User login
Charity case
A 45-year-old comes to the emergency department because of abdominal pain for the last few months. She has been belching and hiccuping, and she has lost 20 pounds.
A 45-year-old comes to the emergency department, where a CT scan shows a large mass in her stomach. There also are enlarged lymph nodes nearby and far away, and the wall of her abdomen is studded with smaller tumors.
A 45-year-old comes to the emergency department and is told she needs to be admitted to the hospital to work this up. It’s likely cancer, she is told, but the only way to confirm the diagnosis is with a biopsy. They do a procedure in which they insert a camera through her mouth and down her esophagus, and they take a sample of the large mass in her stomach. It is cancer – and it’s widely metastatic.
The resident on her team sends me a text. She wants to know: “What do you do in cases like this?”
Except she is not asking for my advice on medical management. The same day the patient is told it’s cancer, she has a confession. She has no insurance.
I read the text, then turn to the oncology case manager sitting next to me. We talk it through and the answer is as I suspected.
“She can be seen in the county clinic,” I text back, and give the name of an oncologist there. “Or, she can apply for emergency Medi-Cal to follow up at our hospital. But that process can take over a month to get approval.”
Except the case manager looks into it further. Actually, she does not qualify for emergency Medi-Cal because she invoked it earlier that year when she had an infection.
When it’s an emergency, hospitals tend to handle this kind of situation well. I’ve seen hospitals absorb the costs of major medical interventions when a person is acutely ill. They call it a charity case, and they cover all the costs of acute illness and treatment when the patient cannot.
But what this person needs is different. The treatment she needs is not emergent. What she needs is a regular oncologist who can give chemotherapy, monitor for side effects, check her blood counts, get regular scans to monitor the disease, and have conversations with her to navigate the bigger questions. What she needs is an ongoing relationship.
That is harder for a hospital to absorb.
I think back to a year ago, when I was volunteering at the free clinic. A 77-year-old man came in complaining of increased urinary frequency. I did a rectal exam, and I felt it: a large, irregular prostate mass. I thought of all I would normally do, down the algorithm of treatment – I’d order a PSA blood test, arrange for him to have a biopsy, likely get a CT scan, then get him back in the clinic to start treatment. But there, I could not do any of that. There, I was lucky when I could get someone a $4 medication. There, all I could do was hand him the truth. “I am concerned you have prostate cancer,” I said.
I remember how he began crying tears of joy. “God bless you,” he said, grabbing my hand. God bless me? For what? For handing him a problem but no solution? For sharing a suspicion of a diagnosis that could kill him but being unable to intervene? Is it really better knowing?
I deliver a lot of bad news in oncology, but I usually get to blame the disease. The cancer is aggressive. The cancer is causing your pain.
What I hate perhaps even more is the other type of bad news: having our hands tied by a system I disagree with – and yet am somehow part of. We can offer X, but not Y. You can be seen in this clinic, but not in that one. This treatment is covered, but that part would be out of pocket. Negotiating what is absolutely necessary and what is preferred.
A 45-year-old comes to the emergency department with abdominal pain. She is told she has metastatic cancer that will take her life in less than 6 months without treatment. She has many questions for me, the inpatient oncology fellow. But they are not about the disease, the prognosis, or the treatment. They are all about insurance options, reimbursement, and cost.
Like everyone with a new devastating diagnosis, she is weighing her options. Except her decisions are weighted with the fear of bankruptcy; her calculus trying to compute the cost of her life.
“I wish things were different,” I say.
Minor details of this story were altered to protect privacy.
Dr. Yurkiewicz is a fellow in hematology and oncology at Stanford (Calif.) University. Follow her on Twitter @ilanayurkiewicz.
A 45-year-old comes to the emergency department because of abdominal pain for the last few months. She has been belching and hiccuping, and she has lost 20 pounds.
A 45-year-old comes to the emergency department, where a CT scan shows a large mass in her stomach. There also are enlarged lymph nodes nearby and far away, and the wall of her abdomen is studded with smaller tumors.
A 45-year-old comes to the emergency department and is told she needs to be admitted to the hospital to work this up. It’s likely cancer, she is told, but the only way to confirm the diagnosis is with a biopsy. They do a procedure in which they insert a camera through her mouth and down her esophagus, and they take a sample of the large mass in her stomach. It is cancer – and it’s widely metastatic.
The resident on her team sends me a text. She wants to know: “What do you do in cases like this?”
Except she is not asking for my advice on medical management. The same day the patient is told it’s cancer, she has a confession. She has no insurance.
I read the text, then turn to the oncology case manager sitting next to me. We talk it through and the answer is as I suspected.
“She can be seen in the county clinic,” I text back, and give the name of an oncologist there. “Or, she can apply for emergency Medi-Cal to follow up at our hospital. But that process can take over a month to get approval.”
Except the case manager looks into it further. Actually, she does not qualify for emergency Medi-Cal because she invoked it earlier that year when she had an infection.
When it’s an emergency, hospitals tend to handle this kind of situation well. I’ve seen hospitals absorb the costs of major medical interventions when a person is acutely ill. They call it a charity case, and they cover all the costs of acute illness and treatment when the patient cannot.
But what this person needs is different. The treatment she needs is not emergent. What she needs is a regular oncologist who can give chemotherapy, monitor for side effects, check her blood counts, get regular scans to monitor the disease, and have conversations with her to navigate the bigger questions. What she needs is an ongoing relationship.
That is harder for a hospital to absorb.
I think back to a year ago, when I was volunteering at the free clinic. A 77-year-old man came in complaining of increased urinary frequency. I did a rectal exam, and I felt it: a large, irregular prostate mass. I thought of all I would normally do, down the algorithm of treatment – I’d order a PSA blood test, arrange for him to have a biopsy, likely get a CT scan, then get him back in the clinic to start treatment. But there, I could not do any of that. There, I was lucky when I could get someone a $4 medication. There, all I could do was hand him the truth. “I am concerned you have prostate cancer,” I said.
I remember how he began crying tears of joy. “God bless you,” he said, grabbing my hand. God bless me? For what? For handing him a problem but no solution? For sharing a suspicion of a diagnosis that could kill him but being unable to intervene? Is it really better knowing?
I deliver a lot of bad news in oncology, but I usually get to blame the disease. The cancer is aggressive. The cancer is causing your pain.
What I hate perhaps even more is the other type of bad news: having our hands tied by a system I disagree with – and yet am somehow part of. We can offer X, but not Y. You can be seen in this clinic, but not in that one. This treatment is covered, but that part would be out of pocket. Negotiating what is absolutely necessary and what is preferred.
A 45-year-old comes to the emergency department with abdominal pain. She is told she has metastatic cancer that will take her life in less than 6 months without treatment. She has many questions for me, the inpatient oncology fellow. But they are not about the disease, the prognosis, or the treatment. They are all about insurance options, reimbursement, and cost.
Like everyone with a new devastating diagnosis, she is weighing her options. Except her decisions are weighted with the fear of bankruptcy; her calculus trying to compute the cost of her life.
“I wish things were different,” I say.
Minor details of this story were altered to protect privacy.
Dr. Yurkiewicz is a fellow in hematology and oncology at Stanford (Calif.) University. Follow her on Twitter @ilanayurkiewicz.
A 45-year-old comes to the emergency department because of abdominal pain for the last few months. She has been belching and hiccuping, and she has lost 20 pounds.
A 45-year-old comes to the emergency department, where a CT scan shows a large mass in her stomach. There also are enlarged lymph nodes nearby and far away, and the wall of her abdomen is studded with smaller tumors.
A 45-year-old comes to the emergency department and is told she needs to be admitted to the hospital to work this up. It’s likely cancer, she is told, but the only way to confirm the diagnosis is with a biopsy. They do a procedure in which they insert a camera through her mouth and down her esophagus, and they take a sample of the large mass in her stomach. It is cancer – and it’s widely metastatic.
The resident on her team sends me a text. She wants to know: “What do you do in cases like this?”
Except she is not asking for my advice on medical management. The same day the patient is told it’s cancer, she has a confession. She has no insurance.
I read the text, then turn to the oncology case manager sitting next to me. We talk it through and the answer is as I suspected.
“She can be seen in the county clinic,” I text back, and give the name of an oncologist there. “Or, she can apply for emergency Medi-Cal to follow up at our hospital. But that process can take over a month to get approval.”
Except the case manager looks into it further. Actually, she does not qualify for emergency Medi-Cal because she invoked it earlier that year when she had an infection.
When it’s an emergency, hospitals tend to handle this kind of situation well. I’ve seen hospitals absorb the costs of major medical interventions when a person is acutely ill. They call it a charity case, and they cover all the costs of acute illness and treatment when the patient cannot.
But what this person needs is different. The treatment she needs is not emergent. What she needs is a regular oncologist who can give chemotherapy, monitor for side effects, check her blood counts, get regular scans to monitor the disease, and have conversations with her to navigate the bigger questions. What she needs is an ongoing relationship.
That is harder for a hospital to absorb.
I think back to a year ago, when I was volunteering at the free clinic. A 77-year-old man came in complaining of increased urinary frequency. I did a rectal exam, and I felt it: a large, irregular prostate mass. I thought of all I would normally do, down the algorithm of treatment – I’d order a PSA blood test, arrange for him to have a biopsy, likely get a CT scan, then get him back in the clinic to start treatment. But there, I could not do any of that. There, I was lucky when I could get someone a $4 medication. There, all I could do was hand him the truth. “I am concerned you have prostate cancer,” I said.
I remember how he began crying tears of joy. “God bless you,” he said, grabbing my hand. God bless me? For what? For handing him a problem but no solution? For sharing a suspicion of a diagnosis that could kill him but being unable to intervene? Is it really better knowing?
I deliver a lot of bad news in oncology, but I usually get to blame the disease. The cancer is aggressive. The cancer is causing your pain.
What I hate perhaps even more is the other type of bad news: having our hands tied by a system I disagree with – and yet am somehow part of. We can offer X, but not Y. You can be seen in this clinic, but not in that one. This treatment is covered, but that part would be out of pocket. Negotiating what is absolutely necessary and what is preferred.
A 45-year-old comes to the emergency department with abdominal pain. She is told she has metastatic cancer that will take her life in less than 6 months without treatment. She has many questions for me, the inpatient oncology fellow. But they are not about the disease, the prognosis, or the treatment. They are all about insurance options, reimbursement, and cost.
Like everyone with a new devastating diagnosis, she is weighing her options. Except her decisions are weighted with the fear of bankruptcy; her calculus trying to compute the cost of her life.
“I wish things were different,” I say.
Minor details of this story were altered to protect privacy.
Dr. Yurkiewicz is a fellow in hematology and oncology at Stanford (Calif.) University. Follow her on Twitter @ilanayurkiewicz.
AHA: Consider obesity as CVD risk factor in children
The American Heart Association has included obesity and severe obesity in its updated scientific statement outlining risk factors and considerations for cardiovascular risk reduction in high-risk pediatric patients.
The scientific statement is an update to a 2006 American Heart Association (AHA) statement, adding details about obesity as an at-risk condition and severe obesity as a moderate-risk condition. Other additions include classifying type 2 diabetes as a high-risk condition and expanding on new risk factors for cardiovascular disease (CVD) among patients who received treatment for childhood cancer.
The AHA said the statement is aimed at pediatric cardiologists, primary care physicians, and subspecialists who care for at-risk pediatric patients, as well as providers who will care for these patients as they transition to adult life.
Obesity
In the AHA scientific statement, Sarah de Ferranti, MD, MPH, of Boston Children’s Hospital, chair of the writing group, and her colleagues, highlighted a 2016 study that identified a twofold to threefold higher risk of CVD-related mortality among patients who were overweight or obese, compared with patients of normal weight (Diabetes Care. 2016 Nov;39[11]:1996-2003).
Patients with obesity and severe obesity are at increased risk of aortic or coronary fatty streaks, dyslipidemia, high blood pressure, hyperglycemia, and insulin resistance, as well as inflammatory and oxidative stress, the AHA writing group noted.
They estimated that approximately 6% of U.S. children aged 2-19 years old are considered severely obese.
After identifying patients with obesity, the writing group said, a “multimodal and graduated approach to treatment” for these patients is generally warranted, with a focus on dietary and lifestyle changes, and use of pharmacotherapy and bariatric surgery if indicated.
However, the authors said therapeutic life change modification “is limited in severe obesity because of small effect size and difficulty with sustainability,” while use of pharmacotherapy for treatment of pediatric obesity remains understudied and medications such as orlistat and metformin offer only modest weight loss.
Bariatric surgery, “the only treatment for severe pediatric obesity consistently associated with clinically meaningful and durable weight loss,” is not consistently offered to patients under 12 years old, they added.
Diabetes
The AHA statement also addresses risks from type 1 (T1D) and type 2 diabetes (T2D). Children with T1D and T2D are at increased risk for dyslipidemia, hypertension, microalbuminuria, and obesity. Annual screening for these patients is indicated, and cardiovascular risk factor reduction can be achieved by managing hyperglycemia, controlling weight gain as a result of medication, and implementing therapeutic lifestyle changes, when possible.
Childhood cancer
As survival rates from childhood cancer have improved, there is a need to address the increased risk of cardiovascular-related mortality (estimated at 8-10 times higher than the general population) as well as cancer relapse, according to the writing group.
Among patients recruited to the Childhood Cancer Survivor Study, there was a 9-fold increase in cerebrovascular accident, 10-fold increased risk of coronary artery disease, and 15-fold increase in heart failure for childhood cancer survivors, compared with their siblings who were cancer free.
Cancer treatments such as radiation exposure are linked to increased rates of myocardial infarction, heart failure, valvular abnormalities, and pericardial disease at a twofold to sixfold higher rate when administered at a greater than 1,500 centigray dose, compared to cancer survivors who did not receive radiation, the authors wrote.
Anthracycline treatment is associated with a dose-dependent increase in the risk of dilated cardiomyopathy, while hematopoietic stem cell transplantation may increase the risk of CVD-related mortality from heart failure, cerebrovascular accident, cardiomyopathy, coronary artery disease, and rhythm disorders.
In treating childhood cancer survivors for CVD risk factors, “a low threshold should be used when considering the initiation of pharmacological agents because of the high risk of these youth,” and standard pharmacotherapies can be used, the authors said. “Treatment of cardiovascular risk factors should consider the cancer therapies the patient has received previously.”
In the AHA statement, Dr. de Ferranti and her colleagues also outlined epidemiology, screening, and treatment data for other cardiovascular risk factors such as familial hypercholesterolemia, Lipoprotein(a), hypertension, chronic kidney disease, congenital heart disease, Kawasaki disease, and heart transplantation.
Some members of the writing group reported research grants from Amgen, Sanofi, the Wisconsin Partnership Program, and the National Institutes of Health. One author reported unpaid consultancies with Novo Nordisk, Orexigen, and Vivus.
SOURCE: de Ferranti SD et al. Circulation. 2019 Feb 25. doi: 10.1161/CIR.0000000000000618.
The American Heart Association has included obesity and severe obesity in its updated scientific statement outlining risk factors and considerations for cardiovascular risk reduction in high-risk pediatric patients.
The scientific statement is an update to a 2006 American Heart Association (AHA) statement, adding details about obesity as an at-risk condition and severe obesity as a moderate-risk condition. Other additions include classifying type 2 diabetes as a high-risk condition and expanding on new risk factors for cardiovascular disease (CVD) among patients who received treatment for childhood cancer.
The AHA said the statement is aimed at pediatric cardiologists, primary care physicians, and subspecialists who care for at-risk pediatric patients, as well as providers who will care for these patients as they transition to adult life.
Obesity
In the AHA scientific statement, Sarah de Ferranti, MD, MPH, of Boston Children’s Hospital, chair of the writing group, and her colleagues, highlighted a 2016 study that identified a twofold to threefold higher risk of CVD-related mortality among patients who were overweight or obese, compared with patients of normal weight (Diabetes Care. 2016 Nov;39[11]:1996-2003).
Patients with obesity and severe obesity are at increased risk of aortic or coronary fatty streaks, dyslipidemia, high blood pressure, hyperglycemia, and insulin resistance, as well as inflammatory and oxidative stress, the AHA writing group noted.
