User login
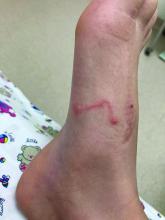
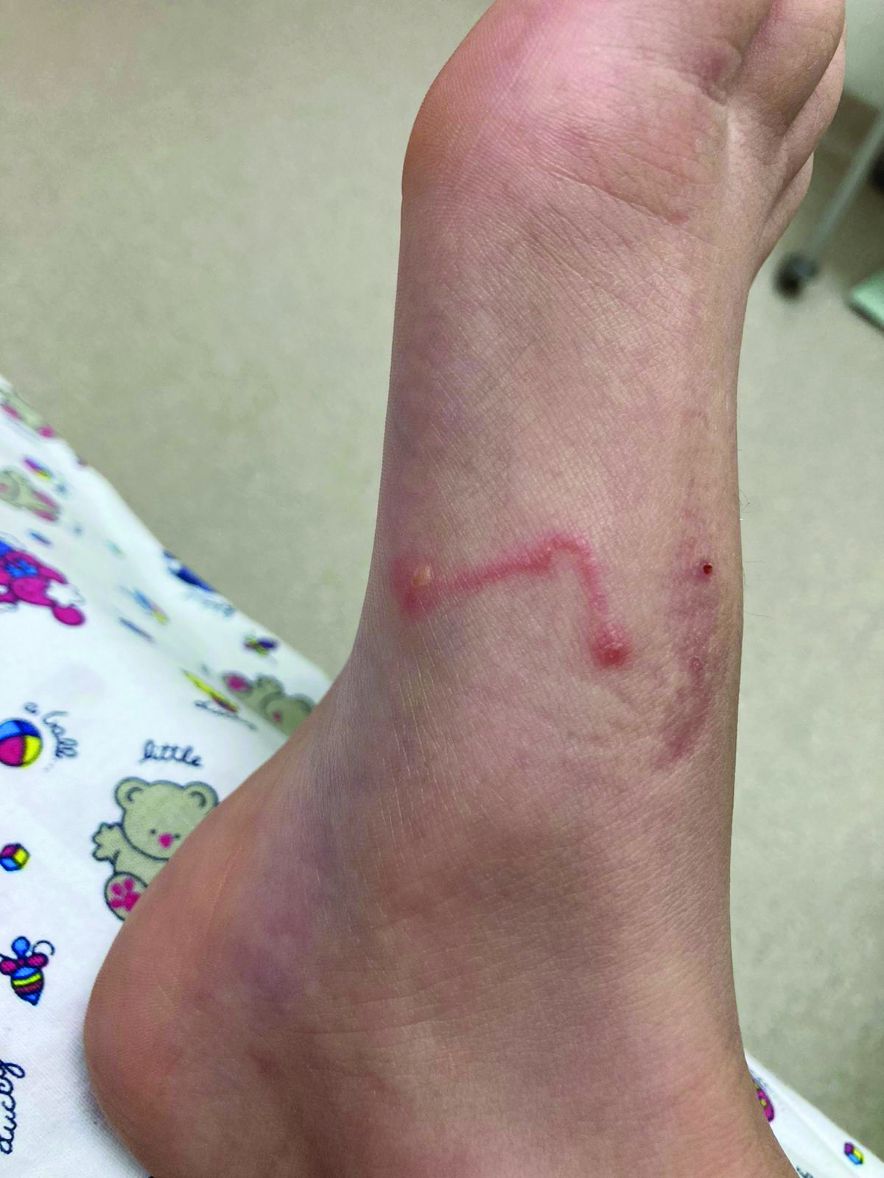
A 9-year-old girl was evaluated for a week-long history of rash on the feet
A complete body examination failed to reveal any other lesions suggestive of a fungal infection. A blood count and urinalysis were within normal limits. She had no lymphadenopathy or hepatosplenomegaly. She was diagnosed with cutaneous larva migrans (CLM) given the clinical appearance of the lesions and the recent travel history.
CLM is a zoonotic infection caused by several hookworms such as Ancylostoma braziliense, Ancylostoma caninum, and Uncinaria stenocephala, as well as human hookworms such as Ancylostoma duodenale and Necator americanus. The hookworms can be present in contaminated soils and sandy beaches on the coastal regions of South America, the Caribbean, the Southeastern United States, Southeast Asia, and Africa.1-5
It is a common disease in the tourist population visiting tropical countries because of exposure to the hookworms in the soil without use of proper foot protection.
The clinical features are of an erythematous linear serpiginous plaque that is pruritic and can progress from millimeters to centimeters in size within a few days to weeks. Vesicles and multiple tracks can also be seen. The most common locations are the feet, buttocks, and thighs.
The larvae in the soil come from eggs excreted in the feces of infected cats and dogs. The infection is caused by direct contact of the larvae with the stratum corneum of the skin creating a burrow and an inflammatory response that will cause erythema, edema, track formation, and pruritus.
Diagnosis is made clinically. Rarely, a skin biopsy is warranted. The differential diagnosis includes tinea pedis, granuloma annulare, larva currens, contact dermatitis, and herpes zoster.
Tinea pedis is a fungal infection of the skin of the feet, commonly localized on the web spaces. The risk factors are a hot and humid environment, prolonged wear of occlusive footwear, excess sweating, and prolonged exposure to water.6 Diagnosis is confirmed by microscopic evaluation of skin scrapings with potassium hydroxide or a fungal culture. The infection is treated with topical antifungal creams and, in severe cases, systemic antifungals. Granuloma annulare is a benign chronic skin condition that presents with annular-shaped lesions. Its etiology is unknown. The lesions may be asymptomatic or mildly pruritic. Localized granuloma annulare typically presents as reddish-brown papules or plaques on the fingers, hands, elbows, dorsal feet, or ankles. The feature distinguishing granuloma annulare from other annular lesions is its absence of scale.
Allergic contact dermatitis is caused by skin exposure to an allergen and a secondary inflammatory response to this material on the skin causing inflammation, vesiculation, and pruritus. Lesions are treated with topical corticosteroids and avoidance of the allergen.
Herpes zoster is caused by a viral infection of the latent varicella-zoster virus. Its reactivation causes the presence of vesicles with an erythematous base that have a dermatomal distribution. The lesions are usually tender. Treatment is recommended to be started within 72 hours of the eruption with antivirals such as acyclovir or valacyclovir.
Cutaneous larva currens is caused by the cutaneous infection with Strongyloides stercoralis. In comparison with CLM, the lesions progress faster, at up to a centimeter within hours.
CLM is usually self-limited. If the patient has multiple lesions or more severe disease, oral albendazole or ivermectin can be prescribed. Other treatments, though not preferred, include freezing and topical thiabendazole solutions.
As our patient had several lesions, oral ivermectin was chosen as treatment and the lesions cleared within a week. Also, she was recommended to always wear shoes when walking on the beach.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Dr. Valderrama is a pediatric dermatologist at Fundación Cardioinfantil, Bogota, Colombia.
References
1. Feldmeier H and Schuster A. Eur J Clin Microbiol Infect Dis. 2012 Jun;31(6):915-8.
2. Jacobson CC and Abel EA. J Am Acad Dermatol. 2007 Jun;56(6):1026-43.
3. Kincaid L et al. Travel Med Infect Dis. 2015 Sep-Oct;13(5):382-7.
4. Gill N et al. Adv Skin Wound Care. 2020 Jul;33(7):356-9.
5. Rodenas-Herranz T et al. Dermatol Ther. 2020 May;33(3):e13316.
6. Pramod K et al. In: StatPearls [Internet]. Treasure Island (Fla): StatPearls Publishing; 2022 Jan.
A complete body examination failed to reveal any other lesions suggestive of a fungal infection. A blood count and urinalysis were within normal limits. She had no lymphadenopathy or hepatosplenomegaly. She was diagnosed with cutaneous larva migrans (CLM) given the clinical appearance of the lesions and the recent travel history.
CLM is a zoonotic infection caused by several hookworms such as Ancylostoma braziliense, Ancylostoma caninum, and Uncinaria stenocephala, as well as human hookworms such as Ancylostoma duodenale and Necator americanus. The hookworms can be present in contaminated soils and sandy beaches on the coastal regions of South America, the Caribbean, the Southeastern United States, Southeast Asia, and Africa.1-5
It is a common disease in the tourist population visiting tropical countries because of exposure to the hookworms in the soil without use of proper foot protection.
The clinical features are of an erythematous linear serpiginous plaque that is pruritic and can progress from millimeters to centimeters in size within a few days to weeks. Vesicles and multiple tracks can also be seen. The most common locations are the feet, buttocks, and thighs.
The larvae in the soil come from eggs excreted in the feces of infected cats and dogs. The infection is caused by direct contact of the larvae with the stratum corneum of the skin creating a burrow and an inflammatory response that will cause erythema, edema, track formation, and pruritus.
Diagnosis is made clinically. Rarely, a skin biopsy is warranted. The differential diagnosis includes tinea pedis, granuloma annulare, larva currens, contact dermatitis, and herpes zoster.
Tinea pedis is a fungal infection of the skin of the feet, commonly localized on the web spaces. The risk factors are a hot and humid environment, prolonged wear of occlusive footwear, excess sweating, and prolonged exposure to water.6 Diagnosis is confirmed by microscopic evaluation of skin scrapings with potassium hydroxide or a fungal culture. The infection is treated with topical antifungal creams and, in severe cases, systemic antifungals. Granuloma annulare is a benign chronic skin condition that presents with annular-shaped lesions. Its etiology is unknown. The lesions may be asymptomatic or mildly pruritic. Localized granuloma annulare typically presents as reddish-brown papules or plaques on the fingers, hands, elbows, dorsal feet, or ankles. The feature distinguishing granuloma annulare from other annular lesions is its absence of scale.
Allergic contact dermatitis is caused by skin exposure to an allergen and a secondary inflammatory response to this material on the skin causing inflammation, vesiculation, and pruritus. Lesions are treated with topical corticosteroids and avoidance of the allergen.
Herpes zoster is caused by a viral infection of the latent varicella-zoster virus. Its reactivation causes the presence of vesicles with an erythematous base that have a dermatomal distribution. The lesions are usually tender. Treatment is recommended to be started within 72 hours of the eruption with antivirals such as acyclovir or valacyclovir.
Cutaneous larva currens is caused by the cutaneous infection with Strongyloides stercoralis. In comparison with CLM, the lesions progress faster, at up to a centimeter within hours.
CLM is usually self-limited. If the patient has multiple lesions or more severe disease, oral albendazole or ivermectin can be prescribed. Other treatments, though not preferred, include freezing and topical thiabendazole solutions.
As our patient had several lesions, oral ivermectin was chosen as treatment and the lesions cleared within a week. Also, she was recommended to always wear shoes when walking on the beach.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Dr. Valderrama is a pediatric dermatologist at Fundación Cardioinfantil, Bogota, Colombia.
References
1. Feldmeier H and Schuster A. Eur J Clin Microbiol Infect Dis. 2012 Jun;31(6):915-8.
2. Jacobson CC and Abel EA. J Am Acad Dermatol. 2007 Jun;56(6):1026-43.
3. Kincaid L et al. Travel Med Infect Dis. 2015 Sep-Oct;13(5):382-7.
4. Gill N et al. Adv Skin Wound Care. 2020 Jul;33(7):356-9.
5. Rodenas-Herranz T et al. Dermatol Ther. 2020 May;33(3):e13316.
6. Pramod K et al. In: StatPearls [Internet]. Treasure Island (Fla): StatPearls Publishing; 2022 Jan.
A complete body examination failed to reveal any other lesions suggestive of a fungal infection. A blood count and urinalysis were within normal limits. She had no lymphadenopathy or hepatosplenomegaly. She was diagnosed with cutaneous larva migrans (CLM) given the clinical appearance of the lesions and the recent travel history.
CLM is a zoonotic infection caused by several hookworms such as Ancylostoma braziliense, Ancylostoma caninum, and Uncinaria stenocephala, as well as human hookworms such as Ancylostoma duodenale and Necator americanus. The hookworms can be present in contaminated soils and sandy beaches on the coastal regions of South America, the Caribbean, the Southeastern United States, Southeast Asia, and Africa.1-5
It is a common disease in the tourist population visiting tropical countries because of exposure to the hookworms in the soil without use of proper foot protection.
The clinical features are of an erythematous linear serpiginous plaque that is pruritic and can progress from millimeters to centimeters in size within a few days to weeks. Vesicles and multiple tracks can also be seen. The most common locations are the feet, buttocks, and thighs.
The larvae in the soil come from eggs excreted in the feces of infected cats and dogs. The infection is caused by direct contact of the larvae with the stratum corneum of the skin creating a burrow and an inflammatory response that will cause erythema, edema, track formation, and pruritus.
Diagnosis is made clinically. Rarely, a skin biopsy is warranted. The differential diagnosis includes tinea pedis, granuloma annulare, larva currens, contact dermatitis, and herpes zoster.
Tinea pedis is a fungal infection of the skin of the feet, commonly localized on the web spaces. The risk factors are a hot and humid environment, prolonged wear of occlusive footwear, excess sweating, and prolonged exposure to water.6 Diagnosis is confirmed by microscopic evaluation of skin scrapings with potassium hydroxide or a fungal culture. The infection is treated with topical antifungal creams and, in severe cases, systemic antifungals. Granuloma annulare is a benign chronic skin condition that presents with annular-shaped lesions. Its etiology is unknown. The lesions may be asymptomatic or mildly pruritic. Localized granuloma annulare typically presents as reddish-brown papules or plaques on the fingers, hands, elbows, dorsal feet, or ankles. The feature distinguishing granuloma annulare from other annular lesions is its absence of scale.
Allergic contact dermatitis is caused by skin exposure to an allergen and a secondary inflammatory response to this material on the skin causing inflammation, vesiculation, and pruritus. Lesions are treated with topical corticosteroids and avoidance of the allergen.
Herpes zoster is caused by a viral infection of the latent varicella-zoster virus. Its reactivation causes the presence of vesicles with an erythematous base that have a dermatomal distribution. The lesions are usually tender. Treatment is recommended to be started within 72 hours of the eruption with antivirals such as acyclovir or valacyclovir.
Cutaneous larva currens is caused by the cutaneous infection with Strongyloides stercoralis. In comparison with CLM, the lesions progress faster, at up to a centimeter within hours.
CLM is usually self-limited. If the patient has multiple lesions or more severe disease, oral albendazole or ivermectin can be prescribed. Other treatments, though not preferred, include freezing and topical thiabendazole solutions.
As our patient had several lesions, oral ivermectin was chosen as treatment and the lesions cleared within a week. Also, she was recommended to always wear shoes when walking on the beach.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Dr. Valderrama is a pediatric dermatologist at Fundación Cardioinfantil, Bogota, Colombia.
References
1. Feldmeier H and Schuster A. Eur J Clin Microbiol Infect Dis. 2012 Jun;31(6):915-8.
2. Jacobson CC and Abel EA. J Am Acad Dermatol. 2007 Jun;56(6):1026-43.
3. Kincaid L et al. Travel Med Infect Dis. 2015 Sep-Oct;13(5):382-7.
4. Gill N et al. Adv Skin Wound Care. 2020 Jul;33(7):356-9.
5. Rodenas-Herranz T et al. Dermatol Ther. 2020 May;33(3):e13316.
6. Pramod K et al. In: StatPearls [Internet]. Treasure Island (Fla): StatPearls Publishing; 2022 Jan.
Her mother reported recent travel to a beachside city in Colombia. A review of systems was negative. She was not taking any other medications or vitamin supplements. There were no pets at home and no other affected family members. Physical exam was notable for an erythematous curvilinear plaque on the feet and a small vesicle.
Risk factors in children linked to stroke as soon as 30s, 40s
In a case-control study, atherosclerotic risk factors were uncommon in childhood and did not appear to be associated with the pathogenesis of arterial ischemic stroke in children or in early young adulthood.
But by the fourth and fifth decades of life, these risk factors were strongly associated with a significant risk for stroke, heightening that risk almost tenfold.
“While strokes in childhood and very early adulthood are not likely caused by atherosclerotic risk factors, it does look like these risk factors increase throughout early and young adulthood and become significant risk factors for stroke in the 30s and 40s,” lead author Sharon N. Poisson, MD, MAS, associate professor of neurology at the University of Colorado at Denver, Aurora, said in an interview.
The findings were published online in JAMA Neurology.
In this study, the researchers focused on arterial ischemic stroke, not hemorrhagic stroke. “We know that high blood pressure, diabetes, smoking, obesity, all of these are risk factors for ischemic stroke, but what we didn’t know is at what age do those atherosclerotic risk factors actually start to cause stroke,” Dr. Poisson said.
To find out more, she and her team did a case control study of data in the Kaiser Permanente Northern California system, which had been accumulating relevant data over a period of 14 years, from Jan. 1, 2000, through Dec. 31, 2014.
The analysis included 141 children and 455 young adults with arterial ischemic stroke and 1,382 age-matched controls.
The children were divided into two age categories: ages 29 days to 9 years and ages 10-19 years.
In the younger group, there were 69 cases of arterial ischemic stroke. In the older age group, there were 72 cases.
Young adults were divided into three age categories: 20-29 years (n = 71 cases), 30-39 years (144 cases), and 40-49 years (240 cases).
Among pediatric controls, 168 children aged 29 days to 9 years (46.5%) and 196 children aged 10-19 years (53.8%) developed arterial ischemic stroke.
There were 121 cases of ischemic stroke among young adult controls aged 20-29 years, 298 cases among controls aged 30-39 years, and 599 cases in those aged 40-49 years.
