User login
Teledermatology follow-up after Mohs surgery gets a thumbs up from patients
SEATTLE – The , according to new findings.
In addition, nearly all patients surveyed (91.4%) were willing to go through electronic follow-up again.
“A big takeaway from our study is that streamlining this process is really essential for successful implementation,” said study author Laura Rezac, MD, a PGY IV dermatology resident at the University of Mississippi, Jackson. “This study demonstrated the flexibility and convenience for both patients and surgeons and can serve as a prototype for future innovation.”
The study results were presented at the annual meeting of the American College of Mohs Surgery.
The role of telehealth has rapidly expanded over the past decade, with its use accelerating during the COVID-19 pandemic and transforming into an indispensable resource. It can be synchronous, Dr. Rezac explained, which is when telehealth happens in live, real-time settings where the patient interacts with a clinician. This usually occurs via phone or video, and providers and patients communicate directly.
Conversely, asynchronous telehealth, also known as “store-and-forward,” is often used for patient intake or follow-up care. For example, in dermatology, a patient can send a photo of a skin condition that is then reviewed by a dermatologist later.
“A pilot survey regarding the adoption of telemedicine in Mohs surgery found that, although most dermatologic surgeons felt that it can play a role, most said that they didn’t plan on using it after the pandemic,” said Dr. Rezac.
The survey, which was reported by this news organization, found that 80% of surveyed surgeons said that they turned to telemedicine during the pandemic, compared with just 23% who relied on the technology prior to the pandemic.
There were numerous perceived barriers to the use of telemedicine, and the one most commonly cited was the uncertainty of how telemedicine fits in the workflow of clinical practice. Other limitations reported were for physical exams (88%), patient response and training (57%), reimbursement concerns (50%), implementation of the technology (37%), regulations such as HIPAA (24%), training of staff (17%), and licensing (8%).
“The survey did identify one key use of telemedicine in Mohs and that was for [postoperative] visits,” she said. “But thus far, a postoperative evaluation after Mohs via an integrated asynchronous ‘store and forward’ teledermatology platform has not yet been evaluated.”
In the study, Dr. Rezac and colleagues sought to evaluate feasibility and efficacy, as well as patient attitudes, using a telemedicine platform for postoperative follow-up. A total of 163 patients who were treated with Mohs at a single academic institution during the 9-month study period (December 2021 through August 2022) responded to a survey and elected to participate in postoperative follow-up using telemedicine.
Dr. Rezac explained how their procedure was implemented for the patient. “On the day of the follow-up, the patient receives a text with a link that takes them to the MyChart website or app on their phone,” she said. “Once they log in, they see that they have a message telling them that they have a teledermatology message waiting for them. When they view it, they are taken to the curated message with instructions and a phone call if they need assistance, and then at the bottom, it shows they have a task to complete, which is the questionnaire.”
The patient will then be prompted to upload photos, which can be taken with their phone camera. The next step is to answer questions regarding the surgical site or pain concerns, and finally, patients are asked to respond to a few short questions about this type of follow-up. Once submitted, then they wait to be contacted by the surgeon.
On the surgeon’s side, these answers come into their EPIC inbox, and they can respond via a MyChart message.
Patient response was overwhelmingly positive, Dr. Rezac noted. Of the patients, 80.4% found the electronic surgery follow-up process to be “easy” or “very easy,” while only 4% found it “difficult” or “very difficult,” she said. “Also, 75.5% preferred electronic follow-up while 17.2% preferred in-person follow-up.”
There were limitations to this study, primarily that the asynchronous method does reduce live interaction, which could be an issue, depending on person’s needs, she pointed out. “But it is easy to schedule a phone call or video call or office visit.”
“The universal barrier is how to adopt it into the workflow, which includes training of staff,” she continued, “But this was a very streamlined process and gave very detailed instructions to the staff. Additionally, widespread use is limited to dermatological proficiency and access, and patients have to be amenable to it, so there is a selection bias since these patients chose to participate.”
Asked to comment on the study, Vishal Patel, MD, director of cutaneous oncology at George Washington University in Washington, said: “The COVID pandemic changed how practices and providers considered follow-up visits for small routine matters. Postoperative visits are often simple and do not require an in-depth, in-person evaluation.” Dr. Patel was not involved with this research.
“This study highlights the comfort of the vast majority of patients to have follow-up postoperative visits conducted via teledermatology – an approach that can help cut overall costs and also increase access for patients who are more in need of in-office care,” he added.
No external funding of the study was reported. Dr. Rezac reported no relevant financial relationships. Dr. Patel is a consultant for Sanofi, Regeneron, and Almirall.
A version of this article originally appeared on Medscape.com.
SEATTLE – The , according to new findings.
In addition, nearly all patients surveyed (91.4%) were willing to go through electronic follow-up again.
“A big takeaway from our study is that streamlining this process is really essential for successful implementation,” said study author Laura Rezac, MD, a PGY IV dermatology resident at the University of Mississippi, Jackson. “This study demonstrated the flexibility and convenience for both patients and surgeons and can serve as a prototype for future innovation.”
The study results were presented at the annual meeting of the American College of Mohs Surgery.
The role of telehealth has rapidly expanded over the past decade, with its use accelerating during the COVID-19 pandemic and transforming into an indispensable resource. It can be synchronous, Dr. Rezac explained, which is when telehealth happens in live, real-time settings where the patient interacts with a clinician. This usually occurs via phone or video, and providers and patients communicate directly.
Conversely, asynchronous telehealth, also known as “store-and-forward,” is often used for patient intake or follow-up care. For example, in dermatology, a patient can send a photo of a skin condition that is then reviewed by a dermatologist later.
“A pilot survey regarding the adoption of telemedicine in Mohs surgery found that, although most dermatologic surgeons felt that it can play a role, most said that they didn’t plan on using it after the pandemic,” said Dr. Rezac.
The survey, which was reported by this news organization, found that 80% of surveyed surgeons said that they turned to telemedicine during the pandemic, compared with just 23% who relied on the technology prior to the pandemic.
There were numerous perceived barriers to the use of telemedicine, and the one most commonly cited was the uncertainty of how telemedicine fits in the workflow of clinical practice. Other limitations reported were for physical exams (88%), patient response and training (57%), reimbursement concerns (50%), implementation of the technology (37%), regulations such as HIPAA (24%), training of staff (17%), and licensing (8%).
“The survey did identify one key use of telemedicine in Mohs and that was for [postoperative] visits,” she said. “But thus far, a postoperative evaluation after Mohs via an integrated asynchronous ‘store and forward’ teledermatology platform has not yet been evaluated.”
In the study, Dr. Rezac and colleagues sought to evaluate feasibility and efficacy, as well as patient attitudes, using a telemedicine platform for postoperative follow-up. A total of 163 patients who were treated with Mohs at a single academic institution during the 9-month study period (December 2021 through August 2022) responded to a survey and elected to participate in postoperative follow-up using telemedicine.
Dr. Rezac explained how their procedure was implemented for the patient. “On the day of the follow-up, the patient receives a text with a link that takes them to the MyChart website or app on their phone,” she said. “Once they log in, they see that they have a message telling them that they have a teledermatology message waiting for them. When they view it, they are taken to the curated message with instructions and a phone call if they need assistance, and then at the bottom, it shows they have a task to complete, which is the questionnaire.”
The patient will then be prompted to upload photos, which can be taken with their phone camera. The next step is to answer questions regarding the surgical site or pain concerns, and finally, patients are asked to respond to a few short questions about this type of follow-up. Once submitted, then they wait to be contacted by the surgeon.
On the surgeon’s side, these answers come into their EPIC inbox, and they can respond via a MyChart message.
Patient response was overwhelmingly positive, Dr. Rezac noted. Of the patients, 80.4% found the electronic surgery follow-up process to be “easy” or “very easy,” while only 4% found it “difficult” or “very difficult,” she said. “Also, 75.5% preferred electronic follow-up while 17.2% preferred in-person follow-up.”
There were limitations to this study, primarily that the asynchronous method does reduce live interaction, which could be an issue, depending on person’s needs, she pointed out. “But it is easy to schedule a phone call or video call or office visit.”
“The universal barrier is how to adopt it into the workflow, which includes training of staff,” she continued, “But this was a very streamlined process and gave very detailed instructions to the staff. Additionally, widespread use is limited to dermatological proficiency and access, and patients have to be amenable to it, so there is a selection bias since these patients chose to participate.”
Asked to comment on the study, Vishal Patel, MD, director of cutaneous oncology at George Washington University in Washington, said: “The COVID pandemic changed how practices and providers considered follow-up visits for small routine matters. Postoperative visits are often simple and do not require an in-depth, in-person evaluation.” Dr. Patel was not involved with this research.
“This study highlights the comfort of the vast majority of patients to have follow-up postoperative visits conducted via teledermatology – an approach that can help cut overall costs and also increase access for patients who are more in need of in-office care,” he added.
No external funding of the study was reported. Dr. Rezac reported no relevant financial relationships. Dr. Patel is a consultant for Sanofi, Regeneron, and Almirall.
A version of this article originally appeared on Medscape.com.
SEATTLE – The , according to new findings.
In addition, nearly all patients surveyed (91.4%) were willing to go through electronic follow-up again.
“A big takeaway from our study is that streamlining this process is really essential for successful implementation,” said study author Laura Rezac, MD, a PGY IV dermatology resident at the University of Mississippi, Jackson. “This study demonstrated the flexibility and convenience for both patients and surgeons and can serve as a prototype for future innovation.”
The study results were presented at the annual meeting of the American College of Mohs Surgery.
The role of telehealth has rapidly expanded over the past decade, with its use accelerating during the COVID-19 pandemic and transforming into an indispensable resource. It can be synchronous, Dr. Rezac explained, which is when telehealth happens in live, real-time settings where the patient interacts with a clinician. This usually occurs via phone or video, and providers and patients communicate directly.
Conversely, asynchronous telehealth, also known as “store-and-forward,” is often used for patient intake or follow-up care. For example, in dermatology, a patient can send a photo of a skin condition that is then reviewed by a dermatologist later.
“A pilot survey regarding the adoption of telemedicine in Mohs surgery found that, although most dermatologic surgeons felt that it can play a role, most said that they didn’t plan on using it after the pandemic,” said Dr. Rezac.
The survey, which was reported by this news organization, found that 80% of surveyed surgeons said that they turned to telemedicine during the pandemic, compared with just 23% who relied on the technology prior to the pandemic.
There were numerous perceived barriers to the use of telemedicine, and the one most commonly cited was the uncertainty of how telemedicine fits in the workflow of clinical practice. Other limitations reported were for physical exams (88%), patient response and training (57%), reimbursement concerns (50%), implementation of the technology (37%), regulations such as HIPAA (24%), training of staff (17%), and licensing (8%).
“The survey did identify one key use of telemedicine in Mohs and that was for [postoperative] visits,” she said. “But thus far, a postoperative evaluation after Mohs via an integrated asynchronous ‘store and forward’ teledermatology platform has not yet been evaluated.”
In the study, Dr. Rezac and colleagues sought to evaluate feasibility and efficacy, as well as patient attitudes, using a telemedicine platform for postoperative follow-up. A total of 163 patients who were treated with Mohs at a single academic institution during the 9-month study period (December 2021 through August 2022) responded to a survey and elected to participate in postoperative follow-up using telemedicine.
Dr. Rezac explained how their procedure was implemented for the patient. “On the day of the follow-up, the patient receives a text with a link that takes them to the MyChart website or app on their phone,” she said. “Once they log in, they see that they have a message telling them that they have a teledermatology message waiting for them. When they view it, they are taken to the curated message with instructions and a phone call if they need assistance, and then at the bottom, it shows they have a task to complete, which is the questionnaire.”
The patient will then be prompted to upload photos, which can be taken with their phone camera. The next step is to answer questions regarding the surgical site or pain concerns, and finally, patients are asked to respond to a few short questions about this type of follow-up. Once submitted, then they wait to be contacted by the surgeon.
On the surgeon’s side, these answers come into their EPIC inbox, and they can respond via a MyChart message.
Patient response was overwhelmingly positive, Dr. Rezac noted. Of the patients, 80.4% found the electronic surgery follow-up process to be “easy” or “very easy,” while only 4% found it “difficult” or “very difficult,” she said. “Also, 75.5% preferred electronic follow-up while 17.2% preferred in-person follow-up.”
There were limitations to this study, primarily that the asynchronous method does reduce live interaction, which could be an issue, depending on person’s needs, she pointed out. “But it is easy to schedule a phone call or video call or office visit.”
“The universal barrier is how to adopt it into the workflow, which includes training of staff,” she continued, “But this was a very streamlined process and gave very detailed instructions to the staff. Additionally, widespread use is limited to dermatological proficiency and access, and patients have to be amenable to it, so there is a selection bias since these patients chose to participate.”
Asked to comment on the study, Vishal Patel, MD, director of cutaneous oncology at George Washington University in Washington, said: “The COVID pandemic changed how practices and providers considered follow-up visits for small routine matters. Postoperative visits are often simple and do not require an in-depth, in-person evaluation.” Dr. Patel was not involved with this research.
“This study highlights the comfort of the vast majority of patients to have follow-up postoperative visits conducted via teledermatology – an approach that can help cut overall costs and also increase access for patients who are more in need of in-office care,” he added.
No external funding of the study was reported. Dr. Rezac reported no relevant financial relationships. Dr. Patel is a consultant for Sanofi, Regeneron, and Almirall.
A version of this article originally appeared on Medscape.com.
AT ACMS 2023
Mohs surgery workforce continues to increase
SEATTLE – At least for now, and that has been the case for the past 5 years.
Using CMS billing codes as a surrogate, the researchers found that there was a steady increase in the number of physicians who billed from 2015 to 2020. With the exception of 2020, which was the height of the COVID-19 pandemic, the number of times that a specific code was billed for increased on average by 4.7% annually.
“Thus, if the attrition rate remains stable, even with changes in board certification and potential payer eligibility restrictions, the number of physicians will continue to increase,” study author Ji Won Ahn, MD, who specializes in dermatology and Mohs surgery at University of Pittsburgh Medical Center, said at the annual meeting of the American College of Mohs Surgery, where she presented the results.
The growth in the number of Mohs surgeons has been fueled by several factors, including a rising incidence of skin cancer as well as the superior cure rates and cosmetic outcomes with the procedure. Reimbursement has been favorable and training pathways have expanded. A 2019 retrospective study reported that there were 2,240 dermatologists who performed Mohs surgery in the United States, with nearly all of them (94.6%) residing in metropolitan areas.
Dr. Ahn explained that it was important to define the workforce because of several new factors that will be affecting it in the future. “With the establishment of Micrographic Surgery and Dermatologic Oncology [MSDO] board certification that went into effect 2 years ago, potential future payer eligibility restrictions may be coming,” she said. “The adequacy of the Mohs surgery workforce is an important consideration.”
Another issue is that new board certification will be limited to fellowship-trained physicians after the first 5 years. “We wanted to compare these numbers with the fellowship numbers,” she said. “Although fellowship numbers are something that the college potentially has the power to change.”
Dr. Ahn and colleagues used the Centers for Medicare & Medicaid Services database to evaluate the use of the Current Procedural Terminology (CPT) code 17311, which is one of the most common billing codes for Mohs micrographic technique. Looking at data from 2015-2020, they found that there was an annual increase in the number of unique national provider identifiers (NPIs) billing for 17311, at an average rate of 75.6 per year.
The total number of times that 17311 was billed also increased from 2015 to 2019 at an average rate of 4.7% per year but declined in 2020 by 8.4%. “Overall, there was an average of 135 new NPIs that appeared and an average of 59.4 NPIs that stopped billing for 17311,” thus, an attrition rate of 59 surgeons, Dr. Ahn explained.
She emphasized that notably, the number of approved MSDO fellowship spots has remained stable since 2016 and is about 92 to 93 per year. “There are about 135 new surgeons and about two-thirds are new fellowship graduates,” she said.
The researchers were also interested in seeing how saturated each surgeon was and looked at the approximate number of cases that they were handling.
Of the physicians who billed 17311 through CMS, over 26% billed less than 100 times and more than 45% billed less than 200 times, and over 80% billed less than 500 times.
“One might be able to conclude that there might be some potential flexibility depending on the future need for surgeons,” she said.
The study was limited by several factors, one being that the researchers looked only at CPT code 17311 and not other designated codes for Mohs surgery. Other factors such as staff and space limitations were not accounted for since only billing data were used.
Dr. Ahn and her team are going to continue their work, and the next steps are to look at geographic trends and monitor for insurance network eligibility changes. “We are currently doing a workforce survey so we can better understand our current workforce rather than just historical data,” she concluded.
Asked to comment on the results, Vishal Patel, MD, assistant professor of dermatology and director of the cutaneous oncology program at George Washington University, Washington, who was not involved with the study, noted that the increase in the “billing rates of the first stage of Mohs micrographic surgery highlights not only the growing skin cancer epidemic, but also the number of providers who are providing these services. This underscores the importance of standardized training guidelines and board certifications of Mohs micrographic surgeons to assure high levels of patient care and the appropriate use of Mohs micrographic surgery,” he said.
No external funding of the study was reported. Dr. Ahn reported no relevant financial relationships. Dr. Patel is a consultant for Sanofi, Regeneron, and Almirall.
A version of this article originally appeared on Medscape.com.
SEATTLE – At least for now, and that has been the case for the past 5 years.
Using CMS billing codes as a surrogate, the researchers found that there was a steady increase in the number of physicians who billed from 2015 to 2020. With the exception of 2020, which was the height of the COVID-19 pandemic, the number of times that a specific code was billed for increased on average by 4.7% annually.
“Thus, if the attrition rate remains stable, even with changes in board certification and potential payer eligibility restrictions, the number of physicians will continue to increase,” study author Ji Won Ahn, MD, who specializes in dermatology and Mohs surgery at University of Pittsburgh Medical Center, said at the annual meeting of the American College of Mohs Surgery, where she presented the results.
The growth in the number of Mohs surgeons has been fueled by several factors, including a rising incidence of skin cancer as well as the superior cure rates and cosmetic outcomes with the procedure. Reimbursement has been favorable and training pathways have expanded. A 2019 retrospective study reported that there were 2,240 dermatologists who performed Mohs surgery in the United States, with nearly all of them (94.6%) residing in metropolitan areas.
Dr. Ahn explained that it was important to define the workforce because of several new factors that will be affecting it in the future. “With the establishment of Micrographic Surgery and Dermatologic Oncology [MSDO] board certification that went into effect 2 years ago, potential future payer eligibility restrictions may be coming,” she said. “The adequacy of the Mohs surgery workforce is an important consideration.”
Another issue is that new board certification will be limited to fellowship-trained physicians after the first 5 years. “We wanted to compare these numbers with the fellowship numbers,” she said. “Although fellowship numbers are something that the college potentially has the power to change.”
Dr. Ahn and colleagues used the Centers for Medicare & Medicaid Services database to evaluate the use of the Current Procedural Terminology (CPT) code 17311, which is one of the most common billing codes for Mohs micrographic technique. Looking at data from 2015-2020, they found that there was an annual increase in the number of unique national provider identifiers (NPIs) billing for 17311, at an average rate of 75.6 per year.
The total number of times that 17311 was billed also increased from 2015 to 2019 at an average rate of 4.7% per year but declined in 2020 by 8.4%. “Overall, there was an average of 135 new NPIs that appeared and an average of 59.4 NPIs that stopped billing for 17311,” thus, an attrition rate of 59 surgeons, Dr. Ahn explained.
She emphasized that notably, the number of approved MSDO fellowship spots has remained stable since 2016 and is about 92 to 93 per year. “There are about 135 new surgeons and about two-thirds are new fellowship graduates,” she said.
The researchers were also interested in seeing how saturated each surgeon was and looked at the approximate number of cases that they were handling.
Of the physicians who billed 17311 through CMS, over 26% billed less than 100 times and more than 45% billed less than 200 times, and over 80% billed less than 500 times.
“One might be able to conclude that there might be some potential flexibility depending on the future need for surgeons,” she said.
The study was limited by several factors, one being that the researchers looked only at CPT code 17311 and not other designated codes for Mohs surgery. Other factors such as staff and space limitations were not accounted for since only billing data were used.
Dr. Ahn and her team are going to continue their work, and the next steps are to look at geographic trends and monitor for insurance network eligibility changes. “We are currently doing a workforce survey so we can better understand our current workforce rather than just historical data,” she concluded.
Asked to comment on the results, Vishal Patel, MD, assistant professor of dermatology and director of the cutaneous oncology program at George Washington University, Washington, who was not involved with the study, noted that the increase in the “billing rates of the first stage of Mohs micrographic surgery highlights not only the growing skin cancer epidemic, but also the number of providers who are providing these services. This underscores the importance of standardized training guidelines and board certifications of Mohs micrographic surgeons to assure high levels of patient care and the appropriate use of Mohs micrographic surgery,” he said.
No external funding of the study was reported. Dr. Ahn reported no relevant financial relationships. Dr. Patel is a consultant for Sanofi, Regeneron, and Almirall.
A version of this article originally appeared on Medscape.com.
SEATTLE – At least for now, and that has been the case for the past 5 years.
Using CMS billing codes as a surrogate, the researchers found that there was a steady increase in the number of physicians who billed from 2015 to 2020. With the exception of 2020, which was the height of the COVID-19 pandemic, the number of times that a specific code was billed for increased on average by 4.7% annually.
“Thus, if the attrition rate remains stable, even with changes in board certification and potential payer eligibility restrictions, the number of physicians will continue to increase,” study author Ji Won Ahn, MD, who specializes in dermatology and Mohs surgery at University of Pittsburgh Medical Center, said at the annual meeting of the American College of Mohs Surgery, where she presented the results.
The growth in the number of Mohs surgeons has been fueled by several factors, including a rising incidence of skin cancer as well as the superior cure rates and cosmetic outcomes with the procedure. Reimbursement has been favorable and training pathways have expanded. A 2019 retrospective study reported that there were 2,240 dermatologists who performed Mohs surgery in the United States, with nearly all of them (94.6%) residing in metropolitan areas.
Dr. Ahn explained that it was important to define the workforce because of several new factors that will be affecting it in the future. “With the establishment of Micrographic Surgery and Dermatologic Oncology [MSDO] board certification that went into effect 2 years ago, potential future payer eligibility restrictions may be coming,” she said. “The adequacy of the Mohs surgery workforce is an important consideration.”
Another issue is that new board certification will be limited to fellowship-trained physicians after the first 5 years. “We wanted to compare these numbers with the fellowship numbers,” she said. “Although fellowship numbers are something that the college potentially has the power to change.”
Dr. Ahn and colleagues used the Centers for Medicare & Medicaid Services database to evaluate the use of the Current Procedural Terminology (CPT) code 17311, which is one of the most common billing codes for Mohs micrographic technique. Looking at data from 2015-2020, they found that there was an annual increase in the number of unique national provider identifiers (NPIs) billing for 17311, at an average rate of 75.6 per year.
The total number of times that 17311 was billed also increased from 2015 to 2019 at an average rate of 4.7% per year but declined in 2020 by 8.4%. “Overall, there was an average of 135 new NPIs that appeared and an average of 59.4 NPIs that stopped billing for 17311,” thus, an attrition rate of 59 surgeons, Dr. Ahn explained.
She emphasized that notably, the number of approved MSDO fellowship spots has remained stable since 2016 and is about 92 to 93 per year. “There are about 135 new surgeons and about two-thirds are new fellowship graduates,” she said.
The researchers were also interested in seeing how saturated each surgeon was and looked at the approximate number of cases that they were handling.
Of the physicians who billed 17311 through CMS, over 26% billed less than 100 times and more than 45% billed less than 200 times, and over 80% billed less than 500 times.
“One might be able to conclude that there might be some potential flexibility depending on the future need for surgeons,” she said.
The study was limited by several factors, one being that the researchers looked only at CPT code 17311 and not other designated codes for Mohs surgery. Other factors such as staff and space limitations were not accounted for since only billing data were used.
Dr. Ahn and her team are going to continue their work, and the next steps are to look at geographic trends and monitor for insurance network eligibility changes. “We are currently doing a workforce survey so we can better understand our current workforce rather than just historical data,” she concluded.
Asked to comment on the results, Vishal Patel, MD, assistant professor of dermatology and director of the cutaneous oncology program at George Washington University, Washington, who was not involved with the study, noted that the increase in the “billing rates of the first stage of Mohs micrographic surgery highlights not only the growing skin cancer epidemic, but also the number of providers who are providing these services. This underscores the importance of standardized training guidelines and board certifications of Mohs micrographic surgeons to assure high levels of patient care and the appropriate use of Mohs micrographic surgery,” he said.
No external funding of the study was reported. Dr. Ahn reported no relevant financial relationships. Dr. Patel is a consultant for Sanofi, Regeneron, and Almirall.
A version of this article originally appeared on Medscape.com.
AT ACMS 2023
Number of cancer survivors with functional limitations doubled in 20 years
Vishal Patel, BS, a student at the Dell Medical School at The University of Texas at Austin, and colleagues identified 51,258 cancer survivors from the National Health Interview Survey, representing a weighted population of approximately 178.8 million from 1999 to 2018.
Most survivors were women (60.2%) and were at least 65 years old (55.4%). In 1999, 3.6 million weighted survivors reported functional limitation. In 2018, the number increased to 8.2 million, a 2.25-fold increase.
The number of survivors who reported no limitations also increased, but not by as much. That group grew 1.34-fold during the study period.
For context, “the 70% prevalence of functional limitation among survivors in 2018 is nearly twice that of the general population,” the authors wrote.
Patients surveyed on function
Functional limitation was defined as “self-reported difficulty performing any of 12 routine physical or social activities without assistance.” Examples of the activities included difficulty sitting for more than 2 hours, difficulty participating in social activities or difficulty pushing or pulling an object the size of a living room chair.
Over the 2 decades analyzed, the adjusted prevalence of functional limitation was highest among survivors of pancreatic cancer (80.3%) and lung cancer (76.5%). Prevalence was lowest for survivors of melanoma (62.2%), breast (61.8%) and prostate (59.5%) cancers.
