User login
For MD-IQ use only
Erythema Nodosum Triggered by a Bite From a Copperhead Snake
The clinical manifestations of snakebites vary based on the species of snake, bite location, and amount and strength of the venom injected. Locally acting toxins in snake venom predominantly consist of enzymes, such as phospholipase A2, that cause local tissue destruction and can result in pain, swelling, blistering, ecchymosis, and tissue necrosis at the site of the bite within hours to days after the bite.1 Systemically acting toxins can target a wide variety of tissues and cause severe systemic complications including paralysis, rhabdomyolysis secondary to muscle damage, coagulopathy, sepsis, and cardiorespiratory failure.2
Although pain and swelling following snakebites typically resolve by 1 month after envenomation, copperhead snakes—a type of pit viper—may cause residual symptoms of pain and swelling lasting for a year or more.3 Additional cutaneous manifestations of copperhead snakebites include wound infections at the bite site, such as cellulitis and necrotizing fasciitis. More devastating complications that have been described following snake envenomation include tissue injury of an entire extremity and development of compartment syndrome, which requires urgent fasciotomy to prevent potential loss of the affected limb.4
Physicians should be aware of the potential complications of snakebites to properly manage and counsel their patients. We describe a 42-year-old woman with tender, erythematous, subcutaneous nodules persisting for 4 months following a copperhead snakebite. A biopsy confirmed the diagnosis of snakebite-associated erythema nodosum (EN).
Case Report
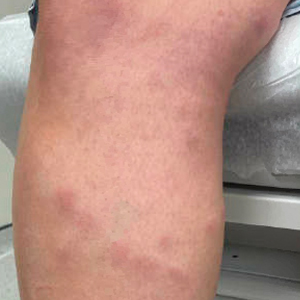
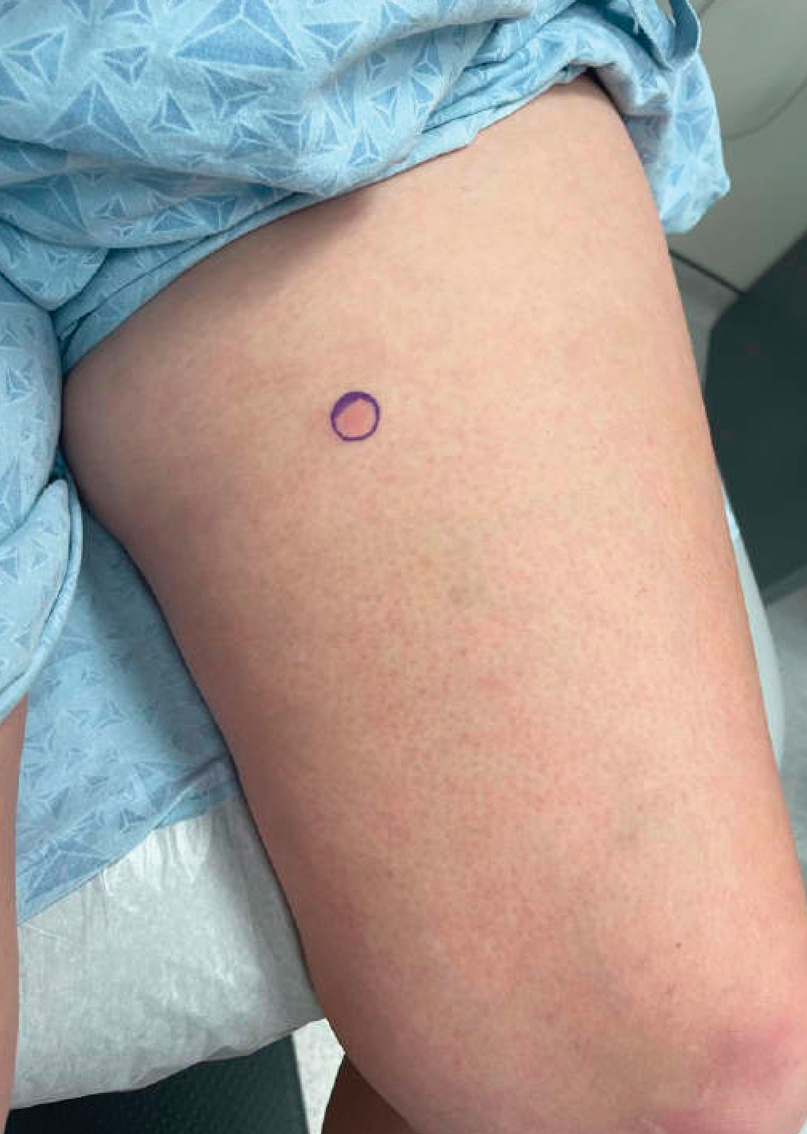
A 42-year-old woman presented to our clinic with progressive tender, pruritic, deep-seated, erythematous nodules in multiple locations on the legs after sustaining a bite by a copperhead snake on the left foot 4 months prior. The lesions tended to fluctuate in intensity. In the days following the bite, she initially developed painful red bumps on the left foot just proximal to the bite site with associated pain and swelling extending up to just below the left knee. She reported no other notable symptoms such as fever, arthralgia, fatigue, or gastrointestinal tract symptoms. Physical examination revealed bilateral pitting edema, which was worse in the left leg, along with multiple deep, palpable, tender subcutaneous nodules with erythematous surface change (Figure 1).
Workup performed by an outside provider over the previous month included 2 venous duplex ultrasounds of the left leg, which showed no signs of deep vein thrombosis. Additionally, the patient underwent lateral and anteroposterior radiographs of the left foot, tibia, and fibula, which showed no evidence of fracture.
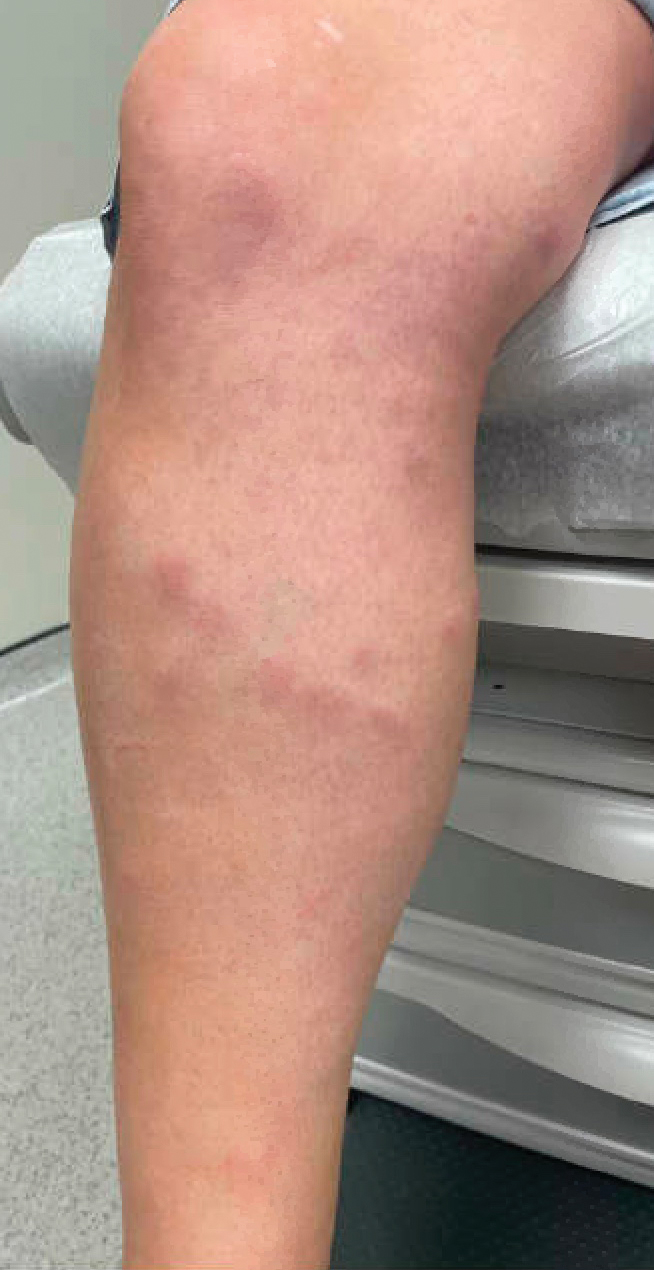
Given the morphology and distribution of the lesions (Figure 2), EN was strongly favored as the cause of the symptoms, and a biopsy confirmed the diagnosis. All immunohistochemical stains including auramine-rhodamine for acid-fast bacilli, Grocott-Gomori methenamine silver for fungal organisms, and Brown and Brenn were negative. Given the waxing and waning course of the lesions, which suggested an active neutrophilic rather than purely chronic granulomatous phase of EN, the patient was treated with colchicine 0.6 mg twice daily for 1 month.
Causes of EN and Clinical Manifestations
Erythema nodosum is a common form of septal panniculitis that can be precipitated by inflammatory conditions, infection, or medications (commonly oral contraceptive pills) but often is idiopathic.5 The acute phase is neutrophilic, with evolution over time to a granulomatous phase. Common etiologies include sarcoidosis; inflammatory bowel disease; and bacterial or fungal infections such as Streptococcus (especially common in children), histoplasmosis, and coccidioidomycosis. The patient was otherwise healthy and was not taking any medications that are known triggers of EN. A PubMed search of articles indexed for MEDLINE in the English-language literature using the terms copperhead snake bite, erythema nodosum snake, and copperhead snake erythema nodosum revealed no reports of EN following a bite from a copperhead snake; however, in one case, an adder bite led to erysipelas, likely due to disturbed blood and lymphatic flow, which then triggered EN.6 Additionally, EN has been reported as a delayed reaction to jellyfish stings.7
Clinical features of EN include the development of tender, erythematous, subcutaneous nodules and plaques most frequently over the pretibial region. Lesions typically evolve from raised, deep-seated nodules into flat indurated plaques over a span of weeks. Occasionally, there is a slight prodromal phase marked by nonspecific symptoms such as fever and arthralgia lasting for 3 to 6 days. Erythema nodosum typically results in spontaneous resolution after 4 to 8 weeks, and management involves treatment of any underlying condition with symptomatic care. Interestingly, our patient experienced persistent symptoms over the course of 4 months, with development of new nodular lesions throughout this time period. The most frequently used drugs for the management of symptomatic EN include nonsteroidal anti-inflammatory drugs, colchicine, and potassium iodide.8 A characteristic histologic finding of the granulomatous phase is the Miescher radial granuloma, which is a septal collection of histiocytes surrounding a cleft.9
Snakebite Reactions
Snakebites can result in a wide range of local and systemic manifestations, as snake venom may contain 20 or more toxins.10 Local complications of pit viper bites include pain, swelling, and fang marks; when examining fang marks, the presence of 2 distinct puncture wounds often indicates envenomation with a poisonous snake, whereas nonvenomous snakebites often result in smaller puncture wounds arranged in an arc. Following bites, pain can develop immediately and spread proximally up the affected limb, which occurred in our patient in the days following the bite. Intense local reactions can occur, as bites often result in intense edema of the affected limb spreading to the trunk in the days to weeks after the bite, occasionally accompanied by regional lymphadenopathy. Some bites can result in local necrosis and secondary bacterial infection caused by organisms in the oral cavity of the culprit snake.
Although they were not present in our patient, snakebites can result in a wide range of systemic toxicities ranging from clotting defects and hemolysis to neurotoxicity, myotoxicity, and nephrotoxicity.10 In severe cases, snake venom can result in disseminated intravascular coagulation, sepsis, and cardiorespiratory collapse.
The eastern copperhead (Agkistrodon contortrix) is a species of venomous snake that is endemic to eastern North America. Copperheads are members of the subfamily Crotalinae in the family Viperidae.11 Reported reactions to copperhead bites include cellulitis, necrotizing fasciitis, compartment syndrome, and tissue necrosis of an entire affected extremity.12,13 Our patient displayed no systemic symptoms to suggest envenomation.
Management of Snakebites
Treatment of snakebites varies based on the constellation and severity of symptoms as well as how recently the envenomation occurred. In urgent cases, antivenom may be administered to prevent further toxicity. In cases of progressive compartment syndrome, emergent surgical procedures such as fasciotomy or amputation are required to prevent further complications. When a superimposed bacterial infection is suspected, broad-spectrum antibiotics are required. Because our patient presented 4 months following the initial bite with isolated cutaneous manifestations, she was treated symptomatically with colchicine for EN.1,2
Final Thoughts
Our patient presented with EN following a bite from a copperhead snake. Physicians should be aware of possible etiologies of EN to evaluate patients who present with new-onset tender subcutaneous nodules. Additionally, physicians should be aware of venomous snakes endemic to their region and also understand the various complications that can result following a snakebite, with the potential for lingering cutaneous manifestations weeks to months following the initial bite.
- Warrell DA. Snake bite. Lancet. 2010;375:77-88. doi:10.1016/S0140-6736(09)61754-2
- White J. Overview of venomous snakes of the world. In: Dart RC, eds. Medical Toxicology. 3rd ed. Lippincott, Williams, & Wilkins; 2004:1543
- Spiller HA, Bosse GM. Prospective study of morbidity associated with snakebite envenomation. J Toxicol Clin Toxicol. 2003;41:125-130. doi:10.1081/clt-120019127
- Scharman EJ, Noffsinger VD. Copperhead snakebites: clinical severity of local effects. Ann Emerg Med. 2001;38:55-61. doi:10.1067/mem.2001.116148
- Hafsi W, Badri T. Erythema nodosum. In: StatPearls. StatPearls Publishing; November 28, 2022. Accessed July 22, 2024. https://www.ncbi.nlm.nih.gov/books/NBK470369/
- Nowowiejska J, Baran A, Flisiak I. Rare coexistence of unilateral erythema nodosum with erysipelas in the area of previous adder bite. Przegl Epidemiol. 2020;74:355-361. doi:10.32394/pe.74.28
- Auerbach PS, Hays JT. Erythema nodosum following a jellyfish sting. J Emerg Med. 1987;5:487-491. doi:10.1016/0736-4679(87)90211-3
- Gilchrist H, Patterson JW. Erythema nodosum and erythema induratum (nodular vasculitis): diagnosis and management. Dermatol Ther. 2010;23:320-327. doi:10.1111/j.1529-8019.2010.01332.x
- Sánchez Yus E, Sanz Vico MD, de Diego V. Miescher’s radial granuloma. a characteristic marker of erythema nodosum. Am J Dermatopathol. 1989;11:434-442. doi:10.1097/00000372-198910000-00005
- Mehta SR, Sashindran VK. Clinical features and management of snake bite. Med J Armed Forces India. 2002;58:247-249. doi:10.1016/S0377-1237(02)80140-X
- Brys AK, Gandolfi BM, Levinson H, et al. Copperhead envenomation resulting in a rare case of hand compartment syndrome and subsequent fasciotomy. Plast Reconstr Surg Glob Open. 2015;3:E396. doi:10.1097/GOX.0000000000000367
- Clark RF, Selden BS, Furbee B. The incidence of wound infection following crotalid envenomation. J Emerg Med. 1993;11:583-586. doi:10.1016/0736-4679(93)90313-v
- Buchanan JT, Thurman J. Crotalidae envenomation. In: StatPearls. StatPearls Publishing; October 3, 2022. Accessed July 22, 2024. https://www.ncbi.nlm.nih.gov/books/NBK551615/
The clinical manifestations of snakebites vary based on the species of snake, bite location, and amount and strength of the venom injected. Locally acting toxins in snake venom predominantly consist of enzymes, such as phospholipase A2, that cause local tissue destruction and can result in pain, swelling, blistering, ecchymosis, and tissue necrosis at the site of the bite within hours to days after the bite.1 Systemically acting toxins can target a wide variety of tissues and cause severe systemic complications including paralysis, rhabdomyolysis secondary to muscle damage, coagulopathy, sepsis, and cardiorespiratory failure.2
Although pain and swelling following snakebites typically resolve by 1 month after envenomation, copperhead snakes—a type of pit viper—may cause residual symptoms of pain and swelling lasting for a year or more.3 Additional cutaneous manifestations of copperhead snakebites include wound infections at the bite site, such as cellulitis and necrotizing fasciitis. More devastating complications that have been described following snake envenomation include tissue injury of an entire extremity and development of compartment syndrome, which requires urgent fasciotomy to prevent potential loss of the affected limb.4
Physicians should be aware of the potential complications of snakebites to properly manage and counsel their patients. We describe a 42-year-old woman with tender, erythematous, subcutaneous nodules persisting for 4 months following a copperhead snakebite. A biopsy confirmed the diagnosis of snakebite-associated erythema nodosum (EN).
Case Report
A 42-year-old woman presented to our clinic with progressive tender, pruritic, deep-seated, erythematous nodules in multiple locations on the legs after sustaining a bite by a copperhead snake on the left foot 4 months prior. The lesions tended to fluctuate in intensity. In the days following the bite, she initially developed painful red bumps on the left foot just proximal to the bite site with associated pain and swelling extending up to just below the left knee. She reported no other notable symptoms such as fever, arthralgia, fatigue, or gastrointestinal tract symptoms. Physical examination revealed bilateral pitting edema, which was worse in the left leg, along with multiple deep, palpable, tender subcutaneous nodules with erythematous surface change (Figure 1).

Workup performed by an outside provider over the previous month included 2 venous duplex ultrasounds of the left leg, which showed no signs of deep vein thrombosis. Additionally, the patient underwent lateral and anteroposterior radiographs of the left foot, tibia, and fibula, which showed no evidence of fracture.
Given the morphology and distribution of the lesions (Figure 2), EN was strongly favored as the cause of the symptoms, and a biopsy confirmed the diagnosis. All immunohistochemical stains including auramine-rhodamine for acid-fast bacilli, Grocott-Gomori methenamine silver for fungal organisms, and Brown and Brenn were negative. Given the waxing and waning course of the lesions, which suggested an active neutrophilic rather than purely chronic granulomatous phase of EN, the patient was treated with colchicine 0.6 mg twice daily for 1 month.

Causes of EN and Clinical Manifestations
Erythema nodosum is a common form of septal panniculitis that can be precipitated by inflammatory conditions, infection, or medications (commonly oral contraceptive pills) but often is idiopathic.5 The acute phase is neutrophilic, with evolution over time to a granulomatous phase. Common etiologies include sarcoidosis; inflammatory bowel disease; and bacterial or fungal infections such as Streptococcus (especially common in children), histoplasmosis, and coccidioidomycosis. The patient was otherwise healthy and was not taking any medications that are known triggers of EN. A PubMed search of articles indexed for MEDLINE in the English-language literature using the terms copperhead snake bite, erythema nodosum snake, and copperhead snake erythema nodosum revealed no reports of EN following a bite from a copperhead snake; however, in one case, an adder bite led to erysipelas, likely due to disturbed blood and lymphatic flow, which then triggered EN.6 Additionally, EN has been reported as a delayed reaction to jellyfish stings.7
Clinical features of EN include the development of tender, erythematous, subcutaneous nodules and plaques most frequently over the pretibial region. Lesions typically evolve from raised, deep-seated nodules into flat indurated plaques over a span of weeks. Occasionally, there is a slight prodromal phase marked by nonspecific symptoms such as fever and arthralgia lasting for 3 to 6 days. Erythema nodosum typically results in spontaneous resolution after 4 to 8 weeks, and management involves treatment of any underlying condition with symptomatic care. Interestingly, our patient experienced persistent symptoms over the course of 4 months, with development of new nodular lesions throughout this time period. The most frequently used drugs for the management of symptomatic EN include nonsteroidal anti-inflammatory drugs, colchicine, and potassium iodide.8 A characteristic histologic finding of the granulomatous phase is the Miescher radial granuloma, which is a septal collection of histiocytes surrounding a cleft.9
Snakebite Reactions
Snakebites can result in a wide range of local and systemic manifestations, as snake venom may contain 20 or more toxins.10 Local complications of pit viper bites include pain, swelling, and fang marks; when examining fang marks, the presence of 2 distinct puncture wounds often indicates envenomation with a poisonous snake, whereas nonvenomous snakebites often result in smaller puncture wounds arranged in an arc. Following bites, pain can develop immediately and spread proximally up the affected limb, which occurred in our patient in the days following the bite. Intense local reactions can occur, as bites often result in intense edema of the affected limb spreading to the trunk in the days to weeks after the bite, occasionally accompanied by regional lymphadenopathy. Some bites can result in local necrosis and secondary bacterial infection caused by organisms in the oral cavity of the culprit snake.
Although they were not present in our patient, snakebites can result in a wide range of systemic toxicities ranging from clotting defects and hemolysis to neurotoxicity, myotoxicity, and nephrotoxicity.10 In severe cases, snake venom can result in disseminated intravascular coagulation, sepsis, and cardiorespiratory collapse.
The eastern copperhead (Agkistrodon contortrix) is a species of venomous snake that is endemic to eastern North America. Copperheads are members of the subfamily Crotalinae in the family Viperidae.11 Reported reactions to copperhead bites include cellulitis, necrotizing fasciitis, compartment syndrome, and tissue necrosis of an entire affected extremity.12,13 Our patient displayed no systemic symptoms to suggest envenomation.
Management of Snakebites
Treatment of snakebites varies based on the constellation and severity of symptoms as well as how recently the envenomation occurred. In urgent cases, antivenom may be administered to prevent further toxicity. In cases of progressive compartment syndrome, emergent surgical procedures such as fasciotomy or amputation are required to prevent further complications. When a superimposed bacterial infection is suspected, broad-spectrum antibiotics are required. Because our patient presented 4 months following the initial bite with isolated cutaneous manifestations, she was treated symptomatically with colchicine for EN.1,2
Final Thoughts
Our patient presented with EN following a bite from a copperhead snake. Physicians should be aware of possible etiologies of EN to evaluate patients who present with new-onset tender subcutaneous nodules. Additionally, physicians should be aware of venomous snakes endemic to their region and also understand the various complications that can result following a snakebite, with the potential for lingering cutaneous manifestations weeks to months following the initial bite.
The clinical manifestations of snakebites vary based on the species of snake, bite location, and amount and strength of the venom injected. Locally acting toxins in snake venom predominantly consist of enzymes, such as phospholipase A2, that cause local tissue destruction and can result in pain, swelling, blistering, ecchymosis, and tissue necrosis at the site of the bite within hours to days after the bite.1 Systemically acting toxins can target a wide variety of tissues and cause severe systemic complications including paralysis, rhabdomyolysis secondary to muscle damage, coagulopathy, sepsis, and cardiorespiratory failure.2
Although pain and swelling following snakebites typically resolve by 1 month after envenomation, copperhead snakes—a type of pit viper—may cause residual symptoms of pain and swelling lasting for a year or more.3 Additional cutaneous manifestations of copperhead snakebites include wound infections at the bite site, such as cellulitis and necrotizing fasciitis. More devastating complications that have been described following snake envenomation include tissue injury of an entire extremity and development of compartment syndrome, which requires urgent fasciotomy to prevent potential loss of the affected limb.4
Physicians should be aware of the potential complications of snakebites to properly manage and counsel their patients. We describe a 42-year-old woman with tender, erythematous, subcutaneous nodules persisting for 4 months following a copperhead snakebite. A biopsy confirmed the diagnosis of snakebite-associated erythema nodosum (EN).
Case Report
A 42-year-old woman presented to our clinic with progressive tender, pruritic, deep-seated, erythematous nodules in multiple locations on the legs after sustaining a bite by a copperhead snake on the left foot 4 months prior. The lesions tended to fluctuate in intensity. In the days following the bite, she initially developed painful red bumps on the left foot just proximal to the bite site with associated pain and swelling extending up to just below the left knee. She reported no other notable symptoms such as fever, arthralgia, fatigue, or gastrointestinal tract symptoms. Physical examination revealed bilateral pitting edema, which was worse in the left leg, along with multiple deep, palpable, tender subcutaneous nodules with erythematous surface change (Figure 1).

Workup performed by an outside provider over the previous month included 2 venous duplex ultrasounds of the left leg, which showed no signs of deep vein thrombosis. Additionally, the patient underwent lateral and anteroposterior radiographs of the left foot, tibia, and fibula, which showed no evidence of fracture.
Given the morphology and distribution of the lesions (Figure 2), EN was strongly favored as the cause of the symptoms, and a biopsy confirmed the diagnosis. All immunohistochemical stains including auramine-rhodamine for acid-fast bacilli, Grocott-Gomori methenamine silver for fungal organisms, and Brown and Brenn were negative. Given the waxing and waning course of the lesions, which suggested an active neutrophilic rather than purely chronic granulomatous phase of EN, the patient was treated with colchicine 0.6 mg twice daily for 1 month.

Causes of EN and Clinical Manifestations
Erythema nodosum is a common form of septal panniculitis that can be precipitated by inflammatory conditions, infection, or medications (commonly oral contraceptive pills) but often is idiopathic.5 The acute phase is neutrophilic, with evolution over time to a granulomatous phase. Common etiologies include sarcoidosis; inflammatory bowel disease; and bacterial or fungal infections such as Streptococcus (especially common in children), histoplasmosis, and coccidioidomycosis. The patient was otherwise healthy and was not taking any medications that are known triggers of EN. A PubMed search of articles indexed for MEDLINE in the English-language literature using the terms copperhead snake bite, erythema nodosum snake, and copperhead snake erythema nodosum revealed no reports of EN following a bite from a copperhead snake; however, in one case, an adder bite led to erysipelas, likely due to disturbed blood and lymphatic flow, which then triggered EN.6 Additionally, EN has been reported as a delayed reaction to jellyfish stings.7
Clinical features of EN include the development of tender, erythematous, subcutaneous nodules and plaques most frequently over the pretibial region. Lesions typically evolve from raised, deep-seated nodules into flat indurated plaques over a span of weeks. Occasionally, there is a slight prodromal phase marked by nonspecific symptoms such as fever and arthralgia lasting for 3 to 6 days. Erythema nodosum typically results in spontaneous resolution after 4 to 8 weeks, and management involves treatment of any underlying condition with symptomatic care. Interestingly, our patient experienced persistent symptoms over the course of 4 months, with development of new nodular lesions throughout this time period. The most frequently used drugs for the management of symptomatic EN include nonsteroidal anti-inflammatory drugs, colchicine, and potassium iodide.8 A characteristic histologic finding of the granulomatous phase is the Miescher radial granuloma, which is a septal collection of histiocytes surrounding a cleft.9
Snakebite Reactions
Snakebites can result in a wide range of local and systemic manifestations, as snake venom may contain 20 or more toxins.10 Local complications of pit viper bites include pain, swelling, and fang marks; when examining fang marks, the presence of 2 distinct puncture wounds often indicates envenomation with a poisonous snake, whereas nonvenomous snakebites often result in smaller puncture wounds arranged in an arc. Following bites, pain can develop immediately and spread proximally up the affected limb, which occurred in our patient in the days following the bite. Intense local reactions can occur, as bites often result in intense edema of the affected limb spreading to the trunk in the days to weeks after the bite, occasionally accompanied by regional lymphadenopathy. Some bites can result in local necrosis and secondary bacterial infection caused by organisms in the oral cavity of the culprit snake.
Although they were not present in our patient, snakebites can result in a wide range of systemic toxicities ranging from clotting defects and hemolysis to neurotoxicity, myotoxicity, and nephrotoxicity.10 In severe cases, snake venom can result in disseminated intravascular coagulation, sepsis, and cardiorespiratory collapse.
The eastern copperhead (Agkistrodon contortrix) is a species of venomous snake that is endemic to eastern North America. Copperheads are members of the subfamily Crotalinae in the family Viperidae.11 Reported reactions to copperhead bites include cellulitis, necrotizing fasciitis, compartment syndrome, and tissue necrosis of an entire affected extremity.12,13 Our patient displayed no systemic symptoms to suggest envenomation.
Management of Snakebites
Treatment of snakebites varies based on the constellation and severity of symptoms as well as how recently the envenomation occurred. In urgent cases, antivenom may be administered to prevent further toxicity. In cases of progressive compartment syndrome, emergent surgical procedures such as fasciotomy or amputation are required to prevent further complications. When a superimposed bacterial infection is suspected, broad-spectrum antibiotics are required. Because our patient presented 4 months following the initial bite with isolated cutaneous manifestations, she was treated symptomatically with colchicine for EN.1,2
Final Thoughts
Our patient presented with EN following a bite from a copperhead snake. Physicians should be aware of possible etiologies of EN to evaluate patients who present with new-onset tender subcutaneous nodules. Additionally, physicians should be aware of venomous snakes endemic to their region and also understand the various complications that can result following a snakebite, with the potential for lingering cutaneous manifestations weeks to months following the initial bite.
- Warrell DA. Snake bite. Lancet. 2010;375:77-88. doi:10.1016/S0140-6736(09)61754-2
- White J. Overview of venomous snakes of the world. In: Dart RC, eds. Medical Toxicology. 3rd ed. Lippincott, Williams, & Wilkins; 2004:1543
- Spiller HA, Bosse GM. Prospective study of morbidity associated with snakebite envenomation. J Toxicol Clin Toxicol. 2003;41:125-130. doi:10.1081/clt-120019127
- Scharman EJ, Noffsinger VD. Copperhead snakebites: clinical severity of local effects. Ann Emerg Med. 2001;38:55-61. doi:10.1067/mem.2001.116148
- Hafsi W, Badri T. Erythema nodosum. In: StatPearls. StatPearls Publishing; November 28, 2022. Accessed July 22, 2024. https://www.ncbi.nlm.nih.gov/books/NBK470369/
- Nowowiejska J, Baran A, Flisiak I. Rare coexistence of unilateral erythema nodosum with erysipelas in the area of previous adder bite. Przegl Epidemiol. 2020;74:355-361. doi:10.32394/pe.74.28
- Auerbach PS, Hays JT. Erythema nodosum following a jellyfish sting. J Emerg Med. 1987;5:487-491. doi:10.1016/0736-4679(87)90211-3
- Gilchrist H, Patterson JW. Erythema nodosum and erythema induratum (nodular vasculitis): diagnosis and management. Dermatol Ther. 2010;23:320-327. doi:10.1111/j.1529-8019.2010.01332.x
- Sánchez Yus E, Sanz Vico MD, de Diego V. Miescher’s radial granuloma. a characteristic marker of erythema nodosum. Am J Dermatopathol. 1989;11:434-442. doi:10.1097/00000372-198910000-00005
- Mehta SR, Sashindran VK. Clinical features and management of snake bite. Med J Armed Forces India. 2002;58:247-249. doi:10.1016/S0377-1237(02)80140-X
- Brys AK, Gandolfi BM, Levinson H, et al. Copperhead envenomation resulting in a rare case of hand compartment syndrome and subsequent fasciotomy. Plast Reconstr Surg Glob Open. 2015;3:E396. doi:10.1097/GOX.0000000000000367
- Clark RF, Selden BS, Furbee B. The incidence of wound infection following crotalid envenomation. J Emerg Med. 1993;11:583-586. doi:10.1016/0736-4679(93)90313-v
- Buchanan JT, Thurman J. Crotalidae envenomation. In: StatPearls. StatPearls Publishing; October 3, 2022. Accessed July 22, 2024. https://www.ncbi.nlm.nih.gov/books/NBK551615/
- Warrell DA. Snake bite. Lancet. 2010;375:77-88. doi:10.1016/S0140-6736(09)61754-2
- White J. Overview of venomous snakes of the world. In: Dart RC, eds. Medical Toxicology. 3rd ed. Lippincott, Williams, & Wilkins; 2004:1543
- Spiller HA, Bosse GM. Prospective study of morbidity associated with snakebite envenomation. J Toxicol Clin Toxicol. 2003;41:125-130. doi:10.1081/clt-120019127
- Scharman EJ, Noffsinger VD. Copperhead snakebites: clinical severity of local effects. Ann Emerg Med. 2001;38:55-61. doi:10.1067/mem.2001.116148
- Hafsi W, Badri T. Erythema nodosum. In: StatPearls. StatPearls Publishing; November 28, 2022. Accessed July 22, 2024. https://www.ncbi.nlm.nih.gov/books/NBK470369/
- Nowowiejska J, Baran A, Flisiak I. Rare coexistence of unilateral erythema nodosum with erysipelas in the area of previous adder bite. Przegl Epidemiol. 2020;74:355-361. doi:10.32394/pe.74.28
- Auerbach PS, Hays JT. Erythema nodosum following a jellyfish sting. J Emerg Med. 1987;5:487-491. doi:10.1016/0736-4679(87)90211-3
- Gilchrist H, Patterson JW. Erythema nodosum and erythema induratum (nodular vasculitis): diagnosis and management. Dermatol Ther. 2010;23:320-327. doi:10.1111/j.1529-8019.2010.01332.x
- Sánchez Yus E, Sanz Vico MD, de Diego V. Miescher’s radial granuloma. a characteristic marker of erythema nodosum. Am J Dermatopathol. 1989;11:434-442. doi:10.1097/00000372-198910000-00005
- Mehta SR, Sashindran VK. Clinical features and management of snake bite. Med J Armed Forces India. 2002;58:247-249. doi:10.1016/S0377-1237(02)80140-X
- Brys AK, Gandolfi BM, Levinson H, et al. Copperhead envenomation resulting in a rare case of hand compartment syndrome and subsequent fasciotomy. Plast Reconstr Surg Glob Open. 2015;3:E396. doi:10.1097/GOX.0000000000000367
- Clark RF, Selden BS, Furbee B. The incidence of wound infection following crotalid envenomation. J Emerg Med. 1993;11:583-586. doi:10.1016/0736-4679(93)90313-v
- Buchanan JT, Thurman J. Crotalidae envenomation. In: StatPearls. StatPearls Publishing; October 3, 2022. Accessed July 22, 2024. https://www.ncbi.nlm.nih.gov/books/NBK551615/
Practice Points
- Erythema nodosum (EN) can occur following snakebites from pit vipers such as the eastern copperhead.
