User login
Could exercise improve bone health in youth with type 1 diabetes?
In a small cross-sectional study of 10- to 16-year-old girls with and without type 1 diabetes, both groups were equally physically active, based on their replies to the bone-specific physical activity questionnaire (BPAQ).
However, among the more sedentary girls (with BPAQ scores below the median), those with type 1 diabetes had worse markers of bone health in imaging tests compared with the girls without diabetes.
the researchers summarize in a poster presented at the annual meeting of the American Society of Bone and Mineral Research.
However, this is early research and further study is needed, the group cautions.
“Ongoing studies with objective measures of physical activity as well as interventional studies will clarify whether increasing physical activity may improve bone health and reduce fracture risk in this vulnerable group,” they conclude.
“If you look at the sedentary kids, there’s a big discrepancy between the kids who have diabetes and the control kids, and that’s if we’re looking at radius or tibia or trabecular bone density or estimated failure load,” senior author Deborah M. Mitchell, MD, said in an interview at the poster session.
However, “when we look at the kids who are more physically active, we’re really not seeing as much difference [in bone health] between the kids with and without diabetes,” said Dr. Mitchell, a pediatric endocrinologist at Massachusetts General Hospital and assistant professor at Harvard Medical School, Boston.
But she also acknowledged, “There’s all sorts of caveats, including that this is retrospective questionnaire data.”
However, if further, rigorous studies confirm these findings, “physical activity is potentially a really effective means of improving bone quality in kids with type 1 diabetes.”
“This study suggests that bone-loading physical activity can substantially improve skeletal health in children with [type 1 diabetes] and should provide hope for patients and their families that they can take some action to prevent or mitigate the effects of diabetes on bone,” coauthor and incoming ASBMR President Mary L. Bouxsein, PhD, told this news organization in an email.
“We interpret these data as an important reason to advocate for increased time in moderate to vigorous bone-loading activity,” said Dr. Bouxsein, professor, department of orthopedic surgery, Harvard Medical School, Boston, “though the ‘dose’ in terms of hours per day or episodes per week to promote optimal bone health is still to be determined.”
“Ongoing debate,” “need stronger proof”
Asked for comment, Laura K. Bachrach, MD, who was not involved with the research, noted: “Activity benefits the development of bone strength through effects on bone geometry more than ‘density,’ and conversely, lack of physical activity can compromise gains in cortical bone diameter and thickness.”
However, “there is ongoing debate about the impact of type 1 diabetes on bone health and the factor(s) determining risk,” Dr. Bachrach, a pediatric endocrinologist at Stanford Children’s Health, Palo Alto, Calif., told this news organization in an email.
The current findings suggest “that physical activity in adolescent girls provided protection against potential adverse effects of type 1 diabetes,” said Dr. Bachrach, who spoke about bone fragility in childhood in a video commentary in 2021.
Study strengths, she noted, “include the rigor and expertise of the investigators, use of multiple surrogate measures that capture bone geometry/microarchitecture, as well as the inclusion of healthy local controls.”
“The study is limited by the cross-sectional design and subjects who opted, or not, to be active,” she added. “Stronger proof of the protective effects of activity on bone health in type 1 diabetes would require a randomized longitudinal intervention study, as alluded to by the authors of the study.”
Hypothesis: Those with type 1 diabetes acquire less bone mass in early 20s
The excess fracture risk in children with type 1 diabetes has been previously reported and is 14%-35% higher than the fracture risk in children without diabetes, Dr. Bouxsein explained. And “between 30% to 50% of kids [with type 1 diabetes] will have a fracture before the age of 18, so the excess fracture risk in diabetes is not clinically obvious,” she added.
However, “several lines of evidence strongly suggest that bone mass and microarchitecture at the time of peak bone mass (early 20s) is a major determinant of fracture risk throughout the lifespan,” she noted.
“Our hypothesis,” Dr. Bouxsein said, “is that the metabolic disruptions of diabetes, when they are present during the acquisition of peak bone mass, interfere with optimal bone development, and therefore may contribute to increased fracture risk later in life.”
Dr. Bachrach agreed that “peak bone strength is achieved by early adulthood, making childhood and adolescence important times to optimize bone health,” and that “peak bone strength is a predictor of lifetime risk of osteoporosis.”
“The diagnosis of pediatric osteoporosis is made when a child or teen sustains a vertebral fracture or femur fracture with minimal or no trauma,” she explained. “The diagnosis can also be made in a pediatric patient with low BMD [bone mineral density] for age in combination with a history of several long-bone fractures.”
Dr. Mitchell noted that type 1 diabetes is associated with a higher risk of fractures, which is sixfold in adults. In another study, she said, the group showed that in 10- to 16-year-old girls who’ve only had diabetes for a few years, “trabecular bone density is lower, they have lower estimated failure load, and longitudinally when we follow them, at least at the radius, we’re seeing bone loss at a relatively young age when we wouldn’t be expecting to see bone loss.”
80 girls enrolled, half had type 1 diabetes
Researchers enrolled 36 girls with type 1 diabetes and 44 girls without type 1 diabetes (controls) who were a mean age of 14.7 years and most (92%) were White. The girls with and without diabetes had similar rates of previous fractures (44% and 51%).
Those with diabetes had been diagnosed at a mean age of 9 years and had had diabetes for a mean of 4.6 years.
Researchers calculated participants’ total BPAQ scores based on type, duration, and frequency of bone-loading activities.
Participants had dual-energy X-ray absorptiometry scans to determine areal bone mineral density (BMD) at the total hip, femoral neck, lumbar spine, and whole body less head.
They also had high-resolution peripheral quantitative computed tomography at the distal tibia and radius to determine volumetric BMD, bone microarchitecture, and estimated bone strength (calculated using microfinite element analysis).
The two groups had similar total BPAQ scores (57.3 and 64.6), with a median score of 49.
BPAQ scores were positively associated with areal BMD at all sites (whole body, lumbar spine, total hip, femoral neck, and 1/3 radius) and with trabecular BMD and estimated failure load at the distal radius and tibia (P < .05 for all, adjusted for bone age).
Among participants with low physical activity (BPAQ below the median), compared with controls, those with type 1 diabetes had 6.6% lower aerial BMD at the lumbar spine (0.868 vs. 0.929 g/cm3; P = .04), 8% lower trabecular volumetric BMD at the distal radius (128.5 vs. 156.8 mg/cm3; P = .01), and 12% lower estimated failure load. Results at the distal tibia were similar.
Next steps
“More observational studies in males and females across a broader age spectrum would be helpful,” Dr. Bachrach noted. “The ‘gold standard’ model would be a long-term randomized controlled activity intervention study.”
“Further studies are underway [in girls and boys] using objective measures of activity including accelerometry and longitudinal observation to help confirm the findings from the current study,” Dr. Bouxsein said. “Ultimately, trials of activity interventions in children with [type 1 diabetes] will be the gold standard to determine to what extent physical activity can mitigate bone disease in [type 1 diabetes],” she agreed.
The study authors and Dr. Bachrach have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a small cross-sectional study of 10- to 16-year-old girls with and without type 1 diabetes, both groups were equally physically active, based on their replies to the bone-specific physical activity questionnaire (BPAQ).
However, among the more sedentary girls (with BPAQ scores below the median), those with type 1 diabetes had worse markers of bone health in imaging tests compared with the girls without diabetes.
the researchers summarize in a poster presented at the annual meeting of the American Society of Bone and Mineral Research.
However, this is early research and further study is needed, the group cautions.
“Ongoing studies with objective measures of physical activity as well as interventional studies will clarify whether increasing physical activity may improve bone health and reduce fracture risk in this vulnerable group,” they conclude.
“If you look at the sedentary kids, there’s a big discrepancy between the kids who have diabetes and the control kids, and that’s if we’re looking at radius or tibia or trabecular bone density or estimated failure load,” senior author Deborah M. Mitchell, MD, said in an interview at the poster session.
However, “when we look at the kids who are more physically active, we’re really not seeing as much difference [in bone health] between the kids with and without diabetes,” said Dr. Mitchell, a pediatric endocrinologist at Massachusetts General Hospital and assistant professor at Harvard Medical School, Boston.
But she also acknowledged, “There’s all sorts of caveats, including that this is retrospective questionnaire data.”
However, if further, rigorous studies confirm these findings, “physical activity is potentially a really effective means of improving bone quality in kids with type 1 diabetes.”
“This study suggests that bone-loading physical activity can substantially improve skeletal health in children with [type 1 diabetes] and should provide hope for patients and their families that they can take some action to prevent or mitigate the effects of diabetes on bone,” coauthor and incoming ASBMR President Mary L. Bouxsein, PhD, told this news organization in an email.
“We interpret these data as an important reason to advocate for increased time in moderate to vigorous bone-loading activity,” said Dr. Bouxsein, professor, department of orthopedic surgery, Harvard Medical School, Boston, “though the ‘dose’ in terms of hours per day or episodes per week to promote optimal bone health is still to be determined.”
“Ongoing debate,” “need stronger proof”
Asked for comment, Laura K. Bachrach, MD, who was not involved with the research, noted: “Activity benefits the development of bone strength through effects on bone geometry more than ‘density,’ and conversely, lack of physical activity can compromise gains in cortical bone diameter and thickness.”
However, “there is ongoing debate about the impact of type 1 diabetes on bone health and the factor(s) determining risk,” Dr. Bachrach, a pediatric endocrinologist at Stanford Children’s Health, Palo Alto, Calif., told this news organization in an email.
The current findings suggest “that physical activity in adolescent girls provided protection against potential adverse effects of type 1 diabetes,” said Dr. Bachrach, who spoke about bone fragility in childhood in a video commentary in 2021.
Study strengths, she noted, “include the rigor and expertise of the investigators, use of multiple surrogate measures that capture bone geometry/microarchitecture, as well as the inclusion of healthy local controls.”
“The study is limited by the cross-sectional design and subjects who opted, or not, to be active,” she added. “Stronger proof of the protective effects of activity on bone health in type 1 diabetes would require a randomized longitudinal intervention study, as alluded to by the authors of the study.”
Hypothesis: Those with type 1 diabetes acquire less bone mass in early 20s
The excess fracture risk in children with type 1 diabetes has been previously reported and is 14%-35% higher than the fracture risk in children without diabetes, Dr. Bouxsein explained. And “between 30% to 50% of kids [with type 1 diabetes] will have a fracture before the age of 18, so the excess fracture risk in diabetes is not clinically obvious,” she added.
However, “several lines of evidence strongly suggest that bone mass and microarchitecture at the time of peak bone mass (early 20s) is a major determinant of fracture risk throughout the lifespan,” she noted.
“Our hypothesis,” Dr. Bouxsein said, “is that the metabolic disruptions of diabetes, when they are present during the acquisition of peak bone mass, interfere with optimal bone development, and therefore may contribute to increased fracture risk later in life.”
Dr. Bachrach agreed that “peak bone strength is achieved by early adulthood, making childhood and adolescence important times to optimize bone health,” and that “peak bone strength is a predictor of lifetime risk of osteoporosis.”
“The diagnosis of pediatric osteoporosis is made when a child or teen sustains a vertebral fracture or femur fracture with minimal or no trauma,” she explained. “The diagnosis can also be made in a pediatric patient with low BMD [bone mineral density] for age in combination with a history of several long-bone fractures.”
Dr. Mitchell noted that type 1 diabetes is associated with a higher risk of fractures, which is sixfold in adults. In another study, she said, the group showed that in 10- to 16-year-old girls who’ve only had diabetes for a few years, “trabecular bone density is lower, they have lower estimated failure load, and longitudinally when we follow them, at least at the radius, we’re seeing bone loss at a relatively young age when we wouldn’t be expecting to see bone loss.”
80 girls enrolled, half had type 1 diabetes
Researchers enrolled 36 girls with type 1 diabetes and 44 girls without type 1 diabetes (controls) who were a mean age of 14.7 years and most (92%) were White. The girls with and without diabetes had similar rates of previous fractures (44% and 51%).
Those with diabetes had been diagnosed at a mean age of 9 years and had had diabetes for a mean of 4.6 years.
Researchers calculated participants’ total BPAQ scores based on type, duration, and frequency of bone-loading activities.
Participants had dual-energy X-ray absorptiometry scans to determine areal bone mineral density (BMD) at the total hip, femoral neck, lumbar spine, and whole body less head.
They also had high-resolution peripheral quantitative computed tomography at the distal tibia and radius to determine volumetric BMD, bone microarchitecture, and estimated bone strength (calculated using microfinite element analysis).
The two groups had similar total BPAQ scores (57.3 and 64.6), with a median score of 49.
BPAQ scores were positively associated with areal BMD at all sites (whole body, lumbar spine, total hip, femoral neck, and 1/3 radius) and with trabecular BMD and estimated failure load at the distal radius and tibia (P < .05 for all, adjusted for bone age).
Among participants with low physical activity (BPAQ below the median), compared with controls, those with type 1 diabetes had 6.6% lower aerial BMD at the lumbar spine (0.868 vs. 0.929 g/cm3; P = .04), 8% lower trabecular volumetric BMD at the distal radius (128.5 vs. 156.8 mg/cm3; P = .01), and 12% lower estimated failure load. Results at the distal tibia were similar.
Next steps
“More observational studies in males and females across a broader age spectrum would be helpful,” Dr. Bachrach noted. “The ‘gold standard’ model would be a long-term randomized controlled activity intervention study.”
“Further studies are underway [in girls and boys] using objective measures of activity including accelerometry and longitudinal observation to help confirm the findings from the current study,” Dr. Bouxsein said. “Ultimately, trials of activity interventions in children with [type 1 diabetes] will be the gold standard to determine to what extent physical activity can mitigate bone disease in [type 1 diabetes],” she agreed.
The study authors and Dr. Bachrach have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a small cross-sectional study of 10- to 16-year-old girls with and without type 1 diabetes, both groups were equally physically active, based on their replies to the bone-specific physical activity questionnaire (BPAQ).
However, among the more sedentary girls (with BPAQ scores below the median), those with type 1 diabetes had worse markers of bone health in imaging tests compared with the girls without diabetes.
the researchers summarize in a poster presented at the annual meeting of the American Society of Bone and Mineral Research.
However, this is early research and further study is needed, the group cautions.
“Ongoing studies with objective measures of physical activity as well as interventional studies will clarify whether increasing physical activity may improve bone health and reduce fracture risk in this vulnerable group,” they conclude.
“If you look at the sedentary kids, there’s a big discrepancy between the kids who have diabetes and the control kids, and that’s if we’re looking at radius or tibia or trabecular bone density or estimated failure load,” senior author Deborah M. Mitchell, MD, said in an interview at the poster session.
However, “when we look at the kids who are more physically active, we’re really not seeing as much difference [in bone health] between the kids with and without diabetes,” said Dr. Mitchell, a pediatric endocrinologist at Massachusetts General Hospital and assistant professor at Harvard Medical School, Boston.
But she also acknowledged, “There’s all sorts of caveats, including that this is retrospective questionnaire data.”
However, if further, rigorous studies confirm these findings, “physical activity is potentially a really effective means of improving bone quality in kids with type 1 diabetes.”
“This study suggests that bone-loading physical activity can substantially improve skeletal health in children with [type 1 diabetes] and should provide hope for patients and their families that they can take some action to prevent or mitigate the effects of diabetes on bone,” coauthor and incoming ASBMR President Mary L. Bouxsein, PhD, told this news organization in an email.
“We interpret these data as an important reason to advocate for increased time in moderate to vigorous bone-loading activity,” said Dr. Bouxsein, professor, department of orthopedic surgery, Harvard Medical School, Boston, “though the ‘dose’ in terms of hours per day or episodes per week to promote optimal bone health is still to be determined.”