Not just a result of living longer
Mr. Patel told this publication that one assumption people might make when they read these results is that people are just living longer with cancer and losing functional ability accordingly.
“But, in fact, we found that the youngest [– those less than 65 years–] actually contributed to this trend more than the oldest people, which means it’s not just [happening], because people are getting older,” he said.
Hispanic and Black individuals had disproportionately higher increases in functional limitation; percentage point increases over the 2 decades were 19.5 for Black people, 25.1 for Hispanic people and 12.5 for White people. There may be a couple of reasons for that, Mr. Patel noted.
Those who are Black or Hispanic tend to have less access to cancer survivorship care for reasons including insurance status and historic health care inequities, he noted.
“The other potential reason is that they have had less access to cancer care historically. And if, 20 years ago Black and Hispanic individuals didn’t have access to some chemotherapies, and now they do, maybe it’s the increased access to care that’s causing these functional limitations. Because chemotherapy can sometimes be very toxic. It may be sort of a catch-up toxicity,” he said.
Quality of life beyond survivorship
Mr. Patel said the results seem to call for building on improved survival rates by tracking and improving function.
“It’s good to celebrate that there are more survivors. But now that we can keep people alive longer, maybe we can shift gears to improving their quality of life,” he said.
The more-than-doubling of functional limitations over 2 decades “is a very sobering trend,” he noted, while pointing out that the functional limitations applied to 8 million people in the United States – people whose needs are not being met.
There’s no sign of the trend stopping, he continued. “We saw no downward trend, only an upward trend.”
Increasingly, including functionality as an endpoint in cancer trials, in addition to improvements in mortality, is one place to start, he added.
“Our findings suggest an urgent need for care teams to understand and address function, for researchers to evaluate function as a core outcome in trials, and for health systems and policy makers to reimagine survivorship care, recognizing the burden of cancer and its treatment on physical, psychosocial, and cognitive function,” the authors wrote in their paper. Limitations of the study include the potential for recall bias, lack of cancer staging or treatment information, and the subjective perception of function.
A coauthor reported personal fees from Astellas, AstraZeneca, AAA, Blue Earth, Janssen, Lantheus, Myovant, Myriad Genetics, Novartis, Telix, and Sanofi, as well as grants from Pfizer and Bayer during the conduct of the study. No other disclosures were reported.
Vishal Patel, BS, a student at the Dell Medical School at The University of Texas at Austin, and colleagues identified 51,258 cancer survivors from the National Health Interview Survey, representing a weighted population of approximately 178.8 million from 1999 to 2018.
Most survivors were women (60.2%) and were at least 65 years old (55.4%). In 1999, 3.6 million weighted survivors reported functional limitation. In 2018, the number increased to 8.2 million, a 2.25-fold increase.
The number of survivors who reported no limitations also increased, but not by as much. That group grew 1.34-fold during the study period.
For context, “the 70% prevalence of functional limitation among survivors in 2018 is nearly twice that of the general population,” the authors wrote.
Patients surveyed on function
Functional limitation was defined as “self-reported difficulty performing any of 12 routine physical or social activities without assistance.” Examples of the activities included difficulty sitting for more than 2 hours, difficulty participating in social activities or difficulty pushing or pulling an object the size of a living room chair.
Over the 2 decades analyzed, the adjusted prevalence of functional limitation was highest among survivors of pancreatic cancer (80.3%) and lung cancer (76.5%). Prevalence was lowest for survivors of melanoma (62.2%), breast (61.8%) and prostate (59.5%) cancers.
Not just a result of living longer
Mr. Patel told this publication that one assumption people might make when they read these results is that people are just living longer with cancer and losing functional ability accordingly.
“But, in fact, we found that the youngest [– those less than 65 years–] actually contributed to this trend more than the oldest people, which means it’s not just [happening], because people are getting older,” he said.
Hispanic and Black individuals had disproportionately higher increases in functional limitation; percentage point increases over the 2 decades were 19.5 for Black people, 25.1 for Hispanic people and 12.5 for White people. There may be a couple of reasons for that, Mr. Patel noted.
Those who are Black or Hispanic tend to have less access to cancer survivorship care for reasons including insurance status and historic health care inequities, he noted.
“The other potential reason is that they have had less access to cancer care historically. And if, 20 years ago Black and Hispanic individuals didn’t have access to some chemotherapies, and now they do, maybe it’s the increased access to care that’s causing these functional limitations. Because chemotherapy can sometimes be very toxic. It may be sort of a catch-up toxicity,” he said.
Quality of life beyond survivorship
Mr. Patel said the results seem to call for building on improved survival rates by tracking and improving function.
“It’s good to celebrate that there are more survivors. But now that we can keep people alive longer, maybe we can shift gears to improving their quality of life,” he said.
The more-than-doubling of functional limitations over 2 decades “is a very sobering trend,” he noted, while pointing out that the functional limitations applied to 8 million people in the United States – people whose needs are not being met.
There’s no sign of the trend stopping, he continued. “We saw no downward trend, only an upward trend.”
Increasingly, including functionality as an endpoint in cancer trials, in addition to improvements in mortality, is one place to start, he added.
“Our findings suggest an urgent need for care teams to understand and address function, for researchers to evaluate function as a core outcome in trials, and for health systems and policy makers to reimagine survivorship care, recognizing the burden of cancer and its treatment on physical, psychosocial, and cognitive function,” the authors wrote in their paper. Limitations of the study include the potential for recall bias, lack of cancer staging or treatment information, and the subjective perception of function.
A coauthor reported personal fees from Astellas, AstraZeneca, AAA, Blue Earth, Janssen, Lantheus, Myovant, Myriad Genetics, Novartis, Telix, and Sanofi, as well as grants from Pfizer and Bayer during the conduct of the study. No other disclosures were reported.
Vishal Patel, BS, a student at the Dell Medical School at The University of Texas at Austin, and colleagues identified 51,258 cancer survivors from the National Health Interview Survey, representing a weighted population of approximately 178.8 million from 1999 to 2018.
Most survivors were women (60.2%) and were at least 65 years old (55.4%). In 1999, 3.6 million weighted survivors reported functional limitation. In 2018, the number increased to 8.2 million, a 2.25-fold increase.
The number of survivors who reported no limitations also increased, but not by as much. That group grew 1.34-fold during the study period.
For context, “the 70% prevalence of functional limitation among survivors in 2018 is nearly twice that of the general population,” the authors wrote.
Patients surveyed on function
Functional limitation was defined as “self-reported difficulty performing any of 12 routine physical or social activities without assistance.” Examples of the activities included difficulty sitting for more than 2 hours, difficulty participating in social activities or difficulty pushing or pulling an object the size of a living room chair.
Over the 2 decades analyzed, the adjusted prevalence of functional limitation was highest among survivors of pancreatic cancer (80.3%) and lung cancer (76.5%). Prevalence was lowest for survivors of melanoma (62.2%), breast (61.8%) and prostate (59.5%) cancers.
Not just a result of living longer
Mr. Patel told this publication that one assumption people might make when they read these results is that people are just living longer with cancer and losing functional ability accordingly.
“But, in fact, we found that the youngest [– those less than 65 years–] actually contributed to this trend more than the oldest people, which means it’s not just [happening], because people are getting older,” he said.
Hispanic and Black individuals had disproportionately higher increases in functional limitation; percentage point increases over the 2 decades were 19.5 for Black people, 25.1 for Hispanic people and 12.5 for White people. There may be a couple of reasons for that, Mr. Patel noted.
Those who are Black or Hispanic tend to have less access to cancer survivorship care for reasons including insurance status and historic health care inequities, he noted.
“The other potential reason is that they have had less access to cancer care historically. And if, 20 years ago Black and Hispanic individuals didn’t have access to some chemotherapies, and now they do, maybe it’s the increased access to care that’s causing these functional limitations. Because chemotherapy can sometimes be very toxic. It may be sort of a catch-up toxicity,” he said.
Quality of life beyond survivorship
Mr. Patel said the results seem to call for building on improved survival rates by tracking and improving function.
“It’s good to celebrate that there are more survivors. But now that we can keep people alive longer, maybe we can shift gears to improving their quality of life,” he said.
The more-than-doubling of functional limitations over 2 decades “is a very sobering trend,” he noted, while pointing out that the functional limitations applied to 8 million people in the United States – people whose needs are not being met.
There’s no sign of the trend stopping, he continued. “We saw no downward trend, only an upward trend.”
Increasingly, including functionality as an endpoint in cancer trials, in addition to improvements in mortality, is one place to start, he added.
“Our findings suggest an urgent need for care teams to understand and address function, for researchers to evaluate function as a core outcome in trials, and for health systems and policy makers to reimagine survivorship care, recognizing the burden of cancer and its treatment on physical, psychosocial, and cognitive function,” the authors wrote in their paper. Limitations of the study include the potential for recall bias, lack of cancer staging or treatment information, and the subjective perception of function.
A coauthor reported personal fees from Astellas, AstraZeneca, AAA, Blue Earth, Janssen, Lantheus, Myovant, Myriad Genetics, Novartis, Telix, and Sanofi, as well as grants from Pfizer and Bayer during the conduct of the study. No other disclosures were reported.
FROM JAMA ONCOLOGY
Study shows higher obesity-related cancer mortality in areas with more fast food
based on data from a new cross-sectional study of more than 3,000 communities.
Although increased healthy eating has been associated with reduced risk of obesity and with reduced cancer incidence and mortality, access to healthier eating remains a challenge in communities with less access to grocery stores and healthy food options (food deserts) and/or easy access to convenience stores and fast food (food swamps), Malcolm Seth Bevel, PhD, of the Medical College of Georgia, Augusta, and colleagues, wrote in their paper, published in JAMA Oncology.
In addition, data on the association between food deserts and swamps and obesity-related cancer mortality are limited, they said.
“We felt that the study was important given the fact that obesity is an epidemic in the United States, and multiple factors contribute to obesity, especially adverse food environments,” Dr. Bevel said in an interview. “Also, I lived in these areas my whole life, and saw how it affected underserved populations. There was a story that needed to be told, so we’re telling it,” he said in an interview.
In a study, the researchers analyzed food access and cancer mortality data from 3,038 counties across the United States. The food access data came from the U.S. Department of Agriculture Food Environment Atlas (FEA) for the years 2012, 2014, 2015, 2017, and 2020. Data on obesity-related cancer mortality came from the Centers for Disease Control and Prevention for the years from 2010 to 2020.
Food desert scores were calculated through data from the FEA, and food swamp scores were based on the ratio of fast-food restaurants and convenience stores to grocery stores and farmers markets in a modification of the Retail Food Environment Index score.
The researchers used an age-adjusted, multiple regression model to determine the association between food desert and food swamp scores and obesity-related cancer mortality rates. Higher food swamp and food desert scores (defined as 20.0 to 58.0 or higher) were used to classify counties as having fewer healthy food resources. The primary outcome was obesity-related cancer mortality, defined as high or low (71.8 or higher per 100,000 individuals and less than 71.8 per 100,000 individuals, respectively).
Overall, high rates of obesity-related cancer mortality were 77% more likely in the counties that met the criteria for high food swamp scores (adjusted odds ratio 1.77). In addition, researchers found a positive dose-response relationship among three levels of both food desert scores and food swamp scores and obesity-related cancer mortality.
A total of 758 counties had obesity-related cancer mortality rates in the highest quartile. Compared to counties with low rates of obesity-related cancer mortality, counties with high rates of obesity-related cancer mortality also had a higher percentage of non-Hispanic Black residents (3.26% vs. 1.77%), higher percentage of adults older than 65 years (15.71% vs. 15.40%), higher rates of adult obesity (33.0% vs. 32.10%), and higher rates of adult diabetes (12.50% vs. 10.70%).
Possible explanations for the results include the lack of interest in grocery stores in neighborhoods with a population with a lower socioeconomic status, which can create a food desert, the researchers wrote in their discussion. “Coupled with the increasing growth rate of fast-food restaurants in recent years and the intentional advertisement of unhealthy foods in urban neighborhoods with [people of lower income], the food desert may transform into a food swamp,” they said.
The findings were limited by several factors including the study design, which did not allow for showing a causal association of food deserts and food swamps with obesity-related cancer mortality, the researchers noted. Other limitations included the use of groups rather than individuals, the potential misclassification of food stores, and the use of county-level data on race, ethnicity, and income, they wrote.
The results indicate that “food swamps appear to be a growing epidemic across the U.S., likely because of systemic issues, and should draw concern and conversation from local and state officials,” the researchers concluded.
Community-level investments can benefit individual health
Dr. Bevel said he was not surprised by the findings, as he has seen firsthand the lack of healthy food options and growth of unhealthy food options, especially for certain populations in certain communities. “Typically, these are people who have lower socioeconomic status, primarily non-Hispanic Black or African American or Hispanic American,” he said “I have watched people have to choose between getting fruits/vegetables versus their medications or running to fast food places to feed their families. What is truly surprising is that we’re not talking about people’s lived environment enough for my taste,” he said.
“I hope that our data and results can inform local and state policymakers to truly invest in all communities, such as funding for community gardens, and realize that adverse food environments, including the barriers in navigating these environments, have significant consequences on real people,” said Dr. Bevel. “Also, I hope that the results can help clinicians realize that a patient’s lived environment can truly affect their obesity and/or obesity-related cancer status; being cognizant of that is the first step in holistic, comprehensive care,” he said.
“One role that oncologists might be able to play in improving patients’ access to healthier food is to create and/or implement healthy lifestyle programs with gardening components to combat the poorest food environments that their patients likely reside in,” said Dr. Bevel. Clinicians also could consider the innovative approach of “food prescriptions” to help reduce the effects of deprived, built environments, he noted.
Looking ahead, next steps for research include determining the severity of association between food swamps and obesity-related cancer by varying factors such as cancer type, and examining any potential racial disparities between people living in these environments and obesity-related cancer, Dr. Bevel added.
Data provide foundation for multilevel interventions
The current study findings “raise a clarion call to elevate the discussion on food availability and access to ensure an equitable emphasis on both the importance of lifestyle factors and the upstream structural, economic, and environmental contexts that shape these behaviors at the individual level,” Karriem S. Watson, DHSc, MS, MPH, of the National Institutes of Health, Bethesda, Md., and Angela Odoms-Young, PhD, of Cornell University, Ithaca, N.Y., wrote in an accompanying editorial.
The findings provide a foundation for studies of obesity-related cancer outcomes that take the community environment into consideration, they added.
The causes of both obesity and cancer are complex, and the study findings suggest that the links between unhealthy food environments and obesity-related cancer may go beyond dietary consumption alone and extend to social and psychological factors, the editorialists noted.
“Whether dealing with the lack of access to healthy foods or an overabundance of unhealthy food, there is a critical need to develop additional research that explores the associations between obesity-related cancer mortality and food inequities,” they concluded.
The study received no outside funding. The researchers and the editorialists had no financial conflicts to disclose.
based on data from a new cross-sectional study of more than 3,000 communities.
Although increased healthy eating has been associated with reduced risk of obesity and with reduced cancer incidence and mortality, access to healthier eating remains a challenge in communities with less access to grocery stores and healthy food options (food deserts) and/or easy access to convenience stores and fast food (food swamps), Malcolm Seth Bevel, PhD, of the Medical College of Georgia, Augusta, and colleagues, wrote in their paper, published in JAMA Oncology.
In addition, data on the association between food deserts and swamps and obesity-related cancer mortality are limited, they said.
“We felt that the study was important given the fact that obesity is an epidemic in the United States, and multiple factors contribute to obesity, especially adverse food environments,” Dr. Bevel said in an interview. “Also, I lived in these areas my whole life, and saw how it affected underserved populations. There was a story that needed to be told, so we’re telling it,” he said in an interview.
In a study, the researchers analyzed food access and cancer mortality data from 3,038 counties across the United States. The food access data came from the U.S. Department of Agriculture Food Environment Atlas (FEA) for the years 2012, 2014, 2015, 2017, and 2020. Data on obesity-related cancer mortality came from the Centers for Disease Control and Prevention for the years from 2010 to 2020.
Food desert scores were calculated through data from the FEA, and food swamp scores were based on the ratio of fast-food restaurants and convenience stores to grocery stores and farmers markets in a modification of the Retail Food Environment Index score.
The researchers used an age-adjusted, multiple regression model to determine the association between food desert and food swamp scores and obesity-related cancer mortality rates. Higher food swamp and food desert scores (defined as 20.0 to 58.0 or higher) were used to classify counties as having fewer healthy food resources. The primary outcome was obesity-related cancer mortality, defined as high or low (71.8 or higher per 100,000 individuals and less than 71.8 per 100,000 individuals, respectively).
Overall, high rates of obesity-related cancer mortality were 77% more likely in the counties that met the criteria for high food swamp scores (adjusted odds ratio 1.77). In addition, researchers found a positive dose-response relationship among three levels of both food desert scores and food swamp scores and obesity-related cancer mortality.
A total of 758 counties had obesity-related cancer mortality rates in the highest quartile. Compared to counties with low rates of obesity-related cancer mortality, counties with high rates of obesity-related cancer mortality also had a higher percentage of non-Hispanic Black residents (3.26% vs. 1.77%), higher percentage of adults older than 65 years (15.71% vs. 15.40%), higher rates of adult obesity (33.0% vs. 32.10%), and higher rates of adult diabetes (12.50% vs. 10.70%).
Possible explanations for the results include the lack of interest in grocery stores in neighborhoods with a population with a lower socioeconomic status, which can create a food desert, the researchers wrote in their discussion. “Coupled with the increasing growth rate of fast-food restaurants in recent years and the intentional advertisement of unhealthy foods in urban neighborhoods with [people of lower income], the food desert may transform into a food swamp,” they said.
The findings were limited by several factors including the study design, which did not allow for showing a causal association of food deserts and food swamps with obesity-related cancer mortality, the researchers noted. Other limitations included the use of groups rather than individuals, the potential misclassification of food stores, and the use of county-level data on race, ethnicity, and income, they wrote.
The results indicate that “food swamps appear to be a growing epidemic across the U.S., likely because of systemic issues, and should draw concern and conversation from local and state officials,” the researchers concluded.
Community-level investments can benefit individual health
Dr. Bevel said he was not surprised by the findings, as he has seen firsthand the lack of healthy food options and growth of unhealthy food options, especially for certain populations in certain communities. “Typically, these are people who have lower socioeconomic status, primarily non-Hispanic Black or African American or Hispanic American,” he said “I have watched people have to choose between getting fruits/vegetables versus their medications or running to fast food places to feed their families. What is truly surprising is that we’re not talking about people’s lived environment enough for my taste,” he said.
“I hope that our data and results can inform local and state policymakers to truly invest in all communities, such as funding for community gardens, and realize that adverse food environments, including the barriers in navigating these environments, have significant consequences on real people,” said Dr. Bevel. “Also, I hope that the results can help clinicians realize that a patient’s lived environment can truly affect their obesity and/or obesity-related cancer status; being cognizant of that is the first step in holistic, comprehensive care,” he said.
“One role that oncologists might be able to play in improving patients’ access to healthier food is to create and/or implement healthy lifestyle programs with gardening components to combat the poorest food environments that their patients likely reside in,” said Dr. Bevel. Clinicians also could consider the innovative approach of “food prescriptions” to help reduce the effects of deprived, built environments, he noted.
Looking ahead, next steps for research include determining the severity of association between food swamps and obesity-related cancer by varying factors such as cancer type, and examining any potential racial disparities between people living in these environments and obesity-related cancer, Dr. Bevel added.
Data provide foundation for multilevel interventions
The current study findings “raise a clarion call to elevate the discussion on food availability and access to ensure an equitable emphasis on both the importance of lifestyle factors and the upstream structural, economic, and environmental contexts that shape these behaviors at the individual level,” Karriem S. Watson, DHSc, MS, MPH, of the National Institutes of Health, Bethesda, Md., and Angela Odoms-Young, PhD, of Cornell University, Ithaca, N.Y., wrote in an accompanying editorial.
The findings provide a foundation for studies of obesity-related cancer outcomes that take the community environment into consideration, they added.
The causes of both obesity and cancer are complex, and the study findings suggest that the links between unhealthy food environments and obesity-related cancer may go beyond dietary consumption alone and extend to social and psychological factors, the editorialists noted.
“Whether dealing with the lack of access to healthy foods or an overabundance of unhealthy food, there is a critical need to develop additional research that explores the associations between obesity-related cancer mortality and food inequities,” they concluded.
The study received no outside funding. The researchers and the editorialists had no financial conflicts to disclose.
based on data from a new cross-sectional study of more than 3,000 communities.
Although increased healthy eating has been associated with reduced risk of obesity and with reduced cancer incidence and mortality, access to healthier eating remains a challenge in communities with less access to grocery stores and healthy food options (food deserts) and/or easy access to convenience stores and fast food (food swamps), Malcolm Seth Bevel, PhD, of the Medical College of Georgia, Augusta, and colleagues, wrote in their paper, published in JAMA Oncology.
In addition, data on the association between food deserts and swamps and obesity-related cancer mortality are limited, they said.
“We felt that the study was important given the fact that obesity is an epidemic in the United States, and multiple factors contribute to obesity, especially adverse food environments,” Dr. Bevel said in an interview. “Also, I lived in these areas my whole life, and saw how it affected underserved populations. There was a story that needed to be told, so we’re telling it,” he said in an interview.
In a study, the researchers analyzed food access and cancer mortality data from 3,038 counties across the United States. The food access data came from the U.S. Department of Agriculture Food Environment Atlas (FEA) for the years 2012, 2014, 2015, 2017, and 2020. Data on obesity-related cancer mortality came from the Centers for Disease Control and Prevention for the years from 2010 to 2020.
Food desert scores were calculated through data from the FEA, and food swamp scores were based on the ratio of fast-food restaurants and convenience stores to grocery stores and farmers markets in a modification of the Retail Food Environment Index score.
The researchers used an age-adjusted, multiple regression model to determine the association between food desert and food swamp scores and obesity-related cancer mortality rates. Higher food swamp and food desert scores (defined as 20.0 to 58.0 or higher) were used to classify counties as having fewer healthy food resources. The primary outcome was obesity-related cancer mortality, defined as high or low (71.8 or higher per 100,000 individuals and less than 71.8 per 100,000 individuals, respectively).
Overall, high rates of obesity-related cancer mortality were 77% more likely in the counties that met the criteria for high food swamp scores (adjusted odds ratio 1.77). In addition, researchers found a positive dose-response relationship among three levels of both food desert scores and food swamp scores and obesity-related cancer mortality.
A total of 758 counties had obesity-related cancer mortality rates in the highest quartile. Compared to counties with low rates of obesity-related cancer mortality, counties with high rates of obesity-related cancer mortality also had a higher percentage of non-Hispanic Black residents (3.26% vs. 1.77%), higher percentage of adults older than 65 years (15.71% vs. 15.40%), higher rates of adult obesity (33.0% vs. 32.10%), and higher rates of adult diabetes (12.50% vs. 10.70%).
Possible explanations for the results include the lack of interest in grocery stores in neighborhoods with a population with a lower socioeconomic status, which can create a food desert, the researchers wrote in their discussion. “Coupled with the increasing growth rate of fast-food restaurants in recent years and the intentional advertisement of unhealthy foods in urban neighborhoods with [people of lower income], the food desert may transform into a food swamp,” they said.
The findings were limited by several factors including the study design, which did not allow for showing a causal association of food deserts and food swamps with obesity-related cancer mortality, the researchers noted. Other limitations included the use of groups rather than individuals, the potential misclassification of food stores, and the use of county-level data on race, ethnicity, and income, they wrote.
The results indicate that “food swamps appear to be a growing epidemic across the U.S., likely because of systemic issues, and should draw concern and conversation from local and state officials,” the researchers concluded.
Community-level investments can benefit individual health
Dr. Bevel said he was not surprised by the findings, as he has seen firsthand the lack of healthy food options and growth of unhealthy food options, especially for certain populations in certain communities. “Typically, these are people who have lower socioeconomic status, primarily non-Hispanic Black or African American or Hispanic American,” he said “I have watched people have to choose between getting fruits/vegetables versus their medications or running to fast food places to feed their families. What is truly surprising is that we’re not talking about people’s lived environment enough for my taste,” he said.
“I hope that our data and results can inform local and state policymakers to truly invest in all communities, such as funding for community gardens, and realize that adverse food environments, including the barriers in navigating these environments, have significant consequences on real people,” said Dr. Bevel. “Also, I hope that the results can help clinicians realize that a patient’s lived environment can truly affect their obesity and/or obesity-related cancer status; being cognizant of that is the first step in holistic, comprehensive care,” he said.
“One role that oncologists might be able to play in improving patients’ access to healthier food is to create and/or implement healthy lifestyle programs with gardening components to combat the poorest food environments that their patients likely reside in,” said Dr. Bevel. Clinicians also could consider the innovative approach of “food prescriptions” to help reduce the effects of deprived, built environments, he noted.
Looking ahead, next steps for research include determining the severity of association between food swamps and obesity-related cancer by varying factors such as cancer type, and examining any potential racial disparities between people living in these environments and obesity-related cancer, Dr. Bevel added.
Data provide foundation for multilevel interventions
The current study findings “raise a clarion call to elevate the discussion on food availability and access to ensure an equitable emphasis on both the importance of lifestyle factors and the upstream structural, economic, and environmental contexts that shape these behaviors at the individual level,” Karriem S. Watson, DHSc, MS, MPH, of the National Institutes of Health, Bethesda, Md., and Angela Odoms-Young, PhD, of Cornell University, Ithaca, N.Y., wrote in an accompanying editorial.
The findings provide a foundation for studies of obesity-related cancer outcomes that take the community environment into consideration, they added.
The causes of both obesity and cancer are complex, and the study findings suggest that the links between unhealthy food environments and obesity-related cancer may go beyond dietary consumption alone and extend to social and psychological factors, the editorialists noted.
“Whether dealing with the lack of access to healthy foods or an overabundance of unhealthy food, there is a critical need to develop additional research that explores the associations between obesity-related cancer mortality and food inequities,” they concluded.