Both childhood cases and controls had a low prevalence of documented diagnoses of atherosclerotic risk factors (ARFs). The odds ratio of having any ARFs on arterial ischemic stroke was 1.87 for ages 0-9 years, and 1.00 for ages 10-19.
However, cases rose with age.
The OR was 2.3 for age range 20-29 years, 3.57 for age range 30-39 years, and 4.91 for age range 40-49 years.
The analysis also showed that the OR associated with multiple ARFs was 5.29 for age range 0-9 years, 2.75 for age range 10-19 years, 7.33 for age range 20-29 years, 9.86 for age range 30-39 years, and 9.35 for age range 40-49 years.
Multiple risk factors were rare in children but became more prevalent with each decade of young adult life.
The presumed cause of arterial ischemic stroke was atherosclerosis. Evidence of atherosclerosis was present in 1.4% of those aged 10-19 years, 8.5% of those aged 20-29 years, 21.5% of those aged 30-39 years, and 42.5% of those aged 40-49 years.
“This study tells us that, while stroke in adolescence and very early adulthood may not be caused by atherosclerotic risk factors, starting to accumulate those risk factors early in life clearly increases the risk of stroke in the 30s and 40s. I hope we can get this message across, because the sooner we can treat the risk factors, the better the outcome,” Dr. Poisson said.
Prevention starts in childhood
Prevention of cardiovascular disease begins in childhood, which is a paradigm shift from the way cardiovascular disease was thought of a couple of decades ago, noted pediatric cardiologist Guilherme Baptista de Faia, MD, from the Ann & Robert H. Lurie Children’s Hospital in Chicago.
“Our guidelines for risk factor reduction in children aim to address how or when do we screen for these risk factors, how or when do we intervene, and do these interventions impact cardiovascular outcomes later in life? This article is part of the mounting research that aims to understand the relationship between childhood cardiovascular risk factors and early cardiovascular disease,” Dr. Baptista de Faia said.
“There has been an interesting progression in our understanding of the impact of CV risk factors early in life. Large cohorts such as Bogalusa Heart Study, Risk in Young Finns Study, Muscatine Study, the Childhood Determinants of Adult Health, CARDIA, and the International Childhood Cardiovascular Cohorts (i3C) have been instrumental in evaluating this question,” he said.
The knowledge that atherosclerotic risk factors in children can lead to acceleration of atherosclerosis in later life opens the door to preventive medicine, said Dr. Baptista de Faia, who was not part of the study.
“This is where preventive medicine comes in. If we can identify the children at increased risk, can we intervene to improve outcomes later in life?” he said. Familial hypercholesterolemia is “a great example of this. We can screen children early in life, there is an effective treatment, and we know from populations studies that early treatment significantly decreases the risk for cardiovascular disease later in life.”
Dr. Poisson reported that she received grants from the National Institutes of Health during the conduct of this study, which was supported by the NIH.
A version of this article first appeared on Medscape.com.
In a case-control study, atherosclerotic risk factors were uncommon in childhood and did not appear to be associated with the pathogenesis of arterial ischemic stroke in children or in early young adulthood.
But by the fourth and fifth decades of life, these risk factors were strongly associated with a significant risk for stroke, heightening that risk almost tenfold.
“While strokes in childhood and very early adulthood are not likely caused by atherosclerotic risk factors, it does look like these risk factors increase throughout early and young adulthood and become significant risk factors for stroke in the 30s and 40s,” lead author Sharon N. Poisson, MD, MAS, associate professor of neurology at the University of Colorado at Denver, Aurora, said in an interview.
The findings were published online in JAMA Neurology.
In this study, the researchers focused on arterial ischemic stroke, not hemorrhagic stroke. “We know that high blood pressure, diabetes, smoking, obesity, all of these are risk factors for ischemic stroke, but what we didn’t know is at what age do those atherosclerotic risk factors actually start to cause stroke,” Dr. Poisson said.
To find out more, she and her team did a case control study of data in the Kaiser Permanente Northern California system, which had been accumulating relevant data over a period of 14 years, from Jan. 1, 2000, through Dec. 31, 2014.
The analysis included 141 children and 455 young adults with arterial ischemic stroke and 1,382 age-matched controls.
The children were divided into two age categories: ages 29 days to 9 years and ages 10-19 years.
In the younger group, there were 69 cases of arterial ischemic stroke. In the older age group, there were 72 cases.
Young adults were divided into three age categories: 20-29 years (n = 71 cases), 30-39 years (144 cases), and 40-49 years (240 cases).
Among pediatric controls, 168 children aged 29 days to 9 years (46.5%) and 196 children aged 10-19 years (53.8%) developed arterial ischemic stroke.
There were 121 cases of ischemic stroke among young adult controls aged 20-29 years, 298 cases among controls aged 30-39 years, and 599 cases in those aged 40-49 years.
Both childhood cases and controls had a low prevalence of documented diagnoses of atherosclerotic risk factors (ARFs). The odds ratio of having any ARFs on arterial ischemic stroke was 1.87 for ages 0-9 years, and 1.00 for ages 10-19.
However, cases rose with age.
The OR was 2.3 for age range 20-29 years, 3.57 for age range 30-39 years, and 4.91 for age range 40-49 years.
The analysis also showed that the OR associated with multiple ARFs was 5.29 for age range 0-9 years, 2.75 for age range 10-19 years, 7.33 for age range 20-29 years, 9.86 for age range 30-39 years, and 9.35 for age range 40-49 years.
Multiple risk factors were rare in children but became more prevalent with each decade of young adult life.
The presumed cause of arterial ischemic stroke was atherosclerosis. Evidence of atherosclerosis was present in 1.4% of those aged 10-19 years, 8.5% of those aged 20-29 years, 21.5% of those aged 30-39 years, and 42.5% of those aged 40-49 years.
“This study tells us that, while stroke in adolescence and very early adulthood may not be caused by atherosclerotic risk factors, starting to accumulate those risk factors early in life clearly increases the risk of stroke in the 30s and 40s. I hope we can get this message across, because the sooner we can treat the risk factors, the better the outcome,” Dr. Poisson said.
Prevention starts in childhood
Prevention of cardiovascular disease begins in childhood, which is a paradigm shift from the way cardiovascular disease was thought of a couple of decades ago, noted pediatric cardiologist Guilherme Baptista de Faia, MD, from the Ann & Robert H. Lurie Children’s Hospital in Chicago.
“Our guidelines for risk factor reduction in children aim to address how or when do we screen for these risk factors, how or when do we intervene, and do these interventions impact cardiovascular outcomes later in life? This article is part of the mounting research that aims to understand the relationship between childhood cardiovascular risk factors and early cardiovascular disease,” Dr. Baptista de Faia said.
“There has been an interesting progression in our understanding of the impact of CV risk factors early in life. Large cohorts such as Bogalusa Heart Study, Risk in Young Finns Study, Muscatine Study, the Childhood Determinants of Adult Health, CARDIA, and the International Childhood Cardiovascular Cohorts (i3C) have been instrumental in evaluating this question,” he said.
The knowledge that atherosclerotic risk factors in children can lead to acceleration of atherosclerosis in later life opens the door to preventive medicine, said Dr. Baptista de Faia, who was not part of the study.
“This is where preventive medicine comes in. If we can identify the children at increased risk, can we intervene to improve outcomes later in life?” he said. Familial hypercholesterolemia is “a great example of this. We can screen children early in life, there is an effective treatment, and we know from populations studies that early treatment significantly decreases the risk for cardiovascular disease later in life.”
Dr. Poisson reported that she received grants from the National Institutes of Health during the conduct of this study, which was supported by the NIH.
A version of this article first appeared on Medscape.com.
In a case-control study, atherosclerotic risk factors were uncommon in childhood and did not appear to be associated with the pathogenesis of arterial ischemic stroke in children or in early young adulthood.
But by the fourth and fifth decades of life, these risk factors were strongly associated with a significant risk for stroke, heightening that risk almost tenfold.
“While strokes in childhood and very early adulthood are not likely caused by atherosclerotic risk factors, it does look like these risk factors increase throughout early and young adulthood and become significant risk factors for stroke in the 30s and 40s,” lead author Sharon N. Poisson, MD, MAS, associate professor of neurology at the University of Colorado at Denver, Aurora, said in an interview.
The findings were published online in JAMA Neurology.
In this study, the researchers focused on arterial ischemic stroke, not hemorrhagic stroke. “We know that high blood pressure, diabetes, smoking, obesity, all of these are risk factors for ischemic stroke, but what we didn’t know is at what age do those atherosclerotic risk factors actually start to cause stroke,” Dr. Poisson said.
To find out more, she and her team did a case control study of data in the Kaiser Permanente Northern California system, which had been accumulating relevant data over a period of 14 years, from Jan. 1, 2000, through Dec. 31, 2014.
The analysis included 141 children and 455 young adults with arterial ischemic stroke and 1,382 age-matched controls.
The children were divided into two age categories: ages 29 days to 9 years and ages 10-19 years.
In the younger group, there were 69 cases of arterial ischemic stroke. In the older age group, there were 72 cases.
Young adults were divided into three age categories: 20-29 years (n = 71 cases), 30-39 years (144 cases), and 40-49 years (240 cases).
Among pediatric controls, 168 children aged 29 days to 9 years (46.5%) and 196 children aged 10-19 years (53.8%) developed arterial ischemic stroke.
There were 121 cases of ischemic stroke among young adult controls aged 20-29 years, 298 cases among controls aged 30-39 years, and 599 cases in those aged 40-49 years.
Both childhood cases and controls had a low prevalence of documented diagnoses of atherosclerotic risk factors (ARFs). The odds ratio of having any ARFs on arterial ischemic stroke was 1.87 for ages 0-9 years, and 1.00 for ages 10-19.
However, cases rose with age.
The OR was 2.3 for age range 20-29 years, 3.57 for age range 30-39 years, and 4.91 for age range 40-49 years.
The analysis also showed that the OR associated with multiple ARFs was 5.29 for age range 0-9 years, 2.75 for age range 10-19 years, 7.33 for age range 20-29 years, 9.86 for age range 30-39 years, and 9.35 for age range 40-49 years.
Multiple risk factors were rare in children but became more prevalent with each decade of young adult life.
The presumed cause of arterial ischemic stroke was atherosclerosis. Evidence of atherosclerosis was present in 1.4% of those aged 10-19 years, 8.5% of those aged 20-29 years, 21.5% of those aged 30-39 years, and 42.5% of those aged 40-49 years.
“This study tells us that, while stroke in adolescence and very early adulthood may not be caused by atherosclerotic risk factors, starting to accumulate those risk factors early in life clearly increases the risk of stroke in the 30s and 40s. I hope we can get this message across, because the sooner we can treat the risk factors, the better the outcome,” Dr. Poisson said.
Prevention starts in childhood
Prevention of cardiovascular disease begins in childhood, which is a paradigm shift from the way cardiovascular disease was thought of a couple of decades ago, noted pediatric cardiologist Guilherme Baptista de Faia, MD, from the Ann & Robert H. Lurie Children’s Hospital in Chicago.
“Our guidelines for risk factor reduction in children aim to address how or when do we screen for these risk factors, how or when do we intervene, and do these interventions impact cardiovascular outcomes later in life? This article is part of the mounting research that aims to understand the relationship between childhood cardiovascular risk factors and early cardiovascular disease,” Dr. Baptista de Faia said.
“There has been an interesting progression in our understanding of the impact of CV risk factors early in life. Large cohorts such as Bogalusa Heart Study, Risk in Young Finns Study, Muscatine Study, the Childhood Determinants of Adult Health, CARDIA, and the International Childhood Cardiovascular Cohorts (i3C) have been instrumental in evaluating this question,” he said.
The knowledge that atherosclerotic risk factors in children can lead to acceleration of atherosclerosis in later life opens the door to preventive medicine, said Dr. Baptista de Faia, who was not part of the study.
“This is where preventive medicine comes in. If we can identify the children at increased risk, can we intervene to improve outcomes later in life?” he said. Familial hypercholesterolemia is “a great example of this. We can screen children early in life, there is an effective treatment, and we know from populations studies that early treatment significantly decreases the risk for cardiovascular disease later in life.”
Dr. Poisson reported that she received grants from the National Institutes of Health during the conduct of this study, which was supported by the NIH.
A version of this article first appeared on Medscape.com.
FROM JAMA NEUROLOGY
Acute otitis media pneumococcal disease burden in children due to serotypes not included in vaccines
My group in Rochester, N.Y., examined the current pneumococcal serotypes causing AOM in children. From our data, we can determine the PCV13 vaccine types that escape prevention and cause AOM and understand what effect to expect from the new pneumococcal conjugate vaccines (PCVs) that will be coming soon. There are limited data from middle ear fluid (MEF) cultures on which to base such analyses. Tympanocentesis is the preferred method for securing MEF for culture and our group is unique in providing such data to the Centers for Disease Control and publishing our results on a periodic basis to inform clinicians.
Pneumococci are the second most common cause of acute otitis media (AOM) since the introduction of pneumococcal conjugate vaccines (PCVs) more than 2 decades ago.1,2 Pneumococcal AOM causes more severe acute disease and more often causes suppurative complications than Haemophilus influenzae, which is the most common cause of AOM. Prevention of pneumococcal AOM will be a highly relevant contributor to cost-effectiveness analyses for the anticipated introduction of PCV15 (Merck) and PCV20 (Pfizer). Both PCV15 and PCV20 have been licensed for adult use; PCV15 licensure for infants and children occurred in June 2022 for invasive pneumococcal disease and is anticipated in the near future for PCV20. They are improvements over PCV13 because they add serotypes that cause invasive pneumococcal diseases, although less so for prevention of AOM, on the basis of our data.
Nasopharyngeal colonization is a necessary pathogenic step in progression to pneumococcal disease. However, not all strains of pneumococci expressing different capsular serotypes are equally virulent and likely to cause disease. In PCV-vaccinated populations, vaccine pressure and antibiotic resistance drive PCV serotype replacement with nonvaccine serotypes (NVTs), gradually reducing the net effectiveness of the vaccines. Therefore, knowledge of prevalent NVTs colonizing the nasopharynx identifies future pneumococcal serotypes most likely to emerge as pathogenic.
We published an effectiveness study of PCV13.3 A relative reduction of 86% in AOM caused by strains expressing PCV13 serotypes was observed in the first few years after PCV13 introduction. The greatest reduction in MEF samples was in serotype 19A, with a relative reduction of 91%. However, over time the vaccine type efficacy of PCV13 against MEF-positive pneumococcal AOM has eroded. There was no clear efficacy against serotype 3, and we still observed cases of serotype 19A and 19F. PCV13 vaccine failures have been even more frequent in Europe (nearly 30% of pneumococcal AOM in Europe is caused by vaccine serotypes) than our data indicate, where about 10% of AOM is caused by PCV13 serotypes.
In our most recent publication covering 2015-2019, we described results from 589 children, aged 6-36 months, from whom we collected 2,042 nasopharyngeal samples.2,4 During AOM, 495 MEF samples from 319 AOM-infected children were collected (during bilateral infections, tympanocentesis was performed in both ears). Whether bacteria were isolated was based per AOM case, not per tap. The average age of children with AOM was 15 months (range 6-31 months). The three most prevalent nasopharyngeal pneumococcal serotypes were 35B, 23B, and 15B/C. Serotype 35B was the most common at AOM visits in both the nasopharynx and MEF samples followed by serotype 15B/C. Nonsusceptibility among pneumococci to penicillin, azithromycin, and multiple other antibiotics was high. Increasing resistance to ceftriaxone was also observed.
Based on our results, if PCV15 (PCV13 + 22F and 33F) effectiveness is identical to PCV13 for the included serotypes and 100% efficacy for the added serotypes is presumed, PCV15 will reduce pneumococcal AOMs by 8%, pneumococcal nasopharyngeal colonization events at onset of AOM by 6%, and pneumococcal nasopharyngeal colonization events during health by 3%. As for the projected reductions brought about by PCV20 (PCV15 + 8, 10A, 11A, 12F, and 15B), presuming serotype 15B is efficacious against serotype 15C and 100% efficacy for the added serotypes, PCV20 will reduce pneumococcal AOMs by 22%, pneumococcal nasopharyngeal colonization events at onset of AOM by 20%, and pneumococcal nasopharyngeal colonization events during health by 3% (Figure).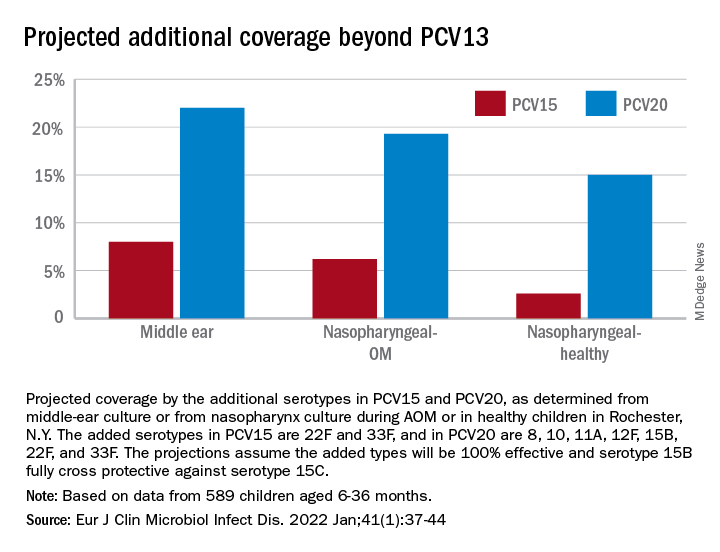
The CDC estimated that, in 2004, pneumococcal disease in the United States caused 4 million illness episodes, 22,000 deaths, 445,000 hospitalizations, 774,000 emergency department visits, 5 million outpatient visits, and 4.1 million outpatient antibiotic prescriptions. Direct medical costs totaled $3.5 billion. Pneumonia (866,000 cases) accounted for 22% of all cases and 72% of pneumococcal costs. AOM and sinusitis (1.5 million cases each) composed 75% of cases and 16% of direct medical costs.5 However, if indirect costs are taken into account, such as work loss by parents of young children, the cost of pneumococcal disease caused by AOM alone may exceed $6 billion annually6 and become dominant in the cost-effectiveness analysis in high-income countries.
