User login
For MD-IQ use only
Asymptomatic Plaque on the Scalp
The Diagnosis: Nevus Comedonicus
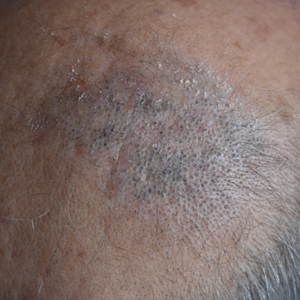
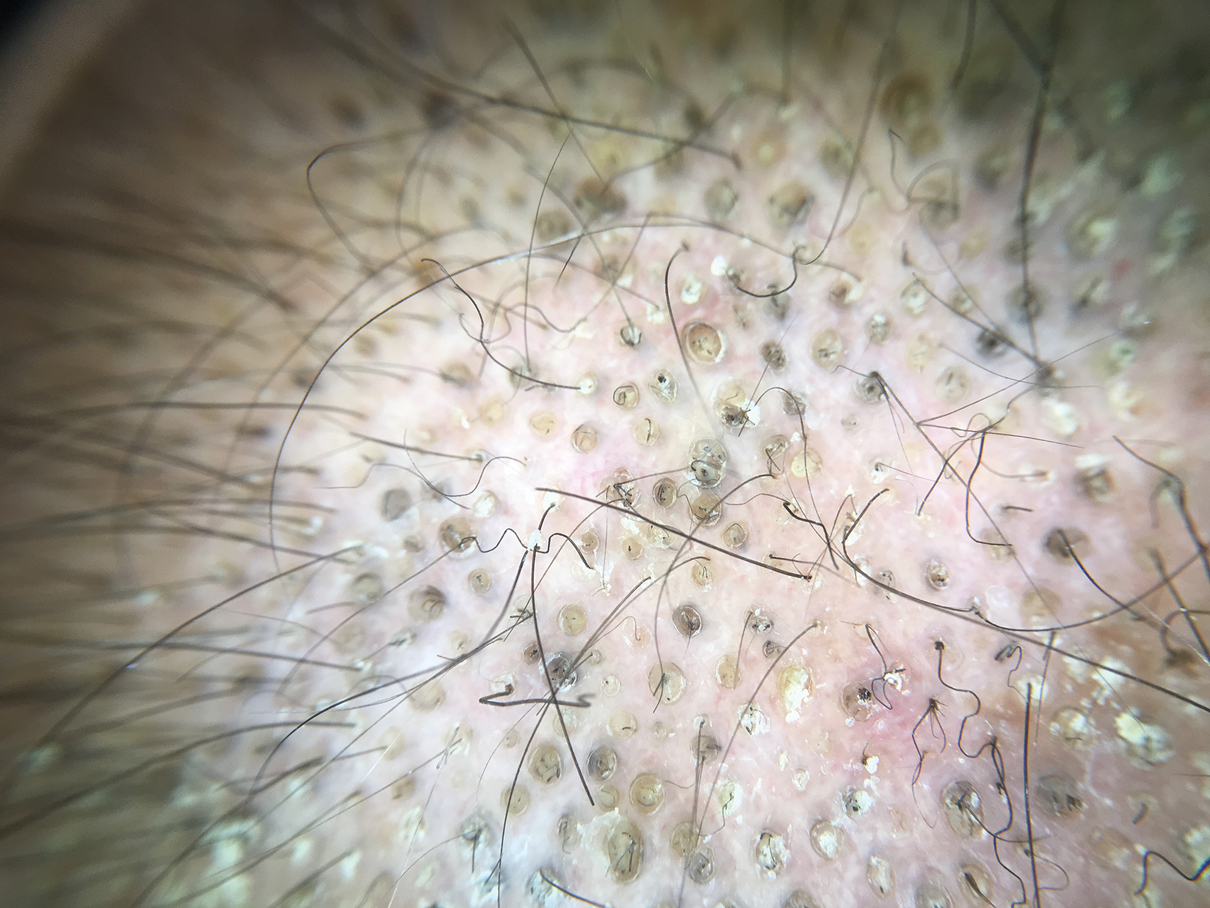
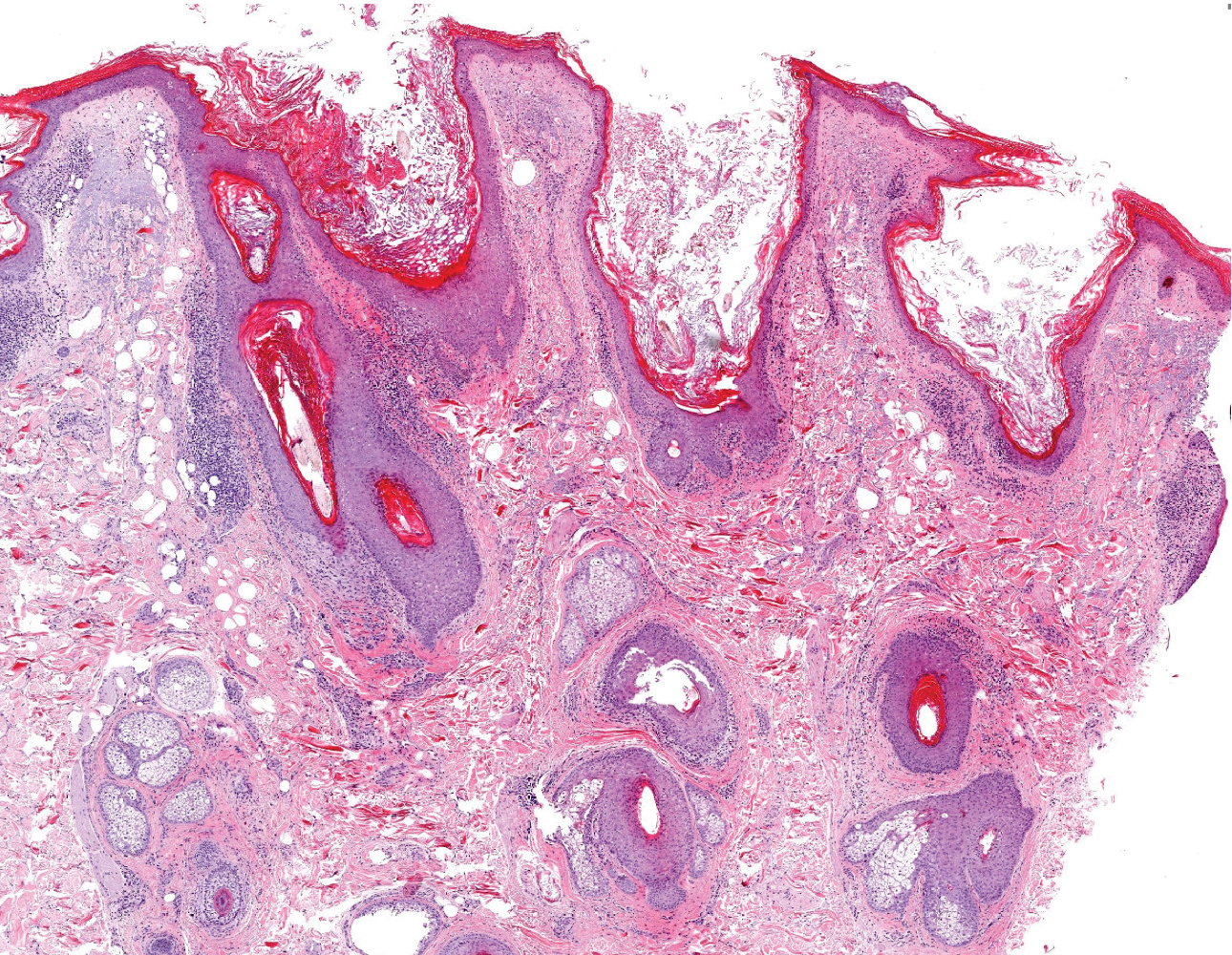
Dermoscopy showed multiple dilated follicular openings plugged with keratinous material (Figure 1). Histopathology revealed dilated follicular infundibula with dilation and orthokeratotic plugging (Figure 2). Routine laboratory tests including complete blood cell count and blood chemistry were within reference range. Thus, on the basis of clinical, dermoscopy, and histopathological findings, a diagnosis of nevus comedonicus (NC) was made. The patient refused treatment for cosmetic reasons.
Nevus comedonicus is a rare hamartoma first described by Kofmann1 in 1895. It is thought to be a developmental defect of the pilosebaceous unit; the resulting structure is unable to produce mature hairs, matrix cells, or sebaceous glands and is capable only of forming soft keratin.2 Clinically, it is characterized by closely grouped papules with hyperkeratotic plugs that mimic comedones. It has a predilection for the face, neck, and trunk area. Nevertheless, scalp involvement rarely has been reported in the literature.2-4 Nevus comedonicus usually appears at birth or during childhood and generally is asymptomatic; however, an inflammatory variant of NC with cyst formation and recurrent infections also has been described.5 Moreover, a syndromic variant was reported and characterized by a combination of NC with ocular, skeletal, or neurological defects.5 Most lesions grow proportionately with age and usually stabilize by late adolescence.2 Our patient's plaque increased in size with age. No triggering factors were found. Although NC usually has a benign course, squamous cell carcinoma arising in NC has been reported.6 Consequently, routine surveillance is necessary.
Diagnosis often is easily made by considering the characteristic morphology of the lesions and the early age of its appearance. However, in atypical NC presentations, acne, seborrheic keratosis, porokeratotic eccrine ostial and dermal duct nevus, folliculotropic mycosis fungoides, Favre-Racouchot syndrome, or familial dyskeratotic comedones should be considered. Dermoscopy has been reported to be useful in the diagnosis of NC. Typical dermoscopy findings are numerous circular and barrel-shaped homogenous areas in light and dark brown shades with remarkable keratin plugs.7,8
Folliculotropic mycosis fungoides is a variant of mycosis fungoides characterized by hair follicle invasion of mature, CD4+, small, lymphoid cells with cerebriform nuclei.9 Patients may present with grouped follicular papules that preferentially involve the head and neck area. It typically occurs in adults but occasionally may affect children. Histopathology is characterized by the presence of folliculotropic infiltrates with variable infiltration of the follicular epithelium, often with sparing of the epidermis. Familial dyskeratotic comedones, rare autosomal-dominant genodermatoses, clinically are characterized by symmetrically scattered comedonelike hyperkeratotic papules. These lesions appear around puberty and show a predilection for the trunk, arms, and face. Histopathology reveals craterlike invaginations filled with keratinous material and evidence of dyskeratosis. Porokeratotic eccrine ostial and dermal duct nevus is a rare adnexal hamartoma with eccrine differentiation. It is characterized by asymptomatic grouped keratotic papules and plaques. The lesions usually present at birth or in childhood and favor the palms and soles. Widespread involvement along Blaschko lines also can occur. Cornoid lamella involving an eccrine duct is the characteristic histopathologic feature of this condition.9
Treatment of NC is essentially reserved for cosmetic reasons or when there are complications such as discomfort or infection. Treatment options include topical corticosteroids, topical retinoids, and keratolytic agents such as ammonium lactate or salicylic acid.10 The use of oral isotretinoin is controversial.2 Surgical excision is useful for localized lesions. Nevus comedonicus, especially occurring at unusual sites such as the scalp, is uncommon. Therefore, a high index of suspicion is required to reach a diagnosis.
- Kofmann S. Ein fall von seltener localisation und verbreitiing von comedonen. Arch Derm Syph. 1895;32:177-178.
- Sikorski D, Parker J, Shwayder T. A boy with an unusual scalp birthmark. Int J Dermatol. 2011;50:670-672.
- Ghaninezhad H, Ehsani AH, Mansoori P, et al. Naevus comedonicus of the scalp. J Eur Acad Dermatol Venereol. 2006;20:184-185.
- Kikkeri N, Priyanka R, Parshawanath H. Nevus comedonicus on scalp: a rare site. Indian J Dermatol. 2015;60:105.
- Happle R. The group of epidermal nevus syndromes. J Am Acad Dermatol. 2010;63:1-22.
- Walling HW, Swick BL. Squamous cell carcinoma arising in nevus comedonicus. Dermatol Surg. 2009;35:144-146.
- Kamin´ska-Winciorek G, S´piewak R. Dermoscopy on nevus comedonicus: a case report and review of the literature. Postepy Dermatol Alergol. 2013;30:252-254.
- Vora R, Kota R, Sheth N. Dermoscopy of nevus comedonicus. Indian Dermatol Online J. 2017;8:388.
- Wang NS, Meola T, Orlow SJ, et al. Porokeratotic eccrine ostial and dermal duct nevus: a report of 2 cases and review of the literature. Am J Dermatopathol. 2009;31:582-586.
- Ferrari B, Taliercio V, Restrepo P, et al. Nevus comedonicus: a case series. Pediatr Dermatol. 2015;32:216-219
The Diagnosis: Nevus Comedonicus
Dermoscopy showed multiple dilated follicular openings plugged with keratinous material (Figure 1). Histopathology revealed dilated follicular infundibula with dilation and orthokeratotic plugging (Figure 2). Routine laboratory tests including complete blood cell count and blood chemistry were within reference range. Thus, on the basis of clinical, dermoscopy, and histopathological findings, a diagnosis of nevus comedonicus (NC) was made. The patient refused treatment for cosmetic reasons.


Nevus comedonicus is a rare hamartoma first described by Kofmann1 in 1895. It is thought to be a developmental defect of the pilosebaceous unit; the resulting structure is unable to produce mature hairs, matrix cells, or sebaceous glands and is capable only of forming soft keratin.2 Clinically, it is characterized by closely grouped papules with hyperkeratotic plugs that mimic comedones. It has a predilection for the face, neck, and trunk area. Nevertheless, scalp involvement rarely has been reported in the literature.2-4 Nevus comedonicus usually appears at birth or during childhood and generally is asymptomatic; however, an inflammatory variant of NC with cyst formation and recurrent infections also has been described.5 Moreover, a syndromic variant was reported and characterized by a combination of NC with ocular, skeletal, or neurological defects.5 Most lesions grow proportionately with age and usually stabilize by late adolescence.2 Our patient's plaque increased in size with age. No triggering factors were found. Although NC usually has a benign course, squamous cell carcinoma arising in NC has been reported.6 Consequently, routine surveillance is necessary.
Diagnosis often is easily made by considering the characteristic morphology of the lesions and the early age of its appearance. However, in atypical NC presentations, acne, seborrheic keratosis, porokeratotic eccrine ostial and dermal duct nevus, folliculotropic mycosis fungoides, Favre-Racouchot syndrome, or familial dyskeratotic comedones should be considered. Dermoscopy has been reported to be useful in the diagnosis of NC. Typical dermoscopy findings are numerous circular and barrel-shaped homogenous areas in light and dark brown shades with remarkable keratin plugs.7,8
Folliculotropic mycosis fungoides is a variant of mycosis fungoides characterized by hair follicle invasion of mature, CD4+, small, lymphoid cells with cerebriform nuclei.9 Patients may present with grouped follicular papules that preferentially involve the head and neck area. It typically occurs in adults but occasionally may affect children. Histopathology is characterized by the presence of folliculotropic infiltrates with variable infiltration of the follicular epithelium, often with sparing of the epidermis. Familial dyskeratotic comedones, rare autosomal-dominant genodermatoses, clinically are characterized by symmetrically scattered comedonelike hyperkeratotic papules. These lesions appear around puberty and show a predilection for the trunk, arms, and face. Histopathology reveals craterlike invaginations filled with keratinous material and evidence of dyskeratosis. Porokeratotic eccrine ostial and dermal duct nevus is a rare adnexal hamartoma with eccrine differentiation. It is characterized by asymptomatic grouped keratotic papules and plaques. The lesions usually present at birth or in childhood and favor the palms and soles. Widespread involvement along Blaschko lines also can occur. Cornoid lamella involving an eccrine duct is the characteristic histopathologic feature of this condition.9
Treatment of NC is essentially reserved for cosmetic reasons or when there are complications such as discomfort or infection. Treatment options include topical corticosteroids, topical retinoids, and keratolytic agents such as ammonium lactate or salicylic acid.10 The use of oral isotretinoin is controversial.2 Surgical excision is useful for localized lesions. Nevus comedonicus, especially occurring at unusual sites such as the scalp, is uncommon. Therefore, a high index of suspicion is required to reach a diagnosis.
The Diagnosis: Nevus Comedonicus
Dermoscopy showed multiple dilated follicular openings plugged with keratinous material (Figure 1). Histopathology revealed dilated follicular infundibula with dilation and orthokeratotic plugging (Figure 2). Routine laboratory tests including complete blood cell count and blood chemistry were within reference range. Thus, on the basis of clinical, dermoscopy, and histopathological findings, a diagnosis of nevus comedonicus (NC) was made. The patient refused treatment for cosmetic reasons.


Nevus comedonicus is a rare hamartoma first described by Kofmann1 in 1895. It is thought to be a developmental defect of the pilosebaceous unit; the resulting structure is unable to produce mature hairs, matrix cells, or sebaceous glands and is capable only of forming soft keratin.2 Clinically, it is characterized by closely grouped papules with hyperkeratotic plugs that mimic comedones. It has a predilection for the face, neck, and trunk area. Nevertheless, scalp involvement rarely has been reported in the literature.2-4 Nevus comedonicus usually appears at birth or during childhood and generally is asymptomatic; however, an inflammatory variant of NC with cyst formation and recurrent infections also has been described.5 Moreover, a syndromic variant was reported and characterized by a combination of NC with ocular, skeletal, or neurological defects.5 Most lesions grow proportionately with age and usually stabilize by late adolescence.2 Our patient's plaque increased in size with age. No triggering factors were found. Although NC usually has a benign course, squamous cell carcinoma arising in NC has been reported.6 Consequently, routine surveillance is necessary.
Diagnosis often is easily made by considering the characteristic morphology of the lesions and the early age of its appearance. However, in atypical NC presentations, acne, seborrheic keratosis, porokeratotic eccrine ostial and dermal duct nevus, folliculotropic mycosis fungoides, Favre-Racouchot syndrome, or familial dyskeratotic comedones should be considered. Dermoscopy has been reported to be useful in the diagnosis of NC. Typical dermoscopy findings are numerous circular and barrel-shaped homogenous areas in light and dark brown shades with remarkable keratin plugs.7,8
Folliculotropic mycosis fungoides is a variant of mycosis fungoides characterized by hair follicle invasion of mature, CD4+, small, lymphoid cells with cerebriform nuclei.9 Patients may present with grouped follicular papules that preferentially involve the head and neck area. It typically occurs in adults but occasionally may affect children. Histopathology is characterized by the presence of folliculotropic infiltrates with variable infiltration of the follicular epithelium, often with sparing of the epidermis. Familial dyskeratotic comedones, rare autosomal-dominant genodermatoses, clinically are characterized by symmetrically scattered comedonelike hyperkeratotic papules. These lesions appear around puberty and show a predilection for the trunk, arms, and face. Histopathology reveals craterlike invaginations filled with keratinous material and evidence of dyskeratosis. Porokeratotic eccrine ostial and dermal duct nevus is a rare adnexal hamartoma with eccrine differentiation. It is characterized by asymptomatic grouped keratotic papules and plaques. The lesions usually present at birth or in childhood and favor the palms and soles. Widespread involvement along Blaschko lines also can occur. Cornoid lamella involving an eccrine duct is the characteristic histopathologic feature of this condition.9
Treatment of NC is essentially reserved for cosmetic reasons or when there are complications such as discomfort or infection. Treatment options include topical corticosteroids, topical retinoids, and keratolytic agents such as ammonium lactate or salicylic acid.10 The use of oral isotretinoin is controversial.2 Surgical excision is useful for localized lesions. Nevus comedonicus, especially occurring at unusual sites such as the scalp, is uncommon. Therefore, a high index of suspicion is required to reach a diagnosis.
