User login
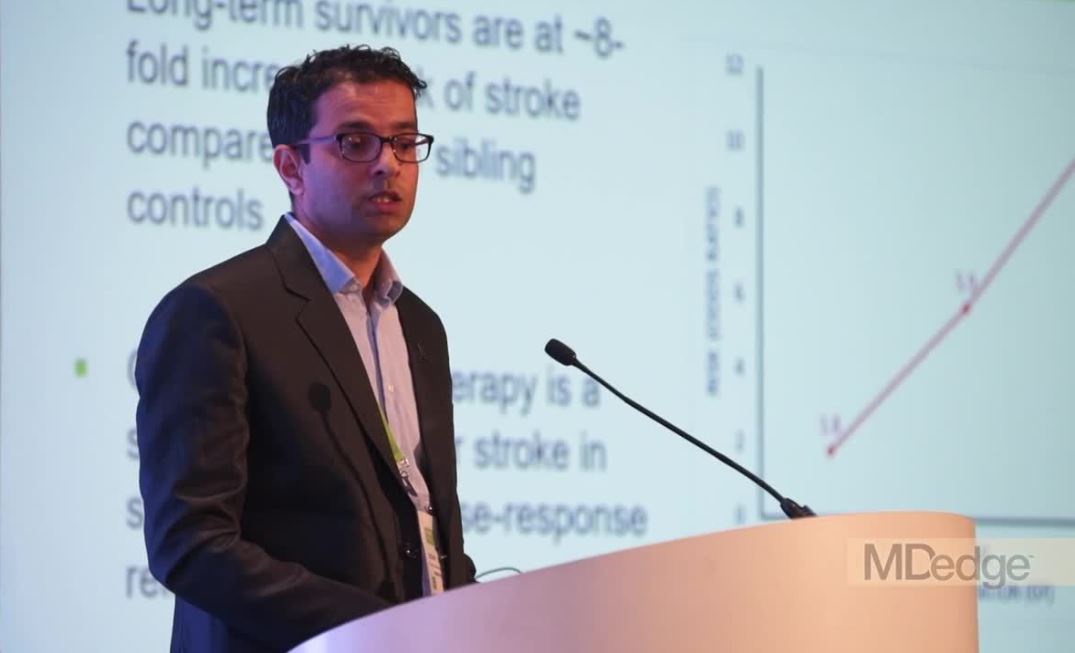
Genetic variant increases stroke risk in childhood cancer survivors
ATLANTA – Adult survivors of childhood cancers are at significantly greater risk than the general population for late-term complications related to therapy, including secondary cancers, cardiovascular disease, and cerebrovascular complications, including ischemic and hemorrhagic strokes.
In particular, childhood cancer survivors have an approximately eightfold higher risk for stroke, compared with their siblings, with a history of cranial irradiation being a strong, dose-dependent risk factor for stroke.
Researchers at St. Jude Children’s Research Hospital in Memphis, Tenn., are conducting a retrospective cohort study with prospective clinical follow-up and ongoing enrollment of childhood cancer survivors who are 5 or more years out of therapy.
The study includes publicly available, whole-genome sequencing data on 4,500 participants. Sifting through these data, Yadav Sapkota, PhD, a clinical research scientist at St. Jude, and his colleagues have identified a genetic variant strongly associated with stroke risk in survivors of European ancestry, and they have replicated the finding in survivors of African ancestry.
In a video interview at the annual meeting of the American Association for Cancer Research, Dr. Sapkota describes his group’s findings and potential research and clinical implications.
The study was sponsored by the National Cancer Institute and ALSAC, the fundraising and awareness organization of St. Jude. Dr. Sapkota declared no conflict of interest.
ATLANTA – Adult survivors of childhood cancers are at significantly greater risk than the general population for late-term complications related to therapy, including secondary cancers, cardiovascular disease, and cerebrovascular complications, including ischemic and hemorrhagic strokes.
In particular, childhood cancer survivors have an approximately eightfold higher risk for stroke, compared with their siblings, with a history of cranial irradiation being a strong, dose-dependent risk factor for stroke.
Researchers at St. Jude Children’s Research Hospital in Memphis, Tenn., are conducting a retrospective cohort study with prospective clinical follow-up and ongoing enrollment of childhood cancer survivors who are 5 or more years out of therapy.
The study includes publicly available, whole-genome sequencing data on 4,500 participants. Sifting through these data, Yadav Sapkota, PhD, a clinical research scientist at St. Jude, and his colleagues have identified a genetic variant strongly associated with stroke risk in survivors of European ancestry, and they have replicated the finding in survivors of African ancestry.
In a video interview at the annual meeting of the American Association for Cancer Research, Dr. Sapkota describes his group’s findings and potential research and clinical implications.
The study was sponsored by the National Cancer Institute and ALSAC, the fundraising and awareness organization of St. Jude. Dr. Sapkota declared no conflict of interest.
ATLANTA – Adult survivors of childhood cancers are at significantly greater risk than the general population for late-term complications related to therapy, including secondary cancers, cardiovascular disease, and cerebrovascular complications, including ischemic and hemorrhagic strokes.
In particular, childhood cancer survivors have an approximately eightfold higher risk for stroke, compared with their siblings, with a history of cranial irradiation being a strong, dose-dependent risk factor for stroke.
Researchers at St. Jude Children’s Research Hospital in Memphis, Tenn., are conducting a retrospective cohort study with prospective clinical follow-up and ongoing enrollment of childhood cancer survivors who are 5 or more years out of therapy.
The study includes publicly available, whole-genome sequencing data on 4,500 participants. Sifting through these data, Yadav Sapkota, PhD, a clinical research scientist at St. Jude, and his colleagues have identified a genetic variant strongly associated with stroke risk in survivors of European ancestry, and they have replicated the finding in survivors of African ancestry.
In a video interview at the annual meeting of the American Association for Cancer Research, Dr. Sapkota describes his group’s findings and potential research and clinical implications.
The study was sponsored by the National Cancer Institute and ALSAC, the fundraising and awareness organization of St. Jude. Dr. Sapkota declared no conflict of interest.
REPORTING FROM AACR 2019
Doctor, will you please lie to me?
“Doctor, I have a question for you.”
“Yes?”
“Have you ever had a patient – I mean, someone like me, someone in my ... state ... get cured without any treatment?”
Mr. B thought he felt too well to have an aggressive cancer infiltrating his liver, abdominal wall, and lungs. He lived an active life, going to church, volunteering, and playing with his grandchildren. But the biopsy results were back. It wasn’t an infection. It wasn’t inflammation. It was stage 4 cancer.
And just like that, his life changed.
His prognosis was likely months, with a best case scenario of several years. But gone were the days of thinking 10, 20, and 30 years ahead.
Mr. B was a spiritual person. He told me this was God’s plan for him. He trusted God to get him through this, so he would not need chemotherapy.
Looking for hope in the face of terrible news is a common reaction. It’s natural. We tend to be optimists, and we look for a silver lining. We look for the “but.”
Patients and doctors alike do this.
“The cancer is metastatic ... but we have chemotherapy that can slow it down.”
“The doctor told me the median survival is 3 months ... but I plan to beat those odds.”
“Your mother is dying ... but we have good medicines to make her comfortable.”
It’s not a bad thing. It’s an adaptive mechanism and positive outlooks can even be associated with better medical outcomes. There’s almost always a “but” if you look hard enough. And in the face of life-changing news, we are incentivized to look really, really hard.
Yet Mr. B caught me off guard because he wasn’t asking me to maintain hope. He was pushing me one step further.
At one point in our conversation, he revealed this clearly. “Doctor, I don’t even care if it’s false hope – just tell me the cancer will disappear.”
Mr. B was asking me to lie to him.
The last thing I wanted was to take away hope, closing the door on a future that truly was clouded in uncertainty. But I also couldn’t look him in the eye and tell him his incurable disease was, in fact, curable.
That doesn’t mean there aren’t ways to phrase things kindly. Patients respond differently to “there’s a 90% chance of survival” and “there’s a 10% chance of death” even though the facts are identical.
When I sense a person like Mr. B is looking to me for hope, I try to frame that initial conversation in the style of the first statement. It’s not dishonest. With an already overwhelming piece of information, it’s choosing to share the most positive version of a narrative that can be told in many different ways and will evolve over time.
Moreover, being hopeful and realistic are not mutually exclusive. Just because someone is seeking the rare chance of a good outcome doesn’t mean he or she doesn’t understand the seriousness of the situation. It is possible to seek out experimental drugs while also considering hospice. It is possible to hope for a plan A while preparing for a plan B. Those are two compatible beliefs, and it’s OK – preferred, even – to hold them at once.
The challenge is avoiding getting pulled into an outlook that is not just positive, not just unlikely – but one with no basis in reality. There’s hope – and then there’s false hope. There’s a positive take – and then there’s lying. It can be a fine line, and with the intention of being kind it can be all too easy to “yes, but” our way into untruths.
The best we can do is set limits with compassion, and understand that telling the truth doesn’t always mean that someone hears it.
“Have you ever had a patient – I mean, someone like me, someone in my ... state ... get cured without any treatment?”
The chances of Mr. B’s cancer responding to chemotherapy were not good but hardly impossible. But without any therapy at all?
“I have not.”
“What about at other places ... in other states or countries. Have you ever heard of a person like me who was cured without treatment?”
“I have not.”
“I guess what I’m asking, Doctor, is ... have you ever seen where the cancer was there and then one day was not ... have you ever seen a miracle?”
Reaching. Grasping.
“I have not.”
“Well,” he said, “Maybe I will be the first.”
We look at each other, pondering this. In my silence is the answer.
Dr. Yurkiewicz is a fellow in hematology and oncology at Stanford (Calif.) University. Follow her on Twitter @ilanayurkiewicz.
“Doctor, I have a question for you.”
“Yes?”
“Have you ever had a patient – I mean, someone like me, someone in my ... state ... get cured without any treatment?”
Mr. B thought he felt too well to have an aggressive cancer infiltrating his liver, abdominal wall, and lungs. He lived an active life, going to church, volunteering, and playing with his grandchildren. But the biopsy results were back. It wasn’t an infection. It wasn’t inflammation. It was stage 4 cancer.
And just like that, his life changed.
His prognosis was likely months, with a best case scenario of several years. But gone were the days of thinking 10, 20, and 30 years ahead.
Mr. B was a spiritual person. He told me this was God’s plan for him. He trusted God to get him through this, so he would not need chemotherapy.
Looking for hope in the face of terrible news is a common reaction. It’s natural. We tend to be optimists, and we look for a silver lining. We look for the “but.”
Patients and doctors alike do this.
“The cancer is metastatic ... but we have chemotherapy that can slow it down.”
“The doctor told me the median survival is 3 months ... but I plan to beat those odds.”
“Your mother is dying ... but we have good medicines to make her comfortable.”
It’s not a bad thing. It’s an adaptive mechanism and positive outlooks can even be associated with better medical outcomes. There’s almost always a “but” if you look hard enough. And in the face of life-changing news, we are incentivized to look really, really hard.
Yet Mr. B caught me off guard because he wasn’t asking me to maintain hope. He was pushing me one step further.
At one point in our conversation, he revealed this clearly. “Doctor, I don’t even care if it’s false hope – just tell me the cancer will disappear.”
Mr. B was asking me to lie to him.
The last thing I wanted was to take away hope, closing the door on a future that truly was clouded in uncertainty. But I also couldn’t look him in the eye and tell him his incurable disease was, in fact, curable.
That doesn’t mean there aren’t ways to phrase things kindly. Patients respond differently to “there’s a 90% chance of survival” and “there’s a 10% chance of death” even though the facts are identical.
When I sense a person like Mr. B is looking to me for hope, I try to frame that initial conversation in the style of the first statement. It’s not dishonest. With an already overwhelming piece of information, it’s choosing to share the most positive version of a narrative that can be told in many different ways and will evolve over time.
Moreover, being hopeful and realistic are not mutually exclusive. Just because someone is seeking the rare chance of a good outcome doesn’t mean he or she doesn’t understand the seriousness of the situation. It is possible to seek out experimental drugs while also considering hospice. It is possible to hope for a plan A while preparing for a plan B. Those are two compatible beliefs, and it’s OK – preferred, even – to hold them at once.
The challenge is avoiding getting pulled into an outlook that is not just positive, not just unlikely – but one with no basis in reality. There’s hope – and then there’s false hope. There’s a positive take – and then there’s lying. It can be a fine line, and with the intention of being kind it can be all too easy to “yes, but” our way into untruths.
The best we can do is set limits with compassion, and understand that telling the truth doesn’t always mean that someone hears it.