Despite widespread use of PCV13, Pneumococcus has shown its resilience under vaccine pressure such that the organism remains a very common AOM pathogen. All-cause AOM has declined modestly and pneumococcal AOM caused by the specific serotypes in PCVs has declined dramatically since the introduction of PCVs. However, the burden of pneumococcal AOM disease is still considerable.
The notion that strains expressing serotypes that were not included in PCV7 were less virulent was proven wrong within a few years after introduction of PCV7, with the emergence of strains expressing serotype 19A, and others. The same cycle occurred after introduction of PCV13. It appears to take about 4 years after introduction of a PCV before peak effectiveness is achieved – which then begins to erode with emergence of NVTs. First, the NVTs are observed to colonize the nasopharynx as commensals and then from among those strains new disease-causing strains emerge.
At the most recent meeting of the International Society of Pneumococci and Pneumococcal Diseases in Toronto in June, many presentations focused on the fact that PCVs elicit highly effective protective serotype-specific antibodies to the capsular polysaccharides of included types. However, 100 serotypes are known. The limitations of PCVs are becoming increasingly apparent. They are costly and consume a large portion of the Vaccines for Children budget. Children in the developing world remain largely unvaccinated because of the high cost. NVTs that have emerged to cause disease vary by country, vary by adult vs. pediatric populations, and are dynamically changing year to year. Forthcoming PCVs of 15 and 20 serotypes will be even more costly than PCV13, will not include many newly emerged serotypes, and will probably likewise encounter “serotype replacement” because of high immune evasion by pneumococci.
When Merck and Pfizer made their decisions on serotype composition for PCV15 and PCV20, respectively, they were based on available data at the time regarding predominant serotypes causing invasive pneumococcal disease in countries that had the best data and would be the market for their products. However, from the time of the decision to licensure of vaccine is many years, and during that time the pneumococcal serotypes have changed, more so for AOM, and I predict more change will occur in the future.
In the past 3 years, Dr. Pichichero has received honoraria from Merck to attend 1-day consulting meetings and his institution has received investigator-initiated research grants to study aspects of PCV15. In the past 3 years, he was reimbursed for expenses to attend the ISPPD meeting in Toronto to present a poster on potential efficacy of PCV20 to prevent complicated AOM.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital.
References
1. Kaur R et al. Pediatrics. 2017;140(3).
2. Kaur R et al. Eur J Clin Microbiol Infect Dis. 2021;41:37-44..
3. Pichichero M et al. Lancet Child Adolesc Health. 2018;2(8):561-8.
4. Zhou F et al. Pediatrics. 2008;121(2):253-60.
5. Huang SS et al. Vaccine. 2011;29(18):3398-412.
6. Casey JR and Pichichero ME. Clin Pediatr (Phila). 2014;53(9):865-73. .
My group in Rochester, N.Y., examined the current pneumococcal serotypes causing AOM in children. From our data, we can determine the PCV13 vaccine types that escape prevention and cause AOM and understand what effect to expect from the new pneumococcal conjugate vaccines (PCVs) that will be coming soon. There are limited data from middle ear fluid (MEF) cultures on which to base such analyses. Tympanocentesis is the preferred method for securing MEF for culture and our group is unique in providing such data to the Centers for Disease Control and publishing our results on a periodic basis to inform clinicians.
Pneumococci are the second most common cause of acute otitis media (AOM) since the introduction of pneumococcal conjugate vaccines (PCVs) more than 2 decades ago.1,2 Pneumococcal AOM causes more severe acute disease and more often causes suppurative complications than Haemophilus influenzae, which is the most common cause of AOM. Prevention of pneumococcal AOM will be a highly relevant contributor to cost-effectiveness analyses for the anticipated introduction of PCV15 (Merck) and PCV20 (Pfizer). Both PCV15 and PCV20 have been licensed for adult use; PCV15 licensure for infants and children occurred in June 2022 for invasive pneumococcal disease and is anticipated in the near future for PCV20. They are improvements over PCV13 because they add serotypes that cause invasive pneumococcal diseases, although less so for prevention of AOM, on the basis of our data.
Nasopharyngeal colonization is a necessary pathogenic step in progression to pneumococcal disease. However, not all strains of pneumococci expressing different capsular serotypes are equally virulent and likely to cause disease. In PCV-vaccinated populations, vaccine pressure and antibiotic resistance drive PCV serotype replacement with nonvaccine serotypes (NVTs), gradually reducing the net effectiveness of the vaccines. Therefore, knowledge of prevalent NVTs colonizing the nasopharynx identifies future pneumococcal serotypes most likely to emerge as pathogenic.
We published an effectiveness study of PCV13.3 A relative reduction of 86% in AOM caused by strains expressing PCV13 serotypes was observed in the first few years after PCV13 introduction. The greatest reduction in MEF samples was in serotype 19A, with a relative reduction of 91%. However, over time the vaccine type efficacy of PCV13 against MEF-positive pneumococcal AOM has eroded. There was no clear efficacy against serotype 3, and we still observed cases of serotype 19A and 19F. PCV13 vaccine failures have been even more frequent in Europe (nearly 30% of pneumococcal AOM in Europe is caused by vaccine serotypes) than our data indicate, where about 10% of AOM is caused by PCV13 serotypes.
In our most recent publication covering 2015-2019, we described results from 589 children, aged 6-36 months, from whom we collected 2,042 nasopharyngeal samples.2,4 During AOM, 495 MEF samples from 319 AOM-infected children were collected (during bilateral infections, tympanocentesis was performed in both ears). Whether bacteria were isolated was based per AOM case, not per tap. The average age of children with AOM was 15 months (range 6-31 months). The three most prevalent nasopharyngeal pneumococcal serotypes were 35B, 23B, and 15B/C. Serotype 35B was the most common at AOM visits in both the nasopharynx and MEF samples followed by serotype 15B/C. Nonsusceptibility among pneumococci to penicillin, azithromycin, and multiple other antibiotics was high. Increasing resistance to ceftriaxone was also observed.
Based on our results, if PCV15 (PCV13 + 22F and 33F) effectiveness is identical to PCV13 for the included serotypes and 100% efficacy for the added serotypes is presumed, PCV15 will reduce pneumococcal AOMs by 8%, pneumococcal nasopharyngeal colonization events at onset of AOM by 6%, and pneumococcal nasopharyngeal colonization events during health by 3%. As for the projected reductions brought about by PCV20 (PCV15 + 8, 10A, 11A, 12F, and 15B), presuming serotype 15B is efficacious against serotype 15C and 100% efficacy for the added serotypes, PCV20 will reduce pneumococcal AOMs by 22%, pneumococcal nasopharyngeal colonization events at onset of AOM by 20%, and pneumococcal nasopharyngeal colonization events during health by 3% (Figure).
The CDC estimated that, in 2004, pneumococcal disease in the United States caused 4 million illness episodes, 22,000 deaths, 445,000 hospitalizations, 774,000 emergency department visits, 5 million outpatient visits, and 4.1 million outpatient antibiotic prescriptions. Direct medical costs totaled $3.5 billion. Pneumonia (866,000 cases) accounted for 22% of all cases and 72% of pneumococcal costs. AOM and sinusitis (1.5 million cases each) composed 75% of cases and 16% of direct medical costs.5 However, if indirect costs are taken into account, such as work loss by parents of young children, the cost of pneumococcal disease caused by AOM alone may exceed $6 billion annually6 and become dominant in the cost-effectiveness analysis in high-income countries.
Despite widespread use of PCV13, Pneumococcus has shown its resilience under vaccine pressure such that the organism remains a very common AOM pathogen. All-cause AOM has declined modestly and pneumococcal AOM caused by the specific serotypes in PCVs has declined dramatically since the introduction of PCVs. However, the burden of pneumococcal AOM disease is still considerable.
The notion that strains expressing serotypes that were not included in PCV7 were less virulent was proven wrong within a few years after introduction of PCV7, with the emergence of strains expressing serotype 19A, and others. The same cycle occurred after introduction of PCV13. It appears to take about 4 years after introduction of a PCV before peak effectiveness is achieved – which then begins to erode with emergence of NVTs. First, the NVTs are observed to colonize the nasopharynx as commensals and then from among those strains new disease-causing strains emerge.
At the most recent meeting of the International Society of Pneumococci and Pneumococcal Diseases in Toronto in June, many presentations focused on the fact that PCVs elicit highly effective protective serotype-specific antibodies to the capsular polysaccharides of included types. However, 100 serotypes are known. The limitations of PCVs are becoming increasingly apparent. They are costly and consume a large portion of the Vaccines for Children budget. Children in the developing world remain largely unvaccinated because of the high cost. NVTs that have emerged to cause disease vary by country, vary by adult vs. pediatric populations, and are dynamically changing year to year. Forthcoming PCVs of 15 and 20 serotypes will be even more costly than PCV13, will not include many newly emerged serotypes, and will probably likewise encounter “serotype replacement” because of high immune evasion by pneumococci.
When Merck and Pfizer made their decisions on serotype composition for PCV15 and PCV20, respectively, they were based on available data at the time regarding predominant serotypes causing invasive pneumococcal disease in countries that had the best data and would be the market for their products. However, from the time of the decision to licensure of vaccine is many years, and during that time the pneumococcal serotypes have changed, more so for AOM, and I predict more change will occur in the future.
In the past 3 years, Dr. Pichichero has received honoraria from Merck to attend 1-day consulting meetings and his institution has received investigator-initiated research grants to study aspects of PCV15. In the past 3 years, he was reimbursed for expenses to attend the ISPPD meeting in Toronto to present a poster on potential efficacy of PCV20 to prevent complicated AOM.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital.
References
1. Kaur R et al. Pediatrics. 2017;140(3).
2. Kaur R et al. Eur J Clin Microbiol Infect Dis. 2021;41:37-44..
3. Pichichero M et al. Lancet Child Adolesc Health. 2018;2(8):561-8.
4. Zhou F et al. Pediatrics. 2008;121(2):253-60.
5. Huang SS et al. Vaccine. 2011;29(18):3398-412.
6. Casey JR and Pichichero ME. Clin Pediatr (Phila). 2014;53(9):865-73. .
My group in Rochester, N.Y., examined the current pneumococcal serotypes causing AOM in children. From our data, we can determine the PCV13 vaccine types that escape prevention and cause AOM and understand what effect to expect from the new pneumococcal conjugate vaccines (PCVs) that will be coming soon. There are limited data from middle ear fluid (MEF) cultures on which to base such analyses. Tympanocentesis is the preferred method for securing MEF for culture and our group is unique in providing such data to the Centers for Disease Control and publishing our results on a periodic basis to inform clinicians.
Pneumococci are the second most common cause of acute otitis media (AOM) since the introduction of pneumococcal conjugate vaccines (PCVs) more than 2 decades ago.1,2 Pneumococcal AOM causes more severe acute disease and more often causes suppurative complications than Haemophilus influenzae, which is the most common cause of AOM. Prevention of pneumococcal AOM will be a highly relevant contributor to cost-effectiveness analyses for the anticipated introduction of PCV15 (Merck) and PCV20 (Pfizer). Both PCV15 and PCV20 have been licensed for adult use; PCV15 licensure for infants and children occurred in June 2022 for invasive pneumococcal disease and is anticipated in the near future for PCV20. They are improvements over PCV13 because they add serotypes that cause invasive pneumococcal diseases, although less so for prevention of AOM, on the basis of our data.
Nasopharyngeal colonization is a necessary pathogenic step in progression to pneumococcal disease. However, not all strains of pneumococci expressing different capsular serotypes are equally virulent and likely to cause disease. In PCV-vaccinated populations, vaccine pressure and antibiotic resistance drive PCV serotype replacement with nonvaccine serotypes (NVTs), gradually reducing the net effectiveness of the vaccines. Therefore, knowledge of prevalent NVTs colonizing the nasopharynx identifies future pneumococcal serotypes most likely to emerge as pathogenic.
We published an effectiveness study of PCV13.3 A relative reduction of 86% in AOM caused by strains expressing PCV13 serotypes was observed in the first few years after PCV13 introduction. The greatest reduction in MEF samples was in serotype 19A, with a relative reduction of 91%. However, over time the vaccine type efficacy of PCV13 against MEF-positive pneumococcal AOM has eroded. There was no clear efficacy against serotype 3, and we still observed cases of serotype 19A and 19F. PCV13 vaccine failures have been even more frequent in Europe (nearly 30% of pneumococcal AOM in Europe is caused by vaccine serotypes) than our data indicate, where about 10% of AOM is caused by PCV13 serotypes.
In our most recent publication covering 2015-2019, we described results from 589 children, aged 6-36 months, from whom we collected 2,042 nasopharyngeal samples.2,4 During AOM, 495 MEF samples from 319 AOM-infected children were collected (during bilateral infections, tympanocentesis was performed in both ears). Whether bacteria were isolated was based per AOM case, not per tap. The average age of children with AOM was 15 months (range 6-31 months). The three most prevalent nasopharyngeal pneumococcal serotypes were 35B, 23B, and 15B/C. Serotype 35B was the most common at AOM visits in both the nasopharynx and MEF samples followed by serotype 15B/C. Nonsusceptibility among pneumococci to penicillin, azithromycin, and multiple other antibiotics was high. Increasing resistance to ceftriaxone was also observed.
Based on our results, if PCV15 (PCV13 + 22F and 33F) effectiveness is identical to PCV13 for the included serotypes and 100% efficacy for the added serotypes is presumed, PCV15 will reduce pneumococcal AOMs by 8%, pneumococcal nasopharyngeal colonization events at onset of AOM by 6%, and pneumococcal nasopharyngeal colonization events during health by 3%. As for the projected reductions brought about by PCV20 (PCV15 + 8, 10A, 11A, 12F, and 15B), presuming serotype 15B is efficacious against serotype 15C and 100% efficacy for the added serotypes, PCV20 will reduce pneumococcal AOMs by 22%, pneumococcal nasopharyngeal colonization events at onset of AOM by 20%, and pneumococcal nasopharyngeal colonization events during health by 3% (Figure).
The CDC estimated that, in 2004, pneumococcal disease in the United States caused 4 million illness episodes, 22,000 deaths, 445,000 hospitalizations, 774,000 emergency department visits, 5 million outpatient visits, and 4.1 million outpatient antibiotic prescriptions. Direct medical costs totaled $3.5 billion. Pneumonia (866,000 cases) accounted for 22% of all cases and 72% of pneumococcal costs. AOM and sinusitis (1.5 million cases each) composed 75% of cases and 16% of direct medical costs.5 However, if indirect costs are taken into account, such as work loss by parents of young children, the cost of pneumococcal disease caused by AOM alone may exceed $6 billion annually6 and become dominant in the cost-effectiveness analysis in high-income countries.
Despite widespread use of PCV13, Pneumococcus has shown its resilience under vaccine pressure such that the organism remains a very common AOM pathogen. All-cause AOM has declined modestly and pneumococcal AOM caused by the specific serotypes in PCVs has declined dramatically since the introduction of PCVs. However, the burden of pneumococcal AOM disease is still considerable.
The notion that strains expressing serotypes that were not included in PCV7 were less virulent was proven wrong within a few years after introduction of PCV7, with the emergence of strains expressing serotype 19A, and others. The same cycle occurred after introduction of PCV13. It appears to take about 4 years after introduction of a PCV before peak effectiveness is achieved – which then begins to erode with emergence of NVTs. First, the NVTs are observed to colonize the nasopharynx as commensals and then from among those strains new disease-causing strains emerge.
At the most recent meeting of the International Society of Pneumococci and Pneumococcal Diseases in Toronto in June, many presentations focused on the fact that PCVs elicit highly effective protective serotype-specific antibodies to the capsular polysaccharides of included types. However, 100 serotypes are known. The limitations of PCVs are becoming increasingly apparent. They are costly and consume a large portion of the Vaccines for Children budget. Children in the developing world remain largely unvaccinated because of the high cost. NVTs that have emerged to cause disease vary by country, vary by adult vs. pediatric populations, and are dynamically changing year to year. Forthcoming PCVs of 15 and 20 serotypes will be even more costly than PCV13, will not include many newly emerged serotypes, and will probably likewise encounter “serotype replacement” because of high immune evasion by pneumococci.
When Merck and Pfizer made their decisions on serotype composition for PCV15 and PCV20, respectively, they were based on available data at the time regarding predominant serotypes causing invasive pneumococcal disease in countries that had the best data and would be the market for their products. However, from the time of the decision to licensure of vaccine is many years, and during that time the pneumococcal serotypes have changed, more so for AOM, and I predict more change will occur in the future.
In the past 3 years, Dr. Pichichero has received honoraria from Merck to attend 1-day consulting meetings and his institution has received investigator-initiated research grants to study aspects of PCV15. In the past 3 years, he was reimbursed for expenses to attend the ISPPD meeting in Toronto to present a poster on potential efficacy of PCV20 to prevent complicated AOM.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital.