- The acute phase of EN is neutrophilic and responds to colchicine. The chronic phase of EN is granulomatous and responds best to rest and elevation as well as nonsteroidal anti-inflammatory drugs and iodides.
Distinguishing Generalized Bullous Fixed Drug Eruption From SJS/TEN: A Retrospective Study on Clinical and Demographic Features
To the Editor:
Generalized bullous fixed drug eruption (GBFDE) is a rare subtype of fixed drug eruption (FDE) that manifests as widespread blisters and erosions following exposure to a causative drug.1 Diagnostic criteria include involvement of at least 3 to 6 anatomic sites—head and neck, anterior trunk, posterior trunk, upper extremities, lower extremities, or genitalia—and more than 10% of the body surface area. It can be challenging to differentiate GBFDE from severe drug rashes such as Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN) due to extensive body surface area involvement of blisters and erosions. Specific features distinguishing GBFDE from SJS/TEN include primary lesions consisting of larger erythematous to dusky, circular plaques that progress to bullae and coalesce into widespread erosions; history of FDE; lack of severe mucosal involvement; and better overall prognosis.2 Treatment typically involves discontinuation of the culprit medication and supportive care; evidence for systemic therapies is not well established.
Our study aimed to characterize the clinical and demographic features of GBFDE in our institution to highlight potential key differences between this diagnosis and SJS/TEN. An electronic medical record search was performed to identify patients who were clinically diagnosed with GBFDE at New York-Presbyterian/Weill Cornell Medical Center (New York, New York) in both outpatient and inpatient settings from January 2015 to December 2022. This retrospective study was approved by the Weill Cornell Medicine institutional review board (#22-05024777).
Ten patients were identified and included in the analysis (eTable). The mean age of the patients was 56 years (range, 39–76 years). Seven (70%) patients had skin of color (non-White) and 6 (60%) were female. The mean body mass index was 35 (range, 20–57), and 7 (70%) patients were clinically obese (body mass index >30). Only 2 (20%) patients had a history of a documented drug eruption (hives and erythema multiforme), and no patients had a history of FDE. Erythematous dusky patches followed by rapid development of blisters were noted within 3 days of drug initiation in 40% (4/10) and within 5 days in 80% (8/10) of patients. Antibiotics were identified as likely inciting agents in 8 (80%) patients. Biopsies were obtained in 3 (30%) patients and all 3 demonstrated cytotoxic CD8+ interface dermatitis with marked epithelial necrosis, neutrophilia, eosinophilia, and melanophage accumulation. Fever was present at initial presentation in only 4 (40%) patients, and only 1 (10%) patient had oral mucosal involvement. All 10 patients had intertriginous involvement (axillae, 90% [9/10]; gluteal cleft, 80% [8/10]; groin, 80% [8/10]; inframammary folds, 20% [2/10]), and there was considerable flank involvement in 9 (90%) patients. All 10 patients had initial erythematous to dusky, circular patches on the trunk and proximal extremities that then denuded most dramatically in the intertriginous areas (Figure). Six (60%) patients received systemic therapy, including 5 patients treated with a single dose of etanercept 50 mg. In patients with continued progression, 1 or 2 additional doses of etanercept 50 mg were administered at 48- to 72-hour intervals until blistering halted. Treatment with etanercept resulted in clinical improvement in all 5 patients, and there were no identifiable adverse events. The mean hospital stay was 19.7 days (range, 1–63 days).
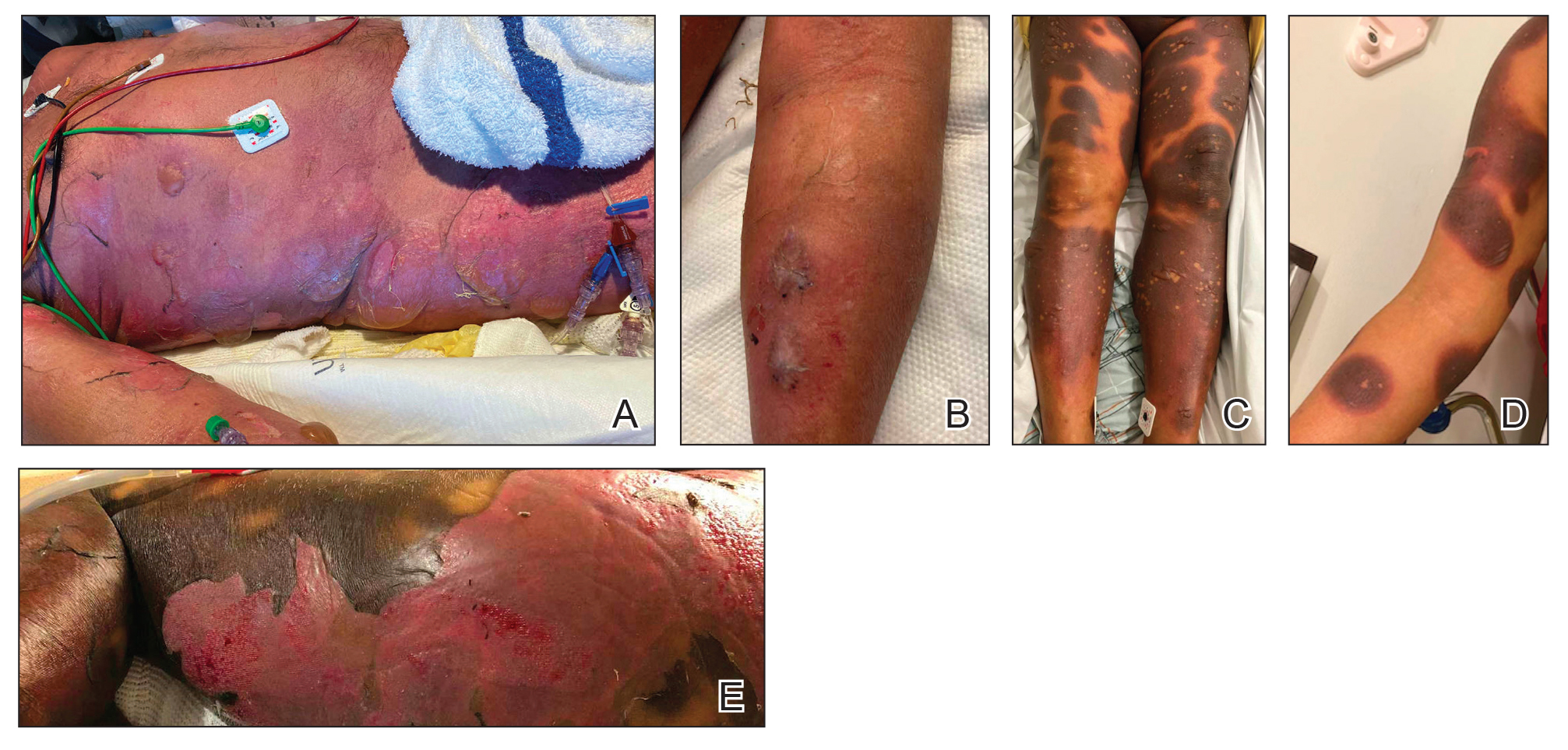
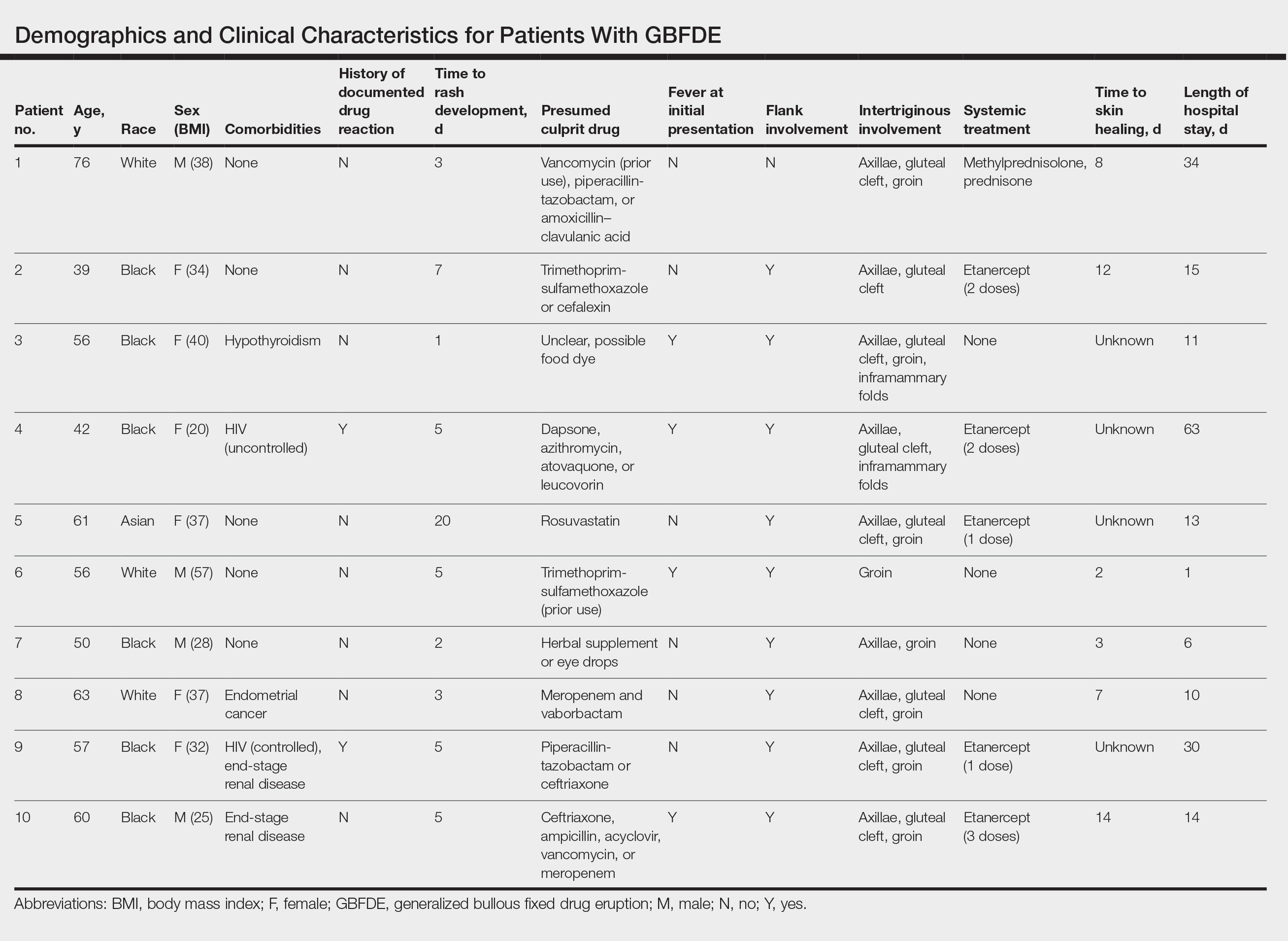
This study highlights notable demographic and clinical features of GBFDE that have not been widely described in the literature. Large erythematous and dusky patches with broad zones of blistering with particular localization to the neck, intertriginous areas, and flanks typically are not described in SJS/TEN and may be helpful in distinguishing these conditions from GBFDE. Mild or complete lack of mucosal and facial involvement as well as more rapid time from drug initiation to rash (as rapid as 1 day) were key factors that aided in distinguishing GBFDE from SJS/TEN in our patients. Although a history of FDE is considered a key characteristic in the diagnosis of GBFDE, none of our patients had a known history of FDE, suggesting GBFDE may be the initial manifestation of FDE in some patients. Histopathology showed similar findings consistent with FDE in the 3 patients in whom a biopsy was performed. The remaining patients were diagnosed clinically based on the presence of distinctive, perfectly circular, dusky plaques present at the periphery of larger denuded areas, which are characteristic of GBFDE. Lower levels of serum granulysin3 have been shown to help distinguish GBFDE from SJS/TEN, but this test is not readily available with time-sensitive results at most institutions, and exact diagnostic ranges for GBFDE vs SJS/TEN are not yet known.
Our study was limited by a small number of patients at a single institution. Another limitation was the retrospective design.
Interestingly, a high proportion of our patients were non-White and clinically obese, which are factors that should be considered for future research. Sixty percent (6/10) of the patients in our study were Black, which is a notable difference from our hospital’s general admission demographics with Black individuals constituting 12% of patients.4 Our study also highlighted the utility of etanercept, which has reported mortality benefits and decreased time to re-epithelialization in other severe blistering cutaneous drug reactions including SJS/TEN,5 as a potential therapeutic option in GBFDE.
It is imperative that clinicians recognize the differences between GBFDE and SJS/TEN, as correct diagnosis is crucial for identifying the most likely causative drug as well as providing accurate prognostic information and may have future therapeutic implications as we further understand the immunologic profiles of these severe blistering drug reactions.
- Patel S, John AM, Handler MZ, et al. Fixed drug eruptions: an update, emphasizing the potentially lethal generalized bullous fixed drug eruption. Am J Clin Dermatol. 2020;21:393-399. doi:10.1007/s40257-020-00505-3
- Anderson HJ, Lee JB. A review of fixed drug eruption with a special focus on generalized bullous fixed drug eruption. Medicina (Kaunas). 2021;57:925. doi:10.3390/medicina57090925
- Cho YT, Lin JW, Chen YC, et al. Generalized bullous fixed drug eruption is distinct from Stevens-Johnson syndrome/toxic epidermal necrolysis by immunohistopathological features. J Am Acad Dermatol. 2014;70:539-548. doi:10.1016/j.jaad.2013.11.015
- Tran T, Shapiro A. New York-Presbyterian 2022 Health Equity Report. New York-Presbyterian; 2023. Accessed July 22, 2024. https://nyp.widen.net/s/jqfbrvrf9p/dalio-center-2022-health-equity-report
- Dreyer SD, Torres J, Stoddard M, et al. Efficacy of etanercept in the treatment of Stevens-Johnson syndrome and toxic epidermal necrolysis. Cutis. 2021;107:E22-E28. doi:10.12788/cutis.0288
To the Editor:
Generalized bullous fixed drug eruption (GBFDE) is a rare subtype of fixed drug eruption (FDE) that manifests as widespread blisters and erosions following exposure to a causative drug.1 Diagnostic criteria include involvement of at least 3 to 6 anatomic sites—head and neck, anterior trunk, posterior trunk, upper extremities, lower extremities, or genitalia—and more than 10% of the body surface area. It can be challenging to differentiate GBFDE from severe drug rashes such as Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN) due to extensive body surface area involvement of blisters and erosions. Specific features distinguishing GBFDE from SJS/TEN include primary lesions consisting of larger erythematous to dusky, circular plaques that progress to bullae and coalesce into widespread erosions; history of FDE; lack of severe mucosal involvement; and better overall prognosis.2 Treatment typically involves discontinuation of the culprit medication and supportive care; evidence for systemic therapies is not well established.
Our study aimed to characterize the clinical and demographic features of GBFDE in our institution to highlight potential key differences between this diagnosis and SJS/TEN. An electronic medical record search was performed to identify patients who were clinically diagnosed with GBFDE at New York-Presbyterian/Weill Cornell Medical Center (New York, New York) in both outpatient and inpatient settings from January 2015 to December 2022. This retrospective study was approved by the Weill Cornell Medicine institutional review board (#22-05024777).
Ten patients were identified and included in the analysis (eTable). The mean age of the patients was 56 years (range, 39–76 years). Seven (70%) patients had skin of color (non-White) and 6 (60%) were female. The mean body mass index was 35 (range, 20–57), and 7 (70%) patients were clinically obese (body mass index >30). Only 2 (20%) patients had a history of a documented drug eruption (hives and erythema multiforme), and no patients had a history of FDE. Erythematous dusky patches followed by rapid development of blisters were noted within 3 days of drug initiation in 40% (4/10) and within 5 days in 80% (8/10) of patients. Antibiotics were identified as likely inciting agents in 8 (80%) patients. Biopsies were obtained in 3 (30%) patients and all 3 demonstrated cytotoxic CD8+ interface dermatitis with marked epithelial necrosis, neutrophilia, eosinophilia, and melanophage accumulation. Fever was present at initial presentation in only 4 (40%) patients, and only 1 (10%) patient had oral mucosal involvement. All 10 patients had intertriginous involvement (axillae, 90% [9/10]; gluteal cleft, 80% [8/10]; groin, 80% [8/10]; inframammary folds, 20% [2/10]), and there was considerable flank involvement in 9 (90%) patients. All 10 patients had initial erythematous to dusky, circular patches on the trunk and proximal extremities that then denuded most dramatically in the intertriginous areas (Figure). Six (60%) patients received systemic therapy, including 5 patients treated with a single dose of etanercept 50 mg. In patients with continued progression, 1 or 2 additional doses of etanercept 50 mg were administered at 48- to 72-hour intervals until blistering halted. Treatment with etanercept resulted in clinical improvement in all 5 patients, and there were no identifiable adverse events. The mean hospital stay was 19.7 days (range, 1–63 days).


This study highlights notable demographic and clinical features of GBFDE that have not been widely described in the literature. Large erythematous and dusky patches with broad zones of blistering with particular localization to the neck, intertriginous areas, and flanks typically are not described in SJS/TEN and may be helpful in distinguishing these conditions from GBFDE. Mild or complete lack of mucosal and facial involvement as well as more rapid time from drug initiation to rash (as rapid as 1 day) were key factors that aided in distinguishing GBFDE from SJS/TEN in our patients. Although a history of FDE is considered a key characteristic in the diagnosis of GBFDE, none of our patients had a known history of FDE, suggesting GBFDE may be the initial manifestation of FDE in some patients. Histopathology showed similar findings consistent with FDE in the 3 patients in whom a biopsy was performed. The remaining patients were diagnosed clinically based on the presence of distinctive, perfectly circular, dusky plaques present at the periphery of larger denuded areas, which are characteristic of GBFDE. Lower levels of serum granulysin3 have been shown to help distinguish GBFDE from SJS/TEN, but this test is not readily available with time-sensitive results at most institutions, and exact diagnostic ranges for GBFDE vs SJS/TEN are not yet known.
Our study was limited by a small number of patients at a single institution. Another limitation was the retrospective design.
Interestingly, a high proportion of our patients were non-White and clinically obese, which are factors that should be considered for future research. Sixty percent (6/10) of the patients in our study were Black, which is a notable difference from our hospital’s general admission demographics with Black individuals constituting 12% of patients.4 Our study also highlighted the utility of etanercept, which has reported mortality benefits and decreased time to re-epithelialization in other severe blistering cutaneous drug reactions including SJS/TEN,5 as a potential therapeutic option in GBFDE.
It is imperative that clinicians recognize the differences between GBFDE and SJS/TEN, as correct diagnosis is crucial for identifying the most likely causative drug as well as providing accurate prognostic information and may have future therapeutic implications as we further understand the immunologic profiles of these severe blistering drug reactions.
To the Editor:
Generalized bullous fixed drug eruption (GBFDE) is a rare subtype of fixed drug eruption (FDE) that manifests as widespread blisters and erosions following exposure to a causative drug.1 Diagnostic criteria include involvement of at least 3 to 6 anatomic sites—head and neck, anterior trunk, posterior trunk, upper extremities, lower extremities, or genitalia—and more than 10% of the body surface area. It can be challenging to differentiate GBFDE from severe drug rashes such as Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN) due to extensive body surface area involvement of blisters and erosions. Specific features distinguishing GBFDE from SJS/TEN include primary lesions consisting of larger erythematous to dusky, circular plaques that progress to bullae and coalesce into widespread erosions; history of FDE; lack of severe mucosal involvement; and better overall prognosis.2 Treatment typically involves discontinuation of the culprit medication and supportive care; evidence for systemic therapies is not well established.
Our study aimed to characterize the clinical and demographic features of GBFDE in our institution to highlight potential key differences between this diagnosis and SJS/TEN. An electronic medical record search was performed to identify patients who were clinically diagnosed with GBFDE at New York-Presbyterian/Weill Cornell Medical Center (New York, New York) in both outpatient and inpatient settings from January 2015 to December 2022. This retrospective study was approved by the Weill Cornell Medicine institutional review board (#22-05024777).
Ten patients were identified and included in the analysis (eTable). The mean age of the patients was 56 years (range, 39–76 years). Seven (70%) patients had skin of color (non-White) and 6 (60%) were female. The mean body mass index was 35 (range, 20–57), and 7 (70%) patients were clinically obese (body mass index >30). Only 2 (20%) patients had a history of a documented drug eruption (hives and erythema multiforme), and no patients had a history of FDE. Erythematous dusky patches followed by rapid development of blisters were noted within 3 days of drug initiation in 40% (4/10) and within 5 days in 80% (8/10) of patients. Antibiotics were identified as likely inciting agents in 8 (80%) patients. Biopsies were obtained in 3 (30%) patients and all 3 demonstrated cytotoxic CD8+ interface dermatitis with marked epithelial necrosis, neutrophilia, eosinophilia, and melanophage accumulation. Fever was present at initial presentation in only 4 (40%) patients, and only 1 (10%) patient had oral mucosal involvement. All 10 patients had intertriginous involvement (axillae, 90% [9/10]; gluteal cleft, 80% [8/10]; groin, 80% [8/10]; inframammary folds, 20% [2/10]), and there was considerable flank involvement in 9 (90%) patients. All 10 patients had initial erythematous to dusky, circular patches on the trunk and proximal extremities that then denuded most dramatically in the intertriginous areas (Figure). Six (60%) patients received systemic therapy, including 5 patients treated with a single dose of etanercept 50 mg. In patients with continued progression, 1 or 2 additional doses of etanercept 50 mg were administered at 48- to 72-hour intervals until blistering halted. Treatment with etanercept resulted in clinical improvement in all 5 patients, and there were no identifiable adverse events. The mean hospital stay was 19.7 days (range, 1–63 days).


This study highlights notable demographic and clinical features of GBFDE that have not been widely described in the literature. Large erythematous and dusky patches with broad zones of blistering with particular localization to the neck, intertriginous areas, and flanks typically are not described in SJS/TEN and may be helpful in distinguishing these conditions from GBFDE. Mild or complete lack of mucosal and facial involvement as well as more rapid time from drug initiation to rash (as rapid as 1 day) were key factors that aided in distinguishing GBFDE from SJS/TEN in our patients. Although a history of FDE is considered a key characteristic in the diagnosis of GBFDE, none of our patients had a known history of FDE, suggesting GBFDE may be the initial manifestation of FDE in some patients. Histopathology showed similar findings consistent with FDE in the 3 patients in whom a biopsy was performed. The remaining patients were diagnosed clinically based on the presence of distinctive, perfectly circular, dusky plaques present at the periphery of larger denuded areas, which are characteristic of GBFDE. Lower levels of serum granulysin3 have been shown to help distinguish GBFDE from SJS/TEN, but this test is not readily available with time-sensitive results at most institutions, and exact diagnostic ranges for GBFDE vs SJS/TEN are not yet known.
Our study was limited by a small number of patients at a single institution. Another limitation was the retrospective design.
Interestingly, a high proportion of our patients were non-White and clinically obese, which are factors that should be considered for future research. Sixty percent (6/10) of the patients in our study were Black, which is a notable difference from our hospital’s general admission demographics with Black individuals constituting 12% of patients.4 Our study also highlighted the utility of etanercept, which has reported mortality benefits and decreased time to re-epithelialization in other severe blistering cutaneous drug reactions including SJS/TEN,5 as a potential therapeutic option in GBFDE.
It is imperative that clinicians recognize the differences between GBFDE and SJS/TEN, as correct diagnosis is crucial for identifying the most likely causative drug as well as providing accurate prognostic information and may have future therapeutic implications as we further understand the immunologic profiles of these severe blistering drug reactions.
- Patel S, John AM, Handler MZ, et al. Fixed drug eruptions: an update, emphasizing the potentially lethal generalized bullous fixed drug eruption. Am J Clin Dermatol. 2020;21:393-399. doi:10.1007/s40257-020-00505-3
- Anderson HJ, Lee JB. A review of fixed drug eruption with a special focus on generalized bullous fixed drug eruption. Medicina (Kaunas). 2021;57:925. doi:10.3390/medicina57090925
- Cho YT, Lin JW, Chen YC, et al. Generalized bullous fixed drug eruption is distinct from Stevens-Johnson syndrome/toxic epidermal necrolysis by immunohistopathological features. J Am Acad Dermatol. 2014;70:539-548. doi:10.1016/j.jaad.2013.11.015
- Tran T, Shapiro A. New York-Presbyterian 2022 Health Equity Report. New York-Presbyterian; 2023. Accessed July 22, 2024. https://nyp.widen.net/s/jqfbrvrf9p/dalio-center-2022-health-equity-report
- Dreyer SD, Torres J, Stoddard M, et al. Efficacy of etanercept in the treatment of Stevens-Johnson syndrome and toxic epidermal necrolysis. Cutis. 2021;107:E22-E28. doi:10.12788/cutis.0288
- Patel S, John AM, Handler MZ, et al. Fixed drug eruptions: an update, emphasizing the potentially lethal generalized bullous fixed drug eruption. Am J Clin Dermatol. 2020;21:393-399. doi:10.1007/s40257-020-00505-3
- Anderson HJ, Lee JB. A review of fixed drug eruption with a special focus on generalized bullous fixed drug eruption. Medicina (Kaunas). 2021;57:925. doi:10.3390/medicina57090925
- Cho YT, Lin JW, Chen YC, et al. Generalized bullous fixed drug eruption is distinct from Stevens-Johnson syndrome/toxic epidermal necrolysis by immunohistopathological features. J Am Acad Dermatol. 2014;70:539-548. doi:10.1016/j.jaad.2013.11.015
- Tran T, Shapiro A. New York-Presbyterian 2022 Health Equity Report. New York-Presbyterian; 2023. Accessed July 22, 2024. https://nyp.widen.net/s/jqfbrvrf9p/dalio-center-2022-health-equity-report
- Dreyer SD, Torres J, Stoddard M, et al. Efficacy of etanercept in the treatment of Stevens-Johnson syndrome and toxic epidermal necrolysis. Cutis. 2021;107:E22-E28. doi:10.12788/cutis.0288
PRACTICE POINTS
- Distinguishing features of generalized bullous fixed
drug eruption (GBFDE) may include truncal and proximal predilection with early intertriginous blistering. - Etanercept is a viable treatment option for GBFDE.
Vulvar Inflammatory Dermatoses: New Approaches for Diagnosis and Treatment
Vulvar dermatoses continue to be an overlooked aspect of medical care, highlighting the necessity for enhanced diagnosis and management of these conditions. Here, we address recent advancements in understanding vulvar inflammatory dermatoses other than lichen sclerosus (LS), which was discussed in a prior Guest Editorial1—specifically vulvovaginal lichen planus (VLP), plasma cell vulvitis (PCV), and vulvar lichen simplex chronicus (LSC).
Vulvar Inflammatory Skin Disease and Quality of Life
There is an increased awareness of the impact vulvar skin disease has on quality of life and its association with anxiety and depression.2-5 Evaluating the burden of vulvar dermatoses remains an active area of research due to its significance in monitoring disease progression and assessing therapeutic effectiveness. Despite the existence of various dermatology quality-of-life assessment tools, many fail to adequately capture the unique impacts of vulvovaginal diseases, such as sexual or urinary dysfunction. The vulvar quality of life index, which was developed and validated by Saunderson et al6 in 2020, consists of a 15-item questionnaire spanning 4 domains: symptoms, anxiety, activities of daily living, and sexuality. This tool has been utilized to gauge treatment response in vulvar conditions and to compare disease burden of various vulvar dermatoses.7,8 Moving forward, integrating this tool into clinical studies on vulvar skin disease holds promise for enhancing our understanding and management of these conditions.
Vulvovaginal Lichen Planus
Vulvovaginal lichen planus is unique among several prevalent vulvar inflammatory skin disorders encountered by dermatologists—primarily due to its erosive form, which can extend to the vagina, resulting in noninfectious vaginitis and potential vaginal stenosis.9,10 Managing VLP poses a notable challenge, even when it is confined to the vulva, as it often proves resistant to topical therapies.11
Evaluation for Vaginal Mucosal Disease—In contrast to LS, which typically spares the vaginal mucosa, VLP can involve mucosal sites.9,12,13 Therefore, it is imperative that all patients with a diagnosis of vulvar VLP undergo evaluation for potential vaginal involvement through speculum examination, wet mount, or vaginal biopsy. Strategies to manage vaginal involvement include use of dilators and pelvic floor physical therapy, lysis of adhesions (if present), topical estrogen, and intravaginal corticosteroids—all tailored to the severity of the disease.9,11,14
Management of VLP—Approximately 20% to 40% of patients with VLP may require systemic therapy for disease management, including those who are younger, those of non-White ethnicity, and those presenting with vulvar pruritus.11 Various systemic immunosuppressants have been used for VLP, with a recent retrospective study revealing similar response rates for both methotrexate and mycophenolate mofetil in the treatment of VLP.15 Another retrospective study found hydroxychloroquine to be safe and effective for VLP but noted a slow onset of action, with approximately 70% responding at 9 months following initiation of therapy.16
Recent attention has shifted to use of targeted therapies for VLP. For instance, apremilast has shown efficacy in a single-center, nonrandomized, open-label pilot study.17 Tildrakizumab, an IL-23 inhibitor, demonstrated efficacy in a case series involving 24 patients with VLP.18 Moreover, recent case reports and series have highlighted the potential of oral Janus kinase (JAK) inhibitors, such as tofacitinib, in VLP treatment.19 Clinical trials are ongoing to evaluate the safety and efficacy of topical ruxolitinib and deucravacitinib (a tyrosine kinase 2 inhibitor) in VLP.20-22 Systemic therapies for VLP currently are used off label, emphasizing the need for future randomized controlled trials to ascertain the optimal therapies for patients affected by erosive and nonerosive forms of this disease.