- Kofmann S. Ein fall von seltener localisation und verbreitiing von comedonen. Arch Derm Syph. 1895;32:177-178.
- Sikorski D, Parker J, Shwayder T. A boy with an unusual scalp birthmark. Int J Dermatol. 2011;50:670-672.
- Ghaninezhad H, Ehsani AH, Mansoori P, et al. Naevus comedonicus of the scalp. J Eur Acad Dermatol Venereol. 2006;20:184-185.
- Kikkeri N, Priyanka R, Parshawanath H. Nevus comedonicus on scalp: a rare site. Indian J Dermatol. 2015;60:105.
- Happle R. The group of epidermal nevus syndromes. J Am Acad Dermatol. 2010;63:1-22.
- Walling HW, Swick BL. Squamous cell carcinoma arising in nevus comedonicus. Dermatol Surg. 2009;35:144-146.
- Kamin´ska-Winciorek G, S´piewak R. Dermoscopy on nevus comedonicus: a case report and review of the literature. Postepy Dermatol Alergol. 2013;30:252-254.
- Vora R, Kota R, Sheth N. Dermoscopy of nevus comedonicus. Indian Dermatol Online J. 2017;8:388.
- Wang NS, Meola T, Orlow SJ, et al. Porokeratotic eccrine ostial and dermal duct nevus: a report of 2 cases and review of the literature. Am J Dermatopathol. 2009;31:582-586.
- Ferrari B, Taliercio V, Restrepo P, et al. Nevus comedonicus: a case series. Pediatr Dermatol. 2015;32:216-219
- Kofmann S. Ein fall von seltener localisation und verbreitiing von comedonen. Arch Derm Syph. 1895;32:177-178.
- Sikorski D, Parker J, Shwayder T. A boy with an unusual scalp birthmark. Int J Dermatol. 2011;50:670-672.
- Ghaninezhad H, Ehsani AH, Mansoori P, et al. Naevus comedonicus of the scalp. J Eur Acad Dermatol Venereol. 2006;20:184-185.
- Kikkeri N, Priyanka R, Parshawanath H. Nevus comedonicus on scalp: a rare site. Indian J Dermatol. 2015;60:105.
- Happle R. The group of epidermal nevus syndromes. J Am Acad Dermatol. 2010;63:1-22.
- Walling HW, Swick BL. Squamous cell carcinoma arising in nevus comedonicus. Dermatol Surg. 2009;35:144-146.
- Kamin´ska-Winciorek G, S´piewak R. Dermoscopy on nevus comedonicus: a case report and review of the literature. Postepy Dermatol Alergol. 2013;30:252-254.
- Vora R, Kota R, Sheth N. Dermoscopy of nevus comedonicus. Indian Dermatol Online J. 2017;8:388.
- Wang NS, Meola T, Orlow SJ, et al. Porokeratotic eccrine ostial and dermal duct nevus: a report of 2 cases and review of the literature. Am J Dermatopathol. 2009;31:582-586.
- Ferrari B, Taliercio V, Restrepo P, et al. Nevus comedonicus: a case series. Pediatr Dermatol. 2015;32:216-219
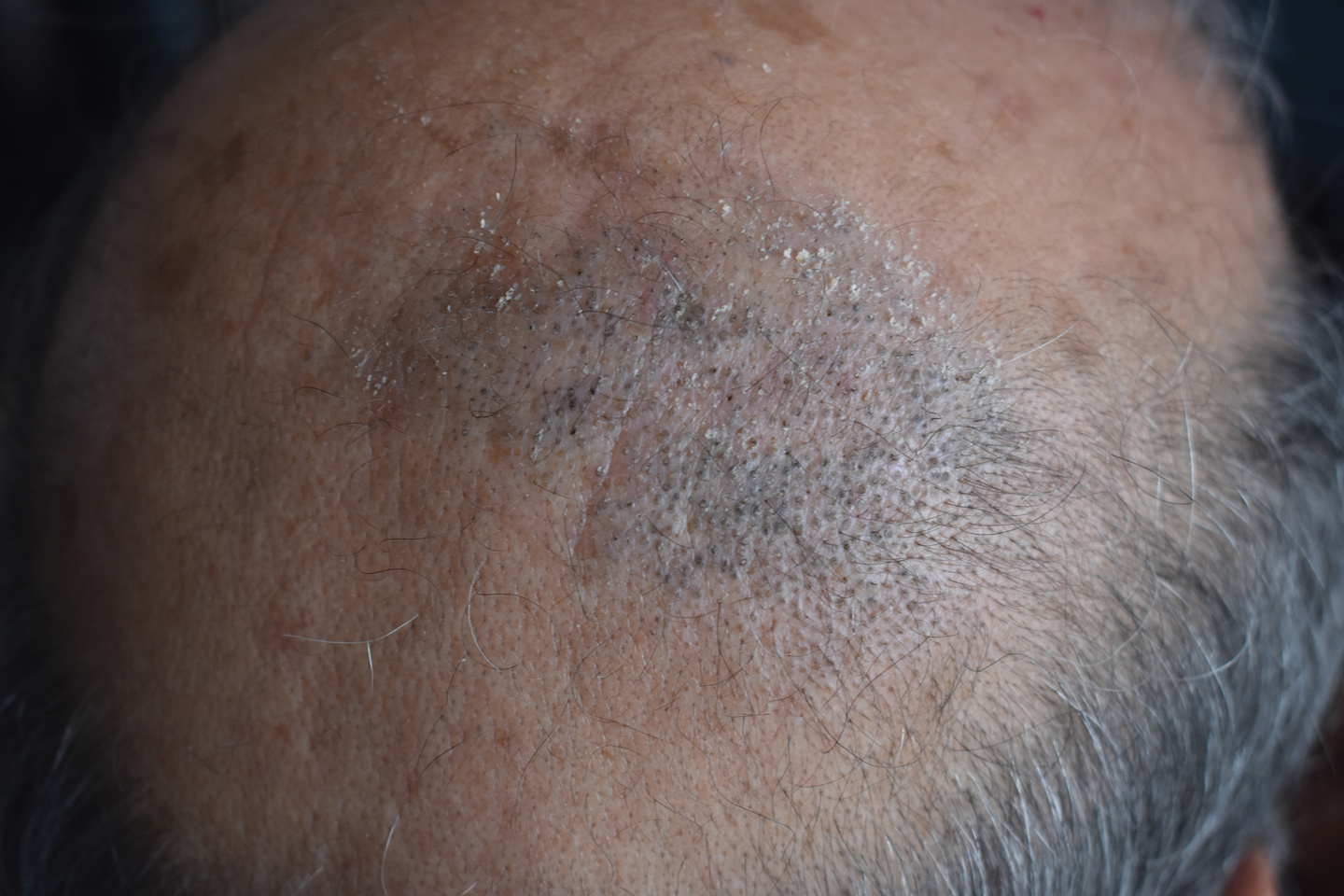
A 50-year-old man presented to the dermatology department with an asymptomatic plaque on the scalp that had been present since childhood. The size of the plaque gradually progressed initially but had notably increased in size in the last 6 months. There was no association with trauma or irritation. There was no family history of similar lesions. Physical examination revealed a 3.0×2.5-cm plaque on the vertex of the scalp consisting of aggregated pits plugged with keratinous material resembling comedones. There were no lesions elsewhere on the body. Dermoscopy and a 4-mm punch biopsy were performed.
Is your job performance being evaluated for the wrong factors?
Most physicians get an annual performance review, and may be either elated, disappointed, or confused with their rating.
But some physicians say the right factors aren’t being evaluated or, in many cases, the performance measures promote efforts that are counterproductive.
“Bonuses are a behaviorist approach,” said Richard Gunderman, MD, professor in the schools of medicine, liberal arts, and philanthropy at Indiana University, Indianapolis. “The presumption is that people will change if they get some money – that they will do what the incentive wants them to do and refrain from what it doesn’t want them to do.”
Dr. Gunderman said this often means just going through the motions to get the bonus, and not sharing goals that only the administration cares about. “The goals might be to lower costs, ensure compliance with regulations or billing requirements, or make patterns of care more uniform. These are not changes that are well tailored to what patients want or how doctors think.”
The bonus is a central feature of the annual review. Merritt Hawkins, the physician search firm, reported that 75% of the physician jobs that it searches for involve some kind of production bonus. Bonuses often make up at least 5% of total compensation, but they can be quite hefty in some specialties.
Having to fulfill measures that they’re not excited about can lead physicians to feel disengaged from their work, Dr. Gunderman said. And this disengagement can contribute to physician burnout, which has climbed to very high rates in recent years.
A 2018 paper by two physician leadership experts explored this problem with bonuses. “A growing consensus [of experts] suggests that quality-incentive pay isn’t paying the dividends first envisioned,” they wrote.
The problem is that the measurements tied to a bonus represent an extrinsic motivation – involving goals that doctors don’t really believe in. Instead, physicians need to be intrinsically motivated. They need to be inspired “to manage their own lives,” “to get better at something,” and “to be a part of a larger cause,” they wrote.
How to develop a better review process
“The best way to motivate improved performance is through purpose and mission,” said Robert Pearl, MD, former CEO of the Permanente Medical Group in California and now a lecturer on strategy at Stanford (Calif.) University.
The review process, Dr. Pearl said, should inspire physicians to do better. The doctors should be asking themselves: “How well did we do in helping maximize the health of all of our patients? And how well did we do in avoiding medical errors, preventing complications, meeting the needs of our patients, and achieving superior quality outcomes?”
When he was CEO of Permanente, the huge physician group that works exclusively for health maintenance organization Kaiser, Dr. Pearl and fellow leaders revamped the review system that all Permanente physicians undergo.
First, the Permanente executives provided all physicians with everyone’s patient-satisfaction data, including their own. That way, each physician could compare performance with others and assess strengths and weaknesses. Then Permanente offered educational programs so that physicians could get help in meeting their goals.
“This approach helped improve quality of care, patient satisfaction, and fulfillment of physicians,” Dr. Pearl said. Kaiser Permanente earned the highest health plan member satisfaction rating by J.D. Power and higher rankings by the National Committee for Quality Assurance.
Permanente does not base the bonus on relative value units but on performance measures that are carefully balanced to avoid too much focus on certain measures. “There needs to be an array of quality measures because doctors deal with a complex set of problems,” Dr. Pearl said. For example, a primary care physician at Permanente is assessed on about 30 different measures.
Physicians are more likely to be successful when you emphasize collaboration. Dr. Pearl said.
Although Permanente physicians are compared with each other, they are not pitted against each other but rather are asked to collaborate. “Physicians are more likely to be successful when you emphasize collaboration,” he said. “They can teach each other. You can be good at some things, and your colleague can be good at others.”
Permanente still has one-on-one yearly evaluations, but much of the assessment work is done in monthly meetings within each department. “There, small groups of doctors look at their data and discuss how each of them can improve,” Dr. Pearl noted.
The 360-degree review is valuable but has some problems
Physicians should be getting a lot more feedback about their behavior than they are actually getting, according to Milton Hammerly, MD, chief medical officer at QualChoice Health Insurance in Little Rock, Ark.
“After residency, you get very little feedback on your work,” said Dr. Hammerly, who used to work for a hospital system. “Annual reviews for physicians focus almost exclusively on outcomes, productivity, and quality metrics, but not on people skills, what is called ‘emotional intelligence.’ ”
Dr. Hammerly said he saw the consequence of this lack of education when he was vice president for medical affairs at the hospital system. He was constantly dealing with physicians who exhibited serious disruptive behavior and had to be disciplined. “If only they had gotten a little help earlier on,” he noted.
Dr. Hammerly said that 360-degree evaluations, which are common in corporations but rarely used for physicians, could benefit the profession. He discovered the 360-degree evaluation when it was used for him at QualChoice, and he has been a fan ever since.
The approach involves collecting evaluations of you from your boss, your peers, and from people who work for you. That is, from 360 degrees around you. These people are asked to rate your strengths and weaknesses in a variety of competencies. In this way, you get feedback from all of your work relationships, not just from your boss.
Ideally, the evaluators are anonymous, and the subject works with a facilitator to process the information. But 360-degree evaluations can be done in all kinds of ways.
Critics of the 360-degree evaluations say the usual anonymity of evaluators allows them to be too harsh. Also, evaluators may be too subjective: What they say about you says more about their own perspective than anything about you.
But many people think 360-degree evaluations are at least going in the right direction, because they focus on people skills rather than just meeting metrics.
Robert Centor, MD, an internist in Birmingham, Ala., and a member of the performance measures committee of the American College of Physicians, said the best way to improve performance is to have conversations about your work with colleagues on the department level. “For example, 20 doctors could meet to discuss a certain issue, such as the need for more vaccinations. That doesn’t have to get rewarded with a bonus payment.”
Dr. Pearl said that “doctors need feedback from their colleagues. Without feedback, how else do you get better? You can only improve if you can know how you’re performing, compared to others.”
A version of this article originally appeared on Medscape.com.
Most physicians get an annual performance review, and may be either elated, disappointed, or confused with their rating.
But some physicians say the right factors aren’t being evaluated or, in many cases, the performance measures promote efforts that are counterproductive.
“Bonuses are a behaviorist approach,” said Richard Gunderman, MD, professor in the schools of medicine, liberal arts, and philanthropy at Indiana University, Indianapolis. “The presumption is that people will change if they get some money – that they will do what the incentive wants them to do and refrain from what it doesn’t want them to do.”
Dr. Gunderman said this often means just going through the motions to get the bonus, and not sharing goals that only the administration cares about. “The goals might be to lower costs, ensure compliance with regulations or billing requirements, or make patterns of care more uniform. These are not changes that are well tailored to what patients want or how doctors think.”
The bonus is a central feature of the annual review. Merritt Hawkins, the physician search firm, reported that 75% of the physician jobs that it searches for involve some kind of production bonus. Bonuses often make up at least 5% of total compensation, but they can be quite hefty in some specialties.
Having to fulfill measures that they’re not excited about can lead physicians to feel disengaged from their work, Dr. Gunderman said. And this disengagement can contribute to physician burnout, which has climbed to very high rates in recent years.
A 2018 paper by two physician leadership experts explored this problem with bonuses. “A growing consensus [of experts] suggests that quality-incentive pay isn’t paying the dividends first envisioned,” they wrote.
The problem is that the measurements tied to a bonus represent an extrinsic motivation – involving goals that doctors don’t really believe in. Instead, physicians need to be intrinsically motivated. They need to be inspired “to manage their own lives,” “to get better at something,” and “to be a part of a larger cause,” they wrote.
How to develop a better review process
“The best way to motivate improved performance is through purpose and mission,” said Robert Pearl, MD, former CEO of the Permanente Medical Group in California and now a lecturer on strategy at Stanford (Calif.) University.
The review process, Dr. Pearl said, should inspire physicians to do better. The doctors should be asking themselves: “How well did we do in helping maximize the health of all of our patients? And how well did we do in avoiding medical errors, preventing complications, meeting the needs of our patients, and achieving superior quality outcomes?”
When he was CEO of Permanente, the huge physician group that works exclusively for health maintenance organization Kaiser, Dr. Pearl and fellow leaders revamped the review system that all Permanente physicians undergo.
First, the Permanente executives provided all physicians with everyone’s patient-satisfaction data, including their own. That way, each physician could compare performance with others and assess strengths and weaknesses. Then Permanente offered educational programs so that physicians could get help in meeting their goals.
“This approach helped improve quality of care, patient satisfaction, and fulfillment of physicians,” Dr. Pearl said. Kaiser Permanente earned the highest health plan member satisfaction rating by J.D. Power and higher rankings by the National Committee for Quality Assurance.
Permanente does not base the bonus on relative value units but on performance measures that are carefully balanced to avoid too much focus on certain measures. “There needs to be an array of quality measures because doctors deal with a complex set of problems,” Dr. Pearl said. For example, a primary care physician at Permanente is assessed on about 30 different measures.
Physicians are more likely to be successful when you emphasize collaboration. Dr. Pearl said.
Although Permanente physicians are compared with each other, they are not pitted against each other but rather are asked to collaborate. “Physicians are more likely to be successful when you emphasize collaboration,” he said. “They can teach each other. You can be good at some things, and your colleague can be good at others.”
Permanente still has one-on-one yearly evaluations, but much of the assessment work is done in monthly meetings within each department. “There, small groups of doctors look at their data and discuss how each of them can improve,” Dr. Pearl noted.
The 360-degree review is valuable but has some problems
Physicians should be getting a lot more feedback about their behavior than they are actually getting, according to Milton Hammerly, MD, chief medical officer at QualChoice Health Insurance in Little Rock, Ark.
“After residency, you get very little feedback on your work,” said Dr. Hammerly, who used to work for a hospital system. “Annual reviews for physicians focus almost exclusively on outcomes, productivity, and quality metrics, but not on people skills, what is called ‘emotional intelligence.’ ”
Dr. Hammerly said he saw the consequence of this lack of education when he was vice president for medical affairs at the hospital system. He was constantly dealing with physicians who exhibited serious disruptive behavior and had to be disciplined. “If only they had gotten a little help earlier on,” he noted.
Dr. Hammerly said that 360-degree evaluations, which are common in corporations but rarely used for physicians, could benefit the profession. He discovered the 360-degree evaluation when it was used for him at QualChoice, and he has been a fan ever since.
The approach involves collecting evaluations of you from your boss, your peers, and from people who work for you. That is, from 360 degrees around you. These people are asked to rate your strengths and weaknesses in a variety of competencies. In this way, you get feedback from all of your work relationships, not just from your boss.
Ideally, the evaluators are anonymous, and the subject works with a facilitator to process the information. But 360-degree evaluations can be done in all kinds of ways.
Critics of the 360-degree evaluations say the usual anonymity of evaluators allows them to be too harsh. Also, evaluators may be too subjective: What they say about you says more about their own perspective than anything about you.
But many people think 360-degree evaluations are at least going in the right direction, because they focus on people skills rather than just meeting metrics.