They estimated that approximately 6% of U.S. children aged 2-19 years old are considered severely obese.
After identifying patients with obesity, the writing group said, a “multimodal and graduated approach to treatment” for these patients is generally warranted, with a focus on dietary and lifestyle changes, and use of pharmacotherapy and bariatric surgery if indicated.
However, the authors said therapeutic life change modification “is limited in severe obesity because of small effect size and difficulty with sustainability,” while use of pharmacotherapy for treatment of pediatric obesity remains understudied and medications such as orlistat and metformin offer only modest weight loss.
Bariatric surgery, “the only treatment for severe pediatric obesity consistently associated with clinically meaningful and durable weight loss,” is not consistently offered to patients under 12 years old, they added.
Diabetes
The AHA statement also addresses risks from type 1 (T1D) and type 2 diabetes (T2D). Children with T1D and T2D are at increased risk for dyslipidemia, hypertension, microalbuminuria, and obesity. Annual screening for these patients is indicated, and cardiovascular risk factor reduction can be achieved by managing hyperglycemia, controlling weight gain as a result of medication, and implementing therapeutic lifestyle changes, when possible.
Childhood cancer
As survival rates from childhood cancer have improved, there is a need to address the increased risk of cardiovascular-related mortality (estimated at 8-10 times higher than the general population) as well as cancer relapse, according to the writing group.
Among patients recruited to the Childhood Cancer Survivor Study, there was a 9-fold increase in cerebrovascular accident, 10-fold increased risk of coronary artery disease, and 15-fold increase in heart failure for childhood cancer survivors, compared with their siblings who were cancer free.
Cancer treatments such as radiation exposure are linked to increased rates of myocardial infarction, heart failure, valvular abnormalities, and pericardial disease at a twofold to sixfold higher rate when administered at a greater than 1,500 centigray dose, compared to cancer survivors who did not receive radiation, the authors wrote.
Anthracycline treatment is associated with a dose-dependent increase in the risk of dilated cardiomyopathy, while hematopoietic stem cell transplantation may increase the risk of CVD-related mortality from heart failure, cerebrovascular accident, cardiomyopathy, coronary artery disease, and rhythm disorders.
In treating childhood cancer survivors for CVD risk factors, “a low threshold should be used when considering the initiation of pharmacological agents because of the high risk of these youth,” and standard pharmacotherapies can be used, the authors said. “Treatment of cardiovascular risk factors should consider the cancer therapies the patient has received previously.”
In the AHA statement, Dr. de Ferranti and her colleagues also outlined epidemiology, screening, and treatment data for other cardiovascular risk factors such as familial hypercholesterolemia, Lipoprotein(a), hypertension, chronic kidney disease, congenital heart disease, Kawasaki disease, and heart transplantation.
Some members of the writing group reported research grants from Amgen, Sanofi, the Wisconsin Partnership Program, and the National Institutes of Health. One author reported unpaid consultancies with Novo Nordisk, Orexigen, and Vivus.
SOURCE: de Ferranti SD et al. Circulation. 2019 Feb 25. doi: 10.1161/CIR.0000000000000618.
The American Heart Association has included obesity and severe obesity in its updated scientific statement outlining risk factors and considerations for cardiovascular risk reduction in high-risk pediatric patients.
The scientific statement is an update to a 2006 American Heart Association (AHA) statement, adding details about obesity as an at-risk condition and severe obesity as a moderate-risk condition. Other additions include classifying type 2 diabetes as a high-risk condition and expanding on new risk factors for cardiovascular disease (CVD) among patients who received treatment for childhood cancer.
The AHA said the statement is aimed at pediatric cardiologists, primary care physicians, and subspecialists who care for at-risk pediatric patients, as well as providers who will care for these patients as they transition to adult life.
Obesity
In the AHA scientific statement, Sarah de Ferranti, MD, MPH, of Boston Children’s Hospital, chair of the writing group, and her colleagues, highlighted a 2016 study that identified a twofold to threefold higher risk of CVD-related mortality among patients who were overweight or obese, compared with patients of normal weight (Diabetes Care. 2016 Nov;39[11]:1996-2003).
Patients with obesity and severe obesity are at increased risk of aortic or coronary fatty streaks, dyslipidemia, high blood pressure, hyperglycemia, and insulin resistance, as well as inflammatory and oxidative stress, the AHA writing group noted.
They estimated that approximately 6% of U.S. children aged 2-19 years old are considered severely obese.
After identifying patients with obesity, the writing group said, a “multimodal and graduated approach to treatment” for these patients is generally warranted, with a focus on dietary and lifestyle changes, and use of pharmacotherapy and bariatric surgery if indicated.
However, the authors said therapeutic life change modification “is limited in severe obesity because of small effect size and difficulty with sustainability,” while use of pharmacotherapy for treatment of pediatric obesity remains understudied and medications such as orlistat and metformin offer only modest weight loss.
Bariatric surgery, “the only treatment for severe pediatric obesity consistently associated with clinically meaningful and durable weight loss,” is not consistently offered to patients under 12 years old, they added.
Diabetes
The AHA statement also addresses risks from type 1 (T1D) and type 2 diabetes (T2D). Children with T1D and T2D are at increased risk for dyslipidemia, hypertension, microalbuminuria, and obesity. Annual screening for these patients is indicated, and cardiovascular risk factor reduction can be achieved by managing hyperglycemia, controlling weight gain as a result of medication, and implementing therapeutic lifestyle changes, when possible.
Childhood cancer
As survival rates from childhood cancer have improved, there is a need to address the increased risk of cardiovascular-related mortality (estimated at 8-10 times higher than the general population) as well as cancer relapse, according to the writing group.
Among patients recruited to the Childhood Cancer Survivor Study, there was a 9-fold increase in cerebrovascular accident, 10-fold increased risk of coronary artery disease, and 15-fold increase in heart failure for childhood cancer survivors, compared with their siblings who were cancer free.
Cancer treatments such as radiation exposure are linked to increased rates of myocardial infarction, heart failure, valvular abnormalities, and pericardial disease at a twofold to sixfold higher rate when administered at a greater than 1,500 centigray dose, compared to cancer survivors who did not receive radiation, the authors wrote.
Anthracycline treatment is associated with a dose-dependent increase in the risk of dilated cardiomyopathy, while hematopoietic stem cell transplantation may increase the risk of CVD-related mortality from heart failure, cerebrovascular accident, cardiomyopathy, coronary artery disease, and rhythm disorders.
In treating childhood cancer survivors for CVD risk factors, “a low threshold should be used when considering the initiation of pharmacological agents because of the high risk of these youth,” and standard pharmacotherapies can be used, the authors said. “Treatment of cardiovascular risk factors should consider the cancer therapies the patient has received previously.”
In the AHA statement, Dr. de Ferranti and her colleagues also outlined epidemiology, screening, and treatment data for other cardiovascular risk factors such as familial hypercholesterolemia, Lipoprotein(a), hypertension, chronic kidney disease, congenital heart disease, Kawasaki disease, and heart transplantation.
Some members of the writing group reported research grants from Amgen, Sanofi, the Wisconsin Partnership Program, and the National Institutes of Health. One author reported unpaid consultancies with Novo Nordisk, Orexigen, and Vivus.
SOURCE: de Ferranti SD et al. Circulation. 2019 Feb 25. doi: 10.1161/CIR.0000000000000618.
FROM CIRCULATION
ICYMI: Rivaroxaban reduces VTE incidence in ambulatory cancer patients
While treatment with rivaroxaban did not significantly reduce venous thromboembolism incidence in high-risk ambulatory patients with cancer over the entire course of a 180-day intervention period (6.0% vs. 8.8% in controls; hazard ratio, 0.66; 95% confidence interval, 0.40-1.09), it did reduce major bleeding incidence while patients were on treatment (2.0% vs. 6.4%; HR, 0.40; 95% CI, 0.20 0.80), according to results from the multicenter, randomized, double-blind, placebo-controlled, parallel-group, phase 3b CASSINI trial published in the New England Journal of Medicine (2019 Feb 20. doi: 10.1056/NEJMoa1814630).
We reported this story at the annual meeting of the American Society of Hematology before it was published in the journal. Find our coverage at the link below.
While treatment with rivaroxaban did not significantly reduce venous thromboembolism incidence in high-risk ambulatory patients with cancer over the entire course of a 180-day intervention period (6.0% vs. 8.8% in controls; hazard ratio, 0.66; 95% confidence interval, 0.40-1.09), it did reduce major bleeding incidence while patients were on treatment (2.0% vs. 6.4%; HR, 0.40; 95% CI, 0.20 0.80), according to results from the multicenter, randomized, double-blind, placebo-controlled, parallel-group, phase 3b CASSINI trial published in the New England Journal of Medicine (2019 Feb 20. doi: 10.1056/NEJMoa1814630).
We reported this story at the annual meeting of the American Society of Hematology before it was published in the journal. Find our coverage at the link below.
While treatment with rivaroxaban did not significantly reduce venous thromboembolism incidence in high-risk ambulatory patients with cancer over the entire course of a 180-day intervention period (6.0% vs. 8.8% in controls; hazard ratio, 0.66; 95% confidence interval, 0.40-1.09), it did reduce major bleeding incidence while patients were on treatment (2.0% vs. 6.4%; HR, 0.40; 95% CI, 0.20 0.80), according to results from the multicenter, randomized, double-blind, placebo-controlled, parallel-group, phase 3b CASSINI trial published in the New England Journal of Medicine (2019 Feb 20. doi: 10.1056/NEJMoa1814630).
We reported this story at the annual meeting of the American Society of Hematology before it was published in the journal. Find our coverage at the link below.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
ADT harms likely limited to men with CV comorbidities
WASHINGTON – The cardiovascular effects of androgen deprivation therapy (ADT) for men with advanced prostate cancer are less severe than once feared, but there is evidence to suggest that men with preexisting heart failure or a history of myocardial infarction could be at excess risk for death from cardiovascular causes when they receive ADT, according to a leading prostate cancer expert.
“I think there are concerns about potential cardiovascular harm of ADT, and I think this has reduced ADT use, despite the fact that we know for most men it improves overall survival,” said Paul Nguyen, MD, a radiation oncologist at the Dana-Farber/Brigham and Women’s Cancer Center in Boston.
“In fact, when we looked recently at men with high-risk prostate cancer, this is a group where overall survival is improved by 50% if they get ADT – so it cuts the risk of death in half – but it turns out that nearly a quarter of those patients are not receiving ADT. I think that the concern about cardiovascular harm and the confusion as to where that data stands is a lot of what’s driving that right now,” he said at the American College of Cardiology’s Advancing the Cardiovascular Care of the Oncology Patient meeting.
Randomized trial data
Dr. Nguyen noted that the evidence suggesting that ADT can increase the risk of death from cardiovascular causes came largely from three major studies:
- A 2006 study of 73,196 Medicare enrollees aged 66 or older, which found that ADT with a gonadotropin-releasing hormone (GnRH) agonist was possibly associated with increased risk of incident diabetes and cardiovascular disease (J Clin Oncol. 2006 Sep 20;24[27]:4448-56.).
- A 2007 analysis of data from the Cancer of the Prostate Strategic Urologic Research Endeavor (CAPSURE) database on 3,262 men treated with radical prostatectomy and 1,630 men treated with radiation or cryotherapy for localized prostate cancer, which found that among those 65 and older the 5-year cumulative incidence of cardiovascular death was 5.5% for patients who received ADT, vs. 2% for those who did not (J Natl Cancer Inst. 2007 Oct 17;99[20]:1516-24).
- A 2007 study of 1,372 men in three randomized trials of radiation therapy with or without androgen suppression therapy up to 8 months in duration, which found that men 65 and older who received 6 months of androgen suppression had significantly shorter times to fatal MIs than did men who did not receive the therapy (J Clin Oncol. 2007;25[17]:2420-5).
These studies, combined with observational data, led to a 2010 consensus statement from the American Heart Association, American Cancer Society, and American Urological Association, with endorsement from the American Society for Radiation Oncology, which stated that “there may be a relation between ADT and cardiovascular events and death.”
Also in 2010, the Food and Drug Administration required new labeling on GnRH agonists warning of “increased risk of diabetes and certain cardiovascular diseases (heart attack, sudden cardiac death, stroke).”
Not unanimous
Two other large randomized studies (J Clin Oncol. 2008 Feb 1;26[4]:585-91 and J Clin Oncol. 2009 Jan 1;27[1]:92-9) and two retrospective studies (J Clin Oncol. 2009 Jul 20;27[21]:3452-8 and J Clin Oncol. 2011 Sep 10;29[26]3510-16) found no excess risk of cardiovascular disease from ADT, Dr. Nguyen said, prompting him and his colleagues to see whether they could get a better estimate of the actual risk.
They did so through a 2011 meta-analysis (JAMA. 2011;306[21]:2359-66) of data on 4,141 patients from eight randomized trials. They found that among patients with unfavorable-risk prostate cancer, ADT was not associated with an increased risk of cardiovascular death, but was associated with lower risks for both prostate-specific and all-cause mortality.
Subpopulations may still be at risk
Dr. Nguyen said that the principal finding of the meta-analysis, while reassuring, “doesn’t let ADT off the hook for metabolic events, diabetes which we know happens, and the possibility of nonfatal cardiac events.”
He noted that while ADT was not associated with cardiovascular disease in clinical trials, observational studies showed significantly increased risk for fatal or non-fatal MI.
One possible explanation for the difference is that observational studies included nonfatal MI, while randomized trials looked only at cardiovascular deaths. It’s also possible that ADT causes harm primarily in men with preexisting comorbidities, who are often excluded from or underrepresented in clinical trials.
Evidence from a 2009 study (JAMA. 2009 Aug 26;302[8]:866-73) showed that among men with clinical stage T1 to T3 noninvasive, nonmetastatic prostate cancer, neoadjuvant hormonal therapy with both a luteinizing hormone-releasing hormone (LHRH) agonist and a nonsteroidal antiandrogen was associated with increased risk for all-cause mortality for those with a history of coronary artery disease–induced heart failure, but not for men with either no comorbidities or only a single comorbidity such as hypertension, hypercholesterolemia, or diabetes.
Clinical considerations
The decision to treat men with prostate cancer with ADT is therefore a balancing act, Dr. Nguyen said.
“As the risk of prostate cancer death goes up, the benefit of ADT goes up. However, as the comorbidity level goes up, the potential cardiovascular harm of ADT goes up,” he said.
For patients at the extreme ends of each continuum, such as a patient with high-risk prostate cancer and no cardiovascular comorbidities or a patient with low-risk cancer but multiple CV risk factors, the decision to give or withhold ADT is relatively simple, he said.
But for patients in between, such as a man with intermediate-risk cancer and one risk factor or a man with high risk disease with multiple comorbidities, the decision is far more complex.
“This where I think the dialogue with the cardiologist really needs to come into this decision,” he said.
Evidence to support the decision comes from retrospective studies suggesting that even men with high-risk prostate cancer have poorer overall survival with ADT if they have a history of heart failure or MI.
For patients with low-risk cancer and diabetes, ADT is associated with worse overall survival, but ADT does not cause additional harm to men with intermediate- to high-risk prostate cancer who have concomitant diabetes, Dr. Nguyen said.
“My view is that ADT has not been shown to increase cardiovascular death in randomized trials, so I think that for the vast majority of patients it probably does not increase cardiovascular deaths. But I think there could very well be a vulnerable 5% of patients who might have an excess risk of cardiovascular death, and I think we have to be careful, but we still have to balance it out against their risks for prostate cancer death,” he said.
Dr. Nguyen reported consulting fees/honoraria from Astellas, Augmenix, Blue Earth Diagnostics, Cota, Dendreon, Ferring Pharmaceuticals, GenomeDx, Janssen, and Nanobiotix.
WASHINGTON – The cardiovascular effects of androgen deprivation therapy (ADT) for men with advanced prostate cancer are less severe than once feared, but there is evidence to suggest that men with preexisting heart failure or a history of myocardial infarction could be at excess risk for death from cardiovascular causes when they receive ADT, according to a leading prostate cancer expert.
