User login
Recently, a woman with advanced colorectal cancer at the University of Minnesota, Minneapolis, was taking capecitabine (Xeloda) in the morning but skipping her evening dose.
Her hands hurt, and she couldn’t open the childproof cap. Her daughter had been doing it for her in the morning but wasn’t around to help out at night.
It was the kind of problem that might have gone on for days or weeks until the next clinic visit, and, even then, be addressed only if the woman remembered to mention it.
But that’s not what happened. The care team realized pretty much right away that she was skipping the p.m. dose because the woman was taking her capecitabine in a gel cap with an adherence sensor.
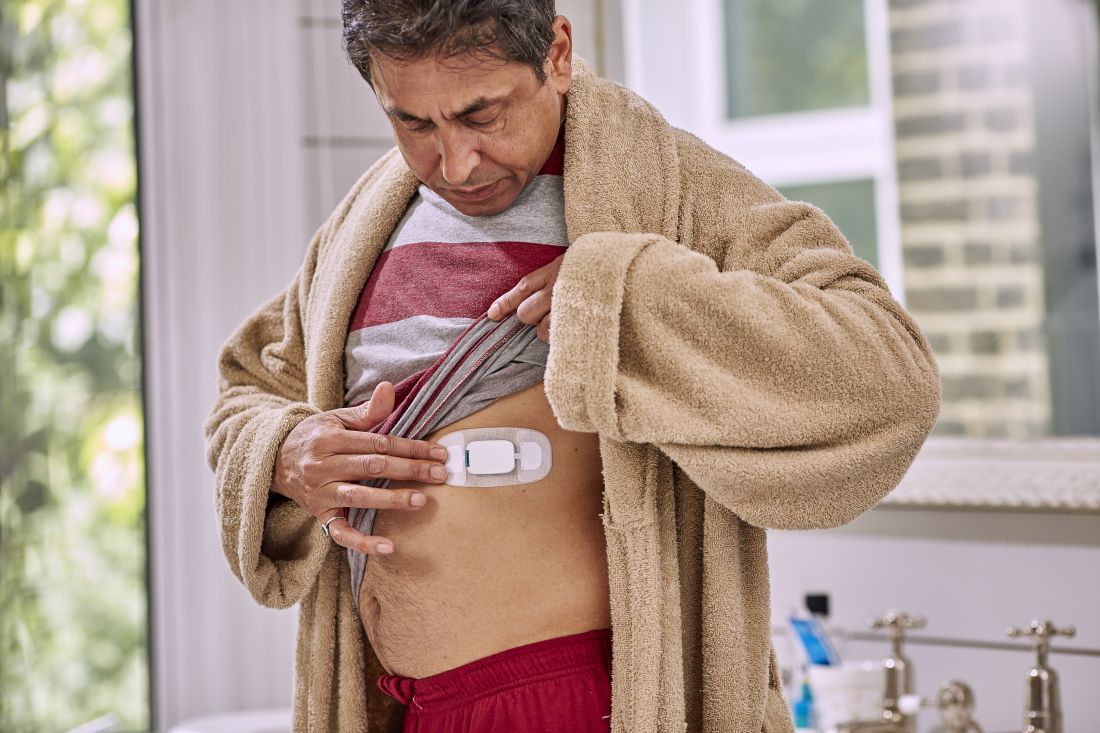
The sensor, a sandwich of copper, silicon, and magnesium in a millimeter square, sent out an electric ping when she took her dose, activated by stomach acid; the ping was picked up by an adhesive bandage patch the woman wore, which relayed the signal to an app on her smartphone; the phone passed it on to a server cloud that the woman had given her providers permission to access.
They monitored her adherence on a Web portal, along with heart rate and activity data, also captured by the patch. The system is called Proteus Discover, from Proteus Digital Health.
Instead of taking days or weeks, the care team quickly realized that she wasn’t taking her evening dose of capecitabine. They contacted her, replaced the childproof cap, and twice-daily dosing resumed.