Plasma Cell Vulvitis
Plasma cell vulvitis is a chronic inflammatory disorder with an unknown etiology that some consider to be a variant of VLP.23 Others have observed an overlap with desquamative inflammatory vaginitis, categorizing PCV as a hemorrhagic vestibulovaginitis.24 Although its classification as a distinct entity remains under scrutiny, studies indicate a predilection for the nonkeratinized or partially keratinized vulva. A systematic review outlining common clinical findings reported that the most common anatomic sites included the vulvar vestibule, periurethral area, and labia minora.23 Additionally, reports have emphasized the association between PCV and other inflammatory vulvar skin conditions, including LS.25
Clinical Variants of PCV—A retrospective review proposed 2 clinical phenotypes for PCV: (1) primary non–lichen-associated PCV and (2) secondary lichen-associated PCV, which is linked to LS.26 The primary form is reported to be restricted to the vestibule, and the authors considered this a vulvar counterpart of atrophic vaginitis due to estrogen deficiency (now known as postmenopausal genitourinary syndrome). The secondary phenotype more commonly involved the vestibular and extravestibular epithelium.26
Management of PCV—Recognizing PCV in the context of LS may be important for identifying comorbid conditions and guiding treatment. However, evidence-based guidelines for PCV treatment are lacking. Commonly reported treatment modalities include clobetasol ointment 0.05% and tacrolimus ointment 0.1%.23 Successful treatment with hydrocortisone suppositories alternating with estradiol vaginal cream was reported in a recent case series.27 Crisaborole also has been reported as a treatment in 1 case of PCV.28 A recent case report found abrocitinib to be effective for the treatment of plasma cell balanitis in the setting of male genital LS,29 but there are limited data on the use of JAK inhibitors for PCV. Further research is necessary to ascertain the incidence, prevalence, clinical subtypes, and optimal management strategies for PCV to effectively treat patients with this condition.
Vulvar LSC
Similar to extragenital LSC, the evaluation of vulvar LSC should prioritize identification of underlying etiologies that contribute to the itch-scratch cycle, which may include psoriasis, atopic dermatitis, neurologic conditions, and allergic or irritant contact dermatitis.30,31 Although treatment strategies may vary based on underlying conditions, we will concentrate on updates in managing vulvar LSC and pruritus associated with an atopic diathesis or resulting from chronic contact dermatitis, which is prevalent in vulvar skin areas. Finally, we highlight some emerging vulvar allergens for consideration in clinical practice.
Management of Vulvar LSC—The advent of targeted therapies, including biologics and small-molecule inhibitors, for atopic dermatitis and prurigo nodularis in recent years presents potential options for treatment of individuals with vulvar LSC. However, studies on the use of these therapies specifically for vulvar LSC are limited, necessitating thorough discussions with patients. Given the debilitating nature of vulvar pruritus that may be seen in vulvar LSC and the potential inadequacy of topical steroids as monotherapy, systemic therapies may serve as alternative options for patients with refractory disease.30
Dupilumab, a dual inhibitor of IL-4 and IL-13 signaling, has shown rapid and sustained disease improvement in patients with atopic dermatitis, prurigo nodularis, and pruritus.32,33 Although data on its role in managing vulvar LSC are scarce, a recent case series reported improvement of vulvar pruritus with dupilumab.34 Similarly, tralokinumab, an IL-13 inhibitor approved by the US Food and Drug Administration (FDA) for atopic dermatitis, has shown efficacy in prurigo nodularis35 and may benefit patients with vulvar LSC, though studies on cutaneous outcomes in those with genital involvement specifically are lacking. Oral JAK inhibitors such as upadacitinib and abrocitinib—both FDA approved for atopic dermatitis—have demonstrated efficacy in treating LSC and itch, potentially serving as management options for vulvar LSC in cases resistant to topical steroids or in which steroid atrophy or other steroid adverse effects may preclude continued use of such agents.36,37 Finally, IL-31 inhibitors such as nemolizumab, which reduced the signs and symptoms of prurigo nodularis in a recent phase 3 clinical trial, may hold utility in addressing vulvar LSC and associated pruritus.38
The topical JAK inhibitor ruxolitinib, which is FDA approved for atopic dermatitis and vitiligo, holds promise for managing LSC on vulvar skin while mitigating the risk for steroid-induced atrophy.39 Additionally, nonsteroidal topicals including roflumilast cream 0.3% and tapinarof cream 1%, both FDA approved for psoriasis, are being evaluated in studies for their safety and efficacy in atopic dermatitis.40,41 These agents may have the potential to improve signs and symptoms of vulvar LSC, but further studies are necessary.
Vulvar Allergens and LSC—When assessing patients with vulvar LSC, it is crucial to recognize that allergic contact dermatitis is a common primary vulvar dermatosis but can coexist with other vulvar dermatoses such as LS.13,30 The vulvar skin’s susceptibly to allergic contact dermatitis is attributed to factors such as a higher ratio of antigen-presenting cells in the vulvar skin, the nonkeratinized nature of certain sites, and frequent contact with potential allergens.42,43 Therefore, incorporating patch testing into the diagnostic process should be considered when evaluating patients with vulvar skin conditions.43
A systemic review identified multiple vulvar allergens, including metals, topical medicaments, fragrances, preservatives, cosmetic constituents, and rubber components that led to contact dermatitis.44 Moreover, a recent analysis of topical preparations recommended by women with LS on social media found a high prevalence of known vulvar allergens in these agents, including botanical extracts/spices.45 Personal-care wipes marketed for vulvar care and hygiene are known to contain a variety of allergens, with a recent study finding numerous allergens in commercially available wipes including fragrances, scented botanicals in the form of essences, oils, fruit juices, and vitamin E.46 These findings underscore the importance of considering potential allergens when caring for patients with vulvar LSC and counseling patients about the potential allergens in many commercially available products that may be recommended on social media sites or by other sources.
Final Thoughts
Vulvar inflammatory dermatoses are becoming increasingly recognized, and there is a need to develop more effective diagnostic and treatment approaches. Recent literature has shed light on some of the challenges in the management of VLP, particularly its resistance to topical therapies and the importance of assessing and managing both cutaneous and vaginal involvement. Efforts have been made to refine the classification of PCV, with studies suggesting a variant that coexists with LS. Although evidence for vulvar-specific treatment of LSC is limited, the emergence of biologics and small-molecule inhibitors that are FDA approved for atopic dermatitis and prurigo nodularis offer promise for certain cases of vulvar LSC and vulvar pruritus. Moreover, recent developments in steroid-sparing topical agents warrant further investigation for their potential efficacy in treating vulvar LSC and possibly other vulvar inflammatory conditions in the future.
- Nguyen B, Kraus C. Vulvar lichen sclerosus: what’s new? Cutis. 2024;113:104-106. doi:10.12788/cutis.0967
- Van De Nieuwenhof HP, Meeuwis KAP, Nieboer TE, et al. The effect of vulvar lichen sclerosus on quality of life and sexual functioning. J Psychosom Obstet Gynaecol. 2010;31:279-284. doi:10.3109/0167482X.2010.507890
- Ranum A, Pearson DR. The impact of genital lichen sclerosus and lichen planus on quality of life: a review. Int J Womens Dermatol. 2022;8:E042. doi:10.1097/JW9.0000000000000042
- Messele F, Hinchee-Rodriguez K, Kraus CN. Vulvar dermatoses and depression: a systematic review of vulvar lichen sclerosus, lichen planus, and lichen simplex chronicus. JAAD Int. 2024;15:15-20. doi:10.1016/j.jdin.2023.10.009
- Choi UE, Nicholson RC, Agrawal P, et al. Involvement of vulva in lichen sclerosus increases the risk of antidepressant and benzodiazepine prescriptions for psychiatric disorder diagnoses. Int J Impot Res. Published online November 16, 2023. doi:10.1038/s41443-023-00793-3
- Saunderson R, Harris V, Yeh R, et al. Vulvar quality of life index (VQLI)—a simple tool to measure quality of life in patients with vulvar disease. Australas J Dermatol. 2020;61:152-157. doi:10.1111/ajd.13235
- Wu M, Kherlopian A, Wijaya M, et al. Quality of life impact and treatment response in vulval disease: comparison of 3 common conditions using the Vulval Quality of Life Index. Australas J Dermatol. 2022;63:E320-E328. doi:10.1111/ajd.13898
- Kherlopian A, Fischer G. Comparing quality of life in women with vulvovaginal lichen planus treated with topical and systemic treatments using the vulvar quality of life index. Australas J Dermatol. 2023;64:E125-E134. doi:10.1111/ajd.14032
- Cooper SM, Haefner HK, Abrahams-Gessel S, et al. Vulvovaginal lichen planus treatment: a survey of current practices. Arch Dermatol. 2008;144:1520-1521. doi:10.1001/archderm.144.11.1520
- Chow MR, Gill N, Alzahrani F, et al. Vulvar lichen planus–induced vulvovaginal stenosis: a case report and review of the literature. SAGE Open Med Case Rep. 2023;11:2050313X231164216. doi:10.1177/2050313X231164216
- Kherlopian A, Fischer G. Identifying predictors of systemic immunosuppressive treatment of vulvovaginal lichen planus: a retrospective cohort study of 122 women. Australas J Dermatol. 2022;63:335-343. doi:10.1111/ajd.13851
- Dunaway S, Tyler K, Kaffenberger, J. Update on treatments for erosive vulvovaginal lichen planus. Int J Dermatol. 2020;59:297-302. doi:10.1111/ijd.14692
- Mauskar MM, Marathe, K, Venkatesan A, et al. Vulvar diseases: conditions in adults and children. J Am Acad Dermatol. 2020;82:1287-1298. doi:10.1016/j.jaad.2019.10.077
- Hinchee-Rodriguez K, Duong A, Kraus CN. Local management strategies for inflammatory vaginitis in dermatologic conditions: suppositories, dilators, and estrogen replacement. JAAD Int. 2022;9:137-138. doi:10.1016/j.jdin.2022.09.004
- Hrin ML, Bowers NL, Feldman SR, et al. Mycophenolate mofetil versus methotrexate for vulvar lichen planus: a 10-year retrospective cohort study demonstrates comparable efficacy and tolerability. J Am Acad Dermatol. 2022;87:436-438. doi:10.1016/j.jaad.2021.08.061
- Vermeer HAB, Rashid H, Esajas MD, et al. The use of hydroxychloroquine as a systemic treatment in erosive lichen planus of the vulva and vagina. Br J Dermatol. 2021;185:201-203. doi:10.1111/bjd.19870
- Skullerud KH, Gjersvik P, Pripp AH, et al. Apremilast for genital erosive lichen planus in women (the AP-GELP Study): study protocol for a randomised placebo-controlled clinical trial. Trials. 2021;22:469. doi:10.1186/s13063-021-05428-w
- Kherlopian A, Fischer G. Successful treatment of vulvovaginal lichen planus with tildrakizumab: a case series of 24 patients. Australas J Dermatol. 2022;63:251-255. doi:10.1111/ajd.13793
- Kassels A, Edwards L, Kraus CN. Treatment of erosive vulvovaginal lichen planus with tofacitinib: a case series. JAAD Case Rep. 2023;40:14-18. doi:10.1016/j.jdcr.2023.08.001
- Wijaya M, Fischer G, Saunderson RB. The efficacy and safety of deucravacitinib compared to methotrexate, in patients with vulvar lichen planus who have failed topical therapy with potent corticosteroids: a study protocol for a single-centre double-blinded randomised controlled trial. Trials. 2024;25:181. doi:10.1186/s13063-024-08022-y
- Brumfiel CM, Patel MH, Severson KJ, et al. Ruxolitinib cream in the treatment of cutaneous lichen planus: a prospective, open-label study. J Invest Dermatol. 2022;142:2109-2116.e4. doi:10.1016/j.jid.2022.01.015
- A study to evaluate the efficacy and safety of ruxolitinib cream in participants with cutaneous lichen planus. ClinicalTrials.gov identifier: NCT05593432. Updated March 12, 2024. Accessed July 12, 2024. https://clinicaltrials.gov/study/NCT05593432
- Sattler S, Elsensohn AN, Mauskar MM, et al. Plasma cell vulvitis: a systematic review. Int J Womens Dermatol. 2021;7:756-762. doi:10.1016/j.ijwd.2021.04.005
- Song M, Day T, Kliman L, et al. Desquamative inflammatory vaginitis and plasma cell vulvitis represent a spectrum of hemorrhagic vestibulovaginitis. J Low Genit Tract Dis. 2022;26:60-67. doi:10.1097/LGT.0000000000000637
- Saeed L, Lee BA, Kraus CN. Tender solitary lesion in vulvar lichen sclerosus. JAAD Case Rep. 2022;23:61-63. doi:10.1016/j.jdcr.2022.01.038
- Wendling J, Plantier F, Moyal-Barracco M. Plasma cell vulvitis: a classification into two clinical phenotypes. J Low Genit Tract Dis. 2023;27:384-389. doi:10.1097/LGT.0000000000000771
- Prestwood CA, Granberry R, Rutherford A, et al. Successful treatment of plasma cell vulvitis: a case series. JAAD Case Rep. 2022;19:37-40. doi:10.1016/j.jdcr.2021.10.023
- He Y, Xu M, Wu M, et al. A case of plasma cell vulvitis successfully treated with crisaborole. J Dermatol. Published online April 1, 2024. doi:10.1111/1346-8138.17205
- Xiong X, Chen R, Wang L, et al. Treatment of plasma cell balanitis associated with male genital lichen sclerosus using abrocitinib. JAAD Case Rep. 2024;46:85-88. doi:10.1016/j.jdcr.2024.02.010
- Stewart KMA. Clinical care of vulvar pruritus, with emphasis on one common cause, lichen simplex chronicus. Dermatol Clin. 2010;28:669-680. doi:10.1016/j.det.2010.08.004
- Rimoin LP, Kwatra SG, Yosipovitch G. Female-specific pruritus from childhood to postmenopause: clinical features, hormonal factors, and treatment considerations. Dermatol Ther. 2013;26:157-167. doi:10.1111/dth.12034
- Simpson EL, Bieber T, Guttman-Yassky E, et al; SOLO 1 and SOLO 2 Investigators. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375:2335-2348. doi:10.1056/NEJMoa1610020
- Yosipovitch G, Mollanazar N, Ständer S, et al. Dupilumab in patients with prurigo nodularis: two randomized, double-blind, placebo-controlled phase 3 trials. Nat Med. 2023;29:1180-1190. doi:10.1038/s41591-023-02320-9
- Gosch M, Cash S, Pichardo R. Vulvar pruritus improved with dupilumab. JSM Sexual Med. 2023;7:1104.
- Pezzolo E, Gambardella A, Guanti M, et al. Tralokinumab shows clinical improvement in patients with prurigo nodularis-like phenotype atopic dermatitis: a multicenter, prospective, open-label case series study. J Am Acad Dermatol. 2023;89:430-432. doi:10.1016/j.jaad.2023.04.056
- Simpson EL, Sinclair R, Forman S, et al. Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE MONO-1): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet. 2020;396:255-266. doi:10.1016/S0140-6736(20)30732-7
- Simpson EL, Papp KA, Blauvelt A, et al. Efficacy and safety of upadacitinib in patients with moderate to severe atopic dermatitis: analysis of follow-up data from the Measure Up 1 and Measure Up 2 randomized clinical trials. JAMA Dermatol. 2022;158:404-413. doi:10.1001/jamadermatol.2022.0029
- Kwatra SG, Yosipovitch G, Legat FJ, et al. Phase 3 trial of nemolizumab in patients with prurigo nodularis. N Engl J Med. 2023;389:1579-1589. doi:10.1056/NEJMoa2301333
- Papp K, Szepietowski JC, Kircik L, et al. Long-term safety and disease control with ruxolitinib cream in atopic dermatitis: results from two phase 3 studies. J Am Acad Dermatol. 2023;88:1008-1016. doi:10.1016/j.jaad.2022.09.060
- Lebwohl MG, Kircik LH, Moore AY, et al. Effect of roflumilast cream vs vehicle cream on chronic plaque psoriasis: the DERMIS-1 and DERMIS-2 randomized clinical trials. JAMA. 2022;328:1073-1084. doi:10.1001/jama.2022.15632
- Lebwohl MG, Gold LS, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229. doi:10.1056/NEJMoa2103629
- O’Gorman SM, Torgerson RR. Allergic contact dermatitis of the vulva. Dermatitis. 2013;24:64-72. doi:10.1097/DER.0b013e318284da33
- Woodruff CM, Trivedi MK, Botto N, et al. Allergic contact dermatitis of the vulva. Dermatitis. 2018;29:233-243. doi:10.1097/DER.0000000000000339
- Vandeweege S, Debaene B, Lapeere H, et al. A systematic review of allergic and irritant contact dermatitis of the vulva: the most important allergens/irritants and the role of patch testing. Contact Dermatitis. 2023;88:249-262. doi:10.1111/cod.14258
- Luu Y, Admani S. Vulvar allergens in topical preparations recommended on social media: a cross-sectional analysis of Facebook groups for lichen sclerosus. Int J Womens Dermatol. 2023;9:E097. doi:10.1097/JW9.0000000000000097
- Newton J, Richardson S, van Oosbre AM, et al. A cross-sectional study of contact allergens in feminine hygiene wipes: a possible cause of vulvar contact dermatitis. Int J Womens Dermatol. 2022;8:E060. doi:10.1097/JW9.0000000000000060
Vulvar dermatoses continue to be an overlooked aspect of medical care, highlighting the necessity for enhanced diagnosis and management of these conditions. Here, we address recent advancements in understanding vulvar inflammatory dermatoses other than lichen sclerosus (LS), which was discussed in a prior Guest Editorial1—specifically vulvovaginal lichen planus (VLP), plasma cell vulvitis (PCV), and vulvar lichen simplex chronicus (LSC).
Vulvar Inflammatory Skin Disease and Quality of Life
There is an increased awareness of the impact vulvar skin disease has on quality of life and its association with anxiety and depression.2-5 Evaluating the burden of vulvar dermatoses remains an active area of research due to its significance in monitoring disease progression and assessing therapeutic effectiveness. Despite the existence of various dermatology quality-of-life assessment tools, many fail to adequately capture the unique impacts of vulvovaginal diseases, such as sexual or urinary dysfunction. The vulvar quality of life index, which was developed and validated by Saunderson et al6 in 2020, consists of a 15-item questionnaire spanning 4 domains: symptoms, anxiety, activities of daily living, and sexuality. This tool has been utilized to gauge treatment response in vulvar conditions and to compare disease burden of various vulvar dermatoses.7,8 Moving forward, integrating this tool into clinical studies on vulvar skin disease holds promise for enhancing our understanding and management of these conditions.
Vulvovaginal Lichen Planus
Vulvovaginal lichen planus is unique among several prevalent vulvar inflammatory skin disorders encountered by dermatologists—primarily due to its erosive form, which can extend to the vagina, resulting in noninfectious vaginitis and potential vaginal stenosis.9,10 Managing VLP poses a notable challenge, even when it is confined to the vulva, as it often proves resistant to topical therapies.11
Evaluation for Vaginal Mucosal Disease—In contrast to LS, which typically spares the vaginal mucosa, VLP can involve mucosal sites.9,12,13 Therefore, it is imperative that all patients with a diagnosis of vulvar VLP undergo evaluation for potential vaginal involvement through speculum examination, wet mount, or vaginal biopsy. Strategies to manage vaginal involvement include use of dilators and pelvic floor physical therapy, lysis of adhesions (if present), topical estrogen, and intravaginal corticosteroids—all tailored to the severity of the disease.9,11,14
Management of VLP—Approximately 20% to 40% of patients with VLP may require systemic therapy for disease management, including those who are younger, those of non-White ethnicity, and those presenting with vulvar pruritus.11 Various systemic immunosuppressants have been used for VLP, with a recent retrospective study revealing similar response rates for both methotrexate and mycophenolate mofetil in the treatment of VLP.15 Another retrospective study found hydroxychloroquine to be safe and effective for VLP but noted a slow onset of action, with approximately 70% responding at 9 months following initiation of therapy.16
Recent attention has shifted to use of targeted therapies for VLP. For instance, apremilast has shown efficacy in a single-center, nonrandomized, open-label pilot study.17 Tildrakizumab, an IL-23 inhibitor, demonstrated efficacy in a case series involving 24 patients with VLP.18 Moreover, recent case reports and series have highlighted the potential of oral Janus kinase (JAK) inhibitors, such as tofacitinib, in VLP treatment.19 Clinical trials are ongoing to evaluate the safety and efficacy of topical ruxolitinib and deucravacitinib (a tyrosine kinase 2 inhibitor) in VLP.20-22 Systemic therapies for VLP currently are used off label, emphasizing the need for future randomized controlled trials to ascertain the optimal therapies for patients affected by erosive and nonerosive forms of this disease.
Plasma Cell Vulvitis
Plasma cell vulvitis is a chronic inflammatory disorder with an unknown etiology that some consider to be a variant of VLP.23 Others have observed an overlap with desquamative inflammatory vaginitis, categorizing PCV as a hemorrhagic vestibulovaginitis.24 Although its classification as a distinct entity remains under scrutiny, studies indicate a predilection for the nonkeratinized or partially keratinized vulva. A systematic review outlining common clinical findings reported that the most common anatomic sites included the vulvar vestibule, periurethral area, and labia minora.23 Additionally, reports have emphasized the association between PCV and other inflammatory vulvar skin conditions, including LS.25
Clinical Variants of PCV—A retrospective review proposed 2 clinical phenotypes for PCV: (1) primary non–lichen-associated PCV and (2) secondary lichen-associated PCV, which is linked to LS.26 The primary form is reported to be restricted to the vestibule, and the authors considered this a vulvar counterpart of atrophic vaginitis due to estrogen deficiency (now known as postmenopausal genitourinary syndrome). The secondary phenotype more commonly involved the vestibular and extravestibular epithelium.26
Management of PCV—Recognizing PCV in the context of LS may be important for identifying comorbid conditions and guiding treatment. However, evidence-based guidelines for PCV treatment are lacking. Commonly reported treatment modalities include clobetasol ointment 0.05% and tacrolimus ointment 0.1%.23 Successful treatment with hydrocortisone suppositories alternating with estradiol vaginal cream was reported in a recent case series.27 Crisaborole also has been reported as a treatment in 1 case of PCV.28 A recent case report found abrocitinib to be effective for the treatment of plasma cell balanitis in the setting of male genital LS,29 but there are limited data on the use of JAK inhibitors for PCV. Further research is necessary to ascertain the incidence, prevalence, clinical subtypes, and optimal management strategies for PCV to effectively treat patients with this condition.
Vulvar LSC
Similar to extragenital LSC, the evaluation of vulvar LSC should prioritize identification of underlying etiologies that contribute to the itch-scratch cycle, which may include psoriasis, atopic dermatitis, neurologic conditions, and allergic or irritant contact dermatitis.30,31 Although treatment strategies may vary based on underlying conditions, we will concentrate on updates in managing vulvar LSC and pruritus associated with an atopic diathesis or resulting from chronic contact dermatitis, which is prevalent in vulvar skin areas. Finally, we highlight some emerging vulvar allergens for consideration in clinical practice.
Management of Vulvar LSC—The advent of targeted therapies, including biologics and small-molecule inhibitors, for atopic dermatitis and prurigo nodularis in recent years presents potential options for treatment of individuals with vulvar LSC. However, studies on the use of these therapies specifically for vulvar LSC are limited, necessitating thorough discussions with patients. Given the debilitating nature of vulvar pruritus that may be seen in vulvar LSC and the potential inadequacy of topical steroids as monotherapy, systemic therapies may serve as alternative options for patients with refractory disease.30
Dupilumab, a dual inhibitor of IL-4 and IL-13 signaling, has shown rapid and sustained disease improvement in patients with atopic dermatitis, prurigo nodularis, and pruritus.32,33 Although data on its role in managing vulvar LSC are scarce, a recent case series reported improvement of vulvar pruritus with dupilumab.34 Similarly, tralokinumab, an IL-13 inhibitor approved by the US Food and Drug Administration (FDA) for atopic dermatitis, has shown efficacy in prurigo nodularis35 and may benefit patients with vulvar LSC, though studies on cutaneous outcomes in those with genital involvement specifically are lacking. Oral JAK inhibitors such as upadacitinib and abrocitinib—both FDA approved for atopic dermatitis—have demonstrated efficacy in treating LSC and itch, potentially serving as management options for vulvar LSC in cases resistant to topical steroids or in which steroid atrophy or other steroid adverse effects may preclude continued use of such agents.36,37 Finally, IL-31 inhibitors such as nemolizumab, which reduced the signs and symptoms of prurigo nodularis in a recent phase 3 clinical trial, may hold utility in addressing vulvar LSC and associated pruritus.38
The topical JAK inhibitor ruxolitinib, which is FDA approved for atopic dermatitis and vitiligo, holds promise for managing LSC on vulvar skin while mitigating the risk for steroid-induced atrophy.39 Additionally, nonsteroidal topicals including roflumilast cream 0.3% and tapinarof cream 1%, both FDA approved for psoriasis, are being evaluated in studies for their safety and efficacy in atopic dermatitis.40,41 These agents may have the potential to improve signs and symptoms of vulvar LSC, but further studies are necessary.
Vulvar Allergens and LSC—When assessing patients with vulvar LSC, it is crucial to recognize that allergic contact dermatitis is a common primary vulvar dermatosis but can coexist with other vulvar dermatoses such as LS.13,30 The vulvar skin’s susceptibly to allergic contact dermatitis is attributed to factors such as a higher ratio of antigen-presenting cells in the vulvar skin, the nonkeratinized nature of certain sites, and frequent contact with potential allergens.42,43 Therefore, incorporating patch testing into the diagnostic process should be considered when evaluating patients with vulvar skin conditions.43
A systemic review identified multiple vulvar allergens, including metals, topical medicaments, fragrances, preservatives, cosmetic constituents, and rubber components that led to contact dermatitis.44 Moreover, a recent analysis of topical preparations recommended by women with LS on social media found a high prevalence of known vulvar allergens in these agents, including botanical extracts/spices.45 Personal-care wipes marketed for vulvar care and hygiene are known to contain a variety of allergens, with a recent study finding numerous allergens in commercially available wipes including fragrances, scented botanicals in the form of essences, oils, fruit juices, and vitamin E.46 These findings underscore the importance of considering potential allergens when caring for patients with vulvar LSC and counseling patients about the potential allergens in many commercially available products that may be recommended on social media sites or by other sources.
Final Thoughts
Vulvar inflammatory dermatoses are becoming increasingly recognized, and there is a need to develop more effective diagnostic and treatment approaches. Recent literature has shed light on some of the challenges in the management of VLP, particularly its resistance to topical therapies and the importance of assessing and managing both cutaneous and vaginal involvement. Efforts have been made to refine the classification of PCV, with studies suggesting a variant that coexists with LS. Although evidence for vulvar-specific treatment of LSC is limited, the emergence of biologics and small-molecule inhibitors that are FDA approved for atopic dermatitis and prurigo nodularis offer promise for certain cases of vulvar LSC and vulvar pruritus. Moreover, recent developments in steroid-sparing topical agents warrant further investigation for their potential efficacy in treating vulvar LSC and possibly other vulvar inflammatory conditions in the future.
Vulvar dermatoses continue to be an overlooked aspect of medical care, highlighting the necessity for enhanced diagnosis and management of these conditions. Here, we address recent advancements in understanding vulvar inflammatory dermatoses other than lichen sclerosus (LS), which was discussed in a prior Guest Editorial1—specifically vulvovaginal lichen planus (VLP), plasma cell vulvitis (PCV), and vulvar lichen simplex chronicus (LSC).
Vulvar Inflammatory Skin Disease and Quality of Life
There is an increased awareness of the impact vulvar skin disease has on quality of life and its association with anxiety and depression.2-5 Evaluating the burden of vulvar dermatoses remains an active area of research due to its significance in monitoring disease progression and assessing therapeutic effectiveness. Despite the existence of various dermatology quality-of-life assessment tools, many fail to adequately capture the unique impacts of vulvovaginal diseases, such as sexual or urinary dysfunction. The vulvar quality of life index, which was developed and validated by Saunderson et al6 in 2020, consists of a 15-item questionnaire spanning 4 domains: symptoms, anxiety, activities of daily living, and sexuality. This tool has been utilized to gauge treatment response in vulvar conditions and to compare disease burden of various vulvar dermatoses.7,8 Moving forward, integrating this tool into clinical studies on vulvar skin disease holds promise for enhancing our understanding and management of these conditions.
Vulvovaginal Lichen Planus
Vulvovaginal lichen planus is unique among several prevalent vulvar inflammatory skin disorders encountered by dermatologists—primarily due to its erosive form, which can extend to the vagina, resulting in noninfectious vaginitis and potential vaginal stenosis.9,10 Managing VLP poses a notable challenge, even when it is confined to the vulva, as it often proves resistant to topical therapies.11
Evaluation for Vaginal Mucosal Disease—In contrast to LS, which typically spares the vaginal mucosa, VLP can involve mucosal sites.9,12,13 Therefore, it is imperative that all patients with a diagnosis of vulvar VLP undergo evaluation for potential vaginal involvement through speculum examination, wet mount, or vaginal biopsy. Strategies to manage vaginal involvement include use of dilators and pelvic floor physical therapy, lysis of adhesions (if present), topical estrogen, and intravaginal corticosteroids—all tailored to the severity of the disease.9,11,14
Management of VLP—Approximately 20% to 40% of patients with VLP may require systemic therapy for disease management, including those who are younger, those of non-White ethnicity, and those presenting with vulvar pruritus.11 Various systemic immunosuppressants have been used for VLP, with a recent retrospective study revealing similar response rates for both methotrexate and mycophenolate mofetil in the treatment of VLP.15 Another retrospective study found hydroxychloroquine to be safe and effective for VLP but noted a slow onset of action, with approximately 70% responding at 9 months following initiation of therapy.16
Recent attention has shifted to use of targeted therapies for VLP. For instance, apremilast has shown efficacy in a single-center, nonrandomized, open-label pilot study.17 Tildrakizumab, an IL-23 inhibitor, demonstrated efficacy in a case series involving 24 patients with VLP.18 Moreover, recent case reports and series have highlighted the potential of oral Janus kinase (JAK) inhibitors, such as tofacitinib, in VLP treatment.19 Clinical trials are ongoing to evaluate the safety and efficacy of topical ruxolitinib and deucravacitinib (a tyrosine kinase 2 inhibitor) in VLP.20-22 Systemic therapies for VLP currently are used off label, emphasizing the need for future randomized controlled trials to ascertain the optimal therapies for patients affected by erosive and nonerosive forms of this disease.
Plasma Cell Vulvitis
Plasma cell vulvitis is a chronic inflammatory disorder with an unknown etiology that some consider to be a variant of VLP.23 Others have observed an overlap with desquamative inflammatory vaginitis, categorizing PCV as a hemorrhagic vestibulovaginitis.24 Although its classification as a distinct entity remains under scrutiny, studies indicate a predilection for the nonkeratinized or partially keratinized vulva. A systematic review outlining common clinical findings reported that the most common anatomic sites included the vulvar vestibule, periurethral area, and labia minora.23 Additionally, reports have emphasized the association between PCV and other inflammatory vulvar skin conditions, including LS.25
Clinical Variants of PCV—A retrospective review proposed 2 clinical phenotypes for PCV: (1) primary non–lichen-associated PCV and (2) secondary lichen-associated PCV, which is linked to LS.26 The primary form is reported to be restricted to the vestibule, and the authors considered this a vulvar counterpart of atrophic vaginitis due to estrogen deficiency (now known as postmenopausal genitourinary syndrome). The secondary phenotype more commonly involved the vestibular and extravestibular epithelium.26
Management of PCV—Recognizing PCV in the context of LS may be important for identifying comorbid conditions and guiding treatment. However, evidence-based guidelines for PCV treatment are lacking. Commonly reported treatment modalities include clobetasol ointment 0.05% and tacrolimus ointment 0.1%.23 Successful treatment with hydrocortisone suppositories alternating with estradiol vaginal cream was reported in a recent case series.27 Crisaborole also has been reported as a treatment in 1 case of PCV.28 A recent case report found abrocitinib to be effective for the treatment of plasma cell balanitis in the setting of male genital LS,29 but there are limited data on the use of JAK inhibitors for PCV. Further research is necessary to ascertain the incidence, prevalence, clinical subtypes, and optimal management strategies for PCV to effectively treat patients with this condition.