“I think there are concerns about potential cardiovascular harm of ADT, and I think this has reduced ADT use, despite the fact that we know for most men it improves overall survival,” said Paul Nguyen, MD, a radiation oncologist at the Dana-Farber/Brigham and Women’s Cancer Center in Boston.
“In fact, when we looked recently at men with high-risk prostate cancer, this is a group where overall survival is improved by 50% if they get ADT – so it cuts the risk of death in half – but it turns out that nearly a quarter of those patients are not receiving ADT. I think that the concern about cardiovascular harm and the confusion as to where that data stands is a lot of what’s driving that right now,” he said at the American College of Cardiology’s Advancing the Cardiovascular Care of the Oncology Patient meeting.
Randomized trial data
Dr. Nguyen noted that the evidence suggesting that ADT can increase the risk of death from cardiovascular causes came largely from three major studies:
- A 2006 study of 73,196 Medicare enrollees aged 66 or older, which found that ADT with a gonadotropin-releasing hormone (GnRH) agonist was possibly associated with increased risk of incident diabetes and cardiovascular disease (J Clin Oncol. 2006 Sep 20;24[27]:4448-56.).
- A 2007 analysis of data from the Cancer of the Prostate Strategic Urologic Research Endeavor (CAPSURE) database on 3,262 men treated with radical prostatectomy and 1,630 men treated with radiation or cryotherapy for localized prostate cancer, which found that among those 65 and older the 5-year cumulative incidence of cardiovascular death was 5.5% for patients who received ADT, vs. 2% for those who did not (J Natl Cancer Inst. 2007 Oct 17;99[20]:1516-24).
- A 2007 study of 1,372 men in three randomized trials of radiation therapy with or without androgen suppression therapy up to 8 months in duration, which found that men 65 and older who received 6 months of androgen suppression had significantly shorter times to fatal MIs than did men who did not receive the therapy (J Clin Oncol. 2007;25[17]:2420-5).
These studies, combined with observational data, led to a 2010 consensus statement from the American Heart Association, American Cancer Society, and American Urological Association, with endorsement from the American Society for Radiation Oncology, which stated that “there may be a relation between ADT and cardiovascular events and death.”
Also in 2010, the Food and Drug Administration required new labeling on GnRH agonists warning of “increased risk of diabetes and certain cardiovascular diseases (heart attack, sudden cardiac death, stroke).”
Not unanimous
Two other large randomized studies (J Clin Oncol. 2008 Feb 1;26[4]:585-91 and J Clin Oncol. 2009 Jan 1;27[1]:92-9) and two retrospective studies (J Clin Oncol. 2009 Jul 20;27[21]:3452-8 and J Clin Oncol. 2011 Sep 10;29[26]3510-16) found no excess risk of cardiovascular disease from ADT, Dr. Nguyen said, prompting him and his colleagues to see whether they could get a better estimate of the actual risk.
They did so through a 2011 meta-analysis (JAMA. 2011;306[21]:2359-66) of data on 4,141 patients from eight randomized trials. They found that among patients with unfavorable-risk prostate cancer, ADT was not associated with an increased risk of cardiovascular death, but was associated with lower risks for both prostate-specific and all-cause mortality.
Subpopulations may still be at risk
Dr. Nguyen said that the principal finding of the meta-analysis, while reassuring, “doesn’t let ADT off the hook for metabolic events, diabetes which we know happens, and the possibility of nonfatal cardiac events.”
He noted that while ADT was not associated with cardiovascular disease in clinical trials, observational studies showed significantly increased risk for fatal or non-fatal MI.
One possible explanation for the difference is that observational studies included nonfatal MI, while randomized trials looked only at cardiovascular deaths. It’s also possible that ADT causes harm primarily in men with preexisting comorbidities, who are often excluded from or underrepresented in clinical trials.
Evidence from a 2009 study (JAMA. 2009 Aug 26;302[8]:866-73) showed that among men with clinical stage T1 to T3 noninvasive, nonmetastatic prostate cancer, neoadjuvant hormonal therapy with both a luteinizing hormone-releasing hormone (LHRH) agonist and a nonsteroidal antiandrogen was associated with increased risk for all-cause mortality for those with a history of coronary artery disease–induced heart failure, but not for men with either no comorbidities or only a single comorbidity such as hypertension, hypercholesterolemia, or diabetes.
Clinical considerations
The decision to treat men with prostate cancer with ADT is therefore a balancing act, Dr. Nguyen said.
“As the risk of prostate cancer death goes up, the benefit of ADT goes up. However, as the comorbidity level goes up, the potential cardiovascular harm of ADT goes up,” he said.
For patients at the extreme ends of each continuum, such as a patient with high-risk prostate cancer and no cardiovascular comorbidities or a patient with low-risk cancer but multiple CV risk factors, the decision to give or withhold ADT is relatively simple, he said.
But for patients in between, such as a man with intermediate-risk cancer and one risk factor or a man with high risk disease with multiple comorbidities, the decision is far more complex.
“This where I think the dialogue with the cardiologist really needs to come into this decision,” he said.
Evidence to support the decision comes from retrospective studies suggesting that even men with high-risk prostate cancer have poorer overall survival with ADT if they have a history of heart failure or MI.
For patients with low-risk cancer and diabetes, ADT is associated with worse overall survival, but ADT does not cause additional harm to men with intermediate- to high-risk prostate cancer who have concomitant diabetes, Dr. Nguyen said.
“My view is that ADT has not been shown to increase cardiovascular death in randomized trials, so I think that for the vast majority of patients it probably does not increase cardiovascular deaths. But I think there could very well be a vulnerable 5% of patients who might have an excess risk of cardiovascular death, and I think we have to be careful, but we still have to balance it out against their risks for prostate cancer death,” he said.
Dr. Nguyen reported consulting fees/honoraria from Astellas, Augmenix, Blue Earth Diagnostics, Cota, Dendreon, Ferring Pharmaceuticals, GenomeDx, Janssen, and Nanobiotix.
WASHINGTON – The cardiovascular effects of androgen deprivation therapy (ADT) for men with advanced prostate cancer are less severe than once feared, but there is evidence to suggest that men with preexisting heart failure or a history of myocardial infarction could be at excess risk for death from cardiovascular causes when they receive ADT, according to a leading prostate cancer expert.
“I think there are concerns about potential cardiovascular harm of ADT, and I think this has reduced ADT use, despite the fact that we know for most men it improves overall survival,” said Paul Nguyen, MD, a radiation oncologist at the Dana-Farber/Brigham and Women’s Cancer Center in Boston.
“In fact, when we looked recently at men with high-risk prostate cancer, this is a group where overall survival is improved by 50% if they get ADT – so it cuts the risk of death in half – but it turns out that nearly a quarter of those patients are not receiving ADT. I think that the concern about cardiovascular harm and the confusion as to where that data stands is a lot of what’s driving that right now,” he said at the American College of Cardiology’s Advancing the Cardiovascular Care of the Oncology Patient meeting.
Randomized trial data
Dr. Nguyen noted that the evidence suggesting that ADT can increase the risk of death from cardiovascular causes came largely from three major studies:
- A 2006 study of 73,196 Medicare enrollees aged 66 or older, which found that ADT with a gonadotropin-releasing hormone (GnRH) agonist was possibly associated with increased risk of incident diabetes and cardiovascular disease (J Clin Oncol. 2006 Sep 20;24[27]:4448-56.).
- A 2007 analysis of data from the Cancer of the Prostate Strategic Urologic Research Endeavor (CAPSURE) database on 3,262 men treated with radical prostatectomy and 1,630 men treated with radiation or cryotherapy for localized prostate cancer, which found that among those 65 and older the 5-year cumulative incidence of cardiovascular death was 5.5% for patients who received ADT, vs. 2% for those who did not (J Natl Cancer Inst. 2007 Oct 17;99[20]:1516-24).
- A 2007 study of 1,372 men in three randomized trials of radiation therapy with or without androgen suppression therapy up to 8 months in duration, which found that men 65 and older who received 6 months of androgen suppression had significantly shorter times to fatal MIs than did men who did not receive the therapy (J Clin Oncol. 2007;25[17]:2420-5).
These studies, combined with observational data, led to a 2010 consensus statement from the American Heart Association, American Cancer Society, and American Urological Association, with endorsement from the American Society for Radiation Oncology, which stated that “there may be a relation between ADT and cardiovascular events and death.”
Also in 2010, the Food and Drug Administration required new labeling on GnRH agonists warning of “increased risk of diabetes and certain cardiovascular diseases (heart attack, sudden cardiac death, stroke).”
Not unanimous
Two other large randomized studies (J Clin Oncol. 2008 Feb 1;26[4]:585-91 and J Clin Oncol. 2009 Jan 1;27[1]:92-9) and two retrospective studies (J Clin Oncol. 2009 Jul 20;27[21]:3452-8 and J Clin Oncol. 2011 Sep 10;29[26]3510-16) found no excess risk of cardiovascular disease from ADT, Dr. Nguyen said, prompting him and his colleagues to see whether they could get a better estimate of the actual risk.
They did so through a 2011 meta-analysis (JAMA. 2011;306[21]:2359-66) of data on 4,141 patients from eight randomized trials. They found that among patients with unfavorable-risk prostate cancer, ADT was not associated with an increased risk of cardiovascular death, but was associated with lower risks for both prostate-specific and all-cause mortality.
Subpopulations may still be at risk
Dr. Nguyen said that the principal finding of the meta-analysis, while reassuring, “doesn’t let ADT off the hook for metabolic events, diabetes which we know happens, and the possibility of nonfatal cardiac events.”
He noted that while ADT was not associated with cardiovascular disease in clinical trials, observational studies showed significantly increased risk for fatal or non-fatal MI.
One possible explanation for the difference is that observational studies included nonfatal MI, while randomized trials looked only at cardiovascular deaths. It’s also possible that ADT causes harm primarily in men with preexisting comorbidities, who are often excluded from or underrepresented in clinical trials.
Evidence from a 2009 study (JAMA. 2009 Aug 26;302[8]:866-73) showed that among men with clinical stage T1 to T3 noninvasive, nonmetastatic prostate cancer, neoadjuvant hormonal therapy with both a luteinizing hormone-releasing hormone (LHRH) agonist and a nonsteroidal antiandrogen was associated with increased risk for all-cause mortality for those with a history of coronary artery disease–induced heart failure, but not for men with either no comorbidities or only a single comorbidity such as hypertension, hypercholesterolemia, or diabetes.
Clinical considerations
The decision to treat men with prostate cancer with ADT is therefore a balancing act, Dr. Nguyen said.
“As the risk of prostate cancer death goes up, the benefit of ADT goes up. However, as the comorbidity level goes up, the potential cardiovascular harm of ADT goes up,” he said.
For patients at the extreme ends of each continuum, such as a patient with high-risk prostate cancer and no cardiovascular comorbidities or a patient with low-risk cancer but multiple CV risk factors, the decision to give or withhold ADT is relatively simple, he said.
But for patients in between, such as a man with intermediate-risk cancer and one risk factor or a man with high risk disease with multiple comorbidities, the decision is far more complex.
“This where I think the dialogue with the cardiologist really needs to come into this decision,” he said.
Evidence to support the decision comes from retrospective studies suggesting that even men with high-risk prostate cancer have poorer overall survival with ADT if they have a history of heart failure or MI.
For patients with low-risk cancer and diabetes, ADT is associated with worse overall survival, but ADT does not cause additional harm to men with intermediate- to high-risk prostate cancer who have concomitant diabetes, Dr. Nguyen said.
“My view is that ADT has not been shown to increase cardiovascular death in randomized trials, so I think that for the vast majority of patients it probably does not increase cardiovascular deaths. But I think there could very well be a vulnerable 5% of patients who might have an excess risk of cardiovascular death, and I think we have to be careful, but we still have to balance it out against their risks for prostate cancer death,” he said.
Dr. Nguyen reported consulting fees/honoraria from Astellas, Augmenix, Blue Earth Diagnostics, Cota, Dendreon, Ferring Pharmaceuticals, GenomeDx, Janssen, and Nanobiotix.
REPORTING FROM ACC CARDIO-ONCOLOGY
Key clinical point: Risk of cardiovascular death should be weighed against proven ADT benefits.
Major finding: ADT-related cardiovascular events appear limited to men with comorbid cardiovascular disease.
Study details: Review of clinical data on the cardiovascular consequences of ADT.
Disclosures: Dr. Nguyen reported consulting fees/honoraria from Astellas, Augmenix, Blue Earth Diagnostics, Cota, Dendreon, Ferring Pharmaceuticals, GenomeDx, Janssen, and Nanobiotix.
Therapy ups breast cancer survivors’ cardiac risks
WASHINGTON – Oncologists and cardiologists need to work hand-in-hand when managing the care of women with breast cancer whose treatment plan includes cardiotoxic therapies and breast irradiation, reported specialists.
Depending on the cancer subtype, women with breast cancer may receive chemotherapy with a cardiotoxic anthracycline such as doxorubicin or epirubicin, or a HER2-targeted agent such as trastuzumab (Herceptin), pertuzumab (Perjeta), or ado-trastuzumab emtansine (Kadcyla).
“The cardiotoxicity related to breast cancer has been a well publicized issue, and chances are your patients know about it and are concerned as well,” Jennifer E. Liu, MD, director of cardiovascular laboratories at Memorial Sloan Kettering Cancer Center in New York, said at the American College of Cardiology’s Advancing the Cardiovascular Care of the Oncology Patient meeting.
Anthracyclines, trastuzumab, and HF
In large adjuvant therapy trials of anthracyclines and trastuzumab in women with breast cancer, doxorubicin alone was associated with an asymptomatic decline in left ventricular ejection fraction (LVEF) of 4% to 11%, and a less than 1% incidence of heart failure (HF), Dr. Liu noted.
When patients received an anthracycline followed by trastuzumab, the incidence of asymptomatic LVEF decline ranged from 4% to 19%, the incidence of clinical HF was 2% to 4%, and the rate of trastuzumab interruption for cardiac adverse events ranged from 5% to 18%.
In comparison, in trials with trastuzumab in combination therapy that did not contain an anthracycline, the risk of cardiovascular complications was lower, with asymptomatic decline in LVEF ranging from 3.2% to 9.4%, and class III/IV HF occurring in just 0.5% of patients. In trials combining trastuzumab and pertuzumab, there were no increases in cardiac toxicity over trastuzumab alone.
Although with longer follow-up, the approximately 4% rate of HF in patients treated with anthracycline-based chemotherapy, paclitaxel, and trastuzumab in the NSABP B-31 trial has not changed significantly; retrospective claims-based studies reflecting daily practice have shown significantly higher rates of HF and or cardiomyopathy, Dr. Liu said.
She cited a 2012 study showing that among 45,537 women with a mean age of 76 years who were treated for breast cancer, the 3-year incidence rates of HF and/or cardiomyopathy were 32% for patients treated with trastuzumab alone, and 41.9% for those treated with an anthracycline followed by trastuzumab. Other, smaller studies also showed lower but significantly elevated risks for the drugs.
The discrepancy between clinical trial and “real world” results may be chalked up to the fact that claims-based data rely on diagnostic codes that may not accurately reflect the actual cardiac diagnosis, and by the fact that clinical trials have strict entry criteria that exclude patients with cardiovascular disease, she said.
Radiation risks
Radiation therapy is associated with a more than 7% increase in major coronary events per Gy of mean heart dose, Dr. Liu noted, citing a 2013 study (N Engl J Med. 2013;368:987-98).
Paul Nguyen, MD, a radiation oncologist at the Dana-Farber/Brigham and Women’s Cancer Center in Boston, said that risk factors for radiation-induced heart disease include anterior or left chest irradiation, cumulative doses above 30 Gy, patient age younger than 50 years, doses of more than 2 Gy per fraction, presence and extent of tumor in or near the heart, lack of radiation shielding, concomitant chemotherapy (especially with anthracyclines), and preexisting cardiovascular disease or risk factors.
