User login
Experts issue health warning about giving melatonin to children
The American Academy of Sleep Medicine has issued a health advisory encouraging parents to talk to a health care professional before giving melatonin or any supplement to children.
“While melatonin can be useful in treating certain sleep-wake disorders, like jet lag, there is much less evidence it can help healthy children or adults fall asleep faster,” Muhammad Adeel Rishi, MD, MBBS, vice chair of the AASM public safety committee, said in a news release.
Spike in poisoning calls
Research previously published in JAMA suggests that the use of melatonin has increased over the past 2 decades among people of all ages.
With this increased use has come a spike in reports of melatonin overdose, calls to poison control centers, and related emergency department visits for children.
Federal data show that the number of U.S. children who unintentionally ingested melatonin supplements jumped 530% from 2012 to 2021.
More than 4,000 of the reported ingestions led to a hospital stay, and 287 children required intensive care.
The AASM notes that next to multivitamins, melatonin is the second most popular “natural” product parents give to their children.
Melatonin is widely available over the counter. It’s marketed as a sleep aid, but there is little evidence that taking it as a supplement is effective in treating insomnia in healthy children, the AASM cautions.
Because it is regulated by the U.S. Food and Drug Administration as a dietary supplement, melatonin receives less oversight. Research shows that the melatonin content in supplements can vary widely, the AASM points out.
In one study, amounts of melatonin ranged from less than one-half to more than four times the amounts stated on the labels. The greatest variability in melatonin content was in chewable tablets, which are most likely to be used for children.
“The availability of melatonin as gummies or chewable tablets makes it more tempting to give to children and more likely for them to overdose,” said Dr. Rishi, a pulmonology, sleep medicine, and critical care specialist at Indiana University Health Physicians, Indianapolis.
“Parents should talk directly with their child’s health care professional before giving their children melatonin products,” he added.
Keep out of reach
The AASM advises that melatonin be managed as any other medication and that it be kept out of reach of children.
Before giving melatonin or any supplement to their children, parents should discuss this decision with a pediatric health care professional.
If use of melatonin is warranted, health care professionals can recommend the appropriate dose and timing in addressing the sleep problem, and they can ensure that the melatonin product that is being used has a USP verified mark.
“Instead of turning to melatonin, parents should encourage children to develop good sleep habits, like setting a regular bedtime and wake time, having a bedtime routine, and limiting screen time as bedtime approaches,” Dr. Rishi said.
A version of this article first appeared on Medscape.com.
The American Academy of Sleep Medicine has issued a health advisory encouraging parents to talk to a health care professional before giving melatonin or any supplement to children.
“While melatonin can be useful in treating certain sleep-wake disorders, like jet lag, there is much less evidence it can help healthy children or adults fall asleep faster,” Muhammad Adeel Rishi, MD, MBBS, vice chair of the AASM public safety committee, said in a news release.
Spike in poisoning calls
Research previously published in JAMA suggests that the use of melatonin has increased over the past 2 decades among people of all ages.
With this increased use has come a spike in reports of melatonin overdose, calls to poison control centers, and related emergency department visits for children.
Federal data show that the number of U.S. children who unintentionally ingested melatonin supplements jumped 530% from 2012 to 2021.
More than 4,000 of the reported ingestions led to a hospital stay, and 287 children required intensive care.
The AASM notes that next to multivitamins, melatonin is the second most popular “natural” product parents give to their children.
Melatonin is widely available over the counter. It’s marketed as a sleep aid, but there is little evidence that taking it as a supplement is effective in treating insomnia in healthy children, the AASM cautions.
Because it is regulated by the U.S. Food and Drug Administration as a dietary supplement, melatonin receives less oversight. Research shows that the melatonin content in supplements can vary widely, the AASM points out.
In one study, amounts of melatonin ranged from less than one-half to more than four times the amounts stated on the labels. The greatest variability in melatonin content was in chewable tablets, which are most likely to be used for children.
“The availability of melatonin as gummies or chewable tablets makes it more tempting to give to children and more likely for them to overdose,” said Dr. Rishi, a pulmonology, sleep medicine, and critical care specialist at Indiana University Health Physicians, Indianapolis.
“Parents should talk directly with their child’s health care professional before giving their children melatonin products,” he added.
Keep out of reach
The AASM advises that melatonin be managed as any other medication and that it be kept out of reach of children.
Before giving melatonin or any supplement to their children, parents should discuss this decision with a pediatric health care professional.
If use of melatonin is warranted, health care professionals can recommend the appropriate dose and timing in addressing the sleep problem, and they can ensure that the melatonin product that is being used has a USP verified mark.
“Instead of turning to melatonin, parents should encourage children to develop good sleep habits, like setting a regular bedtime and wake time, having a bedtime routine, and limiting screen time as bedtime approaches,” Dr. Rishi said.
A version of this article first appeared on Medscape.com.
The American Academy of Sleep Medicine has issued a health advisory encouraging parents to talk to a health care professional before giving melatonin or any supplement to children.
“While melatonin can be useful in treating certain sleep-wake disorders, like jet lag, there is much less evidence it can help healthy children or adults fall asleep faster,” Muhammad Adeel Rishi, MD, MBBS, vice chair of the AASM public safety committee, said in a news release.
Spike in poisoning calls
Research previously published in JAMA suggests that the use of melatonin has increased over the past 2 decades among people of all ages.
With this increased use has come a spike in reports of melatonin overdose, calls to poison control centers, and related emergency department visits for children.
Federal data show that the number of U.S. children who unintentionally ingested melatonin supplements jumped 530% from 2012 to 2021.
More than 4,000 of the reported ingestions led to a hospital stay, and 287 children required intensive care.
The AASM notes that next to multivitamins, melatonin is the second most popular “natural” product parents give to their children.
Melatonin is widely available over the counter. It’s marketed as a sleep aid, but there is little evidence that taking it as a supplement is effective in treating insomnia in healthy children, the AASM cautions.
Because it is regulated by the U.S. Food and Drug Administration as a dietary supplement, melatonin receives less oversight. Research shows that the melatonin content in supplements can vary widely, the AASM points out.
In one study, amounts of melatonin ranged from less than one-half to more than four times the amounts stated on the labels. The greatest variability in melatonin content was in chewable tablets, which are most likely to be used for children.
“The availability of melatonin as gummies or chewable tablets makes it more tempting to give to children and more likely for them to overdose,” said Dr. Rishi, a pulmonology, sleep medicine, and critical care specialist at Indiana University Health Physicians, Indianapolis.
“Parents should talk directly with their child’s health care professional before giving their children melatonin products,” he added.
Keep out of reach
The AASM advises that melatonin be managed as any other medication and that it be kept out of reach of children.
Before giving melatonin or any supplement to their children, parents should discuss this decision with a pediatric health care professional.
If use of melatonin is warranted, health care professionals can recommend the appropriate dose and timing in addressing the sleep problem, and they can ensure that the melatonin product that is being used has a USP verified mark.
“Instead of turning to melatonin, parents should encourage children to develop good sleep habits, like setting a regular bedtime and wake time, having a bedtime routine, and limiting screen time as bedtime approaches,” Dr. Rishi said.
A version of this article first appeared on Medscape.com.
Childhood cow’s milk allergy raises health care costs
Managing children’s cow’s milk allergy is costly to families and to health care systems, largely owing to costs of prescriptions, according to an industry-sponsored study based on data from the United Kingdom.
“This large cohort study provides novel evidence of a significant health economic burden of cow’s milk allergy in children,” Abbie L. Cawood, PhD, RNutr, MICR, head of scientific affairs at Nutricia Ltd in Trowbridge, England, and colleagues wrote in Clinical and Translational Allergy.
“Management of cow’s milk allergy necessitates the exclusion of cow’s milk protein from the diet. Whilst breastmilk remains the ideal nutrient source in infants with cow’s milk allergy, infants who are not exclusively breastfed require a hypoallergenic formula,” added Dr. Cawood, a visiting research fellow at University of Southampton, and her coauthors.
Cow’s milk allergy, an immune‐mediated response to one or more proteins in cow’s milk, is one of the most common childhood food allergies and affects 2%-5% of infants in Europe. Management involves avoiding cow’s milk protein and treating possible related gastrointestinal, skin, respiratory, and other allergic conditions, the authors explained.
In their retrospective matched cohort study, Dr. Cawood and colleagues turned to The Health Improvement Network (THIN), a Cegedim Rx proprietary database of 2.9 million anonymized active patient records. They extracted data from nearly 7,000 case records covering 5 years (2015-2020).
They examined medication prescriptions and health care professional contacts based on diagnosis read-codes and hypoallergenic formula prescriptions and compared health care costs for children with cow’s milk allergy with the costs for those without.
They matched 3,499 children aged 1 year or younger who had confirmed or suspected cow’s milk allergy with the same number of children without cow’s milk allergy. Around half of the participants were boys, and the mean observation period was 4.2 years.
Children with cow’s milk allergy need more, costly health care
The researchers found:
- Medications were prescribed to significantly more children with cow’s milk allergy (CMA), at a higher rate, than to those without CMA. In particular, prescriptions for antireflux medication increased by almost 500%.
- Children with CMA needed significantly more health care contacts and at a higher rate than those without CMA.
- CMA was linked with additional potential health care costs of £1381.53 per person per year. Assuming a 2.5% prevalence from the estimated 2%-5% CMA prevalence range and extrapolating to the UK infant population, CMA may have added more than £25.7 million in annual health care costs nationwide.
“Several conditions in infancy necessitate the elimination of cow milk–based formulas and require extensively hydrolyzed or amino acid formulas or, if preferred or able, exclusive breast milk,” Kara E. Coffey, MD, assistant professor of pediatrics at the University of Pittsburgh, said by email.
“This study shows that, regardless of the reason for cow milk–based avoidance, these infants require more healthcare service utilizations (clinic visits, nutritional assessments, prescriptions) than [do] their peers, which is certainly a commitment of a lot of time and money for their families to ensure their ability to grow and thrive,” added Dr. Coffey, who was not involved in the study.
Jodi A. Shroba, MSN, APRN, CPNP, the coordinator for the Food Allergy Program at Children’s Mercy Kansas City, Mo., did not find these numbers surprising.
“Children with food allergies typically have other atopic comorbidities that require more visits to primary care physicians and specialists and more prescriptions,” Ms. Shroba, who was not involved in the study, said by email.
“An intriguing statement is that the U.K. guidelines recommend the involvement of a dietitian for children with cow’s milk allergy,” she noted. “In the United States, having a dietitian involved would be a wonderful addition to care, as avoidance of cow’s milk can cause nutritional and growth deficiencies. But not all healthcare practices have those resources available.
“The higher rate of antibiotic use and the almost 500% increase of antireflux prescriptions by the children with cow’s milk allergy warrant additional research,” she added.
Nutricia Ltd. funded the study. Dr. Cawood and one coauthor are employed by Nutricia, and all other coauthors have been employees of or have other financial relationships with Nutricia. One coauthor is employed by Cegedim Rx, which was funded for this research by Nutricia. Ms. Shroba and Dr. Coffey report no conflicts of interest with the study.
A version of this article first appeared on Medscape.com.
Managing children’s cow’s milk allergy is costly to families and to health care systems, largely owing to costs of prescriptions, according to an industry-sponsored study based on data from the United Kingdom.
“This large cohort study provides novel evidence of a significant health economic burden of cow’s milk allergy in children,” Abbie L. Cawood, PhD, RNutr, MICR, head of scientific affairs at Nutricia Ltd in Trowbridge, England, and colleagues wrote in Clinical and Translational Allergy.
“Management of cow’s milk allergy necessitates the exclusion of cow’s milk protein from the diet. Whilst breastmilk remains the ideal nutrient source in infants with cow’s milk allergy, infants who are not exclusively breastfed require a hypoallergenic formula,” added Dr. Cawood, a visiting research fellow at University of Southampton, and her coauthors.
Cow’s milk allergy, an immune‐mediated response to one or more proteins in cow’s milk, is one of the most common childhood food allergies and affects 2%-5% of infants in Europe. Management involves avoiding cow’s milk protein and treating possible related gastrointestinal, skin, respiratory, and other allergic conditions, the authors explained.
In their retrospective matched cohort study, Dr. Cawood and colleagues turned to The Health Improvement Network (THIN), a Cegedim Rx proprietary database of 2.9 million anonymized active patient records. They extracted data from nearly 7,000 case records covering 5 years (2015-2020).
They examined medication prescriptions and health care professional contacts based on diagnosis read-codes and hypoallergenic formula prescriptions and compared health care costs for children with cow’s milk allergy with the costs for those without.
They matched 3,499 children aged 1 year or younger who had confirmed or suspected cow’s milk allergy with the same number of children without cow’s milk allergy. Around half of the participants were boys, and the mean observation period was 4.2 years.
Children with cow’s milk allergy need more, costly health care
The researchers found:
- Medications were prescribed to significantly more children with cow’s milk allergy (CMA), at a higher rate, than to those without CMA. In particular, prescriptions for antireflux medication increased by almost 500%.
- Children with CMA needed significantly more health care contacts and at a higher rate than those without CMA.
- CMA was linked with additional potential health care costs of £1381.53 per person per year. Assuming a 2.5% prevalence from the estimated 2%-5% CMA prevalence range and extrapolating to the UK infant population, CMA may have added more than £25.7 million in annual health care costs nationwide.
“Several conditions in infancy necessitate the elimination of cow milk–based formulas and require extensively hydrolyzed or amino acid formulas or, if preferred or able, exclusive breast milk,” Kara E. Coffey, MD, assistant professor of pediatrics at the University of Pittsburgh, said by email.
“This study shows that, regardless of the reason for cow milk–based avoidance, these infants require more healthcare service utilizations (clinic visits, nutritional assessments, prescriptions) than [do] their peers, which is certainly a commitment of a lot of time and money for their families to ensure their ability to grow and thrive,” added Dr. Coffey, who was not involved in the study.
Jodi A. Shroba, MSN, APRN, CPNP, the coordinator for the Food Allergy Program at Children’s Mercy Kansas City, Mo., did not find these numbers surprising.
“Children with food allergies typically have other atopic comorbidities that require more visits to primary care physicians and specialists and more prescriptions,” Ms. Shroba, who was not involved in the study, said by email.
“An intriguing statement is that the U.K. guidelines recommend the involvement of a dietitian for children with cow’s milk allergy,” she noted. “In the United States, having a dietitian involved would be a wonderful addition to care, as avoidance of cow’s milk can cause nutritional and growth deficiencies. But not all healthcare practices have those resources available.
“The higher rate of antibiotic use and the almost 500% increase of antireflux prescriptions by the children with cow’s milk allergy warrant additional research,” she added.
Nutricia Ltd. funded the study. Dr. Cawood and one coauthor are employed by Nutricia, and all other coauthors have been employees of or have other financial relationships with Nutricia. One coauthor is employed by Cegedim Rx, which was funded for this research by Nutricia. Ms. Shroba and Dr. Coffey report no conflicts of interest with the study.
A version of this article first appeared on Medscape.com.
Managing children’s cow’s milk allergy is costly to families and to health care systems, largely owing to costs of prescriptions, according to an industry-sponsored study based on data from the United Kingdom.
“This large cohort study provides novel evidence of a significant health economic burden of cow’s milk allergy in children,” Abbie L. Cawood, PhD, RNutr, MICR, head of scientific affairs at Nutricia Ltd in Trowbridge, England, and colleagues wrote in Clinical and Translational Allergy.
“Management of cow’s milk allergy necessitates the exclusion of cow’s milk protein from the diet. Whilst breastmilk remains the ideal nutrient source in infants with cow’s milk allergy, infants who are not exclusively breastfed require a hypoallergenic formula,” added Dr. Cawood, a visiting research fellow at University of Southampton, and her coauthors.
Cow’s milk allergy, an immune‐mediated response to one or more proteins in cow’s milk, is one of the most common childhood food allergies and affects 2%-5% of infants in Europe. Management involves avoiding cow’s milk protein and treating possible related gastrointestinal, skin, respiratory, and other allergic conditions, the authors explained.
In their retrospective matched cohort study, Dr. Cawood and colleagues turned to The Health Improvement Network (THIN), a Cegedim Rx proprietary database of 2.9 million anonymized active patient records. They extracted data from nearly 7,000 case records covering 5 years (2015-2020).
They examined medication prescriptions and health care professional contacts based on diagnosis read-codes and hypoallergenic formula prescriptions and compared health care costs for children with cow’s milk allergy with the costs for those without.
They matched 3,499 children aged 1 year or younger who had confirmed or suspected cow’s milk allergy with the same number of children without cow’s milk allergy. Around half of the participants were boys, and the mean observation period was 4.2 years.
Children with cow’s milk allergy need more, costly health care
The researchers found:
- Medications were prescribed to significantly more children with cow’s milk allergy (CMA), at a higher rate, than to those without CMA. In particular, prescriptions for antireflux medication increased by almost 500%.
- Children with CMA needed significantly more health care contacts and at a higher rate than those without CMA.
- CMA was linked with additional potential health care costs of £1381.53 per person per year. Assuming a 2.5% prevalence from the estimated 2%-5% CMA prevalence range and extrapolating to the UK infant population, CMA may have added more than £25.7 million in annual health care costs nationwide.
“Several conditions in infancy necessitate the elimination of cow milk–based formulas and require extensively hydrolyzed or amino acid formulas or, if preferred or able, exclusive breast milk,” Kara E. Coffey, MD, assistant professor of pediatrics at the University of Pittsburgh, said by email.
“This study shows that, regardless of the reason for cow milk–based avoidance, these infants require more healthcare service utilizations (clinic visits, nutritional assessments, prescriptions) than [do] their peers, which is certainly a commitment of a lot of time and money for their families to ensure their ability to grow and thrive,” added Dr. Coffey, who was not involved in the study.
Jodi A. Shroba, MSN, APRN, CPNP, the coordinator for the Food Allergy Program at Children’s Mercy Kansas City, Mo., did not find these numbers surprising.
“Children with food allergies typically have other atopic comorbidities that require more visits to primary care physicians and specialists and more prescriptions,” Ms. Shroba, who was not involved in the study, said by email.
“An intriguing statement is that the U.K. guidelines recommend the involvement of a dietitian for children with cow’s milk allergy,” she noted. “In the United States, having a dietitian involved would be a wonderful addition to care, as avoidance of cow’s milk can cause nutritional and growth deficiencies. But not all healthcare practices have those resources available.
“The higher rate of antibiotic use and the almost 500% increase of antireflux prescriptions by the children with cow’s milk allergy warrant additional research,” she added.
Nutricia Ltd. funded the study. Dr. Cawood and one coauthor are employed by Nutricia, and all other coauthors have been employees of or have other financial relationships with Nutricia. One coauthor is employed by Cegedim Rx, which was funded for this research by Nutricia. Ms. Shroba and Dr. Coffey report no conflicts of interest with the study.
A version of this article first appeared on Medscape.com.
FROM CLINICAL AND TRANSLATIONAL ALLERGY
Dr. Birds-n-Bees: How physicians are taking up the sex ed slack
An athletic coach stands in front of a packed gym full of high school students.
“Don’t have sex,” he instructs, “because you will get pregnant and die. Don’t have sex in the missionary position. Don’t have sex standing up. Just don’t do it, promise? Okay, everybody take some rubbers.”
Sad to say, this scene from the 2004 movie “Mean Girls” bears a striking resemblance to the actual sex education courses taught in schools across the United States today. In fact, things may have gotten measurably worse.