Vulvar LSC
Similar to extragenital LSC, the evaluation of vulvar LSC should prioritize identification of underlying etiologies that contribute to the itch-scratch cycle, which may include psoriasis, atopic dermatitis, neurologic conditions, and allergic or irritant contact dermatitis.30,31 Although treatment strategies may vary based on underlying conditions, we will concentrate on updates in managing vulvar LSC and pruritus associated with an atopic diathesis or resulting from chronic contact dermatitis, which is prevalent in vulvar skin areas. Finally, we highlight some emerging vulvar allergens for consideration in clinical practice.
Management of Vulvar LSC—The advent of targeted therapies, including biologics and small-molecule inhibitors, for atopic dermatitis and prurigo nodularis in recent years presents potential options for treatment of individuals with vulvar LSC. However, studies on the use of these therapies specifically for vulvar LSC are limited, necessitating thorough discussions with patients. Given the debilitating nature of vulvar pruritus that may be seen in vulvar LSC and the potential inadequacy of topical steroids as monotherapy, systemic therapies may serve as alternative options for patients with refractory disease.30
Dupilumab, a dual inhibitor of IL-4 and IL-13 signaling, has shown rapid and sustained disease improvement in patients with atopic dermatitis, prurigo nodularis, and pruritus.32,33 Although data on its role in managing vulvar LSC are scarce, a recent case series reported improvement of vulvar pruritus with dupilumab.34 Similarly, tralokinumab, an IL-13 inhibitor approved by the US Food and Drug Administration (FDA) for atopic dermatitis, has shown efficacy in prurigo nodularis35 and may benefit patients with vulvar LSC, though studies on cutaneous outcomes in those with genital involvement specifically are lacking. Oral JAK inhibitors such as upadacitinib and abrocitinib—both FDA approved for atopic dermatitis—have demonstrated efficacy in treating LSC and itch, potentially serving as management options for vulvar LSC in cases resistant to topical steroids or in which steroid atrophy or other steroid adverse effects may preclude continued use of such agents.36,37 Finally, IL-31 inhibitors such as nemolizumab, which reduced the signs and symptoms of prurigo nodularis in a recent phase 3 clinical trial, may hold utility in addressing vulvar LSC and associated pruritus.38
The topical JAK inhibitor ruxolitinib, which is FDA approved for atopic dermatitis and vitiligo, holds promise for managing LSC on vulvar skin while mitigating the risk for steroid-induced atrophy.39 Additionally, nonsteroidal topicals including roflumilast cream 0.3% and tapinarof cream 1%, both FDA approved for psoriasis, are being evaluated in studies for their safety and efficacy in atopic dermatitis.40,41 These agents may have the potential to improve signs and symptoms of vulvar LSC, but further studies are necessary.
Vulvar Allergens and LSC—When assessing patients with vulvar LSC, it is crucial to recognize that allergic contact dermatitis is a common primary vulvar dermatosis but can coexist with other vulvar dermatoses such as LS.13,30 The vulvar skin’s susceptibly to allergic contact dermatitis is attributed to factors such as a higher ratio of antigen-presenting cells in the vulvar skin, the nonkeratinized nature of certain sites, and frequent contact with potential allergens.42,43 Therefore, incorporating patch testing into the diagnostic process should be considered when evaluating patients with vulvar skin conditions.43
A systemic review identified multiple vulvar allergens, including metals, topical medicaments, fragrances, preservatives, cosmetic constituents, and rubber components that led to contact dermatitis.44 Moreover, a recent analysis of topical preparations recommended by women with LS on social media found a high prevalence of known vulvar allergens in these agents, including botanical extracts/spices.45 Personal-care wipes marketed for vulvar care and hygiene are known to contain a variety of allergens, with a recent study finding numerous allergens in commercially available wipes including fragrances, scented botanicals in the form of essences, oils, fruit juices, and vitamin E.46 These findings underscore the importance of considering potential allergens when caring for patients with vulvar LSC and counseling patients about the potential allergens in many commercially available products that may be recommended on social media sites or by other sources.
Final Thoughts
Vulvar inflammatory dermatoses are becoming increasingly recognized, and there is a need to develop more effective diagnostic and treatment approaches. Recent literature has shed light on some of the challenges in the management of VLP, particularly its resistance to topical therapies and the importance of assessing and managing both cutaneous and vaginal involvement. Efforts have been made to refine the classification of PCV, with studies suggesting a variant that coexists with LS. Although evidence for vulvar-specific treatment of LSC is limited, the emergence of biologics and small-molecule inhibitors that are FDA approved for atopic dermatitis and prurigo nodularis offer promise for certain cases of vulvar LSC and vulvar pruritus. Moreover, recent developments in steroid-sparing topical agents warrant further investigation for their potential efficacy in treating vulvar LSC and possibly other vulvar inflammatory conditions in the future.
- Nguyen B, Kraus C. Vulvar lichen sclerosus: what’s new? Cutis. 2024;113:104-106. doi:10.12788/cutis.0967
- Van De Nieuwenhof HP, Meeuwis KAP, Nieboer TE, et al. The effect of vulvar lichen sclerosus on quality of life and sexual functioning. J Psychosom Obstet Gynaecol. 2010;31:279-284. doi:10.3109/0167482X.2010.507890
- Ranum A, Pearson DR. The impact of genital lichen sclerosus and lichen planus on quality of life: a review. Int J Womens Dermatol. 2022;8:E042. doi:10.1097/JW9.0000000000000042
- Messele F, Hinchee-Rodriguez K, Kraus CN. Vulvar dermatoses and depression: a systematic review of vulvar lichen sclerosus, lichen planus, and lichen simplex chronicus. JAAD Int. 2024;15:15-20. doi:10.1016/j.jdin.2023.10.009
- Choi UE, Nicholson RC, Agrawal P, et al. Involvement of vulva in lichen sclerosus increases the risk of antidepressant and benzodiazepine prescriptions for psychiatric disorder diagnoses. Int J Impot Res. Published online November 16, 2023. doi:10.1038/s41443-023-00793-3
- Saunderson R, Harris V, Yeh R, et al. Vulvar quality of life index (VQLI)—a simple tool to measure quality of life in patients with vulvar disease. Australas J Dermatol. 2020;61:152-157. doi:10.1111/ajd.13235
- Wu M, Kherlopian A, Wijaya M, et al. Quality of life impact and treatment response in vulval disease: comparison of 3 common conditions using the Vulval Quality of Life Index. Australas J Dermatol. 2022;63:E320-E328. doi:10.1111/ajd.13898
- Kherlopian A, Fischer G. Comparing quality of life in women with vulvovaginal lichen planus treated with topical and systemic treatments using the vulvar quality of life index. Australas J Dermatol. 2023;64:E125-E134. doi:10.1111/ajd.14032
- Cooper SM, Haefner HK, Abrahams-Gessel S, et al. Vulvovaginal lichen planus treatment: a survey of current practices. Arch Dermatol. 2008;144:1520-1521. doi:10.1001/archderm.144.11.1520
- Chow MR, Gill N, Alzahrani F, et al. Vulvar lichen planus–induced vulvovaginal stenosis: a case report and review of the literature. SAGE Open Med Case Rep. 2023;11:2050313X231164216. doi:10.1177/2050313X231164216
- Kherlopian A, Fischer G. Identifying predictors of systemic immunosuppressive treatment of vulvovaginal lichen planus: a retrospective cohort study of 122 women. Australas J Dermatol. 2022;63:335-343. doi:10.1111/ajd.13851
- Dunaway S, Tyler K, Kaffenberger, J. Update on treatments for erosive vulvovaginal lichen planus. Int J Dermatol. 2020;59:297-302. doi:10.1111/ijd.14692
- Mauskar MM, Marathe, K, Venkatesan A, et al. Vulvar diseases: conditions in adults and children. J Am Acad Dermatol. 2020;82:1287-1298. doi:10.1016/j.jaad.2019.10.077
- Hinchee-Rodriguez K, Duong A, Kraus CN. Local management strategies for inflammatory vaginitis in dermatologic conditions: suppositories, dilators, and estrogen replacement. JAAD Int. 2022;9:137-138. doi:10.1016/j.jdin.2022.09.004
- Hrin ML, Bowers NL, Feldman SR, et al. Mycophenolate mofetil versus methotrexate for vulvar lichen planus: a 10-year retrospective cohort study demonstrates comparable efficacy and tolerability. J Am Acad Dermatol. 2022;87:436-438. doi:10.1016/j.jaad.2021.08.061
- Vermeer HAB, Rashid H, Esajas MD, et al. The use of hydroxychloroquine as a systemic treatment in erosive lichen planus of the vulva and vagina. Br J Dermatol. 2021;185:201-203. doi:10.1111/bjd.19870
- Skullerud KH, Gjersvik P, Pripp AH, et al. Apremilast for genital erosive lichen planus in women (the AP-GELP Study): study protocol for a randomised placebo-controlled clinical trial. Trials. 2021;22:469. doi:10.1186/s13063-021-05428-w
- Kherlopian A, Fischer G. Successful treatment of vulvovaginal lichen planus with tildrakizumab: a case series of 24 patients. Australas J Dermatol. 2022;63:251-255. doi:10.1111/ajd.13793
- Kassels A, Edwards L, Kraus CN. Treatment of erosive vulvovaginal lichen planus with tofacitinib: a case series. JAAD Case Rep. 2023;40:14-18. doi:10.1016/j.jdcr.2023.08.001
- Wijaya M, Fischer G, Saunderson RB. The efficacy and safety of deucravacitinib compared to methotrexate, in patients with vulvar lichen planus who have failed topical therapy with potent corticosteroids: a study protocol for a single-centre double-blinded randomised controlled trial. Trials. 2024;25:181. doi:10.1186/s13063-024-08022-y
- Brumfiel CM, Patel MH, Severson KJ, et al. Ruxolitinib cream in the treatment of cutaneous lichen planus: a prospective, open-label study. J Invest Dermatol. 2022;142:2109-2116.e4. doi:10.1016/j.jid.2022.01.015
- A study to evaluate the efficacy and safety of ruxolitinib cream in participants with cutaneous lichen planus. ClinicalTrials.gov identifier: NCT05593432. Updated March 12, 2024. Accessed July 12, 2024. https://clinicaltrials.gov/study/NCT05593432
- Sattler S, Elsensohn AN, Mauskar MM, et al. Plasma cell vulvitis: a systematic review. Int J Womens Dermatol. 2021;7:756-762. doi:10.1016/j.ijwd.2021.04.005
- Song M, Day T, Kliman L, et al. Desquamative inflammatory vaginitis and plasma cell vulvitis represent a spectrum of hemorrhagic vestibulovaginitis. J Low Genit Tract Dis. 2022;26:60-67. doi:10.1097/LGT.0000000000000637
- Saeed L, Lee BA, Kraus CN. Tender solitary lesion in vulvar lichen sclerosus. JAAD Case Rep. 2022;23:61-63. doi:10.1016/j.jdcr.2022.01.038
- Wendling J, Plantier F, Moyal-Barracco M. Plasma cell vulvitis: a classification into two clinical phenotypes. J Low Genit Tract Dis. 2023;27:384-389. doi:10.1097/LGT.0000000000000771
- Prestwood CA, Granberry R, Rutherford A, et al. Successful treatment of plasma cell vulvitis: a case series. JAAD Case Rep. 2022;19:37-40. doi:10.1016/j.jdcr.2021.10.023
- He Y, Xu M, Wu M, et al. A case of plasma cell vulvitis successfully treated with crisaborole. J Dermatol. Published online April 1, 2024. doi:10.1111/1346-8138.17205
- Xiong X, Chen R, Wang L, et al. Treatment of plasma cell balanitis associated with male genital lichen sclerosus using abrocitinib. JAAD Case Rep. 2024;46:85-88. doi:10.1016/j.jdcr.2024.02.010
- Stewart KMA. Clinical care of vulvar pruritus, with emphasis on one common cause, lichen simplex chronicus. Dermatol Clin. 2010;28:669-680. doi:10.1016/j.det.2010.08.004
- Rimoin LP, Kwatra SG, Yosipovitch G. Female-specific pruritus from childhood to postmenopause: clinical features, hormonal factors, and treatment considerations. Dermatol Ther. 2013;26:157-167. doi:10.1111/dth.12034
- Simpson EL, Bieber T, Guttman-Yassky E, et al; SOLO 1 and SOLO 2 Investigators. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375:2335-2348. doi:10.1056/NEJMoa1610020
- Yosipovitch G, Mollanazar N, Ständer S, et al. Dupilumab in patients with prurigo nodularis: two randomized, double-blind, placebo-controlled phase 3 trials. Nat Med. 2023;29:1180-1190. doi:10.1038/s41591-023-02320-9
- Gosch M, Cash S, Pichardo R. Vulvar pruritus improved with dupilumab. JSM Sexual Med. 2023;7:1104.
- Pezzolo E, Gambardella A, Guanti M, et al. Tralokinumab shows clinical improvement in patients with prurigo nodularis-like phenotype atopic dermatitis: a multicenter, prospective, open-label case series study. J Am Acad Dermatol. 2023;89:430-432. doi:10.1016/j.jaad.2023.04.056
- Simpson EL, Sinclair R, Forman S, et al. Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE MONO-1): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet. 2020;396:255-266. doi:10.1016/S0140-6736(20)30732-7
- Simpson EL, Papp KA, Blauvelt A, et al. Efficacy and safety of upadacitinib in patients with moderate to severe atopic dermatitis: analysis of follow-up data from the Measure Up 1 and Measure Up 2 randomized clinical trials. JAMA Dermatol. 2022;158:404-413. doi:10.1001/jamadermatol.2022.0029
- Kwatra SG, Yosipovitch G, Legat FJ, et al. Phase 3 trial of nemolizumab in patients with prurigo nodularis. N Engl J Med. 2023;389:1579-1589. doi:10.1056/NEJMoa2301333
- Papp K, Szepietowski JC, Kircik L, et al. Long-term safety and disease control with ruxolitinib cream in atopic dermatitis: results from two phase 3 studies. J Am Acad Dermatol. 2023;88:1008-1016. doi:10.1016/j.jaad.2022.09.060
- Lebwohl MG, Kircik LH, Moore AY, et al. Effect of roflumilast cream vs vehicle cream on chronic plaque psoriasis: the DERMIS-1 and DERMIS-2 randomized clinical trials. JAMA. 2022;328:1073-1084. doi:10.1001/jama.2022.15632
- Lebwohl MG, Gold LS, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229. doi:10.1056/NEJMoa2103629
- O’Gorman SM, Torgerson RR. Allergic contact dermatitis of the vulva. Dermatitis. 2013;24:64-72. doi:10.1097/DER.0b013e318284da33
- Woodruff CM, Trivedi MK, Botto N, et al. Allergic contact dermatitis of the vulva. Dermatitis. 2018;29:233-243. doi:10.1097/DER.0000000000000339
- Vandeweege S, Debaene B, Lapeere H, et al. A systematic review of allergic and irritant contact dermatitis of the vulva: the most important allergens/irritants and the role of patch testing. Contact Dermatitis. 2023;88:249-262. doi:10.1111/cod.14258
- Luu Y, Admani S. Vulvar allergens in topical preparations recommended on social media: a cross-sectional analysis of Facebook groups for lichen sclerosus. Int J Womens Dermatol. 2023;9:E097. doi:10.1097/JW9.0000000000000097
- Newton J, Richardson S, van Oosbre AM, et al. A cross-sectional study of contact allergens in feminine hygiene wipes: a possible cause of vulvar contact dermatitis. Int J Womens Dermatol. 2022;8:E060. doi:10.1097/JW9.0000000000000060
- Nguyen B, Kraus C. Vulvar lichen sclerosus: what’s new? Cutis. 2024;113:104-106. doi:10.12788/cutis.0967
- Van De Nieuwenhof HP, Meeuwis KAP, Nieboer TE, et al. The effect of vulvar lichen sclerosus on quality of life and sexual functioning. J Psychosom Obstet Gynaecol. 2010;31:279-284. doi:10.3109/0167482X.2010.507890
- Ranum A, Pearson DR. The impact of genital lichen sclerosus and lichen planus on quality of life: a review. Int J Womens Dermatol. 2022;8:E042. doi:10.1097/JW9.0000000000000042
- Messele F, Hinchee-Rodriguez K, Kraus CN. Vulvar dermatoses and depression: a systematic review of vulvar lichen sclerosus, lichen planus, and lichen simplex chronicus. JAAD Int. 2024;15:15-20. doi:10.1016/j.jdin.2023.10.009
- Choi UE, Nicholson RC, Agrawal P, et al. Involvement of vulva in lichen sclerosus increases the risk of antidepressant and benzodiazepine prescriptions for psychiatric disorder diagnoses. Int J Impot Res. Published online November 16, 2023. doi:10.1038/s41443-023-00793-3
- Saunderson R, Harris V, Yeh R, et al. Vulvar quality of life index (VQLI)—a simple tool to measure quality of life in patients with vulvar disease. Australas J Dermatol. 2020;61:152-157. doi:10.1111/ajd.13235
- Wu M, Kherlopian A, Wijaya M, et al. Quality of life impact and treatment response in vulval disease: comparison of 3 common conditions using the Vulval Quality of Life Index. Australas J Dermatol. 2022;63:E320-E328. doi:10.1111/ajd.13898
- Kherlopian A, Fischer G. Comparing quality of life in women with vulvovaginal lichen planus treated with topical and systemic treatments using the vulvar quality of life index. Australas J Dermatol. 2023;64:E125-E134. doi:10.1111/ajd.14032
- Cooper SM, Haefner HK, Abrahams-Gessel S, et al. Vulvovaginal lichen planus treatment: a survey of current practices. Arch Dermatol. 2008;144:1520-1521. doi:10.1001/archderm.144.11.1520
- Chow MR, Gill N, Alzahrani F, et al. Vulvar lichen planus–induced vulvovaginal stenosis: a case report and review of the literature. SAGE Open Med Case Rep. 2023;11:2050313X231164216. doi:10.1177/2050313X231164216
- Kherlopian A, Fischer G. Identifying predictors of systemic immunosuppressive treatment of vulvovaginal lichen planus: a retrospective cohort study of 122 women. Australas J Dermatol. 2022;63:335-343. doi:10.1111/ajd.13851
- Dunaway S, Tyler K, Kaffenberger, J. Update on treatments for erosive vulvovaginal lichen planus. Int J Dermatol. 2020;59:297-302. doi:10.1111/ijd.14692
- Mauskar MM, Marathe, K, Venkatesan A, et al. Vulvar diseases: conditions in adults and children. J Am Acad Dermatol. 2020;82:1287-1298. doi:10.1016/j.jaad.2019.10.077
- Hinchee-Rodriguez K, Duong A, Kraus CN. Local management strategies for inflammatory vaginitis in dermatologic conditions: suppositories, dilators, and estrogen replacement. JAAD Int. 2022;9:137-138. doi:10.1016/j.jdin.2022.09.004
- Hrin ML, Bowers NL, Feldman SR, et al. Mycophenolate mofetil versus methotrexate for vulvar lichen planus: a 10-year retrospective cohort study demonstrates comparable efficacy and tolerability. J Am Acad Dermatol. 2022;87:436-438. doi:10.1016/j.jaad.2021.08.061
- Vermeer HAB, Rashid H, Esajas MD, et al. The use of hydroxychloroquine as a systemic treatment in erosive lichen planus of the vulva and vagina. Br J Dermatol. 2021;185:201-203. doi:10.1111/bjd.19870
- Skullerud KH, Gjersvik P, Pripp AH, et al. Apremilast for genital erosive lichen planus in women (the AP-GELP Study): study protocol for a randomised placebo-controlled clinical trial. Trials. 2021;22:469. doi:10.1186/s13063-021-05428-w
- Kherlopian A, Fischer G. Successful treatment of vulvovaginal lichen planus with tildrakizumab: a case series of 24 patients. Australas J Dermatol. 2022;63:251-255. doi:10.1111/ajd.13793
- Kassels A, Edwards L, Kraus CN. Treatment of erosive vulvovaginal lichen planus with tofacitinib: a case series. JAAD Case Rep. 2023;40:14-18. doi:10.1016/j.jdcr.2023.08.001
- Wijaya M, Fischer G, Saunderson RB. The efficacy and safety of deucravacitinib compared to methotrexate, in patients with vulvar lichen planus who have failed topical therapy with potent corticosteroids: a study protocol for a single-centre double-blinded randomised controlled trial. Trials. 2024;25:181. doi:10.1186/s13063-024-08022-y
- Brumfiel CM, Patel MH, Severson KJ, et al. Ruxolitinib cream in the treatment of cutaneous lichen planus: a prospective, open-label study. J Invest Dermatol. 2022;142:2109-2116.e4. doi:10.1016/j.jid.2022.01.015
- A study to evaluate the efficacy and safety of ruxolitinib cream in participants with cutaneous lichen planus. ClinicalTrials.gov identifier: NCT05593432. Updated March 12, 2024. Accessed July 12, 2024. https://clinicaltrials.gov/study/NCT05593432
- Sattler S, Elsensohn AN, Mauskar MM, et al. Plasma cell vulvitis: a systematic review. Int J Womens Dermatol. 2021;7:756-762. doi:10.1016/j.ijwd.2021.04.005
- Song M, Day T, Kliman L, et al. Desquamative inflammatory vaginitis and plasma cell vulvitis represent a spectrum of hemorrhagic vestibulovaginitis. J Low Genit Tract Dis. 2022;26:60-67. doi:10.1097/LGT.0000000000000637
- Saeed L, Lee BA, Kraus CN. Tender solitary lesion in vulvar lichen sclerosus. JAAD Case Rep. 2022;23:61-63. doi:10.1016/j.jdcr.2022.01.038
- Wendling J, Plantier F, Moyal-Barracco M. Plasma cell vulvitis: a classification into two clinical phenotypes. J Low Genit Tract Dis. 2023;27:384-389. doi:10.1097/LGT.0000000000000771
- Prestwood CA, Granberry R, Rutherford A, et al. Successful treatment of plasma cell vulvitis: a case series. JAAD Case Rep. 2022;19:37-40. doi:10.1016/j.jdcr.2021.10.023
- He Y, Xu M, Wu M, et al. A case of plasma cell vulvitis successfully treated with crisaborole. J Dermatol. Published online April 1, 2024. doi:10.1111/1346-8138.17205
- Xiong X, Chen R, Wang L, et al. Treatment of plasma cell balanitis associated with male genital lichen sclerosus using abrocitinib. JAAD Case Rep. 2024;46:85-88. doi:10.1016/j.jdcr.2024.02.010
- Stewart KMA. Clinical care of vulvar pruritus, with emphasis on one common cause, lichen simplex chronicus. Dermatol Clin. 2010;28:669-680. doi:10.1016/j.det.2010.08.004
- Rimoin LP, Kwatra SG, Yosipovitch G. Female-specific pruritus from childhood to postmenopause: clinical features, hormonal factors, and treatment considerations. Dermatol Ther. 2013;26:157-167. doi:10.1111/dth.12034
- Simpson EL, Bieber T, Guttman-Yassky E, et al; SOLO 1 and SOLO 2 Investigators. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375:2335-2348. doi:10.1056/NEJMoa1610020
- Yosipovitch G, Mollanazar N, Ständer S, et al. Dupilumab in patients with prurigo nodularis: two randomized, double-blind, placebo-controlled phase 3 trials. Nat Med. 2023;29:1180-1190. doi:10.1038/s41591-023-02320-9
- Gosch M, Cash S, Pichardo R. Vulvar pruritus improved with dupilumab. JSM Sexual Med. 2023;7:1104.
- Pezzolo E, Gambardella A, Guanti M, et al. Tralokinumab shows clinical improvement in patients with prurigo nodularis-like phenotype atopic dermatitis: a multicenter, prospective, open-label case series study. J Am Acad Dermatol. 2023;89:430-432. doi:10.1016/j.jaad.2023.04.056
- Simpson EL, Sinclair R, Forman S, et al. Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE MONO-1): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet. 2020;396:255-266. doi:10.1016/S0140-6736(20)30732-7
- Simpson EL, Papp KA, Blauvelt A, et al. Efficacy and safety of upadacitinib in patients with moderate to severe atopic dermatitis: analysis of follow-up data from the Measure Up 1 and Measure Up 2 randomized clinical trials. JAMA Dermatol. 2022;158:404-413. doi:10.1001/jamadermatol.2022.0029
- Kwatra SG, Yosipovitch G, Legat FJ, et al. Phase 3 trial of nemolizumab in patients with prurigo nodularis. N Engl J Med. 2023;389:1579-1589. doi:10.1056/NEJMoa2301333
- Papp K, Szepietowski JC, Kircik L, et al. Long-term safety and disease control with ruxolitinib cream in atopic dermatitis: results from two phase 3 studies. J Am Acad Dermatol. 2023;88:1008-1016. doi:10.1016/j.jaad.2022.09.060
- Lebwohl MG, Kircik LH, Moore AY, et al. Effect of roflumilast cream vs vehicle cream on chronic plaque psoriasis: the DERMIS-1 and DERMIS-2 randomized clinical trials. JAMA. 2022;328:1073-1084. doi:10.1001/jama.2022.15632
- Lebwohl MG, Gold LS, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229. doi:10.1056/NEJMoa2103629
- O’Gorman SM, Torgerson RR. Allergic contact dermatitis of the vulva. Dermatitis. 2013;24:64-72. doi:10.1097/DER.0b013e318284da33
- Woodruff CM, Trivedi MK, Botto N, et al. Allergic contact dermatitis of the vulva. Dermatitis. 2018;29:233-243. doi:10.1097/DER.0000000000000339
- Vandeweege S, Debaene B, Lapeere H, et al. A systematic review of allergic and irritant contact dermatitis of the vulva: the most important allergens/irritants and the role of patch testing. Contact Dermatitis. 2023;88:249-262. doi:10.1111/cod.14258
- Luu Y, Admani S. Vulvar allergens in topical preparations recommended on social media: a cross-sectional analysis of Facebook groups for lichen sclerosus. Int J Womens Dermatol. 2023;9:E097. doi:10.1097/JW9.0000000000000097
- Newton J, Richardson S, van Oosbre AM, et al. A cross-sectional study of contact allergens in feminine hygiene wipes: a possible cause of vulvar contact dermatitis. Int J Womens Dermatol. 2022;8:E060. doi:10.1097/JW9.0000000000000060
Investing in Future Discovery
The field of GI is rapidly evolving, fueled by new scientific discoveries leading to improved understanding of disease mechanisms and more effective treatment approaches for patients with digestive and liver diseases. But there are many challenges confronting the pipeline of early-career investigators essential to future discovery, most notably a constrained funding environment leading to decreased protected time for research during these critical early years.
Foundation awards, such as those funded by the AGA Research Foundation, play a pivotal role in supporting the career development of promising young investigators in basic, translational, clinical, and health services research and ensure that we have a strong pipeline of independent investigators to stimulate ongoing discovery and innovation in our field. This year, the AGA Research Foundation distributed $2.6 million in funding to 76 investigators, including six coveted Research Scholar Awards awarded to early-career investigators. These promising young researchers represent the best and the brightest in our field — I hope you enjoy learning more about them in the pages of this issue and will join me in continuing to support the Foundation and its work under the leadership of Dr. Michael Camilleri.
Also including a study investigating the impact of H pylori eradication on esophageal cancer risk. We also highlight several important studies relating to eosinophilic esophagitis, including a recent RCT published in The New England Journal of Medicine demonstrating the effectiveness of dupilumab in treatment of PPI-refractory pediatric EoE. Our August Member Spotlight features Dr. Neelendu Dey of Fred Hutchinson Cancer Center, who shares his perspectives on pursuing a career as a physician-scientist and chronicles his research focused on harnessing the microbiome for cancer prevention.
Finally, our quarterly In Focus column from The New Gastroenterologist provides practical advice regarding how best to evaluate patients with chronic bloating symptoms, a frequent presentation in our GI clinics. As always, thanks for reading and please don’t hesitate to reach out with suggestions for future coverage.
Megan A. Adams, MD, JD, MSc
Editor in Chief
The field of GI is rapidly evolving, fueled by new scientific discoveries leading to improved understanding of disease mechanisms and more effective treatment approaches for patients with digestive and liver diseases. But there are many challenges confronting the pipeline of early-career investigators essential to future discovery, most notably a constrained funding environment leading to decreased protected time for research during these critical early years.
Foundation awards, such as those funded by the AGA Research Foundation, play a pivotal role in supporting the career development of promising young investigators in basic, translational, clinical, and health services research and ensure that we have a strong pipeline of independent investigators to stimulate ongoing discovery and innovation in our field. This year, the AGA Research Foundation distributed $2.6 million in funding to 76 investigators, including six coveted Research Scholar Awards awarded to early-career investigators. These promising young researchers represent the best and the brightest in our field — I hope you enjoy learning more about them in the pages of this issue and will join me in continuing to support the Foundation and its work under the leadership of Dr. Michael Camilleri.
Also including a study investigating the impact of H pylori eradication on esophageal cancer risk. We also highlight several important studies relating to eosinophilic esophagitis, including a recent RCT published in The New England Journal of Medicine demonstrating the effectiveness of dupilumab in treatment of PPI-refractory pediatric EoE. Our August Member Spotlight features Dr. Neelendu Dey of Fred Hutchinson Cancer Center, who shares his perspectives on pursuing a career as a physician-scientist and chronicles his research focused on harnessing the microbiome for cancer prevention.
Finally, our quarterly In Focus column from The New Gastroenterologist provides practical advice regarding how best to evaluate patients with chronic bloating symptoms, a frequent presentation in our GI clinics. As always, thanks for reading and please don’t hesitate to reach out with suggestions for future coverage.
Megan A. Adams, MD, JD, MSc
Editor in Chief
The field of GI is rapidly evolving, fueled by new scientific discoveries leading to improved understanding of disease mechanisms and more effective treatment approaches for patients with digestive and liver diseases. But there are many challenges confronting the pipeline of early-career investigators essential to future discovery, most notably a constrained funding environment leading to decreased protected time for research during these critical early years.
Foundation awards, such as those funded by the AGA Research Foundation, play a pivotal role in supporting the career development of promising young investigators in basic, translational, clinical, and health services research and ensure that we have a strong pipeline of independent investigators to stimulate ongoing discovery and innovation in our field. This year, the AGA Research Foundation distributed $2.6 million in funding to 76 investigators, including six coveted Research Scholar Awards awarded to early-career investigators. These promising young researchers represent the best and the brightest in our field — I hope you enjoy learning more about them in the pages of this issue and will join me in continuing to support the Foundation and its work under the leadership of Dr. Michael Camilleri.
Also including a study investigating the impact of H pylori eradication on esophageal cancer risk. We also highlight several important studies relating to eosinophilic esophagitis, including a recent RCT published in The New England Journal of Medicine demonstrating the effectiveness of dupilumab in treatment of PPI-refractory pediatric EoE. Our August Member Spotlight features Dr. Neelendu Dey of Fred Hutchinson Cancer Center, who shares his perspectives on pursuing a career as a physician-scientist and chronicles his research focused on harnessing the microbiome for cancer prevention.
