User login
For MD-IQ use only
COVID-19 pandemic dictates reconsideration of pemphigus therapy
The Dedee F. Murrell, MD, said at the virtual annual meeting of the American Academy of Dermatology.
Together with physicians from the Mayo Clinic, Alexandria (Egypt) University, and Tehran (Iran) University, she recently published updated expert guidance for treatment of this severe, potentially fatal mucocutaneous autoimmune blistering disease, in a letter to the editor in the Journal of the American Academy of Dermatology. She presented some of the key recommendations at AAD 2020.
First off, rituximab (Rituxan), the only Food and Drug Administration–approved medication for moderate to severe pemphigus vulgaris and a biologic considered first-line therapy prepandemic, is ill-advised during the COVID-19 era. Its mechanism of benefit is through B-cell depletion. This is an irreversible effect, and reconstitution of B-cell immunity takes 6-12 months. The absence of this immunologic protection for such a long time poses potentially serious problems for pemphigus patients who become infected with SARS-CoV-2.
Also, the opportunity to administer intravenous infusions of the biologic becomes unpredictable during pandemic surges, when limitations on nonemergent medical care may be necessary, noted Dr. Murrell, professor of dermatology at the University of New South Wales and head of dermatology at St. George University Hospital, both in Sydney.
“We have taken the approach of postponing rituximab infusions temporarily, with the aim of delaying peak patient immunosuppression during peak COVID-19 incidence to reduce the risk of adverse outcomes,” Dr. Murrell and coauthors wrote in the letter (J Am Acad Dermatol. 2020 Jun;82[6]:e235-6).
The other traditional go-to therapy for pemphigus is corticosteroids. They’re effective, fast acting, and relatively inexpensive. But their nonselective immunosuppressive action boosts infection risk in general, and more specifically it increases the risk of developing severe forms of COVID-19 should a patient become infected with SARS-CoV-2.
“A basic therapeutic principle with particular importance during the pandemic is that glucocorticoids and steroid-sparing immunosuppressive agents, such as azathioprine and mycophenolate mofetil, should be tapered to the lowest effective dose. In active COVID-19 infection, immunosuppressive steroid-sparing medications should be discontinued when possible, although glucocorticoid cessation often cannot be considered due to risk for adrenal insufficiency,” the authors continued.
“Effective as adjuvant treatment in both pemphigus and COVID-19,intravenous immunoglobulin supports immunity and therefore may be useful in this setting,” they wrote. It’s not immunosuppressive, and, they noted, there’s good-quality evidence from a Japanese randomized, double-blind, controlled trial that a 5-day course of intravenous immunoglobulin is effective therapy for pemphigus (J Am Acad Dermatol. 2009 Apr;60[4]:595-603).
Moreover, intravenous immunoglobulin is also reportedly effective in severe COVID-19 (Open Forum Infect Dis. 2020 Mar 21. doi: 10.1093/ofid/ofaa102.).
Another option is to consider enrolling a patient with moderate or severe pemphigus vulgaris or foliaceus in the ongoing pivotal phase 3, international, double-blind, placebo-controlled PEGASUS trial of rilzabrutinib, a promising oral reversible Bruton tyrosine kinase inhibitor. The medication has a short half-life and a self-limited immunomodulatory effect. Moreover, the trial is set up for remote patient visits on an outpatient basis via teledermatology, so the 65-week study can continue despite the pandemic. Both newly diagnosed and relapsing patients are eligible for the trial, headed by Dr. Murrell. At AAD 2020 she reported encouraging results from a phase 2b trial of rilzabrutinib.
She is a consultant to Principia Biopharma, sponsor of the PEGASUS trial, and has received institutional research grants from numerous pharmaceutical companies.
The Dedee F. Murrell, MD, said at the virtual annual meeting of the American Academy of Dermatology.
Together with physicians from the Mayo Clinic, Alexandria (Egypt) University, and Tehran (Iran) University, she recently published updated expert guidance for treatment of this severe, potentially fatal mucocutaneous autoimmune blistering disease, in a letter to the editor in the Journal of the American Academy of Dermatology. She presented some of the key recommendations at AAD 2020.
First off, rituximab (Rituxan), the only Food and Drug Administration–approved medication for moderate to severe pemphigus vulgaris and a biologic considered first-line therapy prepandemic, is ill-advised during the COVID-19 era. Its mechanism of benefit is through B-cell depletion. This is an irreversible effect, and reconstitution of B-cell immunity takes 6-12 months. The absence of this immunologic protection for such a long time poses potentially serious problems for pemphigus patients who become infected with SARS-CoV-2.
Also, the opportunity to administer intravenous infusions of the biologic becomes unpredictable during pandemic surges, when limitations on nonemergent medical care may be necessary, noted Dr. Murrell, professor of dermatology at the University of New South Wales and head of dermatology at St. George University Hospital, both in Sydney.
“We have taken the approach of postponing rituximab infusions temporarily, with the aim of delaying peak patient immunosuppression during peak COVID-19 incidence to reduce the risk of adverse outcomes,” Dr. Murrell and coauthors wrote in the letter (J Am Acad Dermatol. 2020 Jun;82[6]:e235-6).
The other traditional go-to therapy for pemphigus is corticosteroids. They’re effective, fast acting, and relatively inexpensive. But their nonselective immunosuppressive action boosts infection risk in general, and more specifically it increases the risk of developing severe forms of COVID-19 should a patient become infected with SARS-CoV-2.
“A basic therapeutic principle with particular importance during the pandemic is that glucocorticoids and steroid-sparing immunosuppressive agents, such as azathioprine and mycophenolate mofetil, should be tapered to the lowest effective dose. In active COVID-19 infection, immunosuppressive steroid-sparing medications should be discontinued when possible, although glucocorticoid cessation often cannot be considered due to risk for adrenal insufficiency,” the authors continued.
“Effective as adjuvant treatment in both pemphigus and COVID-19,intravenous immunoglobulin supports immunity and therefore may be useful in this setting,” they wrote. It’s not immunosuppressive, and, they noted, there’s good-quality evidence from a Japanese randomized, double-blind, controlled trial that a 5-day course of intravenous immunoglobulin is effective therapy for pemphigus (J Am Acad Dermatol. 2009 Apr;60[4]:595-603).
Moreover, intravenous immunoglobulin is also reportedly effective in severe COVID-19 (Open Forum Infect Dis. 2020 Mar 21. doi: 10.1093/ofid/ofaa102.).
Another option is to consider enrolling a patient with moderate or severe pemphigus vulgaris or foliaceus in the ongoing pivotal phase 3, international, double-blind, placebo-controlled PEGASUS trial of rilzabrutinib, a promising oral reversible Bruton tyrosine kinase inhibitor. The medication has a short half-life and a self-limited immunomodulatory effect. Moreover, the trial is set up for remote patient visits on an outpatient basis via teledermatology, so the 65-week study can continue despite the pandemic. Both newly diagnosed and relapsing patients are eligible for the trial, headed by Dr. Murrell. At AAD 2020 she reported encouraging results from a phase 2b trial of rilzabrutinib.
She is a consultant to Principia Biopharma, sponsor of the PEGASUS trial, and has received institutional research grants from numerous pharmaceutical companies.
The Dedee F. Murrell, MD, said at the virtual annual meeting of the American Academy of Dermatology.
Together with physicians from the Mayo Clinic, Alexandria (Egypt) University, and Tehran (Iran) University, she recently published updated expert guidance for treatment of this severe, potentially fatal mucocutaneous autoimmune blistering disease, in a letter to the editor in the Journal of the American Academy of Dermatology. She presented some of the key recommendations at AAD 2020.
First off, rituximab (Rituxan), the only Food and Drug Administration–approved medication for moderate to severe pemphigus vulgaris and a biologic considered first-line therapy prepandemic, is ill-advised during the COVID-19 era. Its mechanism of benefit is through B-cell depletion. This is an irreversible effect, and reconstitution of B-cell immunity takes 6-12 months. The absence of this immunologic protection for such a long time poses potentially serious problems for pemphigus patients who become infected with SARS-CoV-2.
Also, the opportunity to administer intravenous infusions of the biologic becomes unpredictable during pandemic surges, when limitations on nonemergent medical care may be necessary, noted Dr. Murrell, professor of dermatology at the University of New South Wales and head of dermatology at St. George University Hospital, both in Sydney.
“We have taken the approach of postponing rituximab infusions temporarily, with the aim of delaying peak patient immunosuppression during peak COVID-19 incidence to reduce the risk of adverse outcomes,” Dr. Murrell and coauthors wrote in the letter (J Am Acad Dermatol. 2020 Jun;82[6]:e235-6).
The other traditional go-to therapy for pemphigus is corticosteroids. They’re effective, fast acting, and relatively inexpensive. But their nonselective immunosuppressive action boosts infection risk in general, and more specifically it increases the risk of developing severe forms of COVID-19 should a patient become infected with SARS-CoV-2.
“A basic therapeutic principle with particular importance during the pandemic is that glucocorticoids and steroid-sparing immunosuppressive agents, such as azathioprine and mycophenolate mofetil, should be tapered to the lowest effective dose. In active COVID-19 infection, immunosuppressive steroid-sparing medications should be discontinued when possible, although glucocorticoid cessation often cannot be considered due to risk for adrenal insufficiency,” the authors continued.
“Effective as adjuvant treatment in both pemphigus and COVID-19,intravenous immunoglobulin supports immunity and therefore may be useful in this setting,” they wrote. It’s not immunosuppressive, and, they noted, there’s good-quality evidence from a Japanese randomized, double-blind, controlled trial that a 5-day course of intravenous immunoglobulin is effective therapy for pemphigus (J Am Acad Dermatol. 2009 Apr;60[4]:595-603).
Moreover, intravenous immunoglobulin is also reportedly effective in severe COVID-19 (Open Forum Infect Dis. 2020 Mar 21. doi: 10.1093/ofid/ofaa102.).
Another option is to consider enrolling a patient with moderate or severe pemphigus vulgaris or foliaceus in the ongoing pivotal phase 3, international, double-blind, placebo-controlled PEGASUS trial of rilzabrutinib, a promising oral reversible Bruton tyrosine kinase inhibitor. The medication has a short half-life and a self-limited immunomodulatory effect. Moreover, the trial is set up for remote patient visits on an outpatient basis via teledermatology, so the 65-week study can continue despite the pandemic. Both newly diagnosed and relapsing patients are eligible for the trial, headed by Dr. Murrell. At AAD 2020 she reported encouraging results from a phase 2b trial of rilzabrutinib.
She is a consultant to Principia Biopharma, sponsor of the PEGASUS trial, and has received institutional research grants from numerous pharmaceutical companies.
FROM AAD 20
PD-1 Signaling in Extramammary Paget Disease
Primary extramammary Paget disease (EMPD) is an adnexal carcinoma of the apocrine gland ducts that presents as an erythematous patch on cutaneous sites rich with apocrine glands.1 Primary EMPD can be in situ or invasive with the potential to become metastatic.2 Treatment of primary EMPD is challenging due to the difficulty of achieving clear surgical margins, as the tumor has microscopic spread throughout the epidermis in a skipping fashion.3 Mohs micrographic surgery is the treatment of choice; however, there is a clinical need to identify additional treatment modalities, especially for patients with unresectable, invasive, or metastatic primary EMPD,4 which partly is due to lack of data to understand the pathogenesis of primary EMPD. Recently, there have been studies investigating the genetic characteristics of EMPD tumors. The interaction between the programmed cell death receptor 1 (PD-1) and its ligand (PD-L1) is one of the pathways recently studied and has been reported to be a potential target in EMPD.5-7 Programmed cell death receptor 1 signaling constitutes an immune checkpoint pathway that regulates the activation of tumor-specific T cells.8 In several malignancies, cancer cells express PD-L1 on their surface to activate PD-1 signaling in T cells as a mechanism to dampen the tumor-specific immune response and evade antitumor immunity.9 Thus, blocking PD-1 signaling widely is used to activate tumor-specific T cells and decrease tumor burden.10 Given the advances of immunotherapy in many neoplasms and the paucity of effective agents to treat EMPD, this article serves to shed light on recent data studying PD-1 signaling in EMPD and highlights the potential clinical use of immunotherapy for EMPD.
EMPD and Its Subtypes
Extramammary Paget disease is a rare adenocarcinoma typically affecting older patients (age >60 years) in cutaneous sites with abundant apocrine glands such as the genital and perianal skin.3 Extramammary Paget disease presents as an erythematous patch and frequently is treated initially as a skin dermatosis, resulting in a delay in diagnosis. Histologically, EMPD is characterized by the presence of single cells or a nest of cells having abundant pale cytoplasm and large vesicular nuclei distributed in the epidermis in a pagetoid fashion.11
Extramammary Paget disease can be primary or secondary; the 2 subtypes behave differently both clinically and prognostically. Although primary EMPD is considered to be an adnexal carcinoma of the apocrine gland ducts, secondary EMPD is considered to be an intraepithelial extension of malignant cells from an underlying internal neoplasm.12 The underlying malignancies usually are located within dermal adnexal glands or organs in the vicinity of the cutaneous lesion, such as the colon in the case of perianal EMPD. Histologically, primary and secondary EMPD can be differentiated based on their immunophenotypic staining profiles. Although all cases of EMPD show positive immunohistochemistry staining for cytokeratin 7, carcinoembryonic antigen, and epithelial membrane antigen, only primary EMPD will additionally stain for GCDFP-15 (gross cystic disease fluid protein 15) and GATA.11 Regardless of the immunohistochemistry stains, every patient newly diagnosed with EMPD deserves a full workup for malignancy screening, including a colonoscopy, cystoscopy, mammography and Papanicolaou test in women, pelvic ultrasound, and computed tomography of the abdomen and pelvis.13
The first-line treatment of EMPD is surgery; however, obtaining clear surgical margins can be a challenge, with high recurrence rates due to the microscopic spread of the disease throughout the epidermis.4 In addition, anatomic location affects the surgical approach and patient survival. Recent studies on EMPD mortality outcomes in women show that mortality is higher in patients with vaginal EMPD than in those with vulvar/labial EMPD, partly due to the sensitive location that makes it difficult to perform wide local excisions.13,14 Assessing the entire margins with tissue preservation using Mohs micrographic surgery has been shown to be successful in decreasing the recurrence rate, especially when coupled with the use of cytokeratin 7 immunohistochemistry.4 Other treatment modalities include radiation, topical imiquimod, and photodynamic therapy.15,16 Regardless of treatment modality, EMPD requires long‐term follow-up to monitor for disease recurrence, regional lymphadenopathy, distant metastasis, or development of an internal malignancy.
The pathogenesis of primary EMPD remains unclear. The tumor is thought to be derived from Toker cells, which are pluripotent adnexal stem cells located in the epidermis that normally give rise to apocrine glands.17 There have been few studies investigating the genetic characteristics of EMPD lesions in an attempt to understand pathogenesis as well as to find druggable targets. Current data for targeted therapy have focused on HER2 (human epidermal growth factor receptor 2) hormone receptor expression,18 ERBB (erythroblastic oncogene B) amplification,19 CDK4 (cyclin-dependent kinase 4)–cyclin D1 signaling,20 and most recently PD-1/PD-L1 pathway.5-7
PD-1 Expression in EMPD: Implication for Immunotherapy
Most tumors display novel antigens that are recognized by the host immune system and thus stimulate cell-mediated and humoral pathways. The immune system naturally provides regulatory immune checkpoints to T cell–mediated immune responses. One of these checkpoints involves the interaction between PD-1 on T cells and its ligand PD-L1 on tumor cells.21 When PD-1 binds to PD-L1 on tumor cells, there is inhibition of T-cell proliferation, a decrease in cytokine production, and induction of T-cell cytolysis.22 The Figure summarizes the dynamics for T-cell regulation.

Naturally, tumor-infiltrating T cells trigger their own inhibition by binding to PD-L1. However, certain tumor cells constitutively upregulate the expression of PD-L1. With that, the tumor cells gain the ability to suppress T cells and avoid T cell–mediated cytotoxicity,23 which is known as the adoptive immune resistance mechanism. There have been several studies in the literature investigating the PD-1 signaling pathway in EMPD as a way to determine if EMPD would be susceptible to immune checkpoint blockade. The success of checkpoint inhibitor immunotherapy generally correlates with increased PD-L1 expression by tumor cells.
One study evaluated the expression of PD-L1 in tumor cells and tumor-infiltrating T cells in 18 cases of EMPD.6 The authors identified that even though tumor cell PD-L1 expression was detected in only 3 (17%) cases, tumor-infiltrating lymphocytes expressed PD-L1 in the majority of the cases analyzed and in all of the cases positive for tumor cell PD-L1.6
Another study evaluated PD-1 and PD-L1 expression in EMPD tumor cells and tumor-associated immune infiltrate.5 They found that PD-1 was expressed heavily by the tumor-associated immune infiltrate in all EMPD cases analyzed. Similar to the previously mentioned study,6 PD-L1 was expressed by tumor cells in a few cases only. Interestingly, they found that the density of CD3 in the tumor-associated immune infiltrate was significantly (P=.049) higher in patients who were alive than in those who died, suggesting the importance of an exuberant T-cell response for survival in EMPD.5
A third study investigated protein expression of the B7 family members as well as PD-1 and PD-L1/2 in 55 EMPD samples. In this study the authors also found that tumor cell PD-L1 was minimal. Interestingly, they also found that tumor cells expressed B7 proteins in the majority of the cases.7
Finally, another study examined activity levels of T cells in EMPD by measuring the number and expression levels of cytotoxic T-cell cytokines.24 The authors first found that EMPD tumors had a significantly higher number of CD8+ tumor-infiltrating lymphocytes compared to peripheral blood (P<.01). These CD8+ tumor-infiltrating lymphocytes also had a significantly higher expression of PD-1 (P<.01). They also found that tumor cells produced an immunosuppressive molecule called indoleamine 2,3-dyoxygenae that functions by suppressing T-cell activity levels. They concluded that in EMPD, tumor-specific T lymphocytes have an exhausted phenotype due to PD-1 activation as well as indoleamine 2,3-dyoxygenase release to the tumor microenvironment.24
These studies highlight that restoring the effector functions of tumor-specific T lymphocytes could be an effective treatment strategy for EMPD. In fact, immunotherapy has been used with success for EMPD in the form of topical immunomodulators such as imiquimod.16,25 More than 40 cases of EMPD treated with imiquimod 5% have been published; of these, only 6 were considered nonresponders,5 which suggests that EMPD may respond to other immunotherapies such as checkpoint inhibitors. It is an exciting time for immunotherapy as more checkpoint inhibitors are being developed. Among the newer agents is cemiplimab, which is a PD-1 inhibitor now US Food and Drug Administration approved for the treatment of locally advanced or metastatic cutaneous squamous cell carcinoma in patients who are not candidates for curative surgery or curative radiation.26 Programmed cell death receptor 1 signaling can serve as a potential target in EMPD, and further studies need to be performed to test the clinical efficacy, especially in unresectable or invasive/metastatic EMPD. As the PD-1 pathway is more studied in EMPD, and as more PD-1 inhibitors get developed, it would be a clinical need to establish clinical studies for PD-1 inhibitors in EMPD.
- Ito T, Kaku-Ito Y, Furue M. The diagnosis and management of extramammary Paget’s disease. Expert Rev Anticancer Ther. 2018;18:543-553.
- van der Zwan JM, Siesling S, Blokx WAM, et al. Invasive extramammary Paget’s disease and the risk for secondary tumours in Europe. Eur J Surg Oncol. 2012;38:214-221.