National data recently published by the Guttmacher Institute showed that adolescents were less likely to receive adequate sex education from 2015 to 2019 than they were in 1995. Only half of kids aged 15-19 received sex education that met minimum standards recommended by the Department of Health & Human Services, and fewer than half were given this information before having sex for the first time. With such a vast learning gap, it is no surprise that the United States has some of the highest rates of teenage pregnancy and sexually transmitted infections in the developed world.
Concerned and motivated by this need for sex education, physicians and other medical professionals are stepping in to fill the void, offering sexual health information through a range of methods to students of all ages (some a lot older than one may think). It is a calling that takes them outside their hospitals and exam rooms into workshops and through educational materials, video, and social media content created from scratch.
“The fact that we’re able to go in and provide factual, scientific, important information that can affect the trajectory of someone’s life is powerful,” said Julia Rossen, part of a contingent of med students at Brown University, Providence, R.I., who now teach sex ed as an elective.
Their goals are not just about protecting health. Many are also teaching about other topics commonly ignored in sex education classes, such as consent, pleasure, LGBTQ+ identities, and cultural competence. There is a mutually beneficial relationship, they say, between their sex education work and their medical practice.
Changing the status quo
A jumble of state laws govern how and when schools should offer sex education courses. Individual school districts often make the final decisions about their content, creating even more inconsistent standards. Only 29 states and the District of Columbia mandate sex education, and 13 of those do not require that it be medically accurate. Abstinence-only education, which has been shown to be ineffective, is exclusively taught in 16 states.
Without formal instruction, many young people must learn about sex from family members, who may be unwilling, or they may share knowledge between themselves, which is often incorrect, or navigate the limitless information and misinformation available on the internet.
The consequences of this were apparent to several medical students at Brown University in 2013. At the time, the rate of teenage pregnancy across Rhode Island was 1 in 100, but in the small city of Central Falls, it was 1 in 25. Aiming to improve this, the group created a comprehensive sex education program for a Central Falls middle school that was taught by medical student volunteers.
The Sex Ed by Brown Med program continues today. It consists of eight in-person sessions. Topics include anatomy, contraception, STIs, sexual decision-making, consent, sexual violence, and sexual and gender identity. Through this program, as well as other factors, the Central Falls teenage pregnancy rate declined to 1.6 in 100 from 2016 to 2020, according to the Rhode Island Department of Health.
“Historically, sexual education has been politicized,” said Ms. Rossen, one of the current program leaders. “It’s been at the discretion of a lot of different factors that aren’t under the control of the communities that are actually receiving the education.”
Among seventh graders, the teachers say they encounter different levels of maturity. But they feel that the kids are more receptive and open with younger adults who, like them, are still students. Some volunteers recall the flaws in their own sex education, particularly regarding topics such as consent and gender and sexual identity, and they believe middle school is the time to begin the sexual health conversation. “By the time you’re talking to college-age students, it’s pretty much too late,” said another group leader, Benjamin Stone.
Mr. Stone feels that practicing having these often-awkward discussions enhances their clinical skills as physicians. “Sex and sexual history are part of the comprehensive medical interview. People want to have these conversations, and they’re looking for someone to open the door. The kids are excited that we’re opening that door for them. And I think patients feel the same way.”
Conquering social media
Opening the door has been more like releasing a floodgate for Danielle Jones, MD, an ob.gyn. physician who is originally from Texas but who moved to New Zealand in 2021. Known on social media as “Mama Doctor Jones,” she has garnered more than 3 million followers across YouTube, TikTok, Twitter, Instagram, and Facebook. Dr. Jones produces short, friendly, entertaining videos on a range of reproductive health and sex education topics. They appeal to an adolescent audience hungry for a trustworthy voice on issues such as,: “5 ‘Strange’ Things Your Vagina Does That Are NORMAL” and “Condom Broke ... Now What?”
Dr. Jones uses her platform to debunk some of the misleading and inaccurate sexual health information being taught in classrooms, by other social media influencers, and that is found on the internet in general. Her no-nonsense-style videos call out such myths as being unable to pee with a tampon in, Plan B emergency contraception causing abortions, and COVID-19 vaccines damaging fertility.
“The way sex ed is done in the U.S. in most places is continuing the taboo by making it a one-time discussion or health class,” said Dr. Jones, “particularly if boys and girls are separated. That doesn’t further communication between people or foster an environment where it’s okay to discuss your body and puberty and changes in sexual health in general. And if you can’t talk about it in educational spaces, you’re certainly not going to be comfortable talking about that in a one-on-one situation with another 16-year-old.”
Taking on other taboos, Dr. Jones has been outspoken about abortion and the consequences of the recent Supreme Court decision, both as an ethical issue and a medical one that endangers lives. Raised in a deeply religious family, Dr. Jones said she was indoctrinated with antiabortion views, and it took time for her thinking to evolve “from a scientific and humanistic standpoint.” While working in a Texas private practice, Dr. Jones described being unable to mention abortion online because of fear of losing her patients and for her own safety.
Now free of those constraints, Dr. Jones feels that her videos can be important resources for teachers who may have little health training. And she is enthusiastic about the complementary relationship between her social media work and her clinical practice. “There are conversations I have all the time in the clinic where patients tell me: ‘Nobody’s ever really had this conversation in this way with me. Thank you for explaining that,’ ” said Dr. Jones. “And then I think: ‘Well, now I’ll have it with a hundred thousand other people too.’ ”
Promoting pleasure
While not an ob.gyn., discussing sexuality with patients has become a focus for Evelin Dacker, MD, a family physician in Salem, Ore. Dr. Dacker is certified in functional medicine, which takes a holistic and integrative approach. During her training she had a sudden realization: Sexuality had not been discussed at any point during her medical education.
“I recognized that this was a huge gap in how we deal with a person as a human,” Dr. Dacker explained. “Since sexuality plays a role in so many aspects of our humanness, not just having sex.”
Dr. Dacker believes in rethinking sexuality as a fundamental part of overall health, as vital as nutrition or blood pressure. Outside her medical practice, she teaches classes and workshops on sexual health and sex positivity for young adults and other physicians. She has also developed an educational framework for sexual health topics. Dr. Dacker said she frequently confronts the idea that sexuality is only about engaging with another person. She disagrees. Using food as a metaphor, she argues that just as the pleasure of eating something is purely for oneself, sexuality belongs to the individual.
Sexuality can also be a tool for pleasure, which Dr. Dacker believes plays an essential role in physical health. “Pleasure is a medicine,” Dr. Dacker said. “I actually prescribe self-pleasure practices to my patients, so they can start owning it within themselves. Make sure you get 7-8 hours of sleep, do some breathing exercises to help bring down your stress, and do self-pleasure so that you can integrate into your body better.”
She added that the impact of prioritizing one’s own desires, needs, and boundaries can transform how people view their sexuality. Her adult students frequently ask: “Why wasn’t I taught this as a teenager?”
Speaking of adult students – An older generation learns new tricks
While the teen cohort is usually the focus, the lack of sex education in previous decades – and the way sexual culture has evolved in that time – have an impact on older groups. Among U.S. adults aged 55 and older, the rate of STIs has more than doubled in the past 10 years, according to the Centers for Disease Control and Prevention. While the majority of STI cases still occur among teenagers and young adults, the consistent increase in STIs among older persons is cause for concern among physicians and researchers.
The issue worries Shannon Dowler, MD, a family physician in western North Carolina and chief medical officer for North Carolina Medicaid. Dr. Dowler, who has practiced in an STI clinic throughout her career, began seeing more and more older adults with chlamydia, herpes, and other STIs. Dowler cites several factors behind the rise, including the growing retirement community population, the availability of pharmaceuticals for sexual dysfunction, and the “hook-up culture” that is active on dating apps, which research shows are regularly used by more than a third of adults older than 55.
Dr. Dowler also sees a lack of communication about sexual health between physicians and their older patients. “Older adults are more likely to be in relationship with their physician outside the exam room, especially if they’re in a small community,” Dr. Dowler said. “Sometimes they aren’t as comfortable sharing what their risks are. But we are guilty in medicine all the time of not asking. We assume someone’s older so they’re not having sex anymore. But, in fact, they are, and we’re not taking the time to say: ‘Let’s talk about your sex life. Are you at risk for anything? Are you having any difficulties with sex?’ We tend to avoid it as a health care culture.”
In contrast, Dr. Dowler said she talks about sexual health with anyone who will listen. She teaches classes in private schools and universities and for church youth groups and other physicians. She often finds that public schools are not interested, which she attributes to fear of her discussing things “outside the rule book.”
Dr. Dowler takes creative approaches. In 2017, she released a hip-hop video, “STD’s Never Get Old,” in which she raps about safe sex for older adults. Her video went viral, was mentioned by several news outlets, and received over 50,000 views on YouTube. Dr. Dowler’s latest project is a book, “Never Too Late: Your Guide to Safer Sex after 60,” which is scheduled for publication on Valentine’s Day, 2023.
“It’s sex ed for seniors,” she explained. “It’s that gym class that some people got – I won’t say everyone got – in high school. This is the version for older adults who didn’t get that. There are new infections now that didn’t exist when they had sex education, if they had sex education.”
A big subject requires a big mission
For others in the sex education field, physicians are allies in their fight against agendas designed to obstruct or erode sex education. Alison Macklin, director of policy and advocacy at SIECUS: Sex Ed for Social Change, formerly the Sexuality Information and Education Council of the United States, sees this struggle playing out in school boards and state legislatures across the country. For every comprehensive sex education bill passed or school district victory, there is yet another blocked proposal or restrictive law somewhere else.
Ms. Macklin urged doctors to get more involved locally and to expand their knowledge of sexual health issues by reaching out to organizations such as Planned Parenthood and to be “hyper vigilant” in their own communities.
“Doctors are trusted. People really respect what they have to say,” Ms. Macklin said. “And this is an important time for them to speak up.”
A version of this article first appeared on Medscape.com.
An athletic coach stands in front of a packed gym full of high school students.
“Don’t have sex,” he instructs, “because you will get pregnant and die. Don’t have sex in the missionary position. Don’t have sex standing up. Just don’t do it, promise? Okay, everybody take some rubbers.”
Sad to say, this scene from the 2004 movie “Mean Girls” bears a striking resemblance to the actual sex education courses taught in schools across the United States today. In fact, things may have gotten measurably worse.
National data recently published by the Guttmacher Institute showed that adolescents were less likely to receive adequate sex education from 2015 to 2019 than they were in 1995. Only half of kids aged 15-19 received sex education that met minimum standards recommended by the Department of Health & Human Services, and fewer than half were given this information before having sex for the first time. With such a vast learning gap, it is no surprise that the United States has some of the highest rates of teenage pregnancy and sexually transmitted infections in the developed world.
Concerned and motivated by this need for sex education, physicians and other medical professionals are stepping in to fill the void, offering sexual health information through a range of methods to students of all ages (some a lot older than one may think). It is a calling that takes them outside their hospitals and exam rooms into workshops and through educational materials, video, and social media content created from scratch.
“The fact that we’re able to go in and provide factual, scientific, important information that can affect the trajectory of someone’s life is powerful,” said Julia Rossen, part of a contingent of med students at Brown University, Providence, R.I., who now teach sex ed as an elective.
Their goals are not just about protecting health. Many are also teaching about other topics commonly ignored in sex education classes, such as consent, pleasure, LGBTQ+ identities, and cultural competence. There is a mutually beneficial relationship, they say, between their sex education work and their medical practice.
Changing the status quo
A jumble of state laws govern how and when schools should offer sex education courses. Individual school districts often make the final decisions about their content, creating even more inconsistent standards. Only 29 states and the District of Columbia mandate sex education, and 13 of those do not require that it be medically accurate. Abstinence-only education, which has been shown to be ineffective, is exclusively taught in 16 states.
Without formal instruction, many young people must learn about sex from family members, who may be unwilling, or they may share knowledge between themselves, which is often incorrect, or navigate the limitless information and misinformation available on the internet.
The consequences of this were apparent to several medical students at Brown University in 2013. At the time, the rate of teenage pregnancy across Rhode Island was 1 in 100, but in the small city of Central Falls, it was 1 in 25. Aiming to improve this, the group created a comprehensive sex education program for a Central Falls middle school that was taught by medical student volunteers.
The Sex Ed by Brown Med program continues today. It consists of eight in-person sessions. Topics include anatomy, contraception, STIs, sexual decision-making, consent, sexual violence, and sexual and gender identity. Through this program, as well as other factors, the Central Falls teenage pregnancy rate declined to 1.6 in 100 from 2016 to 2020, according to the Rhode Island Department of Health.
“Historically, sexual education has been politicized,” said Ms. Rossen, one of the current program leaders. “It’s been at the discretion of a lot of different factors that aren’t under the control of the communities that are actually receiving the education.”
Among seventh graders, the teachers say they encounter different levels of maturity. But they feel that the kids are more receptive and open with younger adults who, like them, are still students. Some volunteers recall the flaws in their own sex education, particularly regarding topics such as consent and gender and sexual identity, and they believe middle school is the time to begin the sexual health conversation. “By the time you’re talking to college-age students, it’s pretty much too late,” said another group leader, Benjamin Stone.
Mr. Stone feels that practicing having these often-awkward discussions enhances their clinical skills as physicians. “Sex and sexual history are part of the comprehensive medical interview. People want to have these conversations, and they’re looking for someone to open the door. The kids are excited that we’re opening that door for them. And I think patients feel the same way.”
Conquering social media
Opening the door has been more like releasing a floodgate for Danielle Jones, MD, an ob.gyn. physician who is originally from Texas but who moved to New Zealand in 2021. Known on social media as “Mama Doctor Jones,” she has garnered more than 3 million followers across YouTube, TikTok, Twitter, Instagram, and Facebook. Dr. Jones produces short, friendly, entertaining videos on a range of reproductive health and sex education topics. They appeal to an adolescent audience hungry for a trustworthy voice on issues such as,: “5 ‘Strange’ Things Your Vagina Does That Are NORMAL” and “Condom Broke ... Now What?”
Dr. Jones uses her platform to debunk some of the misleading and inaccurate sexual health information being taught in classrooms, by other social media influencers, and that is found on the internet in general. Her no-nonsense-style videos call out such myths as being unable to pee with a tampon in, Plan B emergency contraception causing abortions, and COVID-19 vaccines damaging fertility.
“The way sex ed is done in the U.S. in most places is continuing the taboo by making it a one-time discussion or health class,” said Dr. Jones, “particularly if boys and girls are separated. That doesn’t further communication between people or foster an environment where it’s okay to discuss your body and puberty and changes in sexual health in general. And if you can’t talk about it in educational spaces, you’re certainly not going to be comfortable talking about that in a one-on-one situation with another 16-year-old.”
Taking on other taboos, Dr. Jones has been outspoken about abortion and the consequences of the recent Supreme Court decision, both as an ethical issue and a medical one that endangers lives. Raised in a deeply religious family, Dr. Jones said she was indoctrinated with antiabortion views, and it took time for her thinking to evolve “from a scientific and humanistic standpoint.” While working in a Texas private practice, Dr. Jones described being unable to mention abortion online because of fear of losing her patients and for her own safety.
Now free of those constraints, Dr. Jones feels that her videos can be important resources for teachers who may have little health training. And she is enthusiastic about the complementary relationship between her social media work and her clinical practice. “There are conversations I have all the time in the clinic where patients tell me: ‘Nobody’s ever really had this conversation in this way with me. Thank you for explaining that,’ ” said Dr. Jones. “And then I think: ‘Well, now I’ll have it with a hundred thousand other people too.’ ”
Promoting pleasure
While not an ob.gyn., discussing sexuality with patients has become a focus for Evelin Dacker, MD, a family physician in Salem, Ore. Dr. Dacker is certified in functional medicine, which takes a holistic and integrative approach. During her training she had a sudden realization: Sexuality had not been discussed at any point during her medical education.
“I recognized that this was a huge gap in how we deal with a person as a human,” Dr. Dacker explained. “Since sexuality plays a role in so many aspects of our humanness, not just having sex.”
Dr. Dacker believes in rethinking sexuality as a fundamental part of overall health, as vital as nutrition or blood pressure. Outside her medical practice, she teaches classes and workshops on sexual health and sex positivity for young adults and other physicians. She has also developed an educational framework for sexual health topics. Dr. Dacker said she frequently confronts the idea that sexuality is only about engaging with another person. She disagrees. Using food as a metaphor, she argues that just as the pleasure of eating something is purely for oneself, sexuality belongs to the individual.
Sexuality can also be a tool for pleasure, which Dr. Dacker believes plays an essential role in physical health. “Pleasure is a medicine,” Dr. Dacker said. “I actually prescribe self-pleasure practices to my patients, so they can start owning it within themselves. Make sure you get 7-8 hours of sleep, do some breathing exercises to help bring down your stress, and do self-pleasure so that you can integrate into your body better.”
She added that the impact of prioritizing one’s own desires, needs, and boundaries can transform how people view their sexuality. Her adult students frequently ask: “Why wasn’t I taught this as a teenager?”
Speaking of adult students – An older generation learns new tricks
While the teen cohort is usually the focus, the lack of sex education in previous decades – and the way sexual culture has evolved in that time – have an impact on older groups. Among U.S. adults aged 55 and older, the rate of STIs has more than doubled in the past 10 years, according to the Centers for Disease Control and Prevention. While the majority of STI cases still occur among teenagers and young adults, the consistent increase in STIs among older persons is cause for concern among physicians and researchers.
The issue worries Shannon Dowler, MD, a family physician in western North Carolina and chief medical officer for North Carolina Medicaid. Dr. Dowler, who has practiced in an STI clinic throughout her career, began seeing more and more older adults with chlamydia, herpes, and other STIs. Dowler cites several factors behind the rise, including the growing retirement community population, the availability of pharmaceuticals for sexual dysfunction, and the “hook-up culture” that is active on dating apps, which research shows are regularly used by more than a third of adults older than 55.
Dr. Dowler also sees a lack of communication about sexual health between physicians and their older patients. “Older adults are more likely to be in relationship with their physician outside the exam room, especially if they’re in a small community,” Dr. Dowler said. “Sometimes they aren’t as comfortable sharing what their risks are. But we are guilty in medicine all the time of not asking. We assume someone’s older so they’re not having sex anymore. But, in fact, they are, and we’re not taking the time to say: ‘Let’s talk about your sex life. Are you at risk for anything? Are you having any difficulties with sex?’ We tend to avoid it as a health care culture.”
In contrast, Dr. Dowler said she talks about sexual health with anyone who will listen. She teaches classes in private schools and universities and for church youth groups and other physicians. She often finds that public schools are not interested, which she attributes to fear of her discussing things “outside the rule book.”
Dr. Dowler takes creative approaches. In 2017, she released a hip-hop video, “STD’s Never Get Old,” in which she raps about safe sex for older adults. Her video went viral, was mentioned by several news outlets, and received over 50,000 views on YouTube. Dr. Dowler’s latest project is a book, “Never Too Late: Your Guide to Safer Sex after 60,” which is scheduled for publication on Valentine’s Day, 2023.
“It’s sex ed for seniors,” she explained. “It’s that gym class that some people got – I won’t say everyone got – in high school. This is the version for older adults who didn’t get that. There are new infections now that didn’t exist when they had sex education, if they had sex education.”
A big subject requires a big mission
For others in the sex education field, physicians are allies in their fight against agendas designed to obstruct or erode sex education. Alison Macklin, director of policy and advocacy at SIECUS: Sex Ed for Social Change, formerly the Sexuality Information and Education Council of the United States, sees this struggle playing out in school boards and state legislatures across the country. For every comprehensive sex education bill passed or school district victory, there is yet another blocked proposal or restrictive law somewhere else.
