User login
Elevated IL-6 linked to complications after major abdominal surgery
On postoperative day 1, elevated interleukin-6 level was associated with postoperative complications, according to a single-center cohort study of patients who had major abdominal surgery.
“Up to 28% of patients undergoing major abdominal surgery experience postoperative complications, including wound infection, sepsis, anastomotic dehiscence, pneumonia,cardiovascular or respiratory events, and mortality” but an accurate means of identifying those in the risk category would contribute the development of prevention stratetgies, the investigators wrote.
Previous studies of cardiothoracic surgery have supported an association of systemic inflammation to poor outcomes. Dr. Thijs Rettig and colleagues at St. Antonius Hospital, Nieuwegein, the Netherlands, sought to clarify if markers of inflammation and major abdominal surgery correlate with outcomes. Their results were published in the July issue of Annals of Surgery.
Researchers conducted a prospective cohort study at a single center using data obtained from the Myocardial Injury and Complications after major abdominal surgery (MICOLON) study. Participants in the MICOLON study were individuals aged 45 years or older who underwent elective major abdominal surgery. Other inclusion criteria included major cardiovascular (CV) risk factors, coronary artery disease, cerebrovascular accident, diabetes, renal insufficiency, atrial fibrillation, left ventricular dysfunction, aortic valve stenosis, or two minor CV risk factors.
Interleukin-6 (IL-6), tumor necrosis factor (TNF)-alpha, and C-reactive protein (CRP) levels were obtained at baseline and postoperative days 1, 3, and 7 in 137 patients. Systemic inflammatory response syndrome (SIRS) scores were calculated within 48 hours of surgery.
Primary endpoints were 30-day mortality, sepsis, pneumonia, wound infection, anastomotic dehiscence, reoperation, new-onset atrial fibrillation, respiratory insufficiency, congestive heart failure, and myocardial infarction. Data were also collected on length of stay and patients were followed up at 30 days postoperatively for further complications.
With a mean age of 68 years, 59% of patients were male and 30% (n = 40) had an ASA score of 3 or higher. Colorectal (50%), gastroesophageal (22%), and pancreatic (10%) surgery were the most common procedures performed. After excluding 2 patients from analysis for elevated baseline IL-6, 135 patients were analyzed.
At least one postoperative complication was observed in 29% (n = 39) of study subjects with a mean onset of 5 days after surgery. Use of preoperative steroids, aspirin, and statins were not associated with complications; however, blood loss and longer surgery duration where associated with worse outcomes.
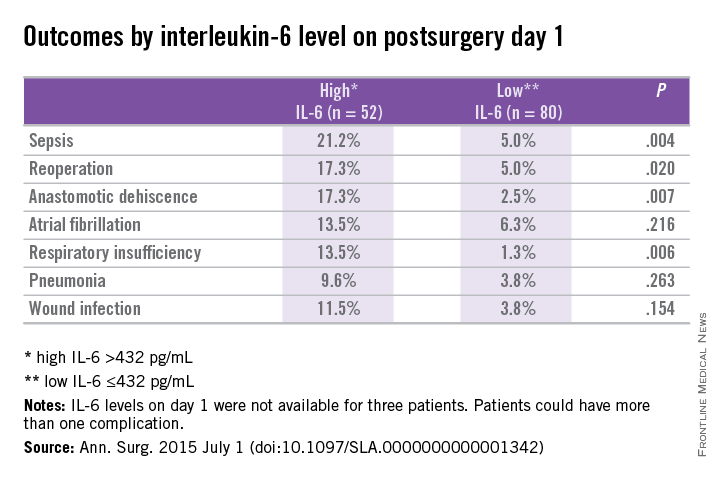
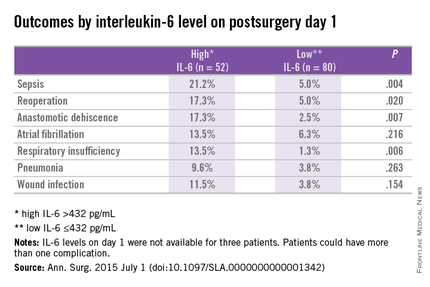
In patients with and without complications, differences in IL-6 levels were observed at day 1 at 596 pg/mL vs. 303 pg/mL (P < .01), day 3 at 128 pg/mL vs. 69 pg/mL (P < .01), and day 7 at 76 pg/mL vs. 27 pg/mL (P = .02).
On day 1, CRP was similar in both groups (90 mg/L vs. 78 mg/L; P = .131), but on days 3 (223 mg/L vs. 131 mg/L; P < .001) and 7 (131 mg/L vs. 63 mg/L; P < .001) differences were observed.
Differences in TNF-alpha were observed between groups on day 7 (0.5 pg/mL vs. 0, P < .01). The two groups demonstrated similar leukocyte counts postoperatively.
Prediction for postoperative complications was associated with an IL-6 of 432 pg/mL at day 1, which was 70% specific and 64% sensitive, and had a positive predictive value (PPV) of 44% and negative predictive value (NPV) of 84%. A longer hospital stay of 12 days vs. 7 days (P < .001) was associated with high IL-6 (> 432 pg/mL) vs. a low IL-6 (< 432 pg/mL) at day 1.
Elevated IL-6 level on postoperative day 1 was independently associated with postoperative complications by multivariant regression analysis (AOR: 3.3; 95% confidence interval, 1.3-8.5; P < .02).
The researchers concluded that an increased IL-6 level on postoperative day 1 was associated with increased length of stay and threefold increased risk of complications after major abdominal surgery. They further continued, “It is plausible that early recognition of postoperative complications optimizes the chance of better outcome. One way to enhance early detection of complications is using inflammatory markers as predictors of outcome.”
The authors reported no conflicts of interest.
On postoperative day 1, elevated interleukin-6 level was associated with postoperative complications, according to a single-center cohort study of patients who had major abdominal surgery.
“Up to 28% of patients undergoing major abdominal surgery experience postoperative complications, including wound infection, sepsis, anastomotic dehiscence, pneumonia,cardiovascular or respiratory events, and mortality” but an accurate means of identifying those in the risk category would contribute the development of prevention stratetgies, the investigators wrote.

Previous studies of cardiothoracic surgery have supported an association of systemic inflammation to poor outcomes. Dr. Thijs Rettig and colleagues at St. Antonius Hospital, Nieuwegein, the Netherlands, sought to clarify if markers of inflammation and major abdominal surgery correlate with outcomes. Their results were published in the July issue of Annals of Surgery.
Researchers conducted a prospective cohort study at a single center using data obtained from the Myocardial Injury and Complications after major abdominal surgery (MICOLON) study. Participants in the MICOLON study were individuals aged 45 years or older who underwent elective major abdominal surgery. Other inclusion criteria included major cardiovascular (CV) risk factors, coronary artery disease, cerebrovascular accident, diabetes, renal insufficiency, atrial fibrillation, left ventricular dysfunction, aortic valve stenosis, or two minor CV risk factors.
Interleukin-6 (IL-6), tumor necrosis factor (TNF)-alpha, and C-reactive protein (CRP) levels were obtained at baseline and postoperative days 1, 3, and 7 in 137 patients. Systemic inflammatory response syndrome (SIRS) scores were calculated within 48 hours of surgery.
Primary endpoints were 30-day mortality, sepsis, pneumonia, wound infection, anastomotic dehiscence, reoperation, new-onset atrial fibrillation, respiratory insufficiency, congestive heart failure, and myocardial infarction. Data were also collected on length of stay and patients were followed up at 30 days postoperatively for further complications.
With a mean age of 68 years, 59% of patients were male and 30% (n = 40) had an ASA score of 3 or higher. Colorectal (50%), gastroesophageal (22%), and pancreatic (10%) surgery were the most common procedures performed. After excluding 2 patients from analysis for elevated baseline IL-6, 135 patients were analyzed.
At least one postoperative complication was observed in 29% (n = 39) of study subjects with a mean onset of 5 days after surgery. Use of preoperative steroids, aspirin, and statins were not associated with complications; however, blood loss and longer surgery duration where associated with worse outcomes.
In patients with and without complications, differences in IL-6 levels were observed at day 1 at 596 pg/mL vs. 303 pg/mL (P < .01), day 3 at 128 pg/mL vs. 69 pg/mL (P < .01), and day 7 at 76 pg/mL vs. 27 pg/mL (P = .02).
On day 1, CRP was similar in both groups (90 mg/L vs. 78 mg/L; P = .131), but on days 3 (223 mg/L vs. 131 mg/L; P < .001) and 7 (131 mg/L vs. 63 mg/L; P < .001) differences were observed.
Differences in TNF-alpha were observed between groups on day 7 (0.5 pg/mL vs. 0, P < .01). The two groups demonstrated similar leukocyte counts postoperatively.
Prediction for postoperative complications was associated with an IL-6 of 432 pg/mL at day 1, which was 70% specific and 64% sensitive, and had a positive predictive value (PPV) of 44% and negative predictive value (NPV) of 84%. A longer hospital stay of 12 days vs. 7 days (P < .001) was associated with high IL-6 (> 432 pg/mL) vs. a low IL-6 (< 432 pg/mL) at day 1.
Elevated IL-6 level on postoperative day 1 was independently associated with postoperative complications by multivariant regression analysis (AOR: 3.3; 95% confidence interval, 1.3-8.5; P < .02).
The researchers concluded that an increased IL-6 level on postoperative day 1 was associated with increased length of stay and threefold increased risk of complications after major abdominal surgery. They further continued, “It is plausible that early recognition of postoperative complications optimizes the chance of better outcome. One way to enhance early detection of complications is using inflammatory markers as predictors of outcome.”
The authors reported no conflicts of interest.
On postoperative day 1, elevated interleukin-6 level was associated with postoperative complications, according to a single-center cohort study of patients who had major abdominal surgery.
“Up to 28% of patients undergoing major abdominal surgery experience postoperative complications, including wound infection, sepsis, anastomotic dehiscence, pneumonia,cardiovascular or respiratory events, and mortality” but an accurate means of identifying those in the risk category would contribute the development of prevention stratetgies, the investigators wrote.