Expanded use in oncology?
Seven other advanced colorectal cancer patients have participated in the University of Minnesota (UM) pilot project, the first use of the device in oncology. “It’s gone so well that it’s annoying to me to not have this for all my patients. I’m already feeling frustrated that I can’t just put this in all the drugs that I give orally,” said Edward Greeno, MD, an oncologist/hematologist at the university, and the Proteus point man.
“You would assume that cancer patients would be hypercompliant, but it turns out they have the same compliance problems” as other patients, plus additional hurdles, he said, including complex regimens and drug toxicity.
“Every patient we have approached so far has been enthusiastic. They might be a little bit annoyed that I know they haven’t been taking their pills, but they are also glad that I am there to hold them responsible and help them. My intention is to roll this out much more broadly,” he said. That just might happen. Dr. Greeno is working with Proteus to roll the sensor out at UM and oncology programs elsewhere.
A slow, careful rollout
The device was cleared by the Food and Drug Administration in 2012. After more than 180,000 ingestions, there have been no safety issues, besides occasional skin irritation from the patch, which is waterproof and meant to be worn for a week, then replaced. It pings if it’s taken off. The sensor is passes through the body like food.
Patients can communicate with providers over the phone app, which also sends reminders when it’s time to take the next pill.
So far, Proteus has worked with ten health systems in the United States, and more are in the works. Commercialization efforts have focused mostly on blood pressure, cholesterol, and type 2 diabetes drugs, the bad boys of drug adherence, but the system has also been piloted for hepatitis C treatment, and trials are underway for HIV preexposure prophylaxis.
Among the company’s many favorable studies, the system has already been demonstrated to be a viable alternative to directly observed therapy in tuberculosis, the current gold-standard, but hugely labor and resource intensive (PLoS One. 2013;8[1]:e53373. doi: 10.1371/journal.pone.0053373).
Proteus can’t be picked up at the local Walgreens. The company works closely with clients and is being careful in its rollout. For one thing, each sensor has to be programed for the specific drug it’s being used with, but also, and as with any new technology, business and payment models are still being worked out.
UM’s partner in the oncology project, Fairview Health Services, pays Proteus when patients hit an adherence rate of 80%, but how much they pay is a proprietary secret. “Most of the cost issue is still not in the public domain,” said Scooter Plowman, MD, the company’s medical director.
In 2017, FDA approved a version of the antipsychotic aripiprazole embedded with the Proteus sensor. The rollout of “Abilify MyCite” by Otsuka Pharmaceuticals has been similarly cautious, under contract with health systems.
“Otsuka has been very smart in the approach they are taking,” Dr. Plowman said.
He wouldn’t give details, but Proteus is in talks with other pharmaceutical makers to bring pills with sensors to the market.
A new fix for an old problem
Proteus isn’t alone in the ingestible event marker (IEM) market. The FDA is reviewing a rival sensor from etectRx, in Gainesville, Fla.
The technology is a little different; the etectRx sensor is a microchip made out of magnesium and silver chloride that’s embedded on the inside of a gel cap. Instead of an electric blip, it sends out a radio wave when activated by stomach acid. It’s larger than the Proteus offering, but still has room to spare in a gel cap.
The signal is stronger, so patients wear a neck pendant instead of a patch to pick it up. The pendant does not capture heart rate or activity data. It’s not meant to be worn continuously and can come off after it pings the system.
The two systems are otherwise similar; etectRx also uses a phone app to relay adherence data to a server cloud clinicians can access, with patient permission. As with Proteus, everything works as long as the phone is on. President and CEO Harry Travis anticipates clearance in 2019.
Adherence is a huge and well-known problem in medicine; only about half of patients take medications as they are prescribed. People end up in the ED or the hospital with problems that might have been avoided. Providers and payers want solutions.
Industry is bringing technology to bear on the problem. The payoff will be huge for the winners; analysts project multiple billion dollar growth in the adherence technology sector.