Ms. Macklin urged doctors to get more involved locally and to expand their knowledge of sexual health issues by reaching out to organizations such as Planned Parenthood and to be “hyper vigilant” in their own communities.
“Doctors are trusted. People really respect what they have to say,” Ms. Macklin said. “And this is an important time for them to speak up.”
A version of this article first appeared on Medscape.com.
An athletic coach stands in front of a packed gym full of high school students.
“Don’t have sex,” he instructs, “because you will get pregnant and die. Don’t have sex in the missionary position. Don’t have sex standing up. Just don’t do it, promise? Okay, everybody take some rubbers.”
Sad to say, this scene from the 2004 movie “Mean Girls” bears a striking resemblance to the actual sex education courses taught in schools across the United States today. In fact, things may have gotten measurably worse.
National data recently published by the Guttmacher Institute showed that adolescents were less likely to receive adequate sex education from 2015 to 2019 than they were in 1995. Only half of kids aged 15-19 received sex education that met minimum standards recommended by the Department of Health & Human Services, and fewer than half were given this information before having sex for the first time. With such a vast learning gap, it is no surprise that the United States has some of the highest rates of teenage pregnancy and sexually transmitted infections in the developed world.
Concerned and motivated by this need for sex education, physicians and other medical professionals are stepping in to fill the void, offering sexual health information through a range of methods to students of all ages (some a lot older than one may think). It is a calling that takes them outside their hospitals and exam rooms into workshops and through educational materials, video, and social media content created from scratch.
“The fact that we’re able to go in and provide factual, scientific, important information that can affect the trajectory of someone’s life is powerful,” said Julia Rossen, part of a contingent of med students at Brown University, Providence, R.I., who now teach sex ed as an elective.
Their goals are not just about protecting health. Many are also teaching about other topics commonly ignored in sex education classes, such as consent, pleasure, LGBTQ+ identities, and cultural competence. There is a mutually beneficial relationship, they say, between their sex education work and their medical practice.
Changing the status quo
A jumble of state laws govern how and when schools should offer sex education courses. Individual school districts often make the final decisions about their content, creating even more inconsistent standards. Only 29 states and the District of Columbia mandate sex education, and 13 of those do not require that it be medically accurate. Abstinence-only education, which has been shown to be ineffective, is exclusively taught in 16 states.
Without formal instruction, many young people must learn about sex from family members, who may be unwilling, or they may share knowledge between themselves, which is often incorrect, or navigate the limitless information and misinformation available on the internet.
The consequences of this were apparent to several medical students at Brown University in 2013. At the time, the rate of teenage pregnancy across Rhode Island was 1 in 100, but in the small city of Central Falls, it was 1 in 25. Aiming to improve this, the group created a comprehensive sex education program for a Central Falls middle school that was taught by medical student volunteers.
The Sex Ed by Brown Med program continues today. It consists of eight in-person sessions. Topics include anatomy, contraception, STIs, sexual decision-making, consent, sexual violence, and sexual and gender identity. Through this program, as well as other factors, the Central Falls teenage pregnancy rate declined to 1.6 in 100 from 2016 to 2020, according to the Rhode Island Department of Health.
“Historically, sexual education has been politicized,” said Ms. Rossen, one of the current program leaders. “It’s been at the discretion of a lot of different factors that aren’t under the control of the communities that are actually receiving the education.”
Among seventh graders, the teachers say they encounter different levels of maturity. But they feel that the kids are more receptive and open with younger adults who, like them, are still students. Some volunteers recall the flaws in their own sex education, particularly regarding topics such as consent and gender and sexual identity, and they believe middle school is the time to begin the sexual health conversation. “By the time you’re talking to college-age students, it’s pretty much too late,” said another group leader, Benjamin Stone.
Mr. Stone feels that practicing having these often-awkward discussions enhances their clinical skills as physicians. “Sex and sexual history are part of the comprehensive medical interview. People want to have these conversations, and they’re looking for someone to open the door. The kids are excited that we’re opening that door for them. And I think patients feel the same way.”
Conquering social media
Opening the door has been more like releasing a floodgate for Danielle Jones, MD, an ob.gyn. physician who is originally from Texas but who moved to New Zealand in 2021. Known on social media as “Mama Doctor Jones,” she has garnered more than 3 million followers across YouTube, TikTok, Twitter, Instagram, and Facebook. Dr. Jones produces short, friendly, entertaining videos on a range of reproductive health and sex education topics. They appeal to an adolescent audience hungry for a trustworthy voice on issues such as,: “5 ‘Strange’ Things Your Vagina Does That Are NORMAL” and “Condom Broke ... Now What?”
Dr. Jones uses her platform to debunk some of the misleading and inaccurate sexual health information being taught in classrooms, by other social media influencers, and that is found on the internet in general. Her no-nonsense-style videos call out such myths as being unable to pee with a tampon in, Plan B emergency contraception causing abortions, and COVID-19 vaccines damaging fertility.
“The way sex ed is done in the U.S. in most places is continuing the taboo by making it a one-time discussion or health class,” said Dr. Jones, “particularly if boys and girls are separated. That doesn’t further communication between people or foster an environment where it’s okay to discuss your body and puberty and changes in sexual health in general. And if you can’t talk about it in educational spaces, you’re certainly not going to be comfortable talking about that in a one-on-one situation with another 16-year-old.”
Taking on other taboos, Dr. Jones has been outspoken about abortion and the consequences of the recent Supreme Court decision, both as an ethical issue and a medical one that endangers lives. Raised in a deeply religious family, Dr. Jones said she was indoctrinated with antiabortion views, and it took time for her thinking to evolve “from a scientific and humanistic standpoint.” While working in a Texas private practice, Dr. Jones described being unable to mention abortion online because of fear of losing her patients and for her own safety.
Now free of those constraints, Dr. Jones feels that her videos can be important resources for teachers who may have little health training. And she is enthusiastic about the complementary relationship between her social media work and her clinical practice. “There are conversations I have all the time in the clinic where patients tell me: ‘Nobody’s ever really had this conversation in this way with me. Thank you for explaining that,’ ” said Dr. Jones. “And then I think: ‘Well, now I’ll have it with a hundred thousand other people too.’ ”
Promoting pleasure
While not an ob.gyn., discussing sexuality with patients has become a focus for Evelin Dacker, MD, a family physician in Salem, Ore. Dr. Dacker is certified in functional medicine, which takes a holistic and integrative approach. During her training she had a sudden realization: Sexuality had not been discussed at any point during her medical education.
“I recognized that this was a huge gap in how we deal with a person as a human,” Dr. Dacker explained. “Since sexuality plays a role in so many aspects of our humanness, not just having sex.”
Dr. Dacker believes in rethinking sexuality as a fundamental part of overall health, as vital as nutrition or blood pressure. Outside her medical practice, she teaches classes and workshops on sexual health and sex positivity for young adults and other physicians. She has also developed an educational framework for sexual health topics. Dr. Dacker said she frequently confronts the idea that sexuality is only about engaging with another person. She disagrees. Using food as a metaphor, she argues that just as the pleasure of eating something is purely for oneself, sexuality belongs to the individual.
Sexuality can also be a tool for pleasure, which Dr. Dacker believes plays an essential role in physical health. “Pleasure is a medicine,” Dr. Dacker said. “I actually prescribe self-pleasure practices to my patients, so they can start owning it within themselves. Make sure you get 7-8 hours of sleep, do some breathing exercises to help bring down your stress, and do self-pleasure so that you can integrate into your body better.”
She added that the impact of prioritizing one’s own desires, needs, and boundaries can transform how people view their sexuality. Her adult students frequently ask: “Why wasn’t I taught this as a teenager?”
Speaking of adult students – An older generation learns new tricks
While the teen cohort is usually the focus, the lack of sex education in previous decades – and the way sexual culture has evolved in that time – have an impact on older groups. Among U.S. adults aged 55 and older, the rate of STIs has more than doubled in the past 10 years, according to the Centers for Disease Control and Prevention. While the majority of STI cases still occur among teenagers and young adults, the consistent increase in STIs among older persons is cause for concern among physicians and researchers.
The issue worries Shannon Dowler, MD, a family physician in western North Carolina and chief medical officer for North Carolina Medicaid. Dr. Dowler, who has practiced in an STI clinic throughout her career, began seeing more and more older adults with chlamydia, herpes, and other STIs. Dowler cites several factors behind the rise, including the growing retirement community population, the availability of pharmaceuticals for sexual dysfunction, and the “hook-up culture” that is active on dating apps, which research shows are regularly used by more than a third of adults older than 55.
Dr. Dowler also sees a lack of communication about sexual health between physicians and their older patients. “Older adults are more likely to be in relationship with their physician outside the exam room, especially if they’re in a small community,” Dr. Dowler said. “Sometimes they aren’t as comfortable sharing what their risks are. But we are guilty in medicine all the time of not asking. We assume someone’s older so they’re not having sex anymore. But, in fact, they are, and we’re not taking the time to say: ‘Let’s talk about your sex life. Are you at risk for anything? Are you having any difficulties with sex?’ We tend to avoid it as a health care culture.”
In contrast, Dr. Dowler said she talks about sexual health with anyone who will listen. She teaches classes in private schools and universities and for church youth groups and other physicians. She often finds that public schools are not interested, which she attributes to fear of her discussing things “outside the rule book.”
Dr. Dowler takes creative approaches. In 2017, she released a hip-hop video, “STD’s Never Get Old,” in which she raps about safe sex for older adults. Her video went viral, was mentioned by several news outlets, and received over 50,000 views on YouTube. Dr. Dowler’s latest project is a book, “Never Too Late: Your Guide to Safer Sex after 60,” which is scheduled for publication on Valentine’s Day, 2023.
“It’s sex ed for seniors,” she explained. “It’s that gym class that some people got – I won’t say everyone got – in high school. This is the version for older adults who didn’t get that. There are new infections now that didn’t exist when they had sex education, if they had sex education.”
A big subject requires a big mission
For others in the sex education field, physicians are allies in their fight against agendas designed to obstruct or erode sex education. Alison Macklin, director of policy and advocacy at SIECUS: Sex Ed for Social Change, formerly the Sexuality Information and Education Council of the United States, sees this struggle playing out in school boards and state legislatures across the country. For every comprehensive sex education bill passed or school district victory, there is yet another blocked proposal or restrictive law somewhere else.
Ms. Macklin urged doctors to get more involved locally and to expand their knowledge of sexual health issues by reaching out to organizations such as Planned Parenthood and to be “hyper vigilant” in their own communities.
“Doctors are trusted. People really respect what they have to say,” Ms. Macklin said. “And this is an important time for them to speak up.”
A version of this article first appeared on Medscape.com.
Visual impairment more common in minority youth
Race, ethnicity, and socioeconomic status are closely associated with visual impairment in adolescents in the United States, researchers have found.
The study showed that adolescents who identified as Black, Mexican-American, of low income, or as non–U.S. citizens were two to three times more likely than White adolescents to report vision problems and to perform worse on objective tests of visual acuity.
Although disparities in visual impairment with respect to ethnic, racial, and socioeconomic status have been described among adults, little research has explored the adolescent population, according to the researchers, whose findings appear in JAMA Ophthalmology.
“The primary motivation behind trying to figure out exactly when these disparities emerge is that it gives us an opportunity to come up with ideas for interventions that can potentially address them before they manifest in a way that they are no longer treatable,” said Isdin Oke, MD, an instructor in ophthalmology at Harvard Medical School, Boston, who led the latest study.
Dr. Oke and his colleagues analyzed the records of 2,833 children and adolescents aged 12 through 18 years (mean, 15.5 years; 49% female) in the National Health and Nutrition Examination Survey. All the participants had completed a visual function questionnaire and had undergone an eye examination. The primary outcomes of the study were subjective (self-reported) poor vision and objective measures of visual function (visual acuity worse than 20/40 in the better-seeing eye).
Of the study participants, 14% were non-Hispanic Black, 11% were Mexican-American, 63% were non-Hispanic White, and 11% were of other race and ethnicity. Five percent of participants were not U.S. citizens, and 19% had a family income below the poverty threshold.
After accounting for potential confounders, self-reported poor vision was more common among Black (odds ratio, 2.85; 95% CI, 2.00-4.05; P < .001), Mexican-American (OR, 2.83; 95% CI, 1.70-4.73; P < .001), and low-income youth (OR, 2.44; 95% CI, 1.63-3.65; P < .001), the researchers report.
The study also found increased odds of visual acuity below 20/40 in the better-seeing eye among Black (OR, 2.13; 95% CI, 1.41-3.24; P = .001) and Mexican-American adolescents (OR, 2.13; 95% CI, 1.39-3.26; P = .001) and non–U.S. citizens (OR, 1.96; 95% CI, 1.10-3.49; P = .02).
Black and Mexican-American adolescents were almost three times more likely to suffer poor subjective visual function and twice as likely to have low objective visual acuity than non-Hispanic White youth, according to the researchers.
“I think it’s something that health care providers, and even the population as a whole, should be more aware of,” Dr. Oke said.
Opportunities for intervention
Dr. Oke said the findings likely reflect the underlying inequities in access to vision care experienced by these persons.
“There are a lot of opportunities for early intervention, whether it’s through vision screening or improving access to vision services for children and adolescents that can really make a big difference over the long term,” he told this news organization.
Michael F. Chiang, MD, director of the National Eye Institute at the National Institutes of Health, Bethesda, Md., agreed that more steps need to be taken to improve access to vision care for all Americans.
“There are good data showing that we don’t have a sufficient number of eye care providers and health-services clinical researchers who come from backgrounds that are currently underrepresented in medicine and science,” Dr. Chiang said. “Therefore, we need to find ways to inspire, recruit, and train a larger number of people to strengthen our vision workforce.”
Other ways to address these gaps in care, he added, include improving understanding of the social determinants of vision health as well as developing ways to improve access to eye care.
“That may include new models of eye care, like telehealth to improve outreach, and will likely need to include what we call implementation science, so clinicians can better adopt these measures,” he said.
Dr. Oke and Dr. Chiang reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Race, ethnicity, and socioeconomic status are closely associated with visual impairment in adolescents in the United States, researchers have found.
The study showed that adolescents who identified as Black, Mexican-American, of low income, or as non–U.S. citizens were two to three times more likely than White adolescents to report vision problems and to perform worse on objective tests of visual acuity.
Although disparities in visual impairment with respect to ethnic, racial, and socioeconomic status have been described among adults, little research has explored the adolescent population, according to the researchers, whose findings appear in JAMA Ophthalmology.
“The primary motivation behind trying to figure out exactly when these disparities emerge is that it gives us an opportunity to come up with ideas for interventions that can potentially address them before they manifest in a way that they are no longer treatable,” said Isdin Oke, MD, an instructor in ophthalmology at Harvard Medical School, Boston, who led the latest study.
Dr. Oke and his colleagues analyzed the records of 2,833 children and adolescents aged 12 through 18 years (mean, 15.5 years; 49% female) in the National Health and Nutrition Examination Survey. All the participants had completed a visual function questionnaire and had undergone an eye examination. The primary outcomes of the study were subjective (self-reported) poor vision and objective measures of visual function (visual acuity worse than 20/40 in the better-seeing eye).
Of the study participants, 14% were non-Hispanic Black, 11% were Mexican-American, 63% were non-Hispanic White, and 11% were of other race and ethnicity. Five percent of participants were not U.S. citizens, and 19% had a family income below the poverty threshold.
After accounting for potential confounders, self-reported poor vision was more common among Black (odds ratio, 2.85; 95% CI, 2.00-4.05; P < .001), Mexican-American (OR, 2.83; 95% CI, 1.70-4.73; P < .001), and low-income youth (OR, 2.44; 95% CI, 1.63-3.65; P < .001), the researchers report.
The study also found increased odds of visual acuity below 20/40 in the better-seeing eye among Black (OR, 2.13; 95% CI, 1.41-3.24; P = .001) and Mexican-American adolescents (OR, 2.13; 95% CI, 1.39-3.26; P = .001) and non–U.S. citizens (OR, 1.96; 95% CI, 1.10-3.49; P = .02).
Black and Mexican-American adolescents were almost three times more likely to suffer poor subjective visual function and twice as likely to have low objective visual acuity than non-Hispanic White youth, according to the researchers.
“I think it’s something that health care providers, and even the population as a whole, should be more aware of,” Dr. Oke said.
Opportunities for intervention
Dr. Oke said the findings likely reflect the underlying inequities in access to vision care experienced by these persons.
“There are a lot of opportunities for early intervention, whether it’s through vision screening or improving access to vision services for children and adolescents that can really make a big difference over the long term,” he told this news organization.
Michael F. Chiang, MD, director of the National Eye Institute at the National Institutes of Health, Bethesda, Md., agreed that more steps need to be taken to improve access to vision care for all Americans.
“There are good data showing that we don’t have a sufficient number of eye care providers and health-services clinical researchers who come from backgrounds that are currently underrepresented in medicine and science,” Dr. Chiang said. “Therefore, we need to find ways to inspire, recruit, and train a larger number of people to strengthen our vision workforce.”
Other ways to address these gaps in care, he added, include improving understanding of the social determinants of vision health as well as developing ways to improve access to eye care.
“That may include new models of eye care, like telehealth to improve outreach, and will likely need to include what we call implementation science, so clinicians can better adopt these measures,” he said.
Dr. Oke and Dr. Chiang reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Race, ethnicity, and socioeconomic status are closely associated with visual impairment in adolescents in the United States, researchers have found.
The study showed that adolescents who identified as Black, Mexican-American, of low income, or as non–U.S. citizens were two to three times more likely than White adolescents to report vision problems and to perform worse on objective tests of visual acuity.
Although disparities in visual impairment with respect to ethnic, racial, and socioeconomic status have been described among adults, little research has explored the adolescent population, according to the researchers, whose findings appear in JAMA Ophthalmology.
“The primary motivation behind trying to figure out exactly when these disparities emerge is that it gives us an opportunity to come up with ideas for interventions that can potentially address them before they manifest in a way that they are no longer treatable,” said Isdin Oke, MD, an instructor in ophthalmology at Harvard Medical School, Boston, who led the latest study.
Dr. Oke and his colleagues analyzed the records of 2,833 children and adolescents aged 12 through 18 years (mean, 15.5 years; 49% female) in the National Health and Nutrition Examination Survey. All the participants had completed a visual function questionnaire and had undergone an eye examination. The primary outcomes of the study were subjective (self-reported) poor vision and objective measures of visual function (visual acuity worse than 20/40 in the better-seeing eye).
Of the study participants, 14% were non-Hispanic Black, 11% were Mexican-American, 63% were non-Hispanic White, and 11% were of other race and ethnicity. Five percent of participants were not U.S. citizens, and 19% had a family income below the poverty threshold.
After accounting for potential confounders, self-reported poor vision was more common among Black (odds ratio, 2.85; 95% CI, 2.00-4.05; P < .001), Mexican-American (OR, 2.83; 95% CI, 1.70-4.73; P < .001), and low-income youth (OR, 2.44; 95% CI, 1.63-3.65; P < .001), the researchers report.
The study also found increased odds of visual acuity below 20/40 in the better-seeing eye among Black (OR, 2.13; 95% CI, 1.41-3.24; P = .001) and Mexican-American adolescents (OR, 2.13; 95% CI, 1.39-3.26; P = .001) and non–U.S. citizens (OR, 1.96; 95% CI, 1.10-3.49; P = .02).
Black and Mexican-American adolescents were almost three times more likely to suffer poor subjective visual function and twice as likely to have low objective visual acuity than non-Hispanic White youth, according to the researchers.
