them toward success within a system by amplifying their strengths and accomplishments and defending and promoting them when necessary. But how can mentoring work if there isn’t a mentor?
For underrepresented groups or marginalized physicians, it too often looks as if there are no mentors who understand the struggles of being a racial or ethnic minority group member or mentors who are even cognizant of those struggles. Mentoring is a practice that occurs within the overarching systems of practice groups, academic departments, hospitals, medicine, and society at large. These systems frequently carry the legacies of bias, discrimination, and exclusion. The mentoring itself that takes place within a biased system risks perpetuating institutional bias, exclusion, or a sense of unworthiness in the mentee. It is stressful for any person with a minority background or even a minority interest to feel that there’s no one to emulate in their immediate working environment. When that is the case, a natural question follows: “Do I even belong here?”
Before departments and psychiatric practices turn to old, surface-level solutions like using mentorship to appear more welcoming to underrepresented groups, leaders must explicitly evaluate their track record of mentorship within their system and determine whether mentorship has been used to protect the status quo or move the culture forward. As mentorship is inherently an imbalanced relationship, there must be department- or group-level reflection about the diversity of mentors and also their examinations of mentors’ own preconceived notions of who will make a “good” mentee.
At the most basic level, leaders can examine whether there are gaps in who is mentored and who is not. Other parts of mentoring relationships should also be examined: a) How can mentoring happen if there is a dearth of underrepresented groups in the department? b) What type of mentoring style is favored? Do departments/groups look for a natural fit between mentor and mentee or are they matched based on interests, ideals, and goals? and c) How is the worthiness for mentorship determined?
One example is the fraught process of evaluating “worthiness” among residents. Prospective mentors frequently divide trainees unofficially into a top-tier candidates, middle-tier performers who may be overlooked, and a bottom tier who are avoided when it comes to mentorship. Because this division is informal and usually based on extremely early perceptions of trainees’ aptitude and openness, the process can be subject to an individual mentor’s conscious and unconscious bias, which then plays a large role in perpetuating systemic racism. When it comes to these informal but often rigid divisions, it can be hard to fall from the top when mentees are buoyed by good will, frequent opportunities to shine, and the mentor’s reputation. Likewise,
Below are three recommendations to consider for improving mentorship within departments:
1) Consider opportunities for senior mentors and potential mentees to interact with one another outside of assigned duties so that some mentorship relationships can be formed organically.
2) Review when mentorship relationships have been ineffective or unsuccessful versus productive and useful for both participants.
3) Increase opportunities for adjunct or former faculty who remain connected to the institution to also be mentors. This approach would open up more possibilities and could increase the diversity of available mentors.
If mentorship is to be part of the armamentarium for promoting equity within academia and workplaces alike, it must be examined and changed to meet the new reality.
Dr. Posada is assistant clinical professor, department of psychiatry and behavioral sciences at George Washington University in Washington. She also serves as staff physician at George Washington Medical Faculty Associates, also in Washington. She disclosed no relevant financial relationships. Dr. Forrester is consultation-liaison psychiatry fellowship training director at the University of Maryland, Baltimore. She disclosed no relevant financial relationships.
Smart health devices – promises and pitfalls
What needs to be done before the data deluge hits the office
Hurricane Sally recently crossed the Gulf of Mexico and landed with torrential rainfalls along the Alabama coast. A little rainfall is important for crops; too much leads to devastation. As physicians, we need data in order to help manage patients’ illnesses and to help to keep them healthy. Our fear though is that too much data provided too quickly may have the opposite effect.
Personal monitoring devices
When I bought my first Fitbit 7 years ago, I was enamored with the technology. The Fitbit was little more than a step tracker, yet I proudly wore its black rubber strap on my wrist. It was my first foray into wearable technology, and it felt quite empowering to have an objective way to track my fitness beyond just using my bathroom scale. Now less than a decade later, that Fitbit looks archaic in comparison with the wrist-top technology currently available.
As I write this, the world’s largest technology company is in the process of releasing its sixth-generation Apple Watch. In addition to acting as a smartphone, this new device, which is barely larger than a postage stamp, offers GPS-based movement tracking, the ability to detect falls, continuous heart rate monitoring, a built-in EKG capable of diagnosing atrial fibrillation, and an oxygen saturation sensor. These features weren’t added thoughtlessly. Apple is marketing this as a health-focused device, with their primary advertising campaign claiming that “the future of health is on your wrist,” and they aren’t the only company making this play.
Along with Apple, Samsung, Withings, Fitbit, and other companies continue to bring products to market that monitor our activity and provide new insights into our health. Typically linked to smartphone-based apps, these devices record all of their measurements for later review, while software helps interpret the findings to make them actionable. From heart rate tracking to sleep analysis, these options now provide access to volumes of data that promise to improve our wellness and change our lives. Of course, those promises will only be fulfilled if our behavior is altered as a consequence of having more detailed information. Whether that will happen remains to be seen.
Health system–linked devices
Major advancements in medical monitoring technology are now enabling physicians to get much deeper insight into their patients’ health status. Internet-connected scales, blood pressure cuffs, and exercise equipment offer the ability to upload information into patient portals and integrate that information into EHRs. New devices provide access to information that previously was impossible to obtain. For example, wearable continuous blood glucose monitors, such as the FreeStyle Libre or DexCom’s G6, allow patients and physicians to follow blood sugar readings 24 hours a day. This provides unprecedented awareness of diabetes control and relieves the pain and inconvenience of finger sticks and blood draws. It also aids with compliance because patients don’t need to remember to check their sugar levels on a schedule.
Other compliance-boosting breakthroughs, such as Bluetooth-enabled asthma inhalers and cellular-connected continuous positive airway pressure machines, assist patients with managing chronic respiratory conditions. Many companies are developing technologies to manage acute conditions as well. One such company, an on-demand telemedicine provider called TytoCare, has developed a $299 suite of instruments that includes a digital stethoscope, thermometer, and camera-based otoscope. In concert with a virtual visit, their providers can remotely use these tools to examine and assess sick individuals. This virtual “laying on of hands” may have sounded like science fiction and likely would have been rejected by patients just a few years ago. Now it is becoming commonplace and will soon be an expectation of many seeking care.
But if we are to be successful, everyone must acknowledge that this revolution in health care brings many challenges along with it. One of those is the deluge of data that connected devices provide.
Information overload
There is such a thing as “too much of a good thing.” Described by journalist David Shenk as “data smog” in his 1997 book of the same name, the idea is clear: There is only so much information we can assimilate.
Even after years of using EHRs and with government-implemented incentives that promote “meaningful use,” physicians are still struggling with EHRs. Additionally, many have expressed frustration with the connectedness that EHRs provide and lament their inability to ever really “leave the office.” As more and more data become available to physicians, the challenge of how to assimilate and act on those data will continue to grow. The addition of patient-provided health statistics will only make information overload worse, with clinicians will feeling an ever-growing burden to know, understand, and act on this information.
Unless we develop systems to sort, filter, and prioritize the flow of information, there is potential for liability from not acting on the amount of virtual information doctors receive. This new risk for already fatigued and overburdened physicians combined with an increase in the amount of virtual information at doctors’ fingertips may lead to the value of patient data being lost.
Dr. Notte is a family physician and chief medical officer of Abington (Pa.) Hospital–Jefferson Health. Follow him on Twitter (@doctornotte). Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington Hospital–Jefferson Health. They have no conflicts related to the content of this piece.
What needs to be done before the data deluge hits the office
What needs to be done before the data deluge hits the office
Hurricane Sally recently crossed the Gulf of Mexico and landed with torrential rainfalls along the Alabama coast. A little rainfall is important for crops; too much leads to devastation. As physicians, we need data in order to help manage patients’ illnesses and to help to keep them healthy. Our fear though is that too much data provided too quickly may have the opposite effect.
Personal monitoring devices
When I bought my first Fitbit 7 years ago, I was enamored with the technology. The Fitbit was little more than a step tracker, yet I proudly wore its black rubber strap on my wrist. It was my first foray into wearable technology, and it felt quite empowering to have an objective way to track my fitness beyond just using my bathroom scale. Now less than a decade later, that Fitbit looks archaic in comparison with the wrist-top technology currently available.
As I write this, the world’s largest technology company is in the process of releasing its sixth-generation Apple Watch. In addition to acting as a smartphone, this new device, which is barely larger than a postage stamp, offers GPS-based movement tracking, the ability to detect falls, continuous heart rate monitoring, a built-in EKG capable of diagnosing atrial fibrillation, and an oxygen saturation sensor. These features weren’t added thoughtlessly. Apple is marketing this as a health-focused device, with their primary advertising campaign claiming that “the future of health is on your wrist,” and they aren’t the only company making this play.
Along with Apple, Samsung, Withings, Fitbit, and other companies continue to bring products to market that monitor our activity and provide new insights into our health. Typically linked to smartphone-based apps, these devices record all of their measurements for later review, while software helps interpret the findings to make them actionable. From heart rate tracking to sleep analysis, these options now provide access to volumes of data that promise to improve our wellness and change our lives. Of course, those promises will only be fulfilled if our behavior is altered as a consequence of having more detailed information. Whether that will happen remains to be seen.
Health system–linked devices
Major advancements in medical monitoring technology are now enabling physicians to get much deeper insight into their patients’ health status. Internet-connected scales, blood pressure cuffs, and exercise equipment offer the ability to upload information into patient portals and integrate that information into EHRs. New devices provide access to information that previously was impossible to obtain. For example, wearable continuous blood glucose monitors, such as the FreeStyle Libre or DexCom’s G6, allow patients and physicians to follow blood sugar readings 24 hours a day. This provides unprecedented awareness of diabetes control and relieves the pain and inconvenience of finger sticks and blood draws. It also aids with compliance because patients don’t need to remember to check their sugar levels on a schedule.
Other compliance-boosting breakthroughs, such as Bluetooth-enabled asthma inhalers and cellular-connected continuous positive airway pressure machines, assist patients with managing chronic respiratory conditions. Many companies are developing technologies to manage acute conditions as well. One such company, an on-demand telemedicine provider called TytoCare, has developed a $299 suite of instruments that includes a digital stethoscope, thermometer, and camera-based otoscope. In concert with a virtual visit, their providers can remotely use these tools to examine and assess sick individuals. This virtual “laying on of hands” may have sounded like science fiction and likely would have been rejected by patients just a few years ago. Now it is becoming commonplace and will soon be an expectation of many seeking care.
But if we are to be successful, everyone must acknowledge that this revolution in health care brings many challenges along with it. One of those is the deluge of data that connected devices provide.
Information overload
There is such a thing as “too much of a good thing.” Described by journalist David Shenk as “data smog” in his 1997 book of the same name, the idea is clear: There is only so much information we can assimilate.
Even after years of using EHRs and with government-implemented incentives that promote “meaningful use,” physicians are still struggling with EHRs. Additionally, many have expressed frustration with the connectedness that EHRs provide and lament their inability to ever really “leave the office.” As more and more data become available to physicians, the challenge of how to assimilate and act on those data will continue to grow. The addition of patient-provided health statistics will only make information overload worse, with clinicians will feeling an ever-growing burden to know, understand, and act on this information.
Unless we develop systems to sort, filter, and prioritize the flow of information, there is potential for liability from not acting on the amount of virtual information doctors receive. This new risk for already fatigued and overburdened physicians combined with an increase in the amount of virtual information at doctors’ fingertips may lead to the value of patient data being lost.
Dr. Notte is a family physician and chief medical officer of Abington (Pa.) Hospital–Jefferson Health. Follow him on Twitter (@doctornotte). Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington Hospital–Jefferson Health. They have no conflicts related to the content of this piece.
Hurricane Sally recently crossed the Gulf of Mexico and landed with torrential rainfalls along the Alabama coast. A little rainfall is important for crops; too much leads to devastation. As physicians, we need data in order to help manage patients’ illnesses and to help to keep them healthy. Our fear though is that too much data provided too quickly may have the opposite effect.
Personal monitoring devices
When I bought my first Fitbit 7 years ago, I was enamored with the technology. The Fitbit was little more than a step tracker, yet I proudly wore its black rubber strap on my wrist. It was my first foray into wearable technology, and it felt quite empowering to have an objective way to track my fitness beyond just using my bathroom scale. Now less than a decade later, that Fitbit looks archaic in comparison with the wrist-top technology currently available.
As I write this, the world’s largest technology company is in the process of releasing its sixth-generation Apple Watch. In addition to acting as a smartphone, this new device, which is barely larger than a postage stamp, offers GPS-based movement tracking, the ability to detect falls, continuous heart rate monitoring, a built-in EKG capable of diagnosing atrial fibrillation, and an oxygen saturation sensor. These features weren’t added thoughtlessly. Apple is marketing this as a health-focused device, with their primary advertising campaign claiming that “the future of health is on your wrist,” and they aren’t the only company making this play.
Along with Apple, Samsung, Withings, Fitbit, and other companies continue to bring products to market that monitor our activity and provide new insights into our health. Typically linked to smartphone-based apps, these devices record all of their measurements for later review, while software helps interpret the findings to make them actionable. From heart rate tracking to sleep analysis, these options now provide access to volumes of data that promise to improve our wellness and change our lives. Of course, those promises will only be fulfilled if our behavior is altered as a consequence of having more detailed information. Whether that will happen remains to be seen.
Health system–linked devices
Major advancements in medical monitoring technology are now enabling physicians to get much deeper insight into their patients’ health status. Internet-connected scales, blood pressure cuffs, and exercise equipment offer the ability to upload information into patient portals and integrate that information into EHRs. New devices provide access to information that previously was impossible to obtain. For example, wearable continuous blood glucose monitors, such as the FreeStyle Libre or DexCom’s G6, allow patients and physicians to follow blood sugar readings 24 hours a day. This provides unprecedented awareness of diabetes control and relieves the pain and inconvenience of finger sticks and blood draws. It also aids with compliance because patients don’t need to remember to check their sugar levels on a schedule.
Other compliance-boosting breakthroughs, such as Bluetooth-enabled asthma inhalers and cellular-connected continuous positive airway pressure machines, assist patients with managing chronic respiratory conditions. Many companies are developing technologies to manage acute conditions as well. One such company, an on-demand telemedicine provider called TytoCare, has developed a $299 suite of instruments that includes a digital stethoscope, thermometer, and camera-based otoscope. In concert with a virtual visit, their providers can remotely use these tools to examine and assess sick individuals. This virtual “laying on of hands” may have sounded like science fiction and likely would have been rejected by patients just a few years ago. Now it is becoming commonplace and will soon be an expectation of many seeking care.
But if we are to be successful, everyone must acknowledge that this revolution in health care brings many challenges along with it. One of those is the deluge of data that connected devices provide.
Information overload
There is such a thing as “too much of a good thing.” Described by journalist David Shenk as “data smog” in his 1997 book of the same name, the idea is clear: There is only so much information we can assimilate.
Even after years of using EHRs and with government-implemented incentives that promote “meaningful use,” physicians are still struggling with EHRs. Additionally, many have expressed frustration with the connectedness that EHRs provide and lament their inability to ever really “leave the office.” As more and more data become available to physicians, the challenge of how to assimilate and act on those data will continue to grow. The addition of patient-provided health statistics will only make information overload worse, with clinicians will feeling an ever-growing burden to know, understand, and act on this information.
Unless we develop systems to sort, filter, and prioritize the flow of information, there is potential for liability from not acting on the amount of virtual information doctors receive. This new risk for already fatigued and overburdened physicians combined with an increase in the amount of virtual information at doctors’ fingertips may lead to the value of patient data being lost.
Dr. Notte is a family physician and chief medical officer of Abington (Pa.) Hospital–Jefferson Health. Follow him on Twitter (@doctornotte). Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington Hospital–Jefferson Health. They have no conflicts related to the content of this piece.
More female specialists, but gender gap persists in pay, survey finds
More female physicians are becoming specialists, a Medscape survey finds, and five specialties have seen particularly large increases during the last 5 years.
Obstetrician/gynecologists and pediatricians had the largest female representation at 58% and those percentages were both up from 50% in 2015, according to the Medscape Female Physician Compensation Report 2020.
Rheumatology saw a dramatic jump in numbers of women from 29% in 2015 to 54% now. Dermatology increased from 32% to 49%, and family medicine rose from 35% to 43% during that time.
Specialist pay gap narrows slightly
The gender gap was the same this year in primary care — women made 25% less ($212,000 vs. $264,000).
The gap in specialists narrowed slightly. Women made 31% less this year ($286,000 vs $375,000) instead of the 33% less reported in last year’s survey, a difference of $89,000 this year.
The gender pay gap was consistent across all race and age groups and was consistent in responses about net worth. Whereas 57% of male physicians had a net worth of $1 million or more, only 40% of female physicians did. Twice as many male physicians as female physicians had a net worth of more than $5 million (10% vs. 5%).
“Many physicians expect the gender pay gap to narrow in the coming years,” John Prescott, MD, chief academic officer of the Association of American Medical Colleges, said in an interview.
“Yet, it is a challenging task, requiring an institutional commitment to transparency, cross-campus collaboration, ongoing communication, dedicated resources, and enlightened leadership,” he said.
Female physicians working in office-based, solo practices made the most overall at $290,000; women in outpatient settings made the least at $223,000.
The survey included more than 4,500 responses. The responses were collected during the early part of the year and do not reflect changes in income expected from the COVID-19 pandemic.
An analysis in Health Affairs, for instance, predicted that primary care practices would lose $67,774 in gross revenue per full-time-equivalent physician in calendar year 2020 because of the pandemic.
Most physicians did not experience a significant financial loss in 2019, but COVID-19 may, at least temporarily, change those answers in next year’s report, physicians predicted.
Women more likely than men to live above their means
More women this year (39%) said they live below their means than answered that way last year (31%). Female physicians were more likely to say they lived above their means than were their male counterparts (8% vs. 6%).
Greenwald Wealth Management in St. Louis Park, Minn., says aiming for putting away 20% of total gross salary is a good financial goal.
Women in this year’s survey spent about 7% less time seeing patients than did their male counterparts (35.9 hours a week vs. 38.8). The average for all physicians was 37.8 hours a week. Add the 15.6 average hours per week physicians spend on paperwork, and they are putting in 53-hour workweeks on average overall.
Asked what parts of their job they found most rewarding, women were more likely than were men to say “gratitude/relationships with patients” (31% vs. 25%). They were less likely than were men to answer that the most rewarding part was “being very good at what I do/finding answers/diagnoses” (22% vs. 25%) or “making good money at a job I like” (9% vs. 13%).
Most female physicians — and physicians overall — said they would choose medicine again. But two specialties saw a substantial increase in that answer.
This year, 79% of those in physical medicine and rehabilitation said they would choose medicine again (compared with 66% last year) and 84% of gastroenterologists answered that way (compared with 76% in 2019).
Psychiatrists, however, were in the group least likely to say they would choose their specialty again along with those in internal medicine, family medicine, and diabetes and endocrinology.
Female physicians in orthopedics, radiology, and dermatology were most likely to choose their specialties again (91% - 92%).
Female physicians were less likely to use physician assistants in their practices than were their male colleagues (31% vs. 38%) but more likely to use NPs (52% vs. 50%). More than a third (38%) of male and female physicians reported they use neither.
A version of this article originally appeared on Medscape.com.
More female physicians are becoming specialists, a Medscape survey finds, and five specialties have seen particularly large increases during the last 5 years.
Obstetrician/gynecologists and pediatricians had the largest female representation at 58% and those percentages were both up from 50% in 2015, according to the Medscape Female Physician Compensation Report 2020.
Rheumatology saw a dramatic jump in numbers of women from 29% in 2015 to 54% now. Dermatology increased from 32% to 49%, and family medicine rose from 35% to 43% during that time.
Specialist pay gap narrows slightly
The gender gap was the same this year in primary care — women made 25% less ($212,000 vs. $264,000).
The gap in specialists narrowed slightly. Women made 31% less this year ($286,000 vs $375,000) instead of the 33% less reported in last year’s survey, a difference of $89,000 this year.
The gender pay gap was consistent across all race and age groups and was consistent in responses about net worth. Whereas 57% of male physicians had a net worth of $1 million or more, only 40% of female physicians did. Twice as many male physicians as female physicians had a net worth of more than $5 million (10% vs. 5%).
“Many physicians expect the gender pay gap to narrow in the coming years,” John Prescott, MD, chief academic officer of the Association of American Medical Colleges, said in an interview.
“Yet, it is a challenging task, requiring an institutional commitment to transparency, cross-campus collaboration, ongoing communication, dedicated resources, and enlightened leadership,” he said.
Female physicians working in office-based, solo practices made the most overall at $290,000; women in outpatient settings made the least at $223,000.
The survey included more than 4,500 responses. The responses were collected during the early part of the year and do not reflect changes in income expected from the COVID-19 pandemic.
An analysis in Health Affairs, for instance, predicted that primary care practices would lose $67,774 in gross revenue per full-time-equivalent physician in calendar year 2020 because of the pandemic.
Most physicians did not experience a significant financial loss in 2019, but COVID-19 may, at least temporarily, change those answers in next year’s report, physicians predicted.
Women more likely than men to live above their means
More women this year (39%) said they live below their means than answered that way last year (31%). Female physicians were more likely to say they lived above their means than were their male counterparts (8% vs. 6%).
Greenwald Wealth Management in St. Louis Park, Minn., says aiming for putting away 20% of total gross salary is a good financial goal.
Women in this year’s survey spent about 7% less time seeing patients than did their male counterparts (35.9 hours a week vs. 38.8). The average for all physicians was 37.8 hours a week. Add the 15.6 average hours per week physicians spend on paperwork, and they are putting in 53-hour workweeks on average overall.
Asked what parts of their job they found most rewarding, women were more likely than were men to say “gratitude/relationships with patients” (31% vs. 25%). They were less likely than were men to answer that the most rewarding part was “being very good at what I do/finding answers/diagnoses” (22% vs. 25%) or “making good money at a job I like” (9% vs. 13%).
Most female physicians — and physicians overall — said they would choose medicine again. But two specialties saw a substantial increase in that answer.
This year, 79% of those in physical medicine and rehabilitation said they would choose medicine again (compared with 66% last year) and 84% of gastroenterologists answered that way (compared with 76% in 2019).
Psychiatrists, however, were in the group least likely to say they would choose their specialty again along with those in internal medicine, family medicine, and diabetes and endocrinology.
Female physicians in orthopedics, radiology, and dermatology were most likely to choose their specialties again (91% - 92%).
Female physicians were less likely to use physician assistants in their practices than were their male colleagues (31% vs. 38%) but more likely to use NPs (52% vs. 50%). More than a third (38%) of male and female physicians reported they use neither.
A version of this article originally appeared on Medscape.com.
More female physicians are becoming specialists, a Medscape survey finds, and five specialties have seen particularly large increases during the last 5 years.
Obstetrician/gynecologists and pediatricians had the largest female representation at 58% and those percentages were both up from 50% in 2015, according to the Medscape Female Physician Compensation Report 2020.
Rheumatology saw a dramatic jump in numbers of women from 29% in 2015 to 54% now. Dermatology increased from 32% to 49%, and family medicine rose from 35% to 43% during that time.
Specialist pay gap narrows slightly
The gender gap was the same this year in primary care — women made 25% less ($212,000 vs. $264,000).
The gap in specialists narrowed slightly. Women made 31% less this year ($286,000 vs $375,000) instead of the 33% less reported in last year’s survey, a difference of $89,000 this year.
The gender pay gap was consistent across all race and age groups and was consistent in responses about net worth. Whereas 57% of male physicians had a net worth of $1 million or more, only 40% of female physicians did. Twice as many male physicians as female physicians had a net worth of more than $5 million (10% vs. 5%).
“Many physicians expect the gender pay gap to narrow in the coming years,” John Prescott, MD, chief academic officer of the Association of American Medical Colleges, said in an interview.
“Yet, it is a challenging task, requiring an institutional commitment to transparency, cross-campus collaboration, ongoing communication, dedicated resources, and enlightened leadership,” he said.
Female physicians working in office-based, solo practices made the most overall at $290,000; women in outpatient settings made the least at $223,000.
The survey included more than 4,500 responses. The responses were collected during the early part of the year and do not reflect changes in income expected from the COVID-19 pandemic.
An analysis in Health Affairs, for instance, predicted that primary care practices would lose $67,774 in gross revenue per full-time-equivalent physician in calendar year 2020 because of the pandemic.
Most physicians did not experience a significant financial loss in 2019, but COVID-19 may, at least temporarily, change those answers in next year’s report, physicians predicted.
Women more likely than men to live above their means
More women this year (39%) said they live below their means than answered that way last year (31%). Female physicians were more likely to say they lived above their means than were their male counterparts (8% vs. 6%).
Greenwald Wealth Management in St. Louis Park, Minn., says aiming for putting away 20% of total gross salary is a good financial goal.
Women in this year’s survey spent about 7% less time seeing patients than did their male counterparts (35.9 hours a week vs. 38.8). The average for all physicians was 37.8 hours a week. Add the 15.6 average hours per week physicians spend on paperwork, and they are putting in 53-hour workweeks on average overall.
Asked what parts of their job they found most rewarding, women were more likely than were men to say “gratitude/relationships with patients” (31% vs. 25%). They were less likely than were men to answer that the most rewarding part was “being very good at what I do/finding answers/diagnoses” (22% vs. 25%) or “making good money at a job I like” (9% vs. 13%).
Most female physicians — and physicians overall — said they would choose medicine again. But two specialties saw a substantial increase in that answer.
This year, 79% of those in physical medicine and rehabilitation said they would choose medicine again (compared with 66% last year) and 84% of gastroenterologists answered that way (compared with 76% in 2019).
Psychiatrists, however, were in the group least likely to say they would choose their specialty again along with those in internal medicine, family medicine, and diabetes and endocrinology.
Female physicians in orthopedics, radiology, and dermatology were most likely to choose their specialties again (91% - 92%).
Female physicians were less likely to use physician assistants in their practices than were their male colleagues (31% vs. 38%) but more likely to use NPs (52% vs. 50%). More than a third (38%) of male and female physicians reported they use neither.
A version of this article originally appeared on Medscape.com.
COVID-19 Screening and Testing Among Patients With Neurologic Dysfunction: The Neuro-COVID-19 Time-out Process and Checklist
From the University of Mississippi Medical Center, Department of Neurology, Division of Neuroscience Intensive Care, Jackson, MS.
Abstract
Objective: To test a coronavirus disease 2019 (COVID-19) screening tool to identify patients who qualify for testing among patients with neurologic dysfunction who are unable to answer the usual screening questions, which could help to prevent unprotected exposure of patients and health care workers to COVID-19.
Methods: The Neuro-COVID-19 Time-out Process and Checklist (NCOT-PC) was implemented at our institution for 1 week as a quality improvement project to improve the pathway for COVID-19 screening and testing among patients with neurologic dysfunction.
Results: A total of 14 new patients were admitted into the neuroscience intensive care unit (NSICU) service during the pilot period. The NCOT-PC was utilized on 9 (64%) patients with neurologic dysfunction; 7 of these patients were found to have a likelihood of requiring testing based on the NCOT-PC and were subsequently screened for COVID-19 testing by contacting the institution’s COVID-19 testing hotline (Med-Com). All these patients were subsequently transitioned into person-under-investigation status based on the determination from Med-Com. The NSICU staff involved were able to utilize NCOT-PC without issues. The NCOT-PC was immediately adopted into the NSICU process.
Conclusion: Use of the NCOT-PC tool was found to be feasible and improved the screening methodology of patients with neurologic dysfunction.
Keywords: coronavirus; health care planning; quality improvement; patient safety; medical decision-making; neuroscience intensive care unit.
The coronavirus disease 2019 (COVID-19) pandemic has altered various standard emergent care pathways. Current recommendations regarding COVID-19 screening for testing involve asking patients about their symptoms, including fever, cough, chest pain, and dyspnea.1 This standard screening method poses a problem when caring for patients with neurologic dysfunction. COVID-19 patients may pre-sent with conditions that affect their ability to answer questions, such as stroke, encephalitis, neuromuscular disorders, or headache, and that may preclude the use of standard screening for testing.2 Patients with acute neurologic dysfunction who cannot undergo standard screening may leave the emergency department (ED) and transition into the neuroscience intensive care unit (NSICU) or any intensive care unit (ICU) without a reliable COVID-19 screening test.
The Protected Code Stroke pathway offers protection in the emergent setting for patients with stroke when their COVID-19 status is unknown.3 A similar process has been applied at our institution for emergent management of patients with cerebrovascular disease (stroke, intracerebral hemorrhage, and subarachnoid hemorrhage). However, the process from the ED after designating “difficult to screen” patients as persons under investigation (PUI) is unclear. The Centers for Disease Control and Prevention (CDC) has delineated the priorities for testing, with not all declared PUIs requiring testing.4 This poses a great challenge, because patients designated as PUIs require the same management as a COVID-19-positive patient, with negative-pressure isolation rooms as well as use of protective personal equipment (PPE), which may not be readily available. It was also recognized that, because the ED staff can be overwhelmed by COVID-19 patients, there may not be enough time to perform detailed screening of patients with neurologic dysfunction and that “reverse masking” may not be done consistently for nonintubated patients. This may place patients and health care workers at risk of unprotected exposure.
Recognizing these challenges, we created a Neuro-COVID-19 Time-out Process and Checklist (NCOT-PC) as a quality improvement project. The aim of this project was to improve and standardize the current process of identifying patients with neurologic dysfunction who require COVID-19 testing to decrease the risk of unprotected exposure of patients and health care workers.
Methods
Patients and Definitions
This quality improvement project was undertaken at the University of Mississippi Medical Center NSICU. Because this was a quality improvement project, an Institutional Review Board exemption was granted.
The NCOT-PC was utilized in consecutive patients with neurologic dysfunction admitted to the NSICU during a period of 1 week. “Neurologic dysfunction” encompasses any neurologic illness affecting the mental status and/or level of alertness, subsequently precluding the ability to reliably screen the patient utilizing standard COVID-19 screening. “Med-Com” at our institution is the equivalent of the national COVID-19 testing hotline, where our institution’s infectious diseases experts screen calls for testing and determine whether testing is warranted. “Unprotected exposure” means exposure to COVID-19 without adequate and appropriate PPE.
Quality Improvement Process
As more PUIs were being admitted to the institution, we used the Plan-Do-Study-Act method for process improvements in the NSICU.5 NSICU stakeholders, including attendings, the nurse manager, and nurse practitioners (NPs), developed an algorithm to facilitate the coordination of the NSICU staff in screening patients to identify those with a high likelihood of needing COVID-19 testing upon arrival in the NSICU (Figure 1). Once the NCOT-PC was finalized, NSICU stakeholders were educated regarding the use of this screening tool.
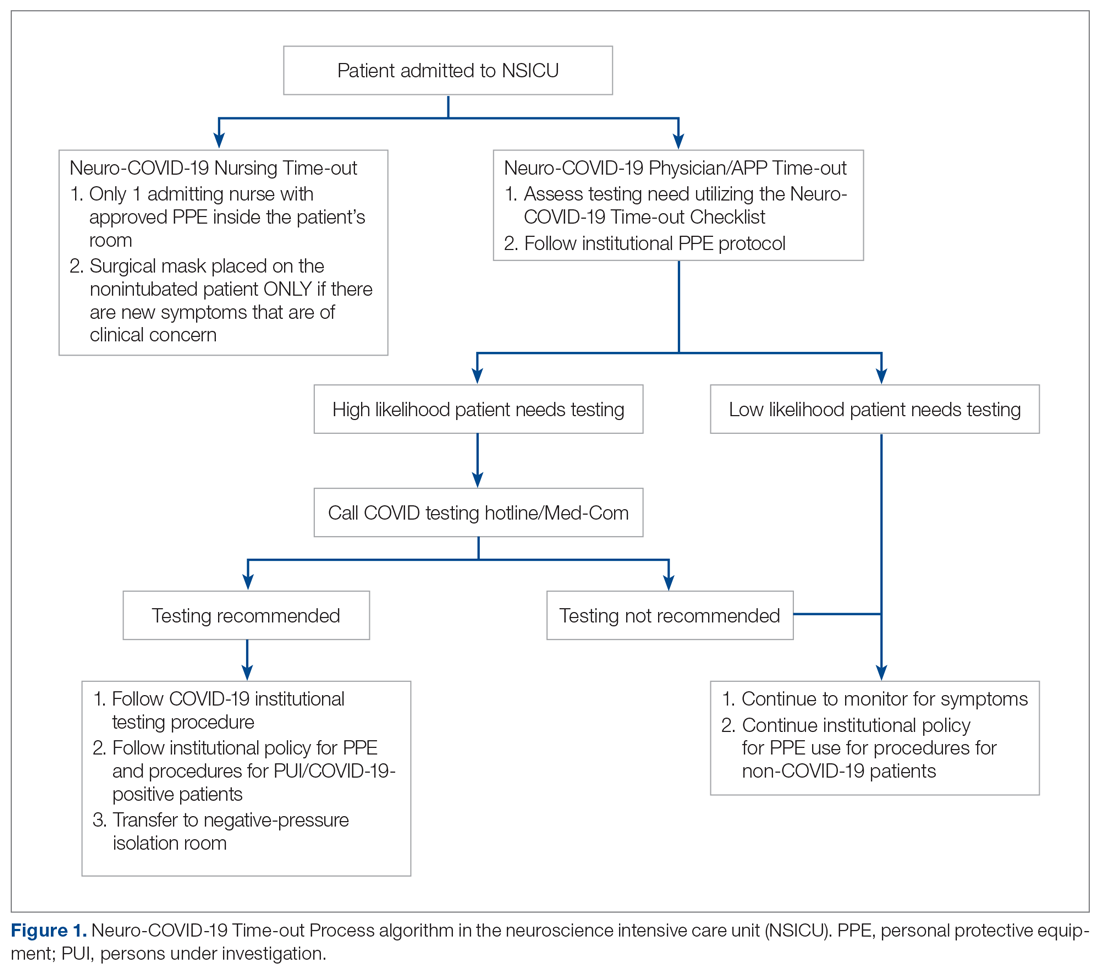
The checklist clinicians review when screening patients is shown in Figure 2. The risk factors comprising the checklist include patient history and clinical and radiographic characteristics that have been shown to be relevant for identifying patients with COVID-19.6,7 The imaging criteria utilize imaging that is part of the standard of care for NSICU patients. For example, computed tomography angiogram of the head and neck performed as part of the acute stroke protocol captures the upper part of the chest. These images are utilized for their incidental findings, such as apical ground-glass opacities and tree-in-bud formation. The risk factors applicable to the patient determine whether the clinician will call Med-Com for testing approval. Institutional COVID-19 processes were then followed accordingly.8 The decision from Med-Com was considered final, and no deviation from institutional policies was allowed.
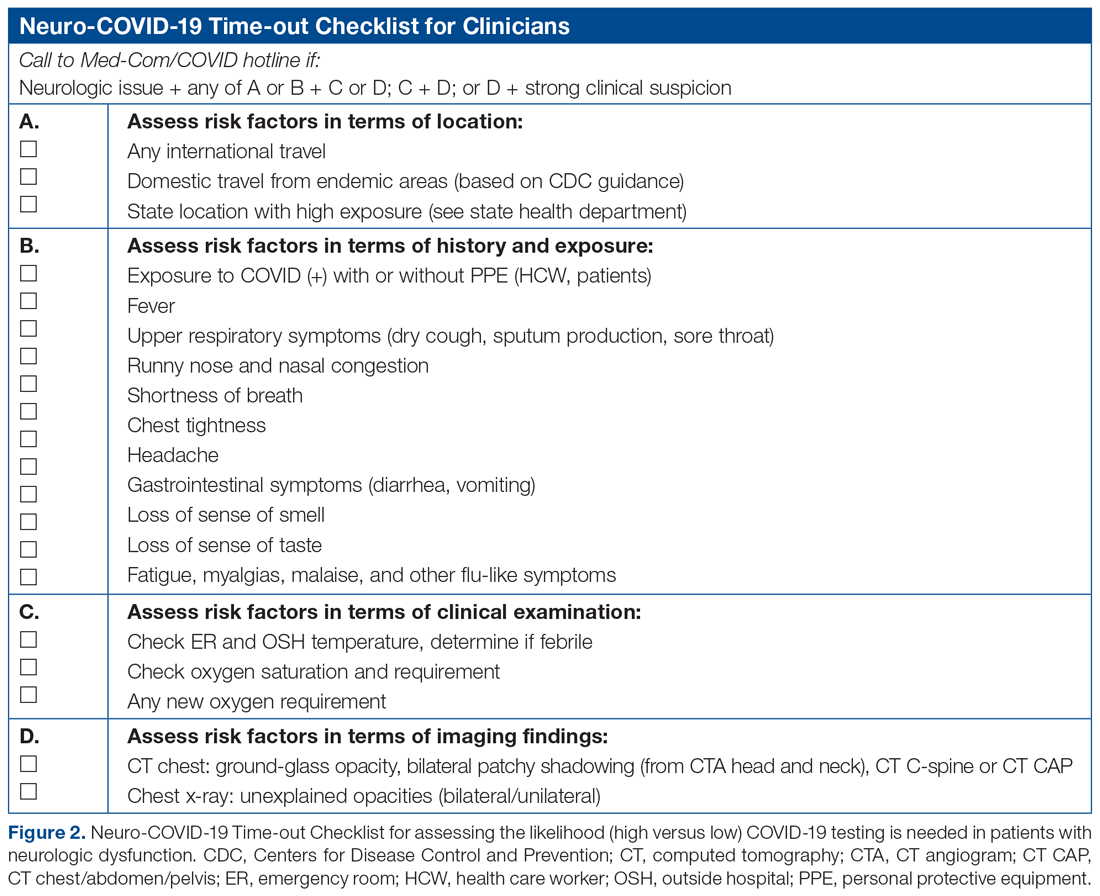
NCOT-PC was utilized for consecutive days for 1 week before re-evaluation of its feasibility and adaptability.
Data Collection and Analysis
Consecutive patients with neurologic dysfunction admitted into the NSICU were assigned nonlinkable patient numbers. No identifiers were collected for the purpose of this project. The primary diagnosis for admission, the neurologic dysfunction that precluded standard screening, and checklist components that the patient fulfilled were collected.
To assess the tool’s feasibility, feedback regarding the ease of use of the NCOT-PC was gathered from the nurses, NPs, charge nurses, fellows, and other attendings. To assess the utility of the NCOT-PC in identifying patients who will be approved for COVID-19 testing, we calculated the proportion of patients who were deemed to have a high likelihood of testing and the proportion of patients who were approved for testing. Descriptive statistics were used, as applicable for the project, to summarize the utility of the NCOT-PC.
Results
We found that the NCOT-PC can be easily used by clinicians. The NSICU staff did not communicate any implementation issues, and since the NCOT-PC was implemented, no problems have been identified.
During the pilot period of the NCOT-PC, 14 new patients were admitted to the NSICU service. Nine (64%) of these had neurologic dysfunction, and the NCOT-PC was used to determine whether Med-Com should be called based on the patients’ likelihood (high vs low) of needing a COVID-19 test. Of those patients with neurologic dysfunction, 7 (78%) were deemed to have a high likelihood of needing a COVID-19 test based on the NCOT-PC. Med-Com was contacted regarding these patients, and all were deemed to require the COVID-19 test by Med-Com and were transitioned into PUI status per institutional policy (Table).
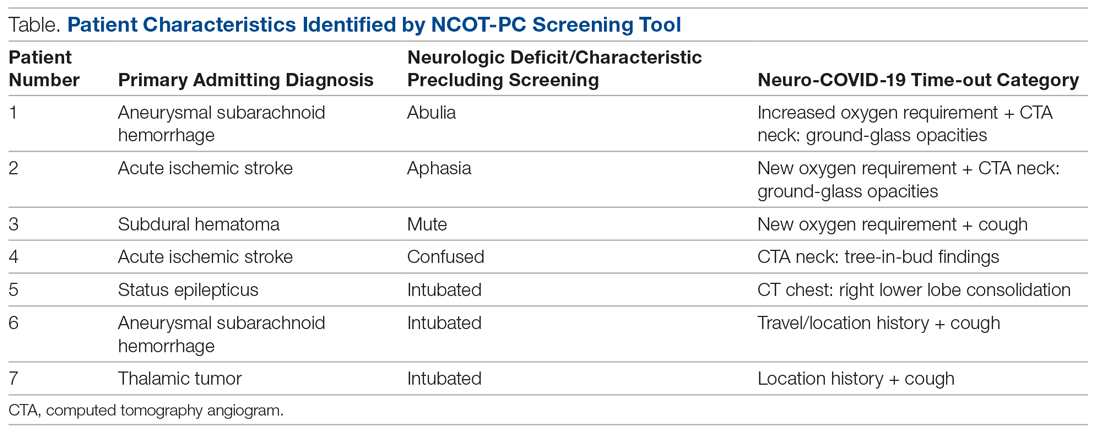
Discussion
The NCOT-PC project improved and standardized the process of identifying and screening patients with neurologic dysfunction for COVID-19 testing. The screening tool is feasible to use, and it decreased inadvertent unprotected exposure of patients and health care workers.
The NCOT-PC was easy to administer. Educating the staff regarding the new process took only a few minutes and involved a meeting with the nurse manager, NPs, fellows, residents, and attendings. We found that this process works well in tandem with the standard institutional processes in place in terms of Protected Code Stroke pathway, PUI isolation, PPE use, and Med-Com screening for COVID-19 testing. Med-Com was called only if the patient fulfilled the checklist criteria. In addition, no extra cost was attributed to implementing the NCOT-PC, since we utilized imaging that was already done as part of the standard of care for patients with neurologic dysfunction.
The standardization of the process of screening for COVID-19 testing among patients with neurologic dysfunction improved patient selection. Before the NCOT-PC, there was no consistency in terms of who should get tested and the reason for testing patients with neurologic dysfunction. Patients can pass through the ED and arrive in the NSICU with an unclear screening status, which may cause inadvertent patient and health care worker exposure to COVID-19. With the NCOT-PC, we have avoided instances of inadvertent staff or patient exposure in the NSICU.
The NCOT-PC was adopted into the NSICU process after the first week it was piloted. Beyond the NSICU, the application of the NCOT-PC can be extended to any patient presentation that precludes standard screening, such as ED and interhospital transfers for stroke codes, trauma codes, code blue, or myocardial infarction codes. In our department, as we started the process of PCS for stroke codes, we included NCOT-PC for stroke patients with neurologic dysfunction.
The results of our initiative are largely limited by the decision-making process of Med-Com when patients are called in for testing. At the time of our project, there were no specific criteria used for patients with altered mental status, except for the standard screening methods, and it was through clinician-to-clinician discussion that testing decisions were made. Another limitation is the short period of time that the NCOT-PC was applied before adoption.
In summary, the NCOT-PC tool improved the screening process for COVID-19 testing in patients with neurologic dysfunction admitted to the NSICU. It was feasible and prevented unprotected staff and patient exposure to COVID-19. The NCOT-PC functionality was compatible with institutional COVID-19 policies in place, which contributed to its overall sustainability.
The Standards for Quality Improvement Reporting Excellence (SQUIRE 2.0) were utilized in preparing this manuscript.9
Acknowledgment: The authors thank the University of Mississippi Medical Center NSICU staff for their input with implementation of the NCOT-PC.
Corresponding author: Prashant A. Natteru, MD, University of Mississippi Medical Center, Department of Neurology, 2500 North State St., Jackson, MS 39216; [email protected].
Financial disclosures: None.
1. Coronavirus disease 2019 (COVID-19) Symptoms. www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html. Accessed April 9, 2020.
2. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:1-9.
3. Khosravani H, Rajendram P, Notario L, et al. Protected code stroke: hyperacute stroke management during the coronavirus disease 2019. (COVID-19) pandemic. Stroke. 2020;51:1891-1895.
4. Coronavirus disease 2019 (COVID-19) evaluation and testing. www.cdc.gov/coronavirus/2019-nCoV/hcp/clinical-criteria.html. Accessed April 9, 2020.
5. Plan-Do-Study-Act Worksheet. Institute for Healthcare Improvement website. www.ihi.org/resources/Pages/Tools/PlanDoStudyActWorksheet.aspx. Accessed March 31,2020.
6. Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. 2020;10.1002/jmv.25728.
7. Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, et al. Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med Infect Dis. 2020;101623.
8. UMMC’s COVID-19 Clinical Processes. www.umc.edu/CoronaVirus/Mississippi-Health-Care-Professionals/Clinical-Resources/Clinical-Resources.html. Accessed April 9, 2020.
9. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): Revised Publication Guidelines from a Detailed Consensus Process. The EQUATOR Network. www.equator-network.org/reporting-guidelines/squire/. Accessed May 12, 2020.
From the University of Mississippi Medical Center, Department of Neurology, Division of Neuroscience Intensive Care, Jackson, MS.
Abstract
Objective: To test a coronavirus disease 2019 (COVID-19) screening tool to identify patients who qualify for testing among patients with neurologic dysfunction who are unable to answer the usual screening questions, which could help to prevent unprotected exposure of patients and health care workers to COVID-19.
Methods: The Neuro-COVID-19 Time-out Process and Checklist (NCOT-PC) was implemented at our institution for 1 week as a quality improvement project to improve the pathway for COVID-19 screening and testing among patients with neurologic dysfunction.
Results: A total of 14 new patients were admitted into the neuroscience intensive care unit (NSICU) service during the pilot period. The NCOT-PC was utilized on 9 (64%) patients with neurologic dysfunction; 7 of these patients were found to have a likelihood of requiring testing based on the NCOT-PC and were subsequently screened for COVID-19 testing by contacting the institution’s COVID-19 testing hotline (Med-Com). All these patients were subsequently transitioned into person-under-investigation status based on the determination from Med-Com. The NSICU staff involved were able to utilize NCOT-PC without issues. The NCOT-PC was immediately adopted into the NSICU process.
Conclusion: Use of the NCOT-PC tool was found to be feasible and improved the screening methodology of patients with neurologic dysfunction.
Keywords: coronavirus; health care planning; quality improvement; patient safety; medical decision-making; neuroscience intensive care unit.
The coronavirus disease 2019 (COVID-19) pandemic has altered various standard emergent care pathways. Current recommendations regarding COVID-19 screening for testing involve asking patients about their symptoms, including fever, cough, chest pain, and dyspnea.1 This standard screening method poses a problem when caring for patients with neurologic dysfunction. COVID-19 patients may pre-sent with conditions that affect their ability to answer questions, such as stroke, encephalitis, neuromuscular disorders, or headache, and that may preclude the use of standard screening for testing.2 Patients with acute neurologic dysfunction who cannot undergo standard screening may leave the emergency department (ED) and transition into the neuroscience intensive care unit (NSICU) or any intensive care unit (ICU) without a reliable COVID-19 screening test.
The Protected Code Stroke pathway offers protection in the emergent setting for patients with stroke when their COVID-19 status is unknown.3 A similar process has been applied at our institution for emergent management of patients with cerebrovascular disease (stroke, intracerebral hemorrhage, and subarachnoid hemorrhage). However, the process from the ED after designating “difficult to screen” patients as persons under investigation (PUI) is unclear. The Centers for Disease Control and Prevention (CDC) has delineated the priorities for testing, with not all declared PUIs requiring testing.4 This poses a great challenge, because patients designated as PUIs require the same management as a COVID-19-positive patient, with negative-pressure isolation rooms as well as use of protective personal equipment (PPE), which may not be readily available. It was also recognized that, because the ED staff can be overwhelmed by COVID-19 patients, there may not be enough time to perform detailed screening of patients with neurologic dysfunction and that “reverse masking” may not be done consistently for nonintubated patients. This may place patients and health care workers at risk of unprotected exposure.
Recognizing these challenges, we created a Neuro-COVID-19 Time-out Process and Checklist (NCOT-PC) as a quality improvement project. The aim of this project was to improve and standardize the current process of identifying patients with neurologic dysfunction who require COVID-19 testing to decrease the risk of unprotected exposure of patients and health care workers.
Methods
Patients and Definitions
This quality improvement project was undertaken at the University of Mississippi Medical Center NSICU. Because this was a quality improvement project, an Institutional Review Board exemption was granted.
The NCOT-PC was utilized in consecutive patients with neurologic dysfunction admitted to the NSICU during a period of 1 week. “Neurologic dysfunction” encompasses any neurologic illness affecting the mental status and/or level of alertness, subsequently precluding the ability to reliably screen the patient utilizing standard COVID-19 screening. “Med-Com” at our institution is the equivalent of the national COVID-19 testing hotline, where our institution’s infectious diseases experts screen calls for testing and determine whether testing is warranted. “Unprotected exposure” means exposure to COVID-19 without adequate and appropriate PPE.
Quality Improvement Process
As more PUIs were being admitted to the institution, we used the Plan-Do-Study-Act method for process improvements in the NSICU.5 NSICU stakeholders, including attendings, the nurse manager, and nurse practitioners (NPs), developed an algorithm to facilitate the coordination of the NSICU staff in screening patients to identify those with a high likelihood of needing COVID-19 testing upon arrival in the NSICU (Figure 1). Once the NCOT-PC was finalized, NSICU stakeholders were educated regarding the use of this screening tool.