“Have you ever had a patient – I mean, someone like me, someone in my ... state ... get cured without any treatment?”
The chances of Mr. B’s cancer responding to chemotherapy were not good but hardly impossible. But without any therapy at all?
“I have not.”
“What about at other places ... in other states or countries. Have you ever heard of a person like me who was cured without treatment?”
“I have not.”
“I guess what I’m asking, Doctor, is ... have you ever seen where the cancer was there and then one day was not ... have you ever seen a miracle?”
Reaching. Grasping.
“I have not.”
“Well,” he said, “Maybe I will be the first.”
We look at each other, pondering this. In my silence is the answer.
Dr. Yurkiewicz is a fellow in hematology and oncology at Stanford (Calif.) University. Follow her on Twitter @ilanayurkiewicz.
“Doctor, I have a question for you.”
“Yes?”
“Have you ever had a patient – I mean, someone like me, someone in my ... state ... get cured without any treatment?”
Mr. B thought he felt too well to have an aggressive cancer infiltrating his liver, abdominal wall, and lungs. He lived an active life, going to church, volunteering, and playing with his grandchildren. But the biopsy results were back. It wasn’t an infection. It wasn’t inflammation. It was stage 4 cancer.
And just like that, his life changed.
His prognosis was likely months, with a best case scenario of several years. But gone were the days of thinking 10, 20, and 30 years ahead.
Mr. B was a spiritual person. He told me this was God’s plan for him. He trusted God to get him through this, so he would not need chemotherapy.
Looking for hope in the face of terrible news is a common reaction. It’s natural. We tend to be optimists, and we look for a silver lining. We look for the “but.”
Patients and doctors alike do this.
“The cancer is metastatic ... but we have chemotherapy that can slow it down.”
“The doctor told me the median survival is 3 months ... but I plan to beat those odds.”
“Your mother is dying ... but we have good medicines to make her comfortable.”
It’s not a bad thing. It’s an adaptive mechanism and positive outlooks can even be associated with better medical outcomes. There’s almost always a “but” if you look hard enough. And in the face of life-changing news, we are incentivized to look really, really hard.
Yet Mr. B caught me off guard because he wasn’t asking me to maintain hope. He was pushing me one step further.
At one point in our conversation, he revealed this clearly. “Doctor, I don’t even care if it’s false hope – just tell me the cancer will disappear.”
Mr. B was asking me to lie to him.
The last thing I wanted was to take away hope, closing the door on a future that truly was clouded in uncertainty. But I also couldn’t look him in the eye and tell him his incurable disease was, in fact, curable.
That doesn’t mean there aren’t ways to phrase things kindly. Patients respond differently to “there’s a 90% chance of survival” and “there’s a 10% chance of death” even though the facts are identical.
When I sense a person like Mr. B is looking to me for hope, I try to frame that initial conversation in the style of the first statement. It’s not dishonest. With an already overwhelming piece of information, it’s choosing to share the most positive version of a narrative that can be told in many different ways and will evolve over time.
Moreover, being hopeful and realistic are not mutually exclusive. Just because someone is seeking the rare chance of a good outcome doesn’t mean he or she doesn’t understand the seriousness of the situation. It is possible to seek out experimental drugs while also considering hospice. It is possible to hope for a plan A while preparing for a plan B. Those are two compatible beliefs, and it’s OK – preferred, even – to hold them at once.
The challenge is avoiding getting pulled into an outlook that is not just positive, not just unlikely – but one with no basis in reality. There’s hope – and then there’s false hope. There’s a positive take – and then there’s lying. It can be a fine line, and with the intention of being kind it can be all too easy to “yes, but” our way into untruths.
The best we can do is set limits with compassion, and understand that telling the truth doesn’t always mean that someone hears it.
“Have you ever had a patient – I mean, someone like me, someone in my ... state ... get cured without any treatment?”
The chances of Mr. B’s cancer responding to chemotherapy were not good but hardly impossible. But without any therapy at all?
“I have not.”
“What about at other places ... in other states or countries. Have you ever heard of a person like me who was cured without treatment?”
“I have not.”
“I guess what I’m asking, Doctor, is ... have you ever seen where the cancer was there and then one day was not ... have you ever seen a miracle?”
Reaching. Grasping.
“I have not.”
“Well,” he said, “Maybe I will be the first.”
We look at each other, pondering this. In my silence is the answer.
Dr. Yurkiewicz is a fellow in hematology and oncology at Stanford (Calif.) University. Follow her on Twitter @ilanayurkiewicz.
Worse survival seen among black patients with MCL
Black non-Hispanic patients with mantle cell lymphoma (MCL) have a lower rate of 5-year overall survival, compared with white non-Hispanic and Hispanic patients, according to a retrospective analysis of more than 18,000 cases.
However, black patients were also most likely to receive treatment at an academic center, which was an independent predictor of better survival, reported Nikesh N. Shah, MD, of Emory University, Atlanta, and his colleagues. This finding suggests that even academic centers still need to focus on overcoming demographic disparities.
“Racial and socioeconomic differences have been reported in many malignancies and certain lymphomas; however, few studies report on disparities in MCL,” the investigators wrote in Clinical Lymphoma, Myeloma & Leukemia. “To our knowledge this is the first such study to assess racial and socioeconomic disparities in this disease.”
The investigators reviewed 18,120 patients with MCL diagnosed between 2004 and 2013; data were drawn from the National Cancer Database. The primary endpoint was overall survival from the time of diagnosis, with analyses conducted to assess various associations with race/ethnicity, facility type, clinical/tumor characteristics, cancer stage, insurance type, and other factors.
Results showed that Hispanic patients had the highest rate of overall survival, at 55.8%, followed by white patients, at 50.1%. Trailing behind these groups were black patients (46.8%) and patients of other races/ethnicities (46.0%).
Along with survival disparities, race/ethnicity was tied to certain clinical and treatment characteristics. Compared with white patients, black patients were more likely to experience B symptoms (28% vs. 25%) and have Medicaid or lack insurance (15% vs. 5%). Black and Hispanic patients were also less likely than white non-Hispanic patients to receive stem cell transplant (13% vs. 10% vs. 10%).
Although black patients were more likely than white patients to receive treatment at an academic center (51% vs. 38%), a factor independently associated with best survival among center types, whatever advantage provided apparently did not exceed disadvantages associated with race.
“We report inferior overall survival in black patients after accounting for socioeconomic status, as seen in other malignancies,” the investigators wrote. “Surprisingly, these patients were more likely to be treated at academic centers, which independently showed improved overall survival in multivariable analysis that controlled for age, disease stage, insurance status, and other socioeconomic factors.”
The researchers cited a number of steps that could help close the survival gap, including providing more comprehensive supportive care between physician visits and enrollment of patients from diverse racial background on clinical trials.
The study was funded by the National Institutes of Health. The researchers reported having no conflicts of interest.
SOURCE: Shah NN et al. Clin Lymphoma Myeloma Leuk. 2019 Mar 11. doi: 10.1016/j.clml.2019.03.006.
Black non-Hispanic patients with mantle cell lymphoma (MCL) have a lower rate of 5-year overall survival, compared with white non-Hispanic and Hispanic patients, according to a retrospective analysis of more than 18,000 cases.
However, black patients were also most likely to receive treatment at an academic center, which was an independent predictor of better survival, reported Nikesh N. Shah, MD, of Emory University, Atlanta, and his colleagues. This finding suggests that even academic centers still need to focus on overcoming demographic disparities.
“Racial and socioeconomic differences have been reported in many malignancies and certain lymphomas; however, few studies report on disparities in MCL,” the investigators wrote in Clinical Lymphoma, Myeloma & Leukemia. “To our knowledge this is the first such study to assess racial and socioeconomic disparities in this disease.”
The investigators reviewed 18,120 patients with MCL diagnosed between 2004 and 2013; data were drawn from the National Cancer Database. The primary endpoint was overall survival from the time of diagnosis, with analyses conducted to assess various associations with race/ethnicity, facility type, clinical/tumor characteristics, cancer stage, insurance type, and other factors.
Results showed that Hispanic patients had the highest rate of overall survival, at 55.8%, followed by white patients, at 50.1%. Trailing behind these groups were black patients (46.8%) and patients of other races/ethnicities (46.0%).
Along with survival disparities, race/ethnicity was tied to certain clinical and treatment characteristics. Compared with white patients, black patients were more likely to experience B symptoms (28% vs. 25%) and have Medicaid or lack insurance (15% vs. 5%). Black and Hispanic patients were also less likely than white non-Hispanic patients to receive stem cell transplant (13% vs. 10% vs. 10%).
Although black patients were more likely than white patients to receive treatment at an academic center (51% vs. 38%), a factor independently associated with best survival among center types, whatever advantage provided apparently did not exceed disadvantages associated with race.
“We report inferior overall survival in black patients after accounting for socioeconomic status, as seen in other malignancies,” the investigators wrote. “Surprisingly, these patients were more likely to be treated at academic centers, which independently showed improved overall survival in multivariable analysis that controlled for age, disease stage, insurance status, and other socioeconomic factors.”
The researchers cited a number of steps that could help close the survival gap, including providing more comprehensive supportive care between physician visits and enrollment of patients from diverse racial background on clinical trials.
The study was funded by the National Institutes of Health. The researchers reported having no conflicts of interest.
SOURCE: Shah NN et al. Clin Lymphoma Myeloma Leuk. 2019 Mar 11. doi: 10.1016/j.clml.2019.03.006.
Black non-Hispanic patients with mantle cell lymphoma (MCL) have a lower rate of 5-year overall survival, compared with white non-Hispanic and Hispanic patients, according to a retrospective analysis of more than 18,000 cases.
However, black patients were also most likely to receive treatment at an academic center, which was an independent predictor of better survival, reported Nikesh N. Shah, MD, of Emory University, Atlanta, and his colleagues. This finding suggests that even academic centers still need to focus on overcoming demographic disparities.
“Racial and socioeconomic differences have been reported in many malignancies and certain lymphomas; however, few studies report on disparities in MCL,” the investigators wrote in Clinical Lymphoma, Myeloma & Leukemia. “To our knowledge this is the first such study to assess racial and socioeconomic disparities in this disease.”
The investigators reviewed 18,120 patients with MCL diagnosed between 2004 and 2013; data were drawn from the National Cancer Database. The primary endpoint was overall survival from the time of diagnosis, with analyses conducted to assess various associations with race/ethnicity, facility type, clinical/tumor characteristics, cancer stage, insurance type, and other factors.
Results showed that Hispanic patients had the highest rate of overall survival, at 55.8%, followed by white patients, at 50.1%. Trailing behind these groups were black patients (46.8%) and patients of other races/ethnicities (46.0%).
Along with survival disparities, race/ethnicity was tied to certain clinical and treatment characteristics. Compared with white patients, black patients were more likely to experience B symptoms (28% vs. 25%) and have Medicaid or lack insurance (15% vs. 5%). Black and Hispanic patients were also less likely than white non-Hispanic patients to receive stem cell transplant (13% vs. 10% vs. 10%).
Although black patients were more likely than white patients to receive treatment at an academic center (51% vs. 38%), a factor independently associated with best survival among center types, whatever advantage provided apparently did not exceed disadvantages associated with race.
“We report inferior overall survival in black patients after accounting for socioeconomic status, as seen in other malignancies,” the investigators wrote. “Surprisingly, these patients were more likely to be treated at academic centers, which independently showed improved overall survival in multivariable analysis that controlled for age, disease stage, insurance status, and other socioeconomic factors.”
The researchers cited a number of steps that could help close the survival gap, including providing more comprehensive supportive care between physician visits and enrollment of patients from diverse racial background on clinical trials.
The study was funded by the National Institutes of Health. The researchers reported having no conflicts of interest.
SOURCE: Shah NN et al. Clin Lymphoma Myeloma Leuk. 2019 Mar 11. doi: 10.1016/j.clml.2019.03.006.
FROM CLINICAL LYMPHOMA, MYELOMA & LEUKEMIA
WISP: Early data provide further support for ISDO in women at high OC risk
HONOLULU – Women at high risk for ovarian cancer who undergo risk-reducing salpingo-oophorectomy (RRSO) or interval salpingectomy with delayed oophorectomy (ISDO) experience significant reductions in cancer distress, according to preliminary findings from the WISP (Women Choosing Surgical Prevention) study.
However, those who choose RRSO experience worse menopausal symptoms and higher rates of regret, Karen H. Lu, MD, reported at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer. Of 183 women enrolled to date in the prospective, nonrandomized, multicenter study, 91 underwent RRSO and 92 underwent ISDO at a median age of 40.9 and 37.5 years, respectively. Significant decreases in cancer-related distress as measured using the Impact of Event scale were seen in both groups at 6-month follow-up after surgery: The mean scores in the RRSO arm were 19.6 at baseline and 10.9 at 6 months, and in the ISDO arm they were 20.8 and 14.0, respectively.