Robert Centor, MD, an internist in Birmingham, Ala., and a member of the performance measures committee of the American College of Physicians, said the best way to improve performance is to have conversations about your work with colleagues on the department level. “For example, 20 doctors could meet to discuss a certain issue, such as the need for more vaccinations. That doesn’t have to get rewarded with a bonus payment.”
Dr. Pearl said that “doctors need feedback from their colleagues. Without feedback, how else do you get better? You can only improve if you can know how you’re performing, compared to others.”
A version of this article originally appeared on Medscape.com.
Most physicians get an annual performance review, and may be either elated, disappointed, or confused with their rating.
But some physicians say the right factors aren’t being evaluated or, in many cases, the performance measures promote efforts that are counterproductive.
“Bonuses are a behaviorist approach,” said Richard Gunderman, MD, professor in the schools of medicine, liberal arts, and philanthropy at Indiana University, Indianapolis. “The presumption is that people will change if they get some money – that they will do what the incentive wants them to do and refrain from what it doesn’t want them to do.”
Dr. Gunderman said this often means just going through the motions to get the bonus, and not sharing goals that only the administration cares about. “The goals might be to lower costs, ensure compliance with regulations or billing requirements, or make patterns of care more uniform. These are not changes that are well tailored to what patients want or how doctors think.”
The bonus is a central feature of the annual review. Merritt Hawkins, the physician search firm, reported that 75% of the physician jobs that it searches for involve some kind of production bonus. Bonuses often make up at least 5% of total compensation, but they can be quite hefty in some specialties.
Having to fulfill measures that they’re not excited about can lead physicians to feel disengaged from their work, Dr. Gunderman said. And this disengagement can contribute to physician burnout, which has climbed to very high rates in recent years.
A 2018 paper by two physician leadership experts explored this problem with bonuses. “A growing consensus [of experts] suggests that quality-incentive pay isn’t paying the dividends first envisioned,” they wrote.
The problem is that the measurements tied to a bonus represent an extrinsic motivation – involving goals that doctors don’t really believe in. Instead, physicians need to be intrinsically motivated. They need to be inspired “to manage their own lives,” “to get better at something,” and “to be a part of a larger cause,” they wrote.
How to develop a better review process
“The best way to motivate improved performance is through purpose and mission,” said Robert Pearl, MD, former CEO of the Permanente Medical Group in California and now a lecturer on strategy at Stanford (Calif.) University.
The review process, Dr. Pearl said, should inspire physicians to do better. The doctors should be asking themselves: “How well did we do in helping maximize the health of all of our patients? And how well did we do in avoiding medical errors, preventing complications, meeting the needs of our patients, and achieving superior quality outcomes?”
When he was CEO of Permanente, the huge physician group that works exclusively for health maintenance organization Kaiser, Dr. Pearl and fellow leaders revamped the review system that all Permanente physicians undergo.
First, the Permanente executives provided all physicians with everyone’s patient-satisfaction data, including their own. That way, each physician could compare performance with others and assess strengths and weaknesses. Then Permanente offered educational programs so that physicians could get help in meeting their goals.
“This approach helped improve quality of care, patient satisfaction, and fulfillment of physicians,” Dr. Pearl said. Kaiser Permanente earned the highest health plan member satisfaction rating by J.D. Power and higher rankings by the National Committee for Quality Assurance.
Permanente does not base the bonus on relative value units but on performance measures that are carefully balanced to avoid too much focus on certain measures. “There needs to be an array of quality measures because doctors deal with a complex set of problems,” Dr. Pearl said. For example, a primary care physician at Permanente is assessed on about 30 different measures.
Physicians are more likely to be successful when you emphasize collaboration. Dr. Pearl said.
Although Permanente physicians are compared with each other, they are not pitted against each other but rather are asked to collaborate. “Physicians are more likely to be successful when you emphasize collaboration,” he said. “They can teach each other. You can be good at some things, and your colleague can be good at others.”
Permanente still has one-on-one yearly evaluations, but much of the assessment work is done in monthly meetings within each department. “There, small groups of doctors look at their data and discuss how each of them can improve,” Dr. Pearl noted.
The 360-degree review is valuable but has some problems
Physicians should be getting a lot more feedback about their behavior than they are actually getting, according to Milton Hammerly, MD, chief medical officer at QualChoice Health Insurance in Little Rock, Ark.
“After residency, you get very little feedback on your work,” said Dr. Hammerly, who used to work for a hospital system. “Annual reviews for physicians focus almost exclusively on outcomes, productivity, and quality metrics, but not on people skills, what is called ‘emotional intelligence.’ ”
Dr. Hammerly said he saw the consequence of this lack of education when he was vice president for medical affairs at the hospital system. He was constantly dealing with physicians who exhibited serious disruptive behavior and had to be disciplined. “If only they had gotten a little help earlier on,” he noted.
Dr. Hammerly said that 360-degree evaluations, which are common in corporations but rarely used for physicians, could benefit the profession. He discovered the 360-degree evaluation when it was used for him at QualChoice, and he has been a fan ever since.
The approach involves collecting evaluations of you from your boss, your peers, and from people who work for you. That is, from 360 degrees around you. These people are asked to rate your strengths and weaknesses in a variety of competencies. In this way, you get feedback from all of your work relationships, not just from your boss.
Ideally, the evaluators are anonymous, and the subject works with a facilitator to process the information. But 360-degree evaluations can be done in all kinds of ways.
Critics of the 360-degree evaluations say the usual anonymity of evaluators allows them to be too harsh. Also, evaluators may be too subjective: What they say about you says more about their own perspective than anything about you.
But many people think 360-degree evaluations are at least going in the right direction, because they focus on people skills rather than just meeting metrics.
Robert Centor, MD, an internist in Birmingham, Ala., and a member of the performance measures committee of the American College of Physicians, said the best way to improve performance is to have conversations about your work with colleagues on the department level. “For example, 20 doctors could meet to discuss a certain issue, such as the need for more vaccinations. That doesn’t have to get rewarded with a bonus payment.”
Dr. Pearl said that “doctors need feedback from their colleagues. Without feedback, how else do you get better? You can only improve if you can know how you’re performing, compared to others.”
A version of this article originally appeared on Medscape.com.
Lessons learned as a gastroenterologist on social media
I have always been a strong believer in meeting patients where they obtain their health information. Early in my clinical training, I realized that patients are exposed to health information through traditional media formats and, increasingly, social media, rather than brief clinical encounters. Unlike traditional media, social media allows individuals the opportunity to post information without a third-party filter. However, this opens the door for untrained individuals to spread misinformation and disinformation. In health care, this could potentially disrupt public health efforts. Even innocent mistakes like overlooking the appropriate clinical context can cause issues. Traditional media outlets also have agendas that may leave certain conditions, therapies, and other facets of health care underrepresented. My belief is that experts should therefore be trained and incentivized to be spokespeople for their own areas of expertise. Furthermore, social media provides a novel opportunity to improve health literacy while humanizing and restoring fading trust in health care.
There are several items to consider before initiating on one’s social media journey: whether you are committed to exploring the space, what one’s purpose is on social media, who the intended target audience is, which platform is most appropriate to serve that purpose and audience, and what potential pitfalls there may be.
The first question to ask oneself is whether you are prepared to devote time to cultivating a social media presence and speak or be heard publicly. Regardless of the platform, a social media presence requires consistency and audience interaction. The decision to partake can be personal; I view social media as an extension of in-person interaction, but not everyone is willing to commit to increased accessibility and visibility. Social media can still be valuable to those who choose to observe and learn rather than post.
Next is what one’s purpose is with being on social media. This can vary from peer education, boosting health literacy for patients, or using social media as a news source, networking tool, or a creative outlet. While my social media activity supports all these, my primary purpose is the distribution of accurate health information as a trained expert. When I started, I was one of few academic gastroenterologists uniquely positioned to bridge the elusive gap between the young, Gen Z crowd and academic medicine. Of similar importance is defining one’s target audience: patients, trainees, colleagues, or the general public.
Because there are numerous social media platforms, and only more to come in the future, it is critical to focus only on platforms that will serve one’s purpose and audience. Additionally, some may find more joy or agility in using one platform over the other. While I am one of the few clinicians who are adept at building communities across multiple rapidly evolving social media platforms, I will be the first to admit that it takes time to fully understand each platform with its ever-growing array of features. I find myself better at some platforms over others and, depending on my goals, I often will shift my focus from one to another.
Each platform has its pros and cons. Twitter is perhaps the most appropriate platform for starters. Easy to use with the least preparation necessary for every post, it also serves as the primary platform for academic discussion among all the popular social media platforms. Over the past few years, hundreds of gastroenterologists have become active on Twitter, which allows for ample networking opportunities and potential collaborations. The space has evolved to house various structured chats and learning opportunities as described by accounts like @MondayNightIBD, @ScopingSundays, #TracingTuesday, and @GIJournal. All major GI journals and societies are also present on Twitter and disseminating the latest information. Now a vestige of the past when text within tweets was not searchable, hashtags were used to curate discussion because searching by hashtag could reveal the latest discussion surrounding a topic and help identify others with a similar interest. Hashtags now remain relevant when crafting tweets, as the strategic inclusion of hashtags can help your content reach those who share an interest. A hashtag ontology was previously published to standardize academic conversation online in gastroenterology. Twitter also boasts features like polls that also help audiences engage.
Twitter has its disadvantages, however. Conversation is often siloed and difficult to reach audiences who don’t already follow you or others associated with you. Tweets disappear quickly in one’s feed and are often not seen by your followers. It lacks the visual appeal of other image- and video-based platforms that tend to attract more members of the general public. (Twitter lags behind these other platforms in monthly users) Other platforms like Facebook, Instagram, YouTube, LinkedIn, and TikTok have other benefits. Facebook may help foster community discussions in groups and business pages are also helpful for practice promotion. Instagram has gained popularity for educational purposes over the past 2 years, given its pairing with imagery and room for a lengthier caption. It has a variety of additional features like the temporary Instagram Stories that last 24 hours (which also allows for polling), question and answer, and livestream options. Other platforms like YouTube and TikTok have greater potential to reach audiences who otherwise would not see your content, with the former having the benefit of being highly searchable and the latter being the social media app with fastest growing popularity.
Having grown up with the Internet-based instant messaging and social media platforms, I have always enjoyed the medium as a way to connect with others. However, productive engagement on these platforms came much later. During a brief stint as part of the ABC News medical unit, I learned how Twitter was used to facilitate weekly chats around a specific topic online. I began exploring my own social media voice, which quickly gave way to live-tweeting medical conferences, hosting and participating Twitter chats myself, and guiding colleagues and professional societies to greater adoption of social media. In an attempt to introduce a divisional social media account during my fellowship, I learned of institutional barriers including antiquated policies that actively dissuaded social media use. I became increasingly involved on committees in our main GI societies after engaging in multiple research projects using social media data looking at how GI journals promote their content online, the associations between social media presence and institutional ranking, social media behavior at medical conferences, and the evolving perspectives of training program leadership regarding social media.
The pitfalls of social media remain a major concern for physicians and employers alike. First and foremost, it is important to review one’s institutional social media policy prior to starting, as individuals are ultimately held to their local policies. Not only can social media activity be a major liability for a health care employer, but also in the general public’s trust in health professionals. Protecting patient privacy and safety are of utmost concern, and physicians must be mindful not to inadvertently reveal patient identity. HIPAA violations are not limited to only naming patients by name or photo; descriptions of procedural cases and posting patient-related images such as radiographs or endoscopic images may reveal patient identity if there are unique details on these images (e.g., a radio-opaque necklace on x-ray or a particular swallowed foreign body).
Another disadvantage of social media is being approached with personal medical questions. I universally decline to answer these inquiries, citing the need to perform a comprehensive review of one’s medical chart and perform an in-person physical exam to fully assess a patient. The distinction between education and advice is subtle, yet important to recognize. Similarly, the need to uphold professionalism online is important. Short messages on social media can be misinterpreted by colleagues and the public. Not only can these interactions be potentially detrimental to one’s career, but it can further erode trust in health care if patients perceive this as fragmentation of the health care system. On platforms that encourage humor and creativity like TikTok, there have also been medical professionals and students publicly criticized and penalized for posting unprofessional content mocking patients.
With the introduction of social media influencers in recent years, some professionals have amassed followings, introducing yet another set of concerns. One is being approached with sponsorship and endorsement offers, as any agreements must be in accordance with institutional policy. As one’s following grows, there may be other concerns of safety both online and in real life. Online concerns include issues with impersonation and use of photos or written content without permission. On the surface this may not seem like a significant concern, but there have been situations where family photos are distributed to intended audiences or one’s likeness is used to endorse a product.
In addition to physical safety, another unintended consequence of social media use is its impact on one’s mental health. As social media tends to be a highlight reel, it is easy to be consumed by comparison with colleagues and their lives on social media, whether it truly reflects one’s actual life or not.
My ability to understand multiple social media platforms and anticipate a growing set of risks and concerns with using social media is what led to my involvement with multiple GI societies and appointment by my institution’s CEO to serve as the first chief medical social media officer. My desire to help other professionals with the journey also led to the formation of the Association for Healthcare Social Media, the first 501(c)(3) nonprofit professional organization devoted to health professionals on social media. There is tremendous opportunity to impact public health through social media, especially with regards to raising awareness about underrepresented conditions and presenting information that is accurate. Many barriers remain to the widespread adoption of social media by health professionals, such as the lack of financial or academic incentives. For now, there is every indication that social media is here to stay, and it will likely continue to play an important role in how we communicate with our patients.
AGA can be found online at @AmerGastroAssn (Facebook, Instagram, and Twitter) and @AGA_Gastro, @AGA_CGH, and @AGA_CMGH (Facebook and Twitter).
Dr. Chiang is assistant professor of medicine, division of gastroenterology & hepatology, director, endoscopic bariatric program, chief medical social media officer, Jefferson Health, Philadelphia, and president, Association for Healthcare Social Media, @austinchiangmd
I have always been a strong believer in meeting patients where they obtain their health information. Early in my clinical training, I realized that patients are exposed to health information through traditional media formats and, increasingly, social media, rather than brief clinical encounters. Unlike traditional media, social media allows individuals the opportunity to post information without a third-party filter. However, this opens the door for untrained individuals to spread misinformation and disinformation. In health care, this could potentially disrupt public health efforts. Even innocent mistakes like overlooking the appropriate clinical context can cause issues. Traditional media outlets also have agendas that may leave certain conditions, therapies, and other facets of health care underrepresented. My belief is that experts should therefore be trained and incentivized to be spokespeople for their own areas of expertise. Furthermore, social media provides a novel opportunity to improve health literacy while humanizing and restoring fading trust in health care.
There are several items to consider before initiating on one’s social media journey: whether you are committed to exploring the space, what one’s purpose is on social media, who the intended target audience is, which platform is most appropriate to serve that purpose and audience, and what potential pitfalls there may be.
The first question to ask oneself is whether you are prepared to devote time to cultivating a social media presence and speak or be heard publicly. Regardless of the platform, a social media presence requires consistency and audience interaction. The decision to partake can be personal; I view social media as an extension of in-person interaction, but not everyone is willing to commit to increased accessibility and visibility. Social media can still be valuable to those who choose to observe and learn rather than post.
Next is what one’s purpose is with being on social media. This can vary from peer education, boosting health literacy for patients, or using social media as a news source, networking tool, or a creative outlet. While my social media activity supports all these, my primary purpose is the distribution of accurate health information as a trained expert. When I started, I was one of few academic gastroenterologists uniquely positioned to bridge the elusive gap between the young, Gen Z crowd and academic medicine. Of similar importance is defining one’s target audience: patients, trainees, colleagues, or the general public.
Because there are numerous social media platforms, and only more to come in the future, it is critical to focus only on platforms that will serve one’s purpose and audience. Additionally, some may find more joy or agility in using one platform over the other. While I am one of the few clinicians who are adept at building communities across multiple rapidly evolving social media platforms, I will be the first to admit that it takes time to fully understand each platform with its ever-growing array of features. I find myself better at some platforms over others and, depending on my goals, I often will shift my focus from one to another.
Each platform has its pros and cons. Twitter is perhaps the most appropriate platform for starters. Easy to use with the least preparation necessary for every post, it also serves as the primary platform for academic discussion among all the popular social media platforms. Over the past few years, hundreds of gastroenterologists have become active on Twitter, which allows for ample networking opportunities and potential collaborations. The space has evolved to house various structured chats and learning opportunities as described by accounts like @MondayNightIBD, @ScopingSundays, #TracingTuesday, and @GIJournal. All major GI journals and societies are also present on Twitter and disseminating the latest information. Now a vestige of the past when text within tweets was not searchable, hashtags were used to curate discussion because searching by hashtag could reveal the latest discussion surrounding a topic and help identify others with a similar interest. Hashtags now remain relevant when crafting tweets, as the strategic inclusion of hashtags can help your content reach those who share an interest. A hashtag ontology was previously published to standardize academic conversation online in gastroenterology. Twitter also boasts features like polls that also help audiences engage.
Twitter has its disadvantages, however. Conversation is often siloed and difficult to reach audiences who don’t already follow you or others associated with you. Tweets disappear quickly in one’s feed and are often not seen by your followers. It lacks the visual appeal of other image- and video-based platforms that tend to attract more members of the general public. (Twitter lags behind these other platforms in monthly users) Other platforms like Facebook, Instagram, YouTube, LinkedIn, and TikTok have other benefits. Facebook may help foster community discussions in groups and business pages are also helpful for practice promotion. Instagram has gained popularity for educational purposes over the past 2 years, given its pairing with imagery and room for a lengthier caption. It has a variety of additional features like the temporary Instagram Stories that last 24 hours (which also allows for polling), question and answer, and livestream options. Other platforms like YouTube and TikTok have greater potential to reach audiences who otherwise would not see your content, with the former having the benefit of being highly searchable and the latter being the social media app with fastest growing popularity.
Having grown up with the Internet-based instant messaging and social media platforms, I have always enjoyed the medium as a way to connect with others. However, productive engagement on these platforms came much later. During a brief stint as part of the ABC News medical unit, I learned how Twitter was used to facilitate weekly chats around a specific topic online. I began exploring my own social media voice, which quickly gave way to live-tweeting medical conferences, hosting and participating Twitter chats myself, and guiding colleagues and professional societies to greater adoption of social media. In an attempt to introduce a divisional social media account during my fellowship, I learned of institutional barriers including antiquated policies that actively dissuaded social media use. I became increasingly involved on committees in our main GI societies after engaging in multiple research projects using social media data looking at how GI journals promote their content online, the associations between social media presence and institutional ranking, social media behavior at medical conferences, and the evolving perspectives of training program leadership regarding social media.
The pitfalls of social media remain a major concern for physicians and employers alike. First and foremost, it is important to review one’s institutional social media policy prior to starting, as individuals are ultimately held to their local policies. Not only can social media activity be a major liability for a health care employer, but also in the general public’s trust in health professionals. Protecting patient privacy and safety are of utmost concern, and physicians must be mindful not to inadvertently reveal patient identity. HIPAA violations are not limited to only naming patients by name or photo; descriptions of procedural cases and posting patient-related images such as radiographs or endoscopic images may reveal patient identity if there are unique details on these images (e.g., a radio-opaque necklace on x-ray or a particular swallowed foreign body).