“Ongoing debate,” “need stronger proof”
Asked for comment, Laura K. Bachrach, MD, who was not involved with the research, noted: “Activity benefits the development of bone strength through effects on bone geometry more than ‘density,’ and conversely, lack of physical activity can compromise gains in cortical bone diameter and thickness.”
However, “there is ongoing debate about the impact of type 1 diabetes on bone health and the factor(s) determining risk,” Dr. Bachrach, a pediatric endocrinologist at Stanford Children’s Health, Palo Alto, Calif., told this news organization in an email.
The current findings suggest “that physical activity in adolescent girls provided protection against potential adverse effects of type 1 diabetes,” said Dr. Bachrach, who spoke about bone fragility in childhood in a video commentary in 2021.
Study strengths, she noted, “include the rigor and expertise of the investigators, use of multiple surrogate measures that capture bone geometry/microarchitecture, as well as the inclusion of healthy local controls.”
“The study is limited by the cross-sectional design and subjects who opted, or not, to be active,” she added. “Stronger proof of the protective effects of activity on bone health in type 1 diabetes would require a randomized longitudinal intervention study, as alluded to by the authors of the study.”
Hypothesis: Those with type 1 diabetes acquire less bone mass in early 20s
The excess fracture risk in children with type 1 diabetes has been previously reported and is 14%-35% higher than the fracture risk in children without diabetes, Dr. Bouxsein explained. And “between 30% to 50% of kids [with type 1 diabetes] will have a fracture before the age of 18, so the excess fracture risk in diabetes is not clinically obvious,” she added.
However, “several lines of evidence strongly suggest that bone mass and microarchitecture at the time of peak bone mass (early 20s) is a major determinant of fracture risk throughout the lifespan,” she noted.
“Our hypothesis,” Dr. Bouxsein said, “is that the metabolic disruptions of diabetes, when they are present during the acquisition of peak bone mass, interfere with optimal bone development, and therefore may contribute to increased fracture risk later in life.”
Dr. Bachrach agreed that “peak bone strength is achieved by early adulthood, making childhood and adolescence important times to optimize bone health,” and that “peak bone strength is a predictor of lifetime risk of osteoporosis.”
“The diagnosis of pediatric osteoporosis is made when a child or teen sustains a vertebral fracture or femur fracture with minimal or no trauma,” she explained. “The diagnosis can also be made in a pediatric patient with low BMD [bone mineral density] for age in combination with a history of several long-bone fractures.”
Dr. Mitchell noted that type 1 diabetes is associated with a higher risk of fractures, which is sixfold in adults. In another study, she said, the group showed that in 10- to 16-year-old girls who’ve only had diabetes for a few years, “trabecular bone density is lower, they have lower estimated failure load, and longitudinally when we follow them, at least at the radius, we’re seeing bone loss at a relatively young age when we wouldn’t be expecting to see bone loss.”
80 girls enrolled, half had type 1 diabetes
Researchers enrolled 36 girls with type 1 diabetes and 44 girls without type 1 diabetes (controls) who were a mean age of 14.7 years and most (92%) were White. The girls with and without diabetes had similar rates of previous fractures (44% and 51%).
Those with diabetes had been diagnosed at a mean age of 9 years and had had diabetes for a mean of 4.6 years.
Researchers calculated participants’ total BPAQ scores based on type, duration, and frequency of bone-loading activities.
Participants had dual-energy X-ray absorptiometry scans to determine areal bone mineral density (BMD) at the total hip, femoral neck, lumbar spine, and whole body less head.
They also had high-resolution peripheral quantitative computed tomography at the distal tibia and radius to determine volumetric BMD, bone microarchitecture, and estimated bone strength (calculated using microfinite element analysis).
The two groups had similar total BPAQ scores (57.3 and 64.6), with a median score of 49.
BPAQ scores were positively associated with areal BMD at all sites (whole body, lumbar spine, total hip, femoral neck, and 1/3 radius) and with trabecular BMD and estimated failure load at the distal radius and tibia (P < .05 for all, adjusted for bone age).
Among participants with low physical activity (BPAQ below the median), compared with controls, those with type 1 diabetes had 6.6% lower aerial BMD at the lumbar spine (0.868 vs. 0.929 g/cm3; P = .04), 8% lower trabecular volumetric BMD at the distal radius (128.5 vs. 156.8 mg/cm3; P = .01), and 12% lower estimated failure load. Results at the distal tibia were similar.
Next steps
“More observational studies in males and females across a broader age spectrum would be helpful,” Dr. Bachrach noted. “The ‘gold standard’ model would be a long-term randomized controlled activity intervention study.”
“Further studies are underway [in girls and boys] using objective measures of activity including accelerometry and longitudinal observation to help confirm the findings from the current study,” Dr. Bouxsein said. “Ultimately, trials of activity interventions in children with [type 1 diabetes] will be the gold standard to determine to what extent physical activity can mitigate bone disease in [type 1 diabetes],” she agreed.
The study authors and Dr. Bachrach have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ASBMR 2022
Children born from frozen embryos may have increased cancer risk
, a large registry study suggests.
The results, however, “should be interpreted cautiously,” the authors noted, given the low number of cancer cases reported among children born using FET.
Still, the findings do “raise concerns considering the increasing use of FET, in particular freeze-all strategies without clear medical indications,” the authors concluded.
The study was published online in PLOS Medicine.
The number of children born after FET has increased globally and even exceeds the number of those born after fresh embryo transfer in many countries. In the United States, for instance, the FET rate has doubled since 2015; FETs constituted almost 80% of all embryo transfers using assisted reproductive technology (ART) without a donor in 2019.
Despite the benefits associated with FET, which include improved embryo survival and higher live birth rates, some previous research has hinted at a higher risk of childhood cancer in this population.
In the current study, researchers from the University of Gothenburg, Sweden, wanted to better understand the risk of childhood cancer following FET. The investigators analyzed data from 171,774 children born via ART, including 22,630 born after FET, as well as roughly 7.7 million children born after spontaneous conception in Denmark, Finland, Norway, and Sweden.
After a mean follow-up of about 10 years, the incidence rate of cancer diagnosed before age 18 years was 16.7 per 100,000 person-years for children born after spontaneous conception (16,184 cases) and 19.3 per 100,000 person-years for children born after ART (329 cases).
The researchers found no increased risk of cancer before age 18 years in the group of children conceived via ART compared with those conceived spontaneously.
However, children born after FET had a significantly higher risk of cancer compared with children born after fresh embryo transfer (adjusted hazard ratio [aHR], 1.59) and spontaneous conception (aHR, 1.65). Specifically with regard to ART, the incidence rate for those born after FET was 30.1 per 100,000 person-years – 48 total cases – compared with 18.8 per 1000,000 person-years after fresh embryo transfer.
Adjustment for macrosomia, birth weight, or major birth defects influenced the association only marginally.
For specific cancer types, children born after FET had more than a twofold higher risk for leukemia in comparison with those born after fresh embryo transfer (aHR, 2.25) and spontaneous conception (aHR, 2.22).
Still, the authors said these results should be interpreted “cautiously,” given the small number of children diagnosed with cancer after FET. The researchers also acknowledged that they do not know why children born after FET would face a higher risk of cancer.
These findings, however, do align with those from a 2019 Dutch population-based study. In the Dutch study, which included more than 24,000 ART-conceived children and more than 23,000 naturally conceived children, the risk of cancer after ART was not higher overall, but it was greater when only those conceived after FET were considered (aHR 1.80); this increased risk, however, was not statistically significant.
“Since the use of FET is substantially increasing, it is important to tease out whether the increased cancer risk is a true risk increase due to the ART procedures using FET, or due to chance or confounding by other factors,” authors of the 2019 Dutch study, Mandy Spaan, PhD, and Flora E. van Leeuwen, PhD, said in an interview.
“But, as childhood cancer is (fortunately) a rare disease, it is very difficult to study this research question among ART children due to limited numbers,” said Dr. Spaan and Dr. van Leeuwen, who are with the Netherlands Cancer Institute.
Given this, the two experts call for additional large population-based cohort studies to investigate the risk of cancer after ART, especially FET, and for a subsequent analysis that pools these data. They hope this strategy “will lead to reliable estimates” and provide information on the risks of FET in comparison with approaches that involve fresh embryos.
The current study had no commercial funding. The study authors as well as Dr. Spaan and Dr. van Leeuwen have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a large registry study suggests.
The results, however, “should be interpreted cautiously,” the authors noted, given the low number of cancer cases reported among children born using FET.
Still, the findings do “raise concerns considering the increasing use of FET, in particular freeze-all strategies without clear medical indications,” the authors concluded.
The study was published online in PLOS Medicine.
The number of children born after FET has increased globally and even exceeds the number of those born after fresh embryo transfer in many countries. In the United States, for instance, the FET rate has doubled since 2015; FETs constituted almost 80% of all embryo transfers using assisted reproductive technology (ART) without a donor in 2019.
Despite the benefits associated with FET, which include improved embryo survival and higher live birth rates, some previous research has hinted at a higher risk of childhood cancer in this population.
In the current study, researchers from the University of Gothenburg, Sweden, wanted to better understand the risk of childhood cancer following FET. The investigators analyzed data from 171,774 children born via ART, including 22,630 born after FET, as well as roughly 7.7 million children born after spontaneous conception in Denmark, Finland, Norway, and Sweden.
After a mean follow-up of about 10 years, the incidence rate of cancer diagnosed before age 18 years was 16.7 per 100,000 person-years for children born after spontaneous conception (16,184 cases) and 19.3 per 100,000 person-years for children born after ART (329 cases).
The researchers found no increased risk of cancer before age 18 years in the group of children conceived via ART compared with those conceived spontaneously.
However, children born after FET had a significantly higher risk of cancer compared with children born after fresh embryo transfer (adjusted hazard ratio [aHR], 1.59) and spontaneous conception (aHR, 1.65). Specifically with regard to ART, the incidence rate for those born after FET was 30.1 per 100,000 person-years – 48 total cases – compared with 18.8 per 1000,000 person-years after fresh embryo transfer.
Adjustment for macrosomia, birth weight, or major birth defects influenced the association only marginally.
For specific cancer types, children born after FET had more than a twofold higher risk for leukemia in comparison with those born after fresh embryo transfer (aHR, 2.25) and spontaneous conception (aHR, 2.22).
Still, the authors said these results should be interpreted “cautiously,” given the small number of children diagnosed with cancer after FET. The researchers also acknowledged that they do not know why children born after FET would face a higher risk of cancer.
These findings, however, do align with those from a 2019 Dutch population-based study. In the Dutch study, which included more than 24,000 ART-conceived children and more than 23,000 naturally conceived children, the risk of cancer after ART was not higher overall, but it was greater when only those conceived after FET were considered (aHR 1.80); this increased risk, however, was not statistically significant.
“Since the use of FET is substantially increasing, it is important to tease out whether the increased cancer risk is a true risk increase due to the ART procedures using FET, or due to chance or confounding by other factors,” authors of the 2019 Dutch study, Mandy Spaan, PhD, and Flora E. van Leeuwen, PhD, said in an interview.
“But, as childhood cancer is (fortunately) a rare disease, it is very difficult to study this research question among ART children due to limited numbers,” said Dr. Spaan and Dr. van Leeuwen, who are with the Netherlands Cancer Institute.
Given this, the two experts call for additional large population-based cohort studies to investigate the risk of cancer after ART, especially FET, and for a subsequent analysis that pools these data. They hope this strategy “will lead to reliable estimates” and provide information on the risks of FET in comparison with approaches that involve fresh embryos.
The current study had no commercial funding. The study authors as well as Dr. Spaan and Dr. van Leeuwen have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a large registry study suggests.
The results, however, “should be interpreted cautiously,” the authors noted, given the low number of cancer cases reported among children born using FET.
Still, the findings do “raise concerns considering the increasing use of FET, in particular freeze-all strategies without clear medical indications,” the authors concluded.
The study was published online in PLOS Medicine.
The number of children born after FET has increased globally and even exceeds the number of those born after fresh embryo transfer in many countries. In the United States, for instance, the FET rate has doubled since 2015; FETs constituted almost 80% of all embryo transfers using assisted reproductive technology (ART) without a donor in 2019.
Despite the benefits associated with FET, which include improved embryo survival and higher live birth rates, some previous research has hinted at a higher risk of childhood cancer in this population.
In the current study, researchers from the University of Gothenburg, Sweden, wanted to better understand the risk of childhood cancer following FET. The investigators analyzed data from 171,774 children born via ART, including 22,630 born after FET, as well as roughly 7.7 million children born after spontaneous conception in Denmark, Finland, Norway, and Sweden.
After a mean follow-up of about 10 years, the incidence rate of cancer diagnosed before age 18 years was 16.7 per 100,000 person-years for children born after spontaneous conception (16,184 cases) and 19.3 per 100,000 person-years for children born after ART (329 cases).
The researchers found no increased risk of cancer before age 18 years in the group of children conceived via ART compared with those conceived spontaneously.
However, children born after FET had a significantly higher risk of cancer compared with children born after fresh embryo transfer (adjusted hazard ratio [aHR], 1.59) and spontaneous conception (aHR, 1.65). Specifically with regard to ART, the incidence rate for those born after FET was 30.1 per 100,000 person-years – 48 total cases – compared with 18.8 per 1000,000 person-years after fresh embryo transfer.
Adjustment for macrosomia, birth weight, or major birth defects influenced the association only marginally.
For specific cancer types, children born after FET had more than a twofold higher risk for leukemia in comparison with those born after fresh embryo transfer (aHR, 2.25) and spontaneous conception (aHR, 2.22).
Still, the authors said these results should be interpreted “cautiously,” given the small number of children diagnosed with cancer after FET. The researchers also acknowledged that they do not know why children born after FET would face a higher risk of cancer.
These findings, however, do align with those from a 2019 Dutch population-based study. In the Dutch study, which included more than 24,000 ART-conceived children and more than 23,000 naturally conceived children, the risk of cancer after ART was not higher overall, but it was greater when only those conceived after FET were considered (aHR 1.80); this increased risk, however, was not statistically significant.
“Since the use of FET is substantially increasing, it is important to tease out whether the increased cancer risk is a true risk increase due to the ART procedures using FET, or due to chance or confounding by other factors,” authors of the 2019 Dutch study, Mandy Spaan, PhD, and Flora E. van Leeuwen, PhD, said in an interview.
“But, as childhood cancer is (fortunately) a rare disease, it is very difficult to study this research question among ART children due to limited numbers,” said Dr. Spaan and Dr. van Leeuwen, who are with the Netherlands Cancer Institute.
Given this, the two experts call for additional large population-based cohort studies to investigate the risk of cancer after ART, especially FET, and for a subsequent analysis that pools these data. They hope this strategy “will lead to reliable estimates” and provide information on the risks of FET in comparison with approaches that involve fresh embryos.
The current study had no commercial funding. The study authors as well as Dr. Spaan and Dr. van Leeuwen have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM PLOS MEDICINE
Children with sickle cell anemia not getting treatments, screening
Fewer than half of children aged 2-16 years with sickle cell anemia are receiving recommended annual screening for stroke, a common complication of the disease, according to a new Vital Signs report from the Centers for Disease Control and Prevention.
Many of these children also are not receiving the recommended medication, hydroxyurea, which can reduce pain and acute chest syndrome and improve anemia and quality of life, according to the report released Sept. 20.
Sickle cell anemia (SCA) is the most severe form of sickle cell disease (SCD), which is a red blood cell disorder that primarily affects Black and African American people in the United States. It is associated with severe complications such as stroke, vison damage, frequent infections, and delayed growth, and a reduction in lifespan of more than 20 years.