The study received no outside funding. The researchers and the editorialists had no financial conflicts to disclose.
FROM JAMA ONCOLOGY
Expert discusses which diets are best, based on the evidence
according to a speaker at the annual meeting of the American College of Physicians.
“Evidence from studies can help clinicians and their patients develop a successful dietary management plan and achieve optimal health,” said internist Michelle Hauser, MD, clinical associate professor at Stanford (Calif.) University. She also discussed evidence-based techniques to support patients in maintaining dietary modifications.
Predominantly plant‐based diets
Popular predominantly plant‐based diets include a Mediterranean diet, healthy vegetarian diet, predominantly whole-food plant‐based (WFPB) diet, and a dietary approach to stop hypertension (DASH).
The DASH diet was originally designed to help patients manage their blood pressure, but evidence suggests that it also can help adults with obesity lose weight. In contrast to the DASH diet, the Mediterranean diet is not low-fat and not very restrictive. Yet the evidence suggests that the Mediterranean diet is not only helpful for losing weight but also can reduce the risk of various chronic diseases, including obesity, type 2 diabetes, cardiovascular disease (CVD), and cancer, Dr. Hauser said. In addition, data suggest that the Mediterranean diet may reduce the risk of all-cause mortality and lower the levels of cholesterol.
“I like to highlight all these protective effects to my patients, because even if their goal is to lose weight, knowing that hard work pays off in additional ways can keep them motivated,” Dr. Hauser stated.
A healthy vegetarian diet and a WFPB diet are similar, and both are helpful in weight loss and management of total cholesterol and LDL‐C levels. Furthermore, healthy vegetarian and WFPB diets may reduce the risk of type 2 diabetes, CVD, and some cancers. Cohort study data suggest that progressively more vegetarian diets are associated with lower BMIs.
“My interpretation of these data is that predominantly plant-based diets rich in whole foods are healthful and can be done in a way that is sustainable for most,” said Dr. Hauser. However, this generally requires a lot of support at the outset to address gaps in knowledge, skills, and other potential barriers.
For example, she referred one obese patient at risk of diabetes and cardiovascular disease to a registered dietitian to develop a dietary plan. The patient also attended a behavioral medicine weight management program to learn strategies such as using smaller plates, and his family attended a healthy cooking class together to improve meal planning and cooking skills.
Time‐restricted feeding
There are numerous variations of time-restricted feeding, commonly referred to as intermittent fasting, but the principles are similar – limiting food intake to a specific window of time each day or week.
Although some studies have shown that time-restricted feeding may help patients reduce adiposity and improve lipid markers, most studies comparing time-restricted feeding to a calorie-restricted diet have shown little to no difference in weight-related outcomes, Dr. Hauser said.
These data suggest that time-restricted feeding may help patients with weight loss only if time restriction helps them reduce calorie intake. She also warned that time-restrictive feeding might cause late-night cravings and might not be helpful in individuals prone to food cravings.
Low‐carbohydrate and ketogenic diets
Losing muscle mass can prevent some people from dieting, but evidence suggests that a high-fat, very low-carbohydrate diet – also called a ketogenic diet – may help patients reduce weight and fat mass while preserving fat‐free mass, Dr. Hauser said.
The evidence regarding the usefulness of a low-carbohydrate (non-keto) diet is less clear because most studies compared it to a low-fat diet, and these two diets might lead to a similar extent of weight loss.
Rating the level of scientific evidence behind different diet options
Nutrition studies do no provide the same level of evidence as drug studies, said Dr. Hauser, because it is easier to conduct a randomized controlled trial of a drug versus placebo. Diets have many more variables, and it also takes much longer to observe most outcomes of a dietary change.
In addition, clinical trials of dietary interventions are typically short and focus on disease markers such as serum lipids and hemoglobin A1c levels. To obtain reliable information on the usefulness of a diet, researchers need to collect detailed health and lifestyle information from hundreds of thousands of people over several decades, which is not always feasible. “This is why meta-analyses of pooled dietary study data are more likely to yield dependable findings,” she noted.
Getting to know patients is essential to help them maintain diet modifications
When developing a diet plan for a patient, it is important to consider the sustainability of a dietary pattern. “The benefits of any healthy dietary change will only last as long as they can be maintained,” said Dr. Hauser. “Counseling someone on choosing an appropriate long-term dietary pattern requires getting to know them – taste preferences, food traditions, barriers, facilitators, food access, and time and cost restrictions.”
In an interview after the session, David Bittleman, MD, an internist at Veterans Affairs San Diego Health Care System, agreed that getting to know patients is essential for successfully advising them on diet.
“I always start developing a diet plan by trying to find out what [a patient’s] diet is like and what their goals are. I need to know what they are already doing in order to make suggestions about what they can do to make their diet healthier,” he said.
When asked about her approach to supporting patients in the long term, Dr. Hauser said that she recommends sequential, gradual changes. Dr. Hauser added that she suggests her patients prioritize implementing dietary changes that they are confident they can maintain.
Dr. Hauser and Dr. Bittleman report no relevant financial relationships.
according to a speaker at the annual meeting of the American College of Physicians.
“Evidence from studies can help clinicians and their patients develop a successful dietary management plan and achieve optimal health,” said internist Michelle Hauser, MD, clinical associate professor at Stanford (Calif.) University. She also discussed evidence-based techniques to support patients in maintaining dietary modifications.
Predominantly plant‐based diets
Popular predominantly plant‐based diets include a Mediterranean diet, healthy vegetarian diet, predominantly whole-food plant‐based (WFPB) diet, and a dietary approach to stop hypertension (DASH).
The DASH diet was originally designed to help patients manage their blood pressure, but evidence suggests that it also can help adults with obesity lose weight. In contrast to the DASH diet, the Mediterranean diet is not low-fat and not very restrictive. Yet the evidence suggests that the Mediterranean diet is not only helpful for losing weight but also can reduce the risk of various chronic diseases, including obesity, type 2 diabetes, cardiovascular disease (CVD), and cancer, Dr. Hauser said. In addition, data suggest that the Mediterranean diet may reduce the risk of all-cause mortality and lower the levels of cholesterol.
“I like to highlight all these protective effects to my patients, because even if their goal is to lose weight, knowing that hard work pays off in additional ways can keep them motivated,” Dr. Hauser stated.
A healthy vegetarian diet and a WFPB diet are similar, and both are helpful in weight loss and management of total cholesterol and LDL‐C levels. Furthermore, healthy vegetarian and WFPB diets may reduce the risk of type 2 diabetes, CVD, and some cancers. Cohort study data suggest that progressively more vegetarian diets are associated with lower BMIs.
“My interpretation of these data is that predominantly plant-based diets rich in whole foods are healthful and can be done in a way that is sustainable for most,” said Dr. Hauser. However, this generally requires a lot of support at the outset to address gaps in knowledge, skills, and other potential barriers.
For example, she referred one obese patient at risk of diabetes and cardiovascular disease to a registered dietitian to develop a dietary plan. The patient also attended a behavioral medicine weight management program to learn strategies such as using smaller plates, and his family attended a healthy cooking class together to improve meal planning and cooking skills.
Time‐restricted feeding
There are numerous variations of time-restricted feeding, commonly referred to as intermittent fasting, but the principles are similar – limiting food intake to a specific window of time each day or week.
Although some studies have shown that time-restricted feeding may help patients reduce adiposity and improve lipid markers, most studies comparing time-restricted feeding to a calorie-restricted diet have shown little to no difference in weight-related outcomes, Dr. Hauser said.
These data suggest that time-restricted feeding may help patients with weight loss only if time restriction helps them reduce calorie intake. She also warned that time-restrictive feeding might cause late-night cravings and might not be helpful in individuals prone to food cravings.
Low‐carbohydrate and ketogenic diets
Losing muscle mass can prevent some people from dieting, but evidence suggests that a high-fat, very low-carbohydrate diet – also called a ketogenic diet – may help patients reduce weight and fat mass while preserving fat‐free mass, Dr. Hauser said.
The evidence regarding the usefulness of a low-carbohydrate (non-keto) diet is less clear because most studies compared it to a low-fat diet, and these two diets might lead to a similar extent of weight loss.
Rating the level of scientific evidence behind different diet options
Nutrition studies do no provide the same level of evidence as drug studies, said Dr. Hauser, because it is easier to conduct a randomized controlled trial of a drug versus placebo. Diets have many more variables, and it also takes much longer to observe most outcomes of a dietary change.
In addition, clinical trials of dietary interventions are typically short and focus on disease markers such as serum lipids and hemoglobin A1c levels. To obtain reliable information on the usefulness of a diet, researchers need to collect detailed health and lifestyle information from hundreds of thousands of people over several decades, which is not always feasible. “This is why meta-analyses of pooled dietary study data are more likely to yield dependable findings,” she noted.
Getting to know patients is essential to help them maintain diet modifications
When developing a diet plan for a patient, it is important to consider the sustainability of a dietary pattern. “The benefits of any healthy dietary change will only last as long as they can be maintained,” said Dr. Hauser. “Counseling someone on choosing an appropriate long-term dietary pattern requires getting to know them – taste preferences, food traditions, barriers, facilitators, food access, and time and cost restrictions.”
In an interview after the session, David Bittleman, MD, an internist at Veterans Affairs San Diego Health Care System, agreed that getting to know patients is essential for successfully advising them on diet.
“I always start developing a diet plan by trying to find out what [a patient’s] diet is like and what their goals are. I need to know what they are already doing in order to make suggestions about what they can do to make their diet healthier,” he said.
When asked about her approach to supporting patients in the long term, Dr. Hauser said that she recommends sequential, gradual changes. Dr. Hauser added that she suggests her patients prioritize implementing dietary changes that they are confident they can maintain.
Dr. Hauser and Dr. Bittleman report no relevant financial relationships.
according to a speaker at the annual meeting of the American College of Physicians.
“Evidence from studies can help clinicians and their patients develop a successful dietary management plan and achieve optimal health,” said internist Michelle Hauser, MD, clinical associate professor at Stanford (Calif.) University. She also discussed evidence-based techniques to support patients in maintaining dietary modifications.
Predominantly plant‐based diets
Popular predominantly plant‐based diets include a Mediterranean diet, healthy vegetarian diet, predominantly whole-food plant‐based (WFPB) diet, and a dietary approach to stop hypertension (DASH).
The DASH diet was originally designed to help patients manage their blood pressure, but evidence suggests that it also can help adults with obesity lose weight. In contrast to the DASH diet, the Mediterranean diet is not low-fat and not very restrictive. Yet the evidence suggests that the Mediterranean diet is not only helpful for losing weight but also can reduce the risk of various chronic diseases, including obesity, type 2 diabetes, cardiovascular disease (CVD), and cancer, Dr. Hauser said. In addition, data suggest that the Mediterranean diet may reduce the risk of all-cause mortality and lower the levels of cholesterol.
“I like to highlight all these protective effects to my patients, because even if their goal is to lose weight, knowing that hard work pays off in additional ways can keep them motivated,” Dr. Hauser stated.
A healthy vegetarian diet and a WFPB diet are similar, and both are helpful in weight loss and management of total cholesterol and LDL‐C levels. Furthermore, healthy vegetarian and WFPB diets may reduce the risk of type 2 diabetes, CVD, and some cancers. Cohort study data suggest that progressively more vegetarian diets are associated with lower BMIs.
“My interpretation of these data is that predominantly plant-based diets rich in whole foods are healthful and can be done in a way that is sustainable for most,” said Dr. Hauser. However, this generally requires a lot of support at the outset to address gaps in knowledge, skills, and other potential barriers.
For example, she referred one obese patient at risk of diabetes and cardiovascular disease to a registered dietitian to develop a dietary plan. The patient also attended a behavioral medicine weight management program to learn strategies such as using smaller plates, and his family attended a healthy cooking class together to improve meal planning and cooking skills.
Time‐restricted feeding
There are numerous variations of time-restricted feeding, commonly referred to as intermittent fasting, but the principles are similar – limiting food intake to a specific window of time each day or week.
Although some studies have shown that time-restricted feeding may help patients reduce adiposity and improve lipid markers, most studies comparing time-restricted feeding to a calorie-restricted diet have shown little to no difference in weight-related outcomes, Dr. Hauser said.
These data suggest that time-restricted feeding may help patients with weight loss only if time restriction helps them reduce calorie intake. She also warned that time-restrictive feeding might cause late-night cravings and might not be helpful in individuals prone to food cravings.
Low‐carbohydrate and ketogenic diets
Losing muscle mass can prevent some people from dieting, but evidence suggests that a high-fat, very low-carbohydrate diet – also called a ketogenic diet – may help patients reduce weight and fat mass while preserving fat‐free mass, Dr. Hauser said.
The evidence regarding the usefulness of a low-carbohydrate (non-keto) diet is less clear because most studies compared it to a low-fat diet, and these two diets might lead to a similar extent of weight loss.
Rating the level of scientific evidence behind different diet options
Nutrition studies do no provide the same level of evidence as drug studies, said Dr. Hauser, because it is easier to conduct a randomized controlled trial of a drug versus placebo. Diets have many more variables, and it also takes much longer to observe most outcomes of a dietary change.
In addition, clinical trials of dietary interventions are typically short and focus on disease markers such as serum lipids and hemoglobin A1c levels. To obtain reliable information on the usefulness of a diet, researchers need to collect detailed health and lifestyle information from hundreds of thousands of people over several decades, which is not always feasible. “This is why meta-analyses of pooled dietary study data are more likely to yield dependable findings,” she noted.
Getting to know patients is essential to help them maintain diet modifications
When developing a diet plan for a patient, it is important to consider the sustainability of a dietary pattern. “The benefits of any healthy dietary change will only last as long as they can be maintained,” said Dr. Hauser. “Counseling someone on choosing an appropriate long-term dietary pattern requires getting to know them – taste preferences, food traditions, barriers, facilitators, food access, and time and cost restrictions.”
In an interview after the session, David Bittleman, MD, an internist at Veterans Affairs San Diego Health Care System, agreed that getting to know patients is essential for successfully advising them on diet.
“I always start developing a diet plan by trying to find out what [a patient’s] diet is like and what their goals are. I need to know what they are already doing in order to make suggestions about what they can do to make their diet healthier,” he said.
When asked about her approach to supporting patients in the long term, Dr. Hauser said that she recommends sequential, gradual changes. Dr. Hauser added that she suggests her patients prioritize implementing dietary changes that they are confident they can maintain.
Dr. Hauser and Dr. Bittleman report no relevant financial relationships.
AT INTERNAL MEDICINE 2023
Cancer pain declines with cannabis use
in a study.
Physician-prescribed cannabis, particularly cannabinoids, has been shown to ease cancer-related pain in adult cancer patients, who often find inadequate pain relief from medications including opioids, Saro Aprikian, MSc, a medical student at the Royal College of Surgeons, Dublin, and colleagues, wrote in their paper.
However, real-world data on the safety and effectiveness of cannabis in the cancer population and the impact on use of other medications are lacking, the researchers said.
In the study, published in BMJ Supportive & Palliative Care, the researchers reviewed data from 358 adults with cancer who were part of a multicenter cannabis registry in Canada between May 2015 and October 2018.
The average age of the patients was 57.6 years, and 48% were men. The top three cancer diagnoses in the study population were genitorurinary, breast, and colorectal.
Pain was the most common reason for obtaining a medical cannabis prescription, cited by 72.4% of patients.
Data were collected at follow-up visits conducted every 3 months over 1 year. Pain was assessed via the Brief Pain Inventory (BPI) and revised Edmonton Symptom Assessment System (ESAS-r) questionnaires and compared to baseline values. Patients rated their pain intensity on a sliding scale of 0 (none) to 10 (worst possible). Pain relief was rated on a scale of 0% (none) to 100% (complete).
Compared to baseline scores, patients showed significant decreases at 3, 6 and 9 months for BPI worst pain (5.5 at baseline, 3.6 for 3, 6, and 9 months) average pain (4.1 at baseline, 2.4, 2.3, and 2.7 for 3, 6, and 9 months, respectively), overall pain severity (2.7 at baseline, 2.3, 2.3, and 2.4 at 3, 6, and 9 months, respectively), and pain interference with daily life (4.3 at baseline, 2.4, 2.2, and 2.4 at 3, 6, and 9 months, respectively; P less than .01 for all four pain measures).
“Pain severity as reported in the ESAS-r decreased significantly at 3-month, 6-month and 9-month follow-ups,” the researchers noted.
In addition, total medication burden based on the medication quantification scale (MQS) and morphine equivalent daily dose (MEDD) were recorded at 3, 6, 9, and 12 months. MQS scores decreased compared to baseline at 3, 6, 9, and 12 months in 10%, 23.5%, 26.2%, and 31.6% of patients, respectively. Also compared with baseline, 11.1%, 31.3%, and 14.3% of patients reported decreases in MEDD scores at 3, 6, and 9 months, respectively.
Overall, products with equal amounts of active ingredients tetrahydrocannabinol (THC) and cannabidiol (CBD) were more effective than were those with a predominance of either THC or CBD, the researchers wrote.
Medical cannabis was well-tolerated; a total of 15 moderate to severe side effects were reported by 11 patients, 13 of which were minor. The most common side effects were sleepiness and fatigue, and five patients discontinued their medical cannabis because of side effects. The two serious side effects reported during the study period – pneumonia and a cardiovascular event – were deemed unlikely related to the patients’ medicinal cannabis use.
The findings were limited by several factors, including the observational design, which prevented conclusions about causality, the researchers noted. Other limitations included the loss of many patients to follow-up and incomplete data on other prescription medications in many cases.
The results support the use of medical cannabis by cancer patients as an adjunct pain relief strategy and a way to potentially reduce the use of other medications such as opioids, the authors concluded.
The study was supported by the Canadian Consortium for the Investigation of Cannabinoids, Collège des Médecins du Québec, and the Canopy Growth Corporation. The researchers had no financial conflicts to disclose.
in a study.
Physician-prescribed cannabis, particularly cannabinoids, has been shown to ease cancer-related pain in adult cancer patients, who often find inadequate pain relief from medications including opioids, Saro Aprikian, MSc, a medical student at the Royal College of Surgeons, Dublin, and colleagues, wrote in their paper.
However, real-world data on the safety and effectiveness of cannabis in the cancer population and the impact on use of other medications are lacking, the researchers said.
In the study, published in BMJ Supportive & Palliative Care, the researchers reviewed data from 358 adults with cancer who were part of a multicenter cannabis registry in Canada between May 2015 and October 2018.
The average age of the patients was 57.6 years, and 48% were men. The top three cancer diagnoses in the study population were genitorurinary, breast, and colorectal.
Pain was the most common reason for obtaining a medical cannabis prescription, cited by 72.4% of patients.
Data were collected at follow-up visits conducted every 3 months over 1 year. Pain was assessed via the Brief Pain Inventory (BPI) and revised Edmonton Symptom Assessment System (ESAS-r) questionnaires and compared to baseline values. Patients rated their pain intensity on a sliding scale of 0 (none) to 10 (worst possible). Pain relief was rated on a scale of 0% (none) to 100% (complete).
Compared to baseline scores, patients showed significant decreases at 3, 6 and 9 months for BPI worst pain (5.5 at baseline, 3.6 for 3, 6, and 9 months) average pain (4.1 at baseline, 2.4, 2.3, and 2.7 for 3, 6, and 9 months, respectively), overall pain severity (2.7 at baseline, 2.3, 2.3, and 2.4 at 3, 6, and 9 months, respectively), and pain interference with daily life (4.3 at baseline, 2.4, 2.2, and 2.4 at 3, 6, and 9 months, respectively; P less than .01 for all four pain measures).
“Pain severity as reported in the ESAS-r decreased significantly at 3-month, 6-month and 9-month follow-ups,” the researchers noted.
In addition, total medication burden based on the medication quantification scale (MQS) and morphine equivalent daily dose (MEDD) were recorded at 3, 6, 9, and 12 months. MQS scores decreased compared to baseline at 3, 6, 9, and 12 months in 10%, 23.5%, 26.2%, and 31.6% of patients, respectively. Also compared with baseline, 11.1%, 31.3%, and 14.3% of patients reported decreases in MEDD scores at 3, 6, and 9 months, respectively.
Overall, products with equal amounts of active ingredients tetrahydrocannabinol (THC) and cannabidiol (CBD) were more effective than were those with a predominance of either THC or CBD, the researchers wrote.
Medical cannabis was well-tolerated; a total of 15 moderate to severe side effects were reported by 11 patients, 13 of which were minor. The most common side effects were sleepiness and fatigue, and five patients discontinued their medical cannabis because of side effects. The two serious side effects reported during the study period – pneumonia and a cardiovascular event – were deemed unlikely related to the patients’ medicinal cannabis use.
The findings were limited by several factors, including the observational design, which prevented conclusions about causality, the researchers noted. Other limitations included the loss of many patients to follow-up and incomplete data on other prescription medications in many cases.
The results support the use of medical cannabis by cancer patients as an adjunct pain relief strategy and a way to potentially reduce the use of other medications such as opioids, the authors concluded.
The study was supported by the Canadian Consortium for the Investigation of Cannabinoids, Collège des Médecins du Québec, and the Canopy Growth Corporation. The researchers had no financial conflicts to disclose.
in a study.
Physician-prescribed cannabis, particularly cannabinoids, has been shown to ease cancer-related pain in adult cancer patients, who often find inadequate pain relief from medications including opioids, Saro Aprikian, MSc, a medical student at the Royal College of Surgeons, Dublin, and colleagues, wrote in their paper.
However, real-world data on the safety and effectiveness of cannabis in the cancer population and the impact on use of other medications are lacking, the researchers said.
In the study, published in BMJ Supportive & Palliative Care, the researchers reviewed data from 358 adults with cancer who were part of a multicenter cannabis registry in Canada between May 2015 and October 2018.
The average age of the patients was 57.6 years, and 48% were men. The top three cancer diagnoses in the study population were genitorurinary, breast, and colorectal.
Pain was the most common reason for obtaining a medical cannabis prescription, cited by 72.4% of patients.
Data were collected at follow-up visits conducted every 3 months over 1 year. Pain was assessed via the Brief Pain Inventory (BPI) and revised Edmonton Symptom Assessment System (ESAS-r) questionnaires and compared to baseline values. Patients rated their pain intensity on a sliding scale of 0 (none) to 10 (worst possible). Pain relief was rated on a scale of 0% (none) to 100% (complete).
Compared to baseline scores, patients showed significant decreases at 3, 6 and 9 months for BPI worst pain (5.5 at baseline, 3.6 for 3, 6, and 9 months) average pain (4.1 at baseline, 2.4, 2.3, and 2.7 for 3, 6, and 9 months, respectively), overall pain severity (2.7 at baseline, 2.3, 2.3, and 2.4 at 3, 6, and 9 months, respectively), and pain interference with daily life (4.3 at baseline, 2.4, 2.2, and 2.4 at 3, 6, and 9 months, respectively; P less than .01 for all four pain measures).
“Pain severity as reported in the ESAS-r decreased significantly at 3-month, 6-month and 9-month follow-ups,” the researchers noted.
In addition, total medication burden based on the medication quantification scale (MQS) and morphine equivalent daily dose (MEDD) were recorded at 3, 6, 9, and 12 months. MQS scores decreased compared to baseline at 3, 6, 9, and 12 months in 10%, 23.5%, 26.2%, and 31.6% of patients, respectively. Also compared with baseline, 11.1%, 31.3%, and 14.3% of patients reported decreases in MEDD scores at 3, 6, and 9 months, respectively.
Overall, products with equal amounts of active ingredients tetrahydrocannabinol (THC) and cannabidiol (CBD) were more effective than were those with a predominance of either THC or CBD, the researchers wrote.
Medical cannabis was well-tolerated; a total of 15 moderate to severe side effects were reported by 11 patients, 13 of which were minor. The most common side effects were sleepiness and fatigue, and five patients discontinued their medical cannabis because of side effects. The two serious side effects reported during the study period – pneumonia and a cardiovascular event – were deemed unlikely related to the patients’ medicinal cannabis use.
The findings were limited by several factors, including the observational design, which prevented conclusions about causality, the researchers noted. Other limitations included the loss of many patients to follow-up and incomplete data on other prescription medications in many cases.
The results support the use of medical cannabis by cancer patients as an adjunct pain relief strategy and a way to potentially reduce the use of other medications such as opioids, the authors concluded.
The study was supported by the Canadian Consortium for the Investigation of Cannabinoids, Collège des Médecins du Québec, and the Canopy Growth Corporation. The researchers had no financial conflicts to disclose.
FROM BMJ SUPPORTIVE & PALLIATIVE CARE
Disparities in Melanoma Demographics, Tumor Stage, and Metastases in Hispanic and Latino Patients: A Retrospective Study
To the Editor:
Melanoma is an aggressive form of skin cancer with a high rate of metastasis and poor prognosis.1 Historically, Hispanic and/or Latino patients have presented with more advanced-stage melanomas and have lower survival rates compared with non-Hispanic and/or non-Latino White patients.2 In this study, we evaluated recent data from the last decade to investigate if disparities in melanoma tumor stage at diagnosis and risk for metastases continue to exist in the Hispanic and/or Latino population.
We conducted a retrospective review of melanoma patients at 2 major medical centers in Los Angeles, California—Keck Medicine of USC and Los Angeles County-USC Medical Center—from January 2010 to January 2020. The data collected from electronic medical records included age at melanoma diagnosis, sex, race and ethnicity, insurance type, Breslow depth of lesion, presence of ulceration, and presence of lymph node or distant metastases. Melanoma tumor stage was determined using the American Joint Committee on Cancer classification. Patients who self-reported their ethnicity as not Hispanic and/or Latino were designated to this group regardless of their reported race. Those patients who reported their ethnicity as not Hispanic and/or Latino and reported their race as White were designated as non-Hispanic and/or non-Latino White. This study was approved by the institutional review board of the University of Southern California (Los Angeles). Data analysis was performed using the Pearson χ2 test, Fisher exact test, and Wilcoxon rank sum test. Statistical significance was determined at P<.05.