Another disadvantage of social media is being approached with personal medical questions. I universally decline to answer these inquiries, citing the need to perform a comprehensive review of one’s medical chart and perform an in-person physical exam to fully assess a patient. The distinction between education and advice is subtle, yet important to recognize. Similarly, the need to uphold professionalism online is important. Short messages on social media can be misinterpreted by colleagues and the public. Not only can these interactions be potentially detrimental to one’s career, but it can further erode trust in health care if patients perceive this as fragmentation of the health care system. On platforms that encourage humor and creativity like TikTok, there have also been medical professionals and students publicly criticized and penalized for posting unprofessional content mocking patients.
With the introduction of social media influencers in recent years, some professionals have amassed followings, introducing yet another set of concerns. One is being approached with sponsorship and endorsement offers, as any agreements must be in accordance with institutional policy. As one’s following grows, there may be other concerns of safety both online and in real life. Online concerns include issues with impersonation and use of photos or written content without permission. On the surface this may not seem like a significant concern, but there have been situations where family photos are distributed to intended audiences or one’s likeness is used to endorse a product.
In addition to physical safety, another unintended consequence of social media use is its impact on one’s mental health. As social media tends to be a highlight reel, it is easy to be consumed by comparison with colleagues and their lives on social media, whether it truly reflects one’s actual life or not.
My ability to understand multiple social media platforms and anticipate a growing set of risks and concerns with using social media is what led to my involvement with multiple GI societies and appointment by my institution’s CEO to serve as the first chief medical social media officer. My desire to help other professionals with the journey also led to the formation of the Association for Healthcare Social Media, the first 501(c)(3) nonprofit professional organization devoted to health professionals on social media. There is tremendous opportunity to impact public health through social media, especially with regards to raising awareness about underrepresented conditions and presenting information that is accurate. Many barriers remain to the widespread adoption of social media by health professionals, such as the lack of financial or academic incentives. For now, there is every indication that social media is here to stay, and it will likely continue to play an important role in how we communicate with our patients.
AGA can be found online at @AmerGastroAssn (Facebook, Instagram, and Twitter) and @AGA_Gastro, @AGA_CGH, and @AGA_CMGH (Facebook and Twitter).
Dr. Chiang is assistant professor of medicine, division of gastroenterology & hepatology, director, endoscopic bariatric program, chief medical social media officer, Jefferson Health, Philadelphia, and president, Association for Healthcare Social Media, @austinchiangmd
I have always been a strong believer in meeting patients where they obtain their health information. Early in my clinical training, I realized that patients are exposed to health information through traditional media formats and, increasingly, social media, rather than brief clinical encounters. Unlike traditional media, social media allows individuals the opportunity to post information without a third-party filter. However, this opens the door for untrained individuals to spread misinformation and disinformation. In health care, this could potentially disrupt public health efforts. Even innocent mistakes like overlooking the appropriate clinical context can cause issues. Traditional media outlets also have agendas that may leave certain conditions, therapies, and other facets of health care underrepresented. My belief is that experts should therefore be trained and incentivized to be spokespeople for their own areas of expertise. Furthermore, social media provides a novel opportunity to improve health literacy while humanizing and restoring fading trust in health care.
There are several items to consider before initiating on one’s social media journey: whether you are committed to exploring the space, what one’s purpose is on social media, who the intended target audience is, which platform is most appropriate to serve that purpose and audience, and what potential pitfalls there may be.
The first question to ask oneself is whether you are prepared to devote time to cultivating a social media presence and speak or be heard publicly. Regardless of the platform, a social media presence requires consistency and audience interaction. The decision to partake can be personal; I view social media as an extension of in-person interaction, but not everyone is willing to commit to increased accessibility and visibility. Social media can still be valuable to those who choose to observe and learn rather than post.
Next is what one’s purpose is with being on social media. This can vary from peer education, boosting health literacy for patients, or using social media as a news source, networking tool, or a creative outlet. While my social media activity supports all these, my primary purpose is the distribution of accurate health information as a trained expert. When I started, I was one of few academic gastroenterologists uniquely positioned to bridge the elusive gap between the young, Gen Z crowd and academic medicine. Of similar importance is defining one’s target audience: patients, trainees, colleagues, or the general public.
Because there are numerous social media platforms, and only more to come in the future, it is critical to focus only on platforms that will serve one’s purpose and audience. Additionally, some may find more joy or agility in using one platform over the other. While I am one of the few clinicians who are adept at building communities across multiple rapidly evolving social media platforms, I will be the first to admit that it takes time to fully understand each platform with its ever-growing array of features. I find myself better at some platforms over others and, depending on my goals, I often will shift my focus from one to another.
Each platform has its pros and cons. Twitter is perhaps the most appropriate platform for starters. Easy to use with the least preparation necessary for every post, it also serves as the primary platform for academic discussion among all the popular social media platforms. Over the past few years, hundreds of gastroenterologists have become active on Twitter, which allows for ample networking opportunities and potential collaborations. The space has evolved to house various structured chats and learning opportunities as described by accounts like @MondayNightIBD, @ScopingSundays, #TracingTuesday, and @GIJournal. All major GI journals and societies are also present on Twitter and disseminating the latest information. Now a vestige of the past when text within tweets was not searchable, hashtags were used to curate discussion because searching by hashtag could reveal the latest discussion surrounding a topic and help identify others with a similar interest. Hashtags now remain relevant when crafting tweets, as the strategic inclusion of hashtags can help your content reach those who share an interest. A hashtag ontology was previously published to standardize academic conversation online in gastroenterology. Twitter also boasts features like polls that also help audiences engage.
Twitter has its disadvantages, however. Conversation is often siloed and difficult to reach audiences who don’t already follow you or others associated with you. Tweets disappear quickly in one’s feed and are often not seen by your followers. It lacks the visual appeal of other image- and video-based platforms that tend to attract more members of the general public. (Twitter lags behind these other platforms in monthly users) Other platforms like Facebook, Instagram, YouTube, LinkedIn, and TikTok have other benefits. Facebook may help foster community discussions in groups and business pages are also helpful for practice promotion. Instagram has gained popularity for educational purposes over the past 2 years, given its pairing with imagery and room for a lengthier caption. It has a variety of additional features like the temporary Instagram Stories that last 24 hours (which also allows for polling), question and answer, and livestream options. Other platforms like YouTube and TikTok have greater potential to reach audiences who otherwise would not see your content, with the former having the benefit of being highly searchable and the latter being the social media app with fastest growing popularity.
Having grown up with the Internet-based instant messaging and social media platforms, I have always enjoyed the medium as a way to connect with others. However, productive engagement on these platforms came much later. During a brief stint as part of the ABC News medical unit, I learned how Twitter was used to facilitate weekly chats around a specific topic online. I began exploring my own social media voice, which quickly gave way to live-tweeting medical conferences, hosting and participating Twitter chats myself, and guiding colleagues and professional societies to greater adoption of social media. In an attempt to introduce a divisional social media account during my fellowship, I learned of institutional barriers including antiquated policies that actively dissuaded social media use. I became increasingly involved on committees in our main GI societies after engaging in multiple research projects using social media data looking at how GI journals promote their content online, the associations between social media presence and institutional ranking, social media behavior at medical conferences, and the evolving perspectives of training program leadership regarding social media.
The pitfalls of social media remain a major concern for physicians and employers alike. First and foremost, it is important to review one’s institutional social media policy prior to starting, as individuals are ultimately held to their local policies. Not only can social media activity be a major liability for a health care employer, but also in the general public’s trust in health professionals. Protecting patient privacy and safety are of utmost concern, and physicians must be mindful not to inadvertently reveal patient identity. HIPAA violations are not limited to only naming patients by name or photo; descriptions of procedural cases and posting patient-related images such as radiographs or endoscopic images may reveal patient identity if there are unique details on these images (e.g., a radio-opaque necklace on x-ray or a particular swallowed foreign body).
Another disadvantage of social media is being approached with personal medical questions. I universally decline to answer these inquiries, citing the need to perform a comprehensive review of one’s medical chart and perform an in-person physical exam to fully assess a patient. The distinction between education and advice is subtle, yet important to recognize. Similarly, the need to uphold professionalism online is important. Short messages on social media can be misinterpreted by colleagues and the public. Not only can these interactions be potentially detrimental to one’s career, but it can further erode trust in health care if patients perceive this as fragmentation of the health care system. On platforms that encourage humor and creativity like TikTok, there have also been medical professionals and students publicly criticized and penalized for posting unprofessional content mocking patients.
With the introduction of social media influencers in recent years, some professionals have amassed followings, introducing yet another set of concerns. One is being approached with sponsorship and endorsement offers, as any agreements must be in accordance with institutional policy. As one’s following grows, there may be other concerns of safety both online and in real life. Online concerns include issues with impersonation and use of photos or written content without permission. On the surface this may not seem like a significant concern, but there have been situations where family photos are distributed to intended audiences or one’s likeness is used to endorse a product.
In addition to physical safety, another unintended consequence of social media use is its impact on one’s mental health. As social media tends to be a highlight reel, it is easy to be consumed by comparison with colleagues and their lives on social media, whether it truly reflects one’s actual life or not.
My ability to understand multiple social media platforms and anticipate a growing set of risks and concerns with using social media is what led to my involvement with multiple GI societies and appointment by my institution’s CEO to serve as the first chief medical social media officer. My desire to help other professionals with the journey also led to the formation of the Association for Healthcare Social Media, the first 501(c)(3) nonprofit professional organization devoted to health professionals on social media. There is tremendous opportunity to impact public health through social media, especially with regards to raising awareness about underrepresented conditions and presenting information that is accurate. Many barriers remain to the widespread adoption of social media by health professionals, such as the lack of financial or academic incentives. For now, there is every indication that social media is here to stay, and it will likely continue to play an important role in how we communicate with our patients.
AGA can be found online at @AmerGastroAssn (Facebook, Instagram, and Twitter) and @AGA_Gastro, @AGA_CGH, and @AGA_CMGH (Facebook and Twitter).
Dr. Chiang is assistant professor of medicine, division of gastroenterology & hepatology, director, endoscopic bariatric program, chief medical social media officer, Jefferson Health, Philadelphia, and president, Association for Healthcare Social Media, @austinchiangmd
Study highlights benefits of integrating dermatology into oncology centers
, according to the results of a retrospective study of 208 adults treated at the Dana-Farber Cancer Institute in Boston, or affiliated sites.
The benefits of prophylactic treatment for treatment-related skin rash in cancer patients are well established, based largely on the Skin Toxicity Evaluation Protocol With Panitumumab (STEPP) trial published in 2012, which led to the development of guidelines for preventing and managing skin toxicity associated with epidermal growth factor receptor inhibitor (EGFRi) treatment, wrote Zizi Yu of Harvard Medical School, Boston, and coauthors. However, they added, “awareness of and adherence to these guidelines among oncology clinicians are thus far poorly understood.” They pointed out that 90% of patients treated with an EGFRi develop cutaneous toxicities, which can affect quality of life, increase the risk of infection, and require dose modification, interruption, or discontinuation of treatment.
In the study, published in JAMA Dermatology, the researchers compared adherence to protocols at Dana-Farber before and after the 2014-2015 initiation of a Skin Toxicities from Anticancer Therapies (STAT) program at Dana-Farber established in 2014 by the department of dermatology.
The study population included 208 adult cancer patients with colorectal cancer, head and neck cancer, or cutaneous squamous cell cancer, treated with at least one dose of cetuximab (Erbitux); the average age of the patients was 62 years and the majority were men. Most had stage IV disease. The STAT program included the integration of 9 oncodermatologists in the head and neck, genitourinary, and cutaneous oncology clinics for 7 of 10 cancer treatment sessions per week, as well as the creation of urgent access time slots in oncodermatology clinics for 10 of 10 sessions per week.
Overall, significantly more patients were treated prophylactically for skin toxicity at the start of cetuximab treatment in 2017 vs. 2012 (47% vs. 25%, P less than .001) after the initiation of a dermatology protocol.
In addition, the preemptive use of tetracycline increased significantly from 45% to 71% (P = .02) between the two time periods, as did the use of topical corticosteroids (from 7% to 57%, P less than .001), while the use of topical antibiotics decreased from 79% to 43% (P = .02). Rates of dose changes or interruptions were significantly lower among those on prophylaxis (5% vs. 19%, P =.01), a 79% lower risk. Patients treated prophylactically were 94% less likely to need a first rescue treatment and 74% less likely to need a second rescue treatment for rash.
The study findings were limited by several factors including the retrospective design, use of data from a single institution, and incomplete documentation of some patients, the researchers noted. However, the results “highlight the value of integrating dermatologic care and education into oncology centers by increasing adherence to evidence-based prophylaxis protocols for rash and appropriate treatment agent selection, which may minimize toxicity-associated chemotherapy interruptions and improve quality of life,” they concluded.
“As novel cancer treatment options for patients continue to develop, and as patients with cancer live longer, the spectrum and prevalence of dermatologic toxic effects will continue to expand,” Bernice Y. Kwong, MD, director of the supportive dermato-oncology program at Stanford (Calif.) University, wrote in an accompanying editorial.
“Dermatologists have a critical and growing opportunity and role to engage in multidisciplinary efforts to provide expert guidance to best manage these cutaneous adverse events to achieve the best outcome for patients with cancer,” she said.
Although the prophylaxis rates at Dana-Farber improved after the establishment of the oncodermatology program, they remained relatively low, “underscoring an opportunity to improve on how to teach, execute, and improve access to oncodermatologic care for patients with cancer,” said Dr. Kwong. Knowledge gaps in the nature of skin toxicity for newer cancer drugs poses another challenge for skin toxicity management in these patients, she added.
However, “timely and consistent access to dermatologic expertise in oncology practices is critical to prevent unnecessary discontinuation of life-saving anticancer therapy, especially as multiple studies have demonstrated that anticancer therapy–associated skin toxicity may be associated with a positive response to anticancer therapy,” she emphasized.
Ms. Yu and one coauthor had no financial conflicts to disclose, the two other authors had several disclosures, outside of the submitted work. Dr. Kwong disclosed serving as a consultant for Genentech and Oncoderm and serving on the advisory board for Kyowa Kirin.
SOURCE: Yu Z et al. JAMA Dermatol. 2020 July 1. doi: 10.1001/jamadermatol.2020.1795. Kwong BY. JAMA Dermatol. 2020 Jul 1. doi: 10.1001/jamadermatol.2020.1794.
, according to the results of a retrospective study of 208 adults treated at the Dana-Farber Cancer Institute in Boston, or affiliated sites.
The benefits of prophylactic treatment for treatment-related skin rash in cancer patients are well established, based largely on the Skin Toxicity Evaluation Protocol With Panitumumab (STEPP) trial published in 2012, which led to the development of guidelines for preventing and managing skin toxicity associated with epidermal growth factor receptor inhibitor (EGFRi) treatment, wrote Zizi Yu of Harvard Medical School, Boston, and coauthors. However, they added, “awareness of and adherence to these guidelines among oncology clinicians are thus far poorly understood.” They pointed out that 90% of patients treated with an EGFRi develop cutaneous toxicities, which can affect quality of life, increase the risk of infection, and require dose modification, interruption, or discontinuation of treatment.
In the study, published in JAMA Dermatology, the researchers compared adherence to protocols at Dana-Farber before and after the 2014-2015 initiation of a Skin Toxicities from Anticancer Therapies (STAT) program at Dana-Farber established in 2014 by the department of dermatology.
The study population included 208 adult cancer patients with colorectal cancer, head and neck cancer, or cutaneous squamous cell cancer, treated with at least one dose of cetuximab (Erbitux); the average age of the patients was 62 years and the majority were men. Most had stage IV disease. The STAT program included the integration of 9 oncodermatologists in the head and neck, genitourinary, and cutaneous oncology clinics for 7 of 10 cancer treatment sessions per week, as well as the creation of urgent access time slots in oncodermatology clinics for 10 of 10 sessions per week.
Overall, significantly more patients were treated prophylactically for skin toxicity at the start of cetuximab treatment in 2017 vs. 2012 (47% vs. 25%, P less than .001) after the initiation of a dermatology protocol.
In addition, the preemptive use of tetracycline increased significantly from 45% to 71% (P = .02) between the two time periods, as did the use of topical corticosteroids (from 7% to 57%, P less than .001), while the use of topical antibiotics decreased from 79% to 43% (P = .02). Rates of dose changes or interruptions were significantly lower among those on prophylaxis (5% vs. 19%, P =.01), a 79% lower risk. Patients treated prophylactically were 94% less likely to need a first rescue treatment and 74% less likely to need a second rescue treatment for rash.
The study findings were limited by several factors including the retrospective design, use of data from a single institution, and incomplete documentation of some patients, the researchers noted. However, the results “highlight the value of integrating dermatologic care and education into oncology centers by increasing adherence to evidence-based prophylaxis protocols for rash and appropriate treatment agent selection, which may minimize toxicity-associated chemotherapy interruptions and improve quality of life,” they concluded.
“As novel cancer treatment options for patients continue to develop, and as patients with cancer live longer, the spectrum and prevalence of dermatologic toxic effects will continue to expand,” Bernice Y. Kwong, MD, director of the supportive dermato-oncology program at Stanford (Calif.) University, wrote in an accompanying editorial.
“Dermatologists have a critical and growing opportunity and role to engage in multidisciplinary efforts to provide expert guidance to best manage these cutaneous adverse events to achieve the best outcome for patients with cancer,” she said.
Although the prophylaxis rates at Dana-Farber improved after the establishment of the oncodermatology program, they remained relatively low, “underscoring an opportunity to improve on how to teach, execute, and improve access to oncodermatologic care for patients with cancer,” said Dr. Kwong. Knowledge gaps in the nature of skin toxicity for newer cancer drugs poses another challenge for skin toxicity management in these patients, she added.
However, “timely and consistent access to dermatologic expertise in oncology practices is critical to prevent unnecessary discontinuation of life-saving anticancer therapy, especially as multiple studies have demonstrated that anticancer therapy–associated skin toxicity may be associated with a positive response to anticancer therapy,” she emphasized.
Ms. Yu and one coauthor had no financial conflicts to disclose, the two other authors had several disclosures, outside of the submitted work. Dr. Kwong disclosed serving as a consultant for Genentech and Oncoderm and serving on the advisory board for Kyowa Kirin.
SOURCE: Yu Z et al. JAMA Dermatol. 2020 July 1. doi: 10.1001/jamadermatol.2020.1795. Kwong BY. JAMA Dermatol. 2020 Jul 1. doi: 10.1001/jamadermatol.2020.1794.
, according to the results of a retrospective study of 208 adults treated at the Dana-Farber Cancer Institute in Boston, or affiliated sites.