SCD affects approximately 100,000 Americans and SCA accounts for about 75% of those cases.
Physician remembers her patients’ pain
In a briefing to reporters in advance of the report’s release, Debra Houry, MD, MPH, the CDC’s acting principal deputy director, recalled “long, tough nights with these young sickle cell warriors” in her career as an emergency department physician.
“[S]eeing children and teens suffering from the severe pain that often accompanies sickle cell anemia was heartbreaking,” she said.
She asked health care providers to confront racism as they build better systems for ensuring optimal treatment for children and adolescents with SCA.
“Health care providers can educate themselves, their colleagues, and their institutions about the specialized needs of people with sickle cell anemia, including how racism inhibits optimal care,” Dr. Houry said.
She said people with SCA report difficulty accessing care and when they do, they often report feeling stigmatized.
Lead author of the report, Laura Schieve, PhD, an epidemiologist with CDC’s National Center on Birth Defects and Developmental Disabilities, and colleagues looked at data from more than 3,300 children with SCA who were continuously enrolled in Medicaid during 2019. The data came from the IBM MarketScan Multi-State Medicaid Database.
Key recommendations issued in 2014
In 2014, the National Heart, Lung, and Blood Institute (NHLBI) issued two key recommendations to prevent or reduce complications in children and adolescents with SCA.
One was annual screening of children and adolescents aged 2-16 years with transcranial Doppler (TCD) ultrasound to identify those at risk for stroke. The second was offering hydroxyurea therapy, which keeps red blood cells from sickling and blocking small blood vessels, to children and adolescents who were at least 9 months old to reduce pain and the risk for several life-threatening complications.
The researchers, however, found that in 2019, only 47% and 38% of children and adolescents aged 2-9 and 10-16 years, respectively, had TCD screening and 38% and 53% of children and adolescents aged 2-9 years and 10-16 years, respectively, used hydroxyurea.
“These complications are preventable – not inevitable. We must do more to help lessen the pain and complications associated with this disease by increasing the number of children who are screened for stroke and using the medication that can help reduce painful episodes,” said Karen Remley, MD, MPH, director of CDC’s National Center on Birth Defects and Developmental Disabilities, said in a press release.
Bridging the gap
Providers, parents, health systems, and governmental agencies all have roles in bringing evidence-based recommended care to young SCA patients, Dr. Houry noted.
Community organizations can also help connect families with resources and tools to increase understanding.
Dr. Schieve pointed to access barriers in that families may have trouble traveling to specialized centers where the TCD screening is given. In addition, appointments for the screening may be limited.
Children taking hydroxyurea must be monitored for the proper dosage of medication, she explained, and that can be logistically challenging as well.
Providers report they often don’t get timely information back from TCD screening programs to keep up with which children need their annual screening.
Overall, the nation lacks providers with expertise in SCD and that can lead to symptoms being dismissed, Dr. Schieve said.
Hematologists and others have a role in advocating for patients with governmental entities to raise awareness of this issue, she added.
It’s also important that electronic health records give prompts and provide information so that all providers who care for a child can track screening and medication for the condition, Dr. Schieve and Dr. Houry said.
New funding for sickle cell data collection
Recent funding to the CDC Sickle Cell Data Collection Program may help more people get appropriate care, Dr. Houry said.
The program is currently active in 11 states and collects data from people all over the United States with SCD to study trends and treatment access for those with the disease.
The data help drive decisions such as where new sickle cell clinics are needed.
“We will expand to more states serving more people affected by this disease,” Dr. Houry said.
The authors declared no relevant financial relationships.
Fewer than half of children aged 2-16 years with sickle cell anemia are receiving recommended annual screening for stroke, a common complication of the disease, according to a new Vital Signs report from the Centers for Disease Control and Prevention.
Many of these children also are not receiving the recommended medication, hydroxyurea, which can reduce pain and acute chest syndrome and improve anemia and quality of life, according to the report released Sept. 20.
Sickle cell anemia (SCA) is the most severe form of sickle cell disease (SCD), which is a red blood cell disorder that primarily affects Black and African American people in the United States. It is associated with severe complications such as stroke, vison damage, frequent infections, and delayed growth, and a reduction in lifespan of more than 20 years.
SCD affects approximately 100,000 Americans and SCA accounts for about 75% of those cases.
Physician remembers her patients’ pain
In a briefing to reporters in advance of the report’s release, Debra Houry, MD, MPH, the CDC’s acting principal deputy director, recalled “long, tough nights with these young sickle cell warriors” in her career as an emergency department physician.
“[S]eeing children and teens suffering from the severe pain that often accompanies sickle cell anemia was heartbreaking,” she said.
She asked health care providers to confront racism as they build better systems for ensuring optimal treatment for children and adolescents with SCA.
“Health care providers can educate themselves, their colleagues, and their institutions about the specialized needs of people with sickle cell anemia, including how racism inhibits optimal care,” Dr. Houry said.
She said people with SCA report difficulty accessing care and when they do, they often report feeling stigmatized.
Lead author of the report, Laura Schieve, PhD, an epidemiologist with CDC’s National Center on Birth Defects and Developmental Disabilities, and colleagues looked at data from more than 3,300 children with SCA who were continuously enrolled in Medicaid during 2019. The data came from the IBM MarketScan Multi-State Medicaid Database.
Key recommendations issued in 2014
In 2014, the National Heart, Lung, and Blood Institute (NHLBI) issued two key recommendations to prevent or reduce complications in children and adolescents with SCA.
One was annual screening of children and adolescents aged 2-16 years with transcranial Doppler (TCD) ultrasound to identify those at risk for stroke. The second was offering hydroxyurea therapy, which keeps red blood cells from sickling and blocking small blood vessels, to children and adolescents who were at least 9 months old to reduce pain and the risk for several life-threatening complications.
The researchers, however, found that in 2019, only 47% and 38% of children and adolescents aged 2-9 and 10-16 years, respectively, had TCD screening and 38% and 53% of children and adolescents aged 2-9 years and 10-16 years, respectively, used hydroxyurea.
“These complications are preventable – not inevitable. We must do more to help lessen the pain and complications associated with this disease by increasing the number of children who are screened for stroke and using the medication that can help reduce painful episodes,” said Karen Remley, MD, MPH, director of CDC’s National Center on Birth Defects and Developmental Disabilities, said in a press release.
Bridging the gap
Providers, parents, health systems, and governmental agencies all have roles in bringing evidence-based recommended care to young SCA patients, Dr. Houry noted.
Community organizations can also help connect families with resources and tools to increase understanding.
Dr. Schieve pointed to access barriers in that families may have trouble traveling to specialized centers where the TCD screening is given. In addition, appointments for the screening may be limited.
Children taking hydroxyurea must be monitored for the proper dosage of medication, she explained, and that can be logistically challenging as well.
Providers report they often don’t get timely information back from TCD screening programs to keep up with which children need their annual screening.
Overall, the nation lacks providers with expertise in SCD and that can lead to symptoms being dismissed, Dr. Schieve said.
Hematologists and others have a role in advocating for patients with governmental entities to raise awareness of this issue, she added.
It’s also important that electronic health records give prompts and provide information so that all providers who care for a child can track screening and medication for the condition, Dr. Schieve and Dr. Houry said.
New funding for sickle cell data collection
Recent funding to the CDC Sickle Cell Data Collection Program may help more people get appropriate care, Dr. Houry said.
The program is currently active in 11 states and collects data from people all over the United States with SCD to study trends and treatment access for those with the disease.
The data help drive decisions such as where new sickle cell clinics are needed.
“We will expand to more states serving more people affected by this disease,” Dr. Houry said.
The authors declared no relevant financial relationships.
Fewer than half of children aged 2-16 years with sickle cell anemia are receiving recommended annual screening for stroke, a common complication of the disease, according to a new Vital Signs report from the Centers for Disease Control and Prevention.
Many of these children also are not receiving the recommended medication, hydroxyurea, which can reduce pain and acute chest syndrome and improve anemia and quality of life, according to the report released Sept. 20.
Sickle cell anemia (SCA) is the most severe form of sickle cell disease (SCD), which is a red blood cell disorder that primarily affects Black and African American people in the United States. It is associated with severe complications such as stroke, vison damage, frequent infections, and delayed growth, and a reduction in lifespan of more than 20 years.
SCD affects approximately 100,000 Americans and SCA accounts for about 75% of those cases.
Physician remembers her patients’ pain
In a briefing to reporters in advance of the report’s release, Debra Houry, MD, MPH, the CDC’s acting principal deputy director, recalled “long, tough nights with these young sickle cell warriors” in her career as an emergency department physician.
“[S]eeing children and teens suffering from the severe pain that often accompanies sickle cell anemia was heartbreaking,” she said.
She asked health care providers to confront racism as they build better systems for ensuring optimal treatment for children and adolescents with SCA.
“Health care providers can educate themselves, their colleagues, and their institutions about the specialized needs of people with sickle cell anemia, including how racism inhibits optimal care,” Dr. Houry said.
She said people with SCA report difficulty accessing care and when they do, they often report feeling stigmatized.
Lead author of the report, Laura Schieve, PhD, an epidemiologist with CDC’s National Center on Birth Defects and Developmental Disabilities, and colleagues looked at data from more than 3,300 children with SCA who were continuously enrolled in Medicaid during 2019. The data came from the IBM MarketScan Multi-State Medicaid Database.
Key recommendations issued in 2014
In 2014, the National Heart, Lung, and Blood Institute (NHLBI) issued two key recommendations to prevent or reduce complications in children and adolescents with SCA.
One was annual screening of children and adolescents aged 2-16 years with transcranial Doppler (TCD) ultrasound to identify those at risk for stroke. The second was offering hydroxyurea therapy, which keeps red blood cells from sickling and blocking small blood vessels, to children and adolescents who were at least 9 months old to reduce pain and the risk for several life-threatening complications.
The researchers, however, found that in 2019, only 47% and 38% of children and adolescents aged 2-9 and 10-16 years, respectively, had TCD screening and 38% and 53% of children and adolescents aged 2-9 years and 10-16 years, respectively, used hydroxyurea.
“These complications are preventable – not inevitable. We must do more to help lessen the pain and complications associated with this disease by increasing the number of children who are screened for stroke and using the medication that can help reduce painful episodes,” said Karen Remley, MD, MPH, director of CDC’s National Center on Birth Defects and Developmental Disabilities, said in a press release.
Bridging the gap
Providers, parents, health systems, and governmental agencies all have roles in bringing evidence-based recommended care to young SCA patients, Dr. Houry noted.
Community organizations can also help connect families with resources and tools to increase understanding.
Dr. Schieve pointed to access barriers in that families may have trouble traveling to specialized centers where the TCD screening is given. In addition, appointments for the screening may be limited.
Children taking hydroxyurea must be monitored for the proper dosage of medication, she explained, and that can be logistically challenging as well.
Providers report they often don’t get timely information back from TCD screening programs to keep up with which children need their annual screening.
Overall, the nation lacks providers with expertise in SCD and that can lead to symptoms being dismissed, Dr. Schieve said.
Hematologists and others have a role in advocating for patients with governmental entities to raise awareness of this issue, she added.
It’s also important that electronic health records give prompts and provide information so that all providers who care for a child can track screening and medication for the condition, Dr. Schieve and Dr. Houry said.
New funding for sickle cell data collection
Recent funding to the CDC Sickle Cell Data Collection Program may help more people get appropriate care, Dr. Houry said.
The program is currently active in 11 states and collects data from people all over the United States with SCD to study trends and treatment access for those with the disease.
The data help drive decisions such as where new sickle cell clinics are needed.
“We will expand to more states serving more people affected by this disease,” Dr. Houry said.
The authors declared no relevant financial relationships.
FROM THE MMWR
Children and COVID: Weekly cases drop to lowest level since April
A hefty decline in new COVID-19 cases among children resulted in the lowest weekly total since late April, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
, making for 2 consecutive weeks of declines after almost 91,000 cases were recorded for the week ending Sept. 1, the AAP and CHA said in their latest COVID report of state-level data.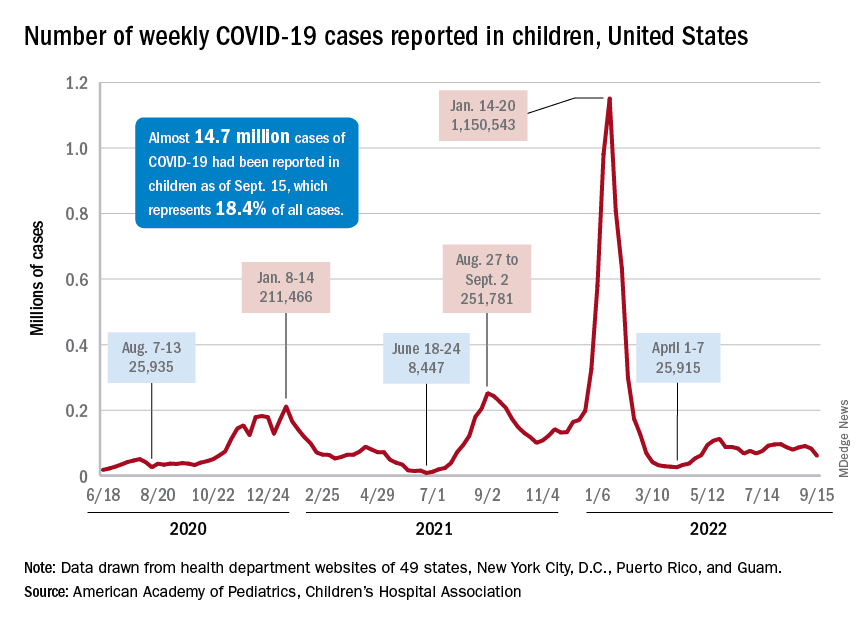
The last time the weekly count was under 60,000 came during the week of April 22-28, when 53,000 were reported by state and territorial health departments in the midst of a 7-week stretch of rising cases. Since that streak ended in mid-May, however, “reported weekly cases have plateaued, fluctuating between a low, now of 60,300 cases and a high of about 112,000,” the AAP noted.
Emergency department visits and hospital admissions, which showed less fluctuation over the summer and more steady rise and fall, have both dropped in recent weeks and are now approaching late May/early June rates, according to data from the Centers for Disease Control and Prevention.
On Sept. 15, for example, ED visits for children under 12 years with diagnosed COVID were just 2.2% of all visits, lower than at any time since May 19 and down from a summer high of 6.8% in late July. Hospital admissions for children aged 0-17 years also rose steadily through June and July, reaching 0.46 per 100,000 population on July 30, but have since slipped to 0.29 per 100,000 as of Sept. 17, the CDC said on its COVID Data Tracker.
Vaccination continues to be a tough sell
Vaccination activity among the most recently eligible age group, in the meantime, remains tepid. Just 6.0% of children under age 5 had received at least one dose of COVID-19 vaccine as of Sept. 13, about 3 months since its final approval in June, and 1.6% were fully vaccinated. For the two older groups of children with separate vaccine approvals, 31.5% of those aged 5-11 years and 43.3% of those aged 12-15 had received at least one dose 3 months after their vaccinations began, the CDC data show.