References
1. Kaur R et al. Pediatrics. 2017;140(3).
2. Kaur R et al. Eur J Clin Microbiol Infect Dis. 2021;41:37-44..
3. Pichichero M et al. Lancet Child Adolesc Health. 2018;2(8):561-8.
4. Zhou F et al. Pediatrics. 2008;121(2):253-60.
5. Huang SS et al. Vaccine. 2011;29(18):3398-412.
6. Casey JR and Pichichero ME. Clin Pediatr (Phila). 2014;53(9):865-73. .
Active shooter drills may be harming children, but doctors offer help
The drills can range from staging lockdowns and sheltering in place to quasi dramas with mock shooters roaming the halls. Although the goals of these training exercises are important, equally important are the potential negative effects of drills on students’ mental health, according to doctors with expertise in pediatrics and mental health.
“Dramatic simulation of an active shooter event at school would be expected to provoke the same stress response as the real thing,” said Peter L. Loper Jr., MD, a pediatrician and psychiatrist, in an interview. “While ensuring their physical safety is very important, we must be intentional about making sure that we are not doing so at the expense of their psychosocial or emotional safety.”
“Children may not be able to differentiate a dramatic drill from a real event,” emphasized Dr. Loper, of the neuropsychiatry and behavioral science departments at the University of South Carolina, Columbia. “The parts of the brain responsible for our flight-fight-or-freeze response would interpret both simulated and real events identically and produce the same neurohormonal stress-response.”
Indeed, a study published in the journal Humanities & Social Sciences Communications suggested children experienced mental health problems related to participating in active shooter drills. In the large study, a team of statisticians from the Georgia Institute of Technology found that students reported a 42% increase in stress and anxiety and a 38.7% increase in depression during the 90 days following active shooter drills, compared with the 90 days before the drills.
The authors of this study, including Mai ElSherief, PhD, drew these conclusions after analyzing 54 million social media posts before and after drills in 114 schools across 33 states. The researchers analyzed the language of the social media posts by teachers, parents, and students and found increased use of the words hope, love, home, school, kids, community, support, and help after the drills. The researchers considered posting with these terms in the aftermath of the drills to be indicative of having high anxiety.
They included examples of how high stress, anxiety, and depression manifested in specific posts from parents in their report. The following is an example of a poster expressing high anxiety and stress: “are we really gonna normalize school shooter drills?! holy sh* there has to be a real way to avoid these tragedies. sh*t like this cannot be normalized. teachers injured after being shot with plastic pellets ‘execution style’ in active shooter drill.”
The authors also shared this post to serve as an example of a person who seems depressed: “and now we are revisiting the trauma on our kids, forcing them to act out school drills monthly. i don’t get why gen x parents buy into this concept wholeheartedly. things need to change.”
The published material did not include posts from students, but the researchers’ analysis of the content of posts overall showed increased concerns for health and increased concerns about death during the period after drills, compared with before drills.
The authors also conducted focus groups in communities in which drills occurred, and many teachers and parents reported anecdotal evidence of children who were nervous long after the drills were over, with some showing extreme reactions such as panic over a standard fire alarm at school. Overall, the results show that school shooter drills can negatively affect school communities over prolonged periods of time, they concluded.
According to a statement from the American Academy of Pediatrics, “there is a need to be cautious about the potential psychological risks and other unintended consequences of directly involving children in live exercises and drills.”
“These risks and consequences are especially a concern when children are deceived and led to believe there is an actual attack and not a drill,” wrote David Schonfeld, MD, the lead author of the statement on Participation of Children and Adolescents in Live Crisis Drills and Exercises, and colleagues.
Managing the fallout
Physicians can help students experiencing mental health problems from these drills, according to doctors interviewed for this piece.
It’s important for providers to know that stress will show up differently in children than in adults, said Chelsea Younghans, MD, a psychiatrist and military officer in Bethesda, Md., in an interview.
“They may see children with headaches, stomach aches, or nonspecific complaints. They may also see children who have not had difficulty with sleep present with nightmares or bed wetting,” she added.
For teens and preteens, validated tools such as the Child PTSD Symptom Scale (CPSS-5) and Child and Adolescent Trauma Screen (CATS) to assess PTSD in youth, may help serve as a starting point for a conversation between providers and their older child population, she noted.
Children who exhibit avoidance or withdrawal behaviors including consistent school refusal, an increase in reassurance-seeking behaviors, or somatic symptoms like vague abdominal pain or headaches that prevent school attendance after participating in a drill, may need more robust mental health services, Dr. Loper noted.
Dr. Schonfeld, who is also director of the National Center for School Crisis and Bereavement at Children’s Hospital Los Angeles, called for health care providers to be available to help children process traumatic reactions to these exercises.
Agreeing with Dr. Schonfeld, Dr. Younghans said: “It is vital to debrief with students and staff after drills, making sure that students have a safe space and ample time to speak with trusted staff. As children will undoubtedly have questions and concerns, creating open lines of communication will help alleviate any traumatic effect these drills may have.”
Communicating with various stakeholders
Experts also gave recommendations for how clinicians communicate with leaders in their area’s school districts and other members of their communities about these training exercises.
“For primary care providers, it is important to establish meaningful relationships within your community and patient population as much as possible,” Dr. Younghans said. “Having a good relationship with the local schools and being part of the conversation can help increase school and community awareness on the impact these drills can have on students and staff,” she added.
For those pediatricians or other health care providers who serve as consultants to schools, Dr. Schonfeld advised they ask about policies related to exercises and drills, such as what are the limits to what children might be exposed to in a drill, and what requirements there might be at the local and state level in terms of frequency and what the drills will and will not involve.
He also noted that clinicians should encourage school leaders to consider the fact that kids may have personal histories of trauma that are completely unknown to the school when they design these exercises.
School staff and health care providers should explain the nature and reasons for drills, invite family members to express concerns, and make accommodations if necessary for some children to participate in drills in a more limited way, noted Dr. Schonfeld, who is also clinical professor of pediatrics at the University of Southern California, Los Angeles.
“I think health care providers should work with legislators, so that if they require a drill, it must be done in a way that is physically and emotionally safe,” he added.
Executing better drills for students’ mental health
Experts also advised on ways to execute these drills that will be least damaging to students.
The AAP statement on Participation of Children and Adolescents in Live Crisis Drills and Exercises, for example, advocates eliminating high-intensity drills, prohibiting deception in drills, and providing accommodations based on children’s vulnerabilities.
Dr. Schonfeld also emphasized, in an interview, that training for an attack need not be extremely realistic to be effective.
“When you are preparing for a crisis, the drills and exercises are for children to practice and develop mastery over something they don’t know how to do fully yet,” said Dr. Schonfeld.
Citing a suggestion from a 2020 report conducted by Everytown for Gun Safety on keeping schools safe from gun violence, Dr. Younghans said, “Schools should be in clear communication with communities and families regarding when drills will be happening,” and advised ensuring that the explanation of drills is developmentally appropriate to the age of the children participating.
The report also recommends conducting drills that do not simulate an actual incident, combining drills with trauma-informed approaches to address students’ well-being during and for a sustained period after the drills, and tracking data on the efficacy and effects of drills.
Dr. Loper suggested ways that clinicians and parents can help navigate the tricky territory of school safety drills.
In his view, they should not be random or unexpected, and anticipatory guidance should be given regarding any visual or auditory stimuli, such as flashing lights or sirens, alarms, or announcements.
“A preventive approach should be utilized to ensure that any child who is experiencing extreme drill-distress be excused from any future disaster drills to prevent retraumatization,” Dr. Loper said.
Physicians interviewed for this piece also provided tips on how to talk about these events with children in a way that is beneficial to their mental health.
“What we want to do is [have a] calm discussion [with kids] about what we are doing and why we are doing it” and guide them through the movements, Dr. Schonfeld said.
When teaching children how to respond to an emergency, some elements of uncertainty need to be discussed. Children need to anticipate “what you might do if you are not in the classroom if something occurs, such as being in the bathroom, or out at recess,” he continued.
Dr. Younghans recommended that parents and staff schedule time to prepare children for the drill and practice in advance, and that behavioral health providers, counselors, and/or primary care providers should be involved in the planning and execution of the drill.
The Georgia Tech study was supported through a grant from Everytown for Gun Safety.
The study authors and experts interviewed for this piece had no financial conflicts to disclose.
The drills can range from staging lockdowns and sheltering in place to quasi dramas with mock shooters roaming the halls. Although the goals of these training exercises are important, equally important are the potential negative effects of drills on students’ mental health, according to doctors with expertise in pediatrics and mental health.
“Dramatic simulation of an active shooter event at school would be expected to provoke the same stress response as the real thing,” said Peter L. Loper Jr., MD, a pediatrician and psychiatrist, in an interview. “While ensuring their physical safety is very important, we must be intentional about making sure that we are not doing so at the expense of their psychosocial or emotional safety.”
“Children may not be able to differentiate a dramatic drill from a real event,” emphasized Dr. Loper, of the neuropsychiatry and behavioral science departments at the University of South Carolina, Columbia. “The parts of the brain responsible for our flight-fight-or-freeze response would interpret both simulated and real events identically and produce the same neurohormonal stress-response.”
Indeed, a study published in the journal Humanities & Social Sciences Communications suggested children experienced mental health problems related to participating in active shooter drills. In the large study, a team of statisticians from the Georgia Institute of Technology found that students reported a 42% increase in stress and anxiety and a 38.7% increase in depression during the 90 days following active shooter drills, compared with the 90 days before the drills.
The authors of this study, including Mai ElSherief, PhD, drew these conclusions after analyzing 54 million social media posts before and after drills in 114 schools across 33 states. The researchers analyzed the language of the social media posts by teachers, parents, and students and found increased use of the words hope, love, home, school, kids, community, support, and help after the drills. The researchers considered posting with these terms in the aftermath of the drills to be indicative of having high anxiety.
They included examples of how high stress, anxiety, and depression manifested in specific posts from parents in their report. The following is an example of a poster expressing high anxiety and stress: “are we really gonna normalize school shooter drills?! holy sh* there has to be a real way to avoid these tragedies. sh*t like this cannot be normalized. teachers injured after being shot with plastic pellets ‘execution style’ in active shooter drill.”
The authors also shared this post to serve as an example of a person who seems depressed: “and now we are revisiting the trauma on our kids, forcing them to act out school drills monthly. i don’t get why gen x parents buy into this concept wholeheartedly. things need to change.”
The published material did not include posts from students, but the researchers’ analysis of the content of posts overall showed increased concerns for health and increased concerns about death during the period after drills, compared with before drills.
The authors also conducted focus groups in communities in which drills occurred, and many teachers and parents reported anecdotal evidence of children who were nervous long after the drills were over, with some showing extreme reactions such as panic over a standard fire alarm at school. Overall, the results show that school shooter drills can negatively affect school communities over prolonged periods of time, they concluded.
According to a statement from the American Academy of Pediatrics, “there is a need to be cautious about the potential psychological risks and other unintended consequences of directly involving children in live exercises and drills.”
“These risks and consequences are especially a concern when children are deceived and led to believe there is an actual attack and not a drill,” wrote David Schonfeld, MD, the lead author of the statement on Participation of Children and Adolescents in Live Crisis Drills and Exercises, and colleagues.
Managing the fallout
Physicians can help students experiencing mental health problems from these drills, according to doctors interviewed for this piece.
It’s important for providers to know that stress will show up differently in children than in adults, said Chelsea Younghans, MD, a psychiatrist and military officer in Bethesda, Md., in an interview.
“They may see children with headaches, stomach aches, or nonspecific complaints. They may also see children who have not had difficulty with sleep present with nightmares or bed wetting,” she added.
For teens and preteens, validated tools such as the Child PTSD Symptom Scale (CPSS-5) and Child and Adolescent Trauma Screen (CATS) to assess PTSD in youth, may help serve as a starting point for a conversation between providers and their older child population, she noted.
Children who exhibit avoidance or withdrawal behaviors including consistent school refusal, an increase in reassurance-seeking behaviors, or somatic symptoms like vague abdominal pain or headaches that prevent school attendance after participating in a drill, may need more robust mental health services, Dr. Loper noted.
Dr. Schonfeld, who is also director of the National Center for School Crisis and Bereavement at Children’s Hospital Los Angeles, called for health care providers to be available to help children process traumatic reactions to these exercises.
Agreeing with Dr. Schonfeld, Dr. Younghans said: “It is vital to debrief with students and staff after drills, making sure that students have a safe space and ample time to speak with trusted staff. As children will undoubtedly have questions and concerns, creating open lines of communication will help alleviate any traumatic effect these drills may have.”
Communicating with various stakeholders
Experts also gave recommendations for how clinicians communicate with leaders in their area’s school districts and other members of their communities about these training exercises.
“For primary care providers, it is important to establish meaningful relationships within your community and patient population as much as possible,” Dr. Younghans said. “Having a good relationship with the local schools and being part of the conversation can help increase school and community awareness on the impact these drills can have on students and staff,” she added.
For those pediatricians or other health care providers who serve as consultants to schools, Dr. Schonfeld advised they ask about policies related to exercises and drills, such as what are the limits to what children might be exposed to in a drill, and what requirements there might be at the local and state level in terms of frequency and what the drills will and will not involve.
He also noted that clinicians should encourage school leaders to consider the fact that kids may have personal histories of trauma that are completely unknown to the school when they design these exercises.
School staff and health care providers should explain the nature and reasons for drills, invite family members to express concerns, and make accommodations if necessary for some children to participate in drills in a more limited way, noted Dr. Schonfeld, who is also clinical professor of pediatrics at the University of Southern California, Los Angeles.
“I think health care providers should work with legislators, so that if they require a drill, it must be done in a way that is physically and emotionally safe,” he added.
Executing better drills for students’ mental health
Experts also advised on ways to execute these drills that will be least damaging to students.
The AAP statement on Participation of Children and Adolescents in Live Crisis Drills and Exercises, for example, advocates eliminating high-intensity drills, prohibiting deception in drills, and providing accommodations based on children’s vulnerabilities.
Dr. Schonfeld also emphasized, in an interview, that training for an attack need not be extremely realistic to be effective.
“When you are preparing for a crisis, the drills and exercises are for children to practice and develop mastery over something they don’t know how to do fully yet,” said Dr. Schonfeld.
Citing a suggestion from a 2020 report conducted by Everytown for Gun Safety on keeping schools safe from gun violence, Dr. Younghans said, “Schools should be in clear communication with communities and families regarding when drills will be happening,” and advised ensuring that the explanation of drills is developmentally appropriate to the age of the children participating.
The report also recommends conducting drills that do not simulate an actual incident, combining drills with trauma-informed approaches to address students’ well-being during and for a sustained period after the drills, and tracking data on the efficacy and effects of drills.
Dr. Loper suggested ways that clinicians and parents can help navigate the tricky territory of school safety drills.
In his view, they should not be random or unexpected, and anticipatory guidance should be given regarding any visual or auditory stimuli, such as flashing lights or sirens, alarms, or announcements.
“A preventive approach should be utilized to ensure that any child who is experiencing extreme drill-distress be excused from any future disaster drills to prevent retraumatization,” Dr. Loper said.
Physicians interviewed for this piece also provided tips on how to talk about these events with children in a way that is beneficial to their mental health.
“What we want to do is [have a] calm discussion [with kids] about what we are doing and why we are doing it” and guide them through the movements, Dr. Schonfeld said.
When teaching children how to respond to an emergency, some elements of uncertainty need to be discussed. Children need to anticipate “what you might do if you are not in the classroom if something occurs, such as being in the bathroom, or out at recess,” he continued.
Dr. Younghans recommended that parents and staff schedule time to prepare children for the drill and practice in advance, and that behavioral health providers, counselors, and/or primary care providers should be involved in the planning and execution of the drill.
The Georgia Tech study was supported through a grant from Everytown for Gun Safety.
The study authors and experts interviewed for this piece had no financial conflicts to disclose.
The drills can range from staging lockdowns and sheltering in place to quasi dramas with mock shooters roaming the halls. Although the goals of these training exercises are important, equally important are the potential negative effects of drills on students’ mental health, according to doctors with expertise in pediatrics and mental health.
“Dramatic simulation of an active shooter event at school would be expected to provoke the same stress response as the real thing,” said Peter L. Loper Jr., MD, a pediatrician and psychiatrist, in an interview. “While ensuring their physical safety is very important, we must be intentional about making sure that we are not doing so at the expense of their psychosocial or emotional safety.”
“Children may not be able to differentiate a dramatic drill from a real event,” emphasized Dr. Loper, of the neuropsychiatry and behavioral science departments at the University of South Carolina, Columbia. “The parts of the brain responsible for our flight-fight-or-freeze response would interpret both simulated and real events identically and produce the same neurohormonal stress-response.”
Indeed, a study published in the journal Humanities & Social Sciences Communications suggested children experienced mental health problems related to participating in active shooter drills. In the large study, a team of statisticians from the Georgia Institute of Technology found that students reported a 42% increase in stress and anxiety and a 38.7% increase in depression during the 90 days following active shooter drills, compared with the 90 days before the drills.
The authors of this study, including Mai ElSherief, PhD, drew these conclusions after analyzing 54 million social media posts before and after drills in 114 schools across 33 states. The researchers analyzed the language of the social media posts by teachers, parents, and students and found increased use of the words hope, love, home, school, kids, community, support, and help after the drills. The researchers considered posting with these terms in the aftermath of the drills to be indicative of having high anxiety.