The benefits of prophylactic treatment for treatment-related skin rash in cancer patients are well established, based largely on the Skin Toxicity Evaluation Protocol With Panitumumab (STEPP) trial published in 2012, which led to the development of guidelines for preventing and managing skin toxicity associated with epidermal growth factor receptor inhibitor (EGFRi) treatment, wrote Zizi Yu of Harvard Medical School, Boston, and coauthors. However, they added, “awareness of and adherence to these guidelines among oncology clinicians are thus far poorly understood.” They pointed out that 90% of patients treated with an EGFRi develop cutaneous toxicities, which can affect quality of life, increase the risk of infection, and require dose modification, interruption, or discontinuation of treatment.
In the study, published in JAMA Dermatology, the researchers compared adherence to protocols at Dana-Farber before and after the 2014-2015 initiation of a Skin Toxicities from Anticancer Therapies (STAT) program at Dana-Farber established in 2014 by the department of dermatology.
The study population included 208 adult cancer patients with colorectal cancer, head and neck cancer, or cutaneous squamous cell cancer, treated with at least one dose of cetuximab (Erbitux); the average age of the patients was 62 years and the majority were men. Most had stage IV disease. The STAT program included the integration of 9 oncodermatologists in the head and neck, genitourinary, and cutaneous oncology clinics for 7 of 10 cancer treatment sessions per week, as well as the creation of urgent access time slots in oncodermatology clinics for 10 of 10 sessions per week.
Overall, significantly more patients were treated prophylactically for skin toxicity at the start of cetuximab treatment in 2017 vs. 2012 (47% vs. 25%, P less than .001) after the initiation of a dermatology protocol.
In addition, the preemptive use of tetracycline increased significantly from 45% to 71% (P = .02) between the two time periods, as did the use of topical corticosteroids (from 7% to 57%, P less than .001), while the use of topical antibiotics decreased from 79% to 43% (P = .02). Rates of dose changes or interruptions were significantly lower among those on prophylaxis (5% vs. 19%, P =.01), a 79% lower risk. Patients treated prophylactically were 94% less likely to need a first rescue treatment and 74% less likely to need a second rescue treatment for rash.
The study findings were limited by several factors including the retrospective design, use of data from a single institution, and incomplete documentation of some patients, the researchers noted. However, the results “highlight the value of integrating dermatologic care and education into oncology centers by increasing adherence to evidence-based prophylaxis protocols for rash and appropriate treatment agent selection, which may minimize toxicity-associated chemotherapy interruptions and improve quality of life,” they concluded.
“As novel cancer treatment options for patients continue to develop, and as patients with cancer live longer, the spectrum and prevalence of dermatologic toxic effects will continue to expand,” Bernice Y. Kwong, MD, director of the supportive dermato-oncology program at Stanford (Calif.) University, wrote in an accompanying editorial.
“Dermatologists have a critical and growing opportunity and role to engage in multidisciplinary efforts to provide expert guidance to best manage these cutaneous adverse events to achieve the best outcome for patients with cancer,” she said.
Although the prophylaxis rates at Dana-Farber improved after the establishment of the oncodermatology program, they remained relatively low, “underscoring an opportunity to improve on how to teach, execute, and improve access to oncodermatologic care for patients with cancer,” said Dr. Kwong. Knowledge gaps in the nature of skin toxicity for newer cancer drugs poses another challenge for skin toxicity management in these patients, she added.
However, “timely and consistent access to dermatologic expertise in oncology practices is critical to prevent unnecessary discontinuation of life-saving anticancer therapy, especially as multiple studies have demonstrated that anticancer therapy–associated skin toxicity may be associated with a positive response to anticancer therapy,” she emphasized.
Ms. Yu and one coauthor had no financial conflicts to disclose, the two other authors had several disclosures, outside of the submitted work. Dr. Kwong disclosed serving as a consultant for Genentech and Oncoderm and serving on the advisory board for Kyowa Kirin.
SOURCE: Yu Z et al. JAMA Dermatol. 2020 July 1. doi: 10.1001/jamadermatol.2020.1795. Kwong BY. JAMA Dermatol. 2020 Jul 1. doi: 10.1001/jamadermatol.2020.1794.
FROM JAMA DERMATOLOGY
Daily Recap: Lifestyle vs. genes in breast cancer showdown; Big pharma sues over insulin affordability law
Here are the stories our MDedge editors across specialties think you need to know about today:
Lifestyle choices may reduce breast cancer risk regardless of genetics
A “favorable” lifestyle was associated with a reduced risk of breast cancer even among women at high genetic risk for the disease in a study of more than 90,000 women, researchers reported.
The findings suggest that, regardless of genetic risk, women may be able to reduce their risk of developing breast cancer by getting adequate levels of exercise; maintaining a healthy weight; and limiting or eliminating use of alcohol, oral contraceptives, and hormone replacement therapy.
“These data should empower patients that they can impact on their overall health and reduce the risk of developing breast cancer,” said William Gradishar, MD, who was not invovled with the study. Read more.
Primary care practices may lose $68K per physician this year
Primary care practices stand to lose almost $68,000 per full-time physician this year as COVID-19 causes care delays and cancellations, researchers estimate. And while some outpatient care has started to rebound to near baseline appointment levels, other ambulatory specialties remain dramatically down from prepandemic rates.
Dermatology and rheumatology visits have recovered, but some specialties have cumulative deficits that are particularly concerning. For example, pediatric visits were down by 47% in the 3 months since March 15, and pulmonology visits were down 45% in that time.
This primary care estimate is without a potential second wave of COVID-19, noted Sanjay Basu, MD, director of research and population health at Collective Health in San Francisco, and colleagues.
“We expect ongoing turbulent times, so having a prospective payment could unleash the capacity for primary care practices to be creative in the way they care for their patients,” Daniel Horn, MD, director of population health and quality at Massachusetts General Hospital in Boston, said in an interview. Read more.
Big pharma sues to block Minnesota insulin affordability law
The Pharmaceutical Research and Manufacturers Association (PhRMA) is suing the state of Minnesota in an attempt to overturn a law that requires insulin makers to provide an emergency supply to individuals free of charge.
In the July 1 filing, PhRMA’s attorneys said the law is unconstitutional. It “order[s] pharmaceutical manufacturers to give insulin to state residents, on the state’s prescribed terms, at no charge to the recipients and without compensating the manufacturers in any way.”
The state has estimated that as many as 30,000 Minnesotans would be eligible for free insulin in the first year of the program. The drugmakers strenuously objected, noting that would mean they would “be compelled to provide 173,800 monthly supplies of free insulin” just in the first year.
“There is nothing in the U.S. Constitution that prevents states from saving the lives of its citizens who are in imminent danger,” said Mayo Clinic hematologist S. Vincent Rajkumar, MD. “The only motives for this lawsuit in my opinion are greed and the worry that other states may also choose to put lives of patients ahead of pharma profits.” Read more.
Despite guidelines, kids get opioids & steroids for pneumonia, sinusitis
A significant percentage of children receive opioids and systemic corticosteroids for pneumonia and sinusitis despite guidelines, according to an analysis of 2016 Medicaid data from South Carolina.
Prescriptions for these drugs were more likely after visits to EDs than after ambulatory visits, researchers reported in Pediatrics.
“Each of the 828 opioid and 2,737 systemic steroid prescriptions in the data set represent a potentially inappropriate prescription,” wrote Karina G. Phang, MD, MPH, of Geisinger Medical Center in Danville, Pa., and colleagues. “These rates appear excessive given that the use of these medications is not supported by available research or recommended in national guidelines.” Read more.
Study supports changing classification of RCC
The definition of stage IV renal cell carcinoma (RCC) should be expanded to include lymph node–positive stage III disease, according to a population-level cohort study published in Cancer.
While patients with lymph node–negative stage III disease had superior overall survival at 5 years, survival rates were similar between patients with node–positive stage III disease and stage IV disease. This supports reclassifying stage III node-positive RCC to stage IV, according to researchers.
“Prior institutional studies have indicated that, among patients with stage III disease, those with lymph node disease have worse oncologic outcomes and experience survival that is similar to that of patients with American Joint Committee on Cancer (AJCC) stage IV disease,” wrote Arnav Srivastava, MD, of Rutgers Cancer Institute of New Jersey, New Brunswick, and colleagues. Read more.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
Lifestyle choices may reduce breast cancer risk regardless of genetics
A “favorable” lifestyle was associated with a reduced risk of breast cancer even among women at high genetic risk for the disease in a study of more than 90,000 women, researchers reported.
The findings suggest that, regardless of genetic risk, women may be able to reduce their risk of developing breast cancer by getting adequate levels of exercise; maintaining a healthy weight; and limiting or eliminating use of alcohol, oral contraceptives, and hormone replacement therapy.
“These data should empower patients that they can impact on their overall health and reduce the risk of developing breast cancer,” said William Gradishar, MD, who was not invovled with the study. Read more.
Primary care practices may lose $68K per physician this year
Primary care practices stand to lose almost $68,000 per full-time physician this year as COVID-19 causes care delays and cancellations, researchers estimate. And while some outpatient care has started to rebound to near baseline appointment levels, other ambulatory specialties remain dramatically down from prepandemic rates.
Dermatology and rheumatology visits have recovered, but some specialties have cumulative deficits that are particularly concerning. For example, pediatric visits were down by 47% in the 3 months since March 15, and pulmonology visits were down 45% in that time.
This primary care estimate is without a potential second wave of COVID-19, noted Sanjay Basu, MD, director of research and population health at Collective Health in San Francisco, and colleagues.
“We expect ongoing turbulent times, so having a prospective payment could unleash the capacity for primary care practices to be creative in the way they care for their patients,” Daniel Horn, MD, director of population health and quality at Massachusetts General Hospital in Boston, said in an interview. Read more.
Big pharma sues to block Minnesota insulin affordability law
The Pharmaceutical Research and Manufacturers Association (PhRMA) is suing the state of Minnesota in an attempt to overturn a law that requires insulin makers to provide an emergency supply to individuals free of charge.
In the July 1 filing, PhRMA’s attorneys said the law is unconstitutional. It “order[s] pharmaceutical manufacturers to give insulin to state residents, on the state’s prescribed terms, at no charge to the recipients and without compensating the manufacturers in any way.”
The state has estimated that as many as 30,000 Minnesotans would be eligible for free insulin in the first year of the program. The drugmakers strenuously objected, noting that would mean they would “be compelled to provide 173,800 monthly supplies of free insulin” just in the first year.
“There is nothing in the U.S. Constitution that prevents states from saving the lives of its citizens who are in imminent danger,” said Mayo Clinic hematologist S. Vincent Rajkumar, MD. “The only motives for this lawsuit in my opinion are greed and the worry that other states may also choose to put lives of patients ahead of pharma profits.” Read more.
Despite guidelines, kids get opioids & steroids for pneumonia, sinusitis
A significant percentage of children receive opioids and systemic corticosteroids for pneumonia and sinusitis despite guidelines, according to an analysis of 2016 Medicaid data from South Carolina.
Prescriptions for these drugs were more likely after visits to EDs than after ambulatory visits, researchers reported in Pediatrics.
“Each of the 828 opioid and 2,737 systemic steroid prescriptions in the data set represent a potentially inappropriate prescription,” wrote Karina G. Phang, MD, MPH, of Geisinger Medical Center in Danville, Pa., and colleagues. “These rates appear excessive given that the use of these medications is not supported by available research or recommended in national guidelines.” Read more.
Study supports changing classification of RCC
The definition of stage IV renal cell carcinoma (RCC) should be expanded to include lymph node–positive stage III disease, according to a population-level cohort study published in Cancer.
While patients with lymph node–negative stage III disease had superior overall survival at 5 years, survival rates were similar between patients with node–positive stage III disease and stage IV disease. This supports reclassifying stage III node-positive RCC to stage IV, according to researchers.
“Prior institutional studies have indicated that, among patients with stage III disease, those with lymph node disease have worse oncologic outcomes and experience survival that is similar to that of patients with American Joint Committee on Cancer (AJCC) stage IV disease,” wrote Arnav Srivastava, MD, of Rutgers Cancer Institute of New Jersey, New Brunswick, and colleagues. Read more.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
Lifestyle choices may reduce breast cancer risk regardless of genetics
A “favorable” lifestyle was associated with a reduced risk of breast cancer even among women at high genetic risk for the disease in a study of more than 90,000 women, researchers reported.
The findings suggest that, regardless of genetic risk, women may be able to reduce their risk of developing breast cancer by getting adequate levels of exercise; maintaining a healthy weight; and limiting or eliminating use of alcohol, oral contraceptives, and hormone replacement therapy.
“These data should empower patients that they can impact on their overall health and reduce the risk of developing breast cancer,” said William Gradishar, MD, who was not invovled with the study. Read more.
Primary care practices may lose $68K per physician this year
Primary care practices stand to lose almost $68,000 per full-time physician this year as COVID-19 causes care delays and cancellations, researchers estimate. And while some outpatient care has started to rebound to near baseline appointment levels, other ambulatory specialties remain dramatically down from prepandemic rates.
Dermatology and rheumatology visits have recovered, but some specialties have cumulative deficits that are particularly concerning. For example, pediatric visits were down by 47% in the 3 months since March 15, and pulmonology visits were down 45% in that time.
This primary care estimate is without a potential second wave of COVID-19, noted Sanjay Basu, MD, director of research and population health at Collective Health in San Francisco, and colleagues.
“We expect ongoing turbulent times, so having a prospective payment could unleash the capacity for primary care practices to be creative in the way they care for their patients,” Daniel Horn, MD, director of population health and quality at Massachusetts General Hospital in Boston, said in an interview. Read more.
Big pharma sues to block Minnesota insulin affordability law
The Pharmaceutical Research and Manufacturers Association (PhRMA) is suing the state of Minnesota in an attempt to overturn a law that requires insulin makers to provide an emergency supply to individuals free of charge.
In the July 1 filing, PhRMA’s attorneys said the law is unconstitutional. It “order[s] pharmaceutical manufacturers to give insulin to state residents, on the state’s prescribed terms, at no charge to the recipients and without compensating the manufacturers in any way.”
The state has estimated that as many as 30,000 Minnesotans would be eligible for free insulin in the first year of the program. The drugmakers strenuously objected, noting that would mean they would “be compelled to provide 173,800 monthly supplies of free insulin” just in the first year.
“There is nothing in the U.S. Constitution that prevents states from saving the lives of its citizens who are in imminent danger,” said Mayo Clinic hematologist S. Vincent Rajkumar, MD. “The only motives for this lawsuit in my opinion are greed and the worry that other states may also choose to put lives of patients ahead of pharma profits.” Read more.
Despite guidelines, kids get opioids & steroids for pneumonia, sinusitis
A significant percentage of children receive opioids and systemic corticosteroids for pneumonia and sinusitis despite guidelines, according to an analysis of 2016 Medicaid data from South Carolina.
Prescriptions for these drugs were more likely after visits to EDs than after ambulatory visits, researchers reported in Pediatrics.
“Each of the 828 opioid and 2,737 systemic steroid prescriptions in the data set represent a potentially inappropriate prescription,” wrote Karina G. Phang, MD, MPH, of Geisinger Medical Center in Danville, Pa., and colleagues. “These rates appear excessive given that the use of these medications is not supported by available research or recommended in national guidelines.” Read more.
Study supports changing classification of RCC
The definition of stage IV renal cell carcinoma (RCC) should be expanded to include lymph node–positive stage III disease, according to a population-level cohort study published in Cancer.
While patients with lymph node–negative stage III disease had superior overall survival at 5 years, survival rates were similar between patients with node–positive stage III disease and stage IV disease. This supports reclassifying stage III node-positive RCC to stage IV, according to researchers.
“Prior institutional studies have indicated that, among patients with stage III disease, those with lymph node disease have worse oncologic outcomes and experience survival that is similar to that of patients with American Joint Committee on Cancer (AJCC) stage IV disease,” wrote Arnav Srivastava, MD, of Rutgers Cancer Institute of New Jersey, New Brunswick, and colleagues. Read more.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Dr. Eric E. Howell assumes new role as CEO of SHM
The Society of Hospital Medicine officially welcomed Eric E. Howell, MD, MHM, as chief executive officer on July 1, 2020. Dr. Howell reports to the Society of Hospital Medicine board of directors and is tasked with ensuring that SHM continues to serve the evolving needs and interests of its members while overseeing the organization’s strategic direction.
“The SHM board of directors is excited to work with Dr. Howell to navigate the future of SHM and of the hospital medicine specialty,” said Danielle Scheurer, MD, MSCR, SFHM, SHM president and chair of the CEO Search Committee. “With his extensive knowledge of the health care landscape and of SHM, Dr. Howell embodies the society’s dedication to empowering hospitalists to be positive change agents in their institutions and in the health care system as a whole.”
Prior to his current role, Dr. Howell served as chief operating officer of SHM for 2 years; in that role, he led senior management’s planning and defined organizational goals to drive growth. As the senior physician adviser to SHM’s Center for Quality Improvement for 5 years, he consulted for the society’s arm that conducts quality improvement programs for hospitalist teams. In addition to being a past president of SHM’s board of directors, he is the course director for the SHM Leadership Academies.
“Now more than ever, SHM has an opportunity to superserve hospitalists and the patients they serve, and I couldn’t be more excited to lead the society into its next chapter,” Dr. Howell said. “Supported by a dedicated member base and innovative staff, I am confident that SHM will continue on its successful path forward and will provide its members with the products, services, and tools that hospitalists need to improve patient care and adapt to the constantly evolving environment.”
In addition to serving in various capacities at SHM, Dr. Howell has served as a professor of medicine in the department of medicine at Johns Hopkins University, Baltimore. He has held multiple titles within the Johns Hopkins medical institutions, including chief of the division of hospital medicine at Johns Hopkins Bayview, section chief of hospital medicine for Johns Hopkins Community Physicians, deputy director of hospital operations for the department of medicine at Johns Hopkins Bayview Medical Center, and chief medical officer of operations at Johns Hopkins Bayview. Dr. Howell joined the Johns Hopkins Bayview hospitalist program in 2000, began the Howard County (Md.) General Hospital hospitalist program in 2010, and oversaw nearly 200 physicians and clinical staff providing patient care in three hospitals. Along with his role as SHM CEO, he will remain a member of the adjunct faculty at Johns Hopkins University.
More recently, Dr. Howell served as chief medical officer for the Baltimore Convention Center Field Hospital, a fully functional, 250-bed hospital created to care for patients in the Baltimore metropolitan area who were suffering from complications from COVID-19.
Dr. Howell received his electrical engineering degree from the University of Maryland, College Park, which has proven instrumental in his mastery of managing and implementing change in the hospital. His research has focused on the relationship between the emergency department and medicine floors, improving communication, throughput and patient outcomes.
The nationwide search process that led to Dr. Howell’s appointment was led by a CEO Search Committee, which included members of the SHM board of directors and was assisted by the executive search firm Spencer Stuart.