The decrease in scores at 6 months did not differ significantly between study arms, but women in the RRSO arm had a significantly increased incidence of hot flashes, night sweats, vaginal dryness, and weight gain after surgery, said Dr. Lu, professor and chair in the department of gynecologic oncology and reproductive medicine and the J. Taylor Wharton Distinguished Chair in Gynecologic Oncology at the University of Texas MD Anderson Cancer Center, Houston.
“There was a significant difference in quality of life physical scores in RRSO versus the salpingectomy arm,” she said, adding that no difference was seen between the groups in quality of life mental scores.
The level of decision regret was significantly higher in the women who underwent RRSO (scores,14.1 vs. 8.7 on the 100-point scale), she noted.
“We hypothesized that use of HRT [hormone replacement therapy] after RRSO may have accounted for this difference, and looked at the two subsets,” she said. “Within the RRSO group, 25% did not go on HRT, and while higher than ISDO scores, these scores did not account for the large difference in decision regret.”
About half of the women had a history of breast cancer.
“Clinically, this agrees with what we see in that women with a prior history of breast cancer have less angst about undergoing RRSO,” she said, adding that 75% of women in the RRSO arm did go on HRT, and “these women represent the majority of our ‘previvors’ – healthy women with no history of cancer who struggle with the RRSO decision and who are able to take HRT.”
“Interestingly, compared to ISDO, RRSO women on HRT had the highest decisional regret,” she said.
An analysis of numerous possible predictors of decisional regret showed that only baseline depression score was a significant independent predictor, she noted.
Study participants are premenopausal women aged 30-50 years who have a high risk of ovarian cancer because of a pathogenic germline mutation in an ovarian cancer predisposition gene, and a normal CA125 and transvaginal ultrasound within 6 months before enrollment. All receive scripted counseling about the recommended age for oophorectomy and can choose their study arm. Planned enrollment at nine participating U.S. centers is 270 women.
The RRSO and ISDO arms at current enrollment are statistically similar with respect to mutation type, education level, breast cancer history, and first/second-degree ovarian cancer, but significantly more women in the ISDO arm had undergone risk-reducing mastectomy (51% vs. 35%), Dr. Lu noted, adding that to date no ovarian cancers have been identified at initial surgery, between surgeries, or at the time of completion oophorectomy in the ISDO patients – although only two women in that arm have undergone completion oophorectomy.
In the RRSO arm, one high-grade serous tubal intraepithelial neoplasia (STIC) was found at the time of surgery in a PALB2 mutation carrier.
“We believe this is the first report of a STIC in a PALB2 carrier. This woman was 39 years old with a strong family history of breast and ovarian cancer,” she said.
The findings suggest that “for these high-risk women who underwent ISDO ... the option encouraged them to undergo limited but potentially lifesaving surgical prophylaxis at an earlier age rather than wait to undergo full surgical prophylaxis at the upper-accepted age limit due to fear of early menopause,” Dr. Lu said.
Safety continues to be closely monitored in this study and the use of ISDO outside of the clinical trial setting is not recommended, she added, noting that an expansion of both WISP and the TUBA trial, for which preliminary results showing similar outcomes were also presented at the SGO meeting, is planned.
Dr. Lu reported having no disclosures.
SOURCE: Lu KH et al. SGO 2019, Late-Breaking Abstract 3.
HONOLULU – Women at high risk for ovarian cancer who undergo risk-reducing salpingo-oophorectomy (RRSO) or interval salpingectomy with delayed oophorectomy (ISDO) experience significant reductions in cancer distress, according to preliminary findings from the WISP (Women Choosing Surgical Prevention) study.
However, those who choose RRSO experience worse menopausal symptoms and higher rates of regret, Karen H. Lu, MD, reported at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer. Of 183 women enrolled to date in the prospective, nonrandomized, multicenter study, 91 underwent RRSO and 92 underwent ISDO at a median age of 40.9 and 37.5 years, respectively. Significant decreases in cancer-related distress as measured using the Impact of Event scale were seen in both groups at 6-month follow-up after surgery: The mean scores in the RRSO arm were 19.6 at baseline and 10.9 at 6 months, and in the ISDO arm they were 20.8 and 14.0, respectively.
The decrease in scores at 6 months did not differ significantly between study arms, but women in the RRSO arm had a significantly increased incidence of hot flashes, night sweats, vaginal dryness, and weight gain after surgery, said Dr. Lu, professor and chair in the department of gynecologic oncology and reproductive medicine and the J. Taylor Wharton Distinguished Chair in Gynecologic Oncology at the University of Texas MD Anderson Cancer Center, Houston.
“There was a significant difference in quality of life physical scores in RRSO versus the salpingectomy arm,” she said, adding that no difference was seen between the groups in quality of life mental scores.
The level of decision regret was significantly higher in the women who underwent RRSO (scores,14.1 vs. 8.7 on the 100-point scale), she noted.
“We hypothesized that use of HRT [hormone replacement therapy] after RRSO may have accounted for this difference, and looked at the two subsets,” she said. “Within the RRSO group, 25% did not go on HRT, and while higher than ISDO scores, these scores did not account for the large difference in decision regret.”
About half of the women had a history of breast cancer.
“Clinically, this agrees with what we see in that women with a prior history of breast cancer have less angst about undergoing RRSO,” she said, adding that 75% of women in the RRSO arm did go on HRT, and “these women represent the majority of our ‘previvors’ – healthy women with no history of cancer who struggle with the RRSO decision and who are able to take HRT.”
“Interestingly, compared to ISDO, RRSO women on HRT had the highest decisional regret,” she said.
An analysis of numerous possible predictors of decisional regret showed that only baseline depression score was a significant independent predictor, she noted.
Study participants are premenopausal women aged 30-50 years who have a high risk of ovarian cancer because of a pathogenic germline mutation in an ovarian cancer predisposition gene, and a normal CA125 and transvaginal ultrasound within 6 months before enrollment. All receive scripted counseling about the recommended age for oophorectomy and can choose their study arm. Planned enrollment at nine participating U.S. centers is 270 women.
The RRSO and ISDO arms at current enrollment are statistically similar with respect to mutation type, education level, breast cancer history, and first/second-degree ovarian cancer, but significantly more women in the ISDO arm had undergone risk-reducing mastectomy (51% vs. 35%), Dr. Lu noted, adding that to date no ovarian cancers have been identified at initial surgery, between surgeries, or at the time of completion oophorectomy in the ISDO patients – although only two women in that arm have undergone completion oophorectomy.
In the RRSO arm, one high-grade serous tubal intraepithelial neoplasia (STIC) was found at the time of surgery in a PALB2 mutation carrier.
“We believe this is the first report of a STIC in a PALB2 carrier. This woman was 39 years old with a strong family history of breast and ovarian cancer,” she said.
The findings suggest that “for these high-risk women who underwent ISDO ... the option encouraged them to undergo limited but potentially lifesaving surgical prophylaxis at an earlier age rather than wait to undergo full surgical prophylaxis at the upper-accepted age limit due to fear of early menopause,” Dr. Lu said.
Safety continues to be closely monitored in this study and the use of ISDO outside of the clinical trial setting is not recommended, she added, noting that an expansion of both WISP and the TUBA trial, for which preliminary results showing similar outcomes were also presented at the SGO meeting, is planned.
Dr. Lu reported having no disclosures.
SOURCE: Lu KH et al. SGO 2019, Late-Breaking Abstract 3.
HONOLULU – Women at high risk for ovarian cancer who undergo risk-reducing salpingo-oophorectomy (RRSO) or interval salpingectomy with delayed oophorectomy (ISDO) experience significant reductions in cancer distress, according to preliminary findings from the WISP (Women Choosing Surgical Prevention) study.
However, those who choose RRSO experience worse menopausal symptoms and higher rates of regret, Karen H. Lu, MD, reported at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer. Of 183 women enrolled to date in the prospective, nonrandomized, multicenter study, 91 underwent RRSO and 92 underwent ISDO at a median age of 40.9 and 37.5 years, respectively. Significant decreases in cancer-related distress as measured using the Impact of Event scale were seen in both groups at 6-month follow-up after surgery: The mean scores in the RRSO arm were 19.6 at baseline and 10.9 at 6 months, and in the ISDO arm they were 20.8 and 14.0, respectively.
The decrease in scores at 6 months did not differ significantly between study arms, but women in the RRSO arm had a significantly increased incidence of hot flashes, night sweats, vaginal dryness, and weight gain after surgery, said Dr. Lu, professor and chair in the department of gynecologic oncology and reproductive medicine and the J. Taylor Wharton Distinguished Chair in Gynecologic Oncology at the University of Texas MD Anderson Cancer Center, Houston.
“There was a significant difference in quality of life physical scores in RRSO versus the salpingectomy arm,” she said, adding that no difference was seen between the groups in quality of life mental scores.
The level of decision regret was significantly higher in the women who underwent RRSO (scores,14.1 vs. 8.7 on the 100-point scale), she noted.
“We hypothesized that use of HRT [hormone replacement therapy] after RRSO may have accounted for this difference, and looked at the two subsets,” she said. “Within the RRSO group, 25% did not go on HRT, and while higher than ISDO scores, these scores did not account for the large difference in decision regret.”
About half of the women had a history of breast cancer.
“Clinically, this agrees with what we see in that women with a prior history of breast cancer have less angst about undergoing RRSO,” she said, adding that 75% of women in the RRSO arm did go on HRT, and “these women represent the majority of our ‘previvors’ – healthy women with no history of cancer who struggle with the RRSO decision and who are able to take HRT.”
“Interestingly, compared to ISDO, RRSO women on HRT had the highest decisional regret,” she said.
An analysis of numerous possible predictors of decisional regret showed that only baseline depression score was a significant independent predictor, she noted.
Study participants are premenopausal women aged 30-50 years who have a high risk of ovarian cancer because of a pathogenic germline mutation in an ovarian cancer predisposition gene, and a normal CA125 and transvaginal ultrasound within 6 months before enrollment. All receive scripted counseling about the recommended age for oophorectomy and can choose their study arm. Planned enrollment at nine participating U.S. centers is 270 women.
The RRSO and ISDO arms at current enrollment are statistically similar with respect to mutation type, education level, breast cancer history, and first/second-degree ovarian cancer, but significantly more women in the ISDO arm had undergone risk-reducing mastectomy (51% vs. 35%), Dr. Lu noted, adding that to date no ovarian cancers have been identified at initial surgery, between surgeries, or at the time of completion oophorectomy in the ISDO patients – although only two women in that arm have undergone completion oophorectomy.
In the RRSO arm, one high-grade serous tubal intraepithelial neoplasia (STIC) was found at the time of surgery in a PALB2 mutation carrier.
“We believe this is the first report of a STIC in a PALB2 carrier. This woman was 39 years old with a strong family history of breast and ovarian cancer,” she said.
The findings suggest that “for these high-risk women who underwent ISDO ... the option encouraged them to undergo limited but potentially lifesaving surgical prophylaxis at an earlier age rather than wait to undergo full surgical prophylaxis at the upper-accepted age limit due to fear of early menopause,” Dr. Lu said.
Safety continues to be closely monitored in this study and the use of ISDO outside of the clinical trial setting is not recommended, she added, noting that an expansion of both WISP and the TUBA trial, for which preliminary results showing similar outcomes were also presented at the SGO meeting, is planned.
Dr. Lu reported having no disclosures.
SOURCE: Lu KH et al. SGO 2019, Late-Breaking Abstract 3.
REPORTING FROM SGO 2019
Preliminary data show similar declines in worry, low regret with RRSO and RRS/RRO
HONOLULU – Women with BRCA1/2 mutations who undergo risk-reducing salpingo-oophorectomy (RRSO) or risk-reducing salpingectomy followed by delayed risk-reducing oophorectomy (RRS/RRO) experience reduced cancer-related worry after surgery and low levels of decision regret, according to preliminary findings from the Dutch multicenter TUBA trial.
Of 384 women included to date in the ongoing trial, 51% carried a BRCA1 mutation and 49% carried a BRCA2 mutation; 72% chose RRS/RRO. A total of 289 completed the 3-month follow-up and 197 completed 1-year follow-up. At 3 and 12 months the decline on the Cancer Worry Scale was similar in the groups after adjusting for age and other baseline characteristics, with 1.9- and 1.4-point declines from baseline scores of 14.4 and 14.0 at 3 months, and 2.2- and 1.0-point declines at 12 months in the RRSO and RRS/RRO groups, respectively, Miranda P. Steenbeek, MD, reported at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer.