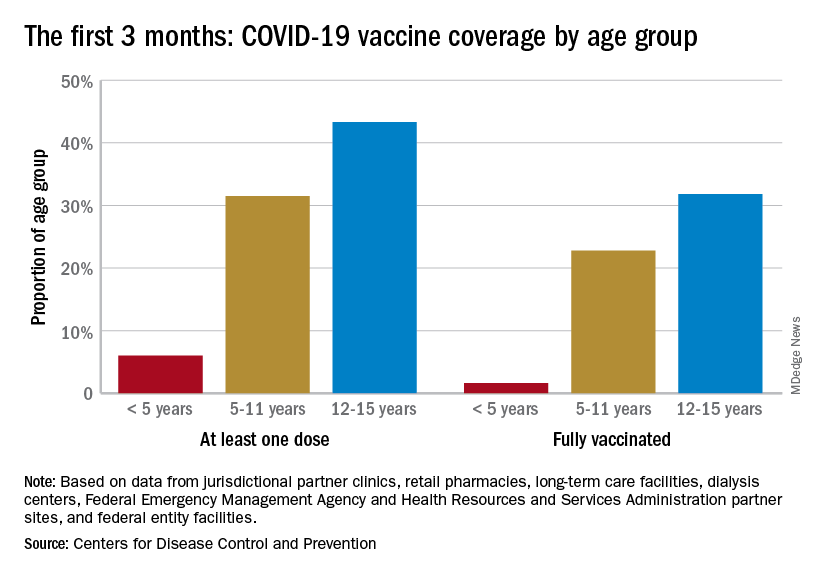
In the 2 weeks ending Sept. 14, almost 59,000 children under age 5 received their initial COVID-19 vaccine dose, as did 28,000 5- to 11-year-olds and 14,000 children aged 12-17. Children under age 5 years represented almost 20% of all Americans getting a first dose during Sept. 1-14, compared with 9.7% for those aged 5-11 and 4.8% for the 12- to 17-year-olds, the CDC said.
At the state level, children under age 5 years in the District of Columbia, where 28% have received at least one dose, and Vermont, at 24%, are the most likely to be vaccinated. The states with the lowest rates in this age group are Alabama, Louisiana, and Mississippi, all of which are at 2%. Vermont and D.C. have the highest rates for ages 5-11 at 70% each, and Alabama (17%) is the lowest, while D.C. (100%), Rhode Island (99%), and Massachusetts (99%) are highest for children aged 12-17 years and Wyoming (41%) is the lowest, the AAP said in a separate report.
A hefty decline in new COVID-19 cases among children resulted in the lowest weekly total since late April, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
, making for 2 consecutive weeks of declines after almost 91,000 cases were recorded for the week ending Sept. 1, the AAP and CHA said in their latest COVID report of state-level data.
The last time the weekly count was under 60,000 came during the week of April 22-28, when 53,000 were reported by state and territorial health departments in the midst of a 7-week stretch of rising cases. Since that streak ended in mid-May, however, “reported weekly cases have plateaued, fluctuating between a low, now of 60,300 cases and a high of about 112,000,” the AAP noted.
Emergency department visits and hospital admissions, which showed less fluctuation over the summer and more steady rise and fall, have both dropped in recent weeks and are now approaching late May/early June rates, according to data from the Centers for Disease Control and Prevention.
On Sept. 15, for example, ED visits for children under 12 years with diagnosed COVID were just 2.2% of all visits, lower than at any time since May 19 and down from a summer high of 6.8% in late July. Hospital admissions for children aged 0-17 years also rose steadily through June and July, reaching 0.46 per 100,000 population on July 30, but have since slipped to 0.29 per 100,000 as of Sept. 17, the CDC said on its COVID Data Tracker.
Vaccination continues to be a tough sell
Vaccination activity among the most recently eligible age group, in the meantime, remains tepid. Just 6.0% of children under age 5 had received at least one dose of COVID-19 vaccine as of Sept. 13, about 3 months since its final approval in June, and 1.6% were fully vaccinated. For the two older groups of children with separate vaccine approvals, 31.5% of those aged 5-11 years and 43.3% of those aged 12-15 had received at least one dose 3 months after their vaccinations began, the CDC data show.

In the 2 weeks ending Sept. 14, almost 59,000 children under age 5 received their initial COVID-19 vaccine dose, as did 28,000 5- to 11-year-olds and 14,000 children aged 12-17. Children under age 5 years represented almost 20% of all Americans getting a first dose during Sept. 1-14, compared with 9.7% for those aged 5-11 and 4.8% for the 12- to 17-year-olds, the CDC said.
At the state level, children under age 5 years in the District of Columbia, where 28% have received at least one dose, and Vermont, at 24%, are the most likely to be vaccinated. The states with the lowest rates in this age group are Alabama, Louisiana, and Mississippi, all of which are at 2%. Vermont and D.C. have the highest rates for ages 5-11 at 70% each, and Alabama (17%) is the lowest, while D.C. (100%), Rhode Island (99%), and Massachusetts (99%) are highest for children aged 12-17 years and Wyoming (41%) is the lowest, the AAP said in a separate report.
A hefty decline in new COVID-19 cases among children resulted in the lowest weekly total since late April, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
, making for 2 consecutive weeks of declines after almost 91,000 cases were recorded for the week ending Sept. 1, the AAP and CHA said in their latest COVID report of state-level data.
The last time the weekly count was under 60,000 came during the week of April 22-28, when 53,000 were reported by state and territorial health departments in the midst of a 7-week stretch of rising cases. Since that streak ended in mid-May, however, “reported weekly cases have plateaued, fluctuating between a low, now of 60,300 cases and a high of about 112,000,” the AAP noted.
Emergency department visits and hospital admissions, which showed less fluctuation over the summer and more steady rise and fall, have both dropped in recent weeks and are now approaching late May/early June rates, according to data from the Centers for Disease Control and Prevention.
On Sept. 15, for example, ED visits for children under 12 years with diagnosed COVID were just 2.2% of all visits, lower than at any time since May 19 and down from a summer high of 6.8% in late July. Hospital admissions for children aged 0-17 years also rose steadily through June and July, reaching 0.46 per 100,000 population on July 30, but have since slipped to 0.29 per 100,000 as of Sept. 17, the CDC said on its COVID Data Tracker.
Vaccination continues to be a tough sell
Vaccination activity among the most recently eligible age group, in the meantime, remains tepid. Just 6.0% of children under age 5 had received at least one dose of COVID-19 vaccine as of Sept. 13, about 3 months since its final approval in June, and 1.6% were fully vaccinated. For the two older groups of children with separate vaccine approvals, 31.5% of those aged 5-11 years and 43.3% of those aged 12-15 had received at least one dose 3 months after their vaccinations began, the CDC data show.

In the 2 weeks ending Sept. 14, almost 59,000 children under age 5 received their initial COVID-19 vaccine dose, as did 28,000 5- to 11-year-olds and 14,000 children aged 12-17. Children under age 5 years represented almost 20% of all Americans getting a first dose during Sept. 1-14, compared with 9.7% for those aged 5-11 and 4.8% for the 12- to 17-year-olds, the CDC said.
At the state level, children under age 5 years in the District of Columbia, where 28% have received at least one dose, and Vermont, at 24%, are the most likely to be vaccinated. The states with the lowest rates in this age group are Alabama, Louisiana, and Mississippi, all of which are at 2%. Vermont and D.C. have the highest rates for ages 5-11 at 70% each, and Alabama (17%) is the lowest, while D.C. (100%), Rhode Island (99%), and Massachusetts (99%) are highest for children aged 12-17 years and Wyoming (41%) is the lowest, the AAP said in a separate report.
Experts debate infant chiropractic care on TikTok
Several chiropractors in the United States are posting TikTok videos of themselves working with newborns, babies, and toddlers, often promoting treatments that aren’t backed by science, according to The Washington Post.
The videos include various devices and treatments, such as vibrating handheld massagers, spinal adjustments, and body movements, which are meant to address colic, constipation, reflux, musculoskeletal problems, and even trauma that babies experience during childbirth.
Chiropractors say the treatments are safe and gentle for babies and are unlike the more strenuous movements associated with adult chiropractic care. However, some doctors have said the videos are concerning because babies have softer bones and looser joints.
“Ultimately, there is no way you’re going to get an improvement in a newborn from a manipulation,” Sean Tabaie, MD, an orthopedic surgeon at Children’s National Hospital, Washington, told the newspaper.
Dr. Tabaie said his colleagues are shocked when he sends them Instagram or TikTok videos of chiropractic clinics treating infants.
“The only thing that you might possibly cause is harm,” he said.
Generally, chiropractors are licensed health professionals who use stretching, pressure, and joint manipulation on the spine to treat patients. Although chiropractic care is typically seen as an “alternative therapy,” some data in adults suggest that chiropractic treatments can help some conditions, such as low back pain.
“To my knowledge, there is little to no evidence that chiropractic care changes the natural history of any disease or condition,” Anthony Stans, MD, a pediatric orthopedic surgeon at Mayo Clinic Children’s Center, Rochester, Minn., told the newspaper. Stans said he would caution parents and recommend against chiropractic treatment for babies.
For some parents, the treatments and TikTok videos seem appealing because they promise relief for problems that traditional medicine can’t always address, especially colic, the newspaper reported. Colic, which features intense and prolonged crying in an otherwise healthy baby, tends to resolve over time without treatment.
Recent studies have attempted to study chiropractic care in infants. In a 2021 study, researchers in Denmark conducted a randomized controlled trial with 186 babies to test light pressure treatments. Although excessive crying was reduced by half an hour in the group that received treatment, the findings weren’t statistically significant in the end.
In a new study, researchers in Spain conducted a randomized trial with 58 babies to test “light touch manual therapy.” The babies who received treatment appeared to cry significantly less, but the parents weren’t “blinded” and were aware of the study’s treatment conditions, which can bias the results.
However, it can be challenging to “get that level of evidence” to support manual therapies such as chiropractic care, Joy Weydert, MD, director of pediatric integrative medicine at the University of Arizona, Tucson, told the newspaper. Certain treatments could help reduce the discomfort of colic or reflux, which can be difficult to measures in infants, she said.
The American Academy of Pediatrics told The Post that it doesn’t have an “official policy” on chiropractic care for infants or toddlers. At the same time, a 2017 report released by the organization concluded that “high-quality evidence” is lacking for spinal manipulation in children.
The American Chiropractic Association said chiropractic treatments are safe and effective for children, yet more research is needed to prove they work.
“We still haven’t been able to demonstrate in the research the effectiveness that we’ve seen clinically,” Jennifer Brocker, president of the group’s Council on Chiropractic Pediatrics, told the newspaper.
“We can’t really say for sure what’s happening,” she said. “It’s sort of like a black box. But what we do know is that, clinically, what we’re doing is effective because we see a change in the symptoms of the child.”
A version of this article first appeared on WebMD.com.
Several chiropractors in the United States are posting TikTok videos of themselves working with newborns, babies, and toddlers, often promoting treatments that aren’t backed by science, according to The Washington Post.
The videos include various devices and treatments, such as vibrating handheld massagers, spinal adjustments, and body movements, which are meant to address colic, constipation, reflux, musculoskeletal problems, and even trauma that babies experience during childbirth.
Chiropractors say the treatments are safe and gentle for babies and are unlike the more strenuous movements associated with adult chiropractic care. However, some doctors have said the videos are concerning because babies have softer bones and looser joints.
“Ultimately, there is no way you’re going to get an improvement in a newborn from a manipulation,” Sean Tabaie, MD, an orthopedic surgeon at Children’s National Hospital, Washington, told the newspaper.
Dr. Tabaie said his colleagues are shocked when he sends them Instagram or TikTok videos of chiropractic clinics treating infants.
“The only thing that you might possibly cause is harm,” he said.
Generally, chiropractors are licensed health professionals who use stretching, pressure, and joint manipulation on the spine to treat patients. Although chiropractic care is typically seen as an “alternative therapy,” some data in adults suggest that chiropractic treatments can help some conditions, such as low back pain.
“To my knowledge, there is little to no evidence that chiropractic care changes the natural history of any disease or condition,” Anthony Stans, MD, a pediatric orthopedic surgeon at Mayo Clinic Children’s Center, Rochester, Minn., told the newspaper. Stans said he would caution parents and recommend against chiropractic treatment for babies.
For some parents, the treatments and TikTok videos seem appealing because they promise relief for problems that traditional medicine can’t always address, especially colic, the newspaper reported. Colic, which features intense and prolonged crying in an otherwise healthy baby, tends to resolve over time without treatment.
Recent studies have attempted to study chiropractic care in infants. In a 2021 study, researchers in Denmark conducted a randomized controlled trial with 186 babies to test light pressure treatments. Although excessive crying was reduced by half an hour in the group that received treatment, the findings weren’t statistically significant in the end.
In a new study, researchers in Spain conducted a randomized trial with 58 babies to test “light touch manual therapy.” The babies who received treatment appeared to cry significantly less, but the parents weren’t “blinded” and were aware of the study’s treatment conditions, which can bias the results.
However, it can be challenging to “get that level of evidence” to support manual therapies such as chiropractic care, Joy Weydert, MD, director of pediatric integrative medicine at the University of Arizona, Tucson, told the newspaper. Certain treatments could help reduce the discomfort of colic or reflux, which can be difficult to measures in infants, she said.
The American Academy of Pediatrics told The Post that it doesn’t have an “official policy” on chiropractic care for infants or toddlers. At the same time, a 2017 report released by the organization concluded that “high-quality evidence” is lacking for spinal manipulation in children.
The American Chiropractic Association said chiropractic treatments are safe and effective for children, yet more research is needed to prove they work.
“We still haven’t been able to demonstrate in the research the effectiveness that we’ve seen clinically,” Jennifer Brocker, president of the group’s Council on Chiropractic Pediatrics, told the newspaper.
“We can’t really say for sure what’s happening,” she said. “It’s sort of like a black box. But what we do know is that, clinically, what we’re doing is effective because we see a change in the symptoms of the child.”
A version of this article first appeared on WebMD.com.
Several chiropractors in the United States are posting TikTok videos of themselves working with newborns, babies, and toddlers, often promoting treatments that aren’t backed by science, according to The Washington Post.
The videos include various devices and treatments, such as vibrating handheld massagers, spinal adjustments, and body movements, which are meant to address colic, constipation, reflux, musculoskeletal problems, and even trauma that babies experience during childbirth.
Chiropractors say the treatments are safe and gentle for babies and are unlike the more strenuous movements associated with adult chiropractic care. However, some doctors have said the videos are concerning because babies have softer bones and looser joints.
“Ultimately, there is no way you’re going to get an improvement in a newborn from a manipulation,” Sean Tabaie, MD, an orthopedic surgeon at Children’s National Hospital, Washington, told the newspaper.
Dr. Tabaie said his colleagues are shocked when he sends them Instagram or TikTok videos of chiropractic clinics treating infants.
“The only thing that you might possibly cause is harm,” he said.
Generally, chiropractors are licensed health professionals who use stretching, pressure, and joint manipulation on the spine to treat patients. Although chiropractic care is typically seen as an “alternative therapy,” some data in adults suggest that chiropractic treatments can help some conditions, such as low back pain.
“To my knowledge, there is little to no evidence that chiropractic care changes the natural history of any disease or condition,” Anthony Stans, MD, a pediatric orthopedic surgeon at Mayo Clinic Children’s Center, Rochester, Minn., told the newspaper. Stans said he would caution parents and recommend against chiropractic treatment for babies.
For some parents, the treatments and TikTok videos seem appealing because they promise relief for problems that traditional medicine can’t always address, especially colic, the newspaper reported. Colic, which features intense and prolonged crying in an otherwise healthy baby, tends to resolve over time without treatment.
Recent studies have attempted to study chiropractic care in infants. In a 2021 study, researchers in Denmark conducted a randomized controlled trial with 186 babies to test light pressure treatments. Although excessive crying was reduced by half an hour in the group that received treatment, the findings weren’t statistically significant in the end.
In a new study, researchers in Spain conducted a randomized trial with 58 babies to test “light touch manual therapy.” The babies who received treatment appeared to cry significantly less, but the parents weren’t “blinded” and were aware of the study’s treatment conditions, which can bias the results.