- Simonds RM, Segal RJ, Sharma A. Extramammary Paget’s disease: a review of the literature. Int J Dermatol. 2019;58:871-879.
- Wollina U, Goldman A, Bieneck A, et al. Surgical treatment for extramammary Paget’s disease. Curr Treat Options Oncol. 2018;19:27.
- Mauzo SH, Tetzlaff MT, Milton DR, et al. Expression of PD-1 and PD-L1 in extramammary Paget disease: implications for immune-targeted therapy. Cancers (Basel). 2019;11:754.
- Fowler MR, Flanigan KL, Googe PB. PD-L1 expression in extramammary Paget disease [published online March 6, 2020]. Am J Dermatopathol. doi:10.1097/dad.0000000000001622.
- Pourmaleki M, Young JH, Socci ND, et al. Extramammary Paget disease shows differential expression of B7 family members B7-H3, B7-H4, PD-L1, PD-L2 and cancer/testis antigens NY-ESO-1 and MAGE-A. Oncotarget. 2019;10:6152-6167.
- Mahoney KM, Freeman GJ, McDermott DF. The next immune-checkpoint inhibitors: PD-1/PD-L1 blockade in melanoma. Clin Ther. 2015;37:764-782.
- Dany M, Nganga R, Chidiac A, et al. Advances in immunotherapy for melanoma management. Hum Vaccines Immunother. 2016;12:2501-2511.
- Richter MD, Hughes GC, Chung SH, et al. Immunologic adverse events from immune checkpoint therapy [published online April 13, 2020]. Best Pract Res Clin Rheumatol. doi:10.1016/j.berh.2020.101511.
- Kang Z, Zhang Q, Zhang Q, et al. Clinical and pathological characteristics of extramammary Paget’s disease: report of 246 Chinese male patients. Int J Clin Exp Pathol. 2015;8:13233-13240.
- Ohara K, Fujisawa Y, Yoshino K, et al. A proposal for a TNM staging system for extramammary Paget disease: retrospective analysis of 301 patients with invasive primary tumors. J Dermatol Sci. 2016;83:234-239.
- Hatta N. Prognostic factors of extramammary Paget’s disease. Curr Treat Options Oncol. 2018;19:47.
- Yao H, Xie M, Fu S, et al. Survival analysis of patients with invasive extramammary Paget disease: implications of anatomic sites. BMC Cancer. 2018;18:403.
- Herrel LA, Weiss AD, Goodman M, et al. Extramammary Paget’s disease in males: survival outcomes in 495 patients. Ann Surg Oncol. 2015;22:1625-1630.
- Sanderson P, Innamaa A, Palmer J, et al. Imiquimod therapy for extramammary Paget’s disease of the vulva: a viable non-surgical alternative. J Obstet Gynaecol. 2013;33:479-483.
- Smith AA. Pre-Paget cells: evidence of keratinocyte origin of extramammary Paget’s disease. Intractable Rare Dis Res. 2019;8:203-205.
- Garganese G, Inzani F, Mantovani G, et al. The vulvar immunohistochemical panel (VIP) project: molecular profiles of vulvar Paget’s disease. J Cancer Res Clin Oncol. 2019;145:2211-2225.
- Dias-Santagata D, Lam Q, Bergethon K, et al. A potential role for targeted therapy in a subset of metastasizing adnexal carcinomas. Mod Pathol. 2011;24:974-982.
- Cohen JM, Granter SR, Werchniak AE. Risk stratification in extramammary Paget disease. Clin Exp Dermatol. 2015;40:473-478.
- Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018;8:1069-1086.
- Shi Y. Regulatory mechanisms of PD-L1 expression in cancer cells. Cancer Immunol Immunother. 2018;67:1481-1489.
- Cui C, Yu B, Jiang Q, et al. The roles of PD-1/PD-L1 and its signalling pathway in gastrointestinal tract cancers. Clin Exp Pharmacol Physiol. 2019;46:3-10.
- Iga N, Otsuka A, Yamamoto Y, et al. Accumulation of exhausted CD8+ T cells in extramammary Paget’s disease. PLoS One. 2019;14:E0211135.
- Frances L, Pascual JC, Leiva-Salinas M, et al. Extramammary Paget disease successfully treated with topical imiquimod 5% and tazarotene. Dermatol Ther. 2014;27:19-20.
- Lee A, Duggan S, Deeks ED. Cemiplimab: a review in advanced cutaneous squamous cell carcinoma. Drugs. 2020;80:813-819.
Primary extramammary Paget disease (EMPD) is an adnexal carcinoma of the apocrine gland ducts that presents as an erythematous patch on cutaneous sites rich with apocrine glands.1 Primary EMPD can be in situ or invasive with the potential to become metastatic.2 Treatment of primary EMPD is challenging due to the difficulty of achieving clear surgical margins, as the tumor has microscopic spread throughout the epidermis in a skipping fashion.3 Mohs micrographic surgery is the treatment of choice; however, there is a clinical need to identify additional treatment modalities, especially for patients with unresectable, invasive, or metastatic primary EMPD,4 which partly is due to lack of data to understand the pathogenesis of primary EMPD. Recently, there have been studies investigating the genetic characteristics of EMPD tumors. The interaction between the programmed cell death receptor 1 (PD-1) and its ligand (PD-L1) is one of the pathways recently studied and has been reported to be a potential target in EMPD.5-7 Programmed cell death receptor 1 signaling constitutes an immune checkpoint pathway that regulates the activation of tumor-specific T cells.8 In several malignancies, cancer cells express PD-L1 on their surface to activate PD-1 signaling in T cells as a mechanism to dampen the tumor-specific immune response and evade antitumor immunity.9 Thus, blocking PD-1 signaling widely is used to activate tumor-specific T cells and decrease tumor burden.10 Given the advances of immunotherapy in many neoplasms and the paucity of effective agents to treat EMPD, this article serves to shed light on recent data studying PD-1 signaling in EMPD and highlights the potential clinical use of immunotherapy for EMPD.
EMPD and Its Subtypes
Extramammary Paget disease is a rare adenocarcinoma typically affecting older patients (age >60 years) in cutaneous sites with abundant apocrine glands such as the genital and perianal skin.3 Extramammary Paget disease presents as an erythematous patch and frequently is treated initially as a skin dermatosis, resulting in a delay in diagnosis. Histologically, EMPD is characterized by the presence of single cells or a nest of cells having abundant pale cytoplasm and large vesicular nuclei distributed in the epidermis in a pagetoid fashion.11
Extramammary Paget disease can be primary or secondary; the 2 subtypes behave differently both clinically and prognostically. Although primary EMPD is considered to be an adnexal carcinoma of the apocrine gland ducts, secondary EMPD is considered to be an intraepithelial extension of malignant cells from an underlying internal neoplasm.12 The underlying malignancies usually are located within dermal adnexal glands or organs in the vicinity of the cutaneous lesion, such as the colon in the case of perianal EMPD. Histologically, primary and secondary EMPD can be differentiated based on their immunophenotypic staining profiles. Although all cases of EMPD show positive immunohistochemistry staining for cytokeratin 7, carcinoembryonic antigen, and epithelial membrane antigen, only primary EMPD will additionally stain for GCDFP-15 (gross cystic disease fluid protein 15) and GATA.11 Regardless of the immunohistochemistry stains, every patient newly diagnosed with EMPD deserves a full workup for malignancy screening, including a colonoscopy, cystoscopy, mammography and Papanicolaou test in women, pelvic ultrasound, and computed tomography of the abdomen and pelvis.13
The first-line treatment of EMPD is surgery; however, obtaining clear surgical margins can be a challenge, with high recurrence rates due to the microscopic spread of the disease throughout the epidermis.4 In addition, anatomic location affects the surgical approach and patient survival. Recent studies on EMPD mortality outcomes in women show that mortality is higher in patients with vaginal EMPD than in those with vulvar/labial EMPD, partly due to the sensitive location that makes it difficult to perform wide local excisions.13,14 Assessing the entire margins with tissue preservation using Mohs micrographic surgery has been shown to be successful in decreasing the recurrence rate, especially when coupled with the use of cytokeratin 7 immunohistochemistry.4 Other treatment modalities include radiation, topical imiquimod, and photodynamic therapy.15,16 Regardless of treatment modality, EMPD requires long‐term follow-up to monitor for disease recurrence, regional lymphadenopathy, distant metastasis, or development of an internal malignancy.
The pathogenesis of primary EMPD remains unclear. The tumor is thought to be derived from Toker cells, which are pluripotent adnexal stem cells located in the epidermis that normally give rise to apocrine glands.17 There have been few studies investigating the genetic characteristics of EMPD lesions in an attempt to understand pathogenesis as well as to find druggable targets. Current data for targeted therapy have focused on HER2 (human epidermal growth factor receptor 2) hormone receptor expression,18 ERBB (erythroblastic oncogene B) amplification,19 CDK4 (cyclin-dependent kinase 4)–cyclin D1 signaling,20 and most recently PD-1/PD-L1 pathway.5-7
PD-1 Expression in EMPD: Implication for Immunotherapy
Most tumors display novel antigens that are recognized by the host immune system and thus stimulate cell-mediated and humoral pathways. The immune system naturally provides regulatory immune checkpoints to T cell–mediated immune responses. One of these checkpoints involves the interaction between PD-1 on T cells and its ligand PD-L1 on tumor cells.21 When PD-1 binds to PD-L1 on tumor cells, there is inhibition of T-cell proliferation, a decrease in cytokine production, and induction of T-cell cytolysis.22 The Figure summarizes the dynamics for T-cell regulation.

Naturally, tumor-infiltrating T cells trigger their own inhibition by binding to PD-L1. However, certain tumor cells constitutively upregulate the expression of PD-L1. With that, the tumor cells gain the ability to suppress T cells and avoid T cell–mediated cytotoxicity,23 which is known as the adoptive immune resistance mechanism. There have been several studies in the literature investigating the PD-1 signaling pathway in EMPD as a way to determine if EMPD would be susceptible to immune checkpoint blockade. The success of checkpoint inhibitor immunotherapy generally correlates with increased PD-L1 expression by tumor cells.
One study evaluated the expression of PD-L1 in tumor cells and tumor-infiltrating T cells in 18 cases of EMPD.6 The authors identified that even though tumor cell PD-L1 expression was detected in only 3 (17%) cases, tumor-infiltrating lymphocytes expressed PD-L1 in the majority of the cases analyzed and in all of the cases positive for tumor cell PD-L1.6
Another study evaluated PD-1 and PD-L1 expression in EMPD tumor cells and tumor-associated immune infiltrate.5 They found that PD-1 was expressed heavily by the tumor-associated immune infiltrate in all EMPD cases analyzed. Similar to the previously mentioned study,6 PD-L1 was expressed by tumor cells in a few cases only. Interestingly, they found that the density of CD3 in the tumor-associated immune infiltrate was significantly (P=.049) higher in patients who were alive than in those who died, suggesting the importance of an exuberant T-cell response for survival in EMPD.5
A third study investigated protein expression of the B7 family members as well as PD-1 and PD-L1/2 in 55 EMPD samples. In this study the authors also found that tumor cell PD-L1 was minimal. Interestingly, they also found that tumor cells expressed B7 proteins in the majority of the cases.7
Finally, another study examined activity levels of T cells in EMPD by measuring the number and expression levels of cytotoxic T-cell cytokines.24 The authors first found that EMPD tumors had a significantly higher number of CD8+ tumor-infiltrating lymphocytes compared to peripheral blood (P<.01). These CD8+ tumor-infiltrating lymphocytes also had a significantly higher expression of PD-1 (P<.01). They also found that tumor cells produced an immunosuppressive molecule called indoleamine 2,3-dyoxygenae that functions by suppressing T-cell activity levels. They concluded that in EMPD, tumor-specific T lymphocytes have an exhausted phenotype due to PD-1 activation as well as indoleamine 2,3-dyoxygenase release to the tumor microenvironment.24
These studies highlight that restoring the effector functions of tumor-specific T lymphocytes could be an effective treatment strategy for EMPD. In fact, immunotherapy has been used with success for EMPD in the form of topical immunomodulators such as imiquimod.16,25 More than 40 cases of EMPD treated with imiquimod 5% have been published; of these, only 6 were considered nonresponders,5 which suggests that EMPD may respond to other immunotherapies such as checkpoint inhibitors. It is an exciting time for immunotherapy as more checkpoint inhibitors are being developed. Among the newer agents is cemiplimab, which is a PD-1 inhibitor now US Food and Drug Administration approved for the treatment of locally advanced or metastatic cutaneous squamous cell carcinoma in patients who are not candidates for curative surgery or curative radiation.26 Programmed cell death receptor 1 signaling can serve as a potential target in EMPD, and further studies need to be performed to test the clinical efficacy, especially in unresectable or invasive/metastatic EMPD. As the PD-1 pathway is more studied in EMPD, and as more PD-1 inhibitors get developed, it would be a clinical need to establish clinical studies for PD-1 inhibitors in EMPD.
Primary extramammary Paget disease (EMPD) is an adnexal carcinoma of the apocrine gland ducts that presents as an erythematous patch on cutaneous sites rich with apocrine glands.1 Primary EMPD can be in situ or invasive with the potential to become metastatic.2 Treatment of primary EMPD is challenging due to the difficulty of achieving clear surgical margins, as the tumor has microscopic spread throughout the epidermis in a skipping fashion.3 Mohs micrographic surgery is the treatment of choice; however, there is a clinical need to identify additional treatment modalities, especially for patients with unresectable, invasive, or metastatic primary EMPD,4 which partly is due to lack of data to understand the pathogenesis of primary EMPD. Recently, there have been studies investigating the genetic characteristics of EMPD tumors. The interaction between the programmed cell death receptor 1 (PD-1) and its ligand (PD-L1) is one of the pathways recently studied and has been reported to be a potential target in EMPD.5-7 Programmed cell death receptor 1 signaling constitutes an immune checkpoint pathway that regulates the activation of tumor-specific T cells.8 In several malignancies, cancer cells express PD-L1 on their surface to activate PD-1 signaling in T cells as a mechanism to dampen the tumor-specific immune response and evade antitumor immunity.9 Thus, blocking PD-1 signaling widely is used to activate tumor-specific T cells and decrease tumor burden.10 Given the advances of immunotherapy in many neoplasms and the paucity of effective agents to treat EMPD, this article serves to shed light on recent data studying PD-1 signaling in EMPD and highlights the potential clinical use of immunotherapy for EMPD.
EMPD and Its Subtypes
Extramammary Paget disease is a rare adenocarcinoma typically affecting older patients (age >60 years) in cutaneous sites with abundant apocrine glands such as the genital and perianal skin.3 Extramammary Paget disease presents as an erythematous patch and frequently is treated initially as a skin dermatosis, resulting in a delay in diagnosis. Histologically, EMPD is characterized by the presence of single cells or a nest of cells having abundant pale cytoplasm and large vesicular nuclei distributed in the epidermis in a pagetoid fashion.11
Extramammary Paget disease can be primary or secondary; the 2 subtypes behave differently both clinically and prognostically. Although primary EMPD is considered to be an adnexal carcinoma of the apocrine gland ducts, secondary EMPD is considered to be an intraepithelial extension of malignant cells from an underlying internal neoplasm.12 The underlying malignancies usually are located within dermal adnexal glands or organs in the vicinity of the cutaneous lesion, such as the colon in the case of perianal EMPD. Histologically, primary and secondary EMPD can be differentiated based on their immunophenotypic staining profiles. Although all cases of EMPD show positive immunohistochemistry staining for cytokeratin 7, carcinoembryonic antigen, and epithelial membrane antigen, only primary EMPD will additionally stain for GCDFP-15 (gross cystic disease fluid protein 15) and GATA.11 Regardless of the immunohistochemistry stains, every patient newly diagnosed with EMPD deserves a full workup for malignancy screening, including a colonoscopy, cystoscopy, mammography and Papanicolaou test in women, pelvic ultrasound, and computed tomography of the abdomen and pelvis.13
The first-line treatment of EMPD is surgery; however, obtaining clear surgical margins can be a challenge, with high recurrence rates due to the microscopic spread of the disease throughout the epidermis.4 In addition, anatomic location affects the surgical approach and patient survival. Recent studies on EMPD mortality outcomes in women show that mortality is higher in patients with vaginal EMPD than in those with vulvar/labial EMPD, partly due to the sensitive location that makes it difficult to perform wide local excisions.13,14 Assessing the entire margins with tissue preservation using Mohs micrographic surgery has been shown to be successful in decreasing the recurrence rate, especially when coupled with the use of cytokeratin 7 immunohistochemistry.4 Other treatment modalities include radiation, topical imiquimod, and photodynamic therapy.15,16 Regardless of treatment modality, EMPD requires long‐term follow-up to monitor for disease recurrence, regional lymphadenopathy, distant metastasis, or development of an internal malignancy.
The pathogenesis of primary EMPD remains unclear. The tumor is thought to be derived from Toker cells, which are pluripotent adnexal stem cells located in the epidermis that normally give rise to apocrine glands.17 There have been few studies investigating the genetic characteristics of EMPD lesions in an attempt to understand pathogenesis as well as to find druggable targets. Current data for targeted therapy have focused on HER2 (human epidermal growth factor receptor 2) hormone receptor expression,18 ERBB (erythroblastic oncogene B) amplification,19 CDK4 (cyclin-dependent kinase 4)–cyclin D1 signaling,20 and most recently PD-1/PD-L1 pathway.5-7
PD-1 Expression in EMPD: Implication for Immunotherapy
Most tumors display novel antigens that are recognized by the host immune system and thus stimulate cell-mediated and humoral pathways. The immune system naturally provides regulatory immune checkpoints to T cell–mediated immune responses. One of these checkpoints involves the interaction between PD-1 on T cells and its ligand PD-L1 on tumor cells.21 When PD-1 binds to PD-L1 on tumor cells, there is inhibition of T-cell proliferation, a decrease in cytokine production, and induction of T-cell cytolysis.22 The Figure summarizes the dynamics for T-cell regulation.

Naturally, tumor-infiltrating T cells trigger their own inhibition by binding to PD-L1. However, certain tumor cells constitutively upregulate the expression of PD-L1. With that, the tumor cells gain the ability to suppress T cells and avoid T cell–mediated cytotoxicity,23 which is known as the adoptive immune resistance mechanism. There have been several studies in the literature investigating the PD-1 signaling pathway in EMPD as a way to determine if EMPD would be susceptible to immune checkpoint blockade. The success of checkpoint inhibitor immunotherapy generally correlates with increased PD-L1 expression by tumor cells.