The final cohort of patients included 79 Hispanic and/or Latino patients and 402 non-Hispanic and/or non-Latino White patients. The median age for the Hispanic and/or Latino group was 54 years and 64 years for the non-Hispanic and/or non-Latino White group (P<.001). There was a greater percentage of females in the Hispanic and/or Latino group compared with the non-Hispanic and/or non-Latino White group (53.2% vs 34.6%)(P=.002). Hispanic and/or Latino patients presented with more advanced tumor stage melanomas (T3: 15.2%; T4: 21.5%) compared with non-Hispanic and/or non-Latino White patients (T3: 8.0%; T4: 10.7%)(P=.004). Furthermore, Hispanic and/or Latino patients had higher rates of lymph node metastases compared with non-Hispanic and/or non-Latino White patients (20.3% vs 7.7% [P<.001]) and higher rates of distant metastases (12.7% vs 5.2% [P=.014])(Table 1). The majority of Hispanic and/or Latino patients had Medicaid (39.2%), while most non-Hispanic and/or non-Latino White patients had a preferred provider organization insurance plan (37.3%) or Medicare (34.3%)(P<.001)(Table 2).
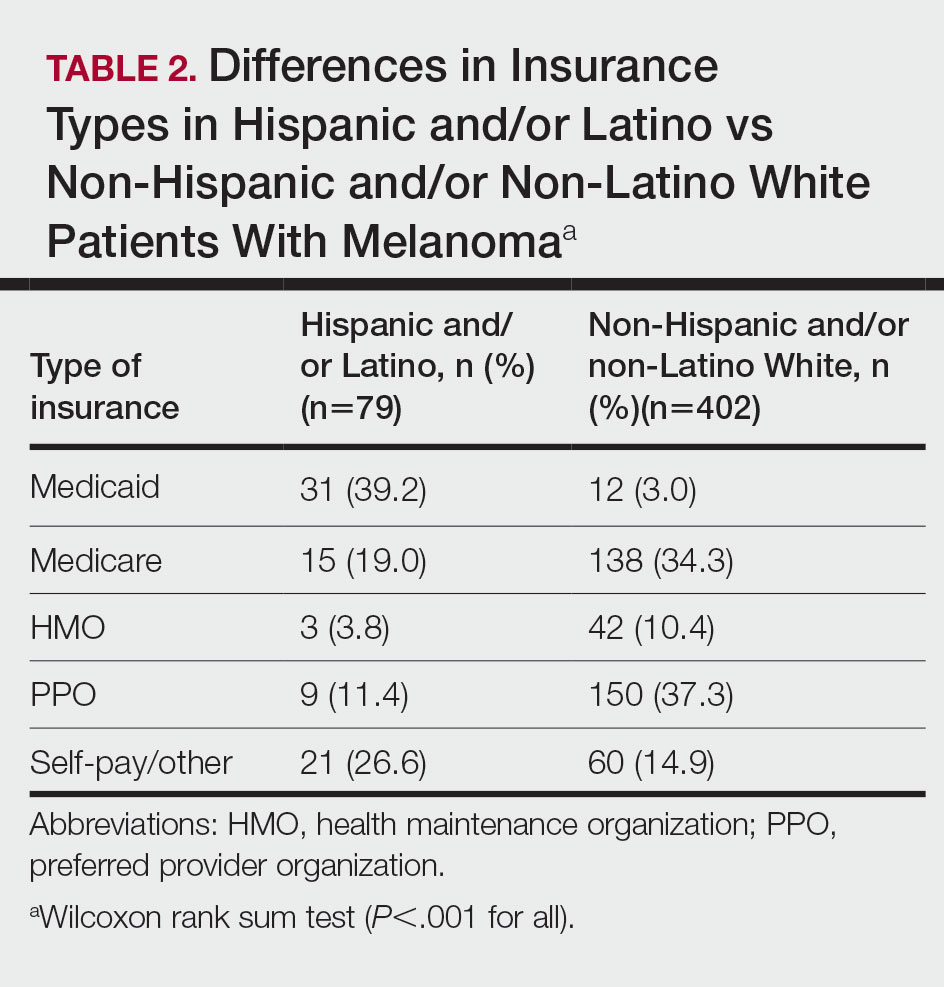
This retrospective study analyzing nearly 10 years of recent melanoma data found that disparities in melanoma diagnosis and treatment continue to exist among Hispanic and/or Latino patients. Compared to non-Hispanic and/or non-Latino White patients, Hispanic and/or Latino patients were diagnosed with melanoma at a younger age and the proportion of females with melanoma was higher. Cormier et al2 also reported that Hispanic patients were younger at melanoma diagnosis, and females represented a larger majority of patients in the Hispanic population compared with the White population. Hispanic and/or Latino patients in our study had more advanced melanoma tumor stage at diagnosis and a higher risk of lymph node and distant metastases, similar to findings reported by Koblinksi et al.3
Our retrospective cohort study demonstrated that the demographics of Hispanic and/or Latino patients with melanoma differ from non-Hispanic and/or non-Latino White patients, specifically with a greater proportion of younger and female patients in the Hispanic and/or Latino population. We also found that Hispanic and/or Latino patients continue to experience worse melanoma outcomes compared with non-Hispanic and/or non-Latino White patients. Further studies are needed to investigate the etiologies behind these health care disparities and potential interventions to address them. In addition, there needs to be increased awareness of the risk for melanoma in Hispanic and/or Latino patients among both health care providers and patients.
Limitations of this study included a smaller sample size of patients from one geographic region. The retrospective design of this study also increased the risk for selection bias, as some of the patients may have had incomplete records or were lost to follow-up. Therefore, the study cohort may not be representative of the general population. Additionally, patients’ skin types could not be determined using standardized tools such as the Fitzpatrick scale, thus we could not assess how patient skin type may have affected melanoma outcomes.
- Aggarwal P, Knabel P, Fleischer AB. United States burden of melanoma and non-melanoma skin cancer from 1990 to 2019. J Am Acad Dermatol. 2021;85:388-395. doi:10.1016/j.jaad.2021.03.109
- Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907. doi:10.1001/archinte.166.17.1907
- Koblinski JE, Maykowski P, Zeitouni NC. Disparities in melanoma stage at diagnosis in Arizona: a 10-year Arizona Cancer Registry study. J Am Acad Dermatol. 2021;84:1776-1779. doi:10.1016/j.jaad.2021.02.045
To the Editor:
Melanoma is an aggressive form of skin cancer with a high rate of metastasis and poor prognosis.1 Historically, Hispanic and/or Latino patients have presented with more advanced-stage melanomas and have lower survival rates compared with non-Hispanic and/or non-Latino White patients.2 In this study, we evaluated recent data from the last decade to investigate if disparities in melanoma tumor stage at diagnosis and risk for metastases continue to exist in the Hispanic and/or Latino population.
We conducted a retrospective review of melanoma patients at 2 major medical centers in Los Angeles, California—Keck Medicine of USC and Los Angeles County-USC Medical Center—from January 2010 to January 2020. The data collected from electronic medical records included age at melanoma diagnosis, sex, race and ethnicity, insurance type, Breslow depth of lesion, presence of ulceration, and presence of lymph node or distant metastases. Melanoma tumor stage was determined using the American Joint Committee on Cancer classification. Patients who self-reported their ethnicity as not Hispanic and/or Latino were designated to this group regardless of their reported race. Those patients who reported their ethnicity as not Hispanic and/or Latino and reported their race as White were designated as non-Hispanic and/or non-Latino White. This study was approved by the institutional review board of the University of Southern California (Los Angeles). Data analysis was performed using the Pearson χ2 test, Fisher exact test, and Wilcoxon rank sum test. Statistical significance was determined at P<.05.

The final cohort of patients included 79 Hispanic and/or Latino patients and 402 non-Hispanic and/or non-Latino White patients. The median age for the Hispanic and/or Latino group was 54 years and 64 years for the non-Hispanic and/or non-Latino White group (P<.001). There was a greater percentage of females in the Hispanic and/or Latino group compared with the non-Hispanic and/or non-Latino White group (53.2% vs 34.6%)(P=.002). Hispanic and/or Latino patients presented with more advanced tumor stage melanomas (T3: 15.2%; T4: 21.5%) compared with non-Hispanic and/or non-Latino White patients (T3: 8.0%; T4: 10.7%)(P=.004). Furthermore, Hispanic and/or Latino patients had higher rates of lymph node metastases compared with non-Hispanic and/or non-Latino White patients (20.3% vs 7.7% [P<.001]) and higher rates of distant metastases (12.7% vs 5.2% [P=.014])(Table 1). The majority of Hispanic and/or Latino patients had Medicaid (39.2%), while most non-Hispanic and/or non-Latino White patients had a preferred provider organization insurance plan (37.3%) or Medicare (34.3%)(P<.001)(Table 2).

This retrospective study analyzing nearly 10 years of recent melanoma data found that disparities in melanoma diagnosis and treatment continue to exist among Hispanic and/or Latino patients. Compared to non-Hispanic and/or non-Latino White patients, Hispanic and/or Latino patients were diagnosed with melanoma at a younger age and the proportion of females with melanoma was higher. Cormier et al2 also reported that Hispanic patients were younger at melanoma diagnosis, and females represented a larger majority of patients in the Hispanic population compared with the White population. Hispanic and/or Latino patients in our study had more advanced melanoma tumor stage at diagnosis and a higher risk of lymph node and distant metastases, similar to findings reported by Koblinksi et al.3
Our retrospective cohort study demonstrated that the demographics of Hispanic and/or Latino patients with melanoma differ from non-Hispanic and/or non-Latino White patients, specifically with a greater proportion of younger and female patients in the Hispanic and/or Latino population. We also found that Hispanic and/or Latino patients continue to experience worse melanoma outcomes compared with non-Hispanic and/or non-Latino White patients. Further studies are needed to investigate the etiologies behind these health care disparities and potential interventions to address them. In addition, there needs to be increased awareness of the risk for melanoma in Hispanic and/or Latino patients among both health care providers and patients.
Limitations of this study included a smaller sample size of patients from one geographic region. The retrospective design of this study also increased the risk for selection bias, as some of the patients may have had incomplete records or were lost to follow-up. Therefore, the study cohort may not be representative of the general population. Additionally, patients’ skin types could not be determined using standardized tools such as the Fitzpatrick scale, thus we could not assess how patient skin type may have affected melanoma outcomes.
To the Editor:
Melanoma is an aggressive form of skin cancer with a high rate of metastasis and poor prognosis.1 Historically, Hispanic and/or Latino patients have presented with more advanced-stage melanomas and have lower survival rates compared with non-Hispanic and/or non-Latino White patients.2 In this study, we evaluated recent data from the last decade to investigate if disparities in melanoma tumor stage at diagnosis and risk for metastases continue to exist in the Hispanic and/or Latino population.
We conducted a retrospective review of melanoma patients at 2 major medical centers in Los Angeles, California—Keck Medicine of USC and Los Angeles County-USC Medical Center—from January 2010 to January 2020. The data collected from electronic medical records included age at melanoma diagnosis, sex, race and ethnicity, insurance type, Breslow depth of lesion, presence of ulceration, and presence of lymph node or distant metastases. Melanoma tumor stage was determined using the American Joint Committee on Cancer classification. Patients who self-reported their ethnicity as not Hispanic and/or Latino were designated to this group regardless of their reported race. Those patients who reported their ethnicity as not Hispanic and/or Latino and reported their race as White were designated as non-Hispanic and/or non-Latino White. This study was approved by the institutional review board of the University of Southern California (Los Angeles). Data analysis was performed using the Pearson χ2 test, Fisher exact test, and Wilcoxon rank sum test. Statistical significance was determined at P<.05.

The final cohort of patients included 79 Hispanic and/or Latino patients and 402 non-Hispanic and/or non-Latino White patients. The median age for the Hispanic and/or Latino group was 54 years and 64 years for the non-Hispanic and/or non-Latino White group (P<.001). There was a greater percentage of females in the Hispanic and/or Latino group compared with the non-Hispanic and/or non-Latino White group (53.2% vs 34.6%)(P=.002). Hispanic and/or Latino patients presented with more advanced tumor stage melanomas (T3: 15.2%; T4: 21.5%) compared with non-Hispanic and/or non-Latino White patients (T3: 8.0%; T4: 10.7%)(P=.004). Furthermore, Hispanic and/or Latino patients had higher rates of lymph node metastases compared with non-Hispanic and/or non-Latino White patients (20.3% vs 7.7% [P<.001]) and higher rates of distant metastases (12.7% vs 5.2% [P=.014])(Table 1). The majority of Hispanic and/or Latino patients had Medicaid (39.2%), while most non-Hispanic and/or non-Latino White patients had a preferred provider organization insurance plan (37.3%) or Medicare (34.3%)(P<.001)(Table 2).

This retrospective study analyzing nearly 10 years of recent melanoma data found that disparities in melanoma diagnosis and treatment continue to exist among Hispanic and/or Latino patients. Compared to non-Hispanic and/or non-Latino White patients, Hispanic and/or Latino patients were diagnosed with melanoma at a younger age and the proportion of females with melanoma was higher. Cormier et al2 also reported that Hispanic patients were younger at melanoma diagnosis, and females represented a larger majority of patients in the Hispanic population compared with the White population. Hispanic and/or Latino patients in our study had more advanced melanoma tumor stage at diagnosis and a higher risk of lymph node and distant metastases, similar to findings reported by Koblinksi et al.3
Our retrospective cohort study demonstrated that the demographics of Hispanic and/or Latino patients with melanoma differ from non-Hispanic and/or non-Latino White patients, specifically with a greater proportion of younger and female patients in the Hispanic and/or Latino population. We also found that Hispanic and/or Latino patients continue to experience worse melanoma outcomes compared with non-Hispanic and/or non-Latino White patients. Further studies are needed to investigate the etiologies behind these health care disparities and potential interventions to address them. In addition, there needs to be increased awareness of the risk for melanoma in Hispanic and/or Latino patients among both health care providers and patients.
Limitations of this study included a smaller sample size of patients from one geographic region. The retrospective design of this study also increased the risk for selection bias, as some of the patients may have had incomplete records or were lost to follow-up. Therefore, the study cohort may not be representative of the general population. Additionally, patients’ skin types could not be determined using standardized tools such as the Fitzpatrick scale, thus we could not assess how patient skin type may have affected melanoma outcomes.
- Aggarwal P, Knabel P, Fleischer AB. United States burden of melanoma and non-melanoma skin cancer from 1990 to 2019. J Am Acad Dermatol. 2021;85:388-395. doi:10.1016/j.jaad.2021.03.109
- Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907. doi:10.1001/archinte.166.17.1907
- Koblinski JE, Maykowski P, Zeitouni NC. Disparities in melanoma stage at diagnosis in Arizona: a 10-year Arizona Cancer Registry study. J Am Acad Dermatol. 2021;84:1776-1779. doi:10.1016/j.jaad.2021.02.045
- Aggarwal P, Knabel P, Fleischer AB. United States burden of melanoma and non-melanoma skin cancer from 1990 to 2019. J Am Acad Dermatol. 2021;85:388-395. doi:10.1016/j.jaad.2021.03.109
- Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907. doi:10.1001/archinte.166.17.1907
- Koblinski JE, Maykowski P, Zeitouni NC. Disparities in melanoma stage at diagnosis in Arizona: a 10-year Arizona Cancer Registry study. J Am Acad Dermatol. 2021;84:1776-1779. doi:10.1016/j.jaad.2021.02.045
Practice Points
- Hispanic and/or Latino patients often present with more advanced-stage melanomas and have decreased survival rates compared with non-Hispanic and/or non-Latino White patients.
- More education and awareness on the risk for melanoma as well as sun-protective behaviors in the Hispanic and/or Latino population is needed among both health care providers and patients to prevent diagnosis of melanoma in later stages and improve outcomes.
Coding the “Spot Check”: Part 1
On January 1, 2021, the Current Procedural Terminology (CPT) evaluation and management (E/M) reporting rules changed dramatically, with “bullet counting” no longer necessary and the coding level now based on either the new medical decision making (MDM) table or time spent on all activities relating to the care of the patient on the day of the encounter.1 This is described in the CPT Professional Edition 2023, a book every practitioner should review annually.2 In particular, every provider should read and reread pages 1 to 14—and beyond if you provide services beyond standard office visits. These changes were made with the intent to simplify the process of documentation and allow a provider to spend more time with patients, though there is still a paucity of data related to whether the new system achieves these aims.
The general rule of reporting work with CPT codes can be simply stated—“Document what you did, do what you documented, and report that which is medically necessary” (David McCafferey, MD, personal communication)—and you should never have any difficulty with audits. Unfortunately, the new system does not let an auditor, who typically lacks a medical degree, audit effectively unless they have a clear understanding of diseases and their stages. Many medical societies, including the American Medical Association3 and American Academy of Dermatology,4 have provided education that focuses on how to report a given vignette, but specific examples of documentation with commentary are uncommon.
To make your documentation more likely to pass audits, explicitly link parts of your documentation to CPT MDM descriptors. We offer scenarios and tips. In part 1 of this series, we discuss how to approach the “spot check,” a commonly encountered chief concern (CC) within dermatology.
A 34-year-old presents with a new spot on the left cheek that seems to be growing and changing shape rapidly. You examine the patient and discuss treatment options. The documentation reads as follows:
• CC: New spot on left cheek that seems to be growing and changing shape rapidly
• History: No family history of skin cancer; concerned about scarring, no blood thinner
• Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy
• Impression: rule out melanoma.
• Plan:
As was the case before 2021, you still need a CC, along with a medically (and medicolegally) appropriate history and physical examination. A diagnostic impression and treatment plan also should be included.
In this situation, reporting is straightforward. There is no separate E/M visit; only the CPT code 11102 for tangential biopsy is reported. An International Classification of Diseases, Tenth Revision code of D48.5 (neoplasm of uncertain behavior of skin) will be included.
Why no E/M code? This is because the biopsy includes preservice and postservice time and work that would be double reported with the E/M. Remember that the preservice work would include any history and physical examination related to the area to be biopsied.
Specifically, preservice work includes:
Inspect and palpate lesion to assess surface size, subcutaneous depth and extension, and whether fixed to underlying structures. Select the most representative and appropriate site to obtain specimen. Examine draining lymph node basins. Discuss need for skin biopsy and biopsy technique options. Describe the tangential biopsy procedure method and expected result and the potential for inconclusive pathology result. Review procedural risks, including bleeding, pain, edema, infection, delayed healing, scarring, and hyper- or hypopigmentation.5
Postservice work includes:
Instruct patient and family on postoperative wound care and dressing changes, as well as problems such as bleeding or pain and restrictions on activities, and follow-up care. Provide prescriptions for pain and antibiotics as necessary. Advise patient and family when results will be available and how they will be communicated. The pathology request form is filled out and signed by the physician. Complete medical record and communicate procedure/results to referring physician as appropriate.5
The Takeaway—Procedure codes include preservice and postservice work. If additional work for the procedure is not documented beyond that, an E/M cannot be included in the encounter.
Scenario 2: What If We Don’t Biopsy?
• CC: New spot on left cheek that seems to be growing and changing shape rapidly.
• History: No family history of skin cancer; concerned about scarring, no blood thinner.
• Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy.
• Impression: rule out melanoma.
• Plan: Review risk, benefits, and alternative options. Schedule biopsy. Discuss unique risk factor of sebaceous peau d’orange skin more prone to contour defects after biopsy.
When determining the coding level for this scenario by MDM, 3 components must be considered: number and complexity of problems addressed at the encounter (column 1), amount and/or complexity of data to be reviewed and analyzed (column 2), and risk of complications and/or morbidity or mortality of patient management (column 3).1 There are no data that are reviewed, so the auditor will assume minimal data to be reviewed and/or analyzed (level 2, row 2 in the MDM table). However, there may be a lot of variation in how an auditor would address the number and complexity of problems (level 1). Consider that you must explicitly state what you are thinking, as an auditor may not know melanoma is a life-threatening diagnosis. From the perspective of the auditor, could this be a:
• Self-limited or minor problem (level 2, or minimal problem in the MDM table)?1
• Stable chronic illness (level 3, or low-level problem)?1
• Undiagnosed new problem with uncertain prognosis (level 4, or moderate level problem)?1
• Acute illness with systemic symptoms (level 4, or moderate level problem)?1
• Acute or chronic illness or injury that poses a threat to life or bodily function (level 5, or high-level problem)?1
• All of the above?
Similarly, there may be variation in how the risk (column 3) would be interpreted in this scenario. The treatment gives no guidance, so the auditor may assume this has a minimal risk of morbidity (level 2) or possibly a low risk of morbidity from additional diagnostic testing or treatment (level 3), as opposed to a moderate risk of morbidity (level 4).1The Takeaway—In the auditor’s mind, this could be a straightforward (CPT codes 99202/99212) or lowlevel (99203/99213) visit as opposed to a moderate-level (99204/99214) visit. From the above documentation, an auditor would not be able to tell what you are thinking, and you can be assured they will not look further into the diagnosis or treatment to learn. That is not their job. So, let us clarify by explicitly stating what you are thinking in the context of the MDM grid.
Modified Scenario 2: A Funny-Looking New Spot With MDM Descriptors to Guide an Auditor
Below are modifications to the documentation for scenario 2 to guide an auditor:
• CC: New spot on left cheek that seems to be growing and changing shape rapidly.
• History: No family history of skin cancer; concerned about scarring, no blood thinner.
• Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy.
• Impression: rule out melanoma
• Plan: Discuss risks, benefits, and alternatives, including biopsy (
In this scenario, the level of MDM is much more clearly documented (as bolded above).
The number and complexity of problems would be an undiagnosed new problem with uncertain prognosis, which would be moderate complexity (column 1, level 4).1 There are no data that are reviewed or analyzed, which would be straightforward (column 2, level 2). For risk, the discussion of the biopsy as part of the diagnostic choices should include discussion of possible scarring, bleeding, pain, and infection, which would be considered best described as a decision regarding minor surgery with identified patient or procedure risk factors, which would make this of moderate complexity (column 3, level 4).1
Importantly, even if the procedure is not chosen as the final treatment plan, the discussion regarding the surgery, including the risks, benefits, and alternatives, can still count toward this category in the MDM table. Therefore, in this scenario with the updated and clarified documentation, this would be reported as CPT code 99204 for a new patient, while an established patient would be 99214.
Scenario 1 Revisited: A Funny-Looking New Spot
Below is scenario 1 with enhanced documentation, now applied to our procedure-only visit.
• CC: New spot on left cheek that seems to be growing and changing shape rapidly.
• History: No family history of skin cancer; concerned about scarring, no blood thinner.
• Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy.
• Impression: rule out melanoma (undiagnosed new problem with uncertain prognosis).
• Plan: Discuss risks, benefits, and alternatives, including biopsy (decision regarding minor surgery with identified patient or procedure risk factors) vs a noninvasive 2 gene expression profiling melanoma rule-out test. Patient wants biopsy. Consent, biopsy via shave technique. Lidocaine hydrochloride 1% with epinephrine, 1 cc, prepare and drape, hemostasis obtained, ointment and bandage applied, and care instructions provided.
This documentation would only allow reporting the biopsy as in Scenario 1, as the decision to perform a 0- or 10-day global procedure is bundled with the procedure if performed on the same date of service.
Final Thoughts
Spot checks are commonly encountered dermatologic visits. With the updated E/M guidelines, clarifying and streamlining your documentation is crucial. In particular, utilizing language that clearly defines number and complexity of problems, amount and/or complexity of data to be reviewed and analyzed, and appropriate risk stratification is crucial to ensuring appropriate reimbursement and minimizing your pain with audits.
- American Medical Association. CPT evaluation and management (E/M) code and guideline changes; 2023. Accessed April 13, 2023. https://www.ama-assn.org/system/files/2023-e-m-descriptors-guidelines.pdf
- American Medical Association. CPT Professional Edition 2023. American Medical Association; 2022.
- American Medical Association. Evaluation and management (E/M) coding. Accessed April 25, 2023. https://www.ama-assn.org/topics/evaluation-and-management-em-coding
- American Academy of Dermatology Association. Coding resource center. Accessed April 13, 2023. https://www.aad.org/member/practice/coding
- American Medical Association. RBVS DataManager Online. Accessed April 13, 2023. https://commerce.ama-assn.org/store/ui/catalog/productDetail?product_id=prod280002&navAction=push
On January 1, 2021, the Current Procedural Terminology (CPT) evaluation and management (E/M) reporting rules changed dramatically, with “bullet counting” no longer necessary and the coding level now based on either the new medical decision making (MDM) table or time spent on all activities relating to the care of the patient on the day of the encounter.1 This is described in the CPT Professional Edition 2023, a book every practitioner should review annually.2 In particular, every provider should read and reread pages 1 to 14—and beyond if you provide services beyond standard office visits. These changes were made with the intent to simplify the process of documentation and allow a provider to spend more time with patients, though there is still a paucity of data related to whether the new system achieves these aims.
The general rule of reporting work with CPT codes can be simply stated—“Document what you did, do what you documented, and report that which is medically necessary” (David McCafferey, MD, personal communication)—and you should never have any difficulty with audits. Unfortunately, the new system does not let an auditor, who typically lacks a medical degree, audit effectively unless they have a clear understanding of diseases and their stages. Many medical societies, including the American Medical Association3 and American Academy of Dermatology,4 have provided education that focuses on how to report a given vignette, but specific examples of documentation with commentary are uncommon.
To make your documentation more likely to pass audits, explicitly link parts of your documentation to CPT MDM descriptors. We offer scenarios and tips. In part 1 of this series, we discuss how to approach the “spot check,” a commonly encountered chief concern (CC) within dermatology.
A 34-year-old presents with a new spot on the left cheek that seems to be growing and changing shape rapidly. You examine the patient and discuss treatment options. The documentation reads as follows:
• CC: New spot on left cheek that seems to be growing and changing shape rapidly
• History: No family history of skin cancer; concerned about scarring, no blood thinner
• Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy
• Impression: rule out melanoma.
• Plan:
As was the case before 2021, you still need a CC, along with a medically (and medicolegally) appropriate history and physical examination. A diagnostic impression and treatment plan also should be included.