The checklist clinicians review when screening patients is shown in Figure 2. The risk factors comprising the checklist include patient history and clinical and radiographic characteristics that have been shown to be relevant for identifying patients with COVID-19.6,7 The imaging criteria utilize imaging that is part of the standard of care for NSICU patients. For example, computed tomography angiogram of the head and neck performed as part of the acute stroke protocol captures the upper part of the chest. These images are utilized for their incidental findings, such as apical ground-glass opacities and tree-in-bud formation. The risk factors applicable to the patient determine whether the clinician will call Med-Com for testing approval. Institutional COVID-19 processes were then followed accordingly.8 The decision from Med-Com was considered final, and no deviation from institutional policies was allowed.

NCOT-PC was utilized for consecutive days for 1 week before re-evaluation of its feasibility and adaptability.
Data Collection and Analysis
Consecutive patients with neurologic dysfunction admitted into the NSICU were assigned nonlinkable patient numbers. No identifiers were collected for the purpose of this project. The primary diagnosis for admission, the neurologic dysfunction that precluded standard screening, and checklist components that the patient fulfilled were collected.
To assess the tool’s feasibility, feedback regarding the ease of use of the NCOT-PC was gathered from the nurses, NPs, charge nurses, fellows, and other attendings. To assess the utility of the NCOT-PC in identifying patients who will be approved for COVID-19 testing, we calculated the proportion of patients who were deemed to have a high likelihood of testing and the proportion of patients who were approved for testing. Descriptive statistics were used, as applicable for the project, to summarize the utility of the NCOT-PC.
Results
We found that the NCOT-PC can be easily used by clinicians. The NSICU staff did not communicate any implementation issues, and since the NCOT-PC was implemented, no problems have been identified.
During the pilot period of the NCOT-PC, 14 new patients were admitted to the NSICU service. Nine (64%) of these had neurologic dysfunction, and the NCOT-PC was used to determine whether Med-Com should be called based on the patients’ likelihood (high vs low) of needing a COVID-19 test. Of those patients with neurologic dysfunction, 7 (78%) were deemed to have a high likelihood of needing a COVID-19 test based on the NCOT-PC. Med-Com was contacted regarding these patients, and all were deemed to require the COVID-19 test by Med-Com and were transitioned into PUI status per institutional policy (Table).

Discussion
The NCOT-PC project improved and standardized the process of identifying and screening patients with neurologic dysfunction for COVID-19 testing. The screening tool is feasible to use, and it decreased inadvertent unprotected exposure of patients and health care workers.
The NCOT-PC was easy to administer. Educating the staff regarding the new process took only a few minutes and involved a meeting with the nurse manager, NPs, fellows, residents, and attendings. We found that this process works well in tandem with the standard institutional processes in place in terms of Protected Code Stroke pathway, PUI isolation, PPE use, and Med-Com screening for COVID-19 testing. Med-Com was called only if the patient fulfilled the checklist criteria. In addition, no extra cost was attributed to implementing the NCOT-PC, since we utilized imaging that was already done as part of the standard of care for patients with neurologic dysfunction.
The standardization of the process of screening for COVID-19 testing among patients with neurologic dysfunction improved patient selection. Before the NCOT-PC, there was no consistency in terms of who should get tested and the reason for testing patients with neurologic dysfunction. Patients can pass through the ED and arrive in the NSICU with an unclear screening status, which may cause inadvertent patient and health care worker exposure to COVID-19. With the NCOT-PC, we have avoided instances of inadvertent staff or patient exposure in the NSICU.
The NCOT-PC was adopted into the NSICU process after the first week it was piloted. Beyond the NSICU, the application of the NCOT-PC can be extended to any patient presentation that precludes standard screening, such as ED and interhospital transfers for stroke codes, trauma codes, code blue, or myocardial infarction codes. In our department, as we started the process of PCS for stroke codes, we included NCOT-PC for stroke patients with neurologic dysfunction.
The results of our initiative are largely limited by the decision-making process of Med-Com when patients are called in for testing. At the time of our project, there were no specific criteria used for patients with altered mental status, except for the standard screening methods, and it was through clinician-to-clinician discussion that testing decisions were made. Another limitation is the short period of time that the NCOT-PC was applied before adoption.
In summary, the NCOT-PC tool improved the screening process for COVID-19 testing in patients with neurologic dysfunction admitted to the NSICU. It was feasible and prevented unprotected staff and patient exposure to COVID-19. The NCOT-PC functionality was compatible with institutional COVID-19 policies in place, which contributed to its overall sustainability.
The Standards for Quality Improvement Reporting Excellence (SQUIRE 2.0) were utilized in preparing this manuscript.9
Acknowledgment: The authors thank the University of Mississippi Medical Center NSICU staff for their input with implementation of the NCOT-PC.
Corresponding author: Prashant A. Natteru, MD, University of Mississippi Medical Center, Department of Neurology, 2500 North State St., Jackson, MS 39216; [email protected].
Financial disclosures: None.
From the University of Mississippi Medical Center, Department of Neurology, Division of Neuroscience Intensive Care, Jackson, MS.
Abstract
Objective: To test a coronavirus disease 2019 (COVID-19) screening tool to identify patients who qualify for testing among patients with neurologic dysfunction who are unable to answer the usual screening questions, which could help to prevent unprotected exposure of patients and health care workers to COVID-19.
Methods: The Neuro-COVID-19 Time-out Process and Checklist (NCOT-PC) was implemented at our institution for 1 week as a quality improvement project to improve the pathway for COVID-19 screening and testing among patients with neurologic dysfunction.
Results: A total of 14 new patients were admitted into the neuroscience intensive care unit (NSICU) service during the pilot period. The NCOT-PC was utilized on 9 (64%) patients with neurologic dysfunction; 7 of these patients were found to have a likelihood of requiring testing based on the NCOT-PC and were subsequently screened for COVID-19 testing by contacting the institution’s COVID-19 testing hotline (Med-Com). All these patients were subsequently transitioned into person-under-investigation status based on the determination from Med-Com. The NSICU staff involved were able to utilize NCOT-PC without issues. The NCOT-PC was immediately adopted into the NSICU process.
Conclusion: Use of the NCOT-PC tool was found to be feasible and improved the screening methodology of patients with neurologic dysfunction.
Keywords: coronavirus; health care planning; quality improvement; patient safety; medical decision-making; neuroscience intensive care unit.
The coronavirus disease 2019 (COVID-19) pandemic has altered various standard emergent care pathways. Current recommendations regarding COVID-19 screening for testing involve asking patients about their symptoms, including fever, cough, chest pain, and dyspnea.1 This standard screening method poses a problem when caring for patients with neurologic dysfunction. COVID-19 patients may pre-sent with conditions that affect their ability to answer questions, such as stroke, encephalitis, neuromuscular disorders, or headache, and that may preclude the use of standard screening for testing.2 Patients with acute neurologic dysfunction who cannot undergo standard screening may leave the emergency department (ED) and transition into the neuroscience intensive care unit (NSICU) or any intensive care unit (ICU) without a reliable COVID-19 screening test.
The Protected Code Stroke pathway offers protection in the emergent setting for patients with stroke when their COVID-19 status is unknown.3 A similar process has been applied at our institution for emergent management of patients with cerebrovascular disease (stroke, intracerebral hemorrhage, and subarachnoid hemorrhage). However, the process from the ED after designating “difficult to screen” patients as persons under investigation (PUI) is unclear. The Centers for Disease Control and Prevention (CDC) has delineated the priorities for testing, with not all declared PUIs requiring testing.4 This poses a great challenge, because patients designated as PUIs require the same management as a COVID-19-positive patient, with negative-pressure isolation rooms as well as use of protective personal equipment (PPE), which may not be readily available. It was also recognized that, because the ED staff can be overwhelmed by COVID-19 patients, there may not be enough time to perform detailed screening of patients with neurologic dysfunction and that “reverse masking” may not be done consistently for nonintubated patients. This may place patients and health care workers at risk of unprotected exposure.
Recognizing these challenges, we created a Neuro-COVID-19 Time-out Process and Checklist (NCOT-PC) as a quality improvement project. The aim of this project was to improve and standardize the current process of identifying patients with neurologic dysfunction who require COVID-19 testing to decrease the risk of unprotected exposure of patients and health care workers.
Methods
Patients and Definitions
This quality improvement project was undertaken at the University of Mississippi Medical Center NSICU. Because this was a quality improvement project, an Institutional Review Board exemption was granted.
The NCOT-PC was utilized in consecutive patients with neurologic dysfunction admitted to the NSICU during a period of 1 week. “Neurologic dysfunction” encompasses any neurologic illness affecting the mental status and/or level of alertness, subsequently precluding the ability to reliably screen the patient utilizing standard COVID-19 screening. “Med-Com” at our institution is the equivalent of the national COVID-19 testing hotline, where our institution’s infectious diseases experts screen calls for testing and determine whether testing is warranted. “Unprotected exposure” means exposure to COVID-19 without adequate and appropriate PPE.
Quality Improvement Process
As more PUIs were being admitted to the institution, we used the Plan-Do-Study-Act method for process improvements in the NSICU.5 NSICU stakeholders, including attendings, the nurse manager, and nurse practitioners (NPs), developed an algorithm to facilitate the coordination of the NSICU staff in screening patients to identify those with a high likelihood of needing COVID-19 testing upon arrival in the NSICU (Figure 1). Once the NCOT-PC was finalized, NSICU stakeholders were educated regarding the use of this screening tool.