Previous studies of cardiothoracic surgery have supported an association of systemic inflammation to poor outcomes. Dr. Thijs Rettig and colleagues at St. Antonius Hospital, Nieuwegein, the Netherlands, sought to clarify if markers of inflammation and major abdominal surgery correlate with outcomes. Their results were published in the July issue of Annals of Surgery.
Researchers conducted a prospective cohort study at a single center using data obtained from the Myocardial Injury and Complications after major abdominal surgery (MICOLON) study. Participants in the MICOLON study were individuals aged 45 years or older who underwent elective major abdominal surgery. Other inclusion criteria included major cardiovascular (CV) risk factors, coronary artery disease, cerebrovascular accident, diabetes, renal insufficiency, atrial fibrillation, left ventricular dysfunction, aortic valve stenosis, or two minor CV risk factors.
Interleukin-6 (IL-6), tumor necrosis factor (TNF)-alpha, and C-reactive protein (CRP) levels were obtained at baseline and postoperative days 1, 3, and 7 in 137 patients. Systemic inflammatory response syndrome (SIRS) scores were calculated within 48 hours of surgery.
Primary endpoints were 30-day mortality, sepsis, pneumonia, wound infection, anastomotic dehiscence, reoperation, new-onset atrial fibrillation, respiratory insufficiency, congestive heart failure, and myocardial infarction. Data were also collected on length of stay and patients were followed up at 30 days postoperatively for further complications.
With a mean age of 68 years, 59% of patients were male and 30% (n = 40) had an ASA score of 3 or higher. Colorectal (50%), gastroesophageal (22%), and pancreatic (10%) surgery were the most common procedures performed. After excluding 2 patients from analysis for elevated baseline IL-6, 135 patients were analyzed.
At least one postoperative complication was observed in 29% (n = 39) of study subjects with a mean onset of 5 days after surgery. Use of preoperative steroids, aspirin, and statins were not associated with complications; however, blood loss and longer surgery duration where associated with worse outcomes.
In patients with and without complications, differences in IL-6 levels were observed at day 1 at 596 pg/mL vs. 303 pg/mL (P < .01), day 3 at 128 pg/mL vs. 69 pg/mL (P < .01), and day 7 at 76 pg/mL vs. 27 pg/mL (P = .02).
On day 1, CRP was similar in both groups (90 mg/L vs. 78 mg/L; P = .131), but on days 3 (223 mg/L vs. 131 mg/L; P < .001) and 7 (131 mg/L vs. 63 mg/L; P < .001) differences were observed.
Differences in TNF-alpha were observed between groups on day 7 (0.5 pg/mL vs. 0, P < .01). The two groups demonstrated similar leukocyte counts postoperatively.
Prediction for postoperative complications was associated with an IL-6 of 432 pg/mL at day 1, which was 70% specific and 64% sensitive, and had a positive predictive value (PPV) of 44% and negative predictive value (NPV) of 84%. A longer hospital stay of 12 days vs. 7 days (P < .001) was associated with high IL-6 (> 432 pg/mL) vs. a low IL-6 (< 432 pg/mL) at day 1.
Elevated IL-6 level on postoperative day 1 was independently associated with postoperative complications by multivariant regression analysis (AOR: 3.3; 95% confidence interval, 1.3-8.5; P < .02).
The researchers concluded that an increased IL-6 level on postoperative day 1 was associated with increased length of stay and threefold increased risk of complications after major abdominal surgery. They further continued, “It is plausible that early recognition of postoperative complications optimizes the chance of better outcome. One way to enhance early detection of complications is using inflammatory markers as predictors of outcome.”
The authors reported no conflicts of interest.
FROM ANNALS OF SURGERY
Key clinical point: Postoperative complications after major abdominal surgery are associated with elevated IL-6.
Major finding: Elevated IL-6 level on postoperative day 1 was independently associated with postoperative complications by multivariant regression analysis (P < .02).
Data source: Prospective cohort study at a single center using data from the Myocardial Injury and Postoperative Complications after major abdominal surgery (MICOLON) study.
Disclosures: The authors reported no conflicts of interest.
Guideline updated on hematopoietic colony-stimulating factors
Hematopoietic colony-stimulating factors should now be considered for patients who are over age 64 years, have diffuse aggressive lymphoma, and are receiving curative chemotherapy (cyclophosphamide, doxorubicin, vincristine, prednisone, and rituximab), particularly those who have comorbidities.
This is one of several recommendations noted in the American Society of Clinical Oncology’s updated practice guidelines, published online in the Journal of Clinical Oncology, on the use of hematopoietic colony-stimulating factors (CSFs) to prevent or treat neutropenia and its complications in adults and children receiving chemotherapy.
This “moderately strong” recommendation is based on a single randomized clinical trial that found pegfilgrastim significantly reduced the risk of febrile neutropenia in this patient population, according to the guidelines (J. Clin. Oncol. 2015 July 13 [doi:10.1200/JCO.2015.62.3488]).
The updated guideline incorporates new evidence from 66 randomized controlled trials and meta-analyses published since its last update in 2006, said cochair Dr. Thomas J. Smith of the Johns Hopkins Sidney Kimmel Comprehensive Cancer Center, Baltimore, and his associates on the update committee.
In addition to pegfilgrastim and filgrastim, the guideline now addresses the use of tbo-filgrastim, filgrastim-sndz, and other biosimilars as they become available. These new agents are effective at preventing chemotherapy-related febrile neutropenia, so the choice of agent depends on convenience, cost, and clinical factors, and in some cases may be dictated by the patient’s treatment schedule. Certain off-label uses of pegfilgrastim can now be considered, such as giving it on the same day as chemotherapy if that is the only feasible timing for some patients.
CSFs should only be used to enable dose-dense chemotherapy regimens “if supported by convincing efficacy data or within an appropriately designed clinical trial” – for example, to support treatment of urothelial cancer or high-risk breast cancer targeted with high-dose-intensity methotrexate, vinblastine, doxorubicin, and cisplatin.
In contrast, the use of CSFs to enable dose-dense chemotherapy for Hodgkin lymphoma is not recommended at this time because the current data supporting such use are limited and conflicting. Similarly, the current evidence strongly argues against giving CSFs to enable dose-dense chemotherapy for other lymphomas, lung cancer, ovarian cancer, osteosarcoma, or sarcoma.
The guideline update was supported by the American Society of Clinical Oncology. Dr. Smith reported stock or other ownership in United Healthcare; his associates reported ties to numerous industry sources.
The full guideline and supplementary material, including slide sets and clinical tools, are available at www.asco.org/guidelines/wbcgf.
Hematopoietic colony-stimulating factors should now be considered for patients who are over age 64 years, have diffuse aggressive lymphoma, and are receiving curative chemotherapy (cyclophosphamide, doxorubicin, vincristine, prednisone, and rituximab), particularly those who have comorbidities.
This is one of several recommendations noted in the American Society of Clinical Oncology’s updated practice guidelines, published online in the Journal of Clinical Oncology, on the use of hematopoietic colony-stimulating factors (CSFs) to prevent or treat neutropenia and its complications in adults and children receiving chemotherapy.
This “moderately strong” recommendation is based on a single randomized clinical trial that found pegfilgrastim significantly reduced the risk of febrile neutropenia in this patient population, according to the guidelines (J. Clin. Oncol. 2015 July 13 [doi:10.1200/JCO.2015.62.3488]).
The updated guideline incorporates new evidence from 66 randomized controlled trials and meta-analyses published since its last update in 2006, said cochair Dr. Thomas J. Smith of the Johns Hopkins Sidney Kimmel Comprehensive Cancer Center, Baltimore, and his associates on the update committee.
In addition to pegfilgrastim and filgrastim, the guideline now addresses the use of tbo-filgrastim, filgrastim-sndz, and other biosimilars as they become available. These new agents are effective at preventing chemotherapy-related febrile neutropenia, so the choice of agent depends on convenience, cost, and clinical factors, and in some cases may be dictated by the patient’s treatment schedule. Certain off-label uses of pegfilgrastim can now be considered, such as giving it on the same day as chemotherapy if that is the only feasible timing for some patients.
CSFs should only be used to enable dose-dense chemotherapy regimens “if supported by convincing efficacy data or within an appropriately designed clinical trial” – for example, to support treatment of urothelial cancer or high-risk breast cancer targeted with high-dose-intensity methotrexate, vinblastine, doxorubicin, and cisplatin.
In contrast, the use of CSFs to enable dose-dense chemotherapy for Hodgkin lymphoma is not recommended at this time because the current data supporting such use are limited and conflicting. Similarly, the current evidence strongly argues against giving CSFs to enable dose-dense chemotherapy for other lymphomas, lung cancer, ovarian cancer, osteosarcoma, or sarcoma.
The guideline update was supported by the American Society of Clinical Oncology. Dr. Smith reported stock or other ownership in United Healthcare; his associates reported ties to numerous industry sources.
The full guideline and supplementary material, including slide sets and clinical tools, are available at www.asco.org/guidelines/wbcgf.
Hematopoietic colony-stimulating factors should now be considered for patients who are over age 64 years, have diffuse aggressive lymphoma, and are receiving curative chemotherapy (cyclophosphamide, doxorubicin, vincristine, prednisone, and rituximab), particularly those who have comorbidities.
This is one of several recommendations noted in the American Society of Clinical Oncology’s updated practice guidelines, published online in the Journal of Clinical Oncology, on the use of hematopoietic colony-stimulating factors (CSFs) to prevent or treat neutropenia and its complications in adults and children receiving chemotherapy.
This “moderately strong” recommendation is based on a single randomized clinical trial that found pegfilgrastim significantly reduced the risk of febrile neutropenia in this patient population, according to the guidelines (J. Clin. Oncol. 2015 July 13 [doi:10.1200/JCO.2015.62.3488]).
The updated guideline incorporates new evidence from 66 randomized controlled trials and meta-analyses published since its last update in 2006, said cochair Dr. Thomas J. Smith of the Johns Hopkins Sidney Kimmel Comprehensive Cancer Center, Baltimore, and his associates on the update committee.