However, it can be challenging to “get that level of evidence” to support manual therapies such as chiropractic care, Joy Weydert, MD, director of pediatric integrative medicine at the University of Arizona, Tucson, told the newspaper. Certain treatments could help reduce the discomfort of colic or reflux, which can be difficult to measures in infants, she said.
The American Academy of Pediatrics told The Post that it doesn’t have an “official policy” on chiropractic care for infants or toddlers. At the same time, a 2017 report released by the organization concluded that “high-quality evidence” is lacking for spinal manipulation in children.
The American Chiropractic Association said chiropractic treatments are safe and effective for children, yet more research is needed to prove they work.
“We still haven’t been able to demonstrate in the research the effectiveness that we’ve seen clinically,” Jennifer Brocker, president of the group’s Council on Chiropractic Pediatrics, told the newspaper.
“We can’t really say for sure what’s happening,” she said. “It’s sort of like a black box. But what we do know is that, clinically, what we’re doing is effective because we see a change in the symptoms of the child.”
A version of this article first appeared on WebMD.com.
Apremilast alleviates severe psoriasis in some children, data show
not controlled by topical therapy, according to the results of a phase 3 trial.
“Unfortunately, there are limited treatment options for pediatric patients with moderate to severe plaque psoriasis” who do not respond to or cannot use topical therapy, said study investigator Anna Belloni Fortina, MD, speaking at the annual meeting of the European Academy of Dermatology and Venereology.
“In this randomized, placebo-controlled trial, oral apremilast demonstrated effectiveness and was well tolerated,” added Dr. Belloni Fortina, of Azienda Ospedale Università Padova (Italy). “I underline oral because for children, oral administration is better than the injection treatment.”
Key findings
Dubbed the SPROUT study, the trial set a primary endpoint of the percentage of children with a Physician’s Global Assessment (sPGA) response after 16 weeks of treatment or placebo. The sPGA is a 5-point scale ranging from 0 (clear) to 4 (severe). The study enrolled children with an sPGA greater than or equal to 3. Response was defined as a sPGA score of 0 or 1, indicating clear or almost clear skin, with at least a 2-point reduction from baseline values.
At week 16, the primary endpoint was met by 33% of 163 children treated with apremilast versus 11% of 82 children who had been given a placebo, a treatment difference of 21.7% (95% confidence interval, 11.2%-32.1%).
A greater proportion of children treated with apremilast also achieved a major secondary endpoint, a 75% or greater reduction in the Psoriasis Area and Severity Index (PASI-75) (45.4% vs. 16.1%), a treatment difference of 29.4% (95% CI, 17.8%-40.9%).
Results unaffected by weight and age
Regarding apremilast, “it’s important to underline that patients were dosed according to their weight,” Dr. Belloni Fortina said.
A dose of 20 mg twice daily was given to children who weighed between 20 kg and less than 50 kg, and a 30-mg twice-daily dose was given to those who weighed greater than or equal to 50 kg.
When the data were analyzed according to weight, proportionately more children on apremilast saw a sPGA response: 47.4% versus 21.8% in the lower weight and dose range and 19.2% versus 1.6% in the higher weight and dose range.
As for PASI-75, a greater proportion of children on apremilast also responded in both the lower and upper weight ranges, a respective 52.4% and 38.7% of patients, compared with 21.4% and 11% of those treated with placebo.
Data were also evaluated according to age, with a younger (aged 6-11 years) and older (age 12-17 years) group. The mean age of children was 12 years overall. Results showed a similar pattern for weight: The psoriasis of more children treated with apremilast was reduced by both measures, sPGA response, and PASI-75.
Safety of apremilast in children
“The overall safety profile during the placebo-controlled phase was comparable with the known safety profile of apremilast,” Dr. Belloni Fontina reported. “No new safety signals were identified.”
The rate of any adverse event was substantially higher in children given the active treatment, however, at 65% versus 41.3% for placebo.
Rates of severe and serious adverse events were low, at around 1.3%, and similar between the groups.
There was also a low rate of withdrawal because of side effects, although this was higher in the apremilast group (3.1% vs. 1.3%).
The primary reason for withdrawal of apremilast treatment were the most commonly reported adverse events: gastrointestinal disorders, including diarrhea, nausea, upper and lower abdominal pain, and vomiting. Headache, pyrexia, and nasopharyngitis were also reported.
Despite being common, most treatment-related adverse effects resolved within 3 days, Dr. Belloni Fontina said.
Expect further data
Further data from the trial are to be expected, because only the 16-week primary endpoint results have been released so far. The trial also included a 36-week extension phase, during which all children who had originally been randomly assigned to placebo were now eligible to be treated with apremilast, and all those who were originally given the active treatment were able to continue. This extension treatment period means that data will be available for a full year of treatment, and there will also be a further 2-week observational follow-up at the end of the trial.
The study was funded by Amgen. Dr. Belloni Fontina reported acting as an investigator and advisory board member for and receiving honoraria from Amgen, Galderma, Leo Pharma, and Pfizer. She also reported speaking on behalf of Pierre-Fabre and Galderma.
A version of this article first appeared on Medscape.com.
not controlled by topical therapy, according to the results of a phase 3 trial.
“Unfortunately, there are limited treatment options for pediatric patients with moderate to severe plaque psoriasis” who do not respond to or cannot use topical therapy, said study investigator Anna Belloni Fortina, MD, speaking at the annual meeting of the European Academy of Dermatology and Venereology.
“In this randomized, placebo-controlled trial, oral apremilast demonstrated effectiveness and was well tolerated,” added Dr. Belloni Fortina, of Azienda Ospedale Università Padova (Italy). “I underline oral because for children, oral administration is better than the injection treatment.”
Key findings
Dubbed the SPROUT study, the trial set a primary endpoint of the percentage of children with a Physician’s Global Assessment (sPGA) response after 16 weeks of treatment or placebo. The sPGA is a 5-point scale ranging from 0 (clear) to 4 (severe). The study enrolled children with an sPGA greater than or equal to 3. Response was defined as a sPGA score of 0 or 1, indicating clear or almost clear skin, with at least a 2-point reduction from baseline values.
At week 16, the primary endpoint was met by 33% of 163 children treated with apremilast versus 11% of 82 children who had been given a placebo, a treatment difference of 21.7% (95% confidence interval, 11.2%-32.1%).
A greater proportion of children treated with apremilast also achieved a major secondary endpoint, a 75% or greater reduction in the Psoriasis Area and Severity Index (PASI-75) (45.4% vs. 16.1%), a treatment difference of 29.4% (95% CI, 17.8%-40.9%).
Results unaffected by weight and age
Regarding apremilast, “it’s important to underline that patients were dosed according to their weight,” Dr. Belloni Fortina said.
A dose of 20 mg twice daily was given to children who weighed between 20 kg and less than 50 kg, and a 30-mg twice-daily dose was given to those who weighed greater than or equal to 50 kg.
When the data were analyzed according to weight, proportionately more children on apremilast saw a sPGA response: 47.4% versus 21.8% in the lower weight and dose range and 19.2% versus 1.6% in the higher weight and dose range.
As for PASI-75, a greater proportion of children on apremilast also responded in both the lower and upper weight ranges, a respective 52.4% and 38.7% of patients, compared with 21.4% and 11% of those treated with placebo.
Data were also evaluated according to age, with a younger (aged 6-11 years) and older (age 12-17 years) group. The mean age of children was 12 years overall. Results showed a similar pattern for weight: The psoriasis of more children treated with apremilast was reduced by both measures, sPGA response, and PASI-75.
Safety of apremilast in children
“The overall safety profile during the placebo-controlled phase was comparable with the known safety profile of apremilast,” Dr. Belloni Fontina reported. “No new safety signals were identified.”
The rate of any adverse event was substantially higher in children given the active treatment, however, at 65% versus 41.3% for placebo.
Rates of severe and serious adverse events were low, at around 1.3%, and similar between the groups.
There was also a low rate of withdrawal because of side effects, although this was higher in the apremilast group (3.1% vs. 1.3%).
The primary reason for withdrawal of apremilast treatment were the most commonly reported adverse events: gastrointestinal disorders, including diarrhea, nausea, upper and lower abdominal pain, and vomiting. Headache, pyrexia, and nasopharyngitis were also reported.
Despite being common, most treatment-related adverse effects resolved within 3 days, Dr. Belloni Fontina said.
Expect further data
Further data from the trial are to be expected, because only the 16-week primary endpoint results have been released so far. The trial also included a 36-week extension phase, during which all children who had originally been randomly assigned to placebo were now eligible to be treated with apremilast, and all those who were originally given the active treatment were able to continue. This extension treatment period means that data will be available for a full year of treatment, and there will also be a further 2-week observational follow-up at the end of the trial.
The study was funded by Amgen. Dr. Belloni Fontina reported acting as an investigator and advisory board member for and receiving honoraria from Amgen, Galderma, Leo Pharma, and Pfizer. She also reported speaking on behalf of Pierre-Fabre and Galderma.
A version of this article first appeared on Medscape.com.
not controlled by topical therapy, according to the results of a phase 3 trial.
“Unfortunately, there are limited treatment options for pediatric patients with moderate to severe plaque psoriasis” who do not respond to or cannot use topical therapy, said study investigator Anna Belloni Fortina, MD, speaking at the annual meeting of the European Academy of Dermatology and Venereology.
“In this randomized, placebo-controlled trial, oral apremilast demonstrated effectiveness and was well tolerated,” added Dr. Belloni Fortina, of Azienda Ospedale Università Padova (Italy). “I underline oral because for children, oral administration is better than the injection treatment.”
Key findings
Dubbed the SPROUT study, the trial set a primary endpoint of the percentage of children with a Physician’s Global Assessment (sPGA) response after 16 weeks of treatment or placebo. The sPGA is a 5-point scale ranging from 0 (clear) to 4 (severe). The study enrolled children with an sPGA greater than or equal to 3. Response was defined as a sPGA score of 0 or 1, indicating clear or almost clear skin, with at least a 2-point reduction from baseline values.
At week 16, the primary endpoint was met by 33% of 163 children treated with apremilast versus 11% of 82 children who had been given a placebo, a treatment difference of 21.7% (95% confidence interval, 11.2%-32.1%).
A greater proportion of children treated with apremilast also achieved a major secondary endpoint, a 75% or greater reduction in the Psoriasis Area and Severity Index (PASI-75) (45.4% vs. 16.1%), a treatment difference of 29.4% (95% CI, 17.8%-40.9%).
Results unaffected by weight and age
Regarding apremilast, “it’s important to underline that patients were dosed according to their weight,” Dr. Belloni Fortina said.
A dose of 20 mg twice daily was given to children who weighed between 20 kg and less than 50 kg, and a 30-mg twice-daily dose was given to those who weighed greater than or equal to 50 kg.
When the data were analyzed according to weight, proportionately more children on apremilast saw a sPGA response: 47.4% versus 21.8% in the lower weight and dose range and 19.2% versus 1.6% in the higher weight and dose range.
As for PASI-75, a greater proportion of children on apremilast also responded in both the lower and upper weight ranges, a respective 52.4% and 38.7% of patients, compared with 21.4% and 11% of those treated with placebo.
Data were also evaluated according to age, with a younger (aged 6-11 years) and older (age 12-17 years) group. The mean age of children was 12 years overall. Results showed a similar pattern for weight: The psoriasis of more children treated with apremilast was reduced by both measures, sPGA response, and PASI-75.
Safety of apremilast in children
“The overall safety profile during the placebo-controlled phase was comparable with the known safety profile of apremilast,” Dr. Belloni Fontina reported. “No new safety signals were identified.”
The rate of any adverse event was substantially higher in children given the active treatment, however, at 65% versus 41.3% for placebo.
Rates of severe and serious adverse events were low, at around 1.3%, and similar between the groups.
There was also a low rate of withdrawal because of side effects, although this was higher in the apremilast group (3.1% vs. 1.3%).
The primary reason for withdrawal of apremilast treatment were the most commonly reported adverse events: gastrointestinal disorders, including diarrhea, nausea, upper and lower abdominal pain, and vomiting. Headache, pyrexia, and nasopharyngitis were also reported.
Despite being common, most treatment-related adverse effects resolved within 3 days, Dr. Belloni Fontina said.
Expect further data
Further data from the trial are to be expected, because only the 16-week primary endpoint results have been released so far. The trial also included a 36-week extension phase, during which all children who had originally been randomly assigned to placebo were now eligible to be treated with apremilast, and all those who were originally given the active treatment were able to continue. This extension treatment period means that data will be available for a full year of treatment, and there will also be a further 2-week observational follow-up at the end of the trial.
The study was funded by Amgen. Dr. Belloni Fontina reported acting as an investigator and advisory board member for and receiving honoraria from Amgen, Galderma, Leo Pharma, and Pfizer. She also reported speaking on behalf of Pierre-Fabre and Galderma.
A version of this article first appeared on Medscape.com.
FROM EADV 2022
Uncombable hair syndrome: One gene, variants responsible for many cases
that manifests during infancy, investigators have reported.
The findings are from a cohort study published in JAMA Dermatology, which involved 107 unrelated children and adults suspected of having UHS, as well as family members, all of whom were recruited from January 2013 to December 2021. Genetic analyses were conducted in Germany from January 2014 to December 2021 with exome sequencing.
Study builds on prior research
Senior author Regina C. Betz, MD, professor of dermatogenetics at the Institute of Human Genetics, University Hospital Bonn, Germany, said that in 2016, she and her coinvestigators authored a study on the molecular genetics of UHS. That study, which involved 18 people with UHS, identified variants in three genes – PADI3, TCHH, and TGM3 – that encode proteins that play a role in the formation of the hair shaft. The investigators described how a deficiency in the shaping and mechanical strengthening of the hair shaft occurs in the UHS phenotype, which is characterized by dry, frizzy, and wiry hair that cannot be combed flat.
As a result of that previous work, “we base the assignment or confirmation of a clinical diagnosis of UHS on molecular genetic diagnostics,” the authors write in the new study, rather than on the clinical appearance of the hair and the physical examination of the patient, with confirmation on microscopical examination of the hair shaft.
Social media as instrument in finding study participants
Following the 2016 study, Dr. Betz and colleagues were contacted by many clinicians and by the public through Facebook and other social media platforms with details about possible cases of UHS, an autosomal recessive disorder. Through these contacts, blood samples, saliva, or DNA was sent to the investigators’ laboratory from 89 unrelated index patients (69 female patients, 20 male patients) suspected of having UHS. This resulted in the identification of pathogenic variants in 69 cases, the investigators write.
“In the first study, we had 18 patients, and then we tried to collect as many as possible” to determine the main mechanism behind UHS, Dr. Betz said. One question is whether there are additional genes responsible for UHS, she noted. “Even now, we are not sure, because in 25% [of cases in the new study], we didn’t find any mutation in the three known genes.”
The current study resulted in the discovery of eight novel pathogenic variants in PADI3, which are responsible for 71.0% (76) of the 107 cases. Of those, “6 were single observations and 2 were observed in 3 and 2 individuals, respectively,” the investigators write.
Children can grow out of this disorder, but it can also persist into adulthood, Dr. Betz noted. Communication that investigators had with parents of the children with UHS revealed that these children are often the targets of bullying by other children, she added.
She and her and colleagues will continue this research and are currently studying adults who have UHS.
Research leads to possible treatment pathways
Jeff Donovan, MD, FRCPC, FAAD, a dermatologist and medical director of the Donovan Hair Clinic in Whistler, British Columbia, described these findings as fundamental to understanding UHS and creating pathways to possible treatments.