The checklist clinicians review when screening patients is shown in Figure 2. The risk factors comprising the checklist include patient history and clinical and radiographic characteristics that have been shown to be relevant for identifying patients with COVID-19.6,7 The imaging criteria utilize imaging that is part of the standard of care for NSICU patients. For example, computed tomography angiogram of the head and neck performed as part of the acute stroke protocol captures the upper part of the chest. These images are utilized for their incidental findings, such as apical ground-glass opacities and tree-in-bud formation. The risk factors applicable to the patient determine whether the clinician will call Med-Com for testing approval. Institutional COVID-19 processes were then followed accordingly.8 The decision from Med-Com was considered final, and no deviation from institutional policies was allowed.

NCOT-PC was utilized for consecutive days for 1 week before re-evaluation of its feasibility and adaptability.
Data Collection and Analysis
Consecutive patients with neurologic dysfunction admitted into the NSICU were assigned nonlinkable patient numbers. No identifiers were collected for the purpose of this project. The primary diagnosis for admission, the neurologic dysfunction that precluded standard screening, and checklist components that the patient fulfilled were collected.
To assess the tool’s feasibility, feedback regarding the ease of use of the NCOT-PC was gathered from the nurses, NPs, charge nurses, fellows, and other attendings. To assess the utility of the NCOT-PC in identifying patients who will be approved for COVID-19 testing, we calculated the proportion of patients who were deemed to have a high likelihood of testing and the proportion of patients who were approved for testing. Descriptive statistics were used, as applicable for the project, to summarize the utility of the NCOT-PC.
Results
We found that the NCOT-PC can be easily used by clinicians. The NSICU staff did not communicate any implementation issues, and since the NCOT-PC was implemented, no problems have been identified.
During the pilot period of the NCOT-PC, 14 new patients were admitted to the NSICU service. Nine (64%) of these had neurologic dysfunction, and the NCOT-PC was used to determine whether Med-Com should be called based on the patients’ likelihood (high vs low) of needing a COVID-19 test. Of those patients with neurologic dysfunction, 7 (78%) were deemed to have a high likelihood of needing a COVID-19 test based on the NCOT-PC. Med-Com was contacted regarding these patients, and all were deemed to require the COVID-19 test by Med-Com and were transitioned into PUI status per institutional policy (Table).

Discussion
The NCOT-PC project improved and standardized the process of identifying and screening patients with neurologic dysfunction for COVID-19 testing. The screening tool is feasible to use, and it decreased inadvertent unprotected exposure of patients and health care workers.
The NCOT-PC was easy to administer. Educating the staff regarding the new process took only a few minutes and involved a meeting with the nurse manager, NPs, fellows, residents, and attendings. We found that this process works well in tandem with the standard institutional processes in place in terms of Protected Code Stroke pathway, PUI isolation, PPE use, and Med-Com screening for COVID-19 testing. Med-Com was called only if the patient fulfilled the checklist criteria. In addition, no extra cost was attributed to implementing the NCOT-PC, since we utilized imaging that was already done as part of the standard of care for patients with neurologic dysfunction.
The standardization of the process of screening for COVID-19 testing among patients with neurologic dysfunction improved patient selection. Before the NCOT-PC, there was no consistency in terms of who should get tested and the reason for testing patients with neurologic dysfunction. Patients can pass through the ED and arrive in the NSICU with an unclear screening status, which may cause inadvertent patient and health care worker exposure to COVID-19. With the NCOT-PC, we have avoided instances of inadvertent staff or patient exposure in the NSICU.
The NCOT-PC was adopted into the NSICU process after the first week it was piloted. Beyond the NSICU, the application of the NCOT-PC can be extended to any patient presentation that precludes standard screening, such as ED and interhospital transfers for stroke codes, trauma codes, code blue, or myocardial infarction codes. In our department, as we started the process of PCS for stroke codes, we included NCOT-PC for stroke patients with neurologic dysfunction.
The results of our initiative are largely limited by the decision-making process of Med-Com when patients are called in for testing. At the time of our project, there were no specific criteria used for patients with altered mental status, except for the standard screening methods, and it was through clinician-to-clinician discussion that testing decisions were made. Another limitation is the short period of time that the NCOT-PC was applied before adoption.
In summary, the NCOT-PC tool improved the screening process for COVID-19 testing in patients with neurologic dysfunction admitted to the NSICU. It was feasible and prevented unprotected staff and patient exposure to COVID-19. The NCOT-PC functionality was compatible with institutional COVID-19 policies in place, which contributed to its overall sustainability.
The Standards for Quality Improvement Reporting Excellence (SQUIRE 2.0) were utilized in preparing this manuscript.9
Acknowledgment: The authors thank the University of Mississippi Medical Center NSICU staff for their input with implementation of the NCOT-PC.
Corresponding author: Prashant A. Natteru, MD, University of Mississippi Medical Center, Department of Neurology, 2500 North State St., Jackson, MS 39216; [email protected].
Financial disclosures: None.
1. Coronavirus disease 2019 (COVID-19) Symptoms. www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html. Accessed April 9, 2020.
2. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:1-9.
3. Khosravani H, Rajendram P, Notario L, et al. Protected code stroke: hyperacute stroke management during the coronavirus disease 2019. (COVID-19) pandemic. Stroke. 2020;51:1891-1895.
4. Coronavirus disease 2019 (COVID-19) evaluation and testing. www.cdc.gov/coronavirus/2019-nCoV/hcp/clinical-criteria.html. Accessed April 9, 2020.
5. Plan-Do-Study-Act Worksheet. Institute for Healthcare Improvement website. www.ihi.org/resources/Pages/Tools/PlanDoStudyActWorksheet.aspx. Accessed March 31,2020.
6. Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. 2020;10.1002/jmv.25728.
7. Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, et al. Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med Infect Dis. 2020;101623.
8. UMMC’s COVID-19 Clinical Processes. www.umc.edu/CoronaVirus/Mississippi-Health-Care-Professionals/Clinical-Resources/Clinical-Resources.html. Accessed April 9, 2020.
9. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): Revised Publication Guidelines from a Detailed Consensus Process. The EQUATOR Network. www.equator-network.org/reporting-guidelines/squire/. Accessed May 12, 2020.
1. Coronavirus disease 2019 (COVID-19) Symptoms. www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html. Accessed April 9, 2020.
2. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:1-9.
3. Khosravani H, Rajendram P, Notario L, et al. Protected code stroke: hyperacute stroke management during the coronavirus disease 2019. (COVID-19) pandemic. Stroke. 2020;51:1891-1895.
4. Coronavirus disease 2019 (COVID-19) evaluation and testing. www.cdc.gov/coronavirus/2019-nCoV/hcp/clinical-criteria.html. Accessed April 9, 2020.
5. Plan-Do-Study-Act Worksheet. Institute for Healthcare Improvement website. www.ihi.org/resources/Pages/Tools/PlanDoStudyActWorksheet.aspx. Accessed March 31,2020.
6. Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. 2020;10.1002/jmv.25728.
7. Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, et al. Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med Infect Dis. 2020;101623.
8. UMMC’s COVID-19 Clinical Processes. www.umc.edu/CoronaVirus/Mississippi-Health-Care-Professionals/Clinical-Resources/Clinical-Resources.html. Accessed April 9, 2020.
9. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): Revised Publication Guidelines from a Detailed Consensus Process. The EQUATOR Network. www.equator-network.org/reporting-guidelines/squire/. Accessed May 12, 2020.
Clinical Utility of Methicillin-Resistant Staphylococcus aureus Polymerase Chain Reaction Nasal Swab Testing in Lower Respiratory Tract Infections
From the Hospital of Central Connecticut, New Britain, CT (Dr. Caulfield and Dr. Shepard); Hartford Hospital, Hartford, CT (Dr. Linder and Dr. Dempsey); and the Hartford HealthCare Research Program, Hartford, CT (Dr. O’Sullivan).
Abstract
- Objective: To assess the utility of methicillin-resistant Staphylococcus aureus (MRSA) polymerase chain reaction (PCR) nasal swab testing in patients with lower respiratory tract infections (LRTI).
- Design and setting: Multicenter, retrospective, electronic chart review conducted within the Hartford HealthCare system.
- Participants: Patients who were treated for LRTIs at the Hospital of Central Connecticut or Hartford Hospital between July 1, 2018, and June 30, 2019.
- Measurements: The primary outcome was anti-MRSA days of therapy (DOT) in patients who underwent MRSA PCR testing versus those who did not. In a subgroup analysis, we compared anti-MRSA DOT among patients with appropriate versus inappropriate utilization of the MRSA PCR test.
- Results: Of the 319 patients treated for LRTIs, 155 (48.6%) had a MRSA PCR ordered, and appropriate utilization occurred in 94 (60.6%) of these patients. Anti-MRSA DOT in the MRSA PCR group (n = 155) was shorter than in the group that did not undergo MRSA PCR testing (n = 164), but this difference did not reach statistical significance (1.68 days [interquartile range {IQR}, 0.80-2.74] vs 1.86 days [IQR, 0.56-3.33], P = 0.458). In the subgroup analysis, anti-MRSA DOT was significantly shorter in the MRSA PCR group with appropriate utilization compared to the inappropriate utilization group (1.16 [IQR, 0.44-1.88] vs 2.68 [IQR, 1.75-3.76], P < 0.001)
- Conclusion: Appropriate utilization of MRSA PCR nasal swab testing can reduce DOT in patients with LRTI. Further education is necessary to expand the appropriate use of the MRSA PCR test across our health system.
Keywords: MRSA; LRTI; pneumonia; antimicrobial stewardship; antibiotic resistance.
More than 300,000 patients were hospitalized with methicillin-resistant Staphylococcus aureus (MRSA) infections in the United States in 2017, and at least 10,000 of these cases resulted in mortality.1 While MRSA infections overall are decreasing, it is crucial to continue to employ antimicrobial stewardship tactics to keep these infections at bay. Recently, strains of S. aureus have become resistant to vancomycin, making this bacterium even more difficult to treat.2
A novel tactic in antimicrobial stewardship involves the use of MRSA polymerase chain reaction (PCR) nasal swab testing to rule out the presence of MRSA in patients with lower respiratory tract infections (LRTI). If used appropriately, this approach may decrease the number of days patients are treated with anti-MRSA antimicrobials. Waiting for cultures to speciate can take up to 72 hours,3 meaning that patients may be exposed to 3 days of unnecessary broad-spectrum antibiotics. Results of MRSA PCR assay of nasal swab specimens can be available in 1 to 2 hours,4 allowing for more rapid de-escalation of therapy. Numerous studies have shown that this test has a negative predictive value (NPV) greater than 95%, indicating that a negative nasal swab result may be useful to guide de-escalation of antibiotic therapy.5-8 The purpose of this study was to assess the utility of MRSA PCR nasal swab testing in patients with LRTI throughout the Hartford HealthCare system.
Methods
Design
This study was a multicenter, retrospective, electronic chart review. It was approved by the Hartford HealthCare Institutional Review Board (HHC-2019-0169).
Selection of Participants
Patients were identified through electronic medical record reports based on ICD-10 codes. Records were categorized into 2 groups: patients who received a MRSA PCR nasal swab testing and patients who did not. Patients who received the MRSA PCR were further categorized by appropriate or inappropriate utilization. Appropriate utilization of the MRSA PCR was defined as MRSA PCR ordered within 48 hours of a new vancomycin or linezolid order, and anti-MRSA therapy discontinued within 24 hours of a negative result. Inappropriate utilization of the MRSA PCR was defined as MRSA PCR ordered more than 48 hours after a new vancomycin or linezolid order, or continuation of anti-MRSA therapy despite a negative MRSA PCR and no other evidence of a MRSA infection.
Patients were included if they met all of the following criteria: age 18 years or older, with no upper age limit; treated for a LRTI, identified by ICD-10 codes (J13-22, J44, J85); treated with empiric antibiotics active against MRSA, specifically vancomycin or linezolid; and treated at the Hospital of Central Connecticut (HOCC) or Hartford Hospital (HH) between July 1, 2018, and June 30, 2019, inclusive. Patients were excluded if they met 1 or more of the following criteria: age less than 18 years old; admitted for 48 hours or fewer or discharged from the emergency department; not treated at either facility; treated before July 1, 2018, or after June 30, 2019; treated for ventilator-associated pneumonia; received anti-MRSA therapy within 30 days prior to admission; or treated for a concurrent bacterial infection requiring anti-MRSA therapy.
Outcome Measures
The primary outcome was anti-MRSA days of therapy (DOT) in patients who underwent MRSA PCR testing compared to patients who did not undergo MRSA PCR testing. A subgroup analysis was completed to compare anti-MRSA DOT within patients in the MRSA PCR group. Patients in the subgroup were categorized by appropriate or inappropriate utilization, and anti-MRSA DOT were compared between these groups. Secondary outcomes that were evaluated included length of stay (LOS), 30-day readmission rate, and incidence of acute kidney injury (AKI). Thirty-day readmission was defined as admission to HOCC, HH, or any institution within Hartford HealthCare within 30 days of discharge. AKI was defined as an increase in serum creatinine by ≥ 0.3 mg/dL in 48 hours, increase ≥ 1.5 times baseline, or a urine volume < 0.5 mL/kg/hr for 6 hours.
Statistical Analyses
The study was powered for the primary outcome, anti-MRSA DOT, with a clinically meaningful difference of 1 day. Group sample sizes of 240 in the MRSA PCR group and 160 in the no MRSA PCR group would have afforded 92% power to detect that difference, if the null hypothesis was that both group means were 4 days and the alternative hypothesis was that the mean of the MRSA PCR group was 3 days, with estimated group standard deviations of 80% of each mean. This estimate used an alpha level of 0.05 with a 2-sided t-test. Among those who received a MRSA PCR test, a clinically meaningful difference between appropriate and inappropriate utilization was 5%.
Descriptive statistics were provided for all variables as a function of the individual hospital and for the combined data set. Continuous data were summarized with means and standard deviations (SD), or with median and interquartile ranges (IQR), depending on distribution. Categorical variables were reported as frequencies, using percentages. All data were evaluated for normality of distribution. Inferential statistics comprised a Student’s t-test to compare normally distributed, continuous data between groups. Nonparametric distributions were compared using a Mann-Whitney U test. Categorical comparisons were made using a Fisher’s exact test for 2×2 tables and a Pearson chi-square test for comparisons involving more than 2 groups.
Since anti-MRSA DOT (primary outcome) and LOS (secondary outcome) are often non-normally distributed, they have been transformed (eg, log or square root, again depending on distribution). Whichever native variable or transformation variable was appropriate was used as the outcome measure in a linear regression model to account for the influence of factors (covariates) that show significant univariate differences. Given the relatively small sample size, a maximum of 10 variables were included in the model. All factors were iterated in a forward regression model (most influential first) until no significant changes were observed.
All calculations were performed with SPSS v. 21 (IBM; Armonk, NY) using an a priori alpha level of 0.05, such that all results yielding P < 0.05 were deemed statistically significant.
Results
Of the 561 patient records reviewed, 319 patients were included and 242 patients were excluded. Reasons for exclusion included 65 patients admitted for a duration of 48 hours or less or discharged from the emergency department; 61 patients having another infection requiring anti-MRSA therapy; 60 patients not having a diagnosis of a LRTI or not receiving anti-MRSA therapy; 52 patients having received anti-MRSA therapy within 30 days prior to admission; and 4 patients treated outside of the specified date range.
Of the 319 patients included, 155 (48.6%) were in the MRSA PCR group and 164 (51.4%) were in the group that did not undergo MRSA PCR (Table 1). Of the 155 patients with a MRSA PCR ordered, the test was utilized appropriately in 94 (60.6%) patients and inappropriately in 61 (39.4%) patients (Table 2). In the MRSA PCR group, 135 patients had a negative result on PCR assay, with 133 of those patients having negative respiratory cultures, resulting in a NPV of 98.5%. Differences in baseline characteristics between the MRSA PCR and no MRSA PCR groups were observed. The patients in the MRSA PCR group appeared to be significantly more ill than those in the no MRSA PCR group, as indicated by statistically significant differences in intensive care unit (ICU) admissions (P = 0.001), positive chest radiographs (P = 0.034), sepsis at time of anti-MRSA initiation (P = 0.013), pulmonary consults placed (P = 0.003), and carbapenem usage (P = 0.047).
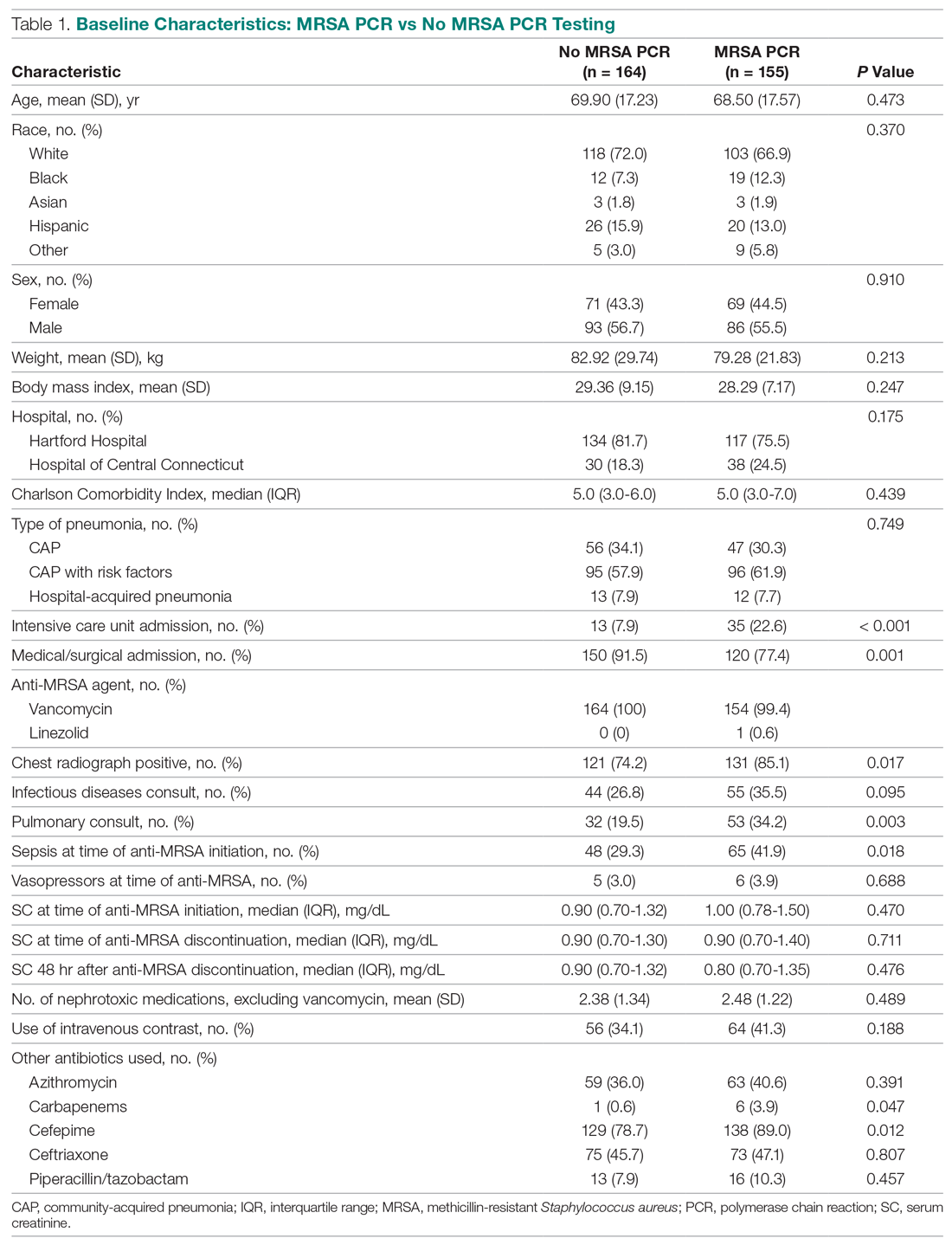
In the subgroup analysis comparing appropriate and inappropriate utilization within the MRSA PCR group, the inappropriate utilization group had significantly higher numbers of infectious diseases consults placed, patients with hospital-acquired pneumonia, and patients with community-acquired pneumonia with risk factors.
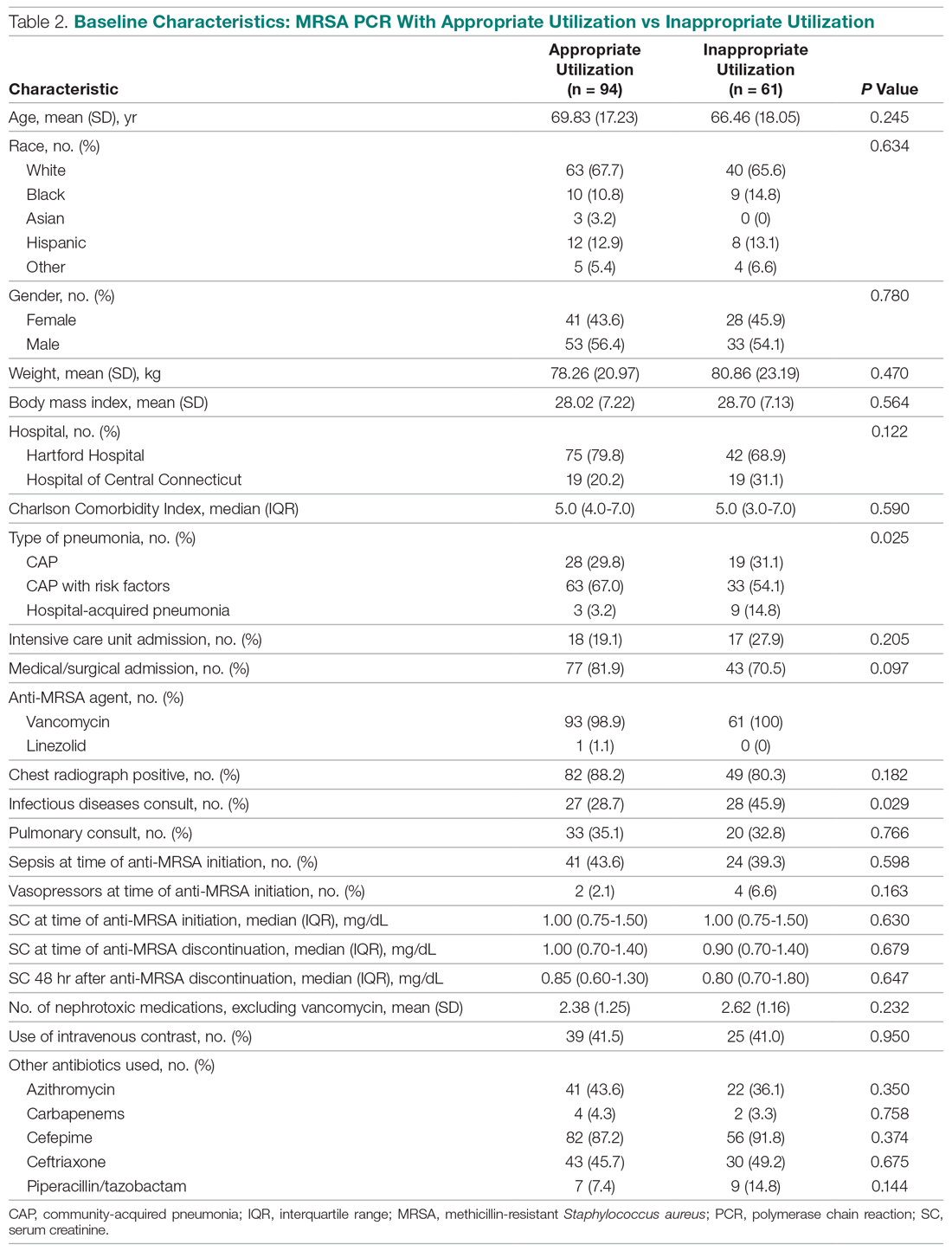
Outcomes
Median anti-MRSA DOT in the MRSA PCR group was shorter than DOT in the no MRSA PCR group, but this difference did not reach statistical significance (1.68 [IQR, 0.80-2.74] vs 1.86 days [IQR, 0.56-3.33], P = 0.458; Table 3). LOS in the MRSA PCR group was longer than in the no MRSA PCR group (6.0 [IQR, 4.0-10.0] vs 5.0 [IQR, 3.0-7.0] days, P = 0.001). There was no difference in 30-day readmissions that were related to the previous visit or incidence of AKI between groups.
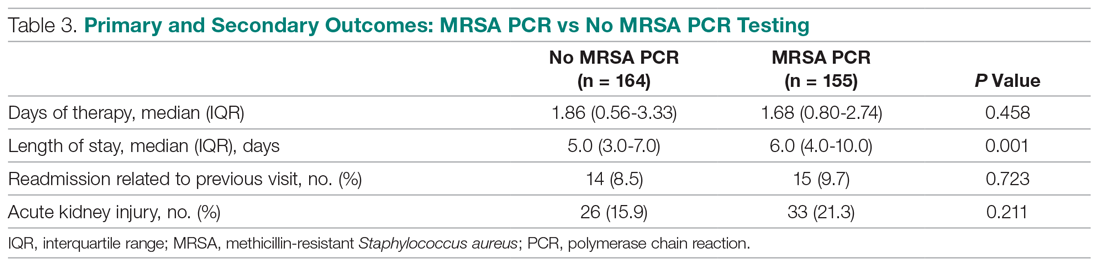
In the subgroup analysis, anti-MRSA DOT in the MRSA PCR group with appropriate utilization was shorter than DOT in the MRSA PCR group with inappropriate utilization (1.16 [IQR, 0.44-1.88] vs 2.68 [IQR, 1.75-3.76] days, P < 0.001; Table 4). LOS in the MRSA PCR group with appropriate utilization was shorter than LOS in the inappropriate utilization group (5.0 [IQR, 4.0-7.0] vs 7.0 [IQR, 5.0-12.0] days, P < 0.001). Thirty-day readmissions that were related to the previous visit were significantly higher in patients in the MRSA PCR group with appropriate utilization (13 vs 2, P = 0.030). There was no difference in incidence of AKI between the groups.
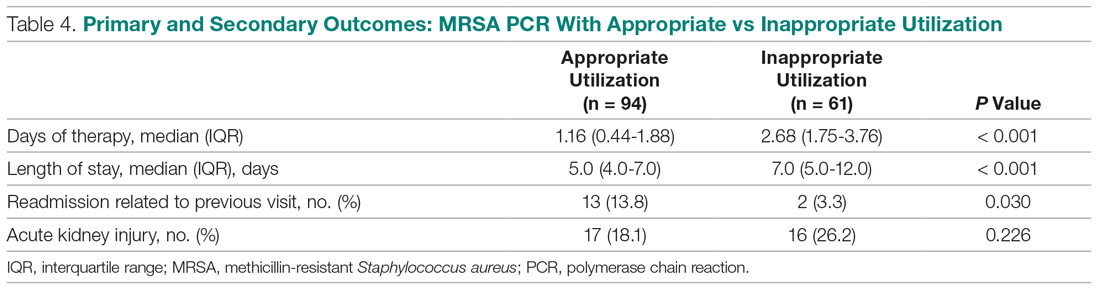
A multivariate analysis was completed to determine whether the sicker MRSA PCR population was confounding outcomes, particularly the secondary outcome of LOS, which was noted to be longer in the MRSA PCR group (Table 5). When comparing LOS in the MRSA PCR and the no MRSA PCR patients, the multivariate analysis showed that admission to the ICU and carbapenem use were associated with a longer LOS (P < 0.001 and P = 0.009, respectively). The incidence of admission to the ICU and carbapenem use were higher in the MRSA PCR group (P = 0.001 and P = 0.047). Therefore, longer LOS in the MRSA PCR patients could be a result of the higher prevalence of ICU admissions and infections requiring carbapenem therapy rather than the result of the MRSA PCR itself.
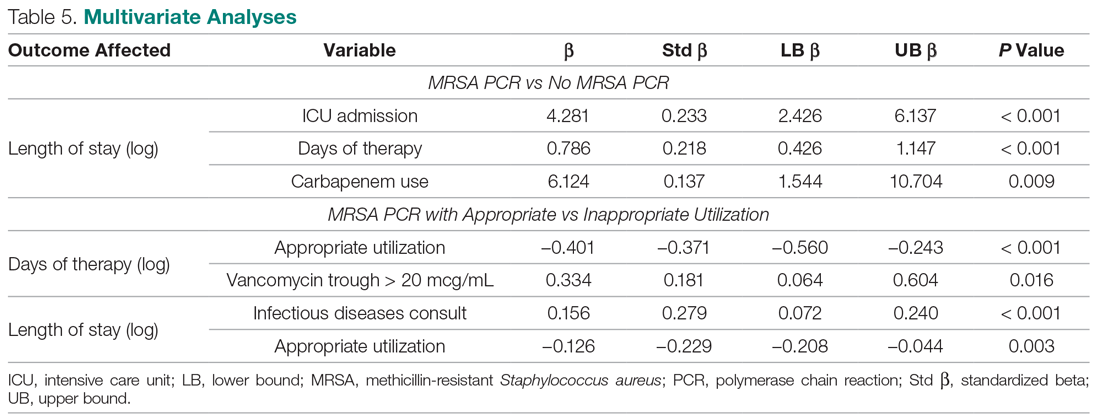
Discussion
A MRSA PCR nasal swab protocol can be used to minimize a patient’s exposure to unnecessary broad-spectrum antibiotics, thereby preventing antimicrobial resistance. Thus, it is important to assess how our health system is utilizing this antimicrobial stewardship tactic. With the MRSA PCR’s high NPV, providers can be confident that MRSA pneumonia is unlikely in the absence of MRSA colonization. Our study established a NPV of 98.5%, which is similar to other studies, all of which have shown NPVs greater than 95%.5-8 Despite the high NPV, this study demonstrated that only 51.4% of patients with LRTI had orders for a MRSA PCR. Of the 155 patients with a MRSA PCR, the test was utilized appropriately only 60.6% of the time. A majority of the inappropriately utilized tests were due to MRSA PCR orders placed more than 48 hours after anti-MRSA therapy initiation. To our knowledge, no other studies have assessed the clinical utility of MRSA PCR nasal swabs as an antimicrobial stewardship tool in a diverse health system; therefore, these results are useful to guide future practices at our institution. There is a clear need for provider and pharmacist education to increase the use of MRSA PCR nasal swab testing for patients with LRTI being treated with anti-MRSA therapy. Additionally, clinician education regarding the initial timing of the MRSA PCR order and the proper utilization of the results of the MRSA PCR likely will benefit patient outcomes at our institution.
When evaluating anti-MRSA DOT, this study demonstrated a reduction of only 0.18 days (about 4 hours) of anti-MRSA therapy in the patients who received MRSA PCR testing compared to the patients without a MRSA PCR ordered. Our anti-MRSA DOT reduction was lower than what has been reported in similar studies. For example, Baby et al found that the use of the MRSA PCR was associated with 46.6 fewer hours of unnecessary antimicrobial treatment. Willis et al evaluated a pharmacist-driven protocol that resulted in a reduction of 1.8 days of anti-MRSA therapy, despite a protocol compliance rate of only 55%.9,10 In our study, the patients in the MRSA PCR group appeared to be significantly more ill than those in the no MRSA PCR group, which may be the reason for the incongruences in our results compared to the current literature. Characteristics such as ICU admissions, positive chest radiographs, sepsis cases, pulmonary consults, and carbapenem usage—all of which are indicative of a sicker population—were more prevalent in the MRSA PCR group. This sicker population could have underestimated the reduction of DOT in the MRSA PCR group compared to the no MRSA PCR group.
After isolating the MRSA PCR patients in the subgroup analysis, anti-MRSA DOT was 1.5 days shorter when the test was appropriately utilized, which is more comparable to what has been reported in the literature.9,10 Only 60.6% of the MRSA PCR patients had their anti-MRSA therapy appropriately managed based on the MRSA PCR. Interestingly, a majority of patients in the inappropriate utilization group had MRSA PCR tests ordered more than 48 hours after beginning anti-MRSA therapy. More prompt and efficient ordering of the MRSA PCR may have resulted in more opportunities for earlier de-escalation of therapy. Due to these factors, the patients in the inappropriate utilization group could have further contributed to the underestimated difference in anti-MRSA DOT between the MRSA PCR and no MRSA PCR patients in the primary outcome. Additionally, there were no notable differences between the appropriate and inappropriate utilization groups, unlike in the MRSA PCR and no MRSA PCR groups, which is why we were able to draw more robust conclusions in the subgroup analysis. Therefore, the subgroup analysis confirmed that if the results of the MRSA PCR are used appropriately to guide anti-MRSA therapy, patients can potentially avoid 36 hours of broad-spectrum antibiotics.
Data on how the utilization of the MRSA PCR nasal swab can affect LOS are limited; however, one study did report a 2.8-day reduction in LOS after implementation of a pharmacist-driven MRSA PCR nasal swab protocol.11 Our study demonstrated that LOS was significantly longer in the MRSA PCR group than in the no MRSA PCR group. This result was likely affected by the aforementioned sicker MRSA PCR population. Our multivariate analysis further confirmed that ICU admissions were associated with a longer LOS, and, given that the MRSA PCR group had a significantly higher ICU population, this likely confounded these results. If our 2 groups had had more evenly distributed characteristics, it is possible that we could have found a shorter LOS in the MRSA PCR group, similar to what is reported in the literature. In the subgroup analysis, LOS was 2 days shorter in the appropriate utilization group compared to the inappropriate utilization group. This further affirms that the results of the MRSA PCR must be used appropriately in order for patient outcomes, like LOS, to benefit.
The effects of the MRSA PCR nasal swab on 30-day readmission rates and incidence of AKI are not well-documented in the literature. One study did report 30-day readmission rates as an outcome, but did not cite any difference after the implementation of a protocol that utilized MRSA PCR nasal swab testing.12 The outcome of AKI is slightly better represented in the literature, but the results are conflicting. Some studies report no difference after the implementation of a MRSA PCR-based protocol,11 and others report a significant decrease in AKI with the use of the MRSA PCR.9 Our study detected no difference in 30-day readmission rates related to the previous admission or in AKI between the MRSA PCR and no MRSA PCR populations. In the subgroup analysis, 30-day readmission rates were significantly higher in the MRSA PCR group with appropriate utilization than in the group with inappropriate utilization; however, our study was not powered to detect a difference in this secondary outcome.
This study had some limitations that may have affected our results. First, this study was a retrospective chart review. Additionally, the baseline characteristics were not well balanced across the different groups. There were sicker patients in the MRSA PCR group, which may have led to an underestimate of the reduction in DOT and LOS in these patients. Finally, we did not include enough patient records to reach power in the MRSA PCR group due to a higher than expected number of patients meeting exclusion criteria. Had we attained sufficient power, there may have been more profound reductions in DOT and LOS.
Conclusion
MRSA infections are a common cause for hospitalization, and there is a growing need for antimicrobial stewardship efforts to limit unnecessary antibiotic usage in order to prevent resistance. As illustrated in our study, appropriate utilization of the MRSA PCR can reduce DOT up to 1.5 days. However, our results suggest that there is room for provider and pharmacist education to increase the use of MRSA PCR nasal swab testing in patients with LRTI receiving anti-MRSA therapy. Further emphasis on the appropriate utilization of the MRSA PCR within our health care system is essential.
Corresponding author: Casey Dempsey, PharmD, BCIDP, 80 Seymour St., Hartford, CT 06106; [email protected].
Financial disclosures: None.
1. Antimicrobial resistance threats. Centers for Disease Control and Prevention web site. www.cdc.gov/drugresistance/biggest-threats.html. Accessed September 9, 2020.
2. Biggest threats and data. Centers for Disease Control and Prevention web site. www.cdc.gov/drugresistance/biggest_threats.html#mrsa. Accessed September 9, 2020.
3. Smith MN, Erdman MJ, Ferreira JA, et al. Clinical utility of methicillin-resistant Staphylococcus aureus nasal polymerase chain reaction assay in critically ill patients with nosocomial pneumonia. J Crit Care. 2017;38:168-171.
4. Giancola SE, Nguyen AT, Le B, et al. Clinical utility of a nasal swab methicillin-resistant Staphylococcus aureus polymerase chain reaction test in intensive and intermediate care unit patients with pneumonia. Diagn Microbiol Infect Dis. 2016;86:307-310.
5. Dangerfield B, Chung A, Webb B, Seville MT. Predictive value of methicillin-resistant Staphylococcus aureus (MRSA) nasal swab PCR assay for MRSA pneumonia. Antimicrob Agents Chemother. 2014;58:859-864.
6. Johnson JA, Wright ME, Sheperd LA, et al. Nasal methicillin-resistant Staphylococcus aureus polymerase chain reaction: a potential use in guiding antibiotic therapy for pneumonia. Perm J. 2015;19: 34-36.
7. Dureau AF, Duclos G, Antonini F, et al. Rapid diagnostic test and use of antibiotic against methicillin-resistant Staphylococcus aureus in adult intensive care unit. Eur J Clin Microbiol Infect Dis. 2017;36:267-272.
8. Tilahun B, Faust AC, McCorstin P, Ortegon A. Nasal colonization and lower respiratory tract infections with methicillin-resistant Staphylococcus aureus. Am J Crit Care. 2015;24:8-12.
9. Baby N, Faust AC, Smith T, et al. Nasal methicillin-resistant Staphylococcus aureus (MRSA) PCR testing reduces the duration of MRSA-targeted therapy in patients with suspected MRSA pneumonia. Antimicrob Agents Chemother. 2017;61:e02432-16.
10. Willis C, Allen B, Tucker C, et al. Impact of a pharmacist-driven methicillin-resistant Staphylococcus aureus surveillance protocol. Am J Health-Syst Pharm. 2017;74:1765-1773.
11. Dadzie P, Dietrich T, Ashurst J. Impact of a pharmacist-driven methicillin-resistant Staphylococcus aureus polymerase chain reaction nasal swab protocol on the de-escalation of empiric vancomycin in patients with pneumonia in a rural healthcare setting. Cureus. 2019;11:e6378
12. Dunaway S, Orwig KW, Arbogast ZQ, et al. Evaluation of a pharmacy-driven methicillin-resistant Staphylococcus aureus surveillance protocol in pneumonia. Int J Clin Pharm. 2018;40;526-532.
From the Hospital of Central Connecticut, New Britain, CT (Dr. Caulfield and Dr. Shepard); Hartford Hospital, Hartford, CT (Dr. Linder and Dr. Dempsey); and the Hartford HealthCare Research Program, Hartford, CT (Dr. O’Sullivan).
Abstract
- Objective: To assess the utility of methicillin-resistant Staphylococcus aureus (MRSA) polymerase chain reaction (PCR) nasal swab testing in patients with lower respiratory tract infections (LRTI).
- Design and setting: Multicenter, retrospective, electronic chart review conducted within the Hartford HealthCare system.
- Participants: Patients who were treated for LRTIs at the Hospital of Central Connecticut or Hartford Hospital between July 1, 2018, and June 30, 2019.
- Measurements: The primary outcome was anti-MRSA days of therapy (DOT) in patients who underwent MRSA PCR testing versus those who did not. In a subgroup analysis, we compared anti-MRSA DOT among patients with appropriate versus inappropriate utilization of the MRSA PCR test.
- Results: Of the 319 patients treated for LRTIs, 155 (48.6%) had a MRSA PCR ordered, and appropriate utilization occurred in 94 (60.6%) of these patients. Anti-MRSA DOT in the MRSA PCR group (n = 155) was shorter than in the group that did not undergo MRSA PCR testing (n = 164), but this difference did not reach statistical significance (1.68 days [interquartile range {IQR}, 0.80-2.74] vs 1.86 days [IQR, 0.56-3.33], P = 0.458). In the subgroup analysis, anti-MRSA DOT was significantly shorter in the MRSA PCR group with appropriate utilization compared to the inappropriate utilization group (1.16 [IQR, 0.44-1.88] vs 2.68 [IQR, 1.75-3.76], P < 0.001)
- Conclusion: Appropriate utilization of MRSA PCR nasal swab testing can reduce DOT in patients with LRTI. Further education is necessary to expand the appropriate use of the MRSA PCR test across our health system.
Keywords: MRSA; LRTI; pneumonia; antimicrobial stewardship; antibiotic resistance.
More than 300,000 patients were hospitalized with methicillin-resistant Staphylococcus aureus (MRSA) infections in the United States in 2017, and at least 10,000 of these cases resulted in mortality.1 While MRSA infections overall are decreasing, it is crucial to continue to employ antimicrobial stewardship tactics to keep these infections at bay. Recently, strains of S. aureus have become resistant to vancomycin, making this bacterium even more difficult to treat.2
A novel tactic in antimicrobial stewardship involves the use of MRSA polymerase chain reaction (PCR) nasal swab testing to rule out the presence of MRSA in patients with lower respiratory tract infections (LRTI). If used appropriately, this approach may decrease the number of days patients are treated with anti-MRSA antimicrobials. Waiting for cultures to speciate can take up to 72 hours,3 meaning that patients may be exposed to 3 days of unnecessary broad-spectrum antibiotics. Results of MRSA PCR assay of nasal swab specimens can be available in 1 to 2 hours,4 allowing for more rapid de-escalation of therapy. Numerous studies have shown that this test has a negative predictive value (NPV) greater than 95%, indicating that a negative nasal swab result may be useful to guide de-escalation of antibiotic therapy.5-8 The purpose of this study was to assess the utility of MRSA PCR nasal swab testing in patients with LRTI throughout the Hartford HealthCare system.
Methods
Design
This study was a multicenter, retrospective, electronic chart review. It was approved by the Hartford HealthCare Institutional Review Board (HHC-2019-0169).
Selection of Participants
Patients were identified through electronic medical record reports based on ICD-10 codes. Records were categorized into 2 groups: patients who received a MRSA PCR nasal swab testing and patients who did not. Patients who received the MRSA PCR were further categorized by appropriate or inappropriate utilization. Appropriate utilization of the MRSA PCR was defined as MRSA PCR ordered within 48 hours of a new vancomycin or linezolid order, and anti-MRSA therapy discontinued within 24 hours of a negative result. Inappropriate utilization of the MRSA PCR was defined as MRSA PCR ordered more than 48 hours after a new vancomycin or linezolid order, or continuation of anti-MRSA therapy despite a negative MRSA PCR and no other evidence of a MRSA infection.
Patients were included if they met all of the following criteria: age 18 years or older, with no upper age limit; treated for a LRTI, identified by ICD-10 codes (J13-22, J44, J85); treated with empiric antibiotics active against MRSA, specifically vancomycin or linezolid; and treated at the Hospital of Central Connecticut (HOCC) or Hartford Hospital (HH) between July 1, 2018, and June 30, 2019, inclusive. Patients were excluded if they met 1 or more of the following criteria: age less than 18 years old; admitted for 48 hours or fewer or discharged from the emergency department; not treated at either facility; treated before July 1, 2018, or after June 30, 2019; treated for ventilator-associated pneumonia; received anti-MRSA therapy within 30 days prior to admission; or treated for a concurrent bacterial infection requiring anti-MRSA therapy.
Outcome Measures
The primary outcome was anti-MRSA days of therapy (DOT) in patients who underwent MRSA PCR testing compared to patients who did not undergo MRSA PCR testing. A subgroup analysis was completed to compare anti-MRSA DOT within patients in the MRSA PCR group. Patients in the subgroup were categorized by appropriate or inappropriate utilization, and anti-MRSA DOT were compared between these groups. Secondary outcomes that were evaluated included length of stay (LOS), 30-day readmission rate, and incidence of acute kidney injury (AKI). Thirty-day readmission was defined as admission to HOCC, HH, or any institution within Hartford HealthCare within 30 days of discharge. AKI was defined as an increase in serum creatinine by ≥ 0.3 mg/dL in 48 hours, increase ≥ 1.5 times baseline, or a urine volume < 0.5 mL/kg/hr for 6 hours.
Statistical Analyses
The study was powered for the primary outcome, anti-MRSA DOT, with a clinically meaningful difference of 1 day. Group sample sizes of 240 in the MRSA PCR group and 160 in the no MRSA PCR group would have afforded 92% power to detect that difference, if the null hypothesis was that both group means were 4 days and the alternative hypothesis was that the mean of the MRSA PCR group was 3 days, with estimated group standard deviations of 80% of each mean. This estimate used an alpha level of 0.05 with a 2-sided t-test. Among those who received a MRSA PCR test, a clinically meaningful difference between appropriate and inappropriate utilization was 5%.
Descriptive statistics were provided for all variables as a function of the individual hospital and for the combined data set. Continuous data were summarized with means and standard deviations (SD), or with median and interquartile ranges (IQR), depending on distribution. Categorical variables were reported as frequencies, using percentages. All data were evaluated for normality of distribution. Inferential statistics comprised a Student’s t-test to compare normally distributed, continuous data between groups. Nonparametric distributions were compared using a Mann-Whitney U test. Categorical comparisons were made using a Fisher’s exact test for 2×2 tables and a Pearson chi-square test for comparisons involving more than 2 groups.
Since anti-MRSA DOT (primary outcome) and LOS (secondary outcome) are often non-normally distributed, they have been transformed (eg, log or square root, again depending on distribution). Whichever native variable or transformation variable was appropriate was used as the outcome measure in a linear regression model to account for the influence of factors (covariates) that show significant univariate differences. Given the relatively small sample size, a maximum of 10 variables were included in the model. All factors were iterated in a forward regression model (most influential first) until no significant changes were observed.
All calculations were performed with SPSS v. 21 (IBM; Armonk, NY) using an a priori alpha level of 0.05, such that all results yielding P < 0.05 were deemed statistically significant.
Results
Of the 561 patient records reviewed, 319 patients were included and 242 patients were excluded. Reasons for exclusion included 65 patients admitted for a duration of 48 hours or less or discharged from the emergency department; 61 patients having another infection requiring anti-MRSA therapy; 60 patients not having a diagnosis of a LRTI or not receiving anti-MRSA therapy; 52 patients having received anti-MRSA therapy within 30 days prior to admission; and 4 patients treated outside of the specified date range.
Of the 319 patients included, 155 (48.6%) were in the MRSA PCR group and 164 (51.4%) were in the group that did not undergo MRSA PCR (Table 1). Of the 155 patients with a MRSA PCR ordered, the test was utilized appropriately in 94 (60.6%) patients and inappropriately in 61 (39.4%) patients (Table 2). In the MRSA PCR group, 135 patients had a negative result on PCR assay, with 133 of those patients having negative respiratory cultures, resulting in a NPV of 98.5%. Differences in baseline characteristics between the MRSA PCR and no MRSA PCR groups were observed. The patients in the MRSA PCR group appeared to be significantly more ill than those in the no MRSA PCR group, as indicated by statistically significant differences in intensive care unit (ICU) admissions (P = 0.001), positive chest radiographs (P = 0.034), sepsis at time of anti-MRSA initiation (P = 0.013), pulmonary consults placed (P = 0.003), and carbapenem usage (P = 0.047).