In addition to pegfilgrastim and filgrastim, the guideline now addresses the use of tbo-filgrastim, filgrastim-sndz, and other biosimilars as they become available. These new agents are effective at preventing chemotherapy-related febrile neutropenia, so the choice of agent depends on convenience, cost, and clinical factors, and in some cases may be dictated by the patient’s treatment schedule. Certain off-label uses of pegfilgrastim can now be considered, such as giving it on the same day as chemotherapy if that is the only feasible timing for some patients.
CSFs should only be used to enable dose-dense chemotherapy regimens “if supported by convincing efficacy data or within an appropriately designed clinical trial” – for example, to support treatment of urothelial cancer or high-risk breast cancer targeted with high-dose-intensity methotrexate, vinblastine, doxorubicin, and cisplatin.
In contrast, the use of CSFs to enable dose-dense chemotherapy for Hodgkin lymphoma is not recommended at this time because the current data supporting such use are limited and conflicting. Similarly, the current evidence strongly argues against giving CSFs to enable dose-dense chemotherapy for other lymphomas, lung cancer, ovarian cancer, osteosarcoma, or sarcoma.
The guideline update was supported by the American Society of Clinical Oncology. Dr. Smith reported stock or other ownership in United Healthcare; his associates reported ties to numerous industry sources.
The full guideline and supplementary material, including slide sets and clinical tools, are available at www.asco.org/guidelines/wbcgf.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Doxorubicin, radiation doses predict heart risk in lymphoma survivors
Adult lymphoma survivors who were treated with autologous hematopoietic stem-cell transplantation had a greater than sixfold increased risk of left ventricular systolic dysfunction compared with controls, according to a study published online in the Journal of Clinical Oncology.
Among 274 adult survivors of Hodgkin or non-Hodgkin lymphoma, 16% had left ventricular systolic dysfunction (LVSD): 11% had overt heart failure (HF) and 5% had asymptomatic LVSD, defined as a left ventricular ejection fraction of less than 50%.Heart symptoms were significantly associated with exposure to doxorubicin at a cumulative dose of 300 mg/m2 or more and with cardiac radiation therapy of more than 30 Gy. Recognizing these patient risk factors allows for more intensive follow-up with the goal of “identification and early treatment of asymptomatic LVSD [which] may prevent the development of HF,” wrote Dr. Klaus Murbraech of Oslo University Hospital and his colleagues (J. Clin. Oncol. 2015 July 13 [doi:10.1200/JCO.2015.60.8125]).
The investigators observed no association between lower-dose cardiac radiation therapy and LVSD. There was only a marginally significant association between the presence of two or more traditional cardiovascular disease risk factors and LVSD.
The cross-sectional multicenter cohort study is the first to assess the prevalence of LVSD, according to Dr. Murbraech and his colleagues. The study included adult survivors of Hodgkin or non-Hodgkin lymphoma, median age 56 years, who underwent autologous stem-cell transplants in Norway from 1987 to 2008. The median observation time was 13 years (range, 4-34 years). The control group consisted of initially healthy patients in an echocardiographic follow-up study. Controls were matched to patients based on age, sex, systolic blood pressure, and body mass index.
The study was supported by the South-Eastern Norway Regional Health Authority and Extrastiftelsen. Dr. Murbraech reported having no disclosures.
Adult lymphoma survivors who were treated with autologous hematopoietic stem-cell transplantation had a greater than sixfold increased risk of left ventricular systolic dysfunction compared with controls, according to a study published online in the Journal of Clinical Oncology.
Among 274 adult survivors of Hodgkin or non-Hodgkin lymphoma, 16% had left ventricular systolic dysfunction (LVSD): 11% had overt heart failure (HF) and 5% had asymptomatic LVSD, defined as a left ventricular ejection fraction of less than 50%.Heart symptoms were significantly associated with exposure to doxorubicin at a cumulative dose of 300 mg/m2 or more and with cardiac radiation therapy of more than 30 Gy. Recognizing these patient risk factors allows for more intensive follow-up with the goal of “identification and early treatment of asymptomatic LVSD [which] may prevent the development of HF,” wrote Dr. Klaus Murbraech of Oslo University Hospital and his colleagues (J. Clin. Oncol. 2015 July 13 [doi:10.1200/JCO.2015.60.8125]).
The investigators observed no association between lower-dose cardiac radiation therapy and LVSD. There was only a marginally significant association between the presence of two or more traditional cardiovascular disease risk factors and LVSD.
The cross-sectional multicenter cohort study is the first to assess the prevalence of LVSD, according to Dr. Murbraech and his colleagues. The study included adult survivors of Hodgkin or non-Hodgkin lymphoma, median age 56 years, who underwent autologous stem-cell transplants in Norway from 1987 to 2008. The median observation time was 13 years (range, 4-34 years). The control group consisted of initially healthy patients in an echocardiographic follow-up study. Controls were matched to patients based on age, sex, systolic blood pressure, and body mass index.
The study was supported by the South-Eastern Norway Regional Health Authority and Extrastiftelsen. Dr. Murbraech reported having no disclosures.
Adult lymphoma survivors who were treated with autologous hematopoietic stem-cell transplantation had a greater than sixfold increased risk of left ventricular systolic dysfunction compared with controls, according to a study published online in the Journal of Clinical Oncology.
Among 274 adult survivors of Hodgkin or non-Hodgkin lymphoma, 16% had left ventricular systolic dysfunction (LVSD): 11% had overt heart failure (HF) and 5% had asymptomatic LVSD, defined as a left ventricular ejection fraction of less than 50%.Heart symptoms were significantly associated with exposure to doxorubicin at a cumulative dose of 300 mg/m2 or more and with cardiac radiation therapy of more than 30 Gy. Recognizing these patient risk factors allows for more intensive follow-up with the goal of “identification and early treatment of asymptomatic LVSD [which] may prevent the development of HF,” wrote Dr. Klaus Murbraech of Oslo University Hospital and his colleagues (J. Clin. Oncol. 2015 July 13 [doi:10.1200/JCO.2015.60.8125]).
The investigators observed no association between lower-dose cardiac radiation therapy and LVSD. There was only a marginally significant association between the presence of two or more traditional cardiovascular disease risk factors and LVSD.
The cross-sectional multicenter cohort study is the first to assess the prevalence of LVSD, according to Dr. Murbraech and his colleagues. The study included adult survivors of Hodgkin or non-Hodgkin lymphoma, median age 56 years, who underwent autologous stem-cell transplants in Norway from 1987 to 2008. The median observation time was 13 years (range, 4-34 years). The control group consisted of initially healthy patients in an echocardiographic follow-up study. Controls were matched to patients based on age, sex, systolic blood pressure, and body mass index.
The study was supported by the South-Eastern Norway Regional Health Authority and Extrastiftelsen. Dr. Murbraech reported having no disclosures.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Lymphoma survivors treated with autologous hematopoietic stem-cell transplantation (auto-HSC) had a significantly higher risk of left ventricular systolic dysfunction than did controls.
Major finding: Treatment with at least 300 mg/m2 cumulative of doxorubicin and with over 30 Gy of cardiac radiation therapy were independent risk factors for LVSD.
Data source: A cross-sectional multicenter cohort study of 274 Hodgkin or non-Hodgkin lymphoma survivors.
Disclosures: Supported by the South-Eastern Norway Regional Health Authority and Extrastiftelsen. Dr. Murbraech reported having no disclosures.
Morbidities linger at least 20 years for young adult cancer survivors
Survivors of young adult cancers had 1.5 times more hospitalizations than did controls in a population-based study spanning 20 years, Dr. Devon P. Richardson and coauthors at the University of Toronto reported online in the Journal of Clinical Oncology.
Investigators compared a cohort of 20,275 patients enrolled in the Ontario Cancer Registry who were aged 20-44 years at the time of diagnosis, and had survived at least 5 years cancer free, with 101,344 noncancer controls selected from a roster of all individuals eligible for the Ontario Health Insurance Plan. Over one-third of survivors were hospitalized during the 20-year study period, and the adjusted relative rate (ARR) of hospitalizations, compared with controls, was 1.51 (95% confidence interval, 1.48-1.54). Hospitalization rates were highest in survivors of upper GI cancers (ARR, 2.49), leukemia (ARR, 2.23), and urologic (ARR, 2.20) cancers, the investigators reported.
In the first three follow-up periods (5-8, 9-11, and 12-14 years after diagnosis), the hospitalization rate in survivors (per 100 person-years) stayed constant at 0.22, whereas the rate decreased significantly in the last two time periods: 0.17 at 15-17 years and 0.15 for 18-20 years after diagnosis (P <.0001). The hospitalization rate among controls was relatively constant during the entire time period, ranging from 0.12 to 0.14, they said.
“The results of our study show that 5-year survivors of cancer diagnosed during young adulthood will have morbidities that increase the number of hospitalizations in this cohort compared with control subjects for at least 20 years after diagnosis,” the authors wrote.
Read the full article here: J. Clin. Oncol. 2015 July 13 (doi:10.1200/JCO.2014.60.1914).
Survivors of young adult cancers had 1.5 times more hospitalizations than did controls in a population-based study spanning 20 years, Dr. Devon P. Richardson and coauthors at the University of Toronto reported online in the Journal of Clinical Oncology.
Investigators compared a cohort of 20,275 patients enrolled in the Ontario Cancer Registry who were aged 20-44 years at the time of diagnosis, and had survived at least 5 years cancer free, with 101,344 noncancer controls selected from a roster of all individuals eligible for the Ontario Health Insurance Plan. Over one-third of survivors were hospitalized during the 20-year study period, and the adjusted relative rate (ARR) of hospitalizations, compared with controls, was 1.51 (95% confidence interval, 1.48-1.54). Hospitalization rates were highest in survivors of upper GI cancers (ARR, 2.49), leukemia (ARR, 2.23), and urologic (ARR, 2.20) cancers, the investigators reported.
In the first three follow-up periods (5-8, 9-11, and 12-14 years after diagnosis), the hospitalization rate in survivors (per 100 person-years) stayed constant at 0.22, whereas the rate decreased significantly in the last two time periods: 0.17 at 15-17 years and 0.15 for 18-20 years after diagnosis (P <.0001). The hospitalization rate among controls was relatively constant during the entire time period, ranging from 0.12 to 0.14, they said.
“The results of our study show that 5-year survivors of cancer diagnosed during young adulthood will have morbidities that increase the number of hospitalizations in this cohort compared with control subjects for at least 20 years after diagnosis,” the authors wrote.