One study evaluated the expression of PD-L1 in tumor cells and tumor-infiltrating T cells in 18 cases of EMPD.6 The authors identified that even though tumor cell PD-L1 expression was detected in only 3 (17%) cases, tumor-infiltrating lymphocytes expressed PD-L1 in the majority of the cases analyzed and in all of the cases positive for tumor cell PD-L1.6
Another study evaluated PD-1 and PD-L1 expression in EMPD tumor cells and tumor-associated immune infiltrate.5 They found that PD-1 was expressed heavily by the tumor-associated immune infiltrate in all EMPD cases analyzed. Similar to the previously mentioned study,6 PD-L1 was expressed by tumor cells in a few cases only. Interestingly, they found that the density of CD3 in the tumor-associated immune infiltrate was significantly (P=.049) higher in patients who were alive than in those who died, suggesting the importance of an exuberant T-cell response for survival in EMPD.5
A third study investigated protein expression of the B7 family members as well as PD-1 and PD-L1/2 in 55 EMPD samples. In this study the authors also found that tumor cell PD-L1 was minimal. Interestingly, they also found that tumor cells expressed B7 proteins in the majority of the cases.7
Finally, another study examined activity levels of T cells in EMPD by measuring the number and expression levels of cytotoxic T-cell cytokines.24 The authors first found that EMPD tumors had a significantly higher number of CD8+ tumor-infiltrating lymphocytes compared to peripheral blood (P<.01). These CD8+ tumor-infiltrating lymphocytes also had a significantly higher expression of PD-1 (P<.01). They also found that tumor cells produced an immunosuppressive molecule called indoleamine 2,3-dyoxygenae that functions by suppressing T-cell activity levels. They concluded that in EMPD, tumor-specific T lymphocytes have an exhausted phenotype due to PD-1 activation as well as indoleamine 2,3-dyoxygenase release to the tumor microenvironment.24
These studies highlight that restoring the effector functions of tumor-specific T lymphocytes could be an effective treatment strategy for EMPD. In fact, immunotherapy has been used with success for EMPD in the form of topical immunomodulators such as imiquimod.16,25 More than 40 cases of EMPD treated with imiquimod 5% have been published; of these, only 6 were considered nonresponders,5 which suggests that EMPD may respond to other immunotherapies such as checkpoint inhibitors. It is an exciting time for immunotherapy as more checkpoint inhibitors are being developed. Among the newer agents is cemiplimab, which is a PD-1 inhibitor now US Food and Drug Administration approved for the treatment of locally advanced or metastatic cutaneous squamous cell carcinoma in patients who are not candidates for curative surgery or curative radiation.26 Programmed cell death receptor 1 signaling can serve as a potential target in EMPD, and further studies need to be performed to test the clinical efficacy, especially in unresectable or invasive/metastatic EMPD. As the PD-1 pathway is more studied in EMPD, and as more PD-1 inhibitors get developed, it would be a clinical need to establish clinical studies for PD-1 inhibitors in EMPD.
- Ito T, Kaku-Ito Y, Furue M. The diagnosis and management of extramammary Paget’s disease. Expert Rev Anticancer Ther. 2018;18:543-553.
- van der Zwan JM, Siesling S, Blokx WAM, et al. Invasive extramammary Paget’s disease and the risk for secondary tumours in Europe. Eur J Surg Oncol. 2012;38:214-221.
- Simonds RM, Segal RJ, Sharma A. Extramammary Paget’s disease: a review of the literature. Int J Dermatol. 2019;58:871-879.
- Wollina U, Goldman A, Bieneck A, et al. Surgical treatment for extramammary Paget’s disease. Curr Treat Options Oncol. 2018;19:27.
- Mauzo SH, Tetzlaff MT, Milton DR, et al. Expression of PD-1 and PD-L1 in extramammary Paget disease: implications for immune-targeted therapy. Cancers (Basel). 2019;11:754.
- Fowler MR, Flanigan KL, Googe PB. PD-L1 expression in extramammary Paget disease [published online March 6, 2020]. Am J Dermatopathol. doi:10.1097/dad.0000000000001622.
- Pourmaleki M, Young JH, Socci ND, et al. Extramammary Paget disease shows differential expression of B7 family members B7-H3, B7-H4, PD-L1, PD-L2 and cancer/testis antigens NY-ESO-1 and MAGE-A. Oncotarget. 2019;10:6152-6167.
- Mahoney KM, Freeman GJ, McDermott DF. The next immune-checkpoint inhibitors: PD-1/PD-L1 blockade in melanoma. Clin Ther. 2015;37:764-782.
- Dany M, Nganga R, Chidiac A, et al. Advances in immunotherapy for melanoma management. Hum Vaccines Immunother. 2016;12:2501-2511.
- Richter MD, Hughes GC, Chung SH, et al. Immunologic adverse events from immune checkpoint therapy [published online April 13, 2020]. Best Pract Res Clin Rheumatol. doi:10.1016/j.berh.2020.101511.
- Kang Z, Zhang Q, Zhang Q, et al. Clinical and pathological characteristics of extramammary Paget’s disease: report of 246 Chinese male patients. Int J Clin Exp Pathol. 2015;8:13233-13240.
- Ohara K, Fujisawa Y, Yoshino K, et al. A proposal for a TNM staging system for extramammary Paget disease: retrospective analysis of 301 patients with invasive primary tumors. J Dermatol Sci. 2016;83:234-239.
- Hatta N. Prognostic factors of extramammary Paget’s disease. Curr Treat Options Oncol. 2018;19:47.
- Yao H, Xie M, Fu S, et al. Survival analysis of patients with invasive extramammary Paget disease: implications of anatomic sites. BMC Cancer. 2018;18:403.
- Herrel LA, Weiss AD, Goodman M, et al. Extramammary Paget’s disease in males: survival outcomes in 495 patients. Ann Surg Oncol. 2015;22:1625-1630.
- Sanderson P, Innamaa A, Palmer J, et al. Imiquimod therapy for extramammary Paget’s disease of the vulva: a viable non-surgical alternative. J Obstet Gynaecol. 2013;33:479-483.
- Smith AA. Pre-Paget cells: evidence of keratinocyte origin of extramammary Paget’s disease. Intractable Rare Dis Res. 2019;8:203-205.
- Garganese G, Inzani F, Mantovani G, et al. The vulvar immunohistochemical panel (VIP) project: molecular profiles of vulvar Paget’s disease. J Cancer Res Clin Oncol. 2019;145:2211-2225.
- Dias-Santagata D, Lam Q, Bergethon K, et al. A potential role for targeted therapy in a subset of metastasizing adnexal carcinomas. Mod Pathol. 2011;24:974-982.
- Cohen JM, Granter SR, Werchniak AE. Risk stratification in extramammary Paget disease. Clin Exp Dermatol. 2015;40:473-478.
- Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018;8:1069-1086.
- Shi Y. Regulatory mechanisms of PD-L1 expression in cancer cells. Cancer Immunol Immunother. 2018;67:1481-1489.
- Cui C, Yu B, Jiang Q, et al. The roles of PD-1/PD-L1 and its signalling pathway in gastrointestinal tract cancers. Clin Exp Pharmacol Physiol. 2019;46:3-10.
- Iga N, Otsuka A, Yamamoto Y, et al. Accumulation of exhausted CD8+ T cells in extramammary Paget’s disease. PLoS One. 2019;14:E0211135.
- Frances L, Pascual JC, Leiva-Salinas M, et al. Extramammary Paget disease successfully treated with topical imiquimod 5% and tazarotene. Dermatol Ther. 2014;27:19-20.
- Lee A, Duggan S, Deeks ED. Cemiplimab: a review in advanced cutaneous squamous cell carcinoma. Drugs. 2020;80:813-819.
- Ito T, Kaku-Ito Y, Furue M. The diagnosis and management of extramammary Paget’s disease. Expert Rev Anticancer Ther. 2018;18:543-553.
- van der Zwan JM, Siesling S, Blokx WAM, et al. Invasive extramammary Paget’s disease and the risk for secondary tumours in Europe. Eur J Surg Oncol. 2012;38:214-221.
- Simonds RM, Segal RJ, Sharma A. Extramammary Paget’s disease: a review of the literature. Int J Dermatol. 2019;58:871-879.
- Wollina U, Goldman A, Bieneck A, et al. Surgical treatment for extramammary Paget’s disease. Curr Treat Options Oncol. 2018;19:27.
- Mauzo SH, Tetzlaff MT, Milton DR, et al. Expression of PD-1 and PD-L1 in extramammary Paget disease: implications for immune-targeted therapy. Cancers (Basel). 2019;11:754.
- Fowler MR, Flanigan KL, Googe PB. PD-L1 expression in extramammary Paget disease [published online March 6, 2020]. Am J Dermatopathol. doi:10.1097/dad.0000000000001622.
- Pourmaleki M, Young JH, Socci ND, et al. Extramammary Paget disease shows differential expression of B7 family members B7-H3, B7-H4, PD-L1, PD-L2 and cancer/testis antigens NY-ESO-1 and MAGE-A. Oncotarget. 2019;10:6152-6167.
- Mahoney KM, Freeman GJ, McDermott DF. The next immune-checkpoint inhibitors: PD-1/PD-L1 blockade in melanoma. Clin Ther. 2015;37:764-782.
- Dany M, Nganga R, Chidiac A, et al. Advances in immunotherapy for melanoma management. Hum Vaccines Immunother. 2016;12:2501-2511.
- Richter MD, Hughes GC, Chung SH, et al. Immunologic adverse events from immune checkpoint therapy [published online April 13, 2020]. Best Pract Res Clin Rheumatol. doi:10.1016/j.berh.2020.101511.
- Kang Z, Zhang Q, Zhang Q, et al. Clinical and pathological characteristics of extramammary Paget’s disease: report of 246 Chinese male patients. Int J Clin Exp Pathol. 2015;8:13233-13240.
- Ohara K, Fujisawa Y, Yoshino K, et al. A proposal for a TNM staging system for extramammary Paget disease: retrospective analysis of 301 patients with invasive primary tumors. J Dermatol Sci. 2016;83:234-239.
- Hatta N. Prognostic factors of extramammary Paget’s disease. Curr Treat Options Oncol. 2018;19:47.
- Yao H, Xie M, Fu S, et al. Survival analysis of patients with invasive extramammary Paget disease: implications of anatomic sites. BMC Cancer. 2018;18:403.
- Herrel LA, Weiss AD, Goodman M, et al. Extramammary Paget’s disease in males: survival outcomes in 495 patients. Ann Surg Oncol. 2015;22:1625-1630.
- Sanderson P, Innamaa A, Palmer J, et al. Imiquimod therapy for extramammary Paget’s disease of the vulva: a viable non-surgical alternative. J Obstet Gynaecol. 2013;33:479-483.
- Smith AA. Pre-Paget cells: evidence of keratinocyte origin of extramammary Paget’s disease. Intractable Rare Dis Res. 2019;8:203-205.
- Garganese G, Inzani F, Mantovani G, et al. The vulvar immunohistochemical panel (VIP) project: molecular profiles of vulvar Paget’s disease. J Cancer Res Clin Oncol. 2019;145:2211-2225.
- Dias-Santagata D, Lam Q, Bergethon K, et al. A potential role for targeted therapy in a subset of metastasizing adnexal carcinomas. Mod Pathol. 2011;24:974-982.
- Cohen JM, Granter SR, Werchniak AE. Risk stratification in extramammary Paget disease. Clin Exp Dermatol. 2015;40:473-478.
- Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018;8:1069-1086.
- Shi Y. Regulatory mechanisms of PD-L1 expression in cancer cells. Cancer Immunol Immunother. 2018;67:1481-1489.
- Cui C, Yu B, Jiang Q, et al. The roles of PD-1/PD-L1 and its signalling pathway in gastrointestinal tract cancers. Clin Exp Pharmacol Physiol. 2019;46:3-10.
- Iga N, Otsuka A, Yamamoto Y, et al. Accumulation of exhausted CD8+ T cells in extramammary Paget’s disease. PLoS One. 2019;14:E0211135.
- Frances L, Pascual JC, Leiva-Salinas M, et al. Extramammary Paget disease successfully treated with topical imiquimod 5% and tazarotene. Dermatol Ther. 2014;27:19-20.
- Lee A, Duggan S, Deeks ED. Cemiplimab: a review in advanced cutaneous squamous cell carcinoma. Drugs. 2020;80:813-819.
Resident Pearls
- Primary extramammary Paget disease (EMPD) is an adnexal carcinoma of the apocrine gland ducts, while secondary EMPD is an extension of malignant cells from an underlying internal neoplasm.
- Surgical margin clearance in EMPD often is problematic, with high recurrence rates indicating the need for additional treatment modalities.
- Programmed cell death receptor 1 (PD-1) signaling can serve as a potential target in EMPD. Further studies and clinical trials are needed to test the efficacy of PD-1 inhibitors in unresectable or invasive/metastatic EMPD.
How to not miss something
It’s a mad, mad, mad world. In California, we seem bent on swelling our curve. We’d just begun bringing our patients back into the office. We felt safe, back to business. Then air raid sirens again. Retreat to the Underground. Minimize waiting room waiting, convert to telephone and video. Do what we can to protect our patients and people.
As doctors, we’ve gotten proficient at being triage nurses, examining each appointment request, and sorting who should be seen in person and who could be cared for virtually. We do it for every clinic now.
My 11 a.m. patient last Thursday was an 83-year-old Filipino man with at least a 13-year history of hand dermatitis (based on his long electronic medical record). He had plenty of betamethasone refills. There were even photos of his large, brown hands in his chart. Grandpa hands, calloused by tending his garden and scarred from fixing bikes, building sheds, and doing oil changes for any nephew or niece who asked. The most recent uploads showed a bit of fingertip fissuring, some lichenified plaques. Not much different than they looked after planting persimmon trees a decade ago. I called him early that morning to offer a phone appointment. Perhaps I could save him from venturing out.
“I see that you have an appointment with me in a few hours. If you’d like, I might be able to help you by phone instead.” “Oh, thank you, doc,” he replied. “It’s so kind of you to call. But doc, I think maybe it is better if I come in to see you.” “Are you sure?” “Oh, yes. I will be careful.”
He checked in at 10:45. When I walked into the room he was wearing a face mask and a face shield – good job! He also had a cane and U.S. Navy Destroyer hat. And on the bottom left of his plastic shield was a sticker decal of a U.S. Navy Chief Petty Officer, dress blue insignia. His hands looked just like the photos: no purpura, plenty of lentigines. Fissures, calluses, lichenified plaques. I touched them. In the unaffected areas, his skin was remarkably soft. What stories these hands told. “I was 20 years in the Navy, doc,” he said. “I would have stayed longer but my wife, who’s younger, wanted me back home.” He talked about his nine grandchildren, some of whom went on to join the navy too – but as officers, he noted with pride. Now he spends his days caring for his wife; she has dementia. He can’t stay long because she’s in the waiting room and is likely to get confused if alone for too long.
We quickly reviewed good hand care. I ordered clobetasol ointment. He was pleased; that seemed to work years ago and he was glad to have it again.
So, why did he need to come in? Clearly I could have done this remotely. “Thank you so much for seeing me, doc,” as he stood to walk out. “Proper inspections have to be done in person, right?” Yes, I thought. Otherwise, you might miss something.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
It’s a mad, mad, mad world. In California, we seem bent on swelling our curve. We’d just begun bringing our patients back into the office. We felt safe, back to business. Then air raid sirens again. Retreat to the Underground. Minimize waiting room waiting, convert to telephone and video. Do what we can to protect our patients and people.
As doctors, we’ve gotten proficient at being triage nurses, examining each appointment request, and sorting who should be seen in person and who could be cared for virtually. We do it for every clinic now.
My 11 a.m. patient last Thursday was an 83-year-old Filipino man with at least a 13-year history of hand dermatitis (based on his long electronic medical record). He had plenty of betamethasone refills. There were even photos of his large, brown hands in his chart. Grandpa hands, calloused by tending his garden and scarred from fixing bikes, building sheds, and doing oil changes for any nephew or niece who asked. The most recent uploads showed a bit of fingertip fissuring, some lichenified plaques. Not much different than they looked after planting persimmon trees a decade ago. I called him early that morning to offer a phone appointment. Perhaps I could save him from venturing out.
“I see that you have an appointment with me in a few hours. If you’d like, I might be able to help you by phone instead.” “Oh, thank you, doc,” he replied. “It’s so kind of you to call. But doc, I think maybe it is better if I come in to see you.” “Are you sure?” “Oh, yes. I will be careful.”
He checked in at 10:45. When I walked into the room he was wearing a face mask and a face shield – good job! He also had a cane and U.S. Navy Destroyer hat. And on the bottom left of his plastic shield was a sticker decal of a U.S. Navy Chief Petty Officer, dress blue insignia. His hands looked just like the photos: no purpura, plenty of lentigines. Fissures, calluses, lichenified plaques. I touched them. In the unaffected areas, his skin was remarkably soft. What stories these hands told. “I was 20 years in the Navy, doc,” he said. “I would have stayed longer but my wife, who’s younger, wanted me back home.” He talked about his nine grandchildren, some of whom went on to join the navy too – but as officers, he noted with pride. Now he spends his days caring for his wife; she has dementia. He can’t stay long because she’s in the waiting room and is likely to get confused if alone for too long.
We quickly reviewed good hand care. I ordered clobetasol ointment. He was pleased; that seemed to work years ago and he was glad to have it again.
So, why did he need to come in? Clearly I could have done this remotely. “Thank you so much for seeing me, doc,” as he stood to walk out. “Proper inspections have to be done in person, right?” Yes, I thought. Otherwise, you might miss something.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
It’s a mad, mad, mad world. In California, we seem bent on swelling our curve. We’d just begun bringing our patients back into the office. We felt safe, back to business. Then air raid sirens again. Retreat to the Underground. Minimize waiting room waiting, convert to telephone and video. Do what we can to protect our patients and people.
As doctors, we’ve gotten proficient at being triage nurses, examining each appointment request, and sorting who should be seen in person and who could be cared for virtually. We do it for every clinic now.
My 11 a.m. patient last Thursday was an 83-year-old Filipino man with at least a 13-year history of hand dermatitis (based on his long electronic medical record). He had plenty of betamethasone refills. There were even photos of his large, brown hands in his chart. Grandpa hands, calloused by tending his garden and scarred from fixing bikes, building sheds, and doing oil changes for any nephew or niece who asked. The most recent uploads showed a bit of fingertip fissuring, some lichenified plaques. Not much different than they looked after planting persimmon trees a decade ago. I called him early that morning to offer a phone appointment. Perhaps I could save him from venturing out.
“I see that you have an appointment with me in a few hours. If you’d like, I might be able to help you by phone instead.” “Oh, thank you, doc,” he replied. “It’s so kind of you to call. But doc, I think maybe it is better if I come in to see you.” “Are you sure?” “Oh, yes. I will be careful.”
He checked in at 10:45. When I walked into the room he was wearing a face mask and a face shield – good job! He also had a cane and U.S. Navy Destroyer hat. And on the bottom left of his plastic shield was a sticker decal of a U.S. Navy Chief Petty Officer, dress blue insignia. His hands looked just like the photos: no purpura, plenty of lentigines. Fissures, calluses, lichenified plaques. I touched them. In the unaffected areas, his skin was remarkably soft. What stories these hands told. “I was 20 years in the Navy, doc,” he said. “I would have stayed longer but my wife, who’s younger, wanted me back home.” He talked about his nine grandchildren, some of whom went on to join the navy too – but as officers, he noted with pride. Now he spends his days caring for his wife; she has dementia. He can’t stay long because she’s in the waiting room and is likely to get confused if alone for too long.
We quickly reviewed good hand care. I ordered clobetasol ointment. He was pleased; that seemed to work years ago and he was glad to have it again.
So, why did he need to come in? Clearly I could have done this remotely. “Thank you so much for seeing me, doc,” as he stood to walk out. “Proper inspections have to be done in person, right?” Yes, I thought. Otherwise, you might miss something.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
Creating a student-staffed family call line to alleviate clinical burden
The coronavirus pandemic has fundamentally altered American health care. At our academic medical center in Brooklyn, a large safety net institution, clinical year medical students are normally integral members of the team consistent with the model of “value-added medical education.”1 With the suspension of clinical rotations on March 13, 2020, a key part of the workforce was suddenly withdrawn while demand skyrocketed.
In response, students self-organized into numerous remote support projects, including the project described below.