The study “identifies more about the genetic basis of this challenging condition,” said Dr. Donovan, who is also clinical instructor in the department of dermatology at the University of British Columbia, Vancouver, and president of the Canadian Hair Loss Foundation. “We really need this type of information in order to have any sort of clue in terms of how to treat it,” he told this news organization.
“In the hair loss world, it’s pretty clear that if you can understand the genetic basis of things, or the basic science of a condition, whether it’s the basic genetics or the basic immunology, you give yourself the best chance to develop good treatments,” said Dr. Donovan.
The article provides advanced genetic information of the condition, such that geneticists can test for at least three markers if they are suspecting UHS, Dr. Donovan observed.
Condition can lead to bullying
Dr. Donovan also commented that UHS can have a detrimental impact on children with regard to socializing with their peers. “Having hair that sticks out and is very full like this is challenging because kids do get teased,” he said.
“It is often the parents who are the most affected” when a child aged 2-5 years has a hair condition such as UHS. But at age 5-9, “children are developing self-identity and an understanding of various aspects of self-esteem and what they look like and what others look like. And that’s where the teasing really starts. And that’s where it does become troublesome.”
Dr. Betz and Dr. Donovan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
that manifests during infancy, investigators have reported.
The findings are from a cohort study published in JAMA Dermatology, which involved 107 unrelated children and adults suspected of having UHS, as well as family members, all of whom were recruited from January 2013 to December 2021. Genetic analyses were conducted in Germany from January 2014 to December 2021 with exome sequencing.
Study builds on prior research
Senior author Regina C. Betz, MD, professor of dermatogenetics at the Institute of Human Genetics, University Hospital Bonn, Germany, said that in 2016, she and her coinvestigators authored a study on the molecular genetics of UHS. That study, which involved 18 people with UHS, identified variants in three genes – PADI3, TCHH, and TGM3 – that encode proteins that play a role in the formation of the hair shaft. The investigators described how a deficiency in the shaping and mechanical strengthening of the hair shaft occurs in the UHS phenotype, which is characterized by dry, frizzy, and wiry hair that cannot be combed flat.
As a result of that previous work, “we base the assignment or confirmation of a clinical diagnosis of UHS on molecular genetic diagnostics,” the authors write in the new study, rather than on the clinical appearance of the hair and the physical examination of the patient, with confirmation on microscopical examination of the hair shaft.
Social media as instrument in finding study participants
Following the 2016 study, Dr. Betz and colleagues were contacted by many clinicians and by the public through Facebook and other social media platforms with details about possible cases of UHS, an autosomal recessive disorder. Through these contacts, blood samples, saliva, or DNA was sent to the investigators’ laboratory from 89 unrelated index patients (69 female patients, 20 male patients) suspected of having UHS. This resulted in the identification of pathogenic variants in 69 cases, the investigators write.
“In the first study, we had 18 patients, and then we tried to collect as many as possible” to determine the main mechanism behind UHS, Dr. Betz said. One question is whether there are additional genes responsible for UHS, she noted. “Even now, we are not sure, because in 25% [of cases in the new study], we didn’t find any mutation in the three known genes.”
The current study resulted in the discovery of eight novel pathogenic variants in PADI3, which are responsible for 71.0% (76) of the 107 cases. Of those, “6 were single observations and 2 were observed in 3 and 2 individuals, respectively,” the investigators write.
Children can grow out of this disorder, but it can also persist into adulthood, Dr. Betz noted. Communication that investigators had with parents of the children with UHS revealed that these children are often the targets of bullying by other children, she added.
She and her and colleagues will continue this research and are currently studying adults who have UHS.
Research leads to possible treatment pathways
Jeff Donovan, MD, FRCPC, FAAD, a dermatologist and medical director of the Donovan Hair Clinic in Whistler, British Columbia, described these findings as fundamental to understanding UHS and creating pathways to possible treatments.
The study “identifies more about the genetic basis of this challenging condition,” said Dr. Donovan, who is also clinical instructor in the department of dermatology at the University of British Columbia, Vancouver, and president of the Canadian Hair Loss Foundation. “We really need this type of information in order to have any sort of clue in terms of how to treat it,” he told this news organization.
“In the hair loss world, it’s pretty clear that if you can understand the genetic basis of things, or the basic science of a condition, whether it’s the basic genetics or the basic immunology, you give yourself the best chance to develop good treatments,” said Dr. Donovan.
The article provides advanced genetic information of the condition, such that geneticists can test for at least three markers if they are suspecting UHS, Dr. Donovan observed.
Condition can lead to bullying
Dr. Donovan also commented that UHS can have a detrimental impact on children with regard to socializing with their peers. “Having hair that sticks out and is very full like this is challenging because kids do get teased,” he said.
“It is often the parents who are the most affected” when a child aged 2-5 years has a hair condition such as UHS. But at age 5-9, “children are developing self-identity and an understanding of various aspects of self-esteem and what they look like and what others look like. And that’s where the teasing really starts. And that’s where it does become troublesome.”
Dr. Betz and Dr. Donovan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
that manifests during infancy, investigators have reported.
The findings are from a cohort study published in JAMA Dermatology, which involved 107 unrelated children and adults suspected of having UHS, as well as family members, all of whom were recruited from January 2013 to December 2021. Genetic analyses were conducted in Germany from January 2014 to December 2021 with exome sequencing.
Study builds on prior research
Senior author Regina C. Betz, MD, professor of dermatogenetics at the Institute of Human Genetics, University Hospital Bonn, Germany, said that in 2016, she and her coinvestigators authored a study on the molecular genetics of UHS. That study, which involved 18 people with UHS, identified variants in three genes – PADI3, TCHH, and TGM3 – that encode proteins that play a role in the formation of the hair shaft. The investigators described how a deficiency in the shaping and mechanical strengthening of the hair shaft occurs in the UHS phenotype, which is characterized by dry, frizzy, and wiry hair that cannot be combed flat.
As a result of that previous work, “we base the assignment or confirmation of a clinical diagnosis of UHS on molecular genetic diagnostics,” the authors write in the new study, rather than on the clinical appearance of the hair and the physical examination of the patient, with confirmation on microscopical examination of the hair shaft.
Social media as instrument in finding study participants
Following the 2016 study, Dr. Betz and colleagues were contacted by many clinicians and by the public through Facebook and other social media platforms with details about possible cases of UHS, an autosomal recessive disorder. Through these contacts, blood samples, saliva, or DNA was sent to the investigators’ laboratory from 89 unrelated index patients (69 female patients, 20 male patients) suspected of having UHS. This resulted in the identification of pathogenic variants in 69 cases, the investigators write.
“In the first study, we had 18 patients, and then we tried to collect as many as possible” to determine the main mechanism behind UHS, Dr. Betz said. One question is whether there are additional genes responsible for UHS, she noted. “Even now, we are not sure, because in 25% [of cases in the new study], we didn’t find any mutation in the three known genes.”
The current study resulted in the discovery of eight novel pathogenic variants in PADI3, which are responsible for 71.0% (76) of the 107 cases. Of those, “6 were single observations and 2 were observed in 3 and 2 individuals, respectively,” the investigators write.
Children can grow out of this disorder, but it can also persist into adulthood, Dr. Betz noted. Communication that investigators had with parents of the children with UHS revealed that these children are often the targets of bullying by other children, she added.
She and her and colleagues will continue this research and are currently studying adults who have UHS.
Research leads to possible treatment pathways
Jeff Donovan, MD, FRCPC, FAAD, a dermatologist and medical director of the Donovan Hair Clinic in Whistler, British Columbia, described these findings as fundamental to understanding UHS and creating pathways to possible treatments.
The study “identifies more about the genetic basis of this challenging condition,” said Dr. Donovan, who is also clinical instructor in the department of dermatology at the University of British Columbia, Vancouver, and president of the Canadian Hair Loss Foundation. “We really need this type of information in order to have any sort of clue in terms of how to treat it,” he told this news organization.
“In the hair loss world, it’s pretty clear that if you can understand the genetic basis of things, or the basic science of a condition, whether it’s the basic genetics or the basic immunology, you give yourself the best chance to develop good treatments,” said Dr. Donovan.
The article provides advanced genetic information of the condition, such that geneticists can test for at least three markers if they are suspecting UHS, Dr. Donovan observed.
Condition can lead to bullying
Dr. Donovan also commented that UHS can have a detrimental impact on children with regard to socializing with their peers. “Having hair that sticks out and is very full like this is challenging because kids do get teased,” he said.
“It is often the parents who are the most affected” when a child aged 2-5 years has a hair condition such as UHS. But at age 5-9, “children are developing self-identity and an understanding of various aspects of self-esteem and what they look like and what others look like. And that’s where the teasing really starts. And that’s where it does become troublesome.”
Dr. Betz and Dr. Donovan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA DERMATOLOGY
Tralokinumab earns EU recommendation to expand age range for atopic dermatitis to include adolescents
Tralokinumab has received a positive opinion from the European Medicine Agency’s Committee for Medicinal Products for Human Use to extend use to adolescents aged 12 years and older with moderate-to-severe atopic dermatitis (AD) who are candidates for systemic therapy, according to a statement from the manufacturer.
The positive CHMP opinion, issued on Sept. 15, recommends extending the use of tralokinumab (Adtralza), an interleukin-13 antagonist, to adolescents aged 12-17 years in the EU. The positive opinion recommends an initial dose of 600 mg administered subcutaneously followed by 300 mg every other week, the dosing recommended for adults.
In December 2021, tralokinumab was approved for adults with moderate to severe AD in the United States, where it is marketed as Adbry. It is also approved for adults in the EU, Great Britain, Canada, the United Arab Emirates, and Switzerland. It is not currently approved for treatment of adolescents in any country, according to the LEO Pharma statement.
A regulatory filing with the U.S. Food and Drug Administration is in progress, the company said, and an additional study of tralokinumab for individuals aged 12 years and older is underway, according to the manufacturer.
The CHMP opinion was supported by data from a phase 3 study (ECZTRA 6) that assessed safety and efficacy of 150-mg or 300-mg doses of tralokinumab, compared with placebo in adolescents with moderate-to-severe AD, the company statement said. The primary outcomes were an Investigator Global Assessment score of clear or almost clear skin (IGA 0/1) and an improvement of at least a 75% on the Eczema Area and Severity Index score (EASI-75). In the study, presented as a poster at a meeting in October 2021, a total of 195 adolescents aged 12-17 with moderate to severe AD who were candidates for systemic therapy were randomly assigned to tralokinumab and 94 to placebo.
At 16 weeks, 21.4% and 17.5% of patients who received 150 mg and 300 mg, respectively, of tralokinumab had IGA scores of 0 or 1, compared with 4.3% of those on placebo (P < .001, P = .002, respectively vs. placebo). In addition, 28.6% and 27.8% of the 150-mg and 300-mg tralokinumab groups, respectively, achieved EASI-75, compared with 6.4% of placebo patients (P < .001, P = .001, respectively, compared with placebo).
Adverse events were similar between the groups, and most were mild or moderate; overall safety profiles were similar to those seen in adult patients.
The European Commission will review the positive opinion and make a final decision.
The research was supported by LEO Pharma.
A version of this article first appeared on Medscape.com.
Tralokinumab has received a positive opinion from the European Medicine Agency’s Committee for Medicinal Products for Human Use to extend use to adolescents aged 12 years and older with moderate-to-severe atopic dermatitis (AD) who are candidates for systemic therapy, according to a statement from the manufacturer.
The positive CHMP opinion, issued on Sept. 15, recommends extending the use of tralokinumab (Adtralza), an interleukin-13 antagonist, to adolescents aged 12-17 years in the EU. The positive opinion recommends an initial dose of 600 mg administered subcutaneously followed by 300 mg every other week, the dosing recommended for adults.
In December 2021, tralokinumab was approved for adults with moderate to severe AD in the United States, where it is marketed as Adbry. It is also approved for adults in the EU, Great Britain, Canada, the United Arab Emirates, and Switzerland. It is not currently approved for treatment of adolescents in any country, according to the LEO Pharma statement.
A regulatory filing with the U.S. Food and Drug Administration is in progress, the company said, and an additional study of tralokinumab for individuals aged 12 years and older is underway, according to the manufacturer.
The CHMP opinion was supported by data from a phase 3 study (ECZTRA 6) that assessed safety and efficacy of 150-mg or 300-mg doses of tralokinumab, compared with placebo in adolescents with moderate-to-severe AD, the company statement said. The primary outcomes were an Investigator Global Assessment score of clear or almost clear skin (IGA 0/1) and an improvement of at least a 75% on the Eczema Area and Severity Index score (EASI-75). In the study, presented as a poster at a meeting in October 2021, a total of 195 adolescents aged 12-17 with moderate to severe AD who were candidates for systemic therapy were randomly assigned to tralokinumab and 94 to placebo.
At 16 weeks, 21.4% and 17.5% of patients who received 150 mg and 300 mg, respectively, of tralokinumab had IGA scores of 0 or 1, compared with 4.3% of those on placebo (P < .001, P = .002, respectively vs. placebo). In addition, 28.6% and 27.8% of the 150-mg and 300-mg tralokinumab groups, respectively, achieved EASI-75, compared with 6.4% of placebo patients (P < .001, P = .001, respectively, compared with placebo).
Adverse events were similar between the groups, and most were mild or moderate; overall safety profiles were similar to those seen in adult patients.
The European Commission will review the positive opinion and make a final decision.
The research was supported by LEO Pharma.
A version of this article first appeared on Medscape.com.
Tralokinumab has received a positive opinion from the European Medicine Agency’s Committee for Medicinal Products for Human Use to extend use to adolescents aged 12 years and older with moderate-to-severe atopic dermatitis (AD) who are candidates for systemic therapy, according to a statement from the manufacturer.
The positive CHMP opinion, issued on Sept. 15, recommends extending the use of tralokinumab (Adtralza), an interleukin-13 antagonist, to adolescents aged 12-17 years in the EU. The positive opinion recommends an initial dose of 600 mg administered subcutaneously followed by 300 mg every other week, the dosing recommended for adults.
In December 2021, tralokinumab was approved for adults with moderate to severe AD in the United States, where it is marketed as Adbry. It is also approved for adults in the EU, Great Britain, Canada, the United Arab Emirates, and Switzerland. It is not currently approved for treatment of adolescents in any country, according to the LEO Pharma statement.
A regulatory filing with the U.S. Food and Drug Administration is in progress, the company said, and an additional study of tralokinumab for individuals aged 12 years and older is underway, according to the manufacturer.
The CHMP opinion was supported by data from a phase 3 study (ECZTRA 6) that assessed safety and efficacy of 150-mg or 300-mg doses of tralokinumab, compared with placebo in adolescents with moderate-to-severe AD, the company statement said. The primary outcomes were an Investigator Global Assessment score of clear or almost clear skin (IGA 0/1) and an improvement of at least a 75% on the Eczema Area and Severity Index score (EASI-75). In the study, presented as a poster at a meeting in October 2021, a total of 195 adolescents aged 12-17 with moderate to severe AD who were candidates for systemic therapy were randomly assigned to tralokinumab and 94 to placebo.
At 16 weeks, 21.4% and 17.5% of patients who received 150 mg and 300 mg, respectively, of tralokinumab had IGA scores of 0 or 1, compared with 4.3% of those on placebo (P < .001, P = .002, respectively vs. placebo). In addition, 28.6% and 27.8% of the 150-mg and 300-mg tralokinumab groups, respectively, achieved EASI-75, compared with 6.4% of placebo patients (P < .001, P = .001, respectively, compared with placebo).
Adverse events were similar between the groups, and most were mild or moderate; overall safety profiles were similar to those seen in adult patients.
The European Commission will review the positive opinion and make a final decision.
The research was supported by LEO Pharma.
A version of this article first appeared on Medscape.com.