“I think it’s something that health care providers, and even the population as a whole, should be more aware of,” Dr. Oke said.
Opportunities for intervention
Dr. Oke said the findings likely reflect the underlying inequities in access to vision care experienced by these persons.
“There are a lot of opportunities for early intervention, whether it’s through vision screening or improving access to vision services for children and adolescents that can really make a big difference over the long term,” he told this news organization.
Michael F. Chiang, MD, director of the National Eye Institute at the National Institutes of Health, Bethesda, Md., agreed that more steps need to be taken to improve access to vision care for all Americans.
“There are good data showing that we don’t have a sufficient number of eye care providers and health-services clinical researchers who come from backgrounds that are currently underrepresented in medicine and science,” Dr. Chiang said. “Therefore, we need to find ways to inspire, recruit, and train a larger number of people to strengthen our vision workforce.”
Other ways to address these gaps in care, he added, include improving understanding of the social determinants of vision health as well as developing ways to improve access to eye care.
“That may include new models of eye care, like telehealth to improve outreach, and will likely need to include what we call implementation science, so clinicians can better adopt these measures,” he said.
Dr. Oke and Dr. Chiang reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
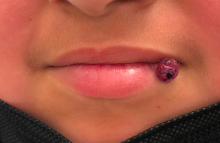
When is an allergic reaction to raw plant food due to tree pollen?
A new guideline aims to help primary care doctors differentiate pollen food syndrome (PFS) – a cross-reactive allergic reaction to certain raw, but not cooked, plant foods – from other food allergies.
The guideline from the British Society of Allergy and Clinical Immunology (BSACI) focuses on birch tree pollen, the major sensitizing PFS allergen in Northern Europe. Providers may be able to diagnose PFS related to birch pollen from clinical history alone, including the foods involved and the rapidity of symptom onset, write lead author Isabel J. Skypala, PhD, RD, of Imperial College London, and her colleagues.
The new BSACI guideline for diagnosis and management of PFS was published in Clinical & Experimental Allergy.
PFS is common and increasingly prevalent
PFS – also called oral allergy syndrome and pollen food allergy syndrome – is common and increasingly prevalent. PFS can begin at any age but usually starts in pollen-sensitized school-age children and adults with seasonal allergic rhinitis.
Symptoms from similar proteins in food
Mild to moderate allergic symptoms develop quickly when people sensitized to birch pollen eat raw plant foods that contain proteins similar to those in the pollen, such as pathogenesis-related protein PR-10. The allergens are broken down by cooking or processing.
Symptoms usually occur immediately or within 15 minutes of eating. Patients may have tingling; itching or soreness in the mouth, throat, or ears; mild lip and oral mucosa angioedema; itchy hands, sneezing, or eye symptoms; tongue or pharynx angioedema; perioral rash; cough; abdominal pain; nausea; and/or worsening of eczema. In children, itch and rash may predominate.
Triggers depend on pollen type
PFS triggers vary depending on a person’s pollen sensitization, which is affected by their geographic area and local dietary habits. In the United Kingdom, almost 70% of birch-allergic adults and more than 40% of birch-allergic children have PFS, the authors write.
Typical triggers include eating apples, stone fruits, kiwis, carrots, celery, hazelnuts, almonds, walnuts, soymilk, and peanuts, as well as peeling potatoes or other root vegetables. Freshly prepared vegetable or fruit smoothies or juices, celery, soymilk, raw nuts, large quantities of roasted nuts, and concentrated nut products can cause more severe reactions.
Diagnostic clinical history
If a patient answers yes to these questions, they almost certainly have PFS, the authors write:
- Are symptoms caused by raw fruits, nuts, carrots, or celery?
- Are the same trigger foods tolerated when they’re cooked well or roasted?
- Do symptoms come immediately or within a few minutes of eating?
- Do symptoms occur in the oropharynx and include tingling, itching, or swelling?
- Does the patient have seasonal allergic rhinitis or sensitization to pollen?
Testing needed for some cases
Allergy tests may be needed for people who report atypical or severe reactions or who also react to cooked or processed plant foods, such as roasted nuts, nuts in foods, fruits or vegetables in juices and smoothies, and soy products other than milk. Tests may also be needed for people who react to foods that are not linked with PFS, such as cashews, pistachios, macadamias, sesame seeds, beans, lentils, and chickpeas.
Whether PFS reactions also occur to roasted hazelnuts, almonds, walnuts, Brazil nuts, or peanuts, either alone or in composite foods such as chocolates, spreads, desserts, and snacks, is unclear.
An oral food challenge to confirm PFS is needed only if the history and diagnostic tests are inconclusive or if the patient is avoiding multiple foods.
Dietary management
PFS is managed by excluding known trigger foods. This becomes challenging for patients with preexisting food allergies and for vegetarians and vegans.
Personalized dietary advice is needed to avoid nutritional imbalance, minimize anxiety and unnecessary food restrictions, and improve quality of life. Reactions after accidental exposure often resolve without medication, and if antihistamines are needed, they rarely require self-injectable devices.
Guideline helpful beyond the United Kingdom and birch pollen
Allyson S. Larkin, MD, associate professor of pediatrics at the University of Pittsburgh School of Medicine, told this news organization in an email that the guideline summarizes in great detail the pathophysiology behind PFS and highlights how component testing may help diagnose patients and manage the condition.
“Patients worry very much about the progression and severity of allergic reactions,” said Dr. Larkin, who was not involved in the guideline development.
“As the authors note, recognizing the nutritional consequences of dietary restrictions is important, and nutrition consults and suitable alternative suggestions are very helpful for these patients, especially for those with food allergy or who are vegetarian or vegan.”
Jill A. Poole, MD, professor of medicine and chief of the Division of Allergy and Immunology at the University of Nebraska College of Medicine, Omaha, noted that PFS, although common, is underrecognized by the public and by health care providers.
“People are not allergic to the specific food, but they are allergic to a seasonal allergen, such as birch tree, that cross-reacts with the food protein, which is typically changed with cooking,” she explained in an email.
“This differs from reactions by those who have moderate to severe allergic food-specific reactions that may include systemic reactions like anaphylaxis from eating certain foods,” she said.
“Importantly, the number of cross-reacting foods with seasonal pollens continues to grow, and the extent of testing has expanded in recent years,” advised Dr. Poole, who also was not involved in the guideline development.
The authors recommend further related research into food immunotherapy and other novel PFS treatments. They also want to raise awareness of factors affecting PFS prevalence, such as increased spread and allergenicity of pollen due to climate change, pollution, the global consumption of previously local traditional foods, and the increase in vegetarian and vegan diets.
The authors, Dr. Larkin, and Dr. Poole report no relevant financial relationships involving this guideline. The guideline was not funded.
A version of this article first appeared on Medscape.com.
A new guideline aims to help primary care doctors differentiate pollen food syndrome (PFS) – a cross-reactive allergic reaction to certain raw, but not cooked, plant foods – from other food allergies.
The guideline from the British Society of Allergy and Clinical Immunology (BSACI) focuses on birch tree pollen, the major sensitizing PFS allergen in Northern Europe. Providers may be able to diagnose PFS related to birch pollen from clinical history alone, including the foods involved and the rapidity of symptom onset, write lead author Isabel J. Skypala, PhD, RD, of Imperial College London, and her colleagues.
The new BSACI guideline for diagnosis and management of PFS was published in Clinical & Experimental Allergy.
PFS is common and increasingly prevalent
PFS – also called oral allergy syndrome and pollen food allergy syndrome – is common and increasingly prevalent. PFS can begin at any age but usually starts in pollen-sensitized school-age children and adults with seasonal allergic rhinitis.
Symptoms from similar proteins in food
Mild to moderate allergic symptoms develop quickly when people sensitized to birch pollen eat raw plant foods that contain proteins similar to those in the pollen, such as pathogenesis-related protein PR-10. The allergens are broken down by cooking or processing.
Symptoms usually occur immediately or within 15 minutes of eating. Patients may have tingling; itching or soreness in the mouth, throat, or ears; mild lip and oral mucosa angioedema; itchy hands, sneezing, or eye symptoms; tongue or pharynx angioedema; perioral rash; cough; abdominal pain; nausea; and/or worsening of eczema. In children, itch and rash may predominate.
Triggers depend on pollen type
PFS triggers vary depending on a person’s pollen sensitization, which is affected by their geographic area and local dietary habits. In the United Kingdom, almost 70% of birch-allergic adults and more than 40% of birch-allergic children have PFS, the authors write.
Typical triggers include eating apples, stone fruits, kiwis, carrots, celery, hazelnuts, almonds, walnuts, soymilk, and peanuts, as well as peeling potatoes or other root vegetables. Freshly prepared vegetable or fruit smoothies or juices, celery, soymilk, raw nuts, large quantities of roasted nuts, and concentrated nut products can cause more severe reactions.
Diagnostic clinical history
If a patient answers yes to these questions, they almost certainly have PFS, the authors write:
- Are symptoms caused by raw fruits, nuts, carrots, or celery?
- Are the same trigger foods tolerated when they’re cooked well or roasted?
- Do symptoms come immediately or within a few minutes of eating?
- Do symptoms occur in the oropharynx and include tingling, itching, or swelling?
- Does the patient have seasonal allergic rhinitis or sensitization to pollen?
Testing needed for some cases
Allergy tests may be needed for people who report atypical or severe reactions or who also react to cooked or processed plant foods, such as roasted nuts, nuts in foods, fruits or vegetables in juices and smoothies, and soy products other than milk. Tests may also be needed for people who react to foods that are not linked with PFS, such as cashews, pistachios, macadamias, sesame seeds, beans, lentils, and chickpeas.
Whether PFS reactions also occur to roasted hazelnuts, almonds, walnuts, Brazil nuts, or peanuts, either alone or in composite foods such as chocolates, spreads, desserts, and snacks, is unclear.
An oral food challenge to confirm PFS is needed only if the history and diagnostic tests are inconclusive or if the patient is avoiding multiple foods.
Dietary management
PFS is managed by excluding known trigger foods. This becomes challenging for patients with preexisting food allergies and for vegetarians and vegans.
Personalized dietary advice is needed to avoid nutritional imbalance, minimize anxiety and unnecessary food restrictions, and improve quality of life. Reactions after accidental exposure often resolve without medication, and if antihistamines are needed, they rarely require self-injectable devices.
Guideline helpful beyond the United Kingdom and birch pollen
Allyson S. Larkin, MD, associate professor of pediatrics at the University of Pittsburgh School of Medicine, told this news organization in an email that the guideline summarizes in great detail the pathophysiology behind PFS and highlights how component testing may help diagnose patients and manage the condition.
“Patients worry very much about the progression and severity of allergic reactions,” said Dr. Larkin, who was not involved in the guideline development.
“As the authors note, recognizing the nutritional consequences of dietary restrictions is important, and nutrition consults and suitable alternative suggestions are very helpful for these patients, especially for those with food allergy or who are vegetarian or vegan.”
Jill A. Poole, MD, professor of medicine and chief of the Division of Allergy and Immunology at the University of Nebraska College of Medicine, Omaha, noted that PFS, although common, is underrecognized by the public and by health care providers.
“People are not allergic to the specific food, but they are allergic to a seasonal allergen, such as birch tree, that cross-reacts with the food protein, which is typically changed with cooking,” she explained in an email.
“This differs from reactions by those who have moderate to severe allergic food-specific reactions that may include systemic reactions like anaphylaxis from eating certain foods,” she said.
“Importantly, the number of cross-reacting foods with seasonal pollens continues to grow, and the extent of testing has expanded in recent years,” advised Dr. Poole, who also was not involved in the guideline development.
The authors recommend further related research into food immunotherapy and other novel PFS treatments. They also want to raise awareness of factors affecting PFS prevalence, such as increased spread and allergenicity of pollen due to climate change, pollution, the global consumption of previously local traditional foods, and the increase in vegetarian and vegan diets.
The authors, Dr. Larkin, and Dr. Poole report no relevant financial relationships involving this guideline. The guideline was not funded.
A version of this article first appeared on Medscape.com.
A new guideline aims to help primary care doctors differentiate pollen food syndrome (PFS) – a cross-reactive allergic reaction to certain raw, but not cooked, plant foods – from other food allergies.
The guideline from the British Society of Allergy and Clinical Immunology (BSACI) focuses on birch tree pollen, the major sensitizing PFS allergen in Northern Europe. Providers may be able to diagnose PFS related to birch pollen from clinical history alone, including the foods involved and the rapidity of symptom onset, write lead author Isabel J. Skypala, PhD, RD, of Imperial College London, and her colleagues.
The new BSACI guideline for diagnosis and management of PFS was published in Clinical & Experimental Allergy.
PFS is common and increasingly prevalent
PFS – also called oral allergy syndrome and pollen food allergy syndrome – is common and increasingly prevalent. PFS can begin at any age but usually starts in pollen-sensitized school-age children and adults with seasonal allergic rhinitis.
Symptoms from similar proteins in food
Mild to moderate allergic symptoms develop quickly when people sensitized to birch pollen eat raw plant foods that contain proteins similar to those in the pollen, such as pathogenesis-related protein PR-10. The allergens are broken down by cooking or processing.
Symptoms usually occur immediately or within 15 minutes of eating. Patients may have tingling; itching or soreness in the mouth, throat, or ears; mild lip and oral mucosa angioedema; itchy hands, sneezing, or eye symptoms; tongue or pharynx angioedema; perioral rash; cough; abdominal pain; nausea; and/or worsening of eczema. In children, itch and rash may predominate.
Triggers depend on pollen type
PFS triggers vary depending on a person’s pollen sensitization, which is affected by their geographic area and local dietary habits. In the United Kingdom, almost 70% of birch-allergic adults and more than 40% of birch-allergic children have PFS, the authors write.
Typical triggers include eating apples, stone fruits, kiwis, carrots, celery, hazelnuts, almonds, walnuts, soymilk, and peanuts, as well as peeling potatoes or other root vegetables. Freshly prepared vegetable or fruit smoothies or juices, celery, soymilk, raw nuts, large quantities of roasted nuts, and concentrated nut products can cause more severe reactions.
Diagnostic clinical history
If a patient answers yes to these questions, they almost certainly have PFS, the authors write:
- Are symptoms caused by raw fruits, nuts, carrots, or celery?
- Are the same trigger foods tolerated when they’re cooked well or roasted?
- Do symptoms come immediately or within a few minutes of eating?
- Do symptoms occur in the oropharynx and include tingling, itching, or swelling?
- Does the patient have seasonal allergic rhinitis or sensitization to pollen?
Testing needed for some cases
Allergy tests may be needed for people who report atypical or severe reactions or who also react to cooked or processed plant foods, such as roasted nuts, nuts in foods, fruits or vegetables in juices and smoothies, and soy products other than milk. Tests may also be needed for people who react to foods that are not linked with PFS, such as cashews, pistachios, macadamias, sesame seeds, beans, lentils, and chickpeas.
Whether PFS reactions also occur to roasted hazelnuts, almonds, walnuts, Brazil nuts, or peanuts, either alone or in composite foods such as chocolates, spreads, desserts, and snacks, is unclear.
An oral food challenge to confirm PFS is needed only if the history and diagnostic tests are inconclusive or if the patient is avoiding multiple foods.
Dietary management
PFS is managed by excluding known trigger foods. This becomes challenging for patients with preexisting food allergies and for vegetarians and vegans.
Personalized dietary advice is needed to avoid nutritional imbalance, minimize anxiety and unnecessary food restrictions, and improve quality of life. Reactions after accidental exposure often resolve without medication, and if antihistamines are needed, they rarely require self-injectable devices.
Guideline helpful beyond the United Kingdom and birch pollen
Allyson S. Larkin, MD, associate professor of pediatrics at the University of Pittsburgh School of Medicine, told this news organization in an email that the guideline summarizes in great detail the pathophysiology behind PFS and highlights how component testing may help diagnose patients and manage the condition.
“Patients worry very much about the progression and severity of allergic reactions,” said Dr. Larkin, who was not involved in the guideline development.
“As the authors note, recognizing the nutritional consequences of dietary restrictions is important, and nutrition consults and suitable alternative suggestions are very helpful for these patients, especially for those with food allergy or who are vegetarian or vegan.”
Jill A. Poole, MD, professor of medicine and chief of the Division of Allergy and Immunology at the University of Nebraska College of Medicine, Omaha, noted that PFS, although common, is underrecognized by the public and by health care providers.
“People are not allergic to the specific food, but they are allergic to a seasonal allergen, such as birch tree, that cross-reacts with the food protein, which is typically changed with cooking,” she explained in an email.
“This differs from reactions by those who have moderate to severe allergic food-specific reactions that may include systemic reactions like anaphylaxis from eating certain foods,” she said.
“Importantly, the number of cross-reacting foods with seasonal pollens continues to grow, and the extent of testing has expanded in recent years,” advised Dr. Poole, who also was not involved in the guideline development.
The authors recommend further related research into food immunotherapy and other novel PFS treatments. They also want to raise awareness of factors affecting PFS prevalence, such as increased spread and allergenicity of pollen due to climate change, pollution, the global consumption of previously local traditional foods, and the increase in vegetarian and vegan diets.
The authors, Dr. Larkin, and Dr. Poole report no relevant financial relationships involving this guideline. The guideline was not funded.
A version of this article first appeared on Medscape.com.
Shift in child hospice care is a lifeline for parents seeking a measure of comfort and hope
POMONA, CALIF. – When you first meet 17-month-old Aaron Martinez, it’s not obvious that something is catastrophically wrong.
What you see is a beautiful little boy with smooth, lustrous skin, an abundance of glossy brown hair, and a disarming smile. What you hear are coos and cries that don’t immediately signal anything is horribly awry.
But his parents, Adriana Pinedo and Hector Martinez, know the truth painfully well.
Although Ms. Pinedo’s doctors and midwife had described the pregnancy as “perfect” for all 9 months, Aaron was born with most of his brain cells dead, the result of two strokes and a massive bleed he sustained while in utero.
Doctors aren’t sure what caused the anomalies that left Aaron with virtually no cognitive function or physical mobility. His voluminous hair hides a head whose circumference is too small for his age. He has epilepsy that triggers multiple seizures each day, and his smile is not always what it seems. “It could be a smile; it could be a seizure,” Ms. Pinedo said.
Shortly after Aaron was born, doctors told Ms. Pinedo, 34, and Mr. Martinez, 35, there was no hope and they should “let nature take its course.” They would learn months later that the doctors had not expected the boy to live more than 5 days. It was on Day 5 that his parents put him in home hospice care, an arrangement that has continued into his second year of life.
The family gets weekly visits from hospice nurses, therapists, social workers, and a chaplain in the cramped one-bedroom apartment they rent from the people who live in the main house on the same lot on a quiet residential street in this Inland Empire city.
One of the main criteria for hospice care, established by Medicare largely for seniors but also applied to children, is a diagnosis of 6 months or less to live. Yet over the course of 17 months, Aaron’s medical team has repeatedly recertified his hospice eligibility.
Under a provision of the 2010 Affordable Care Act, children enrolled in Medicaid or the Children’s Health Insurance Program are allowed, unlike adults, to be in hospice while continuing to receive curative or life-extending care. Commercial insurers are not required to cover this “concurrent care,” but many now do.
More than a decade since its inception, concurrent care is widely credited with improving the quality of life for many terminally ill children, easing stress on the family and, in some cases, sustaining hope for a cure. But the arrangement can contribute to a painful dilemma for parents like Ms. Pinedo and Mr. Martinez, who are torn between their fierce commitment to their son and the futility of knowing that his condition leaves him with no future worth hoping for.