In the subgroup analysis comparing appropriate and inappropriate utilization within the MRSA PCR group, the inappropriate utilization group had significantly higher numbers of infectious diseases consults placed, patients with hospital-acquired pneumonia, and patients with community-acquired pneumonia with risk factors.

Outcomes
Median anti-MRSA DOT in the MRSA PCR group was shorter than DOT in the no MRSA PCR group, but this difference did not reach statistical significance (1.68 [IQR, 0.80-2.74] vs 1.86 days [IQR, 0.56-3.33], P = 0.458; Table 3). LOS in the MRSA PCR group was longer than in the no MRSA PCR group (6.0 [IQR, 4.0-10.0] vs 5.0 [IQR, 3.0-7.0] days, P = 0.001). There was no difference in 30-day readmissions that were related to the previous visit or incidence of AKI between groups.

In the subgroup analysis, anti-MRSA DOT in the MRSA PCR group with appropriate utilization was shorter than DOT in the MRSA PCR group with inappropriate utilization (1.16 [IQR, 0.44-1.88] vs 2.68 [IQR, 1.75-3.76] days, P < 0.001; Table 4). LOS in the MRSA PCR group with appropriate utilization was shorter than LOS in the inappropriate utilization group (5.0 [IQR, 4.0-7.0] vs 7.0 [IQR, 5.0-12.0] days, P < 0.001). Thirty-day readmissions that were related to the previous visit were significantly higher in patients in the MRSA PCR group with appropriate utilization (13 vs 2, P = 0.030). There was no difference in incidence of AKI between the groups.

A multivariate analysis was completed to determine whether the sicker MRSA PCR population was confounding outcomes, particularly the secondary outcome of LOS, which was noted to be longer in the MRSA PCR group (Table 5). When comparing LOS in the MRSA PCR and the no MRSA PCR patients, the multivariate analysis showed that admission to the ICU and carbapenem use were associated with a longer LOS (P < 0.001 and P = 0.009, respectively). The incidence of admission to the ICU and carbapenem use were higher in the MRSA PCR group (P = 0.001 and P = 0.047). Therefore, longer LOS in the MRSA PCR patients could be a result of the higher prevalence of ICU admissions and infections requiring carbapenem therapy rather than the result of the MRSA PCR itself.