In this situation, reporting is straightforward. There is no separate E/M visit; only the CPT code 11102 for tangential biopsy is reported. An International Classification of Diseases, Tenth Revision code of D48.5 (neoplasm of uncertain behavior of skin) will be included.
Why no E/M code? This is because the biopsy includes preservice and postservice time and work that would be double reported with the E/M. Remember that the preservice work would include any history and physical examination related to the area to be biopsied.
Specifically, preservice work includes:
Inspect and palpate lesion to assess surface size, subcutaneous depth and extension, and whether fixed to underlying structures. Select the most representative and appropriate site to obtain specimen. Examine draining lymph node basins. Discuss need for skin biopsy and biopsy technique options. Describe the tangential biopsy procedure method and expected result and the potential for inconclusive pathology result. Review procedural risks, including bleeding, pain, edema, infection, delayed healing, scarring, and hyper- or hypopigmentation.5
Postservice work includes:
Instruct patient and family on postoperative wound care and dressing changes, as well as problems such as bleeding or pain and restrictions on activities, and follow-up care. Provide prescriptions for pain and antibiotics as necessary. Advise patient and family when results will be available and how they will be communicated. The pathology request form is filled out and signed by the physician. Complete medical record and communicate procedure/results to referring physician as appropriate.5
The Takeaway—Procedure codes include preservice and postservice work. If additional work for the procedure is not documented beyond that, an E/M cannot be included in the encounter.
Scenario 2: What If We Don’t Biopsy?
• CC: New spot on left cheek that seems to be growing and changing shape rapidly.
• History: No family history of skin cancer; concerned about scarring, no blood thinner.
• Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy.
• Impression: rule out melanoma.
• Plan: Review risk, benefits, and alternative options. Schedule biopsy. Discuss unique risk factor of sebaceous peau d’orange skin more prone to contour defects after biopsy.
When determining the coding level for this scenario by MDM, 3 components must be considered: number and complexity of problems addressed at the encounter (column 1), amount and/or complexity of data to be reviewed and analyzed (column 2), and risk of complications and/or morbidity or mortality of patient management (column 3).1 There are no data that are reviewed, so the auditor will assume minimal data to be reviewed and/or analyzed (level 2, row 2 in the MDM table). However, there may be a lot of variation in how an auditor would address the number and complexity of problems (level 1). Consider that you must explicitly state what you are thinking, as an auditor may not know melanoma is a life-threatening diagnosis. From the perspective of the auditor, could this be a:
• Self-limited or minor problem (level 2, or minimal problem in the MDM table)?1
• Stable chronic illness (level 3, or low-level problem)?1
• Undiagnosed new problem with uncertain prognosis (level 4, or moderate level problem)?1
• Acute illness with systemic symptoms (level 4, or moderate level problem)?1
• Acute or chronic illness or injury that poses a threat to life or bodily function (level 5, or high-level problem)?1
• All of the above?
Similarly, there may be variation in how the risk (column 3) would be interpreted in this scenario. The treatment gives no guidance, so the auditor may assume this has a minimal risk of morbidity (level 2) or possibly a low risk of morbidity from additional diagnostic testing or treatment (level 3), as opposed to a moderate risk of morbidity (level 4).1The Takeaway—In the auditor’s mind, this could be a straightforward (CPT codes 99202/99212) or lowlevel (99203/99213) visit as opposed to a moderate-level (99204/99214) visit. From the above documentation, an auditor would not be able to tell what you are thinking, and you can be assured they will not look further into the diagnosis or treatment to learn. That is not their job. So, let us clarify by explicitly stating what you are thinking in the context of the MDM grid.
Modified Scenario 2: A Funny-Looking New Spot With MDM Descriptors to Guide an Auditor
Below are modifications to the documentation for scenario 2 to guide an auditor:
• CC: New spot on left cheek that seems to be growing and changing shape rapidly.
• History: No family history of skin cancer; concerned about scarring, no blood thinner.
• Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy.
• Impression: rule out melanoma
• Plan: Discuss risks, benefits, and alternatives, including biopsy (
In this scenario, the level of MDM is much more clearly documented (as bolded above).
The number and complexity of problems would be an undiagnosed new problem with uncertain prognosis, which would be moderate complexity (column 1, level 4).1 There are no data that are reviewed or analyzed, which would be straightforward (column 2, level 2). For risk, the discussion of the biopsy as part of the diagnostic choices should include discussion of possible scarring, bleeding, pain, and infection, which would be considered best described as a decision regarding minor surgery with identified patient or procedure risk factors, which would make this of moderate complexity (column 3, level 4).1
Importantly, even if the procedure is not chosen as the final treatment plan, the discussion regarding the surgery, including the risks, benefits, and alternatives, can still count toward this category in the MDM table. Therefore, in this scenario with the updated and clarified documentation, this would be reported as CPT code 99204 for a new patient, while an established patient would be 99214.
Scenario 1 Revisited: A Funny-Looking New Spot
Below is scenario 1 with enhanced documentation, now applied to our procedure-only visit.
• CC: New spot on left cheek that seems to be growing and changing shape rapidly.
• History: No family history of skin cancer; concerned about scarring, no blood thinner.
• Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy.
• Impression: rule out melanoma (undiagnosed new problem with uncertain prognosis).
• Plan: Discuss risks, benefits, and alternatives, including biopsy (decision regarding minor surgery with identified patient or procedure risk factors) vs a noninvasive 2 gene expression profiling melanoma rule-out test. Patient wants biopsy. Consent, biopsy via shave technique. Lidocaine hydrochloride 1% with epinephrine, 1 cc, prepare and drape, hemostasis obtained, ointment and bandage applied, and care instructions provided.
This documentation would only allow reporting the biopsy as in Scenario 1, as the decision to perform a 0- or 10-day global procedure is bundled with the procedure if performed on the same date of service.
Final Thoughts
Spot checks are commonly encountered dermatologic visits. With the updated E/M guidelines, clarifying and streamlining your documentation is crucial. In particular, utilizing language that clearly defines number and complexity of problems, amount and/or complexity of data to be reviewed and analyzed, and appropriate risk stratification is crucial to ensuring appropriate reimbursement and minimizing your pain with audits.
On January 1, 2021, the Current Procedural Terminology (CPT) evaluation and management (E/M) reporting rules changed dramatically, with “bullet counting” no longer necessary and the coding level now based on either the new medical decision making (MDM) table or time spent on all activities relating to the care of the patient on the day of the encounter.1 This is described in the CPT Professional Edition 2023, a book every practitioner should review annually.2 In particular, every provider should read and reread pages 1 to 14—and beyond if you provide services beyond standard office visits. These changes were made with the intent to simplify the process of documentation and allow a provider to spend more time with patients, though there is still a paucity of data related to whether the new system achieves these aims.
The general rule of reporting work with CPT codes can be simply stated—“Document what you did, do what you documented, and report that which is medically necessary” (David McCafferey, MD, personal communication)—and you should never have any difficulty with audits. Unfortunately, the new system does not let an auditor, who typically lacks a medical degree, audit effectively unless they have a clear understanding of diseases and their stages. Many medical societies, including the American Medical Association3 and American Academy of Dermatology,4 have provided education that focuses on how to report a given vignette, but specific examples of documentation with commentary are uncommon.
To make your documentation more likely to pass audits, explicitly link parts of your documentation to CPT MDM descriptors. We offer scenarios and tips. In part 1 of this series, we discuss how to approach the “spot check,” a commonly encountered chief concern (CC) within dermatology.
A 34-year-old presents with a new spot on the left cheek that seems to be growing and changing shape rapidly. You examine the patient and discuss treatment options. The documentation reads as follows:
• CC: New spot on left cheek that seems to be growing and changing shape rapidly
• History: No family history of skin cancer; concerned about scarring, no blood thinner
• Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy
• Impression: rule out melanoma.
• Plan:
As was the case before 2021, you still need a CC, along with a medically (and medicolegally) appropriate history and physical examination. A diagnostic impression and treatment plan also should be included.
In this situation, reporting is straightforward. There is no separate E/M visit; only the CPT code 11102 for tangential biopsy is reported. An International Classification of Diseases, Tenth Revision code of D48.5 (neoplasm of uncertain behavior of skin) will be included.
Why no E/M code? This is because the biopsy includes preservice and postservice time and work that would be double reported with the E/M. Remember that the preservice work would include any history and physical examination related to the area to be biopsied.
Specifically, preservice work includes:
Inspect and palpate lesion to assess surface size, subcutaneous depth and extension, and whether fixed to underlying structures. Select the most representative and appropriate site to obtain specimen. Examine draining lymph node basins. Discuss need for skin biopsy and biopsy technique options. Describe the tangential biopsy procedure method and expected result and the potential for inconclusive pathology result. Review procedural risks, including bleeding, pain, edema, infection, delayed healing, scarring, and hyper- or hypopigmentation.5
Postservice work includes:
Instruct patient and family on postoperative wound care and dressing changes, as well as problems such as bleeding or pain and restrictions on activities, and follow-up care. Provide prescriptions for pain and antibiotics as necessary. Advise patient and family when results will be available and how they will be communicated. The pathology request form is filled out and signed by the physician. Complete medical record and communicate procedure/results to referring physician as appropriate.5
The Takeaway—Procedure codes include preservice and postservice work. If additional work for the procedure is not documented beyond that, an E/M cannot be included in the encounter.
Scenario 2: What If We Don’t Biopsy?
• CC: New spot on left cheek that seems to be growing and changing shape rapidly.
• History: No family history of skin cancer; concerned about scarring, no blood thinner.
• Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy.
• Impression: rule out melanoma.
• Plan: Review risk, benefits, and alternative options. Schedule biopsy. Discuss unique risk factor of sebaceous peau d’orange skin more prone to contour defects after biopsy.
When determining the coding level for this scenario by MDM, 3 components must be considered: number and complexity of problems addressed at the encounter (column 1), amount and/or complexity of data to be reviewed and analyzed (column 2), and risk of complications and/or morbidity or mortality of patient management (column 3).1 There are no data that are reviewed, so the auditor will assume minimal data to be reviewed and/or analyzed (level 2, row 2 in the MDM table). However, there may be a lot of variation in how an auditor would address the number and complexity of problems (level 1). Consider that you must explicitly state what you are thinking, as an auditor may not know melanoma is a life-threatening diagnosis. From the perspective of the auditor, could this be a:
• Self-limited or minor problem (level 2, or minimal problem in the MDM table)?1
• Stable chronic illness (level 3, or low-level problem)?1
• Undiagnosed new problem with uncertain prognosis (level 4, or moderate level problem)?1
• Acute illness with systemic symptoms (level 4, or moderate level problem)?1
• Acute or chronic illness or injury that poses a threat to life or bodily function (level 5, or high-level problem)?1
• All of the above?
Similarly, there may be variation in how the risk (column 3) would be interpreted in this scenario. The treatment gives no guidance, so the auditor may assume this has a minimal risk of morbidity (level 2) or possibly a low risk of morbidity from additional diagnostic testing or treatment (level 3), as opposed to a moderate risk of morbidity (level 4).1The Takeaway—In the auditor’s mind, this could be a straightforward (CPT codes 99202/99212) or lowlevel (99203/99213) visit as opposed to a moderate-level (99204/99214) visit. From the above documentation, an auditor would not be able to tell what you are thinking, and you can be assured they will not look further into the diagnosis or treatment to learn. That is not their job. So, let us clarify by explicitly stating what you are thinking in the context of the MDM grid.
Modified Scenario 2: A Funny-Looking New Spot With MDM Descriptors to Guide an Auditor
Below are modifications to the documentation for scenario 2 to guide an auditor:
• CC: New spot on left cheek that seems to be growing and changing shape rapidly.
• History: No family history of skin cancer; concerned about scarring, no blood thinner.
• Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy.
• Impression: rule out melanoma
• Plan: Discuss risks, benefits, and alternatives, including biopsy (
In this scenario, the level of MDM is much more clearly documented (as bolded above).
The number and complexity of problems would be an undiagnosed new problem with uncertain prognosis, which would be moderate complexity (column 1, level 4).1 There are no data that are reviewed or analyzed, which would be straightforward (column 2, level 2). For risk, the discussion of the biopsy as part of the diagnostic choices should include discussion of possible scarring, bleeding, pain, and infection, which would be considered best described as a decision regarding minor surgery with identified patient or procedure risk factors, which would make this of moderate complexity (column 3, level 4).1
Importantly, even if the procedure is not chosen as the final treatment plan, the discussion regarding the surgery, including the risks, benefits, and alternatives, can still count toward this category in the MDM table. Therefore, in this scenario with the updated and clarified documentation, this would be reported as CPT code 99204 for a new patient, while an established patient would be 99214.
Scenario 1 Revisited: A Funny-Looking New Spot
Below is scenario 1 with enhanced documentation, now applied to our procedure-only visit.
• CC: New spot on left cheek that seems to be growing and changing shape rapidly.
• History: No family history of skin cancer; concerned about scarring, no blood thinner.
• Examination: Irregular tan to brown to black 8-mm macule. No lymphadenopathy.
• Impression: rule out melanoma (undiagnosed new problem with uncertain prognosis).
• Plan: Discuss risks, benefits, and alternatives, including biopsy (decision regarding minor surgery with identified patient or procedure risk factors) vs a noninvasive 2 gene expression profiling melanoma rule-out test. Patient wants biopsy. Consent, biopsy via shave technique. Lidocaine hydrochloride 1% with epinephrine, 1 cc, prepare and drape, hemostasis obtained, ointment and bandage applied, and care instructions provided.
This documentation would only allow reporting the biopsy as in Scenario 1, as the decision to perform a 0- or 10-day global procedure is bundled with the procedure if performed on the same date of service.
Final Thoughts
Spot checks are commonly encountered dermatologic visits. With the updated E/M guidelines, clarifying and streamlining your documentation is crucial. In particular, utilizing language that clearly defines number and complexity of problems, amount and/or complexity of data to be reviewed and analyzed, and appropriate risk stratification is crucial to ensuring appropriate reimbursement and minimizing your pain with audits.
- American Medical Association. CPT evaluation and management (E/M) code and guideline changes; 2023. Accessed April 13, 2023. https://www.ama-assn.org/system/files/2023-e-m-descriptors-guidelines.pdf
- American Medical Association. CPT Professional Edition 2023. American Medical Association; 2022.
- American Medical Association. Evaluation and management (E/M) coding. Accessed April 25, 2023. https://www.ama-assn.org/topics/evaluation-and-management-em-coding
- American Academy of Dermatology Association. Coding resource center. Accessed April 13, 2023. https://www.aad.org/member/practice/coding
- American Medical Association. RBVS DataManager Online. Accessed April 13, 2023. https://commerce.ama-assn.org/store/ui/catalog/productDetail?product_id=prod280002&navAction=push
- American Medical Association. CPT evaluation and management (E/M) code and guideline changes; 2023. Accessed April 13, 2023. https://www.ama-assn.org/system/files/2023-e-m-descriptors-guidelines.pdf
- American Medical Association. CPT Professional Edition 2023. American Medical Association; 2022.
- American Medical Association. Evaluation and management (E/M) coding. Accessed April 25, 2023. https://www.ama-assn.org/topics/evaluation-and-management-em-coding
- American Academy of Dermatology Association. Coding resource center. Accessed April 13, 2023. https://www.aad.org/member/practice/coding
- American Medical Association. RBVS DataManager Online. Accessed April 13, 2023. https://commerce.ama-assn.org/store/ui/catalog/productDetail?product_id=prod280002&navAction=push
Practice Points
- Clear documentation that reflects your thought process is an important component of effective coding and billing.
- Include Current Procedural Terminology–defined language within documentation to help ensure appropriate reimbursement and decrease the risk of audits.
Gene Expression Profiling for Melanoma Prognosis: Going Beyond What We See With Our Eyes
Dermatology certainly is the most visual medical specialty. In the current era of powerful electronic imaging and laboratory techniques, the skills of physical diagnosis seem to have become less important in medicine—not so in dermatology, in which the experienced clinician is able to identify many conditions by simply looking at the skin. Of course, dermatologists do heavily rely on dermatopathologists to microscopically visualize biopsies to distinguish diseases. Even as we acknowledge the dominant role of visual recognition, there is increasing progress in making clinical determinations based on molecular events. The era of genomic dermatology is here.
The Genodermatoses
There are more than 500 dermatologic conditions resulting from heritable mutational events.1 The rarity of most of these diseases and variability in phenotypic manifestations presents considerable diagnostic challenges, typically the province of a select group of clinical pediatric dermatologists whose abilities have been developed by experience.2 However, the addition of genomic analysis has now made reliable identification more accessible to a wider group of clinicians.3 The Human Genome Project was arguably the most successful health policy endeavor in human history, promoting the development of massive automated, information theory–driven applications to analyze DNA sequences.4 We all think of DNA analysis as the ultimate means to detect mutations by sequencing whole exomes—and in fact the entire genome of affected individuals searching for mutations—but DNA sequencing often is insufficient to detect mutations in noncoding regions of genes and to identify abnormalities of gene expression (eg, splice variants). Building on the advances in high-throughput nucleic acid sequencing and massive computerized analysis, the field has now taken a quantum leap further to sequence transcribed RNA to detect abnormalities.5
The techniques are straightforward: RNA is isolated and reverse transcribed to complementary DNA. The complementary DNA is amplified and then processed by high-throughput sequencers. The sequences are then identified by computer algorithms. It is possible to fully define the transcriptomes of multiple genes, even reaching the threshold of resolution of gene expression emanating from a single cell.6
Studying Gene Expression for Malignant Melanoma
As much as we rely on visual interpretations, we acknowledge that many conditions look very similar, whether to the naked eye or under the microscope. This is true for rare diseases but also for the rashes we routinely see. A group of investigators recently used RNA transcriptome sequencing to analyze differences between atopic dermatitis and psoriasis, permitting better differentiation of these 2 common conditions.7
One of the greatest challenges confronting dermatologists and their dermatopathologist partners is to distinguish malignant melanoma from benign nevi.8 Despite staining for a number of molecular markers, some lesions defy histopathology, such as distinguishing benign and malignant Spitz nevi; however, recent work on RNA transcriptomes suggests that gene expression may increase confidence in assessing atypical Spitz nevi.9 A 23-gene expression panel has yielded a sensitivity of 91.5% and a specificity of 92.5% in differentiating benign nevi from malignant melanoma.10
From the Research Laboratory to Routine Clinical Use
Undoubtedly, it is a large step from proof-of-concept studies to accepted clinical use. The ultimate achievement for a laboratory technique is to enter approved clinical use. Gene expression panels have now been approved by numerous third-party insurers to help predict future clinical evolution of biopsied melanomas. Although early in situ melanomas are eminently curable by wide excision, lesions that have more concerning characteristics (eg, depth >0.8 mm, ulceration) may progress to metastatic disease. The gratifying success of checkpoint inhibitor therapy has improved the previously dismal outlook for advanced melanomas.11 Dermatologists search for clues to suggest which patients may benefit from adjuvant therapy. Sentinel lymph node biopsy (SLNB) has been a standard-of-care technique to help make this determination.12
It has now been demonstrated that gene expression array analysis can provide evidence complementing SLNB results or even independent of SLNB results. In extensive validation studies, a 31-gene expression panel analyzing initial melanoma biopsy specimens showed predictive value for later recurrence and development of metastatic disease.13,14 The gene expression studies have identified patients with negative SLNBs who have gone on to develop metastatic melanomas.15 It has been suggested that gene expression panel diagnosis may reduce the need for invasive SLNBs in patients in whom the surgical procedure may involve risk.16
Looking to the Future
The progress of science is the result of many small steps building on prior work. The terms breakthrough and game changer in medicine have been popularized by the media and rarely are valid. On the contrary, sequential development of methods over many years has preceded the acclaimed successes of medical research; for example, the best-known medical breakthrough—that of Salk’s inactivated polio vaccine—was preceded by the use of an inactivated polio vaccine by Brodie and Park17 in 1935. However, it was the development of tissue culture of poliomyelitis virus by Enders et al18 that provided the methodology to Salk’s group to produce their inactivated polio vaccine.
The ability to go beyond our visual senses will be of great importance in characterizing the variability of skin diseases, especially in skin of color patients; for example, acral melanoma is perhaps the primary melanocytic malignancy in darker-skinned patients and is the target of RNA transcriptomic research.19 Progress is continuing on gene therapy for a growing number of skin conditions.20,21 In vivo correction of abnormal genes is being attempted for a number of inherited cutaneous diseases,22 notably for disorders of skin fragility.23 For now, we welcome the addition of genomic capabilities to the visual practice of dermatology and the capability to go beyond that which we can see with our eyes.
- Feramisco JD, Sadreyev RI, Murray ML, et al. Phenotypic and enotypic analyses of genetic skin disease through the Online Mendelian Inheritance in Man (OMIM) database. J Investig Derm. 2009;129:2628-2636.
- Parker JC, Rangu S, Grand KL, et al. Genetic skin disorders: the value of a multidisciplinary clinic. Am J Med Genet A. 2021;185:1159-1167.
- Richert B, Smits G. Clinical and molecular diagnosis of genodermatoses: review and perspectives. J Eur Acad Dermatol Venereol. 2023;37:488-500.
- Green ED, Watson JD, Collins FS. Human genome project: twenty-five years of big biology. Nature. 2015;526:29-31.
- Saeidian AH, Youssefian L, Vahidnezhad H, et al. Research techniques made simple: whole-transcriptome sequencing by RNA-seq for diagnosis of monogenic disorders. J Invest Dermatol. 2020;140:1117-1126.e1.
- Deutsch A, McLellan BN, Shinoda K. Single-cell transcriptomics in dermatology. JAAD Int. 2020;1:182-188.
- Liu Y, Wang H, Taylor M, et al. Classification of human chronic inflammatory skin disease based on single-cell immune profiling [published online April 15, 2022]. Sci Immunol. doi:10.1126/sciimmunol.abl9165
- Reimann JDR, Salim S, Velazquez EF, et al. Comparison of melanoma gene expression score with histopathology, fluorescence in situ hybridization, and SNP array for the classification of melanocytic neoplasms. Mod Pathol. 2018;31:1733-1743.
- Hillen LM, Geybels MS, Spassova I, et al. A digital mRNA expression signature to classify challenging spitzoid melanocytic neoplasms. FEBS Open Bio. 2020;10:1326-1341.
- Clarke LE, Flake DD 2nd, Busam K, et al. An independent validation of a gene expression signature to differentiate malignant melanoma from benign melanocytic nevi. Cancer. 2017;123:617-628.
- Stege H, Haist M, Nikfarjam U, et al. The status of adjuvant and neoadjuvant melanoma therapy, new developments and upcoming challenges. Target Oncol. 2021;16:537-552.
- Morrison S, Han D. Re-evaluation of sentinel lymph node biopsy for melanoma. Curr Treat Options Oncol. 2021;22:22.
- Gerami P, Cook RW, Russell MC, et al. Gene expression profiling for molecular staging of cutaneous melanoma in patients with sentinel lymph node biopsy. J Am Acad Dermatol. 2015;72:780-785.e3.
- Keller J, Schwartz TL, Lizalek JM, et al. Prospective validation of the prognostic 31-gene expression profiling test in primary cutaneous melanoma. Cancer Med. 2019;8:2205-2212.
- Gastman BR, Gerami P, Kurley SJ, et al. Identification of patients at risk for metastasis using a prognostic 31-gene expression profile in subpopulations of melanoma patients with favorable outcomes by standard criteria. J Am Acad Dermatol. 2019;80:149-157.
- Vetto JT, Hsueh EC, Gastman BR, et al. Guidance of sentinel lymph node biopsy decisions in patients with T1-T2 melanoma using gene expression profiling. Future Oncol. 2019;15:1207-1217.
- Brodie M, Park W. Active immunization against poliomyelitis. JAMA. 1935;105:1089-1093.
- Enders JF, Weller TH, Robbins FC. Cultivation of the Lansing strain of poliomyelitis virus in cultures of various human embryonic tissues. Science. 1949;109:85-87.
- Li J, Smalley I, Chen Z, et al. Single-cell characterization of the cellular landscape of acral melanoma identifies novel targets for immunotherapy. Clin Cancer Res. 2022;28:2131-2146.
- Gorell E, Nguyen N, Lane A, et al. Gene therapy for skin diseases. Cold Spring Harb Perspect Med. 2014;4:A015149.
- Cavazza A, Mavilio F. Gene therapy of skin adhesion disorders (mini review). Curr Pharm Biotechnol. 2012;13:1868-1876.
- Abdul-Wahab A, Qasim W, McGrath JA. Gene therapies for inherited skin disorders. Semin Cutan Med Surg. 2014;33:83-90.
- Bilousova G. Gene therapy for skin fragility diseases: the new generation. J Invest Dermatol. 2019;139:1634-1637.
Dermatology certainly is the most visual medical specialty. In the current era of powerful electronic imaging and laboratory techniques, the skills of physical diagnosis seem to have become less important in medicine—not so in dermatology, in which the experienced clinician is able to identify many conditions by simply looking at the skin. Of course, dermatologists do heavily rely on dermatopathologists to microscopically visualize biopsies to distinguish diseases. Even as we acknowledge the dominant role of visual recognition, there is increasing progress in making clinical determinations based on molecular events. The era of genomic dermatology is here.