Most companies, however, are pinning their hopes on indirect approaches, bottle caps that ping when opened, for instance, or coaching apps for smart phones. IEMs seem to be ahead of the curve.
Guardian angel or watchdog?
Whether that’s a good thing or bad thing depends on who you talk to, but patients do seem more likely to take their medications if a sensor is on board.
Dr. Plowman said he thinks IEMs improve adherence because, with their own health at stake, patients want to do better, and IEM systems provide the extra help they need, complete with positive feedback.
But patients also know they are being watched. The technology is barely off the ground, but concerns have already been raised about surveillance. It’s not hard to imagine insurance companies demanding proof of adherence before paying for expensive drugs. There are privacy concerns as well; everything is encrypted with IEMs, but hackers are clever.
Dr. Plowman and Mr. Travis acknowledged the concerns, and also that there’s no way to know how IEMs – if they take off – will play out in coming decades; it’s a lot like the Internet in 1992.
The intent is for the systems to remain voluntary, as they are now, perhaps with inducements for patients to use them, maybe lower insurance premiums.
“There is something inherently personal about swallowable data,” Dr. Plowman said. “It’s something we take tremendous efforts to protect.” As for compulsory use, “we take enormous strides to prevent that. It’s a major priority.”
“Always, there will be an opt-out” option, said Mr. Travis.
It’s important to consider the potential for IEMs to move medicine forward. When patients with acute bone fractures in one study, for instance, were sent home with the usual handful of oxycodone tablets, it turned out that they only took a median of six. Researchers knew that because the subjects took their oxycodone in an etectRx capsule. It’s was an important insight in the midst of an opioid epidemic (Anesth Analg. 2017 Dec;125[6]:2105-12).
Dr. Greeno is an adviser for Proteus; the company covers his travel costs.
Recently, a woman with advanced colorectal cancer at the University of Minnesota, Minneapolis, was taking capecitabine (Xeloda) in the morning but skipping her evening dose.
Her hands hurt, and she couldn’t open the childproof cap. Her daughter had been doing it for her in the morning but wasn’t around to help out at night.
It was the kind of problem that might have gone on for days or weeks until the next clinic visit, and, even then, be addressed only if the woman remembered to mention it.
But that’s not what happened. The care team realized pretty much right away that she was skipping the p.m. dose because the woman was taking her capecitabine in a gel cap with an adherence sensor.
The sensor, a sandwich of copper, silicon, and magnesium in a millimeter square, sent out an electric ping when she took her dose, activated by stomach acid; the ping was picked up by an adhesive bandage patch the woman wore, which relayed the signal to an app on her smartphone; the phone passed it on to a server cloud that the woman had given her providers permission to access.
They monitored her adherence on a Web portal, along with heart rate and activity data, also captured by the patch. The system is called Proteus Discover, from Proteus Digital Health.
Instead of taking days or weeks, the care team quickly realized that she wasn’t taking her evening dose of capecitabine. They contacted her, replaced the childproof cap, and twice-daily dosing resumed.
Expanded use in oncology?
Seven other advanced colorectal cancer patients have participated in the University of Minnesota (UM) pilot project, the first use of the device in oncology. “It’s gone so well that it’s annoying to me to not have this for all my patients. I’m already feeling frustrated that I can’t just put this in all the drugs that I give orally,” said Edward Greeno, MD, an oncologist/hematologist at the university, and the Proteus point man.
“You would assume that cancer patients would be hypercompliant, but it turns out they have the same compliance problems” as other patients, plus additional hurdles, he said, including complex regimens and drug toxicity.
“Every patient we have approached so far has been enthusiastic. They might be a little bit annoyed that I know they haven’t been taking their pills, but they are also glad that I am there to hold them responsible and help them. My intention is to roll this out much more broadly,” he said. That just might happen. Dr. Greeno is working with Proteus to roll the sensor out at UM and oncology programs elsewhere.