Under infection control regulations, a “no-visitor” policy was instituted. Concurrently, the dramatic increase in patient volume left clinicians unable to regularly update patients’ families. To address this gap, a family contact line was created.
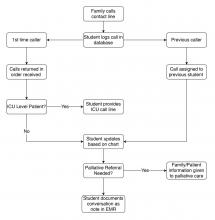
A dedicated phone number was distributed to key hospital personnel to share with families seeking information. The work flow for returning calls is shown in the figure. After verifying patient information and the caller’s relation, students provide updates based on chart review. Calls are prefaced with the disclaimer that students are not part of the treatment team and can only give information that is accessible via the electronic medical record.
Students created a phone script in conjunction with faculty, as well as a referral system for those seeking specific information from other departments. This script undergoes daily revision after the student huddle to address new issues. Flow of information is bidirectional: students relay patient updates as well as quarantine precautions and obtain past medical history. This proved essential during the surge of patients, unknown to the hospital and frequently altered, arriving by ambulance. Students document these conversations in the EMR, including family concerns and whether immediate provider follow-up is needed.
Two key limitations were quickly addressed: First, patients requiring ICU-level care have fluctuating courses, and an update based solely on chart review is insufficient. In response, students worked with intensivist teams to create a dedicated call line staffed by providers.
Second, conversations regarding goals of care and end of life concerns were beyond students’ scope. Together with palliative care teams, students developed criteria for flagging families for follow-up by a consulting palliative care attending.
Through working the call line, students received a crash course in empathetically communicating over the phone. Particularly during the worst of the surge, families were afraid and often frustrated at the lack of communication up to that point. Navigating these emotions, learning how to update family members while removed from the teams, and educating callers on quarantine precautions and other concerns was a valuable learning experience.
As students, we have been exposed to many of the realities of communicating as a physician. Relaying updates and prognosis to family while also providing emotional support is not something we are taught in medical school, but is something we will be expected to handle our first night on the wards as an intern. This experience has prepared us well for that and has illuminated missing parts of the medical school curriculum we are working on emphasizing moving forward.
Over the first 2 weeks, students put in 848 volunteer-hours, making 1,438 calls which reached 1,114 different families. We hope our experience proves instructive for other academic medical centers facing similar concerns in coming months. This model allows medical students to be directly involved in patient care during this crisis and shifts these time-intensive conversations away from overwhelmed primary medical teams.
Reference
1. Gonzalo JD et al. Value-added clinical systems learning roles for 355 medical students that transform education and health: A guide for building partnerships between 356 medical schools and health systems. Acad Med. 2017;92(5):602-7.
Ms. Jaiman is an MD candidate at State University of New York, Brooklyn and a PhD candidate at the National Center of Biological Sciences in Bangalore, India. Mr. Hessburg is an MD/PhD candidate at State University of New York, Brooklyn. Dr. Egelko is a recent graduate of State University of New York, Brooklyn.
The coronavirus pandemic has fundamentally altered American health care. At our academic medical center in Brooklyn, a large safety net institution, clinical year medical students are normally integral members of the team consistent with the model of “value-added medical education.”1 With the suspension of clinical rotations on March 13, 2020, a key part of the workforce was suddenly withdrawn while demand skyrocketed.
In response, students self-organized into numerous remote support projects, including the project described below.
Under infection control regulations, a “no-visitor” policy was instituted. Concurrently, the dramatic increase in patient volume left clinicians unable to regularly update patients’ families. To address this gap, a family contact line was created.
A dedicated phone number was distributed to key hospital personnel to share with families seeking information. The work flow for returning calls is shown in the figure. After verifying patient information and the caller’s relation, students provide updates based on chart review. Calls are prefaced with the disclaimer that students are not part of the treatment team and can only give information that is accessible via the electronic medical record.
Students created a phone script in conjunction with faculty, as well as a referral system for those seeking specific information from other departments. This script undergoes daily revision after the student huddle to address new issues. Flow of information is bidirectional: students relay patient updates as well as quarantine precautions and obtain past medical history. This proved essential during the surge of patients, unknown to the hospital and frequently altered, arriving by ambulance. Students document these conversations in the EMR, including family concerns and whether immediate provider follow-up is needed.
Two key limitations were quickly addressed: First, patients requiring ICU-level care have fluctuating courses, and an update based solely on chart review is insufficient. In response, students worked with intensivist teams to create a dedicated call line staffed by providers.
Second, conversations regarding goals of care and end of life concerns were beyond students’ scope. Together with palliative care teams, students developed criteria for flagging families for follow-up by a consulting palliative care attending.
Through working the call line, students received a crash course in empathetically communicating over the phone. Particularly during the worst of the surge, families were afraid and often frustrated at the lack of communication up to that point. Navigating these emotions, learning how to update family members while removed from the teams, and educating callers on quarantine precautions and other concerns was a valuable learning experience.
As students, we have been exposed to many of the realities of communicating as a physician. Relaying updates and prognosis to family while also providing emotional support is not something we are taught in medical school, but is something we will be expected to handle our first night on the wards as an intern. This experience has prepared us well for that and has illuminated missing parts of the medical school curriculum we are working on emphasizing moving forward.
Over the first 2 weeks, students put in 848 volunteer-hours, making 1,438 calls which reached 1,114 different families. We hope our experience proves instructive for other academic medical centers facing similar concerns in coming months. This model allows medical students to be directly involved in patient care during this crisis and shifts these time-intensive conversations away from overwhelmed primary medical teams.
Reference
1. Gonzalo JD et al. Value-added clinical systems learning roles for 355 medical students that transform education and health: A guide for building partnerships between 356 medical schools and health systems. Acad Med. 2017;92(5):602-7.
Ms. Jaiman is an MD candidate at State University of New York, Brooklyn and a PhD candidate at the National Center of Biological Sciences in Bangalore, India. Mr. Hessburg is an MD/PhD candidate at State University of New York, Brooklyn. Dr. Egelko is a recent graduate of State University of New York, Brooklyn.
The coronavirus pandemic has fundamentally altered American health care. At our academic medical center in Brooklyn, a large safety net institution, clinical year medical students are normally integral members of the team consistent with the model of “value-added medical education.”1 With the suspension of clinical rotations on March 13, 2020, a key part of the workforce was suddenly withdrawn while demand skyrocketed.
In response, students self-organized into numerous remote support projects, including the project described below.
Under infection control regulations, a “no-visitor” policy was instituted. Concurrently, the dramatic increase in patient volume left clinicians unable to regularly update patients’ families. To address this gap, a family contact line was created.
A dedicated phone number was distributed to key hospital personnel to share with families seeking information. The work flow for returning calls is shown in the figure. After verifying patient information and the caller’s relation, students provide updates based on chart review. Calls are prefaced with the disclaimer that students are not part of the treatment team and can only give information that is accessible via the electronic medical record.
Students created a phone script in conjunction with faculty, as well as a referral system for those seeking specific information from other departments. This script undergoes daily revision after the student huddle to address new issues. Flow of information is bidirectional: students relay patient updates as well as quarantine precautions and obtain past medical history. This proved essential during the surge of patients, unknown to the hospital and frequently altered, arriving by ambulance. Students document these conversations in the EMR, including family concerns and whether immediate provider follow-up is needed.
Two key limitations were quickly addressed: First, patients requiring ICU-level care have fluctuating courses, and an update based solely on chart review is insufficient. In response, students worked with intensivist teams to create a dedicated call line staffed by providers.
Second, conversations regarding goals of care and end of life concerns were beyond students’ scope. Together with palliative care teams, students developed criteria for flagging families for follow-up by a consulting palliative care attending.
Through working the call line, students received a crash course in empathetically communicating over the phone. Particularly during the worst of the surge, families were afraid and often frustrated at the lack of communication up to that point. Navigating these emotions, learning how to update family members while removed from the teams, and educating callers on quarantine precautions and other concerns was a valuable learning experience.
As students, we have been exposed to many of the realities of communicating as a physician. Relaying updates and prognosis to family while also providing emotional support is not something we are taught in medical school, but is something we will be expected to handle our first night on the wards as an intern. This experience has prepared us well for that and has illuminated missing parts of the medical school curriculum we are working on emphasizing moving forward.
Over the first 2 weeks, students put in 848 volunteer-hours, making 1,438 calls which reached 1,114 different families. We hope our experience proves instructive for other academic medical centers facing similar concerns in coming months. This model allows medical students to be directly involved in patient care during this crisis and shifts these time-intensive conversations away from overwhelmed primary medical teams.
Reference
1. Gonzalo JD et al. Value-added clinical systems learning roles for 355 medical students that transform education and health: A guide for building partnerships between 356 medical schools and health systems. Acad Med. 2017;92(5):602-7.
Ms. Jaiman is an MD candidate at State University of New York, Brooklyn and a PhD candidate at the National Center of Biological Sciences in Bangalore, India. Mr. Hessburg is an MD/PhD candidate at State University of New York, Brooklyn. Dr. Egelko is a recent graduate of State University of New York, Brooklyn.
AGA News
Rep. Suzan DelBene (D-Wash.) leads prior authorization reform
As a member of the powerful Ways and Means Committee, which has jurisdiction over the Medicare program, Rep. DelBene has worked closely with the American Gastroenterological Association.
When Rep. DelBene was first elected to Congress in 2012, we met with her to share AGA’s policy priorities. We knew instantly that we had a voice for many of our issues. Rep. DelBene started her career as a young investigator before continuing her education and launching a career in the biotechnology industry. From her firsthand experience, she understands the need for investments in National Institutes of Health research and for access to and coverage of colorectal cancer screenings since a member of her family had the disease.
Since Rep. DelBene has been in office, she has taken the lead on several policy priorities affecting our profession, including patient access and protections and regulatory relief. Rep. DelBene is the lead Democratic sponsor of H.R. 3107, the Improving Seniors’ Timely Access to Care Act, legislation that would streamline prior authorization in Medicare Advantage plans. The legislation hit a milestone of securing 218 cosponsors in the House, which is a majority of the members. We look forward to continuing to work with Rep. DelBene on advancing AGA’s policy priorities.
Featured microbiome investigator: Josephine Ni, MD
We’re checking in with a rising star in microbiome research: Dr. Josephine Ni from the University of Pennsylvania, Philadelphia.
Dr. Ni is an instructor of medicine at the University of Pennsylvania, and 2017 recipient of the AGA–Takeda Pharmaceuticals Research Scholar Award in IBD from the AGA Research Foundation.
Congrats to Dr. Ni! While Dr. Ni’s AGA Research Scholar Award concludes at the end of June 2020, we’re proud to share that she has secured two significant grants to continue her work: an NIH KO8 grant and a Burroughs Welcome Fund Award. We catch up with Dr. Ni in the Q&A below.
How would you sum up your research in one sentence?
I am interested in better understanding bacterial colonization of the healthy and inflamed intestinal tract; specifically, my current research focuses on characterizing the role of biofilm formation on intestinal colonization.
What effect do you hope your research will have on patients?
I hope that my work on understanding intestinal colonization will allow us to engineer the microbiota in predictable ways, which will pave the way to exclude enteropathogens, deliver specific compounds, and prevent dysbiosis.
What inspired you to focus your research career on the gut microbiome?
Being able to use data and observations from patient cohorts to generate research hypotheses and then translate those hypotheses into mouse models to explore mechanisms has been a very gratifying experience that I learned from my mentor, Gary Wu, MD. There is still so much to learn about the effects of the microbiome on intestinal health and I’m excited to be a part of this process.
What recent publication from your lab best represents your work if anyone wants to learn more?
Ni J et al. A role for bacterial urease in gut dysbiosis and Crohn’s disease. Sci Transl Med. 2017 Nov 15;9(416):eaah6888.
Gastroenterology invites submissions for an issue focused on colorectal cancer
Share your innovative basic and clinical research for consideration.
The past decade has seen significant milestones in our understanding of the epidemiology, clinical and genetic risk factors, and underlying biological mechanisms of colorectal cancer. This progress has also emphasized the need for further advances. To this end, Gastroenterology will publish a thematic issue in honor of Colorectal Cancer (CRC) Awareness Month in March 2021. The aim is to cover research highlighting novel pathways with human correlates, discoveries related to clinical interventions, clinical trials, and high-profile epidemiologic studies.
Help drive progress of CRC understanding and care by contributing your work. Enhanced promotion of the full issue and automatic indexing of your article to PubMed will increase the visibility of your research in the scientific community and beyond.
Submit your research through Gastroenterology‘s streamlined submission system: www.editorialmanager.com/gastro by Sept. 30, 2020. Original articles and brief communications are welcome.
For more information, please contact Gastroenterology’s Managing Editor, Christopher Lowe, at [email protected].
AGA journals select editorial fellows for 2020-2021 academic year
The AGA journals Gastroenterology, Clinical Gastroenterology and Hepatology (CGH), and Cellular and Molecular Gastroenterology and Hepatology (CMGH) recently selected the recipients of their editorial fellowships, which runs from July 2020 through June 2021. The editorial fellowship program is in its fourth year.
The editorial fellows for each journal are:
Gastroenterology
Ruben Colman, MD
Cincinnati Children’s Hospital Medical Center
John Gubatan, MD
Stanford (Calif.) University Medical Center
CGH
Blake Jones, MD
University of Colorado at Denver, Aurora
Nikhil Thiruvengadam, MD
University of California, San Francisco
CMGH
Samuel Hinman, PhD
University of Washington, Seattle
The editorial fellows will be mentored on the journals’ editorial processes, including peer review and the publication process from manuscript submission to acceptance. They will participate in discussions and conferences with the boards of editors and work closely with the AGA editorial staff. Additionally, the fellows will participate in AGA’s new reviewer education program and will also be offered the opportunity to contribute content to their respective journals.
The journals’ board of editors and editorial staff congratulate the fellows and are excited to work with them over the next year.
AGA welcomes new president, M. Bishr Omary, MD, PhD, AGAF
M. Bishr Omary, MD, PhD, AGAF, will begin his term as the 115th president of the AGA Institute on June 1, 2020.
Dr. Omary, an international leader in GI biology and physiology, currently serves as senior vice chancellor for academic affairs and research for Rutgers Biomedical and Health Sciences schools, centers, and institutes at Rutgers University, Newark, N.J.
Eldest of three siblings, Dr. Omary was born and raised to Syrian parents in New York. After his father obtained his MS degree in political science from Columbia University in New York, the family returned to Damascus, Syria, where his father worked in the Ministry of Urban Planning. The family emigrated to the United States in 1968.
“I am eternally grateful to my parents from whom I learned the meaning of hard work and unconditional love. The opportunities in the U.S. open so many doors, compared with many other countries, including Syria then and especially now given the ongoing 9-year civil war that has ravaged the country,” shared Dr. Omary.
When asked about how he will approach his presidency during a global COVID-19 pandemic, Dr. Omary expressed his commitment to urgently working with and for patients, as well as our community of gastroenterologists, researchers, trainees, and other AGA members, to overcome the disruptions created by the pandemic and ultimately be in a better place than we were before. Dr. Omary holds steadfast to AGA’s vision, a world free from digestive diseases.
Dr. Omary’s primary focus, as an internationally recognized biomedical investigator, is understanding the mechanism and developing therapies for several diseases including lipodystrophies, acute liver failure, and porphyrias. He served as chief of gastroenterology and hepatology at Stanford University, then chair of physiology and chief scientific officer while at the University of Michigan, Ann Arbor, before moving to Rutgers.
Dr. Omary has been a long-time AGA leader, most notably chairing the AGA Institute Research Awards Panel and serving as senior associate editor (2006-2011) then editor in chief (2011-2016) of Gastroenterology, AGA’s premier journal.
Dr. Omary has been on the AGA Governing Board for 2 years as vice president then president-elect; his term as AGA president concludes May 2021.
Rep. Suzan DelBene (D-Wash.) leads prior authorization reform
As a member of the powerful Ways and Means Committee, which has jurisdiction over the Medicare program, Rep. DelBene has worked closely with the American Gastroenterological Association.
When Rep. DelBene was first elected to Congress in 2012, we met with her to share AGA’s policy priorities. We knew instantly that we had a voice for many of our issues. Rep. DelBene started her career as a young investigator before continuing her education and launching a career in the biotechnology industry. From her firsthand experience, she understands the need for investments in National Institutes of Health research and for access to and coverage of colorectal cancer screenings since a member of her family had the disease.
Since Rep. DelBene has been in office, she has taken the lead on several policy priorities affecting our profession, including patient access and protections and regulatory relief. Rep. DelBene is the lead Democratic sponsor of H.R. 3107, the Improving Seniors’ Timely Access to Care Act, legislation that would streamline prior authorization in Medicare Advantage plans. The legislation hit a milestone of securing 218 cosponsors in the House, which is a majority of the members. We look forward to continuing to work with Rep. DelBene on advancing AGA’s policy priorities.
Featured microbiome investigator: Josephine Ni, MD
We’re checking in with a rising star in microbiome research: Dr. Josephine Ni from the University of Pennsylvania, Philadelphia.
Dr. Ni is an instructor of medicine at the University of Pennsylvania, and 2017 recipient of the AGA–Takeda Pharmaceuticals Research Scholar Award in IBD from the AGA Research Foundation.
Congrats to Dr. Ni! While Dr. Ni’s AGA Research Scholar Award concludes at the end of June 2020, we’re proud to share that she has secured two significant grants to continue her work: an NIH KO8 grant and a Burroughs Welcome Fund Award. We catch up with Dr. Ni in the Q&A below.
How would you sum up your research in one sentence?
I am interested in better understanding bacterial colonization of the healthy and inflamed intestinal tract; specifically, my current research focuses on characterizing the role of biofilm formation on intestinal colonization.
What effect do you hope your research will have on patients?
I hope that my work on understanding intestinal colonization will allow us to engineer the microbiota in predictable ways, which will pave the way to exclude enteropathogens, deliver specific compounds, and prevent dysbiosis.
What inspired you to focus your research career on the gut microbiome?
Being able to use data and observations from patient cohorts to generate research hypotheses and then translate those hypotheses into mouse models to explore mechanisms has been a very gratifying experience that I learned from my mentor, Gary Wu, MD. There is still so much to learn about the effects of the microbiome on intestinal health and I’m excited to be a part of this process.
What recent publication from your lab best represents your work if anyone wants to learn more?
Ni J et al. A role for bacterial urease in gut dysbiosis and Crohn’s disease. Sci Transl Med. 2017 Nov 15;9(416):eaah6888.
Gastroenterology invites submissions for an issue focused on colorectal cancer
Share your innovative basic and clinical research for consideration.
The past decade has seen significant milestones in our understanding of the epidemiology, clinical and genetic risk factors, and underlying biological mechanisms of colorectal cancer. This progress has also emphasized the need for further advances. To this end, Gastroenterology will publish a thematic issue in honor of Colorectal Cancer (CRC) Awareness Month in March 2021. The aim is to cover research highlighting novel pathways with human correlates, discoveries related to clinical interventions, clinical trials, and high-profile epidemiologic studies.
Help drive progress of CRC understanding and care by contributing your work. Enhanced promotion of the full issue and automatic indexing of your article to PubMed will increase the visibility of your research in the scientific community and beyond.
Submit your research through Gastroenterology‘s streamlined submission system: www.editorialmanager.com/gastro by Sept. 30, 2020. Original articles and brief communications are welcome.
For more information, please contact Gastroenterology’s Managing Editor, Christopher Lowe, at [email protected].
AGA journals select editorial fellows for 2020-2021 academic year
The AGA journals Gastroenterology, Clinical Gastroenterology and Hepatology (CGH), and Cellular and Molecular Gastroenterology and Hepatology (CMGH) recently selected the recipients of their editorial fellowships, which runs from July 2020 through June 2021. The editorial fellowship program is in its fourth year.