The Genodermatoses
There are more than 500 dermatologic conditions resulting from heritable mutational events.1 The rarity of most of these diseases and variability in phenotypic manifestations presents considerable diagnostic challenges, typically the province of a select group of clinical pediatric dermatologists whose abilities have been developed by experience.2 However, the addition of genomic analysis has now made reliable identification more accessible to a wider group of clinicians.3 The Human Genome Project was arguably the most successful health policy endeavor in human history, promoting the development of massive automated, information theory–driven applications to analyze DNA sequences.4 We all think of DNA analysis as the ultimate means to detect mutations by sequencing whole exomes—and in fact the entire genome of affected individuals searching for mutations—but DNA sequencing often is insufficient to detect mutations in noncoding regions of genes and to identify abnormalities of gene expression (eg, splice variants). Building on the advances in high-throughput nucleic acid sequencing and massive computerized analysis, the field has now taken a quantum leap further to sequence transcribed RNA to detect abnormalities.5
The techniques are straightforward: RNA is isolated and reverse transcribed to complementary DNA. The complementary DNA is amplified and then processed by high-throughput sequencers. The sequences are then identified by computer algorithms. It is possible to fully define the transcriptomes of multiple genes, even reaching the threshold of resolution of gene expression emanating from a single cell.6
Studying Gene Expression for Malignant Melanoma
As much as we rely on visual interpretations, we acknowledge that many conditions look very similar, whether to the naked eye or under the microscope. This is true for rare diseases but also for the rashes we routinely see. A group of investigators recently used RNA transcriptome sequencing to analyze differences between atopic dermatitis and psoriasis, permitting better differentiation of these 2 common conditions.7
One of the greatest challenges confronting dermatologists and their dermatopathologist partners is to distinguish malignant melanoma from benign nevi.8 Despite staining for a number of molecular markers, some lesions defy histopathology, such as distinguishing benign and malignant Spitz nevi; however, recent work on RNA transcriptomes suggests that gene expression may increase confidence in assessing atypical Spitz nevi.9 A 23-gene expression panel has yielded a sensitivity of 91.5% and a specificity of 92.5% in differentiating benign nevi from malignant melanoma.10
From the Research Laboratory to Routine Clinical Use
Undoubtedly, it is a large step from proof-of-concept studies to accepted clinical use. The ultimate achievement for a laboratory technique is to enter approved clinical use. Gene expression panels have now been approved by numerous third-party insurers to help predict future clinical evolution of biopsied melanomas. Although early in situ melanomas are eminently curable by wide excision, lesions that have more concerning characteristics (eg, depth >0.8 mm, ulceration) may progress to metastatic disease. The gratifying success of checkpoint inhibitor therapy has improved the previously dismal outlook for advanced melanomas.11 Dermatologists search for clues to suggest which patients may benefit from adjuvant therapy. Sentinel lymph node biopsy (SLNB) has been a standard-of-care technique to help make this determination.12
It has now been demonstrated that gene expression array analysis can provide evidence complementing SLNB results or even independent of SLNB results. In extensive validation studies, a 31-gene expression panel analyzing initial melanoma biopsy specimens showed predictive value for later recurrence and development of metastatic disease.13,14 The gene expression studies have identified patients with negative SLNBs who have gone on to develop metastatic melanomas.15 It has been suggested that gene expression panel diagnosis may reduce the need for invasive SLNBs in patients in whom the surgical procedure may involve risk.16
Looking to the Future
The progress of science is the result of many small steps building on prior work. The terms breakthrough and game changer in medicine have been popularized by the media and rarely are valid. On the contrary, sequential development of methods over many years has preceded the acclaimed successes of medical research; for example, the best-known medical breakthrough—that of Salk’s inactivated polio vaccine—was preceded by the use of an inactivated polio vaccine by Brodie and Park17 in 1935. However, it was the development of tissue culture of poliomyelitis virus by Enders et al18 that provided the methodology to Salk’s group to produce their inactivated polio vaccine.
The ability to go beyond our visual senses will be of great importance in characterizing the variability of skin diseases, especially in skin of color patients; for example, acral melanoma is perhaps the primary melanocytic malignancy in darker-skinned patients and is the target of RNA transcriptomic research.19 Progress is continuing on gene therapy for a growing number of skin conditions.20,21 In vivo correction of abnormal genes is being attempted for a number of inherited cutaneous diseases,22 notably for disorders of skin fragility.23 For now, we welcome the addition of genomic capabilities to the visual practice of dermatology and the capability to go beyond that which we can see with our eyes.
Dermatology certainly is the most visual medical specialty. In the current era of powerful electronic imaging and laboratory techniques, the skills of physical diagnosis seem to have become less important in medicine—not so in dermatology, in which the experienced clinician is able to identify many conditions by simply looking at the skin. Of course, dermatologists do heavily rely on dermatopathologists to microscopically visualize biopsies to distinguish diseases. Even as we acknowledge the dominant role of visual recognition, there is increasing progress in making clinical determinations based on molecular events. The era of genomic dermatology is here.
The Genodermatoses
There are more than 500 dermatologic conditions resulting from heritable mutational events.1 The rarity of most of these diseases and variability in phenotypic manifestations presents considerable diagnostic challenges, typically the province of a select group of clinical pediatric dermatologists whose abilities have been developed by experience.2 However, the addition of genomic analysis has now made reliable identification more accessible to a wider group of clinicians.3 The Human Genome Project was arguably the most successful health policy endeavor in human history, promoting the development of massive automated, information theory–driven applications to analyze DNA sequences.4 We all think of DNA analysis as the ultimate means to detect mutations by sequencing whole exomes—and in fact the entire genome of affected individuals searching for mutations—but DNA sequencing often is insufficient to detect mutations in noncoding regions of genes and to identify abnormalities of gene expression (eg, splice variants). Building on the advances in high-throughput nucleic acid sequencing and massive computerized analysis, the field has now taken a quantum leap further to sequence transcribed RNA to detect abnormalities.5
The techniques are straightforward: RNA is isolated and reverse transcribed to complementary DNA. The complementary DNA is amplified and then processed by high-throughput sequencers. The sequences are then identified by computer algorithms. It is possible to fully define the transcriptomes of multiple genes, even reaching the threshold of resolution of gene expression emanating from a single cell.6
Studying Gene Expression for Malignant Melanoma
As much as we rely on visual interpretations, we acknowledge that many conditions look very similar, whether to the naked eye or under the microscope. This is true for rare diseases but also for the rashes we routinely see. A group of investigators recently used RNA transcriptome sequencing to analyze differences between atopic dermatitis and psoriasis, permitting better differentiation of these 2 common conditions.7
One of the greatest challenges confronting dermatologists and their dermatopathologist partners is to distinguish malignant melanoma from benign nevi.8 Despite staining for a number of molecular markers, some lesions defy histopathology, such as distinguishing benign and malignant Spitz nevi; however, recent work on RNA transcriptomes suggests that gene expression may increase confidence in assessing atypical Spitz nevi.9 A 23-gene expression panel has yielded a sensitivity of 91.5% and a specificity of 92.5% in differentiating benign nevi from malignant melanoma.10
From the Research Laboratory to Routine Clinical Use
Undoubtedly, it is a large step from proof-of-concept studies to accepted clinical use. The ultimate achievement for a laboratory technique is to enter approved clinical use. Gene expression panels have now been approved by numerous third-party insurers to help predict future clinical evolution of biopsied melanomas. Although early in situ melanomas are eminently curable by wide excision, lesions that have more concerning characteristics (eg, depth >0.8 mm, ulceration) may progress to metastatic disease. The gratifying success of checkpoint inhibitor therapy has improved the previously dismal outlook for advanced melanomas.11 Dermatologists search for clues to suggest which patients may benefit from adjuvant therapy. Sentinel lymph node biopsy (SLNB) has been a standard-of-care technique to help make this determination.12
It has now been demonstrated that gene expression array analysis can provide evidence complementing SLNB results or even independent of SLNB results. In extensive validation studies, a 31-gene expression panel analyzing initial melanoma biopsy specimens showed predictive value for later recurrence and development of metastatic disease.13,14 The gene expression studies have identified patients with negative SLNBs who have gone on to develop metastatic melanomas.15 It has been suggested that gene expression panel diagnosis may reduce the need for invasive SLNBs in patients in whom the surgical procedure may involve risk.16
Looking to the Future
The progress of science is the result of many small steps building on prior work. The terms breakthrough and game changer in medicine have been popularized by the media and rarely are valid. On the contrary, sequential development of methods over many years has preceded the acclaimed successes of medical research; for example, the best-known medical breakthrough—that of Salk’s inactivated polio vaccine—was preceded by the use of an inactivated polio vaccine by Brodie and Park17 in 1935. However, it was the development of tissue culture of poliomyelitis virus by Enders et al18 that provided the methodology to Salk’s group to produce their inactivated polio vaccine.
The ability to go beyond our visual senses will be of great importance in characterizing the variability of skin diseases, especially in skin of color patients; for example, acral melanoma is perhaps the primary melanocytic malignancy in darker-skinned patients and is the target of RNA transcriptomic research.19 Progress is continuing on gene therapy for a growing number of skin conditions.20,21 In vivo correction of abnormal genes is being attempted for a number of inherited cutaneous diseases,22 notably for disorders of skin fragility.23 For now, we welcome the addition of genomic capabilities to the visual practice of dermatology and the capability to go beyond that which we can see with our eyes.
- Feramisco JD, Sadreyev RI, Murray ML, et al. Phenotypic and enotypic analyses of genetic skin disease through the Online Mendelian Inheritance in Man (OMIM) database. J Investig Derm. 2009;129:2628-2636.
- Parker JC, Rangu S, Grand KL, et al. Genetic skin disorders: the value of a multidisciplinary clinic. Am J Med Genet A. 2021;185:1159-1167.
- Richert B, Smits G. Clinical and molecular diagnosis of genodermatoses: review and perspectives. J Eur Acad Dermatol Venereol. 2023;37:488-500.
- Green ED, Watson JD, Collins FS. Human genome project: twenty-five years of big biology. Nature. 2015;526:29-31.
- Saeidian AH, Youssefian L, Vahidnezhad H, et al. Research techniques made simple: whole-transcriptome sequencing by RNA-seq for diagnosis of monogenic disorders. J Invest Dermatol. 2020;140:1117-1126.e1.
- Deutsch A, McLellan BN, Shinoda K. Single-cell transcriptomics in dermatology. JAAD Int. 2020;1:182-188.
- Liu Y, Wang H, Taylor M, et al. Classification of human chronic inflammatory skin disease based on single-cell immune profiling [published online April 15, 2022]. Sci Immunol. doi:10.1126/sciimmunol.abl9165
- Reimann JDR, Salim S, Velazquez EF, et al. Comparison of melanoma gene expression score with histopathology, fluorescence in situ hybridization, and SNP array for the classification of melanocytic neoplasms. Mod Pathol. 2018;31:1733-1743.
- Hillen LM, Geybels MS, Spassova I, et al. A digital mRNA expression signature to classify challenging spitzoid melanocytic neoplasms. FEBS Open Bio. 2020;10:1326-1341.
- Clarke LE, Flake DD 2nd, Busam K, et al. An independent validation of a gene expression signature to differentiate malignant melanoma from benign melanocytic nevi. Cancer. 2017;123:617-628.
- Stege H, Haist M, Nikfarjam U, et al. The status of adjuvant and neoadjuvant melanoma therapy, new developments and upcoming challenges. Target Oncol. 2021;16:537-552.
- Morrison S, Han D. Re-evaluation of sentinel lymph node biopsy for melanoma. Curr Treat Options Oncol. 2021;22:22.
- Gerami P, Cook RW, Russell MC, et al. Gene expression profiling for molecular staging of cutaneous melanoma in patients with sentinel lymph node biopsy. J Am Acad Dermatol. 2015;72:780-785.e3.
- Keller J, Schwartz TL, Lizalek JM, et al. Prospective validation of the prognostic 31-gene expression profiling test in primary cutaneous melanoma. Cancer Med. 2019;8:2205-2212.
- Gastman BR, Gerami P, Kurley SJ, et al. Identification of patients at risk for metastasis using a prognostic 31-gene expression profile in subpopulations of melanoma patients with favorable outcomes by standard criteria. J Am Acad Dermatol. 2019;80:149-157.
- Vetto JT, Hsueh EC, Gastman BR, et al. Guidance of sentinel lymph node biopsy decisions in patients with T1-T2 melanoma using gene expression profiling. Future Oncol. 2019;15:1207-1217.
- Brodie M, Park W. Active immunization against poliomyelitis. JAMA. 1935;105:1089-1093.
- Enders JF, Weller TH, Robbins FC. Cultivation of the Lansing strain of poliomyelitis virus in cultures of various human embryonic tissues. Science. 1949;109:85-87.
- Li J, Smalley I, Chen Z, et al. Single-cell characterization of the cellular landscape of acral melanoma identifies novel targets for immunotherapy. Clin Cancer Res. 2022;28:2131-2146.
- Gorell E, Nguyen N, Lane A, et al. Gene therapy for skin diseases. Cold Spring Harb Perspect Med. 2014;4:A015149.
- Cavazza A, Mavilio F. Gene therapy of skin adhesion disorders (mini review). Curr Pharm Biotechnol. 2012;13:1868-1876.
- Abdul-Wahab A, Qasim W, McGrath JA. Gene therapies for inherited skin disorders. Semin Cutan Med Surg. 2014;33:83-90.
- Bilousova G. Gene therapy for skin fragility diseases: the new generation. J Invest Dermatol. 2019;139:1634-1637.
- Feramisco JD, Sadreyev RI, Murray ML, et al. Phenotypic and enotypic analyses of genetic skin disease through the Online Mendelian Inheritance in Man (OMIM) database. J Investig Derm. 2009;129:2628-2636.
- Parker JC, Rangu S, Grand KL, et al. Genetic skin disorders: the value of a multidisciplinary clinic. Am J Med Genet A. 2021;185:1159-1167.
- Richert B, Smits G. Clinical and molecular diagnosis of genodermatoses: review and perspectives. J Eur Acad Dermatol Venereol. 2023;37:488-500.
- Green ED, Watson JD, Collins FS. Human genome project: twenty-five years of big biology. Nature. 2015;526:29-31.
- Saeidian AH, Youssefian L, Vahidnezhad H, et al. Research techniques made simple: whole-transcriptome sequencing by RNA-seq for diagnosis of monogenic disorders. J Invest Dermatol. 2020;140:1117-1126.e1.
- Deutsch A, McLellan BN, Shinoda K. Single-cell transcriptomics in dermatology. JAAD Int. 2020;1:182-188.
- Liu Y, Wang H, Taylor M, et al. Classification of human chronic inflammatory skin disease based on single-cell immune profiling [published online April 15, 2022]. Sci Immunol. doi:10.1126/sciimmunol.abl9165
- Reimann JDR, Salim S, Velazquez EF, et al. Comparison of melanoma gene expression score with histopathology, fluorescence in situ hybridization, and SNP array for the classification of melanocytic neoplasms. Mod Pathol. 2018;31:1733-1743.
- Hillen LM, Geybels MS, Spassova I, et al. A digital mRNA expression signature to classify challenging spitzoid melanocytic neoplasms. FEBS Open Bio. 2020;10:1326-1341.
- Clarke LE, Flake DD 2nd, Busam K, et al. An independent validation of a gene expression signature to differentiate malignant melanoma from benign melanocytic nevi. Cancer. 2017;123:617-628.
- Stege H, Haist M, Nikfarjam U, et al. The status of adjuvant and neoadjuvant melanoma therapy, new developments and upcoming challenges. Target Oncol. 2021;16:537-552.
- Morrison S, Han D. Re-evaluation of sentinel lymph node biopsy for melanoma. Curr Treat Options Oncol. 2021;22:22.
- Gerami P, Cook RW, Russell MC, et al. Gene expression profiling for molecular staging of cutaneous melanoma in patients with sentinel lymph node biopsy. J Am Acad Dermatol. 2015;72:780-785.e3.
- Keller J, Schwartz TL, Lizalek JM, et al. Prospective validation of the prognostic 31-gene expression profiling test in primary cutaneous melanoma. Cancer Med. 2019;8:2205-2212.
- Gastman BR, Gerami P, Kurley SJ, et al. Identification of patients at risk for metastasis using a prognostic 31-gene expression profile in subpopulations of melanoma patients with favorable outcomes by standard criteria. J Am Acad Dermatol. 2019;80:149-157.
- Vetto JT, Hsueh EC, Gastman BR, et al. Guidance of sentinel lymph node biopsy decisions in patients with T1-T2 melanoma using gene expression profiling. Future Oncol. 2019;15:1207-1217.
- Brodie M, Park W. Active immunization against poliomyelitis. JAMA. 1935;105:1089-1093.
- Enders JF, Weller TH, Robbins FC. Cultivation of the Lansing strain of poliomyelitis virus in cultures of various human embryonic tissues. Science. 1949;109:85-87.
- Li J, Smalley I, Chen Z, et al. Single-cell characterization of the cellular landscape of acral melanoma identifies novel targets for immunotherapy. Clin Cancer Res. 2022;28:2131-2146.
- Gorell E, Nguyen N, Lane A, et al. Gene therapy for skin diseases. Cold Spring Harb Perspect Med. 2014;4:A015149.
- Cavazza A, Mavilio F. Gene therapy of skin adhesion disorders (mini review). Curr Pharm Biotechnol. 2012;13:1868-1876.
- Abdul-Wahab A, Qasim W, McGrath JA. Gene therapies for inherited skin disorders. Semin Cutan Med Surg. 2014;33:83-90.
- Bilousova G. Gene therapy for skin fragility diseases: the new generation. J Invest Dermatol. 2019;139:1634-1637.
Artificial Intelligence vs Medical Providers in the Dermoscopic Diagnosis of Melanoma
The incidence of skin cancer continues to increase, and it is by far the most common malignancy in the United States. Based on the sheer incidence and prevalence of skin cancer, early detection and treatment are critical. Looking at melanoma alone, the 5-year survival rate is greater than 99% when detected early but falls to 71% when the disease reaches the lymph nodes and 32% with metastasis to distant organs.1 Furthermore, a 2018 study found stage I melanoma patients who were treated 4 months after biopsy had a 41% increased risk of death compared with those treated within the first month.2 However, many patients are not seen by a dermatologist first for examination of suspicious skin lesions and instead are referred by a general practitioner or primary care mid-level provider. Therefore, many patients experience a longer time to diagnosis or treatment, which directly correlates with survival rate.
Dermoscopy is a noninvasive diagnostic tool for skin lesions, including melanoma. Using a handheld dermoscope (or dermatoscope), a transilluminating light source magnifies skin lesions and allows for the visualization of subsurface skin structures within the epidermis, dermoepidermal junction, and papillary dermis.3 Dermoscopy has been shown to improve a dermatologist’s accuracy in diagnosing malignant melanoma vs clinical evaluation with the unaided eye.4,5 More recently, dermoscopy has been digitized, allowing for the collection and documentation of case photographs. Dermoscopy also has expanded past the scope of dermatologists and has become increasingly useful in primary care.6 Among family physicians, dermoscopy also has been shown to have a higher sensitivity for melanoma detection compared to gross examination.7 Therefore, both the increased diagnostic performance of malignant melanoma using a dermoscope and the expanded use of dermoscopy in medical care validate the evaluation of an artificial intelligence (AI) algorithm in diagnosing malignant melanoma using dermoscopic images.
Triage (Triage Technologies Inc) is an AI application that uses a web interface and combines a pretrained convolutional neural network (CNN) with a reinforcement learning agent as a question-answering model. The CNN algorithm can classify 133 different skin diseases, 7 of which it is able to classify using dermoscopic images. This study sought to evaluate the performance of Triage’s dermoscopic classifier in identifying lesions as benign or malignant to determine whether AI could assist in the triage of skin cancer cases to shorten time to diagnosis.
Materials and Methods
The MClass-D test set from the International Skin Imaging Collaboration was assessed by both AI and practicing medical providers. The set was composed of 80 benign nevi and 20 biopsy-verified malignant melanomas. Board-certified US dermatologists (n=23), family physicians (n=7), and primary care mid-level providers (n=12)(ie, nurse practitioners, physician assistants) were asked to label the images as benign or malignant. The results from the medical providers were then compared to the performance of the AI application by looking at the sensitivity, specificity, accuracy, positive predictive value (PPV), and negative predictive value (NPV). Statistical significance was determined with a 1 sample t test run through RStudio (Posit Software, PBC), and P<.05 was considered significant.
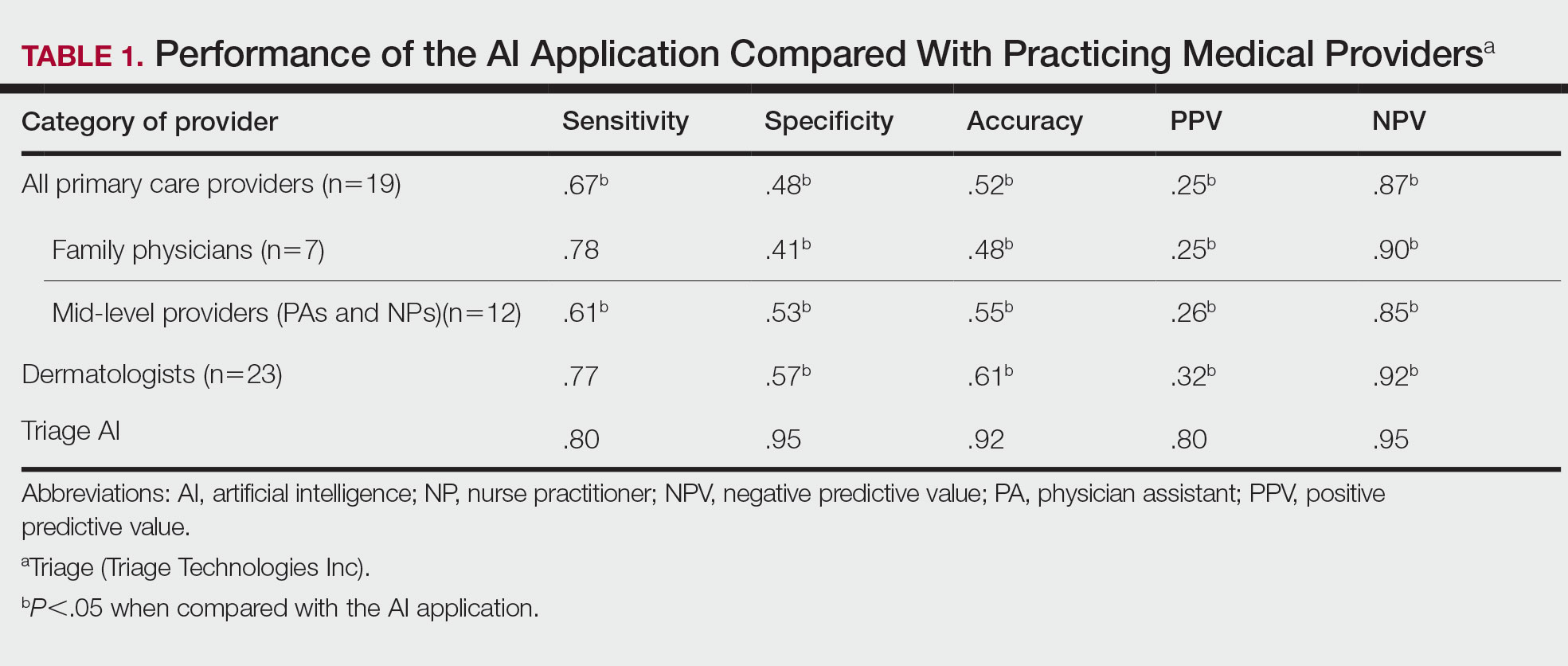
Results
The AI application performed extremely well in differentiating between benign nevi and malignant melanomas, with a sensitivity of 80%, specificity of 95%, accuracy of 92%, PPV of 80%, and NPV of 95% (Table 1). When compared with practicing medical providers, the AI performed significantly better in almost all categories (P<.05)(Figure 1). With all medical providers combined, the AI had significantly higher accuracy, sensitivity, and specificity (P<.05). The accuracy of the individual medical providers ranged from 32% to 78%.
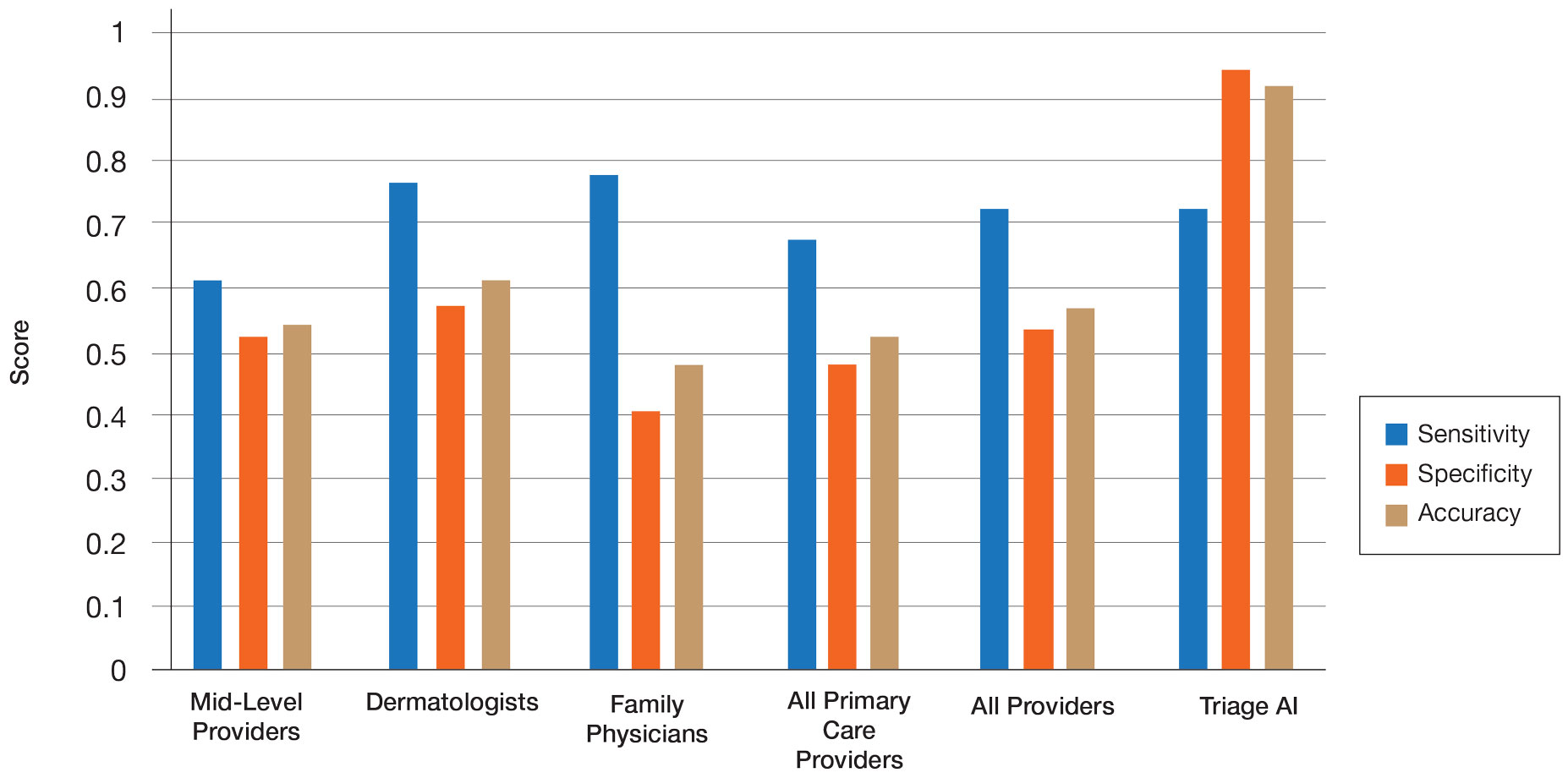
Compared with dermatologists, the AI was significantly more specific and accurate and demonstrated a higher PPV and NPV (P<.05). There was no significant difference between the AI and dermatologists in sensitivity or labeling the true malignant lesions as malignant. The dermatologists who participated had been practicing from 1.5 years to 44 years, with an average of 16 years of dermatologic experience. There was no correlation between years practicing and performance in determining the malignancy of lesions. Of 14 dermatologists, dermoscopy was used daily by 10 and occasionally by 3, but only 6 dermatologists had any formal training. Dermatologists who used dermoscopy averaged 11 years of use.