A slow, careful rollout
The device was cleared by the Food and Drug Administration in 2012. After more than 180,000 ingestions, there have been no safety issues, besides occasional skin irritation from the patch, which is waterproof and meant to be worn for a week, then replaced. It pings if it’s taken off. The sensor is passes through the body like food.
Patients can communicate with providers over the phone app, which also sends reminders when it’s time to take the next pill.
So far, Proteus has worked with ten health systems in the United States, and more are in the works. Commercialization efforts have focused mostly on blood pressure, cholesterol, and type 2 diabetes drugs, the bad boys of drug adherence, but the system has also been piloted for hepatitis C treatment, and trials are underway for HIV preexposure prophylaxis.
Among the company’s many favorable studies, the system has already been demonstrated to be a viable alternative to directly observed therapy in tuberculosis, the current gold-standard, but hugely labor and resource intensive (PLoS One. 2013;8[1]:e53373. doi: 10.1371/journal.pone.0053373).
Proteus can’t be picked up at the local Walgreens. The company works closely with clients and is being careful in its rollout. For one thing, each sensor has to be programed for the specific drug it’s being used with, but also, and as with any new technology, business and payment models are still being worked out.
UM’s partner in the oncology project, Fairview Health Services, pays Proteus when patients hit an adherence rate of 80%, but how much they pay is a proprietary secret. “Most of the cost issue is still not in the public domain,” said Scooter Plowman, MD, the company’s medical director.
In 2017, FDA approved a version of the antipsychotic aripiprazole embedded with the Proteus sensor. The rollout of “Abilify MyCite” by Otsuka Pharmaceuticals has been similarly cautious, under contract with health systems.
“Otsuka has been very smart in the approach they are taking,” Dr. Plowman said.
He wouldn’t give details, but Proteus is in talks with other pharmaceutical makers to bring pills with sensors to the market.
A new fix for an old problem
Proteus isn’t alone in the ingestible event marker (IEM) market. The FDA is reviewing a rival sensor from etectRx, in Gainesville, Fla.
The technology is a little different; the etectRx sensor is a microchip made out of magnesium and silver chloride that’s embedded on the inside of a gel cap. Instead of an electric blip, it sends out a radio wave when activated by stomach acid. It’s larger than the Proteus offering, but still has room to spare in a gel cap.
The signal is stronger, so patients wear a neck pendant instead of a patch to pick it up. The pendant does not capture heart rate or activity data. It’s not meant to be worn continuously and can come off after it pings the system.
The two systems are otherwise similar; etectRx also uses a phone app to relay adherence data to a server cloud clinicians can access, with patient permission. As with Proteus, everything works as long as the phone is on. President and CEO Harry Travis anticipates clearance in 2019.
Adherence is a huge and well-known problem in medicine; only about half of patients take medications as they are prescribed. People end up in the ED or the hospital with problems that might have been avoided. Providers and payers want solutions.
Industry is bringing technology to bear on the problem. The payoff will be huge for the winners; analysts project multiple billion dollar growth in the adherence technology sector.
Most companies, however, are pinning their hopes on indirect approaches, bottle caps that ping when opened, for instance, or coaching apps for smart phones. IEMs seem to be ahead of the curve.
Guardian angel or watchdog?
Whether that’s a good thing or bad thing depends on who you talk to, but patients do seem more likely to take their medications if a sensor is on board.
Dr. Plowman said he thinks IEMs improve adherence because, with their own health at stake, patients want to do better, and IEM systems provide the extra help they need, complete with positive feedback.
But patients also know they are being watched. The technology is barely off the ground, but concerns have already been raised about surveillance. It’s not hard to imagine insurance companies demanding proof of adherence before paying for expensive drugs. There are privacy concerns as well; everything is encrypted with IEMs, but hackers are clever.
Dr. Plowman and Mr. Travis acknowledged the concerns, and also that there’s no way to know how IEMs – if they take off – will play out in coming decades; it’s a lot like the Internet in 1992.