AAP guidance helps distinguish bleeding disorders from abuse
In some cases, bruising or bleeding from bleeding disorders may look like signs of child abuse, but new guidance may help clinicians distinguish one from the other.
On Sept. 19 the American Academy of Pediatrics published two reports – a clinical report and a technical report – in the October 2022 issue of Pediatrics on evaluating for bleeding disorders when child abuse is suspected.
The reports were written by the AAP Section on Hematology/Oncology and the AAP Council on Child Abuse and Neglect.
One doesn’t rule out the other
The reports emphasize that laboratory testing of bleeding cannot always rule out abuse, just as a history of trauma (accidental or nonaccidental) may not rule out a bleeding disorder or other medical condition.
In the clinical report, led by James Anderst, MD, MSCI, with the division of child adversity and resilience, Children’s Mercy Hospital, University of Missouri–Kansas City, the researchers note that infants are at especially high risk of abusive bruising/bleeding, but bleeding disorders may also present in infancy.
The authors give an example of a situation when taking a thorough history won’t necessarily rule out a bleeding disorder: Male infants who have been circumcised with no significant bleeding issues may still have a bleeding disorder. Therefore, laboratory evaluations are often needed to detect disordered bleeding.
Children’s medications should be documented, the authors note, because certain drugs, such as nonsteroidal anti-inflammatory drugs, some antibiotics, antiepileptics, and herbal supplements, can affect tests that might be used to detect bleeding disorders.
Likewise, asking about restrictive or unusual diets or alternative therapies is important as some could increase the likelihood of bleeding/bruising.
Signs that bleeding disorder is not likely
The authors advise that, if a child has any of the following, an evaluation for a bleeding disorder is generally not needed:
- Caregivers’ description of trauma sufficiently explains the bruising.
- The child or an independent witness can provide a history of abuse or nonabusive trauma that explains the bruising.
- The outline of the bruising follows an object or hand pattern.
- The location of the bruising is on the ears, neck, or genitals.
“Bruising to the ears, neck, or genitals is rarely seen in either accidental injuries or in children with bleeding disorders,” the authors write.
Specification of which locations for injuries are more indicative of abuse in both mobile and immobile children was among the most important information from the paper, Seattle pediatrician Timothy Joos, MD, said in an interview.
Also very helpful, he said, was the listing of which tests should be done if bruising looks like potential abuse.
The authors write that if bruising is concerning for abuse that necessitates evaluation for bleeding disorders, the following tests should be done: PT (prothrombin time); aPTT (activated partial thromboplastin time); von Willebrand Factor (VWF) activity (Ristocetin cofactor); factor VIII activity level; factor IX activity level; and a complete blood count, including platelets.
“I think that’s what a lot of us suspected, but there’s not a lot of summary evidence regarding that until now,” Dr. Joos said.
Case-by-case decisions on when to test
The decision on whether to evaluate for a bleeding disorder may be made case by case.
If there is no obvious known trauma or intracranial hemorrhage (ICH), particularly subdural hematoma (SDH) in a nonmobile child, abuse should be suspected, the authors write.
They acknowledge that children can have ICH, such as a small SDH or an epidural hematoma, under the point of impact from a short fall.
“However,” the authors write, “short falls rarely result in significant brain injury.”
Conditions may affect screening tests
Screening tests for bleeding disorders can be falsely positive or falsely negative, the authors caution in the technical report, led by Shannon Carpenter, MD, MS, with the department of pediatrics, University of Missouri–Kansas City.
- If coagulation laboratory test specimens sit in a hot metal box all day, for instance, factor levels may be falsely low, the authors explain.
- Conversely, factors such as VWF and factor VIII are acute-phase reactants and factor levels will be deceptively high if blood specimens are taken in a stressful time.
- Patients who have a traumatic brain injury often show temporary coagulopathy that does not signal a congenital disorder.
Vitamin K deficiency
The technical report explains that if an infant, typically younger than 6 months, presents with bleeding/bruising that raises flags for abuse and has a long PT, clinicians should confirm vitamin K was provided at birth and/or testing for vitamin K deficiency should be performed.
Not all states require vitamin K to be administered at birth and some parents refuse it. Deficiency can lead to bleeding in the skin or from mucosal surfaces from circumcision, generalized ecchymoses, and large intramuscular hemorrhages or ICH.
When infants don’t get vitamin K at birth, vitamin K deficiency bleeding (VKDB) is seen most often in the first days of life, the technical report states. It can also occur 1-3 months after birth.
“Late VKDB occurs from the first month to 3 months after birth,” the authors write. “This deficiency is more prevalent in breast-fed babies, because human milk contains less vitamin K than does cow milk.”
Overall, the authors write, extensive lab tests are usually not necessary, given the rarity of most bleeding disorders and specific clinical factors that decrease the odds that a bleeding disorder caused the child’s findings.
Dr. Joos said the decisions described in this paper are the kind that can keep pediatricians up at night.
“Any kind of guidance is helpful in these difficult cases,” he said. “These are scenarios that can often happen in the middle of the night, and you’re often struggling with evidence or past experience that can help you make some of these decisions.”
Authors of the reports and Dr. Joos declared no relevant financial relationships.
In some cases, bruising or bleeding from bleeding disorders may look like signs of child abuse, but new guidance may help clinicians distinguish one from the other.
On Sept. 19 the American Academy of Pediatrics published two reports – a clinical report and a technical report – in the October 2022 issue of Pediatrics on evaluating for bleeding disorders when child abuse is suspected.
The reports were written by the AAP Section on Hematology/Oncology and the AAP Council on Child Abuse and Neglect.
One doesn’t rule out the other
The reports emphasize that laboratory testing of bleeding cannot always rule out abuse, just as a history of trauma (accidental or nonaccidental) may not rule out a bleeding disorder or other medical condition.
In the clinical report, led by James Anderst, MD, MSCI, with the division of child adversity and resilience, Children’s Mercy Hospital, University of Missouri–Kansas City, the researchers note that infants are at especially high risk of abusive bruising/bleeding, but bleeding disorders may also present in infancy.
The authors give an example of a situation when taking a thorough history won’t necessarily rule out a bleeding disorder: Male infants who have been circumcised with no significant bleeding issues may still have a bleeding disorder. Therefore, laboratory evaluations are often needed to detect disordered bleeding.
Children’s medications should be documented, the authors note, because certain drugs, such as nonsteroidal anti-inflammatory drugs, some antibiotics, antiepileptics, and herbal supplements, can affect tests that might be used to detect bleeding disorders.
Likewise, asking about restrictive or unusual diets or alternative therapies is important as some could increase the likelihood of bleeding/bruising.
Signs that bleeding disorder is not likely
The authors advise that, if a child has any of the following, an evaluation for a bleeding disorder is generally not needed:
- Caregivers’ description of trauma sufficiently explains the bruising.
- The child or an independent witness can provide a history of abuse or nonabusive trauma that explains the bruising.
- The outline of the bruising follows an object or hand pattern.
- The location of the bruising is on the ears, neck, or genitals.
“Bruising to the ears, neck, or genitals is rarely seen in either accidental injuries or in children with bleeding disorders,” the authors write.
Specification of which locations for injuries are more indicative of abuse in both mobile and immobile children was among the most important information from the paper, Seattle pediatrician Timothy Joos, MD, said in an interview.
Also very helpful, he said, was the listing of which tests should be done if bruising looks like potential abuse.
The authors write that if bruising is concerning for abuse that necessitates evaluation for bleeding disorders, the following tests should be done: PT (prothrombin time); aPTT (activated partial thromboplastin time); von Willebrand Factor (VWF) activity (Ristocetin cofactor); factor VIII activity level; factor IX activity level; and a complete blood count, including platelets.
“I think that’s what a lot of us suspected, but there’s not a lot of summary evidence regarding that until now,” Dr. Joos said.
Case-by-case decisions on when to test
The decision on whether to evaluate for a bleeding disorder may be made case by case.
If there is no obvious known trauma or intracranial hemorrhage (ICH), particularly subdural hematoma (SDH) in a nonmobile child, abuse should be suspected, the authors write.
They acknowledge that children can have ICH, such as a small SDH or an epidural hematoma, under the point of impact from a short fall.
“However,” the authors write, “short falls rarely result in significant brain injury.”
Conditions may affect screening tests
Screening tests for bleeding disorders can be falsely positive or falsely negative, the authors caution in the technical report, led by Shannon Carpenter, MD, MS, with the department of pediatrics, University of Missouri–Kansas City.
- If coagulation laboratory test specimens sit in a hot metal box all day, for instance, factor levels may be falsely low, the authors explain.
- Conversely, factors such as VWF and factor VIII are acute-phase reactants and factor levels will be deceptively high if blood specimens are taken in a stressful time.
- Patients who have a traumatic brain injury often show temporary coagulopathy that does not signal a congenital disorder.
Vitamin K deficiency
The technical report explains that if an infant, typically younger than 6 months, presents with bleeding/bruising that raises flags for abuse and has a long PT, clinicians should confirm vitamin K was provided at birth and/or testing for vitamin K deficiency should be performed.
Not all states require vitamin K to be administered at birth and some parents refuse it. Deficiency can lead to bleeding in the skin or from mucosal surfaces from circumcision, generalized ecchymoses, and large intramuscular hemorrhages or ICH.
When infants don’t get vitamin K at birth, vitamin K deficiency bleeding (VKDB) is seen most often in the first days of life, the technical report states. It can also occur 1-3 months after birth.
“Late VKDB occurs from the first month to 3 months after birth,” the authors write. “This deficiency is more prevalent in breast-fed babies, because human milk contains less vitamin K than does cow milk.”
Overall, the authors write, extensive lab tests are usually not necessary, given the rarity of most bleeding disorders and specific clinical factors that decrease the odds that a bleeding disorder caused the child’s findings.
Dr. Joos said the decisions described in this paper are the kind that can keep pediatricians up at night.
“Any kind of guidance is helpful in these difficult cases,” he said. “These are scenarios that can often happen in the middle of the night, and you’re often struggling with evidence or past experience that can help you make some of these decisions.”
Authors of the reports and Dr. Joos declared no relevant financial relationships.
In some cases, bruising or bleeding from bleeding disorders may look like signs of child abuse, but new guidance may help clinicians distinguish one from the other.
On Sept. 19 the American Academy of Pediatrics published two reports – a clinical report and a technical report – in the October 2022 issue of Pediatrics on evaluating for bleeding disorders when child abuse is suspected.
The reports were written by the AAP Section on Hematology/Oncology and the AAP Council on Child Abuse and Neglect.
One doesn’t rule out the other
The reports emphasize that laboratory testing of bleeding cannot always rule out abuse, just as a history of trauma (accidental or nonaccidental) may not rule out a bleeding disorder or other medical condition.
In the clinical report, led by James Anderst, MD, MSCI, with the division of child adversity and resilience, Children’s Mercy Hospital, University of Missouri–Kansas City, the researchers note that infants are at especially high risk of abusive bruising/bleeding, but bleeding disorders may also present in infancy.
The authors give an example of a situation when taking a thorough history won’t necessarily rule out a bleeding disorder: Male infants who have been circumcised with no significant bleeding issues may still have a bleeding disorder. Therefore, laboratory evaluations are often needed to detect disordered bleeding.
Children’s medications should be documented, the authors note, because certain drugs, such as nonsteroidal anti-inflammatory drugs, some antibiotics, antiepileptics, and herbal supplements, can affect tests that might be used to detect bleeding disorders.
Likewise, asking about restrictive or unusual diets or alternative therapies is important as some could increase the likelihood of bleeding/bruising.
Signs that bleeding disorder is not likely
The authors advise that, if a child has any of the following, an evaluation for a bleeding disorder is generally not needed:
- Caregivers’ description of trauma sufficiently explains the bruising.
- The child or an independent witness can provide a history of abuse or nonabusive trauma that explains the bruising.
- The outline of the bruising follows an object or hand pattern.
- The location of the bruising is on the ears, neck, or genitals.
“Bruising to the ears, neck, or genitals is rarely seen in either accidental injuries or in children with bleeding disorders,” the authors write.
Specification of which locations for injuries are more indicative of abuse in both mobile and immobile children was among the most important information from the paper, Seattle pediatrician Timothy Joos, MD, said in an interview.
Also very helpful, he said, was the listing of which tests should be done if bruising looks like potential abuse.
The authors write that if bruising is concerning for abuse that necessitates evaluation for bleeding disorders, the following tests should be done: PT (prothrombin time); aPTT (activated partial thromboplastin time); von Willebrand Factor (VWF) activity (Ristocetin cofactor); factor VIII activity level; factor IX activity level; and a complete blood count, including platelets.
“I think that’s what a lot of us suspected, but there’s not a lot of summary evidence regarding that until now,” Dr. Joos said.
Case-by-case decisions on when to test
The decision on whether to evaluate for a bleeding disorder may be made case by case.
If there is no obvious known trauma or intracranial hemorrhage (ICH), particularly subdural hematoma (SDH) in a nonmobile child, abuse should be suspected, the authors write.
They acknowledge that children can have ICH, such as a small SDH or an epidural hematoma, under the point of impact from a short fall.
“However,” the authors write, “short falls rarely result in significant brain injury.”
Conditions may affect screening tests
Screening tests for bleeding disorders can be falsely positive or falsely negative, the authors caution in the technical report, led by Shannon Carpenter, MD, MS, with the department of pediatrics, University of Missouri–Kansas City.
- If coagulation laboratory test specimens sit in a hot metal box all day, for instance, factor levels may be falsely low, the authors explain.
- Conversely, factors such as VWF and factor VIII are acute-phase reactants and factor levels will be deceptively high if blood specimens are taken in a stressful time.
- Patients who have a traumatic brain injury often show temporary coagulopathy that does not signal a congenital disorder.
Vitamin K deficiency
The technical report explains that if an infant, typically younger than 6 months, presents with bleeding/bruising that raises flags for abuse and has a long PT, clinicians should confirm vitamin K was provided at birth and/or testing for vitamin K deficiency should be performed.
Not all states require vitamin K to be administered at birth and some parents refuse it. Deficiency can lead to bleeding in the skin or from mucosal surfaces from circumcision, generalized ecchymoses, and large intramuscular hemorrhages or ICH.
When infants don’t get vitamin K at birth, vitamin K deficiency bleeding (VKDB) is seen most often in the first days of life, the technical report states. It can also occur 1-3 months after birth.
“Late VKDB occurs from the first month to 3 months after birth,” the authors write. “This deficiency is more prevalent in breast-fed babies, because human milk contains less vitamin K than does cow milk.”
Overall, the authors write, extensive lab tests are usually not necessary, given the rarity of most bleeding disorders and specific clinical factors that decrease the odds that a bleeding disorder caused the child’s findings.
Dr. Joos said the decisions described in this paper are the kind that can keep pediatricians up at night.
“Any kind of guidance is helpful in these difficult cases,” he said. “These are scenarios that can often happen in the middle of the night, and you’re often struggling with evidence or past experience that can help you make some of these decisions.”
Authors of the reports and Dr. Joos declared no relevant financial relationships.
FROM PEDIATRICS
WPATH removes age limits from transgender treatment guidelines
Long-awaited global transgender care guidelines have dropped, with no recommendations regarding age limits for treatment and surgery in teenagers but acknowledging the complexity of dealing with such adolescents amid lack of longitudinal research on the impact of transitioning gender.
The World Professional Association of Transgender Health published its latest standards of care (SOC8) as it opens its annual meeting on Sept. 16 in Montreal.
These are “the most comprehensive set of guidelines ever produced to assist health care professionals around the world in support of transgender and gender diverse adults, adolescents, and children who are taking steps to live their lives authentically,” wrote WPATH President Walter Bouman, MD, PhD, and WPATH President-Elect Marci Bowers, MD, in a news release.