The AI also performed significantly better than the primary care providers, including both family physicians and mid-level providers (P<.05). With the family physicians and mid-level provider scores combined, the AI showed a statistically significantly better performance in all categories examined, including sensitivity, specificity, accuracy, PPV, and NPV (P<.05). However, when compared with family physicians alone, the AI did not demonstrate a statistically significant difference in sensitivity.
Comment
Automatic Visual Recognition Development—The AI application we studied was developed by dermatologists as a tool to assist in the screening of skin lesions suspicious for melanoma or a benign neoplasm.8 Developing AI applications that can reliably recognize objects in photographs has been the subject of considerable research. Notable progress in automatic visual recognition was shown in 2012 when a deep learning model won the ImageNet object recognition challenge and outperformed competing approaches by a large margin.9,10 The ImageNet competition, which has been held annually since 2010, required participants to build a visual classification system that distinguished among 1000 object categories using 1.2 million labeled images as training data. In 2017, participants developed automated visual systems that surpassed the estimated human performance.11 Given this success, the organization decided to deliver a more challenging competition involving 3D imaging—Medical ImageNet, a petabyte-scale, cloud-based, open repository project—with goals including image classification and annotation.12
Convolutional Neural Networks—Convolutional neural networks are computer system architectures commonly employed for making predictions from images.13 Convolutional neural networks are based on a set of layers of learned filters that perform convolution, a mathematical operation that reflects the relationship between the 2 functions. The main algorithm that makes the learning possible is called backpropagation, wherein an error is computed at the output and distributed backward through the neural network’s layers.14 Although CNNs and backpropagation methods have existed since 1989, recent technologic advances have allowed for deep learning–based algorithms to be widely integrated with everyday applications.15 Advances in computational power in the form of graphics processing units and parallelization, the existence of large data sets such as the ImageNet database, and the rise of software frameworks have allowed for quick prototyping and deployment of deep learning models.16,17
Convolutional neural networks have demonstrated potential to excel at a wide range of visual tasks. In dermatology, visual recognition methods often rely on using either a pretrained CNN as a feature extractor for further classification or fine-tuning a pretrained network on dermoscopic images.18-20 In 2017, a model was trained on 130,000 clinical images of benign and malignant skin lesions. Its performance was found to be in line with that of 21 US board-certified dermatology experts when diagnosing skin cancers from clinical images confirmed by biopsy.21
Triage—The AI application Triage is composed of several components contained in a web interface (Figure 2). To use the interface, the user must sign up and upload a photograph to the website. The image first passes through a gated-logic visual classifier that rejects any images that do not contain a visible skin condition. If the image contains a skin condition, the image is passed to a skin classifier that predicts the probability of the image containing 1 of 133 classes of skin conditions, 7 of which the application can diagnose with a dermoscopic image.
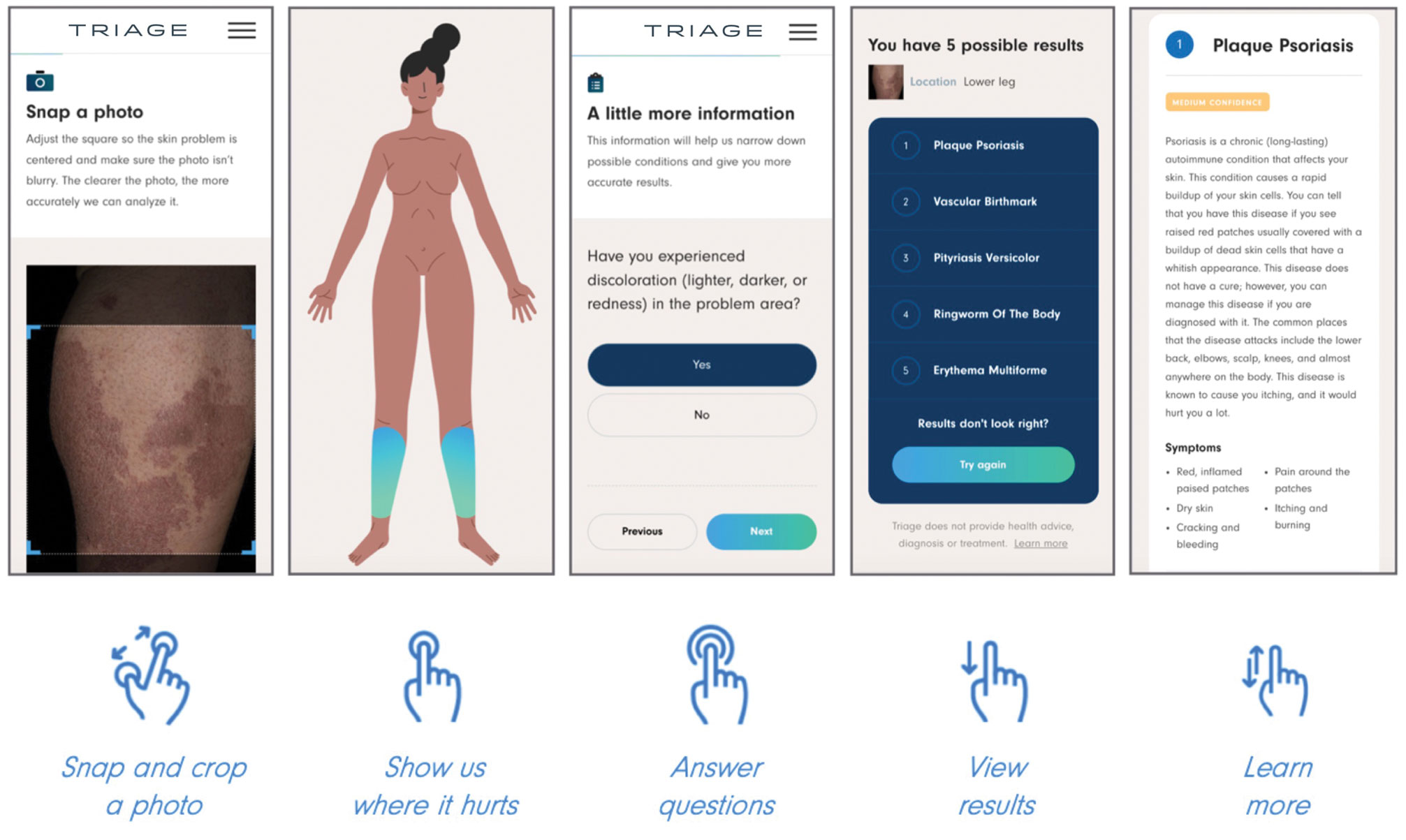
The AI application uses several techniques when training a CNN model. To address skin condition class imbalances (when more examples exist for 1 class than the others) in the training data, additional weights are applied to mistakes made on underrepresented classes, which encourages the model to better detect cases with low prevalence in the data set. Data augmentation techniques such as rotating, zooming, and flipping the training images are applied to allow the model to become more familiar with variability in the input images. Convolutional neural networks are trained using a well-known neural network optimization method called Stochastic gradient descent with momentum.22
The final predictions are refined by a question-and-answer system that encodes dermatology knowledge and is currently under active development. Finally, the top k most probable conditions are displayed to the user, where k≤5. An initial prototype of the system was described in a published research paper in the 2019 medical imaging workshop of the Neural Information Systems conference.23
The prototype demonstrated that combining a pretrained CNN with a reinforcement learning agent as a question-answering model increased the classification confidence and accuracy of its visual symptom checker and decreased the average number of questions asked to narrow down the differential diagnosis. The reinforcement learning approach increases the accuracy more than 20% compared with the CNN-only approach, which only uses visual information to predict the condition.23
This application’s current visual question-answering system is trained on a diverse set of data that includes more than 20 years of clinical encounters and user-uploaded cases submitted by more than 150,000 patients and 10,000 clinicians in more than 150 countries. All crowdsourced images used for training the dermoscopy classifier are biopsy-verified images contributed by dermatologists. These data are made up of case photographs that are tagged with metadata around the patient’s age, sex, symptoms, and diagnoses. The CNN algorithm used covers 133 skin disease classes, representing 588 clinical conditions. It also can automatically detect 7 malignant, premalignant, and benign dermoscopic categories, which is the focus of this study (Table 2). Diagnoses are verified by patient response to treatment, biopsy results, and dermatologist consensus.
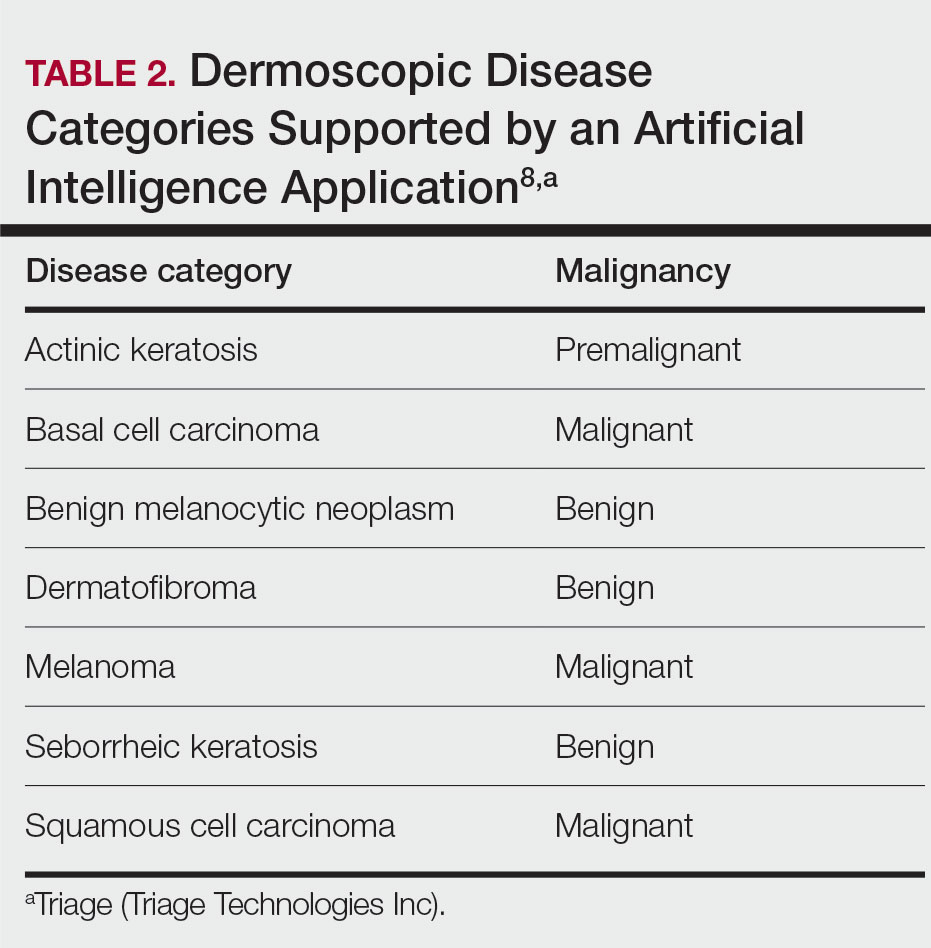
In addition to having improved performance, supporting more than 130 disease classes, and having a diverse data set, the application used has beat competing technologies.20,24 The application currently is available on the internet in more than 30 countries after it received Health Canada Class I medical device approval and the CE mark in Europe.
Can AI Reliably Detect Melanoma?—In our study, of the lesions labeled benign, the higher PPV and NPV of the AI algorithm means that the lesions were more reliably true benign lesions, and the lesions labeled as malignant were more likely to be true malignant lesions. Therefore, the diagnosis given by the AI compared with the medical provider was significantly more likely to be correct. These findings demonstrate that this AI application can reliably detect malignant melanoma using dermoscopic images. However, this study was limited by the small sample size of medical providers. Further studies are necessary to assess whether the high diagnostic accuracy of the application translates to expedited referrals and a decrease in unnecessary biopsies.
Dermoscopy Training—This study looked at dermoscopic images instead of gross examination, as is often done in clinic, which draws into question the dermoscopic training dermatologists receive. The diagnostic accuracy using dermoscopic images has been shown to be higher than evaluation with the naked eye.5,6 However, there currently is no standard for dermoscopic training in dermatology residencies, and education varies widely.25 These data suggest that there may be a lack of dermoscopic training among dermatologists, which could accentuate the difference in performance between dermatologists and AI. Most primary care providers also lack formal dermoscopy training. Although dermoscopy has been shown to increase the diagnostic efficacy of primary care providers, this increase does not become apparent until the medical provider has had years of formal training in addition to clinical experience, which is not commonly provided in the medical training that primary care providers receive.8,26
Conclusion
It is anticipated that AI will shape the future of medicine and become incorporated into daily practice.27 Artificial intelligence will not replace physicians but rather assist clinicians and help to streamline medical care. Clinicians will take on the role of interpreting AI output and integrate it into patient care. With this advancement, it is important to highlight that for AI to improve the quality, efficiency, and accessibility of health care, clinicians must be equipped with the right training.27-29
- Cancer facts & figures 2023. American Cancer Society. Accessed April 20, 2023. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2023/2023-cancer-facts-and-figures.pdf
- Conic RZ, Cabrera CI, Khorana AA, et al. Determination of the impact of melanoma surgical timing on survival using the National Cancer Database. J Am Acad Dermatol. 2018;78:40-46.e7. doi:10.1016/j.jaad.2017.08.039
- Lallas A, Zalaudek I, Argenziano G, et al. Dermoscopy in general dermatology. Dermatol Clin. 2013;31:679-694, x. doi:10.1016/j.det.2013.06.008
- Bafounta M-L, Beauchet A, Aegerter P, et al. Is dermoscopy (epiluminescence microscopy) useful for the diagnosis of melanoma?: results of a meta-analysis using techniques adapted to the evaluation of diagnostic tests. Arch Dermatol. 2001;137:1343-1350. doi:10.1001/archderm.137.10.1343
- Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676. doi:10.1111/j.1365-2133.2008.08713.x
- Marghoob AA, Usatine RP, Jaimes N. Dermoscopy for the family physician. Am Fam Physician. 2013;88:441-450.
- Herschorn A. Dermoscopy for melanoma detection in family practice. Can Fam Physician. 2012;58:740-745, e372-8.
- Instructions for use for the Triage app. Triage website. Accessed April 20, 2023. https://www.triage.com/pdf/en/Instructions%20for%20Use.pdf
- Krizhevsky A, Sutskever I, Hinton GE. ImageNet classification with deep convolutional neural networks. In: Pereira F, Burges CJC, Bottou L, et al, eds. Advances in Neural Information Processing Systems. Vol 25. Curran Associates, Inc; 2012. Accessed April 17, 2023. https://proceedings.neurips.cc/paper/2012/file/c399862d3b9d6b76c8436e924a68c45b-Paper.pdf
- Russakovsky O, Deng J, Su H, et al. ImageNet large scale visualrecognition challenge. Int J Comput Vis. 2015;115:211-252. doi:10.1007/s11263-015-0816-y
- Hu J, Shen L, Albanie S, et al. Squeeze-and-excitation networks. IEEE Trans Patt Anal Mach Intell. 2020;42:2011-2023. doi:10.1109/TPAMI.2019.2913372
- Medical image net-radiology informatics. Stanford University Center for Artificial Intelligence in Medicine & Imaging website. Accessed April 20, 2023. https://aimi.stanford.edu/medical-imagenet
- LeCun Y, Bengio Y, Hinton G. Deep learning. Nature. 2015;521:436-444. doi:10.1038/nature14539
- Le Cun Yet al. A theoretical framework for back-propagation. In:Touretzky D, Honton G, Sejnowski T, eds. Proceedings of the 1988 Connect Models Summer School. Morgan Kaufmann; 1988:21-28.
- Lecun Y, Bottou L, Bengio Y, et al. Gradient-based learning applied to document recognition. Proc IEEE. 1998;86:2278-2324. doi:10.1109/5.726791
- Chollet E. About Keras. Keras website. Accessed April 21, 2023. https://keras.io/about/
- Introduction to TensorFlow. TensorFlow website. Accessed April 21, 2023. https://www.tensorflow.org/learn
- Kawahara J, BenTaieb A, Hamarneh G. Deep features to classify skin lesions. 2016 IEEE 13th International Symposium on Biomedical Imaging. 2016. doi:10.1109/ISBI.2016.7493528
- Lopez AR, Giro-i-Nieto X, Burdick J, et al. Skin lesion classification from dermoscopic images using deep learning techniques. doi:10.2316/P.2017.852-053
- Codella NCF, Nguyen QB, Pankanti S, et al. Deep learning ensembles for melanoma recognition in dermoscopy images. IBM J Res Dev. 2017;61:1-28. doi:10.1147/JRD.2017.2708299
- Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542:115-118. doi:10.1038/nature21056
- Sutskever I, Martens J, Dahl G, et al. On the importance of initialization and momentum in deep learning. ICML’13: Proceedings of the 30th International Conference on International Conference on Machine Learning. 2013;28:1139-1147.
- Akrout M, Farahmand AM, Jarmain T, et al. Improving skin condition classification with a visual symptom checker trained using reinforcement learning. In: Medical Image Computing and Computer Assisted Intervention – MICCAI 2019: 22nd International Conference. October 13-17, 2019. Shenzhen, China. Proceedings, Part IV. Springer-Verlag; 549-557. doi:10.1007/978-3-030-32251-9_60
- Liu Y, Jain A, Eng C, et al. A deep learning system for differential diagnosis of skin diseases. Nat Med. 2020;26:900-908. doi:10.1038/s41591-020-0842-3
- Fried LJ, Tan A, Berry EG, et al. Dermoscopy proficiency expectations for US dermatology resident physicians: results of a modified delphi survey of pigmented lesion experts. JAMA Dermatol. 2021;157:189-197. doi:10.1001/jamadermatol.2020.5213
- Fee JA, McGrady FP, Rosendahl C, et al. Training primary care physicians in dermoscopy for skin cancer detection: a scoping review. J Cancer Educ. 2020;35:643-650. doi:10.1007/s13187-019-01647-7
- James CA, Wachter RM, Woolliscroft JO. Preparing clinicians for a clinical world influenced by artificial intelligence. JAMA. 2022;327:1333-1334. doi:10.1001/jama.2022.3580
- Yu K-H, Beam AL, Kohane IS. Artificial intelligence in healthcare. Nat Biomed Eng. 2018;2:719-731. doi:10.1038/s41551-018-0305-z
- Chen M, Decary M. Artificial intelligence in healthcare: an essential guide for health leaders. Healthc Manag Forum. 2020;33:10-18. doi:10.1177/0840470419873123
The incidence of skin cancer continues to increase, and it is by far the most common malignancy in the United States. Based on the sheer incidence and prevalence of skin cancer, early detection and treatment are critical. Looking at melanoma alone, the 5-year survival rate is greater than 99% when detected early but falls to 71% when the disease reaches the lymph nodes and 32% with metastasis to distant organs.1 Furthermore, a 2018 study found stage I melanoma patients who were treated 4 months after biopsy had a 41% increased risk of death compared with those treated within the first month.2 However, many patients are not seen by a dermatologist first for examination of suspicious skin lesions and instead are referred by a general practitioner or primary care mid-level provider. Therefore, many patients experience a longer time to diagnosis or treatment, which directly correlates with survival rate.
Dermoscopy is a noninvasive diagnostic tool for skin lesions, including melanoma. Using a handheld dermoscope (or dermatoscope), a transilluminating light source magnifies skin lesions and allows for the visualization of subsurface skin structures within the epidermis, dermoepidermal junction, and papillary dermis.3 Dermoscopy has been shown to improve a dermatologist’s accuracy in diagnosing malignant melanoma vs clinical evaluation with the unaided eye.4,5 More recently, dermoscopy has been digitized, allowing for the collection and documentation of case photographs. Dermoscopy also has expanded past the scope of dermatologists and has become increasingly useful in primary care.6 Among family physicians, dermoscopy also has been shown to have a higher sensitivity for melanoma detection compared to gross examination.7 Therefore, both the increased diagnostic performance of malignant melanoma using a dermoscope and the expanded use of dermoscopy in medical care validate the evaluation of an artificial intelligence (AI) algorithm in diagnosing malignant melanoma using dermoscopic images.
Triage (Triage Technologies Inc) is an AI application that uses a web interface and combines a pretrained convolutional neural network (CNN) with a reinforcement learning agent as a question-answering model. The CNN algorithm can classify 133 different skin diseases, 7 of which it is able to classify using dermoscopic images. This study sought to evaluate the performance of Triage’s dermoscopic classifier in identifying lesions as benign or malignant to determine whether AI could assist in the triage of skin cancer cases to shorten time to diagnosis.
Materials and Methods
The MClass-D test set from the International Skin Imaging Collaboration was assessed by both AI and practicing medical providers. The set was composed of 80 benign nevi and 20 biopsy-verified malignant melanomas. Board-certified US dermatologists (n=23), family physicians (n=7), and primary care mid-level providers (n=12)(ie, nurse practitioners, physician assistants) were asked to label the images as benign or malignant. The results from the medical providers were then compared to the performance of the AI application by looking at the sensitivity, specificity, accuracy, positive predictive value (PPV), and negative predictive value (NPV). Statistical significance was determined with a 1 sample t test run through RStudio (Posit Software, PBC), and P<.05 was considered significant.

Results
The AI application performed extremely well in differentiating between benign nevi and malignant melanomas, with a sensitivity of 80%, specificity of 95%, accuracy of 92%, PPV of 80%, and NPV of 95% (Table 1). When compared with practicing medical providers, the AI performed significantly better in almost all categories (P<.05)(Figure 1). With all medical providers combined, the AI had significantly higher accuracy, sensitivity, and specificity (P<.05). The accuracy of the individual medical providers ranged from 32% to 78%.

Compared with dermatologists, the AI was significantly more specific and accurate and demonstrated a higher PPV and NPV (P<.05). There was no significant difference between the AI and dermatologists in sensitivity or labeling the true malignant lesions as malignant. The dermatologists who participated had been practicing from 1.5 years to 44 years, with an average of 16 years of dermatologic experience. There was no correlation between years practicing and performance in determining the malignancy of lesions. Of 14 dermatologists, dermoscopy was used daily by 10 and occasionally by 3, but only 6 dermatologists had any formal training. Dermatologists who used dermoscopy averaged 11 years of use.
The AI also performed significantly better than the primary care providers, including both family physicians and mid-level providers (P<.05). With the family physicians and mid-level provider scores combined, the AI showed a statistically significantly better performance in all categories examined, including sensitivity, specificity, accuracy, PPV, and NPV (P<.05). However, when compared with family physicians alone, the AI did not demonstrate a statistically significant difference in sensitivity.
Comment
Automatic Visual Recognition Development—The AI application we studied was developed by dermatologists as a tool to assist in the screening of skin lesions suspicious for melanoma or a benign neoplasm.8 Developing AI applications that can reliably recognize objects in photographs has been the subject of considerable research. Notable progress in automatic visual recognition was shown in 2012 when a deep learning model won the ImageNet object recognition challenge and outperformed competing approaches by a large margin.9,10 The ImageNet competition, which has been held annually since 2010, required participants to build a visual classification system that distinguished among 1000 object categories using 1.2 million labeled images as training data. In 2017, participants developed automated visual systems that surpassed the estimated human performance.11 Given this success, the organization decided to deliver a more challenging competition involving 3D imaging—Medical ImageNet, a petabyte-scale, cloud-based, open repository project—with goals including image classification and annotation.12
Convolutional Neural Networks—Convolutional neural networks are computer system architectures commonly employed for making predictions from images.13 Convolutional neural networks are based on a set of layers of learned filters that perform convolution, a mathematical operation that reflects the relationship between the 2 functions. The main algorithm that makes the learning possible is called backpropagation, wherein an error is computed at the output and distributed backward through the neural network’s layers.14 Although CNNs and backpropagation methods have existed since 1989, recent technologic advances have allowed for deep learning–based algorithms to be widely integrated with everyday applications.15 Advances in computational power in the form of graphics processing units and parallelization, the existence of large data sets such as the ImageNet database, and the rise of software frameworks have allowed for quick prototyping and deployment of deep learning models.16,17
Convolutional neural networks have demonstrated potential to excel at a wide range of visual tasks. In dermatology, visual recognition methods often rely on using either a pretrained CNN as a feature extractor for further classification or fine-tuning a pretrained network on dermoscopic images.18-20 In 2017, a model was trained on 130,000 clinical images of benign and malignant skin lesions. Its performance was found to be in line with that of 21 US board-certified dermatology experts when diagnosing skin cancers from clinical images confirmed by biopsy.21
Triage—The AI application Triage is composed of several components contained in a web interface (Figure 2). To use the interface, the user must sign up and upload a photograph to the website. The image first passes through a gated-logic visual classifier that rejects any images that do not contain a visible skin condition. If the image contains a skin condition, the image is passed to a skin classifier that predicts the probability of the image containing 1 of 133 classes of skin conditions, 7 of which the application can diagnose with a dermoscopic image.