The intent is for the systems to remain voluntary, as they are now, perhaps with inducements for patients to use them, maybe lower insurance premiums.
“There is something inherently personal about swallowable data,” Dr. Plowman said. “It’s something we take tremendous efforts to protect.” As for compulsory use, “we take enormous strides to prevent that. It’s a major priority.”
“Always, there will be an opt-out” option, said Mr. Travis.
It’s important to consider the potential for IEMs to move medicine forward. When patients with acute bone fractures in one study, for instance, were sent home with the usual handful of oxycodone tablets, it turned out that they only took a median of six. Researchers knew that because the subjects took their oxycodone in an etectRx capsule. It’s was an important insight in the midst of an opioid epidemic (Anesth Analg. 2017 Dec;125[6]:2105-12).
Dr. Greeno is an adviser for Proteus; the company covers his travel costs.
Recently, a woman with advanced colorectal cancer at the University of Minnesota, Minneapolis, was taking capecitabine (Xeloda) in the morning but skipping her evening dose.
Her hands hurt, and she couldn’t open the childproof cap. Her daughter had been doing it for her in the morning but wasn’t around to help out at night.
It was the kind of problem that might have gone on for days or weeks until the next clinic visit, and, even then, be addressed only if the woman remembered to mention it.
But that’s not what happened. The care team realized pretty much right away that she was skipping the p.m. dose because the woman was taking her capecitabine in a gel cap with an adherence sensor.
The sensor, a sandwich of copper, silicon, and magnesium in a millimeter square, sent out an electric ping when she took her dose, activated by stomach acid; the ping was picked up by an adhesive bandage patch the woman wore, which relayed the signal to an app on her smartphone; the phone passed it on to a server cloud that the woman had given her providers permission to access.
They monitored her adherence on a Web portal, along with heart rate and activity data, also captured by the patch. The system is called Proteus Discover, from Proteus Digital Health.
Instead of taking days or weeks, the care team quickly realized that she wasn’t taking her evening dose of capecitabine. They contacted her, replaced the childproof cap, and twice-daily dosing resumed.
Expanded use in oncology?
Seven other advanced colorectal cancer patients have participated in the University of Minnesota (UM) pilot project, the first use of the device in oncology. “It’s gone so well that it’s annoying to me to not have this for all my patients. I’m already feeling frustrated that I can’t just put this in all the drugs that I give orally,” said Edward Greeno, MD, an oncologist/hematologist at the university, and the Proteus point man.
“You would assume that cancer patients would be hypercompliant, but it turns out they have the same compliance problems” as other patients, plus additional hurdles, he said, including complex regimens and drug toxicity.
“Every patient we have approached so far has been enthusiastic. They might be a little bit annoyed that I know they haven’t been taking their pills, but they are also glad that I am there to hold them responsible and help them. My intention is to roll this out much more broadly,” he said. That just might happen. Dr. Greeno is working with Proteus to roll the sensor out at UM and oncology programs elsewhere.
A slow, careful rollout
The device was cleared by the Food and Drug Administration in 2012. After more than 180,000 ingestions, there have been no safety issues, besides occasional skin irritation from the patch, which is waterproof and meant to be worn for a week, then replaced. It pings if it’s taken off. The sensor is passes through the body like food.
Patients can communicate with providers over the phone app, which also sends reminders when it’s time to take the next pill.
So far, Proteus has worked with ten health systems in the United States, and more are in the works. Commercialization efforts have focused mostly on blood pressure, cholesterol, and type 2 diabetes drugs, the bad boys of drug adherence, but the system has also been piloted for hepatitis C treatment, and trials are underway for HIV preexposure prophylaxis.
Among the company’s many favorable studies, the system has already been demonstrated to be a viable alternative to directly observed therapy in tuberculosis, the current gold-standard, but hugely labor and resource intensive (PLoS One. 2013;8[1]:e53373. doi: 10.1371/journal.pone.0053373).