Discussion
A MRSA PCR nasal swab protocol can be used to minimize a patient’s exposure to unnecessary broad-spectrum antibiotics, thereby preventing antimicrobial resistance. Thus, it is important to assess how our health system is utilizing this antimicrobial stewardship tactic. With the MRSA PCR’s high NPV, providers can be confident that MRSA pneumonia is unlikely in the absence of MRSA colonization. Our study established a NPV of 98.5%, which is similar to other studies, all of which have shown NPVs greater than 95%.5-8 Despite the high NPV, this study demonstrated that only 51.4% of patients with LRTI had orders for a MRSA PCR. Of the 155 patients with a MRSA PCR, the test was utilized appropriately only 60.6% of the time. A majority of the inappropriately utilized tests were due to MRSA PCR orders placed more than 48 hours after anti-MRSA therapy initiation. To our knowledge, no other studies have assessed the clinical utility of MRSA PCR nasal swabs as an antimicrobial stewardship tool in a diverse health system; therefore, these results are useful to guide future practices at our institution. There is a clear need for provider and pharmacist education to increase the use of MRSA PCR nasal swab testing for patients with LRTI being treated with anti-MRSA therapy. Additionally, clinician education regarding the initial timing of the MRSA PCR order and the proper utilization of the results of the MRSA PCR likely will benefit patient outcomes at our institution.
When evaluating anti-MRSA DOT, this study demonstrated a reduction of only 0.18 days (about 4 hours) of anti-MRSA therapy in the patients who received MRSA PCR testing compared to the patients without a MRSA PCR ordered. Our anti-MRSA DOT reduction was lower than what has been reported in similar studies. For example, Baby et al found that the use of the MRSA PCR was associated with 46.6 fewer hours of unnecessary antimicrobial treatment. Willis et al evaluated a pharmacist-driven protocol that resulted in a reduction of 1.8 days of anti-MRSA therapy, despite a protocol compliance rate of only 55%.9,10 In our study, the patients in the MRSA PCR group appeared to be significantly more ill than those in the no MRSA PCR group, which may be the reason for the incongruences in our results compared to the current literature. Characteristics such as ICU admissions, positive chest radiographs, sepsis cases, pulmonary consults, and carbapenem usage—all of which are indicative of a sicker population—were more prevalent in the MRSA PCR group. This sicker population could have underestimated the reduction of DOT in the MRSA PCR group compared to the no MRSA PCR group.
After isolating the MRSA PCR patients in the subgroup analysis, anti-MRSA DOT was 1.5 days shorter when the test was appropriately utilized, which is more comparable to what has been reported in the literature.9,10 Only 60.6% of the MRSA PCR patients had their anti-MRSA therapy appropriately managed based on the MRSA PCR. Interestingly, a majority of patients in the inappropriate utilization group had MRSA PCR tests ordered more than 48 hours after beginning anti-MRSA therapy. More prompt and efficient ordering of the MRSA PCR may have resulted in more opportunities for earlier de-escalation of therapy. Due to these factors, the patients in the inappropriate utilization group could have further contributed to the underestimated difference in anti-MRSA DOT between the MRSA PCR and no MRSA PCR patients in the primary outcome. Additionally, there were no notable differences between the appropriate and inappropriate utilization groups, unlike in the MRSA PCR and no MRSA PCR groups, which is why we were able to draw more robust conclusions in the subgroup analysis. Therefore, the subgroup analysis confirmed that if the results of the MRSA PCR are used appropriately to guide anti-MRSA therapy, patients can potentially avoid 36 hours of broad-spectrum antibiotics.
Data on how the utilization of the MRSA PCR nasal swab can affect LOS are limited; however, one study did report a 2.8-day reduction in LOS after implementation of a pharmacist-driven MRSA PCR nasal swab protocol.11 Our study demonstrated that LOS was significantly longer in the MRSA PCR group than in the no MRSA PCR group. This result was likely affected by the aforementioned sicker MRSA PCR population. Our multivariate analysis further confirmed that ICU admissions were associated with a longer LOS, and, given that the MRSA PCR group had a significantly higher ICU population, this likely confounded these results. If our 2 groups had had more evenly distributed characteristics, it is possible that we could have found a shorter LOS in the MRSA PCR group, similar to what is reported in the literature. In the subgroup analysis, LOS was 2 days shorter in the appropriate utilization group compared to the inappropriate utilization group. This further affirms that the results of the MRSA PCR must be used appropriately in order for patient outcomes, like LOS, to benefit.
The effects of the MRSA PCR nasal swab on 30-day readmission rates and incidence of AKI are not well-documented in the literature. One study did report 30-day readmission rates as an outcome, but did not cite any difference after the implementation of a protocol that utilized MRSA PCR nasal swab testing.12 The outcome of AKI is slightly better represented in the literature, but the results are conflicting. Some studies report no difference after the implementation of a MRSA PCR-based protocol,11 and others report a significant decrease in AKI with the use of the MRSA PCR.9 Our study detected no difference in 30-day readmission rates related to the previous admission or in AKI between the MRSA PCR and no MRSA PCR populations. In the subgroup analysis, 30-day readmission rates were significantly higher in the MRSA PCR group with appropriate utilization than in the group with inappropriate utilization; however, our study was not powered to detect a difference in this secondary outcome.
This study had some limitations that may have affected our results. First, this study was a retrospective chart review. Additionally, the baseline characteristics were not well balanced across the different groups. There were sicker patients in the MRSA PCR group, which may have led to an underestimate of the reduction in DOT and LOS in these patients. Finally, we did not include enough patient records to reach power in the MRSA PCR group due to a higher than expected number of patients meeting exclusion criteria. Had we attained sufficient power, there may have been more profound reductions in DOT and LOS.
Conclusion
MRSA infections are a common cause for hospitalization, and there is a growing need for antimicrobial stewardship efforts to limit unnecessary antibiotic usage in order to prevent resistance. As illustrated in our study, appropriate utilization of the MRSA PCR can reduce DOT up to 1.5 days. However, our results suggest that there is room for provider and pharmacist education to increase the use of MRSA PCR nasal swab testing in patients with LRTI receiving anti-MRSA therapy. Further emphasis on the appropriate utilization of the MRSA PCR within our health care system is essential.
Corresponding author: Casey Dempsey, PharmD, BCIDP, 80 Seymour St., Hartford, CT 06106; [email protected].
Financial disclosures: None.
From the Hospital of Central Connecticut, New Britain, CT (Dr. Caulfield and Dr. Shepard); Hartford Hospital, Hartford, CT (Dr. Linder and Dr. Dempsey); and the Hartford HealthCare Research Program, Hartford, CT (Dr. O’Sullivan).
Abstract
- Objective: To assess the utility of methicillin-resistant Staphylococcus aureus (MRSA) polymerase chain reaction (PCR) nasal swab testing in patients with lower respiratory tract infections (LRTI).
- Design and setting: Multicenter, retrospective, electronic chart review conducted within the Hartford HealthCare system.
- Participants: Patients who were treated for LRTIs at the Hospital of Central Connecticut or Hartford Hospital between July 1, 2018, and June 30, 2019.
- Measurements: The primary outcome was anti-MRSA days of therapy (DOT) in patients who underwent MRSA PCR testing versus those who did not. In a subgroup analysis, we compared anti-MRSA DOT among patients with appropriate versus inappropriate utilization of the MRSA PCR test.
- Results: Of the 319 patients treated for LRTIs, 155 (48.6%) had a MRSA PCR ordered, and appropriate utilization occurred in 94 (60.6%) of these patients. Anti-MRSA DOT in the MRSA PCR group (n = 155) was shorter than in the group that did not undergo MRSA PCR testing (n = 164), but this difference did not reach statistical significance (1.68 days [interquartile range {IQR}, 0.80-2.74] vs 1.86 days [IQR, 0.56-3.33], P = 0.458). In the subgroup analysis, anti-MRSA DOT was significantly shorter in the MRSA PCR group with appropriate utilization compared to the inappropriate utilization group (1.16 [IQR, 0.44-1.88] vs 2.68 [IQR, 1.75-3.76], P < 0.001)
- Conclusion: Appropriate utilization of MRSA PCR nasal swab testing can reduce DOT in patients with LRTI. Further education is necessary to expand the appropriate use of the MRSA PCR test across our health system.
Keywords: MRSA; LRTI; pneumonia; antimicrobial stewardship; antibiotic resistance.
More than 300,000 patients were hospitalized with methicillin-resistant Staphylococcus aureus (MRSA) infections in the United States in 2017, and at least 10,000 of these cases resulted in mortality.1 While MRSA infections overall are decreasing, it is crucial to continue to employ antimicrobial stewardship tactics to keep these infections at bay. Recently, strains of S. aureus have become resistant to vancomycin, making this bacterium even more difficult to treat.2
A novel tactic in antimicrobial stewardship involves the use of MRSA polymerase chain reaction (PCR) nasal swab testing to rule out the presence of MRSA in patients with lower respiratory tract infections (LRTI). If used appropriately, this approach may decrease the number of days patients are treated with anti-MRSA antimicrobials. Waiting for cultures to speciate can take up to 72 hours,3 meaning that patients may be exposed to 3 days of unnecessary broad-spectrum antibiotics. Results of MRSA PCR assay of nasal swab specimens can be available in 1 to 2 hours,4 allowing for more rapid de-escalation of therapy. Numerous studies have shown that this test has a negative predictive value (NPV) greater than 95%, indicating that a negative nasal swab result may be useful to guide de-escalation of antibiotic therapy.5-8 The purpose of this study was to assess the utility of MRSA PCR nasal swab testing in patients with LRTI throughout the Hartford HealthCare system.
Methods
Design
This study was a multicenter, retrospective, electronic chart review. It was approved by the Hartford HealthCare Institutional Review Board (HHC-2019-0169).
Selection of Participants
Patients were identified through electronic medical record reports based on ICD-10 codes. Records were categorized into 2 groups: patients who received a MRSA PCR nasal swab testing and patients who did not. Patients who received the MRSA PCR were further categorized by appropriate or inappropriate utilization. Appropriate utilization of the MRSA PCR was defined as MRSA PCR ordered within 48 hours of a new vancomycin or linezolid order, and anti-MRSA therapy discontinued within 24 hours of a negative result. Inappropriate utilization of the MRSA PCR was defined as MRSA PCR ordered more than 48 hours after a new vancomycin or linezolid order, or continuation of anti-MRSA therapy despite a negative MRSA PCR and no other evidence of a MRSA infection.
Patients were included if they met all of the following criteria: age 18 years or older, with no upper age limit; treated for a LRTI, identified by ICD-10 codes (J13-22, J44, J85); treated with empiric antibiotics active against MRSA, specifically vancomycin or linezolid; and treated at the Hospital of Central Connecticut (HOCC) or Hartford Hospital (HH) between July 1, 2018, and June 30, 2019, inclusive. Patients were excluded if they met 1 or more of the following criteria: age less than 18 years old; admitted for 48 hours or fewer or discharged from the emergency department; not treated at either facility; treated before July 1, 2018, or after June 30, 2019; treated for ventilator-associated pneumonia; received anti-MRSA therapy within 30 days prior to admission; or treated for a concurrent bacterial infection requiring anti-MRSA therapy.
Outcome Measures
The primary outcome was anti-MRSA days of therapy (DOT) in patients who underwent MRSA PCR testing compared to patients who did not undergo MRSA PCR testing. A subgroup analysis was completed to compare anti-MRSA DOT within patients in the MRSA PCR group. Patients in the subgroup were categorized by appropriate or inappropriate utilization, and anti-MRSA DOT were compared between these groups. Secondary outcomes that were evaluated included length of stay (LOS), 30-day readmission rate, and incidence of acute kidney injury (AKI). Thirty-day readmission was defined as admission to HOCC, HH, or any institution within Hartford HealthCare within 30 days of discharge. AKI was defined as an increase in serum creatinine by ≥ 0.3 mg/dL in 48 hours, increase ≥ 1.5 times baseline, or a urine volume < 0.5 mL/kg/hr for 6 hours.
Statistical Analyses
The study was powered for the primary outcome, anti-MRSA DOT, with a clinically meaningful difference of 1 day. Group sample sizes of 240 in the MRSA PCR group and 160 in the no MRSA PCR group would have afforded 92% power to detect that difference, if the null hypothesis was that both group means were 4 days and the alternative hypothesis was that the mean of the MRSA PCR group was 3 days, with estimated group standard deviations of 80% of each mean. This estimate used an alpha level of 0.05 with a 2-sided t-test. Among those who received a MRSA PCR test, a clinically meaningful difference between appropriate and inappropriate utilization was 5%.
Descriptive statistics were provided for all variables as a function of the individual hospital and for the combined data set. Continuous data were summarized with means and standard deviations (SD), or with median and interquartile ranges (IQR), depending on distribution. Categorical variables were reported as frequencies, using percentages. All data were evaluated for normality of distribution. Inferential statistics comprised a Student’s t-test to compare normally distributed, continuous data between groups. Nonparametric distributions were compared using a Mann-Whitney U test. Categorical comparisons were made using a Fisher’s exact test for 2×2 tables and a Pearson chi-square test for comparisons involving more than 2 groups.
Since anti-MRSA DOT (primary outcome) and LOS (secondary outcome) are often non-normally distributed, they have been transformed (eg, log or square root, again depending on distribution). Whichever native variable or transformation variable was appropriate was used as the outcome measure in a linear regression model to account for the influence of factors (covariates) that show significant univariate differences. Given the relatively small sample size, a maximum of 10 variables were included in the model. All factors were iterated in a forward regression model (most influential first) until no significant changes were observed.
All calculations were performed with SPSS v. 21 (IBM; Armonk, NY) using an a priori alpha level of 0.05, such that all results yielding P < 0.05 were deemed statistically significant.
Results
Of the 561 patient records reviewed, 319 patients were included and 242 patients were excluded. Reasons for exclusion included 65 patients admitted for a duration of 48 hours or less or discharged from the emergency department; 61 patients having another infection requiring anti-MRSA therapy; 60 patients not having a diagnosis of a LRTI or not receiving anti-MRSA therapy; 52 patients having received anti-MRSA therapy within 30 days prior to admission; and 4 patients treated outside of the specified date range.
Of the 319 patients included, 155 (48.6%) were in the MRSA PCR group and 164 (51.4%) were in the group that did not undergo MRSA PCR (Table 1). Of the 155 patients with a MRSA PCR ordered, the test was utilized appropriately in 94 (60.6%) patients and inappropriately in 61 (39.4%) patients (Table 2). In the MRSA PCR group, 135 patients had a negative result on PCR assay, with 133 of those patients having negative respiratory cultures, resulting in a NPV of 98.5%. Differences in baseline characteristics between the MRSA PCR and no MRSA PCR groups were observed. The patients in the MRSA PCR group appeared to be significantly more ill than those in the no MRSA PCR group, as indicated by statistically significant differences in intensive care unit (ICU) admissions (P = 0.001), positive chest radiographs (P = 0.034), sepsis at time of anti-MRSA initiation (P = 0.013), pulmonary consults placed (P = 0.003), and carbapenem usage (P = 0.047).

In the subgroup analysis comparing appropriate and inappropriate utilization within the MRSA PCR group, the inappropriate utilization group had significantly higher numbers of infectious diseases consults placed, patients with hospital-acquired pneumonia, and patients with community-acquired pneumonia with risk factors.

Outcomes
Median anti-MRSA DOT in the MRSA PCR group was shorter than DOT in the no MRSA PCR group, but this difference did not reach statistical significance (1.68 [IQR, 0.80-2.74] vs 1.86 days [IQR, 0.56-3.33], P = 0.458; Table 3). LOS in the MRSA PCR group was longer than in the no MRSA PCR group (6.0 [IQR, 4.0-10.0] vs 5.0 [IQR, 3.0-7.0] days, P = 0.001). There was no difference in 30-day readmissions that were related to the previous visit or incidence of AKI between groups.

In the subgroup analysis, anti-MRSA DOT in the MRSA PCR group with appropriate utilization was shorter than DOT in the MRSA PCR group with inappropriate utilization (1.16 [IQR, 0.44-1.88] vs 2.68 [IQR, 1.75-3.76] days, P < 0.001; Table 4). LOS in the MRSA PCR group with appropriate utilization was shorter than LOS in the inappropriate utilization group (5.0 [IQR, 4.0-7.0] vs 7.0 [IQR, 5.0-12.0] days, P < 0.001). Thirty-day readmissions that were related to the previous visit were significantly higher in patients in the MRSA PCR group with appropriate utilization (13 vs 2, P = 0.030). There was no difference in incidence of AKI between the groups.

A multivariate analysis was completed to determine whether the sicker MRSA PCR population was confounding outcomes, particularly the secondary outcome of LOS, which was noted to be longer in the MRSA PCR group (Table 5). When comparing LOS in the MRSA PCR and the no MRSA PCR patients, the multivariate analysis showed that admission to the ICU and carbapenem use were associated with a longer LOS (P < 0.001 and P = 0.009, respectively). The incidence of admission to the ICU and carbapenem use were higher in the MRSA PCR group (P = 0.001 and P = 0.047). Therefore, longer LOS in the MRSA PCR patients could be a result of the higher prevalence of ICU admissions and infections requiring carbapenem therapy rather than the result of the MRSA PCR itself.