Read the full article here: J. Clin. Oncol. 2015 July 13 (doi:10.1200/JCO.2014.60.1914).
Survivors of young adult cancers had 1.5 times more hospitalizations than did controls in a population-based study spanning 20 years, Dr. Devon P. Richardson and coauthors at the University of Toronto reported online in the Journal of Clinical Oncology.
Investigators compared a cohort of 20,275 patients enrolled in the Ontario Cancer Registry who were aged 20-44 years at the time of diagnosis, and had survived at least 5 years cancer free, with 101,344 noncancer controls selected from a roster of all individuals eligible for the Ontario Health Insurance Plan. Over one-third of survivors were hospitalized during the 20-year study period, and the adjusted relative rate (ARR) of hospitalizations, compared with controls, was 1.51 (95% confidence interval, 1.48-1.54). Hospitalization rates were highest in survivors of upper GI cancers (ARR, 2.49), leukemia (ARR, 2.23), and urologic (ARR, 2.20) cancers, the investigators reported.
In the first three follow-up periods (5-8, 9-11, and 12-14 years after diagnosis), the hospitalization rate in survivors (per 100 person-years) stayed constant at 0.22, whereas the rate decreased significantly in the last two time periods: 0.17 at 15-17 years and 0.15 for 18-20 years after diagnosis (P <.0001). The hospitalization rate among controls was relatively constant during the entire time period, ranging from 0.12 to 0.14, they said.
“The results of our study show that 5-year survivors of cancer diagnosed during young adulthood will have morbidities that increase the number of hospitalizations in this cohort compared with control subjects for at least 20 years after diagnosis,” the authors wrote.
Read the full article here: J. Clin. Oncol. 2015 July 13 (doi:10.1200/JCO.2014.60.1914).
High VTE recurrence risk persists for at least 3 years
TORONTO – The risk of recurrence following an initial episode of venous thromboembolism is highest in the first 3 months, but remains high for up to 3 years, according to findings from a population-based study involving 2,989 adults.
Over a mean of 23 months (median, 30 months), there were 329 VTE recurrences in the study subjects. Cumulative incidence rates were 5.1% at 3 months, and 14.5% at 3 years. The corresponding rates were 8.7% and 24.8% among those with active cancer, 5.2% and 13.0% among those with provoked VTE, and 3.8% and 13.1% among those with unprovoked VTE, Dr. Wei Huang reported at the International Society on Thrombosis and Haemostasis congress.
Independent predictors of recurrence within 3 years after the index event were active cancer with chemotherapy (hazard ratio, 2.59), active cancer without chemotherapy (HR, 1.59), hypercoagulable state (HR, 2.53) superficial thrombophlebitis (HR, 1.62), varicose vein stripping (HR, 1.75), and inferior vena cava (IVC) filter placement (HR, 2.04), said Dr. Huang of the University of Massachusetts, Worcester.
Individuals included in the study were all residents of the Worcester Metropolitan Statistical Area (WMSA) who had a validated diagnosis of acute first-time deep vein thrombosis and/or pulmonary embolism in a hospital or ambulatory care center that provided short-term care for WMSA residents between 1999 and 2009. Medical records and national and local death registry data were reviewed to examine outcomes up to 3 years after the index event.
Subjects were adults with a mean age of 64 years; 44% were men, and 94% where white. Pulmonary embolism with or without deep vein thrombosis occurred in 42%, and 17% of cases were associated with cancer, 43% involved provoked VTE, and 40% involved unprovoked VTE.
Provoked VTE was defined as VTE occurring within 3 months of a prior surgery, pregnancy, trauma, fracture, or hospitalization in patients without presence of active cancer.
Though limited by the lack of information about variations in physician practices across regions, and by the high proportion of white resident in the WMSA, which both raise questions about whether the findings are generalizable to the U.S. population, the identification of these predictors could allow for improved estimation of risk for individual patients, and may aid in the design of new interventional studies, Dr. Huang concluded.
This study was supported by the National Institutes of Health.
TORONTO – The risk of recurrence following an initial episode of venous thromboembolism is highest in the first 3 months, but remains high for up to 3 years, according to findings from a population-based study involving 2,989 adults.
Over a mean of 23 months (median, 30 months), there were 329 VTE recurrences in the study subjects. Cumulative incidence rates were 5.1% at 3 months, and 14.5% at 3 years. The corresponding rates were 8.7% and 24.8% among those with active cancer, 5.2% and 13.0% among those with provoked VTE, and 3.8% and 13.1% among those with unprovoked VTE, Dr. Wei Huang reported at the International Society on Thrombosis and Haemostasis congress.
Independent predictors of recurrence within 3 years after the index event were active cancer with chemotherapy (hazard ratio, 2.59), active cancer without chemotherapy (HR, 1.59), hypercoagulable state (HR, 2.53) superficial thrombophlebitis (HR, 1.62), varicose vein stripping (HR, 1.75), and inferior vena cava (IVC) filter placement (HR, 2.04), said Dr. Huang of the University of Massachusetts, Worcester.
Individuals included in the study were all residents of the Worcester Metropolitan Statistical Area (WMSA) who had a validated diagnosis of acute first-time deep vein thrombosis and/or pulmonary embolism in a hospital or ambulatory care center that provided short-term care for WMSA residents between 1999 and 2009. Medical records and national and local death registry data were reviewed to examine outcomes up to 3 years after the index event.
Subjects were adults with a mean age of 64 years; 44% were men, and 94% where white. Pulmonary embolism with or without deep vein thrombosis occurred in 42%, and 17% of cases were associated with cancer, 43% involved provoked VTE, and 40% involved unprovoked VTE.
Provoked VTE was defined as VTE occurring within 3 months of a prior surgery, pregnancy, trauma, fracture, or hospitalization in patients without presence of active cancer.
Though limited by the lack of information about variations in physician practices across regions, and by the high proportion of white resident in the WMSA, which both raise questions about whether the findings are generalizable to the U.S. population, the identification of these predictors could allow for improved estimation of risk for individual patients, and may aid in the design of new interventional studies, Dr. Huang concluded.
This study was supported by the National Institutes of Health.
TORONTO – The risk of recurrence following an initial episode of venous thromboembolism is highest in the first 3 months, but remains high for up to 3 years, according to findings from a population-based study involving 2,989 adults.
Over a mean of 23 months (median, 30 months), there were 329 VTE recurrences in the study subjects. Cumulative incidence rates were 5.1% at 3 months, and 14.5% at 3 years. The corresponding rates were 8.7% and 24.8% among those with active cancer, 5.2% and 13.0% among those with provoked VTE, and 3.8% and 13.1% among those with unprovoked VTE, Dr. Wei Huang reported at the International Society on Thrombosis and Haemostasis congress.
Independent predictors of recurrence within 3 years after the index event were active cancer with chemotherapy (hazard ratio, 2.59), active cancer without chemotherapy (HR, 1.59), hypercoagulable state (HR, 2.53) superficial thrombophlebitis (HR, 1.62), varicose vein stripping (HR, 1.75), and inferior vena cava (IVC) filter placement (HR, 2.04), said Dr. Huang of the University of Massachusetts, Worcester.
Individuals included in the study were all residents of the Worcester Metropolitan Statistical Area (WMSA) who had a validated diagnosis of acute first-time deep vein thrombosis and/or pulmonary embolism in a hospital or ambulatory care center that provided short-term care for WMSA residents between 1999 and 2009. Medical records and national and local death registry data were reviewed to examine outcomes up to 3 years after the index event.
Subjects were adults with a mean age of 64 years; 44% were men, and 94% where white. Pulmonary embolism with or without deep vein thrombosis occurred in 42%, and 17% of cases were associated with cancer, 43% involved provoked VTE, and 40% involved unprovoked VTE.
Provoked VTE was defined as VTE occurring within 3 months of a prior surgery, pregnancy, trauma, fracture, or hospitalization in patients without presence of active cancer.
Though limited by the lack of information about variations in physician practices across regions, and by the high proportion of white resident in the WMSA, which both raise questions about whether the findings are generalizable to the U.S. population, the identification of these predictors could allow for improved estimation of risk for individual patients, and may aid in the design of new interventional studies, Dr. Huang concluded.
This study was supported by the National Institutes of Health.
AT THE 2015 ISTH CONGRESS
Key clinical point: The risk of recurrence following an initial episode of venous thromboembolism is highest in the first 3 months, but remains high for up to 3 years, according to findings from a population-based study involving 2,989 adults.
Major finding: Active cancer with chemotherapy was the strongest predictor of VTE recurrence (hazard ratio, 2.59).
Data source: Population-based surveillance of 2,989 adults patients.
Disclosures: The National Institutes of Health supported the study.
Adolescent cancer survivors report more emotional, neurocognitive impairment than do siblings
Adult survivors of cancer who were diagnosed between the ages of 11 and 21 years self-reported higher rates of impairment in emotional and neurocognitive outcomes compared with their sibling counterparts, according to researchers.
Compared with siblings, survivors reported greater anxiety (odds ratio [OR], 2.0; 95% CI, 1.17-3.43), somatization (2.36; 1.55-3.60), and depression (1.55; 1.04-2.30). Higher rates of neurocognitive problems included task efficiency (1.72; 1.21-2.43), emotional regulation (1.74; 1.26-2.40), and memory (1.44; 1.09-1.89). Survivors were significantly less likely to be employed (P < .001).
Previous reports have shown that survivors of childhood cancers have increased risk for impaired neurocognitive functioning, but this is the first study to examine outcomes in survivors who were diagnosed during adolescence and early young adulthood.
“Cancer treatment during this time has the potential to interfere with adolescents’ separation from caregivers, autonomy with regard to planning social and academic schedules, participation in social activities, and maintaining privacy, particularly of their bodies,” wrote Dr. Pinki Prasad, assistant professor of pediatrics at Louisiana State University, New Orleans.