They included examples of how high stress, anxiety, and depression manifested in specific posts from parents in their report. The following is an example of a poster expressing high anxiety and stress: “are we really gonna normalize school shooter drills?! holy sh* there has to be a real way to avoid these tragedies. sh*t like this cannot be normalized. teachers injured after being shot with plastic pellets ‘execution style’ in active shooter drill.”
The authors also shared this post to serve as an example of a person who seems depressed: “and now we are revisiting the trauma on our kids, forcing them to act out school drills monthly. i don’t get why gen x parents buy into this concept wholeheartedly. things need to change.”
The published material did not include posts from students, but the researchers’ analysis of the content of posts overall showed increased concerns for health and increased concerns about death during the period after drills, compared with before drills.
The authors also conducted focus groups in communities in which drills occurred, and many teachers and parents reported anecdotal evidence of children who were nervous long after the drills were over, with some showing extreme reactions such as panic over a standard fire alarm at school. Overall, the results show that school shooter drills can negatively affect school communities over prolonged periods of time, they concluded.
According to a statement from the American Academy of Pediatrics, “there is a need to be cautious about the potential psychological risks and other unintended consequences of directly involving children in live exercises and drills.”
“These risks and consequences are especially a concern when children are deceived and led to believe there is an actual attack and not a drill,” wrote David Schonfeld, MD, the lead author of the statement on Participation of Children and Adolescents in Live Crisis Drills and Exercises, and colleagues.
Managing the fallout
Physicians can help students experiencing mental health problems from these drills, according to doctors interviewed for this piece.
It’s important for providers to know that stress will show up differently in children than in adults, said Chelsea Younghans, MD, a psychiatrist and military officer in Bethesda, Md., in an interview.
“They may see children with headaches, stomach aches, or nonspecific complaints. They may also see children who have not had difficulty with sleep present with nightmares or bed wetting,” she added.
For teens and preteens, validated tools such as the Child PTSD Symptom Scale (CPSS-5) and Child and Adolescent Trauma Screen (CATS) to assess PTSD in youth, may help serve as a starting point for a conversation between providers and their older child population, she noted.
Children who exhibit avoidance or withdrawal behaviors including consistent school refusal, an increase in reassurance-seeking behaviors, or somatic symptoms like vague abdominal pain or headaches that prevent school attendance after participating in a drill, may need more robust mental health services, Dr. Loper noted.
Dr. Schonfeld, who is also director of the National Center for School Crisis and Bereavement at Children’s Hospital Los Angeles, called for health care providers to be available to help children process traumatic reactions to these exercises.
Agreeing with Dr. Schonfeld, Dr. Younghans said: “It is vital to debrief with students and staff after drills, making sure that students have a safe space and ample time to speak with trusted staff. As children will undoubtedly have questions and concerns, creating open lines of communication will help alleviate any traumatic effect these drills may have.”
Communicating with various stakeholders
Experts also gave recommendations for how clinicians communicate with leaders in their area’s school districts and other members of their communities about these training exercises.
“For primary care providers, it is important to establish meaningful relationships within your community and patient population as much as possible,” Dr. Younghans said. “Having a good relationship with the local schools and being part of the conversation can help increase school and community awareness on the impact these drills can have on students and staff,” she added.
For those pediatricians or other health care providers who serve as consultants to schools, Dr. Schonfeld advised they ask about policies related to exercises and drills, such as what are the limits to what children might be exposed to in a drill, and what requirements there might be at the local and state level in terms of frequency and what the drills will and will not involve.
He also noted that clinicians should encourage school leaders to consider the fact that kids may have personal histories of trauma that are completely unknown to the school when they design these exercises.
School staff and health care providers should explain the nature and reasons for drills, invite family members to express concerns, and make accommodations if necessary for some children to participate in drills in a more limited way, noted Dr. Schonfeld, who is also clinical professor of pediatrics at the University of Southern California, Los Angeles.
“I think health care providers should work with legislators, so that if they require a drill, it must be done in a way that is physically and emotionally safe,” he added.
Executing better drills for students’ mental health
Experts also advised on ways to execute these drills that will be least damaging to students.
The AAP statement on Participation of Children and Adolescents in Live Crisis Drills and Exercises, for example, advocates eliminating high-intensity drills, prohibiting deception in drills, and providing accommodations based on children’s vulnerabilities.
Dr. Schonfeld also emphasized, in an interview, that training for an attack need not be extremely realistic to be effective.
“When you are preparing for a crisis, the drills and exercises are for children to practice and develop mastery over something they don’t know how to do fully yet,” said Dr. Schonfeld.
Citing a suggestion from a 2020 report conducted by Everytown for Gun Safety on keeping schools safe from gun violence, Dr. Younghans said, “Schools should be in clear communication with communities and families regarding when drills will be happening,” and advised ensuring that the explanation of drills is developmentally appropriate to the age of the children participating.
The report also recommends conducting drills that do not simulate an actual incident, combining drills with trauma-informed approaches to address students’ well-being during and for a sustained period after the drills, and tracking data on the efficacy and effects of drills.
Dr. Loper suggested ways that clinicians and parents can help navigate the tricky territory of school safety drills.
In his view, they should not be random or unexpected, and anticipatory guidance should be given regarding any visual or auditory stimuli, such as flashing lights or sirens, alarms, or announcements.
“A preventive approach should be utilized to ensure that any child who is experiencing extreme drill-distress be excused from any future disaster drills to prevent retraumatization,” Dr. Loper said.
Physicians interviewed for this piece also provided tips on how to talk about these events with children in a way that is beneficial to their mental health.
“What we want to do is [have a] calm discussion [with kids] about what we are doing and why we are doing it” and guide them through the movements, Dr. Schonfeld said.
When teaching children how to respond to an emergency, some elements of uncertainty need to be discussed. Children need to anticipate “what you might do if you are not in the classroom if something occurs, such as being in the bathroom, or out at recess,” he continued.
Dr. Younghans recommended that parents and staff schedule time to prepare children for the drill and practice in advance, and that behavioral health providers, counselors, and/or primary care providers should be involved in the planning and execution of the drill.
The Georgia Tech study was supported through a grant from Everytown for Gun Safety.
The study authors and experts interviewed for this piece had no financial conflicts to disclose.
Biosimilar-to-biosimilar switches deemed safe and effective, systematic review reveals
Switching from one biosimilar medication to another is safe and effective, a new systematic review indicates, even though this clinical practice is not governed by current health authority regulations or guidance.
“No reduction in effectiveness or increase in adverse events was detected in biosimilar-to-biosimilar switching studies conducted to date,” the review’s authors noted in their study, published online in BioDrugs.
“The possibility of multiple switches between biosimilars of the same reference biologic is already a reality, and these types of switches are expected to become more common in the future. ... Although it is not covered by current health authority regulations or guidance,” added the authors, led by Hillel P. Cohen, PhD, executive director of scientific affairs at Sandoz, a division of Novartis.
The researchers searched electronic databases through December 2021 and found 23 observational studies that met their search criteria, of which 13 were published in peer-reviewed journals; the remainder appeared in abstract form. The studies totaled 3,657 patients. The researchers did not identify any randomized clinical trials.
“The studies were heterogeneous in size, design, and endpoints, providing data on safety, effectiveness, immunogenicity, pharmacokinetics, patient retention, patient and physician perceptions, and drug-use patterns,” the authors wrote.
The authors found that the majority of studies evaluated switches between biosimilars of infliximab, but they also identified switches between biosimilars of adalimumab, etanercept, and rituximab.
“Some health care providers are hesitant to switch patients from one biosimilar to another biosimilar because of a perceived lack of clinical data on such switches,” Dr. Cohen said in an interview.
The review’s findings – that there were no clinically relevant differences when switching patients from one biosimilar to another – are consistent with the science, Dr. Cohen said. “Physicians should have confidence that the data demonstrate that safety and effectiveness are not impacted if patients switch from one biosimilar to another biosimilar of the same reference biologic,” he said.
Currently, the published data include biosimilars to only four reference biologics. “However, I anticipate additional biosimilar-to-biosimilar switching data will become available in the future,” Dr. Cohen said. “In fact, several new studies have been published in recent months, after the cut-off date for inclusion in our systematic review.”
Switching common in rheumatology, dermatology, and gastroenterology
Biosimilar-to-biosimilar switching was observed most commonly in rheumatology practice, but also was seen in the specialties of dermatology and gastroenterology.
Jeffrey Weinberg, MD, clinical professor of dermatology, Icahn School of Medicine at Mount Sinai, New York City, said in an interview that the study is among the best to date showing that switching biosimilars does not compromise efficacy or safety.
“I would hypothesize that the interchangeability would apply to psoriasis patients,” Dr. Weinberg said. However, “over the next few years, we will have an increasing number of biosimilars for an increasing number of different molecules. We will need to be vigilant to observe if similar behavior is observed with the biosimilars yet to come.”
Keith Choate, MD, PhD, professor of dermatology, pathology, and genetics, and associate dean for physician-scientist development at Yale University, New Haven, Conn., said that biosimilars have comparable efficacy to the branded medication they replace. “If response is lost to an individual agent, we would not typically then switch to a biosimilar, but would favor another class of therapy or a distinct therapeutic which targets the same pathway.”
When physicians prescribe a biosimilar for rheumatoid arthritis or psoriatic arthritis, in 9 out 10 people, “it’s going to work as well, and it’s not going to cause any more side effects,” said Stanford Shoor, MD, clinical professor of medicine and rheumatology, Stanford (Calif.) University.
The systematic review, even within its limitations, reinforces confidence in the antitumor necrosis factor biosimilars, said Jean-Frederic Colombel, MD, codirector of the Feinstein Inflammatory Bowel Disease Clinical Center at Mount Sinai, New York, and professor of medicine, division of gastroenterology, Icahn School of Medicine at Mount Sinai.
“Still, studies with longer follow-up are needed,” Dr. Colombel said, adding that the remaining questions relate to the efficacy and safety of switching multiple times, which will likely occur in the near future. There will be a “need to provide information to the patient regarding what originator or biosimilar(s) he has been exposed to during the course of his disease.”
Switching will increasingly become the norm, said Miguel Regueiro, MD, chair of the Digestive Disease & Surgery Institute, Cleveland Clinic. In his clinical practice, he has the most experience with Crohn’s disease and ulcerative colitis, and biosimilar-to-biosimilar infliximab switches. “Unless there are data that emerge, I have no concerns with this.”
He added that it’s an “interesting study that affirms my findings in clinical practice – that one can switch from a biosimilar to biosimilar (of the same reference product).”
The review’s results also make sense from an economic standpoint, said Rajat Bhatt, MD, owner of Prime Rheumatology in Richmond, Tex., and an adjunct faculty member at Caribbean Medical University, Willemstad, Curaçao. “Switching to biosimilars will result in cost savings for the health care system.” Patients on certain insurances also will save by switching to a biosimilar with a lower copay.
However, the review is limited by a relatively small number of studies that have provided primary data on this topic, and most of these were switching from infliximab to a biosimilar for inflammatory bowel disease, said Alfred Kim, MD, PhD, an adult rheumatologist at Barnes-Jewish Hospital, St. Louis, and assistant professor of medicine at Washington University in St. Louis.
As with any meta-analysis evaluating a small number of studies, “broad applicability to all conditions and reference/biosimilar pair can only be assumed. Also, many of the studies used for this meta-analysis are observational, which can introduce a variety of biases that can be difficult to adjust for,” Dr. Kim said. “Nevertheless, these analyses are an important first step in validating the [Food and Drug Administration’s] approach to evaluating biosimilars, as the clinical outcomes are consistent between different biosimilars.”
This systematic review is not enough to prove that all patients will do fine when switching from one biosimilar to another, said Florence Aslinia, MD, a gastroenterologist at the University of Kansas Health System in Kansas City. It’s possible that some patients may not do as well, she said, noting that, in one study of patients with inflammatory bowel disease, 10% of patients on a biosimilar infliximab needed to switch back to the originator infliximab (Remicade, Janssen) because of side effects attributed to the biosimilar. The same thing may or may not happen with biosimilar-to-biosimilar switching, and it requires further study.
The authors did not receive any funding for writing this review. Dr. Cohen is an employee of Sandoz, a division of Novartis. He may own stock in Novartis. Two coauthors are also employees of Sandoz. The other three coauthors reported having financial relationships with numerous pharmaceutical companies, including Sandoz and/or Novartis. Dr. Colombel reported financial relationships with many pharmaceutical companies, including Novartis and other manufacturers of biosimilars. Dr. Regueiro reports financial relationships with numerous pharmaceutical companies, including some manufacturers of biosimilars. Dr. Weinberg reported financial relationships with Celgene, AbbVie, Eli Lilly, and Novartis. Kim reports financial relationships with GlaxoSmithKline, Pfizer, and AstraZeneca. Dr. Aslinia, Dr. Shoor, Dr. Choate, and Dr. Bhatt reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Switching from one biosimilar medication to another is safe and effective, a new systematic review indicates, even though this clinical practice is not governed by current health authority regulations or guidance.
“No reduction in effectiveness or increase in adverse events was detected in biosimilar-to-biosimilar switching studies conducted to date,” the review’s authors noted in their study, published online in BioDrugs.
“The possibility of multiple switches between biosimilars of the same reference biologic is already a reality, and these types of switches are expected to become more common in the future. ... Although it is not covered by current health authority regulations or guidance,” added the authors, led by Hillel P. Cohen, PhD, executive director of scientific affairs at Sandoz, a division of Novartis.
The researchers searched electronic databases through December 2021 and found 23 observational studies that met their search criteria, of which 13 were published in peer-reviewed journals; the remainder appeared in abstract form. The studies totaled 3,657 patients. The researchers did not identify any randomized clinical trials.
“The studies were heterogeneous in size, design, and endpoints, providing data on safety, effectiveness, immunogenicity, pharmacokinetics, patient retention, patient and physician perceptions, and drug-use patterns,” the authors wrote.
The authors found that the majority of studies evaluated switches between biosimilars of infliximab, but they also identified switches between biosimilars of adalimumab, etanercept, and rituximab.
“Some health care providers are hesitant to switch patients from one biosimilar to another biosimilar because of a perceived lack of clinical data on such switches,” Dr. Cohen said in an interview.
The review’s findings – that there were no clinically relevant differences when switching patients from one biosimilar to another – are consistent with the science, Dr. Cohen said. “Physicians should have confidence that the data demonstrate that safety and effectiveness are not impacted if patients switch from one biosimilar to another biosimilar of the same reference biologic,” he said.
Currently, the published data include biosimilars to only four reference biologics. “However, I anticipate additional biosimilar-to-biosimilar switching data will become available in the future,” Dr. Cohen said. “In fact, several new studies have been published in recent months, after the cut-off date for inclusion in our systematic review.”
Switching common in rheumatology, dermatology, and gastroenterology
Biosimilar-to-biosimilar switching was observed most commonly in rheumatology practice, but also was seen in the specialties of dermatology and gastroenterology.
Jeffrey Weinberg, MD, clinical professor of dermatology, Icahn School of Medicine at Mount Sinai, New York City, said in an interview that the study is among the best to date showing that switching biosimilars does not compromise efficacy or safety.
“I would hypothesize that the interchangeability would apply to psoriasis patients,” Dr. Weinberg said. However, “over the next few years, we will have an increasing number of biosimilars for an increasing number of different molecules. We will need to be vigilant to observe if similar behavior is observed with the biosimilars yet to come.”
Keith Choate, MD, PhD, professor of dermatology, pathology, and genetics, and associate dean for physician-scientist development at Yale University, New Haven, Conn., said that biosimilars have comparable efficacy to the branded medication they replace. “If response is lost to an individual agent, we would not typically then switch to a biosimilar, but would favor another class of therapy or a distinct therapeutic which targets the same pathway.”
When physicians prescribe a biosimilar for rheumatoid arthritis or psoriatic arthritis, in 9 out 10 people, “it’s going to work as well, and it’s not going to cause any more side effects,” said Stanford Shoor, MD, clinical professor of medicine and rheumatology, Stanford (Calif.) University.
The systematic review, even within its limitations, reinforces confidence in the antitumor necrosis factor biosimilars, said Jean-Frederic Colombel, MD, codirector of the Feinstein Inflammatory Bowel Disease Clinical Center at Mount Sinai, New York, and professor of medicine, division of gastroenterology, Icahn School of Medicine at Mount Sinai.
“Still, studies with longer follow-up are needed,” Dr. Colombel said, adding that the remaining questions relate to the efficacy and safety of switching multiple times, which will likely occur in the near future. There will be a “need to provide information to the patient regarding what originator or biosimilar(s) he has been exposed to during the course of his disease.”
Switching will increasingly become the norm, said Miguel Regueiro, MD, chair of the Digestive Disease & Surgery Institute, Cleveland Clinic. In his clinical practice, he has the most experience with Crohn’s disease and ulcerative colitis, and biosimilar-to-biosimilar infliximab switches. “Unless there are data that emerge, I have no concerns with this.”
He added that it’s an “interesting study that affirms my findings in clinical practice – that one can switch from a biosimilar to biosimilar (of the same reference product).”
The review’s results also make sense from an economic standpoint, said Rajat Bhatt, MD, owner of Prime Rheumatology in Richmond, Tex., and an adjunct faculty member at Caribbean Medical University, Willemstad, Curaçao. “Switching to biosimilars will result in cost savings for the health care system.” Patients on certain insurances also will save by switching to a biosimilar with a lower copay.
However, the review is limited by a relatively small number of studies that have provided primary data on this topic, and most of these were switching from infliximab to a biosimilar for inflammatory bowel disease, said Alfred Kim, MD, PhD, an adult rheumatologist at Barnes-Jewish Hospital, St. Louis, and assistant professor of medicine at Washington University in St. Louis.