The editorial fellows for each journal are:
Gastroenterology
Ruben Colman, MD
Cincinnati Children’s Hospital Medical Center
John Gubatan, MD
Stanford (Calif.) University Medical Center
CGH
Blake Jones, MD
University of Colorado at Denver, Aurora
Nikhil Thiruvengadam, MD
University of California, San Francisco
CMGH
Samuel Hinman, PhD
University of Washington, Seattle
The editorial fellows will be mentored on the journals’ editorial processes, including peer review and the publication process from manuscript submission to acceptance. They will participate in discussions and conferences with the boards of editors and work closely with the AGA editorial staff. Additionally, the fellows will participate in AGA’s new reviewer education program and will also be offered the opportunity to contribute content to their respective journals.
The journals’ board of editors and editorial staff congratulate the fellows and are excited to work with them over the next year.
AGA welcomes new president, M. Bishr Omary, MD, PhD, AGAF
M. Bishr Omary, MD, PhD, AGAF, will begin his term as the 115th president of the AGA Institute on June 1, 2020.
Dr. Omary, an international leader in GI biology and physiology, currently serves as senior vice chancellor for academic affairs and research for Rutgers Biomedical and Health Sciences schools, centers, and institutes at Rutgers University, Newark, N.J.
Eldest of three siblings, Dr. Omary was born and raised to Syrian parents in New York. After his father obtained his MS degree in political science from Columbia University in New York, the family returned to Damascus, Syria, where his father worked in the Ministry of Urban Planning. The family emigrated to the United States in 1968.
“I am eternally grateful to my parents from whom I learned the meaning of hard work and unconditional love. The opportunities in the U.S. open so many doors, compared with many other countries, including Syria then and especially now given the ongoing 9-year civil war that has ravaged the country,” shared Dr. Omary.
When asked about how he will approach his presidency during a global COVID-19 pandemic, Dr. Omary expressed his commitment to urgently working with and for patients, as well as our community of gastroenterologists, researchers, trainees, and other AGA members, to overcome the disruptions created by the pandemic and ultimately be in a better place than we were before. Dr. Omary holds steadfast to AGA’s vision, a world free from digestive diseases.
Dr. Omary’s primary focus, as an internationally recognized biomedical investigator, is understanding the mechanism and developing therapies for several diseases including lipodystrophies, acute liver failure, and porphyrias. He served as chief of gastroenterology and hepatology at Stanford University, then chair of physiology and chief scientific officer while at the University of Michigan, Ann Arbor, before moving to Rutgers.
Dr. Omary has been a long-time AGA leader, most notably chairing the AGA Institute Research Awards Panel and serving as senior associate editor (2006-2011) then editor in chief (2011-2016) of Gastroenterology, AGA’s premier journal.
Dr. Omary has been on the AGA Governing Board for 2 years as vice president then president-elect; his term as AGA president concludes May 2021.
Rep. Suzan DelBene (D-Wash.) leads prior authorization reform
As a member of the powerful Ways and Means Committee, which has jurisdiction over the Medicare program, Rep. DelBene has worked closely with the American Gastroenterological Association.
When Rep. DelBene was first elected to Congress in 2012, we met with her to share AGA’s policy priorities. We knew instantly that we had a voice for many of our issues. Rep. DelBene started her career as a young investigator before continuing her education and launching a career in the biotechnology industry. From her firsthand experience, she understands the need for investments in National Institutes of Health research and for access to and coverage of colorectal cancer screenings since a member of her family had the disease.
Since Rep. DelBene has been in office, she has taken the lead on several policy priorities affecting our profession, including patient access and protections and regulatory relief. Rep. DelBene is the lead Democratic sponsor of H.R. 3107, the Improving Seniors’ Timely Access to Care Act, legislation that would streamline prior authorization in Medicare Advantage plans. The legislation hit a milestone of securing 218 cosponsors in the House, which is a majority of the members. We look forward to continuing to work with Rep. DelBene on advancing AGA’s policy priorities.
Featured microbiome investigator: Josephine Ni, MD
We’re checking in with a rising star in microbiome research: Dr. Josephine Ni from the University of Pennsylvania, Philadelphia.
Dr. Ni is an instructor of medicine at the University of Pennsylvania, and 2017 recipient of the AGA–Takeda Pharmaceuticals Research Scholar Award in IBD from the AGA Research Foundation.
Congrats to Dr. Ni! While Dr. Ni’s AGA Research Scholar Award concludes at the end of June 2020, we’re proud to share that she has secured two significant grants to continue her work: an NIH KO8 grant and a Burroughs Welcome Fund Award. We catch up with Dr. Ni in the Q&A below.
How would you sum up your research in one sentence?
I am interested in better understanding bacterial colonization of the healthy and inflamed intestinal tract; specifically, my current research focuses on characterizing the role of biofilm formation on intestinal colonization.
What effect do you hope your research will have on patients?
I hope that my work on understanding intestinal colonization will allow us to engineer the microbiota in predictable ways, which will pave the way to exclude enteropathogens, deliver specific compounds, and prevent dysbiosis.
What inspired you to focus your research career on the gut microbiome?
Being able to use data and observations from patient cohorts to generate research hypotheses and then translate those hypotheses into mouse models to explore mechanisms has been a very gratifying experience that I learned from my mentor, Gary Wu, MD. There is still so much to learn about the effects of the microbiome on intestinal health and I’m excited to be a part of this process.
What recent publication from your lab best represents your work if anyone wants to learn more?
Ni J et al. A role for bacterial urease in gut dysbiosis and Crohn’s disease. Sci Transl Med. 2017 Nov 15;9(416):eaah6888.
Gastroenterology invites submissions for an issue focused on colorectal cancer
Share your innovative basic and clinical research for consideration.
The past decade has seen significant milestones in our understanding of the epidemiology, clinical and genetic risk factors, and underlying biological mechanisms of colorectal cancer. This progress has also emphasized the need for further advances. To this end, Gastroenterology will publish a thematic issue in honor of Colorectal Cancer (CRC) Awareness Month in March 2021. The aim is to cover research highlighting novel pathways with human correlates, discoveries related to clinical interventions, clinical trials, and high-profile epidemiologic studies.
Help drive progress of CRC understanding and care by contributing your work. Enhanced promotion of the full issue and automatic indexing of your article to PubMed will increase the visibility of your research in the scientific community and beyond.
Submit your research through Gastroenterology‘s streamlined submission system: www.editorialmanager.com/gastro by Sept. 30, 2020. Original articles and brief communications are welcome.
For more information, please contact Gastroenterology’s Managing Editor, Christopher Lowe, at [email protected].
AGA journals select editorial fellows for 2020-2021 academic year
The AGA journals Gastroenterology, Clinical Gastroenterology and Hepatology (CGH), and Cellular and Molecular Gastroenterology and Hepatology (CMGH) recently selected the recipients of their editorial fellowships, which runs from July 2020 through June 2021. The editorial fellowship program is in its fourth year.
The editorial fellows for each journal are:
Gastroenterology
Ruben Colman, MD
Cincinnati Children’s Hospital Medical Center
John Gubatan, MD
Stanford (Calif.) University Medical Center
CGH
Blake Jones, MD
University of Colorado at Denver, Aurora
Nikhil Thiruvengadam, MD
University of California, San Francisco
CMGH
Samuel Hinman, PhD
University of Washington, Seattle
The editorial fellows will be mentored on the journals’ editorial processes, including peer review and the publication process from manuscript submission to acceptance. They will participate in discussions and conferences with the boards of editors and work closely with the AGA editorial staff. Additionally, the fellows will participate in AGA’s new reviewer education program and will also be offered the opportunity to contribute content to their respective journals.
The journals’ board of editors and editorial staff congratulate the fellows and are excited to work with them over the next year.
AGA welcomes new president, M. Bishr Omary, MD, PhD, AGAF
M. Bishr Omary, MD, PhD, AGAF, will begin his term as the 115th president of the AGA Institute on June 1, 2020.
Dr. Omary, an international leader in GI biology and physiology, currently serves as senior vice chancellor for academic affairs and research for Rutgers Biomedical and Health Sciences schools, centers, and institutes at Rutgers University, Newark, N.J.
Eldest of three siblings, Dr. Omary was born and raised to Syrian parents in New York. After his father obtained his MS degree in political science from Columbia University in New York, the family returned to Damascus, Syria, where his father worked in the Ministry of Urban Planning. The family emigrated to the United States in 1968.
“I am eternally grateful to my parents from whom I learned the meaning of hard work and unconditional love. The opportunities in the U.S. open so many doors, compared with many other countries, including Syria then and especially now given the ongoing 9-year civil war that has ravaged the country,” shared Dr. Omary.
When asked about how he will approach his presidency during a global COVID-19 pandemic, Dr. Omary expressed his commitment to urgently working with and for patients, as well as our community of gastroenterologists, researchers, trainees, and other AGA members, to overcome the disruptions created by the pandemic and ultimately be in a better place than we were before. Dr. Omary holds steadfast to AGA’s vision, a world free from digestive diseases.
Dr. Omary’s primary focus, as an internationally recognized biomedical investigator, is understanding the mechanism and developing therapies for several diseases including lipodystrophies, acute liver failure, and porphyrias. He served as chief of gastroenterology and hepatology at Stanford University, then chair of physiology and chief scientific officer while at the University of Michigan, Ann Arbor, before moving to Rutgers.
Dr. Omary has been a long-time AGA leader, most notably chairing the AGA Institute Research Awards Panel and serving as senior associate editor (2006-2011) then editor in chief (2011-2016) of Gastroenterology, AGA’s premier journal.
Dr. Omary has been on the AGA Governing Board for 2 years as vice president then president-elect; his term as AGA president concludes May 2021.
About one-third of older Americans receive shingles vaccine
The number of Americans aged 60 years and older who report receiving shingles vaccination had risen steadily since 2008 and has leveled off during the past few years, new data from the Centers for Disease Control and Prevention’s (CDC’s) National Center for Health Statistics reveal.
The proportion of people in this age group who were vaccinated rose from 6.7% in 2008 to 34.5% in 2018, for example.
Emily Terlizzi, MPH, told Medscape Medical News.
The report was published online July 9 in NCHS Data Brief.
Similar rates for men and women
Rates of people who reported receiving at least one vaccination with Zostavax (Merck) or Shingrix (GlaxoSmithKline) varied by factors that included Hispanic origin, education, and family income. An unexpected finding was that rates did not vary significantly between men and women.
“One finding that I would say surprised me was that, although the percentage who had ever received a shingles vaccine among women aged 60 and over was higher than that among men in this age group, this difference was not statistically significant,” said Ms. Terlizzi, a health statistician in the Data Analysis and Quality Assurance Branch, Division of Health Interview Statistics, the CDC National Center for Health Statistics. In 2018, for example, 35.4% of women and 33.5% of men reported ever receiving a shingles vaccine.
The similarity of rates was less of a surprise to Len Horovitz, MD, a pulmonary specialist at Lenox Hill Hospital in New York, who was not affiliated with the report. “In my anecdotal experience, I don’t see a preponderance of one sex getting shingles more than another. It’s pretty evenly distributed,” he said in an interview.
Ms. Terlizzi and coauthor Lindsey I. Black, MPH, say their findings align with prior research. However, they noted: “Our report uses more recent data from a large, nationally representative data source to update these estimates and describe these disparities.” Data come from results of the annual National Health Interview Survey of households nationwide.
Multiple factors explain vaccination differences
Non-Hispanic White adults were more likely to report receiving the vaccine than were Hispanic and non-Hispanic Black survey respondents. Non-Hispanic White adults were about twice as likely to report vaccination – 38.6% – compared with 19.5% of Hispanic adults and 18.8% of non-Hispanic Black adults.
The disparity in vaccination by race was “disappointing news,” Kenneth E. Schmader, MD, said in an interview.
“The health disparity with regard to lower vaccination rates in Hispanic and non-Hispanic Black populations is reported with other vaccines as well and points to the need for better efforts to vaccinate Hispanic and non-Hispanic Black populations,” added Dr. Schmader, a professor of medicine at Duke University in Durham, N.C.
On a positive note, “It was good to see increasing use of shingles vaccination over time, given how devastating zoster can be in older adults and the fact that the vaccines are effective,” said Dr. Schmader, who also serves on the working groups for the Herpes Zoster, Influenza and General Adult Immunization Guidelines for the CDC Advisory Committee on Immunization Practices (ACIP).
Self-reports of receiving vaccination increased in association with higher education and family income levels. For example, 39.9% of respondents who had more than a high school diploma or GED (General Educational Development) reported receiving the shingles vaccine. In contrast, only 21.2% of people with lower educational attainment reported receiving a vaccine.
In terms of income, 20.4% of poor adults reported being vaccinated, compared with 38.4% of adults who were not poor.
The investigators also evaluated the data by geographic region. They found that rates of vaccinations varied from 26.3% in the East South Central part of the United States (which includes Tennessee, Kentucky, and Alabama) to 42.8% in the West North Central region (which includes the Dakotas, Minnesota, and Nebraska).
Clinical and research considerations
For most of the decade evaluated in the study, ACIP recommended vaccination against shingles for Americans aged 60 years and older. The current findings, therefore, do not account for ACIP’s expanding its recommendations in 2017 to include adults aged 50 years and older.
Zostavax is expected to be discontinued this year. It was the only shingles vaccine available before the approval of Shingrix in 2018. The shift to a single product could alter vaccination patterns further.
Ms. Terlizzi plans to continue monitoring trends to “see what changes occur in the next few years,” she said.
Compliance a concern
Data on vaccination rates for shingles are important given the large proportion of the population at risk, Dr. Horovitz said. “People over age 50 who have had chickenpox have a one third chance over their lifetimes to get shingles. That is a lot of people.”
Multiple factors could be contributing to the fact that vaccination rates have hovered around 34% in recent years, he said. “Whenever you see variations in vaccination rates, you have to think about cultural differences and questions about differences in access, accessibility, and attitudes. Attitudes toward vaccines vary widely – from people who don’t believe in vaccination to people who are eager to take vaccinations.
“I don’t know how to dissect all that out of these data,” he added.
Compliance with recommendations also contributes to vaccination rates, Dr. Horovitz said. The fact that in about 10% of people, a flulike syndrome develops the day after being vaccinated with Shingrix can cause some to postpone or rethink immunization, he added. In addition, Shingrix requires two shots. “People have to come back, and that always sets up an issue with recalling someone.”
Marketplace shortages of the Shingrix vaccine could also contribute to lower vaccination rates. However, Dr. Horovitz said that, in his practice, availability was only a problem during the first year after approval in 2017.
On a related note, manufacturer GlaxoSmithKline announced that a decrease in vaccination demand during the COVID-19 pandemic has allowed the supply to catch up. Shingrix no longer qualifies for the CDC’s shortages list, according to a July 9 report.
Ms. Terlizzi, Dr. Horovitz, and Dr. Schmader have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The number of Americans aged 60 years and older who report receiving shingles vaccination had risen steadily since 2008 and has leveled off during the past few years, new data from the Centers for Disease Control and Prevention’s (CDC’s) National Center for Health Statistics reveal.
The proportion of people in this age group who were vaccinated rose from 6.7% in 2008 to 34.5% in 2018, for example.
Emily Terlizzi, MPH, told Medscape Medical News.
The report was published online July 9 in NCHS Data Brief.
Similar rates for men and women
Rates of people who reported receiving at least one vaccination with Zostavax (Merck) or Shingrix (GlaxoSmithKline) varied by factors that included Hispanic origin, education, and family income. An unexpected finding was that rates did not vary significantly between men and women.
“One finding that I would say surprised me was that, although the percentage who had ever received a shingles vaccine among women aged 60 and over was higher than that among men in this age group, this difference was not statistically significant,” said Ms. Terlizzi, a health statistician in the Data Analysis and Quality Assurance Branch, Division of Health Interview Statistics, the CDC National Center for Health Statistics. In 2018, for example, 35.4% of women and 33.5% of men reported ever receiving a shingles vaccine.
The similarity of rates was less of a surprise to Len Horovitz, MD, a pulmonary specialist at Lenox Hill Hospital in New York, who was not affiliated with the report. “In my anecdotal experience, I don’t see a preponderance of one sex getting shingles more than another. It’s pretty evenly distributed,” he said in an interview.
Ms. Terlizzi and coauthor Lindsey I. Black, MPH, say their findings align with prior research. However, they noted: “Our report uses more recent data from a large, nationally representative data source to update these estimates and describe these disparities.” Data come from results of the annual National Health Interview Survey of households nationwide.
Multiple factors explain vaccination differences
Non-Hispanic White adults were more likely to report receiving the vaccine than were Hispanic and non-Hispanic Black survey respondents. Non-Hispanic White adults were about twice as likely to report vaccination – 38.6% – compared with 19.5% of Hispanic adults and 18.8% of non-Hispanic Black adults.
The disparity in vaccination by race was “disappointing news,” Kenneth E. Schmader, MD, said in an interview.
“The health disparity with regard to lower vaccination rates in Hispanic and non-Hispanic Black populations is reported with other vaccines as well and points to the need for better efforts to vaccinate Hispanic and non-Hispanic Black populations,” added Dr. Schmader, a professor of medicine at Duke University in Durham, N.C.
On a positive note, “It was good to see increasing use of shingles vaccination over time, given how devastating zoster can be in older adults and the fact that the vaccines are effective,” said Dr. Schmader, who also serves on the working groups for the Herpes Zoster, Influenza and General Adult Immunization Guidelines for the CDC Advisory Committee on Immunization Practices (ACIP).
Self-reports of receiving vaccination increased in association with higher education and family income levels. For example, 39.9% of respondents who had more than a high school diploma or GED (General Educational Development) reported receiving the shingles vaccine. In contrast, only 21.2% of people with lower educational attainment reported receiving a vaccine.
In terms of income, 20.4% of poor adults reported being vaccinated, compared with 38.4% of adults who were not poor.
The investigators also evaluated the data by geographic region. They found that rates of vaccinations varied from 26.3% in the East South Central part of the United States (which includes Tennessee, Kentucky, and Alabama) to 42.8% in the West North Central region (which includes the Dakotas, Minnesota, and Nebraska).
Clinical and research considerations
For most of the decade evaluated in the study, ACIP recommended vaccination against shingles for Americans aged 60 years and older. The current findings, therefore, do not account for ACIP’s expanding its recommendations in 2017 to include adults aged 50 years and older.
Zostavax is expected to be discontinued this year. It was the only shingles vaccine available before the approval of Shingrix in 2018. The shift to a single product could alter vaccination patterns further.
Ms. Terlizzi plans to continue monitoring trends to “see what changes occur in the next few years,” she said.
Compliance a concern
Data on vaccination rates for shingles are important given the large proportion of the population at risk, Dr. Horovitz said. “People over age 50 who have had chickenpox have a one third chance over their lifetimes to get shingles. That is a lot of people.”
Multiple factors could be contributing to the fact that vaccination rates have hovered around 34% in recent years, he said. “Whenever you see variations in vaccination rates, you have to think about cultural differences and questions about differences in access, accessibility, and attitudes. Attitudes toward vaccines vary widely – from people who don’t believe in vaccination to people who are eager to take vaccinations.
“I don’t know how to dissect all that out of these data,” he added.
Compliance with recommendations also contributes to vaccination rates, Dr. Horovitz said. The fact that in about 10% of people, a flulike syndrome develops the day after being vaccinated with Shingrix can cause some to postpone or rethink immunization, he added. In addition, Shingrix requires two shots. “People have to come back, and that always sets up an issue with recalling someone.”