Dr. Howell succeeds Laurence Wellikson, MD, MHM, who helped in founding the Society of Hospital Medicine, its first and only CEO since 2000 prior to Dr. Howell’s appointment.
The Society of Hospital Medicine officially welcomed Eric E. Howell, MD, MHM, as chief executive officer on July 1, 2020. Dr. Howell reports to the Society of Hospital Medicine board of directors and is tasked with ensuring that SHM continues to serve the evolving needs and interests of its members while overseeing the organization’s strategic direction.
“The SHM board of directors is excited to work with Dr. Howell to navigate the future of SHM and of the hospital medicine specialty,” said Danielle Scheurer, MD, MSCR, SFHM, SHM president and chair of the CEO Search Committee. “With his extensive knowledge of the health care landscape and of SHM, Dr. Howell embodies the society’s dedication to empowering hospitalists to be positive change agents in their institutions and in the health care system as a whole.”
Prior to his current role, Dr. Howell served as chief operating officer of SHM for 2 years; in that role, he led senior management’s planning and defined organizational goals to drive growth. As the senior physician adviser to SHM’s Center for Quality Improvement for 5 years, he consulted for the society’s arm that conducts quality improvement programs for hospitalist teams. In addition to being a past president of SHM’s board of directors, he is the course director for the SHM Leadership Academies.
“Now more than ever, SHM has an opportunity to superserve hospitalists and the patients they serve, and I couldn’t be more excited to lead the society into its next chapter,” Dr. Howell said. “Supported by a dedicated member base and innovative staff, I am confident that SHM will continue on its successful path forward and will provide its members with the products, services, and tools that hospitalists need to improve patient care and adapt to the constantly evolving environment.”
In addition to serving in various capacities at SHM, Dr. Howell has served as a professor of medicine in the department of medicine at Johns Hopkins University, Baltimore. He has held multiple titles within the Johns Hopkins medical institutions, including chief of the division of hospital medicine at Johns Hopkins Bayview, section chief of hospital medicine for Johns Hopkins Community Physicians, deputy director of hospital operations for the department of medicine at Johns Hopkins Bayview Medical Center, and chief medical officer of operations at Johns Hopkins Bayview. Dr. Howell joined the Johns Hopkins Bayview hospitalist program in 2000, began the Howard County (Md.) General Hospital hospitalist program in 2010, and oversaw nearly 200 physicians and clinical staff providing patient care in three hospitals. Along with his role as SHM CEO, he will remain a member of the adjunct faculty at Johns Hopkins University.
More recently, Dr. Howell served as chief medical officer for the Baltimore Convention Center Field Hospital, a fully functional, 250-bed hospital created to care for patients in the Baltimore metropolitan area who were suffering from complications from COVID-19.
Dr. Howell received his electrical engineering degree from the University of Maryland, College Park, which has proven instrumental in his mastery of managing and implementing change in the hospital. His research has focused on the relationship between the emergency department and medicine floors, improving communication, throughput and patient outcomes.
The nationwide search process that led to Dr. Howell’s appointment was led by a CEO Search Committee, which included members of the SHM board of directors and was assisted by the executive search firm Spencer Stuart.
Dr. Howell succeeds Laurence Wellikson, MD, MHM, who helped in founding the Society of Hospital Medicine, its first and only CEO since 2000 prior to Dr. Howell’s appointment.
The Society of Hospital Medicine officially welcomed Eric E. Howell, MD, MHM, as chief executive officer on July 1, 2020. Dr. Howell reports to the Society of Hospital Medicine board of directors and is tasked with ensuring that SHM continues to serve the evolving needs and interests of its members while overseeing the organization’s strategic direction.
“The SHM board of directors is excited to work with Dr. Howell to navigate the future of SHM and of the hospital medicine specialty,” said Danielle Scheurer, MD, MSCR, SFHM, SHM president and chair of the CEO Search Committee. “With his extensive knowledge of the health care landscape and of SHM, Dr. Howell embodies the society’s dedication to empowering hospitalists to be positive change agents in their institutions and in the health care system as a whole.”
Prior to his current role, Dr. Howell served as chief operating officer of SHM for 2 years; in that role, he led senior management’s planning and defined organizational goals to drive growth. As the senior physician adviser to SHM’s Center for Quality Improvement for 5 years, he consulted for the society’s arm that conducts quality improvement programs for hospitalist teams. In addition to being a past president of SHM’s board of directors, he is the course director for the SHM Leadership Academies.
“Now more than ever, SHM has an opportunity to superserve hospitalists and the patients they serve, and I couldn’t be more excited to lead the society into its next chapter,” Dr. Howell said. “Supported by a dedicated member base and innovative staff, I am confident that SHM will continue on its successful path forward and will provide its members with the products, services, and tools that hospitalists need to improve patient care and adapt to the constantly evolving environment.”
In addition to serving in various capacities at SHM, Dr. Howell has served as a professor of medicine in the department of medicine at Johns Hopkins University, Baltimore. He has held multiple titles within the Johns Hopkins medical institutions, including chief of the division of hospital medicine at Johns Hopkins Bayview, section chief of hospital medicine for Johns Hopkins Community Physicians, deputy director of hospital operations for the department of medicine at Johns Hopkins Bayview Medical Center, and chief medical officer of operations at Johns Hopkins Bayview. Dr. Howell joined the Johns Hopkins Bayview hospitalist program in 2000, began the Howard County (Md.) General Hospital hospitalist program in 2010, and oversaw nearly 200 physicians and clinical staff providing patient care in three hospitals. Along with his role as SHM CEO, he will remain a member of the adjunct faculty at Johns Hopkins University.
More recently, Dr. Howell served as chief medical officer for the Baltimore Convention Center Field Hospital, a fully functional, 250-bed hospital created to care for patients in the Baltimore metropolitan area who were suffering from complications from COVID-19.
Dr. Howell received his electrical engineering degree from the University of Maryland, College Park, which has proven instrumental in his mastery of managing and implementing change in the hospital. His research has focused on the relationship between the emergency department and medicine floors, improving communication, throughput and patient outcomes.
The nationwide search process that led to Dr. Howell’s appointment was led by a CEO Search Committee, which included members of the SHM board of directors and was assisted by the executive search firm Spencer Stuart.
Dr. Howell succeeds Laurence Wellikson, MD, MHM, who helped in founding the Society of Hospital Medicine, its first and only CEO since 2000 prior to Dr. Howell’s appointment.
Daily Recap: Migraine affects pregnancy planning; FDA okays urothelial carcinoma therapy
Here are the stories our MDedge editors across specialties think you need to know about today:
Migraine is often a deciding factor in pregnancy planning
Migraine can significantly influence a woman’s decision to have children, new research shows.
Results from a multicenter study of more than 600 women showed that, among participants with migraine, those who were younger, had menstrual migraine, or had chronic migraine were more likely to decide to not become pregnant.
“Women who avoided pregnancy due to migraine were most concerned that migraine would make raising a child difficult, that the migraine medications they take would have a negative impact on their child’s development, and that their migraine pattern would worsen during or just after pregnancy,” said study investigator Ryotaro Ishii, MD, PhD, a visiting scientist at Mayo Clinic in Phoenix.
The findings were presented at the virtual annual meeting of the American Headache Society. Read more.
FDA approves avelumab as maintenance for urothelial carcinoma
The Food and Drug Administration has approved avelumab (Bavencio) as a maintenance treatment for patients with locally advanced or metastatic urothelial carcinoma (UC) that has not progressed after first-line platinum-containing chemotherapy.
The new maintenance therapy indication for avelumab is based on efficacy demonstrated in the JAVELIN Bladder 100 trial. Results from this trial were presented as part of the American Society of Clinical Oncology virtual scientific program.
The new indication adds to avelumab use in other patient populations, including people with locally advanced or metastatic UC who experience disease progression during or following platinum-containing chemotherapy. The FDA also previously approved avelumab for patients who experienced UC progression within 12 months of neoadjuvant or adjuvant treatment with platinum-containing chemotherapy. The FDA first approved marketing of avelumab in 2017. Other uses include treatment of metastatic Merkel cell carcinoma and first-line treatment of advanced renal cell carcinoma in combination with axitinib. Read more.
Lifestyle changes may explain skin lesions in pandemic-era patients
Two European prospective case series found no direct association between skin lesions on the hands and feet and SARS-CoV-2 in young people, which raises questions about other contributing factors, such as lockdown conditions, which may be clarified with additional research. The study appeared in JAMA Dermatology.
Meanwhile, data from the American Academy of Dermatology and a recent paper from the British Journal of Dermatology suggest a real association exists, at in least some patients.
“It’s going to be true that most patients with toe lesions are PCR [polymerase chain reaction]-negative because it tends to be a late phenomenon when patients are no longer shedding virus,” explained Lindy P. Fox, MD, professor of dermatology at the University of California, San Francisco, who was not an author of either study. Read more.
Take-home test strips allow drug users to detect fentanyl
Illicit drug users seem to overwhelmingly appreciate being able to use take-home test strips to detect the presence of dangerous fentanyl in opioids and other drugs, a new study finds.
More than 95% said they’d use the inexpensive strips again.
“These tests accurately detect fentanyl in the drug supply, and they can be a valuable addition to other drug prevention strategies,” the study’s lead author and addiction medicine specialist Sukhpreet Klaire, MD, of the British Columbia Center on Substance Use in Vancouver, said in an interview.
Dr. Klaire presented the study findings at the virtual annual meeting of the College on Problems of Drug Dependence. Read more.
New data back use of medical cannabis for epilepsy, pain, anxiety
Two new studies offer positive news about medical cannabis, suggesting that marijuana products improve physical and cognitive symptoms, boost quality of life, and rarely produce signs of problematic use.
In one study, patients with epilepsy who used medical cannabis were nearly half as likely to have needed an emergency department visit within the last 30 days as was a control group. In the other study, just 3 of 54 subjects who used medical cannabis showed signs of possible cannabis use disorder (CUD) over 12 months.
The findings show that “there is improvement in a range of outcome variables, and the adverse effects seem to be minimal, compared to what we might have hypothesized based on the bulk of the literature on the negative effects of cannabis on health outcomes,” cannabis researcher Ziva Cooper, PhD, of the University of California at Los Angeles, said in an interview. Dr. Cooper moderated a session about the studies at the virtual annual meeting of the College on Problems of Drug Dependence. Read more.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
Migraine is often a deciding factor in pregnancy planning
Migraine can significantly influence a woman’s decision to have children, new research shows.
Results from a multicenter study of more than 600 women showed that, among participants with migraine, those who were younger, had menstrual migraine, or had chronic migraine were more likely to decide to not become pregnant.
“Women who avoided pregnancy due to migraine were most concerned that migraine would make raising a child difficult, that the migraine medications they take would have a negative impact on their child’s development, and that their migraine pattern would worsen during or just after pregnancy,” said study investigator Ryotaro Ishii, MD, PhD, a visiting scientist at Mayo Clinic in Phoenix.
The findings were presented at the virtual annual meeting of the American Headache Society. Read more.
FDA approves avelumab as maintenance for urothelial carcinoma
The Food and Drug Administration has approved avelumab (Bavencio) as a maintenance treatment for patients with locally advanced or metastatic urothelial carcinoma (UC) that has not progressed after first-line platinum-containing chemotherapy.
The new maintenance therapy indication for avelumab is based on efficacy demonstrated in the JAVELIN Bladder 100 trial. Results from this trial were presented as part of the American Society of Clinical Oncology virtual scientific program.
The new indication adds to avelumab use in other patient populations, including people with locally advanced or metastatic UC who experience disease progression during or following platinum-containing chemotherapy. The FDA also previously approved avelumab for patients who experienced UC progression within 12 months of neoadjuvant or adjuvant treatment with platinum-containing chemotherapy. The FDA first approved marketing of avelumab in 2017. Other uses include treatment of metastatic Merkel cell carcinoma and first-line treatment of advanced renal cell carcinoma in combination with axitinib. Read more.
Lifestyle changes may explain skin lesions in pandemic-era patients
Two European prospective case series found no direct association between skin lesions on the hands and feet and SARS-CoV-2 in young people, which raises questions about other contributing factors, such as lockdown conditions, which may be clarified with additional research. The study appeared in JAMA Dermatology.
Meanwhile, data from the American Academy of Dermatology and a recent paper from the British Journal of Dermatology suggest a real association exists, at in least some patients.
“It’s going to be true that most patients with toe lesions are PCR [polymerase chain reaction]-negative because it tends to be a late phenomenon when patients are no longer shedding virus,” explained Lindy P. Fox, MD, professor of dermatology at the University of California, San Francisco, who was not an author of either study. Read more.
Take-home test strips allow drug users to detect fentanyl
Illicit drug users seem to overwhelmingly appreciate being able to use take-home test strips to detect the presence of dangerous fentanyl in opioids and other drugs, a new study finds.
More than 95% said they’d use the inexpensive strips again.
“These tests accurately detect fentanyl in the drug supply, and they can be a valuable addition to other drug prevention strategies,” the study’s lead author and addiction medicine specialist Sukhpreet Klaire, MD, of the British Columbia Center on Substance Use in Vancouver, said in an interview.
Dr. Klaire presented the study findings at the virtual annual meeting of the College on Problems of Drug Dependence. Read more.
New data back use of medical cannabis for epilepsy, pain, anxiety
Two new studies offer positive news about medical cannabis, suggesting that marijuana products improve physical and cognitive symptoms, boost quality of life, and rarely produce signs of problematic use.
In one study, patients with epilepsy who used medical cannabis were nearly half as likely to have needed an emergency department visit within the last 30 days as was a control group. In the other study, just 3 of 54 subjects who used medical cannabis showed signs of possible cannabis use disorder (CUD) over 12 months.
The findings show that “there is improvement in a range of outcome variables, and the adverse effects seem to be minimal, compared to what we might have hypothesized based on the bulk of the literature on the negative effects of cannabis on health outcomes,” cannabis researcher Ziva Cooper, PhD, of the University of California at Los Angeles, said in an interview. Dr. Cooper moderated a session about the studies at the virtual annual meeting of the College on Problems of Drug Dependence. Read more.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
Migraine is often a deciding factor in pregnancy planning
Migraine can significantly influence a woman’s decision to have children, new research shows.
Results from a multicenter study of more than 600 women showed that, among participants with migraine, those who were younger, had menstrual migraine, or had chronic migraine were more likely to decide to not become pregnant.
“Women who avoided pregnancy due to migraine were most concerned that migraine would make raising a child difficult, that the migraine medications they take would have a negative impact on their child’s development, and that their migraine pattern would worsen during or just after pregnancy,” said study investigator Ryotaro Ishii, MD, PhD, a visiting scientist at Mayo Clinic in Phoenix.
The findings were presented at the virtual annual meeting of the American Headache Society. Read more.
FDA approves avelumab as maintenance for urothelial carcinoma
The Food and Drug Administration has approved avelumab (Bavencio) as a maintenance treatment for patients with locally advanced or metastatic urothelial carcinoma (UC) that has not progressed after first-line platinum-containing chemotherapy.
The new maintenance therapy indication for avelumab is based on efficacy demonstrated in the JAVELIN Bladder 100 trial. Results from this trial were presented as part of the American Society of Clinical Oncology virtual scientific program.
The new indication adds to avelumab use in other patient populations, including people with locally advanced or metastatic UC who experience disease progression during or following platinum-containing chemotherapy. The FDA also previously approved avelumab for patients who experienced UC progression within 12 months of neoadjuvant or adjuvant treatment with platinum-containing chemotherapy. The FDA first approved marketing of avelumab in 2017. Other uses include treatment of metastatic Merkel cell carcinoma and first-line treatment of advanced renal cell carcinoma in combination with axitinib. Read more.
Lifestyle changes may explain skin lesions in pandemic-era patients
Two European prospective case series found no direct association between skin lesions on the hands and feet and SARS-CoV-2 in young people, which raises questions about other contributing factors, such as lockdown conditions, which may be clarified with additional research. The study appeared in JAMA Dermatology.
Meanwhile, data from the American Academy of Dermatology and a recent paper from the British Journal of Dermatology suggest a real association exists, at in least some patients.
“It’s going to be true that most patients with toe lesions are PCR [polymerase chain reaction]-negative because it tends to be a late phenomenon when patients are no longer shedding virus,” explained Lindy P. Fox, MD, professor of dermatology at the University of California, San Francisco, who was not an author of either study. Read more.
Take-home test strips allow drug users to detect fentanyl
Illicit drug users seem to overwhelmingly appreciate being able to use take-home test strips to detect the presence of dangerous fentanyl in opioids and other drugs, a new study finds.
More than 95% said they’d use the inexpensive strips again.
“These tests accurately detect fentanyl in the drug supply, and they can be a valuable addition to other drug prevention strategies,” the study’s lead author and addiction medicine specialist Sukhpreet Klaire, MD, of the British Columbia Center on Substance Use in Vancouver, said in an interview.
Dr. Klaire presented the study findings at the virtual annual meeting of the College on Problems of Drug Dependence. Read more.
New data back use of medical cannabis for epilepsy, pain, anxiety
Two new studies offer positive news about medical cannabis, suggesting that marijuana products improve physical and cognitive symptoms, boost quality of life, and rarely produce signs of problematic use.
In one study, patients with epilepsy who used medical cannabis were nearly half as likely to have needed an emergency department visit within the last 30 days as was a control group. In the other study, just 3 of 54 subjects who used medical cannabis showed signs of possible cannabis use disorder (CUD) over 12 months.
The findings show that “there is improvement in a range of outcome variables, and the adverse effects seem to be minimal, compared to what we might have hypothesized based on the bulk of the literature on the negative effects of cannabis on health outcomes,” cannabis researcher Ziva Cooper, PhD, of the University of California at Los Angeles, said in an interview. Dr. Cooper moderated a session about the studies at the virtual annual meeting of the College on Problems of Drug Dependence. Read more.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Worrisome health disparities among transgender adults
Background: The transgender population historically has not been identified in population research. Little is known about their health care needs.
Study design: Survey review.
Setting: Large, continuously operative health survey.
Synopsis: The Centers for Disease Control and Prevention added an optional Sexual Orientation and Gender Identity module to the Behavioral Risk Factor Surveillance System in 2014. Compared with non–transgender responders, transgender adults (0.55% of responders) were more likely to report “fair” or “poor” health status (24.5% vs. 18.2%), were more likely to have experienced severe mental distress in the last 30 days (20.3% vs. 11.6), and were more likely to be physically inactive (35% vs. 25.6%), smoke cigarettes (19.2% vs. 16.3%), and lack health care coverage (20.1% vs. 14.6%).
Bottom line: Transgender adults report worse physical and mental health status. Physicians should consider these disparities during screening and treatment.
Citation: Baker K. Findings from the Behavioral Risk Factor Surveillance System on health-related quality of life among U.S. transgender adults, 2014-2017. JAMA Intern Med. 2019 Apr 22. doi: 10.1001/jamainternmed.2018.7931.