The SOC8 is the first update to guidance on the treatment of transgender individuals in 10 years and appears online in the International Journal of Transgender Health.
For the first time, the association wrote a chapter dedicated to transgender and gender-diverse adolescents – distinct from the child chapter.
The complexity of treating adolescents
WPATH officials said that this was owed to exponential growth in adolescent referral rates, more research on adolescent gender diversity–related care, and the unique developmental and care issues of this age group.
Until recently, there was limited information regarding the prevalence of gender diversity among adolescents. Studies from high-school samples indicate much higher rates than was earlier thought, with reports of up to 1.2% of participants identifying as transgender and up to 2.7% or more (for example, 7%-9%) experiencing some level of self-reported gender diversity, WPATH said.
The new chapter “applies to adolescents from the start of puberty until the legal age of majority (in most cases 18 years),” it stated.
However, WPATH did not go as far as to recommend lowering the age at which youth can receive cross-sex hormone therapy or gender-affirming surgeries, as earlier decreed in a draft of the guidelines. That draft suggested that young people could receive hormone therapy at age 14 years and surgeries for double mastectomies at age 15 years and for genital reassignment at age 17 years.
The exception was phalloplasty – surgery to construct a penis in female-to-male individuals – which WPATH stressed should not be performed under the age of 18 years owing to its complexity.
Now, the final SOC8 emphasizes that each transgender adolescent is unique, and decisions must be made on an individual basis, with no recommendations on specific ages for any treatment. This could be interpreted in many ways.
The SOC8 also acknowledges the “very rare” regret of individuals who have transitioned to the opposite gender and then changed their minds.
“[Health care] providers may consider the possibility an adolescent may regret gender-affirming decisions made during adolescence, and a young person will want to stop treatment and return to living in the birth-assigned gender role in the future. Providers may discuss this topic in a collaborative and trusting manner with the adolescent and their parents/caregivers before gender-affirming medical treatments are started,” it states.
WPATH, in addition, stressed the importance of counseling and supporting regretting patients, many who “expressed difficulties finding help during their detransition process and reported their detransition was an isolating experience during which they did not receive either sufficient or appropriate support.”
Although it doesn’t put a firm figure on the rate of regret overall, in its chapter on surgery, WPATH estimates that 0.3%-3.8% of transgender individuals regret gender-affirming surgery.
SOC8 also acknowledges “A pattern of uneven ratios by assigned sex has been reported in gender clinics, with assigned female-at-birth patients initiating care 2.5-7.1 times more frequently” than patients who were assigned male at birth.
And WPATH states in SOC8 that another phenomenon is the growing number of adolescents seeking care who had not previously experienced or expressed gender diversity during their childhood years.
It goes on to cite the 2018 paper of Lisa Littman, MD, MPH, now president of the Institute for Comprehensive Gender Dysphoria Research. Dr. Littman coined the term, “rapid-onset gender dysphoria” to describe this phenomenon; SOC8 refrains from using this phrase, but does acknowledge: “For a select subgroup of young people, susceptibility to social influence impacting gender may be an important differential to consider.”
SOC8 recommends that before any medical or surgical treatment is considered, health care professionals “undertake a comprehensive biopsychosocial assessment of adolescents who present with gender identity-related concerns and seek medical/surgical transition-related care.”
And it specifically mentions that transgender adolescents “show high rates of autism spectrum disorder/characteristics,” and notes that “other neurodevelopmental presentations and/or mental health challenges may also be present, (e.g., ADHD, intellectual disability, and psychotic disorders).”
Who uses WPATH to guide care? This is ‘a big unknown’
WPATH is an umbrella organization with offshoots in most Western nations, such as USPATH in the United States, EPATH in Europe, and AUSPATH and NZPATH in Australia and New Zealand.
However, it is not the only organization to issue guidance on the care of transgender individuals; several specialties take care of this patient population, including, but not limited to: pediatricians, endocrinologists, psychiatrists, psychologists and plastic surgeons.
The extent to which any health care professional, or professional body, follows WPATH guidance is extremely varied.
“There is nothing binding clinicians to the SOC, and the SOC is so broad and vague that anyone can say they’re following it but according to their own biases and interpretation,” Aaron Kimberly, a trans man and mental health clinician from the Gender Dysphoria Alliance, said in an interview.
In North America, some clinics practice full “informed consent” with no assessment and prescriptions at the first visit, Mr. Kimberly said, whereas others do comprehensive assessments.
“I think SOC should be observed. It shouldn’t just be people going rogue,” Erica Anderson, a clinical psychologist in Berkeley, Calif., former president of USPATH, and former member of WPATH, who is herself transgender, said in an interview. “The reason there are standards of care is because hundreds of scientists have weighed in – is it perfect? No. We have a long way to go. But you can’t just ignore whatever it is that we know and let people make their own decisions.”
A version of this article first appeared on Medscape.com.
Long-awaited global transgender care guidelines have dropped, with no recommendations regarding age limits for treatment and surgery in teenagers but acknowledging the complexity of dealing with such adolescents amid lack of longitudinal research on the impact of transitioning gender.
The World Professional Association of Transgender Health published its latest standards of care (SOC8) as it opens its annual meeting on Sept. 16 in Montreal.
These are “the most comprehensive set of guidelines ever produced to assist health care professionals around the world in support of transgender and gender diverse adults, adolescents, and children who are taking steps to live their lives authentically,” wrote WPATH President Walter Bouman, MD, PhD, and WPATH President-Elect Marci Bowers, MD, in a news release.
The SOC8 is the first update to guidance on the treatment of transgender individuals in 10 years and appears online in the International Journal of Transgender Health.
For the first time, the association wrote a chapter dedicated to transgender and gender-diverse adolescents – distinct from the child chapter.
The complexity of treating adolescents
WPATH officials said that this was owed to exponential growth in adolescent referral rates, more research on adolescent gender diversity–related care, and the unique developmental and care issues of this age group.
Until recently, there was limited information regarding the prevalence of gender diversity among adolescents. Studies from high-school samples indicate much higher rates than was earlier thought, with reports of up to 1.2% of participants identifying as transgender and up to 2.7% or more (for example, 7%-9%) experiencing some level of self-reported gender diversity, WPATH said.
The new chapter “applies to adolescents from the start of puberty until the legal age of majority (in most cases 18 years),” it stated.
However, WPATH did not go as far as to recommend lowering the age at which youth can receive cross-sex hormone therapy or gender-affirming surgeries, as earlier decreed in a draft of the guidelines. That draft suggested that young people could receive hormone therapy at age 14 years and surgeries for double mastectomies at age 15 years and for genital reassignment at age 17 years.
The exception was phalloplasty – surgery to construct a penis in female-to-male individuals – which WPATH stressed should not be performed under the age of 18 years owing to its complexity.
Now, the final SOC8 emphasizes that each transgender adolescent is unique, and decisions must be made on an individual basis, with no recommendations on specific ages for any treatment. This could be interpreted in many ways.
The SOC8 also acknowledges the “very rare” regret of individuals who have transitioned to the opposite gender and then changed their minds.
“[Health care] providers may consider the possibility an adolescent may regret gender-affirming decisions made during adolescence, and a young person will want to stop treatment and return to living in the birth-assigned gender role in the future. Providers may discuss this topic in a collaborative and trusting manner with the adolescent and their parents/caregivers before gender-affirming medical treatments are started,” it states.
WPATH, in addition, stressed the importance of counseling and supporting regretting patients, many who “expressed difficulties finding help during their detransition process and reported their detransition was an isolating experience during which they did not receive either sufficient or appropriate support.”
Although it doesn’t put a firm figure on the rate of regret overall, in its chapter on surgery, WPATH estimates that 0.3%-3.8% of transgender individuals regret gender-affirming surgery.
SOC8 also acknowledges “A pattern of uneven ratios by assigned sex has been reported in gender clinics, with assigned female-at-birth patients initiating care 2.5-7.1 times more frequently” than patients who were assigned male at birth.
And WPATH states in SOC8 that another phenomenon is the growing number of adolescents seeking care who had not previously experienced or expressed gender diversity during their childhood years.
It goes on to cite the 2018 paper of Lisa Littman, MD, MPH, now president of the Institute for Comprehensive Gender Dysphoria Research. Dr. Littman coined the term, “rapid-onset gender dysphoria” to describe this phenomenon; SOC8 refrains from using this phrase, but does acknowledge: “For a select subgroup of young people, susceptibility to social influence impacting gender may be an important differential to consider.”
SOC8 recommends that before any medical or surgical treatment is considered, health care professionals “undertake a comprehensive biopsychosocial assessment of adolescents who present with gender identity-related concerns and seek medical/surgical transition-related care.”
And it specifically mentions that transgender adolescents “show high rates of autism spectrum disorder/characteristics,” and notes that “other neurodevelopmental presentations and/or mental health challenges may also be present, (e.g., ADHD, intellectual disability, and psychotic disorders).”
Who uses WPATH to guide care? This is ‘a big unknown’
WPATH is an umbrella organization with offshoots in most Western nations, such as USPATH in the United States, EPATH in Europe, and AUSPATH and NZPATH in Australia and New Zealand.
However, it is not the only organization to issue guidance on the care of transgender individuals; several specialties take care of this patient population, including, but not limited to: pediatricians, endocrinologists, psychiatrists, psychologists and plastic surgeons.
The extent to which any health care professional, or professional body, follows WPATH guidance is extremely varied.
“There is nothing binding clinicians to the SOC, and the SOC is so broad and vague that anyone can say they’re following it but according to their own biases and interpretation,” Aaron Kimberly, a trans man and mental health clinician from the Gender Dysphoria Alliance, said in an interview.
In North America, some clinics practice full “informed consent” with no assessment and prescriptions at the first visit, Mr. Kimberly said, whereas others do comprehensive assessments.
“I think SOC should be observed. It shouldn’t just be people going rogue,” Erica Anderson, a clinical psychologist in Berkeley, Calif., former president of USPATH, and former member of WPATH, who is herself transgender, said in an interview. “The reason there are standards of care is because hundreds of scientists have weighed in – is it perfect? No. We have a long way to go. But you can’t just ignore whatever it is that we know and let people make their own decisions.”
A version of this article first appeared on Medscape.com.
Long-awaited global transgender care guidelines have dropped, with no recommendations regarding age limits for treatment and surgery in teenagers but acknowledging the complexity of dealing with such adolescents amid lack of longitudinal research on the impact of transitioning gender.
The World Professional Association of Transgender Health published its latest standards of care (SOC8) as it opens its annual meeting on Sept. 16 in Montreal.
These are “the most comprehensive set of guidelines ever produced to assist health care professionals around the world in support of transgender and gender diverse adults, adolescents, and children who are taking steps to live their lives authentically,” wrote WPATH President Walter Bouman, MD, PhD, and WPATH President-Elect Marci Bowers, MD, in a news release.
The SOC8 is the first update to guidance on the treatment of transgender individuals in 10 years and appears online in the International Journal of Transgender Health.
For the first time, the association wrote a chapter dedicated to transgender and gender-diverse adolescents – distinct from the child chapter.
The complexity of treating adolescents
WPATH officials said that this was owed to exponential growth in adolescent referral rates, more research on adolescent gender diversity–related care, and the unique developmental and care issues of this age group.
Until recently, there was limited information regarding the prevalence of gender diversity among adolescents. Studies from high-school samples indicate much higher rates than was earlier thought, with reports of up to 1.2% of participants identifying as transgender and up to 2.7% or more (for example, 7%-9%) experiencing some level of self-reported gender diversity, WPATH said.
The new chapter “applies to adolescents from the start of puberty until the legal age of majority (in most cases 18 years),” it stated.
However, WPATH did not go as far as to recommend lowering the age at which youth can receive cross-sex hormone therapy or gender-affirming surgeries, as earlier decreed in a draft of the guidelines. That draft suggested that young people could receive hormone therapy at age 14 years and surgeries for double mastectomies at age 15 years and for genital reassignment at age 17 years.
The exception was phalloplasty – surgery to construct a penis in female-to-male individuals – which WPATH stressed should not be performed under the age of 18 years owing to its complexity.
Now, the final SOC8 emphasizes that each transgender adolescent is unique, and decisions must be made on an individual basis, with no recommendations on specific ages for any treatment. This could be interpreted in many ways.
The SOC8 also acknowledges the “very rare” regret of individuals who have transitioned to the opposite gender and then changed their minds.
“[Health care] providers may consider the possibility an adolescent may regret gender-affirming decisions made during adolescence, and a young person will want to stop treatment and return to living in the birth-assigned gender role in the future. Providers may discuss this topic in a collaborative and trusting manner with the adolescent and their parents/caregivers before gender-affirming medical treatments are started,” it states.
WPATH, in addition, stressed the importance of counseling and supporting regretting patients, many who “expressed difficulties finding help during their detransition process and reported their detransition was an isolating experience during which they did not receive either sufficient or appropriate support.”
Although it doesn’t put a firm figure on the rate of regret overall, in its chapter on surgery, WPATH estimates that 0.3%-3.8% of transgender individuals regret gender-affirming surgery.
SOC8 also acknowledges “A pattern of uneven ratios by assigned sex has been reported in gender clinics, with assigned female-at-birth patients initiating care 2.5-7.1 times more frequently” than patients who were assigned male at birth.
And WPATH states in SOC8 that another phenomenon is the growing number of adolescents seeking care who had not previously experienced or expressed gender diversity during their childhood years.
It goes on to cite the 2018 paper of Lisa Littman, MD, MPH, now president of the Institute for Comprehensive Gender Dysphoria Research. Dr. Littman coined the term, “rapid-onset gender dysphoria” to describe this phenomenon; SOC8 refrains from using this phrase, but does acknowledge: “For a select subgroup of young people, susceptibility to social influence impacting gender may be an important differential to consider.”
SOC8 recommends that before any medical or surgical treatment is considered, health care professionals “undertake a comprehensive biopsychosocial assessment of adolescents who present with gender identity-related concerns and seek medical/surgical transition-related care.”
And it specifically mentions that transgender adolescents “show high rates of autism spectrum disorder/characteristics,” and notes that “other neurodevelopmental presentations and/or mental health challenges may also be present, (e.g., ADHD, intellectual disability, and psychotic disorders).”
Who uses WPATH to guide care? This is ‘a big unknown’
WPATH is an umbrella organization with offshoots in most Western nations, such as USPATH in the United States, EPATH in Europe, and AUSPATH and NZPATH in Australia and New Zealand.
However, it is not the only organization to issue guidance on the care of transgender individuals; several specialties take care of this patient population, including, but not limited to: pediatricians, endocrinologists, psychiatrists, psychologists and plastic surgeons.
The extent to which any health care professional, or professional body, follows WPATH guidance is extremely varied.
“There is nothing binding clinicians to the SOC, and the SOC is so broad and vague that anyone can say they’re following it but according to their own biases and interpretation,” Aaron Kimberly, a trans man and mental health clinician from the Gender Dysphoria Alliance, said in an interview.
In North America, some clinics practice full “informed consent” with no assessment and prescriptions at the first visit, Mr. Kimberly said, whereas others do comprehensive assessments.
“I think SOC should be observed. It shouldn’t just be people going rogue,” Erica Anderson, a clinical psychologist in Berkeley, Calif., former president of USPATH, and former member of WPATH, who is herself transgender, said in an interview. “The reason there are standards of care is because hundreds of scientists have weighed in – is it perfect? No. We have a long way to go. But you can’t just ignore whatever it is that we know and let people make their own decisions.”
A version of this article first appeared on Medscape.com.
FROM THE INTERNATIONAL JOURNAL OF TRANSGENDER HEALTH