The mean scores on the 100-point Decision Regret Scale at 1-year follow-up were low at 13.4 and 13.0 after RRSO and RRS, respectively.
“But it is interesting that there is one group of women who underwent standard salpingo-oophorectomy, but weren’t using hormone replacement therapy [HRT], as these women had a higher level of decision regret [mean score, 18.8],” said Dr. Steenbeek of Radboud University Nijmegen, the Netherlands. “It is interesting to see how these results will develop over time.”
Current recommendations for preventive surgery in woman at increased risk because of BRCA1/2 mutations call for RRSO around age 40 years. However, an innovative approach using RRS followed by RRO has emerged as recent data indicate the fallopian tube, rather than the ovary, as the origin of high-grade serous ovarian cancer, she explained.
The TUBA trial was launched at 13 Dutch oncology centers in 2015, and plans to enroll 510 BRCA1/2 mutation carriers. Participants choose either standard RRSO within current guidelines (at age 35-40 years for BRCA1 carriers, age 40-45 years for BRCA2 carriers) or the innovative RRS approach followed by RRO at up to 5 years after the current guideline age (at age 40-45 years for BRCA1 carriers, age 45-50 years for BRCA2 carriers), she said.
These early findings show that baseline levels of cancer worry are higher among women choosing RRSO, which might explain the slightly larger postsurgery decline in worry in the RRSO group, Dr. Steenbeek said, noting that the higher level of regret with RRSO without HRT might be related to more severe menopausal complaints in that subset of patients.
“Based on these results we cannot conclude whether or not salpingectomy is a safe alternative to for salpingo-oophorectomy, and therefore it is very important to not perform salpingectomy with delayed oophorectomy outside the safety of a clinical and control of a clinical trial,” she said, adding that a report on the quality of life data from the study is expected in 2020.
Dr. Steenbeek reported having no disclosures.
SOURCE: Steenbeek MP et al. SGO 2019, Abstract LB2.
HONOLULU – Women with BRCA1/2 mutations who undergo risk-reducing salpingo-oophorectomy (RRSO) or risk-reducing salpingectomy followed by delayed risk-reducing oophorectomy (RRS/RRO) experience reduced cancer-related worry after surgery and low levels of decision regret, according to preliminary findings from the Dutch multicenter TUBA trial.
Of 384 women included to date in the ongoing trial, 51% carried a BRCA1 mutation and 49% carried a BRCA2 mutation; 72% chose RRS/RRO. A total of 289 completed the 3-month follow-up and 197 completed 1-year follow-up. At 3 and 12 months the decline on the Cancer Worry Scale was similar in the groups after adjusting for age and other baseline characteristics, with 1.9- and 1.4-point declines from baseline scores of 14.4 and 14.0 at 3 months, and 2.2- and 1.0-point declines at 12 months in the RRSO and RRS/RRO groups, respectively, Miranda P. Steenbeek, MD, reported at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer.
The mean scores on the 100-point Decision Regret Scale at 1-year follow-up were low at 13.4 and 13.0 after RRSO and RRS, respectively.
“But it is interesting that there is one group of women who underwent standard salpingo-oophorectomy, but weren’t using hormone replacement therapy [HRT], as these women had a higher level of decision regret [mean score, 18.8],” said Dr. Steenbeek of Radboud University Nijmegen, the Netherlands. “It is interesting to see how these results will develop over time.”
Current recommendations for preventive surgery in woman at increased risk because of BRCA1/2 mutations call for RRSO around age 40 years. However, an innovative approach using RRS followed by RRO has emerged as recent data indicate the fallopian tube, rather than the ovary, as the origin of high-grade serous ovarian cancer, she explained.
The TUBA trial was launched at 13 Dutch oncology centers in 2015, and plans to enroll 510 BRCA1/2 mutation carriers. Participants choose either standard RRSO within current guidelines (at age 35-40 years for BRCA1 carriers, age 40-45 years for BRCA2 carriers) or the innovative RRS approach followed by RRO at up to 5 years after the current guideline age (at age 40-45 years for BRCA1 carriers, age 45-50 years for BRCA2 carriers), she said.
These early findings show that baseline levels of cancer worry are higher among women choosing RRSO, which might explain the slightly larger postsurgery decline in worry in the RRSO group, Dr. Steenbeek said, noting that the higher level of regret with RRSO without HRT might be related to more severe menopausal complaints in that subset of patients.
“Based on these results we cannot conclude whether or not salpingectomy is a safe alternative to for salpingo-oophorectomy, and therefore it is very important to not perform salpingectomy with delayed oophorectomy outside the safety of a clinical and control of a clinical trial,” she said, adding that a report on the quality of life data from the study is expected in 2020.
Dr. Steenbeek reported having no disclosures.
SOURCE: Steenbeek MP et al. SGO 2019, Abstract LB2.
HONOLULU – Women with BRCA1/2 mutations who undergo risk-reducing salpingo-oophorectomy (RRSO) or risk-reducing salpingectomy followed by delayed risk-reducing oophorectomy (RRS/RRO) experience reduced cancer-related worry after surgery and low levels of decision regret, according to preliminary findings from the Dutch multicenter TUBA trial.
Of 384 women included to date in the ongoing trial, 51% carried a BRCA1 mutation and 49% carried a BRCA2 mutation; 72% chose RRS/RRO. A total of 289 completed the 3-month follow-up and 197 completed 1-year follow-up. At 3 and 12 months the decline on the Cancer Worry Scale was similar in the groups after adjusting for age and other baseline characteristics, with 1.9- and 1.4-point declines from baseline scores of 14.4 and 14.0 at 3 months, and 2.2- and 1.0-point declines at 12 months in the RRSO and RRS/RRO groups, respectively, Miranda P. Steenbeek, MD, reported at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer.
The mean scores on the 100-point Decision Regret Scale at 1-year follow-up were low at 13.4 and 13.0 after RRSO and RRS, respectively.
“But it is interesting that there is one group of women who underwent standard salpingo-oophorectomy, but weren’t using hormone replacement therapy [HRT], as these women had a higher level of decision regret [mean score, 18.8],” said Dr. Steenbeek of Radboud University Nijmegen, the Netherlands. “It is interesting to see how these results will develop over time.”
Current recommendations for preventive surgery in woman at increased risk because of BRCA1/2 mutations call for RRSO around age 40 years. However, an innovative approach using RRS followed by RRO has emerged as recent data indicate the fallopian tube, rather than the ovary, as the origin of high-grade serous ovarian cancer, she explained.
The TUBA trial was launched at 13 Dutch oncology centers in 2015, and plans to enroll 510 BRCA1/2 mutation carriers. Participants choose either standard RRSO within current guidelines (at age 35-40 years for BRCA1 carriers, age 40-45 years for BRCA2 carriers) or the innovative RRS approach followed by RRO at up to 5 years after the current guideline age (at age 40-45 years for BRCA1 carriers, age 45-50 years for BRCA2 carriers), she said.
These early findings show that baseline levels of cancer worry are higher among women choosing RRSO, which might explain the slightly larger postsurgery decline in worry in the RRSO group, Dr. Steenbeek said, noting that the higher level of regret with RRSO without HRT might be related to more severe menopausal complaints in that subset of patients.
“Based on these results we cannot conclude whether or not salpingectomy is a safe alternative to for salpingo-oophorectomy, and therefore it is very important to not perform salpingectomy with delayed oophorectomy outside the safety of a clinical and control of a clinical trial,” she said, adding that a report on the quality of life data from the study is expected in 2020.
Dr. Steenbeek reported having no disclosures.
SOURCE: Steenbeek MP et al. SGO 2019, Abstract LB2.
REPORTING FROM SGO 2019
‘Difficult’ discussions reduced anxiety, depression in life-limiting cancer patients
A program to encourage difficult discussions between seriously ill patients and their oncologists reduced anxiety and depression in a recent randomized trial, but its impact on patient-centered outcomes were uncertain.
Goal-concordant care and peacefulness at the end of life, the coprimary study outcomes, were not significantly different between patients who received the quality improvement intervention and controls in the study, which included 91 clinicians providing care for 278 patients with advanced cancer.
However, it’s not clear whether the intervention, known as the Serious Illness Care Program (SICP), failed to improve those outcomes, or if there simply weren’t enough patients in the trial to detect a meaningful difference, according to investigators led by Rachelle Bernacki, MD, of Brigham and Women’s Hospital and the Harvard School of Public Health, Boston.
“Our challenges reflect the need in our field for patient-centered measures of communication that are agreed upon, validated, and demonstrably sensitive to communication interventions,” wrote Dr. Bernacki and her coinvestigators in a report on the study published in JAMA Internal Medicine.
However, the SICP intervention did clearly result in a larger number of serious-illness conversations that occurred earlier and were of higher quality, the investigators wrote in a separate report published in JAMA Oncology. In medical records reviewed after the patients’ deaths, 96% of those who received the intervention had a documented serious-illness conversation with their oncology clinician, compared with 79% of controls (P = .005), according to that report.
The conversations among SICP recipients occurred a median of 2.4 months earlier than controls, and had a greater focus on values and goals, prognosis and understanding of illness, and treatment preferences.
These outcomes are reassuring, since patients “want, require, and deserve” conversations about serious illness, regardless of their impact on measurable outcomes, the authors of an editorial published in JAMA Oncology wrote.
The SICP intervention included a communication guide for clinicians, who also participated in a 2.5-hour training session designed to improve their serious-illness conversation skills. Other aspects of the program for clinicians included email reminders before outpatient visits, a specialized EMR template, and personal coaching. The program also included patient tools, including a letter introducing the intervention and a guide for continuing the conversation with their family.
The study did not demonstrate a significant difference in peacefulness, as measured by the validated Peace, Equanimity, and Acceptance in the Cancer Experience questionnaire, or in goal-concordant care, which was measured by asking patients to select goals of importance, and then asking caregivers to rate whether those goals had been met at the end of life.
However, patients in the SICP group reported less anxiety and depression 14 weeks into the trial, according to the investigators. The proportion of patients reporting moderate to severe anxiety at that time point was 10.2% for the intervention group versus 5.0% for controls (P = .05), while the proportion reporting depression symptoms was 20.8% for the intervention versus 10.6% for controls (P = .04).
The anxiety reduction was maintained at 24 weeks, though the depression reduction was not, the investigators wrote, adding that there were no differences in survival between arms.
Taken together, these results suggest that oncology clinicians can discuss difficult topics without causing harm, and with potential benefit, the investigators wrote in a discussion of their results.
“Further development of serious illness communication interventions will require more reliable and well-accepted patient-centered outcome measures and additional testing of the effect on patients throughout their illness trajectory,” they concluded.
Dr. Bernacki reported no disclosures. Coauthor Susan D. Block, MD, reported compensation from Up to Date and Atul A. Gawande, MD, MPH, reported receiving compensation from health care writing and media and is employed by a health care venture formed by Amazon, Berkshire Hathaway, and JPMorgan Chase.
SOURCE: Bernacki R et al. JAMA Intern Med. 2018 Mar 14. doi: 10.1001/jamainternmed.2019.0077.
While results of this rigorous and innovative clinical trial are disappointing because of an apparent lack of impact on the primary outcomes of care, oncologists still must initiate serious illness conversations with advanced cancer patients at risk of dying in the foreseeable future, according to the authors of an editorial.
Doing so is important “not because this will necessarily improve outcomes, but because patients want, require, and deserve to know what is coming,” wrote Belinda E. Kiely, MBBS, PhD, FRACP, and Martin R. Stockler, MBBS, MSc, FRACP.
Those difficult conversations should not stop at discussing the limits of care, but should include a discussion of the patient’s preferences, priorities, and values, Dr. Kiely and Dr. Stockler wrote, adding that they should be documented in the EMR to ensure they are accessible to other health care providers.
“If nothing else, oncologists should be reassured that having these conversations is unlikely to increase anxiety or depression in their patients,” wrote the editorial authors, referencing the significantly reduced incidence of those secondary endpoints in the study.
However, conversations alone may not be enough to improve other patient-centered outcomes, based on the inability of this trial to demonstrate significant improvements in goal-centered care or peacefulness at the end of life.
Moreover, building this Serious Illness Care Program intervention into a health system could be complicated and may require significant resources.
“Simple, pragmatic, and effective tactics are needed to ensure greater generalizability and widespread applicability of such programs,” the authors concluded.