Finally, our quarterly In Focus column from The New Gastroenterologist provides practical advice regarding how best to evaluate patients with chronic bloating symptoms, a frequent presentation in our GI clinics. As always, thanks for reading and please don’t hesitate to reach out with suggestions for future coverage.
Megan A. Adams, MD, JD, MSc
Editor in Chief
Immunotherapy May Be Overused in Dying Patients With Cancer
Chemotherapy has fallen out of favor for treating cancer toward the end of life. The toxicity is too high, and the benefit, if any, is often too low.
Immunotherapy, however, has been taking its place.
This means “there are patients who are getting immunotherapy who shouldn’t,” said Yale University, New Haven, Connecticut, surgical oncologist Sajid Khan, MD, senior investigator on a recent study that highlighted the growing use of these agents in patients’ last month of life.
What’s driving this trend, and how can oncologists avoid overtreatment with immunotherapy at the end of life?
The N-of-1 Patient
With immunotherapy at the end of life, “each of us has had our N-of-1” where a patient bounces back with a remarkable and durable response, said Don Dizon, MD, a gynecologic oncologist at Brown University, Providence, Rhode Island.
He recalled a patient with sarcoma who did not respond to chemotherapy. But after Dr. Dizon started her on immunotherapy, everything turned around. She has now been in remission for 8 years and counting.
The possibility of an unexpected or remarkable responder is seductive. And the improved safety of immunotherapy over chemotherapy adds to the allure.
Meanwhile, patients are often desperate. It’s rare for someone to be ready to stop treatment, Dr. Dizon said. Everybody “hopes that they’re going to be the exceptional responder.”
At the end of the day, the question often becomes: “Why not try immunotherapy? What’s there to lose?”
This thinking may be prompting broader use of immunotherapy in late-stage disease, even in instances with no Food and Drug Administration indication and virtually no supportive data, such as for metastatic ovarian cancer, Dr. Dizon said.
Back to Earth
The problem with the hopeful approach is that end-of-life turnarounds with immunotherapy are rare, and there’s no way at the moment to predict who will have one, said Laura Petrillo, MD, a palliative care physician at Massachusetts General Hospital, Boston.
Even though immunotherapy generally comes with fewer adverse events than chemotherapy, catastrophic side effects are still possible.
Dr. Petrillo recalled a 95-year-old woman with metastatic cancer who was largely asymptomatic.
She had a qualifying mutation for a checkpoint inhibitor, so her oncologist started her on one. The patient never bounced back from the severe colitis the agent caused, and she died of complications in the hospital.
Although such reactions with immunotherapy are uncommon, less serious problems caused by the agents can still have a major impact on a person’s quality of life. Low-grade diarrhea, for instance, may not sound too bad, but in a patient’s daily life, it can translate to six or more episodes a day.
Even with no side effects, prescribing immunotherapy can mean that patients with limited time left spend a good portion of it at an infusion clinic instead of at home. These patients are also less likely to be referred to hospice and more likely to be admitted to and die in the hospital.
And with treatments that can cost $20,000 per dose, financial toxicity becomes a big concern.
In short, some of the reasons why chemotherapy is not recommended at the end of life also apply to immunotherapy, Dr. Petrillo said.
Prescribing Decisions
Recent research highlights the growing use of immunotherapy at the end of life.
Dr. Khan’s retrospective study found, for instance, that the percentage of patients starting immunotherapy in the last 30 days of life increased by about fourfold to fivefold over the study period for the three cancers analyzed — stage IV melanoma, lung, and kidney cancers.
Among the population that died within 30 days, the percentage receiving immunotherapy increased over the study periods — 0.8%-4.3% for melanoma, 0.9%-3.2% for NSCLC, and 0.5%-2.6% for kidney cell carcinoma — prompting the conclusion that immunotherapy prescriptions in the last month of life are on the rise.
Prescribing immunotherapy in patients who ultimately died within 1 month occurred more frequently at low-volume, nonacademic centers than at academic or high-volume centers, and outcomes varied by practice setting.
Patients had better survival outcomes overall when receiving immunotherapy at academic or high-volume centers — a finding Dr. Khan said is worth investigating further. Possible explanations include better management of severe immune-related side effects at larger centers and more caution when prescribing immunotherapy to “borderline” candidates, such as those with several comorbidities.
Importantly, given the retrospective design, Dr. Khan and colleagues already knew which patients prescribed immunotherapy died within 30 days of initiating treatment.
More specifically, 5192 of 71,204 patients who received immunotherapy (7.3%) died within a month of initiating therapy, while 66,012 (92.7%) lived beyond that point.
The study, however, did not assess how the remaining 92.7% who lived beyond 30 days fared on immunotherapy and the differences between those who lived less than 30 days and those who survived longer.
Knowing the outcome of patients at the outset of the analysis still leaves open the question of when immunotherapy can extend life and when it can’t for the patient in front of you.
To avoid overtreating at the end of life, it’s important to have “the same standard that you have for giving chemotherapy. You have to treat it with the same respect,” said Moshe Chasky, MD, a community medical oncologist with Alliance Cancer Specialists in Philadelphia, Pennsylvania. “You can’t just be throwing” immunotherapy around “at the end of life.”
While there are no clear predictors of risk and benefit, there are some factors to help guide decisions.
As with chemotherapy, Dr. Petrillo said performance status is key. Dr. Petrillo and colleagues found that median overall survival with immune checkpoint inhibitors for advanced non–small cell lung cancer was 14.3 months in patients with an Eastern Cooperative Oncology Group performance score of 0-1 but only 4.5 months with scores of ≥ 2.
Dr. Khan also found that immunotherapy survival is, unsurprisingly, worse in patients with high metastatic burdens and more comorbidities.
“You should still consider immunotherapy for metastatic melanoma, non–small cell lung cancer, and renal cell carcinoma,” Dr. Khan said. The message here is to “think twice before using” it, especially in comorbid patients with widespread metastases.
“Just because something can be done doesn’t always mean it should be done,” he said.
At Yale, when Dr. Khan works, immunotherapy decisions are considered by a multidisciplinary tumor board. At Mass General, immunotherapy has generally moved to the frontline setting, and the hospital no longer prescribes checkpoint inhibitors to hospitalized patients because the cost is too high relative to the potential benefit, Dr. Petrillo explained.
Still, with all the uncertainties about risk and benefit, counseling patients is a challenge. Dr. Dizon called it “the epitome of shared decision-making.”
Dr. Petrillo noted that it’s critical not to counsel patients based solely on the anecdotal patients who do surprisingly well.
“It’s hard to mention that and not have that be what somebody anchors on,” she said. But that speaks to “how desperate people can feel, how hopeful they can be.”
Dr. Khan, Dr. Petrillo, and Dr. Chasky all reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Chemotherapy has fallen out of favor for treating cancer toward the end of life. The toxicity is too high, and the benefit, if any, is often too low.
Immunotherapy, however, has been taking its place.
This means “there are patients who are getting immunotherapy who shouldn’t,” said Yale University, New Haven, Connecticut, surgical oncologist Sajid Khan, MD, senior investigator on a recent study that highlighted the growing use of these agents in patients’ last month of life.
What’s driving this trend, and how can oncologists avoid overtreatment with immunotherapy at the end of life?
The N-of-1 Patient
With immunotherapy at the end of life, “each of us has had our N-of-1” where a patient bounces back with a remarkable and durable response, said Don Dizon, MD, a gynecologic oncologist at Brown University, Providence, Rhode Island.
He recalled a patient with sarcoma who did not respond to chemotherapy. But after Dr. Dizon started her on immunotherapy, everything turned around. She has now been in remission for 8 years and counting.
The possibility of an unexpected or remarkable responder is seductive. And the improved safety of immunotherapy over chemotherapy adds to the allure.
Meanwhile, patients are often desperate. It’s rare for someone to be ready to stop treatment, Dr. Dizon said. Everybody “hopes that they’re going to be the exceptional responder.”
At the end of the day, the question often becomes: “Why not try immunotherapy? What’s there to lose?”
This thinking may be prompting broader use of immunotherapy in late-stage disease, even in instances with no Food and Drug Administration indication and virtually no supportive data, such as for metastatic ovarian cancer, Dr. Dizon said.
Back to Earth
The problem with the hopeful approach is that end-of-life turnarounds with immunotherapy are rare, and there’s no way at the moment to predict who will have one, said Laura Petrillo, MD, a palliative care physician at Massachusetts General Hospital, Boston.
Even though immunotherapy generally comes with fewer adverse events than chemotherapy, catastrophic side effects are still possible.
Dr. Petrillo recalled a 95-year-old woman with metastatic cancer who was largely asymptomatic.
She had a qualifying mutation for a checkpoint inhibitor, so her oncologist started her on one. The patient never bounced back from the severe colitis the agent caused, and she died of complications in the hospital.
Although such reactions with immunotherapy are uncommon, less serious problems caused by the agents can still have a major impact on a person’s quality of life. Low-grade diarrhea, for instance, may not sound too bad, but in a patient’s daily life, it can translate to six or more episodes a day.
Even with no side effects, prescribing immunotherapy can mean that patients with limited time left spend a good portion of it at an infusion clinic instead of at home. These patients are also less likely to be referred to hospice and more likely to be admitted to and die in the hospital.
And with treatments that can cost $20,000 per dose, financial toxicity becomes a big concern.
In short, some of the reasons why chemotherapy is not recommended at the end of life also apply to immunotherapy, Dr. Petrillo said.
Prescribing Decisions
Recent research highlights the growing use of immunotherapy at the end of life.
Dr. Khan’s retrospective study found, for instance, that the percentage of patients starting immunotherapy in the last 30 days of life increased by about fourfold to fivefold over the study period for the three cancers analyzed — stage IV melanoma, lung, and kidney cancers.
Among the population that died within 30 days, the percentage receiving immunotherapy increased over the study periods — 0.8%-4.3% for melanoma, 0.9%-3.2% for NSCLC, and 0.5%-2.6% for kidney cell carcinoma — prompting the conclusion that immunotherapy prescriptions in the last month of life are on the rise.
Prescribing immunotherapy in patients who ultimately died within 1 month occurred more frequently at low-volume, nonacademic centers than at academic or high-volume centers, and outcomes varied by practice setting.
Patients had better survival outcomes overall when receiving immunotherapy at academic or high-volume centers — a finding Dr. Khan said is worth investigating further. Possible explanations include better management of severe immune-related side effects at larger centers and more caution when prescribing immunotherapy to “borderline” candidates, such as those with several comorbidities.
Importantly, given the retrospective design, Dr. Khan and colleagues already knew which patients prescribed immunotherapy died within 30 days of initiating treatment.
More specifically, 5192 of 71,204 patients who received immunotherapy (7.3%) died within a month of initiating therapy, while 66,012 (92.7%) lived beyond that point.
The study, however, did not assess how the remaining 92.7% who lived beyond 30 days fared on immunotherapy and the differences between those who lived less than 30 days and those who survived longer.
Knowing the outcome of patients at the outset of the analysis still leaves open the question of when immunotherapy can extend life and when it can’t for the patient in front of you.
To avoid overtreating at the end of life, it’s important to have “the same standard that you have for giving chemotherapy. You have to treat it with the same respect,” said Moshe Chasky, MD, a community medical oncologist with Alliance Cancer Specialists in Philadelphia, Pennsylvania. “You can’t just be throwing” immunotherapy around “at the end of life.”
While there are no clear predictors of risk and benefit, there are some factors to help guide decisions.
As with chemotherapy, Dr. Petrillo said performance status is key. Dr. Petrillo and colleagues found that median overall survival with immune checkpoint inhibitors for advanced non–small cell lung cancer was 14.3 months in patients with an Eastern Cooperative Oncology Group performance score of 0-1 but only 4.5 months with scores of ≥ 2.
Dr. Khan also found that immunotherapy survival is, unsurprisingly, worse in patients with high metastatic burdens and more comorbidities.
“You should still consider immunotherapy for metastatic melanoma, non–small cell lung cancer, and renal cell carcinoma,” Dr. Khan said. The message here is to “think twice before using” it, especially in comorbid patients with widespread metastases.
“Just because something can be done doesn’t always mean it should be done,” he said.
At Yale, when Dr. Khan works, immunotherapy decisions are considered by a multidisciplinary tumor board. At Mass General, immunotherapy has generally moved to the frontline setting, and the hospital no longer prescribes checkpoint inhibitors to hospitalized patients because the cost is too high relative to the potential benefit, Dr. Petrillo explained.
Still, with all the uncertainties about risk and benefit, counseling patients is a challenge. Dr. Dizon called it “the epitome of shared decision-making.”
Dr. Petrillo noted that it’s critical not to counsel patients based solely on the anecdotal patients who do surprisingly well.
“It’s hard to mention that and not have that be what somebody anchors on,” she said. But that speaks to “how desperate people can feel, how hopeful they can be.”
Dr. Khan, Dr. Petrillo, and Dr. Chasky all reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Chemotherapy has fallen out of favor for treating cancer toward the end of life. The toxicity is too high, and the benefit, if any, is often too low.
Immunotherapy, however, has been taking its place.
This means “there are patients who are getting immunotherapy who shouldn’t,” said Yale University, New Haven, Connecticut, surgical oncologist Sajid Khan, MD, senior investigator on a recent study that highlighted the growing use of these agents in patients’ last month of life.
What’s driving this trend, and how can oncologists avoid overtreatment with immunotherapy at the end of life?
The N-of-1 Patient
With immunotherapy at the end of life, “each of us has had our N-of-1” where a patient bounces back with a remarkable and durable response, said Don Dizon, MD, a gynecologic oncologist at Brown University, Providence, Rhode Island.
He recalled a patient with sarcoma who did not respond to chemotherapy. But after Dr. Dizon started her on immunotherapy, everything turned around. She has now been in remission for 8 years and counting.
The possibility of an unexpected or remarkable responder is seductive. And the improved safety of immunotherapy over chemotherapy adds to the allure.
Meanwhile, patients are often desperate. It’s rare for someone to be ready to stop treatment, Dr. Dizon said. Everybody “hopes that they’re going to be the exceptional responder.”
At the end of the day, the question often becomes: “Why not try immunotherapy? What’s there to lose?”
This thinking may be prompting broader use of immunotherapy in late-stage disease, even in instances with no Food and Drug Administration indication and virtually no supportive data, such as for metastatic ovarian cancer, Dr. Dizon said.
Back to Earth
The problem with the hopeful approach is that end-of-life turnarounds with immunotherapy are rare, and there’s no way at the moment to predict who will have one, said Laura Petrillo, MD, a palliative care physician at Massachusetts General Hospital, Boston.
Even though immunotherapy generally comes with fewer adverse events than chemotherapy, catastrophic side effects are still possible.
Dr. Petrillo recalled a 95-year-old woman with metastatic cancer who was largely asymptomatic.
She had a qualifying mutation for a checkpoint inhibitor, so her oncologist started her on one. The patient never bounced back from the severe colitis the agent caused, and she died of complications in the hospital.
Although such reactions with immunotherapy are uncommon, less serious problems caused by the agents can still have a major impact on a person’s quality of life. Low-grade diarrhea, for instance, may not sound too bad, but in a patient’s daily life, it can translate to six or more episodes a day.
Even with no side effects, prescribing immunotherapy can mean that patients with limited time left spend a good portion of it at an infusion clinic instead of at home. These patients are also less likely to be referred to hospice and more likely to be admitted to and die in the hospital.
And with treatments that can cost $20,000 per dose, financial toxicity becomes a big concern.
In short, some of the reasons why chemotherapy is not recommended at the end of life also apply to immunotherapy, Dr. Petrillo said.
Prescribing Decisions
Recent research highlights the growing use of immunotherapy at the end of life.
Dr. Khan’s retrospective study found, for instance, that the percentage of patients starting immunotherapy in the last 30 days of life increased by about fourfold to fivefold over the study period for the three cancers analyzed — stage IV melanoma, lung, and kidney cancers.
Among the population that died within 30 days, the percentage receiving immunotherapy increased over the study periods — 0.8%-4.3% for melanoma, 0.9%-3.2% for NSCLC, and 0.5%-2.6% for kidney cell carcinoma — prompting the conclusion that immunotherapy prescriptions in the last month of life are on the rise.
Prescribing immunotherapy in patients who ultimately died within 1 month occurred more frequently at low-volume, nonacademic centers than at academic or high-volume centers, and outcomes varied by practice setting.
Patients had better survival outcomes overall when receiving immunotherapy at academic or high-volume centers — a finding Dr. Khan said is worth investigating further. Possible explanations include better management of severe immune-related side effects at larger centers and more caution when prescribing immunotherapy to “borderline” candidates, such as those with several comorbidities.
Importantly, given the retrospective design, Dr. Khan and colleagues already knew which patients prescribed immunotherapy died within 30 days of initiating treatment.
More specifically, 5192 of 71,204 patients who received immunotherapy (7.3%) died within a month of initiating therapy, while 66,012 (92.7%) lived beyond that point.
The study, however, did not assess how the remaining 92.7% who lived beyond 30 days fared on immunotherapy and the differences between those who lived less than 30 days and those who survived longer.
Knowing the outcome of patients at the outset of the analysis still leaves open the question of when immunotherapy can extend life and when it can’t for the patient in front of you.
To avoid overtreating at the end of life, it’s important to have “the same standard that you have for giving chemotherapy. You have to treat it with the same respect,” said Moshe Chasky, MD, a community medical oncologist with Alliance Cancer Specialists in Philadelphia, Pennsylvania. “You can’t just be throwing” immunotherapy around “at the end of life.”
While there are no clear predictors of risk and benefit, there are some factors to help guide decisions.
As with chemotherapy, Dr. Petrillo said performance status is key. Dr. Petrillo and colleagues found that median overall survival with immune checkpoint inhibitors for advanced non–small cell lung cancer was 14.3 months in patients with an Eastern Cooperative Oncology Group performance score of 0-1 but only 4.5 months with scores of ≥ 2.
Dr. Khan also found that immunotherapy survival is, unsurprisingly, worse in patients with high metastatic burdens and more comorbidities.
“You should still consider immunotherapy for metastatic melanoma, non–small cell lung cancer, and renal cell carcinoma,” Dr. Khan said. The message here is to “think twice before using” it, especially in comorbid patients with widespread metastases.
“Just because something can be done doesn’t always mean it should be done,” he said.
At Yale, when Dr. Khan works, immunotherapy decisions are considered by a multidisciplinary tumor board. At Mass General, immunotherapy has generally moved to the frontline setting, and the hospital no longer prescribes checkpoint inhibitors to hospitalized patients because the cost is too high relative to the potential benefit, Dr. Petrillo explained.
Still, with all the uncertainties about risk and benefit, counseling patients is a challenge. Dr. Dizon called it “the epitome of shared decision-making.”
Dr. Petrillo noted that it’s critical not to counsel patients based solely on the anecdotal patients who do surprisingly well.
“It’s hard to mention that and not have that be what somebody anchors on,” she said. But that speaks to “how desperate people can feel, how hopeful they can be.”
Dr. Khan, Dr. Petrillo, and Dr. Chasky all reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
How Clinicians Can Help Patients Navigate Psychedelics/Microdosing
Peter Grinspoon, MD, has some advice for clinicians when patients ask questions about microdosing of psychedelics: Keep the lines of communication open — and don’t be judgmental.
“If you’re dismissive or critical or sound like you’re judging them, then the patients just clam up,” said Dr. Grinspoon, a professor of medicine at Harvard Medical School and a primary care physician at Massachusetts General Hospital, both in Boston.
Psychedelic drugs are still illegal in the majority of states despite the growth of public interest in and use of these substances. That growth is evidenced by a flurry of workshops, reports, law enforcement seizures, and pressure by Congressional members for the Food and Drug Administration to approve new psychedelic drugs, just in the past year.
A recent study in JAMA Health Forum showed a nearly 14-fold increase in Google searches — from 7.9 to 105.6 per 10 million nationwide — for the term “microdosing” and related wording, between 2015 and 2023.
Two states — Oregon and Colorado — have decriminalized certain psychedelic drugs and are in various stages of establishing regulations and centers for prospective clients. Almost two dozen localities, like Ann Arbor, Michigan, have decriminalized psychedelic drugs. A handful of states have active legislation to decriminalize use, while others have bills that never made it out of committee.
But no definitive studies have reported that microdosing produces positive mental effects at a higher rate than placebo, according to Dr. Grinspoon. So
“We’re in this renaissance where everybody is idealizing these medications, as opposed to 20 years ago when we were in the war on drugs and everybody was dismissing them,” Dr. Grinspoon said. “The truth is somewhere in between.”
The Science
Microdosing is defined as taking doses of 1/5 to 1/20 of the conventional recreational amount, which might include a dried psilocybin mushroom, lysergic acid diethylamide, or 3,4-methylenedioxymethamphetamine. But even that much may be neither effective nor safe.
Dr. Grinspoon said clinicians should tell patients that psychedelics may cause harm, although the drugs are relatively nontoxic and are not addictive. An illegally obtained psilocybin could cause negative reactions, especially if the drug has been adulterated with other substances and if the actual dose is higher than what was indicated by the seller.
He noted that people have different reactions to psychedelics, just as they have to prescription medications. He cited one example of a woman who microdosed and could not sleep for 2 weeks afterward. Only recently have randomized, double-blinded studies begun on benefits and harms.
Researchers have also begun investigating whether long-term microdosing of psilocybin could lead to valvular heart disease (VHD), said Kevin Yang, MD, a psychiatry resident at the University of California San Diego School of Medicine. A recent review of evidence concluded that microdosing various psychedelics over a period of months can lead to drug-induced VHD.
“It’s extremely important to emphasize with patients that not only do we not know if it works or not, we also don’t really know how safe it is,” Dr. Yang said.
Dr. Yang also said clinicians should consider referring patients to a mental health professional, and especially those that may have expertise in psychedelic therapies.
One of those experts is Rachel Yehuda, PhD, director of the Center for Psychedelic Psychotherapy and Trauma Research at Icahn School of Medicine at Mount Sinai in New York City. She said therapists should be able to assess the patient’s perceived need for microdosing and “invite reflections about why current approaches are falling short.”
“I would also not actively discourage it either but remain curious until both of you have a better understanding of the reasons for seeking this out and potential alternative strategies for obtaining more therapeutic benefits,” she said. “I think it is really important to study the effects of both micro- and macrodosing of psychedelics but not move in advance of the data.”
Navigating Legality
Recent ballot measures in Oregon and Colorado directed the states to develop regulated and licensed psilocybin-assisted therapy centers for legal “trips.” Oregon’s first center was opened in 2023, and Colorado is now developing its own licensing model.
According to the Oregon Health Authority, the centers are not medical facilities, and prescription or referral from a medical professional is not required.
The Oregon Academy of Family Physicians (OAFP) has yet to release guidance to clinicians on how to talk to their patients about these drugs or potential interest in visiting a licensed therapy center.
However, Betsy Boyd-Flynn, executive director of OAFP, said the organization is working on continuing medical education for what the average family physician needs to know if a patient asks about use.
“We suspect that many of our members have interest and want to learn more,” she said.
Dr. Grinspoon said clinicians should talk with patients about legality during these conversations.
“The big question I get is: ‘I really want to try microdosing, but how do I obtain the mushrooms?’ ” he said. “You can’t really as a physician tell them to do anything illegal. So you tell them to be safe, be careful, and to use their judgment.”
Patients who want to pursue microdosing who do not live in Oregon have two legal and safe options, Dr. Grinspoon said: Enroll in a clinical study or find a facility in a state or country — such as Oregon or Jamaica — that offers microdosing with psilocybin.
Clinicians also should warn their patients that the consequences of obtaining illicit psilocybin could exacerbate the mental health stresses they are seeking to alleviate.
“It’s going to get worse if they get tangled up with law enforcement or take something that’s contaminated and they get real sick,” he said.
Lisa Gillespie contributed reporting to this story. A version of this article appeared on Medscape.com.
Peter Grinspoon, MD, has some advice for clinicians when patients ask questions about microdosing of psychedelics: Keep the lines of communication open — and don’t be judgmental.
“If you’re dismissive or critical or sound like you’re judging them, then the patients just clam up,” said Dr. Grinspoon, a professor of medicine at Harvard Medical School and a primary care physician at Massachusetts General Hospital, both in Boston.
Psychedelic drugs are still illegal in the majority of states despite the growth of public interest in and use of these substances. That growth is evidenced by a flurry of workshops, reports, law enforcement seizures, and pressure by Congressional members for the Food and Drug Administration to approve new psychedelic drugs, just in the past year.
A recent study in JAMA Health Forum showed a nearly 14-fold increase in Google searches — from 7.9 to 105.6 per 10 million nationwide — for the term “microdosing” and related wording, between 2015 and 2023.
Two states — Oregon and Colorado — have decriminalized certain psychedelic drugs and are in various stages of establishing regulations and centers for prospective clients. Almost two dozen localities, like Ann Arbor, Michigan, have decriminalized psychedelic drugs. A handful of states have active legislation to decriminalize use, while others have bills that never made it out of committee.
But no definitive studies have reported that microdosing produces positive mental effects at a higher rate than placebo, according to Dr. Grinspoon. So
“We’re in this renaissance where everybody is idealizing these medications, as opposed to 20 years ago when we were in the war on drugs and everybody was dismissing them,” Dr. Grinspoon said. “The truth is somewhere in between.”
The Science
Microdosing is defined as taking doses of 1/5 to 1/20 of the conventional recreational amount, which might include a dried psilocybin mushroom, lysergic acid diethylamide, or 3,4-methylenedioxymethamphetamine. But even that much may be neither effective nor safe.
Dr. Grinspoon said clinicians should tell patients that psychedelics may cause harm, although the drugs are relatively nontoxic and are not addictive. An illegally obtained psilocybin could cause negative reactions, especially if the drug has been adulterated with other substances and if the actual dose is higher than what was indicated by the seller.
He noted that people have different reactions to psychedelics, just as they have to prescription medications. He cited one example of a woman who microdosed and could not sleep for 2 weeks afterward. Only recently have randomized, double-blinded studies begun on benefits and harms.
Researchers have also begun investigating whether long-term microdosing of psilocybin could lead to valvular heart disease (VHD), said Kevin Yang, MD, a psychiatry resident at the University of California San Diego School of Medicine. A recent review of evidence concluded that microdosing various psychedelics over a period of months can lead to drug-induced VHD.
“It’s extremely important to emphasize with patients that not only do we not know if it works or not, we also don’t really know how safe it is,” Dr. Yang said.
Dr. Yang also said clinicians should consider referring patients to a mental health professional, and especially those that may have expertise in psychedelic therapies.
One of those experts is Rachel Yehuda, PhD, director of the Center for Psychedelic Psychotherapy and Trauma Research at Icahn School of Medicine at Mount Sinai in New York City. She said therapists should be able to assess the patient’s perceived need for microdosing and “invite reflections about why current approaches are falling short.”
“I would also not actively discourage it either but remain curious until both of you have a better understanding of the reasons for seeking this out and potential alternative strategies for obtaining more therapeutic benefits,” she said. “I think it is really important to study the effects of both micro- and macrodosing of psychedelics but not move in advance of the data.”
Navigating Legality
Recent ballot measures in Oregon and Colorado directed the states to develop regulated and licensed psilocybin-assisted therapy centers for legal “trips.” Oregon’s first center was opened in 2023, and Colorado is now developing its own licensing model.
According to the Oregon Health Authority, the centers are not medical facilities, and prescription or referral from a medical professional is not required.
The Oregon Academy of Family Physicians (OAFP) has yet to release guidance to clinicians on how to talk to their patients about these drugs or potential interest in visiting a licensed therapy center.
However, Betsy Boyd-Flynn, executive director of OAFP, said the organization is working on continuing medical education for what the average family physician needs to know if a patient asks about use.
“We suspect that many of our members have interest and want to learn more,” she said.
Dr. Grinspoon said clinicians should talk with patients about legality during these conversations.
“The big question I get is: ‘I really want to try microdosing, but how do I obtain the mushrooms?’ ” he said. “You can’t really as a physician tell them to do anything illegal. So you tell them to be safe, be careful, and to use their judgment.”
Patients who want to pursue microdosing who do not live in Oregon have two legal and safe options, Dr. Grinspoon said: Enroll in a clinical study or find a facility in a state or country — such as Oregon or Jamaica — that offers microdosing with psilocybin.
Clinicians also should warn their patients that the consequences of obtaining illicit psilocybin could exacerbate the mental health stresses they are seeking to alleviate.
“It’s going to get worse if they get tangled up with law enforcement or take something that’s contaminated and they get real sick,” he said.
Lisa Gillespie contributed reporting to this story. A version of this article appeared on Medscape.com.
Peter Grinspoon, MD, has some advice for clinicians when patients ask questions about microdosing of psychedelics: Keep the lines of communication open — and don’t be judgmental.
“If you’re dismissive or critical or sound like you’re judging them, then the patients just clam up,” said Dr. Grinspoon, a professor of medicine at Harvard Medical School and a primary care physician at Massachusetts General Hospital, both in Boston.
Psychedelic drugs are still illegal in the majority of states despite the growth of public interest in and use of these substances. That growth is evidenced by a flurry of workshops, reports, law enforcement seizures, and pressure by Congressional members for the Food and Drug Administration to approve new psychedelic drugs, just in the past year.
A recent study in JAMA Health Forum showed a nearly 14-fold increase in Google searches — from 7.9 to 105.6 per 10 million nationwide — for the term “microdosing” and related wording, between 2015 and 2023.
Two states — Oregon and Colorado — have decriminalized certain psychedelic drugs and are in various stages of establishing regulations and centers for prospective clients. Almost two dozen localities, like Ann Arbor, Michigan, have decriminalized psychedelic drugs. A handful of states have active legislation to decriminalize use, while others have bills that never made it out of committee.
But no definitive studies have reported that microdosing produces positive mental effects at a higher rate than placebo, according to Dr. Grinspoon. So
“We’re in this renaissance where everybody is idealizing these medications, as opposed to 20 years ago when we were in the war on drugs and everybody was dismissing them,” Dr. Grinspoon said. “The truth is somewhere in between.”
The Science
Microdosing is defined as taking doses of 1/5 to 1/20 of the conventional recreational amount, which might include a dried psilocybin mushroom, lysergic acid diethylamide, or 3,4-methylenedioxymethamphetamine. But even that much may be neither effective nor safe.
Dr. Grinspoon said clinicians should tell patients that psychedelics may cause harm, although the drugs are relatively nontoxic and are not addictive. An illegally obtained psilocybin could cause negative reactions, especially if the drug has been adulterated with other substances and if the actual dose is higher than what was indicated by the seller.
He noted that people have different reactions to psychedelics, just as they have to prescription medications. He cited one example of a woman who microdosed and could not sleep for 2 weeks afterward. Only recently have randomized, double-blinded studies begun on benefits and harms.
Researchers have also begun investigating whether long-term microdosing of psilocybin could lead to valvular heart disease (VHD), said Kevin Yang, MD, a psychiatry resident at the University of California San Diego School of Medicine. A recent review of evidence concluded that microdosing various psychedelics over a period of months can lead to drug-induced VHD.
“It’s extremely important to emphasize with patients that not only do we not know if it works or not, we also don’t really know how safe it is,” Dr. Yang said.
Dr. Yang also said clinicians should consider referring patients to a mental health professional, and especially those that may have expertise in psychedelic therapies.
One of those experts is Rachel Yehuda, PhD, director of the Center for Psychedelic Psychotherapy and Trauma Research at Icahn School of Medicine at Mount Sinai in New York City. She said therapists should be able to assess the patient’s perceived need for microdosing and “invite reflections about why current approaches are falling short.”