As with any meta-analysis evaluating a small number of studies, “broad applicability to all conditions and reference/biosimilar pair can only be assumed. Also, many of the studies used for this meta-analysis are observational, which can introduce a variety of biases that can be difficult to adjust for,” Dr. Kim said. “Nevertheless, these analyses are an important first step in validating the [Food and Drug Administration’s] approach to evaluating biosimilars, as the clinical outcomes are consistent between different biosimilars.”
This systematic review is not enough to prove that all patients will do fine when switching from one biosimilar to another, said Florence Aslinia, MD, a gastroenterologist at the University of Kansas Health System in Kansas City. It’s possible that some patients may not do as well, she said, noting that, in one study of patients with inflammatory bowel disease, 10% of patients on a biosimilar infliximab needed to switch back to the originator infliximab (Remicade, Janssen) because of side effects attributed to the biosimilar. The same thing may or may not happen with biosimilar-to-biosimilar switching, and it requires further study.
The authors did not receive any funding for writing this review. Dr. Cohen is an employee of Sandoz, a division of Novartis. He may own stock in Novartis. Two coauthors are also employees of Sandoz. The other three coauthors reported having financial relationships with numerous pharmaceutical companies, including Sandoz and/or Novartis. Dr. Colombel reported financial relationships with many pharmaceutical companies, including Novartis and other manufacturers of biosimilars. Dr. Regueiro reports financial relationships with numerous pharmaceutical companies, including some manufacturers of biosimilars. Dr. Weinberg reported financial relationships with Celgene, AbbVie, Eli Lilly, and Novartis. Kim reports financial relationships with GlaxoSmithKline, Pfizer, and AstraZeneca. Dr. Aslinia, Dr. Shoor, Dr. Choate, and Dr. Bhatt reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Switching from one biosimilar medication to another is safe and effective, a new systematic review indicates, even though this clinical practice is not governed by current health authority regulations or guidance.
“No reduction in effectiveness or increase in adverse events was detected in biosimilar-to-biosimilar switching studies conducted to date,” the review’s authors noted in their study, published online in BioDrugs.
“The possibility of multiple switches between biosimilars of the same reference biologic is already a reality, and these types of switches are expected to become more common in the future. ... Although it is not covered by current health authority regulations or guidance,” added the authors, led by Hillel P. Cohen, PhD, executive director of scientific affairs at Sandoz, a division of Novartis.
The researchers searched electronic databases through December 2021 and found 23 observational studies that met their search criteria, of which 13 were published in peer-reviewed journals; the remainder appeared in abstract form. The studies totaled 3,657 patients. The researchers did not identify any randomized clinical trials.
“The studies were heterogeneous in size, design, and endpoints, providing data on safety, effectiveness, immunogenicity, pharmacokinetics, patient retention, patient and physician perceptions, and drug-use patterns,” the authors wrote.
The authors found that the majority of studies evaluated switches between biosimilars of infliximab, but they also identified switches between biosimilars of adalimumab, etanercept, and rituximab.
“Some health care providers are hesitant to switch patients from one biosimilar to another biosimilar because of a perceived lack of clinical data on such switches,” Dr. Cohen said in an interview.
The review’s findings – that there were no clinically relevant differences when switching patients from one biosimilar to another – are consistent with the science, Dr. Cohen said. “Physicians should have confidence that the data demonstrate that safety and effectiveness are not impacted if patients switch from one biosimilar to another biosimilar of the same reference biologic,” he said.
Currently, the published data include biosimilars to only four reference biologics. “However, I anticipate additional biosimilar-to-biosimilar switching data will become available in the future,” Dr. Cohen said. “In fact, several new studies have been published in recent months, after the cut-off date for inclusion in our systematic review.”
Switching common in rheumatology, dermatology, and gastroenterology
Biosimilar-to-biosimilar switching was observed most commonly in rheumatology practice, but also was seen in the specialties of dermatology and gastroenterology.
Jeffrey Weinberg, MD, clinical professor of dermatology, Icahn School of Medicine at Mount Sinai, New York City, said in an interview that the study is among the best to date showing that switching biosimilars does not compromise efficacy or safety.
“I would hypothesize that the interchangeability would apply to psoriasis patients,” Dr. Weinberg said. However, “over the next few years, we will have an increasing number of biosimilars for an increasing number of different molecules. We will need to be vigilant to observe if similar behavior is observed with the biosimilars yet to come.”
Keith Choate, MD, PhD, professor of dermatology, pathology, and genetics, and associate dean for physician-scientist development at Yale University, New Haven, Conn., said that biosimilars have comparable efficacy to the branded medication they replace. “If response is lost to an individual agent, we would not typically then switch to a biosimilar, but would favor another class of therapy or a distinct therapeutic which targets the same pathway.”
When physicians prescribe a biosimilar for rheumatoid arthritis or psoriatic arthritis, in 9 out 10 people, “it’s going to work as well, and it’s not going to cause any more side effects,” said Stanford Shoor, MD, clinical professor of medicine and rheumatology, Stanford (Calif.) University.
The systematic review, even within its limitations, reinforces confidence in the antitumor necrosis factor biosimilars, said Jean-Frederic Colombel, MD, codirector of the Feinstein Inflammatory Bowel Disease Clinical Center at Mount Sinai, New York, and professor of medicine, division of gastroenterology, Icahn School of Medicine at Mount Sinai.
“Still, studies with longer follow-up are needed,” Dr. Colombel said, adding that the remaining questions relate to the efficacy and safety of switching multiple times, which will likely occur in the near future. There will be a “need to provide information to the patient regarding what originator or biosimilar(s) he has been exposed to during the course of his disease.”
Switching will increasingly become the norm, said Miguel Regueiro, MD, chair of the Digestive Disease & Surgery Institute, Cleveland Clinic. In his clinical practice, he has the most experience with Crohn’s disease and ulcerative colitis, and biosimilar-to-biosimilar infliximab switches. “Unless there are data that emerge, I have no concerns with this.”
He added that it’s an “interesting study that affirms my findings in clinical practice – that one can switch from a biosimilar to biosimilar (of the same reference product).”
The review’s results also make sense from an economic standpoint, said Rajat Bhatt, MD, owner of Prime Rheumatology in Richmond, Tex., and an adjunct faculty member at Caribbean Medical University, Willemstad, Curaçao. “Switching to biosimilars will result in cost savings for the health care system.” Patients on certain insurances also will save by switching to a biosimilar with a lower copay.
However, the review is limited by a relatively small number of studies that have provided primary data on this topic, and most of these were switching from infliximab to a biosimilar for inflammatory bowel disease, said Alfred Kim, MD, PhD, an adult rheumatologist at Barnes-Jewish Hospital, St. Louis, and assistant professor of medicine at Washington University in St. Louis.
As with any meta-analysis evaluating a small number of studies, “broad applicability to all conditions and reference/biosimilar pair can only be assumed. Also, many of the studies used for this meta-analysis are observational, which can introduce a variety of biases that can be difficult to adjust for,” Dr. Kim said. “Nevertheless, these analyses are an important first step in validating the [Food and Drug Administration’s] approach to evaluating biosimilars, as the clinical outcomes are consistent between different biosimilars.”
This systematic review is not enough to prove that all patients will do fine when switching from one biosimilar to another, said Florence Aslinia, MD, a gastroenterologist at the University of Kansas Health System in Kansas City. It’s possible that some patients may not do as well, she said, noting that, in one study of patients with inflammatory bowel disease, 10% of patients on a biosimilar infliximab needed to switch back to the originator infliximab (Remicade, Janssen) because of side effects attributed to the biosimilar. The same thing may or may not happen with biosimilar-to-biosimilar switching, and it requires further study.
The authors did not receive any funding for writing this review. Dr. Cohen is an employee of Sandoz, a division of Novartis. He may own stock in Novartis. Two coauthors are also employees of Sandoz. The other three coauthors reported having financial relationships with numerous pharmaceutical companies, including Sandoz and/or Novartis. Dr. Colombel reported financial relationships with many pharmaceutical companies, including Novartis and other manufacturers of biosimilars. Dr. Regueiro reports financial relationships with numerous pharmaceutical companies, including some manufacturers of biosimilars. Dr. Weinberg reported financial relationships with Celgene, AbbVie, Eli Lilly, and Novartis. Kim reports financial relationships with GlaxoSmithKline, Pfizer, and AstraZeneca. Dr. Aslinia, Dr. Shoor, Dr. Choate, and Dr. Bhatt reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM BIODRUGS
Growing pains? ... Rubbish
I’m pretty sure my ancestors came from Europe. And, as far as I know, I have no relatives in Australia. But, I must have some cosmic relationship with the Land Down Under because as I review articles for these columns I have an uncanny attraction to those coming out of Australia. Most of them are about sleep, one of my obsessions, and in general they address simple questions that no one has thought to ask.
My most recent Australia-based nugget appeared in the August edition of Pediatrics.
The researchers in Sidney were seeking to define “growing pains” by embarking on an extensive review of the medical literature. Beginning with thousands of articles, they winnowed these down to 145 studies. They found “there was extremely poor consensus between studies.” The most consistent components were the lower limb, bilaterality, evening onset, a normal physical assessment, and an episodic or recurrent course. However, all of these factors were mentioned in 50% or less of the articles they reviewed. The investigators wisely concluded that clinicians “should be wary of relying on the diagnosis to direct treatment decisions.”
This may seem like one small step for pediatrics. You may have reassured parents that none of your patients ever died of “growing pains” and the condition would eventually resolve. Hopefully, you were correct and that your case rate fatality is zero. But I suspect it wouldn’t take too long to unearth a wealth of malpractices cases in which another pediatrician’s patient died with an illness whose eventual discovery was tragically delayed by a period of false reassurance and diagnosis that the child merely had growing pains.
I can’t remember which of my sage instructors told me to never use “growing pains” as a diagnosis. It may have just been something I stumbled upon as my clinical experience grew. While holding firm to my commitment to never use it as a diagnosis, it became abundantly clear that I was seeing a large group of children (toddlers to early adolescents) who were experiencing lower leg pains in the early evening, often bad enough to wake them.
It took a bit longer to discover that most often these painful episodes occurred in children who were acutely or chronically sleep deprived. Occasionally, the pain would come on days in which the child had been unusually physically active. However, in most cases there was little correlation with lower limb activity.
I will admit that my observations were colored by my growing obsession that sleep deprivation is the root of many evils, including the phenomenon known as attention-deficit/hyperactivity disorder. I was even bold enough to include it in my one of the books I have written (Is My Child Overtired? Simon & Schuster, 2001). Nonetheless, I am still convinced that every investigation of a child with evening leg pains should include a thorough history of the child’s sleep history.
The bottom line is that these Australian researchers have done us a great favor with their research. However, I think they should have made a bolder statement in their conclusion. It is clear to me that “growing pains” should be removed as a diagnosis and no longer be reimbursed by third-party payers.
The void created by that action should spur some research into a better-defined diagnosis of the condition. If you want to use my tack and label it “nocturnal leg pains of childhood” and suggest better sleep hygiene, I will be flattered. But more importantly, take the time to take a good history, do a thorough exam, and then follow up, follow up, follow up, until the problem resolves.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
I’m pretty sure my ancestors came from Europe. And, as far as I know, I have no relatives in Australia. But, I must have some cosmic relationship with the Land Down Under because as I review articles for these columns I have an uncanny attraction to those coming out of Australia. Most of them are about sleep, one of my obsessions, and in general they address simple questions that no one has thought to ask.
My most recent Australia-based nugget appeared in the August edition of Pediatrics.
The researchers in Sidney were seeking to define “growing pains” by embarking on an extensive review of the medical literature. Beginning with thousands of articles, they winnowed these down to 145 studies. They found “there was extremely poor consensus between studies.” The most consistent components were the lower limb, bilaterality, evening onset, a normal physical assessment, and an episodic or recurrent course. However, all of these factors were mentioned in 50% or less of the articles they reviewed. The investigators wisely concluded that clinicians “should be wary of relying on the diagnosis to direct treatment decisions.”
This may seem like one small step for pediatrics. You may have reassured parents that none of your patients ever died of “growing pains” and the condition would eventually resolve. Hopefully, you were correct and that your case rate fatality is zero. But I suspect it wouldn’t take too long to unearth a wealth of malpractices cases in which another pediatrician’s patient died with an illness whose eventual discovery was tragically delayed by a period of false reassurance and diagnosis that the child merely had growing pains.
I can’t remember which of my sage instructors told me to never use “growing pains” as a diagnosis. It may have just been something I stumbled upon as my clinical experience grew. While holding firm to my commitment to never use it as a diagnosis, it became abundantly clear that I was seeing a large group of children (toddlers to early adolescents) who were experiencing lower leg pains in the early evening, often bad enough to wake them.
It took a bit longer to discover that most often these painful episodes occurred in children who were acutely or chronically sleep deprived. Occasionally, the pain would come on days in which the child had been unusually physically active. However, in most cases there was little correlation with lower limb activity.
I will admit that my observations were colored by my growing obsession that sleep deprivation is the root of many evils, including the phenomenon known as attention-deficit/hyperactivity disorder. I was even bold enough to include it in my one of the books I have written (Is My Child Overtired? Simon & Schuster, 2001). Nonetheless, I am still convinced that every investigation of a child with evening leg pains should include a thorough history of the child’s sleep history.
The bottom line is that these Australian researchers have done us a great favor with their research. However, I think they should have made a bolder statement in their conclusion. It is clear to me that “growing pains” should be removed as a diagnosis and no longer be reimbursed by third-party payers.
The void created by that action should spur some research into a better-defined diagnosis of the condition. If you want to use my tack and label it “nocturnal leg pains of childhood” and suggest better sleep hygiene, I will be flattered. But more importantly, take the time to take a good history, do a thorough exam, and then follow up, follow up, follow up, until the problem resolves.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
I’m pretty sure my ancestors came from Europe. And, as far as I know, I have no relatives in Australia. But, I must have some cosmic relationship with the Land Down Under because as I review articles for these columns I have an uncanny attraction to those coming out of Australia. Most of them are about sleep, one of my obsessions, and in general they address simple questions that no one has thought to ask.
My most recent Australia-based nugget appeared in the August edition of Pediatrics.
The researchers in Sidney were seeking to define “growing pains” by embarking on an extensive review of the medical literature. Beginning with thousands of articles, they winnowed these down to 145 studies. They found “there was extremely poor consensus between studies.” The most consistent components were the lower limb, bilaterality, evening onset, a normal physical assessment, and an episodic or recurrent course. However, all of these factors were mentioned in 50% or less of the articles they reviewed. The investigators wisely concluded that clinicians “should be wary of relying on the diagnosis to direct treatment decisions.”
This may seem like one small step for pediatrics. You may have reassured parents that none of your patients ever died of “growing pains” and the condition would eventually resolve. Hopefully, you were correct and that your case rate fatality is zero. But I suspect it wouldn’t take too long to unearth a wealth of malpractices cases in which another pediatrician’s patient died with an illness whose eventual discovery was tragically delayed by a period of false reassurance and diagnosis that the child merely had growing pains.
I can’t remember which of my sage instructors told me to never use “growing pains” as a diagnosis. It may have just been something I stumbled upon as my clinical experience grew. While holding firm to my commitment to never use it as a diagnosis, it became abundantly clear that I was seeing a large group of children (toddlers to early adolescents) who were experiencing lower leg pains in the early evening, often bad enough to wake them.
It took a bit longer to discover that most often these painful episodes occurred in children who were acutely or chronically sleep deprived. Occasionally, the pain would come on days in which the child had been unusually physically active. However, in most cases there was little correlation with lower limb activity.
I will admit that my observations were colored by my growing obsession that sleep deprivation is the root of many evils, including the phenomenon known as attention-deficit/hyperactivity disorder. I was even bold enough to include it in my one of the books I have written (Is My Child Overtired? Simon & Schuster, 2001). Nonetheless, I am still convinced that every investigation of a child with evening leg pains should include a thorough history of the child’s sleep history.
The bottom line is that these Australian researchers have done us a great favor with their research. However, I think they should have made a bolder statement in their conclusion. It is clear to me that “growing pains” should be removed as a diagnosis and no longer be reimbursed by third-party payers.
The void created by that action should spur some research into a better-defined diagnosis of the condition. If you want to use my tack and label it “nocturnal leg pains of childhood” and suggest better sleep hygiene, I will be flattered. But more importantly, take the time to take a good history, do a thorough exam, and then follow up, follow up, follow up, until the problem resolves.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
NYC switching children’s COVID vaccine sites to monkeypox
The city health department said demand for children’s COVID vaccines had been on the downswing at the clinics, which opened in late June. Meanwhile, monkeypox cases have increased, with the city declaring it a public health emergency July 30.
“We always planned to transition vaccination for very young children to providers,” the city’s health department said in a statement, according to Spectrum News NY1. “Due to the ongoing monkeypox emergency, we transitioned some of these sites to administer monkeypox vaccine.”
All the COVID vaccine sites for children will close by Aug. 14, Spectrum News NY1 said. It’s unclear if the other sites will transition to monkeypox vaccine.
No appointments for children’s COVID vaccinations had to be canceled, the city said. The plan is that children now needing the COVID vaccine can go to doctors, pharmacies, or the health department clinics.
Manhattan City Councilwoman Gale Brewer urged the health department to keep the kids’ COVID vaccine sites open through the fall.
“I strongly urge you to maintain these family-friendly sites, at least until mid-September so that children who are going to day care and school can get vaccinated,” Brewer wrote. City schools open Sept. 8
Ms. Brewer noted that the city-run sites administered the Moderna vaccines, while many doctors and neighborhood health clinics use the Pfizer vaccine. That could be a problem for a child that had not finished the Moderna regimen or for families that prefer Moderna.