Discussion
A MRSA PCR nasal swab protocol can be used to minimize a patient’s exposure to unnecessary broad-spectrum antibiotics, thereby preventing antimicrobial resistance. Thus, it is important to assess how our health system is utilizing this antimicrobial stewardship tactic. With the MRSA PCR’s high NPV, providers can be confident that MRSA pneumonia is unlikely in the absence of MRSA colonization. Our study established a NPV of 98.5%, which is similar to other studies, all of which have shown NPVs greater than 95%.5-8 Despite the high NPV, this study demonstrated that only 51.4% of patients with LRTI had orders for a MRSA PCR. Of the 155 patients with a MRSA PCR, the test was utilized appropriately only 60.6% of the time. A majority of the inappropriately utilized tests were due to MRSA PCR orders placed more than 48 hours after anti-MRSA therapy initiation. To our knowledge, no other studies have assessed the clinical utility of MRSA PCR nasal swabs as an antimicrobial stewardship tool in a diverse health system; therefore, these results are useful to guide future practices at our institution. There is a clear need for provider and pharmacist education to increase the use of MRSA PCR nasal swab testing for patients with LRTI being treated with anti-MRSA therapy. Additionally, clinician education regarding the initial timing of the MRSA PCR order and the proper utilization of the results of the MRSA PCR likely will benefit patient outcomes at our institution.
When evaluating anti-MRSA DOT, this study demonstrated a reduction of only 0.18 days (about 4 hours) of anti-MRSA therapy in the patients who received MRSA PCR testing compared to the patients without a MRSA PCR ordered. Our anti-MRSA DOT reduction was lower than what has been reported in similar studies. For example, Baby et al found that the use of the MRSA PCR was associated with 46.6 fewer hours of unnecessary antimicrobial treatment. Willis et al evaluated a pharmacist-driven protocol that resulted in a reduction of 1.8 days of anti-MRSA therapy, despite a protocol compliance rate of only 55%.9,10 In our study, the patients in the MRSA PCR group appeared to be significantly more ill than those in the no MRSA PCR group, which may be the reason for the incongruences in our results compared to the current literature. Characteristics such as ICU admissions, positive chest radiographs, sepsis cases, pulmonary consults, and carbapenem usage—all of which are indicative of a sicker population—were more prevalent in the MRSA PCR group. This sicker population could have underestimated the reduction of DOT in the MRSA PCR group compared to the no MRSA PCR group.
After isolating the MRSA PCR patients in the subgroup analysis, anti-MRSA DOT was 1.5 days shorter when the test was appropriately utilized, which is more comparable to what has been reported in the literature.9,10 Only 60.6% of the MRSA PCR patients had their anti-MRSA therapy appropriately managed based on the MRSA PCR. Interestingly, a majority of patients in the inappropriate utilization group had MRSA PCR tests ordered more than 48 hours after beginning anti-MRSA therapy. More prompt and efficient ordering of the MRSA PCR may have resulted in more opportunities for earlier de-escalation of therapy. Due to these factors, the patients in the inappropriate utilization group could have further contributed to the underestimated difference in anti-MRSA DOT between the MRSA PCR and no MRSA PCR patients in the primary outcome. Additionally, there were no notable differences between the appropriate and inappropriate utilization groups, unlike in the MRSA PCR and no MRSA PCR groups, which is why we were able to draw more robust conclusions in the subgroup analysis. Therefore, the subgroup analysis confirmed that if the results of the MRSA PCR are used appropriately to guide anti-MRSA therapy, patients can potentially avoid 36 hours of broad-spectrum antibiotics.
Data on how the utilization of the MRSA PCR nasal swab can affect LOS are limited; however, one study did report a 2.8-day reduction in LOS after implementation of a pharmacist-driven MRSA PCR nasal swab protocol.11 Our study demonstrated that LOS was significantly longer in the MRSA PCR group than in the no MRSA PCR group. This result was likely affected by the aforementioned sicker MRSA PCR population. Our multivariate analysis further confirmed that ICU admissions were associated with a longer LOS, and, given that the MRSA PCR group had a significantly higher ICU population, this likely confounded these results. If our 2 groups had had more evenly distributed characteristics, it is possible that we could have found a shorter LOS in the MRSA PCR group, similar to what is reported in the literature. In the subgroup analysis, LOS was 2 days shorter in the appropriate utilization group compared to the inappropriate utilization group. This further affirms that the results of the MRSA PCR must be used appropriately in order for patient outcomes, like LOS, to benefit.
The effects of the MRSA PCR nasal swab on 30-day readmission rates and incidence of AKI are not well-documented in the literature. One study did report 30-day readmission rates as an outcome, but did not cite any difference after the implementation of a protocol that utilized MRSA PCR nasal swab testing.12 The outcome of AKI is slightly better represented in the literature, but the results are conflicting. Some studies report no difference after the implementation of a MRSA PCR-based protocol,11 and others report a significant decrease in AKI with the use of the MRSA PCR.9 Our study detected no difference in 30-day readmission rates related to the previous admission or in AKI between the MRSA PCR and no MRSA PCR populations. In the subgroup analysis, 30-day readmission rates were significantly higher in the MRSA PCR group with appropriate utilization than in the group with inappropriate utilization; however, our study was not powered to detect a difference in this secondary outcome.
This study had some limitations that may have affected our results. First, this study was a retrospective chart review. Additionally, the baseline characteristics were not well balanced across the different groups. There were sicker patients in the MRSA PCR group, which may have led to an underestimate of the reduction in DOT and LOS in these patients. Finally, we did not include enough patient records to reach power in the MRSA PCR group due to a higher than expected number of patients meeting exclusion criteria. Had we attained sufficient power, there may have been more profound reductions in DOT and LOS.
Conclusion
MRSA infections are a common cause for hospitalization, and there is a growing need for antimicrobial stewardship efforts to limit unnecessary antibiotic usage in order to prevent resistance. As illustrated in our study, appropriate utilization of the MRSA PCR can reduce DOT up to 1.5 days. However, our results suggest that there is room for provider and pharmacist education to increase the use of MRSA PCR nasal swab testing in patients with LRTI receiving anti-MRSA therapy. Further emphasis on the appropriate utilization of the MRSA PCR within our health care system is essential.
Corresponding author: Casey Dempsey, PharmD, BCIDP, 80 Seymour St., Hartford, CT 06106; [email protected].
Financial disclosures: None.
1. Antimicrobial resistance threats. Centers for Disease Control and Prevention web site. www.cdc.gov/drugresistance/biggest-threats.html. Accessed September 9, 2020.
2. Biggest threats and data. Centers for Disease Control and Prevention web site. www.cdc.gov/drugresistance/biggest_threats.html#mrsa. Accessed September 9, 2020.
3. Smith MN, Erdman MJ, Ferreira JA, et al. Clinical utility of methicillin-resistant Staphylococcus aureus nasal polymerase chain reaction assay in critically ill patients with nosocomial pneumonia. J Crit Care. 2017;38:168-171.
4. Giancola SE, Nguyen AT, Le B, et al. Clinical utility of a nasal swab methicillin-resistant Staphylococcus aureus polymerase chain reaction test in intensive and intermediate care unit patients with pneumonia. Diagn Microbiol Infect Dis. 2016;86:307-310.
5. Dangerfield B, Chung A, Webb B, Seville MT. Predictive value of methicillin-resistant Staphylococcus aureus (MRSA) nasal swab PCR assay for MRSA pneumonia. Antimicrob Agents Chemother. 2014;58:859-864.
6. Johnson JA, Wright ME, Sheperd LA, et al. Nasal methicillin-resistant Staphylococcus aureus polymerase chain reaction: a potential use in guiding antibiotic therapy for pneumonia. Perm J. 2015;19: 34-36.
7. Dureau AF, Duclos G, Antonini F, et al. Rapid diagnostic test and use of antibiotic against methicillin-resistant Staphylococcus aureus in adult intensive care unit. Eur J Clin Microbiol Infect Dis. 2017;36:267-272.
8. Tilahun B, Faust AC, McCorstin P, Ortegon A. Nasal colonization and lower respiratory tract infections with methicillin-resistant Staphylococcus aureus. Am J Crit Care. 2015;24:8-12.
9. Baby N, Faust AC, Smith T, et al. Nasal methicillin-resistant Staphylococcus aureus (MRSA) PCR testing reduces the duration of MRSA-targeted therapy in patients with suspected MRSA pneumonia. Antimicrob Agents Chemother. 2017;61:e02432-16.
10. Willis C, Allen B, Tucker C, et al. Impact of a pharmacist-driven methicillin-resistant Staphylococcus aureus surveillance protocol. Am J Health-Syst Pharm. 2017;74:1765-1773.
11. Dadzie P, Dietrich T, Ashurst J. Impact of a pharmacist-driven methicillin-resistant Staphylococcus aureus polymerase chain reaction nasal swab protocol on the de-escalation of empiric vancomycin in patients with pneumonia in a rural healthcare setting. Cureus. 2019;11:e6378
12. Dunaway S, Orwig KW, Arbogast ZQ, et al. Evaluation of a pharmacy-driven methicillin-resistant Staphylococcus aureus surveillance protocol in pneumonia. Int J Clin Pharm. 2018;40;526-532.
1. Antimicrobial resistance threats. Centers for Disease Control and Prevention web site. www.cdc.gov/drugresistance/biggest-threats.html. Accessed September 9, 2020.
2. Biggest threats and data. Centers for Disease Control and Prevention web site. www.cdc.gov/drugresistance/biggest_threats.html#mrsa. Accessed September 9, 2020.
3. Smith MN, Erdman MJ, Ferreira JA, et al. Clinical utility of methicillin-resistant Staphylococcus aureus nasal polymerase chain reaction assay in critically ill patients with nosocomial pneumonia. J Crit Care. 2017;38:168-171.
4. Giancola SE, Nguyen AT, Le B, et al. Clinical utility of a nasal swab methicillin-resistant Staphylococcus aureus polymerase chain reaction test in intensive and intermediate care unit patients with pneumonia. Diagn Microbiol Infect Dis. 2016;86:307-310.
5. Dangerfield B, Chung A, Webb B, Seville MT. Predictive value of methicillin-resistant Staphylococcus aureus (MRSA) nasal swab PCR assay for MRSA pneumonia. Antimicrob Agents Chemother. 2014;58:859-864.
6. Johnson JA, Wright ME, Sheperd LA, et al. Nasal methicillin-resistant Staphylococcus aureus polymerase chain reaction: a potential use in guiding antibiotic therapy for pneumonia. Perm J. 2015;19: 34-36.
7. Dureau AF, Duclos G, Antonini F, et al. Rapid diagnostic test and use of antibiotic against methicillin-resistant Staphylococcus aureus in adult intensive care unit. Eur J Clin Microbiol Infect Dis. 2017;36:267-272.
8. Tilahun B, Faust AC, McCorstin P, Ortegon A. Nasal colonization and lower respiratory tract infections with methicillin-resistant Staphylococcus aureus. Am J Crit Care. 2015;24:8-12.
9. Baby N, Faust AC, Smith T, et al. Nasal methicillin-resistant Staphylococcus aureus (MRSA) PCR testing reduces the duration of MRSA-targeted therapy in patients with suspected MRSA pneumonia. Antimicrob Agents Chemother. 2017;61:e02432-16.
10. Willis C, Allen B, Tucker C, et al. Impact of a pharmacist-driven methicillin-resistant Staphylococcus aureus surveillance protocol. Am J Health-Syst Pharm. 2017;74:1765-1773.
11. Dadzie P, Dietrich T, Ashurst J. Impact of a pharmacist-driven methicillin-resistant Staphylococcus aureus polymerase chain reaction nasal swab protocol on the de-escalation of empiric vancomycin in patients with pneumonia in a rural healthcare setting. Cureus. 2019;11:e6378
12. Dunaway S, Orwig KW, Arbogast ZQ, et al. Evaluation of a pharmacy-driven methicillin-resistant Staphylococcus aureus surveillance protocol in pneumonia. Int J Clin Pharm. 2018;40;526-532.
Effect of a Smartphone App Plus an Accelerometer on Physical Activity and Functional Recovery During Hospitalization After Orthopedic Surgery
Study Overview
Objective. To investigate the potential of Hospital Fit (a smartphone application with an accelerometer) to enhance physical activity levels and functional recovery following orthopedic surgery.
Design. Nonrandomized, quasi-experimental pilot study.
Settings and participants. Patients scheduled for an elective total knee arthroplasty (TKA) or total hip arthroplasty (THA) at the orthopedic ward of Maastricht University Medical Center in Maastricht, the Netherlands, were invited to participate. Patients scheduled for surgery between January 2017 and December 2018 were recruited for the control group at a rate of 1 patient per week (due to a limited number of accelerometers available). After development of Hospital Fit was completed in December 2018 (and sufficient accelerators had become available), patients scheduled for surgery between February 2019 and May 2019 were recruited for the intervention group. The ratio of patients included in the control and intervention group was set at 2:1, respectively.
At preoperative physiotherapy screenings (scheduled 6 weeks before surgery), patients received verbal and written information about the study. Patients were eligible if they met the following inclusion criteria: receiving physiotherapy after elective TKA or THA; able to walk independently 2 weeks prior to surgery, as scored on the Functional Ambulation Categories (FAC > 3); were expected to be discharged to their own home; were aged 18 years and older; and had a sufficient understanding of the Dutch language. Exclusion criteria were: the presence of contraindications to walking or wearing an accelerometer on the upper leg; admission to the intensive care unit; impaired cognition (delirium/dementia), as reported by the attending doctor; a life expectancy of less than 3 months; and previous participation in this study. Patients were contacted on the day of their surgery, and written informed consent was obtained prior to the initiation of any study activities.
Intervention. Once enrolled, all patients followed a standardized clinical care pathway for TKA or THA (see original article for additional details). Postoperative physiotherapy was administered to all participating patients, starting within 4 hours after surgery. The physiotherapy treatment was aimed at increasing physical activity levels and enhancing functional recovery. Control group patients only received physiotherapy (twice daily, 30 minutes per session) and had their physical activity levels monitored with an accelerometer, without receiving feedback, until functional recovery was achieved, as measured with the modified Iowa Level of Assistance Scale (mILAS). Intervention group patients used Hospital Fit in addition to physiotherapy. Hospital Fit consists of a smartphone-based app, connected to a MOX activity monitor via Bluetooth (device contains a tri-axial accelerometer sensor in a small waterproof housing attached to the upper leg). Hospital Fit enables objective activity monitoring, provides patients and their physiotherapists insights and real-time feedback on the number of minutes spent standing and walking per day, and offers a tailored exercise program supported by videos aimed at stimulating self-management.
Measures. The primary outcome measure was the time spent physically active (total number of minutes standing and walking) per day until discharge. Physical activity was monitored 24 hours a day; days with ≥ 20 hours of wear time were considered valid measurement days and were included in the analysis. After the last treatment session, the accelerometer was removed, and the raw tri-axial accelerometer data were uploaded and processed to classify minutes as “active” (standing and walking) or “sedentary” (lying and sitting). The secondary outcome measures were the achievement of functional recovery on postoperative day 1 (POD1). Functional recovery was assessed by the physiotherapist during each treatment session using the mILAS and was reported in the electronic health record. In the intervention group, it was also reported in the app. The achievement of functional recovery on POD1 was defined as having reached a total mILAS-score of 0 on or before POD1, using a dichotomized outcome (0 = mILAS = 0 > POD1; 1 = mILAS = 0 ≤ POD1).
The independent variables measured were: Hospital Fit use (control versus the intervention group), age, sex, body mass index (BMI), type of surgery (TKA or THA), and comorbidities assessed by the American Society of Anesthesiologists (ASA) classification (ASA class ≤ 2 versus ASA class = 3; a higher score indicates being less fit for surgery). The medical and demographic data measured were the type of walking aid used and length of stay, with the day of surgery being defined as day 1.
Analysis. Data analysis was performed according to the intention-to-treat principle. Missing values were not substituted; drop-outs were not replaced. Descriptive statistics were presented as means (SD) or as 95% confidence intervals (CI) for continuous variables. The median and interquartile ranges (IQR) were used to present non-normally distributed data. The frequencies and percentages were used to present categorical variables. A multiple linear regression analysis was performed to determine the association between the time spent physically active per day and Hospital Fit use, corrected for potential confounding factors (age, sex, BMI, ASA class, and type of surgery). A multiple logistic regression analysis was performed additionally to determine the association between the achievement of functional recovery on POD1 and Hospital Fit use, corrected for potential confounding factors. For all statistical analyses, the level of significance was set at P < 0.05. All statistical analyses were performed using SPSS (version 23.0.0.2; IBM Corporation, Armonk, NY).
Main results. Ninety-seven patients were recruited; after excluding 9 patients because of missing data, 88 were included for analysis, with 61 (69%) in the control group and 27 (31%) in the intervention group. A median (IQR) number of 1.00 (0) valid measurement days (≥ 20 hr wear time) was collected. Physical activity data for 84 patients (95%) was available on POD1 (n = 61 control group, n = 23 intervention group). On postoperative day 2 (POD2), the majority of patients were discharged (n = 61, 69%), and data for only 23 patients (26%) were available (n = 17 control, n = 6 intervention). From postoperative day 3 to day 7, data of valid measurement days were available for just 1 patient (intervention group). Due to the large reduction in valid measurement days from POD2 onward, data from these days were not included in the analysis.
Results of the multiple linear regression analysis showed that, corrected for age, patients who used Hospital Fit stood and walked an average of 28.43 minutes (95% CI, 5.55-51.32) more on POD1 than patients who did not use Hospital Fit. Also, the model showed that an increase in age led to a decrease in the number of minutes standing and walking on POD1. The results of the multiple logistic regression analysis also showed that, corrected for ASA class, the odds of achieving functional recovery on POD1 were 3.08 times higher (95% CI, 1.14-8.31) for patients who used Hospital Fit compared to patients who did not use Hospital Fit. Including ASA class in the model shows that a lower ASA class increased the odds ratio for a functional recovery on POD1.
Conclusion. A smartphone app combined with an accelerometer demonstrates the potential to enhance patients’ physical activity levels and functional recovery during hospitalization following joint replacement surgery.
Commentary
Although the beneficial effects of physical activity during hospitalization after surgery are well documented, patients continue to spend between 92% and 96% of their time lying or sitting.1-3 Therefore, strategies aimed at increasing the amount of time spent standing and walking are needed. Postoperative physiotherapy aims to enhance physical activity levels and functional recovery of activities of daily living, which are essential to function independently at home.4-7 Physiotherapists may be able to advise patients more effectively on their physical activity behavior if continuous physical activity monitoring with real-time feedback is implemented in standard care. Although mobile health (mHealth) tools are being used to monitor physical activity in support of outpatient physiotherapy within the orthopedic rehabilitation pathway,8-10 there is currently no mHealth tool available that offers hospitalized patients and their physiotherapists essential strategies to enhance their physical activity levels and support their recovery process. In addition, because hospitalized patients frequently use walking aids and often have impaired gait, the algorithm of most available activity monitors is not validated for use in this population.
This study, therefore, is an important contribution to the literature, as it describes a preliminary evaluation of a novel mHealth tool—Hospital Fit—consisting of a smartphone application connected to an accelerometer whose algorithm has been validated to differentiate between lying/sitting and standing/walking among hospitalized patients. Briefly, results from this study showed an increase in the time spent standing and walking, as well as higher odds of functional recovery on POD1 from the introduction of Hospital Fit. While guidelines on the recommended amount of physical activity during hospitalization do not yet exist, an average improvement of 28 minutes (39%) standing and walking on POD1 can be considered a clinically relevant contribution to prevent the negative effects of inactivity.
This study has limitations, particularly related to the study design, which is acknowledged by the authors. The current study was a nonrandomized, quasi-experimental pilot study implemented at a single medical center, and therefore, the results have limited generalizability and more importantly, may not only be attributable to the introduction of Hospital Fit. In addition, as there was lag in patient recruitment where patients were initially selected for the control group over the course of 1 year, followed by selection of patients for the intervention group over 4 months (once Hospital Fit was developed), it is possible that awareness on the importance of physical activity during hospitalization increased among patients and health care professionals, which may have resulted in a bias in favor of the intervention group (and thus a potentially slight overestimation of results). Also, as individual functionalities of Hospital Fit were not investigated, relationships between each functionality and physical activity could not be established. As the authors indicated, future research is needed to determine the effectiveness of Hospital Fit (ie, a larger, cluster randomized controlled trial in a population of hospitalized patients with a longer length of stay). This study design would also enable investigation of the effect of individual functionalities of Hospital Fit on physical activity.
Applications for Clinical Practice
mHealth tools have the potential to increase patient awareness, support personalized care, and stimulate self-management. This study highlights the potential for a novel mHealth tool—Hospital Fit—to improve the amount of physical activity and shorten the time to functional recovery in hospitalized patients following orthopedic surgery. Further, mHealth tools like Hospital Fit may have a greater impact when the hospital stay of a patient permits the use of the tool for a longer period of time. More broadly, continuous objective monitoring through mHealth tools may provide patients and their physiotherapists enhanced and more detailed data to support and create more personalized recovery goals and related strategies.
Katrina F. Mateo, PhD, MPH
1. Brown CJ, Roth DL, Allman RM. Validation of use of wireless monitors to measure levels of mobility during hospitalization. J Rehabil Res Dev. 2008;45:551-558.
2. Pedersen MM, Bodilsen AC, Petersen J, et al. Twenty-four-hour mobility during acute hospitalization in older medical patients. J Gerontol Ser A Biol Sci Med Sci. 2013;68:331–337.
3. Evensen S, Sletvold O, Lydersen S, Taraldsen K. Physical activity among hospitalized older adults – an observational study. BMC Geriatr. 2017;17:110.
4. Engdal M, Foss OA, Taraldsen K, et al. Daily physical activity in total hip arthroplasty patients undergoing different surgical approaches: a cohort study. Am J Phys Med Rehabil. 2017;96:473-478.
5. Hoogeboom TJ, Dronkers JJ, Hulzebos EH, van Meeteren NL. Merits of exercise therapy before and after major surgery. Curr Opin Anaesthesiol. 2014;27:161-166.
6. Hoogeboom TJ, van Meeteren NL, Schank K, et al. Risk factors for delayed inpatient functional recovery after total knee arthroplasty. Biomed Res Int. 2015:2015:167643.
7. Lenssen AF, Crijns YH, Waltje EM, et al. Efficiency of immediate postoperative inpatient physical therapy following total knee arthroplasty: an RCT. BMC Musculoskelet Disord. 2006;7:71.
8. Ramkumar PN, Haeberle HS, Ramanathan D, et al. Remote patient monitoring using mobile health for total knee arthroplasty: validation of a wearable and machine learning-based surveillance platform. J Arthroplast. 2019;34:2253-2259.
9. Ramkumar PN, Haeberle HS, Bloomfield MR, et al. Artificial Intelligence and arthroplasty at a single institution: Real-world applications of machine learning to big data, value-based care, mobile health, and remote patient monitoring. J Arthroplast. 2019;34:2204-2209.
10. Correia FD, Nogueira A, Magalhães I, et al, et al. Medium-term outcomes of digital versus conventional home-based rehabilitation after total knee arthroplasty: prospective, parallel-group feasibility study. JMIR Rehabil Assist Technol. 2019;6:e13111.
Study Overview
Objective. To investigate the potential of Hospital Fit (a smartphone application with an accelerometer) to enhance physical activity levels and functional recovery following orthopedic surgery.
Design. Nonrandomized, quasi-experimental pilot study.
Settings and participants. Patients scheduled for an elective total knee arthroplasty (TKA) or total hip arthroplasty (THA) at the orthopedic ward of Maastricht University Medical Center in Maastricht, the Netherlands, were invited to participate. Patients scheduled for surgery between January 2017 and December 2018 were recruited for the control group at a rate of 1 patient per week (due to a limited number of accelerometers available). After development of Hospital Fit was completed in December 2018 (and sufficient accelerators had become available), patients scheduled for surgery between February 2019 and May 2019 were recruited for the intervention group. The ratio of patients included in the control and intervention group was set at 2:1, respectively.
At preoperative physiotherapy screenings (scheduled 6 weeks before surgery), patients received verbal and written information about the study. Patients were eligible if they met the following inclusion criteria: receiving physiotherapy after elective TKA or THA; able to walk independently 2 weeks prior to surgery, as scored on the Functional Ambulation Categories (FAC > 3); were expected to be discharged to their own home; were aged 18 years and older; and had a sufficient understanding of the Dutch language. Exclusion criteria were: the presence of contraindications to walking or wearing an accelerometer on the upper leg; admission to the intensive care unit; impaired cognition (delirium/dementia), as reported by the attending doctor; a life expectancy of less than 3 months; and previous participation in this study. Patients were contacted on the day of their surgery, and written informed consent was obtained prior to the initiation of any study activities.
Intervention. Once enrolled, all patients followed a standardized clinical care pathway for TKA or THA (see original article for additional details). Postoperative physiotherapy was administered to all participating patients, starting within 4 hours after surgery. The physiotherapy treatment was aimed at increasing physical activity levels and enhancing functional recovery. Control group patients only received physiotherapy (twice daily, 30 minutes per session) and had their physical activity levels monitored with an accelerometer, without receiving feedback, until functional recovery was achieved, as measured with the modified Iowa Level of Assistance Scale (mILAS). Intervention group patients used Hospital Fit in addition to physiotherapy. Hospital Fit consists of a smartphone-based app, connected to a MOX activity monitor via Bluetooth (device contains a tri-axial accelerometer sensor in a small waterproof housing attached to the upper leg). Hospital Fit enables objective activity monitoring, provides patients and their physiotherapists insights and real-time feedback on the number of minutes spent standing and walking per day, and offers a tailored exercise program supported by videos aimed at stimulating self-management.
Measures. The primary outcome measure was the time spent physically active (total number of minutes standing and walking) per day until discharge. Physical activity was monitored 24 hours a day; days with ≥ 20 hours of wear time were considered valid measurement days and were included in the analysis. After the last treatment session, the accelerometer was removed, and the raw tri-axial accelerometer data were uploaded and processed to classify minutes as “active” (standing and walking) or “sedentary” (lying and sitting). The secondary outcome measures were the achievement of functional recovery on postoperative day 1 (POD1). Functional recovery was assessed by the physiotherapist during each treatment session using the mILAS and was reported in the electronic health record. In the intervention group, it was also reported in the app. The achievement of functional recovery on POD1 was defined as having reached a total mILAS-score of 0 on or before POD1, using a dichotomized outcome (0 = mILAS = 0 > POD1; 1 = mILAS = 0 ≤ POD1).
The independent variables measured were: Hospital Fit use (control versus the intervention group), age, sex, body mass index (BMI), type of surgery (TKA or THA), and comorbidities assessed by the American Society of Anesthesiologists (ASA) classification (ASA class ≤ 2 versus ASA class = 3; a higher score indicates being less fit for surgery). The medical and demographic data measured were the type of walking aid used and length of stay, with the day of surgery being defined as day 1.
Analysis. Data analysis was performed according to the intention-to-treat principle. Missing values were not substituted; drop-outs were not replaced. Descriptive statistics were presented as means (SD) or as 95% confidence intervals (CI) for continuous variables. The median and interquartile ranges (IQR) were used to present non-normally distributed data. The frequencies and percentages were used to present categorical variables. A multiple linear regression analysis was performed to determine the association between the time spent physically active per day and Hospital Fit use, corrected for potential confounding factors (age, sex, BMI, ASA class, and type of surgery). A multiple logistic regression analysis was performed additionally to determine the association between the achievement of functional recovery on POD1 and Hospital Fit use, corrected for potential confounding factors. For all statistical analyses, the level of significance was set at P < 0.05. All statistical analyses were performed using SPSS (version 23.0.0.2; IBM Corporation, Armonk, NY).
Main results. Ninety-seven patients were recruited; after excluding 9 patients because of missing data, 88 were included for analysis, with 61 (69%) in the control group and 27 (31%) in the intervention group. A median (IQR) number of 1.00 (0) valid measurement days (≥ 20 hr wear time) was collected. Physical activity data for 84 patients (95%) was available on POD1 (n = 61 control group, n = 23 intervention group). On postoperative day 2 (POD2), the majority of patients were discharged (n = 61, 69%), and data for only 23 patients (26%) were available (n = 17 control, n = 6 intervention). From postoperative day 3 to day 7, data of valid measurement days were available for just 1 patient (intervention group). Due to the large reduction in valid measurement days from POD2 onward, data from these days were not included in the analysis.
Results of the multiple linear regression analysis showed that, corrected for age, patients who used Hospital Fit stood and walked an average of 28.43 minutes (95% CI, 5.55-51.32) more on POD1 than patients who did not use Hospital Fit. Also, the model showed that an increase in age led to a decrease in the number of minutes standing and walking on POD1. The results of the multiple logistic regression analysis also showed that, corrected for ASA class, the odds of achieving functional recovery on POD1 were 3.08 times higher (95% CI, 1.14-8.31) for patients who used Hospital Fit compared to patients who did not use Hospital Fit. Including ASA class in the model shows that a lower ASA class increased the odds ratio for a functional recovery on POD1.
Conclusion. A smartphone app combined with an accelerometer demonstrates the potential to enhance patients’ physical activity levels and functional recovery during hospitalization following joint replacement surgery.
Commentary
Although the beneficial effects of physical activity during hospitalization after surgery are well documented, patients continue to spend between 92% and 96% of their time lying or sitting.1-3 Therefore, strategies aimed at increasing the amount of time spent standing and walking are needed. Postoperative physiotherapy aims to enhance physical activity levels and functional recovery of activities of daily living, which are essential to function independently at home.4-7 Physiotherapists may be able to advise patients more effectively on their physical activity behavior if continuous physical activity monitoring with real-time feedback is implemented in standard care. Although mobile health (mHealth) tools are being used to monitor physical activity in support of outpatient physiotherapy within the orthopedic rehabilitation pathway,8-10 there is currently no mHealth tool available that offers hospitalized patients and their physiotherapists essential strategies to enhance their physical activity levels and support their recovery process. In addition, because hospitalized patients frequently use walking aids and often have impaired gait, the algorithm of most available activity monitors is not validated for use in this population.
This study, therefore, is an important contribution to the literature, as it describes a preliminary evaluation of a novel mHealth tool—Hospital Fit—consisting of a smartphone application connected to an accelerometer whose algorithm has been validated to differentiate between lying/sitting and standing/walking among hospitalized patients. Briefly, results from this study showed an increase in the time spent standing and walking, as well as higher odds of functional recovery on POD1 from the introduction of Hospital Fit. While guidelines on the recommended amount of physical activity during hospitalization do not yet exist, an average improvement of 28 minutes (39%) standing and walking on POD1 can be considered a clinically relevant contribution to prevent the negative effects of inactivity.
This study has limitations, particularly related to the study design, which is acknowledged by the authors. The current study was a nonrandomized, quasi-experimental pilot study implemented at a single medical center, and therefore, the results have limited generalizability and more importantly, may not only be attributable to the introduction of Hospital Fit. In addition, as there was lag in patient recruitment where patients were initially selected for the control group over the course of 1 year, followed by selection of patients for the intervention group over 4 months (once Hospital Fit was developed), it is possible that awareness on the importance of physical activity during hospitalization increased among patients and health care professionals, which may have resulted in a bias in favor of the intervention group (and thus a potentially slight overestimation of results). Also, as individual functionalities of Hospital Fit were not investigated, relationships between each functionality and physical activity could not be established. As the authors indicated, future research is needed to determine the effectiveness of Hospital Fit (ie, a larger, cluster randomized controlled trial in a population of hospitalized patients with a longer length of stay). This study design would also enable investigation of the effect of individual functionalities of Hospital Fit on physical activity.
Applications for Clinical Practice
mHealth tools have the potential to increase patient awareness, support personalized care, and stimulate self-management. This study highlights the potential for a novel mHealth tool—Hospital Fit—to improve the amount of physical activity and shorten the time to functional recovery in hospitalized patients following orthopedic surgery. Further, mHealth tools like Hospital Fit may have a greater impact when the hospital stay of a patient permits the use of the tool for a longer period of time. More broadly, continuous objective monitoring through mHealth tools may provide patients and their physiotherapists enhanced and more detailed data to support and create more personalized recovery goals and related strategies.
Katrina F. Mateo, PhD, MPH
Study Overview
Objective. To investigate the potential of Hospital Fit (a smartphone application with an accelerometer) to enhance physical activity levels and functional recovery following orthopedic surgery.
Design. Nonrandomized, quasi-experimental pilot study.
Settings and participants. Patients scheduled for an elective total knee arthroplasty (TKA) or total hip arthroplasty (THA) at the orthopedic ward of Maastricht University Medical Center in Maastricht, the Netherlands, were invited to participate. Patients scheduled for surgery between January 2017 and December 2018 were recruited for the control group at a rate of 1 patient per week (due to a limited number of accelerometers available). After development of Hospital Fit was completed in December 2018 (and sufficient accelerators had become available), patients scheduled for surgery between February 2019 and May 2019 were recruited for the intervention group. The ratio of patients included in the control and intervention group was set at 2:1, respectively.
At preoperative physiotherapy screenings (scheduled 6 weeks before surgery), patients received verbal and written information about the study. Patients were eligible if they met the following inclusion criteria: receiving physiotherapy after elective TKA or THA; able to walk independently 2 weeks prior to surgery, as scored on the Functional Ambulation Categories (FAC > 3); were expected to be discharged to their own home; were aged 18 years and older; and had a sufficient understanding of the Dutch language. Exclusion criteria were: the presence of contraindications to walking or wearing an accelerometer on the upper leg; admission to the intensive care unit; impaired cognition (delirium/dementia), as reported by the attending doctor; a life expectancy of less than 3 months; and previous participation in this study. Patients were contacted on the day of their surgery, and written informed consent was obtained prior to the initiation of any study activities.