Dr. Kiely and Dr. Stockler are with the National Health and Medical Research Council Clinical Trials Centre at the University of Sydney. Their editorial appears in JAMA Oncology. Dr. Stockler reported grants outside the submitted work from Astellas, Amgen, AstraZeneca, Cancer Australia, Celgene, Bionomics, Bayer, Medivation, Merck, National Health and Medical Research Council Australia, Pfizer, Roche, Sanofi, and Tilray.
While results of this rigorous and innovative clinical trial are disappointing because of an apparent lack of impact on the primary outcomes of care, oncologists still must initiate serious illness conversations with advanced cancer patients at risk of dying in the foreseeable future, according to the authors of an editorial.
Doing so is important “not because this will necessarily improve outcomes, but because patients want, require, and deserve to know what is coming,” wrote Belinda E. Kiely, MBBS, PhD, FRACP, and Martin R. Stockler, MBBS, MSc, FRACP.
Those difficult conversations should not stop at discussing the limits of care, but should include a discussion of the patient’s preferences, priorities, and values, Dr. Kiely and Dr. Stockler wrote, adding that they should be documented in the EMR to ensure they are accessible to other health care providers.
“If nothing else, oncologists should be reassured that having these conversations is unlikely to increase anxiety or depression in their patients,” wrote the editorial authors, referencing the significantly reduced incidence of those secondary endpoints in the study.
However, conversations alone may not be enough to improve other patient-centered outcomes, based on the inability of this trial to demonstrate significant improvements in goal-centered care or peacefulness at the end of life.
Moreover, building this Serious Illness Care Program intervention into a health system could be complicated and may require significant resources.
“Simple, pragmatic, and effective tactics are needed to ensure greater generalizability and widespread applicability of such programs,” the authors concluded.
Dr. Kiely and Dr. Stockler are with the National Health and Medical Research Council Clinical Trials Centre at the University of Sydney. Their editorial appears in JAMA Oncology. Dr. Stockler reported grants outside the submitted work from Astellas, Amgen, AstraZeneca, Cancer Australia, Celgene, Bionomics, Bayer, Medivation, Merck, National Health and Medical Research Council Australia, Pfizer, Roche, Sanofi, and Tilray.
While results of this rigorous and innovative clinical trial are disappointing because of an apparent lack of impact on the primary outcomes of care, oncologists still must initiate serious illness conversations with advanced cancer patients at risk of dying in the foreseeable future, according to the authors of an editorial.
Doing so is important “not because this will necessarily improve outcomes, but because patients want, require, and deserve to know what is coming,” wrote Belinda E. Kiely, MBBS, PhD, FRACP, and Martin R. Stockler, MBBS, MSc, FRACP.
Those difficult conversations should not stop at discussing the limits of care, but should include a discussion of the patient’s preferences, priorities, and values, Dr. Kiely and Dr. Stockler wrote, adding that they should be documented in the EMR to ensure they are accessible to other health care providers.
“If nothing else, oncologists should be reassured that having these conversations is unlikely to increase anxiety or depression in their patients,” wrote the editorial authors, referencing the significantly reduced incidence of those secondary endpoints in the study.
However, conversations alone may not be enough to improve other patient-centered outcomes, based on the inability of this trial to demonstrate significant improvements in goal-centered care or peacefulness at the end of life.
Moreover, building this Serious Illness Care Program intervention into a health system could be complicated and may require significant resources.
“Simple, pragmatic, and effective tactics are needed to ensure greater generalizability and widespread applicability of such programs,” the authors concluded.
Dr. Kiely and Dr. Stockler are with the National Health and Medical Research Council Clinical Trials Centre at the University of Sydney. Their editorial appears in JAMA Oncology. Dr. Stockler reported grants outside the submitted work from Astellas, Amgen, AstraZeneca, Cancer Australia, Celgene, Bionomics, Bayer, Medivation, Merck, National Health and Medical Research Council Australia, Pfizer, Roche, Sanofi, and Tilray.
A program to encourage difficult discussions between seriously ill patients and their oncologists reduced anxiety and depression in a recent randomized trial, but its impact on patient-centered outcomes were uncertain.
Goal-concordant care and peacefulness at the end of life, the coprimary study outcomes, were not significantly different between patients who received the quality improvement intervention and controls in the study, which included 91 clinicians providing care for 278 patients with advanced cancer.
However, it’s not clear whether the intervention, known as the Serious Illness Care Program (SICP), failed to improve those outcomes, or if there simply weren’t enough patients in the trial to detect a meaningful difference, according to investigators led by Rachelle Bernacki, MD, of Brigham and Women’s Hospital and the Harvard School of Public Health, Boston.
“Our challenges reflect the need in our field for patient-centered measures of communication that are agreed upon, validated, and demonstrably sensitive to communication interventions,” wrote Dr. Bernacki and her coinvestigators in a report on the study published in JAMA Internal Medicine.
However, the SICP intervention did clearly result in a larger number of serious-illness conversations that occurred earlier and were of higher quality, the investigators wrote in a separate report published in JAMA Oncology. In medical records reviewed after the patients’ deaths, 96% of those who received the intervention had a documented serious-illness conversation with their oncology clinician, compared with 79% of controls (P = .005), according to that report.
The conversations among SICP recipients occurred a median of 2.4 months earlier than controls, and had a greater focus on values and goals, prognosis and understanding of illness, and treatment preferences.
These outcomes are reassuring, since patients “want, require, and deserve” conversations about serious illness, regardless of their impact on measurable outcomes, the authors of an editorial published in JAMA Oncology wrote.
The SICP intervention included a communication guide for clinicians, who also participated in a 2.5-hour training session designed to improve their serious-illness conversation skills. Other aspects of the program for clinicians included email reminders before outpatient visits, a specialized EMR template, and personal coaching. The program also included patient tools, including a letter introducing the intervention and a guide for continuing the conversation with their family.
The study did not demonstrate a significant difference in peacefulness, as measured by the validated Peace, Equanimity, and Acceptance in the Cancer Experience questionnaire, or in goal-concordant care, which was measured by asking patients to select goals of importance, and then asking caregivers to rate whether those goals had been met at the end of life.
However, patients in the SICP group reported less anxiety and depression 14 weeks into the trial, according to the investigators. The proportion of patients reporting moderate to severe anxiety at that time point was 10.2% for the intervention group versus 5.0% for controls (P = .05), while the proportion reporting depression symptoms was 20.8% for the intervention versus 10.6% for controls (P = .04).
The anxiety reduction was maintained at 24 weeks, though the depression reduction was not, the investigators wrote, adding that there were no differences in survival between arms.
Taken together, these results suggest that oncology clinicians can discuss difficult topics without causing harm, and with potential benefit, the investigators wrote in a discussion of their results.
“Further development of serious illness communication interventions will require more reliable and well-accepted patient-centered outcome measures and additional testing of the effect on patients throughout their illness trajectory,” they concluded.
Dr. Bernacki reported no disclosures. Coauthor Susan D. Block, MD, reported compensation from Up to Date and Atul A. Gawande, MD, MPH, reported receiving compensation from health care writing and media and is employed by a health care venture formed by Amazon, Berkshire Hathaway, and JPMorgan Chase.
SOURCE: Bernacki R et al. JAMA Intern Med. 2018 Mar 14. doi: 10.1001/jamainternmed.2019.0077.
A program to encourage difficult discussions between seriously ill patients and their oncologists reduced anxiety and depression in a recent randomized trial, but its impact on patient-centered outcomes were uncertain.
Goal-concordant care and peacefulness at the end of life, the coprimary study outcomes, were not significantly different between patients who received the quality improvement intervention and controls in the study, which included 91 clinicians providing care for 278 patients with advanced cancer.
However, it’s not clear whether the intervention, known as the Serious Illness Care Program (SICP), failed to improve those outcomes, or if there simply weren’t enough patients in the trial to detect a meaningful difference, according to investigators led by Rachelle Bernacki, MD, of Brigham and Women’s Hospital and the Harvard School of Public Health, Boston.
“Our challenges reflect the need in our field for patient-centered measures of communication that are agreed upon, validated, and demonstrably sensitive to communication interventions,” wrote Dr. Bernacki and her coinvestigators in a report on the study published in JAMA Internal Medicine.
However, the SICP intervention did clearly result in a larger number of serious-illness conversations that occurred earlier and were of higher quality, the investigators wrote in a separate report published in JAMA Oncology. In medical records reviewed after the patients’ deaths, 96% of those who received the intervention had a documented serious-illness conversation with their oncology clinician, compared with 79% of controls (P = .005), according to that report.
The conversations among SICP recipients occurred a median of 2.4 months earlier than controls, and had a greater focus on values and goals, prognosis and understanding of illness, and treatment preferences.
These outcomes are reassuring, since patients “want, require, and deserve” conversations about serious illness, regardless of their impact on measurable outcomes, the authors of an editorial published in JAMA Oncology wrote.
The SICP intervention included a communication guide for clinicians, who also participated in a 2.5-hour training session designed to improve their serious-illness conversation skills. Other aspects of the program for clinicians included email reminders before outpatient visits, a specialized EMR template, and personal coaching. The program also included patient tools, including a letter introducing the intervention and a guide for continuing the conversation with their family.
The study did not demonstrate a significant difference in peacefulness, as measured by the validated Peace, Equanimity, and Acceptance in the Cancer Experience questionnaire, or in goal-concordant care, which was measured by asking patients to select goals of importance, and then asking caregivers to rate whether those goals had been met at the end of life.
However, patients in the SICP group reported less anxiety and depression 14 weeks into the trial, according to the investigators. The proportion of patients reporting moderate to severe anxiety at that time point was 10.2% for the intervention group versus 5.0% for controls (P = .05), while the proportion reporting depression symptoms was 20.8% for the intervention versus 10.6% for controls (P = .04).
The anxiety reduction was maintained at 24 weeks, though the depression reduction was not, the investigators wrote, adding that there were no differences in survival between arms.
Taken together, these results suggest that oncology clinicians can discuss difficult topics without causing harm, and with potential benefit, the investigators wrote in a discussion of their results.
“Further development of serious illness communication interventions will require more reliable and well-accepted patient-centered outcome measures and additional testing of the effect on patients throughout their illness trajectory,” they concluded.
Dr. Bernacki reported no disclosures. Coauthor Susan D. Block, MD, reported compensation from Up to Date and Atul A. Gawande, MD, MPH, reported receiving compensation from health care writing and media and is employed by a health care venture formed by Amazon, Berkshire Hathaway, and JPMorgan Chase.
SOURCE: Bernacki R et al. JAMA Intern Med. 2018 Mar 14. doi: 10.1001/jamainternmed.2019.0077.
FROM JAMA INTERNAL MEDICINE
MI, strokes spike during 30 days after cancer diagnosis
HONOLULU – During the first month after a new cancer diagnosis, patients face a substantially elevated risk for an arterial thromboembolic event – an MI or stroke – consistent with the well-described increased risk newly diagnosed cancer patients face from venous thromboembolism, based on findings from a prospective study of more than 4,000 people.
In the new study, 836 patients newly diagnosed with cancer had a 480% increased rate of a fatal or nonfatal MI or stroke during the 30 days following their diagnosis, compared with 3,339 matched people without cancer and after adjustment for baseline differences in demographics and cardiovascular risk factors, Babak B. Navi, MD, said while presenting a poster at the International Stroke Conference sponsored by the American Heart Association.
An additional analysis that focused on 210 of the 836 patients with incident cancer who had any of seven of the cancers known to pose the highest venous thromboembolism risk (lymphoma; gynecologic cancer; or cancer of the pancreas, stomach, lung, bladder, or testes) showed an 1,750% greater rate of incident MI or stroke during the first 30 days after diagnosis, compared with matched people without cancer, reported Dr. Navi, chief of the stroke and hospital neurology at Weill Cornell Medicine, New York.
In contrast, during both the period 1-3 months after the cancer diagnosis and more than 3 months after, the rate of MI or stroke among recently diagnosed cancer patients was not significantly different from the rate in comparator individuals, although the data showed modest trends toward more arterial thromboembolic events after a month, and the lack of statistically significant differences may have been a power issue, Dr. Navi suggested.
The reasons for this acutely increased risk for arterial thromboembolic events, as well as the early spike in venous thromboembolic events, are not completely clear, but they likely result from factors released by tumors, effects from the drugs that patients receive for cancer treatment, stress, and interruption of antithrombotic treatment. Dr. Navi fingered cancer-induced hypercoagulability as likely the biggest culprit. It may now be reasonable to test the idea of treating newly diagnosed cancer patients with agents that could reduce their risk for MI or stroke, such as aspirin or a statin, he said in an interview.