For patients with breast cancer, the risk of developing radiation-induced heart disease has diminished considerably with the adoption of heart-sparing techniques over the last several decades, including 3-D conformal techniques, intensity-modulated radiation therapy, proton beam therapy, novel patient positioning techniques that allow radiation only to the cancer-involved breast, and deep inspiration breath holds in which the radiation beam is gated to turn on only when the patient is holding a deep breath, Dr. Nguyen noted.
Treatment options for LVEF decline
The package insert for trastuzumab recommends withholding the drug for a minimum of 4 weeks if the patient has a 16% or greater decline in LVEF from baseline, or a 10% or greater decline from baseline to below the lower limit of normal. The insert recommends LVEF monitoring every 3 or 4 weeks, and says that trastuzumab can be resumed if LVEF improves to above the lower limit of normal with an absolute decrease from baseline of not more than 15%. The insert also states, however, that “the safety of continuation or resumption of trastuzumab in patients with trastuzumab induced LV dysfunction has never been studied, “ Dr. Liu noted.
She cited an American Society of Clinical Oncology guideline on the prevention and monitoring of cardiac dysfunction in survivors of adult cancers, which states in part that the decision to continue or discontinue cancer therapy in patients with evidence of cardiac dysfunction “made by the oncologist, should be informed by close collaboration with a cardiologist, fully evaluating the clinical circumstances and considering the risks and benefits of continuation of therapy responsible for the cardiac dysfunction.”
“I want to emphasize the importance of accepting and managing cardiovascular risk in patients priors to and during potentially cardiotoxic therapy. To optimize cardiologic and oncologic outcomes, we need to avoid or minimize treatment interruptions of life-saving therapy, and mitigate cardiac events with aggressive cardiovascular risk-factor modification,” Dr. Liu said.
She called for development of better risk stratification tools to tailor cardiac surveillance during therapy, based on both patient-specific and treatment-specific risk factors.
Dr. Liu reported nothing to disclose. Dr. Nguyen reported consulting fees/honoraria from Astellas, Augmenix, Blue Earth Diagnostics. Cota, Dendreon, Ferring Pharmaceuticals. GenomeDx, Janssen, and Nanobiotix.
WASHINGTON – Oncologists and cardiologists need to work hand-in-hand when managing the care of women with breast cancer whose treatment plan includes cardiotoxic therapies and breast irradiation, reported specialists.
Depending on the cancer subtype, women with breast cancer may receive chemotherapy with a cardiotoxic anthracycline such as doxorubicin or epirubicin, or a HER2-targeted agent such as trastuzumab (Herceptin), pertuzumab (Perjeta), or ado-trastuzumab emtansine (Kadcyla).
“The cardiotoxicity related to breast cancer has been a well publicized issue, and chances are your patients know about it and are concerned as well,” Jennifer E. Liu, MD, director of cardiovascular laboratories at Memorial Sloan Kettering Cancer Center in New York, said at the American College of Cardiology’s Advancing the Cardiovascular Care of the Oncology Patient meeting.
Anthracyclines, trastuzumab, and HF
In large adjuvant therapy trials of anthracyclines and trastuzumab in women with breast cancer, doxorubicin alone was associated with an asymptomatic decline in left ventricular ejection fraction (LVEF) of 4% to 11%, and a less than 1% incidence of heart failure (HF), Dr. Liu noted.
When patients received an anthracycline followed by trastuzumab, the incidence of asymptomatic LVEF decline ranged from 4% to 19%, the incidence of clinical HF was 2% to 4%, and the rate of trastuzumab interruption for cardiac adverse events ranged from 5% to 18%.
In comparison, in trials with trastuzumab in combination therapy that did not contain an anthracycline, the risk of cardiovascular complications was lower, with asymptomatic decline in LVEF ranging from 3.2% to 9.4%, and class III/IV HF occurring in just 0.5% of patients. In trials combining trastuzumab and pertuzumab, there were no increases in cardiac toxicity over trastuzumab alone.
Although with longer follow-up, the approximately 4% rate of HF in patients treated with anthracycline-based chemotherapy, paclitaxel, and trastuzumab in the NSABP B-31 trial has not changed significantly; retrospective claims-based studies reflecting daily practice have shown significantly higher rates of HF and or cardiomyopathy, Dr. Liu said.
She cited a 2012 study showing that among 45,537 women with a mean age of 76 years who were treated for breast cancer, the 3-year incidence rates of HF and/or cardiomyopathy were 32% for patients treated with trastuzumab alone, and 41.9% for those treated with an anthracycline followed by trastuzumab. Other, smaller studies also showed lower but significantly elevated risks for the drugs.
The discrepancy between clinical trial and “real world” results may be chalked up to the fact that claims-based data rely on diagnostic codes that may not accurately reflect the actual cardiac diagnosis, and by the fact that clinical trials have strict entry criteria that exclude patients with cardiovascular disease, she said.
Radiation risks
Radiation therapy is associated with a more than 7% increase in major coronary events per Gy of mean heart dose, Dr. Liu noted, citing a 2013 study (N Engl J Med. 2013;368:987-98).
Paul Nguyen, MD, a radiation oncologist at the Dana-Farber/Brigham and Women’s Cancer Center in Boston, said that risk factors for radiation-induced heart disease include anterior or left chest irradiation, cumulative doses above 30 Gy, patient age younger than 50 years, doses of more than 2 Gy per fraction, presence and extent of tumor in or near the heart, lack of radiation shielding, concomitant chemotherapy (especially with anthracyclines), and preexisting cardiovascular disease or risk factors.
For patients with breast cancer, the risk of developing radiation-induced heart disease has diminished considerably with the adoption of heart-sparing techniques over the last several decades, including 3-D conformal techniques, intensity-modulated radiation therapy, proton beam therapy, novel patient positioning techniques that allow radiation only to the cancer-involved breast, and deep inspiration breath holds in which the radiation beam is gated to turn on only when the patient is holding a deep breath, Dr. Nguyen noted.
Treatment options for LVEF decline
The package insert for trastuzumab recommends withholding the drug for a minimum of 4 weeks if the patient has a 16% or greater decline in LVEF from baseline, or a 10% or greater decline from baseline to below the lower limit of normal. The insert recommends LVEF monitoring every 3 or 4 weeks, and says that trastuzumab can be resumed if LVEF improves to above the lower limit of normal with an absolute decrease from baseline of not more than 15%. The insert also states, however, that “the safety of continuation or resumption of trastuzumab in patients with trastuzumab induced LV dysfunction has never been studied, “ Dr. Liu noted.
She cited an American Society of Clinical Oncology guideline on the prevention and monitoring of cardiac dysfunction in survivors of adult cancers, which states in part that the decision to continue or discontinue cancer therapy in patients with evidence of cardiac dysfunction “made by the oncologist, should be informed by close collaboration with a cardiologist, fully evaluating the clinical circumstances and considering the risks and benefits of continuation of therapy responsible for the cardiac dysfunction.”
“I want to emphasize the importance of accepting and managing cardiovascular risk in patients priors to and during potentially cardiotoxic therapy. To optimize cardiologic and oncologic outcomes, we need to avoid or minimize treatment interruptions of life-saving therapy, and mitigate cardiac events with aggressive cardiovascular risk-factor modification,” Dr. Liu said.
She called for development of better risk stratification tools to tailor cardiac surveillance during therapy, based on both patient-specific and treatment-specific risk factors.
Dr. Liu reported nothing to disclose. Dr. Nguyen reported consulting fees/honoraria from Astellas, Augmenix, Blue Earth Diagnostics. Cota, Dendreon, Ferring Pharmaceuticals. GenomeDx, Janssen, and Nanobiotix.
WASHINGTON – Oncologists and cardiologists need to work hand-in-hand when managing the care of women with breast cancer whose treatment plan includes cardiotoxic therapies and breast irradiation, reported specialists.
Depending on the cancer subtype, women with breast cancer may receive chemotherapy with a cardiotoxic anthracycline such as doxorubicin or epirubicin, or a HER2-targeted agent such as trastuzumab (Herceptin), pertuzumab (Perjeta), or ado-trastuzumab emtansine (Kadcyla).
“The cardiotoxicity related to breast cancer has been a well publicized issue, and chances are your patients know about it and are concerned as well,” Jennifer E. Liu, MD, director of cardiovascular laboratories at Memorial Sloan Kettering Cancer Center in New York, said at the American College of Cardiology’s Advancing the Cardiovascular Care of the Oncology Patient meeting.
Anthracyclines, trastuzumab, and HF
In large adjuvant therapy trials of anthracyclines and trastuzumab in women with breast cancer, doxorubicin alone was associated with an asymptomatic decline in left ventricular ejection fraction (LVEF) of 4% to 11%, and a less than 1% incidence of heart failure (HF), Dr. Liu noted.
When patients received an anthracycline followed by trastuzumab, the incidence of asymptomatic LVEF decline ranged from 4% to 19%, the incidence of clinical HF was 2% to 4%, and the rate of trastuzumab interruption for cardiac adverse events ranged from 5% to 18%.
In comparison, in trials with trastuzumab in combination therapy that did not contain an anthracycline, the risk of cardiovascular complications was lower, with asymptomatic decline in LVEF ranging from 3.2% to 9.4%, and class III/IV HF occurring in just 0.5% of patients. In trials combining trastuzumab and pertuzumab, there were no increases in cardiac toxicity over trastuzumab alone.
Although with longer follow-up, the approximately 4% rate of HF in patients treated with anthracycline-based chemotherapy, paclitaxel, and trastuzumab in the NSABP B-31 trial has not changed significantly; retrospective claims-based studies reflecting daily practice have shown significantly higher rates of HF and or cardiomyopathy, Dr. Liu said.
She cited a 2012 study showing that among 45,537 women with a mean age of 76 years who were treated for breast cancer, the 3-year incidence rates of HF and/or cardiomyopathy were 32% for patients treated with trastuzumab alone, and 41.9% for those treated with an anthracycline followed by trastuzumab. Other, smaller studies also showed lower but significantly elevated risks for the drugs.
The discrepancy between clinical trial and “real world” results may be chalked up to the fact that claims-based data rely on diagnostic codes that may not accurately reflect the actual cardiac diagnosis, and by the fact that clinical trials have strict entry criteria that exclude patients with cardiovascular disease, she said.
Radiation risks
Radiation therapy is associated with a more than 7% increase in major coronary events per Gy of mean heart dose, Dr. Liu noted, citing a 2013 study (N Engl J Med. 2013;368:987-98).
Paul Nguyen, MD, a radiation oncologist at the Dana-Farber/Brigham and Women’s Cancer Center in Boston, said that risk factors for radiation-induced heart disease include anterior or left chest irradiation, cumulative doses above 30 Gy, patient age younger than 50 years, doses of more than 2 Gy per fraction, presence and extent of tumor in or near the heart, lack of radiation shielding, concomitant chemotherapy (especially with anthracyclines), and preexisting cardiovascular disease or risk factors.
For patients with breast cancer, the risk of developing radiation-induced heart disease has diminished considerably with the adoption of heart-sparing techniques over the last several decades, including 3-D conformal techniques, intensity-modulated radiation therapy, proton beam therapy, novel patient positioning techniques that allow radiation only to the cancer-involved breast, and deep inspiration breath holds in which the radiation beam is gated to turn on only when the patient is holding a deep breath, Dr. Nguyen noted.
Treatment options for LVEF decline
The package insert for trastuzumab recommends withholding the drug for a minimum of 4 weeks if the patient has a 16% or greater decline in LVEF from baseline, or a 10% or greater decline from baseline to below the lower limit of normal. The insert recommends LVEF monitoring every 3 or 4 weeks, and says that trastuzumab can be resumed if LVEF improves to above the lower limit of normal with an absolute decrease from baseline of not more than 15%. The insert also states, however, that “the safety of continuation or resumption of trastuzumab in patients with trastuzumab induced LV dysfunction has never been studied, “ Dr. Liu noted.
She cited an American Society of Clinical Oncology guideline on the prevention and monitoring of cardiac dysfunction in survivors of adult cancers, which states in part that the decision to continue or discontinue cancer therapy in patients with evidence of cardiac dysfunction “made by the oncologist, should be informed by close collaboration with a cardiologist, fully evaluating the clinical circumstances and considering the risks and benefits of continuation of therapy responsible for the cardiac dysfunction.”
“I want to emphasize the importance of accepting and managing cardiovascular risk in patients priors to and during potentially cardiotoxic therapy. To optimize cardiologic and oncologic outcomes, we need to avoid or minimize treatment interruptions of life-saving therapy, and mitigate cardiac events with aggressive cardiovascular risk-factor modification,” Dr. Liu said.
She called for development of better risk stratification tools to tailor cardiac surveillance during therapy, based on both patient-specific and treatment-specific risk factors.
Dr. Liu reported nothing to disclose. Dr. Nguyen reported consulting fees/honoraria from Astellas, Augmenix, Blue Earth Diagnostics. Cota, Dendreon, Ferring Pharmaceuticals. GenomeDx, Janssen, and Nanobiotix.
REPORTING FROM ACC CARDIO-ONCOLOGY
Key clinical point: Oncologists should work with cardiologists to mitigate heart disease risk.
Major finding: Anthracyclines followed by trastuzumab significantly increase risk of HF.
Study details: Review of risk for heart disease in breast cancer survivors.
Disclosures: Dr. Liu reported nothing to disclose. Dr. Nguyen reported consulting fees/honoraria from Astellas, Augmenix, Blue Earth Diagnostics, Cota, Dendreon, Ferring Pharmaceuticals, GenomeDx, Janssen, and Nanobiotix.
Cloud of inconsistency hangs over cannabis data
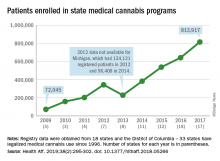
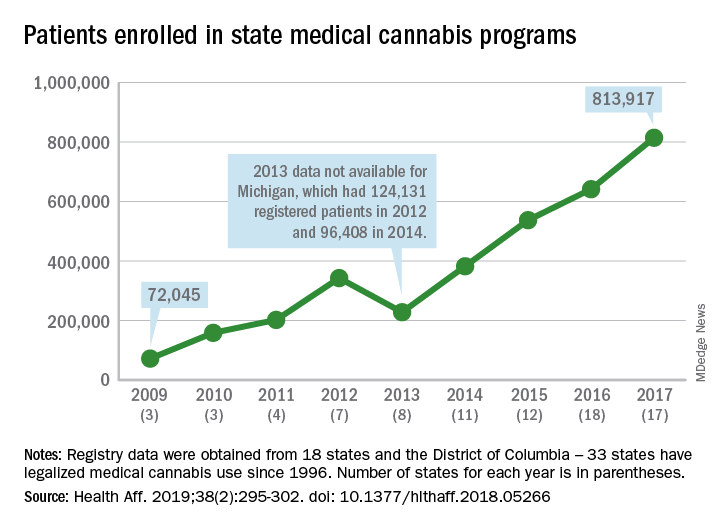
More people are using medical cannabis as it becomes legal in more states, but the lack of standardization in states’ data collection hindered investigators’ efforts to track that use.
Legalized medical cannabis is now available in 33 states and the District of Columbia, and the number of users has risen from just over 72,000 in 2009 to almost 814,000 in 2017. That 814,000, however, covers only 16 states and D.C., since 1 state (Connecticut) does not publish reports on medical cannabis use, 12 did not have statistics available, 2 (New York and Vermont) didn’t report data for 2017, and 2 (California and Maine) have voluntary registries that are unlikely to be accurate, according to Kevin F. Boehnke, PhD, of the University of Michigan, Ann Arbor, and his associates.
Michigan had the largest reported number of patients enrolled in its medical cannabis program in 2017, almost 270,000. California – the state with the oldest medical cannabis legislation (passed in 1996) and the largest overall population but a voluntary cannabis registry – reported its highest number of enrollees, 12,659, in 2009-2010, the investigators said. Colorado had more than 116,000 patients in its medical cannabis program in 2010 (Health Aff. 2019;38[2]:295-302).
The “many inconsistencies in data quality across states [suggest] the need for further standardization of data collection. Such standardization would add transparency to understanding how medical cannabis programs are used, which would help guide both research and policy needs,” Dr. Boehnke and his associates wrote.