According to the city health department, 2,130 people in New York City had tested positive for monkeypox as of Aug. 12.
On Friday, the city announced 9,000 additional monkeypox vaccines would be made available the morning of Aug. 13.
A version of this article first appeared on WebMD.com.
The city health department said demand for children’s COVID vaccines had been on the downswing at the clinics, which opened in late June. Meanwhile, monkeypox cases have increased, with the city declaring it a public health emergency July 30.
“We always planned to transition vaccination for very young children to providers,” the city’s health department said in a statement, according to Spectrum News NY1. “Due to the ongoing monkeypox emergency, we transitioned some of these sites to administer monkeypox vaccine.”
All the COVID vaccine sites for children will close by Aug. 14, Spectrum News NY1 said. It’s unclear if the other sites will transition to monkeypox vaccine.
No appointments for children’s COVID vaccinations had to be canceled, the city said. The plan is that children now needing the COVID vaccine can go to doctors, pharmacies, or the health department clinics.
Manhattan City Councilwoman Gale Brewer urged the health department to keep the kids’ COVID vaccine sites open through the fall.
“I strongly urge you to maintain these family-friendly sites, at least until mid-September so that children who are going to day care and school can get vaccinated,” Brewer wrote. City schools open Sept. 8
Ms. Brewer noted that the city-run sites administered the Moderna vaccines, while many doctors and neighborhood health clinics use the Pfizer vaccine. That could be a problem for a child that had not finished the Moderna regimen or for families that prefer Moderna.
According to the city health department, 2,130 people in New York City had tested positive for monkeypox as of Aug. 12.
On Friday, the city announced 9,000 additional monkeypox vaccines would be made available the morning of Aug. 13.
A version of this article first appeared on WebMD.com.
The city health department said demand for children’s COVID vaccines had been on the downswing at the clinics, which opened in late June. Meanwhile, monkeypox cases have increased, with the city declaring it a public health emergency July 30.
“We always planned to transition vaccination for very young children to providers,” the city’s health department said in a statement, according to Spectrum News NY1. “Due to the ongoing monkeypox emergency, we transitioned some of these sites to administer monkeypox vaccine.”
All the COVID vaccine sites for children will close by Aug. 14, Spectrum News NY1 said. It’s unclear if the other sites will transition to monkeypox vaccine.
No appointments for children’s COVID vaccinations had to be canceled, the city said. The plan is that children now needing the COVID vaccine can go to doctors, pharmacies, or the health department clinics.
Manhattan City Councilwoman Gale Brewer urged the health department to keep the kids’ COVID vaccine sites open through the fall.
“I strongly urge you to maintain these family-friendly sites, at least until mid-September so that children who are going to day care and school can get vaccinated,” Brewer wrote. City schools open Sept. 8
Ms. Brewer noted that the city-run sites administered the Moderna vaccines, while many doctors and neighborhood health clinics use the Pfizer vaccine. That could be a problem for a child that had not finished the Moderna regimen or for families that prefer Moderna.
According to the city health department, 2,130 people in New York City had tested positive for monkeypox as of Aug. 12.
On Friday, the city announced 9,000 additional monkeypox vaccines would be made available the morning of Aug. 13.
A version of this article first appeared on WebMD.com.
What are growing pains? Turns out no one really knows
Just about every child hears it growing up: An ache in the leg? “Growing pains.” A dull pain in the side? “Growing pains.”
The catch-all phrase for random pains that children and teens have is so common that it even inspired the name of a 1980s sitcom. Yet when scientists dug into the evidence to find out what growing pains actually are, they found out that no one really knows. The definitions were as random and all over the place as the very pains that kids complain about, the researchers report in the journal Pediatrics.
Although some studies have suggested that up to a third of children have growing pains, the term has long seemed more like folk medicine than an actual medical diagnosis. Even so, parents, teachers, and doctors frequently use it when they have no other obvious answer to a particular pain a child or teen might describe.
A group of researchers at the University of Sydney in Australia wanted to find out if there was any research offering a more precise definition or criteria. They combed through eight databases for any papers that mentioned growing pains or growth pains in children or adolescents. They found 145 studies and set out to look for common ground: Where do growing pains occur? At what age do they start? Are there any patterns? Risk factors? Common clinical features? Relationships to particular activities?
What they found was that there is “no consensus whatsoever as to what growing pains really are, what they mean, how they’re defined, and how they should be diagnosed,” coauthor Steven J. Kamper, PhD, explained in a video about the findings. “The definitions were really variable, really vague, and sometimes downright contradictory,” he said. “Some studies would suggest growing pains happen in the arms, some in the lower limbs only. Some said it was about muscles, some about joints.”
The closest thing to consistency that they found was that exactly half the studies mentioned the pain being in the lower limbs. Nearly half (48%) described it as happening in the evening or nighttime, 42% said it was recurring, 35% reported it as occurring in youths with an otherwise normal physical exam, and 31% said the pain occurred on both sides of the body. Besides these, no other common feature was mentioned in more than 30% of the studies.
“Really curiously,” Dr. Kamper said, “more than 80% said nothing about the age at which these growing pains come on.” And 93% of the studies didn’t even mention growth as being related to the pain at all.
Several studies did acknowledge that the cause of growing pains is unknown, and several others considered it a diagnosis of exclusion – that is, it’s the diagnosis when everything else has been ruled out.
But that’s hardly a satisfactory explanation for kids and their families, so the researchers drew the only reasonable conclusion they could from what they found: “We think it’s important that the term is not used without some qualification or clarification, whether by researchers or clinicians,” Dr. Kamper said.
A version of this article first appeared on WebMD.com.
Just about every child hears it growing up: An ache in the leg? “Growing pains.” A dull pain in the side? “Growing pains.”
The catch-all phrase for random pains that children and teens have is so common that it even inspired the name of a 1980s sitcom. Yet when scientists dug into the evidence to find out what growing pains actually are, they found out that no one really knows. The definitions were as random and all over the place as the very pains that kids complain about, the researchers report in the journal Pediatrics.
Although some studies have suggested that up to a third of children have growing pains, the term has long seemed more like folk medicine than an actual medical diagnosis. Even so, parents, teachers, and doctors frequently use it when they have no other obvious answer to a particular pain a child or teen might describe.
A group of researchers at the University of Sydney in Australia wanted to find out if there was any research offering a more precise definition or criteria. They combed through eight databases for any papers that mentioned growing pains or growth pains in children or adolescents. They found 145 studies and set out to look for common ground: Where do growing pains occur? At what age do they start? Are there any patterns? Risk factors? Common clinical features? Relationships to particular activities?
What they found was that there is “no consensus whatsoever as to what growing pains really are, what they mean, how they’re defined, and how they should be diagnosed,” coauthor Steven J. Kamper, PhD, explained in a video about the findings. “The definitions were really variable, really vague, and sometimes downright contradictory,” he said. “Some studies would suggest growing pains happen in the arms, some in the lower limbs only. Some said it was about muscles, some about joints.”
The closest thing to consistency that they found was that exactly half the studies mentioned the pain being in the lower limbs. Nearly half (48%) described it as happening in the evening or nighttime, 42% said it was recurring, 35% reported it as occurring in youths with an otherwise normal physical exam, and 31% said the pain occurred on both sides of the body. Besides these, no other common feature was mentioned in more than 30% of the studies.
“Really curiously,” Dr. Kamper said, “more than 80% said nothing about the age at which these growing pains come on.” And 93% of the studies didn’t even mention growth as being related to the pain at all.
Several studies did acknowledge that the cause of growing pains is unknown, and several others considered it a diagnosis of exclusion – that is, it’s the diagnosis when everything else has been ruled out.
But that’s hardly a satisfactory explanation for kids and their families, so the researchers drew the only reasonable conclusion they could from what they found: “We think it’s important that the term is not used without some qualification or clarification, whether by researchers or clinicians,” Dr. Kamper said.
A version of this article first appeared on WebMD.com.
Just about every child hears it growing up: An ache in the leg? “Growing pains.” A dull pain in the side? “Growing pains.”
The catch-all phrase for random pains that children and teens have is so common that it even inspired the name of a 1980s sitcom. Yet when scientists dug into the evidence to find out what growing pains actually are, they found out that no one really knows. The definitions were as random and all over the place as the very pains that kids complain about, the researchers report in the journal Pediatrics.
Although some studies have suggested that up to a third of children have growing pains, the term has long seemed more like folk medicine than an actual medical diagnosis. Even so, parents, teachers, and doctors frequently use it when they have no other obvious answer to a particular pain a child or teen might describe.
A group of researchers at the University of Sydney in Australia wanted to find out if there was any research offering a more precise definition or criteria. They combed through eight databases for any papers that mentioned growing pains or growth pains in children or adolescents. They found 145 studies and set out to look for common ground: Where do growing pains occur? At what age do they start? Are there any patterns? Risk factors? Common clinical features? Relationships to particular activities?
What they found was that there is “no consensus whatsoever as to what growing pains really are, what they mean, how they’re defined, and how they should be diagnosed,” coauthor Steven J. Kamper, PhD, explained in a video about the findings. “The definitions were really variable, really vague, and sometimes downright contradictory,” he said. “Some studies would suggest growing pains happen in the arms, some in the lower limbs only. Some said it was about muscles, some about joints.”
The closest thing to consistency that they found was that exactly half the studies mentioned the pain being in the lower limbs. Nearly half (48%) described it as happening in the evening or nighttime, 42% said it was recurring, 35% reported it as occurring in youths with an otherwise normal physical exam, and 31% said the pain occurred on both sides of the body. Besides these, no other common feature was mentioned in more than 30% of the studies.
“Really curiously,” Dr. Kamper said, “more than 80% said nothing about the age at which these growing pains come on.” And 93% of the studies didn’t even mention growth as being related to the pain at all.
Several studies did acknowledge that the cause of growing pains is unknown, and several others considered it a diagnosis of exclusion – that is, it’s the diagnosis when everything else has been ruled out.
But that’s hardly a satisfactory explanation for kids and their families, so the researchers drew the only reasonable conclusion they could from what they found: “We think it’s important that the term is not used without some qualification or clarification, whether by researchers or clinicians,” Dr. Kamper said.
A version of this article first appeared on WebMD.com.
Two deaths from liver failure linked to spinal muscular atrophy drug
, according to a statement issued by the drug›s manufacturer.
The patients were 4 months and 28 months of age and lived in Russia and Kazakhstan. They died 5-6 weeks after infusion with Zolgensma and approximately 1-10 days after the initiation of a corticosteroid taper.
These are the first known fatal cases of acute liver failure associated with the drug, which the company notes was a known side effect included in the product label and in a boxed warning in the United States.
“Following two recent patient fatalities, and in alignment with health authorities, we will be updating the labeling to specify that fatal acute liver failure has been reported,” the statement reads.
“While this is important safety information, it is not a new safety signal,” it adds.
Rare genetic disorder
SMA is a rare genetic disorder that affects about 1 in 10,000 newborns. Patients with SMA lack a working copy of the survival motor neuron 1 (SMN1) gene, which encodes a protein called SMN that is critical for the maintenance and function of motor neurons.
Without this protein, motor neurons eventually die, causing debilitating and progressive muscle weakness that affects the ability to walk, eat, and breathe.
Zolgensma, a one-time gene replacement therapy delivered via intravenous infusion, replaces the function of the missing or nonworking SMN1 gene with a new, working copy of the SMN1 gene.
The first gene therapy treatment for SMA, it was approved by the U.S. Food and Drug Administration in 2019 for patients with SMA up to 2 years of age. It is also the most expensive drug in the world, costing about $2.1 million for a one-time treatment.
“We have notified health authorities in all markets where Zolgensma is used, including FDA, and are communicating to relevant healthcare professionals as an additional step in markets where this action is supported by health authorities,” the manufacturer’s statement says.
Studies have suggested that the treatment›s effects persist more than 5 years after infusion.
Clinical trials currently underway by Novartis are studying the drug’s long-term efficacy and safety and its potential use in older patients.
The company is also leading the phase 3 clinical trial STEER to test intrathecal (IT) administration of the drug in patients ages 2-18 years who have type 2 SMA.
That trial began late last year after the FDA lifted a 2-year partial hold on an earlier study. The FDA halted the STRONG trial in 2019, citing concerns from animal studies that IT administration may result in dorsal root ganglia injury. The partial hold was released last fall following positive study results in nonhuman primates.
None of the current trials will be affected by the two deaths reported this week, according to a Novartis spokesperson.
A version of this article first appeared on Medscape.com.
, according to a statement issued by the drug›s manufacturer.
The patients were 4 months and 28 months of age and lived in Russia and Kazakhstan. They died 5-6 weeks after infusion with Zolgensma and approximately 1-10 days after the initiation of a corticosteroid taper.
These are the first known fatal cases of acute liver failure associated with the drug, which the company notes was a known side effect included in the product label and in a boxed warning in the United States.
“Following two recent patient fatalities, and in alignment with health authorities, we will be updating the labeling to specify that fatal acute liver failure has been reported,” the statement reads.
“While this is important safety information, it is not a new safety signal,” it adds.
Rare genetic disorder
SMA is a rare genetic disorder that affects about 1 in 10,000 newborns. Patients with SMA lack a working copy of the survival motor neuron 1 (SMN1) gene, which encodes a protein called SMN that is critical for the maintenance and function of motor neurons.
Without this protein, motor neurons eventually die, causing debilitating and progressive muscle weakness that affects the ability to walk, eat, and breathe.
Zolgensma, a one-time gene replacement therapy delivered via intravenous infusion, replaces the function of the missing or nonworking SMN1 gene with a new, working copy of the SMN1 gene.
The first gene therapy treatment for SMA, it was approved by the U.S. Food and Drug Administration in 2019 for patients with SMA up to 2 years of age. It is also the most expensive drug in the world, costing about $2.1 million for a one-time treatment.
“We have notified health authorities in all markets where Zolgensma is used, including FDA, and are communicating to relevant healthcare professionals as an additional step in markets where this action is supported by health authorities,” the manufacturer’s statement says.
Studies have suggested that the treatment›s effects persist more than 5 years after infusion.
Clinical trials currently underway by Novartis are studying the drug’s long-term efficacy and safety and its potential use in older patients.
The company is also leading the phase 3 clinical trial STEER to test intrathecal (IT) administration of the drug in patients ages 2-18 years who have type 2 SMA.
That trial began late last year after the FDA lifted a 2-year partial hold on an earlier study. The FDA halted the STRONG trial in 2019, citing concerns from animal studies that IT administration may result in dorsal root ganglia injury. The partial hold was released last fall following positive study results in nonhuman primates.
None of the current trials will be affected by the two deaths reported this week, according to a Novartis spokesperson.
A version of this article first appeared on Medscape.com.
, according to a statement issued by the drug›s manufacturer.
The patients were 4 months and 28 months of age and lived in Russia and Kazakhstan. They died 5-6 weeks after infusion with Zolgensma and approximately 1-10 days after the initiation of a corticosteroid taper.
These are the first known fatal cases of acute liver failure associated with the drug, which the company notes was a known side effect included in the product label and in a boxed warning in the United States.
“Following two recent patient fatalities, and in alignment with health authorities, we will be updating the labeling to specify that fatal acute liver failure has been reported,” the statement reads.
“While this is important safety information, it is not a new safety signal,” it adds.
Rare genetic disorder
SMA is a rare genetic disorder that affects about 1 in 10,000 newborns. Patients with SMA lack a working copy of the survival motor neuron 1 (SMN1) gene, which encodes a protein called SMN that is critical for the maintenance and function of motor neurons.
Without this protein, motor neurons eventually die, causing debilitating and progressive muscle weakness that affects the ability to walk, eat, and breathe.
Zolgensma, a one-time gene replacement therapy delivered via intravenous infusion, replaces the function of the missing or nonworking SMN1 gene with a new, working copy of the SMN1 gene.
The first gene therapy treatment for SMA, it was approved by the U.S. Food and Drug Administration in 2019 for patients with SMA up to 2 years of age. It is also the most expensive drug in the world, costing about $2.1 million for a one-time treatment.
“We have notified health authorities in all markets where Zolgensma is used, including FDA, and are communicating to relevant healthcare professionals as an additional step in markets where this action is supported by health authorities,” the manufacturer’s statement says.
Studies have suggested that the treatment›s effects persist more than 5 years after infusion.
Clinical trials currently underway by Novartis are studying the drug’s long-term efficacy and safety and its potential use in older patients.
The company is also leading the phase 3 clinical trial STEER to test intrathecal (IT) administration of the drug in patients ages 2-18 years who have type 2 SMA.
That trial began late last year after the FDA lifted a 2-year partial hold on an earlier study. The FDA halted the STRONG trial in 2019, citing concerns from animal studies that IT administration may result in dorsal root ganglia injury. The partial hold was released last fall following positive study results in nonhuman primates.
None of the current trials will be affected by the two deaths reported this week, according to a Novartis spokesperson.
A version of this article first appeared on Medscape.com.
Dermatologists share vitiligo breakthrough news with patients
For the first time, patients with vitiligo who have long lived with patches of skin that are without pigment can now have even skin tones on their faces and other bodily regions with a Food and Drug Administration–approved, easy-to-use topical treatment.
In July, , the most common form of the disease.
Topical ruxolitinib was first approved in September 2021 for atopic dermatitis, and dermatologists are already writing prescriptions for its new vitiligo indication.