The new analysis used data collected in the REGARDS (Reasons for Geographic and Racial Differences in Stroke) study, which during 2003-2007 enrolled more than 30,000 U.S. residents who were at least 45 years old. Dr. Navi and his associates used data collected from all REGARDS participants who developed incident cancer and had continuous Medicare coverage for at least 1 year before entering REGARDS, excluding those with cancer before enrollment. They used the Medicare records to identify the cancer diagnoses, and matched each of these people with four similar but cancer-free people enrolled in the study. Follow-up continued through September 2015. The average age of this REGARDS subgroup at enrollment was 72 years old, and nearly half were women. The incident cancers included 640 patients with a solid tumor, 71 with hematologic cancers, 13 with brain tumors, and 112 with an unknown primary cancer site.
Dr. Navi and his associates designed this study to validate previously reported findings of increased arterial thromboembolic events in newly diagnosed cancer patients from studies of insurance claims databases. Although the increased risk for venous thromboembolism in cancer patients is already well established, documenting a similar risk for arterial events is important because they are “generally more impactful for patients than venous thromboembolism,” Dr. Navi said.
REGARDS has received no commercial funding. Dr. Navi reported no disclosures.
SOURCE: Navi BB et al. Stroke. 2019 Feb;50(Suppl_1): Abstract WMP53.
HONOLULU – During the first month after a new cancer diagnosis, patients face a substantially elevated risk for an arterial thromboembolic event – an MI or stroke – consistent with the well-described increased risk newly diagnosed cancer patients face from venous thromboembolism, based on findings from a prospective study of more than 4,000 people.
In the new study, 836 patients newly diagnosed with cancer had a 480% increased rate of a fatal or nonfatal MI or stroke during the 30 days following their diagnosis, compared with 3,339 matched people without cancer and after adjustment for baseline differences in demographics and cardiovascular risk factors, Babak B. Navi, MD, said while presenting a poster at the International Stroke Conference sponsored by the American Heart Association.
An additional analysis that focused on 210 of the 836 patients with incident cancer who had any of seven of the cancers known to pose the highest venous thromboembolism risk (lymphoma; gynecologic cancer; or cancer of the pancreas, stomach, lung, bladder, or testes) showed an 1,750% greater rate of incident MI or stroke during the first 30 days after diagnosis, compared with matched people without cancer, reported Dr. Navi, chief of the stroke and hospital neurology at Weill Cornell Medicine, New York.
In contrast, during both the period 1-3 months after the cancer diagnosis and more than 3 months after, the rate of MI or stroke among recently diagnosed cancer patients was not significantly different from the rate in comparator individuals, although the data showed modest trends toward more arterial thromboembolic events after a month, and the lack of statistically significant differences may have been a power issue, Dr. Navi suggested.
The reasons for this acutely increased risk for arterial thromboembolic events, as well as the early spike in venous thromboembolic events, are not completely clear, but they likely result from factors released by tumors, effects from the drugs that patients receive for cancer treatment, stress, and interruption of antithrombotic treatment. Dr. Navi fingered cancer-induced hypercoagulability as likely the biggest culprit. It may now be reasonable to test the idea of treating newly diagnosed cancer patients with agents that could reduce their risk for MI or stroke, such as aspirin or a statin, he said in an interview.
The new analysis used data collected in the REGARDS (Reasons for Geographic and Racial Differences in Stroke) study, which during 2003-2007 enrolled more than 30,000 U.S. residents who were at least 45 years old. Dr. Navi and his associates used data collected from all REGARDS participants who developed incident cancer and had continuous Medicare coverage for at least 1 year before entering REGARDS, excluding those with cancer before enrollment. They used the Medicare records to identify the cancer diagnoses, and matched each of these people with four similar but cancer-free people enrolled in the study. Follow-up continued through September 2015. The average age of this REGARDS subgroup at enrollment was 72 years old, and nearly half were women. The incident cancers included 640 patients with a solid tumor, 71 with hematologic cancers, 13 with brain tumors, and 112 with an unknown primary cancer site.
Dr. Navi and his associates designed this study to validate previously reported findings of increased arterial thromboembolic events in newly diagnosed cancer patients from studies of insurance claims databases. Although the increased risk for venous thromboembolism in cancer patients is already well established, documenting a similar risk for arterial events is important because they are “generally more impactful for patients than venous thromboembolism,” Dr. Navi said.
REGARDS has received no commercial funding. Dr. Navi reported no disclosures.
SOURCE: Navi BB et al. Stroke. 2019 Feb;50(Suppl_1): Abstract WMP53.
HONOLULU – During the first month after a new cancer diagnosis, patients face a substantially elevated risk for an arterial thromboembolic event – an MI or stroke – consistent with the well-described increased risk newly diagnosed cancer patients face from venous thromboembolism, based on findings from a prospective study of more than 4,000 people.
In the new study, 836 patients newly diagnosed with cancer had a 480% increased rate of a fatal or nonfatal MI or stroke during the 30 days following their diagnosis, compared with 3,339 matched people without cancer and after adjustment for baseline differences in demographics and cardiovascular risk factors, Babak B. Navi, MD, said while presenting a poster at the International Stroke Conference sponsored by the American Heart Association.
An additional analysis that focused on 210 of the 836 patients with incident cancer who had any of seven of the cancers known to pose the highest venous thromboembolism risk (lymphoma; gynecologic cancer; or cancer of the pancreas, stomach, lung, bladder, or testes) showed an 1,750% greater rate of incident MI or stroke during the first 30 days after diagnosis, compared with matched people without cancer, reported Dr. Navi, chief of the stroke and hospital neurology at Weill Cornell Medicine, New York.
In contrast, during both the period 1-3 months after the cancer diagnosis and more than 3 months after, the rate of MI or stroke among recently diagnosed cancer patients was not significantly different from the rate in comparator individuals, although the data showed modest trends toward more arterial thromboembolic events after a month, and the lack of statistically significant differences may have been a power issue, Dr. Navi suggested.
The reasons for this acutely increased risk for arterial thromboembolic events, as well as the early spike in venous thromboembolic events, are not completely clear, but they likely result from factors released by tumors, effects from the drugs that patients receive for cancer treatment, stress, and interruption of antithrombotic treatment. Dr. Navi fingered cancer-induced hypercoagulability as likely the biggest culprit. It may now be reasonable to test the idea of treating newly diagnosed cancer patients with agents that could reduce their risk for MI or stroke, such as aspirin or a statin, he said in an interview.
The new analysis used data collected in the REGARDS (Reasons for Geographic and Racial Differences in Stroke) study, which during 2003-2007 enrolled more than 30,000 U.S. residents who were at least 45 years old. Dr. Navi and his associates used data collected from all REGARDS participants who developed incident cancer and had continuous Medicare coverage for at least 1 year before entering REGARDS, excluding those with cancer before enrollment. They used the Medicare records to identify the cancer diagnoses, and matched each of these people with four similar but cancer-free people enrolled in the study. Follow-up continued through September 2015. The average age of this REGARDS subgroup at enrollment was 72 years old, and nearly half were women. The incident cancers included 640 patients with a solid tumor, 71 with hematologic cancers, 13 with brain tumors, and 112 with an unknown primary cancer site.
Dr. Navi and his associates designed this study to validate previously reported findings of increased arterial thromboembolic events in newly diagnosed cancer patients from studies of insurance claims databases. Although the increased risk for venous thromboembolism in cancer patients is already well established, documenting a similar risk for arterial events is important because they are “generally more impactful for patients than venous thromboembolism,” Dr. Navi said.
REGARDS has received no commercial funding. Dr. Navi reported no disclosures.
SOURCE: Navi BB et al. Stroke. 2019 Feb;50(Suppl_1): Abstract WMP53.
REPORTING FROM ISC 2019
KD025 produces durable responses in patients with cGVHD
HOUSTON – The according to a speaker at the Transplantation & Cellular Therapy Meetings.
In an ongoing phase 2 trial (NCT02841995), KD025 produced an overall response rate of 59%. Responses occurred in all affected organ systems, and 72% of responders experienced an improvement in Lee Symptom Scale (LSS) score.
The median duration of response was 28 weeks, but durability data are still maturing, according to Madan Jagasia, MBBS, of Vanderbilt University in Nashville, Tenn.
Dr. Jagasia and his colleagues evaluated KD025 in 54 adults who had persistent, active cGVHD after at least 2 months of steroid therapy. Sixty-seven percent of patients had received at least two prior lines of therapy, and 48% had involvement in four or more organs.
KD025 was given at three doses: 200 mg once daily (cohort 1), 200 mg twice daily (cohort 2), and 400 mg once daily (cohort 3). Patients also received glucocorticoid therapy, with or without calcineurin inhibitor therapy.
Cohort 1 included 17 patients who had a median age of 50 years (range, 20-63). They received KD025 for a median of 37 weeks, and six patients were still receiving KD025 at last follow-up.
Cohort 2 included 16 patients who had a median age of 55 years (range, 30-75). They received KD025 for a median of 33 weeks, and three patients were still receiving KD025 at last follow-up.
Cohort 3 included 21 patients who had a median age of 46 years (range, 25-75). They received KD025 for a median of 27 weeks, and 11 patients were still receiving KD025 at last follow-up.
The ORR was 59% (32/54) across the study, 65% (11/17) in cohort 1, 63% (10/16) in cohort 2, and 52% (11/21) in cohort 3. Three patients in cohort 3 didn’t reach the first response assessment, so the ORR in response-evaluable patients was 61% (11/18).
The ORR was 58% in patients who had received two or more prior lines of therapy, 55% in patients with severe cGVHD, and 62% in patients who had four or more organs involved.
“Responses were observed across all affected organ systems,” Dr. Jagasia said. “CRs [complete responses] were seen in all organs except the lung, and there were two partial responses observed in the lung.”
The median duration of response was 28 weeks. Eighty-two percent of responders in cohort 1, 50% of responders in cohort 2, and 36% of responders in cohort 3 had responses lasting 20 weeks or more.
“Keep in mind, the median duration of follow-up in cohort 3 is still short, and the durability data will continue to mature,” Dr. Jagasia said.
Most responders (72%) had at least a 7-point reduction in LSS score. Considering responders and nonresponders together, 65% of patients in cohort 1, 56% in cohort 2, and 52% in cohort 3 had an improvement in LSS score.
In all, 69% of patients stopped or reduced their use of steroids or other immunosuppressants. Seven patients completely discontinued steroids.
Forty-four percent of patients (n = 24) had a treatment-related adverse event (AE), but none of these AEs were considered serious. Four percent of patients (n = 2) had a related AE that led to treatment discontinuation, and 15% (n = 8) had grade 3/4 related AEs.
“The AEs were, overall, consistent with those expected in chronic graft-versus-host disease patients receiving corticosteroids,” Dr. Jagasia said. “Most important to note, there was no apparent increased risk of infection. Specifically, there was no CMV [cytomegalovirus] infection.”
The most common AEs were upper respiratory tract infection, fatigue, nausea, AST/ALT increase, and diarrhea. The most common grade 3/4 AEs were gamma-glutamyltransferase increase, hyperglycemia, anemia, and dyspnea.
There were three on-study deaths, all considered unrelated to KD025. One patient died of leukemia relapse, one died of lung infection, and one died of cardiac arrest.
Dr. Jagasia presented these results at the Transplantation & Cellular Therapy Meetings, held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research. At the meeting, the American Society for Blood and Marrow Transplantation announced a new name for the society: the American Society for Transplantation and Cellular Therapy (ASTCT).
Dr. Jagasia reported relationships with Kadmon Holdings, Mallinckrodt, and Janssen. The trial was sponsored by Kadmon Holdings.
SOURCE: Jagasia M et al. TCT 2019, Abstract 36.
HOUSTON – The according to a speaker at the Transplantation & Cellular Therapy Meetings.
In an ongoing phase 2 trial (NCT02841995), KD025 produced an overall response rate of 59%. Responses occurred in all affected organ systems, and 72% of responders experienced an improvement in Lee Symptom Scale (LSS) score.
The median duration of response was 28 weeks, but durability data are still maturing, according to Madan Jagasia, MBBS, of Vanderbilt University in Nashville, Tenn.
Dr. Jagasia and his colleagues evaluated KD025 in 54 adults who had persistent, active cGVHD after at least 2 months of steroid therapy. Sixty-seven percent of patients had received at least two prior lines of therapy, and 48% had involvement in four or more organs.
KD025 was given at three doses: 200 mg once daily (cohort 1), 200 mg twice daily (cohort 2), and 400 mg once daily (cohort 3). Patients also received glucocorticoid therapy, with or without calcineurin inhibitor therapy.
Cohort 1 included 17 patients who had a median age of 50 years (range, 20-63). They received KD025 for a median of 37 weeks, and six patients were still receiving KD025 at last follow-up.