Survivors who were diagnosed with CNS tumors or leukemia during adolescence reported rates of emotional distress and neurocognitive dysfunction similar to rates of those diagnosed during early childhood, whereas diagnoses of lymphoma/sarcoma during adolescence resulted in lower risk of impairment compared with early childhood diagnoses. This may be due to the fact that the leukemia/CNS tumor group was more likely to receive cranial radiation therapy, a predictor of neurocognitive late effects, Dr. Prasad and colleagues wrote (J. Clin. Oncol. 2015 July 6 [doi:10.1200/JCO.2014.57.7528]).
Among those diagnosed with lymphomas or sarcomas during adolescence, treatment with corticosteroids was associated with greater risk of self-reported difficulties with somatization, anxiety, task efficiency, and memory.
The Childhood Cancer Survivor Study (CCSS) is a multicenter, retrospective cohort study that comprised 2,589 survivors who were diagnosed from 1970 through 1986 when they were between the ages 11 and 21 years, and 360 sibling counterparts. Participants completed the Brief Symptom Inventory–18, which measures symptoms of emotional distress, and the CCSS Neurocognitive Questionnaire.
The authors noted that the results indicate “high rates of self-reported impairment in neurocognitive function and psychological distress that are associated with limitation in development of adult social milestones,” and that further follow-up with survivors of adolescent and early young adult cancers may be necessary.
Adult survivors of cancer who were diagnosed between the ages of 11 and 21 years self-reported higher rates of impairment in emotional and neurocognitive outcomes compared with their sibling counterparts, according to researchers.
Compared with siblings, survivors reported greater anxiety (odds ratio [OR], 2.0; 95% CI, 1.17-3.43), somatization (2.36; 1.55-3.60), and depression (1.55; 1.04-2.30). Higher rates of neurocognitive problems included task efficiency (1.72; 1.21-2.43), emotional regulation (1.74; 1.26-2.40), and memory (1.44; 1.09-1.89). Survivors were significantly less likely to be employed (P < .001).
Previous reports have shown that survivors of childhood cancers have increased risk for impaired neurocognitive functioning, but this is the first study to examine outcomes in survivors who were diagnosed during adolescence and early young adulthood.
“Cancer treatment during this time has the potential to interfere with adolescents’ separation from caregivers, autonomy with regard to planning social and academic schedules, participation in social activities, and maintaining privacy, particularly of their bodies,” wrote Dr. Pinki Prasad, assistant professor of pediatrics at Louisiana State University, New Orleans.
Survivors who were diagnosed with CNS tumors or leukemia during adolescence reported rates of emotional distress and neurocognitive dysfunction similar to rates of those diagnosed during early childhood, whereas diagnoses of lymphoma/sarcoma during adolescence resulted in lower risk of impairment compared with early childhood diagnoses. This may be due to the fact that the leukemia/CNS tumor group was more likely to receive cranial radiation therapy, a predictor of neurocognitive late effects, Dr. Prasad and colleagues wrote (J. Clin. Oncol. 2015 July 6 [doi:10.1200/JCO.2014.57.7528]).
Among those diagnosed with lymphomas or sarcomas during adolescence, treatment with corticosteroids was associated with greater risk of self-reported difficulties with somatization, anxiety, task efficiency, and memory.
The Childhood Cancer Survivor Study (CCSS) is a multicenter, retrospective cohort study that comprised 2,589 survivors who were diagnosed from 1970 through 1986 when they were between the ages 11 and 21 years, and 360 sibling counterparts. Participants completed the Brief Symptom Inventory–18, which measures symptoms of emotional distress, and the CCSS Neurocognitive Questionnaire.
The authors noted that the results indicate “high rates of self-reported impairment in neurocognitive function and psychological distress that are associated with limitation in development of adult social milestones,” and that further follow-up with survivors of adolescent and early young adult cancers may be necessary.
Adult survivors of cancer who were diagnosed between the ages of 11 and 21 years self-reported higher rates of impairment in emotional and neurocognitive outcomes compared with their sibling counterparts, according to researchers.
Compared with siblings, survivors reported greater anxiety (odds ratio [OR], 2.0; 95% CI, 1.17-3.43), somatization (2.36; 1.55-3.60), and depression (1.55; 1.04-2.30). Higher rates of neurocognitive problems included task efficiency (1.72; 1.21-2.43), emotional regulation (1.74; 1.26-2.40), and memory (1.44; 1.09-1.89). Survivors were significantly less likely to be employed (P < .001).
Previous reports have shown that survivors of childhood cancers have increased risk for impaired neurocognitive functioning, but this is the first study to examine outcomes in survivors who were diagnosed during adolescence and early young adulthood.
“Cancer treatment during this time has the potential to interfere with adolescents’ separation from caregivers, autonomy with regard to planning social and academic schedules, participation in social activities, and maintaining privacy, particularly of their bodies,” wrote Dr. Pinki Prasad, assistant professor of pediatrics at Louisiana State University, New Orleans.
Survivors who were diagnosed with CNS tumors or leukemia during adolescence reported rates of emotional distress and neurocognitive dysfunction similar to rates of those diagnosed during early childhood, whereas diagnoses of lymphoma/sarcoma during adolescence resulted in lower risk of impairment compared with early childhood diagnoses. This may be due to the fact that the leukemia/CNS tumor group was more likely to receive cranial radiation therapy, a predictor of neurocognitive late effects, Dr. Prasad and colleagues wrote (J. Clin. Oncol. 2015 July 6 [doi:10.1200/JCO.2014.57.7528]).
Among those diagnosed with lymphomas or sarcomas during adolescence, treatment with corticosteroids was associated with greater risk of self-reported difficulties with somatization, anxiety, task efficiency, and memory.
The Childhood Cancer Survivor Study (CCSS) is a multicenter, retrospective cohort study that comprised 2,589 survivors who were diagnosed from 1970 through 1986 when they were between the ages 11 and 21 years, and 360 sibling counterparts. Participants completed the Brief Symptom Inventory–18, which measures symptoms of emotional distress, and the CCSS Neurocognitive Questionnaire.
The authors noted that the results indicate “high rates of self-reported impairment in neurocognitive function and psychological distress that are associated with limitation in development of adult social milestones,” and that further follow-up with survivors of adolescent and early young adult cancers may be necessary.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Adult cancer survivors diagnosed between ages 11 and 21 years self-reported higher rates of emotional distress and neurocognitive dysfunction compared with sibling counterparts.
Major finding: Compared with siblings, survivors reported greater anxiety (odds ratio, 2.0; 95% CI, 1.17-3.43), somatization (2.36; 1.55-3.60), and depression (1.55; 1.04-2.30). Higher rates of neurocognitive problems included task efficiency (1.72; 1.21-2.43), emotional regulation (1.74; 1.26-2.40), and memory (1.44; 1.09-1.89).
Data source: The Childhood Cancer Survivor Study, a multicenter, retrospective cohort study of 2,589 survivors diagnosed from 1970 through 1986 when they were between the ages 11 and 21 years, and 360 sibling counterparts.
Disclosures: The National Cancer Institute supported the study. Dr. Prasad reported having no disclosures.
Hospital clinicians commonly work while sick
The vast majority of doctors and other trained medical professionals at a hospital went to work while sick within the past year, even though they realized the risk that decision places on patients, according to a recent study.
In fact, almost 1 in 10 hospital clinicians worked while sick at least five times in the past year, primarily because of staffing concerns or not wanting to let colleagues down, reported Julia Szymczak, Ph.D., and her associates at the Children’s Hospital of Philadelphia (JAMA Pediatr. 2015 July 6 [doi: 10.1001/jamapediatrics.2015.0684]).
“A combination of closed- and open-ended questions illustrated that the decision to work while sick was shaped by systems-level and sociocultural factors that interacted to cause our respondents to work while symptomatic, despite recognizing that this choice may put patients and colleagues at risk,” the authors wrote.
Of 929 surveys sent out, 538 clinicians completed them, which included 280 of 459 physicians (61%) and 256 of 470 advanced-practice clinicians (54.5%). The advanced-practice clinicians included registered nurses, physician assistants, clinical nurse specialists, registered nurse anesthetists, and certified nurse midwives. Of those who responded, 15.7% worked in intensive care, 13.1% in surgery, 12.5% in general pediatrics, and 44.8% in another pediatric subspecialty.
Although 95.3% of respondents believed working while sick put patients at risk, 83.1% reported having done so at least once in the past year. Further, that proportion included 52% of all respondents who reported coming to work sick twice in the past year and 9.3% who worked while ill at least five times in the past year.
Nearly a third of respondents said they would work even if they had diarrhea (30%), while 16% said they would work with a fever, and 55.6% would work with acute respiratory symptoms, including cough, congestion, rhinorrhea, and sore throat.
But doctors were more likely than other professionals to say they would go to work with these symptoms: 38.9% of doctors would work despite diarrhea, compared with 19.9% of advanced-practice clinicians. Doctors and advanced-practice clinicians would also work with acute respiratory symptoms (60% vs. 50.8%, respectively), a fever only (21.8% vs. 9.8%), and fever and chills with body aches (18.6% vs. 10.9%, all P < .03).
Nearly every respondent (98.7%) said they worked despite being sick because they did not want to let their colleagues down, just as almost all of them worried the hospital would not have enough staff (94.9%) or that they would let their patients down (92.5%).
Smaller majorities of respondents also worked because others also work while sick (65%), worried their colleagues would ostracize them (64%) if they didn’t work, were concerned about their patients’ continuity of care (63.8%), had unsupportive leadership (56.2%), or believed they could not be easily replaced (52.6%).
Among the 316 respondents who filled in additional reasons, 64.9% said they had a very hard time finding someone to cover their shift, 61.1% described a strong cultural norm to work unless extremely sick, and 57% expressed uncertainty about what is considered “too sick to work.”
The Centers for Disease Control and Prevention funded the research. The authors reported no disclosures.
For centuries, a guiding principle for health care workers has been primum non nocere, or first do no harm. However, health care workers do exactly that when they work with patients while ill themselves with contagious infections. Even common but untreatable infectious like enterovirus and respiratory syncytial virus can prove deadly to immunocompromised patients.
The propensity to work while ill is influenced by cultural trends. In past years, many ill physicians worked even to the point of receiving intravenous fluids while on the job; working while sick was regarded as a badge of courage. Dr. Szymczak and colleagues identified as an issue the absence of an effective sick relief system that has sufficient flexibility to “staff up” during high rates of health care worker illness. Sick relief systems and policies need to be clear regarding when health care workers should stay away from work, how patient coverage will be ensured, and the availability of and access to paid sick leave.