Marketplace shortages of the Shingrix vaccine could also contribute to lower vaccination rates. However, Dr. Horovitz said that, in his practice, availability was only a problem during the first year after approval in 2017.
On a related note, manufacturer GlaxoSmithKline announced that a decrease in vaccination demand during the COVID-19 pandemic has allowed the supply to catch up. Shingrix no longer qualifies for the CDC’s shortages list, according to a July 9 report.
Ms. Terlizzi, Dr. Horovitz, and Dr. Schmader have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The number of Americans aged 60 years and older who report receiving shingles vaccination had risen steadily since 2008 and has leveled off during the past few years, new data from the Centers for Disease Control and Prevention’s (CDC’s) National Center for Health Statistics reveal.
The proportion of people in this age group who were vaccinated rose from 6.7% in 2008 to 34.5% in 2018, for example.
Emily Terlizzi, MPH, told Medscape Medical News.
The report was published online July 9 in NCHS Data Brief.
Similar rates for men and women
Rates of people who reported receiving at least one vaccination with Zostavax (Merck) or Shingrix (GlaxoSmithKline) varied by factors that included Hispanic origin, education, and family income. An unexpected finding was that rates did not vary significantly between men and women.
“One finding that I would say surprised me was that, although the percentage who had ever received a shingles vaccine among women aged 60 and over was higher than that among men in this age group, this difference was not statistically significant,” said Ms. Terlizzi, a health statistician in the Data Analysis and Quality Assurance Branch, Division of Health Interview Statistics, the CDC National Center for Health Statistics. In 2018, for example, 35.4% of women and 33.5% of men reported ever receiving a shingles vaccine.
The similarity of rates was less of a surprise to Len Horovitz, MD, a pulmonary specialist at Lenox Hill Hospital in New York, who was not affiliated with the report. “In my anecdotal experience, I don’t see a preponderance of one sex getting shingles more than another. It’s pretty evenly distributed,” he said in an interview.
Ms. Terlizzi and coauthor Lindsey I. Black, MPH, say their findings align with prior research. However, they noted: “Our report uses more recent data from a large, nationally representative data source to update these estimates and describe these disparities.” Data come from results of the annual National Health Interview Survey of households nationwide.
Multiple factors explain vaccination differences
Non-Hispanic White adults were more likely to report receiving the vaccine than were Hispanic and non-Hispanic Black survey respondents. Non-Hispanic White adults were about twice as likely to report vaccination – 38.6% – compared with 19.5% of Hispanic adults and 18.8% of non-Hispanic Black adults.
The disparity in vaccination by race was “disappointing news,” Kenneth E. Schmader, MD, said in an interview.
“The health disparity with regard to lower vaccination rates in Hispanic and non-Hispanic Black populations is reported with other vaccines as well and points to the need for better efforts to vaccinate Hispanic and non-Hispanic Black populations,” added Dr. Schmader, a professor of medicine at Duke University in Durham, N.C.
On a positive note, “It was good to see increasing use of shingles vaccination over time, given how devastating zoster can be in older adults and the fact that the vaccines are effective,” said Dr. Schmader, who also serves on the working groups for the Herpes Zoster, Influenza and General Adult Immunization Guidelines for the CDC Advisory Committee on Immunization Practices (ACIP).
Self-reports of receiving vaccination increased in association with higher education and family income levels. For example, 39.9% of respondents who had more than a high school diploma or GED (General Educational Development) reported receiving the shingles vaccine. In contrast, only 21.2% of people with lower educational attainment reported receiving a vaccine.
In terms of income, 20.4% of poor adults reported being vaccinated, compared with 38.4% of adults who were not poor.
The investigators also evaluated the data by geographic region. They found that rates of vaccinations varied from 26.3% in the East South Central part of the United States (which includes Tennessee, Kentucky, and Alabama) to 42.8% in the West North Central region (which includes the Dakotas, Minnesota, and Nebraska).
Clinical and research considerations
For most of the decade evaluated in the study, ACIP recommended vaccination against shingles for Americans aged 60 years and older. The current findings, therefore, do not account for ACIP’s expanding its recommendations in 2017 to include adults aged 50 years and older.
Zostavax is expected to be discontinued this year. It was the only shingles vaccine available before the approval of Shingrix in 2018. The shift to a single product could alter vaccination patterns further.
Ms. Terlizzi plans to continue monitoring trends to “see what changes occur in the next few years,” she said.
Compliance a concern
Data on vaccination rates for shingles are important given the large proportion of the population at risk, Dr. Horovitz said. “People over age 50 who have had chickenpox have a one third chance over their lifetimes to get shingles. That is a lot of people.”
Multiple factors could be contributing to the fact that vaccination rates have hovered around 34% in recent years, he said. “Whenever you see variations in vaccination rates, you have to think about cultural differences and questions about differences in access, accessibility, and attitudes. Attitudes toward vaccines vary widely – from people who don’t believe in vaccination to people who are eager to take vaccinations.
“I don’t know how to dissect all that out of these data,” he added.
Compliance with recommendations also contributes to vaccination rates, Dr. Horovitz said. The fact that in about 10% of people, a flulike syndrome develops the day after being vaccinated with Shingrix can cause some to postpone or rethink immunization, he added. In addition, Shingrix requires two shots. “People have to come back, and that always sets up an issue with recalling someone.”
Marketplace shortages of the Shingrix vaccine could also contribute to lower vaccination rates. However, Dr. Horovitz said that, in his practice, availability was only a problem during the first year after approval in 2017.
On a related note, manufacturer GlaxoSmithKline announced that a decrease in vaccination demand during the COVID-19 pandemic has allowed the supply to catch up. Shingrix no longer qualifies for the CDC’s shortages list, according to a July 9 report.
Ms. Terlizzi, Dr. Horovitz, and Dr. Schmader have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Calculations of an academic hospitalist
The term “academic hospitalist” has come to mean more than a mere affiliation to an academic medical center (AMC). Academic hospitalists perform various clinical roles like staffing house staff teams, covering nonteaching services, critical care services, procedure teams, night services, medical consultation, and comanagement services.
Over the last decade, academic hospitalists have successfully managed many nonclinical roles in areas like research, medical unit leadership, faculty development, faculty affairs, quality, safety, informatics, utilization review, clinical documentation, throughput, group management, hospital administration, and educational leadership. The role of an academic hospital is as clear as a chocolate martini these days. Here we present some recent trends in academic hospital medicine.
Compensation
From SHM’s State of Hospital Medicine report (SoHM)2014 to 2018 data, the median compensation for U.S. academic hospitalists has risen by an average of 5.15% every year, although increases vary by rank.1 From 2016 to 2018, clinical instructors saw the most significant growth, 11.23% per year, suggesting a need to remain competitive for junior hospitalists. Compensation also varies by geographic area, with the Southern region reporting the highest compensation. Over the last decade, academic hospitalists received, on average, a 28%-35% lower salary, compared with community hospitalists.
Patient population and census
Lower patient encounters and compensation of the academic hospitalists poses the chicken or the egg dilemma. In the 2018 SoHM report, academic hospitalists had an average of 17% fewer encounters. Of note, AMC patients tend to have higher complexity, as measured by the Case Mix Index (CMI – the average diagnosis-related group weight of a hospital).2 A higher CMI is a surrogate marker for the diagnostic diversity, clinical complexity, and resource needs of the patient population in the hospital.
Productivity and financial metrics
The financial bottom line is a critical aspect, and as a report in the Journal of Hospital Medicine described, all health care executives look at business metrics while making decisions.3 Below are some significant academic and community comparisons from SoHM 2018.
- Collections, encounters, and wRVUs (work relative value units) were highly correlated. All of them were lower for academic hospitalists, corroborating the fact that they see a smaller number of patients. Clinical full-time equivalents (cFTE) is a vernacular of how much of the faculty time is devoted to clinical activities. The academic data from SoHM achieves the same target, as it is standardized to 100% billable clinical activity, so the fact that many academic hospitalists do not work a full-time clinical schedule is not a factor in their lower production.
- Charges had a smaller gap likely because of sicker patients in AMCs. The higher acuity difference can also explain 12% higher wRVU/encounter for academic hospitalists.
- The wRVU/encounter ratio can indicate a few patterns: high acuity of patients in AMCs, higher levels of evaluation and management documentation, or both. As the encounters and charges have the same percentage differences, we would place our bets on the former.
- Compensation per encounter and compensation per wRVU showed that academic hospitalists do get a slight advantage.
CMI and wRVUs
Although the SoHM does not capture information on patient acuity or CMI, we speculate that the relationship between CMI and wRVUs may be more or less linear at lower levels of acuity. However, once level III E/M billing is achieved (assuming there is no critical care provided), wRVUs/encounter plateau, even as acuity continues to increase. This plateau effect may be seen more often in high-acuity AMC settings than in community hospitals.
So, in our opinion, compensation models based solely on wRVU production would not do justice for hospitalists in AMC settings since these models would fail to capture the extra work involved with very-high-acuity patients. SoHM 2018 shows the financial support per wRVU for AMC is $45.81, and for the community is $41.28, an 11% difference. We think the higher financial support per wRVU for academic practices may be related to the lost wRVU potential of caring for very-high-acuity patients.
Conclusion
In an academic setting, hospitalists are reforming the field of hospital medicine and defining the ways we could deliver care. They are the pillars of collaboration, education, research, innovation, quality, and safety. It would be increasingly crucial for academic hospitalist leaders to use comparative metrics from SoHM to advocate for their group. The bottom line can be explained by the title of the qualitative study in JHM referenced above: “Collaboration, not calculation.”3
Dr. Chadha is division chief for the division of hospital medicine at the University of Kentucky Healthcare, Lexington. He actively leads efforts of recruiting, scheduling, practice analysis, and operation of the group. He is a first-time member of the practice analysis committee. Ms. Dede is division administrator for the division of hospital medicine at the University of Kentucky Healthcare. She prepares and manages budgets, liaisons with the downstream revenue teams, and contributes to the building of academic compensation models. She is serving in the practice administrators committee for the second year and is currently vice chair of the Executive Council for the Practice Administrators special interest group.
References
1. State of Hospital Medicine Report. https://www.hospitalmedicine.org/practice-management/shms-state-of-hospital-medicine/
2. Deloitte Center for Health Solutions. Academic Medical Centers: Joining forces with community providers for broad benefits and positive outcomes. 2015. https://www2.deloitte.com/us/en/pages/life-sciences-and-health-care/articles/academic-medical-centers-consolidation.html
3. White AA et al. Collaboration, not calculation: A qualitative study of how hospital executives value hospital medicine groups. J Hosp Med. 2019;14(10):662‐7.
The term “academic hospitalist” has come to mean more than a mere affiliation to an academic medical center (AMC). Academic hospitalists perform various clinical roles like staffing house staff teams, covering nonteaching services, critical care services, procedure teams, night services, medical consultation, and comanagement services.
Over the last decade, academic hospitalists have successfully managed many nonclinical roles in areas like research, medical unit leadership, faculty development, faculty affairs, quality, safety, informatics, utilization review, clinical documentation, throughput, group management, hospital administration, and educational leadership. The role of an academic hospital is as clear as a chocolate martini these days. Here we present some recent trends in academic hospital medicine.
Compensation
From SHM’s State of Hospital Medicine report (SoHM)2014 to 2018 data, the median compensation for U.S. academic hospitalists has risen by an average of 5.15% every year, although increases vary by rank.1 From 2016 to 2018, clinical instructors saw the most significant growth, 11.23% per year, suggesting a need to remain competitive for junior hospitalists. Compensation also varies by geographic area, with the Southern region reporting the highest compensation. Over the last decade, academic hospitalists received, on average, a 28%-35% lower salary, compared with community hospitalists.
Patient population and census
Lower patient encounters and compensation of the academic hospitalists poses the chicken or the egg dilemma. In the 2018 SoHM report, academic hospitalists had an average of 17% fewer encounters. Of note, AMC patients tend to have higher complexity, as measured by the Case Mix Index (CMI – the average diagnosis-related group weight of a hospital).2 A higher CMI is a surrogate marker for the diagnostic diversity, clinical complexity, and resource needs of the patient population in the hospital.
Productivity and financial metrics
The financial bottom line is a critical aspect, and as a report in the Journal of Hospital Medicine described, all health care executives look at business metrics while making decisions.3 Below are some significant academic and community comparisons from SoHM 2018.
- Collections, encounters, and wRVUs (work relative value units) were highly correlated. All of them were lower for academic hospitalists, corroborating the fact that they see a smaller number of patients. Clinical full-time equivalents (cFTE) is a vernacular of how much of the faculty time is devoted to clinical activities. The academic data from SoHM achieves the same target, as it is standardized to 100% billable clinical activity, so the fact that many academic hospitalists do not work a full-time clinical schedule is not a factor in their lower production.
- Charges had a smaller gap likely because of sicker patients in AMCs. The higher acuity difference can also explain 12% higher wRVU/encounter for academic hospitalists.
- The wRVU/encounter ratio can indicate a few patterns: high acuity of patients in AMCs, higher levels of evaluation and management documentation, or both. As the encounters and charges have the same percentage differences, we would place our bets on the former.
- Compensation per encounter and compensation per wRVU showed that academic hospitalists do get a slight advantage.
CMI and wRVUs
Although the SoHM does not capture information on patient acuity or CMI, we speculate that the relationship between CMI and wRVUs may be more or less linear at lower levels of acuity. However, once level III E/M billing is achieved (assuming there is no critical care provided), wRVUs/encounter plateau, even as acuity continues to increase. This plateau effect may be seen more often in high-acuity AMC settings than in community hospitals.
So, in our opinion, compensation models based solely on wRVU production would not do justice for hospitalists in AMC settings since these models would fail to capture the extra work involved with very-high-acuity patients. SoHM 2018 shows the financial support per wRVU for AMC is $45.81, and for the community is $41.28, an 11% difference. We think the higher financial support per wRVU for academic practices may be related to the lost wRVU potential of caring for very-high-acuity patients.
Conclusion
In an academic setting, hospitalists are reforming the field of hospital medicine and defining the ways we could deliver care. They are the pillars of collaboration, education, research, innovation, quality, and safety. It would be increasingly crucial for academic hospitalist leaders to use comparative metrics from SoHM to advocate for their group. The bottom line can be explained by the title of the qualitative study in JHM referenced above: “Collaboration, not calculation.”3
Dr. Chadha is division chief for the division of hospital medicine at the University of Kentucky Healthcare, Lexington. He actively leads efforts of recruiting, scheduling, practice analysis, and operation of the group. He is a first-time member of the practice analysis committee. Ms. Dede is division administrator for the division of hospital medicine at the University of Kentucky Healthcare. She prepares and manages budgets, liaisons with the downstream revenue teams, and contributes to the building of academic compensation models. She is serving in the practice administrators committee for the second year and is currently vice chair of the Executive Council for the Practice Administrators special interest group.
References
1. State of Hospital Medicine Report. https://www.hospitalmedicine.org/practice-management/shms-state-of-hospital-medicine/
2. Deloitte Center for Health Solutions. Academic Medical Centers: Joining forces with community providers for broad benefits and positive outcomes. 2015. https://www2.deloitte.com/us/en/pages/life-sciences-and-health-care/articles/academic-medical-centers-consolidation.html
3. White AA et al. Collaboration, not calculation: A qualitative study of how hospital executives value hospital medicine groups. J Hosp Med. 2019;14(10):662‐7.
The term “academic hospitalist” has come to mean more than a mere affiliation to an academic medical center (AMC). Academic hospitalists perform various clinical roles like staffing house staff teams, covering nonteaching services, critical care services, procedure teams, night services, medical consultation, and comanagement services.
Over the last decade, academic hospitalists have successfully managed many nonclinical roles in areas like research, medical unit leadership, faculty development, faculty affairs, quality, safety, informatics, utilization review, clinical documentation, throughput, group management, hospital administration, and educational leadership. The role of an academic hospital is as clear as a chocolate martini these days. Here we present some recent trends in academic hospital medicine.
Compensation
From SHM’s State of Hospital Medicine report (SoHM)2014 to 2018 data, the median compensation for U.S. academic hospitalists has risen by an average of 5.15% every year, although increases vary by rank.1 From 2016 to 2018, clinical instructors saw the most significant growth, 11.23% per year, suggesting a need to remain competitive for junior hospitalists. Compensation also varies by geographic area, with the Southern region reporting the highest compensation. Over the last decade, academic hospitalists received, on average, a 28%-35% lower salary, compared with community hospitalists.
Patient population and census
Lower patient encounters and compensation of the academic hospitalists poses the chicken or the egg dilemma. In the 2018 SoHM report, academic hospitalists had an average of 17% fewer encounters. Of note, AMC patients tend to have higher complexity, as measured by the Case Mix Index (CMI – the average diagnosis-related group weight of a hospital).2 A higher CMI is a surrogate marker for the diagnostic diversity, clinical complexity, and resource needs of the patient population in the hospital.
Productivity and financial metrics
The financial bottom line is a critical aspect, and as a report in the Journal of Hospital Medicine described, all health care executives look at business metrics while making decisions.3 Below are some significant academic and community comparisons from SoHM 2018.
- Collections, encounters, and wRVUs (work relative value units) were highly correlated. All of them were lower for academic hospitalists, corroborating the fact that they see a smaller number of patients. Clinical full-time equivalents (cFTE) is a vernacular of how much of the faculty time is devoted to clinical activities. The academic data from SoHM achieves the same target, as it is standardized to 100% billable clinical activity, so the fact that many academic hospitalists do not work a full-time clinical schedule is not a factor in their lower production.
- Charges had a smaller gap likely because of sicker patients in AMCs. The higher acuity difference can also explain 12% higher wRVU/encounter for academic hospitalists.
- The wRVU/encounter ratio can indicate a few patterns: high acuity of patients in AMCs, higher levels of evaluation and management documentation, or both. As the encounters and charges have the same percentage differences, we would place our bets on the former.
- Compensation per encounter and compensation per wRVU showed that academic hospitalists do get a slight advantage.
CMI and wRVUs
Although the SoHM does not capture information on patient acuity or CMI, we speculate that the relationship between CMI and wRVUs may be more or less linear at lower levels of acuity. However, once level III E/M billing is achieved (assuming there is no critical care provided), wRVUs/encounter plateau, even as acuity continues to increase. This plateau effect may be seen more often in high-acuity AMC settings than in community hospitals.
So, in our opinion, compensation models based solely on wRVU production would not do justice for hospitalists in AMC settings since these models would fail to capture the extra work involved with very-high-acuity patients. SoHM 2018 shows the financial support per wRVU for AMC is $45.81, and for the community is $41.28, an 11% difference. We think the higher financial support per wRVU for academic practices may be related to the lost wRVU potential of caring for very-high-acuity patients.
Conclusion
In an academic setting, hospitalists are reforming the field of hospital medicine and defining the ways we could deliver care. They are the pillars of collaboration, education, research, innovation, quality, and safety. It would be increasingly crucial for academic hospitalist leaders to use comparative metrics from SoHM to advocate for their group. The bottom line can be explained by the title of the qualitative study in JHM referenced above: “Collaboration, not calculation.”3
Dr. Chadha is division chief for the division of hospital medicine at the University of Kentucky Healthcare, Lexington. He actively leads efforts of recruiting, scheduling, practice analysis, and operation of the group. He is a first-time member of the practice analysis committee. Ms. Dede is division administrator for the division of hospital medicine at the University of Kentucky Healthcare. She prepares and manages budgets, liaisons with the downstream revenue teams, and contributes to the building of academic compensation models. She is serving in the practice administrators committee for the second year and is currently vice chair of the Executive Council for the Practice Administrators special interest group.