Proteus can’t be picked up at the local Walgreens. The company works closely with clients and is being careful in its rollout. For one thing, each sensor has to be programed for the specific drug it’s being used with, but also, and as with any new technology, business and payment models are still being worked out.
UM’s partner in the oncology project, Fairview Health Services, pays Proteus when patients hit an adherence rate of 80%, but how much they pay is a proprietary secret. “Most of the cost issue is still not in the public domain,” said Scooter Plowman, MD, the company’s medical director.
In 2017, FDA approved a version of the antipsychotic aripiprazole embedded with the Proteus sensor. The rollout of “Abilify MyCite” by Otsuka Pharmaceuticals has been similarly cautious, under contract with health systems.
“Otsuka has been very smart in the approach they are taking,” Dr. Plowman said.
He wouldn’t give details, but Proteus is in talks with other pharmaceutical makers to bring pills with sensors to the market.
A new fix for an old problem
Proteus isn’t alone in the ingestible event marker (IEM) market. The FDA is reviewing a rival sensor from etectRx, in Gainesville, Fla.
The technology is a little different; the etectRx sensor is a microchip made out of magnesium and silver chloride that’s embedded on the inside of a gel cap. Instead of an electric blip, it sends out a radio wave when activated by stomach acid. It’s larger than the Proteus offering, but still has room to spare in a gel cap.
The signal is stronger, so patients wear a neck pendant instead of a patch to pick it up. The pendant does not capture heart rate or activity data. It’s not meant to be worn continuously and can come off after it pings the system.
The two systems are otherwise similar; etectRx also uses a phone app to relay adherence data to a server cloud clinicians can access, with patient permission. As with Proteus, everything works as long as the phone is on. President and CEO Harry Travis anticipates clearance in 2019.
Adherence is a huge and well-known problem in medicine; only about half of patients take medications as they are prescribed. People end up in the ED or the hospital with problems that might have been avoided. Providers and payers want solutions.
Industry is bringing technology to bear on the problem. The payoff will be huge for the winners; analysts project multiple billion dollar growth in the adherence technology sector.
Most companies, however, are pinning their hopes on indirect approaches, bottle caps that ping when opened, for instance, or coaching apps for smart phones. IEMs seem to be ahead of the curve.
Guardian angel or watchdog?
Whether that’s a good thing or bad thing depends on who you talk to, but patients do seem more likely to take their medications if a sensor is on board.
Dr. Plowman said he thinks IEMs improve adherence because, with their own health at stake, patients want to do better, and IEM systems provide the extra help they need, complete with positive feedback.
But patients also know they are being watched. The technology is barely off the ground, but concerns have already been raised about surveillance. It’s not hard to imagine insurance companies demanding proof of adherence before paying for expensive drugs. There are privacy concerns as well; everything is encrypted with IEMs, but hackers are clever.
Dr. Plowman and Mr. Travis acknowledged the concerns, and also that there’s no way to know how IEMs – if they take off – will play out in coming decades; it’s a lot like the Internet in 1992.
The intent is for the systems to remain voluntary, as they are now, perhaps with inducements for patients to use them, maybe lower insurance premiums.
“There is something inherently personal about swallowable data,” Dr. Plowman said. “It’s something we take tremendous efforts to protect.” As for compulsory use, “we take enormous strides to prevent that. It’s a major priority.”
“Always, there will be an opt-out” option, said Mr. Travis.
It’s important to consider the potential for IEMs to move medicine forward. When patients with acute bone fractures in one study, for instance, were sent home with the usual handful of oxycodone tablets, it turned out that they only took a median of six. Researchers knew that because the subjects took their oxycodone in an etectRx capsule. It’s was an important insight in the midst of an opioid epidemic (Anesth Analg. 2017 Dec;125[6]:2105-12).
Dr. Greeno is an adviser for Proteus; the company covers his travel costs.