“We could lose a life, but if he continues to live this way, we’ll lose three,” said Ms. Pinedo. “There’s no quality of life for him or for us.”
Aaron’s doctors now say he could conceivably live for years. His body hasn’t stopped growing since he was born. He’s in the 96th percentile for height for his age, and his weight is about average.
His parents have talked about “graduating” him from hospice. But he is never stable for long, and they welcome the visits from their hospice team. The seizures, sometimes 30 a day, are a persistent assault on his brain and, as he grows, the medications intended to control them must be changed or the doses recalibrated. He is at continual risk of gastrointestinal problems and potentially deadly fluid buildup in his lungs.
Ms. Pinedo, who works from home for a nonprofit public health organization, spends much of her time with Aaron, while Mr. Martinez works as a landscaper. She has chosen to live in the moment, she said, because otherwise her mind wanders to a future in which either “he could die – or he won’t, and I’ll end up changing the diapers of a 40-year-old man.” Either of those “are going to suck.”
While cancer is one of the major illnesses afflicting children in hospice, many others, like Aaron, have rare congenital defects, severe neurological impairments, or uncommon metabolic deficiencies.
“We have diseases that families tell us are 1 of 10 cases in the world,” said Glen Komatsu, MD, medical director of Torrance, Calif.–based TrinityKids Care, which provides home hospice services to Aaron and more than 70 other kids in Los Angeles and Orange counties.
In the years leading up to the ACA’s implementation, pediatric health advocates lobbied hard for the concurrent care provision. Without the possibility of life-extending care or hope for a cure, many parents refused to put their terminally ill kids in hospice, thinking it was tantamount to giving up on them. That meant the whole family missed out on the support hospice can provide, not just pain relief and comfort for the dying child, but emotional and spiritual care for parents and siblings under extreme duress.
TrinityKids Care, run by the large national Catholic health system Providence, doesn’t just send nurses, social workers, and chaplains into homes. For patients able to participate, and their siblings, it also offers art and science projects, exercise classes, movies, and music. During the pandemic, these activities have been conducted via Zoom, and volunteers deliver needed supplies to the children’s homes.
The ability to get treatments that prolong their lives is a major reason children in concurrent care are more likely than adults to outlive the 6-months-to-live diagnosis required for hospice.
“Concurrent care, by its very intention, very clearly is going to extend their lives, and by extending their lives they’re no longer going to be hospice-eligible if you use the 6-month life expectancy criteria,” said David Steinhorn, MD, a pediatric intensive care physician in Virginia, who has helped develop numerous children’s hospice programs across the United States.
Another factor is that kids, even sick ones, are simply more robust than many older people.
“Sick kids are often otherwise healthy, except for one organ,” said Debra Lotstein, MD, chief of the division of comfort and palliative care at Children’s Hospital Los Angeles. “They may have cancer in their body, but their hearts are good and their lungs are good, compared to a 90-year-old who at baseline is just not as resilient.”
All of Aaron Martinez’s vital organs, except for his brain, seem to be working. “There have been times when we’ve brought him in, and the nurse looks at the chart and looks at him, and she can’t believe it’s that child,” said Mr. Martinez.
When kids live past the 6-month life expectancy, they must be recertified to stay in hospice. In many cases, Dr. Steinhorn said, he is willing to recertify his pediatric patients indefinitely.
Even with doctors advocating for them, it’s not always easy for children to get into hospice care. Most hospices care primarily for adults and are reluctant to take kids.
“The hospice will say: ‘We don’t have the capacity to treat children. Our nurses aren’t trained. It’s different. We just can’t do it,’ ” said Lori Butterworth, cofounder of the Children’s Hospice and Palliative Care Coalition of California in Watsonville. “The other reason is not wanting to, because it’s existentially devastating and sad and hard.”
Finances also play a role. Home hospice care is paid at a per diem rate set by Medicare – slightly over $200 a day for the first 2 months, about $161 a day after that – and it is typically the same for kids and adults. Children, particularly those with rare conditions, often require more intensive and innovative care, so the per diem doesn’t stretch as far.
The concurrent care provision has made taking pediatric patients more viable for hospice organizations, Dr. Steinhorn and others said. Under the ACA, many of the expenses for certain medications and medical services can be shifted to the patient’s primary insurance, leaving hospices responsible for pain relief and comfort care.
Even so, the relatively small number of kids who die each year from protracted ailments hardly makes pediatric hospice an appealing line of business in an industry craving growth, especially one in which private equity investors are active and seeking a big payday.
In California, only 21 of 1,336 hospices reported having a specialized pediatric hospice program, and 59 said they served at least one patient under age 21, according to an analysis of 2020 state data by Cordt Kassner, CEO of Hospice Analytics in Colorado Springs.
Hospice providers that do cater to children often face a more basic challenge: Even with the possibility of concurrent care, many parents still equate hospice with acceptance of death. That was the case initially for Matt and Reese Sonnen, Los Angeles residents whose daughter, Layla, was born with a seizure disorder that had no name: Her brain had simply failed to develop in the womb, and an MRI showed “fluid taking up space where the brain wasn’t,” her mother said.
When Layla’s team first mentioned hospice, “I was in the car on my phone, and I almost crashed the car,” Mrs. Sonnen recalled. “The first thought that came to mind was: ‘It is just the end,’ but we felt she was nowhere near it, because she was strong, she was mighty. She was my little girl. She was going to get through this.”
About 3 months later, as Layla’s nervous system deteriorated, causing her to writhe in pain, her parents agreed to enroll her in hospice with TrinityKids Care. She died weeks later, not long after her second birthday. She was in her mother’s arms, with Mr. Sonnen close by.
“All of a sudden, Layla breathed out a big rush of air. The nurse looked at me and said: ‘That was her last breath.’ I was literally breathing in her last breath,” Mrs. Sonnen recounted. “I never wanted to breathe again, because now I felt I had her in my lungs. Don’t make me laugh, don’t make me exhale.”
Layla’s parents have no regrets about their decision to put her in hospice. “It was the absolute right decision, and in hindsight we should have done it sooner,” Mr. Sonnen said. “She was suffering, and we had blinders on.”
Ms. Pinedo said she is “infinitely grateful” for hospice, despite the heartache of Aaron’s condition. Sometimes the social worker will stop by, she said, just to say hello and drop off a latte, a small gesture that can feel very uplifting. “They’ve been our lifeline,” she said.
Ms. Pinedo talks about a friend of hers with a healthy baby, also named Aaron, who is pregnant with her second child. “All the stuff that was on our list, they’re living. And I love them dearly. But it’s almost hard to look, because it’s like looking at the stuff that you didn’t get. It’s like Christmas Day, staring through the window at the neighbor’s house, and you’re sitting there in the cold.”
Yet she seems palpably torn between that bleak remorse and the unconditional love parents feel toward their children. At one point, Ms. Pinedo interrupted herself midsentence and turned to her son, who was in Mr. Martinez’s arms: “Yes, Papi, you are so stinking cute, and you are still my dream come true.”
This story was produced by KHN, which publishes California Healthline, an editorially independent service of the California Health Care Foundation. KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
POMONA, CALIF. – When you first meet 17-month-old Aaron Martinez, it’s not obvious that something is catastrophically wrong.
What you see is a beautiful little boy with smooth, lustrous skin, an abundance of glossy brown hair, and a disarming smile. What you hear are coos and cries that don’t immediately signal anything is horribly awry.
But his parents, Adriana Pinedo and Hector Martinez, know the truth painfully well.
Although Ms. Pinedo’s doctors and midwife had described the pregnancy as “perfect” for all 9 months, Aaron was born with most of his brain cells dead, the result of two strokes and a massive bleed he sustained while in utero.
Doctors aren’t sure what caused the anomalies that left Aaron with virtually no cognitive function or physical mobility. His voluminous hair hides a head whose circumference is too small for his age. He has epilepsy that triggers multiple seizures each day, and his smile is not always what it seems. “It could be a smile; it could be a seizure,” Ms. Pinedo said.
Shortly after Aaron was born, doctors told Ms. Pinedo, 34, and Mr. Martinez, 35, there was no hope and they should “let nature take its course.” They would learn months later that the doctors had not expected the boy to live more than 5 days. It was on Day 5 that his parents put him in home hospice care, an arrangement that has continued into his second year of life.
The family gets weekly visits from hospice nurses, therapists, social workers, and a chaplain in the cramped one-bedroom apartment they rent from the people who live in the main house on the same lot on a quiet residential street in this Inland Empire city.
One of the main criteria for hospice care, established by Medicare largely for seniors but also applied to children, is a diagnosis of 6 months or less to live. Yet over the course of 17 months, Aaron’s medical team has repeatedly recertified his hospice eligibility.
Under a provision of the 2010 Affordable Care Act, children enrolled in Medicaid or the Children’s Health Insurance Program are allowed, unlike adults, to be in hospice while continuing to receive curative or life-extending care. Commercial insurers are not required to cover this “concurrent care,” but many now do.
More than a decade since its inception, concurrent care is widely credited with improving the quality of life for many terminally ill children, easing stress on the family and, in some cases, sustaining hope for a cure. But the arrangement can contribute to a painful dilemma for parents like Ms. Pinedo and Mr. Martinez, who are torn between their fierce commitment to their son and the futility of knowing that his condition leaves him with no future worth hoping for.
“We could lose a life, but if he continues to live this way, we’ll lose three,” said Ms. Pinedo. “There’s no quality of life for him or for us.”
Aaron’s doctors now say he could conceivably live for years. His body hasn’t stopped growing since he was born. He’s in the 96th percentile for height for his age, and his weight is about average.
His parents have talked about “graduating” him from hospice. But he is never stable for long, and they welcome the visits from their hospice team. The seizures, sometimes 30 a day, are a persistent assault on his brain and, as he grows, the medications intended to control them must be changed or the doses recalibrated. He is at continual risk of gastrointestinal problems and potentially deadly fluid buildup in his lungs.
Ms. Pinedo, who works from home for a nonprofit public health organization, spends much of her time with Aaron, while Mr. Martinez works as a landscaper. She has chosen to live in the moment, she said, because otherwise her mind wanders to a future in which either “he could die – or he won’t, and I’ll end up changing the diapers of a 40-year-old man.” Either of those “are going to suck.”
While cancer is one of the major illnesses afflicting children in hospice, many others, like Aaron, have rare congenital defects, severe neurological impairments, or uncommon metabolic deficiencies.
“We have diseases that families tell us are 1 of 10 cases in the world,” said Glen Komatsu, MD, medical director of Torrance, Calif.–based TrinityKids Care, which provides home hospice services to Aaron and more than 70 other kids in Los Angeles and Orange counties.
In the years leading up to the ACA’s implementation, pediatric health advocates lobbied hard for the concurrent care provision. Without the possibility of life-extending care or hope for a cure, many parents refused to put their terminally ill kids in hospice, thinking it was tantamount to giving up on them. That meant the whole family missed out on the support hospice can provide, not just pain relief and comfort for the dying child, but emotional and spiritual care for parents and siblings under extreme duress.
TrinityKids Care, run by the large national Catholic health system Providence, doesn’t just send nurses, social workers, and chaplains into homes. For patients able to participate, and their siblings, it also offers art and science projects, exercise classes, movies, and music. During the pandemic, these activities have been conducted via Zoom, and volunteers deliver needed supplies to the children’s homes.
The ability to get treatments that prolong their lives is a major reason children in concurrent care are more likely than adults to outlive the 6-months-to-live diagnosis required for hospice.
“Concurrent care, by its very intention, very clearly is going to extend their lives, and by extending their lives they’re no longer going to be hospice-eligible if you use the 6-month life expectancy criteria,” said David Steinhorn, MD, a pediatric intensive care physician in Virginia, who has helped develop numerous children’s hospice programs across the United States.
Another factor is that kids, even sick ones, are simply more robust than many older people.
“Sick kids are often otherwise healthy, except for one organ,” said Debra Lotstein, MD, chief of the division of comfort and palliative care at Children’s Hospital Los Angeles. “They may have cancer in their body, but their hearts are good and their lungs are good, compared to a 90-year-old who at baseline is just not as resilient.”
All of Aaron Martinez’s vital organs, except for his brain, seem to be working. “There have been times when we’ve brought him in, and the nurse looks at the chart and looks at him, and she can’t believe it’s that child,” said Mr. Martinez.
When kids live past the 6-month life expectancy, they must be recertified to stay in hospice. In many cases, Dr. Steinhorn said, he is willing to recertify his pediatric patients indefinitely.
Even with doctors advocating for them, it’s not always easy for children to get into hospice care. Most hospices care primarily for adults and are reluctant to take kids.
“The hospice will say: ‘We don’t have the capacity to treat children. Our nurses aren’t trained. It’s different. We just can’t do it,’ ” said Lori Butterworth, cofounder of the Children’s Hospice and Palliative Care Coalition of California in Watsonville. “The other reason is not wanting to, because it’s existentially devastating and sad and hard.”
Finances also play a role. Home hospice care is paid at a per diem rate set by Medicare – slightly over $200 a day for the first 2 months, about $161 a day after that – and it is typically the same for kids and adults. Children, particularly those with rare conditions, often require more intensive and innovative care, so the per diem doesn’t stretch as far.
The concurrent care provision has made taking pediatric patients more viable for hospice organizations, Dr. Steinhorn and others said. Under the ACA, many of the expenses for certain medications and medical services can be shifted to the patient’s primary insurance, leaving hospices responsible for pain relief and comfort care.
Even so, the relatively small number of kids who die each year from protracted ailments hardly makes pediatric hospice an appealing line of business in an industry craving growth, especially one in which private equity investors are active and seeking a big payday.
In California, only 21 of 1,336 hospices reported having a specialized pediatric hospice program, and 59 said they served at least one patient under age 21, according to an analysis of 2020 state data by Cordt Kassner, CEO of Hospice Analytics in Colorado Springs.
Hospice providers that do cater to children often face a more basic challenge: Even with the possibility of concurrent care, many parents still equate hospice with acceptance of death. That was the case initially for Matt and Reese Sonnen, Los Angeles residents whose daughter, Layla, was born with a seizure disorder that had no name: Her brain had simply failed to develop in the womb, and an MRI showed “fluid taking up space where the brain wasn’t,” her mother said.
When Layla’s team first mentioned hospice, “I was in the car on my phone, and I almost crashed the car,” Mrs. Sonnen recalled. “The first thought that came to mind was: ‘It is just the end,’ but we felt she was nowhere near it, because she was strong, she was mighty. She was my little girl. She was going to get through this.”
About 3 months later, as Layla’s nervous system deteriorated, causing her to writhe in pain, her parents agreed to enroll her in hospice with TrinityKids Care. She died weeks later, not long after her second birthday. She was in her mother’s arms, with Mr. Sonnen close by.
“All of a sudden, Layla breathed out a big rush of air. The nurse looked at me and said: ‘That was her last breath.’ I was literally breathing in her last breath,” Mrs. Sonnen recounted. “I never wanted to breathe again, because now I felt I had her in my lungs. Don’t make me laugh, don’t make me exhale.”
Layla’s parents have no regrets about their decision to put her in hospice. “It was the absolute right decision, and in hindsight we should have done it sooner,” Mr. Sonnen said. “She was suffering, and we had blinders on.”
Ms. Pinedo said she is “infinitely grateful” for hospice, despite the heartache of Aaron’s condition. Sometimes the social worker will stop by, she said, just to say hello and drop off a latte, a small gesture that can feel very uplifting. “They’ve been our lifeline,” she said.
Ms. Pinedo talks about a friend of hers with a healthy baby, also named Aaron, who is pregnant with her second child. “All the stuff that was on our list, they’re living. And I love them dearly. But it’s almost hard to look, because it’s like looking at the stuff that you didn’t get. It’s like Christmas Day, staring through the window at the neighbor’s house, and you’re sitting there in the cold.”
Yet she seems palpably torn between that bleak remorse and the unconditional love parents feel toward their children. At one point, Ms. Pinedo interrupted herself midsentence and turned to her son, who was in Mr. Martinez’s arms: “Yes, Papi, you are so stinking cute, and you are still my dream come true.”
This story was produced by KHN, which publishes California Healthline, an editorially independent service of the California Health Care Foundation. KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
POMONA, CALIF. – When you first meet 17-month-old Aaron Martinez, it’s not obvious that something is catastrophically wrong.
What you see is a beautiful little boy with smooth, lustrous skin, an abundance of glossy brown hair, and a disarming smile. What you hear are coos and cries that don’t immediately signal anything is horribly awry.
But his parents, Adriana Pinedo and Hector Martinez, know the truth painfully well.
Although Ms. Pinedo’s doctors and midwife had described the pregnancy as “perfect” for all 9 months, Aaron was born with most of his brain cells dead, the result of two strokes and a massive bleed he sustained while in utero.
Doctors aren’t sure what caused the anomalies that left Aaron with virtually no cognitive function or physical mobility. His voluminous hair hides a head whose circumference is too small for his age. He has epilepsy that triggers multiple seizures each day, and his smile is not always what it seems. “It could be a smile; it could be a seizure,” Ms. Pinedo said.
Shortly after Aaron was born, doctors told Ms. Pinedo, 34, and Mr. Martinez, 35, there was no hope and they should “let nature take its course.” They would learn months later that the doctors had not expected the boy to live more than 5 days. It was on Day 5 that his parents put him in home hospice care, an arrangement that has continued into his second year of life.
The family gets weekly visits from hospice nurses, therapists, social workers, and a chaplain in the cramped one-bedroom apartment they rent from the people who live in the main house on the same lot on a quiet residential street in this Inland Empire city.
One of the main criteria for hospice care, established by Medicare largely for seniors but also applied to children, is a diagnosis of 6 months or less to live. Yet over the course of 17 months, Aaron’s medical team has repeatedly recertified his hospice eligibility.
Under a provision of the 2010 Affordable Care Act, children enrolled in Medicaid or the Children’s Health Insurance Program are allowed, unlike adults, to be in hospice while continuing to receive curative or life-extending care. Commercial insurers are not required to cover this “concurrent care,” but many now do.
More than a decade since its inception, concurrent care is widely credited with improving the quality of life for many terminally ill children, easing stress on the family and, in some cases, sustaining hope for a cure. But the arrangement can contribute to a painful dilemma for parents like Ms. Pinedo and Mr. Martinez, who are torn between their fierce commitment to their son and the futility of knowing that his condition leaves him with no future worth hoping for.
“We could lose a life, but if he continues to live this way, we’ll lose three,” said Ms. Pinedo. “There’s no quality of life for him or for us.”
Aaron’s doctors now say he could conceivably live for years. His body hasn’t stopped growing since he was born. He’s in the 96th percentile for height for his age, and his weight is about average.
His parents have talked about “graduating” him from hospice. But he is never stable for long, and they welcome the visits from their hospice team. The seizures, sometimes 30 a day, are a persistent assault on his brain and, as he grows, the medications intended to control them must be changed or the doses recalibrated. He is at continual risk of gastrointestinal problems and potentially deadly fluid buildup in his lungs.
Ms. Pinedo, who works from home for a nonprofit public health organization, spends much of her time with Aaron, while Mr. Martinez works as a landscaper. She has chosen to live in the moment, she said, because otherwise her mind wanders to a future in which either “he could die – or he won’t, and I’ll end up changing the diapers of a 40-year-old man.” Either of those “are going to suck.”
While cancer is one of the major illnesses afflicting children in hospice, many others, like Aaron, have rare congenital defects, severe neurological impairments, or uncommon metabolic deficiencies.
“We have diseases that families tell us are 1 of 10 cases in the world,” said Glen Komatsu, MD, medical director of Torrance, Calif.–based TrinityKids Care, which provides home hospice services to Aaron and more than 70 other kids in Los Angeles and Orange counties.