“The FDA approval of ruxolitinib for repigmentation of vitiligo is historic and groundbreaking,” Seemal R. Desai, MD, a dermatologist at the University of Texas Southwestern Medical Center, Dallas, told this news organization.
The news brings hope to patients 12 years and older who suffer from the psychosocial effects of the disease, which is estimated to affect 1.9 million to 2.8 million adults in the United States.
The announcement followed FDA approval a month earlier of another dermatologic milestone – an oral JAK inhibitor, baricitinib, which became the first treatment for patients with alopecia areata.
For Dr. Desai, the ruxolitinib news is personal. His brother, also a physician, has lived a lifetime with vitiligo. His family experience, Dr. Desai said, showed him “what a disease like this can do to a person psychologically.”
Dr. Desai said his early exposure helped lead to his own decision to dedicate his career to pigmentary diseases.
His brother won’t personally benefit from the cream because his skin has been completely depigmented and repigmentation is not of interest to him, Dr. Desai said. But both brothers are excited as physicians. “It’s really quite an emotional moment,” he said.
Getting the news to patients
As dermatologists introduce the topical treatment to patients, common questions center on why this cream is different and whether it is safe.
David Rosmarin, MD, vice chair of research and education, department of dermatology, Tufts Medical Center, Boston, led the Topical Ruxolitinib Evaluation in Vitiligo Study 1 and 2 (TruE-V1, TruE-V2), conducted in North America and Europe.
He summarized some key findings.
“If patients have involvement on the face, trunk, or extremities, the data show that about half the patients at 52 weeks will get half or more of their pigment back,” he said in an interview. Results for the face alone are even better. “Half the patients will get 75% or more pigment back in the face,” Dr. Rosmarin said.
In addition, analysis of subgroups shows benefit for all patients. “Patients seem to respond similarly well across all subgroups – across gender, sex, age, ethnicity, and race,” Dr. Rosmarin said.
However, anatomic region matters, he pointed out. Skin of the head and neck responds the best, followed by skin of the trunk and extremities. The hands and feet are the most difficult to repigment because there are few hair follicles, which help enable repigmentation.
He added that it’s important to understand patients’ goals, and dermatologists shouldn’t assume that all who have vitiligo will want to undergo repigmentation. They may be interested in the new treatment but may not want it for themselves, he explained.
Explaining risks
Patients may ask about the boxed warning on the label that lists risk of heart attack, stroke, cancer, infections, blood clots, and death. Dermatologists can explain that the warning pertains to the whole JAK class and was based on patients with rheumatoid arthritis, Dr. Rosmarin said.
He added, “We didn’t see a signal for heart attack and stroke for patients using the topical. But it’s still important to discuss the label as the FDA states it.”
There are two main side effects, Dr. Rosmarin said: acne (about 6% of treated patients get it, and it’s usually mild) and application-site reactions. “Luckily, the medication has a tendency not to sting or burn, which is not the case with some of our other treatments. It’s very well tolerated,” he said.
Patients should also know that repigmentation can take time, because initially, the immune system is directed to calm down with treatment, and then pigment must travel back to the affected sites.
Some patients may have a response in as early as 2-3 months, and others need more time, Dr. Rosmarin said.
Treatment responses among adolescents have been particularly good. Responses regarding the skin of the face have been similar to those of adults. “However, on the body, they respond even better,” Dr. Rosmarin said. “About 60% achieve 50% or more repigmentation on the whole body.”
It’s important that ruxolitinib has been approved for persons aged 12 years and older, he said, because “about half the patients will develop vitiligo by the age of 20.”
Approval and insurance coverage
FDA approval will help with reimbursement for the expensive treatment.
The label indicates that patients should not use more than one 60-g tube a week. Currently, the out-of-pocket cost for one tube can be close to $2,000, according to GoodRx.
Raj Chovatiya, MD, PhD, assistant professor of dermatology and director of the Center for Eczema and Itch at Northwestern University, Chicago, said that in recent years, vitiligo patients, aware that their condition could be treated by JAK inhibitors, have been paying out of pocket at compounding pharmacies, which take oral versions of the medication and compound them into topical formulations.
Unlike baricitinib, which is used to treat severe alopecia areata, and other oral JAK inhibitors, testing for TB and hepatitis is not required for initiating treatment with ruxolitinib, so no delay is necessary, Dr. Chovatiya said.
He noted, however, that patients with vitiligo may have given up on effective care after experiencing little or no improvement with topical corticosteroids, phototherapy, or topical calcineurin inhibitors.
“They end up losing steam, are less motivated on therapy, and are lost to care,” he said.
Dermatologists, he said, may need to proactively find these patients and tell them the good news. “Now that we have really good targeted therapeutic options, it’s really up to us to figure out how to bring these people back to the clinic and educate them,” Dr. Chovatiya said.
Unanswered questions to address
Some questions are still unanswered, lead study author Dr. Rosmarin said.
Two big questions are how long people will need to continue using ruxolitinib cream and whether depigmentation will recur if people stop using it.
Another aspect of therapy being studied is whether the cream will be even more effective in combination with other treatments.
“The main combination we think about is ruxolitinib with phototherapy – a light treatment – because light could stimulate those pigment cells,” Dr. Rosmarin said,
He noted that light therapy was included in phase 2 testing and that patients did respond. “What we need and what’s planned is a larger study looking at the combination to see whether it is synergistic or not. The longer patients use the cream, the more benefit we see,” Dr. Rosmarin said.
Dr. Desai has served as an investigator and/or consultant to several companies, including Incyte. Dr. Rosmarin has received honoraria as a consultant and has received research support from Incyte, and has served as a paid speaker for Incyte, as well as other companies.. Dr. Chovatiya has served as an advisory board member, consultant, and/or investigator for companies that include Incyte.
For the first time, patients with vitiligo who have long lived with patches of skin that are without pigment can now have even skin tones on their faces and other bodily regions with a Food and Drug Administration–approved, easy-to-use topical treatment.
In July, , the most common form of the disease.
Topical ruxolitinib was first approved in September 2021 for atopic dermatitis, and dermatologists are already writing prescriptions for its new vitiligo indication.
“The FDA approval of ruxolitinib for repigmentation of vitiligo is historic and groundbreaking,” Seemal R. Desai, MD, a dermatologist at the University of Texas Southwestern Medical Center, Dallas, told this news organization.
The news brings hope to patients 12 years and older who suffer from the psychosocial effects of the disease, which is estimated to affect 1.9 million to 2.8 million adults in the United States.
The announcement followed FDA approval a month earlier of another dermatologic milestone – an oral JAK inhibitor, baricitinib, which became the first treatment for patients with alopecia areata.
For Dr. Desai, the ruxolitinib news is personal. His brother, also a physician, has lived a lifetime with vitiligo. His family experience, Dr. Desai said, showed him “what a disease like this can do to a person psychologically.”
Dr. Desai said his early exposure helped lead to his own decision to dedicate his career to pigmentary diseases.
His brother won’t personally benefit from the cream because his skin has been completely depigmented and repigmentation is not of interest to him, Dr. Desai said. But both brothers are excited as physicians. “It’s really quite an emotional moment,” he said.
Getting the news to patients
As dermatologists introduce the topical treatment to patients, common questions center on why this cream is different and whether it is safe.
David Rosmarin, MD, vice chair of research and education, department of dermatology, Tufts Medical Center, Boston, led the Topical Ruxolitinib Evaluation in Vitiligo Study 1 and 2 (TruE-V1, TruE-V2), conducted in North America and Europe.
He summarized some key findings.
“If patients have involvement on the face, trunk, or extremities, the data show that about half the patients at 52 weeks will get half or more of their pigment back,” he said in an interview. Results for the face alone are even better. “Half the patients will get 75% or more pigment back in the face,” Dr. Rosmarin said.
In addition, analysis of subgroups shows benefit for all patients. “Patients seem to respond similarly well across all subgroups – across gender, sex, age, ethnicity, and race,” Dr. Rosmarin said.
However, anatomic region matters, he pointed out. Skin of the head and neck responds the best, followed by skin of the trunk and extremities. The hands and feet are the most difficult to repigment because there are few hair follicles, which help enable repigmentation.
He added that it’s important to understand patients’ goals, and dermatologists shouldn’t assume that all who have vitiligo will want to undergo repigmentation. They may be interested in the new treatment but may not want it for themselves, he explained.
Explaining risks
Patients may ask about the boxed warning on the label that lists risk of heart attack, stroke, cancer, infections, blood clots, and death. Dermatologists can explain that the warning pertains to the whole JAK class and was based on patients with rheumatoid arthritis, Dr. Rosmarin said.
He added, “We didn’t see a signal for heart attack and stroke for patients using the topical. But it’s still important to discuss the label as the FDA states it.”
There are two main side effects, Dr. Rosmarin said: acne (about 6% of treated patients get it, and it’s usually mild) and application-site reactions. “Luckily, the medication has a tendency not to sting or burn, which is not the case with some of our other treatments. It’s very well tolerated,” he said.
Patients should also know that repigmentation can take time, because initially, the immune system is directed to calm down with treatment, and then pigment must travel back to the affected sites.
Some patients may have a response in as early as 2-3 months, and others need more time, Dr. Rosmarin said.
Treatment responses among adolescents have been particularly good. Responses regarding the skin of the face have been similar to those of adults. “However, on the body, they respond even better,” Dr. Rosmarin said. “About 60% achieve 50% or more repigmentation on the whole body.”
It’s important that ruxolitinib has been approved for persons aged 12 years and older, he said, because “about half the patients will develop vitiligo by the age of 20.”
Approval and insurance coverage
FDA approval will help with reimbursement for the expensive treatment.
The label indicates that patients should not use more than one 60-g tube a week. Currently, the out-of-pocket cost for one tube can be close to $2,000, according to GoodRx.
Raj Chovatiya, MD, PhD, assistant professor of dermatology and director of the Center for Eczema and Itch at Northwestern University, Chicago, said that in recent years, vitiligo patients, aware that their condition could be treated by JAK inhibitors, have been paying out of pocket at compounding pharmacies, which take oral versions of the medication and compound them into topical formulations.
Unlike baricitinib, which is used to treat severe alopecia areata, and other oral JAK inhibitors, testing for TB and hepatitis is not required for initiating treatment with ruxolitinib, so no delay is necessary, Dr. Chovatiya said.
He noted, however, that patients with vitiligo may have given up on effective care after experiencing little or no improvement with topical corticosteroids, phototherapy, or topical calcineurin inhibitors.
“They end up losing steam, are less motivated on therapy, and are lost to care,” he said.
Dermatologists, he said, may need to proactively find these patients and tell them the good news. “Now that we have really good targeted therapeutic options, it’s really up to us to figure out how to bring these people back to the clinic and educate them,” Dr. Chovatiya said.
Unanswered questions to address
Some questions are still unanswered, lead study author Dr. Rosmarin said.
Two big questions are how long people will need to continue using ruxolitinib cream and whether depigmentation will recur if people stop using it.
Another aspect of therapy being studied is whether the cream will be even more effective in combination with other treatments.
“The main combination we think about is ruxolitinib with phototherapy – a light treatment – because light could stimulate those pigment cells,” Dr. Rosmarin said,
He noted that light therapy was included in phase 2 testing and that patients did respond. “What we need and what’s planned is a larger study looking at the combination to see whether it is synergistic or not. The longer patients use the cream, the more benefit we see,” Dr. Rosmarin said.
Dr. Desai has served as an investigator and/or consultant to several companies, including Incyte. Dr. Rosmarin has received honoraria as a consultant and has received research support from Incyte, and has served as a paid speaker for Incyte, as well as other companies.. Dr. Chovatiya has served as an advisory board member, consultant, and/or investigator for companies that include Incyte.
For the first time, patients with vitiligo who have long lived with patches of skin that are without pigment can now have even skin tones on their faces and other bodily regions with a Food and Drug Administration–approved, easy-to-use topical treatment.
In July, , the most common form of the disease.
Topical ruxolitinib was first approved in September 2021 for atopic dermatitis, and dermatologists are already writing prescriptions for its new vitiligo indication.
“The FDA approval of ruxolitinib for repigmentation of vitiligo is historic and groundbreaking,” Seemal R. Desai, MD, a dermatologist at the University of Texas Southwestern Medical Center, Dallas, told this news organization.
The news brings hope to patients 12 years and older who suffer from the psychosocial effects of the disease, which is estimated to affect 1.9 million to 2.8 million adults in the United States.
The announcement followed FDA approval a month earlier of another dermatologic milestone – an oral JAK inhibitor, baricitinib, which became the first treatment for patients with alopecia areata.
For Dr. Desai, the ruxolitinib news is personal. His brother, also a physician, has lived a lifetime with vitiligo. His family experience, Dr. Desai said, showed him “what a disease like this can do to a person psychologically.”
Dr. Desai said his early exposure helped lead to his own decision to dedicate his career to pigmentary diseases.
His brother won’t personally benefit from the cream because his skin has been completely depigmented and repigmentation is not of interest to him, Dr. Desai said. But both brothers are excited as physicians. “It’s really quite an emotional moment,” he said.
Getting the news to patients
As dermatologists introduce the topical treatment to patients, common questions center on why this cream is different and whether it is safe.
David Rosmarin, MD, vice chair of research and education, department of dermatology, Tufts Medical Center, Boston, led the Topical Ruxolitinib Evaluation in Vitiligo Study 1 and 2 (TruE-V1, TruE-V2), conducted in North America and Europe.
He summarized some key findings.
“If patients have involvement on the face, trunk, or extremities, the data show that about half the patients at 52 weeks will get half or more of their pigment back,” he said in an interview. Results for the face alone are even better. “Half the patients will get 75% or more pigment back in the face,” Dr. Rosmarin said.
In addition, analysis of subgroups shows benefit for all patients. “Patients seem to respond similarly well across all subgroups – across gender, sex, age, ethnicity, and race,” Dr. Rosmarin said.
However, anatomic region matters, he pointed out. Skin of the head and neck responds the best, followed by skin of the trunk and extremities. The hands and feet are the most difficult to repigment because there are few hair follicles, which help enable repigmentation.
He added that it’s important to understand patients’ goals, and dermatologists shouldn’t assume that all who have vitiligo will want to undergo repigmentation. They may be interested in the new treatment but may not want it for themselves, he explained.
Explaining risks
Patients may ask about the boxed warning on the label that lists risk of heart attack, stroke, cancer, infections, blood clots, and death. Dermatologists can explain that the warning pertains to the whole JAK class and was based on patients with rheumatoid arthritis, Dr. Rosmarin said.
He added, “We didn’t see a signal for heart attack and stroke for patients using the topical. But it’s still important to discuss the label as the FDA states it.”
There are two main side effects, Dr. Rosmarin said: acne (about 6% of treated patients get it, and it’s usually mild) and application-site reactions. “Luckily, the medication has a tendency not to sting or burn, which is not the case with some of our other treatments. It’s very well tolerated,” he said.
Patients should also know that repigmentation can take time, because initially, the immune system is directed to calm down with treatment, and then pigment must travel back to the affected sites.
Some patients may have a response in as early as 2-3 months, and others need more time, Dr. Rosmarin said.
Treatment responses among adolescents have been particularly good. Responses regarding the skin of the face have been similar to those of adults. “However, on the body, they respond even better,” Dr. Rosmarin said. “About 60% achieve 50% or more repigmentation on the whole body.”
It’s important that ruxolitinib has been approved for persons aged 12 years and older, he said, because “about half the patients will develop vitiligo by the age of 20.”
Approval and insurance coverage
FDA approval will help with reimbursement for the expensive treatment.
The label indicates that patients should not use more than one 60-g tube a week. Currently, the out-of-pocket cost for one tube can be close to $2,000, according to GoodRx.
Raj Chovatiya, MD, PhD, assistant professor of dermatology and director of the Center for Eczema and Itch at Northwestern University, Chicago, said that in recent years, vitiligo patients, aware that their condition could be treated by JAK inhibitors, have been paying out of pocket at compounding pharmacies, which take oral versions of the medication and compound them into topical formulations.
Unlike baricitinib, which is used to treat severe alopecia areata, and other oral JAK inhibitors, testing for TB and hepatitis is not required for initiating treatment with ruxolitinib, so no delay is necessary, Dr. Chovatiya said.
He noted, however, that patients with vitiligo may have given up on effective care after experiencing little or no improvement with topical corticosteroids, phototherapy, or topical calcineurin inhibitors.
“They end up losing steam, are less motivated on therapy, and are lost to care,” he said.
Dermatologists, he said, may need to proactively find these patients and tell them the good news. “Now that we have really good targeted therapeutic options, it’s really up to us to figure out how to bring these people back to the clinic and educate them,” Dr. Chovatiya said.
Unanswered questions to address
Some questions are still unanswered, lead study author Dr. Rosmarin said.
Two big questions are how long people will need to continue using ruxolitinib cream and whether depigmentation will recur if people stop using it.
Another aspect of therapy being studied is whether the cream will be even more effective in combination with other treatments.
“The main combination we think about is ruxolitinib with phototherapy – a light treatment – because light could stimulate those pigment cells,” Dr. Rosmarin said,
He noted that light therapy was included in phase 2 testing and that patients did respond. “What we need and what’s planned is a larger study looking at the combination to see whether it is synergistic or not. The longer patients use the cream, the more benefit we see,” Dr. Rosmarin said.
Dr. Desai has served as an investigator and/or consultant to several companies, including Incyte. Dr. Rosmarin has received honoraria as a consultant and has received research support from Incyte, and has served as a paid speaker for Incyte, as well as other companies.. Dr. Chovatiya has served as an advisory board member, consultant, and/or investigator for companies that include Incyte.