Determining what constitutes being too sick to work is complicated and lacks a sufficient evidence base. Using a system that bases work restrictions on the presence of key symptoms may add clarity and enable health care workers to recognize when they need to stay home.
Creating a safer and more equitable system of sick leave for health care workers requires a culture change in many institutions to decrease the stigma – internal and external – associated with health care worker illness. Identifying solutions to prioritize patient safety must factor in workforce demands and variability in patient census and emphasize flexibility. Strong administrative and physician leadership and creativity are essential to support appropriate sick leave and ensure adequate staffing. Hospital leadership must ensure that the culture supports a paid sick leave policy that is adequate and nonpunitive.
These comments are selected from an accompanying editorial (JAMA Pediatr. 2015 July 6 [doi:10.1001/jamapediatrics.2015.0994]), written by Dr. Jeffrey R. Starke of the department of pediatrics at Baylor College of Medicine in Houston, and Dr. Mary Anne Jackson of the division of infectious diseases at Children’s Mercy Hospital, University of Missouri–Kansas City. Dr. Starke and Dr. Jackson reported no disclosures.
For centuries, a guiding principle for health care workers has been primum non nocere, or first do no harm. However, health care workers do exactly that when they work with patients while ill themselves with contagious infections. Even common but untreatable infectious like enterovirus and respiratory syncytial virus can prove deadly to immunocompromised patients.
The propensity to work while ill is influenced by cultural trends. In past years, many ill physicians worked even to the point of receiving intravenous fluids while on the job; working while sick was regarded as a badge of courage. Dr. Szymczak and colleagues identified as an issue the absence of an effective sick relief system that has sufficient flexibility to “staff up” during high rates of health care worker illness. Sick relief systems and policies need to be clear regarding when health care workers should stay away from work, how patient coverage will be ensured, and the availability of and access to paid sick leave.
Determining what constitutes being too sick to work is complicated and lacks a sufficient evidence base. Using a system that bases work restrictions on the presence of key symptoms may add clarity and enable health care workers to recognize when they need to stay home.
Creating a safer and more equitable system of sick leave for health care workers requires a culture change in many institutions to decrease the stigma – internal and external – associated with health care worker illness. Identifying solutions to prioritize patient safety must factor in workforce demands and variability in patient census and emphasize flexibility. Strong administrative and physician leadership and creativity are essential to support appropriate sick leave and ensure adequate staffing. Hospital leadership must ensure that the culture supports a paid sick leave policy that is adequate and nonpunitive.
These comments are selected from an accompanying editorial (JAMA Pediatr. 2015 July 6 [doi:10.1001/jamapediatrics.2015.0994]), written by Dr. Jeffrey R. Starke of the department of pediatrics at Baylor College of Medicine in Houston, and Dr. Mary Anne Jackson of the division of infectious diseases at Children’s Mercy Hospital, University of Missouri–Kansas City. Dr. Starke and Dr. Jackson reported no disclosures.
For centuries, a guiding principle for health care workers has been primum non nocere, or first do no harm. However, health care workers do exactly that when they work with patients while ill themselves with contagious infections. Even common but untreatable infectious like enterovirus and respiratory syncytial virus can prove deadly to immunocompromised patients.
The propensity to work while ill is influenced by cultural trends. In past years, many ill physicians worked even to the point of receiving intravenous fluids while on the job; working while sick was regarded as a badge of courage. Dr. Szymczak and colleagues identified as an issue the absence of an effective sick relief system that has sufficient flexibility to “staff up” during high rates of health care worker illness. Sick relief systems and policies need to be clear regarding when health care workers should stay away from work, how patient coverage will be ensured, and the availability of and access to paid sick leave.
Determining what constitutes being too sick to work is complicated and lacks a sufficient evidence base. Using a system that bases work restrictions on the presence of key symptoms may add clarity and enable health care workers to recognize when they need to stay home.
Creating a safer and more equitable system of sick leave for health care workers requires a culture change in many institutions to decrease the stigma – internal and external – associated with health care worker illness. Identifying solutions to prioritize patient safety must factor in workforce demands and variability in patient census and emphasize flexibility. Strong administrative and physician leadership and creativity are essential to support appropriate sick leave and ensure adequate staffing. Hospital leadership must ensure that the culture supports a paid sick leave policy that is adequate and nonpunitive.
These comments are selected from an accompanying editorial (JAMA Pediatr. 2015 July 6 [doi:10.1001/jamapediatrics.2015.0994]), written by Dr. Jeffrey R. Starke of the department of pediatrics at Baylor College of Medicine in Houston, and Dr. Mary Anne Jackson of the division of infectious diseases at Children’s Mercy Hospital, University of Missouri–Kansas City. Dr. Starke and Dr. Jackson reported no disclosures.
The vast majority of doctors and other trained medical professionals at a hospital went to work while sick within the past year, even though they realized the risk that decision places on patients, according to a recent study.
In fact, almost 1 in 10 hospital clinicians worked while sick at least five times in the past year, primarily because of staffing concerns or not wanting to let colleagues down, reported Julia Szymczak, Ph.D., and her associates at the Children’s Hospital of Philadelphia (JAMA Pediatr. 2015 July 6 [doi: 10.1001/jamapediatrics.2015.0684]).
“A combination of closed- and open-ended questions illustrated that the decision to work while sick was shaped by systems-level and sociocultural factors that interacted to cause our respondents to work while symptomatic, despite recognizing that this choice may put patients and colleagues at risk,” the authors wrote.
Of 929 surveys sent out, 538 clinicians completed them, which included 280 of 459 physicians (61%) and 256 of 470 advanced-practice clinicians (54.5%). The advanced-practice clinicians included registered nurses, physician assistants, clinical nurse specialists, registered nurse anesthetists, and certified nurse midwives. Of those who responded, 15.7% worked in intensive care, 13.1% in surgery, 12.5% in general pediatrics, and 44.8% in another pediatric subspecialty.
Although 95.3% of respondents believed working while sick put patients at risk, 83.1% reported having done so at least once in the past year. Further, that proportion included 52% of all respondents who reported coming to work sick twice in the past year and 9.3% who worked while ill at least five times in the past year.
Nearly a third of respondents said they would work even if they had diarrhea (30%), while 16% said they would work with a fever, and 55.6% would work with acute respiratory symptoms, including cough, congestion, rhinorrhea, and sore throat.
But doctors were more likely than other professionals to say they would go to work with these symptoms: 38.9% of doctors would work despite diarrhea, compared with 19.9% of advanced-practice clinicians. Doctors and advanced-practice clinicians would also work with acute respiratory symptoms (60% vs. 50.8%, respectively), a fever only (21.8% vs. 9.8%), and fever and chills with body aches (18.6% vs. 10.9%, all P < .03).
Nearly every respondent (98.7%) said they worked despite being sick because they did not want to let their colleagues down, just as almost all of them worried the hospital would not have enough staff (94.9%) or that they would let their patients down (92.5%).
Smaller majorities of respondents also worked because others also work while sick (65%), worried their colleagues would ostracize them (64%) if they didn’t work, were concerned about their patients’ continuity of care (63.8%), had unsupportive leadership (56.2%), or believed they could not be easily replaced (52.6%).
Among the 316 respondents who filled in additional reasons, 64.9% said they had a very hard time finding someone to cover their shift, 61.1% described a strong cultural norm to work unless extremely sick, and 57% expressed uncertainty about what is considered “too sick to work.”
The Centers for Disease Control and Prevention funded the research. The authors reported no disclosures.
The vast majority of doctors and other trained medical professionals at a hospital went to work while sick within the past year, even though they realized the risk that decision places on patients, according to a recent study.
In fact, almost 1 in 10 hospital clinicians worked while sick at least five times in the past year, primarily because of staffing concerns or not wanting to let colleagues down, reported Julia Szymczak, Ph.D., and her associates at the Children’s Hospital of Philadelphia (JAMA Pediatr. 2015 July 6 [doi: 10.1001/jamapediatrics.2015.0684]).
“A combination of closed- and open-ended questions illustrated that the decision to work while sick was shaped by systems-level and sociocultural factors that interacted to cause our respondents to work while symptomatic, despite recognizing that this choice may put patients and colleagues at risk,” the authors wrote.
Of 929 surveys sent out, 538 clinicians completed them, which included 280 of 459 physicians (61%) and 256 of 470 advanced-practice clinicians (54.5%). The advanced-practice clinicians included registered nurses, physician assistants, clinical nurse specialists, registered nurse anesthetists, and certified nurse midwives. Of those who responded, 15.7% worked in intensive care, 13.1% in surgery, 12.5% in general pediatrics, and 44.8% in another pediatric subspecialty.
Although 95.3% of respondents believed working while sick put patients at risk, 83.1% reported having done so at least once in the past year. Further, that proportion included 52% of all respondents who reported coming to work sick twice in the past year and 9.3% who worked while ill at least five times in the past year.
Nearly a third of respondents said they would work even if they had diarrhea (30%), while 16% said they would work with a fever, and 55.6% would work with acute respiratory symptoms, including cough, congestion, rhinorrhea, and sore throat.
But doctors were more likely than other professionals to say they would go to work with these symptoms: 38.9% of doctors would work despite diarrhea, compared with 19.9% of advanced-practice clinicians. Doctors and advanced-practice clinicians would also work with acute respiratory symptoms (60% vs. 50.8%, respectively), a fever only (21.8% vs. 9.8%), and fever and chills with body aches (18.6% vs. 10.9%, all P < .03).
Nearly every respondent (98.7%) said they worked despite being sick because they did not want to let their colleagues down, just as almost all of them worried the hospital would not have enough staff (94.9%) or that they would let their patients down (92.5%).
Smaller majorities of respondents also worked because others also work while sick (65%), worried their colleagues would ostracize them (64%) if they didn’t work, were concerned about their patients’ continuity of care (63.8%), had unsupportive leadership (56.2%), or believed they could not be easily replaced (52.6%).
Among the 316 respondents who filled in additional reasons, 64.9% said they had a very hard time finding someone to cover their shift, 61.1% described a strong cultural norm to work unless extremely sick, and 57% expressed uncertainty about what is considered “too sick to work.”