“I would also not actively discourage it either but remain curious until both of you have a better understanding of the reasons for seeking this out and potential alternative strategies for obtaining more therapeutic benefits,” she said. “I think it is really important to study the effects of both micro- and macrodosing of psychedelics but not move in advance of the data.”
Navigating Legality
Recent ballot measures in Oregon and Colorado directed the states to develop regulated and licensed psilocybin-assisted therapy centers for legal “trips.” Oregon’s first center was opened in 2023, and Colorado is now developing its own licensing model.
According to the Oregon Health Authority, the centers are not medical facilities, and prescription or referral from a medical professional is not required.
The Oregon Academy of Family Physicians (OAFP) has yet to release guidance to clinicians on how to talk to their patients about these drugs or potential interest in visiting a licensed therapy center.
However, Betsy Boyd-Flynn, executive director of OAFP, said the organization is working on continuing medical education for what the average family physician needs to know if a patient asks about use.
“We suspect that many of our members have interest and want to learn more,” she said.
Dr. Grinspoon said clinicians should talk with patients about legality during these conversations.
“The big question I get is: ‘I really want to try microdosing, but how do I obtain the mushrooms?’ ” he said. “You can’t really as a physician tell them to do anything illegal. So you tell them to be safe, be careful, and to use their judgment.”
Patients who want to pursue microdosing who do not live in Oregon have two legal and safe options, Dr. Grinspoon said: Enroll in a clinical study or find a facility in a state or country — such as Oregon or Jamaica — that offers microdosing with psilocybin.
Clinicians also should warn their patients that the consequences of obtaining illicit psilocybin could exacerbate the mental health stresses they are seeking to alleviate.
“It’s going to get worse if they get tangled up with law enforcement or take something that’s contaminated and they get real sick,” he said.
Lisa Gillespie contributed reporting to this story. A version of this article appeared on Medscape.com.
Can Addressing Depression Reduce Chemo Toxicity in Older Adults?
TOPLINE:
METHODOLOGY:
- Researchers conducted a secondary analysis of a randomized controlled trial to evaluate whether greater reductions in grade 3 chemotherapy-related toxicities occurred with geriatric assessment-driven interventions vs standard care.
- A total of 605 patients aged 65 years and older with any stage of solid malignancy were included, with 402 randomized to the intervention arm and 203 to the standard-of-care arm.
- Mental health was assessed using the Mental Health Inventory 13, and chemotherapy toxicity was graded by the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0.
- Patients in the intervention arm received recommendations from a multidisciplinary team based on their baseline GA, while those in the standard-of-care arm received only the baseline assessment results.
- The study was conducted at City of Hope National Medical Center in Duarte, California, and patients were followed throughout treatment or for up to 6 months from starting chemotherapy.
TAKEAWAY:
- According to the authors, patients with depression had increased chemotherapy toxicity in the standard-of-care arm (70.7% vs 54.3%; P = .02) but not in the GA-driven intervention arm (54.3% vs 48.5%; P = .27).
- The association between depression and chemotherapy toxicity was also seen after adjustment for the Cancer and Aging Research Group toxicity score (odds ratio, [OR], 1.98; 95% CI, 1.07-3.65) and for demographic, disease, and treatment factors (OR, 2.00; 95% CI, 1.03-3.85).
- No significant association was found between anxiety and chemotherapy toxicity in either the standard-of-care arm (univariate OR, 1.07; 95% CI, 0.61-1.88) or the GA-driven intervention arm (univariate OR, 1.15; 95% CI, 0.78-1.71).
- The authors stated that depression was associated with increased odds of hematologic-only toxicities (OR, 2.50; 95% CI, 1.13-5.56) in the standard-of-care arm.
- An analysis of a small subgroup found associations between elevated anxiety symptoms and increased risk for hematologic and nonhematologic chemotherapy toxicities.
IN PRACTICE:
“The current study showed that elevated depression symptoms are associated with increased risk of severe chemotherapy toxicities in older adults with cancer. This risk was mitigated in those in the GA intervention arm, which suggests that addressing elevated depression symptoms may lower the risk of toxicities,” the authors wrote. “Overall, elevated anxiety symptoms were not associated with risk for severe chemotherapy toxicity.”
SOURCE:
Reena V. Jayani, MD, MSCI, of Vanderbilt University Medical Center in Nashville, Tennessee, was the first and corresponding author for this paper. This study was published online August 4, 2024, in Cancer.
LIMITATIONS:
The thresholds for depression and anxiety used in the Mental Health Inventory 13 were based on an English-speaking population, which may not be fully applicable to Chinese- and Spanish-speaking patients included in the study. Depression and anxiety were not evaluated by a mental health professional or with a structured interview to assess formal diagnostic criteria. Psychiatric medication used at the time of baseline GA was not included in the analysis. The study is a secondary analysis of a randomized controlled trial, and it is not known which components of the interventions affected mental health.
DISCLOSURES:
This research project was supported by the UniHealth Foundation, the City of Hope Center for Cancer and Aging, and the National Institutes of Health. One coauthor disclosed receiving institutional research funding from AstraZeneca and Brooklyn ImmunoTherapeutics and consulting for multiple pharmaceutical companies, including AbbVie, Adagene, and Bayer HealthCare Pharmaceuticals. William Dale, MD, PhD, of City of Hope National Medical Center, served as senior author and a principal investigator. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Researchers conducted a secondary analysis of a randomized controlled trial to evaluate whether greater reductions in grade 3 chemotherapy-related toxicities occurred with geriatric assessment-driven interventions vs standard care.
- A total of 605 patients aged 65 years and older with any stage of solid malignancy were included, with 402 randomized to the intervention arm and 203 to the standard-of-care arm.
- Mental health was assessed using the Mental Health Inventory 13, and chemotherapy toxicity was graded by the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0.
- Patients in the intervention arm received recommendations from a multidisciplinary team based on their baseline GA, while those in the standard-of-care arm received only the baseline assessment results.
- The study was conducted at City of Hope National Medical Center in Duarte, California, and patients were followed throughout treatment or for up to 6 months from starting chemotherapy.
TAKEAWAY:
- According to the authors, patients with depression had increased chemotherapy toxicity in the standard-of-care arm (70.7% vs 54.3%; P = .02) but not in the GA-driven intervention arm (54.3% vs 48.5%; P = .27).
- The association between depression and chemotherapy toxicity was also seen after adjustment for the Cancer and Aging Research Group toxicity score (odds ratio, [OR], 1.98; 95% CI, 1.07-3.65) and for demographic, disease, and treatment factors (OR, 2.00; 95% CI, 1.03-3.85).
- No significant association was found between anxiety and chemotherapy toxicity in either the standard-of-care arm (univariate OR, 1.07; 95% CI, 0.61-1.88) or the GA-driven intervention arm (univariate OR, 1.15; 95% CI, 0.78-1.71).
- The authors stated that depression was associated with increased odds of hematologic-only toxicities (OR, 2.50; 95% CI, 1.13-5.56) in the standard-of-care arm.
- An analysis of a small subgroup found associations between elevated anxiety symptoms and increased risk for hematologic and nonhematologic chemotherapy toxicities.
IN PRACTICE:
“The current study showed that elevated depression symptoms are associated with increased risk of severe chemotherapy toxicities in older adults with cancer. This risk was mitigated in those in the GA intervention arm, which suggests that addressing elevated depression symptoms may lower the risk of toxicities,” the authors wrote. “Overall, elevated anxiety symptoms were not associated with risk for severe chemotherapy toxicity.”
SOURCE:
Reena V. Jayani, MD, MSCI, of Vanderbilt University Medical Center in Nashville, Tennessee, was the first and corresponding author for this paper. This study was published online August 4, 2024, in Cancer.
LIMITATIONS:
The thresholds for depression and anxiety used in the Mental Health Inventory 13 were based on an English-speaking population, which may not be fully applicable to Chinese- and Spanish-speaking patients included in the study. Depression and anxiety were not evaluated by a mental health professional or with a structured interview to assess formal diagnostic criteria. Psychiatric medication used at the time of baseline GA was not included in the analysis. The study is a secondary analysis of a randomized controlled trial, and it is not known which components of the interventions affected mental health.
DISCLOSURES:
This research project was supported by the UniHealth Foundation, the City of Hope Center for Cancer and Aging, and the National Institutes of Health. One coauthor disclosed receiving institutional research funding from AstraZeneca and Brooklyn ImmunoTherapeutics and consulting for multiple pharmaceutical companies, including AbbVie, Adagene, and Bayer HealthCare Pharmaceuticals. William Dale, MD, PhD, of City of Hope National Medical Center, served as senior author and a principal investigator. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Researchers conducted a secondary analysis of a randomized controlled trial to evaluate whether greater reductions in grade 3 chemotherapy-related toxicities occurred with geriatric assessment-driven interventions vs standard care.
- A total of 605 patients aged 65 years and older with any stage of solid malignancy were included, with 402 randomized to the intervention arm and 203 to the standard-of-care arm.
- Mental health was assessed using the Mental Health Inventory 13, and chemotherapy toxicity was graded by the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0.
- Patients in the intervention arm received recommendations from a multidisciplinary team based on their baseline GA, while those in the standard-of-care arm received only the baseline assessment results.
- The study was conducted at City of Hope National Medical Center in Duarte, California, and patients were followed throughout treatment or for up to 6 months from starting chemotherapy.
TAKEAWAY:
- According to the authors, patients with depression had increased chemotherapy toxicity in the standard-of-care arm (70.7% vs 54.3%; P = .02) but not in the GA-driven intervention arm (54.3% vs 48.5%; P = .27).
- The association between depression and chemotherapy toxicity was also seen after adjustment for the Cancer and Aging Research Group toxicity score (odds ratio, [OR], 1.98; 95% CI, 1.07-3.65) and for demographic, disease, and treatment factors (OR, 2.00; 95% CI, 1.03-3.85).
- No significant association was found between anxiety and chemotherapy toxicity in either the standard-of-care arm (univariate OR, 1.07; 95% CI, 0.61-1.88) or the GA-driven intervention arm (univariate OR, 1.15; 95% CI, 0.78-1.71).
- The authors stated that depression was associated with increased odds of hematologic-only toxicities (OR, 2.50; 95% CI, 1.13-5.56) in the standard-of-care arm.
- An analysis of a small subgroup found associations between elevated anxiety symptoms and increased risk for hematologic and nonhematologic chemotherapy toxicities.
IN PRACTICE:
“The current study showed that elevated depression symptoms are associated with increased risk of severe chemotherapy toxicities in older adults with cancer. This risk was mitigated in those in the GA intervention arm, which suggests that addressing elevated depression symptoms may lower the risk of toxicities,” the authors wrote. “Overall, elevated anxiety symptoms were not associated with risk for severe chemotherapy toxicity.”
SOURCE:
Reena V. Jayani, MD, MSCI, of Vanderbilt University Medical Center in Nashville, Tennessee, was the first and corresponding author for this paper. This study was published online August 4, 2024, in Cancer.
LIMITATIONS:
The thresholds for depression and anxiety used in the Mental Health Inventory 13 were based on an English-speaking population, which may not be fully applicable to Chinese- and Spanish-speaking patients included in the study. Depression and anxiety were not evaluated by a mental health professional or with a structured interview to assess formal diagnostic criteria. Psychiatric medication used at the time of baseline GA was not included in the analysis. The study is a secondary analysis of a randomized controlled trial, and it is not known which components of the interventions affected mental health.
DISCLOSURES:
This research project was supported by the UniHealth Foundation, the City of Hope Center for Cancer and Aging, and the National Institutes of Health. One coauthor disclosed receiving institutional research funding from AstraZeneca and Brooklyn ImmunoTherapeutics and consulting for multiple pharmaceutical companies, including AbbVie, Adagene, and Bayer HealthCare Pharmaceuticals. William Dale, MD, PhD, of City of Hope National Medical Center, served as senior author and a principal investigator. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Tool Can Help Predict Futile Surgery in Pancreatic Cancer
TOPLINE:
METHODOLOGY:
- Immediate resection is associated with a high incidence of postoperative complications and disease recurrence within a year of surgery in patients with pancreatic ductal adenocarcinoma. Predicting which patients likely won’t benefit from upfront pancreatectomy is important.
- To identify preoperative risk factors for futile pancreatectomy, researchers evaluated 1426 patients (median age, 69 years; 53.2% men) with anatomically resectable pancreatic ductal adenocarcinoma who underwent pancreatic resection between January 2010 and December 2021.
- The patients were divided into derivation (n = 885) and validation (n = 541) cohorts.
- The primary outcome was the rate of futile upfront pancreatectomy, defined as death or disease recurrence within 6 months of surgery. Patients were divided into three risk categories — low, intermediate, and high risk — each with escalating likelihoods of futile resection, worse pathological features, and worse outcomes.
- The secondary endpoint was to develop criteria for surgical candidacy, setting a futility likelihood threshold of < 20%. This threshold corresponds to the lower bound of the 95% confidence interval (CI) for postneoadjuvant resection rates (resection rate, 0.90; 95% CI, 0.80-1.01) from recent meta-analyses.
TAKEAWAY:
- The futility rate for pancreatectomy was 18.9% — 19.2% in the development cohort and 18.6% in the validation cohort. Three independent risk factors for futile resection included American Society of Anesthesiologists (ASA) class (95% CI for coefficients, 0.68-0.87), preoperative cancer antigen 19.9 serum levels (95% CI for coefficients, 0.05-0.75), and radiologic tumor size (95% CI for coefficients, 0.28-0.46).
- Using these independent risk factors, the predictive model demonstrated adequate calibration and discrimination in both the derivation and validation cohorts.
- The researchers then identified three risk groups. In the derivation cohort, the rate of futile pancreatectomy was 9.2% in the low-risk group, 18.0% in the intermediate-risk group, and 28.7% in the high-risk group (P < .001 for trend). In the validation cohort, the futility rate was 10.9% in the low-risk group, 20.2% in the intermediate-risk group, and 29.2% in the high-risk group (P < .001 for trend).
- Researchers identified four conditions associated with a futility likelihood below 20%, where larger tumor size is paired with lower cancer antigen 19.9 levels (defined as cancer antigen 19.9–adjusted-to-size). Patients who met these criteria experienced significantly longer disease-free survival (median 18.4 months vs 11.2 months) and overall survival (38.5 months vs 22.1 months).
IN PRACTICE:
“Although the study provides an easy-to-use calculator for clinical decision-making, there are some methodological limitations,” according to the authors of accompanying commentary. These limitations include failing to accurately describe how ASA class, cancer antigen 19.9 level, and tumor size were chosen for the model. “While we do not think the model is yet ready for standard clinical use, it may prove to be a viable tool if tested in future randomized trials comparing the neoadjuvant approach to upfront surgery in resectable pancreatic cancer,” the editorialists added.
SOURCE:
This study, led by Stefano Crippa, MD, PhD, Division of Pancreatic Surgery, Pancreas Translational and Clinical Research Center, San Raffaele Scientific Institute, Vita-Salute San Raffaele University, Milan, Italy, and the accompanying commentary were published online in JAMA Surgery.
LIMITATIONS:
In addition to the limitations noted by the editorialists, others include the study’s retrospective design, which could introduce bias. Because preoperative imaging was not revised, the assigned resectability classes could show variability. Institutional differences existed in the selection process for upfront pancreatectomy. The model cannot be applied to cancer antigen 19.9 nonsecretors and was not externally validated.
DISCLOSURES:
The Italian Association for Cancer Research Special Program in Metastatic Disease and Italian Ministry of Health/Italian Foundation for the Research of Pancreatic Diseases supported the study in the form of a grant. Two authors reported receiving personal fees outside the submitted work. No other disclosures were reported.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Immediate resection is associated with a high incidence of postoperative complications and disease recurrence within a year of surgery in patients with pancreatic ductal adenocarcinoma. Predicting which patients likely won’t benefit from upfront pancreatectomy is important.
- To identify preoperative risk factors for futile pancreatectomy, researchers evaluated 1426 patients (median age, 69 years; 53.2% men) with anatomically resectable pancreatic ductal adenocarcinoma who underwent pancreatic resection between January 2010 and December 2021.
- The patients were divided into derivation (n = 885) and validation (n = 541) cohorts.
- The primary outcome was the rate of futile upfront pancreatectomy, defined as death or disease recurrence within 6 months of surgery. Patients were divided into three risk categories — low, intermediate, and high risk — each with escalating likelihoods of futile resection, worse pathological features, and worse outcomes.
- The secondary endpoint was to develop criteria for surgical candidacy, setting a futility likelihood threshold of < 20%. This threshold corresponds to the lower bound of the 95% confidence interval (CI) for postneoadjuvant resection rates (resection rate, 0.90; 95% CI, 0.80-1.01) from recent meta-analyses.
TAKEAWAY:
- The futility rate for pancreatectomy was 18.9% — 19.2% in the development cohort and 18.6% in the validation cohort. Three independent risk factors for futile resection included American Society of Anesthesiologists (ASA) class (95% CI for coefficients, 0.68-0.87), preoperative cancer antigen 19.9 serum levels (95% CI for coefficients, 0.05-0.75), and radiologic tumor size (95% CI for coefficients, 0.28-0.46).
- Using these independent risk factors, the predictive model demonstrated adequate calibration and discrimination in both the derivation and validation cohorts.
- The researchers then identified three risk groups. In the derivation cohort, the rate of futile pancreatectomy was 9.2% in the low-risk group, 18.0% in the intermediate-risk group, and 28.7% in the high-risk group (P < .001 for trend). In the validation cohort, the futility rate was 10.9% in the low-risk group, 20.2% in the intermediate-risk group, and 29.2% in the high-risk group (P < .001 for trend).
- Researchers identified four conditions associated with a futility likelihood below 20%, where larger tumor size is paired with lower cancer antigen 19.9 levels (defined as cancer antigen 19.9–adjusted-to-size). Patients who met these criteria experienced significantly longer disease-free survival (median 18.4 months vs 11.2 months) and overall survival (38.5 months vs 22.1 months).
IN PRACTICE:
“Although the study provides an easy-to-use calculator for clinical decision-making, there are some methodological limitations,” according to the authors of accompanying commentary. These limitations include failing to accurately describe how ASA class, cancer antigen 19.9 level, and tumor size were chosen for the model. “While we do not think the model is yet ready for standard clinical use, it may prove to be a viable tool if tested in future randomized trials comparing the neoadjuvant approach to upfront surgery in resectable pancreatic cancer,” the editorialists added.
SOURCE:
This study, led by Stefano Crippa, MD, PhD, Division of Pancreatic Surgery, Pancreas Translational and Clinical Research Center, San Raffaele Scientific Institute, Vita-Salute San Raffaele University, Milan, Italy, and the accompanying commentary were published online in JAMA Surgery.
LIMITATIONS:
In addition to the limitations noted by the editorialists, others include the study’s retrospective design, which could introduce bias. Because preoperative imaging was not revised, the assigned resectability classes could show variability. Institutional differences existed in the selection process for upfront pancreatectomy. The model cannot be applied to cancer antigen 19.9 nonsecretors and was not externally validated.
DISCLOSURES:
The Italian Association for Cancer Research Special Program in Metastatic Disease and Italian Ministry of Health/Italian Foundation for the Research of Pancreatic Diseases supported the study in the form of a grant. Two authors reported receiving personal fees outside the submitted work. No other disclosures were reported.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Immediate resection is associated with a high incidence of postoperative complications and disease recurrence within a year of surgery in patients with pancreatic ductal adenocarcinoma. Predicting which patients likely won’t benefit from upfront pancreatectomy is important.
- To identify preoperative risk factors for futile pancreatectomy, researchers evaluated 1426 patients (median age, 69 years; 53.2% men) with anatomically resectable pancreatic ductal adenocarcinoma who underwent pancreatic resection between January 2010 and December 2021.
- The patients were divided into derivation (n = 885) and validation (n = 541) cohorts.
- The primary outcome was the rate of futile upfront pancreatectomy, defined as death or disease recurrence within 6 months of surgery. Patients were divided into three risk categories — low, intermediate, and high risk — each with escalating likelihoods of futile resection, worse pathological features, and worse outcomes.
- The secondary endpoint was to develop criteria for surgical candidacy, setting a futility likelihood threshold of < 20%. This threshold corresponds to the lower bound of the 95% confidence interval (CI) for postneoadjuvant resection rates (resection rate, 0.90; 95% CI, 0.80-1.01) from recent meta-analyses.
TAKEAWAY:
- The futility rate for pancreatectomy was 18.9% — 19.2% in the development cohort and 18.6% in the validation cohort. Three independent risk factors for futile resection included American Society of Anesthesiologists (ASA) class (95% CI for coefficients, 0.68-0.87), preoperative cancer antigen 19.9 serum levels (95% CI for coefficients, 0.05-0.75), and radiologic tumor size (95% CI for coefficients, 0.28-0.46).
- Using these independent risk factors, the predictive model demonstrated adequate calibration and discrimination in both the derivation and validation cohorts.
- The researchers then identified three risk groups. In the derivation cohort, the rate of futile pancreatectomy was 9.2% in the low-risk group, 18.0% in the intermediate-risk group, and 28.7% in the high-risk group (P < .001 for trend). In the validation cohort, the futility rate was 10.9% in the low-risk group, 20.2% in the intermediate-risk group, and 29.2% in the high-risk group (P < .001 for trend).
- Researchers identified four conditions associated with a futility likelihood below 20%, where larger tumor size is paired with lower cancer antigen 19.9 levels (defined as cancer antigen 19.9–adjusted-to-size). Patients who met these criteria experienced significantly longer disease-free survival (median 18.4 months vs 11.2 months) and overall survival (38.5 months vs 22.1 months).
IN PRACTICE:
“Although the study provides an easy-to-use calculator for clinical decision-making, there are some methodological limitations,” according to the authors of accompanying commentary. These limitations include failing to accurately describe how ASA class, cancer antigen 19.9 level, and tumor size were chosen for the model. “While we do not think the model is yet ready for standard clinical use, it may prove to be a viable tool if tested in future randomized trials comparing the neoadjuvant approach to upfront surgery in resectable pancreatic cancer,” the editorialists added.
SOURCE:
This study, led by Stefano Crippa, MD, PhD, Division of Pancreatic Surgery, Pancreas Translational and Clinical Research Center, San Raffaele Scientific Institute, Vita-Salute San Raffaele University, Milan, Italy, and the accompanying commentary were published online in JAMA Surgery.
LIMITATIONS:
In addition to the limitations noted by the editorialists, others include the study’s retrospective design, which could introduce bias. Because preoperative imaging was not revised, the assigned resectability classes could show variability. Institutional differences existed in the selection process for upfront pancreatectomy. The model cannot be applied to cancer antigen 19.9 nonsecretors and was not externally validated.
DISCLOSURES:
The Italian Association for Cancer Research Special Program in Metastatic Disease and Italian Ministry of Health/Italian Foundation for the Research of Pancreatic Diseases supported the study in the form of a grant. Two authors reported receiving personal fees outside the submitted work. No other disclosures were reported.
A version of this article first appeared on Medscape.com.
Impact of a Pharmacist-Led Emergency Department Urinary Tract Infection Aftercare Program
The emergency department (ED) is estimated to provide half of all medical care in the United States, serving as a conduit between ambulatory care and inpatient settings.1 According to the Centers for Disease Control and Prevention, around 11 million antibiotic prescriptions were written in EDs in 2021.2 A previous study conducted at a US Department of Veterans (VA) Affairs medical center found that about 40% of all antimicrobial use in the ED was inappropriate.3 The ED is a critical and high-yield space for antimicrobial stewardship efforts.4
Urinary tract infections (UTIs) are one of the most common reasons for ED visits.4 In 2018, there were about 3 million UTI discharge diagnoses reported in the US.5 Diagnosis and management of UTIs can vary depending on patient sex, upper or lower urinary tract involvement, and the severity of the infection.6 Most UTIs are uncomplicated and can be safely treated with oral antibiotics at home; however, if mismanaged, they can lead to increased morbidity and mortality.6
Antimicrobial prescribing in the ED is predominantly empiric with challenges such as diverse patient needs, rising antimicrobial resistance, and limited microbiologic data at the time of discharge.6 The lack of a standardized process for urine culture follow-up after discharge represents another major complicating factor in the outpatient management of UTIs. Studies have shown that ED pharmacists play a vital role in providing quality follow-up care by optimizing antimicrobial use, resulting in improved patient outcomes in various infectious syndromes, including UTIs.7-13
Program Description
In June 2021, the VA Greater Los Angeles Healthcare System (VAGLAHS) piloted an ED pharmacist-led aftercare program to optimize postdischarge antimicrobial therapy management of UTIs. After a patient is discharged from the ED, the clinical pharmacist reviews urine culture results, interprets available antimicrobial susceptibility, conducts patient interviews, adjusts for patient-specific factors, and addresses potential antibiotic-associated adverse events. The ED pharmacist is then responsible for managing therapy changes in consultation with an ED health care practitioner (HCP).
Methods
This single center, retrospective chart review included veterans who were discharged with an oral antibiotic for UTI treatment from the VAGLAHS ED and evaluated by clinical pharmacists between June 1, 2021, and June 30, 2022. For patients with multiple ED visits, only the initial ED encounter was reviewed. Patients were excluded if they had a complicated UTI diagnosis requiring intravenous antibiotics or if they were admitted to the hospital. Data were generated through the Corporate Data Warehouse by VAGLAHS Pharmacy Informatics Service. Each patient was assigned a random number using the Microsoft Excel formula =RAND( ) and then sorted in chronological order to ensure randomization at baseline prior to data collection.
The primary aim of this quality improvement project was to characterize the impact of ED pharmacist-led interventions by evaluating the proportion of empiric to targeted therapy adjustments, antibiotic therapy discontinuation, and unmodified index treatment. The secondary objectives evaluated time to ED pharmacist aftercare follow-up, days of antibiotic exposure avoided, 30-day ED visits related to a urinary source, and transition of care documentation. Descriptive statistics were performed; median and IQR were calculated in Microsoft Excel.
Results
A total of 548 ED UTI encounters were identified, including 449 patients with an index ED UTI aftercare follow-up evaluation. Of the 246 randomly screened patients, 200 veterans met inclusion criteria. The median age of included patients was 73 years and most (83.0%) were male (Table 1). One hundred thirty-two patients (66.0%) had a cystitis diagnosis, followed by complicated UTI (14.0%) and catheter-associated UTI (11.0%). The most frequently isolated uropathogen was Escherichia coli (30.5%). ß-lactams were prescribed for empiric treatment to 121 patients (60.5%), followed by 36 fluoroquinolones prescriptions (18.0%). The median treatment duration was 7 days.
The median time to ED pharmacist UTI aftercare evaluation was 2 days (Table 2). Sixty-seven cases required pharmacist intervention, which included 34 transitions to targeted therapy (17.0%) and 33 antibiotic discontinuations (16.5%). A total of 144 days of antibiotic exposure was avoided (ie, days antibiotic was prescribed minus days therapy administered). The majority of cases without modification to index therapy were due to appropriate empiric treatment selection (49.0%). Twelve (6.0%) patients had a subsequent urinary-related ED visit within 30 days due to 8 cases of persistent and/or worsening urinary symptoms (66.7%) and 2 cases of recurrent UTI (16.7%).
Discussion
Outpatient antibiotic prescribing for UTI management in the ED is challenging due to the absence of microbiologic data at time of diagnosis and lack of consistent transition of care follow-up.6 The VAGLAHS ED UTI aftercare program piloted a pharmacist-driven protocol for review of all urine cultures and optimization of antibiotic therapy.
Most ED UTI discharges that did not require pharmacist intervention had empiric treatment selection active against the clinical isolates. This suggests that the ED prescribing practices concur with theVAGLAHS antibiogram and treatment guidelines. Clinical pharmacists intervened in about one-third of UTI cases, which included modification or discontinuation of therapy. Further review of these cases demonstrated that about half of those with a subsequent 30-day ED visit related to a urinary source had therapy modification. Most patients with a 30-day ED visit had persistent and/or worsening urinary symptoms, prompting further exploratory workup.
Although this project did not evaluate time from urine culture results to aftercare review, the VAGLAHS ED pharmacists had a median follow-up time of 48 hours. This timeline mirrors the typical duration for urine culture results, suggesting that the pilot program allowed for real time pharmacist review and intervention. Consequently, this initiative resulted in the avoidance of 144 unnecessary days of antibiotic exposure.
While the current protocol highlights the work that ED pharmacists provide postdischarge, there are additional opportunities for pharmacist intervention. For example, one-third of these clinical encounters were completed without HCP notification, indicating an ongoing need to ensure continuity of care. Additionally, all 16 patients diagnosed with asymptomatic bacteriuria were discharged with an oral antibiotic, highlighting an opportunity to further optimize antibiotic prescribing prior to discharge. ED pharmacists continue to play an important role in mitigating inappropriate and unnecessary antibiotic use, which will reduce antibiotic-related adverse drug reactions, Clostridioides difficile infection, and antimicrobial resistance.
Limitations
Inconsistent and incomplete documentation of clinical data in the electronic health record made the characterization of patient encounters challenging. Furthermore, ED HCPs varying clinical practices may have impacted the heterogeneity of UTI diagnosis and management at VAGLAHS.
Conclusions
Implementation of an ED pharmacist-driven UTI aftercare program at VAGLAHS reduced unnecessary antimicrobial exposure, improved antibiotic management, and ensured continuity of care postdischarge. Findings from our project implicate possible future pharmacist involvement predischarge, such as targeting inappropriate asymptomatic bacteriuria treatment.14-16 This pilot program suggested the feasibility of integrating antimicrobial stewardship practices within the ED setting in an ongoing effort to improve the quality of care for veterans.