Cohort 2 included 16 patients who had a median age of 55 years (range, 30-75). They received KD025 for a median of 33 weeks, and three patients were still receiving KD025 at last follow-up.
Cohort 3 included 21 patients who had a median age of 46 years (range, 25-75). They received KD025 for a median of 27 weeks, and 11 patients were still receiving KD025 at last follow-up.
The ORR was 59% (32/54) across the study, 65% (11/17) in cohort 1, 63% (10/16) in cohort 2, and 52% (11/21) in cohort 3. Three patients in cohort 3 didn’t reach the first response assessment, so the ORR in response-evaluable patients was 61% (11/18).
The ORR was 58% in patients who had received two or more prior lines of therapy, 55% in patients with severe cGVHD, and 62% in patients who had four or more organs involved.
“Responses were observed across all affected organ systems,” Dr. Jagasia said. “CRs [complete responses] were seen in all organs except the lung, and there were two partial responses observed in the lung.”
The median duration of response was 28 weeks. Eighty-two percent of responders in cohort 1, 50% of responders in cohort 2, and 36% of responders in cohort 3 had responses lasting 20 weeks or more.
“Keep in mind, the median duration of follow-up in cohort 3 is still short, and the durability data will continue to mature,” Dr. Jagasia said.
Most responders (72%) had at least a 7-point reduction in LSS score. Considering responders and nonresponders together, 65% of patients in cohort 1, 56% in cohort 2, and 52% in cohort 3 had an improvement in LSS score.
In all, 69% of patients stopped or reduced their use of steroids or other immunosuppressants. Seven patients completely discontinued steroids.
Forty-four percent of patients (n = 24) had a treatment-related adverse event (AE), but none of these AEs were considered serious. Four percent of patients (n = 2) had a related AE that led to treatment discontinuation, and 15% (n = 8) had grade 3/4 related AEs.
“The AEs were, overall, consistent with those expected in chronic graft-versus-host disease patients receiving corticosteroids,” Dr. Jagasia said. “Most important to note, there was no apparent increased risk of infection. Specifically, there was no CMV [cytomegalovirus] infection.”
The most common AEs were upper respiratory tract infection, fatigue, nausea, AST/ALT increase, and diarrhea. The most common grade 3/4 AEs were gamma-glutamyltransferase increase, hyperglycemia, anemia, and dyspnea.
There were three on-study deaths, all considered unrelated to KD025. One patient died of leukemia relapse, one died of lung infection, and one died of cardiac arrest.
Dr. Jagasia presented these results at the Transplantation & Cellular Therapy Meetings, held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research. At the meeting, the American Society for Blood and Marrow Transplantation announced a new name for the society: the American Society for Transplantation and Cellular Therapy (ASTCT).
Dr. Jagasia reported relationships with Kadmon Holdings, Mallinckrodt, and Janssen. The trial was sponsored by Kadmon Holdings.
SOURCE: Jagasia M et al. TCT 2019, Abstract 36.
HOUSTON – The according to a speaker at the Transplantation & Cellular Therapy Meetings.
In an ongoing phase 2 trial (NCT02841995), KD025 produced an overall response rate of 59%. Responses occurred in all affected organ systems, and 72% of responders experienced an improvement in Lee Symptom Scale (LSS) score.
The median duration of response was 28 weeks, but durability data are still maturing, according to Madan Jagasia, MBBS, of Vanderbilt University in Nashville, Tenn.
Dr. Jagasia and his colleagues evaluated KD025 in 54 adults who had persistent, active cGVHD after at least 2 months of steroid therapy. Sixty-seven percent of patients had received at least two prior lines of therapy, and 48% had involvement in four or more organs.
KD025 was given at three doses: 200 mg once daily (cohort 1), 200 mg twice daily (cohort 2), and 400 mg once daily (cohort 3). Patients also received glucocorticoid therapy, with or without calcineurin inhibitor therapy.
Cohort 1 included 17 patients who had a median age of 50 years (range, 20-63). They received KD025 for a median of 37 weeks, and six patients were still receiving KD025 at last follow-up.
Cohort 2 included 16 patients who had a median age of 55 years (range, 30-75). They received KD025 for a median of 33 weeks, and three patients were still receiving KD025 at last follow-up.
Cohort 3 included 21 patients who had a median age of 46 years (range, 25-75). They received KD025 for a median of 27 weeks, and 11 patients were still receiving KD025 at last follow-up.
The ORR was 59% (32/54) across the study, 65% (11/17) in cohort 1, 63% (10/16) in cohort 2, and 52% (11/21) in cohort 3. Three patients in cohort 3 didn’t reach the first response assessment, so the ORR in response-evaluable patients was 61% (11/18).
The ORR was 58% in patients who had received two or more prior lines of therapy, 55% in patients with severe cGVHD, and 62% in patients who had four or more organs involved.
“Responses were observed across all affected organ systems,” Dr. Jagasia said. “CRs [complete responses] were seen in all organs except the lung, and there were two partial responses observed in the lung.”
The median duration of response was 28 weeks. Eighty-two percent of responders in cohort 1, 50% of responders in cohort 2, and 36% of responders in cohort 3 had responses lasting 20 weeks or more.
“Keep in mind, the median duration of follow-up in cohort 3 is still short, and the durability data will continue to mature,” Dr. Jagasia said.
Most responders (72%) had at least a 7-point reduction in LSS score. Considering responders and nonresponders together, 65% of patients in cohort 1, 56% in cohort 2, and 52% in cohort 3 had an improvement in LSS score.
In all, 69% of patients stopped or reduced their use of steroids or other immunosuppressants. Seven patients completely discontinued steroids.
Forty-four percent of patients (n = 24) had a treatment-related adverse event (AE), but none of these AEs were considered serious. Four percent of patients (n = 2) had a related AE that led to treatment discontinuation, and 15% (n = 8) had grade 3/4 related AEs.
“The AEs were, overall, consistent with those expected in chronic graft-versus-host disease patients receiving corticosteroids,” Dr. Jagasia said. “Most important to note, there was no apparent increased risk of infection. Specifically, there was no CMV [cytomegalovirus] infection.”
The most common AEs were upper respiratory tract infection, fatigue, nausea, AST/ALT increase, and diarrhea. The most common grade 3/4 AEs were gamma-glutamyltransferase increase, hyperglycemia, anemia, and dyspnea.
There were three on-study deaths, all considered unrelated to KD025. One patient died of leukemia relapse, one died of lung infection, and one died of cardiac arrest.
Dr. Jagasia presented these results at the Transplantation & Cellular Therapy Meetings, held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research. At the meeting, the American Society for Blood and Marrow Transplantation announced a new name for the society: the American Society for Transplantation and Cellular Therapy (ASTCT).
Dr. Jagasia reported relationships with Kadmon Holdings, Mallinckrodt, and Janssen. The trial was sponsored by Kadmon Holdings.
SOURCE: Jagasia M et al. TCT 2019, Abstract 36.
REPORTING FROM TCT 2019
Sperm counts largely stable after adjuvant treatment of clinical stage I testicular cancer
Adjuvant treatments appear to have no significant detrimental long-term effects on sperm count in men being treated for clinical stage I testicular cancer, results of a recent investigation suggest.
Sperm number and concentration were largely stable over time in patients who received a round of chemotherapy or radiation to lymph nodes following orchiectomy, according to results of the 182-patient study.
Investigators said they still offer sperm banking before orchiectomy, since some patients will have low sperm counts prior to orchiectomy that persist after the procedure.
Moreover, the type of testicular cancer and the potential need for other postorchiectomy treatments are often “unknown factors” that underscore the importance of sperm banking, said the researchers, led by Kristina Weibring, MD, of Karolinska University Hospital, Stockholm.
“Assisted reproductive measures may be necessary for these patients regardless of any treatment given,” the researchers noted. The report is in Annals of Oncology.
The lack of effect on sperm counts in this study stands in contrast to previous studies, which clearly show the detrimental effects of multiple chemotherapy cycles on sperm recovery, the investigators said.
Their study comprised 182 patients 18-50 years of age with clinical stage I testicular cancer who underwent unilateral orchiectomy. Depending on tumor characteristics, the patients then received one cycle of adjuvant carboplatin, one cycle of a bleomycin, etoposide, and cisplatin (BEP) regimen, surveillance, or adjuvant radiotherapy to the infradiaphragmal para-aortic and ipsilateral iliac lymph nodes. Sperm samples were obtained at 6, 12, 24, 36, and 60 months after the completion of treatment.
While there was a transient drop in the radiation-treated patients at the 6-month evaluation, mean total sperm number otherwise increased over time in all groups, according to the investigators’ report.
Similarly, mean sperm concentration significantly increased from baseline to 12 months post treatment in the surveillance, BEP, and carboplatin groups, with a nonsignificant decrease in the radiotherapy group, they said in the report.
There were generally no significant differences in sperm count or concentration for the treatments, compared with surveillance, beyond a significant decrease in mean sperm count for radiation versus surveillance, they added.
There were likewise no significant changes in sperm measures for seminoma and nonseminoma patients at any point over the 5 years of evaluation, reported data show.
“With the results of this study, we can now inform our patients that adjuvant chemotherapy does not seem to affect the testicular function,” Dr. Weibring and her colleagues concluded.
The authors reported that they had no conflicts of interest related to the study, which was supported by the Swedish Cancer Society, among other sources.
SOURCE: Weibring K et al. Ann Oncol. 2019 Feb 25. doi: 10.1093/annonc/mdz017/5348526.
Adjuvant treatments appear to have no significant detrimental long-term effects on sperm count in men being treated for clinical stage I testicular cancer, results of a recent investigation suggest.
Sperm number and concentration were largely stable over time in patients who received a round of chemotherapy or radiation to lymph nodes following orchiectomy, according to results of the 182-patient study.
Investigators said they still offer sperm banking before orchiectomy, since some patients will have low sperm counts prior to orchiectomy that persist after the procedure.
Moreover, the type of testicular cancer and the potential need for other postorchiectomy treatments are often “unknown factors” that underscore the importance of sperm banking, said the researchers, led by Kristina Weibring, MD, of Karolinska University Hospital, Stockholm.
“Assisted reproductive measures may be necessary for these patients regardless of any treatment given,” the researchers noted. The report is in Annals of Oncology.
The lack of effect on sperm counts in this study stands in contrast to previous studies, which clearly show the detrimental effects of multiple chemotherapy cycles on sperm recovery, the investigators said.
Their study comprised 182 patients 18-50 years of age with clinical stage I testicular cancer who underwent unilateral orchiectomy. Depending on tumor characteristics, the patients then received one cycle of adjuvant carboplatin, one cycle of a bleomycin, etoposide, and cisplatin (BEP) regimen, surveillance, or adjuvant radiotherapy to the infradiaphragmal para-aortic and ipsilateral iliac lymph nodes. Sperm samples were obtained at 6, 12, 24, 36, and 60 months after the completion of treatment.
While there was a transient drop in the radiation-treated patients at the 6-month evaluation, mean total sperm number otherwise increased over time in all groups, according to the investigators’ report.
Similarly, mean sperm concentration significantly increased from baseline to 12 months post treatment in the surveillance, BEP, and carboplatin groups, with a nonsignificant decrease in the radiotherapy group, they said in the report.
There were generally no significant differences in sperm count or concentration for the treatments, compared with surveillance, beyond a significant decrease in mean sperm count for radiation versus surveillance, they added.
There were likewise no significant changes in sperm measures for seminoma and nonseminoma patients at any point over the 5 years of evaluation, reported data show.
“With the results of this study, we can now inform our patients that adjuvant chemotherapy does not seem to affect the testicular function,” Dr. Weibring and her colleagues concluded.
The authors reported that they had no conflicts of interest related to the study, which was supported by the Swedish Cancer Society, among other sources.
SOURCE: Weibring K et al. Ann Oncol. 2019 Feb 25. doi: 10.1093/annonc/mdz017/5348526.
Adjuvant treatments appear to have no significant detrimental long-term effects on sperm count in men being treated for clinical stage I testicular cancer, results of a recent investigation suggest.
Sperm number and concentration were largely stable over time in patients who received a round of chemotherapy or radiation to lymph nodes following orchiectomy, according to results of the 182-patient study.
Investigators said they still offer sperm banking before orchiectomy, since some patients will have low sperm counts prior to orchiectomy that persist after the procedure.
Moreover, the type of testicular cancer and the potential need for other postorchiectomy treatments are often “unknown factors” that underscore the importance of sperm banking, said the researchers, led by Kristina Weibring, MD, of Karolinska University Hospital, Stockholm.
“Assisted reproductive measures may be necessary for these patients regardless of any treatment given,” the researchers noted. The report is in Annals of Oncology.