Intervention. Once enrolled, all patients followed a standardized clinical care pathway for TKA or THA (see original article for additional details). Postoperative physiotherapy was administered to all participating patients, starting within 4 hours after surgery. The physiotherapy treatment was aimed at increasing physical activity levels and enhancing functional recovery. Control group patients only received physiotherapy (twice daily, 30 minutes per session) and had their physical activity levels monitored with an accelerometer, without receiving feedback, until functional recovery was achieved, as measured with the modified Iowa Level of Assistance Scale (mILAS). Intervention group patients used Hospital Fit in addition to physiotherapy. Hospital Fit consists of a smartphone-based app, connected to a MOX activity monitor via Bluetooth (device contains a tri-axial accelerometer sensor in a small waterproof housing attached to the upper leg). Hospital Fit enables objective activity monitoring, provides patients and their physiotherapists insights and real-time feedback on the number of minutes spent standing and walking per day, and offers a tailored exercise program supported by videos aimed at stimulating self-management.
Measures. The primary outcome measure was the time spent physically active (total number of minutes standing and walking) per day until discharge. Physical activity was monitored 24 hours a day; days with ≥ 20 hours of wear time were considered valid measurement days and were included in the analysis. After the last treatment session, the accelerometer was removed, and the raw tri-axial accelerometer data were uploaded and processed to classify minutes as “active” (standing and walking) or “sedentary” (lying and sitting). The secondary outcome measures were the achievement of functional recovery on postoperative day 1 (POD1). Functional recovery was assessed by the physiotherapist during each treatment session using the mILAS and was reported in the electronic health record. In the intervention group, it was also reported in the app. The achievement of functional recovery on POD1 was defined as having reached a total mILAS-score of 0 on or before POD1, using a dichotomized outcome (0 = mILAS = 0 > POD1; 1 = mILAS = 0 ≤ POD1).
The independent variables measured were: Hospital Fit use (control versus the intervention group), age, sex, body mass index (BMI), type of surgery (TKA or THA), and comorbidities assessed by the American Society of Anesthesiologists (ASA) classification (ASA class ≤ 2 versus ASA class = 3; a higher score indicates being less fit for surgery). The medical and demographic data measured were the type of walking aid used and length of stay, with the day of surgery being defined as day 1.
Analysis. Data analysis was performed according to the intention-to-treat principle. Missing values were not substituted; drop-outs were not replaced. Descriptive statistics were presented as means (SD) or as 95% confidence intervals (CI) for continuous variables. The median and interquartile ranges (IQR) were used to present non-normally distributed data. The frequencies and percentages were used to present categorical variables. A multiple linear regression analysis was performed to determine the association between the time spent physically active per day and Hospital Fit use, corrected for potential confounding factors (age, sex, BMI, ASA class, and type of surgery). A multiple logistic regression analysis was performed additionally to determine the association between the achievement of functional recovery on POD1 and Hospital Fit use, corrected for potential confounding factors. For all statistical analyses, the level of significance was set at P < 0.05. All statistical analyses were performed using SPSS (version 23.0.0.2; IBM Corporation, Armonk, NY).
Main results. Ninety-seven patients were recruited; after excluding 9 patients because of missing data, 88 were included for analysis, with 61 (69%) in the control group and 27 (31%) in the intervention group. A median (IQR) number of 1.00 (0) valid measurement days (≥ 20 hr wear time) was collected. Physical activity data for 84 patients (95%) was available on POD1 (n = 61 control group, n = 23 intervention group). On postoperative day 2 (POD2), the majority of patients were discharged (n = 61, 69%), and data for only 23 patients (26%) were available (n = 17 control, n = 6 intervention). From postoperative day 3 to day 7, data of valid measurement days were available for just 1 patient (intervention group). Due to the large reduction in valid measurement days from POD2 onward, data from these days were not included in the analysis.
Results of the multiple linear regression analysis showed that, corrected for age, patients who used Hospital Fit stood and walked an average of 28.43 minutes (95% CI, 5.55-51.32) more on POD1 than patients who did not use Hospital Fit. Also, the model showed that an increase in age led to a decrease in the number of minutes standing and walking on POD1. The results of the multiple logistic regression analysis also showed that, corrected for ASA class, the odds of achieving functional recovery on POD1 were 3.08 times higher (95% CI, 1.14-8.31) for patients who used Hospital Fit compared to patients who did not use Hospital Fit. Including ASA class in the model shows that a lower ASA class increased the odds ratio for a functional recovery on POD1.
Conclusion. A smartphone app combined with an accelerometer demonstrates the potential to enhance patients’ physical activity levels and functional recovery during hospitalization following joint replacement surgery.
Commentary
Although the beneficial effects of physical activity during hospitalization after surgery are well documented, patients continue to spend between 92% and 96% of their time lying or sitting.1-3 Therefore, strategies aimed at increasing the amount of time spent standing and walking are needed. Postoperative physiotherapy aims to enhance physical activity levels and functional recovery of activities of daily living, which are essential to function independently at home.4-7 Physiotherapists may be able to advise patients more effectively on their physical activity behavior if continuous physical activity monitoring with real-time feedback is implemented in standard care. Although mobile health (mHealth) tools are being used to monitor physical activity in support of outpatient physiotherapy within the orthopedic rehabilitation pathway,8-10 there is currently no mHealth tool available that offers hospitalized patients and their physiotherapists essential strategies to enhance their physical activity levels and support their recovery process. In addition, because hospitalized patients frequently use walking aids and often have impaired gait, the algorithm of most available activity monitors is not validated for use in this population.
This study, therefore, is an important contribution to the literature, as it describes a preliminary evaluation of a novel mHealth tool—Hospital Fit—consisting of a smartphone application connected to an accelerometer whose algorithm has been validated to differentiate between lying/sitting and standing/walking among hospitalized patients. Briefly, results from this study showed an increase in the time spent standing and walking, as well as higher odds of functional recovery on POD1 from the introduction of Hospital Fit. While guidelines on the recommended amount of physical activity during hospitalization do not yet exist, an average improvement of 28 minutes (39%) standing and walking on POD1 can be considered a clinically relevant contribution to prevent the negative effects of inactivity.
This study has limitations, particularly related to the study design, which is acknowledged by the authors. The current study was a nonrandomized, quasi-experimental pilot study implemented at a single medical center, and therefore, the results have limited generalizability and more importantly, may not only be attributable to the introduction of Hospital Fit. In addition, as there was lag in patient recruitment where patients were initially selected for the control group over the course of 1 year, followed by selection of patients for the intervention group over 4 months (once Hospital Fit was developed), it is possible that awareness on the importance of physical activity during hospitalization increased among patients and health care professionals, which may have resulted in a bias in favor of the intervention group (and thus a potentially slight overestimation of results). Also, as individual functionalities of Hospital Fit were not investigated, relationships between each functionality and physical activity could not be established. As the authors indicated, future research is needed to determine the effectiveness of Hospital Fit (ie, a larger, cluster randomized controlled trial in a population of hospitalized patients with a longer length of stay). This study design would also enable investigation of the effect of individual functionalities of Hospital Fit on physical activity.
Applications for Clinical Practice
mHealth tools have the potential to increase patient awareness, support personalized care, and stimulate self-management. This study highlights the potential for a novel mHealth tool—Hospital Fit—to improve the amount of physical activity and shorten the time to functional recovery in hospitalized patients following orthopedic surgery. Further, mHealth tools like Hospital Fit may have a greater impact when the hospital stay of a patient permits the use of the tool for a longer period of time. More broadly, continuous objective monitoring through mHealth tools may provide patients and their physiotherapists enhanced and more detailed data to support and create more personalized recovery goals and related strategies.
Katrina F. Mateo, PhD, MPH
1. Brown CJ, Roth DL, Allman RM. Validation of use of wireless monitors to measure levels of mobility during hospitalization. J Rehabil Res Dev. 2008;45:551-558.
2. Pedersen MM, Bodilsen AC, Petersen J, et al. Twenty-four-hour mobility during acute hospitalization in older medical patients. J Gerontol Ser A Biol Sci Med Sci. 2013;68:331–337.
3. Evensen S, Sletvold O, Lydersen S, Taraldsen K. Physical activity among hospitalized older adults – an observational study. BMC Geriatr. 2017;17:110.
4. Engdal M, Foss OA, Taraldsen K, et al. Daily physical activity in total hip arthroplasty patients undergoing different surgical approaches: a cohort study. Am J Phys Med Rehabil. 2017;96:473-478.
5. Hoogeboom TJ, Dronkers JJ, Hulzebos EH, van Meeteren NL. Merits of exercise therapy before and after major surgery. Curr Opin Anaesthesiol. 2014;27:161-166.
6. Hoogeboom TJ, van Meeteren NL, Schank K, et al. Risk factors for delayed inpatient functional recovery after total knee arthroplasty. Biomed Res Int. 2015:2015:167643.
7. Lenssen AF, Crijns YH, Waltje EM, et al. Efficiency of immediate postoperative inpatient physical therapy following total knee arthroplasty: an RCT. BMC Musculoskelet Disord. 2006;7:71.
8. Ramkumar PN, Haeberle HS, Ramanathan D, et al. Remote patient monitoring using mobile health for total knee arthroplasty: validation of a wearable and machine learning-based surveillance platform. J Arthroplast. 2019;34:2253-2259.
9. Ramkumar PN, Haeberle HS, Bloomfield MR, et al. Artificial Intelligence and arthroplasty at a single institution: Real-world applications of machine learning to big data, value-based care, mobile health, and remote patient monitoring. J Arthroplast. 2019;34:2204-2209.
10. Correia FD, Nogueira A, Magalhães I, et al, et al. Medium-term outcomes of digital versus conventional home-based rehabilitation after total knee arthroplasty: prospective, parallel-group feasibility study. JMIR Rehabil Assist Technol. 2019;6:e13111.
1. Brown CJ, Roth DL, Allman RM. Validation of use of wireless monitors to measure levels of mobility during hospitalization. J Rehabil Res Dev. 2008;45:551-558.
2. Pedersen MM, Bodilsen AC, Petersen J, et al. Twenty-four-hour mobility during acute hospitalization in older medical patients. J Gerontol Ser A Biol Sci Med Sci. 2013;68:331–337.
3. Evensen S, Sletvold O, Lydersen S, Taraldsen K. Physical activity among hospitalized older adults – an observational study. BMC Geriatr. 2017;17:110.
4. Engdal M, Foss OA, Taraldsen K, et al. Daily physical activity in total hip arthroplasty patients undergoing different surgical approaches: a cohort study. Am J Phys Med Rehabil. 2017;96:473-478.
5. Hoogeboom TJ, Dronkers JJ, Hulzebos EH, van Meeteren NL. Merits of exercise therapy before and after major surgery. Curr Opin Anaesthesiol. 2014;27:161-166.
6. Hoogeboom TJ, van Meeteren NL, Schank K, et al. Risk factors for delayed inpatient functional recovery after total knee arthroplasty. Biomed Res Int. 2015:2015:167643.
7. Lenssen AF, Crijns YH, Waltje EM, et al. Efficiency of immediate postoperative inpatient physical therapy following total knee arthroplasty: an RCT. BMC Musculoskelet Disord. 2006;7:71.
8. Ramkumar PN, Haeberle HS, Ramanathan D, et al. Remote patient monitoring using mobile health for total knee arthroplasty: validation of a wearable and machine learning-based surveillance platform. J Arthroplast. 2019;34:2253-2259.
9. Ramkumar PN, Haeberle HS, Bloomfield MR, et al. Artificial Intelligence and arthroplasty at a single institution: Real-world applications of machine learning to big data, value-based care, mobile health, and remote patient monitoring. J Arthroplast. 2019;34:2204-2209.
10. Correia FD, Nogueira A, Magalhães I, et al, et al. Medium-term outcomes of digital versus conventional home-based rehabilitation after total knee arthroplasty: prospective, parallel-group feasibility study. JMIR Rehabil Assist Technol. 2019;6:e13111.
Without Ginsburg, judicial threats to the ACA, reproductive rights heighten
On Feb. 27, 2018, I got an email from the Heritage Foundation that alerted me to a news conference that afternoon held by Republican attorneys general of Texas and other states. It was referred to only as a “discussion about the Affordable Care Act lawsuit.”
I sent the following note to my editor: “I’m off to the Hill anyway. I could stop by this. You never know what it might morph into.”
Few people took that case very seriously – barely a handful of reporters attended the news conference. But it has now “morphed into” the latest existential threat to the Affordable Care Act, scheduled for oral arguments at the Supreme Court a week after the general election in November. And with the death of Justice Ruth Bader Ginsburg on Friday, that case could well morph into the threat that brings down the law in its entirety.
Democrats are raising alarms about the future of the law without Ms. Ginsburg. House Speaker Nancy Pelosi, speaking on ABC’s “This Week” Sunday morning, said that part of the strategy by President Trump and Senate Republicans to quickly fill her seat was to help undermine the ACA.
“The president is rushing to make some kind of a decision because … Nov. 10 is when the arguments begin on the Affordable Care Act,” she said. “He doesn’t want to crush the virus. He wants to crush the Affordable Care Act.”
Ms. Ginsburg’s death could throw an already chaotic general election campaign during a pandemic into even more turmoil.
Let’s take them one at a time.
The ACA under fire – again
The GOP attorneys general argued in February 2018 that the Republican-sponsored tax cut bill Congress passed two months earlier had rendered the ACA unconstitutional by reducing to zero the ACA’s penalty for not having insurance. They based their argument on Chief Justice John Roberts’ 2012 conclusion that the ACA was valid, interpreting that penalty as a constitutionally appropriate tax.
Most legal scholars, including several who challenged the law before the Supreme Court in 2012 and again in 2015, find the argument that the entire law should fall to be unconvincing. “If courts invalidate an entire law merely because Congress eliminates or revises one part, as happened here, that may well inhibit necessary reform of federal legislation in the future by turning it into an ‘all or nothing’ proposition,” wrote a group of conservative and liberal law professors in a brief filed in the case.
Still, in December 2018, U.S. District Judge Reed O’Connor in Texas accepted the GOP argument and declared the law unconstitutional. In December 2019, a three-judge 5th Circuit appeals court panel in New Orleans agreed that without the penalty the requirement to buy insurance is unconstitutional. But it sent the case back to Mr. O’Connor to suggest that perhaps the entire law need not fall.
Not wanting to wait the months or years that reconsideration would take, Democratic attorneys general defending the ACA asked the Supreme Court to hear the case this year. (Democrats are defending the law in court because the Trump administration decided to support the GOP attorneys general’s case.) The court agreed to take the case but scheduled arguments for the week after the November election.
While the fate of the ACA was and is a live political issue, few legal observers were terribly worried about the legal outcome of the case, now known as Texas v. California, if only because the case seemed much weaker than the 2012 and 2015 cases in which Mr. Roberts joined the court’s four liberals. In the 2015 case, which challenged the validity of federal tax subsidies helping millions of Americans buy health insurance on the ACA’s marketplaces, both Mr. Roberts and now-retired Justice Anthony Kennedy voted to uphold the law.
But without Ms. Ginsburg, the case could wind up in a 4-4 tie, even if Mr. Roberts supports the law’s constitutionality. That could let the lower-court ruling stand, although it would not be binding on other courts outside of the 5th Circuit. The court could also put off the arguments or, if the Republican Senate replaces Ms. Ginsburg with another conservative justice before arguments are heard, Republicans could secure a 5-4 ruling against the law. Some court observers argue that Justice Brett Kavanaugh has not favored invalidating an entire statute if only part of it is flawed and might not approve overturning the ACA. Still, what started out as an effort to energize Republican voters for the 2018 midterms after Congress failed to “repeal and replace” the health law in 2017 could end up throwing the nation’s entire health system into chaos.
At least 20 million Americans – and likely many more who sought coverage since the start of the coronavirus pandemic — who buy insurance through the ACA marketplaces or have Medicaid through the law’s expansion could lose coverage right away. Many millions more would lose the law’s popular protections guaranteeing coverage for people with preexisting health conditions, including those who have had COVID-19.
Adult children under age 26 years would no longer be guaranteed the right to remain on their parents’ health plans, and Medicare patients would lose enhanced prescription drug coverage. Women would lose guaranteed access to birth control at no out-of-pocket cost.
But a sudden elimination would affect more than just health care consumers. Insurance companies, drug companies, hospitals, and doctors have all changed the way they do business because of incentives and penalties in the health law. If it’s struck down, many of the “rules of the road” would literally be wiped away, including billing and payment mechanisms.
A new Democratic president could not drop the lawsuit because the Trump administration is not the plaintiff (the GOP attorneys general are). But a Democratic Congress and president could in theory make the entire issue go away by reinstating the penalty for failure to have insurance, even at a minimal amount. However, as far as the health law goes, for now, nothing is a sure thing.
As Nicholas Bagley, a law professor at the University of Michigan, Ann Arbor, who specializes in health issues, tweeted: “Among other things, the Affordable Care Act now dangles from a thread.”
Reproductive rights
A woman’s right to abortion – and even to birth control – also has been hanging by a thread at the high court for more than a decade. This past term, Mr. Roberts joined the liberals to invalidate a Louisiana law that would have closed most of the state’s abortion clinics, but he made it clear it was not a vote for abortion rights. The Louisiana law was too similar to a Texas law the court (without his vote) struck down in 2016, Mr. Roberts argued.
Ms. Ginsburg had been a stalwart supporter of reproductive freedom for women. In her nearly 3 decades on the court, she always voted with backers of abortion rights and birth control and led the dissenters in 2007 when the court upheld a federal ban on a specific abortion procedure.
Adding a justice opposed to abortion to the bench – which is what Trump has promised his supporters – would almost certainly tilt the court in favor of far more dramatic restrictions on the procedure and possibly an overturn of the landmark 1973 ruling Roe v. Wade.
But not only is abortion on the line: The court in recent years has repeatedly ruled that employers with religious objections can refuse to provide contraception.
And waiting in the lower-court pipeline are cases involving federal funding of Planned Parenthood in both the Medicaid and federal family planning programs, and the ability of individual health workers to decline to participate in abortion and other procedures.
For Ms. Ginsburg, those issues came down to a clear question of a woman’s guarantee of equal status under the law.
“Women, it is now acknowledged, have the talent, capacity, and right ‘to participate equally in the economic and social life of the Nation,’ ” she wrote in her dissent in that 2007 abortion case. “Their ability to realize their full potential, the Court recognized, is intimately connected to ‘their ability to control their reproductive lives.’ ”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
On Feb. 27, 2018, I got an email from the Heritage Foundation that alerted me to a news conference that afternoon held by Republican attorneys general of Texas and other states. It was referred to only as a “discussion about the Affordable Care Act lawsuit.”
I sent the following note to my editor: “I’m off to the Hill anyway. I could stop by this. You never know what it might morph into.”
Few people took that case very seriously – barely a handful of reporters attended the news conference. But it has now “morphed into” the latest existential threat to the Affordable Care Act, scheduled for oral arguments at the Supreme Court a week after the general election in November. And with the death of Justice Ruth Bader Ginsburg on Friday, that case could well morph into the threat that brings down the law in its entirety.
Democrats are raising alarms about the future of the law without Ms. Ginsburg. House Speaker Nancy Pelosi, speaking on ABC’s “This Week” Sunday morning, said that part of the strategy by President Trump and Senate Republicans to quickly fill her seat was to help undermine the ACA.
“The president is rushing to make some kind of a decision because … Nov. 10 is when the arguments begin on the Affordable Care Act,” she said. “He doesn’t want to crush the virus. He wants to crush the Affordable Care Act.”
Ms. Ginsburg’s death could throw an already chaotic general election campaign during a pandemic into even more turmoil.
Let’s take them one at a time.
The ACA under fire – again
The GOP attorneys general argued in February 2018 that the Republican-sponsored tax cut bill Congress passed two months earlier had rendered the ACA unconstitutional by reducing to zero the ACA’s penalty for not having insurance. They based their argument on Chief Justice John Roberts’ 2012 conclusion that the ACA was valid, interpreting that penalty as a constitutionally appropriate tax.
Most legal scholars, including several who challenged the law before the Supreme Court in 2012 and again in 2015, find the argument that the entire law should fall to be unconvincing. “If courts invalidate an entire law merely because Congress eliminates or revises one part, as happened here, that may well inhibit necessary reform of federal legislation in the future by turning it into an ‘all or nothing’ proposition,” wrote a group of conservative and liberal law professors in a brief filed in the case.
Still, in December 2018, U.S. District Judge Reed O’Connor in Texas accepted the GOP argument and declared the law unconstitutional. In December 2019, a three-judge 5th Circuit appeals court panel in New Orleans agreed that without the penalty the requirement to buy insurance is unconstitutional. But it sent the case back to Mr. O’Connor to suggest that perhaps the entire law need not fall.
Not wanting to wait the months or years that reconsideration would take, Democratic attorneys general defending the ACA asked the Supreme Court to hear the case this year. (Democrats are defending the law in court because the Trump administration decided to support the GOP attorneys general’s case.) The court agreed to take the case but scheduled arguments for the week after the November election.
While the fate of the ACA was and is a live political issue, few legal observers were terribly worried about the legal outcome of the case, now known as Texas v. California, if only because the case seemed much weaker than the 2012 and 2015 cases in which Mr. Roberts joined the court’s four liberals. In the 2015 case, which challenged the validity of federal tax subsidies helping millions of Americans buy health insurance on the ACA’s marketplaces, both Mr. Roberts and now-retired Justice Anthony Kennedy voted to uphold the law.
But without Ms. Ginsburg, the case could wind up in a 4-4 tie, even if Mr. Roberts supports the law’s constitutionality. That could let the lower-court ruling stand, although it would not be binding on other courts outside of the 5th Circuit. The court could also put off the arguments or, if the Republican Senate replaces Ms. Ginsburg with another conservative justice before arguments are heard, Republicans could secure a 5-4 ruling against the law. Some court observers argue that Justice Brett Kavanaugh has not favored invalidating an entire statute if only part of it is flawed and might not approve overturning the ACA. Still, what started out as an effort to energize Republican voters for the 2018 midterms after Congress failed to “repeal and replace” the health law in 2017 could end up throwing the nation’s entire health system into chaos.
At least 20 million Americans – and likely many more who sought coverage since the start of the coronavirus pandemic — who buy insurance through the ACA marketplaces or have Medicaid through the law’s expansion could lose coverage right away. Many millions more would lose the law’s popular protections guaranteeing coverage for people with preexisting health conditions, including those who have had COVID-19.
Adult children under age 26 years would no longer be guaranteed the right to remain on their parents’ health plans, and Medicare patients would lose enhanced prescription drug coverage. Women would lose guaranteed access to birth control at no out-of-pocket cost.
But a sudden elimination would affect more than just health care consumers. Insurance companies, drug companies, hospitals, and doctors have all changed the way they do business because of incentives and penalties in the health law. If it’s struck down, many of the “rules of the road” would literally be wiped away, including billing and payment mechanisms.
A new Democratic president could not drop the lawsuit because the Trump administration is not the plaintiff (the GOP attorneys general are). But a Democratic Congress and president could in theory make the entire issue go away by reinstating the penalty for failure to have insurance, even at a minimal amount. However, as far as the health law goes, for now, nothing is a sure thing.
As Nicholas Bagley, a law professor at the University of Michigan, Ann Arbor, who specializes in health issues, tweeted: “Among other things, the Affordable Care Act now dangles from a thread.”
Reproductive rights
A woman’s right to abortion – and even to birth control – also has been hanging by a thread at the high court for more than a decade. This past term, Mr. Roberts joined the liberals to invalidate a Louisiana law that would have closed most of the state’s abortion clinics, but he made it clear it was not a vote for abortion rights. The Louisiana law was too similar to a Texas law the court (without his vote) struck down in 2016, Mr. Roberts argued.
Ms. Ginsburg had been a stalwart supporter of reproductive freedom for women. In her nearly 3 decades on the court, she always voted with backers of abortion rights and birth control and led the dissenters in 2007 when the court upheld a federal ban on a specific abortion procedure.
Adding a justice opposed to abortion to the bench – which is what Trump has promised his supporters – would almost certainly tilt the court in favor of far more dramatic restrictions on the procedure and possibly an overturn of the landmark 1973 ruling Roe v. Wade.
But not only is abortion on the line: The court in recent years has repeatedly ruled that employers with religious objections can refuse to provide contraception.
And waiting in the lower-court pipeline are cases involving federal funding of Planned Parenthood in both the Medicaid and federal family planning programs, and the ability of individual health workers to decline to participate in abortion and other procedures.
For Ms. Ginsburg, those issues came down to a clear question of a woman’s guarantee of equal status under the law.
“Women, it is now acknowledged, have the talent, capacity, and right ‘to participate equally in the economic and social life of the Nation,’ ” she wrote in her dissent in that 2007 abortion case. “Their ability to realize their full potential, the Court recognized, is intimately connected to ‘their ability to control their reproductive lives.’ ”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
On Feb. 27, 2018, I got an email from the Heritage Foundation that alerted me to a news conference that afternoon held by Republican attorneys general of Texas and other states. It was referred to only as a “discussion about the Affordable Care Act lawsuit.”
I sent the following note to my editor: “I’m off to the Hill anyway. I could stop by this. You never know what it might morph into.”
Few people took that case very seriously – barely a handful of reporters attended the news conference. But it has now “morphed into” the latest existential threat to the Affordable Care Act, scheduled for oral arguments at the Supreme Court a week after the general election in November. And with the death of Justice Ruth Bader Ginsburg on Friday, that case could well morph into the threat that brings down the law in its entirety.
Democrats are raising alarms about the future of the law without Ms. Ginsburg. House Speaker Nancy Pelosi, speaking on ABC’s “This Week” Sunday morning, said that part of the strategy by President Trump and Senate Republicans to quickly fill her seat was to help undermine the ACA.
“The president is rushing to make some kind of a decision because … Nov. 10 is when the arguments begin on the Affordable Care Act,” she said. “He doesn’t want to crush the virus. He wants to crush the Affordable Care Act.”
Ms. Ginsburg’s death could throw an already chaotic general election campaign during a pandemic into even more turmoil.
Let’s take them one at a time.
The ACA under fire – again
The GOP attorneys general argued in February 2018 that the Republican-sponsored tax cut bill Congress passed two months earlier had rendered the ACA unconstitutional by reducing to zero the ACA’s penalty for not having insurance. They based their argument on Chief Justice John Roberts’ 2012 conclusion that the ACA was valid, interpreting that penalty as a constitutionally appropriate tax.
Most legal scholars, including several who challenged the law before the Supreme Court in 2012 and again in 2015, find the argument that the entire law should fall to be unconvincing. “If courts invalidate an entire law merely because Congress eliminates or revises one part, as happened here, that may well inhibit necessary reform of federal legislation in the future by turning it into an ‘all or nothing’ proposition,” wrote a group of conservative and liberal law professors in a brief filed in the case.
Still, in December 2018, U.S. District Judge Reed O’Connor in Texas accepted the GOP argument and declared the law unconstitutional. In December 2019, a three-judge 5th Circuit appeals court panel in New Orleans agreed that without the penalty the requirement to buy insurance is unconstitutional. But it sent the case back to Mr. O’Connor to suggest that perhaps the entire law need not fall.
Not wanting to wait the months or years that reconsideration would take, Democratic attorneys general defending the ACA asked the Supreme Court to hear the case this year. (Democrats are defending the law in court because the Trump administration decided to support the GOP attorneys general’s case.) The court agreed to take the case but scheduled arguments for the week after the November election.
While the fate of the ACA was and is a live political issue, few legal observers were terribly worried about the legal outcome of the case, now known as Texas v. California, if only because the case seemed much weaker than the 2012 and 2015 cases in which Mr. Roberts joined the court’s four liberals. In the 2015 case, which challenged the validity of federal tax subsidies helping millions of Americans buy health insurance on the ACA’s marketplaces, both Mr. Roberts and now-retired Justice Anthony Kennedy voted to uphold the law.
But without Ms. Ginsburg, the case could wind up in a 4-4 tie, even if Mr. Roberts supports the law’s constitutionality. That could let the lower-court ruling stand, although it would not be binding on other courts outside of the 5th Circuit. The court could also put off the arguments or, if the Republican Senate replaces Ms. Ginsburg with another conservative justice before arguments are heard, Republicans could secure a 5-4 ruling against the law. Some court observers argue that Justice Brett Kavanaugh has not favored invalidating an entire statute if only part of it is flawed and might not approve overturning the ACA. Still, what started out as an effort to energize Republican voters for the 2018 midterms after Congress failed to “repeal and replace” the health law in 2017 could end up throwing the nation’s entire health system into chaos.
At least 20 million Americans – and likely many more who sought coverage since the start of the coronavirus pandemic — who buy insurance through the ACA marketplaces or have Medicaid through the law’s expansion could lose coverage right away. Many millions more would lose the law’s popular protections guaranteeing coverage for people with preexisting health conditions, including those who have had COVID-19.
Adult children under age 26 years would no longer be guaranteed the right to remain on their parents’ health plans, and Medicare patients would lose enhanced prescription drug coverage. Women would lose guaranteed access to birth control at no out-of-pocket cost.
But a sudden elimination would affect more than just health care consumers. Insurance companies, drug companies, hospitals, and doctors have all changed the way they do business because of incentives and penalties in the health law. If it’s struck down, many of the “rules of the road” would literally be wiped away, including billing and payment mechanisms.
A new Democratic president could not drop the lawsuit because the Trump administration is not the plaintiff (the GOP attorneys general are). But a Democratic Congress and president could in theory make the entire issue go away by reinstating the penalty for failure to have insurance, even at a minimal amount. However, as far as the health law goes, for now, nothing is a sure thing.
As Nicholas Bagley, a law professor at the University of Michigan, Ann Arbor, who specializes in health issues, tweeted: “Among other things, the Affordable Care Act now dangles from a thread.”
Reproductive rights
A woman’s right to abortion – and even to birth control – also has been hanging by a thread at the high court for more than a decade. This past term, Mr. Roberts joined the liberals to invalidate a Louisiana law that would have closed most of the state’s abortion clinics, but he made it clear it was not a vote for abortion rights. The Louisiana law was too similar to a Texas law the court (without his vote) struck down in 2016, Mr. Roberts argued.
Ms. Ginsburg had been a stalwart supporter of reproductive freedom for women. In her nearly 3 decades on the court, she always voted with backers of abortion rights and birth control and led the dissenters in 2007 when the court upheld a federal ban on a specific abortion procedure.
Adding a justice opposed to abortion to the bench – which is what Trump has promised his supporters – would almost certainly tilt the court in favor of far more dramatic restrictions on the procedure and possibly an overturn of the landmark 1973 ruling Roe v. Wade.
But not only is abortion on the line: The court in recent years has repeatedly ruled that employers with religious objections can refuse to provide contraception.
And waiting in the lower-court pipeline are cases involving federal funding of Planned Parenthood in both the Medicaid and federal family planning programs, and the ability of individual health workers to decline to participate in abortion and other procedures.
For Ms. Ginsburg, those issues came down to a clear question of a woman’s guarantee of equal status under the law.
“Women, it is now acknowledged, have the talent, capacity, and right ‘to participate equally in the economic and social life of the Nation,’ ” she wrote in her dissent in that 2007 abortion case. “Their ability to realize their full potential, the Court recognized, is intimately connected to ‘their ability to control their reproductive lives.’ ”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Physician reimbursement 2021: Who are the big winners?
Amid all the chaos and problems caused by COVID-19, one might hope that physicians would get a break on their complicated payment-reporting programs.
But that’s not the case: The government recently released the 2021 proposed rule for the Quality Payment Program (QPP), often referred to by its most popular participation track, the Merit-Based Incentive Payment System (MIPS). The program, which launched in 2017, gets annual updates, and this year is no different.
Some good news has made primary care and some other physicians happy.
The government’s proposal includes significant changes to reimbursement for all physicians.
Specialties that rely heavily on office-based E/M services are delighted at this change. Those include internists, family physicians, neurologists, pulmonologists, dermatologists, and all other specialties that rely heavily on office encounters.
According to the estimates from the Centers for Medicare & Medicaid Services (CMS), endocrinologists and rheumatologists are the big winners, at 17% and 16% projected increases, respectively. The government has been pushing to make this shift in reimbursement from surgeries and procedures to office visits for years. Although some physicians may celebrate the change, others will not.
The reimbursement plan for professional services depends on budget neutrality, meaning that the budget increases need to be counterbalanced by budget declines. Specialties that rely heavily on procedures and surgeries will suffer losses. These corresponding reductions felt by proceduralists and surgeons will counterbalance the good fortune of physicians who rely on office visits for the bulk of their revenue. Radiologists, for example, are projected by CMS to experience a 11% downturn, and cardiac surgeons face a 9% decline.
These consequences are significant. The 2021 shift may be the single biggest transfer of reimbursement in the history of the scale, which was adopted in the early 1990s.
If the change affected only Medicare reimbursement, perhaps it would be less significant. Because the majority of private payers use the government’s scale – the resource-based relative value scale – the impact will reverberate across physicians’ bottom lines. Given the state of many physicians’ finances, driven by the pandemic, this may send some affected physicians into a downward spiral.
The boost to E/M reimbursement – which represents approximately 20% of the overall Medicare payout to physicians each year – puts downward pressure on the professional services conversion factor as well.
For 2021, it is proposed to be $32.2605, representing a decrease of $3.83 from the 2020 conversion factor of $36.0896. The resultant conversion factor – which serves as a multiplier applied to the relative value unit to come up with the payment – effectively reduces payments to physicians across the board by 10.6%. Thus, even those who enjoy the benefits of the new E/M increases will see the potential reimbursement high point cut down.