The Centers for Disease Control and Prevention funded the research. The authors reported no disclosures.
FROM PEDIATRICS
Key clinical point: A majority of hospital doctors and other clinicians work while sick.
Major finding: 83.1% of doctors and advanced-practice clinicians worked while ill at least once in the past year; 95.3% recognized the risk to patients and colleagues.
Data source: The findings are based on a cross-sectional, anonymous survey of 280 attending physicians and 256 advanced-practice clinicians at the Children’s Hospital of Philadelphia from January 2014 to March 2014.
Disclosures: The research was funded by the Centers for Disease Control and Prevention. The authors reported no disclosures.
Naloxone lotion improves disabling itch in CTCL
VANCOUVER, B.C. – Naloxone lotion appears to be a safe and effective treatment for the severe chronic itching that occurs in most patients with cutaneous T-cell lymphoma, Dr. Madeleine Duvic reported at the World Congress of Dermatology.
A major unmet need exists for better treatments for pruritis in CTCL. Antihistamines are generally ineffective. Chemotherapeutic agents provide little relief. Moreover, it has been estimated that up to half of all patients with CTCL die as a result of systemic infections arising secondary to pruritic skin excoriations, according to Dr. Duvic, professor of medicine and dermatology at the University of Texas and MD Anderson Cancer Center, Houston.
Naloxone is a pure opiate antagonist with no agonist effects. Naloxone lotion is an investigational agent that has received orphan drug status for treatment of pruritis in CTCL and a fast-track evaluation designation from the Food and Drug Administration.
Dr. Duvic presented a double-blind, vehicle-controlled, multicenter, crossover study involving 15 CTCL patients with severe itching. They were assigned to apply naloxone lotion 0.5% or its vehicle four times daily for 8 days and then cross over to the other regimen for another 8 days following a washout period.
After 8 days of naloxone lotion, patients reported a mean 66% reduction in itch severity from baseline on a visual analog scale, a significantly better result than the 45% reduction on vehicle.
The study suffered from small numbers, as four patients withdrew during part 1 while on vehicle, two dropped out while on naloxone lotion, and one was excluded for a concomitant medication violation. Of the nine patients available for Physician Global Assessment, seven were rated better or much better. Seven of the nine patients also rated themselves as globally better or much better while on naloxone lotion. These ratings were numerically better than while patients were on vehicle.
Adverse events were limited to two cases of mild or moderate application-site erythema.
The study was funded by Elorac. Dr. Duvic reported having no financial conflicts. She noted that the University of Texas received a research grant to conduct the study.
VANCOUVER, B.C. – Naloxone lotion appears to be a safe and effective treatment for the severe chronic itching that occurs in most patients with cutaneous T-cell lymphoma, Dr. Madeleine Duvic reported at the World Congress of Dermatology.
A major unmet need exists for better treatments for pruritis in CTCL. Antihistamines are generally ineffective. Chemotherapeutic agents provide little relief. Moreover, it has been estimated that up to half of all patients with CTCL die as a result of systemic infections arising secondary to pruritic skin excoriations, according to Dr. Duvic, professor of medicine and dermatology at the University of Texas and MD Anderson Cancer Center, Houston.
Naloxone is a pure opiate antagonist with no agonist effects. Naloxone lotion is an investigational agent that has received orphan drug status for treatment of pruritis in CTCL and a fast-track evaluation designation from the Food and Drug Administration.
Dr. Duvic presented a double-blind, vehicle-controlled, multicenter, crossover study involving 15 CTCL patients with severe itching. They were assigned to apply naloxone lotion 0.5% or its vehicle four times daily for 8 days and then cross over to the other regimen for another 8 days following a washout period.
After 8 days of naloxone lotion, patients reported a mean 66% reduction in itch severity from baseline on a visual analog scale, a significantly better result than the 45% reduction on vehicle.
The study suffered from small numbers, as four patients withdrew during part 1 while on vehicle, two dropped out while on naloxone lotion, and one was excluded for a concomitant medication violation. Of the nine patients available for Physician Global Assessment, seven were rated better or much better. Seven of the nine patients also rated themselves as globally better or much better while on naloxone lotion. These ratings were numerically better than while patients were on vehicle.
Adverse events were limited to two cases of mild or moderate application-site erythema.
The study was funded by Elorac. Dr. Duvic reported having no financial conflicts. She noted that the University of Texas received a research grant to conduct the study.
VANCOUVER, B.C. – Naloxone lotion appears to be a safe and effective treatment for the severe chronic itching that occurs in most patients with cutaneous T-cell lymphoma, Dr. Madeleine Duvic reported at the World Congress of Dermatology.
A major unmet need exists for better treatments for pruritis in CTCL. Antihistamines are generally ineffective. Chemotherapeutic agents provide little relief. Moreover, it has been estimated that up to half of all patients with CTCL die as a result of systemic infections arising secondary to pruritic skin excoriations, according to Dr. Duvic, professor of medicine and dermatology at the University of Texas and MD Anderson Cancer Center, Houston.
Naloxone is a pure opiate antagonist with no agonist effects. Naloxone lotion is an investigational agent that has received orphan drug status for treatment of pruritis in CTCL and a fast-track evaluation designation from the Food and Drug Administration.
Dr. Duvic presented a double-blind, vehicle-controlled, multicenter, crossover study involving 15 CTCL patients with severe itching. They were assigned to apply naloxone lotion 0.5% or its vehicle four times daily for 8 days and then cross over to the other regimen for another 8 days following a washout period.
After 8 days of naloxone lotion, patients reported a mean 66% reduction in itch severity from baseline on a visual analog scale, a significantly better result than the 45% reduction on vehicle.
The study suffered from small numbers, as four patients withdrew during part 1 while on vehicle, two dropped out while on naloxone lotion, and one was excluded for a concomitant medication violation. Of the nine patients available for Physician Global Assessment, seven were rated better or much better. Seven of the nine patients also rated themselves as globally better or much better while on naloxone lotion. These ratings were numerically better than while patients were on vehicle.
Adverse events were limited to two cases of mild or moderate application-site erythema.
The study was funded by Elorac. Dr. Duvic reported having no financial conflicts. She noted that the University of Texas received a research grant to conduct the study.
AT WCD 2015
Key clinical point: Naloxone lotion shows promise for the severe pruritis that accompanies cutaneous T-cell lymphoma.
Major finding: Patients with cutaneous T-cell lymphoma reported an absolute 21% greater reduction in pruritis with naloxone lotion than with its vehicle.
Data source: This was a 15-patient, multicenter, double-blind, crossover study.
Disclosures: The study was funded by Elorac. The presenter reported having no financial conflicts.
Faster ipilimumab infusion for solid cancers proves safe
Thirty-minute infusions of ipilimumab appear to be as safe as standard 90-minute infusions for patients with metastatic melanoma and other solid tumors, with “an acceptably low incidence of infusion-related reactions,” investigators reported online in the Journal of Clinical Oncology.
The approved dose for ipilimumab is 3 mg/kg infused over 90 minutes – a time period intentionally selected to be conservative when use of this monoclonal antibody began, “but not based on any specific data of which we are aware,” said Dr. Parisa Momtaz of Memorial Sloan-Kettering Cancer Center, New York, and her associates.
“Now, with extensive experience with the drug, we are in a position to reassess this guideline,” they noted.
Recent clinical trials have assessed a 10-mg/kg dose infused over 90 minutes. “We reasoned that in these patients, the standard dose of 3 mg/kg had been administered in the first 27 minutes. This suggested that a standard 3-mg/kg dose of ipilimumab might be safely administered over 30 minutes, potentially leading to improved efficiency and convenience” for patients and treatment centers alike, the investigators said.
Dr. Momtaz and her associates retrospectively assessed the incidence of infusion-related reactions among 595 patients treated at their center with either dose of the agent during a 5-year period, focusing on grade 2 and 3 symptoms of flushing, chills, pruritus, rash, nausea, dyspnea, cough, bronchospasm, fever, malaise, headache, hypotension, diaphoresis, tachycardia, and pain. The proportions of patients who had such reactions were not significantly different between the 138 who received the 10-mg/kg dose (4.3%) and the 457 who received the 3-mg/kg dose (2.2%). The standard approach at Sloan-Kettering was then changed to 30-minute rather than 90-minute infusion times for ipilimumab (J. Clin. Oncol. 2015 June 29; [doi:10.1200/JCO.2015.61.0030]).
The investigators then prospectively assessed infusion-related reactions among the next 120 patients who received 30-minute infusions of 3 mg/kg ipilimumab during the ensuing 14 months. The rate of reactions was 5.8% in this cohort, which was comparable to the previously observed rates and deemed to be “acceptably low.” These reactions typically were mild to moderate, responded to immediately with the administration of diphenhydramine and/or corticosteroids, and were never dose limiting, they noted.
Thirty-minute infusions of ipilimumab appear to be as safe as standard 90-minute infusions for patients with metastatic melanoma and other solid tumors, with “an acceptably low incidence of infusion-related reactions,” investigators reported online in the Journal of Clinical Oncology.
The approved dose for ipilimumab is 3 mg/kg infused over 90 minutes – a time period intentionally selected to be conservative when use of this monoclonal antibody began, “but not based on any specific data of which we are aware,” said Dr. Parisa Momtaz of Memorial Sloan-Kettering Cancer Center, New York, and her associates.
“Now, with extensive experience with the drug, we are in a position to reassess this guideline,” they noted.
Recent clinical trials have assessed a 10-mg/kg dose infused over 90 minutes. “We reasoned that in these patients, the standard dose of 3 mg/kg had been administered in the first 27 minutes. This suggested that a standard 3-mg/kg dose of ipilimumab might be safely administered over 30 minutes, potentially leading to improved efficiency and convenience” for patients and treatment centers alike, the investigators said.