The lack of effect on sperm counts in this study stands in contrast to previous studies, which clearly show the detrimental effects of multiple chemotherapy cycles on sperm recovery, the investigators said.
Their study comprised 182 patients 18-50 years of age with clinical stage I testicular cancer who underwent unilateral orchiectomy. Depending on tumor characteristics, the patients then received one cycle of adjuvant carboplatin, one cycle of a bleomycin, etoposide, and cisplatin (BEP) regimen, surveillance, or adjuvant radiotherapy to the infradiaphragmal para-aortic and ipsilateral iliac lymph nodes. Sperm samples were obtained at 6, 12, 24, 36, and 60 months after the completion of treatment.
While there was a transient drop in the radiation-treated patients at the 6-month evaluation, mean total sperm number otherwise increased over time in all groups, according to the investigators’ report.
Similarly, mean sperm concentration significantly increased from baseline to 12 months post treatment in the surveillance, BEP, and carboplatin groups, with a nonsignificant decrease in the radiotherapy group, they said in the report.
There were generally no significant differences in sperm count or concentration for the treatments, compared with surveillance, beyond a significant decrease in mean sperm count for radiation versus surveillance, they added.
There were likewise no significant changes in sperm measures for seminoma and nonseminoma patients at any point over the 5 years of evaluation, reported data show.
“With the results of this study, we can now inform our patients that adjuvant chemotherapy does not seem to affect the testicular function,” Dr. Weibring and her colleagues concluded.
The authors reported that they had no conflicts of interest related to the study, which was supported by the Swedish Cancer Society, among other sources.
SOURCE: Weibring K et al. Ann Oncol. 2019 Feb 25. doi: 10.1093/annonc/mdz017/5348526.
FROM ANNALS OF ONCOLOGY
Diet appears to play an important role in response to anti-PD-1 cancer immunotherapy
Diet plays an important role in patient response to anti-programmed death-1 (PD-1) cancer immunotherapy, preliminary findings from a gut microbiome study involving 146 melanoma patients suggest.
Specifically, a high-fiber diet was associated with a more diverse gut microbiome and with improved response to anti-PD-1 therapy, whereas a diet high in sugar and processed meat was associated with fewer of the gut bacteria known to be associated with improved response, Christine Spencer, PhD, reported during a press conference highlighting data to be presented at the upcoming American Association for Cancer Research (AACR) annual meeting in Atlanta.
“We found that patients who reported eating high-fiber diets were about five times as likely to respond to anti-PD-1 checkpoint blockade immunotherapy (odds ratio vs. low-fiber diet, 5.3),” said Dr. Spencer, a research scientist at the Parker Institute for Cancer Immunotherapy.
Notably, more than 40% of patients reported taking probiotics, and those, surprisingly, were also associated with reduced gut microbiome diversity, she said.
For this study, Dr. Spencer and her colleagues at the University of Texas MD Anderson Cancer Center, Houston, analyzed prospectively collected fecal samples from 146 melanoma patients, and collected baseline diet information via the National Cancer Institute dietary screener questionnaire, as well as information about probiotic and antibiotic use, in a subset of 113 who were initiating therapy at MD Anderson. Those patients were then followed to assess therapy response.
“Our early data suggest that different foods and supplements may impact response to cancer immunotherapy in patients, and we think this is likely mediated by the gut microbiome,” she said.
Since only 20%-30% of cancer patients respond to immunotherapy, the findings hint at potential approaches for improving gut microbiome diversity, and thus response to anti-PD-1 cancer immunotherapy.
“Eat your high-fiber foods: fruits, vegetables, and whole grains – lots of different kinds and lots of them,” she said. “High-fiber diets have been linked to health benefits in several other contexts, and this study, although preliminary, shows fiber is linked to more favorable gut microbiome in patients, and better response to cancer immunotherapy.”
Conversations about the use of probiotic supplements also are important, she said.
“A lot of people have the perception that probiotics will provide health benefits, but that might not be the case in cancer patients. We’re not saying all of them are bad, but the message is that these factors have never before been studied in patients on immunotherapy, and our data suggest for the first time that they could matter,” she said, noting that future directions include validation of the findings in larger cohorts.
Some of that work has already been done, and updated results will be reported at the AACR meeting.
AACR president and press conference comoderator Elizabeth M. Jaffee, MD, said the findings highlight an “exciting area that’s emerging in cancer research right now.”
Although microbiome research is in its infancy, the MD Anderson group and others are “really making headway,” said Dr. Jaffee, professor of oncology and deputy director of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University, Baltimore.
“It’s exciting ... to learn from your study that patients and healthy individuals can also be empowered through diet to control cancer development, and also how they can be empowered to influence favorable response to our therapies,” she said, cautioning, however, that “this is early and certainly we need more research in this area.”
Also of note, the findings show that gut bacteria affect cancers that aren’t necessarily deriving from the gut.
“So the microbiome has importance in, probably, many different cancers and their response to therapy – and possibly in the development of those cancers, so those are areas of research that we need to prioritize in future work, as well,” she said.
This study was sponsored by the Melanoma Research Alliance, the MD Anderson Melanoma Moonshot, the Miriam and Jim Mulva Fund for Melanoma Research, and the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation. Dr. Spencer disclosed that she is a contributor to U.S. patent application (PCT/US17/53.717) submitted by MD Anderson Cancer Center that covers methods to enhance immune checkpoint blockade responses by modulating the microbiome.
SOURCE: Spencer C et al. AACR 2019, Abstract preview.
Diet plays an important role in patient response to anti-programmed death-1 (PD-1) cancer immunotherapy, preliminary findings from a gut microbiome study involving 146 melanoma patients suggest.
Specifically, a high-fiber diet was associated with a more diverse gut microbiome and with improved response to anti-PD-1 therapy, whereas a diet high in sugar and processed meat was associated with fewer of the gut bacteria known to be associated with improved response, Christine Spencer, PhD, reported during a press conference highlighting data to be presented at the upcoming American Association for Cancer Research (AACR) annual meeting in Atlanta.
“We found that patients who reported eating high-fiber diets were about five times as likely to respond to anti-PD-1 checkpoint blockade immunotherapy (odds ratio vs. low-fiber diet, 5.3),” said Dr. Spencer, a research scientist at the Parker Institute for Cancer Immunotherapy.
Notably, more than 40% of patients reported taking probiotics, and those, surprisingly, were also associated with reduced gut microbiome diversity, she said.
For this study, Dr. Spencer and her colleagues at the University of Texas MD Anderson Cancer Center, Houston, analyzed prospectively collected fecal samples from 146 melanoma patients, and collected baseline diet information via the National Cancer Institute dietary screener questionnaire, as well as information about probiotic and antibiotic use, in a subset of 113 who were initiating therapy at MD Anderson. Those patients were then followed to assess therapy response.
“Our early data suggest that different foods and supplements may impact response to cancer immunotherapy in patients, and we think this is likely mediated by the gut microbiome,” she said.
Since only 20%-30% of cancer patients respond to immunotherapy, the findings hint at potential approaches for improving gut microbiome diversity, and thus response to anti-PD-1 cancer immunotherapy.
“Eat your high-fiber foods: fruits, vegetables, and whole grains – lots of different kinds and lots of them,” she said. “High-fiber diets have been linked to health benefits in several other contexts, and this study, although preliminary, shows fiber is linked to more favorable gut microbiome in patients, and better response to cancer immunotherapy.”
Conversations about the use of probiotic supplements also are important, she said.
“A lot of people have the perception that probiotics will provide health benefits, but that might not be the case in cancer patients. We’re not saying all of them are bad, but the message is that these factors have never before been studied in patients on immunotherapy, and our data suggest for the first time that they could matter,” she said, noting that future directions include validation of the findings in larger cohorts.
Some of that work has already been done, and updated results will be reported at the AACR meeting.
AACR president and press conference comoderator Elizabeth M. Jaffee, MD, said the findings highlight an “exciting area that’s emerging in cancer research right now.”
Although microbiome research is in its infancy, the MD Anderson group and others are “really making headway,” said Dr. Jaffee, professor of oncology and deputy director of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University, Baltimore.
“It’s exciting ... to learn from your study that patients and healthy individuals can also be empowered through diet to control cancer development, and also how they can be empowered to influence favorable response to our therapies,” she said, cautioning, however, that “this is early and certainly we need more research in this area.”
Also of note, the findings show that gut bacteria affect cancers that aren’t necessarily deriving from the gut.
“So the microbiome has importance in, probably, many different cancers and their response to therapy – and possibly in the development of those cancers, so those are areas of research that we need to prioritize in future work, as well,” she said.
This study was sponsored by the Melanoma Research Alliance, the MD Anderson Melanoma Moonshot, the Miriam and Jim Mulva Fund for Melanoma Research, and the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation. Dr. Spencer disclosed that she is a contributor to U.S. patent application (PCT/US17/53.717) submitted by MD Anderson Cancer Center that covers methods to enhance immune checkpoint blockade responses by modulating the microbiome.
SOURCE: Spencer C et al. AACR 2019, Abstract preview.
Diet plays an important role in patient response to anti-programmed death-1 (PD-1) cancer immunotherapy, preliminary findings from a gut microbiome study involving 146 melanoma patients suggest.
Specifically, a high-fiber diet was associated with a more diverse gut microbiome and with improved response to anti-PD-1 therapy, whereas a diet high in sugar and processed meat was associated with fewer of the gut bacteria known to be associated with improved response, Christine Spencer, PhD, reported during a press conference highlighting data to be presented at the upcoming American Association for Cancer Research (AACR) annual meeting in Atlanta.
“We found that patients who reported eating high-fiber diets were about five times as likely to respond to anti-PD-1 checkpoint blockade immunotherapy (odds ratio vs. low-fiber diet, 5.3),” said Dr. Spencer, a research scientist at the Parker Institute for Cancer Immunotherapy.
Notably, more than 40% of patients reported taking probiotics, and those, surprisingly, were also associated with reduced gut microbiome diversity, she said.
For this study, Dr. Spencer and her colleagues at the University of Texas MD Anderson Cancer Center, Houston, analyzed prospectively collected fecal samples from 146 melanoma patients, and collected baseline diet information via the National Cancer Institute dietary screener questionnaire, as well as information about probiotic and antibiotic use, in a subset of 113 who were initiating therapy at MD Anderson. Those patients were then followed to assess therapy response.
“Our early data suggest that different foods and supplements may impact response to cancer immunotherapy in patients, and we think this is likely mediated by the gut microbiome,” she said.
Since only 20%-30% of cancer patients respond to immunotherapy, the findings hint at potential approaches for improving gut microbiome diversity, and thus response to anti-PD-1 cancer immunotherapy.
“Eat your high-fiber foods: fruits, vegetables, and whole grains – lots of different kinds and lots of them,” she said. “High-fiber diets have been linked to health benefits in several other contexts, and this study, although preliminary, shows fiber is linked to more favorable gut microbiome in patients, and better response to cancer immunotherapy.”
Conversations about the use of probiotic supplements also are important, she said.
“A lot of people have the perception that probiotics will provide health benefits, but that might not be the case in cancer patients. We’re not saying all of them are bad, but the message is that these factors have never before been studied in patients on immunotherapy, and our data suggest for the first time that they could matter,” she said, noting that future directions include validation of the findings in larger cohorts.
Some of that work has already been done, and updated results will be reported at the AACR meeting.
AACR president and press conference comoderator Elizabeth M. Jaffee, MD, said the findings highlight an “exciting area that’s emerging in cancer research right now.”
Although microbiome research is in its infancy, the MD Anderson group and others are “really making headway,” said Dr. Jaffee, professor of oncology and deputy director of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University, Baltimore.
“It’s exciting ... to learn from your study that patients and healthy individuals can also be empowered through diet to control cancer development, and also how they can be empowered to influence favorable response to our therapies,” she said, cautioning, however, that “this is early and certainly we need more research in this area.”
Also of note, the findings show that gut bacteria affect cancers that aren’t necessarily deriving from the gut.
“So the microbiome has importance in, probably, many different cancers and their response to therapy – and possibly in the development of those cancers, so those are areas of research that we need to prioritize in future work, as well,” she said.
This study was sponsored by the Melanoma Research Alliance, the MD Anderson Melanoma Moonshot, the Miriam and Jim Mulva Fund for Melanoma Research, and the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation. Dr. Spencer disclosed that she is a contributor to U.S. patent application (PCT/US17/53.717) submitted by MD Anderson Cancer Center that covers methods to enhance immune checkpoint blockade responses by modulating the microbiome.
SOURCE: Spencer C et al. AACR 2019, Abstract preview.