Dr. Hoegh is a hospitalist at the University of Colorado at Denver, Aurora.
Background: The transgender population historically has not been identified in population research. Little is known about their health care needs.
Study design: Survey review.
Setting: Large, continuously operative health survey.
Synopsis: The Centers for Disease Control and Prevention added an optional Sexual Orientation and Gender Identity module to the Behavioral Risk Factor Surveillance System in 2014. Compared with non–transgender responders, transgender adults (0.55% of responders) were more likely to report “fair” or “poor” health status (24.5% vs. 18.2%), were more likely to have experienced severe mental distress in the last 30 days (20.3% vs. 11.6), and were more likely to be physically inactive (35% vs. 25.6%), smoke cigarettes (19.2% vs. 16.3%), and lack health care coverage (20.1% vs. 14.6%).
Bottom line: Transgender adults report worse physical and mental health status. Physicians should consider these disparities during screening and treatment.
Citation: Baker K. Findings from the Behavioral Risk Factor Surveillance System on health-related quality of life among U.S. transgender adults, 2014-2017. JAMA Intern Med. 2019 Apr 22. doi: 10.1001/jamainternmed.2018.7931.
Dr. Hoegh is a hospitalist at the University of Colorado at Denver, Aurora.
Background: The transgender population historically has not been identified in population research. Little is known about their health care needs.
Study design: Survey review.
Setting: Large, continuously operative health survey.
Synopsis: The Centers for Disease Control and Prevention added an optional Sexual Orientation and Gender Identity module to the Behavioral Risk Factor Surveillance System in 2014. Compared with non–transgender responders, transgender adults (0.55% of responders) were more likely to report “fair” or “poor” health status (24.5% vs. 18.2%), were more likely to have experienced severe mental distress in the last 30 days (20.3% vs. 11.6), and were more likely to be physically inactive (35% vs. 25.6%), smoke cigarettes (19.2% vs. 16.3%), and lack health care coverage (20.1% vs. 14.6%).
Bottom line: Transgender adults report worse physical and mental health status. Physicians should consider these disparities during screening and treatment.
Citation: Baker K. Findings from the Behavioral Risk Factor Surveillance System on health-related quality of life among U.S. transgender adults, 2014-2017. JAMA Intern Med. 2019 Apr 22. doi: 10.1001/jamainternmed.2018.7931.
Dr. Hoegh is a hospitalist at the University of Colorado at Denver, Aurora.
Navigating a pandemic: The importance of preparedness in independent GI practices
It was early March, and our second day of advocacy on Capitol Hill with the Digestive Health Physicians Association (DHPA) was cut short when congressional offices were shuttered because of the COVID-19 pandemic. Sitting with several of my GI physician colleagues from across the country, we knew that our practices, our patients, and our communities would be impacted by the coronavirus. None of us could have known the extent.
We also didn’t know in that moment that our advocacy work through DHPA would be one of the most important factors in ensuring that our practices were prepared to weather the pandemic. Our membership, legal counsel, and legislative lobbyists helped us remain informed about new legislation and regulations and ensured that we had much-needed access to government resources.
Just a few months into what is now the COVID-19 pandemic, independent GI practice leaders have learned a lot about how to strengthen our practices to respond to future crises – and what early-career GIs should look for in the practices they are considering.
First and foremost, practice leadership is key. One thing most successful GI practices have in common is that they hire really smart executives and administrative teams who excel at taking care of the business side of things so that physicians like me can do what we do best: treat patients.
Stay informed about state and federal policies
As a member of DHPA, Capital Digestive Care was well positioned to keep up to date on the government response to the coronavirus and the support it provided to small businesses and to health care providers.
Over the past 5 years, DHPA physician leaders have established strong relationships with our elected federal leaders. During our Capitol Hill visits in early March, we discussed the coronavirus in addition to our policy priorities.
The relationships we’ve built with policymakers have helped us educate them about how private practices were being affected and make the case that it was crucial to include private practices in health care stimulus packages.
Without this federal financial support, many medical groups may have had to close their doors – leaving a large gap in care once the pandemic subsides.
In addition to the federal government’s financial support, our policy advocacy efforts kept us informed about federal health agencies’ decisions on telehealth coverage. We were able to educate our physicians and staff about state and federal adjustments to telehealth rules for the pandemic, on the guidelines for elective procedures, on employee furlough and leave rules, as well as other congressional and state actions that would impact our practice.
You can’t be an independent physician without being open to learning about the business of health care and understanding how health policies affect your ability to practice medicine and care for people in your community. Every early-career physician who is looking to join a practice should ask how its leadership remains informed about health policy at the state and federal levels.
Make plans, be flexible
Implementing telehealth was critical in responding to the coronavirus pandemic. We were able to get up and running quickly on telemedicine because we had already invested in telehealth and had conducted a pilot of the platform with a smaller group of providers well before the pandemic hit.
In March, we were able to expand the telehealth platform to accommodate virtual visits by all of our providers. We also had to figure out how to shift our employees to telework, develop remote desktop and VPN solutions, and make sure that our scheduling and revenue cycle team members were fully operational.
The overriding goal was the safety of patients, staff, and our providers while continuing to provide medical care. Our inflammatory bowel disease patients needing visits to receive medication infusions took over an entire office so that there could be appropriate spacing and limited contacts with staff and other patients.
Our administrators knew early on that we needed a back-up plan and worked with physicians and providers doing telehealth visits to provide the flexibility to switch to Centers for Medicare & Medicaid–approved platforms (including Facetime) for those instances in which patients were uncomfortable using our main platform or when it was strained by bandwidth issues – a common challenge with any platform. Virtual check-in and check-out procedures were developed utilizing our usual office staff from remote locations.
For patients who had indications for gastroenterology procedures, we established a prioritization system, based on state guidelines, for those that were needed urgently or routinely as our endoscopy centers began to reopen. Safety measures were put into place including screening questionnaires, preprocedure COVID rt-PCT testing, personal protective equipment, and workflow changes to achieve social distancing.
As an early-career GI physician who is considering private practice, you’ll likely have several conversations with administrative leaders when deciding what practice to join. Ask about how the practice responded to COVID-19, and what processes it has in place to prepare for future emergencies.
During the early weeks of the pandemic, the CDC Board of Managers met two to three times per week. Task forces to discuss office operations and planning for ambulatory surgery center opening were established with participation by nearly every provider and manager. Communication between all providers and managers was important to decrease the obvious anxiety everyone was experiencing.
Old financial models may no longer work
Most practices develop budgets based on historical data. We quickly figured out that budgets from historical forecasts no longer worked and that we needed to understand the impact to budgets almost in real time.
We immediately looked to conserve cash and reduce expenses, requesting that our large vendors extend payment terms or provide a period of forbearance. We looked at everything from our EMR costs to lab supplies and everything in between.
Changing how we modeled our budgets and reducing costs made some of our hard decisions less difficult. While we had to furlough staff, our models for reducing physician compensation and lowering our costs allowed us to create a model for the return to work that included the use of paid time off and paid health care for our furloughed employees.
Our operations team also set up systems to gather information that was needed to apply for and report on federal loans and grants. They also set up ways to track revenue per visit and appeals for denied telehealth and other services in an effort to create new models and budgets as COVID-19 progressed. The revenue cycle team focused on unpaid older accounts receivable.
Focused on the future
It’s an understatement to say that COVID-19 has forever changed the practice of medicine. The health care industry will need to transform.
For some time now, GI practices have discussed the consequence of disruptive innovation affecting utilization of endoscopic procedures. We were looking at technology that might eventually replace office personnel. No one was thinking about a pandemic that would cause nearly overnight closure of endoscopy suites and curtail the entire in-office administrative workforce. The coronavirus pandemic is likely to be the catalyst that brings many innovations into the mainstream.
We’ll most likely see a transition to the virtual medical office for those visits that don’t require a patient to see a physician in person. This will make online scheduling and registration, on-demand messaging, and remote patient monitoring and chronic care management necessities.
We may also see more rapid adoption of technologies that allow information from health trackers and wearables to be integrated into EMRs that easily follow the patient from physician to physician. Administrative support and patient assistance from remote locations will become the norm.
Inquiring about how practices plan for emergencies and how their leadership thinks about the future of gastroenterology is a great way to show that you’re thinking holistically about health care delivery and how medicine is practiced now and in the future.
So much has changed in the decades I’ve been practicing medicine and so much is yet to change. As early-career GI physicians who are familiar with new technologies, you are in a great position to lead the practices you join into the future of gastroenterology.
Dr. Weinstein is president and CEO of Capital Digestive Care and the immediate past president of the Digestive Health Physicians Association.
It was early March, and our second day of advocacy on Capitol Hill with the Digestive Health Physicians Association (DHPA) was cut short when congressional offices were shuttered because of the COVID-19 pandemic. Sitting with several of my GI physician colleagues from across the country, we knew that our practices, our patients, and our communities would be impacted by the coronavirus. None of us could have known the extent.
We also didn’t know in that moment that our advocacy work through DHPA would be one of the most important factors in ensuring that our practices were prepared to weather the pandemic. Our membership, legal counsel, and legislative lobbyists helped us remain informed about new legislation and regulations and ensured that we had much-needed access to government resources.
Just a few months into what is now the COVID-19 pandemic, independent GI practice leaders have learned a lot about how to strengthen our practices to respond to future crises – and what early-career GIs should look for in the practices they are considering.
First and foremost, practice leadership is key. One thing most successful GI practices have in common is that they hire really smart executives and administrative teams who excel at taking care of the business side of things so that physicians like me can do what we do best: treat patients.
Stay informed about state and federal policies
As a member of DHPA, Capital Digestive Care was well positioned to keep up to date on the government response to the coronavirus and the support it provided to small businesses and to health care providers.
Over the past 5 years, DHPA physician leaders have established strong relationships with our elected federal leaders. During our Capitol Hill visits in early March, we discussed the coronavirus in addition to our policy priorities.
The relationships we’ve built with policymakers have helped us educate them about how private practices were being affected and make the case that it was crucial to include private practices in health care stimulus packages.
Without this federal financial support, many medical groups may have had to close their doors – leaving a large gap in care once the pandemic subsides.
In addition to the federal government’s financial support, our policy advocacy efforts kept us informed about federal health agencies’ decisions on telehealth coverage. We were able to educate our physicians and staff about state and federal adjustments to telehealth rules for the pandemic, on the guidelines for elective procedures, on employee furlough and leave rules, as well as other congressional and state actions that would impact our practice.
You can’t be an independent physician without being open to learning about the business of health care and understanding how health policies affect your ability to practice medicine and care for people in your community. Every early-career physician who is looking to join a practice should ask how its leadership remains informed about health policy at the state and federal levels.
Make plans, be flexible
Implementing telehealth was critical in responding to the coronavirus pandemic. We were able to get up and running quickly on telemedicine because we had already invested in telehealth and had conducted a pilot of the platform with a smaller group of providers well before the pandemic hit.
In March, we were able to expand the telehealth platform to accommodate virtual visits by all of our providers. We also had to figure out how to shift our employees to telework, develop remote desktop and VPN solutions, and make sure that our scheduling and revenue cycle team members were fully operational.
The overriding goal was the safety of patients, staff, and our providers while continuing to provide medical care. Our inflammatory bowel disease patients needing visits to receive medication infusions took over an entire office so that there could be appropriate spacing and limited contacts with staff and other patients.
Our administrators knew early on that we needed a back-up plan and worked with physicians and providers doing telehealth visits to provide the flexibility to switch to Centers for Medicare & Medicaid–approved platforms (including Facetime) for those instances in which patients were uncomfortable using our main platform or when it was strained by bandwidth issues – a common challenge with any platform. Virtual check-in and check-out procedures were developed utilizing our usual office staff from remote locations.
For patients who had indications for gastroenterology procedures, we established a prioritization system, based on state guidelines, for those that were needed urgently or routinely as our endoscopy centers began to reopen. Safety measures were put into place including screening questionnaires, preprocedure COVID rt-PCT testing, personal protective equipment, and workflow changes to achieve social distancing.
As an early-career GI physician who is considering private practice, you’ll likely have several conversations with administrative leaders when deciding what practice to join. Ask about how the practice responded to COVID-19, and what processes it has in place to prepare for future emergencies.
During the early weeks of the pandemic, the CDC Board of Managers met two to three times per week. Task forces to discuss office operations and planning for ambulatory surgery center opening were established with participation by nearly every provider and manager. Communication between all providers and managers was important to decrease the obvious anxiety everyone was experiencing.
Old financial models may no longer work
Most practices develop budgets based on historical data. We quickly figured out that budgets from historical forecasts no longer worked and that we needed to understand the impact to budgets almost in real time.
We immediately looked to conserve cash and reduce expenses, requesting that our large vendors extend payment terms or provide a period of forbearance. We looked at everything from our EMR costs to lab supplies and everything in between.
Changing how we modeled our budgets and reducing costs made some of our hard decisions less difficult. While we had to furlough staff, our models for reducing physician compensation and lowering our costs allowed us to create a model for the return to work that included the use of paid time off and paid health care for our furloughed employees.
Our operations team also set up systems to gather information that was needed to apply for and report on federal loans and grants. They also set up ways to track revenue per visit and appeals for denied telehealth and other services in an effort to create new models and budgets as COVID-19 progressed. The revenue cycle team focused on unpaid older accounts receivable.
Focused on the future
It’s an understatement to say that COVID-19 has forever changed the practice of medicine. The health care industry will need to transform.
For some time now, GI practices have discussed the consequence of disruptive innovation affecting utilization of endoscopic procedures. We were looking at technology that might eventually replace office personnel. No one was thinking about a pandemic that would cause nearly overnight closure of endoscopy suites and curtail the entire in-office administrative workforce. The coronavirus pandemic is likely to be the catalyst that brings many innovations into the mainstream.
We’ll most likely see a transition to the virtual medical office for those visits that don’t require a patient to see a physician in person. This will make online scheduling and registration, on-demand messaging, and remote patient monitoring and chronic care management necessities.
We may also see more rapid adoption of technologies that allow information from health trackers and wearables to be integrated into EMRs that easily follow the patient from physician to physician. Administrative support and patient assistance from remote locations will become the norm.
Inquiring about how practices plan for emergencies and how their leadership thinks about the future of gastroenterology is a great way to show that you’re thinking holistically about health care delivery and how medicine is practiced now and in the future.
So much has changed in the decades I’ve been practicing medicine and so much is yet to change. As early-career GI physicians who are familiar with new technologies, you are in a great position to lead the practices you join into the future of gastroenterology.
Dr. Weinstein is president and CEO of Capital Digestive Care and the immediate past president of the Digestive Health Physicians Association.
It was early March, and our second day of advocacy on Capitol Hill with the Digestive Health Physicians Association (DHPA) was cut short when congressional offices were shuttered because of the COVID-19 pandemic. Sitting with several of my GI physician colleagues from across the country, we knew that our practices, our patients, and our communities would be impacted by the coronavirus. None of us could have known the extent.
We also didn’t know in that moment that our advocacy work through DHPA would be one of the most important factors in ensuring that our practices were prepared to weather the pandemic. Our membership, legal counsel, and legislative lobbyists helped us remain informed about new legislation and regulations and ensured that we had much-needed access to government resources.
Just a few months into what is now the COVID-19 pandemic, independent GI practice leaders have learned a lot about how to strengthen our practices to respond to future crises – and what early-career GIs should look for in the practices they are considering.
First and foremost, practice leadership is key. One thing most successful GI practices have in common is that they hire really smart executives and administrative teams who excel at taking care of the business side of things so that physicians like me can do what we do best: treat patients.
Stay informed about state and federal policies
As a member of DHPA, Capital Digestive Care was well positioned to keep up to date on the government response to the coronavirus and the support it provided to small businesses and to health care providers.
Over the past 5 years, DHPA physician leaders have established strong relationships with our elected federal leaders. During our Capitol Hill visits in early March, we discussed the coronavirus in addition to our policy priorities.
The relationships we’ve built with policymakers have helped us educate them about how private practices were being affected and make the case that it was crucial to include private practices in health care stimulus packages.
Without this federal financial support, many medical groups may have had to close their doors – leaving a large gap in care once the pandemic subsides.
In addition to the federal government’s financial support, our policy advocacy efforts kept us informed about federal health agencies’ decisions on telehealth coverage. We were able to educate our physicians and staff about state and federal adjustments to telehealth rules for the pandemic, on the guidelines for elective procedures, on employee furlough and leave rules, as well as other congressional and state actions that would impact our practice.
You can’t be an independent physician without being open to learning about the business of health care and understanding how health policies affect your ability to practice medicine and care for people in your community. Every early-career physician who is looking to join a practice should ask how its leadership remains informed about health policy at the state and federal levels.
Make plans, be flexible
Implementing telehealth was critical in responding to the coronavirus pandemic. We were able to get up and running quickly on telemedicine because we had already invested in telehealth and had conducted a pilot of the platform with a smaller group of providers well before the pandemic hit.
In March, we were able to expand the telehealth platform to accommodate virtual visits by all of our providers. We also had to figure out how to shift our employees to telework, develop remote desktop and VPN solutions, and make sure that our scheduling and revenue cycle team members were fully operational.
The overriding goal was the safety of patients, staff, and our providers while continuing to provide medical care. Our inflammatory bowel disease patients needing visits to receive medication infusions took over an entire office so that there could be appropriate spacing and limited contacts with staff and other patients.
Our administrators knew early on that we needed a back-up plan and worked with physicians and providers doing telehealth visits to provide the flexibility to switch to Centers for Medicare & Medicaid–approved platforms (including Facetime) for those instances in which patients were uncomfortable using our main platform or when it was strained by bandwidth issues – a common challenge with any platform. Virtual check-in and check-out procedures were developed utilizing our usual office staff from remote locations.
For patients who had indications for gastroenterology procedures, we established a prioritization system, based on state guidelines, for those that were needed urgently or routinely as our endoscopy centers began to reopen. Safety measures were put into place including screening questionnaires, preprocedure COVID rt-PCT testing, personal protective equipment, and workflow changes to achieve social distancing.
As an early-career GI physician who is considering private practice, you’ll likely have several conversations with administrative leaders when deciding what practice to join. Ask about how the practice responded to COVID-19, and what processes it has in place to prepare for future emergencies.
During the early weeks of the pandemic, the CDC Board of Managers met two to three times per week. Task forces to discuss office operations and planning for ambulatory surgery center opening were established with participation by nearly every provider and manager. Communication between all providers and managers was important to decrease the obvious anxiety everyone was experiencing.
Old financial models may no longer work
Most practices develop budgets based on historical data. We quickly figured out that budgets from historical forecasts no longer worked and that we needed to understand the impact to budgets almost in real time.
We immediately looked to conserve cash and reduce expenses, requesting that our large vendors extend payment terms or provide a period of forbearance. We looked at everything from our EMR costs to lab supplies and everything in between.