In the years leading up to the ACA’s implementation, pediatric health advocates lobbied hard for the concurrent care provision. Without the possibility of life-extending care or hope for a cure, many parents refused to put their terminally ill kids in hospice, thinking it was tantamount to giving up on them. That meant the whole family missed out on the support hospice can provide, not just pain relief and comfort for the dying child, but emotional and spiritual care for parents and siblings under extreme duress.
TrinityKids Care, run by the large national Catholic health system Providence, doesn’t just send nurses, social workers, and chaplains into homes. For patients able to participate, and their siblings, it also offers art and science projects, exercise classes, movies, and music. During the pandemic, these activities have been conducted via Zoom, and volunteers deliver needed supplies to the children’s homes.
The ability to get treatments that prolong their lives is a major reason children in concurrent care are more likely than adults to outlive the 6-months-to-live diagnosis required for hospice.
“Concurrent care, by its very intention, very clearly is going to extend their lives, and by extending their lives they’re no longer going to be hospice-eligible if you use the 6-month life expectancy criteria,” said David Steinhorn, MD, a pediatric intensive care physician in Virginia, who has helped develop numerous children’s hospice programs across the United States.
Another factor is that kids, even sick ones, are simply more robust than many older people.
“Sick kids are often otherwise healthy, except for one organ,” said Debra Lotstein, MD, chief of the division of comfort and palliative care at Children’s Hospital Los Angeles. “They may have cancer in their body, but their hearts are good and their lungs are good, compared to a 90-year-old who at baseline is just not as resilient.”
All of Aaron Martinez’s vital organs, except for his brain, seem to be working. “There have been times when we’ve brought him in, and the nurse looks at the chart and looks at him, and she can’t believe it’s that child,” said Mr. Martinez.
When kids live past the 6-month life expectancy, they must be recertified to stay in hospice. In many cases, Dr. Steinhorn said, he is willing to recertify his pediatric patients indefinitely.
Even with doctors advocating for them, it’s not always easy for children to get into hospice care. Most hospices care primarily for adults and are reluctant to take kids.
“The hospice will say: ‘We don’t have the capacity to treat children. Our nurses aren’t trained. It’s different. We just can’t do it,’ ” said Lori Butterworth, cofounder of the Children’s Hospice and Palliative Care Coalition of California in Watsonville. “The other reason is not wanting to, because it’s existentially devastating and sad and hard.”
Finances also play a role. Home hospice care is paid at a per diem rate set by Medicare – slightly over $200 a day for the first 2 months, about $161 a day after that – and it is typically the same for kids and adults. Children, particularly those with rare conditions, often require more intensive and innovative care, so the per diem doesn’t stretch as far.
The concurrent care provision has made taking pediatric patients more viable for hospice organizations, Dr. Steinhorn and others said. Under the ACA, many of the expenses for certain medications and medical services can be shifted to the patient’s primary insurance, leaving hospices responsible for pain relief and comfort care.
Even so, the relatively small number of kids who die each year from protracted ailments hardly makes pediatric hospice an appealing line of business in an industry craving growth, especially one in which private equity investors are active and seeking a big payday.
In California, only 21 of 1,336 hospices reported having a specialized pediatric hospice program, and 59 said they served at least one patient under age 21, according to an analysis of 2020 state data by Cordt Kassner, CEO of Hospice Analytics in Colorado Springs.
Hospice providers that do cater to children often face a more basic challenge: Even with the possibility of concurrent care, many parents still equate hospice with acceptance of death. That was the case initially for Matt and Reese Sonnen, Los Angeles residents whose daughter, Layla, was born with a seizure disorder that had no name: Her brain had simply failed to develop in the womb, and an MRI showed “fluid taking up space where the brain wasn’t,” her mother said.
When Layla’s team first mentioned hospice, “I was in the car on my phone, and I almost crashed the car,” Mrs. Sonnen recalled. “The first thought that came to mind was: ‘It is just the end,’ but we felt she was nowhere near it, because she was strong, she was mighty. She was my little girl. She was going to get through this.”
About 3 months later, as Layla’s nervous system deteriorated, causing her to writhe in pain, her parents agreed to enroll her in hospice with TrinityKids Care. She died weeks later, not long after her second birthday. She was in her mother’s arms, with Mr. Sonnen close by.
“All of a sudden, Layla breathed out a big rush of air. The nurse looked at me and said: ‘That was her last breath.’ I was literally breathing in her last breath,” Mrs. Sonnen recounted. “I never wanted to breathe again, because now I felt I had her in my lungs. Don’t make me laugh, don’t make me exhale.”
Layla’s parents have no regrets about their decision to put her in hospice. “It was the absolute right decision, and in hindsight we should have done it sooner,” Mr. Sonnen said. “She was suffering, and we had blinders on.”
Ms. Pinedo said she is “infinitely grateful” for hospice, despite the heartache of Aaron’s condition. Sometimes the social worker will stop by, she said, just to say hello and drop off a latte, a small gesture that can feel very uplifting. “They’ve been our lifeline,” she said.
Ms. Pinedo talks about a friend of hers with a healthy baby, also named Aaron, who is pregnant with her second child. “All the stuff that was on our list, they’re living. And I love them dearly. But it’s almost hard to look, because it’s like looking at the stuff that you didn’t get. It’s like Christmas Day, staring through the window at the neighbor’s house, and you’re sitting there in the cold.”
Yet she seems palpably torn between that bleak remorse and the unconditional love parents feel toward their children. At one point, Ms. Pinedo interrupted herself midsentence and turned to her son, who was in Mr. Martinez’s arms: “Yes, Papi, you are so stinking cute, and you are still my dream come true.”
This story was produced by KHN, which publishes California Healthline, an editorially independent service of the California Health Care Foundation. KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
FDA OKs sodium thiosulfate injection to reduce ototoxicity risk in children with cancer
This approval makes sodium thiosulfate the first and only treatment FDA-approved in this area.
“Historically, there have been no approved treatments for preventing cisplatin-induced hearing loss,” said David R. Freyer, DO, of Children’s Hospital Los Angeles and primary investigator of one of the two trials, COG ACCL0431. The FDA’s approval “addresses an enormous unmet need for many children and young adults.”
The approval was based on safety and efficacy data from two multicenter open-label, randomized controlled phase 3 trials – SIOPEL 6 and COG ACCL0431 – comparing sodium thiosulfate plus a cisplatin-based regimen to a cisplatin-based regimen alone in pediatric patients. SIOPEL 6 included patients with standard risk hepatoblastoma, and COG ACCL0431 included pediatric patients with solid tumors.
In both studies, the incidence of hearing loss was significantly lower in the sodium thiosulfate group, compared with the cisplatin-only group. In SIOPEL 6, hearing loss of grade 1 or higher occurred in 33% of children (18 of 55) in the cisplatin–sodium thiosulfate group and 63% (29 of 46) in the cisplatin-only group, indicating a 48% lower incidence of hearing loss for those receiving sodium thiosulfate. In COG ACCL0431, hearing loss was identified in 28.6% of patients (14 of 49) receiving sodium thiosulfate, compared with 56.4% (31 of 55) in the control group, indicating a 69% lower risk for hearing loss in the sodium thiosulfate group.
The FDA reported the same overall trend but highlighted slightly different figures. In SIOPEL 6, hearing loss incidence occurred in 39% of patients (24 of 61) in the sodium thiosulfate arm versus 68% (36 of 53) in the control group; in COG ACCL0431, hearing loss incidence occurred among 44% of patients (17 of 39) in the sodium thiosulfate group versus 58% (22 of 38) in the control group.
The recommended dose is based on surface area according to body weight. Sodium thiosulfate is administered as an intravenous infusion over 15 minutes following cisplatin infusions that are 1 to 6 hours in duration.
Serious adverse reactions occurred in 40% of patients who received cisplatin–sodium thiosulfate in SIOPEL 6 and 36% of these patients in COG ACCL0431. The most common adverse reactions in the trials included vomiting, infection, nausea, decreased hemoglobin, hypernatremia, and hypokalemia.
A version of this article first appeared on Medscape.com.
This approval makes sodium thiosulfate the first and only treatment FDA-approved in this area.
“Historically, there have been no approved treatments for preventing cisplatin-induced hearing loss,” said David R. Freyer, DO, of Children’s Hospital Los Angeles and primary investigator of one of the two trials, COG ACCL0431. The FDA’s approval “addresses an enormous unmet need for many children and young adults.”
The approval was based on safety and efficacy data from two multicenter open-label, randomized controlled phase 3 trials – SIOPEL 6 and COG ACCL0431 – comparing sodium thiosulfate plus a cisplatin-based regimen to a cisplatin-based regimen alone in pediatric patients. SIOPEL 6 included patients with standard risk hepatoblastoma, and COG ACCL0431 included pediatric patients with solid tumors.
In both studies, the incidence of hearing loss was significantly lower in the sodium thiosulfate group, compared with the cisplatin-only group. In SIOPEL 6, hearing loss of grade 1 or higher occurred in 33% of children (18 of 55) in the cisplatin–sodium thiosulfate group and 63% (29 of 46) in the cisplatin-only group, indicating a 48% lower incidence of hearing loss for those receiving sodium thiosulfate. In COG ACCL0431, hearing loss was identified in 28.6% of patients (14 of 49) receiving sodium thiosulfate, compared with 56.4% (31 of 55) in the control group, indicating a 69% lower risk for hearing loss in the sodium thiosulfate group.
The FDA reported the same overall trend but highlighted slightly different figures. In SIOPEL 6, hearing loss incidence occurred in 39% of patients (24 of 61) in the sodium thiosulfate arm versus 68% (36 of 53) in the control group; in COG ACCL0431, hearing loss incidence occurred among 44% of patients (17 of 39) in the sodium thiosulfate group versus 58% (22 of 38) in the control group.
The recommended dose is based on surface area according to body weight. Sodium thiosulfate is administered as an intravenous infusion over 15 minutes following cisplatin infusions that are 1 to 6 hours in duration.
Serious adverse reactions occurred in 40% of patients who received cisplatin–sodium thiosulfate in SIOPEL 6 and 36% of these patients in COG ACCL0431. The most common adverse reactions in the trials included vomiting, infection, nausea, decreased hemoglobin, hypernatremia, and hypokalemia.
A version of this article first appeared on Medscape.com.
This approval makes sodium thiosulfate the first and only treatment FDA-approved in this area.
“Historically, there have been no approved treatments for preventing cisplatin-induced hearing loss,” said David R. Freyer, DO, of Children’s Hospital Los Angeles and primary investigator of one of the two trials, COG ACCL0431. The FDA’s approval “addresses an enormous unmet need for many children and young adults.”
The approval was based on safety and efficacy data from two multicenter open-label, randomized controlled phase 3 trials – SIOPEL 6 and COG ACCL0431 – comparing sodium thiosulfate plus a cisplatin-based regimen to a cisplatin-based regimen alone in pediatric patients. SIOPEL 6 included patients with standard risk hepatoblastoma, and COG ACCL0431 included pediatric patients with solid tumors.
In both studies, the incidence of hearing loss was significantly lower in the sodium thiosulfate group, compared with the cisplatin-only group. In SIOPEL 6, hearing loss of grade 1 or higher occurred in 33% of children (18 of 55) in the cisplatin–sodium thiosulfate group and 63% (29 of 46) in the cisplatin-only group, indicating a 48% lower incidence of hearing loss for those receiving sodium thiosulfate. In COG ACCL0431, hearing loss was identified in 28.6% of patients (14 of 49) receiving sodium thiosulfate, compared with 56.4% (31 of 55) in the control group, indicating a 69% lower risk for hearing loss in the sodium thiosulfate group.
The FDA reported the same overall trend but highlighted slightly different figures. In SIOPEL 6, hearing loss incidence occurred in 39% of patients (24 of 61) in the sodium thiosulfate arm versus 68% (36 of 53) in the control group; in COG ACCL0431, hearing loss incidence occurred among 44% of patients (17 of 39) in the sodium thiosulfate group versus 58% (22 of 38) in the control group.
The recommended dose is based on surface area according to body weight. Sodium thiosulfate is administered as an intravenous infusion over 15 minutes following cisplatin infusions that are 1 to 6 hours in duration.
Serious adverse reactions occurred in 40% of patients who received cisplatin–sodium thiosulfate in SIOPEL 6 and 36% of these patients in COG ACCL0431. The most common adverse reactions in the trials included vomiting, infection, nausea, decreased hemoglobin, hypernatremia, and hypokalemia.
A version of this article first appeared on Medscape.com.
FDA warns against cooking chicken in NyQuil
Called the “sleepy chicken challenge,” the trend tells people to cook chicken in NyQuil or similar over-the-counter cough and cold medications, which include ingredients such as acetaminophen, dextromethorphan, and doxylamine.
“The challenge sounds silly and unappetizing – and it is. But it could also be very unsafe,” the FDA said. “Boiling a medication can make it much more concentrated and change its properties in other ways.”
Even if someone doesn’t plan to eat the chicken, inhaling the vapors of the medication while it cooks could cause high levels of the drug to enter the body.
“It could also hurt your lungs,” the FDA said. “Put simply: Someone could take a dangerously high amount of the cough and cold medicine without even realizing it.”
This isn’t the first time that social media challenges involving medicine have gone viral. In a 2020 TikTok challenge, people were encouraged to take large doses of the allergy medicine diphenhydramine, called the “Benadryl challenge,” to cause hallucinations. The FDA received several reports of teens who were hospitalized or died, and it issued a warning about taking high doses of the drug.
“These video challenges, which often target youths, can harm people – and even cause death,” the FDA said. “Nonprescription (also called over-the-counter or OTC) drugs are readily available in many homes, making these challenges even more risky.”
In the latest warning, the FDA provided several ways for parents to make it less likely for children to do the social media challenges, such as locking up prescription and over-the-counter medications to prevent accidental overdoses. The FDA also encouraged parents and guardians to have open conversations with their children.
“Sit down with your children and discuss the dangers of misusing drugs and how social media trends can lead to real, sometimes irreversible, damage,” the FDA said. “Remind your children that overdoses can occur with OTC drugs as well as with prescription drugs.”
Following the FDA warning, the American Academy of Pediatrics also issued an advisory about social media trends. Some challenges, such as the ALS ice bucket challenge or the mannequin challenge, can be fun and positive activities. But medication-related challenges, such as the sleepy chicken and Benadryl challenges, can cause serious heart problems, seizures, coma, and even death.
“Teens’ brains are still developing. The part of the brain that handles rational thought, the prefrontal cortex, is not fully developed until the mid-20s,” the American Academy of Pediatrics said. “This means teens are naturally more impulsive and likely to act before thinking through all of the ramifications.”
Social media rewards outrageous behavior, it wrote, and the more outrageous the behavior, the more likely someone will get more engagement online.
“It’s a quick moving, impulsive environment, and the fear of losing out is real for teens,” the academy said. “What they will focus on is that a popular kid in class did this and got hundreds of likes and comments.”
The academy suggested that parents and guardians talk with teens about which challenges are trending on social media and at school.
“Sometimes kids are more willing to talk about their peers than themselves,” it said. “Asking questions about school trends, friends and fads may yield more answers than direct questions about their own activities.”
A version of this article first appeared on WebMD.com.
Called the “sleepy chicken challenge,” the trend tells people to cook chicken in NyQuil or similar over-the-counter cough and cold medications, which include ingredients such as acetaminophen, dextromethorphan, and doxylamine.
“The challenge sounds silly and unappetizing – and it is. But it could also be very unsafe,” the FDA said. “Boiling a medication can make it much more concentrated and change its properties in other ways.”
Even if someone doesn’t plan to eat the chicken, inhaling the vapors of the medication while it cooks could cause high levels of the drug to enter the body.
“It could also hurt your lungs,” the FDA said. “Put simply: Someone could take a dangerously high amount of the cough and cold medicine without even realizing it.”
This isn’t the first time that social media challenges involving medicine have gone viral. In a 2020 TikTok challenge, people were encouraged to take large doses of the allergy medicine diphenhydramine, called the “Benadryl challenge,” to cause hallucinations. The FDA received several reports of teens who were hospitalized or died, and it issued a warning about taking high doses of the drug.
“These video challenges, which often target youths, can harm people – and even cause death,” the FDA said. “Nonprescription (also called over-the-counter or OTC) drugs are readily available in many homes, making these challenges even more risky.”
In the latest warning, the FDA provided several ways for parents to make it less likely for children to do the social media challenges, such as locking up prescription and over-the-counter medications to prevent accidental overdoses. The FDA also encouraged parents and guardians to have open conversations with their children.
“Sit down with your children and discuss the dangers of misusing drugs and how social media trends can lead to real, sometimes irreversible, damage,” the FDA said. “Remind your children that overdoses can occur with OTC drugs as well as with prescription drugs.”
Following the FDA warning, the American Academy of Pediatrics also issued an advisory about social media trends. Some challenges, such as the ALS ice bucket challenge or the mannequin challenge, can be fun and positive activities. But medication-related challenges, such as the sleepy chicken and Benadryl challenges, can cause serious heart problems, seizures, coma, and even death.
“Teens’ brains are still developing. The part of the brain that handles rational thought, the prefrontal cortex, is not fully developed until the mid-20s,” the American Academy of Pediatrics said. “This means teens are naturally more impulsive and likely to act before thinking through all of the ramifications.”
Social media rewards outrageous behavior, it wrote, and the more outrageous the behavior, the more likely someone will get more engagement online.
“It’s a quick moving, impulsive environment, and the fear of losing out is real for teens,” the academy said. “What they will focus on is that a popular kid in class did this and got hundreds of likes and comments.”
The academy suggested that parents and guardians talk with teens about which challenges are trending on social media and at school.
“Sometimes kids are more willing to talk about their peers than themselves,” it said. “Asking questions about school trends, friends and fads may yield more answers than direct questions about their own activities.”
A version of this article first appeared on WebMD.com.
Called the “sleepy chicken challenge,” the trend tells people to cook chicken in NyQuil or similar over-the-counter cough and cold medications, which include ingredients such as acetaminophen, dextromethorphan, and doxylamine.
“The challenge sounds silly and unappetizing – and it is. But it could also be very unsafe,” the FDA said. “Boiling a medication can make it much more concentrated and change its properties in other ways.”
Even if someone doesn’t plan to eat the chicken, inhaling the vapors of the medication while it cooks could cause high levels of the drug to enter the body.
“It could also hurt your lungs,” the FDA said. “Put simply: Someone could take a dangerously high amount of the cough and cold medicine without even realizing it.”
This isn’t the first time that social media challenges involving medicine have gone viral. In a 2020 TikTok challenge, people were encouraged to take large doses of the allergy medicine diphenhydramine, called the “Benadryl challenge,” to cause hallucinations. The FDA received several reports of teens who were hospitalized or died, and it issued a warning about taking high doses of the drug.
“These video challenges, which often target youths, can harm people – and even cause death,” the FDA said. “Nonprescription (also called over-the-counter or OTC) drugs are readily available in many homes, making these challenges even more risky.”
In the latest warning, the FDA provided several ways for parents to make it less likely for children to do the social media challenges, such as locking up prescription and over-the-counter medications to prevent accidental overdoses. The FDA also encouraged parents and guardians to have open conversations with their children.
“Sit down with your children and discuss the dangers of misusing drugs and how social media trends can lead to real, sometimes irreversible, damage,” the FDA said. “Remind your children that overdoses can occur with OTC drugs as well as with prescription drugs.”