Before launching into the changes in store for 2021, it’s good to determine whether you are an eligible clinician: You need to have more than $90,000 in Medicare Part B charges per year, see more than 200 Medicare Part B patients per year, and provide 200 or more covered professional services to Part B patients.
The program is voluntary, but there are steep penalties for eligible clinicians who don’t participate. For the 2021 reporting year, a 9% penalty will be imposed on Medicare reimbursement in 2023 in the event of participation failure. You can verify your participation status here; you’ll need your National Provider Identifier to run the search, but it takes only seconds to determine your eligibility.
A 9% penalty is a pretty big hit to your income. With 9% at stake, eligible clinicians need to actively engage in the program. Although there have been changes, the basic four-category system remains the same for the MIPS track, as follows: quality, cost, improvement activities, and promoting interoperability.
The four category weights, used to evaluate performance, are changing in 2021. Cost category weight goes up by 5 percentage points, to be 20% of the clinician’s score, and the quality category goes down by 5 percentage points to contribute 40% to the weight. Promoting interoperability remains 25% of the score, with improvement activities constituting the final 15%.
Other key changes include the following:
- The CMS’s Web interface for submission for quality measures will be shuttered in 2021. Users of this submission method will have to find and use another way to report their quality measures.
- Quality measures will be scored against pre-COVID benchmarks in lieu of comparisons with the 2020 reporting year; 206 quality measures are proposed for 2021, compared with the current list of 219.
- Telehealth will be incorporated in the cost category by updates to the measure specifications for the episode-based and total per capita cost measures.
- A new health information exchange measure is added to the promoting interoperability category, and “incorporating” replaces “reconciling” in the reporting requirement “Support Electronic Referral Loops by Receiving and Incorporating Health Information.”
To avoid the 9% penalty, eligible clinicians must earn 50 points in 2021, up from 45 in the current year. Achieving “exceptional performance” remains at 85 points. This elevated level of engagement allows access to a pot of money Congress set aside for high performers.
Many physicians feel that too much work is required to earn the “paltry” bonuses; even a perfect score of 100 has only resulted in bonuses of 1.88% and 1.68%, respectively, in the past 2 years. That includes the $500 million allocation that Congress set aside; this extra funding to reward exceptional performance is only available for the first 6 years of the law. Although the 2019 scores have been released to participants, CMS has not yet announced the overall national average, but it’s expected to be minimal.
The combination of meager payouts and a diminishing funding mechanism has physicians questioning participation altogether. My recent conversations with physicians who qualify for the program revealed their intention to participate, but only at a level to achieve the minimum threshold of 45 points this year and 50 in 2021. With so little upside, it’s impossible to make a business case to aim for the stars.
Perhaps the biggest change in 2021, however, is that the program is not making the previously planned switch to MIPS Value Pathways (MVPs). MVPs were designed to align the four performance categories around a specialty, medical condition, or patient population.
CMS introduced MVPs by giving an example of diabetes: “Endocrinologist reports same ‘foundation’ of PI [promoting interoperability] and population health measures as all other clinicians but now has a MIPS Value Pathway with measures and activities that focus on diabetes prevention and treatment.” CMS had expected MVPs to launch in 2021 for all program participants; because of the pandemic, CMS announced an extension for at least 1 year. This comes as a relief to physicians who are just trying to keep the lights on given the financial pressures brought on by the pandemic.
MVPs, however, will be incorporated into the MIPS Alternative Payment Model (APM) participation segment. This will affect many physicians because this is the path that accountable care organizations (ACOs) have taken. If you are part of an ACO and you report through it, you’ll see some more changes than your colleagues in 2021.
The good news is that ACOs that participate in MIPS and the Medicare Shared Savings Program will have to report only once to satisfy the requirements for both programs. The construct for this new APM-based program is called the “APM Performance Pathway.” This pathway incorporates six population health–based measures that cross-cut specialties.
CMS is also proposing that telemedicine reimbursement will become permanent. As of now, telemedicine services will only be paid when a public health emergency has been declared. This ability to reimburse physicians for telemedicine would end when the current public health emergency is over. CMS is proposing to extend reimbursement beyond the pandemic, which will benefit all physicians who perform these remote encounters.
The CMS proposal would also make some other requirements easier to achieve. The use of codes 99495 and 99496 – the transitional care management codes – is expanding by reducing several key accompanying-services restrictions. Before the public health emergency, there were constraints related to scope of practice; the proposal would extend the ability of advanced practice providers to order diagnostic tests, even after the public health emergency ends.
Furthermore, the proposal reduces restrictions related to billing for remote physiologic monitoring services and outlines the possibility of a new, higher-paying virtual visit code.
Although the Quality Payment Program will undergo some changes, they are minor. Be aware of the requirement to hit the 50-point mark to avoid the steep penalties, however. Perhaps greater benefit will be achieved through the government’s continued push to refine the reimbursement system. As a result of budget neutrality, however, these changes will boost some physicians while resulting in losses for others.
The government’s proposed changes are not final, and there is a period during which they are accepting comments on the proposal; the final rule will be announced in November.
If you want to wash your hands of this now, apply for the 2020 performance year hardship for the Quality Payment Program. The application is now open and available through December 31, 2020; completing it will release you of any program requirements in 2020 (and avoid that hefty 9% penalty on your 2022 reimbursement).
This way, you won’t have to concern yourself with any of these rules until next year; the government’s extension of this “get out of jail free” card is a welcome relief for physicians who are frustrated by the regulatory burdens despite the pressure exerted by COVID. Spending 15 minutes to complete this form is well worth your time and may eliminate much of your worry.
A version of this article originally appeared on Medscape.com.
Amid all the chaos and problems caused by COVID-19, one might hope that physicians would get a break on their complicated payment-reporting programs.
But that’s not the case: The government recently released the 2021 proposed rule for the Quality Payment Program (QPP), often referred to by its most popular participation track, the Merit-Based Incentive Payment System (MIPS). The program, which launched in 2017, gets annual updates, and this year is no different.
Some good news has made primary care and some other physicians happy.
The government’s proposal includes significant changes to reimbursement for all physicians.
Specialties that rely heavily on office-based E/M services are delighted at this change. Those include internists, family physicians, neurologists, pulmonologists, dermatologists, and all other specialties that rely heavily on office encounters.
According to the estimates from the Centers for Medicare & Medicaid Services (CMS), endocrinologists and rheumatologists are the big winners, at 17% and 16% projected increases, respectively. The government has been pushing to make this shift in reimbursement from surgeries and procedures to office visits for years. Although some physicians may celebrate the change, others will not.
The reimbursement plan for professional services depends on budget neutrality, meaning that the budget increases need to be counterbalanced by budget declines. Specialties that rely heavily on procedures and surgeries will suffer losses. These corresponding reductions felt by proceduralists and surgeons will counterbalance the good fortune of physicians who rely on office visits for the bulk of their revenue. Radiologists, for example, are projected by CMS to experience a 11% downturn, and cardiac surgeons face a 9% decline.
These consequences are significant. The 2021 shift may be the single biggest transfer of reimbursement in the history of the scale, which was adopted in the early 1990s.
If the change affected only Medicare reimbursement, perhaps it would be less significant. Because the majority of private payers use the government’s scale – the resource-based relative value scale – the impact will reverberate across physicians’ bottom lines. Given the state of many physicians’ finances, driven by the pandemic, this may send some affected physicians into a downward spiral.
The boost to E/M reimbursement – which represents approximately 20% of the overall Medicare payout to physicians each year – puts downward pressure on the professional services conversion factor as well.
For 2021, it is proposed to be $32.2605, representing a decrease of $3.83 from the 2020 conversion factor of $36.0896. The resultant conversion factor – which serves as a multiplier applied to the relative value unit to come up with the payment – effectively reduces payments to physicians across the board by 10.6%. Thus, even those who enjoy the benefits of the new E/M increases will see the potential reimbursement high point cut down.
Before launching into the changes in store for 2021, it’s good to determine whether you are an eligible clinician: You need to have more than $90,000 in Medicare Part B charges per year, see more than 200 Medicare Part B patients per year, and provide 200 or more covered professional services to Part B patients.
The program is voluntary, but there are steep penalties for eligible clinicians who don’t participate. For the 2021 reporting year, a 9% penalty will be imposed on Medicare reimbursement in 2023 in the event of participation failure. You can verify your participation status here; you’ll need your National Provider Identifier to run the search, but it takes only seconds to determine your eligibility.
A 9% penalty is a pretty big hit to your income. With 9% at stake, eligible clinicians need to actively engage in the program. Although there have been changes, the basic four-category system remains the same for the MIPS track, as follows: quality, cost, improvement activities, and promoting interoperability.
The four category weights, used to evaluate performance, are changing in 2021. Cost category weight goes up by 5 percentage points, to be 20% of the clinician’s score, and the quality category goes down by 5 percentage points to contribute 40% to the weight. Promoting interoperability remains 25% of the score, with improvement activities constituting the final 15%.
Other key changes include the following:
- The CMS’s Web interface for submission for quality measures will be shuttered in 2021. Users of this submission method will have to find and use another way to report their quality measures.
- Quality measures will be scored against pre-COVID benchmarks in lieu of comparisons with the 2020 reporting year; 206 quality measures are proposed for 2021, compared with the current list of 219.
- Telehealth will be incorporated in the cost category by updates to the measure specifications for the episode-based and total per capita cost measures.
- A new health information exchange measure is added to the promoting interoperability category, and “incorporating” replaces “reconciling” in the reporting requirement “Support Electronic Referral Loops by Receiving and Incorporating Health Information.”
To avoid the 9% penalty, eligible clinicians must earn 50 points in 2021, up from 45 in the current year. Achieving “exceptional performance” remains at 85 points. This elevated level of engagement allows access to a pot of money Congress set aside for high performers.
Many physicians feel that too much work is required to earn the “paltry” bonuses; even a perfect score of 100 has only resulted in bonuses of 1.88% and 1.68%, respectively, in the past 2 years. That includes the $500 million allocation that Congress set aside; this extra funding to reward exceptional performance is only available for the first 6 years of the law. Although the 2019 scores have been released to participants, CMS has not yet announced the overall national average, but it’s expected to be minimal.
The combination of meager payouts and a diminishing funding mechanism has physicians questioning participation altogether. My recent conversations with physicians who qualify for the program revealed their intention to participate, but only at a level to achieve the minimum threshold of 45 points this year and 50 in 2021. With so little upside, it’s impossible to make a business case to aim for the stars.
Perhaps the biggest change in 2021, however, is that the program is not making the previously planned switch to MIPS Value Pathways (MVPs). MVPs were designed to align the four performance categories around a specialty, medical condition, or patient population.
CMS introduced MVPs by giving an example of diabetes: “Endocrinologist reports same ‘foundation’ of PI [promoting interoperability] and population health measures as all other clinicians but now has a MIPS Value Pathway with measures and activities that focus on diabetes prevention and treatment.” CMS had expected MVPs to launch in 2021 for all program participants; because of the pandemic, CMS announced an extension for at least 1 year. This comes as a relief to physicians who are just trying to keep the lights on given the financial pressures brought on by the pandemic.
MVPs, however, will be incorporated into the MIPS Alternative Payment Model (APM) participation segment. This will affect many physicians because this is the path that accountable care organizations (ACOs) have taken. If you are part of an ACO and you report through it, you’ll see some more changes than your colleagues in 2021.
The good news is that ACOs that participate in MIPS and the Medicare Shared Savings Program will have to report only once to satisfy the requirements for both programs. The construct for this new APM-based program is called the “APM Performance Pathway.” This pathway incorporates six population health–based measures that cross-cut specialties.
CMS is also proposing that telemedicine reimbursement will become permanent. As of now, telemedicine services will only be paid when a public health emergency has been declared. This ability to reimburse physicians for telemedicine would end when the current public health emergency is over. CMS is proposing to extend reimbursement beyond the pandemic, which will benefit all physicians who perform these remote encounters.
The CMS proposal would also make some other requirements easier to achieve. The use of codes 99495 and 99496 – the transitional care management codes – is expanding by reducing several key accompanying-services restrictions. Before the public health emergency, there were constraints related to scope of practice; the proposal would extend the ability of advanced practice providers to order diagnostic tests, even after the public health emergency ends.
Furthermore, the proposal reduces restrictions related to billing for remote physiologic monitoring services and outlines the possibility of a new, higher-paying virtual visit code.
Although the Quality Payment Program will undergo some changes, they are minor. Be aware of the requirement to hit the 50-point mark to avoid the steep penalties, however. Perhaps greater benefit will be achieved through the government’s continued push to refine the reimbursement system. As a result of budget neutrality, however, these changes will boost some physicians while resulting in losses for others.
The government’s proposed changes are not final, and there is a period during which they are accepting comments on the proposal; the final rule will be announced in November.
If you want to wash your hands of this now, apply for the 2020 performance year hardship for the Quality Payment Program. The application is now open and available through December 31, 2020; completing it will release you of any program requirements in 2020 (and avoid that hefty 9% penalty on your 2022 reimbursement).
This way, you won’t have to concern yourself with any of these rules until next year; the government’s extension of this “get out of jail free” card is a welcome relief for physicians who are frustrated by the regulatory burdens despite the pressure exerted by COVID. Spending 15 minutes to complete this form is well worth your time and may eliminate much of your worry.
A version of this article originally appeared on Medscape.com.
Amid all the chaos and problems caused by COVID-19, one might hope that physicians would get a break on their complicated payment-reporting programs.
But that’s not the case: The government recently released the 2021 proposed rule for the Quality Payment Program (QPP), often referred to by its most popular participation track, the Merit-Based Incentive Payment System (MIPS). The program, which launched in 2017, gets annual updates, and this year is no different.
Some good news has made primary care and some other physicians happy.
The government’s proposal includes significant changes to reimbursement for all physicians.
Specialties that rely heavily on office-based E/M services are delighted at this change. Those include internists, family physicians, neurologists, pulmonologists, dermatologists, and all other specialties that rely heavily on office encounters.
According to the estimates from the Centers for Medicare & Medicaid Services (CMS), endocrinologists and rheumatologists are the big winners, at 17% and 16% projected increases, respectively. The government has been pushing to make this shift in reimbursement from surgeries and procedures to office visits for years. Although some physicians may celebrate the change, others will not.
The reimbursement plan for professional services depends on budget neutrality, meaning that the budget increases need to be counterbalanced by budget declines. Specialties that rely heavily on procedures and surgeries will suffer losses. These corresponding reductions felt by proceduralists and surgeons will counterbalance the good fortune of physicians who rely on office visits for the bulk of their revenue. Radiologists, for example, are projected by CMS to experience a 11% downturn, and cardiac surgeons face a 9% decline.
These consequences are significant. The 2021 shift may be the single biggest transfer of reimbursement in the history of the scale, which was adopted in the early 1990s.
If the change affected only Medicare reimbursement, perhaps it would be less significant. Because the majority of private payers use the government’s scale – the resource-based relative value scale – the impact will reverberate across physicians’ bottom lines. Given the state of many physicians’ finances, driven by the pandemic, this may send some affected physicians into a downward spiral.
The boost to E/M reimbursement – which represents approximately 20% of the overall Medicare payout to physicians each year – puts downward pressure on the professional services conversion factor as well.
For 2021, it is proposed to be $32.2605, representing a decrease of $3.83 from the 2020 conversion factor of $36.0896. The resultant conversion factor – which serves as a multiplier applied to the relative value unit to come up with the payment – effectively reduces payments to physicians across the board by 10.6%. Thus, even those who enjoy the benefits of the new E/M increases will see the potential reimbursement high point cut down.
Before launching into the changes in store for 2021, it’s good to determine whether you are an eligible clinician: You need to have more than $90,000 in Medicare Part B charges per year, see more than 200 Medicare Part B patients per year, and provide 200 or more covered professional services to Part B patients.
The program is voluntary, but there are steep penalties for eligible clinicians who don’t participate. For the 2021 reporting year, a 9% penalty will be imposed on Medicare reimbursement in 2023 in the event of participation failure. You can verify your participation status here; you’ll need your National Provider Identifier to run the search, but it takes only seconds to determine your eligibility.
A 9% penalty is a pretty big hit to your income. With 9% at stake, eligible clinicians need to actively engage in the program. Although there have been changes, the basic four-category system remains the same for the MIPS track, as follows: quality, cost, improvement activities, and promoting interoperability.
The four category weights, used to evaluate performance, are changing in 2021. Cost category weight goes up by 5 percentage points, to be 20% of the clinician’s score, and the quality category goes down by 5 percentage points to contribute 40% to the weight. Promoting interoperability remains 25% of the score, with improvement activities constituting the final 15%.
Other key changes include the following:
- The CMS’s Web interface for submission for quality measures will be shuttered in 2021. Users of this submission method will have to find and use another way to report their quality measures.
- Quality measures will be scored against pre-COVID benchmarks in lieu of comparisons with the 2020 reporting year; 206 quality measures are proposed for 2021, compared with the current list of 219.
- Telehealth will be incorporated in the cost category by updates to the measure specifications for the episode-based and total per capita cost measures.
- A new health information exchange measure is added to the promoting interoperability category, and “incorporating” replaces “reconciling” in the reporting requirement “Support Electronic Referral Loops by Receiving and Incorporating Health Information.”
To avoid the 9% penalty, eligible clinicians must earn 50 points in 2021, up from 45 in the current year. Achieving “exceptional performance” remains at 85 points. This elevated level of engagement allows access to a pot of money Congress set aside for high performers.
Many physicians feel that too much work is required to earn the “paltry” bonuses; even a perfect score of 100 has only resulted in bonuses of 1.88% and 1.68%, respectively, in the past 2 years. That includes the $500 million allocation that Congress set aside; this extra funding to reward exceptional performance is only available for the first 6 years of the law. Although the 2019 scores have been released to participants, CMS has not yet announced the overall national average, but it’s expected to be minimal.
The combination of meager payouts and a diminishing funding mechanism has physicians questioning participation altogether. My recent conversations with physicians who qualify for the program revealed their intention to participate, but only at a level to achieve the minimum threshold of 45 points this year and 50 in 2021. With so little upside, it’s impossible to make a business case to aim for the stars.
Perhaps the biggest change in 2021, however, is that the program is not making the previously planned switch to MIPS Value Pathways (MVPs). MVPs were designed to align the four performance categories around a specialty, medical condition, or patient population.
CMS introduced MVPs by giving an example of diabetes: “Endocrinologist reports same ‘foundation’ of PI [promoting interoperability] and population health measures as all other clinicians but now has a MIPS Value Pathway with measures and activities that focus on diabetes prevention and treatment.” CMS had expected MVPs to launch in 2021 for all program participants; because of the pandemic, CMS announced an extension for at least 1 year. This comes as a relief to physicians who are just trying to keep the lights on given the financial pressures brought on by the pandemic.
MVPs, however, will be incorporated into the MIPS Alternative Payment Model (APM) participation segment. This will affect many physicians because this is the path that accountable care organizations (ACOs) have taken. If you are part of an ACO and you report through it, you’ll see some more changes than your colleagues in 2021.
The good news is that ACOs that participate in MIPS and the Medicare Shared Savings Program will have to report only once to satisfy the requirements for both programs. The construct for this new APM-based program is called the “APM Performance Pathway.” This pathway incorporates six population health–based measures that cross-cut specialties.
CMS is also proposing that telemedicine reimbursement will become permanent. As of now, telemedicine services will only be paid when a public health emergency has been declared. This ability to reimburse physicians for telemedicine would end when the current public health emergency is over. CMS is proposing to extend reimbursement beyond the pandemic, which will benefit all physicians who perform these remote encounters.
The CMS proposal would also make some other requirements easier to achieve. The use of codes 99495 and 99496 – the transitional care management codes – is expanding by reducing several key accompanying-services restrictions. Before the public health emergency, there were constraints related to scope of practice; the proposal would extend the ability of advanced practice providers to order diagnostic tests, even after the public health emergency ends.
Furthermore, the proposal reduces restrictions related to billing for remote physiologic monitoring services and outlines the possibility of a new, higher-paying virtual visit code.
Although the Quality Payment Program will undergo some changes, they are minor. Be aware of the requirement to hit the 50-point mark to avoid the steep penalties, however. Perhaps greater benefit will be achieved through the government’s continued push to refine the reimbursement system. As a result of budget neutrality, however, these changes will boost some physicians while resulting in losses for others.
The government’s proposed changes are not final, and there is a period during which they are accepting comments on the proposal; the final rule will be announced in November.
If you want to wash your hands of this now, apply for the 2020 performance year hardship for the Quality Payment Program. The application is now open and available through December 31, 2020; completing it will release you of any program requirements in 2020 (and avoid that hefty 9% penalty on your 2022 reimbursement).
This way, you won’t have to concern yourself with any of these rules until next year; the government’s extension of this “get out of jail free” card is a welcome relief for physicians who are frustrated by the regulatory burdens despite the pressure exerted by COVID. Spending 15 minutes to complete this form is well worth your time and may eliminate much of your worry.
A version of this article originally appeared on Medscape.com.
Too many patient call messages
In a recent study published in the Journal of the American Medical Informatics Association that used EHR logs, researchers found that “Clinicians with the highest volume of patient call messages have almost 4 times the odds of burnout compared with clinicians with the fewest.” And they discovered that “No other workload measures were significantly associated with burnout.” Like the majority of papers I skim through, it states the obvious. Doesn’t it makes sense that the busiest of providers should be more vulnerable to stress related symptoms? But is that really true for every provider? Being “busy” doesn’t guarantee that you are productive nor does it mean that the stuff you are doing while you are busy is fulfilling or rewarding either emotionally or financially. Certainly, slogging through a long list of patient call messages at the end of the day does qualify as being busy, but it is more likely to generate anger and frustration than it is fulfillment.
Just because you have a large practice, does that mean that you will necessarily have more messages to review and calls to return than a provider with a smaller practice. Maybe you manage your practice and your time so well that you actually have fewer messages and calls to return and, therefore, are less vulnerable to burnout.
There are three general strategies that you might be employing that result in fewer messages and calls that require your response. It may be that you have developed a handbook of frequently asked questions and trained your staff to use it as a reference in a way that reduces the number of messages that filter to you. Creating this triage book and finding the right personnel took time, but it didn’t necessarily mean that you had to hire staff with extensive training, which can be expensive. In-house training of raw talent that has demonstrated common sense and good communication skills can be cost effective and rewarding. You probably already have discovered that continued attention to quality control is an important part of this strategy. Included in your handbook you may have included a clearcut triage system for the questions that the staff can’t answer. Is it a question you must answer (a) as soon as you finish with this patient, (b) before lunch, or (c) at the end of the day? (Category (c) is of course strongly discouraged).
The second general group of strategies you may be using to keep your calls and messages to a minimum is anticipatory guidance. As you wrap up each visit, are you anticipating what calls it might generate? This of course depends on the nature of the problem and the personality of the patient. From your experience you can probably predict most of the questions that are likely going to crop up after the patient arrives home. Preemptively answering these before patients leave and providing a personalized handout that you discuss with them may easily be saving you two or three calls a day. Because you can’t anticipate every question, you have found that promising a follow-up call in a day or 2 encourages the patients to hold their questions and wait for you or your assistant to call.
Finally, you may have discovered long ago that in many cases it is easier and more efficient to see the patient rather than having your staff spend half their time building and maintaining a communication wall around you. This is particularly true if, during the initial contact with your office, the patients have made it clear that they would like to be seen. This strategy is based on commons sense, but for many physicians and their office staff it may require a dramatic shift in attitude. You may have needed to become more comfortable squeezing in short visits at which the goal is to simply begin the dual processes of anxiety relief and diagnosis. In the beginning, you may have had to frequently remind your staff that their primary goal is patient satisfaction and not protecting you from seeing “too many” patients. Ironically, by being over protective, they may have been contributing to burnout when simply cutting to the chase and having the patient come in to be seen would have generated fewer stress-producing calls and messages.
Enabling a system that generates an excess of patient messages is looking for trouble.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
In a recent study published in the Journal of the American Medical Informatics Association that used EHR logs, researchers found that “Clinicians with the highest volume of patient call messages have almost 4 times the odds of burnout compared with clinicians with the fewest.” And they discovered that “No other workload measures were significantly associated with burnout.” Like the majority of papers I skim through, it states the obvious. Doesn’t it makes sense that the busiest of providers should be more vulnerable to stress related symptoms? But is that really true for every provider? Being “busy” doesn’t guarantee that you are productive nor does it mean that the stuff you are doing while you are busy is fulfilling or rewarding either emotionally or financially. Certainly, slogging through a long list of patient call messages at the end of the day does qualify as being busy, but it is more likely to generate anger and frustration than it is fulfillment.
Just because you have a large practice, does that mean that you will necessarily have more messages to review and calls to return than a provider with a smaller practice. Maybe you manage your practice and your time so well that you actually have fewer messages and calls to return and, therefore, are less vulnerable to burnout.
There are three general strategies that you might be employing that result in fewer messages and calls that require your response. It may be that you have developed a handbook of frequently asked questions and trained your staff to use it as a reference in a way that reduces the number of messages that filter to you. Creating this triage book and finding the right personnel took time, but it didn’t necessarily mean that you had to hire staff with extensive training, which can be expensive. In-house training of raw talent that has demonstrated common sense and good communication skills can be cost effective and rewarding. You probably already have discovered that continued attention to quality control is an important part of this strategy. Included in your handbook you may have included a clearcut triage system for the questions that the staff can’t answer. Is it a question you must answer (a) as soon as you finish with this patient, (b) before lunch, or (c) at the end of the day? (Category (c) is of course strongly discouraged).
The second general group of strategies you may be using to keep your calls and messages to a minimum is anticipatory guidance. As you wrap up each visit, are you anticipating what calls it might generate? This of course depends on the nature of the problem and the personality of the patient. From your experience you can probably predict most of the questions that are likely going to crop up after the patient arrives home. Preemptively answering these before patients leave and providing a personalized handout that you discuss with them may easily be saving you two or three calls a day. Because you can’t anticipate every question, you have found that promising a follow-up call in a day or 2 encourages the patients to hold their questions and wait for you or your assistant to call.
Finally, you may have discovered long ago that in many cases it is easier and more efficient to see the patient rather than having your staff spend half their time building and maintaining a communication wall around you. This is particularly true if, during the initial contact with your office, the patients have made it clear that they would like to be seen. This strategy is based on commons sense, but for many physicians and their office staff it may require a dramatic shift in attitude. You may have needed to become more comfortable squeezing in short visits at which the goal is to simply begin the dual processes of anxiety relief and diagnosis. In the beginning, you may have had to frequently remind your staff that their primary goal is patient satisfaction and not protecting you from seeing “too many” patients. Ironically, by being over protective, they may have been contributing to burnout when simply cutting to the chase and having the patient come in to be seen would have generated fewer stress-producing calls and messages.
Enabling a system that generates an excess of patient messages is looking for trouble.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
In a recent study published in the Journal of the American Medical Informatics Association that used EHR logs, researchers found that “Clinicians with the highest volume of patient call messages have almost 4 times the odds of burnout compared with clinicians with the fewest.” And they discovered that “No other workload measures were significantly associated with burnout.” Like the majority of papers I skim through, it states the obvious. Doesn’t it makes sense that the busiest of providers should be more vulnerable to stress related symptoms? But is that really true for every provider? Being “busy” doesn’t guarantee that you are productive nor does it mean that the stuff you are doing while you are busy is fulfilling or rewarding either emotionally or financially. Certainly, slogging through a long list of patient call messages at the end of the day does qualify as being busy, but it is more likely to generate anger and frustration than it is fulfillment.
Just because you have a large practice, does that mean that you will necessarily have more messages to review and calls to return than a provider with a smaller practice. Maybe you manage your practice and your time so well that you actually have fewer messages and calls to return and, therefore, are less vulnerable to burnout.
There are three general strategies that you might be employing that result in fewer messages and calls that require your response. It may be that you have developed a handbook of frequently asked questions and trained your staff to use it as a reference in a way that reduces the number of messages that filter to you. Creating this triage book and finding the right personnel took time, but it didn’t necessarily mean that you had to hire staff with extensive training, which can be expensive. In-house training of raw talent that has demonstrated common sense and good communication skills can be cost effective and rewarding. You probably already have discovered that continued attention to quality control is an important part of this strategy. Included in your handbook you may have included a clearcut triage system for the questions that the staff can’t answer. Is it a question you must answer (a) as soon as you finish with this patient, (b) before lunch, or (c) at the end of the day? (Category (c) is of course strongly discouraged).
The second general group of strategies you may be using to keep your calls and messages to a minimum is anticipatory guidance. As you wrap up each visit, are you anticipating what calls it might generate? This of course depends on the nature of the problem and the personality of the patient. From your experience you can probably predict most of the questions that are likely going to crop up after the patient arrives home. Preemptively answering these before patients leave and providing a personalized handout that you discuss with them may easily be saving you two or three calls a day. Because you can’t anticipate every question, you have found that promising a follow-up call in a day or 2 encourages the patients to hold their questions and wait for you or your assistant to call.
Finally, you may have discovered long ago that in many cases it is easier and more efficient to see the patient rather than having your staff spend half their time building and maintaining a communication wall around you. This is particularly true if, during the initial contact with your office, the patients have made it clear that they would like to be seen. This strategy is based on commons sense, but for many physicians and their office staff it may require a dramatic shift in attitude. You may have needed to become more comfortable squeezing in short visits at which the goal is to simply begin the dual processes of anxiety relief and diagnosis. In the beginning, you may have had to frequently remind your staff that their primary goal is patient satisfaction and not protecting you from seeing “too many” patients. Ironically, by being over protective, they may have been contributing to burnout when simply cutting to the chase and having the patient come in to be seen would have generated fewer stress-producing calls and messages.
Enabling a system that generates an excess of patient messages is looking for trouble.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
