The AI application uses several techniques when training a CNN model. To address skin condition class imbalances (when more examples exist for 1 class than the others) in the training data, additional weights are applied to mistakes made on underrepresented classes, which encourages the model to better detect cases with low prevalence in the data set. Data augmentation techniques such as rotating, zooming, and flipping the training images are applied to allow the model to become more familiar with variability in the input images. Convolutional neural networks are trained using a well-known neural network optimization method called Stochastic gradient descent with momentum.22
The final predictions are refined by a question-and-answer system that encodes dermatology knowledge and is currently under active development. Finally, the top k most probable conditions are displayed to the user, where k≤5. An initial prototype of the system was described in a published research paper in the 2019 medical imaging workshop of the Neural Information Systems conference.23
The prototype demonstrated that combining a pretrained CNN with a reinforcement learning agent as a question-answering model increased the classification confidence and accuracy of its visual symptom checker and decreased the average number of questions asked to narrow down the differential diagnosis. The reinforcement learning approach increases the accuracy more than 20% compared with the CNN-only approach, which only uses visual information to predict the condition.23
This application’s current visual question-answering system is trained on a diverse set of data that includes more than 20 years of clinical encounters and user-uploaded cases submitted by more than 150,000 patients and 10,000 clinicians in more than 150 countries. All crowdsourced images used for training the dermoscopy classifier are biopsy-verified images contributed by dermatologists. These data are made up of case photographs that are tagged with metadata around the patient’s age, sex, symptoms, and diagnoses. The CNN algorithm used covers 133 skin disease classes, representing 588 clinical conditions. It also can automatically detect 7 malignant, premalignant, and benign dermoscopic categories, which is the focus of this study (Table 2). Diagnoses are verified by patient response to treatment, biopsy results, and dermatologist consensus.

In addition to having improved performance, supporting more than 130 disease classes, and having a diverse data set, the application used has beat competing technologies.20,24 The application currently is available on the internet in more than 30 countries after it received Health Canada Class I medical device approval and the CE mark in Europe.
Can AI Reliably Detect Melanoma?—In our study, of the lesions labeled benign, the higher PPV and NPV of the AI algorithm means that the lesions were more reliably true benign lesions, and the lesions labeled as malignant were more likely to be true malignant lesions. Therefore, the diagnosis given by the AI compared with the medical provider was significantly more likely to be correct. These findings demonstrate that this AI application can reliably detect malignant melanoma using dermoscopic images. However, this study was limited by the small sample size of medical providers. Further studies are necessary to assess whether the high diagnostic accuracy of the application translates to expedited referrals and a decrease in unnecessary biopsies.
Dermoscopy Training—This study looked at dermoscopic images instead of gross examination, as is often done in clinic, which draws into question the dermoscopic training dermatologists receive. The diagnostic accuracy using dermoscopic images has been shown to be higher than evaluation with the naked eye.5,6 However, there currently is no standard for dermoscopic training in dermatology residencies, and education varies widely.25 These data suggest that there may be a lack of dermoscopic training among dermatologists, which could accentuate the difference in performance between dermatologists and AI. Most primary care providers also lack formal dermoscopy training. Although dermoscopy has been shown to increase the diagnostic efficacy of primary care providers, this increase does not become apparent until the medical provider has had years of formal training in addition to clinical experience, which is not commonly provided in the medical training that primary care providers receive.8,26
Conclusion
It is anticipated that AI will shape the future of medicine and become incorporated into daily practice.27 Artificial intelligence will not replace physicians but rather assist clinicians and help to streamline medical care. Clinicians will take on the role of interpreting AI output and integrate it into patient care. With this advancement, it is important to highlight that for AI to improve the quality, efficiency, and accessibility of health care, clinicians must be equipped with the right training.27-29
The incidence of skin cancer continues to increase, and it is by far the most common malignancy in the United States. Based on the sheer incidence and prevalence of skin cancer, early detection and treatment are critical. Looking at melanoma alone, the 5-year survival rate is greater than 99% when detected early but falls to 71% when the disease reaches the lymph nodes and 32% with metastasis to distant organs.1 Furthermore, a 2018 study found stage I melanoma patients who were treated 4 months after biopsy had a 41% increased risk of death compared with those treated within the first month.2 However, many patients are not seen by a dermatologist first for examination of suspicious skin lesions and instead are referred by a general practitioner or primary care mid-level provider. Therefore, many patients experience a longer time to diagnosis or treatment, which directly correlates with survival rate.
Dermoscopy is a noninvasive diagnostic tool for skin lesions, including melanoma. Using a handheld dermoscope (or dermatoscope), a transilluminating light source magnifies skin lesions and allows for the visualization of subsurface skin structures within the epidermis, dermoepidermal junction, and papillary dermis.3 Dermoscopy has been shown to improve a dermatologist’s accuracy in diagnosing malignant melanoma vs clinical evaluation with the unaided eye.4,5 More recently, dermoscopy has been digitized, allowing for the collection and documentation of case photographs. Dermoscopy also has expanded past the scope of dermatologists and has become increasingly useful in primary care.6 Among family physicians, dermoscopy also has been shown to have a higher sensitivity for melanoma detection compared to gross examination.7 Therefore, both the increased diagnostic performance of malignant melanoma using a dermoscope and the expanded use of dermoscopy in medical care validate the evaluation of an artificial intelligence (AI) algorithm in diagnosing malignant melanoma using dermoscopic images.
Triage (Triage Technologies Inc) is an AI application that uses a web interface and combines a pretrained convolutional neural network (CNN) with a reinforcement learning agent as a question-answering model. The CNN algorithm can classify 133 different skin diseases, 7 of which it is able to classify using dermoscopic images. This study sought to evaluate the performance of Triage’s dermoscopic classifier in identifying lesions as benign or malignant to determine whether AI could assist in the triage of skin cancer cases to shorten time to diagnosis.
Materials and Methods
The MClass-D test set from the International Skin Imaging Collaboration was assessed by both AI and practicing medical providers. The set was composed of 80 benign nevi and 20 biopsy-verified malignant melanomas. Board-certified US dermatologists (n=23), family physicians (n=7), and primary care mid-level providers (n=12)(ie, nurse practitioners, physician assistants) were asked to label the images as benign or malignant. The results from the medical providers were then compared to the performance of the AI application by looking at the sensitivity, specificity, accuracy, positive predictive value (PPV), and negative predictive value (NPV). Statistical significance was determined with a 1 sample t test run through RStudio (Posit Software, PBC), and P<.05 was considered significant.

Results
The AI application performed extremely well in differentiating between benign nevi and malignant melanomas, with a sensitivity of 80%, specificity of 95%, accuracy of 92%, PPV of 80%, and NPV of 95% (Table 1). When compared with practicing medical providers, the AI performed significantly better in almost all categories (P<.05)(Figure 1). With all medical providers combined, the AI had significantly higher accuracy, sensitivity, and specificity (P<.05). The accuracy of the individual medical providers ranged from 32% to 78%.

Compared with dermatologists, the AI was significantly more specific and accurate and demonstrated a higher PPV and NPV (P<.05). There was no significant difference between the AI and dermatologists in sensitivity or labeling the true malignant lesions as malignant. The dermatologists who participated had been practicing from 1.5 years to 44 years, with an average of 16 years of dermatologic experience. There was no correlation between years practicing and performance in determining the malignancy of lesions. Of 14 dermatologists, dermoscopy was used daily by 10 and occasionally by 3, but only 6 dermatologists had any formal training. Dermatologists who used dermoscopy averaged 11 years of use.
The AI also performed significantly better than the primary care providers, including both family physicians and mid-level providers (P<.05). With the family physicians and mid-level provider scores combined, the AI showed a statistically significantly better performance in all categories examined, including sensitivity, specificity, accuracy, PPV, and NPV (P<.05). However, when compared with family physicians alone, the AI did not demonstrate a statistically significant difference in sensitivity.
Comment
Automatic Visual Recognition Development—The AI application we studied was developed by dermatologists as a tool to assist in the screening of skin lesions suspicious for melanoma or a benign neoplasm.8 Developing AI applications that can reliably recognize objects in photographs has been the subject of considerable research. Notable progress in automatic visual recognition was shown in 2012 when a deep learning model won the ImageNet object recognition challenge and outperformed competing approaches by a large margin.9,10 The ImageNet competition, which has been held annually since 2010, required participants to build a visual classification system that distinguished among 1000 object categories using 1.2 million labeled images as training data. In 2017, participants developed automated visual systems that surpassed the estimated human performance.11 Given this success, the organization decided to deliver a more challenging competition involving 3D imaging—Medical ImageNet, a petabyte-scale, cloud-based, open repository project—with goals including image classification and annotation.12
Convolutional Neural Networks—Convolutional neural networks are computer system architectures commonly employed for making predictions from images.13 Convolutional neural networks are based on a set of layers of learned filters that perform convolution, a mathematical operation that reflects the relationship between the 2 functions. The main algorithm that makes the learning possible is called backpropagation, wherein an error is computed at the output and distributed backward through the neural network’s layers.14 Although CNNs and backpropagation methods have existed since 1989, recent technologic advances have allowed for deep learning–based algorithms to be widely integrated with everyday applications.15 Advances in computational power in the form of graphics processing units and parallelization, the existence of large data sets such as the ImageNet database, and the rise of software frameworks have allowed for quick prototyping and deployment of deep learning models.16,17
Convolutional neural networks have demonstrated potential to excel at a wide range of visual tasks. In dermatology, visual recognition methods often rely on using either a pretrained CNN as a feature extractor for further classification or fine-tuning a pretrained network on dermoscopic images.18-20 In 2017, a model was trained on 130,000 clinical images of benign and malignant skin lesions. Its performance was found to be in line with that of 21 US board-certified dermatology experts when diagnosing skin cancers from clinical images confirmed by biopsy.21
Triage—The AI application Triage is composed of several components contained in a web interface (Figure 2). To use the interface, the user must sign up and upload a photograph to the website. The image first passes through a gated-logic visual classifier that rejects any images that do not contain a visible skin condition. If the image contains a skin condition, the image is passed to a skin classifier that predicts the probability of the image containing 1 of 133 classes of skin conditions, 7 of which the application can diagnose with a dermoscopic image.

The AI application uses several techniques when training a CNN model. To address skin condition class imbalances (when more examples exist for 1 class than the others) in the training data, additional weights are applied to mistakes made on underrepresented classes, which encourages the model to better detect cases with low prevalence in the data set. Data augmentation techniques such as rotating, zooming, and flipping the training images are applied to allow the model to become more familiar with variability in the input images. Convolutional neural networks are trained using a well-known neural network optimization method called Stochastic gradient descent with momentum.22
The final predictions are refined by a question-and-answer system that encodes dermatology knowledge and is currently under active development. Finally, the top k most probable conditions are displayed to the user, where k≤5. An initial prototype of the system was described in a published research paper in the 2019 medical imaging workshop of the Neural Information Systems conference.23
The prototype demonstrated that combining a pretrained CNN with a reinforcement learning agent as a question-answering model increased the classification confidence and accuracy of its visual symptom checker and decreased the average number of questions asked to narrow down the differential diagnosis. The reinforcement learning approach increases the accuracy more than 20% compared with the CNN-only approach, which only uses visual information to predict the condition.23
This application’s current visual question-answering system is trained on a diverse set of data that includes more than 20 years of clinical encounters and user-uploaded cases submitted by more than 150,000 patients and 10,000 clinicians in more than 150 countries. All crowdsourced images used for training the dermoscopy classifier are biopsy-verified images contributed by dermatologists. These data are made up of case photographs that are tagged with metadata around the patient’s age, sex, symptoms, and diagnoses. The CNN algorithm used covers 133 skin disease classes, representing 588 clinical conditions. It also can automatically detect 7 malignant, premalignant, and benign dermoscopic categories, which is the focus of this study (Table 2). Diagnoses are verified by patient response to treatment, biopsy results, and dermatologist consensus.

In addition to having improved performance, supporting more than 130 disease classes, and having a diverse data set, the application used has beat competing technologies.20,24 The application currently is available on the internet in more than 30 countries after it received Health Canada Class I medical device approval and the CE mark in Europe.
Can AI Reliably Detect Melanoma?—In our study, of the lesions labeled benign, the higher PPV and NPV of the AI algorithm means that the lesions were more reliably true benign lesions, and the lesions labeled as malignant were more likely to be true malignant lesions. Therefore, the diagnosis given by the AI compared with the medical provider was significantly more likely to be correct. These findings demonstrate that this AI application can reliably detect malignant melanoma using dermoscopic images. However, this study was limited by the small sample size of medical providers. Further studies are necessary to assess whether the high diagnostic accuracy of the application translates to expedited referrals and a decrease in unnecessary biopsies.
Dermoscopy Training—This study looked at dermoscopic images instead of gross examination, as is often done in clinic, which draws into question the dermoscopic training dermatologists receive. The diagnostic accuracy using dermoscopic images has been shown to be higher than evaluation with the naked eye.5,6 However, there currently is no standard for dermoscopic training in dermatology residencies, and education varies widely.25 These data suggest that there may be a lack of dermoscopic training among dermatologists, which could accentuate the difference in performance between dermatologists and AI. Most primary care providers also lack formal dermoscopy training. Although dermoscopy has been shown to increase the diagnostic efficacy of primary care providers, this increase does not become apparent until the medical provider has had years of formal training in addition to clinical experience, which is not commonly provided in the medical training that primary care providers receive.8,26
Conclusion
It is anticipated that AI will shape the future of medicine and become incorporated into daily practice.27 Artificial intelligence will not replace physicians but rather assist clinicians and help to streamline medical care. Clinicians will take on the role of interpreting AI output and integrate it into patient care. With this advancement, it is important to highlight that for AI to improve the quality, efficiency, and accessibility of health care, clinicians must be equipped with the right training.27-29
- Cancer facts & figures 2023. American Cancer Society. Accessed April 20, 2023. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2023/2023-cancer-facts-and-figures.pdf
- Conic RZ, Cabrera CI, Khorana AA, et al. Determination of the impact of melanoma surgical timing on survival using the National Cancer Database. J Am Acad Dermatol. 2018;78:40-46.e7. doi:10.1016/j.jaad.2017.08.039
- Lallas A, Zalaudek I, Argenziano G, et al. Dermoscopy in general dermatology. Dermatol Clin. 2013;31:679-694, x. doi:10.1016/j.det.2013.06.008
- Bafounta M-L, Beauchet A, Aegerter P, et al. Is dermoscopy (epiluminescence microscopy) useful for the diagnosis of melanoma?: results of a meta-analysis using techniques adapted to the evaluation of diagnostic tests. Arch Dermatol. 2001;137:1343-1350. doi:10.1001/archderm.137.10.1343
- Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676. doi:10.1111/j.1365-2133.2008.08713.x
- Marghoob AA, Usatine RP, Jaimes N. Dermoscopy for the family physician. Am Fam Physician. 2013;88:441-450.
- Herschorn A. Dermoscopy for melanoma detection in family practice. Can Fam Physician. 2012;58:740-745, e372-8.
- Instructions for use for the Triage app. Triage website. Accessed April 20, 2023. https://www.triage.com/pdf/en/Instructions%20for%20Use.pdf
- Krizhevsky A, Sutskever I, Hinton GE. ImageNet classification with deep convolutional neural networks. In: Pereira F, Burges CJC, Bottou L, et al, eds. Advances in Neural Information Processing Systems. Vol 25. Curran Associates, Inc; 2012. Accessed April 17, 2023. https://proceedings.neurips.cc/paper/2012/file/c399862d3b9d6b76c8436e924a68c45b-Paper.pdf
- Russakovsky O, Deng J, Su H, et al. ImageNet large scale visualrecognition challenge. Int J Comput Vis. 2015;115:211-252. doi:10.1007/s11263-015-0816-y
- Hu J, Shen L, Albanie S, et al. Squeeze-and-excitation networks. IEEE Trans Patt Anal Mach Intell. 2020;42:2011-2023. doi:10.1109/TPAMI.2019.2913372
- Medical image net-radiology informatics. Stanford University Center for Artificial Intelligence in Medicine & Imaging website. Accessed April 20, 2023. https://aimi.stanford.edu/medical-imagenet
- LeCun Y, Bengio Y, Hinton G. Deep learning. Nature. 2015;521:436-444. doi:10.1038/nature14539
- Le Cun Yet al. A theoretical framework for back-propagation. In:Touretzky D, Honton G, Sejnowski T, eds. Proceedings of the 1988 Connect Models Summer School. Morgan Kaufmann; 1988:21-28.
- Lecun Y, Bottou L, Bengio Y, et al. Gradient-based learning applied to document recognition. Proc IEEE. 1998;86:2278-2324. doi:10.1109/5.726791
- Chollet E. About Keras. Keras website. Accessed April 21, 2023. https://keras.io/about/
- Introduction to TensorFlow. TensorFlow website. Accessed April 21, 2023. https://www.tensorflow.org/learn
- Kawahara J, BenTaieb A, Hamarneh G. Deep features to classify skin lesions. 2016 IEEE 13th International Symposium on Biomedical Imaging. 2016. doi:10.1109/ISBI.2016.7493528
- Lopez AR, Giro-i-Nieto X, Burdick J, et al. Skin lesion classification from dermoscopic images using deep learning techniques. doi:10.2316/P.2017.852-053
- Codella NCF, Nguyen QB, Pankanti S, et al. Deep learning ensembles for melanoma recognition in dermoscopy images. IBM J Res Dev. 2017;61:1-28. doi:10.1147/JRD.2017.2708299
- Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542:115-118. doi:10.1038/nature21056
- Sutskever I, Martens J, Dahl G, et al. On the importance of initialization and momentum in deep learning. ICML’13: Proceedings of the 30th International Conference on International Conference on Machine Learning. 2013;28:1139-1147.
- Akrout M, Farahmand AM, Jarmain T, et al. Improving skin condition classification with a visual symptom checker trained using reinforcement learning. In: Medical Image Computing and Computer Assisted Intervention – MICCAI 2019: 22nd International Conference. October 13-17, 2019. Shenzhen, China. Proceedings, Part IV. Springer-Verlag; 549-557. doi:10.1007/978-3-030-32251-9_60
- Liu Y, Jain A, Eng C, et al. A deep learning system for differential diagnosis of skin diseases. Nat Med. 2020;26:900-908. doi:10.1038/s41591-020-0842-3
- Fried LJ, Tan A, Berry EG, et al. Dermoscopy proficiency expectations for US dermatology resident physicians: results of a modified delphi survey of pigmented lesion experts. JAMA Dermatol. 2021;157:189-197. doi:10.1001/jamadermatol.2020.5213
- Fee JA, McGrady FP, Rosendahl C, et al. Training primary care physicians in dermoscopy for skin cancer detection: a scoping review. J Cancer Educ. 2020;35:643-650. doi:10.1007/s13187-019-01647-7
- James CA, Wachter RM, Woolliscroft JO. Preparing clinicians for a clinical world influenced by artificial intelligence. JAMA. 2022;327:1333-1334. doi:10.1001/jama.2022.3580
- Yu K-H, Beam AL, Kohane IS. Artificial intelligence in healthcare. Nat Biomed Eng. 2018;2:719-731. doi:10.1038/s41551-018-0305-z
- Chen M, Decary M. Artificial intelligence in healthcare: an essential guide for health leaders. Healthc Manag Forum. 2020;33:10-18. doi:10.1177/0840470419873123
- Cancer facts & figures 2023. American Cancer Society. Accessed April 20, 2023. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2023/2023-cancer-facts-and-figures.pdf
- Conic RZ, Cabrera CI, Khorana AA, et al. Determination of the impact of melanoma surgical timing on survival using the National Cancer Database. J Am Acad Dermatol. 2018;78:40-46.e7. doi:10.1016/j.jaad.2017.08.039
- Lallas A, Zalaudek I, Argenziano G, et al. Dermoscopy in general dermatology. Dermatol Clin. 2013;31:679-694, x. doi:10.1016/j.det.2013.06.008
- Bafounta M-L, Beauchet A, Aegerter P, et al. Is dermoscopy (epiluminescence microscopy) useful for the diagnosis of melanoma?: results of a meta-analysis using techniques adapted to the evaluation of diagnostic tests. Arch Dermatol. 2001;137:1343-1350. doi:10.1001/archderm.137.10.1343
- Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676. doi:10.1111/j.1365-2133.2008.08713.x
- Marghoob AA, Usatine RP, Jaimes N. Dermoscopy for the family physician. Am Fam Physician. 2013;88:441-450.
- Herschorn A. Dermoscopy for melanoma detection in family practice. Can Fam Physician. 2012;58:740-745, e372-8.
- Instructions for use for the Triage app. Triage website. Accessed April 20, 2023. https://www.triage.com/pdf/en/Instructions%20for%20Use.pdf
- Krizhevsky A, Sutskever I, Hinton GE. ImageNet classification with deep convolutional neural networks. In: Pereira F, Burges CJC, Bottou L, et al, eds. Advances in Neural Information Processing Systems. Vol 25. Curran Associates, Inc; 2012. Accessed April 17, 2023. https://proceedings.neurips.cc/paper/2012/file/c399862d3b9d6b76c8436e924a68c45b-Paper.pdf
- Russakovsky O, Deng J, Su H, et al. ImageNet large scale visualrecognition challenge. Int J Comput Vis. 2015;115:211-252. doi:10.1007/s11263-015-0816-y
- Hu J, Shen L, Albanie S, et al. Squeeze-and-excitation networks. IEEE Trans Patt Anal Mach Intell. 2020;42:2011-2023. doi:10.1109/TPAMI.2019.2913372
- Medical image net-radiology informatics. Stanford University Center for Artificial Intelligence in Medicine & Imaging website. Accessed April 20, 2023. https://aimi.stanford.edu/medical-imagenet
- LeCun Y, Bengio Y, Hinton G. Deep learning. Nature. 2015;521:436-444. doi:10.1038/nature14539
- Le Cun Yet al. A theoretical framework for back-propagation. In:Touretzky D, Honton G, Sejnowski T, eds. Proceedings of the 1988 Connect Models Summer School. Morgan Kaufmann; 1988:21-28.
- Lecun Y, Bottou L, Bengio Y, et al. Gradient-based learning applied to document recognition. Proc IEEE. 1998;86:2278-2324. doi:10.1109/5.726791
- Chollet E. About Keras. Keras website. Accessed April 21, 2023. https://keras.io/about/
- Introduction to TensorFlow. TensorFlow website. Accessed April 21, 2023. https://www.tensorflow.org/learn
- Kawahara J, BenTaieb A, Hamarneh G. Deep features to classify skin lesions. 2016 IEEE 13th International Symposium on Biomedical Imaging. 2016. doi:10.1109/ISBI.2016.7493528
- Lopez AR, Giro-i-Nieto X, Burdick J, et al. Skin lesion classification from dermoscopic images using deep learning techniques. doi:10.2316/P.2017.852-053
- Codella NCF, Nguyen QB, Pankanti S, et al. Deep learning ensembles for melanoma recognition in dermoscopy images. IBM J Res Dev. 2017;61:1-28. doi:10.1147/JRD.2017.2708299
- Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542:115-118. doi:10.1038/nature21056
- Sutskever I, Martens J, Dahl G, et al. On the importance of initialization and momentum in deep learning. ICML’13: Proceedings of the 30th International Conference on International Conference on Machine Learning. 2013;28:1139-1147.
- Akrout M, Farahmand AM, Jarmain T, et al. Improving skin condition classification with a visual symptom checker trained using reinforcement learning. In: Medical Image Computing and Computer Assisted Intervention – MICCAI 2019: 22nd International Conference. October 13-17, 2019. Shenzhen, China. Proceedings, Part IV. Springer-Verlag; 549-557. doi:10.1007/978-3-030-32251-9_60
- Liu Y, Jain A, Eng C, et al. A deep learning system for differential diagnosis of skin diseases. Nat Med. 2020;26:900-908. doi:10.1038/s41591-020-0842-3
- Fried LJ, Tan A, Berry EG, et al. Dermoscopy proficiency expectations for US dermatology resident physicians: results of a modified delphi survey of pigmented lesion experts. JAMA Dermatol. 2021;157:189-197. doi:10.1001/jamadermatol.2020.5213
- Fee JA, McGrady FP, Rosendahl C, et al. Training primary care physicians in dermoscopy for skin cancer detection: a scoping review. J Cancer Educ. 2020;35:643-650. doi:10.1007/s13187-019-01647-7
- James CA, Wachter RM, Woolliscroft JO. Preparing clinicians for a clinical world influenced by artificial intelligence. JAMA. 2022;327:1333-1334. doi:10.1001/jama.2022.3580
- Yu K-H, Beam AL, Kohane IS. Artificial intelligence in healthcare. Nat Biomed Eng. 2018;2:719-731. doi:10.1038/s41551-018-0305-z
- Chen M, Decary M. Artificial intelligence in healthcare: an essential guide for health leaders. Healthc Manag Forum. 2020;33:10-18. doi:10.1177/0840470419873123
Practice Points
- Artificial intelligence (AI) has the potential to facilitate the diagnosis of pigmented lesions and expedite the management of malignant melanoma.
- Further studies should be done to see if the high diagnostic accuracy of the AI application we studied translates to a decrease in unnecessary biopsies or expedited referral for pigmented lesions.
- The large variability of formal dermoscopy training among board-certified dermatologists may contribute to the decreased ability to identify pigmented lesions with dermoscopic imaging compared to AI.