Changing how we modeled our budgets and reducing costs made some of our hard decisions less difficult. While we had to furlough staff, our models for reducing physician compensation and lowering our costs allowed us to create a model for the return to work that included the use of paid time off and paid health care for our furloughed employees.
Our operations team also set up systems to gather information that was needed to apply for and report on federal loans and grants. They also set up ways to track revenue per visit and appeals for denied telehealth and other services in an effort to create new models and budgets as COVID-19 progressed. The revenue cycle team focused on unpaid older accounts receivable.
Focused on the future
It’s an understatement to say that COVID-19 has forever changed the practice of medicine. The health care industry will need to transform.
For some time now, GI practices have discussed the consequence of disruptive innovation affecting utilization of endoscopic procedures. We were looking at technology that might eventually replace office personnel. No one was thinking about a pandemic that would cause nearly overnight closure of endoscopy suites and curtail the entire in-office administrative workforce. The coronavirus pandemic is likely to be the catalyst that brings many innovations into the mainstream.
We’ll most likely see a transition to the virtual medical office for those visits that don’t require a patient to see a physician in person. This will make online scheduling and registration, on-demand messaging, and remote patient monitoring and chronic care management necessities.
We may also see more rapid adoption of technologies that allow information from health trackers and wearables to be integrated into EMRs that easily follow the patient from physician to physician. Administrative support and patient assistance from remote locations will become the norm.
Inquiring about how practices plan for emergencies and how their leadership thinks about the future of gastroenterology is a great way to show that you’re thinking holistically about health care delivery and how medicine is practiced now and in the future.
So much has changed in the decades I’ve been practicing medicine and so much is yet to change. As early-career GI physicians who are familiar with new technologies, you are in a great position to lead the practices you join into the future of gastroenterology.
Dr. Weinstein is president and CEO of Capital Digestive Care and the immediate past president of the Digestive Health Physicians Association.
‘I can’t breathe’: Health inequity and state-sanctioned violence
One might immediately think of the deaths of Eric Garner, George Floyd, or even the fictional character Radio Raheem from Spike Lee’s critically acclaimed film, “Do the Right Thing,” when they hear the words “I can’t breathe.” These words are a cry for help. The deaths of these unarmed black men is devastating and has led to a state of rage, palpable pain, and protest across the world.
However, in this moment, I am talking about the health inequity exposed by the COVID-19 pandemic. Whether it be acute respiratory distress syndrome (ARDS) secondary to severe COVID-19, or the subsequent hypercoagulable state of COVID-19 that leads to venous thromboembolism, many black people in this country are left breathless. Many black patients who had no employee-based health insurance also had no primary care physician to order a SARS-CoV2 PCR lab test for them. Many of these patients have preexisting conditions, such as asthma from living in redlined communities affected by environmental racism. Many grew up in food deserts, where no fresh-produce store was interested enough to set up shop in their neighborhoods. They have been eating fast food since early childhood, as a fast-food burger is still cheaper than a salad. The result is obesity, an epidemic that can lead to diabetes mellitus, hypertension that can lead to coronary artery disease, stroke, and end-stage renal disease.
Earlier in my career, I once had a colleague gleefully tell me that all black people drank Kool-Aid while in discussion of the effects of high-sugar diets in our patients; this colleague was sure I would agree. Not all black people drink Kool-Aid. Secondary to my fear of the backlash that can come from the discomfort of “white fragility” that Robin DiAngelo describes in her New York Times bestseller by the same name, ”White Fragility: Why It’s So Hard for White People to Talk About Racism,” I refrained from expressing my own hurt, and I did not offer explicit correction. I, instead, took a serious pause. That pause, which lasted only minutes, seemed to last 400 years. It was a brief reflection of the 400 years of systemic racism seeping into everyday life. This included the circumstances that would lead to the health inequities that result in the health disparities from which many black patients suffer. It is that same systemic racism that could create two America’s in which my colleague might not have to know the historic context in which that question could be hurtful. I retorted with modified shock and a chuckle so that I could muster up enough strength to repeat what was said and leave it open for reflection. The goal was for my colleague to realize the obvious implicit bias that lingered, despite intention. The chuckle was also to cover my pain.
Whether we know it or not, we all carry some form of implicit bias, regardless of race, class, gender, ethnicity, sexual preference, or socioeconomic status. In this case, it is the same implicit bias that causes physicians to ignore some black patients when they have said that they are in pain. A groundbreaking April 2016 article in Proceedings of the National Academy of Sciences, “Racial Bias in Pain Assessment and Treatment Recommendations, and False Beliefs about Biological Differences Between Blacks and Whites” (doi: 10.1073/pnas.1516047113), revealed that racial disparities in pain assessment and treatment recommendations can be directly connected to the racial bias of the provider. It could be possible that this phenomenon has affected black patients who have walked into clinics and emergency departments and said, “I’m short of breath. I think that I might have coronavirus and need to be tested.” It may be that same implicit bias that has cut the air supply to a patient encounter. Instead of inquiring further, the patient might be met with minimum questions while their provider obtains their history and physical. Assumptions and blame on behavior and lack of personal responsibility secretly replace questions that could have been asked. Differentials between exacerbations and other etiologies are not explored. Could that patient have been sent home without a SARS-CoV2 polymerase chain reaction test? Well, what if the tests were in short supply? Sometimes they may have been sent home without a chest x-ray. In most cases, there are no funds to send them home with a pulse oximeter.
The act of assuming a person’s story that we consider to be one dimensional is always dangerous – and even more so during this pandemic. That person we can relate to – secondary to a cool pop culture moment, a TikTok song, or a negative stereotype – is not one dimensional. That assumption and that stereotype can make room for implicit bias. That same implicit bias is the knee on a neck of any marginalized patient. Implicit bias is the choke hold that slowly removes the light and life from a person who has a story, who has a family, and who has been an essential worker who can’t work from home. That person is telling us that they can’t breathe, but sometimes the only things seen are comorbidities through a misinformed or biased lens that suggest an assumed lack of personal responsibility. In a May 2020 New England Journal of Medicine perspective, “Racial health disparities and Covid-19” (doi: 10.1056/NEJMp2012910), Merlin Chowkwanyun, PhD, MPH, and Adolph L. Reed Jr., PhD, caution us against creating race-based explanations for presumed behavioral patterns.
Systemic racism has created the myth that the playing field has been leveled since the end of enslavement. It hasn’t. That black man, woman, or nonbinary person is telling you “I can’t breathe. I’m tired. I’m short of breath ... I have a cough ... I’m feeling weak these days, Doc.” However, implicit bias is still that knee that won’t let up. It has not let up. Communities with lower-income black and Hispanic patients have already seen local hospitals and frontline workers fight to save their lives while losing their own to COVID-19. We all witnessed the battle for scarce resources and PPE [personal protective equipment]. In contrast, some wealthy neighborhoods have occupants who most likely have access to a primary care physician and more testing centers.
As we reexamine ourselves and look at these cases of police brutality against unarmed black men, women, and children with the appropriate shame and outrage, let us reflect upon the privileges that we enjoy. Let us find our voice as we speak up for black lives. Let us look deeply into the history of medicine as it relates to black patients by reading “Medical Apartheid: The Dark History of Medical Experimentation on Black Americans from Colonial Times to the Present” by Harriet A. Washington. Let us examine that painful legacy, which, while having moments of good intention, still carries the stain of indifference, racism, neglect, and even experimentation without informed consent.
Why should we do these things? Because some of our black patients have also yelled or whispered, “I can’t breathe,” and we were not always listening either.
Dr. Ajala is a hospitalist and associate site director for education at Grady Memorial Hospital in Atlanta. She is a member of the executive council for SHM’s Care for Vulnerable Populations special interest group.
One might immediately think of the deaths of Eric Garner, George Floyd, or even the fictional character Radio Raheem from Spike Lee’s critically acclaimed film, “Do the Right Thing,” when they hear the words “I can’t breathe.” These words are a cry for help. The deaths of these unarmed black men is devastating and has led to a state of rage, palpable pain, and protest across the world.
However, in this moment, I am talking about the health inequity exposed by the COVID-19 pandemic. Whether it be acute respiratory distress syndrome (ARDS) secondary to severe COVID-19, or the subsequent hypercoagulable state of COVID-19 that leads to venous thromboembolism, many black people in this country are left breathless. Many black patients who had no employee-based health insurance also had no primary care physician to order a SARS-CoV2 PCR lab test for them. Many of these patients have preexisting conditions, such as asthma from living in redlined communities affected by environmental racism. Many grew up in food deserts, where no fresh-produce store was interested enough to set up shop in their neighborhoods. They have been eating fast food since early childhood, as a fast-food burger is still cheaper than a salad. The result is obesity, an epidemic that can lead to diabetes mellitus, hypertension that can lead to coronary artery disease, stroke, and end-stage renal disease.
Earlier in my career, I once had a colleague gleefully tell me that all black people drank Kool-Aid while in discussion of the effects of high-sugar diets in our patients; this colleague was sure I would agree. Not all black people drink Kool-Aid. Secondary to my fear of the backlash that can come from the discomfort of “white fragility” that Robin DiAngelo describes in her New York Times bestseller by the same name, ”White Fragility: Why It’s So Hard for White People to Talk About Racism,” I refrained from expressing my own hurt, and I did not offer explicit correction. I, instead, took a serious pause. That pause, which lasted only minutes, seemed to last 400 years. It was a brief reflection of the 400 years of systemic racism seeping into everyday life. This included the circumstances that would lead to the health inequities that result in the health disparities from which many black patients suffer. It is that same systemic racism that could create two America’s in which my colleague might not have to know the historic context in which that question could be hurtful. I retorted with modified shock and a chuckle so that I could muster up enough strength to repeat what was said and leave it open for reflection. The goal was for my colleague to realize the obvious implicit bias that lingered, despite intention. The chuckle was also to cover my pain.
Whether we know it or not, we all carry some form of implicit bias, regardless of race, class, gender, ethnicity, sexual preference, or socioeconomic status. In this case, it is the same implicit bias that causes physicians to ignore some black patients when they have said that they are in pain. A groundbreaking April 2016 article in Proceedings of the National Academy of Sciences, “Racial Bias in Pain Assessment and Treatment Recommendations, and False Beliefs about Biological Differences Between Blacks and Whites” (doi: 10.1073/pnas.1516047113), revealed that racial disparities in pain assessment and treatment recommendations can be directly connected to the racial bias of the provider. It could be possible that this phenomenon has affected black patients who have walked into clinics and emergency departments and said, “I’m short of breath. I think that I might have coronavirus and need to be tested.” It may be that same implicit bias that has cut the air supply to a patient encounter. Instead of inquiring further, the patient might be met with minimum questions while their provider obtains their history and physical. Assumptions and blame on behavior and lack of personal responsibility secretly replace questions that could have been asked. Differentials between exacerbations and other etiologies are not explored. Could that patient have been sent home without a SARS-CoV2 polymerase chain reaction test? Well, what if the tests were in short supply? Sometimes they may have been sent home without a chest x-ray. In most cases, there are no funds to send them home with a pulse oximeter.
The act of assuming a person’s story that we consider to be one dimensional is always dangerous – and even more so during this pandemic. That person we can relate to – secondary to a cool pop culture moment, a TikTok song, or a negative stereotype – is not one dimensional. That assumption and that stereotype can make room for implicit bias. That same implicit bias is the knee on a neck of any marginalized patient. Implicit bias is the choke hold that slowly removes the light and life from a person who has a story, who has a family, and who has been an essential worker who can’t work from home. That person is telling us that they can’t breathe, but sometimes the only things seen are comorbidities through a misinformed or biased lens that suggest an assumed lack of personal responsibility. In a May 2020 New England Journal of Medicine perspective, “Racial health disparities and Covid-19” (doi: 10.1056/NEJMp2012910), Merlin Chowkwanyun, PhD, MPH, and Adolph L. Reed Jr., PhD, caution us against creating race-based explanations for presumed behavioral patterns.
Systemic racism has created the myth that the playing field has been leveled since the end of enslavement. It hasn’t. That black man, woman, or nonbinary person is telling you “I can’t breathe. I’m tired. I’m short of breath ... I have a cough ... I’m feeling weak these days, Doc.” However, implicit bias is still that knee that won’t let up. It has not let up. Communities with lower-income black and Hispanic patients have already seen local hospitals and frontline workers fight to save their lives while losing their own to COVID-19. We all witnessed the battle for scarce resources and PPE [personal protective equipment]. In contrast, some wealthy neighborhoods have occupants who most likely have access to a primary care physician and more testing centers.
As we reexamine ourselves and look at these cases of police brutality against unarmed black men, women, and children with the appropriate shame and outrage, let us reflect upon the privileges that we enjoy. Let us find our voice as we speak up for black lives. Let us look deeply into the history of medicine as it relates to black patients by reading “Medical Apartheid: The Dark History of Medical Experimentation on Black Americans from Colonial Times to the Present” by Harriet A. Washington. Let us examine that painful legacy, which, while having moments of good intention, still carries the stain of indifference, racism, neglect, and even experimentation without informed consent.
Why should we do these things? Because some of our black patients have also yelled or whispered, “I can’t breathe,” and we were not always listening either.
Dr. Ajala is a hospitalist and associate site director for education at Grady Memorial Hospital in Atlanta. She is a member of the executive council for SHM’s Care for Vulnerable Populations special interest group.
One might immediately think of the deaths of Eric Garner, George Floyd, or even the fictional character Radio Raheem from Spike Lee’s critically acclaimed film, “Do the Right Thing,” when they hear the words “I can’t breathe.” These words are a cry for help. The deaths of these unarmed black men is devastating and has led to a state of rage, palpable pain, and protest across the world.
However, in this moment, I am talking about the health inequity exposed by the COVID-19 pandemic. Whether it be acute respiratory distress syndrome (ARDS) secondary to severe COVID-19, or the subsequent hypercoagulable state of COVID-19 that leads to venous thromboembolism, many black people in this country are left breathless. Many black patients who had no employee-based health insurance also had no primary care physician to order a SARS-CoV2 PCR lab test for them. Many of these patients have preexisting conditions, such as asthma from living in redlined communities affected by environmental racism. Many grew up in food deserts, where no fresh-produce store was interested enough to set up shop in their neighborhoods. They have been eating fast food since early childhood, as a fast-food burger is still cheaper than a salad. The result is obesity, an epidemic that can lead to diabetes mellitus, hypertension that can lead to coronary artery disease, stroke, and end-stage renal disease.
Earlier in my career, I once had a colleague gleefully tell me that all black people drank Kool-Aid while in discussion of the effects of high-sugar diets in our patients; this colleague was sure I would agree. Not all black people drink Kool-Aid. Secondary to my fear of the backlash that can come from the discomfort of “white fragility” that Robin DiAngelo describes in her New York Times bestseller by the same name, ”White Fragility: Why It’s So Hard for White People to Talk About Racism,” I refrained from expressing my own hurt, and I did not offer explicit correction. I, instead, took a serious pause. That pause, which lasted only minutes, seemed to last 400 years. It was a brief reflection of the 400 years of systemic racism seeping into everyday life. This included the circumstances that would lead to the health inequities that result in the health disparities from which many black patients suffer. It is that same systemic racism that could create two America’s in which my colleague might not have to know the historic context in which that question could be hurtful. I retorted with modified shock and a chuckle so that I could muster up enough strength to repeat what was said and leave it open for reflection. The goal was for my colleague to realize the obvious implicit bias that lingered, despite intention. The chuckle was also to cover my pain.
Whether we know it or not, we all carry some form of implicit bias, regardless of race, class, gender, ethnicity, sexual preference, or socioeconomic status. In this case, it is the same implicit bias that causes physicians to ignore some black patients when they have said that they are in pain. A groundbreaking April 2016 article in Proceedings of the National Academy of Sciences, “Racial Bias in Pain Assessment and Treatment Recommendations, and False Beliefs about Biological Differences Between Blacks and Whites” (doi: 10.1073/pnas.1516047113), revealed that racial disparities in pain assessment and treatment recommendations can be directly connected to the racial bias of the provider. It could be possible that this phenomenon has affected black patients who have walked into clinics and emergency departments and said, “I’m short of breath. I think that I might have coronavirus and need to be tested.” It may be that same implicit bias that has cut the air supply to a patient encounter. Instead of inquiring further, the patient might be met with minimum questions while their provider obtains their history and physical. Assumptions and blame on behavior and lack of personal responsibility secretly replace questions that could have been asked. Differentials between exacerbations and other etiologies are not explored. Could that patient have been sent home without a SARS-CoV2 polymerase chain reaction test? Well, what if the tests were in short supply? Sometimes they may have been sent home without a chest x-ray. In most cases, there are no funds to send them home with a pulse oximeter.
The act of assuming a person’s story that we consider to be one dimensional is always dangerous – and even more so during this pandemic. That person we can relate to – secondary to a cool pop culture moment, a TikTok song, or a negative stereotype – is not one dimensional. That assumption and that stereotype can make room for implicit bias. That same implicit bias is the knee on a neck of any marginalized patient. Implicit bias is the choke hold that slowly removes the light and life from a person who has a story, who has a family, and who has been an essential worker who can’t work from home. That person is telling us that they can’t breathe, but sometimes the only things seen are comorbidities through a misinformed or biased lens that suggest an assumed lack of personal responsibility. In a May 2020 New England Journal of Medicine perspective, “Racial health disparities and Covid-19” (doi: 10.1056/NEJMp2012910), Merlin Chowkwanyun, PhD, MPH, and Adolph L. Reed Jr., PhD, caution us against creating race-based explanations for presumed behavioral patterns.
Systemic racism has created the myth that the playing field has been leveled since the end of enslavement. It hasn’t. That black man, woman, or nonbinary person is telling you “I can’t breathe. I’m tired. I’m short of breath ... I have a cough ... I’m feeling weak these days, Doc.” However, implicit bias is still that knee that won’t let up. It has not let up. Communities with lower-income black and Hispanic patients have already seen local hospitals and frontline workers fight to save their lives while losing their own to COVID-19. We all witnessed the battle for scarce resources and PPE [personal protective equipment]. In contrast, some wealthy neighborhoods have occupants who most likely have access to a primary care physician and more testing centers.
As we reexamine ourselves and look at these cases of police brutality against unarmed black men, women, and children with the appropriate shame and outrage, let us reflect upon the privileges that we enjoy. Let us find our voice as we speak up for black lives. Let us look deeply into the history of medicine as it relates to black patients by reading “Medical Apartheid: The Dark History of Medical Experimentation on Black Americans from Colonial Times to the Present” by Harriet A. Washington. Let us examine that painful legacy, which, while having moments of good intention, still carries the stain of indifference, racism, neglect, and even experimentation without informed consent.
Why should we do these things? Because some of our black patients have also yelled or whispered, “I can’t breathe,” and we were not always listening either.
Dr. Ajala is a hospitalist and associate site director for education at Grady Memorial Hospital in Atlanta. She is a member of the executive council for SHM’s Care for Vulnerable Populations special interest group.