1. Marcozzi D, Carr B, Liferidge A, Baehr N, Browne B.. Trends in the contribution of emergency departments to the provision of hospital-associated health care in the USA. Int J Health Serv. 2018;48(2):267–288. doi:10.1177/0020731417734498
2. Centers for Disease Control and Prevention. Outpatient antibiotic prescriptions — United States, 2021. Updated October 4, 2022. Accessed May 22, 2024. https://archive.cdc.gov/#/details?url=https://www.cdc.gov/antibiotic-use/data/report-2021.html
3. Timbrook TT, Caffrey AR, Ovalle A, et al. Assessments of opportunities to improve antibiotic prescribing in an emergency department: a period prevalence survey. Infect Dis Ther. 2017;6(4):497-505. doi:10.1007/s40121-017-0175-9
4. Pulia M, Redwood R, May L. Antimicrobial stewardship in the emergency department. Emerg Med Clin North. 2018;36(4):853-872. doi:10.1016/j.emc.2018.06.012
5. Weiss A, Jiang H. Most frequent reasons for emergency department visits, 2018. December 16, 2021. Accessed May 22, 2024. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb286-ED-Frequent-Conditions-2018.pdf
6. Abrahamian FM, Moran GJ, Talan DA. Urinary tract infections in the emergency department. Infect Dis Clin North Am. 2008;22(1):73-87. doi:10.1016/j.idc.2007.10.002
7. Dumkow LE, Kenney RM, MacDonald NC, Carreno JJ, Malhotra MK, Davis SL. Impact of a multidisciplinary culture follow-up program of antimicrobial therapy in the emergency department. Infect Dis Ther. 2014;3(1):45-53. doi:10.1007/s40121-014-0026-x
8. Davis LC, Covey RB, Weston JS, Hu BB, Laine GA. Pharmacist-driven antimicrobial optimization in the emergency department. Am J Health Syst Pharm. 2016;73(5 Suppl 1):S49-S56. doi:10.2146/sp150036
9. Lingenfelter E, Darkin Z, Fritz K, Youngquist S, Madsen T, Fix M. ED pharmacist monitoring of provider antibiotic selection aids appropriate treatment for outpatient UTI. Am J Emerg Med. 2016;34(8):1600-1603. doi:10.1016/j.ajem.2016.05.076
10. Zhang X, Rowan N, Pflugeisen BM, Alajbegovic S. Urine culture guided antibiotic interventions: a pharmacist driven antimicrobial stewardship effort in the ED. Am J Emerg Med. 2017;35(4):594-598. doi:10.1016/j.ajem.2016.12.036
11. Percival KM, Valenti KM, Schmittling SE, Strader BD, Lopez RR, Bergman SJ. Impact of an antimicrobial stewardship intervention on urinary tract infection treatment in the ED. Am J Emerg Med. 2015;33(9):1129-1133. doi:10.1016/j.ajem.2015.04.067
12. Almulhim AS, Aldayyen A, Yenina K, Chiappini A, Khan TM. Optimization of antibiotic selection in the emergency department for urine culture follow ups, a retrospective pre-post intervention study: clinical pharmacist efforts. J Pharm Policy Pract. 2019;12(1):8. Published online April 9, 2019. doi:10.1186/s40545-019-0168-z
13. Stoll K, Feltz E, Ebert S. Pharmacist-driven implementation of outpatient antibiotic prescribing algorithms improves guideline adherence in the emergency department. J Pharm Pract. 2021;34(6):875-881. doi:10.1177/0897190020930979
14. Petty LA, Vaughn VM, Flanders SA, et al. Assessment of testing and treatment of asymptomatic bacteriuria initiated in the emergency department. Open Forum Infect Dis. 2020;7(12):ofaa537. Published online November 3, 2020. doi:10.1093/ofid/ofaa537
15. Ingalls EM, Veillette JJ, Olson J, et al. Impact of a multifaceted intervention on antibiotic prescribing for cystitis and asymptomatic bacteriuria in 23 community hospital emergency departments. Hosp Pharm. 2023;58(4):401-407. doi:10.1177/00185787231159578
16. Daniel M, Keller S, Mozafarihashjin M, Pahwa A, Soong C. An implementation guide to reducing overtreatment of asymptomatic bacteriuria. JAMA Intern Med. 2018;178(2):271-276.doi:10.1001/jamainternmed.2017.7290
The emergency department (ED) is estimated to provide half of all medical care in the United States, serving as a conduit between ambulatory care and inpatient settings.1 According to the Centers for Disease Control and Prevention, around 11 million antibiotic prescriptions were written in EDs in 2021.2 A previous study conducted at a US Department of Veterans (VA) Affairs medical center found that about 40% of all antimicrobial use in the ED was inappropriate.3 The ED is a critical and high-yield space for antimicrobial stewardship efforts.4
Urinary tract infections (UTIs) are one of the most common reasons for ED visits.4 In 2018, there were about 3 million UTI discharge diagnoses reported in the US.5 Diagnosis and management of UTIs can vary depending on patient sex, upper or lower urinary tract involvement, and the severity of the infection.6 Most UTIs are uncomplicated and can be safely treated with oral antibiotics at home; however, if mismanaged, they can lead to increased morbidity and mortality.6
Antimicrobial prescribing in the ED is predominantly empiric with challenges such as diverse patient needs, rising antimicrobial resistance, and limited microbiologic data at the time of discharge.6 The lack of a standardized process for urine culture follow-up after discharge represents another major complicating factor in the outpatient management of UTIs. Studies have shown that ED pharmacists play a vital role in providing quality follow-up care by optimizing antimicrobial use, resulting in improved patient outcomes in various infectious syndromes, including UTIs.7-13
Program Description
In June 2021, the VA Greater Los Angeles Healthcare System (VAGLAHS) piloted an ED pharmacist-led aftercare program to optimize postdischarge antimicrobial therapy management of UTIs. After a patient is discharged from the ED, the clinical pharmacist reviews urine culture results, interprets available antimicrobial susceptibility, conducts patient interviews, adjusts for patient-specific factors, and addresses potential antibiotic-associated adverse events. The ED pharmacist is then responsible for managing therapy changes in consultation with an ED health care practitioner (HCP).
Methods
This single center, retrospective chart review included veterans who were discharged with an oral antibiotic for UTI treatment from the VAGLAHS ED and evaluated by clinical pharmacists between June 1, 2021, and June 30, 2022. For patients with multiple ED visits, only the initial ED encounter was reviewed. Patients were excluded if they had a complicated UTI diagnosis requiring intravenous antibiotics or if they were admitted to the hospital. Data were generated through the Corporate Data Warehouse by VAGLAHS Pharmacy Informatics Service. Each patient was assigned a random number using the Microsoft Excel formula =RAND( ) and then sorted in chronological order to ensure randomization at baseline prior to data collection.
The primary aim of this quality improvement project was to characterize the impact of ED pharmacist-led interventions by evaluating the proportion of empiric to targeted therapy adjustments, antibiotic therapy discontinuation, and unmodified index treatment. The secondary objectives evaluated time to ED pharmacist aftercare follow-up, days of antibiotic exposure avoided, 30-day ED visits related to a urinary source, and transition of care documentation. Descriptive statistics were performed; median and IQR were calculated in Microsoft Excel.
Results
A total of 548 ED UTI encounters were identified, including 449 patients with an index ED UTI aftercare follow-up evaluation. Of the 246 randomly screened patients, 200 veterans met inclusion criteria. The median age of included patients was 73 years and most (83.0%) were male (Table 1). One hundred thirty-two patients (66.0%) had a cystitis diagnosis, followed by complicated UTI (14.0%) and catheter-associated UTI (11.0%). The most frequently isolated uropathogen was Escherichia coli (30.5%). ß-lactams were prescribed for empiric treatment to 121 patients (60.5%), followed by 36 fluoroquinolones prescriptions (18.0%). The median treatment duration was 7 days.
The median time to ED pharmacist UTI aftercare evaluation was 2 days (Table 2). Sixty-seven cases required pharmacist intervention, which included 34 transitions to targeted therapy (17.0%) and 33 antibiotic discontinuations (16.5%). A total of 144 days of antibiotic exposure was avoided (ie, days antibiotic was prescribed minus days therapy administered). The majority of cases without modification to index therapy were due to appropriate empiric treatment selection (49.0%). Twelve (6.0%) patients had a subsequent urinary-related ED visit within 30 days due to 8 cases of persistent and/or worsening urinary symptoms (66.7%) and 2 cases of recurrent UTI (16.7%).
Discussion
Outpatient antibiotic prescribing for UTI management in the ED is challenging due to the absence of microbiologic data at time of diagnosis and lack of consistent transition of care follow-up.6 The VAGLAHS ED UTI aftercare program piloted a pharmacist-driven protocol for review of all urine cultures and optimization of antibiotic therapy.
Most ED UTI discharges that did not require pharmacist intervention had empiric treatment selection active against the clinical isolates. This suggests that the ED prescribing practices concur with theVAGLAHS antibiogram and treatment guidelines. Clinical pharmacists intervened in about one-third of UTI cases, which included modification or discontinuation of therapy. Further review of these cases demonstrated that about half of those with a subsequent 30-day ED visit related to a urinary source had therapy modification. Most patients with a 30-day ED visit had persistent and/or worsening urinary symptoms, prompting further exploratory workup.
Although this project did not evaluate time from urine culture results to aftercare review, the VAGLAHS ED pharmacists had a median follow-up time of 48 hours. This timeline mirrors the typical duration for urine culture results, suggesting that the pilot program allowed for real time pharmacist review and intervention. Consequently, this initiative resulted in the avoidance of 144 unnecessary days of antibiotic exposure.
While the current protocol highlights the work that ED pharmacists provide postdischarge, there are additional opportunities for pharmacist intervention. For example, one-third of these clinical encounters were completed without HCP notification, indicating an ongoing need to ensure continuity of care. Additionally, all 16 patients diagnosed with asymptomatic bacteriuria were discharged with an oral antibiotic, highlighting an opportunity to further optimize antibiotic prescribing prior to discharge. ED pharmacists continue to play an important role in mitigating inappropriate and unnecessary antibiotic use, which will reduce antibiotic-related adverse drug reactions, Clostridioides difficile infection, and antimicrobial resistance.
Limitations
Inconsistent and incomplete documentation of clinical data in the electronic health record made the characterization of patient encounters challenging. Furthermore, ED HCPs varying clinical practices may have impacted the heterogeneity of UTI diagnosis and management at VAGLAHS.
Conclusions
Implementation of an ED pharmacist-driven UTI aftercare program at VAGLAHS reduced unnecessary antimicrobial exposure, improved antibiotic management, and ensured continuity of care postdischarge. Findings from our project implicate possible future pharmacist involvement predischarge, such as targeting inappropriate asymptomatic bacteriuria treatment.14-16 This pilot program suggested the feasibility of integrating antimicrobial stewardship practices within the ED setting in an ongoing effort to improve the quality of care for veterans.
The emergency department (ED) is estimated to provide half of all medical care in the United States, serving as a conduit between ambulatory care and inpatient settings.1 According to the Centers for Disease Control and Prevention, around 11 million antibiotic prescriptions were written in EDs in 2021.2 A previous study conducted at a US Department of Veterans (VA) Affairs medical center found that about 40% of all antimicrobial use in the ED was inappropriate.3 The ED is a critical and high-yield space for antimicrobial stewardship efforts.4
Urinary tract infections (UTIs) are one of the most common reasons for ED visits.4 In 2018, there were about 3 million UTI discharge diagnoses reported in the US.5 Diagnosis and management of UTIs can vary depending on patient sex, upper or lower urinary tract involvement, and the severity of the infection.6 Most UTIs are uncomplicated and can be safely treated with oral antibiotics at home; however, if mismanaged, they can lead to increased morbidity and mortality.6
Antimicrobial prescribing in the ED is predominantly empiric with challenges such as diverse patient needs, rising antimicrobial resistance, and limited microbiologic data at the time of discharge.6 The lack of a standardized process for urine culture follow-up after discharge represents another major complicating factor in the outpatient management of UTIs. Studies have shown that ED pharmacists play a vital role in providing quality follow-up care by optimizing antimicrobial use, resulting in improved patient outcomes in various infectious syndromes, including UTIs.7-13
Program Description
In June 2021, the VA Greater Los Angeles Healthcare System (VAGLAHS) piloted an ED pharmacist-led aftercare program to optimize postdischarge antimicrobial therapy management of UTIs. After a patient is discharged from the ED, the clinical pharmacist reviews urine culture results, interprets available antimicrobial susceptibility, conducts patient interviews, adjusts for patient-specific factors, and addresses potential antibiotic-associated adverse events. The ED pharmacist is then responsible for managing therapy changes in consultation with an ED health care practitioner (HCP).
Methods
This single center, retrospective chart review included veterans who were discharged with an oral antibiotic for UTI treatment from the VAGLAHS ED and evaluated by clinical pharmacists between June 1, 2021, and June 30, 2022. For patients with multiple ED visits, only the initial ED encounter was reviewed. Patients were excluded if they had a complicated UTI diagnosis requiring intravenous antibiotics or if they were admitted to the hospital. Data were generated through the Corporate Data Warehouse by VAGLAHS Pharmacy Informatics Service. Each patient was assigned a random number using the Microsoft Excel formula =RAND( ) and then sorted in chronological order to ensure randomization at baseline prior to data collection.
The primary aim of this quality improvement project was to characterize the impact of ED pharmacist-led interventions by evaluating the proportion of empiric to targeted therapy adjustments, antibiotic therapy discontinuation, and unmodified index treatment. The secondary objectives evaluated time to ED pharmacist aftercare follow-up, days of antibiotic exposure avoided, 30-day ED visits related to a urinary source, and transition of care documentation. Descriptive statistics were performed; median and IQR were calculated in Microsoft Excel.
Results
A total of 548 ED UTI encounters were identified, including 449 patients with an index ED UTI aftercare follow-up evaluation. Of the 246 randomly screened patients, 200 veterans met inclusion criteria. The median age of included patients was 73 years and most (83.0%) were male (Table 1). One hundred thirty-two patients (66.0%) had a cystitis diagnosis, followed by complicated UTI (14.0%) and catheter-associated UTI (11.0%). The most frequently isolated uropathogen was Escherichia coli (30.5%). ß-lactams were prescribed for empiric treatment to 121 patients (60.5%), followed by 36 fluoroquinolones prescriptions (18.0%). The median treatment duration was 7 days.
The median time to ED pharmacist UTI aftercare evaluation was 2 days (Table 2). Sixty-seven cases required pharmacist intervention, which included 34 transitions to targeted therapy (17.0%) and 33 antibiotic discontinuations (16.5%). A total of 144 days of antibiotic exposure was avoided (ie, days antibiotic was prescribed minus days therapy administered). The majority of cases without modification to index therapy were due to appropriate empiric treatment selection (49.0%). Twelve (6.0%) patients had a subsequent urinary-related ED visit within 30 days due to 8 cases of persistent and/or worsening urinary symptoms (66.7%) and 2 cases of recurrent UTI (16.7%).
Discussion
Outpatient antibiotic prescribing for UTI management in the ED is challenging due to the absence of microbiologic data at time of diagnosis and lack of consistent transition of care follow-up.6 The VAGLAHS ED UTI aftercare program piloted a pharmacist-driven protocol for review of all urine cultures and optimization of antibiotic therapy.
Most ED UTI discharges that did not require pharmacist intervention had empiric treatment selection active against the clinical isolates. This suggests that the ED prescribing practices concur with theVAGLAHS antibiogram and treatment guidelines. Clinical pharmacists intervened in about one-third of UTI cases, which included modification or discontinuation of therapy. Further review of these cases demonstrated that about half of those with a subsequent 30-day ED visit related to a urinary source had therapy modification. Most patients with a 30-day ED visit had persistent and/or worsening urinary symptoms, prompting further exploratory workup.
Although this project did not evaluate time from urine culture results to aftercare review, the VAGLAHS ED pharmacists had a median follow-up time of 48 hours. This timeline mirrors the typical duration for urine culture results, suggesting that the pilot program allowed for real time pharmacist review and intervention. Consequently, this initiative resulted in the avoidance of 144 unnecessary days of antibiotic exposure.
While the current protocol highlights the work that ED pharmacists provide postdischarge, there are additional opportunities for pharmacist intervention. For example, one-third of these clinical encounters were completed without HCP notification, indicating an ongoing need to ensure continuity of care. Additionally, all 16 patients diagnosed with asymptomatic bacteriuria were discharged with an oral antibiotic, highlighting an opportunity to further optimize antibiotic prescribing prior to discharge. ED pharmacists continue to play an important role in mitigating inappropriate and unnecessary antibiotic use, which will reduce antibiotic-related adverse drug reactions, Clostridioides difficile infection, and antimicrobial resistance.
Limitations
Inconsistent and incomplete documentation of clinical data in the electronic health record made the characterization of patient encounters challenging. Furthermore, ED HCPs varying clinical practices may have impacted the heterogeneity of UTI diagnosis and management at VAGLAHS.
Conclusions
Implementation of an ED pharmacist-driven UTI aftercare program at VAGLAHS reduced unnecessary antimicrobial exposure, improved antibiotic management, and ensured continuity of care postdischarge. Findings from our project implicate possible future pharmacist involvement predischarge, such as targeting inappropriate asymptomatic bacteriuria treatment.14-16 This pilot program suggested the feasibility of integrating antimicrobial stewardship practices within the ED setting in an ongoing effort to improve the quality of care for veterans.
1. Marcozzi D, Carr B, Liferidge A, Baehr N, Browne B.. Trends in the contribution of emergency departments to the provision of hospital-associated health care in the USA. Int J Health Serv. 2018;48(2):267–288. doi:10.1177/0020731417734498
2. Centers for Disease Control and Prevention. Outpatient antibiotic prescriptions — United States, 2021. Updated October 4, 2022. Accessed May 22, 2024. https://archive.cdc.gov/#/details?url=https://www.cdc.gov/antibiotic-use/data/report-2021.html
3. Timbrook TT, Caffrey AR, Ovalle A, et al. Assessments of opportunities to improve antibiotic prescribing in an emergency department: a period prevalence survey. Infect Dis Ther. 2017;6(4):497-505. doi:10.1007/s40121-017-0175-9
4. Pulia M, Redwood R, May L. Antimicrobial stewardship in the emergency department. Emerg Med Clin North. 2018;36(4):853-872. doi:10.1016/j.emc.2018.06.012
5. Weiss A, Jiang H. Most frequent reasons for emergency department visits, 2018. December 16, 2021. Accessed May 22, 2024. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb286-ED-Frequent-Conditions-2018.pdf
6. Abrahamian FM, Moran GJ, Talan DA. Urinary tract infections in the emergency department. Infect Dis Clin North Am. 2008;22(1):73-87. doi:10.1016/j.idc.2007.10.002
7. Dumkow LE, Kenney RM, MacDonald NC, Carreno JJ, Malhotra MK, Davis SL. Impact of a multidisciplinary culture follow-up program of antimicrobial therapy in the emergency department. Infect Dis Ther. 2014;3(1):45-53. doi:10.1007/s40121-014-0026-x
8. Davis LC, Covey RB, Weston JS, Hu BB, Laine GA. Pharmacist-driven antimicrobial optimization in the emergency department. Am J Health Syst Pharm. 2016;73(5 Suppl 1):S49-S56. doi:10.2146/sp150036
9. Lingenfelter E, Darkin Z, Fritz K, Youngquist S, Madsen T, Fix M. ED pharmacist monitoring of provider antibiotic selection aids appropriate treatment for outpatient UTI. Am J Emerg Med. 2016;34(8):1600-1603. doi:10.1016/j.ajem.2016.05.076
10. Zhang X, Rowan N, Pflugeisen BM, Alajbegovic S. Urine culture guided antibiotic interventions: a pharmacist driven antimicrobial stewardship effort in the ED. Am J Emerg Med. 2017;35(4):594-598. doi:10.1016/j.ajem.2016.12.036
11. Percival KM, Valenti KM, Schmittling SE, Strader BD, Lopez RR, Bergman SJ. Impact of an antimicrobial stewardship intervention on urinary tract infection treatment in the ED. Am J Emerg Med. 2015;33(9):1129-1133. doi:10.1016/j.ajem.2015.04.067
12. Almulhim AS, Aldayyen A, Yenina K, Chiappini A, Khan TM. Optimization of antibiotic selection in the emergency department for urine culture follow ups, a retrospective pre-post intervention study: clinical pharmacist efforts. J Pharm Policy Pract. 2019;12(1):8. Published online April 9, 2019. doi:10.1186/s40545-019-0168-z
13. Stoll K, Feltz E, Ebert S. Pharmacist-driven implementation of outpatient antibiotic prescribing algorithms improves guideline adherence in the emergency department. J Pharm Pract. 2021;34(6):875-881. doi:10.1177/0897190020930979
14. Petty LA, Vaughn VM, Flanders SA, et al. Assessment of testing and treatment of asymptomatic bacteriuria initiated in the emergency department. Open Forum Infect Dis. 2020;7(12):ofaa537. Published online November 3, 2020. doi:10.1093/ofid/ofaa537
15. Ingalls EM, Veillette JJ, Olson J, et al. Impact of a multifaceted intervention on antibiotic prescribing for cystitis and asymptomatic bacteriuria in 23 community hospital emergency departments. Hosp Pharm. 2023;58(4):401-407. doi:10.1177/00185787231159578
16. Daniel M, Keller S, Mozafarihashjin M, Pahwa A, Soong C. An implementation guide to reducing overtreatment of asymptomatic bacteriuria. JAMA Intern Med. 2018;178(2):271-276.doi:10.1001/jamainternmed.2017.7290
1. Marcozzi D, Carr B, Liferidge A, Baehr N, Browne B.. Trends in the contribution of emergency departments to the provision of hospital-associated health care in the USA. Int J Health Serv. 2018;48(2):267–288. doi:10.1177/0020731417734498
2. Centers for Disease Control and Prevention. Outpatient antibiotic prescriptions — United States, 2021. Updated October 4, 2022. Accessed May 22, 2024. https://archive.cdc.gov/#/details?url=https://www.cdc.gov/antibiotic-use/data/report-2021.html
3. Timbrook TT, Caffrey AR, Ovalle A, et al. Assessments of opportunities to improve antibiotic prescribing in an emergency department: a period prevalence survey. Infect Dis Ther. 2017;6(4):497-505. doi:10.1007/s40121-017-0175-9
4. Pulia M, Redwood R, May L. Antimicrobial stewardship in the emergency department. Emerg Med Clin North. 2018;36(4):853-872. doi:10.1016/j.emc.2018.06.012
5. Weiss A, Jiang H. Most frequent reasons for emergency department visits, 2018. December 16, 2021. Accessed May 22, 2024. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb286-ED-Frequent-Conditions-2018.pdf
6. Abrahamian FM, Moran GJ, Talan DA. Urinary tract infections in the emergency department. Infect Dis Clin North Am. 2008;22(1):73-87. doi:10.1016/j.idc.2007.10.002
7. Dumkow LE, Kenney RM, MacDonald NC, Carreno JJ, Malhotra MK, Davis SL. Impact of a multidisciplinary culture follow-up program of antimicrobial therapy in the emergency department. Infect Dis Ther. 2014;3(1):45-53. doi:10.1007/s40121-014-0026-x
8. Davis LC, Covey RB, Weston JS, Hu BB, Laine GA. Pharmacist-driven antimicrobial optimization in the emergency department. Am J Health Syst Pharm. 2016;73(5 Suppl 1):S49-S56. doi:10.2146/sp150036
9. Lingenfelter E, Darkin Z, Fritz K, Youngquist S, Madsen T, Fix M. ED pharmacist monitoring of provider antibiotic selection aids appropriate treatment for outpatient UTI. Am J Emerg Med. 2016;34(8):1600-1603. doi:10.1016/j.ajem.2016.05.076
10. Zhang X, Rowan N, Pflugeisen BM, Alajbegovic S. Urine culture guided antibiotic interventions: a pharmacist driven antimicrobial stewardship effort in the ED. Am J Emerg Med. 2017;35(4):594-598. doi:10.1016/j.ajem.2016.12.036
11. Percival KM, Valenti KM, Schmittling SE, Strader BD, Lopez RR, Bergman SJ. Impact of an antimicrobial stewardship intervention on urinary tract infection treatment in the ED. Am J Emerg Med. 2015;33(9):1129-1133. doi:10.1016/j.ajem.2015.04.067
12. Almulhim AS, Aldayyen A, Yenina K, Chiappini A, Khan TM. Optimization of antibiotic selection in the emergency department for urine culture follow ups, a retrospective pre-post intervention study: clinical pharmacist efforts. J Pharm Policy Pract. 2019;12(1):8. Published online April 9, 2019. doi:10.1186/s40545-019-0168-z
13. Stoll K, Feltz E, Ebert S. Pharmacist-driven implementation of outpatient antibiotic prescribing algorithms improves guideline adherence in the emergency department. J Pharm Pract. 2021;34(6):875-881. doi:10.1177/0897190020930979
14. Petty LA, Vaughn VM, Flanders SA, et al. Assessment of testing and treatment of asymptomatic bacteriuria initiated in the emergency department. Open Forum Infect Dis. 2020;7(12):ofaa537. Published online November 3, 2020. doi:10.1093/ofid/ofaa537
15. Ingalls EM, Veillette JJ, Olson J, et al. Impact of a multifaceted intervention on antibiotic prescribing for cystitis and asymptomatic bacteriuria in 23 community hospital emergency departments. Hosp Pharm. 2023;58(4):401-407. doi:10.1177/00185787231159578
16. Daniel M, Keller S, Mozafarihashjin M, Pahwa A, Soong C. An implementation guide to reducing overtreatment of asymptomatic bacteriuria. JAMA Intern Med. 2018;178(2):271-276.doi:10.1001/jamainternmed.2017.7290
A Crisis in Scope: Recruitment and Retention Challenges Reported by VA Gastroenterology Section Chiefs
Veterans have a high burden of digestive diseases, and gastroenterologists are needed for the diagnosis and management of these conditions.1-4 According to the Veterans Health Administration (VHA) Workforce Management and Consulting (WMC) office, the physician specialties with the greatest shortages are psychiatry, primary care, and gastroenterology.5 The VHA estimates it must hire 70 new gastroenterologists annually between fiscal years 2023 and 2027 to provide timely digestive care.5
Filling these positions will be increasingly difficult as competition for gastroenterologists is fierce. A recent Merritt Hawkins review states, “Gastroenterologists were the most in-demand type of provider during the 2022 review period.”6 In 2022, the median annual salary for US gastroenterologists was reported to be $561,375.7 Currently, the US Department of Veterans Affairs (VA) has an aggregate annual pay limit of $400,000 for all federal employees and cannot compete based on salary alone.
Retention of existing VA gastroenterologists also is challenging. The WMC has reported that 21.6% of VA gastroenterologists are eligible to retire, and in 2021, 8.2% left the VA to retire or seek non-VA positions.5 While not specific to the VA, a survey of practicing gastroenterologists conducted by the American College of Gastroenterology found a 49% burnout rate among respondents.8 Factors contributing to burnout at all career stages included administrative nonclinical work and a lack of clinical support staff.8 Burnout is also linked with higher rates of medical errors, interpersonal conflicts, and patient dissatisfaction. Burnout is more common among those with an innate strong sense of purpose and responsibility for their patients, characteristics we have observed in our VA colleagues.9
As members of the Section Chief Subcommittee of the VA Gastroenterology Field Advisory Board (GI FAB), we are passionate about providing outstanding gastroenterology care to US veterans, and we are alarmed at the struggles we are observing with recruiting and retaining a qualified national gastroenterology physician workforce. As such, we set out to survey the VA gastroenterology section chief community to gain insights into recruitment and retention challenges they have faced and identify potential solutions to these problems.
Methods
The GI FAB Section Chief Subcommittee developed a survey on gastroenterologist recruitment and retention using Microsoft Forms (Appendix). A link to the survey, which included 11 questions about facility location, current vacancies, and free text responses on barriers to recruitment and retention and potential solutions, was sent via email to all gastroenterology section chiefs on the National Gastroenterology and Hepatology Program Office’s email list of section chiefs on January 31, 2023. A reminder to complete the survey was sent to all section chiefs on February 8, 2023. Survey responses were aggregated and analyzed by the authors using descriptive statistics.
Results
The VA gastroenterologist recruitment and retention survey was emailed to 131 gastroenterology section chiefs and completed by 55 respondents (42%) (Figure). Of the responding section chiefs, 36 (65%) reported gastroenterologist vacancies at their facilities. Seventeen respondents (47%) reported a single vacancy, 12 (33%) reported 2 vacancies, 4 (11%) reported 3 vacancies, and 3 (8%) reported 4 vacancies. Of the sites with reported vacancies, 32 (89%) reported a need for a general gastroenterologist, 12 (33%) reported a need for a hepatologist, 11 (31%) reported a need for an advanced endoscopist, 9 (25%) reported a need for a gastroenterologist with specialized expertise in inflammatory bowel diseases, and 1 (3%) reported a need for a gastrointestinal motility specialist.
Numerous barriers to the recruitment and retention of gastroenterologists were reported. Given the large number of respondents that reported a unique barrier (ie, being the only respondents to report the barrier), a decision was made to include only barriers to recruitment and retention that were reported by at least 2 sites (Table). While there were some common themes, the reported barriers to retention differed from those to recruitment. The most reported barriers to recruitment were 46 respondents who noted salary, 23 reported human resources-related challenges, and 12 reported location. Respondents also noted various retention barriers, including 32 respondents who reported salary barriers; 13 reported administrative burden barriers, 6 reported medical center leadership, and 4 reported burnout.
Survey respondents provided multiple recommendations on how the VA can best support the recruitment and retention of gastroenterologists. The most frequent recommendations were to increase financial compensation by increasing the current aggregate salary cap to > $400,000, increasing the use of recruitment and retention incentives, and ensuring that gastroenterology is on the national Educational Debt Reduction Program (EDRP) list, which facilitates student loan repayment. It was recommended that a third-party company assist with hiring to overcome perceived issues with human resources. Additionally, there were multiple recommendations for improving administrative and clinical support. These included mandating how many support staff should be assigned to each gastroenterologist and providing best practice recommendations for support staff so that gastroenterologists can focus on physician-level work. Recommendations also included having a dedicated gastroenterology practice manager, nurse care coordinators, a colorectal cancer screening/surveillance coordinator, sufficient medical support assistants, and quality improvement personnel tracking ongoing professional practice evaluation data. Survey respondents also highlighted specific suggestions for recruiting recent graduates. These included offering a 4-day work week, as recent graduates place a premium on work-life balance, and ensuring gastroenterologists have individual offices. One respondent commented that gastroenterology fellows seeing VA gastroenterology attendings in cramped, shared offices, contrasted with private practice gastroenterologists in large private offices, may contribute to choosing private practice over joining the VA.
Discussion
Gastroenterology is currently listed by VHA WMC as 1 of the top 3 medical specialties in the VA with the most physician shortages.5 Working as a physician in the VA has long been recognized to have many benefits. First and foremost, many physicians are motivated by the VA mission to serve veterans, as this offers personal fulfillment and other intangible benefits. In addition, the VA can provide work-life balance, which is often not possible in fee-for-service settings, with patient panels and call volumes typically lower than in comparable private hospital settings. Moreover, VA physicians have outstanding teaching opportunities, as the VA is the largest supporter of medical education, with postgraduate trainees rotating through > 150 VA medical centers. Likewise, the VA offers a variety of student loan repayment programs (eg, the Specialty Education Loan Repayment Program and the EDRP). The VA offers research funding such as the Cooperative Studies Programs or program project funding, and rewards in parallel with the National Institute of Health (eg, career development awards, or merit review awards) and other grants. VA researchers have conducted many landmark studies that continue to shape the practice of gastroenterology and hepatology. From the earliest studies to demonstrate the effectiveness of screening colonoscopy, to the largest ongoing clinical trial in US history to assess the effectiveness of fecal immunochemical testing (FIT) vs screening colonoscopy.10-12 The VA has also led the field in the study of gastroesophageal reflux disease, hepatitis C treatment, and liver cancer screening.13-15 VA physicians also benefit from participation in the Federal Employee Retirement System, including its pension system.
These benefits apply to all medical specialties, making the VA a potentially appealing workplace for gastroenterologists. However, recent trends indicate that recruitment and retention of gastroenterologists is increasingly challenging, as the VA gastroenterology workforce grew by 5.0% in fiscal year (FY) 2020 and 1.8% in FY 2021. However, it was on track to end FY 2022 with a loss (-1.1%).5 It must be noted that this trend is not limited to the VA, and the National Center for Health Workforce Analysis predicts that gastroenterology will remain among the highest projected specialty shortages. Driven by increased demand for digestive health care services, more physicians nearing traditional retirement age, and substantially higher rates of burnout after the COVID-19 pandemic.16 All these factors are likely to result in an increasingly competitive market for gastroenterology, highlight the growing differences between VA and non-VA positions, and may augment the impact of differences for the individual gastroenterologist weighing employment options within and outside the VA.
The survey responses from VA gastroenterology section chiefs help identify potential impediments to the successful recruitment and retention in the specialty. Noncompetitive salary was the most significant barrier to the successful recruitment of gastroenterologists, identified by 46 of 55 respondents. According to a 2022 Medical Group Management Association report, the median annual salary for US gastroenterologists was $561,375.7 According to internal VA WMC data, the median 2022 VA gastroenterologist salary ranged between $287,976 and $346,435, depending on facility complexity level, excluding recruitment, retention, or relocation bonuses; performance pay; or cash awards. The current aggregate salary cap of $400,000 indicates that the VHA will likely be increasingly noncompetitive in the coming years unless novel pay authorizations are implemented.