Following the FDA warning, the American Academy of Pediatrics also issued an advisory about social media trends. Some challenges, such as the ALS ice bucket challenge or the mannequin challenge, can be fun and positive activities. But medication-related challenges, such as the sleepy chicken and Benadryl challenges, can cause serious heart problems, seizures, coma, and even death.
“Teens’ brains are still developing. The part of the brain that handles rational thought, the prefrontal cortex, is not fully developed until the mid-20s,” the American Academy of Pediatrics said. “This means teens are naturally more impulsive and likely to act before thinking through all of the ramifications.”
Social media rewards outrageous behavior, it wrote, and the more outrageous the behavior, the more likely someone will get more engagement online.
“It’s a quick moving, impulsive environment, and the fear of losing out is real for teens,” the academy said. “What they will focus on is that a popular kid in class did this and got hundreds of likes and comments.”
The academy suggested that parents and guardians talk with teens about which challenges are trending on social media and at school.
“Sometimes kids are more willing to talk about their peers than themselves,” it said. “Asking questions about school trends, friends and fads may yield more answers than direct questions about their own activities.”
A version of this article first appeared on WebMD.com.
A 10-year-old with a red bump on her lower lip
The patient’s history and examination are consistent with a diagnosis of pyogenic granuloma. Specifically, the history of rapid growth, friable nature, associated bleeding, and hemorrhagic crusting point to pyogenic granuloma as the most likely diagnosis.
Pyogenic granuloma is an acquired benign vascular growth of the skin or mucous membranes.1 It most frequently occurs in children and young adults and most commonly affects the skin of the head, trunk, and extremities.2 Common mucosal sites include the gingiva, lips, and tongue.2 The etiology of pyogenic granuloma is unknown, though it is thought to be a process akin to the overgrowth of granulation tissue.3,4 Expression of angiogenic factors and subsequent vascular hyperplasia are also implicated as key players in the pathogenesis of pyogenic granuloma.1,4 In addition, several associated factors and inciting triggers have been proposed including trauma, infections, and hormonal fluctuations.3-5 However, the majority of patients do not report predisposing factors or a history of prior trauma at the site.3,6
Clinically, pyogenic granuloma usually presents as a painless, erythematous, dome-shaped friable papule or nodule that easily bleeds and may ulcerate. It typically undergoes a period of growth over weeks to months followed by stabilization. Occasionally, pyogenic granulomas will spontaneously involute, though most do not.7 Pyogenic granuloma may occur within an existing capillary malformation, such as a port wine stain, spontaneously or as a sequela of laser treatment.8,9 Diagnosis of pyogenic granuloma can typically be made clinically on the basis of history and exam. Dermoscopic evaluation of pyogenic granuloma will reveal a homogeneous papule with a surrounding white-brown collarette, and potentially white intersecting lines.10 Histopathologic evaluation may be necessary to differentiate lesions from conditions that may mimic pyogenic granuloma.
What’s on the differential?
The differential diagnosis for pyogenic granuloma consists of Spitz nevus, cherry hemangioma, amelanotic melanoma, and glomus tumor.
Spitz nevus
Spitz nevus (spindle and epithelial cell nevus) is a benign melanocytic lesion that classically appears as a sharply circumscribed, smooth, dome-shaped, pink-red, or brown papule or plaque. There is typically a history of rapid growth over several months followed by stabilization. It usually presents in childhood or adolescence and is most commonly located on the face and extremities. While there are similarities in the appearance of Spitz nevi and pyogenic granuloma, Spitz nevi are not usually friable nor associated with bleeding as in our patient. Furthermore, on dermoscopy, Spitz nevus typically exhibits a starburst pattern with regularly distributed dotted vessels, or a peripheral globular pattern with reticular depigmentation. The definitive diagnosis of Spitz nevi relies on histopathologic evaluation, which is critical for discriminating Spitz nevi from melanoma.
Cherry hemangioma
Cherry angiomas are the most common type of acquired benign vascular proliferation. They present as small, bright red or violaceous macules or papules. However, they typically appear in early to midadulthood and increase in number with age. The age of our patient and solitary presentation of the lesion make this diagnosis unlikely. In addition, cherry angiomas are not usually associated with bleeding. It is important to note that, depending on the age of the patient, pyogenic granuloma may also be confused with infantile hemangioma. Infantile hemangiomas may become bright red papules, nodules, or plaques that appear in early infancy. They characteristically involute, which does not typically happen with pyogenic granuloma.
Amelanotic melanoma
Amelanotic melanoma is an uncommon variant of melanoma with little to no pigmentation. It may appear as a skin-colored to light-brown, pink, or red macule, papule, or nodule. The lesion may be asymmetric with irregular and well-defined borders. The variable and uncharacteristic appearance of this melanoma variant makes it diagnostically challenging and it is often confused with benign lesions including pyogenic granuloma. Dermoscopy can help distinguish amelanotic melanoma from other benign conditions, and will reveal areas of pink to white, polymorphous vessels and crystalline structures. However, ultimately biopsy and histopathological evaluation is necessary for accurate diagnosis.
Glomus tumor
Glomus tumors are rare, benign neoplasms originating from cells of the glomus body that presents as a red-purple, vascular papule or nodule. They are usually found in areas rich in glomus bodies, such as the subungual regions, fingertips, palms, wrists, and forearms. Glomus tumors are typically associated with tenderness, paroxysmal pain, and cold sensitivity. They do not bleed or ulcerate. While pyogenic granuloma may be confused for glomus tumor when present on the fingers or extremities, the location of the lesion in our patient is not consistent with a diagnosis of glomus tumor.
Management and disease course
Management with procedural or topical interventions is usually pursued for pyogenic granuloma because of frequent bleeding and ulceration of lesions. The most common approach is simple excision by a scoop or shave technique, with or without curettage and most commonly with electrocautery of the base. Other options include full-thickness excision, destruction with laser therapy, cryotherapy, or topical treatments (for example, timolol).11 Lesion recurrence can occur with both surgical and nonsurgical management.11 Regardless of management technique, it is useful to obtain histopathologic evaluation of tissue for accurate diagnosis.
Our patient underwent surgical destruction of her lower-lip lesion with shave excision followed by electrocautery. The surgical specimen was sent for pathology, which confirmed the diagnosis of pyogenic granuloma. The patient experienced no complications from the procedure and did not have recurrence of the lesion.
Ms. Sui is a research associate in the department of dermatology, division of pediatric and adolescent dermatology, University of California, San Diego, and Rady Children’s Hospital, San Diego. Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Neither Ms. Sui nor Dr. Eichenfield have any relevant financial disclosures.
References
1. Lin RL and Janniger CK. Cutis. 2004 Oct;74(4):229-33.
2. Harris MN et al. J Am Acad Dermatol. 2000 Jun;42(6):1012-6.
3. Pagliai KA and Cohen BA. Pediatr Dermatol. 2004 Jan-Feb;21(1):10-3.
4. Kamal R et al. J Oral Maxillofac Pathol. 2012 Jan;16(1):79-82.
5. Requena L and Sangueza OP. J Am Acad Dermatol. 1997 Dec;37(6):887-919.
6. Patrice SJ et al. Pediatr Dermatol. 1991 Dec;8(4):267-76.
7. Luba MC et al. Am Fam Physician. 2003 Feb 15;67(4):729-38.
8. Swerlick RA and Cooper PH. J Am Acad Dermatol. 1983 May;8(5):627-30.
9. Sheehan DJ and Lesher JL Jr. Cutis. 2004 Mar;73(3):175-80.
10. Zaballos P et al. Br J Dermatol. 2006 Jun;154(6):1108-11.
11. Lee J et al. J Plast Reconstr Aesthet Surg. 2011 Sep;64(9):1216-20. .
The patient’s history and examination are consistent with a diagnosis of pyogenic granuloma. Specifically, the history of rapid growth, friable nature, associated bleeding, and hemorrhagic crusting point to pyogenic granuloma as the most likely diagnosis.
Pyogenic granuloma is an acquired benign vascular growth of the skin or mucous membranes.1 It most frequently occurs in children and young adults and most commonly affects the skin of the head, trunk, and extremities.2 Common mucosal sites include the gingiva, lips, and tongue.2 The etiology of pyogenic granuloma is unknown, though it is thought to be a process akin to the overgrowth of granulation tissue.3,4 Expression of angiogenic factors and subsequent vascular hyperplasia are also implicated as key players in the pathogenesis of pyogenic granuloma.1,4 In addition, several associated factors and inciting triggers have been proposed including trauma, infections, and hormonal fluctuations.3-5 However, the majority of patients do not report predisposing factors or a history of prior trauma at the site.3,6
Clinically, pyogenic granuloma usually presents as a painless, erythematous, dome-shaped friable papule or nodule that easily bleeds and may ulcerate. It typically undergoes a period of growth over weeks to months followed by stabilization. Occasionally, pyogenic granulomas will spontaneously involute, though most do not.7 Pyogenic granuloma may occur within an existing capillary malformation, such as a port wine stain, spontaneously or as a sequela of laser treatment.8,9 Diagnosis of pyogenic granuloma can typically be made clinically on the basis of history and exam. Dermoscopic evaluation of pyogenic granuloma will reveal a homogeneous papule with a surrounding white-brown collarette, and potentially white intersecting lines.10 Histopathologic evaluation may be necessary to differentiate lesions from conditions that may mimic pyogenic granuloma.
What’s on the differential?
The differential diagnosis for pyogenic granuloma consists of Spitz nevus, cherry hemangioma, amelanotic melanoma, and glomus tumor.
Spitz nevus
Spitz nevus (spindle and epithelial cell nevus) is a benign melanocytic lesion that classically appears as a sharply circumscribed, smooth, dome-shaped, pink-red, or brown papule or plaque. There is typically a history of rapid growth over several months followed by stabilization. It usually presents in childhood or adolescence and is most commonly located on the face and extremities. While there are similarities in the appearance of Spitz nevi and pyogenic granuloma, Spitz nevi are not usually friable nor associated with bleeding as in our patient. Furthermore, on dermoscopy, Spitz nevus typically exhibits a starburst pattern with regularly distributed dotted vessels, or a peripheral globular pattern with reticular depigmentation. The definitive diagnosis of Spitz nevi relies on histopathologic evaluation, which is critical for discriminating Spitz nevi from melanoma.
Cherry hemangioma
Cherry angiomas are the most common type of acquired benign vascular proliferation. They present as small, bright red or violaceous macules or papules. However, they typically appear in early to midadulthood and increase in number with age. The age of our patient and solitary presentation of the lesion make this diagnosis unlikely. In addition, cherry angiomas are not usually associated with bleeding. It is important to note that, depending on the age of the patient, pyogenic granuloma may also be confused with infantile hemangioma. Infantile hemangiomas may become bright red papules, nodules, or plaques that appear in early infancy. They characteristically involute, which does not typically happen with pyogenic granuloma.
Amelanotic melanoma
Amelanotic melanoma is an uncommon variant of melanoma with little to no pigmentation. It may appear as a skin-colored to light-brown, pink, or red macule, papule, or nodule. The lesion may be asymmetric with irregular and well-defined borders. The variable and uncharacteristic appearance of this melanoma variant makes it diagnostically challenging and it is often confused with benign lesions including pyogenic granuloma. Dermoscopy can help distinguish amelanotic melanoma from other benign conditions, and will reveal areas of pink to white, polymorphous vessels and crystalline structures. However, ultimately biopsy and histopathological evaluation is necessary for accurate diagnosis.
Glomus tumor
Glomus tumors are rare, benign neoplasms originating from cells of the glomus body that presents as a red-purple, vascular papule or nodule. They are usually found in areas rich in glomus bodies, such as the subungual regions, fingertips, palms, wrists, and forearms. Glomus tumors are typically associated with tenderness, paroxysmal pain, and cold sensitivity. They do not bleed or ulcerate. While pyogenic granuloma may be confused for glomus tumor when present on the fingers or extremities, the location of the lesion in our patient is not consistent with a diagnosis of glomus tumor.
Management and disease course
Management with procedural or topical interventions is usually pursued for pyogenic granuloma because of frequent bleeding and ulceration of lesions. The most common approach is simple excision by a scoop or shave technique, with or without curettage and most commonly with electrocautery of the base. Other options include full-thickness excision, destruction with laser therapy, cryotherapy, or topical treatments (for example, timolol).11 Lesion recurrence can occur with both surgical and nonsurgical management.11 Regardless of management technique, it is useful to obtain histopathologic evaluation of tissue for accurate diagnosis.
Our patient underwent surgical destruction of her lower-lip lesion with shave excision followed by electrocautery. The surgical specimen was sent for pathology, which confirmed the diagnosis of pyogenic granuloma. The patient experienced no complications from the procedure and did not have recurrence of the lesion.
Ms. Sui is a research associate in the department of dermatology, division of pediatric and adolescent dermatology, University of California, San Diego, and Rady Children’s Hospital, San Diego. Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Neither Ms. Sui nor Dr. Eichenfield have any relevant financial disclosures.
References
1. Lin RL and Janniger CK. Cutis. 2004 Oct;74(4):229-33.
2. Harris MN et al. J Am Acad Dermatol. 2000 Jun;42(6):1012-6.
3. Pagliai KA and Cohen BA. Pediatr Dermatol. 2004 Jan-Feb;21(1):10-3.
4. Kamal R et al. J Oral Maxillofac Pathol. 2012 Jan;16(1):79-82.
5. Requena L and Sangueza OP. J Am Acad Dermatol. 1997 Dec;37(6):887-919.
6. Patrice SJ et al. Pediatr Dermatol. 1991 Dec;8(4):267-76.
7. Luba MC et al. Am Fam Physician. 2003 Feb 15;67(4):729-38.
8. Swerlick RA and Cooper PH. J Am Acad Dermatol. 1983 May;8(5):627-30.
9. Sheehan DJ and Lesher JL Jr. Cutis. 2004 Mar;73(3):175-80.
10. Zaballos P et al. Br J Dermatol. 2006 Jun;154(6):1108-11.
11. Lee J et al. J Plast Reconstr Aesthet Surg. 2011 Sep;64(9):1216-20. .
The patient’s history and examination are consistent with a diagnosis of pyogenic granuloma. Specifically, the history of rapid growth, friable nature, associated bleeding, and hemorrhagic crusting point to pyogenic granuloma as the most likely diagnosis.
Pyogenic granuloma is an acquired benign vascular growth of the skin or mucous membranes.1 It most frequently occurs in children and young adults and most commonly affects the skin of the head, trunk, and extremities.2 Common mucosal sites include the gingiva, lips, and tongue.2 The etiology of pyogenic granuloma is unknown, though it is thought to be a process akin to the overgrowth of granulation tissue.3,4 Expression of angiogenic factors and subsequent vascular hyperplasia are also implicated as key players in the pathogenesis of pyogenic granuloma.1,4 In addition, several associated factors and inciting triggers have been proposed including trauma, infections, and hormonal fluctuations.3-5 However, the majority of patients do not report predisposing factors or a history of prior trauma at the site.3,6
Clinically, pyogenic granuloma usually presents as a painless, erythematous, dome-shaped friable papule or nodule that easily bleeds and may ulcerate. It typically undergoes a period of growth over weeks to months followed by stabilization. Occasionally, pyogenic granulomas will spontaneously involute, though most do not.7 Pyogenic granuloma may occur within an existing capillary malformation, such as a port wine stain, spontaneously or as a sequela of laser treatment.8,9 Diagnosis of pyogenic granuloma can typically be made clinically on the basis of history and exam. Dermoscopic evaluation of pyogenic granuloma will reveal a homogeneous papule with a surrounding white-brown collarette, and potentially white intersecting lines.10 Histopathologic evaluation may be necessary to differentiate lesions from conditions that may mimic pyogenic granuloma.
What’s on the differential?
The differential diagnosis for pyogenic granuloma consists of Spitz nevus, cherry hemangioma, amelanotic melanoma, and glomus tumor.
Spitz nevus
Spitz nevus (spindle and epithelial cell nevus) is a benign melanocytic lesion that classically appears as a sharply circumscribed, smooth, dome-shaped, pink-red, or brown papule or plaque. There is typically a history of rapid growth over several months followed by stabilization. It usually presents in childhood or adolescence and is most commonly located on the face and extremities. While there are similarities in the appearance of Spitz nevi and pyogenic granuloma, Spitz nevi are not usually friable nor associated with bleeding as in our patient. Furthermore, on dermoscopy, Spitz nevus typically exhibits a starburst pattern with regularly distributed dotted vessels, or a peripheral globular pattern with reticular depigmentation. The definitive diagnosis of Spitz nevi relies on histopathologic evaluation, which is critical for discriminating Spitz nevi from melanoma.
Cherry hemangioma
Cherry angiomas are the most common type of acquired benign vascular proliferation. They present as small, bright red or violaceous macules or papules. However, they typically appear in early to midadulthood and increase in number with age. The age of our patient and solitary presentation of the lesion make this diagnosis unlikely. In addition, cherry angiomas are not usually associated with bleeding. It is important to note that, depending on the age of the patient, pyogenic granuloma may also be confused with infantile hemangioma. Infantile hemangiomas may become bright red papules, nodules, or plaques that appear in early infancy. They characteristically involute, which does not typically happen with pyogenic granuloma.
Amelanotic melanoma
Amelanotic melanoma is an uncommon variant of melanoma with little to no pigmentation. It may appear as a skin-colored to light-brown, pink, or red macule, papule, or nodule. The lesion may be asymmetric with irregular and well-defined borders. The variable and uncharacteristic appearance of this melanoma variant makes it diagnostically challenging and it is often confused with benign lesions including pyogenic granuloma. Dermoscopy can help distinguish amelanotic melanoma from other benign conditions, and will reveal areas of pink to white, polymorphous vessels and crystalline structures. However, ultimately biopsy and histopathological evaluation is necessary for accurate diagnosis.
Glomus tumor
Glomus tumors are rare, benign neoplasms originating from cells of the glomus body that presents as a red-purple, vascular papule or nodule. They are usually found in areas rich in glomus bodies, such as the subungual regions, fingertips, palms, wrists, and forearms. Glomus tumors are typically associated with tenderness, paroxysmal pain, and cold sensitivity. They do not bleed or ulcerate. While pyogenic granuloma may be confused for glomus tumor when present on the fingers or extremities, the location of the lesion in our patient is not consistent with a diagnosis of glomus tumor.
Management and disease course
Management with procedural or topical interventions is usually pursued for pyogenic granuloma because of frequent bleeding and ulceration of lesions. The most common approach is simple excision by a scoop or shave technique, with or without curettage and most commonly with electrocautery of the base. Other options include full-thickness excision, destruction with laser therapy, cryotherapy, or topical treatments (for example, timolol).11 Lesion recurrence can occur with both surgical and nonsurgical management.11 Regardless of management technique, it is useful to obtain histopathologic evaluation of tissue for accurate diagnosis.
Our patient underwent surgical destruction of her lower-lip lesion with shave excision followed by electrocautery. The surgical specimen was sent for pathology, which confirmed the diagnosis of pyogenic granuloma. The patient experienced no complications from the procedure and did not have recurrence of the lesion.
Ms. Sui is a research associate in the department of dermatology, division of pediatric and adolescent dermatology, University of California, San Diego, and Rady Children’s Hospital, San Diego. Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Neither Ms. Sui nor Dr. Eichenfield have any relevant financial disclosures.