References
1. State of Hospital Medicine Report. https://www.hospitalmedicine.org/practice-management/shms-state-of-hospital-medicine/
2. Deloitte Center for Health Solutions. Academic Medical Centers: Joining forces with community providers for broad benefits and positive outcomes. 2015. https://www2.deloitte.com/us/en/pages/life-sciences-and-health-care/articles/academic-medical-centers-consolidation.html
3. White AA et al. Collaboration, not calculation: A qualitative study of how hospital executives value hospital medicine groups. J Hosp Med. 2019;14(10):662‐7.
Calendar
For more information about upcoming events and award deadlines, please visit http://agau.gastro.org and http://www.gastro.org/research-funding.
UPCOMING EVENTS
Aug. 13-14, Sept. 16-17, and Oct. 7-8, 2020
2-Day, In-Depth Coding and Billing Seminar
Become a certified GI coder with a 2-day, in-depth training course provided by McVey Associates.
Baltimore, Md. (Aug. 13-14); Atlanta, Ga. (Sept. 16-17); Las Vegas, Nev. (Oct. 7-8)
Aug. 15-16, 2020
2020 Principles of GI for the NP and PA
Because of COVID-19, the American Gastroenterological Association has transitioned the 2020 Principles of GI for the NP and PA course from a live meeting to a virtual course. The virtual course will provide you with team-based expert guidance on managing GI patients through case-based learning from faculty who are seasoned physicians and advanced practice providers. Register at https://bit.ly/38oeK4C.
AWARD DEADLINES
AGA-Pilot Research Award
This award provides $30,000 for 1 year to recipients at any career stage researching new directions in gastroenterology- or hepatology-related areas.
Application deadline: Sept. 2, 2020
AGA-Medtronic Pilot Research Award in Technology Innovation
This award provides $30,000 for 1 year to independent investigators at any career stage to support the research and development of novel devices or technologies that will potentially impact the diagnosis or treatment of digestive disease.
Application deadline: Sept. 2, 2020
AGA–Takeda Pharmaceuticals Research Scholar Award in Celiac Disease
This award provides $100,000 per year for 3 years (totaling $300,000) to early-career faculty (i.e., investigator, instructor, research associate, or equivalent) working toward an independent career in celiac disease research.
Application deadline: Nov. 9, 2020
AGA Research Scholar Award (RSA)
This award provides $100,000 per year for 3 years (totaling $300,000) to early-career faculty (i.e., investigator, instructor, research associate, or equivalent) working toward an independent career in digestive disease research.
Application deadline: Nov. 9, 2020
AGA–Takeda Pharmaceuticals Research Scholar Award in Inflammatory Bowel Disease
This award provides $100,000 per year for 3 years (totaling $300,000) to early-career faculty (i.e., investigator, instructor, research associate, or equivalent) working toward an independent career in inflammatory bowel disease (IBD) research.
Application deadline: Nov. 9, 2020
AGA–Moti L. & Kamla Rustgi International Travel Awards
This $750 travel award provides support to early career (i.e., 35 years or younger at the time of Digestive Disease Week® [DDW]) basic, translational, or clinical investigators residing outside North America to offset travel and related expenses to attend DDW.
Application deadline: Feb. 24, 2021
AGA Student Abstract Award
This $500 travel award supports recipients who are graduate students, medical students, or medical residents (residents up to postgraduate year 3) giving abstract-based oral or poster presentations at Digestive Disease Week® (DDW). The top-scoring abstract will be designated the Student Abstract of the Year and receive a $1,000 award.
Application deadline: Feb 26, 2021
AGA Fellow Abstract Award
This $500 travel award supports recipients who are MD, PhD, or equivalent fellows giving abstract-based oral or poster presentations at Digestive Disease Week® (DDW). The top-scoring abstract will be designated the Fellow Abstract of the Year and receive a $1,000 award.
Application deadline: Feb. 24, 2021
For more information about upcoming events and award deadlines, please visit http://agau.gastro.org and http://www.gastro.org/research-funding.
UPCOMING EVENTS
Aug. 13-14, Sept. 16-17, and Oct. 7-8, 2020
2-Day, In-Depth Coding and Billing Seminar
Become a certified GI coder with a 2-day, in-depth training course provided by McVey Associates.
Baltimore, Md. (Aug. 13-14); Atlanta, Ga. (Sept. 16-17); Las Vegas, Nev. (Oct. 7-8)
Aug. 15-16, 2020
2020 Principles of GI for the NP and PA
Because of COVID-19, the American Gastroenterological Association has transitioned the 2020 Principles of GI for the NP and PA course from a live meeting to a virtual course. The virtual course will provide you with team-based expert guidance on managing GI patients through case-based learning from faculty who are seasoned physicians and advanced practice providers. Register at https://bit.ly/38oeK4C.
AWARD DEADLINES
AGA-Pilot Research Award
This award provides $30,000 for 1 year to recipients at any career stage researching new directions in gastroenterology- or hepatology-related areas.
Application deadline: Sept. 2, 2020
AGA-Medtronic Pilot Research Award in Technology Innovation
This award provides $30,000 for 1 year to independent investigators at any career stage to support the research and development of novel devices or technologies that will potentially impact the diagnosis or treatment of digestive disease.
Application deadline: Sept. 2, 2020
AGA–Takeda Pharmaceuticals Research Scholar Award in Celiac Disease
This award provides $100,000 per year for 3 years (totaling $300,000) to early-career faculty (i.e., investigator, instructor, research associate, or equivalent) working toward an independent career in celiac disease research.
Application deadline: Nov. 9, 2020
AGA Research Scholar Award (RSA)
This award provides $100,000 per year for 3 years (totaling $300,000) to early-career faculty (i.e., investigator, instructor, research associate, or equivalent) working toward an independent career in digestive disease research.
Application deadline: Nov. 9, 2020
AGA–Takeda Pharmaceuticals Research Scholar Award in Inflammatory Bowel Disease
This award provides $100,000 per year for 3 years (totaling $300,000) to early-career faculty (i.e., investigator, instructor, research associate, or equivalent) working toward an independent career in inflammatory bowel disease (IBD) research.
Application deadline: Nov. 9, 2020
AGA–Moti L. & Kamla Rustgi International Travel Awards
This $750 travel award provides support to early career (i.e., 35 years or younger at the time of Digestive Disease Week® [DDW]) basic, translational, or clinical investigators residing outside North America to offset travel and related expenses to attend DDW.
Application deadline: Feb. 24, 2021
AGA Student Abstract Award
This $500 travel award supports recipients who are graduate students, medical students, or medical residents (residents up to postgraduate year 3) giving abstract-based oral or poster presentations at Digestive Disease Week® (DDW). The top-scoring abstract will be designated the Student Abstract of the Year and receive a $1,000 award.
Application deadline: Feb 26, 2021
AGA Fellow Abstract Award
This $500 travel award supports recipients who are MD, PhD, or equivalent fellows giving abstract-based oral or poster presentations at Digestive Disease Week® (DDW). The top-scoring abstract will be designated the Fellow Abstract of the Year and receive a $1,000 award.
Application deadline: Feb. 24, 2021
For more information about upcoming events and award deadlines, please visit http://agau.gastro.org and http://www.gastro.org/research-funding.
UPCOMING EVENTS
Aug. 13-14, Sept. 16-17, and Oct. 7-8, 2020
2-Day, In-Depth Coding and Billing Seminar
Become a certified GI coder with a 2-day, in-depth training course provided by McVey Associates.
Baltimore, Md. (Aug. 13-14); Atlanta, Ga. (Sept. 16-17); Las Vegas, Nev. (Oct. 7-8)
Aug. 15-16, 2020
2020 Principles of GI for the NP and PA
Because of COVID-19, the American Gastroenterological Association has transitioned the 2020 Principles of GI for the NP and PA course from a live meeting to a virtual course. The virtual course will provide you with team-based expert guidance on managing GI patients through case-based learning from faculty who are seasoned physicians and advanced practice providers. Register at https://bit.ly/38oeK4C.
AWARD DEADLINES
AGA-Pilot Research Award
This award provides $30,000 for 1 year to recipients at any career stage researching new directions in gastroenterology- or hepatology-related areas.
Application deadline: Sept. 2, 2020
AGA-Medtronic Pilot Research Award in Technology Innovation
This award provides $30,000 for 1 year to independent investigators at any career stage to support the research and development of novel devices or technologies that will potentially impact the diagnosis or treatment of digestive disease.
Application deadline: Sept. 2, 2020
AGA–Takeda Pharmaceuticals Research Scholar Award in Celiac Disease
This award provides $100,000 per year for 3 years (totaling $300,000) to early-career faculty (i.e., investigator, instructor, research associate, or equivalent) working toward an independent career in celiac disease research.
Application deadline: Nov. 9, 2020
AGA Research Scholar Award (RSA)
This award provides $100,000 per year for 3 years (totaling $300,000) to early-career faculty (i.e., investigator, instructor, research associate, or equivalent) working toward an independent career in digestive disease research.
Application deadline: Nov. 9, 2020
AGA–Takeda Pharmaceuticals Research Scholar Award in Inflammatory Bowel Disease
This award provides $100,000 per year for 3 years (totaling $300,000) to early-career faculty (i.e., investigator, instructor, research associate, or equivalent) working toward an independent career in inflammatory bowel disease (IBD) research.
Application deadline: Nov. 9, 2020
AGA–Moti L. & Kamla Rustgi International Travel Awards
This $750 travel award provides support to early career (i.e., 35 years or younger at the time of Digestive Disease Week® [DDW]) basic, translational, or clinical investigators residing outside North America to offset travel and related expenses to attend DDW.
Application deadline: Feb. 24, 2021
AGA Student Abstract Award
This $500 travel award supports recipients who are graduate students, medical students, or medical residents (residents up to postgraduate year 3) giving abstract-based oral or poster presentations at Digestive Disease Week® (DDW). The top-scoring abstract will be designated the Student Abstract of the Year and receive a $1,000 award.
Application deadline: Feb 26, 2021
AGA Fellow Abstract Award
This $500 travel award supports recipients who are MD, PhD, or equivalent fellows giving abstract-based oral or poster presentations at Digestive Disease Week® (DDW). The top-scoring abstract will be designated the Fellow Abstract of the Year and receive a $1,000 award.
Application deadline: Feb. 24, 2021
A 72-year-old with an acute, pruritic, bullous eruption involving his right pretibial extremity
Localized bullous pemphigoid
with a predilection in the elderly population.1
Localized variants of bullous pemphigoid (BP) are rare and have been reported to arise at sites of mechanical trauma, prior radiation, lymphedema, surgical scars, burns, fistulas, and ostomies.1-3 Although the mechanism remains unclear, the Koebner phenomenon is thought to induce dysregulation of immunologic and vascular factors in sites of mechanical shear and trauma in susceptible individuals.3
Localized BP is an important entity for the dermatologist to be familiar with, as the diagnosis is often delayed. The localized, well-defined skin lesions frequently mimic contact dermatitis. In fact, previous reports have shown the most likely misdiagnosis of localized BP is acute allergic contact dermatitis, stasis dermatitis, and eczematous dermatitis.4,5
In this patient, histopathologic examination of a biopsy revealed a subepidermal blister with numerous eosinophils. Direct immunofluorescence study of perilesional skin showed strong linear IgG and C3 deposits at the basal membrane level. Serum level of autoantibody to BP180 antigen was elevated. Bacterial culture was positive for Staphylococcus aureus. These findings were suggestive of unilateral, localized BP with superimposed bacterial infection. Initial treatment with an extended course of doxycycline 200 mg twice daily, topical triamcinolone 0.1% ointment twice daily with compression therapy, and leg elevation led to clinical improvement with healing of previous lesions on the leg. At follow-up 3 weeks later, the patient had continued to develop new bullous lesions on the trunk and upper thighs. He was subsequently started on systemic immunosuppressive therapy for generalized bullous pemphigoid.
Importantly, localized BP generally follows a more benign disease course, although long-term follow-up is recommended for monitoring given the potential risk of developing the generalized form of BP of approximately 15%.3 Topical corticosteroids and oral antibiotics are recommended as the first-line therapy in these patients, with an escalated systemic therapy if needed for disease progression.3,5
Our case represents an important differential diagnosis to consider when evaluating an acute localized bullous eruption in an elderly patient.
Dr. Cusick and Dr. Dolohanty are with the department of dermatology, University of Rochester (N.Y.), and provided the case and photo. Donna Bilu Martin, MD, edited the column.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Kohroh K et al. J Dermatol. 2007 Jul;34(7):482-5.
2. Nguyen T et al. Dermatology 2014;229(2):88-96.
3. Sen BB et al. Indian J Dermatol Venereol Leprol. 2013;79(4):554.
4. Salomon RJ et al. Arch Dermatol. 1987 Mar;123(3):389-92.
5. Tran JT, Mutasim DF. Int J Dermatol. 2005 Nov;44(11):942-5.
Localized bullous pemphigoid
with a predilection in the elderly population.1
Localized variants of bullous pemphigoid (BP) are rare and have been reported to arise at sites of mechanical trauma, prior radiation, lymphedema, surgical scars, burns, fistulas, and ostomies.1-3 Although the mechanism remains unclear, the Koebner phenomenon is thought to induce dysregulation of immunologic and vascular factors in sites of mechanical shear and trauma in susceptible individuals.3
Localized BP is an important entity for the dermatologist to be familiar with, as the diagnosis is often delayed. The localized, well-defined skin lesions frequently mimic contact dermatitis. In fact, previous reports have shown the most likely misdiagnosis of localized BP is acute allergic contact dermatitis, stasis dermatitis, and eczematous dermatitis.4,5
In this patient, histopathologic examination of a biopsy revealed a subepidermal blister with numerous eosinophils. Direct immunofluorescence study of perilesional skin showed strong linear IgG and C3 deposits at the basal membrane level. Serum level of autoantibody to BP180 antigen was elevated. Bacterial culture was positive for Staphylococcus aureus. These findings were suggestive of unilateral, localized BP with superimposed bacterial infection. Initial treatment with an extended course of doxycycline 200 mg twice daily, topical triamcinolone 0.1% ointment twice daily with compression therapy, and leg elevation led to clinical improvement with healing of previous lesions on the leg. At follow-up 3 weeks later, the patient had continued to develop new bullous lesions on the trunk and upper thighs. He was subsequently started on systemic immunosuppressive therapy for generalized bullous pemphigoid.
Importantly, localized BP generally follows a more benign disease course, although long-term follow-up is recommended for monitoring given the potential risk of developing the generalized form of BP of approximately 15%.3 Topical corticosteroids and oral antibiotics are recommended as the first-line therapy in these patients, with an escalated systemic therapy if needed for disease progression.3,5
Our case represents an important differential diagnosis to consider when evaluating an acute localized bullous eruption in an elderly patient.
Dr. Cusick and Dr. Dolohanty are with the department of dermatology, University of Rochester (N.Y.), and provided the case and photo. Donna Bilu Martin, MD, edited the column.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Kohroh K et al. J Dermatol. 2007 Jul;34(7):482-5.
2. Nguyen T et al. Dermatology 2014;229(2):88-96.
3. Sen BB et al. Indian J Dermatol Venereol Leprol. 2013;79(4):554.
4. Salomon RJ et al. Arch Dermatol. 1987 Mar;123(3):389-92.
5. Tran JT, Mutasim DF. Int J Dermatol. 2005 Nov;44(11):942-5.
Localized bullous pemphigoid
with a predilection in the elderly population.1
Localized variants of bullous pemphigoid (BP) are rare and have been reported to arise at sites of mechanical trauma, prior radiation, lymphedema, surgical scars, burns, fistulas, and ostomies.1-3 Although the mechanism remains unclear, the Koebner phenomenon is thought to induce dysregulation of immunologic and vascular factors in sites of mechanical shear and trauma in susceptible individuals.3
Localized BP is an important entity for the dermatologist to be familiar with, as the diagnosis is often delayed. The localized, well-defined skin lesions frequently mimic contact dermatitis. In fact, previous reports have shown the most likely misdiagnosis of localized BP is acute allergic contact dermatitis, stasis dermatitis, and eczematous dermatitis.4,5
In this patient, histopathologic examination of a biopsy revealed a subepidermal blister with numerous eosinophils. Direct immunofluorescence study of perilesional skin showed strong linear IgG and C3 deposits at the basal membrane level. Serum level of autoantibody to BP180 antigen was elevated. Bacterial culture was positive for Staphylococcus aureus. These findings were suggestive of unilateral, localized BP with superimposed bacterial infection. Initial treatment with an extended course of doxycycline 200 mg twice daily, topical triamcinolone 0.1% ointment twice daily with compression therapy, and leg elevation led to clinical improvement with healing of previous lesions on the leg. At follow-up 3 weeks later, the patient had continued to develop new bullous lesions on the trunk and upper thighs. He was subsequently started on systemic immunosuppressive therapy for generalized bullous pemphigoid.
Importantly, localized BP generally follows a more benign disease course, although long-term follow-up is recommended for monitoring given the potential risk of developing the generalized form of BP of approximately 15%.3 Topical corticosteroids and oral antibiotics are recommended as the first-line therapy in these patients, with an escalated systemic therapy if needed for disease progression.3,5
Our case represents an important differential diagnosis to consider when evaluating an acute localized bullous eruption in an elderly patient.
Dr. Cusick and Dr. Dolohanty are with the department of dermatology, University of Rochester (N.Y.), and provided the case and photo. Donna Bilu Martin, MD, edited the column.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Kohroh K et al. J Dermatol. 2007 Jul;34(7):482-5.
2. Nguyen T et al. Dermatology 2014;229(2):88-96.
3. Sen BB et al. Indian J Dermatol Venereol Leprol. 2013;79(4):554.
4. Salomon RJ et al. Arch Dermatol. 1987 Mar;123(3):389-92.
5. Tran JT, Mutasim DF. Int J Dermatol. 2005 Nov;44(11):942-5.
How to Obtain a Dermatology Residency: A Guide Targeted to Underrepresented in Medicine Medical Students
There has been increasing attention focused on the lack of diversity within dermatology academic and residency programs.1-6 Several factors have been identified as contributing to this narrow pipeline of qualified applicants, including lack of mentorship, delayed exposure to the field, implicit bias, and lack of an overall holistic review of applications with overemphasis on board scores.1,5 In an effort to provide guidance to underrepresented in medicine (UIM) students who are interested in dermatology, the Skin of Color Society (SOCS) has created a detailed, step-by-step guide on how to obtain a position in a dermatology residency program,7 which was modeled after a similar resource created by the American Academy of Orthopaedic Surgeons.8 Here, we highlight the main SOCS recommendations to help guide medical students through a systematic approach to becoming successful applicants for dermatology residency.
Start Early
Competitive fields such as dermatology require intentional efforts starting at the beginning of medical school. Regardless of what specialty is right for you, begin by constructing a well-rounded application for residency immediately. Start by shadowing dermatologists and attending Grand Rounds held in your institution’s dermatology department to ensure that this field is right for you. Students are encouraged to meet with academic advisors and upperclassmen to seek guidance on gaining early exposure to dermatology at their home institutions (or nearby programs) during their first year. As a platform for learning about community-based dermatology activities, join your school’s Dermatology Interest Group, keeping in mind that an executive position in such a group can help foster relationships with faculty and residents of the dermatology department. A long-term commitment to community service also contributes to your depth as an applicant. Getting involved early helps students uncover health disparities in medicine and allows time to formulate ideas to implement change. Forming a well-rounded application mandates maintaining good academic standing, and students should prioritize mastering the curriculum, excelling in clinical rotations, and studying for the US Medical Licensing Examination (USMLE).