More consistency was seen in the reasons for using medical cannabis. Chronic pain made up 62.2% of all qualifying conditions reported by patients during 1999-2016, with the annual average varying between 33.3% and 73%. Multiple sclerosis spasticity symptoms had the second-highest number of reports over the study period, followed by chemotherapy-induced nausea and vomiting, posttraumatic stress disorder, and cancer, they reported.
The investigators also looked at the appropriateness of cannabis and determined that its use in 85.5% of patient-reported conditions was “supported by conclusive or substantial evidence of therapeutic effectiveness, according to the 2017 National Academies report” on the health effects of cannabis.
“We believe not only that it is inappropriate for cannabis to remain a Schedule I substance, but also that state and federal policy makers should begin evaluating evidence-based ways for safely integrating cannabis research and products into the health care system,” they concluded.
SOURCE: Boehnke KF et al. Health Aff. 2019;38(2):295-302.
More people are using medical cannabis as it becomes legal in more states, but the lack of standardization in states’ data collection hindered investigators’ efforts to track that use.
Legalized medical cannabis is now available in 33 states and the District of Columbia, and the number of users has risen from just over 72,000 in 2009 to almost 814,000 in 2017. That 814,000, however, covers only 16 states and D.C., since 1 state (Connecticut) does not publish reports on medical cannabis use, 12 did not have statistics available, 2 (New York and Vermont) didn’t report data for 2017, and 2 (California and Maine) have voluntary registries that are unlikely to be accurate, according to Kevin F. Boehnke, PhD, of the University of Michigan, Ann Arbor, and his associates.
Michigan had the largest reported number of patients enrolled in its medical cannabis program in 2017, almost 270,000. California – the state with the oldest medical cannabis legislation (passed in 1996) and the largest overall population but a voluntary cannabis registry – reported its highest number of enrollees, 12,659, in 2009-2010, the investigators said. Colorado had more than 116,000 patients in its medical cannabis program in 2010 (Health Aff. 2019;38[2]:295-302).
The “many inconsistencies in data quality across states [suggest] the need for further standardization of data collection. Such standardization would add transparency to understanding how medical cannabis programs are used, which would help guide both research and policy needs,” Dr. Boehnke and his associates wrote.
More consistency was seen in the reasons for using medical cannabis. Chronic pain made up 62.2% of all qualifying conditions reported by patients during 1999-2016, with the annual average varying between 33.3% and 73%. Multiple sclerosis spasticity symptoms had the second-highest number of reports over the study period, followed by chemotherapy-induced nausea and vomiting, posttraumatic stress disorder, and cancer, they reported.
The investigators also looked at the appropriateness of cannabis and determined that its use in 85.5% of patient-reported conditions was “supported by conclusive or substantial evidence of therapeutic effectiveness, according to the 2017 National Academies report” on the health effects of cannabis.
“We believe not only that it is inappropriate for cannabis to remain a Schedule I substance, but also that state and federal policy makers should begin evaluating evidence-based ways for safely integrating cannabis research and products into the health care system,” they concluded.
SOURCE: Boehnke KF et al. Health Aff. 2019;38(2):295-302.
More people are using medical cannabis as it becomes legal in more states, but the lack of standardization in states’ data collection hindered investigators’ efforts to track that use.
Legalized medical cannabis is now available in 33 states and the District of Columbia, and the number of users has risen from just over 72,000 in 2009 to almost 814,000 in 2017. That 814,000, however, covers only 16 states and D.C., since 1 state (Connecticut) does not publish reports on medical cannabis use, 12 did not have statistics available, 2 (New York and Vermont) didn’t report data for 2017, and 2 (California and Maine) have voluntary registries that are unlikely to be accurate, according to Kevin F. Boehnke, PhD, of the University of Michigan, Ann Arbor, and his associates.
Michigan had the largest reported number of patients enrolled in its medical cannabis program in 2017, almost 270,000. California – the state with the oldest medical cannabis legislation (passed in 1996) and the largest overall population but a voluntary cannabis registry – reported its highest number of enrollees, 12,659, in 2009-2010, the investigators said. Colorado had more than 116,000 patients in its medical cannabis program in 2010 (Health Aff. 2019;38[2]:295-302).
The “many inconsistencies in data quality across states [suggest] the need for further standardization of data collection. Such standardization would add transparency to understanding how medical cannabis programs are used, which would help guide both research and policy needs,” Dr. Boehnke and his associates wrote.
More consistency was seen in the reasons for using medical cannabis. Chronic pain made up 62.2% of all qualifying conditions reported by patients during 1999-2016, with the annual average varying between 33.3% and 73%. Multiple sclerosis spasticity symptoms had the second-highest number of reports over the study period, followed by chemotherapy-induced nausea and vomiting, posttraumatic stress disorder, and cancer, they reported.
The investigators also looked at the appropriateness of cannabis and determined that its use in 85.5% of patient-reported conditions was “supported by conclusive or substantial evidence of therapeutic effectiveness, according to the 2017 National Academies report” on the health effects of cannabis.
“We believe not only that it is inappropriate for cannabis to remain a Schedule I substance, but also that state and federal policy makers should begin evaluating evidence-based ways for safely integrating cannabis research and products into the health care system,” they concluded.
SOURCE: Boehnke KF et al. Health Aff. 2019;38(2):295-302.
FROM HEALTH AFFAIRS
HHS effort aims to end new HIV cases within 10 years
WASHINGTON – Leaders from five federal agencies came together to announce the framework for a bold new national initiative that aims to eliminate new cases of HIV infection in the United States within 10 years. The announcement came the day after President Trump’s State of the Union address, which highlighted the new effort.
“HIV has cost America too much for too long,” said Adm. Brett Giroir, MD, assistant secretary for health at the Department of Health & Human Services, in a press briefing. In addition to the 700,000 U.S. lives the disease has claimed since 1981, “We are at high risk of another 400,000 becoming infected over the next decade,” with about 40,000 new infections still occurring every year, he said.
Dr. Giroir will lead a coordinated effort among HHS, the Centers for Disease Control, the National Institutes of Health, the Health Resources and Services Administration, and the Indian Health Service. The goals are to reduce new cases of HIV by 50% within 5 years, and by 90% within 10 years.
These 48 counties, together with Washington and San Juan, Puerto Rico, accounted for more than half of the new HIV diagnoses in 2016 and 2017, said Dr. Giroir.
“This is a laser-focused program targeting counties where infection is the highest,” said CDC Director Robert R. Redfield, MD. “We propose to deploy personnel, resources, and strategies” in these targeted areas to maximize not just diagnosis and treatment but also to reach those at risk for HIV to enroll them in preexposure prophylaxis (PrEP) regimens, he said.
In addition to the targeted counties, seven states in the rural South as well as Native American and Alaskan Native populations also will receive intensified education, diagnostic, and treatment services. The targeted states are Alabama, Arkansas, Kentucky, Mississippi, Missouri, Oklahoma, and South Carolina.
George Sigounas, PhD, administrator or the Health Resources and Services Administration, said that existing community health centers will be especially important in reaching rural underserved and marginalized populations. Currently, he said, HRSA supports 12,000 service delivery sites across the country that are already delivering care to 27 million individuals. “These sites will play a major expanded role in providing PrEP to those who are at the greatest risk of contracting HIV,” said Dr. Sigounas.
Among the currently existing resources that will be leveraged are services provided by the Ryan White HIV/AIDS program, which already provides HIV primary medical care and support services through a network of grants to states and local government and community organizations. About half of the people currently diagnosed with HIV in the United States receive services through this program now.
The NIH maintains a geographically distributed network of Centers for AIDS Research that also will be folded into the new initiative.
In his remarks, Anthony S. Fauci, MD, director of the NIH’s National Center for Allergy and Infectious Diseases, pointed out that, “Treatment and detection are wrapped together, because treated individuals can’t transmit HIV” if they are adherent to antiretroviral medication use and achieve an undetectable viral load, he said. “If you get everyone who’s infected on antiretrovirals and give those who need it PrEP, you can theoretically end the epidemic as we know it – and that is our goal.”
Dr. Fauci went on to say that implementation science will play a key role in achieving a targeted and coordinated approach. “We will work closely with our colleagues to make sure the implementation is done well. We have lessons learned; we will do better and better,” he said.
The nuts and bolts of the program include a four-pronged strategy to diagnose individuals as early as possible after infection, to initiate prompt, effective, and sustained treatment, to protect those at risk for HIV by proven means including PrEP, and to provide rapid response when new HIV clusters are identified. A reimagining of current and future personnel into an “HIV health force” will put teams on the ground in each jurisdiction to carry out the initiative.
Though the goal is to provide PrEP to every at-risk individual, Dr. Fauci said that current modeling shows that if PrEP reaches 50%-60% in the at-risk population, new infections can be reduced by 90%. He added, “PrEP works. The efficacy is well over 90%.”
Funding details were not released at the press briefing; Dr. Giroir said that figures will be released by the Office of Management and Budget as part of the 2020 budget cycle. He confirmed, however, that new funds will be allocated for the effort, rather than a mere reshuffling of existing fund and resources.
Several of the leaders acknowledged the problem of stigma and marginalization that many individuals living with or at risk for HIV face, since men who have sex with men, transgender people, sex workers, and those with opioid use disorder all fall into this category.
“Every American deserves to be treated with respect and dignity. We will vigorously enforce all laws on the books about discrimination,” said Rear Adm. Michael Weahkee, MD, principal deputy director of the Indian Health Service. This is especially important in Native American communities “where everybody knows everybody,” he said, and it’s vitally important to include individual and community education in the efforts.
Dr. Redfield concurred, adding that “Dr. Fauci and I have been engaged in HIV since 1981. We have witnessed firsthand the negative impact that stigma can have on our capacity to practice public health. The transgender population, in particular, needs to be reached out to. We need to be able to address in a comprehensive way how to destigmatize the HIV population.”
WASHINGTON – Leaders from five federal agencies came together to announce the framework for a bold new national initiative that aims to eliminate new cases of HIV infection in the United States within 10 years. The announcement came the day after President Trump’s State of the Union address, which highlighted the new effort.

“HIV has cost America too much for too long,” said Adm. Brett Giroir, MD, assistant secretary for health at the Department of Health & Human Services, in a press briefing. In addition to the 700,000 U.S. lives the disease has claimed since 1981, “We are at high risk of another 400,000 becoming infected over the next decade,” with about 40,000 new infections still occurring every year, he said.
Dr. Giroir will lead a coordinated effort among HHS, the Centers for Disease Control, the National Institutes of Health, the Health Resources and Services Administration, and the Indian Health Service. The goals are to reduce new cases of HIV by 50% within 5 years, and by 90% within 10 years.
These 48 counties, together with Washington and San Juan, Puerto Rico, accounted for more than half of the new HIV diagnoses in 2016 and 2017, said Dr. Giroir.
“This is a laser-focused program targeting counties where infection is the highest,” said CDC Director Robert R. Redfield, MD. “We propose to deploy personnel, resources, and strategies” in these targeted areas to maximize not just diagnosis and treatment but also to reach those at risk for HIV to enroll them in preexposure prophylaxis (PrEP) regimens, he said.
In addition to the targeted counties, seven states in the rural South as well as Native American and Alaskan Native populations also will receive intensified education, diagnostic, and treatment services. The targeted states are Alabama, Arkansas, Kentucky, Mississippi, Missouri, Oklahoma, and South Carolina.
George Sigounas, PhD, administrator or the Health Resources and Services Administration, said that existing community health centers will be especially important in reaching rural underserved and marginalized populations. Currently, he said, HRSA supports 12,000 service delivery sites across the country that are already delivering care to 27 million individuals. “These sites will play a major expanded role in providing PrEP to those who are at the greatest risk of contracting HIV,” said Dr. Sigounas.
Among the currently existing resources that will be leveraged are services provided by the Ryan White HIV/AIDS program, which already provides HIV primary medical care and support services through a network of grants to states and local government and community organizations. About half of the people currently diagnosed with HIV in the United States receive services through this program now.
The NIH maintains a geographically distributed network of Centers for AIDS Research that also will be folded into the new initiative.
In his remarks, Anthony S. Fauci, MD, director of the NIH’s National Center for Allergy and Infectious Diseases, pointed out that, “Treatment and detection are wrapped together, because treated individuals can’t transmit HIV” if they are adherent to antiretroviral medication use and achieve an undetectable viral load, he said. “If you get everyone who’s infected on antiretrovirals and give those who need it PrEP, you can theoretically end the epidemic as we know it – and that is our goal.”
Dr. Fauci went on to say that implementation science will play a key role in achieving a targeted and coordinated approach. “We will work closely with our colleagues to make sure the implementation is done well. We have lessons learned; we will do better and better,” he said.
The nuts and bolts of the program include a four-pronged strategy to diagnose individuals as early as possible after infection, to initiate prompt, effective, and sustained treatment, to protect those at risk for HIV by proven means including PrEP, and to provide rapid response when new HIV clusters are identified. A reimagining of current and future personnel into an “HIV health force” will put teams on the ground in each jurisdiction to carry out the initiative.
Though the goal is to provide PrEP to every at-risk individual, Dr. Fauci said that current modeling shows that if PrEP reaches 50%-60% in the at-risk population, new infections can be reduced by 90%. He added, “PrEP works. The efficacy is well over 90%.”
Funding details were not released at the press briefing; Dr. Giroir said that figures will be released by the Office of Management and Budget as part of the 2020 budget cycle. He confirmed, however, that new funds will be allocated for the effort, rather than a mere reshuffling of existing fund and resources.
Several of the leaders acknowledged the problem of stigma and marginalization that many individuals living with or at risk for HIV face, since men who have sex with men, transgender people, sex workers, and those with opioid use disorder all fall into this category.
“Every American deserves to be treated with respect and dignity. We will vigorously enforce all laws on the books about discrimination,” said Rear Adm. Michael Weahkee, MD, principal deputy director of the Indian Health Service. This is especially important in Native American communities “where everybody knows everybody,” he said, and it’s vitally important to include individual and community education in the efforts.
Dr. Redfield concurred, adding that “Dr. Fauci and I have been engaged in HIV since 1981. We have witnessed firsthand the negative impact that stigma can have on our capacity to practice public health. The transgender population, in particular, needs to be reached out to. We need to be able to address in a comprehensive way how to destigmatize the HIV population.”
WASHINGTON – Leaders from five federal agencies came together to announce the framework for a bold new national initiative that aims to eliminate new cases of HIV infection in the United States within 10 years. The announcement came the day after President Trump’s State of the Union address, which highlighted the new effort.

“HIV has cost America too much for too long,” said Adm. Brett Giroir, MD, assistant secretary for health at the Department of Health & Human Services, in a press briefing. In addition to the 700,000 U.S. lives the disease has claimed since 1981, “We are at high risk of another 400,000 becoming infected over the next decade,” with about 40,000 new infections still occurring every year, he said.
Dr. Giroir will lead a coordinated effort among HHS, the Centers for Disease Control, the National Institutes of Health, the Health Resources and Services Administration, and the Indian Health Service. The goals are to reduce new cases of HIV by 50% within 5 years, and by 90% within 10 years.
These 48 counties, together with Washington and San Juan, Puerto Rico, accounted for more than half of the new HIV diagnoses in 2016 and 2017, said Dr. Giroir.
“This is a laser-focused program targeting counties where infection is the highest,” said CDC Director Robert R. Redfield, MD. “We propose to deploy personnel, resources, and strategies” in these targeted areas to maximize not just diagnosis and treatment but also to reach those at risk for HIV to enroll them in preexposure prophylaxis (PrEP) regimens, he said.
In addition to the targeted counties, seven states in the rural South as well as Native American and Alaskan Native populations also will receive intensified education, diagnostic, and treatment services. The targeted states are Alabama, Arkansas, Kentucky, Mississippi, Missouri, Oklahoma, and South Carolina.