Suboptimal human resources were the second most commonly cited impediment to recruiting gastroenterologists. Many section chiefs expressed frustration with the inefficient and slow administrative process of onboarding new gastroenterologists, which may take many months and not infrequently results in losing candidates to competing entities. While this issue is specific to recruitment, recurring and long-standing vacancies can increase work burdens, complicate logistics for remaining faculty, and may also negatively impact retention. One potential opportunity to improve VHA competitiveness is to streamline the administrative component of recruitment and optimize human resources support. The use of a third-party hiring company also should be considered.
Survey responses also indicated that administrative burden and insufficient support staff were significant retention challenges. Several respondents described a lack of efficient endoscopy workflow and delegation of simple administrative tasks to gastroenterologists as more likely in units without proper task distribution. Importantly, these shortcomings occur at the expense of workload-generating activities and career-enhancing opportunities.
While burnout rates among VA gastroenterologists have not been documented systematically, they likely correlate with workplace frustration and jeopardizegastroenterologist retention. Successful retention of gastroenterologists as highly trained medical professionals is more likely in workplaces that are vertically organized, efficient, and use physicians at the top of their skill level.
Conclusions
The VA offers the opportunity for a rewarding lifelong career in gastroenterology. The fulfillment of serving veterans, teaching future health care leaders, performing impactful research, and having job security is invaluable. Despite the tremendous benefits, this survey supports improving VA recruitment and retention strategies for the high-demand gastroenterology specialty. Improved salary parity is needed for workforce maintenance and recruitment, as is improved administrative and clinical support to maintain the high level of care our veterans deserve.
1. Shin A, Xu H, Imperiale TF. The prevalence, humanistic burden, and health care impact of irritable bowel syndrome among united states veterans. Clin Gastroenterol Hepatol. 2023;21(4):1061-1069.e1. doi:10.1016/j.cgh.2022.08.005.
2. Kent KG. Prevalence of gastrointestinal disease in US military veterans under outpatient care at the veterans health administration. SAGE Open Med. 2021;9:20503121211049112. doi:10.1177/20503121211049112
3. Beste LA, Leipertz SL, Green PK, Dominitz JA, Ross D, Ioannou GN. Trends in burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US veterans, 2001-2013. Gastroenterology. 2015;149(6):1471-e18. doi:10.1053/j.gastro.2015.07.056
4. Zullig LL, Sims KJ, McNeil R, et al. Cancer incidence among patients of the U.S. veterans affairs health care system: 2010 update. Mil Med. 2017;182(7):e1883-e1891. doi:10.7205/MILMED-D-16-00371
5. VHA Physician Workforce Resources Blueprint. US Dept of Veterans Affairs. https://dvagov.sharepoint.com/sites/WMCPortal/WFP/Documents/Reports/VHA Physician Workforce Resources Blueprint FY 23-27.pdf [Source not verified]
6. AMN Healthcare. 2022 Review of Physician and Advanced Practitioner Recruiting Incentives. Accessed June 12, 2024. https://www1.amnhealthcare.com/l/123142/2022-07-13/q6ywxg/123142/1657737392vyuONaZZ/mha2022incentivesurgraphic.pdf
7. Medical Group Management Association. MGMA DataDive Provider Compensation Data. Accessed June 12, 2024. https://www.mgma.com/datadive/provider-compensation
8. Anderson JC, Bilal M, Burke CA, et al. Burnout among US gastroenterologists and fellows in training: identifying contributing factors and offering solutions. J Clin Gastroenterol. 2023;57(10):1063-1069. doi:10.1097/MCG.0000000000001781
9. Lacy BE, Chan JL. Physician burnout: the hidden health care crisis. Clin Gastroenterol Hepatol. 2018;16(3):311-317. doi:10.1016/j.cgh.2017.06.043
10. Lieberman DA, Weiss DG, Bond JH, Ahnen DJ, Garewal H, Chejfec G. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. Veterans affairs cooperative study group 380. N Engl J Med. 2000;343(3):162-168. doi:10.1056/NEJM200007203430301
11. Lieberman DA, Weiss DG; Veterans Affairs Cooperative Study Group 380. One-time screening for colorectal cancer with combined fecal occult-blood testing and examination of the distal colon. N Engl J Med. 2001;345(8):555-560. doi:10.1056/NEJMoa010328
12. Robertson DJ, Dominitz JA, Beed A, et al. Baseline features and reasons for nonparticipation in the colonoscopy versus fecal immunochemical test in reducing mortality from colorectal cancer (CONFIRM) study, a colorectal cancer screening trial. JAMA Netw Open. 2023;6(7):e2321730. doi:10.1001/jamanetworkopen.2023.21730
13. Spechler SJ, Hunter JG, Jones KM, et al. Randomized trial of medical versus surgical treatment for refractory heartburn. N Engl J Med. 2019;381(16):1513-1523. doi:10.1056/NEJMoa1811424
14. Beste LA, Green PK, Berry K, Kogut MJ, Allison SK, Ioannou GN. Effectiveness of hepatitis C antiviral treatment in a USA cohort of veteran patients with hepatocellular carcinoma. J Hepatol. 2017;67(1):32-39. doi:10.1016/j.jhep.2017.02.027
15. US Department of Veterans Affairs. Veterans affairs cooperative studies program (CSP). CSP #2023. Updated July 2022. Accessed June 12, 2024. https://www.vacsp.research.va.gov/CSP_2023/CSP_2023.asp
16. US Health Resources & Services Administration. Workforce projections. Accessed June 12, 2024. https://data.hrsa.gov/topics/health-workforce/workforce-projections
Veterans have a high burden of digestive diseases, and gastroenterologists are needed for the diagnosis and management of these conditions.1-4 According to the Veterans Health Administration (VHA) Workforce Management and Consulting (WMC) office, the physician specialties with the greatest shortages are psychiatry, primary care, and gastroenterology.5 The VHA estimates it must hire 70 new gastroenterologists annually between fiscal years 2023 and 2027 to provide timely digestive care.5
Filling these positions will be increasingly difficult as competition for gastroenterologists is fierce. A recent Merritt Hawkins review states, “Gastroenterologists were the most in-demand type of provider during the 2022 review period.”6 In 2022, the median annual salary for US gastroenterologists was reported to be $561,375.7 Currently, the US Department of Veterans Affairs (VA) has an aggregate annual pay limit of $400,000 for all federal employees and cannot compete based on salary alone.
Retention of existing VA gastroenterologists also is challenging. The WMC has reported that 21.6% of VA gastroenterologists are eligible to retire, and in 2021, 8.2% left the VA to retire or seek non-VA positions.5 While not specific to the VA, a survey of practicing gastroenterologists conducted by the American College of Gastroenterology found a 49% burnout rate among respondents.8 Factors contributing to burnout at all career stages included administrative nonclinical work and a lack of clinical support staff.8 Burnout is also linked with higher rates of medical errors, interpersonal conflicts, and patient dissatisfaction. Burnout is more common among those with an innate strong sense of purpose and responsibility for their patients, characteristics we have observed in our VA colleagues.9
As members of the Section Chief Subcommittee of the VA Gastroenterology Field Advisory Board (GI FAB), we are passionate about providing outstanding gastroenterology care to US veterans, and we are alarmed at the struggles we are observing with recruiting and retaining a qualified national gastroenterology physician workforce. As such, we set out to survey the VA gastroenterology section chief community to gain insights into recruitment and retention challenges they have faced and identify potential solutions to these problems.
Methods
The GI FAB Section Chief Subcommittee developed a survey on gastroenterologist recruitment and retention using Microsoft Forms (Appendix). A link to the survey, which included 11 questions about facility location, current vacancies, and free text responses on barriers to recruitment and retention and potential solutions, was sent via email to all gastroenterology section chiefs on the National Gastroenterology and Hepatology Program Office’s email list of section chiefs on January 31, 2023. A reminder to complete the survey was sent to all section chiefs on February 8, 2023. Survey responses were aggregated and analyzed by the authors using descriptive statistics.
Results
The VA gastroenterologist recruitment and retention survey was emailed to 131 gastroenterology section chiefs and completed by 55 respondents (42%) (Figure). Of the responding section chiefs, 36 (65%) reported gastroenterologist vacancies at their facilities. Seventeen respondents (47%) reported a single vacancy, 12 (33%) reported 2 vacancies, 4 (11%) reported 3 vacancies, and 3 (8%) reported 4 vacancies. Of the sites with reported vacancies, 32 (89%) reported a need for a general gastroenterologist, 12 (33%) reported a need for a hepatologist, 11 (31%) reported a need for an advanced endoscopist, 9 (25%) reported a need for a gastroenterologist with specialized expertise in inflammatory bowel diseases, and 1 (3%) reported a need for a gastrointestinal motility specialist.
Numerous barriers to the recruitment and retention of gastroenterologists were reported. Given the large number of respondents that reported a unique barrier (ie, being the only respondents to report the barrier), a decision was made to include only barriers to recruitment and retention that were reported by at least 2 sites (Table). While there were some common themes, the reported barriers to retention differed from those to recruitment. The most reported barriers to recruitment were 46 respondents who noted salary, 23 reported human resources-related challenges, and 12 reported location. Respondents also noted various retention barriers, including 32 respondents who reported salary barriers; 13 reported administrative burden barriers, 6 reported medical center leadership, and 4 reported burnout.
Survey respondents provided multiple recommendations on how the VA can best support the recruitment and retention of gastroenterologists. The most frequent recommendations were to increase financial compensation by increasing the current aggregate salary cap to > $400,000, increasing the use of recruitment and retention incentives, and ensuring that gastroenterology is on the national Educational Debt Reduction Program (EDRP) list, which facilitates student loan repayment. It was recommended that a third-party company assist with hiring to overcome perceived issues with human resources. Additionally, there were multiple recommendations for improving administrative and clinical support. These included mandating how many support staff should be assigned to each gastroenterologist and providing best practice recommendations for support staff so that gastroenterologists can focus on physician-level work. Recommendations also included having a dedicated gastroenterology practice manager, nurse care coordinators, a colorectal cancer screening/surveillance coordinator, sufficient medical support assistants, and quality improvement personnel tracking ongoing professional practice evaluation data. Survey respondents also highlighted specific suggestions for recruiting recent graduates. These included offering a 4-day work week, as recent graduates place a premium on work-life balance, and ensuring gastroenterologists have individual offices. One respondent commented that gastroenterology fellows seeing VA gastroenterology attendings in cramped, shared offices, contrasted with private practice gastroenterologists in large private offices, may contribute to choosing private practice over joining the VA.
Discussion
Gastroenterology is currently listed by VHA WMC as 1 of the top 3 medical specialties in the VA with the most physician shortages.5 Working as a physician in the VA has long been recognized to have many benefits. First and foremost, many physicians are motivated by the VA mission to serve veterans, as this offers personal fulfillment and other intangible benefits. In addition, the VA can provide work-life balance, which is often not possible in fee-for-service settings, with patient panels and call volumes typically lower than in comparable private hospital settings. Moreover, VA physicians have outstanding teaching opportunities, as the VA is the largest supporter of medical education, with postgraduate trainees rotating through > 150 VA medical centers. Likewise, the VA offers a variety of student loan repayment programs (eg, the Specialty Education Loan Repayment Program and the EDRP). The VA offers research funding such as the Cooperative Studies Programs or program project funding, and rewards in parallel with the National Institute of Health (eg, career development awards, or merit review awards) and other grants. VA researchers have conducted many landmark studies that continue to shape the practice of gastroenterology and hepatology. From the earliest studies to demonstrate the effectiveness of screening colonoscopy, to the largest ongoing clinical trial in US history to assess the effectiveness of fecal immunochemical testing (FIT) vs screening colonoscopy.10-12 The VA has also led the field in the study of gastroesophageal reflux disease, hepatitis C treatment, and liver cancer screening.13-15 VA physicians also benefit from participation in the Federal Employee Retirement System, including its pension system.
These benefits apply to all medical specialties, making the VA a potentially appealing workplace for gastroenterologists. However, recent trends indicate that recruitment and retention of gastroenterologists is increasingly challenging, as the VA gastroenterology workforce grew by 5.0% in fiscal year (FY) 2020 and 1.8% in FY 2021. However, it was on track to end FY 2022 with a loss (-1.1%).5 It must be noted that this trend is not limited to the VA, and the National Center for Health Workforce Analysis predicts that gastroenterology will remain among the highest projected specialty shortages. Driven by increased demand for digestive health care services, more physicians nearing traditional retirement age, and substantially higher rates of burnout after the COVID-19 pandemic.16 All these factors are likely to result in an increasingly competitive market for gastroenterology, highlight the growing differences between VA and non-VA positions, and may augment the impact of differences for the individual gastroenterologist weighing employment options within and outside the VA.
The survey responses from VA gastroenterology section chiefs help identify potential impediments to the successful recruitment and retention in the specialty. Noncompetitive salary was the most significant barrier to the successful recruitment of gastroenterologists, identified by 46 of 55 respondents. According to a 2022 Medical Group Management Association report, the median annual salary for US gastroenterologists was $561,375.7 According to internal VA WMC data, the median 2022 VA gastroenterologist salary ranged between $287,976 and $346,435, depending on facility complexity level, excluding recruitment, retention, or relocation bonuses; performance pay; or cash awards. The current aggregate salary cap of $400,000 indicates that the VHA will likely be increasingly noncompetitive in the coming years unless novel pay authorizations are implemented.
Suboptimal human resources were the second most commonly cited impediment to recruiting gastroenterologists. Many section chiefs expressed frustration with the inefficient and slow administrative process of onboarding new gastroenterologists, which may take many months and not infrequently results in losing candidates to competing entities. While this issue is specific to recruitment, recurring and long-standing vacancies can increase work burdens, complicate logistics for remaining faculty, and may also negatively impact retention. One potential opportunity to improve VHA competitiveness is to streamline the administrative component of recruitment and optimize human resources support. The use of a third-party hiring company also should be considered.
Survey responses also indicated that administrative burden and insufficient support staff were significant retention challenges. Several respondents described a lack of efficient endoscopy workflow and delegation of simple administrative tasks to gastroenterologists as more likely in units without proper task distribution. Importantly, these shortcomings occur at the expense of workload-generating activities and career-enhancing opportunities.
While burnout rates among VA gastroenterologists have not been documented systematically, they likely correlate with workplace frustration and jeopardizegastroenterologist retention. Successful retention of gastroenterologists as highly trained medical professionals is more likely in workplaces that are vertically organized, efficient, and use physicians at the top of their skill level.
Conclusions
The VA offers the opportunity for a rewarding lifelong career in gastroenterology. The fulfillment of serving veterans, teaching future health care leaders, performing impactful research, and having job security is invaluable. Despite the tremendous benefits, this survey supports improving VA recruitment and retention strategies for the high-demand gastroenterology specialty. Improved salary parity is needed for workforce maintenance and recruitment, as is improved administrative and clinical support to maintain the high level of care our veterans deserve.
Veterans have a high burden of digestive diseases, and gastroenterologists are needed for the diagnosis and management of these conditions.1-4 According to the Veterans Health Administration (VHA) Workforce Management and Consulting (WMC) office, the physician specialties with the greatest shortages are psychiatry, primary care, and gastroenterology.5 The VHA estimates it must hire 70 new gastroenterologists annually between fiscal years 2023 and 2027 to provide timely digestive care.5
Filling these positions will be increasingly difficult as competition for gastroenterologists is fierce. A recent Merritt Hawkins review states, “Gastroenterologists were the most in-demand type of provider during the 2022 review period.”6 In 2022, the median annual salary for US gastroenterologists was reported to be $561,375.7 Currently, the US Department of Veterans Affairs (VA) has an aggregate annual pay limit of $400,000 for all federal employees and cannot compete based on salary alone.
Retention of existing VA gastroenterologists also is challenging. The WMC has reported that 21.6% of VA gastroenterologists are eligible to retire, and in 2021, 8.2% left the VA to retire or seek non-VA positions.5 While not specific to the VA, a survey of practicing gastroenterologists conducted by the American College of Gastroenterology found a 49% burnout rate among respondents.8 Factors contributing to burnout at all career stages included administrative nonclinical work and a lack of clinical support staff.8 Burnout is also linked with higher rates of medical errors, interpersonal conflicts, and patient dissatisfaction. Burnout is more common among those with an innate strong sense of purpose and responsibility for their patients, characteristics we have observed in our VA colleagues.9
As members of the Section Chief Subcommittee of the VA Gastroenterology Field Advisory Board (GI FAB), we are passionate about providing outstanding gastroenterology care to US veterans, and we are alarmed at the struggles we are observing with recruiting and retaining a qualified national gastroenterology physician workforce. As such, we set out to survey the VA gastroenterology section chief community to gain insights into recruitment and retention challenges they have faced and identify potential solutions to these problems.
Methods
The GI FAB Section Chief Subcommittee developed a survey on gastroenterologist recruitment and retention using Microsoft Forms (Appendix). A link to the survey, which included 11 questions about facility location, current vacancies, and free text responses on barriers to recruitment and retention and potential solutions, was sent via email to all gastroenterology section chiefs on the National Gastroenterology and Hepatology Program Office’s email list of section chiefs on January 31, 2023. A reminder to complete the survey was sent to all section chiefs on February 8, 2023. Survey responses were aggregated and analyzed by the authors using descriptive statistics.
Results
The VA gastroenterologist recruitment and retention survey was emailed to 131 gastroenterology section chiefs and completed by 55 respondents (42%) (Figure). Of the responding section chiefs, 36 (65%) reported gastroenterologist vacancies at their facilities. Seventeen respondents (47%) reported a single vacancy, 12 (33%) reported 2 vacancies, 4 (11%) reported 3 vacancies, and 3 (8%) reported 4 vacancies. Of the sites with reported vacancies, 32 (89%) reported a need for a general gastroenterologist, 12 (33%) reported a need for a hepatologist, 11 (31%) reported a need for an advanced endoscopist, 9 (25%) reported a need for a gastroenterologist with specialized expertise in inflammatory bowel diseases, and 1 (3%) reported a need for a gastrointestinal motility specialist.
Numerous barriers to the recruitment and retention of gastroenterologists were reported. Given the large number of respondents that reported a unique barrier (ie, being the only respondents to report the barrier), a decision was made to include only barriers to recruitment and retention that were reported by at least 2 sites (Table). While there were some common themes, the reported barriers to retention differed from those to recruitment. The most reported barriers to recruitment were 46 respondents who noted salary, 23 reported human resources-related challenges, and 12 reported location. Respondents also noted various retention barriers, including 32 respondents who reported salary barriers; 13 reported administrative burden barriers, 6 reported medical center leadership, and 4 reported burnout.
Survey respondents provided multiple recommendations on how the VA can best support the recruitment and retention of gastroenterologists. The most frequent recommendations were to increase financial compensation by increasing the current aggregate salary cap to > $400,000, increasing the use of recruitment and retention incentives, and ensuring that gastroenterology is on the national Educational Debt Reduction Program (EDRP) list, which facilitates student loan repayment. It was recommended that a third-party company assist with hiring to overcome perceived issues with human resources. Additionally, there were multiple recommendations for improving administrative and clinical support. These included mandating how many support staff should be assigned to each gastroenterologist and providing best practice recommendations for support staff so that gastroenterologists can focus on physician-level work. Recommendations also included having a dedicated gastroenterology practice manager, nurse care coordinators, a colorectal cancer screening/surveillance coordinator, sufficient medical support assistants, and quality improvement personnel tracking ongoing professional practice evaluation data. Survey respondents also highlighted specific suggestions for recruiting recent graduates. These included offering a 4-day work week, as recent graduates place a premium on work-life balance, and ensuring gastroenterologists have individual offices. One respondent commented that gastroenterology fellows seeing VA gastroenterology attendings in cramped, shared offices, contrasted with private practice gastroenterologists in large private offices, may contribute to choosing private practice over joining the VA.
Discussion
Gastroenterology is currently listed by VHA WMC as 1 of the top 3 medical specialties in the VA with the most physician shortages.5 Working as a physician in the VA has long been recognized to have many benefits. First and foremost, many physicians are motivated by the VA mission to serve veterans, as this offers personal fulfillment and other intangible benefits. In addition, the VA can provide work-life balance, which is often not possible in fee-for-service settings, with patient panels and call volumes typically lower than in comparable private hospital settings. Moreover, VA physicians have outstanding teaching opportunities, as the VA is the largest supporter of medical education, with postgraduate trainees rotating through > 150 VA medical centers. Likewise, the VA offers a variety of student loan repayment programs (eg, the Specialty Education Loan Repayment Program and the EDRP). The VA offers research funding such as the Cooperative Studies Programs or program project funding, and rewards in parallel with the National Institute of Health (eg, career development awards, or merit review awards) and other grants. VA researchers have conducted many landmark studies that continue to shape the practice of gastroenterology and hepatology. From the earliest studies to demonstrate the effectiveness of screening colonoscopy, to the largest ongoing clinical trial in US history to assess the effectiveness of fecal immunochemical testing (FIT) vs screening colonoscopy.10-12 The VA has also led the field in the study of gastroesophageal reflux disease, hepatitis C treatment, and liver cancer screening.13-15 VA physicians also benefit from participation in the Federal Employee Retirement System, including its pension system.
These benefits apply to all medical specialties, making the VA a potentially appealing workplace for gastroenterologists. However, recent trends indicate that recruitment and retention of gastroenterologists is increasingly challenging, as the VA gastroenterology workforce grew by 5.0% in fiscal year (FY) 2020 and 1.8% in FY 2021. However, it was on track to end FY 2022 with a loss (-1.1%).5 It must be noted that this trend is not limited to the VA, and the National Center for Health Workforce Analysis predicts that gastroenterology will remain among the highest projected specialty shortages. Driven by increased demand for digestive health care services, more physicians nearing traditional retirement age, and substantially higher rates of burnout after the COVID-19 pandemic.16 All these factors are likely to result in an increasingly competitive market for gastroenterology, highlight the growing differences between VA and non-VA positions, and may augment the impact of differences for the individual gastroenterologist weighing employment options within and outside the VA.
The survey responses from VA gastroenterology section chiefs help identify potential impediments to the successful recruitment and retention in the specialty. Noncompetitive salary was the most significant barrier to the successful recruitment of gastroenterologists, identified by 46 of 55 respondents. According to a 2022 Medical Group Management Association report, the median annual salary for US gastroenterologists was $561,375.7 According to internal VA WMC data, the median 2022 VA gastroenterologist salary ranged between $287,976 and $346,435, depending on facility complexity level, excluding recruitment, retention, or relocation bonuses; performance pay; or cash awards. The current aggregate salary cap of $400,000 indicates that the VHA will likely be increasingly noncompetitive in the coming years unless novel pay authorizations are implemented.
Suboptimal human resources were the second most commonly cited impediment to recruiting gastroenterologists. Many section chiefs expressed frustration with the inefficient and slow administrative process of onboarding new gastroenterologists, which may take many months and not infrequently results in losing candidates to competing entities. While this issue is specific to recruitment, recurring and long-standing vacancies can increase work burdens, complicate logistics for remaining faculty, and may also negatively impact retention. One potential opportunity to improve VHA competitiveness is to streamline the administrative component of recruitment and optimize human resources support. The use of a third-party hiring company also should be considered.
Survey responses also indicated that administrative burden and insufficient support staff were significant retention challenges. Several respondents described a lack of efficient endoscopy workflow and delegation of simple administrative tasks to gastroenterologists as more likely in units without proper task distribution. Importantly, these shortcomings occur at the expense of workload-generating activities and career-enhancing opportunities.
While burnout rates among VA gastroenterologists have not been documented systematically, they likely correlate with workplace frustration and jeopardizegastroenterologist retention. Successful retention of gastroenterologists as highly trained medical professionals is more likely in workplaces that are vertically organized, efficient, and use physicians at the top of their skill level.
Conclusions
The VA offers the opportunity for a rewarding lifelong career in gastroenterology. The fulfillment of serving veterans, teaching future health care leaders, performing impactful research, and having job security is invaluable. Despite the tremendous benefits, this survey supports improving VA recruitment and retention strategies for the high-demand gastroenterology specialty. Improved salary parity is needed for workforce maintenance and recruitment, as is improved administrative and clinical support to maintain the high level of care our veterans deserve.
1. Shin A, Xu H, Imperiale TF. The prevalence, humanistic burden, and health care impact of irritable bowel syndrome among united states veterans. Clin Gastroenterol Hepatol. 2023;21(4):1061-1069.e1. doi:10.1016/j.cgh.2022.08.005.
2. Kent KG. Prevalence of gastrointestinal disease in US military veterans under outpatient care at the veterans health administration. SAGE Open Med. 2021;9:20503121211049112. doi:10.1177/20503121211049112
3. Beste LA, Leipertz SL, Green PK, Dominitz JA, Ross D, Ioannou GN. Trends in burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US veterans, 2001-2013. Gastroenterology. 2015;149(6):1471-e18. doi:10.1053/j.gastro.2015.07.056
4. Zullig LL, Sims KJ, McNeil R, et al. Cancer incidence among patients of the U.S. veterans affairs health care system: 2010 update. Mil Med. 2017;182(7):e1883-e1891. doi:10.7205/MILMED-D-16-00371
5. VHA Physician Workforce Resources Blueprint. US Dept of Veterans Affairs. https://dvagov.sharepoint.com/sites/WMCPortal/WFP/Documents/Reports/VHA Physician Workforce Resources Blueprint FY 23-27.pdf [Source not verified]
6. AMN Healthcare. 2022 Review of Physician and Advanced Practitioner Recruiting Incentives. Accessed June 12, 2024. https://www1.amnhealthcare.com/l/123142/2022-07-13/q6ywxg/123142/1657737392vyuONaZZ/mha2022incentivesurgraphic.pdf
7. Medical Group Management Association. MGMA DataDive Provider Compensation Data. Accessed June 12, 2024. https://www.mgma.com/datadive/provider-compensation
8. Anderson JC, Bilal M, Burke CA, et al. Burnout among US gastroenterologists and fellows in training: identifying contributing factors and offering solutions. J Clin Gastroenterol. 2023;57(10):1063-1069. doi:10.1097/MCG.0000000000001781
9. Lacy BE, Chan JL. Physician burnout: the hidden health care crisis. Clin Gastroenterol Hepatol. 2018;16(3):311-317. doi:10.1016/j.cgh.2017.06.043
10. Lieberman DA, Weiss DG, Bond JH, Ahnen DJ, Garewal H, Chejfec G. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. Veterans affairs cooperative study group 380. N Engl J Med. 2000;343(3):162-168. doi:10.1056/NEJM200007203430301
11. Lieberman DA, Weiss DG; Veterans Affairs Cooperative Study Group 380. One-time screening for colorectal cancer with combined fecal occult-blood testing and examination of the distal colon. N Engl J Med. 2001;345(8):555-560. doi:10.1056/NEJMoa010328
12. Robertson DJ, Dominitz JA, Beed A, et al. Baseline features and reasons for nonparticipation in the colonoscopy versus fecal immunochemical test in reducing mortality from colorectal cancer (CONFIRM) study, a colorectal cancer screening trial. JAMA Netw Open. 2023;6(7):e2321730. doi:10.1001/jamanetworkopen.2023.21730
13. Spechler SJ, Hunter JG, Jones KM, et al. Randomized trial of medical versus surgical treatment for refractory heartburn. N Engl J Med. 2019;381(16):1513-1523. doi:10.1056/NEJMoa1811424
14. Beste LA, Green PK, Berry K, Kogut MJ, Allison SK, Ioannou GN. Effectiveness of hepatitis C antiviral treatment in a USA cohort of veteran patients with hepatocellular carcinoma. J Hepatol. 2017;67(1):32-39. doi:10.1016/j.jhep.2017.02.027
15. US Department of Veterans Affairs. Veterans affairs cooperative studies program (CSP). CSP #2023. Updated July 2022. Accessed June 12, 2024. https://www.vacsp.research.va.gov/CSP_2023/CSP_2023.asp
16. US Health Resources & Services Administration. Workforce projections. Accessed June 12, 2024. https://data.hrsa.gov/topics/health-workforce/workforce-projections
1. Shin A, Xu H, Imperiale TF. The prevalence, humanistic burden, and health care impact of irritable bowel syndrome among united states veterans. Clin Gastroenterol Hepatol. 2023;21(4):1061-1069.e1. doi:10.1016/j.cgh.2022.08.005.
2. Kent KG. Prevalence of gastrointestinal disease in US military veterans under outpatient care at the veterans health administration. SAGE Open Med. 2021;9:20503121211049112. doi:10.1177/20503121211049112
3. Beste LA, Leipertz SL, Green PK, Dominitz JA, Ross D, Ioannou GN. Trends in burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US veterans, 2001-2013. Gastroenterology. 2015;149(6):1471-e18. doi:10.1053/j.gastro.2015.07.056
4. Zullig LL, Sims KJ, McNeil R, et al. Cancer incidence among patients of the U.S. veterans affairs health care system: 2010 update. Mil Med. 2017;182(7):e1883-e1891. doi:10.7205/MILMED-D-16-00371
5. VHA Physician Workforce Resources Blueprint. US Dept of Veterans Affairs. https://dvagov.sharepoint.com/sites/WMCPortal/WFP/Documents/Reports/VHA Physician Workforce Resources Blueprint FY 23-27.pdf [Source not verified]
6. AMN Healthcare. 2022 Review of Physician and Advanced Practitioner Recruiting Incentives. Accessed June 12, 2024. https://www1.amnhealthcare.com/l/123142/2022-07-13/q6ywxg/123142/1657737392vyuONaZZ/mha2022incentivesurgraphic.pdf
7. Medical Group Management Association. MGMA DataDive Provider Compensation Data. Accessed June 12, 2024. https://www.mgma.com/datadive/provider-compensation
8. Anderson JC, Bilal M, Burke CA, et al. Burnout among US gastroenterologists and fellows in training: identifying contributing factors and offering solutions. J Clin Gastroenterol. 2023;57(10):1063-1069. doi:10.1097/MCG.0000000000001781
9. Lacy BE, Chan JL. Physician burnout: the hidden health care crisis. Clin Gastroenterol Hepatol. 2018;16(3):311-317. doi:10.1016/j.cgh.2017.06.043
10. Lieberman DA, Weiss DG, Bond JH, Ahnen DJ, Garewal H, Chejfec G. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. Veterans affairs cooperative study group 380. N Engl J Med. 2000;343(3):162-168. doi:10.1056/NEJM200007203430301
11. Lieberman DA, Weiss DG; Veterans Affairs Cooperative Study Group 380. One-time screening for colorectal cancer with combined fecal occult-blood testing and examination of the distal colon. N Engl J Med. 2001;345(8):555-560. doi:10.1056/NEJMoa010328
12. Robertson DJ, Dominitz JA, Beed A, et al. Baseline features and reasons for nonparticipation in the colonoscopy versus fecal immunochemical test in reducing mortality from colorectal cancer (CONFIRM) study, a colorectal cancer screening trial. JAMA Netw Open. 2023;6(7):e2321730. doi:10.1001/jamanetworkopen.2023.21730
13. Spechler SJ, Hunter JG, Jones KM, et al. Randomized trial of medical versus surgical treatment for refractory heartburn. N Engl J Med. 2019;381(16):1513-1523. doi:10.1056/NEJMoa1811424
14. Beste LA, Green PK, Berry K, Kogut MJ, Allison SK, Ioannou GN. Effectiveness of hepatitis C antiviral treatment in a USA cohort of veteran patients with hepatocellular carcinoma. J Hepatol. 2017;67(1):32-39. doi:10.1016/j.jhep.2017.02.027
15. US Department of Veterans Affairs. Veterans affairs cooperative studies program (CSP). CSP #2023. Updated July 2022. Accessed June 12, 2024. https://www.vacsp.research.va.gov/CSP_2023/CSP_2023.asp
16. US Health Resources & Services Administration. Workforce projections. Accessed June 12, 2024. https://data.hrsa.gov/topics/health-workforce/workforce-projections