References
1. Lin RL and Janniger CK. Cutis. 2004 Oct;74(4):229-33.
2. Harris MN et al. J Am Acad Dermatol. 2000 Jun;42(6):1012-6.
3. Pagliai KA and Cohen BA. Pediatr Dermatol. 2004 Jan-Feb;21(1):10-3.
4. Kamal R et al. J Oral Maxillofac Pathol. 2012 Jan;16(1):79-82.
5. Requena L and Sangueza OP. J Am Acad Dermatol. 1997 Dec;37(6):887-919.
6. Patrice SJ et al. Pediatr Dermatol. 1991 Dec;8(4):267-76.
7. Luba MC et al. Am Fam Physician. 2003 Feb 15;67(4):729-38.
8. Swerlick RA and Cooper PH. J Am Acad Dermatol. 1983 May;8(5):627-30.
9. Sheehan DJ and Lesher JL Jr. Cutis. 2004 Mar;73(3):175-80.
10. Zaballos P et al. Br J Dermatol. 2006 Jun;154(6):1108-11.
11. Lee J et al. J Plast Reconstr Aesthet Surg. 2011 Sep;64(9):1216-20. .
Formula may be right for infants, but experts warn that toddlers don’t need it
Formulas for toddlers are a burgeoning business in the United States: Sales of the drinks more than doubled in recent years as companies convinced parents that their little ones needed the liquid boost. But many experts warn that these products, designed for children ages 1-3, fill no nutritional needs beyond what is available in a typical toddler diet, are subject to less regulation than infant formula, and are expensive.
In addition, some parents feed the toddler versions to infants even though they do not meet federal standards for infant formula and may not provide babies with adequate nutrients to sustain their growth.
Pediatricians and federal health officials say that when most children turn 1, they can begin drinking cow milk or an unsweetened plant-based milk substitute. In a 2019 “consensus” statement, the American Academy of Pediatrics and other health and nutrition organizations recommended against using toddler formulas, saying “they offer no unique nutritional value beyond what could be obtained with healthy foods; furthermore, they may contribute added sugars to the diet.” The toddler formulas often contain sweeteners and fats that add calories.
Some of the same companies that produce infant formula – including Enfamil, Gerber, and Similac – also make toddler formulas, as do some smaller, boutique brands that advertise that they have organic or other special qualities. Toddler formulas are available nearly everywhere infant formulas are sold and are marketed as providing extra nutrients to help children’s brain, immune system, and eye development, among other benefits. They are different from medical formulas prescribed for children with specific needs.
A 2020 study found that sales of toddler formula in the United States rose to $92 million in 2015 from $39 million in 2006.
Parents are often confused by the marketing for the formulas, according to a study led by Jennifer Harris, PhD, a marketing and public health researcher at the University of Connecticut, Hartford. She found that 60% of caregivers falsely believed toddler formulas have nutrients that toddlers can’t get from other foods.
Anthony Porto, MD, MPH, a pediatric gastroenterologist and pediatrics professor at Yale University, New Haven, Conn., said he is concerned these products could be giving toddlers more nutrients and calories than they need. Unlike what’s designed for infants, toddler formula has no nutritional regulations: Experts say standardizing a supplement to toddlers’ diets is impossible because no two children are alike.
In focus groups, Dr. Harris said, parents report feeding their children toddler formula to fill nutritional gaps when a child isn’t eating enough, a common concern among parents.
“Infants are often voracious eaters,” said Stephen Daniels, MD, chair of pediatrics at Children’s Hospital Colorado, Aurora. But at around a year of age, children’s growth plateaus, he said, and “they’re suddenly not hungry in the way they used to be anymore.” That can worry parents, he added, but “it’s a completely normal phenomenon.”
If parents have concerns about their children’s diet, Dr. Daniels said, they should consult a pediatrician or family doctor.
Blanche Lincoln, president of the Infant Nutrition Council of America, which represents the makers of Enfamil, Gerber, Similac, and store brands, said in an email that the toddler formulas can be helpful because they can fill “nutritional gaps during this period of transition to table foods.” Ms. Lincoln, a former U.S. senator from Arkansas, said the drinks “help contribute to the specific nutritional needs of toddlers by providing energy and important nutrients, as well as essential vitamins and minerals during this important period of growth and development.”
But toddler formula isn’t being ingested by toddlers alone – it’s also being fed to infants. In a recent study, Dr. Porto and colleagues found that 5% of infants’ parents reported giving their babies drinks marketed for the older age group. And Dr. Harris’ research indicated that 22% of parents of infants older than 6 months had fed their babies toddler formula in the previous month. Both studies were conducted before the recent infant formula shortage, which may have exacerbated the problem.
“Infant formulas and toddler formulas tend to be next to each other in the supermarket,” Dr. Harris said. “They look similar, but the toddler formulas are cheaper than the infant formulas. So people confuse them, and they grab the wrong one. Or they think: ‘Oh, this one is less expensive. I’ll get this one instead.’ ”
According to an email from Food and Drug Administration spokesperson Lindsay Haake, toddler drinks do not meet the definition of infant formula, so they are not subject to the same requirements. That means they do not have to undergo the clinical trials and pathogen safety testing that the infant versions do. “Unlike infant formulas, toddler formulas are not necessary to meet the nutritional needs of their intended consumers,” Ms. Haake said.
In a statement to KHN, the Infant Nutrition Council of America said: “Toddler drinks have a distinctive use and nutritional makeup from infant formula; the two are not interchangeable. The labeling of toddler nutritional drinks explicitly identifies the product as a toddler drink intended for children 12 months and older on the front of the package label.”
However, several expensive toddler formula brands made by smaller companies – often advertised as being made from goat milk, A2 whole milk (which lacks one common milk protein), or vegan ingredients that aren’t soy – do meet nutritional requirements for infants, and some advertise that.
Dr. Harris argued that this confuses parents, too, and shouldn’t be allowed. Just because a toddler formula has the nutritional ingredients required by the FDA for infant formula doesn’t mean it has met the other tests required of infant formula.
Federal regulators have not forced any of the companies to withdraw those products. In an email, FDA spokesperson Marianna Naum said: “The FDA does not comment on potential compliance actions.”
One company, Nature’s One, whose toddler formulas are named “Baby’s Only,” received warning letters a decade ago from the FDA about marketing them for infants. That case was closed in 2016. The company’s website says that Baby’s Only formula “meets nutrient requirements for infant” and that “Baby’s Only Organic® can be served up to 3 years of age.” Critics say that language implies the formula is fine for babies younger than 1. The company’s website and its Instagram account feature customer testimonials from parents who report feeding the formula to their infants, as well as pictures of infants drinking it.
Jay Highman, CEO and president of Nature’s One, said that Baby’s Only is clearly labeled as a toddler formula and that the back of the can states that “Baby’s Only is intended for a toddler 1 year of age or older OR when directed by a health care professional.” He also said that since the company launched in 1999, its formulas have met all the nutritional, manufacturing, and safety standards required of infant formula even though they don’t have to. “We behaved like we are an infant formula, but we were selling it as a toddler formula.”
He said that the clinical trials required by the FDA are a huge barrier to bringing a new infant formula to market and that many other countries don’t require a clinical trial. Baby’s Only recently completed a clinical trial, and the company expects to be able to sell it as an infant formula soon.
Yet pediatricians and nutritional experts continue to caution parents about using the toddler drinks. “There’s no question that infant formula is very important in the first year of life,” Dr. Daniels said. But he doesn’t recommend the toddler version “because it’s not that useful, because it’s confusing, because it’s expensive.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Formulas for toddlers are a burgeoning business in the United States: Sales of the drinks more than doubled in recent years as companies convinced parents that their little ones needed the liquid boost. But many experts warn that these products, designed for children ages 1-3, fill no nutritional needs beyond what is available in a typical toddler diet, are subject to less regulation than infant formula, and are expensive.
In addition, some parents feed the toddler versions to infants even though they do not meet federal standards for infant formula and may not provide babies with adequate nutrients to sustain their growth.
Pediatricians and federal health officials say that when most children turn 1, they can begin drinking cow milk or an unsweetened plant-based milk substitute. In a 2019 “consensus” statement, the American Academy of Pediatrics and other health and nutrition organizations recommended against using toddler formulas, saying “they offer no unique nutritional value beyond what could be obtained with healthy foods; furthermore, they may contribute added sugars to the diet.” The toddler formulas often contain sweeteners and fats that add calories.
Some of the same companies that produce infant formula – including Enfamil, Gerber, and Similac – also make toddler formulas, as do some smaller, boutique brands that advertise that they have organic or other special qualities. Toddler formulas are available nearly everywhere infant formulas are sold and are marketed as providing extra nutrients to help children’s brain, immune system, and eye development, among other benefits. They are different from medical formulas prescribed for children with specific needs.
A 2020 study found that sales of toddler formula in the United States rose to $92 million in 2015 from $39 million in 2006.
Parents are often confused by the marketing for the formulas, according to a study led by Jennifer Harris, PhD, a marketing and public health researcher at the University of Connecticut, Hartford. She found that 60% of caregivers falsely believed toddler formulas have nutrients that toddlers can’t get from other foods.
Anthony Porto, MD, MPH, a pediatric gastroenterologist and pediatrics professor at Yale University, New Haven, Conn., said he is concerned these products could be giving toddlers more nutrients and calories than they need. Unlike what’s designed for infants, toddler formula has no nutritional regulations: Experts say standardizing a supplement to toddlers’ diets is impossible because no two children are alike.
In focus groups, Dr. Harris said, parents report feeding their children toddler formula to fill nutritional gaps when a child isn’t eating enough, a common concern among parents.
“Infants are often voracious eaters,” said Stephen Daniels, MD, chair of pediatrics at Children’s Hospital Colorado, Aurora. But at around a year of age, children’s growth plateaus, he said, and “they’re suddenly not hungry in the way they used to be anymore.” That can worry parents, he added, but “it’s a completely normal phenomenon.”
If parents have concerns about their children’s diet, Dr. Daniels said, they should consult a pediatrician or family doctor.
Blanche Lincoln, president of the Infant Nutrition Council of America, which represents the makers of Enfamil, Gerber, Similac, and store brands, said in an email that the toddler formulas can be helpful because they can fill “nutritional gaps during this period of transition to table foods.” Ms. Lincoln, a former U.S. senator from Arkansas, said the drinks “help contribute to the specific nutritional needs of toddlers by providing energy and important nutrients, as well as essential vitamins and minerals during this important period of growth and development.”
But toddler formula isn’t being ingested by toddlers alone – it’s also being fed to infants. In a recent study, Dr. Porto and colleagues found that 5% of infants’ parents reported giving their babies drinks marketed for the older age group. And Dr. Harris’ research indicated that 22% of parents of infants older than 6 months had fed their babies toddler formula in the previous month. Both studies were conducted before the recent infant formula shortage, which may have exacerbated the problem.
“Infant formulas and toddler formulas tend to be next to each other in the supermarket,” Dr. Harris said. “They look similar, but the toddler formulas are cheaper than the infant formulas. So people confuse them, and they grab the wrong one. Or they think: ‘Oh, this one is less expensive. I’ll get this one instead.’ ”
According to an email from Food and Drug Administration spokesperson Lindsay Haake, toddler drinks do not meet the definition of infant formula, so they are not subject to the same requirements. That means they do not have to undergo the clinical trials and pathogen safety testing that the infant versions do. “Unlike infant formulas, toddler formulas are not necessary to meet the nutritional needs of their intended consumers,” Ms. Haake said.
In a statement to KHN, the Infant Nutrition Council of America said: “Toddler drinks have a distinctive use and nutritional makeup from infant formula; the two are not interchangeable. The labeling of toddler nutritional drinks explicitly identifies the product as a toddler drink intended for children 12 months and older on the front of the package label.”
However, several expensive toddler formula brands made by smaller companies – often advertised as being made from goat milk, A2 whole milk (which lacks one common milk protein), or vegan ingredients that aren’t soy – do meet nutritional requirements for infants, and some advertise that.
Dr. Harris argued that this confuses parents, too, and shouldn’t be allowed. Just because a toddler formula has the nutritional ingredients required by the FDA for infant formula doesn’t mean it has met the other tests required of infant formula.
Federal regulators have not forced any of the companies to withdraw those products. In an email, FDA spokesperson Marianna Naum said: “The FDA does not comment on potential compliance actions.”
One company, Nature’s One, whose toddler formulas are named “Baby’s Only,” received warning letters a decade ago from the FDA about marketing them for infants. That case was closed in 2016. The company’s website says that Baby’s Only formula “meets nutrient requirements for infant” and that “Baby’s Only Organic® can be served up to 3 years of age.” Critics say that language implies the formula is fine for babies younger than 1. The company’s website and its Instagram account feature customer testimonials from parents who report feeding the formula to their infants, as well as pictures of infants drinking it.
Jay Highman, CEO and president of Nature’s One, said that Baby’s Only is clearly labeled as a toddler formula and that the back of the can states that “Baby’s Only is intended for a toddler 1 year of age or older OR when directed by a health care professional.” He also said that since the company launched in 1999, its formulas have met all the nutritional, manufacturing, and safety standards required of infant formula even though they don’t have to. “We behaved like we are an infant formula, but we were selling it as a toddler formula.”
He said that the clinical trials required by the FDA are a huge barrier to bringing a new infant formula to market and that many other countries don’t require a clinical trial. Baby’s Only recently completed a clinical trial, and the company expects to be able to sell it as an infant formula soon.
Yet pediatricians and nutritional experts continue to caution parents about using the toddler drinks. “There’s no question that infant formula is very important in the first year of life,” Dr. Daniels said. But he doesn’t recommend the toddler version “because it’s not that useful, because it’s confusing, because it’s expensive.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Formulas for toddlers are a burgeoning business in the United States: Sales of the drinks more than doubled in recent years as companies convinced parents that their little ones needed the liquid boost. But many experts warn that these products, designed for children ages 1-3, fill no nutritional needs beyond what is available in a typical toddler diet, are subject to less regulation than infant formula, and are expensive.
In addition, some parents feed the toddler versions to infants even though they do not meet federal standards for infant formula and may not provide babies with adequate nutrients to sustain their growth.
Pediatricians and federal health officials say that when most children turn 1, they can begin drinking cow milk or an unsweetened plant-based milk substitute. In a 2019 “consensus” statement, the American Academy of Pediatrics and other health and nutrition organizations recommended against using toddler formulas, saying “they offer no unique nutritional value beyond what could be obtained with healthy foods; furthermore, they may contribute added sugars to the diet.” The toddler formulas often contain sweeteners and fats that add calories.
Some of the same companies that produce infant formula – including Enfamil, Gerber, and Similac – also make toddler formulas, as do some smaller, boutique brands that advertise that they have organic or other special qualities. Toddler formulas are available nearly everywhere infant formulas are sold and are marketed as providing extra nutrients to help children’s brain, immune system, and eye development, among other benefits. They are different from medical formulas prescribed for children with specific needs.
A 2020 study found that sales of toddler formula in the United States rose to $92 million in 2015 from $39 million in 2006.
Parents are often confused by the marketing for the formulas, according to a study led by Jennifer Harris, PhD, a marketing and public health researcher at the University of Connecticut, Hartford. She found that 60% of caregivers falsely believed toddler formulas have nutrients that toddlers can’t get from other foods.
Anthony Porto, MD, MPH, a pediatric gastroenterologist and pediatrics professor at Yale University, New Haven, Conn., said he is concerned these products could be giving toddlers more nutrients and calories than they need. Unlike what’s designed for infants, toddler formula has no nutritional regulations: Experts say standardizing a supplement to toddlers’ diets is impossible because no two children are alike.
In focus groups, Dr. Harris said, parents report feeding their children toddler formula to fill nutritional gaps when a child isn’t eating enough, a common concern among parents.
“Infants are often voracious eaters,” said Stephen Daniels, MD, chair of pediatrics at Children’s Hospital Colorado, Aurora. But at around a year of age, children’s growth plateaus, he said, and “they’re suddenly not hungry in the way they used to be anymore.” That can worry parents, he added, but “it’s a completely normal phenomenon.”
If parents have concerns about their children’s diet, Dr. Daniels said, they should consult a pediatrician or family doctor.
Blanche Lincoln, president of the Infant Nutrition Council of America, which represents the makers of Enfamil, Gerber, Similac, and store brands, said in an email that the toddler formulas can be helpful because they can fill “nutritional gaps during this period of transition to table foods.” Ms. Lincoln, a former U.S. senator from Arkansas, said the drinks “help contribute to the specific nutritional needs of toddlers by providing energy and important nutrients, as well as essential vitamins and minerals during this important period of growth and development.”
But toddler formula isn’t being ingested by toddlers alone – it’s also being fed to infants. In a recent study, Dr. Porto and colleagues found that 5% of infants’ parents reported giving their babies drinks marketed for the older age group. And Dr. Harris’ research indicated that 22% of parents of infants older than 6 months had fed their babies toddler formula in the previous month. Both studies were conducted before the recent infant formula shortage, which may have exacerbated the problem.
“Infant formulas and toddler formulas tend to be next to each other in the supermarket,” Dr. Harris said. “They look similar, but the toddler formulas are cheaper than the infant formulas. So people confuse them, and they grab the wrong one. Or they think: ‘Oh, this one is less expensive. I’ll get this one instead.’ ”
According to an email from Food and Drug Administration spokesperson Lindsay Haake, toddler drinks do not meet the definition of infant formula, so they are not subject to the same requirements. That means they do not have to undergo the clinical trials and pathogen safety testing that the infant versions do. “Unlike infant formulas, toddler formulas are not necessary to meet the nutritional needs of their intended consumers,” Ms. Haake said.
In a statement to KHN, the Infant Nutrition Council of America said: “Toddler drinks have a distinctive use and nutritional makeup from infant formula; the two are not interchangeable. The labeling of toddler nutritional drinks explicitly identifies the product as a toddler drink intended for children 12 months and older on the front of the package label.”
However, several expensive toddler formula brands made by smaller companies – often advertised as being made from goat milk, A2 whole milk (which lacks one common milk protein), or vegan ingredients that aren’t soy – do meet nutritional requirements for infants, and some advertise that.
Dr. Harris argued that this confuses parents, too, and shouldn’t be allowed. Just because a toddler formula has the nutritional ingredients required by the FDA for infant formula doesn’t mean it has met the other tests required of infant formula.
Federal regulators have not forced any of the companies to withdraw those products. In an email, FDA spokesperson Marianna Naum said: “The FDA does not comment on potential compliance actions.”
One company, Nature’s One, whose toddler formulas are named “Baby’s Only,” received warning letters a decade ago from the FDA about marketing them for infants. That case was closed in 2016. The company’s website says that Baby’s Only formula “meets nutrient requirements for infant” and that “Baby’s Only Organic® can be served up to 3 years of age.” Critics say that language implies the formula is fine for babies younger than 1. The company’s website and its Instagram account feature customer testimonials from parents who report feeding the formula to their infants, as well as pictures of infants drinking it.
Jay Highman, CEO and president of Nature’s One, said that Baby’s Only is clearly labeled as a toddler formula and that the back of the can states that “Baby’s Only is intended for a toddler 1 year of age or older OR when directed by a health care professional.” He also said that since the company launched in 1999, its formulas have met all the nutritional, manufacturing, and safety standards required of infant formula even though they don’t have to. “We behaved like we are an infant formula, but we were selling it as a toddler formula.”
He said that the clinical trials required by the FDA are a huge barrier to bringing a new infant formula to market and that many other countries don’t require a clinical trial. Baby’s Only recently completed a clinical trial, and the company expects to be able to sell it as an infant formula soon.
Yet pediatricians and nutritional experts continue to caution parents about using the toddler drinks. “There’s no question that infant formula is very important in the first year of life,” Dr. Daniels said. But he doesn’t recommend the toddler version “because it’s not that useful, because it’s confusing, because it’s expensive.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.