Dr. Momtaz and her associates retrospectively assessed the incidence of infusion-related reactions among 595 patients treated at their center with either dose of the agent during a 5-year period, focusing on grade 2 and 3 symptoms of flushing, chills, pruritus, rash, nausea, dyspnea, cough, bronchospasm, fever, malaise, headache, hypotension, diaphoresis, tachycardia, and pain. The proportions of patients who had such reactions were not significantly different between the 138 who received the 10-mg/kg dose (4.3%) and the 457 who received the 3-mg/kg dose (2.2%). The standard approach at Sloan-Kettering was then changed to 30-minute rather than 90-minute infusion times for ipilimumab (J. Clin. Oncol. 2015 June 29; [doi:10.1200/JCO.2015.61.0030]).
The investigators then prospectively assessed infusion-related reactions among the next 120 patients who received 30-minute infusions of 3 mg/kg ipilimumab during the ensuing 14 months. The rate of reactions was 5.8% in this cohort, which was comparable to the previously observed rates and deemed to be “acceptably low.” These reactions typically were mild to moderate, responded to immediately with the administration of diphenhydramine and/or corticosteroids, and were never dose limiting, they noted.
Thirty-minute infusions of ipilimumab appear to be as safe as standard 90-minute infusions for patients with metastatic melanoma and other solid tumors, with “an acceptably low incidence of infusion-related reactions,” investigators reported online in the Journal of Clinical Oncology.
The approved dose for ipilimumab is 3 mg/kg infused over 90 minutes – a time period intentionally selected to be conservative when use of this monoclonal antibody began, “but not based on any specific data of which we are aware,” said Dr. Parisa Momtaz of Memorial Sloan-Kettering Cancer Center, New York, and her associates.
“Now, with extensive experience with the drug, we are in a position to reassess this guideline,” they noted.
Recent clinical trials have assessed a 10-mg/kg dose infused over 90 minutes. “We reasoned that in these patients, the standard dose of 3 mg/kg had been administered in the first 27 minutes. This suggested that a standard 3-mg/kg dose of ipilimumab might be safely administered over 30 minutes, potentially leading to improved efficiency and convenience” for patients and treatment centers alike, the investigators said.
Dr. Momtaz and her associates retrospectively assessed the incidence of infusion-related reactions among 595 patients treated at their center with either dose of the agent during a 5-year period, focusing on grade 2 and 3 symptoms of flushing, chills, pruritus, rash, nausea, dyspnea, cough, bronchospasm, fever, malaise, headache, hypotension, diaphoresis, tachycardia, and pain. The proportions of patients who had such reactions were not significantly different between the 138 who received the 10-mg/kg dose (4.3%) and the 457 who received the 3-mg/kg dose (2.2%). The standard approach at Sloan-Kettering was then changed to 30-minute rather than 90-minute infusion times for ipilimumab (J. Clin. Oncol. 2015 June 29; [doi:10.1200/JCO.2015.61.0030]).
The investigators then prospectively assessed infusion-related reactions among the next 120 patients who received 30-minute infusions of 3 mg/kg ipilimumab during the ensuing 14 months. The rate of reactions was 5.8% in this cohort, which was comparable to the previously observed rates and deemed to be “acceptably low.” These reactions typically were mild to moderate, responded to immediately with the administration of diphenhydramine and/or corticosteroids, and were never dose limiting, they noted.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: 30-minute infusions of ipilimumab were as safe as conventional 90-minute infusions in patients with metastatic melanoma and other solid tumors.
Major finding: The proportion of patients who had infusion-related reactions was not significantly different between the 138 who received the 10 mg/kg dose (4.3%) and the 457 who received the 3 mg/kg dose (2.2%) of ipilimumab.
Data source: A retrospective analysis of infusion-related reactions in 595 patients who had 90-minute infusions and a prospective analysis of such reactions in 120 patients who had 30-minute infusions.
Disclosures: This study was supported in part by the John K. Figge Fund. Dr. Momtaz reported having no financial disclosures; her associates reported numerous ties to industry sources.
Urea cream bests antioxidant ointment to prevent chemo-induced hand-foot syndrome
Urea cream is more effective at preventing hand-foot syndrome (HFS) than is a medical ointment high in antioxidants for patients receiving capecitabine, according to a randomized phase III study published online in the Journal of Clinical Oncology.
During a 6-week treatment period, 17 of 76 patients (22.4%) who used 10% urea cream experienced HFS, compared with 30 of 76 patients (39.5%) who used the new ointment Mapisal, which has been available on the German market since 2011 (odds ratio 2.37; 95% CI 1.14 to 4.84; P = .02). The distribution of HFS grades within the groups was similar, with the majority having grade 1 and about 6% experiencing grade 3 HFS. A secondary endpoint, time to develop HFS, was significantly longer in the urea cream group (J. Clin. Oncol. 2015 June 29 [doi:10.1200/JCO.2014.60.4587]).
The result was unexpected. Mapisal contains several antioxidants and oil extracts and exhibits a high radical protection factor, which was hypothesized to be of benefit due to a decline in antioxidative capacity of the skin in patients using capecitabine. “The most striking explanation for the observed ineffectiveness of Mapisal is that either the hypothesis – that is, that Mapisal’s crucial mode of action against HFS is to act as an antioxidant against free radicals – is incorrect, or that this is not the main mechanism of HFS development, at least in the case of capecitabine,” wrote Dr. Ralf-Dieter Hofheinz of University Hospital Mannheim, Germany, and colleagues.
Between 2012 and 2013, the study enrolled 160 patients with GI tumors or breast cancer who were being treated with capecitabine, and 152 were randomly assigned to receive prophylactic treatment with either Mapisal or 10% urea cream for a 6-week period. Aside from HFS, adverse events were similar between the groups. After the treatment period, skin-related quality of life was significantly lower in the Mapisal vs. urea group.
The results of this study, along with findings from a previous phase II study demonstrating activity of urea cream in patients treated with sorafenib, supports the idea that urea cream is an appropriate prophylaxis for HFS and a reasonable standard for future investigations, the researchers noted.
Urea cream is more effective at preventing hand-foot syndrome (HFS) than is a medical ointment high in antioxidants for patients receiving capecitabine, according to a randomized phase III study published online in the Journal of Clinical Oncology.
During a 6-week treatment period, 17 of 76 patients (22.4%) who used 10% urea cream experienced HFS, compared with 30 of 76 patients (39.5%) who used the new ointment Mapisal, which has been available on the German market since 2011 (odds ratio 2.37; 95% CI 1.14 to 4.84; P = .02). The distribution of HFS grades within the groups was similar, with the majority having grade 1 and about 6% experiencing grade 3 HFS. A secondary endpoint, time to develop HFS, was significantly longer in the urea cream group (J. Clin. Oncol. 2015 June 29 [doi:10.1200/JCO.2014.60.4587]).
The result was unexpected. Mapisal contains several antioxidants and oil extracts and exhibits a high radical protection factor, which was hypothesized to be of benefit due to a decline in antioxidative capacity of the skin in patients using capecitabine. “The most striking explanation for the observed ineffectiveness of Mapisal is that either the hypothesis – that is, that Mapisal’s crucial mode of action against HFS is to act as an antioxidant against free radicals – is incorrect, or that this is not the main mechanism of HFS development, at least in the case of capecitabine,” wrote Dr. Ralf-Dieter Hofheinz of University Hospital Mannheim, Germany, and colleagues.
Between 2012 and 2013, the study enrolled 160 patients with GI tumors or breast cancer who were being treated with capecitabine, and 152 were randomly assigned to receive prophylactic treatment with either Mapisal or 10% urea cream for a 6-week period. Aside from HFS, adverse events were similar between the groups. After the treatment period, skin-related quality of life was significantly lower in the Mapisal vs. urea group.
The results of this study, along with findings from a previous phase II study demonstrating activity of urea cream in patients treated with sorafenib, supports the idea that urea cream is an appropriate prophylaxis for HFS and a reasonable standard for future investigations, the researchers noted.
Urea cream is more effective at preventing hand-foot syndrome (HFS) than is a medical ointment high in antioxidants for patients receiving capecitabine, according to a randomized phase III study published online in the Journal of Clinical Oncology.
During a 6-week treatment period, 17 of 76 patients (22.4%) who used 10% urea cream experienced HFS, compared with 30 of 76 patients (39.5%) who used the new ointment Mapisal, which has been available on the German market since 2011 (odds ratio 2.37; 95% CI 1.14 to 4.84; P = .02). The distribution of HFS grades within the groups was similar, with the majority having grade 1 and about 6% experiencing grade 3 HFS. A secondary endpoint, time to develop HFS, was significantly longer in the urea cream group (J. Clin. Oncol. 2015 June 29 [doi:10.1200/JCO.2014.60.4587]).
The result was unexpected. Mapisal contains several antioxidants and oil extracts and exhibits a high radical protection factor, which was hypothesized to be of benefit due to a decline in antioxidative capacity of the skin in patients using capecitabine. “The most striking explanation for the observed ineffectiveness of Mapisal is that either the hypothesis – that is, that Mapisal’s crucial mode of action against HFS is to act as an antioxidant against free radicals – is incorrect, or that this is not the main mechanism of HFS development, at least in the case of capecitabine,” wrote Dr. Ralf-Dieter Hofheinz of University Hospital Mannheim, Germany, and colleagues.
Between 2012 and 2013, the study enrolled 160 patients with GI tumors or breast cancer who were being treated with capecitabine, and 152 were randomly assigned to receive prophylactic treatment with either Mapisal or 10% urea cream for a 6-week period. Aside from HFS, adverse events were similar between the groups. After the treatment period, skin-related quality of life was significantly lower in the Mapisal vs. urea group.
The results of this study, along with findings from a previous phase II study demonstrating activity of urea cream in patients treated with sorafenib, supports the idea that urea cream is an appropriate prophylaxis for HFS and a reasonable standard for future investigations, the researchers noted.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Urea cream was superior to Mapisal, an antioxidant-containing ointment, in preventing hand-foot syndrome (HFS) among patients receiving capecitabine.
Major finding: A significantly higher proportion of patients using Mapisal experienced HFS compared with urea cream: 39.5% vs. 22.4% (P = .02).
Data source: Between 2012 and 2013, the randomized phase III trial included 152 patients with GI tumors or breast cancer who received HFS prophylactic treatment for 6 weeks.
Disclosures: Medac supported the study. Dr. Hofheinz disclosed ties with Medac, Roche, Amgen, Merck, Eli Lilly, Sanofi, and Bayer AG.