George Sigounas, PhD, administrator or the Health Resources and Services Administration, said that existing community health centers will be especially important in reaching rural underserved and marginalized populations. Currently, he said, HRSA supports 12,000 service delivery sites across the country that are already delivering care to 27 million individuals. “These sites will play a major expanded role in providing PrEP to those who are at the greatest risk of contracting HIV,” said Dr. Sigounas.
Among the currently existing resources that will be leveraged are services provided by the Ryan White HIV/AIDS program, which already provides HIV primary medical care and support services through a network of grants to states and local government and community organizations. About half of the people currently diagnosed with HIV in the United States receive services through this program now.
The NIH maintains a geographically distributed network of Centers for AIDS Research that also will be folded into the new initiative.
In his remarks, Anthony S. Fauci, MD, director of the NIH’s National Center for Allergy and Infectious Diseases, pointed out that, “Treatment and detection are wrapped together, because treated individuals can’t transmit HIV” if they are adherent to antiretroviral medication use and achieve an undetectable viral load, he said. “If you get everyone who’s infected on antiretrovirals and give those who need it PrEP, you can theoretically end the epidemic as we know it – and that is our goal.”
Dr. Fauci went on to say that implementation science will play a key role in achieving a targeted and coordinated approach. “We will work closely with our colleagues to make sure the implementation is done well. We have lessons learned; we will do better and better,” he said.
The nuts and bolts of the program include a four-pronged strategy to diagnose individuals as early as possible after infection, to initiate prompt, effective, and sustained treatment, to protect those at risk for HIV by proven means including PrEP, and to provide rapid response when new HIV clusters are identified. A reimagining of current and future personnel into an “HIV health force” will put teams on the ground in each jurisdiction to carry out the initiative.
Though the goal is to provide PrEP to every at-risk individual, Dr. Fauci said that current modeling shows that if PrEP reaches 50%-60% in the at-risk population, new infections can be reduced by 90%. He added, “PrEP works. The efficacy is well over 90%.”
Funding details were not released at the press briefing; Dr. Giroir said that figures will be released by the Office of Management and Budget as part of the 2020 budget cycle. He confirmed, however, that new funds will be allocated for the effort, rather than a mere reshuffling of existing fund and resources.
Several of the leaders acknowledged the problem of stigma and marginalization that many individuals living with or at risk for HIV face, since men who have sex with men, transgender people, sex workers, and those with opioid use disorder all fall into this category.
“Every American deserves to be treated with respect and dignity. We will vigorously enforce all laws on the books about discrimination,” said Rear Adm. Michael Weahkee, MD, principal deputy director of the Indian Health Service. This is especially important in Native American communities “where everybody knows everybody,” he said, and it’s vitally important to include individual and community education in the efforts.
Dr. Redfield concurred, adding that “Dr. Fauci and I have been engaged in HIV since 1981. We have witnessed firsthand the negative impact that stigma can have on our capacity to practice public health. The transgender population, in particular, needs to be reached out to. We need to be able to address in a comprehensive way how to destigmatize the HIV population.”
FROM A HEALTH AND HUMAN SERVICES BRIEFING
Immunotherapy’s cardiac effects require early monitoring, management
WASHINGTON – Unquestionably, immunotherapy is revolutionizing the care of patients with various solid tumors and hematologic malignancies.
But it’s equally true that there’s no such thing as either a free lunch or a cancer therapy free of side effects, whether it’s increased risk for heart failure associated with anthracycline-based chemotherapy, or inflammatory conditions, arrhythmias, and thromboembolic events associated with immune checkpoint inhibitors, said R. Frank Cornell, MD, of Vanderbilt University Medical Center in Nashville, Tenn.
“Early awareness and intervention is critical for improved outcomes, and a multidisciplinary approach between oncology, cardiology, the clinic nurse, and other health care providers is critical in managing these patients with these complicated therapies,” he said at the American College of Cardiology’s Advancing the Cardiovascular Care of the Oncology Patient meeting.
Checkpoint inhibitors and the heart
Toxicities associated with immune checkpoint inhibitors such as the programmed death 1/ligand 1 (PD-1/PD-L1) inhibitors nivolumab (Opdivo) and pembrolizumab (Keytruda) and the cytotoxic T-lymphocyte antigen 4 antibody ipilimumab (Yervoy) tend to mimic autoimmune conditions, Dr. Cornell said.
Cardiovascular events associated with these agents, while uncommon, include myocarditis, pericarditis, arrhythmias, impaired ventricular function with heart failure, vasculitis, and venous thromboembolism, he said, citing an American Society of Clinical Oncology (ASCO) clinical practice guideline (J Clin Oncol 2018;36[17]:1714-68).
Dr. Cornell described the case of a 63-year-old woman with disseminated metastatic melanoma who presented to the emergency department 10 days after starting on combination therapy with ipilimumab and nivolumab. She had developed shortness of breath, pleuritic chest pain, and a mild cough for 1 or 2 days.
Her cardiac laboratory markers had been normal at baseline, but were markedly elevated on presentation, and electrocardiograms showed complete heart block and subsequent ventricular tachycardia.
The patient was started on high-dose prednisone, but she died in hospital, and an autopsy showed that the cause of death was infiltration into the myocardium of CD3-positive and CD8-positive T lymphocytes.
“So how do we manage this? This is a good opportunity, I think, for further cardiology and oncology collaboration to develop more robust guidelines for what we can do to best prevent this,” Dr. Cornell said.
Patients started on the ipilimumab/nivolumab combination should be tested weekly for cardiac troponin, creatine kinase (CK) and CK-muscle/brain (CK-MB) weekly for the first 3-4 weeks of therapy. Therapy should be stopped if troponin levels continue to rise, and the patient should be started on high-dose steroids, he said.
The role of other anti-inflammatory agents such as infliximab (Remicade and biosimilars) is unclear and needs further study, he added.
Dr. Cornell cited a 2018 letter to The Lancet by Javid J. Moslehi, MD, and colleagues from Vanderbilt describing an increase in reports of fatal myocarditis among patients treated with checkpoint inhibitors.
“We highlight the high mortality rate with severe immune checkpoint inhibitor–related myocarditis, which is more frequent with combination PD-1 and CTLA-4 blockade, but can also occur with monotherapy. Myocarditis was observed across immune checkpoint inhibitor regimens, although it remains too early to determine whether the incidence differs between use of anti-PD1 and anti-PD-L1 drugs. Furthermore, this condition occurs early on during therapy and across cancer types,” they wrote.
Most of the patients had no preexisting cardiovascular disease, and most were not taking medications for hypertension, cardiovascular disease, or diabetes.
CAR-T cells and cardiac disease
The primary cardiac complications associated with CAR-T cell therapy are related to the cytokine release syndrome (CRS), a condition marked by progressive elevation in inflammatory cytokines that in turn leads to marked elevations in C-reactive protein (CRP), interferon gamma, tumor necrosis factor al, and release of pro-inflammatory cytokines including interleukin (IL) 6, IL-10, IL-12, and IL-1 beta.
In rare instances, CRS can lead to disseminated intravascular coagulation (DIC), capillary leak syndrome, and a hemophagocytic lymphohistiocytosis-like (HLH) syndrome, Dr. Cornell said.
Package inserts for the two Food and Drug Administration–approved CAR-T cell products, axicabtagene ciloleucel (Yescarta) and tisagenlecleucel (Kymriah) show that each was associated in clinical trials with a high incidence of CRS.
Among patients treated with axicabtagene ciloleucel, 94% developed CRS, which was grade 3 or greater in severity in 13%. The median time to onset was 2 days, and the median duration was 7 days. Cardiovascular adverse events included grade 3 or greater tachycardia in 2%, arrhythmias in 7%, edema in 1%, dyspnea in 3%, pleural effusion in 2%, hypotension in 15%, hypertension in 6%, and thrombosis in 1%.
Among patients treated with tisagenlecleucel, 79% treated for B-cell acute lymphoblastic leukemia (B-ALL) and 74% treated for diffuse large B cell lymphoma (DLBCL) developed CRS, which was grade 3 or greater in 49% and 23% of patients, respectively. The median time to onset was 3 days, and the median duration of CRS was 8 days.
Cardiovascular adverse events of grade 3 or greater among these patients included tachycardia in 4%, fluid overload in 7%, edema in 1%, dyspnea in 12%, pulmonary edema in 4%, hypotension in 22%, and hypertension in 6%.
Risk factors for CRS include high pre-infusion tumor burden, active infections, and concurrent inflammatory processes, Dr. Cornell said.
Prevention of cardiovascular complications of CAR-T cell therapy requires management of CRS. Patients with grade 2 or greater CRS should receive the anti-IL-6 agent tocilizumab (Actemra) 8 mg/kg intravenously over 1 hour to a maximum dose of 800 mg. Tocilizumab infusions can be repeated every 8 hours as needed if the patient is not responsive to intravenous fluids or increasing supplement oxygen, but should be limited to a maximum of three doses over 24 hours, and a maximum total of four doses.
Patients with grade 3 CRS should also receive intravenous methylprednisolone 1 mg/kg twice daily or the equivalent amount of dexamethasone, with corticosteroids continued until the severity of CRS is grade 1 or less, then tapered over 3 days,
Patients with grade 4 CRS should also receive IV methylprednisolone 1,000 mg per day for 3 days, and if symptoms improve, continue management as per grade 3, Dr. Cornell said.
Dr. Cornell reported having nothing to disclose.
WASHINGTON – Unquestionably, immunotherapy is revolutionizing the care of patients with various solid tumors and hematologic malignancies.
But it’s equally true that there’s no such thing as either a free lunch or a cancer therapy free of side effects, whether it’s increased risk for heart failure associated with anthracycline-based chemotherapy, or inflammatory conditions, arrhythmias, and thromboembolic events associated with immune checkpoint inhibitors, said R. Frank Cornell, MD, of Vanderbilt University Medical Center in Nashville, Tenn.
“Early awareness and intervention is critical for improved outcomes, and a multidisciplinary approach between oncology, cardiology, the clinic nurse, and other health care providers is critical in managing these patients with these complicated therapies,” he said at the American College of Cardiology’s Advancing the Cardiovascular Care of the Oncology Patient meeting.
Checkpoint inhibitors and the heart
Toxicities associated with immune checkpoint inhibitors such as the programmed death 1/ligand 1 (PD-1/PD-L1) inhibitors nivolumab (Opdivo) and pembrolizumab (Keytruda) and the cytotoxic T-lymphocyte antigen 4 antibody ipilimumab (Yervoy) tend to mimic autoimmune conditions, Dr. Cornell said.
Cardiovascular events associated with these agents, while uncommon, include myocarditis, pericarditis, arrhythmias, impaired ventricular function with heart failure, vasculitis, and venous thromboembolism, he said, citing an American Society of Clinical Oncology (ASCO) clinical practice guideline (J Clin Oncol 2018;36[17]:1714-68).
Dr. Cornell described the case of a 63-year-old woman with disseminated metastatic melanoma who presented to the emergency department 10 days after starting on combination therapy with ipilimumab and nivolumab. She had developed shortness of breath, pleuritic chest pain, and a mild cough for 1 or 2 days.
Her cardiac laboratory markers had been normal at baseline, but were markedly elevated on presentation, and electrocardiograms showed complete heart block and subsequent ventricular tachycardia.
The patient was started on high-dose prednisone, but she died in hospital, and an autopsy showed that the cause of death was infiltration into the myocardium of CD3-positive and CD8-positive T lymphocytes.
“So how do we manage this? This is a good opportunity, I think, for further cardiology and oncology collaboration to develop more robust guidelines for what we can do to best prevent this,” Dr. Cornell said.
Patients started on the ipilimumab/nivolumab combination should be tested weekly for cardiac troponin, creatine kinase (CK) and CK-muscle/brain (CK-MB) weekly for the first 3-4 weeks of therapy. Therapy should be stopped if troponin levels continue to rise, and the patient should be started on high-dose steroids, he said.
The role of other anti-inflammatory agents such as infliximab (Remicade and biosimilars) is unclear and needs further study, he added.
Dr. Cornell cited a 2018 letter to The Lancet by Javid J. Moslehi, MD, and colleagues from Vanderbilt describing an increase in reports of fatal myocarditis among patients treated with checkpoint inhibitors.
“We highlight the high mortality rate with severe immune checkpoint inhibitor–related myocarditis, which is more frequent with combination PD-1 and CTLA-4 blockade, but can also occur with monotherapy. Myocarditis was observed across immune checkpoint inhibitor regimens, although it remains too early to determine whether the incidence differs between use of anti-PD1 and anti-PD-L1 drugs. Furthermore, this condition occurs early on during therapy and across cancer types,” they wrote.
Most of the patients had no preexisting cardiovascular disease, and most were not taking medications for hypertension, cardiovascular disease, or diabetes.
CAR-T cells and cardiac disease
The primary cardiac complications associated with CAR-T cell therapy are related to the cytokine release syndrome (CRS), a condition marked by progressive elevation in inflammatory cytokines that in turn leads to marked elevations in C-reactive protein (CRP), interferon gamma, tumor necrosis factor al, and release of pro-inflammatory cytokines including interleukin (IL) 6, IL-10, IL-12, and IL-1 beta.
In rare instances, CRS can lead to disseminated intravascular coagulation (DIC), capillary leak syndrome, and a hemophagocytic lymphohistiocytosis-like (HLH) syndrome, Dr. Cornell said.
Package inserts for the two Food and Drug Administration–approved CAR-T cell products, axicabtagene ciloleucel (Yescarta) and tisagenlecleucel (Kymriah) show that each was associated in clinical trials with a high incidence of CRS.
Among patients treated with axicabtagene ciloleucel, 94% developed CRS, which was grade 3 or greater in severity in 13%. The median time to onset was 2 days, and the median duration was 7 days. Cardiovascular adverse events included grade 3 or greater tachycardia in 2%, arrhythmias in 7%, edema in 1%, dyspnea in 3%, pleural effusion in 2%, hypotension in 15%, hypertension in 6%, and thrombosis in 1%.
Among patients treated with tisagenlecleucel, 79% treated for B-cell acute lymphoblastic leukemia (B-ALL) and 74% treated for diffuse large B cell lymphoma (DLBCL) developed CRS, which was grade 3 or greater in 49% and 23% of patients, respectively. The median time to onset was 3 days, and the median duration of CRS was 8 days.
Cardiovascular adverse events of grade 3 or greater among these patients included tachycardia in 4%, fluid overload in 7%, edema in 1%, dyspnea in 12%, pulmonary edema in 4%, hypotension in 22%, and hypertension in 6%.
Risk factors for CRS include high pre-infusion tumor burden, active infections, and concurrent inflammatory processes, Dr. Cornell said.
Prevention of cardiovascular complications of CAR-T cell therapy requires management of CRS. Patients with grade 2 or greater CRS should receive the anti-IL-6 agent tocilizumab (Actemra) 8 mg/kg intravenously over 1 hour to a maximum dose of 800 mg. Tocilizumab infusions can be repeated every 8 hours as needed if the patient is not responsive to intravenous fluids or increasing supplement oxygen, but should be limited to a maximum of three doses over 24 hours, and a maximum total of four doses.
Patients with grade 3 CRS should also receive intravenous methylprednisolone 1 mg/kg twice daily or the equivalent amount of dexamethasone, with corticosteroids continued until the severity of CRS is grade 1 or less, then tapered over 3 days,
Patients with grade 4 CRS should also receive IV methylprednisolone 1,000 mg per day for 3 days, and if symptoms improve, continue management as per grade 3, Dr. Cornell said.
Dr. Cornell reported having nothing to disclose.
WASHINGTON – Unquestionably, immunotherapy is revolutionizing the care of patients with various solid tumors and hematologic malignancies.
But it’s equally true that there’s no such thing as either a free lunch or a cancer therapy free of side effects, whether it’s increased risk for heart failure associated with anthracycline-based chemotherapy, or inflammatory conditions, arrhythmias, and thromboembolic events associated with immune checkpoint inhibitors, said R. Frank Cornell, MD, of Vanderbilt University Medical Center in Nashville, Tenn.
“Early awareness and intervention is critical for improved outcomes, and a multidisciplinary approach between oncology, cardiology, the clinic nurse, and other health care providers is critical in managing these patients with these complicated therapies,” he said at the American College of Cardiology’s Advancing the Cardiovascular Care of the Oncology Patient meeting.