Choose a Mentor
The summer between your first and second years of medical school is an opportune time to explore research opportunities. Students successfully complete research by taking ownership of a project, efficiently meeting deadlines, maintaining contact with research mentors by quickly responding to emails, and producing quality work. Research outside of dermatology also is valued. Research mentors often provide future letters of recommendation, so commit to doing an outstanding job. For those finding it difficult to locate a mentor, consider searching the American Academy of Dermatology (AAD)(https://www.aad.org/mentorship/) or SOCS (https://skinofcolorsociety.org/) websites. The AAD has an established Diversity Mentorship Program (https://www.aad.org/member/career/volunteer/diversity-mentorship) that provides members with direct guidance from dermatologists for 4 weeks. Students use this time to conduct research, learn more about the specialty, and foster a relationship with their mentor. Students can apply any year of medical school; however, the typical awardee usually is a third-year or fourth-year student. The AAD may provide a stipend to help offset expenses.
Prepare for Boards
Second year is a continuation of the agenda set forth in first year, now with the focus shifting toward board preparation and excelling in clinical core didactics and rotations. According to data from the 2018 National Resident Matching Program,9 the mean USMLE Step 1 score for US allopathic senior medical students who matched into dermatology was 249 compared to 241 who did not match into dermatology. However, the mean score is just that—a mean—and people have matched with lower scores. Do not be intimidated by this number; instead, be driven to commit the time and resources to master the content and do your personal best on the USMLE Step examinations. Given the shift in some programs for earlier clinical exposure and postponement of boards until the third year, the recommendations in this timeline can be catered to fit a medical student’s specific situation.
Build Your Application
The third year of medical school is a busy year. Prepare for third-year clinical rotations by speaking with upperclassmen and clinical preceptors as you progress through your rotations. Evaluations and recommendations are weighed heavily by residency program directors, as this information is used to ascertain your clinical abilities. Seek feedback from your preceptors early and often with a sincere attempt to integrate suggested improvements. Schedule a dermatology rotation at your home institution after completing the core rotations. Although they are not required, applicants may complete away rotations early in their fourth year; the application period for visiting student learning opportunities typically opens April 1 of the third year, if not earlier. Free resources are available to help prepare for your dermatology rotations. Start by reviewing the Basic Dermatology Curriculum on the AAD website (https://www.aad.org/member/education/residents/bdc). Make contributions to
Interviewing for Residency
During your fourth year of medical school, you will be completing dermatology rotations, submitting your applications through the Electronic Residency Application Service, and interviewing with residency programs. When deciding which programs to apply to, consider referencing the American Medical Association Residency and Fellowship Database (https://freida.ama-assn.org/Freida/#/). Also keep in mind that, depending on your competitiveness, you should expect to receive 1 interview for every 10 programs you apply to, thus the application process can be quite costly. It is highly encouraged that you ask for letters of recommendation prior to August 15 and that you submit your applications by September 15. Complete mock interviews with a mentor and research commonly asked questions. Prior to your interview day, you want to spend time researching the program, browsing faculty publications, and reviewing your application. Dress in a comfortable suit, shoes, and minimal accessories; arrive early knowing that your interview begins even before you meet your interviewer, so treat everyone you meet with respect. Refrain from speaking to anyone in a casual way and have questions prepared to ask each interviewer. After your interviews, be sure to write thank you notes or emails if a program does not specifically discourage postinterview communication. Continuous efforts will improve your success in obtaining a dermatology residency position.
Final Thoughts
Recent articles have underscored and emphasized the importance of diversity in our field, with a call to action to find meaningful and overdue solutions.2,6 We acknowledge the important role that mentors play in providing timely, honest, and encouraging guidance to UIM students interested in careers in dermatology. We hope to provide readily available and detailed guidance to these students on how they can present themselves as excellent and qualified applicants through this summary and other platforms.
Acknowledgment
The authors would like to thank the members of the SOCS Diversity Task Force for their assistance in creating the original guide.
- Chen A, Shinkai K. Rethinking how we select dermatology applicants—turning the tide. JAMA Dermatol. 2017;153:259-260.
- Granstein RD, Cornelius L, Shinkai K. Diversity in dermatology—a call for action. JAMA Dermatol. 2017;153:499-500.
- Imadojemu S, James WD. Increasing African American representation in dermatology. JAMA Dermatol. 2016;152:15-16.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Pritchett EN, Pandya AG, Ferguson NN, et al. Diversity in dermatology: roadmap for improvement. J Am Acad Dermatol. 2018;79:337-341.
- Taylor SC. Meeting the unique dermatologic needs of black patients [published online August 21, 2019]. JAMA Dermatol. doi:10.1001/jamadermatol.2019.1963.
- Skin of Color Society. How to obtain a position in a dermatology residency program. https://skinofcolorsociety.org/wp-content/uploads/2019/10/How-to-Obtain-a-Position-in-a-Dermatology-Residency-Program-10-08-2019.pdf. Accessed June 24, 2020.
- American Academy of Orthopaedic Surgeons. How to obtain an orthopedic residency by the American Academy of Orthopaedic Surgeons. https://www.aaos.org/globalassets/about/diversity/how-to-obtain-an-orthopaedic-residency.pdf. Accessed June 24, 2020.
- Results and Data—2018 Main Residency Match. Washington, DC: National Resident Matching Program; 2018. Published April 2018. Accessed June 24, 2020.
There has been increasing attention focused on the lack of diversity within dermatology academic and residency programs.1-6 Several factors have been identified as contributing to this narrow pipeline of qualified applicants, including lack of mentorship, delayed exposure to the field, implicit bias, and lack of an overall holistic review of applications with overemphasis on board scores.1,5 In an effort to provide guidance to underrepresented in medicine (UIM) students who are interested in dermatology, the Skin of Color Society (SOCS) has created a detailed, step-by-step guide on how to obtain a position in a dermatology residency program,7 which was modeled after a similar resource created by the American Academy of Orthopaedic Surgeons.8 Here, we highlight the main SOCS recommendations to help guide medical students through a systematic approach to becoming successful applicants for dermatology residency.
Start Early
Competitive fields such as dermatology require intentional efforts starting at the beginning of medical school. Regardless of what specialty is right for you, begin by constructing a well-rounded application for residency immediately. Start by shadowing dermatologists and attending Grand Rounds held in your institution’s dermatology department to ensure that this field is right for you. Students are encouraged to meet with academic advisors and upperclassmen to seek guidance on gaining early exposure to dermatology at their home institutions (or nearby programs) during their first year. As a platform for learning about community-based dermatology activities, join your school’s Dermatology Interest Group, keeping in mind that an executive position in such a group can help foster relationships with faculty and residents of the dermatology department. A long-term commitment to community service also contributes to your depth as an applicant. Getting involved early helps students uncover health disparities in medicine and allows time to formulate ideas to implement change. Forming a well-rounded application mandates maintaining good academic standing, and students should prioritize mastering the curriculum, excelling in clinical rotations, and studying for the US Medical Licensing Examination (USMLE).
Choose a Mentor
The summer between your first and second years of medical school is an opportune time to explore research opportunities. Students successfully complete research by taking ownership of a project, efficiently meeting deadlines, maintaining contact with research mentors by quickly responding to emails, and producing quality work. Research outside of dermatology also is valued. Research mentors often provide future letters of recommendation, so commit to doing an outstanding job. For those finding it difficult to locate a mentor, consider searching the American Academy of Dermatology (AAD)(https://www.aad.org/mentorship/) or SOCS (https://skinofcolorsociety.org/) websites. The AAD has an established Diversity Mentorship Program (https://www.aad.org/member/career/volunteer/diversity-mentorship) that provides members with direct guidance from dermatologists for 4 weeks. Students use this time to conduct research, learn more about the specialty, and foster a relationship with their mentor. Students can apply any year of medical school; however, the typical awardee usually is a third-year or fourth-year student. The AAD may provide a stipend to help offset expenses.
Prepare for Boards
Second year is a continuation of the agenda set forth in first year, now with the focus shifting toward board preparation and excelling in clinical core didactics and rotations. According to data from the 2018 National Resident Matching Program,9 the mean USMLE Step 1 score for US allopathic senior medical students who matched into dermatology was 249 compared to 241 who did not match into dermatology. However, the mean score is just that—a mean—and people have matched with lower scores. Do not be intimidated by this number; instead, be driven to commit the time and resources to master the content and do your personal best on the USMLE Step examinations. Given the shift in some programs for earlier clinical exposure and postponement of boards until the third year, the recommendations in this timeline can be catered to fit a medical student’s specific situation.
Build Your Application
The third year of medical school is a busy year. Prepare for third-year clinical rotations by speaking with upperclassmen and clinical preceptors as you progress through your rotations. Evaluations and recommendations are weighed heavily by residency program directors, as this information is used to ascertain your clinical abilities. Seek feedback from your preceptors early and often with a sincere attempt to integrate suggested improvements. Schedule a dermatology rotation at your home institution after completing the core rotations. Although they are not required, applicants may complete away rotations early in their fourth year; the application period for visiting student learning opportunities typically opens April 1 of the third year, if not earlier. Free resources are available to help prepare for your dermatology rotations. Start by reviewing the Basic Dermatology Curriculum on the AAD website (https://www.aad.org/member/education/residents/bdc). Make contributions to
Interviewing for Residency
During your fourth year of medical school, you will be completing dermatology rotations, submitting your applications through the Electronic Residency Application Service, and interviewing with residency programs. When deciding which programs to apply to, consider referencing the American Medical Association Residency and Fellowship Database (https://freida.ama-assn.org/Freida/#/). Also keep in mind that, depending on your competitiveness, you should expect to receive 1 interview for every 10 programs you apply to, thus the application process can be quite costly. It is highly encouraged that you ask for letters of recommendation prior to August 15 and that you submit your applications by September 15. Complete mock interviews with a mentor and research commonly asked questions. Prior to your interview day, you want to spend time researching the program, browsing faculty publications, and reviewing your application. Dress in a comfortable suit, shoes, and minimal accessories; arrive early knowing that your interview begins even before you meet your interviewer, so treat everyone you meet with respect. Refrain from speaking to anyone in a casual way and have questions prepared to ask each interviewer. After your interviews, be sure to write thank you notes or emails if a program does not specifically discourage postinterview communication. Continuous efforts will improve your success in obtaining a dermatology residency position.
Final Thoughts
Recent articles have underscored and emphasized the importance of diversity in our field, with a call to action to find meaningful and overdue solutions.2,6 We acknowledge the important role that mentors play in providing timely, honest, and encouraging guidance to UIM students interested in careers in dermatology. We hope to provide readily available and detailed guidance to these students on how they can present themselves as excellent and qualified applicants through this summary and other platforms.
Acknowledgment
The authors would like to thank the members of the SOCS Diversity Task Force for their assistance in creating the original guide.
There has been increasing attention focused on the lack of diversity within dermatology academic and residency programs.1-6 Several factors have been identified as contributing to this narrow pipeline of qualified applicants, including lack of mentorship, delayed exposure to the field, implicit bias, and lack of an overall holistic review of applications with overemphasis on board scores.1,5 In an effort to provide guidance to underrepresented in medicine (UIM) students who are interested in dermatology, the Skin of Color Society (SOCS) has created a detailed, step-by-step guide on how to obtain a position in a dermatology residency program,7 which was modeled after a similar resource created by the American Academy of Orthopaedic Surgeons.8 Here, we highlight the main SOCS recommendations to help guide medical students through a systematic approach to becoming successful applicants for dermatology residency.
Start Early
Competitive fields such as dermatology require intentional efforts starting at the beginning of medical school. Regardless of what specialty is right for you, begin by constructing a well-rounded application for residency immediately. Start by shadowing dermatologists and attending Grand Rounds held in your institution’s dermatology department to ensure that this field is right for you. Students are encouraged to meet with academic advisors and upperclassmen to seek guidance on gaining early exposure to dermatology at their home institutions (or nearby programs) during their first year. As a platform for learning about community-based dermatology activities, join your school’s Dermatology Interest Group, keeping in mind that an executive position in such a group can help foster relationships with faculty and residents of the dermatology department. A long-term commitment to community service also contributes to your depth as an applicant. Getting involved early helps students uncover health disparities in medicine and allows time to formulate ideas to implement change. Forming a well-rounded application mandates maintaining good academic standing, and students should prioritize mastering the curriculum, excelling in clinical rotations, and studying for the US Medical Licensing Examination (USMLE).
Choose a Mentor
The summer between your first and second years of medical school is an opportune time to explore research opportunities. Students successfully complete research by taking ownership of a project, efficiently meeting deadlines, maintaining contact with research mentors by quickly responding to emails, and producing quality work. Research outside of dermatology also is valued. Research mentors often provide future letters of recommendation, so commit to doing an outstanding job. For those finding it difficult to locate a mentor, consider searching the American Academy of Dermatology (AAD)(https://www.aad.org/mentorship/) or SOCS (https://skinofcolorsociety.org/) websites. The AAD has an established Diversity Mentorship Program (https://www.aad.org/member/career/volunteer/diversity-mentorship) that provides members with direct guidance from dermatologists for 4 weeks. Students use this time to conduct research, learn more about the specialty, and foster a relationship with their mentor. Students can apply any year of medical school; however, the typical awardee usually is a third-year or fourth-year student. The AAD may provide a stipend to help offset expenses.
Prepare for Boards
Second year is a continuation of the agenda set forth in first year, now with the focus shifting toward board preparation and excelling in clinical core didactics and rotations. According to data from the 2018 National Resident Matching Program,9 the mean USMLE Step 1 score for US allopathic senior medical students who matched into dermatology was 249 compared to 241 who did not match into dermatology. However, the mean score is just that—a mean—and people have matched with lower scores. Do not be intimidated by this number; instead, be driven to commit the time and resources to master the content and do your personal best on the USMLE Step examinations. Given the shift in some programs for earlier clinical exposure and postponement of boards until the third year, the recommendations in this timeline can be catered to fit a medical student’s specific situation.
Build Your Application
The third year of medical school is a busy year. Prepare for third-year clinical rotations by speaking with upperclassmen and clinical preceptors as you progress through your rotations. Evaluations and recommendations are weighed heavily by residency program directors, as this information is used to ascertain your clinical abilities. Seek feedback from your preceptors early and often with a sincere attempt to integrate suggested improvements. Schedule a dermatology rotation at your home institution after completing the core rotations. Although they are not required, applicants may complete away rotations early in their fourth year; the application period for visiting student learning opportunities typically opens April 1 of the third year, if not earlier. Free resources are available to help prepare for your dermatology rotations. Start by reviewing the Basic Dermatology Curriculum on the AAD website (https://www.aad.org/member/education/residents/bdc). Make contributions to
Interviewing for Residency
During your fourth year of medical school, you will be completing dermatology rotations, submitting your applications through the Electronic Residency Application Service, and interviewing with residency programs. When deciding which programs to apply to, consider referencing the American Medical Association Residency and Fellowship Database (https://freida.ama-assn.org/Freida/#/). Also keep in mind that, depending on your competitiveness, you should expect to receive 1 interview for every 10 programs you apply to, thus the application process can be quite costly. It is highly encouraged that you ask for letters of recommendation prior to August 15 and that you submit your applications by September 15. Complete mock interviews with a mentor and research commonly asked questions. Prior to your interview day, you want to spend time researching the program, browsing faculty publications, and reviewing your application. Dress in a comfortable suit, shoes, and minimal accessories; arrive early knowing that your interview begins even before you meet your interviewer, so treat everyone you meet with respect. Refrain from speaking to anyone in a casual way and have questions prepared to ask each interviewer. After your interviews, be sure to write thank you notes or emails if a program does not specifically discourage postinterview communication. Continuous efforts will improve your success in obtaining a dermatology residency position.
Final Thoughts
Recent articles have underscored and emphasized the importance of diversity in our field, with a call to action to find meaningful and overdue solutions.2,6 We acknowledge the important role that mentors play in providing timely, honest, and encouraging guidance to UIM students interested in careers in dermatology. We hope to provide readily available and detailed guidance to these students on how they can present themselves as excellent and qualified applicants through this summary and other platforms.
Acknowledgment
The authors would like to thank the members of the SOCS Diversity Task Force for their assistance in creating the original guide.
- Chen A, Shinkai K. Rethinking how we select dermatology applicants—turning the tide. JAMA Dermatol. 2017;153:259-260.
- Granstein RD, Cornelius L, Shinkai K. Diversity in dermatology—a call for action. JAMA Dermatol. 2017;153:499-500.
- Imadojemu S, James WD. Increasing African American representation in dermatology. JAMA Dermatol. 2016;152:15-16.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Pritchett EN, Pandya AG, Ferguson NN, et al. Diversity in dermatology: roadmap for improvement. J Am Acad Dermatol. 2018;79:337-341.
- Taylor SC. Meeting the unique dermatologic needs of black patients [published online August 21, 2019]. JAMA Dermatol. doi:10.1001/jamadermatol.2019.1963.
- Skin of Color Society. How to obtain a position in a dermatology residency program. https://skinofcolorsociety.org/wp-content/uploads/2019/10/How-to-Obtain-a-Position-in-a-Dermatology-Residency-Program-10-08-2019.pdf. Accessed June 24, 2020.
- American Academy of Orthopaedic Surgeons. How to obtain an orthopedic residency by the American Academy of Orthopaedic Surgeons. https://www.aaos.org/globalassets/about/diversity/how-to-obtain-an-orthopaedic-residency.pdf. Accessed June 24, 2020.
- Results and Data—2018 Main Residency Match. Washington, DC: National Resident Matching Program; 2018. Published April 2018. Accessed June 24, 2020.
- Chen A, Shinkai K. Rethinking how we select dermatology applicants—turning the tide. JAMA Dermatol. 2017;153:259-260.
- Granstein RD, Cornelius L, Shinkai K. Diversity in dermatology—a call for action. JAMA Dermatol. 2017;153:499-500.
- Imadojemu S, James WD. Increasing African American representation in dermatology. JAMA Dermatol. 2016;152:15-16.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Pritchett EN, Pandya AG, Ferguson NN, et al. Diversity in dermatology: roadmap for improvement. J Am Acad Dermatol. 2018;79:337-341.
- Taylor SC. Meeting the unique dermatologic needs of black patients [published online August 21, 2019]. JAMA Dermatol. doi:10.1001/jamadermatol.2019.1963.
- Skin of Color Society. How to obtain a position in a dermatology residency program. https://skinofcolorsociety.org/wp-content/uploads/2019/10/How-to-Obtain-a-Position-in-a-Dermatology-Residency-Program-10-08-2019.pdf. Accessed June 24, 2020.
- American Academy of Orthopaedic Surgeons. How to obtain an orthopedic residency by the American Academy of Orthopaedic Surgeons. https://www.aaos.org/globalassets/about/diversity/how-to-obtain-an-orthopaedic-residency.pdf. Accessed June 24, 2020.
- Results and Data—2018 Main Residency Match. Washington, DC: National Resident Matching Program; 2018. Published April 2018. Accessed June 24, 2020.
Practice Points
- Students interested in dermatology are encouraged to seek mentorship, strive for their academic best, and maintain their unique personal interests that make them a well-rounded applicant.
- Increasing diversity in dermatology requires initiative from students as well as dermatologists who are willing to mentor and sponsor.