Checkpoint inhibitors and the heart
Toxicities associated with immune checkpoint inhibitors such as the programmed death 1/ligand 1 (PD-1/PD-L1) inhibitors nivolumab (Opdivo) and pembrolizumab (Keytruda) and the cytotoxic T-lymphocyte antigen 4 antibody ipilimumab (Yervoy) tend to mimic autoimmune conditions, Dr. Cornell said.
Cardiovascular events associated with these agents, while uncommon, include myocarditis, pericarditis, arrhythmias, impaired ventricular function with heart failure, vasculitis, and venous thromboembolism, he said, citing an American Society of Clinical Oncology (ASCO) clinical practice guideline (J Clin Oncol 2018;36[17]:1714-68).
Dr. Cornell described the case of a 63-year-old woman with disseminated metastatic melanoma who presented to the emergency department 10 days after starting on combination therapy with ipilimumab and nivolumab. She had developed shortness of breath, pleuritic chest pain, and a mild cough for 1 or 2 days.
Her cardiac laboratory markers had been normal at baseline, but were markedly elevated on presentation, and electrocardiograms showed complete heart block and subsequent ventricular tachycardia.
The patient was started on high-dose prednisone, but she died in hospital, and an autopsy showed that the cause of death was infiltration into the myocardium of CD3-positive and CD8-positive T lymphocytes.
“So how do we manage this? This is a good opportunity, I think, for further cardiology and oncology collaboration to develop more robust guidelines for what we can do to best prevent this,” Dr. Cornell said.
Patients started on the ipilimumab/nivolumab combination should be tested weekly for cardiac troponin, creatine kinase (CK) and CK-muscle/brain (CK-MB) weekly for the first 3-4 weeks of therapy. Therapy should be stopped if troponin levels continue to rise, and the patient should be started on high-dose steroids, he said.
The role of other anti-inflammatory agents such as infliximab (Remicade and biosimilars) is unclear and needs further study, he added.
Dr. Cornell cited a 2018 letter to The Lancet by Javid J. Moslehi, MD, and colleagues from Vanderbilt describing an increase in reports of fatal myocarditis among patients treated with checkpoint inhibitors.
“We highlight the high mortality rate with severe immune checkpoint inhibitor–related myocarditis, which is more frequent with combination PD-1 and CTLA-4 blockade, but can also occur with monotherapy. Myocarditis was observed across immune checkpoint inhibitor regimens, although it remains too early to determine whether the incidence differs between use of anti-PD1 and anti-PD-L1 drugs. Furthermore, this condition occurs early on during therapy and across cancer types,” they wrote.
Most of the patients had no preexisting cardiovascular disease, and most were not taking medications for hypertension, cardiovascular disease, or diabetes.
CAR-T cells and cardiac disease
The primary cardiac complications associated with CAR-T cell therapy are related to the cytokine release syndrome (CRS), a condition marked by progressive elevation in inflammatory cytokines that in turn leads to marked elevations in C-reactive protein (CRP), interferon gamma, tumor necrosis factor al, and release of pro-inflammatory cytokines including interleukin (IL) 6, IL-10, IL-12, and IL-1 beta.
In rare instances, CRS can lead to disseminated intravascular coagulation (DIC), capillary leak syndrome, and a hemophagocytic lymphohistiocytosis-like (HLH) syndrome, Dr. Cornell said.
Package inserts for the two Food and Drug Administration–approved CAR-T cell products, axicabtagene ciloleucel (Yescarta) and tisagenlecleucel (Kymriah) show that each was associated in clinical trials with a high incidence of CRS.
Among patients treated with axicabtagene ciloleucel, 94% developed CRS, which was grade 3 or greater in severity in 13%. The median time to onset was 2 days, and the median duration was 7 days. Cardiovascular adverse events included grade 3 or greater tachycardia in 2%, arrhythmias in 7%, edema in 1%, dyspnea in 3%, pleural effusion in 2%, hypotension in 15%, hypertension in 6%, and thrombosis in 1%.
Among patients treated with tisagenlecleucel, 79% treated for B-cell acute lymphoblastic leukemia (B-ALL) and 74% treated for diffuse large B cell lymphoma (DLBCL) developed CRS, which was grade 3 or greater in 49% and 23% of patients, respectively. The median time to onset was 3 days, and the median duration of CRS was 8 days.
Cardiovascular adverse events of grade 3 or greater among these patients included tachycardia in 4%, fluid overload in 7%, edema in 1%, dyspnea in 12%, pulmonary edema in 4%, hypotension in 22%, and hypertension in 6%.
Risk factors for CRS include high pre-infusion tumor burden, active infections, and concurrent inflammatory processes, Dr. Cornell said.
Prevention of cardiovascular complications of CAR-T cell therapy requires management of CRS. Patients with grade 2 or greater CRS should receive the anti-IL-6 agent tocilizumab (Actemra) 8 mg/kg intravenously over 1 hour to a maximum dose of 800 mg. Tocilizumab infusions can be repeated every 8 hours as needed if the patient is not responsive to intravenous fluids or increasing supplement oxygen, but should be limited to a maximum of three doses over 24 hours, and a maximum total of four doses.
Patients with grade 3 CRS should also receive intravenous methylprednisolone 1 mg/kg twice daily or the equivalent amount of dexamethasone, with corticosteroids continued until the severity of CRS is grade 1 or less, then tapered over 3 days,
Patients with grade 4 CRS should also receive IV methylprednisolone 1,000 mg per day for 3 days, and if symptoms improve, continue management as per grade 3, Dr. Cornell said.
Dr. Cornell reported having nothing to disclose.
REPORTING FROM ACC CARDIO-ONCOLOGY
Key clinical point: Monitor for cardiac symptoms and treat or interrupt immunotherapy as needed.
Major finding: Immune checkpoint inhibitors and CAR T-cell therapies are associated with distinct cardiovascular adverse events.
Study details: Review of strategies for managing the cardiovascular consequences of cancer immunotherapies.
Disclosures: Dr. Cornell reported having nothing to disclose.
Clinical relevance of tamoxifen pharmacogenetics in question
The clinical relevance of tamoxifen pharmacogenetics and therapeutic drug monitoring has been challenged, according to findings from an observational study.
“The objective of the prospective CYPTAM study was to associate endoxifen concentrations and CYP2D6 genotypes with clinical outcome[s] in patients with early-stage breast cancer receiving tamoxifen,” wrote Anabel Sanchez-Spitman, PharmD, of the Leiden University Medical Center, the Netherlands, and her colleagues in the Journal of Clinical Oncology.
“Although, in a retrospective study, an endoxifen threshold of 5.9 ng/mL for efficacy [has been] described, confirmation based on prospective studies is lacking,” they wrote.
The researchers followed 667 patients with early-stage breast malignancy who were treated with 20 mg of oral tamoxifen daily.
Dr. Sanchez-Spitman and her colleagues performed CYP2D6 genotype testing and measured blood levels of endoxifen, an active metabolite of tamoxifen, in study participants. Both of these measures were linked with relapse-free survival via statistical analysis.
After analysis, no significant association was found between endoxifen concentrations (P = .691) or CYP2D6 genotyping (P = .799) and relapse-free survival. These data were the same in both univariable and multivariable regression analysis.
“Neither categorizing endoxifen concentrations into quartiles nor using 5.9 ng/mL as threshold altered these results,” they wrote.
The authors acknowledged that a key limitation of the study was the absence of tamoxifen adherence testing, but the availability of persistence data was well collected, lessening this drawback.
“Our data do not justify therapeutic drug monitoring based on endoxifen concentrations in patients with breast cancer receiving tamoxifen,” they concluded.
The study was supported by grant funding provided by ZOLEON. The authors reported no conflicts of interest.
SOURCE: Sanchez-Spitman A et al. J Clin Oncol. 2019 Jan 24. doi: 10.1200/JCO.18.00307.
The clinical relevance of tamoxifen pharmacogenetics and therapeutic drug monitoring has been challenged, according to findings from an observational study.
“The objective of the prospective CYPTAM study was to associate endoxifen concentrations and CYP2D6 genotypes with clinical outcome[s] in patients with early-stage breast cancer receiving tamoxifen,” wrote Anabel Sanchez-Spitman, PharmD, of the Leiden University Medical Center, the Netherlands, and her colleagues in the Journal of Clinical Oncology.
“Although, in a retrospective study, an endoxifen threshold of 5.9 ng/mL for efficacy [has been] described, confirmation based on prospective studies is lacking,” they wrote.
The researchers followed 667 patients with early-stage breast malignancy who were treated with 20 mg of oral tamoxifen daily.
Dr. Sanchez-Spitman and her colleagues performed CYP2D6 genotype testing and measured blood levels of endoxifen, an active metabolite of tamoxifen, in study participants. Both of these measures were linked with relapse-free survival via statistical analysis.
After analysis, no significant association was found between endoxifen concentrations (P = .691) or CYP2D6 genotyping (P = .799) and relapse-free survival. These data were the same in both univariable and multivariable regression analysis.
“Neither categorizing endoxifen concentrations into quartiles nor using 5.9 ng/mL as threshold altered these results,” they wrote.
The authors acknowledged that a key limitation of the study was the absence of tamoxifen adherence testing, but the availability of persistence data was well collected, lessening this drawback.
“Our data do not justify therapeutic drug monitoring based on endoxifen concentrations in patients with breast cancer receiving tamoxifen,” they concluded.
The study was supported by grant funding provided by ZOLEON. The authors reported no conflicts of interest.
SOURCE: Sanchez-Spitman A et al. J Clin Oncol. 2019 Jan 24. doi: 10.1200/JCO.18.00307.
The clinical relevance of tamoxifen pharmacogenetics and therapeutic drug monitoring has been challenged, according to findings from an observational study.
“The objective of the prospective CYPTAM study was to associate endoxifen concentrations and CYP2D6 genotypes with clinical outcome[s] in patients with early-stage breast cancer receiving tamoxifen,” wrote Anabel Sanchez-Spitman, PharmD, of the Leiden University Medical Center, the Netherlands, and her colleagues in the Journal of Clinical Oncology.
“Although, in a retrospective study, an endoxifen threshold of 5.9 ng/mL for efficacy [has been] described, confirmation based on prospective studies is lacking,” they wrote.
The researchers followed 667 patients with early-stage breast malignancy who were treated with 20 mg of oral tamoxifen daily.
Dr. Sanchez-Spitman and her colleagues performed CYP2D6 genotype testing and measured blood levels of endoxifen, an active metabolite of tamoxifen, in study participants. Both of these measures were linked with relapse-free survival via statistical analysis.
After analysis, no significant association was found between endoxifen concentrations (P = .691) or CYP2D6 genotyping (P = .799) and relapse-free survival. These data were the same in both univariable and multivariable regression analysis.
“Neither categorizing endoxifen concentrations into quartiles nor using 5.9 ng/mL as threshold altered these results,” they wrote.
The authors acknowledged that a key limitation of the study was the absence of tamoxifen adherence testing, but the availability of persistence data was well collected, lessening this drawback.
“Our data do not justify therapeutic drug monitoring based on endoxifen concentrations in patients with breast cancer receiving tamoxifen,” they concluded.
The study was supported by grant funding provided by ZOLEON. The authors reported no conflicts of interest.
SOURCE: Sanchez-Spitman A et al. J Clin Oncol. 2019 Jan 24. doi: 10.1200/JCO.18.00307.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point:
Major finding: An endoxifen concentration of greater than 5.9 ng/mL was not linked with clinical efficacy in patients with breast malignancy.
Study details: A prospective study of 667 patients with early-stage breast malignancy treated with adjuvant tamoxifen.
Disclosures: The study was supported by grant funding provided by ZOLEON. The authors reported no conflicts of interest.
Source: Sanchez-Spitman A et al. J Clin Oncol. 2019 Jan 24. doi: 10.1200/JCO.18.00307.
Guardian angel or watchdog? Pills of capecitabine contain sensors
Recently, a woman with advanced colorectal cancer at the University of Minnesota, Minneapolis, was taking capecitabine (Xeloda) in the morning but skipping her evening dose.
Her hands hurt, and she couldn’t open the childproof cap. Her daughter had been doing it for her in the morning but wasn’t around to help out at night.
It was the kind of problem that might have gone on for days or weeks until the next clinic visit, and, even then, be addressed only if the woman remembered to mention it.
But that’s not what happened. The care team realized pretty much right away that she was skipping the p.m. dose because the woman was taking her capecitabine in a gel cap with an adherence sensor.
The sensor, a sandwich of copper, silicon, and magnesium in a millimeter square, sent out an electric ping when she took her dose, activated by stomach acid; the ping was picked up by an adhesive bandage patch the woman wore, which relayed the signal to an app on her smartphone; the phone passed it on to a server cloud that the woman had given her providers permission to access.
They monitored her adherence on a Web portal, along with heart rate and activity data, also captured by the patch. The system is called Proteus Discover, from Proteus Digital Health.
Instead of taking days or weeks, the care team quickly realized that she wasn’t taking her evening dose of capecitabine. They contacted her, replaced the childproof cap, and twice-daily dosing resumed.
Expanded use in oncology?
Seven other advanced colorectal cancer patients have participated in the University of Minnesota (UM) pilot project, the first use of the device in oncology. “It’s gone so well that it’s annoying to me to not have this for all my patients. I’m already feeling frustrated that I can’t just put this in all the drugs that I give orally,” said Edward Greeno, MD, an oncologist/hematologist at the university, and the Proteus point man.
“You would assume that cancer patients would be hypercompliant, but it turns out they have the same compliance problems” as other patients, plus additional hurdles, he said, including complex regimens and drug toxicity.
“Every patient we have approached so far has been enthusiastic. They might be a little bit annoyed that I know they haven’t been taking their pills, but they are also glad that I am there to hold them responsible and help them. My intention is to roll this out much more broadly,” he said. That just might happen. Dr. Greeno is working with Proteus to roll the sensor out at UM and oncology programs elsewhere.
A slow, careful rollout
The device was cleared by the Food and Drug Administration in 2012. After more than 180,000 ingestions, there have been no safety issues, besides occasional skin irritation from the patch, which is waterproof and meant to be worn for a week, then replaced. It pings if it’s taken off. The sensor is passes through the body like food.
Patients can communicate with providers over the phone app, which also sends reminders when it’s time to take the next pill.
So far, Proteus has worked with ten health systems in the United States, and more are in the works. Commercialization efforts have focused mostly on blood pressure, cholesterol, and type 2 diabetes drugs, the bad boys of drug adherence, but the system has also been piloted for hepatitis C treatment, and trials are underway for HIV preexposure prophylaxis.
Among the company’s many favorable studies, the system has already been demonstrated to be a viable alternative to directly observed therapy in tuberculosis, the current gold-standard, but hugely labor and resource intensive (PLoS One. 2013;8[1]:e53373. doi: 10.1371/journal.pone.0053373).
Proteus can’t be picked up at the local Walgreens. The company works closely with clients and is being careful in its rollout. For one thing, each sensor has to be programed for the specific drug it’s being used with, but also, and as with any new technology, business and payment models are still being worked out.
UM’s partner in the oncology project, Fairview Health Services, pays Proteus when patients hit an adherence rate of 80%, but how much they pay is a proprietary secret. “Most of the cost issue is still not in the public domain,” said Scooter Plowman, MD, the company’s medical director.
In 2017, FDA approved a version of the antipsychotic aripiprazole embedded with the Proteus sensor. The rollout of “Abilify MyCite” by Otsuka Pharmaceuticals has been similarly cautious, under contract with health systems.
“Otsuka has been very smart in the approach they are taking,” Dr. Plowman said.
He wouldn’t give details, but Proteus is in talks with other pharmaceutical makers to bring pills with sensors to the market.
A new fix for an old problem
Proteus isn’t alone in the ingestible event marker (IEM) market. The FDA is reviewing a rival sensor from etectRx, in Gainesville, Fla.
The technology is a little different; the etectRx sensor is a microchip made out of magnesium and silver chloride that’s embedded on the inside of a gel cap. Instead of an electric blip, it sends out a radio wave when activated by stomach acid. It’s larger than the Proteus offering, but still has room to spare in a gel cap.
The signal is stronger, so patients wear a neck pendant instead of a patch to pick it up. The pendant does not capture heart rate or activity data. It’s not meant to be worn continuously and can come off after it pings the system.
The two systems are otherwise similar; etectRx also uses a phone app to relay adherence data to a server cloud clinicians can access, with patient permission. As with Proteus, everything works as long as the phone is on. President and CEO Harry Travis anticipates clearance in 2019.
Adherence is a huge and well-known problem in medicine; only about half of patients take medications as they are prescribed. People end up in the ED or the hospital with problems that might have been avoided. Providers and payers want solutions.
Industry is bringing technology to bear on the problem. The payoff will be huge for the winners; analysts project multiple billion dollar growth in the adherence technology sector.
Most companies, however, are pinning their hopes on indirect approaches, bottle caps that ping when opened, for instance, or coaching apps for smart phones. IEMs seem to be ahead of the curve.
Guardian angel or watchdog?
Whether that’s a good thing or bad thing depends on who you talk to, but patients do seem more likely to take their medications if a sensor is on board.
Dr. Plowman said he thinks IEMs improve adherence because, with their own health at stake, patients want to do better, and IEM systems provide the extra help they need, complete with positive feedback.
But patients also know they are being watched. The technology is barely off the ground, but concerns have already been raised about surveillance. It’s not hard to imagine insurance companies demanding proof of adherence before paying for expensive drugs. There are privacy concerns as well; everything is encrypted with IEMs, but hackers are clever.
Dr. Plowman and Mr. Travis acknowledged the concerns, and also that there’s no way to know how IEMs – if they take off – will play out in coming decades; it’s a lot like the Internet in 1992.
The intent is for the systems to remain voluntary, as they are now, perhaps with inducements for patients to use them, maybe lower insurance premiums.
“There is something inherently personal about swallowable data,” Dr. Plowman said. “It’s something we take tremendous efforts to protect.” As for compulsory use, “we take enormous strides to prevent that. It’s a major priority.”
“Always, there will be an opt-out” option, said Mr. Travis.
It’s important to consider the potential for IEMs to move medicine forward. When patients with acute bone fractures in one study, for instance, were sent home with the usual handful of oxycodone tablets, it turned out that they only took a median of six. Researchers knew that because the subjects took their oxycodone in an etectRx capsule. It’s was an important insight in the midst of an opioid epidemic (Anesth Analg. 2017 Dec;125[6]:2105-12).
Dr. Greeno is an adviser for Proteus; the company covers his travel costs.
Recently, a woman with advanced colorectal cancer at the University of Minnesota, Minneapolis, was taking capecitabine (Xeloda) in the morning but skipping her evening dose.
Her hands hurt, and she couldn’t open the childproof cap. Her daughter had been doing it for her in the morning but wasn’t around to help out at night.
It was the kind of problem that might have gone on for days or weeks until the next clinic visit, and, even then, be addressed only if the woman remembered to mention it.
But that’s not what happened. The care team realized pretty much right away that she was skipping the p.m. dose because the woman was taking her capecitabine in a gel cap with an adherence sensor.
The sensor, a sandwich of copper, silicon, and magnesium in a millimeter square, sent out an electric ping when she took her dose, activated by stomach acid; the ping was picked up by an adhesive bandage patch the woman wore, which relayed the signal to an app on her smartphone; the phone passed it on to a server cloud that the woman had given her providers permission to access.
They monitored her adherence on a Web portal, along with heart rate and activity data, also captured by the patch. The system is called Proteus Discover, from Proteus Digital Health.
Instead of taking days or weeks, the care team quickly realized that she wasn’t taking her evening dose of capecitabine. They contacted her, replaced the childproof cap, and twice-daily dosing resumed.
Expanded use in oncology?
Seven other advanced colorectal cancer patients have participated in the University of Minnesota (UM) pilot project, the first use of the device in oncology. “It’s gone so well that it’s annoying to me to not have this for all my patients. I’m already feeling frustrated that I can’t just put this in all the drugs that I give orally,” said Edward Greeno, MD, an oncologist/hematologist at the university, and the Proteus point man.
“You would assume that cancer patients would be hypercompliant, but it turns out they have the same compliance problems” as other patients, plus additional hurdles, he said, including complex regimens and drug toxicity.
“Every patient we have approached so far has been enthusiastic. They might be a little bit annoyed that I know they haven’t been taking their pills, but they are also glad that I am there to hold them responsible and help them. My intention is to roll this out much more broadly,” he said. That just might happen. Dr. Greeno is working with Proteus to roll the sensor out at UM and oncology programs elsewhere.
A slow, careful rollout
The device was cleared by the Food and Drug Administration in 2012. After more than 180,000 ingestions, there have been no safety issues, besides occasional skin irritation from the patch, which is waterproof and meant to be worn for a week, then replaced. It pings if it’s taken off. The sensor is passes through the body like food.
Patients can communicate with providers over the phone app, which also sends reminders when it’s time to take the next pill.
So far, Proteus has worked with ten health systems in the United States, and more are in the works. Commercialization efforts have focused mostly on blood pressure, cholesterol, and type 2 diabetes drugs, the bad boys of drug adherence, but the system has also been piloted for hepatitis C treatment, and trials are underway for HIV preexposure prophylaxis.
Among the company’s many favorable studies, the system has already been demonstrated to be a viable alternative to directly observed therapy in tuberculosis, the current gold-standard, but hugely labor and resource intensive (PLoS One. 2013;8[1]:e53373. doi: 10.1371/journal.pone.0053373).
Proteus can’t be picked up at the local Walgreens. The company works closely with clients and is being careful in its rollout. For one thing, each sensor has to be programed for the specific drug it’s being used with, but also, and as with any new technology, business and payment models are still being worked out.
UM’s partner in the oncology project, Fairview Health Services, pays Proteus when patients hit an adherence rate of 80%, but how much they pay is a proprietary secret. “Most of the cost issue is still not in the public domain,” said Scooter Plowman, MD, the company’s medical director.
In 2017, FDA approved a version of the antipsychotic aripiprazole embedded with the Proteus sensor. The rollout of “Abilify MyCite” by Otsuka Pharmaceuticals has been similarly cautious, under contract with health systems.
“Otsuka has been very smart in the approach they are taking,” Dr. Plowman said.
He wouldn’t give details, but Proteus is in talks with other pharmaceutical makers to bring pills with sensors to the market.
A new fix for an old problem
Proteus isn’t alone in the ingestible event marker (IEM) market. The FDA is reviewing a rival sensor from etectRx, in Gainesville, Fla.
The technology is a little different; the etectRx sensor is a microchip made out of magnesium and silver chloride that’s embedded on the inside of a gel cap. Instead of an electric blip, it sends out a radio wave when activated by stomach acid. It’s larger than the Proteus offering, but still has room to spare in a gel cap.
The signal is stronger, so patients wear a neck pendant instead of a patch to pick it up. The pendant does not capture heart rate or activity data. It’s not meant to be worn continuously and can come off after it pings the system.
The two systems are otherwise similar; etectRx also uses a phone app to relay adherence data to a server cloud clinicians can access, with patient permission. As with Proteus, everything works as long as the phone is on. President and CEO Harry Travis anticipates clearance in 2019.
Adherence is a huge and well-known problem in medicine; only about half of patients take medications as they are prescribed. People end up in the ED or the hospital with problems that might have been avoided. Providers and payers want solutions.
Industry is bringing technology to bear on the problem. The payoff will be huge for the winners; analysts project multiple billion dollar growth in the adherence technology sector.
Most companies, however, are pinning their hopes on indirect approaches, bottle caps that ping when opened, for instance, or coaching apps for smart phones. IEMs seem to be ahead of the curve.
Guardian angel or watchdog?
Whether that’s a good thing or bad thing depends on who you talk to, but patients do seem more likely to take their medications if a sensor is on board.
Dr. Plowman said he thinks IEMs improve adherence because, with their own health at stake, patients want to do better, and IEM systems provide the extra help they need, complete with positive feedback.
But patients also know they are being watched. The technology is barely off the ground, but concerns have already been raised about surveillance. It’s not hard to imagine insurance companies demanding proof of adherence before paying for expensive drugs. There are privacy concerns as well; everything is encrypted with IEMs, but hackers are clever.
Dr. Plowman and Mr. Travis acknowledged the concerns, and also that there’s no way to know how IEMs – if they take off – will play out in coming decades; it’s a lot like the Internet in 1992.
The intent is for the systems to remain voluntary, as they are now, perhaps with inducements for patients to use them, maybe lower insurance premiums.
“There is something inherently personal about swallowable data,” Dr. Plowman said. “It’s something we take tremendous efforts to protect.” As for compulsory use, “we take enormous strides to prevent that. It’s a major priority.”
“Always, there will be an opt-out” option, said Mr. Travis.
It’s important to consider the potential for IEMs to move medicine forward. When patients with acute bone fractures in one study, for instance, were sent home with the usual handful of oxycodone tablets, it turned out that they only took a median of six. Researchers knew that because the subjects took their oxycodone in an etectRx capsule. It’s was an important insight in the midst of an opioid epidemic (Anesth Analg. 2017 Dec;125[6]:2105-12).
Dr. Greeno is an adviser for Proteus; the company covers his travel costs.
Recently, a woman with advanced colorectal cancer at the University of Minnesota, Minneapolis, was taking capecitabine (Xeloda) in the morning but skipping her evening dose.
Her hands hurt, and she couldn’t open the childproof cap. Her daughter had been doing it for her in the morning but wasn’t around to help out at night.
It was the kind of problem that might have gone on for days or weeks until the next clinic visit, and, even then, be addressed only if the woman remembered to mention it.
But that’s not what happened. The care team realized pretty much right away that she was skipping the p.m. dose because the woman was taking her capecitabine in a gel cap with an adherence sensor.
The sensor, a sandwich of copper, silicon, and magnesium in a millimeter square, sent out an electric ping when she took her dose, activated by stomach acid; the ping was picked up by an adhesive bandage patch the woman wore, which relayed the signal to an app on her smartphone; the phone passed it on to a server cloud that the woman had given her providers permission to access.
They monitored her adherence on a Web portal, along with heart rate and activity data, also captured by the patch. The system is called Proteus Discover, from Proteus Digital Health.
Instead of taking days or weeks, the care team quickly realized that she wasn’t taking her evening dose of capecitabine. They contacted her, replaced the childproof cap, and twice-daily dosing resumed.
Expanded use in oncology?
Seven other advanced colorectal cancer patients have participated in the University of Minnesota (UM) pilot project, the first use of the device in oncology. “It’s gone so well that it’s annoying to me to not have this for all my patients. I’m already feeling frustrated that I can’t just put this in all the drugs that I give orally,” said Edward Greeno, MD, an oncologist/hematologist at the university, and the Proteus point man.
“You would assume that cancer patients would be hypercompliant, but it turns out they have the same compliance problems” as other patients, plus additional hurdles, he said, including complex regimens and drug toxicity.
“Every patient we have approached so far has been enthusiastic. They might be a little bit annoyed that I know they haven’t been taking their pills, but they are also glad that I am there to hold them responsible and help them. My intention is to roll this out much more broadly,” he said. That just might happen. Dr. Greeno is working with Proteus to roll the sensor out at UM and oncology programs elsewhere.
A slow, careful rollout
The device was cleared by the Food and Drug Administration in 2012. After more than 180,000 ingestions, there have been no safety issues, besides occasional skin irritation from the patch, which is waterproof and meant to be worn for a week, then replaced. It pings if it’s taken off. The sensor is passes through the body like food.
Patients can communicate with providers over the phone app, which also sends reminders when it’s time to take the next pill.
So far, Proteus has worked with ten health systems in the United States, and more are in the works. Commercialization efforts have focused mostly on blood pressure, cholesterol, and type 2 diabetes drugs, the bad boys of drug adherence, but the system has also been piloted for hepatitis C treatment, and trials are underway for HIV preexposure prophylaxis.
Among the company’s many favorable studies, the system has already been demonstrated to be a viable alternative to directly observed therapy in tuberculosis, the current gold-standard, but hugely labor and resource intensive (PLoS One. 2013;8[1]:e53373. doi: 10.1371/journal.pone.0053373).
Proteus can’t be picked up at the local Walgreens. The company works closely with clients and is being careful in its rollout. For one thing, each sensor has to be programed for the specific drug it’s being used with, but also, and as with any new technology, business and payment models are still being worked out.
UM’s partner in the oncology project, Fairview Health Services, pays Proteus when patients hit an adherence rate of 80%, but how much they pay is a proprietary secret. “Most of the cost issue is still not in the public domain,” said Scooter Plowman, MD, the company’s medical director.
In 2017, FDA approved a version of the antipsychotic aripiprazole embedded with the Proteus sensor. The rollout of “Abilify MyCite” by Otsuka Pharmaceuticals has been similarly cautious, under contract with health systems.
“Otsuka has been very smart in the approach they are taking,” Dr. Plowman said.
He wouldn’t give details, but Proteus is in talks with other pharmaceutical makers to bring pills with sensors to the market.
A new fix for an old problem
Proteus isn’t alone in the ingestible event marker (IEM) market. The FDA is reviewing a rival sensor from etectRx, in Gainesville, Fla.
The technology is a little different; the etectRx sensor is a microchip made out of magnesium and silver chloride that’s embedded on the inside of a gel cap. Instead of an electric blip, it sends out a radio wave when activated by stomach acid. It’s larger than the Proteus offering, but still has room to spare in a gel cap.
The signal is stronger, so patients wear a neck pendant instead of a patch to pick it up. The pendant does not capture heart rate or activity data. It’s not meant to be worn continuously and can come off after it pings the system.
The two systems are otherwise similar; etectRx also uses a phone app to relay adherence data to a server cloud clinicians can access, with patient permission. As with Proteus, everything works as long as the phone is on. President and CEO Harry Travis anticipates clearance in 2019.
Adherence is a huge and well-known problem in medicine; only about half of patients take medications as they are prescribed. People end up in the ED or the hospital with problems that might have been avoided. Providers and payers want solutions.
Industry is bringing technology to bear on the problem. The payoff will be huge for the winners; analysts project multiple billion dollar growth in the adherence technology sector.
Most companies, however, are pinning their hopes on indirect approaches, bottle caps that ping when opened, for instance, or coaching apps for smart phones. IEMs seem to be ahead of the curve.
Guardian angel or watchdog?
Whether that’s a good thing or bad thing depends on who you talk to, but patients do seem more likely to take their medications if a sensor is on board.
Dr. Plowman said he thinks IEMs improve adherence because, with their own health at stake, patients want to do better, and IEM systems provide the extra help they need, complete with positive feedback.
But patients also know they are being watched. The technology is barely off the ground, but concerns have already been raised about surveillance. It’s not hard to imagine insurance companies demanding proof of adherence before paying for expensive drugs. There are privacy concerns as well; everything is encrypted with IEMs, but hackers are clever.
Dr. Plowman and Mr. Travis acknowledged the concerns, and also that there’s no way to know how IEMs – if they take off – will play out in coming decades; it’s a lot like the Internet in 1992.
The intent is for the systems to remain voluntary, as they are now, perhaps with inducements for patients to use them, maybe lower insurance premiums.
“There is something inherently personal about swallowable data,” Dr. Plowman said. “It’s something we take tremendous efforts to protect.” As for compulsory use, “we take enormous strides to prevent that. It’s a major priority.”
“Always, there will be an opt-out” option, said Mr. Travis.
It’s important to consider the potential for IEMs to move medicine forward. When patients with acute bone fractures in one study, for instance, were sent home with the usual handful of oxycodone tablets, it turned out that they only took a median of six. Researchers knew that because the subjects took their oxycodone in an etectRx capsule. It’s was an important insight in the midst of an opioid epidemic (Anesth Analg. 2017 Dec;125[6]:2105-12).
Dr. Greeno is an adviser for Proteus; the company covers his travel costs.